User login
Findings could change breast cancer risk management
The findings come from two large studies, both published on Jan. 20 in the New England Journal of Medicine.
The two articles are “extraordinary” for broadening and validating the genomic panel to help screen women at risk for breast cancer in the future, commented Eric Topol, MD, professor of molecular medicine, Scripps Research, La Jolla, Calif., and Medscape editor in chief.
“Traditionally, genetic testing of inherited breast cancer genes has focused on women at high risk who have a strong family history of breast cancer or those who were diagnosed at an early age, such as under 45 years,” commented the lead investigator of one of the studies, Fergus Couch, PhD, a pathologist at the Mayo Clinic, Rochester, Minn.
“[Although] the risk of developing breast cancer is generally lower for women without a family history of the disease ... when we looked at all women, we found that 30% of breast cancer mutations occurred in women who are not high risk,” he said.
In both studies, mutations or variants in eight genes – BRCA1, BRCA2, PALB2, BARD1, RAD51C, RAD51D, ATM, and CHEK2 – were found to be significantly associated with breast cancer risk.
However, the distribution of mutations among women with breast cancer differed from the distribution among unaffected women, noted Steven Narod, MD, from the Women’s College Research Institute, Toronto, in an accompanying editorial.
“What this means to clinicians, now that we are expanding the use of gene-panel testing to include unaffected women with a moderate risk of breast cancer in the family history, is that our time will increasingly be spent counseling women with CHEK2 and ATM mutations,” he wrote. Currently, these two are “clumped in with ‘other genes.’ ... Most of the pretest discussion is currently focused on the implications of finding a BRCA1 or BRCA2 mutation.”
The new findings may lead to new risk management strategies, he suggested. “Most breast cancers that occur in women with a mutation in ATM or CHEK2 are estrogen receptor positive, so these women may be candidates for antiestrogen therapies such as tamoxifen, raloxifene, or aromatase inhibitors,” he wrote.
Dr. Narod observed that, for now, the management of most women with either mutation will consist of screening alone, starting with MRI at age 40 years.
The medical community is not ready yet to expand genetic screening to the general population, cautions Walton Taylor, MD, past president of the American Society of Breast Surgeons.
The ASBrS currently recommends that all patients with breast cancer as well as those at high risk for breast cancer be offered genetic testing. “All women at risk should be tested, and all patients with pathogenic variants need to be managed appropriately – it saves lives,” Dr. Taylor emphasized.
However, “unaffected people with no family history do not need genetic testing at this time,” he said in an interview.
As to what physicians might do to better manage patients with mutations that predispose to breast cancer, Dr. Taylor said, “It’s surprisingly easy.”
Every genetic testing company provides genetic counselors to guide patients through next steps, Dr. Taylor pointed out, and most cancer patients have nurse navigators who make sure patients get tested and followed appropriately.
Members of the ASBrS follow the National Comprehensive Cancer Network guidelines when they identify carriers of a pathogenic variant. Dr. Taylor said these are very useful guidelines for virtually all mutations identified thus far.
“This research is not necessarily new, but it is confirmatory for what we are doing, and that helps us make sure we are going down the right pathway,” Dr. Taylor said. “It confirms that what we think is right is right – and that matters,.”
CARRIERS consortium findings
The study led by Dr. Couch was carried out by the Cancer Risk Estimates Related to Susceptibility (CARRIERS) consortium. It involved analyzing data from 17 epidemiology studies that focused on women in the general population who develop breast cancer. For the studies, which were conducted in the United States, pathogenic variants in 28 cancer-predisposition genes were sequenced from 32,247 women with breast cancer (case patients) and 32,544 unaffected women (control persons).
In the overall CARRIERS analysis, the prevalence of pathogenic variants in 12 clinically actionable genes was 5.03% among case patients and 1.63% among control persons. The prevalence was similar in non-Hispanic White women, non-Hispanic Black women, and Hispanic case patients, as well as control persons, they added. The prevalence of pathogenic variants among Asian American case patients was lower, at only 1.64%.
Among patients who had breast cancer, the most common pathogenic variants included BRCA2, which occurred in 1.29% of case patients, followed by CHEK2, at a prevalence of 1.08%, and BRCA1, at a prevalence of 0.85%.
Mutations in BRCA1 increased the risk for breast cancer more than 7.5-fold; mutations in BRCA2 increased that risk more than fivefold, the investigators stated.
Mutations in PALB2 increased the risk of breast cancer approximately fourfold, they added.
