User login
When the benchwarmer is a slugger
I still, on occasion, use Felbatol (felbamate).
Thirty years since its explosive entrance to the market, then even more explosive collapse, it remains, in my opinion, the most effective of the second generation of anti-seizure medications. Arguably, even more effective than any of the third generation, too.
That’s not to say I use a lot of it. I don’t. It’s like handling unstable dynamite. Tremendous power, but also an above-average degree of risk. Even after things hit the fan with it in the mid-90s, I remember one of my epilepsy clinic attendings telling me, “This is a home-run drug. In refractory patients you might see some benefit by adding another agent, but with this one, you could stop their seizures and hit it out of the park.”
Like most neurologists, I use other epilepsy options first and second line. But sometimes you get the patient who’s failed the usual ones. Then I start to think about Felbatol. I explain the situation to the patients and their families and let them make the final decision. I worry and watch labs very closely for a while. I probably have no more than three to five patients on it in the practice. But when it works, it’s amazing stuff.
Now, let’s jump ahead to 2021. The year of Aduhelm (and several similar agents racing up behind it).
None of these drugs are even close to hitting home runs. For that matter, I’m not convinced they’re even able to get a man on base. To stretch my baseball analogy a bit, imagine watching a game by looking only at the RBI and ERA stats changing. The numbers change slightly, but you have no evidence that either team is winning. Which is, after all, the whole point.
And, to some extent, that’s the basis of Aduhelm’s approval, and likely the same standards its competitors will be held to.
Although they treat different conditions, and are chemically unrelated, the similarities between Felbatol and the currently advancing bunch of monoclonal antibody (MAB) agents for Alzheimer’s disease make an interesting contrast.
Unlike Felbatol’s proven efficacy for epilepsy, the current MABs offer minimal statistically significant clinical benefit for Alzheimer’s disease. At the same time the risk of amyloid-related imaging abnormalities (ARIA) and its complications with them is significantly higher than that of either of Felbatol’s known, potentially lethal, idiosyncratic effects.
With those odds, In medicine, every day is an exercise in working through the risks and benefits of each patient’s individual situation.
As I’ve stated before, I’m not in the grandstand rooting for these Alzheimer’s drugs to fail. I’ve lost a few family members, and certainly my share of patients, to dementia. I’d be thrilled, and more than willing to prescribe it, if something truly effective came along for it.
Nor do I take any kind of pleasure in the recent news that, because of Aduhelm’s failings, around 1,000 Biogen employees will lose their jobs. I feel terrible for them, as most had nothing to do with the decision to forge ahead with the product. More may soon follow at other companies working with similar agents.
Here we are, though, going into 2022. I’m still, albeit rarely, writing for Felbatol 30 years after it came to market for one reason: It works. But it seems pretty unlikely that future neurologists in 2052 will say the same about the current crops of MABs for Alzheimer’s disease.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I still, on occasion, use Felbatol (felbamate).
Thirty years since its explosive entrance to the market, then even more explosive collapse, it remains, in my opinion, the most effective of the second generation of anti-seizure medications. Arguably, even more effective than any of the third generation, too.
That’s not to say I use a lot of it. I don’t. It’s like handling unstable dynamite. Tremendous power, but also an above-average degree of risk. Even after things hit the fan with it in the mid-90s, I remember one of my epilepsy clinic attendings telling me, “This is a home-run drug. In refractory patients you might see some benefit by adding another agent, but with this one, you could stop their seizures and hit it out of the park.”
Like most neurologists, I use other epilepsy options first and second line. But sometimes you get the patient who’s failed the usual ones. Then I start to think about Felbatol. I explain the situation to the patients and their families and let them make the final decision. I worry and watch labs very closely for a while. I probably have no more than three to five patients on it in the practice. But when it works, it’s amazing stuff.
Now, let’s jump ahead to 2021. The year of Aduhelm (and several similar agents racing up behind it).
None of these drugs are even close to hitting home runs. For that matter, I’m not convinced they’re even able to get a man on base. To stretch my baseball analogy a bit, imagine watching a game by looking only at the RBI and ERA stats changing. The numbers change slightly, but you have no evidence that either team is winning. Which is, after all, the whole point.
And, to some extent, that’s the basis of Aduhelm’s approval, and likely the same standards its competitors will be held to.
Although they treat different conditions, and are chemically unrelated, the similarities between Felbatol and the currently advancing bunch of monoclonal antibody (MAB) agents for Alzheimer’s disease make an interesting contrast.
Unlike Felbatol’s proven efficacy for epilepsy, the current MABs offer minimal statistically significant clinical benefit for Alzheimer’s disease. At the same time the risk of amyloid-related imaging abnormalities (ARIA) and its complications with them is significantly higher than that of either of Felbatol’s known, potentially lethal, idiosyncratic effects.
With those odds, In medicine, every day is an exercise in working through the risks and benefits of each patient’s individual situation.
As I’ve stated before, I’m not in the grandstand rooting for these Alzheimer’s drugs to fail. I’ve lost a few family members, and certainly my share of patients, to dementia. I’d be thrilled, and more than willing to prescribe it, if something truly effective came along for it.
Nor do I take any kind of pleasure in the recent news that, because of Aduhelm’s failings, around 1,000 Biogen employees will lose their jobs. I feel terrible for them, as most had nothing to do with the decision to forge ahead with the product. More may soon follow at other companies working with similar agents.
Here we are, though, going into 2022. I’m still, albeit rarely, writing for Felbatol 30 years after it came to market for one reason: It works. But it seems pretty unlikely that future neurologists in 2052 will say the same about the current crops of MABs for Alzheimer’s disease.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I still, on occasion, use Felbatol (felbamate).
Thirty years since its explosive entrance to the market, then even more explosive collapse, it remains, in my opinion, the most effective of the second generation of anti-seizure medications. Arguably, even more effective than any of the third generation, too.
That’s not to say I use a lot of it. I don’t. It’s like handling unstable dynamite. Tremendous power, but also an above-average degree of risk. Even after things hit the fan with it in the mid-90s, I remember one of my epilepsy clinic attendings telling me, “This is a home-run drug. In refractory patients you might see some benefit by adding another agent, but with this one, you could stop their seizures and hit it out of the park.”
Like most neurologists, I use other epilepsy options first and second line. But sometimes you get the patient who’s failed the usual ones. Then I start to think about Felbatol. I explain the situation to the patients and their families and let them make the final decision. I worry and watch labs very closely for a while. I probably have no more than three to five patients on it in the practice. But when it works, it’s amazing stuff.
Now, let’s jump ahead to 2021. The year of Aduhelm (and several similar agents racing up behind it).
None of these drugs are even close to hitting home runs. For that matter, I’m not convinced they’re even able to get a man on base. To stretch my baseball analogy a bit, imagine watching a game by looking only at the RBI and ERA stats changing. The numbers change slightly, but you have no evidence that either team is winning. Which is, after all, the whole point.
And, to some extent, that’s the basis of Aduhelm’s approval, and likely the same standards its competitors will be held to.
Although they treat different conditions, and are chemically unrelated, the similarities between Felbatol and the currently advancing bunch of monoclonal antibody (MAB) agents for Alzheimer’s disease make an interesting contrast.
Unlike Felbatol’s proven efficacy for epilepsy, the current MABs offer minimal statistically significant clinical benefit for Alzheimer’s disease. At the same time the risk of amyloid-related imaging abnormalities (ARIA) and its complications with them is significantly higher than that of either of Felbatol’s known, potentially lethal, idiosyncratic effects.
With those odds, In medicine, every day is an exercise in working through the risks and benefits of each patient’s individual situation.
As I’ve stated before, I’m not in the grandstand rooting for these Alzheimer’s drugs to fail. I’ve lost a few family members, and certainly my share of patients, to dementia. I’d be thrilled, and more than willing to prescribe it, if something truly effective came along for it.
Nor do I take any kind of pleasure in the recent news that, because of Aduhelm’s failings, around 1,000 Biogen employees will lose their jobs. I feel terrible for them, as most had nothing to do with the decision to forge ahead with the product. More may soon follow at other companies working with similar agents.
Here we are, though, going into 2022. I’m still, albeit rarely, writing for Felbatol 30 years after it came to market for one reason: It works. But it seems pretty unlikely that future neurologists in 2052 will say the same about the current crops of MABs for Alzheimer’s disease.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Treatment of opioid use disorder in hospitalized patients
An opportunity for impact
Case
A 35-year-old woman with opioid use disorder (OUD) presents with fever, left arm redness, and swelling. She is admitted to the hospital for cellulitis treatment. On the day after admission she becomes agitated and develops nausea, diarrhea, and generalized pain. Opioid withdrawal is suspected. How should her opioid use be addressed while in the hospital?
Brief overview of the issue
Since 1999, there have been more than 800,000 deaths related to drug overdose in the United States, and in 2019 more than 70% of drug overdose deaths involved an opioid.1,2 Although effective treatments for OUD exist, less than 20% of those with OUD are engaged in treatment.3
In America, 4%-11% of hospitalized patients have OUD. Hospitalized patients with OUD often experience stigma surrounding their disease, and many inpatient clinicians lack knowledge regarding the care of patients with OUD. As a result, withdrawal symptoms may go untreated, which can erode trust in the medical system and contribute to patients’ leaving the hospital before their primary medical issue is fully addressed. Therefore, it is essential that inpatient clinicians be familiar with the management of this complex and vulnerable patient population. Initiating treatment for OUD in the hospital setting is feasible and effective, and can lead to increased engagement in OUD treatment even after the hospital stay.
Overview of the data
Assessing patients with suspected OUD
Assessment for OUD starts with an in-depth opioid use history including frequency, amount, and method of administration. Clinicians should gather information regarding use of other substances or nonprescribed medications, and take thorough psychiatric and social histories. A formal diagnosis of OUD can be made using the Fifth Edition Diagnostic and Statistical Manual for Mental Disorders (DSM-5) diagnostic criteria.
Recognizing and managing opioid withdrawal
OUD in hospitalized patients often becomes apparent when patients develop signs and symptoms of withdrawal. Decreasing physical discomfort related to withdrawal can allow inpatient clinicians to address the condition for which the patient was hospitalized, help to strengthen the patient-clinician relationship, and provide an opportunity to discuss long-term OUD treatment.
Signs and symptoms of opioid withdrawal include anxiety, restlessness, irritability, generalized pain, rhinorrhea, yawning, lacrimation, piloerection, anorexia, and nausea. Withdrawal can last days to weeks, depending on the half-life of the opioid that was used. Opioids with shorter half-lives, such as heroin or oxycodone, cause withdrawal with earlier onset and shorter duration than do opioids with longer half-lives, such as methadone. The degree of withdrawal can be quantified with validated tools, such as the Clinical Opiate Withdrawal Scale (COWS).
Treatment of opioid withdrawal should generally include the use of an opioid agonist such as methadone or buprenorphine. A 2017 Cochrane meta-analysis found methadone or buprenorphine to be more effective than clonidine in alleviating symptoms of withdrawal and in retaining patients in treatment.4 Clonidine, an alpha2-adrenergic agonist that binds to receptors in the locus coeruleus, does not alleviate opioid cravings, but may be used as an adjunctive treatment for associated autonomic withdrawal symptoms. Other adjunctive medications include analgesics, antiemetics, antidiarrheals, and antihistamines.
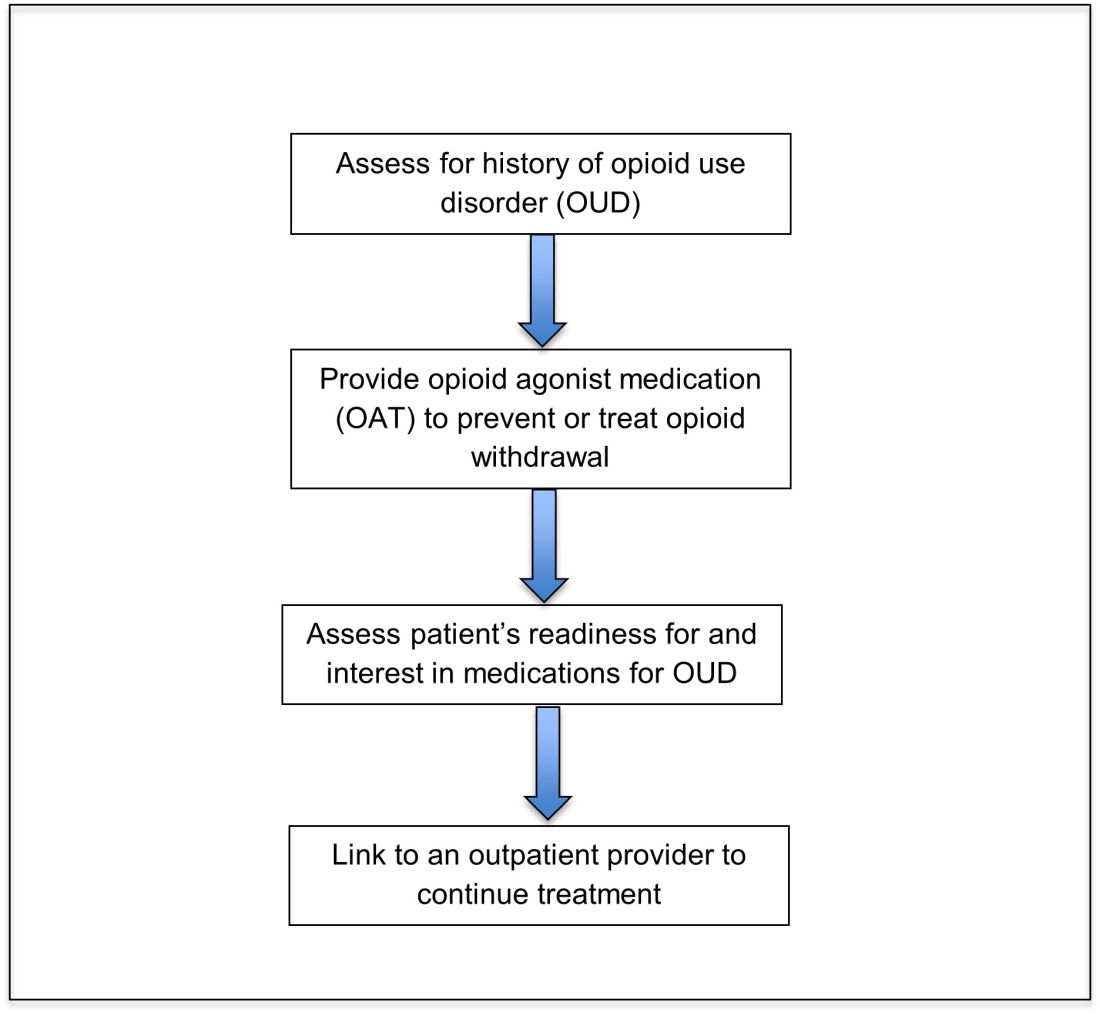
Opioid agonist treatment for opioid use disorder
Opioid agonist treatment (OAT) with methadone or buprenorphine is associated with decreased mortality, opioid use, and infectious complications, but remains underutilized.5 Hospitalized patients with OUD are frequently managed with a rapid opioid detoxification and then discharged without continued OUD treatment. Detoxification alone can lead to a relapse rate as high as 90%.6 Patients are at increased risk for overdose after withdrawal due to loss of tolerance. Inpatient clinicians can close this OUD treatment gap by familiarizing themselves with OAT and offering to initiate OAT for maintenance treatment in interested patients. In one study, patients started on buprenorphine while hospitalized were more likely to be engaged in treatment and less likely to report drug use at follow-up, compared to patients who were referred without starting the medication.7
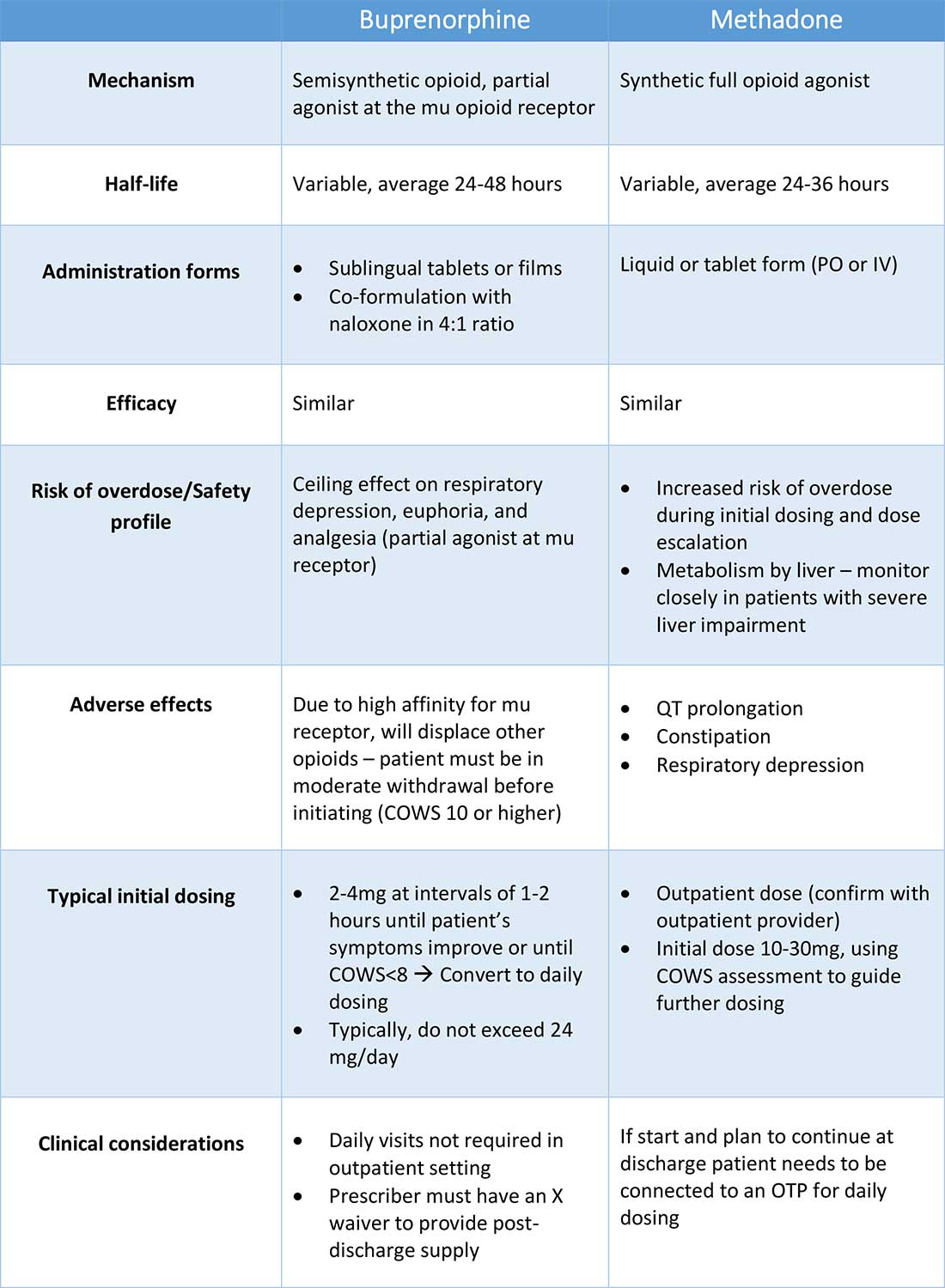
Buprenorphine
Buprenorphine is a partial agonist at the mu opioid receptor that can be ordered in the inpatient setting by any clinician. In the outpatient setting only DATA 2000 waivered clinicians can prescribe buprenorphine.8 Buprenorphine is most commonly coformulated with naloxone, an opioid antagonist, and is available in sublingual films or tablets. The naloxone component is not bioavailable when taken sublingually but becomes bioavailable if the drug is injected intravenously, leading to acute withdrawal.
Buprenorphine has a higher affinity for the mu opioid receptor than most opioids. If administered while other opioids are still present, it will displace the other opioid from the receptor but only partially stimulate the receptor, which can cause precipitated withdrawal. Buprenorphine initiation can start when the COWS score reflects moderate withdrawal. Many institutions use a threshold of 8-12 on the COWS scale. Typical dosing is 2-4 mg of buprenorphine at intervals of 1-2 hours as needed until the COWS score is less than 8, up to a maximum of 16 mg on day 1. The total dose from day 1 may be given as a daily dose beginning on day 2, up to a maximum total daily dose of 24 mg.
In recent years, a method of initiating buprenorphine called “micro-dosing” has gained traction. Very small doses of buprenorphine are given while a patient is receiving other opioids, thereby reducing the risk of precipitated withdrawal. This method can be helpful for patients who cannot tolerate withdrawal or who have recently taken long-acting opioids such as methadone. Such protocols should be utilized only at centers where consultation with an addiction specialist or experienced clinician is possible.
Despite evidence of buprenorphine’s efficacy, there are barriers to prescribing it. Physicians and advanced practitioners must be granted a waiver from the Drug Enforcement Administration to prescribe buprenorphine to outpatients. As of 2017, less than 10% of primary care physicians had obtained waivers.9 However, inpatient clinicians without a waiver can order buprenorphine and initiate treatment. Best practice is to do so with a specific plan for continuation at discharge. We encourage inpatient clinicians to obtain a waiver, so that a prescription can be given at discharge to bridge the patient to a first appointment with a community clinician who can continue treatment. As of April 27, 2021, providers treating fewer than 30 patients with OUD at one time may obtain a waiver without additional training.10
Methadone
Methadone is a full agonist at the mu opioid receptor. In the hospital setting, methadone can be ordered by any clinician to prevent and treat withdrawal. Commonly, doses of 10 mg can be given using the COWS score to guide the need for additional dosing. The patient can be reassessed every 1-2 hours to ensure that symptoms are improving, and that there is no sign of oversedation before giving additional methadone. For most patients, withdrawal can be managed with 20-40 mg of methadone daily.
In contrast to buprenorphine, methadone will not precipitate withdrawal and can be initiated even when patients are not yet showing withdrawal symptoms. Outpatient methadone treatment for OUD is federally regulated and can be delivered only in opioid treatment programs (OTPs).
Choosing methadone or buprenorphine in the inpatient setting
The choice between buprenorphine and methadone should take into consideration several factors, including patient preference, treatment history, and available outpatient treatment programs, which may vary widely by geographic region. Some patients benefit from the higher level of support and counseling available at OTPs. Methadone is available at all OTPs, and the availability of buprenorphine in this setting is increasing. Other patients may prefer the convenience and flexibility of buprenorphine treatment in an outpatient office setting.
Some patients have prior negative experiences with OAT. These can include prior precipitated withdrawal with buprenorphine induction, or negative experiences with the structure of OTPs. Clinicians are encouraged to provide counseling if patients have a history of precipitated withdrawal to assure them that this can be avoided with proper dosing. Clinicians should be familiar with available treatment options in their community and can refer to the Substance Abuse and Mental Health Services Administration (SAMHSA) website to locate OTPs and buprenorphine prescribers.
Polypharmacy and safety
If combined with benzodiazepines, alcohol, or other sedating agents, methadone or buprenorphine can increase risk of overdose. However, OUD treatment should not be withheld because of other substance use. Clinicians initiating treatment should counsel patients on the risk of concomitant substance use and provide overdose prevention education.
A brief note on naltrexone
Naltrexone, an opioid antagonist, is used more commonly in outpatient addiction treatment than in the inpatient setting, but inpatient clinicians should be aware of its use. It is available in oral and long-acting injectable formulations. Its utility in the inpatient setting may be limited as safe administration requires 7-10 days of opioid abstinence.
Discharge planning
Patients with OUD or who are started on OAT during a hospitalization should be linked to continued outpatient treatment. Before discharge it is best to ensure vaccinations for HAV, HBV, pneumococcus, and tetanus are up to date, and perform screening for HIV, hepatitis C, tuberculosis, and sexually transmitted infections if appropriate. All patients with OUD should be prescribed or provided with take-home naloxone for overdose reversal. Patients can also be referred to syringe service programs for additional harm reduction counseling and services.
Application of the data to our patient
For our patient, either methadone or buprenorphine could be used to treat her withdrawal. The COWS score should be used to assess withdrawal severity, and to guide appropriate timing of medication initiation. If she wishes to continue OAT after discharge, she should be linked to a clinician who can engage her in ongoing medical care. Prior to discharge she should also receive relevant vaccines and screening for infectious diseases as outlined above, as well as take-home naloxone (or a prescription).
