User login
Coping with a shattered immune system: COVID and beyond
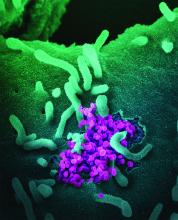
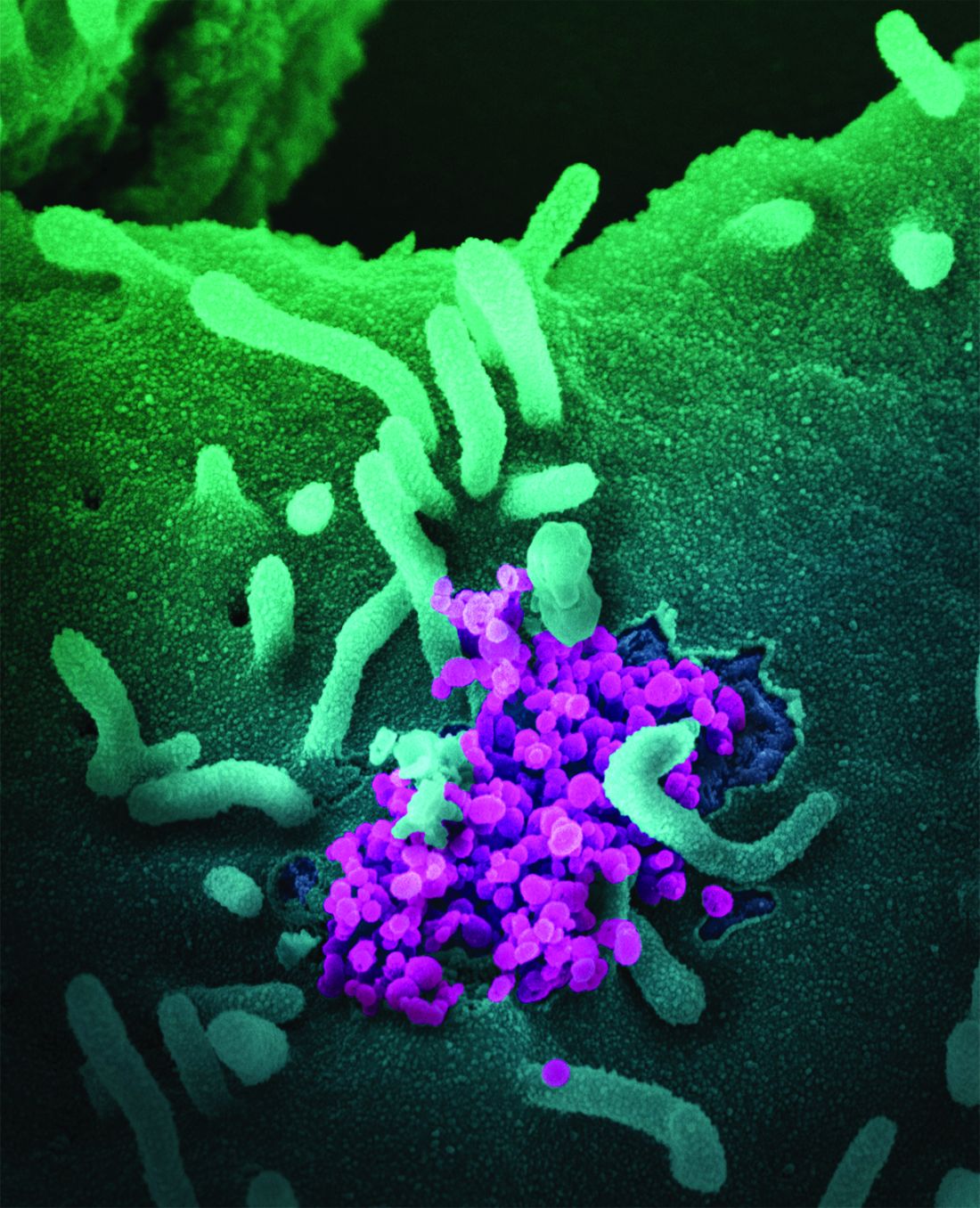
The co-opting and weakening of the immune system by hematologic malignancies and many of their treatments – and the blunting of the immune system’s response to vaccines – may be more salient during the COVID-19 pandemic than ever before.
Hematologic malignancies have been associated in large cancer-and-COVID-19 registries with more severe COVID-19 outcomes than solid tumors, and COVID-19 mRNA vaccines have yielded suboptimal responses across multiple studies. Clinicians and researchers have no shortage of questions, like what is the optimal timing of vaccines relative to cancer-directed therapy? What is the durability and impact of the immune response? What is the status of the immune system in patients who do not produce antispike antibodies after COVID-19 vaccination?
Moreover, will there be novel nonvaccine strategies – such as antibody cocktails or convalescent plasma – to ensure protection against COVID-19 and other future viral threats? And what really defines immunocompromise today and moving forward?
“We don’t know what we don’t know,” said Jeremy L. Warner, MD, associate professor of medicine (hematology/oncology) and biomedical informatics at Vanderbilt University, Nashville, Tenn., and cofounder of the international COVID-19 and Cancer Consortium. “The immune system is incredibly complex and there are numerous defenses, in addition to the humoral response that we routinely measure.”
Another of the pressing pandemic-time questions for infectious disease specialists working in cancer centers concerns a different infectious threat: measles. “There is a lot of concern in this space about the reported drop in childhood vaccinations and the possibility of measles outbreaks as a follow-up to COVID-19,” said Steven A. Pergam, MD, MPH, associate professor in the vaccine and infectious disease division and the clinical research division of the Fred Hutchinson Cancer Research Center, Seattle Cancer Care Alliance.
Whether recipients of hematopoietic cell transplantation (HCT) and cellular therapy should be revaccinated earlier than 2 years post treatment is a question worthy of preemptive discussion, he said.
What about timing?
“A silver lining of the pandemic is that it’s improving our understanding of response to vaccinations and outcomes with respiratory viruses in patients with hematologic malignancies,” said Samuel Rubinstein, MD, of the division of hematology at the University of North Carolina at Chapel Hill. “We’re going to learn a lot more about how to ensure that our patients are optimally protected from respiratory viruses.”
Dr. Rubinstein focuses on plasma cell disorders, mostly multiple myeloma, and routinely explains to patients consenting to use daratumumab, an anti-CD38 monoclonal antibody, or a BCMA-directed therapy, that these therapies “in particular probably do impair vaccine immune response.”
He has handled the timing of the COVID-19 vaccines – currently boosters, in most cases – as he has with influenza and other immunizations such as the pneumococcal vaccine, administering the vaccines agnostic to therapy unless the patient is about to start daratumumab or a BCMA-directed therapy. In this case, he considers vaccinating and waiting 2 weeks (for an immune response to occur) before starting therapy.
However, “if I have any concern that a delay will result in suboptimal cancer control, then I don’t wait,” Dr. Rubinstein said. Poor control of a primary malignancy has been consistently associated with worse COVID-19–specific outcomes in cancer–COVID-19 studies, he said, including an analysis of almost 5,000 patients recorded to the COVID-19 and Cancer Consortium .1
(The analysis also documented that patients with a hematologic malignancy had an odds ratio of higher COVID-19 severity of 1.7, compared with patients with a solid tumor, and an odds ratio of 30-day mortality of 1.44.)
Ideally, said Dr. Warner, patients will get vaccinated with the COVID-19 vaccines or others, “before starting on any cytotoxic chemotherapy and when they do not have low blood counts or perhaps autoimmune complications of immunotherapy.” However, “perfect being the enemy of good, it’s better to get vaccinated than to wait for the exact ideal time.”
Peter Paul Yu, MD, physician-in-chief at Hartford (Conn.) Healthcare Cancer Institute, said that for most patients, there’s no evidence to support an optimal timing of vaccine administration during the chemotherapy cycle. “We looked into that [to guide administration of the COVID-19 vaccines], thinking there might be some data about influenza vaccination,” he said. “But there isn’t much. … And if we make things more complicated than the evidence suggests, we may have fewer people getting vaccinations.”
The National Comprehensive Cancer Network offered several timing recommendations in its August 2021 COVID-19 vaccination guidance – mainly that patients receiving intensive cytotoxic chemotherapy (such as those on cytarabine/anthracycline-based induction regimens for acute myeloid leukemia) delay COVID-19 vaccination until absolute neutrophil count recovery, and that patients on long-term maintenance therapy (for instance, targeted agents for chronic lymphocytic leukemia or myeloproliferative neoplasms) be vaccinated as soon as possible.
Vaccination should be delayed for at least 3 months, the NCCN noted, following HCT or engineered cell therapy (for example, chimeric antigen receptor [CAR] T cells) “in order to maximize vaccine efficacy.”
More known unknowns
The tempered efficacy of the COVID-19 vaccines in patients with hematologic malignancies “has been shown in multiple studies of multiple myeloma, chronic lymphocytic leukemia (CLL), and other malignancies, and we know it’s true in transplant,” said Dr. Pergam.
In a study of 67 patients with hematologic malignancies at the University of Pittsburgh Medical Center Hillman Cancer Center, for instance, 46.3% did not generate IgG antibodies against the SARS-CoV-2 spike protein receptor–binding domain after completing their two-dose mRNA vaccine series. Patients with B-cell CLL were especially unlikely to develop antibodies.2A much larger study of more than 1,400 patients from investigators at the Mayo Clinics in Rochester, Minn., and Jacksonville, Fla., found that approximately 25% of all patients with hematologic malignancies did not produce antispike IgG antibodies, and that those with the most common B-cell malignancies had the lowest rate of seropositivity (44%-79%).3There’s a clear but challenging delineation between antibody testing in the research space and in clinical practice, however. Various national and cancer societies recommended earlier this year against routine postvaccine serological monitoring outside of clinical trials, and the sources interviewed for this story all emphasized that antibody titer measurements should not guide decisions about boosters or about the precautions advised for patients.
Titers checked at a single point in time do not capture the kinetics, multidimensional nature, or durability of an immune response, Dr. Warner said. “There are papers out there that say zero patients with CCL seroconverted … but they do still have some immunity, and maybe even a lot of immunity.”
Antibody testing can create a false sense of security, or a false sense of dread, he said. Yet in practice, the use of serological monitoring “has been all over the place [with] no consistency … and decisions probably being made at the individual clinic level or health system level,” he said.
To a lesser degree, so have definitions of what composes significant immunocompromise in the context of COVID-19 vaccine eligibility. “The question comes up, what does immunocompromised really mean?” said Dr. Yu, whose institution is a member of the Memorial Sloan Kettering (MSK) Cancer Alliance.
As of September, the MSK Cancer Center had taken a more granular approach to describing moderate to severe immunocompromise than did the Centers for Disease Control and Prevention. The CDC said this level of immunocompromise includes people receiving active cancer treatment for tumors or cancers of the blood, and those who’ve received a stem cell transplant within the past 2 years. MSK extended the recommendation, as it concerns hematologic malignancies, to patients who are within 12 months after treatment with B-cell depleting drugs, patients who have been treated for blood cancers within the last 6 months, and patients who received CAR T therapy within the past 2 years.
Dr. Yu, who was not involved in creating the MSK recommendations for third COVID-19 vaccines, said that he has been thinking more broadly during the pandemic about the notion of immunocompetence. “It’s my opinion that patients with hematologic malignancies, even if they’re not on treatment, are not fully immune competent,” he said. This includes patients with CLL stage 0 and patients with plasma cell dyscrasias who don’t yet meet the criteria for multiple myeloma but have a monoclonal gammopathy, and those with lower-risk myelodysplastic syndromes, he said.
“We’re seeing [variable] recommendations based on expert opinion, and I think that’s justifiable in such a dynamic situation,” Dr. Yu said. “I would [even] argue it’s desirable so we can learn from different approaches” and collect more rigorous observational data.
Immunocompetence needs to be “viewed in the context of the threat,” he added. “COVID changes the equation. … What’s immunocompromised in my mind has changed [from prepandemic times].”
Preparing for measles
Measles lit up on Dr. Pergam’s radar screen in 2019, when an outbreak occurred in nearby Clark County, Wash. This and other outbreaks in New York, California, and other states highlighted declines in measles herd immunity in the United States and prompted him to investigate the seroprevalence of measles antibodies in the Fred Hutchinson Cancer Research Center’s outpatient population.
Of 959 consecutive patients seen at the center, they found, 25% lacked protective antibodies for measles. For patients with hematologic malignancies and those with a history of HCT, seroprevalence was worse: 37% and 54%, respectively, were without the IgG antibodies.4 Measles “is the most contagious human virus we have at the moment,” he said, and “revaccinating people is hard when it comes to cancer because it is a live virus vaccine.”
Vaccine hesitancy, a rise in nonmedical exemptions, and other factors were threatening herd immunity before the pandemic began. Now, with declines in routine childhood medical visits and other vaccination opportunities and resources here and in other countries – and declining immunization rates documented by the CDC in May 2021 – the pandemic has made measles outbreaks more likely, he said. (Measles outbreaks in West Africa on the tail end of the Ebola outbreak in 2014-2015 caused more deaths in children than Ebola, he noted.)
The first priority is vaccination “cocooning,” a strategy that has long been important for patients with hematologic malignancies. But it also possible, Dr. Pergam said, that in the setting of any future community transmission, revaccination for HCT recipients could occur earlier than the standard 2-year post-transplantation recommendation.
In a 2019 position statement endorsed by the American Society for Transplantation and Cellular Therapy, Dr. Pergam and other infectious disease physicians and oncologists provide criteria for considering early revaccination on a case-by-case basis for patients on minimal immunosuppressive therapy who are at least 1-year post transplantation.5
“Our thinking was that there may be lower-risk patients to whom we could offer the vaccine” – patients for whom the risk of developing measles might outweigh the risk of potential vaccine-related complications, he said.
And if there were community cases, he added, there might be a place for testing antibody levels in post-transplant patients, however imperfect the window to immunity may be. “We’re thinking through potential scenarios,” he said. “Oncologists should think about measles again and have it on their back burner.”
References
1. Grivas P et al. Ann Oncol. 2021 Jun;32(6):787-800.
2. Agha ME et al. Open Forum Infect Dis. 2021 July;8(7):ofab353.
3. Greenberger LM et al. Cancer Cell. 2021 Aug 9;39(8):1031-3.
4. Marquis SR et al. JAMA Netw Open. 2021 July;4(7):e2118508.
5. Pergam SA et al. Biol Blood Marrow Transplant. 2019 Nov;25:e321-30.
The co-opting and weakening of the immune system by hematologic malignancies and many of their treatments – and the blunting of the immune system’s response to vaccines – may be more salient during the COVID-19 pandemic than ever before.
Hematologic malignancies have been associated in large cancer-and-COVID-19 registries with more severe COVID-19 outcomes than solid tumors, and COVID-19 mRNA vaccines have yielded suboptimal responses across multiple studies. Clinicians and researchers have no shortage of questions, like what is the optimal timing of vaccines relative to cancer-directed therapy? What is the durability and impact of the immune response? What is the status of the immune system in patients who do not produce antispike antibodies after COVID-19 vaccination?
Moreover, will there be novel nonvaccine strategies – such as antibody cocktails or convalescent plasma – to ensure protection against COVID-19 and other future viral threats? And what really defines immunocompromise today and moving forward?
“We don’t know what we don’t know,” said Jeremy L. Warner, MD, associate professor of medicine (hematology/oncology) and biomedical informatics at Vanderbilt University, Nashville, Tenn., and cofounder of the international COVID-19 and Cancer Consortium. “The immune system is incredibly complex and there are numerous defenses, in addition to the humoral response that we routinely measure.”
Another of the pressing pandemic-time questions for infectious disease specialists working in cancer centers concerns a different infectious threat: measles. “There is a lot of concern in this space about the reported drop in childhood vaccinations and the possibility of measles outbreaks as a follow-up to COVID-19,” said Steven A. Pergam, MD, MPH, associate professor in the vaccine and infectious disease division and the clinical research division of the Fred Hutchinson Cancer Research Center, Seattle Cancer Care Alliance.
Whether recipients of hematopoietic cell transplantation (HCT) and cellular therapy should be revaccinated earlier than 2 years post treatment is a question worthy of preemptive discussion, he said.
What about timing?
“A silver lining of the pandemic is that it’s improving our understanding of response to vaccinations and outcomes with respiratory viruses in patients with hematologic malignancies,” said Samuel Rubinstein, MD, of the division of hematology at the University of North Carolina at Chapel Hill. “We’re going to learn a lot more about how to ensure that our patients are optimally protected from respiratory viruses.”
Dr. Rubinstein focuses on plasma cell disorders, mostly multiple myeloma, and routinely explains to patients consenting to use daratumumab, an anti-CD38 monoclonal antibody, or a BCMA-directed therapy, that these therapies “in particular probably do impair vaccine immune response.”
He has handled the timing of the COVID-19 vaccines – currently boosters, in most cases – as he has with influenza and other immunizations such as the pneumococcal vaccine, administering the vaccines agnostic to therapy unless the patient is about to start daratumumab or a BCMA-directed therapy. In this case, he considers vaccinating and waiting 2 weeks (for an immune response to occur) before starting therapy.
However, “if I have any concern that a delay will result in suboptimal cancer control, then I don’t wait,” Dr. Rubinstein said. Poor control of a primary malignancy has been consistently associated with worse COVID-19–specific outcomes in cancer–COVID-19 studies, he said, including an analysis of almost 5,000 patients recorded to the COVID-19 and Cancer Consortium .1
(The analysis also documented that patients with a hematologic malignancy had an odds ratio of higher COVID-19 severity of 1.7, compared with patients with a solid tumor, and an odds ratio of 30-day mortality of 1.44.)
Ideally, said Dr. Warner, patients will get vaccinated with the COVID-19 vaccines or others, “before starting on any cytotoxic chemotherapy and when they do not have low blood counts or perhaps autoimmune complications of immunotherapy.” However, “perfect being the enemy of good, it’s better to get vaccinated than to wait for the exact ideal time.”
Peter Paul Yu, MD, physician-in-chief at Hartford (Conn.) Healthcare Cancer Institute, said that for most patients, there’s no evidence to support an optimal timing of vaccine administration during the chemotherapy cycle. “We looked into that [to guide administration of the COVID-19 vaccines], thinking there might be some data about influenza vaccination,” he said. “But there isn’t much. … And if we make things more complicated than the evidence suggests, we may have fewer people getting vaccinations.”
The National Comprehensive Cancer Network offered several timing recommendations in its August 2021 COVID-19 vaccination guidance – mainly that patients receiving intensive cytotoxic chemotherapy (such as those on cytarabine/anthracycline-based induction regimens for acute myeloid leukemia) delay COVID-19 vaccination until absolute neutrophil count recovery, and that patients on long-term maintenance therapy (for instance, targeted agents for chronic lymphocytic leukemia or myeloproliferative neoplasms) be vaccinated as soon as possible.
Vaccination should be delayed for at least 3 months, the NCCN noted, following HCT or engineered cell therapy (for example, chimeric antigen receptor [CAR] T cells) “in order to maximize vaccine efficacy.”
More known unknowns
The tempered efficacy of the COVID-19 vaccines in patients with hematologic malignancies “has been shown in multiple studies of multiple myeloma, chronic lymphocytic leukemia (CLL), and other malignancies, and we know it’s true in transplant,” said Dr. Pergam.
In a study of 67 patients with hematologic malignancies at the University of Pittsburgh Medical Center Hillman Cancer Center, for instance, 46.3% did not generate IgG antibodies against the SARS-CoV-2 spike protein receptor–binding domain after completing their two-dose mRNA vaccine series. Patients with B-cell CLL were especially unlikely to develop antibodies.2A much larger study of more than 1,400 patients from investigators at the Mayo Clinics in Rochester, Minn., and Jacksonville, Fla., found that approximately 25% of all patients with hematologic malignancies did not produce antispike IgG antibodies, and that those with the most common B-cell malignancies had the lowest rate of seropositivity (44%-79%).3There’s a clear but challenging delineation between antibody testing in the research space and in clinical practice, however. Various national and cancer societies recommended earlier this year against routine postvaccine serological monitoring outside of clinical trials, and the sources interviewed for this story all emphasized that antibody titer measurements should not guide decisions about boosters or about the precautions advised for patients.
Titers checked at a single point in time do not capture the kinetics, multidimensional nature, or durability of an immune response, Dr. Warner said. “There are papers out there that say zero patients with CCL seroconverted … but they do still have some immunity, and maybe even a lot of immunity.”
Antibody testing can create a false sense of security, or a false sense of dread, he said. Yet in practice, the use of serological monitoring “has been all over the place [with] no consistency … and decisions probably being made at the individual clinic level or health system level,” he said.
To a lesser degree, so have definitions of what composes significant immunocompromise in the context of COVID-19 vaccine eligibility. “The question comes up, what does immunocompromised really mean?” said Dr. Yu, whose institution is a member of the Memorial Sloan Kettering (MSK) Cancer Alliance.
