User login
Clinical Edge Journal Scan Commentary: PsA December 2021
Research published in November has provided us with insights on the impact of psoriatic arthritis (PsA) as well as treatment outcomes. Although PsA often affects women of child-bearing age, data on pregnancy outcomes in PsA is scarce. To evaluate pregnancy outcomes in patients with severe PsA, Remaeus et al1 conducted a Swedish nationwide register-based cohort study of births from Jul 1 2007 to Dec 31 2017. A total of 921 PsA- pregnancies and 9210 non-PsA-pregnancies (matched on maternal age, year, and parity) were identified. Pregnancy in PsA vs. non-PsA women were associated with increased risk for preterm birth (adjusted odds ratio [aOR] 1.69; 95% CI 1.27-2.24), elective cesarean delivery (CD; aOR 1.77; 95% CI 1.43-2.20), and emergency CD (aOR 1.42; 95% CI 1.10-1.84) with the risk even more pronounced in pregnancies in women with PsA with exposure to antirheumatic treatment any time before or during pregnancy (surrogate for disease severity- preterm birth: aOR 1.98; 95% CI 1.27-2.86; elective CD: aOR 1.96; 95% CI 1.47-2.63; and emergency CD: aOR 1.67; 95% CI 1.18-2.36). Thus, pregnant women with PsA, particularly those requiring antirheumatic treatment, are at increased risk for adverse pregnancy outcomes and therefore should be counselled appropriately.
Depression is a well-known comorbidity of PsA. However, little is known about the impact of the COVID-19 pandemic on the prevalence of depressive symptoms in PsA patients. Engelbrecht et al2 evaluated 89 patients with PsA participating in the German multicenter RheumaDatenRhePort registry. Symptoms of depression were assessed using the Patient Health Questionnaire-2 (PHQ-2). The majority of patients scored <2 on the PHQ-2 indicating that they did not have depressive symptoms during (85.39%) and prior to (83.15%) the pandemic. The prevalence of depressive symptoms was not significantly different before and during the pandemic, irrespective of disease activity. Thus, contrary to expectations, the COVID-19 pandemic did not increase the occurrence of depressive symptoms among patients with PsA.
With regard to longer-term treatment efficacy and safety of recently approved advanced therapies for PsA, McInnes et al reported 2-year results from the from the Phase-3 DISCOVER-2 trial that included 739 biologic-naive patients with active PsA. At week 100, ACR20 response was achieved by 76%, 74%, and 68% of patients who initially were randomized to receive guselkumab every 4 weeks, every 8 weeks, or placebo, respectively, indicating a durable response. No new safety signals were identified. The 56-week efficacy and safety results from SELECT-PsA 1 trial with upadacitinib reported by McInnes et al4,5 showed that of 1705 patients randomized, 1419 (83.2%) completed 56 weeks of treatment. A higher proportion of patients achieved ACR20 response with upadacitinib (15 mg, 74.4%; 30 mg, 74.7%) vs. adalimumab (68.5%; P = .046) at week 56. No new safety signals were identified.
Safety, especially risk of infection, remains a significant concern when treating patients with biologics, especially tumor necrosis factor inhibitors (TNFi). Patients with rheumatoid arthritis (RA) are known to have a higher risk of infection, but data are scarce regarding the risk of serious infections in patients with PsA treated with TNFi and the comparative risk of infection in TNFi-treated RA patients versus patients with PsA. Using data from 1,352 and 1,007 patients with RA and PsA, respectively, followed in the prospective multi-center NORwegian-Disease Modifying Anti-Rheumatic Drug (NOR-DMARD) registry, Christensen et al report that patients with PsA vs. RA had a lower risk of contracting serious infections (adjusted hazard ratio 0.65; P = .025).
References
- Remaeus K et al. Pregnancy outcomes in women with psoriatic arthritis with respect to presence and timing of antirheumatic treatment. Arthritis Rheumatol. 2021(Oct 20).
- Englbrecht M et al. Prevalence of depressive symptoms in patients with psoriatic arthritis: have numbers changed during the COVID-19 pandemic? Front Med (Lausanne). 2021(Nov 1);8:74826
- McInnes IB et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through 2 years: results from a phase 3, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arthritis Rheumatol. 2021(Nov 1).
- McInnes IB et al. Upadacitinib in patients with psoriatic arthritis and an inadequate response to non-biological therapy: 56-week data from the phase 3 SELECT-PsA 1 study. RMD Open. 2021;7:e001838 (Oct 18).
- Christensen IE et al. Serious infections in patients with rheumatoid arthritis and psoriatic arthritis treated with tumour necrosis factor inhibitors: data from register linkage of the NOR-DMARD study. Ann Rheum Dis. 2021(Oct 8). Correction: Upadacitinib in patients with psoriatic arthritis and an inadequate response to non-biological therapy: 56-week data from the phase 3 SELECT-PsA 1 study. RMD Open. 2021 Nov;7(3):e001838corr1.
Research published in November has provided us with insights on the impact of psoriatic arthritis (PsA) as well as treatment outcomes. Although PsA often affects women of child-bearing age, data on pregnancy outcomes in PsA is scarce. To evaluate pregnancy outcomes in patients with severe PsA, Remaeus et al1 conducted a Swedish nationwide register-based cohort study of births from Jul 1 2007 to Dec 31 2017. A total of 921 PsA- pregnancies and 9210 non-PsA-pregnancies (matched on maternal age, year, and parity) were identified. Pregnancy in PsA vs. non-PsA women were associated with increased risk for preterm birth (adjusted odds ratio [aOR] 1.69; 95% CI 1.27-2.24), elective cesarean delivery (CD; aOR 1.77; 95% CI 1.43-2.20), and emergency CD (aOR 1.42; 95% CI 1.10-1.84) with the risk even more pronounced in pregnancies in women with PsA with exposure to antirheumatic treatment any time before or during pregnancy (surrogate for disease severity- preterm birth: aOR 1.98; 95% CI 1.27-2.86; elective CD: aOR 1.96; 95% CI 1.47-2.63; and emergency CD: aOR 1.67; 95% CI 1.18-2.36). Thus, pregnant women with PsA, particularly those requiring antirheumatic treatment, are at increased risk for adverse pregnancy outcomes and therefore should be counselled appropriately.
Depression is a well-known comorbidity of PsA. However, little is known about the impact of the COVID-19 pandemic on the prevalence of depressive symptoms in PsA patients. Engelbrecht et al2 evaluated 89 patients with PsA participating in the German multicenter RheumaDatenRhePort registry. Symptoms of depression were assessed using the Patient Health Questionnaire-2 (PHQ-2). The majority of patients scored <2 on the PHQ-2 indicating that they did not have depressive symptoms during (85.39%) and prior to (83.15%) the pandemic. The prevalence of depressive symptoms was not significantly different before and during the pandemic, irrespective of disease activity. Thus, contrary to expectations, the COVID-19 pandemic did not increase the occurrence of depressive symptoms among patients with PsA.
With regard to longer-term treatment efficacy and safety of recently approved advanced therapies for PsA, McInnes et al reported 2-year results from the from the Phase-3 DISCOVER-2 trial that included 739 biologic-naive patients with active PsA. At week 100, ACR20 response was achieved by 76%, 74%, and 68% of patients who initially were randomized to receive guselkumab every 4 weeks, every 8 weeks, or placebo, respectively, indicating a durable response. No new safety signals were identified. The 56-week efficacy and safety results from SELECT-PsA 1 trial with upadacitinib reported by McInnes et al4,5 showed that of 1705 patients randomized, 1419 (83.2%) completed 56 weeks of treatment. A higher proportion of patients achieved ACR20 response with upadacitinib (15 mg, 74.4%; 30 mg, 74.7%) vs. adalimumab (68.5%; P = .046) at week 56. No new safety signals were identified.
Safety, especially risk of infection, remains a significant concern when treating patients with biologics, especially tumor necrosis factor inhibitors (TNFi). Patients with rheumatoid arthritis (RA) are known to have a higher risk of infection, but data are scarce regarding the risk of serious infections in patients with PsA treated with TNFi and the comparative risk of infection in TNFi-treated RA patients versus patients with PsA. Using data from 1,352 and 1,007 patients with RA and PsA, respectively, followed in the prospective multi-center NORwegian-Disease Modifying Anti-Rheumatic Drug (NOR-DMARD) registry, Christensen et al report that patients with PsA vs. RA had a lower risk of contracting serious infections (adjusted hazard ratio 0.65; P = .025).
References
- Remaeus K et al. Pregnancy outcomes in women with psoriatic arthritis with respect to presence and timing of antirheumatic treatment. Arthritis Rheumatol. 2021(Oct 20).
- Englbrecht M et al. Prevalence of depressive symptoms in patients with psoriatic arthritis: have numbers changed during the COVID-19 pandemic? Front Med (Lausanne). 2021(Nov 1);8:74826
- McInnes IB et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through 2 years: results from a phase 3, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arthritis Rheumatol. 2021(Nov 1).
- McInnes IB et al. Upadacitinib in patients with psoriatic arthritis and an inadequate response to non-biological therapy: 56-week data from the phase 3 SELECT-PsA 1 study. RMD Open. 2021;7:e001838 (Oct 18).
- Christensen IE et al. Serious infections in patients with rheumatoid arthritis and psoriatic arthritis treated with tumour necrosis factor inhibitors: data from register linkage of the NOR-DMARD study. Ann Rheum Dis. 2021(Oct 8). Correction: Upadacitinib in patients with psoriatic arthritis and an inadequate response to non-biological therapy: 56-week data from the phase 3 SELECT-PsA 1 study. RMD Open. 2021 Nov;7(3):e001838corr1.
Research published in November has provided us with insights on the impact of psoriatic arthritis (PsA) as well as treatment outcomes. Although PsA often affects women of child-bearing age, data on pregnancy outcomes in PsA is scarce. To evaluate pregnancy outcomes in patients with severe PsA, Remaeus et al1 conducted a Swedish nationwide register-based cohort study of births from Jul 1 2007 to Dec 31 2017. A total of 921 PsA- pregnancies and 9210 non-PsA-pregnancies (matched on maternal age, year, and parity) were identified. Pregnancy in PsA vs. non-PsA women were associated with increased risk for preterm birth (adjusted odds ratio [aOR] 1.69; 95% CI 1.27-2.24), elective cesarean delivery (CD; aOR 1.77; 95% CI 1.43-2.20), and emergency CD (aOR 1.42; 95% CI 1.10-1.84) with the risk even more pronounced in pregnancies in women with PsA with exposure to antirheumatic treatment any time before or during pregnancy (surrogate for disease severity- preterm birth: aOR 1.98; 95% CI 1.27-2.86; elective CD: aOR 1.96; 95% CI 1.47-2.63; and emergency CD: aOR 1.67; 95% CI 1.18-2.36). Thus, pregnant women with PsA, particularly those requiring antirheumatic treatment, are at increased risk for adverse pregnancy outcomes and therefore should be counselled appropriately.
Depression is a well-known comorbidity of PsA. However, little is known about the impact of the COVID-19 pandemic on the prevalence of depressive symptoms in PsA patients. Engelbrecht et al2 evaluated 89 patients with PsA participating in the German multicenter RheumaDatenRhePort registry. Symptoms of depression were assessed using the Patient Health Questionnaire-2 (PHQ-2). The majority of patients scored <2 on the PHQ-2 indicating that they did not have depressive symptoms during (85.39%) and prior to (83.15%) the pandemic. The prevalence of depressive symptoms was not significantly different before and during the pandemic, irrespective of disease activity. Thus, contrary to expectations, the COVID-19 pandemic did not increase the occurrence of depressive symptoms among patients with PsA.
With regard to longer-term treatment efficacy and safety of recently approved advanced therapies for PsA, McInnes et al reported 2-year results from the from the Phase-3 DISCOVER-2 trial that included 739 biologic-naive patients with active PsA. At week 100, ACR20 response was achieved by 76%, 74%, and 68% of patients who initially were randomized to receive guselkumab every 4 weeks, every 8 weeks, or placebo, respectively, indicating a durable response. No new safety signals were identified. The 56-week efficacy and safety results from SELECT-PsA 1 trial with upadacitinib reported by McInnes et al4,5 showed that of 1705 patients randomized, 1419 (83.2%) completed 56 weeks of treatment. A higher proportion of patients achieved ACR20 response with upadacitinib (15 mg, 74.4%; 30 mg, 74.7%) vs. adalimumab (68.5%; P = .046) at week 56. No new safety signals were identified.
Safety, especially risk of infection, remains a significant concern when treating patients with biologics, especially tumor necrosis factor inhibitors (TNFi). Patients with rheumatoid arthritis (RA) are known to have a higher risk of infection, but data are scarce regarding the risk of serious infections in patients with PsA treated with TNFi and the comparative risk of infection in TNFi-treated RA patients versus patients with PsA. Using data from 1,352 and 1,007 patients with RA and PsA, respectively, followed in the prospective multi-center NORwegian-Disease Modifying Anti-Rheumatic Drug (NOR-DMARD) registry, Christensen et al report that patients with PsA vs. RA had a lower risk of contracting serious infections (adjusted hazard ratio 0.65; P = .025).
References
- Remaeus K et al. Pregnancy outcomes in women with psoriatic arthritis with respect to presence and timing of antirheumatic treatment. Arthritis Rheumatol. 2021(Oct 20).
- Englbrecht M et al. Prevalence of depressive symptoms in patients with psoriatic arthritis: have numbers changed during the COVID-19 pandemic? Front Med (Lausanne). 2021(Nov 1);8:74826
- McInnes IB et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through 2 years: results from a phase 3, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arthritis Rheumatol. 2021(Nov 1).
- McInnes IB et al. Upadacitinib in patients with psoriatic arthritis and an inadequate response to non-biological therapy: 56-week data from the phase 3 SELECT-PsA 1 study. RMD Open. 2021;7:e001838 (Oct 18).
- Christensen IE et al. Serious infections in patients with rheumatoid arthritis and psoriatic arthritis treated with tumour necrosis factor inhibitors: data from register linkage of the NOR-DMARD study. Ann Rheum Dis. 2021(Oct 8). Correction: Upadacitinib in patients with psoriatic arthritis and an inadequate response to non-biological therapy: 56-week data from the phase 3 SELECT-PsA 1 study. RMD Open. 2021 Nov;7(3):e001838corr1.
Clinical Edge Journal Scan Commentary: CAP December 2021
The virtual elimination of Haemophilus influenzae type B as a respiratory pathogen, recognition of Mycoplasma pneumoniae as a common cause of pneumonia in older children and young adults, and atypical pathogens in elderly adults have recently changed our selection of initial empiric antimicrobial therapy for lower respiratory tract infections in some patients. It is increasingly important to use such information since narrow-spectrum antibiotics for empiric therapy of moderately severe community acquired pneumonia (CAP) should be standard therapy.1 On the other hand, the addition of doxycycline to a beta-lactam antibiotic has recently been shown to improve outcomes of CAP in elderly adults.2 Along with advanced age, male gender is also a risk factor for treatment failure of moderately severe CAP,3 so should be taken into consideration in management decisions.
If a patient with mild CAP does not respond to initial antibacterial therapy, the most likely explanation is a viral cause. Other bacterial causes might also be considered, such as Staphylococcus aureus, multi-resistant pneumococcus, coliforms, ampicillin-resistant H. influenzae, fungi, or anaerobes depending on clinical and laboratory factors.
New, rapid diagnostic tests are also useful in making clinical decisions and are particularly important for children who are unable to produce sputum for examination and whose small airways limit use of bronchoscopy. Recent studies have shown that heparin-binding protein (HBP) predicts disease progression in children with severe CAP, directing the physician to do further testing of microbiologic etiology.4
Treatment of pneumonia is usually empiric. If Chlamydia pneumoniae or M. pneumoniae is suspected as the responsible pathogen, azithromycin should be used as primary therapy. Quinolones or tetracycline-based antibiotics can be considered when macrolides are not tolerated. In children with community-acquired pneumonia (CAP) discharged from emergency departments or inpatient wards, findings from a trial including 814 children > 6 months old with CAP found that a lower dose and shorter course of amoxicillin was not inferior compared to higher doses and longer courses. The children were randomly assigned 1:1 after hospital discharge to receive one of the 4 possible combinations of amoxicillin dose (35-50 or 70-90 mg/kg) and duration (3 or 7 days). The results indicated that further outpatient treatment with amoxicillin at the lower dose was not inferior to a higher dose, and a 3-day treatment course was not inferior to a 7-day treatment course.5
Pneumococcal urinary antigen testing (PUAT) has recently been shown to direct narrow spectrum antibiotic therapy when positive in children or allow earlier de-escalation from broad spectrum antibiotics.6
When Staphylococcus aureus is suspected, methicillin resistance (MRSA) must be considered. Vancomycin has been standard therapy for this pathogen unless clindamycin susceptibility is documented. A recent study showed that the newer cephalosporin, ceftaroline, used as monotherapy or in combination with a macrolide or quinolone resulted in a lower hospital mortality rate than standard therapy with vancomycin or combination antibiotics.7
Other data used to determine probable causes of CAP include associated clinical signs and symptoms, chest x-ray findings, and diagnostic laboratory tests. Sputum is rarely produced by children during episodes of pneumonia, so the usual common step in the management of adult severe pneumonias, Gram stain examination of sputum is eliminated.
Antibiotics are selected primarily on the basis of age and severity of illness. Duration of therapy is 7 to 10 days for uncomplicated CAP. Once the causative agent is identified by culture or with one of the rapid antigen detection assays, specific therapy may be readily selected.
Treatment of pneumonia is usually empiric but the preference for narrow spectrum antibiotics should be emphasized .1 Amoxicillin for children and a quinolone for adults is the usual therapy.
If a patient with mild CAP does not respond to initial antibacterial therapy, the most likely explanation is a viral cause but for severely ill patients, other bacterial etiologies should also be considered, particularly MRSA where the addition of caftaroline would be considered.7
References
- Schweitzer VA et al. Narrow-spectrum antibiotics for community-acquired pneumonia in Dutch adults (CAP-PACT): a cross-sectional, stepped-wedge, cluster-randomised, non-inferiority, antimicrobial stewardship intervention trial. Lancet Infect Dis. 2021(Oct 7).
- Uddin M et al. Effectiveness of Beta-Lactam plus Doxycycline for Patients Hospitalized with Community-Acquired Pneumonia Clin Infect Dis. 2021;ciab863 (Nov 9).
- Dinh A et al. Factors Associated With Treatment Failure in Moderately Severe Community-Acquired Pneumonia: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw Open. 2021;4(10):e2129566 (Oct 15).
- Huang C et al. Heparin-Binding Protein in Critically Ill Children With Severe Community-Acquired Pneumonia. Front Pediatr. 2021 (Oct 28).
- Bielicki JA et al. Effect of Amoxicillin Dose and Treatment Duration on the Need for Antibiotic Re-treatment in Children With Community-Acquired Pneumonia: The CAP-IT Randomized Clinical Trial. JAMA. 2021;326(17):1713-1724 (Nov 2).
- Greenfield A et al. Impact of Streptococcus pneumoniae Urinary Antigen Testing in Patients with Community-acquired Pneumonia Admitted within a Large Academic Health System. Open Forum Infect Dis. 2021;ofab522 (Oct 22).
- Cilloniz C et al. Impact on in-hospital mortality of ceftaroline versus standard of care in community-acquired pneumonia: a propensity-matched analysis. Eur J Clin Microbiol Infect Dis. 2021 (Nov 12).
The virtual elimination of Haemophilus influenzae type B as a respiratory pathogen, recognition of Mycoplasma pneumoniae as a common cause of pneumonia in older children and young adults, and atypical pathogens in elderly adults have recently changed our selection of initial empiric antimicrobial therapy for lower respiratory tract infections in some patients. It is increasingly important to use such information since narrow-spectrum antibiotics for empiric therapy of moderately severe community acquired pneumonia (CAP) should be standard therapy.1 On the other hand, the addition of doxycycline to a beta-lactam antibiotic has recently been shown to improve outcomes of CAP in elderly adults.2 Along with advanced age, male gender is also a risk factor for treatment failure of moderately severe CAP,3 so should be taken into consideration in management decisions.
If a patient with mild CAP does not respond to initial antibacterial therapy, the most likely explanation is a viral cause. Other bacterial causes might also be considered, such as Staphylococcus aureus, multi-resistant pneumococcus, coliforms, ampicillin-resistant H. influenzae, fungi, or anaerobes depending on clinical and laboratory factors.
