User login
Cutting dementia risk in AFib: Does rhythm control strategy matter?
The risk for dementia goes up in patients with atrial fibrillation (AFib), but some evidence suggests that risk can be blunted with therapies that restore sinus rhythm. However, a new cohort study suggests that the treatment effect’s magnitude might depend on the rhythm control strategy. It hinted that AFib catheter ablation might be more effective than pharmacologic rhythm control alone at cutting the risk for dementia.
The case-matched study of more than 38,000 adults with AFib saw a 41% reduction (P < .0001) in risk for dementia among those who underwent catheter ablation after attempted rhythm control with antiarrhythmic drugs (AAD), compared with those managed with pharmacologic rhythm control therapy alone.
The observational study comprising 20 years of data comes with big limitations and can’t say for sure whether catheter ablation is better than AAD-only at cutting the dementia risk in AFib. But it and other evidence support the idea, which has yet to be explored in a randomized fashion.
In a secondary finding, the analysis showed a similar reduction in dementia risk from catheter ablation, compared with AAD, in women and in men by 40% and 45%, respectively (P < .0001 for both). The findings are particularly relevant “given the higher life-long risk of dementia among women and the lower likelihood that women will be offered ablation, which has been demonstrated repeatedly,” Emily P. Zeitler, MD, MHS, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire, told this news organization. “I think this is another reason to try to be more generous in offering ablation to women.”
Management of AFib certainly evolved in important ways from 2000 to 2021, the period covered by the study. But a sensitivity analysis based on data from 2010 to 2021 showed “no meaningful differences” in the results, said Dr. Zeitler, who is slated to present the findings April 30 at the Heart Rhythm Society 2022 Scientific Sessions, conducted virtually and live in San Francisco.
Dr. Zeitler acknowledged that the observational study, even with its propensity-matched ablation and AAD cohorts, can only hint at a preference for ablation over AAD for lowering risk for AFib-associated dementia. “We know there’s unmeasured and unfixable confounding between those two groups, so we see this really as hypothesis-generating.”
It was “a well-done analysis,” and the conclusion that the dementia risk was lower with catheter ablation is “absolutely correct,” but only as far as the study and its limitations allow, agreed David Conen, MD, MPH, McMaster University, Hamilton, Ontario, who is not a coauthor.
“Even with propensity matching, you can get rid of some sorts of confounding, but you can never get rid of all selection bias issues.” That, he said when interviewed, takes randomized trials.
Dr. Conen, who is studying cognitive decline in AFib as a SWISS-AF trial principal investigator, pointed to a secondary finding of the analysis as evidence for such confounding. He said the ablation group’s nearly 50% drop (P < .0001) in competing risk for death, compared with patients managed with AAD, isn’t plausible.
The finding “strongly suggests these people were healthier and that there’s some sort of selection bias. They were at lower risk of death, they were at lower risk of dementia, and they were probably also at lower risk of stroke, myocardial infarction, thrombosis, and cancer because they were just probably a little healthier than the others,” Dr. Conen said. The ablation and AAD groups “were two very different populations from the get-go.”
The analysis was based on U.S. insurance and Medicare claims data from AFib patients who either underwent catheter ablation after at least one AAD trial or filled prescriptions for at least two different antiarrhythmic agents in the year after AFib diagnosis. Patients with history of dementia, catheter or surgical AFib ablation, or a valve procedure were excluded.
The ablation and AAD-only groups each consisted of 19,066 patients after propensity matching, and the groups were balanced with respect to age, sex, type of insurance, CHA2DS2-VASc scores, and use of renin-angiotensin-system inhibitors, oral anticoagulants, and antiplatelets.
The overall risk for dementia was 1.9% for the ablation group and 3.3% for AAD-only patients (hazard ratio, 0.59; 95% confidence interval, 0.52-0.67). Corresponding HRs by sex were 0.55 (95% CI, 0.46-0.66) for men and 0.60 (95% CI, 0.50-0.72) for women.
The competing risk for death was also significantly decreased in the ablation group (HR, 0.51; 95% CI, 0.46-0.55).
Dr. Zeitler pointed to a randomized trial now in the early stages called Neurocognition and Greater Maintenance of Sinus Rhythm in Atrial Fibrillation, or NOGGIN-AF, which will explore relationships between rhythm control therapy and dementia in patients with AFib, whether catheter ablation or AAD can mitigate that risk, and whether either strategy works better than the other, among other goals.
“I’m optimistic,” she said, “and I think it’s going to add to the growing motivations to get patients ablated more quickly and more broadly.”
The analysis was funded by Biosense-Webster. Dr. Zeitler discloses consulting for Biosense-Webster and Arena Pharmaceuticals (now Pfizer); fees for speaking from Medtronic; and receiving research support from Boston Scientific, Sanofi, and Biosense-Webster. Dr. Conen has previously reported receiving speaker fees from Servier Canada.
A version of this article first appeared on Medscape.com.
The risk for dementia goes up in patients with atrial fibrillation (AFib), but some evidence suggests that risk can be blunted with therapies that restore sinus rhythm. However, a new cohort study suggests that the treatment effect’s magnitude might depend on the rhythm control strategy. It hinted that AFib catheter ablation might be more effective than pharmacologic rhythm control alone at cutting the risk for dementia.
The case-matched study of more than 38,000 adults with AFib saw a 41% reduction (P < .0001) in risk for dementia among those who underwent catheter ablation after attempted rhythm control with antiarrhythmic drugs (AAD), compared with those managed with pharmacologic rhythm control therapy alone.
The observational study comprising 20 years of data comes with big limitations and can’t say for sure whether catheter ablation is better than AAD-only at cutting the dementia risk in AFib. But it and other evidence support the idea, which has yet to be explored in a randomized fashion.
In a secondary finding, the analysis showed a similar reduction in dementia risk from catheter ablation, compared with AAD, in women and in men by 40% and 45%, respectively (P < .0001 for both). The findings are particularly relevant “given the higher life-long risk of dementia among women and the lower likelihood that women will be offered ablation, which has been demonstrated repeatedly,” Emily P. Zeitler, MD, MHS, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire, told this news organization. “I think this is another reason to try to be more generous in offering ablation to women.”
Management of AFib certainly evolved in important ways from 2000 to 2021, the period covered by the study. But a sensitivity analysis based on data from 2010 to 2021 showed “no meaningful differences” in the results, said Dr. Zeitler, who is slated to present the findings April 30 at the Heart Rhythm Society 2022 Scientific Sessions, conducted virtually and live in San Francisco.
Dr. Zeitler acknowledged that the observational study, even with its propensity-matched ablation and AAD cohorts, can only hint at a preference for ablation over AAD for lowering risk for AFib-associated dementia. “We know there’s unmeasured and unfixable confounding between those two groups, so we see this really as hypothesis-generating.”
It was “a well-done analysis,” and the conclusion that the dementia risk was lower with catheter ablation is “absolutely correct,” but only as far as the study and its limitations allow, agreed David Conen, MD, MPH, McMaster University, Hamilton, Ontario, who is not a coauthor.
“Even with propensity matching, you can get rid of some sorts of confounding, but you can never get rid of all selection bias issues.” That, he said when interviewed, takes randomized trials.
Dr. Conen, who is studying cognitive decline in AFib as a SWISS-AF trial principal investigator, pointed to a secondary finding of the analysis as evidence for such confounding. He said the ablation group’s nearly 50% drop (P < .0001) in competing risk for death, compared with patients managed with AAD, isn’t plausible.
The finding “strongly suggests these people were healthier and that there’s some sort of selection bias. They were at lower risk of death, they were at lower risk of dementia, and they were probably also at lower risk of stroke, myocardial infarction, thrombosis, and cancer because they were just probably a little healthier than the others,” Dr. Conen said. The ablation and AAD groups “were two very different populations from the get-go.”
The analysis was based on U.S. insurance and Medicare claims data from AFib patients who either underwent catheter ablation after at least one AAD trial or filled prescriptions for at least two different antiarrhythmic agents in the year after AFib diagnosis. Patients with history of dementia, catheter or surgical AFib ablation, or a valve procedure were excluded.
The ablation and AAD-only groups each consisted of 19,066 patients after propensity matching, and the groups were balanced with respect to age, sex, type of insurance, CHA2DS2-VASc scores, and use of renin-angiotensin-system inhibitors, oral anticoagulants, and antiplatelets.
The overall risk for dementia was 1.9% for the ablation group and 3.3% for AAD-only patients (hazard ratio, 0.59; 95% confidence interval, 0.52-0.67). Corresponding HRs by sex were 0.55 (95% CI, 0.46-0.66) for men and 0.60 (95% CI, 0.50-0.72) for women.
The competing risk for death was also significantly decreased in the ablation group (HR, 0.51; 95% CI, 0.46-0.55).
Dr. Zeitler pointed to a randomized trial now in the early stages called Neurocognition and Greater Maintenance of Sinus Rhythm in Atrial Fibrillation, or NOGGIN-AF, which will explore relationships between rhythm control therapy and dementia in patients with AFib, whether catheter ablation or AAD can mitigate that risk, and whether either strategy works better than the other, among other goals.
“I’m optimistic,” she said, “and I think it’s going to add to the growing motivations to get patients ablated more quickly and more broadly.”
The analysis was funded by Biosense-Webster. Dr. Zeitler discloses consulting for Biosense-Webster and Arena Pharmaceuticals (now Pfizer); fees for speaking from Medtronic; and receiving research support from Boston Scientific, Sanofi, and Biosense-Webster. Dr. Conen has previously reported receiving speaker fees from Servier Canada.
A version of this article first appeared on Medscape.com.
The risk for dementia goes up in patients with atrial fibrillation (AFib), but some evidence suggests that risk can be blunted with therapies that restore sinus rhythm. However, a new cohort study suggests that the treatment effect’s magnitude might depend on the rhythm control strategy. It hinted that AFib catheter ablation might be more effective than pharmacologic rhythm control alone at cutting the risk for dementia.
The case-matched study of more than 38,000 adults with AFib saw a 41% reduction (P < .0001) in risk for dementia among those who underwent catheter ablation after attempted rhythm control with antiarrhythmic drugs (AAD), compared with those managed with pharmacologic rhythm control therapy alone.
The observational study comprising 20 years of data comes with big limitations and can’t say for sure whether catheter ablation is better than AAD-only at cutting the dementia risk in AFib. But it and other evidence support the idea, which has yet to be explored in a randomized fashion.
In a secondary finding, the analysis showed a similar reduction in dementia risk from catheter ablation, compared with AAD, in women and in men by 40% and 45%, respectively (P < .0001 for both). The findings are particularly relevant “given the higher life-long risk of dementia among women and the lower likelihood that women will be offered ablation, which has been demonstrated repeatedly,” Emily P. Zeitler, MD, MHS, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire, told this news organization. “I think this is another reason to try to be more generous in offering ablation to women.”
Management of AFib certainly evolved in important ways from 2000 to 2021, the period covered by the study. But a sensitivity analysis based on data from 2010 to 2021 showed “no meaningful differences” in the results, said Dr. Zeitler, who is slated to present the findings April 30 at the Heart Rhythm Society 2022 Scientific Sessions, conducted virtually and live in San Francisco.
Dr. Zeitler acknowledged that the observational study, even with its propensity-matched ablation and AAD cohorts, can only hint at a preference for ablation over AAD for lowering risk for AFib-associated dementia. “We know there’s unmeasured and unfixable confounding between those two groups, so we see this really as hypothesis-generating.”
It was “a well-done analysis,” and the conclusion that the dementia risk was lower with catheter ablation is “absolutely correct,” but only as far as the study and its limitations allow, agreed David Conen, MD, MPH, McMaster University, Hamilton, Ontario, who is not a coauthor.
“Even with propensity matching, you can get rid of some sorts of confounding, but you can never get rid of all selection bias issues.” That, he said when interviewed, takes randomized trials.
Dr. Conen, who is studying cognitive decline in AFib as a SWISS-AF trial principal investigator, pointed to a secondary finding of the analysis as evidence for such confounding. He said the ablation group’s nearly 50% drop (P < .0001) in competing risk for death, compared with patients managed with AAD, isn’t plausible.
The finding “strongly suggests these people were healthier and that there’s some sort of selection bias. They were at lower risk of death, they were at lower risk of dementia, and they were probably also at lower risk of stroke, myocardial infarction, thrombosis, and cancer because they were just probably a little healthier than the others,” Dr. Conen said. The ablation and AAD groups “were two very different populations from the get-go.”
The analysis was based on U.S. insurance and Medicare claims data from AFib patients who either underwent catheter ablation after at least one AAD trial or filled prescriptions for at least two different antiarrhythmic agents in the year after AFib diagnosis. Patients with history of dementia, catheter or surgical AFib ablation, or a valve procedure were excluded.
The ablation and AAD-only groups each consisted of 19,066 patients after propensity matching, and the groups were balanced with respect to age, sex, type of insurance, CHA2DS2-VASc scores, and use of renin-angiotensin-system inhibitors, oral anticoagulants, and antiplatelets.
The overall risk for dementia was 1.9% for the ablation group and 3.3% for AAD-only patients (hazard ratio, 0.59; 95% confidence interval, 0.52-0.67). Corresponding HRs by sex were 0.55 (95% CI, 0.46-0.66) for men and 0.60 (95% CI, 0.50-0.72) for women.
The competing risk for death was also significantly decreased in the ablation group (HR, 0.51; 95% CI, 0.46-0.55).
Dr. Zeitler pointed to a randomized trial now in the early stages called Neurocognition and Greater Maintenance of Sinus Rhythm in Atrial Fibrillation, or NOGGIN-AF, which will explore relationships between rhythm control therapy and dementia in patients with AFib, whether catheter ablation or AAD can mitigate that risk, and whether either strategy works better than the other, among other goals.
“I’m optimistic,” she said, “and I think it’s going to add to the growing motivations to get patients ablated more quickly and more broadly.”
The analysis was funded by Biosense-Webster. Dr. Zeitler discloses consulting for Biosense-Webster and Arena Pharmaceuticals (now Pfizer); fees for speaking from Medtronic; and receiving research support from Boston Scientific, Sanofi, and Biosense-Webster. Dr. Conen has previously reported receiving speaker fees from Servier Canada.
A version of this article first appeared on Medscape.com.
