User login
The future of Advanced Practice Providers (APPs) in sleep clinics and telemedicine post-pandemic
Loretta J. Colvin, APRN, ACNP-BC, is a Nurse Practitioner at Sleep Services, SSM Health Medical Group, specializing in the treatment of sleep disorders. Her focus is on insomnia, narcolepsy, obstructive sleep apnea, parasomnias, periodic leg movement disorder, restless leg syndrome, sleep disorders, snoring and telehealth.
Q: As a nurse practitioner and a specialist at a clinic focused on identifying sleep related disorders, what do you feel is the overall value of such a clinic as a therapeutic need for patients?
Ms. Colvin: Well, for us in sleep, we've experienced a growth in our field, and anticipate further growth. We know that many people remain undiagnosed with their sleep disorders. So, we see our role as filling a need for the public in providing more personalized and specialized care for those that have a sleep problem.
Patients get to us via many routes, including self-referral or referral by their primary care provider or specialist. We help these patients identify their sleep problems, guide them in testing and treatment decisions, and provide on-going treatment support.
Currently, there are two areas in which there is a particular need. With current stressors impacting our society, there is an increased need for patients to discuss their insomnia or sleep difficulty concerns with a healthcare provider. We are seeing more patients bring up insomnia concerns to their primary care providers or coming directly to our sleep clinic for discussion of these concerns. As the recognition of the importance of diagnosing and treatment sleep apnea continues to grow, we see more patients coming to us for care, including those with less obvious symptoms but high risk of sleep apnea due to their comorbid diseases.
Q: Given current predictions that the outpatient health care structure will change and the number of APRNs and PAs will increase, what is your perspective on role and utilization of APPs, as well as the need to plan for the future care of patients with sleep disorders?
Ms. Colvin: First, let me talk about the national landscape with regards to structure. Nationally, the APP role and the number of professionals in that role is growing, so that's going to help meet some of the needs of our society for providing health care, whether it be primary care or specialty care, like I do, or acute care in the hospital. At present, there are a significant number of places where we fill a needed role but within the area of sleep, there is a likely to be an increase in need for APPs as a result of attrition in the field as well as a shift in how many physicians are available to provide care in our ever-expanding specialty. What we then need to do is figure out how do we train these Providers in a specialty? This is not a specialty that is a large part of our basic education, so how do we train people into that specialty? And how do we prepare them for their role and ensure that we are offering opportunities to expand their capabilities over time?
An example of this can be seen with regards to the opportunities now being offered in telehealth in behavioral sleep medicine and in working with conditions like insomnia, or with people who have difficulty in trying to adjust to Continuous Positive Airway Pressure (CPAP) therapy and might need additional assistance and coaching.
Within my organization, physicians will refer patients directly to me who have struggled to use PAP in the past. With specialized guidance, these past struggles can often be overcome thanks to improvements in our equipment and technology, patient knowledge and acceptance combined with personalized specialty care. I have had good success with helping patients who were not successful using PAP 10 or 20 years ago to become successful through guidance and coaching. When we encounter anxiety or claustrophobia with PAP, we can incorporate behavioral sleep principles into the patient’s care to help them better acclimate to PAP therapy or consider alternative therapies.
Q: With more than 70 million US adults affected by sleep disorders and a growing number of clinicians and sleep practitioners gaining expertise in virtual models for diagnosis and treatments, what is your approach to using the telehealth to provide the same level of support, education, and therapy at home versus in the office?
Ms. Colvin: The pandemic definitely pushed us farther along in how we use telehealth. Before the pandemic, I was utilizing it in small increments, but there were some limits as a result of either regulation or reimbursement that caused it to not be included as a larger part of our program. However, now that we've experienced this shift with the pandemic, our health system has invested more in technology, and exposed more patients to the experience, I think telehealth and our usage of it will be different once we come out on the other side of this public health crisis. So, now we must decide, how are we going to use this mode as an alternative model of care?
I see two main focuses for us in sleep—expanding patient access and patient convenience. As technology improves, it will expand access for patients with less accessibility to technology and the internet, such as those in rural areas. And with smartphones becoming even more readily available and more capable of doing virtual care, we see potential to reach out and treat patients who we would otherwise not be able to offer treatment to.
Patient convenience is also very important. With virtual visits, we may be able to keep patients from having to leave work as they may be able to just ‘pop out’ at lunch, have a visit, and then go right back to work. Doing so also helps if patients have care giving responsibilities as they don't have to, for example, find a babysitter to come in for an office visit as they can make the necessary arrangements from home.
In lockstep with patient access and convenience, I am interested to see how telehealth, over time, manifests for patients with disabilities but we are already seeing the benefits of its application within this population.
I have a patient who is confined to a wheelchair so for this patient to get to a visit requires significant planning time to get into the van and be driven to the clinic by his caregiver, who has to schedule time off from work. So, it is not an easy process for this patient to come in and see me for a quick visit. With telehealth, this simple visit doesn’t have to be a whole- or half-day affair as it can be a quick check-in. If an in-person visit is warranted, we can always arrange that but usually we can accomplish what we need to on video and audio.
Another example is with those patients with hearing impairments. Depending on the impairment, certain patients may be able to use Bluetooth or audio enhancement with their hearing aids and can actually hear me better in a video environment than they can in the clinic; especially at this point in time as we are masked when in the office.
Q: In what ways do you think the telemedicine diagnosis process might be impacted post-pandemic?
Ms. Colvin: At-home sleep testing became available several years ago, but it has a limited role as it is specifically for the diagnosis of sleep apnea in the uncomplicated patient. Telehealth offers some convenience in enabling patients to be tested in their home and it is also more affordable for the patient and for insurance. In fact, this is seen as one of the disruptors in our field that will continue to expand in the appropriate patient populations. But we will always have to acknowledge that it won't serve all patient needs because our more complex patients still need to come in for in-person testing.
Q: Overall, in what ways do sleep professionals support the value of having a specialist care model versus a generalist PCP model to perform patient care within the US as well as other countries?
Ms. Colvin: From my view, I see that our PCPs are already stretched thin in their ability to provide easily accessible care and I think it would be difficult for them to also provide the specialty care that patients with sleep disorders need. Some of the less complex patients might be able to stay within a primary care environment but as technology, as well as the software training that is required to be able to communicate with the devices our patients use for treatment or for diagnosis, continues to become more complex, it can become difficult to manage through the primary care environment.
The question then goes back to, how can we be as accessible as possible in an underserved area or where the specialty clinic is not easy to access? I think this is where telehealth may give us new options for expanding access to patients who can use the technology that is available.
Loretta J. Colvin, APRN, ACNP-BC, is a Nurse Practitioner at Sleep Services, SSM Health Medical Group, specializing in the treatment of sleep disorders. Her focus is on insomnia, narcolepsy, obstructive sleep apnea, parasomnias, periodic leg movement disorder, restless leg syndrome, sleep disorders, snoring and telehealth.
Q: As a nurse practitioner and a specialist at a clinic focused on identifying sleep related disorders, what do you feel is the overall value of such a clinic as a therapeutic need for patients?
Ms. Colvin: Well, for us in sleep, we've experienced a growth in our field, and anticipate further growth. We know that many people remain undiagnosed with their sleep disorders. So, we see our role as filling a need for the public in providing more personalized and specialized care for those that have a sleep problem.
Patients get to us via many routes, including self-referral or referral by their primary care provider or specialist. We help these patients identify their sleep problems, guide them in testing and treatment decisions, and provide on-going treatment support.
Currently, there are two areas in which there is a particular need. With current stressors impacting our society, there is an increased need for patients to discuss their insomnia or sleep difficulty concerns with a healthcare provider. We are seeing more patients bring up insomnia concerns to their primary care providers or coming directly to our sleep clinic for discussion of these concerns. As the recognition of the importance of diagnosing and treatment sleep apnea continues to grow, we see more patients coming to us for care, including those with less obvious symptoms but high risk of sleep apnea due to their comorbid diseases.
Q: Given current predictions that the outpatient health care structure will change and the number of APRNs and PAs will increase, what is your perspective on role and utilization of APPs, as well as the need to plan for the future care of patients with sleep disorders?
Ms. Colvin: First, let me talk about the national landscape with regards to structure. Nationally, the APP role and the number of professionals in that role is growing, so that's going to help meet some of the needs of our society for providing health care, whether it be primary care or specialty care, like I do, or acute care in the hospital. At present, there are a significant number of places where we fill a needed role but within the area of sleep, there is a likely to be an increase in need for APPs as a result of attrition in the field as well as a shift in how many physicians are available to provide care in our ever-expanding specialty. What we then need to do is figure out how do we train these Providers in a specialty? This is not a specialty that is a large part of our basic education, so how do we train people into that specialty? And how do we prepare them for their role and ensure that we are offering opportunities to expand their capabilities over time?
An example of this can be seen with regards to the opportunities now being offered in telehealth in behavioral sleep medicine and in working with conditions like insomnia, or with people who have difficulty in trying to adjust to Continuous Positive Airway Pressure (CPAP) therapy and might need additional assistance and coaching.
Within my organization, physicians will refer patients directly to me who have struggled to use PAP in the past. With specialized guidance, these past struggles can often be overcome thanks to improvements in our equipment and technology, patient knowledge and acceptance combined with personalized specialty care. I have had good success with helping patients who were not successful using PAP 10 or 20 years ago to become successful through guidance and coaching. When we encounter anxiety or claustrophobia with PAP, we can incorporate behavioral sleep principles into the patient’s care to help them better acclimate to PAP therapy or consider alternative therapies.
Q: With more than 70 million US adults affected by sleep disorders and a growing number of clinicians and sleep practitioners gaining expertise in virtual models for diagnosis and treatments, what is your approach to using the telehealth to provide the same level of support, education, and therapy at home versus in the office?
Ms. Colvin: The pandemic definitely pushed us farther along in how we use telehealth. Before the pandemic, I was utilizing it in small increments, but there were some limits as a result of either regulation or reimbursement that caused it to not be included as a larger part of our program. However, now that we've experienced this shift with the pandemic, our health system has invested more in technology, and exposed more patients to the experience, I think telehealth and our usage of it will be different once we come out on the other side of this public health crisis. So, now we must decide, how are we going to use this mode as an alternative model of care?
I see two main focuses for us in sleep—expanding patient access and patient convenience. As technology improves, it will expand access for patients with less accessibility to technology and the internet, such as those in rural areas. And with smartphones becoming even more readily available and more capable of doing virtual care, we see potential to reach out and treat patients who we would otherwise not be able to offer treatment to.
Patient convenience is also very important. With virtual visits, we may be able to keep patients from having to leave work as they may be able to just ‘pop out’ at lunch, have a visit, and then go right back to work. Doing so also helps if patients have care giving responsibilities as they don't have to, for example, find a babysitter to come in for an office visit as they can make the necessary arrangements from home.
In lockstep with patient access and convenience, I am interested to see how telehealth, over time, manifests for patients with disabilities but we are already seeing the benefits of its application within this population.
I have a patient who is confined to a wheelchair so for this patient to get to a visit requires significant planning time to get into the van and be driven to the clinic by his caregiver, who has to schedule time off from work. So, it is not an easy process for this patient to come in and see me for a quick visit. With telehealth, this simple visit doesn’t have to be a whole- or half-day affair as it can be a quick check-in. If an in-person visit is warranted, we can always arrange that but usually we can accomplish what we need to on video and audio.
Another example is with those patients with hearing impairments. Depending on the impairment, certain patients may be able to use Bluetooth or audio enhancement with their hearing aids and can actually hear me better in a video environment than they can in the clinic; especially at this point in time as we are masked when in the office.
Q: In what ways do you think the telemedicine diagnosis process might be impacted post-pandemic?
Ms. Colvin: At-home sleep testing became available several years ago, but it has a limited role as it is specifically for the diagnosis of sleep apnea in the uncomplicated patient. Telehealth offers some convenience in enabling patients to be tested in their home and it is also more affordable for the patient and for insurance. In fact, this is seen as one of the disruptors in our field that will continue to expand in the appropriate patient populations. But we will always have to acknowledge that it won't serve all patient needs because our more complex patients still need to come in for in-person testing.
Q: Overall, in what ways do sleep professionals support the value of having a specialist care model versus a generalist PCP model to perform patient care within the US as well as other countries?
Ms. Colvin: From my view, I see that our PCPs are already stretched thin in their ability to provide easily accessible care and I think it would be difficult for them to also provide the specialty care that patients with sleep disorders need. Some of the less complex patients might be able to stay within a primary care environment but as technology, as well as the software training that is required to be able to communicate with the devices our patients use for treatment or for diagnosis, continues to become more complex, it can become difficult to manage through the primary care environment.
The question then goes back to, how can we be as accessible as possible in an underserved area or where the specialty clinic is not easy to access? I think this is where telehealth may give us new options for expanding access to patients who can use the technology that is available.
Loretta J. Colvin, APRN, ACNP-BC, is a Nurse Practitioner at Sleep Services, SSM Health Medical Group, specializing in the treatment of sleep disorders. Her focus is on insomnia, narcolepsy, obstructive sleep apnea, parasomnias, periodic leg movement disorder, restless leg syndrome, sleep disorders, snoring and telehealth.
Q: As a nurse practitioner and a specialist at a clinic focused on identifying sleep related disorders, what do you feel is the overall value of such a clinic as a therapeutic need for patients?
Ms. Colvin: Well, for us in sleep, we've experienced a growth in our field, and anticipate further growth. We know that many people remain undiagnosed with their sleep disorders. So, we see our role as filling a need for the public in providing more personalized and specialized care for those that have a sleep problem.
Patients get to us via many routes, including self-referral or referral by their primary care provider or specialist. We help these patients identify their sleep problems, guide them in testing and treatment decisions, and provide on-going treatment support.
Currently, there are two areas in which there is a particular need. With current stressors impacting our society, there is an increased need for patients to discuss their insomnia or sleep difficulty concerns with a healthcare provider. We are seeing more patients bring up insomnia concerns to their primary care providers or coming directly to our sleep clinic for discussion of these concerns. As the recognition of the importance of diagnosing and treatment sleep apnea continues to grow, we see more patients coming to us for care, including those with less obvious symptoms but high risk of sleep apnea due to their comorbid diseases.
Q: Given current predictions that the outpatient health care structure will change and the number of APRNs and PAs will increase, what is your perspective on role and utilization of APPs, as well as the need to plan for the future care of patients with sleep disorders?
Ms. Colvin: First, let me talk about the national landscape with regards to structure. Nationally, the APP role and the number of professionals in that role is growing, so that's going to help meet some of the needs of our society for providing health care, whether it be primary care or specialty care, like I do, or acute care in the hospital. At present, there are a significant number of places where we fill a needed role but within the area of sleep, there is a likely to be an increase in need for APPs as a result of attrition in the field as well as a shift in how many physicians are available to provide care in our ever-expanding specialty. What we then need to do is figure out how do we train these Providers in a specialty? This is not a specialty that is a large part of our basic education, so how do we train people into that specialty? And how do we prepare them for their role and ensure that we are offering opportunities to expand their capabilities over time?
An example of this can be seen with regards to the opportunities now being offered in telehealth in behavioral sleep medicine and in working with conditions like insomnia, or with people who have difficulty in trying to adjust to Continuous Positive Airway Pressure (CPAP) therapy and might need additional assistance and coaching.
Within my organization, physicians will refer patients directly to me who have struggled to use PAP in the past. With specialized guidance, these past struggles can often be overcome thanks to improvements in our equipment and technology, patient knowledge and acceptance combined with personalized specialty care. I have had good success with helping patients who were not successful using PAP 10 or 20 years ago to become successful through guidance and coaching. When we encounter anxiety or claustrophobia with PAP, we can incorporate behavioral sleep principles into the patient’s care to help them better acclimate to PAP therapy or consider alternative therapies.
Q: With more than 70 million US adults affected by sleep disorders and a growing number of clinicians and sleep practitioners gaining expertise in virtual models for diagnosis and treatments, what is your approach to using the telehealth to provide the same level of support, education, and therapy at home versus in the office?
Ms. Colvin: The pandemic definitely pushed us farther along in how we use telehealth. Before the pandemic, I was utilizing it in small increments, but there were some limits as a result of either regulation or reimbursement that caused it to not be included as a larger part of our program. However, now that we've experienced this shift with the pandemic, our health system has invested more in technology, and exposed more patients to the experience, I think telehealth and our usage of it will be different once we come out on the other side of this public health crisis. So, now we must decide, how are we going to use this mode as an alternative model of care?
I see two main focuses for us in sleep—expanding patient access and patient convenience. As technology improves, it will expand access for patients with less accessibility to technology and the internet, such as those in rural areas. And with smartphones becoming even more readily available and more capable of doing virtual care, we see potential to reach out and treat patients who we would otherwise not be able to offer treatment to.
Patient convenience is also very important. With virtual visits, we may be able to keep patients from having to leave work as they may be able to just ‘pop out’ at lunch, have a visit, and then go right back to work. Doing so also helps if patients have care giving responsibilities as they don't have to, for example, find a babysitter to come in for an office visit as they can make the necessary arrangements from home.
In lockstep with patient access and convenience, I am interested to see how telehealth, over time, manifests for patients with disabilities but we are already seeing the benefits of its application within this population.
I have a patient who is confined to a wheelchair so for this patient to get to a visit requires significant planning time to get into the van and be driven to the clinic by his caregiver, who has to schedule time off from work. So, it is not an easy process for this patient to come in and see me for a quick visit. With telehealth, this simple visit doesn’t have to be a whole- or half-day affair as it can be a quick check-in. If an in-person visit is warranted, we can always arrange that but usually we can accomplish what we need to on video and audio.
Another example is with those patients with hearing impairments. Depending on the impairment, certain patients may be able to use Bluetooth or audio enhancement with their hearing aids and can actually hear me better in a video environment than they can in the clinic; especially at this point in time as we are masked when in the office.
Q: In what ways do you think the telemedicine diagnosis process might be impacted post-pandemic?
Ms. Colvin: At-home sleep testing became available several years ago, but it has a limited role as it is specifically for the diagnosis of sleep apnea in the uncomplicated patient. Telehealth offers some convenience in enabling patients to be tested in their home and it is also more affordable for the patient and for insurance. In fact, this is seen as one of the disruptors in our field that will continue to expand in the appropriate patient populations. But we will always have to acknowledge that it won't serve all patient needs because our more complex patients still need to come in for in-person testing.
Q: Overall, in what ways do sleep professionals support the value of having a specialist care model versus a generalist PCP model to perform patient care within the US as well as other countries?
Ms. Colvin: From my view, I see that our PCPs are already stretched thin in their ability to provide easily accessible care and I think it would be difficult for them to also provide the specialty care that patients with sleep disorders need. Some of the less complex patients might be able to stay within a primary care environment but as technology, as well as the software training that is required to be able to communicate with the devices our patients use for treatment or for diagnosis, continues to become more complex, it can become difficult to manage through the primary care environment.
The question then goes back to, how can we be as accessible as possible in an underserved area or where the specialty clinic is not easy to access? I think this is where telehealth may give us new options for expanding access to patients who can use the technology that is available.
Four police suicides in the aftermath of the Capitol siege: What can we learn?
Officer Scott Davis is a passionate man who thinks and talks quickly. As a member of the Special Events Team for Montgomery County, Maryland, he was already staging in Rockville, outside of Washington, D.C., when the call came in last Jan. 6 to move their unit to the U.S. Capitol.