New, rapid diagnostic tests are also useful in making clinical decisions and are particularly important for children who are unable to produce sputum for examination and whose small airways limit use of bronchoscopy. Recent studies have shown that heparin-binding protein (HBP) predicts disease progression in children with severe CAP, directing the physician to do further testing of microbiologic etiology.4
Treatment of pneumonia is usually empiric. If Chlamydia pneumoniae or M. pneumoniae is suspected as the responsible pathogen, azithromycin should be used as primary therapy. Quinolones or tetracycline-based antibiotics can be considered when macrolides are not tolerated. In children with community-acquired pneumonia (CAP) discharged from emergency departments or inpatient wards, findings from a trial including 814 children > 6 months old with CAP found that a lower dose and shorter course of amoxicillin was not inferior compared to higher doses and longer courses. The children were randomly assigned 1:1 after hospital discharge to receive one of the 4 possible combinations of amoxicillin dose (35-50 or 70-90 mg/kg) and duration (3 or 7 days). The results indicated that further outpatient treatment with amoxicillin at the lower dose was not inferior to a higher dose, and a 3-day treatment course was not inferior to a 7-day treatment course.5
Pneumococcal urinary antigen testing (PUAT) has recently been shown to direct narrow spectrum antibiotic therapy when positive in children or allow earlier de-escalation from broad spectrum antibiotics.6
When Staphylococcus aureus is suspected, methicillin resistance (MRSA) must be considered. Vancomycin has been standard therapy for this pathogen unless clindamycin susceptibility is documented. A recent study showed that the newer cephalosporin, ceftaroline, used as monotherapy or in combination with a macrolide or quinolone resulted in a lower hospital mortality rate than standard therapy with vancomycin or combination antibiotics.7
Other data used to determine probable causes of CAP include associated clinical signs and symptoms, chest x-ray findings, and diagnostic laboratory tests. Sputum is rarely produced by children during episodes of pneumonia, so the usual common step in the management of adult severe pneumonias, Gram stain examination of sputum is eliminated.
Antibiotics are selected primarily on the basis of age and severity of illness. Duration of therapy is 7 to 10 days for uncomplicated CAP. Once the causative agent is identified by culture or with one of the rapid antigen detection assays, specific therapy may be readily selected.
Treatment of pneumonia is usually empiric but the preference for narrow spectrum antibiotics should be emphasized .1 Amoxicillin for children and a quinolone for adults is the usual therapy.
If a patient with mild CAP does not respond to initial antibacterial therapy, the most likely explanation is a viral cause but for severely ill patients, other bacterial etiologies should also be considered, particularly MRSA where the addition of caftaroline would be considered.7
References
- Schweitzer VA et al. Narrow-spectrum antibiotics for community-acquired pneumonia in Dutch adults (CAP-PACT): a cross-sectional, stepped-wedge, cluster-randomised, non-inferiority, antimicrobial stewardship intervention trial. Lancet Infect Dis. 2021(Oct 7).
- Uddin M et al. Effectiveness of Beta-Lactam plus Doxycycline for Patients Hospitalized with Community-Acquired Pneumonia Clin Infect Dis. 2021;ciab863 (Nov 9).
- Dinh A et al. Factors Associated With Treatment Failure in Moderately Severe Community-Acquired Pneumonia: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw Open. 2021;4(10):e2129566 (Oct 15).
- Huang C et al. Heparin-Binding Protein in Critically Ill Children With Severe Community-Acquired Pneumonia. Front Pediatr. 2021 (Oct 28).
- Bielicki JA et al. Effect of Amoxicillin Dose and Treatment Duration on the Need for Antibiotic Re-treatment in Children With Community-Acquired Pneumonia: The CAP-IT Randomized Clinical Trial. JAMA. 2021;326(17):1713-1724 (Nov 2).
- Greenfield A et al. Impact of Streptococcus pneumoniae Urinary Antigen Testing in Patients with Community-acquired Pneumonia Admitted within a Large Academic Health System. Open Forum Infect Dis. 2021;ofab522 (Oct 22).
- Cilloniz C et al. Impact on in-hospital mortality of ceftaroline versus standard of care in community-acquired pneumonia: a propensity-matched analysis. Eur J Clin Microbiol Infect Dis. 2021 (Nov 12).
The virtual elimination of Haemophilus influenzae type B as a respiratory pathogen, recognition of Mycoplasma pneumoniae as a common cause of pneumonia in older children and young adults, and atypical pathogens in elderly adults have recently changed our selection of initial empiric antimicrobial therapy for lower respiratory tract infections in some patients. It is increasingly important to use such information since narrow-spectrum antibiotics for empiric therapy of moderately severe community acquired pneumonia (CAP) should be standard therapy.1 On the other hand, the addition of doxycycline to a beta-lactam antibiotic has recently been shown to improve outcomes of CAP in elderly adults.2 Along with advanced age, male gender is also a risk factor for treatment failure of moderately severe CAP,3 so should be taken into consideration in management decisions.
If a patient with mild CAP does not respond to initial antibacterial therapy, the most likely explanation is a viral cause. Other bacterial causes might also be considered, such as Staphylococcus aureus, multi-resistant pneumococcus, coliforms, ampicillin-resistant H. influenzae, fungi, or anaerobes depending on clinical and laboratory factors.
New, rapid diagnostic tests are also useful in making clinical decisions and are particularly important for children who are unable to produce sputum for examination and whose small airways limit use of bronchoscopy. Recent studies have shown that heparin-binding protein (HBP) predicts disease progression in children with severe CAP, directing the physician to do further testing of microbiologic etiology.4
Treatment of pneumonia is usually empiric. If Chlamydia pneumoniae or M. pneumoniae is suspected as the responsible pathogen, azithromycin should be used as primary therapy. Quinolones or tetracycline-based antibiotics can be considered when macrolides are not tolerated. In children with community-acquired pneumonia (CAP) discharged from emergency departments or inpatient wards, findings from a trial including 814 children > 6 months old with CAP found that a lower dose and shorter course of amoxicillin was not inferior compared to higher doses and longer courses. The children were randomly assigned 1:1 after hospital discharge to receive one of the 4 possible combinations of amoxicillin dose (35-50 or 70-90 mg/kg) and duration (3 or 7 days). The results indicated that further outpatient treatment with amoxicillin at the lower dose was not inferior to a higher dose, and a 3-day treatment course was not inferior to a 7-day treatment course.5
Pneumococcal urinary antigen testing (PUAT) has recently been shown to direct narrow spectrum antibiotic therapy when positive in children or allow earlier de-escalation from broad spectrum antibiotics.6
When Staphylococcus aureus is suspected, methicillin resistance (MRSA) must be considered. Vancomycin has been standard therapy for this pathogen unless clindamycin susceptibility is documented. A recent study showed that the newer cephalosporin, ceftaroline, used as monotherapy or in combination with a macrolide or quinolone resulted in a lower hospital mortality rate than standard therapy with vancomycin or combination antibiotics.7
Other data used to determine probable causes of CAP include associated clinical signs and symptoms, chest x-ray findings, and diagnostic laboratory tests. Sputum is rarely produced by children during episodes of pneumonia, so the usual common step in the management of adult severe pneumonias, Gram stain examination of sputum is eliminated.
Antibiotics are selected primarily on the basis of age and severity of illness. Duration of therapy is 7 to 10 days for uncomplicated CAP. Once the causative agent is identified by culture or with one of the rapid antigen detection assays, specific therapy may be readily selected.
Treatment of pneumonia is usually empiric but the preference for narrow spectrum antibiotics should be emphasized .1 Amoxicillin for children and a quinolone for adults is the usual therapy.
If a patient with mild CAP does not respond to initial antibacterial therapy, the most likely explanation is a viral cause but for severely ill patients, other bacterial etiologies should also be considered, particularly MRSA where the addition of caftaroline would be considered.7
References
- Schweitzer VA et al. Narrow-spectrum antibiotics for community-acquired pneumonia in Dutch adults (CAP-PACT): a cross-sectional, stepped-wedge, cluster-randomised, non-inferiority, antimicrobial stewardship intervention trial. Lancet Infect Dis. 2021(Oct 7).
- Uddin M et al. Effectiveness of Beta-Lactam plus Doxycycline for Patients Hospitalized with Community-Acquired Pneumonia Clin Infect Dis. 2021;ciab863 (Nov 9).
- Dinh A et al. Factors Associated With Treatment Failure in Moderately Severe Community-Acquired Pneumonia: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw Open. 2021;4(10):e2129566 (Oct 15).
- Huang C et al. Heparin-Binding Protein in Critically Ill Children With Severe Community-Acquired Pneumonia. Front Pediatr. 2021 (Oct 28).
- Bielicki JA et al. Effect of Amoxicillin Dose and Treatment Duration on the Need for Antibiotic Re-treatment in Children With Community-Acquired Pneumonia: The CAP-IT Randomized Clinical Trial. JAMA. 2021;326(17):1713-1724 (Nov 2).
- Greenfield A et al. Impact of Streptococcus pneumoniae Urinary Antigen Testing in Patients with Community-acquired Pneumonia Admitted within a Large Academic Health System. Open Forum Infect Dis. 2021;ofab522 (Oct 22).
- Cilloniz C et al. Impact on in-hospital mortality of ceftaroline versus standard of care in community-acquired pneumonia: a propensity-matched analysis. Eur J Clin Microbiol Infect Dis. 2021 (Nov 12).
Boom in sports betting spurs new guidance on gambling disorder
Amid growing concerns about the impact of increased legalized online sports gambling, the American Psychiatric Association has issued an updated guide on gambling disorder.
The guide provides expert guidance based on current research and provides information on the etiology, psychopathology, neurobiology, and treatment of the disorder.
“For doctors who might think of gambling as either innocuous behavior or simply equivalent to, say, an alcohol problem, this guide not only shows the complexity and seriousness of gambling disorder but also evidence-based treatments that may help people actually get better,” the guide’s coeditor, Jon E. Grant, MD, MPH, JD, professor of psychiatry at the University of Chicago, said in an interview.
Online sports betting is booming. “It has really taken off” in recent years and is now a multibillion dollar industry worldwide, Dr. Grant added.
A recent CBS News report highlights a record volume of legally placed online sports bets during the first week of this year’s NFL season. All told, 26 states now have legalized sports betting.
said Dr. Grant. “They realized they could stay home, stay safe, and still gamble, so there was an uptick in that movement.”
However, the popularity of online gambling is also a sign of the times. “A whole generation of young adults have been raised on the Internet. A lot of companies realize this is not a market that would ever go to a land-based casino, so they essentially took their product to the young people,” said Dr. Grant.
Gambling meets technology
In addition to football, online gamblers can bet on other sports, including horse racing, or participate in “fantasy” sports where users assemble virtual teams of stand-ins for real professional players. There are also online casinos where users can play such things as blackjack and roulette.
The new guide devotes a chapter to online gambling and the complex interplay between gambling and technology. It highlights the growth of interactive platforms, the role of new player experiences and reward structures, and the integration into other online activities, such as social media.
Other chapters explore the interface between gambling and the legal system and differences in gender and between age groups.
There is also information on advances in treatments. Although there are no Food and Drug Administration–approved drugs for gambling disorder, new evidence supports the use of certain agents for this disorder, said Dr. Grant.
These include naltrexone, which has long been used for alcohol and drug addiction, and over-the-counter N-acetylcysteine (NAC), an amino acid that affects the reward system in the brain and has been used for cocaine and marijuana addiction.
Research also suggests that brief-format cognitive-behavioral therapy may be effective for gambling disorder, said Dr. Grant.
An estimated 1% of the population has such a disorder, which involves repeated, problem gambling with sufferers struggling to control their gambling behavior. Gambling disorder is associated with decreased self-esteem, comorbid substance abuse disorders, financial and legal difficulties, relationship and family stress, and suicidality.
Early intervention is key
Most gamblers don’t have a diagnosable disorder and can participate in the pastime without any long-term harm. However, some will show signs of problem gambling, Dr. Grant noted.
“We believe that’s where interventions may have an even bigger impact,” said Dr. Grant. “We want to get people early on in the illness.” He added that gambling “runs along a continuum” from simply dabbling to serious addiction.
Whereas previous versions of the DSM put gambling in an impulse control category, the latest version – DSM-5 – recognizes gambling as an addiction alongside substances.
“That shows greater awareness of the biological connection to substance addiction,” said Dr. Grant. “It’s important for clinicians who are screening substance use disorder folks to make sure they include gambling in that screening.”
The guide includes information on available screening and assessment instruments for diagnosing gambling disorder and for monitoring symptom changes.
Many clinicians may be unaware of the personal and social consequences of gambling disorder and its implications for public health. The new guide provides a detailed look at the effects of gambling on society and families, as well as on individual health and well-being.
A version of this article first appeared on Medscape.com.
Amid growing concerns about the impact of increased legalized online sports gambling, the American Psychiatric Association has issued an updated guide on gambling disorder.
The guide provides expert guidance based on current research and provides information on the etiology, psychopathology, neurobiology, and treatment of the disorder.
“For doctors who might think of gambling as either innocuous behavior or simply equivalent to, say, an alcohol problem, this guide not only shows the complexity and seriousness of gambling disorder but also evidence-based treatments that may help people actually get better,” the guide’s coeditor, Jon E. Grant, MD, MPH, JD, professor of psychiatry at the University of Chicago, said in an interview.
Online sports betting is booming. “It has really taken off” in recent years and is now a multibillion dollar industry worldwide, Dr. Grant added.
A recent CBS News report highlights a record volume of legally placed online sports bets during the first week of this year’s NFL season. All told, 26 states now have legalized sports betting.
said Dr. Grant. “They realized they could stay home, stay safe, and still gamble, so there was an uptick in that movement.”
However, the popularity of online gambling is also a sign of the times. “A whole generation of young adults have been raised on the Internet. A lot of companies realize this is not a market that would ever go to a land-based casino, so they essentially took their product to the young people,” said Dr. Grant.
Gambling meets technology
In addition to football, online gamblers can bet on other sports, including horse racing, or participate in “fantasy” sports where users assemble virtual teams of stand-ins for real professional players. There are also online casinos where users can play such things as blackjack and roulette.
The new guide devotes a chapter to online gambling and the complex interplay between gambling and technology. It highlights the growth of interactive platforms, the role of new player experiences and reward structures, and the integration into other online activities, such as social media.
Other chapters explore the interface between gambling and the legal system and differences in gender and between age groups.
There is also information on advances in treatments. Although there are no Food and Drug Administration–approved drugs for gambling disorder, new evidence supports the use of certain agents for this disorder, said Dr. Grant.
These include naltrexone, which has long been used for alcohol and drug addiction, and over-the-counter N-acetylcysteine (NAC), an amino acid that affects the reward system in the brain and has been used for cocaine and marijuana addiction.
Research also suggests that brief-format cognitive-behavioral therapy may be effective for gambling disorder, said Dr. Grant.
An estimated 1% of the population has such a disorder, which involves repeated, problem gambling with sufferers struggling to control their gambling behavior. Gambling disorder is associated with decreased self-esteem, comorbid substance abuse disorders, financial and legal difficulties, relationship and family stress, and suicidality.
Early intervention is key
Most gamblers don’t have a diagnosable disorder and can participate in the pastime without any long-term harm. However, some will show signs of problem gambling, Dr. Grant noted.
“We believe that’s where interventions may have an even bigger impact,” said Dr. Grant. “We want to get people early on in the illness.” He added that gambling “runs along a continuum” from simply dabbling to serious addiction.
Whereas previous versions of the DSM put gambling in an impulse control category, the latest version – DSM-5 – recognizes gambling as an addiction alongside substances.
“That shows greater awareness of the biological connection to substance addiction,” said Dr. Grant. “It’s important for clinicians who are screening substance use disorder folks to make sure they include gambling in that screening.”
The guide includes information on available screening and assessment instruments for diagnosing gambling disorder and for monitoring symptom changes.
Many clinicians may be unaware of the personal and social consequences of gambling disorder and its implications for public health. The new guide provides a detailed look at the effects of gambling on society and families, as well as on individual health and well-being.
A version of this article first appeared on Medscape.com.
Amid growing concerns about the impact of increased legalized online sports gambling, the American Psychiatric Association has issued an updated guide on gambling disorder.
The guide provides expert guidance based on current research and provides information on the etiology, psychopathology, neurobiology, and treatment of the disorder.
“For doctors who might think of gambling as either innocuous behavior or simply equivalent to, say, an alcohol problem, this guide not only shows the complexity and seriousness of gambling disorder but also evidence-based treatments that may help people actually get better,” the guide’s coeditor, Jon E. Grant, MD, MPH, JD, professor of psychiatry at the University of Chicago, said in an interview.
Online sports betting is booming. “It has really taken off” in recent years and is now a multibillion dollar industry worldwide, Dr. Grant added.
A recent CBS News report highlights a record volume of legally placed online sports bets during the first week of this year’s NFL season. All told, 26 states now have legalized sports betting.
said Dr. Grant. “They realized they could stay home, stay safe, and still gamble, so there was an uptick in that movement.”
However, the popularity of online gambling is also a sign of the times. “A whole generation of young adults have been raised on the Internet. A lot of companies realize this is not a market that would ever go to a land-based casino, so they essentially took their product to the young people,” said Dr. Grant.
Gambling meets technology
In addition to football, online gamblers can bet on other sports, including horse racing, or participate in “fantasy” sports where users assemble virtual teams of stand-ins for real professional players. There are also online casinos where users can play such things as blackjack and roulette.
The new guide devotes a chapter to online gambling and the complex interplay between gambling and technology. It highlights the growth of interactive platforms, the role of new player experiences and reward structures, and the integration into other online activities, such as social media.
Other chapters explore the interface between gambling and the legal system and differences in gender and between age groups.
There is also information on advances in treatments. Although there are no Food and Drug Administration–approved drugs for gambling disorder, new evidence supports the use of certain agents for this disorder, said Dr. Grant.
These include naltrexone, which has long been used for alcohol and drug addiction, and over-the-counter N-acetylcysteine (NAC), an amino acid that affects the reward system in the brain and has been used for cocaine and marijuana addiction.
Research also suggests that brief-format cognitive-behavioral therapy may be effective for gambling disorder, said Dr. Grant.
An estimated 1% of the population has such a disorder, which involves repeated, problem gambling with sufferers struggling to control their gambling behavior. Gambling disorder is associated with decreased self-esteem, comorbid substance abuse disorders, financial and legal difficulties, relationship and family stress, and suicidality.
Early intervention is key
Most gamblers don’t have a diagnosable disorder and can participate in the pastime without any long-term harm. However, some will show signs of problem gambling, Dr. Grant noted.
“We believe that’s where interventions may have an even bigger impact,” said Dr. Grant. “We want to get people early on in the illness.” He added that gambling “runs along a continuum” from simply dabbling to serious addiction.
Whereas previous versions of the DSM put gambling in an impulse control category, the latest version – DSM-5 – recognizes gambling as an addiction alongside substances.
“That shows greater awareness of the biological connection to substance addiction,” said Dr. Grant. “It’s important for clinicians who are screening substance use disorder folks to make sure they include gambling in that screening.”
The guide includes information on available screening and assessment instruments for diagnosing gambling disorder and for monitoring symptom changes.
Many clinicians may be unaware of the personal and social consequences of gambling disorder and its implications for public health. The new guide provides a detailed look at the effects of gambling on society and families, as well as on individual health and well-being.
A version of this article first appeared on Medscape.com.
Cardiologist positive for Omicron after London conference
Elad Maor, MD, an interventional cardiologist at Sheba Medical Centre near Tel Aviv, posted on Twitter on Nov. 30: “What a mess! Came back from a conference in London. With a mask and 3 Pfizer vaccines I managed to get Omicron.”
Dr. Maor traveled to London on November 19 to attend the PCR London Valves 2021 conference held at the ExCeL Centre Nov. 21-23. He stayed four nights at a hotel in north London and took public transport to and from the ExCeL Centre in East London each day of the meeting. He returned to Israel on the evening of Nov. 23.
Dr. Maor, 45, who has received three doses of the Pfizer COVID-19 vaccine, had two PCR tests in the United Kingdom – on November 20 and 21 in line with travel requirements – and another PCR test upon arriving back in Israel in the early hours of Nov. 24. All three tests were negative.
He began experiencing symptoms within days and tested positive on Nov. 27. His symptoms have been mild so far, and he said he was feeling “better” at the time of his tweet on Nov. 30.
Dr. Maor believes he was infected during his trip to London. “The only reasonable explanation is that I got infected on the last day of the meeting – maybe at the airport, maybe at the meeting,” he told The Guardian newspaper.
Although his wife accompanied him to London, neither she nor any of his 3 children have experienced symptoms or tested positive for COVID-19. But Dr. Maor believes he has passed the infection to a 69-year-old colleague in Israel who has since tested positive for the Omicron variant. The colleague, who has also received three vaccine doses, is understood to have mild symptoms at present.