Children and COVID: New cases up for third straight week
Moderna submitted a request to the Food and Drug administration for emergency use authorization of its COVID-19 vaccine in children under the age of 6 years, according to this news organization, and Pfizer/BioNTech officially applied for authorization of a booster dose in children aged 5-11, the companies announced.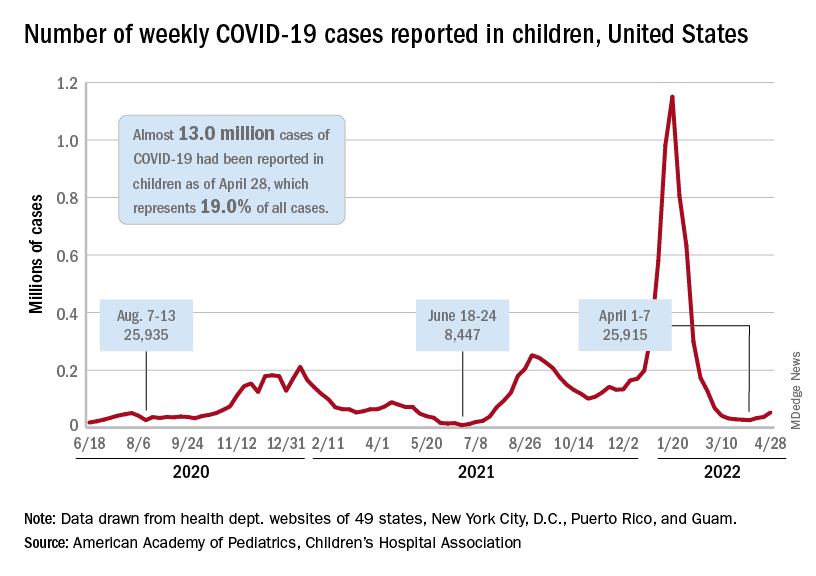
The FDA has tentatively scheduled meetings of its Vaccines and Related Biological Products Advisory Committee in June to consider the applications, saying that it “understands the urgency to authorize a vaccine for age groups who are not currently eligible for vaccination and will work diligently to complete our evaluation of the data. Should any of the submissions be completed in a timely manner and the data support a clear path forward following our evaluation, the FDA will act quickly” to convene the necessary meetings.
The need for greater access to vaccines seems to be increasing, as new pediatric COVID cases rose for the third consecutive week. April 22-28 saw over 53,000 new cases reported in children, up 43.5% from the previous week and up 105% since cases started rising again after dipping under 26,000 during the week of April 1-7, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
Hospital admissions involving diagnosed COVID also ticked up over the latter half of April, although the most recent 7-day average (April 24-30) of 112 per day was lower than the 117 reported for the previous week (April 17-23), the Centers for Disease Control and Prevention said, also noting that figures for the latest week “should be interpreted with caution.”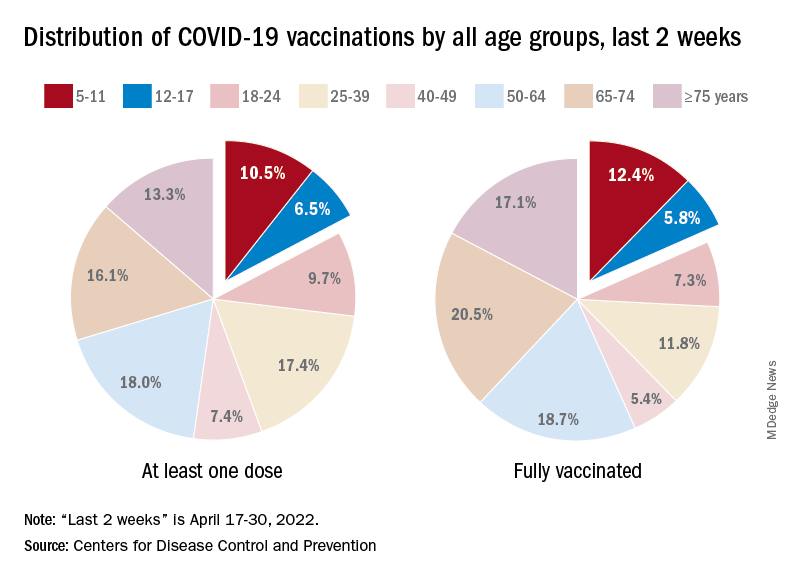
Vaccinations also were up slightly in children aged 5-11 years, with 52,000 receiving their first dose during the week of April 21-27, compared with 48,000 the week before. There was a slight dip, however, among 12- to 17-year-olds, who received 34,000 first doses during April 21-27, versus 35,000 the previous week, the AAP said in a separate report.
Cumulatively, almost 69% of all children aged 12-17 years have received at least one dose of the COVID-19 vaccine and 59% are fully vaccinated. Those aged 5-11 are well short of those figures, with just over 35% having received at least one dose and 28.5% fully vaccinated, the CDC said on its COVID Data Tracker.
A look at recent activity shows that children are not gaining on adults, who are much more likely to be vaccinated – full vaccination in those aged 50-64, for example, is 80%. During the 2 weeks from April 17-30, the 5- to 11-year-olds represented 10.5% of those who had initiated a first dose and 12.4% of those who gained full-vaccination status, both of which were well below the oldest age groups, the CDC reported.
Moderna submitted a request to the Food and Drug administration for emergency use authorization of its COVID-19 vaccine in children under the age of 6 years, according to this news organization, and Pfizer/BioNTech officially applied for authorization of a booster dose in children aged 5-11, the companies announced.
The FDA has tentatively scheduled meetings of its Vaccines and Related Biological Products Advisory Committee in June to consider the applications, saying that it “understands the urgency to authorize a vaccine for age groups who are not currently eligible for vaccination and will work diligently to complete our evaluation of the data. Should any of the submissions be completed in a timely manner and the data support a clear path forward following our evaluation, the FDA will act quickly” to convene the necessary meetings.
The need for greater access to vaccines seems to be increasing, as new pediatric COVID cases rose for the third consecutive week. April 22-28 saw over 53,000 new cases reported in children, up 43.5% from the previous week and up 105% since cases started rising again after dipping under 26,000 during the week of April 1-7, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
Hospital admissions involving diagnosed COVID also ticked up over the latter half of April, although the most recent 7-day average (April 24-30) of 112 per day was lower than the 117 reported for the previous week (April 17-23), the Centers for Disease Control and Prevention said, also noting that figures for the latest week “should be interpreted with caution.”
Vaccinations also were up slightly in children aged 5-11 years, with 52,000 receiving their first dose during the week of April 21-27, compared with 48,000 the week before. There was a slight dip, however, among 12- to 17-year-olds, who received 34,000 first doses during April 21-27, versus 35,000 the previous week, the AAP said in a separate report.
Cumulatively, almost 69% of all children aged 12-17 years have received at least one dose of the COVID-19 vaccine and 59% are fully vaccinated. Those aged 5-11 are well short of those figures, with just over 35% having received at least one dose and 28.5% fully vaccinated, the CDC said on its COVID Data Tracker.
A look at recent activity shows that children are not gaining on adults, who are much more likely to be vaccinated – full vaccination in those aged 50-64, for example, is 80%. During the 2 weeks from April 17-30, the 5- to 11-year-olds represented 10.5% of those who had initiated a first dose and 12.4% of those who gained full-vaccination status, both of which were well below the oldest age groups, the CDC reported.
Moderna submitted a request to the Food and Drug administration for emergency use authorization of its COVID-19 vaccine in children under the age of 6 years, according to this news organization, and Pfizer/BioNTech officially applied for authorization of a booster dose in children aged 5-11, the companies announced.
The FDA has tentatively scheduled meetings of its Vaccines and Related Biological Products Advisory Committee in June to consider the applications, saying that it “understands the urgency to authorize a vaccine for age groups who are not currently eligible for vaccination and will work diligently to complete our evaluation of the data. Should any of the submissions be completed in a timely manner and the data support a clear path forward following our evaluation, the FDA will act quickly” to convene the necessary meetings.
The need for greater access to vaccines seems to be increasing, as new pediatric COVID cases rose for the third consecutive week. April 22-28 saw over 53,000 new cases reported in children, up 43.5% from the previous week and up 105% since cases started rising again after dipping under 26,000 during the week of April 1-7, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
Hospital admissions involving diagnosed COVID also ticked up over the latter half of April, although the most recent 7-day average (April 24-30) of 112 per day was lower than the 117 reported for the previous week (April 17-23), the Centers for Disease Control and Prevention said, also noting that figures for the latest week “should be interpreted with caution.”
Vaccinations also were up slightly in children aged 5-11 years, with 52,000 receiving their first dose during the week of April 21-27, compared with 48,000 the week before. There was a slight dip, however, among 12- to 17-year-olds, who received 34,000 first doses during April 21-27, versus 35,000 the previous week, the AAP said in a separate report.
Cumulatively, almost 69% of all children aged 12-17 years have received at least one dose of the COVID-19 vaccine and 59% are fully vaccinated. Those aged 5-11 are well short of those figures, with just over 35% having received at least one dose and 28.5% fully vaccinated, the CDC said on its COVID Data Tracker.
A look at recent activity shows that children are not gaining on adults, who are much more likely to be vaccinated – full vaccination in those aged 50-64, for example, is 80%. During the 2 weeks from April 17-30, the 5- to 11-year-olds represented 10.5% of those who had initiated a first dose and 12.4% of those who gained full-vaccination status, both of which were well below the oldest age groups, the CDC reported.
New research holds promise for fighting obesity, says expert
Caroline Apovian, MD, codirector of the Center for Weight Management and Wellness at Brigham and Women’s Hospital, described some of the new insights about obesity she has gained during her talk at the annual meeting of the American College of Physicians.
“When I was a medical student a while back, I learned that fat tissue just sat there and stored fat,” she said. “Now we know it’s an endocrine organ.”
This tissue secretes hormones, such as leptin, and other factors that have an array of effects on the brain, pancreas, heart, liver, and muscles. Moreover, it has plasticity, with the ability to change, constantly adjusting our metabolism as nutrient supply and demand changes, she continued.
Obesity leads to a decline in this plasticity, leading to fibrosis and inflammation and other problems. These changes can further impair the function of adipose tissue, leading to metabolic disease. But the central role of adipose tissue, and its dynamic nature, presents an opportunity for treatment, Dr. Apovian said, during her talk.
Hints to why obesity has become more common
More than 42% of the U.S. population – “unbelievably,” Dr. Apovian said – is obese, meaning they have a BMI over 30, according to the Centers for Disease Control and Prevention. That’s up by about 25% since 1960, although calories eaten hasn’t increased, and physical activity has increased somewhat, she said.
The root cause is still a bit of a mystery, but according to “good hints and clues” from animal models that are starting to be translated to the study of human obesity, “it has to do with epigenetics and how our brains and our bodies are perceiving the environment,” she noted, during her presentation.
“Our genes haven’t changed. Our environment has changed,” she said.
The industrialization of the food supply, the use of pesticides and preservatives, the dawn of fast food have all combined, most likely, to do “a number on our bodies,” Dr. Apovian said.
But not all hope is lost thanks to new research, Dr. Apovian suggested.
New treatments show promise for helping patients’ obesity
New research that has increased Dr. Apovian’s understanding of the sophisticated role of adipose tissue may be helpful for treating patients with obesity, offering more targets for intervention, she told the audience.
Some treatment avenues already identified have started producing results, Dr. Apovian noted.
Gastric bypass surgery typically leads to a loss of 25% of body weight, but is often shunned by patients, she said. “With such a great surgical procedure, we still only do 256,000 procedures and we have millions of Americans with a BMI over 30.”
Weight control with obsessive dieting, meal-planning and calorie-counting, “can be done, but it’s really hard,” Dr. Apovian noted.
More appealing therapies targeting hormones and appetite suppression have produced impressive results. Recently approved semaglutide produced 14% weight loss, compared with about 2% for placebo, she said.
Results just released for tirzepatide, a dual agonist of gut hormones GLP-1 and GIP, show a 22% total weight loss, compared with about 2% for placebo, with about 56% of patients losing more than 20% of their body weight, Dr. Apovian said.
Referencing studies finding that several hormones are altered during weight loss, she predicted that targeting multiple hormones with drug treatment will also be necessary for best results.
But, she noted, “we’re treating obesity now with one- or two-drug combos.”
Medication costs are too high for many patients
Isis Smith, MD, an internist at University Medical Center in New Orleans, said in an interview that the cost of the most effective medications – which are not covered by Medicaid – means that many of her patients don’t have access to these treatments.
“We’re talking about $1,000 a month. And so there is no way they can afford [them]. I can prescribe phentermine [but] unless a patient has another indication, Medicaid will not pay for it,” she explained.
“I love hearing about all of the new developments. ... It’s interesting to hear, but we need to get insurance to pay so that I can actually prescribe,” Dr. Smith noted.
Dr. Apovian reports financial relationships with Xeno Biosciences, Cowen, Allergan, Novo Nordisk, Abbott Nutrition, and other companies.
Caroline Apovian, MD, codirector of the Center for Weight Management and Wellness at Brigham and Women’s Hospital, described some of the new insights about obesity she has gained during her talk at the annual meeting of the American College of Physicians.
“When I was a medical student a while back, I learned that fat tissue just sat there and stored fat,” she said. “Now we know it’s an endocrine organ.”
This tissue secretes hormones, such as leptin, and other factors that have an array of effects on the brain, pancreas, heart, liver, and muscles. Moreover, it has plasticity, with the ability to change, constantly adjusting our metabolism as nutrient supply and demand changes, she continued.
Obesity leads to a decline in this plasticity, leading to fibrosis and inflammation and other problems. These changes can further impair the function of adipose tissue, leading to metabolic disease. But the central role of adipose tissue, and its dynamic nature, presents an opportunity for treatment, Dr. Apovian said, during her talk.
Hints to why obesity has become more common
More than 42% of the U.S. population – “unbelievably,” Dr. Apovian said – is obese, meaning they have a BMI over 30, according to the Centers for Disease Control and Prevention. That’s up by about 25% since 1960, although calories eaten hasn’t increased, and physical activity has increased somewhat, she said.
The root cause is still a bit of a mystery, but according to “good hints and clues” from animal models that are starting to be translated to the study of human obesity, “it has to do with epigenetics and how our brains and our bodies are perceiving the environment,” she noted, during her presentation.
“Our genes haven’t changed. Our environment has changed,” she said.
The industrialization of the food supply, the use of pesticides and preservatives, the dawn of fast food have all combined, most likely, to do “a number on our bodies,” Dr. Apovian said.
But not all hope is lost thanks to new research, Dr. Apovian suggested.
New treatments show promise for helping patients’ obesity
New research that has increased Dr. Apovian’s understanding of the sophisticated role of adipose tissue may be helpful for treating patients with obesity, offering more targets for intervention, she told the audience.
Some treatment avenues already identified have started producing results, Dr. Apovian noted.
Gastric bypass surgery typically leads to a loss of 25% of body weight, but is often shunned by patients, she said. “With such a great surgical procedure, we still only do 256,000 procedures and we have millions of Americans with a BMI over 30.”
Weight control with obsessive dieting, meal-planning and calorie-counting, “can be done, but it’s really hard,” Dr. Apovian noted.
More appealing therapies targeting hormones and appetite suppression have produced impressive results. Recently approved semaglutide produced 14% weight loss, compared with about 2% for placebo, she said.
Results just released for tirzepatide, a dual agonist of gut hormones GLP-1 and GIP, show a 22% total weight loss, compared with about 2% for placebo, with about 56% of patients losing more than 20% of their body weight, Dr. Apovian said.
Referencing studies finding that several hormones are altered during weight loss, she predicted that targeting multiple hormones with drug treatment will also be necessary for best results.
But, she noted, “we’re treating obesity now with one- or two-drug combos.”
Medication costs are too high for many patients
Isis Smith, MD, an internist at University Medical Center in New Orleans, said in an interview that the cost of the most effective medications – which are not covered by Medicaid – means that many of her patients don’t have access to these treatments.
“We’re talking about $1,000 a month. And so there is no way they can afford [them]. I can prescribe phentermine [but] unless a patient has another indication, Medicaid will not pay for it,” she explained.
