User login
Single-use endoscopy: Here to stay?
Single-use endoscopes are becoming increasingly common, and economic and regulatory factors are driving growth and innovation in this field. Those were some of the messages presented at a session on innovations in endoscope devices at the 2022 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
“We’ve seen a shift in the need for more disposable technologies to support overall environmental parameters,” Brian Sanders, director of market development for GI at Ambu, explained during his presentation. “The more complicated these designs became, the more challenging they became to clean.”
Mr. Sanders highlighted some of the advantages of single-use scopes. There are no repair costs, and fewer storage and supply costs. They are more convenient since there is no need to wait for a scope to be cleaned. And the Food and Drug Administration has supported moving to single-use duodenoscopes. He also surmised that consumers might prefer single-use endoscopes since the risk of infection is likely to be lower than with reusable endoscopes.
Still, it can be difficult to get a full understanding of the costs of reusable versus single-use devices. Costs may be spread out across departments within a facility, and can include capital costs, repairs, reprocessing, consumables, and opportunity costs that occur due to delays. “[Many] categories are not transparent because they are hosted with cross-lateral budgets throughout the facility, so this is a messy web,” said Mr. Sanders.
Furthermore, findings from a meta-analysis of bronchoscopes indicate a 15.2% contamination rate, and an infection rate attributable to reusable bronchoscopes of 2.8%, with an average treatment cost of $11,788.
“When you consider all of the drivers we’re seeing in the endoscope arena, it’s our strong belief that, within the next 10 years, most endoscopes being utilized in medical practice will turn to single use,” Mr. Sanders continued.
During the Q&A period following the presentation, the discussion turned to the environmental impact of single-use devices. “We do a lot of endoscopies. If we start moving into single-use gastroscopes and colonoscopes, how do we process them? Can we recycle them?” asked panel moderator Sushovan Guha, MD, PhD, professor of medicine at McGovern Medical School and codirector of the Center for Interventional Gastroenterology at UTHealth Science Center, Houston.
The question drew a response from panelist Katie Eckerline, EUS group manager at Boston Scientific. She noted that the water and chemicals used in reprocessing the company’s EXALT single-use duodenoscope device are important, as is the requirement for personal protective equipment. “[T]here’s an underappreciated environmental impact that comes along with scope reprocessing because it’s not happening directly in the rooms, and this is often overlooked,” replied Ms. Eckerline.
She noted that Boston Scientific has taken steps to make EXALT duodenoscopes recyclable. The company sends used scopes to a third-party company that autoclaves them and separates the plastic from the electronics and metals. The electronics and metals can be repurposed for nonmedical use, and the plastic is recycled.
However, while Boston Scientific offers this recycling option for free, and hospital administrators and physicians often bring up the issue of environmental impact during negotiations, “only about 25% or 30% of the customers who are using EXALT choose to implement [recycling],” Ms. Eckerline explained.
Sanders noted that the FDA updated its guidance on April 4, encouraging transition to duodenoscopes that are fully disposable or have disposable components. The revision was based on new interim information from postmarketing surveillance studies, which showed that duodenoscopes with a removable component to facilitate cleaning had a contamination rate of 0.5%, compared with rates as high as 6% in older models. “It’s really almost forcing our hand at this point to move to some type of disposable option,” said Mr. Sanders.
Dr. Guha has consulted for Medtronic.
Single-use endoscopes are becoming increasingly common, and economic and regulatory factors are driving growth and innovation in this field. Those were some of the messages presented at a session on innovations in endoscope devices at the 2022 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
“We’ve seen a shift in the need for more disposable technologies to support overall environmental parameters,” Brian Sanders, director of market development for GI at Ambu, explained during his presentation. “The more complicated these designs became, the more challenging they became to clean.”
Mr. Sanders highlighted some of the advantages of single-use scopes. There are no repair costs, and fewer storage and supply costs. They are more convenient since there is no need to wait for a scope to be cleaned. And the Food and Drug Administration has supported moving to single-use duodenoscopes. He also surmised that consumers might prefer single-use endoscopes since the risk of infection is likely to be lower than with reusable endoscopes.
Still, it can be difficult to get a full understanding of the costs of reusable versus single-use devices. Costs may be spread out across departments within a facility, and can include capital costs, repairs, reprocessing, consumables, and opportunity costs that occur due to delays. “[Many] categories are not transparent because they are hosted with cross-lateral budgets throughout the facility, so this is a messy web,” said Mr. Sanders.
Furthermore, findings from a meta-analysis of bronchoscopes indicate a 15.2% contamination rate, and an infection rate attributable to reusable bronchoscopes of 2.8%, with an average treatment cost of $11,788.
“When you consider all of the drivers we’re seeing in the endoscope arena, it’s our strong belief that, within the next 10 years, most endoscopes being utilized in medical practice will turn to single use,” Mr. Sanders continued.
During the Q&A period following the presentation, the discussion turned to the environmental impact of single-use devices. “We do a lot of endoscopies. If we start moving into single-use gastroscopes and colonoscopes, how do we process them? Can we recycle them?” asked panel moderator Sushovan Guha, MD, PhD, professor of medicine at McGovern Medical School and codirector of the Center for Interventional Gastroenterology at UTHealth Science Center, Houston.
The question drew a response from panelist Katie Eckerline, EUS group manager at Boston Scientific. She noted that the water and chemicals used in reprocessing the company’s EXALT single-use duodenoscope device are important, as is the requirement for personal protective equipment. “[T]here’s an underappreciated environmental impact that comes along with scope reprocessing because it’s not happening directly in the rooms, and this is often overlooked,” replied Ms. Eckerline.
She noted that Boston Scientific has taken steps to make EXALT duodenoscopes recyclable. The company sends used scopes to a third-party company that autoclaves them and separates the plastic from the electronics and metals. The electronics and metals can be repurposed for nonmedical use, and the plastic is recycled.
However, while Boston Scientific offers this recycling option for free, and hospital administrators and physicians often bring up the issue of environmental impact during negotiations, “only about 25% or 30% of the customers who are using EXALT choose to implement [recycling],” Ms. Eckerline explained.
Sanders noted that the FDA updated its guidance on April 4, encouraging transition to duodenoscopes that are fully disposable or have disposable components. The revision was based on new interim information from postmarketing surveillance studies, which showed that duodenoscopes with a removable component to facilitate cleaning had a contamination rate of 0.5%, compared with rates as high as 6% in older models. “It’s really almost forcing our hand at this point to move to some type of disposable option,” said Mr. Sanders.
Dr. Guha has consulted for Medtronic.
Single-use endoscopes are becoming increasingly common, and economic and regulatory factors are driving growth and innovation in this field. Those were some of the messages presented at a session on innovations in endoscope devices at the 2022 AGA Tech Summit, sponsored by the AGA Center for GI Innovation and Technology.
“We’ve seen a shift in the need for more disposable technologies to support overall environmental parameters,” Brian Sanders, director of market development for GI at Ambu, explained during his presentation. “The more complicated these designs became, the more challenging they became to clean.”
Mr. Sanders highlighted some of the advantages of single-use scopes. There are no repair costs, and fewer storage and supply costs. They are more convenient since there is no need to wait for a scope to be cleaned. And the Food and Drug Administration has supported moving to single-use duodenoscopes. He also surmised that consumers might prefer single-use endoscopes since the risk of infection is likely to be lower than with reusable endoscopes.
Still, it can be difficult to get a full understanding of the costs of reusable versus single-use devices. Costs may be spread out across departments within a facility, and can include capital costs, repairs, reprocessing, consumables, and opportunity costs that occur due to delays. “[Many] categories are not transparent because they are hosted with cross-lateral budgets throughout the facility, so this is a messy web,” said Mr. Sanders.
Furthermore, findings from a meta-analysis of bronchoscopes indicate a 15.2% contamination rate, and an infection rate attributable to reusable bronchoscopes of 2.8%, with an average treatment cost of $11,788.
“When you consider all of the drivers we’re seeing in the endoscope arena, it’s our strong belief that, within the next 10 years, most endoscopes being utilized in medical practice will turn to single use,” Mr. Sanders continued.
During the Q&A period following the presentation, the discussion turned to the environmental impact of single-use devices. “We do a lot of endoscopies. If we start moving into single-use gastroscopes and colonoscopes, how do we process them? Can we recycle them?” asked panel moderator Sushovan Guha, MD, PhD, professor of medicine at McGovern Medical School and codirector of the Center for Interventional Gastroenterology at UTHealth Science Center, Houston.
The question drew a response from panelist Katie Eckerline, EUS group manager at Boston Scientific. She noted that the water and chemicals used in reprocessing the company’s EXALT single-use duodenoscope device are important, as is the requirement for personal protective equipment. “[T]here’s an underappreciated environmental impact that comes along with scope reprocessing because it’s not happening directly in the rooms, and this is often overlooked,” replied Ms. Eckerline.
She noted that Boston Scientific has taken steps to make EXALT duodenoscopes recyclable. The company sends used scopes to a third-party company that autoclaves them and separates the plastic from the electronics and metals. The electronics and metals can be repurposed for nonmedical use, and the plastic is recycled.
However, while Boston Scientific offers this recycling option for free, and hospital administrators and physicians often bring up the issue of environmental impact during negotiations, “only about 25% or 30% of the customers who are using EXALT choose to implement [recycling],” Ms. Eckerline explained.
Sanders noted that the FDA updated its guidance on April 4, encouraging transition to duodenoscopes that are fully disposable or have disposable components. The revision was based on new interim information from postmarketing surveillance studies, which showed that duodenoscopes with a removable component to facilitate cleaning had a contamination rate of 0.5%, compared with rates as high as 6% in older models. “It’s really almost forcing our hand at this point to move to some type of disposable option,” said Mr. Sanders.
Dr. Guha has consulted for Medtronic.
FROM THE 2022 AGA TECH SUMMIT
Focal hair loss
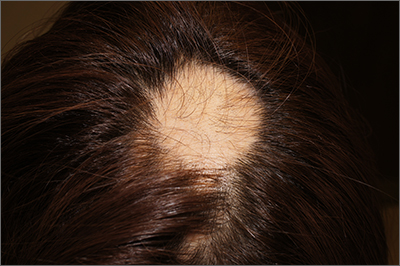
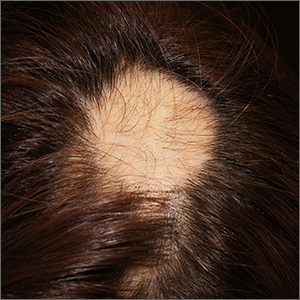
The findings of smooth, round alopecia occurring rapidly without associated scarring, pain, or itching, is consistent with the diagnosis of alopecia areata.
Alopecia areata is a common autoimmune disease caused by T lymphocytes targeting hair follicles and resulting in rapid and nonscarring hair loss. It is usually self-resolving and about 2% of all individuals are affected at some point during their lifetime, with an average age of onset of 33 years.1 Some patients may progress to loss of all scalp hair (alopecia totalis) or all hair on the scalp and body (alopecia universalis).1
It is important to inspect a patient’s scalp, face, and body for more subtle areas of loss that could signal other disorders, such as lichen planopilaris, discoid lupus, or telogen effluvium. It is worth noting that alopecia areata is not associated with scalp lesions, crusting, or scars without follicles. Such findings should be further investigated with a 4-mm punch biopsy of affected and adjacent follicular units. Carefully labeling biopsy specimens as scalp specimens for hair loss will aid in a correct histopathologic diagnosis.
Systematic data comparing treatments for alopecia areata are lacking. For localized disease, topical or intradermal triamcinolone injections at a concentration of 5 to 10 mg/mL, with about 0.1 mL to 0.05 mL injected every square centimeter of affected area (up to 40 mg per visit), can provide rapid regrowth.1 Within 4 months of the monthly injections, 63% of patients experience complete regrowth.1 Despite this favorable outcome, there is also a high rate of recurrence.
For more widespread disease, contact immunotherapy with squaric acid dibutyl ester or diphencyprone can provoke a low-grade contact allergy and induce antigenic completion. This therapy is painless but can be itchy; medications must be compounded and titrated to activity.
The patient in this case opted to receive monthly triamcinolone injections in an undiluted concentration of 10 mg/mL for 3 months, at which point she experienced excellent hair regrowth. A small patch of recurrence was noted a year later and treated twice with monthly triamcinolone injections.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Darwin E, Hirt PA, Fertig R, et al. Alopecia areata: review of epidemiology, clinical features, pathogenesis, and new treatment options. Int J Trichology. 2018;10:51-60. doi: 10.4103/ijt.ijt_99_17

The findings of smooth, round alopecia occurring rapidly without associated scarring, pain, or itching, is consistent with the diagnosis of alopecia areata.
Alopecia areata is a common autoimmune disease caused by T lymphocytes targeting hair follicles and resulting in rapid and nonscarring hair loss. It is usually self-resolving and about 2% of all individuals are affected at some point during their lifetime, with an average age of onset of 33 years.1 Some patients may progress to loss of all scalp hair (alopecia totalis) or all hair on the scalp and body (alopecia universalis).1
It is important to inspect a patient’s scalp, face, and body for more subtle areas of loss that could signal other disorders, such as lichen planopilaris, discoid lupus, or telogen effluvium. It is worth noting that alopecia areata is not associated with scalp lesions, crusting, or scars without follicles. Such findings should be further investigated with a 4-mm punch biopsy of affected and adjacent follicular units. Carefully labeling biopsy specimens as scalp specimens for hair loss will aid in a correct histopathologic diagnosis.
Systematic data comparing treatments for alopecia areata are lacking. For localized disease, topical or intradermal triamcinolone injections at a concentration of 5 to 10 mg/mL, with about 0.1 mL to 0.05 mL injected every square centimeter of affected area (up to 40 mg per visit), can provide rapid regrowth.1 Within 4 months of the monthly injections, 63% of patients experience complete regrowth.1 Despite this favorable outcome, there is also a high rate of recurrence.
For more widespread disease, contact immunotherapy with squaric acid dibutyl ester or diphencyprone can provoke a low-grade contact allergy and induce antigenic completion. This therapy is painless but can be itchy; medications must be compounded and titrated to activity.
The patient in this case opted to receive monthly triamcinolone injections in an undiluted concentration of 10 mg/mL for 3 months, at which point she experienced excellent hair regrowth. A small patch of recurrence was noted a year later and treated twice with monthly triamcinolone injections.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

The findings of smooth, round alopecia occurring rapidly without associated scarring, pain, or itching, is consistent with the diagnosis of alopecia areata.
