User login
Androgen annihilation strategy prolongs rPFS in mCRPC
Adding the androgen receptor antagonist to standard care – abiraterone acetate and prednisone – prolonged radiographic progression-free survival (rPFS) by 6.0 months at the trial’s primary analysis and by 7.4 months at the trial’s final analysis. Adverse events were consistent with the drug’s known safety profile.
These findings were reported at the 2021 Genitourinary Cancers Symposium (Abstract 9).
“mCRPC is frequently driven by activated androgen receptors and elevated intratumoral androgens,” said investigator Dana E. Rathkopf, MD, of Memorial Sloan Kettering Cancer Center, New York.
Therefore, androgen annihilation using agents with distinct mechanisms that target both pathways is attractive.
With this in mind, investigators conducted the ACIS trial. They enrolled 982 patients who had mCRPC that had progressed on androgen deprivation therapy but who had not received chemotherapy or androgen-signaling inhibitors for castration-resistant disease.
Patients were randomized evenly to apalutamide or placebo, each given with abiraterone plus prednisone. All patients continued their ongoing androgen deprivation therapy.
Study outcomes
The trial met its primary endpoint, Dr. Rathkopf reported. In the primary analysis, conducted at a median follow-up of 25.7 months, the median investigator-assessed rPFS was 22.6 months with apalutamide and 16.6 months with placebo (hazard ratio, 0.69; P < .0001).
Results held up at the final analysis, conducted at a median follow-up of 54.8 months. At that time, the median investigator-assessed rPFS was 24.0 months with apalutamide and 16.6 months with placebo (HR, 0.70; 95% confidence interval, 0.60-0.83). The median overall survival was 36.2 months and 33.7 months, respectively, a nonsignificant difference.
For both rPFS and overall survival, there were trends toward benefit in two clinical subgroups typically having poorer prognosis – men with visceral metastases and men aged 75 years and older. In analyses of biomarkers, benefit was greater in men whose tumors were luminal subtype and in patients who had average or high androgen receptor activity.
The apalutamide and placebo groups did not differ significantly on time to second PFS, initiation of cytotoxic chemotherapy, chronic opioid use, and pain progression. However, apalutamide therapy increased the percentage of men who achieved a confirmed decline of at least 50% in prostate-specific antigen (PSA) level (79.5% vs. 72.9%) and an undetectable PSA level at any time during treatment (24.6% vs. 19.2%).
Apalutamide was associated with a higher rate of grade 3/4 treatment-emergent adverse events (63.3% vs. 56.2%), including fatigue, hypertension, rash, cardiac disorders, and fracture/osteoporosis.
Health-related quality of life declined over time in both treatment groups, although not to a clinically meaningful extent.
“Clinical and biomarker subgroups identified in this analysis will need further exploration to better delineate who might benefit most from the addition of apalutamide to abiraterone and prednisone in mCRPC,” Dr. Rathkopf said, noting that she currently looks at the whole picture when deciding whether to use the combination.
“It’s not just luminal subtype or Gleason grade or age. You have to look at all of these variables together. There are definitely patients that are more suited to a more aggressive approach early on,” she elaborated. “And some patients want to be more aggressive. A progression-free survival gain of 6 or 7 months up front is meaningful to them. A longer time to progression and a more profound decline in PSA will allow them to possibly enjoy their life more during this treatment period, balanced against whatever toxicities we may see with the combination.”
Practice changing?
To its merit, the ACIS trial was large; used an active, standard-of-care comparator; and had a blinded design, said invited discussant Joshi J. Alumkal, MD, of the Rogel Cancer Center at the University of Michigan, Ann Arbor.
However, “because of the increase in toxicity, cost, similar radiographic progression-free survival 2, and the lack of overall survival benefit at this time, and in light of the clinical insights from other studies with combined or sequential ARSI [androgen receptor signaling inhibitor] treatment, I do not believe results from ACIS change practice at this time,” he said.
Additional research into the varied molecular pathways driving this disease will be essential for tailoring therapy to improve clinical outcomes for various patient subsets, Dr. Alumkal maintained.
“To move the needle in CRPC, it is important to understand the biology in those patients who derive the least benefit from ARSI treatment,” he elaborated. “Understanding the key drivers in these tumors may provide a roadmap for how to address the most aggressive subsets of CRPC tumors that appear to do quite poorly, even with ARSI escalation as done in SPARTAN or ACIS.”
The ACIS study was funded by Janssen Research and Development. Dr. Rathkopf disclosed relationships with AstraZeneca, Bayer, Janssen, Celgene, Ferring, Genentech/Roche, Medivation, Millennium, Novartis, Taiho Pharmaceutical, Takeda, and TRACON Pharma. Dr. Alumkal disclosed relationships with Dendreon, Merck Sharpe & Dohme, Aragon Pharmaceuticals, Astellas Pharma, Gilead Sciences, and Zenith Epigenetics.
Adding the androgen receptor antagonist to standard care – abiraterone acetate and prednisone – prolonged radiographic progression-free survival (rPFS) by 6.0 months at the trial’s primary analysis and by 7.4 months at the trial’s final analysis. Adverse events were consistent with the drug’s known safety profile.
These findings were reported at the 2021 Genitourinary Cancers Symposium (Abstract 9).
“mCRPC is frequently driven by activated androgen receptors and elevated intratumoral androgens,” said investigator Dana E. Rathkopf, MD, of Memorial Sloan Kettering Cancer Center, New York.
Therefore, androgen annihilation using agents with distinct mechanisms that target both pathways is attractive.
With this in mind, investigators conducted the ACIS trial. They enrolled 982 patients who had mCRPC that had progressed on androgen deprivation therapy but who had not received chemotherapy or androgen-signaling inhibitors for castration-resistant disease.
Patients were randomized evenly to apalutamide or placebo, each given with abiraterone plus prednisone. All patients continued their ongoing androgen deprivation therapy.
Study outcomes
The trial met its primary endpoint, Dr. Rathkopf reported. In the primary analysis, conducted at a median follow-up of 25.7 months, the median investigator-assessed rPFS was 22.6 months with apalutamide and 16.6 months with placebo (hazard ratio, 0.69; P < .0001).
Results held up at the final analysis, conducted at a median follow-up of 54.8 months. At that time, the median investigator-assessed rPFS was 24.0 months with apalutamide and 16.6 months with placebo (HR, 0.70; 95% confidence interval, 0.60-0.83). The median overall survival was 36.2 months and 33.7 months, respectively, a nonsignificant difference.
For both rPFS and overall survival, there were trends toward benefit in two clinical subgroups typically having poorer prognosis – men with visceral metastases and men aged 75 years and older. In analyses of biomarkers, benefit was greater in men whose tumors were luminal subtype and in patients who had average or high androgen receptor activity.
The apalutamide and placebo groups did not differ significantly on time to second PFS, initiation of cytotoxic chemotherapy, chronic opioid use, and pain progression. However, apalutamide therapy increased the percentage of men who achieved a confirmed decline of at least 50% in prostate-specific antigen (PSA) level (79.5% vs. 72.9%) and an undetectable PSA level at any time during treatment (24.6% vs. 19.2%).
Apalutamide was associated with a higher rate of grade 3/4 treatment-emergent adverse events (63.3% vs. 56.2%), including fatigue, hypertension, rash, cardiac disorders, and fracture/osteoporosis.
Health-related quality of life declined over time in both treatment groups, although not to a clinically meaningful extent.
“Clinical and biomarker subgroups identified in this analysis will need further exploration to better delineate who might benefit most from the addition of apalutamide to abiraterone and prednisone in mCRPC,” Dr. Rathkopf said, noting that she currently looks at the whole picture when deciding whether to use the combination.
“It’s not just luminal subtype or Gleason grade or age. You have to look at all of these variables together. There are definitely patients that are more suited to a more aggressive approach early on,” she elaborated. “And some patients want to be more aggressive. A progression-free survival gain of 6 or 7 months up front is meaningful to them. A longer time to progression and a more profound decline in PSA will allow them to possibly enjoy their life more during this treatment period, balanced against whatever toxicities we may see with the combination.”
Practice changing?
To its merit, the ACIS trial was large; used an active, standard-of-care comparator; and had a blinded design, said invited discussant Joshi J. Alumkal, MD, of the Rogel Cancer Center at the University of Michigan, Ann Arbor.
However, “because of the increase in toxicity, cost, similar radiographic progression-free survival 2, and the lack of overall survival benefit at this time, and in light of the clinical insights from other studies with combined or sequential ARSI [androgen receptor signaling inhibitor] treatment, I do not believe results from ACIS change practice at this time,” he said.
Additional research into the varied molecular pathways driving this disease will be essential for tailoring therapy to improve clinical outcomes for various patient subsets, Dr. Alumkal maintained.
“To move the needle in CRPC, it is important to understand the biology in those patients who derive the least benefit from ARSI treatment,” he elaborated. “Understanding the key drivers in these tumors may provide a roadmap for how to address the most aggressive subsets of CRPC tumors that appear to do quite poorly, even with ARSI escalation as done in SPARTAN or ACIS.”
The ACIS study was funded by Janssen Research and Development. Dr. Rathkopf disclosed relationships with AstraZeneca, Bayer, Janssen, Celgene, Ferring, Genentech/Roche, Medivation, Millennium, Novartis, Taiho Pharmaceutical, Takeda, and TRACON Pharma. Dr. Alumkal disclosed relationships with Dendreon, Merck Sharpe & Dohme, Aragon Pharmaceuticals, Astellas Pharma, Gilead Sciences, and Zenith Epigenetics.
Adding the androgen receptor antagonist to standard care – abiraterone acetate and prednisone – prolonged radiographic progression-free survival (rPFS) by 6.0 months at the trial’s primary analysis and by 7.4 months at the trial’s final analysis. Adverse events were consistent with the drug’s known safety profile.
These findings were reported at the 2021 Genitourinary Cancers Symposium (Abstract 9).
“mCRPC is frequently driven by activated androgen receptors and elevated intratumoral androgens,” said investigator Dana E. Rathkopf, MD, of Memorial Sloan Kettering Cancer Center, New York.
Therefore, androgen annihilation using agents with distinct mechanisms that target both pathways is attractive.
With this in mind, investigators conducted the ACIS trial. They enrolled 982 patients who had mCRPC that had progressed on androgen deprivation therapy but who had not received chemotherapy or androgen-signaling inhibitors for castration-resistant disease.
Patients were randomized evenly to apalutamide or placebo, each given with abiraterone plus prednisone. All patients continued their ongoing androgen deprivation therapy.
Study outcomes
The trial met its primary endpoint, Dr. Rathkopf reported. In the primary analysis, conducted at a median follow-up of 25.7 months, the median investigator-assessed rPFS was 22.6 months with apalutamide and 16.6 months with placebo (hazard ratio, 0.69; P < .0001).
Results held up at the final analysis, conducted at a median follow-up of 54.8 months. At that time, the median investigator-assessed rPFS was 24.0 months with apalutamide and 16.6 months with placebo (HR, 0.70; 95% confidence interval, 0.60-0.83). The median overall survival was 36.2 months and 33.7 months, respectively, a nonsignificant difference.
For both rPFS and overall survival, there were trends toward benefit in two clinical subgroups typically having poorer prognosis – men with visceral metastases and men aged 75 years and older. In analyses of biomarkers, benefit was greater in men whose tumors were luminal subtype and in patients who had average or high androgen receptor activity.
The apalutamide and placebo groups did not differ significantly on time to second PFS, initiation of cytotoxic chemotherapy, chronic opioid use, and pain progression. However, apalutamide therapy increased the percentage of men who achieved a confirmed decline of at least 50% in prostate-specific antigen (PSA) level (79.5% vs. 72.9%) and an undetectable PSA level at any time during treatment (24.6% vs. 19.2%).
Apalutamide was associated with a higher rate of grade 3/4 treatment-emergent adverse events (63.3% vs. 56.2%), including fatigue, hypertension, rash, cardiac disorders, and fracture/osteoporosis.
Health-related quality of life declined over time in both treatment groups, although not to a clinically meaningful extent.
“Clinical and biomarker subgroups identified in this analysis will need further exploration to better delineate who might benefit most from the addition of apalutamide to abiraterone and prednisone in mCRPC,” Dr. Rathkopf said, noting that she currently looks at the whole picture when deciding whether to use the combination.
“It’s not just luminal subtype or Gleason grade or age. You have to look at all of these variables together. There are definitely patients that are more suited to a more aggressive approach early on,” she elaborated. “And some patients want to be more aggressive. A progression-free survival gain of 6 or 7 months up front is meaningful to them. A longer time to progression and a more profound decline in PSA will allow them to possibly enjoy their life more during this treatment period, balanced against whatever toxicities we may see with the combination.”
Practice changing?
To its merit, the ACIS trial was large; used an active, standard-of-care comparator; and had a blinded design, said invited discussant Joshi J. Alumkal, MD, of the Rogel Cancer Center at the University of Michigan, Ann Arbor.
However, “because of the increase in toxicity, cost, similar radiographic progression-free survival 2, and the lack of overall survival benefit at this time, and in light of the clinical insights from other studies with combined or sequential ARSI [androgen receptor signaling inhibitor] treatment, I do not believe results from ACIS change practice at this time,” he said.
Additional research into the varied molecular pathways driving this disease will be essential for tailoring therapy to improve clinical outcomes for various patient subsets, Dr. Alumkal maintained.
“To move the needle in CRPC, it is important to understand the biology in those patients who derive the least benefit from ARSI treatment,” he elaborated. “Understanding the key drivers in these tumors may provide a roadmap for how to address the most aggressive subsets of CRPC tumors that appear to do quite poorly, even with ARSI escalation as done in SPARTAN or ACIS.”
The ACIS study was funded by Janssen Research and Development. Dr. Rathkopf disclosed relationships with AstraZeneca, Bayer, Janssen, Celgene, Ferring, Genentech/Roche, Medivation, Millennium, Novartis, Taiho Pharmaceutical, Takeda, and TRACON Pharma. Dr. Alumkal disclosed relationships with Dendreon, Merck Sharpe & Dohme, Aragon Pharmaceuticals, Astellas Pharma, Gilead Sciences, and Zenith Epigenetics.
FROM GUCS 2021
Declines in PSA screening may account for rise in metastatic prostate cancers
Between 2008 and 2016, the mean incidence of prostate cancers that were metastatic at diagnosis increased from 6.4 to 9.0 per 100,000 men. During the same period, the mean percentage of men undergoing PSA screening decreased from 61.8% to 50.5%, Vidit Sharma, MD, reported in a poster session at the 2021 Genitourinary Cancers Symposium (Abstract 228).
A random-effects linear regression model demonstrated that longitudinal reductions across states in PSA screening were indeed associated with increased age-adjusted incidence of metastatic prostate cancer, said Dr. Sharma, the lead author of the study and a health services fellow in urologic oncology at the University of California, Los Angeles.
The regression coefficient per 100,000 men was 14.9, confirming that states with greater declines in screening had greater increases in prostate cancers that were metastatic at diagnosis, he added, noting that, “overall, variation in PSA screening explained 27% of the longitudinal variation in metastatic disease at diagnosis.”
Dr. Sharma and colleagues had reviewed North American Association of Central Cancer Registries data from 2002 to 2016 for each state and extracted survey-weighted PSA screening estimates from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System. The researchers noted wide variations in screening across states, but they said across-the-board declines were evident beginning in 2010, marking a “worrisome consequence that needs attention.”
Robert Dreicer, MD, deputy director of the University of Virginia Cancer Center, Charlottesville, agreed, noting in a press statement that the findings suggest reduced PSA screening may come at the cost of more men presenting with metastatic disease.
“Patients should discuss the risks and benefits associated with PSA screening with their doctor to identify the best approach for them,” Dr. Dreicer said.
PSA screening has been shown to reduce prostate cancer metastasis and mortality, but screening has also been linked to overdiagnosis and overtreatment of prostate cancer. As a result, the U.S. Preventive Services Task Force (USPSTF) “found insufficient evidence to recommend PSA screening in 2008 and later recommended against PSA screening in 2012,” Dr. Sharma said.
Several studies subsequently showed a rise in metastatic prostate cancer diagnosis, but the role of PSA screening reductions in those findings was unclear. In 2018, the USPSTF updated its recommendations, stating that men aged 55-69 years should make “an individual decision about whether to be screened after a conversation with their clinician about the potential benefits and harms.”
The task force recommended against PSA screening in men older than 70 years.
The current study “strengthens the epidemiological evidence that reductions in PSA screening may be responsible for at least some of the increase in metastatic prostate cancer diagnoses,” Dr. Sharma said. He added that he and his coauthors support shared decision-making policies to optimize PSA screening approaches to reduce the incidence of metastatic prostate cancer, such as those recommended in the 2018 USPSTF update.
Dr. Sharma disclosed research funding from the Veterans Affairs Health Services Research & Development Fellowship. He and his colleagues had no other disclosures.
Between 2008 and 2016, the mean incidence of prostate cancers that were metastatic at diagnosis increased from 6.4 to 9.0 per 100,000 men. During the same period, the mean percentage of men undergoing PSA screening decreased from 61.8% to 50.5%, Vidit Sharma, MD, reported in a poster session at the 2021 Genitourinary Cancers Symposium (Abstract 228).
A random-effects linear regression model demonstrated that longitudinal reductions across states in PSA screening were indeed associated with increased age-adjusted incidence of metastatic prostate cancer, said Dr. Sharma, the lead author of the study and a health services fellow in urologic oncology at the University of California, Los Angeles.
The regression coefficient per 100,000 men was 14.9, confirming that states with greater declines in screening had greater increases in prostate cancers that were metastatic at diagnosis, he added, noting that, “overall, variation in PSA screening explained 27% of the longitudinal variation in metastatic disease at diagnosis.”
Dr. Sharma and colleagues had reviewed North American Association of Central Cancer Registries data from 2002 to 2016 for each state and extracted survey-weighted PSA screening estimates from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System. The researchers noted wide variations in screening across states, but they said across-the-board declines were evident beginning in 2010, marking a “worrisome consequence that needs attention.”
Robert Dreicer, MD, deputy director of the University of Virginia Cancer Center, Charlottesville, agreed, noting in a press statement that the findings suggest reduced PSA screening may come at the cost of more men presenting with metastatic disease.
“Patients should discuss the risks and benefits associated with PSA screening with their doctor to identify the best approach for them,” Dr. Dreicer said.
PSA screening has been shown to reduce prostate cancer metastasis and mortality, but screening has also been linked to overdiagnosis and overtreatment of prostate cancer. As a result, the U.S. Preventive Services Task Force (USPSTF) “found insufficient evidence to recommend PSA screening in 2008 and later recommended against PSA screening in 2012,” Dr. Sharma said.
Several studies subsequently showed a rise in metastatic prostate cancer diagnosis, but the role of PSA screening reductions in those findings was unclear. In 2018, the USPSTF updated its recommendations, stating that men aged 55-69 years should make “an individual decision about whether to be screened after a conversation with their clinician about the potential benefits and harms.”
The task force recommended against PSA screening in men older than 70 years.
The current study “strengthens the epidemiological evidence that reductions in PSA screening may be responsible for at least some of the increase in metastatic prostate cancer diagnoses,” Dr. Sharma said. He added that he and his coauthors support shared decision-making policies to optimize PSA screening approaches to reduce the incidence of metastatic prostate cancer, such as those recommended in the 2018 USPSTF update.
Dr. Sharma disclosed research funding from the Veterans Affairs Health Services Research & Development Fellowship. He and his colleagues had no other disclosures.
Between 2008 and 2016, the mean incidence of prostate cancers that were metastatic at diagnosis increased from 6.4 to 9.0 per 100,000 men. During the same period, the mean percentage of men undergoing PSA screening decreased from 61.8% to 50.5%, Vidit Sharma, MD, reported in a poster session at the 2021 Genitourinary Cancers Symposium (Abstract 228).
A random-effects linear regression model demonstrated that longitudinal reductions across states in PSA screening were indeed associated with increased age-adjusted incidence of metastatic prostate cancer, said Dr. Sharma, the lead author of the study and a health services fellow in urologic oncology at the University of California, Los Angeles.
The regression coefficient per 100,000 men was 14.9, confirming that states with greater declines in screening had greater increases in prostate cancers that were metastatic at diagnosis, he added, noting that, “overall, variation in PSA screening explained 27% of the longitudinal variation in metastatic disease at diagnosis.”
Dr. Sharma and colleagues had reviewed North American Association of Central Cancer Registries data from 2002 to 2016 for each state and extracted survey-weighted PSA screening estimates from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System. The researchers noted wide variations in screening across states, but they said across-the-board declines were evident beginning in 2010, marking a “worrisome consequence that needs attention.”
Robert Dreicer, MD, deputy director of the University of Virginia Cancer Center, Charlottesville, agreed, noting in a press statement that the findings suggest reduced PSA screening may come at the cost of more men presenting with metastatic disease.
“Patients should discuss the risks and benefits associated with PSA screening with their doctor to identify the best approach for them,” Dr. Dreicer said.
PSA screening has been shown to reduce prostate cancer metastasis and mortality, but screening has also been linked to overdiagnosis and overtreatment of prostate cancer. As a result, the U.S. Preventive Services Task Force (USPSTF) “found insufficient evidence to recommend PSA screening in 2008 and later recommended against PSA screening in 2012,” Dr. Sharma said.
Several studies subsequently showed a rise in metastatic prostate cancer diagnosis, but the role of PSA screening reductions in those findings was unclear. In 2018, the USPSTF updated its recommendations, stating that men aged 55-69 years should make “an individual decision about whether to be screened after a conversation with their clinician about the potential benefits and harms.”
The task force recommended against PSA screening in men older than 70 years.
