User login
Puppy love: Is losing a pet too hard for children?
The big news in the Wilkoff household is that Marilyn and I will be celebrating the arrival of a granddog into our nuclear family. Our younger daughter and her husband will be welcoming into their home a golden retriever puppy the first week in March. This may not seem like big news to some families and is certainly a step down on the priority list to the arrival of the four grandchildren that we already claim on our resume. But, you must understand that no one in our family has ever owned a dog.
Although my wife’s family had a dog, she apparently never really bonded with the canine. My pleas and occasional whining from our three children to get a dog were always met with my wife’s concerns about cleanliness and hygiene. We did have an antisocial cat who lived under a bed in the guest room or in the basement. His passing after 16 years when the kids were in college was not an event marked with any emotion beyond relief.
I think I harbored an unspoken concern about how I and our children might respond emotionally and psychologically to the inevitable death of what would likely have become our family’s best friend. Dispatching a belly-up goldfish after a month or two is small potatoes compared to putting down a tail-wagging, frisbee-catching, four-footed member of the family.
It turns out that my concerns about the mental health of our children may not have been unfounded. A recently published study from the Harvard Medical School and Massachusetts General Hospital found that children who had experienced the death of a loved pet were more likely to exhibit symptoms of psychopathology than were those who had loved a pet who was still alive (Crawford et al. Eur Child Adolesc Psychiatry. 2020 Sep 10. doi: 10.1007/s00787-020-01594-5). The observed effect of the loss was more pronounced in boys. There was also no statistical difference between the psychopathology symptoms of those children who had loved and lost and those children who had never loved a pet.
By the time I left for college I had grown up with five different dogs. I had endured the loss of sweet Mary, the boxer, when we moved to a small apartment and had to send her to a “farm.” I had watched 2-year-old Blackie experience a seizure that heralded his fatal bout with distemper. I shared the struggle with my parents as we made the decision to send my much loved inveterate car chasing “Butch” back to the pound.
However, I survived these losses and wonder whether they in some way prepared me for some of the emotional challenges that would come later in life. This study from Harvard sampled only children from birth to age 8 years. For those of us in primary care a more interesting study might be one that looked for any long-term associations between pet loss as a young child with adolescent and adult mental health. With the surge in pet ownership that has surfaced during the pandemic, there should be an abundance of clinical material to mine. The Harvard researchers’ findings should make us aware of the potential for psychopathology in a child who has suffered the loss of a pet. Each family must decide whether the plusses of pet ownership are worth the risk. However, I side with Tennyson who said it is better to have loved and lost than never to have loved at all.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
The big news in the Wilkoff household is that Marilyn and I will be celebrating the arrival of a granddog into our nuclear family. Our younger daughter and her husband will be welcoming into their home a golden retriever puppy the first week in March. This may not seem like big news to some families and is certainly a step down on the priority list to the arrival of the four grandchildren that we already claim on our resume. But, you must understand that no one in our family has ever owned a dog.
Although my wife’s family had a dog, she apparently never really bonded with the canine. My pleas and occasional whining from our three children to get a dog were always met with my wife’s concerns about cleanliness and hygiene. We did have an antisocial cat who lived under a bed in the guest room or in the basement. His passing after 16 years when the kids were in college was not an event marked with any emotion beyond relief.
I think I harbored an unspoken concern about how I and our children might respond emotionally and psychologically to the inevitable death of what would likely have become our family’s best friend. Dispatching a belly-up goldfish after a month or two is small potatoes compared to putting down a tail-wagging, frisbee-catching, four-footed member of the family.
It turns out that my concerns about the mental health of our children may not have been unfounded. A recently published study from the Harvard Medical School and Massachusetts General Hospital found that children who had experienced the death of a loved pet were more likely to exhibit symptoms of psychopathology than were those who had loved a pet who was still alive (Crawford et al. Eur Child Adolesc Psychiatry. 2020 Sep 10. doi: 10.1007/s00787-020-01594-5). The observed effect of the loss was more pronounced in boys. There was also no statistical difference between the psychopathology symptoms of those children who had loved and lost and those children who had never loved a pet.
By the time I left for college I had grown up with five different dogs. I had endured the loss of sweet Mary, the boxer, when we moved to a small apartment and had to send her to a “farm.” I had watched 2-year-old Blackie experience a seizure that heralded his fatal bout with distemper. I shared the struggle with my parents as we made the decision to send my much loved inveterate car chasing “Butch” back to the pound.
However, I survived these losses and wonder whether they in some way prepared me for some of the emotional challenges that would come later in life. This study from Harvard sampled only children from birth to age 8 years. For those of us in primary care a more interesting study might be one that looked for any long-term associations between pet loss as a young child with adolescent and adult mental health. With the surge in pet ownership that has surfaced during the pandemic, there should be an abundance of clinical material to mine. The Harvard researchers’ findings should make us aware of the potential for psychopathology in a child who has suffered the loss of a pet. Each family must decide whether the plusses of pet ownership are worth the risk. However, I side with Tennyson who said it is better to have loved and lost than never to have loved at all.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
The big news in the Wilkoff household is that Marilyn and I will be celebrating the arrival of a granddog into our nuclear family. Our younger daughter and her husband will be welcoming into their home a golden retriever puppy the first week in March. This may not seem like big news to some families and is certainly a step down on the priority list to the arrival of the four grandchildren that we already claim on our resume. But, you must understand that no one in our family has ever owned a dog.
Although my wife’s family had a dog, she apparently never really bonded with the canine. My pleas and occasional whining from our three children to get a dog were always met with my wife’s concerns about cleanliness and hygiene. We did have an antisocial cat who lived under a bed in the guest room or in the basement. His passing after 16 years when the kids were in college was not an event marked with any emotion beyond relief.
I think I harbored an unspoken concern about how I and our children might respond emotionally and psychologically to the inevitable death of what would likely have become our family’s best friend. Dispatching a belly-up goldfish after a month or two is small potatoes compared to putting down a tail-wagging, frisbee-catching, four-footed member of the family.
It turns out that my concerns about the mental health of our children may not have been unfounded. A recently published study from the Harvard Medical School and Massachusetts General Hospital found that children who had experienced the death of a loved pet were more likely to exhibit symptoms of psychopathology than were those who had loved a pet who was still alive (Crawford et al. Eur Child Adolesc Psychiatry. 2020 Sep 10. doi: 10.1007/s00787-020-01594-5). The observed effect of the loss was more pronounced in boys. There was also no statistical difference between the psychopathology symptoms of those children who had loved and lost and those children who had never loved a pet.
By the time I left for college I had grown up with five different dogs. I had endured the loss of sweet Mary, the boxer, when we moved to a small apartment and had to send her to a “farm.” I had watched 2-year-old Blackie experience a seizure that heralded his fatal bout with distemper. I shared the struggle with my parents as we made the decision to send my much loved inveterate car chasing “Butch” back to the pound.
However, I survived these losses and wonder whether they in some way prepared me for some of the emotional challenges that would come later in life. This study from Harvard sampled only children from birth to age 8 years. For those of us in primary care a more interesting study might be one that looked for any long-term associations between pet loss as a young child with adolescent and adult mental health. With the surge in pet ownership that has surfaced during the pandemic, there should be an abundance of clinical material to mine. The Harvard researchers’ findings should make us aware of the potential for psychopathology in a child who has suffered the loss of a pet. Each family must decide whether the plusses of pet ownership are worth the risk. However, I side with Tennyson who said it is better to have loved and lost than never to have loved at all.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Glucosuria Is Not Always Due to Diabetes
Familial renal glucosuria is an uncommon, rarely documented condition wherein the absence of other renal or endocrine conditions and with a normal serum glucose level, glucosuria persists due to an isolated defect in the nephron’s proximal tubule. Seemingly, in these patients, the body’s physiologic function mimics that of sodiumglucose cotransporter-2 (SGLT2)-inhibiting medications with the glucose cotransporter being selectively targeted for promoting renal excretion of glucose. This has implications for the patient’s prospective development of hyperglycemic diseases, urinary tract infections (UTIs), and potentially even cardiovascular disease. Though it is a generally asymptomatic condition, it is one that seasoned clinicians should investigate given the future impacts and considerations required for their patients.
Case Presentation
Mr. A was a 28-year-old male with no medical history nor prescription medication use who presented to the nephrology clinic at Eglin Air Force Base, Florida, in June 2019 for a workup of asymptomatic glucosuria. The condition was discovered on a routine urinalysis in October 2015 at the initial presentation at Eglin Air Force Base, when the patient was being evaluated by his primary care physician for acute, benign headache with fever and chills. Urinalysis testing was performed in October 2015 and resulted in a urine glucose of 500 mg/dL (2+). He was directed to the emergency department for further evaluation, reciprocating the results.
On further laboratory testing in October 2015, his blood glucose was normal at 75 mg/dL; hemoglobin A1c was 5.5%. On repeat urinalysis 2 weeks later, his urinary glucose was found to be 500 mg/dL (2+). Each time, the elevated urinary glucose was the only abnormal finding: There was no concurrent hematuria, proteinuria, or ketonuria. The patient reported he had no associated symptoms, including nausea, vomiting, abdominal pain, dysuria, polyuria, and increased thirst. He was not taking any prescription medications, including SGLT2 inhibitors. His presenting headache and fever resolved with supportive care and was considered unrelated to his additional workup.
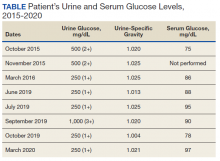
A diagnostic evaluation ensued from 2015 to 2020, including follow-up urinalyses, metabolic panels, complete blood counts, urine protein electrophoresis (UPEP), urine creatinine, urine electrolytes, 25-OH vitamin D level, κ/λ light chain panel, and serum protein electrophoresis (SPEP). The results of all diagnostic workup throughout the entirety of his evaluation were found to be normal. In 2020, his 25-OH vitamin D level was borderline low at 29.4 ng/mL. His κ/λ ratio was normal at 1.65, and his serum albumin protein electrophoresis was 4.74 g/dL, marginally elevated, but his SPEP and UPEP were normal, as were urine protein levels, total gamma globulin, and no monoclonal gamma spike noted on pathology review. Serum uric acid, and urine phosphorous were both normal. His serum creatinine and electrolytes were all within normal limits. Over the 5 years of intermittent monitoring, the maximum amount of glucosuria was 1,000 mg/dL (3+) and the minimum was 250 mg/dL (1+). There was a gap of monitoring from March 2016 until June 2019 due to the patient receiving care from offsite health care providers without shared documentation of specific laboratory values, but notes documenting persistent glucosuria (Table).
Analysis
Building the initial differential diagnosis for this patient began with confirming that he had isolated glucosuria, and not glucosuria secondary to elevated serum glucose. Additionally, conditions related to generalized proximal tubule dysfunction, acute or chronic impaired renal function, and neoplasms, including multiple myeloma (MM), were eliminated because this patient did not have the other specific findings associated with these conditions.
Proximal tubulopathies, including proximal renal tubular acidosis (type 2) and Fanconi syndrome, was initially a leading diagnosis in this patient. Isolated proximal renal tubular acidosis (RTA) (type 2) is uncommon and pathophysiologically involves reduced proximal tubular reabsorption of bicarbonate, resulting in low serum bicarbonate and metabolic acidosis. Patients with isolated proximal RTA (type 2) typically present in infancy with failure to thrive, tachypnea, recurrent vomiting, and feeding difficulties. These symptoms do not meet our patient’s clinical presentation. Fanconi syndrome involves a specific disruption in the proximal tubular apical sodium uptake mechanism affecting the transmembrane sodium gradient and the sodium-potassium- ATPase pump. Fanconi syndrome, therefore, would not only present with glucosuria, but also classically with proteinuria, hypophosphatemia, hypokalemia, and a hyperchloremic metabolic acidosis.
Chronic or acute renal disease may present with glucosuria, but one would expect additional findings including elevated serum creatinine, elevated urinary creatinine, 25-OH vitamin D deficiency, or anemia of chronic disease. Other potential diagnoses included MM and similar neoplasms. MM also would present with glucosuria with proteinuria, an elevated κ/λ light chain ratio, and an elevated SPEP and concern for bone lytic lesions, which were not present. A related disorder, monoclonal gammopathy of renal significance (MGRS), akin to monoclonal gammopathy of unknown significance (MGUS), presents with proteinuria with evidence of renal injury. While this patient had a marginally elevated κ/λ light chain ratio, the remainder of his SPEP and UPEP were normal, and evaluation by a hematologist/ oncologist and pathology review of laboratory findings confirmed no additional evidence for MM, including no monoclonal γ spike. With no evidence of renal injury with a normal serum creatinine and glomerular filtration rate, MGRS was eliminated from the differential as it did not meet the International Myeloma Working Group diagnostic criteria.1 The elevated κ/λ ratio with normal renal function is attributed to polyclonal immunoglobulin elevation, which may occur more commonly with uncomplicated acute viral illnesses.
Diagnosis
The differential homed in on a targeted defect in the proximal tubular SGLT2 gene as the final diagnosis causing isolated glucosuria. Familial renal glucosuria (FRG), a condition caused by a mutation in the SLC5A2 gene that codes for the SGLT2 has been identified in the literature as causing cases with nearly identical presentations to this patient.2,3 This condition is often found in otherwise healthy, asymptomatic patients in whom isolated glucosuria was identified on routine urinalysis testing.
Due to isolated case reports sharing this finding and the asymptomatic nature of the condition, specific data pertaining to its prevalence are not available. Case studies of other affected individuals have not noted adverse effects (AEs), such as UTIs or hypotension specifically.2,3 The patient was referred for genetic testing for this gene mutation; however, he was unable to obtain the test due to lack of insurance coverage. Mr. A has no other family members that have been evaluated for or identified as having this condition. Despite the name, FRG has an unknown inheritance pattern and is attributed to a variety of missense mutations in the SLC5A2 gene.4,5
Discussion
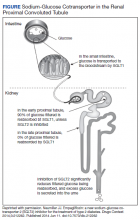
The SGLT2 gene believed to be mutated in this patient has recently become wellknown. The inhibition of the SGLT2 transport protein has become an important tool in the management of type 2 diabetes mellitus (T2DM) independent of the insulin pathway. The SGLT2 in the proximal convoluted tubule of the kidney reabsorbs the majority, 98%, of the renal glucose for reabsorption, and the remaining glucose is reabsorbed by the SGLT2 gene in the more distal portion of the proximal tubule in healthy individuals.4,6 The normal renal threshold for glucose reabsorption in a patient with a normal glomerular filtration rate is equivalent to a serum glucose concentration of 180 mg/dL, even higher in patients with T2DM due to upregulation of the SGLT2 inhibitors. SGLT2 inhibitors, such as canagliflozin, dapagliflozin, and empagliflozin, selectively inhibit this cotransporter, reducing the threshold from 40 to 120 mg/dL, thereby significantly increasing the renal excretion of glucose.4 The patient’s mutation in question and clinical presentation aligned with a naturally occurring mimicry of this drug’s mechanism of action (Figure).
