User login
Don’t give up on relentless youth depression
As pediatricians, we are acutely aware of the increase in depression in our teen patients. Lifetime prevalence is now approaching 20%, and we are doing our best to help.
The Guidelines for Adolescent Depression in Primary Care (GLAD-PC, 2018) has advice on screening and primary care provider (PCP) management, verifying our role in care. But GLAD-PC also advises “referral to a mental health specialist” in patient scenarios we see multiple times per week. Even when patients are willing and able to go, mental health specialists are in short supply or have months-long waiting lists. What should we do to help the more severely depressed adolescent when immediate referral is not possible? What should we expect of specialist care for what is called treatment-resistant or treatment-refractory depression (TRD)?
To know what to do for a youth with TRD, first you need to know what constitutes an adequate trial of treatment. After diagnosis of major depressive disorder (MDD) from a validated screening tool or an interview based on DSM-5 criteria and an appropriate assessment (as described in GLAD-PC), patients and parents need education on symptoms, course, prognosis including suicide risk, and treatment options. Known TRD risk factors, besides longer or greater depression severity, anhedonia, and poor global functioning, can benefit from being specifically addressed: trauma, bullying, comorbid anxiety or substance use, subsyndromal mania, insomnia, hypothyroidism, nutritional deficiencies from eating disorders, certain genetic variants, LGBTQ identification, family conflict, and parental depression. Screening and assessment for suicidal ideation/attempts is needed initially and in follow-up as MDD increases risk of suicide 30 times.
PCPs can manage mild depression with regular visits every 1-2 weeks for active support for 6-8 weeks. Advise all depressed youth on healthy eating, adequate sleep and exercise, pleasurable activities, and refraining from substance use. With a full response (50%+ reduction in symptom score from baseline), monthly monitoring for symptoms, suicidality, and stressors (phone/televisits suffice) should continue for 6-24 months as half recur. Monitoring with ratings by both youth and parent are recommended and may be required by insurers. Scores below cutoff suggest “remission,” although functioning must be considered. Youth report symptoms best but parents may better report improved functioning and affect that can precede symptom reduction.
If there is no initial response (< 25% decrease in symptom score) or a partial response (25%-49% decrease), PCPs should begin treatment as for moderate depression with either a selective serotonin reuptake inhibitor (SSRI) or psychotherapy. Use of both has the best evidence; cognitive behavior therapy (CBT) and interpersonal psychotherapy for adolescents are equally effective.
Side effects from SSRIs are almost universal with GI upset, headaches, and sexual dysfunction most common, but activation (increased agitation or irritability) may occur. Educate patients about these and encourage tolerating them as they tend to subside in weeks, allowing continuation of these most effective medicines. Activation rarely indicates true mania, which would require stopping and referral.
Moderate depression with only comorbid anxiety may be addressed by PCPs with problem-focused supportive counseling and SSRIs, but mental health consultation or referral also are appropriate. Fluoxetine starting at 5-10 mg/day has best evidence and Food and Drug Administration approval for MDD from age 8. Starting at a higher dose may increase risk of suicidal ideation. Alternatively, escitalopram is FDA approved for MDD at age 12 starting at 10 mg/day, although meta-analyses do not distinguish effectiveness within the SSRI class. Although benefit usually appears within 2 weeks, a trial of at least 4 weeks should be used to assess effect.
If after 4 weeks, the SSRI is tolerated but has little or no response, reassess the diagnosis, try a different SSRI, e.g. sertraline, and add CBT (combined SSRI+CBT has an advantage). To switch SSRIs, reduce the first every 1-2 weeks (by 10-20 mg for fluoxetine; 5-10 for escitalopram) to reduce side effects. If overlapping, the replacement SSRI may start midway in the wean at low dose with patients educated about serotonin syndrome. If instead there was a partial response to the initial SSRI, progressively increase the dose (by 10 mg for fluoxetine or 5 mg for escitalopram monthly) as indicated by symptom change up to the maximum (60-80 mg fluoxetine or 20 mg escitalopram), if needed, and maintain for another 4 weeks. Alternatively, or in addition, start psychotherapy or ask to change current therapy, as therapy focus makes a difference in effect. Initial CBT focus on anxiety acts fastest when anxiety is comorbid.
Once a regimen produces a response, maintain it for 16-20 weeks, the longer for more severe depression. Although three-fourths of mildly to moderately depressed youth are late responders, emerging near 6 weeks, a rapid initial response is associated with better outcome. The recommended 8 weeks on a final tolerated dose constituting an adequate trial before changing may be shortened to 6 weeks in severe unremitting cases. Youth not remitting by 12 weeks should be offered alternative treatment. Referral is recommended for moderately severe depression with comorbidity or severe depression but also for unresponsive moderate depression or by family or clinician preference.
Treatment-resistant depression is defined as “clinically impairing depression symptoms despite an adequate trial of an evidence-based psychotherapy and an antidepressant with grade A evidence (fluoxetine, escitalopram, or sertraline),” sequentially or together; treatment-refractory depression comprises the above with failure on at least two antidepressants, with at least one being grade A. Unfortunately, TRD occurs in 30%-40% of children and remission is only 30%. Low adherence based on pill counts (> 30% missed) or with therapy (fewer than nine visits) should be considered in treatment failures.
With manageable factors addressed, the next step for TRD is treatment augmentation. The best evidence-based augmentation for TRD is CBT; 55% of those receiving CBT responded within 12 weeks. TRD augmentations and interventions with evidence in adults have either no evidence of effect in children (SNRIs, lithium), no randomized controlled trials, or support only from small suggestive studies, e.g., antipsychotics, 16 g/day omega-3 fatty acid supplementation, folic acid supplementation, repetitive transcranial magnetic stimulation, electroconvulsive therapy, or ketamine. Prompt referral to a child psychiatrist is essential for youth classified as TRD as earlier more aggressive treatment may avoid the long-term morbidity of chronic depression.
Fortunately, a meta-analysis of studies showed that PCP medication management visits with monitoring could improve outcomes, even for TRD.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Reference
Dwyer J et al. Annual research review: Defining and treating pediatric treatment-resistant depression. J Child Psychol Psychiatry. 2020 March;61(3):312-32.
As pediatricians, we are acutely aware of the increase in depression in our teen patients. Lifetime prevalence is now approaching 20%, and we are doing our best to help.
The Guidelines for Adolescent Depression in Primary Care (GLAD-PC, 2018) has advice on screening and primary care provider (PCP) management, verifying our role in care. But GLAD-PC also advises “referral to a mental health specialist” in patient scenarios we see multiple times per week. Even when patients are willing and able to go, mental health specialists are in short supply or have months-long waiting lists. What should we do to help the more severely depressed adolescent when immediate referral is not possible? What should we expect of specialist care for what is called treatment-resistant or treatment-refractory depression (TRD)?
To know what to do for a youth with TRD, first you need to know what constitutes an adequate trial of treatment. After diagnosis of major depressive disorder (MDD) from a validated screening tool or an interview based on DSM-5 criteria and an appropriate assessment (as described in GLAD-PC), patients and parents need education on symptoms, course, prognosis including suicide risk, and treatment options. Known TRD risk factors, besides longer or greater depression severity, anhedonia, and poor global functioning, can benefit from being specifically addressed: trauma, bullying, comorbid anxiety or substance use, subsyndromal mania, insomnia, hypothyroidism, nutritional deficiencies from eating disorders, certain genetic variants, LGBTQ identification, family conflict, and parental depression. Screening and assessment for suicidal ideation/attempts is needed initially and in follow-up as MDD increases risk of suicide 30 times.
PCPs can manage mild depression with regular visits every 1-2 weeks for active support for 6-8 weeks. Advise all depressed youth on healthy eating, adequate sleep and exercise, pleasurable activities, and refraining from substance use. With a full response (50%+ reduction in symptom score from baseline), monthly monitoring for symptoms, suicidality, and stressors (phone/televisits suffice) should continue for 6-24 months as half recur. Monitoring with ratings by both youth and parent are recommended and may be required by insurers. Scores below cutoff suggest “remission,” although functioning must be considered. Youth report symptoms best but parents may better report improved functioning and affect that can precede symptom reduction.
If there is no initial response (< 25% decrease in symptom score) or a partial response (25%-49% decrease), PCPs should begin treatment as for moderate depression with either a selective serotonin reuptake inhibitor (SSRI) or psychotherapy. Use of both has the best evidence; cognitive behavior therapy (CBT) and interpersonal psychotherapy for adolescents are equally effective.
Side effects from SSRIs are almost universal with GI upset, headaches, and sexual dysfunction most common, but activation (increased agitation or irritability) may occur. Educate patients about these and encourage tolerating them as they tend to subside in weeks, allowing continuation of these most effective medicines. Activation rarely indicates true mania, which would require stopping and referral.
Moderate depression with only comorbid anxiety may be addressed by PCPs with problem-focused supportive counseling and SSRIs, but mental health consultation or referral also are appropriate. Fluoxetine starting at 5-10 mg/day has best evidence and Food and Drug Administration approval for MDD from age 8. Starting at a higher dose may increase risk of suicidal ideation. Alternatively, escitalopram is FDA approved for MDD at age 12 starting at 10 mg/day, although meta-analyses do not distinguish effectiveness within the SSRI class. Although benefit usually appears within 2 weeks, a trial of at least 4 weeks should be used to assess effect.
If after 4 weeks, the SSRI is tolerated but has little or no response, reassess the diagnosis, try a different SSRI, e.g. sertraline, and add CBT (combined SSRI+CBT has an advantage). To switch SSRIs, reduce the first every 1-2 weeks (by 10-20 mg for fluoxetine; 5-10 for escitalopram) to reduce side effects. If overlapping, the replacement SSRI may start midway in the wean at low dose with patients educated about serotonin syndrome. If instead there was a partial response to the initial SSRI, progressively increase the dose (by 10 mg for fluoxetine or 5 mg for escitalopram monthly) as indicated by symptom change up to the maximum (60-80 mg fluoxetine or 20 mg escitalopram), if needed, and maintain for another 4 weeks. Alternatively, or in addition, start psychotherapy or ask to change current therapy, as therapy focus makes a difference in effect. Initial CBT focus on anxiety acts fastest when anxiety is comorbid.
Once a regimen produces a response, maintain it for 16-20 weeks, the longer for more severe depression. Although three-fourths of mildly to moderately depressed youth are late responders, emerging near 6 weeks, a rapid initial response is associated with better outcome. The recommended 8 weeks on a final tolerated dose constituting an adequate trial before changing may be shortened to 6 weeks in severe unremitting cases. Youth not remitting by 12 weeks should be offered alternative treatment. Referral is recommended for moderately severe depression with comorbidity or severe depression but also for unresponsive moderate depression or by family or clinician preference.
Treatment-resistant depression is defined as “clinically impairing depression symptoms despite an adequate trial of an evidence-based psychotherapy and an antidepressant with grade A evidence (fluoxetine, escitalopram, or sertraline),” sequentially or together; treatment-refractory depression comprises the above with failure on at least two antidepressants, with at least one being grade A. Unfortunately, TRD occurs in 30%-40% of children and remission is only 30%. Low adherence based on pill counts (> 30% missed) or with therapy (fewer than nine visits) should be considered in treatment failures.
With manageable factors addressed, the next step for TRD is treatment augmentation. The best evidence-based augmentation for TRD is CBT; 55% of those receiving CBT responded within 12 weeks. TRD augmentations and interventions with evidence in adults have either no evidence of effect in children (SNRIs, lithium), no randomized controlled trials, or support only from small suggestive studies, e.g., antipsychotics, 16 g/day omega-3 fatty acid supplementation, folic acid supplementation, repetitive transcranial magnetic stimulation, electroconvulsive therapy, or ketamine. Prompt referral to a child psychiatrist is essential for youth classified as TRD as earlier more aggressive treatment may avoid the long-term morbidity of chronic depression.
Fortunately, a meta-analysis of studies showed that PCP medication management visits with monitoring could improve outcomes, even for TRD.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Reference
Dwyer J et al. Annual research review: Defining and treating pediatric treatment-resistant depression. J Child Psychol Psychiatry. 2020 March;61(3):312-32.
As pediatricians, we are acutely aware of the increase in depression in our teen patients. Lifetime prevalence is now approaching 20%, and we are doing our best to help.
The Guidelines for Adolescent Depression in Primary Care (GLAD-PC, 2018) has advice on screening and primary care provider (PCP) management, verifying our role in care. But GLAD-PC also advises “referral to a mental health specialist” in patient scenarios we see multiple times per week. Even when patients are willing and able to go, mental health specialists are in short supply or have months-long waiting lists. What should we do to help the more severely depressed adolescent when immediate referral is not possible? What should we expect of specialist care for what is called treatment-resistant or treatment-refractory depression (TRD)?
To know what to do for a youth with TRD, first you need to know what constitutes an adequate trial of treatment. After diagnosis of major depressive disorder (MDD) from a validated screening tool or an interview based on DSM-5 criteria and an appropriate assessment (as described in GLAD-PC), patients and parents need education on symptoms, course, prognosis including suicide risk, and treatment options. Known TRD risk factors, besides longer or greater depression severity, anhedonia, and poor global functioning, can benefit from being specifically addressed: trauma, bullying, comorbid anxiety or substance use, subsyndromal mania, insomnia, hypothyroidism, nutritional deficiencies from eating disorders, certain genetic variants, LGBTQ identification, family conflict, and parental depression. Screening and assessment for suicidal ideation/attempts is needed initially and in follow-up as MDD increases risk of suicide 30 times.
PCPs can manage mild depression with regular visits every 1-2 weeks for active support for 6-8 weeks. Advise all depressed youth on healthy eating, adequate sleep and exercise, pleasurable activities, and refraining from substance use. With a full response (50%+ reduction in symptom score from baseline), monthly monitoring for symptoms, suicidality, and stressors (phone/televisits suffice) should continue for 6-24 months as half recur. Monitoring with ratings by both youth and parent are recommended and may be required by insurers. Scores below cutoff suggest “remission,” although functioning must be considered. Youth report symptoms best but parents may better report improved functioning and affect that can precede symptom reduction.
If there is no initial response (< 25% decrease in symptom score) or a partial response (25%-49% decrease), PCPs should begin treatment as for moderate depression with either a selective serotonin reuptake inhibitor (SSRI) or psychotherapy. Use of both has the best evidence; cognitive behavior therapy (CBT) and interpersonal psychotherapy for adolescents are equally effective.
Side effects from SSRIs are almost universal with GI upset, headaches, and sexual dysfunction most common, but activation (increased agitation or irritability) may occur. Educate patients about these and encourage tolerating them as they tend to subside in weeks, allowing continuation of these most effective medicines. Activation rarely indicates true mania, which would require stopping and referral.
Moderate depression with only comorbid anxiety may be addressed by PCPs with problem-focused supportive counseling and SSRIs, but mental health consultation or referral also are appropriate. Fluoxetine starting at 5-10 mg/day has best evidence and Food and Drug Administration approval for MDD from age 8. Starting at a higher dose may increase risk of suicidal ideation. Alternatively, escitalopram is FDA approved for MDD at age 12 starting at 10 mg/day, although meta-analyses do not distinguish effectiveness within the SSRI class. Although benefit usually appears within 2 weeks, a trial of at least 4 weeks should be used to assess effect.
If after 4 weeks, the SSRI is tolerated but has little or no response, reassess the diagnosis, try a different SSRI, e.g. sertraline, and add CBT (combined SSRI+CBT has an advantage). To switch SSRIs, reduce the first every 1-2 weeks (by 10-20 mg for fluoxetine; 5-10 for escitalopram) to reduce side effects. If overlapping, the replacement SSRI may start midway in the wean at low dose with patients educated about serotonin syndrome. If instead there was a partial response to the initial SSRI, progressively increase the dose (by 10 mg for fluoxetine or 5 mg for escitalopram monthly) as indicated by symptom change up to the maximum (60-80 mg fluoxetine or 20 mg escitalopram), if needed, and maintain for another 4 weeks. Alternatively, or in addition, start psychotherapy or ask to change current therapy, as therapy focus makes a difference in effect. Initial CBT focus on anxiety acts fastest when anxiety is comorbid.
Once a regimen produces a response, maintain it for 16-20 weeks, the longer for more severe depression. Although three-fourths of mildly to moderately depressed youth are late responders, emerging near 6 weeks, a rapid initial response is associated with better outcome. The recommended 8 weeks on a final tolerated dose constituting an adequate trial before changing may be shortened to 6 weeks in severe unremitting cases. Youth not remitting by 12 weeks should be offered alternative treatment. Referral is recommended for moderately severe depression with comorbidity or severe depression but also for unresponsive moderate depression or by family or clinician preference.
Treatment-resistant depression is defined as “clinically impairing depression symptoms despite an adequate trial of an evidence-based psychotherapy and an antidepressant with grade A evidence (fluoxetine, escitalopram, or sertraline),” sequentially or together; treatment-refractory depression comprises the above with failure on at least two antidepressants, with at least one being grade A. Unfortunately, TRD occurs in 30%-40% of children and remission is only 30%. Low adherence based on pill counts (> 30% missed) or with therapy (fewer than nine visits) should be considered in treatment failures.
With manageable factors addressed, the next step for TRD is treatment augmentation. The best evidence-based augmentation for TRD is CBT; 55% of those receiving CBT responded within 12 weeks. TRD augmentations and interventions with evidence in adults have either no evidence of effect in children (SNRIs, lithium), no randomized controlled trials, or support only from small suggestive studies, e.g., antipsychotics, 16 g/day omega-3 fatty acid supplementation, folic acid supplementation, repetitive transcranial magnetic stimulation, electroconvulsive therapy, or ketamine. Prompt referral to a child psychiatrist is essential for youth classified as TRD as earlier more aggressive treatment may avoid the long-term morbidity of chronic depression.
Fortunately, a meta-analysis of studies showed that PCP medication management visits with monitoring could improve outcomes, even for TRD.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Reference
Dwyer J et al. Annual research review: Defining and treating pediatric treatment-resistant depression. J Child Psychol Psychiatry. 2020 March;61(3):312-32.
‘Highest survival’ with combo immunotherapy in advanced melanoma
An researchers say.
Nearly half the patients treated with nivolumab (Opdivo) and ipilimumab (Yervoy) were alive at 6½ years. Within this group, 77% had not received further systemic treatment after coming off the study drugs.
After a minimum follow-up of 77 months, median overall survival was 72.1 months in patients on the combination, which was more than three times longer than the 19.9 months with ipilimumab alone (hazard ratio, 0.52; 95% confidence interval, 0.43-0.64) and twice as long as the 36.9 months with nivolumab alone (HR, 0.84; 95% CI, 0.67-1.04).
The results represent the longest median overall survival seen in a phase 3 trial of advanced melanoma and are evidence of “a substantial development in the melanoma treatment landscape versus the standard median survival of 8 months a decade ago,” researchers wrote in a study published online in the Journal of Clinical Oncology.
However, lead author Jedd D. Wolchok, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, noted that the study was not designed to compare nivolumab alone with the combination. “It wasn’t powered for that. [But] what we can say is that the highest survival was in the combination group,” Dr. Wolchok told this news organization.
Dr. Wolchok cautioned that the combination therapy is not currently standard of care. “PD-1 blockade – either nivolumab or the combination – are both excellent options for care,” he added. “I can’t tell you that one of them is the standard of care because that’s too complex of a decision.”
For example, he explained, “for a patient who only has lung metastases, a single-agent PD-1 blockade might be sufficient. But if it has spread to other organs, such as the liver or bones, which are more difficult to treat, that’s when we often reach for the combination.”
Other factors that weigh into the therapeutic decision are the patient’s performance status and their so-called clinical reserve for tolerating side effects. “The likelihood of having a high-grade side effect with the combination is more than twice that of the single agent,” Dr. Wolchok said.
Until 2011, only two therapies were approved for metastatic melanoma: Chemotherapy with dacarbazine and immunotherapy with high-dose interleukin-2, neither of which was very effective at prolonging life. But patient survival changed with the advent of targeted therapies and immunotherapy. Some patients are now living for years, and as the current study shows, many have surpassed the 5-year mark and are treatment free.