Bottom line
Inpatient clinicians can play a pivotal role in patients’ lives by ensuring that patients with OUD receive OAT and are connected to outpatient care at discharge.
Dr. Linker is assistant professor in the division of hospital medicine, Icahn School of Medicine at Mount Sinai, New York. Ms. Hirt, Mr. Fine, and Mr. Villasanivis are medical students at the Icahn School of Medicine at Mount Sinai. Dr. Wang is assistant professor in the division of general internal medicine, Icahn School of Medicine at Mount Sinai. Dr. Herscher is assistant professor in the division of hospital medicine, Icahn School of Medicine at Mount Sinai.
References
1. Wide-ranging online data for epidemiologic research (WONDER). Atlanta, GA: CDC, National Center for Health Statistics; 2020. Available at http://wonder.cdc.gov.
2. Mattson CL et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths – United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70:202-7. doi: 10.15585/mmwr.mm7006a4.
3. Wakeman SE et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020 Feb 5;3(2):e1920622. doi: 10.1001/jamanetworkopen.2019.20622.
4. Gowing L et al. Buprenorphine for managing opioid withdrawal. Cochrane Database Syst Rev. 2017 Feb;2017(2):CD002025. doi: 10.1002/14651858.CD002025.pub5.
5. Sordo L et al. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ. 2017 Apr 26;357:j1550. doi: 10.1136/bmj.j1550.
6. Smyth BP et al. Lapse and relapse following inpatient treatment of opiate dependence. Ir Med J. 2010 Jun;103(6):176-9. Available at www.drugsandalcohol.ie/13405.
7. Liebschutz JM. Buprenorphine treatment for hospitalized, opioid-dependent patients: A randomized clinical trial. JAMA Intern Med. 2014 Aug;174(8):1369-76. doi: 10.1001/jamainternmed.2014.2556.
8. Substance Abuse and Mental Health Services Administration. (Aug 20, 2020) Statutes, Regulations, and Guidelines.
9. McBain RK et al. Growth and distribution of buprenorphine-waivered providers in the United States, 2007-2017. Ann Intern Med. 2020;172(7):504-6. doi: 10.7326/M19-2403.
10. HHS releases new buprenorphine practice guidelines, expanding access to treatment for opioid use disorder. Apr 27, 2021.
11. Herscher M et al. Diagnosis and management of opioid use disorder in hospitalized patients. Med Clin North Am. 2020 Jul;104(4):695-708. doi: 10.1016/j.mcna.2020.03.003.
Additional reading
Winetsky D. Expanding treatment opportunities for hospitalized patients with opioid use disorders. J Hosp Med. 2018 Jan;13(1):62-4. doi: 10.12788/jhm.2861.
Donroe JH. Caring for patients with opioid use disorder in the hospital. Can Med Assoc J. 2016 Dec 6;188(17-18):1232-9. doi: 10.1503/cmaj.160290.
Herscher M et al. Diagnosis and management of opioid use disorder in hospitalized patients. Med Clin North Am. 2020 Jul;104(4):695-708. doi: 10.1016/j.mcna.2020.03.003.
Key points
- Most patients with OUD are not engaged in evidence-based treatment. Clinicians have an opportunity to utilize the inpatient stay as a ‘reachable moment’ to engage patients with OUD in evidence-based treatment.
- Buprenorphine and methadone are effective opioid agonist medications used to treat OUD, and clinicians with the appropriate knowledge base can initiate either during the inpatient encounter, and link the patient to OUD treatment after the hospital stay.
Quiz
Caring for hospitalized patients with OUD
Most patients with OUD are not engaged in effective treatment. Hospitalization can be a ‘reachable moment’ to engage patients with OUD in evidence-based treatment.
1. Which is an effective and evidence-based medication for treating opioid withdrawal and OUD?
a) Naltrexone.
b) Buprenorphine.
c) Opioid detoxification.
d) Clonidine.
Explanation: Buprenorphine is effective for alleviating symptoms of withdrawal as well as for the long-term treatment of OUD. While naltrexone is also used to treat OUD, it is not useful for treating withdrawal. Clonidine can be a useful adjunctive medication for treating withdrawal but is not a long-term treatment for OUD. Nonpharmacologic detoxification is not an effective treatment for OUD and is associated with high relapse rates.
2. What scale can be used during a hospital stay to monitor patients with OUD at risk of opioid withdrawal, and to aid in buprenorphine initiation?
a) CIWA score.
b) PADUA score.
c) COWS score.
d) 4T score.
Explanation: COWS is the “clinical opiate withdrawal scale.” The COWS score should be calculated by a trained provider, and includes objective parameters (such as pulse) and subjective symptoms (such as GI upset, bone/joint aches.) It is recommended that agonist therapy be started when the COWS score is consistent with moderate withdrawal.
3. How can clinicians reliably find out if there are outpatient resources/clinics for patients with OUD in their area?
a) No way to find this out without personal knowledge.
b) Hospital providers and patients can visit www.samhsa.gov/find-help/national-helpline or call 1-800-662-HELP (4357) to find options for treatment for substance use disorders in their areas.
c) Dial “0” on any phone and ask.
d) Ask around at your hospital.
Explanation: The Substance Abuse and Mental Health Services Administration (SAMHSA) is an agency in the U.S. Department of Health and Human Services that is engaged in public health efforts to reduce the impact of substance abuse and mental illness on local communities. The agency’s website has helpful information about resources for substance use treatment.
4. Patients with OUD should be prescribed and given training about what medication that can be lifesaving when given during an opioid overdose?
a) Aspirin.
b) Naloxone.
c) Naltrexone.
d) Clonidine.
Explanation: Naloxone can be life-saving in the setting of an overdose. Best practice is to provide naloxone and training to patients with OUD.
5. When patients take buprenorphine soon after taking other opioids, there is concern for the development of which reaction:
a) Precipitated withdrawal.
b) Opioid overdose.
c) Allergic reaction.
d) Intoxication.
Explanation: Administering buprenorphine soon after taking other opioids can cause precipitated withdrawal, as buprenorphine binds with higher affinity to the mu receptor than many opioids. Precipitated withdrawal causes severe discomfort and can be dangerous for patients.
An opportunity for impact
An opportunity for impact
Case
A 35-year-old woman with opioid use disorder (OUD) presents with fever, left arm redness, and swelling. She is admitted to the hospital for cellulitis treatment. On the day after admission she becomes agitated and develops nausea, diarrhea, and generalized pain. Opioid withdrawal is suspected. How should her opioid use be addressed while in the hospital?
Brief overview of the issue
Since 1999, there have been more than 800,000 deaths related to drug overdose in the United States, and in 2019 more than 70% of drug overdose deaths involved an opioid.1,2 Although effective treatments for OUD exist, less than 20% of those with OUD are engaged in treatment.3
In America, 4%-11% of hospitalized patients have OUD. Hospitalized patients with OUD often experience stigma surrounding their disease, and many inpatient clinicians lack knowledge regarding the care of patients with OUD. As a result, withdrawal symptoms may go untreated, which can erode trust in the medical system and contribute to patients’ leaving the hospital before their primary medical issue is fully addressed. Therefore, it is essential that inpatient clinicians be familiar with the management of this complex and vulnerable patient population. Initiating treatment for OUD in the hospital setting is feasible and effective, and can lead to increased engagement in OUD treatment even after the hospital stay.
Overview of the data
Assessing patients with suspected OUD
Assessment for OUD starts with an in-depth opioid use history including frequency, amount, and method of administration. Clinicians should gather information regarding use of other substances or nonprescribed medications, and take thorough psychiatric and social histories. A formal diagnosis of OUD can be made using the Fifth Edition Diagnostic and Statistical Manual for Mental Disorders (DSM-5) diagnostic criteria.
Recognizing and managing opioid withdrawal
OUD in hospitalized patients often becomes apparent when patients develop signs and symptoms of withdrawal. Decreasing physical discomfort related to withdrawal can allow inpatient clinicians to address the condition for which the patient was hospitalized, help to strengthen the patient-clinician relationship, and provide an opportunity to discuss long-term OUD treatment.
Signs and symptoms of opioid withdrawal include anxiety, restlessness, irritability, generalized pain, rhinorrhea, yawning, lacrimation, piloerection, anorexia, and nausea. Withdrawal can last days to weeks, depending on the half-life of the opioid that was used. Opioids with shorter half-lives, such as heroin or oxycodone, cause withdrawal with earlier onset and shorter duration than do opioids with longer half-lives, such as methadone. The degree of withdrawal can be quantified with validated tools, such as the Clinical Opiate Withdrawal Scale (COWS).
Treatment of opioid withdrawal should generally include the use of an opioid agonist such as methadone or buprenorphine. A 2017 Cochrane meta-analysis found methadone or buprenorphine to be more effective than clonidine in alleviating symptoms of withdrawal and in retaining patients in treatment.4 Clonidine, an alpha2-adrenergic agonist that binds to receptors in the locus coeruleus, does not alleviate opioid cravings, but may be used as an adjunctive treatment for associated autonomic withdrawal symptoms. Other adjunctive medications include analgesics, antiemetics, antidiarrheals, and antihistamines.

Opioid agonist treatment for opioid use disorder
Opioid agonist treatment (OAT) with methadone or buprenorphine is associated with decreased mortality, opioid use, and infectious complications, but remains underutilized.5 Hospitalized patients with OUD are frequently managed with a rapid opioid detoxification and then discharged without continued OUD treatment. Detoxification alone can lead to a relapse rate as high as 90%.6 Patients are at increased risk for overdose after withdrawal due to loss of tolerance. Inpatient clinicians can close this OUD treatment gap by familiarizing themselves with OAT and offering to initiate OAT for maintenance treatment in interested patients. In one study, patients started on buprenorphine while hospitalized were more likely to be engaged in treatment and less likely to report drug use at follow-up, compared to patients who were referred without starting the medication.7
Buprenorphine
Buprenorphine is a partial agonist at the mu opioid receptor that can be ordered in the inpatient setting by any clinician. In the outpatient setting only DATA 2000 waivered clinicians can prescribe buprenorphine.8 Buprenorphine is most commonly coformulated with naloxone, an opioid antagonist, and is available in sublingual films or tablets. The naloxone component is not bioavailable when taken sublingually but becomes bioavailable if the drug is injected intravenously, leading to acute withdrawal.
Buprenorphine has a higher affinity for the mu opioid receptor than most opioids. If administered while other opioids are still present, it will displace the other opioid from the receptor but only partially stimulate the receptor, which can cause precipitated withdrawal. Buprenorphine initiation can start when the COWS score reflects moderate withdrawal. Many institutions use a threshold of 8-12 on the COWS scale. Typical dosing is 2-4 mg of buprenorphine at intervals of 1-2 hours as needed until the COWS score is less than 8, up to a maximum of 16 mg on day 1. The total dose from day 1 may be given as a daily dose beginning on day 2, up to a maximum total daily dose of 24 mg.
In recent years, a method of initiating buprenorphine called “micro-dosing” has gained traction. Very small doses of buprenorphine are given while a patient is receiving other opioids, thereby reducing the risk of precipitated withdrawal. This method can be helpful for patients who cannot tolerate withdrawal or who have recently taken long-acting opioids such as methadone. Such protocols should be utilized only at centers where consultation with an addiction specialist or experienced clinician is possible.
Despite evidence of buprenorphine’s efficacy, there are barriers to prescribing it. Physicians and advanced practitioners must be granted a waiver from the Drug Enforcement Administration to prescribe buprenorphine to outpatients. As of 2017, less than 10% of primary care physicians had obtained waivers.9 However, inpatient clinicians without a waiver can order buprenorphine and initiate treatment. Best practice is to do so with a specific plan for continuation at discharge. We encourage inpatient clinicians to obtain a waiver, so that a prescription can be given at discharge to bridge the patient to a first appointment with a community clinician who can continue treatment. As of April 27, 2021, providers treating fewer than 30 patients with OUD at one time may obtain a waiver without additional training.10
Methadone
Methadone is a full agonist at the mu opioid receptor. In the hospital setting, methadone can be ordered by any clinician to prevent and treat withdrawal. Commonly, doses of 10 mg can be given using the COWS score to guide the need for additional dosing. The patient can be reassessed every 1-2 hours to ensure that symptoms are improving, and that there is no sign of oversedation before giving additional methadone. For most patients, withdrawal can be managed with 20-40 mg of methadone daily.
In contrast to buprenorphine, methadone will not precipitate withdrawal and can be initiated even when patients are not yet showing withdrawal symptoms. Outpatient methadone treatment for OUD is federally regulated and can be delivered only in opioid treatment programs (OTPs).
Choosing methadone or buprenorphine in the inpatient setting
The choice between buprenorphine and methadone should take into consideration several factors, including patient preference, treatment history, and available outpatient treatment programs, which may vary widely by geographic region. Some patients benefit from the higher level of support and counseling available at OTPs. Methadone is available at all OTPs, and the availability of buprenorphine in this setting is increasing. Other patients may prefer the convenience and flexibility of buprenorphine treatment in an outpatient office setting.
Some patients have prior negative experiences with OAT. These can include prior precipitated withdrawal with buprenorphine induction, or negative experiences with the structure of OTPs. Clinicians are encouraged to provide counseling if patients have a history of precipitated withdrawal to assure them that this can be avoided with proper dosing. Clinicians should be familiar with available treatment options in their community and can refer to the Substance Abuse and Mental Health Services Administration (SAMHSA) website to locate OTPs and buprenorphine prescribers.

Polypharmacy and safety
If combined with benzodiazepines, alcohol, or other sedating agents, methadone or buprenorphine can increase risk of overdose. However, OUD treatment should not be withheld because of other substance use. Clinicians initiating treatment should counsel patients on the risk of concomitant substance use and provide overdose prevention education.
A brief note on naltrexone
Naltrexone, an opioid antagonist, is used more commonly in outpatient addiction treatment than in the inpatient setting, but inpatient clinicians should be aware of its use. It is available in oral and long-acting injectable formulations. Its utility in the inpatient setting may be limited as safe administration requires 7-10 days of opioid abstinence.
Discharge planning
Patients with OUD or who are started on OAT during a hospitalization should be linked to continued outpatient treatment. Before discharge it is best to ensure vaccinations for HAV, HBV, pneumococcus, and tetanus are up to date, and perform screening for HIV, hepatitis C, tuberculosis, and sexually transmitted infections if appropriate. All patients with OUD should be prescribed or provided with take-home naloxone for overdose reversal. Patients can also be referred to syringe service programs for additional harm reduction counseling and services.
Application of the data to our patient
For our patient, either methadone or buprenorphine could be used to treat her withdrawal. The COWS score should be used to assess withdrawal severity, and to guide appropriate timing of medication initiation. If she wishes to continue OAT after discharge, she should be linked to a clinician who can engage her in ongoing medical care. Prior to discharge she should also receive relevant vaccines and screening for infectious diseases as outlined above, as well as take-home naloxone (or a prescription).
Bottom line
Inpatient clinicians can play a pivotal role in patients’ lives by ensuring that patients with OUD receive OAT and are connected to outpatient care at discharge.
Dr. Linker is assistant professor in the division of hospital medicine, Icahn School of Medicine at Mount Sinai, New York. Ms. Hirt, Mr. Fine, and Mr. Villasanivis are medical students at the Icahn School of Medicine at Mount Sinai. Dr. Wang is assistant professor in the division of general internal medicine, Icahn School of Medicine at Mount Sinai. Dr. Herscher is assistant professor in the division of hospital medicine, Icahn School of Medicine at Mount Sinai.
References
1. Wide-ranging online data for epidemiologic research (WONDER). Atlanta, GA: CDC, National Center for Health Statistics; 2020. Available at http://wonder.cdc.gov.
2. Mattson CL et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths – United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70:202-7. doi: 10.15585/mmwr.mm7006a4.
3. Wakeman SE et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020 Feb 5;3(2):e1920622. doi: 10.1001/jamanetworkopen.2019.20622.
4. Gowing L et al. Buprenorphine for managing opioid withdrawal. Cochrane Database Syst Rev. 2017 Feb;2017(2):CD002025. doi: 10.1002/14651858.CD002025.pub5.
5. Sordo L et al. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ. 2017 Apr 26;357:j1550. doi: 10.1136/bmj.j1550.
6. Smyth BP et al. Lapse and relapse following inpatient treatment of opiate dependence. Ir Med J. 2010 Jun;103(6):176-9. Available at www.drugsandalcohol.ie/13405.
7. Liebschutz JM. Buprenorphine treatment for hospitalized, opioid-dependent patients: A randomized clinical trial. JAMA Intern Med. 2014 Aug;174(8):1369-76. doi: 10.1001/jamainternmed.2014.2556.
8. Substance Abuse and Mental Health Services Administration. (Aug 20, 2020) Statutes, Regulations, and Guidelines.
9. McBain RK et al. Growth and distribution of buprenorphine-waivered providers in the United States, 2007-2017. Ann Intern Med. 2020;172(7):504-6. doi: 10.7326/M19-2403.
10. HHS releases new buprenorphine practice guidelines, expanding access to treatment for opioid use disorder. Apr 27, 2021.
11. Herscher M et al. Diagnosis and management of opioid use disorder in hospitalized patients. Med Clin North Am. 2020 Jul;104(4):695-708. doi: 10.1016/j.mcna.2020.03.003.
Additional reading
Winetsky D. Expanding treatment opportunities for hospitalized patients with opioid use disorders. J Hosp Med. 2018 Jan;13(1):62-4. doi: 10.12788/jhm.2861.
Donroe JH. Caring for patients with opioid use disorder in the hospital. Can Med Assoc J. 2016 Dec 6;188(17-18):1232-9. doi: 10.1503/cmaj.160290.
Herscher M et al. Diagnosis and management of opioid use disorder in hospitalized patients. Med Clin North Am. 2020 Jul;104(4):695-708. doi: 10.1016/j.mcna.2020.03.003.
Key points
- Most patients with OUD are not engaged in evidence-based treatment. Clinicians have an opportunity to utilize the inpatient stay as a ‘reachable moment’ to engage patients with OUD in evidence-based treatment.
- Buprenorphine and methadone are effective opioid agonist medications used to treat OUD, and clinicians with the appropriate knowledge base can initiate either during the inpatient encounter, and link the patient to OUD treatment after the hospital stay.
Quiz
Caring for hospitalized patients with OUD
Most patients with OUD are not engaged in effective treatment. Hospitalization can be a ‘reachable moment’ to engage patients with OUD in evidence-based treatment.
1. Which is an effective and evidence-based medication for treating opioid withdrawal and OUD?
a) Naltrexone.
b) Buprenorphine.
c) Opioid detoxification.
d) Clonidine.
Explanation: Buprenorphine is effective for alleviating symptoms of withdrawal as well as for the long-term treatment of OUD. While naltrexone is also used to treat OUD, it is not useful for treating withdrawal. Clonidine can be a useful adjunctive medication for treating withdrawal but is not a long-term treatment for OUD. Nonpharmacologic detoxification is not an effective treatment for OUD and is associated with high relapse rates.
2. What scale can be used during a hospital stay to monitor patients with OUD at risk of opioid withdrawal, and to aid in buprenorphine initiation?
a) CIWA score.
b) PADUA score.
c) COWS score.
d) 4T score.
Explanation: COWS is the “clinical opiate withdrawal scale.” The COWS score should be calculated by a trained provider, and includes objective parameters (such as pulse) and subjective symptoms (such as GI upset, bone/joint aches.) It is recommended that agonist therapy be started when the COWS score is consistent with moderate withdrawal.
3. How can clinicians reliably find out if there are outpatient resources/clinics for patients with OUD in their area?
a) No way to find this out without personal knowledge.
b) Hospital providers and patients can visit www.samhsa.gov/find-help/national-helpline or call 1-800-662-HELP (4357) to find options for treatment for substance use disorders in their areas.
c) Dial “0” on any phone and ask.
d) Ask around at your hospital.
Explanation: The Substance Abuse and Mental Health Services Administration (SAMHSA) is an agency in the U.S. Department of Health and Human Services that is engaged in public health efforts to reduce the impact of substance abuse and mental illness on local communities. The agency’s website has helpful information about resources for substance use treatment.
4. Patients with OUD should be prescribed and given training about what medication that can be lifesaving when given during an opioid overdose?
a) Aspirin.
b) Naloxone.
c) Naltrexone.
d) Clonidine.
Explanation: Naloxone can be life-saving in the setting of an overdose. Best practice is to provide naloxone and training to patients with OUD.
5. When patients take buprenorphine soon after taking other opioids, there is concern for the development of which reaction:
a) Precipitated withdrawal.
b) Opioid overdose.
c) Allergic reaction.
d) Intoxication.
Explanation: Administering buprenorphine soon after taking other opioids can cause precipitated withdrawal, as buprenorphine binds with higher affinity to the mu receptor than many opioids. Precipitated withdrawal causes severe discomfort and can be dangerous for patients.
Case
A 35-year-old woman with opioid use disorder (OUD) presents with fever, left arm redness, and swelling. She is admitted to the hospital for cellulitis treatment. On the day after admission she becomes agitated and develops nausea, diarrhea, and generalized pain. Opioid withdrawal is suspected. How should her opioid use be addressed while in the hospital?
Brief overview of the issue
Since 1999, there have been more than 800,000 deaths related to drug overdose in the United States, and in 2019 more than 70% of drug overdose deaths involved an opioid.1,2 Although effective treatments for OUD exist, less than 20% of those with OUD are engaged in treatment.3
In America, 4%-11% of hospitalized patients have OUD. Hospitalized patients with OUD often experience stigma surrounding their disease, and many inpatient clinicians lack knowledge regarding the care of patients with OUD. As a result, withdrawal symptoms may go untreated, which can erode trust in the medical system and contribute to patients’ leaving the hospital before their primary medical issue is fully addressed. Therefore, it is essential that inpatient clinicians be familiar with the management of this complex and vulnerable patient population. Initiating treatment for OUD in the hospital setting is feasible and effective, and can lead to increased engagement in OUD treatment even after the hospital stay.
Overview of the data
Assessing patients with suspected OUD
Assessment for OUD starts with an in-depth opioid use history including frequency, amount, and method of administration. Clinicians should gather information regarding use of other substances or nonprescribed medications, and take thorough psychiatric and social histories. A formal diagnosis of OUD can be made using the Fifth Edition Diagnostic and Statistical Manual for Mental Disorders (DSM-5) diagnostic criteria.
Recognizing and managing opioid withdrawal
OUD in hospitalized patients often becomes apparent when patients develop signs and symptoms of withdrawal. Decreasing physical discomfort related to withdrawal can allow inpatient clinicians to address the condition for which the patient was hospitalized, help to strengthen the patient-clinician relationship, and provide an opportunity to discuss long-term OUD treatment.
Signs and symptoms of opioid withdrawal include anxiety, restlessness, irritability, generalized pain, rhinorrhea, yawning, lacrimation, piloerection, anorexia, and nausea. Withdrawal can last days to weeks, depending on the half-life of the opioid that was used. Opioids with shorter half-lives, such as heroin or oxycodone, cause withdrawal with earlier onset and shorter duration than do opioids with longer half-lives, such as methadone. The degree of withdrawal can be quantified with validated tools, such as the Clinical Opiate Withdrawal Scale (COWS).
Treatment of opioid withdrawal should generally include the use of an opioid agonist such as methadone or buprenorphine. A 2017 Cochrane meta-analysis found methadone or buprenorphine to be more effective than clonidine in alleviating symptoms of withdrawal and in retaining patients in treatment.4 Clonidine, an alpha2-adrenergic agonist that binds to receptors in the locus coeruleus, does not alleviate opioid cravings, but may be used as an adjunctive treatment for associated autonomic withdrawal symptoms. Other adjunctive medications include analgesics, antiemetics, antidiarrheals, and antihistamines.