The case suggests that the Omicron variant of COVID-19 may have been circulating in the United Kingdom earlier than previously thought.
Implications for in-person conferences
It will also inevitably lead to questions about the safety of face-to-face conferences, which are only just starting to get underway again.
The PCR Valves 2021 meeting had more than 1,250 on-site attendees as well as 2,400 or more joining online, according to figures on its website. Dr. Maor said he did not have any issues with the conference organizers, who required proof of vaccination before entry. But he posted a photograph on his Twitter account of a crowded auditorium with many delegates not wearing masks.
The conference subsequently posted an announcement on its website alerting delegates that one of the attendees had tested positive for COVID-19 after returning to their home country. It reads: “Since the reported case comes less than a week after the end of PCR London Valves, we want to inform you so that you may decide the best course of action, for yourself, if any.” It does not mention that the case was the Omicron variant.
Patrick Jolly, strategic and market development director of the conference, commented: “As you may imagine, the health, safety and well-being of everyone who visited PCR London Valves was our number-one priority. All protocols mandated by the U.K. government were put in place. Anyone entering the congress center had to present a valid health pass and were requested to wear a mask. Hydro-alcoholic gel and masks were made readily available for all participants and disposal bins for used protective equipment were provided.”
Mr. Jolly also noted: “To date – more than 9 days after the end of PCR London Valves – we have had no report of any other case of participants testing positive who attended PCR London Valves.”
He said the EuroPCR organization believes that medical conferences are safe to be held in person.
“With the above sanitary requirements and protocols, and no complacency in their enforcement, we believe strongly that medical conferences can take place, as the benefits of in-person medical conferences are obvious for the concerned medical communities,” Mr. Jolly added.
But what about other meetings happening imminently and planning in-person attendance?
Eileen Murray, executive director of the American Epilepsy Society (AES), whose annual 5-day meeting starts today at Chicago’s McCormick Place Convention Center, said in an interview that the health, safety, and well-being of everyone attending is a priority.
“Vaccinations are required, with no exceptions, to anyone attending the in-person event,” Ms. Murray said. “AES is using the CLEAR HealthPass to verify identity and vaccination status for our attendees. No one who cannot verify identity and vaccination requirement will be permitted to attend the in-person event.”
She noted that masks will also be required except in limited circumstances when actively eating or drinking, or for a faculty member when actively presenting at a lecture or panel. “Anyone not adhering to the mask policy will be asked to leave the meeting and will be denied readmission to the meeting with no refund,” she said.
“These guidelines were developed in accordance with the latest public health guidance and AES will continue to follow that guidance as any updates are made with the emergence of the Omicron variant,” Ms. Murray added.
Also commenting on this issue, a spokesperson for the American Heart Association, which has its large annual international stroke meeting planned for in-person attendance in New Orleans in February, said: “As we have throughout the pandemic, the American Heart Association is closely monitoring conditions and following the guidance of the CDC as well as state and local health departments related to all in-person meetings.”
“Our upcoming International Stroke Conference, February 9-11, is planned as an in-person and digital experience which allows us the ultimate flexibility to address changing pandemic conditions. The health, safety, and well-being of our volunteers, members, and attendees from around the world remains our number-one priority,” the AHA spokesperson added.
But some COVID-19 experts are taking a more cautious view.
Rowland Kao, PhD, an expert in infectious disease dynamics at the University of Edinburgh, United Kingdom, expressed concern about such large in-person conferences.
“We know that the Omicron variant appears to be spreading rapidly, with a recent preprint also telling us that the reinfection rate appears to be higher in South Africa. Should this be borne out, then the evidence would support that our reliance on a combination of vaccine-induced and natural immunity may be compromised by the Omicron variant,” he commented.
“We already know that extended contact indoors provides an additional risk, and so large meetings of this type have the potential to create extended risks. Until we know the extent to which Omicron causes severe illness, we should be extra cautious about these high-risk settings,” Dr. Kao commented.
A version of this article first appeared on Medscape.com.
Elad Maor, MD, an interventional cardiologist at Sheba Medical Centre near Tel Aviv, posted on Twitter on Nov. 30: “What a mess! Came back from a conference in London. With a mask and 3 Pfizer vaccines I managed to get Omicron.”
Dr. Maor traveled to London on November 19 to attend the PCR London Valves 2021 conference held at the ExCeL Centre Nov. 21-23. He stayed four nights at a hotel in north London and took public transport to and from the ExCeL Centre in East London each day of the meeting. He returned to Israel on the evening of Nov. 23.
Dr. Maor, 45, who has received three doses of the Pfizer COVID-19 vaccine, had two PCR tests in the United Kingdom – on November 20 and 21 in line with travel requirements – and another PCR test upon arriving back in Israel in the early hours of Nov. 24. All three tests were negative.
He began experiencing symptoms within days and tested positive on Nov. 27. His symptoms have been mild so far, and he said he was feeling “better” at the time of his tweet on Nov. 30.
Dr. Maor believes he was infected during his trip to London. “The only reasonable explanation is that I got infected on the last day of the meeting – maybe at the airport, maybe at the meeting,” he told The Guardian newspaper.
Although his wife accompanied him to London, neither she nor any of his 3 children have experienced symptoms or tested positive for COVID-19. But Dr. Maor believes he has passed the infection to a 69-year-old colleague in Israel who has since tested positive for the Omicron variant. The colleague, who has also received three vaccine doses, is understood to have mild symptoms at present.
The case suggests that the Omicron variant of COVID-19 may have been circulating in the United Kingdom earlier than previously thought.
Implications for in-person conferences
It will also inevitably lead to questions about the safety of face-to-face conferences, which are only just starting to get underway again.
The PCR Valves 2021 meeting had more than 1,250 on-site attendees as well as 2,400 or more joining online, according to figures on its website. Dr. Maor said he did not have any issues with the conference organizers, who required proof of vaccination before entry. But he posted a photograph on his Twitter account of a crowded auditorium with many delegates not wearing masks.
The conference subsequently posted an announcement on its website alerting delegates that one of the attendees had tested positive for COVID-19 after returning to their home country. It reads: “Since the reported case comes less than a week after the end of PCR London Valves, we want to inform you so that you may decide the best course of action, for yourself, if any.” It does not mention that the case was the Omicron variant.
Patrick Jolly, strategic and market development director of the conference, commented: “As you may imagine, the health, safety and well-being of everyone who visited PCR London Valves was our number-one priority. All protocols mandated by the U.K. government were put in place. Anyone entering the congress center had to present a valid health pass and were requested to wear a mask. Hydro-alcoholic gel and masks were made readily available for all participants and disposal bins for used protective equipment were provided.”
Mr. Jolly also noted: “To date – more than 9 days after the end of PCR London Valves – we have had no report of any other case of participants testing positive who attended PCR London Valves.”
He said the EuroPCR organization believes that medical conferences are safe to be held in person.
“With the above sanitary requirements and protocols, and no complacency in their enforcement, we believe strongly that medical conferences can take place, as the benefits of in-person medical conferences are obvious for the concerned medical communities,” Mr. Jolly added.
But what about other meetings happening imminently and planning in-person attendance?
Eileen Murray, executive director of the American Epilepsy Society (AES), whose annual 5-day meeting starts today at Chicago’s McCormick Place Convention Center, said in an interview that the health, safety, and well-being of everyone attending is a priority.
“Vaccinations are required, with no exceptions, to anyone attending the in-person event,” Ms. Murray said. “AES is using the CLEAR HealthPass to verify identity and vaccination status for our attendees. No one who cannot verify identity and vaccination requirement will be permitted to attend the in-person event.”
She noted that masks will also be required except in limited circumstances when actively eating or drinking, or for a faculty member when actively presenting at a lecture or panel. “Anyone not adhering to the mask policy will be asked to leave the meeting and will be denied readmission to the meeting with no refund,” she said.
“These guidelines were developed in accordance with the latest public health guidance and AES will continue to follow that guidance as any updates are made with the emergence of the Omicron variant,” Ms. Murray added.
Also commenting on this issue, a spokesperson for the American Heart Association, which has its large annual international stroke meeting planned for in-person attendance in New Orleans in February, said: “As we have throughout the pandemic, the American Heart Association is closely monitoring conditions and following the guidance of the CDC as well as state and local health departments related to all in-person meetings.”
“Our upcoming International Stroke Conference, February 9-11, is planned as an in-person and digital experience which allows us the ultimate flexibility to address changing pandemic conditions. The health, safety, and well-being of our volunteers, members, and attendees from around the world remains our number-one priority,” the AHA spokesperson added.
But some COVID-19 experts are taking a more cautious view.
Rowland Kao, PhD, an expert in infectious disease dynamics at the University of Edinburgh, United Kingdom, expressed concern about such large in-person conferences.
“We know that the Omicron variant appears to be spreading rapidly, with a recent preprint also telling us that the reinfection rate appears to be higher in South Africa. Should this be borne out, then the evidence would support that our reliance on a combination of vaccine-induced and natural immunity may be compromised by the Omicron variant,” he commented.
“We already know that extended contact indoors provides an additional risk, and so large meetings of this type have the potential to create extended risks. Until we know the extent to which Omicron causes severe illness, we should be extra cautious about these high-risk settings,” Dr. Kao commented.
A version of this article first appeared on Medscape.com.
Elad Maor, MD, an interventional cardiologist at Sheba Medical Centre near Tel Aviv, posted on Twitter on Nov. 30: “What a mess! Came back from a conference in London. With a mask and 3 Pfizer vaccines I managed to get Omicron.”
Dr. Maor traveled to London on November 19 to attend the PCR London Valves 2021 conference held at the ExCeL Centre Nov. 21-23. He stayed four nights at a hotel in north London and took public transport to and from the ExCeL Centre in East London each day of the meeting. He returned to Israel on the evening of Nov. 23.
Dr. Maor, 45, who has received three doses of the Pfizer COVID-19 vaccine, had two PCR tests in the United Kingdom – on November 20 and 21 in line with travel requirements – and another PCR test upon arriving back in Israel in the early hours of Nov. 24. All three tests were negative.
He began experiencing symptoms within days and tested positive on Nov. 27. His symptoms have been mild so far, and he said he was feeling “better” at the time of his tweet on Nov. 30.
Dr. Maor believes he was infected during his trip to London. “The only reasonable explanation is that I got infected on the last day of the meeting – maybe at the airport, maybe at the meeting,” he told The Guardian newspaper.
Although his wife accompanied him to London, neither she nor any of his 3 children have experienced symptoms or tested positive for COVID-19. But Dr. Maor believes he has passed the infection to a 69-year-old colleague in Israel who has since tested positive for the Omicron variant. The colleague, who has also received three vaccine doses, is understood to have mild symptoms at present.
The case suggests that the Omicron variant of COVID-19 may have been circulating in the United Kingdom earlier than previously thought.
Implications for in-person conferences
It will also inevitably lead to questions about the safety of face-to-face conferences, which are only just starting to get underway again.
The PCR Valves 2021 meeting had more than 1,250 on-site attendees as well as 2,400 or more joining online, according to figures on its website. Dr. Maor said he did not have any issues with the conference organizers, who required proof of vaccination before entry. But he posted a photograph on his Twitter account of a crowded auditorium with many delegates not wearing masks.
The conference subsequently posted an announcement on its website alerting delegates that one of the attendees had tested positive for COVID-19 after returning to their home country. It reads: “Since the reported case comes less than a week after the end of PCR London Valves, we want to inform you so that you may decide the best course of action, for yourself, if any.” It does not mention that the case was the Omicron variant.
Patrick Jolly, strategic and market development director of the conference, commented: “As you may imagine, the health, safety and well-being of everyone who visited PCR London Valves was our number-one priority. All protocols mandated by the U.K. government were put in place. Anyone entering the congress center had to present a valid health pass and were requested to wear a mask. Hydro-alcoholic gel and masks were made readily available for all participants and disposal bins for used protective equipment were provided.”
Mr. Jolly also noted: “To date – more than 9 days after the end of PCR London Valves – we have had no report of any other case of participants testing positive who attended PCR London Valves.”
He said the EuroPCR organization believes that medical conferences are safe to be held in person.
“With the above sanitary requirements and protocols, and no complacency in their enforcement, we believe strongly that medical conferences can take place, as the benefits of in-person medical conferences are obvious for the concerned medical communities,” Mr. Jolly added.
But what about other meetings happening imminently and planning in-person attendance?
Eileen Murray, executive director of the American Epilepsy Society (AES), whose annual 5-day meeting starts today at Chicago’s McCormick Place Convention Center, said in an interview that the health, safety, and well-being of everyone attending is a priority.
“Vaccinations are required, with no exceptions, to anyone attending the in-person event,” Ms. Murray said. “AES is using the CLEAR HealthPass to verify identity and vaccination status for our attendees. No one who cannot verify identity and vaccination requirement will be permitted to attend the in-person event.”
She noted that masks will also be required except in limited circumstances when actively eating or drinking, or for a faculty member when actively presenting at a lecture or panel. “Anyone not adhering to the mask policy will be asked to leave the meeting and will be denied readmission to the meeting with no refund,” she said.
“These guidelines were developed in accordance with the latest public health guidance and AES will continue to follow that guidance as any updates are made with the emergence of the Omicron variant,” Ms. Murray added.
Also commenting on this issue, a spokesperson for the American Heart Association, which has its large annual international stroke meeting planned for in-person attendance in New Orleans in February, said: “As we have throughout the pandemic, the American Heart Association is closely monitoring conditions and following the guidance of the CDC as well as state and local health departments related to all in-person meetings.”
“Our upcoming International Stroke Conference, February 9-11, is planned as an in-person and digital experience which allows us the ultimate flexibility to address changing pandemic conditions. The health, safety, and well-being of our volunteers, members, and attendees from around the world remains our number-one priority,” the AHA spokesperson added.
But some COVID-19 experts are taking a more cautious view.
Rowland Kao, PhD, an expert in infectious disease dynamics at the University of Edinburgh, United Kingdom, expressed concern about such large in-person conferences.
“We know that the Omicron variant appears to be spreading rapidly, with a recent preprint also telling us that the reinfection rate appears to be higher in South Africa. Should this be borne out, then the evidence would support that our reliance on a combination of vaccine-induced and natural immunity may be compromised by the Omicron variant,” he commented.
“We already know that extended contact indoors provides an additional risk, and so large meetings of this type have the potential to create extended risks. Until we know the extent to which Omicron causes severe illness, we should be extra cautious about these high-risk settings,” Dr. Kao commented.
A version of this article first appeared on Medscape.com.
Clinical Edge Journal Scan Commentary: COVID-19 December 2021
A second study answered a question I am often asked about neurological sequalae such as Guillain Barre syndrome among patients with COVID-19 infection, compared to risk of the same from vaccines. Patone et al linked country wide data from English National Immunisation (NIMS) Database of COVID-19 vaccinations with patient level data to examine incidence of neurological adverse events such acute central nervous system (CNS) demyelinating events, encephalitis meningitis and myelitis, Guillain–Barré syndrome, Bell’s palsy, myasthenic disorders, hemorrhagic stroke and subarachnoid hemorrhage in the 28 days following either having a positive SARS-CoV-2 test, or neither ChAdOx1nCoV-19 or BNT162b2 vaccines. The study reported increased incidence risk ratios (IRR) of hospitalization or death related to all of the aforementioned neurological events in patients with SARS-CoV-2 infection, particularly in the time right after diagnosis. There was a small increase in IRR for Guillain-Barre syndrome (IRR, 2.90; 95% confidence interval (CI): 2.15–3.92 at 15–21 days after vaccination) and Bell’s palsy (IRR, 1.29; 95% CI: 1.08–1.56 at 15–21 days) with ChAdOx1nCoV-19. However, this was lower than what was seen after a positive SARS-CoV-2 test (Guillain–Barré syndrome (IRR, 5.25; 95% CI: 3.00–9.18) and Bell’s Palsy, (IRR, 1.34; 95% CI: 0.91–1.97). There was a slightly increased association seen between hemorrhagic stroke and the first dose of BNT162b2, with IRR at 1–7 days (IRR, 1.27; 95% CI: 1.02–1.59) and 15–21 days (IRR, 1.38; 95% CI: 1.12–1.71). However, this risk was dwarfed compared to risk for hemorrhagic stroke seen up to 7 days after a positive SARS-CoV-2 test (IRR, 12.42; 95% CI: 7.73–19.95 at day 0; IRR, 2.01; 95% CI: 1.29–3.15 at 1–7 days). The results highlight immense increase of neurological events after SARS-CoV-2 infection.
Lastly, the RECOVERY trial reported out results of colchicine treatment arm, where 5,610 patients were assigned to standard of care (SOC) with colchicine compared to 5,730 who just received standard of care. The ongoing RECOVERY trial has been an incredibly power tool in helping identify both some effective treatments as well as shedding light on the limited utility of others. No significant differences were seen between the treatment and SOC only arms in all-cause mortality (rate ratio [RR], 1.01; P = .77), the probability of being discharged alive within 28 days (RR, 0.98; P = .44), or the risk of progressing to invasive mechanical ventilation or death (RR, 1.02; P = .47). The large sample size as well as the well-controlled design provides good evidence that colchicine will not make the COVID-19 treatment arsenal.
A second study answered a question I am often asked about neurological sequalae such as Guillain Barre syndrome among patients with COVID-19 infection, compared to risk of the same from vaccines. Patone et al linked country wide data from English National Immunisation (NIMS) Database of COVID-19 vaccinations with patient level data to examine incidence of neurological adverse events such acute central nervous system (CNS) demyelinating events, encephalitis meningitis and myelitis, Guillain–Barré syndrome, Bell’s palsy, myasthenic disorders, hemorrhagic stroke and subarachnoid hemorrhage in the 28 days following either having a positive SARS-CoV-2 test, or neither ChAdOx1nCoV-19 or BNT162b2 vaccines. The study reported increased incidence risk ratios (IRR) of hospitalization or death related to all of the aforementioned neurological events in patients with SARS-CoV-2 infection, particularly in the time right after diagnosis. There was a small increase in IRR for Guillain-Barre syndrome (IRR, 2.90; 95% confidence interval (CI): 2.15–3.92 at 15–21 days after vaccination) and Bell’s palsy (IRR, 1.29; 95% CI: 1.08–1.56 at 15–21 days) with ChAdOx1nCoV-19. However, this was lower than what was seen after a positive SARS-CoV-2 test (Guillain–Barré syndrome (IRR, 5.25; 95% CI: 3.00–9.18) and Bell’s Palsy, (IRR, 1.34; 95% CI: 0.91–1.97). There was a slightly increased association seen between hemorrhagic stroke and the first dose of BNT162b2, with IRR at 1–7 days (IRR, 1.27; 95% CI: 1.02–1.59) and 15–21 days (IRR, 1.38; 95% CI: 1.12–1.71). However, this risk was dwarfed compared to risk for hemorrhagic stroke seen up to 7 days after a positive SARS-CoV-2 test (IRR, 12.42; 95% CI: 7.73–19.95 at day 0; IRR, 2.01; 95% CI: 1.29–3.15 at 1–7 days). The results highlight immense increase of neurological events after SARS-CoV-2 infection.
Lastly, the RECOVERY trial reported out results of colchicine treatment arm, where 5,610 patients were assigned to standard of care (SOC) with colchicine compared to 5,730 who just received standard of care. The ongoing RECOVERY trial has been an incredibly power tool in helping identify both some effective treatments as well as shedding light on the limited utility of others. No significant differences were seen between the treatment and SOC only arms in all-cause mortality (rate ratio [RR], 1.01; P = .77), the probability of being discharged alive within 28 days (RR, 0.98; P = .44), or the risk of progressing to invasive mechanical ventilation or death (RR, 1.02; P = .47). The large sample size as well as the well-controlled design provides good evidence that colchicine will not make the COVID-19 treatment arsenal.