As of September, the MSK Cancer Center had taken a more granular approach to describing moderate to severe immunocompromise than did the Centers for Disease Control and Prevention. The CDC said this level of immunocompromise includes people receiving active cancer treatment for tumors or cancers of the blood, and those who’ve received a stem cell transplant within the past 2 years. MSK extended the recommendation, as it concerns hematologic malignancies, to patients who are within 12 months after treatment with B-cell depleting drugs, patients who have been treated for blood cancers within the last 6 months, and patients who received CAR T therapy within the past 2 years.
Dr. Yu, who was not involved in creating the MSK recommendations for third COVID-19 vaccines, said that he has been thinking more broadly during the pandemic about the notion of immunocompetence. “It’s my opinion that patients with hematologic malignancies, even if they’re not on treatment, are not fully immune competent,” he said. This includes patients with CLL stage 0 and patients with plasma cell dyscrasias who don’t yet meet the criteria for multiple myeloma but have a monoclonal gammopathy, and those with lower-risk myelodysplastic syndromes, he said.
“We’re seeing [variable] recommendations based on expert opinion, and I think that’s justifiable in such a dynamic situation,” Dr. Yu said. “I would [even] argue it’s desirable so we can learn from different approaches” and collect more rigorous observational data.
Immunocompetence needs to be “viewed in the context of the threat,” he added. “COVID changes the equation. … What’s immunocompromised in my mind has changed [from prepandemic times].”
Preparing for measles
Measles lit up on Dr. Pergam’s radar screen in 2019, when an outbreak occurred in nearby Clark County, Wash. This and other outbreaks in New York, California, and other states highlighted declines in measles herd immunity in the United States and prompted him to investigate the seroprevalence of measles antibodies in the Fred Hutchinson Cancer Research Center’s outpatient population.
Of 959 consecutive patients seen at the center, they found, 25% lacked protective antibodies for measles. For patients with hematologic malignancies and those with a history of HCT, seroprevalence was worse: 37% and 54%, respectively, were without the IgG antibodies.4 Measles “is the most contagious human virus we have at the moment,” he said, and “revaccinating people is hard when it comes to cancer because it is a live virus vaccine.”
Vaccine hesitancy, a rise in nonmedical exemptions, and other factors were threatening herd immunity before the pandemic began. Now, with declines in routine childhood medical visits and other vaccination opportunities and resources here and in other countries – and declining immunization rates documented by the CDC in May 2021 – the pandemic has made measles outbreaks more likely, he said. (Measles outbreaks in West Africa on the tail end of the Ebola outbreak in 2014-2015 caused more deaths in children than Ebola, he noted.)
The first priority is vaccination “cocooning,” a strategy that has long been important for patients with hematologic malignancies. But it also possible, Dr. Pergam said, that in the setting of any future community transmission, revaccination for HCT recipients could occur earlier than the standard 2-year post-transplantation recommendation.
In a 2019 position statement endorsed by the American Society for Transplantation and Cellular Therapy, Dr. Pergam and other infectious disease physicians and oncologists provide criteria for considering early revaccination on a case-by-case basis for patients on minimal immunosuppressive therapy who are at least 1-year post transplantation.5
“Our thinking was that there may be lower-risk patients to whom we could offer the vaccine” – patients for whom the risk of developing measles might outweigh the risk of potential vaccine-related complications, he said.
And if there were community cases, he added, there might be a place for testing antibody levels in post-transplant patients, however imperfect the window to immunity may be. “We’re thinking through potential scenarios,” he said. “Oncologists should think about measles again and have it on their back burner.”
References
1. Grivas P et al. Ann Oncol. 2021 Jun;32(6):787-800.
2. Agha ME et al. Open Forum Infect Dis. 2021 July;8(7):ofab353.
3. Greenberger LM et al. Cancer Cell. 2021 Aug 9;39(8):1031-3.
4. Marquis SR et al. JAMA Netw Open. 2021 July;4(7):e2118508.
5. Pergam SA et al. Biol Blood Marrow Transplant. 2019 Nov;25:e321-30.
The co-opting and weakening of the immune system by hematologic malignancies and many of their treatments – and the blunting of the immune system’s response to vaccines – may be more salient during the COVID-19 pandemic than ever before.
Hematologic malignancies have been associated in large cancer-and-COVID-19 registries with more severe COVID-19 outcomes than solid tumors, and COVID-19 mRNA vaccines have yielded suboptimal responses across multiple studies. Clinicians and researchers have no shortage of questions, like what is the optimal timing of vaccines relative to cancer-directed therapy? What is the durability and impact of the immune response? What is the status of the immune system in patients who do not produce antispike antibodies after COVID-19 vaccination?
Moreover, will there be novel nonvaccine strategies – such as antibody cocktails or convalescent plasma – to ensure protection against COVID-19 and other future viral threats? And what really defines immunocompromise today and moving forward?
“We don’t know what we don’t know,” said Jeremy L. Warner, MD, associate professor of medicine (hematology/oncology) and biomedical informatics at Vanderbilt University, Nashville, Tenn., and cofounder of the international COVID-19 and Cancer Consortium. “The immune system is incredibly complex and there are numerous defenses, in addition to the humoral response that we routinely measure.”
Another of the pressing pandemic-time questions for infectious disease specialists working in cancer centers concerns a different infectious threat: measles. “There is a lot of concern in this space about the reported drop in childhood vaccinations and the possibility of measles outbreaks as a follow-up to COVID-19,” said Steven A. Pergam, MD, MPH, associate professor in the vaccine and infectious disease division and the clinical research division of the Fred Hutchinson Cancer Research Center, Seattle Cancer Care Alliance.
Whether recipients of hematopoietic cell transplantation (HCT) and cellular therapy should be revaccinated earlier than 2 years post treatment is a question worthy of preemptive discussion, he said.
What about timing?
“A silver lining of the pandemic is that it’s improving our understanding of response to vaccinations and outcomes with respiratory viruses in patients with hematologic malignancies,” said Samuel Rubinstein, MD, of the division of hematology at the University of North Carolina at Chapel Hill. “We’re going to learn a lot more about how to ensure that our patients are optimally protected from respiratory viruses.”
Dr. Rubinstein focuses on plasma cell disorders, mostly multiple myeloma, and routinely explains to patients consenting to use daratumumab, an anti-CD38 monoclonal antibody, or a BCMA-directed therapy, that these therapies “in particular probably do impair vaccine immune response.”
He has handled the timing of the COVID-19 vaccines – currently boosters, in most cases – as he has with influenza and other immunizations such as the pneumococcal vaccine, administering the vaccines agnostic to therapy unless the patient is about to start daratumumab or a BCMA-directed therapy. In this case, he considers vaccinating and waiting 2 weeks (for an immune response to occur) before starting therapy.
However, “if I have any concern that a delay will result in suboptimal cancer control, then I don’t wait,” Dr. Rubinstein said. Poor control of a primary malignancy has been consistently associated with worse COVID-19–specific outcomes in cancer–COVID-19 studies, he said, including an analysis of almost 5,000 patients recorded to the COVID-19 and Cancer Consortium .1
(The analysis also documented that patients with a hematologic malignancy had an odds ratio of higher COVID-19 severity of 1.7, compared with patients with a solid tumor, and an odds ratio of 30-day mortality of 1.44.)
Ideally, said Dr. Warner, patients will get vaccinated with the COVID-19 vaccines or others, “before starting on any cytotoxic chemotherapy and when they do not have low blood counts or perhaps autoimmune complications of immunotherapy.” However, “perfect being the enemy of good, it’s better to get vaccinated than to wait for the exact ideal time.”
Peter Paul Yu, MD, physician-in-chief at Hartford (Conn.) Healthcare Cancer Institute, said that for most patients, there’s no evidence to support an optimal timing of vaccine administration during the chemotherapy cycle. “We looked into that [to guide administration of the COVID-19 vaccines], thinking there might be some data about influenza vaccination,” he said. “But there isn’t much. … And if we make things more complicated than the evidence suggests, we may have fewer people getting vaccinations.”
The National Comprehensive Cancer Network offered several timing recommendations in its August 2021 COVID-19 vaccination guidance – mainly that patients receiving intensive cytotoxic chemotherapy (such as those on cytarabine/anthracycline-based induction regimens for acute myeloid leukemia) delay COVID-19 vaccination until absolute neutrophil count recovery, and that patients on long-term maintenance therapy (for instance, targeted agents for chronic lymphocytic leukemia or myeloproliferative neoplasms) be vaccinated as soon as possible.
Vaccination should be delayed for at least 3 months, the NCCN noted, following HCT or engineered cell therapy (for example, chimeric antigen receptor [CAR] T cells) “in order to maximize vaccine efficacy.”
More known unknowns
The tempered efficacy of the COVID-19 vaccines in patients with hematologic malignancies “has been shown in multiple studies of multiple myeloma, chronic lymphocytic leukemia (CLL), and other malignancies, and we know it’s true in transplant,” said Dr. Pergam.
In a study of 67 patients with hematologic malignancies at the University of Pittsburgh Medical Center Hillman Cancer Center, for instance, 46.3% did not generate IgG antibodies against the SARS-CoV-2 spike protein receptor–binding domain after completing their two-dose mRNA vaccine series. Patients with B-cell CLL were especially unlikely to develop antibodies.2A much larger study of more than 1,400 patients from investigators at the Mayo Clinics in Rochester, Minn., and Jacksonville, Fla., found that approximately 25% of all patients with hematologic malignancies did not produce antispike IgG antibodies, and that those with the most common B-cell malignancies had the lowest rate of seropositivity (44%-79%).3There’s a clear but challenging delineation between antibody testing in the research space and in clinical practice, however. Various national and cancer societies recommended earlier this year against routine postvaccine serological monitoring outside of clinical trials, and the sources interviewed for this story all emphasized that antibody titer measurements should not guide decisions about boosters or about the precautions advised for patients.
Titers checked at a single point in time do not capture the kinetics, multidimensional nature, or durability of an immune response, Dr. Warner said. “There are papers out there that say zero patients with CCL seroconverted … but they do still have some immunity, and maybe even a lot of immunity.”
Antibody testing can create a false sense of security, or a false sense of dread, he said. Yet in practice, the use of serological monitoring “has been all over the place [with] no consistency … and decisions probably being made at the individual clinic level or health system level,” he said.
To a lesser degree, so have definitions of what composes significant immunocompromise in the context of COVID-19 vaccine eligibility. “The question comes up, what does immunocompromised really mean?” said Dr. Yu, whose institution is a member of the Memorial Sloan Kettering (MSK) Cancer Alliance.
As of September, the MSK Cancer Center had taken a more granular approach to describing moderate to severe immunocompromise than did the Centers for Disease Control and Prevention. The CDC said this level of immunocompromise includes people receiving active cancer treatment for tumors or cancers of the blood, and those who’ve received a stem cell transplant within the past 2 years. MSK extended the recommendation, as it concerns hematologic malignancies, to patients who are within 12 months after treatment with B-cell depleting drugs, patients who have been treated for blood cancers within the last 6 months, and patients who received CAR T therapy within the past 2 years.
Dr. Yu, who was not involved in creating the MSK recommendations for third COVID-19 vaccines, said that he has been thinking more broadly during the pandemic about the notion of immunocompetence. “It’s my opinion that patients with hematologic malignancies, even if they’re not on treatment, are not fully immune competent,” he said. This includes patients with CLL stage 0 and patients with plasma cell dyscrasias who don’t yet meet the criteria for multiple myeloma but have a monoclonal gammopathy, and those with lower-risk myelodysplastic syndromes, he said.
“We’re seeing [variable] recommendations based on expert opinion, and I think that’s justifiable in such a dynamic situation,” Dr. Yu said. “I would [even] argue it’s desirable so we can learn from different approaches” and collect more rigorous observational data.
Immunocompetence needs to be “viewed in the context of the threat,” he added. “COVID changes the equation. … What’s immunocompromised in my mind has changed [from prepandemic times].”
Preparing for measles
Measles lit up on Dr. Pergam’s radar screen in 2019, when an outbreak occurred in nearby Clark County, Wash. This and other outbreaks in New York, California, and other states highlighted declines in measles herd immunity in the United States and prompted him to investigate the seroprevalence of measles antibodies in the Fred Hutchinson Cancer Research Center’s outpatient population.
Of 959 consecutive patients seen at the center, they found, 25% lacked protective antibodies for measles. For patients with hematologic malignancies and those with a history of HCT, seroprevalence was worse: 37% and 54%, respectively, were without the IgG antibodies.4 Measles “is the most contagious human virus we have at the moment,” he said, and “revaccinating people is hard when it comes to cancer because it is a live virus vaccine.”
Vaccine hesitancy, a rise in nonmedical exemptions, and other factors were threatening herd immunity before the pandemic began. Now, with declines in routine childhood medical visits and other vaccination opportunities and resources here and in other countries – and declining immunization rates documented by the CDC in May 2021 – the pandemic has made measles outbreaks more likely, he said. (Measles outbreaks in West Africa on the tail end of the Ebola outbreak in 2014-2015 caused more deaths in children than Ebola, he noted.)
The first priority is vaccination “cocooning,” a strategy that has long been important for patients with hematologic malignancies. But it also possible, Dr. Pergam said, that in the setting of any future community transmission, revaccination for HCT recipients could occur earlier than the standard 2-year post-transplantation recommendation.
In a 2019 position statement endorsed by the American Society for Transplantation and Cellular Therapy, Dr. Pergam and other infectious disease physicians and oncologists provide criteria for considering early revaccination on a case-by-case basis for patients on minimal immunosuppressive therapy who are at least 1-year post transplantation.5
“Our thinking was that there may be lower-risk patients to whom we could offer the vaccine” – patients for whom the risk of developing measles might outweigh the risk of potential vaccine-related complications, he said.
And if there were community cases, he added, there might be a place for testing antibody levels in post-transplant patients, however imperfect the window to immunity may be. “We’re thinking through potential scenarios,” he said. “Oncologists should think about measles again and have it on their back burner.”
References
1. Grivas P et al. Ann Oncol. 2021 Jun;32(6):787-800.
2. Agha ME et al. Open Forum Infect Dis. 2021 July;8(7):ofab353.
3. Greenberger LM et al. Cancer Cell. 2021 Aug 9;39(8):1031-3.
4. Marquis SR et al. JAMA Netw Open. 2021 July;4(7):e2118508.
5. Pergam SA et al. Biol Blood Marrow Transplant. 2019 Nov;25:e321-30.
Precision medicine: A new approach to AML, other blood cancers
The emergence of precision medicine has ushered in a groundbreaking era for the treatment of myeloid malignancies, with the ability to integrate individual molecular data into patient care.
Over the past decade, insights from research focusing on the mutations driving the malignant transformation of myeloid cells have provided the basis for the development of novel targeted therapies.1 With the recent U.S. Food and Drug Administration approval of several novel therapies for different acute myeloid leukemia (AML) indications, the current treatment landscape for AML is evolving rapidly.2
In addition, there has been substantial progress in the development of novel therapeutic strategies for other myeloid neoplasms, with numerous molecularly based therapies in early clinical trials in myeloproliferative neoplasms (MPNs) and myelodysplastic syndromes (MDSs). These advancements have been translated into optimized algorithms for diagnosis, prognostication, and treatment.
AML: Historical perspective
AML comprises a heterogeneous group of blood cell malignancies that require different treatment approaches and confer different prognoses.2 These include acute promyelocytic leukemia (APL) and core binding factor (CBF) AML, both of which have high rates of remission and prolonged survival. The remaining non-APL, non-CBF types can be divided by their cytogenetic-molecular profiles, as well as fitness for intensive chemotherapy. AML can also arise secondary to other myeloid neoplasms, especially after exposure to hypomethylating agents (HMAs), chemotherapy, or irradiation as prior treatment for the primary malignancy.
Historically, anthracycline- and cytarabine-based chemotherapy with or without allogeneic hematopoietic stem-cell transplant (allo-HSCT) was the standard of care in AML treatment with curative intent.1 In the palliative setting, low-dose cytarabine or HMAs were also treatment options. Despite 5 decades of clinical use of these options, researchers have continued to evaluate different dosing schedules of cytosine arabinoside (cytarabine or ara-C) and daunorubicin – the first two agents approved for the treatment of AML – during induction and consolidation treatment phases.
However, recent discoveries have led to the clinical development of targeted agents directed at isocitrate dehydrogenase (IDH), FMS-like tyrosine kinase 3 (FLT3), and BCL2.2 These developments, and the highly anticipated combinations arising from them, continue to challenge traditional treatment approaches, raising the question of whether intensive chemotherapy should remain the optimal standard of care.
Novel therapeutics in AML
Since 2017, several new therapies have been approved for the treatment of AML, including gemtuzumab ozogamicin, two FLT3 inhibitors (gilteritinib and midostaurin), two IDH inhibitors (ivosidenib and enasidenib), a BCL2 inhibitor (venetoclax), an oral HMA agent (azacitidine), a hedgehog inhibitor (glasdegib), and a liposomal formulation of CPX351. In addition, oral decitabine/cedazuridine may be used as an alternative oral HMA in AML, but it is currently the only FDA-approved treatment for chronic myelomonocytic leukemia (CMML) and MDS.2 Because AML subsets are very heterogeneous, an open question remains about how to best integrate these new agents into frontline and salvage combination regimens.
Acute promyelocytic leukemia
APL composes 5%-10% of AML and is characterized by the cytogenetic translocation between chromosomes 15 and 17, which leads to the PML-RAR alpha fusion oncogene and its encoded oncoprotein.2 Two therapies, all-trans retinoic acid (ATRA) and arsenic trioxide, when administered in combination with chemotherapy during induction, have been shown to improve outcomes in APL. At present, the combination of idarubicin and ATRA is the standard-of-care treatment for APL. In addition, patients with high-risk disease have been shown to benefit from the addition of gemtuzumab ozogamicin or anthracyclines.
Core binding factor AML
CBF AML includes patients with the cytogenetic-molecular subsets of inversion 16. Chemotherapy combined with gemtuzumab ozogamicin results in cure rates of 75% or higher and an estimated 5-year survival of 75%. Fludarabine, high-dose cytarabine, and gemtuzumab ozogamicin during induction and consolidation, and an alternative treatment modality (for example, allo-HSCT), for persistent minimal residual disease (MRD) in patients who achieve complete response (CR) is a commonly used regimen. Patients who cannot tolerate this regimen or who have persistent MRD may be treated with an HMA (for instance, decitabine or azacitidine) in combination with venetoclax and gemtuzumab ozogamicin, with the treatment duration adjusted according to MRD status or for 12 months or longer.
Mutations, such as N/KRAS (30%-50%), KIT (25%-30%), and FLT3 (15%-20%), also occur in CBF AML. Targeted agents may also be considered in some cases (for example, dasatinib or avapritinib for KIT mutations; FLT3 inhibitors for FLT3 mutations).
Intensive chemotherapy in younger/fit AML
Several AML regimens have demonstrated better outcomes than the conventional “3 + 7 regimen” (3 days of daunorubicin plus 7 days of cytarabine). Recently, the treatment paradigm has shifted from intensive chemotherapy alone to multidrug combination regimens, including regimens that incorporate targeted therapies, such as FLT3 inhibitors in FLT3-mutated AML, and venetoclax and/or IDH inhibitors as indicated. In addition, the recent FDA approval of oral azacitidine as maintenance therapy for patients in first CR (CR duration, 4 months or less; patients unable to complete the curative intensive chemotherapy) may allow for expanded combination regimens.
Older/unfit patients with AML: Low-intensity therapy
Prior to 2000, the majority of older/unfit patients with AML were offered supportive/palliative treatment. Today, the HMAs azacitidine and decitabine are the most commonly used drugs for the treatment of older/unfit AML. Recently, the FDA approved an oral formulation of decitabine plus oral cedazuridine for the treatment of CMML and MDS. This could provide an opportunity to investigate and develop an effective oral therapy regimen for older/unfit AML, such as oral decitabine/cedazuridine in combination with venetoclax, which may ease administration and improve quality of life for patients in CR post induction in the community setting.
Other studies have shown benefit for combining an HMA with venetoclax in patients with TP53-mutated AML. In addition, triplet regimens may also improve outcomes, with combinations such as HMA plus FLT3 inhibitor (for instance, midostaurin or gilteritinib) with or without venetoclax now being investigated. However, the potential increased risk of myelosuppression also needs to be considered with use of triplet regimens. The results of these and other combinatorial trials are greatly anticipated.
Two oral IDH inhibitors, ivosidenib (IDH1 inhibitor) and enasidenib (IDH2 inhibitor) were recently FDA approved as monotherapy for the treatment of IDH-mutated AML. Combination regimens of IDH inhibitors with chemotherapy are currently being investigated in patients with IDH-mutated AML and appear promising based on preliminary data demonstrating improved response rates and event-free survival.
Other FDA-approved therapies in AML
CPX-351 is a nanoscale liposome with a fixed 5:1 molar ratio of cytarabine and daunorubicin. Results from a phase 3 trial showed that CPX-351 resulted in higher response rates and longer survival compared with 3 + 7 chemotherapy in patients with secondary AML, a subgroup of patients with a very poor prognosis. Additional studies are ongoing, combining CPX-351 with gemtuzumab ozogamicin, venetoclax, and other targeted agents.