Alopecia areata is a common autoimmune disease caused by T lymphocytes targeting hair follicles and resulting in rapid and nonscarring hair loss. It is usually self-resolving and about 2% of all individuals are affected at some point during their lifetime, with an average age of onset of 33 years.1 Some patients may progress to loss of all scalp hair (alopecia totalis) or all hair on the scalp and body (alopecia universalis).1
It is important to inspect a patient’s scalp, face, and body for more subtle areas of loss that could signal other disorders, such as lichen planopilaris, discoid lupus, or telogen effluvium. It is worth noting that alopecia areata is not associated with scalp lesions, crusting, or scars without follicles. Such findings should be further investigated with a 4-mm punch biopsy of affected and adjacent follicular units. Carefully labeling biopsy specimens as scalp specimens for hair loss will aid in a correct histopathologic diagnosis.
Systematic data comparing treatments for alopecia areata are lacking. For localized disease, topical or intradermal triamcinolone injections at a concentration of 5 to 10 mg/mL, with about 0.1 mL to 0.05 mL injected every square centimeter of affected area (up to 40 mg per visit), can provide rapid regrowth.1 Within 4 months of the monthly injections, 63% of patients experience complete regrowth.1 Despite this favorable outcome, there is also a high rate of recurrence.
For more widespread disease, contact immunotherapy with squaric acid dibutyl ester or diphencyprone can provoke a low-grade contact allergy and induce antigenic completion. This therapy is painless but can be itchy; medications must be compounded and titrated to activity.
The patient in this case opted to receive monthly triamcinolone injections in an undiluted concentration of 10 mg/mL for 3 months, at which point she experienced excellent hair regrowth. A small patch of recurrence was noted a year later and treated twice with monthly triamcinolone injections.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Darwin E, Hirt PA, Fertig R, et al. Alopecia areata: review of epidemiology, clinical features, pathogenesis, and new treatment options. Int J Trichology. 2018;10:51-60. doi: 10.4103/ijt.ijt_99_17
1. Darwin E, Hirt PA, Fertig R, et al. Alopecia areata: review of epidemiology, clinical features, pathogenesis, and new treatment options. Int J Trichology. 2018;10:51-60. doi: 10.4103/ijt.ijt_99_17
Mood instability in childhood as a precursor to bipolar disorder
Mood instability, or sudden, unpredictable, and frequent shifts in emotional states, characterizes many types of psychiatric disorder, including attention-deficit/hyperactivity disorder (ADHD), personality disorders, depression, and posttraumatic stress disorder. To say that individuals with bipolar disorder (BD) have mood instability sounds like a tautology. Nonetheless, mood instability has particular relevance to BD: Many patients have irregular or labile moods even when they are between major episodes of mania and depression.1
Children of parents with BD who have high levels of mood instability are at particularly high risk for developing BD (types I or II) in late adolescence or early adulthood.2 The following case provides an illustration:
Patrick, age 14, entered treatment with diagnoses of ADHD and other specified bipolar disorder. His mother felt that his behavior resembled that of his father, who had been treated for manic episodes. During the COVID-19 pandemic, Patrick had become increasingly difficult at home, with significant oppositionality, impulsive behavior, and difficulty following through on school assignments or household tasks. His mother’s most significant complaints concerned Patrick’s sudden outbursts of anger and abrupt verbal abuse when she asked him to stop playing video games. When interrupted, he cursed loudly and sometimes turned violent; he had broken a window and a door at home and had on one occasion physically attacked his younger brother. Patrick agreed that he became angry at times, but felt that others provoked him. When queried about depression, he described anxiety and worry. He was unable to describe a particular trigger for his anxiety except for being interrupted in online games with his friends, which made him “feel like a total loser.”
His mother reported that Patrick had multiple 1- to 2-day intervals in which he became “really silly, laughing at nothing,” talking rapidly, jumping from one topic to another, and becoming annoyed when others didn’t share his enthusiasm. In these activated intervals, he slept little and seemed to be full of energy; his mother would hear him talking loudly into his phone throughout the night. During one such interval he had become verbally aggressive with a peer, which had ruined their friendship. Both Patrick and his mother reported that they had been fighting constantly and, in her words, “our house has become a war zone.”
In our recent article in the Journal of the American Academy of Child and Adolescent Psychiatry,3 my coauthors and I examined the association between parents’ ratings of mood instability and clinicians’ longitudinal ratings of symptoms and functioning among youth (ages 9-17 years) who were at high risk for BD. The participants met DSM-5 diagnostic criteria for major depressive disorder or other specified BD, defined as recurrent and brief periods of elevation and activation that did not meet syndromal mania or hypomania criteria. All participants had at least one first- or second-degree family member with a history of BD I or II. Following a period of evaluation, participants were randomly assigned to one of two 4-month psychological therapies: Family-focused therapy (12 sessions of psychoeducation, communication training, and problem-solving skills training) or enhanced usual care (6 sessions of family and individual psychoeducation and support). They also received pharmacological management from study-affiliated psychiatrists when warranted.
We measured mood instability at intake and every 4-6 months over an average of 2 years (range 0-255 weeks). We used a brief parent questionnaire – the Children’s Affective Lability Scale4 – which enables measurement of lability on the dimensions of elevation or activation (e.g., bursts of silliness or hilarity, excessive familiarity with others), irritability (e.g., temper outbursts), or anxious-depression (e.g., sudden bouts of crying).
Over the 1- to 4-year period of follow-up, mood instability was associated with poor prognosis indicators in high-risk youth: Being younger, having younger ages at first symptom onset, being diagnosed with other specified BD (vs. major depression), and having more complex patterns of comorbid disorders. Mood instability tracked closely with levels of mania, depression, and global functioning over the follow-up. There was a temporal pathway between a diagnosis of other specified bipolar disorder at intake and higher levels of mood instability at follow-up, which in turn predicted higher levels of parent/child conflict. High levels of mood lability may lead to isolation from peers and tension within family relationships, which may fuel further children’s expressions of frustration, rage, depression, or impulsive behavior.
Youth with higher levels of mood instability required more complex medication regimens over 1 year than did those with lower instability. There was an overall reduction in mood instability as children aged (or spent more time in treatment). Over the 1- to 4-year follow-up, family-focused therapy was associated with longer intervals prior to new mood episodes than was enhanced usual care, but reductions in mood instability were independent of the type of psychosocial treatment assigned to children.
The participants in this study could not be followed long enough to determine whether levels of mood instability were associated with the later development of syndromal BD. Other studies, however, have documented this relationship. Large-scale longitudinal studies of high-risk children find that measures of mood lability – along with early onset manic symptoms, depression, anxiety, and a family history of mania or hypomania – can be combined to calculate the risk that any individual child will develop BD I or II over the next 5-8 years.2,5
Clinicians should include measurement of the severity and psychosocial determinants of persistent mood shifts in youth under their care, particularly those with a family history of BD. Mood instability is associated with more severe symptom trajectories, more social isolation, and greater distress and conflict within the family. It may require a greater intensity of both pharmacological and psychosocial treatments to treat existing symptoms and functional impairments, and to prevent further mood deterioration.
Dr. Miklowitz is Distinguished Professor of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Semel Institute for Neuroscience and Human Behavior. He is the author of “The Bipolar Disorder Survival Guide, 3rd Ed.” (New York: Guilford Press, 2019) and “Bipolar Disorder: A Family-Focused Treatment Approach, 2nd Ed” (New York: Guilford Press, 2010). He has no conflicts of interest to disclose. Contact Dr. Miklowitz at [email protected].
References
1. Bonsall MB, et al. Nonlinear time-series approaches in characterizing mood stability and mood instability in bipolar disorder. Proc Biol Sci. Mar 7 2012;279(1730):916-24. doi: 10.1098/rspb.2011.1246.
2. Hafeman DM, et al. Toward the definition of a bipolar prodrome: Dimensional predictors of bipolar spectrum disorders in at-risk youths. Am J Psychiatry. 2016;173(7):695-704. doi: 10.1176/appi.ajp.2015.15040414.
3. Miklowitz DJ, et al. Mood instability in youth at high risk for bipolar disorder. J Am Acad Child Adol Psychiatry. 2022 Mar 17;S0890-8567(22)00118-6. doi: 10.1016/j.jaac.2022.03.009.
4. Gerson AC, et al. The Children’s Affective Lability Scale: a psychometric evaluation of reliability. Psychiatry Res. Dec 20 1996;65(3):189-98. doi: 10.1016/s0165-1781(96)02851-x.
5. Birmaher B, et al. A risk calculator to predict the individual risk of conversion from subthreshold bipolar symptoms to bipolar disorder I or II in youth. J Am Acad Child Adol Psychiatry. 2018;57(10):755-63. doi: 10.1016/j.jaac.2018.05.023.
Mood instability, or sudden, unpredictable, and frequent shifts in emotional states, characterizes many types of psychiatric disorder, including attention-deficit/hyperactivity disorder (ADHD), personality disorders, depression, and posttraumatic stress disorder. To say that individuals with bipolar disorder (BD) have mood instability sounds like a tautology. Nonetheless, mood instability has particular relevance to BD: Many patients have irregular or labile moods even when they are between major episodes of mania and depression.1
Children of parents with BD who have high levels of mood instability are at particularly high risk for developing BD (types I or II) in late adolescence or early adulthood.2 The following case provides an illustration:
Patrick, age 14, entered treatment with diagnoses of ADHD and other specified bipolar disorder. His mother felt that his behavior resembled that of his father, who had been treated for manic episodes. During the COVID-19 pandemic, Patrick had become increasingly difficult at home, with significant oppositionality, impulsive behavior, and difficulty following through on school assignments or household tasks. His mother’s most significant complaints concerned Patrick’s sudden outbursts of anger and abrupt verbal abuse when she asked him to stop playing video games. When interrupted, he cursed loudly and sometimes turned violent; he had broken a window and a door at home and had on one occasion physically attacked his younger brother. Patrick agreed that he became angry at times, but felt that others provoked him. When queried about depression, he described anxiety and worry. He was unable to describe a particular trigger for his anxiety except for being interrupted in online games with his friends, which made him “feel like a total loser.”
His mother reported that Patrick had multiple 1- to 2-day intervals in which he became “really silly, laughing at nothing,” talking rapidly, jumping from one topic to another, and becoming annoyed when others didn’t share his enthusiasm. In these activated intervals, he slept little and seemed to be full of energy; his mother would hear him talking loudly into his phone throughout the night. During one such interval he had become verbally aggressive with a peer, which had ruined their friendship. Both Patrick and his mother reported that they had been fighting constantly and, in her words, “our house has become a war zone.”
In our recent article in the Journal of the American Academy of Child and Adolescent Psychiatry,3 my coauthors and I examined the association between parents’ ratings of mood instability and clinicians’ longitudinal ratings of symptoms and functioning among youth (ages 9-17 years) who were at high risk for BD. The participants met DSM-5 diagnostic criteria for major depressive disorder or other specified BD, defined as recurrent and brief periods of elevation and activation that did not meet syndromal mania or hypomania criteria. All participants had at least one first- or second-degree family member with a history of BD I or II. Following a period of evaluation, participants were randomly assigned to one of two 4-month psychological therapies: Family-focused therapy (12 sessions of psychoeducation, communication training, and problem-solving skills training) or enhanced usual care (6 sessions of family and individual psychoeducation and support). They also received pharmacological management from study-affiliated psychiatrists when warranted.
We measured mood instability at intake and every 4-6 months over an average of 2 years (range 0-255 weeks). We used a brief parent questionnaire – the Children’s Affective Lability Scale4 – which enables measurement of lability on the dimensions of elevation or activation (e.g., bursts of silliness or hilarity, excessive familiarity with others), irritability (e.g., temper outbursts), or anxious-depression (e.g., sudden bouts of crying).
Over the 1- to 4-year period of follow-up, mood instability was associated with poor prognosis indicators in high-risk youth: Being younger, having younger ages at first symptom onset, being diagnosed with other specified BD (vs. major depression), and having more complex patterns of comorbid disorders. Mood instability tracked closely with levels of mania, depression, and global functioning over the follow-up. There was a temporal pathway between a diagnosis of other specified bipolar disorder at intake and higher levels of mood instability at follow-up, which in turn predicted higher levels of parent/child conflict. High levels of mood lability may lead to isolation from peers and tension within family relationships, which may fuel further children’s expressions of frustration, rage, depression, or impulsive behavior.
Youth with higher levels of mood instability required more complex medication regimens over 1 year than did those with lower instability. There was an overall reduction in mood instability as children aged (or spent more time in treatment). Over the 1- to 4-year follow-up, family-focused therapy was associated with longer intervals prior to new mood episodes than was enhanced usual care, but reductions in mood instability were independent of the type of psychosocial treatment assigned to children.
The participants in this study could not be followed long enough to determine whether levels of mood instability were associated with the later development of syndromal BD. Other studies, however, have documented this relationship. Large-scale longitudinal studies of high-risk children find that measures of mood lability – along with early onset manic symptoms, depression, anxiety, and a family history of mania or hypomania – can be combined to calculate the risk that any individual child will develop BD I or II over the next 5-8 years.2,5
Clinicians should include measurement of the severity and psychosocial determinants of persistent mood shifts in youth under their care, particularly those with a family history of BD. Mood instability is associated with more severe symptom trajectories, more social isolation, and greater distress and conflict within the family. It may require a greater intensity of both pharmacological and psychosocial treatments to treat existing symptoms and functional impairments, and to prevent further mood deterioration.
Dr. Miklowitz is Distinguished Professor of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Semel Institute for Neuroscience and Human Behavior. He is the author of “The Bipolar Disorder Survival Guide, 3rd Ed.” (New York: Guilford Press, 2019) and “Bipolar Disorder: A Family-Focused Treatment Approach, 2nd Ed” (New York: Guilford Press, 2010). He has no conflicts of interest to disclose. Contact Dr. Miklowitz at [email protected].
References
1. Bonsall MB, et al. Nonlinear time-series approaches in characterizing mood stability and mood instability in bipolar disorder. Proc Biol Sci. Mar 7 2012;279(1730):916-24. doi: 10.1098/rspb.2011.1246.
2. Hafeman DM, et al. Toward the definition of a bipolar prodrome: Dimensional predictors of bipolar spectrum disorders in at-risk youths. Am J Psychiatry. 2016;173(7):695-704. doi: 10.1176/appi.ajp.2015.15040414.
3. Miklowitz DJ, et al. Mood instability in youth at high risk for bipolar disorder. J Am Acad Child Adol Psychiatry. 2022 Mar 17;S0890-8567(22)00118-6. doi: 10.1016/j.jaac.2022.03.009.
4. Gerson AC, et al. The Children’s Affective Lability Scale: a psychometric evaluation of reliability. Psychiatry Res. Dec 20 1996;65(3):189-98. doi: 10.1016/s0165-1781(96)02851-x.
5. Birmaher B, et al. A risk calculator to predict the individual risk of conversion from subthreshold bipolar symptoms to bipolar disorder I or II in youth. J Am Acad Child Adol Psychiatry. 2018;57(10):755-63. doi: 10.1016/j.jaac.2018.05.023.