The current study “strengthens the epidemiological evidence that reductions in PSA screening may be responsible for at least some of the increase in metastatic prostate cancer diagnoses,” Dr. Sharma said. He added that he and his coauthors support shared decision-making policies to optimize PSA screening approaches to reduce the incidence of metastatic prostate cancer, such as those recommended in the 2018 USPSTF update.
Dr. Sharma disclosed research funding from the Veterans Affairs Health Services Research & Development Fellowship. He and his colleagues had no other disclosures.
FROM GUCS 2021
Clozapine still underused in refractory schizophrenia
With the exception of clozapine, the selection of an antipsychotic medication for acute treatment is driven by side effects.
That’s a key pearl of wisdom that Stephen R. Marder, MD, shared during a discussion of key criteria for choosing an antipsychotic for patients with schizophrenia.
“It’s a decision that can have huge consequences, both to an individual’s mental health and their physical health,” Dr. Marder said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “If a patient did well and liked a prior antipsychotic, that’s usually evidence that they’ll respond again. That’s been shown numerous times. Aside from that, the largest consideration is usually adverse effects.”
In a multiple-treatments meta-analysis that compared the efficacy and tolerability of 15 antipsychotic drugs in schizophrenia, researchers found that an overall positive change in symptoms occurred with clozapine, compared with any other drug.
“Clozapine is not just the most effective antipsychotic for patients who are treatment resistant; it’s also the most effective antipsychotic in general populations,” said Dr. Marder, the Daniel X. Freedman Professor of Psychiatry at the Semel Institute for Neuroscience and Human Behavior at the University of California, Los Angeles. “The next most effective antipsychotic is amisulpride, which is not available in the U.S., although there’s a company that’s developing a formulation of amisulpride. After that, the 95% confidence intervals overlap, and the differences are probably related not to their true effectiveness but to other circumstances.”
For example, he continued, risperidone and olanzapine were developed in the 1990s. They were always compared with haloperidol and they tended to work a little bit better. “The drugs developed later on in clinical trials tended to be used in patients who were more treatment resistant,” he said. “Aside from clozapine, the differences in effectiveness are relatively small. But the differences in side effects are large.”
The meta-analysis found that haloperidol stood out as the antipsychotic most likely to cause extrapyramidal side effects. Olanzapine and clozapine stood out as causing the most weight gain, while ziprasidone and lurasidone were less likely to cause weight gain. In addition, risperidone, paliperidone, and haloperidol tended to cause the greatest elevation of prolactin levels, while aripiprazole was found to reduce prolactin levels.
“This becomes an important issue, particularly in young people when one is worried about galactorrhea in women or gynecomastia in men, which sometimes happens with risperidone or haloperidol, and to a lesser extent, sexual dysfunction,” said Dr. Marder, who is also director of the VISN 22 Mental Illness Research, Education, and Clinical Center for the Department of Veterans Affairs. “Sedation is a major consideration for clozapine and chlorpromazine, but less for other antipsychotics.”
When do you know if you’ve selected the right medication for your patient? According to a meta-analysis of 42 studies involving 7,450 patients, improvement tends to occur within the first 2 weeks of treatment. “Which means Dr. Marder said. “This has been consequential because it provides guidance for clinicians to make decisions.”
Symptoms that are likely to improve in the first couple of days include agitation and psychomotor excitement. Improvement in psychotic symptoms typically occurs in the following order: those with thought disorder symptoms tend to develop more organized thinking, those with hallucinations tend to experience a decrease the intensity and frequency of their episodes, and those with well-ingrained delusions “tend to experience fewer misinterpretations,” Dr. Marder said. “They may feel less suspicious and they may talk less about delusions.”
Dr. Marder makes it a point to evaluate the antipsychotic response of patients in 2-3 weeks. “If it’s a partial response, continue a bit longer,” he advised. “It it’s no response, switch. And, of course, if the drug isn’t tolerated well, switch.”
He advised against thinking that patients can easily be categorized as being strong responders or nonresponders. Instead, he favors viewing responsiveness to an antipsychotic along a continuum. “Ten to fifteen percent of patients will fail to remit even at first exposure to an antipsychotic medication, but it’s more common that patients will be partial responders,” Dr. Marder said. “One will have to determine whether that response is adequate or not. There’s also the idea that patients sometimes respond vigorously to an antipsychotic early on. For example, first-episode patients tend to respond very well, and they respond at substantially lower doses. But I set a high criteria that we really want patients on an antipsychotic to respond well, to being in a remission that they can live with, not just to be partially remitted.”
In an analysis of response rates, 244 patients with first-episode schizophrenia moved through two antipsychotic trials, followed by a trial with clozapine. For the first two trials, treatment consisted of risperidone followed by olanzapine, or vice versa. About 75% of patients on either drug showed an initial response. “Among those who did not respond in the first trial but were switched to either drug, the response rate was very low, averaging about 16%,” Dr. Marder said. “In other words, if somebody responds poorly to risperidone, they’re not likely to respond to olanzapine, or vice versa. I think this is true among nearly all of the antipsychotic drugs that are available. Patients tend to have sort of an idiosyncratic ability to respond to a nonclozapine antipsychotic. They may respond to one better than the other, but oftentimes they won’t respond well.” When patients in the trial were switched to clozapine, 75% showed an adequate response.
Based on the study findings and on his own clinical practice, Dr. Marder recommends trying one or two antipsychotics before prescribing clozapine. “If they haven’t responded in a couple of weeks, it’s probably good to change them to another antipsychotic,” he said. “If the patient is responding poorly they should go on to clozapine, which I think is very underutilized.”
In late 2019, the Food and Drug Administration approved lumateperone, a presynaptic D2 partial agonist and a postsynaptic D2 antagonist, for the treatment of schizophrenia in adults. “Its dopamine blockage doesn’t lead to increased dopamine, so it seems to work differently than other antipsychotics,” Dr. Marder said. “It’s effective at lower D2 affinity, which is similar to drugs like clozapine, and it has greater 5 HT2A:D2 antagonism.” It appears to have a relatively benign safety profile, including minimal weight gain, minimal metabolic adverse effects, and minimal extrapyramidal effects. “However, I think the jury’s out,” he added. “There is very little information about head-to-head comparisons between lumateperone and other antipsychotics.”
The new kid on the block is the Alkermes agent AKLS 3831, a combination drug of olanzapine-samidorphan, for the treatment of adults with schizophrenia and adults with bipolar I disorder. In December 2020, the FDA accepted the company’s New Drug Application and set the Prescription Drug User Fee Act target action date of June 1, 2021. Results from a phase 2 trial demonstrated mitigation of olanzapine-induced weight gain with the opioid antagonist samidorphan. “This is not a weight-loss drug,” Dr. Marder said. “It’s just a formulation that causes less weight gain. For patients who do well on olanzapine, putting them on this combination may be helpful in preventing weight gain.”
Dr. Marder disclosed that he has served as a consultant for AbbVie, Allergan, Boehringer Ingelheim, Forum, Genentech, Lundbeck, Neurocrine, Otsuka, Roche, Sunovion, Takeda, Targacept, and Teva. He has also received research support from Boehringer Ingelheim, Neurocrine, and Takeda, and is a section editor for UpToDate.
With the exception of clozapine, the selection of an antipsychotic medication for acute treatment is driven by side effects.
That’s a key pearl of wisdom that Stephen R. Marder, MD, shared during a discussion of key criteria for choosing an antipsychotic for patients with schizophrenia.
“It’s a decision that can have huge consequences, both to an individual’s mental health and their physical health,” Dr. Marder said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “If a patient did well and liked a prior antipsychotic, that’s usually evidence that they’ll respond again. That’s been shown numerous times. Aside from that, the largest consideration is usually adverse effects.”
In a multiple-treatments meta-analysis that compared the efficacy and tolerability of 15 antipsychotic drugs in schizophrenia, researchers found that an overall positive change in symptoms occurred with clozapine, compared with any other drug.
“Clozapine is not just the most effective antipsychotic for patients who are treatment resistant; it’s also the most effective antipsychotic in general populations,” said Dr. Marder, the Daniel X. Freedman Professor of Psychiatry at the Semel Institute for Neuroscience and Human Behavior at the University of California, Los Angeles. “The next most effective antipsychotic is amisulpride, which is not available in the U.S., although there’s a company that’s developing a formulation of amisulpride. After that, the 95% confidence intervals overlap, and the differences are probably related not to their true effectiveness but to other circumstances.”
For example, he continued, risperidone and olanzapine were developed in the 1990s. They were always compared with haloperidol and they tended to work a little bit better. “The drugs developed later on in clinical trials tended to be used in patients who were more treatment resistant,” he said. “Aside from clozapine, the differences in effectiveness are relatively small. But the differences in side effects are large.”
The meta-analysis found that haloperidol stood out as the antipsychotic most likely to cause extrapyramidal side effects. Olanzapine and clozapine stood out as causing the most weight gain, while ziprasidone and lurasidone were less likely to cause weight gain. In addition, risperidone, paliperidone, and haloperidol tended to cause the greatest elevation of prolactin levels, while aripiprazole was found to reduce prolactin levels.
“This becomes an important issue, particularly in young people when one is worried about galactorrhea in women or gynecomastia in men, which sometimes happens with risperidone or haloperidol, and to a lesser extent, sexual dysfunction,” said Dr. Marder, who is also director of the VISN 22 Mental Illness Research, Education, and Clinical Center for the Department of Veterans Affairs. “Sedation is a major consideration for clozapine and chlorpromazine, but less for other antipsychotics.”
When do you know if you’ve selected the right medication for your patient? According to a meta-analysis of 42 studies involving 7,450 patients, improvement tends to occur within the first 2 weeks of treatment. “Which means Dr. Marder said. “This has been consequential because it provides guidance for clinicians to make decisions.”
Symptoms that are likely to improve in the first couple of days include agitation and psychomotor excitement. Improvement in psychotic symptoms typically occurs in the following order: those with thought disorder symptoms tend to develop more organized thinking, those with hallucinations tend to experience a decrease the intensity and frequency of their episodes, and those with well-ingrained delusions “tend to experience fewer misinterpretations,” Dr. Marder said. “They may feel less suspicious and they may talk less about delusions.”
Dr. Marder makes it a point to evaluate the antipsychotic response of patients in 2-3 weeks. “If it’s a partial response, continue a bit longer,” he advised. “It it’s no response, switch. And, of course, if the drug isn’t tolerated well, switch.”
He advised against thinking that patients can easily be categorized as being strong responders or nonresponders. Instead, he favors viewing responsiveness to an antipsychotic along a continuum. “Ten to fifteen percent of patients will fail to remit even at first exposure to an antipsychotic medication, but it’s more common that patients will be partial responders,” Dr. Marder said. “One will have to determine whether that response is adequate or not. There’s also the idea that patients sometimes respond vigorously to an antipsychotic early on. For example, first-episode patients tend to respond very well, and they respond at substantially lower doses. But I set a high criteria that we really want patients on an antipsychotic to respond well, to being in a remission that they can live with, not just to be partially remitted.”
In an analysis of response rates, 244 patients with first-episode schizophrenia moved through two antipsychotic trials, followed by a trial with clozapine. For the first two trials, treatment consisted of risperidone followed by olanzapine, or vice versa. About 75% of patients on either drug showed an initial response. “Among those who did not respond in the first trial but were switched to either drug, the response rate was very low, averaging about 16%,” Dr. Marder said. “In other words, if somebody responds poorly to risperidone, they’re not likely to respond to olanzapine, or vice versa. I think this is true among nearly all of the antipsychotic drugs that are available. Patients tend to have sort of an idiosyncratic ability to respond to a nonclozapine antipsychotic. They may respond to one better than the other, but oftentimes they won’t respond well.” When patients in the trial were switched to clozapine, 75% showed an adequate response.
Based on the study findings and on his own clinical practice, Dr. Marder recommends trying one or two antipsychotics before prescribing clozapine. “If they haven’t responded in a couple of weeks, it’s probably good to change them to another antipsychotic,” he said. “If the patient is responding poorly they should go on to clozapine, which I think is very underutilized.”
In late 2019, the Food and Drug Administration approved lumateperone, a presynaptic D2 partial agonist and a postsynaptic D2 antagonist, for the treatment of schizophrenia in adults. “Its dopamine blockage doesn’t lead to increased dopamine, so it seems to work differently than other antipsychotics,” Dr. Marder said. “It’s effective at lower D2 affinity, which is similar to drugs like clozapine, and it has greater 5 HT2A:D2 antagonism.” It appears to have a relatively benign safety profile, including minimal weight gain, minimal metabolic adverse effects, and minimal extrapyramidal effects. “However, I think the jury’s out,” he added. “There is very little information about head-to-head comparisons between lumateperone and other antipsychotics.”
The new kid on the block is the Alkermes agent AKLS 3831, a combination drug of olanzapine-samidorphan, for the treatment of adults with schizophrenia and adults with bipolar I disorder. In December 2020, the FDA accepted the company’s New Drug Application and set the Prescription Drug User Fee Act target action date of June 1, 2021. Results from a phase 2 trial demonstrated mitigation of olanzapine-induced weight gain with the opioid antagonist samidorphan. “This is not a weight-loss drug,” Dr. Marder said. “It’s just a formulation that causes less weight gain. For patients who do well on olanzapine, putting them on this combination may be helpful in preventing weight gain.”
Dr. Marder disclosed that he has served as a consultant for AbbVie, Allergan, Boehringer Ingelheim, Forum, Genentech, Lundbeck, Neurocrine, Otsuka, Roche, Sunovion, Takeda, Targacept, and Teva. He has also received research support from Boehringer Ingelheim, Neurocrine, and Takeda, and is a section editor for UpToDate.
With the exception of clozapine, the selection of an antipsychotic medication for acute treatment is driven by side effects.
That’s a key pearl of wisdom that Stephen R. Marder, MD, shared during a discussion of key criteria for choosing an antipsychotic for patients with schizophrenia.
“It’s a decision that can have huge consequences, both to an individual’s mental health and their physical health,” Dr. Marder said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “If a patient did well and liked a prior antipsychotic, that’s usually evidence that they’ll respond again. That’s been shown numerous times. Aside from that, the largest consideration is usually adverse effects.”
In a multiple-treatments meta-analysis that compared the efficacy and tolerability of 15 antipsychotic drugs in schizophrenia, researchers found that an overall positive change in symptoms occurred with clozapine, compared with any other drug.
“Clozapine is not just the most effective antipsychotic for patients who are treatment resistant; it’s also the most effective antipsychotic in general populations,” said Dr. Marder, the Daniel X. Freedman Professor of Psychiatry at the Semel Institute for Neuroscience and Human Behavior at the University of California, Los Angeles. “The next most effective antipsychotic is amisulpride, which is not available in the U.S., although there’s a company that’s developing a formulation of amisulpride. After that, the 95% confidence intervals overlap, and the differences are probably related not to their true effectiveness but to other circumstances.”
For example, he continued, risperidone and olanzapine were developed in the 1990s. They were always compared with haloperidol and they tended to work a little bit better. “The drugs developed later on in clinical trials tended to be used in patients who were more treatment resistant,” he said. “Aside from clozapine, the differences in effectiveness are relatively small. But the differences in side effects are large.”
The meta-analysis found that haloperidol stood out as the antipsychotic most likely to cause extrapyramidal side effects. Olanzapine and clozapine stood out as causing the most weight gain, while ziprasidone and lurasidone were less likely to cause weight gain. In addition, risperidone, paliperidone, and haloperidol tended to cause the greatest elevation of prolactin levels, while aripiprazole was found to reduce prolactin levels.
“This becomes an important issue, particularly in young people when one is worried about galactorrhea in women or gynecomastia in men, which sometimes happens with risperidone or haloperidol, and to a lesser extent, sexual dysfunction,” said Dr. Marder, who is also director of the VISN 22 Mental Illness Research, Education, and Clinical Center for the Department of Veterans Affairs. “Sedation is a major consideration for clozapine and chlorpromazine, but less for other antipsychotics.”
When do you know if you’ve selected the right medication for your patient? According to a meta-analysis of 42 studies involving 7,450 patients, improvement tends to occur within the first 2 weeks of treatment. “Which means Dr. Marder said. “This has been consequential because it provides guidance for clinicians to make decisions.”
Symptoms that are likely to improve in the first couple of days include agitation and psychomotor excitement. Improvement in psychotic symptoms typically occurs in the following order: those with thought disorder symptoms tend to develop more organized thinking, those with hallucinations tend to experience a decrease the intensity and frequency of their episodes, and those with well-ingrained delusions “tend to experience fewer misinterpretations,” Dr. Marder said. “They may feel less suspicious and they may talk less about delusions.”
Dr. Marder makes it a point to evaluate the antipsychotic response of patients in 2-3 weeks. “If it’s a partial response, continue a bit longer,” he advised. “It it’s no response, switch. And, of course, if the drug isn’t tolerated well, switch.”
He advised against thinking that patients can easily be categorized as being strong responders or nonresponders. Instead, he favors viewing responsiveness to an antipsychotic along a continuum. “Ten to fifteen percent of patients will fail to remit even at first exposure to an antipsychotic medication, but it’s more common that patients will be partial responders,” Dr. Marder said. “One will have to determine whether that response is adequate or not. There’s also the idea that patients sometimes respond vigorously to an antipsychotic early on. For example, first-episode patients tend to respond very well, and they respond at substantially lower doses. But I set a high criteria that we really want patients on an antipsychotic to respond well, to being in a remission that they can live with, not just to be partially remitted.”
In an analysis of response rates, 244 patients with first-episode schizophrenia moved through two antipsychotic trials, followed by a trial with clozapine. For the first two trials, treatment consisted of risperidone followed by olanzapine, or vice versa. About 75% of patients on either drug showed an initial response. “Among those who did not respond in the first trial but were switched to either drug, the response rate was very low, averaging about 16%,” Dr. Marder said. “In other words, if somebody responds poorly to risperidone, they’re not likely to respond to olanzapine, or vice versa. I think this is true among nearly all of the antipsychotic drugs that are available. Patients tend to have sort of an idiosyncratic ability to respond to a nonclozapine antipsychotic. They may respond to one better than the other, but oftentimes they won’t respond well.” When patients in the trial were switched to clozapine, 75% showed an adequate response.
Based on the study findings and on his own clinical practice, Dr. Marder recommends trying one or two antipsychotics before prescribing clozapine. “If they haven’t responded in a couple of weeks, it’s probably good to change them to another antipsychotic,” he said. “If the patient is responding poorly they should go on to clozapine, which I think is very underutilized.”
In late 2019, the Food and Drug Administration approved lumateperone, a presynaptic D2 partial agonist and a postsynaptic D2 antagonist, for the treatment of schizophrenia in adults. “Its dopamine blockage doesn’t lead to increased dopamine, so it seems to work differently than other antipsychotics,” Dr. Marder said. “It’s effective at lower D2 affinity, which is similar to drugs like clozapine, and it has greater 5 HT2A:D2 antagonism.” It appears to have a relatively benign safety profile, including minimal weight gain, minimal metabolic adverse effects, and minimal extrapyramidal effects. “However, I think the jury’s out,” he added. “There is very little information about head-to-head comparisons between lumateperone and other antipsychotics.”
The new kid on the block is the Alkermes agent AKLS 3831, a combination drug of olanzapine-samidorphan, for the treatment of adults with schizophrenia and adults with bipolar I disorder. In December 2020, the FDA accepted the company’s New Drug Application and set the Prescription Drug User Fee Act target action date of June 1, 2021. Results from a phase 2 trial demonstrated mitigation of olanzapine-induced weight gain with the opioid antagonist samidorphan. “This is not a weight-loss drug,” Dr. Marder said. “It’s just a formulation that causes less weight gain. For patients who do well on olanzapine, putting them on this combination may be helpful in preventing weight gain.”
Dr. Marder disclosed that he has served as a consultant for AbbVie, Allergan, Boehringer Ingelheim, Forum, Genentech, Lundbeck, Neurocrine, Otsuka, Roche, Sunovion, Takeda, Targacept, and Teva. He has also received research support from Boehringer Ingelheim, Neurocrine, and Takeda, and is a section editor for UpToDate.
FROM NPA 2021
Steroid and immunoglobulin standard of care for MIS-C
The combination of methylprednisolone and intravenous immunoglobulins works better than intravenous immunoglobulins alone for multisystem inflammatory syndrome in children (MIS-C), researchers say.
“I’m not sure it’s the best treatment because we have not studied every possible treatment,” François Angoulvant, MD, PhD, told this news organization, “but right now, it’s the standard of care.”
Dr. Angoulvant, a professor of pediatrics at University of Paris, and colleagues published a comparison of the two treatments in the Journal of the American Medical Association.
A small percentage of children infected with SARS-CoV-2 develop MIS-C about 2 to 4 weeks later. It is considered a separate disease entity from COVID-19 and is associated with persistent fever, digestive symptoms, rash, bilateral nonpurulent conjunctivitis, mucocutaneous inflammation signs, and frequent cardiovascular involvement. In more than 60% of cases, it leads to hemodynamic failure, with acute cardiac dysfunction.
Because MIS-C resembles Kawasaki disease, clinicians modeled their treatment on that condition and started with immunoglobulins alone, Dr. Angoulvant said.
Based on expert opinion, the National Health Service in the United Kingdom published a consensus statement in Sept. listing immunoglobulins alone as the first-line treatment.
But anecdotal reports have emerged that combining the immunoglobulins with a corticosteroid worked better. To investigate this possibility, Dr. Angoulvant and colleagues analyzed records of MIS-C cases in France, where physicians are required to report all suspected cases of MIS-C to the French National Public Health Agency.
Among the 181 cases they scrutinized, 111 fulfilled the World Health Organization criteria for MIS-C. Of these, the researchers were able to match 64 patients who had received immunoglobulins alone with 32 who had received the combined therapy and could be matched using propensity scores.