“I love hearing about all of the new developments. ... It’s interesting to hear, but we need to get insurance to pay so that I can actually prescribe,” Dr. Smith noted.
Dr. Apovian reports financial relationships with Xeno Biosciences, Cowen, Allergan, Novo Nordisk, Abbott Nutrition, and other companies.
Caroline Apovian, MD, codirector of the Center for Weight Management and Wellness at Brigham and Women’s Hospital, described some of the new insights about obesity she has gained during her talk at the annual meeting of the American College of Physicians.
“When I was a medical student a while back, I learned that fat tissue just sat there and stored fat,” she said. “Now we know it’s an endocrine organ.”
This tissue secretes hormones, such as leptin, and other factors that have an array of effects on the brain, pancreas, heart, liver, and muscles. Moreover, it has plasticity, with the ability to change, constantly adjusting our metabolism as nutrient supply and demand changes, she continued.
Obesity leads to a decline in this plasticity, leading to fibrosis and inflammation and other problems. These changes can further impair the function of adipose tissue, leading to metabolic disease. But the central role of adipose tissue, and its dynamic nature, presents an opportunity for treatment, Dr. Apovian said, during her talk.
Hints to why obesity has become more common
More than 42% of the U.S. population – “unbelievably,” Dr. Apovian said – is obese, meaning they have a BMI over 30, according to the Centers for Disease Control and Prevention. That’s up by about 25% since 1960, although calories eaten hasn’t increased, and physical activity has increased somewhat, she said.
The root cause is still a bit of a mystery, but according to “good hints and clues” from animal models that are starting to be translated to the study of human obesity, “it has to do with epigenetics and how our brains and our bodies are perceiving the environment,” she noted, during her presentation.
“Our genes haven’t changed. Our environment has changed,” she said.
The industrialization of the food supply, the use of pesticides and preservatives, the dawn of fast food have all combined, most likely, to do “a number on our bodies,” Dr. Apovian said.
But not all hope is lost thanks to new research, Dr. Apovian suggested.
New treatments show promise for helping patients’ obesity
New research that has increased Dr. Apovian’s understanding of the sophisticated role of adipose tissue may be helpful for treating patients with obesity, offering more targets for intervention, she told the audience.
Some treatment avenues already identified have started producing results, Dr. Apovian noted.
Gastric bypass surgery typically leads to a loss of 25% of body weight, but is often shunned by patients, she said. “With such a great surgical procedure, we still only do 256,000 procedures and we have millions of Americans with a BMI over 30.”
Weight control with obsessive dieting, meal-planning and calorie-counting, “can be done, but it’s really hard,” Dr. Apovian noted.
More appealing therapies targeting hormones and appetite suppression have produced impressive results. Recently approved semaglutide produced 14% weight loss, compared with about 2% for placebo, she said.
Results just released for tirzepatide, a dual agonist of gut hormones GLP-1 and GIP, show a 22% total weight loss, compared with about 2% for placebo, with about 56% of patients losing more than 20% of their body weight, Dr. Apovian said.
Referencing studies finding that several hormones are altered during weight loss, she predicted that targeting multiple hormones with drug treatment will also be necessary for best results.
But, she noted, “we’re treating obesity now with one- or two-drug combos.”
Medication costs are too high for many patients
Isis Smith, MD, an internist at University Medical Center in New Orleans, said in an interview that the cost of the most effective medications – which are not covered by Medicaid – means that many of her patients don’t have access to these treatments.
“We’re talking about $1,000 a month. And so there is no way they can afford [them]. I can prescribe phentermine [but] unless a patient has another indication, Medicaid will not pay for it,” she explained.
“I love hearing about all of the new developments. ... It’s interesting to hear, but we need to get insurance to pay so that I can actually prescribe,” Dr. Smith noted.
Dr. Apovian reports financial relationships with Xeno Biosciences, Cowen, Allergan, Novo Nordisk, Abbott Nutrition, and other companies.
AT INTERNAL MEDICINE 2022
Air pollution is a seizure trigger for patients with epilepsy
, a unique new study suggests.
The link between daily outdoor CO exposure and seizure risk was particularly evident for subclinical seizures – those in patients with abnormal electroencephalography (EEG) signals but no clinical symptoms.
“Our findings suggest that people with epilepsy should avoid high CO exposure to reduce potential seizure risk,” said study investigator Zhuying Chen, PhD candidate, department of biomedical engineering, University of Melbourne.
The study was published online in Epilepsia.
Pollution’s impact on brain health
Emerging evidence indicates air pollution affects brain health and may increase the risk of hospitalization or outpatient visits for epilepsy. However, little is known about the effect of pollution on the occurrence of epileptic seizures.
The study used two independent long-term seizure datasets – the NeuroVista (NV) study and the Seer App seizure diary (SD). In the NeuroVista study, researchers recorded continuous intracranial iEEG from patients with refractory focal epilepsy who had been implanted with a personal seizure advisory device that wirelessly recorded seizures on an external device.
The SD dataset included diaries documenting self-reported seizures, seizure cycles, and medication adherence.
Researchers collected data on hourly concentrations of outdoor CO, nitrogen dioxide (NO2), particulate matter of 10 μm or less in diameter (PM10), ozone (O3), and sulfur dioxide (SO2). The levels were measured at air quality monitoring stations in Australia.
Investigators aggregated hourly observations into daily mean data. All daily concentrations of CO and SO2 and at least 95% of daily concentrations of NO2, O3, and PM10 were within Australian air quality standards, said Mr. Chen.
The study included 49 participants, with epilepsy data on 15 patients in the NeuroVista study and on 34 from the SD dataset.
Overall, 6,692 epileptic seizures on 3,639 seizure days were recorded during 23,349 follow-up days from 2010 to 2012 (NV dataset) and 2018 to 2021 (SD dataset).
The investigators found a significant positive association between CO concentrations and epileptic seizure risks. The relative risk (RR) was 1.04 (95% confidence interval, 1.01–1.07; P < .01) for an interquartile range (IQR) increase of CO (0.13 parts per million).
Sex differences
There were no significant relationships for the other four air pollutants. However, Mr. Chen noted that Australia has very low air pollution levels; most usually are within World Health Organization air quality guidelines.
“Our findings may not be generalized to other countries with high air pollution levels,” said Mr. Chen. He noted that the relatively small number of patients in the study may limit the statistical power to detect some associations.
The study showed that females had a significantly increased risk of epileptic seizures when exposed to elevated CO (RR, 1.05; 95% CI, 1.01–1.08; P < .05) and NO2 (RR, 1.09; 95% CI, 1.01–1.16; P < .05) concentrations. There were no significant associations in males for any air pollutants.
Differences in outdoor activities and behaviors such as smoking and exercise may lead to variations in environmental exposure and help explain the sex differences, said Mr. Chen. These differences may also be due to the study’s limited sample size.
Analyzing the two datasets separately, the researchers found there was a significant association between CO concentration and epileptic seizure risk in the NV dataset (RR, 1.10; 95% CI, 1.03–1.17; P < .01).
There were no significant associations in the SD dataset for any air pollutants. This may be because only clinical seizures – those associated with evident symptoms – are self-reported, said Mr. Chen. He also noted that seizure diaries may be unreliable.
In the NV dataset, the epileptic seizure risk was significantly increased when only subclinical seizures were considered (RR, 1.20; 95% CI, 1.12–1.28; P < .001) for an IQR increase of CO concentration.
The risk was significantly decreased by 13% for subclinical seizures with an IQR increase of PM10 and by 9% for subclinical seizures with an IQR increase of SO2 concentrations.
These negative associations should be interpreted with caution, inasmuch as the associations were not robust in subsequent subgroup and sensitivity analyses, said Mr. Chen.
There were no significant associations when considering clinical seizures for any air pollutants.
The positive association for subclinical but not clinical seizures suggests that low-level CO exposure may not be strong enough to directly trigger clinical seizures, said Mr. Chen.
Although previous research has demonstrated adverse neurologic effects of exposure to air pollutants, most studies were based on hospital databases or registers. Thus, they may have missed seizures that did not lead to hospital admission.
Unclear mechanism
The exact mechanisms linking air pollution to seizures are unclear but probably involve the synergistic interaction of multiple pathways, said Mr. Chen. “Air pollution may affect brain metabolism, alter the immune response of the brain, and induce oxidative stress and neuroinflammation, causing the brain to be more susceptible to seizures,” he noted.
This is the first study to investigate seizure rates through intracranial EEG signals and self-reported seizure diaries. It’s also the first to look into the impact of pollutants at low concentration levels on subclinical seizures.
However, the study has some limitations. Self-reported seizures in the SD dataset might underestimate the influence of air pollution on seizures. The study used postal codes as proxies for exposure to pollution, which could introduce measurement errors and underestimate associations.
In addition, Mr. Chen noted that seizures from the NeuroVista dataset were recorded from patients with drug-resistant focal epilepsy. “Whether our findings can be generalized to other epilepsy types needs further investigation.”
The study could have important clinical and public health implications. For example, said Mr. Chen, it’s possible that seizure risk could be reduced through behavioral interventions, such as avoiding being outside or using an air filtration system when pollutant levels are high.
“Clinicians could counsel their patients to avoid the potential risk of high carbon monoxide exposure,” he said.
CO exposure could be a new factor for seizure risk forecasting, which could reduce the uncertainty of seizures and help guide epilepsy management, Mr. Chen added.
The study was supported by the Melbourne Monash Consciousness Research Seed Funding and an Australian National Health and Medical Research Council Ideas grant. Mr. Chen has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a unique new study suggests.
The link between daily outdoor CO exposure and seizure risk was particularly evident for subclinical seizures – those in patients with abnormal electroencephalography (EEG) signals but no clinical symptoms.
“Our findings suggest that people with epilepsy should avoid high CO exposure to reduce potential seizure risk,” said study investigator Zhuying Chen, PhD candidate, department of biomedical engineering, University of Melbourne.
The study was published online in Epilepsia.
Pollution’s impact on brain health
Emerging evidence indicates air pollution affects brain health and may increase the risk of hospitalization or outpatient visits for epilepsy. However, little is known about the effect of pollution on the occurrence of epileptic seizures.
The study used two independent long-term seizure datasets – the NeuroVista (NV) study and the Seer App seizure diary (SD). In the NeuroVista study, researchers recorded continuous intracranial iEEG from patients with refractory focal epilepsy who had been implanted with a personal seizure advisory device that wirelessly recorded seizures on an external device.
The SD dataset included diaries documenting self-reported seizures, seizure cycles, and medication adherence.
Researchers collected data on hourly concentrations of outdoor CO, nitrogen dioxide (NO2), particulate matter of 10 μm or less in diameter (PM10), ozone (O3), and sulfur dioxide (SO2). The levels were measured at air quality monitoring stations in Australia.
Investigators aggregated hourly observations into daily mean data. All daily concentrations of CO and SO2 and at least 95% of daily concentrations of NO2, O3, and PM10 were within Australian air quality standards, said Mr. Chen.
The study included 49 participants, with epilepsy data on 15 patients in the NeuroVista study and on 34 from the SD dataset.
Overall, 6,692 epileptic seizures on 3,639 seizure days were recorded during 23,349 follow-up days from 2010 to 2012 (NV dataset) and 2018 to 2021 (SD dataset).
The investigators found a significant positive association between CO concentrations and epileptic seizure risks. The relative risk (RR) was 1.04 (95% confidence interval, 1.01–1.07; P < .01) for an interquartile range (IQR) increase of CO (0.13 parts per million).
Sex differences
There were no significant relationships for the other four air pollutants. However, Mr. Chen noted that Australia has very low air pollution levels; most usually are within World Health Organization air quality guidelines.
“Our findings may not be generalized to other countries with high air pollution levels,” said Mr. Chen. He noted that the relatively small number of patients in the study may limit the statistical power to detect some associations.
The study showed that females had a significantly increased risk of epileptic seizures when exposed to elevated CO (RR, 1.05; 95% CI, 1.01–1.08; P < .05) and NO2 (RR, 1.09; 95% CI, 1.01–1.16; P < .05) concentrations. There were no significant associations in males for any air pollutants.
Differences in outdoor activities and behaviors such as smoking and exercise may lead to variations in environmental exposure and help explain the sex differences, said Mr. Chen. These differences may also be due to the study’s limited sample size.
Analyzing the two datasets separately, the researchers found there was a significant association between CO concentration and epileptic seizure risk in the NV dataset (RR, 1.10; 95% CI, 1.03–1.17; P < .01).
There were no significant associations in the SD dataset for any air pollutants. This may be because only clinical seizures – those associated with evident symptoms – are self-reported, said Mr. Chen. He also noted that seizure diaries may be unreliable.
In the NV dataset, the epileptic seizure risk was significantly increased when only subclinical seizures were considered (RR, 1.20; 95% CI, 1.12–1.28; P < .001) for an IQR increase of CO concentration.
The risk was significantly decreased by 13% for subclinical seizures with an IQR increase of PM10 and by 9% for subclinical seizures with an IQR increase of SO2 concentrations.
These negative associations should be interpreted with caution, inasmuch as the associations were not robust in subsequent subgroup and sensitivity analyses, said Mr. Chen.
There were no significant associations when considering clinical seizures for any air pollutants.
The positive association for subclinical but not clinical seizures suggests that low-level CO exposure may not be strong enough to directly trigger clinical seizures, said Mr. Chen.
Although previous research has demonstrated adverse neurologic effects of exposure to air pollutants, most studies were based on hospital databases or registers. Thus, they may have missed seizures that did not lead to hospital admission.
Unclear mechanism
The exact mechanisms linking air pollution to seizures are unclear but probably involve the synergistic interaction of multiple pathways, said Mr. Chen. “Air pollution may affect brain metabolism, alter the immune response of the brain, and induce oxidative stress and neuroinflammation, causing the brain to be more susceptible to seizures,” he noted.
This is the first study to investigate seizure rates through intracranial EEG signals and self-reported seizure diaries. It’s also the first to look into the impact of pollutants at low concentration levels on subclinical seizures.
However, the study has some limitations. Self-reported seizures in the SD dataset might underestimate the influence of air pollution on seizures. The study used postal codes as proxies for exposure to pollution, which could introduce measurement errors and underestimate associations.
In addition, Mr. Chen noted that seizures from the NeuroVista dataset were recorded from patients with drug-resistant focal epilepsy. “Whether our findings can be generalized to other epilepsy types needs further investigation.”
The study could have important clinical and public health implications. For example, said Mr. Chen, it’s possible that seizure risk could be reduced through behavioral interventions, such as avoiding being outside or using an air filtration system when pollutant levels are high.
“Clinicians could counsel their patients to avoid the potential risk of high carbon monoxide exposure,” he said.
CO exposure could be a new factor for seizure risk forecasting, which could reduce the uncertainty of seizures and help guide epilepsy management, Mr. Chen added.
The study was supported by the Melbourne Monash Consciousness Research Seed Funding and an Australian National Health and Medical Research Council Ideas grant. Mr. Chen has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a unique new study suggests.
The link between daily outdoor CO exposure and seizure risk was particularly evident for subclinical seizures – those in patients with abnormal electroencephalography (EEG) signals but no clinical symptoms.
