User login
Is prescribing stimulants OK for comorbid opioid use disorder, ADHD?
A growing number of patients with opioid use disorder (OUD) have a diagnosis of comorbid attention-deficit/hyperactivity disorder (ADHD), raising issues about whether it’s appropriate to prescribe stimulants in this patient population.
One new study showed that from 2007-2017, there was a threefold increase in OUD and comorbid ADHD and that a significant number of these patients received prescription stimulants.
“This is the beginning stages of looking at whether or not there are risks of prescribing stimulants to patients who are on medications for opioid use disorder,” investigator Tae Woo (Ted) Park, MD, assistant professor, department of psychiatry, University of Pittsburgh School of Medicine, told this news organization.
“More and more people are being identified with ADHD, and we need to do more research on the best way to manage this patient group,” Dr. Park added.
The findings were presented at the annual meeting of the American Academy of Addiction Psychiatry.
Biological connection?
Dr. Park is not convinced there is “an actual biological connection” between ADHD and OUD, noting that there are many reasons why patients with ADHD may be more prone to developing such a disorder.
Perhaps they did not get an ADHD diagnosis as a child, “which led to impairment in their ability to be successful at school and then in a job,” which in turn predisposed them to having a substance use disorder, said Dr. Park.
From previous research and his own clinical experience, ADHD can significantly affect quality of life and “cause increased impairment” in patients with a substance use disorder, he added.
Interestingly, there’s evidence suggesting patients treated for ADHD early in life are less likely to develop a substance use disorder later on, he said.
The “gold standard” treatment for ADHD is a prescription stimulant, which carries its own addiction risks. “So the issue is about whether or not to prescribe risky medications and how to weigh the risks and benefits,” said Dr. Park.
From a private health insurance database, researchers examined records for patients aged 18-64 years who were receiving medication for OUD, including buprenorphine, methadone, or naltrexone, from 2007-2017.
In the study sample, about 17,000 individuals were receiving stimulants, and 156,000 were not receiving these drugs. The largest percentage of participants in both groups was in the age-18-to-25 category.
About 35% of those receiving stimulants had ADHD, and about the same percentage had a mood disorder diagnosis.
Percentage of co-occurring ADHD and OUD increased from more than 4% in 2007 to more than 14% in 2017. The prevalence of stimulant use plus medication for OUD also increased during that time.
The increase in ADHD diagnoses may reflect growing identification of the condition, Dr. Park noted. As the opioid problem became more apparent and additional treatments made available, “there were more health care contacts, more assessments, and more diagnoses, including of ADHD,” he said.
Risks versus benefits
Stimulants may also be risky in patients with OUD. Results from another study presented at the AAAP meeting showed these drugs were associated with an increased chance of poisoning in patients receiving buprenorphine.
However, Dr. Park is skeptical the combination of stimulants and buprenorphine “leads to a biological risk of overdose.” He used a hypothetical scenario where other factors play into the connection: A patient gets a prescription stimulant, becomes addicted, then starts using street or illicit stimulants, which leads to a relapse on opioids, and then to an overdose.
Dr. Park noted that the same study that found an increased poisoning risk in stimulant users also found that patients tend to stay on buprenorphine treatment, providing protection against overdose.
“So there are risks and benefits of prescribing these medications, and it becomes tricky to know whether to prescribe them or not,” he said.
While stimulants are by far the best treatment for ADHD, atomoxetine (Strattera), a nonstimulant medication with antidepressant effects is another option, Dr. Park said.
He added that a limitation of his study was that very few individuals in the database received methadone.
A version of this article first appeared on Medscape.com.
A growing number of patients with opioid use disorder (OUD) have a diagnosis of comorbid attention-deficit/hyperactivity disorder (ADHD), raising issues about whether it’s appropriate to prescribe stimulants in this patient population.
One new study showed that from 2007-2017, there was a threefold increase in OUD and comorbid ADHD and that a significant number of these patients received prescription stimulants.
“This is the beginning stages of looking at whether or not there are risks of prescribing stimulants to patients who are on medications for opioid use disorder,” investigator Tae Woo (Ted) Park, MD, assistant professor, department of psychiatry, University of Pittsburgh School of Medicine, told this news organization.
“More and more people are being identified with ADHD, and we need to do more research on the best way to manage this patient group,” Dr. Park added.
The findings were presented at the annual meeting of the American Academy of Addiction Psychiatry.
Biological connection?
Dr. Park is not convinced there is “an actual biological connection” between ADHD and OUD, noting that there are many reasons why patients with ADHD may be more prone to developing such a disorder.
Perhaps they did not get an ADHD diagnosis as a child, “which led to impairment in their ability to be successful at school and then in a job,” which in turn predisposed them to having a substance use disorder, said Dr. Park.
From previous research and his own clinical experience, ADHD can significantly affect quality of life and “cause increased impairment” in patients with a substance use disorder, he added.
Interestingly, there’s evidence suggesting patients treated for ADHD early in life are less likely to develop a substance use disorder later on, he said.
The “gold standard” treatment for ADHD is a prescription stimulant, which carries its own addiction risks. “So the issue is about whether or not to prescribe risky medications and how to weigh the risks and benefits,” said Dr. Park.
From a private health insurance database, researchers examined records for patients aged 18-64 years who were receiving medication for OUD, including buprenorphine, methadone, or naltrexone, from 2007-2017.
In the study sample, about 17,000 individuals were receiving stimulants, and 156,000 were not receiving these drugs. The largest percentage of participants in both groups was in the age-18-to-25 category.
About 35% of those receiving stimulants had ADHD, and about the same percentage had a mood disorder diagnosis.
Percentage of co-occurring ADHD and OUD increased from more than 4% in 2007 to more than 14% in 2017. The prevalence of stimulant use plus medication for OUD also increased during that time.
The increase in ADHD diagnoses may reflect growing identification of the condition, Dr. Park noted. As the opioid problem became more apparent and additional treatments made available, “there were more health care contacts, more assessments, and more diagnoses, including of ADHD,” he said.
Risks versus benefits
Stimulants may also be risky in patients with OUD. Results from another study presented at the AAAP meeting showed these drugs were associated with an increased chance of poisoning in patients receiving buprenorphine.
However, Dr. Park is skeptical the combination of stimulants and buprenorphine “leads to a biological risk of overdose.” He used a hypothetical scenario where other factors play into the connection: A patient gets a prescription stimulant, becomes addicted, then starts using street or illicit stimulants, which leads to a relapse on opioids, and then to an overdose.
Dr. Park noted that the same study that found an increased poisoning risk in stimulant users also found that patients tend to stay on buprenorphine treatment, providing protection against overdose.
“So there are risks and benefits of prescribing these medications, and it becomes tricky to know whether to prescribe them or not,” he said.
While stimulants are by far the best treatment for ADHD, atomoxetine (Strattera), a nonstimulant medication with antidepressant effects is another option, Dr. Park said.
He added that a limitation of his study was that very few individuals in the database received methadone.
A version of this article first appeared on Medscape.com.
A growing number of patients with opioid use disorder (OUD) have a diagnosis of comorbid attention-deficit/hyperactivity disorder (ADHD), raising issues about whether it’s appropriate to prescribe stimulants in this patient population.
One new study showed that from 2007-2017, there was a threefold increase in OUD and comorbid ADHD and that a significant number of these patients received prescription stimulants.
“This is the beginning stages of looking at whether or not there are risks of prescribing stimulants to patients who are on medications for opioid use disorder,” investigator Tae Woo (Ted) Park, MD, assistant professor, department of psychiatry, University of Pittsburgh School of Medicine, told this news organization.
“More and more people are being identified with ADHD, and we need to do more research on the best way to manage this patient group,” Dr. Park added.
The findings were presented at the annual meeting of the American Academy of Addiction Psychiatry.
Biological connection?
Dr. Park is not convinced there is “an actual biological connection” between ADHD and OUD, noting that there are many reasons why patients with ADHD may be more prone to developing such a disorder.
Perhaps they did not get an ADHD diagnosis as a child, “which led to impairment in their ability to be successful at school and then in a job,” which in turn predisposed them to having a substance use disorder, said Dr. Park.
From previous research and his own clinical experience, ADHD can significantly affect quality of life and “cause increased impairment” in patients with a substance use disorder, he added.
Interestingly, there’s evidence suggesting patients treated for ADHD early in life are less likely to develop a substance use disorder later on, he said.
The “gold standard” treatment for ADHD is a prescription stimulant, which carries its own addiction risks. “So the issue is about whether or not to prescribe risky medications and how to weigh the risks and benefits,” said Dr. Park.
From a private health insurance database, researchers examined records for patients aged 18-64 years who were receiving medication for OUD, including buprenorphine, methadone, or naltrexone, from 2007-2017.
In the study sample, about 17,000 individuals were receiving stimulants, and 156,000 were not receiving these drugs. The largest percentage of participants in both groups was in the age-18-to-25 category.
About 35% of those receiving stimulants had ADHD, and about the same percentage had a mood disorder diagnosis.
Percentage of co-occurring ADHD and OUD increased from more than 4% in 2007 to more than 14% in 2017. The prevalence of stimulant use plus medication for OUD also increased during that time.
The increase in ADHD diagnoses may reflect growing identification of the condition, Dr. Park noted. As the opioid problem became more apparent and additional treatments made available, “there were more health care contacts, more assessments, and more diagnoses, including of ADHD,” he said.
Risks versus benefits
Stimulants may also be risky in patients with OUD. Results from another study presented at the AAAP meeting showed these drugs were associated with an increased chance of poisoning in patients receiving buprenorphine.
However, Dr. Park is skeptical the combination of stimulants and buprenorphine “leads to a biological risk of overdose.” He used a hypothetical scenario where other factors play into the connection: A patient gets a prescription stimulant, becomes addicted, then starts using street or illicit stimulants, which leads to a relapse on opioids, and then to an overdose.
Dr. Park noted that the same study that found an increased poisoning risk in stimulant users also found that patients tend to stay on buprenorphine treatment, providing protection against overdose.
“So there are risks and benefits of prescribing these medications, and it becomes tricky to know whether to prescribe them or not,” he said.
While stimulants are by far the best treatment for ADHD, atomoxetine (Strattera), a nonstimulant medication with antidepressant effects is another option, Dr. Park said.
He added that a limitation of his study was that very few individuals in the database received methadone.
A version of this article first appeared on Medscape.com.
FROM AAAP 2021
Average-risk women with dense breasts—What breast screening is appropriate?
Text copyright DenseBreast-info.org.
Answer
A. For women with extremely dense breasts who are not otherwise at increased risk for breast cancer, screening magnetic resonance imaging (MRI) is preferred, plus her mammogram or tomosynthesis. If MRI is not an option, consider ultrasonography or contrast-enhanced mammography.
The same screening considerations apply to women with heterogeneously dense breasts; however, there is limited capacity for MRI or even ultrasound screening at many facilities. Research supports MRI in dense breasts, and abbreviated, lower-cost protocols have been validated that address some of the barriers to MRI.1 Although not yet widely available, abbreviated MRI will likely have a greater role in screening women with dense breasts who are not high risk. It is important to note that preauthorization from insurance may be required for screening MRI, and in most US states, deductibles and copays apply.
The exam
Contrast-enhanced MRI requires IV injection of gadolinium-based contrast to look at the anatomy and blood flow patterns of the breast tissue. The patient lies face down with the breasts placed in two rectangular openings, or “coils.” The exam takes place inside the tunnel of the scanner, with the head facing out.After initial images are obtained, the contrast agent is injected into a vein in the arm, and additional images are taken, which will show areas of enhancement. The exam takes about 20 to 40 minutes. An “abbreviated” MRI can be performed for screening in some centers, which uses fewer sequences and takes about 10 minutes.
Benefits
At least 40% of cancers are missed on mammography in women with dense breasts.2 MRI is the most widely studied technique using a contrast agent, and it produces the highest additional cancer detection of all the supplemental technologies to date, yielding, in the first year, 10-16 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Berg et al.3). The cancer-detection benefit is seen across all breast density categories, even among average-risk women.4 There is no ionizing radiation, and it has been shown to reduce the rate of interval cancers (those detected due to symptoms after a negative screening mammogram), as well as the rate of late-stage disease. Axillary lymph nodes can be examined at the same screening exam.
While tomosynthesis improves cancer detection in women with fatty breasts, scattered fibroglandular breast tissue, and heterogeneously dense breasts, it does not significantly improve cancer detection in women with extremely dense breasts.5,6 Current American Cancer Society and National Comprehensive Cancer Network guidelines recommend annual screening MRI for women at high risk for breast cancer (regardless of breast density); however, increasingly, research supports the effectiveness of MRI in women with dense breasts who are otherwise considered average risk. A large randomized controlled trial in the Netherlands compared outcomes in women with extremely dense breasts invited to have screening MRI after negative mammography to those assigned to continue receiving screening mammography only. The incremental cancer detection rate was 16.5 per 1,000 (79/4,783) women screened with MRI in the first round7 and 6 per 1,000 women screened in the second round 2 years later.8 The interval cancer rate was 0.8 per 1,000 (4/4,783) women screened with MRI, compared with 4.9 per 1,000 (16/3,278) women who declined MRI and received mammography only.7
Screening ultrasound will show up to 3 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Vourtsis and Berg9 and Berg and Vourtsis10), far lower than the added cancer-detection rate of MRI. Consider screening ultrasound for women who cannot tolerate or access screening MRI.11 Contrast-enhanced mammography (CEM) uses iodinated contrast (as in computed tomography). CEM is not widely available but appears to show cancer-detection similar to MRI. For further discussion, see Berg et al’s 2021 review.3
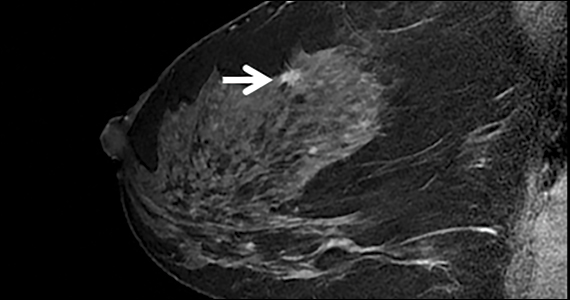
The FIGURE shows an example of an invasive cancer depicted on contrast-enhanced MRI in a 53-year-old woman with dense breasts and a family history of breast cancer that was not visible on tomosynthesis, even in retrospect, due to masking by dense tissue.

Considerations
Breast MRI increases callbacks even after mammography and ultrasound; however, such false alarms are reduced in subsequent screening rounds. MRI cannot be performed in women who have certain metal implants— some pacemakers or spinal fixation rods—and is not recommended for pregnant women. Claustrophobia may be an issue for some women. MRI is expensive and requires IV contrast. Gadolinium is known to accumulate in the brain, although the long-term effects of this are unknown and no harm has been shown.●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323:746-756. doi: 10.1001 /jama.2020.0572
- Kerlikowske K, Zhu W, Tosteson AN, et al. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med. 2015;162:673-681. doi: 10.7326/M14-1465.
- Berg WA, Rafferty EA, Friedewald SM, Hruska CB, Rahbar H. Screening Algorithms in Dense Breasts: AJR Expert Panel Narrative Review. AJR Am J Roentgenol. 2021;216:275-294. doi: 10.2214/AJR.20.24436.
- Kuhl CK, Strobel K, Bieling H, et al. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283:361-370. doi: 10.1148/radiol.2016161444.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786. doi: 10.1001/jama.2016.1708.
- Osteras BH, Martinsen ACT, Gullien R, et al. Digital mammography versus breast tomosynthesis: impact of breast density on diagnostic performance in population-based screening. Radiology. 2019;293:60-68. doi: 10.1148 /radiol.2019190425.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102. doi: 10.1056/NEJMoa1903986.
- Veenhuizen SGA, de Lange SV, Bakker MF, et al. Supplemental breast MRI for women with extremely dense breasts: results of the second screening round of the DENSE trial. Radiology. 2021;299:278-286. doi: 10.1148/radiol.2021203633.
- Vourtsis A, Berg WA. Breast density implications and supplemental screening. Eur Radiol. 2019;29:1762-1777. doi: 10.1007/s00330-018-5668-8.
- Berg WA, Vourtsis A. Screening ultrasound using handheld or automated technique in women with dense breasts. J Breast Imaging. 2019;1:283-296.
- National Comprehensive Cancer Network. Breast Cancer Screening and Diagnosis (Version 1.2021). https://www.nccn. org/professionals/physician_gls/pdf/breast-screening.pdf. Accessed November 18, 2021.
Text copyright DenseBreast-info.org.
Answer
A. For women with extremely dense breasts who are not otherwise at increased risk for breast cancer, screening magnetic resonance imaging (MRI) is preferred, plus her mammogram or tomosynthesis. If MRI is not an option, consider ultrasonography or contrast-enhanced mammography.
The same screening considerations apply to women with heterogeneously dense breasts; however, there is limited capacity for MRI or even ultrasound screening at many facilities. Research supports MRI in dense breasts, and abbreviated, lower-cost protocols have been validated that address some of the barriers to MRI.1 Although not yet widely available, abbreviated MRI will likely have a greater role in screening women with dense breasts who are not high risk. It is important to note that preauthorization from insurance may be required for screening MRI, and in most US states, deductibles and copays apply.
The exam
Contrast-enhanced MRI requires IV injection of gadolinium-based contrast to look at the anatomy and blood flow patterns of the breast tissue. The patient lies face down with the breasts placed in two rectangular openings, or “coils.” The exam takes place inside the tunnel of the scanner, with the head facing out.After initial images are obtained, the contrast agent is injected into a vein in the arm, and additional images are taken, which will show areas of enhancement. The exam takes about 20 to 40 minutes. An “abbreviated” MRI can be performed for screening in some centers, which uses fewer sequences and takes about 10 minutes.
Benefits
At least 40% of cancers are missed on mammography in women with dense breasts.2 MRI is the most widely studied technique using a contrast agent, and it produces the highest additional cancer detection of all the supplemental technologies to date, yielding, in the first year, 10-16 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Berg et al.3). The cancer-detection benefit is seen across all breast density categories, even among average-risk women.4 There is no ionizing radiation, and it has been shown to reduce the rate of interval cancers (those detected due to symptoms after a negative screening mammogram), as well as the rate of late-stage disease. Axillary lymph nodes can be examined at the same screening exam.
While tomosynthesis improves cancer detection in women with fatty breasts, scattered fibroglandular breast tissue, and heterogeneously dense breasts, it does not significantly improve cancer detection in women with extremely dense breasts.5,6 Current American Cancer Society and National Comprehensive Cancer Network guidelines recommend annual screening MRI for women at high risk for breast cancer (regardless of breast density); however, increasingly, research supports the effectiveness of MRI in women with dense breasts who are otherwise considered average risk. A large randomized controlled trial in the Netherlands compared outcomes in women with extremely dense breasts invited to have screening MRI after negative mammography to those assigned to continue receiving screening mammography only. The incremental cancer detection rate was 16.5 per 1,000 (79/4,783) women screened with MRI in the first round7 and 6 per 1,000 women screened in the second round 2 years later.8 The interval cancer rate was 0.8 per 1,000 (4/4,783) women screened with MRI, compared with 4.9 per 1,000 (16/3,278) women who declined MRI and received mammography only.7
Screening ultrasound will show up to 3 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Vourtsis and Berg9 and Berg and Vourtsis10), far lower than the added cancer-detection rate of MRI. Consider screening ultrasound for women who cannot tolerate or access screening MRI.11 Contrast-enhanced mammography (CEM) uses iodinated contrast (as in computed tomography). CEM is not widely available but appears to show cancer-detection similar to MRI. For further discussion, see Berg et al’s 2021 review.3
The FIGURE shows an example of an invasive cancer depicted on contrast-enhanced MRI in a 53-year-old woman with dense breasts and a family history of breast cancer that was not visible on tomosynthesis, even in retrospect, due to masking by dense tissue.

Considerations
Breast MRI increases callbacks even after mammography and ultrasound; however, such false alarms are reduced in subsequent screening rounds. MRI cannot be performed in women who have certain metal implants— some pacemakers or spinal fixation rods—and is not recommended for pregnant women. Claustrophobia may be an issue for some women. MRI is expensive and requires IV contrast. Gadolinium is known to accumulate in the brain, although the long-term effects of this are unknown and no harm has been shown.●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
Text copyright DenseBreast-info.org.