Results from a phase 2 trial led to the FDA approval of the hedgehog inhibitor glasdegib when given with low-dose cytarabine. The combination improved survival compared with low-dose cytarabine alone in older/unfit AML and high-risk MDS. However, because of poor survival relative to venetoclax-based combinations, glasdegib is not widely used in clinical practice; other trials exploring combinations with azacitidine and with intensive chemotherapy are ongoing.
Expert perspectives: Future of AML therapy
Amir T. Fathi, MD, associate professor of medicine at Harvard Medical School, Boston, and Farhad Ravandi, MD, professor of medicine at the University of Texas MD Anderson Cancer Center, Houston, are coauthors of a recent review that summarized the current treatment landscape in AML, including areas of evolving research.1
“In the next several years, I am hopeful there will be a series of regulatory approvals of novel, effective agents for myeloid malignancies,” Dr. Fathi explained. “Even if approvals are not as numerous as we’ve seen in AML, any additional effective options would be very welcome.”
Dr. Ravandi also noted that increased understanding of the biology underlying myeloid neoplasms has helped to develop novel therapies.
“As we’ve increased our understanding of the biology of these blood cancers, particularly the mechanisms of leukemogenesis and neoplastic change, we’ve been able to develop more effective therapies in AML,” Dr. Ravandi said.
“In the future, we are likely to see a similar trend in other myeloid neoplasms, such as MDSs and MPNs, as we better understand their underlying pathogenesis,” he further explained.
They both acknowledged that the future treatment paradigm in AML will focus on maximizing the potential of new drug approvals, largely through the development of new combination regimens; however, this could be limited by timely validation and regulatory concerns as the disease has become increasingly segmented into smaller subgroups, each with access to a variety of potentially effective therapies.
Dr. Fathi reported consulting/advisory services for Agios, BMS/Celgene, Astellas, and a variety of other pharmaceutical and biotechnology companies. He also reported receiving research support from Agios, BMS/Celgene, and AbbVie. Dr. Ravandi reported no conflicts of interest.
References
1. Westermann J and Bullinger L. Cancer Biol. 2021 April;S1044-579X(21)00084-5.
2. Kantarjian HM et al. Clin Lymphoma Myeloma Leuk. 2021 Sept;21(9):580-97.
The emergence of precision medicine has ushered in a groundbreaking era for the treatment of myeloid malignancies, with the ability to integrate individual molecular data into patient care.
Over the past decade, insights from research focusing on the mutations driving the malignant transformation of myeloid cells have provided the basis for the development of novel targeted therapies.1 With the recent U.S. Food and Drug Administration approval of several novel therapies for different acute myeloid leukemia (AML) indications, the current treatment landscape for AML is evolving rapidly.2
In addition, there has been substantial progress in the development of novel therapeutic strategies for other myeloid neoplasms, with numerous molecularly based therapies in early clinical trials in myeloproliferative neoplasms (MPNs) and myelodysplastic syndromes (MDSs). These advancements have been translated into optimized algorithms for diagnosis, prognostication, and treatment.
AML: Historical perspective
AML comprises a heterogeneous group of blood cell malignancies that require different treatment approaches and confer different prognoses.2 These include acute promyelocytic leukemia (APL) and core binding factor (CBF) AML, both of which have high rates of remission and prolonged survival. The remaining non-APL, non-CBF types can be divided by their cytogenetic-molecular profiles, as well as fitness for intensive chemotherapy. AML can also arise secondary to other myeloid neoplasms, especially after exposure to hypomethylating agents (HMAs), chemotherapy, or irradiation as prior treatment for the primary malignancy.
Historically, anthracycline- and cytarabine-based chemotherapy with or without allogeneic hematopoietic stem-cell transplant (allo-HSCT) was the standard of care in AML treatment with curative intent.1 In the palliative setting, low-dose cytarabine or HMAs were also treatment options. Despite 5 decades of clinical use of these options, researchers have continued to evaluate different dosing schedules of cytosine arabinoside (cytarabine or ara-C) and daunorubicin – the first two agents approved for the treatment of AML – during induction and consolidation treatment phases.
However, recent discoveries have led to the clinical development of targeted agents directed at isocitrate dehydrogenase (IDH), FMS-like tyrosine kinase 3 (FLT3), and BCL2.2 These developments, and the highly anticipated combinations arising from them, continue to challenge traditional treatment approaches, raising the question of whether intensive chemotherapy should remain the optimal standard of care.
Novel therapeutics in AML
Since 2017, several new therapies have been approved for the treatment of AML, including gemtuzumab ozogamicin, two FLT3 inhibitors (gilteritinib and midostaurin), two IDH inhibitors (ivosidenib and enasidenib), a BCL2 inhibitor (venetoclax), an oral HMA agent (azacitidine), a hedgehog inhibitor (glasdegib), and a liposomal formulation of CPX351. In addition, oral decitabine/cedazuridine may be used as an alternative oral HMA in AML, but it is currently the only FDA-approved treatment for chronic myelomonocytic leukemia (CMML) and MDS.2 Because AML subsets are very heterogeneous, an open question remains about how to best integrate these new agents into frontline and salvage combination regimens.
Acute promyelocytic leukemia
APL composes 5%-10% of AML and is characterized by the cytogenetic translocation between chromosomes 15 and 17, which leads to the PML-RAR alpha fusion oncogene and its encoded oncoprotein.2 Two therapies, all-trans retinoic acid (ATRA) and arsenic trioxide, when administered in combination with chemotherapy during induction, have been shown to improve outcomes in APL. At present, the combination of idarubicin and ATRA is the standard-of-care treatment for APL. In addition, patients with high-risk disease have been shown to benefit from the addition of gemtuzumab ozogamicin or anthracyclines.
Core binding factor AML
CBF AML includes patients with the cytogenetic-molecular subsets of inversion 16. Chemotherapy combined with gemtuzumab ozogamicin results in cure rates of 75% or higher and an estimated 5-year survival of 75%. Fludarabine, high-dose cytarabine, and gemtuzumab ozogamicin during induction and consolidation, and an alternative treatment modality (for example, allo-HSCT), for persistent minimal residual disease (MRD) in patients who achieve complete response (CR) is a commonly used regimen. Patients who cannot tolerate this regimen or who have persistent MRD may be treated with an HMA (for instance, decitabine or azacitidine) in combination with venetoclax and gemtuzumab ozogamicin, with the treatment duration adjusted according to MRD status or for 12 months or longer.
Mutations, such as N/KRAS (30%-50%), KIT (25%-30%), and FLT3 (15%-20%), also occur in CBF AML. Targeted agents may also be considered in some cases (for example, dasatinib or avapritinib for KIT mutations; FLT3 inhibitors for FLT3 mutations).
Intensive chemotherapy in younger/fit AML
Several AML regimens have demonstrated better outcomes than the conventional “3 + 7 regimen” (3 days of daunorubicin plus 7 days of cytarabine). Recently, the treatment paradigm has shifted from intensive chemotherapy alone to multidrug combination regimens, including regimens that incorporate targeted therapies, such as FLT3 inhibitors in FLT3-mutated AML, and venetoclax and/or IDH inhibitors as indicated. In addition, the recent FDA approval of oral azacitidine as maintenance therapy for patients in first CR (CR duration, 4 months or less; patients unable to complete the curative intensive chemotherapy) may allow for expanded combination regimens.
Older/unfit patients with AML: Low-intensity therapy
Prior to 2000, the majority of older/unfit patients with AML were offered supportive/palliative treatment. Today, the HMAs azacitidine and decitabine are the most commonly used drugs for the treatment of older/unfit AML. Recently, the FDA approved an oral formulation of decitabine plus oral cedazuridine for the treatment of CMML and MDS. This could provide an opportunity to investigate and develop an effective oral therapy regimen for older/unfit AML, such as oral decitabine/cedazuridine in combination with venetoclax, which may ease administration and improve quality of life for patients in CR post induction in the community setting.
Other studies have shown benefit for combining an HMA with venetoclax in patients with TP53-mutated AML. In addition, triplet regimens may also improve outcomes, with combinations such as HMA plus FLT3 inhibitor (for instance, midostaurin or gilteritinib) with or without venetoclax now being investigated. However, the potential increased risk of myelosuppression also needs to be considered with use of triplet regimens. The results of these and other combinatorial trials are greatly anticipated.
Two oral IDH inhibitors, ivosidenib (IDH1 inhibitor) and enasidenib (IDH2 inhibitor) were recently FDA approved as monotherapy for the treatment of IDH-mutated AML. Combination regimens of IDH inhibitors with chemotherapy are currently being investigated in patients with IDH-mutated AML and appear promising based on preliminary data demonstrating improved response rates and event-free survival.
Other FDA-approved therapies in AML
CPX-351 is a nanoscale liposome with a fixed 5:1 molar ratio of cytarabine and daunorubicin. Results from a phase 3 trial showed that CPX-351 resulted in higher response rates and longer survival compared with 3 + 7 chemotherapy in patients with secondary AML, a subgroup of patients with a very poor prognosis. Additional studies are ongoing, combining CPX-351 with gemtuzumab ozogamicin, venetoclax, and other targeted agents.
Results from a phase 2 trial led to the FDA approval of the hedgehog inhibitor glasdegib when given with low-dose cytarabine. The combination improved survival compared with low-dose cytarabine alone in older/unfit AML and high-risk MDS. However, because of poor survival relative to venetoclax-based combinations, glasdegib is not widely used in clinical practice; other trials exploring combinations with azacitidine and with intensive chemotherapy are ongoing.
Expert perspectives: Future of AML therapy
Amir T. Fathi, MD, associate professor of medicine at Harvard Medical School, Boston, and Farhad Ravandi, MD, professor of medicine at the University of Texas MD Anderson Cancer Center, Houston, are coauthors of a recent review that summarized the current treatment landscape in AML, including areas of evolving research.1
“In the next several years, I am hopeful there will be a series of regulatory approvals of novel, effective agents for myeloid malignancies,” Dr. Fathi explained. “Even if approvals are not as numerous as we’ve seen in AML, any additional effective options would be very welcome.”
Dr. Ravandi also noted that increased understanding of the biology underlying myeloid neoplasms has helped to develop novel therapies.
“As we’ve increased our understanding of the biology of these blood cancers, particularly the mechanisms of leukemogenesis and neoplastic change, we’ve been able to develop more effective therapies in AML,” Dr. Ravandi said.
“In the future, we are likely to see a similar trend in other myeloid neoplasms, such as MDSs and MPNs, as we better understand their underlying pathogenesis,” he further explained.
They both acknowledged that the future treatment paradigm in AML will focus on maximizing the potential of new drug approvals, largely through the development of new combination regimens; however, this could be limited by timely validation and regulatory concerns as the disease has become increasingly segmented into smaller subgroups, each with access to a variety of potentially effective therapies.
Dr. Fathi reported consulting/advisory services for Agios, BMS/Celgene, Astellas, and a variety of other pharmaceutical and biotechnology companies. He also reported receiving research support from Agios, BMS/Celgene, and AbbVie. Dr. Ravandi reported no conflicts of interest.
References
1. Westermann J and Bullinger L. Cancer Biol. 2021 April;S1044-579X(21)00084-5.
2. Kantarjian HM et al. Clin Lymphoma Myeloma Leuk. 2021 Sept;21(9):580-97.
The emergence of precision medicine has ushered in a groundbreaking era for the treatment of myeloid malignancies, with the ability to integrate individual molecular data into patient care.
Over the past decade, insights from research focusing on the mutations driving the malignant transformation of myeloid cells have provided the basis for the development of novel targeted therapies.1 With the recent U.S. Food and Drug Administration approval of several novel therapies for different acute myeloid leukemia (AML) indications, the current treatment landscape for AML is evolving rapidly.2
In addition, there has been substantial progress in the development of novel therapeutic strategies for other myeloid neoplasms, with numerous molecularly based therapies in early clinical trials in myeloproliferative neoplasms (MPNs) and myelodysplastic syndromes (MDSs). These advancements have been translated into optimized algorithms for diagnosis, prognostication, and treatment.
AML: Historical perspective
AML comprises a heterogeneous group of blood cell malignancies that require different treatment approaches and confer different prognoses.2 These include acute promyelocytic leukemia (APL) and core binding factor (CBF) AML, both of which have high rates of remission and prolonged survival. The remaining non-APL, non-CBF types can be divided by their cytogenetic-molecular profiles, as well as fitness for intensive chemotherapy. AML can also arise secondary to other myeloid neoplasms, especially after exposure to hypomethylating agents (HMAs), chemotherapy, or irradiation as prior treatment for the primary malignancy.
Historically, anthracycline- and cytarabine-based chemotherapy with or without allogeneic hematopoietic stem-cell transplant (allo-HSCT) was the standard of care in AML treatment with curative intent.1 In the palliative setting, low-dose cytarabine or HMAs were also treatment options. Despite 5 decades of clinical use of these options, researchers have continued to evaluate different dosing schedules of cytosine arabinoside (cytarabine or ara-C) and daunorubicin – the first two agents approved for the treatment of AML – during induction and consolidation treatment phases.
However, recent discoveries have led to the clinical development of targeted agents directed at isocitrate dehydrogenase (IDH), FMS-like tyrosine kinase 3 (FLT3), and BCL2.2 These developments, and the highly anticipated combinations arising from them, continue to challenge traditional treatment approaches, raising the question of whether intensive chemotherapy should remain the optimal standard of care.
Novel therapeutics in AML
Since 2017, several new therapies have been approved for the treatment of AML, including gemtuzumab ozogamicin, two FLT3 inhibitors (gilteritinib and midostaurin), two IDH inhibitors (ivosidenib and enasidenib), a BCL2 inhibitor (venetoclax), an oral HMA agent (azacitidine), a hedgehog inhibitor (glasdegib), and a liposomal formulation of CPX351. In addition, oral decitabine/cedazuridine may be used as an alternative oral HMA in AML, but it is currently the only FDA-approved treatment for chronic myelomonocytic leukemia (CMML) and MDS.2 Because AML subsets are very heterogeneous, an open question remains about how to best integrate these new agents into frontline and salvage combination regimens.
Acute promyelocytic leukemia
APL composes 5%-10% of AML and is characterized by the cytogenetic translocation between chromosomes 15 and 17, which leads to the PML-RAR alpha fusion oncogene and its encoded oncoprotein.2 Two therapies, all-trans retinoic acid (ATRA) and arsenic trioxide, when administered in combination with chemotherapy during induction, have been shown to improve outcomes in APL. At present, the combination of idarubicin and ATRA is the standard-of-care treatment for APL. In addition, patients with high-risk disease have been shown to benefit from the addition of gemtuzumab ozogamicin or anthracyclines.
Core binding factor AML
CBF AML includes patients with the cytogenetic-molecular subsets of inversion 16. Chemotherapy combined with gemtuzumab ozogamicin results in cure rates of 75% or higher and an estimated 5-year survival of 75%. Fludarabine, high-dose cytarabine, and gemtuzumab ozogamicin during induction and consolidation, and an alternative treatment modality (for example, allo-HSCT), for persistent minimal residual disease (MRD) in patients who achieve complete response (CR) is a commonly used regimen. Patients who cannot tolerate this regimen or who have persistent MRD may be treated with an HMA (for instance, decitabine or azacitidine) in combination with venetoclax and gemtuzumab ozogamicin, with the treatment duration adjusted according to MRD status or for 12 months or longer.
Mutations, such as N/KRAS (30%-50%), KIT (25%-30%), and FLT3 (15%-20%), also occur in CBF AML. Targeted agents may also be considered in some cases (for example, dasatinib or avapritinib for KIT mutations; FLT3 inhibitors for FLT3 mutations).
Intensive chemotherapy in younger/fit AML
Several AML regimens have demonstrated better outcomes than the conventional “3 + 7 regimen” (3 days of daunorubicin plus 7 days of cytarabine). Recently, the treatment paradigm has shifted from intensive chemotherapy alone to multidrug combination regimens, including regimens that incorporate targeted therapies, such as FLT3 inhibitors in FLT3-mutated AML, and venetoclax and/or IDH inhibitors as indicated. In addition, the recent FDA approval of oral azacitidine as maintenance therapy for patients in first CR (CR duration, 4 months or less; patients unable to complete the curative intensive chemotherapy) may allow for expanded combination regimens.
Older/unfit patients with AML: Low-intensity therapy
Prior to 2000, the majority of older/unfit patients with AML were offered supportive/palliative treatment. Today, the HMAs azacitidine and decitabine are the most commonly used drugs for the treatment of older/unfit AML. Recently, the FDA approved an oral formulation of decitabine plus oral cedazuridine for the treatment of CMML and MDS. This could provide an opportunity to investigate and develop an effective oral therapy regimen for older/unfit AML, such as oral decitabine/cedazuridine in combination with venetoclax, which may ease administration and improve quality of life for patients in CR post induction in the community setting.
Other studies have shown benefit for combining an HMA with venetoclax in patients with TP53-mutated AML. In addition, triplet regimens may also improve outcomes, with combinations such as HMA plus FLT3 inhibitor (for instance, midostaurin or gilteritinib) with or without venetoclax now being investigated. However, the potential increased risk of myelosuppression also needs to be considered with use of triplet regimens. The results of these and other combinatorial trials are greatly anticipated.
Two oral IDH inhibitors, ivosidenib (IDH1 inhibitor) and enasidenib (IDH2 inhibitor) were recently FDA approved as monotherapy for the treatment of IDH-mutated AML. Combination regimens of IDH inhibitors with chemotherapy are currently being investigated in patients with IDH-mutated AML and appear promising based on preliminary data demonstrating improved response rates and event-free survival.
Other FDA-approved therapies in AML
CPX-351 is a nanoscale liposome with a fixed 5:1 molar ratio of cytarabine and daunorubicin. Results from a phase 3 trial showed that CPX-351 resulted in higher response rates and longer survival compared with 3 + 7 chemotherapy in patients with secondary AML, a subgroup of patients with a very poor prognosis. Additional studies are ongoing, combining CPX-351 with gemtuzumab ozogamicin, venetoclax, and other targeted agents.
Results from a phase 2 trial led to the FDA approval of the hedgehog inhibitor glasdegib when given with low-dose cytarabine. The combination improved survival compared with low-dose cytarabine alone in older/unfit AML and high-risk MDS. However, because of poor survival relative to venetoclax-based combinations, glasdegib is not widely used in clinical practice; other trials exploring combinations with azacitidine and with intensive chemotherapy are ongoing.
Expert perspectives: Future of AML therapy
Amir T. Fathi, MD, associate professor of medicine at Harvard Medical School, Boston, and Farhad Ravandi, MD, professor of medicine at the University of Texas MD Anderson Cancer Center, Houston, are coauthors of a recent review that summarized the current treatment landscape in AML, including areas of evolving research.1
“In the next several years, I am hopeful there will be a series of regulatory approvals of novel, effective agents for myeloid malignancies,” Dr. Fathi explained. “Even if approvals are not as numerous as we’ve seen in AML, any additional effective options would be very welcome.”
Dr. Ravandi also noted that increased understanding of the biology underlying myeloid neoplasms has helped to develop novel therapies.
“As we’ve increased our understanding of the biology of these blood cancers, particularly the mechanisms of leukemogenesis and neoplastic change, we’ve been able to develop more effective therapies in AML,” Dr. Ravandi said.
“In the future, we are likely to see a similar trend in other myeloid neoplasms, such as MDSs and MPNs, as we better understand their underlying pathogenesis,” he further explained.
They both acknowledged that the future treatment paradigm in AML will focus on maximizing the potential of new drug approvals, largely through the development of new combination regimens; however, this could be limited by timely validation and regulatory concerns as the disease has become increasingly segmented into smaller subgroups, each with access to a variety of potentially effective therapies.
Dr. Fathi reported consulting/advisory services for Agios, BMS/Celgene, Astellas, and a variety of other pharmaceutical and biotechnology companies. He also reported receiving research support from Agios, BMS/Celgene, and AbbVie. Dr. Ravandi reported no conflicts of interest.
References
1. Westermann J and Bullinger L. Cancer Biol. 2021 April;S1044-579X(21)00084-5.
2. Kantarjian HM et al. Clin Lymphoma Myeloma Leuk. 2021 Sept;21(9):580-97.
NORD: Approaching rare cancers through a diversity lens
The National Organization for Rare Disorders (NORD® ) advocates for all rare disease patients, no matter their race, ethnicity, religion, color, national origin, age, disability, sexual orientation, gender identity, etc. Since its inception in 1983, NORD has advocated for marginalized individuals – people living with rare diseases, diagnosed and undiagnosed – who were excluded from conventional clinical care, research, and drug development.
People living with rare diseases often experience a long and arduous journey to diagnosis, due to a dearth of information in medical textbooks, lack of knowledge in the clinical setting, lack of research, and lack of FDA-approved treatments. Furthermore, a substantial amount of research has shown inequity in access to and quality of health care for marginalized groups, especially Black, Brown, indigenous and people of color (BIPOC). The barriers to be accurately diagnosed and provided quality care by specialists already poses threats to quality and length of life of the community at large, but the additional barriers faced by BIPOC communities have deadly consequences due to the lack of access to culturally proficient health care and access to rare disease specialists, in addition to socioeconomic considerations (i.e., insurance access and medical literacy).