“Our findings suggest that people with epilepsy should avoid high CO exposure to reduce potential seizure risk,” said study investigator Zhuying Chen, PhD candidate, department of biomedical engineering, University of Melbourne.
The study was published online in Epilepsia.
Pollution’s impact on brain health
Emerging evidence indicates air pollution affects brain health and may increase the risk of hospitalization or outpatient visits for epilepsy. However, little is known about the effect of pollution on the occurrence of epileptic seizures.
The study used two independent long-term seizure datasets – the NeuroVista (NV) study and the Seer App seizure diary (SD). In the NeuroVista study, researchers recorded continuous intracranial iEEG from patients with refractory focal epilepsy who had been implanted with a personal seizure advisory device that wirelessly recorded seizures on an external device.
The SD dataset included diaries documenting self-reported seizures, seizure cycles, and medication adherence.
Researchers collected data on hourly concentrations of outdoor CO, nitrogen dioxide (NO2), particulate matter of 10 μm or less in diameter (PM10), ozone (O3), and sulfur dioxide (SO2). The levels were measured at air quality monitoring stations in Australia.
Investigators aggregated hourly observations into daily mean data. All daily concentrations of CO and SO2 and at least 95% of daily concentrations of NO2, O3, and PM10 were within Australian air quality standards, said Mr. Chen.
The study included 49 participants, with epilepsy data on 15 patients in the NeuroVista study and on 34 from the SD dataset.
Overall, 6,692 epileptic seizures on 3,639 seizure days were recorded during 23,349 follow-up days from 2010 to 2012 (NV dataset) and 2018 to 2021 (SD dataset).
The investigators found a significant positive association between CO concentrations and epileptic seizure risks. The relative risk (RR) was 1.04 (95% confidence interval, 1.01–1.07; P < .01) for an interquartile range (IQR) increase of CO (0.13 parts per million).
Sex differences
There were no significant relationships for the other four air pollutants. However, Mr. Chen noted that Australia has very low air pollution levels; most usually are within World Health Organization air quality guidelines.
“Our findings may not be generalized to other countries with high air pollution levels,” said Mr. Chen. He noted that the relatively small number of patients in the study may limit the statistical power to detect some associations.
The study showed that females had a significantly increased risk of epileptic seizures when exposed to elevated CO (RR, 1.05; 95% CI, 1.01–1.08; P < .05) and NO2 (RR, 1.09; 95% CI, 1.01–1.16; P < .05) concentrations. There were no significant associations in males for any air pollutants.
Differences in outdoor activities and behaviors such as smoking and exercise may lead to variations in environmental exposure and help explain the sex differences, said Mr. Chen. These differences may also be due to the study’s limited sample size.
Analyzing the two datasets separately, the researchers found there was a significant association between CO concentration and epileptic seizure risk in the NV dataset (RR, 1.10; 95% CI, 1.03–1.17; P < .01).
There were no significant associations in the SD dataset for any air pollutants. This may be because only clinical seizures – those associated with evident symptoms – are self-reported, said Mr. Chen. He also noted that seizure diaries may be unreliable.
In the NV dataset, the epileptic seizure risk was significantly increased when only subclinical seizures were considered (RR, 1.20; 95% CI, 1.12–1.28; P < .001) for an IQR increase of CO concentration.
The risk was significantly decreased by 13% for subclinical seizures with an IQR increase of PM10 and by 9% for subclinical seizures with an IQR increase of SO2 concentrations.
These negative associations should be interpreted with caution, inasmuch as the associations were not robust in subsequent subgroup and sensitivity analyses, said Mr. Chen.
There were no significant associations when considering clinical seizures for any air pollutants.
The positive association for subclinical but not clinical seizures suggests that low-level CO exposure may not be strong enough to directly trigger clinical seizures, said Mr. Chen.
Although previous research has demonstrated adverse neurologic effects of exposure to air pollutants, most studies were based on hospital databases or registers. Thus, they may have missed seizures that did not lead to hospital admission.
Unclear mechanism
The exact mechanisms linking air pollution to seizures are unclear but probably involve the synergistic interaction of multiple pathways, said Mr. Chen. “Air pollution may affect brain metabolism, alter the immune response of the brain, and induce oxidative stress and neuroinflammation, causing the brain to be more susceptible to seizures,” he noted.
This is the first study to investigate seizure rates through intracranial EEG signals and self-reported seizure diaries. It’s also the first to look into the impact of pollutants at low concentration levels on subclinical seizures.
However, the study has some limitations. Self-reported seizures in the SD dataset might underestimate the influence of air pollution on seizures. The study used postal codes as proxies for exposure to pollution, which could introduce measurement errors and underestimate associations.
In addition, Mr. Chen noted that seizures from the NeuroVista dataset were recorded from patients with drug-resistant focal epilepsy. “Whether our findings can be generalized to other epilepsy types needs further investigation.”
The study could have important clinical and public health implications. For example, said Mr. Chen, it’s possible that seizure risk could be reduced through behavioral interventions, such as avoiding being outside or using an air filtration system when pollutant levels are high.
“Clinicians could counsel their patients to avoid the potential risk of high carbon monoxide exposure,” he said.
CO exposure could be a new factor for seizure risk forecasting, which could reduce the uncertainty of seizures and help guide epilepsy management, Mr. Chen added.
The study was supported by the Melbourne Monash Consciousness Research Seed Funding and an Australian National Health and Medical Research Council Ideas grant. Mr. Chen has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
WHO, UNICEF warn about increased risk of measles outbreaks
The World Health Organization and United Nations International Children’s Emergency Fund are warning about a heightened risk of measles spreading and triggering larger outbreaks in 2022.
Worldwide cases are up nearly 80% so far over 2021, the groups reported. More than 17,300 measles cases were reported worldwide in January and February, compared with 9,600 cases at the beginning of 2021.
In the last 12 months, there have been 21 “large and disruptive” measles outbreaks, particularly in Africa and the East Mediterranean region. The actual numbers are likely higher because of underreporting and disruptions to surveillance systems.
“Pandemic-related disruptions, increasing inequalities in access to vaccines, and the diversion of resources from routine immunization are leaving too many children without protection against measles and other vaccine-preventable diseases,” the organizations said.
As cities and countries relax COVID-19 restrictions, measles outbreaks are becoming more likely, they noted.
“It is encouraging that people in many communities are beginning to feel protected enough from COVID-19 to return to more social activities. But doing so in places where children are not receiving routine vaccination creates the perfect storm for the spread of a disease like measles,” Catherine Russell, executive director for UNICEF, said in the statement.
In the past year, the largest measles outbreaks have occurred in Somalia, Yemen, Nigeria, Afghanistan, and Ethiopia. The main reason for outbreaks is a lack measles vaccine coverage, the organizations said.
About 23 million children missed childhood vaccinations in 2020, the groups said. Childhood vaccination campaigns were hindered because of the COVID-19 pandemic and conflicts in Ukraine, Ethiopia, Somalia, and Afghanistan.
Overall, 57 campaigns targeting vaccine-preventable diseases across 43 countries that were scheduled to take place since the beginning of the COVID-19 pandemic are still postponed, the groups said, which could affect 203 million people. Among those, 19 are measles campaigns, which could put 73 million children at risk of measles because of missed vaccinations.
Vaccine coverage of 95% or higher with two doses of the measles vaccine can provide protection, according to the organizations. But the five countries that had the highest measles cases in the last year had first-dose coverage between 46% and 68%.
In the United States, measles vaccinations in kindergarten students dropped from about 95% to 93.9% for the 2020-2021 school year, according to CNN.
Vaccination coverage also dropped from 95% to 93.6% for diphtheria, tetanus, acellular pertussis, and varicella. Even though the decreases appear small, it means tens of thousands of children across the United States started school without their common childhood vaccinations, the Centers for Disease Control and Prevention said.
“We are concerned that missed routine vaccinations could leave children vulnerable to preventable diseases like measles and whooping cough, which are extremely contagious and can be very serious, especially for babies and young children,” Shannon Stokley, DrPH, deputy director of the CDC’s immunization services division, told CNN.
The numbers show a “concerning decline in childhood immunizations that began in March 2020,” she said.
A version of this article first appeared on WebMD.com.
The World Health Organization and United Nations International Children’s Emergency Fund are warning about a heightened risk of measles spreading and triggering larger outbreaks in 2022.
Worldwide cases are up nearly 80% so far over 2021, the groups reported. More than 17,300 measles cases were reported worldwide in January and February, compared with 9,600 cases at the beginning of 2021.
In the last 12 months, there have been 21 “large and disruptive” measles outbreaks, particularly in Africa and the East Mediterranean region. The actual numbers are likely higher because of underreporting and disruptions to surveillance systems.
“Pandemic-related disruptions, increasing inequalities in access to vaccines, and the diversion of resources from routine immunization are leaving too many children without protection against measles and other vaccine-preventable diseases,” the organizations said.
As cities and countries relax COVID-19 restrictions, measles outbreaks are becoming more likely, they noted.
“It is encouraging that people in many communities are beginning to feel protected enough from COVID-19 to return to more social activities. But doing so in places where children are not receiving routine vaccination creates the perfect storm for the spread of a disease like measles,” Catherine Russell, executive director for UNICEF, said in the statement.
In the past year, the largest measles outbreaks have occurred in Somalia, Yemen, Nigeria, Afghanistan, and Ethiopia. The main reason for outbreaks is a lack measles vaccine coverage, the organizations said.
About 23 million children missed childhood vaccinations in 2020, the groups said. Childhood vaccination campaigns were hindered because of the COVID-19 pandemic and conflicts in Ukraine, Ethiopia, Somalia, and Afghanistan.
Overall, 57 campaigns targeting vaccine-preventable diseases across 43 countries that were scheduled to take place since the beginning of the COVID-19 pandemic are still postponed, the groups said, which could affect 203 million people. Among those, 19 are measles campaigns, which could put 73 million children at risk of measles because of missed vaccinations.
Vaccine coverage of 95% or higher with two doses of the measles vaccine can provide protection, according to the organizations. But the five countries that had the highest measles cases in the last year had first-dose coverage between 46% and 68%.
In the United States, measles vaccinations in kindergarten students dropped from about 95% to 93.9% for the 2020-2021 school year, according to CNN.
Vaccination coverage also dropped from 95% to 93.6% for diphtheria, tetanus, acellular pertussis, and varicella. Even though the decreases appear small, it means tens of thousands of children across the United States started school without their common childhood vaccinations, the Centers for Disease Control and Prevention said.
“We are concerned that missed routine vaccinations could leave children vulnerable to preventable diseases like measles and whooping cough, which are extremely contagious and can be very serious, especially for babies and young children,” Shannon Stokley, DrPH, deputy director of the CDC’s immunization services division, told CNN.
The numbers show a “concerning decline in childhood immunizations that began in March 2020,” she said.
A version of this article first appeared on WebMD.com.
The World Health Organization and United Nations International Children’s Emergency Fund are warning about a heightened risk of measles spreading and triggering larger outbreaks in 2022.
Worldwide cases are up nearly 80% so far over 2021, the groups reported. More than 17,300 measles cases were reported worldwide in January and February, compared with 9,600 cases at the beginning of 2021.
In the last 12 months, there have been 21 “large and disruptive” measles outbreaks, particularly in Africa and the East Mediterranean region. The actual numbers are likely higher because of underreporting and disruptions to surveillance systems.
“Pandemic-related disruptions, increasing inequalities in access to vaccines, and the diversion of resources from routine immunization are leaving too many children without protection against measles and other vaccine-preventable diseases,” the organizations said.
As cities and countries relax COVID-19 restrictions, measles outbreaks are becoming more likely, they noted.
“It is encouraging that people in many communities are beginning to feel protected enough from COVID-19 to return to more social activities. But doing so in places where children are not receiving routine vaccination creates the perfect storm for the spread of a disease like measles,” Catherine Russell, executive director for UNICEF, said in the statement.
In the past year, the largest measles outbreaks have occurred in Somalia, Yemen, Nigeria, Afghanistan, and Ethiopia. The main reason for outbreaks is a lack measles vaccine coverage, the organizations said.
About 23 million children missed childhood vaccinations in 2020, the groups said. Childhood vaccination campaigns were hindered because of the COVID-19 pandemic and conflicts in Ukraine, Ethiopia, Somalia, and Afghanistan.
Overall, 57 campaigns targeting vaccine-preventable diseases across 43 countries that were scheduled to take place since the beginning of the COVID-19 pandemic are still postponed, the groups said, which could affect 203 million people. Among those, 19 are measles campaigns, which could put 73 million children at risk of measles because of missed vaccinations.
Vaccine coverage of 95% or higher with two doses of the measles vaccine can provide protection, according to the organizations. But the five countries that had the highest measles cases in the last year had first-dose coverage between 46% and 68%.
In the United States, measles vaccinations in kindergarten students dropped from about 95% to 93.9% for the 2020-2021 school year, according to CNN.
Vaccination coverage also dropped from 95% to 93.6% for diphtheria, tetanus, acellular pertussis, and varicella. Even though the decreases appear small, it means tens of thousands of children across the United States started school without their common childhood vaccinations, the Centers for Disease Control and Prevention said.
“We are concerned that missed routine vaccinations could leave children vulnerable to preventable diseases like measles and whooping cough, which are extremely contagious and can be very serious, especially for babies and young children,” Shannon Stokley, DrPH, deputy director of the CDC’s immunization services division, told CNN.
The numbers show a “concerning decline in childhood immunizations that began in March 2020,” she said.
A version of this article first appeared on WebMD.com.
Nurses, med staff voice their heartache about California nurse suicide
The Santa Clara Police Department “thoroughly investigated” a report April 27 at Kaiser Permanente Santa Clara Medical Center that a male nurse in the emergency department died from a self-inflicted gunshot wound and ruled it a suicide, according to Wahid Kazem, assistant chief of police.
“This tragic event occurred in a closed room that is not used for patient care, adjacent to the emergency department. No other staff or patients were threatened,” according to Rakesh Chaudhary, MD, physician-in-chief of the medical center.
He added that the emergency department remained open for walk-in patients during the investigation, but ambulances were temporarily diverted to nearby hospitals. “The Santa Clara Police Department and our staff immediately took precautions to isolate the affected area and avoid impact to patient care.”
In terms of the effect on those closer to the victim, Dr. Chaudhary said, “Our hearts go out to the family, friends, and coworkers affected by this terrible loss. Our teams are on site providing emotional support and resources for staff.”
Neither the police nor the hospital released the victim’s name. “Out of respect for the privacy of our colleague and their family, we cannot provide any additional details,” Dr. Chaudhary said.
Among those who tweeted reactions to the news the past few days was someone who worked with the victim, according to the post: “My heart goes out to my coworker who thought he had no one to lean on and to my good friend who had to witness this tragedy. Love my ER fam.”
A male critical care RN tweeted: “My heart hurts for the nurse, his loved ones, and colleagues. Anyone working in ER understands the unique stress that we’ve been under. This is so tragic.”
While others cited the need for more mental health services to care for nurses, a psychiatrist on Twitter added, “Nurses are not OK and pizza and pats on the back aren’t going to fix it. This affects all of us.”