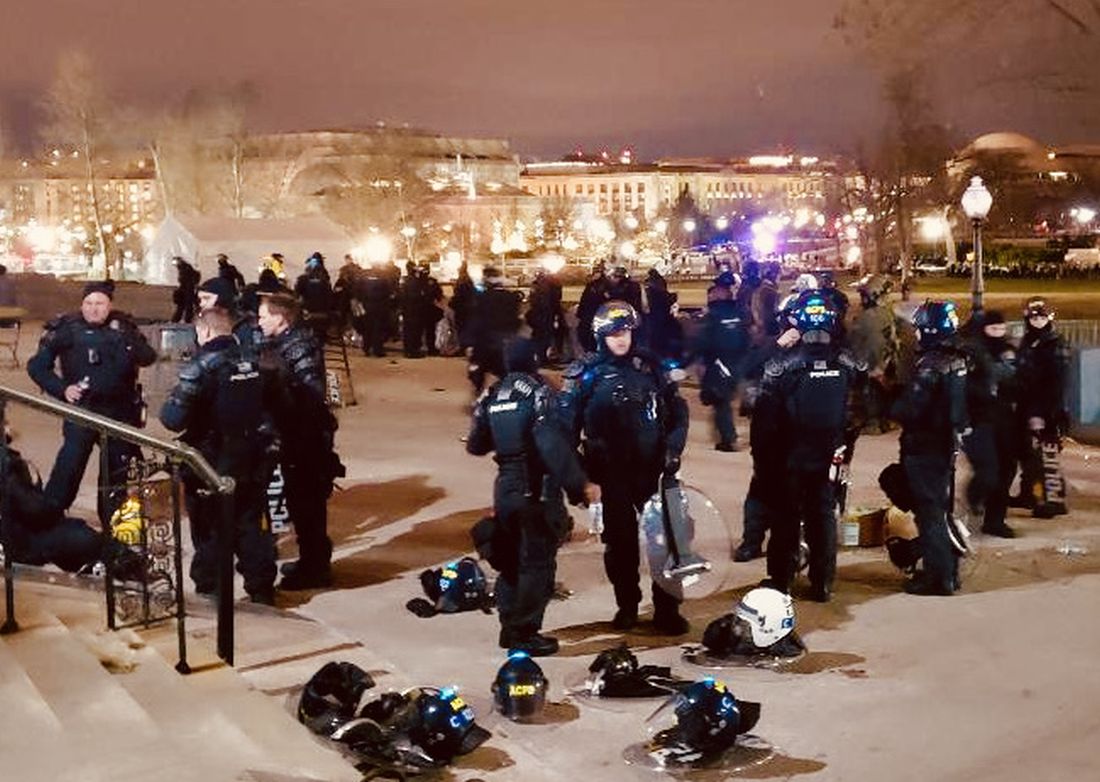
“It was surreal,” said Mr. Davis. “There were people from all different groups at the Capitol that day. Many people were trying to get out, but others surrounded us. They called us ‘human race traitors.’ And then I heard someone say, ‘It’s good you brought your shields, we’ll carry your bodies out on them.’”
Mr. Davis described hours of mayhem during which he was hit with bear spray, a brick, a chair, and a metal rod. One of the members of Mr. Davis’ unit remains on leave with a head injury nearly 9 months after the siege.
“It went on for 3 hours, but it felt like 15 minutes. Then, all of a sudden, it was over.”
For the members of law enforcement at the Capitol that day, the repercussions are still being felt, perhaps most notably in the case of the four officers who subsequently died of suicide. Three of the officers were with the Metropolitan Police Department of the District of Columbia and one worked for the Capitol Police Department.
Police officers are subjected to traumas on a regular basis and often placed in circumstances where their lives are in danger. Yet four suicides within a short time – all connected to a single event – is particularly shocking and tragic, even more so for how little attention it has garnered to date.
What contributes to the high rate of suicide among officers?
Scott Silverii, PhD, a former police officer and author of Broken and Blue: A Policeman’s Guide to Health, Hope, and Healing, commented that he “wouldn’t be surprised if there are more suicides to come.” This stems not only from the experiences of that day but also the elevated risk for suicide that law enforcement officers already experienced prior to the Capitol riots. Suicide remains a rare event, with a national all-population average of 13.9 per 100,000 citizens. But as Dr. Silverii noted, more officers die by suicide each year than are killed in the line of duty.
“Suicide is a big part of police culture – officers are doers and fixers, and it is seen as being more honorable to take yourself out of the equation than it is to ask for help,” he said. “Most officers come in with past pain, and this is a situation where they are being overwhelmed and under-respected. At the same time, police culture is a closed culture, and it is not friendly to researchers.”
Another contributor is the frequency with which law enforcement officers are exposed to trauma, according to Vernon Herron, Director of Officer Safety and Wellness for the Baltimore City Police.
Mr. Herron said, citing the psychiatric and addiction issues that officers commonly experience.
Protecting the protectors
Mr. Herron and others are working to address these problems head-on.
“We are trying to identify employees exposed to trauma and to offer counseling and intervention,” he said, “Otherwise, everything else will fall short.”
Yet implementing such measures is no easy task, given the lack of a central oversight organization for law enforcement, said Sheldon Greenberg, PhD, a former police officer and professor of management in the School of Education at Johns Hopkins University, Baltimore.
“In the United States there is no such thing as ‘The Police.’ There is no one in a position to set policy, standards, or training mandates nationally,” he said. “There are approximately 18,000 police and sheriff departments in the country, and many of them are small. No one can compel law enforcement agencies to implement officer wellness and suicide prevention programs, make counseling available to officers, or train supervisors and peers to identify suicide ideation.”
Dr. Greenberg said a further barrier to helping police officers considering self-harm is posed by the fact that even if they do seek out counseling, there is no guarantee that it will remain confidential.
“Support personnel have an obligation to report an officer who is thinking about committing suicide,” he said. “Many officers are concerned about this lack of confidentiality and that they may be branded if they seek help.”
Although Dr. Greenberg said many police officers are self-professed “action junkies,” even their unusually high capacity for stress is often tested by the realities of the job.
“Increasing demands for service, shortages of personnel, misinformation about police, COVID-19, talk about restructuring policing with little concrete direction, increased exposure to violence, greater numbers of vulnerable people, and more take a toll over time,” he lamented. “In addition, we are in a recruiting crisis in law enforcement, and there are no standards to ensure the quality of psychological screening provided to applicants. Many officers will go through their entire career and never be screened again. We know little about the stresses and strains that officers bring to the job.”
After the siege
It is not clear how many police officers were present at the Capitol on Jan. 6. During the chaos of the day, reinforcements to the Capitol Police Department arrived from Washington D.C., Maryland, and Virginia, but no official numbers on responders were obtained; Mr. Davis thought it was likely that there were at least 1,000 law enforcement officers present. Those who did respond sustained an estimated 100 injuries, including an officer who died the next day. Of the officers who died by suicide, one died 3 days after, another died 9 days later, and two more died in July – numbers that contradict the notion that this is some coincidence. Officer Alexander Kettering, a colleague of Mr. Davis who has been with Montgomery County Police for 15 years, was among those tasked with protecting the Capitol on Jan. 6. The chaos, violence, and destruction of the day has stuck with him and continues to occupy his thoughts.
“I had a front-row seat to the whole thing. It was overwhelming, and I’ve never seen people this angry,” said Mr. Kettering. “There were people up on the veranda and on the scaffolding set up for the inauguration. They were smashing windows and throwing things into the crowd. It was insane. There were decent people coming up to us and saying they would pray for us, then others calling us traitors, telling us to stand down and join them.”
In the aftermath of the Capitol siege, Mr. Kettering watched in dismay as the narrative of the day’s events began to warp.
“At first there was a consensus that what happened was so wrong, and then the politics took over. People were saying it wasn’t as bad as the media said, that it really wasn’t that violent and those speaking out are traitors or political operatives. I relive it every day, and it’s hard to escape, even in casual conversation.”
He added that the days’ events were compounded by the already heightened tensions surrounding the national debate around policing.
“It’s been 18 months of stress, of anti-police movements, and there is a fine line between addressing police brutality and being anti-police,” Mr. Kettering said, noting that the aforementioned issues have all contributed to the ongoing struggles his fellow officers are experiencing.
“It’s not a thing for cops to talk about how an event affected them,” he said. “A lot of officers have just shut down. People have careers and pensions to protect, and every time we stop a motorist, something could go wrong, even if we do everything right. There are mixed signals: They tell us, ‘Defend but don’t defend.’”
His colleague, Mr. Davis, said that officers “need more support from politicians,” noting that he felt particularly insulted by a comment made by a Montgomery County public official who accused the officers present at the Capitol of racism. “And finally, we feel a little betrayed by the public.”
More questions than answers from the Capitol’s day of chaos
What about the events of Jan. 6 led to the suicides of four law enforcement officers and what can be done to prevent more deaths in the future? There are the individual factors of each man’s personal history, circumstances, and vulnerabilities, including the sense of being personally endangered, witnessing trauma, and direct injury – one officer who died of suicide had sustained a head injury that day.
We don’t know if the officers went into the event with preexisting mental illness or addiction or if the day’s events precipitated psychiatric episodes. And with all the partisan anger surrounding the presidential election, we don’t know if each officer’s political beliefs amplified his distress over what occurred in a social media climate where police are being faulted by all sides.
When multiple suicides occur in a community, there is always concern about a “copycat” phenomena. These concerns are made more difficult to address, however, given the police culture of taboo and stigma associated with getting professional help, difficulty accessing care, and career repercussions for speaking openly about suicidal thoughts and mental health issues.
Finally, there is the current political agenda that leaves officers feeling unsupported, fearful of negative outcomes, and unappreciated. The Capitol siege in particular embodied a great deal of national distress and confusion over basic issues of truth, justice, and perceptions of reality in our polarized society.
Can we move to a place where those who enforce laws have easy access to treatment, free from stigma? Can we encourage a culture that does not tolerate brutality or racism, while also refusing to label all police as bad and lending support to their mission? Can we be more attuned to the repercussions of circumstances where officers are witnesses to trauma, are endangered themselves, and would benefit from acknowledgment of their distress?
Time will tell if our anti-police pendulum swings back. In the meantime, these four suicides among people defending our country remain tragically overlooked.
Dinah Miller, MD, is coauthor of Committed: The Battle Over Involuntary Psychiatric Care (Johns Hopkins University Press, 2016). She has a private practice in Baltimore and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University. A version of this article first appeared on Medscape.com.
Officer Scott Davis is a passionate man who thinks and talks quickly. As a member of the Special Events Team for Montgomery County, Maryland, he was already staging in Rockville, outside of Washington, D.C., when the call came in last Jan. 6 to move their unit to the U.S. Capitol.
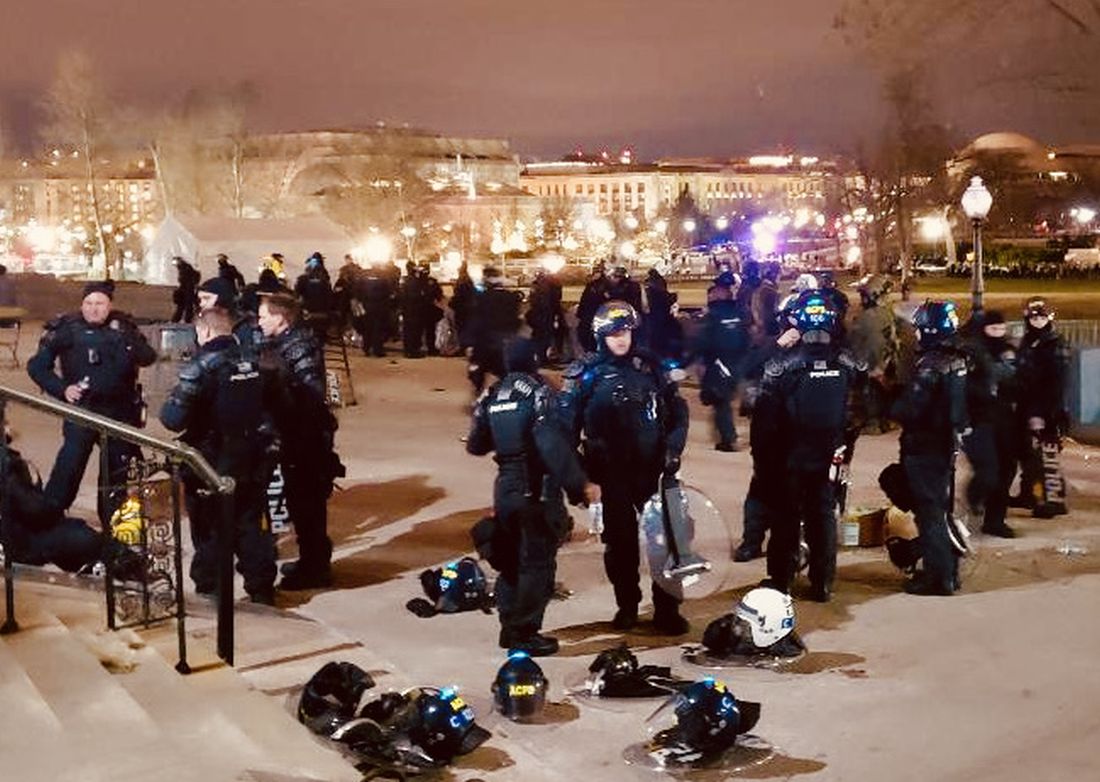
“It was surreal,” said Mr. Davis. “There were people from all different groups at the Capitol that day. Many people were trying to get out, but others surrounded us. They called us ‘human race traitors.’ And then I heard someone say, ‘It’s good you brought your shields, we’ll carry your bodies out on them.’”
Mr. Davis described hours of mayhem during which he was hit with bear spray, a brick, a chair, and a metal rod. One of the members of Mr. Davis’ unit remains on leave with a head injury nearly 9 months after the siege.
“It went on for 3 hours, but it felt like 15 minutes. Then, all of a sudden, it was over.”
For the members of law enforcement at the Capitol that day, the repercussions are still being felt, perhaps most notably in the case of the four officers who subsequently died of suicide. Three of the officers were with the Metropolitan Police Department of the District of Columbia and one worked for the Capitol Police Department.
Police officers are subjected to traumas on a regular basis and often placed in circumstances where their lives are in danger. Yet four suicides within a short time – all connected to a single event – is particularly shocking and tragic, even more so for how little attention it has garnered to date.
What contributes to the high rate of suicide among officers?
Scott Silverii, PhD, a former police officer and author of Broken and Blue: A Policeman’s Guide to Health, Hope, and Healing, commented that he “wouldn’t be surprised if there are more suicides to come.” This stems not only from the experiences of that day but also the elevated risk for suicide that law enforcement officers already experienced prior to the Capitol riots. Suicide remains a rare event, with a national all-population average of 13.9 per 100,000 citizens. But as Dr. Silverii noted, more officers die by suicide each year than are killed in the line of duty.
“Suicide is a big part of police culture – officers are doers and fixers, and it is seen as being more honorable to take yourself out of the equation than it is to ask for help,” he said. “Most officers come in with past pain, and this is a situation where they are being overwhelmed and under-respected. At the same time, police culture is a closed culture, and it is not friendly to researchers.”
Another contributor is the frequency with which law enforcement officers are exposed to trauma, according to Vernon Herron, Director of Officer Safety and Wellness for the Baltimore City Police.
Mr. Herron said, citing the psychiatric and addiction issues that officers commonly experience.
Protecting the protectors
Mr. Herron and others are working to address these problems head-on.
“We are trying to identify employees exposed to trauma and to offer counseling and intervention,” he said, “Otherwise, everything else will fall short.”
Yet implementing such measures is no easy task, given the lack of a central oversight organization for law enforcement, said Sheldon Greenberg, PhD, a former police officer and professor of management in the School of Education at Johns Hopkins University, Baltimore.
“In the United States there is no such thing as ‘The Police.’ There is no one in a position to set policy, standards, or training mandates nationally,” he said. “There are approximately 18,000 police and sheriff departments in the country, and many of them are small. No one can compel law enforcement agencies to implement officer wellness and suicide prevention programs, make counseling available to officers, or train supervisors and peers to identify suicide ideation.”
Dr. Greenberg said a further barrier to helping police officers considering self-harm is posed by the fact that even if they do seek out counseling, there is no guarantee that it will remain confidential.
“Support personnel have an obligation to report an officer who is thinking about committing suicide,” he said. “Many officers are concerned about this lack of confidentiality and that they may be branded if they seek help.”
Although Dr. Greenberg said many police officers are self-professed “action junkies,” even their unusually high capacity for stress is often tested by the realities of the job.
“Increasing demands for service, shortages of personnel, misinformation about police, COVID-19, talk about restructuring policing with little concrete direction, increased exposure to violence, greater numbers of vulnerable people, and more take a toll over time,” he lamented. “In addition, we are in a recruiting crisis in law enforcement, and there are no standards to ensure the quality of psychological screening provided to applicants. Many officers will go through their entire career and never be screened again. We know little about the stresses and strains that officers bring to the job.”
After the siege
It is not clear how many police officers were present at the Capitol on Jan. 6. During the chaos of the day, reinforcements to the Capitol Police Department arrived from Washington D.C., Maryland, and Virginia, but no official numbers on responders were obtained; Mr. Davis thought it was likely that there were at least 1,000 law enforcement officers present. Those who did respond sustained an estimated 100 injuries, including an officer who died the next day. Of the officers who died by suicide, one died 3 days after, another died 9 days later, and two more died in July – numbers that contradict the notion that this is some coincidence. Officer Alexander Kettering, a colleague of Mr. Davis who has been with Montgomery County Police for 15 years, was among those tasked with protecting the Capitol on Jan. 6. The chaos, violence, and destruction of the day has stuck with him and continues to occupy his thoughts.
“I had a front-row seat to the whole thing. It was overwhelming, and I’ve never seen people this angry,” said Mr. Kettering. “There were people up on the veranda and on the scaffolding set up for the inauguration. They were smashing windows and throwing things into the crowd. It was insane. There were decent people coming up to us and saying they would pray for us, then others calling us traitors, telling us to stand down and join them.”
In the aftermath of the Capitol siege, Mr. Kettering watched in dismay as the narrative of the day’s events began to warp.
“At first there was a consensus that what happened was so wrong, and then the politics took over. People were saying it wasn’t as bad as the media said, that it really wasn’t that violent and those speaking out are traitors or political operatives. I relive it every day, and it’s hard to escape, even in casual conversation.”
He added that the days’ events were compounded by the already heightened tensions surrounding the national debate around policing.
“It’s been 18 months of stress, of anti-police movements, and there is a fine line between addressing police brutality and being anti-police,” Mr. Kettering said, noting that the aforementioned issues have all contributed to the ongoing struggles his fellow officers are experiencing.
“It’s not a thing for cops to talk about how an event affected them,” he said. “A lot of officers have just shut down. People have careers and pensions to protect, and every time we stop a motorist, something could go wrong, even if we do everything right. There are mixed signals: They tell us, ‘Defend but don’t defend.’”
His colleague, Mr. Davis, said that officers “need more support from politicians,” noting that he felt particularly insulted by a comment made by a Montgomery County public official who accused the officers present at the Capitol of racism. “And finally, we feel a little betrayed by the public.”
More questions than answers from the Capitol’s day of chaos
What about the events of Jan. 6 led to the suicides of four law enforcement officers and what can be done to prevent more deaths in the future? There are the individual factors of each man’s personal history, circumstances, and vulnerabilities, including the sense of being personally endangered, witnessing trauma, and direct injury – one officer who died of suicide had sustained a head injury that day.
We don’t know if the officers went into the event with preexisting mental illness or addiction or if the day’s events precipitated psychiatric episodes. And with all the partisan anger surrounding the presidential election, we don’t know if each officer’s political beliefs amplified his distress over what occurred in a social media climate where police are being faulted by all sides.
When multiple suicides occur in a community, there is always concern about a “copycat” phenomena. These concerns are made more difficult to address, however, given the police culture of taboo and stigma associated with getting professional help, difficulty accessing care, and career repercussions for speaking openly about suicidal thoughts and mental health issues.
Finally, there is the current political agenda that leaves officers feeling unsupported, fearful of negative outcomes, and unappreciated. The Capitol siege in particular embodied a great deal of national distress and confusion over basic issues of truth, justice, and perceptions of reality in our polarized society.
Can we move to a place where those who enforce laws have easy access to treatment, free from stigma? Can we encourage a culture that does not tolerate brutality or racism, while also refusing to label all police as bad and lending support to their mission? Can we be more attuned to the repercussions of circumstances where officers are witnesses to trauma, are endangered themselves, and would benefit from acknowledgment of their distress?
Time will tell if our anti-police pendulum swings back. In the meantime, these four suicides among people defending our country remain tragically overlooked.
Dinah Miller, MD, is coauthor of Committed: The Battle Over Involuntary Psychiatric Care (Johns Hopkins University Press, 2016). She has a private practice in Baltimore and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University. A version of this article first appeared on Medscape.com.
Officer Scott Davis is a passionate man who thinks and talks quickly. As a member of the Special Events Team for Montgomery County, Maryland, he was already staging in Rockville, outside of Washington, D.C., when the call came in last Jan. 6 to move their unit to the U.S. Capitol.