The updated CheckMate 067 analysis included patients with previously untreated, unresectable stage III/IV melanoma who were randomly assigned to receive nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks (four doses) followed by nivolumab 3 mg/kg every 2 weeks (n = 314), nivolumab 3 mg/kg every 2 weeks (n = 316), or ipilimumab 3 mg/ kg every 3 weeks (four doses; n = 315).
The authors reported the 5-year overall survival rates from the trial, published in the New England Journal of Medicine in 2019 – 52% with the combination, 44% with nivolumab alone, and 26% with ipilimumab alone.
In the updated study, overall survival at 6½ years had dropped slightly to 49%, 42%, and 23%, respectively. Patients with BRAF-mutant tumors had overall survival rates of 57%, 43%, and 25% versus 46%, 42%, and 22% in those with BRAF wild-type tumors.
Overall, median investigator-assessed progression-free survival was 11.5 months with the combination, 6.9 months with nivolumab alone, and 2.9 months with ipilimumab.
The new analysis also evaluated melanoma-specific survival (MSS), which removes competing causes of deaths from the long-term follow-up. The MSS was not reached in the combination group, and was 58.7 months in the nivolumab group and 21.9 months for ipilimumab, with MSS rates at 6.5 years of 56%, 48%, and 27%, respectively.
No new safety signals were detected, but there was more immune-mediated toxicity in the combination group, the researchers reported.
“The patients will continue to be followed,” said Dr. Wolchok, “And data are still being collected.”
The trial was supported by Bristol-Myers Squibb, the National Cancer Institute, and the National Institute for Health Research Royal Marsden–Institute of Cancer Research Biomedical Research Centre. Dr. Wolchok and coauthors reported relationships with Bristol-Myers Squibb and other drugmakers.
A version of this article first appeared on Medscape.com.
An researchers say.
Nearly half the patients treated with nivolumab (Opdivo) and ipilimumab (Yervoy) were alive at 6½ years. Within this group, 77% had not received further systemic treatment after coming off the study drugs.
After a minimum follow-up of 77 months, median overall survival was 72.1 months in patients on the combination, which was more than three times longer than the 19.9 months with ipilimumab alone (hazard ratio, 0.52; 95% confidence interval, 0.43-0.64) and twice as long as the 36.9 months with nivolumab alone (HR, 0.84; 95% CI, 0.67-1.04).
The results represent the longest median overall survival seen in a phase 3 trial of advanced melanoma and are evidence of “a substantial development in the melanoma treatment landscape versus the standard median survival of 8 months a decade ago,” researchers wrote in a study published online in the Journal of Clinical Oncology.
However, lead author Jedd D. Wolchok, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, noted that the study was not designed to compare nivolumab alone with the combination. “It wasn’t powered for that. [But] what we can say is that the highest survival was in the combination group,” Dr. Wolchok told this news organization.
Dr. Wolchok cautioned that the combination therapy is not currently standard of care. “PD-1 blockade – either nivolumab or the combination – are both excellent options for care,” he added. “I can’t tell you that one of them is the standard of care because that’s too complex of a decision.”
For example, he explained, “for a patient who only has lung metastases, a single-agent PD-1 blockade might be sufficient. But if it has spread to other organs, such as the liver or bones, which are more difficult to treat, that’s when we often reach for the combination.”
Other factors that weigh into the therapeutic decision are the patient’s performance status and their so-called clinical reserve for tolerating side effects. “The likelihood of having a high-grade side effect with the combination is more than twice that of the single agent,” Dr. Wolchok said.
Until 2011, only two therapies were approved for metastatic melanoma: Chemotherapy with dacarbazine and immunotherapy with high-dose interleukin-2, neither of which was very effective at prolonging life. But patient survival changed with the advent of targeted therapies and immunotherapy. Some patients are now living for years, and as the current study shows, many have surpassed the 5-year mark and are treatment free.
The updated CheckMate 067 analysis included patients with previously untreated, unresectable stage III/IV melanoma who were randomly assigned to receive nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks (four doses) followed by nivolumab 3 mg/kg every 2 weeks (n = 314), nivolumab 3 mg/kg every 2 weeks (n = 316), or ipilimumab 3 mg/ kg every 3 weeks (four doses; n = 315).
The authors reported the 5-year overall survival rates from the trial, published in the New England Journal of Medicine in 2019 – 52% with the combination, 44% with nivolumab alone, and 26% with ipilimumab alone.
In the updated study, overall survival at 6½ years had dropped slightly to 49%, 42%, and 23%, respectively. Patients with BRAF-mutant tumors had overall survival rates of 57%, 43%, and 25% versus 46%, 42%, and 22% in those with BRAF wild-type tumors.
Overall, median investigator-assessed progression-free survival was 11.5 months with the combination, 6.9 months with nivolumab alone, and 2.9 months with ipilimumab.
The new analysis also evaluated melanoma-specific survival (MSS), which removes competing causes of deaths from the long-term follow-up. The MSS was not reached in the combination group, and was 58.7 months in the nivolumab group and 21.9 months for ipilimumab, with MSS rates at 6.5 years of 56%, 48%, and 27%, respectively.
No new safety signals were detected, but there was more immune-mediated toxicity in the combination group, the researchers reported.
“The patients will continue to be followed,” said Dr. Wolchok, “And data are still being collected.”
The trial was supported by Bristol-Myers Squibb, the National Cancer Institute, and the National Institute for Health Research Royal Marsden–Institute of Cancer Research Biomedical Research Centre. Dr. Wolchok and coauthors reported relationships with Bristol-Myers Squibb and other drugmakers.
A version of this article first appeared on Medscape.com.
An researchers say.
Nearly half the patients treated with nivolumab (Opdivo) and ipilimumab (Yervoy) were alive at 6½ years. Within this group, 77% had not received further systemic treatment after coming off the study drugs.
After a minimum follow-up of 77 months, median overall survival was 72.1 months in patients on the combination, which was more than three times longer than the 19.9 months with ipilimumab alone (hazard ratio, 0.52; 95% confidence interval, 0.43-0.64) and twice as long as the 36.9 months with nivolumab alone (HR, 0.84; 95% CI, 0.67-1.04).
The results represent the longest median overall survival seen in a phase 3 trial of advanced melanoma and are evidence of “a substantial development in the melanoma treatment landscape versus the standard median survival of 8 months a decade ago,” researchers wrote in a study published online in the Journal of Clinical Oncology.
However, lead author Jedd D. Wolchok, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, noted that the study was not designed to compare nivolumab alone with the combination. “It wasn’t powered for that. [But] what we can say is that the highest survival was in the combination group,” Dr. Wolchok told this news organization.
Dr. Wolchok cautioned that the combination therapy is not currently standard of care. “PD-1 blockade – either nivolumab or the combination – are both excellent options for care,” he added. “I can’t tell you that one of them is the standard of care because that’s too complex of a decision.”
For example, he explained, “for a patient who only has lung metastases, a single-agent PD-1 blockade might be sufficient. But if it has spread to other organs, such as the liver or bones, which are more difficult to treat, that’s when we often reach for the combination.”
Other factors that weigh into the therapeutic decision are the patient’s performance status and their so-called clinical reserve for tolerating side effects. “The likelihood of having a high-grade side effect with the combination is more than twice that of the single agent,” Dr. Wolchok said.
Until 2011, only two therapies were approved for metastatic melanoma: Chemotherapy with dacarbazine and immunotherapy with high-dose interleukin-2, neither of which was very effective at prolonging life. But patient survival changed with the advent of targeted therapies and immunotherapy. Some patients are now living for years, and as the current study shows, many have surpassed the 5-year mark and are treatment free.
The updated CheckMate 067 analysis included patients with previously untreated, unresectable stage III/IV melanoma who were randomly assigned to receive nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks (four doses) followed by nivolumab 3 mg/kg every 2 weeks (n = 314), nivolumab 3 mg/kg every 2 weeks (n = 316), or ipilimumab 3 mg/ kg every 3 weeks (four doses; n = 315).
The authors reported the 5-year overall survival rates from the trial, published in the New England Journal of Medicine in 2019 – 52% with the combination, 44% with nivolumab alone, and 26% with ipilimumab alone.
In the updated study, overall survival at 6½ years had dropped slightly to 49%, 42%, and 23%, respectively. Patients with BRAF-mutant tumors had overall survival rates of 57%, 43%, and 25% versus 46%, 42%, and 22% in those with BRAF wild-type tumors.
Overall, median investigator-assessed progression-free survival was 11.5 months with the combination, 6.9 months with nivolumab alone, and 2.9 months with ipilimumab.
The new analysis also evaluated melanoma-specific survival (MSS), which removes competing causes of deaths from the long-term follow-up. The MSS was not reached in the combination group, and was 58.7 months in the nivolumab group and 21.9 months for ipilimumab, with MSS rates at 6.5 years of 56%, 48%, and 27%, respectively.
No new safety signals were detected, but there was more immune-mediated toxicity in the combination group, the researchers reported.
“The patients will continue to be followed,” said Dr. Wolchok, “And data are still being collected.”
The trial was supported by Bristol-Myers Squibb, the National Cancer Institute, and the National Institute for Health Research Royal Marsden–Institute of Cancer Research Biomedical Research Centre. Dr. Wolchok and coauthors reported relationships with Bristol-Myers Squibb and other drugmakers.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
AMA president calls on Congress to stabilize Medicare payments to physicians
Physician practices around the country took an unprecedented financial hit with the arrival of the COVID-19 pandemic in March 2020. Recent research from the American Medical Association reveals an estimated pandemic-related shortfall in Medicare physician fee spending of $13.9 billion, or a 14% reduction, across all states and all major specialties in 2020.
While the report pointed to a “strong recovery” in May and June, that recovery stalled in the second half of 2020, and spending never returned to pre–COVID-19 levels.
“Physicians experienced a significant and sustained drop in Medicare revenue during the first 10 months of the pandemic,” said AMA President Gerald Harmon, MD, in a statement. “Medical practices that have not buckled under financial strain continue to be stretched clinically, emotionally, and fiscally as the pandemic persists. Yet physicians face an array of planned cuts that would reduce Medicare physician payments by nearly 10% for 2022.”
The reduction in the Medicare physician fee schedule payments means providers may face payment cuts of more than 9% starting Jan. 1, 2022, when the cuts take effect. That is, unless Congress makes changes.
Medicare physician fee schedule spending on telehealth stood at $4.1 billion, or 5% of the total Medicare spent in 2020. From March 16 to June 30, $1.8 billion of this amount was on telehealth, while $1.1 billion came in during third and fourth quarters of 2020, respectively, per the report.
According to AMA’s research:
- Medicare physician fee schedule spending for 2020, relative to expected 2020 spending, dipped 32% between March 16 and June 30; spending was down during the last 6 months of the year by between 9% and 10%.
- The care settings hit the worst were ambulatory surgical centers, outpatient hospitals, and physician offices; the next worst off were hospital emergency departments, inpatient hospitals, and skilled nursing facilities.
- The specialties that fared worst included physical therapists (-28%), opthamologists (-19%), podiatrists (-18%), and dermatologists (-18%).
- Cumulative spending was down the most in Minnesota (-22%), Maine (-19%), and New York (-19%); less affected states included Idaho (-9%), Oklahoma (-9%), and South Carolina (9%).
AMA: Budget neutrality hurting physicians’ financial stability
Dr. Harmon is calling for financial stability in Medicare spending. In particular, the AMA is “strongly urging Congress to avert the planned payment cuts,” he said in a statement.
The challenge: The Medicare physician fee schedule is currently “budget neutral,” meaning that the budget is fixed, Dr. Harmon, a family medicine specialist in South Carolina, told this news organization.
“If you rob from Peter to pay Paul, Paul is going to be less efficient or less rewarded. It continues to be that there’s always a ‘pay for’ in these things. So budget neutrality is probably one of the first things we need to address,” he said.
Lack of routine care expected to affect health outcomes
The result of reduced screening and treatment during the pandemic could be as many as 10,000 excess deaths due to cancers of the breast and colon during the next 10 years, wrote Norman Sharpless, MD, director of the National Cancer Institute, in Science in June. Combined, breast cancer and colon cancer account for one-sixth of all cancers in the U.S., he wrote.
In addition, blood pressure control has gotten worse since the start of the pandemic, said Michael Rakotz, MD, FAHA, FAAFP, vice president of improving health outcomes at the AMA, in an AMA blog post.
Dr. Harmon’s advice for physician practices on getting patients in for routine care:
- Educate the area’s largest employers to encourage their employees.
- Engage with hospital employees, since hospitals are often the largest employers in many communities.
- Partner with health insurers.
- Show up at athletic events, which is a particularly good fit for “small town America,” said Dr. Harmon.
The AMA’s research doesn’t consider reimbursement from other public and private payers. It also doesn’t account for funding sources such as Provider Relief Fund grants, Paycheck Protection Program loans, and the temporary suspension of the Medicare sequester, per the report.
A version of this article first appeared on Medscape.com.
Physician practices around the country took an unprecedented financial hit with the arrival of the COVID-19 pandemic in March 2020. Recent research from the American Medical Association reveals an estimated pandemic-related shortfall in Medicare physician fee spending of $13.9 billion, or a 14% reduction, across all states and all major specialties in 2020.
While the report pointed to a “strong recovery” in May and June, that recovery stalled in the second half of 2020, and spending never returned to pre–COVID-19 levels.
“Physicians experienced a significant and sustained drop in Medicare revenue during the first 10 months of the pandemic,” said AMA President Gerald Harmon, MD, in a statement. “Medical practices that have not buckled under financial strain continue to be stretched clinically, emotionally, and fiscally as the pandemic persists. Yet physicians face an array of planned cuts that would reduce Medicare physician payments by nearly 10% for 2022.”
The reduction in the Medicare physician fee schedule payments means providers may face payment cuts of more than 9% starting Jan. 1, 2022, when the cuts take effect. That is, unless Congress makes changes.
Medicare physician fee schedule spending on telehealth stood at $4.1 billion, or 5% of the total Medicare spent in 2020. From March 16 to June 30, $1.8 billion of this amount was on telehealth, while $1.1 billion came in during third and fourth quarters of 2020, respectively, per the report.
According to AMA’s research:
- Medicare physician fee schedule spending for 2020, relative to expected 2020 spending, dipped 32% between March 16 and June 30; spending was down during the last 6 months of the year by between 9% and 10%.
- The care settings hit the worst were ambulatory surgical centers, outpatient hospitals, and physician offices; the next worst off were hospital emergency departments, inpatient hospitals, and skilled nursing facilities.
- The specialties that fared worst included physical therapists (-28%), opthamologists (-19%), podiatrists (-18%), and dermatologists (-18%).
- Cumulative spending was down the most in Minnesota (-22%), Maine (-19%), and New York (-19%); less affected states included Idaho (-9%), Oklahoma (-9%), and South Carolina (9%).
AMA: Budget neutrality hurting physicians’ financial stability
Dr. Harmon is calling for financial stability in Medicare spending. In particular, the AMA is “strongly urging Congress to avert the planned payment cuts,” he said in a statement.
The challenge: The Medicare physician fee schedule is currently “budget neutral,” meaning that the budget is fixed, Dr. Harmon, a family medicine specialist in South Carolina, told this news organization.
“If you rob from Peter to pay Paul, Paul is going to be less efficient or less rewarded. It continues to be that there’s always a ‘pay for’ in these things. So budget neutrality is probably one of the first things we need to address,” he said.
Lack of routine care expected to affect health outcomes
The result of reduced screening and treatment during the pandemic could be as many as 10,000 excess deaths due to cancers of the breast and colon during the next 10 years, wrote Norman Sharpless, MD, director of the National Cancer Institute, in Science in June. Combined, breast cancer and colon cancer account for one-sixth of all cancers in the U.S., he wrote.
In addition, blood pressure control has gotten worse since the start of the pandemic, said Michael Rakotz, MD, FAHA, FAAFP, vice president of improving health outcomes at the AMA, in an AMA blog post.
Dr. Harmon’s advice for physician practices on getting patients in for routine care:
- Educate the area’s largest employers to encourage their employees.
- Engage with hospital employees, since hospitals are often the largest employers in many communities.
- Partner with health insurers.
- Show up at athletic events, which is a particularly good fit for “small town America,” said Dr. Harmon.
The AMA’s research doesn’t consider reimbursement from other public and private payers. It also doesn’t account for funding sources such as Provider Relief Fund grants, Paycheck Protection Program loans, and the temporary suspension of the Medicare sequester, per the report.
A version of this article first appeared on Medscape.com.
Physician practices around the country took an unprecedented financial hit with the arrival of the COVID-19 pandemic in March 2020. Recent research from the American Medical Association reveals an estimated pandemic-related shortfall in Medicare physician fee spending of $13.9 billion, or a 14% reduction, across all states and all major specialties in 2020.
While the report pointed to a “strong recovery” in May and June, that recovery stalled in the second half of 2020, and spending never returned to pre–COVID-19 levels.
“Physicians experienced a significant and sustained drop in Medicare revenue during the first 10 months of the pandemic,” said AMA President Gerald Harmon, MD, in a statement. “Medical practices that have not buckled under financial strain continue to be stretched clinically, emotionally, and fiscally as the pandemic persists. Yet physicians face an array of planned cuts that would reduce Medicare physician payments by nearly 10% for 2022.”
The reduction in the Medicare physician fee schedule payments means providers may face payment cuts of more than 9% starting Jan. 1, 2022, when the cuts take effect. That is, unless Congress makes changes.
Medicare physician fee schedule spending on telehealth stood at $4.1 billion, or 5% of the total Medicare spent in 2020. From March 16 to June 30, $1.8 billion of this amount was on telehealth, while $1.1 billion came in during third and fourth quarters of 2020, respectively, per the report.
According to AMA’s research:
- Medicare physician fee schedule spending for 2020, relative to expected 2020 spending, dipped 32% between March 16 and June 30; spending was down during the last 6 months of the year by between 9% and 10%.
- The care settings hit the worst were ambulatory surgical centers, outpatient hospitals, and physician offices; the next worst off were hospital emergency departments, inpatient hospitals, and skilled nursing facilities.
- The specialties that fared worst included physical therapists (-28%), opthamologists (-19%), podiatrists (-18%), and dermatologists (-18%).
- Cumulative spending was down the most in Minnesota (-22%), Maine (-19%), and New York (-19%); less affected states included Idaho (-9%), Oklahoma (-9%), and South Carolina (9%).
AMA: Budget neutrality hurting physicians’ financial stability
Dr. Harmon is calling for financial stability in Medicare spending. In particular, the AMA is “strongly urging Congress to avert the planned payment cuts,” he said in a statement.
The challenge: The Medicare physician fee schedule is currently “budget neutral,” meaning that the budget is fixed, Dr. Harmon, a family medicine specialist in South Carolina, told this news organization.
“If you rob from Peter to pay Paul, Paul is going to be less efficient or less rewarded. It continues to be that there’s always a ‘pay for’ in these things. So budget neutrality is probably one of the first things we need to address,” he said.
Lack of routine care expected to affect health outcomes
The result of reduced screening and treatment during the pandemic could be as many as 10,000 excess deaths due to cancers of the breast and colon during the next 10 years, wrote Norman Sharpless, MD, director of the National Cancer Institute, in Science in June. Combined, breast cancer and colon cancer account for one-sixth of all cancers in the U.S., he wrote.
In addition, blood pressure control has gotten worse since the start of the pandemic, said Michael Rakotz, MD, FAHA, FAAFP, vice president of improving health outcomes at the AMA, in an AMA blog post.
Dr. Harmon’s advice for physician practices on getting patients in for routine care:
- Educate the area’s largest employers to encourage their employees.
- Engage with hospital employees, since hospitals are often the largest employers in many communities.
- Partner with health insurers.
- Show up at athletic events, which is a particularly good fit for “small town America,” said Dr. Harmon.
The AMA’s research doesn’t consider reimbursement from other public and private payers. It also doesn’t account for funding sources such as Provider Relief Fund grants, Paycheck Protection Program loans, and the temporary suspension of the Medicare sequester, per the report.
A version of this article first appeared on Medscape.com.
Physicians may be overprescribing immunotherapy for unfit cancer patients
.