Mood instability, or sudden, unpredictable, and frequent shifts in emotional states, characterizes many types of psychiatric disorder, including attention-deficit/hyperactivity disorder (ADHD), personality disorders, depression, and posttraumatic stress disorder. To say that individuals with bipolar disorder (BD) have mood instability sounds like a tautology. Nonetheless, mood instability has particular relevance to BD: Many patients have irregular or labile moods even when they are between major episodes of mania and depression.1
Children of parents with BD who have high levels of mood instability are at particularly high risk for developing BD (types I or II) in late adolescence or early adulthood.2 The following case provides an illustration:
Patrick, age 14, entered treatment with diagnoses of ADHD and other specified bipolar disorder. His mother felt that his behavior resembled that of his father, who had been treated for manic episodes. During the COVID-19 pandemic, Patrick had become increasingly difficult at home, with significant oppositionality, impulsive behavior, and difficulty following through on school assignments or household tasks. His mother’s most significant complaints concerned Patrick’s sudden outbursts of anger and abrupt verbal abuse when she asked him to stop playing video games. When interrupted, he cursed loudly and sometimes turned violent; he had broken a window and a door at home and had on one occasion physically attacked his younger brother. Patrick agreed that he became angry at times, but felt that others provoked him. When queried about depression, he described anxiety and worry. He was unable to describe a particular trigger for his anxiety except for being interrupted in online games with his friends, which made him “feel like a total loser.”
His mother reported that Patrick had multiple 1- to 2-day intervals in which he became “really silly, laughing at nothing,” talking rapidly, jumping from one topic to another, and becoming annoyed when others didn’t share his enthusiasm. In these activated intervals, he slept little and seemed to be full of energy; his mother would hear him talking loudly into his phone throughout the night. During one such interval he had become verbally aggressive with a peer, which had ruined their friendship. Both Patrick and his mother reported that they had been fighting constantly and, in her words, “our house has become a war zone.”
In our recent article in the Journal of the American Academy of Child and Adolescent Psychiatry,3 my coauthors and I examined the association between parents’ ratings of mood instability and clinicians’ longitudinal ratings of symptoms and functioning among youth (ages 9-17 years) who were at high risk for BD. The participants met DSM-5 diagnostic criteria for major depressive disorder or other specified BD, defined as recurrent and brief periods of elevation and activation that did not meet syndromal mania or hypomania criteria. All participants had at least one first- or second-degree family member with a history of BD I or II. Following a period of evaluation, participants were randomly assigned to one of two 4-month psychological therapies: Family-focused therapy (12 sessions of psychoeducation, communication training, and problem-solving skills training) or enhanced usual care (6 sessions of family and individual psychoeducation and support). They also received pharmacological management from study-affiliated psychiatrists when warranted.
We measured mood instability at intake and every 4-6 months over an average of 2 years (range 0-255 weeks). We used a brief parent questionnaire – the Children’s Affective Lability Scale4 – which enables measurement of lability on the dimensions of elevation or activation (e.g., bursts of silliness or hilarity, excessive familiarity with others), irritability (e.g., temper outbursts), or anxious-depression (e.g., sudden bouts of crying).
Over the 1- to 4-year period of follow-up, mood instability was associated with poor prognosis indicators in high-risk youth: Being younger, having younger ages at first symptom onset, being diagnosed with other specified BD (vs. major depression), and having more complex patterns of comorbid disorders. Mood instability tracked closely with levels of mania, depression, and global functioning over the follow-up. There was a temporal pathway between a diagnosis of other specified bipolar disorder at intake and higher levels of mood instability at follow-up, which in turn predicted higher levels of parent/child conflict. High levels of mood lability may lead to isolation from peers and tension within family relationships, which may fuel further children’s expressions of frustration, rage, depression, or impulsive behavior.
Youth with higher levels of mood instability required more complex medication regimens over 1 year than did those with lower instability. There was an overall reduction in mood instability as children aged (or spent more time in treatment). Over the 1- to 4-year follow-up, family-focused therapy was associated with longer intervals prior to new mood episodes than was enhanced usual care, but reductions in mood instability were independent of the type of psychosocial treatment assigned to children.
The participants in this study could not be followed long enough to determine whether levels of mood instability were associated with the later development of syndromal BD. Other studies, however, have documented this relationship. Large-scale longitudinal studies of high-risk children find that measures of mood lability – along with early onset manic symptoms, depression, anxiety, and a family history of mania or hypomania – can be combined to calculate the risk that any individual child will develop BD I or II over the next 5-8 years.2,5
Clinicians should include measurement of the severity and psychosocial determinants of persistent mood shifts in youth under their care, particularly those with a family history of BD. Mood instability is associated with more severe symptom trajectories, more social isolation, and greater distress and conflict within the family. It may require a greater intensity of both pharmacological and psychosocial treatments to treat existing symptoms and functional impairments, and to prevent further mood deterioration.
Dr. Miklowitz is Distinguished Professor of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, Semel Institute for Neuroscience and Human Behavior. He is the author of “The Bipolar Disorder Survival Guide, 3rd Ed.” (New York: Guilford Press, 2019) and “Bipolar Disorder: A Family-Focused Treatment Approach, 2nd Ed” (New York: Guilford Press, 2010). He has no conflicts of interest to disclose. Contact Dr. Miklowitz at [email protected].
References
1. Bonsall MB, et al. Nonlinear time-series approaches in characterizing mood stability and mood instability in bipolar disorder. Proc Biol Sci. Mar 7 2012;279(1730):916-24. doi: 10.1098/rspb.2011.1246.
2. Hafeman DM, et al. Toward the definition of a bipolar prodrome: Dimensional predictors of bipolar spectrum disorders in at-risk youths. Am J Psychiatry. 2016;173(7):695-704. doi: 10.1176/appi.ajp.2015.15040414.
3. Miklowitz DJ, et al. Mood instability in youth at high risk for bipolar disorder. J Am Acad Child Adol Psychiatry. 2022 Mar 17;S0890-8567(22)00118-6. doi: 10.1016/j.jaac.2022.03.009.
4. Gerson AC, et al. The Children’s Affective Lability Scale: a psychometric evaluation of reliability. Psychiatry Res. Dec 20 1996;65(3):189-98. doi: 10.1016/s0165-1781(96)02851-x.
5. Birmaher B, et al. A risk calculator to predict the individual risk of conversion from subthreshold bipolar symptoms to bipolar disorder I or II in youth. J Am Acad Child Adol Psychiatry. 2018;57(10):755-63. doi: 10.1016/j.jaac.2018.05.023.
Endoscopic ultrasound survives the sharks at AGA Tech Summit
After a 3-year, pandemic-induced hiatus, the American Gastroenterological Association’s Tech Summit returned to a live meeting in San Francisco. As usual, the highlight of the 2-day event, which is sponsored by the AGA Center for GI Innovation and Technology, was the Shark Tank, where selected companies presented lightning-round overviews of their technology and business plans. A panel of sharks and the audience voted for their favorite.
The contestants presented technologies such as a cell phone app to improve gut health (Agora Health), a polypectomy suite (IzoMed), an implantable weight-loss device (Lean Medical), a device to alleviate gastric obstruction in pancreatic cancer (Myka Labs), a pill designed to map out the gastrointestinal system to aid in diagnosis (Rock West Medical Devices), and an endoscopic ultrasound device (EndoSound).
Six finalists were selected from 20 submissions, and EndoSound was the winner. According to Raman Muthusamy, MD, medical director of endoscopy at UCLA Health and professor of clinical medicine at the University of California, Los Angeles, and past chair of the AGA Center for GI Innovation and Technology, the quality of presentations and the sophistication of the companies have increased year after year. “This was really the very best,” said Dr. Muthusamy.
Both the judges and the audience chose EndoSound. Endoscopic ultrasound (EUS) focuses on diagnosis and treatment of chest and abdomen disorders, particularly the pancreas. The EndoSound device attaches to an upper endoscope and converts it to a fully therapeutic endoscope that can perform all standard EUS procedures. Moreover, it does not use an elevator, which has been linked to infection risk.
Most clinical facilities lack EUS capability: 97% of ambulatory surgical centers and 80% of hospitals. EUS systems have hardly changed since the late 20th century, and they cost about $450,000. The projected cost of the EndoSound device is closer to $50,000.
“Just like colonoscopies and upper endoscopies, most endoscopic ultrasounds ought to be done in surgical centers. The idea that they can do them efficiently, and at lower cost and greater convenience to their patients and themselves, seems to me the way everything is going, and the way this procedure ought to go as well. The only obstacle to that has been the cost of the equipment. If we can take away that obstacle, then people who are already doing procedures in hospitals where it’s not convenient and not efficient, will be able to do the procedures in surgical centers,” said Stephen Steinberg, MD, founder and President of EndoSound.
“It’s a radical redesign. You’ve cut cost and you’ve cut space. And it’s something that could be put on at a moment’s notice. Rather than referring the patient for [ultrasound], it could allow you to do it on the spot, and perhaps save a second trip for a patient. It allows flexibility in terms of site of service,” said Dr. Muthusamy.
Dr. Muthusamy called it a “godsend” for low-resource institutions in the United States or abroad who have the expertise, but not the equipment, to perform EUS. “There’s no question that more EUS procedures could be done than are currently being done because of issues of availability, and this device takes a significant step to alleviate that.”
The Food and Drug Administration has granted a breakthrough device designation to EndoSound, which allows the company to forgo human clinical trials to support the application. “We’re hoping and expecting to have our application in the beginning of the fourth quarter, and with a little bit of luck to be approved by the end of the year. That’s our goal,” said Dr. Steinberg.
The technology started out as a challenge that Dr. Steinberg set for himself. His career overlapped with some of the earliest innovators of therapeutic endoscopy. “They were the stars. I wasn’t, but I was there,” said Dr. Steinberg. In his practice, Dr. Steinberg was doing procedures that included endoscopic ultrasound.
By the new millennium, EUS had gained a lot of interest, but there was a problem. “It was expensive, and it could only be done in hospitals. I started wondering if we couldn’t get it into a different environment by having a simpler solution,” said Dr. Steinberg.
But success didn’t come quickly. “I started drawing on the back of napkins to see if there wasn’t some solution,” said Dr. Steinberg. It wasn’t until a serendipitous meeting occurred that the concept took shape. Dr. Steinberg’s wife was the CEO and provost of Oregon Health Sciences University, Portland, as well as head of the technology transfer program. Dr. Steinberg’s practice, however, was in Florida so he commuted to Oregon every weekend.
One day, she told him about a presentation by Scott Corbett, MD. “My wife said: ‘Hey, they’re doing ultrasound. Why don’t you come and sit in [on the meeting] because I don’t know anything about it.’ [Dr.] Corbett was working with Sonivate, a point-of-care ultrasound company that was developing an ultrasound that could be placed over the end of the finger, to be used in battlefield triage. I thought, well, if you could put it on a finger, why couldn’t you put it on a scope? So, Scott and I got to talking, and went through a couple of iterations that didn’t work, and then finally came up with one that seemed like it was suitable.”
The device has been tested in five animal models with 20 EUS physicians who concluded that the images were equivalent to legacy devices and that they could be adopted quickly. The company also presented results from a human study that demonstrated noninferiority to the latest EUS system from Pentax.
Dr. Steinberg is an employee and stockholder of Sonivate. Dr. Muthusamy has no relevant financial disclosures. The 2022 AGA Tech Summit was supported by independent grants from Castle Biosciences, Medtronic, Boston Scientific, Exact Sciences, Olympus, 3-D Matrix, Apollo Endosurgery, Motus GI Holdings, STERIS Endoscopy, Cook Medical, FUJIFILM Healthcare Americas, and Virgo.
This article was updated 5/10/22.
*Correction, 5/17/22: An earlier version of this article stated that Geneoscopy was a finalist in the competition. It was not. Also, EndoSound should have been listed as a finalist in this paragraph.
After a 3-year, pandemic-induced hiatus, the American Gastroenterological Association’s Tech Summit returned to a live meeting in San Francisco. As usual, the highlight of the 2-day event, which is sponsored by the AGA Center for GI Innovation and Technology, was the Shark Tank, where selected companies presented lightning-round overviews of their technology and business plans. A panel of sharks and the audience voted for their favorite.
The contestants presented technologies such as a cell phone app to improve gut health (Agora Health), a polypectomy suite (IzoMed), an implantable weight-loss device (Lean Medical), a device to alleviate gastric obstruction in pancreatic cancer (Myka Labs), a pill designed to map out the gastrointestinal system to aid in diagnosis (Rock West Medical Devices), and an endoscopic ultrasound device (EndoSound).
Six finalists were selected from 20 submissions, and EndoSound was the winner. According to Raman Muthusamy, MD, medical director of endoscopy at UCLA Health and professor of clinical medicine at the University of California, Los Angeles, and past chair of the AGA Center for GI Innovation and Technology, the quality of presentations and the sophistication of the companies have increased year after year. “This was really the very best,” said Dr. Muthusamy.
Both the judges and the audience chose EndoSound. Endoscopic ultrasound (EUS) focuses on diagnosis and treatment of chest and abdomen disorders, particularly the pancreas. The EndoSound device attaches to an upper endoscope and converts it to a fully therapeutic endoscope that can perform all standard EUS procedures. Moreover, it does not use an elevator, which has been linked to infection risk.
Most clinical facilities lack EUS capability: 97% of ambulatory surgical centers and 80% of hospitals. EUS systems have hardly changed since the late 20th century, and they cost about $450,000. The projected cost of the EndoSound device is closer to $50,000.
“Just like colonoscopies and upper endoscopies, most endoscopic ultrasounds ought to be done in surgical centers. The idea that they can do them efficiently, and at lower cost and greater convenience to their patients and themselves, seems to me the way everything is going, and the way this procedure ought to go as well. The only obstacle to that has been the cost of the equipment. If we can take away that obstacle, then people who are already doing procedures in hospitals where it’s not convenient and not efficient, will be able to do the procedures in surgical centers,” said Stephen Steinberg, MD, founder and President of EndoSound.
“It’s a radical redesign. You’ve cut cost and you’ve cut space. And it’s something that could be put on at a moment’s notice. Rather than referring the patient for [ultrasound], it could allow you to do it on the spot, and perhaps save a second trip for a patient. It allows flexibility in terms of site of service,” said Dr. Muthusamy.
Dr. Muthusamy called it a “godsend” for low-resource institutions in the United States or abroad who have the expertise, but not the equipment, to perform EUS. “There’s no question that more EUS procedures could be done than are currently being done because of issues of availability, and this device takes a significant step to alleviate that.”
The Food and Drug Administration has granted a breakthrough device designation to EndoSound, which allows the company to forgo human clinical trials to support the application. “We’re hoping and expecting to have our application in the beginning of the fourth quarter, and with a little bit of luck to be approved by the end of the year. That’s our goal,” said Dr. Steinberg.
The technology started out as a challenge that Dr. Steinberg set for himself. His career overlapped with some of the earliest innovators of therapeutic endoscopy. “They were the stars. I wasn’t, but I was there,” said Dr. Steinberg. In his practice, Dr. Steinberg was doing procedures that included endoscopic ultrasound.