A second study answered a question I am often asked about neurological sequalae such as Guillain Barre syndrome among patients with COVID-19 infection, compared to risk of the same from vaccines. Patone et al linked country wide data from English National Immunisation (NIMS) Database of COVID-19 vaccinations with patient level data to examine incidence of neurological adverse events such acute central nervous system (CNS) demyelinating events, encephalitis meningitis and myelitis, Guillain–Barré syndrome, Bell’s palsy, myasthenic disorders, hemorrhagic stroke and subarachnoid hemorrhage in the 28 days following either having a positive SARS-CoV-2 test, or neither ChAdOx1nCoV-19 or BNT162b2 vaccines. The study reported increased incidence risk ratios (IRR) of hospitalization or death related to all of the aforementioned neurological events in patients with SARS-CoV-2 infection, particularly in the time right after diagnosis. There was a small increase in IRR for Guillain-Barre syndrome (IRR, 2.90; 95% confidence interval (CI): 2.15–3.92 at 15–21 days after vaccination) and Bell’s palsy (IRR, 1.29; 95% CI: 1.08–1.56 at 15–21 days) with ChAdOx1nCoV-19. However, this was lower than what was seen after a positive SARS-CoV-2 test (Guillain–Barré syndrome (IRR, 5.25; 95% CI: 3.00–9.18) and Bell’s Palsy, (IRR, 1.34; 95% CI: 0.91–1.97). There was a slightly increased association seen between hemorrhagic stroke and the first dose of BNT162b2, with IRR at 1–7 days (IRR, 1.27; 95% CI: 1.02–1.59) and 15–21 days (IRR, 1.38; 95% CI: 1.12–1.71). However, this risk was dwarfed compared to risk for hemorrhagic stroke seen up to 7 days after a positive SARS-CoV-2 test (IRR, 12.42; 95% CI: 7.73–19.95 at day 0; IRR, 2.01; 95% CI: 1.29–3.15 at 1–7 days). The results highlight immense increase of neurological events after SARS-CoV-2 infection.
Lastly, the RECOVERY trial reported out results of colchicine treatment arm, where 5,610 patients were assigned to standard of care (SOC) with colchicine compared to 5,730 who just received standard of care. The ongoing RECOVERY trial has been an incredibly power tool in helping identify both some effective treatments as well as shedding light on the limited utility of others. No significant differences were seen between the treatment and SOC only arms in all-cause mortality (rate ratio [RR], 1.01; P = .77), the probability of being discharged alive within 28 days (RR, 0.98; P = .44), or the risk of progressing to invasive mechanical ventilation or death (RR, 1.02; P = .47). The large sample size as well as the well-controlled design provides good evidence that colchicine will not make the COVID-19 treatment arsenal.
ASH meeting: Diversity, inclusion, immunotherapy, and COVID-19
In 2021, the American Society of Hematology will be hosting its annual meeting in a hybrid format. Content will be presented both live and in person at the Georgia World Congress Center in Atlanta and also online for those who can’t or don’t want to be there in person.
Inevitably during the ongoing pandemic, the meeting will contain key sessions on COVID-19 in hematology, including a plenary presentation outlining a biologic mechanism for the increased coagulopathy with SARS-CoV-2 infections.
In addition, there will be a scientific symposium on COVID-19 vaccination in immunocompromised patients and a special moderated session summarizing nine abstracts on the science of thrombosis in COVID-19, outcomes in patients with hematologic disease, and vaccine responses.
And speaking of COVID, lest anyone forget, annual meeting attendees will be required to be fully vaccinated and masked. Free COVID-19 testing will be available at stations situated throughout the convention center.
Diversifying care
chair of the ASH committee on communications and chief of the division of hematology at the Sylvester Comprehensive Cancer Center at the University of Miami.
For example, investigators at Massachusetts General Hospital in Boston will present new data on code-status transitions among patients with poor-prognosis high-risk acute myeloid leukemia (AML) who are approaching the end of life. Their findings suggest that physician-patient discussions about the goals of care may occur too late in the course of illness for many patients (abstract 109).
“While there have been many advances in the treatment of acute myeloid leukemia, and in fact there has been significant progress even among high-risk patients, addressing end-of-life issues is an often neglected area,” commented briefing participant Martin A. Tallman, MD, from Memorial Sloan Kettering Cancer Center, New York, who is also the current ASH president.
On a more upbeat note, Dr. Tallman also pointed to the results of the phase 3, randomized AGILE trial as an example of progress in AML, especially for patients with newly diagnosed high-risk disease who have mutations in IDH1. This trial investigated a new approach to treatment, with a combination of the combination of the IDH1 inhibitor ivosidenib (Tibsovo) and azacitidine, and compared it with azacitidine alone. The investigators assessed impact on event-free survival, overall survival, and clinical responses (abstract 697).
Dr. Tallman also highlighted abstracts touching on racial, social, and socioeconomic contributors to health care disparities among children with acute lymphoblastic leukemia (ALL; abstract 211) and on clinical trial enrollment characteristics and outcomes for Black and Hispanic adolescents and young adults with ALL (abstract 337).
Immunotherapy advances
Some of the most eagerly awaited abstracts will be highlighting advances in immunotherapy for hematologic malignancies, and these were previewed by Cynthia E. Dunbar, MD, ASH secretary and chief of the Translational Stem Cell Biology Branch within the Intramural Research Program of the National Heart, Lung, and Blood Institute in Bethesda, Md.
These abstracts include the primary analysis of the ZUMA-7 trial, a randomized, phase 3 study comparing the chimeric antigen receptor T-cell (CAR T) construct axicabtagene ciloleucel (axi-cel; Yescarta) with standard of care in patients with relapsed or refractory large B-cell lymphomas (LBCLs; abstract 2) and the interim analysis of the randomized, phase 3 Transform Study comparing the CAR T construct lisocabtagene maralecleucl (liso-cel; Breyanzi) with salvage chemotherapy in patients with relapsed/refractory LBCL (abstract 91).
“Over 500 patients were enrolled in the two studies, and both abstracts report significantly longer survival without relapse in the CAR T arm – for instance, fourfold higher in ZUMA-7, compared to standard of care,” Dr. Dunbar said at the briefing.
“These abstracts provide really critical information to patients, their treating physicians, and the payers who are trying to decide whether use of these expensive, complex, and potentially toxic CAR T-cell therapies are justified, compared to standard therapy,” she said.
Dr. Dunbar also highlighted an abstract on the addition of the anti-CD38 monoclonal antibody isatuximab (Sarclisa) to lenalidomide, bortezomib, and dexamethasone as induction therapy for patients with newly diagnosed multiple myeloma who are eligible for stem cell transplantation (abstract 463).
“The authors report that patients on the isatuximab arm had significantly fewer tumor cells following treatment,” Dr. Dunbar said. “We have come a long way beyond treating myeloma with a single drug, with remissions now measured in many years instead of 1 or 2 following initiation of treatment, and this abstract is another demonstration that novel combinations of multiple agents are really making a difference in this very debilitating disease.”
She also cited an abstract (abstract 127) on monotherapy with the novel bispecific T-cell–engaging monoclonal antibody mosunetuzumab for treatment of patients with follicular lymphoma that has relapsed or is refractory to at least two prior lines of therapy.
Old disorders, new insights
Other abstracts highlighted at the premeeting press briefing included a study that found a high prevalence of monoclonal gammopathy in persons at risk for multiple myeloma (abstract 152) and another with the surprising finding that clonal hematopoiesis, a risk factor myeloid malignancies, may be protective against Alzheimer’s disease (abstract 5).
In addition, a long-term follow-up study of patients with transfusion-dependent beta-thalassemia treated with gene therapy showed that some patients have become transfusion independent and iron homeostasis was restored (abstract 573).
Presentations from CDC and FDA
Dr. Sekeres highlighted other events of interest scheduled for ASH 2021, including a Grassroots Network Lunch featuring a discussion with Rochelle Walensky, MD, MPH, director of the Centers for Disease Control and Prevention in Atlanta, and a joint symposium between ASH and the Food and Drug Administration on newly approved drugs in hematology.
Dr. Sekeres has disclosed consulting/advising for Novartis, Takea/Millennium, and Bristol-Myers Squibb. Dr. Dunbar reported no relevant conflicts of interest. Dr. Tallman disclosed consulting/advising with and research funding from multiple entities.
A version of this article first appeared on Medscape.com.
In 2021, the American Society of Hematology will be hosting its annual meeting in a hybrid format. Content will be presented both live and in person at the Georgia World Congress Center in Atlanta and also online for those who can’t or don’t want to be there in person.
Inevitably during the ongoing pandemic, the meeting will contain key sessions on COVID-19 in hematology, including a plenary presentation outlining a biologic mechanism for the increased coagulopathy with SARS-CoV-2 infections.
In addition, there will be a scientific symposium on COVID-19 vaccination in immunocompromised patients and a special moderated session summarizing nine abstracts on the science of thrombosis in COVID-19, outcomes in patients with hematologic disease, and vaccine responses.
And speaking of COVID, lest anyone forget, annual meeting attendees will be required to be fully vaccinated and masked. Free COVID-19 testing will be available at stations situated throughout the convention center.
Diversifying care
chair of the ASH committee on communications and chief of the division of hematology at the Sylvester Comprehensive Cancer Center at the University of Miami.
For example, investigators at Massachusetts General Hospital in Boston will present new data on code-status transitions among patients with poor-prognosis high-risk acute myeloid leukemia (AML) who are approaching the end of life. Their findings suggest that physician-patient discussions about the goals of care may occur too late in the course of illness for many patients (abstract 109).
“While there have been many advances in the treatment of acute myeloid leukemia, and in fact there has been significant progress even among high-risk patients, addressing end-of-life issues is an often neglected area,” commented briefing participant Martin A. Tallman, MD, from Memorial Sloan Kettering Cancer Center, New York, who is also the current ASH president.
On a more upbeat note, Dr. Tallman also pointed to the results of the phase 3, randomized AGILE trial as an example of progress in AML, especially for patients with newly diagnosed high-risk disease who have mutations in IDH1. This trial investigated a new approach to treatment, with a combination of the combination of the IDH1 inhibitor ivosidenib (Tibsovo) and azacitidine, and compared it with azacitidine alone. The investigators assessed impact on event-free survival, overall survival, and clinical responses (abstract 697).
Dr. Tallman also highlighted abstracts touching on racial, social, and socioeconomic contributors to health care disparities among children with acute lymphoblastic leukemia (ALL; abstract 211) and on clinical trial enrollment characteristics and outcomes for Black and Hispanic adolescents and young adults with ALL (abstract 337).
Immunotherapy advances
Some of the most eagerly awaited abstracts will be highlighting advances in immunotherapy for hematologic malignancies, and these were previewed by Cynthia E. Dunbar, MD, ASH secretary and chief of the Translational Stem Cell Biology Branch within the Intramural Research Program of the National Heart, Lung, and Blood Institute in Bethesda, Md.
These abstracts include the primary analysis of the ZUMA-7 trial, a randomized, phase 3 study comparing the chimeric antigen receptor T-cell (CAR T) construct axicabtagene ciloleucel (axi-cel; Yescarta) with standard of care in patients with relapsed or refractory large B-cell lymphomas (LBCLs; abstract 2) and the interim analysis of the randomized, phase 3 Transform Study comparing the CAR T construct lisocabtagene maralecleucl (liso-cel; Breyanzi) with salvage chemotherapy in patients with relapsed/refractory LBCL (abstract 91).
“Over 500 patients were enrolled in the two studies, and both abstracts report significantly longer survival without relapse in the CAR T arm – for instance, fourfold higher in ZUMA-7, compared to standard of care,” Dr. Dunbar said at the briefing.
“These abstracts provide really critical information to patients, their treating physicians, and the payers who are trying to decide whether use of these expensive, complex, and potentially toxic CAR T-cell therapies are justified, compared to standard therapy,” she said.
Dr. Dunbar also highlighted an abstract on the addition of the anti-CD38 monoclonal antibody isatuximab (Sarclisa) to lenalidomide, bortezomib, and dexamethasone as induction therapy for patients with newly diagnosed multiple myeloma who are eligible for stem cell transplantation (abstract 463).
“The authors report that patients on the isatuximab arm had significantly fewer tumor cells following treatment,” Dr. Dunbar said. “We have come a long way beyond treating myeloma with a single drug, with remissions now measured in many years instead of 1 or 2 following initiation of treatment, and this abstract is another demonstration that novel combinations of multiple agents are really making a difference in this very debilitating disease.”
She also cited an abstract (abstract 127) on monotherapy with the novel bispecific T-cell–engaging monoclonal antibody mosunetuzumab for treatment of patients with follicular lymphoma that has relapsed or is refractory to at least two prior lines of therapy.
Old disorders, new insights
Other abstracts highlighted at the premeeting press briefing included a study that found a high prevalence of monoclonal gammopathy in persons at risk for multiple myeloma (abstract 152) and another with the surprising finding that clonal hematopoiesis, a risk factor myeloid malignancies, may be protective against Alzheimer’s disease (abstract 5).
In addition, a long-term follow-up study of patients with transfusion-dependent beta-thalassemia treated with gene therapy showed that some patients have become transfusion independent and iron homeostasis was restored (abstract 573).
Presentations from CDC and FDA
Dr. Sekeres highlighted other events of interest scheduled for ASH 2021, including a Grassroots Network Lunch featuring a discussion with Rochelle Walensky, MD, MPH, director of the Centers for Disease Control and Prevention in Atlanta, and a joint symposium between ASH and the Food and Drug Administration on newly approved drugs in hematology.
Dr. Sekeres has disclosed consulting/advising for Novartis, Takea/Millennium, and Bristol-Myers Squibb. Dr. Dunbar reported no relevant conflicts of interest. Dr. Tallman disclosed consulting/advising with and research funding from multiple entities.
A version of this article first appeared on Medscape.com.
In 2021, the American Society of Hematology will be hosting its annual meeting in a hybrid format. Content will be presented both live and in person at the Georgia World Congress Center in Atlanta and also online for those who can’t or don’t want to be there in person.
Inevitably during the ongoing pandemic, the meeting will contain key sessions on COVID-19 in hematology, including a plenary presentation outlining a biologic mechanism for the increased coagulopathy with SARS-CoV-2 infections.
In addition, there will be a scientific symposium on COVID-19 vaccination in immunocompromised patients and a special moderated session summarizing nine abstracts on the science of thrombosis in COVID-19, outcomes in patients with hematologic disease, and vaccine responses.
And speaking of COVID, lest anyone forget, annual meeting attendees will be required to be fully vaccinated and masked. Free COVID-19 testing will be available at stations situated throughout the convention center.
Diversifying care
chair of the ASH committee on communications and chief of the division of hematology at the Sylvester Comprehensive Cancer Center at the University of Miami.
For example, investigators at Massachusetts General Hospital in Boston will present new data on code-status transitions among patients with poor-prognosis high-risk acute myeloid leukemia (AML) who are approaching the end of life. Their findings suggest that physician-patient discussions about the goals of care may occur too late in the course of illness for many patients (abstract 109).
“While there have been many advances in the treatment of acute myeloid leukemia, and in fact there has been significant progress even among high-risk patients, addressing end-of-life issues is an often neglected area,” commented briefing participant Martin A. Tallman, MD, from Memorial Sloan Kettering Cancer Center, New York, who is also the current ASH president.
On a more upbeat note, Dr. Tallman also pointed to the results of the phase 3, randomized AGILE trial as an example of progress in AML, especially for patients with newly diagnosed high-risk disease who have mutations in IDH1. This trial investigated a new approach to treatment, with a combination of the combination of the IDH1 inhibitor ivosidenib (Tibsovo) and azacitidine, and compared it with azacitidine alone. The investigators assessed impact on event-free survival, overall survival, and clinical responses (abstract 697).
Dr. Tallman also highlighted abstracts touching on racial, social, and socioeconomic contributors to health care disparities among children with acute lymphoblastic leukemia (ALL; abstract 211) and on clinical trial enrollment characteristics and outcomes for Black and Hispanic adolescents and young adults with ALL (abstract 337).
Immunotherapy advances
Some of the most eagerly awaited abstracts will be highlighting advances in immunotherapy for hematologic malignancies, and these were previewed by Cynthia E. Dunbar, MD, ASH secretary and chief of the Translational Stem Cell Biology Branch within the Intramural Research Program of the National Heart, Lung, and Blood Institute in Bethesda, Md.
These abstracts include the primary analysis of the ZUMA-7 trial, a randomized, phase 3 study comparing the chimeric antigen receptor T-cell (CAR T) construct axicabtagene ciloleucel (axi-cel; Yescarta) with standard of care in patients with relapsed or refractory large B-cell lymphomas (LBCLs; abstract 2) and the interim analysis of the randomized, phase 3 Transform Study comparing the CAR T construct lisocabtagene maralecleucl (liso-cel; Breyanzi) with salvage chemotherapy in patients with relapsed/refractory LBCL (abstract 91).
“Over 500 patients were enrolled in the two studies, and both abstracts report significantly longer survival without relapse in the CAR T arm – for instance, fourfold higher in ZUMA-7, compared to standard of care,” Dr. Dunbar said at the briefing.
“These abstracts provide really critical information to patients, their treating physicians, and the payers who are trying to decide whether use of these expensive, complex, and potentially toxic CAR T-cell therapies are justified, compared to standard therapy,” she said.
Dr. Dunbar also highlighted an abstract on the addition of the anti-CD38 monoclonal antibody isatuximab (Sarclisa) to lenalidomide, bortezomib, and dexamethasone as induction therapy for patients with newly diagnosed multiple myeloma who are eligible for stem cell transplantation (abstract 463).
“The authors report that patients on the isatuximab arm had significantly fewer tumor cells following treatment,” Dr. Dunbar said. “We have come a long way beyond treating myeloma with a single drug, with remissions now measured in many years instead of 1 or 2 following initiation of treatment, and this abstract is another demonstration that novel combinations of multiple agents are really making a difference in this very debilitating disease.”
She also cited an abstract (abstract 127) on monotherapy with the novel bispecific T-cell–engaging monoclonal antibody mosunetuzumab for treatment of patients with follicular lymphoma that has relapsed or is refractory to at least two prior lines of therapy.
Old disorders, new insights
Other abstracts highlighted at the premeeting press briefing included a study that found a high prevalence of monoclonal gammopathy in persons at risk for multiple myeloma (abstract 152) and another with the surprising finding that clonal hematopoiesis, a risk factor myeloid malignancies, may be protective against Alzheimer’s disease (abstract 5).
In addition, a long-term follow-up study of patients with transfusion-dependent beta-thalassemia treated with gene therapy showed that some patients have become transfusion independent and iron homeostasis was restored (abstract 573).
Presentations from CDC and FDA
Dr. Sekeres highlighted other events of interest scheduled for ASH 2021, including a Grassroots Network Lunch featuring a discussion with Rochelle Walensky, MD, MPH, director of the Centers for Disease Control and Prevention in Atlanta, and a joint symposium between ASH and the Food and Drug Administration on newly approved drugs in hematology.
Dr. Sekeres has disclosed consulting/advising for Novartis, Takea/Millennium, and Bristol-Myers Squibb. Dr. Dunbar reported no relevant conflicts of interest. Dr. Tallman disclosed consulting/advising with and research funding from multiple entities.
A version of this article first appeared on Medscape.com.
Successful COVID-19 Surge Management With Monoclonal Antibody Infusion in Emergency Department Patients
From the Center for Artificial Intelligence in Diagnostic Medicine, University of California, Irvine, CA (Drs. Chow and Chang, Mazaya Soundara), University of California Irvine School of Medicine, Irvine, CA (Ruchi Desai), Division of Infectious Diseases, University of California, Irvine, CA (Dr. Gohil), and the Department of Medicine and Hospital Medicine Program, University of California, Irvine, CA (Dr. Amin).
Background: The COVID-19 pandemic has placed substantial strain on hospital resources and has been responsible for more than 733 000 deaths in the United States. The US Food and Drug Administration has granted emergency use authorization (EUA) for monoclonal antibody (mAb) therapy in the US for patients with early-stage high-risk COVID-19.
Methods: In this retrospective cohort study, we studied the emergency department (ED) during a massive COVID-19 surge in Orange County, California, from December 4, 2020, to January 29, 2021, as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high-risk COVID-19 patients. All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs, presented with 1 or more mild or moderate symptom, and met EUA criteria for mAb treatment. The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19.
Results: We identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the treatment period. Of these patients, 239 control patients and 63 treatment patients met EUA criteria. Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03).
Conclusion: Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Keywords: COVID-19; mAb; bamlanivimab; surge management.
Since December 2019, the novel pathogen SARS-CoV-2 has spread rapidly, culminating in a pandemic that has caused more than 4.9 million deaths worldwide and claimed more than 733 000 lives in the United States.1 The scale of the COVID-19 pandemic has placed an immense strain on hospital resources, including personal protective equipment (PPE), beds, ventilators and personnel.2,3 A previous analysis demonstrated that hospital capacity strain is associated with increased mortality and worsened health outcomes.4 A more recent analysis in light of the COVID-19 pandemic found that strains on critical care capacity were associated with increased COVID-19 intensive care unit (ICU) mortality.5 While more studies are needed to understand the association between hospital resources and COVID-19 mortality, efforts to decrease COVID-19 hospitalizations by early targeted treatment of patients in outpatient and emergency department (ED) settings may help to relieve the burden on hospital personnel and resources and decrease subsequent mortality.