Mental health support was listed as a prime demand of striking workers recently at Stanford (Calif.) Health Care and Lucile Packard Children’s Hospital in Palo Alto, about a half hour away from Santa Clara Medical Center.
The nurses’ strike ended May 2 with an agreement between the health systems and the Committee for Recognition of Nursing Achievement union representing the nurses. The contract includes improvements to existing benefits supporting nurses’ health and well-being, according to a StanfordPackardVoice.com newsletter updating the negotiations.
Earlier this year, an intensive care unit RN from Stanford, Michael Odell, reportedly walked off his shift and was found dead 2 days later in San Francisco by the Alameda County Sheriff’s Office dive team. No foul play was suspected and the incident was believed to be a suicide.
A version of this article first appeared on Medscape.com.
The Santa Clara Police Department “thoroughly investigated” a report April 27 at Kaiser Permanente Santa Clara Medical Center that a male nurse in the emergency department died from a self-inflicted gunshot wound and ruled it a suicide, according to Wahid Kazem, assistant chief of police.
“This tragic event occurred in a closed room that is not used for patient care, adjacent to the emergency department. No other staff or patients were threatened,” according to Rakesh Chaudhary, MD, physician-in-chief of the medical center.
He added that the emergency department remained open for walk-in patients during the investigation, but ambulances were temporarily diverted to nearby hospitals. “The Santa Clara Police Department and our staff immediately took precautions to isolate the affected area and avoid impact to patient care.”
In terms of the effect on those closer to the victim, Dr. Chaudhary said, “Our hearts go out to the family, friends, and coworkers affected by this terrible loss. Our teams are on site providing emotional support and resources for staff.”
Neither the police nor the hospital released the victim’s name. “Out of respect for the privacy of our colleague and their family, we cannot provide any additional details,” Dr. Chaudhary said.
Among those who tweeted reactions to the news the past few days was someone who worked with the victim, according to the post: “My heart goes out to my coworker who thought he had no one to lean on and to my good friend who had to witness this tragedy. Love my ER fam.”
A male critical care RN tweeted: “My heart hurts for the nurse, his loved ones, and colleagues. Anyone working in ER understands the unique stress that we’ve been under. This is so tragic.”
While others cited the need for more mental health services to care for nurses, a psychiatrist on Twitter added, “Nurses are not OK and pizza and pats on the back aren’t going to fix it. This affects all of us.”
Mental health support was listed as a prime demand of striking workers recently at Stanford (Calif.) Health Care and Lucile Packard Children’s Hospital in Palo Alto, about a half hour away from Santa Clara Medical Center.
The nurses’ strike ended May 2 with an agreement between the health systems and the Committee for Recognition of Nursing Achievement union representing the nurses. The contract includes improvements to existing benefits supporting nurses’ health and well-being, according to a StanfordPackardVoice.com newsletter updating the negotiations.
Earlier this year, an intensive care unit RN from Stanford, Michael Odell, reportedly walked off his shift and was found dead 2 days later in San Francisco by the Alameda County Sheriff’s Office dive team. No foul play was suspected and the incident was believed to be a suicide.
A version of this article first appeared on Medscape.com.
The Santa Clara Police Department “thoroughly investigated” a report April 27 at Kaiser Permanente Santa Clara Medical Center that a male nurse in the emergency department died from a self-inflicted gunshot wound and ruled it a suicide, according to Wahid Kazem, assistant chief of police.
“This tragic event occurred in a closed room that is not used for patient care, adjacent to the emergency department. No other staff or patients were threatened,” according to Rakesh Chaudhary, MD, physician-in-chief of the medical center.
He added that the emergency department remained open for walk-in patients during the investigation, but ambulances were temporarily diverted to nearby hospitals. “The Santa Clara Police Department and our staff immediately took precautions to isolate the affected area and avoid impact to patient care.”
In terms of the effect on those closer to the victim, Dr. Chaudhary said, “Our hearts go out to the family, friends, and coworkers affected by this terrible loss. Our teams are on site providing emotional support and resources for staff.”
Neither the police nor the hospital released the victim’s name. “Out of respect for the privacy of our colleague and their family, we cannot provide any additional details,” Dr. Chaudhary said.
Among those who tweeted reactions to the news the past few days was someone who worked with the victim, according to the post: “My heart goes out to my coworker who thought he had no one to lean on and to my good friend who had to witness this tragedy. Love my ER fam.”
A male critical care RN tweeted: “My heart hurts for the nurse, his loved ones, and colleagues. Anyone working in ER understands the unique stress that we’ve been under. This is so tragic.”
While others cited the need for more mental health services to care for nurses, a psychiatrist on Twitter added, “Nurses are not OK and pizza and pats on the back aren’t going to fix it. This affects all of us.”
Mental health support was listed as a prime demand of striking workers recently at Stanford (Calif.) Health Care and Lucile Packard Children’s Hospital in Palo Alto, about a half hour away from Santa Clara Medical Center.
The nurses’ strike ended May 2 with an agreement between the health systems and the Committee for Recognition of Nursing Achievement union representing the nurses. The contract includes improvements to existing benefits supporting nurses’ health and well-being, according to a StanfordPackardVoice.com newsletter updating the negotiations.
Earlier this year, an intensive care unit RN from Stanford, Michael Odell, reportedly walked off his shift and was found dead 2 days later in San Francisco by the Alameda County Sheriff’s Office dive team. No foul play was suspected and the incident was believed to be a suicide.
A version of this article first appeared on Medscape.com.
Unexplained hepatitis cases in children reported in 10 U.S. states, more than 200 worldwide
Health officials are investigating at least 30 cases of severe hepatitis in children across 10 U.S. states. The Minnesota Department of Health received two reports of severe hepatitis, one in an infant and another in a 2-year-old, the Associated Press reported on April 30. One child was treated “several months ago” and required a liver transplant, according to the article.
Worldwide cases surpass 200, including 34 cases in the United Kingdom, the U.K. Health Security Agency announced on April 29. Most cases have occurred in the United Kingdom, but there have been more than 55 probable and confirmed hepatitis cases in children in 12 countries in the European Union or the European Economic Area. Cases have also been identified in Asia, with both Japan and Singapore reporting one case each of acute hepatitis, Bloomberg reported. Additionally, three children in Indonesia died from acute hepatitis in April, but the total number of cases in that country was not available.
Although the total number of worldwide cases remains small, the severity of the cases – as well as their unexplained cause – have health officials on alert, said David Lee Thomas, MD, MPH, of the Viral Hepatitis Center at Johns Hopkins Medicine in Baltimore. “There are some kids who would have died if not for liver transplants.”
In the United States, the only confirmed cases are in Alabama, where nine patients were admitted for severe hepatitis between October 2021 and February 2022. Beyond the two suspected cases in Minnesota, health officials are investigating at least 19 other potential cases in eight states, according to NBC News: Delaware (1), Georgia, Illinois (3), Louisiana (1), New York, North Carolina (2), Tennessee (6), and Wisconsin (4). (New York and Georgia did not specify the number of cases being investigated.)
Reported cases have occurred in patients aged between 1 month and 16 years old. Globally, at least 17 patients have needed liver transplants, according to a World Health Organization alert on April 23. While WHO officials said there has been at least one death globally linked to hepatitis, that does not include the three deaths in Indonesia. One death has also been reported in Wisconsin, but the state’s Department of Health Services did not confirm whether this death was included in the WHO announcement.
The cause of these severe hepatitis cases has yet to be identified, but these cases have tested negative for more common viruses that can cause hepatitis in children. There is no link between these cases and COVID-19 vaccination, according to WHO, because most affected children have not been vaccinated.
Adenovirus is a possible contributing factor in these cases, as many of the cases in Europe tested positive for the virus. In an analysis of the nine Alabama cases released by the Centers for Disease Control and Prevention, adenovirus was detected in the blood samples of all nine children. Five of the nine children tested positive for adenovirus type 41, which is a common cause of acute gastroenteritis in children. While the six liver biopsies performed showed varying degrees of hepatitis, there were “no viral inclusions observed, no immunohistochemical evidence of adenovirus, or no viral particles identified by electron microscopy,” according to the report. None of the children tested positive for COVID-19 or had a documented history of previous COVID-19 infection.
“At this time, we believe that adenovirus may be the cause for these reported cases, but other potential environmental and situational factors are still being investigated,” the CDC said in a media statement. The CDC added that the report was specific to the nine Alabama cases, and that the agency is working to investigate other potential cases with state and local public health officials.
While the “growing consensus” among experts is that adenovirus could be behind these severe cases, there are many unanswered questions, Dr. Thomas added, such as why this strain of adenovirus causes such severe hepatitis, and why the liver biopsies do not show classic signs of viral infection. That information will come as investigations continue.
“From a provider point of view, if you have a child with an unexplained liver problem, report it to the CDC,” he advised. “Right now, we have to learn more about [these cases],” and that requires more research like the investigations in Alabama, he noted.
A version of this article first appeared on Medscape.com.
Health officials are investigating at least 30 cases of severe hepatitis in children across 10 U.S. states. The Minnesota Department of Health received two reports of severe hepatitis, one in an infant and another in a 2-year-old, the Associated Press reported on April 30. One child was treated “several months ago” and required a liver transplant, according to the article.
Worldwide cases surpass 200, including 34 cases in the United Kingdom, the U.K. Health Security Agency announced on April 29. Most cases have occurred in the United Kingdom, but there have been more than 55 probable and confirmed hepatitis cases in children in 12 countries in the European Union or the European Economic Area. Cases have also been identified in Asia, with both Japan and Singapore reporting one case each of acute hepatitis, Bloomberg reported. Additionally, three children in Indonesia died from acute hepatitis in April, but the total number of cases in that country was not available.
Although the total number of worldwide cases remains small, the severity of the cases – as well as their unexplained cause – have health officials on alert, said David Lee Thomas, MD, MPH, of the Viral Hepatitis Center at Johns Hopkins Medicine in Baltimore. “There are some kids who would have died if not for liver transplants.”
In the United States, the only confirmed cases are in Alabama, where nine patients were admitted for severe hepatitis between October 2021 and February 2022. Beyond the two suspected cases in Minnesota, health officials are investigating at least 19 other potential cases in eight states, according to NBC News: Delaware (1), Georgia, Illinois (3), Louisiana (1), New York, North Carolina (2), Tennessee (6), and Wisconsin (4). (New York and Georgia did not specify the number of cases being investigated.)
Reported cases have occurred in patients aged between 1 month and 16 years old. Globally, at least 17 patients have needed liver transplants, according to a World Health Organization alert on April 23. While WHO officials said there has been at least one death globally linked to hepatitis, that does not include the three deaths in Indonesia. One death has also been reported in Wisconsin, but the state’s Department of Health Services did not confirm whether this death was included in the WHO announcement.
The cause of these severe hepatitis cases has yet to be identified, but these cases have tested negative for more common viruses that can cause hepatitis in children. There is no link between these cases and COVID-19 vaccination, according to WHO, because most affected children have not been vaccinated.
Adenovirus is a possible contributing factor in these cases, as many of the cases in Europe tested positive for the virus. In an analysis of the nine Alabama cases released by the Centers for Disease Control and Prevention, adenovirus was detected in the blood samples of all nine children. Five of the nine children tested positive for adenovirus type 41, which is a common cause of acute gastroenteritis in children. While the six liver biopsies performed showed varying degrees of hepatitis, there were “no viral inclusions observed, no immunohistochemical evidence of adenovirus, or no viral particles identified by electron microscopy,” according to the report. None of the children tested positive for COVID-19 or had a documented history of previous COVID-19 infection.
“At this time, we believe that adenovirus may be the cause for these reported cases, but other potential environmental and situational factors are still being investigated,” the CDC said in a media statement. The CDC added that the report was specific to the nine Alabama cases, and that the agency is working to investigate other potential cases with state and local public health officials.
While the “growing consensus” among experts is that adenovirus could be behind these severe cases, there are many unanswered questions, Dr. Thomas added, such as why this strain of adenovirus causes such severe hepatitis, and why the liver biopsies do not show classic signs of viral infection. That information will come as investigations continue.
“From a provider point of view, if you have a child with an unexplained liver problem, report it to the CDC,” he advised. “Right now, we have to learn more about [these cases],” and that requires more research like the investigations in Alabama, he noted.
A version of this article first appeared on Medscape.com.
Health officials are investigating at least 30 cases of severe hepatitis in children across 10 U.S. states. The Minnesota Department of Health received two reports of severe hepatitis, one in an infant and another in a 2-year-old, the Associated Press reported on April 30. One child was treated “several months ago” and required a liver transplant, according to the article.
Worldwide cases surpass 200, including 34 cases in the United Kingdom, the U.K. Health Security Agency announced on April 29. Most cases have occurred in the United Kingdom, but there have been more than 55 probable and confirmed hepatitis cases in children in 12 countries in the European Union or the European Economic Area. Cases have also been identified in Asia, with both Japan and Singapore reporting one case each of acute hepatitis, Bloomberg reported. Additionally, three children in Indonesia died from acute hepatitis in April, but the total number of cases in that country was not available.
Although the total number of worldwide cases remains small, the severity of the cases – as well as their unexplained cause – have health officials on alert, said David Lee Thomas, MD, MPH, of the Viral Hepatitis Center at Johns Hopkins Medicine in Baltimore. “There are some kids who would have died if not for liver transplants.”
In the United States, the only confirmed cases are in Alabama, where nine patients were admitted for severe hepatitis between October 2021 and February 2022. Beyond the two suspected cases in Minnesota, health officials are investigating at least 19 other potential cases in eight states, according to NBC News: Delaware (1), Georgia, Illinois (3), Louisiana (1), New York, North Carolina (2), Tennessee (6), and Wisconsin (4). (New York and Georgia did not specify the number of cases being investigated.)
Reported cases have occurred in patients aged between 1 month and 16 years old. Globally, at least 17 patients have needed liver transplants, according to a World Health Organization alert on April 23. While WHO officials said there has been at least one death globally linked to hepatitis, that does not include the three deaths in Indonesia. One death has also been reported in Wisconsin, but the state’s Department of Health Services did not confirm whether this death was included in the WHO announcement.
The cause of these severe hepatitis cases has yet to be identified, but these cases have tested negative for more common viruses that can cause hepatitis in children. There is no link between these cases and COVID-19 vaccination, according to WHO, because most affected children have not been vaccinated.
Adenovirus is a possible contributing factor in these cases, as many of the cases in Europe tested positive for the virus. In an analysis of the nine Alabama cases released by the Centers for Disease Control and Prevention, adenovirus was detected in the blood samples of all nine children. Five of the nine children tested positive for adenovirus type 41, which is a common cause of acute gastroenteritis in children. While the six liver biopsies performed showed varying degrees of hepatitis, there were “no viral inclusions observed, no immunohistochemical evidence of adenovirus, or no viral particles identified by electron microscopy,” according to the report. None of the children tested positive for COVID-19 or had a documented history of previous COVID-19 infection.
“At this time, we believe that adenovirus may be the cause for these reported cases, but other potential environmental and situational factors are still being investigated,” the CDC said in a media statement. The CDC added that the report was specific to the nine Alabama cases, and that the agency is working to investigate other potential cases with state and local public health officials.