Arguably, one of the more significant benefits to using this new class of oral antihyperglycemics, aside from the noninferior glycemic control compared with that of other first-line agents, is the added metabolic benefit. To date, SGLT2 inhibitors have been found to decrease blood pressure in all studies of the medications and promote moderate weight loss.7 SGLT2 inhibitors have not only demonstrated significant cardiovascular (CV) benefits, linked with the aforementioned metabolic benefits, but also have reduced hospitalizations for heart failure in patients with T2DM and those without.7 The EMPA-REG OUTCOME trial showed a 38% relative risk reduction in CV events in empagliflozin vs placebo.4,8 However, it is unknown whether patients with the SLC5A2 mutation also benefit from these CV benefits akin to the SGLT2 inhibiting medications, and it is and worthy of studying via longterm follow-up with patients similar to this.
This SLC5A2 mutation causing FRG selectively inhibiting SGLT2 function effectively causes this patient’s natural physiology to mimic that of these new oral antihyperglycemic medications. Patients with FRG should be counseled regarding this condition and the implications it has on their overall health. At this time, there is no formal recommendation for short-term or longterm management of patients with FRG; observation and routine preventive care monitoring based on US Preventive Services Task Force screening recommendations apply to this population in line with the general population.
This condition is not known to be associated with hypotension or hypoglycemia, and to some extent, it can be theorized that patients with this condition may have inherent protection of development of hyperglycemia. 4 Akin to patients on SGLT2 inhibitors, these patients may be at an increased risk of UTIs and genital infections, including mycotic infections due to glycemic-related imbalance in the normal flora of the urinary tract.9 Other serious AEs of SGLT2 inhibitors, such as diabetic ketoacidosis, osteoporosis and related fractures, and acute pancreatitis, should be shared with FRG patients, though they are unlikely to be at increased risk for this condition in the setting of normal serum glucose and electrolyte levels. Notably, the osteoporosis risk is small, and specific other risk factors pertinent to individual patient’s medical history, and canagliflozin exclusively. If a patient with FRG develops T2DM after diagnosis, it is imperative that they inform physicians of their condition, because SGLT2-inhibiting drugs will be ineffective in this subset of patients, necessitating increased clinical judgment in selecting an appropriate antihyperglycemic agent in this population.
Conclusions
FRG is an uncommon diagnosis of exclusion that presents with isolated glucosuria in the setting of normal serum glucose. The patient generally presents asymptomatically with a urinalysis completed for other reasons, and the patient may or may not have a family history of similar findings. The condition is of particular interest given that its SGLT2 mutation mimics the effect of SGLT2 inhibitors used for T2DM. More monitoring of patients with this condition will be required for documentation regarding long-term implications, including development of further renal disease, T2DM, or CV disease.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12). doi:10.1016/s1470-2045(14)70442-5
2. Calado J, Sznajer Y, Metzger D, et al. Twenty-one additional cases of familial renal glucosuria: absence of genetic heterogeneity, high prevalence of private mutations and further evidence of volume depletion. Nephrol Dial Transplant. 2008;23(12):3874-3879. doi.org/10.1093/ndt/gfn386
3. Kim KM, Kwon SK, Kim HY. A case of isolated glycosuria mediated by an SLC5A2 gene mutation and characterized by postprandial heavy glycosuria without salt wasting. Electrolyte Blood Press. 2016;14(2):35-37. doi:10.5049/EBP.2016.14.2.35
4. Hsia DS, Grove O, Cefalu WT. An update on sodiumglucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73-79. doi:10.1097/MED.0000000000000311
5. Kleta R. Renal glucosuria due to SGLT2 mutations. Mol Genet Metab. 2004;82(1):56-58. doi:10.1016/j.ymgme.2004.01.018
6. Neumiller JJ. Empagliflozin: a new sodium-glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. Drugs Context. 2014;3:212262. doi:10.7573/dic.212262
7. Raz I, Cernea S, Cahn A. SGLT2 inhibitors for primary prevention of cardiovascular events. J Diabetes. 2020;12(1):5- 7. doi:10.1111/1753-0407.13004
8. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/nejmoa1504720
9. Mcgill JB, Subramanian S. Safety of sodium-glucose cotransporter 2 inhibitors. Am J Cardiol. 2019;124(suppl 1):S45-S52. doi:10.1016/j.amjcard.2019.10.029
Familial renal glucosuria is an uncommon, rarely documented condition wherein the absence of other renal or endocrine conditions and with a normal serum glucose level, glucosuria persists due to an isolated defect in the nephron’s proximal tubule. Seemingly, in these patients, the body’s physiologic function mimics that of sodiumglucose cotransporter-2 (SGLT2)-inhibiting medications with the glucose cotransporter being selectively targeted for promoting renal excretion of glucose. This has implications for the patient’s prospective development of hyperglycemic diseases, urinary tract infections (UTIs), and potentially even cardiovascular disease. Though it is a generally asymptomatic condition, it is one that seasoned clinicians should investigate given the future impacts and considerations required for their patients.
Case Presentation
Mr. A was a 28-year-old male with no medical history nor prescription medication use who presented to the nephrology clinic at Eglin Air Force Base, Florida, in June 2019 for a workup of asymptomatic glucosuria. The condition was discovered on a routine urinalysis in October 2015 at the initial presentation at Eglin Air Force Base, when the patient was being evaluated by his primary care physician for acute, benign headache with fever and chills. Urinalysis testing was performed in October 2015 and resulted in a urine glucose of 500 mg/dL (2+). He was directed to the emergency department for further evaluation, reciprocating the results.
On further laboratory testing in October 2015, his blood glucose was normal at 75 mg/dL; hemoglobin A1c was 5.5%. On repeat urinalysis 2 weeks later, his urinary glucose was found to be 500 mg/dL (2+). Each time, the elevated urinary glucose was the only abnormal finding: There was no concurrent hematuria, proteinuria, or ketonuria. The patient reported he had no associated symptoms, including nausea, vomiting, abdominal pain, dysuria, polyuria, and increased thirst. He was not taking any prescription medications, including SGLT2 inhibitors. His presenting headache and fever resolved with supportive care and was considered unrelated to his additional workup.
A diagnostic evaluation ensued from 2015 to 2020, including follow-up urinalyses, metabolic panels, complete blood counts, urine protein electrophoresis (UPEP), urine creatinine, urine electrolytes, 25-OH vitamin D level, κ/λ light chain panel, and serum protein electrophoresis (SPEP). The results of all diagnostic workup throughout the entirety of his evaluation were found to be normal. In 2020, his 25-OH vitamin D level was borderline low at 29.4 ng/mL. His κ/λ ratio was normal at 1.65, and his serum albumin protein electrophoresis was 4.74 g/dL, marginally elevated, but his SPEP and UPEP were normal, as were urine protein levels, total gamma globulin, and no monoclonal gamma spike noted on pathology review. Serum uric acid, and urine phosphorous were both normal. His serum creatinine and electrolytes were all within normal limits. Over the 5 years of intermittent monitoring, the maximum amount of glucosuria was 1,000 mg/dL (3+) and the minimum was 250 mg/dL (1+). There was a gap of monitoring from March 2016 until June 2019 due to the patient receiving care from offsite health care providers without shared documentation of specific laboratory values, but notes documenting persistent glucosuria (Table).
Analysis
Building the initial differential diagnosis for this patient began with confirming that he had isolated glucosuria, and not glucosuria secondary to elevated serum glucose. Additionally, conditions related to generalized proximal tubule dysfunction, acute or chronic impaired renal function, and neoplasms, including multiple myeloma (MM), were eliminated because this patient did not have the other specific findings associated with these conditions.
Proximal tubulopathies, including proximal renal tubular acidosis (type 2) and Fanconi syndrome, was initially a leading diagnosis in this patient. Isolated proximal renal tubular acidosis (RTA) (type 2) is uncommon and pathophysiologically involves reduced proximal tubular reabsorption of bicarbonate, resulting in low serum bicarbonate and metabolic acidosis. Patients with isolated proximal RTA (type 2) typically present in infancy with failure to thrive, tachypnea, recurrent vomiting, and feeding difficulties. These symptoms do not meet our patient’s clinical presentation. Fanconi syndrome involves a specific disruption in the proximal tubular apical sodium uptake mechanism affecting the transmembrane sodium gradient and the sodium-potassium- ATPase pump. Fanconi syndrome, therefore, would not only present with glucosuria, but also classically with proteinuria, hypophosphatemia, hypokalemia, and a hyperchloremic metabolic acidosis.
Chronic or acute renal disease may present with glucosuria, but one would expect additional findings including elevated serum creatinine, elevated urinary creatinine, 25-OH vitamin D deficiency, or anemia of chronic disease. Other potential diagnoses included MM and similar neoplasms. MM also would present with glucosuria with proteinuria, an elevated κ/λ light chain ratio, and an elevated SPEP and concern for bone lytic lesions, which were not present. A related disorder, monoclonal gammopathy of renal significance (MGRS), akin to monoclonal gammopathy of unknown significance (MGUS), presents with proteinuria with evidence of renal injury. While this patient had a marginally elevated κ/λ light chain ratio, the remainder of his SPEP and UPEP were normal, and evaluation by a hematologist/ oncologist and pathology review of laboratory findings confirmed no additional evidence for MM, including no monoclonal γ spike. With no evidence of renal injury with a normal serum creatinine and glomerular filtration rate, MGRS was eliminated from the differential as it did not meet the International Myeloma Working Group diagnostic criteria.1 The elevated κ/λ ratio with normal renal function is attributed to polyclonal immunoglobulin elevation, which may occur more commonly with uncomplicated acute viral illnesses.
Diagnosis
The differential homed in on a targeted defect in the proximal tubular SGLT2 gene as the final diagnosis causing isolated glucosuria. Familial renal glucosuria (FRG), a condition caused by a mutation in the SLC5A2 gene that codes for the SGLT2 has been identified in the literature as causing cases with nearly identical presentations to this patient.2,3 This condition is often found in otherwise healthy, asymptomatic patients in whom isolated glucosuria was identified on routine urinalysis testing.
Due to isolated case reports sharing this finding and the asymptomatic nature of the condition, specific data pertaining to its prevalence are not available. Case studies of other affected individuals have not noted adverse effects (AEs), such as UTIs or hypotension specifically.2,3 The patient was referred for genetic testing for this gene mutation; however, he was unable to obtain the test due to lack of insurance coverage. Mr. A has no other family members that have been evaluated for or identified as having this condition. Despite the name, FRG has an unknown inheritance pattern and is attributed to a variety of missense mutations in the SLC5A2 gene.4,5
Discussion
The SGLT2 gene believed to be mutated in this patient has recently become wellknown. The inhibition of the SGLT2 transport protein has become an important tool in the management of type 2 diabetes mellitus (T2DM) independent of the insulin pathway. The SGLT2 in the proximal convoluted tubule of the kidney reabsorbs the majority, 98%, of the renal glucose for reabsorption, and the remaining glucose is reabsorbed by the SGLT2 gene in the more distal portion of the proximal tubule in healthy individuals.4,6 The normal renal threshold for glucose reabsorption in a patient with a normal glomerular filtration rate is equivalent to a serum glucose concentration of 180 mg/dL, even higher in patients with T2DM due to upregulation of the SGLT2 inhibitors. SGLT2 inhibitors, such as canagliflozin, dapagliflozin, and empagliflozin, selectively inhibit this cotransporter, reducing the threshold from 40 to 120 mg/dL, thereby significantly increasing the renal excretion of glucose.4 The patient’s mutation in question and clinical presentation aligned with a naturally occurring mimicry of this drug’s mechanism of action (Figure).
Arguably, one of the more significant benefits to using this new class of oral antihyperglycemics, aside from the noninferior glycemic control compared with that of other first-line agents, is the added metabolic benefit. To date, SGLT2 inhibitors have been found to decrease blood pressure in all studies of the medications and promote moderate weight loss.7 SGLT2 inhibitors have not only demonstrated significant cardiovascular (CV) benefits, linked with the aforementioned metabolic benefits, but also have reduced hospitalizations for heart failure in patients with T2DM and those without.7 The EMPA-REG OUTCOME trial showed a 38% relative risk reduction in CV events in empagliflozin vs placebo.4,8 However, it is unknown whether patients with the SLC5A2 mutation also benefit from these CV benefits akin to the SGLT2 inhibiting medications, and it is and worthy of studying via longterm follow-up with patients similar to this.
This SLC5A2 mutation causing FRG selectively inhibiting SGLT2 function effectively causes this patient’s natural physiology to mimic that of these new oral antihyperglycemic medications. Patients with FRG should be counseled regarding this condition and the implications it has on their overall health. At this time, there is no formal recommendation for short-term or longterm management of patients with FRG; observation and routine preventive care monitoring based on US Preventive Services Task Force screening recommendations apply to this population in line with the general population.
This condition is not known to be associated with hypotension or hypoglycemia, and to some extent, it can be theorized that patients with this condition may have inherent protection of development of hyperglycemia. 4 Akin to patients on SGLT2 inhibitors, these patients may be at an increased risk of UTIs and genital infections, including mycotic infections due to glycemic-related imbalance in the normal flora of the urinary tract.9 Other serious AEs of SGLT2 inhibitors, such as diabetic ketoacidosis, osteoporosis and related fractures, and acute pancreatitis, should be shared with FRG patients, though they are unlikely to be at increased risk for this condition in the setting of normal serum glucose and electrolyte levels. Notably, the osteoporosis risk is small, and specific other risk factors pertinent to individual patient’s medical history, and canagliflozin exclusively. If a patient with FRG develops T2DM after diagnosis, it is imperative that they inform physicians of their condition, because SGLT2-inhibiting drugs will be ineffective in this subset of patients, necessitating increased clinical judgment in selecting an appropriate antihyperglycemic agent in this population.
