User login
A Contrasting Dark Background for Nail Sampling
Practice Gap
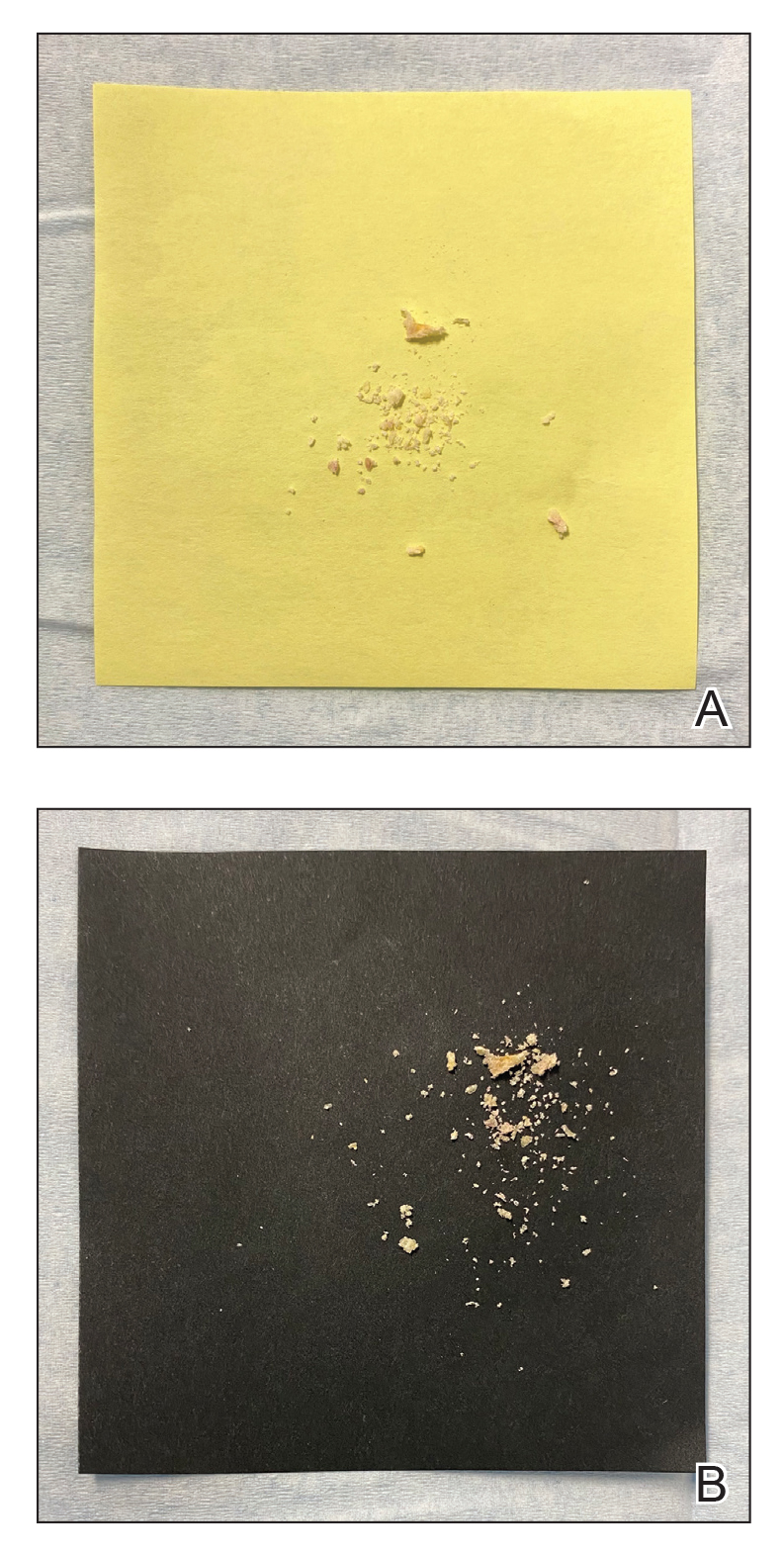
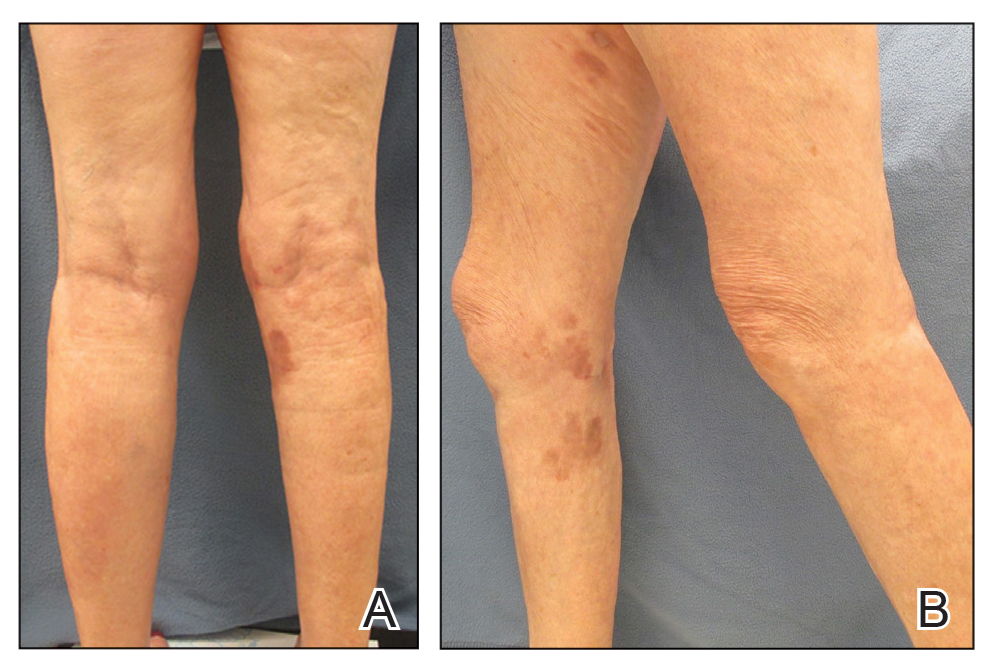
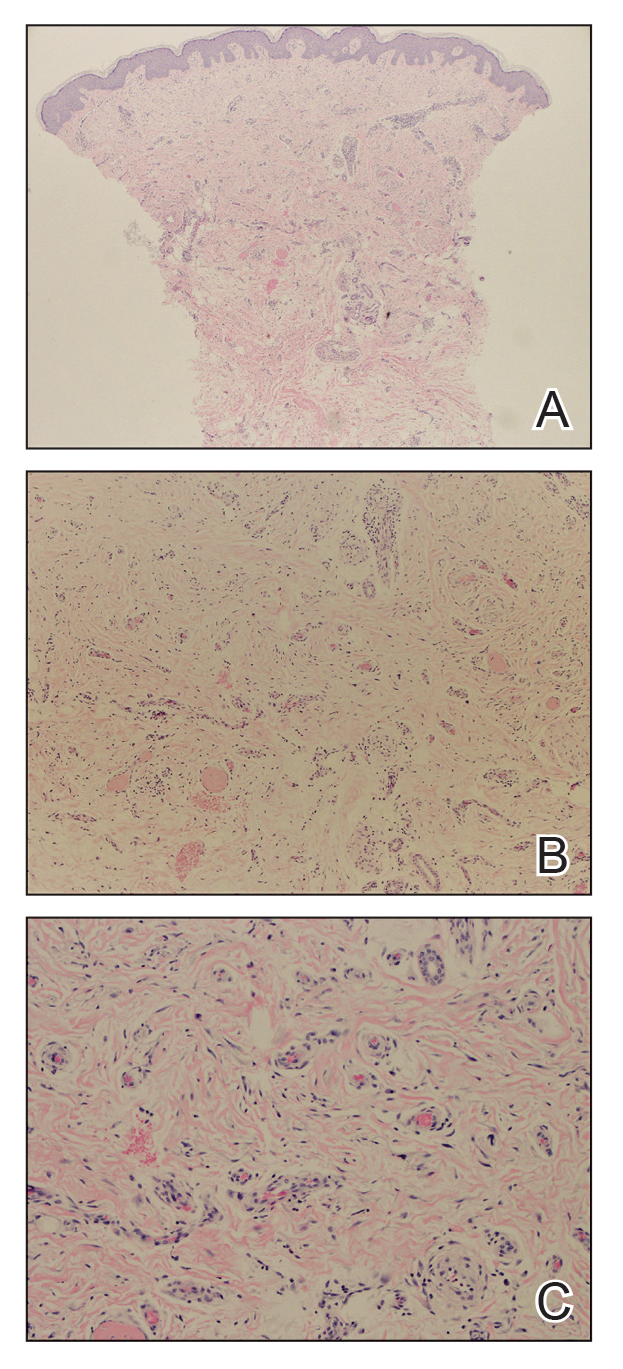
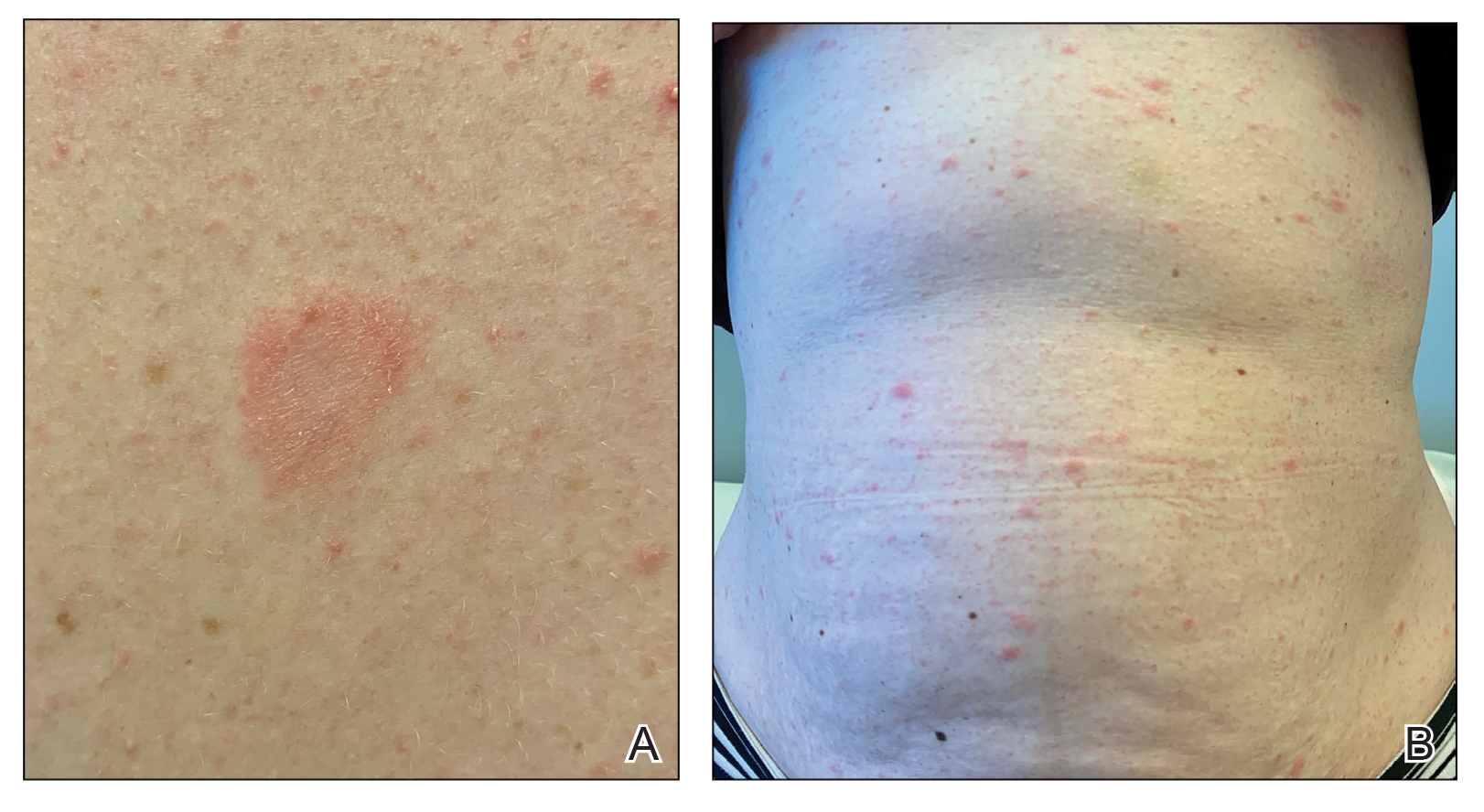
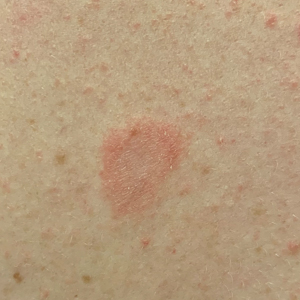
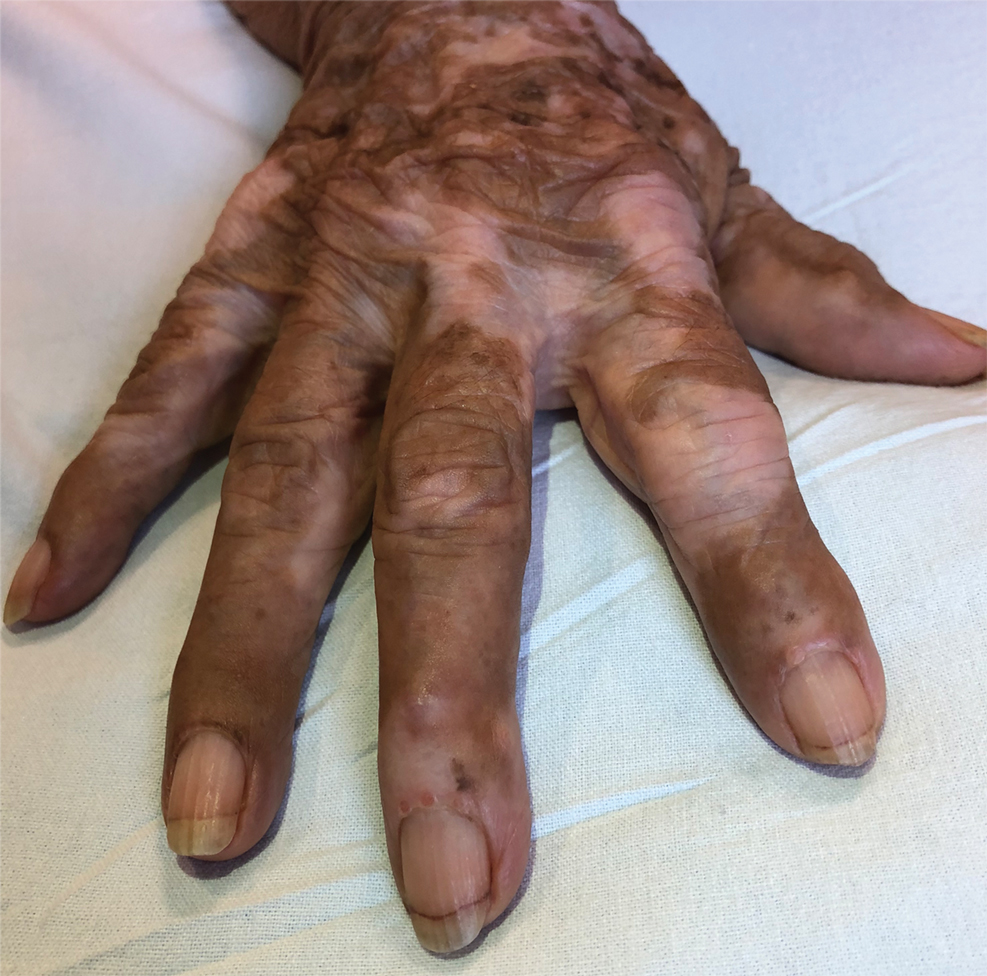
Mycologic testing is necessary and cost-effective1 for appropriate diagnosis and treatment of onychomycosis. Empiric treatment of onychodystrophy for presumed onychomycosis can result in misdiagnosis, treatment failure, or potential adverse effects caused by medications.2 Collection of ample subungual debris facilitates the sensitivity and specificity of fungal culture and fungal polymerase chain reaction. However, the naturally pale hue of subungual debris makes specimen estimation challenging, particularly when using a similarly light-colored gauze or piece of paper for collection (Figure, A).
The Technique
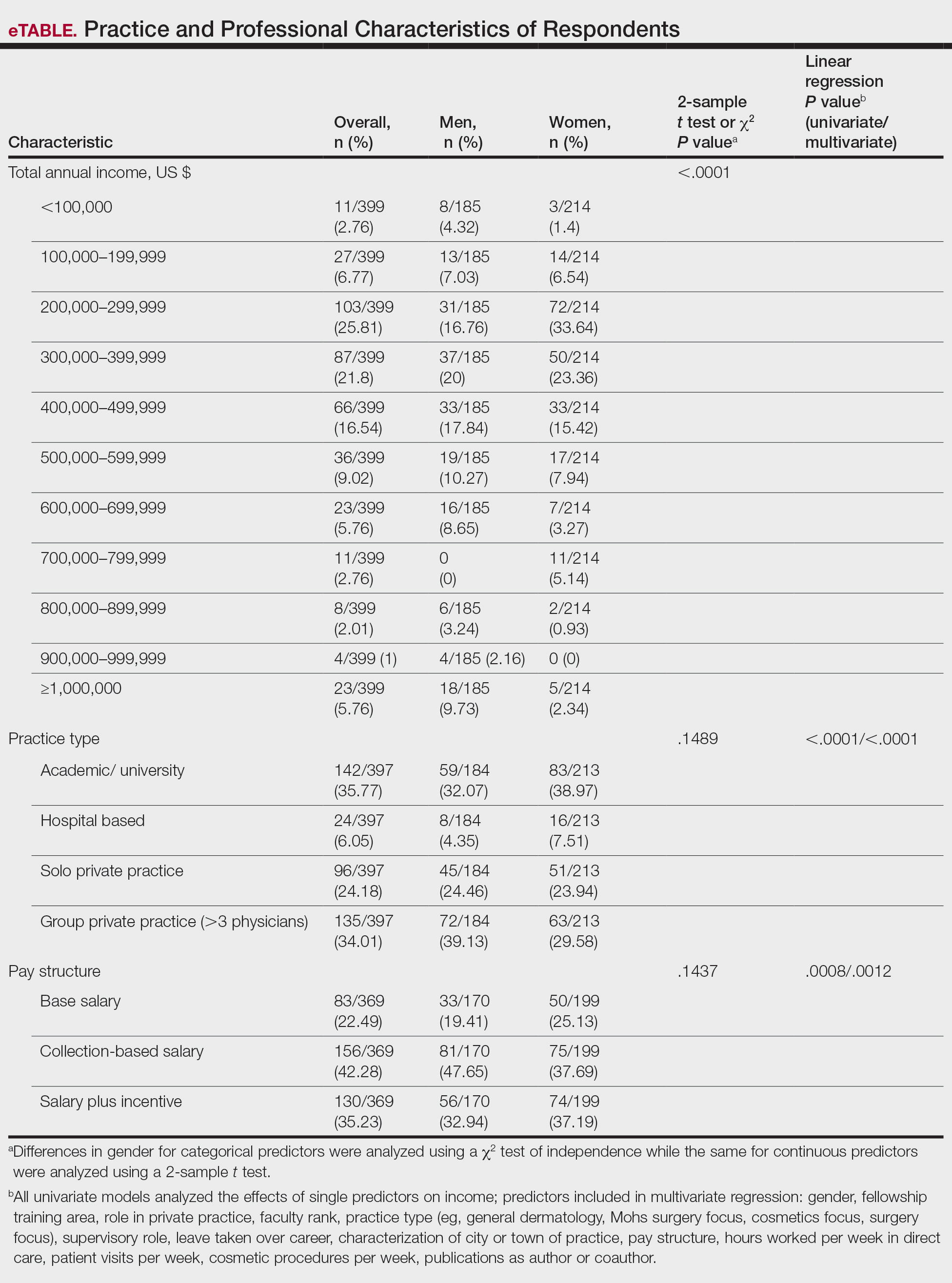
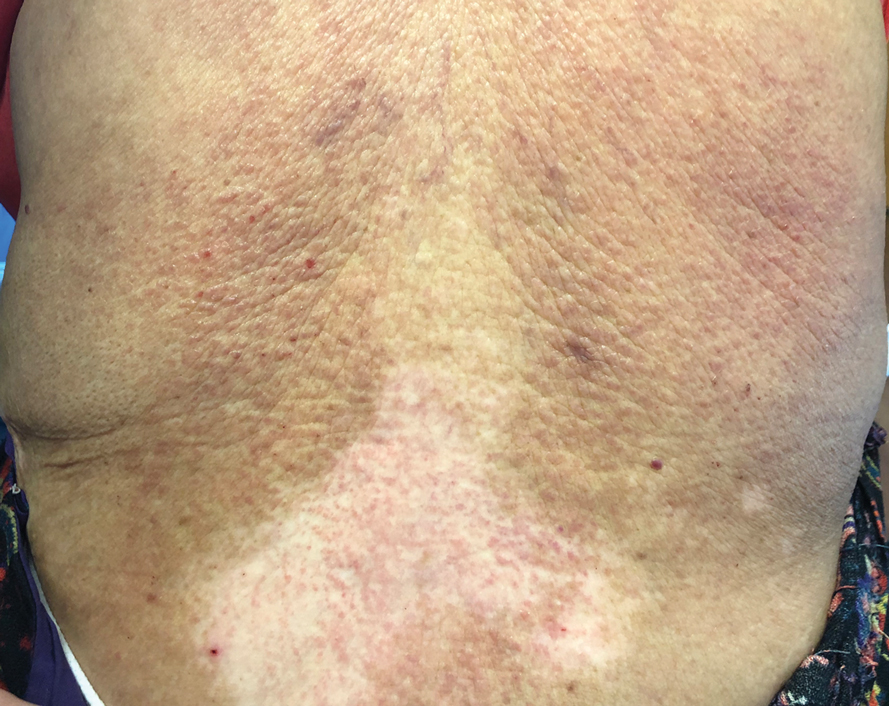
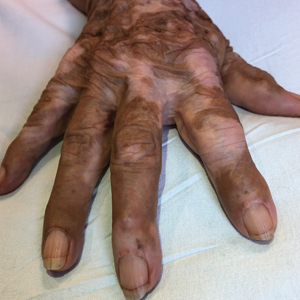
A sheet from a black sticky notepad (widely available and cost-effective) can be adapted for making a diagnosis of onychomycosis (Figure, B).
Practical Implication
Use of a dark background that contrasts with light-hued nail debris is valuable to ensure an adequate specimen for fungal culture and polymerase chain reaction.
- Gupta AK, Versteeg SG, Shear NH. Confirmatory testing prior to initiating onychomycosis therapy is cost effective. J Cutan Med Surg. 2018;22:129-141. doi:10.1177/1203475417733461
- Lipner SR, Scher RK. Onychomycosis—a small step for quality of care. Curr Med Res Opin. 2016;32:865-867. doi:10.1185/03007995.2016.1147026
Practice Gap
Mycologic testing is necessary and cost-effective1 for appropriate diagnosis and treatment of onychomycosis. Empiric treatment of onychodystrophy for presumed onychomycosis can result in misdiagnosis, treatment failure, or potential adverse effects caused by medications.2 Collection of ample subungual debris facilitates the sensitivity and specificity of fungal culture and fungal polymerase chain reaction. However, the naturally pale hue of subungual debris makes specimen estimation challenging, particularly when using a similarly light-colored gauze or piece of paper for collection (Figure, A).
The Technique
A sheet from a black sticky notepad (widely available and cost-effective) can be adapted for making a diagnosis of onychomycosis (Figure, B).
Practical Implication
Use of a dark background that contrasts with light-hued nail debris is valuable to ensure an adequate specimen for fungal culture and polymerase chain reaction.
Practice Gap
Mycologic testing is necessary and cost-effective1 for appropriate diagnosis and treatment of onychomycosis. Empiric treatment of onychodystrophy for presumed onychomycosis can result in misdiagnosis, treatment failure, or potential adverse effects caused by medications.2 Collection of ample subungual debris facilitates the sensitivity and specificity of fungal culture and fungal polymerase chain reaction. However, the naturally pale hue of subungual debris makes specimen estimation challenging, particularly when using a similarly light-colored gauze or piece of paper for collection (Figure, A).
The Technique
A sheet from a black sticky notepad (widely available and cost-effective) can be adapted for making a diagnosis of onychomycosis (Figure, B).
Practical Implication
Use of a dark background that contrasts with light-hued nail debris is valuable to ensure an adequate specimen for fungal culture and polymerase chain reaction.
- Gupta AK, Versteeg SG, Shear NH. Confirmatory testing prior to initiating onychomycosis therapy is cost effective. J Cutan Med Surg. 2018;22:129-141. doi:10.1177/1203475417733461
- Lipner SR, Scher RK. Onychomycosis—a small step for quality of care. Curr Med Res Opin. 2016;32:865-867. doi:10.1185/03007995.2016.1147026
- Gupta AK, Versteeg SG, Shear NH. Confirmatory testing prior to initiating onychomycosis therapy is cost effective. J Cutan Med Surg. 2018;22:129-141. doi:10.1177/1203475417733461
- Lipner SR, Scher RK. Onychomycosis—a small step for quality of care. Curr Med Res Opin. 2016;32:865-867. doi:10.1185/03007995.2016.1147026
Gender Disparities in Income Among Board-Certified Dermatologists
Although the number of female graduates from US medical schools has steadily increased,1 several studies since the 1970s indicate that a disparity exists in salary, academic rank, and promotion among female and male physicians across multiple specialties.2-8 Proposed explanations include women working fewer hours, having lower productivity rates, undernegotiating compensation, and underbilling for the same services. However, when controlling for variables such as time, experience, specialty, rank, and research activities, this gap unequivocally persists. There are limited data on this topic in dermatology, a field in which women comprise more than half of the working population.6,7 Most analyses of gender disparities in dermatology are based on data primarily from academic dermatologists, which may not be representative of the larger population of dermatologists.8,9 The purpose of this study is to determine if an income disparity exists between male and female physicians in dermatology, including those in private practice and those who are specialty trained.
Methods
Population—We performed a cross-sectional self-reported survey to examine compensation of male and female board-certified dermatologists (MDs/DOs). Several populations of dermatologists were surveyed in August and September 2018. Approximately 20% of the members of the American Academy of Dermatology were randomly selected and sent a link to the survey. Additionally, a survey link was emailed to members of the Association of Professors of Dermatology, American College of Mohs Surgery, and American Society for Dermatologic Surgery. A link to the survey also was published on “The Board Certified Dermatologists” Facebook group.
Statistical Analysis—Descriptive statistics were used to summarize the distribution of variables overall and within gender (male or female). Not all respondents completed every section, and duplicates and incomplete responses were removed. Variables were compared between genders using t tests (continuous), the Pearson χ2 test (nominal), or the Cochran-Mantel-Haenszel test (ordinal). For categorical variables with small cell counts, an exact χ2 test for small samples was used. For continuous variables, t test P values were calculated using either pooled or Satterthwaithe approximation.
To analyze the effect of different variables on total income using multivariate and univariate linear regression, the income variable was transformed into a continuous variable by using midpoints of the categories. Univariate linear regression was used to assess the effect and significance of each variable on total annual income. Variables that were found to have a P value of less than .05 (α=.05) were deemed as significant predictors of total annual income. These variables were added to a multivariate linear regression model to determine their effect on income when adjusting for other significant (and approaching significance) factors. In addition, variables that were found to have a P value of less than .2 (α=.05) were added to the multivariate linear regression model to assess significance of these specific variables when adjusting for other factors. In this way, we tested and accounted for a multitude of variables as potential sources of confounding.
Results
Demographics—Our survey was emailed to 3079 members of the American Academy of Dermatology, and 277 responses were received. Approximately 144 additional responses were obtained collectively from links sent to the directories of the Association of Professors of Dermatology, American College of Mohs Surgery, and American Society for Dermatologic Surgery and from social media. Of these respondents, 53.65% (213/397) were female and 46.35% (184/397) were male. When stratifying by race/ethnicity, 77.33% identified as White; 13.85% identified as Asian; 6.3% identified as Black or African American, Hispanic/Latino, and Native American; and 2.52% chose not to respond. Although most male and female respondents were White, a significantly higher proportion of female respondents identified as Asian or Black/African American/Hispanic/Latino/Native American (P=.0006). We found that race/ethnicity did not significantly impact income (P=.2736). All US Census regions were represented in this study, and geographic distribution as well as population density of practice location (ie, rural, suburban, urban setting) did not differ significantly between males and females (P=.5982 and P=.1007, respectively) and did not significantly impact income (P=.3225 and P=.10663, respectively).
Income—Total annual income was defined as the aggregate sum of all types of financial compensation received in 1 calendar year (eg, salary, bonuses, benefits) and was elicited as an ordinal variable in income brackets of US $100,000. Overall, χ2 analysis showed a statistically significant difference in annual total income between male and female dermatologists (P<.0001), with a higher proportion of males in the highest pay bracket (Figure). Gender remained a statistically significant predictor of income on both univariate and multivariate linear regression analyses (P=.0002 and P<.0001, respectively), indicating that gender has a significant impact on compensation, even after controlling for other variables (eTable). Of note, males in this sample were on average older and in practice longer than females (approximately 6 years, P<.0001). However, when univariate linear regression was performed, both age (P=.8281) and number of years since residency or fellowship completion (P=.8743) were not significant predictors of income.
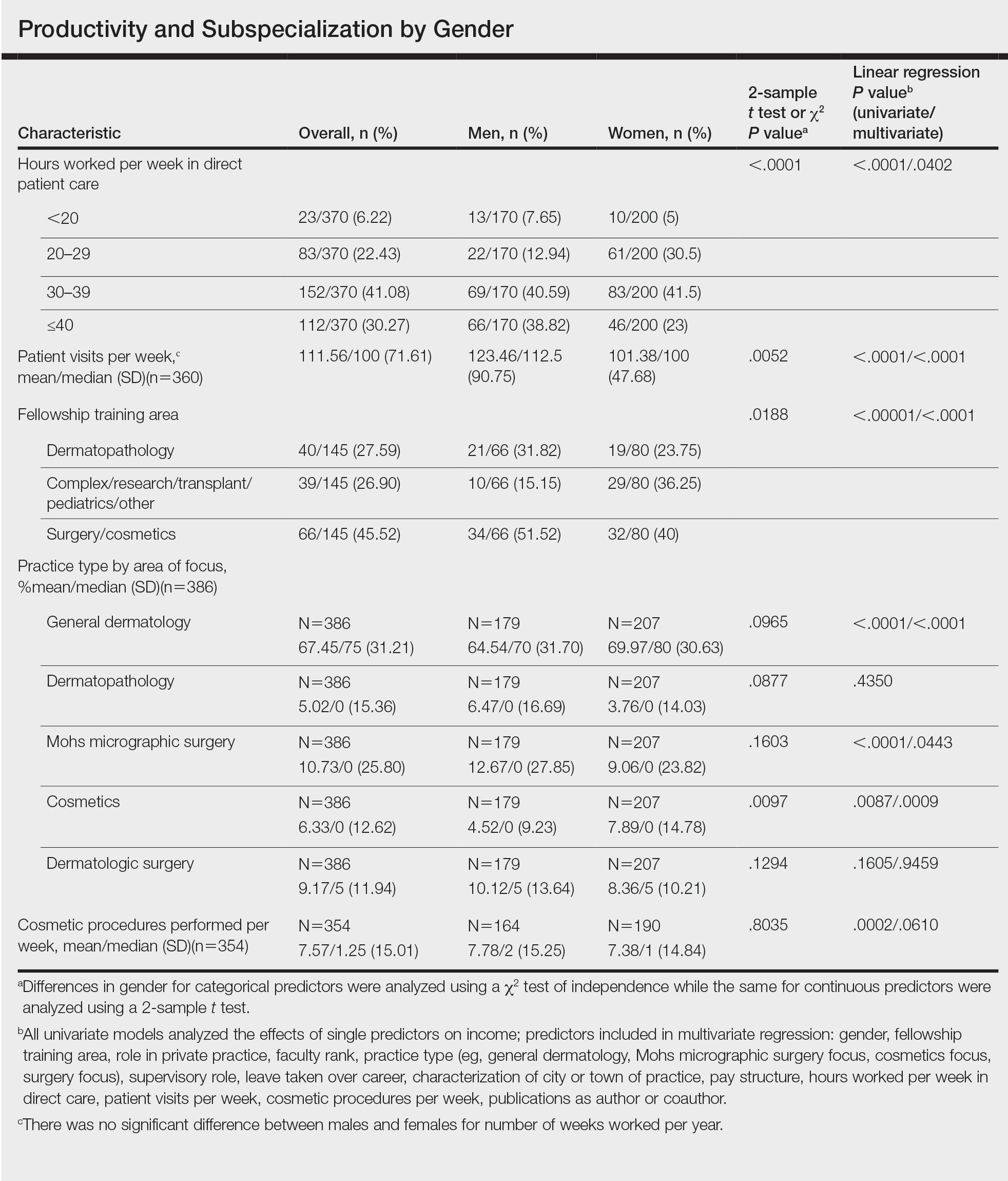
Practice Type—There were no statistically significant differences between men and women in practice type (P=.1489), including academic/university, hospital based, and solo and group private practice; pay structure (P=.1437), including base salary, collection-based salary, or salary plus incentive; holding a supervisory role (P=.0846); or having ownership of a practice (P=.3565)(eTable). Most respondents were in solo or group private practice (58.2%) and had a component of productivity-based compensation (77.5%). In addition, 62% of private practice dermatologists (133/212) had an ownership interest in their practice. As expected, univariate and multivariate regression analyses showed that practice type, pay structure, supervisory roles, and employee vs ownership roles were significant predictors of income (P<.05)(eTable).