Opioid agonist treatment for opioid use disorder
Opioid agonist treatment (OAT) with methadone or buprenorphine is associated with decreased mortality, opioid use, and infectious complications, but remains underutilized.5 Hospitalized patients with OUD are frequently managed with a rapid opioid detoxification and then discharged without continued OUD treatment. Detoxification alone can lead to a relapse rate as high as 90%.6 Patients are at increased risk for overdose after withdrawal due to loss of tolerance. Inpatient clinicians can close this OUD treatment gap by familiarizing themselves with OAT and offering to initiate OAT for maintenance treatment in interested patients. In one study, patients started on buprenorphine while hospitalized were more likely to be engaged in treatment and less likely to report drug use at follow-up, compared to patients who were referred without starting the medication.7
Buprenorphine
Buprenorphine is a partial agonist at the mu opioid receptor that can be ordered in the inpatient setting by any clinician. In the outpatient setting only DATA 2000 waivered clinicians can prescribe buprenorphine.8 Buprenorphine is most commonly coformulated with naloxone, an opioid antagonist, and is available in sublingual films or tablets. The naloxone component is not bioavailable when taken sublingually but becomes bioavailable if the drug is injected intravenously, leading to acute withdrawal.
Buprenorphine has a higher affinity for the mu opioid receptor than most opioids. If administered while other opioids are still present, it will displace the other opioid from the receptor but only partially stimulate the receptor, which can cause precipitated withdrawal. Buprenorphine initiation can start when the COWS score reflects moderate withdrawal. Many institutions use a threshold of 8-12 on the COWS scale. Typical dosing is 2-4 mg of buprenorphine at intervals of 1-2 hours as needed until the COWS score is less than 8, up to a maximum of 16 mg on day 1. The total dose from day 1 may be given as a daily dose beginning on day 2, up to a maximum total daily dose of 24 mg.
In recent years, a method of initiating buprenorphine called “micro-dosing” has gained traction. Very small doses of buprenorphine are given while a patient is receiving other opioids, thereby reducing the risk of precipitated withdrawal. This method can be helpful for patients who cannot tolerate withdrawal or who have recently taken long-acting opioids such as methadone. Such protocols should be utilized only at centers where consultation with an addiction specialist or experienced clinician is possible.
Despite evidence of buprenorphine’s efficacy, there are barriers to prescribing it. Physicians and advanced practitioners must be granted a waiver from the Drug Enforcement Administration to prescribe buprenorphine to outpatients. As of 2017, less than 10% of primary care physicians had obtained waivers.9 However, inpatient clinicians without a waiver can order buprenorphine and initiate treatment. Best practice is to do so with a specific plan for continuation at discharge. We encourage inpatient clinicians to obtain a waiver, so that a prescription can be given at discharge to bridge the patient to a first appointment with a community clinician who can continue treatment. As of April 27, 2021, providers treating fewer than 30 patients with OUD at one time may obtain a waiver without additional training.10
Methadone
Methadone is a full agonist at the mu opioid receptor. In the hospital setting, methadone can be ordered by any clinician to prevent and treat withdrawal. Commonly, doses of 10 mg can be given using the COWS score to guide the need for additional dosing. The patient can be reassessed every 1-2 hours to ensure that symptoms are improving, and that there is no sign of oversedation before giving additional methadone. For most patients, withdrawal can be managed with 20-40 mg of methadone daily.
In contrast to buprenorphine, methadone will not precipitate withdrawal and can be initiated even when patients are not yet showing withdrawal symptoms. Outpatient methadone treatment for OUD is federally regulated and can be delivered only in opioid treatment programs (OTPs).
Choosing methadone or buprenorphine in the inpatient setting
The choice between buprenorphine and methadone should take into consideration several factors, including patient preference, treatment history, and available outpatient treatment programs, which may vary widely by geographic region. Some patients benefit from the higher level of support and counseling available at OTPs. Methadone is available at all OTPs, and the availability of buprenorphine in this setting is increasing. Other patients may prefer the convenience and flexibility of buprenorphine treatment in an outpatient office setting.
Some patients have prior negative experiences with OAT. These can include prior precipitated withdrawal with buprenorphine induction, or negative experiences with the structure of OTPs. Clinicians are encouraged to provide counseling if patients have a history of precipitated withdrawal to assure them that this can be avoided with proper dosing. Clinicians should be familiar with available treatment options in their community and can refer to the Substance Abuse and Mental Health Services Administration (SAMHSA) website to locate OTPs and buprenorphine prescribers.

Polypharmacy and safety
If combined with benzodiazepines, alcohol, or other sedating agents, methadone or buprenorphine can increase risk of overdose. However, OUD treatment should not be withheld because of other substance use. Clinicians initiating treatment should counsel patients on the risk of concomitant substance use and provide overdose prevention education.
A brief note on naltrexone
Naltrexone, an opioid antagonist, is used more commonly in outpatient addiction treatment than in the inpatient setting, but inpatient clinicians should be aware of its use. It is available in oral and long-acting injectable formulations. Its utility in the inpatient setting may be limited as safe administration requires 7-10 days of opioid abstinence.
Discharge planning
Patients with OUD or who are started on OAT during a hospitalization should be linked to continued outpatient treatment. Before discharge it is best to ensure vaccinations for HAV, HBV, pneumococcus, and tetanus are up to date, and perform screening for HIV, hepatitis C, tuberculosis, and sexually transmitted infections if appropriate. All patients with OUD should be prescribed or provided with take-home naloxone for overdose reversal. Patients can also be referred to syringe service programs for additional harm reduction counseling and services.
Application of the data to our patient
For our patient, either methadone or buprenorphine could be used to treat her withdrawal. The COWS score should be used to assess withdrawal severity, and to guide appropriate timing of medication initiation. If she wishes to continue OAT after discharge, she should be linked to a clinician who can engage her in ongoing medical care. Prior to discharge she should also receive relevant vaccines and screening for infectious diseases as outlined above, as well as take-home naloxone (or a prescription).
Bottom line
Inpatient clinicians can play a pivotal role in patients’ lives by ensuring that patients with OUD receive OAT and are connected to outpatient care at discharge.
Dr. Linker is assistant professor in the division of hospital medicine, Icahn School of Medicine at Mount Sinai, New York. Ms. Hirt, Mr. Fine, and Mr. Villasanivis are medical students at the Icahn School of Medicine at Mount Sinai. Dr. Wang is assistant professor in the division of general internal medicine, Icahn School of Medicine at Mount Sinai. Dr. Herscher is assistant professor in the division of hospital medicine, Icahn School of Medicine at Mount Sinai.
References
1. Wide-ranging online data for epidemiologic research (WONDER). Atlanta, GA: CDC, National Center for Health Statistics; 2020. Available at http://wonder.cdc.gov.
2. Mattson CL et al. Trends and geographic patterns in drug and synthetic opioid overdose deaths – United States, 2013-2019. MMWR Morb Mortal Wkly Rep. 2021;70:202-7. doi: 10.15585/mmwr.mm7006a4.
3. Wakeman SE et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020 Feb 5;3(2):e1920622. doi: 10.1001/jamanetworkopen.2019.20622.
4. Gowing L et al. Buprenorphine for managing opioid withdrawal. Cochrane Database Syst Rev. 2017 Feb;2017(2):CD002025. doi: 10.1002/14651858.CD002025.pub5.
5. Sordo L et al. Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. BMJ. 2017 Apr 26;357:j1550. doi: 10.1136/bmj.j1550.
6. Smyth BP et al. Lapse and relapse following inpatient treatment of opiate dependence. Ir Med J. 2010 Jun;103(6):176-9. Available at www.drugsandalcohol.ie/13405.
7. Liebschutz JM. Buprenorphine treatment for hospitalized, opioid-dependent patients: A randomized clinical trial. JAMA Intern Med. 2014 Aug;174(8):1369-76. doi: 10.1001/jamainternmed.2014.2556.
8. Substance Abuse and Mental Health Services Administration. (Aug 20, 2020) Statutes, Regulations, and Guidelines.
9. McBain RK et al. Growth and distribution of buprenorphine-waivered providers in the United States, 2007-2017. Ann Intern Med. 2020;172(7):504-6. doi: 10.7326/M19-2403.
10. HHS releases new buprenorphine practice guidelines, expanding access to treatment for opioid use disorder. Apr 27, 2021.
11. Herscher M et al. Diagnosis and management of opioid use disorder in hospitalized patients. Med Clin North Am. 2020 Jul;104(4):695-708. doi: 10.1016/j.mcna.2020.03.003.
Additional reading
Winetsky D. Expanding treatment opportunities for hospitalized patients with opioid use disorders. J Hosp Med. 2018 Jan;13(1):62-4. doi: 10.12788/jhm.2861.
Donroe JH. Caring for patients with opioid use disorder in the hospital. Can Med Assoc J. 2016 Dec 6;188(17-18):1232-9. doi: 10.1503/cmaj.160290.
Herscher M et al. Diagnosis and management of opioid use disorder in hospitalized patients. Med Clin North Am. 2020 Jul;104(4):695-708. doi: 10.1016/j.mcna.2020.03.003.
Key points
- Most patients with OUD are not engaged in evidence-based treatment. Clinicians have an opportunity to utilize the inpatient stay as a ‘reachable moment’ to engage patients with OUD in evidence-based treatment.
- Buprenorphine and methadone are effective opioid agonist medications used to treat OUD, and clinicians with the appropriate knowledge base can initiate either during the inpatient encounter, and link the patient to OUD treatment after the hospital stay.
Quiz
Caring for hospitalized patients with OUD
Most patients with OUD are not engaged in effective treatment. Hospitalization can be a ‘reachable moment’ to engage patients with OUD in evidence-based treatment.
1. Which is an effective and evidence-based medication for treating opioid withdrawal and OUD?
a) Naltrexone.
b) Buprenorphine.
c) Opioid detoxification.
d) Clonidine.
Explanation: Buprenorphine is effective for alleviating symptoms of withdrawal as well as for the long-term treatment of OUD. While naltrexone is also used to treat OUD, it is not useful for treating withdrawal. Clonidine can be a useful adjunctive medication for treating withdrawal but is not a long-term treatment for OUD. Nonpharmacologic detoxification is not an effective treatment for OUD and is associated with high relapse rates.
2. What scale can be used during a hospital stay to monitor patients with OUD at risk of opioid withdrawal, and to aid in buprenorphine initiation?
a) CIWA score.
b) PADUA score.
c) COWS score.
d) 4T score.
Explanation: COWS is the “clinical opiate withdrawal scale.” The COWS score should be calculated by a trained provider, and includes objective parameters (such as pulse) and subjective symptoms (such as GI upset, bone/joint aches.) It is recommended that agonist therapy be started when the COWS score is consistent with moderate withdrawal.
3. How can clinicians reliably find out if there are outpatient resources/clinics for patients with OUD in their area?
a) No way to find this out without personal knowledge.
b) Hospital providers and patients can visit www.samhsa.gov/find-help/national-helpline or call 1-800-662-HELP (4357) to find options for treatment for substance use disorders in their areas.
c) Dial “0” on any phone and ask.
d) Ask around at your hospital.
Explanation: The Substance Abuse and Mental Health Services Administration (SAMHSA) is an agency in the U.S. Department of Health and Human Services that is engaged in public health efforts to reduce the impact of substance abuse and mental illness on local communities. The agency’s website has helpful information about resources for substance use treatment.
4. Patients with OUD should be prescribed and given training about what medication that can be lifesaving when given during an opioid overdose?
a) Aspirin.
b) Naloxone.
c) Naltrexone.
d) Clonidine.
Explanation: Naloxone can be life-saving in the setting of an overdose. Best practice is to provide naloxone and training to patients with OUD.
5. When patients take buprenorphine soon after taking other opioids, there is concern for the development of which reaction:
a) Precipitated withdrawal.
b) Opioid overdose.
c) Allergic reaction.
d) Intoxication.
Explanation: Administering buprenorphine soon after taking other opioids can cause precipitated withdrawal, as buprenorphine binds with higher affinity to the mu receptor than many opioids. Precipitated withdrawal causes severe discomfort and can be dangerous for patients.
Is it OK to just be satisfied?
It is possible to talk to a patient for a brief moment and just know if he or she is a satisficer or a maximizer. A “satisficer” when presented with treatment options will invariably say: “I’ll do whatever you say, Doctor.” A “maximizer,” in contrast, would like a printed copy of treatment choices, then would seek a second opinion before ultimately buying an UpToDate subscription to research treatments for him or herself.
.
This notion that we have tendencies toward maximizing or satisficing is thanks to Nobel Memorial Prize winner and all-around smart guy, Herbert A. Simon, PhD. Dr. Simon recognized that, although each person might be expected to make optimal decisions to benefit himself or herself, this is practically impossible. To do so would require an infinite amount of time and energy. He found therefore that we actually exhibit “bounded rationality;” that is, we make the best decision given the limits of time, the price of acquiring information, and even our cognitive abilities. The amount of effort we give to make a decision also depends on the situation: You might be very invested in choosing the right spouse, but not at all invested in choosing soup or salad. (Although, we all have friends who are: “Um, is there any thyme in the soup?”)
You’ll certainly recognize that people have different set points on the spectrum between being a satisficer, one who will take the first option that meets a standard, and a maximizer, one who will seek and accept only the best, even if choosing is at great cost. There are risks and benefits of each. In getting the best job, maximizers might be more successful, but satisficers seem to be happier.
How much this extends into other spheres of life is unclear. It is clear, though, that the work of choosing can come at a cost.
The psychologist Barry Schwartz, PhD, believes that, in general, having more choices leads to more anxiety, not more contentment. For example, which Christmas tree lot would you rather visit: One with hundreds of trees of half a dozen varieties? Or one with just a few trees each of Balsam and Douglas Firs? Dr. Schwartz would argue that you might waste an entire afternoon in the first lot only to bring it home and have remorse when you realize it’s a little lopsided. Or let’s say your child applied to all the Ivy League and Public Ivy schools and also threw in all the top liberal arts colleges. The anxiety of selecting the best and the terror that the “best one” might not choose him or her could be overwhelming. A key lesson is that more in life is by chance than we realize, including how straight your tree is and who gets into Princeton this year. Yet, our expectation that things will work out perfectly if only we maximize is ubiquitous. That confidence in our ability to choose correctly is, however, unwarranted. Better to do your best and know that your tree will be festive and there are many colleges which would lead to a happy life than to fret in choosing and then suffer from dashed expectations. Sometimes good enough is good enough.
Being a satisficer or maximizer is probably somewhat fixed, a personality trait, like being extroverted or conscientious. Yet, having insight can be helpful. If choosing a restaurant in Manhattan becomes an actual project for you with spreadsheets and your own statistical analysis, then go for it! Just know that if that process causes you angst and apprehension, then there is another way. Go to Eleven Madison Park, just because I say so. You might have the best dinner of your life or maybe not. At least by not choosing you’ll have the gift of time to spend picking out a tree instead.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
It is possible to talk to a patient for a brief moment and just know if he or she is a satisficer or a maximizer. A “satisficer” when presented with treatment options will invariably say: “I’ll do whatever you say, Doctor.” A “maximizer,” in contrast, would like a printed copy of treatment choices, then would seek a second opinion before ultimately buying an UpToDate subscription to research treatments for him or herself.
.
This notion that we have tendencies toward maximizing or satisficing is thanks to Nobel Memorial Prize winner and all-around smart guy, Herbert A. Simon, PhD. Dr. Simon recognized that, although each person might be expected to make optimal decisions to benefit himself or herself, this is practically impossible. To do so would require an infinite amount of time and energy. He found therefore that we actually exhibit “bounded rationality;” that is, we make the best decision given the limits of time, the price of acquiring information, and even our cognitive abilities. The amount of effort we give to make a decision also depends on the situation: You might be very invested in choosing the right spouse, but not at all invested in choosing soup or salad. (Although, we all have friends who are: “Um, is there any thyme in the soup?”)
You’ll certainly recognize that people have different set points on the spectrum between being a satisficer, one who will take the first option that meets a standard, and a maximizer, one who will seek and accept only the best, even if choosing is at great cost. There are risks and benefits of each. In getting the best job, maximizers might be more successful, but satisficers seem to be happier.
How much this extends into other spheres of life is unclear. It is clear, though, that the work of choosing can come at a cost.
The psychologist Barry Schwartz, PhD, believes that, in general, having more choices leads to more anxiety, not more contentment. For example, which Christmas tree lot would you rather visit: One with hundreds of trees of half a dozen varieties? Or one with just a few trees each of Balsam and Douglas Firs? Dr. Schwartz would argue that you might waste an entire afternoon in the first lot only to bring it home and have remorse when you realize it’s a little lopsided. Or let’s say your child applied to all the Ivy League and Public Ivy schools and also threw in all the top liberal arts colleges. The anxiety of selecting the best and the terror that the “best one” might not choose him or her could be overwhelming. A key lesson is that more in life is by chance than we realize, including how straight your tree is and who gets into Princeton this year. Yet, our expectation that things will work out perfectly if only we maximize is ubiquitous. That confidence in our ability to choose correctly is, however, unwarranted. Better to do your best and know that your tree will be festive and there are many colleges which would lead to a happy life than to fret in choosing and then suffer from dashed expectations. Sometimes good enough is good enough.
Being a satisficer or maximizer is probably somewhat fixed, a personality trait, like being extroverted or conscientious. Yet, having insight can be helpful. If choosing a restaurant in Manhattan becomes an actual project for you with spreadsheets and your own statistical analysis, then go for it! Just know that if that process causes you angst and apprehension, then there is another way. Go to Eleven Madison Park, just because I say so. You might have the best dinner of your life or maybe not. At least by not choosing you’ll have the gift of time to spend picking out a tree instead.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
It is possible to talk to a patient for a brief moment and just know if he or she is a satisficer or a maximizer. A “satisficer” when presented with treatment options will invariably say: “I’ll do whatever you say, Doctor.” A “maximizer,” in contrast, would like a printed copy of treatment choices, then would seek a second opinion before ultimately buying an UpToDate subscription to research treatments for him or herself.
.
This notion that we have tendencies toward maximizing or satisficing is thanks to Nobel Memorial Prize winner and all-around smart guy, Herbert A. Simon, PhD. Dr. Simon recognized that, although each person might be expected to make optimal decisions to benefit himself or herself, this is practically impossible. To do so would require an infinite amount of time and energy. He found therefore that we actually exhibit “bounded rationality;” that is, we make the best decision given the limits of time, the price of acquiring information, and even our cognitive abilities. The amount of effort we give to make a decision also depends on the situation: You might be very invested in choosing the right spouse, but not at all invested in choosing soup or salad. (Although, we all have friends who are: “Um, is there any thyme in the soup?”)
You’ll certainly recognize that people have different set points on the spectrum between being a satisficer, one who will take the first option that meets a standard, and a maximizer, one who will seek and accept only the best, even if choosing is at great cost. There are risks and benefits of each. In getting the best job, maximizers might be more successful, but satisficers seem to be happier.
How much this extends into other spheres of life is unclear. It is clear, though, that the work of choosing can come at a cost.
The psychologist Barry Schwartz, PhD, believes that, in general, having more choices leads to more anxiety, not more contentment. For example, which Christmas tree lot would you rather visit: One with hundreds of trees of half a dozen varieties? Or one with just a few trees each of Balsam and Douglas Firs? Dr. Schwartz would argue that you might waste an entire afternoon in the first lot only to bring it home and have remorse when you realize it’s a little lopsided. Or let’s say your child applied to all the Ivy League and Public Ivy schools and also threw in all the top liberal arts colleges. The anxiety of selecting the best and the terror that the “best one” might not choose him or her could be overwhelming. A key lesson is that more in life is by chance than we realize, including how straight your tree is and who gets into Princeton this year. Yet, our expectation that things will work out perfectly if only we maximize is ubiquitous. That confidence in our ability to choose correctly is, however, unwarranted. Better to do your best and know that your tree will be festive and there are many colleges which would lead to a happy life than to fret in choosing and then suffer from dashed expectations. Sometimes good enough is good enough.
Being a satisficer or maximizer is probably somewhat fixed, a personality trait, like being extroverted or conscientious. Yet, having insight can be helpful. If choosing a restaurant in Manhattan becomes an actual project for you with spreadsheets and your own statistical analysis, then go for it! Just know that if that process causes you angst and apprehension, then there is another way. Go to Eleven Madison Park, just because I say so. You might have the best dinner of your life or maybe not. At least by not choosing you’ll have the gift of time to spend picking out a tree instead.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
ADHD Pathophysiology
When surgery is the next step in treating endometriosis—know your patient’s priorities and how to optimize long-term pain relief
Cara R. King, DO, MS, is a member of the Cleveland Clinic Section of Minimally Invasive Gynecologic Surgery (MIGS). She is the Director of Benign Gynecologic Surgery, and Associate Program Director of the MIGS Fellowship, and Director of Innovation for the Women’s Health Institute. She is a member of the American Association of Gynecologic Laparoscopists (AAGL), the Society of Gynecologic Surgeons (SGS), American College of Surgeons (ACS), and the American Congress of Obstetricians and Gynecologists (ACOG).
Q: How much of your surgical practice is dedicated to patients with endometriosis?
Dr. King: The majority of my practice is dedicated to treating women with endometriosis. I practice at the Cleveland Clinic in Cleveland, Ohio, which is a high-volume referral center, so many of my patients are coming to me for endometriosis or pelvic pain-type symptoms. For most of my patients, I serve as a consultant, which means it's not their initial visit for this issue. I'm often seeing patients who have not found relief through alternate medical or surgical treatments and typically, have more deeply infiltrating or complex endometriosis disease.
Q: How do you make the treatment decision with patients that surgery is the next or proper needed step?
Dr. King: This decision depends on the goals and priorities of each of my patients. I don't have a one-size-fits-all type approach as every patient's journey and unique experiences vary. Ultimately, deciding on the available options and order of treatment depends on the patient's symptoms and priorities. I always start with a thorough history, including a detailed physical exam. The pelvic exam includes evaluation of the bladder, bowel, pelvic floor muscles, nerves, as well as the gynecologic organs including vagina, uterus, cervix and adnexa. If I palpate a nodule on the uterosacral ligaments or behind the cervix, I will sometimes perform a rectovaginal exam to assess for deeply infiltrating bowel disease. Various imaging modalities, including a transvaginal ultrasound or an MRI, can be helpful to further characterize the disease. This allows us to create a treatment plan that best aligns with the patients’ priorities and goals. As a general rule, surgery is usually indicated if empiric options have failed or if they desire definitive diagnosis; meaning the patient is still having pain symptoms despite conservative options or if they have failed or are intolerant to medical options. Some patients are not candidates for medical therapy, such as those who desire pregnancy or who are trying to conceive, so medical options wouldn't be an option for these patients. For patients who prefer an immediate diagnosis, surgical intervention may also be the best option. When I see initial consults for patients who haven't previously seen an endometriosis specialist, if they're not trying to conceive and if they are candidates for medical therapy, I think that's a reasonable first step. We must understand that medications are not curative, they are merely suppressive for endometriosis, so when patients come to me that have been on medical therapy for more than 3 months without pain improvement, and they haven't been offered a surgical approach, diagnostic laparoscopy is often the next best step.
Q: Please detail the presurgical discussion, or the consent process, that would allow you to go beyond the agreed-to procedure, if necessary?
Dr. King: Endometriosis is extremely unique in that you sometimes cannot tell how deeply infiltrative the disease is until you start excising it. So, my consent process and discussions are substantial parts of all patient presurgical conversations. This is crucial for understanding how comfortable the patient is with more aggressive surgery and to fully understand each individual’s symptoms and priorities. I spend a significant amount of time talking to patients about their exact goals for surgery and I conduct a thorough workup before we get into the operating room so that when coupled with a proper physical exam and detailed imaging, the element for surprise, such as finding disease that is much more advanced than you had thought, is decreased. Understanding your patient's symptoms as well as how aggressive they want you to be with regards to surgery is of utmost importance. The more accurate the description that I have of the type of disease that we're working with allows me to talk about all possibilities that could occur before the patients get into the operating room so that we can ensure expectations are met, for the patient and for the surgeon.
Q: Do you have any protocols to share with the audience that relate to limiting reoperation for residual disease?
Dr. King: Conducting a thorough history and physical exam in addition to having detailed imaging is crucial to optimize success. That said, there are times when imaging may appear “normal” when endometriosis is actually present, which is why it is of utmost importance to listen to your patient’s history. With deeply infiltrating endometriosis, superficially, if you look at the peritoneum, it can sometimes appear as if the disease is not that invasive. Again, endometriosis is unique in that until you start excising it, sometimes you don’t know the extent of the infiltration. So, having detailed imaging is going to allow for better mapping of the endometriosis beforehand which will allow you to properly focus in on those areas and enhance preoperative counseling.