Answer
A. For women with extremely dense breasts who are not otherwise at increased risk for breast cancer, screening magnetic resonance imaging (MRI) is preferred, plus her mammogram or tomosynthesis. If MRI is not an option, consider ultrasonography or contrast-enhanced mammography.
The same screening considerations apply to women with heterogeneously dense breasts; however, there is limited capacity for MRI or even ultrasound screening at many facilities. Research supports MRI in dense breasts, and abbreviated, lower-cost protocols have been validated that address some of the barriers to MRI.1 Although not yet widely available, abbreviated MRI will likely have a greater role in screening women with dense breasts who are not high risk. It is important to note that preauthorization from insurance may be required for screening MRI, and in most US states, deductibles and copays apply.
The exam
Contrast-enhanced MRI requires IV injection of gadolinium-based contrast to look at the anatomy and blood flow patterns of the breast tissue. The patient lies face down with the breasts placed in two rectangular openings, or “coils.” The exam takes place inside the tunnel of the scanner, with the head facing out.After initial images are obtained, the contrast agent is injected into a vein in the arm, and additional images are taken, which will show areas of enhancement. The exam takes about 20 to 40 minutes. An “abbreviated” MRI can be performed for screening in some centers, which uses fewer sequences and takes about 10 minutes.
Benefits
At least 40% of cancers are missed on mammography in women with dense breasts.2 MRI is the most widely studied technique using a contrast agent, and it produces the highest additional cancer detection of all the supplemental technologies to date, yielding, in the first year, 10-16 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Berg et al.3). The cancer-detection benefit is seen across all breast density categories, even among average-risk women.4 There is no ionizing radiation, and it has been shown to reduce the rate of interval cancers (those detected due to symptoms after a negative screening mammogram), as well as the rate of late-stage disease. Axillary lymph nodes can be examined at the same screening exam.
While tomosynthesis improves cancer detection in women with fatty breasts, scattered fibroglandular breast tissue, and heterogeneously dense breasts, it does not significantly improve cancer detection in women with extremely dense breasts.5,6 Current American Cancer Society and National Comprehensive Cancer Network guidelines recommend annual screening MRI for women at high risk for breast cancer (regardless of breast density); however, increasingly, research supports the effectiveness of MRI in women with dense breasts who are otherwise considered average risk. A large randomized controlled trial in the Netherlands compared outcomes in women with extremely dense breasts invited to have screening MRI after negative mammography to those assigned to continue receiving screening mammography only. The incremental cancer detection rate was 16.5 per 1,000 (79/4,783) women screened with MRI in the first round7 and 6 per 1,000 women screened in the second round 2 years later.8 The interval cancer rate was 0.8 per 1,000 (4/4,783) women screened with MRI, compared with 4.9 per 1,000 (16/3,278) women who declined MRI and received mammography only.7
Screening ultrasound will show up to 3 additional cancers per 1,000 women screened after mammography/tomosynthesis (reviewed in Vourtsis and Berg9 and Berg and Vourtsis10), far lower than the added cancer-detection rate of MRI. Consider screening ultrasound for women who cannot tolerate or access screening MRI.11 Contrast-enhanced mammography (CEM) uses iodinated contrast (as in computed tomography). CEM is not widely available but appears to show cancer-detection similar to MRI. For further discussion, see Berg et al’s 2021 review.3
The FIGURE shows an example of an invasive cancer depicted on contrast-enhanced MRI in a 53-year-old woman with dense breasts and a family history of breast cancer that was not visible on tomosynthesis, even in retrospect, due to masking by dense tissue.

Considerations
Breast MRI increases callbacks even after mammography and ultrasound; however, such false alarms are reduced in subsequent screening rounds. MRI cannot be performed in women who have certain metal implants— some pacemakers or spinal fixation rods—and is not recommended for pregnant women. Claustrophobia may be an issue for some women. MRI is expensive and requires IV contrast. Gadolinium is known to accumulate in the brain, although the long-term effects of this are unknown and no harm has been shown.●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323:746-756. doi: 10.1001 /jama.2020.0572
- Kerlikowske K, Zhu W, Tosteson AN, et al. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med. 2015;162:673-681. doi: 10.7326/M14-1465.
- Berg WA, Rafferty EA, Friedewald SM, Hruska CB, Rahbar H. Screening Algorithms in Dense Breasts: AJR Expert Panel Narrative Review. AJR Am J Roentgenol. 2021;216:275-294. doi: 10.2214/AJR.20.24436.
- Kuhl CK, Strobel K, Bieling H, et al. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283:361-370. doi: 10.1148/radiol.2016161444.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786. doi: 10.1001/jama.2016.1708.
- Osteras BH, Martinsen ACT, Gullien R, et al. Digital mammography versus breast tomosynthesis: impact of breast density on diagnostic performance in population-based screening. Radiology. 2019;293:60-68. doi: 10.1148 /radiol.2019190425.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102. doi: 10.1056/NEJMoa1903986.
- Veenhuizen SGA, de Lange SV, Bakker MF, et al. Supplemental breast MRI for women with extremely dense breasts: results of the second screening round of the DENSE trial. Radiology. 2021;299:278-286. doi: 10.1148/radiol.2021203633.
- Vourtsis A, Berg WA. Breast density implications and supplemental screening. Eur Radiol. 2019;29:1762-1777. doi: 10.1007/s00330-018-5668-8.
- Berg WA, Vourtsis A. Screening ultrasound using handheld or automated technique in women with dense breasts. J Breast Imaging. 2019;1:283-296.
- National Comprehensive Cancer Network. Breast Cancer Screening and Diagnosis (Version 1.2021). https://www.nccn. org/professionals/physician_gls/pdf/breast-screening.pdf. Accessed November 18, 2021.
- Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening. JAMA. 2020;323:746-756. doi: 10.1001 /jama.2020.0572
- Kerlikowske K, Zhu W, Tosteson AN, et al. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med. 2015;162:673-681. doi: 10.7326/M14-1465.
- Berg WA, Rafferty EA, Friedewald SM, Hruska CB, Rahbar H. Screening Algorithms in Dense Breasts: AJR Expert Panel Narrative Review. AJR Am J Roentgenol. 2021;216:275-294. doi: 10.2214/AJR.20.24436.
- Kuhl CK, Strobel K, Bieling H, et al. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283:361-370. doi: 10.1148/radiol.2016161444.
- Rafferty EA, Durand MA, Conant EF, et al. Breast cancer screening using tomosynthesis and digital mammography in dense and nondense breasts. JAMA. 2016;315:1784-1786. doi: 10.1001/jama.2016.1708.
- Osteras BH, Martinsen ACT, Gullien R, et al. Digital mammography versus breast tomosynthesis: impact of breast density on diagnostic performance in population-based screening. Radiology. 2019;293:60-68. doi: 10.1148 /radiol.2019190425.
- Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381:2091-2102. doi: 10.1056/NEJMoa1903986.
- Veenhuizen SGA, de Lange SV, Bakker MF, et al. Supplemental breast MRI for women with extremely dense breasts: results of the second screening round of the DENSE trial. Radiology. 2021;299:278-286. doi: 10.1148/radiol.2021203633.
- Vourtsis A, Berg WA. Breast density implications and supplemental screening. Eur Radiol. 2019;29:1762-1777. doi: 10.1007/s00330-018-5668-8.
- Berg WA, Vourtsis A. Screening ultrasound using handheld or automated technique in women with dense breasts. J Breast Imaging. 2019;1:283-296.
- National Comprehensive Cancer Network. Breast Cancer Screening and Diagnosis (Version 1.2021). https://www.nccn. org/professionals/physician_gls/pdf/breast-screening.pdf. Accessed November 18, 2021.
Quiz developed in collaboration with 
Outrage over dapagliflozin withdrawal for type 1 diabetes in EU
In a shocking, yet low-key, announcement, the sodium-glucose transporter 2 (SGLT2) inhibitor dapagliflozin (Forxiga, AstraZeneca) has been withdrawn from the market in all EU countries for the indication of type 1 diabetes.
This includes withdrawal in the U.K., which was part of the EU when dapagliflozin was approved for type 1 diabetes in 2019, but following Brexit, is no longer.
AstraZeneca said the decision is not motivated by safety concerns but points nevertheless to an increased risk of diabetic ketoacidosis (DKA) associated with SGLT2 inhibitors in those with type 1 diabetes, which it said might cause “confusion” among physicians using the drug to treat numerous other indications for which this agent is now approved.
DKA is a potentially dangerous side effect resulting from acid build-up in the blood and is normally accompanied by very high glucose levels. DKA is flagged as a potential side effect in type 2 diabetes but is more common in those with type 1 diabetes. It can also occur as “euglycemic” DKA, which is ketosis but with relatively normal glucose levels (and therefore harder for patients to detect). Euglycemic DKA is thought to be more of a risk in those with type 1 diabetes than in those with type 2 diabetes.
One charity believes concerns around safety are the underlying factor for the withdrawal of dapagliflozin for type 1 diabetes in Europe, suggesting that AstraZeneca might not want to risk income from more lucrative indications – such as type 2 diabetes with much larger patient populations – because of potential concerns from doctors, who may be deterred from prescribing the drug due to concerns about DKA.
JDRF International, a leading global type 1 diabetes charity, called on AstraZeneca in a statement “to explain to people affected by type 1 diabetes why the drug has been withdrawn.”
It added that dapagliflozin is the “only other drug besides insulin” to be licensed in Europe for the treatment of type 1 diabetes and represents a “major advancement since the discovery of insulin 100 years ago.”
Karen Addington, U.K. Chief Executive of JDRF, said it is “appalling” that the drug has been withdrawn, as “many people with type 1 are finding it an effective and useful tool to help manage their glucose levels.”
SGLT2 inhibitors never approved for type 1 diabetes in U.S.
Dapagliflozin and other drugs from the SGLT2 inhibitor class had already been approved for the treatment of type 2 diabetes for a number of years when dapagliflozin was approved in early 2019 for the treatment of adults with type 1 diabetes meeting certain criteria by the European Medicines Agency (EMA), which at that time included the U.K. in its remit, based on data from the DEPICT series of phase 3 trials.
SGLT2 inhibitors have also recently shown benefit in other indications, such as heart failure and chronic kidney disease – even in the absence of diabetes – leaving some to label them a new class of wonder drugs.
Following the 2019 EU approval for type 1 diabetes, dapagliflozin was subsequently recommended for this use on the National Health Service (NHS) in England and Wales and was accompanied by guidance from the National Institute for Health and Care Excellence (NICE), which has now had to be withdrawn.
Of note, dapagliflozin was never approved for use in type 1 diabetes in the United States (where it is known as Farxiga), with the U.S. Food and Drug Administration turning it down in July 2019.
An advisory panel for the FDA also later turned down another SGLT2 inhibitor for type 1 diabetes, empagliflozin (Jardiance, Boehringer Ingelheim) in Nov. 2019, as reported by this news organization.
Discontinuation ‘not due to safety concerns,’ says AZ
The announcement to discontinue dapagliflozin for the indication of type 1 diabetes in certain adults just two and a half years after its approval in the EU comes as a big surprise, especially as it was made with little fanfare just last month.
In the U.K., AstraZeneca sent a letter to health care professionals on Nov. 2 stating that, from Oct. 25, dapagliflozin 5 mg was “no longer authorized” for the treatment of type 1 diabetes and “should no longer be used” in this patient population.
However, it underlined that other indications for dapagliflozin 5 mg and 10 mg were “not affected by this licensing change,” and it remains available for adults with type 2 diabetes, as well as for the management of symptomatic chronic heart feature with reduced ejection fraction (HFrEF) and chronic kidney disease (CKD).
In the letter, sent by Tom Keith-Roach, country president of AstraZeneca UK, the company asserts that the removal of the type 1 diabetes indication from dapagliflozin is “not due to any safety concern” with the drug “in any indication, including type 1 diabetes.”
It nevertheless goes on to highlight that DKA is a known common side effect of dapagliflozin in type 1 diabetes and, following the announcement, “additional risk minimization measures ... will no longer be available.”
In a separate statement, AstraZeneca said that the decision to remove the indication was made “voluntarily” and had been “agreed” with the Medicines and Healthcare products Regulatory Agency (MHRA) in Great Britain and the equivalent body in Northern Ireland.
“It follows discussions regarding product information changes needed post-approval for dapagliflozin 5 mg specific to type 1 diabetes,” the company said, “which might cause confusion” among physicians treating patients with type 2 diabetes, chronic heart feature with reduced ejection fraction, or CKD.
AstraZeneca told this news organization that similar communications about the withdrawal were issued to health care agencies and health care professionals in all countries of the EU.
‘Appalling, devastating, disappointing’ for patients
The announcement has been met with disappointment in some quarters and outrage in others, and questions have been raised as to the explanation given by AstraZeneca for the drug’s withdrawal.
“Although only a small number of people with type 1 diabetes have been using dapagliflozin, we know that those who have been using it will have been benefitting from tighter control of their condition,” Simon O’Neill, director of health intelligence and professional liaison at Diabetes UK, told this news organization.
“It’s disappointing that these people will now need to go back to the drawing board and will have to work with their clinical team to find other ways of better managing their condition.”
Mr. O’Neill said it was “disappointing that AstraZeneca and the MHRA were unable to find a workable solution to allow people living with type 1 diabetes to continue using the drug safely without leading to confusion for clinicians or people living with type 2 diabetes, who also use it.”
Sanjoy Dutta, JDRF International vice president of research, added that the news is “devastating.”
“The impending negative impact of removing a drug like dapagliflozin from any market can be detrimental in the potential for other national medical ruling boards to have confidence in approving it for their citizens,” he added.
“We stand with our type 1 diabetes communities across the globe in demanding an explanation to clarify this removal.”
Why not an educational campaign about DKA risk?
In an interview, Hilary Nathan, policy & communications director at JDRF International, explained that the charity has its theories as to why dapagliflozin has been withdrawn for type 1 diabetes.
What AstraZeneca is saying, “and what we don’t agree with them on,” is that the “black triangle” warning that has to be put onto the drug due to the increased risk of DKA in type 1 diabetes is “misunderstood by health care practitioners” outside of that specialty and that “by having that black triangle, it will inhibit take-up in those other markets.”
In other words, “there will be less desire to prescribe it,” ventured Ms. Nathan.
She continued: “For us, we feel that if a medicine is deemed safe and efficacious, it should not be withdrawn because of other patient constituencies.”
“We asked: ‘Why can’t you do an educational awareness campaign about the black triangle?’ And the might of AstraZeneca said it would be too big a task.”
Ms. Nathan was also surprised at how the drug could be withdrawn without any warning or real explanation.
“How is it possible that, when a drug is approved there are multiple stakeholders that are involved in putting forward views and experiences – both from the clinical and patient advocacy communities, as well as obviously the pharmaceutical community – yet [a drug] can be withdrawn by a ... company that may well have conflicts of interest around commercial take-up.”
She added: “I feel that there are potentially motives around the withdrawal that AstraZeneca are still not being clear about.”
Perhaps a further clue as to the real motives behind the withdrawal can be found in an announcement, just last week, by the British MHRA.
“The decision by the marketing authorization holder to voluntarily withdraw the indication in type 1 diabetes followed commercial considerations due to a specific European-wide regulatory requirement for this authorization,” it said.
“The decision was not driven by any new safety concerns, such as the already known increased risk of DKA in type 1 diabetes compared with type 2 diabetes.”
Separately, a new in-depth investigation into when Johnson & Johnson, which markets another SGLT2 inhibitor, canagliflozin (Invokana), first knew that its agent was associated with DKA has revealed multiple discrepancies in staff accounts. Some claim the company knew as early as 2010 that canagliflozin – first approved for type 2 diabetes in the United States in 2013 – could increase the risk of DKA. It was not until May 2015 that the FDA first issued a warning about the potential risk of DKA associated with use of SGLT2 inhibitors, with the EMA following suit a month later. In Dec. 2015, the FDA updated the labels for all SGLT2 inhibitors approved in the United States at that time – canagliflozin, empagliflozin, and dapagliflozin – to include the risks for ketoacidosis (and urinary tract infections).
Forxiga (dapagliflozin) is manufactured by AstraZeneca. No relevant financial relationships declared.
A version of this article first appeared on Medscape.com.
In a shocking, yet low-key, announcement, the sodium-glucose transporter 2 (SGLT2) inhibitor dapagliflozin (Forxiga, AstraZeneca) has been withdrawn from the market in all EU countries for the indication of type 1 diabetes.
This includes withdrawal in the U.K., which was part of the EU when dapagliflozin was approved for type 1 diabetes in 2019, but following Brexit, is no longer.
AstraZeneca said the decision is not motivated by safety concerns but points nevertheless to an increased risk of diabetic ketoacidosis (DKA) associated with SGLT2 inhibitors in those with type 1 diabetes, which it said might cause “confusion” among physicians using the drug to treat numerous other indications for which this agent is now approved.
DKA is a potentially dangerous side effect resulting from acid build-up in the blood and is normally accompanied by very high glucose levels. DKA is flagged as a potential side effect in type 2 diabetes but is more common in those with type 1 diabetes. It can also occur as “euglycemic” DKA, which is ketosis but with relatively normal glucose levels (and therefore harder for patients to detect). Euglycemic DKA is thought to be more of a risk in those with type 1 diabetes than in those with type 2 diabetes.
One charity believes concerns around safety are the underlying factor for the withdrawal of dapagliflozin for type 1 diabetes in Europe, suggesting that AstraZeneca might not want to risk income from more lucrative indications – such as type 2 diabetes with much larger patient populations – because of potential concerns from doctors, who may be deterred from prescribing the drug due to concerns about DKA.
JDRF International, a leading global type 1 diabetes charity, called on AstraZeneca in a statement “to explain to people affected by type 1 diabetes why the drug has been withdrawn.”
It added that dapagliflozin is the “only other drug besides insulin” to be licensed in Europe for the treatment of type 1 diabetes and represents a “major advancement since the discovery of insulin 100 years ago.”
Karen Addington, U.K. Chief Executive of JDRF, said it is “appalling” that the drug has been withdrawn, as “many people with type 1 are finding it an effective and useful tool to help manage their glucose levels.”
SGLT2 inhibitors never approved for type 1 diabetes in U.S.
Dapagliflozin and other drugs from the SGLT2 inhibitor class had already been approved for the treatment of type 2 diabetes for a number of years when dapagliflozin was approved in early 2019 for the treatment of adults with type 1 diabetes meeting certain criteria by the European Medicines Agency (EMA), which at that time included the U.K. in its remit, based on data from the DEPICT series of phase 3 trials.
SGLT2 inhibitors have also recently shown benefit in other indications, such as heart failure and chronic kidney disease – even in the absence of diabetes – leaving some to label them a new class of wonder drugs.
Following the 2019 EU approval for type 1 diabetes, dapagliflozin was subsequently recommended for this use on the National Health Service (NHS) in England and Wales and was accompanied by guidance from the National Institute for Health and Care Excellence (NICE), which has now had to be withdrawn.
Of note, dapagliflozin was never approved for use in type 1 diabetes in the United States (where it is known as Farxiga), with the U.S. Food and Drug Administration turning it down in July 2019.
An advisory panel for the FDA also later turned down another SGLT2 inhibitor for type 1 diabetes, empagliflozin (Jardiance, Boehringer Ingelheim) in Nov. 2019, as reported by this news organization.
Discontinuation ‘not due to safety concerns,’ says AZ
The announcement to discontinue dapagliflozin for the indication of type 1 diabetes in certain adults just two and a half years after its approval in the EU comes as a big surprise, especially as it was made with little fanfare just last month.
In the U.K., AstraZeneca sent a letter to health care professionals on Nov. 2 stating that, from Oct. 25, dapagliflozin 5 mg was “no longer authorized” for the treatment of type 1 diabetes and “should no longer be used” in this patient population.
However, it underlined that other indications for dapagliflozin 5 mg and 10 mg were “not affected by this licensing change,” and it remains available for adults with type 2 diabetes, as well as for the management of symptomatic chronic heart feature with reduced ejection fraction (HFrEF) and chronic kidney disease (CKD).