Current therapeutic options focus on inpatient management of patients who progress to acute respiratory illness while patients with mild presentations are managed with outpatient monitoring, even those at high risk for progression. At the moment, only remdesivir, a viral RNA-dependent RNA polymerase inhibitor, has been approved by the US Food and Drug Administration (FDA) for treatment of hospitalized COVID-19 patients.6 However, in November 2020, the FDA granted emergency use authorization (EUA) for monoclonal antibodies (mAbs), monotherapy, and combination therapy in a broad range of early-stage, high-risk patients.7-9 Neutralizing mAbs include bamlanivimab (LY-CoV555), etesevimab (LY-CoV016), sotrovimab (VIR-7831), and casirivimab/imdevimab (REGN-COV2). These anti–spike protein antibodies prevent viral attachment to the human angiotensin-converting enzyme 2 receptor (hACE2) and subsequently prevent viral entry.10 mAb therapy has been shown to be effective in substantially reducing viral load, hospitalizations, and ED visits.11
Despite these promising results, uptake of mAb therapy has been slow, with more than 600 000 available doses remaining unused as of mid-January 2021, despite very high infection rates across the United States.12 In addition to the logistical challenges associated with intravenous (IV) therapy in the ambulatory setting, identifying, notifying, and scheduling appointments for ambulatory patients hamper efficient delivery to high-risk patients and limit access to underserved patients without primary care providers. For patients not treated in the ambulatory setting, the ED may serve as an ideal location for early implementation of mAb treatment in high-risk patients with mild to moderate COVID-19.
The University of California, Irvine (UCI) Medical Center is not only the major premium academic medical center in Orange County, California, but also the primary safety net hospital for vulnerable populations in Orange County. During the surge period from December 2020 through January 2021, we were over 100% capacity and had built an onsite mobile hospital to expand the number of beds available. Given the severity of the impact of COVID-19 on our resources, implementing a strategy to reduce hospital admissions, patient death, and subsequent ED visits was imperative. Our goal was to implement a strategy on the front end through the ED to optimize care for patients and reduce the strain on hospital resources.
We sought to study the ED during this massive surge as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high risk COVID-19 patients.
Methods
We conducted a retrospective cohort study (approved by UCI institutional review board) of sequential COVID-19 adult patients who were evaluated and discharged from the ED between December 4, 2020, and January 29, 2021, and received bamlanivimab treatment (cases) compared with a nontreatment group (control) of ED patients.
Using the UCI electronic medical record (EMR) system, we identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the months of December 2020 and January 2021. All patients included in this study met the EUA criteria for mAb therapy. According to the Centers for Disease Control and Prevention (CDC), during the period of this study, patients met EUA criteria if they had mild to moderate COVID-19, a positive direct SARS-CoV-2 viral testing, and a high risk for progressing to severe COVID-19 or hospitalization.13 High risk for progressing to severe COVID-19 and/or hospitalization is defined as meeting at least 1 of the following criteria: a body mass index of 35 or higher, chronic kidney disease (CKD), diabetes, immunosuppressive disease, currently receiving immunosuppressive treatment, aged 65 years or older, aged 55 years or older and have cardiovascular disease or hypertension, or chronic obstructive pulmonary disease (COPD)/other chronic respiratory diseases.13 All patients in the ED who met EUA criteria were offered mAb treatment; those who accepted the treatment were included in the treatment group, and those who refused were included in the control group.
All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs and presented with 1 or more mild or moderate symptom, defined as: fever, cough, sore throat, malaise, headache, muscle pain, gastrointestinal symptoms, or shortness of breath. We excluded patients admitted to the hospital on that ED visit and those discharged to hospice. In addition, we excluded patients who presented 2 weeks after symptom onset and those who did not meet EUA criteria. Demographic data (age and gender) and comorbid conditions were obtained by EMR review. Comorbid conditions obtained included diabetes, hypertension, cardiovascular disease, coronary artery disease, CKD/end-stage renal disease (ESRD), COPD, obesity, and immunocompromised status.
Bamlanivimab infusion therapy in the ED followed CDC guidelines. Each patient received 700 mg of bamlanivimab diluted in 0.9% sodium chloride and administered as a single IV infusion. We established protocols to give patients IV immunoglobulin (IVIG) infusions directly in the ED.
The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19 within 90 days of initial ED visit. Each patient was only counted once. Data analysis and statistical tests were conducted using SPSS statistical software (SPSS Inc). Treatment effects were compared using χ2 test with an α level of 0.05. A t test was used for continuous variables, including age. A P value of less than .05 was considered significant.
Results
We screened a total of 1351 patients with COVID-19. Of these, 1278 patients did not receive treatment with bamlanivimab. Two hundred thirty-nine patients met inclusion criteria and were included in the control group. Seventy-three patients were treated with bamlanivimab in the ED; 63 of these patients met EUA criteria and comprised the treatment group (Figure 1).
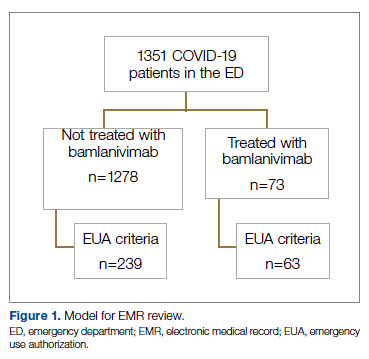
Demographic details of the trial groups are provided in Table 1. The median age of the treatment group was 61 years (interquartile range [IQR], 55-73), while the median age of the control group was 57 years (IQR, 48-68). The difference in median age between the treatment and control individuals was significantly different (P = .03). There was no significant difference found in terms of gender between the control and treatment groups (P = .07). In addition, no significant difference was seen among racial and ethnic groups in the control and treatment groups. Comorbidities and demographics of all patients in the treatment and control groups are provided in Table 1. The only comorbidity that was found to be significantly different between the treatment and control groups was CKD/ESRD. Among those treated with bamlanivimab, 20.6% (13/63) had CKD/ESRD compared with 10.5% (25/239) in the control group (P = .02).
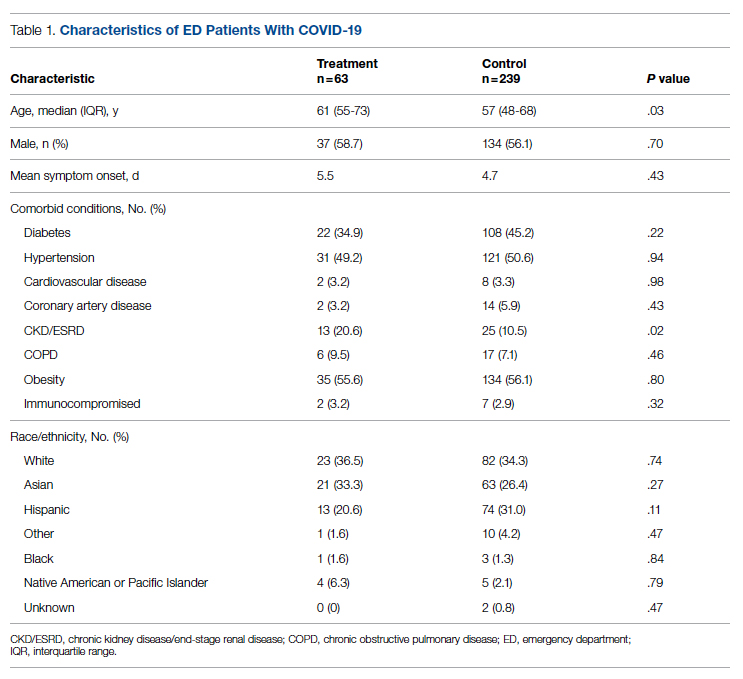
Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03) (Table 2).
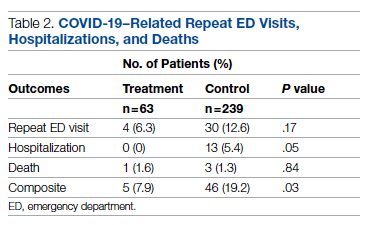
While the primary outcome of overall improvement was significantly different between the 2 groups, comparison of the individual components, including subsequent ED visits, hospitalizations, or death, were not significant. No treatment patients were hospitalized, compared with 5.4% (13/239) in the control group (P = .05). In the treatment group, 6.3% (4/63) returned to the ED compared with 12.6% (30/239) of the control group (P = .17). Finally, 1.6% (1/63) of the treatment group had a subsequent death that was due to COVID-19 compared with 1.3% (3/239) in the control group (P = .84) (Figure 2).
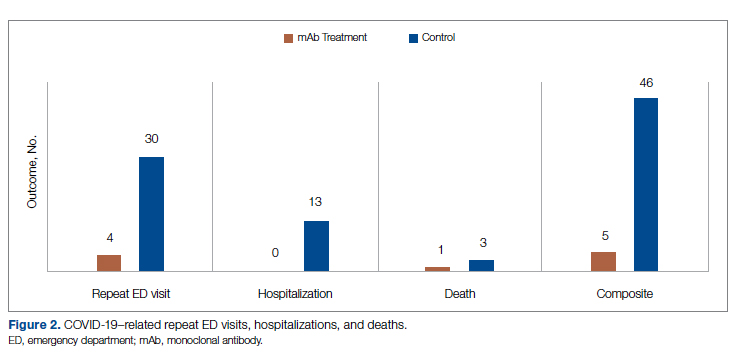
Discussion
In this retrospective cohort study, we observed a significant difference in rates of COVID-19 patients requiring repeat ED visits, hospitalizations, and deaths among those who received bamlanivimab compared with those who did not. Our study focused on high-risk patients with mild or moderate COVID-19, a unique subset of individuals who would normally be followed and treated via outpatient monitoring. We propose that treating high-risk patients earlier in their disease process with mAb therapy can have a major impact on overall outcomes, as defined by decreased subsequent hospitalizations, ED visits, and death.
Compared to clinical trials such as BLAZE-1 or REGN-COV2, every patient in this trial had at least 1 high-risk characteristic.9,11 This may explain why a greater proportion of our patients in both the control and treatment groups had subsequent hospitalization, ED visits, and deaths. COVID-19 patients seen in the ED may be a uniquely self-selected population of individuals likely to benefit from mAb therapy since they may be more likely to be sicker, have more comorbidities, or have less readily available primary care access for testing and treatment.14
Despite conducting a thorough literature review, we were unable to find any similar studies describing the ED as an appropriate setting for mAb treatment in patients with COVID-19. Multiple studies have used outpatient clinics as a setting for mAb treatment, and 1 retrospective analysis found that neutralizing mAb treatment in COVID-19 patients in an outpatient setting reduced hospital utilization.15 However, many Americans do not have access to primary care, with 1 study finding that only 75% of Americans had an identified source of primary care in 2015.16 Obstacles to primary care access include disabilities, lack of health insurance, language-related barriers, race/ethnicity, and homelessness.17 Barriers to access for primary care services and timely care make these populations more likely to frequent the ED.17 This makes the ED a unique location for early and targeted treatment of COVID-19 patients with a high risk for progression to severe COVID-19.
During surge periods in the COVID-19 pandemic, many hospitals met capacity or superseded their capacity for patients, with 4423 hospitals reporting more than 90% of hospital beds occupied and 2591 reporting more than 90% of ICU beds occupied during the peak surge week of January 1, 2021, to January 7, 2021.18 The main goals of lockdowns and masking have been to decrease the transmission of COVID-19 and hopefully flatten the curve to alleviate the burden on hospitals and decrease patient mortality. However, in surge situations when hospitals have already been pushed to their limits, we need to find ways to circumvent these shortages. This was particularly true at our academic medical center during the surge period of December 2020 through January 2021, necessitating the need for an innovative approach to improve patient outcomes and reduce the strain on resources. Utilizing the ED and implementing early treatment strategies with mAbs, especially during a surge crisis, can decrease severity of illness, hospitalizations, and deaths, as demonstrated in our article.
This study had several limitations. First, it is plausible that some ED patients may have gone to a different hospital after discharge from the UCI ED rather than returning to our institution. Given the constraints of using the EMR, we were only able to assess hospitalizations and subsequent ED visits at UCI. Second, there were 2 confounding variables identified when analyzing the demographic differences between the control and treatment group among those who met EUA criteria. The median age among those in the treatment group was greater than those in the control group (P = .03), and the proportion of individuals with CKD/ESRD was also greater in those in the treatment group (P = .02). It is well known that older patients and those with renal disease have higher incidences of morbidity and mortality. Achieving statistically significant differences overall between control and treatment groups despite greater numbers of older individuals and patients with renal disease in the treatment group supports our strategy and the usage of mAb.19,20
Finally, as of April 16, 2021, the FDA revoked EUA for bamlanivimab when administered alone. However, alternative mAb therapies remain available under the EUA, including REGEN-COV (casirivimab and imdevimab), sotrovimab, and the combination therapy of bamlanivimab and etesevimab.21 This decision was made in light of the increased frequency of resistant variants of SARS-CoV-2 with bamlanivimab treatment alone.21 Our study was conducted prior to this announcement. However, as treatment with other mAbs is still permissible, we believe our findings can translate to treatment with mAbs in general. In fact, combination therapy with bamlanivimab and etesevimab has been found to be more effective than monotherapy alone, suggesting that our results may be even more robust with combination mAb therapy.11 Overall, while additional studies are needed with larger sample sizes and combination mAb treatment to fully elucidate the impact of administering mAb treatment in the ED, our results suggest that targeting ED patients for mAb treatment may be an effective strategy to prevent the composite end point of repeat ED visits, hospitalizations, or deaths.
Conclusion
Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Corresponding author: Alpesh Amin, MD, MBA, Department of Medicine and Hospital Medicine Program, University of California, Irvine, 333 City Tower West, Ste 500, Orange, CA 92868; [email protected].
Financial disclosures: This manuscript was generously supported by multiple donors, including the Mehra Family, the Yang Family, and the Chao Family. Dr. Amin reported serving as Principal Investigator or Co-Investigator of clinical trials sponsored by NIH/NIAID, NeuroRX Pharma, Pulmotect, Blade Therapeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, OctaPharma, Fulcrum Therapeutics, and Alexion, unrelated to the present study. He has served as speaker and/or consultant for BMS, Pfizer, BI, Portola, Sunovion, Mylan, Salix, Alexion, AstraZeneca, Novartis, Nabriva, Paratek, Bayer, Tetraphase, Achaogen La Jolla, Ferring, Seres, Millennium, PeraHealth, HeartRite, Aseptiscope, and Sprightly, unrelated to the present study.
1. Global map. Johns Hopkins University & Medicine Coronavirus Resource Center. Updated November 9, 2021. Accessed November 9, 2021. https://coronavirus.jhu.edu/map.html
2. Truog RD, Mitchell C, Daley GQ. The toughest triage — allocating ventilators in a pandemic. N Engl J Med. 2020;382(21):1973-1975. doi:10.1056/NEJMp2005689
3. Cavallo JJ, Donoho DA, Forman HP. Hospital capacity and operations in the coronavirus disease 2019 (COVID-19) pandemic—planning for the Nth patient. JAMA Health Forum. 2020;1(3):e200345. doi:10.1001/jamahealthforum.2020.0345
4. Eriksson CO, Stoner RC, Eden KB, et al. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. doi:10.1007/s11606-016-3936-3
5. Bravata DM, Perkins AJ, Myers LJ, et al. Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021;4(1):e2034266. doi:10.1001/jamanetworkopen.2020.34266
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19);1813-1826. doi:10.1056/NEJMoa2007764
7. Coronavirus (COVID-19) update: FDA authorizes monoclonal antibody for treatment of COVID-19. US Food & Drug Administration. November 9, 2020. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19
8. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
9. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
10. Chen X, Li R, Pan Z, et al. Human monoclonal antibodies block the binding of SARS-CoV-2 spike protein to angiotensin converting enzyme 2 receptor. Cell Mol Immunol. 2020;17(6):647-649. doi:10.1038/s41423-020-0426-7
11. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
12. Toy S, Walker J, Evans M. Highly touted monoclonal antibody therapies sit unused in hospitals The Wall Street Journal. December 27, 2020. Accessed November 9, 2021. https://www.wsj.com/articles/highly-touted-monoclonal-antibody-therapies-sit-unused-in-hospitals-11609087364
13. Anti-SARS-CoV-2 monoclonal antibodies. NIH COVID-19 Treatment Guidelines. Updated October 19, 2021. Accessed November 9, 2021. https://www.covid19treatmentguidelines.nih.gov/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/
14. Langellier BA. Policy recommendations to address high risk of COVID-19 among immigrants. Am J Public Health. 2020;110(8):1137-1139. doi:10.2105/AJPH.2020.305792
15. Verderese J P, Stepanova M, Lam B, et al. Neutralizing monoclonal antibody treatment reduces hospitalization for mild and moderate COVID-19: a real-world experience. Clin Infect Dis. 2021;ciab579. doi:10.1093/cid/ciab579
16. Levine DM, Linder JA, Landon BE. Characteristics of Americans with primary care and changes over time, 2002-2015. JAMA Intern Med. 2020;180(3):463-466. doi:10.1001/jamainternmed.2019.6282
17. Rust G, Ye J, Daniels E, et al. Practical barriers to timely primary care access: impact on adult use of emergency department services. Arch Intern Med. 2008;168(15):1705-1710. doi:10.1001/archinte.168.15.1705
18. COVID-19 Hospitalization Tracking Project: analysis of HHS data. University of Minnesota. Carlson School of Management. Accessed November 9, 2021. https://carlsonschool.umn.edu/mili-misrc-covid19-tracking-project
19. Zare˛bska-Michaluk D, Jaroszewicz J, Rogalska M, et al. Impact of kidney failure on the severity of COVID-19. J Clin Med. 2021;10(9):2042. doi:10.3390/jcm10092042
20. Shahid Z, Kalayanamitra R, McClafferty B, et al. COVID‐19 and older adults: what we know. J Am Geriatr Soc. 2020;68(5):926-929. doi:10.1111/jgs.16472
21. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. US Food & Drug Administration. April 16, 2021. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
From the Center for Artificial Intelligence in Diagnostic Medicine, University of California, Irvine, CA (Drs. Chow and Chang, Mazaya Soundara), University of California Irvine School of Medicine, Irvine, CA (Ruchi Desai), Division of Infectious Diseases, University of California, Irvine, CA (Dr. Gohil), and the Department of Medicine and Hospital Medicine Program, University of California, Irvine, CA (Dr. Amin).
Background: The COVID-19 pandemic has placed substantial strain on hospital resources and has been responsible for more than 733 000 deaths in the United States. The US Food and Drug Administration has granted emergency use authorization (EUA) for monoclonal antibody (mAb) therapy in the US for patients with early-stage high-risk COVID-19.
Methods: In this retrospective cohort study, we studied the emergency department (ED) during a massive COVID-19 surge in Orange County, California, from December 4, 2020, to January 29, 2021, as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high-risk COVID-19 patients. All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs, presented with 1 or more mild or moderate symptom, and met EUA criteria for mAb treatment. The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19.
Results: We identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the treatment period. Of these patients, 239 control patients and 63 treatment patients met EUA criteria. Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03).
Conclusion: Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Keywords: COVID-19; mAb; bamlanivimab; surge management.
Since December 2019, the novel pathogen SARS-CoV-2 has spread rapidly, culminating in a pandemic that has caused more than 4.9 million deaths worldwide and claimed more than 733 000 lives in the United States.1 The scale of the COVID-19 pandemic has placed an immense strain on hospital resources, including personal protective equipment (PPE), beds, ventilators and personnel.2,3 A previous analysis demonstrated that hospital capacity strain is associated with increased mortality and worsened health outcomes.4 A more recent analysis in light of the COVID-19 pandemic found that strains on critical care capacity were associated with increased COVID-19 intensive care unit (ICU) mortality.5 While more studies are needed to understand the association between hospital resources and COVID-19 mortality, efforts to decrease COVID-19 hospitalizations by early targeted treatment of patients in outpatient and emergency department (ED) settings may help to relieve the burden on hospital personnel and resources and decrease subsequent mortality.
Current therapeutic options focus on inpatient management of patients who progress to acute respiratory illness while patients with mild presentations are managed with outpatient monitoring, even those at high risk for progression. At the moment, only remdesivir, a viral RNA-dependent RNA polymerase inhibitor, has been approved by the US Food and Drug Administration (FDA) for treatment of hospitalized COVID-19 patients.6 However, in November 2020, the FDA granted emergency use authorization (EUA) for monoclonal antibodies (mAbs), monotherapy, and combination therapy in a broad range of early-stage, high-risk patients.7-9 Neutralizing mAbs include bamlanivimab (LY-CoV555), etesevimab (LY-CoV016), sotrovimab (VIR-7831), and casirivimab/imdevimab (REGN-COV2). These anti–spike protein antibodies prevent viral attachment to the human angiotensin-converting enzyme 2 receptor (hACE2) and subsequently prevent viral entry.10 mAb therapy has been shown to be effective in substantially reducing viral load, hospitalizations, and ED visits.11
Despite these promising results, uptake of mAb therapy has been slow, with more than 600 000 available doses remaining unused as of mid-January 2021, despite very high infection rates across the United States.12 In addition to the logistical challenges associated with intravenous (IV) therapy in the ambulatory setting, identifying, notifying, and scheduling appointments for ambulatory patients hamper efficient delivery to high-risk patients and limit access to underserved patients without primary care providers. For patients not treated in the ambulatory setting, the ED may serve as an ideal location for early implementation of mAb treatment in high-risk patients with mild to moderate COVID-19.
