User login
Clinical Edge Journal Scan Commentary: AML December 2021
This month, the phase II randomized study of azacitidine vs. azacitidine + enasidenib was reported by Dinardo et al.1 The primary endpoint was overall response rate. The study included 101 adult patients with newly diagnosed mutant-IDH2 acute myeloid leukemia (AML) who were ineligible for intensive chemotherapy. They were randomly assigned to enasidenib and azacitidine (Ena-AZA, n = 68) or azacitidine alone (AZA, n = 33). The overall response rate was significantly higher in patients receiving Ena-AZA vs. AZA (74% vs. 36%; odds ratio 4.9; P = .0003). More importantly, the rates of complete remission/complete remission with partial hematologic recovery was higher with Ena-AZA vs. AZA (57% vs. 18% P = .0001). The duration of response was 24.1 months vs 9.9 months for the Ena-AZA vs AZA group. Despite the higher and more durable responses with Ena+AZA, there was no difference in overall survival. The authors attributed that to patients receiving other effective therapies after disease progression. Overall patients tolerated the combination therapy well. The most frequent grade 3 or 4 events in the Ena-AZA vs. AZA groups were thrombocytopenia (37% vs. 19%) and neutropenia (37% vs. 25%).
Another study from the NCRI AML 16 trial demonstrated that reduced intensity conditioning (RIC) transplant in first remission vs. chemotherapy alone improved survival in older patients with AML who lacked favorable risk cytogenetics and were considered fit for intensive treatment. During a median follow-up of 60 months from remission, patients receiving RIC transplant vs. no transplant had superior survival (37% vs. 20%; hazard ratio [HR] 0.67; P < .001). Survival benefit with transplant in first remission vs. chemotherapy alone was observed across all Wheatley risk groups (adjusted HR 0.68; P < .001). The trial including 932 patients (age 60-70 years) with AML who entered remission and lacked favorable risk. Of these, 144 underwent RIC transplants from either matched sibling donors (n = 52) or matched unrelated donors (n = 92).
The outcome of patients with AML and central nervous system (CNS) involvement at presentation was reported by Ganzel et al. In that study, the investigators evaluated the incidence of CNS involvement in 11 consecutive Eastern Cooperative Oncology Group-American College of Radiology Imaging Network (ECOG-ACRIN) clinical trials. The incidence of CNS incolvement was 1.111 % (36 of 3240 patients). CNS involvement had no effect on remission rates or survival.
References
- Dinardo CD et al. Enasidenib plus azacitidine versus azacitidine alone in patients with newly diagnosed, mutant-IDH2 acute myeloid leukaemia (AG221-AML-005): a single-arm, phase 1b and randomised, phase 2 trial. Lancet Oncol. 2021;22(11):P1597-1608 (Nov 1).
- Russell NH et al. Outcomes of older patients aged 60 to 70 years undergoing reduced intensity transplant for acute myeloblastic leukemia: results of the NCRI acute myeloid leukemia 16 trial. Haematologica. 2021(Oct 14).
- Ganzel C et al. CNS involvement in AML at diagnosis is rare and does not affect response or survival: data from 11 ECOG-ACRIN trials. Blood Adv. 2021(Nov 12);5(22):4560-4568. doi: 10.1182/bloodadvances.2021004999.
This month, the phase II randomized study of azacitidine vs. azacitidine + enasidenib was reported by Dinardo et al.1 The primary endpoint was overall response rate. The study included 101 adult patients with newly diagnosed mutant-IDH2 acute myeloid leukemia (AML) who were ineligible for intensive chemotherapy. They were randomly assigned to enasidenib and azacitidine (Ena-AZA, n = 68) or azacitidine alone (AZA, n = 33). The overall response rate was significantly higher in patients receiving Ena-AZA vs. AZA (74% vs. 36%; odds ratio 4.9; P = .0003). More importantly, the rates of complete remission/complete remission with partial hematologic recovery was higher with Ena-AZA vs. AZA (57% vs. 18% P = .0001). The duration of response was 24.1 months vs 9.9 months for the Ena-AZA vs AZA group. Despite the higher and more durable responses with Ena+AZA, there was no difference in overall survival. The authors attributed that to patients receiving other effective therapies after disease progression. Overall patients tolerated the combination therapy well. The most frequent grade 3 or 4 events in the Ena-AZA vs. AZA groups were thrombocytopenia (37% vs. 19%) and neutropenia (37% vs. 25%).
Another study from the NCRI AML 16 trial demonstrated that reduced intensity conditioning (RIC) transplant in first remission vs. chemotherapy alone improved survival in older patients with AML who lacked favorable risk cytogenetics and were considered fit for intensive treatment. During a median follow-up of 60 months from remission, patients receiving RIC transplant vs. no transplant had superior survival (37% vs. 20%; hazard ratio [HR] 0.67; P < .001). Survival benefit with transplant in first remission vs. chemotherapy alone was observed across all Wheatley risk groups (adjusted HR 0.68; P < .001). The trial including 932 patients (age 60-70 years) with AML who entered remission and lacked favorable risk. Of these, 144 underwent RIC transplants from either matched sibling donors (n = 52) or matched unrelated donors (n = 92).
The outcome of patients with AML and central nervous system (CNS) involvement at presentation was reported by Ganzel et al. In that study, the investigators evaluated the incidence of CNS involvement in 11 consecutive Eastern Cooperative Oncology Group-American College of Radiology Imaging Network (ECOG-ACRIN) clinical trials. The incidence of CNS incolvement was 1.111 % (36 of 3240 patients). CNS involvement had no effect on remission rates or survival.
References
- Dinardo CD et al. Enasidenib plus azacitidine versus azacitidine alone in patients with newly diagnosed, mutant-IDH2 acute myeloid leukaemia (AG221-AML-005): a single-arm, phase 1b and randomised, phase 2 trial. Lancet Oncol. 2021;22(11):P1597-1608 (Nov 1).
- Russell NH et al. Outcomes of older patients aged 60 to 70 years undergoing reduced intensity transplant for acute myeloblastic leukemia: results of the NCRI acute myeloid leukemia 16 trial. Haematologica. 2021(Oct 14).
- Ganzel C et al. CNS involvement in AML at diagnosis is rare and does not affect response or survival: data from 11 ECOG-ACRIN trials. Blood Adv. 2021(Nov 12);5(22):4560-4568. doi: 10.1182/bloodadvances.2021004999.
This month, the phase II randomized study of azacitidine vs. azacitidine + enasidenib was reported by Dinardo et al.1 The primary endpoint was overall response rate. The study included 101 adult patients with newly diagnosed mutant-IDH2 acute myeloid leukemia (AML) who were ineligible for intensive chemotherapy. They were randomly assigned to enasidenib and azacitidine (Ena-AZA, n = 68) or azacitidine alone (AZA, n = 33). The overall response rate was significantly higher in patients receiving Ena-AZA vs. AZA (74% vs. 36%; odds ratio 4.9; P = .0003). More importantly, the rates of complete remission/complete remission with partial hematologic recovery was higher with Ena-AZA vs. AZA (57% vs. 18% P = .0001). The duration of response was 24.1 months vs 9.9 months for the Ena-AZA vs AZA group. Despite the higher and more durable responses with Ena+AZA, there was no difference in overall survival. The authors attributed that to patients receiving other effective therapies after disease progression. Overall patients tolerated the combination therapy well. The most frequent grade 3 or 4 events in the Ena-AZA vs. AZA groups were thrombocytopenia (37% vs. 19%) and neutropenia (37% vs. 25%).
Another study from the NCRI AML 16 trial demonstrated that reduced intensity conditioning (RIC) transplant in first remission vs. chemotherapy alone improved survival in older patients with AML who lacked favorable risk cytogenetics and were considered fit for intensive treatment. During a median follow-up of 60 months from remission, patients receiving RIC transplant vs. no transplant had superior survival (37% vs. 20%; hazard ratio [HR] 0.67; P < .001). Survival benefit with transplant in first remission vs. chemotherapy alone was observed across all Wheatley risk groups (adjusted HR 0.68; P < .001). The trial including 932 patients (age 60-70 years) with AML who entered remission and lacked favorable risk. Of these, 144 underwent RIC transplants from either matched sibling donors (n = 52) or matched unrelated donors (n = 92).
The outcome of patients with AML and central nervous system (CNS) involvement at presentation was reported by Ganzel et al. In that study, the investigators evaluated the incidence of CNS involvement in 11 consecutive Eastern Cooperative Oncology Group-American College of Radiology Imaging Network (ECOG-ACRIN) clinical trials. The incidence of CNS incolvement was 1.111 % (36 of 3240 patients). CNS involvement had no effect on remission rates or survival.
References
- Dinardo CD et al. Enasidenib plus azacitidine versus azacitidine alone in patients with newly diagnosed, mutant-IDH2 acute myeloid leukaemia (AG221-AML-005): a single-arm, phase 1b and randomised, phase 2 trial. Lancet Oncol. 2021;22(11):P1597-1608 (Nov 1).
- Russell NH et al. Outcomes of older patients aged 60 to 70 years undergoing reduced intensity transplant for acute myeloblastic leukemia: results of the NCRI acute myeloid leukemia 16 trial. Haematologica. 2021(Oct 14).
- Ganzel C et al. CNS involvement in AML at diagnosis is rare and does not affect response or survival: data from 11 ECOG-ACRIN trials. Blood Adv. 2021(Nov 12);5(22):4560-4568. doi: 10.1182/bloodadvances.2021004999.
Clinical Edge Journal Scan Commentary: CML December 2021
Patients who adhere to their tyrosine kinase inhibitor (TKI) medication regimen will achieve CML survival goals that would be expected for their age group. Imatinib is a first generation TKI inhibitor. In a recent post hoc analysis of the ADAGIO study patients, Obeng-Kusi M et al1 showed that compared with 90% adherence, a 100% adherence to imatinib was associated with a 2-fold increase in achieving or maintaining treatment response in patients with chronic myeloid leukemia (CML), highlighting the urgent need to assess and promote patient adherence. There is virtually no margin for nonadherence, if the objective is to optimize the likelihood of treatment response, and a minimal margin to avoid impaired treatment response.
On the same topic, Davis TC et al.2also recently reported a study of suboptimal adherence to TKI. From the 86 patients with CML studied, almost 17.9% of participants reported nonadherence, i.e., missing at least 1 dose of CML medication in the previous week. The main reason that patients reported nonadherence were side effects, a busy schedule, and the difficulty of complying daily with the TKI regimen.
Cardiovascular adverse events (AE) have been described with TKI treatment at different rates, but cardiovascular risk stratification was not included in the design of many trials. Baggio D et al3 in a retrospective study included 88 patients with CML treated with any TKI and a median follow up of 3.8 months. They described the rates of major cardiovascular AEs by combining age, history of prior cardiovascular diseases, and Framingham risk score, along with additional insights from coronary artery calcium scoring (CACS). The authors found cardiovascular AEs in 0%, 10%, and 19%, of the low-, intermediate-, and high-risk groups respectively. By using CACS score they were able to reclassify patients from intermediate to low risk and none of those patients experienced a major adverse cardiovascular event.
The use of kinase domain mutation analysis at diagnosis is not a recommended practice by the National Comprehensive Cancer Network (NCCN) or the European Leukemia Net (ELN), but is reserved for use in patents who failed first or subsequent lines of therapies. Furthermore, previous publications in the topic have showed discordant results. More recently, the use of ultra-deep sequencing has detected low-frequency genetic mutations with high sensitivity. Park H et al.4 recently described the most common mutations found in a population of 50 CML patients treated with nilotinib. V299 L mutation associated with dasatinib resistance and nilotinib sensitivity were observed in 98% of patients. Two uncommon mutations S417Y and the V371A were associated with reduced molecular response.
References
- Obeng-Kusi M et al. No margin for non-adherence: Probabilistic Kaplan-Meier modeling of imatinib non-adherence and treatment response in CML (ADAGIO study). Leuk Res. 2021;111:106734 (Oct 21).
- Davis TC et al. Assessment of oral chemotherapy nonadherence in chronic myeloid leukemia patients using brief measures in community cancer clinics: a pilot study. Int J Environ Res Public Health. 2021;18(21):11045 (Oct 21).
- Baggio D et al. Prediction of cardiovascular events in patients with chronic myeloid leukaemia using baseline risk factors and coronary artery calcium scoring. Intern Med J. 2021;51(10):1736-40 (Oct 18).
- Park H et al. Ultra-deep sequencing mutation analysis of the BCR/ABL1 kinase domain in newly diagnosed chronic myeloid leukemia patients. Leuk Res. 2021;111:106728 (Oct 15).
Patients who adhere to their tyrosine kinase inhibitor (TKI) medication regimen will achieve CML survival goals that would be expected for their age group. Imatinib is a first generation TKI inhibitor. In a recent post hoc analysis of the ADAGIO study patients, Obeng-Kusi M et al1 showed that compared with 90% adherence, a 100% adherence to imatinib was associated with a 2-fold increase in achieving or maintaining treatment response in patients with chronic myeloid leukemia (CML), highlighting the urgent need to assess and promote patient adherence. There is virtually no margin for nonadherence, if the objective is to optimize the likelihood of treatment response, and a minimal margin to avoid impaired treatment response.
On the same topic, Davis TC et al.2also recently reported a study of suboptimal adherence to TKI. From the 86 patients with CML studied, almost 17.9% of participants reported nonadherence, i.e., missing at least 1 dose of CML medication in the previous week. The main reason that patients reported nonadherence were side effects, a busy schedule, and the difficulty of complying daily with the TKI regimen.
Cardiovascular adverse events (AE) have been described with TKI treatment at different rates, but cardiovascular risk stratification was not included in the design of many trials. Baggio D et al3 in a retrospective study included 88 patients with CML treated with any TKI and a median follow up of 3.8 months. They described the rates of major cardiovascular AEs by combining age, history of prior cardiovascular diseases, and Framingham risk score, along with additional insights from coronary artery calcium scoring (CACS). The authors found cardiovascular AEs in 0%, 10%, and 19%, of the low-, intermediate-, and high-risk groups respectively. By using CACS score they were able to reclassify patients from intermediate to low risk and none of those patients experienced a major adverse cardiovascular event.
The use of kinase domain mutation analysis at diagnosis is not a recommended practice by the National Comprehensive Cancer Network (NCCN) or the European Leukemia Net (ELN), but is reserved for use in patents who failed first or subsequent lines of therapies. Furthermore, previous publications in the topic have showed discordant results. More recently, the use of ultra-deep sequencing has detected low-frequency genetic mutations with high sensitivity. Park H et al.4 recently described the most common mutations found in a population of 50 CML patients treated with nilotinib. V299 L mutation associated with dasatinib resistance and nilotinib sensitivity were observed in 98% of patients. Two uncommon mutations S417Y and the V371A were associated with reduced molecular response.
References
- Obeng-Kusi M et al. No margin for non-adherence: Probabilistic Kaplan-Meier modeling of imatinib non-adherence and treatment response in CML (ADAGIO study). Leuk Res. 2021;111:106734 (Oct 21).
- Davis TC et al. Assessment of oral chemotherapy nonadherence in chronic myeloid leukemia patients using brief measures in community cancer clinics: a pilot study. Int J Environ Res Public Health. 2021;18(21):11045 (Oct 21).
- Baggio D et al. Prediction of cardiovascular events in patients with chronic myeloid leukaemia using baseline risk factors and coronary artery calcium scoring. Intern Med J. 2021;51(10):1736-40 (Oct 18).
- Park H et al. Ultra-deep sequencing mutation analysis of the BCR/ABL1 kinase domain in newly diagnosed chronic myeloid leukemia patients. Leuk Res. 2021;111:106728 (Oct 15).
Patients who adhere to their tyrosine kinase inhibitor (TKI) medication regimen will achieve CML survival goals that would be expected for their age group. Imatinib is a first generation TKI inhibitor. In a recent post hoc analysis of the ADAGIO study patients, Obeng-Kusi M et al1 showed that compared with 90% adherence, a 100% adherence to imatinib was associated with a 2-fold increase in achieving or maintaining treatment response in patients with chronic myeloid leukemia (CML), highlighting the urgent need to assess and promote patient adherence. There is virtually no margin for nonadherence, if the objective is to optimize the likelihood of treatment response, and a minimal margin to avoid impaired treatment response.
On the same topic, Davis TC et al.2also recently reported a study of suboptimal adherence to TKI. From the 86 patients with CML studied, almost 17.9% of participants reported nonadherence, i.e., missing at least 1 dose of CML medication in the previous week. The main reason that patients reported nonadherence were side effects, a busy schedule, and the difficulty of complying daily with the TKI regimen.
Cardiovascular adverse events (AE) have been described with TKI treatment at different rates, but cardiovascular risk stratification was not included in the design of many trials. Baggio D et al3 in a retrospective study included 88 patients with CML treated with any TKI and a median follow up of 3.8 months. They described the rates of major cardiovascular AEs by combining age, history of prior cardiovascular diseases, and Framingham risk score, along with additional insights from coronary artery calcium scoring (CACS). The authors found cardiovascular AEs in 0%, 10%, and 19%, of the low-, intermediate-, and high-risk groups respectively. By using CACS score they were able to reclassify patients from intermediate to low risk and none of those patients experienced a major adverse cardiovascular event.
The use of kinase domain mutation analysis at diagnosis is not a recommended practice by the National Comprehensive Cancer Network (NCCN) or the European Leukemia Net (ELN), but is reserved for use in patents who failed first or subsequent lines of therapies. Furthermore, previous publications in the topic have showed discordant results. More recently, the use of ultra-deep sequencing has detected low-frequency genetic mutations with high sensitivity. Park H et al.4 recently described the most common mutations found in a population of 50 CML patients treated with nilotinib. V299 L mutation associated with dasatinib resistance and nilotinib sensitivity were observed in 98% of patients. Two uncommon mutations S417Y and the V371A were associated with reduced molecular response.
References
- Obeng-Kusi M et al. No margin for non-adherence: Probabilistic Kaplan-Meier modeling of imatinib non-adherence and treatment response in CML (ADAGIO study). Leuk Res. 2021;111:106734 (Oct 21).
- Davis TC et al. Assessment of oral chemotherapy nonadherence in chronic myeloid leukemia patients using brief measures in community cancer clinics: a pilot study. Int J Environ Res Public Health. 2021;18(21):11045 (Oct 21).
- Baggio D et al. Prediction of cardiovascular events in patients with chronic myeloid leukaemia using baseline risk factors and coronary artery calcium scoring. Intern Med J. 2021;51(10):1736-40 (Oct 18).
- Park H et al. Ultra-deep sequencing mutation analysis of the BCR/ABL1 kinase domain in newly diagnosed chronic myeloid leukemia patients. Leuk Res. 2021;111:106728 (Oct 15).
Apixaban outmatches rivaroxaban for VTE in study
Recurrent venous thromboembolism (VTE) – a composite of pulmonary embolism and deep vein thrombosis – was the primary effectiveness outcome in the retrospective analysis of new-user data from almost 40,000 patients, which was published in Annals of Internal Medicine. Safety was evaluated through a composite of intracranial and gastrointestinal bleeding.
After a median follow-up of 102 days in the apixaban group and 105 days in the rivaroxaban group, apixaban demonstrated superiority for both primary outcomes.
These real-world findings may guide selection of initial anticoagulant therapy, reported lead author Ghadeer K. Dawwas, PhD, MSc, MBA, of the University of Pennsylvania, Philadelphia, and colleagues.
“Randomized clinical trials comparing apixaban with rivaroxaban in patients with VTE are under way (for example, COBRRA (NCT03266783),” the investigators wrote. “Until the results from these trials become available (The estimated completion date for COBRRA is December 2023.), observational studies that use existing data can provide evidence on the effectiveness and safety of these alternatives to inform clinical practice.”
In the new research, apixaban was associated with a 23% lower rate of recurrent VTE (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87), including a 15% lower rate of deep vein thrombosis and a 41% lower rate of pulmonary embolism. Apixaban was associated with 40% fewer bleeding events (HR, 0.60; 95% CI, 0.53-0.69]), including a 40% lower rate of GI bleeding and a 46% lower rate of intracranial bleeding.
The study involved 37,236 patients with VTE, all of whom were diagnosed in at least one inpatient encounter and initiated direct oral anticoagulant (DOAC) therapy within 30 days, according to Optum’s deidentified Clinformatics Data Mart Database. Patients were evenly split into apixaban and rivaroxaban groups, with 18,618 individuals in each. Propensity score matching was used to minimize differences in baseline characteristics.
Apixaban was associated with an absolute reduction in recurrent VTE of 0.6% and 1.1% over 2 and 6 months, respectively, as well as reductions in bleeding of 1.1% and 1.5% over the same respective time periods.
The investigators noted that these findings were maintained in various sensitivity and subgroup analyses, including a model in which patients with VTE who had transient risk factors were compared with VTE patients exhibiting chronic risk factors.