In the letter, sent by Tom Keith-Roach, country president of AstraZeneca UK, the company asserts that the removal of the type 1 diabetes indication from dapagliflozin is “not due to any safety concern” with the drug “in any indication, including type 1 diabetes.”
It nevertheless goes on to highlight that DKA is a known common side effect of dapagliflozin in type 1 diabetes and, following the announcement, “additional risk minimization measures ... will no longer be available.”
In a separate statement, AstraZeneca said that the decision to remove the indication was made “voluntarily” and had been “agreed” with the Medicines and Healthcare products Regulatory Agency (MHRA) in Great Britain and the equivalent body in Northern Ireland.
“It follows discussions regarding product information changes needed post-approval for dapagliflozin 5 mg specific to type 1 diabetes,” the company said, “which might cause confusion” among physicians treating patients with type 2 diabetes, chronic heart feature with reduced ejection fraction, or CKD.
AstraZeneca told this news organization that similar communications about the withdrawal were issued to health care agencies and health care professionals in all countries of the EU.
‘Appalling, devastating, disappointing’ for patients
The announcement has been met with disappointment in some quarters and outrage in others, and questions have been raised as to the explanation given by AstraZeneca for the drug’s withdrawal.
“Although only a small number of people with type 1 diabetes have been using dapagliflozin, we know that those who have been using it will have been benefitting from tighter control of their condition,” Simon O’Neill, director of health intelligence and professional liaison at Diabetes UK, told this news organization.
“It’s disappointing that these people will now need to go back to the drawing board and will have to work with their clinical team to find other ways of better managing their condition.”
Mr. O’Neill said it was “disappointing that AstraZeneca and the MHRA were unable to find a workable solution to allow people living with type 1 diabetes to continue using the drug safely without leading to confusion for clinicians or people living with type 2 diabetes, who also use it.”
Sanjoy Dutta, JDRF International vice president of research, added that the news is “devastating.”
“The impending negative impact of removing a drug like dapagliflozin from any market can be detrimental in the potential for other national medical ruling boards to have confidence in approving it for their citizens,” he added.
“We stand with our type 1 diabetes communities across the globe in demanding an explanation to clarify this removal.”
Why not an educational campaign about DKA risk?
In an interview, Hilary Nathan, policy & communications director at JDRF International, explained that the charity has its theories as to why dapagliflozin has been withdrawn for type 1 diabetes.
What AstraZeneca is saying, “and what we don’t agree with them on,” is that the “black triangle” warning that has to be put onto the drug due to the increased risk of DKA in type 1 diabetes is “misunderstood by health care practitioners” outside of that specialty and that “by having that black triangle, it will inhibit take-up in those other markets.”
In other words, “there will be less desire to prescribe it,” ventured Ms. Nathan.
She continued: “For us, we feel that if a medicine is deemed safe and efficacious, it should not be withdrawn because of other patient constituencies.”
“We asked: ‘Why can’t you do an educational awareness campaign about the black triangle?’ And the might of AstraZeneca said it would be too big a task.”
Ms. Nathan was also surprised at how the drug could be withdrawn without any warning or real explanation.
“How is it possible that, when a drug is approved there are multiple stakeholders that are involved in putting forward views and experiences – both from the clinical and patient advocacy communities, as well as obviously the pharmaceutical community – yet [a drug] can be withdrawn by a ... company that may well have conflicts of interest around commercial take-up.”
She added: “I feel that there are potentially motives around the withdrawal that AstraZeneca are still not being clear about.”
Perhaps a further clue as to the real motives behind the withdrawal can be found in an announcement, just last week, by the British MHRA.
“The decision by the marketing authorization holder to voluntarily withdraw the indication in type 1 diabetes followed commercial considerations due to a specific European-wide regulatory requirement for this authorization,” it said.
“The decision was not driven by any new safety concerns, such as the already known increased risk of DKA in type 1 diabetes compared with type 2 diabetes.”
Separately, a new in-depth investigation into when Johnson & Johnson, which markets another SGLT2 inhibitor, canagliflozin (Invokana), first knew that its agent was associated with DKA has revealed multiple discrepancies in staff accounts. Some claim the company knew as early as 2010 that canagliflozin – first approved for type 2 diabetes in the United States in 2013 – could increase the risk of DKA. It was not until May 2015 that the FDA first issued a warning about the potential risk of DKA associated with use of SGLT2 inhibitors, with the EMA following suit a month later. In Dec. 2015, the FDA updated the labels for all SGLT2 inhibitors approved in the United States at that time – canagliflozin, empagliflozin, and dapagliflozin – to include the risks for ketoacidosis (and urinary tract infections).
Forxiga (dapagliflozin) is manufactured by AstraZeneca. No relevant financial relationships declared.
A version of this article first appeared on Medscape.com.
In a shocking, yet low-key, announcement, the sodium-glucose transporter 2 (SGLT2) inhibitor dapagliflozin (Forxiga, AstraZeneca) has been withdrawn from the market in all EU countries for the indication of type 1 diabetes.
This includes withdrawal in the U.K., which was part of the EU when dapagliflozin was approved for type 1 diabetes in 2019, but following Brexit, is no longer.
AstraZeneca said the decision is not motivated by safety concerns but points nevertheless to an increased risk of diabetic ketoacidosis (DKA) associated with SGLT2 inhibitors in those with type 1 diabetes, which it said might cause “confusion” among physicians using the drug to treat numerous other indications for which this agent is now approved.
DKA is a potentially dangerous side effect resulting from acid build-up in the blood and is normally accompanied by very high glucose levels. DKA is flagged as a potential side effect in type 2 diabetes but is more common in those with type 1 diabetes. It can also occur as “euglycemic” DKA, which is ketosis but with relatively normal glucose levels (and therefore harder for patients to detect). Euglycemic DKA is thought to be more of a risk in those with type 1 diabetes than in those with type 2 diabetes.
One charity believes concerns around safety are the underlying factor for the withdrawal of dapagliflozin for type 1 diabetes in Europe, suggesting that AstraZeneca might not want to risk income from more lucrative indications – such as type 2 diabetes with much larger patient populations – because of potential concerns from doctors, who may be deterred from prescribing the drug due to concerns about DKA.
JDRF International, a leading global type 1 diabetes charity, called on AstraZeneca in a statement “to explain to people affected by type 1 diabetes why the drug has been withdrawn.”
It added that dapagliflozin is the “only other drug besides insulin” to be licensed in Europe for the treatment of type 1 diabetes and represents a “major advancement since the discovery of insulin 100 years ago.”
Karen Addington, U.K. Chief Executive of JDRF, said it is “appalling” that the drug has been withdrawn, as “many people with type 1 are finding it an effective and useful tool to help manage their glucose levels.”
SGLT2 inhibitors never approved for type 1 diabetes in U.S.
Dapagliflozin and other drugs from the SGLT2 inhibitor class had already been approved for the treatment of type 2 diabetes for a number of years when dapagliflozin was approved in early 2019 for the treatment of adults with type 1 diabetes meeting certain criteria by the European Medicines Agency (EMA), which at that time included the U.K. in its remit, based on data from the DEPICT series of phase 3 trials.
SGLT2 inhibitors have also recently shown benefit in other indications, such as heart failure and chronic kidney disease – even in the absence of diabetes – leaving some to label them a new class of wonder drugs.
Following the 2019 EU approval for type 1 diabetes, dapagliflozin was subsequently recommended for this use on the National Health Service (NHS) in England and Wales and was accompanied by guidance from the National Institute for Health and Care Excellence (NICE), which has now had to be withdrawn.
Of note, dapagliflozin was never approved for use in type 1 diabetes in the United States (where it is known as Farxiga), with the U.S. Food and Drug Administration turning it down in July 2019.
An advisory panel for the FDA also later turned down another SGLT2 inhibitor for type 1 diabetes, empagliflozin (Jardiance, Boehringer Ingelheim) in Nov. 2019, as reported by this news organization.
Discontinuation ‘not due to safety concerns,’ says AZ
The announcement to discontinue dapagliflozin for the indication of type 1 diabetes in certain adults just two and a half years after its approval in the EU comes as a big surprise, especially as it was made with little fanfare just last month.
In the U.K., AstraZeneca sent a letter to health care professionals on Nov. 2 stating that, from Oct. 25, dapagliflozin 5 mg was “no longer authorized” for the treatment of type 1 diabetes and “should no longer be used” in this patient population.
However, it underlined that other indications for dapagliflozin 5 mg and 10 mg were “not affected by this licensing change,” and it remains available for adults with type 2 diabetes, as well as for the management of symptomatic chronic heart feature with reduced ejection fraction (HFrEF) and chronic kidney disease (CKD).
In the letter, sent by Tom Keith-Roach, country president of AstraZeneca UK, the company asserts that the removal of the type 1 diabetes indication from dapagliflozin is “not due to any safety concern” with the drug “in any indication, including type 1 diabetes.”
It nevertheless goes on to highlight that DKA is a known common side effect of dapagliflozin in type 1 diabetes and, following the announcement, “additional risk minimization measures ... will no longer be available.”
In a separate statement, AstraZeneca said that the decision to remove the indication was made “voluntarily” and had been “agreed” with the Medicines and Healthcare products Regulatory Agency (MHRA) in Great Britain and the equivalent body in Northern Ireland.
“It follows discussions regarding product information changes needed post-approval for dapagliflozin 5 mg specific to type 1 diabetes,” the company said, “which might cause confusion” among physicians treating patients with type 2 diabetes, chronic heart feature with reduced ejection fraction, or CKD.
AstraZeneca told this news organization that similar communications about the withdrawal were issued to health care agencies and health care professionals in all countries of the EU.
‘Appalling, devastating, disappointing’ for patients
The announcement has been met with disappointment in some quarters and outrage in others, and questions have been raised as to the explanation given by AstraZeneca for the drug’s withdrawal.
“Although only a small number of people with type 1 diabetes have been using dapagliflozin, we know that those who have been using it will have been benefitting from tighter control of their condition,” Simon O’Neill, director of health intelligence and professional liaison at Diabetes UK, told this news organization.
“It’s disappointing that these people will now need to go back to the drawing board and will have to work with their clinical team to find other ways of better managing their condition.”
Mr. O’Neill said it was “disappointing that AstraZeneca and the MHRA were unable to find a workable solution to allow people living with type 1 diabetes to continue using the drug safely without leading to confusion for clinicians or people living with type 2 diabetes, who also use it.”
Sanjoy Dutta, JDRF International vice president of research, added that the news is “devastating.”
“The impending negative impact of removing a drug like dapagliflozin from any market can be detrimental in the potential for other national medical ruling boards to have confidence in approving it for their citizens,” he added.
“We stand with our type 1 diabetes communities across the globe in demanding an explanation to clarify this removal.”
Why not an educational campaign about DKA risk?
In an interview, Hilary Nathan, policy & communications director at JDRF International, explained that the charity has its theories as to why dapagliflozin has been withdrawn for type 1 diabetes.
What AstraZeneca is saying, “and what we don’t agree with them on,” is that the “black triangle” warning that has to be put onto the drug due to the increased risk of DKA in type 1 diabetes is “misunderstood by health care practitioners” outside of that specialty and that “by having that black triangle, it will inhibit take-up in those other markets.”
In other words, “there will be less desire to prescribe it,” ventured Ms. Nathan.
She continued: “For us, we feel that if a medicine is deemed safe and efficacious, it should not be withdrawn because of other patient constituencies.”
“We asked: ‘Why can’t you do an educational awareness campaign about the black triangle?’ And the might of AstraZeneca said it would be too big a task.”
Ms. Nathan was also surprised at how the drug could be withdrawn without any warning or real explanation.
“How is it possible that, when a drug is approved there are multiple stakeholders that are involved in putting forward views and experiences – both from the clinical and patient advocacy communities, as well as obviously the pharmaceutical community – yet [a drug] can be withdrawn by a ... company that may well have conflicts of interest around commercial take-up.”
She added: “I feel that there are potentially motives around the withdrawal that AstraZeneca are still not being clear about.”
Perhaps a further clue as to the real motives behind the withdrawal can be found in an announcement, just last week, by the British MHRA.
“The decision by the marketing authorization holder to voluntarily withdraw the indication in type 1 diabetes followed commercial considerations due to a specific European-wide regulatory requirement for this authorization,” it said.
“The decision was not driven by any new safety concerns, such as the already known increased risk of DKA in type 1 diabetes compared with type 2 diabetes.”
Separately, a new in-depth investigation into when Johnson & Johnson, which markets another SGLT2 inhibitor, canagliflozin (Invokana), first knew that its agent was associated with DKA has revealed multiple discrepancies in staff accounts. Some claim the company knew as early as 2010 that canagliflozin – first approved for type 2 diabetes in the United States in 2013 – could increase the risk of DKA. It was not until May 2015 that the FDA first issued a warning about the potential risk of DKA associated with use of SGLT2 inhibitors, with the EMA following suit a month later. In Dec. 2015, the FDA updated the labels for all SGLT2 inhibitors approved in the United States at that time – canagliflozin, empagliflozin, and dapagliflozin – to include the risks for ketoacidosis (and urinary tract infections).
Forxiga (dapagliflozin) is manufactured by AstraZeneca. No relevant financial relationships declared.
A version of this article first appeared on Medscape.com.
Infectious disease pop quiz: Clinical challenges for the ObGyn
In this question-and-answer article (the second in a series), our objective is to reinforce for the clinician several practical points of management for common infectious diseases. The principal references for the answers to the questions are 2 textbook chapters written by Dr. Duff.1,2 Other pertinent references are included in the text.
9. For uncomplicated chlamydia infection in a pregnant woman, what is the most appropriate treatment?
Uncomplicated chlamydia infection in a pregnant woman should be treated with a single 1,000-mg oral dose of azithromycin. An acceptable alternative is amoxicillin
500 mg orally 3 times daily for 7 days.
In a nonpregnant patient, doxycycline 100 mg orally twice daily for 7 days is also an appropriate alternative. However, doxycycline is relatively expensive and may not be well tolerated because of gastrointestinal adverse effects. (Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64[RR3]:1-137.)
10. What are the characteristic mucocutaneous lesions of primary, secondary, and tertiary syphilis?
The characteristic mucosal lesion of primary syphilis is the painless chancre. The usual mucocutaneous manifestations of secondary syphilis are maculopapular lesions (red or violet in color) on the palms and soles, mucous patches on the oral membranes, and condyloma lata on the genitalia. The classic mucocutaneous lesion of tertiary syphilis is the gumma.
Other serious manifestations of advanced syphilis include central nervous system abnormalities, such as tabes dorsalis, the Argyll Robertson pupil, and dementia, and cardiac abnormalities, such as aortitis, which can lead to a dissecting aneurysm of the aortic root. (Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64[RR3]:1-137.)
11. In a pregnant woman with a history of recurrent herpes simplex virus infection, what is the best way to prevent an outbreak of lesions near term?
Obstetric patients with a history of recurrent herpes simplex infection should be treated with acyclovir 400 mg orally 3 times daily from 36 weeks until delivery. This
regimen significantly reduces the likelihood of a recurrent outbreak near the time of delivery, which if it occurred, would necessitate a cesarean delivery. In patients at increased risk for preterm delivery, the prophylactic regimen should be started earlier.
Valacyclovir, 500 mg orally twice daily, is an acceptable alternative but is significantly more expensive.
Continue to: 12. What are the best office-based tests for the diagnosis of bacterial vaginosis?...
12. What are the best office-based tests for the diagnosis of bacterial vaginosis?
In patients with bacterial vaginosis, the vaginal pH typically is elevated in the range of 4.5. When a drop of potassium hydroxide solution is added to the vaginal secretions, a characteristic fishlike (amine) odor is liberated (positive “whiff test”). With saline microscopy, the key findings are a relative absence of lactobacilli in the background, an abundance of small cocci and bacilli, and the presence of clue cells, which are epithelial cells studded with bacteria along their
outer margin.
13. For a moderately ill pregnant woman, what is the most appropriate antibiotic combination for inpatient treatment of community-acquired pneumonia?
This patient should be treated with intravenous ceftriaxone (2 g every 24 hours) plus oral or intravenous azithromycin. The appropriate oral dose of azithromycin is 500 mg on day 1, then 250 mg daily for 4 doses. The appropriate intravenous dose of azithromycin is 500 mg every 24 hours. The goal is to provide appropriate coverage for the most likely pathogens: Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and mycoplasmas. (Antibacterial drugs for community-acquired pneumonia. Med Lett Drugs Ther. 2021:63:10-14. Postma DF, van Werkoven CH, van Eldin LJ, et al; CAP-START Study Group. Antibiotic treatment strategies for community acquired pneumonia in adults. N Engl J Med. 2015;372: 1312-1323.)
14. What tests are best for the diagnosis of COVID-19 infection?
The 2 key diagnostic tests for COVID-19 infection are detecting antigen in nasopharyngeal washings or saliva by nucleic acid amplification tests and identifying groundglass opacities on computed tomography imaging of the chest. (Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383:2451-2460.)
15. What is the most appropriate treatment for a pregnant woman who is moderately to severely ill with COVID-19 infection?
Moderately to severely ill pregnant women with COVID-19 infection should be hospitalized and treated with supplementary oxygen, remdesivir, and dexamethasone. Other possible therapies include inhaled nitric oxide, baricitinib (a Janus kinase inhibitor), and tocilizumab (an anti-interleukin 6 receptor antibody). (RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693704. Kalil AC, Patterson TF, Mehta AK, et al; ACTT-2 Study Group. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795-807. Berlin DA, Gulick RM, Martinez FJ, et al. Severe COVID19. N Engl J Med. 2020;383;2451-2460.)
16. What is the best test for the diagnosis of acute hepatitis A infection?
The single best test for the diagnosis of acute hepatitis A infection is detection of immunoglobulin M (IgM)–specific antibody to the virus.
17. What are the best tests for identification of a patient with chronic hepatitis B infection?
Patients with chronic hepatitis B infection typically test positive for the hepatitis B surface antigen (HBsAg) and for IgG antibody to the hepatitis B core antigen (HBcAg). In addition, they also may test positive for the hepatitis B e antigen (HBeAg), and their viral load can be quantified by polymerase chain reaction (PCR) when significant antigenemia is present. The presence of the e antigen indicates a high rate of viral replication and a corresponding high rate of infectivity.
18. What antenatal treatment is indicated in a pregnant woman at 28 weeks’ gestation who has a hepatitis B viral load of 2 million copies/mL?
This patient has a markedly elevated viral load and is at significantly increased risk of transmitting hepatitis B infection to her neonate even if the infant receives hepatitis B immune globulin immediately after birth and quickly begins the hepatitis B vaccine series. Daily antenatal treatment with tenofovir (300 mg daily) from 28 weeks until delivery will significantly reduce the risk of perinatal transmission.
19. Should a postpartum patient with chronic hepatitis C infection be discouraged from breastfeeding her infant?
Hepatitis C is not a contraindication to breastfeeding. Although the virus has been identified in breast milk, the risk of transmission to the infant is exceedingly low.
20. What are the principal microorganisms that cause puerperal mastitis?
Staphylococci and Streptococcus viridans are the 2 dominant microorganisms that cause puerperal mastitis. For the initial treatment of mastitis, the drug of choice is dicloxacillin sodium (500 mg orally every 6 to 8 hours for 7 to 10 days). If the patient has a mild allergy to penicillin, cephalexin (500 mg orally every 6 to 8 hours for 7 to 10 days) is an appropriate alternative. If the allergy to penicillin is severe or if methicillin-resistant Staphylococcus aureus (MRSA) infection is suspected, either clindamycin (300 mg orally twice daily for 7 to 10 days) or trimethoprim-sulfamethoxazole double strength orally twice daily for 7 to 10 days should be used. ●
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
In this question-and-answer article (the second in a series), our objective is to reinforce for the clinician several practical points of management for common infectious diseases. The principal references for the answers to the questions are 2 textbook chapters written by Dr. Duff.1,2 Other pertinent references are included in the text.
9. For uncomplicated chlamydia infection in a pregnant woman, what is the most appropriate treatment?
Uncomplicated chlamydia infection in a pregnant woman should be treated with a single 1,000-mg oral dose of azithromycin. An acceptable alternative is amoxicillin
500 mg orally 3 times daily for 7 days.