My second level of advice is to know your limits with regards to surgical complexity and your laparoscopic skills. For instance, if an endometrioma is present on imaging, you will most likely encounter peritoneal disease and fibrosis below that ovary on the pelvic side wall adjacent to the ureter. If you are not comfortable excising this disease, you should consider referring the patient to an advanced pelvic surgeon. When you see certain characteristics on imaging, understanding what the disease process will look like when you get in there and understanding your own skill level at which you can safely and efficiently perform that dissection is very important. And if you do not have that skill level or if you are still working on detailed knowledge of retroperitoneal anatomy, then the opportunity exists to build up your team; consider including another subspecialist within GYN or urology, colorectal surgery, or cardiothoracic surgery, if you are working with diaphragmatic endometriosis. Loading your boat will allow you to safely and efficiently remove as much of the disease as you can and decrease the risk of leaving any behind. You could also consider video based surgical coaching to further enhance your own laparoscopic skills and surgical performance when treating this complex disease.
Q: How do you approach postsurgical management to maximize the pain-free period for patients?
Dr. King: We know that the best intervention for pain relief is complete excision of endometriosis. By performing a complete excision, we know that this procedure will prolong the length of time for pain-free interval. So, getting as close as possible to a complete excision is going to be the first step. It is also important to treat alternate sources of pain that can be impacted by endometriosis such as spasm of the pelvic floor muscles or central sensitization. While it is difficult to say whether recurrent endometriosis pain is secondary to reactivation of residual disease as opposed to new disease, we do know that complete excision provides longer relief. Assuming surgery has relieved a majority of or, all of the endometriosis associated pain, then the main strategy that we can use to postoperatively maximize that pain-free period is to minimize ovulation. This is typically accomplished with hormonal suppression. It is worth nothing that this isn't indicated for all patients and it is not mandatory as we, again, must be mindful of the patient's goals and priorities. But a recent systematic review did find that when we start hormonal suppression within 6 weeks of our endometriosis surgery, there is a significant reduction in recurrent endometriosis pain scores for up to one year postoperative. Currently, there are no non-hormonal medications that we can offer, nor do we have any interventions to alter genetics or immune aspects of the disease, though it is hoped such could possibly become available in the near future. At the current point in time, hormonal suppressive options are typically the best route but again, I want to reiterate that medications are suppressive and are not curative. And with regards to details of medical options, pulling in patient preference, financial aspects, underlying comorbidities, and long-term reproductive plans, are factors that are important to consider when making weighing decision.
Cara R. King, DO, MS, is a member of the Cleveland Clinic Section of Minimally Invasive Gynecologic Surgery (MIGS). She is the Director of Benign Gynecologic Surgery, and Associate Program Director of the MIGS Fellowship, and Director of Innovation for the Women’s Health Institute. She is a member of the American Association of Gynecologic Laparoscopists (AAGL), the Society of Gynecologic Surgeons (SGS), American College of Surgeons (ACS), and the American Congress of Obstetricians and Gynecologists (ACOG).
Q: How much of your surgical practice is dedicated to patients with endometriosis?
Dr. King: The majority of my practice is dedicated to treating women with endometriosis. I practice at the Cleveland Clinic in Cleveland, Ohio, which is a high-volume referral center, so many of my patients are coming to me for endometriosis or pelvic pain-type symptoms. For most of my patients, I serve as a consultant, which means it's not their initial visit for this issue. I'm often seeing patients who have not found relief through alternate medical or surgical treatments and typically, have more deeply infiltrating or complex endometriosis disease.
Q: How do you make the treatment decision with patients that surgery is the next or proper needed step?
Dr. King: This decision depends on the goals and priorities of each of my patients. I don't have a one-size-fits-all type approach as every patient's journey and unique experiences vary. Ultimately, deciding on the available options and order of treatment depends on the patient's symptoms and priorities. I always start with a thorough history, including a detailed physical exam. The pelvic exam includes evaluation of the bladder, bowel, pelvic floor muscles, nerves, as well as the gynecologic organs including vagina, uterus, cervix and adnexa. If I palpate a nodule on the uterosacral ligaments or behind the cervix, I will sometimes perform a rectovaginal exam to assess for deeply infiltrating bowel disease. Various imaging modalities, including a transvaginal ultrasound or an MRI, can be helpful to further characterize the disease. This allows us to create a treatment plan that best aligns with the patients’ priorities and goals. As a general rule, surgery is usually indicated if empiric options have failed or if they desire definitive diagnosis; meaning the patient is still having pain symptoms despite conservative options or if they have failed or are intolerant to medical options. Some patients are not candidates for medical therapy, such as those who desire pregnancy or who are trying to conceive, so medical options wouldn't be an option for these patients. For patients who prefer an immediate diagnosis, surgical intervention may also be the best option. When I see initial consults for patients who haven't previously seen an endometriosis specialist, if they're not trying to conceive and if they are candidates for medical therapy, I think that's a reasonable first step. We must understand that medications are not curative, they are merely suppressive for endometriosis, so when patients come to me that have been on medical therapy for more than 3 months without pain improvement, and they haven't been offered a surgical approach, diagnostic laparoscopy is often the next best step.
Q: Please detail the presurgical discussion, or the consent process, that would allow you to go beyond the agreed-to procedure, if necessary?
Dr. King: Endometriosis is extremely unique in that you sometimes cannot tell how deeply infiltrative the disease is until you start excising it. So, my consent process and discussions are substantial parts of all patient presurgical conversations. This is crucial for understanding how comfortable the patient is with more aggressive surgery and to fully understand each individual’s symptoms and priorities. I spend a significant amount of time talking to patients about their exact goals for surgery and I conduct a thorough workup before we get into the operating room so that when coupled with a proper physical exam and detailed imaging, the element for surprise, such as finding disease that is much more advanced than you had thought, is decreased. Understanding your patient's symptoms as well as how aggressive they want you to be with regards to surgery is of utmost importance. The more accurate the description that I have of the type of disease that we're working with allows me to talk about all possibilities that could occur before the patients get into the operating room so that we can ensure expectations are met, for the patient and for the surgeon.
Q: Do you have any protocols to share with the audience that relate to limiting reoperation for residual disease?
Dr. King: Conducting a thorough history and physical exam in addition to having detailed imaging is crucial to optimize success. That said, there are times when imaging may appear “normal” when endometriosis is actually present, which is why it is of utmost importance to listen to your patient’s history. With deeply infiltrating endometriosis, superficially, if you look at the peritoneum, it can sometimes appear as if the disease is not that invasive. Again, endometriosis is unique in that until you start excising it, sometimes you don’t know the extent of the infiltration. So, having detailed imaging is going to allow for better mapping of the endometriosis beforehand which will allow you to properly focus in on those areas and enhance preoperative counseling.
My second level of advice is to know your limits with regards to surgical complexity and your laparoscopic skills. For instance, if an endometrioma is present on imaging, you will most likely encounter peritoneal disease and fibrosis below that ovary on the pelvic side wall adjacent to the ureter. If you are not comfortable excising this disease, you should consider referring the patient to an advanced pelvic surgeon. When you see certain characteristics on imaging, understanding what the disease process will look like when you get in there and understanding your own skill level at which you can safely and efficiently perform that dissection is very important. And if you do not have that skill level or if you are still working on detailed knowledge of retroperitoneal anatomy, then the opportunity exists to build up your team; consider including another subspecialist within GYN or urology, colorectal surgery, or cardiothoracic surgery, if you are working with diaphragmatic endometriosis. Loading your boat will allow you to safely and efficiently remove as much of the disease as you can and decrease the risk of leaving any behind. You could also consider video based surgical coaching to further enhance your own laparoscopic skills and surgical performance when treating this complex disease.
Q: How do you approach postsurgical management to maximize the pain-free period for patients?
Dr. King: We know that the best intervention for pain relief is complete excision of endometriosis. By performing a complete excision, we know that this procedure will prolong the length of time for pain-free interval. So, getting as close as possible to a complete excision is going to be the first step. It is also important to treat alternate sources of pain that can be impacted by endometriosis such as spasm of the pelvic floor muscles or central sensitization. While it is difficult to say whether recurrent endometriosis pain is secondary to reactivation of residual disease as opposed to new disease, we do know that complete excision provides longer relief. Assuming surgery has relieved a majority of or, all of the endometriosis associated pain, then the main strategy that we can use to postoperatively maximize that pain-free period is to minimize ovulation. This is typically accomplished with hormonal suppression. It is worth nothing that this isn't indicated for all patients and it is not mandatory as we, again, must be mindful of the patient's goals and priorities. But a recent systematic review did find that when we start hormonal suppression within 6 weeks of our endometriosis surgery, there is a significant reduction in recurrent endometriosis pain scores for up to one year postoperative. Currently, there are no non-hormonal medications that we can offer, nor do we have any interventions to alter genetics or immune aspects of the disease, though it is hoped such could possibly become available in the near future. At the current point in time, hormonal suppressive options are typically the best route but again, I want to reiterate that medications are suppressive and are not curative. And with regards to details of medical options, pulling in patient preference, financial aspects, underlying comorbidities, and long-term reproductive plans, are factors that are important to consider when making weighing decision.
Cara R. King, DO, MS, is a member of the Cleveland Clinic Section of Minimally Invasive Gynecologic Surgery (MIGS). She is the Director of Benign Gynecologic Surgery, and Associate Program Director of the MIGS Fellowship, and Director of Innovation for the Women’s Health Institute. She is a member of the American Association of Gynecologic Laparoscopists (AAGL), the Society of Gynecologic Surgeons (SGS), American College of Surgeons (ACS), and the American Congress of Obstetricians and Gynecologists (ACOG).
Q: How much of your surgical practice is dedicated to patients with endometriosis?
Dr. King: The majority of my practice is dedicated to treating women with endometriosis. I practice at the Cleveland Clinic in Cleveland, Ohio, which is a high-volume referral center, so many of my patients are coming to me for endometriosis or pelvic pain-type symptoms. For most of my patients, I serve as a consultant, which means it's not their initial visit for this issue. I'm often seeing patients who have not found relief through alternate medical or surgical treatments and typically, have more deeply infiltrating or complex endometriosis disease.
Q: How do you make the treatment decision with patients that surgery is the next or proper needed step?
Dr. King: This decision depends on the goals and priorities of each of my patients. I don't have a one-size-fits-all type approach as every patient's journey and unique experiences vary. Ultimately, deciding on the available options and order of treatment depends on the patient's symptoms and priorities. I always start with a thorough history, including a detailed physical exam. The pelvic exam includes evaluation of the bladder, bowel, pelvic floor muscles, nerves, as well as the gynecologic organs including vagina, uterus, cervix and adnexa. If I palpate a nodule on the uterosacral ligaments or behind the cervix, I will sometimes perform a rectovaginal exam to assess for deeply infiltrating bowel disease. Various imaging modalities, including a transvaginal ultrasound or an MRI, can be helpful to further characterize the disease. This allows us to create a treatment plan that best aligns with the patients’ priorities and goals. As a general rule, surgery is usually indicated if empiric options have failed or if they desire definitive diagnosis; meaning the patient is still having pain symptoms despite conservative options or if they have failed or are intolerant to medical options. Some patients are not candidates for medical therapy, such as those who desire pregnancy or who are trying to conceive, so medical options wouldn't be an option for these patients. For patients who prefer an immediate diagnosis, surgical intervention may also be the best option. When I see initial consults for patients who haven't previously seen an endometriosis specialist, if they're not trying to conceive and if they are candidates for medical therapy, I think that's a reasonable first step. We must understand that medications are not curative, they are merely suppressive for endometriosis, so when patients come to me that have been on medical therapy for more than 3 months without pain improvement, and they haven't been offered a surgical approach, diagnostic laparoscopy is often the next best step.
Q: Please detail the presurgical discussion, or the consent process, that would allow you to go beyond the agreed-to procedure, if necessary?
Dr. King: Endometriosis is extremely unique in that you sometimes cannot tell how deeply infiltrative the disease is until you start excising it. So, my consent process and discussions are substantial parts of all patient presurgical conversations. This is crucial for understanding how comfortable the patient is with more aggressive surgery and to fully understand each individual’s symptoms and priorities. I spend a significant amount of time talking to patients about their exact goals for surgery and I conduct a thorough workup before we get into the operating room so that when coupled with a proper physical exam and detailed imaging, the element for surprise, such as finding disease that is much more advanced than you had thought, is decreased. Understanding your patient's symptoms as well as how aggressive they want you to be with regards to surgery is of utmost importance. The more accurate the description that I have of the type of disease that we're working with allows me to talk about all possibilities that could occur before the patients get into the operating room so that we can ensure expectations are met, for the patient and for the surgeon.
Q: Do you have any protocols to share with the audience that relate to limiting reoperation for residual disease?
Dr. King: Conducting a thorough history and physical exam in addition to having detailed imaging is crucial to optimize success. That said, there are times when imaging may appear “normal” when endometriosis is actually present, which is why it is of utmost importance to listen to your patient’s history. With deeply infiltrating endometriosis, superficially, if you look at the peritoneum, it can sometimes appear as if the disease is not that invasive. Again, endometriosis is unique in that until you start excising it, sometimes you don’t know the extent of the infiltration. So, having detailed imaging is going to allow for better mapping of the endometriosis beforehand which will allow you to properly focus in on those areas and enhance preoperative counseling.
My second level of advice is to know your limits with regards to surgical complexity and your laparoscopic skills. For instance, if an endometrioma is present on imaging, you will most likely encounter peritoneal disease and fibrosis below that ovary on the pelvic side wall adjacent to the ureter. If you are not comfortable excising this disease, you should consider referring the patient to an advanced pelvic surgeon. When you see certain characteristics on imaging, understanding what the disease process will look like when you get in there and understanding your own skill level at which you can safely and efficiently perform that dissection is very important. And if you do not have that skill level or if you are still working on detailed knowledge of retroperitoneal anatomy, then the opportunity exists to build up your team; consider including another subspecialist within GYN or urology, colorectal surgery, or cardiothoracic surgery, if you are working with diaphragmatic endometriosis. Loading your boat will allow you to safely and efficiently remove as much of the disease as you can and decrease the risk of leaving any behind. You could also consider video based surgical coaching to further enhance your own laparoscopic skills and surgical performance when treating this complex disease.
Q: How do you approach postsurgical management to maximize the pain-free period for patients?
Dr. King: We know that the best intervention for pain relief is complete excision of endometriosis. By performing a complete excision, we know that this procedure will prolong the length of time for pain-free interval. So, getting as close as possible to a complete excision is going to be the first step. It is also important to treat alternate sources of pain that can be impacted by endometriosis such as spasm of the pelvic floor muscles or central sensitization. While it is difficult to say whether recurrent endometriosis pain is secondary to reactivation of residual disease as opposed to new disease, we do know that complete excision provides longer relief. Assuming surgery has relieved a majority of or, all of the endometriosis associated pain, then the main strategy that we can use to postoperatively maximize that pain-free period is to minimize ovulation. This is typically accomplished with hormonal suppression. It is worth nothing that this isn't indicated for all patients and it is not mandatory as we, again, must be mindful of the patient's goals and priorities. But a recent systematic review did find that when we start hormonal suppression within 6 weeks of our endometriosis surgery, there is a significant reduction in recurrent endometriosis pain scores for up to one year postoperative. Currently, there are no non-hormonal medications that we can offer, nor do we have any interventions to alter genetics or immune aspects of the disease, though it is hoped such could possibly become available in the near future. At the current point in time, hormonal suppressive options are typically the best route but again, I want to reiterate that medications are suppressive and are not curative. And with regards to details of medical options, pulling in patient preference, financial aspects, underlying comorbidities, and long-term reproductive plans, are factors that are important to consider when making weighing decision.
Infant milk allergy guidelines promote overdiagnosis, study says
International guidelines developed to help nonspecialists diagnose cow’s milk allergy (CMA) lead providers to attribute normal infant symptoms to CMA and result in overdiagnosis, say authors of a study published online in Clinical & Experimental Allergy.
Lead author Rosie Vincent, MBChB, with Population Health Sciences at University of Bristol, United Kingdom, told this news organization their study shows that symptoms listed in the international Milk Allergy in Primary Care (iMAP) guidelines as indicative of non-immunoglobulin E (IgE)-mediated milk allergy are very common in a baby’s first year. Examples include vomiting, regurgitating milk, loose or more frequent stools, colic, and irritability.
Findings come from performing a secondary analysis of data from 1,303 infants from the EAT study, a population-based, randomized controlled trial in the U.K. that looked at whether introducing allergenic foods into an infant’s diet reduced the risk of developing an allergy to that food.
In an indication of how common the symptoms in the guidelines (published in 2017 and 2019) are found in all infants, nearly three-fourths (74%) of participants reported at least two mild-to-moderate symptoms, and 9% reported at least two severe symptoms in at least one month between 3 and 12 months of age. Data were not available for younger infants.
However, the prevalence of non–IgE-mediated CMA is thought to be less than 1% in infants in European countries, the study states.
In the study, two or more non-IgE CMA mild-to-moderate symptoms were reported by 25% of families, and 1.4% reported severe symptoms each month between ages 3 and 12 months.
“These symptoms peaked at 38%, with at least two mild-to-moderate symptoms and 4.3% with at least two severe symptoms at 3 months, when participants were not directly consuming cow’s milk,” Ms. Vincent said.
Researchers write that at 6 months there was no significant difference in the proportion of children with at least two symptoms between those consuming and not consuming cow’s milk.
Consequences of misdiagnosis
Overdiagnosing milk allergy can lead to additional costs, unnecessary tests, and less breastfeeding, she said.
Cow’s milk protein is commonly found in standard infant formula or in milk-containing foods.
The authors note that “small levels of lactoglobulin are found in breastmilk; however, the quantities are below the threshold likely to trigger a reaction in more than 99% of infants with IgE-mediated cow’s milk allergy.”
Misdiagnosis is likely to result in increasing prescriptions of unwarranted specialized formula, with increased cost to patients and health care systems, and use of unvalidated allergy tests, Ms. Vincent said.
Ms. Vincent added that even the suggestion that cow’s milk protein delivered through breast milk might be inducing symptoms could lead a mother to stop breastfeeding.
The authors also note that in reviewing recent CMA guidelines, “three of nine CMA guidelines were directly supported by formula manufacturers or marketing consultants, and 81% of all guideline authors reported a conflict of interest with formula manufacturers.”
Heather Cassell, MD, a pediatric allergy and immunology specialist with Banner Health and a clinical associate professor of pediatrics at the University of Arizona College of Medicine in Tucson, told this news organization the conflicts of interest in milk allergy research and guidelines have been a long-standing problem.
She said historically there has been a big push “that people who can afford formula should be paying for formula. That was 100% marketed by the formula companies.”
“We have formula companies bringing us samples to encourage pediatricians to use the formula early if we’re concerned about a milk protein allergy,” Dr. Cassell said.
As for the overdiagnosis of milk allergy, she said reintroduction of cow’s milk later is one way to improve diagnosis to see if the child no longer has a reaction. However, she points out that in this study, only 21% of parents reintroduced cow’s milk.
“Really, it should be closer to 100%, with the exception of the babies who are having severe symptoms,” Dr. Cassell said. “You don’t want to keep a baby from progressing with their diet.”
She said families and providers need to look at several contextual clues before they land on a milk allergy label.
“It’s not just about reflux, it’s not just about a baby spitting up. Happy babies spit up and there’s nothing that needs to be done because they will eventually grow out of it,” Dr. Cassell stressed.
She said significant irritability with blood in the stool might warrant more concern. “I think the [emphasis] needs to be on retrying the food another time,” she suggested.
Ms. Vincent pointed out that there is no quick or easy test to diagnose non–IgE-mediated cow’s milk allergy.
“We need further research to identify what symptoms are much more likely to point to a diagnosis,” she said.
Although the researchers used iMAP guidelines, they write that results are likely to apply to other CMA guidelines, because they list similar symptoms and signs.
The study was funded by the International Society of Atopic Dermatitis. Ms. Vincent reports receiving a 3-month research fellowship award from Pfizer and support from the NIHR School for Primary Care Research. Other authors’ financial disclosures are available with the full text. Dr. Cassell reports that the University of Arizona School of Medicine is a trial site for testing a patch to help with diagnosing milk protein allergy in infants.
A version of this article first appeared on Medscape.com.
International guidelines developed to help nonspecialists diagnose cow’s milk allergy (CMA) lead providers to attribute normal infant symptoms to CMA and result in overdiagnosis, say authors of a study published online in Clinical & Experimental Allergy.
Lead author Rosie Vincent, MBChB, with Population Health Sciences at University of Bristol, United Kingdom, told this news organization their study shows that symptoms listed in the international Milk Allergy in Primary Care (iMAP) guidelines as indicative of non-immunoglobulin E (IgE)-mediated milk allergy are very common in a baby’s first year. Examples include vomiting, regurgitating milk, loose or more frequent stools, colic, and irritability.
Findings come from performing a secondary analysis of data from 1,303 infants from the EAT study, a population-based, randomized controlled trial in the U.K. that looked at whether introducing allergenic foods into an infant’s diet reduced the risk of developing an allergy to that food.
In an indication of how common the symptoms in the guidelines (published in 2017 and 2019) are found in all infants, nearly three-fourths (74%) of participants reported at least two mild-to-moderate symptoms, and 9% reported at least two severe symptoms in at least one month between 3 and 12 months of age. Data were not available for younger infants.
However, the prevalence of non–IgE-mediated CMA is thought to be less than 1% in infants in European countries, the study states.
In the study, two or more non-IgE CMA mild-to-moderate symptoms were reported by 25% of families, and 1.4% reported severe symptoms each month between ages 3 and 12 months.
“These symptoms peaked at 38%, with at least two mild-to-moderate symptoms and 4.3% with at least two severe symptoms at 3 months, when participants were not directly consuming cow’s milk,” Ms. Vincent said.
Researchers write that at 6 months there was no significant difference in the proportion of children with at least two symptoms between those consuming and not consuming cow’s milk.
Consequences of misdiagnosis
Overdiagnosing milk allergy can lead to additional costs, unnecessary tests, and less breastfeeding, she said.
Cow’s milk protein is commonly found in standard infant formula or in milk-containing foods.
The authors note that “small levels of lactoglobulin are found in breastmilk; however, the quantities are below the threshold likely to trigger a reaction in more than 99% of infants with IgE-mediated cow’s milk allergy.”
Misdiagnosis is likely to result in increasing prescriptions of unwarranted specialized formula, with increased cost to patients and health care systems, and use of unvalidated allergy tests, Ms. Vincent said.
Ms. Vincent added that even the suggestion that cow’s milk protein delivered through breast milk might be inducing symptoms could lead a mother to stop breastfeeding.
The authors also note that in reviewing recent CMA guidelines, “three of nine CMA guidelines were directly supported by formula manufacturers or marketing consultants, and 81% of all guideline authors reported a conflict of interest with formula manufacturers.”
Heather Cassell, MD, a pediatric allergy and immunology specialist with Banner Health and a clinical associate professor of pediatrics at the University of Arizona College of Medicine in Tucson, told this news organization the conflicts of interest in milk allergy research and guidelines have been a long-standing problem.
She said historically there has been a big push “that people who can afford formula should be paying for formula. That was 100% marketed by the formula companies.”
“We have formula companies bringing us samples to encourage pediatricians to use the formula early if we’re concerned about a milk protein allergy,” Dr. Cassell said.
As for the overdiagnosis of milk allergy, she said reintroduction of cow’s milk later is one way to improve diagnosis to see if the child no longer has a reaction. However, she points out that in this study, only 21% of parents reintroduced cow’s milk.
“Really, it should be closer to 100%, with the exception of the babies who are having severe symptoms,” Dr. Cassell said. “You don’t want to keep a baby from progressing with their diet.”
She said families and providers need to look at several contextual clues before they land on a milk allergy label.
“It’s not just about reflux, it’s not just about a baby spitting up. Happy babies spit up and there’s nothing that needs to be done because they will eventually grow out of it,” Dr. Cassell stressed.