“These findings suggest that apixaban has superior effectiveness and safety, compared with rivaroxaban and may provide guidance to clinicians and patients regarding selection of an anticoagulant for treatment of VTE,” Dr. Dawwas and colleagues concluded.
Study may have missed some nuance in possible outcomes, according to vascular surgeon
Thomas Wakefield, MD, a vascular surgeon and a professor of surgery at the University of Michigan Health Frankel Cardiovascular Center, Ann Arbor, generally agreed with the investigators’ conclusion, although he noted that DOAC selection may also be influenced by other considerations.
“The results of this study suggest that, when choosing an agent for an individual patient, apixaban does appear to have an advantage over rivaroxaban related to recurrent VTE and bleeding,” Dr. Wakefield said in an interview. “One must keep in mind that these are not the only factors that are considered when choosing an agent and these are not the only two DOACs available. For example, rivaroxaban is given once per day while apixaban is given twice per day, and rivaroxaban has been shown to be successful in the treatment of other thrombotic disorders.”
Dr. Wakefield also pointed out that the study may have missed some nuance in possible outcomes.
“The current study looked at severe outcomes that resulted in inpatient hospitalization, so the generalization to strictly outpatient treatment and less severe outcomes cannot be inferred,” he said.
Damon E. Houghton, MD, of the department of medicine and a consultant in the department of vascular medicine and hematology at Mayo Clinic, Rochester, Minn., called the study a “very nice analysis,” highlighting the large sample size.
“The results are not a reason to abandon rivaroxaban altogether, but do suggest that, when otherwise appropriate for a patient, apixaban should be the first choice,” Dr. Houghton said in a written comment. “Hopefully this analysis will encourage more payers to create financial incentives that facilitate the use of apixaban in more patients.”
Randomized trial needed, says hematologist
Colleen Edwards, MD, of the departments of medicine, hematology, and medical oncology, at the Icahn School of Medicine at Mount Sinai, New York, had a more guarded view of the findings than Dr. Wakefield and Dr. Houghton.
“[The investigators] certainly seem to be doing a lot of statistical gymnastics in this paper,” Dr. Edwards said in an interview. “They used all kinds of surrogates in place of real data that you would get from a randomized trial.”
For example, Dr. Edwards noted the use of prescription refills as a surrogate for medication adherence, and emphasized that inpatient observational data may not reflect outpatient therapy.
“Inpatients are constantly missing their medicines all the time,” she said. “They’re holding it for procedures, they’re NPO, they’re off the floor, so they missed their medicine. So it’s just a very different patient population than the outpatient population, which is where venous thromboembolism is treated now, by and large.”
Although Dr. Edwards suggested that the findings might guide treatment selection “a little bit,” she noted that insurance constraints and costs play a greater role, and ultimately concluded that a randomized trial is needed to materially alter clinical decision-making.
“I think we really have to wait for randomized trial before we abandon our other choices,” she said.
The investigators disclosed relationships with Merck, Celgene, UCB, and others. Dr. Wakefield reported awaiting disclosures. Dr. Houghton and Dr. Edwards reported no relevant conflicts of interest.
Recurrent venous thromboembolism (VTE) – a composite of pulmonary embolism and deep vein thrombosis – was the primary effectiveness outcome in the retrospective analysis of new-user data from almost 40,000 patients, which was published in Annals of Internal Medicine. Safety was evaluated through a composite of intracranial and gastrointestinal bleeding.
After a median follow-up of 102 days in the apixaban group and 105 days in the rivaroxaban group, apixaban demonstrated superiority for both primary outcomes.
These real-world findings may guide selection of initial anticoagulant therapy, reported lead author Ghadeer K. Dawwas, PhD, MSc, MBA, of the University of Pennsylvania, Philadelphia, and colleagues.
“Randomized clinical trials comparing apixaban with rivaroxaban in patients with VTE are under way (for example, COBRRA (NCT03266783),” the investigators wrote. “Until the results from these trials become available (The estimated completion date for COBRRA is December 2023.), observational studies that use existing data can provide evidence on the effectiveness and safety of these alternatives to inform clinical practice.”
In the new research, apixaban was associated with a 23% lower rate of recurrent VTE (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87), including a 15% lower rate of deep vein thrombosis and a 41% lower rate of pulmonary embolism. Apixaban was associated with 40% fewer bleeding events (HR, 0.60; 95% CI, 0.53-0.69]), including a 40% lower rate of GI bleeding and a 46% lower rate of intracranial bleeding.
The study involved 37,236 patients with VTE, all of whom were diagnosed in at least one inpatient encounter and initiated direct oral anticoagulant (DOAC) therapy within 30 days, according to Optum’s deidentified Clinformatics Data Mart Database. Patients were evenly split into apixaban and rivaroxaban groups, with 18,618 individuals in each. Propensity score matching was used to minimize differences in baseline characteristics.
Apixaban was associated with an absolute reduction in recurrent VTE of 0.6% and 1.1% over 2 and 6 months, respectively, as well as reductions in bleeding of 1.1% and 1.5% over the same respective time periods.
The investigators noted that these findings were maintained in various sensitivity and subgroup analyses, including a model in which patients with VTE who had transient risk factors were compared with VTE patients exhibiting chronic risk factors.
“These findings suggest that apixaban has superior effectiveness and safety, compared with rivaroxaban and may provide guidance to clinicians and patients regarding selection of an anticoagulant for treatment of VTE,” Dr. Dawwas and colleagues concluded.
Study may have missed some nuance in possible outcomes, according to vascular surgeon
Thomas Wakefield, MD, a vascular surgeon and a professor of surgery at the University of Michigan Health Frankel Cardiovascular Center, Ann Arbor, generally agreed with the investigators’ conclusion, although he noted that DOAC selection may also be influenced by other considerations.
“The results of this study suggest that, when choosing an agent for an individual patient, apixaban does appear to have an advantage over rivaroxaban related to recurrent VTE and bleeding,” Dr. Wakefield said in an interview. “One must keep in mind that these are not the only factors that are considered when choosing an agent and these are not the only two DOACs available. For example, rivaroxaban is given once per day while apixaban is given twice per day, and rivaroxaban has been shown to be successful in the treatment of other thrombotic disorders.”
Dr. Wakefield also pointed out that the study may have missed some nuance in possible outcomes.
“The current study looked at severe outcomes that resulted in inpatient hospitalization, so the generalization to strictly outpatient treatment and less severe outcomes cannot be inferred,” he said.
Damon E. Houghton, MD, of the department of medicine and a consultant in the department of vascular medicine and hematology at Mayo Clinic, Rochester, Minn., called the study a “very nice analysis,” highlighting the large sample size.
“The results are not a reason to abandon rivaroxaban altogether, but do suggest that, when otherwise appropriate for a patient, apixaban should be the first choice,” Dr. Houghton said in a written comment. “Hopefully this analysis will encourage more payers to create financial incentives that facilitate the use of apixaban in more patients.”
Randomized trial needed, says hematologist
Colleen Edwards, MD, of the departments of medicine, hematology, and medical oncology, at the Icahn School of Medicine at Mount Sinai, New York, had a more guarded view of the findings than Dr. Wakefield and Dr. Houghton.
“[The investigators] certainly seem to be doing a lot of statistical gymnastics in this paper,” Dr. Edwards said in an interview. “They used all kinds of surrogates in place of real data that you would get from a randomized trial.”
For example, Dr. Edwards noted the use of prescription refills as a surrogate for medication adherence, and emphasized that inpatient observational data may not reflect outpatient therapy.
“Inpatients are constantly missing their medicines all the time,” she said. “They’re holding it for procedures, they’re NPO, they’re off the floor, so they missed their medicine. So it’s just a very different patient population than the outpatient population, which is where venous thromboembolism is treated now, by and large.”
Although Dr. Edwards suggested that the findings might guide treatment selection “a little bit,” she noted that insurance constraints and costs play a greater role, and ultimately concluded that a randomized trial is needed to materially alter clinical decision-making.
“I think we really have to wait for randomized trial before we abandon our other choices,” she said.
The investigators disclosed relationships with Merck, Celgene, UCB, and others. Dr. Wakefield reported awaiting disclosures. Dr. Houghton and Dr. Edwards reported no relevant conflicts of interest.
Recurrent venous thromboembolism (VTE) – a composite of pulmonary embolism and deep vein thrombosis – was the primary effectiveness outcome in the retrospective analysis of new-user data from almost 40,000 patients, which was published in Annals of Internal Medicine. Safety was evaluated through a composite of intracranial and gastrointestinal bleeding.
After a median follow-up of 102 days in the apixaban group and 105 days in the rivaroxaban group, apixaban demonstrated superiority for both primary outcomes.
These real-world findings may guide selection of initial anticoagulant therapy, reported lead author Ghadeer K. Dawwas, PhD, MSc, MBA, of the University of Pennsylvania, Philadelphia, and colleagues.
“Randomized clinical trials comparing apixaban with rivaroxaban in patients with VTE are under way (for example, COBRRA (NCT03266783),” the investigators wrote. “Until the results from these trials become available (The estimated completion date for COBRRA is December 2023.), observational studies that use existing data can provide evidence on the effectiveness and safety of these alternatives to inform clinical practice.”
In the new research, apixaban was associated with a 23% lower rate of recurrent VTE (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87), including a 15% lower rate of deep vein thrombosis and a 41% lower rate of pulmonary embolism. Apixaban was associated with 40% fewer bleeding events (HR, 0.60; 95% CI, 0.53-0.69]), including a 40% lower rate of GI bleeding and a 46% lower rate of intracranial bleeding.
The study involved 37,236 patients with VTE, all of whom were diagnosed in at least one inpatient encounter and initiated direct oral anticoagulant (DOAC) therapy within 30 days, according to Optum’s deidentified Clinformatics Data Mart Database. Patients were evenly split into apixaban and rivaroxaban groups, with 18,618 individuals in each. Propensity score matching was used to minimize differences in baseline characteristics.
Apixaban was associated with an absolute reduction in recurrent VTE of 0.6% and 1.1% over 2 and 6 months, respectively, as well as reductions in bleeding of 1.1% and 1.5% over the same respective time periods.
The investigators noted that these findings were maintained in various sensitivity and subgroup analyses, including a model in which patients with VTE who had transient risk factors were compared with VTE patients exhibiting chronic risk factors.
“These findings suggest that apixaban has superior effectiveness and safety, compared with rivaroxaban and may provide guidance to clinicians and patients regarding selection of an anticoagulant for treatment of VTE,” Dr. Dawwas and colleagues concluded.
Study may have missed some nuance in possible outcomes, according to vascular surgeon
Thomas Wakefield, MD, a vascular surgeon and a professor of surgery at the University of Michigan Health Frankel Cardiovascular Center, Ann Arbor, generally agreed with the investigators’ conclusion, although he noted that DOAC selection may also be influenced by other considerations.
“The results of this study suggest that, when choosing an agent for an individual patient, apixaban does appear to have an advantage over rivaroxaban related to recurrent VTE and bleeding,” Dr. Wakefield said in an interview. “One must keep in mind that these are not the only factors that are considered when choosing an agent and these are not the only two DOACs available. For example, rivaroxaban is given once per day while apixaban is given twice per day, and rivaroxaban has been shown to be successful in the treatment of other thrombotic disorders.”
Dr. Wakefield also pointed out that the study may have missed some nuance in possible outcomes.
“The current study looked at severe outcomes that resulted in inpatient hospitalization, so the generalization to strictly outpatient treatment and less severe outcomes cannot be inferred,” he said.
Damon E. Houghton, MD, of the department of medicine and a consultant in the department of vascular medicine and hematology at Mayo Clinic, Rochester, Minn., called the study a “very nice analysis,” highlighting the large sample size.
“The results are not a reason to abandon rivaroxaban altogether, but do suggest that, when otherwise appropriate for a patient, apixaban should be the first choice,” Dr. Houghton said in a written comment. “Hopefully this analysis will encourage more payers to create financial incentives that facilitate the use of apixaban in more patients.”
Randomized trial needed, says hematologist
Colleen Edwards, MD, of the departments of medicine, hematology, and medical oncology, at the Icahn School of Medicine at Mount Sinai, New York, had a more guarded view of the findings than Dr. Wakefield and Dr. Houghton.
“[The investigators] certainly seem to be doing a lot of statistical gymnastics in this paper,” Dr. Edwards said in an interview. “They used all kinds of surrogates in place of real data that you would get from a randomized trial.”
For example, Dr. Edwards noted the use of prescription refills as a surrogate for medication adherence, and emphasized that inpatient observational data may not reflect outpatient therapy.
“Inpatients are constantly missing their medicines all the time,” she said. “They’re holding it for procedures, they’re NPO, they’re off the floor, so they missed their medicine. So it’s just a very different patient population than the outpatient population, which is where venous thromboembolism is treated now, by and large.”
Although Dr. Edwards suggested that the findings might guide treatment selection “a little bit,” she noted that insurance constraints and costs play a greater role, and ultimately concluded that a randomized trial is needed to materially alter clinical decision-making.
“I think we really have to wait for randomized trial before we abandon our other choices,” she said.
The investigators disclosed relationships with Merck, Celgene, UCB, and others. Dr. Wakefield reported awaiting disclosures. Dr. Houghton and Dr. Edwards reported no relevant conflicts of interest.
FROM ANNALS OF INTERNAL MEDICINE
Tender Subcutaneous Nodule in a Prepubescent Boy
The Diagnosis: Dermatomyofibroma
Dermatomyofibroma is an uncommon, benign, cutaneous mesenchymal neoplasm composed of fibroblasts and myofibroblasts.1-3 This skin tumor was first described in 1991 by Hugel4 in the German literature as plaquelike fibromatosis. Pediatric dermatomyofibromas are exceedingly rare, with pediatric patients ranging in age from infants to teenagers.1
Clinically, dermatomyofibromas appear as long-standing, isolated, ill-demarcated, flesh-colored, slightly hyperpigmented or erythematous nodules or plaques that may be raised or indurated.1 Dermatomyofibromas may present with constant mild pain or pruritus, though in most cases the lesions are asymptomatic.1,3 The clinical presentation of dermatomyofibroma has a few distinct differences in children compared to adults. In adulthood, dermatomyofibroma has a strong female predominance and most commonly is located on the shoulder and adjacent upper body regions, including the axilla, neck, upper arm, and upper trunk.1-3 In childhood, the majority of dermatomyofibromas occur in young boys and usually are located on the neck with other upper body regions occurring less frequently.1,2 A shared characteristic includes the tendency for dermatomyofibromas to have an initial period of enlargement followed by stabilization or slow growth.1 Reported pediatric lesions have ranged in size from 4 to 60 mm with an average size of 14.9 mm (median, 12 mm).2
The diagnosis of dermatomyofibroma is based on histopathologic features in addition to clinical presentation. Histology from punch biopsy usually reveals a noninvasive dermal proliferation of bland, uniform, slender spindle cells oriented parallel to the overlying epidermis with increased and fragmented elastic fibers.1,3 Infiltration into the mid or deep dermis is common. The adnexal structures usually are spared; the stroma contains collagen and increased small blood vessels; and there typically is no inflammatory infiltrate, except for occasional scattered mast cells.2 Cytologically, the monomorphic spindleshaped tumor cells have an ill-defined, pale, eosinophilic cytoplasm and nuclei that are elongated with tapered edges.3 Dermatomyofibroma has a variable immunohistochemical profile, as it may stain focally positive for CD34 or smooth muscle actin, with occasional staining of factor XIIIa, desmin, calponin, or vimentin.1-3 Normal to increased levels of often fragmented elastic fibers is a helpful clue in distinguishing dermatomyofibroma from dermatofibroma, hypertrophic scar, dermatofibrosarcoma protuberans, and pilar leiomyoma, in which elastic fibers typically are reduced.3 Differential diagnoses of mesenchymal tumors in children include desmoid fibromatosis, connective tissue nevus, myofibromatosis, and smooth muscle hamartoma.1
A punch biopsy with clinical observation and followup is recommended for the management of lesions in cosmetically sensitive areas or in very young children who may not tolerate surgery. In symptomatic or cosmetically unappealing cases of dermatomyofibroma, simple surgical excision remains a viable treatment option. Recurrence is uncommon, even if only partially excised, and no instances of metastasis have been reported.1-5