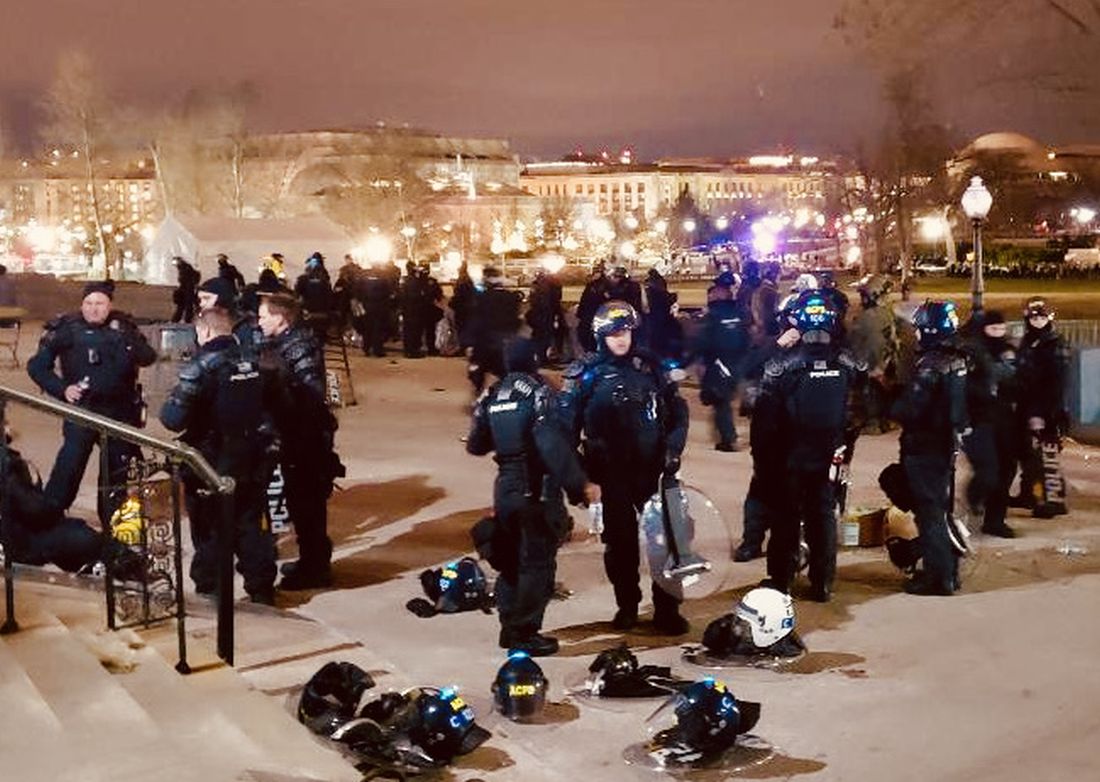
“It was surreal,” said Mr. Davis. “There were people from all different groups at the Capitol that day. Many people were trying to get out, but others surrounded us. They called us ‘human race traitors.’ And then I heard someone say, ‘It’s good you brought your shields, we’ll carry your bodies out on them.’”
Mr. Davis described hours of mayhem during which he was hit with bear spray, a brick, a chair, and a metal rod. One of the members of Mr. Davis’ unit remains on leave with a head injury nearly 9 months after the siege.
“It went on for 3 hours, but it felt like 15 minutes. Then, all of a sudden, it was over.”
For the members of law enforcement at the Capitol that day, the repercussions are still being felt, perhaps most notably in the case of the four officers who subsequently died of suicide. Three of the officers were with the Metropolitan Police Department of the District of Columbia and one worked for the Capitol Police Department.
Police officers are subjected to traumas on a regular basis and often placed in circumstances where their lives are in danger. Yet four suicides within a short time – all connected to a single event – is particularly shocking and tragic, even more so for how little attention it has garnered to date.
What contributes to the high rate of suicide among officers?
Scott Silverii, PhD, a former police officer and author of Broken and Blue: A Policeman’s Guide to Health, Hope, and Healing, commented that he “wouldn’t be surprised if there are more suicides to come.” This stems not only from the experiences of that day but also the elevated risk for suicide that law enforcement officers already experienced prior to the Capitol riots. Suicide remains a rare event, with a national all-population average of 13.9 per 100,000 citizens. But as Dr. Silverii noted, more officers die by suicide each year than are killed in the line of duty.
“Suicide is a big part of police culture – officers are doers and fixers, and it is seen as being more honorable to take yourself out of the equation than it is to ask for help,” he said. “Most officers come in with past pain, and this is a situation where they are being overwhelmed and under-respected. At the same time, police culture is a closed culture, and it is not friendly to researchers.”
Another contributor is the frequency with which law enforcement officers are exposed to trauma, according to Vernon Herron, Director of Officer Safety and Wellness for the Baltimore City Police.
Mr. Herron said, citing the psychiatric and addiction issues that officers commonly experience.
Protecting the protectors
Mr. Herron and others are working to address these problems head-on.
“We are trying to identify employees exposed to trauma and to offer counseling and intervention,” he said, “Otherwise, everything else will fall short.”
Yet implementing such measures is no easy task, given the lack of a central oversight organization for law enforcement, said Sheldon Greenberg, PhD, a former police officer and professor of management in the School of Education at Johns Hopkins University, Baltimore.
“In the United States there is no such thing as ‘The Police.’ There is no one in a position to set policy, standards, or training mandates nationally,” he said. “There are approximately 18,000 police and sheriff departments in the country, and many of them are small. No one can compel law enforcement agencies to implement officer wellness and suicide prevention programs, make counseling available to officers, or train supervisors and peers to identify suicide ideation.”
Dr. Greenberg said a further barrier to helping police officers considering self-harm is posed by the fact that even if they do seek out counseling, there is no guarantee that it will remain confidential.
“Support personnel have an obligation to report an officer who is thinking about committing suicide,” he said. “Many officers are concerned about this lack of confidentiality and that they may be branded if they seek help.”
Although Dr. Greenberg said many police officers are self-professed “action junkies,” even their unusually high capacity for stress is often tested by the realities of the job.
“Increasing demands for service, shortages of personnel, misinformation about police, COVID-19, talk about restructuring policing with little concrete direction, increased exposure to violence, greater numbers of vulnerable people, and more take a toll over time,” he lamented. “In addition, we are in a recruiting crisis in law enforcement, and there are no standards to ensure the quality of psychological screening provided to applicants. Many officers will go through their entire career and never be screened again. We know little about the stresses and strains that officers bring to the job.”
After the siege
It is not clear how many police officers were present at the Capitol on Jan. 6. During the chaos of the day, reinforcements to the Capitol Police Department arrived from Washington D.C., Maryland, and Virginia, but no official numbers on responders were obtained; Mr. Davis thought it was likely that there were at least 1,000 law enforcement officers present. Those who did respond sustained an estimated 100 injuries, including an officer who died the next day. Of the officers who died by suicide, one died 3 days after, another died 9 days later, and two more died in July – numbers that contradict the notion that this is some coincidence. Officer Alexander Kettering, a colleague of Mr. Davis who has been with Montgomery County Police for 15 years, was among those tasked with protecting the Capitol on Jan. 6. The chaos, violence, and destruction of the day has stuck with him and continues to occupy his thoughts.
“I had a front-row seat to the whole thing. It was overwhelming, and I’ve never seen people this angry,” said Mr. Kettering. “There were people up on the veranda and on the scaffolding set up for the inauguration. They were smashing windows and throwing things into the crowd. It was insane. There were decent people coming up to us and saying they would pray for us, then others calling us traitors, telling us to stand down and join them.”
In the aftermath of the Capitol siege, Mr. Kettering watched in dismay as the narrative of the day’s events began to warp.
“At first there was a consensus that what happened was so wrong, and then the politics took over. People were saying it wasn’t as bad as the media said, that it really wasn’t that violent and those speaking out are traitors or political operatives. I relive it every day, and it’s hard to escape, even in casual conversation.”
He added that the days’ events were compounded by the already heightened tensions surrounding the national debate around policing.
“It’s been 18 months of stress, of anti-police movements, and there is a fine line between addressing police brutality and being anti-police,” Mr. Kettering said, noting that the aforementioned issues have all contributed to the ongoing struggles his fellow officers are experiencing.
“It’s not a thing for cops to talk about how an event affected them,” he said. “A lot of officers have just shut down. People have careers and pensions to protect, and every time we stop a motorist, something could go wrong, even if we do everything right. There are mixed signals: They tell us, ‘Defend but don’t defend.’”
His colleague, Mr. Davis, said that officers “need more support from politicians,” noting that he felt particularly insulted by a comment made by a Montgomery County public official who accused the officers present at the Capitol of racism. “And finally, we feel a little betrayed by the public.”
More questions than answers from the Capitol’s day of chaos
What about the events of Jan. 6 led to the suicides of four law enforcement officers and what can be done to prevent more deaths in the future? There are the individual factors of each man’s personal history, circumstances, and vulnerabilities, including the sense of being personally endangered, witnessing trauma, and direct injury – one officer who died of suicide had sustained a head injury that day.
We don’t know if the officers went into the event with preexisting mental illness or addiction or if the day’s events precipitated psychiatric episodes. And with all the partisan anger surrounding the presidential election, we don’t know if each officer’s political beliefs amplified his distress over what occurred in a social media climate where police are being faulted by all sides.
When multiple suicides occur in a community, there is always concern about a “copycat” phenomena. These concerns are made more difficult to address, however, given the police culture of taboo and stigma associated with getting professional help, difficulty accessing care, and career repercussions for speaking openly about suicidal thoughts and mental health issues.
Finally, there is the current political agenda that leaves officers feeling unsupported, fearful of negative outcomes, and unappreciated. The Capitol siege in particular embodied a great deal of national distress and confusion over basic issues of truth, justice, and perceptions of reality in our polarized society.
Can we move to a place where those who enforce laws have easy access to treatment, free from stigma? Can we encourage a culture that does not tolerate brutality or racism, while also refusing to label all police as bad and lending support to their mission? Can we be more attuned to the repercussions of circumstances where officers are witnesses to trauma, are endangered themselves, and would benefit from acknowledgment of their distress?
Time will tell if our anti-police pendulum swings back. In the meantime, these four suicides among people defending our country remain tragically overlooked.
Dinah Miller, MD, is coauthor of Committed: The Battle Over Involuntary Psychiatric Care (Johns Hopkins University Press, 2016). She has a private practice in Baltimore and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University. A version of this article first appeared on Medscape.com.
Advocates seek to reframe masks as a disability accommodation
As governors and legislatures in states such as Texas, Florida, South Carolina, and Arkansas have banned schools and other entities from implementing mask mandates, disability rights advocates have pushed back. In federal civil rights lawsuits, they argue that bans on mask mandates violate antidiscrimination laws protecting people with disabilities.
For unvaccinated and immunosuppressed individuals, masks can provide crucial protection from SARS-CoV-2.
argues Mical Raz, MD, PhD, a professor at the University of Rochester (N.Y.) and a physician at Strong Memorial Hospital, also in Rochester, New York, in an article published in JAMA with coauthor Doron Dorfman, LLB, JSD.
This news organization talked with Dr. Raz about approaching mask requirements as disability accommodation during the COVID-19 pandemic. The following interview was lightly edited for length and clarity.
How did you come to think about mask requirements as a form of disability accommodation?
I saw a tweet from a professor at a university who said they couldn’t ask students about their vaccination status or to wear a mask. All agency was removed from the professor to take care of and protect themselves. I thought, well, that can’t be right. And ostensibly, that would be particularly dangerous for somebody with immunosuppression for whom the vaccine is not adequately protective. So, I called my friend, Doron Dorfman, and asked him to help me think through the legal part of this. We fleshed it out and wrote the article that same night.
How novel is it to view accommodations for people who are immunosuppressed through the lens of disability accommodation?
I think there has not been enough focus during the pandemic on individuals with disabilities or on how disability law can be mobilized during this pandemic to help supplement the public health law. This framework should be used a lot more because it’s good for everybody, not just for individuals with disabilities.
For example, take what’s called the “curb effect.” If you expand sidewalks, yes, it helps individuals who use a wheelchair. But it also helps me as a mom with a stroller. It helps somebody with a shopping cart, or a kid with a bike. If we adopt policies that are inclusive to those who are disadvantaged, it’s good for everybody. We should always strive to be an inclusive society, not just because it’s the right thing to do but because it really makes our society better.
How can mask requirements be used as a form of disability accommodation, as you argue in the JAMA article?
The ADA requires employers to provide reasonable accommodations for disability. In this case, the disability is your immunosuppressive status. We have an abundance of evidence showing individuals who are immunocompromised and vaccinated are still inadequately protected from the SARS-CoV-2 virus. So, there is absolute data to show individuals with immunosuppression have a disability that requires accommodation.
The ADA has a mandate requiring employers to adjust or modify policies in order to accommodate a disability. There are certain situations in which you cannot or do not need to accommodate a disability, when it would fundamentally alter the kind of employment you offer or if it’s an undue burden or hardship. But given that we’ve been wearing masks and working remotely for a year now, arguing that somehow these accommodations are no longer possible seems disingenuous.
In that way, allowing a person who’s immunocompromised to require those around them to mask is a form of modified protective policies. And in this case, those policies line up with a public health good, masking in the face of the highly contagious Delta variant ravaging our country right now.
In your view, can this argument be used in the mask debates happening right now across the country?
This argument can and should be useful for a couple of different lawsuits that are now underway in different states. I hope our article will provide further support for those suits. And I hope in school board hearings, when parents and teachers are talking about their concerns, this could be one way to argue for why we should allow mask mandates in classes. I’ve received emails from parents who said they’re going to bring this article to their school board hearing.
I also hope this could shift the narrative around the pandemic. Instead of focusing on individual responsibility – I got my vaccine shot so I’m fine – let’s focus on how we create an inclusive environment where we protect everybody, including those who cannot be vaccinated because of age or disability, or those who are vaccinated but inadequately protected because of their underlying conditions.
In the JAMA article, you talk about how our pandemic response has focused on individual health and how that individual focus can be ableist. Can you explain that point?
I think this idea that we just make our choices – like whether to get vaccinated or wear a mask, or not – and live with it really perpetuates a highly individualistic and ableist mindset. It doesn’t consider the people I admit to the hospital who are vaccinated but have a heart transplant and didn’t mount the sufficient immune response. Or even the people who chose not to be vaccinated because they were exposed to hours and hours of misinformation on TV.
We like to individualize everything, focusing on personal responsibility and choices, but a pandemic is one of those moments where everybody’s choices affect everybody else. Laying responsibility at the doorstep of each person, rather than thinking about what steps we as a society could be taking, is cheap and politically expedient. There is no public health rationale behind the bans on mask requirements in states like Texas, Iowa, and Florida. These choices are about politics. And the price is always borne by the most disadvantaged among us.
A version of this article first appeared on Medscape.com.
As governors and legislatures in states such as Texas, Florida, South Carolina, and Arkansas have banned schools and other entities from implementing mask mandates, disability rights advocates have pushed back. In federal civil rights lawsuits, they argue that bans on mask mandates violate antidiscrimination laws protecting people with disabilities.
For unvaccinated and immunosuppressed individuals, masks can provide crucial protection from SARS-CoV-2.
argues Mical Raz, MD, PhD, a professor at the University of Rochester (N.Y.) and a physician at Strong Memorial Hospital, also in Rochester, New York, in an article published in JAMA with coauthor Doron Dorfman, LLB, JSD.
This news organization talked with Dr. Raz about approaching mask requirements as disability accommodation during the COVID-19 pandemic. The following interview was lightly edited for length and clarity.
How did you come to think about mask requirements as a form of disability accommodation?
I saw a tweet from a professor at a university who said they couldn’t ask students about their vaccination status or to wear a mask. All agency was removed from the professor to take care of and protect themselves. I thought, well, that can’t be right. And ostensibly, that would be particularly dangerous for somebody with immunosuppression for whom the vaccine is not adequately protective. So, I called my friend, Doron Dorfman, and asked him to help me think through the legal part of this. We fleshed it out and wrote the article that same night.
How novel is it to view accommodations for people who are immunosuppressed through the lens of disability accommodation?
I think there has not been enough focus during the pandemic on individuals with disabilities or on how disability law can be mobilized during this pandemic to help supplement the public health law. This framework should be used a lot more because it’s good for everybody, not just for individuals with disabilities.
For example, take what’s called the “curb effect.” If you expand sidewalks, yes, it helps individuals who use a wheelchair. But it also helps me as a mom with a stroller. It helps somebody with a shopping cart, or a kid with a bike. If we adopt policies that are inclusive to those who are disadvantaged, it’s good for everybody. We should always strive to be an inclusive society, not just because it’s the right thing to do but because it really makes our society better.
How can mask requirements be used as a form of disability accommodation, as you argue in the JAMA article?
The ADA requires employers to provide reasonable accommodations for disability. In this case, the disability is your immunosuppressive status. We have an abundance of evidence showing individuals who are immunocompromised and vaccinated are still inadequately protected from the SARS-CoV-2 virus. So, there is absolute data to show individuals with immunosuppression have a disability that requires accommodation.
The ADA has a mandate requiring employers to adjust or modify policies in order to accommodate a disability. There are certain situations in which you cannot or do not need to accommodate a disability, when it would fundamentally alter the kind of employment you offer or if it’s an undue burden or hardship. But given that we’ve been wearing masks and working remotely for a year now, arguing that somehow these accommodations are no longer possible seems disingenuous.
In that way, allowing a person who’s immunocompromised to require those around them to mask is a form of modified protective policies. And in this case, those policies line up with a public health good, masking in the face of the highly contagious Delta variant ravaging our country right now.
In your view, can this argument be used in the mask debates happening right now across the country?
This argument can and should be useful for a couple of different lawsuits that are now underway in different states. I hope our article will provide further support for those suits. And I hope in school board hearings, when parents and teachers are talking about their concerns, this could be one way to argue for why we should allow mask mandates in classes. I’ve received emails from parents who said they’re going to bring this article to their school board hearing.
I also hope this could shift the narrative around the pandemic. Instead of focusing on individual responsibility – I got my vaccine shot so I’m fine – let’s focus on how we create an inclusive environment where we protect everybody, including those who cannot be vaccinated because of age or disability, or those who are vaccinated but inadequately protected because of their underlying conditions.
In the JAMA article, you talk about how our pandemic response has focused on individual health and how that individual focus can be ableist. Can you explain that point?
I think this idea that we just make our choices – like whether to get vaccinated or wear a mask, or not – and live with it really perpetuates a highly individualistic and ableist mindset. It doesn’t consider the people I admit to the hospital who are vaccinated but have a heart transplant and didn’t mount the sufficient immune response. Or even the people who chose not to be vaccinated because they were exposed to hours and hours of misinformation on TV.
We like to individualize everything, focusing on personal responsibility and choices, but a pandemic is one of those moments where everybody’s choices affect everybody else. Laying responsibility at the doorstep of each person, rather than thinking about what steps we as a society could be taking, is cheap and politically expedient. There is no public health rationale behind the bans on mask requirements in states like Texas, Iowa, and Florida. These choices are about politics. And the price is always borne by the most disadvantaged among us.
A version of this article first appeared on Medscape.com.
As governors and legislatures in states such as Texas, Florida, South Carolina, and Arkansas have banned schools and other entities from implementing mask mandates, disability rights advocates have pushed back. In federal civil rights lawsuits, they argue that bans on mask mandates violate antidiscrimination laws protecting people with disabilities.
For unvaccinated and immunosuppressed individuals, masks can provide crucial protection from SARS-CoV-2.
argues Mical Raz, MD, PhD, a professor at the University of Rochester (N.Y.) and a physician at Strong Memorial Hospital, also in Rochester, New York, in an article published in JAMA with coauthor Doron Dorfman, LLB, JSD.
This news organization talked with Dr. Raz about approaching mask requirements as disability accommodation during the COVID-19 pandemic. The following interview was lightly edited for length and clarity.
How did you come to think about mask requirements as a form of disability accommodation?
I saw a tweet from a professor at a university who said they couldn’t ask students about their vaccination status or to wear a mask. All agency was removed from the professor to take care of and protect themselves. I thought, well, that can’t be right. And ostensibly, that would be particularly dangerous for somebody with immunosuppression for whom the vaccine is not adequately protective. So, I called my friend, Doron Dorfman, and asked him to help me think through the legal part of this. We fleshed it out and wrote the article that same night.
How novel is it to view accommodations for people who are immunosuppressed through the lens of disability accommodation?
I think there has not been enough focus during the pandemic on individuals with disabilities or on how disability law can be mobilized during this pandemic to help supplement the public health law. This framework should be used a lot more because it’s good for everybody, not just for individuals with disabilities.
For example, take what’s called the “curb effect.” If you expand sidewalks, yes, it helps individuals who use a wheelchair. But it also helps me as a mom with a stroller. It helps somebody with a shopping cart, or a kid with a bike. If we adopt policies that are inclusive to those who are disadvantaged, it’s good for everybody. We should always strive to be an inclusive society, not just because it’s the right thing to do but because it really makes our society better.
How can mask requirements be used as a form of disability accommodation, as you argue in the JAMA article?
The ADA requires employers to provide reasonable accommodations for disability. In this case, the disability is your immunosuppressive status. We have an abundance of evidence showing individuals who are immunocompromised and vaccinated are still inadequately protected from the SARS-CoV-2 virus. So, there is absolute data to show individuals with immunosuppression have a disability that requires accommodation.