In addition to further delayed diagnosis and inequities in care, people of color are consistently underrepresented in clinical trials and registries, resulting in a lack of diversity in clinical studies and some mystery in how effective therapies will be across diverse populations of patients. Because many rare diseases are genetic and certain genetic conditions disproportionately affect communities of color, having a vast majority of white participants creates significant knowledge gaps that can affect patient care and drug development and effectiveness.
Unfortunately, when looking at the rare cancer population within the rare disease community, the same problems persist. Of the approximately 7,000 known rare diseases, 1 more than 500 are rare cancers, 2 and combined, all rare cancers account for slightly more than one out of ever four cancer diagnoses each year and one out of every four cancer-related deaths. 3 Black people have the highest death rate and shortest survival of any racial/ethnic group in the U.S. for most cancers, and Black men have the highest cancer incidence rate. 4 NORD and the NORD Rare Cancer Coalition™, including 27 rare cancer member organizations, are committed to shining a light on the causes of these inequities for rare cancer patients, including but not limited to systemic racism, economic disparities, cultural differences, and issues concerning access to quality health care and inclusive research.
To raise awareness of rare cancers and the issues rare cancer patients face throughout the diagnostic odyssey, in seeking and receiving specialized care and in advocating for awareness, and increased research and drug development, NORD and the NORD Rare Cancer Coalition spearheaded Rare Cancer Day, observed annually on September 30. Through the universal hashtag campaign #RareCancerDay and our social media toolkit of infographics and messaging, NORD brings together the global community of advocates to promote awareness of rare cancers and provide opportunities to educate patients, caregivers, clinicians, and researchers. NORD hosted a free webinar for the rare disease community, Rare Cancers: Breaking Down Barriers to Diagnosis, Treatment and Research, to explore rare cancer challenges and offer insights to assist those who are impacted. Throughout August and September, NORD highlighted the powerful, important, and inspiring stories of the rare cancer community on the NORD blog.
In addition, the 2021 NORD Rare Diseases + Orphan Products Breakthrough Summit, held October 18 and 19, featured a breakout session and a follow-up discussion group on Advancing Rare Cancer Awareness & Education Among Healthcare Professionals. These sessions explored educational gaps and approaches for increasing awareness and delivering quality education for healthcare professionals in optimizing care for rare cancer patients, genomic testing, personalized medicine, and collaboration with researchers and patient advocacy groups.
This issue of Rare Diseases Report: Cancers helps us further our mission to foster the identification, treatment, and cure of rare disorders through programs of education, advocacy, research, and patient services, as well as the work of NORD’s Rare Cancer Coalition™ which aims to unite NORD member organizations working in rare cancers to collaborate on issues facing the greater rare cancer community.
NORD remains steadfastly committed to advocating for all rare disease patients, no matter their race, ethnicity, religion, color, national origin, age, disability, sexual orientation, gender identity, parental status, marital status, political affiliation, gender expression, mental illness, socioeconomic status or background, neuro(a)typicality, or physical appearance. NORD’s work includes advocating for rare cancer patients, raising awareness of rare cancer patients, sharing the stories of people living with rare cancers, and educating patients, caregivers and healthcare professionals about accurate diagnosis, quality care, advancements in research, and available treatment options. Learn more at rarediseases.org.
Rebecca Aune
Director of Education Programs
Debbie Drell
Director of Membership
References
1. Genetic and Rare Diseases Information Center; National Center for Advancing Translational Sciences; FAQs About Rare Diseases; 11/30/2017. https://rarediseases.info.nih.gov/diseases/pages/31/faqs-about-rare-diseases
2. Genetic and Rare Diseases Information Center; National Center for Advancing Translational Sciences; Rare Cancers; 1/25/2019. https://www.youtube.com/watch?v=ES5KylRT1qY, https://rarediseases.info.nih.gov/diseases/diseases-by-category/1/rare-cancers, or https://rarediseases.info.nih.gov/diseases/diseases-by-category/1/rare-cancers
3. NIH National Cancer Institute. Rare Cancer Statistics | Did You Know? [Video]. Youtube. https://www.youtube.com/watch?v=ES5KylRT1qY&t=155s. Published April 5, 2018. Accessed Oct. 20, 2021.
4. American Cancer Society. Cancer Facts; Figures for African Americans 2019-2021. Atlanta: American Cancer Society, 2019. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-facts-and-figures-for-african-americans/cancer-facts-and-figures-for-african-americans-2019-2021.pdf1.
The National Organization for Rare Disorders (NORD® ) advocates for all rare disease patients, no matter their race, ethnicity, religion, color, national origin, age, disability, sexual orientation, gender identity, etc. Since its inception in 1983, NORD has advocated for marginalized individuals – people living with rare diseases, diagnosed and undiagnosed – who were excluded from conventional clinical care, research, and drug development.
People living with rare diseases often experience a long and arduous journey to diagnosis, due to a dearth of information in medical textbooks, lack of knowledge in the clinical setting, lack of research, and lack of FDA-approved treatments. Furthermore, a substantial amount of research has shown inequity in access to and quality of health care for marginalized groups, especially Black, Brown, indigenous and people of color (BIPOC). The barriers to be accurately diagnosed and provided quality care by specialists already poses threats to quality and length of life of the community at large, but the additional barriers faced by BIPOC communities have deadly consequences due to the lack of access to culturally proficient health care and access to rare disease specialists, in addition to socioeconomic considerations (i.e., insurance access and medical literacy).
In addition to further delayed diagnosis and inequities in care, people of color are consistently underrepresented in clinical trials and registries, resulting in a lack of diversity in clinical studies and some mystery in how effective therapies will be across diverse populations of patients. Because many rare diseases are genetic and certain genetic conditions disproportionately affect communities of color, having a vast majority of white participants creates significant knowledge gaps that can affect patient care and drug development and effectiveness.
Unfortunately, when looking at the rare cancer population within the rare disease community, the same problems persist. Of the approximately 7,000 known rare diseases, 1 more than 500 are rare cancers, 2 and combined, all rare cancers account for slightly more than one out of ever four cancer diagnoses each year and one out of every four cancer-related deaths. 3 Black people have the highest death rate and shortest survival of any racial/ethnic group in the U.S. for most cancers, and Black men have the highest cancer incidence rate. 4 NORD and the NORD Rare Cancer Coalition™, including 27 rare cancer member organizations, are committed to shining a light on the causes of these inequities for rare cancer patients, including but not limited to systemic racism, economic disparities, cultural differences, and issues concerning access to quality health care and inclusive research.
To raise awareness of rare cancers and the issues rare cancer patients face throughout the diagnostic odyssey, in seeking and receiving specialized care and in advocating for awareness, and increased research and drug development, NORD and the NORD Rare Cancer Coalition spearheaded Rare Cancer Day, observed annually on September 30. Through the universal hashtag campaign #RareCancerDay and our social media toolkit of infographics and messaging, NORD brings together the global community of advocates to promote awareness of rare cancers and provide opportunities to educate patients, caregivers, clinicians, and researchers. NORD hosted a free webinar for the rare disease community, Rare Cancers: Breaking Down Barriers to Diagnosis, Treatment and Research, to explore rare cancer challenges and offer insights to assist those who are impacted. Throughout August and September, NORD highlighted the powerful, important, and inspiring stories of the rare cancer community on the NORD blog.
In addition, the 2021 NORD Rare Diseases + Orphan Products Breakthrough Summit, held October 18 and 19, featured a breakout session and a follow-up discussion group on Advancing Rare Cancer Awareness & Education Among Healthcare Professionals. These sessions explored educational gaps and approaches for increasing awareness and delivering quality education for healthcare professionals in optimizing care for rare cancer patients, genomic testing, personalized medicine, and collaboration with researchers and patient advocacy groups.
This issue of Rare Diseases Report: Cancers helps us further our mission to foster the identification, treatment, and cure of rare disorders through programs of education, advocacy, research, and patient services, as well as the work of NORD’s Rare Cancer Coalition™ which aims to unite NORD member organizations working in rare cancers to collaborate on issues facing the greater rare cancer community.
NORD remains steadfastly committed to advocating for all rare disease patients, no matter their race, ethnicity, religion, color, national origin, age, disability, sexual orientation, gender identity, parental status, marital status, political affiliation, gender expression, mental illness, socioeconomic status or background, neuro(a)typicality, or physical appearance. NORD’s work includes advocating for rare cancer patients, raising awareness of rare cancer patients, sharing the stories of people living with rare cancers, and educating patients, caregivers and healthcare professionals about accurate diagnosis, quality care, advancements in research, and available treatment options. Learn more at rarediseases.org.
Rebecca Aune
Director of Education Programs
Debbie Drell
Director of Membership
References
1. Genetic and Rare Diseases Information Center; National Center for Advancing Translational Sciences; FAQs About Rare Diseases; 11/30/2017. https://rarediseases.info.nih.gov/diseases/pages/31/faqs-about-rare-diseases
2. Genetic and Rare Diseases Information Center; National Center for Advancing Translational Sciences; Rare Cancers; 1/25/2019. https://www.youtube.com/watch?v=ES5KylRT1qY, https://rarediseases.info.nih.gov/diseases/diseases-by-category/1/rare-cancers, or https://rarediseases.info.nih.gov/diseases/diseases-by-category/1/rare-cancers
3. NIH National Cancer Institute. Rare Cancer Statistics | Did You Know? [Video]. Youtube. https://www.youtube.com/watch?v=ES5KylRT1qY&t=155s. Published April 5, 2018. Accessed Oct. 20, 2021.
4. American Cancer Society. Cancer Facts; Figures for African Americans 2019-2021. Atlanta: American Cancer Society, 2019. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-facts-and-figures-for-african-americans/cancer-facts-and-figures-for-african-americans-2019-2021.pdf1.
The National Organization for Rare Disorders (NORD® ) advocates for all rare disease patients, no matter their race, ethnicity, religion, color, national origin, age, disability, sexual orientation, gender identity, etc. Since its inception in 1983, NORD has advocated for marginalized individuals – people living with rare diseases, diagnosed and undiagnosed – who were excluded from conventional clinical care, research, and drug development.
People living with rare diseases often experience a long and arduous journey to diagnosis, due to a dearth of information in medical textbooks, lack of knowledge in the clinical setting, lack of research, and lack of FDA-approved treatments. Furthermore, a substantial amount of research has shown inequity in access to and quality of health care for marginalized groups, especially Black, Brown, indigenous and people of color (BIPOC). The barriers to be accurately diagnosed and provided quality care by specialists already poses threats to quality and length of life of the community at large, but the additional barriers faced by BIPOC communities have deadly consequences due to the lack of access to culturally proficient health care and access to rare disease specialists, in addition to socioeconomic considerations (i.e., insurance access and medical literacy).
In addition to further delayed diagnosis and inequities in care, people of color are consistently underrepresented in clinical trials and registries, resulting in a lack of diversity in clinical studies and some mystery in how effective therapies will be across diverse populations of patients. Because many rare diseases are genetic and certain genetic conditions disproportionately affect communities of color, having a vast majority of white participants creates significant knowledge gaps that can affect patient care and drug development and effectiveness.
Unfortunately, when looking at the rare cancer population within the rare disease community, the same problems persist. Of the approximately 7,000 known rare diseases, 1 more than 500 are rare cancers, 2 and combined, all rare cancers account for slightly more than one out of ever four cancer diagnoses each year and one out of every four cancer-related deaths. 3 Black people have the highest death rate and shortest survival of any racial/ethnic group in the U.S. for most cancers, and Black men have the highest cancer incidence rate. 4 NORD and the NORD Rare Cancer Coalition™, including 27 rare cancer member organizations, are committed to shining a light on the causes of these inequities for rare cancer patients, including but not limited to systemic racism, economic disparities, cultural differences, and issues concerning access to quality health care and inclusive research.
To raise awareness of rare cancers and the issues rare cancer patients face throughout the diagnostic odyssey, in seeking and receiving specialized care and in advocating for awareness, and increased research and drug development, NORD and the NORD Rare Cancer Coalition spearheaded Rare Cancer Day, observed annually on September 30. Through the universal hashtag campaign #RareCancerDay and our social media toolkit of infographics and messaging, NORD brings together the global community of advocates to promote awareness of rare cancers and provide opportunities to educate patients, caregivers, clinicians, and researchers. NORD hosted a free webinar for the rare disease community, Rare Cancers: Breaking Down Barriers to Diagnosis, Treatment and Research, to explore rare cancer challenges and offer insights to assist those who are impacted. Throughout August and September, NORD highlighted the powerful, important, and inspiring stories of the rare cancer community on the NORD blog.
In addition, the 2021 NORD Rare Diseases + Orphan Products Breakthrough Summit, held October 18 and 19, featured a breakout session and a follow-up discussion group on Advancing Rare Cancer Awareness & Education Among Healthcare Professionals. These sessions explored educational gaps and approaches for increasing awareness and delivering quality education for healthcare professionals in optimizing care for rare cancer patients, genomic testing, personalized medicine, and collaboration with researchers and patient advocacy groups.
This issue of Rare Diseases Report: Cancers helps us further our mission to foster the identification, treatment, and cure of rare disorders through programs of education, advocacy, research, and patient services, as well as the work of NORD’s Rare Cancer Coalition™ which aims to unite NORD member organizations working in rare cancers to collaborate on issues facing the greater rare cancer community.
NORD remains steadfastly committed to advocating for all rare disease patients, no matter their race, ethnicity, religion, color, national origin, age, disability, sexual orientation, gender identity, parental status, marital status, political affiliation, gender expression, mental illness, socioeconomic status or background, neuro(a)typicality, or physical appearance. NORD’s work includes advocating for rare cancer patients, raising awareness of rare cancer patients, sharing the stories of people living with rare cancers, and educating patients, caregivers and healthcare professionals about accurate diagnosis, quality care, advancements in research, and available treatment options. Learn more at rarediseases.org.
Rebecca Aune
Director of Education Programs
Debbie Drell
Director of Membership
References
1. Genetic and Rare Diseases Information Center; National Center for Advancing Translational Sciences; FAQs About Rare Diseases; 11/30/2017. https://rarediseases.info.nih.gov/diseases/pages/31/faqs-about-rare-diseases
2. Genetic and Rare Diseases Information Center; National Center for Advancing Translational Sciences; Rare Cancers; 1/25/2019. https://www.youtube.com/watch?v=ES5KylRT1qY, https://rarediseases.info.nih.gov/diseases/diseases-by-category/1/rare-cancers, or https://rarediseases.info.nih.gov/diseases/diseases-by-category/1/rare-cancers
3. NIH National Cancer Institute. Rare Cancer Statistics | Did You Know? [Video]. Youtube. https://www.youtube.com/watch?v=ES5KylRT1qY&t=155s. Published April 5, 2018. Accessed Oct. 20, 2021.
4. American Cancer Society. Cancer Facts; Figures for African Americans 2019-2021. Atlanta: American Cancer Society, 2019. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-facts-and-figures-for-african-americans/cancer-facts-and-figures-for-african-americans-2019-2021.pdf1.
Myeloid patients respond robustly to Moderna COVID vaccine
Factors including age, gender, race, disease status, lower-intensity active treatment, baseline neutrophil and lymphocyte counts, and past history of stem cell transplant had no effects on seroconversion in the study, which, despite its small numbers, is one of the largest series to date among patients with myeloid cancers. The findings were reported at the annual meeting of the American Society of Hematology.
COVID vaccination “appears to induce a strong antibody response” in patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS), unlike with B-cell malignancies. “It indicates we should be aggressive about vaccinating such patients,” said senior investigator Jeffrey Lancet, MD, a blood cancer specialist at Moffitt, when he presented the findings at the meeting.
Presentation moderator Laura Michaelis, MD, a hematologist-oncologist at the Medical College of Wisconsin, Milwaukee, agreed.
The “strong antibody response in this group,” coupled with its high risk for severe COVID, “confirm the importance of these patients getting vaccinated,” she said.
Thirty patients with AML and 16 with MDS were included in the review. Most patients were in remission at the time of vaccination, but a third were in active treatment, including six on hypomethylating agents, six on targeted therapies, two on luspatercept, and one on lenalidomide. Thirty-two patients (69.6%) were a median of 17 months past allogeneic stem cell transplant.
Overall, 69.6% of patients developed IgG against spike proteins after the first shot and 95.7% of patients after the second dose, with a large increase in titer levels from the first to the second dose, from a mean of 315 AU/mL to 3,806.5 AU/mL following the second dose.
“Lab and clinical variables did not affect the antibody positivity rate after the second dose,” but patients on steroids and other immunosuppressants seemed less likely to respond to the first shot, Dr. Lancet said.
The study, conducted in early 2021, did not include acutely ill patients or those undergoing cheomotherapy induction and other aggressive treatments, because such patients were not being vaccinated at Moffitt during the study period.
The investigators measured anti-spike IgG by ELISA at baseline, then again about a month after the first shot and a month after the second shot.
Side effects were common and typically mild, including injection site pain, fatigue, headache, and arm swelling. Two patients with AML relapsed after vaccination.
Patients were a median of 68 years old when they were vaccinated; 58.7% were men; and almost all of the subjects were White. The median time from diagnosis to the first shot was 2 years.
The next step in the project is to study the timing of vaccination and response to it among patients on aggressive treatment and to perform neutralizing antibody assays to correlate IgG response with protection from COVID.
No funding was reported for the study. Investigators had numerous industry ties, including Dr. Lancet, a consultant for Celgene/BMS, Millenium Pharma/Takeda, AbbVie, and other firms. Dr. Michaelis didn’t have any disclosures.
[email protected]
Factors including age, gender, race, disease status, lower-intensity active treatment, baseline neutrophil and lymphocyte counts, and past history of stem cell transplant had no effects on seroconversion in the study, which, despite its small numbers, is one of the largest series to date among patients with myeloid cancers. The findings were reported at the annual meeting of the American Society of Hematology.
COVID vaccination “appears to induce a strong antibody response” in patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS), unlike with B-cell malignancies. “It indicates we should be aggressive about vaccinating such patients,” said senior investigator Jeffrey Lancet, MD, a blood cancer specialist at Moffitt, when he presented the findings at the meeting.
Presentation moderator Laura Michaelis, MD, a hematologist-oncologist at the Medical College of Wisconsin, Milwaukee, agreed.
The “strong antibody response in this group,” coupled with its high risk for severe COVID, “confirm the importance of these patients getting vaccinated,” she said.
Thirty patients with AML and 16 with MDS were included in the review. Most patients were in remission at the time of vaccination, but a third were in active treatment, including six on hypomethylating agents, six on targeted therapies, two on luspatercept, and one on lenalidomide. Thirty-two patients (69.6%) were a median of 17 months past allogeneic stem cell transplant.
Overall, 69.6% of patients developed IgG against spike proteins after the first shot and 95.7% of patients after the second dose, with a large increase in titer levels from the first to the second dose, from a mean of 315 AU/mL to 3,806.5 AU/mL following the second dose.
“Lab and clinical variables did not affect the antibody positivity rate after the second dose,” but patients on steroids and other immunosuppressants seemed less likely to respond to the first shot, Dr. Lancet said.
The study, conducted in early 2021, did not include acutely ill patients or those undergoing cheomotherapy induction and other aggressive treatments, because such patients were not being vaccinated at Moffitt during the study period.
The investigators measured anti-spike IgG by ELISA at baseline, then again about a month after the first shot and a month after the second shot.
Side effects were common and typically mild, including injection site pain, fatigue, headache, and arm swelling. Two patients with AML relapsed after vaccination.
Patients were a median of 68 years old when they were vaccinated; 58.7% were men; and almost all of the subjects were White. The median time from diagnosis to the first shot was 2 years.
The next step in the project is to study the timing of vaccination and response to it among patients on aggressive treatment and to perform neutralizing antibody assays to correlate IgG response with protection from COVID.
No funding was reported for the study. Investigators had numerous industry ties, including Dr. Lancet, a consultant for Celgene/BMS, Millenium Pharma/Takeda, AbbVie, and other firms. Dr. Michaelis didn’t have any disclosures.
[email protected]
Factors including age, gender, race, disease status, lower-intensity active treatment, baseline neutrophil and lymphocyte counts, and past history of stem cell transplant had no effects on seroconversion in the study, which, despite its small numbers, is one of the largest series to date among patients with myeloid cancers. The findings were reported at the annual meeting of the American Society of Hematology.
COVID vaccination “appears to induce a strong antibody response” in patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS), unlike with B-cell malignancies. “It indicates we should be aggressive about vaccinating such patients,” said senior investigator Jeffrey Lancet, MD, a blood cancer specialist at Moffitt, when he presented the findings at the meeting.
Presentation moderator Laura Michaelis, MD, a hematologist-oncologist at the Medical College of Wisconsin, Milwaukee, agreed.
The “strong antibody response in this group,” coupled with its high risk for severe COVID, “confirm the importance of these patients getting vaccinated,” she said.
Thirty patients with AML and 16 with MDS were included in the review. Most patients were in remission at the time of vaccination, but a third were in active treatment, including six on hypomethylating agents, six on targeted therapies, two on luspatercept, and one on lenalidomide. Thirty-two patients (69.6%) were a median of 17 months past allogeneic stem cell transplant.