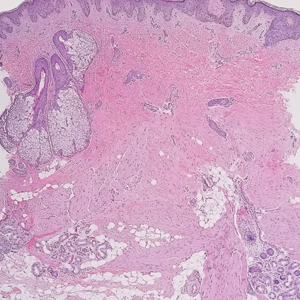
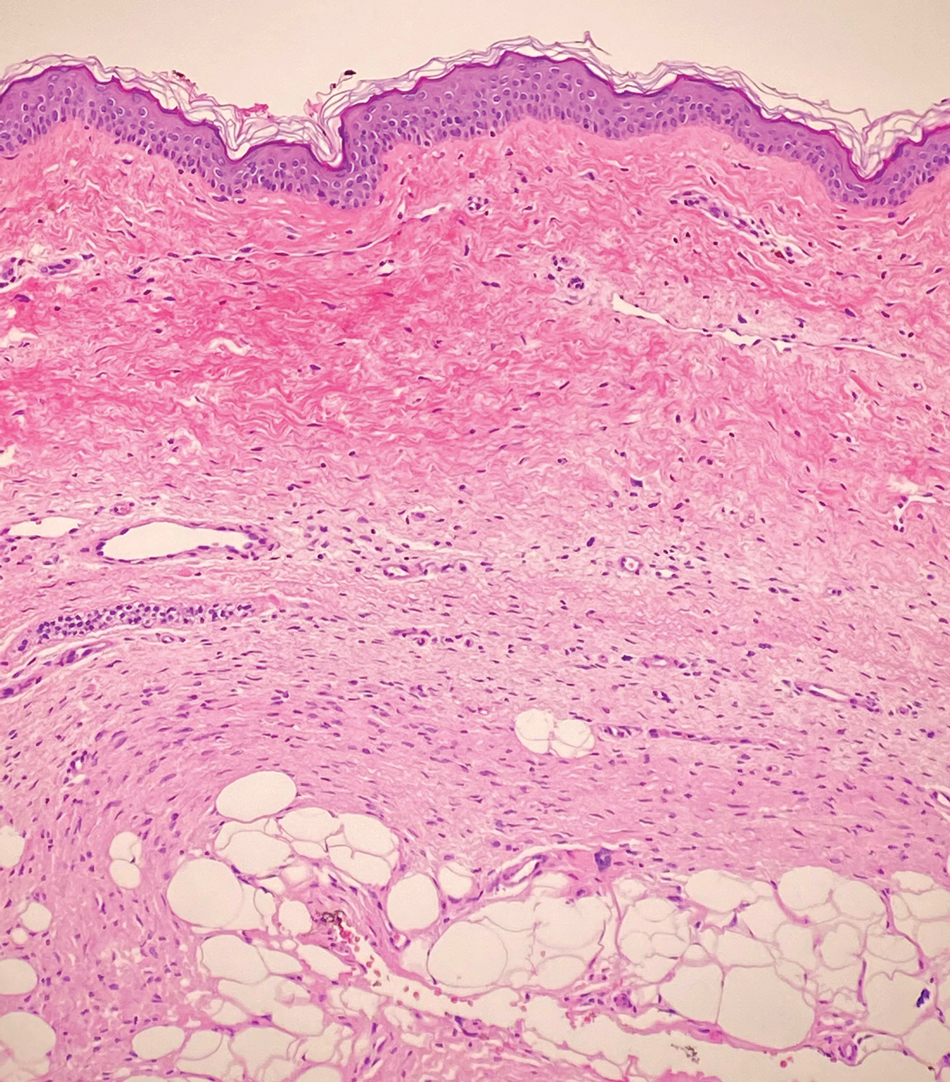
Dermatomyofibromas may be mistaken for several other entities both benign and malignant. For example, the benign dermatofibroma is the second most common fibrohistiocytic tumor of the skin and presents as a firm, nontender, minimally elevated to dome-shaped papule that usually measures less than or equal to 1 cm in diameter with or without overlying skin changes.5,6 It primarily is seen in adults with a slight female predominance and favors the lower extremities.5 Patients usually are asymptomatic but often report a history of local trauma at the lesion site.6 Histologically, dermatofibroma is characterized by a nodular dermal proliferation of spindleshaped fibrous cells and histiocytes in a storiform pattern (Figure 1).6 Epidermal induction with acanthosis overlying the tumor often is found with occasional basilar hyperpigmentation.5 Dermatofibroma also characteristically has trapped collagen (“collagen balls”) seen at the periphery.5,6
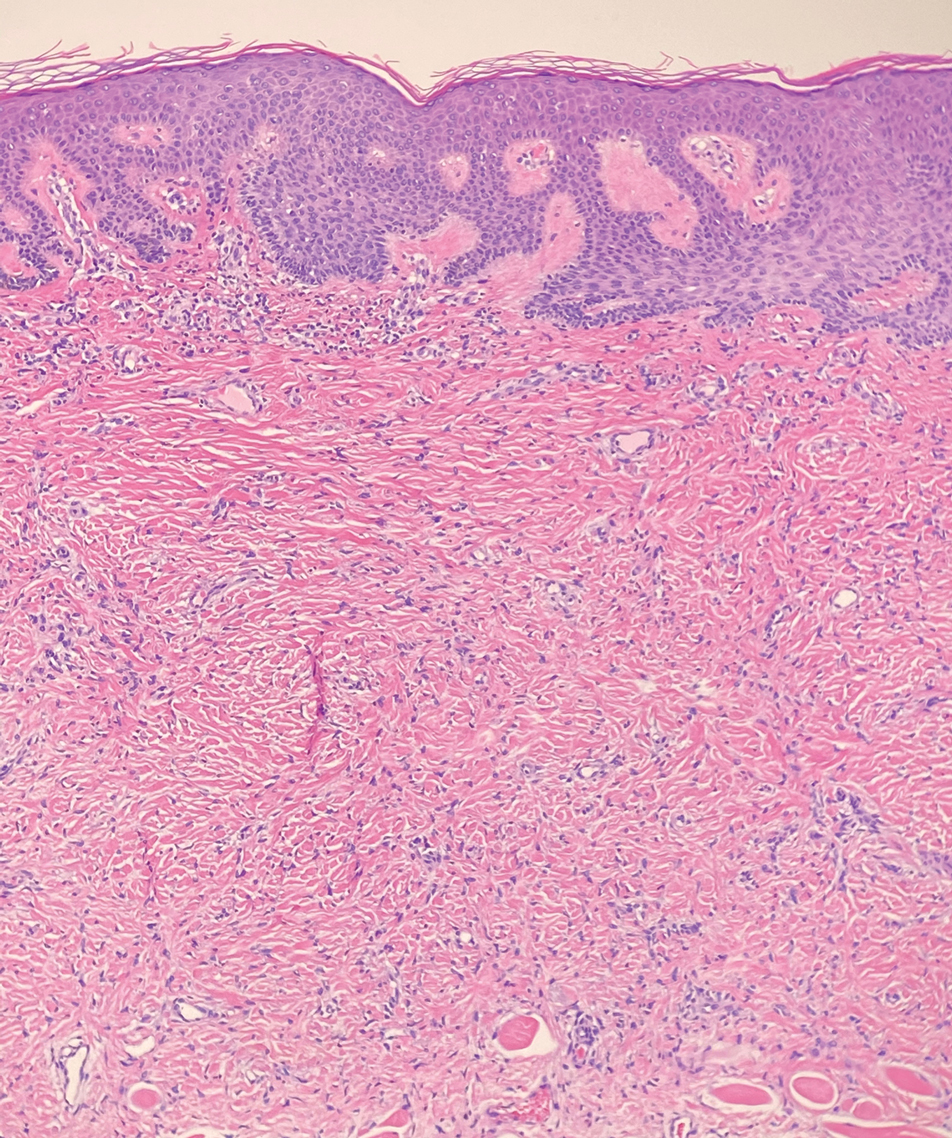
Piloleiomyomas are benign smooth muscle tumors arising from arrector pili muscles that may be solitary or multiple.5 Clinically, they typically present as firm, reddish-brown to flesh-colored papules or nodules that develop more commonly in adulthood.5,7 Piloleiomyomas favor the extremities and trunk, particularly the shoulder, and can be associated with spontaneous or induced pain. Histologically, piloleiomyomas are well circumscribed and centered within the reticular dermis situated closely to hair follicles (Figure 2).5 They are composed of numerous interlacing fascicles or whorls of smooth muscle cells with abundant eosinophilic cytoplasm and blunt-ended, cigar-shaped nuclei.5,7
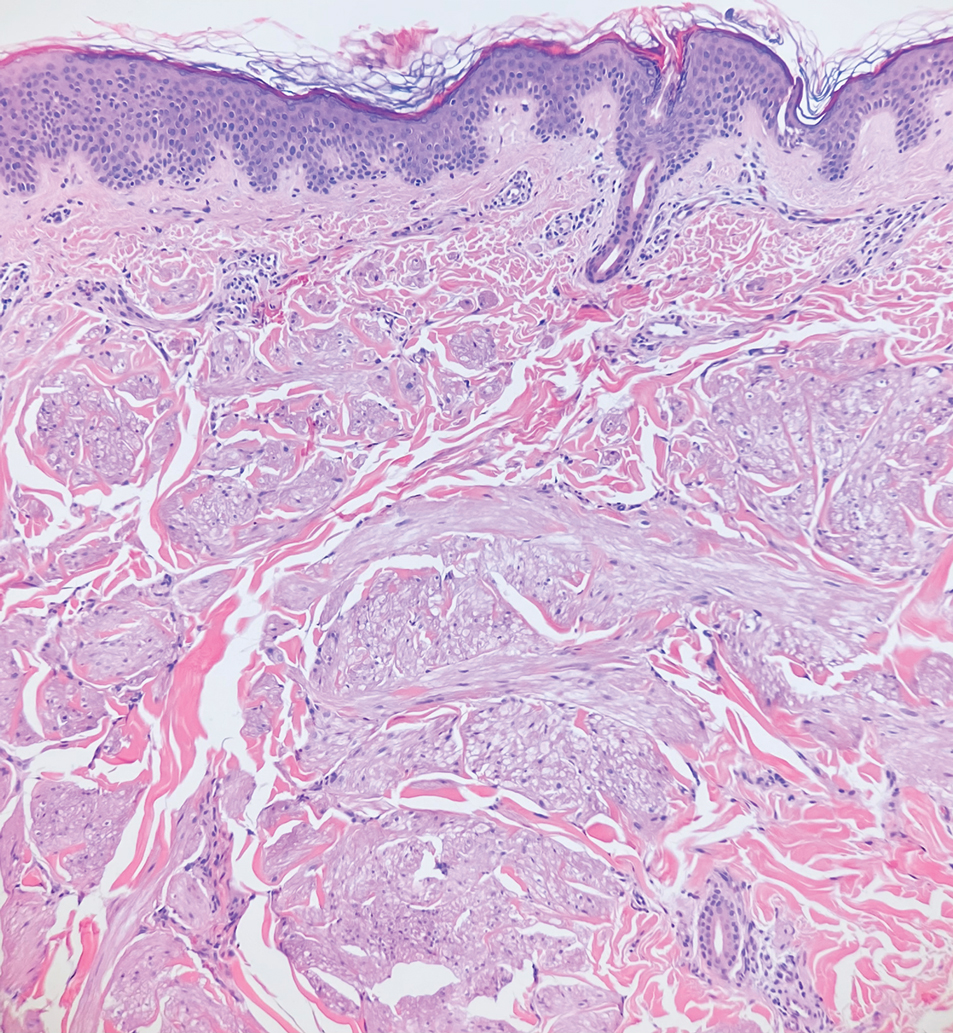
Solitary cutaneous myofibroma is a benign fibrous tumor found in adolescents and adults and is the counterpart to infantile myofibromatosis.8 Clinically, myofibromas typically present as painless, slow-growing, firm nodules with an occasional bluish hue. Histologically, solitary cutaneous myofibromas appear in a biphasic pattern, with hemangiopericytomatous components as well as spindle cells arranged in short bundles and fascicles resembling leiomyoma (Figure 3). The spindle cells also have abundant eosinophilic cytoplasm with short plump nuclei; the random, irregularly intersecting angles can be used to help differentiate myofibromas from smooth muscle lesions.8 Solitary cutaneous myofibroma is in the differential diagnosis for dermatomyofibroma because of their shared myofibroblastic nature.9
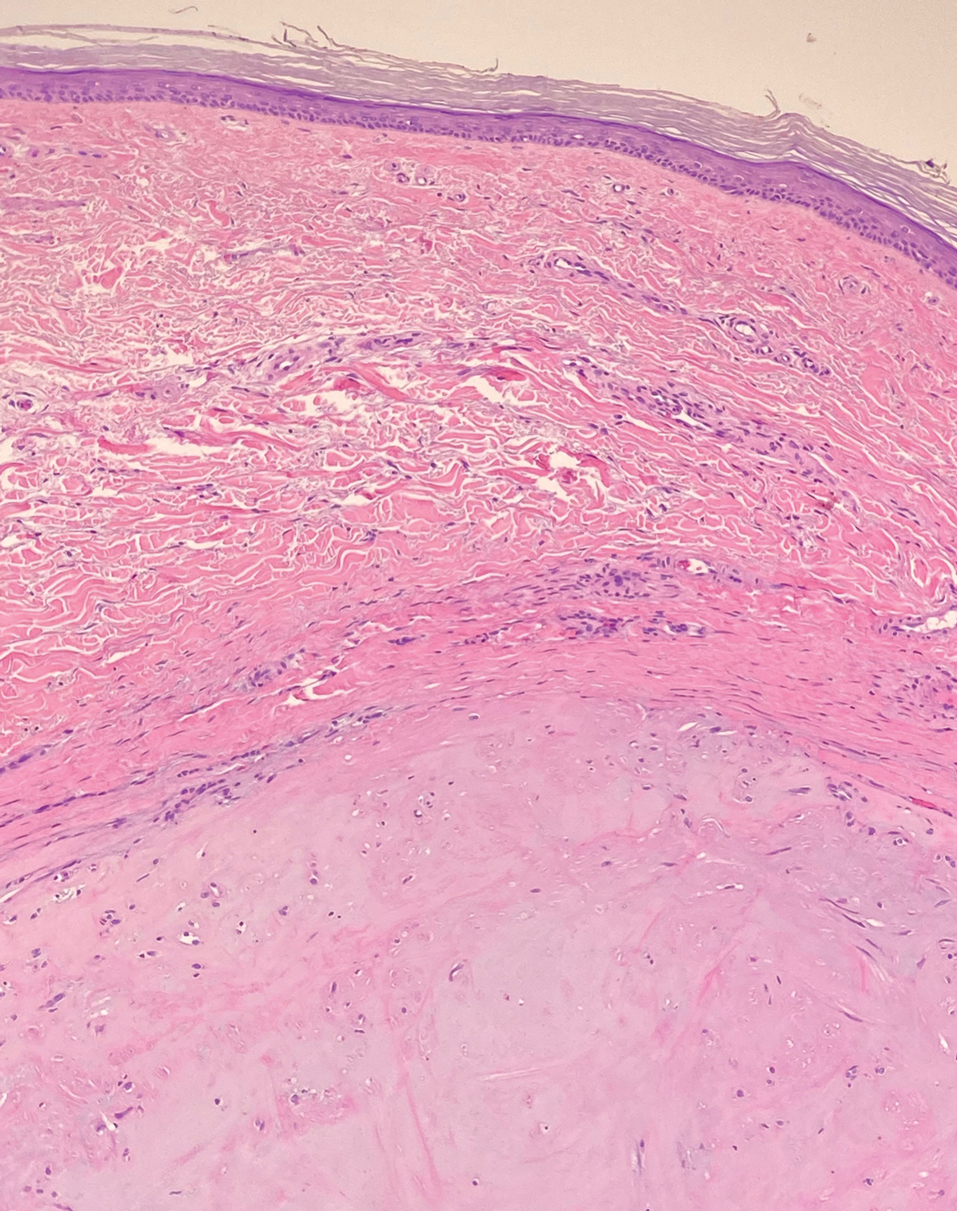
Dermatofibrosarcoma protuberans (DFSP) is an uncommon, locally invasive sarcoma with a high recurrence rate that favors young to middle-aged adults, with rare childhood onset reported.5,10,11 Clinically, DFSP typically presents as an asymptomatic, slow-growing, firm, flesh-colored, indurated plaque that develops into a violaceous to reddish-brown nodule.5 The atrophic variant of DFSP is characterized by a nonprotuberant lesion and can be especially difficult to distinguish from other entities such as dermatomyofibroma.11 The majority of DFSP lesions occur on the trunk, particularly in the shoulder or pelvic region.5 Histologically, early plaque lesions are comprised of monomorphic spindle cells arranged in long fascicles (parallel to the skin surface), infiltrating adnexal structures, and subcutaneous adipocytes in a multilayered honeycomb pattern; the spindle cells of late nodular lesions are arranged in short fascicles in a matted or storiform pattern (Figure 4).5,10 Early stages of DFSP as well as variations in childhood-onset DFSP can easily be misdiagnosed and incompletely excised.5
- Ma JE, Wieland CN, Tollefson MM. Dermatomyofibromas arising in children: report of two new cases and review of the literature. Pediatr Dermatol. 2017;34:347-351.
- Tardio JC, Azorin D, Hernandez-Nunez A, et al. Dermatomyofibromas presenting in pediatric patients: clinicopathologic characteristics and differential diagnosis. J Cutan Pathol. 2011;38:967-972.
- Mentzel T, Kutzner H. Dermatomyofibroma: clinicopathologic and immunohistochemical analysis of 56 cases and reappraisal of a rare and distinct cutaneous neoplasm. Am J Dermatopathol. 2009;31:44-49.
- Hugel H. Plaque-like dermal fibromatosis. Hautarzt. 1991;42:223-226.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. WB Saunders Co; 2012.
- Myers DJ, Fillman EP. Dermatofibroma. StatPearls [Internet]. StatPearls Publishing; 2020.
- Dilek N, Yuksel D, Sehitoglu I, et al. Cutaneous leiomyoma in a child: a case report. Oncol Lett. 2013;5:1163-1164.
- Roh HS, Paek JO, Yu HJ, et al. Solitary cutaneous myofibroma on the sole: an unusual localization. Ann Dermatol. 2012;24:220-222.
- Weedon D, Strutton G, Rubin AI, et al. Weedon’s Skin Pathology. Churchill Livingstone/Elsevier; 2010.
- Mendenhall WM, Zlotecki RA, Scarborough MT. Dermatofibrosarcoma protuberans. Cancer. 2004;101:2503-2508.
- Akay BN, Unlu E, Erdem C, et al. Dermatoscopic findings of atrophic dermatofibrosarcoma protuberans. Dermatol Pract Concept. 2015;5:71-73.
The Diagnosis: Dermatomyofibroma
Dermatomyofibroma is an uncommon, benign, cutaneous mesenchymal neoplasm composed of fibroblasts and myofibroblasts.1-3 This skin tumor was first described in 1991 by Hugel4 in the German literature as plaquelike fibromatosis. Pediatric dermatomyofibromas are exceedingly rare, with pediatric patients ranging in age from infants to teenagers.1
Clinically, dermatomyofibromas appear as long-standing, isolated, ill-demarcated, flesh-colored, slightly hyperpigmented or erythematous nodules or plaques that may be raised or indurated.1 Dermatomyofibromas may present with constant mild pain or pruritus, though in most cases the lesions are asymptomatic.1,3 The clinical presentation of dermatomyofibroma has a few distinct differences in children compared to adults. In adulthood, dermatomyofibroma has a strong female predominance and most commonly is located on the shoulder and adjacent upper body regions, including the axilla, neck, upper arm, and upper trunk.1-3 In childhood, the majority of dermatomyofibromas occur in young boys and usually are located on the neck with other upper body regions occurring less frequently.1,2 A shared characteristic includes the tendency for dermatomyofibromas to have an initial period of enlargement followed by stabilization or slow growth.1 Reported pediatric lesions have ranged in size from 4 to 60 mm with an average size of 14.9 mm (median, 12 mm).2
The diagnosis of dermatomyofibroma is based on histopathologic features in addition to clinical presentation. Histology from punch biopsy usually reveals a noninvasive dermal proliferation of bland, uniform, slender spindle cells oriented parallel to the overlying epidermis with increased and fragmented elastic fibers.1,3 Infiltration into the mid or deep dermis is common. The adnexal structures usually are spared; the stroma contains collagen and increased small blood vessels; and there typically is no inflammatory infiltrate, except for occasional scattered mast cells.2 Cytologically, the monomorphic spindleshaped tumor cells have an ill-defined, pale, eosinophilic cytoplasm and nuclei that are elongated with tapered edges.3 Dermatomyofibroma has a variable immunohistochemical profile, as it may stain focally positive for CD34 or smooth muscle actin, with occasional staining of factor XIIIa, desmin, calponin, or vimentin.1-3 Normal to increased levels of often fragmented elastic fibers is a helpful clue in distinguishing dermatomyofibroma from dermatofibroma, hypertrophic scar, dermatofibrosarcoma protuberans, and pilar leiomyoma, in which elastic fibers typically are reduced.3 Differential diagnoses of mesenchymal tumors in children include desmoid fibromatosis, connective tissue nevus, myofibromatosis, and smooth muscle hamartoma.1
A punch biopsy with clinical observation and followup is recommended for the management of lesions in cosmetically sensitive areas or in very young children who may not tolerate surgery. In symptomatic or cosmetically unappealing cases of dermatomyofibroma, simple surgical excision remains a viable treatment option. Recurrence is uncommon, even if only partially excised, and no instances of metastasis have been reported.1-5
Dermatomyofibromas may be mistaken for several other entities both benign and malignant. For example, the benign dermatofibroma is the second most common fibrohistiocytic tumor of the skin and presents as a firm, nontender, minimally elevated to dome-shaped papule that usually measures less than or equal to 1 cm in diameter with or without overlying skin changes.5,6 It primarily is seen in adults with a slight female predominance and favors the lower extremities.5 Patients usually are asymptomatic but often report a history of local trauma at the lesion site.6 Histologically, dermatofibroma is characterized by a nodular dermal proliferation of spindleshaped fibrous cells and histiocytes in a storiform pattern (Figure 1).6 Epidermal induction with acanthosis overlying the tumor often is found with occasional basilar hyperpigmentation.5 Dermatofibroma also characteristically has trapped collagen (“collagen balls”) seen at the periphery.5,6

Piloleiomyomas are benign smooth muscle tumors arising from arrector pili muscles that may be solitary or multiple.5 Clinically, they typically present as firm, reddish-brown to flesh-colored papules or nodules that develop more commonly in adulthood.5,7 Piloleiomyomas favor the extremities and trunk, particularly the shoulder, and can be associated with spontaneous or induced pain. Histologically, piloleiomyomas are well circumscribed and centered within the reticular dermis situated closely to hair follicles (Figure 2).5 They are composed of numerous interlacing fascicles or whorls of smooth muscle cells with abundant eosinophilic cytoplasm and blunt-ended, cigar-shaped nuclei.5,7

Solitary cutaneous myofibroma is a benign fibrous tumor found in adolescents and adults and is the counterpart to infantile myofibromatosis.8 Clinically, myofibromas typically present as painless, slow-growing, firm nodules with an occasional bluish hue. Histologically, solitary cutaneous myofibromas appear in a biphasic pattern, with hemangiopericytomatous components as well as spindle cells arranged in short bundles and fascicles resembling leiomyoma (Figure 3). The spindle cells also have abundant eosinophilic cytoplasm with short plump nuclei; the random, irregularly intersecting angles can be used to help differentiate myofibromas from smooth muscle lesions.8 Solitary cutaneous myofibroma is in the differential diagnosis for dermatomyofibroma because of their shared myofibroblastic nature.9

Dermatofibrosarcoma protuberans (DFSP) is an uncommon, locally invasive sarcoma with a high recurrence rate that favors young to middle-aged adults, with rare childhood onset reported.5,10,11 Clinically, DFSP typically presents as an asymptomatic, slow-growing, firm, flesh-colored, indurated plaque that develops into a violaceous to reddish-brown nodule.5 The atrophic variant of DFSP is characterized by a nonprotuberant lesion and can be especially difficult to distinguish from other entities such as dermatomyofibroma.11 The majority of DFSP lesions occur on the trunk, particularly in the shoulder or pelvic region.5 Histologically, early plaque lesions are comprised of monomorphic spindle cells arranged in long fascicles (parallel to the skin surface), infiltrating adnexal structures, and subcutaneous adipocytes in a multilayered honeycomb pattern; the spindle cells of late nodular lesions are arranged in short fascicles in a matted or storiform pattern (Figure 4).5,10 Early stages of DFSP as well as variations in childhood-onset DFSP can easily be misdiagnosed and incompletely excised.5

The Diagnosis: Dermatomyofibroma
Dermatomyofibroma is an uncommon, benign, cutaneous mesenchymal neoplasm composed of fibroblasts and myofibroblasts.1-3 This skin tumor was first described in 1991 by Hugel4 in the German literature as plaquelike fibromatosis. Pediatric dermatomyofibromas are exceedingly rare, with pediatric patients ranging in age from infants to teenagers.1
Clinically, dermatomyofibromas appear as long-standing, isolated, ill-demarcated, flesh-colored, slightly hyperpigmented or erythematous nodules or plaques that may be raised or indurated.1 Dermatomyofibromas may present with constant mild pain or pruritus, though in most cases the lesions are asymptomatic.1,3 The clinical presentation of dermatomyofibroma has a few distinct differences in children compared to adults. In adulthood, dermatomyofibroma has a strong female predominance and most commonly is located on the shoulder and adjacent upper body regions, including the axilla, neck, upper arm, and upper trunk.1-3 In childhood, the majority of dermatomyofibromas occur in young boys and usually are located on the neck with other upper body regions occurring less frequently.1,2 A shared characteristic includes the tendency for dermatomyofibromas to have an initial period of enlargement followed by stabilization or slow growth.1 Reported pediatric lesions have ranged in size from 4 to 60 mm with an average size of 14.9 mm (median, 12 mm).2
The diagnosis of dermatomyofibroma is based on histopathologic features in addition to clinical presentation. Histology from punch biopsy usually reveals a noninvasive dermal proliferation of bland, uniform, slender spindle cells oriented parallel to the overlying epidermis with increased and fragmented elastic fibers.1,3 Infiltration into the mid or deep dermis is common. The adnexal structures usually are spared; the stroma contains collagen and increased small blood vessels; and there typically is no inflammatory infiltrate, except for occasional scattered mast cells.2 Cytologically, the monomorphic spindleshaped tumor cells have an ill-defined, pale, eosinophilic cytoplasm and nuclei that are elongated with tapered edges.3 Dermatomyofibroma has a variable immunohistochemical profile, as it may stain focally positive for CD34 or smooth muscle actin, with occasional staining of factor XIIIa, desmin, calponin, or vimentin.1-3 Normal to increased levels of often fragmented elastic fibers is a helpful clue in distinguishing dermatomyofibroma from dermatofibroma, hypertrophic scar, dermatofibrosarcoma protuberans, and pilar leiomyoma, in which elastic fibers typically are reduced.3 Differential diagnoses of mesenchymal tumors in children include desmoid fibromatosis, connective tissue nevus, myofibromatosis, and smooth muscle hamartoma.1
A punch biopsy with clinical observation and followup is recommended for the management of lesions in cosmetically sensitive areas or in very young children who may not tolerate surgery. In symptomatic or cosmetically unappealing cases of dermatomyofibroma, simple surgical excision remains a viable treatment option. Recurrence is uncommon, even if only partially excised, and no instances of metastasis have been reported.1-5
Dermatomyofibromas may be mistaken for several other entities both benign and malignant. For example, the benign dermatofibroma is the second most common fibrohistiocytic tumor of the skin and presents as a firm, nontender, minimally elevated to dome-shaped papule that usually measures less than or equal to 1 cm in diameter with or without overlying skin changes.5,6 It primarily is seen in adults with a slight female predominance and favors the lower extremities.5 Patients usually are asymptomatic but often report a history of local trauma at the lesion site.6 Histologically, dermatofibroma is characterized by a nodular dermal proliferation of spindleshaped fibrous cells and histiocytes in a storiform pattern (Figure 1).6 Epidermal induction with acanthosis overlying the tumor often is found with occasional basilar hyperpigmentation.5 Dermatofibroma also characteristically has trapped collagen (“collagen balls”) seen at the periphery.5,6

Piloleiomyomas are benign smooth muscle tumors arising from arrector pili muscles that may be solitary or multiple.5 Clinically, they typically present as firm, reddish-brown to flesh-colored papules or nodules that develop more commonly in adulthood.5,7 Piloleiomyomas favor the extremities and trunk, particularly the shoulder, and can be associated with spontaneous or induced pain. Histologically, piloleiomyomas are well circumscribed and centered within the reticular dermis situated closely to hair follicles (Figure 2).5 They are composed of numerous interlacing fascicles or whorls of smooth muscle cells with abundant eosinophilic cytoplasm and blunt-ended, cigar-shaped nuclei.5,7

Solitary cutaneous myofibroma is a benign fibrous tumor found in adolescents and adults and is the counterpart to infantile myofibromatosis.8 Clinically, myofibromas typically present as painless, slow-growing, firm nodules with an occasional bluish hue. Histologically, solitary cutaneous myofibromas appear in a biphasic pattern, with hemangiopericytomatous components as well as spindle cells arranged in short bundles and fascicles resembling leiomyoma (Figure 3). The spindle cells also have abundant eosinophilic cytoplasm with short plump nuclei; the random, irregularly intersecting angles can be used to help differentiate myofibromas from smooth muscle lesions.8 Solitary cutaneous myofibroma is in the differential diagnosis for dermatomyofibroma because of their shared myofibroblastic nature.9

Dermatofibrosarcoma protuberans (DFSP) is an uncommon, locally invasive sarcoma with a high recurrence rate that favors young to middle-aged adults, with rare childhood onset reported.5,10,11 Clinically, DFSP typically presents as an asymptomatic, slow-growing, firm, flesh-colored, indurated plaque that develops into a violaceous to reddish-brown nodule.5 The atrophic variant of DFSP is characterized by a nonprotuberant lesion and can be especially difficult to distinguish from other entities such as dermatomyofibroma.11 The majority of DFSP lesions occur on the trunk, particularly in the shoulder or pelvic region.5 Histologically, early plaque lesions are comprised of monomorphic spindle cells arranged in long fascicles (parallel to the skin surface), infiltrating adnexal structures, and subcutaneous adipocytes in a multilayered honeycomb pattern; the spindle cells of late nodular lesions are arranged in short fascicles in a matted or storiform pattern (Figure 4).5,10 Early stages of DFSP as well as variations in childhood-onset DFSP can easily be misdiagnosed and incompletely excised.5

- Ma JE, Wieland CN, Tollefson MM. Dermatomyofibromas arising in children: report of two new cases and review of the literature. Pediatr Dermatol. 2017;34:347-351.