She said significant irritability with blood in the stool might warrant more concern. “I think the [emphasis] needs to be on retrying the food another time,” she suggested.
Ms. Vincent pointed out that there is no quick or easy test to diagnose non–IgE-mediated cow’s milk allergy.
“We need further research to identify what symptoms are much more likely to point to a diagnosis,” she said.
Although the researchers used iMAP guidelines, they write that results are likely to apply to other CMA guidelines, because they list similar symptoms and signs.
The study was funded by the International Society of Atopic Dermatitis. Ms. Vincent reports receiving a 3-month research fellowship award from Pfizer and support from the NIHR School for Primary Care Research. Other authors’ financial disclosures are available with the full text. Dr. Cassell reports that the University of Arizona School of Medicine is a trial site for testing a patch to help with diagnosing milk protein allergy in infants.
A version of this article first appeared on Medscape.com.
International guidelines developed to help nonspecialists diagnose cow’s milk allergy (CMA) lead providers to attribute normal infant symptoms to CMA and result in overdiagnosis, say authors of a study published online in Clinical & Experimental Allergy.
Lead author Rosie Vincent, MBChB, with Population Health Sciences at University of Bristol, United Kingdom, told this news organization their study shows that symptoms listed in the international Milk Allergy in Primary Care (iMAP) guidelines as indicative of non-immunoglobulin E (IgE)-mediated milk allergy are very common in a baby’s first year. Examples include vomiting, regurgitating milk, loose or more frequent stools, colic, and irritability.
Findings come from performing a secondary analysis of data from 1,303 infants from the EAT study, a population-based, randomized controlled trial in the U.K. that looked at whether introducing allergenic foods into an infant’s diet reduced the risk of developing an allergy to that food.
In an indication of how common the symptoms in the guidelines (published in 2017 and 2019) are found in all infants, nearly three-fourths (74%) of participants reported at least two mild-to-moderate symptoms, and 9% reported at least two severe symptoms in at least one month between 3 and 12 months of age. Data were not available for younger infants.
However, the prevalence of non–IgE-mediated CMA is thought to be less than 1% in infants in European countries, the study states.
In the study, two or more non-IgE CMA mild-to-moderate symptoms were reported by 25% of families, and 1.4% reported severe symptoms each month between ages 3 and 12 months.
“These symptoms peaked at 38%, with at least two mild-to-moderate symptoms and 4.3% with at least two severe symptoms at 3 months, when participants were not directly consuming cow’s milk,” Ms. Vincent said.
Researchers write that at 6 months there was no significant difference in the proportion of children with at least two symptoms between those consuming and not consuming cow’s milk.
Consequences of misdiagnosis
Overdiagnosing milk allergy can lead to additional costs, unnecessary tests, and less breastfeeding, she said.
Cow’s milk protein is commonly found in standard infant formula or in milk-containing foods.
The authors note that “small levels of lactoglobulin are found in breastmilk; however, the quantities are below the threshold likely to trigger a reaction in more than 99% of infants with IgE-mediated cow’s milk allergy.”
Misdiagnosis is likely to result in increasing prescriptions of unwarranted specialized formula, with increased cost to patients and health care systems, and use of unvalidated allergy tests, Ms. Vincent said.
Ms. Vincent added that even the suggestion that cow’s milk protein delivered through breast milk might be inducing symptoms could lead a mother to stop breastfeeding.
The authors also note that in reviewing recent CMA guidelines, “three of nine CMA guidelines were directly supported by formula manufacturers or marketing consultants, and 81% of all guideline authors reported a conflict of interest with formula manufacturers.”
Heather Cassell, MD, a pediatric allergy and immunology specialist with Banner Health and a clinical associate professor of pediatrics at the University of Arizona College of Medicine in Tucson, told this news organization the conflicts of interest in milk allergy research and guidelines have been a long-standing problem.
She said historically there has been a big push “that people who can afford formula should be paying for formula. That was 100% marketed by the formula companies.”
“We have formula companies bringing us samples to encourage pediatricians to use the formula early if we’re concerned about a milk protein allergy,” Dr. Cassell said.
As for the overdiagnosis of milk allergy, she said reintroduction of cow’s milk later is one way to improve diagnosis to see if the child no longer has a reaction. However, she points out that in this study, only 21% of parents reintroduced cow’s milk.
“Really, it should be closer to 100%, with the exception of the babies who are having severe symptoms,” Dr. Cassell said. “You don’t want to keep a baby from progressing with their diet.”
She said families and providers need to look at several contextual clues before they land on a milk allergy label.
“It’s not just about reflux, it’s not just about a baby spitting up. Happy babies spit up and there’s nothing that needs to be done because they will eventually grow out of it,” Dr. Cassell stressed.
She said significant irritability with blood in the stool might warrant more concern. “I think the [emphasis] needs to be on retrying the food another time,” she suggested.
Ms. Vincent pointed out that there is no quick or easy test to diagnose non–IgE-mediated cow’s milk allergy.
“We need further research to identify what symptoms are much more likely to point to a diagnosis,” she said.
Although the researchers used iMAP guidelines, they write that results are likely to apply to other CMA guidelines, because they list similar symptoms and signs.
The study was funded by the International Society of Atopic Dermatitis. Ms. Vincent reports receiving a 3-month research fellowship award from Pfizer and support from the NIHR School for Primary Care Research. Other authors’ financial disclosures are available with the full text. Dr. Cassell reports that the University of Arizona School of Medicine is a trial site for testing a patch to help with diagnosing milk protein allergy in infants.
A version of this article first appeared on Medscape.com.
FROM CLINICAL & EXPERIMENTAL ALLERGY
Physician gender pay gap isn’t news; health inequity is rampant
A recent study examined projected career earnings between the genders in a largely community-based physician population, finding a difference of about $2 million in career earnings. That a gender pay gap exists in medicine is not news – but the manner in which this study was done, the investigators’ ability to control for a number of confounding variables, and the size of the study group (over 80,000) are newsworthy.
Some of the key findings include that gender pay gaps start with your first job, and you never close the gap, even as you gain experience and efficiency. Also, the more highly remunerated your specialty, the larger the gap. The gender pay gap joins a growing list of inequities within health care. Although physician compensation is not the most important, given that nearly all physicians are well-paid, and we have much more significant inequities that lead to direct patient harm, the reasons for this discrepancy warrant further consideration.
When I was first being educated about social inequity as part of work in social determinants of health, I made the error of using “inequality” and “inequity” interchangeably. The subtle yet important difference between the two terms was quickly described to me. Inequality is a gastroenterologist getting paid more money to do a colonoscopy than a family physician. Inequity is a female gastroenterologist getting paid less than a male gastroenterologist. Global Health Europe boldly identifies that “inequity is the result of failure.” In looking at the inequity inherent in the gender pay gap, I consider what failed and why.
I’m currently making a major career change, leaving an executive leadership position to return to full-time clinical practice. There is a significant pay decrease that will accompany this change because I am in a primary care specialty. Beyond that, I am considering two employment contracts from different systems to do a similar clinical role.
One of the questions my husband asked was which will pay more over the long run. This is difficult to discern because the compensation formula each health system uses is different, even though they are based on standard national benchmarking data. It is possible that women, in general, are like I am and look for factors other than compensation to make a job decision – assuming, like I do, that it will be close enough to not matter or is generally fair. In fact, while compensation is most certainly a consideration for me, once I determined that it was likely to be in the same ballpark, I stopped comparing. Even as the sole breadwinner in our family, I take this (probably faulty) approach.
It’s time to reconsider how we pay physicians
Women may be more likely to gloss over compensation details that men evaluate and negotiate carefully. To change this, women must first take responsibility for being an active, informed, and engaged part of compensation negotiations. In addition, employers who value gender pay equity must negotiate in good faith, keeping in mind the well-described vulnerabilities in discussions about pay. Finally, male and female mentors and leaders should actively coach female physicians on how to approach these conversations with confidence and skill.
In primary care, female physicians spend, on average, about 15% more time with their patients during a visit. Despite spending as much time in clinic seeing patients per week, they see fewer patients, thereby generating less revenue. For compensation plans that are based on productivity, the extra time spent costs money. In this case, it costs the female physicians lost compensation.
The way in which women are more likely to practice medicine, which includes the amount of time they spend with patients, may affect clinical outcomes without directly increasing productivity. A 2017 study demonstrated that elderly patients had lower rates of mortality and readmission when cared for by a female rather than a male physician. These findings require health systems to critically evaluate what compensation plans value and to promote an appropriate balance between quality of care, quantity of care, and style of care.
Although I’ve seen gender pay inequity as blatant as two different salaries for physicians doing the same work – one male and one female – I think this is uncommon. Like many forms of inequity, the outputs are often related to a failed system rather than solely a series of individual failures. Making compensation formulas gender-blind is an important step – but it is only the first step, not the last. Recognizing that the structure of a compensation formula may be biased toward a style of medical practice more likely to be espoused by one gender is necessary as well.
The data, including the findings of this recent study, clearly identify the gender pay gap that exists in medicine, as it does in many other fields, and that it is not explainable solely by differences in specialties, work hours, family status, or title.
To address the inequity, it is imperative that women engage with employers and leaders to both understand and develop skills around effective and appropriate compensation negotiation. Recognizing that compensation plans, especially those built on productivity models, may fail to place adequate value on gender-specific practice styles.
Jennifer Frank is a family physician, physician leader, wife, and mother in Northeast Wisconsin.
A version of this article first appeared on Medscape.com.
A recent study examined projected career earnings between the genders in a largely community-based physician population, finding a difference of about $2 million in career earnings. That a gender pay gap exists in medicine is not news – but the manner in which this study was done, the investigators’ ability to control for a number of confounding variables, and the size of the study group (over 80,000) are newsworthy.
Some of the key findings include that gender pay gaps start with your first job, and you never close the gap, even as you gain experience and efficiency. Also, the more highly remunerated your specialty, the larger the gap. The gender pay gap joins a growing list of inequities within health care. Although physician compensation is not the most important, given that nearly all physicians are well-paid, and we have much more significant inequities that lead to direct patient harm, the reasons for this discrepancy warrant further consideration.
When I was first being educated about social inequity as part of work in social determinants of health, I made the error of using “inequality” and “inequity” interchangeably. The subtle yet important difference between the two terms was quickly described to me. Inequality is a gastroenterologist getting paid more money to do a colonoscopy than a family physician. Inequity is a female gastroenterologist getting paid less than a male gastroenterologist. Global Health Europe boldly identifies that “inequity is the result of failure.” In looking at the inequity inherent in the gender pay gap, I consider what failed and why.
I’m currently making a major career change, leaving an executive leadership position to return to full-time clinical practice. There is a significant pay decrease that will accompany this change because I am in a primary care specialty. Beyond that, I am considering two employment contracts from different systems to do a similar clinical role.
One of the questions my husband asked was which will pay more over the long run. This is difficult to discern because the compensation formula each health system uses is different, even though they are based on standard national benchmarking data. It is possible that women, in general, are like I am and look for factors other than compensation to make a job decision – assuming, like I do, that it will be close enough to not matter or is generally fair. In fact, while compensation is most certainly a consideration for me, once I determined that it was likely to be in the same ballpark, I stopped comparing. Even as the sole breadwinner in our family, I take this (probably faulty) approach.
It’s time to reconsider how we pay physicians
Women may be more likely to gloss over compensation details that men evaluate and negotiate carefully. To change this, women must first take responsibility for being an active, informed, and engaged part of compensation negotiations. In addition, employers who value gender pay equity must negotiate in good faith, keeping in mind the well-described vulnerabilities in discussions about pay. Finally, male and female mentors and leaders should actively coach female physicians on how to approach these conversations with confidence and skill.
In primary care, female physicians spend, on average, about 15% more time with their patients during a visit. Despite spending as much time in clinic seeing patients per week, they see fewer patients, thereby generating less revenue. For compensation plans that are based on productivity, the extra time spent costs money. In this case, it costs the female physicians lost compensation.
The way in which women are more likely to practice medicine, which includes the amount of time they spend with patients, may affect clinical outcomes without directly increasing productivity. A 2017 study demonstrated that elderly patients had lower rates of mortality and readmission when cared for by a female rather than a male physician. These findings require health systems to critically evaluate what compensation plans value and to promote an appropriate balance between quality of care, quantity of care, and style of care.
Although I’ve seen gender pay inequity as blatant as two different salaries for physicians doing the same work – one male and one female – I think this is uncommon. Like many forms of inequity, the outputs are often related to a failed system rather than solely a series of individual failures. Making compensation formulas gender-blind is an important step – but it is only the first step, not the last. Recognizing that the structure of a compensation formula may be biased toward a style of medical practice more likely to be espoused by one gender is necessary as well.
The data, including the findings of this recent study, clearly identify the gender pay gap that exists in medicine, as it does in many other fields, and that it is not explainable solely by differences in specialties, work hours, family status, or title.
To address the inequity, it is imperative that women engage with employers and leaders to both understand and develop skills around effective and appropriate compensation negotiation. Recognizing that compensation plans, especially those built on productivity models, may fail to place adequate value on gender-specific practice styles.
Jennifer Frank is a family physician, physician leader, wife, and mother in Northeast Wisconsin.
A version of this article first appeared on Medscape.com.
A recent study examined projected career earnings between the genders in a largely community-based physician population, finding a difference of about $2 million in career earnings. That a gender pay gap exists in medicine is not news – but the manner in which this study was done, the investigators’ ability to control for a number of confounding variables, and the size of the study group (over 80,000) are newsworthy.
Some of the key findings include that gender pay gaps start with your first job, and you never close the gap, even as you gain experience and efficiency. Also, the more highly remunerated your specialty, the larger the gap. The gender pay gap joins a growing list of inequities within health care. Although physician compensation is not the most important, given that nearly all physicians are well-paid, and we have much more significant inequities that lead to direct patient harm, the reasons for this discrepancy warrant further consideration.
When I was first being educated about social inequity as part of work in social determinants of health, I made the error of using “inequality” and “inequity” interchangeably. The subtle yet important difference between the two terms was quickly described to me. Inequality is a gastroenterologist getting paid more money to do a colonoscopy than a family physician. Inequity is a female gastroenterologist getting paid less than a male gastroenterologist. Global Health Europe boldly identifies that “inequity is the result of failure.” In looking at the inequity inherent in the gender pay gap, I consider what failed and why.
I’m currently making a major career change, leaving an executive leadership position to return to full-time clinical practice. There is a significant pay decrease that will accompany this change because I am in a primary care specialty. Beyond that, I am considering two employment contracts from different systems to do a similar clinical role.
One of the questions my husband asked was which will pay more over the long run. This is difficult to discern because the compensation formula each health system uses is different, even though they are based on standard national benchmarking data. It is possible that women, in general, are like I am and look for factors other than compensation to make a job decision – assuming, like I do, that it will be close enough to not matter or is generally fair. In fact, while compensation is most certainly a consideration for me, once I determined that it was likely to be in the same ballpark, I stopped comparing. Even as the sole breadwinner in our family, I take this (probably faulty) approach.
It’s time to reconsider how we pay physicians
Women may be more likely to gloss over compensation details that men evaluate and negotiate carefully. To change this, women must first take responsibility for being an active, informed, and engaged part of compensation negotiations. In addition, employers who value gender pay equity must negotiate in good faith, keeping in mind the well-described vulnerabilities in discussions about pay. Finally, male and female mentors and leaders should actively coach female physicians on how to approach these conversations with confidence and skill.
In primary care, female physicians spend, on average, about 15% more time with their patients during a visit. Despite spending as much time in clinic seeing patients per week, they see fewer patients, thereby generating less revenue. For compensation plans that are based on productivity, the extra time spent costs money. In this case, it costs the female physicians lost compensation.
The way in which women are more likely to practice medicine, which includes the amount of time they spend with patients, may affect clinical outcomes without directly increasing productivity. A 2017 study demonstrated that elderly patients had lower rates of mortality and readmission when cared for by a female rather than a male physician. These findings require health systems to critically evaluate what compensation plans value and to promote an appropriate balance between quality of care, quantity of care, and style of care.
Although I’ve seen gender pay inequity as blatant as two different salaries for physicians doing the same work – one male and one female – I think this is uncommon. Like many forms of inequity, the outputs are often related to a failed system rather than solely a series of individual failures. Making compensation formulas gender-blind is an important step – but it is only the first step, not the last. Recognizing that the structure of a compensation formula may be biased toward a style of medical practice more likely to be espoused by one gender is necessary as well.
The data, including the findings of this recent study, clearly identify the gender pay gap that exists in medicine, as it does in many other fields, and that it is not explainable solely by differences in specialties, work hours, family status, or title.
To address the inequity, it is imperative that women engage with employers and leaders to both understand and develop skills around effective and appropriate compensation negotiation. Recognizing that compensation plans, especially those built on productivity models, may fail to place adequate value on gender-specific practice styles.
Jennifer Frank is a family physician, physician leader, wife, and mother in Northeast Wisconsin.
A version of this article first appeared on Medscape.com.
Alternative rheumatology practice models aim to avoid traditional limitations
Elizabeth Ortiz, MD, knew she needed a change. Working at an academic county clinic, she was often worn down and pulled in different directions. “When I thought about what I really liked about my job, it was patient care and spending time with my patients, which I wasn’t able to do,” Dr. Ortiz said during the annual meeting of the American College of Rheumatology.
She’d heard of direct or concierge care but wasn’t sure if it was a good fit for her. COVID-19 offered a catalyst of sorts for a move to a new care model.
Ten weeks after she moved to Dallas, the pandemic hit full force. Seeing how telehealth was taking off, Dr. Ortiz began crafting a new model of care, a hybrid of telemedicine and house calls that offered multiple venues to connect with patients. The practice is just a year old, and “it’s working and it’s a constant experiment,” said Dr. Ortiz, who offers membership plans and prepaid appointments. She also does “a la carte” visits where established patients can see her at a one-off price. Her goal is to achieve 100% membership.
Although she operates through a direct pay and cash-only model, only recently has she become comfortable with the word “concierge.” There’s a preconceived notion of what that word means, she said.
Direct care: A definition
Following the trend of some primary care practices, more rheumatologists who are dissatisfied with the status quo are embracing these models of care.
Direct and concierge care are often mentioned in tandem, but there are nuanced differences. Direct specialty care removes third-party payers to protect the best interests of patients, according to Diana Girnita, MD, founder and CEO of Rheumatologist OnCall, a direct care practice. Her patient base hails from rural and urban areas in least 10 states. She also created a Facebook group for specialists in direct care and is the cofounder of the Direct Specialty Care Alliance.
Direct care offers a membership fee and additional fees for “as needed” services. “As the physician, I do not have to be contracted to an insurance company to see patients. I contract directly with patients. It is the patient’s choice to contract with an insurance and use the insurance for ancillary services and medication,” Dr. Girnita said. Patients with out-of-network benefits can claim the insurance to cover part of the consultation cost, she added.
In concierge or retainer medicine, a patient pays an annual or monthly fee or retainer to get access to the physician practice. In addition to this fee, the practice can bill the patient’s insurance for consultations or other services. “The concierge model does not eliminate the sub payer. You still contract with the patient’s insurance,” explained Dr. Girnita.
Physicians who establish these models sometimes do a hybrid of cash only and insurance. Micah Yu, MD, who practices rheumatology in Newport Beach, Calif., only takes Medicare. “Otherwise, patients are private pay. I am mainly fee for service, so patients are paying me for my time,” he said.
By tailoring their patient base and services, adopters find they have more time to spend with patients. “In my model, I spend 30 minutes for follow-up and 1 hour for new patients,” Dr. Yu said.
Limitations of traditional care
Carrying insurance doesn’t guarantee you the right care, Dr. Girnita said. Wait times to see a rheumatologist range from 4 to 6 months. For physicians who contract with insurance companies, reimbursement for services isn’t always paid promptly and decreases every year. A new cut in reimbursement is expected for rheumatology services in 2022.
Patients in direct care “pay a small amount for memberships that cover the cost of their visits and the time physicians spend in coordinating their additional care between the visits. The cost of the visits is always transparent,” Dr. Girnita said.
Irene Kazmers, MD, a solo private rheumatology practitioner in northern Michigan, was seeing 20-plus patients a day before she made the leap to a concierge model. “The paperwork and administrative burdens of practicing rheumatology as a solo [physician] have mushroomed in the last 10 years,” she said during the ACR meeting. She and staff were spending an inordinate amount of time on prior authorizations, step therapy requirements, electronic health record documentation, and other administrative burdens.
Reimbursements from payers have progressively declined as administrative challenges have necessitated more staff. “I was struggling to maintain an ample financial margin,” she said.
Improved communication, unlimited visits
Dr. Kazmers attests that the transition to the concierge model has enabled and fostered a higher level of communication and specialty care for her patients.
Patients who enroll in the practice pay an annual membership fee and get access to her personal cell phone number and email address. “If they need an urgent appointment, it is typically arranged the same or next day,” she said in an interview. “Visits are not as rushed as in the traditional model, conducive to incorporating beneficial integrative medicine modalities such as dietary, exercise, and mind-body approaches as appropriate, in addition to state-of-the-art treatment.”
She also has more time to coordinate care with her patients’ primary care providers and other care team professionals and to give patients feedback on lab and study results.
Dr. Girnita has ramped down from 28 to 15 patients a day. She’s able to spend 60 minutes for new patients and 30 minutes for follow-ups. Like Dr. Kazmers, she feels she has more time to address patient needs and listen to their concerns.
She’s kept her hospital affiliations but finds that she doesn’t have to go to the hospital as much as she used to. Direct care “reduces hospital visits because physicians significantly have much more time to spend with the patient and address the needs of the patient.” A patient with a gout flare, for example, may end up in the hospital under traditional care because there’s no room in the physician’s schedule to address the patient’s needs.
Dr. Girnita recalled when she assisted a patient who had developed inflammatory arthritis and was desperate to see a doctor. The patient had good insurance, but appointments in her area weren’t available for at least 6 months. “Her primary care physician called me. I saw her and provided her with the appropriate care. A couple of months later she is doing great.”
What insurance does and doesn’t cover
Many patients who seek out direct or concierge models retain their insurance. At least 90% of Dr. Girnita’s patients have insurance with high deductibles. The other 10% have other types of insurance or no insurance.
Ellen McKnight, MD, who has a hybrid rheumatology practice in Pensacola, Fla., still accepts commercial insurance, but has opted out of Medicare. Her patients mostly come from rural areas in Florida, and their insurance situations vary widely. “In my practice, I estimate that 65% have insurance and 35% do not. Most of my patients have commercial insurance, and a substantial portion, about 40%, are just paying cash. My cash pay patients have Medicare, HMOs, and others are uninsured,” she said in an interview.
Direct care practices may continue to bill traditional insurance for items like visits, injections, and ultrasound.
Dr. Girnita’s patients have the option of submitting a “superbill” or invoice to insurance companies for patients to be reimbursed by their insurance for the cost of the visit. It contains the CPT code for the visit along with the ICD-10 codes for diagnoses. “I use a company called Reimbursify to help patients submit their invoice to their insurance company,” Dr. Girnita said.
Dr. Ortiz takes a different approach, offering superbills for consults and individual appointments, but not for patients enrolled in her membership program.
Some in the payer industry contend that direct care arrangements increase costs and distort risk pools. If most direct care patients already have a comprehensive health insurance policy, it’s likely they’re being billed twice for services, said David Allen, spokesperson for America’s Health Insurance Plans.
“Duplicative payments inflate the cost of care at a convenience to the providers and increase the cost of insurance premiums when insurers receive bills for those same services from providers. In other words, patients are being double billed,” Mr. Allen said.
These providers are assuming risk without state insurance oversight or regulations to ensure patient protections and safeguards are in place, he continued. “If utilization of services outpaces capacity, the provider may ultimately be unable to provide the amount of care expected by the patient because their practice agreed to unlimited visits and services with little or no restrictions.”
Eliminating ‘surprise’ bills
Adopters of direct care/concierge services counter that it’s the insurance and pharmaceutical companies driving up costs. Patients – especially those who have high-deductible plans – save money through these models. “In the direct care model, doctors have worked out advocacy for patients that are unsurpassed. Insurance companies don’t do that,” Dr. McKnight said.