In a nonpregnant patient, doxycycline 100 mg orally twice daily for 7 days is also an appropriate alternative. However, doxycycline is relatively expensive and may not be well tolerated because of gastrointestinal adverse effects. (Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64[RR3]:1-137.)
10. What are the characteristic mucocutaneous lesions of primary, secondary, and tertiary syphilis?
The characteristic mucosal lesion of primary syphilis is the painless chancre. The usual mucocutaneous manifestations of secondary syphilis are maculopapular lesions (red or violet in color) on the palms and soles, mucous patches on the oral membranes, and condyloma lata on the genitalia. The classic mucocutaneous lesion of tertiary syphilis is the gumma.
Other serious manifestations of advanced syphilis include central nervous system abnormalities, such as tabes dorsalis, the Argyll Robertson pupil, and dementia, and cardiac abnormalities, such as aortitis, which can lead to a dissecting aneurysm of the aortic root. (Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64[RR3]:1-137.)
11. In a pregnant woman with a history of recurrent herpes simplex virus infection, what is the best way to prevent an outbreak of lesions near term?
Obstetric patients with a history of recurrent herpes simplex infection should be treated with acyclovir 400 mg orally 3 times daily from 36 weeks until delivery. This
regimen significantly reduces the likelihood of a recurrent outbreak near the time of delivery, which if it occurred, would necessitate a cesarean delivery. In patients at increased risk for preterm delivery, the prophylactic regimen should be started earlier.
Valacyclovir, 500 mg orally twice daily, is an acceptable alternative but is significantly more expensive.
Continue to: 12. What are the best office-based tests for the diagnosis of bacterial vaginosis?...
12. What are the best office-based tests for the diagnosis of bacterial vaginosis?
In patients with bacterial vaginosis, the vaginal pH typically is elevated in the range of 4.5. When a drop of potassium hydroxide solution is added to the vaginal secretions, a characteristic fishlike (amine) odor is liberated (positive “whiff test”). With saline microscopy, the key findings are a relative absence of lactobacilli in the background, an abundance of small cocci and bacilli, and the presence of clue cells, which are epithelial cells studded with bacteria along their
outer margin.
13. For a moderately ill pregnant woman, what is the most appropriate antibiotic combination for inpatient treatment of community-acquired pneumonia?
This patient should be treated with intravenous ceftriaxone (2 g every 24 hours) plus oral or intravenous azithromycin. The appropriate oral dose of azithromycin is 500 mg on day 1, then 250 mg daily for 4 doses. The appropriate intravenous dose of azithromycin is 500 mg every 24 hours. The goal is to provide appropriate coverage for the most likely pathogens: Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and mycoplasmas. (Antibacterial drugs for community-acquired pneumonia. Med Lett Drugs Ther. 2021:63:10-14. Postma DF, van Werkoven CH, van Eldin LJ, et al; CAP-START Study Group. Antibiotic treatment strategies for community acquired pneumonia in adults. N Engl J Med. 2015;372: 1312-1323.)
14. What tests are best for the diagnosis of COVID-19 infection?
The 2 key diagnostic tests for COVID-19 infection are detecting antigen in nasopharyngeal washings or saliva by nucleic acid amplification tests and identifying groundglass opacities on computed tomography imaging of the chest. (Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383:2451-2460.)
15. What is the most appropriate treatment for a pregnant woman who is moderately to severely ill with COVID-19 infection?
Moderately to severely ill pregnant women with COVID-19 infection should be hospitalized and treated with supplementary oxygen, remdesivir, and dexamethasone. Other possible therapies include inhaled nitric oxide, baricitinib (a Janus kinase inhibitor), and tocilizumab (an anti-interleukin 6 receptor antibody). (RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693704. Kalil AC, Patterson TF, Mehta AK, et al; ACTT-2 Study Group. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795-807. Berlin DA, Gulick RM, Martinez FJ, et al. Severe COVID19. N Engl J Med. 2020;383;2451-2460.)
16. What is the best test for the diagnosis of acute hepatitis A infection?
The single best test for the diagnosis of acute hepatitis A infection is detection of immunoglobulin M (IgM)–specific antibody to the virus.
17. What are the best tests for identification of a patient with chronic hepatitis B infection?
Patients with chronic hepatitis B infection typically test positive for the hepatitis B surface antigen (HBsAg) and for IgG antibody to the hepatitis B core antigen (HBcAg). In addition, they also may test positive for the hepatitis B e antigen (HBeAg), and their viral load can be quantified by polymerase chain reaction (PCR) when significant antigenemia is present. The presence of the e antigen indicates a high rate of viral replication and a corresponding high rate of infectivity.
18. What antenatal treatment is indicated in a pregnant woman at 28 weeks’ gestation who has a hepatitis B viral load of 2 million copies/mL?
This patient has a markedly elevated viral load and is at significantly increased risk of transmitting hepatitis B infection to her neonate even if the infant receives hepatitis B immune globulin immediately after birth and quickly begins the hepatitis B vaccine series. Daily antenatal treatment with tenofovir (300 mg daily) from 28 weeks until delivery will significantly reduce the risk of perinatal transmission.
19. Should a postpartum patient with chronic hepatitis C infection be discouraged from breastfeeding her infant?
Hepatitis C is not a contraindication to breastfeeding. Although the virus has been identified in breast milk, the risk of transmission to the infant is exceedingly low.
20. What are the principal microorganisms that cause puerperal mastitis?
Staphylococci and Streptococcus viridans are the 2 dominant microorganisms that cause puerperal mastitis. For the initial treatment of mastitis, the drug of choice is dicloxacillin sodium (500 mg orally every 6 to 8 hours for 7 to 10 days). If the patient has a mild allergy to penicillin, cephalexin (500 mg orally every 6 to 8 hours for 7 to 10 days) is an appropriate alternative. If the allergy to penicillin is severe or if methicillin-resistant Staphylococcus aureus (MRSA) infection is suspected, either clindamycin (300 mg orally twice daily for 7 to 10 days) or trimethoprim-sulfamethoxazole double strength orally twice daily for 7 to 10 days should be used. ●
In this question-and-answer article (the second in a series), our objective is to reinforce for the clinician several practical points of management for common infectious diseases. The principal references for the answers to the questions are 2 textbook chapters written by Dr. Duff.1,2 Other pertinent references are included in the text.
9. For uncomplicated chlamydia infection in a pregnant woman, what is the most appropriate treatment?
Uncomplicated chlamydia infection in a pregnant woman should be treated with a single 1,000-mg oral dose of azithromycin. An acceptable alternative is amoxicillin
500 mg orally 3 times daily for 7 days.
In a nonpregnant patient, doxycycline 100 mg orally twice daily for 7 days is also an appropriate alternative. However, doxycycline is relatively expensive and may not be well tolerated because of gastrointestinal adverse effects. (Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64[RR3]:1-137.)
10. What are the characteristic mucocutaneous lesions of primary, secondary, and tertiary syphilis?
The characteristic mucosal lesion of primary syphilis is the painless chancre. The usual mucocutaneous manifestations of secondary syphilis are maculopapular lesions (red or violet in color) on the palms and soles, mucous patches on the oral membranes, and condyloma lata on the genitalia. The classic mucocutaneous lesion of tertiary syphilis is the gumma.
Other serious manifestations of advanced syphilis include central nervous system abnormalities, such as tabes dorsalis, the Argyll Robertson pupil, and dementia, and cardiac abnormalities, such as aortitis, which can lead to a dissecting aneurysm of the aortic root. (Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64[RR3]:1-137.)
11. In a pregnant woman with a history of recurrent herpes simplex virus infection, what is the best way to prevent an outbreak of lesions near term?
Obstetric patients with a history of recurrent herpes simplex infection should be treated with acyclovir 400 mg orally 3 times daily from 36 weeks until delivery. This
regimen significantly reduces the likelihood of a recurrent outbreak near the time of delivery, which if it occurred, would necessitate a cesarean delivery. In patients at increased risk for preterm delivery, the prophylactic regimen should be started earlier.
Valacyclovir, 500 mg orally twice daily, is an acceptable alternative but is significantly more expensive.
Continue to: 12. What are the best office-based tests for the diagnosis of bacterial vaginosis?...
12. What are the best office-based tests for the diagnosis of bacterial vaginosis?
In patients with bacterial vaginosis, the vaginal pH typically is elevated in the range of 4.5. When a drop of potassium hydroxide solution is added to the vaginal secretions, a characteristic fishlike (amine) odor is liberated (positive “whiff test”). With saline microscopy, the key findings are a relative absence of lactobacilli in the background, an abundance of small cocci and bacilli, and the presence of clue cells, which are epithelial cells studded with bacteria along their
outer margin.
13. For a moderately ill pregnant woman, what is the most appropriate antibiotic combination for inpatient treatment of community-acquired pneumonia?
This patient should be treated with intravenous ceftriaxone (2 g every 24 hours) plus oral or intravenous azithromycin. The appropriate oral dose of azithromycin is 500 mg on day 1, then 250 mg daily for 4 doses. The appropriate intravenous dose of azithromycin is 500 mg every 24 hours. The goal is to provide appropriate coverage for the most likely pathogens: Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and mycoplasmas. (Antibacterial drugs for community-acquired pneumonia. Med Lett Drugs Ther. 2021:63:10-14. Postma DF, van Werkoven CH, van Eldin LJ, et al; CAP-START Study Group. Antibiotic treatment strategies for community acquired pneumonia in adults. N Engl J Med. 2015;372: 1312-1323.)
14. What tests are best for the diagnosis of COVID-19 infection?
The 2 key diagnostic tests for COVID-19 infection are detecting antigen in nasopharyngeal washings or saliva by nucleic acid amplification tests and identifying groundglass opacities on computed tomography imaging of the chest. (Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383:2451-2460.)
15. What is the most appropriate treatment for a pregnant woman who is moderately to severely ill with COVID-19 infection?
Moderately to severely ill pregnant women with COVID-19 infection should be hospitalized and treated with supplementary oxygen, remdesivir, and dexamethasone. Other possible therapies include inhaled nitric oxide, baricitinib (a Janus kinase inhibitor), and tocilizumab (an anti-interleukin 6 receptor antibody). (RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693704. Kalil AC, Patterson TF, Mehta AK, et al; ACTT-2 Study Group. Baricitinib plus remdesivir for hospitalized adults with COVID-19. N Engl J Med. 2021;384:795-807. Berlin DA, Gulick RM, Martinez FJ, et al. Severe COVID19. N Engl J Med. 2020;383;2451-2460.)
16. What is the best test for the diagnosis of acute hepatitis A infection?
The single best test for the diagnosis of acute hepatitis A infection is detection of immunoglobulin M (IgM)–specific antibody to the virus.
17. What are the best tests for identification of a patient with chronic hepatitis B infection?
Patients with chronic hepatitis B infection typically test positive for the hepatitis B surface antigen (HBsAg) and for IgG antibody to the hepatitis B core antigen (HBcAg). In addition, they also may test positive for the hepatitis B e antigen (HBeAg), and their viral load can be quantified by polymerase chain reaction (PCR) when significant antigenemia is present. The presence of the e antigen indicates a high rate of viral replication and a corresponding high rate of infectivity.
18. What antenatal treatment is indicated in a pregnant woman at 28 weeks’ gestation who has a hepatitis B viral load of 2 million copies/mL?
This patient has a markedly elevated viral load and is at significantly increased risk of transmitting hepatitis B infection to her neonate even if the infant receives hepatitis B immune globulin immediately after birth and quickly begins the hepatitis B vaccine series. Daily antenatal treatment with tenofovir (300 mg daily) from 28 weeks until delivery will significantly reduce the risk of perinatal transmission.
19. Should a postpartum patient with chronic hepatitis C infection be discouraged from breastfeeding her infant?
Hepatitis C is not a contraindication to breastfeeding. Although the virus has been identified in breast milk, the risk of transmission to the infant is exceedingly low.
20. What are the principal microorganisms that cause puerperal mastitis?
Staphylococci and Streptococcus viridans are the 2 dominant microorganisms that cause puerperal mastitis. For the initial treatment of mastitis, the drug of choice is dicloxacillin sodium (500 mg orally every 6 to 8 hours for 7 to 10 days). If the patient has a mild allergy to penicillin, cephalexin (500 mg orally every 6 to 8 hours for 7 to 10 days) is an appropriate alternative. If the allergy to penicillin is severe or if methicillin-resistant Staphylococcus aureus (MRSA) infection is suspected, either clindamycin (300 mg orally twice daily for 7 to 10 days) or trimethoprim-sulfamethoxazole double strength orally twice daily for 7 to 10 days should be used. ●
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Duff P. Maternal and perinatal infections: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2021:1124-1146.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TJ, et al. Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
Ginger for migraine: A new review
in patients who do not want to use or don’t have access to prescription medications, new data suggest.
Conducted by investigators at the National Institute of Mental Health and Neurosciences, Bangalore, India, the review showed ginger root can relieve migraine-related pain, nausea, and vomiting. However, the evidence does not support ginger’s use as a first-line therapy for acute migraine or for migraine prevention.
Study author Chittaranjan Andrade, MD, professor of clinical psychopharmacology and neurotoxicology at the institute, said in an interview that the evidence base is still “too small” to support formal clinical recommendations. However, he added, ginger can be considered as a viable “home-remedy option” for acute migraine.
The review was published online Dec. 2 in The Journal of Clinical Psychiatry.
Potential uses
Used for centuries in traditional medicine, much of the preclinical and clinical research has examined the potential of raw ginger, ginger extracts, and ginger constituents to prevent and treat a wide range of medical conditions. These include nausea and vomiting associated with pregnancy, chemotherapy, postoperative states, motion sickness, and other diseases and disorders, said Dr. Andrade.
Ginger has “long been recommended as an effective home remedy for the acute treatment of migraine, relieving both headache and the associated nausea,” Dr. Andrade noted.
One recommended recipe is stirring half a teaspoon of ground ginger into a glass of water and drinking the “ginger juice,” while another is to drink hot tea made from a teaspoon of freshly ground ginger.
“Patients with a number of common ailments, including migraine, are sometimes caught without medicines; or they may have poor access to medicines,” Dr. Andrade said. “I came across a reference to the use of ginger for migraine in a book on home remedies and I thought that if the research literature supports the use of ginger for migraine episodes, such patients could benefit.”
Large treatment gap
The review and meta-analysis included three randomized controlled trials with 227 patients looking at ginger versus placebo for the treatment.
One of the studies investigated the therapeutic efficacy of a specific proprietary formulation of ginger, combined with feverfew, while two trials were independent of industry.
Of these two, one examined the benefit of add-on dry ginger extract (400 mg; 5% active gingerols) in 50 patients who were also taking ketoprofen to treat migraine episodes, while the other examined the 3-month efficacy of daily dry ginger extract for migraine prophylaxis in 107 patients.
The two studies that examined the therapeutic efficacy of ginger versus placebo showed ginger reduced mean pain scores at 2 hours (mean difference, –1.27 [95% confidence interval, –1.46 to 1,07]) and also increased the proportion of patients who were pain free at 2 hours (RR, 1.79 [1.04 to 3.09]). In addition, compared to placebo, ginger halved the risk of migraine-related nausea and vomiting in all of the studies and was not associated with an increased risk of adverse events.
One RCT investigated prophylactic efficacy and found it to be more effective than placebo in bringing a ≥ 50% reduction in the frequency of monthly migraine episodes (in 42% versus 39% of patients, respectively), but the difference was not deemed statistically significant. In addition, there were no significant differences between the groups in days of pain, severe pain, days requiring use of analgesics, number of migraine episodes, and maximum duration of migraine episodes.
Dr. Andrade noted that ginger has many chemical constituents, including phenolic compounds, terpenes, polysaccharides, lipids, and organic acids of which 6-shogaol, 6-gingerol, and 10-dehydrogingerdione “may be important.”
It also has antioxidant and anti-inflammatory effects, lowering prostaglandins, and reducing several serum lipid and glycemic measures. Additionally, it has “putative” vasculoprotective effects, he added.
“Ginger has a large number of chemical constituents and we do not know which of these, separately or in combination, will help relieve migraine,” he said. “We won’t know the answer unless clinical trials are conducted with the individual constituents rather than with ginger extract.” He compared this to the study of omega-3 fatty acids rather than fish and nuts for various neuropsychiatric or cardiovascular indications.
Nevertheless, given the high global prevalence of migraine and the “large treatment gap [of migraine] in primary care,” it could be common for many affected patients to experience episodes of migraine headache “without recourse to recommended pharmacologic relief,” he noted. “In such cases, the availability of a simple home remedy, such as ginger, could be helpful.”
‘Good additional tool’
Commenting on the study for this news organization, Jessica Ailani, MD, director, MedStar Georgetown Headache Center and professor of clinical neurology, MedStar Georgetown University Hospital, Washington, said that for “people with migraine who are seeking treatment with minimal side effects that they can obtain without counsel of a health care provider, ginger is a good additional tool to have.”
Dr. Ailani, vice cochair of strategic planning in the MedStar department of neurology, who was not involved with the study, said that clinicians can “consider suggesting ginger to patients with migraine that have associated nausea who are interested in nonpharmacologic ways to treat symptoms.”
Since there are “many other effective ways to treat migraine,” she advises “conversing with the patient about speed of onset of efficacy, along with tolerability, and return of migraine symptoms as important factors to evaluate when choosing and staying with a treatment.”
Also commenting on the study for this news organization, Nada Hindiyeh, MD, clinical associate professor, department of neurology, Stanford (Calif.) University, called it a “nice summary of the objective research available for the use of ginger in acute and preventive treatment of migraine.”
Although there is insufficient literature evaluating ginger alone in migraine treatment, so “no definitive conclusions can be drawn,” since it appears to be safe and “somewhat helpful for migraine-associated nausea and vomiting and possibly in frequency of migraine reduction, it remains a considerable alternative for those seeking nonprescription options,” said Dr. Hindiyeh, who was not involved with the study.
Dr. Andrade publishes an e-newsletter supported by Sun Pharmaceuticals, with payments made to charities. He has received payments for developing educational materials for scientific initiatives and programs. Dr. Ailani reports honoraria for independent consulting from various pharmaceutical companies and clinical trial grants to her institution from the American Migraine Foundation, Allergan, Biohaven, Eli Lilly, Satsuma, and Zosano. Dr. Hindiyeh discloses no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in patients who do not want to use or don’t have access to prescription medications, new data suggest.
Conducted by investigators at the National Institute of Mental Health and Neurosciences, Bangalore, India, the review showed ginger root can relieve migraine-related pain, nausea, and vomiting. However, the evidence does not support ginger’s use as a first-line therapy for acute migraine or for migraine prevention.
Study author Chittaranjan Andrade, MD, professor of clinical psychopharmacology and neurotoxicology at the institute, said in an interview that the evidence base is still “too small” to support formal clinical recommendations. However, he added, ginger can be considered as a viable “home-remedy option” for acute migraine.
The review was published online Dec. 2 in The Journal of Clinical Psychiatry.
Potential uses
Used for centuries in traditional medicine, much of the preclinical and clinical research has examined the potential of raw ginger, ginger extracts, and ginger constituents to prevent and treat a wide range of medical conditions. These include nausea and vomiting associated with pregnancy, chemotherapy, postoperative states, motion sickness, and other diseases and disorders, said Dr. Andrade.
Ginger has “long been recommended as an effective home remedy for the acute treatment of migraine, relieving both headache and the associated nausea,” Dr. Andrade noted.
One recommended recipe is stirring half a teaspoon of ground ginger into a glass of water and drinking the “ginger juice,” while another is to drink hot tea made from a teaspoon of freshly ground ginger.
“Patients with a number of common ailments, including migraine, are sometimes caught without medicines; or they may have poor access to medicines,” Dr. Andrade said. “I came across a reference to the use of ginger for migraine in a book on home remedies and I thought that if the research literature supports the use of ginger for migraine episodes, such patients could benefit.”
Large treatment gap
The review and meta-analysis included three randomized controlled trials with 227 patients looking at ginger versus placebo for the treatment.