The University of California, Irvine (UCI) Medical Center is not only the major premium academic medical center in Orange County, California, but also the primary safety net hospital for vulnerable populations in Orange County. During the surge period from December 2020 through January 2021, we were over 100% capacity and had built an onsite mobile hospital to expand the number of beds available. Given the severity of the impact of COVID-19 on our resources, implementing a strategy to reduce hospital admissions, patient death, and subsequent ED visits was imperative. Our goal was to implement a strategy on the front end through the ED to optimize care for patients and reduce the strain on hospital resources.
We sought to study the ED during this massive surge as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high risk COVID-19 patients.
Methods
We conducted a retrospective cohort study (approved by UCI institutional review board) of sequential COVID-19 adult patients who were evaluated and discharged from the ED between December 4, 2020, and January 29, 2021, and received bamlanivimab treatment (cases) compared with a nontreatment group (control) of ED patients.
Using the UCI electronic medical record (EMR) system, we identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the months of December 2020 and January 2021. All patients included in this study met the EUA criteria for mAb therapy. According to the Centers for Disease Control and Prevention (CDC), during the period of this study, patients met EUA criteria if they had mild to moderate COVID-19, a positive direct SARS-CoV-2 viral testing, and a high risk for progressing to severe COVID-19 or hospitalization.13 High risk for progressing to severe COVID-19 and/or hospitalization is defined as meeting at least 1 of the following criteria: a body mass index of 35 or higher, chronic kidney disease (CKD), diabetes, immunosuppressive disease, currently receiving immunosuppressive treatment, aged 65 years or older, aged 55 years or older and have cardiovascular disease or hypertension, or chronic obstructive pulmonary disease (COPD)/other chronic respiratory diseases.13 All patients in the ED who met EUA criteria were offered mAb treatment; those who accepted the treatment were included in the treatment group, and those who refused were included in the control group.
All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs and presented with 1 or more mild or moderate symptom, defined as: fever, cough, sore throat, malaise, headache, muscle pain, gastrointestinal symptoms, or shortness of breath. We excluded patients admitted to the hospital on that ED visit and those discharged to hospice. In addition, we excluded patients who presented 2 weeks after symptom onset and those who did not meet EUA criteria. Demographic data (age and gender) and comorbid conditions were obtained by EMR review. Comorbid conditions obtained included diabetes, hypertension, cardiovascular disease, coronary artery disease, CKD/end-stage renal disease (ESRD), COPD, obesity, and immunocompromised status.
Bamlanivimab infusion therapy in the ED followed CDC guidelines. Each patient received 700 mg of bamlanivimab diluted in 0.9% sodium chloride and administered as a single IV infusion. We established protocols to give patients IV immunoglobulin (IVIG) infusions directly in the ED.
The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19 within 90 days of initial ED visit. Each patient was only counted once. Data analysis and statistical tests were conducted using SPSS statistical software (SPSS Inc). Treatment effects were compared using χ2 test with an α level of 0.05. A t test was used for continuous variables, including age. A P value of less than .05 was considered significant.
Results
We screened a total of 1351 patients with COVID-19. Of these, 1278 patients did not receive treatment with bamlanivimab. Two hundred thirty-nine patients met inclusion criteria and were included in the control group. Seventy-three patients were treated with bamlanivimab in the ED; 63 of these patients met EUA criteria and comprised the treatment group (Figure 1).

Demographic details of the trial groups are provided in Table 1. The median age of the treatment group was 61 years (interquartile range [IQR], 55-73), while the median age of the control group was 57 years (IQR, 48-68). The difference in median age between the treatment and control individuals was significantly different (P = .03). There was no significant difference found in terms of gender between the control and treatment groups (P = .07). In addition, no significant difference was seen among racial and ethnic groups in the control and treatment groups. Comorbidities and demographics of all patients in the treatment and control groups are provided in Table 1. The only comorbidity that was found to be significantly different between the treatment and control groups was CKD/ESRD. Among those treated with bamlanivimab, 20.6% (13/63) had CKD/ESRD compared with 10.5% (25/239) in the control group (P = .02).

Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03) (Table 2).

While the primary outcome of overall improvement was significantly different between the 2 groups, comparison of the individual components, including subsequent ED visits, hospitalizations, or death, were not significant. No treatment patients were hospitalized, compared with 5.4% (13/239) in the control group (P = .05). In the treatment group, 6.3% (4/63) returned to the ED compared with 12.6% (30/239) of the control group (P = .17). Finally, 1.6% (1/63) of the treatment group had a subsequent death that was due to COVID-19 compared with 1.3% (3/239) in the control group (P = .84) (Figure 2).

Discussion
In this retrospective cohort study, we observed a significant difference in rates of COVID-19 patients requiring repeat ED visits, hospitalizations, and deaths among those who received bamlanivimab compared with those who did not. Our study focused on high-risk patients with mild or moderate COVID-19, a unique subset of individuals who would normally be followed and treated via outpatient monitoring. We propose that treating high-risk patients earlier in their disease process with mAb therapy can have a major impact on overall outcomes, as defined by decreased subsequent hospitalizations, ED visits, and death.
Compared to clinical trials such as BLAZE-1 or REGN-COV2, every patient in this trial had at least 1 high-risk characteristic.9,11 This may explain why a greater proportion of our patients in both the control and treatment groups had subsequent hospitalization, ED visits, and deaths. COVID-19 patients seen in the ED may be a uniquely self-selected population of individuals likely to benefit from mAb therapy since they may be more likely to be sicker, have more comorbidities, or have less readily available primary care access for testing and treatment.14
Despite conducting a thorough literature review, we were unable to find any similar studies describing the ED as an appropriate setting for mAb treatment in patients with COVID-19. Multiple studies have used outpatient clinics as a setting for mAb treatment, and 1 retrospective analysis found that neutralizing mAb treatment in COVID-19 patients in an outpatient setting reduced hospital utilization.15 However, many Americans do not have access to primary care, with 1 study finding that only 75% of Americans had an identified source of primary care in 2015.16 Obstacles to primary care access include disabilities, lack of health insurance, language-related barriers, race/ethnicity, and homelessness.17 Barriers to access for primary care services and timely care make these populations more likely to frequent the ED.17 This makes the ED a unique location for early and targeted treatment of COVID-19 patients with a high risk for progression to severe COVID-19.
During surge periods in the COVID-19 pandemic, many hospitals met capacity or superseded their capacity for patients, with 4423 hospitals reporting more than 90% of hospital beds occupied and 2591 reporting more than 90% of ICU beds occupied during the peak surge week of January 1, 2021, to January 7, 2021.18 The main goals of lockdowns and masking have been to decrease the transmission of COVID-19 and hopefully flatten the curve to alleviate the burden on hospitals and decrease patient mortality. However, in surge situations when hospitals have already been pushed to their limits, we need to find ways to circumvent these shortages. This was particularly true at our academic medical center during the surge period of December 2020 through January 2021, necessitating the need for an innovative approach to improve patient outcomes and reduce the strain on resources. Utilizing the ED and implementing early treatment strategies with mAbs, especially during a surge crisis, can decrease severity of illness, hospitalizations, and deaths, as demonstrated in our article.
This study had several limitations. First, it is plausible that some ED patients may have gone to a different hospital after discharge from the UCI ED rather than returning to our institution. Given the constraints of using the EMR, we were only able to assess hospitalizations and subsequent ED visits at UCI. Second, there were 2 confounding variables identified when analyzing the demographic differences between the control and treatment group among those who met EUA criteria. The median age among those in the treatment group was greater than those in the control group (P = .03), and the proportion of individuals with CKD/ESRD was also greater in those in the treatment group (P = .02). It is well known that older patients and those with renal disease have higher incidences of morbidity and mortality. Achieving statistically significant differences overall between control and treatment groups despite greater numbers of older individuals and patients with renal disease in the treatment group supports our strategy and the usage of mAb.19,20
Finally, as of April 16, 2021, the FDA revoked EUA for bamlanivimab when administered alone. However, alternative mAb therapies remain available under the EUA, including REGEN-COV (casirivimab and imdevimab), sotrovimab, and the combination therapy of bamlanivimab and etesevimab.21 This decision was made in light of the increased frequency of resistant variants of SARS-CoV-2 with bamlanivimab treatment alone.21 Our study was conducted prior to this announcement. However, as treatment with other mAbs is still permissible, we believe our findings can translate to treatment with mAbs in general. In fact, combination therapy with bamlanivimab and etesevimab has been found to be more effective than monotherapy alone, suggesting that our results may be even more robust with combination mAb therapy.11 Overall, while additional studies are needed with larger sample sizes and combination mAb treatment to fully elucidate the impact of administering mAb treatment in the ED, our results suggest that targeting ED patients for mAb treatment may be an effective strategy to prevent the composite end point of repeat ED visits, hospitalizations, or deaths.
Conclusion
Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Corresponding author: Alpesh Amin, MD, MBA, Department of Medicine and Hospital Medicine Program, University of California, Irvine, 333 City Tower West, Ste 500, Orange, CA 92868; [email protected].
Financial disclosures: This manuscript was generously supported by multiple donors, including the Mehra Family, the Yang Family, and the Chao Family. Dr. Amin reported serving as Principal Investigator or Co-Investigator of clinical trials sponsored by NIH/NIAID, NeuroRX Pharma, Pulmotect, Blade Therapeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, OctaPharma, Fulcrum Therapeutics, and Alexion, unrelated to the present study. He has served as speaker and/or consultant for BMS, Pfizer, BI, Portola, Sunovion, Mylan, Salix, Alexion, AstraZeneca, Novartis, Nabriva, Paratek, Bayer, Tetraphase, Achaogen La Jolla, Ferring, Seres, Millennium, PeraHealth, HeartRite, Aseptiscope, and Sprightly, unrelated to the present study.
From the Center for Artificial Intelligence in Diagnostic Medicine, University of California, Irvine, CA (Drs. Chow and Chang, Mazaya Soundara), University of California Irvine School of Medicine, Irvine, CA (Ruchi Desai), Division of Infectious Diseases, University of California, Irvine, CA (Dr. Gohil), and the Department of Medicine and Hospital Medicine Program, University of California, Irvine, CA (Dr. Amin).
Background: The COVID-19 pandemic has placed substantial strain on hospital resources and has been responsible for more than 733 000 deaths in the United States. The US Food and Drug Administration has granted emergency use authorization (EUA) for monoclonal antibody (mAb) therapy in the US for patients with early-stage high-risk COVID-19.
Methods: In this retrospective cohort study, we studied the emergency department (ED) during a massive COVID-19 surge in Orange County, California, from December 4, 2020, to January 29, 2021, as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high-risk COVID-19 patients. All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs, presented with 1 or more mild or moderate symptom, and met EUA criteria for mAb treatment. The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19.
Results: We identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the treatment period. Of these patients, 239 control patients and 63 treatment patients met EUA criteria. Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03).
Conclusion: Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Keywords: COVID-19; mAb; bamlanivimab; surge management.
Since December 2019, the novel pathogen SARS-CoV-2 has spread rapidly, culminating in a pandemic that has caused more than 4.9 million deaths worldwide and claimed more than 733 000 lives in the United States.1 The scale of the COVID-19 pandemic has placed an immense strain on hospital resources, including personal protective equipment (PPE), beds, ventilators and personnel.2,3 A previous analysis demonstrated that hospital capacity strain is associated with increased mortality and worsened health outcomes.4 A more recent analysis in light of the COVID-19 pandemic found that strains on critical care capacity were associated with increased COVID-19 intensive care unit (ICU) mortality.5 While more studies are needed to understand the association between hospital resources and COVID-19 mortality, efforts to decrease COVID-19 hospitalizations by early targeted treatment of patients in outpatient and emergency department (ED) settings may help to relieve the burden on hospital personnel and resources and decrease subsequent mortality.
Current therapeutic options focus on inpatient management of patients who progress to acute respiratory illness while patients with mild presentations are managed with outpatient monitoring, even those at high risk for progression. At the moment, only remdesivir, a viral RNA-dependent RNA polymerase inhibitor, has been approved by the US Food and Drug Administration (FDA) for treatment of hospitalized COVID-19 patients.6 However, in November 2020, the FDA granted emergency use authorization (EUA) for monoclonal antibodies (mAbs), monotherapy, and combination therapy in a broad range of early-stage, high-risk patients.7-9 Neutralizing mAbs include bamlanivimab (LY-CoV555), etesevimab (LY-CoV016), sotrovimab (VIR-7831), and casirivimab/imdevimab (REGN-COV2). These anti–spike protein antibodies prevent viral attachment to the human angiotensin-converting enzyme 2 receptor (hACE2) and subsequently prevent viral entry.10 mAb therapy has been shown to be effective in substantially reducing viral load, hospitalizations, and ED visits.11
Despite these promising results, uptake of mAb therapy has been slow, with more than 600 000 available doses remaining unused as of mid-January 2021, despite very high infection rates across the United States.12 In addition to the logistical challenges associated with intravenous (IV) therapy in the ambulatory setting, identifying, notifying, and scheduling appointments for ambulatory patients hamper efficient delivery to high-risk patients and limit access to underserved patients without primary care providers. For patients not treated in the ambulatory setting, the ED may serve as an ideal location for early implementation of mAb treatment in high-risk patients with mild to moderate COVID-19.
The University of California, Irvine (UCI) Medical Center is not only the major premium academic medical center in Orange County, California, but also the primary safety net hospital for vulnerable populations in Orange County. During the surge period from December 2020 through January 2021, we were over 100% capacity and had built an onsite mobile hospital to expand the number of beds available. Given the severity of the impact of COVID-19 on our resources, implementing a strategy to reduce hospital admissions, patient death, and subsequent ED visits was imperative. Our goal was to implement a strategy on the front end through the ED to optimize care for patients and reduce the strain on hospital resources.
We sought to study the ED during this massive surge as a potential setting for efficient mAb delivery by evaluating the impact of bamlanivimab use in high risk COVID-19 patients.
Methods
We conducted a retrospective cohort study (approved by UCI institutional review board) of sequential COVID-19 adult patients who were evaluated and discharged from the ED between December 4, 2020, and January 29, 2021, and received bamlanivimab treatment (cases) compared with a nontreatment group (control) of ED patients.
Using the UCI electronic medical record (EMR) system, we identified 1278 ED patients with COVID-19 not treated with bamlanivimab and 73 patients with COVID-19 treated with bamlanivimab during the months of December 2020 and January 2021. All patients included in this study met the EUA criteria for mAb therapy. According to the Centers for Disease Control and Prevention (CDC), during the period of this study, patients met EUA criteria if they had mild to moderate COVID-19, a positive direct SARS-CoV-2 viral testing, and a high risk for progressing to severe COVID-19 or hospitalization.13 High risk for progressing to severe COVID-19 and/or hospitalization is defined as meeting at least 1 of the following criteria: a body mass index of 35 or higher, chronic kidney disease (CKD), diabetes, immunosuppressive disease, currently receiving immunosuppressive treatment, aged 65 years or older, aged 55 years or older and have cardiovascular disease or hypertension, or chronic obstructive pulmonary disease (COPD)/other chronic respiratory diseases.13 All patients in the ED who met EUA criteria were offered mAb treatment; those who accepted the treatment were included in the treatment group, and those who refused were included in the control group.
All patients included in this study had positive results on nucleic acid amplification detection from nasopharyngeal or throat swabs and presented with 1 or more mild or moderate symptom, defined as: fever, cough, sore throat, malaise, headache, muscle pain, gastrointestinal symptoms, or shortness of breath. We excluded patients admitted to the hospital on that ED visit and those discharged to hospice. In addition, we excluded patients who presented 2 weeks after symptom onset and those who did not meet EUA criteria. Demographic data (age and gender) and comorbid conditions were obtained by EMR review. Comorbid conditions obtained included diabetes, hypertension, cardiovascular disease, coronary artery disease, CKD/end-stage renal disease (ESRD), COPD, obesity, and immunocompromised status.
Bamlanivimab infusion therapy in the ED followed CDC guidelines. Each patient received 700 mg of bamlanivimab diluted in 0.9% sodium chloride and administered as a single IV infusion. We established protocols to give patients IV immunoglobulin (IVIG) infusions directly in the ED.
The primary outcome analyzed among this cohort of ED patients was overall improvement, which included subsequent ED/hospital visits, inpatient hospitalization, and death related to COVID-19 within 90 days of initial ED visit. Each patient was only counted once. Data analysis and statistical tests were conducted using SPSS statistical software (SPSS Inc). Treatment effects were compared using χ2 test with an α level of 0.05. A t test was used for continuous variables, including age. A P value of less than .05 was considered significant.
Results
We screened a total of 1351 patients with COVID-19. Of these, 1278 patients did not receive treatment with bamlanivimab. Two hundred thirty-nine patients met inclusion criteria and were included in the control group. Seventy-three patients were treated with bamlanivimab in the ED; 63 of these patients met EUA criteria and comprised the treatment group (Figure 1).

Demographic details of the trial groups are provided in Table 1. The median age of the treatment group was 61 years (interquartile range [IQR], 55-73), while the median age of the control group was 57 years (IQR, 48-68). The difference in median age between the treatment and control individuals was significantly different (P = .03). There was no significant difference found in terms of gender between the control and treatment groups (P = .07). In addition, no significant difference was seen among racial and ethnic groups in the control and treatment groups. Comorbidities and demographics of all patients in the treatment and control groups are provided in Table 1. The only comorbidity that was found to be significantly different between the treatment and control groups was CKD/ESRD. Among those treated with bamlanivimab, 20.6% (13/63) had CKD/ESRD compared with 10.5% (25/239) in the control group (P = .02).

Overall, 7.9% (5/63) of patients receiving bamlanivimab had a subsequent ED/hospital visit, hospitalization, or death compared with 19.2% (46/239) in the control group (P = .03) (Table 2).

While the primary outcome of overall improvement was significantly different between the 2 groups, comparison of the individual components, including subsequent ED visits, hospitalizations, or death, were not significant. No treatment patients were hospitalized, compared with 5.4% (13/239) in the control group (P = .05). In the treatment group, 6.3% (4/63) returned to the ED compared with 12.6% (30/239) of the control group (P = .17). Finally, 1.6% (1/63) of the treatment group had a subsequent death that was due to COVID-19 compared with 1.3% (3/239) in the control group (P = .84) (Figure 2).

Discussion
In this retrospective cohort study, we observed a significant difference in rates of COVID-19 patients requiring repeat ED visits, hospitalizations, and deaths among those who received bamlanivimab compared with those who did not. Our study focused on high-risk patients with mild or moderate COVID-19, a unique subset of individuals who would normally be followed and treated via outpatient monitoring. We propose that treating high-risk patients earlier in their disease process with mAb therapy can have a major impact on overall outcomes, as defined by decreased subsequent hospitalizations, ED visits, and death.
Compared to clinical trials such as BLAZE-1 or REGN-COV2, every patient in this trial had at least 1 high-risk characteristic.9,11 This may explain why a greater proportion of our patients in both the control and treatment groups had subsequent hospitalization, ED visits, and deaths. COVID-19 patients seen in the ED may be a uniquely self-selected population of individuals likely to benefit from mAb therapy since they may be more likely to be sicker, have more comorbidities, or have less readily available primary care access for testing and treatment.14
Despite conducting a thorough literature review, we were unable to find any similar studies describing the ED as an appropriate setting for mAb treatment in patients with COVID-19. Multiple studies have used outpatient clinics as a setting for mAb treatment, and 1 retrospective analysis found that neutralizing mAb treatment in COVID-19 patients in an outpatient setting reduced hospital utilization.15 However, many Americans do not have access to primary care, with 1 study finding that only 75% of Americans had an identified source of primary care in 2015.16 Obstacles to primary care access include disabilities, lack of health insurance, language-related barriers, race/ethnicity, and homelessness.17 Barriers to access for primary care services and timely care make these populations more likely to frequent the ED.17 This makes the ED a unique location for early and targeted treatment of COVID-19 patients with a high risk for progression to severe COVID-19.