Consumers know up front what the price is for other services. When you go to a restaurant, you always look on the menu to see what the price is for a bottle of wine or steak, Dr. Girnita said. “Only in the medical field you don’t know anything. And you’re shocked about the price you must pay.” Not many practices list their prices on their website, although federal rules seek to further increase price transparency in hospitals and among insurers.
Patients will sometimes get a “surprise” bill for their visit, laboratory, or imaging tests. According to Dr. Girnita, “that doesn’t happen in my practice. I discuss all prices with them before they get to the lab or MRI. I don’t charge copayments or anything extra.” Without a copayment – usually $50-$75 for specialist services – or a surprise bill, patients are always paying less, she said.
Costs through insurance are oftentimes higher, she continued. For routine lab work, a patient in a direct care practice pays about $30-$40. If they request this work through a lab, they’re likely to pay $150. “Think about an MRI. Through a direct care practice like mine, you pay $450-$700. In a hospital setting, you pay at least $5,000.”
Patients with high-deductible insurance plans often pay thousands of dollars before meeting their deductible, Dr. Girnita and others noted. A patient with this type of plan may pay $250 for a vitamin D lab if they haven’t met their deductible, Dr. McKnight explained. “With direct care, you’ll be paying $12.50.”
Dr. Girnita said her members get excellent discounts for labs and imaging. In the direct care models, physicians can help with this by contracting directly with labs, imaging centers, and independent pharmacies, giving patients access to affordable and transparent prices for their medical care.
What patients pay for services
In direct and concierge care membership models, coverage for services and fees vary widely from practice to practice.
Dr. Girnita offers several membership options. One package, which is $199 a month, is for patients with stable symptoms that guarantee continuity of care. It includes four visits a year and immediate access to the practice in case of emergency (including two additional urgent visits). “This works for a lot of patients. They consider that affordable, and they have all the benefits of a concierge practice. They can have direct communication with me, and they have guaranteed continuity of care,” Dr. Girnita said.
The other model, which is $299 per month, is for patients who need monthly contact with the rheumatologist for visits, telephone and email communications, urgent appointments, integrative medicine consultations, and many other benefits. For 1-hour consultations, Dr. Girnita charges $399.
Dr. Ortiz, who offers a direct pay model, charges $899 for an initial consult, which covers 3.5 hours of her time. “We do an hour of telemedicine, and we do a house call, which is 1.5-2 hours.” She follows up with a telehealth visit. Labs and x-rays are not included and go through the patient’s insurance.
Once the consult takes place, she assesses what a patient needs and offers them either a 6- or 12-month membership, which includes unlimited visits.
Patients can also buy a prepaid, six-appointment package with a 12% discount. Dr. Ortiz prices her telehealth visits at $350 and house calls at $550.
Dr. McKnight’s cash-only model for established patients offers four visits a year, reducing the fee for each visit. For example, a patient will pay $95 for the first visit, then $90, $85, and $80 for subsequent visits.
Accessing medications through direct care
One challenge with this model is finding affordable medications for patients outside of insurance.
Insurance dictates what’s covered, leaving fewer options for patients, Dr. McKnight said. “You have to jump through hoops, and there’s prior authorizations.” For a condition like severe osteoporosis, treatment should start sequentially with the true bone builders first, then move on to a medication like alendronate (Fosamax).
“Insurers will make you go to Fosamax first and then fail it,” she said. This results in the patient potentially developing worsening bone loss or possibly even sustaining a fracture.
Prior authorization requirements demand excessive staff time and effort, Dr. Kazmers said. This can translate to more than $90,000 a year in human resource costs for rheumatologists, who often deal with many specialty drug authorizations. “Every practice needs to hire staff to handle prior authorizations. We receive no compensation for this from the pharmaceutical companies and middlemen who ultimately profit from this cumbersome process,” she added.
Among the two big classes for rheumatology patients, conventional synthetic disease-modifying antirheumatic drugs (DMARDs) are the most widely available. Pharmacies can offer DMARDs for cash, although some are limited in terms of where they can ship, Dr. Girnita said.
The other class, biologic DMARDs, are the most expensive medications rheumatologists use for conditions such as rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis.
With biologics, it’s more difficult, as they’re very expensive, typically $6,000 a month or more, sources told this news organization.
“Unfortunately, we can’t partner at this time with pharmaceutical companies that produce biologics or independent pharmacies,” Dr. Girnita said. Physicians can’t control biologic prices either. “Insurance companies and pharmacy benefit managers have the control on these prices.”
Physicians can direct patients to multiple resources where they can find assistance.
Biologics companies that offer patient assistance programs can sometimes offer medications for free, while others offer savings cards or copay cards, “which helps a lot,” Dr. Girnita said. She assists her patients by filing some of the paperwork necessary to qualify for these programs, and the patients submit the rest.
“For these companies to help the patient, they need the patient’s financial information,” she said. “But I do most of that work; I complete the forms and send to the company and justify need for the medication.”
What’s ahead for direct specialty care
While some patients have benefited, others have had to seek alternatives as their doctors transition to alternative models.
Not everyone can afford the concierge retainer fee, said Dr. Kazmers, who practices in a rural area of Michigan, where rheumatologists are scarce. Enrollment in her concierge practice filled months before the switchover from her traditional practice took place. There are 70 patients on a waiting list.
Patients who elect not to enroll in the concierge practice need to find another source of rheumatology care. This is a downside to the practice transition, she acknowledged. “The closest rheumatologist taking new patients is a 3- to 4-hour drive away, which simply reflects the shortage of medical school graduates choosing to go into rheumatology in the United States,” Dr. Kazmers added.
One physician caring for thousands of chronically ill, complicated patients within systems that don’t allow them the time to really care for their patients threatens to make the access problem worse, Dr. Ortiz said. The direct care/concierge model offers an alternative for the provider “and is a way to keep providers in the workforce, who may otherwise consider leaving.”
Direct care/concierge medicine isn’t for all doctors. But for Dr. Kazmers, it’s the best option for her at this point in her career. “I’ve been practicing for 45 years in various models, including academic positions and private practice employment. I have worked for years in settings accepting Medicaid. I understand that if every rheumatologist went concierge tomorrow, this would constrict access to needed specialty care. But in my case, it provided a viable alternative to closing the practice’s doors altogether.”
Ultimately, the U.S. medical system needs more rheumatologists and other specialists. “If you really want to increase the service, then Medicare or other sources should support opening more residency and fellowship spots for medical graduates to pursue,” Dr. Girnita said.
Other solutions call for more systemic and institutional changes, such as expanding rheumatology divisions and faculties at institutions that train fellows and addressing medical school debt, Dr. Ortiz said.
Some practices see themselves branching out from individual patient care and partnering with local businesses to provide care for employees. That’s the future for direct specialty care, said Dr. Girnita, who’s been in discussions with a few employers to make such arrangements.
The direct primary care community has already started to contract with employers. “Their employees get care they need for just a fraction of the cost. These discussions are arising more and more,” she said.
A version of this article first appeared on Medscape.com.
Elizabeth Ortiz, MD, knew she needed a change. Working at an academic county clinic, she was often worn down and pulled in different directions. “When I thought about what I really liked about my job, it was patient care and spending time with my patients, which I wasn’t able to do,” Dr. Ortiz said during the annual meeting of the American College of Rheumatology.
She’d heard of direct or concierge care but wasn’t sure if it was a good fit for her. COVID-19 offered a catalyst of sorts for a move to a new care model.
Ten weeks after she moved to Dallas, the pandemic hit full force. Seeing how telehealth was taking off, Dr. Ortiz began crafting a new model of care, a hybrid of telemedicine and house calls that offered multiple venues to connect with patients. The practice is just a year old, and “it’s working and it’s a constant experiment,” said Dr. Ortiz, who offers membership plans and prepaid appointments. She also does “a la carte” visits where established patients can see her at a one-off price. Her goal is to achieve 100% membership.
Although she operates through a direct pay and cash-only model, only recently has she become comfortable with the word “concierge.” There’s a preconceived notion of what that word means, she said.
Direct care: A definition
Following the trend of some primary care practices, more rheumatologists who are dissatisfied with the status quo are embracing these models of care.
Direct and concierge care are often mentioned in tandem, but there are nuanced differences. Direct specialty care removes third-party payers to protect the best interests of patients, according to Diana Girnita, MD, founder and CEO of Rheumatologist OnCall, a direct care practice. Her patient base hails from rural and urban areas in least 10 states. She also created a Facebook group for specialists in direct care and is the cofounder of the Direct Specialty Care Alliance.
Direct care offers a membership fee and additional fees for “as needed” services. “As the physician, I do not have to be contracted to an insurance company to see patients. I contract directly with patients. It is the patient’s choice to contract with an insurance and use the insurance for ancillary services and medication,” Dr. Girnita said. Patients with out-of-network benefits can claim the insurance to cover part of the consultation cost, she added.
In concierge or retainer medicine, a patient pays an annual or monthly fee or retainer to get access to the physician practice. In addition to this fee, the practice can bill the patient’s insurance for consultations or other services. “The concierge model does not eliminate the sub payer. You still contract with the patient’s insurance,” explained Dr. Girnita.
Physicians who establish these models sometimes do a hybrid of cash only and insurance. Micah Yu, MD, who practices rheumatology in Newport Beach, Calif., only takes Medicare. “Otherwise, patients are private pay. I am mainly fee for service, so patients are paying me for my time,” he said.
By tailoring their patient base and services, adopters find they have more time to spend with patients. “In my model, I spend 30 minutes for follow-up and 1 hour for new patients,” Dr. Yu said.
Limitations of traditional care
Carrying insurance doesn’t guarantee you the right care, Dr. Girnita said. Wait times to see a rheumatologist range from 4 to 6 months. For physicians who contract with insurance companies, reimbursement for services isn’t always paid promptly and decreases every year. A new cut in reimbursement is expected for rheumatology services in 2022.
Patients in direct care “pay a small amount for memberships that cover the cost of their visits and the time physicians spend in coordinating their additional care between the visits. The cost of the visits is always transparent,” Dr. Girnita said.
Irene Kazmers, MD, a solo private rheumatology practitioner in northern Michigan, was seeing 20-plus patients a day before she made the leap to a concierge model. “The paperwork and administrative burdens of practicing rheumatology as a solo [physician] have mushroomed in the last 10 years,” she said during the ACR meeting. She and staff were spending an inordinate amount of time on prior authorizations, step therapy requirements, electronic health record documentation, and other administrative burdens.
Reimbursements from payers have progressively declined as administrative challenges have necessitated more staff. “I was struggling to maintain an ample financial margin,” she said.
Improved communication, unlimited visits
Dr. Kazmers attests that the transition to the concierge model has enabled and fostered a higher level of communication and specialty care for her patients.
Patients who enroll in the practice pay an annual membership fee and get access to her personal cell phone number and email address. “If they need an urgent appointment, it is typically arranged the same or next day,” she said in an interview. “Visits are not as rushed as in the traditional model, conducive to incorporating beneficial integrative medicine modalities such as dietary, exercise, and mind-body approaches as appropriate, in addition to state-of-the-art treatment.”
She also has more time to coordinate care with her patients’ primary care providers and other care team professionals and to give patients feedback on lab and study results.
Dr. Girnita has ramped down from 28 to 15 patients a day. She’s able to spend 60 minutes for new patients and 30 minutes for follow-ups. Like Dr. Kazmers, she feels she has more time to address patient needs and listen to their concerns.
She’s kept her hospital affiliations but finds that she doesn’t have to go to the hospital as much as she used to. Direct care “reduces hospital visits because physicians significantly have much more time to spend with the patient and address the needs of the patient.” A patient with a gout flare, for example, may end up in the hospital under traditional care because there’s no room in the physician’s schedule to address the patient’s needs.
Dr. Girnita recalled when she assisted a patient who had developed inflammatory arthritis and was desperate to see a doctor. The patient had good insurance, but appointments in her area weren’t available for at least 6 months. “Her primary care physician called me. I saw her and provided her with the appropriate care. A couple of months later she is doing great.”
What insurance does and doesn’t cover
Many patients who seek out direct or concierge models retain their insurance. At least 90% of Dr. Girnita’s patients have insurance with high deductibles. The other 10% have other types of insurance or no insurance.
Ellen McKnight, MD, who has a hybrid rheumatology practice in Pensacola, Fla., still accepts commercial insurance, but has opted out of Medicare. Her patients mostly come from rural areas in Florida, and their insurance situations vary widely. “In my practice, I estimate that 65% have insurance and 35% do not. Most of my patients have commercial insurance, and a substantial portion, about 40%, are just paying cash. My cash pay patients have Medicare, HMOs, and others are uninsured,” she said in an interview.
Direct care practices may continue to bill traditional insurance for items like visits, injections, and ultrasound.
Dr. Girnita’s patients have the option of submitting a “superbill” or invoice to insurance companies for patients to be reimbursed by their insurance for the cost of the visit. It contains the CPT code for the visit along with the ICD-10 codes for diagnoses. “I use a company called Reimbursify to help patients submit their invoice to their insurance company,” Dr. Girnita said.
Dr. Ortiz takes a different approach, offering superbills for consults and individual appointments, but not for patients enrolled in her membership program.
Some in the payer industry contend that direct care arrangements increase costs and distort risk pools. If most direct care patients already have a comprehensive health insurance policy, it’s likely they’re being billed twice for services, said David Allen, spokesperson for America’s Health Insurance Plans.
“Duplicative payments inflate the cost of care at a convenience to the providers and increase the cost of insurance premiums when insurers receive bills for those same services from providers. In other words, patients are being double billed,” Mr. Allen said.
These providers are assuming risk without state insurance oversight or regulations to ensure patient protections and safeguards are in place, he continued. “If utilization of services outpaces capacity, the provider may ultimately be unable to provide the amount of care expected by the patient because their practice agreed to unlimited visits and services with little or no restrictions.”
Eliminating ‘surprise’ bills
Adopters of direct care/concierge services counter that it’s the insurance and pharmaceutical companies driving up costs. Patients – especially those who have high-deductible plans – save money through these models. “In the direct care model, doctors have worked out advocacy for patients that are unsurpassed. Insurance companies don’t do that,” Dr. McKnight said.
Consumers know up front what the price is for other services. When you go to a restaurant, you always look on the menu to see what the price is for a bottle of wine or steak, Dr. Girnita said. “Only in the medical field you don’t know anything. And you’re shocked about the price you must pay.” Not many practices list their prices on their website, although federal rules seek to further increase price transparency in hospitals and among insurers.
Patients will sometimes get a “surprise” bill for their visit, laboratory, or imaging tests. According to Dr. Girnita, “that doesn’t happen in my practice. I discuss all prices with them before they get to the lab or MRI. I don’t charge copayments or anything extra.” Without a copayment – usually $50-$75 for specialist services – or a surprise bill, patients are always paying less, she said.
Costs through insurance are oftentimes higher, she continued. For routine lab work, a patient in a direct care practice pays about $30-$40. If they request this work through a lab, they’re likely to pay $150. “Think about an MRI. Through a direct care practice like mine, you pay $450-$700. In a hospital setting, you pay at least $5,000.”
Patients with high-deductible insurance plans often pay thousands of dollars before meeting their deductible, Dr. Girnita and others noted. A patient with this type of plan may pay $250 for a vitamin D lab if they haven’t met their deductible, Dr. McKnight explained. “With direct care, you’ll be paying $12.50.”
Dr. Girnita said her members get excellent discounts for labs and imaging. In the direct care models, physicians can help with this by contracting directly with labs, imaging centers, and independent pharmacies, giving patients access to affordable and transparent prices for their medical care.
What patients pay for services
In direct and concierge care membership models, coverage for services and fees vary widely from practice to practice.
Dr. Girnita offers several membership options. One package, which is $199 a month, is for patients with stable symptoms that guarantee continuity of care. It includes four visits a year and immediate access to the practice in case of emergency (including two additional urgent visits). “This works for a lot of patients. They consider that affordable, and they have all the benefits of a concierge practice. They can have direct communication with me, and they have guaranteed continuity of care,” Dr. Girnita said.
The other model, which is $299 per month, is for patients who need monthly contact with the rheumatologist for visits, telephone and email communications, urgent appointments, integrative medicine consultations, and many other benefits. For 1-hour consultations, Dr. Girnita charges $399.
Dr. Ortiz, who offers a direct pay model, charges $899 for an initial consult, which covers 3.5 hours of her time. “We do an hour of telemedicine, and we do a house call, which is 1.5-2 hours.” She follows up with a telehealth visit. Labs and x-rays are not included and go through the patient’s insurance.
Once the consult takes place, she assesses what a patient needs and offers them either a 6- or 12-month membership, which includes unlimited visits.
Patients can also buy a prepaid, six-appointment package with a 12% discount. Dr. Ortiz prices her telehealth visits at $350 and house calls at $550.
Dr. McKnight’s cash-only model for established patients offers four visits a year, reducing the fee for each visit. For example, a patient will pay $95 for the first visit, then $90, $85, and $80 for subsequent visits.
Accessing medications through direct care
One challenge with this model is finding affordable medications for patients outside of insurance.
Insurance dictates what’s covered, leaving fewer options for patients, Dr. McKnight said. “You have to jump through hoops, and there’s prior authorizations.” For a condition like severe osteoporosis, treatment should start sequentially with the true bone builders first, then move on to a medication like alendronate (Fosamax).
“Insurers will make you go to Fosamax first and then fail it,” she said. This results in the patient potentially developing worsening bone loss or possibly even sustaining a fracture.
Prior authorization requirements demand excessive staff time and effort, Dr. Kazmers said. This can translate to more than $90,000 a year in human resource costs for rheumatologists, who often deal with many specialty drug authorizations. “Every practice needs to hire staff to handle prior authorizations. We receive no compensation for this from the pharmaceutical companies and middlemen who ultimately profit from this cumbersome process,” she added.
Among the two big classes for rheumatology patients, conventional synthetic disease-modifying antirheumatic drugs (DMARDs) are the most widely available. Pharmacies can offer DMARDs for cash, although some are limited in terms of where they can ship, Dr. Girnita said.
The other class, biologic DMARDs, are the most expensive medications rheumatologists use for conditions such as rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis.
With biologics, it’s more difficult, as they’re very expensive, typically $6,000 a month or more, sources told this news organization.
“Unfortunately, we can’t partner at this time with pharmaceutical companies that produce biologics or independent pharmacies,” Dr. Girnita said. Physicians can’t control biologic prices either. “Insurance companies and pharmacy benefit managers have the control on these prices.”
Physicians can direct patients to multiple resources where they can find assistance.
Biologics companies that offer patient assistance programs can sometimes offer medications for free, while others offer savings cards or copay cards, “which helps a lot,” Dr. Girnita said. She assists her patients by filing some of the paperwork necessary to qualify for these programs, and the patients submit the rest.
“For these companies to help the patient, they need the patient’s financial information,” she said. “But I do most of that work; I complete the forms and send to the company and justify need for the medication.”
What’s ahead for direct specialty care
While some patients have benefited, others have had to seek alternatives as their doctors transition to alternative models.
Not everyone can afford the concierge retainer fee, said Dr. Kazmers, who practices in a rural area of Michigan, where rheumatologists are scarce. Enrollment in her concierge practice filled months before the switchover from her traditional practice took place. There are 70 patients on a waiting list.
Patients who elect not to enroll in the concierge practice need to find another source of rheumatology care. This is a downside to the practice transition, she acknowledged. “The closest rheumatologist taking new patients is a 3- to 4-hour drive away, which simply reflects the shortage of medical school graduates choosing to go into rheumatology in the United States,” Dr. Kazmers added.
One physician caring for thousands of chronically ill, complicated patients within systems that don’t allow them the time to really care for their patients threatens to make the access problem worse, Dr. Ortiz said. The direct care/concierge model offers an alternative for the provider “and is a way to keep providers in the workforce, who may otherwise consider leaving.”
Direct care/concierge medicine isn’t for all doctors. But for Dr. Kazmers, it’s the best option for her at this point in her career. “I’ve been practicing for 45 years in various models, including academic positions and private practice employment. I have worked for years in settings accepting Medicaid. I understand that if every rheumatologist went concierge tomorrow, this would constrict access to needed specialty care. But in my case, it provided a viable alternative to closing the practice’s doors altogether.”
Ultimately, the U.S. medical system needs more rheumatologists and other specialists. “If you really want to increase the service, then Medicare or other sources should support opening more residency and fellowship spots for medical graduates to pursue,” Dr. Girnita said.
Other solutions call for more systemic and institutional changes, such as expanding rheumatology divisions and faculties at institutions that train fellows and addressing medical school debt, Dr. Ortiz said.
Some practices see themselves branching out from individual patient care and partnering with local businesses to provide care for employees. That’s the future for direct specialty care, said Dr. Girnita, who’s been in discussions with a few employers to make such arrangements.
The direct primary care community has already started to contract with employers. “Their employees get care they need for just a fraction of the cost. These discussions are arising more and more,” she said.
A version of this article first appeared on Medscape.com.
Elizabeth Ortiz, MD, knew she needed a change. Working at an academic county clinic, she was often worn down and pulled in different directions. “When I thought about what I really liked about my job, it was patient care and spending time with my patients, which I wasn’t able to do,” Dr. Ortiz said during the annual meeting of the American College of Rheumatology.
She’d heard of direct or concierge care but wasn’t sure if it was a good fit for her. COVID-19 offered a catalyst of sorts for a move to a new care model.
Ten weeks after she moved to Dallas, the pandemic hit full force. Seeing how telehealth was taking off, Dr. Ortiz began crafting a new model of care, a hybrid of telemedicine and house calls that offered multiple venues to connect with patients. The practice is just a year old, and “it’s working and it’s a constant experiment,” said Dr. Ortiz, who offers membership plans and prepaid appointments. She also does “a la carte” visits where established patients can see her at a one-off price. Her goal is to achieve 100% membership.
Although she operates through a direct pay and cash-only model, only recently has she become comfortable with the word “concierge.” There’s a preconceived notion of what that word means, she said.
Direct care: A definition
Following the trend of some primary care practices, more rheumatologists who are dissatisfied with the status quo are embracing these models of care.
Direct and concierge care are often mentioned in tandem, but there are nuanced differences. Direct specialty care removes third-party payers to protect the best interests of patients, according to Diana Girnita, MD, founder and CEO of Rheumatologist OnCall, a direct care practice. Her patient base hails from rural and urban areas in least 10 states. She also created a Facebook group for specialists in direct care and is the cofounder of the Direct Specialty Care Alliance.
Direct care offers a membership fee and additional fees for “as needed” services. “As the physician, I do not have to be contracted to an insurance company to see patients. I contract directly with patients. It is the patient’s choice to contract with an insurance and use the insurance for ancillary services and medication,” Dr. Girnita said. Patients with out-of-network benefits can claim the insurance to cover part of the consultation cost, she added.
In concierge or retainer medicine, a patient pays an annual or monthly fee or retainer to get access to the physician practice. In addition to this fee, the practice can bill the patient’s insurance for consultations or other services. “The concierge model does not eliminate the sub payer. You still contract with the patient’s insurance,” explained Dr. Girnita.
Physicians who establish these models sometimes do a hybrid of cash only and insurance. Micah Yu, MD, who practices rheumatology in Newport Beach, Calif., only takes Medicare. “Otherwise, patients are private pay. I am mainly fee for service, so patients are paying me for my time,” he said.
By tailoring their patient base and services, adopters find they have more time to spend with patients. “In my model, I spend 30 minutes for follow-up and 1 hour for new patients,” Dr. Yu said.
Limitations of traditional care
Carrying insurance doesn’t guarantee you the right care, Dr. Girnita said. Wait times to see a rheumatologist range from 4 to 6 months. For physicians who contract with insurance companies, reimbursement for services isn’t always paid promptly and decreases every year. A new cut in reimbursement is expected for rheumatology services in 2022.
Patients in direct care “pay a small amount for memberships that cover the cost of their visits and the time physicians spend in coordinating their additional care between the visits. The cost of the visits is always transparent,” Dr. Girnita said.
Irene Kazmers, MD, a solo private rheumatology practitioner in northern Michigan, was seeing 20-plus patients a day before she made the leap to a concierge model. “The paperwork and administrative burdens of practicing rheumatology as a solo [physician] have mushroomed in the last 10 years,” she said during the ACR meeting. She and staff were spending an inordinate amount of time on prior authorizations, step therapy requirements, electronic health record documentation, and other administrative burdens.