The researchers defined treatment failure as persistence of fever for 2 days after the start of therapy or recurrence of fever within a week. By this measure, the combination treatment failed in only 9% of cases while immunoglobulins alone failed in 38% of cases. The difference was statistically significant (P = .008). Most of those for whom these treatments failed received second-line treatments such as steroids or biological agents.
Patients treated with the combination therapy also had a lower risk of secondary acute left ventricular dysfunction (odds ratio, 0.20; 95% confidence interval, 0.06-0.66) and a lower risk of needing hemodynamic support (OR, 0.21; 95% CI, 0.06-0.76).
Those receiving the combination therapy spent a mean of 4 days in the pediatric intensive care unit compared with 6 days for those receiving immunoglobulins alone. (Difference in days, −2.4; 95% CI, −4.0 to −0.7; P = .005).
There are few drawbacks to the combination approach, Dr. Angoulvant said, as the side effects of corticosteroids are generally not severe and they can be anticipated because this class of medications has been used for many years.
The study raises the question of whether corticosteroids might work as well by themselves, but it could not be answered with this database as no one is using that approach in France, Dr. Angoulvant said. “I hope other teams around the world could bring us the answer.”
In the United States, most physicians appear to already be using the combination therapy, said David Teachey, MD, an associate professor of pediatrics at the Children’s Hospital of Philadelphia and the University of Pennsylvania, Philadelphia.
The reduction in time in pediatric intensive care and the reduced risk of cardiac dysfunction are important findings, he said.
This retrospective study falls short of the evidence provided by a randomized clinical trial, Dr. Teachey noted. But he acknowledged that few families would agree to participate in such a trial as they would have to take a chance that the sick children would receive a less effective therapy than what they would otherwise get. “It’s hard to [talk] about a therapy reduction,” he told this news organization.
Given that impediment, he agreed with Dr. Angoulvant that the current study and others like it may provide the best data available pointing to a treatment approach for MIS-C.
The study received an unrestricted grant from Pfizer. The French COVID-19 Paediatric Inflammation Consortium received an unrestricted grant from the Square Foundation (Grandir–Fonds de Solidarité pour L’Enfance). Dr. Angoulvant and Dr. Teachey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The combination of methylprednisolone and intravenous immunoglobulins works better than intravenous immunoglobulins alone for multisystem inflammatory syndrome in children (MIS-C), researchers say.
“I’m not sure it’s the best treatment because we have not studied every possible treatment,” François Angoulvant, MD, PhD, told this news organization, “but right now, it’s the standard of care.”
Dr. Angoulvant, a professor of pediatrics at University of Paris, and colleagues published a comparison of the two treatments in the Journal of the American Medical Association.
A small percentage of children infected with SARS-CoV-2 develop MIS-C about 2 to 4 weeks later. It is considered a separate disease entity from COVID-19 and is associated with persistent fever, digestive symptoms, rash, bilateral nonpurulent conjunctivitis, mucocutaneous inflammation signs, and frequent cardiovascular involvement. In more than 60% of cases, it leads to hemodynamic failure, with acute cardiac dysfunction.
Because MIS-C resembles Kawasaki disease, clinicians modeled their treatment on that condition and started with immunoglobulins alone, Dr. Angoulvant said.
Based on expert opinion, the National Health Service in the United Kingdom published a consensus statement in Sept. listing immunoglobulins alone as the first-line treatment.
But anecdotal reports have emerged that combining the immunoglobulins with a corticosteroid worked better. To investigate this possibility, Dr. Angoulvant and colleagues analyzed records of MIS-C cases in France, where physicians are required to report all suspected cases of MIS-C to the French National Public Health Agency.
Among the 181 cases they scrutinized, 111 fulfilled the World Health Organization criteria for MIS-C. Of these, the researchers were able to match 64 patients who had received immunoglobulins alone with 32 who had received the combined therapy and could be matched using propensity scores.
The researchers defined treatment failure as persistence of fever for 2 days after the start of therapy or recurrence of fever within a week. By this measure, the combination treatment failed in only 9% of cases while immunoglobulins alone failed in 38% of cases. The difference was statistically significant (P = .008). Most of those for whom these treatments failed received second-line treatments such as steroids or biological agents.
Patients treated with the combination therapy also had a lower risk of secondary acute left ventricular dysfunction (odds ratio, 0.20; 95% confidence interval, 0.06-0.66) and a lower risk of needing hemodynamic support (OR, 0.21; 95% CI, 0.06-0.76).
Those receiving the combination therapy spent a mean of 4 days in the pediatric intensive care unit compared with 6 days for those receiving immunoglobulins alone. (Difference in days, −2.4; 95% CI, −4.0 to −0.7; P = .005).
There are few drawbacks to the combination approach, Dr. Angoulvant said, as the side effects of corticosteroids are generally not severe and they can be anticipated because this class of medications has been used for many years.
The study raises the question of whether corticosteroids might work as well by themselves, but it could not be answered with this database as no one is using that approach in France, Dr. Angoulvant said. “I hope other teams around the world could bring us the answer.”
In the United States, most physicians appear to already be using the combination therapy, said David Teachey, MD, an associate professor of pediatrics at the Children’s Hospital of Philadelphia and the University of Pennsylvania, Philadelphia.
The reduction in time in pediatric intensive care and the reduced risk of cardiac dysfunction are important findings, he said.
This retrospective study falls short of the evidence provided by a randomized clinical trial, Dr. Teachey noted. But he acknowledged that few families would agree to participate in such a trial as they would have to take a chance that the sick children would receive a less effective therapy than what they would otherwise get. “It’s hard to [talk] about a therapy reduction,” he told this news organization.
Given that impediment, he agreed with Dr. Angoulvant that the current study and others like it may provide the best data available pointing to a treatment approach for MIS-C.
The study received an unrestricted grant from Pfizer. The French COVID-19 Paediatric Inflammation Consortium received an unrestricted grant from the Square Foundation (Grandir–Fonds de Solidarité pour L’Enfance). Dr. Angoulvant and Dr. Teachey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The combination of methylprednisolone and intravenous immunoglobulins works better than intravenous immunoglobulins alone for multisystem inflammatory syndrome in children (MIS-C), researchers say.
“I’m not sure it’s the best treatment because we have not studied every possible treatment,” François Angoulvant, MD, PhD, told this news organization, “but right now, it’s the standard of care.”
Dr. Angoulvant, a professor of pediatrics at University of Paris, and colleagues published a comparison of the two treatments in the Journal of the American Medical Association.
A small percentage of children infected with SARS-CoV-2 develop MIS-C about 2 to 4 weeks later. It is considered a separate disease entity from COVID-19 and is associated with persistent fever, digestive symptoms, rash, bilateral nonpurulent conjunctivitis, mucocutaneous inflammation signs, and frequent cardiovascular involvement. In more than 60% of cases, it leads to hemodynamic failure, with acute cardiac dysfunction.
Because MIS-C resembles Kawasaki disease, clinicians modeled their treatment on that condition and started with immunoglobulins alone, Dr. Angoulvant said.
Based on expert opinion, the National Health Service in the United Kingdom published a consensus statement in Sept. listing immunoglobulins alone as the first-line treatment.
But anecdotal reports have emerged that combining the immunoglobulins with a corticosteroid worked better. To investigate this possibility, Dr. Angoulvant and colleagues analyzed records of MIS-C cases in France, where physicians are required to report all suspected cases of MIS-C to the French National Public Health Agency.
Among the 181 cases they scrutinized, 111 fulfilled the World Health Organization criteria for MIS-C. Of these, the researchers were able to match 64 patients who had received immunoglobulins alone with 32 who had received the combined therapy and could be matched using propensity scores.
The researchers defined treatment failure as persistence of fever for 2 days after the start of therapy or recurrence of fever within a week. By this measure, the combination treatment failed in only 9% of cases while immunoglobulins alone failed in 38% of cases. The difference was statistically significant (P = .008). Most of those for whom these treatments failed received second-line treatments such as steroids or biological agents.
Patients treated with the combination therapy also had a lower risk of secondary acute left ventricular dysfunction (odds ratio, 0.20; 95% confidence interval, 0.06-0.66) and a lower risk of needing hemodynamic support (OR, 0.21; 95% CI, 0.06-0.76).
Those receiving the combination therapy spent a mean of 4 days in the pediatric intensive care unit compared with 6 days for those receiving immunoglobulins alone. (Difference in days, −2.4; 95% CI, −4.0 to −0.7; P = .005).
There are few drawbacks to the combination approach, Dr. Angoulvant said, as the side effects of corticosteroids are generally not severe and they can be anticipated because this class of medications has been used for many years.
The study raises the question of whether corticosteroids might work as well by themselves, but it could not be answered with this database as no one is using that approach in France, Dr. Angoulvant said. “I hope other teams around the world could bring us the answer.”
In the United States, most physicians appear to already be using the combination therapy, said David Teachey, MD, an associate professor of pediatrics at the Children’s Hospital of Philadelphia and the University of Pennsylvania, Philadelphia.
The reduction in time in pediatric intensive care and the reduced risk of cardiac dysfunction are important findings, he said.
This retrospective study falls short of the evidence provided by a randomized clinical trial, Dr. Teachey noted. But he acknowledged that few families would agree to participate in such a trial as they would have to take a chance that the sick children would receive a less effective therapy than what they would otherwise get. “It’s hard to [talk] about a therapy reduction,” he told this news organization.
Given that impediment, he agreed with Dr. Angoulvant that the current study and others like it may provide the best data available pointing to a treatment approach for MIS-C.
The study received an unrestricted grant from Pfizer. The French COVID-19 Paediatric Inflammation Consortium received an unrestricted grant from the Square Foundation (Grandir–Fonds de Solidarité pour L’Enfance). Dr. Angoulvant and Dr. Teachey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Stump Pemphigoid Demonstrating Circulating Anti–BP180 and BP230 Antibodies
To the Editor:
Bullous pemphigoid (BP) is a rare complication of lower limb amputation. Termed stump pemphigoid, it previously was described as a late complication arising on the stumps of leg amputees and tends to remain localized. We describe a case of stump pemphigoid presenting with an urticarial prodromal phase without generalized progression, confirmed by serum assay for circulating anti–basement membrane antibodies.
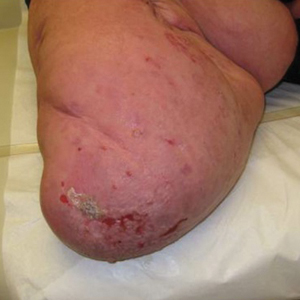
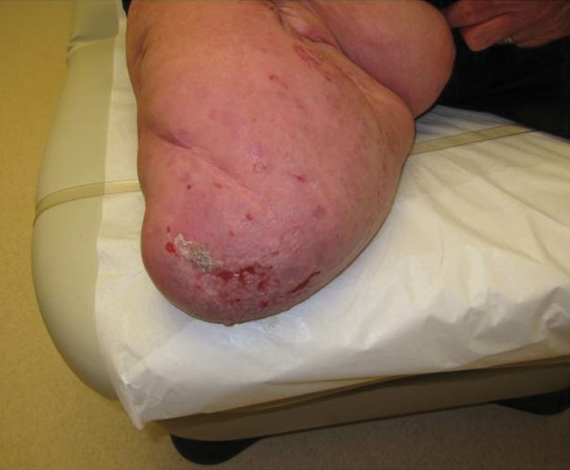
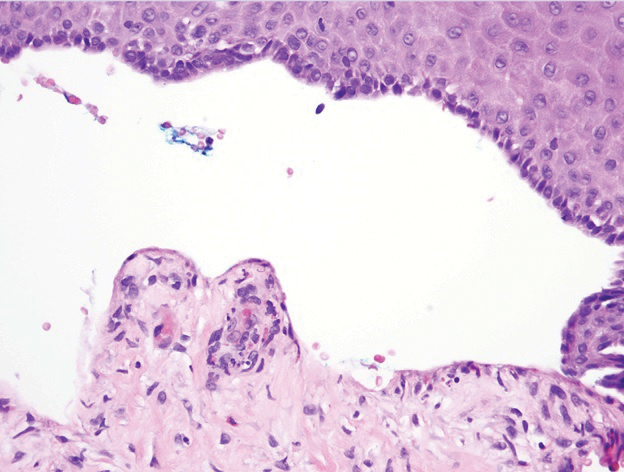
A 62-year-old man with a history of a right above-knee amputation initially presented with erythema as well as coalescing erosions and ulcers with fluid-filled vesicles and bullae on the amputation stump (Figure 1). The amputation was performed 15 years prior after a motorcycle accident. A skin biopsy of a vesicle on the amputation stump revealed subepidermal and focal intraepidermal clefting with hemorrhage and rare inflammatory cells composed of neutrophils and eosinophils (Figure 2). A tissue direct immunofluorescence test demonstrated linear C3 and IgG deposition along the dermoepidermal junction. Serum enzyme-linked immunosorbent assay (ELISA) demonstrated an anti-BP180 IgG of 50.90 U/mL and anti-BP230 IgG of 129.40 U/mL (reference range, <9.00 U/mL [for both]).
Topical clobetasol led to only modest improvement of blistering on the stump. Minor frictional trauma related to his leg prosthesis continued to trigger new vesicles and bullae on the stump. Oral prednisone 0.5 mg/kg daily was administered and tapered slowly over the course of 6 months. He also received oral niacinamide and doxycycline. He was completely clear after 3 weeks of initiating treatment and remained clear while prednisone was slowly tapered. One month after stopping prednisone he had recurrence of blisters on the stump only after he resumed wearing his prosthesis. Mycophenolate mofetil was started at a dosage of 1 g twice daily while he refrained from wearing the prosthesis. After 3 months he was able to wear the prosthesis without developing blisters. Two years after the initial presentation, repeat serum ELISA demonstrated normalization of the anti-BP180 IgG and anti-BP230 IgG titers. Thirty months after the initial presentation, mycophenolate mofetil was tapered and discontinued. The patient remained blisterfree and continued to wear his leg prosthesis without further blistering.
Amputees experience a high rate of skin complications on their stump,1 including friction blisters, shear injury, contact dermatitis, infections, and autoimmune blistering disorders (ie, BP, epidermolysis bullosa acquisita). The etiology of stump pemphigoid is not entirely understood but could be related to exposure of structural components of the hemidesmosome (eg, BP230, BP180), leading to autoantibody production as a consequence of either the underlying limb injury or from recurrent trauma related to limb prosthetics.2
Two previously reported cases of stump pemphigoid demonstrated a positive direct immunofluorescence antibody test.3,4 Another case demonstrated the presence of circulating IgG antibodies on indirect immunofluorescence to salt-split skin.5 We report a case of stump pemphigoid confirmed by presence of circulating anti–basement membrane antibodies on ELISA, supporting its use in the diagnostic workup and monitoring treatment response.
- Colgecen E, Korkmaz M, Ozyurt K, et al. A clinical evaluation of skin disorders of lower limb amputation sites. Int J Dermatol. 2016;55:468-472.
- Lo Schiavo A, Ruocco E, Brancaccio G, et al. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol. 2013;31:391-399.
- Reilly GD, Boulton AJ, Harrington CI. Stump pemphigoid: a new complication of the amputee. Br Med J (Clin Res Ed). 1983;287:875-876.
- de Jong MC, Kardaun SH, Tupker RA, et al. Immunomapping in localized bullous pemphigoid. Hautarzt. 1989;40:226-230.
- Brodell RT, Korman NJ. Stump pemphigoid. Cutis. 1996;57:245-246.
To the Editor:
Bullous pemphigoid (BP) is a rare complication of lower limb amputation. Termed stump pemphigoid, it previously was described as a late complication arising on the stumps of leg amputees and tends to remain localized. We describe a case of stump pemphigoid presenting with an urticarial prodromal phase without generalized progression, confirmed by serum assay for circulating anti–basement membrane antibodies.
A 62-year-old man with a history of a right above-knee amputation initially presented with erythema as well as coalescing erosions and ulcers with fluid-filled vesicles and bullae on the amputation stump (Figure 1). The amputation was performed 15 years prior after a motorcycle accident. A skin biopsy of a vesicle on the amputation stump revealed subepidermal and focal intraepidermal clefting with hemorrhage and rare inflammatory cells composed of neutrophils and eosinophils (Figure 2). A tissue direct immunofluorescence test demonstrated linear C3 and IgG deposition along the dermoepidermal junction. Serum enzyme-linked immunosorbent assay (ELISA) demonstrated an anti-BP180 IgG of 50.90 U/mL and anti-BP230 IgG of 129.40 U/mL (reference range, <9.00 U/mL [for both]).


Topical clobetasol led to only modest improvement of blistering on the stump. Minor frictional trauma related to his leg prosthesis continued to trigger new vesicles and bullae on the stump. Oral prednisone 0.5 mg/kg daily was administered and tapered slowly over the course of 6 months. He also received oral niacinamide and doxycycline. He was completely clear after 3 weeks of initiating treatment and remained clear while prednisone was slowly tapered. One month after stopping prednisone he had recurrence of blisters on the stump only after he resumed wearing his prosthesis. Mycophenolate mofetil was started at a dosage of 1 g twice daily while he refrained from wearing the prosthesis. After 3 months he was able to wear the prosthesis without developing blisters. Two years after the initial presentation, repeat serum ELISA demonstrated normalization of the anti-BP180 IgG and anti-BP230 IgG titers. Thirty months after the initial presentation, mycophenolate mofetil was tapered and discontinued. The patient remained blisterfree and continued to wear his leg prosthesis without further blistering.
Amputees experience a high rate of skin complications on their stump,1 including friction blisters, shear injury, contact dermatitis, infections, and autoimmune blistering disorders (ie, BP, epidermolysis bullosa acquisita). The etiology of stump pemphigoid is not entirely understood but could be related to exposure of structural components of the hemidesmosome (eg, BP230, BP180), leading to autoantibody production as a consequence of either the underlying limb injury or from recurrent trauma related to limb prosthetics.2
Two previously reported cases of stump pemphigoid demonstrated a positive direct immunofluorescence antibody test.3,4 Another case demonstrated the presence of circulating IgG antibodies on indirect immunofluorescence to salt-split skin.5 We report a case of stump pemphigoid confirmed by presence of circulating anti–basement membrane antibodies on ELISA, supporting its use in the diagnostic workup and monitoring treatment response.
To the Editor:
Bullous pemphigoid (BP) is a rare complication of lower limb amputation. Termed stump pemphigoid, it previously was described as a late complication arising on the stumps of leg amputees and tends to remain localized. We describe a case of stump pemphigoid presenting with an urticarial prodromal phase without generalized progression, confirmed by serum assay for circulating anti–basement membrane antibodies.
A 62-year-old man with a history of a right above-knee amputation initially presented with erythema as well as coalescing erosions and ulcers with fluid-filled vesicles and bullae on the amputation stump (Figure 1). The amputation was performed 15 years prior after a motorcycle accident. A skin biopsy of a vesicle on the amputation stump revealed subepidermal and focal intraepidermal clefting with hemorrhage and rare inflammatory cells composed of neutrophils and eosinophils (Figure 2). A tissue direct immunofluorescence test demonstrated linear C3 and IgG deposition along the dermoepidermal junction. Serum enzyme-linked immunosorbent assay (ELISA) demonstrated an anti-BP180 IgG of 50.90 U/mL and anti-BP230 IgG of 129.40 U/mL (reference range, <9.00 U/mL [for both]).


Topical clobetasol led to only modest improvement of blistering on the stump. Minor frictional trauma related to his leg prosthesis continued to trigger new vesicles and bullae on the stump. Oral prednisone 0.5 mg/kg daily was administered and tapered slowly over the course of 6 months. He also received oral niacinamide and doxycycline. He was completely clear after 3 weeks of initiating treatment and remained clear while prednisone was slowly tapered. One month after stopping prednisone he had recurrence of blisters on the stump only after he resumed wearing his prosthesis. Mycophenolate mofetil was started at a dosage of 1 g twice daily while he refrained from wearing the prosthesis. After 3 months he was able to wear the prosthesis without developing blisters. Two years after the initial presentation, repeat serum ELISA demonstrated normalization of the anti-BP180 IgG and anti-BP230 IgG titers. Thirty months after the initial presentation, mycophenolate mofetil was tapered and discontinued. The patient remained blisterfree and continued to wear his leg prosthesis without further blistering.
Amputees experience a high rate of skin complications on their stump,1 including friction blisters, shear injury, contact dermatitis, infections, and autoimmune blistering disorders (ie, BP, epidermolysis bullosa acquisita). The etiology of stump pemphigoid is not entirely understood but could be related to exposure of structural components of the hemidesmosome (eg, BP230, BP180), leading to autoantibody production as a consequence of either the underlying limb injury or from recurrent trauma related to limb prosthetics.2
Two previously reported cases of stump pemphigoid demonstrated a positive direct immunofluorescence antibody test.3,4 Another case demonstrated the presence of circulating IgG antibodies on indirect immunofluorescence to salt-split skin.5 We report a case of stump pemphigoid confirmed by presence of circulating anti–basement membrane antibodies on ELISA, supporting its use in the diagnostic workup and monitoring treatment response.
- Colgecen E, Korkmaz M, Ozyurt K, et al. A clinical evaluation of skin disorders of lower limb amputation sites. Int J Dermatol. 2016;55:468-472.
- Lo Schiavo A, Ruocco E, Brancaccio G, et al. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol. 2013;31:391-399.
- Reilly GD, Boulton AJ, Harrington CI. Stump pemphigoid: a new complication of the amputee. Br Med J (Clin Res Ed). 1983;287:875-876.
- de Jong MC, Kardaun SH, Tupker RA, et al. Immunomapping in localized bullous pemphigoid. Hautarzt. 1989;40:226-230.