- Tardio JC, Azorin D, Hernandez-Nunez A, et al. Dermatomyofibromas presenting in pediatric patients: clinicopathologic characteristics and differential diagnosis. J Cutan Pathol. 2011;38:967-972.
- Mentzel T, Kutzner H. Dermatomyofibroma: clinicopathologic and immunohistochemical analysis of 56 cases and reappraisal of a rare and distinct cutaneous neoplasm. Am J Dermatopathol. 2009;31:44-49.
- Hugel H. Plaque-like dermal fibromatosis. Hautarzt. 1991;42:223-226.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. WB Saunders Co; 2012.
- Myers DJ, Fillman EP. Dermatofibroma. StatPearls [Internet]. StatPearls Publishing; 2020.
- Dilek N, Yuksel D, Sehitoglu I, et al. Cutaneous leiomyoma in a child: a case report. Oncol Lett. 2013;5:1163-1164.
- Roh HS, Paek JO, Yu HJ, et al. Solitary cutaneous myofibroma on the sole: an unusual localization. Ann Dermatol. 2012;24:220-222.
- Weedon D, Strutton G, Rubin AI, et al. Weedon’s Skin Pathology. Churchill Livingstone/Elsevier; 2010.
- Mendenhall WM, Zlotecki RA, Scarborough MT. Dermatofibrosarcoma protuberans. Cancer. 2004;101:2503-2508.
- Akay BN, Unlu E, Erdem C, et al. Dermatoscopic findings of atrophic dermatofibrosarcoma protuberans. Dermatol Pract Concept. 2015;5:71-73.
- Ma JE, Wieland CN, Tollefson MM. Dermatomyofibromas arising in children: report of two new cases and review of the literature. Pediatr Dermatol. 2017;34:347-351.
- Tardio JC, Azorin D, Hernandez-Nunez A, et al. Dermatomyofibromas presenting in pediatric patients: clinicopathologic characteristics and differential diagnosis. J Cutan Pathol. 2011;38:967-972.
- Mentzel T, Kutzner H. Dermatomyofibroma: clinicopathologic and immunohistochemical analysis of 56 cases and reappraisal of a rare and distinct cutaneous neoplasm. Am J Dermatopathol. 2009;31:44-49.
- Hugel H. Plaque-like dermal fibromatosis. Hautarzt. 1991;42:223-226.
- Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. WB Saunders Co; 2012.
- Myers DJ, Fillman EP. Dermatofibroma. StatPearls [Internet]. StatPearls Publishing; 2020.
- Dilek N, Yuksel D, Sehitoglu I, et al. Cutaneous leiomyoma in a child: a case report. Oncol Lett. 2013;5:1163-1164.
- Roh HS, Paek JO, Yu HJ, et al. Solitary cutaneous myofibroma on the sole: an unusual localization. Ann Dermatol. 2012;24:220-222.
- Weedon D, Strutton G, Rubin AI, et al. Weedon’s Skin Pathology. Churchill Livingstone/Elsevier; 2010.
- Mendenhall WM, Zlotecki RA, Scarborough MT. Dermatofibrosarcoma protuberans. Cancer. 2004;101:2503-2508.
- Akay BN, Unlu E, Erdem C, et al. Dermatoscopic findings of atrophic dermatofibrosarcoma protuberans. Dermatol Pract Concept. 2015;5:71-73.
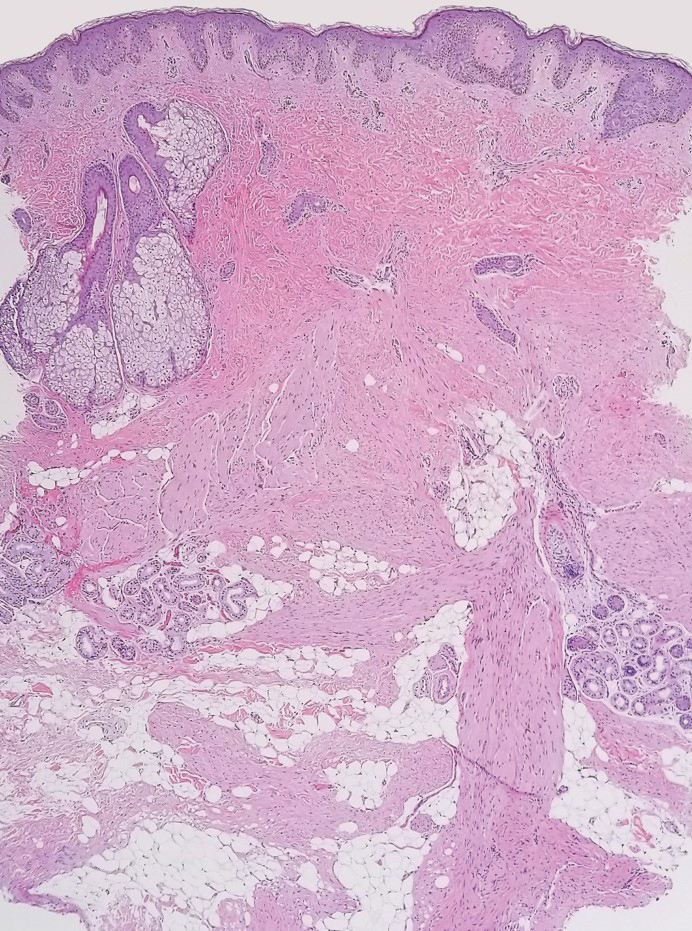

A 12-year-old boy with olive skin presented with a tender subcutaneous nodule on the back of 6 months’ duration. He reported the lesion initially grew rapidly with increasing pain for approximately 3 months with subsequent stabilization in size and modest resolution of his symptoms. Physical examination revealed a solitary, 15-mm, ill-defined, indurated, tender, subcutaneous nodule with subtle overlying hyperpigmentation on the left side of the upper back. Hematoxylin and eosin staining of a 4-mm punch biopsy revealed a nonencapsulated mass of monomorphic eosinophilic spindle cells organized into fascicles arranged predominantly parallel to the skin surface. The mass extended from the mid reticular dermis to the upper subcutis, sparing adnexal structures.
Care via video teleconferencing can be as effective as in-person for some conditions
This was a finding of a new study published in Annals of Internal Medicine involving a review of literature on video teleconferencing (VTC) visits, which was authored by Jordan Albritton, PhD, MPH and his colleagues.
The authors found generally comparable patient outcomes as well as no differences in health care use, patient satisfaction, and quality of life when visits conducted using VTC were compared with usual care.
While VTC may work best for monitoring patients with chronic conditions, it can also be effective for acute care, said Dr. Albritton, who is a research public health analyst at RTI International in Research Triangle Park, N.C., in an interview.
The investigators analyzed 20 randomized controlled trials of at least 50 patients and acceptable risk of bias in which VTC was used either for main or adjunct care delivery. Published from 2013 to 2019, these studies looked at care for diabetes and pain management, as well as some respiratory, neurologic, and cardiovascular conditions. Studies comparing VTC with usual care that did not involve any added in-person care were more likely to favor the VTC group, the investigators found.
“We excluded conditions such as substance use disorders, maternal care, and weight management for which there was sufficient prior evidence of the benefit of VTC,” Dr. Albritton said in an interview. “But I don’t think our results would have been substantially different if we had included these other diseases. We found general evidence in the literature that VTC is effective for a broader range of conditions.”
In some cases, such as if changes in a patient’s condition triggered an automatic virtual visit, the author said he thinks VTC may lead to even greater effectiveness.
“The doctor and patient could figure out on the spot what’s going on and perhaps change the medication,” Dr. Albritton explained.
In general agreement is Julia L. Frydman, MD, assistant professor in the Brookdale Department of Geriatric and Palliative Medicine at Icahn School of Medicine at Mount Sinai in New York, who was not involved in the RTI research.
“Telemedicine has promise across many medical subspecialties, and what we need now are more studies to understand the perspectives of patients, caregivers, and clinicians as well as the impact of telemedicine on health outcomes and healthcare utilization.”
In acknowledgment of their utility, video visits are on the rise in the United States. A 2020 survey found that 22% of patients and 80% of physicians reported having participated in a video visit, three times the rate of the previous year. The authors noted that policy changes enacted to support telehealth strategies during the pandemic are expected to remain in place, and although patients are returning to in-person care, the virtual visit market will likely continue growing.
Increased telemedicine use by older adults
“We’ve seen an exciting expansion of telemedicine use among older adults, and we need to focus on continuing to meet their needs,” Dr. Frydman said.
In a recent study of televisits during the pandemic, Dr. Frydman’s group found a fivefold greater uptake of remote consultations by seniors – from 5% to 25%. Although in-person visits were far more common among older adults.
A specific advantage of video-based over audio-only telehealth, noted Dr. Albritton, is that physicians can directly observe patients in their home environment. Sharing that view is Deepa Iyengar, MBBS/MD,MPH, professor of family medicine at McGovern Medical School at The University of Texas Health Science Center at Houston, where, she said, “the pandemic has put VTC use into overdrive.”
According to Dr. Iyengar, who was not involved in the RTI research, the video component definitely represents value-added over phone calls. “You can pick up visual cues on video that you might not see if the patient came in and you can see what the home environment is like – whether there are a lot of loose rugs on the floor or broken or missing light bulbs,” she said in an interview.
‘VTC is here to stay’
In other parts of the country, doctors are finding virtual care useful – and more common. “VTC is here to stay, for sure – the horse is out of the barn,” said Cheryl L. Wilkes, MD, an internist at Northwestern Medicine and assistant professor of medicine at Northwestern University in Chicago. “The RTI study shows no harm from VTC and also shows it may even improve clinical outcomes.”
Video visits can also save patients high parking fees at clinics and spare the sick or elderly from having to hire caregivers to bring them into the office or from having to walk blocks in dangerous weather conditions, she added. “And I can do a virtual visit on the fly or at night when a relative or caregiver is home from work to be there with the patient.”
In addition to being beneficial for following up with patients with chronic diseases such as hypertension or diabetes, VTC may be able to replace some visits that have traditionally required hands-on care, said Dr. Wilkes.
She said she knows a cardiologist who has refined a process whereby a patient – say, one who may have edema – is asked to perform a maneuver via VTC and then display the result to the doctor: The doctor says, “put your leg up and press on it hard for 10 seconds and then show me what it looks like,” according to Dr. Wilkes.
The key now is to identify the best persons across specialties from neurology to rheumatology to videotape ways they’ve created to help their patients participate virtually in consults traditionally done at the office, Dr. Wilkes noted.
But some conditions will always require palpation and the use of a stethoscope, according Dr. Iyengar.
“If someone has an ulcer, I have to be able to feel it,” she said.
And while some maternity care can be given virtually – for instance, if a mother-to be develops a bad cold – hands-on obstetrical care to check the position and health of the baby obviously has to be done in person. “So VTC is definitely going to be a welcome addition but not a replacement,” Dr. Iyengar said.
Gaps in research on VTC visits
Many questions remain regarding the overall usefulness of VTC visits for certain patient groups, according to the authors.
They highlighted, for example, the dearth of data on subgroups or on underserved and vulnerable populations, with no head-to-head studies identified in their review. In addition, they found no studies examining VTC versus usual care for patients with concurrent conditions or on its effect on health equity and disparities.
“It’s now our job to understand the ongoing barriers to telemedicine access, including the digital divide and the usability of telemedicine platforms, and design interventions that overcome them,” Dr. Frydman said. “At the same time, we need to make sure we’re understanding and respecting the preferences of older adults in terms of how they access health care.”
This study was supported by the Patient-Centered Outcomes Research Institute (PCORI). Dr. Albritton is employed by RTI International, the contractor responsible for conducting the research and developing the manuscript. Several coauthors disclosed support from or contracts with PCORI. One coauthor’s spouse holds stock in private health companies. Dr. Frydman, Dr. Iyengar, and Dr. Wilkes disclosed no competing interests relevant to their comments.
This was a finding of a new study published in Annals of Internal Medicine involving a review of literature on video teleconferencing (VTC) visits, which was authored by Jordan Albritton, PhD, MPH and his colleagues.
The authors found generally comparable patient outcomes as well as no differences in health care use, patient satisfaction, and quality of life when visits conducted using VTC were compared with usual care.
While VTC may work best for monitoring patients with chronic conditions, it can also be effective for acute care, said Dr. Albritton, who is a research public health analyst at RTI International in Research Triangle Park, N.C., in an interview.
The investigators analyzed 20 randomized controlled trials of at least 50 patients and acceptable risk of bias in which VTC was used either for main or adjunct care delivery. Published from 2013 to 2019, these studies looked at care for diabetes and pain management, as well as some respiratory, neurologic, and cardiovascular conditions. Studies comparing VTC with usual care that did not involve any added in-person care were more likely to favor the VTC group, the investigators found.
“We excluded conditions such as substance use disorders, maternal care, and weight management for which there was sufficient prior evidence of the benefit of VTC,” Dr. Albritton said in an interview. “But I don’t think our results would have been substantially different if we had included these other diseases. We found general evidence in the literature that VTC is effective for a broader range of conditions.”
In some cases, such as if changes in a patient’s condition triggered an automatic virtual visit, the author said he thinks VTC may lead to even greater effectiveness.
“The doctor and patient could figure out on the spot what’s going on and perhaps change the medication,” Dr. Albritton explained.
In general agreement is Julia L. Frydman, MD, assistant professor in the Brookdale Department of Geriatric and Palliative Medicine at Icahn School of Medicine at Mount Sinai in New York, who was not involved in the RTI research.
“Telemedicine has promise across many medical subspecialties, and what we need now are more studies to understand the perspectives of patients, caregivers, and clinicians as well as the impact of telemedicine on health outcomes and healthcare utilization.”
In acknowledgment of their utility, video visits are on the rise in the United States. A 2020 survey found that 22% of patients and 80% of physicians reported having participated in a video visit, three times the rate of the previous year. The authors noted that policy changes enacted to support telehealth strategies during the pandemic are expected to remain in place, and although patients are returning to in-person care, the virtual visit market will likely continue growing.
Increased telemedicine use by older adults
“We’ve seen an exciting expansion of telemedicine use among older adults, and we need to focus on continuing to meet their needs,” Dr. Frydman said.
In a recent study of televisits during the pandemic, Dr. Frydman’s group found a fivefold greater uptake of remote consultations by seniors – from 5% to 25%. Although in-person visits were far more common among older adults.
A specific advantage of video-based over audio-only telehealth, noted Dr. Albritton, is that physicians can directly observe patients in their home environment. Sharing that view is Deepa Iyengar, MBBS/MD,MPH, professor of family medicine at McGovern Medical School at The University of Texas Health Science Center at Houston, where, she said, “the pandemic has put VTC use into overdrive.”
According to Dr. Iyengar, who was not involved in the RTI research, the video component definitely represents value-added over phone calls. “You can pick up visual cues on video that you might not see if the patient came in and you can see what the home environment is like – whether there are a lot of loose rugs on the floor or broken or missing light bulbs,” she said in an interview.
‘VTC is here to stay’
In other parts of the country, doctors are finding virtual care useful – and more common. “VTC is here to stay, for sure – the horse is out of the barn,” said Cheryl L. Wilkes, MD, an internist at Northwestern Medicine and assistant professor of medicine at Northwestern University in Chicago. “The RTI study shows no harm from VTC and also shows it may even improve clinical outcomes.”