Conclusions
FRG is an uncommon diagnosis of exclusion that presents with isolated glucosuria in the setting of normal serum glucose. The patient generally presents asymptomatically with a urinalysis completed for other reasons, and the patient may or may not have a family history of similar findings. The condition is of particular interest given that its SGLT2 mutation mimics the effect of SGLT2 inhibitors used for T2DM. More monitoring of patients with this condition will be required for documentation regarding long-term implications, including development of further renal disease, T2DM, or CV disease.
Familial renal glucosuria is an uncommon, rarely documented condition wherein the absence of other renal or endocrine conditions and with a normal serum glucose level, glucosuria persists due to an isolated defect in the nephron’s proximal tubule. Seemingly, in these patients, the body’s physiologic function mimics that of sodiumglucose cotransporter-2 (SGLT2)-inhibiting medications with the glucose cotransporter being selectively targeted for promoting renal excretion of glucose. This has implications for the patient’s prospective development of hyperglycemic diseases, urinary tract infections (UTIs), and potentially even cardiovascular disease. Though it is a generally asymptomatic condition, it is one that seasoned clinicians should investigate given the future impacts and considerations required for their patients.
Case Presentation
Mr. A was a 28-year-old male with no medical history nor prescription medication use who presented to the nephrology clinic at Eglin Air Force Base, Florida, in June 2019 for a workup of asymptomatic glucosuria. The condition was discovered on a routine urinalysis in October 2015 at the initial presentation at Eglin Air Force Base, when the patient was being evaluated by his primary care physician for acute, benign headache with fever and chills. Urinalysis testing was performed in October 2015 and resulted in a urine glucose of 500 mg/dL (2+). He was directed to the emergency department for further evaluation, reciprocating the results.
On further laboratory testing in October 2015, his blood glucose was normal at 75 mg/dL; hemoglobin A1c was 5.5%. On repeat urinalysis 2 weeks later, his urinary glucose was found to be 500 mg/dL (2+). Each time, the elevated urinary glucose was the only abnormal finding: There was no concurrent hematuria, proteinuria, or ketonuria. The patient reported he had no associated symptoms, including nausea, vomiting, abdominal pain, dysuria, polyuria, and increased thirst. He was not taking any prescription medications, including SGLT2 inhibitors. His presenting headache and fever resolved with supportive care and was considered unrelated to his additional workup.
A diagnostic evaluation ensued from 2015 to 2020, including follow-up urinalyses, metabolic panels, complete blood counts, urine protein electrophoresis (UPEP), urine creatinine, urine electrolytes, 25-OH vitamin D level, κ/λ light chain panel, and serum protein electrophoresis (SPEP). The results of all diagnostic workup throughout the entirety of his evaluation were found to be normal. In 2020, his 25-OH vitamin D level was borderline low at 29.4 ng/mL. His κ/λ ratio was normal at 1.65, and his serum albumin protein electrophoresis was 4.74 g/dL, marginally elevated, but his SPEP and UPEP were normal, as were urine protein levels, total gamma globulin, and no monoclonal gamma spike noted on pathology review. Serum uric acid, and urine phosphorous were both normal. His serum creatinine and electrolytes were all within normal limits. Over the 5 years of intermittent monitoring, the maximum amount of glucosuria was 1,000 mg/dL (3+) and the minimum was 250 mg/dL (1+). There was a gap of monitoring from March 2016 until June 2019 due to the patient receiving care from offsite health care providers without shared documentation of specific laboratory values, but notes documenting persistent glucosuria (Table).
Analysis
Building the initial differential diagnosis for this patient began with confirming that he had isolated glucosuria, and not glucosuria secondary to elevated serum glucose. Additionally, conditions related to generalized proximal tubule dysfunction, acute or chronic impaired renal function, and neoplasms, including multiple myeloma (MM), were eliminated because this patient did not have the other specific findings associated with these conditions.
Proximal tubulopathies, including proximal renal tubular acidosis (type 2) and Fanconi syndrome, was initially a leading diagnosis in this patient. Isolated proximal renal tubular acidosis (RTA) (type 2) is uncommon and pathophysiologically involves reduced proximal tubular reabsorption of bicarbonate, resulting in low serum bicarbonate and metabolic acidosis. Patients with isolated proximal RTA (type 2) typically present in infancy with failure to thrive, tachypnea, recurrent vomiting, and feeding difficulties. These symptoms do not meet our patient’s clinical presentation. Fanconi syndrome involves a specific disruption in the proximal tubular apical sodium uptake mechanism affecting the transmembrane sodium gradient and the sodium-potassium- ATPase pump. Fanconi syndrome, therefore, would not only present with glucosuria, but also classically with proteinuria, hypophosphatemia, hypokalemia, and a hyperchloremic metabolic acidosis.
Chronic or acute renal disease may present with glucosuria, but one would expect additional findings including elevated serum creatinine, elevated urinary creatinine, 25-OH vitamin D deficiency, or anemia of chronic disease. Other potential diagnoses included MM and similar neoplasms. MM also would present with glucosuria with proteinuria, an elevated κ/λ light chain ratio, and an elevated SPEP and concern for bone lytic lesions, which were not present. A related disorder, monoclonal gammopathy of renal significance (MGRS), akin to monoclonal gammopathy of unknown significance (MGUS), presents with proteinuria with evidence of renal injury. While this patient had a marginally elevated κ/λ light chain ratio, the remainder of his SPEP and UPEP were normal, and evaluation by a hematologist/ oncologist and pathology review of laboratory findings confirmed no additional evidence for MM, including no monoclonal γ spike. With no evidence of renal injury with a normal serum creatinine and glomerular filtration rate, MGRS was eliminated from the differential as it did not meet the International Myeloma Working Group diagnostic criteria.1 The elevated κ/λ ratio with normal renal function is attributed to polyclonal immunoglobulin elevation, which may occur more commonly with uncomplicated acute viral illnesses.
Diagnosis
The differential homed in on a targeted defect in the proximal tubular SGLT2 gene as the final diagnosis causing isolated glucosuria. Familial renal glucosuria (FRG), a condition caused by a mutation in the SLC5A2 gene that codes for the SGLT2 has been identified in the literature as causing cases with nearly identical presentations to this patient.2,3 This condition is often found in otherwise healthy, asymptomatic patients in whom isolated glucosuria was identified on routine urinalysis testing.
Due to isolated case reports sharing this finding and the asymptomatic nature of the condition, specific data pertaining to its prevalence are not available. Case studies of other affected individuals have not noted adverse effects (AEs), such as UTIs or hypotension specifically.2,3 The patient was referred for genetic testing for this gene mutation; however, he was unable to obtain the test due to lack of insurance coverage. Mr. A has no other family members that have been evaluated for or identified as having this condition. Despite the name, FRG has an unknown inheritance pattern and is attributed to a variety of missense mutations in the SLC5A2 gene.4,5
Discussion
The SGLT2 gene believed to be mutated in this patient has recently become wellknown. The inhibition of the SGLT2 transport protein has become an important tool in the management of type 2 diabetes mellitus (T2DM) independent of the insulin pathway. The SGLT2 in the proximal convoluted tubule of the kidney reabsorbs the majority, 98%, of the renal glucose for reabsorption, and the remaining glucose is reabsorbed by the SGLT2 gene in the more distal portion of the proximal tubule in healthy individuals.4,6 The normal renal threshold for glucose reabsorption in a patient with a normal glomerular filtration rate is equivalent to a serum glucose concentration of 180 mg/dL, even higher in patients with T2DM due to upregulation of the SGLT2 inhibitors. SGLT2 inhibitors, such as canagliflozin, dapagliflozin, and empagliflozin, selectively inhibit this cotransporter, reducing the threshold from 40 to 120 mg/dL, thereby significantly increasing the renal excretion of glucose.4 The patient’s mutation in question and clinical presentation aligned with a naturally occurring mimicry of this drug’s mechanism of action (Figure).
Arguably, one of the more significant benefits to using this new class of oral antihyperglycemics, aside from the noninferior glycemic control compared with that of other first-line agents, is the added metabolic benefit. To date, SGLT2 inhibitors have been found to decrease blood pressure in all studies of the medications and promote moderate weight loss.7 SGLT2 inhibitors have not only demonstrated significant cardiovascular (CV) benefits, linked with the aforementioned metabolic benefits, but also have reduced hospitalizations for heart failure in patients with T2DM and those without.7 The EMPA-REG OUTCOME trial showed a 38% relative risk reduction in CV events in empagliflozin vs placebo.4,8 However, it is unknown whether patients with the SLC5A2 mutation also benefit from these CV benefits akin to the SGLT2 inhibiting medications, and it is and worthy of studying via longterm follow-up with patients similar to this.
This SLC5A2 mutation causing FRG selectively inhibiting SGLT2 function effectively causes this patient’s natural physiology to mimic that of these new oral antihyperglycemic medications. Patients with FRG should be counseled regarding this condition and the implications it has on their overall health. At this time, there is no formal recommendation for short-term or longterm management of patients with FRG; observation and routine preventive care monitoring based on US Preventive Services Task Force screening recommendations apply to this population in line with the general population.
This condition is not known to be associated with hypotension or hypoglycemia, and to some extent, it can be theorized that patients with this condition may have inherent protection of development of hyperglycemia. 4 Akin to patients on SGLT2 inhibitors, these patients may be at an increased risk of UTIs and genital infections, including mycotic infections due to glycemic-related imbalance in the normal flora of the urinary tract.9 Other serious AEs of SGLT2 inhibitors, such as diabetic ketoacidosis, osteoporosis and related fractures, and acute pancreatitis, should be shared with FRG patients, though they are unlikely to be at increased risk for this condition in the setting of normal serum glucose and electrolyte levels. Notably, the osteoporosis risk is small, and specific other risk factors pertinent to individual patient’s medical history, and canagliflozin exclusively. If a patient with FRG develops T2DM after diagnosis, it is imperative that they inform physicians of their condition, because SGLT2-inhibiting drugs will be ineffective in this subset of patients, necessitating increased clinical judgment in selecting an appropriate antihyperglycemic agent in this population.
Conclusions
FRG is an uncommon diagnosis of exclusion that presents with isolated glucosuria in the setting of normal serum glucose. The patient generally presents asymptomatically with a urinalysis completed for other reasons, and the patient may or may not have a family history of similar findings. The condition is of particular interest given that its SGLT2 mutation mimics the effect of SGLT2 inhibitors used for T2DM. More monitoring of patients with this condition will be required for documentation regarding long-term implications, including development of further renal disease, T2DM, or CV disease.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12). doi:10.1016/s1470-2045(14)70442-5
2. Calado J, Sznajer Y, Metzger D, et al. Twenty-one additional cases of familial renal glucosuria: absence of genetic heterogeneity, high prevalence of private mutations and further evidence of volume depletion. Nephrol Dial Transplant. 2008;23(12):3874-3879. doi.org/10.1093/ndt/gfn386
3. Kim KM, Kwon SK, Kim HY. A case of isolated glycosuria mediated by an SLC5A2 gene mutation and characterized by postprandial heavy glycosuria without salt wasting. Electrolyte Blood Press. 2016;14(2):35-37. doi:10.5049/EBP.2016.14.2.35
4. Hsia DS, Grove O, Cefalu WT. An update on sodiumglucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73-79. doi:10.1097/MED.0000000000000311
5. Kleta R. Renal glucosuria due to SGLT2 mutations. Mol Genet Metab. 2004;82(1):56-58. doi:10.1016/j.ymgme.2004.01.018
6. Neumiller JJ. Empagliflozin: a new sodium-glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. Drugs Context. 2014;3:212262. doi:10.7573/dic.212262
7. Raz I, Cernea S, Cahn A. SGLT2 inhibitors for primary prevention of cardiovascular events. J Diabetes. 2020;12(1):5- 7. doi:10.1111/1753-0407.13004
8. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/nejmoa1504720
9. Mcgill JB, Subramanian S. Safety of sodium-glucose cotransporter 2 inhibitors. Am J Cardiol. 2019;124(suppl 1):S45-S52. doi:10.1016/j.amjcard.2019.10.029
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12). doi:10.1016/s1470-2045(14)70442-5
2. Calado J, Sznajer Y, Metzger D, et al. Twenty-one additional cases of familial renal glucosuria: absence of genetic heterogeneity, high prevalence of private mutations and further evidence of volume depletion. Nephrol Dial Transplant. 2008;23(12):3874-3879. doi.org/10.1093/ndt/gfn386
3. Kim KM, Kwon SK, Kim HY. A case of isolated glycosuria mediated by an SLC5A2 gene mutation and characterized by postprandial heavy glycosuria without salt wasting. Electrolyte Blood Press. 2016;14(2):35-37. doi:10.5049/EBP.2016.14.2.35
4. Hsia DS, Grove O, Cefalu WT. An update on sodiumglucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24(1):73-79. doi:10.1097/MED.0000000000000311
5. Kleta R. Renal glucosuria due to SGLT2 mutations. Mol Genet Metab. 2004;82(1):56-58. doi:10.1016/j.ymgme.2004.01.018
6. Neumiller JJ. Empagliflozin: a new sodium-glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. Drugs Context. 2014;3:212262. doi:10.7573/dic.212262
7. Raz I, Cernea S, Cahn A. SGLT2 inhibitors for primary prevention of cardiovascular events. J Diabetes. 2020;12(1):5- 7. doi:10.1111/1753-0407.13004
8. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/nejmoa1504720
9. Mcgill JB, Subramanian S. Safety of sodium-glucose cotransporter 2 inhibitors. Am J Cardiol. 2019;124(suppl 1):S45-S52. doi:10.1016/j.amjcard.2019.10.029
Reminders of our mortality can come when physicians least expect it
This time of year I spend weekend afternoons in my hot tub, catching up on medical journals, CME, paperbacks, and generally anything worth reading that shows up in my mailbox.
One of those items was the alumni news from my medical school. As usual, I leafed through it, reading articles of interest and glancing at updates on any classmates that were featured.
Then I stopped.
There, in the back of the magazine, was an obituary on the first of my classmates to pass (that I’m aware of).
I reread it a few times in disbelief. Maybe it was on her taking a new job or being promoted, and was in the wrong section. Nope.