- Brodell RT, Korman NJ. Stump pemphigoid. Cutis. 1996;57:245-246.
- Colgecen E, Korkmaz M, Ozyurt K, et al. A clinical evaluation of skin disorders of lower limb amputation sites. Int J Dermatol. 2016;55:468-472.
- Lo Schiavo A, Ruocco E, Brancaccio G, et al. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol. 2013;31:391-399.
- Reilly GD, Boulton AJ, Harrington CI. Stump pemphigoid: a new complication of the amputee. Br Med J (Clin Res Ed). 1983;287:875-876.
- de Jong MC, Kardaun SH, Tupker RA, et al. Immunomapping in localized bullous pemphigoid. Hautarzt. 1989;40:226-230.
- Brodell RT, Korman NJ. Stump pemphigoid. Cutis. 1996;57:245-246.
Practice Points
- Bullous pemphigoid (BP) can mimic friction blisters and should be considered in amputees who present with vesicles and bullae on their amputation stump.
- Circulating anti–basement membrane antibodies BP230 and BP180 IgG may aid in diagnosis when skin biopsy results are equivocal and also may be helpful in gauging treatment response.
Scrub Typhus in Chile
To the Editor:
Scrub typhus (ST) is an infection caused by Orientia tsutsugamushi (genus Rickettsia), which is transmitted by the larvae of trombiculid mites, commonly called chiggers. The disease mainly has been described in Asia in an area known as the Tsutsugamushi Triangle, delineated by Pakistan, eastern Russia, and northern Australia. Although this classic distribution remains, recent reports have documented 1 case in the Arabian Peninsula1 and more than 16 cases in southern Chile.2-4 The first case in Chile was published in 2011 from Chiloé Island.2 To date, no other cases have been reported in the Americas.1-6
We describe a new case of ST from Chiloé Island and compare it to the first case reported in Chile in 2011.2 Both patients showed the typical clinical manifestation, but because ST has become an increasingly suspected disease in southern regions of Chile, new cases are now easily diagnosed. This infection is diagnosed mainly by skin lesions; therefore, dermatologists should be aware of this diagnosis when presented with a febrile rash.
A 67-year-old man from the city of Punta Arenas presented to the emergency department with a dark necrotic lesion on the right foot of 1 week’s duration. The patient later developed a generalized pruritic rash and fever. He also reported muscle pain, headache, cough, night sweats, and odynophagia. He reported recent travel to a rural area in the northern part of Chiloé Island, where he came into contact with firewood and participated in outdoor activities. He had no other relevant medical history.
Physical examination revealed a temperature of 38 °C and a macular rash, with some papules distributed mainly on the face, trunk, and proximal extremities (Figure 1). He had a necrotic eschar on the dorsum of the right foot, with an erythematous halo (tache noire)(Figure 2).
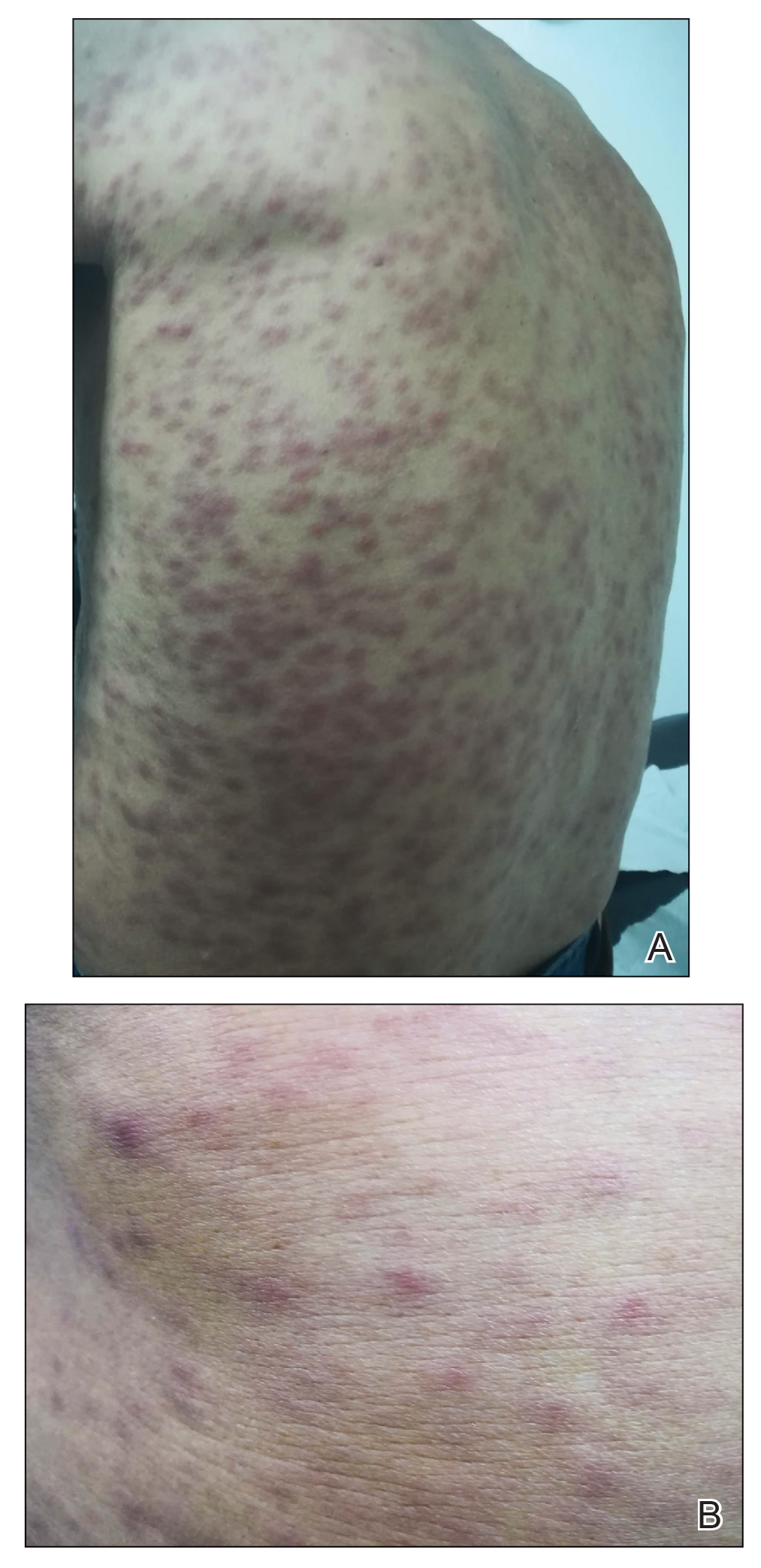
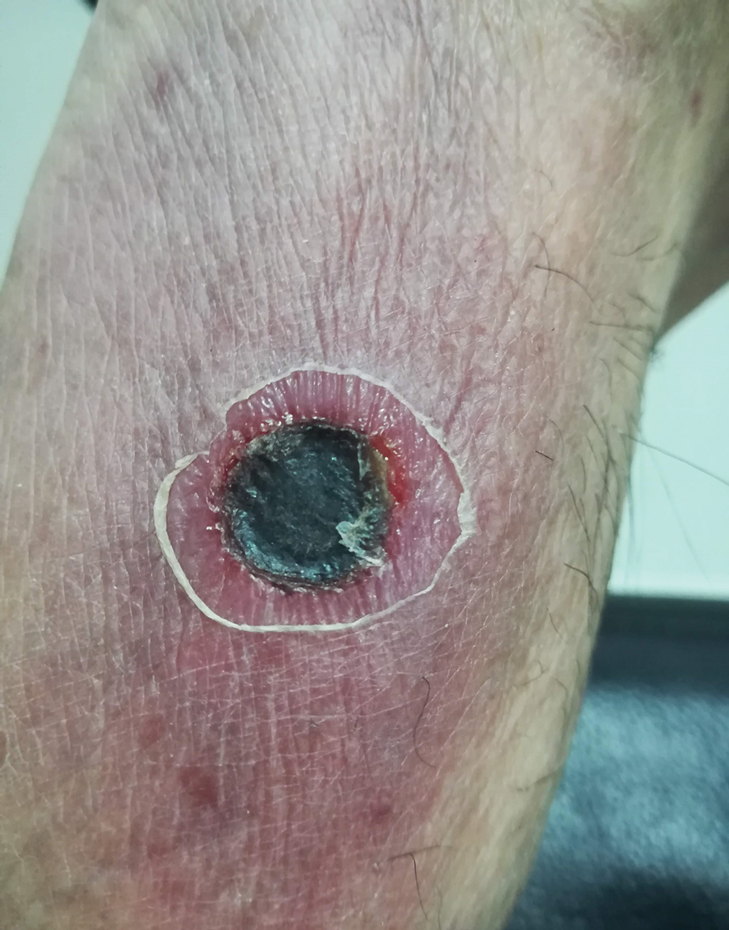
A complete blood cell count, urinalysis, and tests of hepatic and renal function were normal. C-reactive protein was elevated 18 times the normal value. Because of high awareness of ST in the region, eschar samples were taken and submitted for serologic testing and polymerase chain reaction (PCR) targeting the 16S rRNA Orientia gene. Empirical treatment with oral doxycycline 100 mg twice daily was started. Polymerase chain reaction analysis showed the presence of Orientia species, confirming the diagnosis of ST. The rash and eschar diminished considerably after 7 days of antibiotic treatment.
Scrub typhus is a high-impact disease in Asia, described mainly in an area known as the Tsutsugamushi Triangle. Recent reports show important epidemiologic changes in the distribution of the disease, with new published reports of cases outside this endemic area—1 in the Arabian peninsula1 and more than 16 in southern Chile.2-4
The disease begins with a painless, erythematous, and usually unnoticed papule at the site of the bite. After 48 to 72 hours, the papule changes to a necrotic form (tache noire), surrounded by a red halo that often is small, similar to a cigarette burn. This lesion is described in 20% to 90% of infected patients in different series.7 Two or 3 days later (1 to 3 weeks after exposure), high fever suddenly develops. Along with fever, a maculopapular rash distributed centrifugally develops, without compromise of the palms or soles. Patients frequently report headache and night sweating. Sometimes, ST is accompanied by muscle or joint pain, red eye, cough, and abdominal pain. Hearing loss and altered mental status less frequently have been reported.5,8
Common laboratory tests can be of use in diagnosis. An elevated C-reactive protein level and a slight to moderate increase in hepatic transaminases should be expected. Thrombocytopenia, leukopenia, and elevation of the lactate dehydrogenase level less frequently are present.5,9
Our case de1monstrated a typical presentation. The patient developed a febrile syndrome with a generalized rash and a tache noire–type eschar associated with muscle pain, headache, cough, night sweats, and odynophagia. Because of epidemiologic changes in the area, the familiar clinical findings, and laboratory confirmation, histologic studies were unnecessary. In cases in which the diagnosis is not evident, skin biopsy could be useful, as in the first case reported in Chile.2
In that first case, the patient initially was hospitalized because of a febrile syndrome; eventually, a necrotic eschar was noticed on his leg. He had been staying on Chiloé Island and reported being bitten by leeches on multiple occasions. Laboratory findings revealed only slightly raised levels of hepatic transaminases and alkaline phosphatase. After a more precise dermatologic evaluation, the eschar of a tache noire, combined with other clinical and laboratory findings, raised suspicion of ST. Because this entity had never been described in Chile, biopsy of the eschar was taken to consider other entities in the differential diagnosis. Biopsy showed necrotizing leukocytoclastic vasculitis in the dermis and subcutaneous tissue, perivascular inflammatory infiltrates comprising lymphocytes and macrophages, and rickettsial microorganisms inside endothelial cells under electron microscopic examination. The specimen was tested for the 16S ribosomal RNA Orientia gene; its presence confirmed the diagnosis.2
Classically, histology from the eschar shows signs of vasculitis and rickettsial microorganisms inside endothelial cells on electron microscopy.2,10 More recent publications describe important necrotic changes within keratinocytes as well as an inflammatory infiltrate comprising antigen-presenting cells, monocytes, macrophages, and dendritic cells. Using high-resolution thin sections with confocal laser scanning microscopy and staining of specific monoclonal antibodies against 56 kDa type-specific surface antigens, the bacteria were found inside antigen-presenting cells, many of them located perivascularly or passing through the endothelium.11
The causal agent in Asia is O tsutsugamushi, an obligate intracellular bacterium (genus Rickettsia). Orientia species are transmitted by larvae of trombiculid mites, commonly called chiggers. The reservoir is believed to be the same as with chiggers, in which some vertebrates become infected and trombiculid mites feed on them.12 Recent studies of Chilean cases have revealed the presence of a novel Orientia species, Candidatus Orientia chiloensis and its vector, trombiculid mites from the Herpetacarus species, Quadraseta species, and Paratrombicula species genera.13,14
A high seroprevalence of Orientia species in dogs was reported in the main cities of Chiloé Island. Rates were higher in rural settings and older dogs. Of 202 specimens, 21.3% were positive for IgG against Orientia species.15
In Chile, most cases of ST came from Chiloé Island; some reports of cases from continental Chilean regions have been published.6 Most cases have occurred in the context of activities that brought the patients in contact with plants and firewood in rural areas during the summer.3-6
The diagnosis of ST is eminently clinical, based on the triad of fever, macular or papular rash, and an inoculation necrotic eschar. The diagnosis is supported by epidemiologic facts and fast recovery after treatment is initiated.16 Although the diagnosis can be established based on a quick recovery in endemic countries, in areas such as Chile where incidence and distribution are not completely known, it is better to confirm the diagnosis with laboratory tests without delaying treatment. Several testing options exist, including serologic techniques (immunofluorescence or enzyme-linked immunosorbent assay), culture, and detection of the genetic material of Orientia species by PCR. Usually, IgM titers initially are negative, and IgG testing requires paired samples (acute and convalescent) to demonstrate seroconversion and therefore acute infection.17 Because culture requires a highly specialized laboratory, it is not frequently used. Polymerase chain reaction is recognized as the best confirmation method due to its high sensitivity and because it remains positive for a few days after treatment has been initiated. The specimen of choice is the eschar because of its high bacterial load. The base of the scar and the buffy coat are useful specimens when the eschar is unavailable.5,17-19
Due to potential complications of ST, empirical treatment with an antibiotic should be started based on clinical facts and never delayed because of diagnostic tests.18 Classically, ST is treated with a member of the tetracycline family, such as doxycycline, which provides a cure rate of 63% to 100% in ST.5
A 2017 systematic review of treatment options for this infection examined 11 studies from Southeast Asia, China, and South Korea (N=957).16 The review mainly compared doxycycline with azithromycin, chloramphenicol, and tetracycline. No significant difference in cure rate was noted in comparing doxycycline with any of the other 3 antibiotics; most of the studies examined were characterized by a moderate level of evidence. Regarding adverse effects, doxycycline showed a few more cases of gastrointestinal intolerance, and in 2 of 4 studies with chloramphenicol, patients presented with leukopenia.16 Several studies compared standard treatment (doxycycline) with rifampicin, telithromycin, erythromycin, and levofloxacin individually; similar cure rates were noted between doxycycline and each of those 4 agents.
Therapeutic failure in ST has been reported in several cases with the use of levofloxacin.20 Evidence for this novel antibiotic is still insufficient. Further studies are needed before rifampicin, telithromycin, erythromycin, or levofloxacin can be considered as options.Scrub typhus usually resolves within a few weeks. Left untreated, the disease can cause complications such as pneumonia, meningoencephalitis, renal failure, and even multiorgan failure and death. Without treatment, mortality is variable. A 2015 systematic review of mortality from untreated ST showed, on average, mortality of 6% (range, 0%–70%).21 When ST is treated, mortality falls to 0% to 30%.22 Cases reported in Chile have neither been lethal nor presented with severe complications.4,5
Scrub typhus is an infectious disease common in Asia, caused by O tsutsugamushi and transmitted by chiggers. It should be suspected when a febrile macular or papular rash and a tache noire appear. The diagnosis can be supported by laboratory findings, such as an elevated C-reactive protein level or a slight increase in the levels of hepatic transaminases, and response to treatment. The diagnosis is confirmed by serology or PCR of a specimen of the eschar. Empiric therapy with antibiotics is mandatory; doxycycline is the first option.
First described in Chile in 2011,2 ST was seen in a patient in whom disease was suspected because of clinical characteristics, laboratory and histologic findings, absence of prior reporting in South America, and confirmation with PCR targeting the 16S ribosomal RNA Orientia gene from specimens of the eschar. By 2020, 60 cases have been confirmed in Chile, not all of them published; there are no other reported cases in South America.
When comparing the first case in Chile2 with our case, we noted that both described classic clinical findings; however, the management approach and diagnostic challenges have evolved over time. Nowadays, ST is highly suspected, so it can be largely recognized and treated, which also provides better understanding of the nature of this disease in Chile. Because this infection is diagnosed mainly by characteristic cutaneous lesions, dermatologists should be aware of its epidemiology, clinical features, and transmission, and they should stay open to the possibility of this (until now) unusual diagnosis in South America.
Acknowledgments
The authors would like to thank the Chilean Rickettsia & Zoonosis Research Group (Thomas Weitzel, MD [Santiago, Chile]; Constanza Martínez-Valdebenito [Santiago, Chile]; and Gerardo Acosta-Jammet, DSc [Valdivia, Chile]), whose study in execution in the country allowed the detection of the case and confirmation by PCR. The authors also thank Juan Carlos Román, MD (Chiloé, Chile) who was part of the team that detected this case.
- Izzard L, Fuller A, Blacksell SD, et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J Clin Microbiol. 2010;48:4404-4409.
- Balcells ME, Rabagliati R, García P, et al. Endemic scrub typhus-like illness, Chile. Emerg Infect Dis. 2011;17:1659-1663.
- Weitzel T, Dittrich S, López J, et al. Endemic scrub typhus in South America. N Engl J Med. 2016;375:954-961.
- Weitzel T, Acosta-Jamett G, Martínez-Valdebenito C, et al. Scrub typhus risk in travelers to southern Chile. Travel Med Infect Dis. 2019;29:78-79.
- Abarca K, Weitzel T, Martínez-Valdebenito C, et al. Scrub typhus, an emerging infectious disease in Chile. Rev Chilena Infectol. 2018;35:696-699.
- Weitzel T, Martínez-Valdebenito C, Acosta-Jamett G, et al. Scrub typhus in continental Chile, 2016-2018. Emerg Infect Dis. 2019;25:1214-1217.
- Guerrant RL, Walker DH, Weller PF, eds. Tropical Infectious Diseases: Principles, Pathogens and Practice. 3rd ed. Elsevier; 2011.
- Mahara F. Rickettsioses in Japan and the Far East. Ann N Y Acad Sci. 2006;1078:60-73.
- Salje J. Orientia tsutsugamushi: a neglected but fascinating obligate intracellular bacterial pathogen. PLoS Pathog. 2017;13:e1006657.
- Lee JS, Park MY, Kim YJ, et al. Histopathological features in both the eschar and erythematous lesions of tsutsugamushi disease: identification of CD30+ cell infiltration in tsutsugamushi disease. Am J Dermatopathol. 2009;31:551-556.
- Paris DH, Phetsouvanh R, Tanganuchitcharnchai A, et al. Orientia tsutsugamushi in human scrub typhus eschars shows tropism for dendritic cells and monocytes rather than endothelium. PLoS Negl Trop Dis. 2012;6:E1466.
- Walker DH. Scrub typhus—scientific neglect, ever-widening impact. N Engl J Med. 2016;375:913-915.
- Acosta-Jamett G, Martínez-Valdebenito C, Beltrami E, et al. Identification of trombiculid mites (Acari: Trombiculidae) on rodents from Chiloé Island and molecular evidence of infection with Orientia species [published online January 23, 2020]. PLoS Negl Trop Dis. doi:10.1371/journal.pntd.0007619
- Martínez-Valdebenito C, Angulo J, et al. Molecular description of a novel Orientia species causing scrub typhus in Chile. Emerg Infect Dis. 2020;26:2148-2156.
- Weitzel T, Jiang J, Acosta-Jamett G, et al. Canine seroprevalence to Orientia species in southern Chile: a cross-sectional survey on the Chiloé Island. PLoS One. 2018;13:e0200362.
- Wee I, Lo A, Rodrigo C. Drug treatment of scrub typhus: a systematic review and meta-analysis of controlled clinical trials. Trans R Soc Trop Med Hyg. 2017;111:336-344.
- Koh GCKW, Maude RJ, Paris DH, et al. Diagnosis of scrub typhus. Am J Trop Med Hyg. 2010;82:368-370.
- Weitzel T, Aylwin M, Martínez-Valdebenito C, et al. Imported scrub typhus: first case in South America and review of the literature. Trop Dis Travel Med Vaccines. 2018;4:10.
- Le Viet N, Laroche M, Thi Pham HL, et al. Use of eschar swabbing for the molecular diagnosis and genotyping of Orientia tsutsugamushi causing scrub typhus in Quang Nam province, Vietnam. 2017;11:e0005397.
- Jang HC, Choi SM, Jang MO, et al. Inappropriateness of quinolone in scrub typhus treatment due to gyrA mutation in Orientia tsutsugamushi Boryong strain. J Korean Med Sci. 2013;28:667-671.
- Taylor AJ, Paris DH, Newton PN. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl Trop Dis. 2015;9:e0003971.
- Bonell A, Lubell Y, Newton PN, et al. Estimating the burden of scrub typhus: a systematic review. PLoS Negl Trop Dis. 2017;11:e0005838.