By the new millennium, EUS had gained a lot of interest, but there was a problem. “It was expensive, and it could only be done in hospitals. I started wondering if we couldn’t get it into a different environment by having a simpler solution,” said Dr. Steinberg.
But success didn’t come quickly. “I started drawing on the back of napkins to see if there wasn’t some solution,” said Dr. Steinberg. It wasn’t until a serendipitous meeting occurred that the concept took shape. Dr. Steinberg’s wife was the CEO and provost of Oregon Health Sciences University, Portland, as well as head of the technology transfer program. Dr. Steinberg’s practice, however, was in Florida so he commuted to Oregon every weekend.
One day, she told him about a presentation by Scott Corbett, MD. “My wife said: ‘Hey, they’re doing ultrasound. Why don’t you come and sit in [on the meeting] because I don’t know anything about it.’ [Dr.] Corbett was working with Sonivate, a point-of-care ultrasound company that was developing an ultrasound that could be placed over the end of the finger, to be used in battlefield triage. I thought, well, if you could put it on a finger, why couldn’t you put it on a scope? So, Scott and I got to talking, and went through a couple of iterations that didn’t work, and then finally came up with one that seemed like it was suitable.”
The device has been tested in five animal models with 20 EUS physicians who concluded that the images were equivalent to legacy devices and that they could be adopted quickly. The company also presented results from a human study that demonstrated noninferiority to the latest EUS system from Pentax.
Dr. Steinberg is an employee and stockholder of Sonivate. Dr. Muthusamy has no relevant financial disclosures. The 2022 AGA Tech Summit was supported by independent grants from Castle Biosciences, Medtronic, Boston Scientific, Exact Sciences, Olympus, 3-D Matrix, Apollo Endosurgery, Motus GI Holdings, STERIS Endoscopy, Cook Medical, FUJIFILM Healthcare Americas, and Virgo.
This article was updated 5/10/22.
*Correction, 5/17/22: An earlier version of this article stated that Geneoscopy was a finalist in the competition. It was not. Also, EndoSound should have been listed as a finalist in this paragraph.
After a 3-year, pandemic-induced hiatus, the American Gastroenterological Association’s Tech Summit returned to a live meeting in San Francisco. As usual, the highlight of the 2-day event, which is sponsored by the AGA Center for GI Innovation and Technology, was the Shark Tank, where selected companies presented lightning-round overviews of their technology and business plans. A panel of sharks and the audience voted for their favorite.
The contestants presented technologies such as a cell phone app to improve gut health (Agora Health), a polypectomy suite (IzoMed), an implantable weight-loss device (Lean Medical), a device to alleviate gastric obstruction in pancreatic cancer (Myka Labs), a pill designed to map out the gastrointestinal system to aid in diagnosis (Rock West Medical Devices), and an endoscopic ultrasound device (EndoSound).
Six finalists were selected from 20 submissions, and EndoSound was the winner. According to Raman Muthusamy, MD, medical director of endoscopy at UCLA Health and professor of clinical medicine at the University of California, Los Angeles, and past chair of the AGA Center for GI Innovation and Technology, the quality of presentations and the sophistication of the companies have increased year after year. “This was really the very best,” said Dr. Muthusamy.
Both the judges and the audience chose EndoSound. Endoscopic ultrasound (EUS) focuses on diagnosis and treatment of chest and abdomen disorders, particularly the pancreas. The EndoSound device attaches to an upper endoscope and converts it to a fully therapeutic endoscope that can perform all standard EUS procedures. Moreover, it does not use an elevator, which has been linked to infection risk.
Most clinical facilities lack EUS capability: 97% of ambulatory surgical centers and 80% of hospitals. EUS systems have hardly changed since the late 20th century, and they cost about $450,000. The projected cost of the EndoSound device is closer to $50,000.
“Just like colonoscopies and upper endoscopies, most endoscopic ultrasounds ought to be done in surgical centers. The idea that they can do them efficiently, and at lower cost and greater convenience to their patients and themselves, seems to me the way everything is going, and the way this procedure ought to go as well. The only obstacle to that has been the cost of the equipment. If we can take away that obstacle, then people who are already doing procedures in hospitals where it’s not convenient and not efficient, will be able to do the procedures in surgical centers,” said Stephen Steinberg, MD, founder and President of EndoSound.
“It’s a radical redesign. You’ve cut cost and you’ve cut space. And it’s something that could be put on at a moment’s notice. Rather than referring the patient for [ultrasound], it could allow you to do it on the spot, and perhaps save a second trip for a patient. It allows flexibility in terms of site of service,” said Dr. Muthusamy.
Dr. Muthusamy called it a “godsend” for low-resource institutions in the United States or abroad who have the expertise, but not the equipment, to perform EUS. “There’s no question that more EUS procedures could be done than are currently being done because of issues of availability, and this device takes a significant step to alleviate that.”
The Food and Drug Administration has granted a breakthrough device designation to EndoSound, which allows the company to forgo human clinical trials to support the application. “We’re hoping and expecting to have our application in the beginning of the fourth quarter, and with a little bit of luck to be approved by the end of the year. That’s our goal,” said Dr. Steinberg.
The technology started out as a challenge that Dr. Steinberg set for himself. His career overlapped with some of the earliest innovators of therapeutic endoscopy. “They were the stars. I wasn’t, but I was there,” said Dr. Steinberg. In his practice, Dr. Steinberg was doing procedures that included endoscopic ultrasound.
By the new millennium, EUS had gained a lot of interest, but there was a problem. “It was expensive, and it could only be done in hospitals. I started wondering if we couldn’t get it into a different environment by having a simpler solution,” said Dr. Steinberg.
But success didn’t come quickly. “I started drawing on the back of napkins to see if there wasn’t some solution,” said Dr. Steinberg. It wasn’t until a serendipitous meeting occurred that the concept took shape. Dr. Steinberg’s wife was the CEO and provost of Oregon Health Sciences University, Portland, as well as head of the technology transfer program. Dr. Steinberg’s practice, however, was in Florida so he commuted to Oregon every weekend.
One day, she told him about a presentation by Scott Corbett, MD. “My wife said: ‘Hey, they’re doing ultrasound. Why don’t you come and sit in [on the meeting] because I don’t know anything about it.’ [Dr.] Corbett was working with Sonivate, a point-of-care ultrasound company that was developing an ultrasound that could be placed over the end of the finger, to be used in battlefield triage. I thought, well, if you could put it on a finger, why couldn’t you put it on a scope? So, Scott and I got to talking, and went through a couple of iterations that didn’t work, and then finally came up with one that seemed like it was suitable.”
The device has been tested in five animal models with 20 EUS physicians who concluded that the images were equivalent to legacy devices and that they could be adopted quickly. The company also presented results from a human study that demonstrated noninferiority to the latest EUS system from Pentax.
Dr. Steinberg is an employee and stockholder of Sonivate. Dr. Muthusamy has no relevant financial disclosures. The 2022 AGA Tech Summit was supported by independent grants from Castle Biosciences, Medtronic, Boston Scientific, Exact Sciences, Olympus, 3-D Matrix, Apollo Endosurgery, Motus GI Holdings, STERIS Endoscopy, Cook Medical, FUJIFILM Healthcare Americas, and Virgo.
This article was updated 5/10/22.
*Correction, 5/17/22: An earlier version of this article stated that Geneoscopy was a finalist in the competition. It was not. Also, EndoSound should have been listed as a finalist in this paragraph.
FROM 2022 AGA TECH SUMMIT
Fecal transfer could be the transplant of youth
Fecal matter may be in the fountain of youth
Yes, you read that headline correctly. New research by scientists at Quadram Institute and the University of East Anglia, both in Norwich, England, supports the claim that transferring fecal microbes might actually have some positive effects on reversing the aging process in the eyes, brain, and gut.
How do they know? Mice, of course. In the study, scientists took the gut microbes from older mice and transferred them into the younger mince. The young mice displayed inflamed signs of aging in their guts, brains, and eyes, which, we all know, decline in function as we age. What happens is a chronic inflammation of cells as we get older that can be found in the brain or gut that leads to a degenerative state over time.
When the older mice received the gut microbes from younger mice, the investigators saw the reverse: Gut, brain, and eye functionality improved. In a way, minimizing the inflammation.
There’s tons of research out there that suggests gut health is the key to a healthy life, but this study points directly to an improvement in brain and vision functionality as a result of the transfer.
Now, we’re not insinuating you get a poo transfer as you reach old age. And the shift to human studies on microbiota replacement therapy is still in the works. But this definitely is a topic to watch and could be a game changer in the age-old quest to bottle youth or at least improve quality of life as we age.
For now, the scientists did find some connections between the beneficial bacteria in the transplants and the human diet that could have similar effects, like changes in the metabolism of certain fats and vitamin that could have effects on the inflammatory cells in the eye and brain.
The more you know!
It’s not lying, it’s preemptive truth
Lying is bad. Bold statement, we know, but a true one. After all, God spent an entire commandment telling people not to do the whole bearing false witness thing, and God is generally known for not joking around. He’s a pretty serious dude.
In case you’ve been wandering around the desert for a while and haven’t had wifi, we have a bit of a misinformation problem these days. People lie all the time about a lot of things, and a lot of people believe the lies. According to new research, however, there are also a lot of people who recognize the lies but accept them anyway because they believe that the lies will become true in the future.
Imagine the following scenario: A friend gets a job he’s not qualified for because he listed a skill he doesn’t have. That’s bad, right? And the people the researchers interviewed agreed, at least initially. But when informed that our friend is planning on obtaining the skill in summer classes in the near future, the study participants became far more willing to excuse the initial lie.
A friend jumping the gun on training he doesn’t have yet is fairly innocuous as far as lying goes, but as the researchers found, this willingness to forgive lies because they could become true extends far further. For example, millions of people do not vote illegally in U.S. elections, nor do White people get approved for mortgages at rates 300% higher than minorities, but when asked to imagine scenarios in which those statements could be true, study participants were less likely to condemn the lie and prevent it from spreading further, especially if their political viewpoints aligned with the respective falsehood.
It seems, then, that while we may aspire to not tell lies, we take after another guy with magic powers who spent too much time in the desert: “What I told you was true, from a certain point of view.”
It tastes like feng shui, but it’s not
You know about biomes. You’ve read about various microbiomes. Allow us to introduce you to the envirome,
The envirome “includes all the natural and man-made elements of our environment throughout the lifespan, notably the built environment,” said Robert Schneider, dean of the College of Integrative Medicine at Maharishi International University. Located in – you guessed it – Fairfield, Iowa, and home of the Fighting Transcendentalists. MAHARISHI RULES!
[Editor’s note: You made that up, right? Well, it really is in Iowa, but they don’t seem to have an athletic program.]
In an effort to maximize the envirome’s potential to improve quality of life, Dr. Schneider and his associates systematically integrated the principles of Maharishi Vastu architecture (MVA) into a comprehensive building system. MVA is “a holistic wellness architectural system that aligns buildings with nature’s intelligence, creating balanced, orderly, and integrated living environments with the goal of improving occupants’ lives,” the university explained in a written statement.
Since “modern medicine now recognizes the powerful effects of the ‘envirome’ on health,” Dr. Schneider said in that statement, the researchers reviewed 40 years’ worth of published studies on MVA’s benefits – an analysis that appears in Global Advances in Health and Medicine.
As far as our homes are concerned, here are some of the things MVA says we should be doing:
- The headboard of a bed should be oriented to the east or south when you sleep. This will improve mental health.
- While sitting at a desk or work area, a person should face east or north to improve brain coherence.
- The main entrance of a house should face east because morning light is superior to afternoon light.
And you were worried about feng shui. Well, forget feng shui. Feng shui is for amateurs. MVA is the way to go. MVA is the GOAT. MAHARISHI RULES!
Fecal matter may be in the fountain of youth
Yes, you read that headline correctly. New research by scientists at Quadram Institute and the University of East Anglia, both in Norwich, England, supports the claim that transferring fecal microbes might actually have some positive effects on reversing the aging process in the eyes, brain, and gut.
How do they know? Mice, of course. In the study, scientists took the gut microbes from older mice and transferred them into the younger mince. The young mice displayed inflamed signs of aging in their guts, brains, and eyes, which, we all know, decline in function as we age. What happens is a chronic inflammation of cells as we get older that can be found in the brain or gut that leads to a degenerative state over time.
When the older mice received the gut microbes from younger mice, the investigators saw the reverse: Gut, brain, and eye functionality improved. In a way, minimizing the inflammation.
There’s tons of research out there that suggests gut health is the key to a healthy life, but this study points directly to an improvement in brain and vision functionality as a result of the transfer.
Now, we’re not insinuating you get a poo transfer as you reach old age. And the shift to human studies on microbiota replacement therapy is still in the works. But this definitely is a topic to watch and could be a game changer in the age-old quest to bottle youth or at least improve quality of life as we age.
For now, the scientists did find some connections between the beneficial bacteria in the transplants and the human diet that could have similar effects, like changes in the metabolism of certain fats and vitamin that could have effects on the inflammatory cells in the eye and brain.
The more you know!
It’s not lying, it’s preemptive truth
Lying is bad. Bold statement, we know, but a true one. After all, God spent an entire commandment telling people not to do the whole bearing false witness thing, and God is generally known for not joking around. He’s a pretty serious dude.
In case you’ve been wandering around the desert for a while and haven’t had wifi, we have a bit of a misinformation problem these days. People lie all the time about a lot of things, and a lot of people believe the lies. According to new research, however, there are also a lot of people who recognize the lies but accept them anyway because they believe that the lies will become true in the future.
Imagine the following scenario: A friend gets a job he’s not qualified for because he listed a skill he doesn’t have. That’s bad, right? And the people the researchers interviewed agreed, at least initially. But when informed that our friend is planning on obtaining the skill in summer classes in the near future, the study participants became far more willing to excuse the initial lie.
A friend jumping the gun on training he doesn’t have yet is fairly innocuous as far as lying goes, but as the researchers found, this willingness to forgive lies because they could become true extends far further. For example, millions of people do not vote illegally in U.S. elections, nor do White people get approved for mortgages at rates 300% higher than minorities, but when asked to imagine scenarios in which those statements could be true, study participants were less likely to condemn the lie and prevent it from spreading further, especially if their political viewpoints aligned with the respective falsehood.
It seems, then, that while we may aspire to not tell lies, we take after another guy with magic powers who spent too much time in the desert: “What I told you was true, from a certain point of view.”
It tastes like feng shui, but it’s not
You know about biomes. You’ve read about various microbiomes. Allow us to introduce you to the envirome,
The envirome “includes all the natural and man-made elements of our environment throughout the lifespan, notably the built environment,” said Robert Schneider, dean of the College of Integrative Medicine at Maharishi International University. Located in – you guessed it – Fairfield, Iowa, and home of the Fighting Transcendentalists. MAHARISHI RULES!