I put the magazine down. She was 1 year younger than me and had gone into internal medicine. Not someone I’d kept in touch with, but certainly was friendly with during those 4 years and frequently chatted with in hallways or between classes. I remember meeting her during the first week of school, when I got her name mixed up with another girl’s in our class. I saw her at parties, meetings, and I think even played doubles tennis with her once, though who we played against I have no idea anymore.
She was at our 20th reunion, and we’d talked for a few minutes. We caught up on our lives since graduation and, as people do at these things, moved on to chat with others.
No details were given as to her death, and it really doesn’t matter.
. For most of each day it’s a fact in the back of our minds, behind the daily activities of working, shopping, doing laundry, commuting, and cooking dinner. After all, it’s really what we do while here that matters, no matter how mundane it may seem.
But sometimes something will push that realization to the front, and make us remember how important every minute really is.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
This time of year I spend weekend afternoons in my hot tub, catching up on medical journals, CME, paperbacks, and generally anything worth reading that shows up in my mailbox.
One of those items was the alumni news from my medical school. As usual, I leafed through it, reading articles of interest and glancing at updates on any classmates that were featured.
Then I stopped.
There, in the back of the magazine, was an obituary on the first of my classmates to pass (that I’m aware of).
I reread it a few times in disbelief. Maybe it was on her taking a new job or being promoted, and was in the wrong section. Nope.
I put the magazine down. She was 1 year younger than me and had gone into internal medicine. Not someone I’d kept in touch with, but certainly was friendly with during those 4 years and frequently chatted with in hallways or between classes. I remember meeting her during the first week of school, when I got her name mixed up with another girl’s in our class. I saw her at parties, meetings, and I think even played doubles tennis with her once, though who we played against I have no idea anymore.
She was at our 20th reunion, and we’d talked for a few minutes. We caught up on our lives since graduation and, as people do at these things, moved on to chat with others.
No details were given as to her death, and it really doesn’t matter.
. For most of each day it’s a fact in the back of our minds, behind the daily activities of working, shopping, doing laundry, commuting, and cooking dinner. After all, it’s really what we do while here that matters, no matter how mundane it may seem.
But sometimes something will push that realization to the front, and make us remember how important every minute really is.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
This time of year I spend weekend afternoons in my hot tub, catching up on medical journals, CME, paperbacks, and generally anything worth reading that shows up in my mailbox.
One of those items was the alumni news from my medical school. As usual, I leafed through it, reading articles of interest and glancing at updates on any classmates that were featured.
Then I stopped.
There, in the back of the magazine, was an obituary on the first of my classmates to pass (that I’m aware of).
I reread it a few times in disbelief. Maybe it was on her taking a new job or being promoted, and was in the wrong section. Nope.
I put the magazine down. She was 1 year younger than me and had gone into internal medicine. Not someone I’d kept in touch with, but certainly was friendly with during those 4 years and frequently chatted with in hallways or between classes. I remember meeting her during the first week of school, when I got her name mixed up with another girl’s in our class. I saw her at parties, meetings, and I think even played doubles tennis with her once, though who we played against I have no idea anymore.
She was at our 20th reunion, and we’d talked for a few minutes. We caught up on our lives since graduation and, as people do at these things, moved on to chat with others.
No details were given as to her death, and it really doesn’t matter.
. For most of each day it’s a fact in the back of our minds, behind the daily activities of working, shopping, doing laundry, commuting, and cooking dinner. After all, it’s really what we do while here that matters, no matter how mundane it may seem.
But sometimes something will push that realization to the front, and make us remember how important every minute really is.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Neoadjuvant immunotherapy shows promise in stage III melanoma
The next dramatic , John M. Kirkwood, MD, predicted at a virtual forum on cutaneous malignancies jointly presented by the Postgraduate Institute for Medicine and Global Academy for Medical Education.
These agents have already demonstrated profound efficacy, first in stage IV metastatic disease and more recently as adjuvant therapy for resected stage III melanoma. Now, there is a great interest in learning whether by prescribing them preoperatively, patients might reduce their risk of advancing to metastatic disease. And neoadjuvant therapy offers an extremely attractive feature: It yields results in an accelerated fashion.
“The major problem with postoperative adjuvant trials in melanoma since 1984 is the long time to maturity. Many of us don’t want to wait the full 9 or 10 years for a full-bore, phase 3 adjuvant trial in stage III melanoma to mature,” explained Dr. Kirkwood, professor of medicine, dermatology, and translational science and coleader of the melanoma and skin cancer program at the University of Pittsburgh. “The opportunity to treat a patient who presents with a bulky lymph node, has a biopsy, and then can be treated for 3 or 6 weeks or sometimes even longer periods with a therapy that’s promising allows us to ask what’s going on in the tumor tissue, what’s going on in the clinical response at 3 or 6 weeks, and if there’s pathological complete or near-complete response under the microscope.”
Because pathological complete response is a strong predictor of relapse-free survival, this neoadjuvant-forward therapeutic strategy has the potential to provide patients and their physicians with an early forecast of likely clinical outcome only 4-6 weeks into treatment. Also, there is both preclinical and clinical evidence that neoadjuvant therapy may offer a survival advantage over adjuvant therapy, perhaps as a result of early treatment of micrometastatic disease. Another benefit of neoadjuvant therapy for melanoma is the resultant tumor shrinkage, which can permit less extensive surgery.
Dr. Kirkwood highlighted a phase 2 clinical trial conducted at the University of Pittsburgh to illustrate the potential of neoadjuvant therapy in melanoma. The ongoing single-arm study includes 32 patients with stage IIIB or IIIC resectable melanoma along with accessible tumor for biopsy and intratumoral injections of CMP-001, a toll-like receptor 9 agonist. According to the Eighth Edition of the American Joint Committee on Cancer staging manual, stage IIIB melanoma has a 10-year mortality of 23%, and stage IIIC disease has 40%.
CMP-001 triggers type 1 interferon production through activation of plasmacytoid dendritic cells. The resultant inflammatory response draws T cells into the tumor to enhance the response to immunotherapy, which in this study was nivolumab (Opdivo), a human programmed death ligand 1 (PD-L1)–blocking antibody. The neoadjuvant regimen consisted of seven once-weekly intratumoral injections of CMP-001, plus three 240-mg doses of nivolumab given at 2-week intervals. This was followed by resection, then 1 year of adjuvant therapy with nivolumab at 480 mg every 4 weeks and intratumoral CMP-001 every 4 weeks.
In an interim analysis, a major pathologic response occurred in an impressive 15 of 21 patients (71%) after 6 weeks of neoadjuvant therapy. Thirteen of the 15 had a pathologic complete response. Encouragingly, no one with a pathologic complete or near-complete response has relapsed to date.
“A pathologic complete response or near-complete response with neoadjuvant therapy appears to be a biomarker of durable disease control and is associated with excellent outcomes,” Dr. Kirkwood observed, adding that the Pittsburgh experience has been mirrored in reports from the Netherlands, Australia, and University of Texas M.D. Anderson Cancer Center, Houston, involving other neoadjuvant agents.
Other potential early biomarkers of favorable outcome with neoadjuvant therapy include CD8+ T cells in the tumor at baseline, tumor mutational burden, T-cell clonality, and a T-cell–inflamed gene-expression profile.
There were no dose-limiting toxicities or delays in surgery related to the neoadjuvant treatment.
Of note, imaging often inaccurately showed only a partial response in patients who actually had a pathologic complete response, meaning totally devoid of tumor, Dr. Kirkwood said.
Corroboration of these findings is planned in the national multicenter ECOG-ACRIN neoadjuvant trial EA6194.
“Consider referring to this trial any patients who present with bulky nodal disease for whom a treatment assessment at 4-6 weeks is desired in order to predict what the outcome may be,” he suggested.
Dr. Kirkwood reported receiving research grants from Amgen, BMS, Castle Biosciences, Checkmate, Immunocore, Iovance, and Novartis and serving as a consultant to a handful of companies.
Global Academy for Medical Education and this news organization are owned by the same company.
The next dramatic , John M. Kirkwood, MD, predicted at a virtual forum on cutaneous malignancies jointly presented by the Postgraduate Institute for Medicine and Global Academy for Medical Education.
These agents have already demonstrated profound efficacy, first in stage IV metastatic disease and more recently as adjuvant therapy for resected stage III melanoma. Now, there is a great interest in learning whether by prescribing them preoperatively, patients might reduce their risk of advancing to metastatic disease. And neoadjuvant therapy offers an extremely attractive feature: It yields results in an accelerated fashion.
“The major problem with postoperative adjuvant trials in melanoma since 1984 is the long time to maturity. Many of us don’t want to wait the full 9 or 10 years for a full-bore, phase 3 adjuvant trial in stage III melanoma to mature,” explained Dr. Kirkwood, professor of medicine, dermatology, and translational science and coleader of the melanoma and skin cancer program at the University of Pittsburgh. “The opportunity to treat a patient who presents with a bulky lymph node, has a biopsy, and then can be treated for 3 or 6 weeks or sometimes even longer periods with a therapy that’s promising allows us to ask what’s going on in the tumor tissue, what’s going on in the clinical response at 3 or 6 weeks, and if there’s pathological complete or near-complete response under the microscope.”
Because pathological complete response is a strong predictor of relapse-free survival, this neoadjuvant-forward therapeutic strategy has the potential to provide patients and their physicians with an early forecast of likely clinical outcome only 4-6 weeks into treatment. Also, there is both preclinical and clinical evidence that neoadjuvant therapy may offer a survival advantage over adjuvant therapy, perhaps as a result of early treatment of micrometastatic disease. Another benefit of neoadjuvant therapy for melanoma is the resultant tumor shrinkage, which can permit less extensive surgery.
Dr. Kirkwood highlighted a phase 2 clinical trial conducted at the University of Pittsburgh to illustrate the potential of neoadjuvant therapy in melanoma. The ongoing single-arm study includes 32 patients with stage IIIB or IIIC resectable melanoma along with accessible tumor for biopsy and intratumoral injections of CMP-001, a toll-like receptor 9 agonist. According to the Eighth Edition of the American Joint Committee on Cancer staging manual, stage IIIB melanoma has a 10-year mortality of 23%, and stage IIIC disease has 40%.
CMP-001 triggers type 1 interferon production through activation of plasmacytoid dendritic cells. The resultant inflammatory response draws T cells into the tumor to enhance the response to immunotherapy, which in this study was nivolumab (Opdivo), a human programmed death ligand 1 (PD-L1)–blocking antibody. The neoadjuvant regimen consisted of seven once-weekly intratumoral injections of CMP-001, plus three 240-mg doses of nivolumab given at 2-week intervals. This was followed by resection, then 1 year of adjuvant therapy with nivolumab at 480 mg every 4 weeks and intratumoral CMP-001 every 4 weeks.
In an interim analysis, a major pathologic response occurred in an impressive 15 of 21 patients (71%) after 6 weeks of neoadjuvant therapy. Thirteen of the 15 had a pathologic complete response. Encouragingly, no one with a pathologic complete or near-complete response has relapsed to date.
“A pathologic complete response or near-complete response with neoadjuvant therapy appears to be a biomarker of durable disease control and is associated with excellent outcomes,” Dr. Kirkwood observed, adding that the Pittsburgh experience has been mirrored in reports from the Netherlands, Australia, and University of Texas M.D. Anderson Cancer Center, Houston, involving other neoadjuvant agents.
Other potential early biomarkers of favorable outcome with neoadjuvant therapy include CD8+ T cells in the tumor at baseline, tumor mutational burden, T-cell clonality, and a T-cell–inflamed gene-expression profile.
There were no dose-limiting toxicities or delays in surgery related to the neoadjuvant treatment.
Of note, imaging often inaccurately showed only a partial response in patients who actually had a pathologic complete response, meaning totally devoid of tumor, Dr. Kirkwood said.
Corroboration of these findings is planned in the national multicenter ECOG-ACRIN neoadjuvant trial EA6194.
“Consider referring to this trial any patients who present with bulky nodal disease for whom a treatment assessment at 4-6 weeks is desired in order to predict what the outcome may be,” he suggested.
Dr. Kirkwood reported receiving research grants from Amgen, BMS, Castle Biosciences, Checkmate, Immunocore, Iovance, and Novartis and serving as a consultant to a handful of companies.
Global Academy for Medical Education and this news organization are owned by the same company.
The next dramatic , John M. Kirkwood, MD, predicted at a virtual forum on cutaneous malignancies jointly presented by the Postgraduate Institute for Medicine and Global Academy for Medical Education.
These agents have already demonstrated profound efficacy, first in stage IV metastatic disease and more recently as adjuvant therapy for resected stage III melanoma. Now, there is a great interest in learning whether by prescribing them preoperatively, patients might reduce their risk of advancing to metastatic disease. And neoadjuvant therapy offers an extremely attractive feature: It yields results in an accelerated fashion.
“The major problem with postoperative adjuvant trials in melanoma since 1984 is the long time to maturity. Many of us don’t want to wait the full 9 or 10 years for a full-bore, phase 3 adjuvant trial in stage III melanoma to mature,” explained Dr. Kirkwood, professor of medicine, dermatology, and translational science and coleader of the melanoma and skin cancer program at the University of Pittsburgh. “The opportunity to treat a patient who presents with a bulky lymph node, has a biopsy, and then can be treated for 3 or 6 weeks or sometimes even longer periods with a therapy that’s promising allows us to ask what’s going on in the tumor tissue, what’s going on in the clinical response at 3 or 6 weeks, and if there’s pathological complete or near-complete response under the microscope.”
Because pathological complete response is a strong predictor of relapse-free survival, this neoadjuvant-forward therapeutic strategy has the potential to provide patients and their physicians with an early forecast of likely clinical outcome only 4-6 weeks into treatment. Also, there is both preclinical and clinical evidence that neoadjuvant therapy may offer a survival advantage over adjuvant therapy, perhaps as a result of early treatment of micrometastatic disease. Another benefit of neoadjuvant therapy for melanoma is the resultant tumor shrinkage, which can permit less extensive surgery.
Dr. Kirkwood highlighted a phase 2 clinical trial conducted at the University of Pittsburgh to illustrate the potential of neoadjuvant therapy in melanoma. The ongoing single-arm study includes 32 patients with stage IIIB or IIIC resectable melanoma along with accessible tumor for biopsy and intratumoral injections of CMP-001, a toll-like receptor 9 agonist. According to the Eighth Edition of the American Joint Committee on Cancer staging manual, stage IIIB melanoma has a 10-year mortality of 23%, and stage IIIC disease has 40%.