To the Editor:
Scrub typhus (ST) is an infection caused by Orientia tsutsugamushi (genus Rickettsia), which is transmitted by the larvae of trombiculid mites, commonly called chiggers. The disease mainly has been described in Asia in an area known as the Tsutsugamushi Triangle, delineated by Pakistan, eastern Russia, and northern Australia. Although this classic distribution remains, recent reports have documented 1 case in the Arabian Peninsula1 and more than 16 cases in southern Chile.2-4 The first case in Chile was published in 2011 from Chiloé Island.2 To date, no other cases have been reported in the Americas.1-6
We describe a new case of ST from Chiloé Island and compare it to the first case reported in Chile in 2011.2 Both patients showed the typical clinical manifestation, but because ST has become an increasingly suspected disease in southern regions of Chile, new cases are now easily diagnosed. This infection is diagnosed mainly by skin lesions; therefore, dermatologists should be aware of this diagnosis when presented with a febrile rash.
A 67-year-old man from the city of Punta Arenas presented to the emergency department with a dark necrotic lesion on the right foot of 1 week’s duration. The patient later developed a generalized pruritic rash and fever. He also reported muscle pain, headache, cough, night sweats, and odynophagia. He reported recent travel to a rural area in the northern part of Chiloé Island, where he came into contact with firewood and participated in outdoor activities. He had no other relevant medical history.
Physical examination revealed a temperature of 38 °C and a macular rash, with some papules distributed mainly on the face, trunk, and proximal extremities (Figure 1). He had a necrotic eschar on the dorsum of the right foot, with an erythematous halo (tache noire)(Figure 2).


A complete blood cell count, urinalysis, and tests of hepatic and renal function were normal. C-reactive protein was elevated 18 times the normal value. Because of high awareness of ST in the region, eschar samples were taken and submitted for serologic testing and polymerase chain reaction (PCR) targeting the 16S rRNA Orientia gene. Empirical treatment with oral doxycycline 100 mg twice daily was started. Polymerase chain reaction analysis showed the presence of Orientia species, confirming the diagnosis of ST. The rash and eschar diminished considerably after 7 days of antibiotic treatment.
Scrub typhus is a high-impact disease in Asia, described mainly in an area known as the Tsutsugamushi Triangle. Recent reports show important epidemiologic changes in the distribution of the disease, with new published reports of cases outside this endemic area—1 in the Arabian peninsula1 and more than 16 in southern Chile.2-4
The disease begins with a painless, erythematous, and usually unnoticed papule at the site of the bite. After 48 to 72 hours, the papule changes to a necrotic form (tache noire), surrounded by a red halo that often is small, similar to a cigarette burn. This lesion is described in 20% to 90% of infected patients in different series.7 Two or 3 days later (1 to 3 weeks after exposure), high fever suddenly develops. Along with fever, a maculopapular rash distributed centrifugally develops, without compromise of the palms or soles. Patients frequently report headache and night sweating. Sometimes, ST is accompanied by muscle or joint pain, red eye, cough, and abdominal pain. Hearing loss and altered mental status less frequently have been reported.5,8
Common laboratory tests can be of use in diagnosis. An elevated C-reactive protein level and a slight to moderate increase in hepatic transaminases should be expected. Thrombocytopenia, leukopenia, and elevation of the lactate dehydrogenase level less frequently are present.5,9
Our case de1monstrated a typical presentation. The patient developed a febrile syndrome with a generalized rash and a tache noire–type eschar associated with muscle pain, headache, cough, night sweats, and odynophagia. Because of epidemiologic changes in the area, the familiar clinical findings, and laboratory confirmation, histologic studies were unnecessary. In cases in which the diagnosis is not evident, skin biopsy could be useful, as in the first case reported in Chile.2
In that first case, the patient initially was hospitalized because of a febrile syndrome; eventually, a necrotic eschar was noticed on his leg. He had been staying on Chiloé Island and reported being bitten by leeches on multiple occasions. Laboratory findings revealed only slightly raised levels of hepatic transaminases and alkaline phosphatase. After a more precise dermatologic evaluation, the eschar of a tache noire, combined with other clinical and laboratory findings, raised suspicion of ST. Because this entity had never been described in Chile, biopsy of the eschar was taken to consider other entities in the differential diagnosis. Biopsy showed necrotizing leukocytoclastic vasculitis in the dermis and subcutaneous tissue, perivascular inflammatory infiltrates comprising lymphocytes and macrophages, and rickettsial microorganisms inside endothelial cells under electron microscopic examination. The specimen was tested for the 16S ribosomal RNA Orientia gene; its presence confirmed the diagnosis.2
Classically, histology from the eschar shows signs of vasculitis and rickettsial microorganisms inside endothelial cells on electron microscopy.2,10 More recent publications describe important necrotic changes within keratinocytes as well as an inflammatory infiltrate comprising antigen-presenting cells, monocytes, macrophages, and dendritic cells. Using high-resolution thin sections with confocal laser scanning microscopy and staining of specific monoclonal antibodies against 56 kDa type-specific surface antigens, the bacteria were found inside antigen-presenting cells, many of them located perivascularly or passing through the endothelium.11
The causal agent in Asia is O tsutsugamushi, an obligate intracellular bacterium (genus Rickettsia). Orientia species are transmitted by larvae of trombiculid mites, commonly called chiggers. The reservoir is believed to be the same as with chiggers, in which some vertebrates become infected and trombiculid mites feed on them.12 Recent studies of Chilean cases have revealed the presence of a novel Orientia species, Candidatus Orientia chiloensis and its vector, trombiculid mites from the Herpetacarus species, Quadraseta species, and Paratrombicula species genera.13,14
A high seroprevalence of Orientia species in dogs was reported in the main cities of Chiloé Island. Rates were higher in rural settings and older dogs. Of 202 specimens, 21.3% were positive for IgG against Orientia species.15
In Chile, most cases of ST came from Chiloé Island; some reports of cases from continental Chilean regions have been published.6 Most cases have occurred in the context of activities that brought the patients in contact with plants and firewood in rural areas during the summer.3-6
The diagnosis of ST is eminently clinical, based on the triad of fever, macular or papular rash, and an inoculation necrotic eschar. The diagnosis is supported by epidemiologic facts and fast recovery after treatment is initiated.16 Although the diagnosis can be established based on a quick recovery in endemic countries, in areas such as Chile where incidence and distribution are not completely known, it is better to confirm the diagnosis with laboratory tests without delaying treatment. Several testing options exist, including serologic techniques (immunofluorescence or enzyme-linked immunosorbent assay), culture, and detection of the genetic material of Orientia species by PCR. Usually, IgM titers initially are negative, and IgG testing requires paired samples (acute and convalescent) to demonstrate seroconversion and therefore acute infection.17 Because culture requires a highly specialized laboratory, it is not frequently used. Polymerase chain reaction is recognized as the best confirmation method due to its high sensitivity and because it remains positive for a few days after treatment has been initiated. The specimen of choice is the eschar because of its high bacterial load. The base of the scar and the buffy coat are useful specimens when the eschar is unavailable.5,17-19
Due to potential complications of ST, empirical treatment with an antibiotic should be started based on clinical facts and never delayed because of diagnostic tests.18 Classically, ST is treated with a member of the tetracycline family, such as doxycycline, which provides a cure rate of 63% to 100% in ST.5
A 2017 systematic review of treatment options for this infection examined 11 studies from Southeast Asia, China, and South Korea (N=957).16 The review mainly compared doxycycline with azithromycin, chloramphenicol, and tetracycline. No significant difference in cure rate was noted in comparing doxycycline with any of the other 3 antibiotics; most of the studies examined were characterized by a moderate level of evidence. Regarding adverse effects, doxycycline showed a few more cases of gastrointestinal intolerance, and in 2 of 4 studies with chloramphenicol, patients presented with leukopenia.16 Several studies compared standard treatment (doxycycline) with rifampicin, telithromycin, erythromycin, and levofloxacin individually; similar cure rates were noted between doxycycline and each of those 4 agents.
Therapeutic failure in ST has been reported in several cases with the use of levofloxacin.20 Evidence for this novel antibiotic is still insufficient. Further studies are needed before rifampicin, telithromycin, erythromycin, or levofloxacin can be considered as options.Scrub typhus usually resolves within a few weeks. Left untreated, the disease can cause complications such as pneumonia, meningoencephalitis, renal failure, and even multiorgan failure and death. Without treatment, mortality is variable. A 2015 systematic review of mortality from untreated ST showed, on average, mortality of 6% (range, 0%–70%).21 When ST is treated, mortality falls to 0% to 30%.22 Cases reported in Chile have neither been lethal nor presented with severe complications.4,5
Scrub typhus is an infectious disease common in Asia, caused by O tsutsugamushi and transmitted by chiggers. It should be suspected when a febrile macular or papular rash and a tache noire appear. The diagnosis can be supported by laboratory findings, such as an elevated C-reactive protein level or a slight increase in the levels of hepatic transaminases, and response to treatment. The diagnosis is confirmed by serology or PCR of a specimen of the eschar. Empiric therapy with antibiotics is mandatory; doxycycline is the first option.
First described in Chile in 2011,2 ST was seen in a patient in whom disease was suspected because of clinical characteristics, laboratory and histologic findings, absence of prior reporting in South America, and confirmation with PCR targeting the 16S ribosomal RNA Orientia gene from specimens of the eschar. By 2020, 60 cases have been confirmed in Chile, not all of them published; there are no other reported cases in South America.
When comparing the first case in Chile2 with our case, we noted that both described classic clinical findings; however, the management approach and diagnostic challenges have evolved over time. Nowadays, ST is highly suspected, so it can be largely recognized and treated, which also provides better understanding of the nature of this disease in Chile. Because this infection is diagnosed mainly by characteristic cutaneous lesions, dermatologists should be aware of its epidemiology, clinical features, and transmission, and they should stay open to the possibility of this (until now) unusual diagnosis in South America.
Acknowledgments
The authors would like to thank the Chilean Rickettsia & Zoonosis Research Group (Thomas Weitzel, MD [Santiago, Chile]; Constanza Martínez-Valdebenito [Santiago, Chile]; and Gerardo Acosta-Jammet, DSc [Valdivia, Chile]), whose study in execution in the country allowed the detection of the case and confirmation by PCR. The authors also thank Juan Carlos Román, MD (Chiloé, Chile) who was part of the team that detected this case.
To the Editor:
Scrub typhus (ST) is an infection caused by Orientia tsutsugamushi (genus Rickettsia), which is transmitted by the larvae of trombiculid mites, commonly called chiggers. The disease mainly has been described in Asia in an area known as the Tsutsugamushi Triangle, delineated by Pakistan, eastern Russia, and northern Australia. Although this classic distribution remains, recent reports have documented 1 case in the Arabian Peninsula1 and more than 16 cases in southern Chile.2-4 The first case in Chile was published in 2011 from Chiloé Island.2 To date, no other cases have been reported in the Americas.1-6
We describe a new case of ST from Chiloé Island and compare it to the first case reported in Chile in 2011.2 Both patients showed the typical clinical manifestation, but because ST has become an increasingly suspected disease in southern regions of Chile, new cases are now easily diagnosed. This infection is diagnosed mainly by skin lesions; therefore, dermatologists should be aware of this diagnosis when presented with a febrile rash.
A 67-year-old man from the city of Punta Arenas presented to the emergency department with a dark necrotic lesion on the right foot of 1 week’s duration. The patient later developed a generalized pruritic rash and fever. He also reported muscle pain, headache, cough, night sweats, and odynophagia. He reported recent travel to a rural area in the northern part of Chiloé Island, where he came into contact with firewood and participated in outdoor activities. He had no other relevant medical history.
Physical examination revealed a temperature of 38 °C and a macular rash, with some papules distributed mainly on the face, trunk, and proximal extremities (Figure 1). He had a necrotic eschar on the dorsum of the right foot, with an erythematous halo (tache noire)(Figure 2).


A complete blood cell count, urinalysis, and tests of hepatic and renal function were normal. C-reactive protein was elevated 18 times the normal value. Because of high awareness of ST in the region, eschar samples were taken and submitted for serologic testing and polymerase chain reaction (PCR) targeting the 16S rRNA Orientia gene. Empirical treatment with oral doxycycline 100 mg twice daily was started. Polymerase chain reaction analysis showed the presence of Orientia species, confirming the diagnosis of ST. The rash and eschar diminished considerably after 7 days of antibiotic treatment.
Scrub typhus is a high-impact disease in Asia, described mainly in an area known as the Tsutsugamushi Triangle. Recent reports show important epidemiologic changes in the distribution of the disease, with new published reports of cases outside this endemic area—1 in the Arabian peninsula1 and more than 16 in southern Chile.2-4
The disease begins with a painless, erythematous, and usually unnoticed papule at the site of the bite. After 48 to 72 hours, the papule changes to a necrotic form (tache noire), surrounded by a red halo that often is small, similar to a cigarette burn. This lesion is described in 20% to 90% of infected patients in different series.7 Two or 3 days later (1 to 3 weeks after exposure), high fever suddenly develops. Along with fever, a maculopapular rash distributed centrifugally develops, without compromise of the palms or soles. Patients frequently report headache and night sweating. Sometimes, ST is accompanied by muscle or joint pain, red eye, cough, and abdominal pain. Hearing loss and altered mental status less frequently have been reported.5,8
Common laboratory tests can be of use in diagnosis. An elevated C-reactive protein level and a slight to moderate increase in hepatic transaminases should be expected. Thrombocytopenia, leukopenia, and elevation of the lactate dehydrogenase level less frequently are present.5,9
Our case de1monstrated a typical presentation. The patient developed a febrile syndrome with a generalized rash and a tache noire–type eschar associated with muscle pain, headache, cough, night sweats, and odynophagia. Because of epidemiologic changes in the area, the familiar clinical findings, and laboratory confirmation, histologic studies were unnecessary. In cases in which the diagnosis is not evident, skin biopsy could be useful, as in the first case reported in Chile.2
In that first case, the patient initially was hospitalized because of a febrile syndrome; eventually, a necrotic eschar was noticed on his leg. He had been staying on Chiloé Island and reported being bitten by leeches on multiple occasions. Laboratory findings revealed only slightly raised levels of hepatic transaminases and alkaline phosphatase. After a more precise dermatologic evaluation, the eschar of a tache noire, combined with other clinical and laboratory findings, raised suspicion of ST. Because this entity had never been described in Chile, biopsy of the eschar was taken to consider other entities in the differential diagnosis. Biopsy showed necrotizing leukocytoclastic vasculitis in the dermis and subcutaneous tissue, perivascular inflammatory infiltrates comprising lymphocytes and macrophages, and rickettsial microorganisms inside endothelial cells under electron microscopic examination. The specimen was tested for the 16S ribosomal RNA Orientia gene; its presence confirmed the diagnosis.2
Classically, histology from the eschar shows signs of vasculitis and rickettsial microorganisms inside endothelial cells on electron microscopy.2,10 More recent publications describe important necrotic changes within keratinocytes as well as an inflammatory infiltrate comprising antigen-presenting cells, monocytes, macrophages, and dendritic cells. Using high-resolution thin sections with confocal laser scanning microscopy and staining of specific monoclonal antibodies against 56 kDa type-specific surface antigens, the bacteria were found inside antigen-presenting cells, many of them located perivascularly or passing through the endothelium.11
The causal agent in Asia is O tsutsugamushi, an obligate intracellular bacterium (genus Rickettsia). Orientia species are transmitted by larvae of trombiculid mites, commonly called chiggers. The reservoir is believed to be the same as with chiggers, in which some vertebrates become infected and trombiculid mites feed on them.12 Recent studies of Chilean cases have revealed the presence of a novel Orientia species, Candidatus Orientia chiloensis and its vector, trombiculid mites from the Herpetacarus species, Quadraseta species, and Paratrombicula species genera.13,14
A high seroprevalence of Orientia species in dogs was reported in the main cities of Chiloé Island. Rates were higher in rural settings and older dogs. Of 202 specimens, 21.3% were positive for IgG against Orientia species.15
In Chile, most cases of ST came from Chiloé Island; some reports of cases from continental Chilean regions have been published.6 Most cases have occurred in the context of activities that brought the patients in contact with plants and firewood in rural areas during the summer.3-6
The diagnosis of ST is eminently clinical, based on the triad of fever, macular or papular rash, and an inoculation necrotic eschar. The diagnosis is supported by epidemiologic facts and fast recovery after treatment is initiated.16 Although the diagnosis can be established based on a quick recovery in endemic countries, in areas such as Chile where incidence and distribution are not completely known, it is better to confirm the diagnosis with laboratory tests without delaying treatment. Several testing options exist, including serologic techniques (immunofluorescence or enzyme-linked immunosorbent assay), culture, and detection of the genetic material of Orientia species by PCR. Usually, IgM titers initially are negative, and IgG testing requires paired samples (acute and convalescent) to demonstrate seroconversion and therefore acute infection.17 Because culture requires a highly specialized laboratory, it is not frequently used. Polymerase chain reaction is recognized as the best confirmation method due to its high sensitivity and because it remains positive for a few days after treatment has been initiated. The specimen of choice is the eschar because of its high bacterial load. The base of the scar and the buffy coat are useful specimens when the eschar is unavailable.5,17-19
Due to potential complications of ST, empirical treatment with an antibiotic should be started based on clinical facts and never delayed because of diagnostic tests.18 Classically, ST is treated with a member of the tetracycline family, such as doxycycline, which provides a cure rate of 63% to 100% in ST.5
A 2017 systematic review of treatment options for this infection examined 11 studies from Southeast Asia, China, and South Korea (N=957).16 The review mainly compared doxycycline with azithromycin, chloramphenicol, and tetracycline. No significant difference in cure rate was noted in comparing doxycycline with any of the other 3 antibiotics; most of the studies examined were characterized by a moderate level of evidence. Regarding adverse effects, doxycycline showed a few more cases of gastrointestinal intolerance, and in 2 of 4 studies with chloramphenicol, patients presented with leukopenia.16 Several studies compared standard treatment (doxycycline) with rifampicin, telithromycin, erythromycin, and levofloxacin individually; similar cure rates were noted between doxycycline and each of those 4 agents.
Therapeutic failure in ST has been reported in several cases with the use of levofloxacin.20 Evidence for this novel antibiotic is still insufficient. Further studies are needed before rifampicin, telithromycin, erythromycin, or levofloxacin can be considered as options.Scrub typhus usually resolves within a few weeks. Left untreated, the disease can cause complications such as pneumonia, meningoencephalitis, renal failure, and even multiorgan failure and death. Without treatment, mortality is variable. A 2015 systematic review of mortality from untreated ST showed, on average, mortality of 6% (range, 0%–70%).21 When ST is treated, mortality falls to 0% to 30%.22 Cases reported in Chile have neither been lethal nor presented with severe complications.4,5
Scrub typhus is an infectious disease common in Asia, caused by O tsutsugamushi and transmitted by chiggers. It should be suspected when a febrile macular or papular rash and a tache noire appear. The diagnosis can be supported by laboratory findings, such as an elevated C-reactive protein level or a slight increase in the levels of hepatic transaminases, and response to treatment. The diagnosis is confirmed by serology or PCR of a specimen of the eschar. Empiric therapy with antibiotics is mandatory; doxycycline is the first option.
First described in Chile in 2011,2 ST was seen in a patient in whom disease was suspected because of clinical characteristics, laboratory and histologic findings, absence of prior reporting in South America, and confirmation with PCR targeting the 16S ribosomal RNA Orientia gene from specimens of the eschar. By 2020, 60 cases have been confirmed in Chile, not all of them published; there are no other reported cases in South America.
When comparing the first case in Chile2 with our case, we noted that both described classic clinical findings; however, the management approach and diagnostic challenges have evolved over time. Nowadays, ST is highly suspected, so it can be largely recognized and treated, which also provides better understanding of the nature of this disease in Chile. Because this infection is diagnosed mainly by characteristic cutaneous lesions, dermatologists should be aware of its epidemiology, clinical features, and transmission, and they should stay open to the possibility of this (until now) unusual diagnosis in South America.
Acknowledgments
The authors would like to thank the Chilean Rickettsia & Zoonosis Research Group (Thomas Weitzel, MD [Santiago, Chile]; Constanza Martínez-Valdebenito [Santiago, Chile]; and Gerardo Acosta-Jammet, DSc [Valdivia, Chile]), whose study in execution in the country allowed the detection of the case and confirmation by PCR. The authors also thank Juan Carlos Román, MD (Chiloé, Chile) who was part of the team that detected this case.
- Izzard L, Fuller A, Blacksell SD, et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J Clin Microbiol. 2010;48:4404-4409.
- Balcells ME, Rabagliati R, García P, et al. Endemic scrub typhus-like illness, Chile. Emerg Infect Dis. 2011;17:1659-1663.
- Weitzel T, Dittrich S, López J, et al. Endemic scrub typhus in South America. N Engl J Med. 2016;375:954-961.
- Weitzel T, Acosta-Jamett G, Martínez-Valdebenito C, et al. Scrub typhus risk in travelers to southern Chile. Travel Med Infect Dis. 2019;29:78-79.
- Abarca K, Weitzel T, Martínez-Valdebenito C, et al. Scrub typhus, an emerging infectious disease in Chile. Rev Chilena Infectol. 2018;35:696-699.
- Weitzel T, Martínez-Valdebenito C, Acosta-Jamett G, et al. Scrub typhus in continental Chile, 2016-2018. Emerg Infect Dis. 2019;25:1214-1217.
- Guerrant RL, Walker DH, Weller PF, eds. Tropical Infectious Diseases: Principles, Pathogens and Practice. 3rd ed. Elsevier; 2011.
- Mahara F. Rickettsioses in Japan and the Far East. Ann N Y Acad Sci. 2006;1078:60-73.
- Salje J. Orientia tsutsugamushi: a neglected but fascinating obligate intracellular bacterial pathogen. PLoS Pathog. 2017;13:e1006657.