Video visits can also save patients high parking fees at clinics and spare the sick or elderly from having to hire caregivers to bring them into the office or from having to walk blocks in dangerous weather conditions, she added. “And I can do a virtual visit on the fly or at night when a relative or caregiver is home from work to be there with the patient.”
In addition to being beneficial for following up with patients with chronic diseases such as hypertension or diabetes, VTC may be able to replace some visits that have traditionally required hands-on care, said Dr. Wilkes.
She said she knows a cardiologist who has refined a process whereby a patient – say, one who may have edema – is asked to perform a maneuver via VTC and then display the result to the doctor: The doctor says, “put your leg up and press on it hard for 10 seconds and then show me what it looks like,” according to Dr. Wilkes.
The key now is to identify the best persons across specialties from neurology to rheumatology to videotape ways they’ve created to help their patients participate virtually in consults traditionally done at the office, Dr. Wilkes noted.
But some conditions will always require palpation and the use of a stethoscope, according Dr. Iyengar.
“If someone has an ulcer, I have to be able to feel it,” she said.
And while some maternity care can be given virtually – for instance, if a mother-to be develops a bad cold – hands-on obstetrical care to check the position and health of the baby obviously has to be done in person. “So VTC is definitely going to be a welcome addition but not a replacement,” Dr. Iyengar said.
Gaps in research on VTC visits
Many questions remain regarding the overall usefulness of VTC visits for certain patient groups, according to the authors.
They highlighted, for example, the dearth of data on subgroups or on underserved and vulnerable populations, with no head-to-head studies identified in their review. In addition, they found no studies examining VTC versus usual care for patients with concurrent conditions or on its effect on health equity and disparities.
“It’s now our job to understand the ongoing barriers to telemedicine access, including the digital divide and the usability of telemedicine platforms, and design interventions that overcome them,” Dr. Frydman said. “At the same time, we need to make sure we’re understanding and respecting the preferences of older adults in terms of how they access health care.”
This study was supported by the Patient-Centered Outcomes Research Institute (PCORI). Dr. Albritton is employed by RTI International, the contractor responsible for conducting the research and developing the manuscript. Several coauthors disclosed support from or contracts with PCORI. One coauthor’s spouse holds stock in private health companies. Dr. Frydman, Dr. Iyengar, and Dr. Wilkes disclosed no competing interests relevant to their comments.
This was a finding of a new study published in Annals of Internal Medicine involving a review of literature on video teleconferencing (VTC) visits, which was authored by Jordan Albritton, PhD, MPH and his colleagues.
The authors found generally comparable patient outcomes as well as no differences in health care use, patient satisfaction, and quality of life when visits conducted using VTC were compared with usual care.
While VTC may work best for monitoring patients with chronic conditions, it can also be effective for acute care, said Dr. Albritton, who is a research public health analyst at RTI International in Research Triangle Park, N.C., in an interview.
The investigators analyzed 20 randomized controlled trials of at least 50 patients and acceptable risk of bias in which VTC was used either for main or adjunct care delivery. Published from 2013 to 2019, these studies looked at care for diabetes and pain management, as well as some respiratory, neurologic, and cardiovascular conditions. Studies comparing VTC with usual care that did not involve any added in-person care were more likely to favor the VTC group, the investigators found.
“We excluded conditions such as substance use disorders, maternal care, and weight management for which there was sufficient prior evidence of the benefit of VTC,” Dr. Albritton said in an interview. “But I don’t think our results would have been substantially different if we had included these other diseases. We found general evidence in the literature that VTC is effective for a broader range of conditions.”
In some cases, such as if changes in a patient’s condition triggered an automatic virtual visit, the author said he thinks VTC may lead to even greater effectiveness.
“The doctor and patient could figure out on the spot what’s going on and perhaps change the medication,” Dr. Albritton explained.
In general agreement is Julia L. Frydman, MD, assistant professor in the Brookdale Department of Geriatric and Palliative Medicine at Icahn School of Medicine at Mount Sinai in New York, who was not involved in the RTI research.
“Telemedicine has promise across many medical subspecialties, and what we need now are more studies to understand the perspectives of patients, caregivers, and clinicians as well as the impact of telemedicine on health outcomes and healthcare utilization.”
In acknowledgment of their utility, video visits are on the rise in the United States. A 2020 survey found that 22% of patients and 80% of physicians reported having participated in a video visit, three times the rate of the previous year. The authors noted that policy changes enacted to support telehealth strategies during the pandemic are expected to remain in place, and although patients are returning to in-person care, the virtual visit market will likely continue growing.
Increased telemedicine use by older adults
“We’ve seen an exciting expansion of telemedicine use among older adults, and we need to focus on continuing to meet their needs,” Dr. Frydman said.
In a recent study of televisits during the pandemic, Dr. Frydman’s group found a fivefold greater uptake of remote consultations by seniors – from 5% to 25%. Although in-person visits were far more common among older adults.
A specific advantage of video-based over audio-only telehealth, noted Dr. Albritton, is that physicians can directly observe patients in their home environment. Sharing that view is Deepa Iyengar, MBBS/MD,MPH, professor of family medicine at McGovern Medical School at The University of Texas Health Science Center at Houston, where, she said, “the pandemic has put VTC use into overdrive.”
According to Dr. Iyengar, who was not involved in the RTI research, the video component definitely represents value-added over phone calls. “You can pick up visual cues on video that you might not see if the patient came in and you can see what the home environment is like – whether there are a lot of loose rugs on the floor or broken or missing light bulbs,” she said in an interview.
‘VTC is here to stay’
In other parts of the country, doctors are finding virtual care useful – and more common. “VTC is here to stay, for sure – the horse is out of the barn,” said Cheryl L. Wilkes, MD, an internist at Northwestern Medicine and assistant professor of medicine at Northwestern University in Chicago. “The RTI study shows no harm from VTC and also shows it may even improve clinical outcomes.”
Video visits can also save patients high parking fees at clinics and spare the sick or elderly from having to hire caregivers to bring them into the office or from having to walk blocks in dangerous weather conditions, she added. “And I can do a virtual visit on the fly or at night when a relative or caregiver is home from work to be there with the patient.”
In addition to being beneficial for following up with patients with chronic diseases such as hypertension or diabetes, VTC may be able to replace some visits that have traditionally required hands-on care, said Dr. Wilkes.
She said she knows a cardiologist who has refined a process whereby a patient – say, one who may have edema – is asked to perform a maneuver via VTC and then display the result to the doctor: The doctor says, “put your leg up and press on it hard for 10 seconds and then show me what it looks like,” according to Dr. Wilkes.
The key now is to identify the best persons across specialties from neurology to rheumatology to videotape ways they’ve created to help their patients participate virtually in consults traditionally done at the office, Dr. Wilkes noted.
But some conditions will always require palpation and the use of a stethoscope, according Dr. Iyengar.
“If someone has an ulcer, I have to be able to feel it,” she said.
And while some maternity care can be given virtually – for instance, if a mother-to be develops a bad cold – hands-on obstetrical care to check the position and health of the baby obviously has to be done in person. “So VTC is definitely going to be a welcome addition but not a replacement,” Dr. Iyengar said.
Gaps in research on VTC visits
Many questions remain regarding the overall usefulness of VTC visits for certain patient groups, according to the authors.
They highlighted, for example, the dearth of data on subgroups or on underserved and vulnerable populations, with no head-to-head studies identified in their review. In addition, they found no studies examining VTC versus usual care for patients with concurrent conditions or on its effect on health equity and disparities.
“It’s now our job to understand the ongoing barriers to telemedicine access, including the digital divide and the usability of telemedicine platforms, and design interventions that overcome them,” Dr. Frydman said. “At the same time, we need to make sure we’re understanding and respecting the preferences of older adults in terms of how they access health care.”
This study was supported by the Patient-Centered Outcomes Research Institute (PCORI). Dr. Albritton is employed by RTI International, the contractor responsible for conducting the research and developing the manuscript. Several coauthors disclosed support from or contracts with PCORI. One coauthor’s spouse holds stock in private health companies. Dr. Frydman, Dr. Iyengar, and Dr. Wilkes disclosed no competing interests relevant to their comments.
FROM ANNALS OF INTERNAL MEDICINE
Hemostatic powder noninferior in nonvariceal upper GI bleeds
TC-325, a bentonite-derived hemostatic powder, was not inferior to standard therapy for the endoscopic management of acute nonvariceal upper GI bleeding, according to a new study.
The findings from the study, lead investigator and study author James Y.W. Lau, MD, of the Prince of Wales Hospital in Hong Kong, said in an interview, suggest TC-325 could “be considered one of the primary endoscopic treatments to actively stop nonvariceal bleeding,” particularly in cases when other therapies prove unsuccessful. The study findings were published in Annals of Internal Medicine.
The study team noted that, after they first reported the use of TC-325 in active bleeding from gastroduodenal ulcers in 2011, there have been other studies of its use with acute nonvariceal upper GI bleeding, but to date there has been only two randomized controlled trials of it as a sole endoscopic treatment option for acute nonvariceal upper GI bleeding. To close this research gap, Dr. Lau and researchers enrolled 224 adult patients with acute bleeding from a nonvariceal source on upper GI endoscopy and randomly assigned these patients to receive either TC-325 (n = 111) or standard hemostatic treatment (n = 113). Standard endoscopic bleeding management consisted of contact thermocoagulation using a heater probe or bipolar probe, or hemoclipping with or without previously injected diluted epinephrine.
Success of assigned treatment was defined by the cessation of active bleeding as well as flattening of the protuberance or vessel with a heater or bipolar probe. For the primary outcome of the study, the investigators assessed the rate of bleeding control within 30 days following randomization. Additionally, the researchers compared the treatment groups to identify differences in the failure to control bleeding during the initial endoscopy and recurrent bleeding following hemostasis.
Treatment groups were even in regard to the proportions of patients with bleeding gastroduodenal ulcers (61.3% vs. 60.2%). A smaller proportion of patients in the TC-325 arm had a history of alcohol use (3.0% vs. 9.8%) and current use of NSAIDs (8.1% vs. 20.4%). The group assigned to TC-325 had more bleeding tumors (20.7% vs. 8.8%) and fewer Dieulafoy lesions (5.4% vs. 14.2%), compared with the standard treatment arm. Additionally, patients in the TC-325 group had a higher median Glasgow-Blatchford Score at hospital admission than the standard endoscopy management group (12 vs. 11, respectively; P < .05).
Although a greater proportion of patients assigned TC-325 had bleeding controlled within 30 days of randomization (90.1% vs. 81.4%; risk difference, 8.7 percentage points; 1-sided 95% CI, 0.95 percentage points), the researchers noted that the lower limit of the confidence interval for treatment difference “did not extend beyond the prespecified noninferiority margin of 10 percentage points, indicating that TC-325 is not inferior to standard treatment in the control of bleeding.”
Fewer failures of hemostasis were observed with TC-325 during index endoscopy (2.7% vs. 9.7%; odds ratio, 0.26; 95% CI, 0.07-0.95). After initial endoscopic control, recurrent bleeding was observed in 9 patients in the TC-325 arm and 10 patients in the standard treatment group.
The authors suggested that the low recurrent bleeding rate in the TC-325 arm may reflect enhanced responsiveness in the predominantly Asian study population, a group with lower parietal cell masses and higher rates of Helicobacter pylori infections. In an accompanying editorial published online in Annals of Internal Medicine, Alan N. Barkun, MD, McGill University and McGill University Health Centre in Montreal, and Ali Alali, MB BCh BAO, in the department of medicine at Kuwait University, Kuwait City, noted that “possible additional reasons for the enhanced effectiveness of TC-325 observed in the current trial may be its varied performance in the patients with nonulcer bleeding.”
No difference was found between the treatment strategies in terms of the need for additional interventions within 30 days. The need for further endoscopic treatment was reported in 7.2% of patients in the TC-325 groups versus 8.8% of patients assigned to standard treatment. In addition, further angiography was required in 1.8% and 3.5% of patients, while further surgery was required in 0.9% of patients treated with TC-325 versus none in the standard treatment group. Each group reported 14 deaths.
Dr. Lau noted that the study enrolled Asian patients who were more responsive to proton pump inhibitor therapy, which may limit the generalizability of the findings. “We also included patients with mixed etiologies,” he added. “Studies that focus on specific lesions would further inform our practice, and larger observational studies are required to understand failures with TC-325.”
Based on the study findings, corresponding editorial author Dr. Barkun wrote that “TC-325 can be used alone in nonvariceal upper gastrointestinal bleeding or as rescue therapy but should be reserved for patients with actively bleeding lesions” and suggests the treatment option “is likely one of the most effective modalities in achieving immediate hemostasis.”
The researchers reported no conflicts of interest with the pharmaceutical industry. The editorialists also reported no disclosures of interest.
TC-325, a bentonite-derived hemostatic powder, was not inferior to standard therapy for the endoscopic management of acute nonvariceal upper GI bleeding, according to a new study.
The findings from the study, lead investigator and study author James Y.W. Lau, MD, of the Prince of Wales Hospital in Hong Kong, said in an interview, suggest TC-325 could “be considered one of the primary endoscopic treatments to actively stop nonvariceal bleeding,” particularly in cases when other therapies prove unsuccessful. The study findings were published in Annals of Internal Medicine.
The study team noted that, after they first reported the use of TC-325 in active bleeding from gastroduodenal ulcers in 2011, there have been other studies of its use with acute nonvariceal upper GI bleeding, but to date there has been only two randomized controlled trials of it as a sole endoscopic treatment option for acute nonvariceal upper GI bleeding. To close this research gap, Dr. Lau and researchers enrolled 224 adult patients with acute bleeding from a nonvariceal source on upper GI endoscopy and randomly assigned these patients to receive either TC-325 (n = 111) or standard hemostatic treatment (n = 113). Standard endoscopic bleeding management consisted of contact thermocoagulation using a heater probe or bipolar probe, or hemoclipping with or without previously injected diluted epinephrine.
Success of assigned treatment was defined by the cessation of active bleeding as well as flattening of the protuberance or vessel with a heater or bipolar probe. For the primary outcome of the study, the investigators assessed the rate of bleeding control within 30 days following randomization. Additionally, the researchers compared the treatment groups to identify differences in the failure to control bleeding during the initial endoscopy and recurrent bleeding following hemostasis.
Treatment groups were even in regard to the proportions of patients with bleeding gastroduodenal ulcers (61.3% vs. 60.2%). A smaller proportion of patients in the TC-325 arm had a history of alcohol use (3.0% vs. 9.8%) and current use of NSAIDs (8.1% vs. 20.4%). The group assigned to TC-325 had more bleeding tumors (20.7% vs. 8.8%) and fewer Dieulafoy lesions (5.4% vs. 14.2%), compared with the standard treatment arm. Additionally, patients in the TC-325 group had a higher median Glasgow-Blatchford Score at hospital admission than the standard endoscopy management group (12 vs. 11, respectively; P < .05).
Although a greater proportion of patients assigned TC-325 had bleeding controlled within 30 days of randomization (90.1% vs. 81.4%; risk difference, 8.7 percentage points; 1-sided 95% CI, 0.95 percentage points), the researchers noted that the lower limit of the confidence interval for treatment difference “did not extend beyond the prespecified noninferiority margin of 10 percentage points, indicating that TC-325 is not inferior to standard treatment in the control of bleeding.”
Fewer failures of hemostasis were observed with TC-325 during index endoscopy (2.7% vs. 9.7%; odds ratio, 0.26; 95% CI, 0.07-0.95). After initial endoscopic control, recurrent bleeding was observed in 9 patients in the TC-325 arm and 10 patients in the standard treatment group.
The authors suggested that the low recurrent bleeding rate in the TC-325 arm may reflect enhanced responsiveness in the predominantly Asian study population, a group with lower parietal cell masses and higher rates of Helicobacter pylori infections. In an accompanying editorial published online in Annals of Internal Medicine, Alan N. Barkun, MD, McGill University and McGill University Health Centre in Montreal, and Ali Alali, MB BCh BAO, in the department of medicine at Kuwait University, Kuwait City, noted that “possible additional reasons for the enhanced effectiveness of TC-325 observed in the current trial may be its varied performance in the patients with nonulcer bleeding.”
No difference was found between the treatment strategies in terms of the need for additional interventions within 30 days. The need for further endoscopic treatment was reported in 7.2% of patients in the TC-325 groups versus 8.8% of patients assigned to standard treatment. In addition, further angiography was required in 1.8% and 3.5% of patients, while further surgery was required in 0.9% of patients treated with TC-325 versus none in the standard treatment group. Each group reported 14 deaths.
Dr. Lau noted that the study enrolled Asian patients who were more responsive to proton pump inhibitor therapy, which may limit the generalizability of the findings. “We also included patients with mixed etiologies,” he added. “Studies that focus on specific lesions would further inform our practice, and larger observational studies are required to understand failures with TC-325.”
Based on the study findings, corresponding editorial author Dr. Barkun wrote that “TC-325 can be used alone in nonvariceal upper gastrointestinal bleeding or as rescue therapy but should be reserved for patients with actively bleeding lesions” and suggests the treatment option “is likely one of the most effective modalities in achieving immediate hemostasis.”
The researchers reported no conflicts of interest with the pharmaceutical industry. The editorialists also reported no disclosures of interest.
TC-325, a bentonite-derived hemostatic powder, was not inferior to standard therapy for the endoscopic management of acute nonvariceal upper GI bleeding, according to a new study.
The findings from the study, lead investigator and study author James Y.W. Lau, MD, of the Prince of Wales Hospital in Hong Kong, said in an interview, suggest TC-325 could “be considered one of the primary endoscopic treatments to actively stop nonvariceal bleeding,” particularly in cases when other therapies prove unsuccessful. The study findings were published in Annals of Internal Medicine.
The study team noted that, after they first reported the use of TC-325 in active bleeding from gastroduodenal ulcers in 2011, there have been other studies of its use with acute nonvariceal upper GI bleeding, but to date there has been only two randomized controlled trials of it as a sole endoscopic treatment option for acute nonvariceal upper GI bleeding. To close this research gap, Dr. Lau and researchers enrolled 224 adult patients with acute bleeding from a nonvariceal source on upper GI endoscopy and randomly assigned these patients to receive either TC-325 (n = 111) or standard hemostatic treatment (n = 113). Standard endoscopic bleeding management consisted of contact thermocoagulation using a heater probe or bipolar probe, or hemoclipping with or without previously injected diluted epinephrine.
Success of assigned treatment was defined by the cessation of active bleeding as well as flattening of the protuberance or vessel with a heater or bipolar probe. For the primary outcome of the study, the investigators assessed the rate of bleeding control within 30 days following randomization. Additionally, the researchers compared the treatment groups to identify differences in the failure to control bleeding during the initial endoscopy and recurrent bleeding following hemostasis.
Treatment groups were even in regard to the proportions of patients with bleeding gastroduodenal ulcers (61.3% vs. 60.2%). A smaller proportion of patients in the TC-325 arm had a history of alcohol use (3.0% vs. 9.8%) and current use of NSAIDs (8.1% vs. 20.4%). The group assigned to TC-325 had more bleeding tumors (20.7% vs. 8.8%) and fewer Dieulafoy lesions (5.4% vs. 14.2%), compared with the standard treatment arm. Additionally, patients in the TC-325 group had a higher median Glasgow-Blatchford Score at hospital admission than the standard endoscopy management group (12 vs. 11, respectively; P < .05).
Although a greater proportion of patients assigned TC-325 had bleeding controlled within 30 days of randomization (90.1% vs. 81.4%; risk difference, 8.7 percentage points; 1-sided 95% CI, 0.95 percentage points), the researchers noted that the lower limit of the confidence interval for treatment difference “did not extend beyond the prespecified noninferiority margin of 10 percentage points, indicating that TC-325 is not inferior to standard treatment in the control of bleeding.”
Fewer failures of hemostasis were observed with TC-325 during index endoscopy (2.7% vs. 9.7%; odds ratio, 0.26; 95% CI, 0.07-0.95). After initial endoscopic control, recurrent bleeding was observed in 9 patients in the TC-325 arm and 10 patients in the standard treatment group.
The authors suggested that the low recurrent bleeding rate in the TC-325 arm may reflect enhanced responsiveness in the predominantly Asian study population, a group with lower parietal cell masses and higher rates of Helicobacter pylori infections. In an accompanying editorial published online in Annals of Internal Medicine, Alan N. Barkun, MD, McGill University and McGill University Health Centre in Montreal, and Ali Alali, MB BCh BAO, in the department of medicine at Kuwait University, Kuwait City, noted that “possible additional reasons for the enhanced effectiveness of TC-325 observed in the current trial may be its varied performance in the patients with nonulcer bleeding.”
No difference was found between the treatment strategies in terms of the need for additional interventions within 30 days. The need for further endoscopic treatment was reported in 7.2% of patients in the TC-325 groups versus 8.8% of patients assigned to standard treatment. In addition, further angiography was required in 1.8% and 3.5% of patients, while further surgery was required in 0.9% of patients treated with TC-325 versus none in the standard treatment group. Each group reported 14 deaths.