Work Productivity—Statistically significant differences were found between men and women in hours worked per week in direct patient care (P<.0001) and in patient visits per week (P=.0052), with a higher percentage of men working more than 40 hours per week and men seeing an average of approximately 22 more patients per week than women. In the subgroup of all dermatologists working more than 40 hours per week, a statistically significant difference in income persisted between males and females (P=.0001). Hours worked per week and patient visits per week were statistically significant predictors of income on both univariate and multivariate regression analyses (P<.05)(Table).
Education and Fellowship Training—No significant difference existed between males and females in type of undergraduate school attended, namely public or private institutions (P=.1090), but a significant difference existed within type of medical school education, with a higher percentage of females attending private medical schools (53.03%) compared to males (38.24%)(P=.0045). However, type of undergraduate or medical school attended had no impact on income (P=.9103). A higher percentage of males (27.32%) completed additional advanced degrees, such as a master of business administration or a master of public health, compared to females (16.9%)(P=.0122). However, the completion of additional advanced degrees had no significant impact on income (P=.2379). No statistical significance existed between males and females in number of residencies completed (P=.3236), and residencies completed had no significant impact on income (P=.4584).
Of 397 respondents, approximately one-third of respondents completed fellowship training (36.5%). Fellowships included dermatopathology, surgery/cosmetics, and other (encompassing complex medical, research, transplant, and pediatric dermatology). Although similar percentages of men and women completed fellowship training, men and women differed significantly by type of fellowship completed (P=.0188). There were similar rates of dermatopathology and surgical fellowship completion between genders but almost 3 times the number of females who completed other fellowships. Type of fellowship training was a statistically significant predictor of income on both univariate and multivariate regression analyses (P<.00001 and P<.0001, respectively).
Work Activity—Respondents were asked to estimate the amount of time devoted to general dermatology, dermatopathology, Mohs micrographic surgery, cosmetics, and dermatologic surgery in their practices (Table). Women devoted a significantly higher average percentage of time to cosmetics (7.89%) compared to men (4.52%)(P=.0097). The number of cosmetic procedures performed per week was not statistically significantly different between men and women (P=.8035) but was a significant factor for income on univariate regression analysis (P=.0002). Time spent performing dermatologic surgery, general dermatology, or Mohs micrographic surgery did not significantly differ between men and women but was found to significantly influence income.
Academic Dermatology—Among the respondents working in academic settings, χ2 analysis identified a significant difference in the faculty rank between males and females, with a tendency for lower academic rank in females (P=.0508). Assistant professorship was comprised of 35% of men vs 51% of women, whereas full professorship consisted of 26% of men but only 13% of women. Academic rank was found to be a significant predictor of income, with higher rank associated with higher income (P<.0001 on univariate regression analysis). However, when adjusting for other factors, academic rank was no longer a significant predictor of income (P=.0840 on multivariate regression analysis). No significant difference existed between men and women in funding received from the National Institutes of Health, conduction of clinical trials, or authorship of scientific publications, and these factors were not found to have a significant impact on income.
Work Leave—Male and female dermatologists showed a statistically significant difference in maternity or Family and Medical Leave Act (FMLA) leave taken over their careers, with 56.03% of females reporting leave taken compared to 6.78% of males (P<.0001). Women reported a significantly higher average number of weeks of maternity or FMLA leave taken over their careers (12.92 weeks) compared to men (2.42 weeks) (P<.0001). However, upon univariate regression analysis, whether or not maternity or FMLA leave was taken over their careers (P=.2005), the number of times that maternity or FMLA leave was taken (P=.4350), and weeks of maternity or FMLA leave taken (P=.4057) were all not significant predictors of income.
Comment
This study sought to investigate the relationship between income and gender in dermatology, and our results demonstrated that statistically significant differences in total annual income exist between male and female dermatologists, with male dermatologists earning a significantly higher income, approximately an additional $80,000. Our results are consistent with other studies of US physician income, which have found a gender gap ranging from $13,399 to $82,000 that persists even when controlling for factors such as specialty choice, practice setting, rank and role in practice, work hours, vacation/leave taken, and others.2-7,10-15
There was a significant difference in rank of male and female academic dermatologists, with fewer females at higher academic ranks. These results are consistent with numerous studies in academic dermatology that show underrepresentation of women at higher academic ranks and leadership positions.8,9,16-18 Poor negotiation may contribute to differences in both rank and income.19,20 There are conflicting data on research productivity of academic dermatologists and length of career, first and senior authorship, and quality and academic impact, all of which add complexity to this topic.8,9,12,16-18,20-23Male and female dermatologists reported significant differences in productivity, with male dermatologists working more hours and seeing more patients per week than female dermatologists. These results are consistent with other studies of dermatologists4,24 and other physicians.12 Regardless, gender was still found to have a significant impact on income even when controlling for differences in productivity and FMLA leave taken. These results are consistent with numerous studies of US physicians that found a gender gap in income even when controlling for hours worked.12,23 Although fellowship training as a whole was found to significantly impact income, our results do not characterize whether the impact on income was positive or negative for each type of fellowship. Fellowship training in specialties such as internal medicine or general surgery likewise has variable effects on income.24,25
A comprehensive survey design and significant data elicited from dermatologists working in private practice for the first time served as the main strengths of this study. Limitations included self-reported design, categorical ranges, and limited sample size in subgroups. Future directions include deeper analysis of subgroups, including fellowship-trained dermatologists, dermatologists working more than 40 hours per week, and female dermatologists by race/ethnicity.
Conclusion
We have demonstrated that self-reported discrepancies in salary between male and female dermatologists exist, with male dermatologists earning a significantly higher annual salary than their female counterparts. This study identified and stratified several career factors that comprise the broad field and practice of dermatology. Even when controlling for these variations, we have demonstrated that gender alone remains a significant predictor of income, indicating that an unexplained income gap between the 2 genders exists in dermatology.
- Association of American Medical Colleges. Table B-2.2: Total Graduates by U.S. Medical School and Sex, 2015-2016 through 2019-2020. December 3, 2020. Accessed October 12, 2021. https://www.aamc.org/download/321532/data/factstableb2-2.pdf
- Willett LL, Halvorsen AJ, McDonald FS, et al. Gender differences in salary of internal medicine residency directors: a national survey. Am J Med. 2015;128:659-665.
- Weeks WB, Wallace AE, Mackenzie TA. Gender differences in anesthesiologists’ annual incomes. Anesthesiology. 2007;106:806-811.
- Weeks WB, Wallace AE. Gender differences in ophthalmologists’ annual incomes. Ophthalmology. 2007;114:1696-1701.
- Singh A, Burke CA, Larive B, et al. Do gender disparities persist in gastroenterology after 10 years of practice? Am J Gastroenterol. 2008;103:1589-1595.
- Desai T, Ali S, Fang X, et al. Equal work for unequal pay: the gender reimbursement gap for healthcare providers in the United States. Postgrad Med J. 2016;92:571-575.
- Jena AB, Olenski AR, Blumenthal DM. Sex differences in physician salary in US public medical schools. JAMA Intern Med. 2016;176:1294-1304.
- John AM, Gupta AB, John ES, et al. A gender-based comparison of promotion and research productivity in academic dermatology. Dermatol Online J. 2016;22:13030/qt1hx610pf.
- Sadeghpour M, Bernstein I, Ko C, et al. Role of sex in academic dermatology: results from a national survey. Arch Dermatol. 2012;148:809-814.
- Gilbert SB, Allshouse A, Skaznik-Wikiel ME. Gender inequality in salaries among reproductive endocrinology and infertility subspecialists in the United States. Fertil Steril. 2019;111:1194-1200.
- Jagsi R, Griffith KA, Stewart A, et al. Gender differences in the salaries of physician researchers. JAMA. 2012;307:2410-2417. doi:10.1001/jama.2012.6183
- Apaydin EA, Chen PGC, Friedberg MW, et al. Differences in physician income by gender in a multiregion survey. J Gen Intern Med. 2018;33:1574-1581.
- Read S, Butkus R, Weissman A, et al. Compensation disparities by gender in internal medicine. Ann Intern Med. 2018;169:658-661.
- Guss ZD, Chen Q, Hu C, et al. Differences in physician compensation between men and women at United States public academic radiation oncology departments. Int J Radiat Oncol Biol Phys. 2019;103:314-319.
- Lo Sasso AT, Richards MR, Chou CF, et al. The $16,819 pay gap for newly trained physicians: the unexplained trend of men earning more than women. Health Aff (Millwood). 2011;30:193-201.
- Shah A, Jalal S, Khosa F. Influences for gender disparity in dermatology in North America. Int J Dermatol. 2018;57:171-176.
- Shi CR, Olbricht S, Vleugels RA, et al. Sex and leadership in academic dermatology: a nationwide survey. J Am Acad Dermatol. 2017;77:782-784.
- Shih AF, Sun W, Yick C, et al. Trends in scholarly productivity of dermatology faculty by academic status and gender. J Am Acad Dermatol. 2019;80:1774-1776.
- Sarfaty S, Kolb D, Barnett R, et al. Negotiation in academic medicine: a necessary career skill. J Womens Health (Larchmt). 2007;16:235-244.
- Jacobson CC, Nguyen JC, Kimball AB. Gender and parenting significantly affect work hours of recent dermatology program graduates. Arch Dermatol. 2004;140:191-196.
- Feramisco JD, Leitenberger JJ, Redfern SI, et al. A gender gap in the dermatology literature? Cross-sectional analysis of manuscript authorship trends in dermatology journals during 3 decades. J Am Acad Dermatol. 2009;60:63-69.
- Bendels MHK, Dietz MC, Brüggmann D, et al. Gender disparities in high-quality dermatology research: a descriptive bibliometric study on scientific authorships. BMJ Open. 2018;8:e020089.
- Seabury SA, Chandra A, Jena AB. Trends in the earnings of male and female health care professionals in the United States, 1987 to 2010. JAMA Intern Med. 2013;173:1748-1750.
- Baimas-George M, Fleischer B, Slakey D, et al. Is it all about the money? Not all surgical subspecialization leads to higher lifetime revenue when compared to general surgery. J Surg Educ. 2017;74:E62-E66.
- Leigh JP, Tancredi D, Jerant A, et al. Lifetime earnings for physicians across specialties. Med Care. 2012;50:1093-1101.
Although the number of female graduates from US medical schools has steadily increased,1 several studies since the 1970s indicate that a disparity exists in salary, academic rank, and promotion among female and male physicians across multiple specialties.2-8 Proposed explanations include women working fewer hours, having lower productivity rates, undernegotiating compensation, and underbilling for the same services. However, when controlling for variables such as time, experience, specialty, rank, and research activities, this gap unequivocally persists. There are limited data on this topic in dermatology, a field in which women comprise more than half of the working population.6,7 Most analyses of gender disparities in dermatology are based on data primarily from academic dermatologists, which may not be representative of the larger population of dermatologists.8,9 The purpose of this study is to determine if an income disparity exists between male and female physicians in dermatology, including those in private practice and those who are specialty trained.
Methods
Population—We performed a cross-sectional self-reported survey to examine compensation of male and female board-certified dermatologists (MDs/DOs). Several populations of dermatologists were surveyed in August and September 2018. Approximately 20% of the members of the American Academy of Dermatology were randomly selected and sent a link to the survey. Additionally, a survey link was emailed to members of the Association of Professors of Dermatology, American College of Mohs Surgery, and American Society for Dermatologic Surgery. A link to the survey also was published on “The Board Certified Dermatologists” Facebook group.
Statistical Analysis—Descriptive statistics were used to summarize the distribution of variables overall and within gender (male or female). Not all respondents completed every section, and duplicates and incomplete responses were removed. Variables were compared between genders using t tests (continuous), the Pearson χ2 test (nominal), or the Cochran-Mantel-Haenszel test (ordinal). For categorical variables with small cell counts, an exact χ2 test for small samples was used. For continuous variables, t test P values were calculated using either pooled or Satterthwaithe approximation.
To analyze the effect of different variables on total income using multivariate and univariate linear regression, the income variable was transformed into a continuous variable by using midpoints of the categories. Univariate linear regression was used to assess the effect and significance of each variable on total annual income. Variables that were found to have a P value of less than .05 (α=.05) were deemed as significant predictors of total annual income. These variables were added to a multivariate linear regression model to determine their effect on income when adjusting for other significant (and approaching significance) factors. In addition, variables that were found to have a P value of less than .2 (α=.05) were added to the multivariate linear regression model to assess significance of these specific variables when adjusting for other factors. In this way, we tested and accounted for a multitude of variables as potential sources of confounding.
Results
Demographics—Our survey was emailed to 3079 members of the American Academy of Dermatology, and 277 responses were received. Approximately 144 additional responses were obtained collectively from links sent to the directories of the Association of Professors of Dermatology, American College of Mohs Surgery, and American Society for Dermatologic Surgery and from social media. Of these respondents, 53.65% (213/397) were female and 46.35% (184/397) were male. When stratifying by race/ethnicity, 77.33% identified as White; 13.85% identified as Asian; 6.3% identified as Black or African American, Hispanic/Latino, and Native American; and 2.52% chose not to respond. Although most male and female respondents were White, a significantly higher proportion of female respondents identified as Asian or Black/African American/Hispanic/Latino/Native American (P=.0006). We found that race/ethnicity did not significantly impact income (P=.2736). All US Census regions were represented in this study, and geographic distribution as well as population density of practice location (ie, rural, suburban, urban setting) did not differ significantly between males and females (P=.5982 and P=.1007, respectively) and did not significantly impact income (P=.3225 and P=.10663, respectively).
Income—Total annual income was defined as the aggregate sum of all types of financial compensation received in 1 calendar year (eg, salary, bonuses, benefits) and was elicited as an ordinal variable in income brackets of US $100,000. Overall, χ2 analysis showed a statistically significant difference in annual total income between male and female dermatologists (P<.0001), with a higher proportion of males in the highest pay bracket (Figure). Gender remained a statistically significant predictor of income on both univariate and multivariate linear regression analyses (P=.0002 and P<.0001, respectively), indicating that gender has a significant impact on compensation, even after controlling for other variables (eTable). Of note, males in this sample were on average older and in practice longer than females (approximately 6 years, P<.0001). However, when univariate linear regression was performed, both age (P=.8281) and number of years since residency or fellowship completion (P=.8743) were not significant predictors of income.
Practice Type—There were no statistically significant differences between men and women in practice type (P=.1489), including academic/university, hospital based, and solo and group private practice; pay structure (P=.1437), including base salary, collection-based salary, or salary plus incentive; holding a supervisory role (P=.0846); or having ownership of a practice (P=.3565)(eTable). Most respondents were in solo or group private practice (58.2%) and had a component of productivity-based compensation (77.5%). In addition, 62% of private practice dermatologists (133/212) had an ownership interest in their practice. As expected, univariate and multivariate regression analyses showed that practice type, pay structure, supervisory roles, and employee vs ownership roles were significant predictors of income (P<.05)(eTable).
Work Productivity—Statistically significant differences were found between men and women in hours worked per week in direct patient care (P<.0001) and in patient visits per week (P=.0052), with a higher percentage of men working more than 40 hours per week and men seeing an average of approximately 22 more patients per week than women. In the subgroup of all dermatologists working more than 40 hours per week, a statistically significant difference in income persisted between males and females (P=.0001). Hours worked per week and patient visits per week were statistically significant predictors of income on both univariate and multivariate regression analyses (P<.05)(Table).
Education and Fellowship Training—No significant difference existed between males and females in type of undergraduate school attended, namely public or private institutions (P=.1090), but a significant difference existed within type of medical school education, with a higher percentage of females attending private medical schools (53.03%) compared to males (38.24%)(P=.0045). However, type of undergraduate or medical school attended had no impact on income (P=.9103). A higher percentage of males (27.32%) completed additional advanced degrees, such as a master of business administration or a master of public health, compared to females (16.9%)(P=.0122). However, the completion of additional advanced degrees had no significant impact on income (P=.2379). No statistical significance existed between males and females in number of residencies completed (P=.3236), and residencies completed had no significant impact on income (P=.4584).
Of 397 respondents, approximately one-third of respondents completed fellowship training (36.5%). Fellowships included dermatopathology, surgery/cosmetics, and other (encompassing complex medical, research, transplant, and pediatric dermatology). Although similar percentages of men and women completed fellowship training, men and women differed significantly by type of fellowship completed (P=.0188). There were similar rates of dermatopathology and surgical fellowship completion between genders but almost 3 times the number of females who completed other fellowships. Type of fellowship training was a statistically significant predictor of income on both univariate and multivariate regression analyses (P<.00001 and P<.0001, respectively).
Work Activity—Respondents were asked to estimate the amount of time devoted to general dermatology, dermatopathology, Mohs micrographic surgery, cosmetics, and dermatologic surgery in their practices (Table). Women devoted a significantly higher average percentage of time to cosmetics (7.89%) compared to men (4.52%)(P=.0097). The number of cosmetic procedures performed per week was not statistically significantly different between men and women (P=.8035) but was a significant factor for income on univariate regression analysis (P=.0002). Time spent performing dermatologic surgery, general dermatology, or Mohs micrographic surgery did not significantly differ between men and women but was found to significantly influence income.
Academic Dermatology—Among the respondents working in academic settings, χ2 analysis identified a significant difference in the faculty rank between males and females, with a tendency for lower academic rank in females (P=.0508). Assistant professorship was comprised of 35% of men vs 51% of women, whereas full professorship consisted of 26% of men but only 13% of women. Academic rank was found to be a significant predictor of income, with higher rank associated with higher income (P<.0001 on univariate regression analysis). However, when adjusting for other factors, academic rank was no longer a significant predictor of income (P=.0840 on multivariate regression analysis). No significant difference existed between men and women in funding received from the National Institutes of Health, conduction of clinical trials, or authorship of scientific publications, and these factors were not found to have a significant impact on income.
Work Leave—Male and female dermatologists showed a statistically significant difference in maternity or Family and Medical Leave Act (FMLA) leave taken over their careers, with 56.03% of females reporting leave taken compared to 6.78% of males (P<.0001). Women reported a significantly higher average number of weeks of maternity or FMLA leave taken over their careers (12.92 weeks) compared to men (2.42 weeks) (P<.0001). However, upon univariate regression analysis, whether or not maternity or FMLA leave was taken over their careers (P=.2005), the number of times that maternity or FMLA leave was taken (P=.4350), and weeks of maternity or FMLA leave taken (P=.4057) were all not significant predictors of income.
Comment
This study sought to investigate the relationship between income and gender in dermatology, and our results demonstrated that statistically significant differences in total annual income exist between male and female dermatologists, with male dermatologists earning a significantly higher income, approximately an additional $80,000. Our results are consistent with other studies of US physician income, which have found a gender gap ranging from $13,399 to $82,000 that persists even when controlling for factors such as specialty choice, practice setting, rank and role in practice, work hours, vacation/leave taken, and others.2-7,10-15
There was a significant difference in rank of male and female academic dermatologists, with fewer females at higher academic ranks. These results are consistent with numerous studies in academic dermatology that show underrepresentation of women at higher academic ranks and leadership positions.8,9,16-18 Poor negotiation may contribute to differences in both rank and income.19,20 There are conflicting data on research productivity of academic dermatologists and length of career, first and senior authorship, and quality and academic impact, all of which add complexity to this topic.8,9,12,16-18,20-23Male and female dermatologists reported significant differences in productivity, with male dermatologists working more hours and seeing more patients per week than female dermatologists. These results are consistent with other studies of dermatologists4,24 and other physicians.12 Regardless, gender was still found to have a significant impact on income even when controlling for differences in productivity and FMLA leave taken. These results are consistent with numerous studies of US physicians that found a gender gap in income even when controlling for hours worked.12,23 Although fellowship training as a whole was found to significantly impact income, our results do not characterize whether the impact on income was positive or negative for each type of fellowship. Fellowship training in specialties such as internal medicine or general surgery likewise has variable effects on income.24,25
A comprehensive survey design and significant data elicited from dermatologists working in private practice for the first time served as the main strengths of this study. Limitations included self-reported design, categorical ranges, and limited sample size in subgroups. Future directions include deeper analysis of subgroups, including fellowship-trained dermatologists, dermatologists working more than 40 hours per week, and female dermatologists by race/ethnicity.
Conclusion
We have demonstrated that self-reported discrepancies in salary between male and female dermatologists exist, with male dermatologists earning a significantly higher annual salary than their female counterparts. This study identified and stratified several career factors that comprise the broad field and practice of dermatology. Even when controlling for these variations, we have demonstrated that gender alone remains a significant predictor of income, indicating that an unexplained income gap between the 2 genders exists in dermatology.
Although the number of female graduates from US medical schools has steadily increased,1 several studies since the 1970s indicate that a disparity exists in salary, academic rank, and promotion among female and male physicians across multiple specialties.2-8 Proposed explanations include women working fewer hours, having lower productivity rates, undernegotiating compensation, and underbilling for the same services. However, when controlling for variables such as time, experience, specialty, rank, and research activities, this gap unequivocally persists. There are limited data on this topic in dermatology, a field in which women comprise more than half of the working population.6,7 Most analyses of gender disparities in dermatology are based on data primarily from academic dermatologists, which may not be representative of the larger population of dermatologists.8,9 The purpose of this study is to determine if an income disparity exists between male and female physicians in dermatology, including those in private practice and those who are specialty trained.
Methods
Population—We performed a cross-sectional self-reported survey to examine compensation of male and female board-certified dermatologists (MDs/DOs). Several populations of dermatologists were surveyed in August and September 2018. Approximately 20% of the members of the American Academy of Dermatology were randomly selected and sent a link to the survey. Additionally, a survey link was emailed to members of the Association of Professors of Dermatology, American College of Mohs Surgery, and American Society for Dermatologic Surgery. A link to the survey also was published on “The Board Certified Dermatologists” Facebook group.
Statistical Analysis—Descriptive statistics were used to summarize the distribution of variables overall and within gender (male or female). Not all respondents completed every section, and duplicates and incomplete responses were removed. Variables were compared between genders using t tests (continuous), the Pearson χ2 test (nominal), or the Cochran-Mantel-Haenszel test (ordinal). For categorical variables with small cell counts, an exact χ2 test for small samples was used. For continuous variables, t test P values were calculated using either pooled or Satterthwaithe approximation.
To analyze the effect of different variables on total income using multivariate and univariate linear regression, the income variable was transformed into a continuous variable by using midpoints of the categories. Univariate linear regression was used to assess the effect and significance of each variable on total annual income. Variables that were found to have a P value of less than .05 (α=.05) were deemed as significant predictors of total annual income. These variables were added to a multivariate linear regression model to determine their effect on income when adjusting for other significant (and approaching significance) factors. In addition, variables that were found to have a P value of less than .2 (α=.05) were added to the multivariate linear regression model to assess significance of these specific variables when adjusting for other factors. In this way, we tested and accounted for a multitude of variables as potential sources of confounding.
Results
Demographics—Our survey was emailed to 3079 members of the American Academy of Dermatology, and 277 responses were received. Approximately 144 additional responses were obtained collectively from links sent to the directories of the Association of Professors of Dermatology, American College of Mohs Surgery, and American Society for Dermatologic Surgery and from social media. Of these respondents, 53.65% (213/397) were female and 46.35% (184/397) were male. When stratifying by race/ethnicity, 77.33% identified as White; 13.85% identified as Asian; 6.3% identified as Black or African American, Hispanic/Latino, and Native American; and 2.52% chose not to respond. Although most male and female respondents were White, a significantly higher proportion of female respondents identified as Asian or Black/African American/Hispanic/Latino/Native American (P=.0006). We found that race/ethnicity did not significantly impact income (P=.2736). All US Census regions were represented in this study, and geographic distribution as well as population density of practice location (ie, rural, suburban, urban setting) did not differ significantly between males and females (P=.5982 and P=.1007, respectively) and did not significantly impact income (P=.3225 and P=.10663, respectively).
Income—Total annual income was defined as the aggregate sum of all types of financial compensation received in 1 calendar year (eg, salary, bonuses, benefits) and was elicited as an ordinal variable in income brackets of US $100,000. Overall, χ2 analysis showed a statistically significant difference in annual total income between male and female dermatologists (P<.0001), with a higher proportion of males in the highest pay bracket (Figure). Gender remained a statistically significant predictor of income on both univariate and multivariate linear regression analyses (P=.0002 and P<.0001, respectively), indicating that gender has a significant impact on compensation, even after controlling for other variables (eTable). Of note, males in this sample were on average older and in practice longer than females (approximately 6 years, P<.0001). However, when univariate linear regression was performed, both age (P=.8281) and number of years since residency or fellowship completion (P=.8743) were not significant predictors of income.
Practice Type—There were no statistically significant differences between men and women in practice type (P=.1489), including academic/university, hospital based, and solo and group private practice; pay structure (P=.1437), including base salary, collection-based salary, or salary plus incentive; holding a supervisory role (P=.0846); or having ownership of a practice (P=.3565)(eTable). Most respondents were in solo or group private practice (58.2%) and had a component of productivity-based compensation (77.5%). In addition, 62% of private practice dermatologists (133/212) had an ownership interest in their practice. As expected, univariate and multivariate regression analyses showed that practice type, pay structure, supervisory roles, and employee vs ownership roles were significant predictors of income (P<.05)(eTable).
Work Productivity—Statistically significant differences were found between men and women in hours worked per week in direct patient care (P<.0001) and in patient visits per week (P=.0052), with a higher percentage of men working more than 40 hours per week and men seeing an average of approximately 22 more patients per week than women. In the subgroup of all dermatologists working more than 40 hours per week, a statistically significant difference in income persisted between males and females (P=.0001). Hours worked per week and patient visits per week were statistically significant predictors of income on both univariate and multivariate regression analyses (P<.05)(Table).
Education and Fellowship Training—No significant difference existed between males and females in type of undergraduate school attended, namely public or private institutions (P=.1090), but a significant difference existed within type of medical school education, with a higher percentage of females attending private medical schools (53.03%) compared to males (38.24%)(P=.0045). However, type of undergraduate or medical school attended had no impact on income (P=.9103). A higher percentage of males (27.32%) completed additional advanced degrees, such as a master of business administration or a master of public health, compared to females (16.9%)(P=.0122). However, the completion of additional advanced degrees had no significant impact on income (P=.2379). No statistical significance existed between males and females in number of residencies completed (P=.3236), and residencies completed had no significant impact on income (P=.4584).
Of 397 respondents, approximately one-third of respondents completed fellowship training (36.5%). Fellowships included dermatopathology, surgery/cosmetics, and other (encompassing complex medical, research, transplant, and pediatric dermatology). Although similar percentages of men and women completed fellowship training, men and women differed significantly by type of fellowship completed (P=.0188). There were similar rates of dermatopathology and surgical fellowship completion between genders but almost 3 times the number of females who completed other fellowships. Type of fellowship training was a statistically significant predictor of income on both univariate and multivariate regression analyses (P<.00001 and P<.0001, respectively).
Work Activity—Respondents were asked to estimate the amount of time devoted to general dermatology, dermatopathology, Mohs micrographic surgery, cosmetics, and dermatologic surgery in their practices (Table). Women devoted a significantly higher average percentage of time to cosmetics (7.89%) compared to men (4.52%)(P=.0097). The number of cosmetic procedures performed per week was not statistically significantly different between men and women (P=.8035) but was a significant factor for income on univariate regression analysis (P=.0002). Time spent performing dermatologic surgery, general dermatology, or Mohs micrographic surgery did not significantly differ between men and women but was found to significantly influence income.
Academic Dermatology—Among the respondents working in academic settings, χ2 analysis identified a significant difference in the faculty rank between males and females, with a tendency for lower academic rank in females (P=.0508). Assistant professorship was comprised of 35% of men vs 51% of women, whereas full professorship consisted of 26% of men but only 13% of women. Academic rank was found to be a significant predictor of income, with higher rank associated with higher income (P<.0001 on univariate regression analysis). However, when adjusting for other factors, academic rank was no longer a significant predictor of income (P=.0840 on multivariate regression analysis). No significant difference existed between men and women in funding received from the National Institutes of Health, conduction of clinical trials, or authorship of scientific publications, and these factors were not found to have a significant impact on income.
Work Leave—Male and female dermatologists showed a statistically significant difference in maternity or Family and Medical Leave Act (FMLA) leave taken over their careers, with 56.03% of females reporting leave taken compared to 6.78% of males (P<.0001). Women reported a significantly higher average number of weeks of maternity or FMLA leave taken over their careers (12.92 weeks) compared to men (2.42 weeks) (P<.0001). However, upon univariate regression analysis, whether or not maternity or FMLA leave was taken over their careers (P=.2005), the number of times that maternity or FMLA leave was taken (P=.4350), and weeks of maternity or FMLA leave taken (P=.4057) were all not significant predictors of income.
Comment
This study sought to investigate the relationship between income and gender in dermatology, and our results demonstrated that statistically significant differences in total annual income exist between male and female dermatologists, with male dermatologists earning a significantly higher income, approximately an additional $80,000. Our results are consistent with other studies of US physician income, which have found a gender gap ranging from $13,399 to $82,000 that persists even when controlling for factors such as specialty choice, practice setting, rank and role in practice, work hours, vacation/leave taken, and others.2-7,10-15
There was a significant difference in rank of male and female academic dermatologists, with fewer females at higher academic ranks. These results are consistent with numerous studies in academic dermatology that show underrepresentation of women at higher academic ranks and leadership positions.8,9,16-18 Poor negotiation may contribute to differences in both rank and income.19,20 There are conflicting data on research productivity of academic dermatologists and length of career, first and senior authorship, and quality and academic impact, all of which add complexity to this topic.8,9,12,16-18,20-23Male and female dermatologists reported significant differences in productivity, with male dermatologists working more hours and seeing more patients per week than female dermatologists. These results are consistent with other studies of dermatologists4,24 and other physicians.12 Regardless, gender was still found to have a significant impact on income even when controlling for differences in productivity and FMLA leave taken. These results are consistent with numerous studies of US physicians that found a gender gap in income even when controlling for hours worked.12,23 Although fellowship training as a whole was found to significantly impact income, our results do not characterize whether the impact on income was positive or negative for each type of fellowship. Fellowship training in specialties such as internal medicine or general surgery likewise has variable effects on income.24,25
A comprehensive survey design and significant data elicited from dermatologists working in private practice for the first time served as the main strengths of this study. Limitations included self-reported design, categorical ranges, and limited sample size in subgroups. Future directions include deeper analysis of subgroups, including fellowship-trained dermatologists, dermatologists working more than 40 hours per week, and female dermatologists by race/ethnicity.