The ADA has a mandate requiring employers to adjust or modify policies in order to accommodate a disability. There are certain situations in which you cannot or do not need to accommodate a disability, when it would fundamentally alter the kind of employment you offer or if it’s an undue burden or hardship. But given that we’ve been wearing masks and working remotely for a year now, arguing that somehow these accommodations are no longer possible seems disingenuous.
In that way, allowing a person who’s immunocompromised to require those around them to mask is a form of modified protective policies. And in this case, those policies line up with a public health good, masking in the face of the highly contagious Delta variant ravaging our country right now.
In your view, can this argument be used in the mask debates happening right now across the country?
This argument can and should be useful for a couple of different lawsuits that are now underway in different states. I hope our article will provide further support for those suits. And I hope in school board hearings, when parents and teachers are talking about their concerns, this could be one way to argue for why we should allow mask mandates in classes. I’ve received emails from parents who said they’re going to bring this article to their school board hearing.
I also hope this could shift the narrative around the pandemic. Instead of focusing on individual responsibility – I got my vaccine shot so I’m fine – let’s focus on how we create an inclusive environment where we protect everybody, including those who cannot be vaccinated because of age or disability, or those who are vaccinated but inadequately protected because of their underlying conditions.
In the JAMA article, you talk about how our pandemic response has focused on individual health and how that individual focus can be ableist. Can you explain that point?
I think this idea that we just make our choices – like whether to get vaccinated or wear a mask, or not – and live with it really perpetuates a highly individualistic and ableist mindset. It doesn’t consider the people I admit to the hospital who are vaccinated but have a heart transplant and didn’t mount the sufficient immune response. Or even the people who chose not to be vaccinated because they were exposed to hours and hours of misinformation on TV.
We like to individualize everything, focusing on personal responsibility and choices, but a pandemic is one of those moments where everybody’s choices affect everybody else. Laying responsibility at the doorstep of each person, rather than thinking about what steps we as a society could be taking, is cheap and politically expedient. There is no public health rationale behind the bans on mask requirements in states like Texas, Iowa, and Florida. These choices are about politics. And the price is always borne by the most disadvantaged among us.
A version of this article first appeared on Medscape.com.
The other epidemic: Violence against health care workers
After working two busy evening hospital shifts, I was eating breakfast with my children when I started reading about physicians confronted and verbally abused during school board meetings for advocating for face masks in school. The pandemic changed course with the Delta variant increasing hospitalizations, and it seems to me the public response to physicians and health care workers also changed.
During the first wave of the pandemic, public support accompanied health care workers’ sacrifices. Nightly applause rang through New York City, there were donations of food, and murals reflected public backing.
We as a nation rallied. We masked up and locked down. We produced vaccines. COVID cases decreased, and by spring, a hint of normalcy bloomed.
Then the virus changed, and the Delta variant spread. Pandemic fatigue set in. Health care workers asked for help with continued masking and increased vaccinations and instead were met with threats. The summer, already made difficult, makes the prospect of winter even more daunting.
This kind of abuse is persistent
Violence against health care workers is not a new dilemma. Stories abound of patients or family members physically attacking, verbally abusing, or harassing health care workers. A 2014 survey reported almost 80% of nurses attacked during their career. Data from the Bureau of Labor Statistics also reveals health care workers experience more nonfatal workplace violence, as compared with other professions.
Nurses, who often spend the most face-to-face time with patients, receive a litany of abuse. A 2019 nursing survey reported 59% of respondents experiencing verbal abuse from patients and more than 43% experiencing verbal abuse from patients’ families. Even more concerning is 23% of survey respondents reporting physical abuse, an increase from 20% in 2018.
Physicians, likewise, are not immune from the same maltreatment. A 2014 physician survey reported more than 71% of physicians in the United States have experienced at least one incident of workplace violence in their careers. Of the physician specialties, the highest rates of violence are in the emergency department and against less experienced physicians. This is likely caused by the higher rates of patient frustration in EDs as a result of long wait times, overcrowding, and boarding while awaiting an inpatient room.
These statistics are disheartening. However, what I find most discouraging is the almost submissive acceptance of this abuse in the health care field as almost 73% of health care workers feel that the abuse is part of the job.
COVID and the increase in violence against health care workers
As the Delta variant spreads, hospitals’ capacity to handle both COVID and non-COVID issues is further strained. Compounding this stress is the public’s pandemic fatigue and the ongoing battles with masking and vaccinations.
In San Antonio, health care workers faced verbal and physical abuse as they enforced masking and visitation restrictions for COVID patients. Online, health care workers, who advocate for masking or vaccination, are often subject to death threats, threats to family members, and verbal abuse on social media. Veiled threats of “we know who you are” and “we will find you” follow physicians who advocate for masking in schools.
This problem is not isolated to the United States. In Italy, a COVID patient spat at health care workers who asked them to wait, resulting in closure of an entire hospital ward. In the United Kingdom, health care workers were subject to the same abuse as those in San Antonio when trying to enforce masking in the hospital. In India, Pakistan, and Spain, a stigma exists against health care workers for being sources of contagion.
The presence of a growing divide between health care workers and those we serve threatens to undermine not only delivery of care but also our response to the pandemic. This is in addition to the mental health burden and compassion fatigue suffered by many health care workers who find their efforts in doubt. An already strained medical system will find it difficult to withstand the loss of its essential workforce.
Standing united against health care worker abuse
Despite the level of discord surrounding COVID-19, it is important that health care workers remain united. An effective response to the increase in violence toward health care workers will greatly depend on how we address the following.
First, we must actively work to combat the spread of misinformation that erodes the public trust in science and medicine. Transparency is paramount. Policy changes and plans for implementation should be open and free of political influence. This remains a challenge due to the CDC’s standing as both a federal and scientific institution. A steadfast and explicit presentation of scientific evidence by the CDC is a vital first step in repairing this trust.
In addition, we must become our own advocates. The passage of HR 1195, the Workplace Violence Prevention for Health Care and Social Service Workers Act, in the House of Representatives with bipartisan support is an indication that the time is ripe for sweeping change. Its supporters include the American Nurses Association, American Psychiatric Nurses Association, National Nurses United, and the American College of Emergency Physicians. Active opposition includes the American Hospital Association, which cites prohibitive cost as a source of objection.
HR 1195 now waits in the U.S. Senate for approval. We should alert local, state, and health system leadership to the violence against health care workers. We should demand increased protection for our most vulnerable colleagues in EDs and hospitals. Our advocacy will produce a paradigm shift away from the acceptance of this abuse.
Lastly, we must be mindful of compassion fatigue and health care worker burnout. Cynicism threatens to take away our greatest strengths of empathy and humanity. In our work environment, we must lift each other up and increase our awareness of when our colleagues need help. Self-care and creative outlets are encouraged. Admittedly, I am blogging as a personal safeguard against compassion fatigue and burnout.
The pandemic will have enduring implications both positive and negative. It is my hope that support for health care workers not only endures but is also enhanced long after the pandemic ends.
Giancarlo Toledanes, DO, is an assistant professor of pediatrics and a pediatric hospitalist at Texas Children’s Hospital and Baylor College of Medicine, both in Houston. He has no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
After working two busy evening hospital shifts, I was eating breakfast with my children when I started reading about physicians confronted and verbally abused during school board meetings for advocating for face masks in school. The pandemic changed course with the Delta variant increasing hospitalizations, and it seems to me the public response to physicians and health care workers also changed.
During the first wave of the pandemic, public support accompanied health care workers’ sacrifices. Nightly applause rang through New York City, there were donations of food, and murals reflected public backing.
We as a nation rallied. We masked up and locked down. We produced vaccines. COVID cases decreased, and by spring, a hint of normalcy bloomed.
Then the virus changed, and the Delta variant spread. Pandemic fatigue set in. Health care workers asked for help with continued masking and increased vaccinations and instead were met with threats. The summer, already made difficult, makes the prospect of winter even more daunting.
This kind of abuse is persistent
Violence against health care workers is not a new dilemma. Stories abound of patients or family members physically attacking, verbally abusing, or harassing health care workers. A 2014 survey reported almost 80% of nurses attacked during their career. Data from the Bureau of Labor Statistics also reveals health care workers experience more nonfatal workplace violence, as compared with other professions.
Nurses, who often spend the most face-to-face time with patients, receive a litany of abuse. A 2019 nursing survey reported 59% of respondents experiencing verbal abuse from patients and more than 43% experiencing verbal abuse from patients’ families. Even more concerning is 23% of survey respondents reporting physical abuse, an increase from 20% in 2018.
Physicians, likewise, are not immune from the same maltreatment. A 2014 physician survey reported more than 71% of physicians in the United States have experienced at least one incident of workplace violence in their careers. Of the physician specialties, the highest rates of violence are in the emergency department and against less experienced physicians. This is likely caused by the higher rates of patient frustration in EDs as a result of long wait times, overcrowding, and boarding while awaiting an inpatient room.
These statistics are disheartening. However, what I find most discouraging is the almost submissive acceptance of this abuse in the health care field as almost 73% of health care workers feel that the abuse is part of the job.
COVID and the increase in violence against health care workers
As the Delta variant spreads, hospitals’ capacity to handle both COVID and non-COVID issues is further strained. Compounding this stress is the public’s pandemic fatigue and the ongoing battles with masking and vaccinations.
In San Antonio, health care workers faced verbal and physical abuse as they enforced masking and visitation restrictions for COVID patients. Online, health care workers, who advocate for masking or vaccination, are often subject to death threats, threats to family members, and verbal abuse on social media. Veiled threats of “we know who you are” and “we will find you” follow physicians who advocate for masking in schools.
This problem is not isolated to the United States. In Italy, a COVID patient spat at health care workers who asked them to wait, resulting in closure of an entire hospital ward. In the United Kingdom, health care workers were subject to the same abuse as those in San Antonio when trying to enforce masking in the hospital. In India, Pakistan, and Spain, a stigma exists against health care workers for being sources of contagion.
The presence of a growing divide between health care workers and those we serve threatens to undermine not only delivery of care but also our response to the pandemic. This is in addition to the mental health burden and compassion fatigue suffered by many health care workers who find their efforts in doubt. An already strained medical system will find it difficult to withstand the loss of its essential workforce.
Standing united against health care worker abuse
Despite the level of discord surrounding COVID-19, it is important that health care workers remain united. An effective response to the increase in violence toward health care workers will greatly depend on how we address the following.
First, we must actively work to combat the spread of misinformation that erodes the public trust in science and medicine. Transparency is paramount. Policy changes and plans for implementation should be open and free of political influence. This remains a challenge due to the CDC’s standing as both a federal and scientific institution. A steadfast and explicit presentation of scientific evidence by the CDC is a vital first step in repairing this trust.
In addition, we must become our own advocates. The passage of HR 1195, the Workplace Violence Prevention for Health Care and Social Service Workers Act, in the House of Representatives with bipartisan support is an indication that the time is ripe for sweeping change. Its supporters include the American Nurses Association, American Psychiatric Nurses Association, National Nurses United, and the American College of Emergency Physicians. Active opposition includes the American Hospital Association, which cites prohibitive cost as a source of objection.
HR 1195 now waits in the U.S. Senate for approval. We should alert local, state, and health system leadership to the violence against health care workers. We should demand increased protection for our most vulnerable colleagues in EDs and hospitals. Our advocacy will produce a paradigm shift away from the acceptance of this abuse.
Lastly, we must be mindful of compassion fatigue and health care worker burnout. Cynicism threatens to take away our greatest strengths of empathy and humanity. In our work environment, we must lift each other up and increase our awareness of when our colleagues need help. Self-care and creative outlets are encouraged. Admittedly, I am blogging as a personal safeguard against compassion fatigue and burnout.
The pandemic will have enduring implications both positive and negative. It is my hope that support for health care workers not only endures but is also enhanced long after the pandemic ends.
Giancarlo Toledanes, DO, is an assistant professor of pediatrics and a pediatric hospitalist at Texas Children’s Hospital and Baylor College of Medicine, both in Houston. He has no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
After working two busy evening hospital shifts, I was eating breakfast with my children when I started reading about physicians confronted and verbally abused during school board meetings for advocating for face masks in school. The pandemic changed course with the Delta variant increasing hospitalizations, and it seems to me the public response to physicians and health care workers also changed.
During the first wave of the pandemic, public support accompanied health care workers’ sacrifices. Nightly applause rang through New York City, there were donations of food, and murals reflected public backing.
We as a nation rallied. We masked up and locked down. We produced vaccines. COVID cases decreased, and by spring, a hint of normalcy bloomed.
Then the virus changed, and the Delta variant spread. Pandemic fatigue set in. Health care workers asked for help with continued masking and increased vaccinations and instead were met with threats. The summer, already made difficult, makes the prospect of winter even more daunting.
This kind of abuse is persistent
Violence against health care workers is not a new dilemma. Stories abound of patients or family members physically attacking, verbally abusing, or harassing health care workers. A 2014 survey reported almost 80% of nurses attacked during their career. Data from the Bureau of Labor Statistics also reveals health care workers experience more nonfatal workplace violence, as compared with other professions.
Nurses, who often spend the most face-to-face time with patients, receive a litany of abuse. A 2019 nursing survey reported 59% of respondents experiencing verbal abuse from patients and more than 43% experiencing verbal abuse from patients’ families. Even more concerning is 23% of survey respondents reporting physical abuse, an increase from 20% in 2018.
Physicians, likewise, are not immune from the same maltreatment. A 2014 physician survey reported more than 71% of physicians in the United States have experienced at least one incident of workplace violence in their careers. Of the physician specialties, the highest rates of violence are in the emergency department and against less experienced physicians. This is likely caused by the higher rates of patient frustration in EDs as a result of long wait times, overcrowding, and boarding while awaiting an inpatient room.
These statistics are disheartening. However, what I find most discouraging is the almost submissive acceptance of this abuse in the health care field as almost 73% of health care workers feel that the abuse is part of the job.
COVID and the increase in violence against health care workers
As the Delta variant spreads, hospitals’ capacity to handle both COVID and non-COVID issues is further strained. Compounding this stress is the public’s pandemic fatigue and the ongoing battles with masking and vaccinations.
In San Antonio, health care workers faced verbal and physical abuse as they enforced masking and visitation restrictions for COVID patients. Online, health care workers, who advocate for masking or vaccination, are often subject to death threats, threats to family members, and verbal abuse on social media. Veiled threats of “we know who you are” and “we will find you” follow physicians who advocate for masking in schools.
This problem is not isolated to the United States. In Italy, a COVID patient spat at health care workers who asked them to wait, resulting in closure of an entire hospital ward. In the United Kingdom, health care workers were subject to the same abuse as those in San Antonio when trying to enforce masking in the hospital. In India, Pakistan, and Spain, a stigma exists against health care workers for being sources of contagion.
The presence of a growing divide between health care workers and those we serve threatens to undermine not only delivery of care but also our response to the pandemic. This is in addition to the mental health burden and compassion fatigue suffered by many health care workers who find their efforts in doubt. An already strained medical system will find it difficult to withstand the loss of its essential workforce.
Standing united against health care worker abuse
Despite the level of discord surrounding COVID-19, it is important that health care workers remain united. An effective response to the increase in violence toward health care workers will greatly depend on how we address the following.
First, we must actively work to combat the spread of misinformation that erodes the public trust in science and medicine. Transparency is paramount. Policy changes and plans for implementation should be open and free of political influence. This remains a challenge due to the CDC’s standing as both a federal and scientific institution. A steadfast and explicit presentation of scientific evidence by the CDC is a vital first step in repairing this trust.
In addition, we must become our own advocates. The passage of HR 1195, the Workplace Violence Prevention for Health Care and Social Service Workers Act, in the House of Representatives with bipartisan support is an indication that the time is ripe for sweeping change. Its supporters include the American Nurses Association, American Psychiatric Nurses Association, National Nurses United, and the American College of Emergency Physicians. Active opposition includes the American Hospital Association, which cites prohibitive cost as a source of objection.
HR 1195 now waits in the U.S. Senate for approval. We should alert local, state, and health system leadership to the violence against health care workers. We should demand increased protection for our most vulnerable colleagues in EDs and hospitals. Our advocacy will produce a paradigm shift away from the acceptance of this abuse.
Lastly, we must be mindful of compassion fatigue and health care worker burnout. Cynicism threatens to take away our greatest strengths of empathy and humanity. In our work environment, we must lift each other up and increase our awareness of when our colleagues need help. Self-care and creative outlets are encouraged. Admittedly, I am blogging as a personal safeguard against compassion fatigue and burnout.
The pandemic will have enduring implications both positive and negative. It is my hope that support for health care workers not only endures but is also enhanced long after the pandemic ends.
Giancarlo Toledanes, DO, is an assistant professor of pediatrics and a pediatric hospitalist at Texas Children’s Hospital and Baylor College of Medicine, both in Houston. He has no relevant financial conflicts of interest.
A version of this article first appeared on Medscape.com.
PA gets prison time for knowingly prescribing unneeded addictive drugs
A U.S. District Judge sentenced William Soyke, 68, of Hanover, Penn., for acting outside the scope of professional practice and not for a legitimate medical purpose, according to the U.S. Attorney’s Office in Maryland. The 37-month prison term will be followed by 3 years of supervised release.
According to the plea agreement, Mr. Soyke worked as a physician assistant with Rosen-Hoffberg Rehabilitation and Pain Management from 2011 to 2018, where he treated patients during follow-up doctor appointments. As a physician assistant, Mr. Soyke had privileges to prescribe controlled substance medications, but was required to operate under a delegation agreement with the Rosen-Hoffberg owners.
In his plea, Mr. Soyke said that he believed the owners, Norman Rosen, MD, and Howard Hoffberg, MD, prescribed excessive levels of opioids. Despite Mr. Soyke’s attempts to lower patient’s prescription doses, both doctors overruled the PA’s opinion, according to the plea agreement. Also, if another health care provider within the practice declined to treat a patient because of the patient’s aberrant behavior – such as failing a drug screening test for illicit drugs or selling their prescriptions – Dr. Rosen and Dr. Hoffberg would assume that patient’s care, the report continued.
As stated in the plea agreement, Mr. Sokye was aware that many of the patients presenting to Rosen-Hoffberg Rehabilitation and Pain Management did not have a legitimate medical need for the oxycodone, fentanyl, alprazolam, and methadone they were being prescribed. Nevertheless, Mr. Soyke issued prescriptions for these drugs to patients without a legitimate medical need and outside the bounds of acceptable medical practice, according to the release.
Mr. Soyke also admitted that in several instances he engaged in sexual, physical contact with female patients who were attempting to get prescriptions, the plea agreement stated. Specifically, Mr. Soyke asked some female customers to engage in a range of motion test, and while they were bending over, he would position himself behind them such that his genitalia would rub against the customers’ buttocks through their clothes. These patients often submitted to this sexual abuse for fear of not getting the medications to which they were addicted, according to the press release.
Although the female patients complained to Dr. Rosen and Dr. Hoffberg about Mr. Soyke’s behavior, the doctors did not fire Mr. Soyke because the PA saw the largest number of patients at the practice and generated significant revenue, according to federal officials.
Dr. Hoffberg, the associate medical director and part-owner of the practice, pleaded guilty in June to accepting kickbacks from pharmaceutical company Insys Therapeutics in exchange for prescribing an opioid drug called Subsys (a fentanyl sublingual spray) marketed by Insys for breakthrough pain in cancer patients for off-label purposes. He will be sentenced in September and faces a maximum of 5 years in federal prison.
Mr. Soyke pled guilty to a federal drug charge in July 2019. In announcing the guilty plea then, U.S. Attorney Robert Hur said, “Opioid overdoses are killing thousands of Marylanders each year, and opioid addiction is fueled by health care providers who prescribe drugs for people without a legitimate medical need. Doctors and other medical professionals who irresponsibly write opioid prescriptions are acting like street-corner drug pushers.”
A version of this article first appeared on Medscape.com.
A U.S. District Judge sentenced William Soyke, 68, of Hanover, Penn., for acting outside the scope of professional practice and not for a legitimate medical purpose, according to the U.S. Attorney’s Office in Maryland. The 37-month prison term will be followed by 3 years of supervised release.
According to the plea agreement, Mr. Soyke worked as a physician assistant with Rosen-Hoffberg Rehabilitation and Pain Management from 2011 to 2018, where he treated patients during follow-up doctor appointments. As a physician assistant, Mr. Soyke had privileges to prescribe controlled substance medications, but was required to operate under a delegation agreement with the Rosen-Hoffberg owners.