CMP-001 triggers type 1 interferon production through activation of plasmacytoid dendritic cells. The resultant inflammatory response draws T cells into the tumor to enhance the response to immunotherapy, which in this study was nivolumab (Opdivo), a human programmed death ligand 1 (PD-L1)–blocking antibody. The neoadjuvant regimen consisted of seven once-weekly intratumoral injections of CMP-001, plus three 240-mg doses of nivolumab given at 2-week intervals. This was followed by resection, then 1 year of adjuvant therapy with nivolumab at 480 mg every 4 weeks and intratumoral CMP-001 every 4 weeks.
In an interim analysis, a major pathologic response occurred in an impressive 15 of 21 patients (71%) after 6 weeks of neoadjuvant therapy. Thirteen of the 15 had a pathologic complete response. Encouragingly, no one with a pathologic complete or near-complete response has relapsed to date.
“A pathologic complete response or near-complete response with neoadjuvant therapy appears to be a biomarker of durable disease control and is associated with excellent outcomes,” Dr. Kirkwood observed, adding that the Pittsburgh experience has been mirrored in reports from the Netherlands, Australia, and University of Texas M.D. Anderson Cancer Center, Houston, involving other neoadjuvant agents.
Other potential early biomarkers of favorable outcome with neoadjuvant therapy include CD8+ T cells in the tumor at baseline, tumor mutational burden, T-cell clonality, and a T-cell–inflamed gene-expression profile.
There were no dose-limiting toxicities or delays in surgery related to the neoadjuvant treatment.
Of note, imaging often inaccurately showed only a partial response in patients who actually had a pathologic complete response, meaning totally devoid of tumor, Dr. Kirkwood said.
Corroboration of these findings is planned in the national multicenter ECOG-ACRIN neoadjuvant trial EA6194.
“Consider referring to this trial any patients who present with bulky nodal disease for whom a treatment assessment at 4-6 weeks is desired in order to predict what the outcome may be,” he suggested.
Dr. Kirkwood reported receiving research grants from Amgen, BMS, Castle Biosciences, Checkmate, Immunocore, Iovance, and Novartis and serving as a consultant to a handful of companies.
Global Academy for Medical Education and this news organization are owned by the same company.
FROM THE CUTANEOUS MALIGNANCIES FORUM
Study: COVID cases have been ‘severely undercounted’
Large numbers of COVID-19 cases have been undetected and unreported, which has resulted in severe undercounting of the total number of people who have been infected during the pandemic, according to a new study published Monday in the journal PLOS ONE.
In the United States, the number of COVID-19 cases is likely three times that of reported cases. According to the study, more than 71 million Americans have contracted the virus during the pandemic, and 7 million were infected or potentially contagious last week.
Public health officials rely on case counts to guide decisions, so the undercounting should be considered while trying to end the pandemic.
“The estimates of actual infections reveal for the first time the true severity of COVID-19 across the U.S. and in countries worldwide,” Jungsik Noh, PhD, a bioinformatics professor at the University of Texas Southwestern Medical Center, said in a statement.
Dr. Noh and colleague Gaudenz Danuser created a computational model that uses machine-learning strategies to estimate the actual number of daily cases in the United States and the 50 most-infected countries.
The model pulls data from the Johns Hopkins University database and the COVID Tracking Project, as well as large-scale surveys conducted by the CDC and several states. The algorithm uses the number of reported deaths, which is thought to be more accurate than the number of lab-confirmed cases, as the basis for calculations.
In 25 of the 50 countries, the “actual” cumulative cases were estimated to be 5-20 times greater than the confirmed cases. In the United States, Belgium, and Brazil, about 10% of the population has contracted the coronavirus, according to the model. At the beginning of February, about 11% of the population in Pennsylvania had current infections, which was the highest rate of any state. About 0.15% of residents in Minnesota had infections, and about 2.5% of residents in New York and Texas had infections.
“Knowing the true severity in different regions will help us effectively fight against the virus spreading,” Dr. Noh said. “The currently infected population is the cause of future infections and deaths. Its actual size in a region is a crucial variable required when determining the severity of COVID-19 and building strategies against regional outbreaks.”
A version of this article first appeared on WebMD.com.
Large numbers of COVID-19 cases have been undetected and unreported, which has resulted in severe undercounting of the total number of people who have been infected during the pandemic, according to a new study published Monday in the journal PLOS ONE.
In the United States, the number of COVID-19 cases is likely three times that of reported cases. According to the study, more than 71 million Americans have contracted the virus during the pandemic, and 7 million were infected or potentially contagious last week.
Public health officials rely on case counts to guide decisions, so the undercounting should be considered while trying to end the pandemic.
“The estimates of actual infections reveal for the first time the true severity of COVID-19 across the U.S. and in countries worldwide,” Jungsik Noh, PhD, a bioinformatics professor at the University of Texas Southwestern Medical Center, said in a statement.
Dr. Noh and colleague Gaudenz Danuser created a computational model that uses machine-learning strategies to estimate the actual number of daily cases in the United States and the 50 most-infected countries.
The model pulls data from the Johns Hopkins University database and the COVID Tracking Project, as well as large-scale surveys conducted by the CDC and several states. The algorithm uses the number of reported deaths, which is thought to be more accurate than the number of lab-confirmed cases, as the basis for calculations.
In 25 of the 50 countries, the “actual” cumulative cases were estimated to be 5-20 times greater than the confirmed cases. In the United States, Belgium, and Brazil, about 10% of the population has contracted the coronavirus, according to the model. At the beginning of February, about 11% of the population in Pennsylvania had current infections, which was the highest rate of any state. About 0.15% of residents in Minnesota had infections, and about 2.5% of residents in New York and Texas had infections.
“Knowing the true severity in different regions will help us effectively fight against the virus spreading,” Dr. Noh said. “The currently infected population is the cause of future infections and deaths. Its actual size in a region is a crucial variable required when determining the severity of COVID-19 and building strategies against regional outbreaks.”
A version of this article first appeared on WebMD.com.
Large numbers of COVID-19 cases have been undetected and unreported, which has resulted in severe undercounting of the total number of people who have been infected during the pandemic, according to a new study published Monday in the journal PLOS ONE.
In the United States, the number of COVID-19 cases is likely three times that of reported cases. According to the study, more than 71 million Americans have contracted the virus during the pandemic, and 7 million were infected or potentially contagious last week.
Public health officials rely on case counts to guide decisions, so the undercounting should be considered while trying to end the pandemic.
“The estimates of actual infections reveal for the first time the true severity of COVID-19 across the U.S. and in countries worldwide,” Jungsik Noh, PhD, a bioinformatics professor at the University of Texas Southwestern Medical Center, said in a statement.
Dr. Noh and colleague Gaudenz Danuser created a computational model that uses machine-learning strategies to estimate the actual number of daily cases in the United States and the 50 most-infected countries.
The model pulls data from the Johns Hopkins University database and the COVID Tracking Project, as well as large-scale surveys conducted by the CDC and several states. The algorithm uses the number of reported deaths, which is thought to be more accurate than the number of lab-confirmed cases, as the basis for calculations.
In 25 of the 50 countries, the “actual” cumulative cases were estimated to be 5-20 times greater than the confirmed cases. In the United States, Belgium, and Brazil, about 10% of the population has contracted the coronavirus, according to the model. At the beginning of February, about 11% of the population in Pennsylvania had current infections, which was the highest rate of any state. About 0.15% of residents in Minnesota had infections, and about 2.5% of residents in New York and Texas had infections.
“Knowing the true severity in different regions will help us effectively fight against the virus spreading,” Dr. Noh said. “The currently infected population is the cause of future infections and deaths. Its actual size in a region is a crucial variable required when determining the severity of COVID-19 and building strategies against regional outbreaks.”
A version of this article first appeared on WebMD.com.
COVID-19: Peginterferon lambda may prevent clinical deterioration, shorten viral shedding
and shorten the duration of viral shedding, according to results of a double-blind, placebo-controlled trial (NCT04354259).
Reductions in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA were greater with peginterferon lambda than with placebo from day 3 onward in the phase 2 study led by Jordan J. Feld, MD, of the Toronto Centre for Liver Disease. The findings were reported in The Lancet Respiratory Medicine.
Fewer side effects
To date in randomized clinical trials, efficacy in treatment of COVID-19 has been shown only for remdesivir and dexamethasone in hospitalized patients, and in an interim analysis of accelerated viral clearance for a monoclonal antibody infusion in outpatients.
Activity against respiratory pathogens has been demonstrated for interferon lambda-1, a type III interferon shown to be involved in innate antiviral responses. Interferons, Dr. Feld and coauthors stated, drive induction of genes with antiviral, antiproliferative and immunoregulatory properties, and early treatment with interferons might halt clinical progression and shorten the duration of viral shedding with reduced onward transmission. In addition, interferon lambdas (type III) use a distinct receptor complex with high expression levels limited to epithelial cells in the lung, liver, and intestine, leading to fewer side effects than other interferons, including avoiding risk of promoting cytokine storm syndrome.
The researchers investigated peginterferon lambda safety and efficacy in treatment of patients with laboratory-confirmed, mild to moderate COVID-19. Sixty patients (median age 46 years, about 60% female, about 50% White) were recruited from outpatient testing centers at six institutions in Toronto, and referred to a single ambulatory site. Patients were randomly assigned 1:1 to a single subcutaneous injection of peginterferon lambda 180 mcg or placebo within 7 days of symptom onset or, if asymptomatic, of their first positive swab. Mean time from symptom onset to injection was about 4.5 days, and about 18.5% were asymptomatic. The primary outcome was the proportion of patients negative for SARS-CoV-2 RNA on day 7 after the injection.
Greater benefit with higher baseline load
A higher baseline SARS-CoV-2 RNA concentration found in the peginterferon lambda group was found to be significantly associated with day 7 clearance (odds ratio [OR] 0.69 [95% confidence interval 0.51-0.87]; P = ·001). In the peginterferon lambda group, also, the mean decline in SARS-CoV-2 RNA was significantly larger than in the placebo group across all time points (days 3, 5, 7, and14). While viral load decline was 0.81 log greater in the treatment group (P = .14) by day 3, viral load decline increased to 1.67 log copies per mL by day 5 (P = .013) and 2.42 log copies per mL by day 7 (P = .0041). At day 14, the viral decline was 1.77 log copies per mL larger in the peginterferon lambda group (P = .048). The investigators pointed out that the difference in viral load decline between groups was greater in patients with high baseline viral load (at or above 106 copies per mL). In the peginterferon lambda high baseline viral load group, the reduction was 7.17 log copies per mL, versus 4.92 log copies per mL in the placebo group (P = .004).
More patients SARS-CoV-2 RNA negative
By day 7, 80% of patients in the peginterferon lambda group were negative for SARS-CoV-2 RNA, compared with 63% in the placebo group (P = .15). After baseline load adjustment, however, the peginterferon lambda treatment was significantly associated with day 7 clearance (OR 4·12 [95% CI 1·15-16·73]; P = .029).
Respiratory symptoms improved faster
Most symptoms in both groups were mild to moderate, without difference in frequency or severity. While symptom improvement was generally similar over time for both groups, respiratory symptoms improved faster with peginterferon lambda, with the effect more pronounced in the high baseline viral load group (OR 5·88 (0·81-42·46; P =. 079).
Laboratory adverse events, similar for both groups, were mild.
“Peginterferon lambda has potential to prevent clinical deterioration and shorten duration of viral shedding,” the investigators concluded.
“This clinical trial is important” because it suggests that a single intravenous dose of peginterferon lambda administered to outpatients with a positive SARS-CoV-2 nasopharyngeal swab speeds reduction of SARS-CoV-2 viral load, David L. Bowton, MD, FCCP, professor emeritus, Wake Forest Baptist Health, Winston-Salem, N.C., said in an interview. He observed that the smaller viral load difference observed at day 14 likely reflects host immune responses.
Dr. Bowton also noted that two placebo group baseline characteristics (five placebo group patients with anti-SARS-CoV-2 S protein IgG antibodies; two times more undetectable SARS-CoV-2 RNA at baseline assessment) would tend to reduce differences between the peginterferon lambda and placebo groups. He added that the study findings were concordant with another phase 2 trial of hospitalized COVID-19 patients receiving inhaled interferon beta-1a.
“Thus, interferons may find a place in the treatment of COVID-19 and perhaps other severe viral illnesses,” Dr. Bowton said.
The study was funded by the Toronto COVID-19 Action Initiative, University of Toronto, and the Ontario First COVID-19 Rapid Research Fund, Toronto General & Western Hospital Foundation.
Dr. Bowton had no disclosures. Disclosures for Dr. Feld and coauthors are listed on the Lancet Respiratory Medicine website.
and shorten the duration of viral shedding, according to results of a double-blind, placebo-controlled trial (NCT04354259).
Reductions in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA were greater with peginterferon lambda than with placebo from day 3 onward in the phase 2 study led by Jordan J. Feld, MD, of the Toronto Centre for Liver Disease. The findings were reported in The Lancet Respiratory Medicine.
Fewer side effects
To date in randomized clinical trials, efficacy in treatment of COVID-19 has been shown only for remdesivir and dexamethasone in hospitalized patients, and in an interim analysis of accelerated viral clearance for a monoclonal antibody infusion in outpatients.
Activity against respiratory pathogens has been demonstrated for interferon lambda-1, a type III interferon shown to be involved in innate antiviral responses. Interferons, Dr. Feld and coauthors stated, drive induction of genes with antiviral, antiproliferative and immunoregulatory properties, and early treatment with interferons might halt clinical progression and shorten the duration of viral shedding with reduced onward transmission. In addition, interferon lambdas (type III) use a distinct receptor complex with high expression levels limited to epithelial cells in the lung, liver, and intestine, leading to fewer side effects than other interferons, including avoiding risk of promoting cytokine storm syndrome.
The researchers investigated peginterferon lambda safety and efficacy in treatment of patients with laboratory-confirmed, mild to moderate COVID-19. Sixty patients (median age 46 years, about 60% female, about 50% White) were recruited from outpatient testing centers at six institutions in Toronto, and referred to a single ambulatory site. Patients were randomly assigned 1:1 to a single subcutaneous injection of peginterferon lambda 180 mcg or placebo within 7 days of symptom onset or, if asymptomatic, of their first positive swab. Mean time from symptom onset to injection was about 4.5 days, and about 18.5% were asymptomatic. The primary outcome was the proportion of patients negative for SARS-CoV-2 RNA on day 7 after the injection.