While the “growing consensus” among experts is that adenovirus could be behind these severe cases, there are many unanswered questions, Dr. Thomas added, such as why this strain of adenovirus causes such severe hepatitis, and why the liver biopsies do not show classic signs of viral infection. That information will come as investigations continue.
“From a provider point of view, if you have a child with an unexplained liver problem, report it to the CDC,” he advised. “Right now, we have to learn more about [these cases],” and that requires more research like the investigations in Alabama, he noted.
A version of this article first appeared on Medscape.com.
Experts show how to reduce school-related sedentary behavior
The Sedentary Behavior Research Network has published new guidelines “to provide guidance to parents, educators, policy makers, researchers, and health care providers” on means to reduce school-related sedentary behavior.
The recommendations, published in the International Journal of Behavioral Nutrition and Physical Activity were written by researchers led by Travis J. Saunders, PhD, associate professor of applied human sciences at the University of Prince Edward Island, Charlottetown. Based on work carried out by a panel of international experts and informed by the best available evidence and stakeholder consultation, “these recommendations will be useful in supporting the physical and mental health, well-being, and academic success of school-age children and youth,” according to the authors.
The key strength of their work, they wrote, is that it is based on robust scientific data and specifically refers to school-related sedentary behaviors, whether these occur during lessons in the classroom or while completing assignments at home. “Existing sedentary behavior guidelines for children and youth target overall sedentary behavior and recreational screen time, without any specific recommendations regarding school-related sedentary behaviors.” The article also mentions the impact of the COVID-19 pandemic. Lack of movement was already a problem in these age groups; social distancing and distance learning over such an extended period only made things worse.
Risks and benefits
Dr. Saunders and colleagues wrote: “The relationships between sedentary behaviors and student health and academic outcomes are complex and likely differ for specific sedentary behaviors.”
While on one hand sedentary behavior may have a significant negative impact on metabolic outcomes, there is evidence that higher durations of homework and reading are associated with better academic achievement among school-aged children.
Another example of this complexity is that screen-based sedentary behaviors (spending time in front of computer screens, TVs, tablets, smartphones) often demonstrate deleterious associations with a range of health outcomes among school-aged children and youth aged 5-18 years, including body composition, cardiometabolic risk, and self-esteem. Yet screen-based devices may offer opportunities for novel pedagogic approaches and student engagement and may increase access to education for some students, especially during the COVID-19 pandemic.
The researchers noted that “many common sedentary activities ... do not have to be sedentary in nature. These behaviors are only considered to be sedentary when combined with both low energy expenditure and a sitting, reclining, or lying posture.” As an example, they pointed out that “active video gaming, or paper-based work at a standing desk, are both ways that common sedentary behaviors can be made nonsedentary.”
One thing’s for sure: Children and teenagers don’t move around all that much.
Data from the 2019 Eye on Health survey found that one of five children (20.3%) had not engaged in any physical activity the day before the survey, almost half (43.5%) had a TV in their bedroom, and about the same number (44.5%) spent more than 2 hours a day in front of a screen.
As for schools, the survey showed that, while 93% had initiatives to promote physical activity, fewer than 30% of these programs involved parents. It should be kept in mind that these are prepandemic numbers.
‘A healthy school day’
The authors recommend the following for reducing school-related sedentary behavior:
- Break up periods of extended sedentary behavior with both scheduled and unscheduled movement breaks: at least once every 30 minutes for ages 5-11 years and at least once every hour for ages 12-18 years. Consider activities that vary in intensity and duration (for example, standing, stretching breaks, moving to another classroom, active lessons, active breaks).
- Incorporate different types of movement into homework whenever possible, and limit sedentary homework to no more than 10 minutes per day per grade level (for example, no more than 10 minutes per day in grade 1, or 60 minutes per day in grade 6).
- Regardless of the location, school-related screen time should be meaningful, mentally or physically active, and serve a specific pedagogic purpose that enhances learning, compared with alternative methods. When school-related screen time is warranted, the following are recommended: limit time on devices, especially for students age 5-11 years; take a device break at least once every 30 minutes; discourage media multitasking in the classroom and while doing homework; and avoid screen-based homework within an hour of bedtime.
- Replace sedentary learning activities with movement-based learning activities (including standing) and replacing screen-based learning activities with non–screen-based learning activities (for example, outdoor lessons) can further support students’ health and well-being.
“Given the important role that schools can play in the promotion of healthy behaviors,” Dr. Saunders and associates wrote, “we encourage national and international public health agencies to consider inclusion of specific recommendations related to the school environment in future sedentary behavior guidelines.”
A version of this article first appeared on Medscape.com.
The Sedentary Behavior Research Network has published new guidelines “to provide guidance to parents, educators, policy makers, researchers, and health care providers” on means to reduce school-related sedentary behavior.
The recommendations, published in the International Journal of Behavioral Nutrition and Physical Activity were written by researchers led by Travis J. Saunders, PhD, associate professor of applied human sciences at the University of Prince Edward Island, Charlottetown. Based on work carried out by a panel of international experts and informed by the best available evidence and stakeholder consultation, “these recommendations will be useful in supporting the physical and mental health, well-being, and academic success of school-age children and youth,” according to the authors.
The key strength of their work, they wrote, is that it is based on robust scientific data and specifically refers to school-related sedentary behaviors, whether these occur during lessons in the classroom or while completing assignments at home. “Existing sedentary behavior guidelines for children and youth target overall sedentary behavior and recreational screen time, without any specific recommendations regarding school-related sedentary behaviors.” The article also mentions the impact of the COVID-19 pandemic. Lack of movement was already a problem in these age groups; social distancing and distance learning over such an extended period only made things worse.
Risks and benefits
Dr. Saunders and colleagues wrote: “The relationships between sedentary behaviors and student health and academic outcomes are complex and likely differ for specific sedentary behaviors.”
While on one hand sedentary behavior may have a significant negative impact on metabolic outcomes, there is evidence that higher durations of homework and reading are associated with better academic achievement among school-aged children.
Another example of this complexity is that screen-based sedentary behaviors (spending time in front of computer screens, TVs, tablets, smartphones) often demonstrate deleterious associations with a range of health outcomes among school-aged children and youth aged 5-18 years, including body composition, cardiometabolic risk, and self-esteem. Yet screen-based devices may offer opportunities for novel pedagogic approaches and student engagement and may increase access to education for some students, especially during the COVID-19 pandemic.
The researchers noted that “many common sedentary activities ... do not have to be sedentary in nature. These behaviors are only considered to be sedentary when combined with both low energy expenditure and a sitting, reclining, or lying posture.” As an example, they pointed out that “active video gaming, or paper-based work at a standing desk, are both ways that common sedentary behaviors can be made nonsedentary.”
One thing’s for sure: Children and teenagers don’t move around all that much.
Data from the 2019 Eye on Health survey found that one of five children (20.3%) had not engaged in any physical activity the day before the survey, almost half (43.5%) had a TV in their bedroom, and about the same number (44.5%) spent more than 2 hours a day in front of a screen.
As for schools, the survey showed that, while 93% had initiatives to promote physical activity, fewer than 30% of these programs involved parents. It should be kept in mind that these are prepandemic numbers.
‘A healthy school day’
The authors recommend the following for reducing school-related sedentary behavior:
- Break up periods of extended sedentary behavior with both scheduled and unscheduled movement breaks: at least once every 30 minutes for ages 5-11 years and at least once every hour for ages 12-18 years. Consider activities that vary in intensity and duration (for example, standing, stretching breaks, moving to another classroom, active lessons, active breaks).
- Incorporate different types of movement into homework whenever possible, and limit sedentary homework to no more than 10 minutes per day per grade level (for example, no more than 10 minutes per day in grade 1, or 60 minutes per day in grade 6).
- Regardless of the location, school-related screen time should be meaningful, mentally or physically active, and serve a specific pedagogic purpose that enhances learning, compared with alternative methods. When school-related screen time is warranted, the following are recommended: limit time on devices, especially for students age 5-11 years; take a device break at least once every 30 minutes; discourage media multitasking in the classroom and while doing homework; and avoid screen-based homework within an hour of bedtime.
- Replace sedentary learning activities with movement-based learning activities (including standing) and replacing screen-based learning activities with non–screen-based learning activities (for example, outdoor lessons) can further support students’ health and well-being.
“Given the important role that schools can play in the promotion of healthy behaviors,” Dr. Saunders and associates wrote, “we encourage national and international public health agencies to consider inclusion of specific recommendations related to the school environment in future sedentary behavior guidelines.”
A version of this article first appeared on Medscape.com.
The Sedentary Behavior Research Network has published new guidelines “to provide guidance to parents, educators, policy makers, researchers, and health care providers” on means to reduce school-related sedentary behavior.
The recommendations, published in the International Journal of Behavioral Nutrition and Physical Activity were written by researchers led by Travis J. Saunders, PhD, associate professor of applied human sciences at the University of Prince Edward Island, Charlottetown. Based on work carried out by a panel of international experts and informed by the best available evidence and stakeholder consultation, “these recommendations will be useful in supporting the physical and mental health, well-being, and academic success of school-age children and youth,” according to the authors.
The key strength of their work, they wrote, is that it is based on robust scientific data and specifically refers to school-related sedentary behaviors, whether these occur during lessons in the classroom or while completing assignments at home. “Existing sedentary behavior guidelines for children and youth target overall sedentary behavior and recreational screen time, without any specific recommendations regarding school-related sedentary behaviors.” The article also mentions the impact of the COVID-19 pandemic. Lack of movement was already a problem in these age groups; social distancing and distance learning over such an extended period only made things worse.
Risks and benefits
Dr. Saunders and colleagues wrote: “The relationships between sedentary behaviors and student health and academic outcomes are complex and likely differ for specific sedentary behaviors.”
While on one hand sedentary behavior may have a significant negative impact on metabolic outcomes, there is evidence that higher durations of homework and reading are associated with better academic achievement among school-aged children.
Another example of this complexity is that screen-based sedentary behaviors (spending time in front of computer screens, TVs, tablets, smartphones) often demonstrate deleterious associations with a range of health outcomes among school-aged children and youth aged 5-18 years, including body composition, cardiometabolic risk, and self-esteem. Yet screen-based devices may offer opportunities for novel pedagogic approaches and student engagement and may increase access to education for some students, especially during the COVID-19 pandemic.
The researchers noted that “many common sedentary activities ... do not have to be sedentary in nature. These behaviors are only considered to be sedentary when combined with both low energy expenditure and a sitting, reclining, or lying posture.” As an example, they pointed out that “active video gaming, or paper-based work at a standing desk, are both ways that common sedentary behaviors can be made nonsedentary.”
One thing’s for sure: Children and teenagers don’t move around all that much.
Data from the 2019 Eye on Health survey found that one of five children (20.3%) had not engaged in any physical activity the day before the survey, almost half (43.5%) had a TV in their bedroom, and about the same number (44.5%) spent more than 2 hours a day in front of a screen.
As for schools, the survey showed that, while 93% had initiatives to promote physical activity, fewer than 30% of these programs involved parents. It should be kept in mind that these are prepandemic numbers.
‘A healthy school day’
The authors recommend the following for reducing school-related sedentary behavior:
- Break up periods of extended sedentary behavior with both scheduled and unscheduled movement breaks: at least once every 30 minutes for ages 5-11 years and at least once every hour for ages 12-18 years. Consider activities that vary in intensity and duration (for example, standing, stretching breaks, moving to another classroom, active lessons, active breaks).
- Incorporate different types of movement into homework whenever possible, and limit sedentary homework to no more than 10 minutes per day per grade level (for example, no more than 10 minutes per day in grade 1, or 60 minutes per day in grade 6).
- Regardless of the location, school-related screen time should be meaningful, mentally or physically active, and serve a specific pedagogic purpose that enhances learning, compared with alternative methods. When school-related screen time is warranted, the following are recommended: limit time on devices, especially for students age 5-11 years; take a device break at least once every 30 minutes; discourage media multitasking in the classroom and while doing homework; and avoid screen-based homework within an hour of bedtime.
- Replace sedentary learning activities with movement-based learning activities (including standing) and replacing screen-based learning activities with non–screen-based learning activities (for example, outdoor lessons) can further support students’ health and well-being.
“Given the important role that schools can play in the promotion of healthy behaviors,” Dr. Saunders and associates wrote, “we encourage national and international public health agencies to consider inclusion of specific recommendations related to the school environment in future sedentary behavior guidelines.”
A version of this article first appeared on Medscape.com.
FROM THE INTERNATIONAL JOURNAL OF BEHAVIORAL NUTRITION AND PHYSICAL ACTIVITY
Premature return to play after concussion has decreased
, according to a recent chart review. Rates of premature return to learn (RTL) are essentially unchanged, however.
“Delay in recovery is the major reason why it’s important not to RTL or RTP prematurely,” said James Carson, MD, associate professor of family and community medicine, University of Toronto.
“That delay in recovery only sets students further back in terms of the stress they get from being delayed with their schoolwork – they could lose their year in school, lose all their social contacts. So, there are a number of psychosocial issues that come into play if recovery is delayed, and that is what premature RTL and premature RTP will do – they delay the student’s recovery,” he emphasized.
The study was published in Canadian Family Physician.
Differences by sex
The study involved 241 students who had 258 distinct cases of SRC. The researchers defined premature RTP and RTL as chart records documenting the relapse, recurrence, or worsening of concussion symptoms that accompanied the patient’s RTP or RTL. Between 2011 and 2016, 26.7% of students had evidence of premature RTP, while 42.6% of them had evidence of premature RTL, the authors noted.
Compared with findings from an earlier survey of data from 2006 to 2011, the incidence of premature RTP dropped by 38.6% (P = .0003). In contrast, symptoms associated with premature RTL dropped by only 4.7% from the previous survey. This change was not statistically significant.
There was also a significant difference between males and females in the proportion of SRC cases with relapse of symptoms. Relapse occurred in 43.4% of female athletes with SRC versus 29.7% of male athletes with SRC (P = .023).
Female athletes also had significantly longer times before being cleared for RTP. The mean time was 74.5 days for females, compared with a mean of 42.3 days for male athletes (P < .001). “The median time to RTP clearance was nearly double [for female athletes] at 49 days versus 25 days [for male athletes],” wrote the authors.
The rate of premature RTL was also higher among secondary school students (48.8%), compared with 28% among elementary students and 42% among postsecondary students.
More concussions coming?
Before the first consensus conference, organized by the Concussion in Sport Group in 2001, management of concussion was based on rating and grading scales that had no medical evidence to support them, said Dr. Carson. After the consensus conference, it was recommended that physicians manage each concussion individually and, when it came to RTP, recommendations were based upon symptom resolution.
In contrast, there was nothing in the literature regarding how student athletes who sustain a concussion should RTL. Some schools made generous accommodations, and others none. This situation changed around 2011, when experts started publishing data about how better to accommodate student athletes who have a temporary disability for which schools need to introduce temporary accommodations to help them recover.
“Recommendations for RTP essentially had a 12-year head-start,” Dr. Carson emphasized, “and RTL had a much slower start.” Unfortunately, Dr. Carson foresees more athletes sustaining concussions as pandemic restrictions ease over the next few months. “As athletes RTP after the pandemic, they just will not be in game shape,” he said.