Conclusion
We have demonstrated that self-reported discrepancies in salary between male and female dermatologists exist, with male dermatologists earning a significantly higher annual salary than their female counterparts. This study identified and stratified several career factors that comprise the broad field and practice of dermatology. Even when controlling for these variations, we have demonstrated that gender alone remains a significant predictor of income, indicating that an unexplained income gap between the 2 genders exists in dermatology.
- Association of American Medical Colleges. Table B-2.2: Total Graduates by U.S. Medical School and Sex, 2015-2016 through 2019-2020. December 3, 2020. Accessed October 12, 2021. https://www.aamc.org/download/321532/data/factstableb2-2.pdf
- Willett LL, Halvorsen AJ, McDonald FS, et al. Gender differences in salary of internal medicine residency directors: a national survey. Am J Med. 2015;128:659-665.
- Weeks WB, Wallace AE, Mackenzie TA. Gender differences in anesthesiologists’ annual incomes. Anesthesiology. 2007;106:806-811.
- Weeks WB, Wallace AE. Gender differences in ophthalmologists’ annual incomes. Ophthalmology. 2007;114:1696-1701.
- Singh A, Burke CA, Larive B, et al. Do gender disparities persist in gastroenterology after 10 years of practice? Am J Gastroenterol. 2008;103:1589-1595.
- Desai T, Ali S, Fang X, et al. Equal work for unequal pay: the gender reimbursement gap for healthcare providers in the United States. Postgrad Med J. 2016;92:571-575.
- Jena AB, Olenski AR, Blumenthal DM. Sex differences in physician salary in US public medical schools. JAMA Intern Med. 2016;176:1294-1304.
- John AM, Gupta AB, John ES, et al. A gender-based comparison of promotion and research productivity in academic dermatology. Dermatol Online J. 2016;22:13030/qt1hx610pf.
- Sadeghpour M, Bernstein I, Ko C, et al. Role of sex in academic dermatology: results from a national survey. Arch Dermatol. 2012;148:809-814.
- Gilbert SB, Allshouse A, Skaznik-Wikiel ME. Gender inequality in salaries among reproductive endocrinology and infertility subspecialists in the United States. Fertil Steril. 2019;111:1194-1200.
- Jagsi R, Griffith KA, Stewart A, et al. Gender differences in the salaries of physician researchers. JAMA. 2012;307:2410-2417. doi:10.1001/jama.2012.6183
- Apaydin EA, Chen PGC, Friedberg MW, et al. Differences in physician income by gender in a multiregion survey. J Gen Intern Med. 2018;33:1574-1581.
- Read S, Butkus R, Weissman A, et al. Compensation disparities by gender in internal medicine. Ann Intern Med. 2018;169:658-661.
- Guss ZD, Chen Q, Hu C, et al. Differences in physician compensation between men and women at United States public academic radiation oncology departments. Int J Radiat Oncol Biol Phys. 2019;103:314-319.
- Lo Sasso AT, Richards MR, Chou CF, et al. The $16,819 pay gap for newly trained physicians: the unexplained trend of men earning more than women. Health Aff (Millwood). 2011;30:193-201.
- Shah A, Jalal S, Khosa F. Influences for gender disparity in dermatology in North America. Int J Dermatol. 2018;57:171-176.
- Shi CR, Olbricht S, Vleugels RA, et al. Sex and leadership in academic dermatology: a nationwide survey. J Am Acad Dermatol. 2017;77:782-784.
- Shih AF, Sun W, Yick C, et al. Trends in scholarly productivity of dermatology faculty by academic status and gender. J Am Acad Dermatol. 2019;80:1774-1776.
- Sarfaty S, Kolb D, Barnett R, et al. Negotiation in academic medicine: a necessary career skill. J Womens Health (Larchmt). 2007;16:235-244.
- Jacobson CC, Nguyen JC, Kimball AB. Gender and parenting significantly affect work hours of recent dermatology program graduates. Arch Dermatol. 2004;140:191-196.
- Feramisco JD, Leitenberger JJ, Redfern SI, et al. A gender gap in the dermatology literature? Cross-sectional analysis of manuscript authorship trends in dermatology journals during 3 decades. J Am Acad Dermatol. 2009;60:63-69.
- Bendels MHK, Dietz MC, Brüggmann D, et al. Gender disparities in high-quality dermatology research: a descriptive bibliometric study on scientific authorships. BMJ Open. 2018;8:e020089.
- Seabury SA, Chandra A, Jena AB. Trends in the earnings of male and female health care professionals in the United States, 1987 to 2010. JAMA Intern Med. 2013;173:1748-1750.
- Baimas-George M, Fleischer B, Slakey D, et al. Is it all about the money? Not all surgical subspecialization leads to higher lifetime revenue when compared to general surgery. J Surg Educ. 2017;74:E62-E66.
- Leigh JP, Tancredi D, Jerant A, et al. Lifetime earnings for physicians across specialties. Med Care. 2012;50:1093-1101.
- Association of American Medical Colleges. Table B-2.2: Total Graduates by U.S. Medical School and Sex, 2015-2016 through 2019-2020. December 3, 2020. Accessed October 12, 2021. https://www.aamc.org/download/321532/data/factstableb2-2.pdf
- Willett LL, Halvorsen AJ, McDonald FS, et al. Gender differences in salary of internal medicine residency directors: a national survey. Am J Med. 2015;128:659-665.
- Weeks WB, Wallace AE, Mackenzie TA. Gender differences in anesthesiologists’ annual incomes. Anesthesiology. 2007;106:806-811.
- Weeks WB, Wallace AE. Gender differences in ophthalmologists’ annual incomes. Ophthalmology. 2007;114:1696-1701.
- Singh A, Burke CA, Larive B, et al. Do gender disparities persist in gastroenterology after 10 years of practice? Am J Gastroenterol. 2008;103:1589-1595.
- Desai T, Ali S, Fang X, et al. Equal work for unequal pay: the gender reimbursement gap for healthcare providers in the United States. Postgrad Med J. 2016;92:571-575.
- Jena AB, Olenski AR, Blumenthal DM. Sex differences in physician salary in US public medical schools. JAMA Intern Med. 2016;176:1294-1304.
- John AM, Gupta AB, John ES, et al. A gender-based comparison of promotion and research productivity in academic dermatology. Dermatol Online J. 2016;22:13030/qt1hx610pf.
- Sadeghpour M, Bernstein I, Ko C, et al. Role of sex in academic dermatology: results from a national survey. Arch Dermatol. 2012;148:809-814.
- Gilbert SB, Allshouse A, Skaznik-Wikiel ME. Gender inequality in salaries among reproductive endocrinology and infertility subspecialists in the United States. Fertil Steril. 2019;111:1194-1200.
- Jagsi R, Griffith KA, Stewart A, et al. Gender differences in the salaries of physician researchers. JAMA. 2012;307:2410-2417. doi:10.1001/jama.2012.6183
- Apaydin EA, Chen PGC, Friedberg MW, et al. Differences in physician income by gender in a multiregion survey. J Gen Intern Med. 2018;33:1574-1581.
- Read S, Butkus R, Weissman A, et al. Compensation disparities by gender in internal medicine. Ann Intern Med. 2018;169:658-661.
- Guss ZD, Chen Q, Hu C, et al. Differences in physician compensation between men and women at United States public academic radiation oncology departments. Int J Radiat Oncol Biol Phys. 2019;103:314-319.
- Lo Sasso AT, Richards MR, Chou CF, et al. The $16,819 pay gap for newly trained physicians: the unexplained trend of men earning more than women. Health Aff (Millwood). 2011;30:193-201.
- Shah A, Jalal S, Khosa F. Influences for gender disparity in dermatology in North America. Int J Dermatol. 2018;57:171-176.
- Shi CR, Olbricht S, Vleugels RA, et al. Sex and leadership in academic dermatology: a nationwide survey. J Am Acad Dermatol. 2017;77:782-784.
- Shih AF, Sun W, Yick C, et al. Trends in scholarly productivity of dermatology faculty by academic status and gender. J Am Acad Dermatol. 2019;80:1774-1776.
- Sarfaty S, Kolb D, Barnett R, et al. Negotiation in academic medicine: a necessary career skill. J Womens Health (Larchmt). 2007;16:235-244.
- Jacobson CC, Nguyen JC, Kimball AB. Gender and parenting significantly affect work hours of recent dermatology program graduates. Arch Dermatol. 2004;140:191-196.
- Feramisco JD, Leitenberger JJ, Redfern SI, et al. A gender gap in the dermatology literature? Cross-sectional analysis of manuscript authorship trends in dermatology journals during 3 decades. J Am Acad Dermatol. 2009;60:63-69.
- Bendels MHK, Dietz MC, Brüggmann D, et al. Gender disparities in high-quality dermatology research: a descriptive bibliometric study on scientific authorships. BMJ Open. 2018;8:e020089.
- Seabury SA, Chandra A, Jena AB. Trends in the earnings of male and female health care professionals in the United States, 1987 to 2010. JAMA Intern Med. 2013;173:1748-1750.
- Baimas-George M, Fleischer B, Slakey D, et al. Is it all about the money? Not all surgical subspecialization leads to higher lifetime revenue when compared to general surgery. J Surg Educ. 2017;74:E62-E66.
- Leigh JP, Tancredi D, Jerant A, et al. Lifetime earnings for physicians across specialties. Med Care. 2012;50:1093-1101.
Practice Points
- In this survey-based cross-sectional study, a statistically significant income disparity between male and female dermatologists was found.
- Although several differences were identified between male and female dermatologists that contribute to income, gender remained a statistically significant predictor of income, and this disparity could not be explained by other factors.
Proper Use and Compliance of Facial Masks During the COVID-19 Pandemic: An Observational Study of Hospitals in New York City
Although the universal use of masks by both health care professionals and the general public now appears routine, widely differing recommendations were distributed by different health organizations early in the pandemic. In April 2020, the World Health Organization (WHO) stated that there was no evidence that healthy individuals wearing a medical mask in the community prevented COVID-19 infection.1 However, these recommendations must be placed in the context of a national shortage of personal protective equipment early in the pandemic. The WHO guidance released on June 5, 2020, recommended continuous use of masks for health care workers in the clinical setting.2 Additional recommendations included mask replacement when wet, soiled, or damaged, and when the wearer touched the mask. The WHO also recommended mask usage by those with underlying medical comorbidities and those living in high population–density areas and in settings where physical distancing was not possible.2
The Centers for Disease Control and Prevention (CDC) officially recommended the use of face coverings for the general public to prevent COVID-19 transmission on April 3, 2020.3 The CDC highlighted that masks should not be worn by children younger than 2 years; individuals with respiratory compromise; and patients who are unconscious, incapacitated, or unable to remove a mask without assistance.4 Medical masks and respirators were only recommended for health care workers. Importantly, masks with valves/vents were not recommended, as respiratory droplets can be emitted, defeating the purpose of source control.4 New York State mandated mask usage in public places starting on April 15, 2020.
These recommendations were based on the hypothesis that COVID-19 transmission occurs primarily via droplets and contact. In reality, SARS-CoV-2 transmission more likely occurs in a continuum from larger droplets to miniscule aerosols expelled from an infected person when talking, coughing, or sneezing.5,6 It should be noted that there was a formal suggestion of the potential for airborne transmission of SARS-CoV-2 by the CDC in a statement on September 18, 2020, that was subsequently retracted 3 days later.7,8 The CDC, reversing their prior recommendations, updated their guidance on October 5, 2020, endorsing prior reports that SARS-CoV-2 can be spread through aerosol transmission.8
Mask usage helps prevent viral spread by all individuals, especially those who are presymptomatic and asymptomatic. Presymptomatic individuals account for approximately 40% to 60% of transmissions, and asymptomatic individuals account for approximately 4% to 30% of infections by some models, which suggest these individuals are the drivers of the pandemic, more so than symptomatic individuals.9-15 Additionally, masking also may in effect reduce the amount of SARS-CoV-2 to which individuals are being exposed in the community.14 Universal masking is a relatively low-cost, low-risk intervention that may provide moderate benefit to the individual but substantial benefit to communities at large.10-13 Universal masking in other countries also has clearly demonstrated major benefits during the pandemic. Implementation of universal masking in Taiwan resulted in only approximately 440 COVID-19 cases and less than 10 deaths, despite a population of 23 million.16 South Korea, having experience with Middle East respiratory syndrome, also was able to quickly institute a mask policy for its citizens, resulting in approximately 94% compliance.17 Moreover, several mathematical models have shown that even imperfect use of masks on a population level can prevent disease transmission and should be instituted.18
Given the importance and potential benefits of mask usage, we investigated compliance and proper utilization of facial masks in New York City (NYC), once the epicenter of the pandemic in the United States. New York City and the rest of New York State experienced more than 1.13 million and 1.46 million cases of COVID-19, respectively, as of early November 2021.19 Nationwide, NYC had the greatest absolute death count of more than 34,634 and the greatest rate of death per 100,000 individuals of 412. In contrast, New York State, excluding NYC, had an absolute death count of more than 21,646 and a death rate per 100,000 individuals of 195 as of early November 2021.19 Now entering 20 months since the first case of COVID-19 in NYC, it continues to be vital for facial mask protocols to be emphasized as part of a comprehensive infection prevention protocol, especially in light of continued vaccine resistance, to help stall continued spread of SARS-CoV-2.20
We seek to show that despite months of policies for universal masking in NYC, there is still considerable mask noncompliance by the general public in health care settings where the use of masks is particularly imperative. We conducted an observational study investigating proper use of face masks of adults entering the main entrance of 4 hospitals located in NYC.
Methods
We observed mask usage in adults entering 4 hospitals in September 2020 (postsurge in NYC and prior to the availability of COVID-19 vaccinations). Hospitals were chosen to represent several types of health care delivery systems available in the United States and included a city, state, federal, and private hospital. Data collection was completed during peak traffic hours (8:00
Mask usage was observed and classified into several categories: correctly fitting mask over the nose and mouth, no face mask, mask usage with nose exposed, mask usage with mouth exposed, mask usage with both nose and mouth exposed (ie, mask on the chin/neck area), loosely fitting mask, vented/valved mask, or other form of face covering (eg, bandana, scarf).
Results
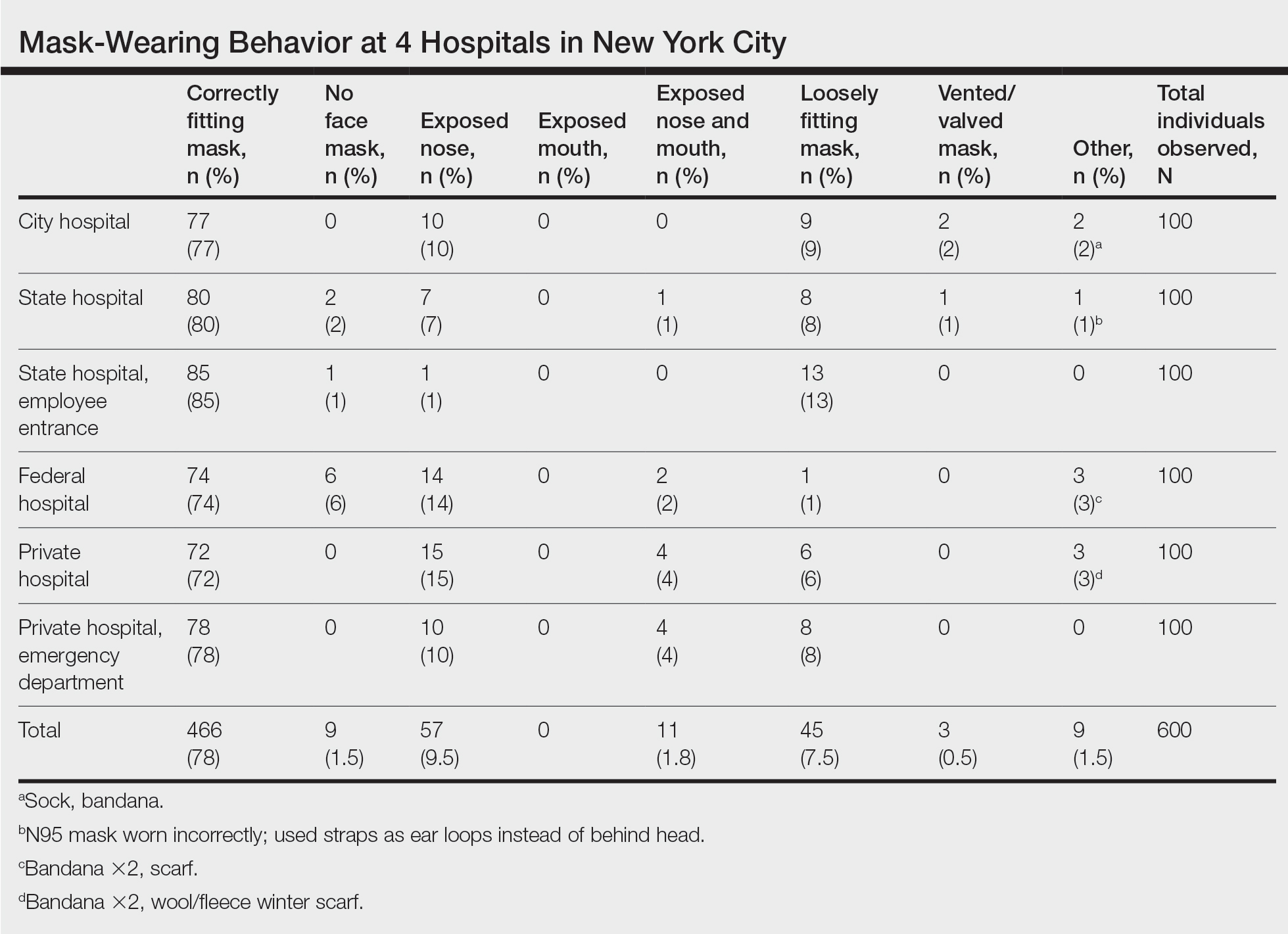
We observed a consistent rate of mask compliance between 72% and 85%, with an average of 78% of the 600 individuals observed wearing correctly fitting masks across the 4 hospitals included in this study (Table). The employee entrance included in this study had the highest compliance rate of 85%. An overall low rate of complete mask noncompliance was observed, with only 9 individuals (1.5%) in the entire study not wearing any mask. The federal hospital had the highest rate of mask noncompliance. We also observed a low rate of nose and mouth exposure, with 1.8% of individuals wearing a mask with the nose and mouth exposed (ie, mask tucked under the chin). No individuals were observed with the mouth exposed but with the nose covered by a mask. Additionally, only 3 individuals (0.5%) wore a mask with a vent/valve. The most common way that masks were worn incorrectly was with the nose exposed, accounting for 9.5% of individuals observed. Overall, only 9 individuals (1.5%) wore a nontraditional face covering, with a bandana being the most commonly observed makeshift mask.
Signage regarding the requirement to wear masks and to social distance was universally instituted at all hospital entry points (both inside and outside the hospital) in this study. However, there were no illustrations demonstrating correct and incorrect forms of mask usage. All signage merely displayed a graphic of a facial mask noting the requirement to wear a mask prior to entering the building. Hospital staff also had face masks available for patients who failed to bring a mask or who wore an inappropriate mask (ie, vented/valved masks).
Comment
Mask Effectiveness—Masks reduce the spread of SARS-CoV-2 by preventing both droplets and potentially virus-bearing aerosols.6,21,22 It has been demonstrated that well-fitted cotton homemade masks and medical masks provide the most effective method of reducing droplet dispersion. Loosely fitted masks as well as bandana-style facial coverings minimally reduce small aerosolized droplets, and an uncovered mouth and nose can disperse particles at a distance much greater than 6 feet.22
Mask Compliance—We report an overall high compliance rate with mask wearing among individuals visiting a hospital; however, compliance was still imperfect. Overall, 78% of observed individuals wore a correctly fitting mask when entering a hospital, even with hospital staff positioned at entry points to ensure proper mask usage. With all the resources available at health care centers, we anticipated a much higher compliance rate for correctly fitting masks at hospital entrances. We hypothesize that given only 78% of individuals showed proper mask compliance in a setting with enforcement by health care personnel, the mask compliance rate in the larger community is likely much lower. It is imperative to enforce continued mask compliance in medical centers and other public areas given notable vaccine noncompliance in certain parts of the country.
Tools to Prevent Disease Transmission—Mask usage by the general public in NYC helped in its response to the COVID-19 pandemic. Yang et al23 demonstrated through mathematical modeling that mask usage in NYC was associated with a 6.6% reduction in transmission overall and a 20% decrease in transmission for individuals 65 years and older during the first month of the universal mask policy going into effect. The authors extrapolated these data during the NYC reopening and found that universal masking reduced transmission by approximately 9% to 11%, accounting for the increase in hours spent outside home quarantine. The authors also hypothesized that if universal masking was as effective in its reduction of transmission for everyone in NYC as it was for older adults, the potential reduction in transmission of SARS-CoV-2 could be as high as 28% to 32%.23
Temperature checks at entrance barricades were standard protocol during the observation period. Although the main purpose of this study was to investigate compliance with and proper use of facial masks in a health care setting, it should be mentioned that, although temperature checks were being done on almost every person entering a hospital, the uniformity and practicality of this intervention has not been backed by substantial evidence. Although many nontouch thermometers are intended to capture a forehead temperature for the most accurate reading, the authors will share that in their observation, medical personnel screening individuals at hospital entrances were observed checking temperatures at any easily accessible body part, such as the forearm, hand, or neck. Furthermore, it has been reported that only approximately 40% of individuals with COVID-19 present with a fever.24 Many hospitals, including the 4 that were included in this investigation, have formal protocols for patients presenting with a fever, especially those presenting to an ambulatory center. Patients are usually instructed to call ahead if they have a fever, and a decision regarding next steps will be discussed with a health care provider. In addition, 1 meta-analysis on the symptoms of COVID-19 suggested that approximately 12% of infected patients are asymptomatic, likely a conservative estimate.25 Although we do not suggest that hospitals stop temperature checks, consistent temperature checks in anatomic locations intended for the specific thermometer used must be employed. Alternatively, a thermographic camera system that could detect heat signatures may be a way to screen faster, only necessitating that those above a threshold be assessed further.
The results of this study suggest that much greater effort is being placed on these temperature checks than on other equally important components of the entrance health assessment. This initial encounter at hospital entrances should serve as an opportunity for education on proper choice and use of masks with clear instructions that masks should not be removed unless directed by a health care provider and in a designated area, such as an examination room. The COVID-19 pandemic in the United States is likely the first time an individual is wearing these types of masks. Reiterating when and how often a mask should be changed (eg, when wet or soiled), how a soiled mask is not an effective mask, how a used mask should be discarded, ways to prevent self-contamination (ie, proper donning and doffing), and the importance of other infection-prevention behaviors—hand hygiene; social distancing; avoidance of touching the eyes, nose, and mouth with unwashed hands; and regular disinfecting of surfaces—should be practiced.11,26-29 Extended use and reuse of masks also can result in transmission of infection.30
Throughout the pandemic, our personal experience is that some patients often overtly refuse to wear a mask, citing underlying respiratory issues. The implications of patients not wearing a mask in a medical office and endangering other patients and staff are beyond the scope of this analysis. We will, however, comment briefly on the evidence behind this common concern. Matuschek et al31 found substantial adverse changes in respiratory rate, oxygen saturation, and CO2 levels in patients with severe chronic obstructive pulmonary disease who were wearing N95 respirators during a 6-minute walk test. Another study by Chan et al32 showed that nonmedical masks in healthy older adults in the community setting had no impact on oxygen saturation. Ultimately, the most effective mask a patient can wear is a mask that will be worn consistently.32
Populations With Limited Access to Masks—The COVID-19 pandemic disproportionately impacted disadvantaged populations, both in socioeconomic status and minority status. A disproportionate number of COVID-19 hospitalizations and deaths occurred in lower-income and minority populations.10 In fact, Lamb et al33 reported that NYC neighborhoods with a larger proportion of uninsured individuals with limited access to health care and overall lower socioeconomic status had a higher rate of SARS-CoV-2 positivity. A retrospective study in Louisiana showed that Black individuals accounted for 77% of hospitalizations and 71% of deaths due to COVID-19 in a population where only 31% of individuals identified as Black.10 Chu et al6 even asserted that policies should be put into place to address equity issues for populations with limited access to masks. We agree that policies should be put into action to ensure that individuals lacking the means to obtain appropriate masks or unable to obtain an adequate supply of masks be provided this new necessity. It has been calculated that the impact of masks in reducing virus transmission would be greatest if mask availability to disadvantaged populations is ensured.18 We support a plan for masks to be covered by government-sponsored health plans.
Study Limitations—Several limitations exist in our study that should be discussed. Although the data collectors observed a large number of individuals, each hospital entrance was only observed for 1 half-day morning session. There may be variations in the number of people wearing a mask at different times of day and different days of the week with fluctuations in hospital traffic. Although data were collected at a variety of hospitals representing the diverse health care delivery models available in the United States, the NYC hospitals included in this study may have different resources available for infection-prevention strategies than hospitals across the country, given NYC’s unique population density and demographics.
Study Strengths—The generalizability of the study should be recognized. Data were collected by all major health care delivery models available in the United States—private, state, city, and federal hospital systems. This study can be easily replicated in other health care delivery systems to further investigate potential gaps in mask usage and infection prevention. Repeating this study in areas where a large portion of the population does not believe in the virus also will likely show lower levels of mask use.
Conclusion
As the country grapples with vaccine hesitancy and with the new variants of SARS-CoV-2, continued universal masking is still imperative. The effectiveness of universal masking has been demonstrated, and with the combination of vaccinations, we can be assured that the world will continue to emerge from the pandemic.
- World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance (6 April 2020). Accessed November 8, 2021. https://apps.who.int/iris/bitstream/handle/10665/331693/WHO-2019-nCov-IPC_Masks-2020.3-eng.pdf?sequence=1ceisAllowed=y
- World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance (5 June 2020). Accessed November 8, 2021. https://apps.who.int/iris/bitstream/handle/10665/332293/WHO- 2019-nCov-IPC_Masks-2020.4-eng.pdf?sequence=1&isAllowed=y
- Fisher KA, Barile JP, Guerin RJ, et al. Factors associated with cloth face covering use among adults during the COVID-19 pandemic—United States, April and May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:933-937.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Considerations for wearing masks (19 April 2021). Accessed November 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover-guidance.html
- Conly J, Seto WH, Pittet D, et al. Use of medical face masks versus particulate respirators as a component of personal protective equipment for health care workers in the context of the COVID-19 pandemic. Antimicrob Resist Infect Control. 2020;9:126.
- Chu DK, Akl EA, Duda S, et al; COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973-1987.
- Huang, P. Coronavirus FAQs: Why can’t the CDC make up its mind about airborne transmission? NPR. September 25, 2020. Accessed November 8, 2021. https://www.npr.org/sections/goatsandsoda/2020/09/25/916624967/coronavirus-faqs-why-cant-the-cdc-make-up-its-mind-about-airborne-transmission
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). How COVID-19 spreads (14 July 2021). Accessed November 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782-793.
- Klompas M, Morris CA, Shenoy ES. Universal masking in the covid-19 era. N Engl J Med. 2020;383:E9.
- Middleton JD, Lopes H. Face masks in the covid-19 crisis: caveats, limits, and priorities. BMJ. 2020;369:m2030.
- Cheng KK, Lam TH, Leung CC. Wearing face masks in the community during the COVID-19 pandemic: altruism and solidarity [published online April 16, 2020]. Lancet. doi:10.1016/S0140-6736(20)30918-1
- Javid B, Weekes MP, Matheson NJ. Covid-19: should the public wear face masks? BMJ. 2020;369:m1442.
- Gandhi M, Beyrer C, Goosby E. Masks do more than protect others during COVID-19: reducing the inoculum of SARS-CoV-2 to protect the wearer. J Gen Intern Med. 2020;35:3063-3066.
- Ngonghala CN, Iboi EA, Gumel AB. Could masks curtail the post-lockdown resurgence of COVID-19 in the US? Math Biosci. 2020;329:108452. doi:10.1016/j.mbs.2020.108452
- Yi-Fong Su V, Yen YF, Yang KY, et al. Masks and medical care: two keys to Taiwan’s success in preventing COVID-19 spread. Travel Med Infect Dis. 2020;38:101780.
- Lim S, Yoon HI, Song KH, et al. Face masks and containment of COVID-19: experience from South Korea. J Hosp Infect. 2020;106:206-207.
- Fisman DN, Greer AL, Tuite AR. Bidirectional impact of imperfect mask use on reproduction number of COVID-19: a next generation matrix approach. Infect Dis Model. 2020;5:405-408.
- Centers for Disease Control and Prevention. COVID data tracker. United States COVID-19 cases, deaths, and laboratory testing (NAATs) by state, territory, and jurisdiction. Accessed July 6, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_totalcases
- Francescani C. Timeline: the first 100 days of New York Gov. Andrew Cuomo’s COVID-19 response. ABC News. June 17, 2020. Accessed November 8, 2021. https://abcnews.go.com/US/News/timeline-100-days-york-gov-andrew-cuomos-covid/story?id=71292880
- Zhang R, Li Y, Zhang AL, et al. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci U S A. 2020;117:14857-14863.
- Verma S, Dhanak M, Frankenfield J. Visualizing the effectiveness of face masks in obstructing respiratory jets. Phys Fluids (1994). 2020;32:061708.
- Yang W, Shaff J, Shaman J. COVID-19 transmission dynamics and effectiveness of public health interventions in New York City during the 2020 spring pandemic wave. medRxiv. Preprint posted online September 9, 2020. doi:10.1101/2020.09.08.20190710
- Zavascki AP, Falci DR. Clinical characteristics of covid-19 in China. N Engl J Med. 2020;382:1859.
- Zhu J, Ji P, Pang J, et al. Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol. 2020;92:1902-1914. doi:10.1002/jmv.25884
- Sommerstein R, Fux CA, Vuichard-Gysin D, et al. Risk of SARS-CoV-2 transmission by aerosols, the rational use of masks, and protection of healthcare workers from COVID-19. Antimicrob Resist Infect Control. 2020;9:100.
- Stone TE, Kunaviktikul W, Omura M, et al. Facemasks and the covid 19 pandemic: what advice should health professionals be giving the general public about the wearing of facemasks? Nurs Health Sci. 2020;22:339-342.
- Tam VC, Tam SY, Poon WK, et al. A reality check on the use of face masks during the COVID-19 outbreak in Hong Kong. EClinicalMedicine. 2020;22:100356.
- Chen YJ, Qin G, Chen J, et al. Comparison of face-touching behaviors before and during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3:e2016924.
- O’Dowd K, Nair KM, Forouzandeh P, et al. Face masks and respirators in the fight against the COVID-19 pandemic: a review of current materials, advances and future perspectives. Materials (Basel). 2020;13:3363.
- Matuschek C, Moll F, Fangerau H, et al. Face masks: benefits and risks during the COVID-19 crisis. Eur J Med Res. 2020;25:32.