Greater benefit with higher baseline load
A higher baseline SARS-CoV-2 RNA concentration found in the peginterferon lambda group was found to be significantly associated with day 7 clearance (odds ratio [OR] 0.69 [95% confidence interval 0.51-0.87]; P = ·001). In the peginterferon lambda group, also, the mean decline in SARS-CoV-2 RNA was significantly larger than in the placebo group across all time points (days 3, 5, 7, and14). While viral load decline was 0.81 log greater in the treatment group (P = .14) by day 3, viral load decline increased to 1.67 log copies per mL by day 5 (P = .013) and 2.42 log copies per mL by day 7 (P = .0041). At day 14, the viral decline was 1.77 log copies per mL larger in the peginterferon lambda group (P = .048). The investigators pointed out that the difference in viral load decline between groups was greater in patients with high baseline viral load (at or above 106 copies per mL). In the peginterferon lambda high baseline viral load group, the reduction was 7.17 log copies per mL, versus 4.92 log copies per mL in the placebo group (P = .004).
More patients SARS-CoV-2 RNA negative
By day 7, 80% of patients in the peginterferon lambda group were negative for SARS-CoV-2 RNA, compared with 63% in the placebo group (P = .15). After baseline load adjustment, however, the peginterferon lambda treatment was significantly associated with day 7 clearance (OR 4·12 [95% CI 1·15-16·73]; P = .029).
Respiratory symptoms improved faster
Most symptoms in both groups were mild to moderate, without difference in frequency or severity. While symptom improvement was generally similar over time for both groups, respiratory symptoms improved faster with peginterferon lambda, with the effect more pronounced in the high baseline viral load group (OR 5·88 (0·81-42·46; P =. 079).
Laboratory adverse events, similar for both groups, were mild.
“Peginterferon lambda has potential to prevent clinical deterioration and shorten duration of viral shedding,” the investigators concluded.
“This clinical trial is important” because it suggests that a single intravenous dose of peginterferon lambda administered to outpatients with a positive SARS-CoV-2 nasopharyngeal swab speeds reduction of SARS-CoV-2 viral load, David L. Bowton, MD, FCCP, professor emeritus, Wake Forest Baptist Health, Winston-Salem, N.C., said in an interview. He observed that the smaller viral load difference observed at day 14 likely reflects host immune responses.
Dr. Bowton also noted that two placebo group baseline characteristics (five placebo group patients with anti-SARS-CoV-2 S protein IgG antibodies; two times more undetectable SARS-CoV-2 RNA at baseline assessment) would tend to reduce differences between the peginterferon lambda and placebo groups. He added that the study findings were concordant with another phase 2 trial of hospitalized COVID-19 patients receiving inhaled interferon beta-1a.
“Thus, interferons may find a place in the treatment of COVID-19 and perhaps other severe viral illnesses,” Dr. Bowton said.
The study was funded by the Toronto COVID-19 Action Initiative, University of Toronto, and the Ontario First COVID-19 Rapid Research Fund, Toronto General & Western Hospital Foundation.
Dr. Bowton had no disclosures. Disclosures for Dr. Feld and coauthors are listed on the Lancet Respiratory Medicine website.
and shorten the duration of viral shedding, according to results of a double-blind, placebo-controlled trial (NCT04354259).
Reductions in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA were greater with peginterferon lambda than with placebo from day 3 onward in the phase 2 study led by Jordan J. Feld, MD, of the Toronto Centre for Liver Disease. The findings were reported in The Lancet Respiratory Medicine.
Fewer side effects
To date in randomized clinical trials, efficacy in treatment of COVID-19 has been shown only for remdesivir and dexamethasone in hospitalized patients, and in an interim analysis of accelerated viral clearance for a monoclonal antibody infusion in outpatients.
Activity against respiratory pathogens has been demonstrated for interferon lambda-1, a type III interferon shown to be involved in innate antiviral responses. Interferons, Dr. Feld and coauthors stated, drive induction of genes with antiviral, antiproliferative and immunoregulatory properties, and early treatment with interferons might halt clinical progression and shorten the duration of viral shedding with reduced onward transmission. In addition, interferon lambdas (type III) use a distinct receptor complex with high expression levels limited to epithelial cells in the lung, liver, and intestine, leading to fewer side effects than other interferons, including avoiding risk of promoting cytokine storm syndrome.
The researchers investigated peginterferon lambda safety and efficacy in treatment of patients with laboratory-confirmed, mild to moderate COVID-19. Sixty patients (median age 46 years, about 60% female, about 50% White) were recruited from outpatient testing centers at six institutions in Toronto, and referred to a single ambulatory site. Patients were randomly assigned 1:1 to a single subcutaneous injection of peginterferon lambda 180 mcg or placebo within 7 days of symptom onset or, if asymptomatic, of their first positive swab. Mean time from symptom onset to injection was about 4.5 days, and about 18.5% were asymptomatic. The primary outcome was the proportion of patients negative for SARS-CoV-2 RNA on day 7 after the injection.
Greater benefit with higher baseline load
A higher baseline SARS-CoV-2 RNA concentration found in the peginterferon lambda group was found to be significantly associated with day 7 clearance (odds ratio [OR] 0.69 [95% confidence interval 0.51-0.87]; P = ·001). In the peginterferon lambda group, also, the mean decline in SARS-CoV-2 RNA was significantly larger than in the placebo group across all time points (days 3, 5, 7, and14). While viral load decline was 0.81 log greater in the treatment group (P = .14) by day 3, viral load decline increased to 1.67 log copies per mL by day 5 (P = .013) and 2.42 log copies per mL by day 7 (P = .0041). At day 14, the viral decline was 1.77 log copies per mL larger in the peginterferon lambda group (P = .048). The investigators pointed out that the difference in viral load decline between groups was greater in patients with high baseline viral load (at or above 106 copies per mL). In the peginterferon lambda high baseline viral load group, the reduction was 7.17 log copies per mL, versus 4.92 log copies per mL in the placebo group (P = .004).
More patients SARS-CoV-2 RNA negative
By day 7, 80% of patients in the peginterferon lambda group were negative for SARS-CoV-2 RNA, compared with 63% in the placebo group (P = .15). After baseline load adjustment, however, the peginterferon lambda treatment was significantly associated with day 7 clearance (OR 4·12 [95% CI 1·15-16·73]; P = .029).
Respiratory symptoms improved faster
Most symptoms in both groups were mild to moderate, without difference in frequency or severity. While symptom improvement was generally similar over time for both groups, respiratory symptoms improved faster with peginterferon lambda, with the effect more pronounced in the high baseline viral load group (OR 5·88 (0·81-42·46; P =. 079).
Laboratory adverse events, similar for both groups, were mild.
“Peginterferon lambda has potential to prevent clinical deterioration and shorten duration of viral shedding,” the investigators concluded.
“This clinical trial is important” because it suggests that a single intravenous dose of peginterferon lambda administered to outpatients with a positive SARS-CoV-2 nasopharyngeal swab speeds reduction of SARS-CoV-2 viral load, David L. Bowton, MD, FCCP, professor emeritus, Wake Forest Baptist Health, Winston-Salem, N.C., said in an interview. He observed that the smaller viral load difference observed at day 14 likely reflects host immune responses.
Dr. Bowton also noted that two placebo group baseline characteristics (five placebo group patients with anti-SARS-CoV-2 S protein IgG antibodies; two times more undetectable SARS-CoV-2 RNA at baseline assessment) would tend to reduce differences between the peginterferon lambda and placebo groups. He added that the study findings were concordant with another phase 2 trial of hospitalized COVID-19 patients receiving inhaled interferon beta-1a.
“Thus, interferons may find a place in the treatment of COVID-19 and perhaps other severe viral illnesses,” Dr. Bowton said.
The study was funded by the Toronto COVID-19 Action Initiative, University of Toronto, and the Ontario First COVID-19 Rapid Research Fund, Toronto General & Western Hospital Foundation.
Dr. Bowton had no disclosures. Disclosures for Dr. Feld and coauthors are listed on the Lancet Respiratory Medicine website.
FROM THE LANCET RESPIRATORY MEDICINE
Neighborhood police complaints tied to Black preterm birth rates
The more complaints of excessive force by police reported by neighborhood residents, the more likely it is that Black pregnant people living in that neighborhood will deliver preterm, according to findings from a new study presented Jan. 28 at the virtual Society for Maternal-Fetal Medicine 2021 Annual Pregnancy Meeting.
“We know there are significant racial disparities in preterm birth which aren’t fully explained by traditional risk factors, like being older, having health problems like high blood pressure, or limited income,” Alexa Freedman, PhD, a postdoctoral fellow at NorthShore University HealthSystem and Northwestern University Institute for Policy Research, Evanston, Ill., told this news organization. “This has left many wondering if there are stressors unique to Black individuals that may be involved,” which has led to past research on the association of preterm birth with neighborhood segregation and historical “redlining” practices.
Black individuals have a substantially higher rate of preterm birth, compared with all other racial and ethnic groups in the US: 13.8% of Black infants born between 2016 and 2018 were preterm, compared with 11.6% among Native Americans – the next highest group – and 9.1% among White women.
“Studies have shown that psychosocial stress contributes to preterm birth disparities, potentially through several physiologic pathways that impact pregnancy outcomes,” Dr. Freedman told attendees. “Pregnant Black individuals have been reported to experience greater psychosocial stress regardless of socioeconomic status, possibly secondary to experiences of racism and discrimination.”
Though past research has examined neighborhood disadvantage and violence as stressors potentially contributing to preterm birth, little data exist on police–community relationships or police violence and pregnancy outcomes, despite being a “particularly salient stressor for Black individuals,” Dr. Freedman said. “Among pregnant Black individuals, prenatal depression has been correlated with concern about negative interactions between youth in their community and police.” To cite one example of the prevalence of racial bias in policing, she noted that “Chicago police are almost 10 times more likely to use force when interacting with a Black individual as compared [with] a White individual.”
The researchers therefore sought to determine whether a relationship existed between preterm birth rates and complaints regarding use of excessive force by police in the same neighborhood. They compiled records on all singleton live births from one Chicago hospital between March 2008 and March 2018, excluding those who lived outside Chicago, had a missing address, listed their race as “other,” or lacked data for specific other confounders.
Assessing police complaints within census blocks
The researchers obtained data on police complaints in Chicago from the Invisible Institute’s Citizen Police Data Project. They focused only on complaints of excessive use of force, “such as unnecessary physical contact and unnecessary display of a weapon,” Dr. Freedman said. They considered a person exposed in the neighborhood if a complaint was reported in her census block in the year leading up to birth. During their study period, more than 6,000 complaints of excessive force were reported across an estimated 70% of the blocks.
The study population had an average age of 31 and included 59.5% White, 12% Black, 20% Hispanic, and 8.5% Asian people. Just over half the pregnancies (55%) were first-time pregnancies, and 3.3% of the population had a history of preterm birth (before 37 weeks). The researchers also gathered data to adjust for the study population’s:
- Age
- Parity (number of times the woman has given birth).
- Population size of census block.
- Exposure to a homicide on the block in the year leading up to birth.
- Socioeconomic status by block (based on a composite of median home value, median income, percentage of a high school diploma, and percentage employed).
“Those who lived in a block with an excessive force complaint were more likely to be Black, more likely to deliver preterm, and more likely to be exposed to homicide,” Dr. Freedman told attendees.
The proportion of pregnant women exposed to police complaints was 15.8%, and 10.2% lived in neighborhoods where a homicide occurred in the year leading up to birth. Within the group exposed to a homicide, 16.5% lived in a neighborhood with an excessive force complaint and 9.1% did not.
Overall, 8.1% of the population gave birth preterm. When stratified by whether or not they lived in a block with an excessive force complaint, the researchers found the proportion of preterm births was higher among those who did than those who did not (9.3% vs. 7.8%).
Both before and after adjusting for confounders, Black people were the only racial/ethnic group who had a significantly increased risk of preterm birth if they lived on a block with a complaint. They were nearly 30% more likely to deliver preterm if an excessive force complaint had been reported nearby (odds ratio, 1.29). The odds of preterm birth were slightly elevated for White people and slightly reduced for Hispanic and Asian people, but none of those associations reached significance.
In a sensitivity analysis comparing 189 Black individuals to themselves, the researchers compared those who had one preterm birth and one term birth. They found that the preterm birth was 32% more likely to occur in a year when an excessive force complaint was filed after adjusting for age and birth order (OR, 1.32; 95% confidence interval, 0.82-2.13).
“Police violence reflects just one component of structural racism,” Dr. Freedman said in an interview. “Our findings highlight the need to more thoroughly consider how these systemic and structural factors contribute to disparities in maternal and fetal health.”
Clinical and policy implications
The clinical implications of these findings focus on the need for obstetric clinical teams to understand patients’ stressors and to provide support and resources, according to Dr. Freedman’s mentor, Ann Borders, MD, MSc, MPH, a maternal-fetal medicine physician at NorthShore and Evanston Hospital and a clinical associate professor at the University of Chicago Pritzker School of Medicine.
“Potential strategies include training on improved listening and respectful patient-centered care, such as provided by the CDC Hear Her campaign, and consideration of universal social determinants of health screening during obstetric care,” Dr. Borders told this news organization..
Though the study included a large sample size and allowed the researchers to control for individual and neighborhood characteristics, Dr. Freedman acknowledged that census blocks may or may not correlate with the way individuals define their own neighborhoods. They also didn’t have the data to assess the quality of prenatal care or the type of preterm birth, but they are developing a qualitative study to determine the best ways of measuring exposure to police violence.
In addition, the researchers’ reliance only on formal police complaints could have underestimated prevalence of excessive force, and the study did not take into account people’s direct experience with police violence; police violence that occurs within a person’s social network; or police violence widely covered in the news.
It wasn’t possible for the researchers to verify whether excessive force actually occurred or whether the force might have been justified, and it instead relied on the fact that someone lodged a complaint because he or she perceived the action as excessive.
Allison Bryant Mantha, MD, MPH, vice chair for Quality, Equity, and Safety at Massachusetts General Hospital in Boston and a board member of SMFM, said she was impressed with the adjustment of homicide exposure as a proxy for neighborhood crime.