In his plea, Mr. Soyke said that he believed the owners, Norman Rosen, MD, and Howard Hoffberg, MD, prescribed excessive levels of opioids. Despite Mr. Soyke’s attempts to lower patient’s prescription doses, both doctors overruled the PA’s opinion, according to the plea agreement. Also, if another health care provider within the practice declined to treat a patient because of the patient’s aberrant behavior – such as failing a drug screening test for illicit drugs or selling their prescriptions – Dr. Rosen and Dr. Hoffberg would assume that patient’s care, the report continued.
As stated in the plea agreement, Mr. Sokye was aware that many of the patients presenting to Rosen-Hoffberg Rehabilitation and Pain Management did not have a legitimate medical need for the oxycodone, fentanyl, alprazolam, and methadone they were being prescribed. Nevertheless, Mr. Soyke issued prescriptions for these drugs to patients without a legitimate medical need and outside the bounds of acceptable medical practice, according to the release.
Mr. Soyke also admitted that in several instances he engaged in sexual, physical contact with female patients who were attempting to get prescriptions, the plea agreement stated. Specifically, Mr. Soyke asked some female customers to engage in a range of motion test, and while they were bending over, he would position himself behind them such that his genitalia would rub against the customers’ buttocks through their clothes. These patients often submitted to this sexual abuse for fear of not getting the medications to which they were addicted, according to the press release.
Although the female patients complained to Dr. Rosen and Dr. Hoffberg about Mr. Soyke’s behavior, the doctors did not fire Mr. Soyke because the PA saw the largest number of patients at the practice and generated significant revenue, according to federal officials.
Dr. Hoffberg, the associate medical director and part-owner of the practice, pleaded guilty in June to accepting kickbacks from pharmaceutical company Insys Therapeutics in exchange for prescribing an opioid drug called Subsys (a fentanyl sublingual spray) marketed by Insys for breakthrough pain in cancer patients for off-label purposes. He will be sentenced in September and faces a maximum of 5 years in federal prison.
Mr. Soyke pled guilty to a federal drug charge in July 2019. In announcing the guilty plea then, U.S. Attorney Robert Hur said, “Opioid overdoses are killing thousands of Marylanders each year, and opioid addiction is fueled by health care providers who prescribe drugs for people without a legitimate medical need. Doctors and other medical professionals who irresponsibly write opioid prescriptions are acting like street-corner drug pushers.”
A version of this article first appeared on Medscape.com.
A U.S. District Judge sentenced William Soyke, 68, of Hanover, Penn., for acting outside the scope of professional practice and not for a legitimate medical purpose, according to the U.S. Attorney’s Office in Maryland. The 37-month prison term will be followed by 3 years of supervised release.
According to the plea agreement, Mr. Soyke worked as a physician assistant with Rosen-Hoffberg Rehabilitation and Pain Management from 2011 to 2018, where he treated patients during follow-up doctor appointments. As a physician assistant, Mr. Soyke had privileges to prescribe controlled substance medications, but was required to operate under a delegation agreement with the Rosen-Hoffberg owners.
In his plea, Mr. Soyke said that he believed the owners, Norman Rosen, MD, and Howard Hoffberg, MD, prescribed excessive levels of opioids. Despite Mr. Soyke’s attempts to lower patient’s prescription doses, both doctors overruled the PA’s opinion, according to the plea agreement. Also, if another health care provider within the practice declined to treat a patient because of the patient’s aberrant behavior – such as failing a drug screening test for illicit drugs or selling their prescriptions – Dr. Rosen and Dr. Hoffberg would assume that patient’s care, the report continued.
As stated in the plea agreement, Mr. Sokye was aware that many of the patients presenting to Rosen-Hoffberg Rehabilitation and Pain Management did not have a legitimate medical need for the oxycodone, fentanyl, alprazolam, and methadone they were being prescribed. Nevertheless, Mr. Soyke issued prescriptions for these drugs to patients without a legitimate medical need and outside the bounds of acceptable medical practice, according to the release.
Mr. Soyke also admitted that in several instances he engaged in sexual, physical contact with female patients who were attempting to get prescriptions, the plea agreement stated. Specifically, Mr. Soyke asked some female customers to engage in a range of motion test, and while they were bending over, he would position himself behind them such that his genitalia would rub against the customers’ buttocks through their clothes. These patients often submitted to this sexual abuse for fear of not getting the medications to which they were addicted, according to the press release.
Although the female patients complained to Dr. Rosen and Dr. Hoffberg about Mr. Soyke’s behavior, the doctors did not fire Mr. Soyke because the PA saw the largest number of patients at the practice and generated significant revenue, according to federal officials.
Dr. Hoffberg, the associate medical director and part-owner of the practice, pleaded guilty in June to accepting kickbacks from pharmaceutical company Insys Therapeutics in exchange for prescribing an opioid drug called Subsys (a fentanyl sublingual spray) marketed by Insys for breakthrough pain in cancer patients for off-label purposes. He will be sentenced in September and faces a maximum of 5 years in federal prison.
Mr. Soyke pled guilty to a federal drug charge in July 2019. In announcing the guilty plea then, U.S. Attorney Robert Hur said, “Opioid overdoses are killing thousands of Marylanders each year, and opioid addiction is fueled by health care providers who prescribe drugs for people without a legitimate medical need. Doctors and other medical professionals who irresponsibly write opioid prescriptions are acting like street-corner drug pushers.”
A version of this article first appeared on Medscape.com.
ACR updates COVID vaccine guidance with booster schedule
Patients on immunosuppressive or immunomodulatory therapy should receive a third dose of either the Pfizer-BioNTech COVID-19 vaccine or the Moderna COVID-19 vaccine at least 28 days after the second dose of either of these two mRNA vaccines, according to updated recommendations from the American College of Rheumatology.
The update follows the Centers for Disease Control and Prevention’s recommendation that certain immunocompromised patients receive a third dose of an mRNA vaccine to reduce their risk of contracting COVID-19.
Individuals receiving the Pfizer vaccine must be aged 12 years and older, while those receiving the Moderna vaccine must be 18 years and older, the ACR emphasized.
“These statements were based upon a dearth of high-quality data and are not intended to replace clinical judgment,” the authors wrote. “Modifications made to treatment plans, particularly in complex rheumatic disease patients, are highly disease, patient, geography, and time specific and, therefore, must be individualized as part of a shared decision-making process.”
The task force recommended using the same mRNA vaccine booster as the patient received for their initial two-dose series when possible, but notes that either mRNA vaccine is acceptable, and recommends the mRNA vaccine for patients who have yet to receive any vaccine because of the availability of the booster. The task force emphasized that they achieved no consensus on recommending a booster mRNA vaccine to patients who received a single dose of Johnson & Johnson vaccine because the safety data are uncertain.
The updated guidance also identifies the Food and Drug Administration’s emergency use authorization in August for the use of REGEN-COV monoclonal antibody treatment for emergency postexposure prophylaxis for COVID-19 in adults and adolescents aged 12 years and older who weigh at least 40 kg and are at increased risk for severe COVID-19, which includes patients receiving immunosuppressive or immunomodulatory therapies other than hydroxychloroquine. Patients who have been exposed to an individual with COVID-19 should discuss this treatment with their health care provider as an added precaution; however, the guidance emphasized that the prophylactic treatment is not a substitute for COVID-19 vaccination.
The recommendations advise clinicians to counsel their patients to refrain from taking certain immunomodulatory or immunosuppressive medications for 1-2 weeks after booster vaccination if disease activity allows, with the exception of glucocorticoids and anticytokines such as tumor necrosis factor inhibitors and others including interleukin-17, IL-12/23, IL-23, IL-1R, IL-6R antagonists, for which the task force did not achieve a consensus recommendation.
The guidance notes that patients on rituximab or other anti-CD20 medications “should discuss the optimal timing [of the booster] with their rheumatology provider” and that some practitioners measure CD19 B cells as a tool with which to time the booster and subsequent rituximab dosing. For those who elect to dose without such information, or for whom such measurement is not available or feasible, provide the booster 2-4 weeks before next anticipated rituximab dose (e.g., at month 5.0 or 5.5 for patients on an every-6-month rituximab dosing schedule).”
There was strong consensus from the task force that health care providers “should not routinely order any lab testing (e.g., antibody tests for IgM and/or IgG to spike or nucleocapsid proteins) to assess immunity to COVID-19 post vaccination, nor to assess the need for vaccination in a yet-unvaccinated person.”
“The updated information from the ACR addresses not only booster vaccination but also other important and practical issues facing rheumatology providers and their patients related to the pandemic,” said task force chair Jeffrey R. Curtis, MD, of the University of Alabama at Birmingham, in an ACR statement announcing the updates.
“Although the guidance is issued in light of the best evidence available, the science regarding COVID-19 vaccination as it affects the practice of rheumatology is undergoing rapid evolution,” he noted. “We need direct evidence such as that from randomized trials to inform the best practices of what we can do to protect our patients from SARS-CoV-2.”
The update retains the current recommendations that rheumatology patients follow all public health guidelines regarding physical distancing and other preventive measures following vaccination, but the task force did not recommend exceeding current public health guidance. “The appropriateness for continued preventive measures (e.g., masking, physical distancing) should be discussed with patients as their rheumatology providers deem appropriate,” they wrote.
The full updated version of the ACR’s COVID-19 Vaccine Clinical Guidance for Patients with Rheumatic and Musculoskeletal Diseases will be published in Arthritis & Rheumatology. The summary was developed by the ACR COVID-19 Vaccine Clinical Guidance Task Force, which included 9 rheumatologists, 2 infectious disease specialists, and 2 public health experts with current or past employment history with the CDC.
The ACR encourages clinicians with questions or concerns to email [email protected] for support.
Patients on immunosuppressive or immunomodulatory therapy should receive a third dose of either the Pfizer-BioNTech COVID-19 vaccine or the Moderna COVID-19 vaccine at least 28 days after the second dose of either of these two mRNA vaccines, according to updated recommendations from the American College of Rheumatology.
The update follows the Centers for Disease Control and Prevention’s recommendation that certain immunocompromised patients receive a third dose of an mRNA vaccine to reduce their risk of contracting COVID-19.
Individuals receiving the Pfizer vaccine must be aged 12 years and older, while those receiving the Moderna vaccine must be 18 years and older, the ACR emphasized.
“These statements were based upon a dearth of high-quality data and are not intended to replace clinical judgment,” the authors wrote. “Modifications made to treatment plans, particularly in complex rheumatic disease patients, are highly disease, patient, geography, and time specific and, therefore, must be individualized as part of a shared decision-making process.”
The task force recommended using the same mRNA vaccine booster as the patient received for their initial two-dose series when possible, but notes that either mRNA vaccine is acceptable, and recommends the mRNA vaccine for patients who have yet to receive any vaccine because of the availability of the booster. The task force emphasized that they achieved no consensus on recommending a booster mRNA vaccine to patients who received a single dose of Johnson & Johnson vaccine because the safety data are uncertain.
The updated guidance also identifies the Food and Drug Administration’s emergency use authorization in August for the use of REGEN-COV monoclonal antibody treatment for emergency postexposure prophylaxis for COVID-19 in adults and adolescents aged 12 years and older who weigh at least 40 kg and are at increased risk for severe COVID-19, which includes patients receiving immunosuppressive or immunomodulatory therapies other than hydroxychloroquine. Patients who have been exposed to an individual with COVID-19 should discuss this treatment with their health care provider as an added precaution; however, the guidance emphasized that the prophylactic treatment is not a substitute for COVID-19 vaccination.
The recommendations advise clinicians to counsel their patients to refrain from taking certain immunomodulatory or immunosuppressive medications for 1-2 weeks after booster vaccination if disease activity allows, with the exception of glucocorticoids and anticytokines such as tumor necrosis factor inhibitors and others including interleukin-17, IL-12/23, IL-23, IL-1R, IL-6R antagonists, for which the task force did not achieve a consensus recommendation.
The guidance notes that patients on rituximab or other anti-CD20 medications “should discuss the optimal timing [of the booster] with their rheumatology provider” and that some practitioners measure CD19 B cells as a tool with which to time the booster and subsequent rituximab dosing. For those who elect to dose without such information, or for whom such measurement is not available or feasible, provide the booster 2-4 weeks before next anticipated rituximab dose (e.g., at month 5.0 or 5.5 for patients on an every-6-month rituximab dosing schedule).”
There was strong consensus from the task force that health care providers “should not routinely order any lab testing (e.g., antibody tests for IgM and/or IgG to spike or nucleocapsid proteins) to assess immunity to COVID-19 post vaccination, nor to assess the need for vaccination in a yet-unvaccinated person.”
“The updated information from the ACR addresses not only booster vaccination but also other important and practical issues facing rheumatology providers and their patients related to the pandemic,” said task force chair Jeffrey R. Curtis, MD, of the University of Alabama at Birmingham, in an ACR statement announcing the updates.
“Although the guidance is issued in light of the best evidence available, the science regarding COVID-19 vaccination as it affects the practice of rheumatology is undergoing rapid evolution,” he noted. “We need direct evidence such as that from randomized trials to inform the best practices of what we can do to protect our patients from SARS-CoV-2.”
The update retains the current recommendations that rheumatology patients follow all public health guidelines regarding physical distancing and other preventive measures following vaccination, but the task force did not recommend exceeding current public health guidance. “The appropriateness for continued preventive measures (e.g., masking, physical distancing) should be discussed with patients as their rheumatology providers deem appropriate,” they wrote.
The full updated version of the ACR’s COVID-19 Vaccine Clinical Guidance for Patients with Rheumatic and Musculoskeletal Diseases will be published in Arthritis & Rheumatology. The summary was developed by the ACR COVID-19 Vaccine Clinical Guidance Task Force, which included 9 rheumatologists, 2 infectious disease specialists, and 2 public health experts with current or past employment history with the CDC.
The ACR encourages clinicians with questions or concerns to email [email protected] for support.
Patients on immunosuppressive or immunomodulatory therapy should receive a third dose of either the Pfizer-BioNTech COVID-19 vaccine or the Moderna COVID-19 vaccine at least 28 days after the second dose of either of these two mRNA vaccines, according to updated recommendations from the American College of Rheumatology.
The update follows the Centers for Disease Control and Prevention’s recommendation that certain immunocompromised patients receive a third dose of an mRNA vaccine to reduce their risk of contracting COVID-19.
Individuals receiving the Pfizer vaccine must be aged 12 years and older, while those receiving the Moderna vaccine must be 18 years and older, the ACR emphasized.
“These statements were based upon a dearth of high-quality data and are not intended to replace clinical judgment,” the authors wrote. “Modifications made to treatment plans, particularly in complex rheumatic disease patients, are highly disease, patient, geography, and time specific and, therefore, must be individualized as part of a shared decision-making process.”
The task force recommended using the same mRNA vaccine booster as the patient received for their initial two-dose series when possible, but notes that either mRNA vaccine is acceptable, and recommends the mRNA vaccine for patients who have yet to receive any vaccine because of the availability of the booster. The task force emphasized that they achieved no consensus on recommending a booster mRNA vaccine to patients who received a single dose of Johnson & Johnson vaccine because the safety data are uncertain.
The updated guidance also identifies the Food and Drug Administration’s emergency use authorization in August for the use of REGEN-COV monoclonal antibody treatment for emergency postexposure prophylaxis for COVID-19 in adults and adolescents aged 12 years and older who weigh at least 40 kg and are at increased risk for severe COVID-19, which includes patients receiving immunosuppressive or immunomodulatory therapies other than hydroxychloroquine. Patients who have been exposed to an individual with COVID-19 should discuss this treatment with their health care provider as an added precaution; however, the guidance emphasized that the prophylactic treatment is not a substitute for COVID-19 vaccination.
The recommendations advise clinicians to counsel their patients to refrain from taking certain immunomodulatory or immunosuppressive medications for 1-2 weeks after booster vaccination if disease activity allows, with the exception of glucocorticoids and anticytokines such as tumor necrosis factor inhibitors and others including interleukin-17, IL-12/23, IL-23, IL-1R, IL-6R antagonists, for which the task force did not achieve a consensus recommendation.
The guidance notes that patients on rituximab or other anti-CD20 medications “should discuss the optimal timing [of the booster] with their rheumatology provider” and that some practitioners measure CD19 B cells as a tool with which to time the booster and subsequent rituximab dosing. For those who elect to dose without such information, or for whom such measurement is not available or feasible, provide the booster 2-4 weeks before next anticipated rituximab dose (e.g., at month 5.0 or 5.5 for patients on an every-6-month rituximab dosing schedule).”
There was strong consensus from the task force that health care providers “should not routinely order any lab testing (e.g., antibody tests for IgM and/or IgG to spike or nucleocapsid proteins) to assess immunity to COVID-19 post vaccination, nor to assess the need for vaccination in a yet-unvaccinated person.”
“The updated information from the ACR addresses not only booster vaccination but also other important and practical issues facing rheumatology providers and their patients related to the pandemic,” said task force chair Jeffrey R. Curtis, MD, of the University of Alabama at Birmingham, in an ACR statement announcing the updates.
“Although the guidance is issued in light of the best evidence available, the science regarding COVID-19 vaccination as it affects the practice of rheumatology is undergoing rapid evolution,” he noted. “We need direct evidence such as that from randomized trials to inform the best practices of what we can do to protect our patients from SARS-CoV-2.”
The update retains the current recommendations that rheumatology patients follow all public health guidelines regarding physical distancing and other preventive measures following vaccination, but the task force did not recommend exceeding current public health guidance. “The appropriateness for continued preventive measures (e.g., masking, physical distancing) should be discussed with patients as their rheumatology providers deem appropriate,” they wrote.
The full updated version of the ACR’s COVID-19 Vaccine Clinical Guidance for Patients with Rheumatic and Musculoskeletal Diseases will be published in Arthritis & Rheumatology. The summary was developed by the ACR COVID-19 Vaccine Clinical Guidance Task Force, which included 9 rheumatologists, 2 infectious disease specialists, and 2 public health experts with current or past employment history with the CDC.
The ACR encourages clinicians with questions or concerns to email [email protected] for support.
ICU infections and all-cause hospital mortality rate
Background: Many articles have been published on sepsis and mortality in ICUs, but there are not many analyzing outcomes in patients with infections, nor types of infections. More information on the infection rate, types of infection, and possible impact on mortality should heighten awareness of infection effects, as well as guide resource allocation and help direct policy development for diagnosis and treatment.
Study design: 24-hour point-prevalence study with longitudinal follow-up.
Setting: ICUs in 1,150 centers in 88 countries.
Synopsis: The study included 15,202 patients who were aged 18 or older (mean, 61.6) within a 24-hour time period on Sept. 13, 2017, who were admitted to the ICU in participating centers and had documented, confirmed, or suspected infection. The investigators looked at prevalence of infection and antibiotic exposure on the study day and the main outcome measure was all cause in-hospital mortality, which was compiled 60 days later. The prevalence of suspected or proven infection in ICUs was 54% (8,135) and that of ICU-acquired infection was 22%. Of confirmed or suspected infection, 65% (5,259) had at least one positive microbiology culture. Of those cultures, 67% were gram-negative and 37% gram-positive bacteria, and 16% were fungal. 70% of ICU patients received at least one antibiotic. The in-hospital mortality rate with proven or suspected infection was 30% (2,404 of 7,936). Multilevel analysis disclosed two independent risk factors for mortality, which were ICU-acquired infections and antibiotic-resistant organisms, specifically, vancomycin-resistant Enterococcus, Klebsiella resistant to beta-lactam antibiotics, and carbapenem-resistant Acinetobacter.
Despite limitations related to being an observational study, 24-hour point evaluation, a centrally controlled database, and different geographic locations, this study elucidated the world-wide prevalence of ICU infection and high hospital-in mortality in those patients.
Bottom line: There is a high prevalence of infection in ICUs: 43%-60% depending on location. This is associated with 30% in-hospital mortality.
Citation: Vincent J-L et al. Prevalance and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020 Mar 24;323(15):1478-87.
Dr. Rogozinska is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
Background: Many articles have been published on sepsis and mortality in ICUs, but there are not many analyzing outcomes in patients with infections, nor types of infections. More information on the infection rate, types of infection, and possible impact on mortality should heighten awareness of infection effects, as well as guide resource allocation and help direct policy development for diagnosis and treatment.
Study design: 24-hour point-prevalence study with longitudinal follow-up.
Setting: ICUs in 1,150 centers in 88 countries.