- Chan NC, Li K, Hirsh J. Peripheral oxygen saturation in older persons wearing nonmedical face masks in community settings. JAMA. 2020;324:2323-2324. doi:10.1001/jama.2020.21905
- , , . Differential COVID‐19 case positivity in New York City neighborhoods: socioeconomic factors and mobility. Influenza Other Respir Viruses. 2021;15:209-217. doi:10.1111/irv.12816
Although the universal use of masks by both health care professionals and the general public now appears routine, widely differing recommendations were distributed by different health organizations early in the pandemic. In April 2020, the World Health Organization (WHO) stated that there was no evidence that healthy individuals wearing a medical mask in the community prevented COVID-19 infection.1 However, these recommendations must be placed in the context of a national shortage of personal protective equipment early in the pandemic. The WHO guidance released on June 5, 2020, recommended continuous use of masks for health care workers in the clinical setting.2 Additional recommendations included mask replacement when wet, soiled, or damaged, and when the wearer touched the mask. The WHO also recommended mask usage by those with underlying medical comorbidities and those living in high population–density areas and in settings where physical distancing was not possible.2
The Centers for Disease Control and Prevention (CDC) officially recommended the use of face coverings for the general public to prevent COVID-19 transmission on April 3, 2020.3 The CDC highlighted that masks should not be worn by children younger than 2 years; individuals with respiratory compromise; and patients who are unconscious, incapacitated, or unable to remove a mask without assistance.4 Medical masks and respirators were only recommended for health care workers. Importantly, masks with valves/vents were not recommended, as respiratory droplets can be emitted, defeating the purpose of source control.4 New York State mandated mask usage in public places starting on April 15, 2020.
These recommendations were based on the hypothesis that COVID-19 transmission occurs primarily via droplets and contact. In reality, SARS-CoV-2 transmission more likely occurs in a continuum from larger droplets to miniscule aerosols expelled from an infected person when talking, coughing, or sneezing.5,6 It should be noted that there was a formal suggestion of the potential for airborne transmission of SARS-CoV-2 by the CDC in a statement on September 18, 2020, that was subsequently retracted 3 days later.7,8 The CDC, reversing their prior recommendations, updated their guidance on October 5, 2020, endorsing prior reports that SARS-CoV-2 can be spread through aerosol transmission.8
Mask usage helps prevent viral spread by all individuals, especially those who are presymptomatic and asymptomatic. Presymptomatic individuals account for approximately 40% to 60% of transmissions, and asymptomatic individuals account for approximately 4% to 30% of infections by some models, which suggest these individuals are the drivers of the pandemic, more so than symptomatic individuals.9-15 Additionally, masking also may in effect reduce the amount of SARS-CoV-2 to which individuals are being exposed in the community.14 Universal masking is a relatively low-cost, low-risk intervention that may provide moderate benefit to the individual but substantial benefit to communities at large.10-13 Universal masking in other countries also has clearly demonstrated major benefits during the pandemic. Implementation of universal masking in Taiwan resulted in only approximately 440 COVID-19 cases and less than 10 deaths, despite a population of 23 million.16 South Korea, having experience with Middle East respiratory syndrome, also was able to quickly institute a mask policy for its citizens, resulting in approximately 94% compliance.17 Moreover, several mathematical models have shown that even imperfect use of masks on a population level can prevent disease transmission and should be instituted.18
Given the importance and potential benefits of mask usage, we investigated compliance and proper utilization of facial masks in New York City (NYC), once the epicenter of the pandemic in the United States. New York City and the rest of New York State experienced more than 1.13 million and 1.46 million cases of COVID-19, respectively, as of early November 2021.19 Nationwide, NYC had the greatest absolute death count of more than 34,634 and the greatest rate of death per 100,000 individuals of 412. In contrast, New York State, excluding NYC, had an absolute death count of more than 21,646 and a death rate per 100,000 individuals of 195 as of early November 2021.19 Now entering 20 months since the first case of COVID-19 in NYC, it continues to be vital for facial mask protocols to be emphasized as part of a comprehensive infection prevention protocol, especially in light of continued vaccine resistance, to help stall continued spread of SARS-CoV-2.20
We seek to show that despite months of policies for universal masking in NYC, there is still considerable mask noncompliance by the general public in health care settings where the use of masks is particularly imperative. We conducted an observational study investigating proper use of face masks of adults entering the main entrance of 4 hospitals located in NYC.
Methods
We observed mask usage in adults entering 4 hospitals in September 2020 (postsurge in NYC and prior to the availability of COVID-19 vaccinations). Hospitals were chosen to represent several types of health care delivery systems available in the United States and included a city, state, federal, and private hospital. Data collection was completed during peak traffic hours (8:00
Mask usage was observed and classified into several categories: correctly fitting mask over the nose and mouth, no face mask, mask usage with nose exposed, mask usage with mouth exposed, mask usage with both nose and mouth exposed (ie, mask on the chin/neck area), loosely fitting mask, vented/valved mask, or other form of face covering (eg, bandana, scarf).
Results
We observed a consistent rate of mask compliance between 72% and 85%, with an average of 78% of the 600 individuals observed wearing correctly fitting masks across the 4 hospitals included in this study (Table). The employee entrance included in this study had the highest compliance rate of 85%. An overall low rate of complete mask noncompliance was observed, with only 9 individuals (1.5%) in the entire study not wearing any mask. The federal hospital had the highest rate of mask noncompliance. We also observed a low rate of nose and mouth exposure, with 1.8% of individuals wearing a mask with the nose and mouth exposed (ie, mask tucked under the chin). No individuals were observed with the mouth exposed but with the nose covered by a mask. Additionally, only 3 individuals (0.5%) wore a mask with a vent/valve. The most common way that masks were worn incorrectly was with the nose exposed, accounting for 9.5% of individuals observed. Overall, only 9 individuals (1.5%) wore a nontraditional face covering, with a bandana being the most commonly observed makeshift mask.
Signage regarding the requirement to wear masks and to social distance was universally instituted at all hospital entry points (both inside and outside the hospital) in this study. However, there were no illustrations demonstrating correct and incorrect forms of mask usage. All signage merely displayed a graphic of a facial mask noting the requirement to wear a mask prior to entering the building. Hospital staff also had face masks available for patients who failed to bring a mask or who wore an inappropriate mask (ie, vented/valved masks).
Comment
Mask Effectiveness—Masks reduce the spread of SARS-CoV-2 by preventing both droplets and potentially virus-bearing aerosols.6,21,22 It has been demonstrated that well-fitted cotton homemade masks and medical masks provide the most effective method of reducing droplet dispersion. Loosely fitted masks as well as bandana-style facial coverings minimally reduce small aerosolized droplets, and an uncovered mouth and nose can disperse particles at a distance much greater than 6 feet.22
Mask Compliance—We report an overall high compliance rate with mask wearing among individuals visiting a hospital; however, compliance was still imperfect. Overall, 78% of observed individuals wore a correctly fitting mask when entering a hospital, even with hospital staff positioned at entry points to ensure proper mask usage. With all the resources available at health care centers, we anticipated a much higher compliance rate for correctly fitting masks at hospital entrances. We hypothesize that given only 78% of individuals showed proper mask compliance in a setting with enforcement by health care personnel, the mask compliance rate in the larger community is likely much lower. It is imperative to enforce continued mask compliance in medical centers and other public areas given notable vaccine noncompliance in certain parts of the country.
Tools to Prevent Disease Transmission—Mask usage by the general public in NYC helped in its response to the COVID-19 pandemic. Yang et al23 demonstrated through mathematical modeling that mask usage in NYC was associated with a 6.6% reduction in transmission overall and a 20% decrease in transmission for individuals 65 years and older during the first month of the universal mask policy going into effect. The authors extrapolated these data during the NYC reopening and found that universal masking reduced transmission by approximately 9% to 11%, accounting for the increase in hours spent outside home quarantine. The authors also hypothesized that if universal masking was as effective in its reduction of transmission for everyone in NYC as it was for older adults, the potential reduction in transmission of SARS-CoV-2 could be as high as 28% to 32%.23
Temperature checks at entrance barricades were standard protocol during the observation period. Although the main purpose of this study was to investigate compliance with and proper use of facial masks in a health care setting, it should be mentioned that, although temperature checks were being done on almost every person entering a hospital, the uniformity and practicality of this intervention has not been backed by substantial evidence. Although many nontouch thermometers are intended to capture a forehead temperature for the most accurate reading, the authors will share that in their observation, medical personnel screening individuals at hospital entrances were observed checking temperatures at any easily accessible body part, such as the forearm, hand, or neck. Furthermore, it has been reported that only approximately 40% of individuals with COVID-19 present with a fever.24 Many hospitals, including the 4 that were included in this investigation, have formal protocols for patients presenting with a fever, especially those presenting to an ambulatory center. Patients are usually instructed to call ahead if they have a fever, and a decision regarding next steps will be discussed with a health care provider. In addition, 1 meta-analysis on the symptoms of COVID-19 suggested that approximately 12% of infected patients are asymptomatic, likely a conservative estimate.25 Although we do not suggest that hospitals stop temperature checks, consistent temperature checks in anatomic locations intended for the specific thermometer used must be employed. Alternatively, a thermographic camera system that could detect heat signatures may be a way to screen faster, only necessitating that those above a threshold be assessed further.
The results of this study suggest that much greater effort is being placed on these temperature checks than on other equally important components of the entrance health assessment. This initial encounter at hospital entrances should serve as an opportunity for education on proper choice and use of masks with clear instructions that masks should not be removed unless directed by a health care provider and in a designated area, such as an examination room. The COVID-19 pandemic in the United States is likely the first time an individual is wearing these types of masks. Reiterating when and how often a mask should be changed (eg, when wet or soiled), how a soiled mask is not an effective mask, how a used mask should be discarded, ways to prevent self-contamination (ie, proper donning and doffing), and the importance of other infection-prevention behaviors—hand hygiene; social distancing; avoidance of touching the eyes, nose, and mouth with unwashed hands; and regular disinfecting of surfaces—should be practiced.11,26-29 Extended use and reuse of masks also can result in transmission of infection.30
Throughout the pandemic, our personal experience is that some patients often overtly refuse to wear a mask, citing underlying respiratory issues. The implications of patients not wearing a mask in a medical office and endangering other patients and staff are beyond the scope of this analysis. We will, however, comment briefly on the evidence behind this common concern. Matuschek et al31 found substantial adverse changes in respiratory rate, oxygen saturation, and CO2 levels in patients with severe chronic obstructive pulmonary disease who were wearing N95 respirators during a 6-minute walk test. Another study by Chan et al32 showed that nonmedical masks in healthy older adults in the community setting had no impact on oxygen saturation. Ultimately, the most effective mask a patient can wear is a mask that will be worn consistently.32
Populations With Limited Access to Masks—The COVID-19 pandemic disproportionately impacted disadvantaged populations, both in socioeconomic status and minority status. A disproportionate number of COVID-19 hospitalizations and deaths occurred in lower-income and minority populations.10 In fact, Lamb et al33 reported that NYC neighborhoods with a larger proportion of uninsured individuals with limited access to health care and overall lower socioeconomic status had a higher rate of SARS-CoV-2 positivity. A retrospective study in Louisiana showed that Black individuals accounted for 77% of hospitalizations and 71% of deaths due to COVID-19 in a population where only 31% of individuals identified as Black.10 Chu et al6 even asserted that policies should be put into place to address equity issues for populations with limited access to masks. We agree that policies should be put into action to ensure that individuals lacking the means to obtain appropriate masks or unable to obtain an adequate supply of masks be provided this new necessity. It has been calculated that the impact of masks in reducing virus transmission would be greatest if mask availability to disadvantaged populations is ensured.18 We support a plan for masks to be covered by government-sponsored health plans.
Study Limitations—Several limitations exist in our study that should be discussed. Although the data collectors observed a large number of individuals, each hospital entrance was only observed for 1 half-day morning session. There may be variations in the number of people wearing a mask at different times of day and different days of the week with fluctuations in hospital traffic. Although data were collected at a variety of hospitals representing the diverse health care delivery models available in the United States, the NYC hospitals included in this study may have different resources available for infection-prevention strategies than hospitals across the country, given NYC’s unique population density and demographics.
Study Strengths—The generalizability of the study should be recognized. Data were collected by all major health care delivery models available in the United States—private, state, city, and federal hospital systems. This study can be easily replicated in other health care delivery systems to further investigate potential gaps in mask usage and infection prevention. Repeating this study in areas where a large portion of the population does not believe in the virus also will likely show lower levels of mask use.
Conclusion
As the country grapples with vaccine hesitancy and with the new variants of SARS-CoV-2, continued universal masking is still imperative. The effectiveness of universal masking has been demonstrated, and with the combination of vaccinations, we can be assured that the world will continue to emerge from the pandemic.
Although the universal use of masks by both health care professionals and the general public now appears routine, widely differing recommendations were distributed by different health organizations early in the pandemic. In April 2020, the World Health Organization (WHO) stated that there was no evidence that healthy individuals wearing a medical mask in the community prevented COVID-19 infection.1 However, these recommendations must be placed in the context of a national shortage of personal protective equipment early in the pandemic. The WHO guidance released on June 5, 2020, recommended continuous use of masks for health care workers in the clinical setting.2 Additional recommendations included mask replacement when wet, soiled, or damaged, and when the wearer touched the mask. The WHO also recommended mask usage by those with underlying medical comorbidities and those living in high population–density areas and in settings where physical distancing was not possible.2
The Centers for Disease Control and Prevention (CDC) officially recommended the use of face coverings for the general public to prevent COVID-19 transmission on April 3, 2020.3 The CDC highlighted that masks should not be worn by children younger than 2 years; individuals with respiratory compromise; and patients who are unconscious, incapacitated, or unable to remove a mask without assistance.4 Medical masks and respirators were only recommended for health care workers. Importantly, masks with valves/vents were not recommended, as respiratory droplets can be emitted, defeating the purpose of source control.4 New York State mandated mask usage in public places starting on April 15, 2020.
These recommendations were based on the hypothesis that COVID-19 transmission occurs primarily via droplets and contact. In reality, SARS-CoV-2 transmission more likely occurs in a continuum from larger droplets to miniscule aerosols expelled from an infected person when talking, coughing, or sneezing.5,6 It should be noted that there was a formal suggestion of the potential for airborne transmission of SARS-CoV-2 by the CDC in a statement on September 18, 2020, that was subsequently retracted 3 days later.7,8 The CDC, reversing their prior recommendations, updated their guidance on October 5, 2020, endorsing prior reports that SARS-CoV-2 can be spread through aerosol transmission.8
Mask usage helps prevent viral spread by all individuals, especially those who are presymptomatic and asymptomatic. Presymptomatic individuals account for approximately 40% to 60% of transmissions, and asymptomatic individuals account for approximately 4% to 30% of infections by some models, which suggest these individuals are the drivers of the pandemic, more so than symptomatic individuals.9-15 Additionally, masking also may in effect reduce the amount of SARS-CoV-2 to which individuals are being exposed in the community.14 Universal masking is a relatively low-cost, low-risk intervention that may provide moderate benefit to the individual but substantial benefit to communities at large.10-13 Universal masking in other countries also has clearly demonstrated major benefits during the pandemic. Implementation of universal masking in Taiwan resulted in only approximately 440 COVID-19 cases and less than 10 deaths, despite a population of 23 million.16 South Korea, having experience with Middle East respiratory syndrome, also was able to quickly institute a mask policy for its citizens, resulting in approximately 94% compliance.17 Moreover, several mathematical models have shown that even imperfect use of masks on a population level can prevent disease transmission and should be instituted.18
Given the importance and potential benefits of mask usage, we investigated compliance and proper utilization of facial masks in New York City (NYC), once the epicenter of the pandemic in the United States. New York City and the rest of New York State experienced more than 1.13 million and 1.46 million cases of COVID-19, respectively, as of early November 2021.19 Nationwide, NYC had the greatest absolute death count of more than 34,634 and the greatest rate of death per 100,000 individuals of 412. In contrast, New York State, excluding NYC, had an absolute death count of more than 21,646 and a death rate per 100,000 individuals of 195 as of early November 2021.19 Now entering 20 months since the first case of COVID-19 in NYC, it continues to be vital for facial mask protocols to be emphasized as part of a comprehensive infection prevention protocol, especially in light of continued vaccine resistance, to help stall continued spread of SARS-CoV-2.20
We seek to show that despite months of policies for universal masking in NYC, there is still considerable mask noncompliance by the general public in health care settings where the use of masks is particularly imperative. We conducted an observational study investigating proper use of face masks of adults entering the main entrance of 4 hospitals located in NYC.
Methods
We observed mask usage in adults entering 4 hospitals in September 2020 (postsurge in NYC and prior to the availability of COVID-19 vaccinations). Hospitals were chosen to represent several types of health care delivery systems available in the United States and included a city, state, federal, and private hospital. Data collection was completed during peak traffic hours (8:00
Mask usage was observed and classified into several categories: correctly fitting mask over the nose and mouth, no face mask, mask usage with nose exposed, mask usage with mouth exposed, mask usage with both nose and mouth exposed (ie, mask on the chin/neck area), loosely fitting mask, vented/valved mask, or other form of face covering (eg, bandana, scarf).
Results
We observed a consistent rate of mask compliance between 72% and 85%, with an average of 78% of the 600 individuals observed wearing correctly fitting masks across the 4 hospitals included in this study (Table). The employee entrance included in this study had the highest compliance rate of 85%. An overall low rate of complete mask noncompliance was observed, with only 9 individuals (1.5%) in the entire study not wearing any mask. The federal hospital had the highest rate of mask noncompliance. We also observed a low rate of nose and mouth exposure, with 1.8% of individuals wearing a mask with the nose and mouth exposed (ie, mask tucked under the chin). No individuals were observed with the mouth exposed but with the nose covered by a mask. Additionally, only 3 individuals (0.5%) wore a mask with a vent/valve. The most common way that masks were worn incorrectly was with the nose exposed, accounting for 9.5% of individuals observed. Overall, only 9 individuals (1.5%) wore a nontraditional face covering, with a bandana being the most commonly observed makeshift mask.
Signage regarding the requirement to wear masks and to social distance was universally instituted at all hospital entry points (both inside and outside the hospital) in this study. However, there were no illustrations demonstrating correct and incorrect forms of mask usage. All signage merely displayed a graphic of a facial mask noting the requirement to wear a mask prior to entering the building. Hospital staff also had face masks available for patients who failed to bring a mask or who wore an inappropriate mask (ie, vented/valved masks).
Comment
Mask Effectiveness—Masks reduce the spread of SARS-CoV-2 by preventing both droplets and potentially virus-bearing aerosols.6,21,22 It has been demonstrated that well-fitted cotton homemade masks and medical masks provide the most effective method of reducing droplet dispersion. Loosely fitted masks as well as bandana-style facial coverings minimally reduce small aerosolized droplets, and an uncovered mouth and nose can disperse particles at a distance much greater than 6 feet.22
Mask Compliance—We report an overall high compliance rate with mask wearing among individuals visiting a hospital; however, compliance was still imperfect. Overall, 78% of observed individuals wore a correctly fitting mask when entering a hospital, even with hospital staff positioned at entry points to ensure proper mask usage. With all the resources available at health care centers, we anticipated a much higher compliance rate for correctly fitting masks at hospital entrances. We hypothesize that given only 78% of individuals showed proper mask compliance in a setting with enforcement by health care personnel, the mask compliance rate in the larger community is likely much lower. It is imperative to enforce continued mask compliance in medical centers and other public areas given notable vaccine noncompliance in certain parts of the country.
Tools to Prevent Disease Transmission—Mask usage by the general public in NYC helped in its response to the COVID-19 pandemic. Yang et al23 demonstrated through mathematical modeling that mask usage in NYC was associated with a 6.6% reduction in transmission overall and a 20% decrease in transmission for individuals 65 years and older during the first month of the universal mask policy going into effect. The authors extrapolated these data during the NYC reopening and found that universal masking reduced transmission by approximately 9% to 11%, accounting for the increase in hours spent outside home quarantine. The authors also hypothesized that if universal masking was as effective in its reduction of transmission for everyone in NYC as it was for older adults, the potential reduction in transmission of SARS-CoV-2 could be as high as 28% to 32%.23
Temperature checks at entrance barricades were standard protocol during the observation period. Although the main purpose of this study was to investigate compliance with and proper use of facial masks in a health care setting, it should be mentioned that, although temperature checks were being done on almost every person entering a hospital, the uniformity and practicality of this intervention has not been backed by substantial evidence. Although many nontouch thermometers are intended to capture a forehead temperature for the most accurate reading, the authors will share that in their observation, medical personnel screening individuals at hospital entrances were observed checking temperatures at any easily accessible body part, such as the forearm, hand, or neck. Furthermore, it has been reported that only approximately 40% of individuals with COVID-19 present with a fever.24 Many hospitals, including the 4 that were included in this investigation, have formal protocols for patients presenting with a fever, especially those presenting to an ambulatory center. Patients are usually instructed to call ahead if they have a fever, and a decision regarding next steps will be discussed with a health care provider. In addition, 1 meta-analysis on the symptoms of COVID-19 suggested that approximately 12% of infected patients are asymptomatic, likely a conservative estimate.25 Although we do not suggest that hospitals stop temperature checks, consistent temperature checks in anatomic locations intended for the specific thermometer used must be employed. Alternatively, a thermographic camera system that could detect heat signatures may be a way to screen faster, only necessitating that those above a threshold be assessed further.
The results of this study suggest that much greater effort is being placed on these temperature checks than on other equally important components of the entrance health assessment. This initial encounter at hospital entrances should serve as an opportunity for education on proper choice and use of masks with clear instructions that masks should not be removed unless directed by a health care provider and in a designated area, such as an examination room. The COVID-19 pandemic in the United States is likely the first time an individual is wearing these types of masks. Reiterating when and how often a mask should be changed (eg, when wet or soiled), how a soiled mask is not an effective mask, how a used mask should be discarded, ways to prevent self-contamination (ie, proper donning and doffing), and the importance of other infection-prevention behaviors—hand hygiene; social distancing; avoidance of touching the eyes, nose, and mouth with unwashed hands; and regular disinfecting of surfaces—should be practiced.11,26-29 Extended use and reuse of masks also can result in transmission of infection.30
Throughout the pandemic, our personal experience is that some patients often overtly refuse to wear a mask, citing underlying respiratory issues. The implications of patients not wearing a mask in a medical office and endangering other patients and staff are beyond the scope of this analysis. We will, however, comment briefly on the evidence behind this common concern. Matuschek et al31 found substantial adverse changes in respiratory rate, oxygen saturation, and CO2 levels in patients with severe chronic obstructive pulmonary disease who were wearing N95 respirators during a 6-minute walk test. Another study by Chan et al32 showed that nonmedical masks in healthy older adults in the community setting had no impact on oxygen saturation. Ultimately, the most effective mask a patient can wear is a mask that will be worn consistently.32
Populations With Limited Access to Masks—The COVID-19 pandemic disproportionately impacted disadvantaged populations, both in socioeconomic status and minority status. A disproportionate number of COVID-19 hospitalizations and deaths occurred in lower-income and minority populations.10 In fact, Lamb et al33 reported that NYC neighborhoods with a larger proportion of uninsured individuals with limited access to health care and overall lower socioeconomic status had a higher rate of SARS-CoV-2 positivity. A retrospective study in Louisiana showed that Black individuals accounted for 77% of hospitalizations and 71% of deaths due to COVID-19 in a population where only 31% of individuals identified as Black.10 Chu et al6 even asserted that policies should be put into place to address equity issues for populations with limited access to masks. We agree that policies should be put into action to ensure that individuals lacking the means to obtain appropriate masks or unable to obtain an adequate supply of masks be provided this new necessity. It has been calculated that the impact of masks in reducing virus transmission would be greatest if mask availability to disadvantaged populations is ensured.18 We support a plan for masks to be covered by government-sponsored health plans.
Study Limitations—Several limitations exist in our study that should be discussed. Although the data collectors observed a large number of individuals, each hospital entrance was only observed for 1 half-day morning session. There may be variations in the number of people wearing a mask at different times of day and different days of the week with fluctuations in hospital traffic. Although data were collected at a variety of hospitals representing the diverse health care delivery models available in the United States, the NYC hospitals included in this study may have different resources available for infection-prevention strategies than hospitals across the country, given NYC’s unique population density and demographics.
Study Strengths—The generalizability of the study should be recognized. Data were collected by all major health care delivery models available in the United States—private, state, city, and federal hospital systems. This study can be easily replicated in other health care delivery systems to further investigate potential gaps in mask usage and infection prevention. Repeating this study in areas where a large portion of the population does not believe in the virus also will likely show lower levels of mask use.
Conclusion
As the country grapples with vaccine hesitancy and with the new variants of SARS-CoV-2, continued universal masking is still imperative. The effectiveness of universal masking has been demonstrated, and with the combination of vaccinations, we can be assured that the world will continue to emerge from the pandemic.
- World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance (6 April 2020). Accessed November 8, 2021. https://apps.who.int/iris/bitstream/handle/10665/331693/WHO-2019-nCov-IPC_Masks-2020.3-eng.pdf?sequence=1ceisAllowed=y
- World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance (5 June 2020). Accessed November 8, 2021. https://apps.who.int/iris/bitstream/handle/10665/332293/WHO- 2019-nCov-IPC_Masks-2020.4-eng.pdf?sequence=1&isAllowed=y
- Fisher KA, Barile JP, Guerin RJ, et al. Factors associated with cloth face covering use among adults during the COVID-19 pandemic—United States, April and May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:933-937.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Considerations for wearing masks (19 April 2021). Accessed November 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover-guidance.html
- Conly J, Seto WH, Pittet D, et al. Use of medical face masks versus particulate respirators as a component of personal protective equipment for health care workers in the context of the COVID-19 pandemic. Antimicrob Resist Infect Control. 2020;9:126.
- Chu DK, Akl EA, Duda S, et al; COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973-1987.
- Huang, P. Coronavirus FAQs: Why can’t the CDC make up its mind about airborne transmission? NPR. September 25, 2020. Accessed November 8, 2021. https://www.npr.org/sections/goatsandsoda/2020/09/25/916624967/coronavirus-faqs-why-cant-the-cdc-make-up-its-mind-about-airborne-transmission
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). How COVID-19 spreads (14 July 2021). Accessed November 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782-793.
- Klompas M, Morris CA, Shenoy ES. Universal masking in the covid-19 era. N Engl J Med. 2020;383:E9.
- Middleton JD, Lopes H. Face masks in the covid-19 crisis: caveats, limits, and priorities. BMJ. 2020;369:m2030.
- Cheng KK, Lam TH, Leung CC. Wearing face masks in the community during the COVID-19 pandemic: altruism and solidarity [published online April 16, 2020]. Lancet. doi:10.1016/S0140-6736(20)30918-1
- Javid B, Weekes MP, Matheson NJ. Covid-19: should the public wear face masks? BMJ. 2020;369:m1442.
- Gandhi M, Beyrer C, Goosby E. Masks do more than protect others during COVID-19: reducing the inoculum of SARS-CoV-2 to protect the wearer. J Gen Intern Med. 2020;35:3063-3066.
- Ngonghala CN, Iboi EA, Gumel AB. Could masks curtail the post-lockdown resurgence of COVID-19 in the US? Math Biosci. 2020;329:108452. doi:10.1016/j.mbs.2020.108452
- Yi-Fong Su V, Yen YF, Yang KY, et al. Masks and medical care: two keys to Taiwan’s success in preventing COVID-19 spread. Travel Med Infect Dis. 2020;38:101780.
- Lim S, Yoon HI, Song KH, et al. Face masks and containment of COVID-19: experience from South Korea. J Hosp Infect. 2020;106:206-207.
- Fisman DN, Greer AL, Tuite AR. Bidirectional impact of imperfect mask use on reproduction number of COVID-19: a next generation matrix approach. Infect Dis Model. 2020;5:405-408.
- Centers for Disease Control and Prevention. COVID data tracker. United States COVID-19 cases, deaths, and laboratory testing (NAATs) by state, territory, and jurisdiction. Accessed July 6, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_totalcases
- Francescani C. Timeline: the first 100 days of New York Gov. Andrew Cuomo’s COVID-19 response. ABC News. June 17, 2020. Accessed November 8, 2021. https://abcnews.go.com/US/News/timeline-100-days-york-gov-andrew-cuomos-covid/story?id=71292880
- Zhang R, Li Y, Zhang AL, et al. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci U S A. 2020;117:14857-14863.
- Verma S, Dhanak M, Frankenfield J. Visualizing the effectiveness of face masks in obstructing respiratory jets. Phys Fluids (1994). 2020;32:061708.
- Yang W, Shaff J, Shaman J. COVID-19 transmission dynamics and effectiveness of public health interventions in New York City during the 2020 spring pandemic wave. medRxiv. Preprint posted online September 9, 2020. doi:10.1101/2020.09.08.20190710
- Zavascki AP, Falci DR. Clinical characteristics of covid-19 in China. N Engl J Med. 2020;382:1859.
- Zhu J, Ji P, Pang J, et al. Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol. 2020;92:1902-1914. doi:10.1002/jmv.25884
- Sommerstein R, Fux CA, Vuichard-Gysin D, et al. Risk of SARS-CoV-2 transmission by aerosols, the rational use of masks, and protection of healthcare workers from COVID-19. Antimicrob Resist Infect Control. 2020;9:100.
- Stone TE, Kunaviktikul W, Omura M, et al. Facemasks and the covid 19 pandemic: what advice should health professionals be giving the general public about the wearing of facemasks? Nurs Health Sci. 2020;22:339-342.
- Tam VC, Tam SY, Poon WK, et al. A reality check on the use of face masks during the COVID-19 outbreak in Hong Kong. EClinicalMedicine. 2020;22:100356.
- Chen YJ, Qin G, Chen J, et al. Comparison of face-touching behaviors before and during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3:e2016924.
- O’Dowd K, Nair KM, Forouzandeh P, et al. Face masks and respirators in the fight against the COVID-19 pandemic: a review of current materials, advances and future perspectives. Materials (Basel). 2020;13:3363.
- Matuschek C, Moll F, Fangerau H, et al. Face masks: benefits and risks during the COVID-19 crisis. Eur J Med Res. 2020;25:32.
- Chan NC, Li K, Hirsh J. Peripheral oxygen saturation in older persons wearing nonmedical face masks in community settings. JAMA. 2020;324:2323-2324. doi:10.1001/jama.2020.21905
- , , . Differential COVID‐19 case positivity in New York City neighborhoods: socioeconomic factors and mobility. Influenza Other Respir Viruses. 2021;15:209-217. doi:10.1111/irv.12816
- World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance (6 April 2020). Accessed November 8, 2021. https://apps.who.int/iris/bitstream/handle/10665/331693/WHO-2019-nCov-IPC_Masks-2020.3-eng.pdf?sequence=1ceisAllowed=y
- World Health Organization. Advice on the use of masks in the context of COVID-19. Interim guidance (5 June 2020). Accessed November 8, 2021. https://apps.who.int/iris/bitstream/handle/10665/332293/WHO- 2019-nCov-IPC_Masks-2020.4-eng.pdf?sequence=1&isAllowed=y
- Fisher KA, Barile JP, Guerin RJ, et al. Factors associated with cloth face covering use among adults during the COVID-19 pandemic—United States, April and May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:933-937.
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). Considerations for wearing masks (19 April 2021). Accessed November 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover-guidance.html
- Conly J, Seto WH, Pittet D, et al. Use of medical face masks versus particulate respirators as a component of personal protective equipment for health care workers in the context of the COVID-19 pandemic. Antimicrob Resist Infect Control. 2020;9:126.
- Chu DK, Akl EA, Duda S, et al; COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet. 2020;395:1973-1987.
- Huang, P. Coronavirus FAQs: Why can’t the CDC make up its mind about airborne transmission? NPR. September 25, 2020. Accessed November 8, 2021. https://www.npr.org/sections/goatsandsoda/2020/09/25/916624967/coronavirus-faqs-why-cant-the-cdc-make-up-its-mind-about-airborne-transmission
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). How COVID-19 spreads (14 July 2021). Accessed November 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782-793.
- Klompas M, Morris CA, Shenoy ES. Universal masking in the covid-19 era. N Engl J Med. 2020;383:E9.
- Middleton JD, Lopes H. Face masks in the covid-19 crisis: caveats, limits, and priorities. BMJ. 2020;369:m2030.