Dr. Lau noted that the study enrolled Asian patients who were more responsive to proton pump inhibitor therapy, which may limit the generalizability of the findings. “We also included patients with mixed etiologies,” he added. “Studies that focus on specific lesions would further inform our practice, and larger observational studies are required to understand failures with TC-325.”
Based on the study findings, corresponding editorial author Dr. Barkun wrote that “TC-325 can be used alone in nonvariceal upper gastrointestinal bleeding or as rescue therapy but should be reserved for patients with actively bleeding lesions” and suggests the treatment option “is likely one of the most effective modalities in achieving immediate hemostasis.”
The researchers reported no conflicts of interest with the pharmaceutical industry. The editorialists also reported no disclosures of interest.
FROM ANNALS OF INTERNAL MEDICINE
The future of training: AGA EndoscopyNow Fellows Forum recap
Introduction
The virtual space has created new opportunities for gastroenterology fellows, but direct conversations about education and career development on the national level have been limited. On Oct. 16, 2021, the American Gastroenterological Association and EndoscopyNow hosted an online Fellows Forum titled “Navigating New Frontiers of Training in Gastroenterology.” Close to 100 fellows attended and had the chance to listen to discussions from a national panel of faculty with expertise in medical education, ask candid questions, and share experiences in breakout rooms specific to their year of training. Reading materials were also provided, which are cited throughout this article. What follows is a rundown of the discussion and points of particular interest for fellows.
What do fellows value?
Dr. Laura Raffals kicked off the event by asking fellows to create word clouds related to their challenges (“Balance” was the most common answer) and joys (“Family”). These answers underscore that, when faced with pressures to be 100% at work and home, it is human connection, particularly family, that sustains us. Fellows, however, worried that spending time with family conflicted with spending time on GI training and that they would be perceived as “that person who always leaves early.”1
Attendees discussed that “there are only 168 hours in a week,” (time is a zero-sum game), and it is important to be self-aware and honest about one’s personal values and commit the commensurate time and energy to those values. Consider personal development exercises.2 Faculty have a crucial role in coaching fellows on time management based on personal values.3
Has COVID-19 reduced fellows’ endoscopic skills?
One brave attendee asked: Is this generation of fellows “weaker” because of limited scoping during the pandemic? Faculty discussed that, even prepandemic, it was “not all about quantity; the quality of exposure matters just as much.” From their perspective, prepared, goal-directed, and helpful fellows would maximize learning during endoscopy blocks (see below). Lawrence Schiller, MD, providing the long view, reassured fellows that with a proactive attitude it all evens out in the end.
Fellows reflected that, although social isolation and burnout were rampant, some individuals stepped up to do extra work, supported colleagues with personal or family health issues, and scoped COVID-positive patients if others could not. In future years, the pandemic will be seen as a case study for those in leadership positions. The decisions that health systems, administrators, and providers made will be remembered, as well as how algorithms for “practice as usual” changed.4
What fellows can do to maximize endoscopic learning (attendings’ perspective):
- Know the patient before the case. Prior endoscopy reports, patient comorbidities, and medical history including details like anticoagulation use or issues with anesthesia.
- Help the work flow (and reduce the attending’s stress level). Consent patients and complete preprocedure paperwork if possible.
- Come into the scope block with a plan. Example: “I want to get to the cecum. The attending can withdraw, and I will take out polyps we find.”
- Ask about decision-making. Example: “Why did you choose to place a clip over that polypectomy site?” or “Why did you choose that instrument and not the other?”
- Give feedback on problematic behavior. Attendings that treat fellows like burdens and undermine fellow scope time should be reported. Fellows may be concerned about being perceived as a “troublemaker,” but discussing these situations with program directors is a civic duty.
How can we improve diversity?
We cannot wait for, and must instead proactively recruit, diverse trainees, as well as create inclusive environments. Mentorship is key. However, recent work showing imbalances in gender of mentor-mentees and extra pressure on women mentors raises concerns about sustainability.5,6 The panel suggested that interested fellows could engage students earlier in the pipeline, participate in community awareness and exposure programs, and dedicate education time to health equity.7 Fellows raised concerns about barriers for international medical graduates, which would require institutional and federal policy changes would to implement change.
How can fellows develop better practice patterns?
Sri Komanduri, MD, focused on complex endoscopic cases.8 Having live video, using polls, and listening to other attendings comment on cases was illuminating and sometimes humbling. The panel discussed that simulation labs could strongly enhance endoscopic skill training, but if unavailable, companies are often willing to sponsor events for teaching purposes.9 If training on specific topics is not offered at an institution, regional weekend courses are also an option.
Raman Muthusamy, MD, MS, discussed his philosophy towards endoscopic complications: be prepared and follow your instinct if something feels off. He and Dr. Ikuo Hirano emphasized the importance of following up with patients after a complication. The panel also suggested that fellows can build quality improvement experience by contributing to GI morbidity and mortality conferences or start them if not already offered.
Where is the future headed?
Amrita Sethi, MD, outlined the trend towards virtual platforms and getting the global GI community involved in education efforts. She pointed out the need for a gold standard on assessing competency in endoscopy. From a practice perspective, implementation of telemedicine in GI merits further study, as so far this technology has been attractive to providers and patients alike. Todd Baron, MD, stressed that newer technologies, including artificial intelligence, will not replace the endoscopist but may reduce the need for screening procedures and instead increase demand for specific diagnostic and therapeutic procedures. He used the examples of therapeutic applications of endoscopic ultrasound and the development of single-use duodenoscopes.
Concerns about transitioning from training to independent practice
During the third-year breakout session, fellows discussed anxieties about starting practice and living up to expectations: “What if it’s my first week and there’s something I can’t do?” Faculty recommended getting to know colleagues at a new institution, being confident in your training, and staying engaged with your own complications.10 Fellows described the surprising amount of time and energy they dedicated to the job search and got counseling from Dr. Schiller, who recommended defining what “success” and “satisfaction” look like (again, defining one’s values). He recommended that, for fellows looking at private practice positions, one should ask: How much autonomy do I want? How much business risk am I willing to accept? Fellows need more formal education on practice management and the “business side” of gastroenterology.11
Conclusions
The 2021 AGA EndoscopyNow forum was unique in its discussion of issues impacting GI fellows. The forum revealed that worries about personal well-being, training quality, and future career prospects have affected fellows everywhere: you are not alone. Presentations and lively conversation between seasoned faculty who reflected on career development, education, and medical management demonstrate the importance of seeking advice from colleagues and mentorship. Based on this event, future sessions with conversations between faculty and fellows to assess needs and set priorities for directions in training would be welcome.
Dr. Liu is a gastroenterology fellow, Northwestern University, Chicago. The author has no conflicts of interest to disclose.
References
1. Katzka DA and Proctor DD. Gastroenterology. 2009;136(4):1147-8.
2. Sull D and Houlder D. Do Your Commitments Match Your Convictions? Harv Bus Rev. 2005 Jan 1. https://hbr.org/2005/01/do-your-commitments-match-your-convictions.
3. Keswani RN et al. Gastroenterology. 2020;159(1):26-9.
4. Sethi A et al. Clin Gastroenterol Hepatol. 2020;18(8):1673-81.
5. Rabinowitz LG et al. Gastrointest Endosc. 2021;93(5):1047-56.e5.
6. Rabinowitz LG et al. Gastrointest Endosc. 2020;91(1):155-61.
7. Lee-Allen J, Shah BJ. Gastroenterology. 2021;160(6):1924-8.
8. Richter JM et al. Am J Gastroenterol. 2016;111(3):348-52.
9. Muthusamy VR and Komanduri S. Clin Gastroenterol Hepatol. 2019 Mar;17(4):580-3.
10. Liu H and Boyatzis RE. Front Psychol. 2021. doi: 10.3389/fpsyg.2021.685829.
11. Amann ST et al. “Words” to practice by: A guide to understand the business vernacular of a healthy practice. https://webfiles.gi.org/links/pm/TheHealthOfMyPracticeToolboxPMCommitteeToolbox.pdf.
Introduction
The virtual space has created new opportunities for gastroenterology fellows, but direct conversations about education and career development on the national level have been limited. On Oct. 16, 2021, the American Gastroenterological Association and EndoscopyNow hosted an online Fellows Forum titled “Navigating New Frontiers of Training in Gastroenterology.” Close to 100 fellows attended and had the chance to listen to discussions from a national panel of faculty with expertise in medical education, ask candid questions, and share experiences in breakout rooms specific to their year of training. Reading materials were also provided, which are cited throughout this article. What follows is a rundown of the discussion and points of particular interest for fellows.
What do fellows value?
Dr. Laura Raffals kicked off the event by asking fellows to create word clouds related to their challenges (“Balance” was the most common answer) and joys (“Family”). These answers underscore that, when faced with pressures to be 100% at work and home, it is human connection, particularly family, that sustains us. Fellows, however, worried that spending time with family conflicted with spending time on GI training and that they would be perceived as “that person who always leaves early.”1
Attendees discussed that “there are only 168 hours in a week,” (time is a zero-sum game), and it is important to be self-aware and honest about one’s personal values and commit the commensurate time and energy to those values. Consider personal development exercises.2 Faculty have a crucial role in coaching fellows on time management based on personal values.3
Has COVID-19 reduced fellows’ endoscopic skills?
One brave attendee asked: Is this generation of fellows “weaker” because of limited scoping during the pandemic? Faculty discussed that, even prepandemic, it was “not all about quantity; the quality of exposure matters just as much.” From their perspective, prepared, goal-directed, and helpful fellows would maximize learning during endoscopy blocks (see below). Lawrence Schiller, MD, providing the long view, reassured fellows that with a proactive attitude it all evens out in the end.
Fellows reflected that, although social isolation and burnout were rampant, some individuals stepped up to do extra work, supported colleagues with personal or family health issues, and scoped COVID-positive patients if others could not. In future years, the pandemic will be seen as a case study for those in leadership positions. The decisions that health systems, administrators, and providers made will be remembered, as well as how algorithms for “practice as usual” changed.4
What fellows can do to maximize endoscopic learning (attendings’ perspective):
- Know the patient before the case. Prior endoscopy reports, patient comorbidities, and medical history including details like anticoagulation use or issues with anesthesia.
- Help the work flow (and reduce the attending’s stress level). Consent patients and complete preprocedure paperwork if possible.
- Come into the scope block with a plan. Example: “I want to get to the cecum. The attending can withdraw, and I will take out polyps we find.”
- Ask about decision-making. Example: “Why did you choose to place a clip over that polypectomy site?” or “Why did you choose that instrument and not the other?”
- Give feedback on problematic behavior. Attendings that treat fellows like burdens and undermine fellow scope time should be reported. Fellows may be concerned about being perceived as a “troublemaker,” but discussing these situations with program directors is a civic duty.
How can we improve diversity?
We cannot wait for, and must instead proactively recruit, diverse trainees, as well as create inclusive environments. Mentorship is key. However, recent work showing imbalances in gender of mentor-mentees and extra pressure on women mentors raises concerns about sustainability.5,6 The panel suggested that interested fellows could engage students earlier in the pipeline, participate in community awareness and exposure programs, and dedicate education time to health equity.7 Fellows raised concerns about barriers for international medical graduates, which would require institutional and federal policy changes would to implement change.
How can fellows develop better practice patterns?
Sri Komanduri, MD, focused on complex endoscopic cases.8 Having live video, using polls, and listening to other attendings comment on cases was illuminating and sometimes humbling. The panel discussed that simulation labs could strongly enhance endoscopic skill training, but if unavailable, companies are often willing to sponsor events for teaching purposes.9 If training on specific topics is not offered at an institution, regional weekend courses are also an option.
Raman Muthusamy, MD, MS, discussed his philosophy towards endoscopic complications: be prepared and follow your instinct if something feels off. He and Dr. Ikuo Hirano emphasized the importance of following up with patients after a complication. The panel also suggested that fellows can build quality improvement experience by contributing to GI morbidity and mortality conferences or start them if not already offered.
Where is the future headed?
Amrita Sethi, MD, outlined the trend towards virtual platforms and getting the global GI community involved in education efforts. She pointed out the need for a gold standard on assessing competency in endoscopy. From a practice perspective, implementation of telemedicine in GI merits further study, as so far this technology has been attractive to providers and patients alike. Todd Baron, MD, stressed that newer technologies, including artificial intelligence, will not replace the endoscopist but may reduce the need for screening procedures and instead increase demand for specific diagnostic and therapeutic procedures. He used the examples of therapeutic applications of endoscopic ultrasound and the development of single-use duodenoscopes.
Concerns about transitioning from training to independent practice
During the third-year breakout session, fellows discussed anxieties about starting practice and living up to expectations: “What if it’s my first week and there’s something I can’t do?” Faculty recommended getting to know colleagues at a new institution, being confident in your training, and staying engaged with your own complications.10 Fellows described the surprising amount of time and energy they dedicated to the job search and got counseling from Dr. Schiller, who recommended defining what “success” and “satisfaction” look like (again, defining one’s values). He recommended that, for fellows looking at private practice positions, one should ask: How much autonomy do I want? How much business risk am I willing to accept? Fellows need more formal education on practice management and the “business side” of gastroenterology.11
Conclusions
The 2021 AGA EndoscopyNow forum was unique in its discussion of issues impacting GI fellows. The forum revealed that worries about personal well-being, training quality, and future career prospects have affected fellows everywhere: you are not alone. Presentations and lively conversation between seasoned faculty who reflected on career development, education, and medical management demonstrate the importance of seeking advice from colleagues and mentorship. Based on this event, future sessions with conversations between faculty and fellows to assess needs and set priorities for directions in training would be welcome.
Dr. Liu is a gastroenterology fellow, Northwestern University, Chicago. The author has no conflicts of interest to disclose.
References
1. Katzka DA and Proctor DD. Gastroenterology. 2009;136(4):1147-8.
2. Sull D and Houlder D. Do Your Commitments Match Your Convictions? Harv Bus Rev. 2005 Jan 1. https://hbr.org/2005/01/do-your-commitments-match-your-convictions.
3. Keswani RN et al. Gastroenterology. 2020;159(1):26-9.
4. Sethi A et al. Clin Gastroenterol Hepatol. 2020;18(8):1673-81.
5. Rabinowitz LG et al. Gastrointest Endosc. 2021;93(5):1047-56.e5.
6. Rabinowitz LG et al. Gastrointest Endosc. 2020;91(1):155-61.
7. Lee-Allen J, Shah BJ. Gastroenterology. 2021;160(6):1924-8.
8. Richter JM et al. Am J Gastroenterol. 2016;111(3):348-52.
9. Muthusamy VR and Komanduri S. Clin Gastroenterol Hepatol. 2019 Mar;17(4):580-3.
10. Liu H and Boyatzis RE. Front Psychol. 2021. doi: 10.3389/fpsyg.2021.685829.
11. Amann ST et al. “Words” to practice by: A guide to understand the business vernacular of a healthy practice. https://webfiles.gi.org/links/pm/TheHealthOfMyPracticeToolboxPMCommitteeToolbox.pdf.
Introduction
The virtual space has created new opportunities for gastroenterology fellows, but direct conversations about education and career development on the national level have been limited. On Oct. 16, 2021, the American Gastroenterological Association and EndoscopyNow hosted an online Fellows Forum titled “Navigating New Frontiers of Training in Gastroenterology.” Close to 100 fellows attended and had the chance to listen to discussions from a national panel of faculty with expertise in medical education, ask candid questions, and share experiences in breakout rooms specific to their year of training. Reading materials were also provided, which are cited throughout this article. What follows is a rundown of the discussion and points of particular interest for fellows.
What do fellows value?
Dr. Laura Raffals kicked off the event by asking fellows to create word clouds related to their challenges (“Balance” was the most common answer) and joys (“Family”). These answers underscore that, when faced with pressures to be 100% at work and home, it is human connection, particularly family, that sustains us. Fellows, however, worried that spending time with family conflicted with spending time on GI training and that they would be perceived as “that person who always leaves early.”1
Attendees discussed that “there are only 168 hours in a week,” (time is a zero-sum game), and it is important to be self-aware and honest about one’s personal values and commit the commensurate time and energy to those values. Consider personal development exercises.2 Faculty have a crucial role in coaching fellows on time management based on personal values.3
Has COVID-19 reduced fellows’ endoscopic skills?
One brave attendee asked: Is this generation of fellows “weaker” because of limited scoping during the pandemic? Faculty discussed that, even prepandemic, it was “not all about quantity; the quality of exposure matters just as much.” From their perspective, prepared, goal-directed, and helpful fellows would maximize learning during endoscopy blocks (see below). Lawrence Schiller, MD, providing the long view, reassured fellows that with a proactive attitude it all evens out in the end.
Fellows reflected that, although social isolation and burnout were rampant, some individuals stepped up to do extra work, supported colleagues with personal or family health issues, and scoped COVID-positive patients if others could not. In future years, the pandemic will be seen as a case study for those in leadership positions. The decisions that health systems, administrators, and providers made will be remembered, as well as how algorithms for “practice as usual” changed.4
What fellows can do to maximize endoscopic learning (attendings’ perspective):
- Know the patient before the case. Prior endoscopy reports, patient comorbidities, and medical history including details like anticoagulation use or issues with anesthesia.
- Help the work flow (and reduce the attending’s stress level). Consent patients and complete preprocedure paperwork if possible.
- Come into the scope block with a plan. Example: “I want to get to the cecum. The attending can withdraw, and I will take out polyps we find.”
- Ask about decision-making. Example: “Why did you choose to place a clip over that polypectomy site?” or “Why did you choose that instrument and not the other?”
- Give feedback on problematic behavior. Attendings that treat fellows like burdens and undermine fellow scope time should be reported. Fellows may be concerned about being perceived as a “troublemaker,” but discussing these situations with program directors is a civic duty.
How can we improve diversity?
We cannot wait for, and must instead proactively recruit, diverse trainees, as well as create inclusive environments. Mentorship is key. However, recent work showing imbalances in gender of mentor-mentees and extra pressure on women mentors raises concerns about sustainability.5,6 The panel suggested that interested fellows could engage students earlier in the pipeline, participate in community awareness and exposure programs, and dedicate education time to health equity.7 Fellows raised concerns about barriers for international medical graduates, which would require institutional and federal policy changes would to implement change.
How can fellows develop better practice patterns?
Sri Komanduri, MD, focused on complex endoscopic cases.8 Having live video, using polls, and listening to other attendings comment on cases was illuminating and sometimes humbling. The panel discussed that simulation labs could strongly enhance endoscopic skill training, but if unavailable, companies are often willing to sponsor events for teaching purposes.9 If training on specific topics is not offered at an institution, regional weekend courses are also an option.
Raman Muthusamy, MD, MS, discussed his philosophy towards endoscopic complications: be prepared and follow your instinct if something feels off. He and Dr. Ikuo Hirano emphasized the importance of following up with patients after a complication. The panel also suggested that fellows can build quality improvement experience by contributing to GI morbidity and mortality conferences or start them if not already offered.
Where is the future headed?
Amrita Sethi, MD, outlined the trend towards virtual platforms and getting the global GI community involved in education efforts. She pointed out the need for a gold standard on assessing competency in endoscopy. From a practice perspective, implementation of telemedicine in GI merits further study, as so far this technology has been attractive to providers and patients alike. Todd Baron, MD, stressed that newer technologies, including artificial intelligence, will not replace the endoscopist but may reduce the need for screening procedures and instead increase demand for specific diagnostic and therapeutic procedures. He used the examples of therapeutic applications of endoscopic ultrasound and the development of single-use duodenoscopes.
Concerns about transitioning from training to independent practice
During the third-year breakout session, fellows discussed anxieties about starting practice and living up to expectations: “What if it’s my first week and there’s something I can’t do?” Faculty recommended getting to know colleagues at a new institution, being confident in your training, and staying engaged with your own complications.10 Fellows described the surprising amount of time and energy they dedicated to the job search and got counseling from Dr. Schiller, who recommended defining what “success” and “satisfaction” look like (again, defining one’s values). He recommended that, for fellows looking at private practice positions, one should ask: How much autonomy do I want? How much business risk am I willing to accept? Fellows need more formal education on practice management and the “business side” of gastroenterology.11
Conclusions
The 2021 AGA EndoscopyNow forum was unique in its discussion of issues impacting GI fellows. The forum revealed that worries about personal well-being, training quality, and future career prospects have affected fellows everywhere: you are not alone. Presentations and lively conversation between seasoned faculty who reflected on career development, education, and medical management demonstrate the importance of seeking advice from colleagues and mentorship. Based on this event, future sessions with conversations between faculty and fellows to assess needs and set priorities for directions in training would be welcome.
Dr. Liu is a gastroenterology fellow, Northwestern University, Chicago. The author has no conflicts of interest to disclose.