The study, by Ravi B. Parikh, MD, an assistant professor of medical ethics and health policy and medicine at the University of Pennsylvania, Philadelphia, is an analysis of patient data from 280 U.S.-based community oncology practices. It included 34,131 patients who received first-line systemic therapy with immune checkpoint inhibitors (ICIs), or other treatment, between January 2014 and December 2019 for newly diagnosed metastatic or recurrent non–small cell lung cancer (NSCLC), urothelial cell cancer (UCC), renal cell cancer (RCC), or hepatocellular carcinoma (HCC). Researchers examined survival outcomes between patients who were eligible to participate in clinical trials with those who were deemed ineligible but may have still received ICIs.
For patients with poor performance status or organ dysfunction, participating in randomized clinical trials for immune checkpoint inhibitors is largely out of reach because of advanced disease, but it is not unusual for these patients to be accepted into clinical trials, a decision sometimes referred to as “desperation oncology,” the authors wrote.
In this study of 34,131 patients, 9,318 were considered ineligible to participate in ICI clinical trials because of advanced disease or organ dysfunction, yet up to 30% of these patients were treated with ICIs by their physician outside of a clinical trial. Dr. Parikh and colleagues found no overall survival differences between patients deemed ineligible for clinical trials, but were ultimately treated with ICI monotherapy, ICI combination therapy, or other treatments at 12 and 36 months. In fact, ICI monotherapy appeared to be harmful within 6 months of starting treatment.
“Clinicians who care for patients with poor performance status or organ dysfunction should be cautious about ICI use and carefully weigh expected survival gains against the potential for early mortality and adverse effects,” the authors wrote. They found the efficacy of ICI treatment alone, or in combination with other treatment, can be worse among trial-ineligible patients than patients who met the criteria for clinical trials.
No survival benefit was found for trial-ineligible patients who were treated with ICI monotherapy or combination therapy. Overall survival rates were similar at 12 and 36 months for both treatment groups. The overall median survival was less than 10 months, but 40% of trial ineligible patients treated with ICIs died within 6 months.
The use of ICIs for patients with poor performance status was found to be associated with lower hospice enrollment, more inpatient deaths, and more treatment during the last month of life. “It is critical to ensure that vulnerable, trial-ineligible patients are not exposed to non–evidence-based therapies that could cause harm and contradict patient goals,” the authors wrote.
The harms of treating unfit patients
The use of immune checkpoint inhibitor monotherapy in trial-ineligible patients is concerning, the authors said, because for patients with UCC and NSCLC, the standard of care is platinum-based chemotherapy. For patients with HCC and RCC, the standard of care is oral anti–vascular endothelial growth factor therapy. Immune checkpoint inhibitors may be prescribed in these cases to avoid side effects associated with other therapies, despite the lack of evidence showing that ICIs are effective in these cases.
“Individuals with poor performance status and/or organ dysfunction are vulnerable to receiving treatments that may not benefit them or cause disproportionately high side effects,” Dr. Parikh said in an interview. “Immunotherapy causes fewer side effects overall and is an attractive option, but there is no good phase 3 evidence that immunotherapy has benefits in this population.
“Physicians are preferentially using immunotherapy for unfit patients despite the fact that these individuals are usually excluded from clinical trials. Trial-ineligible patients – despite making up 30% of the cancer population – are different from patients studied in clinical trials. They are generally sicker, older and more prone to treatment adverse effects (including death), However, excluding these groups means that we don’t have good data on what treatments could benefit this vulnerable group. Thus, we are usually left to extrapolating results from healthier patients to unhealthy patients which risks giving them the wrong treatment,” he said.
A review that looked at immunotherapy in older adults suggested that, while those aged 65 or older represent most cancer patients, they are under-represented in clinical trials, including studies that led to approval of immunotherapy agents. A 2019 report suggested that, while 11 pivotal phase 3, randomized clinical trials have estimated the activity of ICIs in locally advanced and advanced NSCLC, each trial excluded patients with poor performance status.
Phase 3 trials needed for patients with poor performance
This retrospective study included 34,131 patients (median age, 70 years; 42% women) of which 27.3% had poor performance status and/or organ dysfunction and were classed as trial ineligible. The researchers assessed the use and overall survival outcomes following first-line ICI and non-ICI therapy that was initiated from January 2014 through December 2019.
Over the course of the study, the proportion of patients receiving ICI monotherapy increased from 0%-30.2% among trial-ineligible patients and from 0.1%-19.4% among eligible patients. However, among trial-ineligible patients, there were no overall survival differences between treatment with ICI monotherapy, ICI combination therapy and non-ICI therapy at 12 and 36 months.
Among trial-ineligible patients, ICI use was linked to a 14%-19% greater risk of death during the first 6 months after ICI initiation, but a 20% lower risk of death among those who survived 6 months after ICI initiation. Further, ICI combination therapy was associated with potential early harm among trial-ineligible patients.
“Phase 3 trials are sorely needed in patients with poor performance status or organ dysfunction so that we can adequately counsel patients who are unfit about expectations with novel cancer therapies,” Dr. Parikh said.
The cohort only included patients who received systemic therapy, which is a limitation of the study, so conclusions cannot be made about the efficacy of systemic therapy versus no systemic therapy in trial-ineligible patients.
Dr. Parikh reported nonfinancial support from Flatiron Health, grants from Humana, personal fees and equity from GNS Healthcare and Onc.AI, along with personal fees from the Cancer Study Group and Nanology outside the submitted work.
.
The study, by Ravi B. Parikh, MD, an assistant professor of medical ethics and health policy and medicine at the University of Pennsylvania, Philadelphia, is an analysis of patient data from 280 U.S.-based community oncology practices. It included 34,131 patients who received first-line systemic therapy with immune checkpoint inhibitors (ICIs), or other treatment, between January 2014 and December 2019 for newly diagnosed metastatic or recurrent non–small cell lung cancer (NSCLC), urothelial cell cancer (UCC), renal cell cancer (RCC), or hepatocellular carcinoma (HCC). Researchers examined survival outcomes between patients who were eligible to participate in clinical trials with those who were deemed ineligible but may have still received ICIs.
For patients with poor performance status or organ dysfunction, participating in randomized clinical trials for immune checkpoint inhibitors is largely out of reach because of advanced disease, but it is not unusual for these patients to be accepted into clinical trials, a decision sometimes referred to as “desperation oncology,” the authors wrote.
In this study of 34,131 patients, 9,318 were considered ineligible to participate in ICI clinical trials because of advanced disease or organ dysfunction, yet up to 30% of these patients were treated with ICIs by their physician outside of a clinical trial. Dr. Parikh and colleagues found no overall survival differences between patients deemed ineligible for clinical trials, but were ultimately treated with ICI monotherapy, ICI combination therapy, or other treatments at 12 and 36 months. In fact, ICI monotherapy appeared to be harmful within 6 months of starting treatment.
“Clinicians who care for patients with poor performance status or organ dysfunction should be cautious about ICI use and carefully weigh expected survival gains against the potential for early mortality and adverse effects,” the authors wrote. They found the efficacy of ICI treatment alone, or in combination with other treatment, can be worse among trial-ineligible patients than patients who met the criteria for clinical trials.
No survival benefit was found for trial-ineligible patients who were treated with ICI monotherapy or combination therapy. Overall survival rates were similar at 12 and 36 months for both treatment groups. The overall median survival was less than 10 months, but 40% of trial ineligible patients treated with ICIs died within 6 months.
The use of ICIs for patients with poor performance status was found to be associated with lower hospice enrollment, more inpatient deaths, and more treatment during the last month of life. “It is critical to ensure that vulnerable, trial-ineligible patients are not exposed to non–evidence-based therapies that could cause harm and contradict patient goals,” the authors wrote.
The harms of treating unfit patients
The use of immune checkpoint inhibitor monotherapy in trial-ineligible patients is concerning, the authors said, because for patients with UCC and NSCLC, the standard of care is platinum-based chemotherapy. For patients with HCC and RCC, the standard of care is oral anti–vascular endothelial growth factor therapy. Immune checkpoint inhibitors may be prescribed in these cases to avoid side effects associated with other therapies, despite the lack of evidence showing that ICIs are effective in these cases.
“Individuals with poor performance status and/or organ dysfunction are vulnerable to receiving treatments that may not benefit them or cause disproportionately high side effects,” Dr. Parikh said in an interview. “Immunotherapy causes fewer side effects overall and is an attractive option, but there is no good phase 3 evidence that immunotherapy has benefits in this population.
“Physicians are preferentially using immunotherapy for unfit patients despite the fact that these individuals are usually excluded from clinical trials. Trial-ineligible patients – despite making up 30% of the cancer population – are different from patients studied in clinical trials. They are generally sicker, older and more prone to treatment adverse effects (including death), However, excluding these groups means that we don’t have good data on what treatments could benefit this vulnerable group. Thus, we are usually left to extrapolating results from healthier patients to unhealthy patients which risks giving them the wrong treatment,” he said.
A review that looked at immunotherapy in older adults suggested that, while those aged 65 or older represent most cancer patients, they are under-represented in clinical trials, including studies that led to approval of immunotherapy agents. A 2019 report suggested that, while 11 pivotal phase 3, randomized clinical trials have estimated the activity of ICIs in locally advanced and advanced NSCLC, each trial excluded patients with poor performance status.
Phase 3 trials needed for patients with poor performance
This retrospective study included 34,131 patients (median age, 70 years; 42% women) of which 27.3% had poor performance status and/or organ dysfunction and were classed as trial ineligible. The researchers assessed the use and overall survival outcomes following first-line ICI and non-ICI therapy that was initiated from January 2014 through December 2019.
Over the course of the study, the proportion of patients receiving ICI monotherapy increased from 0%-30.2% among trial-ineligible patients and from 0.1%-19.4% among eligible patients. However, among trial-ineligible patients, there were no overall survival differences between treatment with ICI monotherapy, ICI combination therapy and non-ICI therapy at 12 and 36 months.
Among trial-ineligible patients, ICI use was linked to a 14%-19% greater risk of death during the first 6 months after ICI initiation, but a 20% lower risk of death among those who survived 6 months after ICI initiation. Further, ICI combination therapy was associated with potential early harm among trial-ineligible patients.
“Phase 3 trials are sorely needed in patients with poor performance status or organ dysfunction so that we can adequately counsel patients who are unfit about expectations with novel cancer therapies,” Dr. Parikh said.
The cohort only included patients who received systemic therapy, which is a limitation of the study, so conclusions cannot be made about the efficacy of systemic therapy versus no systemic therapy in trial-ineligible patients.
Dr. Parikh reported nonfinancial support from Flatiron Health, grants from Humana, personal fees and equity from GNS Healthcare and Onc.AI, along with personal fees from the Cancer Study Group and Nanology outside the submitted work.
.
The study, by Ravi B. Parikh, MD, an assistant professor of medical ethics and health policy and medicine at the University of Pennsylvania, Philadelphia, is an analysis of patient data from 280 U.S.-based community oncology practices. It included 34,131 patients who received first-line systemic therapy with immune checkpoint inhibitors (ICIs), or other treatment, between January 2014 and December 2019 for newly diagnosed metastatic or recurrent non–small cell lung cancer (NSCLC), urothelial cell cancer (UCC), renal cell cancer (RCC), or hepatocellular carcinoma (HCC). Researchers examined survival outcomes between patients who were eligible to participate in clinical trials with those who were deemed ineligible but may have still received ICIs.
For patients with poor performance status or organ dysfunction, participating in randomized clinical trials for immune checkpoint inhibitors is largely out of reach because of advanced disease, but it is not unusual for these patients to be accepted into clinical trials, a decision sometimes referred to as “desperation oncology,” the authors wrote.
In this study of 34,131 patients, 9,318 were considered ineligible to participate in ICI clinical trials because of advanced disease or organ dysfunction, yet up to 30% of these patients were treated with ICIs by their physician outside of a clinical trial. Dr. Parikh and colleagues found no overall survival differences between patients deemed ineligible for clinical trials, but were ultimately treated with ICI monotherapy, ICI combination therapy, or other treatments at 12 and 36 months. In fact, ICI monotherapy appeared to be harmful within 6 months of starting treatment.
“Clinicians who care for patients with poor performance status or organ dysfunction should be cautious about ICI use and carefully weigh expected survival gains against the potential for early mortality and adverse effects,” the authors wrote. They found the efficacy of ICI treatment alone, or in combination with other treatment, can be worse among trial-ineligible patients than patients who met the criteria for clinical trials.
No survival benefit was found for trial-ineligible patients who were treated with ICI monotherapy or combination therapy. Overall survival rates were similar at 12 and 36 months for both treatment groups. The overall median survival was less than 10 months, but 40% of trial ineligible patients treated with ICIs died within 6 months.
The use of ICIs for patients with poor performance status was found to be associated with lower hospice enrollment, more inpatient deaths, and more treatment during the last month of life. “It is critical to ensure that vulnerable, trial-ineligible patients are not exposed to non–evidence-based therapies that could cause harm and contradict patient goals,” the authors wrote.
The harms of treating unfit patients
The use of immune checkpoint inhibitor monotherapy in trial-ineligible patients is concerning, the authors said, because for patients with UCC and NSCLC, the standard of care is platinum-based chemotherapy. For patients with HCC and RCC, the standard of care is oral anti–vascular endothelial growth factor therapy. Immune checkpoint inhibitors may be prescribed in these cases to avoid side effects associated with other therapies, despite the lack of evidence showing that ICIs are effective in these cases.
“Individuals with poor performance status and/or organ dysfunction are vulnerable to receiving treatments that may not benefit them or cause disproportionately high side effects,” Dr. Parikh said in an interview. “Immunotherapy causes fewer side effects overall and is an attractive option, but there is no good phase 3 evidence that immunotherapy has benefits in this population.
“Physicians are preferentially using immunotherapy for unfit patients despite the fact that these individuals are usually excluded from clinical trials. Trial-ineligible patients – despite making up 30% of the cancer population – are different from patients studied in clinical trials. They are generally sicker, older and more prone to treatment adverse effects (including death), However, excluding these groups means that we don’t have good data on what treatments could benefit this vulnerable group. Thus, we are usually left to extrapolating results from healthier patients to unhealthy patients which risks giving them the wrong treatment,” he said.
A review that looked at immunotherapy in older adults suggested that, while those aged 65 or older represent most cancer patients, they are under-represented in clinical trials, including studies that led to approval of immunotherapy agents. A 2019 report suggested that, while 11 pivotal phase 3, randomized clinical trials have estimated the activity of ICIs in locally advanced and advanced NSCLC, each trial excluded patients with poor performance status.
Phase 3 trials needed for patients with poor performance
This retrospective study included 34,131 patients (median age, 70 years; 42% women) of which 27.3% had poor performance status and/or organ dysfunction and were classed as trial ineligible. The researchers assessed the use and overall survival outcomes following first-line ICI and non-ICI therapy that was initiated from January 2014 through December 2019.
Over the course of the study, the proportion of patients receiving ICI monotherapy increased from 0%-30.2% among trial-ineligible patients and from 0.1%-19.4% among eligible patients. However, among trial-ineligible patients, there were no overall survival differences between treatment with ICI monotherapy, ICI combination therapy and non-ICI therapy at 12 and 36 months.
Among trial-ineligible patients, ICI use was linked to a 14%-19% greater risk of death during the first 6 months after ICI initiation, but a 20% lower risk of death among those who survived 6 months after ICI initiation. Further, ICI combination therapy was associated with potential early harm among trial-ineligible patients.
“Phase 3 trials are sorely needed in patients with poor performance status or organ dysfunction so that we can adequately counsel patients who are unfit about expectations with novel cancer therapies,” Dr. Parikh said.
The cohort only included patients who received systemic therapy, which is a limitation of the study, so conclusions cannot be made about the efficacy of systemic therapy versus no systemic therapy in trial-ineligible patients.
Dr. Parikh reported nonfinancial support from Flatiron Health, grants from Humana, personal fees and equity from GNS Healthcare and Onc.AI, along with personal fees from the Cancer Study Group and Nanology outside the submitted work.
FROM JAMA ONCOLOGY
The importance of self-compassion for hospitalists
A mindful way relate to ourselves
Physicians, clinicians, providers, healers, and now heroes, are some of the names we have been given throughout history. These titles bring together a universal concept in medicine that all human beings deserve compassion, understanding, and care. However, as health care providers we forget to show ourselves the same compassion we bestow upon others.
Self-compassion is a new way of relating to ourselves. As clinicians, we are trained investigators, delving deeper into what our patient is thinking and feeling. “Tell me more about that. How does that make you feel? That must have been (very painful/scary/frustrating).” These are a few statements we learned in patient interviewing to actively engage with patients, build rapport, solidify trust, validate their concerns, and ultimately obtain the information needed to diagnose and heal.
We know the importance of looking beyond the surface, as more often than not a deeper inspection reveals more to the story. We have uncovered cracks in the foundation, erosion of the roof, worn out siding, and a glimpse into the complexities that make up each individual. We look at our patients, loved ones, and the world with night-vision lenses to uncover what is deeper.
Clinicians are good at directing compassion toward others, but not as good at giving it to themselves.1 Many health care providers may see self-compassion as soft, weak, selfish, or unnecessary. However, mindful self-compassion is a positive practice that opens a pathway for healing, personal growth, and protection against the negative consequences of self-judgment, isolation, anxiety, burnout, and depression.
What is self-compassion?
Kristin Neff, PhD, an associate professor in educational psychology at the University of Texas at Austin, was the first to academically define self-compassion. Self-compassion brings together three core elements – kindness, humanity, and mindfulness.2 Self-compassion involves acting the same way toward yourself when you are having a difficult time as you would toward another person. Instead of mercilessly judging and criticizing yourself for self-perceived inadequacies or shortcomings, self-compassion allows you to ask yourself: “How can I give myself comfort and care in this moment?”
Mindfulness acknowledges a painful experience without resistance or judgment, while being present in the moment with things as they are. Self-compassion provides the emotional safety needed to mindfully open to our pain, disappointments, and defeats. Mindfulness and self-compassion both allow us to live with more acceptance toward ourselves and our lives. Mindfulness asks: “What am I experiencing right now?” Self-compassion asks: “What do I need right now?” When you feel compassion for yourself or another, you recognize that suffering, failure, and imperfection are all part of the shared human experience.
The physiology of self-compassion
When we practice self-compassion, we feel safe and cared for because there is a physiological pathway that explains this response. Self-compassion helps down-regulate the stress response (fight-flight-freeze). When we are triggered by a threat to our self-concept, we are likely to do one, two, or all of three things: we fight ourselves (self-criticism – often our first reaction when things go wrong), we flee from others (isolation), or we freeze (rumination).
Feeling threatened puts stress on the mind and body, and chronic stress leads to anxiety and depression, which hinders emotional and physical well-being. With self-criticism, we are both the attacker and the attacked. When we practice self-compassion, we are deactivating the threat-defense system and activating the care system, releasing oxytocin and endorphins, which reduce stress and increase feelings of safety and security.3
Why is self-compassion important to provider well-being?
Research has shown that individuals who are more self-compassionate tend to have greater happiness, life satisfaction, and motivation; better relationships and physical health; and less anxiety and depression. They also have the resilience needed to cope with stressful life events. The more we practice being kind and compassionate with ourselves, the more we’ll increase the habit of self-compassion, and extend compassion to our patients and loved ones in daily life.4
Why is self-compassion important? When we experience a setback at work or in life, we can become defensive, accuse others, or blame ourselves, especially when we are already under immense stress. These responses are not helpful, productive, or effective to the situation or our personal well-being. Although in the moment it may feel good to be reactive, it is a short-lived feeling that we trade for the longer-lasting effects of learning, resilience, and personal growth. Self-compassion teaches us to connect with our inner imperfections, and what makes us human, as to err is human.
To cultivate a habit of self-compassion itself, it is important to understand that self-compassion is a practice of goodwill, not good feelings. Self-compassion is aimed at the alleviation of suffering, but it does not erase any pain and suffering that does exist. The truth is, we can’t always control external forces – the events of 2020-2021 are a perfect example of this. As a result, we cannot utilize self-compassion as a practice to make our pain disappear or suppress strong emotions.
Instead, self-compassion helps us cultivate the resilience needed to mindfully acknowledge and accept a painful moment or experience, while reminding us to embrace ourselves with kindness and care in response. This builds our internal foundation with support, love, and self-care, while providing the optimal conditions for growth, resilience, and transformation
Self-compassion and the backdraft phenomenon
When you start the practice of self-compassion, you may experience backdraft, a phenomenon in which pain initially increases.5 Backdraft is similar to the stages of grief or when the flames of a burning house become larger when a door is opened and oxygen surges in. Practicing self-compassion may cause a tidal wave of emotions to come to the forefront, but it is likely that if this happens, it needs to happen.