- Cheng KK, Lam TH, Leung CC. Wearing face masks in the community during the COVID-19 pandemic: altruism and solidarity [published online April 16, 2020]. Lancet. doi:10.1016/S0140-6736(20)30918-1
- Javid B, Weekes MP, Matheson NJ. Covid-19: should the public wear face masks? BMJ. 2020;369:m1442.
- Gandhi M, Beyrer C, Goosby E. Masks do more than protect others during COVID-19: reducing the inoculum of SARS-CoV-2 to protect the wearer. J Gen Intern Med. 2020;35:3063-3066.
- Ngonghala CN, Iboi EA, Gumel AB. Could masks curtail the post-lockdown resurgence of COVID-19 in the US? Math Biosci. 2020;329:108452. doi:10.1016/j.mbs.2020.108452
- Yi-Fong Su V, Yen YF, Yang KY, et al. Masks and medical care: two keys to Taiwan’s success in preventing COVID-19 spread. Travel Med Infect Dis. 2020;38:101780.
- Lim S, Yoon HI, Song KH, et al. Face masks and containment of COVID-19: experience from South Korea. J Hosp Infect. 2020;106:206-207.
- Fisman DN, Greer AL, Tuite AR. Bidirectional impact of imperfect mask use on reproduction number of COVID-19: a next generation matrix approach. Infect Dis Model. 2020;5:405-408.
- Centers for Disease Control and Prevention. COVID data tracker. United States COVID-19 cases, deaths, and laboratory testing (NAATs) by state, territory, and jurisdiction. Accessed July 6, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_totalcases
- Francescani C. Timeline: the first 100 days of New York Gov. Andrew Cuomo’s COVID-19 response. ABC News. June 17, 2020. Accessed November 8, 2021. https://abcnews.go.com/US/News/timeline-100-days-york-gov-andrew-cuomos-covid/story?id=71292880
- Zhang R, Li Y, Zhang AL, et al. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci U S A. 2020;117:14857-14863.
- Verma S, Dhanak M, Frankenfield J. Visualizing the effectiveness of face masks in obstructing respiratory jets. Phys Fluids (1994). 2020;32:061708.
- Yang W, Shaff J, Shaman J. COVID-19 transmission dynamics and effectiveness of public health interventions in New York City during the 2020 spring pandemic wave. medRxiv. Preprint posted online September 9, 2020. doi:10.1101/2020.09.08.20190710
- Zavascki AP, Falci DR. Clinical characteristics of covid-19 in China. N Engl J Med. 2020;382:1859.
- Zhu J, Ji P, Pang J, et al. Clinical characteristics of 3062 COVID-19 patients: a meta-analysis. J Med Virol. 2020;92:1902-1914. doi:10.1002/jmv.25884
- Sommerstein R, Fux CA, Vuichard-Gysin D, et al. Risk of SARS-CoV-2 transmission by aerosols, the rational use of masks, and protection of healthcare workers from COVID-19. Antimicrob Resist Infect Control. 2020;9:100.
- Stone TE, Kunaviktikul W, Omura M, et al. Facemasks and the covid 19 pandemic: what advice should health professionals be giving the general public about the wearing of facemasks? Nurs Health Sci. 2020;22:339-342.
- Tam VC, Tam SY, Poon WK, et al. A reality check on the use of face masks during the COVID-19 outbreak in Hong Kong. EClinicalMedicine. 2020;22:100356.
- Chen YJ, Qin G, Chen J, et al. Comparison of face-touching behaviors before and during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3:e2016924.
- O’Dowd K, Nair KM, Forouzandeh P, et al. Face masks and respirators in the fight against the COVID-19 pandemic: a review of current materials, advances and future perspectives. Materials (Basel). 2020;13:3363.
- Matuschek C, Moll F, Fangerau H, et al. Face masks: benefits and risks during the COVID-19 crisis. Eur J Med Res. 2020;25:32.
- Chan NC, Li K, Hirsh J. Peripheral oxygen saturation in older persons wearing nonmedical face masks in community settings. JAMA. 2020;324:2323-2324. doi:10.1001/jama.2020.21905
- , , . Differential COVID‐19 case positivity in New York City neighborhoods: socioeconomic factors and mobility. Influenza Other Respir Viruses. 2021;15:209-217. doi:10.1111/irv.12816
Practice Points
- Enormous financial and human resources have been utilized by health care systems to prevent the spread of COVID-19 in health care settings, including universal temperature checks, clinical symptom triage, and masking policies. Despite these mitigation practices, mask noncompliance continues to be a major problem in hospitals.
- Mask compliance among 600 individuals entering 4 New York City hospitals was observed to be 78%, despite months of policies for universal masking and the city’s high mortality rates during the first COVID-19 wave.
- Masks have been shown to reduce the spread of COVID-19, and proper mask compliance is an important issue that must be addressed by health care administrations and governmental agencies.
Nephrogenic Systemic Fibrosis in the Setting of Transient Renal Insufficiency
Nephrogenic systemic fibrosis (NSF) is a rare debilitating disorder characterized by dermal plaques, joint contractures, and fibrosis of the skin with possible involvement of muscles and internal organs.1-3 Originally identified in 1997 as nephrogenic fibrosing dermopathy to describe its characteristic cutaneous thickening and hardening, the name was changed to NSF to more accurately reflect the noncutaneous manifestations present in other organ tissues.2,4,5 Nephrogenic systemic fibrosis occurs in patients with a history of renal insufficiency and exposure to gadolinium-based contrast agents (GBCAs) used in magnetic resonance angiography and magnetic resonance imaging. There is no predilection for age, sex, or ethnicity.
Nephrogenic systemic fibrosis may develop over a period of days to several weeks. However, there have been cases of NSF developing 10 years after gadolinium exposure.2 In most cases, patients have a history of severe chronic renal disease requiring hemodialysis. There have been a few reported cases of NSF occurring in patients with resolved acute kidney injury or resolved acute on chronic renal disease.1,6-10 We present a case of NSF occurring in a patient with resolved transient renal insufficiency and no history of chronic renal disease.
Case Report
A 68-year-old woman presented with new dark, painless, pink plaques on the right thigh and calf. The patient stated the condition started and got worse after she was hospitalized 12 years prior for lower extremity cellulitis, sepsis, and acute renal failure. The patient developed complications during that hospital stay and underwent a renal biopsy and renal artery embolization requiring use of a GBCA. After the procedure, she noticed skin hardening in the extremities and decreased mobility in both legs while she was still in the hospital. It was thought that the lower leg changes were due to cellulitis. Therefore, when the renal issues resolved, she was discharged. Her skin and joint changes remained stable until 6 years later when she noticed new pink plaques appearing. Her medical history was positive for breast cancer, which was surgically and medically treated 16 years prior to presentation.
On presentation, physical examination revealed dark pink, hyperpigmented plaques on the right leg and a firm hypopigmented broad linear plaque on the right forearm. Palpation of the legs revealed thickened sclerotic plaques from the thighs down to the ankles (Figure 1). The plaques were not tender to palpation. She did have a decreased range of motion with eversion and inversion of the feet and ankles.
Biopsies from the right medial leg and right volar forearm showed increased bland dermal spindle cellularity associated with numerous round to ovoid osteoid aggregates encircling elastic fibers and surrounded by osteoblasts (Figure 2). CD34 immunohistochemistry showed general retention of staining within the dermal fibroblast population, and elastin stain showed general retention of elastic fiber bundles and thickening.
Laboratory workup included a complete blood cell count, comprehensive metabolic panel, thyroid-stimulating hormone level, and serum protein electrophoresis; results were all within reference range. The patient also had a urine element profile from an outside provider 1 month after presenting to our office that showed an elevated urine gadolinium level of 4.146 μg/g (reference range, 0–0.019 μg/g). The patient’s skin lesions have remained stable, and she is now working with physical therapy to help with her range of motion.
Comment
Gadolinium Causing Fibrosis—The incidence of NSF varies according to the severity of renal impairment, dosage level of GBCA used, and the history of GBCA use. In patients with normal renal function, gadolinium is excreted within 90 minutes. In patients with severe renal disease, the half-life can increase to up to 34.3 hours.11 Reduced renal clearance and increased half-life of gadolinium lead to prolonged excretion, causing the GBCA to become unstable and dissociate into its constituents, leading to tissue deposition of Gd3+ cations. This dissociation is thought to be due to differences in the stability of the various chelation complexes among the different formulations of GBCAs.12 The mechanism by which the dissociated gadolinium causes the fibrosis in the skin or other organs of the body is still unknown. Furthermore, even patients with normal renal function who undergo repeated administration of GBCA have been found to have higher levels of Gd3+ in their tissues, even in the absence of symptoms.13
Diagnosing NSF—In 2011, Girardi et al14 created a clinical and histopathological scoring system to help diagnose NSF. Clinical findings can be broken down into major criteria and minor criteria. Major criteria consist of patterned plaques, joint contractures, cobblestoning, marked induration, or peau d’orange change. Minor criteria consist of puckering, linear banding, superficial plaques or patches, dermal papules, and scleral plaques. Histopathologic findings include increased dermal cellularity (score +1), CD34+ cells with tram tracking (score +1), thickened or thin collagen bundles (score +1), preserved elastic fibers (score −1), septal involvement (score +1), and osseous metaplasia (score +3)(eTable).14
Differential Diagnosis—The differential diagnosis of NSF includes scleromyxedema, scleroderma, eosinophilic fasciitis, eosinophilia-myalgia syndrome, lipodermatosclerosis, morphea, and chronic graft-vs-host disease. Histopathologic examination of scleromyxedema can look identical to NSF. Therefore, a review of the patient’s medical history, prior hospitalizations, and prior gadolinium exposure is important. Appropriate laboratory workups should be ordered to rule out the other differential diagnoses.
NSF and Kidney Injury—A PubMed search of articles indexed for MEDLINE using the terms NSF with kidney injury revealed 7 cases of NSF occurring in patients who either had resolved acute kidney injury or resolved acute on chronic kidney disease.1,6-10 Of those cases, 3 reported NSF occurring in patients with completely resolved acute kidney injury.6,7,10 One of those cases involved a 65-year-old man who developed acute renal failure due to acute tubular necrosis.7 He had no history of renal disease prior to hospitalization. His skin lesions continued to improve as his renal function normalized back to baseline after discharge.7 The second case involved a 42-year-old man who had repeated exposure to GBCAs during a brief period of acute kidney injury.6 Nephrogenic systemic fibrosis developed after his renal function normalized. The authors did not mention if there was clinical improvement.6 The third case involved a 22-year-old man who developed acute renal failure after ingestion of hair dye. He did not have a history of chronic renal disease, and as he recovered from the acute kidney injury, almost all of the skin lesions cleared after 1 year.10
Our patient did not have a history of chronic renal disease when she presented to the hospital for sepsis and acute tubular necrosis. Unlike 2 of the prior cases, she did not notice improvement of the skin lesions as the renal function returned to baseline. She continued to experience changes in the skin, even up to 5 years after, and then stabilized. Throughout that time, her renal function was normal. Interestingly, despite having a normal creatinine level, the patient had an elevated gadolinium level on the urine gadolinium test, which typically is not a standard test for NSF. However, the elevated value does shed light on the persistence of gadolinium in the patient despite her exposure having been more than 10 years earlier.
Treatment of NSF—There is no gold standard treatment of NSF, and reversing the fibrosis has proven to be difficult. Avoidance of GBCAs in acute kidney injury or chronic severe renal disease, as recommended by the US Food and Drug Administration, is key to preventing this debilitating disease.15 Restoration of renal function is essential for excreting the gadolinium and improvement in NSF.12 Physical and occupational therapy can improve joint mobility. Therapies such as extracorporeal photopheresis, sodium thiosulfate, pentoxifylline, glucocorticoids, plasmapheresis, intravenous immunoglobulin, cyclophosphamide, imatinib mesylate, intralesional interferon alfa, topical calcipotriene, corticosteroids, and UVA1 light therapy have been used with varying results.12 It has been suggested that renal transplantation can stop the progression of NSF. However, in the cases we reviewed, renal transplantation would not have benefited those patients because their renal function normalized.6,7,10 Additionally, even though our patient’s renal function normalized after discharge from the hospital, she continued to see more skin lesions developing, likely due to the accumulated gadolinium that was already in her tissue. The possibility of chelation therapy to remove the gadolinium has been proposed. In 1 case study involving deferoxamine injected intramuscularly in a patient with NSF, the urine excretion of gadolinium increased almost 2-fold, but there was no change in the serum concentration level of gadolinium or improvement in the patient’s clinical symptoms.16 We anticipate that our patient’s symptoms will slowly improve, as her body is still excreting the gadolinium. Our patient also was added to the International NSF Registry that was created by Dr. Shawn E. Cowper at the Yale School of Medicine (New Haven, Connecticut).
Conclusion
We report a rare case of NSF occurring in a patient with resolved acute kidney injury and no history of chronic renal disease. Our patient initially did not improve after her renal function normalized, as she continued to develop lesions 10 years after the exposure. Her elevated urine gadolinium excretion level also sheds light on the persistence of gadolinium in her body despite her normal renal function 10 years after her exposure. Although her clinical symptoms have stabilized, our case reiterates the complex pathology of this entity and challenge regarding treatment options. Physicians should be aware that NSF can still occur in healthy patients with no chronic renal disease who have had an episode of acute renal insufficiency along with exposure to a GBCA.
- Cowper SE, Su LD, Bhawan J, et al. Nephrogenic fibrosing dermopathy. Am J Dermatopathol. 2001;23:383-393.
- Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104-1108.
- Larson KN, Gagnon AL, Darling MD, et al. Nephrogenic systemic fibrosis manifesting a decade after exposure to gadolinium. JAMA Dermatol. 2015;151:1117-1120.
- Mendoza FA, Artlett CM, Sandorfi N, et al. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35:238-249.
- Ting WW, Stone MS, Madison KC, et al. Nephrogenic fibrosing dermopathy with systemic involvement. Arch Dermatol. 2003;139:903-906.
- Lu CF, Hsiao CH, Tjiu JW. Nephrogenic systemic fibrosis developed after recovery from acute renal failure: gadolinium as a possible aetiological factor. J Eur Acad Dermatol Venereol. 2009;23:339-340.
- Cassis TB, Jackson JM, Sonnier GB, et al. Nephrogenic fibrosing dermopathy in a patient with acute renal failure never requiring dialysis. Int J Dermatol. 2006;45:56-59.
- Swartz RD, Crofford LJ, Phan SH, et al. Nephrogenic fibrosing dermopathy: a novel cutaneous fibrosing disorder in patients with renal failure. Am J Med. 2003;114:563-572.
- Mackay-Wiggan JM, Cohen DJ, Hardy MA, et al. Nephrogenic fibrosing dermopathy (scleromyxedema-like illness of renal disease). J Am Acad Dermatol. 2003;48:55-60.
- Reddy IS, Somani VK, Swarnalata G, et al. Nephrogenic systemic fibrosis following hair-dye ingestion induced acute renal failure. Indian J Dermatol Venereol Leprol. 2006;76:400-403.
- Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17:2359-2362.
- Cheong BYC, Muthupillai R. Nephrogenic systemic fibrosis: a concise review for cardiologists. Texas Heart Inst J. 2010;37:508-515.
- Rogosnitzky M, Branch S. Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms. BioMetals. 2016;29:365-376.
- Girardi M, Kay J, Elston DM, et al. Nephrogenic systemic fibrosis: clinicopathological definition and workup recommendations. J Am Acad Dermatol. 2011;65:1095-1106.
- US Food and Drug Administration. FDA Drug Safety Communication: new warnings for using gadolinium-based contrast agents in patients with kidney dysfunction. Updated February 6, 2018. Accessed November 22, 2021. http://www.fda.gov/Drugs/DrugSafety/ucm223966.htm
- Leung N, Pittelkow MR, Lee CU, et al. Chelation of gadolinium with deferoxamine in a patient with nephrogenic systemic fibrosis. NDT Plus. 2009;2:309-311.
Nephrogenic systemic fibrosis (NSF) is a rare debilitating disorder characterized by dermal plaques, joint contractures, and fibrosis of the skin with possible involvement of muscles and internal organs.1-3 Originally identified in 1997 as nephrogenic fibrosing dermopathy to describe its characteristic cutaneous thickening and hardening, the name was changed to NSF to more accurately reflect the noncutaneous manifestations present in other organ tissues.2,4,5 Nephrogenic systemic fibrosis occurs in patients with a history of renal insufficiency and exposure to gadolinium-based contrast agents (GBCAs) used in magnetic resonance angiography and magnetic resonance imaging. There is no predilection for age, sex, or ethnicity.
Nephrogenic systemic fibrosis may develop over a period of days to several weeks. However, there have been cases of NSF developing 10 years after gadolinium exposure.2 In most cases, patients have a history of severe chronic renal disease requiring hemodialysis. There have been a few reported cases of NSF occurring in patients with resolved acute kidney injury or resolved acute on chronic renal disease.1,6-10 We present a case of NSF occurring in a patient with resolved transient renal insufficiency and no history of chronic renal disease.
Case Report
A 68-year-old woman presented with new dark, painless, pink plaques on the right thigh and calf. The patient stated the condition started and got worse after she was hospitalized 12 years prior for lower extremity cellulitis, sepsis, and acute renal failure. The patient developed complications during that hospital stay and underwent a renal biopsy and renal artery embolization requiring use of a GBCA. After the procedure, she noticed skin hardening in the extremities and decreased mobility in both legs while she was still in the hospital. It was thought that the lower leg changes were due to cellulitis. Therefore, when the renal issues resolved, she was discharged. Her skin and joint changes remained stable until 6 years later when she noticed new pink plaques appearing. Her medical history was positive for breast cancer, which was surgically and medically treated 16 years prior to presentation.
On presentation, physical examination revealed dark pink, hyperpigmented plaques on the right leg and a firm hypopigmented broad linear plaque on the right forearm. Palpation of the legs revealed thickened sclerotic plaques from the thighs down to the ankles (Figure 1). The plaques were not tender to palpation. She did have a decreased range of motion with eversion and inversion of the feet and ankles.
Biopsies from the right medial leg and right volar forearm showed increased bland dermal spindle cellularity associated with numerous round to ovoid osteoid aggregates encircling elastic fibers and surrounded by osteoblasts (Figure 2). CD34 immunohistochemistry showed general retention of staining within the dermal fibroblast population, and elastin stain showed general retention of elastic fiber bundles and thickening.
Laboratory workup included a complete blood cell count, comprehensive metabolic panel, thyroid-stimulating hormone level, and serum protein electrophoresis; results were all within reference range. The patient also had a urine element profile from an outside provider 1 month after presenting to our office that showed an elevated urine gadolinium level of 4.146 μg/g (reference range, 0–0.019 μg/g). The patient’s skin lesions have remained stable, and she is now working with physical therapy to help with her range of motion.
Comment
Gadolinium Causing Fibrosis—The incidence of NSF varies according to the severity of renal impairment, dosage level of GBCA used, and the history of GBCA use. In patients with normal renal function, gadolinium is excreted within 90 minutes. In patients with severe renal disease, the half-life can increase to up to 34.3 hours.11 Reduced renal clearance and increased half-life of gadolinium lead to prolonged excretion, causing the GBCA to become unstable and dissociate into its constituents, leading to tissue deposition of Gd3+ cations. This dissociation is thought to be due to differences in the stability of the various chelation complexes among the different formulations of GBCAs.12 The mechanism by which the dissociated gadolinium causes the fibrosis in the skin or other organs of the body is still unknown. Furthermore, even patients with normal renal function who undergo repeated administration of GBCA have been found to have higher levels of Gd3+ in their tissues, even in the absence of symptoms.13
Diagnosing NSF—In 2011, Girardi et al14 created a clinical and histopathological scoring system to help diagnose NSF. Clinical findings can be broken down into major criteria and minor criteria. Major criteria consist of patterned plaques, joint contractures, cobblestoning, marked induration, or peau d’orange change. Minor criteria consist of puckering, linear banding, superficial plaques or patches, dermal papules, and scleral plaques. Histopathologic findings include increased dermal cellularity (score +1), CD34+ cells with tram tracking (score +1), thickened or thin collagen bundles (score +1), preserved elastic fibers (score −1), septal involvement (score +1), and osseous metaplasia (score +3)(eTable).14
Differential Diagnosis—The differential diagnosis of NSF includes scleromyxedema, scleroderma, eosinophilic fasciitis, eosinophilia-myalgia syndrome, lipodermatosclerosis, morphea, and chronic graft-vs-host disease. Histopathologic examination of scleromyxedema can look identical to NSF. Therefore, a review of the patient’s medical history, prior hospitalizations, and prior gadolinium exposure is important. Appropriate laboratory workups should be ordered to rule out the other differential diagnoses.
NSF and Kidney Injury—A PubMed search of articles indexed for MEDLINE using the terms NSF with kidney injury revealed 7 cases of NSF occurring in patients who either had resolved acute kidney injury or resolved acute on chronic kidney disease.1,6-10 Of those cases, 3 reported NSF occurring in patients with completely resolved acute kidney injury.6,7,10 One of those cases involved a 65-year-old man who developed acute renal failure due to acute tubular necrosis.7 He had no history of renal disease prior to hospitalization. His skin lesions continued to improve as his renal function normalized back to baseline after discharge.7 The second case involved a 42-year-old man who had repeated exposure to GBCAs during a brief period of acute kidney injury.6 Nephrogenic systemic fibrosis developed after his renal function normalized. The authors did not mention if there was clinical improvement.6 The third case involved a 22-year-old man who developed acute renal failure after ingestion of hair dye. He did not have a history of chronic renal disease, and as he recovered from the acute kidney injury, almost all of the skin lesions cleared after 1 year.10
Our patient did not have a history of chronic renal disease when she presented to the hospital for sepsis and acute tubular necrosis. Unlike 2 of the prior cases, she did not notice improvement of the skin lesions as the renal function returned to baseline. She continued to experience changes in the skin, even up to 5 years after, and then stabilized. Throughout that time, her renal function was normal. Interestingly, despite having a normal creatinine level, the patient had an elevated gadolinium level on the urine gadolinium test, which typically is not a standard test for NSF. However, the elevated value does shed light on the persistence of gadolinium in the patient despite her exposure having been more than 10 years earlier.
Treatment of NSF—There is no gold standard treatment of NSF, and reversing the fibrosis has proven to be difficult. Avoidance of GBCAs in acute kidney injury or chronic severe renal disease, as recommended by the US Food and Drug Administration, is key to preventing this debilitating disease.15 Restoration of renal function is essential for excreting the gadolinium and improvement in NSF.12 Physical and occupational therapy can improve joint mobility. Therapies such as extracorporeal photopheresis, sodium thiosulfate, pentoxifylline, glucocorticoids, plasmapheresis, intravenous immunoglobulin, cyclophosphamide, imatinib mesylate, intralesional interferon alfa, topical calcipotriene, corticosteroids, and UVA1 light therapy have been used with varying results.12 It has been suggested that renal transplantation can stop the progression of NSF. However, in the cases we reviewed, renal transplantation would not have benefited those patients because their renal function normalized.6,7,10 Additionally, even though our patient’s renal function normalized after discharge from the hospital, she continued to see more skin lesions developing, likely due to the accumulated gadolinium that was already in her tissue. The possibility of chelation therapy to remove the gadolinium has been proposed. In 1 case study involving deferoxamine injected intramuscularly in a patient with NSF, the urine excretion of gadolinium increased almost 2-fold, but there was no change in the serum concentration level of gadolinium or improvement in the patient’s clinical symptoms.16 We anticipate that our patient’s symptoms will slowly improve, as her body is still excreting the gadolinium. Our patient also was added to the International NSF Registry that was created by Dr. Shawn E. Cowper at the Yale School of Medicine (New Haven, Connecticut).
Conclusion
We report a rare case of NSF occurring in a patient with resolved acute kidney injury and no history of chronic renal disease. Our patient initially did not improve after her renal function normalized, as she continued to develop lesions 10 years after the exposure. Her elevated urine gadolinium excretion level also sheds light on the persistence of gadolinium in her body despite her normal renal function 10 years after her exposure. Although her clinical symptoms have stabilized, our case reiterates the complex pathology of this entity and challenge regarding treatment options. Physicians should be aware that NSF can still occur in healthy patients with no chronic renal disease who have had an episode of acute renal insufficiency along with exposure to a GBCA.
Nephrogenic systemic fibrosis (NSF) is a rare debilitating disorder characterized by dermal plaques, joint contractures, and fibrosis of the skin with possible involvement of muscles and internal organs.1-3 Originally identified in 1997 as nephrogenic fibrosing dermopathy to describe its characteristic cutaneous thickening and hardening, the name was changed to NSF to more accurately reflect the noncutaneous manifestations present in other organ tissues.2,4,5 Nephrogenic systemic fibrosis occurs in patients with a history of renal insufficiency and exposure to gadolinium-based contrast agents (GBCAs) used in magnetic resonance angiography and magnetic resonance imaging. There is no predilection for age, sex, or ethnicity.
Nephrogenic systemic fibrosis may develop over a period of days to several weeks. However, there have been cases of NSF developing 10 years after gadolinium exposure.2 In most cases, patients have a history of severe chronic renal disease requiring hemodialysis. There have been a few reported cases of NSF occurring in patients with resolved acute kidney injury or resolved acute on chronic renal disease.1,6-10 We present a case of NSF occurring in a patient with resolved transient renal insufficiency and no history of chronic renal disease.
Case Report
A 68-year-old woman presented with new dark, painless, pink plaques on the right thigh and calf. The patient stated the condition started and got worse after she was hospitalized 12 years prior for lower extremity cellulitis, sepsis, and acute renal failure. The patient developed complications during that hospital stay and underwent a renal biopsy and renal artery embolization requiring use of a GBCA. After the procedure, she noticed skin hardening in the extremities and decreased mobility in both legs while she was still in the hospital. It was thought that the lower leg changes were due to cellulitis. Therefore, when the renal issues resolved, she was discharged. Her skin and joint changes remained stable until 6 years later when she noticed new pink plaques appearing. Her medical history was positive for breast cancer, which was surgically and medically treated 16 years prior to presentation.
On presentation, physical examination revealed dark pink, hyperpigmented plaques on the right leg and a firm hypopigmented broad linear plaque on the right forearm. Palpation of the legs revealed thickened sclerotic plaques from the thighs down to the ankles (Figure 1). The plaques were not tender to palpation. She did have a decreased range of motion with eversion and inversion of the feet and ankles.
Biopsies from the right medial leg and right volar forearm showed increased bland dermal spindle cellularity associated with numerous round to ovoid osteoid aggregates encircling elastic fibers and surrounded by osteoblasts (Figure 2). CD34 immunohistochemistry showed general retention of staining within the dermal fibroblast population, and elastin stain showed general retention of elastic fiber bundles and thickening.
Laboratory workup included a complete blood cell count, comprehensive metabolic panel, thyroid-stimulating hormone level, and serum protein electrophoresis; results were all within reference range. The patient also had a urine element profile from an outside provider 1 month after presenting to our office that showed an elevated urine gadolinium level of 4.146 μg/g (reference range, 0–0.019 μg/g). The patient’s skin lesions have remained stable, and she is now working with physical therapy to help with her range of motion.
Comment
Gadolinium Causing Fibrosis—The incidence of NSF varies according to the severity of renal impairment, dosage level of GBCA used, and the history of GBCA use. In patients with normal renal function, gadolinium is excreted within 90 minutes. In patients with severe renal disease, the half-life can increase to up to 34.3 hours.11 Reduced renal clearance and increased half-life of gadolinium lead to prolonged excretion, causing the GBCA to become unstable and dissociate into its constituents, leading to tissue deposition of Gd3+ cations. This dissociation is thought to be due to differences in the stability of the various chelation complexes among the different formulations of GBCAs.12 The mechanism by which the dissociated gadolinium causes the fibrosis in the skin or other organs of the body is still unknown. Furthermore, even patients with normal renal function who undergo repeated administration of GBCA have been found to have higher levels of Gd3+ in their tissues, even in the absence of symptoms.13
Diagnosing NSF—In 2011, Girardi et al14 created a clinical and histopathological scoring system to help diagnose NSF. Clinical findings can be broken down into major criteria and minor criteria. Major criteria consist of patterned plaques, joint contractures, cobblestoning, marked induration, or peau d’orange change. Minor criteria consist of puckering, linear banding, superficial plaques or patches, dermal papules, and scleral plaques. Histopathologic findings include increased dermal cellularity (score +1), CD34+ cells with tram tracking (score +1), thickened or thin collagen bundles (score +1), preserved elastic fibers (score −1), septal involvement (score +1), and osseous metaplasia (score +3)(eTable).14
Differential Diagnosis—The differential diagnosis of NSF includes scleromyxedema, scleroderma, eosinophilic fasciitis, eosinophilia-myalgia syndrome, lipodermatosclerosis, morphea, and chronic graft-vs-host disease. Histopathologic examination of scleromyxedema can look identical to NSF. Therefore, a review of the patient’s medical history, prior hospitalizations, and prior gadolinium exposure is important. Appropriate laboratory workups should be ordered to rule out the other differential diagnoses.
NSF and Kidney Injury—A PubMed search of articles indexed for MEDLINE using the terms NSF with kidney injury revealed 7 cases of NSF occurring in patients who either had resolved acute kidney injury or resolved acute on chronic kidney disease.1,6-10 Of those cases, 3 reported NSF occurring in patients with completely resolved acute kidney injury.6,7,10 One of those cases involved a 65-year-old man who developed acute renal failure due to acute tubular necrosis.7 He had no history of renal disease prior to hospitalization. His skin lesions continued to improve as his renal function normalized back to baseline after discharge.7 The second case involved a 42-year-old man who had repeated exposure to GBCAs during a brief period of acute kidney injury.6 Nephrogenic systemic fibrosis developed after his renal function normalized. The authors did not mention if there was clinical improvement.6 The third case involved a 22-year-old man who developed acute renal failure after ingestion of hair dye. He did not have a history of chronic renal disease, and as he recovered from the acute kidney injury, almost all of the skin lesions cleared after 1 year.10
Our patient did not have a history of chronic renal disease when she presented to the hospital for sepsis and acute tubular necrosis. Unlike 2 of the prior cases, she did not notice improvement of the skin lesions as the renal function returned to baseline. She continued to experience changes in the skin, even up to 5 years after, and then stabilized. Throughout that time, her renal function was normal. Interestingly, despite having a normal creatinine level, the patient had an elevated gadolinium level on the urine gadolinium test, which typically is not a standard test for NSF. However, the elevated value does shed light on the persistence of gadolinium in the patient despite her exposure having been more than 10 years earlier.
Treatment of NSF—There is no gold standard treatment of NSF, and reversing the fibrosis has proven to be difficult. Avoidance of GBCAs in acute kidney injury or chronic severe renal disease, as recommended by the US Food and Drug Administration, is key to preventing this debilitating disease.15 Restoration of renal function is essential for excreting the gadolinium and improvement in NSF.12 Physical and occupational therapy can improve joint mobility. Therapies such as extracorporeal photopheresis, sodium thiosulfate, pentoxifylline, glucocorticoids, plasmapheresis, intravenous immunoglobulin, cyclophosphamide, imatinib mesylate, intralesional interferon alfa, topical calcipotriene, corticosteroids, and UVA1 light therapy have been used with varying results.12 It has been suggested that renal transplantation can stop the progression of NSF. However, in the cases we reviewed, renal transplantation would not have benefited those patients because their renal function normalized.6,7,10 Additionally, even though our patient’s renal function normalized after discharge from the hospital, she continued to see more skin lesions developing, likely due to the accumulated gadolinium that was already in her tissue. The possibility of chelation therapy to remove the gadolinium has been proposed. In 1 case study involving deferoxamine injected intramuscularly in a patient with NSF, the urine excretion of gadolinium increased almost 2-fold, but there was no change in the serum concentration level of gadolinium or improvement in the patient’s clinical symptoms.16 We anticipate that our patient’s symptoms will slowly improve, as her body is still excreting the gadolinium. Our patient also was added to the International NSF Registry that was created by Dr. Shawn E. Cowper at the Yale School of Medicine (New Haven, Connecticut).