- Lee JS, Park MY, Kim YJ, et al. Histopathological features in both the eschar and erythematous lesions of tsutsugamushi disease: identification of CD30+ cell infiltration in tsutsugamushi disease. Am J Dermatopathol. 2009;31:551-556.
- Paris DH, Phetsouvanh R, Tanganuchitcharnchai A, et al. Orientia tsutsugamushi in human scrub typhus eschars shows tropism for dendritic cells and monocytes rather than endothelium. PLoS Negl Trop Dis. 2012;6:E1466.
- Walker DH. Scrub typhus—scientific neglect, ever-widening impact. N Engl J Med. 2016;375:913-915.
- Acosta-Jamett G, Martínez-Valdebenito C, Beltrami E, et al. Identification of trombiculid mites (Acari: Trombiculidae) on rodents from Chiloé Island and molecular evidence of infection with Orientia species [published online January 23, 2020]. PLoS Negl Trop Dis. doi:10.1371/journal.pntd.0007619
- Martínez-Valdebenito C, Angulo J, et al. Molecular description of a novel Orientia species causing scrub typhus in Chile. Emerg Infect Dis. 2020;26:2148-2156.
- Weitzel T, Jiang J, Acosta-Jamett G, et al. Canine seroprevalence to Orientia species in southern Chile: a cross-sectional survey on the Chiloé Island. PLoS One. 2018;13:e0200362.
- Wee I, Lo A, Rodrigo C. Drug treatment of scrub typhus: a systematic review and meta-analysis of controlled clinical trials. Trans R Soc Trop Med Hyg. 2017;111:336-344.
- Koh GCKW, Maude RJ, Paris DH, et al. Diagnosis of scrub typhus. Am J Trop Med Hyg. 2010;82:368-370.
- Weitzel T, Aylwin M, Martínez-Valdebenito C, et al. Imported scrub typhus: first case in South America and review of the literature. Trop Dis Travel Med Vaccines. 2018;4:10.
- Le Viet N, Laroche M, Thi Pham HL, et al. Use of eschar swabbing for the molecular diagnosis and genotyping of Orientia tsutsugamushi causing scrub typhus in Quang Nam province, Vietnam. 2017;11:e0005397.
- Jang HC, Choi SM, Jang MO, et al. Inappropriateness of quinolone in scrub typhus treatment due to gyrA mutation in Orientia tsutsugamushi Boryong strain. J Korean Med Sci. 2013;28:667-671.
- Taylor AJ, Paris DH, Newton PN. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl Trop Dis. 2015;9:e0003971.
- Bonell A, Lubell Y, Newton PN, et al. Estimating the burden of scrub typhus: a systematic review. PLoS Negl Trop Dis. 2017;11:e0005838.
- Izzard L, Fuller A, Blacksell SD, et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J Clin Microbiol. 2010;48:4404-4409.
- Balcells ME, Rabagliati R, García P, et al. Endemic scrub typhus-like illness, Chile. Emerg Infect Dis. 2011;17:1659-1663.
- Weitzel T, Dittrich S, López J, et al. Endemic scrub typhus in South America. N Engl J Med. 2016;375:954-961.
- Weitzel T, Acosta-Jamett G, Martínez-Valdebenito C, et al. Scrub typhus risk in travelers to southern Chile. Travel Med Infect Dis. 2019;29:78-79.
- Abarca K, Weitzel T, Martínez-Valdebenito C, et al. Scrub typhus, an emerging infectious disease in Chile. Rev Chilena Infectol. 2018;35:696-699.
- Weitzel T, Martínez-Valdebenito C, Acosta-Jamett G, et al. Scrub typhus in continental Chile, 2016-2018. Emerg Infect Dis. 2019;25:1214-1217.
- Guerrant RL, Walker DH, Weller PF, eds. Tropical Infectious Diseases: Principles, Pathogens and Practice. 3rd ed. Elsevier; 2011.
- Mahara F. Rickettsioses in Japan and the Far East. Ann N Y Acad Sci. 2006;1078:60-73.
- Salje J. Orientia tsutsugamushi: a neglected but fascinating obligate intracellular bacterial pathogen. PLoS Pathog. 2017;13:e1006657.
- Lee JS, Park MY, Kim YJ, et al. Histopathological features in both the eschar and erythematous lesions of tsutsugamushi disease: identification of CD30+ cell infiltration in tsutsugamushi disease. Am J Dermatopathol. 2009;31:551-556.
- Paris DH, Phetsouvanh R, Tanganuchitcharnchai A, et al. Orientia tsutsugamushi in human scrub typhus eschars shows tropism for dendritic cells and monocytes rather than endothelium. PLoS Negl Trop Dis. 2012;6:E1466.
- Walker DH. Scrub typhus—scientific neglect, ever-widening impact. N Engl J Med. 2016;375:913-915.
- Acosta-Jamett G, Martínez-Valdebenito C, Beltrami E, et al. Identification of trombiculid mites (Acari: Trombiculidae) on rodents from Chiloé Island and molecular evidence of infection with Orientia species [published online January 23, 2020]. PLoS Negl Trop Dis. doi:10.1371/journal.pntd.0007619
- Martínez-Valdebenito C, Angulo J, et al. Molecular description of a novel Orientia species causing scrub typhus in Chile. Emerg Infect Dis. 2020;26:2148-2156.
- Weitzel T, Jiang J, Acosta-Jamett G, et al. Canine seroprevalence to Orientia species in southern Chile: a cross-sectional survey on the Chiloé Island. PLoS One. 2018;13:e0200362.
- Wee I, Lo A, Rodrigo C. Drug treatment of scrub typhus: a systematic review and meta-analysis of controlled clinical trials. Trans R Soc Trop Med Hyg. 2017;111:336-344.
- Koh GCKW, Maude RJ, Paris DH, et al. Diagnosis of scrub typhus. Am J Trop Med Hyg. 2010;82:368-370.
- Weitzel T, Aylwin M, Martínez-Valdebenito C, et al. Imported scrub typhus: first case in South America and review of the literature. Trop Dis Travel Med Vaccines. 2018;4:10.
- Le Viet N, Laroche M, Thi Pham HL, et al. Use of eschar swabbing for the molecular diagnosis and genotyping of Orientia tsutsugamushi causing scrub typhus in Quang Nam province, Vietnam. 2017;11:e0005397.
- Jang HC, Choi SM, Jang MO, et al. Inappropriateness of quinolone in scrub typhus treatment due to gyrA mutation in Orientia tsutsugamushi Boryong strain. J Korean Med Sci. 2013;28:667-671.
- Taylor AJ, Paris DH, Newton PN. A systematic review of mortality from untreated scrub typhus (Orientia tsutsugamushi). PLoS Negl Trop Dis. 2015;9:e0003971.
- Bonell A, Lubell Y, Newton PN, et al. Estimating the burden of scrub typhus: a systematic review. PLoS Negl Trop Dis. 2017;11:e0005838.
Practice Points
- Scrub typhus is clinically suspected in patients who present with a febrile macular or papular rash and a characteristic necrotic eschar known as tache noire while residing in or traveling to rural areas.
- Scrub typhus can lead to serious complications. Due to its changing epidemiology, dermatologists outside the usual area of distribution should be aware in the event that new cases emerge.
Menopause transition affects heart health risks
Menopause is a key time to monitor women for the development or increase of cardiovascular risk factors, according to a new consensus statement developed by the Task Force on Gender of the European Society of Cardiology and a multidisciplinary ESC working group on Women’s Health in Menopause.
“After menopause, traditional cardiovascular risk factors are adversely affected – particularly hypertension,” wrote Angela H.E.M. Maas, MD, of Radboud University Medical Center, Nijmegen, Netherlands, and colleagues.
“Since the first ESC consensus paper on the management of cardiovascular risk in perimenopausal women was published in 2007, we have a greater understanding on the role of female-specific risk factors for cardiovascular disease (CVD),” they said.
In a consensus statement published in the European Heart Journal, the authors presented clinical guidance for diagnosis and management of cardiovascular risk factors during the menopause transition. The transition to menopause increases a woman’s risk for developing several CVD risk factors, including central adiposity, increased insulin resistance, a proatherogenic lipid profile, and autonomic dysfunction that can contribute to increased heart rate variability, according to the statement.
Estrogen changes may affect ischemic disease
In general, obstructive coronary artery disease (CAD) strikes women later than men, but coronary vasomotor conditions are a common cause of ischemic heart disease in women with or without CAD, the authors noted.
“Lower estrogen levels after menopause are related to altered vascular function, enhanced inflammation, and up-regulation of other hormonal systems such as the renin–angiotensin–aldosterone system, the sympathetic nervous system, and reduced nitric oxide–dependent vasodilation,” they wrote. They recommended use of the coronary artery calcium score for screening middle-aged women who are symptomatic or at intermediate cardiovascular risk.
The transition to menopause causes changes in lipid profiles, and a rise in blood pressure in particular “may be both a direct effect of hormonal changes on the vasculature and metabolic changes with aging,” but hypertension in early post menopause is “often poorly managed,” the authors noted.
Compared with asymptomatic women, women who suffer from severe menopausal symptoms often have increased cardiovascular disease risk factors. For example, the Women’s Health Initiative (WHI) study showed a 48% increased risk of incident diabetes at follow-up in women with severe symptoms of hot flashes and night sweats, the authors wrote. Clinicians should also be aware of the increased immune reactivity that occurs during and after menopause and the increased CVD risk associated with autoimmune and endocrine disorders, they said.
Multiple strategies to reduce risk
Strategies to address the cardiovascular risk in menopause include assessing glucose, lipid levels, and blood pressure during the transition to menopause, according to the statement.
In addition, they recommended increasing employer awareness of menopause, as changes may interfere with working ability. A healthy lifestyle including healthy diet and regular exercise can help reduce cardiovascular risks and relieve symptoms. Menopausal hormone therapy (MHT) may be indicated to relieve symptoms, including symptoms of depression, and provide cardioprotection for younger women around the time of menopause, according to the statement.
However, “MHT is not recommended in women at high CV risk and after a previous CVD event,” and all women should be assessed for cardiovascular risk factors before starting MHT, they emphasized.
Results raise awareness of cardiovascular health and menopause link
“Over the past 20 years, our knowledge of how menopause might contribute to cardiovascular disease has dramatically evolved,” said Samar El Khoudary, MD, of the University of Pittsburg, in an interview.
“We have accumulated data that consistently point to the menopause transition as a time of change in cardiovascular health. As such, there is a compelling need to discuss the implications of the accumulating body of literature on this topic,” she said. “The goal is to raise awareness for both health care providers and women of the significant adverse cardiovascular health changes accompanying the menopause transition and to point out the importance of adopting prevention strategies early during this stage,” she explained.
The impact of the hormonal changes of menopause on CVD risk “is very complex,” Dr. El Khoudary said. “Until now, we could not prove that using estrogen therapy is cardioprotective,” she emphasized. “Studies point to the need to consider the timing of hormone use, as well as types and route of administration,” she noted. “The truth is that, although the menopause transition is associated with an acceleration in CVD risk, the exact mechanism still is not completely clear. Hormone changes contribute, but they are not the ultimate contributor,” she added.
Research gaps include data on lifestyle and behavioral interventions
“Irrespective of the accumulating findings showing adverse changes in multiple cardiovascular health parameters, as women transition through menopause, we do not have data documenting current status of ideal cardiovascular health components during the menopause transition among women,” said Dr. El Khoudary. “The limited data we have [suggest] that a very small proportion of women transitioning through menopause eat a healthy diet (less than 20%) or practice physical activity (about7.2%) at a level that matches the current recommendations,” she noted.
“Lifestyle and behavioral interventions are critical to maintain a healthy heart and reduce heart disease; we do not have adequate randomized clinical trials testing these interventions specifically during the menopause transition,” she said.
“Similarly, we are in need of randomized clinical trials of therapeutic interventions such as lipid-lowering medications and menopause hormone therapy in women transitioning through menopause,” said Dr. El Khoudary. “This high-risk population has not been the focus of previous clinical trials, leaving us with questions of how the results from these studies might apply to women during the menopause transition,” she said.
Consensus invites collaboration
“I commend the group for putting together a statement that crosses practice and specialty boundaries,” said Lubna Pal, MD, of Yale School of Medicine, New Haven, Conn., in an interview. Although the statement does not present novel information, it “has the power of unifying the various providers by bringing focus on the individual elements spanning a woman’s life that cumulatively determine her lifetime health risk,” she said. Preeclampsia may be a risk factor for cardiovascular disease later in life, and events in reproductive age may determine a woman’s trajectory during the transition to menopause and beyond, Dr. Pal noted.
“The consensus statement will likely be read by internists and family medicine providers as well as ob.gyns.; it encourages all those involved in caring for female patients to take on the responsibility of ‘passing on the baton,’ such that all women who are deemed at an enhanced risk for cardiovascular disease are assured due diligence in care through stringent surveillance and timely interventions,” said Dr. Pal. “It is a call for the various providers who care for women at distinct stages of life to work together toward a shared goal of optimizing every woman’s health across her lifespan,” she said.
“More research is needed for us to better understand the mechanisms at play” in the development of cardiovascular risk and in understanding the continuity of changes across women’s lifespans, Dr. Pal said. “We have associations, but not much information about causation,” she emphasized. However, the statement promotes the dissemination of information about women’s health and sensitizes providers to the potential and the power of preventive care. “We should be much more liberal and loud in holding conversations about risk quantification and risk reduction, and this statement is a resounding effort toward identifying and mitigating long-term cardiovascular risk, even if only through promoting a healthier lifestyle in those deemed at risk,” she added.
The statement received no outside funding. Lead author Dr. Maas had no financial conflicts to disclose. Dr. El Khoudary had no financial conflicts to disclose. Dr. Pal had no relevant financial conflicts to disclose.
Menopause is a key time to monitor women for the development or increase of cardiovascular risk factors, according to a new consensus statement developed by the Task Force on Gender of the European Society of Cardiology and a multidisciplinary ESC working group on Women’s Health in Menopause.
“After menopause, traditional cardiovascular risk factors are adversely affected – particularly hypertension,” wrote Angela H.E.M. Maas, MD, of Radboud University Medical Center, Nijmegen, Netherlands, and colleagues.
“Since the first ESC consensus paper on the management of cardiovascular risk in perimenopausal women was published in 2007, we have a greater understanding on the role of female-specific risk factors for cardiovascular disease (CVD),” they said.
In a consensus statement published in the European Heart Journal, the authors presented clinical guidance for diagnosis and management of cardiovascular risk factors during the menopause transition. The transition to menopause increases a woman’s risk for developing several CVD risk factors, including central adiposity, increased insulin resistance, a proatherogenic lipid profile, and autonomic dysfunction that can contribute to increased heart rate variability, according to the statement.
Estrogen changes may affect ischemic disease
In general, obstructive coronary artery disease (CAD) strikes women later than men, but coronary vasomotor conditions are a common cause of ischemic heart disease in women with or without CAD, the authors noted.
“Lower estrogen levels after menopause are related to altered vascular function, enhanced inflammation, and up-regulation of other hormonal systems such as the renin–angiotensin–aldosterone system, the sympathetic nervous system, and reduced nitric oxide–dependent vasodilation,” they wrote. They recommended use of the coronary artery calcium score for screening middle-aged women who are symptomatic or at intermediate cardiovascular risk.
The transition to menopause causes changes in lipid profiles, and a rise in blood pressure in particular “may be both a direct effect of hormonal changes on the vasculature and metabolic changes with aging,” but hypertension in early post menopause is “often poorly managed,” the authors noted.
Compared with asymptomatic women, women who suffer from severe menopausal symptoms often have increased cardiovascular disease risk factors. For example, the Women’s Health Initiative (WHI) study showed a 48% increased risk of incident diabetes at follow-up in women with severe symptoms of hot flashes and night sweats, the authors wrote. Clinicians should also be aware of the increased immune reactivity that occurs during and after menopause and the increased CVD risk associated with autoimmune and endocrine disorders, they said.
Multiple strategies to reduce risk
Strategies to address the cardiovascular risk in menopause include assessing glucose, lipid levels, and blood pressure during the transition to menopause, according to the statement.
In addition, they recommended increasing employer awareness of menopause, as changes may interfere with working ability. A healthy lifestyle including healthy diet and regular exercise can help reduce cardiovascular risks and relieve symptoms. Menopausal hormone therapy (MHT) may be indicated to relieve symptoms, including symptoms of depression, and provide cardioprotection for younger women around the time of menopause, according to the statement.
However, “MHT is not recommended in women at high CV risk and after a previous CVD event,” and all women should be assessed for cardiovascular risk factors before starting MHT, they emphasized.
Results raise awareness of cardiovascular health and menopause link
“Over the past 20 years, our knowledge of how menopause might contribute to cardiovascular disease has dramatically evolved,” said Samar El Khoudary, MD, of the University of Pittsburg, in an interview.
“We have accumulated data that consistently point to the menopause transition as a time of change in cardiovascular health. As such, there is a compelling need to discuss the implications of the accumulating body of literature on this topic,” she said. “The goal is to raise awareness for both health care providers and women of the significant adverse cardiovascular health changes accompanying the menopause transition and to point out the importance of adopting prevention strategies early during this stage,” she explained.
The impact of the hormonal changes of menopause on CVD risk “is very complex,” Dr. El Khoudary said. “Until now, we could not prove that using estrogen therapy is cardioprotective,” she emphasized. “Studies point to the need to consider the timing of hormone use, as well as types and route of administration,” she noted. “The truth is that, although the menopause transition is associated with an acceleration in CVD risk, the exact mechanism still is not completely clear. Hormone changes contribute, but they are not the ultimate contributor,” she added.
Research gaps include data on lifestyle and behavioral interventions
“Irrespective of the accumulating findings showing adverse changes in multiple cardiovascular health parameters, as women transition through menopause, we do not have data documenting current status of ideal cardiovascular health components during the menopause transition among women,” said Dr. El Khoudary. “The limited data we have [suggest] that a very small proportion of women transitioning through menopause eat a healthy diet (less than 20%) or practice physical activity (about7.2%) at a level that matches the current recommendations,” she noted.
“Lifestyle and behavioral interventions are critical to maintain a healthy heart and reduce heart disease; we do not have adequate randomized clinical trials testing these interventions specifically during the menopause transition,” she said.
“Similarly, we are in need of randomized clinical trials of therapeutic interventions such as lipid-lowering medications and menopause hormone therapy in women transitioning through menopause,” said Dr. El Khoudary. “This high-risk population has not been the focus of previous clinical trials, leaving us with questions of how the results from these studies might apply to women during the menopause transition,” she said.
Consensus invites collaboration
“I commend the group for putting together a statement that crosses practice and specialty boundaries,” said Lubna Pal, MD, of Yale School of Medicine, New Haven, Conn., in an interview. Although the statement does not present novel information, it “has the power of unifying the various providers by bringing focus on the individual elements spanning a woman’s life that cumulatively determine her lifetime health risk,” she said. Preeclampsia may be a risk factor for cardiovascular disease later in life, and events in reproductive age may determine a woman’s trajectory during the transition to menopause and beyond, Dr. Pal noted.
“The consensus statement will likely be read by internists and family medicine providers as well as ob.gyns.; it encourages all those involved in caring for female patients to take on the responsibility of ‘passing on the baton,’ such that all women who are deemed at an enhanced risk for cardiovascular disease are assured due diligence in care through stringent surveillance and timely interventions,” said Dr. Pal. “It is a call for the various providers who care for women at distinct stages of life to work together toward a shared goal of optimizing every woman’s health across her lifespan,” she said.
“More research is needed for us to better understand the mechanisms at play” in the development of cardiovascular risk and in understanding the continuity of changes across women’s lifespans, Dr. Pal said. “We have associations, but not much information about causation,” she emphasized. However, the statement promotes the dissemination of information about women’s health and sensitizes providers to the potential and the power of preventive care. “We should be much more liberal and loud in holding conversations about risk quantification and risk reduction, and this statement is a resounding effort toward identifying and mitigating long-term cardiovascular risk, even if only through promoting a healthier lifestyle in those deemed at risk,” she added.
The statement received no outside funding. Lead author Dr. Maas had no financial conflicts to disclose. Dr. El Khoudary had no financial conflicts to disclose. Dr. Pal had no relevant financial conflicts to disclose.
Menopause is a key time to monitor women for the development or increase of cardiovascular risk factors, according to a new consensus statement developed by the Task Force on Gender of the European Society of Cardiology and a multidisciplinary ESC working group on Women’s Health in Menopause.
“After menopause, traditional cardiovascular risk factors are adversely affected – particularly hypertension,” wrote Angela H.E.M. Maas, MD, of Radboud University Medical Center, Nijmegen, Netherlands, and colleagues.
“Since the first ESC consensus paper on the management of cardiovascular risk in perimenopausal women was published in 2007, we have a greater understanding on the role of female-specific risk factors for cardiovascular disease (CVD),” they said.
In a consensus statement published in the European Heart Journal, the authors presented clinical guidance for diagnosis and management of cardiovascular risk factors during the menopause transition. The transition to menopause increases a woman’s risk for developing several CVD risk factors, including central adiposity, increased insulin resistance, a proatherogenic lipid profile, and autonomic dysfunction that can contribute to increased heart rate variability, according to the statement.
Estrogen changes may affect ischemic disease
In general, obstructive coronary artery disease (CAD) strikes women later than men, but coronary vasomotor conditions are a common cause of ischemic heart disease in women with or without CAD, the authors noted.
“Lower estrogen levels after menopause are related to altered vascular function, enhanced inflammation, and up-regulation of other hormonal systems such as the renin–angiotensin–aldosterone system, the sympathetic nervous system, and reduced nitric oxide–dependent vasodilation,” they wrote. They recommended use of the coronary artery calcium score for screening middle-aged women who are symptomatic or at intermediate cardiovascular risk.