Imagine yourself in a room with two versions of yourself. To the left is your best self that you present to the world, standing tall, organized, well kept, and without any noticeable imperfections. To the right, is the deepest part of your being, laying on the floor, filled with raw emotions – sadness, fear, anger, and love. This version of yourself is vulnerable, open, honest, and imperfect. When looking at each version of yourself, which one is the real you? The right? The left? Maybe it’s both?
Imagine what would happen if you walked over to the version of yourself on the right, sat down, and provided it comfort, and embraced yourself with love and kindness. What would happen if you gave that version of yourself a hug? Seeing your true self, with all the layers peeled away, at the very core of your being, vulnerable, and possibly broken, is a powerful and gut-wrenching experience. It may hurt at first, but once we embrace our own pain and suffering, that is where mindfulness and self-compassion intersect to begin the path to healing. It takes more strength and courage to be the version of ourselves on the right than the version on the left.
What is not self compassion?
Self-compassion is not self-pity, weakness, self-esteem, or selfishness. When individuals feel self-pity, they become immersed in their own problems and feel that they are the only ones in the world who are suffering. Self-compassion makes us more willing to accept, experience, and acknowledge difficult feelings with kindness. This paradoxically helps us process and let go of these feelings without long-term negative consequences, and with a better ability to recognize the suffering of others.
Self-compassion allows us to be our own inner ally and strengthens our ability to cope successfully when life gets hard. Self-compassion will not make you weak and vulnerable. It is a reliable source of inner strength that enhances resilience when faced with difficulties. Research shows self-compassionate people are better able to cope with tough situations like divorce, trauma, and crisis.
Self-compassion and self-esteem are important to well-being; however, they are not the same. Self-esteem refers to a judgment or evaluation of our sense of self-worth, perceived value, or how much we like ourselves. While self-compassion relates to the changing landscape of who we are with kindness and acceptance – especially in times when we feel useless, inadequate, or hopeless – self-esteem allows for greater self-clarity, independent of external circumstances, and acknowledges that all human beings deserve compassion and understanding, not because they possess certain traits or have a certain perceive valued, but because we share the human experience and the human condition of imperfection. Finally, self-compassion is not selfish, as practicing it helps people sustain the act of caring for others and decrease caregiver burnout.6,7
Strategies to practice self-compassion
There are many ways to practice self-compassion. Here are a few experiences created by Dr. Neff, a leader in the field.8
Experience 1: How would you treat a friend?
How do you think things might change if you responded to yourself in the same way you typically respond to a close friend when he or she is suffering? Why not try treating yourself like a good friend and see what happens.
Take out a sheet of paper and write down your answer to the following questions:
- First, think about times when a close friend feels really bad about him or herself or is really struggling in some way. How would you respond to your friend in this situation (especially when you’re at your best)? Write down what you typically do and say and note the tone in which you typically talk to your friends.
- Second, think about times when you feel bad about yourself or are struggling. How do you typically respond to yourself in these situations? Write down what you typically do and say, and note the tone in which you typically talk to your friends.
- Did you notice a difference? If so, ask yourself why. What factors or fears come into play that lead you to treat yourself and others so differently?
- Please write down how you think things might change if you responded to yourself in the same way you typically respond to a close friend when you’re suffering.
Experience 2: Take a self-compassion break
This practice can be used any time of day or night, with others or alone. It will help you remember to evoke the three aspects of self-compassion when you need it most.
Think of a situation in your life that is difficult, that is causing you stress. Call the situation to mind, and if you feel comfortable, allow yourself to experience these feelings and emotions, without judgment and without altering them to what you think they should be.
- Say to yourself one of the following: “This is a difficult moment,” “This is a moment of suffering,” “This is stress,” “This hurts,” or “Ouch.” Doing this step is “mindfulness”: A willingness to observe negative thoughts and emotions with openness and clarity, so that they are held in mindful awareness, without judgment.
- Find your equilibrium of observation with thoughts and feelings. Try not to suppress or deny them and try not to get caught up and swept away by them.
- Remind yourself of the shared human experience. Recognize that suffering and personal difficulty is something that we all go through rather than being something that happens to “me” alone. Remind yourself that “other people feel this way,” “I’m not alone,” and “we all have struggles in life.”
- Be kind to yourself and ask: “What do I need to hear right now to express kindness to myself?” Is there a phrase that speaks to you in your particular situation? For example: “May I give myself the compassion that I need; may I learn to accept myself as I am; may I forgive myself; may I be strong; may I be patient.” There is no wrong answer.
Exercise 3: Explore self-compassion through writing
Everybody has something about themselves that they don’t like; something that causes them to feel shame, to feel insecure, or not “good enough.” This exercise will help you write a letter to yourself about this issue from a place of acceptance and compassion. It can feel uncomfortable at first, but it gets easier with practice.
- Write about an issue you have that makes you feel inadequate or bad about yourself (physical appearance, work, or relationship issue) What emotions do you experience when you think about this aspect of yourself? Try to only feel your emotions exactly as they are – no more, no less – and then write about them.
- Write a letter as if you were talking to a dearly beloved friend who was struggling with the same concerns as you and has the same strengths and weaknesses as you. How would you convey deep compassion, especially for the pain you feel when they judge themselves so harshly? What would you write to your friend to remind them that they are only human, that all people have both strengths and weaknesses? As you write, try to infuse your letter with a strong sense of acceptance, kindness, caring, and desire for their health and happiness.
- After writing the letter, put it aside for a little while. Then come back and read it again, really letting the words sink in. Feel the compassion as it pours into you, soothing and comforting you. Love, connection, and acceptance are a part of your human right. To claim them you need only look within yourself.
Experience 4: Taking care of the caregiver
We work in the very stressful time of the COVID pandemic. As medical providers, we are caregivers to our patients and our families. Yet, we do not give ourselves time to rest, recover, and recharge. Remember, to care for others, you cannot pour from an empty cup.
- Give yourself permission to meet your own needs, recognizing that this will not only enhance your quality of life, it will also enhance your ability to be there for those that rely on you. Our time is limited but self-care can occur both at work and outside of work.
- When you are “off the clock,” be off the clock! Turn off notifications, don’t check email, and be present in your personal lives. If you are constantly answering patient calls or nursing questions until 10 p.m., that means your health care system is in need of an upgrade, as you need the appropriate coverage to give you time to care for yourself, just as well as you care for your patients.
- While at work you can practice self-care. Take 2 minutes to practice relaxation breathing. Take 1 minute to show yourself or another person gratitude. Take 5 minutes before you start writing your notes for the day to listen to relaxing music or a mindful podcast. Take 3 minutes to share three good things that happened in the day with your family or colleagues. Take 5-10 minutes to do chair yoga. Take a self-compassion break.
- Implement a 5-minute wellness break into your group’s daily function with some of the previous mentioned examples. This will allow you to care for and nurture yourself, while also caring for and nurturing others in an environment that cultivates your wellness goals.
As a hospitalist, I can attest that I did not show myself self-compassion nearly as often as I showed compassion to others. I am my own worst critic and my training taught me to suffer in silence, and not seek out others who are experiencing the same thing for fear of being perceived as weak, inadequate, or flawed.
This false notion that we need to always be tough, strong, and without emotion in order to be taken seriously, to advance, or be held in high regard is rubbish and only perpetuated by accepting it. In order to change the culture of medicine, we have to change the way we think and behave. I have practiced self-compassion exercises and it has enhanced my perspective to see that many of us are going through varying degrees of the same thing. It has shown me the positive effects on my inner being and my life. If you are ready to try something new that will benefit your psychological and emotional well-being, and help you through pain, suffering, struggles, and crisis, you have nothing to lose. Be the change, and show yourself self-compassion.
In summary, self-compassion is an attitude of warmth, curiosity, connection, and care. Learning to become more self-compassionate is a process of moving from striving to change our experience and ourselves toward embracing who we are already.9 The practice of self-compassion is giving ourselves what we need in the moment. Even if we are not ready, or it is too painful to fully accept or embrace, we can still plant the seeds that will, with time and patience, grow and bloom.
When we are mindful of our struggles, when we respond to ourselves with compassion, kindness, and give ourselves support in times of difficulty, we learn to embrace ourselves and our lives, our inner and outer imperfections, and provide ourselves with the strength needed to thrive in the most precarious and difficult situations. With self-compassion, we give the world the best of us, instead of what is left of us.
Dr. Williams is vice president of the Hampton Roads chapter of the Society of Hospital Medicine. She is a hospitalist at Sentara Careplex Hospital in Hampton, Va., where she also serves as vice president of the medical executive committee.
References
1. Sanchez-Reilly S et al. Caring for oneself to care for others: Physicians and their self-care. J Community Support Oncol. 2013;11(2):75-81. doi: 10.12788/j.suponc.0003.
2. Neff K. Self-Compassion: An Alternative Conceptualization of a Healthy Attitude Toward Oneself. Self Identity. 2010;2(2):85-101. doi: 10.1080/15298860309032.
3. Neff K et al. The forest and the trees: Examining the association of self-compassion and its positive and negative components with psychological functioning. Self Identity. 2018;17(6):627-45. doi: 10.1080/15298868.2018.1436587.
4. Zessin U et al. The relationship between self-compassion and well-being: A meta-analysis. Appl Psychol Health Well-Being. 2015;7(3):340-64. doi: 10.1111/aphw.12051.
5. Warren R et al. Self-criticism and self-compassion: Risk and resilience. Current Psychiatry. 2016 Dec;15(12):18-21,24-28,32.
6. Neff K. The Five Myths of Self-Compassion. Greater Good Magazine. 2015 Sep 30. https://greatergood.berkeley.edu/article/item/the_five_myths_of_self_compassion.
7. Neff KD and Germer CK. A pilot study and randomized controlled trial of the mindful self-compassion program. J Clin Psychol. 2013 Jan;69(1):28-44. doi: 10.1002/jclp.21923.
8. Neff K. Self-Compassion Guided Meditations and Exercises. https://self-compassion.org/category/exercises/#exercises.
9. Germer C and Neff KD. Mindful Self-Compassion (MSC), in “The handbook of mindfulness-based programs.” (London: Routledge, 2019, pp. 357-67).
A mindful way relate to ourselves
A mindful way relate to ourselves
Physicians, clinicians, providers, healers, and now heroes, are some of the names we have been given throughout history. These titles bring together a universal concept in medicine that all human beings deserve compassion, understanding, and care. However, as health care providers we forget to show ourselves the same compassion we bestow upon others.
Self-compassion is a new way of relating to ourselves. As clinicians, we are trained investigators, delving deeper into what our patient is thinking and feeling. “Tell me more about that. How does that make you feel? That must have been (very painful/scary/frustrating).” These are a few statements we learned in patient interviewing to actively engage with patients, build rapport, solidify trust, validate their concerns, and ultimately obtain the information needed to diagnose and heal.
We know the importance of looking beyond the surface, as more often than not a deeper inspection reveals more to the story. We have uncovered cracks in the foundation, erosion of the roof, worn out siding, and a glimpse into the complexities that make up each individual. We look at our patients, loved ones, and the world with night-vision lenses to uncover what is deeper.
Clinicians are good at directing compassion toward others, but not as good at giving it to themselves.1 Many health care providers may see self-compassion as soft, weak, selfish, or unnecessary. However, mindful self-compassion is a positive practice that opens a pathway for healing, personal growth, and protection against the negative consequences of self-judgment, isolation, anxiety, burnout, and depression.
What is self-compassion?
Kristin Neff, PhD, an associate professor in educational psychology at the University of Texas at Austin, was the first to academically define self-compassion. Self-compassion brings together three core elements – kindness, humanity, and mindfulness.2 Self-compassion involves acting the same way toward yourself when you are having a difficult time as you would toward another person. Instead of mercilessly judging and criticizing yourself for self-perceived inadequacies or shortcomings, self-compassion allows you to ask yourself: “How can I give myself comfort and care in this moment?”
Mindfulness acknowledges a painful experience without resistance or judgment, while being present in the moment with things as they are. Self-compassion provides the emotional safety needed to mindfully open to our pain, disappointments, and defeats. Mindfulness and self-compassion both allow us to live with more acceptance toward ourselves and our lives. Mindfulness asks: “What am I experiencing right now?” Self-compassion asks: “What do I need right now?” When you feel compassion for yourself or another, you recognize that suffering, failure, and imperfection are all part of the shared human experience.
The physiology of self-compassion
When we practice self-compassion, we feel safe and cared for because there is a physiological pathway that explains this response. Self-compassion helps down-regulate the stress response (fight-flight-freeze). When we are triggered by a threat to our self-concept, we are likely to do one, two, or all of three things: we fight ourselves (self-criticism – often our first reaction when things go wrong), we flee from others (isolation), or we freeze (rumination).
Feeling threatened puts stress on the mind and body, and chronic stress leads to anxiety and depression, which hinders emotional and physical well-being. With self-criticism, we are both the attacker and the attacked. When we practice self-compassion, we are deactivating the threat-defense system and activating the care system, releasing oxytocin and endorphins, which reduce stress and increase feelings of safety and security.3
Why is self-compassion important to provider well-being?
Research has shown that individuals who are more self-compassionate tend to have greater happiness, life satisfaction, and motivation; better relationships and physical health; and less anxiety and depression. They also have the resilience needed to cope with stressful life events. The more we practice being kind and compassionate with ourselves, the more we’ll increase the habit of self-compassion, and extend compassion to our patients and loved ones in daily life.4
Why is self-compassion important? When we experience a setback at work or in life, we can become defensive, accuse others, or blame ourselves, especially when we are already under immense stress. These responses are not helpful, productive, or effective to the situation or our personal well-being. Although in the moment it may feel good to be reactive, it is a short-lived feeling that we trade for the longer-lasting effects of learning, resilience, and personal growth. Self-compassion teaches us to connect with our inner imperfections, and what makes us human, as to err is human.
To cultivate a habit of self-compassion itself, it is important to understand that self-compassion is a practice of goodwill, not good feelings. Self-compassion is aimed at the alleviation of suffering, but it does not erase any pain and suffering that does exist. The truth is, we can’t always control external forces – the events of 2020-2021 are a perfect example of this. As a result, we cannot utilize self-compassion as a practice to make our pain disappear or suppress strong emotions.
Instead, self-compassion helps us cultivate the resilience needed to mindfully acknowledge and accept a painful moment or experience, while reminding us to embrace ourselves with kindness and care in response. This builds our internal foundation with support, love, and self-care, while providing the optimal conditions for growth, resilience, and transformation
Self-compassion and the backdraft phenomenon
When you start the practice of self-compassion, you may experience backdraft, a phenomenon in which pain initially increases.5 Backdraft is similar to the stages of grief or when the flames of a burning house become larger when a door is opened and oxygen surges in. Practicing self-compassion may cause a tidal wave of emotions to come to the forefront, but it is likely that if this happens, it needs to happen.
Imagine yourself in a room with two versions of yourself. To the left is your best self that you present to the world, standing tall, organized, well kept, and without any noticeable imperfections. To the right, is the deepest part of your being, laying on the floor, filled with raw emotions – sadness, fear, anger, and love. This version of yourself is vulnerable, open, honest, and imperfect. When looking at each version of yourself, which one is the real you? The right? The left? Maybe it’s both?
Imagine what would happen if you walked over to the version of yourself on the right, sat down, and provided it comfort, and embraced yourself with love and kindness. What would happen if you gave that version of yourself a hug? Seeing your true self, with all the layers peeled away, at the very core of your being, vulnerable, and possibly broken, is a powerful and gut-wrenching experience. It may hurt at first, but once we embrace our own pain and suffering, that is where mindfulness and self-compassion intersect to begin the path to healing. It takes more strength and courage to be the version of ourselves on the right than the version on the left.
What is not self compassion?
Self-compassion is not self-pity, weakness, self-esteem, or selfishness. When individuals feel self-pity, they become immersed in their own problems and feel that they are the only ones in the world who are suffering. Self-compassion makes us more willing to accept, experience, and acknowledge difficult feelings with kindness. This paradoxically helps us process and let go of these feelings without long-term negative consequences, and with a better ability to recognize the suffering of others.
Self-compassion allows us to be our own inner ally and strengthens our ability to cope successfully when life gets hard. Self-compassion will not make you weak and vulnerable. It is a reliable source of inner strength that enhances resilience when faced with difficulties. Research shows self-compassionate people are better able to cope with tough situations like divorce, trauma, and crisis.
Self-compassion and self-esteem are important to well-being; however, they are not the same. Self-esteem refers to a judgment or evaluation of our sense of self-worth, perceived value, or how much we like ourselves. While self-compassion relates to the changing landscape of who we are with kindness and acceptance – especially in times when we feel useless, inadequate, or hopeless – self-esteem allows for greater self-clarity, independent of external circumstances, and acknowledges that all human beings deserve compassion and understanding, not because they possess certain traits or have a certain perceive valued, but because we share the human experience and the human condition of imperfection. Finally, self-compassion is not selfish, as practicing it helps people sustain the act of caring for others and decrease caregiver burnout.6,7
Strategies to practice self-compassion
There are many ways to practice self-compassion. Here are a few experiences created by Dr. Neff, a leader in the field.8
Experience 1: How would you treat a friend?
How do you think things might change if you responded to yourself in the same way you typically respond to a close friend when he or she is suffering? Why not try treating yourself like a good friend and see what happens.
Take out a sheet of paper and write down your answer to the following questions:
- First, think about times when a close friend feels really bad about him or herself or is really struggling in some way. How would you respond to your friend in this situation (especially when you’re at your best)? Write down what you typically do and say and note the tone in which you typically talk to your friends.
- Second, think about times when you feel bad about yourself or are struggling. How do you typically respond to yourself in these situations? Write down what you typically do and say, and note the tone in which you typically talk to your friends.
- Did you notice a difference? If so, ask yourself why. What factors or fears come into play that lead you to treat yourself and others so differently?
- Please write down how you think things might change if you responded to yourself in the same way you typically respond to a close friend when you’re suffering.
Experience 2: Take a self-compassion break
This practice can be used any time of day or night, with others or alone. It will help you remember to evoke the three aspects of self-compassion when you need it most.
Think of a situation in your life that is difficult, that is causing you stress. Call the situation to mind, and if you feel comfortable, allow yourself to experience these feelings and emotions, without judgment and without altering them to what you think they should be.
- Say to yourself one of the following: “This is a difficult moment,” “This is a moment of suffering,” “This is stress,” “This hurts,” or “Ouch.” Doing this step is “mindfulness”: A willingness to observe negative thoughts and emotions with openness and clarity, so that they are held in mindful awareness, without judgment.
- Find your equilibrium of observation with thoughts and feelings. Try not to suppress or deny them and try not to get caught up and swept away by them.
- Remind yourself of the shared human experience. Recognize that suffering and personal difficulty is something that we all go through rather than being something that happens to “me” alone. Remind yourself that “other people feel this way,” “I’m not alone,” and “we all have struggles in life.”
- Be kind to yourself and ask: “What do I need to hear right now to express kindness to myself?” Is there a phrase that speaks to you in your particular situation? For example: “May I give myself the compassion that I need; may I learn to accept myself as I am; may I forgive myself; may I be strong; may I be patient.” There is no wrong answer.
Exercise 3: Explore self-compassion through writing
Everybody has something about themselves that they don’t like; something that causes them to feel shame, to feel insecure, or not “good enough.” This exercise will help you write a letter to yourself about this issue from a place of acceptance and compassion. It can feel uncomfortable at first, but it gets easier with practice.
- Write about an issue you have that makes you feel inadequate or bad about yourself (physical appearance, work, or relationship issue) What emotions do you experience when you think about this aspect of yourself? Try to only feel your emotions exactly as they are – no more, no less – and then write about them.
- Write a letter as if you were talking to a dearly beloved friend who was struggling with the same concerns as you and has the same strengths and weaknesses as you. How would you convey deep compassion, especially for the pain you feel when they judge themselves so harshly? What would you write to your friend to remind them that they are only human, that all people have both strengths and weaknesses? As you write, try to infuse your letter with a strong sense of acceptance, kindness, caring, and desire for their health and happiness.