Overall, 69.6% of patients developed IgG against spike proteins after the first shot and 95.7% of patients after the second dose, with a large increase in titer levels from the first to the second dose, from a mean of 315 AU/mL to 3,806.5 AU/mL following the second dose.
“Lab and clinical variables did not affect the antibody positivity rate after the second dose,” but patients on steroids and other immunosuppressants seemed less likely to respond to the first shot, Dr. Lancet said.
The study, conducted in early 2021, did not include acutely ill patients or those undergoing cheomotherapy induction and other aggressive treatments, because such patients were not being vaccinated at Moffitt during the study period.
The investigators measured anti-spike IgG by ELISA at baseline, then again about a month after the first shot and a month after the second shot.
Side effects were common and typically mild, including injection site pain, fatigue, headache, and arm swelling. Two patients with AML relapsed after vaccination.
Patients were a median of 68 years old when they were vaccinated; 58.7% were men; and almost all of the subjects were White. The median time from diagnosis to the first shot was 2 years.
The next step in the project is to study the timing of vaccination and response to it among patients on aggressive treatment and to perform neutralizing antibody assays to correlate IgG response with protection from COVID.
No funding was reported for the study. Investigators had numerous industry ties, including Dr. Lancet, a consultant for Celgene/BMS, Millenium Pharma/Takeda, AbbVie, and other firms. Dr. Michaelis didn’t have any disclosures.
[email protected]
FROM ASH 2021
‘Outstanding data’: Mosunetuzumab in r/r follicular lymphoma
An experimental bi-specific monoclonal antibody known as mosunetuzumab has induced high response rates and long-duration responses as monotherapy for patients with heavily pretreated, relapsed or refractory follicular lymphoma in a phase 2 expansion study.
At a median follow-up of 18.3 months, 54 of 90 patients (60%) had a complete response, and 18 (20%) had a partial response after treatment with mosunetuzumab, reported L. Elizabeth Budde, MD, PhD, from City of Hope Comprehensive Cancer Center in Duarte, Calif.
In contrast, the complete response rate for historical controls was just 14% (P < .0001), Dr. Budde noted.
“We have seen deep and durable responses in heavily pretreated, high-risk relapsed/refractory follicular lymphoma patients with fixed-duration treatment. We also observed a very favorable tolerability profile, with most cytokine release syndrome confined to cycle 1 and low grade, and treatment administration is without mandatory hospitalization,” she commented.
Budde was speaking at a press briefing prior to her presentation of the data at the annual meeting of the American Society of Hematology (ASH), held in a hybrid live/virtual format.
The manufacturer, Genentech, said in a statement that based on these “highly positive results,” it plans to submit the new data to the U.S. Food and Drug Administration (FDA) in the near future for approval consideration.
If approved, mosunetuzumab has the potential to be a first-in-class CD20xCD3 T-cell engaging bispecific antibody in non-Hodgkin lymphoma, the company added.
“Outstanding” data
A lymphoma specialist who was not involved in the study told this news organization that he was favorably impressed by the findings.
“To me, the single-agent data looks really outstanding, with a response rate of 80%, a complete response rate of 60%, and a median duration of response of 23 months, and really very acceptable rates of cytokine release syndrome,” commented Brad S. Kahl, MD, from the Siteman Cancer Center and Washington University School of Medicine in St. Louis.
“I think as a single agent — if it does get approval — it will be a really valuable addition to the armamentarium in follicular lymphoma,” he said.
Dr. Kahl pointed to a separate phase 1b study, also presented at the meeting, suggesting that the combination of mosunetuzumab and lenalidomide (Revlimid) was safe and showed promising antitumor activity in patients with follicular lymphoma that has relapsed after at least 1 line of therapy.
“I’m very interested to see how mosunetuzumab plus lenalidomide pans out in the long run,” he said.
Study details
Mosunetuzumab engages T cells and redirects them to eliminate malignant B cells. It has the potential to be used as an off-the-shelf product, Dr. Budde said.
In the single-arm phase 2 expansion trial, Dr. Budde and colleagues enrolled 90 patients with grades 1 to 3a follicular lymphoma whose disease relapsed or was refractory to at least two prior lines of therapy, including at least one anti-CD20 monoclonal antibody, and at least one alkylating agent.
Patients were treated with step-up dosing for the first 21-day cycle to mitigate the cytokine release syndrome. They then received eight cycles if they had a complete response, and 17 cycles if they had a partial response or stable disease after eight cycles.
The primary endpoint was complete response rate by independent review, which was 60%, and the overall response rate (ORR), a secondary efficacy endpoint, was 80%.
There were no significant differences in CR or ORR rates among subgroups according to patient age, number of prior lines of therapy, relapsed or refractory disease to last prior line of therapy, double-refractory disease, or disease progression within 24 months of primary therapy.
The median duration of response among all responders was 22.8 months, with a median time to first response of 1.4 months. The 12- and 18-months event-free rates were 62% and 57%, respectively.
The safety profile was manageable, Dr. Budde said, with grade 3 or 4 drug-related adverse events occurring in about half of patients, and serious adverse events occurring in a third.
There were two deaths during the study, but neither was judged to be related to mosunetuzumab, and there were only two events leading to drug discontinuation.
Cytokine release syndrome (CRS) of any grade occurred in 40 patients (44.4%), but only 1 patient each had a grade 3 or 4 CR. The median time to CRS onset was 5.2 hours in cycle 1, and 26.6 hours in subsequent cycles. The median duration of CRS was 3 days. Ten patients had CRS managed with corticosteroids, and seven had it managed with tocilizumab.
Immune effector cell-associated neurotoxicity syndrome (ICANS) events were infrequent, and all were grade 1 or 2 in severity.
The study was supported by Genentech. Dr. Budde disclosed consulting for the company and others. Dr. Kahl has previously disclosed financial considerations with AbbVie.
A version of this article first appeared on Medscape.com.
This article was updated 12/12/21.
An experimental bi-specific monoclonal antibody known as mosunetuzumab has induced high response rates and long-duration responses as monotherapy for patients with heavily pretreated, relapsed or refractory follicular lymphoma in a phase 2 expansion study.
At a median follow-up of 18.3 months, 54 of 90 patients (60%) had a complete response, and 18 (20%) had a partial response after treatment with mosunetuzumab, reported L. Elizabeth Budde, MD, PhD, from City of Hope Comprehensive Cancer Center in Duarte, Calif.
In contrast, the complete response rate for historical controls was just 14% (P < .0001), Dr. Budde noted.
“We have seen deep and durable responses in heavily pretreated, high-risk relapsed/refractory follicular lymphoma patients with fixed-duration treatment. We also observed a very favorable tolerability profile, with most cytokine release syndrome confined to cycle 1 and low grade, and treatment administration is without mandatory hospitalization,” she commented.
Budde was speaking at a press briefing prior to her presentation of the data at the annual meeting of the American Society of Hematology (ASH), held in a hybrid live/virtual format.
The manufacturer, Genentech, said in a statement that based on these “highly positive results,” it plans to submit the new data to the U.S. Food and Drug Administration (FDA) in the near future for approval consideration.
If approved, mosunetuzumab has the potential to be a first-in-class CD20xCD3 T-cell engaging bispecific antibody in non-Hodgkin lymphoma, the company added.
“Outstanding” data
A lymphoma specialist who was not involved in the study told this news organization that he was favorably impressed by the findings.
“To me, the single-agent data looks really outstanding, with a response rate of 80%, a complete response rate of 60%, and a median duration of response of 23 months, and really very acceptable rates of cytokine release syndrome,” commented Brad S. Kahl, MD, from the Siteman Cancer Center and Washington University School of Medicine in St. Louis.
“I think as a single agent — if it does get approval — it will be a really valuable addition to the armamentarium in follicular lymphoma,” he said.
Dr. Kahl pointed to a separate phase 1b study, also presented at the meeting, suggesting that the combination of mosunetuzumab and lenalidomide (Revlimid) was safe and showed promising antitumor activity in patients with follicular lymphoma that has relapsed after at least 1 line of therapy.
“I’m very interested to see how mosunetuzumab plus lenalidomide pans out in the long run,” he said.
Study details
Mosunetuzumab engages T cells and redirects them to eliminate malignant B cells. It has the potential to be used as an off-the-shelf product, Dr. Budde said.
In the single-arm phase 2 expansion trial, Dr. Budde and colleagues enrolled 90 patients with grades 1 to 3a follicular lymphoma whose disease relapsed or was refractory to at least two prior lines of therapy, including at least one anti-CD20 monoclonal antibody, and at least one alkylating agent.
Patients were treated with step-up dosing for the first 21-day cycle to mitigate the cytokine release syndrome. They then received eight cycles if they had a complete response, and 17 cycles if they had a partial response or stable disease after eight cycles.
The primary endpoint was complete response rate by independent review, which was 60%, and the overall response rate (ORR), a secondary efficacy endpoint, was 80%.
There were no significant differences in CR or ORR rates among subgroups according to patient age, number of prior lines of therapy, relapsed or refractory disease to last prior line of therapy, double-refractory disease, or disease progression within 24 months of primary therapy.
The median duration of response among all responders was 22.8 months, with a median time to first response of 1.4 months. The 12- and 18-months event-free rates were 62% and 57%, respectively.
The safety profile was manageable, Dr. Budde said, with grade 3 or 4 drug-related adverse events occurring in about half of patients, and serious adverse events occurring in a third.
There were two deaths during the study, but neither was judged to be related to mosunetuzumab, and there were only two events leading to drug discontinuation.
Cytokine release syndrome (CRS) of any grade occurred in 40 patients (44.4%), but only 1 patient each had a grade 3 or 4 CR. The median time to CRS onset was 5.2 hours in cycle 1, and 26.6 hours in subsequent cycles. The median duration of CRS was 3 days. Ten patients had CRS managed with corticosteroids, and seven had it managed with tocilizumab.
Immune effector cell-associated neurotoxicity syndrome (ICANS) events were infrequent, and all were grade 1 or 2 in severity.
The study was supported by Genentech. Dr. Budde disclosed consulting for the company and others. Dr. Kahl has previously disclosed financial considerations with AbbVie.
A version of this article first appeared on Medscape.com.
This article was updated 12/12/21.
An experimental bi-specific monoclonal antibody known as mosunetuzumab has induced high response rates and long-duration responses as monotherapy for patients with heavily pretreated, relapsed or refractory follicular lymphoma in a phase 2 expansion study.
At a median follow-up of 18.3 months, 54 of 90 patients (60%) had a complete response, and 18 (20%) had a partial response after treatment with mosunetuzumab, reported L. Elizabeth Budde, MD, PhD, from City of Hope Comprehensive Cancer Center in Duarte, Calif.
In contrast, the complete response rate for historical controls was just 14% (P < .0001), Dr. Budde noted.
“We have seen deep and durable responses in heavily pretreated, high-risk relapsed/refractory follicular lymphoma patients with fixed-duration treatment. We also observed a very favorable tolerability profile, with most cytokine release syndrome confined to cycle 1 and low grade, and treatment administration is without mandatory hospitalization,” she commented.
Budde was speaking at a press briefing prior to her presentation of the data at the annual meeting of the American Society of Hematology (ASH), held in a hybrid live/virtual format.
The manufacturer, Genentech, said in a statement that based on these “highly positive results,” it plans to submit the new data to the U.S. Food and Drug Administration (FDA) in the near future for approval consideration.
If approved, mosunetuzumab has the potential to be a first-in-class CD20xCD3 T-cell engaging bispecific antibody in non-Hodgkin lymphoma, the company added.
“Outstanding” data
A lymphoma specialist who was not involved in the study told this news organization that he was favorably impressed by the findings.
“To me, the single-agent data looks really outstanding, with a response rate of 80%, a complete response rate of 60%, and a median duration of response of 23 months, and really very acceptable rates of cytokine release syndrome,” commented Brad S. Kahl, MD, from the Siteman Cancer Center and Washington University School of Medicine in St. Louis.
“I think as a single agent — if it does get approval — it will be a really valuable addition to the armamentarium in follicular lymphoma,” he said.
Dr. Kahl pointed to a separate phase 1b study, also presented at the meeting, suggesting that the combination of mosunetuzumab and lenalidomide (Revlimid) was safe and showed promising antitumor activity in patients with follicular lymphoma that has relapsed after at least 1 line of therapy.
“I’m very interested to see how mosunetuzumab plus lenalidomide pans out in the long run,” he said.
Study details
Mosunetuzumab engages T cells and redirects them to eliminate malignant B cells. It has the potential to be used as an off-the-shelf product, Dr. Budde said.
In the single-arm phase 2 expansion trial, Dr. Budde and colleagues enrolled 90 patients with grades 1 to 3a follicular lymphoma whose disease relapsed or was refractory to at least two prior lines of therapy, including at least one anti-CD20 monoclonal antibody, and at least one alkylating agent.
Patients were treated with step-up dosing for the first 21-day cycle to mitigate the cytokine release syndrome. They then received eight cycles if they had a complete response, and 17 cycles if they had a partial response or stable disease after eight cycles.
The primary endpoint was complete response rate by independent review, which was 60%, and the overall response rate (ORR), a secondary efficacy endpoint, was 80%.
There were no significant differences in CR or ORR rates among subgroups according to patient age, number of prior lines of therapy, relapsed or refractory disease to last prior line of therapy, double-refractory disease, or disease progression within 24 months of primary therapy.
The median duration of response among all responders was 22.8 months, with a median time to first response of 1.4 months. The 12- and 18-months event-free rates were 62% and 57%, respectively.
The safety profile was manageable, Dr. Budde said, with grade 3 or 4 drug-related adverse events occurring in about half of patients, and serious adverse events occurring in a third.
There were two deaths during the study, but neither was judged to be related to mosunetuzumab, and there were only two events leading to drug discontinuation.
Cytokine release syndrome (CRS) of any grade occurred in 40 patients (44.4%), but only 1 patient each had a grade 3 or 4 CR. The median time to CRS onset was 5.2 hours in cycle 1, and 26.6 hours in subsequent cycles. The median duration of CRS was 3 days. Ten patients had CRS managed with corticosteroids, and seven had it managed with tocilizumab.
Immune effector cell-associated neurotoxicity syndrome (ICANS) events were infrequent, and all were grade 1 or 2 in severity.
The study was supported by Genentech. Dr. Budde disclosed consulting for the company and others. Dr. Kahl has previously disclosed financial considerations with AbbVie.
A version of this article first appeared on Medscape.com.
This article was updated 12/12/21.
AT ASH 2021
For leukemias, COVID-19 death risks tied to poor prognoses, ICU deferrals
, results of an American Society of Hematology (ASH) COVID-19 registry study suggest.
Rates of severe COVID-19 were significantly higher among patients who had active disease or neutropenia at the time of their COVID-19 diagnosis. Mortality related to COVID-19 was linked to neutropenia, primary disease prognosis of less than 6 months, and deferral of recommended ICU care, study results show.
By contrast, mortality was not associated with active primary disease or its treatment, according to researcher Pinkal Desai, MD, MPH.
Taken together, these findings provide preliminary evidence to support the use of aggressive supportive treatment of COVID-19 in patients with acute leukemias and myelodysplastic syndromes, said Dr. Desai, a hematologist-oncologist with Weill Cornell Medicine and NewYork-Presbyterian in New York.
“If desired by patients, aggressive support for hospitalized patients with COVID-19 is appropriate, regardless of remission status, given the results of our study,” Dr. Desai said in a press conference during the annual meeting of the American Society of Hematology.
In non-cancer patient populations, advanced age and cytopenias have been associated with mortality related to COVID-19, Dr. Desai said. Likewise, patients with acute leukemias and myelodysplastic syndrome are generally older and have disease- or treatment-related cytopenias, which might affect the severity of and mortality from COVID-19, she added.
With that concern in mind, Dr. Desai and co-investigators looked at predictors of severe COVID-19 disease and death among patients in the ASH Research Collaborative (ASH RC) COVID-19 Registry for Hematology.
This registry was started in the early days of the pandemic to provide real-time observational COVID-19 data to clinicians, according to an ASH news release.
The analysis by Dr. Desai and co-authors included 257 patients with COVID-19 as determined by their physician, including 135 with a primary diagnosis of acute myeloid leukemia, 82 with acute lymphocytic leukemia, and 40 with myelodysplastic syndromes. Sixty percent of the patients were hospitalized due to COVID-19.
At the time of COVID-19 diagnosis, 46% of patients were in remission, and 44% had active disease, according to the report.
Both neutropenia and active disease status at COVID-19 diagnosis were linked to severe COVID-19, defined as ICU admission due to a COVID-19-related reason, according to results of multivariable analysis. Among patients with severe COVID-19, 67% had active disease, meaning just 33% were in remission, Dr. Desai noted.
In multivariable analysis, two factors were significantly associated with mortality, she added: having an estimated pre-COVID-19 prognosis from the primary disease of less than 6 months, and deferral of ICU care when it was recommended to the patient.
Mortality was 21% overall, higher than would be expected in a non-cancer population, Dr. Desai said. For patients with COVID-19 requiring hospitalization, the mortality rate was 34% and for those patients who did go to the ICU, the mortality rate was 68%.
By contrast, there was no significant association between mortality and active disease as compared to disease in remission, Dr. Desai noted in her presentation. Likewise, mortality was not associated with active treatment at the time of COVID-19 diagnosis as compared to no treatment.
Gwen Nichols, MD, executive vice president and chief medical officer of the Leukemia & Lymphoma Society, New York, said those are reassuring data for patients with acute leukemias and myelodysplastic syndromes and their healthcare providers.
“From our point of view, it helps us say, ‘do not stop your treatment because of worries about COVID-19—it’s more important that you treat your cancer,” Dr. Nichols said in an interview. “We now know we can help people through COVID-19, and I think this is just really important data to back that up,” she added.
Dr. Desai provided disclosures related to Agios, Kura Oncology, and Bristol Myers Squibb (consultancy), and to Janssen R&D and Astex (research funding).
, results of an American Society of Hematology (ASH) COVID-19 registry study suggest.
Rates of severe COVID-19 were significantly higher among patients who had active disease or neutropenia at the time of their COVID-19 diagnosis. Mortality related to COVID-19 was linked to neutropenia, primary disease prognosis of less than 6 months, and deferral of recommended ICU care, study results show.
By contrast, mortality was not associated with active primary disease or its treatment, according to researcher Pinkal Desai, MD, MPH.
Taken together, these findings provide preliminary evidence to support the use of aggressive supportive treatment of COVID-19 in patients with acute leukemias and myelodysplastic syndromes, said Dr. Desai, a hematologist-oncologist with Weill Cornell Medicine and NewYork-Presbyterian in New York.
“If desired by patients, aggressive support for hospitalized patients with COVID-19 is appropriate, regardless of remission status, given the results of our study,” Dr. Desai said in a press conference during the annual meeting of the American Society of Hematology.
In non-cancer patient populations, advanced age and cytopenias have been associated with mortality related to COVID-19, Dr. Desai said. Likewise, patients with acute leukemias and myelodysplastic syndrome are generally older and have disease- or treatment-related cytopenias, which might affect the severity of and mortality from COVID-19, she added.
With that concern in mind, Dr. Desai and co-investigators looked at predictors of severe COVID-19 disease and death among patients in the ASH Research Collaborative (ASH RC) COVID-19 Registry for Hematology.
This registry was started in the early days of the pandemic to provide real-time observational COVID-19 data to clinicians, according to an ASH news release.
The analysis by Dr. Desai and co-authors included 257 patients with COVID-19 as determined by their physician, including 135 with a primary diagnosis of acute myeloid leukemia, 82 with acute lymphocytic leukemia, and 40 with myelodysplastic syndromes. Sixty percent of the patients were hospitalized due to COVID-19.
At the time of COVID-19 diagnosis, 46% of patients were in remission, and 44% had active disease, according to the report.
Both neutropenia and active disease status at COVID-19 diagnosis were linked to severe COVID-19, defined as ICU admission due to a COVID-19-related reason, according to results of multivariable analysis. Among patients with severe COVID-19, 67% had active disease, meaning just 33% were in remission, Dr. Desai noted.
In multivariable analysis, two factors were significantly associated with mortality, she added: having an estimated pre-COVID-19 prognosis from the primary disease of less than 6 months, and deferral of ICU care when it was recommended to the patient.
Mortality was 21% overall, higher than would be expected in a non-cancer population, Dr. Desai said. For patients with COVID-19 requiring hospitalization, the mortality rate was 34% and for those patients who did go to the ICU, the mortality rate was 68%.
By contrast, there was no significant association between mortality and active disease as compared to disease in remission, Dr. Desai noted in her presentation. Likewise, mortality was not associated with active treatment at the time of COVID-19 diagnosis as compared to no treatment.
Gwen Nichols, MD, executive vice president and chief medical officer of the Leukemia & Lymphoma Society, New York, said those are reassuring data for patients with acute leukemias and myelodysplastic syndromes and their healthcare providers.
“From our point of view, it helps us say, ‘do not stop your treatment because of worries about COVID-19—it’s more important that you treat your cancer,” Dr. Nichols said in an interview. “We now know we can help people through COVID-19, and I think this is just really important data to back that up,” she added.