Reimbursements from payers have progressively declined as administrative challenges have necessitated more staff. “I was struggling to maintain an ample financial margin,” she said.
Improved communication, unlimited visits
Dr. Kazmers attests that the transition to the concierge model has enabled and fostered a higher level of communication and specialty care for her patients.
Patients who enroll in the practice pay an annual membership fee and get access to her personal cell phone number and email address. “If they need an urgent appointment, it is typically arranged the same or next day,” she said in an interview. “Visits are not as rushed as in the traditional model, conducive to incorporating beneficial integrative medicine modalities such as dietary, exercise, and mind-body approaches as appropriate, in addition to state-of-the-art treatment.”
She also has more time to coordinate care with her patients’ primary care providers and other care team professionals and to give patients feedback on lab and study results.
Dr. Girnita has ramped down from 28 to 15 patients a day. She’s able to spend 60 minutes for new patients and 30 minutes for follow-ups. Like Dr. Kazmers, she feels she has more time to address patient needs and listen to their concerns.
She’s kept her hospital affiliations but finds that she doesn’t have to go to the hospital as much as she used to. Direct care “reduces hospital visits because physicians significantly have much more time to spend with the patient and address the needs of the patient.” A patient with a gout flare, for example, may end up in the hospital under traditional care because there’s no room in the physician’s schedule to address the patient’s needs.
Dr. Girnita recalled when she assisted a patient who had developed inflammatory arthritis and was desperate to see a doctor. The patient had good insurance, but appointments in her area weren’t available for at least 6 months. “Her primary care physician called me. I saw her and provided her with the appropriate care. A couple of months later she is doing great.”
What insurance does and doesn’t cover
Many patients who seek out direct or concierge models retain their insurance. At least 90% of Dr. Girnita’s patients have insurance with high deductibles. The other 10% have other types of insurance or no insurance.
Ellen McKnight, MD, who has a hybrid rheumatology practice in Pensacola, Fla., still accepts commercial insurance, but has opted out of Medicare. Her patients mostly come from rural areas in Florida, and their insurance situations vary widely. “In my practice, I estimate that 65% have insurance and 35% do not. Most of my patients have commercial insurance, and a substantial portion, about 40%, are just paying cash. My cash pay patients have Medicare, HMOs, and others are uninsured,” she said in an interview.
Direct care practices may continue to bill traditional insurance for items like visits, injections, and ultrasound.
Dr. Girnita’s patients have the option of submitting a “superbill” or invoice to insurance companies for patients to be reimbursed by their insurance for the cost of the visit. It contains the CPT code for the visit along with the ICD-10 codes for diagnoses. “I use a company called Reimbursify to help patients submit their invoice to their insurance company,” Dr. Girnita said.
Dr. Ortiz takes a different approach, offering superbills for consults and individual appointments, but not for patients enrolled in her membership program.
Some in the payer industry contend that direct care arrangements increase costs and distort risk pools. If most direct care patients already have a comprehensive health insurance policy, it’s likely they’re being billed twice for services, said David Allen, spokesperson for America’s Health Insurance Plans.
“Duplicative payments inflate the cost of care at a convenience to the providers and increase the cost of insurance premiums when insurers receive bills for those same services from providers. In other words, patients are being double billed,” Mr. Allen said.
These providers are assuming risk without state insurance oversight or regulations to ensure patient protections and safeguards are in place, he continued. “If utilization of services outpaces capacity, the provider may ultimately be unable to provide the amount of care expected by the patient because their practice agreed to unlimited visits and services with little or no restrictions.”
Eliminating ‘surprise’ bills
Adopters of direct care/concierge services counter that it’s the insurance and pharmaceutical companies driving up costs. Patients – especially those who have high-deductible plans – save money through these models. “In the direct care model, doctors have worked out advocacy for patients that are unsurpassed. Insurance companies don’t do that,” Dr. McKnight said.
Consumers know up front what the price is for other services. When you go to a restaurant, you always look on the menu to see what the price is for a bottle of wine or steak, Dr. Girnita said. “Only in the medical field you don’t know anything. And you’re shocked about the price you must pay.” Not many practices list their prices on their website, although federal rules seek to further increase price transparency in hospitals and among insurers.
Patients will sometimes get a “surprise” bill for their visit, laboratory, or imaging tests. According to Dr. Girnita, “that doesn’t happen in my practice. I discuss all prices with them before they get to the lab or MRI. I don’t charge copayments or anything extra.” Without a copayment – usually $50-$75 for specialist services – or a surprise bill, patients are always paying less, she said.
Costs through insurance are oftentimes higher, she continued. For routine lab work, a patient in a direct care practice pays about $30-$40. If they request this work through a lab, they’re likely to pay $150. “Think about an MRI. Through a direct care practice like mine, you pay $450-$700. In a hospital setting, you pay at least $5,000.”
Patients with high-deductible insurance plans often pay thousands of dollars before meeting their deductible, Dr. Girnita and others noted. A patient with this type of plan may pay $250 for a vitamin D lab if they haven’t met their deductible, Dr. McKnight explained. “With direct care, you’ll be paying $12.50.”
Dr. Girnita said her members get excellent discounts for labs and imaging. In the direct care models, physicians can help with this by contracting directly with labs, imaging centers, and independent pharmacies, giving patients access to affordable and transparent prices for their medical care.
What patients pay for services
In direct and concierge care membership models, coverage for services and fees vary widely from practice to practice.
Dr. Girnita offers several membership options. One package, which is $199 a month, is for patients with stable symptoms that guarantee continuity of care. It includes four visits a year and immediate access to the practice in case of emergency (including two additional urgent visits). “This works for a lot of patients. They consider that affordable, and they have all the benefits of a concierge practice. They can have direct communication with me, and they have guaranteed continuity of care,” Dr. Girnita said.
The other model, which is $299 per month, is for patients who need monthly contact with the rheumatologist for visits, telephone and email communications, urgent appointments, integrative medicine consultations, and many other benefits. For 1-hour consultations, Dr. Girnita charges $399.
Dr. Ortiz, who offers a direct pay model, charges $899 for an initial consult, which covers 3.5 hours of her time. “We do an hour of telemedicine, and we do a house call, which is 1.5-2 hours.” She follows up with a telehealth visit. Labs and x-rays are not included and go through the patient’s insurance.
Once the consult takes place, she assesses what a patient needs and offers them either a 6- or 12-month membership, which includes unlimited visits.
Patients can also buy a prepaid, six-appointment package with a 12% discount. Dr. Ortiz prices her telehealth visits at $350 and house calls at $550.
Dr. McKnight’s cash-only model for established patients offers four visits a year, reducing the fee for each visit. For example, a patient will pay $95 for the first visit, then $90, $85, and $80 for subsequent visits.
Accessing medications through direct care
One challenge with this model is finding affordable medications for patients outside of insurance.
Insurance dictates what’s covered, leaving fewer options for patients, Dr. McKnight said. “You have to jump through hoops, and there’s prior authorizations.” For a condition like severe osteoporosis, treatment should start sequentially with the true bone builders first, then move on to a medication like alendronate (Fosamax).
“Insurers will make you go to Fosamax first and then fail it,” she said. This results in the patient potentially developing worsening bone loss or possibly even sustaining a fracture.
Prior authorization requirements demand excessive staff time and effort, Dr. Kazmers said. This can translate to more than $90,000 a year in human resource costs for rheumatologists, who often deal with many specialty drug authorizations. “Every practice needs to hire staff to handle prior authorizations. We receive no compensation for this from the pharmaceutical companies and middlemen who ultimately profit from this cumbersome process,” she added.
Among the two big classes for rheumatology patients, conventional synthetic disease-modifying antirheumatic drugs (DMARDs) are the most widely available. Pharmacies can offer DMARDs for cash, although some are limited in terms of where they can ship, Dr. Girnita said.
The other class, biologic DMARDs, are the most expensive medications rheumatologists use for conditions such as rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis.
With biologics, it’s more difficult, as they’re very expensive, typically $6,000 a month or more, sources told this news organization.
“Unfortunately, we can’t partner at this time with pharmaceutical companies that produce biologics or independent pharmacies,” Dr. Girnita said. Physicians can’t control biologic prices either. “Insurance companies and pharmacy benefit managers have the control on these prices.”
Physicians can direct patients to multiple resources where they can find assistance.
Biologics companies that offer patient assistance programs can sometimes offer medications for free, while others offer savings cards or copay cards, “which helps a lot,” Dr. Girnita said. She assists her patients by filing some of the paperwork necessary to qualify for these programs, and the patients submit the rest.
“For these companies to help the patient, they need the patient’s financial information,” she said. “But I do most of that work; I complete the forms and send to the company and justify need for the medication.”
What’s ahead for direct specialty care
While some patients have benefited, others have had to seek alternatives as their doctors transition to alternative models.
Not everyone can afford the concierge retainer fee, said Dr. Kazmers, who practices in a rural area of Michigan, where rheumatologists are scarce. Enrollment in her concierge practice filled months before the switchover from her traditional practice took place. There are 70 patients on a waiting list.
Patients who elect not to enroll in the concierge practice need to find another source of rheumatology care. This is a downside to the practice transition, she acknowledged. “The closest rheumatologist taking new patients is a 3- to 4-hour drive away, which simply reflects the shortage of medical school graduates choosing to go into rheumatology in the United States,” Dr. Kazmers added.
One physician caring for thousands of chronically ill, complicated patients within systems that don’t allow them the time to really care for their patients threatens to make the access problem worse, Dr. Ortiz said. The direct care/concierge model offers an alternative for the provider “and is a way to keep providers in the workforce, who may otherwise consider leaving.”
Direct care/concierge medicine isn’t for all doctors. But for Dr. Kazmers, it’s the best option for her at this point in her career. “I’ve been practicing for 45 years in various models, including academic positions and private practice employment. I have worked for years in settings accepting Medicaid. I understand that if every rheumatologist went concierge tomorrow, this would constrict access to needed specialty care. But in my case, it provided a viable alternative to closing the practice’s doors altogether.”
Ultimately, the U.S. medical system needs more rheumatologists and other specialists. “If you really want to increase the service, then Medicare or other sources should support opening more residency and fellowship spots for medical graduates to pursue,” Dr. Girnita said.
Other solutions call for more systemic and institutional changes, such as expanding rheumatology divisions and faculties at institutions that train fellows and addressing medical school debt, Dr. Ortiz said.
Some practices see themselves branching out from individual patient care and partnering with local businesses to provide care for employees. That’s the future for direct specialty care, said Dr. Girnita, who’s been in discussions with a few employers to make such arrangements.
The direct primary care community has already started to contract with employers. “Their employees get care they need for just a fraction of the cost. These discussions are arising more and more,” she said.
A version of this article first appeared on Medscape.com.
A case-based framework for de-escalating conflict
Hospital medicine can be a demanding and fast-paced environment where resources are stretched thin, with both clinicians and patients stressed. A hospitalist’s role is dynamic, serving as an advocate, leader, or role model while working with interdisciplinary and diverse teams for the welfare of the patient. This constellation of pressures makes a degree of conflict inevitable.
Often, an unexpected scenario can render the hospitalist uncertain and yet the hospitalist’s response can escalate or deescalate conflict. The multiple roles that a hospitalist represents may buckle to the single role of advocating for themselves, a colleague, or a patient in a tense scenario. When this happens, many hospitalists feel disempowered to respond.
De-escalation is a practical skill that involves being calm, respectful, and open minded toward the other person, while also maintaining boundaries. Here we provide case-based tips and skills that highlight the role for de-escalation.
Questions to ask yourself in midst of conflict:
- How did the problematic behavior make you feel?
- What will be your approach in handling this?
- When should you address this?
- What is the outcome you are hoping to achieve?
- What is the outcome the other person is hoping to achieve?
Case 1
There is a female physician rounding with your team. Introductions were made at the start of a patient encounter. The patient repeatedly calls the female physician by her first name and refers to a male colleague as “doctor.”
Commentary: This scenario is commonly encountered by women who are physicians. They may be mistaken for the nurse, a technician, or a housekeeper. This exacerbates inequality and impostor syndrome as women can feel unheard, undervalued, and not recognized for their expertise and achievements. It can be challenging for a woman to reaffirm herself as she worries that the patient will not respect her or will think that she is being aggressive.
Approach: It is vital to interject by firmly reintroducing the female physician by her correct title. If you are the subject of this scenario, you may interject by firmly reintroducing yourself. If the patient or a colleague continues to refer to her by her first name, it is appropriate to say, “Please call her Dr. XYZ.” There is likely another female colleague or trainee nearby that will view this scenario as a model for setting boundaries.
To prevent similar future situations, consistently refer to all peers by their title in front of patients and peers in all professional settings (such as lectures, luncheons, etc.) to establish this as a cultural norm. Also, utilize hospital badges that clearly display roles in large letters.
Case 2
During sign out from a colleague, the colleague repeatedly refers to a patient hospitalized with sickle cell disease as a “frequent flyer” and “drug seeker,” and then remarks, “you know how these patients are.”
Commentary: A situation like this raises concerns about bias and stereotyping. Everyone has implicit bias. Recognizing and acknowledging when implicit bias affects objectivity in patient care is vital to providing appropriate care. It can be intimidating to broach this subject with a colleague as it may cause the colleague to become defensive and uncomfortable as revealing another person’s bias can be difficult. But physicians owe it to a patient’s wellbeing to remain objective and to prevent future colleagues from providing subpar care as a result.
Approach: In this case, saying, “Sometimes my previous experiences can affect my thinking. Will you explain what behaviors the patient has shown this admission that are concerning to you? This will allow me to grasp the complexity of the situation.” Another strategy is to share that there are new recommendations for how to use language about patients with sickle cell disease and patients who require opioids as a part of their treatment plan. Your hospitalist group could have a journal club on how bias affects patients and about the best practices in the care of people with sickle cell disease. A next step could be to build a quality improvement project to review the care of patients hospitalized for sickle cell disease or opioid use.
Case 3
You are conducting bedside rounds with your team. Your intern, a person of color, begins to present. The patient interjects by requesting that the intern leave as he “does not want a foreigner taking care” of him.
Commentary: Requests like this can be shocking. The team leader has a responsibility to immediately act to ensure the psychological safety of the team. Ideally, your response should set firm boundaries and expectations that support the learner as a valued and respected clinician and allow the intern to complete the presentation. In this scenario, regardless of the response the patient takes, it is vital to maintain a safe environment for the trainee. It is crucial to debrief with the team immediately after as an exchange of thoughts and emotions in a safe space can allow for everyone to feel welcome. Additionally, this debrief can provide insights to the team leader of how to address similar situations in the future. The opportunity to allow the intern to no longer follow the patient should be offered, and if the intern opts to no longer follow the patient, accommodations should be made.
Approach: “This physician is a member of the medical team, and we are all working together to provide you with the best care. Everyone on this team is an equal. We value diversity of our team members as it allows us to take care of all our patients. We respect you and expect respect for each member of the team. If you feel that you are unable to respect our team members right now, we will leave for now and return later.” To ensure the patient is provided with appropriate care, be sure to debrief with the patient’s nurse.
Conclusion
These scenarios represent some of the many complex interpersonal challenges hospitalists encounter. These approaches are suggestions that are open to improvement as de-escalation of a conflict is a critical and evolving skill and practice.
For more tips on managing conflict, consider reading “Crucial Conversations” by Kerry Patterson and colleagues. These skills can provide the tools we need to recenter ourselves when we are in the midst of these challenging situations.
Dr. Rawal is clinical assistant professor of medicine at the University of Pittsburgh Medical Center. Dr. Ashford is assistant professor and program director in the department of internal medicine/pediatrics at the University of Nebraska Medical Center, Omaha. Dr. Lee and Dr. Barrett are based in the department of internal medicine, University of New Mexico School of Medicine, Albuquerque. This article is sponsored by the SHM Physicians in Training (PIT) committee, which submits quarterly content to The Hospitalist on topics relevant to trainees and early career hospitalists.
Hospital medicine can be a demanding and fast-paced environment where resources are stretched thin, with both clinicians and patients stressed. A hospitalist’s role is dynamic, serving as an advocate, leader, or role model while working with interdisciplinary and diverse teams for the welfare of the patient. This constellation of pressures makes a degree of conflict inevitable.
Often, an unexpected scenario can render the hospitalist uncertain and yet the hospitalist’s response can escalate or deescalate conflict. The multiple roles that a hospitalist represents may buckle to the single role of advocating for themselves, a colleague, or a patient in a tense scenario. When this happens, many hospitalists feel disempowered to respond.
De-escalation is a practical skill that involves being calm, respectful, and open minded toward the other person, while also maintaining boundaries. Here we provide case-based tips and skills that highlight the role for de-escalation.
Questions to ask yourself in midst of conflict:
- How did the problematic behavior make you feel?
- What will be your approach in handling this?
- When should you address this?
- What is the outcome you are hoping to achieve?
- What is the outcome the other person is hoping to achieve?
Case 1
There is a female physician rounding with your team. Introductions were made at the start of a patient encounter. The patient repeatedly calls the female physician by her first name and refers to a male colleague as “doctor.”
Commentary: This scenario is commonly encountered by women who are physicians. They may be mistaken for the nurse, a technician, or a housekeeper. This exacerbates inequality and impostor syndrome as women can feel unheard, undervalued, and not recognized for their expertise and achievements. It can be challenging for a woman to reaffirm herself as she worries that the patient will not respect her or will think that she is being aggressive.
Approach: It is vital to interject by firmly reintroducing the female physician by her correct title. If you are the subject of this scenario, you may interject by firmly reintroducing yourself. If the patient or a colleague continues to refer to her by her first name, it is appropriate to say, “Please call her Dr. XYZ.” There is likely another female colleague or trainee nearby that will view this scenario as a model for setting boundaries.
To prevent similar future situations, consistently refer to all peers by their title in front of patients and peers in all professional settings (such as lectures, luncheons, etc.) to establish this as a cultural norm. Also, utilize hospital badges that clearly display roles in large letters.
Case 2
During sign out from a colleague, the colleague repeatedly refers to a patient hospitalized with sickle cell disease as a “frequent flyer” and “drug seeker,” and then remarks, “you know how these patients are.”
Commentary: A situation like this raises concerns about bias and stereotyping. Everyone has implicit bias. Recognizing and acknowledging when implicit bias affects objectivity in patient care is vital to providing appropriate care. It can be intimidating to broach this subject with a colleague as it may cause the colleague to become defensive and uncomfortable as revealing another person’s bias can be difficult. But physicians owe it to a patient’s wellbeing to remain objective and to prevent future colleagues from providing subpar care as a result.
Approach: In this case, saying, “Sometimes my previous experiences can affect my thinking. Will you explain what behaviors the patient has shown this admission that are concerning to you? This will allow me to grasp the complexity of the situation.” Another strategy is to share that there are new recommendations for how to use language about patients with sickle cell disease and patients who require opioids as a part of their treatment plan. Your hospitalist group could have a journal club on how bias affects patients and about the best practices in the care of people with sickle cell disease. A next step could be to build a quality improvement project to review the care of patients hospitalized for sickle cell disease or opioid use.
Case 3
You are conducting bedside rounds with your team. Your intern, a person of color, begins to present. The patient interjects by requesting that the intern leave as he “does not want a foreigner taking care” of him.
Commentary: Requests like this can be shocking. The team leader has a responsibility to immediately act to ensure the psychological safety of the team. Ideally, your response should set firm boundaries and expectations that support the learner as a valued and respected clinician and allow the intern to complete the presentation. In this scenario, regardless of the response the patient takes, it is vital to maintain a safe environment for the trainee. It is crucial to debrief with the team immediately after as an exchange of thoughts and emotions in a safe space can allow for everyone to feel welcome. Additionally, this debrief can provide insights to the team leader of how to address similar situations in the future. The opportunity to allow the intern to no longer follow the patient should be offered, and if the intern opts to no longer follow the patient, accommodations should be made.
Approach: “This physician is a member of the medical team, and we are all working together to provide you with the best care. Everyone on this team is an equal. We value diversity of our team members as it allows us to take care of all our patients. We respect you and expect respect for each member of the team. If you feel that you are unable to respect our team members right now, we will leave for now and return later.” To ensure the patient is provided with appropriate care, be sure to debrief with the patient’s nurse.
Conclusion
These scenarios represent some of the many complex interpersonal challenges hospitalists encounter. These approaches are suggestions that are open to improvement as de-escalation of a conflict is a critical and evolving skill and practice.
For more tips on managing conflict, consider reading “Crucial Conversations” by Kerry Patterson and colleagues. These skills can provide the tools we need to recenter ourselves when we are in the midst of these challenging situations.
Dr. Rawal is clinical assistant professor of medicine at the University of Pittsburgh Medical Center. Dr. Ashford is assistant professor and program director in the department of internal medicine/pediatrics at the University of Nebraska Medical Center, Omaha. Dr. Lee and Dr. Barrett are based in the department of internal medicine, University of New Mexico School of Medicine, Albuquerque. This article is sponsored by the SHM Physicians in Training (PIT) committee, which submits quarterly content to The Hospitalist on topics relevant to trainees and early career hospitalists.
Hospital medicine can be a demanding and fast-paced environment where resources are stretched thin, with both clinicians and patients stressed. A hospitalist’s role is dynamic, serving as an advocate, leader, or role model while working with interdisciplinary and diverse teams for the welfare of the patient. This constellation of pressures makes a degree of conflict inevitable.
Often, an unexpected scenario can render the hospitalist uncertain and yet the hospitalist’s response can escalate or deescalate conflict. The multiple roles that a hospitalist represents may buckle to the single role of advocating for themselves, a colleague, or a patient in a tense scenario. When this happens, many hospitalists feel disempowered to respond.
De-escalation is a practical skill that involves being calm, respectful, and open minded toward the other person, while also maintaining boundaries. Here we provide case-based tips and skills that highlight the role for de-escalation.
Questions to ask yourself in midst of conflict:
- How did the problematic behavior make you feel?
- What will be your approach in handling this?
- When should you address this?
- What is the outcome you are hoping to achieve?
- What is the outcome the other person is hoping to achieve?
Case 1
There is a female physician rounding with your team. Introductions were made at the start of a patient encounter. The patient repeatedly calls the female physician by her first name and refers to a male colleague as “doctor.”
Commentary: This scenario is commonly encountered by women who are physicians. They may be mistaken for the nurse, a technician, or a housekeeper. This exacerbates inequality and impostor syndrome as women can feel unheard, undervalued, and not recognized for their expertise and achievements. It can be challenging for a woman to reaffirm herself as she worries that the patient will not respect her or will think that she is being aggressive.
Approach: It is vital to interject by firmly reintroducing the female physician by her correct title. If you are the subject of this scenario, you may interject by firmly reintroducing yourself. If the patient or a colleague continues to refer to her by her first name, it is appropriate to say, “Please call her Dr. XYZ.” There is likely another female colleague or trainee nearby that will view this scenario as a model for setting boundaries.
To prevent similar future situations, consistently refer to all peers by their title in front of patients and peers in all professional settings (such as lectures, luncheons, etc.) to establish this as a cultural norm. Also, utilize hospital badges that clearly display roles in large letters.
Case 2
During sign out from a colleague, the colleague repeatedly refers to a patient hospitalized with sickle cell disease as a “frequent flyer” and “drug seeker,” and then remarks, “you know how these patients are.”
Commentary: A situation like this raises concerns about bias and stereotyping. Everyone has implicit bias. Recognizing and acknowledging when implicit bias affects objectivity in patient care is vital to providing appropriate care. It can be intimidating to broach this subject with a colleague as it may cause the colleague to become defensive and uncomfortable as revealing another person’s bias can be difficult. But physicians owe it to a patient’s wellbeing to remain objective and to prevent future colleagues from providing subpar care as a result.
Approach: In this case, saying, “Sometimes my previous experiences can affect my thinking. Will you explain what behaviors the patient has shown this admission that are concerning to you? This will allow me to grasp the complexity of the situation.” Another strategy is to share that there are new recommendations for how to use language about patients with sickle cell disease and patients who require opioids as a part of their treatment plan. Your hospitalist group could have a journal club on how bias affects patients and about the best practices in the care of people with sickle cell disease. A next step could be to build a quality improvement project to review the care of patients hospitalized for sickle cell disease or opioid use.