- After writing the letter, put it aside for a little while. Then come back and read it again, really letting the words sink in. Feel the compassion as it pours into you, soothing and comforting you. Love, connection, and acceptance are a part of your human right. To claim them you need only look within yourself.
Experience 4: Taking care of the caregiver
We work in the very stressful time of the COVID pandemic. As medical providers, we are caregivers to our patients and our families. Yet, we do not give ourselves time to rest, recover, and recharge. Remember, to care for others, you cannot pour from an empty cup.
- Give yourself permission to meet your own needs, recognizing that this will not only enhance your quality of life, it will also enhance your ability to be there for those that rely on you. Our time is limited but self-care can occur both at work and outside of work.
- When you are “off the clock,” be off the clock! Turn off notifications, don’t check email, and be present in your personal lives. If you are constantly answering patient calls or nursing questions until 10 p.m., that means your health care system is in need of an upgrade, as you need the appropriate coverage to give you time to care for yourself, just as well as you care for your patients.
- While at work you can practice self-care. Take 2 minutes to practice relaxation breathing. Take 1 minute to show yourself or another person gratitude. Take 5 minutes before you start writing your notes for the day to listen to relaxing music or a mindful podcast. Take 3 minutes to share three good things that happened in the day with your family or colleagues. Take 5-10 minutes to do chair yoga. Take a self-compassion break.
- Implement a 5-minute wellness break into your group’s daily function with some of the previous mentioned examples. This will allow you to care for and nurture yourself, while also caring for and nurturing others in an environment that cultivates your wellness goals.
As a hospitalist, I can attest that I did not show myself self-compassion nearly as often as I showed compassion to others. I am my own worst critic and my training taught me to suffer in silence, and not seek out others who are experiencing the same thing for fear of being perceived as weak, inadequate, or flawed.
This false notion that we need to always be tough, strong, and without emotion in order to be taken seriously, to advance, or be held in high regard is rubbish and only perpetuated by accepting it. In order to change the culture of medicine, we have to change the way we think and behave. I have practiced self-compassion exercises and it has enhanced my perspective to see that many of us are going through varying degrees of the same thing. It has shown me the positive effects on my inner being and my life. If you are ready to try something new that will benefit your psychological and emotional well-being, and help you through pain, suffering, struggles, and crisis, you have nothing to lose. Be the change, and show yourself self-compassion.
In summary, self-compassion is an attitude of warmth, curiosity, connection, and care. Learning to become more self-compassionate is a process of moving from striving to change our experience and ourselves toward embracing who we are already.9 The practice of self-compassion is giving ourselves what we need in the moment. Even if we are not ready, or it is too painful to fully accept or embrace, we can still plant the seeds that will, with time and patience, grow and bloom.
When we are mindful of our struggles, when we respond to ourselves with compassion, kindness, and give ourselves support in times of difficulty, we learn to embrace ourselves and our lives, our inner and outer imperfections, and provide ourselves with the strength needed to thrive in the most precarious and difficult situations. With self-compassion, we give the world the best of us, instead of what is left of us.
Dr. Williams is vice president of the Hampton Roads chapter of the Society of Hospital Medicine. She is a hospitalist at Sentara Careplex Hospital in Hampton, Va., where she also serves as vice president of the medical executive committee.
References
1. Sanchez-Reilly S et al. Caring for oneself to care for others: Physicians and their self-care. J Community Support Oncol. 2013;11(2):75-81. doi: 10.12788/j.suponc.0003.
2. Neff K. Self-Compassion: An Alternative Conceptualization of a Healthy Attitude Toward Oneself. Self Identity. 2010;2(2):85-101. doi: 10.1080/15298860309032.
3. Neff K et al. The forest and the trees: Examining the association of self-compassion and its positive and negative components with psychological functioning. Self Identity. 2018;17(6):627-45. doi: 10.1080/15298868.2018.1436587.
4. Zessin U et al. The relationship between self-compassion and well-being: A meta-analysis. Appl Psychol Health Well-Being. 2015;7(3):340-64. doi: 10.1111/aphw.12051.
5. Warren R et al. Self-criticism and self-compassion: Risk and resilience. Current Psychiatry. 2016 Dec;15(12):18-21,24-28,32.
6. Neff K. The Five Myths of Self-Compassion. Greater Good Magazine. 2015 Sep 30. https://greatergood.berkeley.edu/article/item/the_five_myths_of_self_compassion.
7. Neff KD and Germer CK. A pilot study and randomized controlled trial of the mindful self-compassion program. J Clin Psychol. 2013 Jan;69(1):28-44. doi: 10.1002/jclp.21923.
8. Neff K. Self-Compassion Guided Meditations and Exercises. https://self-compassion.org/category/exercises/#exercises.
9. Germer C and Neff KD. Mindful Self-Compassion (MSC), in “The handbook of mindfulness-based programs.” (London: Routledge, 2019, pp. 357-67).
Physicians, clinicians, providers, healers, and now heroes, are some of the names we have been given throughout history. These titles bring together a universal concept in medicine that all human beings deserve compassion, understanding, and care. However, as health care providers we forget to show ourselves the same compassion we bestow upon others.
Self-compassion is a new way of relating to ourselves. As clinicians, we are trained investigators, delving deeper into what our patient is thinking and feeling. “Tell me more about that. How does that make you feel? That must have been (very painful/scary/frustrating).” These are a few statements we learned in patient interviewing to actively engage with patients, build rapport, solidify trust, validate their concerns, and ultimately obtain the information needed to diagnose and heal.
We know the importance of looking beyond the surface, as more often than not a deeper inspection reveals more to the story. We have uncovered cracks in the foundation, erosion of the roof, worn out siding, and a glimpse into the complexities that make up each individual. We look at our patients, loved ones, and the world with night-vision lenses to uncover what is deeper.
Clinicians are good at directing compassion toward others, but not as good at giving it to themselves.1 Many health care providers may see self-compassion as soft, weak, selfish, or unnecessary. However, mindful self-compassion is a positive practice that opens a pathway for healing, personal growth, and protection against the negative consequences of self-judgment, isolation, anxiety, burnout, and depression.
What is self-compassion?
Kristin Neff, PhD, an associate professor in educational psychology at the University of Texas at Austin, was the first to academically define self-compassion. Self-compassion brings together three core elements – kindness, humanity, and mindfulness.2 Self-compassion involves acting the same way toward yourself when you are having a difficult time as you would toward another person. Instead of mercilessly judging and criticizing yourself for self-perceived inadequacies or shortcomings, self-compassion allows you to ask yourself: “How can I give myself comfort and care in this moment?”
Mindfulness acknowledges a painful experience without resistance or judgment, while being present in the moment with things as they are. Self-compassion provides the emotional safety needed to mindfully open to our pain, disappointments, and defeats. Mindfulness and self-compassion both allow us to live with more acceptance toward ourselves and our lives. Mindfulness asks: “What am I experiencing right now?” Self-compassion asks: “What do I need right now?” When you feel compassion for yourself or another, you recognize that suffering, failure, and imperfection are all part of the shared human experience.
The physiology of self-compassion
When we practice self-compassion, we feel safe and cared for because there is a physiological pathway that explains this response. Self-compassion helps down-regulate the stress response (fight-flight-freeze). When we are triggered by a threat to our self-concept, we are likely to do one, two, or all of three things: we fight ourselves (self-criticism – often our first reaction when things go wrong), we flee from others (isolation), or we freeze (rumination).
Feeling threatened puts stress on the mind and body, and chronic stress leads to anxiety and depression, which hinders emotional and physical well-being. With self-criticism, we are both the attacker and the attacked. When we practice self-compassion, we are deactivating the threat-defense system and activating the care system, releasing oxytocin and endorphins, which reduce stress and increase feelings of safety and security.3
Why is self-compassion important to provider well-being?
Research has shown that individuals who are more self-compassionate tend to have greater happiness, life satisfaction, and motivation; better relationships and physical health; and less anxiety and depression. They also have the resilience needed to cope with stressful life events. The more we practice being kind and compassionate with ourselves, the more we’ll increase the habit of self-compassion, and extend compassion to our patients and loved ones in daily life.4
Why is self-compassion important? When we experience a setback at work or in life, we can become defensive, accuse others, or blame ourselves, especially when we are already under immense stress. These responses are not helpful, productive, or effective to the situation or our personal well-being. Although in the moment it may feel good to be reactive, it is a short-lived feeling that we trade for the longer-lasting effects of learning, resilience, and personal growth. Self-compassion teaches us to connect with our inner imperfections, and what makes us human, as to err is human.
To cultivate a habit of self-compassion itself, it is important to understand that self-compassion is a practice of goodwill, not good feelings. Self-compassion is aimed at the alleviation of suffering, but it does not erase any pain and suffering that does exist. The truth is, we can’t always control external forces – the events of 2020-2021 are a perfect example of this. As a result, we cannot utilize self-compassion as a practice to make our pain disappear or suppress strong emotions.
Instead, self-compassion helps us cultivate the resilience needed to mindfully acknowledge and accept a painful moment or experience, while reminding us to embrace ourselves with kindness and care in response. This builds our internal foundation with support, love, and self-care, while providing the optimal conditions for growth, resilience, and transformation
Self-compassion and the backdraft phenomenon
When you start the practice of self-compassion, you may experience backdraft, a phenomenon in which pain initially increases.5 Backdraft is similar to the stages of grief or when the flames of a burning house become larger when a door is opened and oxygen surges in. Practicing self-compassion may cause a tidal wave of emotions to come to the forefront, but it is likely that if this happens, it needs to happen.
Imagine yourself in a room with two versions of yourself. To the left is your best self that you present to the world, standing tall, organized, well kept, and without any noticeable imperfections. To the right, is the deepest part of your being, laying on the floor, filled with raw emotions – sadness, fear, anger, and love. This version of yourself is vulnerable, open, honest, and imperfect. When looking at each version of yourself, which one is the real you? The right? The left? Maybe it’s both?
Imagine what would happen if you walked over to the version of yourself on the right, sat down, and provided it comfort, and embraced yourself with love and kindness. What would happen if you gave that version of yourself a hug? Seeing your true self, with all the layers peeled away, at the very core of your being, vulnerable, and possibly broken, is a powerful and gut-wrenching experience. It may hurt at first, but once we embrace our own pain and suffering, that is where mindfulness and self-compassion intersect to begin the path to healing. It takes more strength and courage to be the version of ourselves on the right than the version on the left.
What is not self compassion?
Self-compassion is not self-pity, weakness, self-esteem, or selfishness. When individuals feel self-pity, they become immersed in their own problems and feel that they are the only ones in the world who are suffering. Self-compassion makes us more willing to accept, experience, and acknowledge difficult feelings with kindness. This paradoxically helps us process and let go of these feelings without long-term negative consequences, and with a better ability to recognize the suffering of others.
Self-compassion allows us to be our own inner ally and strengthens our ability to cope successfully when life gets hard. Self-compassion will not make you weak and vulnerable. It is a reliable source of inner strength that enhances resilience when faced with difficulties. Research shows self-compassionate people are better able to cope with tough situations like divorce, trauma, and crisis.
Self-compassion and self-esteem are important to well-being; however, they are not the same. Self-esteem refers to a judgment or evaluation of our sense of self-worth, perceived value, or how much we like ourselves. While self-compassion relates to the changing landscape of who we are with kindness and acceptance – especially in times when we feel useless, inadequate, or hopeless – self-esteem allows for greater self-clarity, independent of external circumstances, and acknowledges that all human beings deserve compassion and understanding, not because they possess certain traits or have a certain perceive valued, but because we share the human experience and the human condition of imperfection. Finally, self-compassion is not selfish, as practicing it helps people sustain the act of caring for others and decrease caregiver burnout.6,7
Strategies to practice self-compassion
There are many ways to practice self-compassion. Here are a few experiences created by Dr. Neff, a leader in the field.8
Experience 1: How would you treat a friend?
How do you think things might change if you responded to yourself in the same way you typically respond to a close friend when he or she is suffering? Why not try treating yourself like a good friend and see what happens.
Take out a sheet of paper and write down your answer to the following questions:
- First, think about times when a close friend feels really bad about him or herself or is really struggling in some way. How would you respond to your friend in this situation (especially when you’re at your best)? Write down what you typically do and say and note the tone in which you typically talk to your friends.
- Second, think about times when you feel bad about yourself or are struggling. How do you typically respond to yourself in these situations? Write down what you typically do and say, and note the tone in which you typically talk to your friends.
- Did you notice a difference? If so, ask yourself why. What factors or fears come into play that lead you to treat yourself and others so differently?
- Please write down how you think things might change if you responded to yourself in the same way you typically respond to a close friend when you’re suffering.
Experience 2: Take a self-compassion break
This practice can be used any time of day or night, with others or alone. It will help you remember to evoke the three aspects of self-compassion when you need it most.
Think of a situation in your life that is difficult, that is causing you stress. Call the situation to mind, and if you feel comfortable, allow yourself to experience these feelings and emotions, without judgment and without altering them to what you think they should be.
- Say to yourself one of the following: “This is a difficult moment,” “This is a moment of suffering,” “This is stress,” “This hurts,” or “Ouch.” Doing this step is “mindfulness”: A willingness to observe negative thoughts and emotions with openness and clarity, so that they are held in mindful awareness, without judgment.
- Find your equilibrium of observation with thoughts and feelings. Try not to suppress or deny them and try not to get caught up and swept away by them.
- Remind yourself of the shared human experience. Recognize that suffering and personal difficulty is something that we all go through rather than being something that happens to “me” alone. Remind yourself that “other people feel this way,” “I’m not alone,” and “we all have struggles in life.”
- Be kind to yourself and ask: “What do I need to hear right now to express kindness to myself?” Is there a phrase that speaks to you in your particular situation? For example: “May I give myself the compassion that I need; may I learn to accept myself as I am; may I forgive myself; may I be strong; may I be patient.” There is no wrong answer.
Exercise 3: Explore self-compassion through writing
Everybody has something about themselves that they don’t like; something that causes them to feel shame, to feel insecure, or not “good enough.” This exercise will help you write a letter to yourself about this issue from a place of acceptance and compassion. It can feel uncomfortable at first, but it gets easier with practice.
- Write about an issue you have that makes you feel inadequate or bad about yourself (physical appearance, work, or relationship issue) What emotions do you experience when you think about this aspect of yourself? Try to only feel your emotions exactly as they are – no more, no less – and then write about them.
- Write a letter as if you were talking to a dearly beloved friend who was struggling with the same concerns as you and has the same strengths and weaknesses as you. How would you convey deep compassion, especially for the pain you feel when they judge themselves so harshly? What would you write to your friend to remind them that they are only human, that all people have both strengths and weaknesses? As you write, try to infuse your letter with a strong sense of acceptance, kindness, caring, and desire for their health and happiness.
- After writing the letter, put it aside for a little while. Then come back and read it again, really letting the words sink in. Feel the compassion as it pours into you, soothing and comforting you. Love, connection, and acceptance are a part of your human right. To claim them you need only look within yourself.
Experience 4: Taking care of the caregiver
We work in the very stressful time of the COVID pandemic. As medical providers, we are caregivers to our patients and our families. Yet, we do not give ourselves time to rest, recover, and recharge. Remember, to care for others, you cannot pour from an empty cup.
- Give yourself permission to meet your own needs, recognizing that this will not only enhance your quality of life, it will also enhance your ability to be there for those that rely on you. Our time is limited but self-care can occur both at work and outside of work.
- When you are “off the clock,” be off the clock! Turn off notifications, don’t check email, and be present in your personal lives. If you are constantly answering patient calls or nursing questions until 10 p.m., that means your health care system is in need of an upgrade, as you need the appropriate coverage to give you time to care for yourself, just as well as you care for your patients.
- While at work you can practice self-care. Take 2 minutes to practice relaxation breathing. Take 1 minute to show yourself or another person gratitude. Take 5 minutes before you start writing your notes for the day to listen to relaxing music or a mindful podcast. Take 3 minutes to share three good things that happened in the day with your family or colleagues. Take 5-10 minutes to do chair yoga. Take a self-compassion break.
- Implement a 5-minute wellness break into your group’s daily function with some of the previous mentioned examples. This will allow you to care for and nurture yourself, while also caring for and nurturing others in an environment that cultivates your wellness goals.
As a hospitalist, I can attest that I did not show myself self-compassion nearly as often as I showed compassion to others. I am my own worst critic and my training taught me to suffer in silence, and not seek out others who are experiencing the same thing for fear of being perceived as weak, inadequate, or flawed.
This false notion that we need to always be tough, strong, and without emotion in order to be taken seriously, to advance, or be held in high regard is rubbish and only perpetuated by accepting it. In order to change the culture of medicine, we have to change the way we think and behave. I have practiced self-compassion exercises and it has enhanced my perspective to see that many of us are going through varying degrees of the same thing. It has shown me the positive effects on my inner being and my life. If you are ready to try something new that will benefit your psychological and emotional well-being, and help you through pain, suffering, struggles, and crisis, you have nothing to lose. Be the change, and show yourself self-compassion.
In summary, self-compassion is an attitude of warmth, curiosity, connection, and care. Learning to become more self-compassionate is a process of moving from striving to change our experience and ourselves toward embracing who we are already.9 The practice of self-compassion is giving ourselves what we need in the moment. Even if we are not ready, or it is too painful to fully accept or embrace, we can still plant the seeds that will, with time and patience, grow and bloom.
When we are mindful of our struggles, when we respond to ourselves with compassion, kindness, and give ourselves support in times of difficulty, we learn to embrace ourselves and our lives, our inner and outer imperfections, and provide ourselves with the strength needed to thrive in the most precarious and difficult situations. With self-compassion, we give the world the best of us, instead of what is left of us.
Dr. Williams is vice president of the Hampton Roads chapter of the Society of Hospital Medicine. She is a hospitalist at Sentara Careplex Hospital in Hampton, Va., where she also serves as vice president of the medical executive committee.
References
1. Sanchez-Reilly S et al. Caring for oneself to care for others: Physicians and their self-care. J Community Support Oncol. 2013;11(2):75-81. doi: 10.12788/j.suponc.0003.
2. Neff K. Self-Compassion: An Alternative Conceptualization of a Healthy Attitude Toward Oneself. Self Identity. 2010;2(2):85-101. doi: 10.1080/15298860309032.
3. Neff K et al. The forest and the trees: Examining the association of self-compassion and its positive and negative components with psychological functioning. Self Identity. 2018;17(6):627-45. doi: 10.1080/15298868.2018.1436587.
4. Zessin U et al. The relationship between self-compassion and well-being: A meta-analysis. Appl Psychol Health Well-Being. 2015;7(3):340-64. doi: 10.1111/aphw.12051.
5. Warren R et al. Self-criticism and self-compassion: Risk and resilience. Current Psychiatry. 2016 Dec;15(12):18-21,24-28,32.
6. Neff K. The Five Myths of Self-Compassion. Greater Good Magazine. 2015 Sep 30. https://greatergood.berkeley.edu/article/item/the_five_myths_of_self_compassion.
7. Neff KD and Germer CK. A pilot study and randomized controlled trial of the mindful self-compassion program. J Clin Psychol. 2013 Jan;69(1):28-44. doi: 10.1002/jclp.21923.
8. Neff K. Self-Compassion Guided Meditations and Exercises. https://self-compassion.org/category/exercises/#exercises.
9. Germer C and Neff KD. Mindful Self-Compassion (MSC), in “The handbook of mindfulness-based programs.” (London: Routledge, 2019, pp. 357-67).
Children and COVID-19: 7 million cases and still counting
Total COVID-19 cases in children surpassed the 7-million mark as new cases rose slightly after the previous week’s decline, according to the American Academy of Pediatrics and the Children’s Hospital Association.