Dr. Desai provided disclosures related to Agios, Kura Oncology, and Bristol Myers Squibb (consultancy), and to Janssen R&D and Astex (research funding).
, results of an American Society of Hematology (ASH) COVID-19 registry study suggest.
Rates of severe COVID-19 were significantly higher among patients who had active disease or neutropenia at the time of their COVID-19 diagnosis. Mortality related to COVID-19 was linked to neutropenia, primary disease prognosis of less than 6 months, and deferral of recommended ICU care, study results show.
By contrast, mortality was not associated with active primary disease or its treatment, according to researcher Pinkal Desai, MD, MPH.
Taken together, these findings provide preliminary evidence to support the use of aggressive supportive treatment of COVID-19 in patients with acute leukemias and myelodysplastic syndromes, said Dr. Desai, a hematologist-oncologist with Weill Cornell Medicine and NewYork-Presbyterian in New York.
“If desired by patients, aggressive support for hospitalized patients with COVID-19 is appropriate, regardless of remission status, given the results of our study,” Dr. Desai said in a press conference during the annual meeting of the American Society of Hematology.
In non-cancer patient populations, advanced age and cytopenias have been associated with mortality related to COVID-19, Dr. Desai said. Likewise, patients with acute leukemias and myelodysplastic syndrome are generally older and have disease- or treatment-related cytopenias, which might affect the severity of and mortality from COVID-19, she added.
With that concern in mind, Dr. Desai and co-investigators looked at predictors of severe COVID-19 disease and death among patients in the ASH Research Collaborative (ASH RC) COVID-19 Registry for Hematology.
This registry was started in the early days of the pandemic to provide real-time observational COVID-19 data to clinicians, according to an ASH news release.
The analysis by Dr. Desai and co-authors included 257 patients with COVID-19 as determined by their physician, including 135 with a primary diagnosis of acute myeloid leukemia, 82 with acute lymphocytic leukemia, and 40 with myelodysplastic syndromes. Sixty percent of the patients were hospitalized due to COVID-19.
At the time of COVID-19 diagnosis, 46% of patients were in remission, and 44% had active disease, according to the report.
Both neutropenia and active disease status at COVID-19 diagnosis were linked to severe COVID-19, defined as ICU admission due to a COVID-19-related reason, according to results of multivariable analysis. Among patients with severe COVID-19, 67% had active disease, meaning just 33% were in remission, Dr. Desai noted.
In multivariable analysis, two factors were significantly associated with mortality, she added: having an estimated pre-COVID-19 prognosis from the primary disease of less than 6 months, and deferral of ICU care when it was recommended to the patient.
Mortality was 21% overall, higher than would be expected in a non-cancer population, Dr. Desai said. For patients with COVID-19 requiring hospitalization, the mortality rate was 34% and for those patients who did go to the ICU, the mortality rate was 68%.
By contrast, there was no significant association between mortality and active disease as compared to disease in remission, Dr. Desai noted in her presentation. Likewise, mortality was not associated with active treatment at the time of COVID-19 diagnosis as compared to no treatment.
Gwen Nichols, MD, executive vice president and chief medical officer of the Leukemia & Lymphoma Society, New York, said those are reassuring data for patients with acute leukemias and myelodysplastic syndromes and their healthcare providers.
“From our point of view, it helps us say, ‘do not stop your treatment because of worries about COVID-19—it’s more important that you treat your cancer,” Dr. Nichols said in an interview. “We now know we can help people through COVID-19, and I think this is just really important data to back that up,” she added.
Dr. Desai provided disclosures related to Agios, Kura Oncology, and Bristol Myers Squibb (consultancy), and to Janssen R&D and Astex (research funding).
FROM ASH 2021
Editor’s Note: Looking forward
In this special report we bring you the latest information on new and ongoing developments in the treatment of a number of cancer types through interviews with leaders in the field. And in this unique time of COVID-19 disease, we provide an update on the effects of the pandemic on immune system issues in this highly vulnerable population of cancer patients. In addition, we feature some of the critical issues of dealing with racial, ethnic, sex, and gender disparities among others in these unique populations. We hope you enjoy the issue.
– Mark S. Lesney, PhD
Editor
In this special report we bring you the latest information on new and ongoing developments in the treatment of a number of cancer types through interviews with leaders in the field. And in this unique time of COVID-19 disease, we provide an update on the effects of the pandemic on immune system issues in this highly vulnerable population of cancer patients. In addition, we feature some of the critical issues of dealing with racial, ethnic, sex, and gender disparities among others in these unique populations. We hope you enjoy the issue.
– Mark S. Lesney, PhD
Editor
In this special report we bring you the latest information on new and ongoing developments in the treatment of a number of cancer types through interviews with leaders in the field. And in this unique time of COVID-19 disease, we provide an update on the effects of the pandemic on immune system issues in this highly vulnerable population of cancer patients. In addition, we feature some of the critical issues of dealing with racial, ethnic, sex, and gender disparities among others in these unique populations. We hope you enjoy the issue.
– Mark S. Lesney, PhD
Editor
Beta-thalassemia gene therapy achieves lasting transfusion independence
, an investigator reported at the annual meeting of the American Society of Hematology.
Among patients who received betibeglogene autotemcel (beti-cel) in a phase 3 trial and enrolled in a long-term follow-up study, nearly 90% achieved durable transfusion independence, according to Alexis A. Thompson, MD, MPH, of the hematology section at the Ann & Robert H. Lurie Children’s Hospital of Chicago.
The median duration of ongoing transfusion independence was nearly 3 years as of this report, which Dr. Thompson described in a press conference at the meeting.
In a subanalysis of this international study, Dr. Thompson and co-investigators reported that in patients who achieve transfusion independence, chelation reduced iron, and iron markers stabilized even after chelation was stopped.
Beyond 2 years post-infusion, no adverse events related to the drug product were seen. This suggested that the therapy has a favorable long-term safety profile, according to Dr. Thompson.
“At this point, we believe that beti-cel is potentially curative for patients with TDT [transfusion-dependent beta-thalassemia],” Dr. Thompson said in the press conference.
This study answers one of the major outstanding questions about beti-cel and iron metabolism, according to Arielle L. Langer, MD, MPH, an instructor in medicine at Harvard Medical School and attending physician for adult thalassemia patients at Brigham and Women’s and Dana Farber Cancer Institute, both in Boston.
“Seeing the restoration of iron metabolism, it really takes us a step closer to really thinking the term ‘cure’ might truly apply,” Dr. Langer said in an interview.
Dr. Langer said she looks forward to “very long-term outcomes” of beti-cel-treated patients to see whether endocrinopathies and other long-term sequelae of TDT are also abated.
“This [study] is a great intermediate point, but really, when we think about how thalassemia harms and kills our patients, we really sometimes measure that in decades,” she said.
Beta-thalassemia is caused by mutations in the beta-globin gene, resulting in reduced levels of hemoglobin. Patients with TDT, the most serious form of the disease, have severe anemia and are often dependent on red blood cell transfusions from infancy onward, Dr. Thompson said.
With chronic transfusions needed to maintain hemoglobin levels, TDT patients inevitably experience iron overload, which can lead to organ damage and can be fatal. Consequently, patients will require lifelong iron chelation therapy, she added.
Beti-cel, an investigational ex vivo gene addition therapy currently under review by the U.S. Food and Drug Administration, involves adding functional copies of a modified form of the beta-globin gene into a patient’s own hematopoietic stem cells. Once those cells are reinfused, patients may produce adult hemoglobin at levels that eliminate the need for transfusions, according to Dr. Thompson.
At the meeting, Dr. Thompson reported on patients from two phase 1/2 and two phase 3 beti-cel clinical trials who subsequently enrolled in LTF-303, a 13-year follow-up study of the gene therapy’s safety and efficacy.
A total of 57 patients were included in this report, making it the largest gene therapy program to date in any blood disorder, according to Dr. Thompson. Before receiving beti-cel, the patients, who had a broad range of thalassemia genotypes, were receiving between 10 and almost 40 red blood cell transfusions per year, she reported.
Patients ranged in age from 5 to 35 years. The median age in the phase 1/2 studies was 20 years, while in the phase 3 studies it was 15 years.
“The early experience in the phase 1/2 trials allowed us to be more comfortable with enrolling more children, and that has actually helped us to understand safety and efficacy and children in the phase 3 setting,” Dr. Thompson said.
Fertility preservation measures had been undertaken by about 59% of patients from the phase 1/2 studies and 71% of patients from the phase 3 studies, the data show.
Among patients from the phase 3 beti-cel studies who could be evaluated, 31 out of 35 (or 89%) achieved durable transfusion independence, according to the investigator.
The median duration of ongoing transfusion independence was 32 months, with a range of about 18 to 49 months, she added.
Dr. Thompson also reported a subanalysis intended to assess iron status in 16 patients who restarted and then stopped chelation. That subanalysis demonstrated iron reduction in response to chelation, and then stabilization of iron markers after chelation was stopped. Post-gene therapy chelation led to reductions in liver iron concentration and serum ferritin that remained relatively stable after chelation was stopped, she said.
Serious adverse events occurred in eight patients in the long-term follow-up study. However, adverse events related to beti-cel have been absent beyond 2 years post-infusion, according to Dr. Thompson, who added that there have been no reported cases of replication-competent lentivirus, no clonal expansion, no insertional oncogenesis, and no malignancies observed.
“Very reassuringly, there have been 2 male patients, one of whom underwent fertility preservation, who report having healthy children with their partners,” she added.
Dr. Thompson provided disclosures related to Baxalta, Biomarin, bluebird bio, Inc., Celgene/BMS, CRISPR Therapeutics, Vertex, Editas, Graphite Bio, Novartis, Agios, Beam, and Global Blood Therapeutics.
, an investigator reported at the annual meeting of the American Society of Hematology.
Among patients who received betibeglogene autotemcel (beti-cel) in a phase 3 trial and enrolled in a long-term follow-up study, nearly 90% achieved durable transfusion independence, according to Alexis A. Thompson, MD, MPH, of the hematology section at the Ann & Robert H. Lurie Children’s Hospital of Chicago.
The median duration of ongoing transfusion independence was nearly 3 years as of this report, which Dr. Thompson described in a press conference at the meeting.
In a subanalysis of this international study, Dr. Thompson and co-investigators reported that in patients who achieve transfusion independence, chelation reduced iron, and iron markers stabilized even after chelation was stopped.
Beyond 2 years post-infusion, no adverse events related to the drug product were seen. This suggested that the therapy has a favorable long-term safety profile, according to Dr. Thompson.
“At this point, we believe that beti-cel is potentially curative for patients with TDT [transfusion-dependent beta-thalassemia],” Dr. Thompson said in the press conference.
This study answers one of the major outstanding questions about beti-cel and iron metabolism, according to Arielle L. Langer, MD, MPH, an instructor in medicine at Harvard Medical School and attending physician for adult thalassemia patients at Brigham and Women’s and Dana Farber Cancer Institute, both in Boston.
“Seeing the restoration of iron metabolism, it really takes us a step closer to really thinking the term ‘cure’ might truly apply,” Dr. Langer said in an interview.
Dr. Langer said she looks forward to “very long-term outcomes” of beti-cel-treated patients to see whether endocrinopathies and other long-term sequelae of TDT are also abated.
“This [study] is a great intermediate point, but really, when we think about how thalassemia harms and kills our patients, we really sometimes measure that in decades,” she said.
Beta-thalassemia is caused by mutations in the beta-globin gene, resulting in reduced levels of hemoglobin. Patients with TDT, the most serious form of the disease, have severe anemia and are often dependent on red blood cell transfusions from infancy onward, Dr. Thompson said.
With chronic transfusions needed to maintain hemoglobin levels, TDT patients inevitably experience iron overload, which can lead to organ damage and can be fatal. Consequently, patients will require lifelong iron chelation therapy, she added.
Beti-cel, an investigational ex vivo gene addition therapy currently under review by the U.S. Food and Drug Administration, involves adding functional copies of a modified form of the beta-globin gene into a patient’s own hematopoietic stem cells. Once those cells are reinfused, patients may produce adult hemoglobin at levels that eliminate the need for transfusions, according to Dr. Thompson.
At the meeting, Dr. Thompson reported on patients from two phase 1/2 and two phase 3 beti-cel clinical trials who subsequently enrolled in LTF-303, a 13-year follow-up study of the gene therapy’s safety and efficacy.
A total of 57 patients were included in this report, making it the largest gene therapy program to date in any blood disorder, according to Dr. Thompson. Before receiving beti-cel, the patients, who had a broad range of thalassemia genotypes, were receiving between 10 and almost 40 red blood cell transfusions per year, she reported.
Patients ranged in age from 5 to 35 years. The median age in the phase 1/2 studies was 20 years, while in the phase 3 studies it was 15 years.
“The early experience in the phase 1/2 trials allowed us to be more comfortable with enrolling more children, and that has actually helped us to understand safety and efficacy and children in the phase 3 setting,” Dr. Thompson said.
Fertility preservation measures had been undertaken by about 59% of patients from the phase 1/2 studies and 71% of patients from the phase 3 studies, the data show.
Among patients from the phase 3 beti-cel studies who could be evaluated, 31 out of 35 (or 89%) achieved durable transfusion independence, according to the investigator.
The median duration of ongoing transfusion independence was 32 months, with a range of about 18 to 49 months, she added.
Dr. Thompson also reported a subanalysis intended to assess iron status in 16 patients who restarted and then stopped chelation. That subanalysis demonstrated iron reduction in response to chelation, and then stabilization of iron markers after chelation was stopped. Post-gene therapy chelation led to reductions in liver iron concentration and serum ferritin that remained relatively stable after chelation was stopped, she said.
Serious adverse events occurred in eight patients in the long-term follow-up study. However, adverse events related to beti-cel have been absent beyond 2 years post-infusion, according to Dr. Thompson, who added that there have been no reported cases of replication-competent lentivirus, no clonal expansion, no insertional oncogenesis, and no malignancies observed.
“Very reassuringly, there have been 2 male patients, one of whom underwent fertility preservation, who report having healthy children with their partners,” she added.
Dr. Thompson provided disclosures related to Baxalta, Biomarin, bluebird bio, Inc., Celgene/BMS, CRISPR Therapeutics, Vertex, Editas, Graphite Bio, Novartis, Agios, Beam, and Global Blood Therapeutics.
, an investigator reported at the annual meeting of the American Society of Hematology.
Among patients who received betibeglogene autotemcel (beti-cel) in a phase 3 trial and enrolled in a long-term follow-up study, nearly 90% achieved durable transfusion independence, according to Alexis A. Thompson, MD, MPH, of the hematology section at the Ann & Robert H. Lurie Children’s Hospital of Chicago.
The median duration of ongoing transfusion independence was nearly 3 years as of this report, which Dr. Thompson described in a press conference at the meeting.
In a subanalysis of this international study, Dr. Thompson and co-investigators reported that in patients who achieve transfusion independence, chelation reduced iron, and iron markers stabilized even after chelation was stopped.
Beyond 2 years post-infusion, no adverse events related to the drug product were seen. This suggested that the therapy has a favorable long-term safety profile, according to Dr. Thompson.
“At this point, we believe that beti-cel is potentially curative for patients with TDT [transfusion-dependent beta-thalassemia],” Dr. Thompson said in the press conference.
This study answers one of the major outstanding questions about beti-cel and iron metabolism, according to Arielle L. Langer, MD, MPH, an instructor in medicine at Harvard Medical School and attending physician for adult thalassemia patients at Brigham and Women’s and Dana Farber Cancer Institute, both in Boston.
“Seeing the restoration of iron metabolism, it really takes us a step closer to really thinking the term ‘cure’ might truly apply,” Dr. Langer said in an interview.
Dr. Langer said she looks forward to “very long-term outcomes” of beti-cel-treated patients to see whether endocrinopathies and other long-term sequelae of TDT are also abated.
“This [study] is a great intermediate point, but really, when we think about how thalassemia harms and kills our patients, we really sometimes measure that in decades,” she said.
Beta-thalassemia is caused by mutations in the beta-globin gene, resulting in reduced levels of hemoglobin. Patients with TDT, the most serious form of the disease, have severe anemia and are often dependent on red blood cell transfusions from infancy onward, Dr. Thompson said.
With chronic transfusions needed to maintain hemoglobin levels, TDT patients inevitably experience iron overload, which can lead to organ damage and can be fatal. Consequently, patients will require lifelong iron chelation therapy, she added.
Beti-cel, an investigational ex vivo gene addition therapy currently under review by the U.S. Food and Drug Administration, involves adding functional copies of a modified form of the beta-globin gene into a patient’s own hematopoietic stem cells. Once those cells are reinfused, patients may produce adult hemoglobin at levels that eliminate the need for transfusions, according to Dr. Thompson.
At the meeting, Dr. Thompson reported on patients from two phase 1/2 and two phase 3 beti-cel clinical trials who subsequently enrolled in LTF-303, a 13-year follow-up study of the gene therapy’s safety and efficacy.
A total of 57 patients were included in this report, making it the largest gene therapy program to date in any blood disorder, according to Dr. Thompson. Before receiving beti-cel, the patients, who had a broad range of thalassemia genotypes, were receiving between 10 and almost 40 red blood cell transfusions per year, she reported.
Patients ranged in age from 5 to 35 years. The median age in the phase 1/2 studies was 20 years, while in the phase 3 studies it was 15 years.
“The early experience in the phase 1/2 trials allowed us to be more comfortable with enrolling more children, and that has actually helped us to understand safety and efficacy and children in the phase 3 setting,” Dr. Thompson said.
Fertility preservation measures had been undertaken by about 59% of patients from the phase 1/2 studies and 71% of patients from the phase 3 studies, the data show.
Among patients from the phase 3 beti-cel studies who could be evaluated, 31 out of 35 (or 89%) achieved durable transfusion independence, according to the investigator.
The median duration of ongoing transfusion independence was 32 months, with a range of about 18 to 49 months, she added.
Dr. Thompson also reported a subanalysis intended to assess iron status in 16 patients who restarted and then stopped chelation. That subanalysis demonstrated iron reduction in response to chelation, and then stabilization of iron markers after chelation was stopped. Post-gene therapy chelation led to reductions in liver iron concentration and serum ferritin that remained relatively stable after chelation was stopped, she said.
Serious adverse events occurred in eight patients in the long-term follow-up study. However, adverse events related to beti-cel have been absent beyond 2 years post-infusion, according to Dr. Thompson, who added that there have been no reported cases of replication-competent lentivirus, no clonal expansion, no insertional oncogenesis, and no malignancies observed.
“Very reassuringly, there have been 2 male patients, one of whom underwent fertility preservation, who report having healthy children with their partners,” she added.
Dr. Thompson provided disclosures related to Baxalta, Biomarin, bluebird bio, Inc., Celgene/BMS, CRISPR Therapeutics, Vertex, Editas, Graphite Bio, Novartis, Agios, Beam, and Global Blood Therapeutics.
FROM ASH 2021
‘Remarkable’ results with CAR T cells could make chemo obsolete
ATLANTA — Chimeric antigen receptor, results of the phase 3 ZUMA-7 and TRANSFORM trials suggest.
In the ZUMA-7 trial, at a median follow-up of 24.9 months, patients randomly assigned to receive CAR T-cell therapy with axicabtagene ciloleucel, or axi-cell (Yescarta) had a median event-free survival (EFS) of 8.3 months, compared with 2 months for patients randomly assigned to standard-of-care chemoimmunotherapy, reported Frederick L. Locke, MD, from the Moffitt Cancer Center in Tampa, Fla.
In TRANSFORM, comparing the CAR T construct lisocabtagene maraleucel, or liso-cel (Breyanzi) with standard-of-care second-line chemotherapy, median EFS was 10.1 months with liso-cel, compared with 2.3 months with standard of care, reported Manali Kamdar, MD, from the University of Colorado Cancer Center in Aurora.
The trials differed slightly in eligibility criteria and other details, but their overall results show great promise for improving second-line therapy for patients with relapsed or refractory LBCL, commented Laurie Sehn, MD, MPH, from the BC Cancer Centre for Lymphoid Cancer in Vancouver, Canada.
“It’s really remarkable that the results are so far in favor of the CAR T-cell therapy that I think it’s inevitable that this will become the standard of care,” Dr. Sehn commented. She was not an investigator in either of the two trials.
Dr. Sehn was speaking at a press briefing here during the annual meeting of the American Society of Hematology. The new data from the two studies were presented at oral sessions, and the results from ZUMA-7 were also simultaneously published in the New England Journal of Medicine.
“For somebody who treats patients with large B-cell lymphoma like I do, it’s incredibly frustrating when patients fail frontline therapy,” Dr. Sehn said. “We come into the second line with more chemotherapy and at higher doses to try and slam things down hard. Particularly for the patients who were enrolled in these studies, which were the worst of the worst — the patients who are either refractory to chemotherapy or relapsed relatively early, within 1 year — it’s not surprising that coming in with a novel approach and a cellular therapy that has a proven curative capacity may have outperformed coming in with more chemotherapy.”
In an interview with this news organization, Dr. Locke said that, based on the findings of the ZUMA-7 trial that he presented, it’s likely that chemotherapy in the second-line setting for relapsed/refractory LBCL will largely fall by the wayside.