“Many might assume that reports of police misconduct might be a marker for a ‘dangerous neighborhood,’ and it was thoughtful of the authors to adjust their analyses for exposure to crime to demonstrate that, even above and beyond crime, reports of police misconduct seem to be associated with adverse outcomes,” Dr. Bryant Mantha, who moderated the session, said in an interview.
Confronting this issue goes beyond what clinicians can do on their own, Dr. Bryant Mantha suggested.
“The greatest change will come with addressing the structural racism that underlies differential exposure to police misconduct in communities in the first place,” she said. “Concurrent with this, however, clinicians may consider adding in an assessment of neighborhood characteristics to include reports of police misconduct as they screen for other social determinants of health. While we do not have intervention studies to demonstrate efficacy, it is not a huge leap to imagine that recognition of this burden in individuals’ lives, plus offering ways to manage stress or seek redress, could be of benefit.”
The research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute on Minority Health and Health Disparities, and the Northwestern Medicine Enterprise Data Warehouse Pilot Data Program. Dr. Freedman, Dr. Borders, and Dr. Bryant Mantha have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The more complaints of excessive force by police reported by neighborhood residents, the more likely it is that Black pregnant people living in that neighborhood will deliver preterm, according to findings from a new study presented Jan. 28 at the virtual Society for Maternal-Fetal Medicine 2021 Annual Pregnancy Meeting.
“We know there are significant racial disparities in preterm birth which aren’t fully explained by traditional risk factors, like being older, having health problems like high blood pressure, or limited income,” Alexa Freedman, PhD, a postdoctoral fellow at NorthShore University HealthSystem and Northwestern University Institute for Policy Research, Evanston, Ill., told this news organization. “This has left many wondering if there are stressors unique to Black individuals that may be involved,” which has led to past research on the association of preterm birth with neighborhood segregation and historical “redlining” practices.
Black individuals have a substantially higher rate of preterm birth, compared with all other racial and ethnic groups in the US: 13.8% of Black infants born between 2016 and 2018 were preterm, compared with 11.6% among Native Americans – the next highest group – and 9.1% among White women.
“Studies have shown that psychosocial stress contributes to preterm birth disparities, potentially through several physiologic pathways that impact pregnancy outcomes,” Dr. Freedman told attendees. “Pregnant Black individuals have been reported to experience greater psychosocial stress regardless of socioeconomic status, possibly secondary to experiences of racism and discrimination.”
Though past research has examined neighborhood disadvantage and violence as stressors potentially contributing to preterm birth, little data exist on police–community relationships or police violence and pregnancy outcomes, despite being a “particularly salient stressor for Black individuals,” Dr. Freedman said. “Among pregnant Black individuals, prenatal depression has been correlated with concern about negative interactions between youth in their community and police.” To cite one example of the prevalence of racial bias in policing, she noted that “Chicago police are almost 10 times more likely to use force when interacting with a Black individual as compared [with] a White individual.”
The researchers therefore sought to determine whether a relationship existed between preterm birth rates and complaints regarding use of excessive force by police in the same neighborhood. They compiled records on all singleton live births from one Chicago hospital between March 2008 and March 2018, excluding those who lived outside Chicago, had a missing address, listed their race as “other,” or lacked data for specific other confounders.
Assessing police complaints within census blocks
The researchers obtained data on police complaints in Chicago from the Invisible Institute’s Citizen Police Data Project. They focused only on complaints of excessive use of force, “such as unnecessary physical contact and unnecessary display of a weapon,” Dr. Freedman said. They considered a person exposed in the neighborhood if a complaint was reported in her census block in the year leading up to birth. During their study period, more than 6,000 complaints of excessive force were reported across an estimated 70% of the blocks.
The study population had an average age of 31 and included 59.5% White, 12% Black, 20% Hispanic, and 8.5% Asian people. Just over half the pregnancies (55%) were first-time pregnancies, and 3.3% of the population had a history of preterm birth (before 37 weeks). The researchers also gathered data to adjust for the study population’s:
- Age
- Parity (number of times the woman has given birth).
- Population size of census block.
- Exposure to a homicide on the block in the year leading up to birth.
- Socioeconomic status by block (based on a composite of median home value, median income, percentage of a high school diploma, and percentage employed).
“Those who lived in a block with an excessive force complaint were more likely to be Black, more likely to deliver preterm, and more likely to be exposed to homicide,” Dr. Freedman told attendees.
The proportion of pregnant women exposed to police complaints was 15.8%, and 10.2% lived in neighborhoods where a homicide occurred in the year leading up to birth. Within the group exposed to a homicide, 16.5% lived in a neighborhood with an excessive force complaint and 9.1% did not.
Overall, 8.1% of the population gave birth preterm. When stratified by whether or not they lived in a block with an excessive force complaint, the researchers found the proportion of preterm births was higher among those who did than those who did not (9.3% vs. 7.8%).
Both before and after adjusting for confounders, Black people were the only racial/ethnic group who had a significantly increased risk of preterm birth if they lived on a block with a complaint. They were nearly 30% more likely to deliver preterm if an excessive force complaint had been reported nearby (odds ratio, 1.29). The odds of preterm birth were slightly elevated for White people and slightly reduced for Hispanic and Asian people, but none of those associations reached significance.
In a sensitivity analysis comparing 189 Black individuals to themselves, the researchers compared those who had one preterm birth and one term birth. They found that the preterm birth was 32% more likely to occur in a year when an excessive force complaint was filed after adjusting for age and birth order (OR, 1.32; 95% confidence interval, 0.82-2.13).
“Police violence reflects just one component of structural racism,” Dr. Freedman said in an interview. “Our findings highlight the need to more thoroughly consider how these systemic and structural factors contribute to disparities in maternal and fetal health.”
Clinical and policy implications
The clinical implications of these findings focus on the need for obstetric clinical teams to understand patients’ stressors and to provide support and resources, according to Dr. Freedman’s mentor, Ann Borders, MD, MSc, MPH, a maternal-fetal medicine physician at NorthShore and Evanston Hospital and a clinical associate professor at the University of Chicago Pritzker School of Medicine.
“Potential strategies include training on improved listening and respectful patient-centered care, such as provided by the CDC Hear Her campaign, and consideration of universal social determinants of health screening during obstetric care,” Dr. Borders told this news organization..
Though the study included a large sample size and allowed the researchers to control for individual and neighborhood characteristics, Dr. Freedman acknowledged that census blocks may or may not correlate with the way individuals define their own neighborhoods. They also didn’t have the data to assess the quality of prenatal care or the type of preterm birth, but they are developing a qualitative study to determine the best ways of measuring exposure to police violence.
In addition, the researchers’ reliance only on formal police complaints could have underestimated prevalence of excessive force, and the study did not take into account people’s direct experience with police violence; police violence that occurs within a person’s social network; or police violence widely covered in the news.
It wasn’t possible for the researchers to verify whether excessive force actually occurred or whether the force might have been justified, and it instead relied on the fact that someone lodged a complaint because he or she perceived the action as excessive.
Allison Bryant Mantha, MD, MPH, vice chair for Quality, Equity, and Safety at Massachusetts General Hospital in Boston and a board member of SMFM, said she was impressed with the adjustment of homicide exposure as a proxy for neighborhood crime.
“Many might assume that reports of police misconduct might be a marker for a ‘dangerous neighborhood,’ and it was thoughtful of the authors to adjust their analyses for exposure to crime to demonstrate that, even above and beyond crime, reports of police misconduct seem to be associated with adverse outcomes,” Dr. Bryant Mantha, who moderated the session, said in an interview.
Confronting this issue goes beyond what clinicians can do on their own, Dr. Bryant Mantha suggested.
“The greatest change will come with addressing the structural racism that underlies differential exposure to police misconduct in communities in the first place,” she said. “Concurrent with this, however, clinicians may consider adding in an assessment of neighborhood characteristics to include reports of police misconduct as they screen for other social determinants of health. While we do not have intervention studies to demonstrate efficacy, it is not a huge leap to imagine that recognition of this burden in individuals’ lives, plus offering ways to manage stress or seek redress, could be of benefit.”
The research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute on Minority Health and Health Disparities, and the Northwestern Medicine Enterprise Data Warehouse Pilot Data Program. Dr. Freedman, Dr. Borders, and Dr. Bryant Mantha have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The more complaints of excessive force by police reported by neighborhood residents, the more likely it is that Black pregnant people living in that neighborhood will deliver preterm, according to findings from a new study presented Jan. 28 at the virtual Society for Maternal-Fetal Medicine 2021 Annual Pregnancy Meeting.
“We know there are significant racial disparities in preterm birth which aren’t fully explained by traditional risk factors, like being older, having health problems like high blood pressure, or limited income,” Alexa Freedman, PhD, a postdoctoral fellow at NorthShore University HealthSystem and Northwestern University Institute for Policy Research, Evanston, Ill., told this news organization. “This has left many wondering if there are stressors unique to Black individuals that may be involved,” which has led to past research on the association of preterm birth with neighborhood segregation and historical “redlining” practices.
Black individuals have a substantially higher rate of preterm birth, compared with all other racial and ethnic groups in the US: 13.8% of Black infants born between 2016 and 2018 were preterm, compared with 11.6% among Native Americans – the next highest group – and 9.1% among White women.
“Studies have shown that psychosocial stress contributes to preterm birth disparities, potentially through several physiologic pathways that impact pregnancy outcomes,” Dr. Freedman told attendees. “Pregnant Black individuals have been reported to experience greater psychosocial stress regardless of socioeconomic status, possibly secondary to experiences of racism and discrimination.”
Though past research has examined neighborhood disadvantage and violence as stressors potentially contributing to preterm birth, little data exist on police–community relationships or police violence and pregnancy outcomes, despite being a “particularly salient stressor for Black individuals,” Dr. Freedman said. “Among pregnant Black individuals, prenatal depression has been correlated with concern about negative interactions between youth in their community and police.” To cite one example of the prevalence of racial bias in policing, she noted that “Chicago police are almost 10 times more likely to use force when interacting with a Black individual as compared [with] a White individual.”
The researchers therefore sought to determine whether a relationship existed between preterm birth rates and complaints regarding use of excessive force by police in the same neighborhood. They compiled records on all singleton live births from one Chicago hospital between March 2008 and March 2018, excluding those who lived outside Chicago, had a missing address, listed their race as “other,” or lacked data for specific other confounders.
Assessing police complaints within census blocks
The researchers obtained data on police complaints in Chicago from the Invisible Institute’s Citizen Police Data Project. They focused only on complaints of excessive use of force, “such as unnecessary physical contact and unnecessary display of a weapon,” Dr. Freedman said. They considered a person exposed in the neighborhood if a complaint was reported in her census block in the year leading up to birth. During their study period, more than 6,000 complaints of excessive force were reported across an estimated 70% of the blocks.
The study population had an average age of 31 and included 59.5% White, 12% Black, 20% Hispanic, and 8.5% Asian people. Just over half the pregnancies (55%) were first-time pregnancies, and 3.3% of the population had a history of preterm birth (before 37 weeks). The researchers also gathered data to adjust for the study population’s:
- Age
- Parity (number of times the woman has given birth).
- Population size of census block.
- Exposure to a homicide on the block in the year leading up to birth.
- Socioeconomic status by block (based on a composite of median home value, median income, percentage of a high school diploma, and percentage employed).
“Those who lived in a block with an excessive force complaint were more likely to be Black, more likely to deliver preterm, and more likely to be exposed to homicide,” Dr. Freedman told attendees.
The proportion of pregnant women exposed to police complaints was 15.8%, and 10.2% lived in neighborhoods where a homicide occurred in the year leading up to birth. Within the group exposed to a homicide, 16.5% lived in a neighborhood with an excessive force complaint and 9.1% did not.
Overall, 8.1% of the population gave birth preterm. When stratified by whether or not they lived in a block with an excessive force complaint, the researchers found the proportion of preterm births was higher among those who did than those who did not (9.3% vs. 7.8%).
Both before and after adjusting for confounders, Black people were the only racial/ethnic group who had a significantly increased risk of preterm birth if they lived on a block with a complaint. They were nearly 30% more likely to deliver preterm if an excessive force complaint had been reported nearby (odds ratio, 1.29). The odds of preterm birth were slightly elevated for White people and slightly reduced for Hispanic and Asian people, but none of those associations reached significance.
In a sensitivity analysis comparing 189 Black individuals to themselves, the researchers compared those who had one preterm birth and one term birth. They found that the preterm birth was 32% more likely to occur in a year when an excessive force complaint was filed after adjusting for age and birth order (OR, 1.32; 95% confidence interval, 0.82-2.13).
“Police violence reflects just one component of structural racism,” Dr. Freedman said in an interview. “Our findings highlight the need to more thoroughly consider how these systemic and structural factors contribute to disparities in maternal and fetal health.”
Clinical and policy implications
The clinical implications of these findings focus on the need for obstetric clinical teams to understand patients’ stressors and to provide support and resources, according to Dr. Freedman’s mentor, Ann Borders, MD, MSc, MPH, a maternal-fetal medicine physician at NorthShore and Evanston Hospital and a clinical associate professor at the University of Chicago Pritzker School of Medicine.
“Potential strategies include training on improved listening and respectful patient-centered care, such as provided by the CDC Hear Her campaign, and consideration of universal social determinants of health screening during obstetric care,” Dr. Borders told this news organization..
Though the study included a large sample size and allowed the researchers to control for individual and neighborhood characteristics, Dr. Freedman acknowledged that census blocks may or may not correlate with the way individuals define their own neighborhoods. They also didn’t have the data to assess the quality of prenatal care or the type of preterm birth, but they are developing a qualitative study to determine the best ways of measuring exposure to police violence.
In addition, the researchers’ reliance only on formal police complaints could have underestimated prevalence of excessive force, and the study did not take into account people’s direct experience with police violence; police violence that occurs within a person’s social network; or police violence widely covered in the news.
It wasn’t possible for the researchers to verify whether excessive force actually occurred or whether the force might have been justified, and it instead relied on the fact that someone lodged a complaint because he or she perceived the action as excessive.
Allison Bryant Mantha, MD, MPH, vice chair for Quality, Equity, and Safety at Massachusetts General Hospital in Boston and a board member of SMFM, said she was impressed with the adjustment of homicide exposure as a proxy for neighborhood crime.