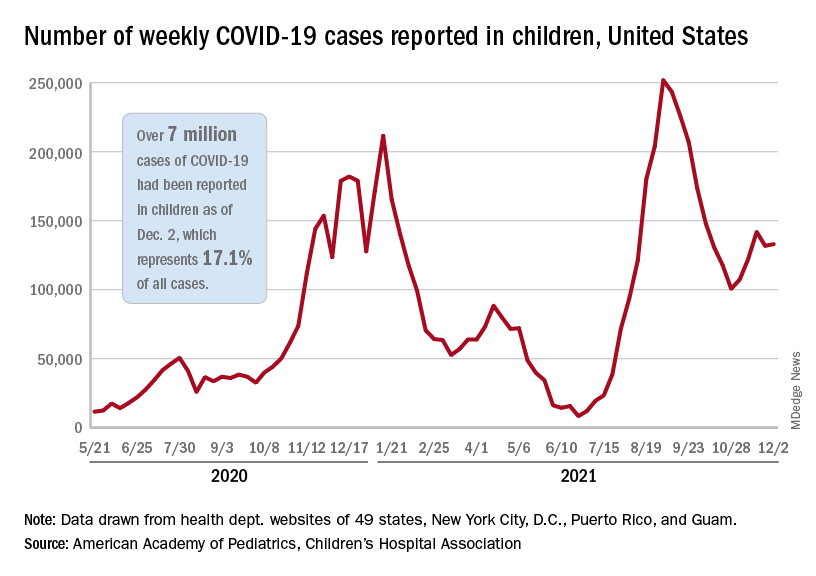
, the AAP and CHA said in their weekly COVID-19 report. New cases had dropped the previous week after 3 straight weeks of increases since late October.
The Centers for Disease Control and Prevention puts the total number of child COVID-19 cases at 6.2 million, but both estimates are based on all-age totals – 40 million for the CDC and 41 million for the AAP/CHA – that are well short of the CDC’s latest cumulative figure, which is now just over 49 million, so the actual figures are undoubtedly higher.
Meanwhile, the 1-month anniversary of 5- to 11-year-olds’ vaccine eligibility brought many completions: 923,000 received their second dose during the week ending Dec. 6, compared with 405,000 the previous week. About 16.9% (4.9 million) of children aged 5-11 have gotten at least one dose of the COVID-19 vaccine thus far, of whom almost 1.5 million children (5.1% of the age group) are now fully vaccinated, the CDC said on its COVID-19 Data Tracker.
The pace of vaccinations, however, is much lower for older children. Weekly numbers for all COVID-19 vaccinations, both first and second doses, dropped from 84,000 (Nov. 23-29) to 70,000 (Nov. 30 to Dec. 6), for those aged 12-17 years. In that group, 61.6% have received at least one dose and 51.8% are fully vaccinated, the CDC said.
The pace of vaccinations varies for younger children as well, when geography is considered. The AAP analyzed the CDC’s data and found that 42% of all 5- to 11-year-olds in Vermont had received at least one dose as of Dec. 1, followed by Massachusetts (33%), Maine (30%), and Rhode Island (28%). At the other end of the vaccination scale are Alabama, Louisiana, Mississippi, and West Virginia, all with 4%, the AAP reported.
As the United States puts 7 million children infected with COVID-19 in its rear view mirror, another milestone is looming ahead: The CDC’s current count of deaths in children is 974.
Total COVID-19 cases in children surpassed the 7-million mark as new cases rose slightly after the previous week’s decline, according to the American Academy of Pediatrics and the Children’s Hospital Association.

, the AAP and CHA said in their weekly COVID-19 report. New cases had dropped the previous week after 3 straight weeks of increases since late October.
The Centers for Disease Control and Prevention puts the total number of child COVID-19 cases at 6.2 million, but both estimates are based on all-age totals – 40 million for the CDC and 41 million for the AAP/CHA – that are well short of the CDC’s latest cumulative figure, which is now just over 49 million, so the actual figures are undoubtedly higher.
Meanwhile, the 1-month anniversary of 5- to 11-year-olds’ vaccine eligibility brought many completions: 923,000 received their second dose during the week ending Dec. 6, compared with 405,000 the previous week. About 16.9% (4.9 million) of children aged 5-11 have gotten at least one dose of the COVID-19 vaccine thus far, of whom almost 1.5 million children (5.1% of the age group) are now fully vaccinated, the CDC said on its COVID-19 Data Tracker.
The pace of vaccinations, however, is much lower for older children. Weekly numbers for all COVID-19 vaccinations, both first and second doses, dropped from 84,000 (Nov. 23-29) to 70,000 (Nov. 30 to Dec. 6), for those aged 12-17 years. In that group, 61.6% have received at least one dose and 51.8% are fully vaccinated, the CDC said.
The pace of vaccinations varies for younger children as well, when geography is considered. The AAP analyzed the CDC’s data and found that 42% of all 5- to 11-year-olds in Vermont had received at least one dose as of Dec. 1, followed by Massachusetts (33%), Maine (30%), and Rhode Island (28%). At the other end of the vaccination scale are Alabama, Louisiana, Mississippi, and West Virginia, all with 4%, the AAP reported.
As the United States puts 7 million children infected with COVID-19 in its rear view mirror, another milestone is looming ahead: The CDC’s current count of deaths in children is 974.
Total COVID-19 cases in children surpassed the 7-million mark as new cases rose slightly after the previous week’s decline, according to the American Academy of Pediatrics and the Children’s Hospital Association.

, the AAP and CHA said in their weekly COVID-19 report. New cases had dropped the previous week after 3 straight weeks of increases since late October.
The Centers for Disease Control and Prevention puts the total number of child COVID-19 cases at 6.2 million, but both estimates are based on all-age totals – 40 million for the CDC and 41 million for the AAP/CHA – that are well short of the CDC’s latest cumulative figure, which is now just over 49 million, so the actual figures are undoubtedly higher.
Meanwhile, the 1-month anniversary of 5- to 11-year-olds’ vaccine eligibility brought many completions: 923,000 received their second dose during the week ending Dec. 6, compared with 405,000 the previous week. About 16.9% (4.9 million) of children aged 5-11 have gotten at least one dose of the COVID-19 vaccine thus far, of whom almost 1.5 million children (5.1% of the age group) are now fully vaccinated, the CDC said on its COVID-19 Data Tracker.
The pace of vaccinations, however, is much lower for older children. Weekly numbers for all COVID-19 vaccinations, both first and second doses, dropped from 84,000 (Nov. 23-29) to 70,000 (Nov. 30 to Dec. 6), for those aged 12-17 years. In that group, 61.6% have received at least one dose and 51.8% are fully vaccinated, the CDC said.
The pace of vaccinations varies for younger children as well, when geography is considered. The AAP analyzed the CDC’s data and found that 42% of all 5- to 11-year-olds in Vermont had received at least one dose as of Dec. 1, followed by Massachusetts (33%), Maine (30%), and Rhode Island (28%). At the other end of the vaccination scale are Alabama, Louisiana, Mississippi, and West Virginia, all with 4%, the AAP reported.
As the United States puts 7 million children infected with COVID-19 in its rear view mirror, another milestone is looming ahead: The CDC’s current count of deaths in children is 974.
Does inadequate sleep increase obesity risk in children?
Evidence summary
Multiple analyses suggest short sleep increases obesity risk
Three recent, large systematic reviews of prospective cohort studies with meta-analyses in infants, children, and adolescents all found associations between short sleep at intake and later excessive weight.
The largest meta-analysis included 42 prospective studies with 75,499 patients ranging in age from infancy to adolescence and with follow-up ranging from 1 to 27 years. In a pooled analysis, short sleep—variously defined across trials and mostly assessed by parental report—was associated with an increased risk of obesity or overweight (relative risk [RR] = 1.58; 95% CI, 1.35-1.85; I2= 92%), compared to normal and long sleep. When the authors adjusted for suspected publication bias using a “trim and fill” method, short sleep remained associated with later overweight or obesity (RR = 1.42; 95% CI, 1.12-1.81). Short sleep was associated with later unhealthy weight status in all age groups: 0 to < 3 years (RR = 1.4; 95% CI, 1.19-1.65); 3 to < 9 years (RR = 1.57; 95% CI, 1.4-1.76);9 to < 12 years (RR = 2.23; 95% CI, 2.18-2.27); and 12 to 18 years (RR = 1.3; 95% CI, 1.11-1.53). In addition to high heterogeneity, limitations of the review included variability in the definition of short sleep, use of parent- or self-reported sleep duration, and variability in classification of overweight and obesity in primary studies.1
A second systematic review and meta-analysis included 25 longitudinal studies (20 of which overlapped with the previously discussed meta-analysis) of children and adolescents (N = 56,584). Patients ranged in age from infancy to 16 years, and follow-up ranged from 6 months to 10 years (mean, 3.4 years). Children and adolescents with the shortest sleep duration were more likely to be overweight or obese at follow-up (pooled odds ratio [OR] = 1.76; 95% CI, 1.39-2.23; I2 = 70.5%) than those with the longest sleep duration. Due to the overlap in studies, the limitations of this analysis were similar to those already mentioned. Lack of a linear association between sleep duration and weight was cited as evidence of possible publication bias; the authors did not attempt to correct for it.2
The third systematic review and meta-analysis included 22 longitudinal studies (18 overlapped with first meta-analysis and 17 with the second) of children and adolescents (N = 24,821) ages 6 months to 18 years. Follow-up ranged from 1 to 27 years. This meta-analysis standardized the categories of sleep duration using recommendations from the Sleep Health Foundation. Patients with short sleep duration had an increased risk of overweight or obesity compared with patients sleeping “normal” or “longer than normal” durations (pooled OR = 2.15; 95% CI, 1.64-2.81; I2 = 67%). The authors indicated that their analysis could have been more robust if information about daytime sleep (ie, napping) had been available, but it was not collected in many of the included studies.3
Accelerometer data quantify the sleep/obesity association
A subsequent cohort study (N = 202) sought to better examine the association between sleep characteristics and adiposity by measuring sleep duration using accelerometers. Toddlers (ages 12 to 26 months) without previous medical history were recruited from early childhood education centers. Patients wore accelerometers for 7 consecutive days and then returned to the clinic after 12 months for collection of biometric information. Researchers measured body morphology with the BMI z-score (ie, the number of standard deviations from the mean). Every additional hour of total sleep time was associated with a 0.12-unit lower BMI z-score (95% CI, –0.23 to –0.01) at 1 year. However, every hour increase in nap duration was associated with a 0.41-unit higher BMI z-score (95% CI, 0.14-0.68).4
Recommendations from others
In 2016, the American Academy of Sleep Medicine (AASM) recommended the following sleep durations (per 24 hours): infants ages 4 to 12 months, 12-16 hours; children 1 to 2 years, 11-14 hours; children 3 to 5 years, 10-13 hours; children 6 to 12 years, 9-12 hours; and teenagers 13 to 18 years, 8-10 hours. The AASM further stated that sleeping the recommended number of hours was associated with better health outcomes, and that sleeping too few hours increased the risk of various health conditions, including obesity.5 In 2015, the American Academy of Pediatrics Committee on Nutrition acknowledged the association between obesity and short sleep duration and recommended that health care professionals counsel parents about age-appropriate sleep guidelines.6
Editor’s takeaway
Studies demonstrate that short sleep duration in pediatric patients is associated with later weight gain. However, associations do not prove a causal link, and other factors may contribute to both weight gain and poor sleep.
1. Miller MA, Kruisbrink M, Wallace J, et al. Sleep duration and incidence of obesity in infants, children, and adolescents: a systematic review and meta-analysis of prospective studies. Sleep. 2018;41:1-19. doi: 10.1093/sleep/zsy018
2. Ruan H, Xun P, Cai W, et al. Habitual sleep duration and risk of childhood obesity: systematic review and dose-response meta-analysis of prospective cohort studies. Sci Rep. 2015;5:16160. doi: 10.1038/srep16160
3. Fatima Y, Doi SA, Mamun AA. Longitudinal impact of sleep on overweight and obesity in children and adolescents: a systematic review and bias-adjusted meta-analysis. Obes Rev. 2015;16:137-149. doi: 10.1111/obr.12245
4. Zhang Z, Pereira JR, Sousa-Sá E, et al. The cross‐sectional and prospective associations between sleep characteristics and adiposity in toddlers: results from the GET UP! study. Pediatr Obes. 2019;14:e1255. doi: 10.1111/ijpo.12557
5. Paruthi S, Brooks LJ, D’Ambrosio C, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016;12:785-786. doi: 10.5664/jcsm.5866
6. Daniels SR, Hassink SG; American Academy of Pediatrics Committee on Nutrition. The role of the pediatrician in primary prevention of obesity. Pediatrics 2015;136:e275-e292. doi: 10.1542/peds.2015-1558
Evidence summary
Multiple analyses suggest short sleep increases obesity risk
Three recent, large systematic reviews of prospective cohort studies with meta-analyses in infants, children, and adolescents all found associations between short sleep at intake and later excessive weight.
The largest meta-analysis included 42 prospective studies with 75,499 patients ranging in age from infancy to adolescence and with follow-up ranging from 1 to 27 years. In a pooled analysis, short sleep—variously defined across trials and mostly assessed by parental report—was associated with an increased risk of obesity or overweight (relative risk [RR] = 1.58; 95% CI, 1.35-1.85; I2= 92%), compared to normal and long sleep. When the authors adjusted for suspected publication bias using a “trim and fill” method, short sleep remained associated with later overweight or obesity (RR = 1.42; 95% CI, 1.12-1.81). Short sleep was associated with later unhealthy weight status in all age groups: 0 to < 3 years (RR = 1.4; 95% CI, 1.19-1.65); 3 to < 9 years (RR = 1.57; 95% CI, 1.4-1.76);9 to < 12 years (RR = 2.23; 95% CI, 2.18-2.27); and 12 to 18 years (RR = 1.3; 95% CI, 1.11-1.53). In addition to high heterogeneity, limitations of the review included variability in the definition of short sleep, use of parent- or self-reported sleep duration, and variability in classification of overweight and obesity in primary studies.1
A second systematic review and meta-analysis included 25 longitudinal studies (20 of which overlapped with the previously discussed meta-analysis) of children and adolescents (N = 56,584). Patients ranged in age from infancy to 16 years, and follow-up ranged from 6 months to 10 years (mean, 3.4 years). Children and adolescents with the shortest sleep duration were more likely to be overweight or obese at follow-up (pooled odds ratio [OR] = 1.76; 95% CI, 1.39-2.23; I2 = 70.5%) than those with the longest sleep duration. Due to the overlap in studies, the limitations of this analysis were similar to those already mentioned. Lack of a linear association between sleep duration and weight was cited as evidence of possible publication bias; the authors did not attempt to correct for it.2
The third systematic review and meta-analysis included 22 longitudinal studies (18 overlapped with first meta-analysis and 17 with the second) of children and adolescents (N = 24,821) ages 6 months to 18 years. Follow-up ranged from 1 to 27 years. This meta-analysis standardized the categories of sleep duration using recommendations from the Sleep Health Foundation. Patients with short sleep duration had an increased risk of overweight or obesity compared with patients sleeping “normal” or “longer than normal” durations (pooled OR = 2.15; 95% CI, 1.64-2.81; I2 = 67%). The authors indicated that their analysis could have been more robust if information about daytime sleep (ie, napping) had been available, but it was not collected in many of the included studies.3
Accelerometer data quantify the sleep/obesity association
A subsequent cohort study (N = 202) sought to better examine the association between sleep characteristics and adiposity by measuring sleep duration using accelerometers. Toddlers (ages 12 to 26 months) without previous medical history were recruited from early childhood education centers. Patients wore accelerometers for 7 consecutive days and then returned to the clinic after 12 months for collection of biometric information. Researchers measured body morphology with the BMI z-score (ie, the number of standard deviations from the mean). Every additional hour of total sleep time was associated with a 0.12-unit lower BMI z-score (95% CI, –0.23 to –0.01) at 1 year. However, every hour increase in nap duration was associated with a 0.41-unit higher BMI z-score (95% CI, 0.14-0.68).4
Recommendations from others
In 2016, the American Academy of Sleep Medicine (AASM) recommended the following sleep durations (per 24 hours): infants ages 4 to 12 months, 12-16 hours; children 1 to 2 years, 11-14 hours; children 3 to 5 years, 10-13 hours; children 6 to 12 years, 9-12 hours; and teenagers 13 to 18 years, 8-10 hours. The AASM further stated that sleeping the recommended number of hours was associated with better health outcomes, and that sleeping too few hours increased the risk of various health conditions, including obesity.5 In 2015, the American Academy of Pediatrics Committee on Nutrition acknowledged the association between obesity and short sleep duration and recommended that health care professionals counsel parents about age-appropriate sleep guidelines.6
Editor’s takeaway
Studies demonstrate that short sleep duration in pediatric patients is associated with later weight gain. However, associations do not prove a causal link, and other factors may contribute to both weight gain and poor sleep.
Evidence summary
Multiple analyses suggest short sleep increases obesity risk
Three recent, large systematic reviews of prospective cohort studies with meta-analyses in infants, children, and adolescents all found associations between short sleep at intake and later excessive weight.
The largest meta-analysis included 42 prospective studies with 75,499 patients ranging in age from infancy to adolescence and with follow-up ranging from 1 to 27 years. In a pooled analysis, short sleep—variously defined across trials and mostly assessed by parental report—was associated with an increased risk of obesity or overweight (relative risk [RR] = 1.58; 95% CI, 1.35-1.85; I2= 92%), compared to normal and long sleep. When the authors adjusted for suspected publication bias using a “trim and fill” method, short sleep remained associated with later overweight or obesity (RR = 1.42; 95% CI, 1.12-1.81). Short sleep was associated with later unhealthy weight status in all age groups: 0 to < 3 years (RR = 1.4; 95% CI, 1.19-1.65); 3 to < 9 years (RR = 1.57; 95% CI, 1.4-1.76);9 to < 12 years (RR = 2.23; 95% CI, 2.18-2.27); and 12 to 18 years (RR = 1.3; 95% CI, 1.11-1.53). In addition to high heterogeneity, limitations of the review included variability in the definition of short sleep, use of parent- or self-reported sleep duration, and variability in classification of overweight and obesity in primary studies.1
A second systematic review and meta-analysis included 25 longitudinal studies (20 of which overlapped with the previously discussed meta-analysis) of children and adolescents (N = 56,584). Patients ranged in age from infancy to 16 years, and follow-up ranged from 6 months to 10 years (mean, 3.4 years). Children and adolescents with the shortest sleep duration were more likely to be overweight or obese at follow-up (pooled odds ratio [OR] = 1.76; 95% CI, 1.39-2.23; I2 = 70.5%) than those with the longest sleep duration. Due to the overlap in studies, the limitations of this analysis were similar to those already mentioned. Lack of a linear association between sleep duration and weight was cited as evidence of possible publication bias; the authors did not attempt to correct for it.2
The third systematic review and meta-analysis included 22 longitudinal studies (18 overlapped with first meta-analysis and 17 with the second) of children and adolescents (N = 24,821) ages 6 months to 18 years. Follow-up ranged from 1 to 27 years. This meta-analysis standardized the categories of sleep duration using recommendations from the Sleep Health Foundation. Patients with short sleep duration had an increased risk of overweight or obesity compared with patients sleeping “normal” or “longer than normal” durations (pooled OR = 2.15; 95% CI, 1.64-2.81; I2 = 67%). The authors indicated that their analysis could have been more robust if information about daytime sleep (ie, napping) had been available, but it was not collected in many of the included studies.3
Accelerometer data quantify the sleep/obesity association
A subsequent cohort study (N = 202) sought to better examine the association between sleep characteristics and adiposity by measuring sleep duration using accelerometers. Toddlers (ages 12 to 26 months) without previous medical history were recruited from early childhood education centers. Patients wore accelerometers for 7 consecutive days and then returned to the clinic after 12 months for collection of biometric information. Researchers measured body morphology with the BMI z-score (ie, the number of standard deviations from the mean). Every additional hour of total sleep time was associated with a 0.12-unit lower BMI z-score (95% CI, –0.23 to –0.01) at 1 year. However, every hour increase in nap duration was associated with a 0.41-unit higher BMI z-score (95% CI, 0.14-0.68).4
Recommendations from others
In 2016, the American Academy of Sleep Medicine (AASM) recommended the following sleep durations (per 24 hours): infants ages 4 to 12 months, 12-16 hours; children 1 to 2 years, 11-14 hours; children 3 to 5 years, 10-13 hours; children 6 to 12 years, 9-12 hours; and teenagers 13 to 18 years, 8-10 hours. The AASM further stated that sleeping the recommended number of hours was associated with better health outcomes, and that sleeping too few hours increased the risk of various health conditions, including obesity.5 In 2015, the American Academy of Pediatrics Committee on Nutrition acknowledged the association between obesity and short sleep duration and recommended that health care professionals counsel parents about age-appropriate sleep guidelines.6
Editor’s takeaway
Studies demonstrate that short sleep duration in pediatric patients is associated with later weight gain. However, associations do not prove a causal link, and other factors may contribute to both weight gain and poor sleep.