The first question is to identify the patients who can tolerate CAR T-cell therapy. “We need to refer these patients to a CAR T-cell center to make that decision. That decision really can’t be made in the local oncologist’s office,” he said. “That being said, there are patients who need urgent therapy, and they may need to get second-line chemotherapy right away.”
“What we know with CAR T cells is that older patients and patients with comorbidities can get these therapies safely, so to me there is no obvious patient who can’t get CAR T-cell therapy,” he added.
Also at the briefing, Dr. Kamdar, who presented the TRANSFORM trial results, remarked that “in my opinion, this is a breakthrough therapy, which has shown superiority over standard of care, in terms of not just efficacy but also an extremely favorable safety profile,” she said at a briefing.
For patients with LBCL for whom first-line therapy has failed, chemoimmunotherapy followed by high-dose chemotherapy and autologous stem cell transplant (ASCT) has been the standard of care, but only about 25% of patients who are candidates for ASCT achieve durable remissions, Dr. Kamdar noted.
Both ZUMA-7 and TRANSFORM were designed to test whether moving CAR T-cell therapy forward into the second line could improve outcomes.
ZUMA-7 results
THE ZUMA-7 trial randomly assigned 180 patients to receive CAR T-cell therapy with axi-cell and 179 patients to standard of care. This consisted of two or three cycles of investigator-selected, protocol-defined chemoimmunotherapy, with patients who had a complete or partial response going onto ASCT.
As noted, the primary endpoint of EFS according to blinded central review favored axi-cel, with 24-month event-free survival rates of 41% vs. 16% for standard of care. The difference translated into a hazard ratio (HR) for progression or death of 0.40 (P < .001).
In all, 65% of patients had a complete response (CR) to axi-cel, compared with 32% with standard of care. The respective overall response rates were 83% and 50% (P < .001).
Dr. Locke pointed out that 94% of the patients assigned to axi-cel received definitive therapy, compared with the 36% of patients in the standard-of-care arm who went on to ASCT.
In an interim analysis, 2-year estimated overall survival was 61% with axi-cel vs. 52% with standard of care, although this difference was not statistically significant.
Median overall survival was not reached with axi-cel, compared with 35.1 months with standard-of-care.
Grade 3 or higher adverse events occurred in 91% of patients with CAR T, and 83% with the standard of care. In the axi-cel arm, 6% of patients had grade 3 or higher cytokine release syndrome (CRS), and 21% had grade 3 or higher neurologic events, although there were no deaths related to CRS or neurologic events.
TRANSFORM results
The TRANSFORM trial had broader eligibility criteria than ZUMA-7, including patients who had diffuse LBCL not otherwise specified (de novo or transformed from indolent NHL), high-grade BCL (double- or triple-hit) with DLBCL histology, follicular lymphoma grade 3B, primary mediastinal LBCL, or T-cell/histocyte-rich LBCL.
A total of 184 patients were randomly assigned, 92 in each group, to receive either liso-cel or standard-of-care. Patients assigned to liso-cel were allowed to have bridging therapy, and crossover to liso-cel was allowed for patients assigned to standard of care who either did not have a response by week 9 after randomization, had disease progression at any time, or started a new antineoplastic therapy after ASCT.
As noted before, the primary endpoint of EFS significantly favored CAR T-cell therapy, with a hazard ratio of 0.349 (P < .0001).
The EFS rates at 6 months were 63.3% with liso-cel vs 33.4% with standard of care, and the EFS rates at 12 months were 44.5% vs. 23.7%, respectively.
“Overall survival data were still immature at the time of this analysis, but show a trend favoring liso-cel, despite crossover,” Dr. Kamdar said.
Grade 3 or higher adverse events (AEs) occurred in 92% of patients on liso-cell and 87% of patients on standard of care. There was one treatment-related death in the liso-cel arm, and two in the standard of care arm, both from grade 3 or higher AEs. Neutropenia, anemia, and thrombocytopenia were the most common treatment-emergent AEs in each group.
ZUMA-7 is supported by Kite. Dr. Locke disclosed serving as a scientific advisor to Kite and relationships with other companies. TRANSFORM is supported by Celgene (BMS). Dr. Kamdar disclosed consultancy fees from BMS and others.
A version of this article first appeared on Medscape.com.
ATLANTA — Chimeric antigen receptor, results of the phase 3 ZUMA-7 and TRANSFORM trials suggest.
In the ZUMA-7 trial, at a median follow-up of 24.9 months, patients randomly assigned to receive CAR T-cell therapy with axicabtagene ciloleucel, or axi-cell (Yescarta) had a median event-free survival (EFS) of 8.3 months, compared with 2 months for patients randomly assigned to standard-of-care chemoimmunotherapy, reported Frederick L. Locke, MD, from the Moffitt Cancer Center in Tampa, Fla.
In TRANSFORM, comparing the CAR T construct lisocabtagene maraleucel, or liso-cel (Breyanzi) with standard-of-care second-line chemotherapy, median EFS was 10.1 months with liso-cel, compared with 2.3 months with standard of care, reported Manali Kamdar, MD, from the University of Colorado Cancer Center in Aurora.
The trials differed slightly in eligibility criteria and other details, but their overall results show great promise for improving second-line therapy for patients with relapsed or refractory LBCL, commented Laurie Sehn, MD, MPH, from the BC Cancer Centre for Lymphoid Cancer in Vancouver, Canada.
“It’s really remarkable that the results are so far in favor of the CAR T-cell therapy that I think it’s inevitable that this will become the standard of care,” Dr. Sehn commented. She was not an investigator in either of the two trials.
Dr. Sehn was speaking at a press briefing here during the annual meeting of the American Society of Hematology. The new data from the two studies were presented at oral sessions, and the results from ZUMA-7 were also simultaneously published in the New England Journal of Medicine.
“For somebody who treats patients with large B-cell lymphoma like I do, it’s incredibly frustrating when patients fail frontline therapy,” Dr. Sehn said. “We come into the second line with more chemotherapy and at higher doses to try and slam things down hard. Particularly for the patients who were enrolled in these studies, which were the worst of the worst — the patients who are either refractory to chemotherapy or relapsed relatively early, within 1 year — it’s not surprising that coming in with a novel approach and a cellular therapy that has a proven curative capacity may have outperformed coming in with more chemotherapy.”
In an interview with this news organization, Dr. Locke said that, based on the findings of the ZUMA-7 trial that he presented, it’s likely that chemotherapy in the second-line setting for relapsed/refractory LBCL will largely fall by the wayside.
The first question is to identify the patients who can tolerate CAR T-cell therapy. “We need to refer these patients to a CAR T-cell center to make that decision. That decision really can’t be made in the local oncologist’s office,” he said. “That being said, there are patients who need urgent therapy, and they may need to get second-line chemotherapy right away.”
“What we know with CAR T cells is that older patients and patients with comorbidities can get these therapies safely, so to me there is no obvious patient who can’t get CAR T-cell therapy,” he added.
Also at the briefing, Dr. Kamdar, who presented the TRANSFORM trial results, remarked that “in my opinion, this is a breakthrough therapy, which has shown superiority over standard of care, in terms of not just efficacy but also an extremely favorable safety profile,” she said at a briefing.
For patients with LBCL for whom first-line therapy has failed, chemoimmunotherapy followed by high-dose chemotherapy and autologous stem cell transplant (ASCT) has been the standard of care, but only about 25% of patients who are candidates for ASCT achieve durable remissions, Dr. Kamdar noted.
Both ZUMA-7 and TRANSFORM were designed to test whether moving CAR T-cell therapy forward into the second line could improve outcomes.
ZUMA-7 results
THE ZUMA-7 trial randomly assigned 180 patients to receive CAR T-cell therapy with axi-cell and 179 patients to standard of care. This consisted of two or three cycles of investigator-selected, protocol-defined chemoimmunotherapy, with patients who had a complete or partial response going onto ASCT.
As noted, the primary endpoint of EFS according to blinded central review favored axi-cel, with 24-month event-free survival rates of 41% vs. 16% for standard of care. The difference translated into a hazard ratio (HR) for progression or death of 0.40 (P < .001).
In all, 65% of patients had a complete response (CR) to axi-cel, compared with 32% with standard of care. The respective overall response rates were 83% and 50% (P < .001).
Dr. Locke pointed out that 94% of the patients assigned to axi-cel received definitive therapy, compared with the 36% of patients in the standard-of-care arm who went on to ASCT.
In an interim analysis, 2-year estimated overall survival was 61% with axi-cel vs. 52% with standard of care, although this difference was not statistically significant.
Median overall survival was not reached with axi-cel, compared with 35.1 months with standard-of-care.
Grade 3 or higher adverse events occurred in 91% of patients with CAR T, and 83% with the standard of care. In the axi-cel arm, 6% of patients had grade 3 or higher cytokine release syndrome (CRS), and 21% had grade 3 or higher neurologic events, although there were no deaths related to CRS or neurologic events.
TRANSFORM results
The TRANSFORM trial had broader eligibility criteria than ZUMA-7, including patients who had diffuse LBCL not otherwise specified (de novo or transformed from indolent NHL), high-grade BCL (double- or triple-hit) with DLBCL histology, follicular lymphoma grade 3B, primary mediastinal LBCL, or T-cell/histocyte-rich LBCL.
A total of 184 patients were randomly assigned, 92 in each group, to receive either liso-cel or standard-of-care. Patients assigned to liso-cel were allowed to have bridging therapy, and crossover to liso-cel was allowed for patients assigned to standard of care who either did not have a response by week 9 after randomization, had disease progression at any time, or started a new antineoplastic therapy after ASCT.
As noted before, the primary endpoint of EFS significantly favored CAR T-cell therapy, with a hazard ratio of 0.349 (P < .0001).
The EFS rates at 6 months were 63.3% with liso-cel vs 33.4% with standard of care, and the EFS rates at 12 months were 44.5% vs. 23.7%, respectively.
“Overall survival data were still immature at the time of this analysis, but show a trend favoring liso-cel, despite crossover,” Dr. Kamdar said.
Grade 3 or higher adverse events (AEs) occurred in 92% of patients on liso-cell and 87% of patients on standard of care. There was one treatment-related death in the liso-cel arm, and two in the standard of care arm, both from grade 3 or higher AEs. Neutropenia, anemia, and thrombocytopenia were the most common treatment-emergent AEs in each group.
ZUMA-7 is supported by Kite. Dr. Locke disclosed serving as a scientific advisor to Kite and relationships with other companies. TRANSFORM is supported by Celgene (BMS). Dr. Kamdar disclosed consultancy fees from BMS and others.
A version of this article first appeared on Medscape.com.
ATLANTA — Chimeric antigen receptor, results of the phase 3 ZUMA-7 and TRANSFORM trials suggest.
In the ZUMA-7 trial, at a median follow-up of 24.9 months, patients randomly assigned to receive CAR T-cell therapy with axicabtagene ciloleucel, or axi-cell (Yescarta) had a median event-free survival (EFS) of 8.3 months, compared with 2 months for patients randomly assigned to standard-of-care chemoimmunotherapy, reported Frederick L. Locke, MD, from the Moffitt Cancer Center in Tampa, Fla.
In TRANSFORM, comparing the CAR T construct lisocabtagene maraleucel, or liso-cel (Breyanzi) with standard-of-care second-line chemotherapy, median EFS was 10.1 months with liso-cel, compared with 2.3 months with standard of care, reported Manali Kamdar, MD, from the University of Colorado Cancer Center in Aurora.
The trials differed slightly in eligibility criteria and other details, but their overall results show great promise for improving second-line therapy for patients with relapsed or refractory LBCL, commented Laurie Sehn, MD, MPH, from the BC Cancer Centre for Lymphoid Cancer in Vancouver, Canada.
“It’s really remarkable that the results are so far in favor of the CAR T-cell therapy that I think it’s inevitable that this will become the standard of care,” Dr. Sehn commented. She was not an investigator in either of the two trials.
Dr. Sehn was speaking at a press briefing here during the annual meeting of the American Society of Hematology. The new data from the two studies were presented at oral sessions, and the results from ZUMA-7 were also simultaneously published in the New England Journal of Medicine.
“For somebody who treats patients with large B-cell lymphoma like I do, it’s incredibly frustrating when patients fail frontline therapy,” Dr. Sehn said. “We come into the second line with more chemotherapy and at higher doses to try and slam things down hard. Particularly for the patients who were enrolled in these studies, which were the worst of the worst — the patients who are either refractory to chemotherapy or relapsed relatively early, within 1 year — it’s not surprising that coming in with a novel approach and a cellular therapy that has a proven curative capacity may have outperformed coming in with more chemotherapy.”
In an interview with this news organization, Dr. Locke said that, based on the findings of the ZUMA-7 trial that he presented, it’s likely that chemotherapy in the second-line setting for relapsed/refractory LBCL will largely fall by the wayside.
The first question is to identify the patients who can tolerate CAR T-cell therapy. “We need to refer these patients to a CAR T-cell center to make that decision. That decision really can’t be made in the local oncologist’s office,” he said. “That being said, there are patients who need urgent therapy, and they may need to get second-line chemotherapy right away.”
“What we know with CAR T cells is that older patients and patients with comorbidities can get these therapies safely, so to me there is no obvious patient who can’t get CAR T-cell therapy,” he added.
Also at the briefing, Dr. Kamdar, who presented the TRANSFORM trial results, remarked that “in my opinion, this is a breakthrough therapy, which has shown superiority over standard of care, in terms of not just efficacy but also an extremely favorable safety profile,” she said at a briefing.
For patients with LBCL for whom first-line therapy has failed, chemoimmunotherapy followed by high-dose chemotherapy and autologous stem cell transplant (ASCT) has been the standard of care, but only about 25% of patients who are candidates for ASCT achieve durable remissions, Dr. Kamdar noted.
Both ZUMA-7 and TRANSFORM were designed to test whether moving CAR T-cell therapy forward into the second line could improve outcomes.
ZUMA-7 results
THE ZUMA-7 trial randomly assigned 180 patients to receive CAR T-cell therapy with axi-cell and 179 patients to standard of care. This consisted of two or three cycles of investigator-selected, protocol-defined chemoimmunotherapy, with patients who had a complete or partial response going onto ASCT.
As noted, the primary endpoint of EFS according to blinded central review favored axi-cel, with 24-month event-free survival rates of 41% vs. 16% for standard of care. The difference translated into a hazard ratio (HR) for progression or death of 0.40 (P < .001).
In all, 65% of patients had a complete response (CR) to axi-cel, compared with 32% with standard of care. The respective overall response rates were 83% and 50% (P < .001).
Dr. Locke pointed out that 94% of the patients assigned to axi-cel received definitive therapy, compared with the 36% of patients in the standard-of-care arm who went on to ASCT.
In an interim analysis, 2-year estimated overall survival was 61% with axi-cel vs. 52% with standard of care, although this difference was not statistically significant.
Median overall survival was not reached with axi-cel, compared with 35.1 months with standard-of-care.
Grade 3 or higher adverse events occurred in 91% of patients with CAR T, and 83% with the standard of care. In the axi-cel arm, 6% of patients had grade 3 or higher cytokine release syndrome (CRS), and 21% had grade 3 or higher neurologic events, although there were no deaths related to CRS or neurologic events.
TRANSFORM results
The TRANSFORM trial had broader eligibility criteria than ZUMA-7, including patients who had diffuse LBCL not otherwise specified (de novo or transformed from indolent NHL), high-grade BCL (double- or triple-hit) with DLBCL histology, follicular lymphoma grade 3B, primary mediastinal LBCL, or T-cell/histocyte-rich LBCL.
A total of 184 patients were randomly assigned, 92 in each group, to receive either liso-cel or standard-of-care. Patients assigned to liso-cel were allowed to have bridging therapy, and crossover to liso-cel was allowed for patients assigned to standard of care who either did not have a response by week 9 after randomization, had disease progression at any time, or started a new antineoplastic therapy after ASCT.
As noted before, the primary endpoint of EFS significantly favored CAR T-cell therapy, with a hazard ratio of 0.349 (P < .0001).
The EFS rates at 6 months were 63.3% with liso-cel vs 33.4% with standard of care, and the EFS rates at 12 months were 44.5% vs. 23.7%, respectively.
“Overall survival data were still immature at the time of this analysis, but show a trend favoring liso-cel, despite crossover,” Dr. Kamdar said.
Grade 3 or higher adverse events (AEs) occurred in 92% of patients on liso-cell and 87% of patients on standard of care. There was one treatment-related death in the liso-cel arm, and two in the standard of care arm, both from grade 3 or higher AEs. Neutropenia, anemia, and thrombocytopenia were the most common treatment-emergent AEs in each group.
ZUMA-7 is supported by Kite. Dr. Locke disclosed serving as a scientific advisor to Kite and relationships with other companies. TRANSFORM is supported by Celgene (BMS). Dr. Kamdar disclosed consultancy fees from BMS and others.
A version of this article first appeared on Medscape.com.
AT ASH 2021
The evolving HER2+ metastatic breast cancer landscape: Novel agents and promising combination therapies
Recent therapeutic advances in HER2-positive metastatic breast cancer (MBC) have begun to reshape the treatment landscape for patients. Since late 2019, the U.S. Food and Drug Administration (FDA) has approved a handful of novel agents for HER2-positive MBC — most notably, the antibody-drug conjugate (ADC) trastuzumab deruxtecan in December 2019 and the tyrosine kinase inhibitors (TKIs) tucatinib and neratinib in 2020. According to the National Cancer Institute›s Surveillance, Epidemiology, and End Results (SEER) program, the 5-year survival rate for patients with advanced disease was already on the rise between 2004 and 2018, and
“I’ve been involved in the HER2 space for a long time and have watched the field evolve,” said Adam Brufsky, MD, PhD, associate chief in the division of hematology/oncology and co-director of the Comprehensive Breast Cancer Center at the University of Pittsburgh School of Medicine. “The fact that we’re now talking about fourth- and fifth-line therapies for HER2-positive MBC represents a major advance in the management of these patients.”
Oncologists are still building on this progress, focusing on designing more targeted therapies as well as studying different combinations of available agents. The main goal of treatment, experts say, is to prolong patients’ systemic response and prevent recurrences, especially in the brain. This news organization spoke to Dr. Brufksy and others about promising agents and therapeutic strategies on the horizon to treat HER2-positive MBC.
Inside emerging ADCs
Because many patients develop resistance to trastuzumab emtansine (T-DM1) — the first FDA-approved ADC in breast cancer — researchers have focused on developing the next generation of ADCs with more potent payloads, different linkers, and distinct mechanisms of action, according to Sayeh Lavasani, MD, MS, a medical oncologist at City of Hope, a comprehensive cancer center in Los Angeles County.
The second-generation ADC trastuzumab deruxtecan showed “really dramatic” results in HER2-positive MBC, demonstrating progression-free survival of 16 months, remarked Kevin Kalinsky, MD, acting associate professor in the department of hematology and medical oncology at Emory University School of Medicine in Atlanta and director of the Glenn Family Breast Center at the Winship Cancer Institute of Emory University. “These outcomes further changed how we treat patients with metastatic disease and prompted considerable excitement over the potential to develop novel ADCs to treat HER2-positive MBC.”
Most recently, two investigational ADCs — trastuzumab duocarmazine (SYD985) and ARX788 — have stood out. The FDA granted fast-track designations to trastuzumab duocarmazine in January 2018 and ARX788 in January 2021. Trastuzumab duocarmazine, the furthest along the pipeline, has shown promising results so far. In June 2021, Netherlands-based biopharmaceutical company Byondis reported preliminary phase 3 data from the TULIP trial. The open-label, randomized phase 3 study enrolled 436 patients with HER2-positive locally advanced or metastatic disease that had progressed on previous anti-HER2 regimens. The company shared early results that trastuzumab duocarmazine achieved its progression-free survival primary endpoint, marking a significant improvement over physician’s choice of chemotherapy, and promised more detailed results to come later this year.
Although only in early-phase trials, ARX788 has also shown robust anti-HER2 activity as well as low toxicity in HER2-positive tumors, according to recent data. The findings from two phase 1 studies, presented at the June 2021 virtual American Society for Clinical Oncology meeting (abstract 1038), revealed an overall response rate of 74% in the breast cancer cohort, but the investigators acknowledged it was too early to report median progression-free survival outcomes. Preclinical data also showed activity in HER2-low and T-DM1–resistant tumors.
Despite the encouraging initial findings, Dr. Kalinsky remains cautiously optimistic about long-term outcomes for both ADCs. “These data are hot off the press, but it’s too soon to know how these two ADCs and others in the pipeline will measure up to approved therapies,” he commented. As experts learn more about the efficacy of these novel ADCs, Dr. Brufsky would also like to better understand resistance mechanisms and how to integrate these agents into current treatment strategies. “The cellular biology of HER2-positive MBC is complicated, and many factors in these tumor cells affect where ADCs are released, how resistance develops, and whether or not resistance to one ADC applies to others,” Dr. Brufsky remarked. “As we gather more data, we’ll understand resistance mechanisms better and begin to figure out where to go with treatment sequencing.”