Synopsis: The study included 15,202 patients who were aged 18 or older (mean, 61.6) within a 24-hour time period on Sept. 13, 2017, who were admitted to the ICU in participating centers and had documented, confirmed, or suspected infection. The investigators looked at prevalence of infection and antibiotic exposure on the study day and the main outcome measure was all cause in-hospital mortality, which was compiled 60 days later. The prevalence of suspected or proven infection in ICUs was 54% (8,135) and that of ICU-acquired infection was 22%. Of confirmed or suspected infection, 65% (5,259) had at least one positive microbiology culture. Of those cultures, 67% were gram-negative and 37% gram-positive bacteria, and 16% were fungal. 70% of ICU patients received at least one antibiotic. The in-hospital mortality rate with proven or suspected infection was 30% (2,404 of 7,936). Multilevel analysis disclosed two independent risk factors for mortality, which were ICU-acquired infections and antibiotic-resistant organisms, specifically, vancomycin-resistant Enterococcus, Klebsiella resistant to beta-lactam antibiotics, and carbapenem-resistant Acinetobacter.
Despite limitations related to being an observational study, 24-hour point evaluation, a centrally controlled database, and different geographic locations, this study elucidated the world-wide prevalence of ICU infection and high hospital-in mortality in those patients.
Bottom line: There is a high prevalence of infection in ICUs: 43%-60% depending on location. This is associated with 30% in-hospital mortality.
Citation: Vincent J-L et al. Prevalance and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020 Mar 24;323(15):1478-87.
Dr. Rogozinska is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
Background: Many articles have been published on sepsis and mortality in ICUs, but there are not many analyzing outcomes in patients with infections, nor types of infections. More information on the infection rate, types of infection, and possible impact on mortality should heighten awareness of infection effects, as well as guide resource allocation and help direct policy development for diagnosis and treatment.
Study design: 24-hour point-prevalence study with longitudinal follow-up.
Setting: ICUs in 1,150 centers in 88 countries.
Synopsis: The study included 15,202 patients who were aged 18 or older (mean, 61.6) within a 24-hour time period on Sept. 13, 2017, who were admitted to the ICU in participating centers and had documented, confirmed, or suspected infection. The investigators looked at prevalence of infection and antibiotic exposure on the study day and the main outcome measure was all cause in-hospital mortality, which was compiled 60 days later. The prevalence of suspected or proven infection in ICUs was 54% (8,135) and that of ICU-acquired infection was 22%. Of confirmed or suspected infection, 65% (5,259) had at least one positive microbiology culture. Of those cultures, 67% were gram-negative and 37% gram-positive bacteria, and 16% were fungal. 70% of ICU patients received at least one antibiotic. The in-hospital mortality rate with proven or suspected infection was 30% (2,404 of 7,936). Multilevel analysis disclosed two independent risk factors for mortality, which were ICU-acquired infections and antibiotic-resistant organisms, specifically, vancomycin-resistant Enterococcus, Klebsiella resistant to beta-lactam antibiotics, and carbapenem-resistant Acinetobacter.
Despite limitations related to being an observational study, 24-hour point evaluation, a centrally controlled database, and different geographic locations, this study elucidated the world-wide prevalence of ICU infection and high hospital-in mortality in those patients.
Bottom line: There is a high prevalence of infection in ICUs: 43%-60% depending on location. This is associated with 30% in-hospital mortality.
Citation: Vincent J-L et al. Prevalance and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020 Mar 24;323(15):1478-87.
Dr. Rogozinska is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
The secret I’ll take to my grave: Doc reveals
An internist will never forget the dark secret his patient revealed during a routine visit – or the grim aftermath.
The patient, who had a progressive, incurable neurological condition, confided that he planned to kill himself. The patient intended to conceal the true manner and make the death look natural.
“[He planned to do it] very carefully at home so no one would know,” said the internist, who remains anonymous. “[He shared] the methods he would use.”
Perhaps more shocking than the patient’s confession was the physician’s response.
“He did not require my help to do what he planned, and I did not try to stop him,” said the internist. “I reported his death as ‘natural causes’ and never told anyone.”
An ob.gyn., for instance, wrote about struggling with whether to tell a father that his newborn baby was not his genetic child. The newborn had a blood type that made it impossible for the father to be biologically related to the infant, the anonymous doctor wrote.
“I told the wife who then informed me she had a lover,” the ob.gyn. said. “I never told the husband.”
It’s uncertain whether carrying the burden of such hidden knowledge affected the physicians involved in these cases. However, in general, secrets can weigh heavily on the minds of those who keep them and can contribute to stress, said Malia Mason, PhD, a psychologist and dean of research at Columbia Business School in New York. Holding onto secrets can cause depression and anxiety, research shows. The more often people think about the secret, the greater the impact, according to a recent study coauthored by Dr. Mason.
“Keeping a secret diminishes well-being,” Dr. Mason said. “It makes people feel socially distant. It lowers relationship satisfaction, and it leads people to feel inauthentic. The reason that secrets do this is because people think about them all the time. The more you think about it, the more you see these consequences.”
Feelings that stem from a secret depend on the content. The more immoral a secret is thought to be, the more people feel ashamed, according to a 2021 analysis of thousands of secrets, reported by Michael L. Slepian, PhD, and Alex Koch, PhD. However, secrets more related to a person’s profession are often internalized differently, the study found. The more a secret fell higher on the profession/goal-oriented dimension, the more people felt they had insight into the secret, according to the analysis. For example, having clear thinking about the secret and/or knowing how to handle it.
“The more shame participants felt from their secret, the more they indicated the secret hurt their well-being,” Dr. Slepian and Dr. Koch wrote in the study. “The more insight participants felt they had into their secret, the less they indicated the secret hurt their well-being.”
Suspicious deaths exposed after investigations
The internist’s account of keeping his patient’s suicide a secret raises many questions, such as how the patient masked his manner of death. The internist did not share any more details about the incident.
Suicides are among the most challenging manners of deaths to certify, according to James Gill, MD, a pathologist and president of the National Association of Medical Examiners. Death investigators must demonstrate intent, meaning the individuals caused the injury to intentionally harm themselves. Fewer than half of people who die by suicide leave a note, Dr. Gill said, so investigators can’t rely on the absence or the presence of a note in making their determination.
A decedent who had cancer or a severe neurological disorder presents further challenges, said Dr. Gill, who serves as chief medical examiner for the state of Connecticut.
“These [deaths] may not be unexpected and may not be reported to the medical examiner/coroner,” Dr. Gill said. “If there is no suspicion and the treating doctor is willing to sign the death certificate, the death will not come under the jurisdiction of the medical examiner.”
Dr. Gill recalled a death his colleague once investigated that appeared to be natural but emerged as something else after a deeper look.
A woman with metastatic breast cancer was about to be discharged from a hospital into hospice the next morning. The night before, she had a “going away” party with friends who came to visit her in the hospital. Shortly after the friends left, the woman was found dead. Because of her condition, she could have died at any time, Dr. Gill said, but she also had a history of depression and hospital staff were suspicious. The death was reported to the medical examiner’s office.
Toxicology testing found markedly elevated concentrations of phenytoin and pentobarbital, neither of which were prescribed during her hospital stay. Dr. Gill said it turned out that the woman and her friends worked at a veterinarian’s office, and the medication they used to euthanize dogs was a combination of phenytoin and pentobarbital.
“The death was certified as a homicide because of the direct actions of another, but a reasonable argument could be made for suicide,” Dr. Gill said.
In a similar case reported in the journal Science & Justice, a 64-year-old cardiologist was found lifeless by his wife after he collapsed near the stairs of his home. Next to his body was a bottle of whiskey and two cups, one that appeared to be used for the alcohol and one with a yellowish liquid smelling of honey. The wife reported that her husband always drank whiskey with honey before bed. The death was initially classified as natural, but after vehement protest by the family, a forensic autopsy was performed.
Prior to the autopsy, death investigators learned the decedent, who was a well-known and successful practitioner in his community, had Parkinson’s disease. At times, he could not sign his prescriptions because of the increasing tremor in his hands, according to the case study. Investigators learned the patient’s mother had also suffered from Parkinson’s, and that her son had witnessed her decline.
The autopsy revealed only nonspecific lesions such as acute stasis of the viscera, moderate pulmonary and cerebral edema, and moderate generalized atheromatosis. Histological examinations did not yield any unusual findings.
An analysis of the beverage containers detected pentobarbital in the yellowish syrup residue of the second cup. Testing of the doctor’s peripheral blood revealed the presence of a metabolite of pentobarbital, ethanol, and traces of phenobarbital. In addition, a urine analysis showed the presence of venlafaxine, an antidepressant, as well as the benzophenone of lorazepam, a sedating benzodiazepine, and metoclopramide, an antiemetic.
Lead author C. Brandt-Casadevall, MD, and colleagues wrote that the levels were clearly compatible with a scenario of a pentobarbital overdose with a lethal outcome.
“... It is obvious that the victim attempted to hide his suicide from his family circle,” Dr. Brandt-Casadevall and colleagues wrote. “Thus, we obtained no evidence indicating that he might have spoken at any point of putting an end to his life. There was no written note. The victim did not wait to be alone at home. Instead, he committed his act in a routine situation: his wife was watching television late at night and he was upstairs, presumably going to sleep. Thus, he had one to two hours at his disposal, and he ingested a very fast-acting drug which would make any attempt at reanimation impossible, even after a brief period of time. This may have induced the physician in charge to believe that the cause of death was cardiac origin, a likely hypothesis given the age of the victim.”
What to do when a terminally ill patient talks suicide
When a terminally ill patient expresses the desire to end his or her life, it’s important to understand that desire is often a result of existential suffering, a sense of hopelessness, and lack of social support, said Lynn A. Jansen, PhD, a bioethicist at the University of Arizona in Tucson.
“The duty of beneficence requires that physicians attempt to provide the support and care that is needed,” said Dr. Jansen. “Here, interdisciplinary teamwork is important and should be utilized. Physicians should refer patients to professionals, such as social workers, pastoral care, psychologists, etc., who are better able to address these issues.”
The rate of desire for a hastened death among terminally ill patients ranges from 17% to 45%, depending on the population studied and how the desire is evaluated, according to an analysis in the Primary Care Companion to the Journal of Clinical Psychiatry. In one study, 14% of about 130 palliative care patients with cancer had a strong desire to quicken the dying process.
In addition, patients with neurologic disorders have a significantly higher suicide rate than that of those without neurologic disorders, a recent JAMA study found. About 1 in 150 patients diagnosed with a neurological disorder dies by suicide, the analysis determined.
A tricky point to remember is that a desire by a terminally ill patient to hasten his or her death by suicide should not be taken by itself to indicate depression, Dr. Jansen noted.
“In principle, such patients can make an autonomous decision to end their lives,” she said. “However, the expression of such a desire is very often associated with depression and forms of suffering that can be effectively addressed by the health care team.”
Physicians can also explore other avenues with the patient such as palliative care or making sure adequate pain relief is available, added Robert Klitzman, MD, professor of psychiatry and academic director of the master of science in bioethics program at Columbia University, New York.
“If they are saying it’s because they are distressed, ethically, a doctor can and should find ways to decrease their distress,” he said.
Of course, those who practice in the U.S. jurisdictions that have physician-assisted-dying laws have different legal and ethical elements to consider. Physicians in these areas have no ethical duty to participate in the process, Dr. Jansen said, but they have a duty to refer patients who express a desire to pursue physician aid-in-dying to another provider who can assist them.
Physician aid-in-dying laws vary somewhat so it’s important that physicians in these areas be aware of their specific statute, Dr. Klitzman said. California, Colorado, Hawaii, Maine, New Jersey, New Mexico, Oregon, Vermont, Washington, and the District of Columbia have these laws.
“In these states, if a terminally ill patient says they don’t want to live anymore, a physician would first decide if this is a result of depression or if it’s a request for physician aid-in-dying,” he said. “Even then, in most cases, the patient would be evaluated by not one, but two different health professionals at two different points. We want to see if it is a consistent decision that the person has made that they want physician aid-in-dying, and not just that they’ve had a bad day or a setback in their treatment.”
In the case of the internist who told no one of his patient’s suicide plan, Dr. Klitzman said he would have dug deeper into the patient’s mindset.
“Not knowing anything about the patient or the doctor, I would have responded differently,” he said. “I think a physician should address why a patient feels that way. They may feel their pain is unbearable, and we potentially offer more pain relief. Maybe the patient shows evidence of having depression, which may be treatable [with medication]. The patient would then feel better and be able to spend quality time with family and loved ones, make sure their affairs are in order, and have a chance to say goodbye.”
A version of this article first appeared on Medscape.com.
An internist will never forget the dark secret his patient revealed during a routine visit – or the grim aftermath.
The patient, who had a progressive, incurable neurological condition, confided that he planned to kill himself. The patient intended to conceal the true manner and make the death look natural.
“[He planned to do it] very carefully at home so no one would know,” said the internist, who remains anonymous. “[He shared] the methods he would use.”
Perhaps more shocking than the patient’s confession was the physician’s response.
“He did not require my help to do what he planned, and I did not try to stop him,” said the internist. “I reported his death as ‘natural causes’ and never told anyone.”
An ob.gyn., for instance, wrote about struggling with whether to tell a father that his newborn baby was not his genetic child. The newborn had a blood type that made it impossible for the father to be biologically related to the infant, the anonymous doctor wrote.
“I told the wife who then informed me she had a lover,” the ob.gyn. said. “I never told the husband.”
It’s uncertain whether carrying the burden of such hidden knowledge affected the physicians involved in these cases. However, in general, secrets can weigh heavily on the minds of those who keep them and can contribute to stress, said Malia Mason, PhD, a psychologist and dean of research at Columbia Business School in New York. Holding onto secrets can cause depression and anxiety, research shows. The more often people think about the secret, the greater the impact, according to a recent study coauthored by Dr. Mason.
“Keeping a secret diminishes well-being,” Dr. Mason said. “It makes people feel socially distant. It lowers relationship satisfaction, and it leads people to feel inauthentic. The reason that secrets do this is because people think about them all the time. The more you think about it, the more you see these consequences.”
Feelings that stem from a secret depend on the content. The more immoral a secret is thought to be, the more people feel ashamed, according to a 2021 analysis of thousands of secrets, reported by Michael L. Slepian, PhD, and Alex Koch, PhD. However, secrets more related to a person’s profession are often internalized differently, the study found. The more a secret fell higher on the profession/goal-oriented dimension, the more people felt they had insight into the secret, according to the analysis. For example, having clear thinking about the secret and/or knowing how to handle it.
“The more shame participants felt from their secret, the more they indicated the secret hurt their well-being,” Dr. Slepian and Dr. Koch wrote in the study. “The more insight participants felt they had into their secret, the less they indicated the secret hurt their well-being.”
Suspicious deaths exposed after investigations
The internist’s account of keeping his patient’s suicide a secret raises many questions, such as how the patient masked his manner of death. The internist did not share any more details about the incident.
Suicides are among the most challenging manners of deaths to certify, according to James Gill, MD, a pathologist and president of the National Association of Medical Examiners. Death investigators must demonstrate intent, meaning the individuals caused the injury to intentionally harm themselves. Fewer than half of people who die by suicide leave a note, Dr. Gill said, so investigators can’t rely on the absence or the presence of a note in making their determination.
A decedent who had cancer or a severe neurological disorder presents further challenges, said Dr. Gill, who serves as chief medical examiner for the state of Connecticut.
“These [deaths] may not be unexpected and may not be reported to the medical examiner/coroner,” Dr. Gill said. “If there is no suspicion and the treating doctor is willing to sign the death certificate, the death will not come under the jurisdiction of the medical examiner.”
Dr. Gill recalled a death his colleague once investigated that appeared to be natural but emerged as something else after a deeper look.
A woman with metastatic breast cancer was about to be discharged from a hospital into hospice the next morning. The night before, she had a “going away” party with friends who came to visit her in the hospital. Shortly after the friends left, the woman was found dead. Because of her condition, she could have died at any time, Dr. Gill said, but she also had a history of depression and hospital staff were suspicious. The death was reported to the medical examiner’s office.
Toxicology testing found markedly elevated concentrations of phenytoin and pentobarbital, neither of which were prescribed during her hospital stay. Dr. Gill said it turned out that the woman and her friends worked at a veterinarian’s office, and the medication they used to euthanize dogs was a combination of phenytoin and pentobarbital.
“The death was certified as a homicide because of the direct actions of another, but a reasonable argument could be made for suicide,” Dr. Gill said.
In a similar case reported in the journal Science & Justice, a 64-year-old cardiologist was found lifeless by his wife after he collapsed near the stairs of his home. Next to his body was a bottle of whiskey and two cups, one that appeared to be used for the alcohol and one with a yellowish liquid smelling of honey. The wife reported that her husband always drank whiskey with honey before bed. The death was initially classified as natural, but after vehement protest by the family, a forensic autopsy was performed.
Prior to the autopsy, death investigators learned the decedent, who was a well-known and successful practitioner in his community, had Parkinson’s disease. At times, he could not sign his prescriptions because of the increasing tremor in his hands, according to the case study. Investigators learned the patient’s mother had also suffered from Parkinson’s, and that her son had witnessed her decline.
The autopsy revealed only nonspecific lesions such as acute stasis of the viscera, moderate pulmonary and cerebral edema, and moderate generalized atheromatosis. Histological examinations did not yield any unusual findings.
An analysis of the beverage containers detected pentobarbital in the yellowish syrup residue of the second cup. Testing of the doctor’s peripheral blood revealed the presence of a metabolite of pentobarbital, ethanol, and traces of phenobarbital. In addition, a urine analysis showed the presence of venlafaxine, an antidepressant, as well as the benzophenone of lorazepam, a sedating benzodiazepine, and metoclopramide, an antiemetic.
Lead author C. Brandt-Casadevall, MD, and colleagues wrote that the levels were clearly compatible with a scenario of a pentobarbital overdose with a lethal outcome.
“... It is obvious that the victim attempted to hide his suicide from his family circle,” Dr. Brandt-Casadevall and colleagues wrote. “Thus, we obtained no evidence indicating that he might have spoken at any point of putting an end to his life. There was no written note. The victim did not wait to be alone at home. Instead, he committed his act in a routine situation: his wife was watching television late at night and he was upstairs, presumably going to sleep. Thus, he had one to two hours at his disposal, and he ingested a very fast-acting drug which would make any attempt at reanimation impossible, even after a brief period of time. This may have induced the physician in charge to believe that the cause of death was cardiac origin, a likely hypothesis given the age of the victim.”
What to do when a terminally ill patient talks suicide
When a terminally ill patient expresses the desire to end his or her life, it’s important to understand that desire is often a result of existential suffering, a sense of hopelessness, and lack of social support, said Lynn A. Jansen, PhD, a bioethicist at the University of Arizona in Tucson.
“The duty of beneficence requires that physicians attempt to provide the support and care that is needed,” said Dr. Jansen. “Here, interdisciplinary teamwork is important and should be utilized. Physicians should refer patients to professionals, such as social workers, pastoral care, psychologists, etc., who are better able to address these issues.”
The rate of desire for a hastened death among terminally ill patients ranges from 17% to 45%, depending on the population studied and how the desire is evaluated, according to an analysis in the Primary Care Companion to the Journal of Clinical Psychiatry. In one study, 14% of about 130 palliative care patients with cancer had a strong desire to quicken the dying process.
In addition, patients with neurologic disorders have a significantly higher suicide rate than that of those without neurologic disorders, a recent JAMA study found. About 1 in 150 patients diagnosed with a neurological disorder dies by suicide, the analysis determined.
A tricky point to remember is that a desire by a terminally ill patient to hasten his or her death by suicide should not be taken by itself to indicate depression, Dr. Jansen noted.
“In principle, such patients can make an autonomous decision to end their lives,” she said. “However, the expression of such a desire is very often associated with depression and forms of suffering that can be effectively addressed by the health care team.”
Physicians can also explore other avenues with the patient such as palliative care or making sure adequate pain relief is available, added Robert Klitzman, MD, professor of psychiatry and academic director of the master of science in bioethics program at Columbia University, New York.
“If they are saying it’s because they are distressed, ethically, a doctor can and should find ways to decrease their distress,” he said.
Of course, those who practice in the U.S. jurisdictions that have physician-assisted-dying laws have different legal and ethical elements to consider. Physicians in these areas have no ethical duty to participate in the process, Dr. Jansen said, but they have a duty to refer patients who express a desire to pursue physician aid-in-dying to another provider who can assist them.
Physician aid-in-dying laws vary somewhat so it’s important that physicians in these areas be aware of their specific statute, Dr. Klitzman said. California, Colorado, Hawaii, Maine, New Jersey, New Mexico, Oregon, Vermont, Washington, and the District of Columbia have these laws.
“In these states, if a terminally ill patient says they don’t want to live anymore, a physician would first decide if this is a result of depression or if it’s a request for physician aid-in-dying,” he said. “Even then, in most cases, the patient would be evaluated by not one, but two different health professionals at two different points. We want to see if it is a consistent decision that the person has made that they want physician aid-in-dying, and not just that they’ve had a bad day or a setback in their treatment.”
In the case of the internist who told no one of his patient’s suicide plan, Dr. Klitzman said he would have dug deeper into the patient’s mindset.