[Editor’s note: You made that up, right? Well, it really is in Iowa, but they don’t seem to have an athletic program.]
In an effort to maximize the envirome’s potential to improve quality of life, Dr. Schneider and his associates systematically integrated the principles of Maharishi Vastu architecture (MVA) into a comprehensive building system. MVA is “a holistic wellness architectural system that aligns buildings with nature’s intelligence, creating balanced, orderly, and integrated living environments with the goal of improving occupants’ lives,” the university explained in a written statement.
Since “modern medicine now recognizes the powerful effects of the ‘envirome’ on health,” Dr. Schneider said in that statement, the researchers reviewed 40 years’ worth of published studies on MVA’s benefits – an analysis that appears in Global Advances in Health and Medicine.
As far as our homes are concerned, here are some of the things MVA says we should be doing:
- The headboard of a bed should be oriented to the east or south when you sleep. This will improve mental health.
- While sitting at a desk or work area, a person should face east or north to improve brain coherence.
- The main entrance of a house should face east because morning light is superior to afternoon light.
And you were worried about feng shui. Well, forget feng shui. Feng shui is for amateurs. MVA is the way to go. MVA is the GOAT. MAHARISHI RULES!
Fecal matter may be in the fountain of youth
Yes, you read that headline correctly. New research by scientists at Quadram Institute and the University of East Anglia, both in Norwich, England, supports the claim that transferring fecal microbes might actually have some positive effects on reversing the aging process in the eyes, brain, and gut.
How do they know? Mice, of course. In the study, scientists took the gut microbes from older mice and transferred them into the younger mince. The young mice displayed inflamed signs of aging in their guts, brains, and eyes, which, we all know, decline in function as we age. What happens is a chronic inflammation of cells as we get older that can be found in the brain or gut that leads to a degenerative state over time.
When the older mice received the gut microbes from younger mice, the investigators saw the reverse: Gut, brain, and eye functionality improved. In a way, minimizing the inflammation.
There’s tons of research out there that suggests gut health is the key to a healthy life, but this study points directly to an improvement in brain and vision functionality as a result of the transfer.
Now, we’re not insinuating you get a poo transfer as you reach old age. And the shift to human studies on microbiota replacement therapy is still in the works. But this definitely is a topic to watch and could be a game changer in the age-old quest to bottle youth or at least improve quality of life as we age.
For now, the scientists did find some connections between the beneficial bacteria in the transplants and the human diet that could have similar effects, like changes in the metabolism of certain fats and vitamin that could have effects on the inflammatory cells in the eye and brain.
The more you know!
It’s not lying, it’s preemptive truth
Lying is bad. Bold statement, we know, but a true one. After all, God spent an entire commandment telling people not to do the whole bearing false witness thing, and God is generally known for not joking around. He’s a pretty serious dude.
In case you’ve been wandering around the desert for a while and haven’t had wifi, we have a bit of a misinformation problem these days. People lie all the time about a lot of things, and a lot of people believe the lies. According to new research, however, there are also a lot of people who recognize the lies but accept them anyway because they believe that the lies will become true in the future.
Imagine the following scenario: A friend gets a job he’s not qualified for because he listed a skill he doesn’t have. That’s bad, right? And the people the researchers interviewed agreed, at least initially. But when informed that our friend is planning on obtaining the skill in summer classes in the near future, the study participants became far more willing to excuse the initial lie.
A friend jumping the gun on training he doesn’t have yet is fairly innocuous as far as lying goes, but as the researchers found, this willingness to forgive lies because they could become true extends far further. For example, millions of people do not vote illegally in U.S. elections, nor do White people get approved for mortgages at rates 300% higher than minorities, but when asked to imagine scenarios in which those statements could be true, study participants were less likely to condemn the lie and prevent it from spreading further, especially if their political viewpoints aligned with the respective falsehood.
It seems, then, that while we may aspire to not tell lies, we take after another guy with magic powers who spent too much time in the desert: “What I told you was true, from a certain point of view.”
It tastes like feng shui, but it’s not
You know about biomes. You’ve read about various microbiomes. Allow us to introduce you to the envirome,
The envirome “includes all the natural and man-made elements of our environment throughout the lifespan, notably the built environment,” said Robert Schneider, dean of the College of Integrative Medicine at Maharishi International University. Located in – you guessed it – Fairfield, Iowa, and home of the Fighting Transcendentalists. MAHARISHI RULES!
[Editor’s note: You made that up, right? Well, it really is in Iowa, but they don’t seem to have an athletic program.]
In an effort to maximize the envirome’s potential to improve quality of life, Dr. Schneider and his associates systematically integrated the principles of Maharishi Vastu architecture (MVA) into a comprehensive building system. MVA is “a holistic wellness architectural system that aligns buildings with nature’s intelligence, creating balanced, orderly, and integrated living environments with the goal of improving occupants’ lives,” the university explained in a written statement.
Since “modern medicine now recognizes the powerful effects of the ‘envirome’ on health,” Dr. Schneider said in that statement, the researchers reviewed 40 years’ worth of published studies on MVA’s benefits – an analysis that appears in Global Advances in Health and Medicine.
As far as our homes are concerned, here are some of the things MVA says we should be doing:
- The headboard of a bed should be oriented to the east or south when you sleep. This will improve mental health.
- While sitting at a desk or work area, a person should face east or north to improve brain coherence.
- The main entrance of a house should face east because morning light is superior to afternoon light.
And you were worried about feng shui. Well, forget feng shui. Feng shui is for amateurs. MVA is the way to go. MVA is the GOAT. MAHARISHI RULES!
Surgery handoffs still a risky juncture in care – but increasing communication can help
It involved a 70-year-old man who had a history of prostate cancer, obstructive sleep apnea, and hernias. In January, he had a surgery for hernia repair. On the 3rd day after the procedure, he was transferred to the hospital medicine service at about 9 p.m. and was on a patient-controlled pump for pain and had abdominal drains. Because of the extensive surgery and because he had begun to walk shortly after the procedure, he wasn’t on thrombosis prevention medication, Dr. Merli explained at the annual meeting of the American College of Physicians.
The day after his transfer he was walking with a physical therapist when he became short of breath, his oxygen saturation dropped, and his heart rate soared. Bilateral pulmonary emboli were found, along with thrombosis in the right leg.
What was remarkable, Dr. Merli noted, was what the patient’s medical record was lacking.
He added, “I think if we start looking at this at our sites, we may find out that communication needs to be improved, and I believe standardized.”
This situation underscores the continuing need to refine handoffs between surgery and hospital medicine, a point in care that is primed for potential errors, the other panelists noted during the session.
Most important information is often not communicated
A 2010 study in pediatrics that looked at intern-to-intern handoffs found that the most important piece of information wasn’t communicated successfully 60% of the time – in other words, more often than not, the person on the receiving end didn’t really understand that crucial part of the scenario. Since then, the literature has been regularly populated with studies attempting to refine handoff procedures.
Lily Ackermann, MD, hospitalist and clinical associate professor of medicine at Jefferson, said in the session that hospitalists need to be sure to reach out to surgery at important junctures in care.
“I would say the No. 1 biggest mistake we make is not calling the surgery attending directly when clinical questions arise,” she said. “I think this is very important – attending [physician in hospital medicine] to attending [physician in surgery].”
Murray Cohen, MD, director of acute care surgery at Jefferson, said he shared that concern.
“We want to be called, we want to be called for our patients,” he said in the session. “And we’re upset when you don’t call for our patients.”
Hospitalists should discuss blood loss, pain management, management of drains, deep vein thrombosis prevention, nutrition, infectious disease concerns, and timing of vaccines post procedure, Dr. Ackermann said during the presentation,
The panelists also emphasized that understanding the follow-up care that surgery was planning after a procedure is important, and to not just expect surgeons to actively follow a patient. They also reminded hospitalists to look at the wounds and make sure they understand how to handle the wounds going forward. Plus, when transferring a patient to surgery, hospitalists should understand when getting someone to surgery is urgent and not to order unnecessary tests as a formality when time is of the essence, they said.
IPASS: a formalized handoff process
The panelists all spoke highly of a formalized handoff process known as IPASS. This acronym reminds physicians to ask specific questions.
The I represents illness severity and calls for asking: “Is the patient stable or unstable?
The P stands for patient summary and is meant to prompt physicians to seek details about the procedure.
The A is for action list, which is meant to remind the physician to get the post-op plan for neurological, cardiovascular, gastrointestinal, and other areas.
The first S is for situational awareness, and calls for asking: What is the biggest concern over the next 24 hours?
The final S represents synthesis by the receiver, prompting a physician to summarize the information he or she has received about the patient.
Natalie Margules, MD, a clinical instructor and hospitalist at Jefferson who did not present in the session, reiterated the value of the IPASS system. Before it was used for handoffs, she said, “I was never taught anything formalized – basically, just ‘Tell them what’s important.’
Dr. Margules noted that she considers the framework’s call for the synthesis to be one of it most useful parts.
Dr. Merli, Dr. Ackermann, and Dr. Cohen reported no relevant financial disclosures.
It involved a 70-year-old man who had a history of prostate cancer, obstructive sleep apnea, and hernias. In January, he had a surgery for hernia repair. On the 3rd day after the procedure, he was transferred to the hospital medicine service at about 9 p.m. and was on a patient-controlled pump for pain and had abdominal drains. Because of the extensive surgery and because he had begun to walk shortly after the procedure, he wasn’t on thrombosis prevention medication, Dr. Merli explained at the annual meeting of the American College of Physicians.
The day after his transfer he was walking with a physical therapist when he became short of breath, his oxygen saturation dropped, and his heart rate soared. Bilateral pulmonary emboli were found, along with thrombosis in the right leg.
What was remarkable, Dr. Merli noted, was what the patient’s medical record was lacking.
He added, “I think if we start looking at this at our sites, we may find out that communication needs to be improved, and I believe standardized.”
This situation underscores the continuing need to refine handoffs between surgery and hospital medicine, a point in care that is primed for potential errors, the other panelists noted during the session.
Most important information is often not communicated
A 2010 study in pediatrics that looked at intern-to-intern handoffs found that the most important piece of information wasn’t communicated successfully 60% of the time – in other words, more often than not, the person on the receiving end didn’t really understand that crucial part of the scenario. Since then, the literature has been regularly populated with studies attempting to refine handoff procedures.
Lily Ackermann, MD, hospitalist and clinical associate professor of medicine at Jefferson, said in the session that hospitalists need to be sure to reach out to surgery at important junctures in care.
“I would say the No. 1 biggest mistake we make is not calling the surgery attending directly when clinical questions arise,” she said. “I think this is very important – attending [physician in hospital medicine] to attending [physician in surgery].”
Murray Cohen, MD, director of acute care surgery at Jefferson, said he shared that concern.
“We want to be called, we want to be called for our patients,” he said in the session. “And we’re upset when you don’t call for our patients.”
Hospitalists should discuss blood loss, pain management, management of drains, deep vein thrombosis prevention, nutrition, infectious disease concerns, and timing of vaccines post procedure, Dr. Ackermann said during the presentation,
The panelists also emphasized that understanding the follow-up care that surgery was planning after a procedure is important, and to not just expect surgeons to actively follow a patient. They also reminded hospitalists to look at the wounds and make sure they understand how to handle the wounds going forward. Plus, when transferring a patient to surgery, hospitalists should understand when getting someone to surgery is urgent and not to order unnecessary tests as a formality when time is of the essence, they said.
IPASS: a formalized handoff process
The panelists all spoke highly of a formalized handoff process known as IPASS. This acronym reminds physicians to ask specific questions.
The I represents illness severity and calls for asking: “Is the patient stable or unstable?
The P stands for patient summary and is meant to prompt physicians to seek details about the procedure.
The A is for action list, which is meant to remind the physician to get the post-op plan for neurological, cardiovascular, gastrointestinal, and other areas.
The first S is for situational awareness, and calls for asking: What is the biggest concern over the next 24 hours?
The final S represents synthesis by the receiver, prompting a physician to summarize the information he or she has received about the patient.
Natalie Margules, MD, a clinical instructor and hospitalist at Jefferson who did not present in the session, reiterated the value of the IPASS system. Before it was used for handoffs, she said, “I was never taught anything formalized – basically, just ‘Tell them what’s important.’
Dr. Margules noted that she considers the framework’s call for the synthesis to be one of it most useful parts.
Dr. Merli, Dr. Ackermann, and Dr. Cohen reported no relevant financial disclosures.
It involved a 70-year-old man who had a history of prostate cancer, obstructive sleep apnea, and hernias. In January, he had a surgery for hernia repair. On the 3rd day after the procedure, he was transferred to the hospital medicine service at about 9 p.m. and was on a patient-controlled pump for pain and had abdominal drains. Because of the extensive surgery and because he had begun to walk shortly after the procedure, he wasn’t on thrombosis prevention medication, Dr. Merli explained at the annual meeting of the American College of Physicians.
The day after his transfer he was walking with a physical therapist when he became short of breath, his oxygen saturation dropped, and his heart rate soared. Bilateral pulmonary emboli were found, along with thrombosis in the right leg.
What was remarkable, Dr. Merli noted, was what the patient’s medical record was lacking.
He added, “I think if we start looking at this at our sites, we may find out that communication needs to be improved, and I believe standardized.”
This situation underscores the continuing need to refine handoffs between surgery and hospital medicine, a point in care that is primed for potential errors, the other panelists noted during the session.
Most important information is often not communicated
A 2010 study in pediatrics that looked at intern-to-intern handoffs found that the most important piece of information wasn’t communicated successfully 60% of the time – in other words, more often than not, the person on the receiving end didn’t really understand that crucial part of the scenario. Since then, the literature has been regularly populated with studies attempting to refine handoff procedures.
Lily Ackermann, MD, hospitalist and clinical associate professor of medicine at Jefferson, said in the session that hospitalists need to be sure to reach out to surgery at important junctures in care.
“I would say the No. 1 biggest mistake we make is not calling the surgery attending directly when clinical questions arise,” she said. “I think this is very important – attending [physician in hospital medicine] to attending [physician in surgery].”
Murray Cohen, MD, director of acute care surgery at Jefferson, said he shared that concern.
“We want to be called, we want to be called for our patients,” he said in the session. “And we’re upset when you don’t call for our patients.”
Hospitalists should discuss blood loss, pain management, management of drains, deep vein thrombosis prevention, nutrition, infectious disease concerns, and timing of vaccines post procedure, Dr. Ackermann said during the presentation,
The panelists also emphasized that understanding the follow-up care that surgery was planning after a procedure is important, and to not just expect surgeons to actively follow a patient. They also reminded hospitalists to look at the wounds and make sure they understand how to handle the wounds going forward. Plus, when transferring a patient to surgery, hospitalists should understand when getting someone to surgery is urgent and not to order unnecessary tests as a formality when time is of the essence, they said.