Case 3
You are conducting bedside rounds with your team. Your intern, a person of color, begins to present. The patient interjects by requesting that the intern leave as he “does not want a foreigner taking care” of him.
Commentary: Requests like this can be shocking. The team leader has a responsibility to immediately act to ensure the psychological safety of the team. Ideally, your response should set firm boundaries and expectations that support the learner as a valued and respected clinician and allow the intern to complete the presentation. In this scenario, regardless of the response the patient takes, it is vital to maintain a safe environment for the trainee. It is crucial to debrief with the team immediately after as an exchange of thoughts and emotions in a safe space can allow for everyone to feel welcome. Additionally, this debrief can provide insights to the team leader of how to address similar situations in the future. The opportunity to allow the intern to no longer follow the patient should be offered, and if the intern opts to no longer follow the patient, accommodations should be made.
Approach: “This physician is a member of the medical team, and we are all working together to provide you with the best care. Everyone on this team is an equal. We value diversity of our team members as it allows us to take care of all our patients. We respect you and expect respect for each member of the team. If you feel that you are unable to respect our team members right now, we will leave for now and return later.” To ensure the patient is provided with appropriate care, be sure to debrief with the patient’s nurse.
Conclusion
These scenarios represent some of the many complex interpersonal challenges hospitalists encounter. These approaches are suggestions that are open to improvement as de-escalation of a conflict is a critical and evolving skill and practice.
For more tips on managing conflict, consider reading “Crucial Conversations” by Kerry Patterson and colleagues. These skills can provide the tools we need to recenter ourselves when we are in the midst of these challenging situations.
Dr. Rawal is clinical assistant professor of medicine at the University of Pittsburgh Medical Center. Dr. Ashford is assistant professor and program director in the department of internal medicine/pediatrics at the University of Nebraska Medical Center, Omaha. Dr. Lee and Dr. Barrett are based in the department of internal medicine, University of New Mexico School of Medicine, Albuquerque. This article is sponsored by the SHM Physicians in Training (PIT) committee, which submits quarterly content to The Hospitalist on topics relevant to trainees and early career hospitalists.
Vegetative Plaques on the Face
THE DIAGNOSIS: Vegetative Majocchi Granuloma
A biopsy and tissue culture showed acute dermal inflammation with granulomatous features and numerous fungal hyphae within the stratum corneum (Figure 1A), which were confirmed on GrocottGomori methenamine-silver staining (Figure 1B). Gram and Fite stains were negative for bacteria. A tissue culture speciated Trichophyton rubrum, which led to a diagnosis of deep dermatophyte infection (Majocchi granuloma) with a highly unusual clinical presentation of vegetative plaques. Predisposing factors included treatment with topical corticosteroids and possibly poor health and nutritional status at baseline. Our patient was treated with fluconazole 200 mg daily for 6 weeks, with near resolution of lesions at 3-week follow-up (Figure 2).
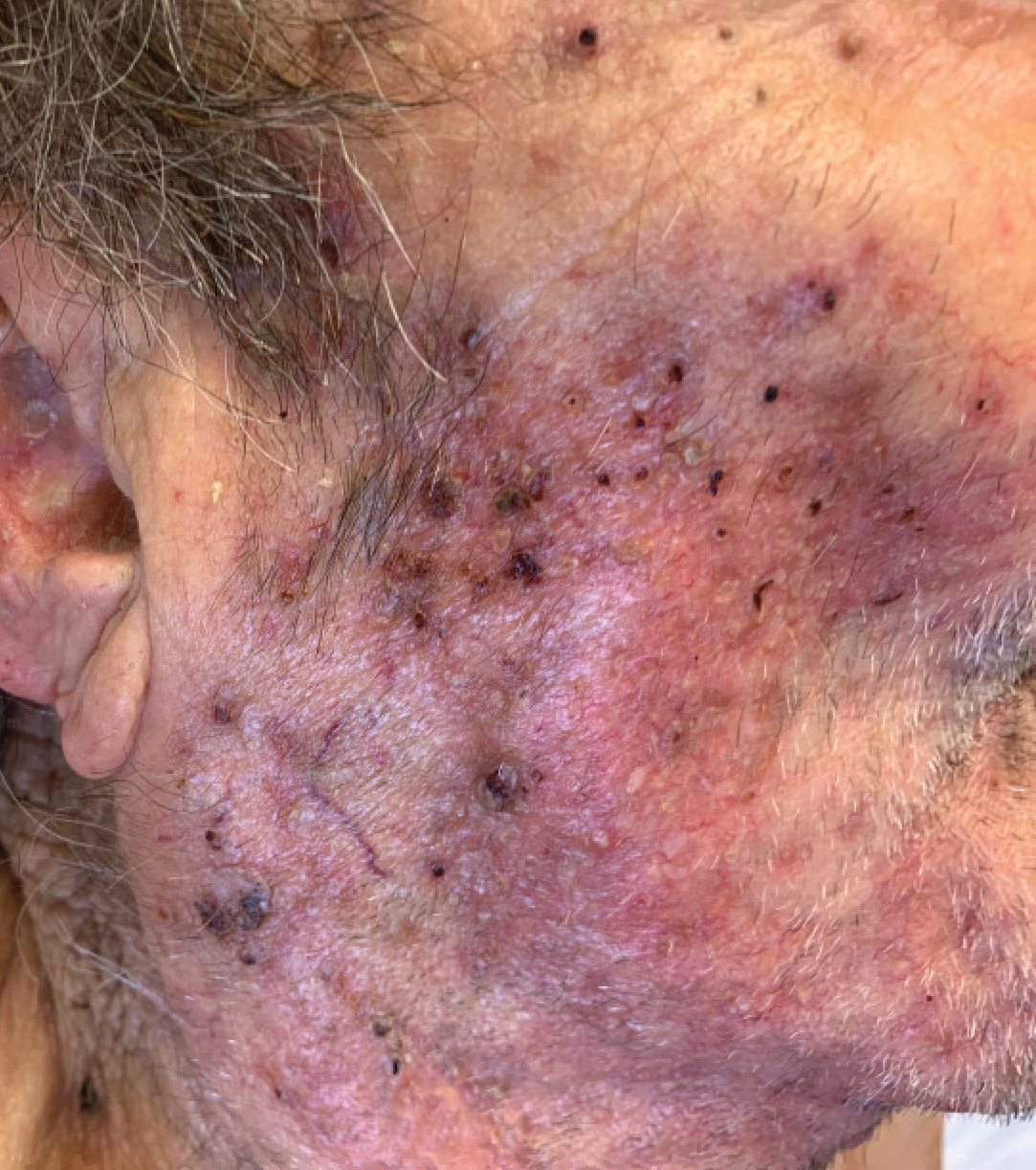
Dermatophytes are a common cause of superficial skin infections. The classic morphology consists of an annular scaly plaque; however, a wide variety of presentations have been observed (eg, verrucous, vesicular, pustular, granulomatous). Therefore, dermatophyte infections often mimic other dermatologic conditions, including atopic dermatitis, rosacea, psoriasis, bacterial abscess, erythema gyratum repens, lupus, granuloma annulare, cutaneous lymphoma, Hailey-Hailey disease, scarring alopecia, and syphilis.1
Notably, when dermatophytes grow downward along hair follicles causing deeper infection, disruption of the follicular wall can lead to an excessive inflammatory response with granulomatous features.2 Risk factors include cutaneous trauma, long-standing infection, immunocompromise, and treatment with topical corticosteroids.3 This disease evolution clinically appears as a nodule or infiltrated plaque, often without scale. The most well-known example is a kerion on the scalp. Elsewhere on the body, lesions often are termed Majocchi granulomas.2
Vegetative plaques, as seen in our patient, are a highly unusual morphology for deep tinea infection. Guanziroli et al4 reported a case of vegetative lesions on the forearm of a 67-year-old immunocompromised man that were successfully treated with a 3-month course of oral terbinafine after Trichophyton verrucosum was isolated. Skorepova et al5 reported a case of pyoderma vegetans triggered by recurrent Trichophyton mentagrophytes on the dorsal hands of a 64-year-old man with immunoglobulin deficiency of unknown etiology. The lesions were successfully treated with a prolonged course of doxycycline, topical triamcinolone, and intravenous immunoglobulin following 2 initial courses of terbinafine.
The differential diagnosis for vegetative lesions includes pemphigus vegetans, a vegetative variant of pyoderma gangrenosum; halogenoderma; and a variety of infections, including dimorphic fungi (histoplasmosis, blastomycosis), blastomycosislike pyoderma (bacterial), and candidiasis.6 These conditions usually can be distinguished based on histopathology. Clinically, pemphigus vegetans presents with pustules and vegetative lesions, as in our patient, but usually is more diffuse and favors the intertriginous areas. Histology likely would reveal foci of acantholysis and eosinophils. Vegetative pyoderma gangrenosum favors the trunk, particularly in sites of surgical trauma. In our patient, no lesions were present near the abdominal surgical sites, and there was no antecedent cribriform ulceration. Halogenoderma was a strong initial consideration given the localization, presence of large pustules, and history of numerous contrast computed tomography studies; however, our patient’s iodine levels were normal. Infectious etiologies including dimorphic fungi and blastomycosislike pyoderma generally are not restricted to the head and neck, and tissue culture helps exclude them. Vegetative lesions may occur in the setting of other infections, and tissue culture may be necessary to differentiate them if histopathology is not suggestive.
Deep dermatophyte infections require treatment with oral antifungals, as topicals do not penetrate adequately into the hair follicles. Exact regimens vary, but generally oral terbinafine or an oral azole (except ketoconazole) is administered for 2 to 6 weeks, with immunocompromise necessitating longer courses.
We present a rare case of vegetative Majocchi granuloma secondary to T rubrum infection. A dermatophyte infection should be included in the differential for vegetative lesions, especially in dense hair-bearing areas such as the beard. Treatment generally is straightforward with oral antifungals.
- Atzori L, Pau M, Aste N, et al. Dermatophyte infections mimicking other skin diseases: a 154-person case survey of tinea atypica in the district of Cagliari (Italy). Int J Dermatol. 2012;51:410-415.
- Ilkit M, Durdu M, Karakas M. Majocchi’s granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457.
- Jevremovic L, Ilijin I, Kostic K, et al. Pyoderma vegetans—a case report. Serbian J Dermatol Venereol. 2017;9:22-28.
- Guanziroli E, Pavia G, Guttadauro A, et al. Deep dermatophytosis caused by Trichophyton verrucosum in an immunosuppressed patient: successful outcome with terbinafine. Mycopathologia. 2019;184:543-545.
- Skorepová M, Stuchlík D. Chronic pyoderma vegetans triggered by Trichophyton mentagrophytes. Mycoses. 2006;49:143-144.
- Reinholz M, Hermans C, Dietrich A, et al. A case of cutaneous vegetating candidiasis in a patient with keratitis-ichthyosis-deafness syndrome. J Eur Acad Dermatol Venereol. 2016;30:537-539.
THE DIAGNOSIS: Vegetative Majocchi Granuloma
A biopsy and tissue culture showed acute dermal inflammation with granulomatous features and numerous fungal hyphae within the stratum corneum (Figure 1A), which were confirmed on GrocottGomori methenamine-silver staining (Figure 1B). Gram and Fite stains were negative for bacteria. A tissue culture speciated Trichophyton rubrum, which led to a diagnosis of deep dermatophyte infection (Majocchi granuloma) with a highly unusual clinical presentation of vegetative plaques. Predisposing factors included treatment with topical corticosteroids and possibly poor health and nutritional status at baseline. Our patient was treated with fluconazole 200 mg daily for 6 weeks, with near resolution of lesions at 3-week follow-up (Figure 2).


Dermatophytes are a common cause of superficial skin infections. The classic morphology consists of an annular scaly plaque; however, a wide variety of presentations have been observed (eg, verrucous, vesicular, pustular, granulomatous). Therefore, dermatophyte infections often mimic other dermatologic conditions, including atopic dermatitis, rosacea, psoriasis, bacterial abscess, erythema gyratum repens, lupus, granuloma annulare, cutaneous lymphoma, Hailey-Hailey disease, scarring alopecia, and syphilis.1
Notably, when dermatophytes grow downward along hair follicles causing deeper infection, disruption of the follicular wall can lead to an excessive inflammatory response with granulomatous features.2 Risk factors include cutaneous trauma, long-standing infection, immunocompromise, and treatment with topical corticosteroids.3 This disease evolution clinically appears as a nodule or infiltrated plaque, often without scale. The most well-known example is a kerion on the scalp. Elsewhere on the body, lesions often are termed Majocchi granulomas.2
Vegetative plaques, as seen in our patient, are a highly unusual morphology for deep tinea infection. Guanziroli et al4 reported a case of vegetative lesions on the forearm of a 67-year-old immunocompromised man that were successfully treated with a 3-month course of oral terbinafine after Trichophyton verrucosum was isolated. Skorepova et al5 reported a case of pyoderma vegetans triggered by recurrent Trichophyton mentagrophytes on the dorsal hands of a 64-year-old man with immunoglobulin deficiency of unknown etiology. The lesions were successfully treated with a prolonged course of doxycycline, topical triamcinolone, and intravenous immunoglobulin following 2 initial courses of terbinafine.
The differential diagnosis for vegetative lesions includes pemphigus vegetans, a vegetative variant of pyoderma gangrenosum; halogenoderma; and a variety of infections, including dimorphic fungi (histoplasmosis, blastomycosis), blastomycosislike pyoderma (bacterial), and candidiasis.6 These conditions usually can be distinguished based on histopathology. Clinically, pemphigus vegetans presents with pustules and vegetative lesions, as in our patient, but usually is more diffuse and favors the intertriginous areas. Histology likely would reveal foci of acantholysis and eosinophils. Vegetative pyoderma gangrenosum favors the trunk, particularly in sites of surgical trauma. In our patient, no lesions were present near the abdominal surgical sites, and there was no antecedent cribriform ulceration. Halogenoderma was a strong initial consideration given the localization, presence of large pustules, and history of numerous contrast computed tomography studies; however, our patient’s iodine levels were normal. Infectious etiologies including dimorphic fungi and blastomycosislike pyoderma generally are not restricted to the head and neck, and tissue culture helps exclude them. Vegetative lesions may occur in the setting of other infections, and tissue culture may be necessary to differentiate them if histopathology is not suggestive.
Deep dermatophyte infections require treatment with oral antifungals, as topicals do not penetrate adequately into the hair follicles. Exact regimens vary, but generally oral terbinafine or an oral azole (except ketoconazole) is administered for 2 to 6 weeks, with immunocompromise necessitating longer courses.
We present a rare case of vegetative Majocchi granuloma secondary to T rubrum infection. A dermatophyte infection should be included in the differential for vegetative lesions, especially in dense hair-bearing areas such as the beard. Treatment generally is straightforward with oral antifungals.
THE DIAGNOSIS: Vegetative Majocchi Granuloma
A biopsy and tissue culture showed acute dermal inflammation with granulomatous features and numerous fungal hyphae within the stratum corneum (Figure 1A), which were confirmed on GrocottGomori methenamine-silver staining (Figure 1B). Gram and Fite stains were negative for bacteria. A tissue culture speciated Trichophyton rubrum, which led to a diagnosis of deep dermatophyte infection (Majocchi granuloma) with a highly unusual clinical presentation of vegetative plaques. Predisposing factors included treatment with topical corticosteroids and possibly poor health and nutritional status at baseline. Our patient was treated with fluconazole 200 mg daily for 6 weeks, with near resolution of lesions at 3-week follow-up (Figure 2).


Dermatophytes are a common cause of superficial skin infections. The classic morphology consists of an annular scaly plaque; however, a wide variety of presentations have been observed (eg, verrucous, vesicular, pustular, granulomatous). Therefore, dermatophyte infections often mimic other dermatologic conditions, including atopic dermatitis, rosacea, psoriasis, bacterial abscess, erythema gyratum repens, lupus, granuloma annulare, cutaneous lymphoma, Hailey-Hailey disease, scarring alopecia, and syphilis.1
Notably, when dermatophytes grow downward along hair follicles causing deeper infection, disruption of the follicular wall can lead to an excessive inflammatory response with granulomatous features.2 Risk factors include cutaneous trauma, long-standing infection, immunocompromise, and treatment with topical corticosteroids.3 This disease evolution clinically appears as a nodule or infiltrated plaque, often without scale. The most well-known example is a kerion on the scalp. Elsewhere on the body, lesions often are termed Majocchi granulomas.2
Vegetative plaques, as seen in our patient, are a highly unusual morphology for deep tinea infection. Guanziroli et al4 reported a case of vegetative lesions on the forearm of a 67-year-old immunocompromised man that were successfully treated with a 3-month course of oral terbinafine after Trichophyton verrucosum was isolated. Skorepova et al5 reported a case of pyoderma vegetans triggered by recurrent Trichophyton mentagrophytes on the dorsal hands of a 64-year-old man with immunoglobulin deficiency of unknown etiology. The lesions were successfully treated with a prolonged course of doxycycline, topical triamcinolone, and intravenous immunoglobulin following 2 initial courses of terbinafine.
The differential diagnosis for vegetative lesions includes pemphigus vegetans, a vegetative variant of pyoderma gangrenosum; halogenoderma; and a variety of infections, including dimorphic fungi (histoplasmosis, blastomycosis), blastomycosislike pyoderma (bacterial), and candidiasis.6 These conditions usually can be distinguished based on histopathology. Clinically, pemphigus vegetans presents with pustules and vegetative lesions, as in our patient, but usually is more diffuse and favors the intertriginous areas. Histology likely would reveal foci of acantholysis and eosinophils. Vegetative pyoderma gangrenosum favors the trunk, particularly in sites of surgical trauma. In our patient, no lesions were present near the abdominal surgical sites, and there was no antecedent cribriform ulceration. Halogenoderma was a strong initial consideration given the localization, presence of large pustules, and history of numerous contrast computed tomography studies; however, our patient’s iodine levels were normal. Infectious etiologies including dimorphic fungi and blastomycosislike pyoderma generally are not restricted to the head and neck, and tissue culture helps exclude them. Vegetative lesions may occur in the setting of other infections, and tissue culture may be necessary to differentiate them if histopathology is not suggestive.
Deep dermatophyte infections require treatment with oral antifungals, as topicals do not penetrate adequately into the hair follicles. Exact regimens vary, but generally oral terbinafine or an oral azole (except ketoconazole) is administered for 2 to 6 weeks, with immunocompromise necessitating longer courses.
We present a rare case of vegetative Majocchi granuloma secondary to T rubrum infection. A dermatophyte infection should be included in the differential for vegetative lesions, especially in dense hair-bearing areas such as the beard. Treatment generally is straightforward with oral antifungals.
- Atzori L, Pau M, Aste N, et al. Dermatophyte infections mimicking other skin diseases: a 154-person case survey of tinea atypica in the district of Cagliari (Italy). Int J Dermatol. 2012;51:410-415.
- Ilkit M, Durdu M, Karakas M. Majocchi’s granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457.
- Jevremovic L, Ilijin I, Kostic K, et al. Pyoderma vegetans—a case report. Serbian J Dermatol Venereol. 2017;9:22-28.
- Guanziroli E, Pavia G, Guttadauro A, et al. Deep dermatophytosis caused by Trichophyton verrucosum in an immunosuppressed patient: successful outcome with terbinafine. Mycopathologia. 2019;184:543-545.
- Skorepová M, Stuchlík D. Chronic pyoderma vegetans triggered by Trichophyton mentagrophytes. Mycoses. 2006;49:143-144.
- Reinholz M, Hermans C, Dietrich A, et al. A case of cutaneous vegetating candidiasis in a patient with keratitis-ichthyosis-deafness syndrome. J Eur Acad Dermatol Venereol. 2016;30:537-539.
- Atzori L, Pau M, Aste N, et al. Dermatophyte infections mimicking other skin diseases: a 154-person case survey of tinea atypica in the district of Cagliari (Italy). Int J Dermatol. 2012;51:410-415.
- Ilkit M, Durdu M, Karakas M. Majocchi’s granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50:449-457.
- Jevremovic L, Ilijin I, Kostic K, et al. Pyoderma vegetans—a case report. Serbian J Dermatol Venereol. 2017;9:22-28.
- Guanziroli E, Pavia G, Guttadauro A, et al. Deep dermatophytosis caused by Trichophyton verrucosum in an immunosuppressed patient: successful outcome with terbinafine. Mycopathologia. 2019;184:543-545.
- Skorepová M, Stuchlík D. Chronic pyoderma vegetans triggered by Trichophyton mentagrophytes. Mycoses. 2006;49:143-144.
- Reinholz M, Hermans C, Dietrich A, et al. A case of cutaneous vegetating candidiasis in a patient with keratitis-ichthyosis-deafness syndrome. J Eur Acad Dermatol Venereol. 2016;30:537-539.
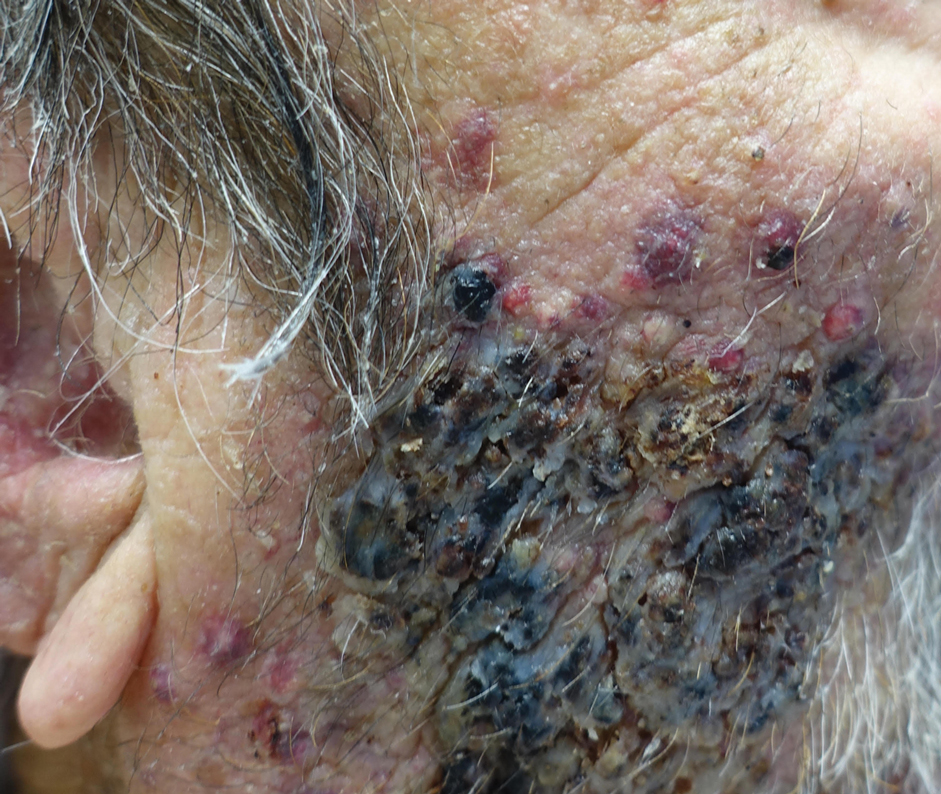
An 86-year-old man was admitted to the hospital for sigmoid colon perforation secondary to ischemic colitis. His medical history consisted of sequelae from atherosclerotic vascular disease. He had no known personal or family history of skin disease. His bowel perforation was surgically repaired, and his clinical status was stabilized, enabling transfer to a transitional care hospital. His course was complicated by delayed healing of the midline abdominal surgical wounds, leading to multiple computed tomography studies with iodinated contrast. One week following arrival at the transitional care hospital, he was noted to have a pustular rash on the face. He was empirically treated with topical steroids, mupirocin, and sulfacetamide. The rash did not improve, and the appearance changed, at which point dermatology was consulted. On evaluation, the patient was afebrile with a normal white blood cell count. Physical examination revealed gray-brown, moist, vegetative plaques on the cheeks with a few large pustules as well as similar-appearing lesions on the neck and upper chest. Attempted removal of a portion of the plaque left an erosion.