“Not knowing anything about the patient or the doctor, I would have responded differently,” he said. “I think a physician should address why a patient feels that way. They may feel their pain is unbearable, and we potentially offer more pain relief. Maybe the patient shows evidence of having depression, which may be treatable [with medication]. The patient would then feel better and be able to spend quality time with family and loved ones, make sure their affairs are in order, and have a chance to say goodbye.”
A version of this article first appeared on Medscape.com.
An internist will never forget the dark secret his patient revealed during a routine visit – or the grim aftermath.
The patient, who had a progressive, incurable neurological condition, confided that he planned to kill himself. The patient intended to conceal the true manner and make the death look natural.
“[He planned to do it] very carefully at home so no one would know,” said the internist, who remains anonymous. “[He shared] the methods he would use.”
Perhaps more shocking than the patient’s confession was the physician’s response.
“He did not require my help to do what he planned, and I did not try to stop him,” said the internist. “I reported his death as ‘natural causes’ and never told anyone.”
An ob.gyn., for instance, wrote about struggling with whether to tell a father that his newborn baby was not his genetic child. The newborn had a blood type that made it impossible for the father to be biologically related to the infant, the anonymous doctor wrote.
“I told the wife who then informed me she had a lover,” the ob.gyn. said. “I never told the husband.”
It’s uncertain whether carrying the burden of such hidden knowledge affected the physicians involved in these cases. However, in general, secrets can weigh heavily on the minds of those who keep them and can contribute to stress, said Malia Mason, PhD, a psychologist and dean of research at Columbia Business School in New York. Holding onto secrets can cause depression and anxiety, research shows. The more often people think about the secret, the greater the impact, according to a recent study coauthored by Dr. Mason.
“Keeping a secret diminishes well-being,” Dr. Mason said. “It makes people feel socially distant. It lowers relationship satisfaction, and it leads people to feel inauthentic. The reason that secrets do this is because people think about them all the time. The more you think about it, the more you see these consequences.”
Feelings that stem from a secret depend on the content. The more immoral a secret is thought to be, the more people feel ashamed, according to a 2021 analysis of thousands of secrets, reported by Michael L. Slepian, PhD, and Alex Koch, PhD. However, secrets more related to a person’s profession are often internalized differently, the study found. The more a secret fell higher on the profession/goal-oriented dimension, the more people felt they had insight into the secret, according to the analysis. For example, having clear thinking about the secret and/or knowing how to handle it.
“The more shame participants felt from their secret, the more they indicated the secret hurt their well-being,” Dr. Slepian and Dr. Koch wrote in the study. “The more insight participants felt they had into their secret, the less they indicated the secret hurt their well-being.”
Suspicious deaths exposed after investigations
The internist’s account of keeping his patient’s suicide a secret raises many questions, such as how the patient masked his manner of death. The internist did not share any more details about the incident.
Suicides are among the most challenging manners of deaths to certify, according to James Gill, MD, a pathologist and president of the National Association of Medical Examiners. Death investigators must demonstrate intent, meaning the individuals caused the injury to intentionally harm themselves. Fewer than half of people who die by suicide leave a note, Dr. Gill said, so investigators can’t rely on the absence or the presence of a note in making their determination.
A decedent who had cancer or a severe neurological disorder presents further challenges, said Dr. Gill, who serves as chief medical examiner for the state of Connecticut.
“These [deaths] may not be unexpected and may not be reported to the medical examiner/coroner,” Dr. Gill said. “If there is no suspicion and the treating doctor is willing to sign the death certificate, the death will not come under the jurisdiction of the medical examiner.”
Dr. Gill recalled a death his colleague once investigated that appeared to be natural but emerged as something else after a deeper look.
A woman with metastatic breast cancer was about to be discharged from a hospital into hospice the next morning. The night before, she had a “going away” party with friends who came to visit her in the hospital. Shortly after the friends left, the woman was found dead. Because of her condition, she could have died at any time, Dr. Gill said, but she also had a history of depression and hospital staff were suspicious. The death was reported to the medical examiner’s office.
Toxicology testing found markedly elevated concentrations of phenytoin and pentobarbital, neither of which were prescribed during her hospital stay. Dr. Gill said it turned out that the woman and her friends worked at a veterinarian’s office, and the medication they used to euthanize dogs was a combination of phenytoin and pentobarbital.
“The death was certified as a homicide because of the direct actions of another, but a reasonable argument could be made for suicide,” Dr. Gill said.
In a similar case reported in the journal Science & Justice, a 64-year-old cardiologist was found lifeless by his wife after he collapsed near the stairs of his home. Next to his body was a bottle of whiskey and two cups, one that appeared to be used for the alcohol and one with a yellowish liquid smelling of honey. The wife reported that her husband always drank whiskey with honey before bed. The death was initially classified as natural, but after vehement protest by the family, a forensic autopsy was performed.
Prior to the autopsy, death investigators learned the decedent, who was a well-known and successful practitioner in his community, had Parkinson’s disease. At times, he could not sign his prescriptions because of the increasing tremor in his hands, according to the case study. Investigators learned the patient’s mother had also suffered from Parkinson’s, and that her son had witnessed her decline.
The autopsy revealed only nonspecific lesions such as acute stasis of the viscera, moderate pulmonary and cerebral edema, and moderate generalized atheromatosis. Histological examinations did not yield any unusual findings.
An analysis of the beverage containers detected pentobarbital in the yellowish syrup residue of the second cup. Testing of the doctor’s peripheral blood revealed the presence of a metabolite of pentobarbital, ethanol, and traces of phenobarbital. In addition, a urine analysis showed the presence of venlafaxine, an antidepressant, as well as the benzophenone of lorazepam, a sedating benzodiazepine, and metoclopramide, an antiemetic.
Lead author C. Brandt-Casadevall, MD, and colleagues wrote that the levels were clearly compatible with a scenario of a pentobarbital overdose with a lethal outcome.
“... It is obvious that the victim attempted to hide his suicide from his family circle,” Dr. Brandt-Casadevall and colleagues wrote. “Thus, we obtained no evidence indicating that he might have spoken at any point of putting an end to his life. There was no written note. The victim did not wait to be alone at home. Instead, he committed his act in a routine situation: his wife was watching television late at night and he was upstairs, presumably going to sleep. Thus, he had one to two hours at his disposal, and he ingested a very fast-acting drug which would make any attempt at reanimation impossible, even after a brief period of time. This may have induced the physician in charge to believe that the cause of death was cardiac origin, a likely hypothesis given the age of the victim.”
What to do when a terminally ill patient talks suicide
When a terminally ill patient expresses the desire to end his or her life, it’s important to understand that desire is often a result of existential suffering, a sense of hopelessness, and lack of social support, said Lynn A. Jansen, PhD, a bioethicist at the University of Arizona in Tucson.
“The duty of beneficence requires that physicians attempt to provide the support and care that is needed,” said Dr. Jansen. “Here, interdisciplinary teamwork is important and should be utilized. Physicians should refer patients to professionals, such as social workers, pastoral care, psychologists, etc., who are better able to address these issues.”
The rate of desire for a hastened death among terminally ill patients ranges from 17% to 45%, depending on the population studied and how the desire is evaluated, according to an analysis in the Primary Care Companion to the Journal of Clinical Psychiatry. In one study, 14% of about 130 palliative care patients with cancer had a strong desire to quicken the dying process.
In addition, patients with neurologic disorders have a significantly higher suicide rate than that of those without neurologic disorders, a recent JAMA study found. About 1 in 150 patients diagnosed with a neurological disorder dies by suicide, the analysis determined.
A tricky point to remember is that a desire by a terminally ill patient to hasten his or her death by suicide should not be taken by itself to indicate depression, Dr. Jansen noted.
“In principle, such patients can make an autonomous decision to end their lives,” she said. “However, the expression of such a desire is very often associated with depression and forms of suffering that can be effectively addressed by the health care team.”
Physicians can also explore other avenues with the patient such as palliative care or making sure adequate pain relief is available, added Robert Klitzman, MD, professor of psychiatry and academic director of the master of science in bioethics program at Columbia University, New York.
“If they are saying it’s because they are distressed, ethically, a doctor can and should find ways to decrease their distress,” he said.
Of course, those who practice in the U.S. jurisdictions that have physician-assisted-dying laws have different legal and ethical elements to consider. Physicians in these areas have no ethical duty to participate in the process, Dr. Jansen said, but they have a duty to refer patients who express a desire to pursue physician aid-in-dying to another provider who can assist them.
Physician aid-in-dying laws vary somewhat so it’s important that physicians in these areas be aware of their specific statute, Dr. Klitzman said. California, Colorado, Hawaii, Maine, New Jersey, New Mexico, Oregon, Vermont, Washington, and the District of Columbia have these laws.
“In these states, if a terminally ill patient says they don’t want to live anymore, a physician would first decide if this is a result of depression or if it’s a request for physician aid-in-dying,” he said. “Even then, in most cases, the patient would be evaluated by not one, but two different health professionals at two different points. We want to see if it is a consistent decision that the person has made that they want physician aid-in-dying, and not just that they’ve had a bad day or a setback in their treatment.”
In the case of the internist who told no one of his patient’s suicide plan, Dr. Klitzman said he would have dug deeper into the patient’s mindset.
“Not knowing anything about the patient or the doctor, I would have responded differently,” he said. “I think a physician should address why a patient feels that way. They may feel their pain is unbearable, and we potentially offer more pain relief. Maybe the patient shows evidence of having depression, which may be treatable [with medication]. The patient would then feel better and be able to spend quality time with family and loved ones, make sure their affairs are in order, and have a chance to say goodbye.”
A version of this article first appeared on Medscape.com.
MRI is a poor disability predictor in secondary progressive MS
, new research suggests. Analysis from the phase 3 ASCEND trial of nearly 900 patients showed that MRI measures were not associated with worsening of scores on the Expanded Disability Status Scale (EDSS), the most widely used physical outcome measure.
The few associations that were shown between MRI measures and clinical outcomes “were with the newer and possibly more sensitive outcomes” – the Timed 25-Foot Walk (T25FW) and Nine-Hole Peg Test (NHPT), wrote the investigators, led by Marcus W. Koch, MD, PhD, associate professor of neurology in the MS program at the University of Calgary, Canada.
However, “it is unclear if these associations are clinically meaningful,” they added.
Worsening on the NHPT at 48 weeks was associated with a 0.86% loss in normalized brain volume; worsening at 96 weeks was associated with a 1.47% loss.
The findings were published online July 26 in the Multiple Sclerosis Journal.
ASCEND data analysis
Although brain volume loss occurs in all forms of MS, it is believed to be particularly relevant in SPMS. Clinical trials often use MRI measures of brain volume as endpoints, likely on the assumption that these measures indicate worsening disability.
However, brain volume loss proceeds slowly. Changes that occur during the typical 2-year study period may not be associated with significant physical or cognitive disability.
In the current study, investigators examined data from the ASCEND trial, which assessed the use of natalizumab for patients with SPMS, to examine these potential associations. Eligible participants in ASCEND were between ages 18 and 58 years, had had SPMS for 2 or more years, had had disability progression during the previous year, and had an EDSS score between 3.0 and 6.5 at baseline.
Participants underwent gadolinium-enhanced cranial MRI at screening and at 24, 48, 72, and 96 weeks. MRI outcomes included normalized brain volume, normalized cortical gray matter volume, and normalized whole gray matter volume. The ASCEND investigators also examined the number and volume of T2 and contrast-enhancing lesions.
The study’s clinical outcomes included scores on the EDSS, T25FW, and NHPT, which were administered at baseline and every 12 weeks thereafter. Participants also underwent the Symbol Digit Modalities Test (SDMT), which is a cognitive assessment, at baseline and every 4 weeks thereafter. In addition, 3-month confirmed disability progression was measured every 12 weeks.
Few significant associations
The investigators’ analysis included 889 patients (61.9% women; median age, 48 years). The median EDSS score at screening was 6.
Brain volume measures decreased consistently during follow-up. Mean volume loss at 96 weeks was about 1%. In contrast, T2 lesion volume changed little during follow-up. The cumulative number of contrast-enhancing lesions and the cumulative number of new or newly enlarging T2 lesions increased steadily during follow-up.
For an increasing number of participants, scores on the EDSS, NHPT, and T25FW worsened significantly during follow-up. Performance on SDMT, however, changed little. Of all the clinical measures, the NHPT was most consistently associated with MRI measures.
Among patients whose NHPT score worsened at 48 weeks, there was greater loss of normalized brain volume (0.86%, P = .02), normalized cortical gray matter volume (1.15%, P = .03), and normalized whole gray matter volume (1.08%, P = .03) than among those whose NHPT score did not worsen.
Among patients whose NHPT score worsened at 96 weeks, there was greater normalized brain volume loss (1.47%, P = .002), greater increase in T2 lesion volume (4.68%, P = .02), and a greater number of cumulative new or newly enlarging T2 lesions (7.81, P = .03) than those whose NHPT score did not worsen.
After adjusting the data for covariables, the investigators found few significant associations between MRI measures and clinical outcomes. Worsening on the EDSS and SDMT was not associated with any MRI outcome.
Important disability contributors missed
The odds ratio of 3-month confirmed worsening on the T25FW at 96 weeks was 2.25 for patients with more than 10 cumulative new or newly enlarging T2 lesions (P = .03). The OR of 3-month confirmed worsening on the NHPT at 96 weeks was 3.04 for patients with more than 10 such lesions (P = .03).
Greater normalized brain volume loss at 48 weeks was associated with a greater risk for worsening disability on the NHPT at 48 and 96 weeks. For patients with a volume loss greater than 1.5%, the OR of worsening NHPT at 96 weeks was 4.69 (P = .05).
Although previous cross-sectional studies have shown correlations between brain volume and cognitive dysfunction, the current investigators found no association between change in SDMT performance and MRI measures.
From the ASCEND dataset, they found that performance on the SDMT unexpectedly improved with time, perhaps because of a practice effect.
“The SDMT may therefore not adequately reflect the steady cognitive decline that people with SPMS experience,” the investigators wrote.
The lack of association between MRI measures and clinical outcomes may indicate that traditional MRI does not measure important contributors to disability, they noted.
“Although the investigated volume measures in this study are currently the most commonly used in clinical trials, newer MRI metrics such as thalamic or corpus callosum atrophy may have a closer relation to clinical outcome,” they added.
‘Interesting and provocative’
Commenting on the findings, E. Ann Yeh, MD, director of the Pediatric MS and Neuroinflammatory Disorders Program at the Hospital for Sick Children, Toronto, called the study “interesting and provocative.”
“Other studies previously have shown associations between disability and progression, but many have been cross-sectional,” said Dr. Yeh, who was not involved with the research.
The current study is longitudinal and analyzes carefully documented follow-up data from a clinical trial, she noted. However, the 2-year follow-up period was short, considering the pace at which whole brain volume change occurs, Dr. Yeh said.
Some patients with MS have greater brain volume loss than others. Because of this variability, researchers often examine a population’s average brain volume loss. “When you look at averages, it makes it more difficult to understand if the larger brain volume losses are actually associated with change,” said Dr. Yeh.
She noted that because the study population had high EDSS scores at baseline, it is not surprising that the NHPT and the T25FW were more strongly associated with change in brain volume than the EDSS was. Large changes in EDSS score probably did not occur during follow-up, she added.
“We’ll continue to use the EDSS, because it’s what we have,” said Dr. Yeh. However, newer measures, such as the NHPT and the T25FW, may provide better information, she said. Similarly, composite measures of cognition, such as the Brief International Cognitive Assessment for MS, may be superior to the SDMT but take longer to administer.
“We need to look more deeply at which MRI measures are the best for predicting outcome and that correlate well in a short period of time,” said Dr. Yeh.
These measures could include specific regional brain volumes “and more advanced measures that look at axonal injury or axonal loss.” Studies with longer follow-up are also necessary, she concluded.
The investigators and Dr. Yeh have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Analysis from the phase 3 ASCEND trial of nearly 900 patients showed that MRI measures were not associated with worsening of scores on the Expanded Disability Status Scale (EDSS), the most widely used physical outcome measure.
The few associations that were shown between MRI measures and clinical outcomes “were with the newer and possibly more sensitive outcomes” – the Timed 25-Foot Walk (T25FW) and Nine-Hole Peg Test (NHPT), wrote the investigators, led by Marcus W. Koch, MD, PhD, associate professor of neurology in the MS program at the University of Calgary, Canada.
However, “it is unclear if these associations are clinically meaningful,” they added.
Worsening on the NHPT at 48 weeks was associated with a 0.86% loss in normalized brain volume; worsening at 96 weeks was associated with a 1.47% loss.
The findings were published online July 26 in the Multiple Sclerosis Journal.
ASCEND data analysis
Although brain volume loss occurs in all forms of MS, it is believed to be particularly relevant in SPMS. Clinical trials often use MRI measures of brain volume as endpoints, likely on the assumption that these measures indicate worsening disability.
However, brain volume loss proceeds slowly. Changes that occur during the typical 2-year study period may not be associated with significant physical or cognitive disability.
In the current study, investigators examined data from the ASCEND trial, which assessed the use of natalizumab for patients with SPMS, to examine these potential associations. Eligible participants in ASCEND were between ages 18 and 58 years, had had SPMS for 2 or more years, had had disability progression during the previous year, and had an EDSS score between 3.0 and 6.5 at baseline.
Participants underwent gadolinium-enhanced cranial MRI at screening and at 24, 48, 72, and 96 weeks. MRI outcomes included normalized brain volume, normalized cortical gray matter volume, and normalized whole gray matter volume. The ASCEND investigators also examined the number and volume of T2 and contrast-enhancing lesions.
The study’s clinical outcomes included scores on the EDSS, T25FW, and NHPT, which were administered at baseline and every 12 weeks thereafter. Participants also underwent the Symbol Digit Modalities Test (SDMT), which is a cognitive assessment, at baseline and every 4 weeks thereafter. In addition, 3-month confirmed disability progression was measured every 12 weeks.
Few significant associations
The investigators’ analysis included 889 patients (61.9% women; median age, 48 years). The median EDSS score at screening was 6.
Brain volume measures decreased consistently during follow-up. Mean volume loss at 96 weeks was about 1%. In contrast, T2 lesion volume changed little during follow-up. The cumulative number of contrast-enhancing lesions and the cumulative number of new or newly enlarging T2 lesions increased steadily during follow-up.
For an increasing number of participants, scores on the EDSS, NHPT, and T25FW worsened significantly during follow-up. Performance on SDMT, however, changed little. Of all the clinical measures, the NHPT was most consistently associated with MRI measures.
Among patients whose NHPT score worsened at 48 weeks, there was greater loss of normalized brain volume (0.86%, P = .02), normalized cortical gray matter volume (1.15%, P = .03), and normalized whole gray matter volume (1.08%, P = .03) than among those whose NHPT score did not worsen.
Among patients whose NHPT score worsened at 96 weeks, there was greater normalized brain volume loss (1.47%, P = .002), greater increase in T2 lesion volume (4.68%, P = .02), and a greater number of cumulative new or newly enlarging T2 lesions (7.81, P = .03) than those whose NHPT score did not worsen.
After adjusting the data for covariables, the investigators found few significant associations between MRI measures and clinical outcomes. Worsening on the EDSS and SDMT was not associated with any MRI outcome.
Important disability contributors missed
The odds ratio of 3-month confirmed worsening on the T25FW at 96 weeks was 2.25 for patients with more than 10 cumulative new or newly enlarging T2 lesions (P = .03). The OR of 3-month confirmed worsening on the NHPT at 96 weeks was 3.04 for patients with more than 10 such lesions (P = .03).
Greater normalized brain volume loss at 48 weeks was associated with a greater risk for worsening disability on the NHPT at 48 and 96 weeks. For patients with a volume loss greater than 1.5%, the OR of worsening NHPT at 96 weeks was 4.69 (P = .05).
Although previous cross-sectional studies have shown correlations between brain volume and cognitive dysfunction, the current investigators found no association between change in SDMT performance and MRI measures.
From the ASCEND dataset, they found that performance on the SDMT unexpectedly improved with time, perhaps because of a practice effect.
“The SDMT may therefore not adequately reflect the steady cognitive decline that people with SPMS experience,” the investigators wrote.
The lack of association between MRI measures and clinical outcomes may indicate that traditional MRI does not measure important contributors to disability, they noted.
“Although the investigated volume measures in this study are currently the most commonly used in clinical trials, newer MRI metrics such as thalamic or corpus callosum atrophy may have a closer relation to clinical outcome,” they added.
‘Interesting and provocative’
Commenting on the findings, E. Ann Yeh, MD, director of the Pediatric MS and Neuroinflammatory Disorders Program at the Hospital for Sick Children, Toronto, called the study “interesting and provocative.”