IPASS: a formalized handoff process
The panelists all spoke highly of a formalized handoff process known as IPASS. This acronym reminds physicians to ask specific questions.
The I represents illness severity and calls for asking: “Is the patient stable or unstable?
The P stands for patient summary and is meant to prompt physicians to seek details about the procedure.
The A is for action list, which is meant to remind the physician to get the post-op plan for neurological, cardiovascular, gastrointestinal, and other areas.
The first S is for situational awareness, and calls for asking: What is the biggest concern over the next 24 hours?
The final S represents synthesis by the receiver, prompting a physician to summarize the information he or she has received about the patient.
Natalie Margules, MD, a clinical instructor and hospitalist at Jefferson who did not present in the session, reiterated the value of the IPASS system. Before it was used for handoffs, she said, “I was never taught anything formalized – basically, just ‘Tell them what’s important.’
Dr. Margules noted that she considers the framework’s call for the synthesis to be one of it most useful parts.
Dr. Merli, Dr. Ackermann, and Dr. Cohen reported no relevant financial disclosures.
AT INTERNAL MEDICINE 2022
Omicron sublineages evade immunity from past infection
A South African study based on blood samples found that the BA.4 and BA.5 sublineages of Omicron were more likely to evade antibodies produced by previous Omicron infections than the immunity provided by vaccinations.
Scientists took blood samples from 39 people infected with Omicron, with 24 people not vaccinated and 15 vaccinated with the Pfizer or the Johnson & Johnson vaccines, Reuters reported.
“The vaccinated group showed about a fivefold higher neutralization capacity ... and should be better protected,” the investigators found, according to Reuters.
There was an eightfold decrease in antibody protection in unvaccinated blood samples when exposed to the subvariants compared to a threefold decrease in the blood samples from vaccinated people.
“Based on neutralization escape, BA.4 and BA.5 have potential to result in a new infection wave,” the investigators found.
The finding is important because health authorities say cases caused by the sublineages are increasing in South Africa to a degree that the nation may be entering a fifth wave of COVID, Reuters said.
Health Minister Joe Phaahla said recently that hospitalizations were increasing but that ICU admissions had not greatly gone up yet.
A version of this article first appeared on WebMD.com.
A South African study based on blood samples found that the BA.4 and BA.5 sublineages of Omicron were more likely to evade antibodies produced by previous Omicron infections than the immunity provided by vaccinations.
Scientists took blood samples from 39 people infected with Omicron, with 24 people not vaccinated and 15 vaccinated with the Pfizer or the Johnson & Johnson vaccines, Reuters reported.
“The vaccinated group showed about a fivefold higher neutralization capacity ... and should be better protected,” the investigators found, according to Reuters.
There was an eightfold decrease in antibody protection in unvaccinated blood samples when exposed to the subvariants compared to a threefold decrease in the blood samples from vaccinated people.
“Based on neutralization escape, BA.4 and BA.5 have potential to result in a new infection wave,” the investigators found.
The finding is important because health authorities say cases caused by the sublineages are increasing in South Africa to a degree that the nation may be entering a fifth wave of COVID, Reuters said.
Health Minister Joe Phaahla said recently that hospitalizations were increasing but that ICU admissions had not greatly gone up yet.
A version of this article first appeared on WebMD.com.
A South African study based on blood samples found that the BA.4 and BA.5 sublineages of Omicron were more likely to evade antibodies produced by previous Omicron infections than the immunity provided by vaccinations.
Scientists took blood samples from 39 people infected with Omicron, with 24 people not vaccinated and 15 vaccinated with the Pfizer or the Johnson & Johnson vaccines, Reuters reported.
“The vaccinated group showed about a fivefold higher neutralization capacity ... and should be better protected,” the investigators found, according to Reuters.
There was an eightfold decrease in antibody protection in unvaccinated blood samples when exposed to the subvariants compared to a threefold decrease in the blood samples from vaccinated people.
“Based on neutralization escape, BA.4 and BA.5 have potential to result in a new infection wave,” the investigators found.
The finding is important because health authorities say cases caused by the sublineages are increasing in South Africa to a degree that the nation may be entering a fifth wave of COVID, Reuters said.
Health Minister Joe Phaahla said recently that hospitalizations were increasing but that ICU admissions had not greatly gone up yet.
A version of this article first appeared on WebMD.com.
Newly defined liver disorder associated with COVID mortality
People with metabolic dysfunction–associated fatty liver disease (MAFLD) – a newly defined condition – may be more likely to die from COVID-19, researchers say.
A cohort of people hospitalized for COVID-19 in Central Military Hospital, Mexico City, who met the criteria for MAFLD died at a higher rate than a control group without fatty liver disease, said Martín Uriel Vázquez-Medina, MSc, a researcher in the National Polytechnic Institute in Mexico City.
Patients who met only the criteria for the traditional classification, nonalcoholic fatty liver disease (NAFLD), also died of COVID-19 at a higher rate than the control group, but the difference was not statistically significant.
“It is important to screen for MAFLD,” Mr. Vázquez-Medina told this news organization. “It’s a new definition, but it has really helped us to identify which patients are going to get worse by COVID-19.”
The study was published in Hepatology Communications.
More evidence for clinical relevance of MAFLD
The finding lends support to an initiative to use MAFLD instead of NAFLD to identify patients whose liver steatosis poses a threat to their health, Mr. Vázquez-Medina said.
NAFLD affects as much as a quarter of the world’s population. No drugs have been approved to treat it. Some researchers have reasoned that the imprecision of the definition of NAFLD could be one reason for the lack of progress in treatment.
“NAFLD is something that doesn’t have positive criteria to be diagnosed,” said Mr. Vázquez-Medina. “You only say NAFLD when you don’t find hepatitis or another disease.”
In an article published in Gastroenterology, an international consensus panel proposed MAFLD as an alternative, arguing that a focus on metabolic dysfunction could more accurately reflect the pathogenesis of the disease and help stratify patients.
Previous research has suggested that patients with MAFLD have a higher risk of atherosclerotic cardiovascular disease and that the prevalence of colorectal adenomas is a higher in these patients, compared with patients with NAFLD.
The high prevalence of MAFLD in Mexico – about 30% – could help explain the country’s high rate of mortality from COVID-19, Mr. Vázquez-Medina said. Almost 6% of people diagnosed with COVID in Mexico have died from it, according to the Johns Hopkins University and Medical Center Coronavirus Resource Center.
Sorting COVID outcomes by liver steatosis
To understand the interaction of MAFLD, NAFLD, liver fibrosis, and COVID-19, Mr. Vázquez-Medina and his colleagues analyzed the records of all patients admitted to the Central Military Hospital with COVID-19 from April 4, 2020, to June 24, 2020.
They excluded patients for whom complete data were lacking or for whom a liver function test was not conducted in the first 24 hours of hospitalization. Also excluded were patients with significant consumption of alcohol (> 30 g/day for men and > 20 g/day for women) and those with a history of autoimmune liver disease, liver cancer, decompensated cirrhosis, platelet disorders, or myopathies.
The remaining patients were divided into three groups – 220 who met the criteria for MAFLD, 79 who met the criteria for NAFLD but not MAFLD, and 60 other patients as a control group.
The researchers defined MAFLD as the presence of liver steatosis detected with a noninvasive method and one of the following: overweight (body mass index, 25-29.9 kg/m2), type 2 diabetes, or the presence of two metabolic abnormalities (blood pressure > 140/90 mm Hg, plasma triglycerides > 150 mg/dL, plasma high-density lipoprotein cholesterol < 40 mg/dL in men and < 50 mg/dL in women, and prediabetes).
They defined NAFLD as the presence of liver steatosis without the other criteria for MAFLD.
The patients with MAFLD were the most likely to be intubated and were the most likely to die (intubation, 44.09%; mortality, 55%), followed by those with NAFLD (intubation, 40.51%; mortality, 51.9%) and those in the control group (intubation, 20%; mortality, 38.33%).
The difference in mortality between the MAFLD group and the control group was statistically significant (P = .02). The mortality difference between the NAFLD and the control group fell just short of statistical significance (P = .07).
For intubation, the difference between the MAFLD and the control group was highly statistically significant (P = .001), and the difference between the NAFLD and the control group was also statistically significant (P = .01)
Patients with advanced fibrosis and either MAFLD or NAFLD were also more likely to die than patients in the control group with advanced fibrosis.
That’s why screening for MAFLD is important, Mr. Vázquez-Medina said.
Next steps and new questions
Future research should examine whether patients with MAFLD have elevated levels of biomarkers for inflammation, such as interleukin 6, Mr. Vázquez-Medina said. A “chronic low proinflammatory state” may be the key to understanding the vulnerability of patients to MAFLD to COVID-19, he speculated.
The metabolic traits associated with MAFLD could explain the higher mortality and intubation rates with COVID, said Rohit Loomba, MD, MHSc, a professor of medicine in the division of gastroenterology at the University of California, San Diego, who was not involved in the study.
“Hypertension, diabetes, and obesity increase the risk of complications from COVID in all patients, whether they have been diagnosed with NAFLD or not,” he told this news organization in an email.
Mr. Vasquez-Medina pointed out that the patients with MAFLD had a higher risk of mortality even after adjusting for age, sex, type 2 diabetes, hypertension, overweight, and obesity (BMI ≥ 30 kg/m2). MAFLD also was more strongly associated with a poor outcome than either hypertension alone or obesity alone. Only age emerged as a significant independent covariate in the study.
Dr. Loomba also questioned whether the regression model used in this study for liver steatosis was “fully reflective of NAFLD.”
The researchers identified liver steatosis with a diagnostic formula that used noninvasive clinical BMI and laboratory tests (alanine aminotransferase), citing a study that found the regression formula was better at diagnosing NAFLD than FibroScan.
Mr. Vázquez-Medina reported no relevant financial relationships. Dr. Loomba serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse Bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen, Madrigal, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89bio, Terns Pharmaceuticals, and Viking Therapeutics. He is co-founder of LipoNexus.
A version of this article first appeared on Medscape.com.
People with metabolic dysfunction–associated fatty liver disease (MAFLD) – a newly defined condition – may be more likely to die from COVID-19, researchers say.
A cohort of people hospitalized for COVID-19 in Central Military Hospital, Mexico City, who met the criteria for MAFLD died at a higher rate than a control group without fatty liver disease, said Martín Uriel Vázquez-Medina, MSc, a researcher in the National Polytechnic Institute in Mexico City.
Patients who met only the criteria for the traditional classification, nonalcoholic fatty liver disease (NAFLD), also died of COVID-19 at a higher rate than the control group, but the difference was not statistically significant.
“It is important to screen for MAFLD,” Mr. Vázquez-Medina told this news organization. “It’s a new definition, but it has really helped us to identify which patients are going to get worse by COVID-19.”
The study was published in Hepatology Communications.
More evidence for clinical relevance of MAFLD
The finding lends support to an initiative to use MAFLD instead of NAFLD to identify patients whose liver steatosis poses a threat to their health, Mr. Vázquez-Medina said.
NAFLD affects as much as a quarter of the world’s population. No drugs have been approved to treat it. Some researchers have reasoned that the imprecision of the definition of NAFLD could be one reason for the lack of progress in treatment.
“NAFLD is something that doesn’t have positive criteria to be diagnosed,” said Mr. Vázquez-Medina. “You only say NAFLD when you don’t find hepatitis or another disease.”
In an article published in Gastroenterology, an international consensus panel proposed MAFLD as an alternative, arguing that a focus on metabolic dysfunction could more accurately reflect the pathogenesis of the disease and help stratify patients.
Previous research has suggested that patients with MAFLD have a higher risk of atherosclerotic cardiovascular disease and that the prevalence of colorectal adenomas is a higher in these patients, compared with patients with NAFLD.
The high prevalence of MAFLD in Mexico – about 30% – could help explain the country’s high rate of mortality from COVID-19, Mr. Vázquez-Medina said. Almost 6% of people diagnosed with COVID in Mexico have died from it, according to the Johns Hopkins University and Medical Center Coronavirus Resource Center.
Sorting COVID outcomes by liver steatosis
To understand the interaction of MAFLD, NAFLD, liver fibrosis, and COVID-19, Mr. Vázquez-Medina and his colleagues analyzed the records of all patients admitted to the Central Military Hospital with COVID-19 from April 4, 2020, to June 24, 2020.
They excluded patients for whom complete data were lacking or for whom a liver function test was not conducted in the first 24 hours of hospitalization. Also excluded were patients with significant consumption of alcohol (> 30 g/day for men and > 20 g/day for women) and those with a history of autoimmune liver disease, liver cancer, decompensated cirrhosis, platelet disorders, or myopathies.
The remaining patients were divided into three groups – 220 who met the criteria for MAFLD, 79 who met the criteria for NAFLD but not MAFLD, and 60 other patients as a control group.
The researchers defined MAFLD as the presence of liver steatosis detected with a noninvasive method and one of the following: overweight (body mass index, 25-29.9 kg/m2), type 2 diabetes, or the presence of two metabolic abnormalities (blood pressure > 140/90 mm Hg, plasma triglycerides > 150 mg/dL, plasma high-density lipoprotein cholesterol < 40 mg/dL in men and < 50 mg/dL in women, and prediabetes).
They defined NAFLD as the presence of liver steatosis without the other criteria for MAFLD.
The patients with MAFLD were the most likely to be intubated and were the most likely to die (intubation, 44.09%; mortality, 55%), followed by those with NAFLD (intubation, 40.51%; mortality, 51.9%) and those in the control group (intubation, 20%; mortality, 38.33%).
The difference in mortality between the MAFLD group and the control group was statistically significant (P = .02). The mortality difference between the NAFLD and the control group fell just short of statistical significance (P = .07).
For intubation, the difference between the MAFLD and the control group was highly statistically significant (P = .001), and the difference between the NAFLD and the control group was also statistically significant (P = .01)
Patients with advanced fibrosis and either MAFLD or NAFLD were also more likely to die than patients in the control group with advanced fibrosis.
That’s why screening for MAFLD is important, Mr. Vázquez-Medina said.
Next steps and new questions
Future research should examine whether patients with MAFLD have elevated levels of biomarkers for inflammation, such as interleukin 6, Mr. Vázquez-Medina said. A “chronic low proinflammatory state” may be the key to understanding the vulnerability of patients to MAFLD to COVID-19, he speculated.
The metabolic traits associated with MAFLD could explain the higher mortality and intubation rates with COVID, said Rohit Loomba, MD, MHSc, a professor of medicine in the division of gastroenterology at the University of California, San Diego, who was not involved in the study.
“Hypertension, diabetes, and obesity increase the risk of complications from COVID in all patients, whether they have been diagnosed with NAFLD or not,” he told this news organization in an email.
Mr. Vasquez-Medina pointed out that the patients with MAFLD had a higher risk of mortality even after adjusting for age, sex, type 2 diabetes, hypertension, overweight, and obesity (BMI ≥ 30 kg/m2). MAFLD also was more strongly associated with a poor outcome than either hypertension alone or obesity alone. Only age emerged as a significant independent covariate in the study.