Conclusion
We report a rare case of NSF occurring in a patient with resolved acute kidney injury and no history of chronic renal disease. Our patient initially did not improve after her renal function normalized, as she continued to develop lesions 10 years after the exposure. Her elevated urine gadolinium excretion level also sheds light on the persistence of gadolinium in her body despite her normal renal function 10 years after her exposure. Although her clinical symptoms have stabilized, our case reiterates the complex pathology of this entity and challenge regarding treatment options. Physicians should be aware that NSF can still occur in healthy patients with no chronic renal disease who have had an episode of acute renal insufficiency along with exposure to a GBCA.
- Cowper SE, Su LD, Bhawan J, et al. Nephrogenic fibrosing dermopathy. Am J Dermatopathol. 2001;23:383-393.
- Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104-1108.
- Larson KN, Gagnon AL, Darling MD, et al. Nephrogenic systemic fibrosis manifesting a decade after exposure to gadolinium. JAMA Dermatol. 2015;151:1117-1120.
- Mendoza FA, Artlett CM, Sandorfi N, et al. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35:238-249.
- Ting WW, Stone MS, Madison KC, et al. Nephrogenic fibrosing dermopathy with systemic involvement. Arch Dermatol. 2003;139:903-906.
- Lu CF, Hsiao CH, Tjiu JW. Nephrogenic systemic fibrosis developed after recovery from acute renal failure: gadolinium as a possible aetiological factor. J Eur Acad Dermatol Venereol. 2009;23:339-340.
- Cassis TB, Jackson JM, Sonnier GB, et al. Nephrogenic fibrosing dermopathy in a patient with acute renal failure never requiring dialysis. Int J Dermatol. 2006;45:56-59.
- Swartz RD, Crofford LJ, Phan SH, et al. Nephrogenic fibrosing dermopathy: a novel cutaneous fibrosing disorder in patients with renal failure. Am J Med. 2003;114:563-572.
- Mackay-Wiggan JM, Cohen DJ, Hardy MA, et al. Nephrogenic fibrosing dermopathy (scleromyxedema-like illness of renal disease). J Am Acad Dermatol. 2003;48:55-60.
- Reddy IS, Somani VK, Swarnalata G, et al. Nephrogenic systemic fibrosis following hair-dye ingestion induced acute renal failure. Indian J Dermatol Venereol Leprol. 2006;76:400-403.
- Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17:2359-2362.
- Cheong BYC, Muthupillai R. Nephrogenic systemic fibrosis: a concise review for cardiologists. Texas Heart Inst J. 2010;37:508-515.
- Rogosnitzky M, Branch S. Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms. BioMetals. 2016;29:365-376.
- Girardi M, Kay J, Elston DM, et al. Nephrogenic systemic fibrosis: clinicopathological definition and workup recommendations. J Am Acad Dermatol. 2011;65:1095-1106.
- US Food and Drug Administration. FDA Drug Safety Communication: new warnings for using gadolinium-based contrast agents in patients with kidney dysfunction. Updated February 6, 2018. Accessed November 22, 2021. http://www.fda.gov/Drugs/DrugSafety/ucm223966.htm
- Leung N, Pittelkow MR, Lee CU, et al. Chelation of gadolinium with deferoxamine in a patient with nephrogenic systemic fibrosis. NDT Plus. 2009;2:309-311.
- Cowper SE, Su LD, Bhawan J, et al. Nephrogenic fibrosing dermopathy. Am J Dermatopathol. 2001;23:383-393.
- Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104-1108.
- Larson KN, Gagnon AL, Darling MD, et al. Nephrogenic systemic fibrosis manifesting a decade after exposure to gadolinium. JAMA Dermatol. 2015;151:1117-1120.
- Mendoza FA, Artlett CM, Sandorfi N, et al. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35:238-249.
- Ting WW, Stone MS, Madison KC, et al. Nephrogenic fibrosing dermopathy with systemic involvement. Arch Dermatol. 2003;139:903-906.
- Lu CF, Hsiao CH, Tjiu JW. Nephrogenic systemic fibrosis developed after recovery from acute renal failure: gadolinium as a possible aetiological factor. J Eur Acad Dermatol Venereol. 2009;23:339-340.
- Cassis TB, Jackson JM, Sonnier GB, et al. Nephrogenic fibrosing dermopathy in a patient with acute renal failure never requiring dialysis. Int J Dermatol. 2006;45:56-59.
- Swartz RD, Crofford LJ, Phan SH, et al. Nephrogenic fibrosing dermopathy: a novel cutaneous fibrosing disorder in patients with renal failure. Am J Med. 2003;114:563-572.
- Mackay-Wiggan JM, Cohen DJ, Hardy MA, et al. Nephrogenic fibrosing dermopathy (scleromyxedema-like illness of renal disease). J Am Acad Dermatol. 2003;48:55-60.
- Reddy IS, Somani VK, Swarnalata G, et al. Nephrogenic systemic fibrosis following hair-dye ingestion induced acute renal failure. Indian J Dermatol Venereol Leprol. 2006;76:400-403.
- Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17:2359-2362.
- Cheong BYC, Muthupillai R. Nephrogenic systemic fibrosis: a concise review for cardiologists. Texas Heart Inst J. 2010;37:508-515.
- Rogosnitzky M, Branch S. Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms. BioMetals. 2016;29:365-376.
- Girardi M, Kay J, Elston DM, et al. Nephrogenic systemic fibrosis: clinicopathological definition and workup recommendations. J Am Acad Dermatol. 2011;65:1095-1106.
- US Food and Drug Administration. FDA Drug Safety Communication: new warnings for using gadolinium-based contrast agents in patients with kidney dysfunction. Updated February 6, 2018. Accessed November 22, 2021. http://www.fda.gov/Drugs/DrugSafety/ucm223966.htm
- Leung N, Pittelkow MR, Lee CU, et al. Chelation of gadolinium with deferoxamine in a patient with nephrogenic systemic fibrosis. NDT Plus. 2009;2:309-311.
Practice Points
- Nephrogenic systemic fibrosis may occur in patients with a history of renal insufficiency and exposure to gadolinium-based contrast agents.
- Nephrogenic systemic fibrosis may develop over a period of days to several years after exposure.
- Symptoms of this rare disease can progress and get worse even after renal function normalizes.
Pityriasis Rosea Associated With COVID-19 Vaccination: A Common Rash Following Administration of a Novel Vaccine
Pityriasis rosea is a papulosquamous eruption that favors the trunk and proximal extremities. It occurs most commonly in adolescents and young adults.1 The rash typically presents with a solitary lesion, known as a “herald patch,” which is followed by a scaly erythematous eruption along the cleavage lines of the skin. The condition is self-limited and often resolves in 6 to 8 weeks. Recent evidence suggests that viral reactivation of human herpesvirus 6 and human herpesvirus 7 may play a role in the development of skin lesions.2 Pityriasis rosea also has been reported following the administration of new medications and vaccinations.1-3 We report a case of a 30-year-old woman who developed pityriasis rosea 3 days after receiving the second dose of the COVID-19 vaccine.
Case Report
A 30-year-old woman presented to the dermatology office for evaluation of a rash on the trunk and upper extremities that had been present for 5 days. She reported an initial solitary lesion on the left upper back, subsequently followed by the appearance of a mildly pruritic rash on the trunk and upper extremities. The rash first appeared 3 days after she received the second dose of the Pfizer-BioNTech COVID-19 vaccine. She was otherwise asymptomatic after vaccination and denied fever, chills, headache, and myalgia. She denied any rash following her first dose of the COVID-19 vaccine, history of known COVID-19 infection or exposures, or new medications. Notably, the patient worked in health care.
Physical examination revealed a 2-cm, erythematous, thin, scaly plaque over the left side of the upper back (Figure, A). Erythematous, scaly, thin papules of varying sizes were distributed along the cleavage lines of the trunk and upper extremities (Figure, B). No biopsy was performed because of the classic clinical presentation of this self-limited condition and the patient’s history of hypertrophic scarring. No additional laboratory workup was performed. She was prescribed triamcinolone cream 0.1% as needed for pruritus and was reassured about the benign nature of this cutaneous eruption.
Comment
A broad spectrum of cutaneous manifestations has been reported in association with acute COVID-19 infection, including a papulovesicular rash, perniolike eruptions, urticaria, livedo reticularis, and petechiae.4 Several cases of pityriasis rosea in association with acute COVID-19 infection also have been reported.5 COVID-19 infection has been linked to reactivation of the herpesvirus, which may explain the connection between acute COVID-19 infection and the development of pityriasis rosea.6 Pityriasis rosea associated with administration of the COVID-19 vaccine is a rare complication with few reports in the literature.7 Similar to our patient, there are reports of pityriasis rosea developing after the second dose of the vaccine, with some patients reporting a reactivation of skin lesions.8 There is a paucity of reports describing pityriasis rosea associated with the influenza vaccine, hepatitis B vaccine, and human papillomavirus vaccine.3 In such cases, the onset of skin lesions was thought to be related to vaccine-induced stimulation of the immune system or a component of the vaccine.
Conclusion
We presented a unique case of pityriasis rosea following COVID-19 vaccination. Because additional laboratory workup and a skin biopsy were not performed, we are unable to infer causation. However, the classic clinical presentation, rash development within 3 days of vaccination, and prior reports of vaccine-associated pityriasis rosea strengthen the aforementioned association. We hope this case adds to the growing understanding of the novel COVID-19 vaccine. As more individuals become vaccinated, both clinicians and patients should be aware of this benign cutaneous eruption that can develop following COVID-19 vaccination.
- Papakostas D, Stavropoulos PG, Papafragkaki D, et al. An atypical case of pityriasis rosea gigantea after influenza vaccination. Case Rep Dermatol. 2014;6:119-123.
- Chen FJ, Chian CP, Chen YF, et al. Pityriasis rosea following influenza (H1N1) vaccination. J Chin Med Assoc. 2011;74:280-282.
- Li A, Li P, Li Y, et al. Recurrent pityriasis rosea: a case report. Hum Vaccin Immunother. 2018;4:1024-1026.
- Ng SM. Prolonged dermatological manifestation 4 weeks following recovery of COVID-19 in a child. BMJ Case Rep. 2020;13:e237056. doi:10.1136/bcr-2020-237056
- Johansen M, Chisolm SS, Aspey LD, et al. Pityriasis rosea in otherwise asymptomatic confirmed COVID-19-positive patients: a report of 2 cases. JAAD Case Rep. 2021;7:93-94.
- Dursun R, Temiz SA. The clinics of HHV-6 infection in COVID-19 pandemic: pityriasis rosea and Kawasaki disease. Dermatol Ther. 2020;33:e13730. doi:10.1111/dth.13730
- Leerunyakul K, Pakornphadungsit K, Suchonwanit P. Case report: pityriasis rosea-like eruption following COVID-19 vaccination [published online September 7, 2021]. Front Med. doi:10.3389/fmed.2021.752443
- Marcantonio-Santa Cruz OY, Vidal-Navarro A, Pesqué D, et al. Pityriasis rosea developing after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:E721-E722. doi:10.1111/jdv.17498
Pityriasis rosea is a papulosquamous eruption that favors the trunk and proximal extremities. It occurs most commonly in adolescents and young adults.1 The rash typically presents with a solitary lesion, known as a “herald patch,” which is followed by a scaly erythematous eruption along the cleavage lines of the skin. The condition is self-limited and often resolves in 6 to 8 weeks. Recent evidence suggests that viral reactivation of human herpesvirus 6 and human herpesvirus 7 may play a role in the development of skin lesions.2 Pityriasis rosea also has been reported following the administration of new medications and vaccinations.1-3 We report a case of a 30-year-old woman who developed pityriasis rosea 3 days after receiving the second dose of the COVID-19 vaccine.
Case Report
A 30-year-old woman presented to the dermatology office for evaluation of a rash on the trunk and upper extremities that had been present for 5 days. She reported an initial solitary lesion on the left upper back, subsequently followed by the appearance of a mildly pruritic rash on the trunk and upper extremities. The rash first appeared 3 days after she received the second dose of the Pfizer-BioNTech COVID-19 vaccine. She was otherwise asymptomatic after vaccination and denied fever, chills, headache, and myalgia. She denied any rash following her first dose of the COVID-19 vaccine, history of known COVID-19 infection or exposures, or new medications. Notably, the patient worked in health care.
Physical examination revealed a 2-cm, erythematous, thin, scaly plaque over the left side of the upper back (Figure, A). Erythematous, scaly, thin papules of varying sizes were distributed along the cleavage lines of the trunk and upper extremities (Figure, B). No biopsy was performed because of the classic clinical presentation of this self-limited condition and the patient’s history of hypertrophic scarring. No additional laboratory workup was performed. She was prescribed triamcinolone cream 0.1% as needed for pruritus and was reassured about the benign nature of this cutaneous eruption.
Comment
A broad spectrum of cutaneous manifestations has been reported in association with acute COVID-19 infection, including a papulovesicular rash, perniolike eruptions, urticaria, livedo reticularis, and petechiae.4 Several cases of pityriasis rosea in association with acute COVID-19 infection also have been reported.5 COVID-19 infection has been linked to reactivation of the herpesvirus, which may explain the connection between acute COVID-19 infection and the development of pityriasis rosea.6 Pityriasis rosea associated with administration of the COVID-19 vaccine is a rare complication with few reports in the literature.7 Similar to our patient, there are reports of pityriasis rosea developing after the second dose of the vaccine, with some patients reporting a reactivation of skin lesions.8 There is a paucity of reports describing pityriasis rosea associated with the influenza vaccine, hepatitis B vaccine, and human papillomavirus vaccine.3 In such cases, the onset of skin lesions was thought to be related to vaccine-induced stimulation of the immune system or a component of the vaccine.
Conclusion
We presented a unique case of pityriasis rosea following COVID-19 vaccination. Because additional laboratory workup and a skin biopsy were not performed, we are unable to infer causation. However, the classic clinical presentation, rash development within 3 days of vaccination, and prior reports of vaccine-associated pityriasis rosea strengthen the aforementioned association. We hope this case adds to the growing understanding of the novel COVID-19 vaccine. As more individuals become vaccinated, both clinicians and patients should be aware of this benign cutaneous eruption that can develop following COVID-19 vaccination.
Pityriasis rosea is a papulosquamous eruption that favors the trunk and proximal extremities. It occurs most commonly in adolescents and young adults.1 The rash typically presents with a solitary lesion, known as a “herald patch,” which is followed by a scaly erythematous eruption along the cleavage lines of the skin. The condition is self-limited and often resolves in 6 to 8 weeks. Recent evidence suggests that viral reactivation of human herpesvirus 6 and human herpesvirus 7 may play a role in the development of skin lesions.2 Pityriasis rosea also has been reported following the administration of new medications and vaccinations.1-3 We report a case of a 30-year-old woman who developed pityriasis rosea 3 days after receiving the second dose of the COVID-19 vaccine.
Case Report
A 30-year-old woman presented to the dermatology office for evaluation of a rash on the trunk and upper extremities that had been present for 5 days. She reported an initial solitary lesion on the left upper back, subsequently followed by the appearance of a mildly pruritic rash on the trunk and upper extremities. The rash first appeared 3 days after she received the second dose of the Pfizer-BioNTech COVID-19 vaccine. She was otherwise asymptomatic after vaccination and denied fever, chills, headache, and myalgia. She denied any rash following her first dose of the COVID-19 vaccine, history of known COVID-19 infection or exposures, or new medications. Notably, the patient worked in health care.
Physical examination revealed a 2-cm, erythematous, thin, scaly plaque over the left side of the upper back (Figure, A). Erythematous, scaly, thin papules of varying sizes were distributed along the cleavage lines of the trunk and upper extremities (Figure, B). No biopsy was performed because of the classic clinical presentation of this self-limited condition and the patient’s history of hypertrophic scarring. No additional laboratory workup was performed. She was prescribed triamcinolone cream 0.1% as needed for pruritus and was reassured about the benign nature of this cutaneous eruption.
Comment
A broad spectrum of cutaneous manifestations has been reported in association with acute COVID-19 infection, including a papulovesicular rash, perniolike eruptions, urticaria, livedo reticularis, and petechiae.4 Several cases of pityriasis rosea in association with acute COVID-19 infection also have been reported.5 COVID-19 infection has been linked to reactivation of the herpesvirus, which may explain the connection between acute COVID-19 infection and the development of pityriasis rosea.6 Pityriasis rosea associated with administration of the COVID-19 vaccine is a rare complication with few reports in the literature.7 Similar to our patient, there are reports of pityriasis rosea developing after the second dose of the vaccine, with some patients reporting a reactivation of skin lesions.8 There is a paucity of reports describing pityriasis rosea associated with the influenza vaccine, hepatitis B vaccine, and human papillomavirus vaccine.3 In such cases, the onset of skin lesions was thought to be related to vaccine-induced stimulation of the immune system or a component of the vaccine.
Conclusion
We presented a unique case of pityriasis rosea following COVID-19 vaccination. Because additional laboratory workup and a skin biopsy were not performed, we are unable to infer causation. However, the classic clinical presentation, rash development within 3 days of vaccination, and prior reports of vaccine-associated pityriasis rosea strengthen the aforementioned association. We hope this case adds to the growing understanding of the novel COVID-19 vaccine. As more individuals become vaccinated, both clinicians and patients should be aware of this benign cutaneous eruption that can develop following COVID-19 vaccination.
- Papakostas D, Stavropoulos PG, Papafragkaki D, et al. An atypical case of pityriasis rosea gigantea after influenza vaccination. Case Rep Dermatol. 2014;6:119-123.
- Chen FJ, Chian CP, Chen YF, et al. Pityriasis rosea following influenza (H1N1) vaccination. J Chin Med Assoc. 2011;74:280-282.
- Li A, Li P, Li Y, et al. Recurrent pityriasis rosea: a case report. Hum Vaccin Immunother. 2018;4:1024-1026.
- Ng SM. Prolonged dermatological manifestation 4 weeks following recovery of COVID-19 in a child. BMJ Case Rep. 2020;13:e237056. doi:10.1136/bcr-2020-237056
- Johansen M, Chisolm SS, Aspey LD, et al. Pityriasis rosea in otherwise asymptomatic confirmed COVID-19-positive patients: a report of 2 cases. JAAD Case Rep. 2021;7:93-94.
- Dursun R, Temiz SA. The clinics of HHV-6 infection in COVID-19 pandemic: pityriasis rosea and Kawasaki disease. Dermatol Ther. 2020;33:e13730. doi:10.1111/dth.13730
- Leerunyakul K, Pakornphadungsit K, Suchonwanit P. Case report: pityriasis rosea-like eruption following COVID-19 vaccination [published online September 7, 2021]. Front Med. doi:10.3389/fmed.2021.752443
- Marcantonio-Santa Cruz OY, Vidal-Navarro A, Pesqué D, et al. Pityriasis rosea developing after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:E721-E722. doi:10.1111/jdv.17498
- Papakostas D, Stavropoulos PG, Papafragkaki D, et al. An atypical case of pityriasis rosea gigantea after influenza vaccination. Case Rep Dermatol. 2014;6:119-123.
- Chen FJ, Chian CP, Chen YF, et al. Pityriasis rosea following influenza (H1N1) vaccination. J Chin Med Assoc. 2011;74:280-282.
- Li A, Li P, Li Y, et al. Recurrent pityriasis rosea: a case report. Hum Vaccin Immunother. 2018;4:1024-1026.
- Ng SM. Prolonged dermatological manifestation 4 weeks following recovery of COVID-19 in a child. BMJ Case Rep. 2020;13:e237056. doi:10.1136/bcr-2020-237056
- Johansen M, Chisolm SS, Aspey LD, et al. Pityriasis rosea in otherwise asymptomatic confirmed COVID-19-positive patients: a report of 2 cases. JAAD Case Rep. 2021;7:93-94.
- Dursun R, Temiz SA. The clinics of HHV-6 infection in COVID-19 pandemic: pityriasis rosea and Kawasaki disease. Dermatol Ther. 2020;33:e13730. doi:10.1111/dth.13730
- Leerunyakul K, Pakornphadungsit K, Suchonwanit P. Case report: pityriasis rosea-like eruption following COVID-19 vaccination [published online September 7, 2021]. Front Med. doi:10.3389/fmed.2021.752443
- Marcantonio-Santa Cruz OY, Vidal-Navarro A, Pesqué D, et al. Pityriasis rosea developing after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:E721-E722. doi:10.1111/jdv.17498
Practice Points
- Clinicians should be aware of the association between COVID-19 vaccination and the development of pityriasis rosea.
- Pityriasis rosea has been linked to reactivation of human herpesvirus 6 and human herpesvirus 7 and has been reported following administration of the influenza and human papillomavirus vaccines.
- Pityriasis rosea is a self-limited, cutaneous eruption that resolves within 6 to 8 weeks, and patients should be educated on the benign nature of this condition.
Periungual Papules in an Elderly Woman
The Diagnosis: Multicentric Reticulohistiocytosis
Te patient presented with pink papules coalescing into plaques on the upper chest and lower back (Figure 1) as well as a characteristic finding of periungual papules with a coral bead appearance. Histopathologic examination revealed a dense infiltrate of epithelioid histiocytes with amphophilic ground-glass cytoplasm in a nodular configuration (Figure 2). This pattern in conjunction with the clinical features seen in our patient was consistent with a diagnosis of multicentric reticulohistiocytosis (MRH).1-3 The cutaneous symptoms were managed with triamcinolone ointment 0.1% twice daily and oral hydroxyzine 10 mg 3 times daily as needed for itching with moderate improvement. She was referred to rheumatology for arthritis management, and the initial cancer screening was negative.
Multicentric reticulohistiocytosis is a rare granulomatous disease characterized by papulonodular cutaneous lesions and severe erosive arthritis. It has an insidious onset and most commonly affects middle-aged women.1 Multicentric reticulohistiocytosis typically presents as rounded pruritic papules or nodules that may be pink, red, or brown primarily affecting the face and distal upper extremities.1,3 Mucosal involvement occurs in more than half of patients and is characterized by multiple erythematous papules and nodules on the oral and nasopharyngeal mucosae that rarely can produce leonine facies.2 A hallmark feature of MRH is the presence of multiple shiny erythematous papules along the proximal and lateral nail folds that take on a coral bead appearance.1,3,4 Furthermore, nail changes such as atrophy, longitudinal ridging, brittleness, and hyperpigmentation can occur secondary to a synovial reaction that disturbs the nail matrix.4,5
Joint involvement precedes cutaneous involvement in most cases of MRH.1,5 Multicentric reticulohistiocytosis is associated with a symmetric destructive arthritis affecting the hands, knees, shoulders, and hips that often is associated with pain, stiffness, and swelling.1,3 The arthritis rapidly progresses in the early stages of the disease but then becomes less active over the subsequent 8 to 10 years.1 It has the potential to develop into arthritis mutilans, an end-stage form of arthritis also seen in psoriatic and rheumatoid arthritis that leads to severe joint deformity and debilitation.1,2
The etiology of MRH still is unknown, but it has an association with underlying malignancy in up to 25% of patients.6 Multicentric reticulohistiocytosis has been reported in the context of a wide variety of malignancies including melanoma; sarcoma; lymphoma; leukemia; and carcinomas of the breast, colon, and lung. In some cases, the diagnosis of MRH may even precede the diagnosis of cancer.3 Multicentric reticulohistiocytosis also may be associated with autoimmune conditions,3 as seen in our patient who had a history of both hypothyroidism and vitiligo.
Histopathologic examination is essential in distinguishing MRH from other autoimmune disorders associated with hand lesions, rash, and arthralgia. Erythema elevatum diutinum is associated with symmetric, violaceous, red or brown papules and plaques located on the extensor surfaces of the extremities and hands; however, histology reveals a leukocytoclastic vasculitis with a mixture of polymorphonuclear leukocytes and lymphocytes.7 Dermatomyositis may present with arthralgia, flattopped, erythematous (Gottron) papules localized over the proximal interphalangeal and distal interphalangeal joints, as well as proximal nail findings. The latter generally presents with periungual erythema associated with dilated capillary loops rather than the discrete orderly papules seen in MRH. Histologic examination of dermatomyositis shows mild epidermal atrophy, vacuolar changes in the basal keratinocyte layer, and a dermal perivascular lymphocytic infiltrate.8 Because MRH initially can present with joint symptoms and hand nodules, it may be confused with rheumatoid arthritis. However, rheumatoid arthritis typically is associated with severe osteopenia and tends to affect the metacarpophalangeal and proximal interphalangeal joints rather than the distal interphalangeal joints that most often are affected in MRH.1 Histologic examination of rheumatoid nodules reveals palisading granulomas surrounding a central area of fibrinoid necrosis.9 Sarcoidosis is a multisystem disease that can present with cutaneous involvement including erythema nodosum, skin plaques, subcutaneous nodules, and papular eruptions in addition to joint lesions.10 Sarcoidosis most frequently involves the lungs, manifesting as diffuse interstitial lung disease with bilateral hilar lymphadenopathy. Furthermore, histologic examination of lesions demonstrates classic noncaseating granulomas containing epithelioid cells, multinucleated giant cells with inclusion bodies, and lymphocytes.11
A skin biopsy is required to establish the diagnosis of MRH. In general, patients with MRH and no underlying malignancy have a good prognosis and respond to anti-inflammatory therapies such as nonsteroidal antiinflammatory drugs and corticosteroids. Other agents including methotrexate, cyclophosphamide, and tumor necrosis factor α inhibitors also have been effective in more severe cases.1,3,12 Finally, in addition to treating the cutaneous manifestations of MRH, it is important to screen patients for underlying malignancies and other autoimmune conditions.
- Tajirian AL, Malik MK, Robinson-Bostom L, et al. Multicentric reticulohistiocytosis. Clin Dermatol. 2006;24:486-492.
- Gold RH, Metzger AL, Mirra JM, et al. Multicentric reticulohistiocytosis (lipoid dermato-arthritis). an erosive polyarthritis with distinctive clinical, roentgenographic and pathologic features. Am J Roentgenol Radium Ther Nucl Med. 1975;124:610-624.
- Luz FB, Gaspar TAP, Kalil-Gaspar N, et al. Multicentric reticulohistiocytosis. J Eur Acad Dermatol Venereol. 2001;15:524-531.
- Barrow MV. The nails in multicentric reticulohistiocytosis. (lipoid dermato-arthritis). Arch Dermatol. 1967;95:200-201.
- Barrow MV, Holubar K. Multicentric reticulohistiocytosis. a review of 33 patients. Medicine (Baltimore). 1969;48:287-305.
- Snow JL, Muller SA. Malignancy-associated multicentric reticulohistiocytosis: a clinical, histological and immunophenotypic study. Br J Dermatol. 1995;133:71-76.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Smith ES, Hallman JR, DeLuca AM, et al. Dermatomyositis: a clinicopathological study of 40 patients. Am J Dermatopathol. 2009; 31:61-67.
- Athanasou NA, Quinn J, Woods CG, et al. Immunohistology of rheumatoid nodules and rheumatoid synovium. Ann Rheum Dis. 1988;47:398-403.
- Yanardag H, Pamuk ON, Karayel T. Cutaneous involvement in sarcoidosis: analysis of the features in 170 patients. Respir Med. 2003;97:978-982.
- Ma Y, Gal A, Koss MN. The pathology of pulmonary sarcoidosis: update. Semin Diagn Pathol. 2007;24:150-161.
- Kovach BT, Calamia KT, Walsh JS, et al. Treatment of multicentric reticulohistiocytosis with etanercept. Arch Dermatol. 2004;140:919-921.
The Diagnosis: Multicentric Reticulohistiocytosis
Te patient presented with pink papules coalescing into plaques on the upper chest and lower back (Figure 1) as well as a characteristic finding of periungual papules with a coral bead appearance. Histopathologic examination revealed a dense infiltrate of epithelioid histiocytes with amphophilic ground-glass cytoplasm in a nodular configuration (Figure 2). This pattern in conjunction with the clinical features seen in our patient was consistent with a diagnosis of multicentric reticulohistiocytosis (MRH).1-3 The cutaneous symptoms were managed with triamcinolone ointment 0.1% twice daily and oral hydroxyzine 10 mg 3 times daily as needed for itching with moderate improvement. She was referred to rheumatology for arthritis management, and the initial cancer screening was negative.
Multicentric reticulohistiocytosis is a rare granulomatous disease characterized by papulonodular cutaneous lesions and severe erosive arthritis. It has an insidious onset and most commonly affects middle-aged women.1 Multicentric reticulohistiocytosis typically presents as rounded pruritic papules or nodules that may be pink, red, or brown primarily affecting the face and distal upper extremities.1,3 Mucosal involvement occurs in more than half of patients and is characterized by multiple erythematous papules and nodules on the oral and nasopharyngeal mucosae that rarely can produce leonine facies.2 A hallmark feature of MRH is the presence of multiple shiny erythematous papules along the proximal and lateral nail folds that take on a coral bead appearance.1,3,4 Furthermore, nail changes such as atrophy, longitudinal ridging, brittleness, and hyperpigmentation can occur secondary to a synovial reaction that disturbs the nail matrix.4,5
Joint involvement precedes cutaneous involvement in most cases of MRH.1,5 Multicentric reticulohistiocytosis is associated with a symmetric destructive arthritis affecting the hands, knees, shoulders, and hips that often is associated with pain, stiffness, and swelling.1,3 The arthritis rapidly progresses in the early stages of the disease but then becomes less active over the subsequent 8 to 10 years.1 It has the potential to develop into arthritis mutilans, an end-stage form of arthritis also seen in psoriatic and rheumatoid arthritis that leads to severe joint deformity and debilitation.1,2
The etiology of MRH still is unknown, but it has an association with underlying malignancy in up to 25% of patients.6 Multicentric reticulohistiocytosis has been reported in the context of a wide variety of malignancies including melanoma; sarcoma; lymphoma; leukemia; and carcinomas of the breast, colon, and lung. In some cases, the diagnosis of MRH may even precede the diagnosis of cancer.3 Multicentric reticulohistiocytosis also may be associated with autoimmune conditions,3 as seen in our patient who had a history of both hypothyroidism and vitiligo.
Histopathologic examination is essential in distinguishing MRH from other autoimmune disorders associated with hand lesions, rash, and arthralgia. Erythema elevatum diutinum is associated with symmetric, violaceous, red or brown papules and plaques located on the extensor surfaces of the extremities and hands; however, histology reveals a leukocytoclastic vasculitis with a mixture of polymorphonuclear leukocytes and lymphocytes.7 Dermatomyositis may present with arthralgia, flattopped, erythematous (Gottron) papules localized over the proximal interphalangeal and distal interphalangeal joints, as well as proximal nail findings. The latter generally presents with periungual erythema associated with dilated capillary loops rather than the discrete orderly papules seen in MRH. Histologic examination of dermatomyositis shows mild epidermal atrophy, vacuolar changes in the basal keratinocyte layer, and a dermal perivascular lymphocytic infiltrate.8 Because MRH initially can present with joint symptoms and hand nodules, it may be confused with rheumatoid arthritis. However, rheumatoid arthritis typically is associated with severe osteopenia and tends to affect the metacarpophalangeal and proximal interphalangeal joints rather than the distal interphalangeal joints that most often are affected in MRH.1 Histologic examination of rheumatoid nodules reveals palisading granulomas surrounding a central area of fibrinoid necrosis.9 Sarcoidosis is a multisystem disease that can present with cutaneous involvement including erythema nodosum, skin plaques, subcutaneous nodules, and papular eruptions in addition to joint lesions.10 Sarcoidosis most frequently involves the lungs, manifesting as diffuse interstitial lung disease with bilateral hilar lymphadenopathy. Furthermore, histologic examination of lesions demonstrates classic noncaseating granulomas containing epithelioid cells, multinucleated giant cells with inclusion bodies, and lymphocytes.11
A skin biopsy is required to establish the diagnosis of MRH. In general, patients with MRH and no underlying malignancy have a good prognosis and respond to anti-inflammatory therapies such as nonsteroidal antiinflammatory drugs and corticosteroids. Other agents including methotrexate, cyclophosphamide, and tumor necrosis factor α inhibitors also have been effective in more severe cases.1,3,12 Finally, in addition to treating the cutaneous manifestations of MRH, it is important to screen patients for underlying malignancies and other autoimmune conditions.