“Other studies previously have shown associations between disability and progression, but many have been cross-sectional,” said Dr. Yeh, who was not involved with the research.
The current study is longitudinal and analyzes carefully documented follow-up data from a clinical trial, she noted. However, the 2-year follow-up period was short, considering the pace at which whole brain volume change occurs, Dr. Yeh said.
Some patients with MS have greater brain volume loss than others. Because of this variability, researchers often examine a population’s average brain volume loss. “When you look at averages, it makes it more difficult to understand if the larger brain volume losses are actually associated with change,” said Dr. Yeh.
She noted that because the study population had high EDSS scores at baseline, it is not surprising that the NHPT and the T25FW were more strongly associated with change in brain volume than the EDSS was. Large changes in EDSS score probably did not occur during follow-up, she added.
“We’ll continue to use the EDSS, because it’s what we have,” said Dr. Yeh. However, newer measures, such as the NHPT and the T25FW, may provide better information, she said. Similarly, composite measures of cognition, such as the Brief International Cognitive Assessment for MS, may be superior to the SDMT but take longer to administer.
“We need to look more deeply at which MRI measures are the best for predicting outcome and that correlate well in a short period of time,” said Dr. Yeh.
These measures could include specific regional brain volumes “and more advanced measures that look at axonal injury or axonal loss.” Studies with longer follow-up are also necessary, she concluded.
The investigators and Dr. Yeh have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Analysis from the phase 3 ASCEND trial of nearly 900 patients showed that MRI measures were not associated with worsening of scores on the Expanded Disability Status Scale (EDSS), the most widely used physical outcome measure.
The few associations that were shown between MRI measures and clinical outcomes “were with the newer and possibly more sensitive outcomes” – the Timed 25-Foot Walk (T25FW) and Nine-Hole Peg Test (NHPT), wrote the investigators, led by Marcus W. Koch, MD, PhD, associate professor of neurology in the MS program at the University of Calgary, Canada.
However, “it is unclear if these associations are clinically meaningful,” they added.
Worsening on the NHPT at 48 weeks was associated with a 0.86% loss in normalized brain volume; worsening at 96 weeks was associated with a 1.47% loss.
The findings were published online July 26 in the Multiple Sclerosis Journal.
ASCEND data analysis
Although brain volume loss occurs in all forms of MS, it is believed to be particularly relevant in SPMS. Clinical trials often use MRI measures of brain volume as endpoints, likely on the assumption that these measures indicate worsening disability.
However, brain volume loss proceeds slowly. Changes that occur during the typical 2-year study period may not be associated with significant physical or cognitive disability.
In the current study, investigators examined data from the ASCEND trial, which assessed the use of natalizumab for patients with SPMS, to examine these potential associations. Eligible participants in ASCEND were between ages 18 and 58 years, had had SPMS for 2 or more years, had had disability progression during the previous year, and had an EDSS score between 3.0 and 6.5 at baseline.
Participants underwent gadolinium-enhanced cranial MRI at screening and at 24, 48, 72, and 96 weeks. MRI outcomes included normalized brain volume, normalized cortical gray matter volume, and normalized whole gray matter volume. The ASCEND investigators also examined the number and volume of T2 and contrast-enhancing lesions.
The study’s clinical outcomes included scores on the EDSS, T25FW, and NHPT, which were administered at baseline and every 12 weeks thereafter. Participants also underwent the Symbol Digit Modalities Test (SDMT), which is a cognitive assessment, at baseline and every 4 weeks thereafter. In addition, 3-month confirmed disability progression was measured every 12 weeks.
Few significant associations
The investigators’ analysis included 889 patients (61.9% women; median age, 48 years). The median EDSS score at screening was 6.
Brain volume measures decreased consistently during follow-up. Mean volume loss at 96 weeks was about 1%. In contrast, T2 lesion volume changed little during follow-up. The cumulative number of contrast-enhancing lesions and the cumulative number of new or newly enlarging T2 lesions increased steadily during follow-up.
For an increasing number of participants, scores on the EDSS, NHPT, and T25FW worsened significantly during follow-up. Performance on SDMT, however, changed little. Of all the clinical measures, the NHPT was most consistently associated with MRI measures.
Among patients whose NHPT score worsened at 48 weeks, there was greater loss of normalized brain volume (0.86%, P = .02), normalized cortical gray matter volume (1.15%, P = .03), and normalized whole gray matter volume (1.08%, P = .03) than among those whose NHPT score did not worsen.
Among patients whose NHPT score worsened at 96 weeks, there was greater normalized brain volume loss (1.47%, P = .002), greater increase in T2 lesion volume (4.68%, P = .02), and a greater number of cumulative new or newly enlarging T2 lesions (7.81, P = .03) than those whose NHPT score did not worsen.
After adjusting the data for covariables, the investigators found few significant associations between MRI measures and clinical outcomes. Worsening on the EDSS and SDMT was not associated with any MRI outcome.
Important disability contributors missed
The odds ratio of 3-month confirmed worsening on the T25FW at 96 weeks was 2.25 for patients with more than 10 cumulative new or newly enlarging T2 lesions (P = .03). The OR of 3-month confirmed worsening on the NHPT at 96 weeks was 3.04 for patients with more than 10 such lesions (P = .03).
Greater normalized brain volume loss at 48 weeks was associated with a greater risk for worsening disability on the NHPT at 48 and 96 weeks. For patients with a volume loss greater than 1.5%, the OR of worsening NHPT at 96 weeks was 4.69 (P = .05).
Although previous cross-sectional studies have shown correlations between brain volume and cognitive dysfunction, the current investigators found no association between change in SDMT performance and MRI measures.
From the ASCEND dataset, they found that performance on the SDMT unexpectedly improved with time, perhaps because of a practice effect.
“The SDMT may therefore not adequately reflect the steady cognitive decline that people with SPMS experience,” the investigators wrote.
The lack of association between MRI measures and clinical outcomes may indicate that traditional MRI does not measure important contributors to disability, they noted.
“Although the investigated volume measures in this study are currently the most commonly used in clinical trials, newer MRI metrics such as thalamic or corpus callosum atrophy may have a closer relation to clinical outcome,” they added.
‘Interesting and provocative’
Commenting on the findings, E. Ann Yeh, MD, director of the Pediatric MS and Neuroinflammatory Disorders Program at the Hospital for Sick Children, Toronto, called the study “interesting and provocative.”
“Other studies previously have shown associations between disability and progression, but many have been cross-sectional,” said Dr. Yeh, who was not involved with the research.
The current study is longitudinal and analyzes carefully documented follow-up data from a clinical trial, she noted. However, the 2-year follow-up period was short, considering the pace at which whole brain volume change occurs, Dr. Yeh said.
Some patients with MS have greater brain volume loss than others. Because of this variability, researchers often examine a population’s average brain volume loss. “When you look at averages, it makes it more difficult to understand if the larger brain volume losses are actually associated with change,” said Dr. Yeh.
She noted that because the study population had high EDSS scores at baseline, it is not surprising that the NHPT and the T25FW were more strongly associated with change in brain volume than the EDSS was. Large changes in EDSS score probably did not occur during follow-up, she added.
“We’ll continue to use the EDSS, because it’s what we have,” said Dr. Yeh. However, newer measures, such as the NHPT and the T25FW, may provide better information, she said. Similarly, composite measures of cognition, such as the Brief International Cognitive Assessment for MS, may be superior to the SDMT but take longer to administer.
“We need to look more deeply at which MRI measures are the best for predicting outcome and that correlate well in a short period of time,” said Dr. Yeh.
These measures could include specific regional brain volumes “and more advanced measures that look at axonal injury or axonal loss.” Studies with longer follow-up are also necessary, she concluded.
The investigators and Dr. Yeh have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From Multiple Sclerosis Journal
Walnuts lowered LDL cholesterol in healthy seniors: WAHA study
Benefit sustained over 2 years
Healthy elderly people who ate a walnut-supplemented diet for 2 years showed significant reductions in LDL cholesterol, according to a randomized study that used imaging to evaluate lipid changes.
“Regularly eating walnuts will lower your LDL cholesterol and improve the quality of LDL particles, rendering them less prone to enter the arterial wall and build up atherosclerosis, and this will occur without unwanted weight gain in spite of the high-fat – healthy vegetable fat, though – content of walnuts,” Emilio Ros, MD, PhD, senior author of the Walnuts and Healthy Aging (WAHA) study, said in an interview.
WAHA is a parallel-group, randomized, controlled trial that followed 636 patients over 2 years at centers in Loma Linda, Calif., and Barcelona. They were randomly assigned to either a walnut-free or walnut-supplemented diet, and every 2 months they were underwent nuclear magnetic resonance spectroscopy and recorded their compliance, toleration, medication changes, and body weight.
The researchers reported “significantly decreased” total cholesterol, LDL cholesterol and intermediate-density lipoprotein cholesterol, along with reductions in total LDL cholesterol particles and small LDL cholesterol particle number in patients on a walnut-supplemented diet, compared with controls. However, triglycerides and HDL cholesterol were unaffected.
Study results
The study reported mean reductions in the following lipid categories among what the researchers called the “walnut group”:
- Total cholesterol, –8.5 mg/dL (95% confidence interval, –11.2 to –5.4), a 4.4% mean reduction.
- LDL-C, –4.3 mg/dL (95% CI, –6.6 to –1.6), a 3.6% reduction.
- Intermediate-density lipoprotein cholesterol, –1.3 mg/dL (95% CI, –1.5 to –1.0] for a 16.8% reduction.
- Total LDL cholesterol particles, a reduction of 4.3%.
- Small LDL cholesterol particle number, a 6.1% decrease.
“WAHA is the largest and longest randomized nut trial to date, which overcomes power limitations of former trials, smaller and of shorter duration,” said Dr. Ros, of the lipid clinic, endocrinology, nutrition service at the Hospital Clinic Villarroel at the University of Barcelona. He noted that studies he has participated in have already shown that the walnut-supplemented diet had beneficial effects on blood pressure, systemic inflammation and endothelial function.
Other strengths of the study, he said, were that it recruited patients from two distinct locations and that it retained 90% of participants over 2 years (the study started out with 708 participants).
Christie Ballantyne, MD, concurred that the size and duration of the study are worth noting. “People always have questions about what they should eat,” said Dr. Ballantyne, chief of cardiology at Baylor College of Medicine and director of cardiovascular disease prevention at Methodist DeBakey Heart Center, both in Houston, said in an interview. “It’s very difficult to do nutritional studies. Most studies are small and short term, so it’s unusual to have study that’s large with 2 years of follow-up. This is a larger-than-usual study.”
Potential study limitations
Dr. Ros did acknowledge some limitations of the study. Participants weren’t blinded, the feeding setting wasn’t controlled, and participants were generally healthy and had average normal lipid profiles because they were taking statins, which may explain the modest lipid improvements in the study. “LDL cholesterol lowering by nuts is related to baseline levels, hence the reduction observed in our study was modest,” he said.
Additionally, because the study group was elderly, the results don’t apply to younger people. “Yet,” Dr. Ros added, “we know from many prior studies that nuts in general and walnuts in particular will lower blood cholesterol regardless of age.”
Dr. Ballantyne noted that the use of nuclear magnetic resonance spectroscopy to evaluate lipid levels involves a methodology that isn’t as systematic or as standardized as the typical lipid profile, and that different systems use proprietary software to interpret results. “That’s a little bit of an issue,” he said. “What exactly do the numbers mean?”
Overall, though, Dr. Ballantyne said the study is a significant addition to the literature. “The study is large, well done, and it confirms the benefits of something we’ve been telling patients is a good choice. It’s useful because there’s so much noise. It is important because there’s tremendous confusion and misinformation about what’s really healthy to eat.”
The study was supported by a grant from the California Walnut Commission (CWC), from which Dr. Ros and two coauthors received research funding through their institutions. Dr. Ros also reported receiving compensation from CWC and serves on a CWC advisory council. The other authors have no relationships to disclose. The WAHA study was supported by a grant from the CWC, from which Dr. Ros and some coinvestigators have received research funding through their institutions. Dr. Ballantyne has no relevant relationships to disclose.
Benefit sustained over 2 years
Benefit sustained over 2 years
Healthy elderly people who ate a walnut-supplemented diet for 2 years showed significant reductions in LDL cholesterol, according to a randomized study that used imaging to evaluate lipid changes.
“Regularly eating walnuts will lower your LDL cholesterol and improve the quality of LDL particles, rendering them less prone to enter the arterial wall and build up atherosclerosis, and this will occur without unwanted weight gain in spite of the high-fat – healthy vegetable fat, though – content of walnuts,” Emilio Ros, MD, PhD, senior author of the Walnuts and Healthy Aging (WAHA) study, said in an interview.
WAHA is a parallel-group, randomized, controlled trial that followed 636 patients over 2 years at centers in Loma Linda, Calif., and Barcelona. They were randomly assigned to either a walnut-free or walnut-supplemented diet, and every 2 months they were underwent nuclear magnetic resonance spectroscopy and recorded their compliance, toleration, medication changes, and body weight.
The researchers reported “significantly decreased” total cholesterol, LDL cholesterol and intermediate-density lipoprotein cholesterol, along with reductions in total LDL cholesterol particles and small LDL cholesterol particle number in patients on a walnut-supplemented diet, compared with controls. However, triglycerides and HDL cholesterol were unaffected.
Study results
The study reported mean reductions in the following lipid categories among what the researchers called the “walnut group”:
- Total cholesterol, –8.5 mg/dL (95% confidence interval, –11.2 to –5.4), a 4.4% mean reduction.
- LDL-C, –4.3 mg/dL (95% CI, –6.6 to –1.6), a 3.6% reduction.
- Intermediate-density lipoprotein cholesterol, –1.3 mg/dL (95% CI, –1.5 to –1.0] for a 16.8% reduction.
- Total LDL cholesterol particles, a reduction of 4.3%.
- Small LDL cholesterol particle number, a 6.1% decrease.
“WAHA is the largest and longest randomized nut trial to date, which overcomes power limitations of former trials, smaller and of shorter duration,” said Dr. Ros, of the lipid clinic, endocrinology, nutrition service at the Hospital Clinic Villarroel at the University of Barcelona. He noted that studies he has participated in have already shown that the walnut-supplemented diet had beneficial effects on blood pressure, systemic inflammation and endothelial function.
Other strengths of the study, he said, were that it recruited patients from two distinct locations and that it retained 90% of participants over 2 years (the study started out with 708 participants).
Christie Ballantyne, MD, concurred that the size and duration of the study are worth noting. “People always have questions about what they should eat,” said Dr. Ballantyne, chief of cardiology at Baylor College of Medicine and director of cardiovascular disease prevention at Methodist DeBakey Heart Center, both in Houston, said in an interview. “It’s very difficult to do nutritional studies. Most studies are small and short term, so it’s unusual to have study that’s large with 2 years of follow-up. This is a larger-than-usual study.”
Potential study limitations
Dr. Ros did acknowledge some limitations of the study. Participants weren’t blinded, the feeding setting wasn’t controlled, and participants were generally healthy and had average normal lipid profiles because they were taking statins, which may explain the modest lipid improvements in the study. “LDL cholesterol lowering by nuts is related to baseline levels, hence the reduction observed in our study was modest,” he said.
Additionally, because the study group was elderly, the results don’t apply to younger people. “Yet,” Dr. Ros added, “we know from many prior studies that nuts in general and walnuts in particular will lower blood cholesterol regardless of age.”
Dr. Ballantyne noted that the use of nuclear magnetic resonance spectroscopy to evaluate lipid levels involves a methodology that isn’t as systematic or as standardized as the typical lipid profile, and that different systems use proprietary software to interpret results. “That’s a little bit of an issue,” he said. “What exactly do the numbers mean?”
Overall, though, Dr. Ballantyne said the study is a significant addition to the literature. “The study is large, well done, and it confirms the benefits of something we’ve been telling patients is a good choice. It’s useful because there’s so much noise. It is important because there’s tremendous confusion and misinformation about what’s really healthy to eat.”
The study was supported by a grant from the California Walnut Commission (CWC), from which Dr. Ros and two coauthors received research funding through their institutions. Dr. Ros also reported receiving compensation from CWC and serves on a CWC advisory council. The other authors have no relationships to disclose. The WAHA study was supported by a grant from the CWC, from which Dr. Ros and some coinvestigators have received research funding through their institutions. Dr. Ballantyne has no relevant relationships to disclose.
Healthy elderly people who ate a walnut-supplemented diet for 2 years showed significant reductions in LDL cholesterol, according to a randomized study that used imaging to evaluate lipid changes.
“Regularly eating walnuts will lower your LDL cholesterol and improve the quality of LDL particles, rendering them less prone to enter the arterial wall and build up atherosclerosis, and this will occur without unwanted weight gain in spite of the high-fat – healthy vegetable fat, though – content of walnuts,” Emilio Ros, MD, PhD, senior author of the Walnuts and Healthy Aging (WAHA) study, said in an interview.
WAHA is a parallel-group, randomized, controlled trial that followed 636 patients over 2 years at centers in Loma Linda, Calif., and Barcelona. They were randomly assigned to either a walnut-free or walnut-supplemented diet, and every 2 months they were underwent nuclear magnetic resonance spectroscopy and recorded their compliance, toleration, medication changes, and body weight.
The researchers reported “significantly decreased” total cholesterol, LDL cholesterol and intermediate-density lipoprotein cholesterol, along with reductions in total LDL cholesterol particles and small LDL cholesterol particle number in patients on a walnut-supplemented diet, compared with controls. However, triglycerides and HDL cholesterol were unaffected.
Study results
The study reported mean reductions in the following lipid categories among what the researchers called the “walnut group”:
- Total cholesterol, –8.5 mg/dL (95% confidence interval, –11.2 to –5.4), a 4.4% mean reduction.
- LDL-C, –4.3 mg/dL (95% CI, –6.6 to –1.6), a 3.6% reduction.
- Intermediate-density lipoprotein cholesterol, –1.3 mg/dL (95% CI, –1.5 to –1.0] for a 16.8% reduction.
- Total LDL cholesterol particles, a reduction of 4.3%.
- Small LDL cholesterol particle number, a 6.1% decrease.
“WAHA is the largest and longest randomized nut trial to date, which overcomes power limitations of former trials, smaller and of shorter duration,” said Dr. Ros, of the lipid clinic, endocrinology, nutrition service at the Hospital Clinic Villarroel at the University of Barcelona. He noted that studies he has participated in have already shown that the walnut-supplemented diet had beneficial effects on blood pressure, systemic inflammation and endothelial function.
Other strengths of the study, he said, were that it recruited patients from two distinct locations and that it retained 90% of participants over 2 years (the study started out with 708 participants).
Christie Ballantyne, MD, concurred that the size and duration of the study are worth noting. “People always have questions about what they should eat,” said Dr. Ballantyne, chief of cardiology at Baylor College of Medicine and director of cardiovascular disease prevention at Methodist DeBakey Heart Center, both in Houston, said in an interview. “It’s very difficult to do nutritional studies. Most studies are small and short term, so it’s unusual to have study that’s large with 2 years of follow-up. This is a larger-than-usual study.”
Potential study limitations
Dr. Ros did acknowledge some limitations of the study. Participants weren’t blinded, the feeding setting wasn’t controlled, and participants were generally healthy and had average normal lipid profiles because they were taking statins, which may explain the modest lipid improvements in the study. “LDL cholesterol lowering by nuts is related to baseline levels, hence the reduction observed in our study was modest,” he said.
Additionally, because the study group was elderly, the results don’t apply to younger people. “Yet,” Dr. Ros added, “we know from many prior studies that nuts in general and walnuts in particular will lower blood cholesterol regardless of age.”
Dr. Ballantyne noted that the use of nuclear magnetic resonance spectroscopy to evaluate lipid levels involves a methodology that isn’t as systematic or as standardized as the typical lipid profile, and that different systems use proprietary software to interpret results. “That’s a little bit of an issue,” he said. “What exactly do the numbers mean?”
Overall, though, Dr. Ballantyne said the study is a significant addition to the literature. “The study is large, well done, and it confirms the benefits of something we’ve been telling patients is a good choice. It’s useful because there’s so much noise. It is important because there’s tremendous confusion and misinformation about what’s really healthy to eat.”
The study was supported by a grant from the California Walnut Commission (CWC), from which Dr. Ros and two coauthors received research funding through their institutions. Dr. Ros also reported receiving compensation from CWC and serves on a CWC advisory council. The other authors have no relationships to disclose. The WAHA study was supported by a grant from the CWC, from which Dr. Ros and some coinvestigators have received research funding through their institutions. Dr. Ballantyne has no relevant relationships to disclose.
FROM CIRCULATION