One of the studies investigated the therapeutic efficacy of a specific proprietary formulation of ginger, combined with feverfew, while two trials were independent of industry.
Of these two, one examined the benefit of add-on dry ginger extract (400 mg; 5% active gingerols) in 50 patients who were also taking ketoprofen to treat migraine episodes, while the other examined the 3-month efficacy of daily dry ginger extract for migraine prophylaxis in 107 patients.
The two studies that examined the therapeutic efficacy of ginger versus placebo showed ginger reduced mean pain scores at 2 hours (mean difference, –1.27 [95% confidence interval, –1.46 to 1,07]) and also increased the proportion of patients who were pain free at 2 hours (RR, 1.79 [1.04 to 3.09]). In addition, compared to placebo, ginger halved the risk of migraine-related nausea and vomiting in all of the studies and was not associated with an increased risk of adverse events.
One RCT investigated prophylactic efficacy and found it to be more effective than placebo in bringing a ≥ 50% reduction in the frequency of monthly migraine episodes (in 42% versus 39% of patients, respectively), but the difference was not deemed statistically significant. In addition, there were no significant differences between the groups in days of pain, severe pain, days requiring use of analgesics, number of migraine episodes, and maximum duration of migraine episodes.
Dr. Andrade noted that ginger has many chemical constituents, including phenolic compounds, terpenes, polysaccharides, lipids, and organic acids of which 6-shogaol, 6-gingerol, and 10-dehydrogingerdione “may be important.”
It also has antioxidant and anti-inflammatory effects, lowering prostaglandins, and reducing several serum lipid and glycemic measures. Additionally, it has “putative” vasculoprotective effects, he added.
“Ginger has a large number of chemical constituents and we do not know which of these, separately or in combination, will help relieve migraine,” he said. “We won’t know the answer unless clinical trials are conducted with the individual constituents rather than with ginger extract.” He compared this to the study of omega-3 fatty acids rather than fish and nuts for various neuropsychiatric or cardiovascular indications.
Nevertheless, given the high global prevalence of migraine and the “large treatment gap [of migraine] in primary care,” it could be common for many affected patients to experience episodes of migraine headache “without recourse to recommended pharmacologic relief,” he noted. “In such cases, the availability of a simple home remedy, such as ginger, could be helpful.”
‘Good additional tool’
Commenting on the study for this news organization, Jessica Ailani, MD, director, MedStar Georgetown Headache Center and professor of clinical neurology, MedStar Georgetown University Hospital, Washington, said that for “people with migraine who are seeking treatment with minimal side effects that they can obtain without counsel of a health care provider, ginger is a good additional tool to have.”
Dr. Ailani, vice cochair of strategic planning in the MedStar department of neurology, who was not involved with the study, said that clinicians can “consider suggesting ginger to patients with migraine that have associated nausea who are interested in nonpharmacologic ways to treat symptoms.”
Since there are “many other effective ways to treat migraine,” she advises “conversing with the patient about speed of onset of efficacy, along with tolerability, and return of migraine symptoms as important factors to evaluate when choosing and staying with a treatment.”
Also commenting on the study for this news organization, Nada Hindiyeh, MD, clinical associate professor, department of neurology, Stanford (Calif.) University, called it a “nice summary of the objective research available for the use of ginger in acute and preventive treatment of migraine.”
Although there is insufficient literature evaluating ginger alone in migraine treatment, so “no definitive conclusions can be drawn,” since it appears to be safe and “somewhat helpful for migraine-associated nausea and vomiting and possibly in frequency of migraine reduction, it remains a considerable alternative for those seeking nonprescription options,” said Dr. Hindiyeh, who was not involved with the study.
Dr. Andrade publishes an e-newsletter supported by Sun Pharmaceuticals, with payments made to charities. He has received payments for developing educational materials for scientific initiatives and programs. Dr. Ailani reports honoraria for independent consulting from various pharmaceutical companies and clinical trial grants to her institution from the American Migraine Foundation, Allergan, Biohaven, Eli Lilly, Satsuma, and Zosano. Dr. Hindiyeh discloses no relevant financial relationships.
A version of this article first appeared on Medscape.com.
in patients who do not want to use or don’t have access to prescription medications, new data suggest.
Conducted by investigators at the National Institute of Mental Health and Neurosciences, Bangalore, India, the review showed ginger root can relieve migraine-related pain, nausea, and vomiting. However, the evidence does not support ginger’s use as a first-line therapy for acute migraine or for migraine prevention.
Study author Chittaranjan Andrade, MD, professor of clinical psychopharmacology and neurotoxicology at the institute, said in an interview that the evidence base is still “too small” to support formal clinical recommendations. However, he added, ginger can be considered as a viable “home-remedy option” for acute migraine.
The review was published online Dec. 2 in The Journal of Clinical Psychiatry.
Potential uses
Used for centuries in traditional medicine, much of the preclinical and clinical research has examined the potential of raw ginger, ginger extracts, and ginger constituents to prevent and treat a wide range of medical conditions. These include nausea and vomiting associated with pregnancy, chemotherapy, postoperative states, motion sickness, and other diseases and disorders, said Dr. Andrade.
Ginger has “long been recommended as an effective home remedy for the acute treatment of migraine, relieving both headache and the associated nausea,” Dr. Andrade noted.
One recommended recipe is stirring half a teaspoon of ground ginger into a glass of water and drinking the “ginger juice,” while another is to drink hot tea made from a teaspoon of freshly ground ginger.
“Patients with a number of common ailments, including migraine, are sometimes caught without medicines; or they may have poor access to medicines,” Dr. Andrade said. “I came across a reference to the use of ginger for migraine in a book on home remedies and I thought that if the research literature supports the use of ginger for migraine episodes, such patients could benefit.”
Large treatment gap
The review and meta-analysis included three randomized controlled trials with 227 patients looking at ginger versus placebo for the treatment.
One of the studies investigated the therapeutic efficacy of a specific proprietary formulation of ginger, combined with feverfew, while two trials were independent of industry.
Of these two, one examined the benefit of add-on dry ginger extract (400 mg; 5% active gingerols) in 50 patients who were also taking ketoprofen to treat migraine episodes, while the other examined the 3-month efficacy of daily dry ginger extract for migraine prophylaxis in 107 patients.
The two studies that examined the therapeutic efficacy of ginger versus placebo showed ginger reduced mean pain scores at 2 hours (mean difference, –1.27 [95% confidence interval, –1.46 to 1,07]) and also increased the proportion of patients who were pain free at 2 hours (RR, 1.79 [1.04 to 3.09]). In addition, compared to placebo, ginger halved the risk of migraine-related nausea and vomiting in all of the studies and was not associated with an increased risk of adverse events.
One RCT investigated prophylactic efficacy and found it to be more effective than placebo in bringing a ≥ 50% reduction in the frequency of monthly migraine episodes (in 42% versus 39% of patients, respectively), but the difference was not deemed statistically significant. In addition, there were no significant differences between the groups in days of pain, severe pain, days requiring use of analgesics, number of migraine episodes, and maximum duration of migraine episodes.
Dr. Andrade noted that ginger has many chemical constituents, including phenolic compounds, terpenes, polysaccharides, lipids, and organic acids of which 6-shogaol, 6-gingerol, and 10-dehydrogingerdione “may be important.”
It also has antioxidant and anti-inflammatory effects, lowering prostaglandins, and reducing several serum lipid and glycemic measures. Additionally, it has “putative” vasculoprotective effects, he added.
“Ginger has a large number of chemical constituents and we do not know which of these, separately or in combination, will help relieve migraine,” he said. “We won’t know the answer unless clinical trials are conducted with the individual constituents rather than with ginger extract.” He compared this to the study of omega-3 fatty acids rather than fish and nuts for various neuropsychiatric or cardiovascular indications.
Nevertheless, given the high global prevalence of migraine and the “large treatment gap [of migraine] in primary care,” it could be common for many affected patients to experience episodes of migraine headache “without recourse to recommended pharmacologic relief,” he noted. “In such cases, the availability of a simple home remedy, such as ginger, could be helpful.”
‘Good additional tool’
Commenting on the study for this news organization, Jessica Ailani, MD, director, MedStar Georgetown Headache Center and professor of clinical neurology, MedStar Georgetown University Hospital, Washington, said that for “people with migraine who are seeking treatment with minimal side effects that they can obtain without counsel of a health care provider, ginger is a good additional tool to have.”
Dr. Ailani, vice cochair of strategic planning in the MedStar department of neurology, who was not involved with the study, said that clinicians can “consider suggesting ginger to patients with migraine that have associated nausea who are interested in nonpharmacologic ways to treat symptoms.”
Since there are “many other effective ways to treat migraine,” she advises “conversing with the patient about speed of onset of efficacy, along with tolerability, and return of migraine symptoms as important factors to evaluate when choosing and staying with a treatment.”
Also commenting on the study for this news organization, Nada Hindiyeh, MD, clinical associate professor, department of neurology, Stanford (Calif.) University, called it a “nice summary of the objective research available for the use of ginger in acute and preventive treatment of migraine.”
Although there is insufficient literature evaluating ginger alone in migraine treatment, so “no definitive conclusions can be drawn,” since it appears to be safe and “somewhat helpful for migraine-associated nausea and vomiting and possibly in frequency of migraine reduction, it remains a considerable alternative for those seeking nonprescription options,” said Dr. Hindiyeh, who was not involved with the study.
Dr. Andrade publishes an e-newsletter supported by Sun Pharmaceuticals, with payments made to charities. He has received payments for developing educational materials for scientific initiatives and programs. Dr. Ailani reports honoraria for independent consulting from various pharmaceutical companies and clinical trial grants to her institution from the American Migraine Foundation, Allergan, Biohaven, Eli Lilly, Satsuma, and Zosano. Dr. Hindiyeh discloses no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
iPLEDGE rollout described as a failure, chaotic, and a disaster
The that launched on Dec. 13, and what can be done to fix it.
By most accounts, the rollout was disastrous, chaotic, and a failure. Dermatologists on Twitter and elsewhere are angry and frustrated, with some calling for a temporary halt to the program until the bugs can be ironed out.
On Twitter Dec. 15, the Academy posted: “Due to the unacceptable situation with #iPLEDGE, the @US_FDA has convened an emergency meeting with AADA representatives tomorrow, December 16.”
The switch to a new platform was met with frustration from physicians, pharmacists, and patients alike. The new website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they attempted to follow instructions to enter information. Calls to obtain support from a live person often required hours on hold, several said.
The new approach to the isotretinoin risk-mitigation program itself isn’t under fire. It was welcomed by dermatologists and others who had long requested the change. Instead of three risk categories (females of reproductive potential, females not of reproductive potential, and males), there are now two (those who can get pregnant and those who cannot). Advocates for the change said it will make the experience more inclusive for transgender patients. The previous categories, some contended, were a barrier to access to care.
Because isotretinoin (Absorica, Amnesteem, Claravis, others), an oral retinoid used to treat severe forms of acne, is teratogenic, with a high risk of birth defects, and has also been associated with other health issues, those who take the medication who are able to get pregnant must take contraceptive precautions. The risk evaluation and mitigation program (REMS), mandated by the FDA, stipulates that physicians, patients, and pharmacists prescribing, using, or dispensing the drug must all be registered with requirements that include the use of two forms of an effective contraceptive and regular pregnancy tests by those capable of becoming pregnant.
A day of frustration
Before navigating the new website, a new log-on name was needed, said Ilona J. Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco. “They made you create a month-day-year date of personal significance.” When she tried to log on, she got locked out, she said in an interview.
The transition from the old website to the new, which Dr. Frieden said is now administered by a different vendor, was done quickly. The previous website shut down Dec. 10, and the new one launched Dec. 13, the first day for the new approach.
“A slower rollout would have helped,” Dr. Frieden said. While she and other dermatologists said they offered input previously on how to make the transition go more smoothly, no one seemed to want that help. “We did have a listening session with the FDA,” Dr. Frieden said. That was before the scheduled meeting of Dec. 16.
Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, also was frustrated with the rollout. “The week before the transition, one of my staff had to call iPLEDGE. They had a 177-minute wait to get to a human.
“They want us to register patients online now instead of signing forms in the office, but the links to view, download, or print don’t work,” Dr. Goldberg said in an interview.
This was after receiving information from the iPLEDGE REMS program, which stated, “The iPLEDGE REMS website will be updated to a modernized platform. All program materials and educational tools will be now available to you at the click of a button.’’
Dr. Goldberg also received calls from three patients who reported that they couldn’t complete the quiz that is required of patients capable of reproducing to demonstrate their comprehension about risk. Without the completed quiz, required monthly, the prescription can’t be refilled.
“It’s chaotic,” said Howa Yeung, MD, assistant professor of dermatology at Emory University, Atlanta. “The change is sudden, it’s a major change in the workflow. The process of reverification [required] is not that hard, but a lot of people have trouble even logging into the platform.”
What would help? To have a human on the phone to help navigate the system, Dr. Yeung said.
The glitches are delaying prescriptions for established patients and new ones as well, Dr. Yeung said. Existing patients who can get pregnant have 7 days after their negative pregnancy test to get their prescription filled. “And over the weekend the website was down,” he said, so that was a 2-day delay.
“The information we have and were told to use doesn’t match what is in their database,” said Mitesh Patel, PharmD, owner of Sunshine Pharmacy in White Plains, N.Y., who said pharmacists are experiencing issues with the new platform similar to those of doctors.
Twitter users had a lot to say, as well. Jack Resneck Jr., MD, professor of dermatology at the University of California, San Francisco, tweeted: “#Accutane has basically been pulled from market by utter incompetence of @SyneosHealth hired by @US_FDA to administer risk mgmt program.”
Dr. Resneck, president-elect of the American Medical Association, noted the crashed website, help line with 6-hour hold times, and patients unable to get the drug.
Adewole Adamson, MD, a dermatologist at the University of Texas, Austin, tweeted, “Dermatologists around the US are BIG mad about the current accutane debacle brought on by @SyneosHealth and @US_FDA. What a disaster for patient care!”
Several called for the FDA to immediately halt the program and let physicians manage the risk until the platform could be improved.
Are fixes in sight?
On Tuesday, Dec. 14, AADA President Kenneth J. Tomecki, MD, issued a statement expressing disappointment about the transition.
“In advance of this transition, the AADA engaged the FDA and the iPLEDGE administrator, Syneos Health, about the numerous workflow concerns raised by dermatologists and how the impending changes would threaten patient access to necessary medication. Those concerns have become a reality across the country and we’re working to ensure patients can maintain safe and appropriate access to the treatment they need.”
The AADA, the statement continues, supports efforts to streamline the program while keeping patient safety and incorporating input from physicians.
“We are very aware of the problems with the implementation of the iPLEDGE program,” FDA spokesperson Charlie Kohler said in an email. “We are continuing to work closely with the isotretinoin manufacturers to ensure that they implement a smoothly functioning iPLEDGE REMS program and that patient care is not interrupted.”
“Syneos Health appreciates the concern about iPLEDGE,” said Gary Gatyas, a spokesperson for Syneos Health. “While Syneos Health does not maintain the iPLEDGE system or contact center, we are doing what we can to help the responsible parties with a resolution.” Meanwhile, he recommended that people contact the call center.
He did not respond immediately to questions about who is responsible for maintaining the system and call center.
Dr. Goldberg, Dr. Frieden, and Dr. Yeung have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The that launched on Dec. 13, and what can be done to fix it.
By most accounts, the rollout was disastrous, chaotic, and a failure. Dermatologists on Twitter and elsewhere are angry and frustrated, with some calling for a temporary halt to the program until the bugs can be ironed out.
On Twitter Dec. 15, the Academy posted: “Due to the unacceptable situation with #iPLEDGE, the @US_FDA has convened an emergency meeting with AADA representatives tomorrow, December 16.”
The switch to a new platform was met with frustration from physicians, pharmacists, and patients alike. The new website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they attempted to follow instructions to enter information. Calls to obtain support from a live person often required hours on hold, several said.
The new approach to the isotretinoin risk-mitigation program itself isn’t under fire. It was welcomed by dermatologists and others who had long requested the change. Instead of three risk categories (females of reproductive potential, females not of reproductive potential, and males), there are now two (those who can get pregnant and those who cannot). Advocates for the change said it will make the experience more inclusive for transgender patients. The previous categories, some contended, were a barrier to access to care.
Because isotretinoin (Absorica, Amnesteem, Claravis, others), an oral retinoid used to treat severe forms of acne, is teratogenic, with a high risk of birth defects, and has also been associated with other health issues, those who take the medication who are able to get pregnant must take contraceptive precautions. The risk evaluation and mitigation program (REMS), mandated by the FDA, stipulates that physicians, patients, and pharmacists prescribing, using, or dispensing the drug must all be registered with requirements that include the use of two forms of an effective contraceptive and regular pregnancy tests by those capable of becoming pregnant.
A day of frustration
Before navigating the new website, a new log-on name was needed, said Ilona J. Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco. “They made you create a month-day-year date of personal significance.” When she tried to log on, she got locked out, she said in an interview.
The transition from the old website to the new, which Dr. Frieden said is now administered by a different vendor, was done quickly. The previous website shut down Dec. 10, and the new one launched Dec. 13, the first day for the new approach.
“A slower rollout would have helped,” Dr. Frieden said. While she and other dermatologists said they offered input previously on how to make the transition go more smoothly, no one seemed to want that help. “We did have a listening session with the FDA,” Dr. Frieden said. That was before the scheduled meeting of Dec. 16.
Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, also was frustrated with the rollout. “The week before the transition, one of my staff had to call iPLEDGE. They had a 177-minute wait to get to a human.
“They want us to register patients online now instead of signing forms in the office, but the links to view, download, or print don’t work,” Dr. Goldberg said in an interview.
This was after receiving information from the iPLEDGE REMS program, which stated, “The iPLEDGE REMS website will be updated to a modernized platform. All program materials and educational tools will be now available to you at the click of a button.’’
Dr. Goldberg also received calls from three patients who reported that they couldn’t complete the quiz that is required of patients capable of reproducing to demonstrate their comprehension about risk. Without the completed quiz, required monthly, the prescription can’t be refilled.
“It’s chaotic,” said Howa Yeung, MD, assistant professor of dermatology at Emory University, Atlanta. “The change is sudden, it’s a major change in the workflow. The process of reverification [required] is not that hard, but a lot of people have trouble even logging into the platform.”
What would help? To have a human on the phone to help navigate the system, Dr. Yeung said.
The glitches are delaying prescriptions for established patients and new ones as well, Dr. Yeung said. Existing patients who can get pregnant have 7 days after their negative pregnancy test to get their prescription filled. “And over the weekend the website was down,” he said, so that was a 2-day delay.
“The information we have and were told to use doesn’t match what is in their database,” said Mitesh Patel, PharmD, owner of Sunshine Pharmacy in White Plains, N.Y., who said pharmacists are experiencing issues with the new platform similar to those of doctors.
Twitter users had a lot to say, as well. Jack Resneck Jr., MD, professor of dermatology at the University of California, San Francisco, tweeted: “#Accutane has basically been pulled from market by utter incompetence of @SyneosHealth hired by @US_FDA to administer risk mgmt program.”
Dr. Resneck, president-elect of the American Medical Association, noted the crashed website, help line with 6-hour hold times, and patients unable to get the drug.
Adewole Adamson, MD, a dermatologist at the University of Texas, Austin, tweeted, “Dermatologists around the US are BIG mad about the current accutane debacle brought on by @SyneosHealth and @US_FDA. What a disaster for patient care!”
Several called for the FDA to immediately halt the program and let physicians manage the risk until the platform could be improved.
Are fixes in sight?
On Tuesday, Dec. 14, AADA President Kenneth J. Tomecki, MD, issued a statement expressing disappointment about the transition.
“In advance of this transition, the AADA engaged the FDA and the iPLEDGE administrator, Syneos Health, about the numerous workflow concerns raised by dermatologists and how the impending changes would threaten patient access to necessary medication. Those concerns have become a reality across the country and we’re working to ensure patients can maintain safe and appropriate access to the treatment they need.”
The AADA, the statement continues, supports efforts to streamline the program while keeping patient safety and incorporating input from physicians.