The Diagnosis: Multicentric Reticulohistiocytosis
Te patient presented with pink papules coalescing into plaques on the upper chest and lower back (Figure 1) as well as a characteristic finding of periungual papules with a coral bead appearance. Histopathologic examination revealed a dense infiltrate of epithelioid histiocytes with amphophilic ground-glass cytoplasm in a nodular configuration (Figure 2). This pattern in conjunction with the clinical features seen in our patient was consistent with a diagnosis of multicentric reticulohistiocytosis (MRH).1-3 The cutaneous symptoms were managed with triamcinolone ointment 0.1% twice daily and oral hydroxyzine 10 mg 3 times daily as needed for itching with moderate improvement. She was referred to rheumatology for arthritis management, and the initial cancer screening was negative.
Multicentric reticulohistiocytosis is a rare granulomatous disease characterized by papulonodular cutaneous lesions and severe erosive arthritis. It has an insidious onset and most commonly affects middle-aged women.1 Multicentric reticulohistiocytosis typically presents as rounded pruritic papules or nodules that may be pink, red, or brown primarily affecting the face and distal upper extremities.1,3 Mucosal involvement occurs in more than half of patients and is characterized by multiple erythematous papules and nodules on the oral and nasopharyngeal mucosae that rarely can produce leonine facies.2 A hallmark feature of MRH is the presence of multiple shiny erythematous papules along the proximal and lateral nail folds that take on a coral bead appearance.1,3,4 Furthermore, nail changes such as atrophy, longitudinal ridging, brittleness, and hyperpigmentation can occur secondary to a synovial reaction that disturbs the nail matrix.4,5
Joint involvement precedes cutaneous involvement in most cases of MRH.1,5 Multicentric reticulohistiocytosis is associated with a symmetric destructive arthritis affecting the hands, knees, shoulders, and hips that often is associated with pain, stiffness, and swelling.1,3 The arthritis rapidly progresses in the early stages of the disease but then becomes less active over the subsequent 8 to 10 years.1 It has the potential to develop into arthritis mutilans, an end-stage form of arthritis also seen in psoriatic and rheumatoid arthritis that leads to severe joint deformity and debilitation.1,2
The etiology of MRH still is unknown, but it has an association with underlying malignancy in up to 25% of patients.6 Multicentric reticulohistiocytosis has been reported in the context of a wide variety of malignancies including melanoma; sarcoma; lymphoma; leukemia; and carcinomas of the breast, colon, and lung. In some cases, the diagnosis of MRH may even precede the diagnosis of cancer.3 Multicentric reticulohistiocytosis also may be associated with autoimmune conditions,3 as seen in our patient who had a history of both hypothyroidism and vitiligo.
Histopathologic examination is essential in distinguishing MRH from other autoimmune disorders associated with hand lesions, rash, and arthralgia. Erythema elevatum diutinum is associated with symmetric, violaceous, red or brown papules and plaques located on the extensor surfaces of the extremities and hands; however, histology reveals a leukocytoclastic vasculitis with a mixture of polymorphonuclear leukocytes and lymphocytes.7 Dermatomyositis may present with arthralgia, flattopped, erythematous (Gottron) papules localized over the proximal interphalangeal and distal interphalangeal joints, as well as proximal nail findings. The latter generally presents with periungual erythema associated with dilated capillary loops rather than the discrete orderly papules seen in MRH. Histologic examination of dermatomyositis shows mild epidermal atrophy, vacuolar changes in the basal keratinocyte layer, and a dermal perivascular lymphocytic infiltrate.8 Because MRH initially can present with joint symptoms and hand nodules, it may be confused with rheumatoid arthritis. However, rheumatoid arthritis typically is associated with severe osteopenia and tends to affect the metacarpophalangeal and proximal interphalangeal joints rather than the distal interphalangeal joints that most often are affected in MRH.1 Histologic examination of rheumatoid nodules reveals palisading granulomas surrounding a central area of fibrinoid necrosis.9 Sarcoidosis is a multisystem disease that can present with cutaneous involvement including erythema nodosum, skin plaques, subcutaneous nodules, and papular eruptions in addition to joint lesions.10 Sarcoidosis most frequently involves the lungs, manifesting as diffuse interstitial lung disease with bilateral hilar lymphadenopathy. Furthermore, histologic examination of lesions demonstrates classic noncaseating granulomas containing epithelioid cells, multinucleated giant cells with inclusion bodies, and lymphocytes.11
A skin biopsy is required to establish the diagnosis of MRH. In general, patients with MRH and no underlying malignancy have a good prognosis and respond to anti-inflammatory therapies such as nonsteroidal antiinflammatory drugs and corticosteroids. Other agents including methotrexate, cyclophosphamide, and tumor necrosis factor α inhibitors also have been effective in more severe cases.1,3,12 Finally, in addition to treating the cutaneous manifestations of MRH, it is important to screen patients for underlying malignancies and other autoimmune conditions.
- Tajirian AL, Malik MK, Robinson-Bostom L, et al. Multicentric reticulohistiocytosis. Clin Dermatol. 2006;24:486-492.
- Gold RH, Metzger AL, Mirra JM, et al. Multicentric reticulohistiocytosis (lipoid dermato-arthritis). an erosive polyarthritis with distinctive clinical, roentgenographic and pathologic features. Am J Roentgenol Radium Ther Nucl Med. 1975;124:610-624.
- Luz FB, Gaspar TAP, Kalil-Gaspar N, et al. Multicentric reticulohistiocytosis. J Eur Acad Dermatol Venereol. 2001;15:524-531.
- Barrow MV. The nails in multicentric reticulohistiocytosis. (lipoid dermato-arthritis). Arch Dermatol. 1967;95:200-201.
- Barrow MV, Holubar K. Multicentric reticulohistiocytosis. a review of 33 patients. Medicine (Baltimore). 1969;48:287-305.
- Snow JL, Muller SA. Malignancy-associated multicentric reticulohistiocytosis: a clinical, histological and immunophenotypic study. Br J Dermatol. 1995;133:71-76.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Smith ES, Hallman JR, DeLuca AM, et al. Dermatomyositis: a clinicopathological study of 40 patients. Am J Dermatopathol. 2009; 31:61-67.
- Athanasou NA, Quinn J, Woods CG, et al. Immunohistology of rheumatoid nodules and rheumatoid synovium. Ann Rheum Dis. 1988;47:398-403.
- Yanardag H, Pamuk ON, Karayel T. Cutaneous involvement in sarcoidosis: analysis of the features in 170 patients. Respir Med. 2003;97:978-982.
- Ma Y, Gal A, Koss MN. The pathology of pulmonary sarcoidosis: update. Semin Diagn Pathol. 2007;24:150-161.
- Kovach BT, Calamia KT, Walsh JS, et al. Treatment of multicentric reticulohistiocytosis with etanercept. Arch Dermatol. 2004;140:919-921.
- Tajirian AL, Malik MK, Robinson-Bostom L, et al. Multicentric reticulohistiocytosis. Clin Dermatol. 2006;24:486-492.
- Gold RH, Metzger AL, Mirra JM, et al. Multicentric reticulohistiocytosis (lipoid dermato-arthritis). an erosive polyarthritis with distinctive clinical, roentgenographic and pathologic features. Am J Roentgenol Radium Ther Nucl Med. 1975;124:610-624.
- Luz FB, Gaspar TAP, Kalil-Gaspar N, et al. Multicentric reticulohistiocytosis. J Eur Acad Dermatol Venereol. 2001;15:524-531.
- Barrow MV. The nails in multicentric reticulohistiocytosis. (lipoid dermato-arthritis). Arch Dermatol. 1967;95:200-201.
- Barrow MV, Holubar K. Multicentric reticulohistiocytosis. a review of 33 patients. Medicine (Baltimore). 1969;48:287-305.
- Snow JL, Muller SA. Malignancy-associated multicentric reticulohistiocytosis: a clinical, histological and immunophenotypic study. Br J Dermatol. 1995;133:71-76.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Smith ES, Hallman JR, DeLuca AM, et al. Dermatomyositis: a clinicopathological study of 40 patients. Am J Dermatopathol. 2009; 31:61-67.
- Athanasou NA, Quinn J, Woods CG, et al. Immunohistology of rheumatoid nodules and rheumatoid synovium. Ann Rheum Dis. 1988;47:398-403.
- Yanardag H, Pamuk ON, Karayel T. Cutaneous involvement in sarcoidosis: analysis of the features in 170 patients. Respir Med. 2003;97:978-982.
- Ma Y, Gal A, Koss MN. The pathology of pulmonary sarcoidosis: update. Semin Diagn Pathol. 2007;24:150-161.
- Kovach BT, Calamia KT, Walsh JS, et al. Treatment of multicentric reticulohistiocytosis with etanercept. Arch Dermatol. 2004;140:919-921.
A 79-year-old woman presented with pruritic papules and plaques on the chest, back, arms, hands, legs, and feet of 1 year’s duration. She reported a history of hypothyroidism, arthritis, and vitiligo but denied a history of cancer. Physical examination showed pink papules coalescing into plaques on the upper chest and lower back as well as lichenified plaques on the forearms and knees. Erythematous papules on the proximal nail folds of the right first and second digits also were noted. Multiple depigmented patches on the hands, wrists, arms, and lower back also were present, and deformities of the hands and bulbous-appearing knees were observed. Results from a complete blood cell count and blood chemistry analyses showed mild anemia but were otherwise normal. Radiography of the right knee showed degenerative changes and periarticular radiolucencies consistent with an inflammatory arthropathy. A 4-mm punch biopsy specimen from the back was obtained for histopathologic examination.
In (K)need of Help
ANSWER
The correct answer is bullous impetigo (choice “a”).
Had the source of the problem been MRSA (choice “b”), there would have been marked tenderness and swelling. Psoriasis vulgaris (choice “c”) was a possibility; however, it almost never manifests with blisters, it rarely comes on as quickly as this patient’s problem did, and it produces scale that is far thicker and more tenacious than that seen in bullous impetigo. Nummular eczema (choice “d”) does not manifest with blisters and would likely have caused itching.
DISCUSSION
Impetigo is one of many common skin diseases—other examples include granuloma annulare and lichen planus—that have bullous variants, which can make diagnosis challenging. Impetigo is easy to diagnose in its more common papulosquamous form. But it also can manifest with flaccid blisters that last only a short time, leaving the round, scaly lesions seen in this case.
Staphylococcus aureus, the organism responsible for bullous impetigo, elaborates serine proteases, which bind to and cleave desmoglein-1. This effectively destroys the connections between skin layers, creating a space that is quickly filled with serum. The level of this separation is typically subcorneal, which allows the formation of a very thin, friable roof for the bulla.
This modern version of impetigo is not considered dangerous, despite its association with staph aureus. In pre-antibiotic times, the predominant organism was streptococcal, some types of which could be nephritogenic—that is, capable of causing Bright disease or, as it is now known, acute post-streptococcal glomerulonephritis. Fortunately, this potentially fatal condition is only rarely seen in modern times.
Two items of note about this case: Bullous impetigo, contrary to what was seen in this patient, typically favors intertriginous areas. And a key factor is the history of atopy, which renders the patient more susceptible to skin infections of all kinds.
Treatment
The patient responded well to topical mupirocin ointment, applied three times a day. In rare instances, impetigo can require an oral antibiotic, such as cephalexin.
ANSWER
The correct answer is bullous impetigo (choice “a”).
Had the source of the problem been MRSA (choice “b”), there would have been marked tenderness and swelling. Psoriasis vulgaris (choice “c”) was a possibility; however, it almost never manifests with blisters, it rarely comes on as quickly as this patient’s problem did, and it produces scale that is far thicker and more tenacious than that seen in bullous impetigo. Nummular eczema (choice “d”) does not manifest with blisters and would likely have caused itching.
DISCUSSION
Impetigo is one of many common skin diseases—other examples include granuloma annulare and lichen planus—that have bullous variants, which can make diagnosis challenging. Impetigo is easy to diagnose in its more common papulosquamous form. But it also can manifest with flaccid blisters that last only a short time, leaving the round, scaly lesions seen in this case.
Staphylococcus aureus, the organism responsible for bullous impetigo, elaborates serine proteases, which bind to and cleave desmoglein-1. This effectively destroys the connections between skin layers, creating a space that is quickly filled with serum. The level of this separation is typically subcorneal, which allows the formation of a very thin, friable roof for the bulla.
This modern version of impetigo is not considered dangerous, despite its association with staph aureus. In pre-antibiotic times, the predominant organism was streptococcal, some types of which could be nephritogenic—that is, capable of causing Bright disease or, as it is now known, acute post-streptococcal glomerulonephritis. Fortunately, this potentially fatal condition is only rarely seen in modern times.
Two items of note about this case: Bullous impetigo, contrary to what was seen in this patient, typically favors intertriginous areas. And a key factor is the history of atopy, which renders the patient more susceptible to skin infections of all kinds.
Treatment
The patient responded well to topical mupirocin ointment, applied three times a day. In rare instances, impetigo can require an oral antibiotic, such as cephalexin.
ANSWER
The correct answer is bullous impetigo (choice “a”).
Had the source of the problem been MRSA (choice “b”), there would have been marked tenderness and swelling. Psoriasis vulgaris (choice “c”) was a possibility; however, it almost never manifests with blisters, it rarely comes on as quickly as this patient’s problem did, and it produces scale that is far thicker and more tenacious than that seen in bullous impetigo. Nummular eczema (choice “d”) does not manifest with blisters and would likely have caused itching.
DISCUSSION
Impetigo is one of many common skin diseases—other examples include granuloma annulare and lichen planus—that have bullous variants, which can make diagnosis challenging. Impetigo is easy to diagnose in its more common papulosquamous form. But it also can manifest with flaccid blisters that last only a short time, leaving the round, scaly lesions seen in this case.
Staphylococcus aureus, the organism responsible for bullous impetigo, elaborates serine proteases, which bind to and cleave desmoglein-1. This effectively destroys the connections between skin layers, creating a space that is quickly filled with serum. The level of this separation is typically subcorneal, which allows the formation of a very thin, friable roof for the bulla.
This modern version of impetigo is not considered dangerous, despite its association with staph aureus. In pre-antibiotic times, the predominant organism was streptococcal, some types of which could be nephritogenic—that is, capable of causing Bright disease or, as it is now known, acute post-streptococcal glomerulonephritis. Fortunately, this potentially fatal condition is only rarely seen in modern times.
Two items of note about this case: Bullous impetigo, contrary to what was seen in this patient, typically favors intertriginous areas. And a key factor is the history of atopy, which renders the patient more susceptible to skin infections of all kinds.
Treatment
The patient responded well to topical mupirocin ointment, applied three times a day. In rare instances, impetigo can require an oral antibiotic, such as cephalexin.
The parents of a 6-year-old boy were quite concerned about several lesions on the child’s left knee. The asymptomatic blisters had first appeared about 2 weeks prior. A topical steroid cream prescribed by the family’s primary care provider had not helped.
No one else in the family was similarly affected. They all were reportedly quite healthy, although all were atopic—prone to seasonal allergies and eczema.
Examination revealed at least 6 round, scaly, red lesions on the patient’s knee, ranging from 3 mm to 3 cm in diameter. According to the parents, these had first appeared as intact blisters. There was little to no tenderness or redness around the lesions, and there was no palpable adenopathy in the groin.
The child was in no distress and was afebrile.
Is it time to change the definition of ‘fully vaccinated’?
As more indoor venues require proof of vaccination for entrance and with winter — as well as omicron, a new COVID variant — looming,
It’s been more than six months since many Americans finished their vaccination course against COVID; statistically, their immunity is waning.
At the same time, cases of infections with the Omicron variant have been reported in at least 17 states, as of Dec. 6. Omicron is distinguished by at least 50 mutations, some of which appear to be associated with increased transmissibility. The World Health Organization dubbed it a variant of concern on Nov. 26.
The Centers for Disease Control and Prevention has recommended that everyone 18 and older get a COVID booster shot, revising its narrower guidance that only people 50 and up “should” get a shot while younger adults could choose whether or not to do so. Scientists assume the additional shots will offer significant protection from the new variant, though they do not know for certain how much.
Anthony Fauci, MD, chief medical adviser to President Joe Biden, during a White House press briefing was unequivocal in advising the public. “Get boosted now,” Dr. Fauci said, adding urgency to the current federal guidance. About a quarter of U.S. adults have received additional vaccine doses.
“The definition of ‘fully vaccinated’ has not changed. That’s, you know, after your second dose of a Pfizer or Moderna vaccine, after your single dose of a Johnson & Johnson vaccine,” said the CDC’s director, Dr. Rochelle Walensky, during a Nov. 30 White House briefing on COVID. “We are absolutely encouraging those who are eligible for a boost six months after those mRNA doses to get your boost. But we are not changing the definition of ‘fully vaccinated’ right now.” A booster is recommended two months after receiving the J&J shot.
But that, she noted, could change: “As that science evolves, we will look at whether we need to update our definition of ‘fully vaccinated.’”
Still, the Democratic governors of Connecticut and New Mexico are sending a different signal in their states, as are some countries — such as Israel, which arguably has been the most aggressive nation in its approach. Some scientists point out that many vaccines involve three doses over six months for robust long-term protection, such as the shot against hepatitis. So “fully vaccinated” may need to include shot No. 3 to be considered a full course.
“In my view, if you were vaccinated more than six months ago, you’re not fully vaccinated,” Connecticut Gov. Ned Lamont said Nov. 18 during a press briefing. He was encouraging everyone to get boosted at that time, even before the federal government authorized extra shots for everyone.
New Mexico Gov. Michelle Lujan Grisham had a similar response in mid-November, saying she defined “fully vaccinated” as receiving three shots of the mRNA type. She also opened up booster eligibility to all of her state residents before the CDC and Food and Drug Administration did.
What do the varying views on the evolving science mean for vaccine requirements imposed on travelers, or by schools or workplaces? And what about businesses that have required patrons to provide proof of vaccination?
Dr. Paul Offit, director of the Vaccine Education Center at the Children’s Hospital of Pennsylvania, said the CDC’s stronger recommendation for everyone to get boosted signals to him that a booster is now part of the vaccine regimen. Yet Dr. Offit, who is also a member of the FDA’s vaccine advisory committee, wrote a joint op-ed this week in which he and two other scientists argued that boosters were not yet needed for everyone and that healthy young people should wait to see whether an Omicron-specific booster might be needed.
“I think when the CDC said they are recommending a third dose, they just made the statement that this is a three-dose vaccine series,” Dr. Offit told KHN. “And, frankly, I think it’s going to throw a wrench into mandates.”
Yet to be determined is whether restaurants or other places of business will look more closely at vaccine cards for the booster.
Dr. Georges Benjamin, executive director of the American Public Health Association, said it’s too early to say. “For now, businesses should stay focused on current guidelines,” he said.
Dr. Marc Siegel, an associate professor of medicine at the George Washington School of Medicine and Health Sciences, in Washington, said the question of whether you are fully vaccinated with just two doses or need a booster is a question of semantics. COVID immunity level is the more important issue.
Dr. Siegel said he thinks more suitable terminology would be to call someone “appropriately” or “adequately” vaccinated against COVID rather than “fully” vaccinated, since it’s possible that more boosters could be needed in the future — making “full vaccination” a moving target.
But, as with so many aspects of the pandemic, ambiguity prevails — both in federal guidance on the definition of “fully vaccinated” and in entrance policies, which vary by state, school and business.
Right now, businesses don’t appear to be checking for boosters, but that could change. So, it may be wise to first check the requirements — lest patrons present a two-shot vaccine passport, only to be turned away as inadequately protected.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
As more indoor venues require proof of vaccination for entrance and with winter — as well as omicron, a new COVID variant — looming,
It’s been more than six months since many Americans finished their vaccination course against COVID; statistically, their immunity is waning.
At the same time, cases of infections with the Omicron variant have been reported in at least 17 states, as of Dec. 6. Omicron is distinguished by at least 50 mutations, some of which appear to be associated with increased transmissibility. The World Health Organization dubbed it a variant of concern on Nov. 26.
The Centers for Disease Control and Prevention has recommended that everyone 18 and older get a COVID booster shot, revising its narrower guidance that only people 50 and up “should” get a shot while younger adults could choose whether or not to do so. Scientists assume the additional shots will offer significant protection from the new variant, though they do not know for certain how much.
Anthony Fauci, MD, chief medical adviser to President Joe Biden, during a White House press briefing was unequivocal in advising the public. “Get boosted now,” Dr. Fauci said, adding urgency to the current federal guidance. About a quarter of U.S. adults have received additional vaccine doses.
“The definition of ‘fully vaccinated’ has not changed. That’s, you know, after your second dose of a Pfizer or Moderna vaccine, after your single dose of a Johnson & Johnson vaccine,” said the CDC’s director, Dr. Rochelle Walensky, during a Nov. 30 White House briefing on COVID. “We are absolutely encouraging those who are eligible for a boost six months after those mRNA doses to get your boost. But we are not changing the definition of ‘fully vaccinated’ right now.” A booster is recommended two months after receiving the J&J shot.
But that, she noted, could change: “As that science evolves, we will look at whether we need to update our definition of ‘fully vaccinated.’”
Still, the Democratic governors of Connecticut and New Mexico are sending a different signal in their states, as are some countries — such as Israel, which arguably has been the most aggressive nation in its approach. Some scientists point out that many vaccines involve three doses over six months for robust long-term protection, such as the shot against hepatitis. So “fully vaccinated” may need to include shot No. 3 to be considered a full course.
“In my view, if you were vaccinated more than six months ago, you’re not fully vaccinated,” Connecticut Gov. Ned Lamont said Nov. 18 during a press briefing. He was encouraging everyone to get boosted at that time, even before the federal government authorized extra shots for everyone.
New Mexico Gov. Michelle Lujan Grisham had a similar response in mid-November, saying she defined “fully vaccinated” as receiving three shots of the mRNA type. She also opened up booster eligibility to all of her state residents before the CDC and Food and Drug Administration did.
What do the varying views on the evolving science mean for vaccine requirements imposed on travelers, or by schools or workplaces? And what about businesses that have required patrons to provide proof of vaccination?
Dr. Paul Offit, director of the Vaccine Education Center at the Children’s Hospital of Pennsylvania, said the CDC’s stronger recommendation for everyone to get boosted signals to him that a booster is now part of the vaccine regimen. Yet Dr. Offit, who is also a member of the FDA’s vaccine advisory committee, wrote a joint op-ed this week in which he and two other scientists argued that boosters were not yet needed for everyone and that healthy young people should wait to see whether an Omicron-specific booster might be needed.
“I think when the CDC said they are recommending a third dose, they just made the statement that this is a three-dose vaccine series,” Dr. Offit told KHN. “And, frankly, I think it’s going to throw a wrench into mandates.”
Yet to be determined is whether restaurants or other places of business will look more closely at vaccine cards for the booster.
Dr. Georges Benjamin, executive director of the American Public Health Association, said it’s too early to say. “For now, businesses should stay focused on current guidelines,” he said.
Dr. Marc Siegel, an associate professor of medicine at the George Washington School of Medicine and Health Sciences, in Washington, said the question of whether you are fully vaccinated with just two doses or need a booster is a question of semantics. COVID immunity level is the more important issue.
Dr. Siegel said he thinks more suitable terminology would be to call someone “appropriately” or “adequately” vaccinated against COVID rather than “fully” vaccinated, since it’s possible that more boosters could be needed in the future — making “full vaccination” a moving target.
But, as with so many aspects of the pandemic, ambiguity prevails — both in federal guidance on the definition of “fully vaccinated” and in entrance policies, which vary by state, school and business.
Right now, businesses don’t appear to be checking for boosters, but that could change. So, it may be wise to first check the requirements — lest patrons present a two-shot vaccine passport, only to be turned away as inadequately protected.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
As more indoor venues require proof of vaccination for entrance and with winter — as well as omicron, a new COVID variant — looming,
It’s been more than six months since many Americans finished their vaccination course against COVID; statistically, their immunity is waning.
At the same time, cases of infections with the Omicron variant have been reported in at least 17 states, as of Dec. 6. Omicron is distinguished by at least 50 mutations, some of which appear to be associated with increased transmissibility. The World Health Organization dubbed it a variant of concern on Nov. 26.
The Centers for Disease Control and Prevention has recommended that everyone 18 and older get a COVID booster shot, revising its narrower guidance that only people 50 and up “should” get a shot while younger adults could choose whether or not to do so. Scientists assume the additional shots will offer significant protection from the new variant, though they do not know for certain how much.
Anthony Fauci, MD, chief medical adviser to President Joe Biden, during a White House press briefing was unequivocal in advising the public. “Get boosted now,” Dr. Fauci said, adding urgency to the current federal guidance. About a quarter of U.S. adults have received additional vaccine doses.
“The definition of ‘fully vaccinated’ has not changed. That’s, you know, after your second dose of a Pfizer or Moderna vaccine, after your single dose of a Johnson & Johnson vaccine,” said the CDC’s director, Dr. Rochelle Walensky, during a Nov. 30 White House briefing on COVID. “We are absolutely encouraging those who are eligible for a boost six months after those mRNA doses to get your boost. But we are not changing the definition of ‘fully vaccinated’ right now.” A booster is recommended two months after receiving the J&J shot.
But that, she noted, could change: “As that science evolves, we will look at whether we need to update our definition of ‘fully vaccinated.’”
Still, the Democratic governors of Connecticut and New Mexico are sending a different signal in their states, as are some countries — such as Israel, which arguably has been the most aggressive nation in its approach. Some scientists point out that many vaccines involve three doses over six months for robust long-term protection, such as the shot against hepatitis. So “fully vaccinated” may need to include shot No. 3 to be considered a full course.
“In my view, if you were vaccinated more than six months ago, you’re not fully vaccinated,” Connecticut Gov. Ned Lamont said Nov. 18 during a press briefing. He was encouraging everyone to get boosted at that time, even before the federal government authorized extra shots for everyone.
New Mexico Gov. Michelle Lujan Grisham had a similar response in mid-November, saying she defined “fully vaccinated” as receiving three shots of the mRNA type. She also opened up booster eligibility to all of her state residents before the CDC and Food and Drug Administration did.
What do the varying views on the evolving science mean for vaccine requirements imposed on travelers, or by schools or workplaces? And what about businesses that have required patrons to provide proof of vaccination?
Dr. Paul Offit, director of the Vaccine Education Center at the Children’s Hospital of Pennsylvania, said the CDC’s stronger recommendation for everyone to get boosted signals to him that a booster is now part of the vaccine regimen. Yet Dr. Offit, who is also a member of the FDA’s vaccine advisory committee, wrote a joint op-ed this week in which he and two other scientists argued that boosters were not yet needed for everyone and that healthy young people should wait to see whether an Omicron-specific booster might be needed.
“I think when the CDC said they are recommending a third dose, they just made the statement that this is a three-dose vaccine series,” Dr. Offit told KHN. “And, frankly, I think it’s going to throw a wrench into mandates.”
Yet to be determined is whether restaurants or other places of business will look more closely at vaccine cards for the booster.
Dr. Georges Benjamin, executive director of the American Public Health Association, said it’s too early to say. “For now, businesses should stay focused on current guidelines,” he said.
Dr. Marc Siegel, an associate professor of medicine at the George Washington School of Medicine and Health Sciences, in Washington, said the question of whether you are fully vaccinated with just two doses or need a booster is a question of semantics. COVID immunity level is the more important issue.
Dr. Siegel said he thinks more suitable terminology would be to call someone “appropriately” or “adequately” vaccinated against COVID rather than “fully” vaccinated, since it’s possible that more boosters could be needed in the future — making “full vaccination” a moving target.
But, as with so many aspects of the pandemic, ambiguity prevails — both in federal guidance on the definition of “fully vaccinated” and in entrance policies, which vary by state, school and business.
Right now, businesses don’t appear to be checking for boosters, but that could change. So, it may be wise to first check the requirements — lest patrons present a two-shot vaccine passport, only to be turned away as inadequately protected.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Apixaban noninferior to low-molecular-weight heparin in cancer-associated VTE
Background: VTE is common in patients with cancer and can lead to serious complications and death. Relatively recently, the use of edoxaban or rivaroxaban was recommended by major guidelines for the treatment of cancer-associated VTE. Previous studies have demonstrated a higher risk of major bleeding when compared with low-molecular-weight heparin. Whether oral apixaban can be safely used in this setting is unknown.
Study design: Randomized, controlled, open-label, noninferiority clinical trial.
Setting: Multinational study with patients enrolled in nine European countries, Israel, and the United States.
Synopsis: Adult patients with confirmed cancer who had a new diagnosis of proximal lower-limb deep vein thrombosis or pulmonary embolism were enrolled in the trial. Of those enrolled, 1,170 patients underwent randomization to receive either oral apixaban twice daily or subcutaneous dalteparin once daily. The primary outcome was recurrent deep vein thrombosis or pulmonary embolism. The principal safety outcome was major bleeding. Researchers followed patients for 7 months after randomization. The primary outcome occurred in 32 of 576 patients (5.6%) in the apixaban group and 46 of 579 patients (7.9%) in the dalteparin group (hazard ratio, 0.63; 95% CI, 0.37-1.07). Major bleeding occurred in 22 patients (3.8%) in the apixaban group and 23 patients (4.0%) in the dalteparin group (HR, 0.82; 95% CI, 0.40-1.69). Limitations were the open-label trial design; the exclusion of patients with primary brain tumors, cerebral metastases, or acute leukemia; and the sample size being powered for the primary outcome, rather than to allow definitive conclusions about bleeding. Additionally, long-term data are needed as patients were followed for only 7 months.
Bottom line: Apixaban was noninferior to subcutaneous dalteparin for the treatment of VTE in patients with cancer and did not increase bleeding.
Citation: Agnelli G et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020 Apr 23;382:1599-607. doi: 10.1056/NEJMoa1915103.
Dr. Hermansen is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: VTE is common in patients with cancer and can lead to serious complications and death. Relatively recently, the use of edoxaban or rivaroxaban was recommended by major guidelines for the treatment of cancer-associated VTE. Previous studies have demonstrated a higher risk of major bleeding when compared with low-molecular-weight heparin. Whether oral apixaban can be safely used in this setting is unknown.
Study design: Randomized, controlled, open-label, noninferiority clinical trial.
Setting: Multinational study with patients enrolled in nine European countries, Israel, and the United States.
Synopsis: Adult patients with confirmed cancer who had a new diagnosis of proximal lower-limb deep vein thrombosis or pulmonary embolism were enrolled in the trial. Of those enrolled, 1,170 patients underwent randomization to receive either oral apixaban twice daily or subcutaneous dalteparin once daily. The primary outcome was recurrent deep vein thrombosis or pulmonary embolism. The principal safety outcome was major bleeding. Researchers followed patients for 7 months after randomization. The primary outcome occurred in 32 of 576 patients (5.6%) in the apixaban group and 46 of 579 patients (7.9%) in the dalteparin group (hazard ratio, 0.63; 95% CI, 0.37-1.07). Major bleeding occurred in 22 patients (3.8%) in the apixaban group and 23 patients (4.0%) in the dalteparin group (HR, 0.82; 95% CI, 0.40-1.69). Limitations were the open-label trial design; the exclusion of patients with primary brain tumors, cerebral metastases, or acute leukemia; and the sample size being powered for the primary outcome, rather than to allow definitive conclusions about bleeding. Additionally, long-term data are needed as patients were followed for only 7 months.
Bottom line: Apixaban was noninferior to subcutaneous dalteparin for the treatment of VTE in patients with cancer and did not increase bleeding.