During surge periods in the COVID-19 pandemic, many hospitals met capacity or superseded their capacity for patients, with 4423 hospitals reporting more than 90% of hospital beds occupied and 2591 reporting more than 90% of ICU beds occupied during the peak surge week of January 1, 2021, to January 7, 2021.18 The main goals of lockdowns and masking have been to decrease the transmission of COVID-19 and hopefully flatten the curve to alleviate the burden on hospitals and decrease patient mortality. However, in surge situations when hospitals have already been pushed to their limits, we need to find ways to circumvent these shortages. This was particularly true at our academic medical center during the surge period of December 2020 through January 2021, necessitating the need for an innovative approach to improve patient outcomes and reduce the strain on resources. Utilizing the ED and implementing early treatment strategies with mAbs, especially during a surge crisis, can decrease severity of illness, hospitalizations, and deaths, as demonstrated in our article.
This study had several limitations. First, it is plausible that some ED patients may have gone to a different hospital after discharge from the UCI ED rather than returning to our institution. Given the constraints of using the EMR, we were only able to assess hospitalizations and subsequent ED visits at UCI. Second, there were 2 confounding variables identified when analyzing the demographic differences between the control and treatment group among those who met EUA criteria. The median age among those in the treatment group was greater than those in the control group (P = .03), and the proportion of individuals with CKD/ESRD was also greater in those in the treatment group (P = .02). It is well known that older patients and those with renal disease have higher incidences of morbidity and mortality. Achieving statistically significant differences overall between control and treatment groups despite greater numbers of older individuals and patients with renal disease in the treatment group supports our strategy and the usage of mAb.19,20
Finally, as of April 16, 2021, the FDA revoked EUA for bamlanivimab when administered alone. However, alternative mAb therapies remain available under the EUA, including REGEN-COV (casirivimab and imdevimab), sotrovimab, and the combination therapy of bamlanivimab and etesevimab.21 This decision was made in light of the increased frequency of resistant variants of SARS-CoV-2 with bamlanivimab treatment alone.21 Our study was conducted prior to this announcement. However, as treatment with other mAbs is still permissible, we believe our findings can translate to treatment with mAbs in general. In fact, combination therapy with bamlanivimab and etesevimab has been found to be more effective than monotherapy alone, suggesting that our results may be even more robust with combination mAb therapy.11 Overall, while additional studies are needed with larger sample sizes and combination mAb treatment to fully elucidate the impact of administering mAb treatment in the ED, our results suggest that targeting ED patients for mAb treatment may be an effective strategy to prevent the composite end point of repeat ED visits, hospitalizations, or deaths.
Conclusion
Targeting ED patients for mAb treatment may be an effective strategy to prevent progression to severe COVID-19 illness and substantially reduce the composite end point of repeat ED visits, hospitalizations, and deaths, especially for individuals of underserved populations who may not have access to ambulatory care.
Corresponding author: Alpesh Amin, MD, MBA, Department of Medicine and Hospital Medicine Program, University of California, Irvine, 333 City Tower West, Ste 500, Orange, CA 92868; [email protected].
Financial disclosures: This manuscript was generously supported by multiple donors, including the Mehra Family, the Yang Family, and the Chao Family. Dr. Amin reported serving as Principal Investigator or Co-Investigator of clinical trials sponsored by NIH/NIAID, NeuroRX Pharma, Pulmotect, Blade Therapeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, OctaPharma, Fulcrum Therapeutics, and Alexion, unrelated to the present study. He has served as speaker and/or consultant for BMS, Pfizer, BI, Portola, Sunovion, Mylan, Salix, Alexion, AstraZeneca, Novartis, Nabriva, Paratek, Bayer, Tetraphase, Achaogen La Jolla, Ferring, Seres, Millennium, PeraHealth, HeartRite, Aseptiscope, and Sprightly, unrelated to the present study.
1. Global map. Johns Hopkins University & Medicine Coronavirus Resource Center. Updated November 9, 2021. Accessed November 9, 2021. https://coronavirus.jhu.edu/map.html
2. Truog RD, Mitchell C, Daley GQ. The toughest triage — allocating ventilators in a pandemic. N Engl J Med. 2020;382(21):1973-1975. doi:10.1056/NEJMp2005689
3. Cavallo JJ, Donoho DA, Forman HP. Hospital capacity and operations in the coronavirus disease 2019 (COVID-19) pandemic—planning for the Nth patient. JAMA Health Forum. 2020;1(3):e200345. doi:10.1001/jamahealthforum.2020.0345
4. Eriksson CO, Stoner RC, Eden KB, et al. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. doi:10.1007/s11606-016-3936-3
5. Bravata DM, Perkins AJ, Myers LJ, et al. Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021;4(1):e2034266. doi:10.1001/jamanetworkopen.2020.34266
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19);1813-1826. doi:10.1056/NEJMoa2007764
7. Coronavirus (COVID-19) update: FDA authorizes monoclonal antibody for treatment of COVID-19. US Food & Drug Administration. November 9, 2020. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19
8. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
9. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
10. Chen X, Li R, Pan Z, et al. Human monoclonal antibodies block the binding of SARS-CoV-2 spike protein to angiotensin converting enzyme 2 receptor. Cell Mol Immunol. 2020;17(6):647-649. doi:10.1038/s41423-020-0426-7
11. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
12. Toy S, Walker J, Evans M. Highly touted monoclonal antibody therapies sit unused in hospitals The Wall Street Journal. December 27, 2020. Accessed November 9, 2021. https://www.wsj.com/articles/highly-touted-monoclonal-antibody-therapies-sit-unused-in-hospitals-11609087364
13. Anti-SARS-CoV-2 monoclonal antibodies. NIH COVID-19 Treatment Guidelines. Updated October 19, 2021. Accessed November 9, 2021. https://www.covid19treatmentguidelines.nih.gov/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/
14. Langellier BA. Policy recommendations to address high risk of COVID-19 among immigrants. Am J Public Health. 2020;110(8):1137-1139. doi:10.2105/AJPH.2020.305792
15. Verderese J P, Stepanova M, Lam B, et al. Neutralizing monoclonal antibody treatment reduces hospitalization for mild and moderate COVID-19: a real-world experience. Clin Infect Dis. 2021;ciab579. doi:10.1093/cid/ciab579
16. Levine DM, Linder JA, Landon BE. Characteristics of Americans with primary care and changes over time, 2002-2015. JAMA Intern Med. 2020;180(3):463-466. doi:10.1001/jamainternmed.2019.6282
17. Rust G, Ye J, Daniels E, et al. Practical barriers to timely primary care access: impact on adult use of emergency department services. Arch Intern Med. 2008;168(15):1705-1710. doi:10.1001/archinte.168.15.1705
18. COVID-19 Hospitalization Tracking Project: analysis of HHS data. University of Minnesota. Carlson School of Management. Accessed November 9, 2021. https://carlsonschool.umn.edu/mili-misrc-covid19-tracking-project
19. Zare˛bska-Michaluk D, Jaroszewicz J, Rogalska M, et al. Impact of kidney failure on the severity of COVID-19. J Clin Med. 2021;10(9):2042. doi:10.3390/jcm10092042
20. Shahid Z, Kalayanamitra R, McClafferty B, et al. COVID‐19 and older adults: what we know. J Am Geriatr Soc. 2020;68(5):926-929. doi:10.1111/jgs.16472
21. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. US Food & Drug Administration. April 16, 2021. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
1. Global map. Johns Hopkins University & Medicine Coronavirus Resource Center. Updated November 9, 2021. Accessed November 9, 2021. https://coronavirus.jhu.edu/map.html
2. Truog RD, Mitchell C, Daley GQ. The toughest triage — allocating ventilators in a pandemic. N Engl J Med. 2020;382(21):1973-1975. doi:10.1056/NEJMp2005689
3. Cavallo JJ, Donoho DA, Forman HP. Hospital capacity and operations in the coronavirus disease 2019 (COVID-19) pandemic—planning for the Nth patient. JAMA Health Forum. 2020;1(3):e200345. doi:10.1001/jamahealthforum.2020.0345
4. Eriksson CO, Stoner RC, Eden KB, et al. The association between hospital capacity strain and inpatient outcomes in highly developed countries: a systematic review. J Gen Intern Med. 2017;32(6):686-696. doi:10.1007/s11606-016-3936-3
5. Bravata DM, Perkins AJ, Myers LJ, et al. Association of intensive care unit patient load and demand with mortality rates in US Department of Veterans Affairs hospitals during the COVID-19 pandemic. JAMA Netw Open. 2021;4(1):e2034266. doi:10.1001/jamanetworkopen.2020.34266
6. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19);1813-1826. doi:10.1056/NEJMoa2007764
7. Coronavirus (COVID-19) update: FDA authorizes monoclonal antibody for treatment of COVID-19. US Food & Drug Administration. November 9, 2020. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19
8. Chen P, Nirula A, Heller B, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. 2021;384(3):229-237. doi:10.1056/NEJMoa2029849
9. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384(3):238-251. doi:10.1056/NEJMoa2035002
10. Chen X, Li R, Pan Z, et al. Human monoclonal antibodies block the binding of SARS-CoV-2 spike protein to angiotensin converting enzyme 2 receptor. Cell Mol Immunol. 2020;17(6):647-649. doi:10.1038/s41423-020-0426-7
11. Gottlieb RL, Nirula A, Chen P, et al. Effect of bamlanivimab as monotherapy or in combination with etesevimab on viral load in patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2021;325(7):632-644. doi:10.1001/jama.2021.0202
12. Toy S, Walker J, Evans M. Highly touted monoclonal antibody therapies sit unused in hospitals The Wall Street Journal. December 27, 2020. Accessed November 9, 2021. https://www.wsj.com/articles/highly-touted-monoclonal-antibody-therapies-sit-unused-in-hospitals-11609087364
13. Anti-SARS-CoV-2 monoclonal antibodies. NIH COVID-19 Treatment Guidelines. Updated October 19, 2021. Accessed November 9, 2021. https://www.covid19treatmentguidelines.nih.gov/anti-sars-cov-2-antibody-products/anti-sars-cov-2-monoclonal-antibodies/
14. Langellier BA. Policy recommendations to address high risk of COVID-19 among immigrants. Am J Public Health. 2020;110(8):1137-1139. doi:10.2105/AJPH.2020.305792
15. Verderese J P, Stepanova M, Lam B, et al. Neutralizing monoclonal antibody treatment reduces hospitalization for mild and moderate COVID-19: a real-world experience. Clin Infect Dis. 2021;ciab579. doi:10.1093/cid/ciab579
16. Levine DM, Linder JA, Landon BE. Characteristics of Americans with primary care and changes over time, 2002-2015. JAMA Intern Med. 2020;180(3):463-466. doi:10.1001/jamainternmed.2019.6282
17. Rust G, Ye J, Daniels E, et al. Practical barriers to timely primary care access: impact on adult use of emergency department services. Arch Intern Med. 2008;168(15):1705-1710. doi:10.1001/archinte.168.15.1705
18. COVID-19 Hospitalization Tracking Project: analysis of HHS data. University of Minnesota. Carlson School of Management. Accessed November 9, 2021. https://carlsonschool.umn.edu/mili-misrc-covid19-tracking-project
19. Zare˛bska-Michaluk D, Jaroszewicz J, Rogalska M, et al. Impact of kidney failure on the severity of COVID-19. J Clin Med. 2021;10(9):2042. doi:10.3390/jcm10092042
20. Shahid Z, Kalayanamitra R, McClafferty B, et al. COVID‐19 and older adults: what we know. J Am Geriatr Soc. 2020;68(5):926-929. doi:10.1111/jgs.16472
21. Coronavirus (COVID-19) update: FDA revokes emergency use authorization for monoclonal antibody bamlanivimab. US Food & Drug Administration. April 16, 2021. Accessed November 9, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab
Antibiotics vs. placebo in acute uncomplicated diverticulitis
Background: Antibiotic therapy is considered the standard of care for acute uncomplicated diverticulitis. Over the past decade, randomized clinical trials have suggested that treatment with antibiotics may be noninferior to observation with supportive care; however, there have not been any blinded, placebo-controlled trials to provide high-quality evidence.
Study design: Placebo-controlled, double-blinded, randomized noninferiority trial.
Setting: Four centers in New Zealand and Australia.
Synopsis: Researchers randomized 180 patients hospitalized for acute uncomplicated diverticulitis with Hinchey 1a CT findings (i.e., phlegmon without abscess) into two groups treated with either antibiotics (intravenous cefuroxime and oral metronidazole followed by oral amoxicillin/clavulanic acid) or placebo for 7 days. Median lengths of stay between the antibiotic (40.0 hours) and placebo (45.8 hours) groups were not significantly different (5.9 hours difference between groups; 95% CI, –3.7 to 15.5; P = .2). Additionally, there were no significant differences in the secondary outcomes of readmission at 7 days and 30 days or in need for procedural intervention, mortality, pain scores at 24 hours, or change in white blood cell count.
Notably, though this study was adequately powered to detect differences in length of stay, it was not powered to detect differences in clinical outcomes, including death or the need for surgery. The exclusion of patients with language barriers raises concerns regarding the generalizability of the results.
Bottom line: Antibiotic therapy does not decrease length of hospital stay when compared with placebo for patients with acute uncomplicated diverticulitis.
Citation: Jaung R et al. Antibiotics do not reduce length of hospital stay for uncomplicated diverticulitis in a pragmatic double-blind randomized trial. Clin Gastroenterol Hepatol. 2020 Mar;S1542-3565(20):30426-2. doi: 10.1016/j.cgh.2020.03.049.
Dr. Elyahu is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Antibiotic therapy is considered the standard of care for acute uncomplicated diverticulitis. Over the past decade, randomized clinical trials have suggested that treatment with antibiotics may be noninferior to observation with supportive care; however, there have not been any blinded, placebo-controlled trials to provide high-quality evidence.
Study design: Placebo-controlled, double-blinded, randomized noninferiority trial.
Setting: Four centers in New Zealand and Australia.
Synopsis: Researchers randomized 180 patients hospitalized for acute uncomplicated diverticulitis with Hinchey 1a CT findings (i.e., phlegmon without abscess) into two groups treated with either antibiotics (intravenous cefuroxime and oral metronidazole followed by oral amoxicillin/clavulanic acid) or placebo for 7 days. Median lengths of stay between the antibiotic (40.0 hours) and placebo (45.8 hours) groups were not significantly different (5.9 hours difference between groups; 95% CI, –3.7 to 15.5; P = .2). Additionally, there were no significant differences in the secondary outcomes of readmission at 7 days and 30 days or in need for procedural intervention, mortality, pain scores at 24 hours, or change in white blood cell count.
Notably, though this study was adequately powered to detect differences in length of stay, it was not powered to detect differences in clinical outcomes, including death or the need for surgery. The exclusion of patients with language barriers raises concerns regarding the generalizability of the results.
Bottom line: Antibiotic therapy does not decrease length of hospital stay when compared with placebo for patients with acute uncomplicated diverticulitis.
Citation: Jaung R et al. Antibiotics do not reduce length of hospital stay for uncomplicated diverticulitis in a pragmatic double-blind randomized trial. Clin Gastroenterol Hepatol. 2020 Mar;S1542-3565(20):30426-2. doi: 10.1016/j.cgh.2020.03.049.
Dr. Elyahu is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Antibiotic therapy is considered the standard of care for acute uncomplicated diverticulitis. Over the past decade, randomized clinical trials have suggested that treatment with antibiotics may be noninferior to observation with supportive care; however, there have not been any blinded, placebo-controlled trials to provide high-quality evidence.
Study design: Placebo-controlled, double-blinded, randomized noninferiority trial.
Setting: Four centers in New Zealand and Australia.
Synopsis: Researchers randomized 180 patients hospitalized for acute uncomplicated diverticulitis with Hinchey 1a CT findings (i.e., phlegmon without abscess) into two groups treated with either antibiotics (intravenous cefuroxime and oral metronidazole followed by oral amoxicillin/clavulanic acid) or placebo for 7 days. Median lengths of stay between the antibiotic (40.0 hours) and placebo (45.8 hours) groups were not significantly different (5.9 hours difference between groups; 95% CI, –3.7 to 15.5; P = .2). Additionally, there were no significant differences in the secondary outcomes of readmission at 7 days and 30 days or in need for procedural intervention, mortality, pain scores at 24 hours, or change in white blood cell count.
Notably, though this study was adequately powered to detect differences in length of stay, it was not powered to detect differences in clinical outcomes, including death or the need for surgery. The exclusion of patients with language barriers raises concerns regarding the generalizability of the results.
Bottom line: Antibiotic therapy does not decrease length of hospital stay when compared with placebo for patients with acute uncomplicated diverticulitis.
Citation: Jaung R et al. Antibiotics do not reduce length of hospital stay for uncomplicated diverticulitis in a pragmatic double-blind randomized trial. Clin Gastroenterol Hepatol. 2020 Mar;S1542-3565(20):30426-2. doi: 10.1016/j.cgh.2020.03.049.
Dr. Elyahu is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Baked milk immunotherapy may help children with cow’s milk allergy
, new research suggests.
The small, ongoing clinical trial has enabled some participants – all of whom reacted to less than a tablespoon of baked milk at baseline – to begin incorporating baked milk products into everyday diets and to eat in restaurants with less fear of allergic reactions, reported study author Jennifer Dantzer, MD, MHS, assistant professor of pediatrics in the division of pediatric allergy, immunology, and rheumatology at Johns Hopkins University in Baltimore.
Cow’s milk is the most common food allergy in young children, and “for many, it’s a constant stressor that’s always there,” Dr. Dantzer said in an interview. “For a lot of families, this impacts where they eat out, if they eat out, and sometimes where they vacation, or a lot of the social activities they do.
“This was a unique group of kids with a very severe milk phenotype who were reactive to teeny doses and may not have qualified or done well with other types of oral immunotherapy,” she added. “Using a modified allergen – baked milk – seems to work. But for now, we think this is something that still needs further research before it’s ready for a clinical setting.”
The study, for which 24-month unblinded results are being tallied, was recently published in the Journal of Allergy and Clinical Immunology .
About 2%-3% of preschool-age children are affected by cow’s milk allergy. Children often outgrow it, but for about 20% of children, it persists into adolescence and adulthood. The only current management approaches are avoidance and emergency medications to treat reactions.
But for those with severe milk allergy who react to even trace amounts of milk in any form, the now-routine clinical practice of introducing baked milk isn’t an option, Dr. Dantzer said. The new trial stood out from prior research by using lower starting doses and a more gradual dose escalation of extensively heated milk to determine if oral immunotherapy could be safer but still effective.
Dr. Dantzer and her team randomly assigned 30 participants (aged 3-18 years) into two blinded groups. For 12 months, one group received baked milk oral immunotherapy (BMOIT), and the other a placebo consisting of tapioca flour. At baseline, for all participants, the milk skin prick test wheal diameter was ≥ 3 mm, and the cow’s milk immunoglobulin E (IgE) level was > 5 kU/L. All the children experienced positive dose-limiting reactions to < 1 tablespoon of baked milk protein but could tolerate at least 3 mg on initial dose escalation.
Measured doses of baked milk and placebo powders were supplied to participants for all doses consumed at home. Participants were given instructions on how to prepare it in cupcake or muffin batter. Over 12 months, doses were gradually increased to a maximum cumulative dose of 4,044 mg baked milk protein, or approximately a half tablespoon.
Researchers collected blood samples for immune studies, and participants or their parents completed quality-of-life questionnaires that asked about food anxiety, social and dietary limitations, emotional impact, risk for accidental ingestion, and allergen avoidance.
Fourteen of 15 participants (93%) in the BMOIT group reached the goal-maintenance dose of 2,000 mg of baked milk protein (about a quarter tablespoon). Of those who completed the 12-month challenge, 11 of 14 (79%) in the BMOIT group tolerated 4,000 mg of baked milk protein, compared to none in the placebo group.
“We anticipated that by starting with really small amounts, we would be able to build up the amount of baked milk these kids could tolerate,” Dr. Dantzer said. “We were very pleased by how many could reach the maximal dose at the end of the first year. Once we get the results of the second year, that will provide a lot of additional detail about how this translates into unheated milk amounts they can tolerate and introduce into their diet at home.”
No significant changes were found in IgE levels over time in either study group. Most in the BMOIT group reported improvement in at least one quality-of-life domain, while more in the placebo group reported improvements in only the emotional impact domain.
Adverse events such as gastrointestinal side effects occurred in both groups of participants, but the vast majority of events were mild, Dr. Dantzer said. Fewer than 1% of dosing-related reactions were severe. Four participants required epinephrine.
“This highlights how this needs to be done by someone comfortable and trained, and not by a family at home on their own,” Dr. Dantzer said. “But potentially in the future, this concept of using a modified allergen could be applied to more kids with milk allergy.”