Dr. Loomba also questioned whether the regression model used in this study for liver steatosis was “fully reflective of NAFLD.”
The researchers identified liver steatosis with a diagnostic formula that used noninvasive clinical BMI and laboratory tests (alanine aminotransferase), citing a study that found the regression formula was better at diagnosing NAFLD than FibroScan.
Mr. Vázquez-Medina reported no relevant financial relationships. Dr. Loomba serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse Bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen, Madrigal, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89bio, Terns Pharmaceuticals, and Viking Therapeutics. He is co-founder of LipoNexus.
A version of this article first appeared on Medscape.com.
People with metabolic dysfunction–associated fatty liver disease (MAFLD) – a newly defined condition – may be more likely to die from COVID-19, researchers say.
A cohort of people hospitalized for COVID-19 in Central Military Hospital, Mexico City, who met the criteria for MAFLD died at a higher rate than a control group without fatty liver disease, said Martín Uriel Vázquez-Medina, MSc, a researcher in the National Polytechnic Institute in Mexico City.
Patients who met only the criteria for the traditional classification, nonalcoholic fatty liver disease (NAFLD), also died of COVID-19 at a higher rate than the control group, but the difference was not statistically significant.
“It is important to screen for MAFLD,” Mr. Vázquez-Medina told this news organization. “It’s a new definition, but it has really helped us to identify which patients are going to get worse by COVID-19.”
The study was published in Hepatology Communications.
More evidence for clinical relevance of MAFLD
The finding lends support to an initiative to use MAFLD instead of NAFLD to identify patients whose liver steatosis poses a threat to their health, Mr. Vázquez-Medina said.
NAFLD affects as much as a quarter of the world’s population. No drugs have been approved to treat it. Some researchers have reasoned that the imprecision of the definition of NAFLD could be one reason for the lack of progress in treatment.
“NAFLD is something that doesn’t have positive criteria to be diagnosed,” said Mr. Vázquez-Medina. “You only say NAFLD when you don’t find hepatitis or another disease.”
In an article published in Gastroenterology, an international consensus panel proposed MAFLD as an alternative, arguing that a focus on metabolic dysfunction could more accurately reflect the pathogenesis of the disease and help stratify patients.
Previous research has suggested that patients with MAFLD have a higher risk of atherosclerotic cardiovascular disease and that the prevalence of colorectal adenomas is a higher in these patients, compared with patients with NAFLD.
The high prevalence of MAFLD in Mexico – about 30% – could help explain the country’s high rate of mortality from COVID-19, Mr. Vázquez-Medina said. Almost 6% of people diagnosed with COVID in Mexico have died from it, according to the Johns Hopkins University and Medical Center Coronavirus Resource Center.
Sorting COVID outcomes by liver steatosis
To understand the interaction of MAFLD, NAFLD, liver fibrosis, and COVID-19, Mr. Vázquez-Medina and his colleagues analyzed the records of all patients admitted to the Central Military Hospital with COVID-19 from April 4, 2020, to June 24, 2020.
They excluded patients for whom complete data were lacking or for whom a liver function test was not conducted in the first 24 hours of hospitalization. Also excluded were patients with significant consumption of alcohol (> 30 g/day for men and > 20 g/day for women) and those with a history of autoimmune liver disease, liver cancer, decompensated cirrhosis, platelet disorders, or myopathies.
The remaining patients were divided into three groups – 220 who met the criteria for MAFLD, 79 who met the criteria for NAFLD but not MAFLD, and 60 other patients as a control group.
The researchers defined MAFLD as the presence of liver steatosis detected with a noninvasive method and one of the following: overweight (body mass index, 25-29.9 kg/m2), type 2 diabetes, or the presence of two metabolic abnormalities (blood pressure > 140/90 mm Hg, plasma triglycerides > 150 mg/dL, plasma high-density lipoprotein cholesterol < 40 mg/dL in men and < 50 mg/dL in women, and prediabetes).
They defined NAFLD as the presence of liver steatosis without the other criteria for MAFLD.
The patients with MAFLD were the most likely to be intubated and were the most likely to die (intubation, 44.09%; mortality, 55%), followed by those with NAFLD (intubation, 40.51%; mortality, 51.9%) and those in the control group (intubation, 20%; mortality, 38.33%).
The difference in mortality between the MAFLD group and the control group was statistically significant (P = .02). The mortality difference between the NAFLD and the control group fell just short of statistical significance (P = .07).
For intubation, the difference between the MAFLD and the control group was highly statistically significant (P = .001), and the difference between the NAFLD and the control group was also statistically significant (P = .01)
Patients with advanced fibrosis and either MAFLD or NAFLD were also more likely to die than patients in the control group with advanced fibrosis.
That’s why screening for MAFLD is important, Mr. Vázquez-Medina said.
Next steps and new questions
Future research should examine whether patients with MAFLD have elevated levels of biomarkers for inflammation, such as interleukin 6, Mr. Vázquez-Medina said. A “chronic low proinflammatory state” may be the key to understanding the vulnerability of patients to MAFLD to COVID-19, he speculated.
The metabolic traits associated with MAFLD could explain the higher mortality and intubation rates with COVID, said Rohit Loomba, MD, MHSc, a professor of medicine in the division of gastroenterology at the University of California, San Diego, who was not involved in the study.
“Hypertension, diabetes, and obesity increase the risk of complications from COVID in all patients, whether they have been diagnosed with NAFLD or not,” he told this news organization in an email.
Mr. Vasquez-Medina pointed out that the patients with MAFLD had a higher risk of mortality even after adjusting for age, sex, type 2 diabetes, hypertension, overweight, and obesity (BMI ≥ 30 kg/m2). MAFLD also was more strongly associated with a poor outcome than either hypertension alone or obesity alone. Only age emerged as a significant independent covariate in the study.
Dr. Loomba also questioned whether the regression model used in this study for liver steatosis was “fully reflective of NAFLD.”
The researchers identified liver steatosis with a diagnostic formula that used noninvasive clinical BMI and laboratory tests (alanine aminotransferase), citing a study that found the regression formula was better at diagnosing NAFLD than FibroScan.
Mr. Vázquez-Medina reported no relevant financial relationships. Dr. Loomba serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse Bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen, Madrigal, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89bio, Terns Pharmaceuticals, and Viking Therapeutics. He is co-founder of LipoNexus.
A version of this article first appeared on Medscape.com.
FROM HEPATOLOGY COMMUNICATIONS
Clinical chest images power up survival prediction in lung cancer
In patients with stage I lung cancer, adding noncancerous features from CT chest imaging predicts overall survival better than clinical characteristics alone, according to a paper published online in the American Journal of Roentgenology.
Modeling that incorporates noncancerous imaging features captured on chest computed tomography (CT) along with clinical features, when calculated before stereotactic body radiation therapy (SBRT) is administered, improves survival prediction, compared with modeling that relies only on clinical features, the authors report.
“The focus of the study was to look at the environment in which the cancer lives,” said senior author Florian J. Fintelmann, MD, radiologist at Massachusetts General Hospital and associate professor of radiology at Harvard Medical School, both in Boston. “This is looking at parameters like the aortic diameter, body composition – that is, the quantification and characterization of adipose tissue and muscle – coronary artery calcifications, and emphysema quantification.”
CT images are used by radiation oncologists to determine where the radiation should be delivered. “There is more information from these images that we can utilize,” he said.
Survival estimates in patients with state I lung cancer now rely on biological age, ECOG (Eastern Cooperative Oncology Group) score, and the presence of comorbidities, Dr. Fintelmann said.
This retrospective investigation involved 282 patients with a median age of 75 years. There were 168 women and 114 men. All patients had stage I lung cancer and were treated with SBRT between January 2009 and June 2017.
Investigators analyzed pre-treatment chest images with CT. They assessed coronary artery calcium (CAC) score (see above image), pulmonary artery (PA)-to-aorta ratio, emphysema, and several measures of body composition (skeletal muscle and adipose tissue). They developed a statistical model to link clinical and imaging features with overall survival.
An elevated CAC score (11-399: HR, 1.83 [95% confidence interval, 1.15-2.91]; ≥ 400: HR, 1.63 [95% CI, 1.01-2.63]), increased PA-to-aorta ratio (HR, 1.33 [95% CI, 1.16-1.52], per 0.1-unit increase) and decreased thoracic skeletal muscle (HR, 0.88 [95% CI, 0.79-0.98], per 10 cm2/m2 increase) were independently associated with shorter overall survival, investigators observed.
In addition, 5-year overall survival was superior for the model that included clinical and imaging features and inferior for the model restricted to only clinical features. Of all features, the one that emerged the most predictive of overall survival was PA-to-aorta ratio.
In this single-center study of stage I lung cancer patients who were undergoing SBRT, increased CAC score, increased PA-to-aorta ratio, and decreased thoracic skeletal muscle index were independently predictive of poorer overall survival.
“Our modeling shows that these imaging features add so much more [to predicting overall survival],” Dr. Fintelmann said. “The strength of this study is that we show the utility [of the model] and how it exceeds the clinical risk prediction that is currently standard of care. We think this will benefit patients in terms of being able to counsel them and better advise them on their medical decisions.”
This proof-of-concept investigation requires external validation, Dr. Fintelmann stressed. “External data for validation is the next step,” he said, noting he and co-investigators welcome data input from other investigators.
Elsie Nguyen, MD, FRCPC, FNASCI, associate professor of radiology, University of Toronto, responded by email that the study shows that imaging features supplement clinical data in predicting overall survival.
“This study demonstrates the value of extracting non–cancer related computed tomography imaging features to build a model that can better predict overall survival as compared to clinical parameters alone (such as age, performance status and co-morbidities) for stage I lung cancer patients treated with SBRT,” Dr. Nguyen wrote.
“Coronary artery calcium score, pulmonary artery-to-aorta ratio, and sarcopenia independently predicted overall survival,” she wrote. “These results are not surprising, as the prognostic value of each of these imaging features has already been established in the literature.”
Dr. Nguyen pointed out the power in the sum of these imaging features to predict overall survival.
“However, the results of this study demonstrate promising results supportive of the notion that combining clinical and imaging data points can help build a more accurate prediction model for overall survival,” she wrote. “This is analogous to the Brock University (in St. Catharines, Ontario) calculator for solitary pulmonary nodules that calculates malignancy risk based on both clinical and imaging data points. However, external validation of these study results at other centers is first required.”
Dr. Fintelmann and Dr. Nguyen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In patients with stage I lung cancer, adding noncancerous features from CT chest imaging predicts overall survival better than clinical characteristics alone, according to a paper published online in the American Journal of Roentgenology.
Modeling that incorporates noncancerous imaging features captured on chest computed tomography (CT) along with clinical features, when calculated before stereotactic body radiation therapy (SBRT) is administered, improves survival prediction, compared with modeling that relies only on clinical features, the authors report.
“The focus of the study was to look at the environment in which the cancer lives,” said senior author Florian J. Fintelmann, MD, radiologist at Massachusetts General Hospital and associate professor of radiology at Harvard Medical School, both in Boston. “This is looking at parameters like the aortic diameter, body composition – that is, the quantification and characterization of adipose tissue and muscle – coronary artery calcifications, and emphysema quantification.”
CT images are used by radiation oncologists to determine where the radiation should be delivered. “There is more information from these images that we can utilize,” he said.
Survival estimates in patients with state I lung cancer now rely on biological age, ECOG (Eastern Cooperative Oncology Group) score, and the presence of comorbidities, Dr. Fintelmann said.
This retrospective investigation involved 282 patients with a median age of 75 years. There were 168 women and 114 men. All patients had stage I lung cancer and were treated with SBRT between January 2009 and June 2017.

Investigators analyzed pre-treatment chest images with CT. They assessed coronary artery calcium (CAC) score (see above image), pulmonary artery (PA)-to-aorta ratio, emphysema, and several measures of body composition (skeletal muscle and adipose tissue). They developed a statistical model to link clinical and imaging features with overall survival.
An elevated CAC score (11-399: HR, 1.83 [95% confidence interval, 1.15-2.91]; ≥ 400: HR, 1.63 [95% CI, 1.01-2.63]), increased PA-to-aorta ratio (HR, 1.33 [95% CI, 1.16-1.52], per 0.1-unit increase) and decreased thoracic skeletal muscle (HR, 0.88 [95% CI, 0.79-0.98], per 10 cm2/m2 increase) were independently associated with shorter overall survival, investigators observed.
In addition, 5-year overall survival was superior for the model that included clinical and imaging features and inferior for the model restricted to only clinical features. Of all features, the one that emerged the most predictive of overall survival was PA-to-aorta ratio.
In this single-center study of stage I lung cancer patients who were undergoing SBRT, increased CAC score, increased PA-to-aorta ratio, and decreased thoracic skeletal muscle index were independently predictive of poorer overall survival.
“Our modeling shows that these imaging features add so much more [to predicting overall survival],” Dr. Fintelmann said. “The strength of this study is that we show the utility [of the model] and how it exceeds the clinical risk prediction that is currently standard of care. We think this will benefit patients in terms of being able to counsel them and better advise them on their medical decisions.”
This proof-of-concept investigation requires external validation, Dr. Fintelmann stressed. “External data for validation is the next step,” he said, noting he and co-investigators welcome data input from other investigators.
Elsie Nguyen, MD, FRCPC, FNASCI, associate professor of radiology, University of Toronto, responded by email that the study shows that imaging features supplement clinical data in predicting overall survival.
“This study demonstrates the value of extracting non–cancer related computed tomography imaging features to build a model that can better predict overall survival as compared to clinical parameters alone (such as age, performance status and co-morbidities) for stage I lung cancer patients treated with SBRT,” Dr. Nguyen wrote.
“Coronary artery calcium score, pulmonary artery-to-aorta ratio, and sarcopenia independently predicted overall survival,” she wrote. “These results are not surprising, as the prognostic value of each of these imaging features has already been established in the literature.”
Dr. Nguyen pointed out the power in the sum of these imaging features to predict overall survival.
“However, the results of this study demonstrate promising results supportive of the notion that combining clinical and imaging data points can help build a more accurate prediction model for overall survival,” she wrote. “This is analogous to the Brock University (in St. Catharines, Ontario) calculator for solitary pulmonary nodules that calculates malignancy risk based on both clinical and imaging data points. However, external validation of these study results at other centers is first required.”
Dr. Fintelmann and Dr. Nguyen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In patients with stage I lung cancer, adding noncancerous features from CT chest imaging predicts overall survival better than clinical characteristics alone, according to a paper published online in the American Journal of Roentgenology.
Modeling that incorporates noncancerous imaging features captured on chest computed tomography (CT) along with clinical features, when calculated before stereotactic body radiation therapy (SBRT) is administered, improves survival prediction, compared with modeling that relies only on clinical features, the authors report.