“Many might assume that reports of police misconduct might be a marker for a ‘dangerous neighborhood,’ and it was thoughtful of the authors to adjust their analyses for exposure to crime to demonstrate that, even above and beyond crime, reports of police misconduct seem to be associated with adverse outcomes,” Dr. Bryant Mantha, who moderated the session, said in an interview.
Confronting this issue goes beyond what clinicians can do on their own, Dr. Bryant Mantha suggested.
“The greatest change will come with addressing the structural racism that underlies differential exposure to police misconduct in communities in the first place,” she said. “Concurrent with this, however, clinicians may consider adding in an assessment of neighborhood characteristics to include reports of police misconduct as they screen for other social determinants of health. While we do not have intervention studies to demonstrate efficacy, it is not a huge leap to imagine that recognition of this burden in individuals’ lives, plus offering ways to manage stress or seek redress, could be of benefit.”
The research was funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute on Minority Health and Health Disparities, and the Northwestern Medicine Enterprise Data Warehouse Pilot Data Program. Dr. Freedman, Dr. Borders, and Dr. Bryant Mantha have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Best of 2020: The RA Report
Read this supplement to Rheumatology News here.
In this issue:
- Synovial biopsy findings drive precision medicine closer to the clinic
- Ready for PRIME time? Newly identified cells predict flares
- Rheumatologists’ prescriptions of biosimilars stagnate
- Methotrexate in terms of ILD concerns, poor adherence, and low-dose AEs 10-13 TNF inhibitors linked to risk for inflammatory CNS events
- Hypersensitivity reactions to biologics differentiated
- Repeat latent TB testing focuses on new risks
Read this supplement to Rheumatology News here.
In this issue:
- Synovial biopsy findings drive precision medicine closer to the clinic
- Ready for PRIME time? Newly identified cells predict flares
- Rheumatologists’ prescriptions of biosimilars stagnate
- Methotrexate in terms of ILD concerns, poor adherence, and low-dose AEs 10-13 TNF inhibitors linked to risk for inflammatory CNS events
- Hypersensitivity reactions to biologics differentiated
- Repeat latent TB testing focuses on new risks
Read this supplement to Rheumatology News here.
In this issue:
- Synovial biopsy findings drive precision medicine closer to the clinic
- Ready for PRIME time? Newly identified cells predict flares
- Rheumatologists’ prescriptions of biosimilars stagnate
- Methotrexate in terms of ILD concerns, poor adherence, and low-dose AEs 10-13 TNF inhibitors linked to risk for inflammatory CNS events
- Hypersensitivity reactions to biologics differentiated
- Repeat latent TB testing focuses on new risks
Dr. Pinilla-Ibarz: Trial supports the use of nilotinib 300mg twice daily as front line therapy for CML
Long term CML follow up clinical trials are quite important tool to monitor the late or new adverse side effects as well as to conform the efficacy in the long run of the drug tested. However, there are not so many examples on CML outside the IRIS trial. Recently, Kantarjian and collaborators had published the 10 years follow up on the ENESTnd trial that compared the use of two doses of nilotinib (300md bid and 400 mg bid) against imatinib (400mg qd). Once again, the study showed a higher cumulative molecular response rates (MMR: 77% vs 79% vs 62% and MR4.5:61% vs61% vs 39%) that translated in a higher proportion of patient candidates for TFR (48% vs 47% vs 29%). Furthermore, nilotinib was associated to lower rates of disease progression compare with imatinib. As previously described there were no differences in terms of PFS or OS between the three arms. In terms of toxicity, although the overall adverse effects were similar, a higher incidence of cardiovascular events were reported (16% vs 23% vs 3%). The incidence of these CVE on the nilotinib arm continue to occur at the same rate each year and were more associated with high and intermediate Framingham risk. Overall, the trial supports the use of nilotinib 300mg twice daily as front line therapy for CML for optimal long terms outcomes with a positive benefit-risk in the context of TFR as treatment goal.
One of the important aspects of the CVE secondary to second generation TKIs is the identification of high-risk populations as well as biomarkers that may help to prevent these episodes. In this regard, Italian investigators recently published a study of 369 patients treated with nilotinib where, besides stratification by the new coronary risk evaluation (SCORE), they measure the lipids levels at various times point since the initiation of therapy and identify cholesterol greater than 200mg/dL and LDL greater than 70 mg/dL as predictors factor for increased risk of CVE as well as a high SCORE risk. The authors suggest an aggressive follow up on lipids levels in patients taking nilotinib and the incorporation of cholesterol lowering medications.
Although the incidence of CVE has been more classically associated with nilotinib and ponatinib, more recent evidence suggests that the use of second generation TKIs in general can increase the risk of CVE in CML patients in comparison with the general population; however, it is hard to study the contribution of the disease itself. Leong and collaborators recently published a very large retrospective analysis of more than 4,000 CML patients that were age and sex matched with 42,000 controls without CML, showing that the incidence of these events is higher before and after the introduction of TKIs in 2001.
Javier Pinilla-Ibarz, MD, PhD
Senior Member
Lymphoma Section Head and
Director of Immunotherapy
Malignant Hematology Department
H. Lee Moffitt Cancer Center & Research Institute
Long term CML follow up clinical trials are quite important tool to monitor the late or new adverse side effects as well as to conform the efficacy in the long run of the drug tested. However, there are not so many examples on CML outside the IRIS trial. Recently, Kantarjian and collaborators had published the 10 years follow up on the ENESTnd trial that compared the use of two doses of nilotinib (300md bid and 400 mg bid) against imatinib (400mg qd). Once again, the study showed a higher cumulative molecular response rates (MMR: 77% vs 79% vs 62% and MR4.5:61% vs61% vs 39%) that translated in a higher proportion of patient candidates for TFR (48% vs 47% vs 29%). Furthermore, nilotinib was associated to lower rates of disease progression compare with imatinib. As previously described there were no differences in terms of PFS or OS between the three arms. In terms of toxicity, although the overall adverse effects were similar, a higher incidence of cardiovascular events were reported (16% vs 23% vs 3%). The incidence of these CVE on the nilotinib arm continue to occur at the same rate each year and were more associated with high and intermediate Framingham risk. Overall, the trial supports the use of nilotinib 300mg twice daily as front line therapy for CML for optimal long terms outcomes with a positive benefit-risk in the context of TFR as treatment goal.
One of the important aspects of the CVE secondary to second generation TKIs is the identification of high-risk populations as well as biomarkers that may help to prevent these episodes. In this regard, Italian investigators recently published a study of 369 patients treated with nilotinib where, besides stratification by the new coronary risk evaluation (SCORE), they measure the lipids levels at various times point since the initiation of therapy and identify cholesterol greater than 200mg/dL and LDL greater than 70 mg/dL as predictors factor for increased risk of CVE as well as a high SCORE risk. The authors suggest an aggressive follow up on lipids levels in patients taking nilotinib and the incorporation of cholesterol lowering medications.
Although the incidence of CVE has been more classically associated with nilotinib and ponatinib, more recent evidence suggests that the use of second generation TKIs in general can increase the risk of CVE in CML patients in comparison with the general population; however, it is hard to study the contribution of the disease itself. Leong and collaborators recently published a very large retrospective analysis of more than 4,000 CML patients that were age and sex matched with 42,000 controls without CML, showing that the incidence of these events is higher before and after the introduction of TKIs in 2001.
Javier Pinilla-Ibarz, MD, PhD
Senior Member
Lymphoma Section Head and
Director of Immunotherapy
Malignant Hematology Department
H. Lee Moffitt Cancer Center & Research Institute
Long term CML follow up clinical trials are quite important tool to monitor the late or new adverse side effects as well as to conform the efficacy in the long run of the drug tested. However, there are not so many examples on CML outside the IRIS trial. Recently, Kantarjian and collaborators had published the 10 years follow up on the ENESTnd trial that compared the use of two doses of nilotinib (300md bid and 400 mg bid) against imatinib (400mg qd). Once again, the study showed a higher cumulative molecular response rates (MMR: 77% vs 79% vs 62% and MR4.5:61% vs61% vs 39%) that translated in a higher proportion of patient candidates for TFR (48% vs 47% vs 29%). Furthermore, nilotinib was associated to lower rates of disease progression compare with imatinib. As previously described there were no differences in terms of PFS or OS between the three arms. In terms of toxicity, although the overall adverse effects were similar, a higher incidence of cardiovascular events were reported (16% vs 23% vs 3%). The incidence of these CVE on the nilotinib arm continue to occur at the same rate each year and were more associated with high and intermediate Framingham risk. Overall, the trial supports the use of nilotinib 300mg twice daily as front line therapy for CML for optimal long terms outcomes with a positive benefit-risk in the context of TFR as treatment goal.
One of the important aspects of the CVE secondary to second generation TKIs is the identification of high-risk populations as well as biomarkers that may help to prevent these episodes. In this regard, Italian investigators recently published a study of 369 patients treated with nilotinib where, besides stratification by the new coronary risk evaluation (SCORE), they measure the lipids levels at various times point since the initiation of therapy and identify cholesterol greater than 200mg/dL and LDL greater than 70 mg/dL as predictors factor for increased risk of CVE as well as a high SCORE risk. The authors suggest an aggressive follow up on lipids levels in patients taking nilotinib and the incorporation of cholesterol lowering medications.
Although the incidence of CVE has been more classically associated with nilotinib and ponatinib, more recent evidence suggests that the use of second generation TKIs in general can increase the risk of CVE in CML patients in comparison with the general population; however, it is hard to study the contribution of the disease itself. Leong and collaborators recently published a very large retrospective analysis of more than 4,000 CML patients that were age and sex matched with 42,000 controls without CML, showing that the incidence of these events is higher before and after the introduction of TKIs in 2001.
Javier Pinilla-Ibarz, MD, PhD
Senior Member
Lymphoma Section Head and
Director of Immunotherapy
Malignant Hematology Department
H. Lee Moffitt Cancer Center & Research Institute
I Spy, Near the Corner of My Eye
ANSWER
The correct answer—the false statement—is that SA has no pathologic implications (choice “a”).
DISCUSSION
SAs are usually benign and occur in 10% to 15% of the population (particularly in children). But they can sometimes be a sign of serious disease such as liver failure, with related esophageal varices, especially if > 3 lesions are present.
SAs are caused by a failure in the sphincter muscle surrounding a dilated cutaneous arteriole, which in turn is caused by increased estrogen levels in the blood. This increase can be related to the estrogen in birth control medications or to pregnancy.
A diseased liver, unable to metabolize estrogen properly, can contribute to increased blood levels of estrogen. For example, about one-third of patients with cirrhosis will develop multiple SAs.
SAs are seen only in the distribution of the superior vena cava. This means that—in addition to manifesting on the face—they can also appear on the arms, hands, trunk, and fingers.
Momentarily fading completely when central pressure is applied is a peculiar trait of SAs and is therefore diagnostic.
TREATMENT
While these lesions do, in fact, usually resolve on their own, treatment attempts are usually highly satisfactory. In my experience, destruction by laser ablation is superior to electrodessication, though recurrences are common. At the time of this presentation, the patient and her mother were still pondering the treatment options.
ANSWER
The correct answer—the false statement—is that SA has no pathologic implications (choice “a”).
DISCUSSION
SAs are usually benign and occur in 10% to 15% of the population (particularly in children). But they can sometimes be a sign of serious disease such as liver failure, with related esophageal varices, especially if > 3 lesions are present.
SAs are caused by a failure in the sphincter muscle surrounding a dilated cutaneous arteriole, which in turn is caused by increased estrogen levels in the blood. This increase can be related to the estrogen in birth control medications or to pregnancy.
A diseased liver, unable to metabolize estrogen properly, can contribute to increased blood levels of estrogen. For example, about one-third of patients with cirrhosis will develop multiple SAs.
SAs are seen only in the distribution of the superior vena cava. This means that—in addition to manifesting on the face—they can also appear on the arms, hands, trunk, and fingers.
Momentarily fading completely when central pressure is applied is a peculiar trait of SAs and is therefore diagnostic.
TREATMENT
While these lesions do, in fact, usually resolve on their own, treatment attempts are usually highly satisfactory. In my experience, destruction by laser ablation is superior to electrodessication, though recurrences are common. At the time of this presentation, the patient and her mother were still pondering the treatment options.
ANSWER
The correct answer—the false statement—is that SA has no pathologic implications (choice “a”).
DISCUSSION
SAs are usually benign and occur in 10% to 15% of the population (particularly in children). But they can sometimes be a sign of serious disease such as liver failure, with related esophageal varices, especially if > 3 lesions are present.
SAs are caused by a failure in the sphincter muscle surrounding a dilated cutaneous arteriole, which in turn is caused by increased estrogen levels in the blood. This increase can be related to the estrogen in birth control medications or to pregnancy.
A diseased liver, unable to metabolize estrogen properly, can contribute to increased blood levels of estrogen. For example, about one-third of patients with cirrhosis will develop multiple SAs.
SAs are seen only in the distribution of the superior vena cava. This means that—in addition to manifesting on the face—they can also appear on the arms, hands, trunk, and fingers.
Momentarily fading completely when central pressure is applied is a peculiar trait of SAs and is therefore diagnostic.
TREATMENT
While these lesions do, in fact, usually resolve on their own, treatment attempts are usually highly satisfactory. In my experience, destruction by laser ablation is superior to electrodessication, though recurrences are common. At the time of this presentation, the patient and her mother were still pondering the treatment options.
About 3 years ago, an asymptomatic lesion appeared on an 8-year-old girl’s right cheek. Because the spot made her feel self-conscious, the child’s mother had tried covering it up with makeup—but the makeup was even more obvious than the spot.
Their primary care provider (PCP) advised them to do nothing, noting that such lesions usually resolve on their own. The PCP did not believe the lesion was indicative of any related health problems. Dissatisfied with this instruction, the mother brings her daughter to dermatology for evaluation.
The girl is in otherwise good health. The lesion in question is a curious, bright red macule consisting of a tiny pinpoint red dot with very narrow “legs” (tiny, slender, red, vascular lines) emanating from the periphery. It is about 7 mm, and the center red dot is about 1 mm in diameter.
Using a dull pencil to create gentle pinpoint pressure causes the whole lesion to instantly and completely fade, only to return fully after pressure is released. The lesion is diagnosed as a typical spider angioma (SA; also known as spider nevi).