1. Miller MA, Kruisbrink M, Wallace J, et al. Sleep duration and incidence of obesity in infants, children, and adolescents: a systematic review and meta-analysis of prospective studies. Sleep. 2018;41:1-19. doi: 10.1093/sleep/zsy018
2. Ruan H, Xun P, Cai W, et al. Habitual sleep duration and risk of childhood obesity: systematic review and dose-response meta-analysis of prospective cohort studies. Sci Rep. 2015;5:16160. doi: 10.1038/srep16160
3. Fatima Y, Doi SA, Mamun AA. Longitudinal impact of sleep on overweight and obesity in children and adolescents: a systematic review and bias-adjusted meta-analysis. Obes Rev. 2015;16:137-149. doi: 10.1111/obr.12245
4. Zhang Z, Pereira JR, Sousa-Sá E, et al. The cross‐sectional and prospective associations between sleep characteristics and adiposity in toddlers: results from the GET UP! study. Pediatr Obes. 2019;14:e1255. doi: 10.1111/ijpo.12557
5. Paruthi S, Brooks LJ, D’Ambrosio C, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016;12:785-786. doi: 10.5664/jcsm.5866
6. Daniels SR, Hassink SG; American Academy of Pediatrics Committee on Nutrition. The role of the pediatrician in primary prevention of obesity. Pediatrics 2015;136:e275-e292. doi: 10.1542/peds.2015-1558
1. Miller MA, Kruisbrink M, Wallace J, et al. Sleep duration and incidence of obesity in infants, children, and adolescents: a systematic review and meta-analysis of prospective studies. Sleep. 2018;41:1-19. doi: 10.1093/sleep/zsy018
2. Ruan H, Xun P, Cai W, et al. Habitual sleep duration and risk of childhood obesity: systematic review and dose-response meta-analysis of prospective cohort studies. Sci Rep. 2015;5:16160. doi: 10.1038/srep16160
3. Fatima Y, Doi SA, Mamun AA. Longitudinal impact of sleep on overweight and obesity in children and adolescents: a systematic review and bias-adjusted meta-analysis. Obes Rev. 2015;16:137-149. doi: 10.1111/obr.12245
4. Zhang Z, Pereira JR, Sousa-Sá E, et al. The cross‐sectional and prospective associations between sleep characteristics and adiposity in toddlers: results from the GET UP! study. Pediatr Obes. 2019;14:e1255. doi: 10.1111/ijpo.12557
5. Paruthi S, Brooks LJ, D’Ambrosio C, et al. Recommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016;12:785-786. doi: 10.5664/jcsm.5866
6. Daniels SR, Hassink SG; American Academy of Pediatrics Committee on Nutrition. The role of the pediatrician in primary prevention of obesity. Pediatrics 2015;136:e275-e292. doi: 10.1542/peds.2015-1558
EVIDENCE-BASED ANSWER:
Yes, a link has been established but not a cause-effect relationship. Shorter reported sleep duration in childhood is associated with an increased risk of overweight or obesity years later (strength of recommendation [SOR]: B, meta-analyses of prospective cohort trials with high heterogeneity). In toddlers, accelerometer documentation of short sleep duration is associated with elevation of body mass index (BMI) at 1-year follow-up (SOR: B, prospective cohort). Adequate sleep is recommended to help prevent excessive weight gain in children (SOR: C, expert opinion).
LGBTQ health care: There is reason to be hopeful
I write a lot about watershed moments in my career, things that proved to be moments of tremendous growth, as a person and as a doctor.
One of these occurred early in my career when I met a new patient with ovarian cancer. When I walked into the exam room, I made eye contact with the woman who was accompanied by a man. I assumed they were married, so I went to her first. I introduced myself, stating that I was here to talk about how best to treat her cancer. She stopped me quickly. “Doctor, I am not the patient,” she said. “He is.”
It was the first time I had cared for a transgender man with ovarian cancer. I recall how awkward the following moments were – for all of us. It was the first time I realized that cancer does not have a gender. Men can get breast cancer. Trans women can get prostate cancer. Trans men can get ovarian cancer.
But even many years later, we are not much further along in how prepared we are as a medical community to care for LGBTQ persons. Lesbian, gay, bisexual, transgender, and queer people are not part of the normal medical school curriculum. For most medical students, LGBTQ health is still approached as an aside – perhaps during an infectious disease clerkship, while learning about STDs and HIV. Students do not learn how to approach the male couple seeking to become parents, STD risk reduction for gays and lesbians, or the trans man with ovarian cancer.
But they should, particularly in light of a 2015 study evaluating bias among U.S. medical students. The analysis found that about 45% of medical students exhibited explicit bias against LGBTQ individuals and 8 in 10 held an implicit bias. Fewer than 20% showed no evidence of bias. This lack of preparedness to treat LGBTQ individuals against a backdrop of bias in the medical community often leads patients to mistrust medicine.
To gain perspective outside of oncology, I spoke to Michelle Forcier (she/they), MD, MPH, assistant dean of admissions and professor of pediatrics at Brown University, Providence, R.I. Dr. Forcier agreed that “LGBTQ/rainbow health has been harmfully treated by the system, by both intention and by ignorance.”
“I have had patients who report that EMTs have tried to look under their clothes to determine their gender and transgender patients who have asked point blank to show a provider the results of gender reassignment surgery, not because it was relevant to the issue at hand, but purely out of curiosity,” Dr. Forcier continued. “Then there are the patients who are addressed by the name on their legal record rather than the name that reflects their actual lived experience and identity. These experiences foster this anticipation that is pervasive in this community, that something will be said or done that doesn’t fit who they are, and that ultimately will out them as ‘other.’ ”
I have also felt this sense of being “other” – something I thought I would be immune to as a physician. I have been asked on multiple occasions what my wife does for a living. Moments like this are always awkward. I’m either forced to come out of the closet yet again, or answer vaguely, as if I should be ashamed of my sexuality.
So, how can we move toward equity? Dr. Forcier explained how she lays the groundwork early. “I love pediatrics because kids know when you are being authentic,” she said. “I say who I am, I use she/they pronouns. I also teach by example. If there are more than just my patient in a room, I say, ‘Let’s go around the room and introduce ourselves’ so all have a chance to tell me who they are and how they have come together. If it’s not clear to me, sometimes I prod: ‘How are you here to support [the patient]?’ ”
The point, according to Dr. Forcier: Don’t make assumptions about relationships when you walk into a room with more than one person. Don’t even make assumptions about who the patient is.
But bringing up gender and sexuality can be awkward. Even I sometimes have a hard time. In oncology, patients are there to talk about their cancer and what can be done about it.
“I think it’s really about how it’s framed,” Dr. Forcier said. “In pediatrics, I might start by prefacing it with ‘I am going to ask you some personal questions, and it might seem invasive, but it’s important for your health care. How do you see yourself in the world? What gender identity fits you the best? Who are you attracted to?’ And then I shut up. Doctors need to learn how to stop and wait, provide the space to answer.”
I can see why understanding our patients more deeply is important. We treat people with cancer, not cancer people. As such, understanding someone more fully includes being cognizant of how they identify.
“I am continuously inspired by my LGBTQ patients who have fought to realize who they are and become their truer selves,” Dr. Forcier said. “They know who they are, and they know what they need. They have learned to demand it, to demand that their rights be respected – both civil and human rights.”
As we look toward a future in medicine where diversity, equity, and inclusion have gained prominence and urgency, I think there is reason to be hopeful. In oncology, one institutional study published in 2017 found that, although only about a third of practicing clinicians surveyed were comfortable treating LGBTQ patients, 92% of them acknowledged our unique needs, 78% wanted more education on how to appropriately care for our community, and 64% wanted to be listed as an LGBTQ-friendly provider.
“As an optimist, I believe that those struggling with homophobia/transphobia are open to doing things better,” Dr. Forcier said. “After all, we all strive to be better doctors. Whether explicit or implicit bias is at play, turning moments where colleagues are being inappropriate and showing them an alternative, more inclusive way to handle things is one mechanism to educate, rather than to shame. The bottom line is simple: You don’t have to be perfect. You just have to try.”
Dr. Dizon is the director of women’s cancers at Lifespan Cancer Institute and director of medical oncology at Rhode Island Hospital, both in Providence. He is also a professor of medicine at Brown University. His research interests are in novel treatments of women’s cancers and issues related to survivorship, particularly as they relate to sexual health after cancer for both men and women.
A version of this article first appeared on Medscape.com.
I write a lot about watershed moments in my career, things that proved to be moments of tremendous growth, as a person and as a doctor.
One of these occurred early in my career when I met a new patient with ovarian cancer. When I walked into the exam room, I made eye contact with the woman who was accompanied by a man. I assumed they were married, so I went to her first. I introduced myself, stating that I was here to talk about how best to treat her cancer. She stopped me quickly. “Doctor, I am not the patient,” she said. “He is.”
It was the first time I had cared for a transgender man with ovarian cancer. I recall how awkward the following moments were – for all of us. It was the first time I realized that cancer does not have a gender. Men can get breast cancer. Trans women can get prostate cancer. Trans men can get ovarian cancer.
But even many years later, we are not much further along in how prepared we are as a medical community to care for LGBTQ persons. Lesbian, gay, bisexual, transgender, and queer people are not part of the normal medical school curriculum. For most medical students, LGBTQ health is still approached as an aside – perhaps during an infectious disease clerkship, while learning about STDs and HIV. Students do not learn how to approach the male couple seeking to become parents, STD risk reduction for gays and lesbians, or the trans man with ovarian cancer.
But they should, particularly in light of a 2015 study evaluating bias among U.S. medical students. The analysis found that about 45% of medical students exhibited explicit bias against LGBTQ individuals and 8 in 10 held an implicit bias. Fewer than 20% showed no evidence of bias. This lack of preparedness to treat LGBTQ individuals against a backdrop of bias in the medical community often leads patients to mistrust medicine.
To gain perspective outside of oncology, I spoke to Michelle Forcier (she/they), MD, MPH, assistant dean of admissions and professor of pediatrics at Brown University, Providence, R.I. Dr. Forcier agreed that “LGBTQ/rainbow health has been harmfully treated by the system, by both intention and by ignorance.”
“I have had patients who report that EMTs have tried to look under their clothes to determine their gender and transgender patients who have asked point blank to show a provider the results of gender reassignment surgery, not because it was relevant to the issue at hand, but purely out of curiosity,” Dr. Forcier continued. “Then there are the patients who are addressed by the name on their legal record rather than the name that reflects their actual lived experience and identity. These experiences foster this anticipation that is pervasive in this community, that something will be said or done that doesn’t fit who they are, and that ultimately will out them as ‘other.’ ”
I have also felt this sense of being “other” – something I thought I would be immune to as a physician. I have been asked on multiple occasions what my wife does for a living. Moments like this are always awkward. I’m either forced to come out of the closet yet again, or answer vaguely, as if I should be ashamed of my sexuality.
So, how can we move toward equity? Dr. Forcier explained how she lays the groundwork early. “I love pediatrics because kids know when you are being authentic,” she said. “I say who I am, I use she/they pronouns. I also teach by example. If there are more than just my patient in a room, I say, ‘Let’s go around the room and introduce ourselves’ so all have a chance to tell me who they are and how they have come together. If it’s not clear to me, sometimes I prod: ‘How are you here to support [the patient]?’ ”
The point, according to Dr. Forcier: Don’t make assumptions about relationships when you walk into a room with more than one person. Don’t even make assumptions about who the patient is.
But bringing up gender and sexuality can be awkward. Even I sometimes have a hard time. In oncology, patients are there to talk about their cancer and what can be done about it.
“I think it’s really about how it’s framed,” Dr. Forcier said. “In pediatrics, I might start by prefacing it with ‘I am going to ask you some personal questions, and it might seem invasive, but it’s important for your health care. How do you see yourself in the world? What gender identity fits you the best? Who are you attracted to?’ And then I shut up. Doctors need to learn how to stop and wait, provide the space to answer.”
I can see why understanding our patients more deeply is important. We treat people with cancer, not cancer people. As such, understanding someone more fully includes being cognizant of how they identify.
“I am continuously inspired by my LGBTQ patients who have fought to realize who they are and become their truer selves,” Dr. Forcier said. “They know who they are, and they know what they need. They have learned to demand it, to demand that their rights be respected – both civil and human rights.”
As we look toward a future in medicine where diversity, equity, and inclusion have gained prominence and urgency, I think there is reason to be hopeful. In oncology, one institutional study published in 2017 found that, although only about a third of practicing clinicians surveyed were comfortable treating LGBTQ patients, 92% of them acknowledged our unique needs, 78% wanted more education on how to appropriately care for our community, and 64% wanted to be listed as an LGBTQ-friendly provider.
“As an optimist, I believe that those struggling with homophobia/transphobia are open to doing things better,” Dr. Forcier said. “After all, we all strive to be better doctors. Whether explicit or implicit bias is at play, turning moments where colleagues are being inappropriate and showing them an alternative, more inclusive way to handle things is one mechanism to educate, rather than to shame. The bottom line is simple: You don’t have to be perfect. You just have to try.”
Dr. Dizon is the director of women’s cancers at Lifespan Cancer Institute and director of medical oncology at Rhode Island Hospital, both in Providence. He is also a professor of medicine at Brown University. His research interests are in novel treatments of women’s cancers and issues related to survivorship, particularly as they relate to sexual health after cancer for both men and women.
A version of this article first appeared on Medscape.com.
I write a lot about watershed moments in my career, things that proved to be moments of tremendous growth, as a person and as a doctor.
One of these occurred early in my career when I met a new patient with ovarian cancer. When I walked into the exam room, I made eye contact with the woman who was accompanied by a man. I assumed they were married, so I went to her first. I introduced myself, stating that I was here to talk about how best to treat her cancer. She stopped me quickly. “Doctor, I am not the patient,” she said. “He is.”
It was the first time I had cared for a transgender man with ovarian cancer. I recall how awkward the following moments were – for all of us. It was the first time I realized that cancer does not have a gender. Men can get breast cancer. Trans women can get prostate cancer. Trans men can get ovarian cancer.
But even many years later, we are not much further along in how prepared we are as a medical community to care for LGBTQ persons. Lesbian, gay, bisexual, transgender, and queer people are not part of the normal medical school curriculum. For most medical students, LGBTQ health is still approached as an aside – perhaps during an infectious disease clerkship, while learning about STDs and HIV. Students do not learn how to approach the male couple seeking to become parents, STD risk reduction for gays and lesbians, or the trans man with ovarian cancer.
But they should, particularly in light of a 2015 study evaluating bias among U.S. medical students. The analysis found that about 45% of medical students exhibited explicit bias against LGBTQ individuals and 8 in 10 held an implicit bias. Fewer than 20% showed no evidence of bias. This lack of preparedness to treat LGBTQ individuals against a backdrop of bias in the medical community often leads patients to mistrust medicine.
To gain perspective outside of oncology, I spoke to Michelle Forcier (she/they), MD, MPH, assistant dean of admissions and professor of pediatrics at Brown University, Providence, R.I. Dr. Forcier agreed that “LGBTQ/rainbow health has been harmfully treated by the system, by both intention and by ignorance.”
“I have had patients who report that EMTs have tried to look under their clothes to determine their gender and transgender patients who have asked point blank to show a provider the results of gender reassignment surgery, not because it was relevant to the issue at hand, but purely out of curiosity,” Dr. Forcier continued. “Then there are the patients who are addressed by the name on their legal record rather than the name that reflects their actual lived experience and identity. These experiences foster this anticipation that is pervasive in this community, that something will be said or done that doesn’t fit who they are, and that ultimately will out them as ‘other.’ ”
I have also felt this sense of being “other” – something I thought I would be immune to as a physician. I have been asked on multiple occasions what my wife does for a living. Moments like this are always awkward. I’m either forced to come out of the closet yet again, or answer vaguely, as if I should be ashamed of my sexuality.
So, how can we move toward equity? Dr. Forcier explained how she lays the groundwork early. “I love pediatrics because kids know when you are being authentic,” she said. “I say who I am, I use she/they pronouns. I also teach by example. If there are more than just my patient in a room, I say, ‘Let’s go around the room and introduce ourselves’ so all have a chance to tell me who they are and how they have come together. If it’s not clear to me, sometimes I prod: ‘How are you here to support [the patient]?’ ”
The point, according to Dr. Forcier: Don’t make assumptions about relationships when you walk into a room with more than one person. Don’t even make assumptions about who the patient is.
But bringing up gender and sexuality can be awkward. Even I sometimes have a hard time. In oncology, patients are there to talk about their cancer and what can be done about it.
“I think it’s really about how it’s framed,” Dr. Forcier said. “In pediatrics, I might start by prefacing it with ‘I am going to ask you some personal questions, and it might seem invasive, but it’s important for your health care. How do you see yourself in the world? What gender identity fits you the best? Who are you attracted to?’ And then I shut up. Doctors need to learn how to stop and wait, provide the space to answer.”
I can see why understanding our patients more deeply is important. We treat people with cancer, not cancer people. As such, understanding someone more fully includes being cognizant of how they identify.
“I am continuously inspired by my LGBTQ patients who have fought to realize who they are and become their truer selves,” Dr. Forcier said. “They know who they are, and they know what they need. They have learned to demand it, to demand that their rights be respected – both civil and human rights.”
As we look toward a future in medicine where diversity, equity, and inclusion have gained prominence and urgency, I think there is reason to be hopeful. In oncology, one institutional study published in 2017 found that, although only about a third of practicing clinicians surveyed were comfortable treating LGBTQ patients, 92% of them acknowledged our unique needs, 78% wanted more education on how to appropriately care for our community, and 64% wanted to be listed as an LGBTQ-friendly provider.
“As an optimist, I believe that those struggling with homophobia/transphobia are open to doing things better,” Dr. Forcier said. “After all, we all strive to be better doctors. Whether explicit or implicit bias is at play, turning moments where colleagues are being inappropriate and showing them an alternative, more inclusive way to handle things is one mechanism to educate, rather than to shame. The bottom line is simple: You don’t have to be perfect. You just have to try.”
Dr. Dizon is the director of women’s cancers at Lifespan Cancer Institute and director of medical oncology at Rhode Island Hospital, both in Providence. He is also a professor of medicine at Brown University. His research interests are in novel treatments of women’s cancers and issues related to survivorship, particularly as they relate to sexual health after cancer for both men and women.
A version of this article first appeared on Medscape.com.
Differences in response to immunotherapy in men versus women
.
In a population-based cohort study, women with advanced melanoma and prior ipilimumab treatment who then received combination nivolumab and ipilimumab immunotherapy had a more than twofold increase in the risk for death in comparison with their male counterparts.
The hazard ratio (HR) for mortality among women versus men treated with the combination immunotherapy after prior ipilimumab treatment was 2.06 (P = .003). No such difference was observed among those receiving single-agent therapy with pembrolizumab or nivolumab (HR for mortality in women vs. men, 0.97; P = .85) or among patients without prior ipilimumab use (HR, 0.85; P = .16).
Women with prior ipilimumab use also had a nearly threefold increase in the risk for death with combination immunotherapy versus with single-agent anti–programmed cell death protein–1 (anti-PD-1) therapy (HR, 2.82), but no such difference was seen among the men in the study.
The findings were published online Dec. 2 in JAMA Network Open.
They come from an analysis of Surveillance, Epidemiology, and End Results (SEERS)–Medicare linked data for 982 men and 387 women with stage III or IV melanoma whose median age was 75 years.
The findings suggest that the patient’s sex should be considered in treatment planning to optimize outcomes, the authors noted.
“These novel findings suggest that, for women with a prior history of ipilimumab, treatment with anti-PD-1 therapy may be preferable to combination therapy, whereas for men, it is unclear which treatment is better,” they wrote.
In a press release, principal author Grace Lu-Yao, PhD, a professor at Thomas Jefferson University, Philadelphia, acknowledged that it remains unclear whether the increased risk for death in women is a result of treatment side effects or lack of efficacy, but she stressed that “this is a powerful signal in real-world data that we need to investigate further.