“The focus of the study was to look at the environment in which the cancer lives,” said senior author Florian J. Fintelmann, MD, radiologist at Massachusetts General Hospital and associate professor of radiology at Harvard Medical School, both in Boston. “This is looking at parameters like the aortic diameter, body composition – that is, the quantification and characterization of adipose tissue and muscle – coronary artery calcifications, and emphysema quantification.”
CT images are used by radiation oncologists to determine where the radiation should be delivered. “There is more information from these images that we can utilize,” he said.
Survival estimates in patients with state I lung cancer now rely on biological age, ECOG (Eastern Cooperative Oncology Group) score, and the presence of comorbidities, Dr. Fintelmann said.
This retrospective investigation involved 282 patients with a median age of 75 years. There were 168 women and 114 men. All patients had stage I lung cancer and were treated with SBRT between January 2009 and June 2017.

Investigators analyzed pre-treatment chest images with CT. They assessed coronary artery calcium (CAC) score (see above image), pulmonary artery (PA)-to-aorta ratio, emphysema, and several measures of body composition (skeletal muscle and adipose tissue). They developed a statistical model to link clinical and imaging features with overall survival.
An elevated CAC score (11-399: HR, 1.83 [95% confidence interval, 1.15-2.91]; ≥ 400: HR, 1.63 [95% CI, 1.01-2.63]), increased PA-to-aorta ratio (HR, 1.33 [95% CI, 1.16-1.52], per 0.1-unit increase) and decreased thoracic skeletal muscle (HR, 0.88 [95% CI, 0.79-0.98], per 10 cm2/m2 increase) were independently associated with shorter overall survival, investigators observed.
In addition, 5-year overall survival was superior for the model that included clinical and imaging features and inferior for the model restricted to only clinical features. Of all features, the one that emerged the most predictive of overall survival was PA-to-aorta ratio.
In this single-center study of stage I lung cancer patients who were undergoing SBRT, increased CAC score, increased PA-to-aorta ratio, and decreased thoracic skeletal muscle index were independently predictive of poorer overall survival.
“Our modeling shows that these imaging features add so much more [to predicting overall survival],” Dr. Fintelmann said. “The strength of this study is that we show the utility [of the model] and how it exceeds the clinical risk prediction that is currently standard of care. We think this will benefit patients in terms of being able to counsel them and better advise them on their medical decisions.”
This proof-of-concept investigation requires external validation, Dr. Fintelmann stressed. “External data for validation is the next step,” he said, noting he and co-investigators welcome data input from other investigators.
Elsie Nguyen, MD, FRCPC, FNASCI, associate professor of radiology, University of Toronto, responded by email that the study shows that imaging features supplement clinical data in predicting overall survival.
“This study demonstrates the value of extracting non–cancer related computed tomography imaging features to build a model that can better predict overall survival as compared to clinical parameters alone (such as age, performance status and co-morbidities) for stage I lung cancer patients treated with SBRT,” Dr. Nguyen wrote.
“Coronary artery calcium score, pulmonary artery-to-aorta ratio, and sarcopenia independently predicted overall survival,” she wrote. “These results are not surprising, as the prognostic value of each of these imaging features has already been established in the literature.”
Dr. Nguyen pointed out the power in the sum of these imaging features to predict overall survival.
“However, the results of this study demonstrate promising results supportive of the notion that combining clinical and imaging data points can help build a more accurate prediction model for overall survival,” she wrote. “This is analogous to the Brock University (in St. Catharines, Ontario) calculator for solitary pulmonary nodules that calculates malignancy risk based on both clinical and imaging data points. However, external validation of these study results at other centers is first required.”
Dr. Fintelmann and Dr. Nguyen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Don’t let FOMI lead to antibiotic overuse
Is fear of missing an infection – call it “FOMI” – leading you to overprescribe antibiotics to your patients?
Inappropriate use of antibiotics can result in adverse events and toxicity, superinfections such as Clostridioides difficile and Methicillin-resistant Staphylococcus aureus, excess mortality and costs, and resistance to the drugs.
All that has been well-known for years, and antibiotic resistance has become a leading public health concern. So why are physicians continuing to overprescribe the drugs?
Speaking at the 2022 annual Internal Medicine Meeting of the American College of Physicians, James “Brad” Cutrell, MD, medical director of antimicrobial stewardship, University of Texas Southwestern Medical Center, Dallas, said clinicians in the United States and elsewhere appear to be falling into a three-part fallacy when it comes to using the drugs: fear of “missing an infection,” coupled with patient expectations that they will leave the office with a prescription and combined with an overemphasis on the potential benefit to the individual at the expense of the risk to society of antibiotic resistance.
Antibiotics are the only drugs that lose their efficacy for all patients over time the more they are used. “For example, if I give a beta blocker to a patient, it’s not going to affect other patients down the road,” Dr. Cutrell said. “It’s not going to lose its efficacy.”
“What we need in medicine is a new culture around antibiotic use,” Dr. Cutrell added. “We need more respect for the dangers of antibiotic misuse and to have confidence in [their] benefits and when they can be used wisely.”
Rampant misuse
Outpatient prescriptions account for at least 60% of antibiotic use in the United States. The rate is even higher in other countries, Dr. Cutrell said during a presentation at the 2022 annual Internal Medicine Meeting of the American College of Physicians.
“About 10% of adult visits and 20% of pediatric visits will result in an antibiotic prescription,” said Dr. Cutrell, noting that prescribing patterns vary widely across the country, with as much as a three-fold difference in some locations. But at least 30% of outpatient antibiotic prescriptions are inappropriately ordered, he said.
“When we look at acute respiratory infections, upwards of 50% are not indicated at all,” he said. Imagine, he added, if the same error rate applied to other medical practices: “What if surgeons were only right 50% of the time, or if the oncologist was only giving the right treatment 50% of the time?”
The most recent Antibiotic Threats Report from the U.S. Centers for Disease Control and Prevention estimated that antibiotic-resistant bacteria and fungi cause more than 2.8 million infections and about 36,000 deaths annually in the United States alone.
How to be a better steward
The core elements for antimicrobial stewardship in the outpatient setting, according to Dr. Cutrell, include making a commitment to optimize prescribing, implementing at least one policy or practice to improve prescribing, monitoring prescribing practices and offering feedback to clinicians, and educating both patients and clinicians.
All that is similar to in-patient stewardship, he said, but outpatient clinicians face a few unique challenges. “Patients are lower acuity, and there is less diagnostic data, and program resources and time are more limited,” he said. Patient satisfaction is also a major driver, and it is also more difficult to measure and track ambulatory antibiotic use.
Interventions have been identified, however, that can help improve stewardship. One is auditing and feedback with peers. “Another [is] commitment posters, which can be placed around the clinic, and that helps set the culture,” he said. “Clinical education and practice guidelines are also important.”
Clinicians should also:
- Observe antibiotic best practices
- Optimize antibiotic selection and dosing
- Practice effective diagnostic stewardship
- Use the shortest duration of therapy necessary
- Avoid antibiotics for inappropriate indications
- Educate patients on when antibiotics are needed
- Follow and become good antibiotic stewardship mentors
“Multiple antibiotic stewardship interventions are effective, particularly those focused on behavioral interventions,” Dr. Cutrell said. “Every provider should follow antibiotic ‘best practices’ and other simple steps to prescribe antibiotics more wisely and to improve patient care.”
Dr. Cutrell reported financial relationships with Gilead Sciences and Regeneron Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Is fear of missing an infection – call it “FOMI” – leading you to overprescribe antibiotics to your patients?
Inappropriate use of antibiotics can result in adverse events and toxicity, superinfections such as Clostridioides difficile and Methicillin-resistant Staphylococcus aureus, excess mortality and costs, and resistance to the drugs.
All that has been well-known for years, and antibiotic resistance has become a leading public health concern. So why are physicians continuing to overprescribe the drugs?
Speaking at the 2022 annual Internal Medicine Meeting of the American College of Physicians, James “Brad” Cutrell, MD, medical director of antimicrobial stewardship, University of Texas Southwestern Medical Center, Dallas, said clinicians in the United States and elsewhere appear to be falling into a three-part fallacy when it comes to using the drugs: fear of “missing an infection,” coupled with patient expectations that they will leave the office with a prescription and combined with an overemphasis on the potential benefit to the individual at the expense of the risk to society of antibiotic resistance.
Antibiotics are the only drugs that lose their efficacy for all patients over time the more they are used. “For example, if I give a beta blocker to a patient, it’s not going to affect other patients down the road,” Dr. Cutrell said. “It’s not going to lose its efficacy.”
“What we need in medicine is a new culture around antibiotic use,” Dr. Cutrell added. “We need more respect for the dangers of antibiotic misuse and to have confidence in [their] benefits and when they can be used wisely.”
Rampant misuse
Outpatient prescriptions account for at least 60% of antibiotic use in the United States. The rate is even higher in other countries, Dr. Cutrell said during a presentation at the 2022 annual Internal Medicine Meeting of the American College of Physicians.
“About 10% of adult visits and 20% of pediatric visits will result in an antibiotic prescription,” said Dr. Cutrell, noting that prescribing patterns vary widely across the country, with as much as a three-fold difference in some locations. But at least 30% of outpatient antibiotic prescriptions are inappropriately ordered, he said.
“When we look at acute respiratory infections, upwards of 50% are not indicated at all,” he said. Imagine, he added, if the same error rate applied to other medical practices: “What if surgeons were only right 50% of the time, or if the oncologist was only giving the right treatment 50% of the time?”
The most recent Antibiotic Threats Report from the U.S. Centers for Disease Control and Prevention estimated that antibiotic-resistant bacteria and fungi cause more than 2.8 million infections and about 36,000 deaths annually in the United States alone.
How to be a better steward
The core elements for antimicrobial stewardship in the outpatient setting, according to Dr. Cutrell, include making a commitment to optimize prescribing, implementing at least one policy or practice to improve prescribing, monitoring prescribing practices and offering feedback to clinicians, and educating both patients and clinicians.
All that is similar to in-patient stewardship, he said, but outpatient clinicians face a few unique challenges. “Patients are lower acuity, and there is less diagnostic data, and program resources and time are more limited,” he said. Patient satisfaction is also a major driver, and it is also more difficult to measure and track ambulatory antibiotic use.
Interventions have been identified, however, that can help improve stewardship. One is auditing and feedback with peers. “Another [is] commitment posters, which can be placed around the clinic, and that helps set the culture,” he said. “Clinical education and practice guidelines are also important.”
Clinicians should also:
- Observe antibiotic best practices
- Optimize antibiotic selection and dosing
- Practice effective diagnostic stewardship
- Use the shortest duration of therapy necessary
- Avoid antibiotics for inappropriate indications
- Educate patients on when antibiotics are needed
- Follow and become good antibiotic stewardship mentors
“Multiple antibiotic stewardship interventions are effective, particularly those focused on behavioral interventions,” Dr. Cutrell said. “Every provider should follow antibiotic ‘best practices’ and other simple steps to prescribe antibiotics more wisely and to improve patient care.”
Dr. Cutrell reported financial relationships with Gilead Sciences and Regeneron Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Is fear of missing an infection – call it “FOMI” – leading you to overprescribe antibiotics to your patients?
Inappropriate use of antibiotics can result in adverse events and toxicity, superinfections such as Clostridioides difficile and Methicillin-resistant Staphylococcus aureus, excess mortality and costs, and resistance to the drugs.
All that has been well-known for years, and antibiotic resistance has become a leading public health concern. So why are physicians continuing to overprescribe the drugs?
Speaking at the 2022 annual Internal Medicine Meeting of the American College of Physicians, James “Brad” Cutrell, MD, medical director of antimicrobial stewardship, University of Texas Southwestern Medical Center, Dallas, said clinicians in the United States and elsewhere appear to be falling into a three-part fallacy when it comes to using the drugs: fear of “missing an infection,” coupled with patient expectations that they will leave the office with a prescription and combined with an overemphasis on the potential benefit to the individual at the expense of the risk to society of antibiotic resistance.
Antibiotics are the only drugs that lose their efficacy for all patients over time the more they are used. “For example, if I give a beta blocker to a patient, it’s not going to affect other patients down the road,” Dr. Cutrell said. “It’s not going to lose its efficacy.”
“What we need in medicine is a new culture around antibiotic use,” Dr. Cutrell added. “We need more respect for the dangers of antibiotic misuse and to have confidence in [their] benefits and when they can be used wisely.”
Rampant misuse
Outpatient prescriptions account for at least 60% of antibiotic use in the United States. The rate is even higher in other countries, Dr. Cutrell said during a presentation at the 2022 annual Internal Medicine Meeting of the American College of Physicians.
“About 10% of adult visits and 20% of pediatric visits will result in an antibiotic prescription,” said Dr. Cutrell, noting that prescribing patterns vary widely across the country, with as much as a three-fold difference in some locations. But at least 30% of outpatient antibiotic prescriptions are inappropriately ordered, he said.
“When we look at acute respiratory infections, upwards of 50% are not indicated at all,” he said. Imagine, he added, if the same error rate applied to other medical practices: “What if surgeons were only right 50% of the time, or if the oncologist was only giving the right treatment 50% of the time?”
The most recent Antibiotic Threats Report from the U.S. Centers for Disease Control and Prevention estimated that antibiotic-resistant bacteria and fungi cause more than 2.8 million infections and about 36,000 deaths annually in the United States alone.
How to be a better steward
The core elements for antimicrobial stewardship in the outpatient setting, according to Dr. Cutrell, include making a commitment to optimize prescribing, implementing at least one policy or practice to improve prescribing, monitoring prescribing practices and offering feedback to clinicians, and educating both patients and clinicians.
All that is similar to in-patient stewardship, he said, but outpatient clinicians face a few unique challenges. “Patients are lower acuity, and there is less diagnostic data, and program resources and time are more limited,” he said. Patient satisfaction is also a major driver, and it is also more difficult to measure and track ambulatory antibiotic use.
Interventions have been identified, however, that can help improve stewardship. One is auditing and feedback with peers. “Another [is] commitment posters, which can be placed around the clinic, and that helps set the culture,” he said. “Clinical education and practice guidelines are also important.”
Clinicians should also:
- Observe antibiotic best practices
- Optimize antibiotic selection and dosing
- Practice effective diagnostic stewardship
- Use the shortest duration of therapy necessary
- Avoid antibiotics for inappropriate indications
- Educate patients on when antibiotics are needed
- Follow and become good antibiotic stewardship mentors
“Multiple antibiotic stewardship interventions are effective, particularly those focused on behavioral interventions,” Dr. Cutrell said. “Every provider should follow antibiotic ‘best practices’ and other simple steps to prescribe antibiotics more wisely and to improve patient care.”
Dr. Cutrell reported financial relationships with Gilead Sciences and Regeneron Pharmaceuticals.
A version of this article first appeared on Medscape.com.
FROM INTERNAL MEDICINE 2022