“In other words, athletes may not have the neuromuscular control to avoid these injuries as easily,” he added. Worse, athletes may not realize they are not quite ready to return to the expected level of participation so quickly. “I believe this scenario will lead to more concussions that will be difficult to manage in the context of an already strained health care system,” said Dr. Carson.
A limitation of the study was that it was difficult to assess whether all patients followed medical advice consistently.
“Very positive shifts”
Commenting on the findings, Nick Reed, PhD, Canada research chair in pediatric concussion and associate professor of occupational science and occupational therapy, University of Toronto, said that sports medicine physicians are seeing “very positive shifts” in concussion awareness and related behaviors such as providing education, support, and accommodations to students within the school environment. “More and more teachers are seeking education to learn what a concussion is and what to do to best support their students with concussion,” he said. Dr. Reed was not involved in the current study.
Indeed, this increasing awareness led to the development of a concussion education tool for teachers – SCHOOLFirst – although Dr. Reed did acknowledge that not all teachers have either the knowledge or the resources they need to optimally support their students with concussion. In the meantime, to reduce the risk of injury, Dr. Reed stressed that it is important for students to wear equipment appropriate for the game being played and to play by the rules.
“It is key to play sports in a way that is fair and respectful and not [engage] in behaviors with the intent of injuring an opponent,” he stressed. It is also important for athletes themselves to know the signs and symptoms of concussion and, if they think they have a concussion, to immediately stop playing, report how they are feeling to a coach, teacher, or parent, and to seek medical assessment to determine if they have a concussion or not.
“The key here is to focus on what the athlete can do after a concussion rather than what they can’t do,” Dr. Reed said. After even a few days of complete rest, students with a concussion can gradually introduce low levels of physical and cognitive activity that won’t make their symptoms worse. This activity can include going back to school with temporary accommodations in place, such as shorter school days and increased rest breaks. “When returning to school and to sport after a concussion, it is important to follow a stepwise and gradual return to activities so that you aren’t doing too much too fast,” Dr. Reed emphasized.
The study was conducted without external funding. Dr. Carson and Dr. Reed reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
, according to a recent chart review. Rates of premature return to learn (RTL) are essentially unchanged, however.
“Delay in recovery is the major reason why it’s important not to RTL or RTP prematurely,” said James Carson, MD, associate professor of family and community medicine, University of Toronto.
“That delay in recovery only sets students further back in terms of the stress they get from being delayed with their schoolwork – they could lose their year in school, lose all their social contacts. So, there are a number of psychosocial issues that come into play if recovery is delayed, and that is what premature RTL and premature RTP will do – they delay the student’s recovery,” he emphasized.
The study was published in Canadian Family Physician.
Differences by sex
The study involved 241 students who had 258 distinct cases of SRC. The researchers defined premature RTP and RTL as chart records documenting the relapse, recurrence, or worsening of concussion symptoms that accompanied the patient’s RTP or RTL. Between 2011 and 2016, 26.7% of students had evidence of premature RTP, while 42.6% of them had evidence of premature RTL, the authors noted.
Compared with findings from an earlier survey of data from 2006 to 2011, the incidence of premature RTP dropped by 38.6% (P = .0003). In contrast, symptoms associated with premature RTL dropped by only 4.7% from the previous survey. This change was not statistically significant.
There was also a significant difference between males and females in the proportion of SRC cases with relapse of symptoms. Relapse occurred in 43.4% of female athletes with SRC versus 29.7% of male athletes with SRC (P = .023).
Female athletes also had significantly longer times before being cleared for RTP. The mean time was 74.5 days for females, compared with a mean of 42.3 days for male athletes (P < .001). “The median time to RTP clearance was nearly double [for female athletes] at 49 days versus 25 days [for male athletes],” wrote the authors.
The rate of premature RTL was also higher among secondary school students (48.8%), compared with 28% among elementary students and 42% among postsecondary students.
More concussions coming?
Before the first consensus conference, organized by the Concussion in Sport Group in 2001, management of concussion was based on rating and grading scales that had no medical evidence to support them, said Dr. Carson. After the consensus conference, it was recommended that physicians manage each concussion individually and, when it came to RTP, recommendations were based upon symptom resolution.
In contrast, there was nothing in the literature regarding how student athletes who sustain a concussion should RTL. Some schools made generous accommodations, and others none. This situation changed around 2011, when experts started publishing data about how better to accommodate student athletes who have a temporary disability for which schools need to introduce temporary accommodations to help them recover.
“Recommendations for RTP essentially had a 12-year head-start,” Dr. Carson emphasized, “and RTL had a much slower start.” Unfortunately, Dr. Carson foresees more athletes sustaining concussions as pandemic restrictions ease over the next few months. “As athletes RTP after the pandemic, they just will not be in game shape,” he said.
“In other words, athletes may not have the neuromuscular control to avoid these injuries as easily,” he added. Worse, athletes may not realize they are not quite ready to return to the expected level of participation so quickly. “I believe this scenario will lead to more concussions that will be difficult to manage in the context of an already strained health care system,” said Dr. Carson.
A limitation of the study was that it was difficult to assess whether all patients followed medical advice consistently.
“Very positive shifts”
Commenting on the findings, Nick Reed, PhD, Canada research chair in pediatric concussion and associate professor of occupational science and occupational therapy, University of Toronto, said that sports medicine physicians are seeing “very positive shifts” in concussion awareness and related behaviors such as providing education, support, and accommodations to students within the school environment. “More and more teachers are seeking education to learn what a concussion is and what to do to best support their students with concussion,” he said. Dr. Reed was not involved in the current study.
Indeed, this increasing awareness led to the development of a concussion education tool for teachers – SCHOOLFirst – although Dr. Reed did acknowledge that not all teachers have either the knowledge or the resources they need to optimally support their students with concussion. In the meantime, to reduce the risk of injury, Dr. Reed stressed that it is important for students to wear equipment appropriate for the game being played and to play by the rules.
“It is key to play sports in a way that is fair and respectful and not [engage] in behaviors with the intent of injuring an opponent,” he stressed. It is also important for athletes themselves to know the signs and symptoms of concussion and, if they think they have a concussion, to immediately stop playing, report how they are feeling to a coach, teacher, or parent, and to seek medical assessment to determine if they have a concussion or not.
“The key here is to focus on what the athlete can do after a concussion rather than what they can’t do,” Dr. Reed said. After even a few days of complete rest, students with a concussion can gradually introduce low levels of physical and cognitive activity that won’t make their symptoms worse. This activity can include going back to school with temporary accommodations in place, such as shorter school days and increased rest breaks. “When returning to school and to sport after a concussion, it is important to follow a stepwise and gradual return to activities so that you aren’t doing too much too fast,” Dr. Reed emphasized.
The study was conducted without external funding. Dr. Carson and Dr. Reed reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
, according to a recent chart review. Rates of premature return to learn (RTL) are essentially unchanged, however.
“Delay in recovery is the major reason why it’s important not to RTL or RTP prematurely,” said James Carson, MD, associate professor of family and community medicine, University of Toronto.
“That delay in recovery only sets students further back in terms of the stress they get from being delayed with their schoolwork – they could lose their year in school, lose all their social contacts. So, there are a number of psychosocial issues that come into play if recovery is delayed, and that is what premature RTL and premature RTP will do – they delay the student’s recovery,” he emphasized.
The study was published in Canadian Family Physician.
Differences by sex
The study involved 241 students who had 258 distinct cases of SRC. The researchers defined premature RTP and RTL as chart records documenting the relapse, recurrence, or worsening of concussion symptoms that accompanied the patient’s RTP or RTL. Between 2011 and 2016, 26.7% of students had evidence of premature RTP, while 42.6% of them had evidence of premature RTL, the authors noted.
Compared with findings from an earlier survey of data from 2006 to 2011, the incidence of premature RTP dropped by 38.6% (P = .0003). In contrast, symptoms associated with premature RTL dropped by only 4.7% from the previous survey. This change was not statistically significant.
There was also a significant difference between males and females in the proportion of SRC cases with relapse of symptoms. Relapse occurred in 43.4% of female athletes with SRC versus 29.7% of male athletes with SRC (P = .023).
Female athletes also had significantly longer times before being cleared for RTP. The mean time was 74.5 days for females, compared with a mean of 42.3 days for male athletes (P < .001). “The median time to RTP clearance was nearly double [for female athletes] at 49 days versus 25 days [for male athletes],” wrote the authors.
The rate of premature RTL was also higher among secondary school students (48.8%), compared with 28% among elementary students and 42% among postsecondary students.
More concussions coming?
Before the first consensus conference, organized by the Concussion in Sport Group in 2001, management of concussion was based on rating and grading scales that had no medical evidence to support them, said Dr. Carson. After the consensus conference, it was recommended that physicians manage each concussion individually and, when it came to RTP, recommendations were based upon symptom resolution.
In contrast, there was nothing in the literature regarding how student athletes who sustain a concussion should RTL. Some schools made generous accommodations, and others none. This situation changed around 2011, when experts started publishing data about how better to accommodate student athletes who have a temporary disability for which schools need to introduce temporary accommodations to help them recover.
“Recommendations for RTP essentially had a 12-year head-start,” Dr. Carson emphasized, “and RTL had a much slower start.” Unfortunately, Dr. Carson foresees more athletes sustaining concussions as pandemic restrictions ease over the next few months. “As athletes RTP after the pandemic, they just will not be in game shape,” he said.
“In other words, athletes may not have the neuromuscular control to avoid these injuries as easily,” he added. Worse, athletes may not realize they are not quite ready to return to the expected level of participation so quickly. “I believe this scenario will lead to more concussions that will be difficult to manage in the context of an already strained health care system,” said Dr. Carson.
A limitation of the study was that it was difficult to assess whether all patients followed medical advice consistently.
“Very positive shifts”
Commenting on the findings, Nick Reed, PhD, Canada research chair in pediatric concussion and associate professor of occupational science and occupational therapy, University of Toronto, said that sports medicine physicians are seeing “very positive shifts” in concussion awareness and related behaviors such as providing education, support, and accommodations to students within the school environment. “More and more teachers are seeking education to learn what a concussion is and what to do to best support their students with concussion,” he said. Dr. Reed was not involved in the current study.
Indeed, this increasing awareness led to the development of a concussion education tool for teachers – SCHOOLFirst – although Dr. Reed did acknowledge that not all teachers have either the knowledge or the resources they need to optimally support their students with concussion. In the meantime, to reduce the risk of injury, Dr. Reed stressed that it is important for students to wear equipment appropriate for the game being played and to play by the rules.
“It is key to play sports in a way that is fair and respectful and not [engage] in behaviors with the intent of injuring an opponent,” he stressed. It is also important for athletes themselves to know the signs and symptoms of concussion and, if they think they have a concussion, to immediately stop playing, report how they are feeling to a coach, teacher, or parent, and to seek medical assessment to determine if they have a concussion or not.
“The key here is to focus on what the athlete can do after a concussion rather than what they can’t do,” Dr. Reed said. After even a few days of complete rest, students with a concussion can gradually introduce low levels of physical and cognitive activity that won’t make their symptoms worse. This activity can include going back to school with temporary accommodations in place, such as shorter school days and increased rest breaks. “When returning to school and to sport after a concussion, it is important to follow a stepwise and gradual return to activities so that you aren’t doing too much too fast,” Dr. Reed emphasized.
The study was conducted without external funding. Dr. Carson and Dr. Reed reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
OIG Finds More Problems in the VA’s Problem-Prone EHR Rollout
Scheduling delays. Disappearing lab orders. Bad links for telehealth appointments. Erroneous medication dispensing. Time-consuming workarounds.
The rollout of the $16 billion electronic health record (EHR) system at the US Department of Veterans Affair (VA) has met some fairly large bumps in the past few years. And now, the VA Office of the Inspector General (OIG) has pronounced on a “range of allegations” at the Mann-Grandstaff VA Medical Center in Spokane, Washington, the first of several hospitals and clinics in the Pacific Northwest set to implement the new system.
VA Inspector General Michael Missal issued 3 reports in mid-March on how the “go-live” process was faring: one on medication management deficiencies, one on care coordination deficiencies, and one on technical issues.
The reports document the OIG’s “concerns” with the new process. According to the technical report, for instance, between October 2020 and March 2021, new EHR users placed more than 38,700 requests for assistance. Of those, 33% were closed without a documented resolution. The OIG also reviewed 210 tickets related to care coordination and found that 1% were closed without a documented resolution.
The OIG said EHR implementation had “created difficulties” for end users in 8 areas:
- Patient record flags, including failures to transfer flags and information related to patients at high risk for suicide;
- Data migration errors leading to inaccurate name, sex, and contact information;
- Scheduling process issues, such as delays in primary care scheduling;
- VA Video Connect problems, including inoperable and misdirected links;
- Referral management deficiencies, including lost or unaddressed referrals;
- Laboratory orders “disappearing” that affected workflow and tracking, and delayed results;
- Patient portal and secure messaging being inaccessible; and
- Documentation processes, including creating additional work and limiting the ability to correctly code patient diagnoses.
The OIG’s technical report identified 5 factors that contributed to the headaches: EHR usability problems, training deficits, interoperability challenges, post–go-live fixes and refinement needs, and problem-resolution process challenges.
The OIG did not identify any associated patient deaths during the inspection but says “future deployment of the new EHR without resolving deficiencies can increase risks to patient safety.”
The technological overhaul has been handled by Cerner. The VA initially awarded Cerner $113 million for EHR modernization, and in 2018 the company secured a 10-year, $10 billion contract to help the VA rebuild its system, similar to the way it did for the US Department of Defense (DoD) with MHS GENESIS. The Cerner DoD project, which has been called “the most lucrative electronic health record contract in history” was launched at the Fairchild Air Force Base in Spokane, Washington, in 2017, and is expected to be operational in more than half of military hospitals and clinics by the end of this year. In 2021, Cerner received an 18-month, $134.1 million task order to deploy the company’s EHR platform at VA medical centers.
This isn’t the first time the VA/Cerner EHR project has hit snags. In 2021, the VA scrapped the schedule, trading it for a 6-month pause after a strategic review ordered by VA Secretary Denis McDonough found problems with governance and management. McDonough told the Senate Veterans’ Affairs Committee that a 3-month internal review had found too many structural problems to warrant continuing the rollout. The sole-source contract with Cerner also raised concerns, as did the influence of 3 confidants of Present Trump on the process. Moreover, cost estimates kept growing—from $10 billion to $16 billion—in part because VA leaders during the Trump administration did not budget for technology and hospital upgrades to allow the new platform to work, according to an article in The Washington Post.
During the senate hearing, committee chair Sen. Jon Tester (D-MT) said, “There’s been damn little accountability. I hope Cerner’s watching this. If they’re not open to making a user-friendly health medical record, they ought to admit it so we can get the money back and start all over.” He told McDonough that the failures were “not all your fault—I don’t know if any of it is your fault.”
“It’s a lot of money you’ve entrusted to us,” McDonough told the committee. The serious problems, he said, were “on us.” He added, “We are taking swift and decisive action to incorporate the management rigor and enterprise jointness required for this program to deliver on its intended purpose: seamless excellence in VA care for veterans. VA’s first implementation of the [project] did not live up to that promise, either for our veterans or for our providers.”