“This data is a wake-up call based on the experience of hundreds of patients on these drugs,” said Dr. Lu-Yao. “This real-world data demonstrates that the results derived from men might not be applicable to women and it is critical to design studies with sufficient power to evaluate treatment effectiveness by sex.”
Relevance for routine practice is unclear
The relevance of the findings for routine practice is unclear, given the median age of the cohort and a lack of data on whether excess mortality was cancer- or toxicity-related or due to another cause, Jeffrey S. Weber, MD, PhD, told this news organization. Dr. Weber is a professor and deputy director of the Laura and Isaac Perlmutter Cancer Center at New York University.
“The study is interesting and detailed, but it is a rather narrowly defined cohort that is over 65 and has a median of age 75, [which is] very different than most melanoma patient cohorts of patients treated with immunotherapy, whose median age is 10 years younger,” Dr. Weber said in an interview.
Furthermore, “in practice, almost no current patients will have been previously treated with ipilimumab and then receive combination immunotherapy,” he said. “Overall, these data would not impact on how I treat patients,” he said.
Gender differences in response
This study is not the first to show a gender-based difference in outcomes after immunotherapy. As previously reported by this news organization, a meta-analysis published in The Lancet Oncology in 2018 showed that immune checkpoint inhibitors are twice as effective as standard cancer therapies in men with advanced solid tumors, compared with their female counterparts.
However, sex-based differences remain under-assessed despite “accumulating evidence of the potential role played by sex in drug effectiveness owing to the biological differences between men and women,” wrote the authors of the latest study in melanoma.
“This lack of attention on the association of sex with the effectiveness of immune checkpoint inhibitor (ICI)–based immunotherapy may have significant negative consequences, especially because these treatments are associated with high toxicity and high treatment cost. For future trials, it would be crucial to examine effect modification by sex,” they added.
The study was funded by the Sidney Kimmel Cancer Center. Dr. Lu-Yao and coauthors have disclosed no relevant financial relationships. Dr. Weber is a regular contributor to Medscape. He reports relationships with Bristol-Myers Squibb, GlaxoSmithKline, Genentech BioOncology, Merck & Co, Novartis, EMD Serono, Celldex, CytomX, Nektar, Roche, Altor, Daiichi-Sankyo, and Eli Lilly and is named on patents filed for biomarkers for ipilimumab and nivolumab.
A version of this article first appeared on Medscape.com.
.
In a population-based cohort study, women with advanced melanoma and prior ipilimumab treatment who then received combination nivolumab and ipilimumab immunotherapy had a more than twofold increase in the risk for death in comparison with their male counterparts.
The hazard ratio (HR) for mortality among women versus men treated with the combination immunotherapy after prior ipilimumab treatment was 2.06 (P = .003). No such difference was observed among those receiving single-agent therapy with pembrolizumab or nivolumab (HR for mortality in women vs. men, 0.97; P = .85) or among patients without prior ipilimumab use (HR, 0.85; P = .16).
Women with prior ipilimumab use also had a nearly threefold increase in the risk for death with combination immunotherapy versus with single-agent anti–programmed cell death protein–1 (anti-PD-1) therapy (HR, 2.82), but no such difference was seen among the men in the study.
The findings were published online Dec. 2 in JAMA Network Open.
They come from an analysis of Surveillance, Epidemiology, and End Results (SEERS)–Medicare linked data for 982 men and 387 women with stage III or IV melanoma whose median age was 75 years.
The findings suggest that the patient’s sex should be considered in treatment planning to optimize outcomes, the authors noted.
“These novel findings suggest that, for women with a prior history of ipilimumab, treatment with anti-PD-1 therapy may be preferable to combination therapy, whereas for men, it is unclear which treatment is better,” they wrote.
In a press release, principal author Grace Lu-Yao, PhD, a professor at Thomas Jefferson University, Philadelphia, acknowledged that it remains unclear whether the increased risk for death in women is a result of treatment side effects or lack of efficacy, but she stressed that “this is a powerful signal in real-world data that we need to investigate further.
“This data is a wake-up call based on the experience of hundreds of patients on these drugs,” said Dr. Lu-Yao. “This real-world data demonstrates that the results derived from men might not be applicable to women and it is critical to design studies with sufficient power to evaluate treatment effectiveness by sex.”
Relevance for routine practice is unclear
The relevance of the findings for routine practice is unclear, given the median age of the cohort and a lack of data on whether excess mortality was cancer- or toxicity-related or due to another cause, Jeffrey S. Weber, MD, PhD, told this news organization. Dr. Weber is a professor and deputy director of the Laura and Isaac Perlmutter Cancer Center at New York University.
“The study is interesting and detailed, but it is a rather narrowly defined cohort that is over 65 and has a median of age 75, [which is] very different than most melanoma patient cohorts of patients treated with immunotherapy, whose median age is 10 years younger,” Dr. Weber said in an interview.
Furthermore, “in practice, almost no current patients will have been previously treated with ipilimumab and then receive combination immunotherapy,” he said. “Overall, these data would not impact on how I treat patients,” he said.
Gender differences in response
This study is not the first to show a gender-based difference in outcomes after immunotherapy. As previously reported by this news organization, a meta-analysis published in The Lancet Oncology in 2018 showed that immune checkpoint inhibitors are twice as effective as standard cancer therapies in men with advanced solid tumors, compared with their female counterparts.
However, sex-based differences remain under-assessed despite “accumulating evidence of the potential role played by sex in drug effectiveness owing to the biological differences between men and women,” wrote the authors of the latest study in melanoma.
“This lack of attention on the association of sex with the effectiveness of immune checkpoint inhibitor (ICI)–based immunotherapy may have significant negative consequences, especially because these treatments are associated with high toxicity and high treatment cost. For future trials, it would be crucial to examine effect modification by sex,” they added.
The study was funded by the Sidney Kimmel Cancer Center. Dr. Lu-Yao and coauthors have disclosed no relevant financial relationships. Dr. Weber is a regular contributor to Medscape. He reports relationships with Bristol-Myers Squibb, GlaxoSmithKline, Genentech BioOncology, Merck & Co, Novartis, EMD Serono, Celldex, CytomX, Nektar, Roche, Altor, Daiichi-Sankyo, and Eli Lilly and is named on patents filed for biomarkers for ipilimumab and nivolumab.
A version of this article first appeared on Medscape.com.
.
In a population-based cohort study, women with advanced melanoma and prior ipilimumab treatment who then received combination nivolumab and ipilimumab immunotherapy had a more than twofold increase in the risk for death in comparison with their male counterparts.
The hazard ratio (HR) for mortality among women versus men treated with the combination immunotherapy after prior ipilimumab treatment was 2.06 (P = .003). No such difference was observed among those receiving single-agent therapy with pembrolizumab or nivolumab (HR for mortality in women vs. men, 0.97; P = .85) or among patients without prior ipilimumab use (HR, 0.85; P = .16).
Women with prior ipilimumab use also had a nearly threefold increase in the risk for death with combination immunotherapy versus with single-agent anti–programmed cell death protein–1 (anti-PD-1) therapy (HR, 2.82), but no such difference was seen among the men in the study.
The findings were published online Dec. 2 in JAMA Network Open.
They come from an analysis of Surveillance, Epidemiology, and End Results (SEERS)–Medicare linked data for 982 men and 387 women with stage III or IV melanoma whose median age was 75 years.
The findings suggest that the patient’s sex should be considered in treatment planning to optimize outcomes, the authors noted.
“These novel findings suggest that, for women with a prior history of ipilimumab, treatment with anti-PD-1 therapy may be preferable to combination therapy, whereas for men, it is unclear which treatment is better,” they wrote.
In a press release, principal author Grace Lu-Yao, PhD, a professor at Thomas Jefferson University, Philadelphia, acknowledged that it remains unclear whether the increased risk for death in women is a result of treatment side effects or lack of efficacy, but she stressed that “this is a powerful signal in real-world data that we need to investigate further.
“This data is a wake-up call based on the experience of hundreds of patients on these drugs,” said Dr. Lu-Yao. “This real-world data demonstrates that the results derived from men might not be applicable to women and it is critical to design studies with sufficient power to evaluate treatment effectiveness by sex.”
Relevance for routine practice is unclear
The relevance of the findings for routine practice is unclear, given the median age of the cohort and a lack of data on whether excess mortality was cancer- or toxicity-related or due to another cause, Jeffrey S. Weber, MD, PhD, told this news organization. Dr. Weber is a professor and deputy director of the Laura and Isaac Perlmutter Cancer Center at New York University.
“The study is interesting and detailed, but it is a rather narrowly defined cohort that is over 65 and has a median of age 75, [which is] very different than most melanoma patient cohorts of patients treated with immunotherapy, whose median age is 10 years younger,” Dr. Weber said in an interview.
Furthermore, “in practice, almost no current patients will have been previously treated with ipilimumab and then receive combination immunotherapy,” he said. “Overall, these data would not impact on how I treat patients,” he said.
Gender differences in response
This study is not the first to show a gender-based difference in outcomes after immunotherapy. As previously reported by this news organization, a meta-analysis published in The Lancet Oncology in 2018 showed that immune checkpoint inhibitors are twice as effective as standard cancer therapies in men with advanced solid tumors, compared with their female counterparts.
However, sex-based differences remain under-assessed despite “accumulating evidence of the potential role played by sex in drug effectiveness owing to the biological differences between men and women,” wrote the authors of the latest study in melanoma.
“This lack of attention on the association of sex with the effectiveness of immune checkpoint inhibitor (ICI)–based immunotherapy may have significant negative consequences, especially because these treatments are associated with high toxicity and high treatment cost. For future trials, it would be crucial to examine effect modification by sex,” they added.
The study was funded by the Sidney Kimmel Cancer Center. Dr. Lu-Yao and coauthors have disclosed no relevant financial relationships. Dr. Weber is a regular contributor to Medscape. He reports relationships with Bristol-Myers Squibb, GlaxoSmithKline, Genentech BioOncology, Merck & Co, Novartis, EMD Serono, Celldex, CytomX, Nektar, Roche, Altor, Daiichi-Sankyo, and Eli Lilly and is named on patents filed for biomarkers for ipilimumab and nivolumab.
A version of this article first appeared on Medscape.com.
International panel backs energy-based devices as first-line treatment of acne scars
International consensus .
Peter R. Shumaker, MD, a dermatologist and dermatologic surgeon at the VA San Diego Healthcare System and one of the authors of the paper, noted that a panel of 24 international experts in dermatology and plastic surgery assembled to develop the recommendations for integrating EBDs into the management of acne scarring.
“The advent of fractional laser technology in the mid-2000s ushered in an exciting new period of exploration and advances in scar treatment with EBDs,” Dr. Shumaker said in an interview. “Despite intense interest and a wealth of available literature, international treatment guidelines and patient access to these potentially life-changing treatments are currently lagging behind our capabilities.”
One of the key recommendations of the paper is that EBDs should have an expanded role in the treatment of acne scars, according to Dr. Shumaker, associate clinical professor of dermatology at the University of California, San Diego. “Panel members were unanimous in their view that EBDs, particularly ablative and nonablative fractional lasers, vascular lasers, and fractional radiofrequency devices, have an important role in the management of acne scars and should be considered a first-line treatment for a variety of scar types,” he said.
The process leading to the recommendations included developing clinical questions, based on input from the panelists and a literature review, and using a two-step modified Delphi method, “an iterative process used to achieve consensus for a defined clinical problem where there is little or conflicting published evidence and where expert opinion is decisive,” the authors wrote. This involved email questionnaires highlighting different topics, including the role of EBDs in mitigating and treating acne scars in patients with active acne, the use of different EBDs for treating different types of acne scars, and considerations in treating skin of color.
The panel noted considerations in the treatment of acne scars in skin of color. “Regardless of the platform, patients with darker skin types may require treatment modifications including: a reduction in fluence/pulse energy; decreased microcolumn density; greater intervals between treatments; longer pulse durations; epidermal cooling with fastidious technique to ensure appropriate cooling, additional cooling in between passes to decrease bulk heating; and pretreatment and posttreatment topical regimens (e.g., retinoids, bleaching creams, etc.) and strict sun precautions,” wrote the authors.
Panelists agreed that there is an absence of large, well-controlled, multicenter comparative trials of various laser and energy treatments for acne scars. “Such trials would be helpful in establishing the relative utility and persistence of benefit of various laser treatments and also in comparing their effectiveness versus that of nonenergy treatments,” the authors noted.
Asked to comment on the paper, Andrei Metelitsa, MD, a dermatologist in Calgary, Alta., and clinical associate professor at the University of Calgary, said the consensus recommendations on EBDs in acne scarring are “providing an international expert perspective, potentially changing a long-perceived paradigm of treatments.”
Dr. Metelitsa pointed out that the authors are taking a solid position with respect to reducing the delay to initiation of laser treatment following isotretinoin therapy. “The authors take a strong stance against the old dogma of postponing laser resurfacing for at least 6 months post isotretinoin,” he said. “According to the authors, there is sufficient evidence to support the idea of safely starting laser therapies, including fractional ablative and nonablative, within 1 month post isotretinoin, much sooner than previously suggested.”
He added that the authors point to the fact most experts utilize vascular lasers, such as pulsed-dye, to treat active acne in combination with medical therapy, thus reducing duration and severity of inflammation and potentially reducing further scar formation. “According to this published consensus, such laser therapies can even be used while the patient is actively treated with isotretinoin,” he said.
Dr. Metelitsa noted that the consensus recommendations outline how the choice of device should be guided by the clinical subtype of acne scars.
Dr. Shumaker, Dr. Metelitsa, and the authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
International consensus .
Peter R. Shumaker, MD, a dermatologist and dermatologic surgeon at the VA San Diego Healthcare System and one of the authors of the paper, noted that a panel of 24 international experts in dermatology and plastic surgery assembled to develop the recommendations for integrating EBDs into the management of acne scarring.
“The advent of fractional laser technology in the mid-2000s ushered in an exciting new period of exploration and advances in scar treatment with EBDs,” Dr. Shumaker said in an interview. “Despite intense interest and a wealth of available literature, international treatment guidelines and patient access to these potentially life-changing treatments are currently lagging behind our capabilities.”
One of the key recommendations of the paper is that EBDs should have an expanded role in the treatment of acne scars, according to Dr. Shumaker, associate clinical professor of dermatology at the University of California, San Diego. “Panel members were unanimous in their view that EBDs, particularly ablative and nonablative fractional lasers, vascular lasers, and fractional radiofrequency devices, have an important role in the management of acne scars and should be considered a first-line treatment for a variety of scar types,” he said.
The process leading to the recommendations included developing clinical questions, based on input from the panelists and a literature review, and using a two-step modified Delphi method, “an iterative process used to achieve consensus for a defined clinical problem where there is little or conflicting published evidence and where expert opinion is decisive,” the authors wrote. This involved email questionnaires highlighting different topics, including the role of EBDs in mitigating and treating acne scars in patients with active acne, the use of different EBDs for treating different types of acne scars, and considerations in treating skin of color.
The panel noted considerations in the treatment of acne scars in skin of color. “Regardless of the platform, patients with darker skin types may require treatment modifications including: a reduction in fluence/pulse energy; decreased microcolumn density; greater intervals between treatments; longer pulse durations; epidermal cooling with fastidious technique to ensure appropriate cooling, additional cooling in between passes to decrease bulk heating; and pretreatment and posttreatment topical regimens (e.g., retinoids, bleaching creams, etc.) and strict sun precautions,” wrote the authors.
Panelists agreed that there is an absence of large, well-controlled, multicenter comparative trials of various laser and energy treatments for acne scars. “Such trials would be helpful in establishing the relative utility and persistence of benefit of various laser treatments and also in comparing their effectiveness versus that of nonenergy treatments,” the authors noted.
Asked to comment on the paper, Andrei Metelitsa, MD, a dermatologist in Calgary, Alta., and clinical associate professor at the University of Calgary, said the consensus recommendations on EBDs in acne scarring are “providing an international expert perspective, potentially changing a long-perceived paradigm of treatments.”
Dr. Metelitsa pointed out that the authors are taking a solid position with respect to reducing the delay to initiation of laser treatment following isotretinoin therapy. “The authors take a strong stance against the old dogma of postponing laser resurfacing for at least 6 months post isotretinoin,” he said. “According to the authors, there is sufficient evidence to support the idea of safely starting laser therapies, including fractional ablative and nonablative, within 1 month post isotretinoin, much sooner than previously suggested.”
He added that the authors point to the fact most experts utilize vascular lasers, such as pulsed-dye, to treat active acne in combination with medical therapy, thus reducing duration and severity of inflammation and potentially reducing further scar formation. “According to this published consensus, such laser therapies can even be used while the patient is actively treated with isotretinoin,” he said.
Dr. Metelitsa noted that the consensus recommendations outline how the choice of device should be guided by the clinical subtype of acne scars.
Dr. Shumaker, Dr. Metelitsa, and the authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
International consensus .
Peter R. Shumaker, MD, a dermatologist and dermatologic surgeon at the VA San Diego Healthcare System and one of the authors of the paper, noted that a panel of 24 international experts in dermatology and plastic surgery assembled to develop the recommendations for integrating EBDs into the management of acne scarring.
“The advent of fractional laser technology in the mid-2000s ushered in an exciting new period of exploration and advances in scar treatment with EBDs,” Dr. Shumaker said in an interview. “Despite intense interest and a wealth of available literature, international treatment guidelines and patient access to these potentially life-changing treatments are currently lagging behind our capabilities.”
One of the key recommendations of the paper is that EBDs should have an expanded role in the treatment of acne scars, according to Dr. Shumaker, associate clinical professor of dermatology at the University of California, San Diego. “Panel members were unanimous in their view that EBDs, particularly ablative and nonablative fractional lasers, vascular lasers, and fractional radiofrequency devices, have an important role in the management of acne scars and should be considered a first-line treatment for a variety of scar types,” he said.
The process leading to the recommendations included developing clinical questions, based on input from the panelists and a literature review, and using a two-step modified Delphi method, “an iterative process used to achieve consensus for a defined clinical problem where there is little or conflicting published evidence and where expert opinion is decisive,” the authors wrote. This involved email questionnaires highlighting different topics, including the role of EBDs in mitigating and treating acne scars in patients with active acne, the use of different EBDs for treating different types of acne scars, and considerations in treating skin of color.
The panel noted considerations in the treatment of acne scars in skin of color. “Regardless of the platform, patients with darker skin types may require treatment modifications including: a reduction in fluence/pulse energy; decreased microcolumn density; greater intervals between treatments; longer pulse durations; epidermal cooling with fastidious technique to ensure appropriate cooling, additional cooling in between passes to decrease bulk heating; and pretreatment and posttreatment topical regimens (e.g., retinoids, bleaching creams, etc.) and strict sun precautions,” wrote the authors.
Panelists agreed that there is an absence of large, well-controlled, multicenter comparative trials of various laser and energy treatments for acne scars. “Such trials would be helpful in establishing the relative utility and persistence of benefit of various laser treatments and also in comparing their effectiveness versus that of nonenergy treatments,” the authors noted.
Asked to comment on the paper, Andrei Metelitsa, MD, a dermatologist in Calgary, Alta., and clinical associate professor at the University of Calgary, said the consensus recommendations on EBDs in acne scarring are “providing an international expert perspective, potentially changing a long-perceived paradigm of treatments.”
Dr. Metelitsa pointed out that the authors are taking a solid position with respect to reducing the delay to initiation of laser treatment following isotretinoin therapy. “The authors take a strong stance against the old dogma of postponing laser resurfacing for at least 6 months post isotretinoin,” he said. “According to the authors, there is sufficient evidence to support the idea of safely starting laser therapies, including fractional ablative and nonablative, within 1 month post isotretinoin, much sooner than previously suggested.”
He added that the authors point to the fact most experts utilize vascular lasers, such as pulsed-dye, to treat active acne in combination with medical therapy, thus reducing duration and severity of inflammation and potentially reducing further scar formation. “According to this published consensus, such laser therapies can even be used while the patient is actively treated with isotretinoin,” he said.
Dr. Metelitsa noted that the consensus recommendations outline how the choice of device should be guided by the clinical subtype of acne scars.
Dr. Shumaker, Dr. Metelitsa, and the authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.