A Montreal-based pediatric allergy specialist who was not involved in the study said the results weren’t surprising. “We’ve known for a good while that the allergenic proteins found in certain foods, or caused by milk in this context, are influenced by the way in which food is processed,” said Christine McCusker, MD, associate professor of pediatrics and director of the division of pediatric allergy, immunology, and dermatology at Montreal Children’s Hospital at McGill University Health Center.
But “having this relatively definitive data that supports what you’re suggesting to patients is obviously the way to optimize your management,” Dr. McCusker said in an interview. “These types of studies are important steps, especially in this age of increased food allergies where many of these things can be dealt with in very young children before their immune systems are fixed.”
Dr. Dantzer and Dr. McCusker agreed that the small size of the study was a limitation, though “waiting for more participants means you don’t always get information out there in a timely manner,” Dr. McCusker said.
She said additional research should focus on preidentifying which children may be prone to severe, lasting food allergies. “If you have a milk allergy that will stay with you the rest of your life and we could maybe modify that outcome with early, targeted intervention, that would be the nirvana of the field,” Dr. McCusker said.
Dr. Dantzer said her research “showed us that oral immunotherapy is an option, but not a perfect option.
“We still need to keep working on other alternatives that can be even safer and potentially work better,” she added.
The study was supported by the Myra Reinhard Family Foundation. Dr. Dantzer and Dr. McCusker report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
The small, ongoing clinical trial has enabled some participants – all of whom reacted to less than a tablespoon of baked milk at baseline – to begin incorporating baked milk products into everyday diets and to eat in restaurants with less fear of allergic reactions, reported study author Jennifer Dantzer, MD, MHS, assistant professor of pediatrics in the division of pediatric allergy, immunology, and rheumatology at Johns Hopkins University in Baltimore.
Cow’s milk is the most common food allergy in young children, and “for many, it’s a constant stressor that’s always there,” Dr. Dantzer said in an interview. “For a lot of families, this impacts where they eat out, if they eat out, and sometimes where they vacation, or a lot of the social activities they do.
“This was a unique group of kids with a very severe milk phenotype who were reactive to teeny doses and may not have qualified or done well with other types of oral immunotherapy,” she added. “Using a modified allergen – baked milk – seems to work. But for now, we think this is something that still needs further research before it’s ready for a clinical setting.”
The study, for which 24-month unblinded results are being tallied, was recently published in the Journal of Allergy and Clinical Immunology .
About 2%-3% of preschool-age children are affected by cow’s milk allergy. Children often outgrow it, but for about 20% of children, it persists into adolescence and adulthood. The only current management approaches are avoidance and emergency medications to treat reactions.
But for those with severe milk allergy who react to even trace amounts of milk in any form, the now-routine clinical practice of introducing baked milk isn’t an option, Dr. Dantzer said. The new trial stood out from prior research by using lower starting doses and a more gradual dose escalation of extensively heated milk to determine if oral immunotherapy could be safer but still effective.
Dr. Dantzer and her team randomly assigned 30 participants (aged 3-18 years) into two blinded groups. For 12 months, one group received baked milk oral immunotherapy (BMOIT), and the other a placebo consisting of tapioca flour. At baseline, for all participants, the milk skin prick test wheal diameter was ≥ 3 mm, and the cow’s milk immunoglobulin E (IgE) level was > 5 kU/L. All the children experienced positive dose-limiting reactions to < 1 tablespoon of baked milk protein but could tolerate at least 3 mg on initial dose escalation.
Measured doses of baked milk and placebo powders were supplied to participants for all doses consumed at home. Participants were given instructions on how to prepare it in cupcake or muffin batter. Over 12 months, doses were gradually increased to a maximum cumulative dose of 4,044 mg baked milk protein, or approximately a half tablespoon.
Researchers collected blood samples for immune studies, and participants or their parents completed quality-of-life questionnaires that asked about food anxiety, social and dietary limitations, emotional impact, risk for accidental ingestion, and allergen avoidance.
Fourteen of 15 participants (93%) in the BMOIT group reached the goal-maintenance dose of 2,000 mg of baked milk protein (about a quarter tablespoon). Of those who completed the 12-month challenge, 11 of 14 (79%) in the BMOIT group tolerated 4,000 mg of baked milk protein, compared to none in the placebo group.
“We anticipated that by starting with really small amounts, we would be able to build up the amount of baked milk these kids could tolerate,” Dr. Dantzer said. “We were very pleased by how many could reach the maximal dose at the end of the first year. Once we get the results of the second year, that will provide a lot of additional detail about how this translates into unheated milk amounts they can tolerate and introduce into their diet at home.”
No significant changes were found in IgE levels over time in either study group. Most in the BMOIT group reported improvement in at least one quality-of-life domain, while more in the placebo group reported improvements in only the emotional impact domain.
Adverse events such as gastrointestinal side effects occurred in both groups of participants, but the vast majority of events were mild, Dr. Dantzer said. Fewer than 1% of dosing-related reactions were severe. Four participants required epinephrine.
“This highlights how this needs to be done by someone comfortable and trained, and not by a family at home on their own,” Dr. Dantzer said. “But potentially in the future, this concept of using a modified allergen could be applied to more kids with milk allergy.”
A Montreal-based pediatric allergy specialist who was not involved in the study said the results weren’t surprising. “We’ve known for a good while that the allergenic proteins found in certain foods, or caused by milk in this context, are influenced by the way in which food is processed,” said Christine McCusker, MD, associate professor of pediatrics and director of the division of pediatric allergy, immunology, and dermatology at Montreal Children’s Hospital at McGill University Health Center.
But “having this relatively definitive data that supports what you’re suggesting to patients is obviously the way to optimize your management,” Dr. McCusker said in an interview. “These types of studies are important steps, especially in this age of increased food allergies where many of these things can be dealt with in very young children before their immune systems are fixed.”
Dr. Dantzer and Dr. McCusker agreed that the small size of the study was a limitation, though “waiting for more participants means you don’t always get information out there in a timely manner,” Dr. McCusker said.
She said additional research should focus on preidentifying which children may be prone to severe, lasting food allergies. “If you have a milk allergy that will stay with you the rest of your life and we could maybe modify that outcome with early, targeted intervention, that would be the nirvana of the field,” Dr. McCusker said.
Dr. Dantzer said her research “showed us that oral immunotherapy is an option, but not a perfect option.
“We still need to keep working on other alternatives that can be even safer and potentially work better,” she added.
The study was supported by the Myra Reinhard Family Foundation. Dr. Dantzer and Dr. McCusker report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
The small, ongoing clinical trial has enabled some participants – all of whom reacted to less than a tablespoon of baked milk at baseline – to begin incorporating baked milk products into everyday diets and to eat in restaurants with less fear of allergic reactions, reported study author Jennifer Dantzer, MD, MHS, assistant professor of pediatrics in the division of pediatric allergy, immunology, and rheumatology at Johns Hopkins University in Baltimore.
Cow’s milk is the most common food allergy in young children, and “for many, it’s a constant stressor that’s always there,” Dr. Dantzer said in an interview. “For a lot of families, this impacts where they eat out, if they eat out, and sometimes where they vacation, or a lot of the social activities they do.
“This was a unique group of kids with a very severe milk phenotype who were reactive to teeny doses and may not have qualified or done well with other types of oral immunotherapy,” she added. “Using a modified allergen – baked milk – seems to work. But for now, we think this is something that still needs further research before it’s ready for a clinical setting.”
The study, for which 24-month unblinded results are being tallied, was recently published in the Journal of Allergy and Clinical Immunology .
About 2%-3% of preschool-age children are affected by cow’s milk allergy. Children often outgrow it, but for about 20% of children, it persists into adolescence and adulthood. The only current management approaches are avoidance and emergency medications to treat reactions.
But for those with severe milk allergy who react to even trace amounts of milk in any form, the now-routine clinical practice of introducing baked milk isn’t an option, Dr. Dantzer said. The new trial stood out from prior research by using lower starting doses and a more gradual dose escalation of extensively heated milk to determine if oral immunotherapy could be safer but still effective.
Dr. Dantzer and her team randomly assigned 30 participants (aged 3-18 years) into two blinded groups. For 12 months, one group received baked milk oral immunotherapy (BMOIT), and the other a placebo consisting of tapioca flour. At baseline, for all participants, the milk skin prick test wheal diameter was ≥ 3 mm, and the cow’s milk immunoglobulin E (IgE) level was > 5 kU/L. All the children experienced positive dose-limiting reactions to < 1 tablespoon of baked milk protein but could tolerate at least 3 mg on initial dose escalation.
Measured doses of baked milk and placebo powders were supplied to participants for all doses consumed at home. Participants were given instructions on how to prepare it in cupcake or muffin batter. Over 12 months, doses were gradually increased to a maximum cumulative dose of 4,044 mg baked milk protein, or approximately a half tablespoon.
Researchers collected blood samples for immune studies, and participants or their parents completed quality-of-life questionnaires that asked about food anxiety, social and dietary limitations, emotional impact, risk for accidental ingestion, and allergen avoidance.
Fourteen of 15 participants (93%) in the BMOIT group reached the goal-maintenance dose of 2,000 mg of baked milk protein (about a quarter tablespoon). Of those who completed the 12-month challenge, 11 of 14 (79%) in the BMOIT group tolerated 4,000 mg of baked milk protein, compared to none in the placebo group.
“We anticipated that by starting with really small amounts, we would be able to build up the amount of baked milk these kids could tolerate,” Dr. Dantzer said. “We were very pleased by how many could reach the maximal dose at the end of the first year. Once we get the results of the second year, that will provide a lot of additional detail about how this translates into unheated milk amounts they can tolerate and introduce into their diet at home.”
No significant changes were found in IgE levels over time in either study group. Most in the BMOIT group reported improvement in at least one quality-of-life domain, while more in the placebo group reported improvements in only the emotional impact domain.
Adverse events such as gastrointestinal side effects occurred in both groups of participants, but the vast majority of events were mild, Dr. Dantzer said. Fewer than 1% of dosing-related reactions were severe. Four participants required epinephrine.
“This highlights how this needs to be done by someone comfortable and trained, and not by a family at home on their own,” Dr. Dantzer said. “But potentially in the future, this concept of using a modified allergen could be applied to more kids with milk allergy.”
A Montreal-based pediatric allergy specialist who was not involved in the study said the results weren’t surprising. “We’ve known for a good while that the allergenic proteins found in certain foods, or caused by milk in this context, are influenced by the way in which food is processed,” said Christine McCusker, MD, associate professor of pediatrics and director of the division of pediatric allergy, immunology, and dermatology at Montreal Children’s Hospital at McGill University Health Center.
But “having this relatively definitive data that supports what you’re suggesting to patients is obviously the way to optimize your management,” Dr. McCusker said in an interview. “These types of studies are important steps, especially in this age of increased food allergies where many of these things can be dealt with in very young children before their immune systems are fixed.”
Dr. Dantzer and Dr. McCusker agreed that the small size of the study was a limitation, though “waiting for more participants means you don’t always get information out there in a timely manner,” Dr. McCusker said.
She said additional research should focus on preidentifying which children may be prone to severe, lasting food allergies. “If you have a milk allergy that will stay with you the rest of your life and we could maybe modify that outcome with early, targeted intervention, that would be the nirvana of the field,” Dr. McCusker said.
Dr. Dantzer said her research “showed us that oral immunotherapy is an option, but not a perfect option.
“We still need to keep working on other alternatives that can be even safer and potentially work better,” she added.
The study was supported by the Myra Reinhard Family Foundation. Dr. Dantzer and Dr. McCusker report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Large analysis confirms safety of nipple-sparing mastectomy
A new analysis of over 22,000 mastectomy patients confirms what smaller studies have indicated: Patients who undergo nipple-sparing mastectomy have overall and disease-free survival similar to that of those who receive a total mastectomy.
When nipple-sparing mastectomy was introduced, many experts felt uneasy about opting for the less invasive procedure, recalled Rosa Hwang, MD, associate medical director for breast surgery at MD Anderson Cancer Center in Houston. “The concern was leaving all this skin,” said Dr. Hwang. “Are you going to leave cancer behind” and increase the risk of local recurrence?
Over the past 2 decades, the number of patients undergoing nipple-sparing mastectomy increased and, in turn, studies began to demonstrate the safety of the procedure.
However, large analyses evaluating long-term outcomes – namely, overall survival and breast cancer-specific survival – of nipple-sparing mastectomy were still lacking.
The latest study, published online Nov. 20 in Annals of Surgical Oncology, compared the long-term prognosis and survival benefits of nipple-sparing to total mastectomy in thousands of women. The analysis, which pulled data from the SEER cancer database, included 5,765 patients who underwent the nipple-sparing procedure and 17,289 patients who had a total mastectomy.
The authors found that overall survival and breast cancer–specific survival were similar for women undergoing nipple-sparing mastectomy and those receiving a total mastectomy. In fact, over the long-term, the nipple-sparing group slightly edged out the total mastectomy group in overall survival (94.61% vs. 93% at 5 years and 86.34% vs. 83.48% at 10 years, respectively) and in breast cancer-specific survival rates (96.16% vs. 95.74% at 5 years, and 92.2% vs. 91.37% at 10 years). The differences, however, were not significant.
The study also found that certain subgroups – including White women, women over age 46, those with a median household income of $70,000 or more, hormone receptor-positive, and HER2 negative – had significantly better overall survival rate with the nipple-sparing procedure (P < .05). However, the authors noted, the survival advantage in the nipple-sparing group did not extend to breast cancer–specific survival.
Dr. Hwang, who was not involved in the current analysis, said the significant overall survival result in the subgroup analysis was surprising because “there’s no biological reason why one would expect that to be true.”
Given that the subgroups did not demonstrate better breast cancer–specific survival, Dr. Hwang believes the overall survival finding may have more to do with comorbidities, which the study did not account for, than type of mastectomy.
When choosing who is eligible for a nipple-sparing mastectomy, “We’re more selective,” Dr. Hwang said. For instance, patients with uncontrolled diabetes or who smoke are unlikely to be candidates. “So, I think it’s possible that medical comorbidities and medical conditions between these groups [were] different.”
According to the authors, coding inconsistencies represent another possible weakness of the study. From 1998 to 2010, “the term ‘nipple-sparing mastectomy’ was coded as a [total mastectomy] with the ‘subcutaneous mastectomy’ code.” It’s possible that some patients receiving the nipple-sparing procedure before 2011 were not appropriately coded in the current study.
Moving forward, a large prospective study that includes comorbidities would be helpful, but overall the study helps validate that “nipple-sparing mastectomy is a safe operation for selected patients,” Dr. Hwang said.
A version of this article first appeared on Medscape.com.
A new analysis of over 22,000 mastectomy patients confirms what smaller studies have indicated: Patients who undergo nipple-sparing mastectomy have overall and disease-free survival similar to that of those who receive a total mastectomy.
When nipple-sparing mastectomy was introduced, many experts felt uneasy about opting for the less invasive procedure, recalled Rosa Hwang, MD, associate medical director for breast surgery at MD Anderson Cancer Center in Houston. “The concern was leaving all this skin,” said Dr. Hwang. “Are you going to leave cancer behind” and increase the risk of local recurrence?
Over the past 2 decades, the number of patients undergoing nipple-sparing mastectomy increased and, in turn, studies began to demonstrate the safety of the procedure.
However, large analyses evaluating long-term outcomes – namely, overall survival and breast cancer-specific survival – of nipple-sparing mastectomy were still lacking.
The latest study, published online Nov. 20 in Annals of Surgical Oncology, compared the long-term prognosis and survival benefits of nipple-sparing to total mastectomy in thousands of women. The analysis, which pulled data from the SEER cancer database, included 5,765 patients who underwent the nipple-sparing procedure and 17,289 patients who had a total mastectomy.
The authors found that overall survival and breast cancer–specific survival were similar for women undergoing nipple-sparing mastectomy and those receiving a total mastectomy. In fact, over the long-term, the nipple-sparing group slightly edged out the total mastectomy group in overall survival (94.61% vs. 93% at 5 years and 86.34% vs. 83.48% at 10 years, respectively) and in breast cancer-specific survival rates (96.16% vs. 95.74% at 5 years, and 92.2% vs. 91.37% at 10 years). The differences, however, were not significant.
The study also found that certain subgroups – including White women, women over age 46, those with a median household income of $70,000 or more, hormone receptor-positive, and HER2 negative – had significantly better overall survival rate with the nipple-sparing procedure (P < .05). However, the authors noted, the survival advantage in the nipple-sparing group did not extend to breast cancer–specific survival.
Dr. Hwang, who was not involved in the current analysis, said the significant overall survival result in the subgroup analysis was surprising because “there’s no biological reason why one would expect that to be true.”
Given that the subgroups did not demonstrate better breast cancer–specific survival, Dr. Hwang believes the overall survival finding may have more to do with comorbidities, which the study did not account for, than type of mastectomy.
When choosing who is eligible for a nipple-sparing mastectomy, “We’re more selective,” Dr. Hwang said. For instance, patients with uncontrolled diabetes or who smoke are unlikely to be candidates. “So, I think it’s possible that medical comorbidities and medical conditions between these groups [were] different.”
According to the authors, coding inconsistencies represent another possible weakness of the study. From 1998 to 2010, “the term ‘nipple-sparing mastectomy’ was coded as a [total mastectomy] with the ‘subcutaneous mastectomy’ code.” It’s possible that some patients receiving the nipple-sparing procedure before 2011 were not appropriately coded in the current study.
Moving forward, a large prospective study that includes comorbidities would be helpful, but overall the study helps validate that “nipple-sparing mastectomy is a safe operation for selected patients,” Dr. Hwang said.
A version of this article first appeared on Medscape.com.
A new analysis of over 22,000 mastectomy patients confirms what smaller studies have indicated: Patients who undergo nipple-sparing mastectomy have overall and disease-free survival similar to that of those who receive a total mastectomy.
When nipple-sparing mastectomy was introduced, many experts felt uneasy about opting for the less invasive procedure, recalled Rosa Hwang, MD, associate medical director for breast surgery at MD Anderson Cancer Center in Houston. “The concern was leaving all this skin,” said Dr. Hwang. “Are you going to leave cancer behind” and increase the risk of local recurrence?
Over the past 2 decades, the number of patients undergoing nipple-sparing mastectomy increased and, in turn, studies began to demonstrate the safety of the procedure.
However, large analyses evaluating long-term outcomes – namely, overall survival and breast cancer-specific survival – of nipple-sparing mastectomy were still lacking.
The latest study, published online Nov. 20 in Annals of Surgical Oncology, compared the long-term prognosis and survival benefits of nipple-sparing to total mastectomy in thousands of women. The analysis, which pulled data from the SEER cancer database, included 5,765 patients who underwent the nipple-sparing procedure and 17,289 patients who had a total mastectomy.
The authors found that overall survival and breast cancer–specific survival were similar for women undergoing nipple-sparing mastectomy and those receiving a total mastectomy. In fact, over the long-term, the nipple-sparing group slightly edged out the total mastectomy group in overall survival (94.61% vs. 93% at 5 years and 86.34% vs. 83.48% at 10 years, respectively) and in breast cancer-specific survival rates (96.16% vs. 95.74% at 5 years, and 92.2% vs. 91.37% at 10 years). The differences, however, were not significant.
The study also found that certain subgroups – including White women, women over age 46, those with a median household income of $70,000 or more, hormone receptor-positive, and HER2 negative – had significantly better overall survival rate with the nipple-sparing procedure (P < .05). However, the authors noted, the survival advantage in the nipple-sparing group did not extend to breast cancer–specific survival.
Dr. Hwang, who was not involved in the current analysis, said the significant overall survival result in the subgroup analysis was surprising because “there’s no biological reason why one would expect that to be true.”
Given that the subgroups did not demonstrate better breast cancer–specific survival, Dr. Hwang believes the overall survival finding may have more to do with comorbidities, which the study did not account for, than type of mastectomy.
When choosing who is eligible for a nipple-sparing mastectomy, “We’re more selective,” Dr. Hwang said. For instance, patients with uncontrolled diabetes or who smoke are unlikely to be candidates. “So, I think it’s possible that medical comorbidities and medical conditions between these groups [were] different.”
According to the authors, coding inconsistencies represent another possible weakness of the study. From 1998 to 2010, “the term ‘nipple-sparing mastectomy’ was coded as a [total mastectomy] with the ‘subcutaneous mastectomy’ code.” It’s possible that some patients receiving the nipple-sparing procedure before 2011 were not appropriately coded in the current study.
Moving forward, a large prospective study that includes comorbidities would be helpful, but overall the study helps validate that “nipple-sparing mastectomy is a safe operation for selected patients,” Dr. Hwang said.
A version of this article first appeared on Medscape.com.