He said he had ordered an overhaul that will include better training for clinical staff, more reliable testing and oversight of Cerner, and a leadership shake-up. He also said he had installed a patient safety team at the Spokane hospital.
Terry Adirim, MD, formerly with the DoD, took over the EHR program in January. In an interview, she said, “[W]e’ve made a substantial number of changes,” such as a new round of training for the hospital’s medical staff. “These deployments are really complex and they’re really hard,” Adirim said, noting that about half of digital medical records programs at private hospitals fail at first. She pointed to the revamped DoD program, which also had its flaws but is running much more smoothly. One of the issues, she said, is that many physicians did not realize that the Cerner system would differ so dramatically from VistA, the system it’s replacing.
The first installment of the rocky rollout left hospital staff confused and worn out. Sen. Patty Murphy (D-WA) said in 2021 that the Spokane staff had filed hundreds of reports of patient safety issues caused by the new system. “Patients are not getting accurate meds. Meds are sent to the wrong address. What used to take a few clicks is now a lot more complicated. Providers are burning out.”
A year later, in a statement, echoing her earlier comments, she said, “We need to put a pause on this rollout right now.”
But Adirim has said the VA is moving ahead with the rollout. The VA has added extra support staff and plans to have physicians from outside the hospital on hand in case things go wrong. According to the Washington Post, Deputy VA Secretary Donald Remy told the OIG that the VA is working to address the outstanding issues and hopes to resolve them by mid-May.
Meanwhile, the beleaguered project ran into another obstacle in early March, when computers went down at Mann-Grandstaff, leading to 20 hours of yet more confusion about medications and surgeries. The VA said the IT system outage also happened at Columbus, Ohio (another of the planned pilot spots). The system was back online the next day, with no known patient safety issues.
Eastern Washington Congresswoman Cathy McMorris Rodgers released a statement saying, “The shutdown of Mann-Grandstaff VA yesterday is another event in a series of challenges that the new electronic health record has created for staff and veterans at the facility. My understanding is that an update made to help the VA’s database for demographic data better communicate with the Cerner system was not performed correctly. Mann-Grandstaff leadership rightly took the system offline until the scope of the problem was understood, so no patients were harmed.”
However, Sen. Murphy called the technical failure “absolutely unacceptable.” In a more recent statement about the rollout, she said, “This is about patient safety and it needs to get fixed—period. VA needs to be upfront about issues like this in real time—Congress absolutely requires transparency when it comes to failures as serious as this. I should be hearing about this from local reporting first.”
If the high-quality care veterans deserve is uncertain at any point, she added, “the rollout should be delayed.” Again.
Scheduling delays. Disappearing lab orders. Bad links for telehealth appointments. Erroneous medication dispensing. Time-consuming workarounds.
The rollout of the $16 billion electronic health record (EHR) system at the US Department of Veterans Affair (VA) has met some fairly large bumps in the past few years. And now, the VA Office of the Inspector General (OIG) has pronounced on a “range of allegations” at the Mann-Grandstaff VA Medical Center in Spokane, Washington, the first of several hospitals and clinics in the Pacific Northwest set to implement the new system.
VA Inspector General Michael Missal issued 3 reports in mid-March on how the “go-live” process was faring: one on medication management deficiencies, one on care coordination deficiencies, and one on technical issues.
The reports document the OIG’s “concerns” with the new process. According to the technical report, for instance, between October 2020 and March 2021, new EHR users placed more than 38,700 requests for assistance. Of those, 33% were closed without a documented resolution. The OIG also reviewed 210 tickets related to care coordination and found that 1% were closed without a documented resolution.
The OIG said EHR implementation had “created difficulties” for end users in 8 areas:
- Patient record flags, including failures to transfer flags and information related to patients at high risk for suicide;
- Data migration errors leading to inaccurate name, sex, and contact information;
- Scheduling process issues, such as delays in primary care scheduling;
- VA Video Connect problems, including inoperable and misdirected links;
- Referral management deficiencies, including lost or unaddressed referrals;
- Laboratory orders “disappearing” that affected workflow and tracking, and delayed results;
- Patient portal and secure messaging being inaccessible; and
- Documentation processes, including creating additional work and limiting the ability to correctly code patient diagnoses.
The OIG’s technical report identified 5 factors that contributed to the headaches: EHR usability problems, training deficits, interoperability challenges, post–go-live fixes and refinement needs, and problem-resolution process challenges.
The OIG did not identify any associated patient deaths during the inspection but says “future deployment of the new EHR without resolving deficiencies can increase risks to patient safety.”
The technological overhaul has been handled by Cerner. The VA initially awarded Cerner $113 million for EHR modernization, and in 2018 the company secured a 10-year, $10 billion contract to help the VA rebuild its system, similar to the way it did for the US Department of Defense (DoD) with MHS GENESIS. The Cerner DoD project, which has been called “the most lucrative electronic health record contract in history” was launched at the Fairchild Air Force Base in Spokane, Washington, in 2017, and is expected to be operational in more than half of military hospitals and clinics by the end of this year. In 2021, Cerner received an 18-month, $134.1 million task order to deploy the company’s EHR platform at VA medical centers.
This isn’t the first time the VA/Cerner EHR project has hit snags. In 2021, the VA scrapped the schedule, trading it for a 6-month pause after a strategic review ordered by VA Secretary Denis McDonough found problems with governance and management. McDonough told the Senate Veterans’ Affairs Committee that a 3-month internal review had found too many structural problems to warrant continuing the rollout. The sole-source contract with Cerner also raised concerns, as did the influence of 3 confidants of Present Trump on the process. Moreover, cost estimates kept growing—from $10 billion to $16 billion—in part because VA leaders during the Trump administration did not budget for technology and hospital upgrades to allow the new platform to work, according to an article in The Washington Post.
During the senate hearing, committee chair Sen. Jon Tester (D-MT) said, “There’s been damn little accountability. I hope Cerner’s watching this. If they’re not open to making a user-friendly health medical record, they ought to admit it so we can get the money back and start all over.” He told McDonough that the failures were “not all your fault—I don’t know if any of it is your fault.”
“It’s a lot of money you’ve entrusted to us,” McDonough told the committee. The serious problems, he said, were “on us.” He added, “We are taking swift and decisive action to incorporate the management rigor and enterprise jointness required for this program to deliver on its intended purpose: seamless excellence in VA care for veterans. VA’s first implementation of the [project] did not live up to that promise, either for our veterans or for our providers.”
He said he had ordered an overhaul that will include better training for clinical staff, more reliable testing and oversight of Cerner, and a leadership shake-up. He also said he had installed a patient safety team at the Spokane hospital.
Terry Adirim, MD, formerly with the DoD, took over the EHR program in January. In an interview, she said, “[W]e’ve made a substantial number of changes,” such as a new round of training for the hospital’s medical staff. “These deployments are really complex and they’re really hard,” Adirim said, noting that about half of digital medical records programs at private hospitals fail at first. She pointed to the revamped DoD program, which also had its flaws but is running much more smoothly. One of the issues, she said, is that many physicians did not realize that the Cerner system would differ so dramatically from VistA, the system it’s replacing.
The first installment of the rocky rollout left hospital staff confused and worn out. Sen. Patty Murphy (D-WA) said in 2021 that the Spokane staff had filed hundreds of reports of patient safety issues caused by the new system. “Patients are not getting accurate meds. Meds are sent to the wrong address. What used to take a few clicks is now a lot more complicated. Providers are burning out.”
A year later, in a statement, echoing her earlier comments, she said, “We need to put a pause on this rollout right now.”
But Adirim has said the VA is moving ahead with the rollout. The VA has added extra support staff and plans to have physicians from outside the hospital on hand in case things go wrong. According to the Washington Post, Deputy VA Secretary Donald Remy told the OIG that the VA is working to address the outstanding issues and hopes to resolve them by mid-May.
Meanwhile, the beleaguered project ran into another obstacle in early March, when computers went down at Mann-Grandstaff, leading to 20 hours of yet more confusion about medications and surgeries. The VA said the IT system outage also happened at Columbus, Ohio (another of the planned pilot spots). The system was back online the next day, with no known patient safety issues.
Eastern Washington Congresswoman Cathy McMorris Rodgers released a statement saying, “The shutdown of Mann-Grandstaff VA yesterday is another event in a series of challenges that the new electronic health record has created for staff and veterans at the facility. My understanding is that an update made to help the VA’s database for demographic data better communicate with the Cerner system was not performed correctly. Mann-Grandstaff leadership rightly took the system offline until the scope of the problem was understood, so no patients were harmed.”
However, Sen. Murphy called the technical failure “absolutely unacceptable.” In a more recent statement about the rollout, she said, “This is about patient safety and it needs to get fixed—period. VA needs to be upfront about issues like this in real time—Congress absolutely requires transparency when it comes to failures as serious as this. I should be hearing about this from local reporting first.”
If the high-quality care veterans deserve is uncertain at any point, she added, “the rollout should be delayed.” Again.
Scheduling delays. Disappearing lab orders. Bad links for telehealth appointments. Erroneous medication dispensing. Time-consuming workarounds.
The rollout of the $16 billion electronic health record (EHR) system at the US Department of Veterans Affair (VA) has met some fairly large bumps in the past few years. And now, the VA Office of the Inspector General (OIG) has pronounced on a “range of allegations” at the Mann-Grandstaff VA Medical Center in Spokane, Washington, the first of several hospitals and clinics in the Pacific Northwest set to implement the new system.
VA Inspector General Michael Missal issued 3 reports in mid-March on how the “go-live” process was faring: one on medication management deficiencies, one on care coordination deficiencies, and one on technical issues.
The reports document the OIG’s “concerns” with the new process. According to the technical report, for instance, between October 2020 and March 2021, new EHR users placed more than 38,700 requests for assistance. Of those, 33% were closed without a documented resolution. The OIG also reviewed 210 tickets related to care coordination and found that 1% were closed without a documented resolution.
The OIG said EHR implementation had “created difficulties” for end users in 8 areas:
- Patient record flags, including failures to transfer flags and information related to patients at high risk for suicide;
- Data migration errors leading to inaccurate name, sex, and contact information;
- Scheduling process issues, such as delays in primary care scheduling;
- VA Video Connect problems, including inoperable and misdirected links;
- Referral management deficiencies, including lost or unaddressed referrals;
- Laboratory orders “disappearing” that affected workflow and tracking, and delayed results;
- Patient portal and secure messaging being inaccessible; and
- Documentation processes, including creating additional work and limiting the ability to correctly code patient diagnoses.
The OIG’s technical report identified 5 factors that contributed to the headaches: EHR usability problems, training deficits, interoperability challenges, post–go-live fixes and refinement needs, and problem-resolution process challenges.
The OIG did not identify any associated patient deaths during the inspection but says “future deployment of the new EHR without resolving deficiencies can increase risks to patient safety.”
The technological overhaul has been handled by Cerner. The VA initially awarded Cerner $113 million for EHR modernization, and in 2018 the company secured a 10-year, $10 billion contract to help the VA rebuild its system, similar to the way it did for the US Department of Defense (DoD) with MHS GENESIS. The Cerner DoD project, which has been called “the most lucrative electronic health record contract in history” was launched at the Fairchild Air Force Base in Spokane, Washington, in 2017, and is expected to be operational in more than half of military hospitals and clinics by the end of this year. In 2021, Cerner received an 18-month, $134.1 million task order to deploy the company’s EHR platform at VA medical centers.
This isn’t the first time the VA/Cerner EHR project has hit snags. In 2021, the VA scrapped the schedule, trading it for a 6-month pause after a strategic review ordered by VA Secretary Denis McDonough found problems with governance and management. McDonough told the Senate Veterans’ Affairs Committee that a 3-month internal review had found too many structural problems to warrant continuing the rollout. The sole-source contract with Cerner also raised concerns, as did the influence of 3 confidants of Present Trump on the process. Moreover, cost estimates kept growing—from $10 billion to $16 billion—in part because VA leaders during the Trump administration did not budget for technology and hospital upgrades to allow the new platform to work, according to an article in The Washington Post.
During the senate hearing, committee chair Sen. Jon Tester (D-MT) said, “There’s been damn little accountability. I hope Cerner’s watching this. If they’re not open to making a user-friendly health medical record, they ought to admit it so we can get the money back and start all over.” He told McDonough that the failures were “not all your fault—I don’t know if any of it is your fault.”
“It’s a lot of money you’ve entrusted to us,” McDonough told the committee. The serious problems, he said, were “on us.” He added, “We are taking swift and decisive action to incorporate the management rigor and enterprise jointness required for this program to deliver on its intended purpose: seamless excellence in VA care for veterans. VA’s first implementation of the [project] did not live up to that promise, either for our veterans or for our providers.”
He said he had ordered an overhaul that will include better training for clinical staff, more reliable testing and oversight of Cerner, and a leadership shake-up. He also said he had installed a patient safety team at the Spokane hospital.
Terry Adirim, MD, formerly with the DoD, took over the EHR program in January. In an interview, she said, “[W]e’ve made a substantial number of changes,” such as a new round of training for the hospital’s medical staff. “These deployments are really complex and they’re really hard,” Adirim said, noting that about half of digital medical records programs at private hospitals fail at first. She pointed to the revamped DoD program, which also had its flaws but is running much more smoothly. One of the issues, she said, is that many physicians did not realize that the Cerner system would differ so dramatically from VistA, the system it’s replacing.
The first installment of the rocky rollout left hospital staff confused and worn out. Sen. Patty Murphy (D-WA) said in 2021 that the Spokane staff had filed hundreds of reports of patient safety issues caused by the new system. “Patients are not getting accurate meds. Meds are sent to the wrong address. What used to take a few clicks is now a lot more complicated. Providers are burning out.”
A year later, in a statement, echoing her earlier comments, she said, “We need to put a pause on this rollout right now.”
But Adirim has said the VA is moving ahead with the rollout. The VA has added extra support staff and plans to have physicians from outside the hospital on hand in case things go wrong. According to the Washington Post, Deputy VA Secretary Donald Remy told the OIG that the VA is working to address the outstanding issues and hopes to resolve them by mid-May.
Meanwhile, the beleaguered project ran into another obstacle in early March, when computers went down at Mann-Grandstaff, leading to 20 hours of yet more confusion about medications and surgeries. The VA said the IT system outage also happened at Columbus, Ohio (another of the planned pilot spots). The system was back online the next day, with no known patient safety issues.
Eastern Washington Congresswoman Cathy McMorris Rodgers released a statement saying, “The shutdown of Mann-Grandstaff VA yesterday is another event in a series of challenges that the new electronic health record has created for staff and veterans at the facility. My understanding is that an update made to help the VA’s database for demographic data better communicate with the Cerner system was not performed correctly. Mann-Grandstaff leadership rightly took the system offline until the scope of the problem was understood, so no patients were harmed.”
However, Sen. Murphy called the technical failure “absolutely unacceptable.” In a more recent statement about the rollout, she said, “This is about patient safety and it needs to get fixed—period. VA needs to be upfront about issues like this in real time—Congress absolutely requires transparency when it comes to failures as serious as this. I should be hearing about this from local reporting first.”
If the high-quality care veterans deserve is uncertain at any point, she added, “the rollout should be delayed.” Again.