References
1. Katzka DA and Proctor DD. Gastroenterology. 2009;136(4):1147-8.
2. Sull D and Houlder D. Do Your Commitments Match Your Convictions? Harv Bus Rev. 2005 Jan 1. https://hbr.org/2005/01/do-your-commitments-match-your-convictions.
3. Keswani RN et al. Gastroenterology. 2020;159(1):26-9.
4. Sethi A et al. Clin Gastroenterol Hepatol. 2020;18(8):1673-81.
5. Rabinowitz LG et al. Gastrointest Endosc. 2021;93(5):1047-56.e5.
6. Rabinowitz LG et al. Gastrointest Endosc. 2020;91(1):155-61.
7. Lee-Allen J, Shah BJ. Gastroenterology. 2021;160(6):1924-8.
8. Richter JM et al. Am J Gastroenterol. 2016;111(3):348-52.
9. Muthusamy VR and Komanduri S. Clin Gastroenterol Hepatol. 2019 Mar;17(4):580-3.
10. Liu H and Boyatzis RE. Front Psychol. 2021. doi: 10.3389/fpsyg.2021.685829.
11. Amann ST et al. “Words” to practice by: A guide to understand the business vernacular of a healthy practice. https://webfiles.gi.org/links/pm/TheHealthOfMyPracticeToolboxPMCommitteeToolbox.pdf.
An expensive lesson
In mid-July my son strained his neck working out at the gym.
It was an obvious generic muscle pull. I told him to take some ibuprofen and use a heating pad. My wife, a nurse, told him the same thing.
Regrettably, while my medical training (hopefully) counts for something with my patients, it doesn’t mean much to my kids. The unqualified opinions of their friends and Google are far more worthwhile, convincing him he had any number of serious injuries.
As a result, while we were at work he went to the emergency department to get checked out. He was evaluated by one of my colleagues who did x-rays and a cervical spine CT. (I figure the last one was because my son kept reminding them I was a doctor). After all the results were in, the ED physician told him he had a muscle strain, and to take ibuprofen and use a heating pad.
Big surprise, huh? I’m sure he was shocked to find out that his old man knew what he was doing. Of course, I didn’t order any tests so the ED doc tops me for that in my son’s mind.
But kids not believing their parents is nothing new, and I can’t claim innocence either from what I remember of being a teenager.
Fast-forward to today. From what I can see, the total bills for his little adventure in modern medicine were around $4,000-$5,000. Granted, I’m well aware that what gets charged has no relationship to what’s actually going to be collected but I’m not going to write about modern medical charges or collections or even defensive medicine. I understand all those, and certainly don’t fault my ED colleague for how he handled it.
Reassurance isn’t cheap, though. When it’s all over, our out-of-pocket share will be roughly $1,000, which we certainly hadn’t planned for in the usually money-tight months of December and January.
That’s a lot of money for ibuprofen and a heating pad (we had both at home, and they’re around $20 total at Target, anyway).
There’s certainly no shortage of research on unnecessary ED visits for minor things, but to me this is a classic example of it. Beyond just the financial cost (which, admittedly, I’m pretty irritated with him about) he tied up a bed and ED staff that someone in more dire circumstances may have needed.
His injury could have been handled at an urgent care, or, even better, just by staying home, listening to us, and using ibuprofen and a heating pad.
, and clarify what constitutes an emergency in the first place. There’s no shortage of urgent cares and other walk-in clinics that are there specifically to handle such things during daylight hours, if they need to be seen at all.
Of course, I can’t change the results of Google searches, or the age-old teenage belief that parents are morons.
But he is going to learn about what constitutes an emergency, and what else that $1,000 could have been used for.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In mid-July my son strained his neck working out at the gym.
It was an obvious generic muscle pull. I told him to take some ibuprofen and use a heating pad. My wife, a nurse, told him the same thing.
Regrettably, while my medical training (hopefully) counts for something with my patients, it doesn’t mean much to my kids. The unqualified opinions of their friends and Google are far more worthwhile, convincing him he had any number of serious injuries.
As a result, while we were at work he went to the emergency department to get checked out. He was evaluated by one of my colleagues who did x-rays and a cervical spine CT. (I figure the last one was because my son kept reminding them I was a doctor). After all the results were in, the ED physician told him he had a muscle strain, and to take ibuprofen and use a heating pad.
Big surprise, huh? I’m sure he was shocked to find out that his old man knew what he was doing. Of course, I didn’t order any tests so the ED doc tops me for that in my son’s mind.
But kids not believing their parents is nothing new, and I can’t claim innocence either from what I remember of being a teenager.
Fast-forward to today. From what I can see, the total bills for his little adventure in modern medicine were around $4,000-$5,000. Granted, I’m well aware that what gets charged has no relationship to what’s actually going to be collected but I’m not going to write about modern medical charges or collections or even defensive medicine. I understand all those, and certainly don’t fault my ED colleague for how he handled it.
Reassurance isn’t cheap, though. When it’s all over, our out-of-pocket share will be roughly $1,000, which we certainly hadn’t planned for in the usually money-tight months of December and January.
That’s a lot of money for ibuprofen and a heating pad (we had both at home, and they’re around $20 total at Target, anyway).
There’s certainly no shortage of research on unnecessary ED visits for minor things, but to me this is a classic example of it. Beyond just the financial cost (which, admittedly, I’m pretty irritated with him about) he tied up a bed and ED staff that someone in more dire circumstances may have needed.
His injury could have been handled at an urgent care, or, even better, just by staying home, listening to us, and using ibuprofen and a heating pad.
, and clarify what constitutes an emergency in the first place. There’s no shortage of urgent cares and other walk-in clinics that are there specifically to handle such things during daylight hours, if they need to be seen at all.
Of course, I can’t change the results of Google searches, or the age-old teenage belief that parents are morons.
But he is going to learn about what constitutes an emergency, and what else that $1,000 could have been used for.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In mid-July my son strained his neck working out at the gym.
It was an obvious generic muscle pull. I told him to take some ibuprofen and use a heating pad. My wife, a nurse, told him the same thing.
Regrettably, while my medical training (hopefully) counts for something with my patients, it doesn’t mean much to my kids. The unqualified opinions of their friends and Google are far more worthwhile, convincing him he had any number of serious injuries.
As a result, while we were at work he went to the emergency department to get checked out. He was evaluated by one of my colleagues who did x-rays and a cervical spine CT. (I figure the last one was because my son kept reminding them I was a doctor). After all the results were in, the ED physician told him he had a muscle strain, and to take ibuprofen and use a heating pad.
Big surprise, huh? I’m sure he was shocked to find out that his old man knew what he was doing. Of course, I didn’t order any tests so the ED doc tops me for that in my son’s mind.
But kids not believing their parents is nothing new, and I can’t claim innocence either from what I remember of being a teenager.
Fast-forward to today. From what I can see, the total bills for his little adventure in modern medicine were around $4,000-$5,000. Granted, I’m well aware that what gets charged has no relationship to what’s actually going to be collected but I’m not going to write about modern medical charges or collections or even defensive medicine. I understand all those, and certainly don’t fault my ED colleague for how he handled it.
Reassurance isn’t cheap, though. When it’s all over, our out-of-pocket share will be roughly $1,000, which we certainly hadn’t planned for in the usually money-tight months of December and January.
That’s a lot of money for ibuprofen and a heating pad (we had both at home, and they’re around $20 total at Target, anyway).
There’s certainly no shortage of research on unnecessary ED visits for minor things, but to me this is a classic example of it. Beyond just the financial cost (which, admittedly, I’m pretty irritated with him about) he tied up a bed and ED staff that someone in more dire circumstances may have needed.
His injury could have been handled at an urgent care, or, even better, just by staying home, listening to us, and using ibuprofen and a heating pad.
, and clarify what constitutes an emergency in the first place. There’s no shortage of urgent cares and other walk-in clinics that are there specifically to handle such things during daylight hours, if they need to be seen at all.
Of course, I can’t change the results of Google searches, or the age-old teenage belief that parents are morons.
But he is going to learn about what constitutes an emergency, and what else that $1,000 could have been used for.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Characterizing Counterfeit Dermatologic Devices Sold on Popular E-commerce Websites
To the Editor:
Approved medical devices on the market are substantial capital investments for practitioners. E-commerce websites, such as Alibaba.com (https://www.alibaba.com/) and DHgate.com (https://www.dhgate.com/), sell sham medical devices at a fraction of the cost of authentic products, with sellers often echoing the same treatment claims as legitimate devices that have been cleared by the US Food and Drug Administration (FDA).
In dermatology, devices claiming to perform cryolipolysis, laser skin resurfacing, radiofrequency skin tightening, and more exist on e-commerce websites. These counterfeit medical devices might differ from legitimate devices in ways that affect patient safety and treatment efficacy.1,2 The degree of difference between counterfeit and legitimate devices remains unknown, and potential harm from so-called knockoff devices needs to be critically examined by providers.
In this exploratory study, we characterize counterfeit listings of devices commonly used in dermatology. Using the trademark name of devices as the key terms, we searched Alibaba.com and DHgate.com for listings of counterfeit products. We recorded the total number of listings; the listing name, catalog number, and unit price; and claims of FDA certification. Characteristics of counterfeit listings were summarized using standard descriptive statistics in Microsoft Excel. Continuous variables were summarized with means and ranges.
Six medical devices that had been cleared by the FDA between 2002 and 2012 for use in dermatology were explored, including systems for picosecond and fractionated lasers, monopolar and bipolar radiofrequency skin tightening, cryolipolysis, and nonablative radiofrequency skin resurfacing. Our search of these 6 representative dermatologic devices revealed 47,055 counterfeit product listings on Alibaba.com and DHgate.com. Upon searching these popular e-commerce websites using the device name as the search term, the number of listings varied considerably between the 2 e-commerce websites for the same device and from device to device on the same e-commerce website. On Alibaba.com, the greatest number of listings resulted for picosecond laser (23,622 listings), fractionated laser (15,269), and radiofrequency skin tightening devices (3555); cryolipolysis and nonablative radiofrequency resurfacing devices had notably fewer listings (35 and 38, respectively). On DHGate.com, a similar trend was noted with the most numerous listings for picosecond and fractionated laser systems (2429 and 1345, respectively).
Among the first 10 listings of products on Alibaba.com and DHgate.com for these 6 devices, 10.7% (11 of 103) had advertised claims of FDA clearance on the listing page. Of 103 counterfeit products, China was the country of origin for 100; South Korea for 2; and Thailand for 1. Unit pricing was heterogeneous between the 2 e-commerce websites for the counterfeit listings; pricing for duplicate fractionated laser systems was particularly dissimilar, with an average price on Alibab.com of US $8105.80 and an average price on DHgate.com of US $3409.14. Even on the same e-commerce website, the range of unit pricing differed greatly for dermatologic devices. For example, among the first 10 listings on Alibaba.com for a fractionated laser system, the price ranged from US $2300 to US $32,000.
Counterfeit medical devices are on the rise in dermatology.1,3 Although devices such as radiofrequency and laser systems had thousands of knockoff listings on 2 e-commerce websites, other devices, such as cryolipolysis and body contouring systems, had fewer listings, suggesting heterogeneity in the prevalence of different counterfeit dermatologic devices on the market.
The varied pricing of the top 10 listings for each product and spurious claims of FDA clearance for some listings highlight the lack of regulatory authority over consistent product information on e-commerce websites. Furthermore, differences between characteristics of counterfeit device listings can impede efforts to trace suppliers and increase the opacity of counterfeit purchasing.
Three criteria have been proposed for a device to be considered counterfeit3:
• The device has no proven safety or efficacy among consumers. For example, the substantial threat of copycat devices in dermatology has been demonstrated by reports of burns caused by fake cryolipolysis devices.2
• The device violates patent rights or copy trademarks. Due to the regional nature of intellectual property rights, country-specific filings of patents and trademarks are required if protections are sought internationally. In this study, counterfeit devices originated in China, South Korea, and Thailand, where patent and trademark protections for the original devices do not extend.
• The device is falsely claimed to have been cleared by the FDA or other clinical regulatory authorities. Legitimate medical devices are subject to rounds of safety and compatibility testing using standards set by regulatory bodies, such as the FDA’s Center for Devices and Radiological Health, the International Organization of Standardization, and the International Electrotechnical Commission. Compliance with these safety standards is lost, however, among unregulated internet sales of medical devices. Our search revealed that 10.7% of the top 10 counterfeit device listings for each product explicitly mentioned FDA clearance in the product description. Among the thousands of listings on e-commerce sites, even a fraction that make spurious FDA-clearance claims can mislead consumers.
The issue of counterfeit medical devices has not gone unrecognized globally. In 2013, the World Health Organization created the Global Surveillance and Monitoring System to unify international efforts for reporting substandard, unlicensed, or falsified medical products.4 Although universal monitoring systems can improve detection of counterfeit products, we highlight the alarming continuing ease of purchasing counterfeit dermatologic devices through e-commerce websites. Due to the widespread nature of counterfeiting across all domains of medicine, the onus of curbing counterfeit dermatologic devices might be on dermatology providers to recognize and report such occurrences.
This exploration of counterfeit dermatologic devices revealed a lack of consistency throughout product listings on 2 popular e-commerce websites, Alibaba.com and DHgate.com. Given the alarming availability of these devices on the internet, practitioners should approach the purchase of any device with concern about counterfeiting. Future avenues of study might explore the prevalence of counterfeit devices used in dermatology practices and offer insight on regulation and consumer safety efforts.
- Wang JV, Zachary CB, Saedi N. Counterfeit esthetic devices and patient safety in dermatology. J Cosmet Dermatol. 2018;17:396-397. doi:10.1111/jocd.12526
- Biesman BS, Patel N. Physician alert: beware of counterfeit medical devices. Lasers Surg Med. 2014;46:528‐530. doi:10.1002/lsm.22275
- Stevens WG, Spring MA, Macias LH. Counterfeit medical devices: the money you save up front will cost you big in the end. Aesthet Surg J. 2014;34:786‐788. doi:10.1177/1090820X14529960
- Pisani E. WHO Global Surveillance and Monitoring System for Substandard and Falsified Medical Products. World Health Organization; 2017. Accessed November 21, 2021. https://www.who.int/medicines/regulation/ssffc/publications/GSMSreport_EN.pdf?ua=1
To the Editor:
Approved medical devices on the market are substantial capital investments for practitioners. E-commerce websites, such as Alibaba.com (https://www.alibaba.com/) and DHgate.com (https://www.dhgate.com/), sell sham medical devices at a fraction of the cost of authentic products, with sellers often echoing the same treatment claims as legitimate devices that have been cleared by the US Food and Drug Administration (FDA).
In dermatology, devices claiming to perform cryolipolysis, laser skin resurfacing, radiofrequency skin tightening, and more exist on e-commerce websites. These counterfeit medical devices might differ from legitimate devices in ways that affect patient safety and treatment efficacy.1,2 The degree of difference between counterfeit and legitimate devices remains unknown, and potential harm from so-called knockoff devices needs to be critically examined by providers.
In this exploratory study, we characterize counterfeit listings of devices commonly used in dermatology. Using the trademark name of devices as the key terms, we searched Alibaba.com and DHgate.com for listings of counterfeit products. We recorded the total number of listings; the listing name, catalog number, and unit price; and claims of FDA certification. Characteristics of counterfeit listings were summarized using standard descriptive statistics in Microsoft Excel. Continuous variables were summarized with means and ranges.
Six medical devices that had been cleared by the FDA between 2002 and 2012 for use in dermatology were explored, including systems for picosecond and fractionated lasers, monopolar and bipolar radiofrequency skin tightening, cryolipolysis, and nonablative radiofrequency skin resurfacing. Our search of these 6 representative dermatologic devices revealed 47,055 counterfeit product listings on Alibaba.com and DHgate.com. Upon searching these popular e-commerce websites using the device name as the search term, the number of listings varied considerably between the 2 e-commerce websites for the same device and from device to device on the same e-commerce website. On Alibaba.com, the greatest number of listings resulted for picosecond laser (23,622 listings), fractionated laser (15,269), and radiofrequency skin tightening devices (3555); cryolipolysis and nonablative radiofrequency resurfacing devices had notably fewer listings (35 and 38, respectively). On DHGate.com, a similar trend was noted with the most numerous listings for picosecond and fractionated laser systems (2429 and 1345, respectively).
Among the first 10 listings of products on Alibaba.com and DHgate.com for these 6 devices, 10.7% (11 of 103) had advertised claims of FDA clearance on the listing page. Of 103 counterfeit products, China was the country of origin for 100; South Korea for 2; and Thailand for 1. Unit pricing was heterogeneous between the 2 e-commerce websites for the counterfeit listings; pricing for duplicate fractionated laser systems was particularly dissimilar, with an average price on Alibab.com of US $8105.80 and an average price on DHgate.com of US $3409.14. Even on the same e-commerce website, the range of unit pricing differed greatly for dermatologic devices. For example, among the first 10 listings on Alibaba.com for a fractionated laser system, the price ranged from US $2300 to US $32,000.
Counterfeit medical devices are on the rise in dermatology.1,3 Although devices such as radiofrequency and laser systems had thousands of knockoff listings on 2 e-commerce websites, other devices, such as cryolipolysis and body contouring systems, had fewer listings, suggesting heterogeneity in the prevalence of different counterfeit dermatologic devices on the market.
The varied pricing of the top 10 listings for each product and spurious claims of FDA clearance for some listings highlight the lack of regulatory authority over consistent product information on e-commerce websites. Furthermore, differences between characteristics of counterfeit device listings can impede efforts to trace suppliers and increase the opacity of counterfeit purchasing.
Three criteria have been proposed for a device to be considered counterfeit3:
• The device has no proven safety or efficacy among consumers. For example, the substantial threat of copycat devices in dermatology has been demonstrated by reports of burns caused by fake cryolipolysis devices.2
• The device violates patent rights or copy trademarks. Due to the regional nature of intellectual property rights, country-specific filings of patents and trademarks are required if protections are sought internationally. In this study, counterfeit devices originated in China, South Korea, and Thailand, where patent and trademark protections for the original devices do not extend.
• The device is falsely claimed to have been cleared by the FDA or other clinical regulatory authorities. Legitimate medical devices are subject to rounds of safety and compatibility testing using standards set by regulatory bodies, such as the FDA’s Center for Devices and Radiological Health, the International Organization of Standardization, and the International Electrotechnical Commission. Compliance with these safety standards is lost, however, among unregulated internet sales of medical devices. Our search revealed that 10.7% of the top 10 counterfeit device listings for each product explicitly mentioned FDA clearance in the product description. Among the thousands of listings on e-commerce sites, even a fraction that make spurious FDA-clearance claims can mislead consumers.
The issue of counterfeit medical devices has not gone unrecognized globally. In 2013, the World Health Organization created the Global Surveillance and Monitoring System to unify international efforts for reporting substandard, unlicensed, or falsified medical products.4 Although universal monitoring systems can improve detection of counterfeit products, we highlight the alarming continuing ease of purchasing counterfeit dermatologic devices through e-commerce websites. Due to the widespread nature of counterfeiting across all domains of medicine, the onus of curbing counterfeit dermatologic devices might be on dermatology providers to recognize and report such occurrences.
This exploration of counterfeit dermatologic devices revealed a lack of consistency throughout product listings on 2 popular e-commerce websites, Alibaba.com and DHgate.com. Given the alarming availability of these devices on the internet, practitioners should approach the purchase of any device with concern about counterfeiting. Future avenues of study might explore the prevalence of counterfeit devices used in dermatology practices and offer insight on regulation and consumer safety efforts.
To the Editor:
Approved medical devices on the market are substantial capital investments for practitioners. E-commerce websites, such as Alibaba.com (https://www.alibaba.com/) and DHgate.com (https://www.dhgate.com/), sell sham medical devices at a fraction of the cost of authentic products, with sellers often echoing the same treatment claims as legitimate devices that have been cleared by the US Food and Drug Administration (FDA).
In dermatology, devices claiming to perform cryolipolysis, laser skin resurfacing, radiofrequency skin tightening, and more exist on e-commerce websites. These counterfeit medical devices might differ from legitimate devices in ways that affect patient safety and treatment efficacy.1,2 The degree of difference between counterfeit and legitimate devices remains unknown, and potential harm from so-called knockoff devices needs to be critically examined by providers.
In this exploratory study, we characterize counterfeit listings of devices commonly used in dermatology. Using the trademark name of devices as the key terms, we searched Alibaba.com and DHgate.com for listings of counterfeit products. We recorded the total number of listings; the listing name, catalog number, and unit price; and claims of FDA certification. Characteristics of counterfeit listings were summarized using standard descriptive statistics in Microsoft Excel. Continuous variables were summarized with means and ranges.