TKIs and beyond
In addition to ADCs, TKIs continue to make their mark in the targeted HER2 therapeutic space. The approvals of tucatinib and neratinib last year represented an important advance in treating HER2-positive MBC, particularly for patients with brain metastases. The HER2CLIMB trial, for instance, found that tucatinib combined with trastuzumab and capecitabine had a 4.5-month overall survival advantage compared with placebo (21.9 vs 17.4) and a median progression-free survival advantage of 5.4 months in patients with active brain metastases (9.5 vs 4.1) and 8.3 months in patients with stable metastases (13.9 vs 5.6).
Given this progress, experts are looking to add new TKIs to the armamentarium. In particular, pyrotinib — already approved in China for treating HER2-positive MBC — has demonstrated significantly longer progression-free survival compared with a standard TKI, lapatinib. The phase 3 PHOEBE trial results, published in The Lancet in early 2021, found a median progression-free survival of 12.5 months in patients randomly assigned to receive pyrotinib plus capecitabine compared with 6.8 months in those receiving lapatinib plus capecitabine. The investigators also reported “manageable toxicity”; diarrhea was the most common grade 3 adverse event, occurring in 31% of the pyrotinib group vs. 8% of the lapatinib group, and overall serious adverse events occurred in 10% of patients receiving pyrotinib vs. 8% of those receiving lapatinib.
More recent data on pyrotinib come from the phase 2 PERMEATE trial, which focused on the safety and efficacy of the agent in patients with advanced disease and brain metastases. The investigators, who presented their findings at the 2021 virtual ASCO meeting (abstract 1037), reported that radiation therapy–naive patients receiving pyrotinib plus capecitabine had an overall response rate of 74.6% in the central nervous system. Patients experiencing progression after whole-brain or stereotactic radiation therapy, however, had a comparatively lower overall response rate of 42.1%.
Similarly, median progression-free survival was much higher in the radiation therapy–naive patients (12.1 vs 5.6 months in the radiation therapy cohort). Similar to the PHOEBE trial, the most common grade 3 adverse event was diarrhea (23.1%), followed by decreased neutrophil and white blood cell counts (12.8% for both), anemia (9%), and hand-foot syndrome (7.7%). The main question for Dr. Kalinsky is how well pyrotinib will ultimately stack up to tucatinib and neratinib. “Pyrotinib — like neratinib — was shown to be superior to lapatinib plus capecitabine , but its role may be limited by its gastrointestinal toxicity,” he said. In addition to research focused on expanding the selection of novel ADCs and TKIs, researchers are also exploring new combinations of approved treatments and whether these combinations can be used earlier in treatment sequencing.
Take the CompassHER2 trials. The ongoing phase 3 trial in patients with high-risk HER2-positive breast cancer and residual disease will explore whether tucatinib plus T-DM1 compared with T-DM1 alone improves overall survival and recurrence-free survival and prevents brain metastases. Another possibility currently under investigation is pairing tucatinib and trastuzumab deruxtecan, instead of T-DM1. “Overall, it’s exciting that we are increasing the number of therapeutic options and combinations,” commented Debu Tripathy, MD, professor and chairman in the department of breast medical oncology at the University of Texas MD Anderson Cancer Center in Houston. “Having more choices allows us to tailor therapies to manage resistance and prolong patients’ responses.”
Curbing brain metastasis, according to Dr. Brufksy, is particularly important, and experts need to explore the extent to which ADCs can penetrate the blood-brain barrier. Already, a subgroup analysis of the DESTINY-Breast01 trial found that trastuzumab deruxtecan appeared to be active in patients with brain metastases. Investigators reported an overall response rate of 58.3% and a median progression-free survival of 18.1 months — results in line with those in the general study cohort — but the study population did not include patients with untreated or progressive brain metastases. A phase 2 study currently under way will examine whether patients with HER2-positive and HER2-low breast cancer who have untreated or progressive brain metastases respond to trastuzumab deruxtecan as well. Ultimately, Dr. Brufksy hopes the recent successes with preventing brain metastases in pediatric acute lymphoblastic leukemia (ALL) foreshadow what›s to come in HER2-positive MBC.
“When we figured out how to treat brain metastases prophylactically in childhood ALL, we saw a huge improvement in the cure rate, which is ultimately my vision for HER2-positive disease,” Dr. Brufsky remarked. “Are there cures for HER2-positive MBC on the horizon? We don’t know yet, but the field has really exploded in recent years.”
A version of this article first appeared on Medscape.com.
Recent therapeutic advances in HER2-positive metastatic breast cancer (MBC) have begun to reshape the treatment landscape for patients. Since late 2019, the U.S. Food and Drug Administration (FDA) has approved a handful of novel agents for HER2-positive MBC — most notably, the antibody-drug conjugate (ADC) trastuzumab deruxtecan in December 2019 and the tyrosine kinase inhibitors (TKIs) tucatinib and neratinib in 2020. According to the National Cancer Institute›s Surveillance, Epidemiology, and End Results (SEER) program, the 5-year survival rate for patients with advanced disease was already on the rise between 2004 and 2018, and
“I’ve been involved in the HER2 space for a long time and have watched the field evolve,” said Adam Brufsky, MD, PhD, associate chief in the division of hematology/oncology and co-director of the Comprehensive Breast Cancer Center at the University of Pittsburgh School of Medicine. “The fact that we’re now talking about fourth- and fifth-line therapies for HER2-positive MBC represents a major advance in the management of these patients.”
Oncologists are still building on this progress, focusing on designing more targeted therapies as well as studying different combinations of available agents. The main goal of treatment, experts say, is to prolong patients’ systemic response and prevent recurrences, especially in the brain. This news organization spoke to Dr. Brufksy and others about promising agents and therapeutic strategies on the horizon to treat HER2-positive MBC.
Inside emerging ADCs
Because many patients develop resistance to trastuzumab emtansine (T-DM1) — the first FDA-approved ADC in breast cancer — researchers have focused on developing the next generation of ADCs with more potent payloads, different linkers, and distinct mechanisms of action, according to Sayeh Lavasani, MD, MS, a medical oncologist at City of Hope, a comprehensive cancer center in Los Angeles County.
The second-generation ADC trastuzumab deruxtecan showed “really dramatic” results in HER2-positive MBC, demonstrating progression-free survival of 16 months, remarked Kevin Kalinsky, MD, acting associate professor in the department of hematology and medical oncology at Emory University School of Medicine in Atlanta and director of the Glenn Family Breast Center at the Winship Cancer Institute of Emory University. “These outcomes further changed how we treat patients with metastatic disease and prompted considerable excitement over the potential to develop novel ADCs to treat HER2-positive MBC.”
Most recently, two investigational ADCs — trastuzumab duocarmazine (SYD985) and ARX788 — have stood out. The FDA granted fast-track designations to trastuzumab duocarmazine in January 2018 and ARX788 in January 2021. Trastuzumab duocarmazine, the furthest along the pipeline, has shown promising results so far. In June 2021, Netherlands-based biopharmaceutical company Byondis reported preliminary phase 3 data from the TULIP trial. The open-label, randomized phase 3 study enrolled 436 patients with HER2-positive locally advanced or metastatic disease that had progressed on previous anti-HER2 regimens. The company shared early results that trastuzumab duocarmazine achieved its progression-free survival primary endpoint, marking a significant improvement over physician’s choice of chemotherapy, and promised more detailed results to come later this year.
Although only in early-phase trials, ARX788 has also shown robust anti-HER2 activity as well as low toxicity in HER2-positive tumors, according to recent data. The findings from two phase 1 studies, presented at the June 2021 virtual American Society for Clinical Oncology meeting (abstract 1038), revealed an overall response rate of 74% in the breast cancer cohort, but the investigators acknowledged it was too early to report median progression-free survival outcomes. Preclinical data also showed activity in HER2-low and T-DM1–resistant tumors.
Despite the encouraging initial findings, Dr. Kalinsky remains cautiously optimistic about long-term outcomes for both ADCs. “These data are hot off the press, but it’s too soon to know how these two ADCs and others in the pipeline will measure up to approved therapies,” he commented. As experts learn more about the efficacy of these novel ADCs, Dr. Brufsky would also like to better understand resistance mechanisms and how to integrate these agents into current treatment strategies. “The cellular biology of HER2-positive MBC is complicated, and many factors in these tumor cells affect where ADCs are released, how resistance develops, and whether or not resistance to one ADC applies to others,” Dr. Brufsky remarked. “As we gather more data, we’ll understand resistance mechanisms better and begin to figure out where to go with treatment sequencing.”
TKIs and beyond
In addition to ADCs, TKIs continue to make their mark in the targeted HER2 therapeutic space. The approvals of tucatinib and neratinib last year represented an important advance in treating HER2-positive MBC, particularly for patients with brain metastases. The HER2CLIMB trial, for instance, found that tucatinib combined with trastuzumab and capecitabine had a 4.5-month overall survival advantage compared with placebo (21.9 vs 17.4) and a median progression-free survival advantage of 5.4 months in patients with active brain metastases (9.5 vs 4.1) and 8.3 months in patients with stable metastases (13.9 vs 5.6).
Given this progress, experts are looking to add new TKIs to the armamentarium. In particular, pyrotinib — already approved in China for treating HER2-positive MBC — has demonstrated significantly longer progression-free survival compared with a standard TKI, lapatinib. The phase 3 PHOEBE trial results, published in The Lancet in early 2021, found a median progression-free survival of 12.5 months in patients randomly assigned to receive pyrotinib plus capecitabine compared with 6.8 months in those receiving lapatinib plus capecitabine. The investigators also reported “manageable toxicity”; diarrhea was the most common grade 3 adverse event, occurring in 31% of the pyrotinib group vs. 8% of the lapatinib group, and overall serious adverse events occurred in 10% of patients receiving pyrotinib vs. 8% of those receiving lapatinib.
More recent data on pyrotinib come from the phase 2 PERMEATE trial, which focused on the safety and efficacy of the agent in patients with advanced disease and brain metastases. The investigators, who presented their findings at the 2021 virtual ASCO meeting (abstract 1037), reported that radiation therapy–naive patients receiving pyrotinib plus capecitabine had an overall response rate of 74.6% in the central nervous system. Patients experiencing progression after whole-brain or stereotactic radiation therapy, however, had a comparatively lower overall response rate of 42.1%.
Similarly, median progression-free survival was much higher in the radiation therapy–naive patients (12.1 vs 5.6 months in the radiation therapy cohort). Similar to the PHOEBE trial, the most common grade 3 adverse event was diarrhea (23.1%), followed by decreased neutrophil and white blood cell counts (12.8% for both), anemia (9%), and hand-foot syndrome (7.7%). The main question for Dr. Kalinsky is how well pyrotinib will ultimately stack up to tucatinib and neratinib. “Pyrotinib — like neratinib — was shown to be superior to lapatinib plus capecitabine , but its role may be limited by its gastrointestinal toxicity,” he said. In addition to research focused on expanding the selection of novel ADCs and TKIs, researchers are also exploring new combinations of approved treatments and whether these combinations can be used earlier in treatment sequencing.
Take the CompassHER2 trials. The ongoing phase 3 trial in patients with high-risk HER2-positive breast cancer and residual disease will explore whether tucatinib plus T-DM1 compared with T-DM1 alone improves overall survival and recurrence-free survival and prevents brain metastases. Another possibility currently under investigation is pairing tucatinib and trastuzumab deruxtecan, instead of T-DM1. “Overall, it’s exciting that we are increasing the number of therapeutic options and combinations,” commented Debu Tripathy, MD, professor and chairman in the department of breast medical oncology at the University of Texas MD Anderson Cancer Center in Houston. “Having more choices allows us to tailor therapies to manage resistance and prolong patients’ responses.”
Curbing brain metastasis, according to Dr. Brufksy, is particularly important, and experts need to explore the extent to which ADCs can penetrate the blood-brain barrier. Already, a subgroup analysis of the DESTINY-Breast01 trial found that trastuzumab deruxtecan appeared to be active in patients with brain metastases. Investigators reported an overall response rate of 58.3% and a median progression-free survival of 18.1 months — results in line with those in the general study cohort — but the study population did not include patients with untreated or progressive brain metastases. A phase 2 study currently under way will examine whether patients with HER2-positive and HER2-low breast cancer who have untreated or progressive brain metastases respond to trastuzumab deruxtecan as well. Ultimately, Dr. Brufksy hopes the recent successes with preventing brain metastases in pediatric acute lymphoblastic leukemia (ALL) foreshadow what›s to come in HER2-positive MBC.
“When we figured out how to treat brain metastases prophylactically in childhood ALL, we saw a huge improvement in the cure rate, which is ultimately my vision for HER2-positive disease,” Dr. Brufsky remarked. “Are there cures for HER2-positive MBC on the horizon? We don’t know yet, but the field has really exploded in recent years.”
A version of this article first appeared on Medscape.com.
Recent therapeutic advances in HER2-positive metastatic breast cancer (MBC) have begun to reshape the treatment landscape for patients. Since late 2019, the U.S. Food and Drug Administration (FDA) has approved a handful of novel agents for HER2-positive MBC — most notably, the antibody-drug conjugate (ADC) trastuzumab deruxtecan in December 2019 and the tyrosine kinase inhibitors (TKIs) tucatinib and neratinib in 2020. According to the National Cancer Institute›s Surveillance, Epidemiology, and End Results (SEER) program, the 5-year survival rate for patients with advanced disease was already on the rise between 2004 and 2018, and
“I’ve been involved in the HER2 space for a long time and have watched the field evolve,” said Adam Brufsky, MD, PhD, associate chief in the division of hematology/oncology and co-director of the Comprehensive Breast Cancer Center at the University of Pittsburgh School of Medicine. “The fact that we’re now talking about fourth- and fifth-line therapies for HER2-positive MBC represents a major advance in the management of these patients.”
Oncologists are still building on this progress, focusing on designing more targeted therapies as well as studying different combinations of available agents. The main goal of treatment, experts say, is to prolong patients’ systemic response and prevent recurrences, especially in the brain. This news organization spoke to Dr. Brufksy and others about promising agents and therapeutic strategies on the horizon to treat HER2-positive MBC.
Inside emerging ADCs
Because many patients develop resistance to trastuzumab emtansine (T-DM1) — the first FDA-approved ADC in breast cancer — researchers have focused on developing the next generation of ADCs with more potent payloads, different linkers, and distinct mechanisms of action, according to Sayeh Lavasani, MD, MS, a medical oncologist at City of Hope, a comprehensive cancer center in Los Angeles County.
The second-generation ADC trastuzumab deruxtecan showed “really dramatic” results in HER2-positive MBC, demonstrating progression-free survival of 16 months, remarked Kevin Kalinsky, MD, acting associate professor in the department of hematology and medical oncology at Emory University School of Medicine in Atlanta and director of the Glenn Family Breast Center at the Winship Cancer Institute of Emory University. “These outcomes further changed how we treat patients with metastatic disease and prompted considerable excitement over the potential to develop novel ADCs to treat HER2-positive MBC.”
Most recently, two investigational ADCs — trastuzumab duocarmazine (SYD985) and ARX788 — have stood out. The FDA granted fast-track designations to trastuzumab duocarmazine in January 2018 and ARX788 in January 2021. Trastuzumab duocarmazine, the furthest along the pipeline, has shown promising results so far. In June 2021, Netherlands-based biopharmaceutical company Byondis reported preliminary phase 3 data from the TULIP trial. The open-label, randomized phase 3 study enrolled 436 patients with HER2-positive locally advanced or metastatic disease that had progressed on previous anti-HER2 regimens. The company shared early results that trastuzumab duocarmazine achieved its progression-free survival primary endpoint, marking a significant improvement over physician’s choice of chemotherapy, and promised more detailed results to come later this year.
Although only in early-phase trials, ARX788 has also shown robust anti-HER2 activity as well as low toxicity in HER2-positive tumors, according to recent data. The findings from two phase 1 studies, presented at the June 2021 virtual American Society for Clinical Oncology meeting (abstract 1038), revealed an overall response rate of 74% in the breast cancer cohort, but the investigators acknowledged it was too early to report median progression-free survival outcomes. Preclinical data also showed activity in HER2-low and T-DM1–resistant tumors.
Despite the encouraging initial findings, Dr. Kalinsky remains cautiously optimistic about long-term outcomes for both ADCs. “These data are hot off the press, but it’s too soon to know how these two ADCs and others in the pipeline will measure up to approved therapies,” he commented. As experts learn more about the efficacy of these novel ADCs, Dr. Brufsky would also like to better understand resistance mechanisms and how to integrate these agents into current treatment strategies. “The cellular biology of HER2-positive MBC is complicated, and many factors in these tumor cells affect where ADCs are released, how resistance develops, and whether or not resistance to one ADC applies to others,” Dr. Brufsky remarked. “As we gather more data, we’ll understand resistance mechanisms better and begin to figure out where to go with treatment sequencing.”
TKIs and beyond
In addition to ADCs, TKIs continue to make their mark in the targeted HER2 therapeutic space. The approvals of tucatinib and neratinib last year represented an important advance in treating HER2-positive MBC, particularly for patients with brain metastases. The HER2CLIMB trial, for instance, found that tucatinib combined with trastuzumab and capecitabine had a 4.5-month overall survival advantage compared with placebo (21.9 vs 17.4) and a median progression-free survival advantage of 5.4 months in patients with active brain metastases (9.5 vs 4.1) and 8.3 months in patients with stable metastases (13.9 vs 5.6).
Given this progress, experts are looking to add new TKIs to the armamentarium. In particular, pyrotinib — already approved in China for treating HER2-positive MBC — has demonstrated significantly longer progression-free survival compared with a standard TKI, lapatinib. The phase 3 PHOEBE trial results, published in The Lancet in early 2021, found a median progression-free survival of 12.5 months in patients randomly assigned to receive pyrotinib plus capecitabine compared with 6.8 months in those receiving lapatinib plus capecitabine. The investigators also reported “manageable toxicity”; diarrhea was the most common grade 3 adverse event, occurring in 31% of the pyrotinib group vs. 8% of the lapatinib group, and overall serious adverse events occurred in 10% of patients receiving pyrotinib vs. 8% of those receiving lapatinib.
More recent data on pyrotinib come from the phase 2 PERMEATE trial, which focused on the safety and efficacy of the agent in patients with advanced disease and brain metastases. The investigators, who presented their findings at the 2021 virtual ASCO meeting (abstract 1037), reported that radiation therapy–naive patients receiving pyrotinib plus capecitabine had an overall response rate of 74.6% in the central nervous system. Patients experiencing progression after whole-brain or stereotactic radiation therapy, however, had a comparatively lower overall response rate of 42.1%.
Similarly, median progression-free survival was much higher in the radiation therapy–naive patients (12.1 vs 5.6 months in the radiation therapy cohort). Similar to the PHOEBE trial, the most common grade 3 adverse event was diarrhea (23.1%), followed by decreased neutrophil and white blood cell counts (12.8% for both), anemia (9%), and hand-foot syndrome (7.7%). The main question for Dr. Kalinsky is how well pyrotinib will ultimately stack up to tucatinib and neratinib. “Pyrotinib — like neratinib — was shown to be superior to lapatinib plus capecitabine , but its role may be limited by its gastrointestinal toxicity,” he said. In addition to research focused on expanding the selection of novel ADCs and TKIs, researchers are also exploring new combinations of approved treatments and whether these combinations can be used earlier in treatment sequencing.
Take the CompassHER2 trials. The ongoing phase 3 trial in patients with high-risk HER2-positive breast cancer and residual disease will explore whether tucatinib plus T-DM1 compared with T-DM1 alone improves overall survival and recurrence-free survival and prevents brain metastases. Another possibility currently under investigation is pairing tucatinib and trastuzumab deruxtecan, instead of T-DM1. “Overall, it’s exciting that we are increasing the number of therapeutic options and combinations,” commented Debu Tripathy, MD, professor and chairman in the department of breast medical oncology at the University of Texas MD Anderson Cancer Center in Houston. “Having more choices allows us to tailor therapies to manage resistance and prolong patients’ responses.”
Curbing brain metastasis, according to Dr. Brufksy, is particularly important, and experts need to explore the extent to which ADCs can penetrate the blood-brain barrier. Already, a subgroup analysis of the DESTINY-Breast01 trial found that trastuzumab deruxtecan appeared to be active in patients with brain metastases. Investigators reported an overall response rate of 58.3% and a median progression-free survival of 18.1 months — results in line with those in the general study cohort — but the study population did not include patients with untreated or progressive brain metastases. A phase 2 study currently under way will examine whether patients with HER2-positive and HER2-low breast cancer who have untreated or progressive brain metastases respond to trastuzumab deruxtecan as well. Ultimately, Dr. Brufksy hopes the recent successes with preventing brain metastases in pediatric acute lymphoblastic leukemia (ALL) foreshadow what›s to come in HER2-positive MBC.
“When we figured out how to treat brain metastases prophylactically in childhood ALL, we saw a huge improvement in the cure rate, which is ultimately my vision for HER2-positive disease,” Dr. Brufsky remarked. “Are there cures for HER2-positive MBC on the horizon? We don’t know yet, but the field has really exploded in recent years.”
A version of this article first appeared on Medscape.com.