“We are very aware of the problems with the implementation of the iPLEDGE program,” FDA spokesperson Charlie Kohler said in an email. “We are continuing to work closely with the isotretinoin manufacturers to ensure that they implement a smoothly functioning iPLEDGE REMS program and that patient care is not interrupted.”
“Syneos Health appreciates the concern about iPLEDGE,” said Gary Gatyas, a spokesperson for Syneos Health. “While Syneos Health does not maintain the iPLEDGE system or contact center, we are doing what we can to help the responsible parties with a resolution.” Meanwhile, he recommended that people contact the call center.
He did not respond immediately to questions about who is responsible for maintaining the system and call center.
Dr. Goldberg, Dr. Frieden, and Dr. Yeung have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The that launched on Dec. 13, and what can be done to fix it.
By most accounts, the rollout was disastrous, chaotic, and a failure. Dermatologists on Twitter and elsewhere are angry and frustrated, with some calling for a temporary halt to the program until the bugs can be ironed out.
On Twitter Dec. 15, the Academy posted: “Due to the unacceptable situation with #iPLEDGE, the @US_FDA has convened an emergency meeting with AADA representatives tomorrow, December 16.”
The switch to a new platform was met with frustration from physicians, pharmacists, and patients alike. The new website crashed repeatedly, with physicians and patients complaining they got locked out or bounced off the platform when they attempted to follow instructions to enter information. Calls to obtain support from a live person often required hours on hold, several said.
The new approach to the isotretinoin risk-mitigation program itself isn’t under fire. It was welcomed by dermatologists and others who had long requested the change. Instead of three risk categories (females of reproductive potential, females not of reproductive potential, and males), there are now two (those who can get pregnant and those who cannot). Advocates for the change said it will make the experience more inclusive for transgender patients. The previous categories, some contended, were a barrier to access to care.
Because isotretinoin (Absorica, Amnesteem, Claravis, others), an oral retinoid used to treat severe forms of acne, is teratogenic, with a high risk of birth defects, and has also been associated with other health issues, those who take the medication who are able to get pregnant must take contraceptive precautions. The risk evaluation and mitigation program (REMS), mandated by the FDA, stipulates that physicians, patients, and pharmacists prescribing, using, or dispensing the drug must all be registered with requirements that include the use of two forms of an effective contraceptive and regular pregnancy tests by those capable of becoming pregnant.
A day of frustration
Before navigating the new website, a new log-on name was needed, said Ilona J. Frieden, MD, chair of the AADA’s iPLEDGE Workgroup and professor of dermatology at the University of California, San Francisco. “They made you create a month-day-year date of personal significance.” When she tried to log on, she got locked out, she said in an interview.
The transition from the old website to the new, which Dr. Frieden said is now administered by a different vendor, was done quickly. The previous website shut down Dec. 10, and the new one launched Dec. 13, the first day for the new approach.
“A slower rollout would have helped,” Dr. Frieden said. While she and other dermatologists said they offered input previously on how to make the transition go more smoothly, no one seemed to want that help. “We did have a listening session with the FDA,” Dr. Frieden said. That was before the scheduled meeting of Dec. 16.
Neil S. Goldberg, MD, a dermatologist in Westchester County, New York, also was frustrated with the rollout. “The week before the transition, one of my staff had to call iPLEDGE. They had a 177-minute wait to get to a human.
“They want us to register patients online now instead of signing forms in the office, but the links to view, download, or print don’t work,” Dr. Goldberg said in an interview.
This was after receiving information from the iPLEDGE REMS program, which stated, “The iPLEDGE REMS website will be updated to a modernized platform. All program materials and educational tools will be now available to you at the click of a button.’’
Dr. Goldberg also received calls from three patients who reported that they couldn’t complete the quiz that is required of patients capable of reproducing to demonstrate their comprehension about risk. Without the completed quiz, required monthly, the prescription can’t be refilled.
“It’s chaotic,” said Howa Yeung, MD, assistant professor of dermatology at Emory University, Atlanta. “The change is sudden, it’s a major change in the workflow. The process of reverification [required] is not that hard, but a lot of people have trouble even logging into the platform.”
What would help? To have a human on the phone to help navigate the system, Dr. Yeung said.
The glitches are delaying prescriptions for established patients and new ones as well, Dr. Yeung said. Existing patients who can get pregnant have 7 days after their negative pregnancy test to get their prescription filled. “And over the weekend the website was down,” he said, so that was a 2-day delay.
“The information we have and were told to use doesn’t match what is in their database,” said Mitesh Patel, PharmD, owner of Sunshine Pharmacy in White Plains, N.Y., who said pharmacists are experiencing issues with the new platform similar to those of doctors.
Twitter users had a lot to say, as well. Jack Resneck Jr., MD, professor of dermatology at the University of California, San Francisco, tweeted: “#Accutane has basically been pulled from market by utter incompetence of @SyneosHealth hired by @US_FDA to administer risk mgmt program.”
Dr. Resneck, president-elect of the American Medical Association, noted the crashed website, help line with 6-hour hold times, and patients unable to get the drug.
Adewole Adamson, MD, a dermatologist at the University of Texas, Austin, tweeted, “Dermatologists around the US are BIG mad about the current accutane debacle brought on by @SyneosHealth and @US_FDA. What a disaster for patient care!”
Several called for the FDA to immediately halt the program and let physicians manage the risk until the platform could be improved.
Are fixes in sight?
On Tuesday, Dec. 14, AADA President Kenneth J. Tomecki, MD, issued a statement expressing disappointment about the transition.
“In advance of this transition, the AADA engaged the FDA and the iPLEDGE administrator, Syneos Health, about the numerous workflow concerns raised by dermatologists and how the impending changes would threaten patient access to necessary medication. Those concerns have become a reality across the country and we’re working to ensure patients can maintain safe and appropriate access to the treatment they need.”
The AADA, the statement continues, supports efforts to streamline the program while keeping patient safety and incorporating input from physicians.
“We are very aware of the problems with the implementation of the iPLEDGE program,” FDA spokesperson Charlie Kohler said in an email. “We are continuing to work closely with the isotretinoin manufacturers to ensure that they implement a smoothly functioning iPLEDGE REMS program and that patient care is not interrupted.”
“Syneos Health appreciates the concern about iPLEDGE,” said Gary Gatyas, a spokesperson for Syneos Health. “While Syneos Health does not maintain the iPLEDGE system or contact center, we are doing what we can to help the responsible parties with a resolution.” Meanwhile, he recommended that people contact the call center.
He did not respond immediately to questions about who is responsible for maintaining the system and call center.
Dr. Goldberg, Dr. Frieden, and Dr. Yeung have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA updates risks, cautions for clotting-bleeding disorder on Janssen COVID-19 vaccine
Updated Janssen/Johnson & Johnson COVID-19 vaccine fact sheets for health care professionals and the general public now include a contraindication to its use in persons with a history of thrombosis with thrombocytopenia after receiving it “or any other adenovirus-vectored COVID-19 vaccine,” the U.S. Food and Drug Administration has announced.
Thrombosis with thrombocytopenia syndrome (TTS) – thrombocytopenia and increased bleeding risk along with documented thrombosis – after administration of the Janssen Ad26.COV2.S vaccine remains rare. But over all age groups, about one in seven cases have been fatal, said the agency.
the provider fact sheet states.
Although TTS associated with the Janssen COVID-19 vaccine has been reported in men and women aged 18 and older, the highest reported rate has been for women aged 30-49, the agency states. The rate in that group has been about 1 case per 100,000 doses administered.
Symptoms of TTS may occur 1-2 weeks after administration of the Janssen COVID-19 vaccine, the FDA says, based on data from the Vaccine Adverse Events Reporting System (VAERS).
Its clinical course shares features with autoimmune heparin-induced thrombocytopenia. In individuals with suspected TTS following receipt of the Janssen COVID-19 vaccine, the agency cautions, the use of heparin “may be harmful and alternative treatments may be needed. Consultation with hematology specialists is strongly recommended.”
The apparent excess risk of TTS remains under investigation, but “the FDA continues to find that the known and potential benefits of the Janssen COVID-19 vaccine outweigh its known and potential risks in individuals 18 years of age and older,” the agency states.
A version of this article first appeared on Medscape.com.
Updated Janssen/Johnson & Johnson COVID-19 vaccine fact sheets for health care professionals and the general public now include a contraindication to its use in persons with a history of thrombosis with thrombocytopenia after receiving it “or any other adenovirus-vectored COVID-19 vaccine,” the U.S. Food and Drug Administration has announced.
Thrombosis with thrombocytopenia syndrome (TTS) – thrombocytopenia and increased bleeding risk along with documented thrombosis – after administration of the Janssen Ad26.COV2.S vaccine remains rare. But over all age groups, about one in seven cases have been fatal, said the agency.
the provider fact sheet states.
Although TTS associated with the Janssen COVID-19 vaccine has been reported in men and women aged 18 and older, the highest reported rate has been for women aged 30-49, the agency states. The rate in that group has been about 1 case per 100,000 doses administered.
Symptoms of TTS may occur 1-2 weeks after administration of the Janssen COVID-19 vaccine, the FDA says, based on data from the Vaccine Adverse Events Reporting System (VAERS).
Its clinical course shares features with autoimmune heparin-induced thrombocytopenia. In individuals with suspected TTS following receipt of the Janssen COVID-19 vaccine, the agency cautions, the use of heparin “may be harmful and alternative treatments may be needed. Consultation with hematology specialists is strongly recommended.”
The apparent excess risk of TTS remains under investigation, but “the FDA continues to find that the known and potential benefits of the Janssen COVID-19 vaccine outweigh its known and potential risks in individuals 18 years of age and older,” the agency states.
A version of this article first appeared on Medscape.com.
Updated Janssen/Johnson & Johnson COVID-19 vaccine fact sheets for health care professionals and the general public now include a contraindication to its use in persons with a history of thrombosis with thrombocytopenia after receiving it “or any other adenovirus-vectored COVID-19 vaccine,” the U.S. Food and Drug Administration has announced.
Thrombosis with thrombocytopenia syndrome (TTS) – thrombocytopenia and increased bleeding risk along with documented thrombosis – after administration of the Janssen Ad26.COV2.S vaccine remains rare. But over all age groups, about one in seven cases have been fatal, said the agency.
the provider fact sheet states.
Although TTS associated with the Janssen COVID-19 vaccine has been reported in men and women aged 18 and older, the highest reported rate has been for women aged 30-49, the agency states. The rate in that group has been about 1 case per 100,000 doses administered.
Symptoms of TTS may occur 1-2 weeks after administration of the Janssen COVID-19 vaccine, the FDA says, based on data from the Vaccine Adverse Events Reporting System (VAERS).
Its clinical course shares features with autoimmune heparin-induced thrombocytopenia. In individuals with suspected TTS following receipt of the Janssen COVID-19 vaccine, the agency cautions, the use of heparin “may be harmful and alternative treatments may be needed. Consultation with hematology specialists is strongly recommended.”
The apparent excess risk of TTS remains under investigation, but “the FDA continues to find that the known and potential benefits of the Janssen COVID-19 vaccine outweigh its known and potential risks in individuals 18 years of age and older,” the agency states.
A version of this article first appeared on Medscape.com.
Cancer risk tied to some manufactured foods
SAN ANTONIO –
The findings were reported in three poster presentations (P1-09-01, P1-09-02 and P3-12-35) at the 2021 San Antonio Breast Cancer Symposium from the ongoing French NutriNet-Santé web-based study of 171,000 people that was launched in France in 2009 to investigate nutrition and health relationships. The authors of the analyses note that while evidence of deleterious health effects has been established for the dietary focus of their studies, and cancer risks have been suspected, strong evidence of a cancer association has been lacking.
Nitrates and nitrites are used in processed meats to increase shelf life and to avoid bacterial growth, said Eloi Chazelas, PhD, Nutritional Epidemiology Research Team (EREN) at Sorbonne Paris Nord University. Dr. Chazelas looked at consumption of nitrites and nitrates through repeated 24 hour dietary records, linked to a comprehensive food composition database. The study’s main outcome measure was adjusted associations between nitrite and nitrate exposures and the risk of cancer (overall and by main cancer sites).
During follow-up, 966 breast and 400 prostate cancers were diagnosed among 3,311 first incident cancer cases. Breast cancer risk was elevated (HR = 1.24 [1.03-1.48], P = 0.02) among higher consumers of nitrates from food additives, especially with potassium nitrate consumption (HR = 1.25 [1.04-1.50], P = 0.01). Elevated prostate cancer risk was associated with nitrites (HR = 1.58 [1.14-2.18], P = 0.008), specifically for sodium nitrite (HR = 1.62 [1.17-2.25], P = 0.004). Nitrates and nitrites from natural sources were not associated significantly with higher cancer risk, Dr. Chazelas said.
He and his team found that food additive nitrates were positively associated with breast cancer risk, and food additive nitrites were positively associated with prostate cancer risk. “While these results need confirmation in other large-scale prospective studies, they provide new insights in a context of lively debate around the ban of nitrite additives in the food industry,” said Dr. Chazelas, who is a doctoral candidate at Sorbonne Paris Nord University.
In “Breast and prostate cancer risk associated with nitrites and nitrates from food additives (P1-09-01),” the study included 102,046 adults from the French NutriNet-Santé prospective cohort (2009-2021). It examined associations between artificial sweetener intakes (total from all dietary sources, the most frequently consumed ones [aspartame e951, acesulfame-K e950 and sucralose e955]) and cancer risk (overall and by sites: breast, prostate and obesity-related cancers).
Overall cancer risk in people who consumed higher amounts of total sweeteners (i.e. above the median exposure in consumers) was elevated (n = 2,527 cases, hazard ratio = 1.12, 95 percent confidence interval = 1.00-1.25, P-trend=0.005), especially for aspartame (HR = 1.20 [1.05-1.38] P = 0.001) and acesulfame-K (HR = 1.18 [1.04-1.34] P = 0.003). Elevated breast cancer risks (among 723 cases) were observed for total sweeteners (HR = 1.25 [1.02-1.53] P = 0.01), for aspartame (HR = 1.33 [1.05-1.69] P = 0.007), and for acesulfame-K (HR = 1.39 [1.11-1.74] P = 0.003). Also, obesity-related cancers (1,509 cases) were increased for total sweeteners (HR = 1.16 [1.00-1.33] P = 0.02), for aspartame (HR = 1.22 [1.02-1.45] P = 0.01) and for acesulfame-K (HR = 1.23 [1.04-1.45] P = 0.01).
Artificial sweeteners are found in more than 10,000 foods and beverages, said Charlotte Debras, a doctoral candidate in nutritional epidemiology at Sorbonne Paris Nord University. “These findings provide important and novel insights for the ongoing re-evaluation of food additive sweeteners by the European Food Safety Authority and other health agencies globally,” she said.
Trans fatty acid intakes and cancer risk
Investigating associations between trans fatty acid intake (total ruminant [rTFAs], industrial [iTFAs], and corresponding specific isomers and cancer risk), the analysis of Gaëlle Wendeu-Foyet, PhD, Sorbonne Paris Nord University, found a total of 3,374 incident cancer cases (982 breast, 405 prostate) in an overall population of 104,909. Dietary intake of total TFAs was associated with higher prostate cancer risk (hazard ration for quartile 4 versus 1: 1.27, 1.11-1.77 P-trend = 0.005). Also, rTFAs were associated with increased overall cancer risk (1.16, 1.02-1.32 P-trend = 0.07), in particular the conjugated linoleic acid isomers (CLA) (1.19, 1.04-1.36 P-trend = 0.04). These associations were specifically observed for breast cancer (rTFAs: 1.35, 1.06-1.72 P-trend = 0.01; CLA: 1.29, 1.00-1.66 P-trend = 0.048), in particular before menopause (rTFAs: 1.68, 1.06-2.67 P-trend = 0.02; CLA: 2.013, 1.25-3.23 P-trend = 0.003). Several iTFAs were associated with overall (1.18, 1.06-1.31 P-trend = 0.02 for transdocosenoic acid), breast (isomer 18:2t: 1.30, 1.06-1.58 P-trend = 0.01; hexadecenoic acid: 1.28, 1.05-1.56 P-trend = 0.02) and prostate (transdocosenoic acid: 1.52, 1.09-2.12 P-trend = 0.07) cancer risks.
“These results support the WHO’s goal of achieving elimination from food supplies of industrially produced TFAs,” Dr. Foyet said. “The consumption of food products containing partially hydrogenated oils should be avoided.”
Nutrition, along with avoiding tobacco intake, is one of the main modifiable risk factors for chronic diseases. “There is a lot at stake in terms of prevention. This requires a combination of actions at the individual level to the public level by informing the public through food labeling,” Ms. Debras said.
It also requires influencing the context in which citizens evolve by encouraging manufacturers to improve their products (pricing policies, commitment charters for product reformulation, etc.), and limiting advertising and marketing for products of poor nutritional quality (especially among children),” she said.
SAN ANTONIO –
The findings were reported in three poster presentations (P1-09-01, P1-09-02 and P3-12-35) at the 2021 San Antonio Breast Cancer Symposium from the ongoing French NutriNet-Santé web-based study of 171,000 people that was launched in France in 2009 to investigate nutrition and health relationships. The authors of the analyses note that while evidence of deleterious health effects has been established for the dietary focus of their studies, and cancer risks have been suspected, strong evidence of a cancer association has been lacking.
Nitrates and nitrites are used in processed meats to increase shelf life and to avoid bacterial growth, said Eloi Chazelas, PhD, Nutritional Epidemiology Research Team (EREN) at Sorbonne Paris Nord University. Dr. Chazelas looked at consumption of nitrites and nitrates through repeated 24 hour dietary records, linked to a comprehensive food composition database. The study’s main outcome measure was adjusted associations between nitrite and nitrate exposures and the risk of cancer (overall and by main cancer sites).
During follow-up, 966 breast and 400 prostate cancers were diagnosed among 3,311 first incident cancer cases. Breast cancer risk was elevated (HR = 1.24 [1.03-1.48], P = 0.02) among higher consumers of nitrates from food additives, especially with potassium nitrate consumption (HR = 1.25 [1.04-1.50], P = 0.01). Elevated prostate cancer risk was associated with nitrites (HR = 1.58 [1.14-2.18], P = 0.008), specifically for sodium nitrite (HR = 1.62 [1.17-2.25], P = 0.004). Nitrates and nitrites from natural sources were not associated significantly with higher cancer risk, Dr. Chazelas said.
He and his team found that food additive nitrates were positively associated with breast cancer risk, and food additive nitrites were positively associated with prostate cancer risk. “While these results need confirmation in other large-scale prospective studies, they provide new insights in a context of lively debate around the ban of nitrite additives in the food industry,” said Dr. Chazelas, who is a doctoral candidate at Sorbonne Paris Nord University.
In “Breast and prostate cancer risk associated with nitrites and nitrates from food additives (P1-09-01),” the study included 102,046 adults from the French NutriNet-Santé prospective cohort (2009-2021). It examined associations between artificial sweetener intakes (total from all dietary sources, the most frequently consumed ones [aspartame e951, acesulfame-K e950 and sucralose e955]) and cancer risk (overall and by sites: breast, prostate and obesity-related cancers).
Overall cancer risk in people who consumed higher amounts of total sweeteners (i.e. above the median exposure in consumers) was elevated (n = 2,527 cases, hazard ratio = 1.12, 95 percent confidence interval = 1.00-1.25, P-trend=0.005), especially for aspartame (HR = 1.20 [1.05-1.38] P = 0.001) and acesulfame-K (HR = 1.18 [1.04-1.34] P = 0.003). Elevated breast cancer risks (among 723 cases) were observed for total sweeteners (HR = 1.25 [1.02-1.53] P = 0.01), for aspartame (HR = 1.33 [1.05-1.69] P = 0.007), and for acesulfame-K (HR = 1.39 [1.11-1.74] P = 0.003). Also, obesity-related cancers (1,509 cases) were increased for total sweeteners (HR = 1.16 [1.00-1.33] P = 0.02), for aspartame (HR = 1.22 [1.02-1.45] P = 0.01) and for acesulfame-K (HR = 1.23 [1.04-1.45] P = 0.01).