Citation: Agnelli G et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020 Apr 23;382:1599-607. doi: 10.1056/NEJMoa1915103.
Dr. Hermansen is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: VTE is common in patients with cancer and can lead to serious complications and death. Relatively recently, the use of edoxaban or rivaroxaban was recommended by major guidelines for the treatment of cancer-associated VTE. Previous studies have demonstrated a higher risk of major bleeding when compared with low-molecular-weight heparin. Whether oral apixaban can be safely used in this setting is unknown.
Study design: Randomized, controlled, open-label, noninferiority clinical trial.
Setting: Multinational study with patients enrolled in nine European countries, Israel, and the United States.
Synopsis: Adult patients with confirmed cancer who had a new diagnosis of proximal lower-limb deep vein thrombosis or pulmonary embolism were enrolled in the trial. Of those enrolled, 1,170 patients underwent randomization to receive either oral apixaban twice daily or subcutaneous dalteparin once daily. The primary outcome was recurrent deep vein thrombosis or pulmonary embolism. The principal safety outcome was major bleeding. Researchers followed patients for 7 months after randomization. The primary outcome occurred in 32 of 576 patients (5.6%) in the apixaban group and 46 of 579 patients (7.9%) in the dalteparin group (hazard ratio, 0.63; 95% CI, 0.37-1.07). Major bleeding occurred in 22 patients (3.8%) in the apixaban group and 23 patients (4.0%) in the dalteparin group (HR, 0.82; 95% CI, 0.40-1.69). Limitations were the open-label trial design; the exclusion of patients with primary brain tumors, cerebral metastases, or acute leukemia; and the sample size being powered for the primary outcome, rather than to allow definitive conclusions about bleeding. Additionally, long-term data are needed as patients were followed for only 7 months.
Bottom line: Apixaban was noninferior to subcutaneous dalteparin for the treatment of VTE in patients with cancer and did not increase bleeding.
Citation: Agnelli G et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020 Apr 23;382:1599-607. doi: 10.1056/NEJMoa1915103.
Dr. Hermansen is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Seven legal risks of promoting unproven COVID-19 treatments
The emergence of COVID-19 has given the medical world a bewildering array of prevention and treatment protocols. Some physicians are advocating treatments that have not been validated by sound scientific studies. This has already led to licensing issues and other disciplinary actions being taken against physicians, pharmacies, and other health care providers across the country.
Medical professionals try their very best to give sound advice to patients. A medical license does not, however, confer immunity from being misled.
The supporting “science” for alternative prevention and treatments may look legitimate, but these claims are often based on anecdotal evidence. Some studies involve small populations, some are meta-analyses of several small or single-case studies, and others are not properly designed, interpreted, or executed in line with U.S. research and requirements. Yet others have been conducted only in nonhuman analogues, such as frogs or mice.
Many people are refusing a vaccine that has been proven to be relatively safe and effective in numerous repeated and validated studies in the best medical centers across the globe – all in favor of less validated alternatives. This can have serious legal consequences.
The crux of the issue
This is not a question of a physician’s first amendment rights. Nor is it a question of advocating for a scientifically valid minority medical opinion. The point of this article is that promoting unproven products, preventives, treatments, and cures can have dire consequences for licensed medical professionals.
On July 29, 2021, the Federation of State Medical Boards’ Board of Directors released a statement in response to a dramatic increase in the dissemination of COVID-19 vaccine misinformation and disinformation by physicians and other health care professionals on social media platforms, online, and in the media. The statement reads as follows:
“Physicians who generate and spread COVID-19 vaccine misinformation or disinformation are risking disciplinary action by state medical boards, including the suspension or revocation of their medical license. Due to their specialized knowledge and training, licensed physicians possess a high degree of public trust and therefore have a powerful platform in society, whether they recognize it or not. They also have an ethical and professional responsibility to practice medicine in the best interests of their patients and must share information that is factual, scientifically grounded, and consensus-driven for the betterment of public health. Spreading inaccurate COVID-19 vaccine information contradicts that responsibility, threatens to further erode public trust in the medical profession, and puts all patients at risk.”
What are the legal consequences?
Medical malpractice
The first consequence to consider is professional liability or medical malpractice. This applies if a patient claims harm as a result of the health care practitioner’s recommendation of an unproven treatment, product, or protocol. For example, strongly discouraging vaccination can result in a wrongful death claim if the patient follows the doctor’s advice, chooses not to vaccinate, contracts COVID-19, and does not recover. Recommending or providing unproven approaches and unapproved treatments is arguably a violation of the standard of care.
The standard of care is grounded in evidence-based medicine: It is commonly defined as the degree of care and skill that would be used by the average physician, who is practicing in his or her relevant specialty, under the same or similar circumstances, given the generally accepted medical knowledge at the time in question.
By way of example, one can see why inhaling peroxide, drinking bleach, or even taking Food and Drug Administration–approved medications that have little or no proven efficacy in treating or preventing COVID-19 is not what the average physician would advocate for under the same or similar circumstances, considering available and commonly accepted medical knowledge. Recommending or providing such treatments can be a breach of the standard of care and can form the basis of a medical malpractice action if, in fact, compensable harm has occurred.
In addition, recommending unproven and unapproved COVID-19 preventives and treatments without appropriate informed consent from patients is arguably also a breach of the standard of care. The claim would be that the patient has not been appropriately informed of the all the known benefits, risks, costs, and other legally required information such as proven efficacy and reasonably available alternatives.
In any event, physicians can rest assured that if a patient is harmed as a result of any of these situations, they’ll probably be answering to someone in the legal system.
Professional licensing action
Regardless of whether there is a medical malpractice action, there is still the potential for a patient complaint to be filed with the state licensing authority on the basis of the same facts and grounds. This can result in an investigation or an administrative complaint against the license of the health care provider.
This is not a mere potential risk. Licensing investigations are underway across the country. Disciplinary licensing actions have already taken place. For example, a Washington Medical Commission panel suspended the license of a physician assistant (PA) on Oct. 12, 2021, after an allegation that his treatment of COVID-19 patients fell below the standard of care. The PA allegedly began a public campaign promoting ivermectin as a curative agent for COVID-19 and prescribed it without adequate examination to at least one person, with no evidence from reliable clinical studies that establish its efficacy in preventing or treating COVID-19.
In licensing claims, alleged violations of failing to comply with the standard of care are usually asserted. These claims may also cite violations of other state statutes that encompass such concepts as negligence; breach of the duty of due care; incompetence; lack of good moral character; and lack of ability to serve the public in a fair, honest, and open manner. A licensing complaint may include alleged violations of statutes that address prescribing protocols, reckless endangerment, failure to supervise, and other issues.
The filing of an administrative complaint is a different animal from a medical malpractice action – they are not even in the same system or branch of government. The focus is not just about what happened to the one patient who complained; it is about protection of the public.
The states’ power to put a clinician on probation, condition, limit, suspend, or revoke the clinician’s license, as well as issue other sanctions such as physician monitoring and fines), is profound. The discipline imposed can upend a clinician’s career and potentially end it entirely.
Administrative discipline determinations are usually available to the public and are required to be reported to all employers (current and future). These discipline determinations are also sent to the National Practitioner Data Bank, other professional clearinghouse organizations (such as the Federation of State Medical Boards), state offices, professional liability insurers, payers with whom the clinician contracts, accreditation and certification organizations, and the clinician’s patients.
Discipline determinations must be promptly reported to licensing agencies in other states where the clinician holds a license, and often results in “sister state” actions because discipline was issued against the clinician in another state. It must be disclosed every time a clinician applies for hospital privileges or new employment. It can result in de-participation from health care insurance programs and can affect board certification, recertification, or accreditation for care programs in which the clinician participates.
In sum, licensing actions can be much worse than medical malpractice judgments and can have longer-term consequences.
Peer review and affected privileges
Recommending, promoting, and providing unapproved or unproven treatments, cures, or preventives to patients may violate hospital/health system, practice group, or surgical center bylaws. This can trigger the peer review process, which serves to improve patient safety and the quality of care.
The peer review process may be commenced because of a concern about the clinician’s compliance with the standard of care; potential patient safety issues; ethical issues; and the clinician’s stability, credibility, or professional competence. Any hospital disciplinary penalty is generally reported to state licensing authorities, which can trigger a licensing investigation. If clinical privileges are affected for a period of more than 30 days, the organization must report the situation to the National Practitioner Data Bank.
Criminal charges
Depending on the facts, a physician or other health care professional could be charged with reckless endangerment, criminal negligence, or manslaughter. If the clinician was assisting someone else who profited from that clinician’s actions, then we can look to a variety of potential federal and state fraud charges as well.
Conviction of a fraud-related felony may also lead to federal health care program and Centers for Medicare & Medicaid Services (CMS) exclusion for several years, and then CMS preclusion that can be imposed for years beyond the conclusion of the statutorily required exclusion.
Breach of contract
Some practice groups or other organizational employers have provisions in employment contracts that treat discipline for this type of conduct as a breach of contract. Because of this, the clinician committing breach may be subject to liquidated damages clauses, forfeiture of monies (such as bonuses or other incentives or rewards), termination of employment, forced withdrawal from ownership status, and being sued for breach of contract to recover damages.
Reputation/credibility damage and the attendant consequences
In regard to hospitals and health care system practice groups, another risk is the loss of referrals and revenue. Local media may air or publish exposés. Such stories may widely publicize the media’s version of the facts – true or not. This can cause immediate reputation and credibility damage within the community and may adversely affect a clinician’s patient base. Any information that is publicly broadcast might attract the attention of licensing and law enforcement authorities and taint potential jurors.
Hospitals and health care systems may pull privileges; post on websites; make official statements about the termination of affiliation; or denounce the clinician’s behavior, conduct, and beliefs as being inconsistent with quality care and patient safety. This causes further damage to a physician’s reputation and credibility.
In a group practice, accusations of this sort, licensing discipline, medical malpractice liability, investigations, loss of privileges, and the other sequelae of this conduct can force the withdrawal of the clinician as a member or shareholder in multiprovider groups. Adverse effects on the financial bottom line, patient referrals, and patient volume and bad press are often the basis for voting a clinician out.
Violation of the COVID-19 Consumer Protection Act of 2020
For the duration of the COVID-19 public health emergency, the FTC COVID-19 Consumer Protection Act makes it unlawful for any person, partnership, or corporation (as those terms are defined broadly in the act) to engage in a deceptive act or practice in or affecting commerce associated with the treatment, cure, prevention, mitigation, or diagnosis of COVID-19 or a government benefit related to COVID-19.
The first enforcement action authorized by this act took place in April 2021 against a chiropractor who promised vitamin treatments and cures for COVID-19. The act provides that such a violation shall be treated as a violation of a rule defining an unfair or deceptive act or practice prescribed under the FTC Act.
Under the act, the FTC is authorized to prescribe “rules that define with specificity acts or practices which are unfair or deceptive acts or practices in or affecting commerce.” Deceptive practices are defined as involving a material representation, omission, or practice that is “likely to mislead a consumer acting reasonably in the circumstances.” An act or practice is unfair if it “causes or is likely to cause substantial injury to consumers which is not reasonably avoidable by consumers themselves and not outweighed by countervailing benefits to consumers or to competition.”
After an investigation, the FTC may initiate an enforcement action using either an administrative or judicial process if it has “reason to believe” that the law has been violated. Violations of some laws may result in injunctive relief or civil monetary penalties, which are adjusted annually for inflation.
In addition, many states have deceptive and unfair trade laws that can be enforced in regard to the recommendation, sale, or provision of unproven or unapproved COVID-19 treatments, cures, and preventives as well.
Conclusion
It is difficult even for intelligent, well-intentioned physicians to know precisely what to believe and what to advocate for in the middle of a pandemic. It seems as though new reports and recommendations for preventing and treating COVID-19 are surfacing on a weekly basis. By far, the safest approach for any medical clinician to take is to advocate for positions that are generally accepted in the medical and scientific community at the time advice is given.
Mr. Whitelaw disclosed no relevant financial relationships. Ms. Janeway disclosed various associations with the Michigan Association for Healthcare Quality and the Greater Houston Society for Healthcare Risk Management. A version of this article first appeared on Medscape.com.
The emergence of COVID-19 has given the medical world a bewildering array of prevention and treatment protocols. Some physicians are advocating treatments that have not been validated by sound scientific studies. This has already led to licensing issues and other disciplinary actions being taken against physicians, pharmacies, and other health care providers across the country.
Medical professionals try their very best to give sound advice to patients. A medical license does not, however, confer immunity from being misled.
The supporting “science” for alternative prevention and treatments may look legitimate, but these claims are often based on anecdotal evidence. Some studies involve small populations, some are meta-analyses of several small or single-case studies, and others are not properly designed, interpreted, or executed in line with U.S. research and requirements. Yet others have been conducted only in nonhuman analogues, such as frogs or mice.
Many people are refusing a vaccine that has been proven to be relatively safe and effective in numerous repeated and validated studies in the best medical centers across the globe – all in favor of less validated alternatives. This can have serious legal consequences.
The crux of the issue
This is not a question of a physician’s first amendment rights. Nor is it a question of advocating for a scientifically valid minority medical opinion. The point of this article is that promoting unproven products, preventives, treatments, and cures can have dire consequences for licensed medical professionals.
On July 29, 2021, the Federation of State Medical Boards’ Board of Directors released a statement in response to a dramatic increase in the dissemination of COVID-19 vaccine misinformation and disinformation by physicians and other health care professionals on social media platforms, online, and in the media. The statement reads as follows:
“Physicians who generate and spread COVID-19 vaccine misinformation or disinformation are risking disciplinary action by state medical boards, including the suspension or revocation of their medical license. Due to their specialized knowledge and training, licensed physicians possess a high degree of public trust and therefore have a powerful platform in society, whether they recognize it or not. They also have an ethical and professional responsibility to practice medicine in the best interests of their patients and must share information that is factual, scientifically grounded, and consensus-driven for the betterment of public health. Spreading inaccurate COVID-19 vaccine information contradicts that responsibility, threatens to further erode public trust in the medical profession, and puts all patients at risk.”
What are the legal consequences?
Medical malpractice
The first consequence to consider is professional liability or medical malpractice. This applies if a patient claims harm as a result of the health care practitioner’s recommendation of an unproven treatment, product, or protocol. For example, strongly discouraging vaccination can result in a wrongful death claim if the patient follows the doctor’s advice, chooses not to vaccinate, contracts COVID-19, and does not recover. Recommending or providing unproven approaches and unapproved treatments is arguably a violation of the standard of care.
The standard of care is grounded in evidence-based medicine: It is commonly defined as the degree of care and skill that would be used by the average physician, who is practicing in his or her relevant specialty, under the same or similar circumstances, given the generally accepted medical knowledge at the time in question.
By way of example, one can see why inhaling peroxide, drinking bleach, or even taking Food and Drug Administration–approved medications that have little or no proven efficacy in treating or preventing COVID-19 is not what the average physician would advocate for under the same or similar circumstances, considering available and commonly accepted medical knowledge. Recommending or providing such treatments can be a breach of the standard of care and can form the basis of a medical malpractice action if, in fact, compensable harm has occurred.
In addition, recommending unproven and unapproved COVID-19 preventives and treatments without appropriate informed consent from patients is arguably also a breach of the standard of care. The claim would be that the patient has not been appropriately informed of the all the known benefits, risks, costs, and other legally required information such as proven efficacy and reasonably available alternatives.
In any event, physicians can rest assured that if a patient is harmed as a result of any of these situations, they’ll probably be answering to someone in the legal system.
Professional licensing action
Regardless of whether there is a medical malpractice action, there is still the potential for a patient complaint to be filed with the state licensing authority on the basis of the same facts and grounds. This can result in an investigation or an administrative complaint against the license of the health care provider.
This is not a mere potential risk. Licensing investigations are underway across the country. Disciplinary licensing actions have already taken place. For example, a Washington Medical Commission panel suspended the license of a physician assistant (PA) on Oct. 12, 2021, after an allegation that his treatment of COVID-19 patients fell below the standard of care. The PA allegedly began a public campaign promoting ivermectin as a curative agent for COVID-19 and prescribed it without adequate examination to at least one person, with no evidence from reliable clinical studies that establish its efficacy in preventing or treating COVID-19.
In licensing claims, alleged violations of failing to comply with the standard of care are usually asserted. These claims may also cite violations of other state statutes that encompass such concepts as negligence; breach of the duty of due care; incompetence; lack of good moral character; and lack of ability to serve the public in a fair, honest, and open manner. A licensing complaint may include alleged violations of statutes that address prescribing protocols, reckless endangerment, failure to supervise, and other issues.
The filing of an administrative complaint is a different animal from a medical malpractice action – they are not even in the same system or branch of government. The focus is not just about what happened to the one patient who complained; it is about protection of the public.
The states’ power to put a clinician on probation, condition, limit, suspend, or revoke the clinician’s license, as well as issue other sanctions such as physician monitoring and fines), is profound. The discipline imposed can upend a clinician’s career and potentially end it entirely.
Administrative discipline determinations are usually available to the public and are required to be reported to all employers (current and future). These discipline determinations are also sent to the National Practitioner Data Bank, other professional clearinghouse organizations (such as the Federation of State Medical Boards), state offices, professional liability insurers, payers with whom the clinician contracts, accreditation and certification organizations, and the clinician’s patients.
Discipline determinations must be promptly reported to licensing agencies in other states where the clinician holds a license, and often results in “sister state” actions because discipline was issued against the clinician in another state. It must be disclosed every time a clinician applies for hospital privileges or new employment. It can result in de-participation from health care insurance programs and can affect board certification, recertification, or accreditation for care programs in which the clinician participates.
In sum, licensing actions can be much worse than medical malpractice judgments and can have longer-term consequences.
Peer review and affected privileges
Recommending, promoting, and providing unapproved or unproven treatments, cures, or preventives to patients may violate hospital/health system, practice group, or surgical center bylaws. This can trigger the peer review process, which serves to improve patient safety and the quality of care.
The peer review process may be commenced because of a concern about the clinician’s compliance with the standard of care; potential patient safety issues; ethical issues; and the clinician’s stability, credibility, or professional competence. Any hospital disciplinary penalty is generally reported to state licensing authorities, which can trigger a licensing investigation. If clinical privileges are affected for a period of more than 30 days, the organization must report the situation to the National Practitioner Data Bank.
Criminal charges
Depending on the facts, a physician or other health care professional could be charged with reckless endangerment, criminal negligence, or manslaughter. If the clinician was assisting someone else who profited from that clinician’s actions, then we can look to a variety of potential federal and state fraud charges as well.
Conviction of a fraud-related felony may also lead to federal health care program and Centers for Medicare & Medicaid Services (CMS) exclusion for several years, and then CMS preclusion that can be imposed for years beyond the conclusion of the statutorily required exclusion.
Breach of contract
Some practice groups or other organizational employers have provisions in employment contracts that treat discipline for this type of conduct as a breach of contract. Because of this, the clinician committing breach may be subject to liquidated damages clauses, forfeiture of monies (such as bonuses or other incentives or rewards), termination of employment, forced withdrawal from ownership status, and being sued for breach of contract to recover damages.
Reputation/credibility damage and the attendant consequences
In regard to hospitals and health care system practice groups, another risk is the loss of referrals and revenue. Local media may air or publish exposés. Such stories may widely publicize the media’s version of the facts – true or not. This can cause immediate reputation and credibility damage within the community and may adversely affect a clinician’s patient base. Any information that is publicly broadcast might attract the attention of licensing and law enforcement authorities and taint potential jurors.
Hospitals and health care systems may pull privileges; post on websites; make official statements about the termination of affiliation; or denounce the clinician’s behavior, conduct, and beliefs as being inconsistent with quality care and patient safety. This causes further damage to a physician’s reputation and credibility.
In a group practice, accusations of this sort, licensing discipline, medical malpractice liability, investigations, loss of privileges, and the other sequelae of this conduct can force the withdrawal of the clinician as a member or shareholder in multiprovider groups. Adverse effects on the financial bottom line, patient referrals, and patient volume and bad press are often the basis for voting a clinician out.
Violation of the COVID-19 Consumer Protection Act of 2020
For the duration of the COVID-19 public health emergency, the FTC COVID-19 Consumer Protection Act makes it unlawful for any person, partnership, or corporation (as those terms are defined broadly in the act) to engage in a deceptive act or practice in or affecting commerce associated with the treatment, cure, prevention, mitigation, or diagnosis of COVID-19 or a government benefit related to COVID-19.
The first enforcement action authorized by this act took place in April 2021 against a chiropractor who promised vitamin treatments and cures for COVID-19. The act provides that such a violation shall be treated as a violation of a rule defining an unfair or deceptive act or practice prescribed under the FTC Act.
Under the act, the FTC is authorized to prescribe “rules that define with specificity acts or practices which are unfair or deceptive acts or practices in or affecting commerce.” Deceptive practices are defined as involving a material representation, omission, or practice that is “likely to mislead a consumer acting reasonably in the circumstances.” An act or practice is unfair if it “causes or is likely to cause substantial injury to consumers which is not reasonably avoidable by consumers themselves and not outweighed by countervailing benefits to consumers or to competition.”
After an investigation, the FTC may initiate an enforcement action using either an administrative or judicial process if it has “reason to believe” that the law has been violated. Violations of some laws may result in injunctive relief or civil monetary penalties, which are adjusted annually for inflation.
In addition, many states have deceptive and unfair trade laws that can be enforced in regard to the recommendation, sale, or provision of unproven or unapproved COVID-19 treatments, cures, and preventives as well.
Conclusion
It is difficult even for intelligent, well-intentioned physicians to know precisely what to believe and what to advocate for in the middle of a pandemic. It seems as though new reports and recommendations for preventing and treating COVID-19 are surfacing on a weekly basis. By far, the safest approach for any medical clinician to take is to advocate for positions that are generally accepted in the medical and scientific community at the time advice is given.
Mr. Whitelaw disclosed no relevant financial relationships. Ms. Janeway disclosed various associations with the Michigan Association for Healthcare Quality and the Greater Houston Society for Healthcare Risk Management. A version of this article first appeared on Medscape.com.
The emergence of COVID-19 has given the medical world a bewildering array of prevention and treatment protocols. Some physicians are advocating treatments that have not been validated by sound scientific studies. This has already led to licensing issues and other disciplinary actions being taken against physicians, pharmacies, and other health care providers across the country.
Medical professionals try their very best to give sound advice to patients. A medical license does not, however, confer immunity from being misled.
The supporting “science” for alternative prevention and treatments may look legitimate, but these claims are often based on anecdotal evidence. Some studies involve small populations, some are meta-analyses of several small or single-case studies, and others are not properly designed, interpreted, or executed in line with U.S. research and requirements. Yet others have been conducted only in nonhuman analogues, such as frogs or mice.
Many people are refusing a vaccine that has been proven to be relatively safe and effective in numerous repeated and validated studies in the best medical centers across the globe – all in favor of less validated alternatives. This can have serious legal consequences.
The crux of the issue
This is not a question of a physician’s first amendment rights. Nor is it a question of advocating for a scientifically valid minority medical opinion. The point of this article is that promoting unproven products, preventives, treatments, and cures can have dire consequences for licensed medical professionals.
On July 29, 2021, the Federation of State Medical Boards’ Board of Directors released a statement in response to a dramatic increase in the dissemination of COVID-19 vaccine misinformation and disinformation by physicians and other health care professionals on social media platforms, online, and in the media. The statement reads as follows:
“Physicians who generate and spread COVID-19 vaccine misinformation or disinformation are risking disciplinary action by state medical boards, including the suspension or revocation of their medical license. Due to their specialized knowledge and training, licensed physicians possess a high degree of public trust and therefore have a powerful platform in society, whether they recognize it or not. They also have an ethical and professional responsibility to practice medicine in the best interests of their patients and must share information that is factual, scientifically grounded, and consensus-driven for the betterment of public health. Spreading inaccurate COVID-19 vaccine information contradicts that responsibility, threatens to further erode public trust in the medical profession, and puts all patients at risk.”
What are the legal consequences?
Medical malpractice
The first consequence to consider is professional liability or medical malpractice. This applies if a patient claims harm as a result of the health care practitioner’s recommendation of an unproven treatment, product, or protocol. For example, strongly discouraging vaccination can result in a wrongful death claim if the patient follows the doctor’s advice, chooses not to vaccinate, contracts COVID-19, and does not recover. Recommending or providing unproven approaches and unapproved treatments is arguably a violation of the standard of care.
The standard of care is grounded in evidence-based medicine: It is commonly defined as the degree of care and skill that would be used by the average physician, who is practicing in his or her relevant specialty, under the same or similar circumstances, given the generally accepted medical knowledge at the time in question.
By way of example, one can see why inhaling peroxide, drinking bleach, or even taking Food and Drug Administration–approved medications that have little or no proven efficacy in treating or preventing COVID-19 is not what the average physician would advocate for under the same or similar circumstances, considering available and commonly accepted medical knowledge. Recommending or providing such treatments can be a breach of the standard of care and can form the basis of a medical malpractice action if, in fact, compensable harm has occurred.
In addition, recommending unproven and unapproved COVID-19 preventives and treatments without appropriate informed consent from patients is arguably also a breach of the standard of care. The claim would be that the patient has not been appropriately informed of the all the known benefits, risks, costs, and other legally required information such as proven efficacy and reasonably available alternatives.
In any event, physicians can rest assured that if a patient is harmed as a result of any of these situations, they’ll probably be answering to someone in the legal system.
Professional licensing action
Regardless of whether there is a medical malpractice action, there is still the potential for a patient complaint to be filed with the state licensing authority on the basis of the same facts and grounds. This can result in an investigation or an administrative complaint against the license of the health care provider.
This is not a mere potential risk. Licensing investigations are underway across the country. Disciplinary licensing actions have already taken place. For example, a Washington Medical Commission panel suspended the license of a physician assistant (PA) on Oct. 12, 2021, after an allegation that his treatment of COVID-19 patients fell below the standard of care. The PA allegedly began a public campaign promoting ivermectin as a curative agent for COVID-19 and prescribed it without adequate examination to at least one person, with no evidence from reliable clinical studies that establish its efficacy in preventing or treating COVID-19.
In licensing claims, alleged violations of failing to comply with the standard of care are usually asserted. These claims may also cite violations of other state statutes that encompass such concepts as negligence; breach of the duty of due care; incompetence; lack of good moral character; and lack of ability to serve the public in a fair, honest, and open manner. A licensing complaint may include alleged violations of statutes that address prescribing protocols, reckless endangerment, failure to supervise, and other issues.
The filing of an administrative complaint is a different animal from a medical malpractice action – they are not even in the same system or branch of government. The focus is not just about what happened to the one patient who complained; it is about protection of the public.
The states’ power to put a clinician on probation, condition, limit, suspend, or revoke the clinician’s license, as well as issue other sanctions such as physician monitoring and fines), is profound. The discipline imposed can upend a clinician’s career and potentially end it entirely.
Administrative discipline determinations are usually available to the public and are required to be reported to all employers (current and future). These discipline determinations are also sent to the National Practitioner Data Bank, other professional clearinghouse organizations (such as the Federation of State Medical Boards), state offices, professional liability insurers, payers with whom the clinician contracts, accreditation and certification organizations, and the clinician’s patients.
Discipline determinations must be promptly reported to licensing agencies in other states where the clinician holds a license, and often results in “sister state” actions because discipline was issued against the clinician in another state. It must be disclosed every time a clinician applies for hospital privileges or new employment. It can result in de-participation from health care insurance programs and can affect board certification, recertification, or accreditation for care programs in which the clinician participates.
In sum, licensing actions can be much worse than medical malpractice judgments and can have longer-term consequences.
Peer review and affected privileges
Recommending, promoting, and providing unapproved or unproven treatments, cures, or preventives to patients may violate hospital/health system, practice group, or surgical center bylaws. This can trigger the peer review process, which serves to improve patient safety and the quality of care.
The peer review process may be commenced because of a concern about the clinician’s compliance with the standard of care; potential patient safety issues; ethical issues; and the clinician’s stability, credibility, or professional competence. Any hospital disciplinary penalty is generally reported to state licensing authorities, which can trigger a licensing investigation. If clinical privileges are affected for a period of more than 30 days, the organization must report the situation to the National Practitioner Data Bank.
Criminal charges
Depending on the facts, a physician or other health care professional could be charged with reckless endangerment, criminal negligence, or manslaughter. If the clinician was assisting someone else who profited from that clinician’s actions, then we can look to a variety of potential federal and state fraud charges as well.
Conviction of a fraud-related felony may also lead to federal health care program and Centers for Medicare & Medicaid Services (CMS) exclusion for several years, and then CMS preclusion that can be imposed for years beyond the conclusion of the statutorily required exclusion.
Breach of contract
Some practice groups or other organizational employers have provisions in employment contracts that treat discipline for this type of conduct as a breach of contract. Because of this, the clinician committing breach may be subject to liquidated damages clauses, forfeiture of monies (such as bonuses or other incentives or rewards), termination of employment, forced withdrawal from ownership status, and being sued for breach of contract to recover damages.
Reputation/credibility damage and the attendant consequences
In regard to hospitals and health care system practice groups, another risk is the loss of referrals and revenue. Local media may air or publish exposés. Such stories may widely publicize the media’s version of the facts – true or not. This can cause immediate reputation and credibility damage within the community and may adversely affect a clinician’s patient base. Any information that is publicly broadcast might attract the attention of licensing and law enforcement authorities and taint potential jurors.
Hospitals and health care systems may pull privileges; post on websites; make official statements about the termination of affiliation; or denounce the clinician’s behavior, conduct, and beliefs as being inconsistent with quality care and patient safety. This causes further damage to a physician’s reputation and credibility.
In a group practice, accusations of this sort, licensing discipline, medical malpractice liability, investigations, loss of privileges, and the other sequelae of this conduct can force the withdrawal of the clinician as a member or shareholder in multiprovider groups. Adverse effects on the financial bottom line, patient referrals, and patient volume and bad press are often the basis for voting a clinician out.
Violation of the COVID-19 Consumer Protection Act of 2020
For the duration of the COVID-19 public health emergency, the FTC COVID-19 Consumer Protection Act makes it unlawful for any person, partnership, or corporation (as those terms are defined broadly in the act) to engage in a deceptive act or practice in or affecting commerce associated with the treatment, cure, prevention, mitigation, or diagnosis of COVID-19 or a government benefit related to COVID-19.
The first enforcement action authorized by this act took place in April 2021 against a chiropractor who promised vitamin treatments and cures for COVID-19. The act provides that such a violation shall be treated as a violation of a rule defining an unfair or deceptive act or practice prescribed under the FTC Act.
Under the act, the FTC is authorized to prescribe “rules that define with specificity acts or practices which are unfair or deceptive acts or practices in or affecting commerce.” Deceptive practices are defined as involving a material representation, omission, or practice that is “likely to mislead a consumer acting reasonably in the circumstances.” An act or practice is unfair if it “causes or is likely to cause substantial injury to consumers which is not reasonably avoidable by consumers themselves and not outweighed by countervailing benefits to consumers or to competition.”
After an investigation, the FTC may initiate an enforcement action using either an administrative or judicial process if it has “reason to believe” that the law has been violated. Violations of some laws may result in injunctive relief or civil monetary penalties, which are adjusted annually for inflation.
In addition, many states have deceptive and unfair trade laws that can be enforced in regard to the recommendation, sale, or provision of unproven or unapproved COVID-19 treatments, cures, and preventives as well.
Conclusion
It is difficult even for intelligent, well-intentioned physicians to know precisely what to believe and what to advocate for in the middle of a pandemic. It seems as though new reports and recommendations for preventing and treating COVID-19 are surfacing on a weekly basis. By far, the safest approach for any medical clinician to take is to advocate for positions that are generally accepted in the medical and scientific community at the time advice is given.
Mr. Whitelaw disclosed no relevant financial relationships. Ms. Janeway disclosed various associations with the Michigan Association for Healthcare Quality and the Greater Houston Society for Healthcare Risk Management. A version of this article first appeared on Medscape.com.