The transition to menopause causes changes in lipid profiles, and a rise in blood pressure in particular “may be both a direct effect of hormonal changes on the vasculature and metabolic changes with aging,” but hypertension in early post menopause is “often poorly managed,” the authors noted.
Compared with asymptomatic women, women who suffer from severe menopausal symptoms often have increased cardiovascular disease risk factors. For example, the Women’s Health Initiative (WHI) study showed a 48% increased risk of incident diabetes at follow-up in women with severe symptoms of hot flashes and night sweats, the authors wrote. Clinicians should also be aware of the increased immune reactivity that occurs during and after menopause and the increased CVD risk associated with autoimmune and endocrine disorders, they said.
Multiple strategies to reduce risk
Strategies to address the cardiovascular risk in menopause include assessing glucose, lipid levels, and blood pressure during the transition to menopause, according to the statement.
In addition, they recommended increasing employer awareness of menopause, as changes may interfere with working ability. A healthy lifestyle including healthy diet and regular exercise can help reduce cardiovascular risks and relieve symptoms. Menopausal hormone therapy (MHT) may be indicated to relieve symptoms, including symptoms of depression, and provide cardioprotection for younger women around the time of menopause, according to the statement.
However, “MHT is not recommended in women at high CV risk and after a previous CVD event,” and all women should be assessed for cardiovascular risk factors before starting MHT, they emphasized.
Results raise awareness of cardiovascular health and menopause link
“Over the past 20 years, our knowledge of how menopause might contribute to cardiovascular disease has dramatically evolved,” said Samar El Khoudary, MD, of the University of Pittsburg, in an interview.
“We have accumulated data that consistently point to the menopause transition as a time of change in cardiovascular health. As such, there is a compelling need to discuss the implications of the accumulating body of literature on this topic,” she said. “The goal is to raise awareness for both health care providers and women of the significant adverse cardiovascular health changes accompanying the menopause transition and to point out the importance of adopting prevention strategies early during this stage,” she explained.
The impact of the hormonal changes of menopause on CVD risk “is very complex,” Dr. El Khoudary said. “Until now, we could not prove that using estrogen therapy is cardioprotective,” she emphasized. “Studies point to the need to consider the timing of hormone use, as well as types and route of administration,” she noted. “The truth is that, although the menopause transition is associated with an acceleration in CVD risk, the exact mechanism still is not completely clear. Hormone changes contribute, but they are not the ultimate contributor,” she added.
Research gaps include data on lifestyle and behavioral interventions
“Irrespective of the accumulating findings showing adverse changes in multiple cardiovascular health parameters, as women transition through menopause, we do not have data documenting current status of ideal cardiovascular health components during the menopause transition among women,” said Dr. El Khoudary. “The limited data we have [suggest] that a very small proportion of women transitioning through menopause eat a healthy diet (less than 20%) or practice physical activity (about7.2%) at a level that matches the current recommendations,” she noted.
“Lifestyle and behavioral interventions are critical to maintain a healthy heart and reduce heart disease; we do not have adequate randomized clinical trials testing these interventions specifically during the menopause transition,” she said.
“Similarly, we are in need of randomized clinical trials of therapeutic interventions such as lipid-lowering medications and menopause hormone therapy in women transitioning through menopause,” said Dr. El Khoudary. “This high-risk population has not been the focus of previous clinical trials, leaving us with questions of how the results from these studies might apply to women during the menopause transition,” she said.
Consensus invites collaboration
“I commend the group for putting together a statement that crosses practice and specialty boundaries,” said Lubna Pal, MD, of Yale School of Medicine, New Haven, Conn., in an interview. Although the statement does not present novel information, it “has the power of unifying the various providers by bringing focus on the individual elements spanning a woman’s life that cumulatively determine her lifetime health risk,” she said. Preeclampsia may be a risk factor for cardiovascular disease later in life, and events in reproductive age may determine a woman’s trajectory during the transition to menopause and beyond, Dr. Pal noted.
“The consensus statement will likely be read by internists and family medicine providers as well as ob.gyns.; it encourages all those involved in caring for female patients to take on the responsibility of ‘passing on the baton,’ such that all women who are deemed at an enhanced risk for cardiovascular disease are assured due diligence in care through stringent surveillance and timely interventions,” said Dr. Pal. “It is a call for the various providers who care for women at distinct stages of life to work together toward a shared goal of optimizing every woman’s health across her lifespan,” she said.
“More research is needed for us to better understand the mechanisms at play” in the development of cardiovascular risk and in understanding the continuity of changes across women’s lifespans, Dr. Pal said. “We have associations, but not much information about causation,” she emphasized. However, the statement promotes the dissemination of information about women’s health and sensitizes providers to the potential and the power of preventive care. “We should be much more liberal and loud in holding conversations about risk quantification and risk reduction, and this statement is a resounding effort toward identifying and mitigating long-term cardiovascular risk, even if only through promoting a healthier lifestyle in those deemed at risk,” she added.
The statement received no outside funding. Lead author Dr. Maas had no financial conflicts to disclose. Dr. El Khoudary had no financial conflicts to disclose. Dr. Pal had no relevant financial conflicts to disclose.
FROM THE EUROPEAN HEART JOURNAL
2021 ACIP adult schedule released
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention has updated its recommended immunization schedule for adults for 2021.
A summary of the annual update was published online Feb. 11 in the CDC’s Morbidity and Mortality Weekly Report and is available in Annals of Internal Medicine and on the CDC website.
It features a special section on vaccination during the pandemic as well as interim recommendations on administering the Pfizer-BioNtech and Moderna COVID-19 vaccines.
The authors, led by Mark S. Freedman, DVM, MPH, DACVPM, of the CDC’s National Center for Immunization and Respiratory Diseases, in Atlanta, note that this year’s recommendations for adults – persons aged 19 years and older – are largely the same as last year’s. “There have been very few changes,” Dr. Freedman said in an interview. “Changes to the schedule tables and notes were made to harmonize to the greatest extent possible the adult and child/adolescent schedules.”
Changes in the schedule include new or updated ACIP recommendations for influenza, hepatitis A, hepatitis B (Hep B), and human papillomavirus (HPV) as well as for meningococcal serogroups A, C, W, and Y (MenACYW) vaccines, meningococcal B (MenB) vaccines, and the zoster vaccine.
Vaccine-specific changes
Influenza
The schedule highlights updates to the composition of several influenza vaccines, which apply to components in both trivalent and quadrivalent formulations.
The cover page abbreviation for live attenuated influenza vaccine (LAIV) was changed to LAIV4. The abbreviation for live recombinant influenza vaccine (RIV) was changed to RIV4.
For individuals with a history of egg allergy who experience reactions other than hives, the following procedural warning has been added: “If using an influenza vaccine other than RIV4 or ccIIV4, administer in medical setting under supervision of health care provider who can recognize and manage severe allergic reactions.”
Zoster
The zoster vaccine live (Zostavax) has been removed from the schedule because it is no longer available in the United States. The recombinant zoster vaccine Shingrix remains available as a 2-dose regimen for adults aged 50 years or older.
HPV
As in previous years, HPV vaccination is routinely recommended for persons aged 11-12 years, with catch-up vaccination for those aged 26 or younger. Catch-up vaccination can be considered with shared decision making for those aged 27 through 45. In this year’s schedule, in the pregnancy column, the color pink, which formerly indicated “delay until after pregnancy,” has been replaced with red and an asterisk, indicating “vaccinate after pregnancy.”
HepB
ACIP continues to recommend vaccination of adults at risk for HepB; however, the text overlay has been changed to read, “2, 3, or 4 doses, depending on vaccine or condition.” Additionally, HepB vaccination is now routinely recommended for adults younger than 60 years with diabetes. For those with diabetes who are older than 60, shared decision making is recommended.
Meningococcal vaccine
ACIP continues to recommend routine vaccination with a quadrivalent meningococcal conjugate vaccine (MenACWY) for persons at increased risk for meningococcal disease caused by serogroups A, C, W, or Y. The MenQuadfi (MenACWY-TT) vaccine, which was first licensed in 2020, has been added to all relevant sections of MenACWY vaccines. For MenACWY booster doses, new text addresses special situations, including outbreaks.
Improvements have been made to text and layout, Dr. Freedman said. An example is the minimizing of specialized text. Other changes were made to ensure more consistent text structure and language. Various fine-tunings of color and positioning were made to the cover page and tables, and the wording of the notes sections was improved.
Vaccination in the pandemic
The updated schedule outlines guidance on the use of COVID-19 vaccines approved by the Food and Drug Administration under emergency use authorization, with interim recommendations for the Pfizer-BioNTech COVID-19 vaccine for people aged 16 and older and the Moderna COVID-19 vaccine for people aged 18 and older.
The authors stress the importance of receiving the recommended routine and catch-up immunizations notwithstanding widespread anxiety about visiting medical offices. Last spring, the CDC reported a dramatic drop in child vaccinations after the declaration of the national emergency in mid-March, a drop attributed to fear of COVID-19 exposure.
“ACIP continued to meet and make recommendations during the pandemic,” Dr. Freedman said. “Our recommendation remains that despite challenges caused by the COVID-19 pandemic, adults and their healthcare providers should follow the recommended vaccine schedule to protect against serious and sometimes deadly diseases.”
Regular vaccines can be safely administered even as COVID-19 retains its grasp on the United States. “Healthcare providers should follow the CDC’s interim guidance for the safe delivery of vaccines during the pandemic, which includes the use of personal protective equipment and physical distancing,” Dr. Freedman said.
Dr. Freedman has disclosed no relevant financial relationships. Coauthor Henry Bernstein, DO, is the editor of the Current Opinion in Pediatrics Office Pediatrics Series, is a Harvard School of Public Health faculty member, and is a member of the data safety and monitoring board for a Takeda study on intrathecal enzymes for Hunter and San Filippo syndromes. Coauthor Kevin Ault, MD, has served on the data safety and monitoring committee for ACI Clinical.
A version of this article first appeared on Medscape.com .
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention has updated its recommended immunization schedule for adults for 2021.
A summary of the annual update was published online Feb. 11 in the CDC’s Morbidity and Mortality Weekly Report and is available in Annals of Internal Medicine and on the CDC website.
It features a special section on vaccination during the pandemic as well as interim recommendations on administering the Pfizer-BioNtech and Moderna COVID-19 vaccines.
The authors, led by Mark S. Freedman, DVM, MPH, DACVPM, of the CDC’s National Center for Immunization and Respiratory Diseases, in Atlanta, note that this year’s recommendations for adults – persons aged 19 years and older – are largely the same as last year’s. “There have been very few changes,” Dr. Freedman said in an interview. “Changes to the schedule tables and notes were made to harmonize to the greatest extent possible the adult and child/adolescent schedules.”
Changes in the schedule include new or updated ACIP recommendations for influenza, hepatitis A, hepatitis B (Hep B), and human papillomavirus (HPV) as well as for meningococcal serogroups A, C, W, and Y (MenACYW) vaccines, meningococcal B (MenB) vaccines, and the zoster vaccine.
Vaccine-specific changes
Influenza
The schedule highlights updates to the composition of several influenza vaccines, which apply to components in both trivalent and quadrivalent formulations.
The cover page abbreviation for live attenuated influenza vaccine (LAIV) was changed to LAIV4. The abbreviation for live recombinant influenza vaccine (RIV) was changed to RIV4.
For individuals with a history of egg allergy who experience reactions other than hives, the following procedural warning has been added: “If using an influenza vaccine other than RIV4 or ccIIV4, administer in medical setting under supervision of health care provider who can recognize and manage severe allergic reactions.”
Zoster
The zoster vaccine live (Zostavax) has been removed from the schedule because it is no longer available in the United States. The recombinant zoster vaccine Shingrix remains available as a 2-dose regimen for adults aged 50 years or older.
HPV
As in previous years, HPV vaccination is routinely recommended for persons aged 11-12 years, with catch-up vaccination for those aged 26 or younger. Catch-up vaccination can be considered with shared decision making for those aged 27 through 45. In this year’s schedule, in the pregnancy column, the color pink, which formerly indicated “delay until after pregnancy,” has been replaced with red and an asterisk, indicating “vaccinate after pregnancy.”
HepB
ACIP continues to recommend vaccination of adults at risk for HepB; however, the text overlay has been changed to read, “2, 3, or 4 doses, depending on vaccine or condition.” Additionally, HepB vaccination is now routinely recommended for adults younger than 60 years with diabetes. For those with diabetes who are older than 60, shared decision making is recommended.
Meningococcal vaccine
ACIP continues to recommend routine vaccination with a quadrivalent meningococcal conjugate vaccine (MenACWY) for persons at increased risk for meningococcal disease caused by serogroups A, C, W, or Y. The MenQuadfi (MenACWY-TT) vaccine, which was first licensed in 2020, has been added to all relevant sections of MenACWY vaccines. For MenACWY booster doses, new text addresses special situations, including outbreaks.
Improvements have been made to text and layout, Dr. Freedman said. An example is the minimizing of specialized text. Other changes were made to ensure more consistent text structure and language. Various fine-tunings of color and positioning were made to the cover page and tables, and the wording of the notes sections was improved.
Vaccination in the pandemic
The updated schedule outlines guidance on the use of COVID-19 vaccines approved by the Food and Drug Administration under emergency use authorization, with interim recommendations for the Pfizer-BioNTech COVID-19 vaccine for people aged 16 and older and the Moderna COVID-19 vaccine for people aged 18 and older.
The authors stress the importance of receiving the recommended routine and catch-up immunizations notwithstanding widespread anxiety about visiting medical offices. Last spring, the CDC reported a dramatic drop in child vaccinations after the declaration of the national emergency in mid-March, a drop attributed to fear of COVID-19 exposure.
“ACIP continued to meet and make recommendations during the pandemic,” Dr. Freedman said. “Our recommendation remains that despite challenges caused by the COVID-19 pandemic, adults and their healthcare providers should follow the recommended vaccine schedule to protect against serious and sometimes deadly diseases.”
Regular vaccines can be safely administered even as COVID-19 retains its grasp on the United States. “Healthcare providers should follow the CDC’s interim guidance for the safe delivery of vaccines during the pandemic, which includes the use of personal protective equipment and physical distancing,” Dr. Freedman said.
Dr. Freedman has disclosed no relevant financial relationships. Coauthor Henry Bernstein, DO, is the editor of the Current Opinion in Pediatrics Office Pediatrics Series, is a Harvard School of Public Health faculty member, and is a member of the data safety and monitoring board for a Takeda study on intrathecal enzymes for Hunter and San Filippo syndromes. Coauthor Kevin Ault, MD, has served on the data safety and monitoring committee for ACI Clinical.
A version of this article first appeared on Medscape.com .
The Advisory Committee on Immunization Practices of the Centers for Disease Control and Prevention has updated its recommended immunization schedule for adults for 2021.
A summary of the annual update was published online Feb. 11 in the CDC’s Morbidity and Mortality Weekly Report and is available in Annals of Internal Medicine and on the CDC website.
It features a special section on vaccination during the pandemic as well as interim recommendations on administering the Pfizer-BioNtech and Moderna COVID-19 vaccines.
The authors, led by Mark S. Freedman, DVM, MPH, DACVPM, of the CDC’s National Center for Immunization and Respiratory Diseases, in Atlanta, note that this year’s recommendations for adults – persons aged 19 years and older – are largely the same as last year’s. “There have been very few changes,” Dr. Freedman said in an interview. “Changes to the schedule tables and notes were made to harmonize to the greatest extent possible the adult and child/adolescent schedules.”
Changes in the schedule include new or updated ACIP recommendations for influenza, hepatitis A, hepatitis B (Hep B), and human papillomavirus (HPV) as well as for meningococcal serogroups A, C, W, and Y (MenACYW) vaccines, meningococcal B (MenB) vaccines, and the zoster vaccine.
Vaccine-specific changes
Influenza
The schedule highlights updates to the composition of several influenza vaccines, which apply to components in both trivalent and quadrivalent formulations.
The cover page abbreviation for live attenuated influenza vaccine (LAIV) was changed to LAIV4. The abbreviation for live recombinant influenza vaccine (RIV) was changed to RIV4.
For individuals with a history of egg allergy who experience reactions other than hives, the following procedural warning has been added: “If using an influenza vaccine other than RIV4 or ccIIV4, administer in medical setting under supervision of health care provider who can recognize and manage severe allergic reactions.”
Zoster
The zoster vaccine live (Zostavax) has been removed from the schedule because it is no longer available in the United States. The recombinant zoster vaccine Shingrix remains available as a 2-dose regimen for adults aged 50 years or older.
HPV
As in previous years, HPV vaccination is routinely recommended for persons aged 11-12 years, with catch-up vaccination for those aged 26 or younger. Catch-up vaccination can be considered with shared decision making for those aged 27 through 45. In this year’s schedule, in the pregnancy column, the color pink, which formerly indicated “delay until after pregnancy,” has been replaced with red and an asterisk, indicating “vaccinate after pregnancy.”
HepB
ACIP continues to recommend vaccination of adults at risk for HepB; however, the text overlay has been changed to read, “2, 3, or 4 doses, depending on vaccine or condition.” Additionally, HepB vaccination is now routinely recommended for adults younger than 60 years with diabetes. For those with diabetes who are older than 60, shared decision making is recommended.
Meningococcal vaccine
ACIP continues to recommend routine vaccination with a quadrivalent meningococcal conjugate vaccine (MenACWY) for persons at increased risk for meningococcal disease caused by serogroups A, C, W, or Y. The MenQuadfi (MenACWY-TT) vaccine, which was first licensed in 2020, has been added to all relevant sections of MenACWY vaccines. For MenACWY booster doses, new text addresses special situations, including outbreaks.
Improvements have been made to text and layout, Dr. Freedman said. An example is the minimizing of specialized text. Other changes were made to ensure more consistent text structure and language. Various fine-tunings of color and positioning were made to the cover page and tables, and the wording of the notes sections was improved.
Vaccination in the pandemic
The updated schedule outlines guidance on the use of COVID-19 vaccines approved by the Food and Drug Administration under emergency use authorization, with interim recommendations for the Pfizer-BioNTech COVID-19 vaccine for people aged 16 and older and the Moderna COVID-19 vaccine for people aged 18 and older.
The authors stress the importance of receiving the recommended routine and catch-up immunizations notwithstanding widespread anxiety about visiting medical offices. Last spring, the CDC reported a dramatic drop in child vaccinations after the declaration of the national emergency in mid-March, a drop attributed to fear of COVID-19 exposure.
“ACIP continued to meet and make recommendations during the pandemic,” Dr. Freedman said. “Our recommendation remains that despite challenges caused by the COVID-19 pandemic, adults and their healthcare providers should follow the recommended vaccine schedule to protect against serious and sometimes deadly diseases.”
Regular vaccines can be safely administered even as COVID-19 retains its grasp on the United States. “Healthcare providers should follow the CDC’s interim guidance for the safe delivery of vaccines during the pandemic, which includes the use of personal protective equipment and physical distancing,” Dr. Freedman said.
Dr. Freedman has disclosed no relevant financial relationships. Coauthor Henry Bernstein, DO, is the editor of the Current Opinion in Pediatrics Office Pediatrics Series, is a Harvard School of Public Health faculty member, and is a member of the data safety and monitoring board for a Takeda study on intrathecal enzymes for Hunter and San Filippo syndromes. Coauthor Kevin Ault, MD, has served on the data safety and monitoring committee for ACI Clinical.
A version of this article first appeared on Medscape.com .
The true measure of cluster headache
Patients with cluster headache face a double whammy: Physicians too often fail to recognize it, and their condition is among the most severe and debilitating among headache types. In fact,
The study’s comparison of cluster headaches to other common painful experiences can help nonsufferers relate to the experience, said Larry Schor, PhD, a coauthor of the paper. “Headache is a terrible word. Bee stings sting, burns burn. [A cluster headache] doesn’t ache. It’s a piercing intensity like you just can’t believe,” said Dr. Schor, professor of psychology at the University of West Georgia, Carrollton, and a cluster headache patient since he first experienced an attack at the age of 21.
The study was published in the January 2021 issue of Headache.
Ranking cluster headaches as worse than experiences such as childbirth or kidney stones is “kind of eye opening, and helps to describe the experience in terms that more people can relate to. I think it helps to share the experience of cluster headache more broadly, because we’re in a situation where cluster headache remains underfunded, and we don’t have enough treatments for it. I think one way to overcome that is to spread awareness of what this problem is, and the impact it has on human life,” said Rashmi Halker Singh, MD, associate professor of neurology at the Mayo Clinic in Scottsdale, Ariz., and deputy editor of Headache. She was not involved in the study.
Dr. Schor called for physicians to consider cluster headache an emergency, because of the severity of pain and also the potential for suicidality. Treatments remain comparatively sparse, but high-flow oxygen can help some patients, and intranasal or intravenous triptans can treat acute pain. In 2018, the Food and Drug Administration approved galcanezumab (Eli Lilly) for prevention of episodic cluster headaches.
But cluster headaches are often misdiagnosed. For many patients, it takes more than a year or even as long as 5 years to get an accurate diagnosis, according to Dr. Schor. Women may be particularly vulnerable to misdiagnosis, because migraines are more common in women. It doesn’t help that many neurologists are taught that cluster headache is primarily a male disease. “Because that idea is so ingrained, I think a lot of women who have cluster headache are probably missed and told they have migraine instead. There are a lot of women who have cluster headache, and that gender difference might not be as big a difference as we were initially taught. We need to do a better job of recognizing cluster headache to better understand what the true prevalence is,” said Dr. Halker Singh.