Artificial sweeteners are found in more than 10,000 foods and beverages, said Charlotte Debras, a doctoral candidate in nutritional epidemiology at Sorbonne Paris Nord University. “These findings provide important and novel insights for the ongoing re-evaluation of food additive sweeteners by the European Food Safety Authority and other health agencies globally,” she said.
Trans fatty acid intakes and cancer risk
Investigating associations between trans fatty acid intake (total ruminant [rTFAs], industrial [iTFAs], and corresponding specific isomers and cancer risk), the analysis of Gaëlle Wendeu-Foyet, PhD, Sorbonne Paris Nord University, found a total of 3,374 incident cancer cases (982 breast, 405 prostate) in an overall population of 104,909. Dietary intake of total TFAs was associated with higher prostate cancer risk (hazard ration for quartile 4 versus 1: 1.27, 1.11-1.77 P-trend = 0.005). Also, rTFAs were associated with increased overall cancer risk (1.16, 1.02-1.32 P-trend = 0.07), in particular the conjugated linoleic acid isomers (CLA) (1.19, 1.04-1.36 P-trend = 0.04). These associations were specifically observed for breast cancer (rTFAs: 1.35, 1.06-1.72 P-trend = 0.01; CLA: 1.29, 1.00-1.66 P-trend = 0.048), in particular before menopause (rTFAs: 1.68, 1.06-2.67 P-trend = 0.02; CLA: 2.013, 1.25-3.23 P-trend = 0.003). Several iTFAs were associated with overall (1.18, 1.06-1.31 P-trend = 0.02 for transdocosenoic acid), breast (isomer 18:2t: 1.30, 1.06-1.58 P-trend = 0.01; hexadecenoic acid: 1.28, 1.05-1.56 P-trend = 0.02) and prostate (transdocosenoic acid: 1.52, 1.09-2.12 P-trend = 0.07) cancer risks.
“These results support the WHO’s goal of achieving elimination from food supplies of industrially produced TFAs,” Dr. Foyet said. “The consumption of food products containing partially hydrogenated oils should be avoided.”
Nutrition, along with avoiding tobacco intake, is one of the main modifiable risk factors for chronic diseases. “There is a lot at stake in terms of prevention. This requires a combination of actions at the individual level to the public level by informing the public through food labeling,” Ms. Debras said.
It also requires influencing the context in which citizens evolve by encouraging manufacturers to improve their products (pricing policies, commitment charters for product reformulation, etc.), and limiting advertising and marketing for products of poor nutritional quality (especially among children),” she said.
SAN ANTONIO –
The findings were reported in three poster presentations (P1-09-01, P1-09-02 and P3-12-35) at the 2021 San Antonio Breast Cancer Symposium from the ongoing French NutriNet-Santé web-based study of 171,000 people that was launched in France in 2009 to investigate nutrition and health relationships. The authors of the analyses note that while evidence of deleterious health effects has been established for the dietary focus of their studies, and cancer risks have been suspected, strong evidence of a cancer association has been lacking.
Nitrates and nitrites are used in processed meats to increase shelf life and to avoid bacterial growth, said Eloi Chazelas, PhD, Nutritional Epidemiology Research Team (EREN) at Sorbonne Paris Nord University. Dr. Chazelas looked at consumption of nitrites and nitrates through repeated 24 hour dietary records, linked to a comprehensive food composition database. The study’s main outcome measure was adjusted associations between nitrite and nitrate exposures and the risk of cancer (overall and by main cancer sites).
During follow-up, 966 breast and 400 prostate cancers were diagnosed among 3,311 first incident cancer cases. Breast cancer risk was elevated (HR = 1.24 [1.03-1.48], P = 0.02) among higher consumers of nitrates from food additives, especially with potassium nitrate consumption (HR = 1.25 [1.04-1.50], P = 0.01). Elevated prostate cancer risk was associated with nitrites (HR = 1.58 [1.14-2.18], P = 0.008), specifically for sodium nitrite (HR = 1.62 [1.17-2.25], P = 0.004). Nitrates and nitrites from natural sources were not associated significantly with higher cancer risk, Dr. Chazelas said.
He and his team found that food additive nitrates were positively associated with breast cancer risk, and food additive nitrites were positively associated with prostate cancer risk. “While these results need confirmation in other large-scale prospective studies, they provide new insights in a context of lively debate around the ban of nitrite additives in the food industry,” said Dr. Chazelas, who is a doctoral candidate at Sorbonne Paris Nord University.
In “Breast and prostate cancer risk associated with nitrites and nitrates from food additives (P1-09-01),” the study included 102,046 adults from the French NutriNet-Santé prospective cohort (2009-2021). It examined associations between artificial sweetener intakes (total from all dietary sources, the most frequently consumed ones [aspartame e951, acesulfame-K e950 and sucralose e955]) and cancer risk (overall and by sites: breast, prostate and obesity-related cancers).
Overall cancer risk in people who consumed higher amounts of total sweeteners (i.e. above the median exposure in consumers) was elevated (n = 2,527 cases, hazard ratio = 1.12, 95 percent confidence interval = 1.00-1.25, P-trend=0.005), especially for aspartame (HR = 1.20 [1.05-1.38] P = 0.001) and acesulfame-K (HR = 1.18 [1.04-1.34] P = 0.003). Elevated breast cancer risks (among 723 cases) were observed for total sweeteners (HR = 1.25 [1.02-1.53] P = 0.01), for aspartame (HR = 1.33 [1.05-1.69] P = 0.007), and for acesulfame-K (HR = 1.39 [1.11-1.74] P = 0.003). Also, obesity-related cancers (1,509 cases) were increased for total sweeteners (HR = 1.16 [1.00-1.33] P = 0.02), for aspartame (HR = 1.22 [1.02-1.45] P = 0.01) and for acesulfame-K (HR = 1.23 [1.04-1.45] P = 0.01).
Artificial sweeteners are found in more than 10,000 foods and beverages, said Charlotte Debras, a doctoral candidate in nutritional epidemiology at Sorbonne Paris Nord University. “These findings provide important and novel insights for the ongoing re-evaluation of food additive sweeteners by the European Food Safety Authority and other health agencies globally,” she said.
Trans fatty acid intakes and cancer risk
Investigating associations between trans fatty acid intake (total ruminant [rTFAs], industrial [iTFAs], and corresponding specific isomers and cancer risk), the analysis of Gaëlle Wendeu-Foyet, PhD, Sorbonne Paris Nord University, found a total of 3,374 incident cancer cases (982 breast, 405 prostate) in an overall population of 104,909. Dietary intake of total TFAs was associated with higher prostate cancer risk (hazard ration for quartile 4 versus 1: 1.27, 1.11-1.77 P-trend = 0.005). Also, rTFAs were associated with increased overall cancer risk (1.16, 1.02-1.32 P-trend = 0.07), in particular the conjugated linoleic acid isomers (CLA) (1.19, 1.04-1.36 P-trend = 0.04). These associations were specifically observed for breast cancer (rTFAs: 1.35, 1.06-1.72 P-trend = 0.01; CLA: 1.29, 1.00-1.66 P-trend = 0.048), in particular before menopause (rTFAs: 1.68, 1.06-2.67 P-trend = 0.02; CLA: 2.013, 1.25-3.23 P-trend = 0.003). Several iTFAs were associated with overall (1.18, 1.06-1.31 P-trend = 0.02 for transdocosenoic acid), breast (isomer 18:2t: 1.30, 1.06-1.58 P-trend = 0.01; hexadecenoic acid: 1.28, 1.05-1.56 P-trend = 0.02) and prostate (transdocosenoic acid: 1.52, 1.09-2.12 P-trend = 0.07) cancer risks.
“These results support the WHO’s goal of achieving elimination from food supplies of industrially produced TFAs,” Dr. Foyet said. “The consumption of food products containing partially hydrogenated oils should be avoided.”
Nutrition, along with avoiding tobacco intake, is one of the main modifiable risk factors for chronic diseases. “There is a lot at stake in terms of prevention. This requires a combination of actions at the individual level to the public level by informing the public through food labeling,” Ms. Debras said.
It also requires influencing the context in which citizens evolve by encouraging manufacturers to improve their products (pricing policies, commitment charters for product reformulation, etc.), and limiting advertising and marketing for products of poor nutritional quality (especially among children),” she said.
FROM SABCS 2021
Breast cancer-related musculoskeletal pain alleviated with acupuncture
SAN ANTONIO –
Both techniques led to clinically meaningful and persistent reduction of pain, but electroacupuncture was more effective in reducing pain severity, according to study author Wanqing Iris Zhi, MD, PhD, of the Breast Medicine Service at Memorial Sloan Kettering Cancer Center in New York.
Among breast cancer survivors, Dr. Zhi said, chronic musculoskeletal pain is common and debilitating. In earlier results of the PEACE (Personalized Electroacupuncture versus Auricular Acupuncture Comparative Effectiveness) trial, both electroacupuncture and auricular acupuncture improved pain control better than usual care in cancer survivors. The comparative effectiveness between electroacupuncture and auricular acupuncture among breast cancer survivors, specifically for chronic musculoskeletal pain, remains unknown.
To evaluate potential differences between electroacupuncture and auricular acupuncture, Dr. Zhi et al. examined data from PEACE, a three-arm, parallel, single center randomized trial investigating electroacupuncture and auricular acupuncture for chronic musculoskeletal pain, compared with usual care. Among 360 cancer survivors in PEACE, mean age in 165 cancer survivors with a primary diagnosis of breast cancer was 60.3 years (35.8 percent non-White) with a mean of 5.4 years since their cancer diagnoses. Patients in both the electroacupuncture and auricular acupuncture groups received 10 weekly treatments. Change in mean Brief Pain Inventory (BPI) pain severity from baseline to week 12 was the primary endpoint, with BPI change to week 24 as a secondary endpoint. Usual care patients, after week 12, could receive 10 electroacupuncture treatments.
The most common locations of chronic musculoskeletal pain, Dr. Zhi observed, were lower back (24 percent), knee/leg (24 percent) and shoulder/elbow (14 percent). About 70 percent of patients were taking pain medication. Both electroacupuncture and auricular acupuncture were associated with clinically meaningful and persistent pain reductions among the evaluated breast cancer survivors. The change in BPI severity from baseline to week 12 was –0.29 (confidence interval, –0.08, 0.28) in the UC group. In the electroacupuncture group it was –2.65 (CI, –3.06, –2.25; P ≤0.001 from baseline) and –2.37 versus usual care (CI, –3.05, –1.68; P ≤0.001 versus UC). For the auricular acupuncture group, the change from baseline was –1.75 (CI, –2.15, –1.35; P ≤0.001 from baseline) and –1.46 versus usual care (CI, –2.14, –0.78; P ≤0.001 versus UC). The difference in BPI pain severity reduction from baseline between electroacupuncture and auricular acupuncture of –0.90 (CI, –1.45, –0.36) was statistically significant (P ≤0.001). Electroacupuncture also reduced pain severity significantly more than auricular acupuncture at week 24 (CI, –0.82, [–1.38, –0.27], P = 0.004).
Dr. Zhi concluded that among breast cancer survivors, although both electroacupuncture and auricular acupuncture were associated with clinically meaningful and persistent pain reduction, electroacupuncture was more effective at reducing pain severity.
She pointed out also that neither surgery type (mastectomy versus lumpectomy; P = 0.83) nor aromatase inhibitor versus tamoxifen versus neither (P = 0.59) was associated with BPI/severity response among electroacupuncture and auricular acupuncture patients.
“Both electroacupuncture and auricular acupuncture are significantly better than usual care, so it suggests that both acupuncture methods can be utilized for treating chronic muscle skeletal pain in breast cancer survivors, but electroacupuncture is preferred,” Dr. Zhi said.
“Auricular acupuncture can be more painful,” said PEACE principal investigator Jun Mao, MD, who is chair of integrative medicine at Memorial Sloan Kettering. “Ten percent of women could not tolerate the ear pain or discomfort. Electroacupuncture is generally well tolerated. People are more relaxed after treatment. If both are available, start with electroacupuncture,” he said.
SAN ANTONIO –
Both techniques led to clinically meaningful and persistent reduction of pain, but electroacupuncture was more effective in reducing pain severity, according to study author Wanqing Iris Zhi, MD, PhD, of the Breast Medicine Service at Memorial Sloan Kettering Cancer Center in New York.
Among breast cancer survivors, Dr. Zhi said, chronic musculoskeletal pain is common and debilitating. In earlier results of the PEACE (Personalized Electroacupuncture versus Auricular Acupuncture Comparative Effectiveness) trial, both electroacupuncture and auricular acupuncture improved pain control better than usual care in cancer survivors. The comparative effectiveness between electroacupuncture and auricular acupuncture among breast cancer survivors, specifically for chronic musculoskeletal pain, remains unknown.
To evaluate potential differences between electroacupuncture and auricular acupuncture, Dr. Zhi et al. examined data from PEACE, a three-arm, parallel, single center randomized trial investigating electroacupuncture and auricular acupuncture for chronic musculoskeletal pain, compared with usual care. Among 360 cancer survivors in PEACE, mean age in 165 cancer survivors with a primary diagnosis of breast cancer was 60.3 years (35.8 percent non-White) with a mean of 5.4 years since their cancer diagnoses. Patients in both the electroacupuncture and auricular acupuncture groups received 10 weekly treatments. Change in mean Brief Pain Inventory (BPI) pain severity from baseline to week 12 was the primary endpoint, with BPI change to week 24 as a secondary endpoint. Usual care patients, after week 12, could receive 10 electroacupuncture treatments.
The most common locations of chronic musculoskeletal pain, Dr. Zhi observed, were lower back (24 percent), knee/leg (24 percent) and shoulder/elbow (14 percent). About 70 percent of patients were taking pain medication. Both electroacupuncture and auricular acupuncture were associated with clinically meaningful and persistent pain reductions among the evaluated breast cancer survivors. The change in BPI severity from baseline to week 12 was –0.29 (confidence interval, –0.08, 0.28) in the UC group. In the electroacupuncture group it was –2.65 (CI, –3.06, –2.25; P ≤0.001 from baseline) and –2.37 versus usual care (CI, –3.05, –1.68; P ≤0.001 versus UC). For the auricular acupuncture group, the change from baseline was –1.75 (CI, –2.15, –1.35; P ≤0.001 from baseline) and –1.46 versus usual care (CI, –2.14, –0.78; P ≤0.001 versus UC). The difference in BPI pain severity reduction from baseline between electroacupuncture and auricular acupuncture of –0.90 (CI, –1.45, –0.36) was statistically significant (P ≤0.001). Electroacupuncture also reduced pain severity significantly more than auricular acupuncture at week 24 (CI, –0.82, [–1.38, –0.27], P = 0.004).
Dr. Zhi concluded that among breast cancer survivors, although both electroacupuncture and auricular acupuncture were associated with clinically meaningful and persistent pain reduction, electroacupuncture was more effective at reducing pain severity.
She pointed out also that neither surgery type (mastectomy versus lumpectomy; P = 0.83) nor aromatase inhibitor versus tamoxifen versus neither (P = 0.59) was associated with BPI/severity response among electroacupuncture and auricular acupuncture patients.
“Both electroacupuncture and auricular acupuncture are significantly better than usual care, so it suggests that both acupuncture methods can be utilized for treating chronic muscle skeletal pain in breast cancer survivors, but electroacupuncture is preferred,” Dr. Zhi said.
“Auricular acupuncture can be more painful,” said PEACE principal investigator Jun Mao, MD, who is chair of integrative medicine at Memorial Sloan Kettering. “Ten percent of women could not tolerate the ear pain or discomfort. Electroacupuncture is generally well tolerated. People are more relaxed after treatment. If both are available, start with electroacupuncture,” he said.
SAN ANTONIO –
Both techniques led to clinically meaningful and persistent reduction of pain, but electroacupuncture was more effective in reducing pain severity, according to study author Wanqing Iris Zhi, MD, PhD, of the Breast Medicine Service at Memorial Sloan Kettering Cancer Center in New York.
Among breast cancer survivors, Dr. Zhi said, chronic musculoskeletal pain is common and debilitating. In earlier results of the PEACE (Personalized Electroacupuncture versus Auricular Acupuncture Comparative Effectiveness) trial, both electroacupuncture and auricular acupuncture improved pain control better than usual care in cancer survivors. The comparative effectiveness between electroacupuncture and auricular acupuncture among breast cancer survivors, specifically for chronic musculoskeletal pain, remains unknown.
To evaluate potential differences between electroacupuncture and auricular acupuncture, Dr. Zhi et al. examined data from PEACE, a three-arm, parallel, single center randomized trial investigating electroacupuncture and auricular acupuncture for chronic musculoskeletal pain, compared with usual care. Among 360 cancer survivors in PEACE, mean age in 165 cancer survivors with a primary diagnosis of breast cancer was 60.3 years (35.8 percent non-White) with a mean of 5.4 years since their cancer diagnoses. Patients in both the electroacupuncture and auricular acupuncture groups received 10 weekly treatments. Change in mean Brief Pain Inventory (BPI) pain severity from baseline to week 12 was the primary endpoint, with BPI change to week 24 as a secondary endpoint. Usual care patients, after week 12, could receive 10 electroacupuncture treatments.
The most common locations of chronic musculoskeletal pain, Dr. Zhi observed, were lower back (24 percent), knee/leg (24 percent) and shoulder/elbow (14 percent). About 70 percent of patients were taking pain medication. Both electroacupuncture and auricular acupuncture were associated with clinically meaningful and persistent pain reductions among the evaluated breast cancer survivors. The change in BPI severity from baseline to week 12 was –0.29 (confidence interval, –0.08, 0.28) in the UC group. In the electroacupuncture group it was –2.65 (CI, –3.06, –2.25; P ≤0.001 from baseline) and –2.37 versus usual care (CI, –3.05, –1.68; P ≤0.001 versus UC). For the auricular acupuncture group, the change from baseline was –1.75 (CI, –2.15, –1.35; P ≤0.001 from baseline) and –1.46 versus usual care (CI, –2.14, –0.78; P ≤0.001 versus UC). The difference in BPI pain severity reduction from baseline between electroacupuncture and auricular acupuncture of –0.90 (CI, –1.45, –0.36) was statistically significant (P ≤0.001). Electroacupuncture also reduced pain severity significantly more than auricular acupuncture at week 24 (CI, –0.82, [–1.38, –0.27], P = 0.004).
Dr. Zhi concluded that among breast cancer survivors, although both electroacupuncture and auricular acupuncture were associated with clinically meaningful and persistent pain reduction, electroacupuncture was more effective at reducing pain severity.
She pointed out also that neither surgery type (mastectomy versus lumpectomy; P = 0.83) nor aromatase inhibitor versus tamoxifen versus neither (P = 0.59) was associated with BPI/severity response among electroacupuncture and auricular acupuncture patients.
“Both electroacupuncture and auricular acupuncture are significantly better than usual care, so it suggests that both acupuncture methods can be utilized for treating chronic muscle skeletal pain in breast cancer survivors, but electroacupuncture is preferred,” Dr. Zhi said.
“Auricular acupuncture can be more painful,” said PEACE principal investigator Jun Mao, MD, who is chair of integrative medicine at Memorial Sloan Kettering. “Ten percent of women could not tolerate the ear pain or discomfort. Electroacupuncture is generally well tolerated. People are more relaxed after treatment. If both are available, start with electroacupuncture,” he said.
FROM SABCS 2021
Doctors as trusted messengers
On a recent Friday, oncologist Christine Berg, MD, devoted 3 hours to a webinar about electrification of heavy- and medium-duty trucks in Maryland.
It’s not the way most cancer specialists choose to spend their time. But Dr. Berg, who is board certified in medical oncology, radiation oncology, and internal medicine, has made air pollution her current focus. Through organizations such as the Public Employees for Environmental Responsibility, she is working to raise awareness of the huge impact it can have on cancer.
“I think oncologists can make a difference,” she said.
That’s why Dr. Berg took a keen interest in a recent study by ProPublica, the nonprofit journalism organization, that identified previously ignored “hot spots of cancer-causing air.” While the ProPublica report gives an incomplete picture of airborne carcinogens, it puts an important spotlight on industrial air pollution, Dr. Berg and other experts say.