Six medical devices that had been cleared by the FDA between 2002 and 2012 for use in dermatology were explored, including systems for picosecond and fractionated lasers, monopolar and bipolar radiofrequency skin tightening, cryolipolysis, and nonablative radiofrequency skin resurfacing. Our search of these 6 representative dermatologic devices revealed 47,055 counterfeit product listings on Alibaba.com and DHgate.com. Upon searching these popular e-commerce websites using the device name as the search term, the number of listings varied considerably between the 2 e-commerce websites for the same device and from device to device on the same e-commerce website. On Alibaba.com, the greatest number of listings resulted for picosecond laser (23,622 listings), fractionated laser (15,269), and radiofrequency skin tightening devices (3555); cryolipolysis and nonablative radiofrequency resurfacing devices had notably fewer listings (35 and 38, respectively). On DHGate.com, a similar trend was noted with the most numerous listings for picosecond and fractionated laser systems (2429 and 1345, respectively).
Among the first 10 listings of products on Alibaba.com and DHgate.com for these 6 devices, 10.7% (11 of 103) had advertised claims of FDA clearance on the listing page. Of 103 counterfeit products, China was the country of origin for 100; South Korea for 2; and Thailand for 1. Unit pricing was heterogeneous between the 2 e-commerce websites for the counterfeit listings; pricing for duplicate fractionated laser systems was particularly dissimilar, with an average price on Alibab.com of US $8105.80 and an average price on DHgate.com of US $3409.14. Even on the same e-commerce website, the range of unit pricing differed greatly for dermatologic devices. For example, among the first 10 listings on Alibaba.com for a fractionated laser system, the price ranged from US $2300 to US $32,000.
Counterfeit medical devices are on the rise in dermatology.1,3 Although devices such as radiofrequency and laser systems had thousands of knockoff listings on 2 e-commerce websites, other devices, such as cryolipolysis and body contouring systems, had fewer listings, suggesting heterogeneity in the prevalence of different counterfeit dermatologic devices on the market.
The varied pricing of the top 10 listings for each product and spurious claims of FDA clearance for some listings highlight the lack of regulatory authority over consistent product information on e-commerce websites. Furthermore, differences between characteristics of counterfeit device listings can impede efforts to trace suppliers and increase the opacity of counterfeit purchasing.
Three criteria have been proposed for a device to be considered counterfeit3:
• The device has no proven safety or efficacy among consumers. For example, the substantial threat of copycat devices in dermatology has been demonstrated by reports of burns caused by fake cryolipolysis devices.2
• The device violates patent rights or copy trademarks. Due to the regional nature of intellectual property rights, country-specific filings of patents and trademarks are required if protections are sought internationally. In this study, counterfeit devices originated in China, South Korea, and Thailand, where patent and trademark protections for the original devices do not extend.
• The device is falsely claimed to have been cleared by the FDA or other clinical regulatory authorities. Legitimate medical devices are subject to rounds of safety and compatibility testing using standards set by regulatory bodies, such as the FDA’s Center for Devices and Radiological Health, the International Organization of Standardization, and the International Electrotechnical Commission. Compliance with these safety standards is lost, however, among unregulated internet sales of medical devices. Our search revealed that 10.7% of the top 10 counterfeit device listings for each product explicitly mentioned FDA clearance in the product description. Among the thousands of listings on e-commerce sites, even a fraction that make spurious FDA-clearance claims can mislead consumers.
The issue of counterfeit medical devices has not gone unrecognized globally. In 2013, the World Health Organization created the Global Surveillance and Monitoring System to unify international efforts for reporting substandard, unlicensed, or falsified medical products.4 Although universal monitoring systems can improve detection of counterfeit products, we highlight the alarming continuing ease of purchasing counterfeit dermatologic devices through e-commerce websites. Due to the widespread nature of counterfeiting across all domains of medicine, the onus of curbing counterfeit dermatologic devices might be on dermatology providers to recognize and report such occurrences.
This exploration of counterfeit dermatologic devices revealed a lack of consistency throughout product listings on 2 popular e-commerce websites, Alibaba.com and DHgate.com. Given the alarming availability of these devices on the internet, practitioners should approach the purchase of any device with concern about counterfeiting. Future avenues of study might explore the prevalence of counterfeit devices used in dermatology practices and offer insight on regulation and consumer safety efforts.
- Wang JV, Zachary CB, Saedi N. Counterfeit esthetic devices and patient safety in dermatology. J Cosmet Dermatol. 2018;17:396-397. doi:10.1111/jocd.12526
- Biesman BS, Patel N. Physician alert: beware of counterfeit medical devices. Lasers Surg Med. 2014;46:528‐530. doi:10.1002/lsm.22275
- Stevens WG, Spring MA, Macias LH. Counterfeit medical devices: the money you save up front will cost you big in the end. Aesthet Surg J. 2014;34:786‐788. doi:10.1177/1090820X14529960
- Pisani E. WHO Global Surveillance and Monitoring System for Substandard and Falsified Medical Products. World Health Organization; 2017. Accessed November 21, 2021. https://www.who.int/medicines/regulation/ssffc/publications/GSMSreport_EN.pdf?ua=1
- Wang JV, Zachary CB, Saedi N. Counterfeit esthetic devices and patient safety in dermatology. J Cosmet Dermatol. 2018;17:396-397. doi:10.1111/jocd.12526
- Biesman BS, Patel N. Physician alert: beware of counterfeit medical devices. Lasers Surg Med. 2014;46:528‐530. doi:10.1002/lsm.22275
- Stevens WG, Spring MA, Macias LH. Counterfeit medical devices: the money you save up front will cost you big in the end. Aesthet Surg J. 2014;34:786‐788. doi:10.1177/1090820X14529960
- Pisani E. WHO Global Surveillance and Monitoring System for Substandard and Falsified Medical Products. World Health Organization; 2017. Accessed November 21, 2021. https://www.who.int/medicines/regulation/ssffc/publications/GSMSreport_EN.pdf?ua=1
Practice Points
- Among thousands of counterfeit dermatologic listings, there is great heterogeneity in the number of listings per different subtypes of dermatologic devices, device descriptions, and unit pricing, along with false claims of US Food and Drug Administration clearance.
- Given the prevalence of counterfeit medical devices readily available for purchase online, dermatology practitioners should be wary of the authenticity of any medical device purchased for clinical use.
Dr. Fauci: HIV advances ‘breathtaking,’ but steadfast focus on disparities needed
Decades before becoming the go-to authority in the United States on the COVID-19 global pandemic, Anthony S. Fauci, MD, found himself witnessing the earliest perplexing cases of what would become another devastating global pandemic – HIV/AIDS. And while extraordinary advances have transformed treatment and prevention, glaring treatment gaps and challenges remain after 40 years.
“I certainly remember those initial MMWRs [the Morbidity and Mortality Weekly Reports] in the summer of 1981 that introduced us to what would turn out to be the most extraordinary and devastating pandemic of an infectious disease up until that time in the modern era,” said Dr. Fauci when addressing the 2021 United States Conference on HIV/AIDS.
“Now, 40 years into it, we are still in the middle of a global pandemic despite the fact that there have been extraordinary advances,” said Dr. Fauci, who is director of the National Institute of Allergy and Infectious Diseases and chief medical advisor to the President of the United States.
Specifically, it was on June 5, 1981, that the Centers for Disease Control and Prevention issued its fateful report on the first five cases of what would soon become known as Acquired Immune Deficiency Syndrome.
By 2020, the 5 cases had grown to 79.3 million HIV infections since the start of the HIV/AIDS pandemic, claiming 36.3 million lives, according to the NAIDS Global AIDS update, Dr. Fauci reported.
At the end of 2020, there were 1.5 million new infections, as many as 37.7 million people living with HIV, and 680,000 deaths, according to the report.
The fact that so many people are living with HIV – and not dying from it – is largely attributable to “breathtaking” advances in treatment, Dr. Fauci said, underscoring the fact that there are now 13 single-tablet, once-daily, antiretroviral (ART) regimens approved in the United States to replace the multidrug cocktail that has long been necessary with HIV treatment.
“I can remember when the combination therapies were first made available, we were giving patients literally dozens of pills of different types each day, but that is no longer the case,” Dr. Fauci said.
“We can say, without hyperbole, that highly effective antiretroviral therapy for HIV is indeed one of the most important biomedical research advances of our era.”
Furthermore, HIV prevention using pre-exposure prophylaxis (PrEP), when used optimally and consistently, has further transformed the HIV landscape with 99% efficacy in preventing sexual HIV acquisition.
Troubling treatment gaps
Despite the advances, disparities and challenges are abundant, Dr. Fauci said.
Notably, the majority of those infected globally – 65% – are concentrated among key populations, including gay men and other men who have sex with men (23%), clients of sex workers (20%), sex workers (11%), people who inject drugs (9%), and transgender people (2%), according to the Joint United Nations Programme on HIV/AIDS.
According to UNAIDS, among the 37.7 million people living with HIV at the end of 2020, 27.5 million were being treated with life-saving ART, leaving a gap of 10.2 million people with HIV who are not receiving the treatment, Dr. Fauci pointed out.
And of those who do receive treatment, retention is suboptimal, with only about 65% of patients in low- and middle-income countries being retained in care at 48 months following ART initiation.
Dr. Fauci underscored encouraging developments that could address some of those problems, notably long-acting ART therapies that, in requiring administration only every 6 months or so, could negate the need for adherence to daily ART therapy.
Likewise, long-acting PrEP provided intermittently over longer periods could prevent transmission.
“We’re looking at [long-acting PrEP] with a great deal of enthusiasm as providing protection with longer durations between doses to get people to essentially have close to 99% protection against HIV acquisition,” Dr. Fauci said.
While several efforts to develop vaccines for HIV in long-term clinical trials have had disappointing results, Dr. Fauci says he stops short of calling them failures.
“We don’t consider the trials to be failures because, in fact, they tell us the way we need to go – which direction,” he said.
“In fact, COVID-19 itself has given us new enthusiasm about the use of vaccine platforms such as mRNA that are now being applied in the vaccine quest for HIV,” Dr. Fauci noted.
Ultimately, “we must steadily and steadfastly move forward to address critical research gaps and unanswered questions [regarding HIV],” Dr. Fauci said. “The scientific advances have been breathtaking and it is up to us to [achieve] greater scientific advances, but also to translate them into something that can be implemented.”
USCHA Executive Director Paul Kawata, MD, commented that he shares Dr. Fauci’s optimism — and his concerns.
“NMAC [formerly the National Minority AIDS Council, which runs USCHA] is very excited about the science,” he said in an interview. “Our ability to make treatment easier should be a pathway to success.”
“Our concern is that we need more implementation science to know if long-acting ART will be used by the communities hardest hit by HIV,” he said.
Dr. Kawata noted that NMAC agrees that vaccine trial “failures” can offer important lessons, “but we are getting impatient,” he said. “Back in the 80s, Secretary Margret Heckler said we would have a vaccine in 5 years.”
Furthermore, ongoing racial disparities, left unaddressed, will hold back meaningful progress in the fight against HIV, he noted. “We are always hopeful, [but] the reality is that race and racism play an outsized role in health outcome in America. Unless we address these inequalities, we will never end HIV.”
NMAC receives funding from Gilead, Viiv, Merck, and Janssen.
A version of this article first appeared on Medscape.com.
Decades before becoming the go-to authority in the United States on the COVID-19 global pandemic, Anthony S. Fauci, MD, found himself witnessing the earliest perplexing cases of what would become another devastating global pandemic – HIV/AIDS. And while extraordinary advances have transformed treatment and prevention, glaring treatment gaps and challenges remain after 40 years.
“I certainly remember those initial MMWRs [the Morbidity and Mortality Weekly Reports] in the summer of 1981 that introduced us to what would turn out to be the most extraordinary and devastating pandemic of an infectious disease up until that time in the modern era,” said Dr. Fauci when addressing the 2021 United States Conference on HIV/AIDS.
“Now, 40 years into it, we are still in the middle of a global pandemic despite the fact that there have been extraordinary advances,” said Dr. Fauci, who is director of the National Institute of Allergy and Infectious Diseases and chief medical advisor to the President of the United States.
Specifically, it was on June 5, 1981, that the Centers for Disease Control and Prevention issued its fateful report on the first five cases of what would soon become known as Acquired Immune Deficiency Syndrome.
By 2020, the 5 cases had grown to 79.3 million HIV infections since the start of the HIV/AIDS pandemic, claiming 36.3 million lives, according to the NAIDS Global AIDS update, Dr. Fauci reported.
At the end of 2020, there were 1.5 million new infections, as many as 37.7 million people living with HIV, and 680,000 deaths, according to the report.
The fact that so many people are living with HIV – and not dying from it – is largely attributable to “breathtaking” advances in treatment, Dr. Fauci said, underscoring the fact that there are now 13 single-tablet, once-daily, antiretroviral (ART) regimens approved in the United States to replace the multidrug cocktail that has long been necessary with HIV treatment.
“I can remember when the combination therapies were first made available, we were giving patients literally dozens of pills of different types each day, but that is no longer the case,” Dr. Fauci said.
“We can say, without hyperbole, that highly effective antiretroviral therapy for HIV is indeed one of the most important biomedical research advances of our era.”
Furthermore, HIV prevention using pre-exposure prophylaxis (PrEP), when used optimally and consistently, has further transformed the HIV landscape with 99% efficacy in preventing sexual HIV acquisition.
Troubling treatment gaps
Despite the advances, disparities and challenges are abundant, Dr. Fauci said.
Notably, the majority of those infected globally – 65% – are concentrated among key populations, including gay men and other men who have sex with men (23%), clients of sex workers (20%), sex workers (11%), people who inject drugs (9%), and transgender people (2%), according to the Joint United Nations Programme on HIV/AIDS.
According to UNAIDS, among the 37.7 million people living with HIV at the end of 2020, 27.5 million were being treated with life-saving ART, leaving a gap of 10.2 million people with HIV who are not receiving the treatment, Dr. Fauci pointed out.
And of those who do receive treatment, retention is suboptimal, with only about 65% of patients in low- and middle-income countries being retained in care at 48 months following ART initiation.
Dr. Fauci underscored encouraging developments that could address some of those problems, notably long-acting ART therapies that, in requiring administration only every 6 months or so, could negate the need for adherence to daily ART therapy.
Likewise, long-acting PrEP provided intermittently over longer periods could prevent transmission.
“We’re looking at [long-acting PrEP] with a great deal of enthusiasm as providing protection with longer durations between doses to get people to essentially have close to 99% protection against HIV acquisition,” Dr. Fauci said.
While several efforts to develop vaccines for HIV in long-term clinical trials have had disappointing results, Dr. Fauci says he stops short of calling them failures.
“We don’t consider the trials to be failures because, in fact, they tell us the way we need to go – which direction,” he said.
“In fact, COVID-19 itself has given us new enthusiasm about the use of vaccine platforms such as mRNA that are now being applied in the vaccine quest for HIV,” Dr. Fauci noted.
Ultimately, “we must steadily and steadfastly move forward to address critical research gaps and unanswered questions [regarding HIV],” Dr. Fauci said. “The scientific advances have been breathtaking and it is up to us to [achieve] greater scientific advances, but also to translate them into something that can be implemented.”
USCHA Executive Director Paul Kawata, MD, commented that he shares Dr. Fauci’s optimism — and his concerns.
“NMAC [formerly the National Minority AIDS Council, which runs USCHA] is very excited about the science,” he said in an interview. “Our ability to make treatment easier should be a pathway to success.”
“Our concern is that we need more implementation science to know if long-acting ART will be used by the communities hardest hit by HIV,” he said.
Dr. Kawata noted that NMAC agrees that vaccine trial “failures” can offer important lessons, “but we are getting impatient,” he said. “Back in the 80s, Secretary Margret Heckler said we would have a vaccine in 5 years.”
Furthermore, ongoing racial disparities, left unaddressed, will hold back meaningful progress in the fight against HIV, he noted. “We are always hopeful, [but] the reality is that race and racism play an outsized role in health outcome in America. Unless we address these inequalities, we will never end HIV.”
NMAC receives funding from Gilead, Viiv, Merck, and Janssen.
A version of this article first appeared on Medscape.com.
Decades before becoming the go-to authority in the United States on the COVID-19 global pandemic, Anthony S. Fauci, MD, found himself witnessing the earliest perplexing cases of what would become another devastating global pandemic – HIV/AIDS. And while extraordinary advances have transformed treatment and prevention, glaring treatment gaps and challenges remain after 40 years.
“I certainly remember those initial MMWRs [the Morbidity and Mortality Weekly Reports] in the summer of 1981 that introduced us to what would turn out to be the most extraordinary and devastating pandemic of an infectious disease up until that time in the modern era,” said Dr. Fauci when addressing the 2021 United States Conference on HIV/AIDS.
“Now, 40 years into it, we are still in the middle of a global pandemic despite the fact that there have been extraordinary advances,” said Dr. Fauci, who is director of the National Institute of Allergy and Infectious Diseases and chief medical advisor to the President of the United States.
Specifically, it was on June 5, 1981, that the Centers for Disease Control and Prevention issued its fateful report on the first five cases of what would soon become known as Acquired Immune Deficiency Syndrome.
By 2020, the 5 cases had grown to 79.3 million HIV infections since the start of the HIV/AIDS pandemic, claiming 36.3 million lives, according to the NAIDS Global AIDS update, Dr. Fauci reported.
At the end of 2020, there were 1.5 million new infections, as many as 37.7 million people living with HIV, and 680,000 deaths, according to the report.
The fact that so many people are living with HIV – and not dying from it – is largely attributable to “breathtaking” advances in treatment, Dr. Fauci said, underscoring the fact that there are now 13 single-tablet, once-daily, antiretroviral (ART) regimens approved in the United States to replace the multidrug cocktail that has long been necessary with HIV treatment.
“I can remember when the combination therapies were first made available, we were giving patients literally dozens of pills of different types each day, but that is no longer the case,” Dr. Fauci said.
“We can say, without hyperbole, that highly effective antiretroviral therapy for HIV is indeed one of the most important biomedical research advances of our era.”
Furthermore, HIV prevention using pre-exposure prophylaxis (PrEP), when used optimally and consistently, has further transformed the HIV landscape with 99% efficacy in preventing sexual HIV acquisition.
Troubling treatment gaps
Despite the advances, disparities and challenges are abundant, Dr. Fauci said.
Notably, the majority of those infected globally – 65% – are concentrated among key populations, including gay men and other men who have sex with men (23%), clients of sex workers (20%), sex workers (11%), people who inject drugs (9%), and transgender people (2%), according to the Joint United Nations Programme on HIV/AIDS.
According to UNAIDS, among the 37.7 million people living with HIV at the end of 2020, 27.5 million were being treated with life-saving ART, leaving a gap of 10.2 million people with HIV who are not receiving the treatment, Dr. Fauci pointed out.
And of those who do receive treatment, retention is suboptimal, with only about 65% of patients in low- and middle-income countries being retained in care at 48 months following ART initiation.
Dr. Fauci underscored encouraging developments that could address some of those problems, notably long-acting ART therapies that, in requiring administration only every 6 months or so, could negate the need for adherence to daily ART therapy.
Likewise, long-acting PrEP provided intermittently over longer periods could prevent transmission.
“We’re looking at [long-acting PrEP] with a great deal of enthusiasm as providing protection with longer durations between doses to get people to essentially have close to 99% protection against HIV acquisition,” Dr. Fauci said.
While several efforts to develop vaccines for HIV in long-term clinical trials have had disappointing results, Dr. Fauci says he stops short of calling them failures.
“We don’t consider the trials to be failures because, in fact, they tell us the way we need to go – which direction,” he said.
“In fact, COVID-19 itself has given us new enthusiasm about the use of vaccine platforms such as mRNA that are now being applied in the vaccine quest for HIV,” Dr. Fauci noted.
Ultimately, “we must steadily and steadfastly move forward to address critical research gaps and unanswered questions [regarding HIV],” Dr. Fauci said. “The scientific advances have been breathtaking and it is up to us to [achieve] greater scientific advances, but also to translate them into something that can be implemented.”
USCHA Executive Director Paul Kawata, MD, commented that he shares Dr. Fauci’s optimism — and his concerns.
“NMAC [formerly the National Minority AIDS Council, which runs USCHA] is very excited about the science,” he said in an interview. “Our ability to make treatment easier should be a pathway to success.”
“Our concern is that we need more implementation science to know if long-acting ART will be used by the communities hardest hit by HIV,” he said.
Dr. Kawata noted that NMAC agrees that vaccine trial “failures” can offer important lessons, “but we are getting impatient,” he said. “Back in the 80s, Secretary Margret Heckler said we would have a vaccine in 5 years.”
Furthermore, ongoing racial disparities, left unaddressed, will hold back meaningful progress in the fight against HIV, he noted. “We are always hopeful, [but] the reality is that race and racism play an outsized role in health outcome in America. Unless we address these inequalities, we will never end HIV.”
NMAC receives funding from Gilead, Viiv, Merck, and Janssen.
A version of this article first appeared on Medscape.com.