She noted that patients with side-locked headache should be evaluated for cluster headache, and asked how long the pain lasts in the absence of medication. “Also ask about the presence of cranial autonomic symptoms, and if they occur in the context of headache pain, and if they are side-locked to the side of the headache. Those are important questions that can tease out cluster headache from other conditions,” said Dr. Halker Singh.
For the survey, the researchers asked 1,604 patients with cluster headache patients to rate pain on a scale of 1 to 10. Cluster headache ranked highest at 9.7, then labor pain (7.2), pancreatitis (7.0), and nephrolithiasis (6.9). Cluster headache pain was ranked at 10.0 by 72.1% of respondents. Those reporting maximal pain or were more likely to have cranial autonomic features in comparison with patients who reported less pain, including conjunctival injection or lacrimation (91% versus 85%), eyelid edema (77% versus 66%), forehead/facial sweating (60% versus 49%), fullness in the ear (47% versus 35%), and miosis or ptosis (85% versus 75%). They had more frequent attacks (4.0 versus 3.5 per day), higher Hopelessness Depression Symptom Questionnaire scores (24.5 versus 21.1), and reduced effectiveness of calcium channel blockers (2.2 versus 2.5 on a 5-point Likert scale). They were more often female (34% versus 24%). (P < .001 for all).
The study received funding from Autonomic Technologies and Cluster Busters. Dr. Schor and Dr. Halker Singh had no relevant financial disclosures.
Patients with cluster headache face a double whammy: Physicians too often fail to recognize it, and their condition is among the most severe and debilitating among headache types. In fact,
The study’s comparison of cluster headaches to other common painful experiences can help nonsufferers relate to the experience, said Larry Schor, PhD, a coauthor of the paper. “Headache is a terrible word. Bee stings sting, burns burn. [A cluster headache] doesn’t ache. It’s a piercing intensity like you just can’t believe,” said Dr. Schor, professor of psychology at the University of West Georgia, Carrollton, and a cluster headache patient since he first experienced an attack at the age of 21.
The study was published in the January 2021 issue of Headache.
Ranking cluster headaches as worse than experiences such as childbirth or kidney stones is “kind of eye opening, and helps to describe the experience in terms that more people can relate to. I think it helps to share the experience of cluster headache more broadly, because we’re in a situation where cluster headache remains underfunded, and we don’t have enough treatments for it. I think one way to overcome that is to spread awareness of what this problem is, and the impact it has on human life,” said Rashmi Halker Singh, MD, associate professor of neurology at the Mayo Clinic in Scottsdale, Ariz., and deputy editor of Headache. She was not involved in the study.
Dr. Schor called for physicians to consider cluster headache an emergency, because of the severity of pain and also the potential for suicidality. Treatments remain comparatively sparse, but high-flow oxygen can help some patients, and intranasal or intravenous triptans can treat acute pain. In 2018, the Food and Drug Administration approved galcanezumab (Eli Lilly) for prevention of episodic cluster headaches.
But cluster headaches are often misdiagnosed. For many patients, it takes more than a year or even as long as 5 years to get an accurate diagnosis, according to Dr. Schor. Women may be particularly vulnerable to misdiagnosis, because migraines are more common in women. It doesn’t help that many neurologists are taught that cluster headache is primarily a male disease. “Because that idea is so ingrained, I think a lot of women who have cluster headache are probably missed and told they have migraine instead. There are a lot of women who have cluster headache, and that gender difference might not be as big a difference as we were initially taught. We need to do a better job of recognizing cluster headache to better understand what the true prevalence is,” said Dr. Halker Singh.
She noted that patients with side-locked headache should be evaluated for cluster headache, and asked how long the pain lasts in the absence of medication. “Also ask about the presence of cranial autonomic symptoms, and if they occur in the context of headache pain, and if they are side-locked to the side of the headache. Those are important questions that can tease out cluster headache from other conditions,” said Dr. Halker Singh.
For the survey, the researchers asked 1,604 patients with cluster headache patients to rate pain on a scale of 1 to 10. Cluster headache ranked highest at 9.7, then labor pain (7.2), pancreatitis (7.0), and nephrolithiasis (6.9). Cluster headache pain was ranked at 10.0 by 72.1% of respondents. Those reporting maximal pain or were more likely to have cranial autonomic features in comparison with patients who reported less pain, including conjunctival injection or lacrimation (91% versus 85%), eyelid edema (77% versus 66%), forehead/facial sweating (60% versus 49%), fullness in the ear (47% versus 35%), and miosis or ptosis (85% versus 75%). They had more frequent attacks (4.0 versus 3.5 per day), higher Hopelessness Depression Symptom Questionnaire scores (24.5 versus 21.1), and reduced effectiveness of calcium channel blockers (2.2 versus 2.5 on a 5-point Likert scale). They were more often female (34% versus 24%). (P < .001 for all).
The study received funding from Autonomic Technologies and Cluster Busters. Dr. Schor and Dr. Halker Singh had no relevant financial disclosures.
Patients with cluster headache face a double whammy: Physicians too often fail to recognize it, and their condition is among the most severe and debilitating among headache types. In fact,
The study’s comparison of cluster headaches to other common painful experiences can help nonsufferers relate to the experience, said Larry Schor, PhD, a coauthor of the paper. “Headache is a terrible word. Bee stings sting, burns burn. [A cluster headache] doesn’t ache. It’s a piercing intensity like you just can’t believe,” said Dr. Schor, professor of psychology at the University of West Georgia, Carrollton, and a cluster headache patient since he first experienced an attack at the age of 21.
The study was published in the January 2021 issue of Headache.
Ranking cluster headaches as worse than experiences such as childbirth or kidney stones is “kind of eye opening, and helps to describe the experience in terms that more people can relate to. I think it helps to share the experience of cluster headache more broadly, because we’re in a situation where cluster headache remains underfunded, and we don’t have enough treatments for it. I think one way to overcome that is to spread awareness of what this problem is, and the impact it has on human life,” said Rashmi Halker Singh, MD, associate professor of neurology at the Mayo Clinic in Scottsdale, Ariz., and deputy editor of Headache. She was not involved in the study.
Dr. Schor called for physicians to consider cluster headache an emergency, because of the severity of pain and also the potential for suicidality. Treatments remain comparatively sparse, but high-flow oxygen can help some patients, and intranasal or intravenous triptans can treat acute pain. In 2018, the Food and Drug Administration approved galcanezumab (Eli Lilly) for prevention of episodic cluster headaches.
But cluster headaches are often misdiagnosed. For many patients, it takes more than a year or even as long as 5 years to get an accurate diagnosis, according to Dr. Schor. Women may be particularly vulnerable to misdiagnosis, because migraines are more common in women. It doesn’t help that many neurologists are taught that cluster headache is primarily a male disease. “Because that idea is so ingrained, I think a lot of women who have cluster headache are probably missed and told they have migraine instead. There are a lot of women who have cluster headache, and that gender difference might not be as big a difference as we were initially taught. We need to do a better job of recognizing cluster headache to better understand what the true prevalence is,” said Dr. Halker Singh.
She noted that patients with side-locked headache should be evaluated for cluster headache, and asked how long the pain lasts in the absence of medication. “Also ask about the presence of cranial autonomic symptoms, and if they occur in the context of headache pain, and if they are side-locked to the side of the headache. Those are important questions that can tease out cluster headache from other conditions,” said Dr. Halker Singh.
For the survey, the researchers asked 1,604 patients with cluster headache patients to rate pain on a scale of 1 to 10. Cluster headache ranked highest at 9.7, then labor pain (7.2), pancreatitis (7.0), and nephrolithiasis (6.9). Cluster headache pain was ranked at 10.0 by 72.1% of respondents. Those reporting maximal pain or were more likely to have cranial autonomic features in comparison with patients who reported less pain, including conjunctival injection or lacrimation (91% versus 85%), eyelid edema (77% versus 66%), forehead/facial sweating (60% versus 49%), fullness in the ear (47% versus 35%), and miosis or ptosis (85% versus 75%). They had more frequent attacks (4.0 versus 3.5 per day), higher Hopelessness Depression Symptom Questionnaire scores (24.5 versus 21.1), and reduced effectiveness of calcium channel blockers (2.2 versus 2.5 on a 5-point Likert scale). They were more often female (34% versus 24%). (P < .001 for all).
The study received funding from Autonomic Technologies and Cluster Busters. Dr. Schor and Dr. Halker Singh had no relevant financial disclosures.
FROM HEADACHE
PPE protected critical care staff from COVID-19 transmission
, a new study has found.
“Other staff, other areas of the hospital, and the wider community are more likely sources of infection,” said lead author Kate El Bouzidi, MRCP, South London Specialist Virology Centre, King’s College Hospital NHS Foundation Trust, London.
She noted that 60% of critical care staff were symptomatic during the first wave of the coronavirus pandemic and 20% were antibody positive, with 10% asymptomatic. “Staff acquisition peaked 3 weeks before the peak of COVID-19 ICU admission, and personal protective equipment (PPE) was effective at preventing transmission from patients.” Working in other areas of the hospital was associated with higher seroprevalence, Dr. El Bouzidi noted.
The findings were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The novel coronavirus was spreading around the world, and when it reached northern Italy, medical authorities began to think in terms of how it might overwhelm the health care system in the United Kingdom, explained Dr. El Bouzidi.
“There was a lot of interest at this time about health care workers who were particularly vulnerable and also about the allocation of resources and rationing of care, particularly in intensive care,” she said. “And this only intensified when our prime minister was admitted to intensive care. About this time, antibody testing also became available.”
The goal of their study was to determine the SARS-CoV-2 seroprevalence in critical care staff, as well as look at the correlation between antibody status, prior swab testing, and COVID-19 symptoms.
The survey was conducted at Kings College Hospital in London, which is a tertiary-care teaching center. The critical care department is one of the largest in the United Kingdom. The authors estimate that more than 800 people worked in the critical care units, and between March and April 2020, more than 2,000 patients with COVID-19 were admitted, of whom 180 required care in the ICU.
“There was good PPE available in the ICU units right from the start,” she said, “and staff testing was available.”
All staff working in the critical care department participated in the study, which required serum samples and completion of a questionnaire. The samples were tested via six different assays to measure receptor-binding domain, nucleoprotein, and tri-spike, with one antibody result determined for each sample.
Of the 625 staff members, 384 (61.4%) had previously reported experiencing symptoms and 124 (19.8%) had sent a swab for testing. COVID-19 infection had been confirmed in 37 of those health care workers (29.8%).
Overall, 21% were positive for SARS-CoV-2 antibodies, of whom 9.9% had been asymptomatic.
“We were surprised to find that 61% of staff reported symptoms they felt could be consistent with COVID-19,” she said, noting that fatigue, headache, and cough were the most common symptoms reported. “Seroprevalence was reported in 31% of symptomatic staff and in 5% of those without symptoms.”
Seroprevalence differed by role in a critical care unit, although it did not significantly differ by factors such as age, sex, ethnicity, or underlying conditions. Consultants, who are senior physicians, were twice as likely to test positive, compared with junior doctors. The reason for this finding is not clear, but it may lie in the nature of their work responsibilities, such as performing more aerosol-generating procedures in the ICU or in other departments.
The investigators looked at the timing of infections and found that they preceded peak of patient admissions by 3 weeks, with peak onset of staff symptoms in early March. At this time, Dr. El Bouzidi noted, there were very few patients with COVID-19 in the hospital, and good PPE was available throughout this time period.
“Staff were unlikely to be infected by ICU patients, and therefore PPE was largely effective,” she said. “Other sources of infection were more likely to be the cause, such as interactions with other staff, meetings, or contact in break rooms. Routine mask-wearing throughout the hospital was only encouraged as of June 15.”
There were several limitations to the study, such as the cross-sectional design, reliance on response/recall, the fact that antibody tests are unlikely to detect all previous infections, and no genomic data were available to confirm infections. Even though the study had limitations, Dr. El Bouzidi concluded that ICU staff are unlikely to contract COVID-19 from patients but that other staff, other areas of the hospital, and the wider community are more likely sources of infection.
These findings, she added, demonstrate that PPE was effective at preventing transmission from patients and that protective measures need to be maintained when staff is away from the bedside.
In commenting on the study, Greg S. Martin, MD, professor of medicine in the division of pulmonary, allergy, critical care and sleep medicine, Emory University, Atlanta, noted that, even though the study was conducted almost a year ago, the results are still relevant with regard to the effectiveness of PPE.
“There was a huge amount of uncertainty about PPE – what was most effective, could we reuse it, how to sterilize it, what about surfaces, and so on,” he said. “Even for people who work in ICU and who are familiar with the environment and familiar with the patients, there was 1,000 times more uncertainty about everything they were doing.”
Dr. Martin believes that the situation has improved. “It’s not that we take COVID more lightly, but I think the staff is more comfortable dealing with it,” he said. “They now know what they need to do on an hourly and daily basis to stay safe. The PPE had become second nature to them now, with all the other precautions.”
, a new study has found.
“Other staff, other areas of the hospital, and the wider community are more likely sources of infection,” said lead author Kate El Bouzidi, MRCP, South London Specialist Virology Centre, King’s College Hospital NHS Foundation Trust, London.
She noted that 60% of critical care staff were symptomatic during the first wave of the coronavirus pandemic and 20% were antibody positive, with 10% asymptomatic. “Staff acquisition peaked 3 weeks before the peak of COVID-19 ICU admission, and personal protective equipment (PPE) was effective at preventing transmission from patients.” Working in other areas of the hospital was associated with higher seroprevalence, Dr. El Bouzidi noted.
The findings were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The novel coronavirus was spreading around the world, and when it reached northern Italy, medical authorities began to think in terms of how it might overwhelm the health care system in the United Kingdom, explained Dr. El Bouzidi.
“There was a lot of interest at this time about health care workers who were particularly vulnerable and also about the allocation of resources and rationing of care, particularly in intensive care,” she said. “And this only intensified when our prime minister was admitted to intensive care. About this time, antibody testing also became available.”
The goal of their study was to determine the SARS-CoV-2 seroprevalence in critical care staff, as well as look at the correlation between antibody status, prior swab testing, and COVID-19 symptoms.
The survey was conducted at Kings College Hospital in London, which is a tertiary-care teaching center. The critical care department is one of the largest in the United Kingdom. The authors estimate that more than 800 people worked in the critical care units, and between March and April 2020, more than 2,000 patients with COVID-19 were admitted, of whom 180 required care in the ICU.
“There was good PPE available in the ICU units right from the start,” she said, “and staff testing was available.”
All staff working in the critical care department participated in the study, which required serum samples and completion of a questionnaire. The samples were tested via six different assays to measure receptor-binding domain, nucleoprotein, and tri-spike, with one antibody result determined for each sample.
Of the 625 staff members, 384 (61.4%) had previously reported experiencing symptoms and 124 (19.8%) had sent a swab for testing. COVID-19 infection had been confirmed in 37 of those health care workers (29.8%).
Overall, 21% were positive for SARS-CoV-2 antibodies, of whom 9.9% had been asymptomatic.
“We were surprised to find that 61% of staff reported symptoms they felt could be consistent with COVID-19,” she said, noting that fatigue, headache, and cough were the most common symptoms reported. “Seroprevalence was reported in 31% of symptomatic staff and in 5% of those without symptoms.”
Seroprevalence differed by role in a critical care unit, although it did not significantly differ by factors such as age, sex, ethnicity, or underlying conditions. Consultants, who are senior physicians, were twice as likely to test positive, compared with junior doctors. The reason for this finding is not clear, but it may lie in the nature of their work responsibilities, such as performing more aerosol-generating procedures in the ICU or in other departments.
The investigators looked at the timing of infections and found that they preceded peak of patient admissions by 3 weeks, with peak onset of staff symptoms in early March. At this time, Dr. El Bouzidi noted, there were very few patients with COVID-19 in the hospital, and good PPE was available throughout this time period.
“Staff were unlikely to be infected by ICU patients, and therefore PPE was largely effective,” she said. “Other sources of infection were more likely to be the cause, such as interactions with other staff, meetings, or contact in break rooms. Routine mask-wearing throughout the hospital was only encouraged as of June 15.”
There were several limitations to the study, such as the cross-sectional design, reliance on response/recall, the fact that antibody tests are unlikely to detect all previous infections, and no genomic data were available to confirm infections. Even though the study had limitations, Dr. El Bouzidi concluded that ICU staff are unlikely to contract COVID-19 from patients but that other staff, other areas of the hospital, and the wider community are more likely sources of infection.
These findings, she added, demonstrate that PPE was effective at preventing transmission from patients and that protective measures need to be maintained when staff is away from the bedside.
In commenting on the study, Greg S. Martin, MD, professor of medicine in the division of pulmonary, allergy, critical care and sleep medicine, Emory University, Atlanta, noted that, even though the study was conducted almost a year ago, the results are still relevant with regard to the effectiveness of PPE.
“There was a huge amount of uncertainty about PPE – what was most effective, could we reuse it, how to sterilize it, what about surfaces, and so on,” he said. “Even for people who work in ICU and who are familiar with the environment and familiar with the patients, there was 1,000 times more uncertainty about everything they were doing.”
Dr. Martin believes that the situation has improved. “It’s not that we take COVID more lightly, but I think the staff is more comfortable dealing with it,” he said. “They now know what they need to do on an hourly and daily basis to stay safe. The PPE had become second nature to them now, with all the other precautions.”
, a new study has found.
“Other staff, other areas of the hospital, and the wider community are more likely sources of infection,” said lead author Kate El Bouzidi, MRCP, South London Specialist Virology Centre, King’s College Hospital NHS Foundation Trust, London.
She noted that 60% of critical care staff were symptomatic during the first wave of the coronavirus pandemic and 20% were antibody positive, with 10% asymptomatic. “Staff acquisition peaked 3 weeks before the peak of COVID-19 ICU admission, and personal protective equipment (PPE) was effective at preventing transmission from patients.” Working in other areas of the hospital was associated with higher seroprevalence, Dr. El Bouzidi noted.
The findings were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The novel coronavirus was spreading around the world, and when it reached northern Italy, medical authorities began to think in terms of how it might overwhelm the health care system in the United Kingdom, explained Dr. El Bouzidi.
“There was a lot of interest at this time about health care workers who were particularly vulnerable and also about the allocation of resources and rationing of care, particularly in intensive care,” she said. “And this only intensified when our prime minister was admitted to intensive care. About this time, antibody testing also became available.”
The goal of their study was to determine the SARS-CoV-2 seroprevalence in critical care staff, as well as look at the correlation between antibody status, prior swab testing, and COVID-19 symptoms.
The survey was conducted at Kings College Hospital in London, which is a tertiary-care teaching center. The critical care department is one of the largest in the United Kingdom. The authors estimate that more than 800 people worked in the critical care units, and between March and April 2020, more than 2,000 patients with COVID-19 were admitted, of whom 180 required care in the ICU.
“There was good PPE available in the ICU units right from the start,” she said, “and staff testing was available.”
All staff working in the critical care department participated in the study, which required serum samples and completion of a questionnaire. The samples were tested via six different assays to measure receptor-binding domain, nucleoprotein, and tri-spike, with one antibody result determined for each sample.
Of the 625 staff members, 384 (61.4%) had previously reported experiencing symptoms and 124 (19.8%) had sent a swab for testing. COVID-19 infection had been confirmed in 37 of those health care workers (29.8%).
Overall, 21% were positive for SARS-CoV-2 antibodies, of whom 9.9% had been asymptomatic.
“We were surprised to find that 61% of staff reported symptoms they felt could be consistent with COVID-19,” she said, noting that fatigue, headache, and cough were the most common symptoms reported. “Seroprevalence was reported in 31% of symptomatic staff and in 5% of those without symptoms.”
Seroprevalence differed by role in a critical care unit, although it did not significantly differ by factors such as age, sex, ethnicity, or underlying conditions. Consultants, who are senior physicians, were twice as likely to test positive, compared with junior doctors. The reason for this finding is not clear, but it may lie in the nature of their work responsibilities, such as performing more aerosol-generating procedures in the ICU or in other departments.
The investigators looked at the timing of infections and found that they preceded peak of patient admissions by 3 weeks, with peak onset of staff symptoms in early March. At this time, Dr. El Bouzidi noted, there were very few patients with COVID-19 in the hospital, and good PPE was available throughout this time period.
“Staff were unlikely to be infected by ICU patients, and therefore PPE was largely effective,” she said. “Other sources of infection were more likely to be the cause, such as interactions with other staff, meetings, or contact in break rooms. Routine mask-wearing throughout the hospital was only encouraged as of June 15.”
There were several limitations to the study, such as the cross-sectional design, reliance on response/recall, the fact that antibody tests are unlikely to detect all previous infections, and no genomic data were available to confirm infections. Even though the study had limitations, Dr. El Bouzidi concluded that ICU staff are unlikely to contract COVID-19 from patients but that other staff, other areas of the hospital, and the wider community are more likely sources of infection.
These findings, she added, demonstrate that PPE was effective at preventing transmission from patients and that protective measures need to be maintained when staff is away from the bedside.
In commenting on the study, Greg S. Martin, MD, professor of medicine in the division of pulmonary, allergy, critical care and sleep medicine, Emory University, Atlanta, noted that, even though the study was conducted almost a year ago, the results are still relevant with regard to the effectiveness of PPE.
“There was a huge amount of uncertainty about PPE – what was most effective, could we reuse it, how to sterilize it, what about surfaces, and so on,” he said. “Even for people who work in ICU and who are familiar with the environment and familiar with the patients, there was 1,000 times more uncertainty about everything they were doing.”
Dr. Martin believes that the situation has improved. “It’s not that we take COVID more lightly, but I think the staff is more comfortable dealing with it,” he said. “They now know what they need to do on an hourly and daily basis to stay safe. The PPE had become second nature to them now, with all the other precautions.”
FROM CCC50