Relying on data from the Environmental Protection Agency’s Risk-Screening Environmental Indicators (RSEI), ProPublica researchers estimated the effects of industrial air pollution around the country and found problems the EPA overlooked, they reported. “The EPA collects data on each individual facility, but it doesn’t consider the excess cancer risk from all of the facilities’ combined emissions,” reporter Lylla Younes and colleagues wrote. “ProPublica did.”
The ProPublica team produced a map of cancer-causing industrial air pollution hot spots. They estimated that 256,000 people in the United States live in areas where incidences of cancer caused by air pollution exceed the EPA’s upper limit of acceptable risk.
While some of the spots are scattered around the country, they are concentrated along the Gulf Coast of Texas and Louisiana. For example, near the Equistar Chemicals Bayport Chemical Plant in Pasadena, Texas, ProPublica calculated the increased risk of cancer at 1 in 220, “46 times the EPA’s acceptable risk.” (The agency defines an acceptable risk as less than a 1 in 10,000 chance of developing cancer.)
Almost all the hot spots with the highest level of risk are in southern United States “known for having weaker environmental regulations,” the report said.
The researchers also identified race as a risk factor. In predominantly Black census tracts, they estimated the risk from toxic air pollution is more than double the risk in predominantly White census tracts. It attributed this pattern to deliberate policies of redlining that segregated neighborhoods and to zoning ordinances that encouraged industry in communities of color.
Measuring risk not straightforward
In response to a query from this news organization, an EPA spokesperson provided a statement saying the RSEI data are not intended for the purpose used by ProPublica. “RSEI does not provide a risk assessment (e.g., excess cancer case estimates),” the statement said. The RSEI data are poorly suited to this purpose because they use “worst-case assumptions about toxicity and potential exposure where data are lacking, and also use simplifying assumptions to reduce the complexity of the calculations,” the statement said.
Instead, the data are meant as a kind of index to compare one place to another, or show changes over time, the agency said. In this way, it can prompt regulators to investigate further. “A more refined assessment is required before any conclusions about health impacts can be drawn.” The agency is working on just such a refined approach, per the EPA statement.
That’s not just bureaucratic stonewalling, said Stan Meiberg, PhD, MA, a former EPA official and director of graduate studies in sustainability at Wake Forest University in Winston-Salem, N.C. “To say that you can speak with great precision, that the risk of individuals getting cancer is 1 in 100, may be a little overstating the date on which that statement is based.”
Risk estimates are improving as citizens gain access to more sophisticated monitoring devices, he said. And the primary point of the ProPublica report, that the EPA has underestimated risk by looking at individual sources of pollution rather than combining them, is not an original one, Dr. Meiberg said. “This is an issue that’s been kicking around for quite some time.”
Still, it’s one that demands attention. EPA regulations have succeeded in reducing the overall risk from industrial air pollution over the past few decades. “But there remain areas of particular geographic concentrations,” he said. “And the ProPublica article hit two of them, which have been the subject of discussion for many years, the Houston Ship Channel area and the Baton Rouge to New Orleans industrial corridor where you have a significant proportion of all the chemical petrochemical industry in the United States.”
Improvements in containment of the pollutants, and changes to the industrial processes that produce them, can also help reduce exposure. These changes should occur in the context of dialogue within the communities exposed to the pollution, Dr. Meiberg said.
The role of cancer-causing airborne particulate matter
But even if measures are perfectly implemented, Joan Schiller, MD, will not breathe easy. An adjunct professor of oncology at the University of Virginia in Charlottesville, Dr. Schiller has researched the role of airborne particulate matter in causing cancer, a correlation barely mentioned in the ProPublica analysis, she pointed out.
Particulate matter contains a wide range of toxic substances, she said. Researchers have focused on particles 2.5 microns in diameter, or PM 2.5. Some studies have indicated that it’s responsible for one in seven deaths from lung cancer, Dr. Schiller said. “Air pollution also causes lung cancer in never smokers, people who’ve never smoked, not just in smokers.”
Power plants and automobile traffic may be more significant sources of PM 2.5 than industry, and wildfires have recently emerged as increasingly important source, a result of climate change and poor forest management, she said.
PM 2.5 doesn’t affect just lung cancer, said Alexandra White, PhD, an investigator at the National Institute of Environmental Health Sciences in Research Triangle Park, N.C. “My work, as well as work of others, is increasingly suggesting that air pollution is also related to breast cancer risk, in particular, air pollution that is arising from traffic related forces.” And more research is needed on other cancers, she said. “I think that the lack of findings of other cancer sites reflects a lack of study.”
Other pollutants not analyzed in the ProPublica report are also correlated to cancer risk. In a recent meta-analysis, researcher Stephan Gabet, PhD, PharmD, and colleagues at the University of Grenoble, France, estimated that 3.15% of new breast cancer cases in that country could be attributed to nitrogen dioxide and 2.15% to PM 10.
Sources of nitrogen dioxide, PM 2.5, and PM 10 in France include automobile traffic, inefficient wood-burning stoves, and coal-burning power plants in neighboring countries, Dr. Gabet said.
A good approach to reducing pollution from road traffic is the implementation of low-emission zones that prohibit the most polluting vehicles, he said. But a 2019 United Kingdom government study found that brake wear, tire wear, and road surface wear account for 72% of the PM 10 and 60% of the PM 2.5 pollution from road traffic, suggesting that a transition to electric vehicles won’t fix the problem. Better yet, is “the promotion of active modes like walking, cycling, etc., because like this, you can bring additional health gains due to the increase in physical activity,” he said.
Oncologists can help their patients reduce their exposure to air pollution, Dr. Schiller said. “If you have lung cancer, air pollution will hasten your demise. It makes you sicker. Oncologists should be telling their patients about this and advising them to move away from air pollution if possible, and also making sure they know to monitor the health of the air.”
On days when air pollution is high, patients may want to avoid exercising outdoors, or stay indoors altogether, Dr. Berg said. Air purifiers and N95 masks may also help.
And physicians can make a difference by speaking out in their communities, Dr. Schiller said. She is inviting oncologists to join a new group, Oncologists Understanding for Climate and Health. Through this group or on their own, oncologists can speak to their local legislatures or city councils in support of measures to reduce pollution, she said. “Doctors are trusted messengers.”
Dr. Berg disclosed affiliations with Grail, Mercy BioAnalytics and Lucid Diagnostics.
On a recent Friday, oncologist Christine Berg, MD, devoted 3 hours to a webinar about electrification of heavy- and medium-duty trucks in Maryland.
It’s not the way most cancer specialists choose to spend their time. But Dr. Berg, who is board certified in medical oncology, radiation oncology, and internal medicine, has made air pollution her current focus. Through organizations such as the Public Employees for Environmental Responsibility, she is working to raise awareness of the huge impact it can have on cancer.
“I think oncologists can make a difference,” she said.
That’s why Dr. Berg took a keen interest in a recent study by ProPublica, the nonprofit journalism organization, that identified previously ignored “hot spots of cancer-causing air.” While the ProPublica report gives an incomplete picture of airborne carcinogens, it puts an important spotlight on industrial air pollution, Dr. Berg and other experts say.
Relying on data from the Environmental Protection Agency’s Risk-Screening Environmental Indicators (RSEI), ProPublica researchers estimated the effects of industrial air pollution around the country and found problems the EPA overlooked, they reported. “The EPA collects data on each individual facility, but it doesn’t consider the excess cancer risk from all of the facilities’ combined emissions,” reporter Lylla Younes and colleagues wrote. “ProPublica did.”
The ProPublica team produced a map of cancer-causing industrial air pollution hot spots. They estimated that 256,000 people in the United States live in areas where incidences of cancer caused by air pollution exceed the EPA’s upper limit of acceptable risk.
While some of the spots are scattered around the country, they are concentrated along the Gulf Coast of Texas and Louisiana. For example, near the Equistar Chemicals Bayport Chemical Plant in Pasadena, Texas, ProPublica calculated the increased risk of cancer at 1 in 220, “46 times the EPA’s acceptable risk.” (The agency defines an acceptable risk as less than a 1 in 10,000 chance of developing cancer.)
Almost all the hot spots with the highest level of risk are in southern United States “known for having weaker environmental regulations,” the report said.
The researchers also identified race as a risk factor. In predominantly Black census tracts, they estimated the risk from toxic air pollution is more than double the risk in predominantly White census tracts. It attributed this pattern to deliberate policies of redlining that segregated neighborhoods and to zoning ordinances that encouraged industry in communities of color.
Measuring risk not straightforward
In response to a query from this news organization, an EPA spokesperson provided a statement saying the RSEI data are not intended for the purpose used by ProPublica. “RSEI does not provide a risk assessment (e.g., excess cancer case estimates),” the statement said. The RSEI data are poorly suited to this purpose because they use “worst-case assumptions about toxicity and potential exposure where data are lacking, and also use simplifying assumptions to reduce the complexity of the calculations,” the statement said.
Instead, the data are meant as a kind of index to compare one place to another, or show changes over time, the agency said. In this way, it can prompt regulators to investigate further. “A more refined assessment is required before any conclusions about health impacts can be drawn.” The agency is working on just such a refined approach, per the EPA statement.
That’s not just bureaucratic stonewalling, said Stan Meiberg, PhD, MA, a former EPA official and director of graduate studies in sustainability at Wake Forest University in Winston-Salem, N.C. “To say that you can speak with great precision, that the risk of individuals getting cancer is 1 in 100, may be a little overstating the date on which that statement is based.”
Risk estimates are improving as citizens gain access to more sophisticated monitoring devices, he said. And the primary point of the ProPublica report, that the EPA has underestimated risk by looking at individual sources of pollution rather than combining them, is not an original one, Dr. Meiberg said. “This is an issue that’s been kicking around for quite some time.”
Still, it’s one that demands attention. EPA regulations have succeeded in reducing the overall risk from industrial air pollution over the past few decades. “But there remain areas of particular geographic concentrations,” he said. “And the ProPublica article hit two of them, which have been the subject of discussion for many years, the Houston Ship Channel area and the Baton Rouge to New Orleans industrial corridor where you have a significant proportion of all the chemical petrochemical industry in the United States.”
Improvements in containment of the pollutants, and changes to the industrial processes that produce them, can also help reduce exposure. These changes should occur in the context of dialogue within the communities exposed to the pollution, Dr. Meiberg said.
The role of cancer-causing airborne particulate matter
But even if measures are perfectly implemented, Joan Schiller, MD, will not breathe easy. An adjunct professor of oncology at the University of Virginia in Charlottesville, Dr. Schiller has researched the role of airborne particulate matter in causing cancer, a correlation barely mentioned in the ProPublica analysis, she pointed out.
Particulate matter contains a wide range of toxic substances, she said. Researchers have focused on particles 2.5 microns in diameter, or PM 2.5. Some studies have indicated that it’s responsible for one in seven deaths from lung cancer, Dr. Schiller said. “Air pollution also causes lung cancer in never smokers, people who’ve never smoked, not just in smokers.”
Power plants and automobile traffic may be more significant sources of PM 2.5 than industry, and wildfires have recently emerged as increasingly important source, a result of climate change and poor forest management, she said.
PM 2.5 doesn’t affect just lung cancer, said Alexandra White, PhD, an investigator at the National Institute of Environmental Health Sciences in Research Triangle Park, N.C. “My work, as well as work of others, is increasingly suggesting that air pollution is also related to breast cancer risk, in particular, air pollution that is arising from traffic related forces.” And more research is needed on other cancers, she said. “I think that the lack of findings of other cancer sites reflects a lack of study.”
Other pollutants not analyzed in the ProPublica report are also correlated to cancer risk. In a recent meta-analysis, researcher Stephan Gabet, PhD, PharmD, and colleagues at the University of Grenoble, France, estimated that 3.15% of new breast cancer cases in that country could be attributed to nitrogen dioxide and 2.15% to PM 10.
Sources of nitrogen dioxide, PM 2.5, and PM 10 in France include automobile traffic, inefficient wood-burning stoves, and coal-burning power plants in neighboring countries, Dr. Gabet said.
A good approach to reducing pollution from road traffic is the implementation of low-emission zones that prohibit the most polluting vehicles, he said. But a 2019 United Kingdom government study found that brake wear, tire wear, and road surface wear account for 72% of the PM 10 and 60% of the PM 2.5 pollution from road traffic, suggesting that a transition to electric vehicles won’t fix the problem. Better yet, is “the promotion of active modes like walking, cycling, etc., because like this, you can bring additional health gains due to the increase in physical activity,” he said.
Oncologists can help their patients reduce their exposure to air pollution, Dr. Schiller said. “If you have lung cancer, air pollution will hasten your demise. It makes you sicker. Oncologists should be telling their patients about this and advising them to move away from air pollution if possible, and also making sure they know to monitor the health of the air.”
On days when air pollution is high, patients may want to avoid exercising outdoors, or stay indoors altogether, Dr. Berg said. Air purifiers and N95 masks may also help.
And physicians can make a difference by speaking out in their communities, Dr. Schiller said. She is inviting oncologists to join a new group, Oncologists Understanding for Climate and Health. Through this group or on their own, oncologists can speak to their local legislatures or city councils in support of measures to reduce pollution, she said. “Doctors are trusted messengers.”
Dr. Berg disclosed affiliations with Grail, Mercy BioAnalytics and Lucid Diagnostics.
On a recent Friday, oncologist Christine Berg, MD, devoted 3 hours to a webinar about electrification of heavy- and medium-duty trucks in Maryland.
It’s not the way most cancer specialists choose to spend their time. But Dr. Berg, who is board certified in medical oncology, radiation oncology, and internal medicine, has made air pollution her current focus. Through organizations such as the Public Employees for Environmental Responsibility, she is working to raise awareness of the huge impact it can have on cancer.
“I think oncologists can make a difference,” she said.
That’s why Dr. Berg took a keen interest in a recent study by ProPublica, the nonprofit journalism organization, that identified previously ignored “hot spots of cancer-causing air.” While the ProPublica report gives an incomplete picture of airborne carcinogens, it puts an important spotlight on industrial air pollution, Dr. Berg and other experts say.
Relying on data from the Environmental Protection Agency’s Risk-Screening Environmental Indicators (RSEI), ProPublica researchers estimated the effects of industrial air pollution around the country and found problems the EPA overlooked, they reported. “The EPA collects data on each individual facility, but it doesn’t consider the excess cancer risk from all of the facilities’ combined emissions,” reporter Lylla Younes and colleagues wrote. “ProPublica did.”
The ProPublica team produced a map of cancer-causing industrial air pollution hot spots. They estimated that 256,000 people in the United States live in areas where incidences of cancer caused by air pollution exceed the EPA’s upper limit of acceptable risk.
While some of the spots are scattered around the country, they are concentrated along the Gulf Coast of Texas and Louisiana. For example, near the Equistar Chemicals Bayport Chemical Plant in Pasadena, Texas, ProPublica calculated the increased risk of cancer at 1 in 220, “46 times the EPA’s acceptable risk.” (The agency defines an acceptable risk as less than a 1 in 10,000 chance of developing cancer.)
Almost all the hot spots with the highest level of risk are in southern United States “known for having weaker environmental regulations,” the report said.
The researchers also identified race as a risk factor. In predominantly Black census tracts, they estimated the risk from toxic air pollution is more than double the risk in predominantly White census tracts. It attributed this pattern to deliberate policies of redlining that segregated neighborhoods and to zoning ordinances that encouraged industry in communities of color.
Measuring risk not straightforward
In response to a query from this news organization, an EPA spokesperson provided a statement saying the RSEI data are not intended for the purpose used by ProPublica. “RSEI does not provide a risk assessment (e.g., excess cancer case estimates),” the statement said. The RSEI data are poorly suited to this purpose because they use “worst-case assumptions about toxicity and potential exposure where data are lacking, and also use simplifying assumptions to reduce the complexity of the calculations,” the statement said.
Instead, the data are meant as a kind of index to compare one place to another, or show changes over time, the agency said. In this way, it can prompt regulators to investigate further. “A more refined assessment is required before any conclusions about health impacts can be drawn.” The agency is working on just such a refined approach, per the EPA statement.
That’s not just bureaucratic stonewalling, said Stan Meiberg, PhD, MA, a former EPA official and director of graduate studies in sustainability at Wake Forest University in Winston-Salem, N.C. “To say that you can speak with great precision, that the risk of individuals getting cancer is 1 in 100, may be a little overstating the date on which that statement is based.”
Risk estimates are improving as citizens gain access to more sophisticated monitoring devices, he said. And the primary point of the ProPublica report, that the EPA has underestimated risk by looking at individual sources of pollution rather than combining them, is not an original one, Dr. Meiberg said. “This is an issue that’s been kicking around for quite some time.”
Still, it’s one that demands attention. EPA regulations have succeeded in reducing the overall risk from industrial air pollution over the past few decades. “But there remain areas of particular geographic concentrations,” he said. “And the ProPublica article hit two of them, which have been the subject of discussion for many years, the Houston Ship Channel area and the Baton Rouge to New Orleans industrial corridor where you have a significant proportion of all the chemical petrochemical industry in the United States.”
Improvements in containment of the pollutants, and changes to the industrial processes that produce them, can also help reduce exposure. These changes should occur in the context of dialogue within the communities exposed to the pollution, Dr. Meiberg said.
The role of cancer-causing airborne particulate matter
But even if measures are perfectly implemented, Joan Schiller, MD, will not breathe easy. An adjunct professor of oncology at the University of Virginia in Charlottesville, Dr. Schiller has researched the role of airborne particulate matter in causing cancer, a correlation barely mentioned in the ProPublica analysis, she pointed out.
Particulate matter contains a wide range of toxic substances, she said. Researchers have focused on particles 2.5 microns in diameter, or PM 2.5. Some studies have indicated that it’s responsible for one in seven deaths from lung cancer, Dr. Schiller said. “Air pollution also causes lung cancer in never smokers, people who’ve never smoked, not just in smokers.”
Power plants and automobile traffic may be more significant sources of PM 2.5 than industry, and wildfires have recently emerged as increasingly important source, a result of climate change and poor forest management, she said.
PM 2.5 doesn’t affect just lung cancer, said Alexandra White, PhD, an investigator at the National Institute of Environmental Health Sciences in Research Triangle Park, N.C. “My work, as well as work of others, is increasingly suggesting that air pollution is also related to breast cancer risk, in particular, air pollution that is arising from traffic related forces.” And more research is needed on other cancers, she said. “I think that the lack of findings of other cancer sites reflects a lack of study.”
Other pollutants not analyzed in the ProPublica report are also correlated to cancer risk. In a recent meta-analysis, researcher Stephan Gabet, PhD, PharmD, and colleagues at the University of Grenoble, France, estimated that 3.15% of new breast cancer cases in that country could be attributed to nitrogen dioxide and 2.15% to PM 10.
Sources of nitrogen dioxide, PM 2.5, and PM 10 in France include automobile traffic, inefficient wood-burning stoves, and coal-burning power plants in neighboring countries, Dr. Gabet said.
A good approach to reducing pollution from road traffic is the implementation of low-emission zones that prohibit the most polluting vehicles, he said. But a 2019 United Kingdom government study found that brake wear, tire wear, and road surface wear account for 72% of the PM 10 and 60% of the PM 2.5 pollution from road traffic, suggesting that a transition to electric vehicles won’t fix the problem. Better yet, is “the promotion of active modes like walking, cycling, etc., because like this, you can bring additional health gains due to the increase in physical activity,” he said.
Oncologists can help their patients reduce their exposure to air pollution, Dr. Schiller said. “If you have lung cancer, air pollution will hasten your demise. It makes you sicker. Oncologists should be telling their patients about this and advising them to move away from air pollution if possible, and also making sure they know to monitor the health of the air.”
On days when air pollution is high, patients may want to avoid exercising outdoors, or stay indoors altogether, Dr. Berg said. Air purifiers and N95 masks may also help.
And physicians can make a difference by speaking out in their communities, Dr. Schiller said. She is inviting oncologists to join a new group, Oncologists Understanding for Climate and Health. Through this group or on their own, oncologists can speak to their local legislatures or city councils in support of measures to reduce pollution, she said. “Doctors are trusted messengers.”
Dr. Berg disclosed affiliations with Grail, Mercy BioAnalytics and Lucid Diagnostics.