Prevalence rates for both BRCA1 and BRCA2 among breast cancer patients declined rapidly after the age of 40. The decline in other variants, including ATM, CHEK2, and PALB2, was limited with increasing age.
Indeed, mutations in all five of these genes were associated with a lifetime absolute risk for breast cancer greater than 20% by the age of 85 years among non-Hispanic Whites.
Pathogenic variants in BRCA1 or BRCA2 yielded a lifetime risk for breast cancer of approximately 50%. Mutations in PALB2 yielded a lifetime breast cancer risk of approximately 32%.
The risk of having a mutation in specific genes varied depending on the type of breast cancer. For example, mutations in BARD1, RAD51C, and RAD51D increased the risk for estrogen receptor (ER)–negative breast cancer as well as triple-negative breast cancer, the authors noted, whereas mutations in ATM, CDH1, and CHEK2 increased the risk for ER-positive breast cancer.
“These refined estimates of the prevalences of pathogenic variants among women with breast cancer in the overall population, as opposed to selected high-risk patients, may inform ongoing discussions regarding testing in patients with breast cancer,” the CARRIERS authors observed.
“The risks of breast cancer associated with pathogenic variants in the genes evaluated in the population-based CARRIERS analysis also provide important information for risk assessment and counseling of women with breast cancer who do not meet high-risk selection criteria,” they suggested.
Similar findings in second study
The second study was conducted by the Breast Cancer Association Consortium under lead author Leila Dorling, PhD, University of Cambridge (England). This group sequenced 34 susceptibility genes from 60,466 women with breast cancer and 53,461 unaffected control persons.
“Protein-truncating variants in five genes (ATM, BRCA1, BRCA2, CHEK2, and PALB2) were associated with a significant risk of breast cancer overall (P < .0001),” the BCAC members reported. “For these genes, odds ratios ranged from 2.10 to 10.57.”
The association between overall breast cancer risk and mutations in seven other genes was more modest, conferring approximately twice the risk for breast cancer overall, although that risk was threefold higher for the TP53 mutation.
For the 12 genes the consortium singled out as being associated with either a significant or a more modest risk for breast cancer, the effect size did not vary significantly between European and Asian women, the authors noted. Again, the risk for ER-positive breast cancer was over two times greater for those who had either the ATM or the CHEK2 mutation. Having mutations in BARD1, BRCA1, BRCA1, PALB2, RAD51C, and RAD51D conferred a higher risk for ER-negative disease than for ER-positive disease.
There was also an association between rare missense variants in six genes – CHEK2, ATM, TP53, BRCA1, CDH1, and RECQL – and overall breast cancer risk, with the clearest evidence being for CHEK2.
“The absolute risk estimates place protein-truncating variants in BRCA1, BRCA2, and PALB2 in the high-risk category and place protein-truncating variants in ATM, BARD1, CHEK2, RAD51CC, and RAD51D in the moderate-risk category,” Dr. Dorling and colleagues reaffirmed.
“These results may guide screening as well as prevention with risk-reducing surgery or medication, in accordance with national guidelines,” the authors suggested.
The CARRIERS study was supported by the National Institutes of Health. The study by Dr. Dorling and colleagues was supported by the European Union Horizon 2020 research and innovation programs, among others. Dr. Narod disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The findings come from two large studies, both published on Jan. 20 in the New England Journal of Medicine.
The two articles are “extraordinary” for broadening and validating the genomic panel to help screen women at risk for breast cancer in the future, commented Eric Topol, MD, professor of molecular medicine, Scripps Research, La Jolla, Calif., and Medscape editor in chief.
“Traditionally, genetic testing of inherited breast cancer genes has focused on women at high risk who have a strong family history of breast cancer or those who were diagnosed at an early age, such as under 45 years,” commented the lead investigator of one of the studies, Fergus Couch, PhD, a pathologist at the Mayo Clinic, Rochester, Minn.
“[Although] the risk of developing breast cancer is generally lower for women without a family history of the disease ... when we looked at all women, we found that 30% of breast cancer mutations occurred in women who are not high risk,” he said.
In both studies, mutations or variants in eight genes – BRCA1, BRCA2, PALB2, BARD1, RAD51C, RAD51D, ATM, and CHEK2 – were found to be significantly associated with breast cancer risk.
However, the distribution of mutations among women with breast cancer differed from the distribution among unaffected women, noted Steven Narod, MD, from the Women’s College Research Institute, Toronto, in an accompanying editorial.
“What this means to clinicians, now that we are expanding the use of gene-panel testing to include unaffected women with a moderate risk of breast cancer in the family history, is that our time will increasingly be spent counseling women with CHEK2 and ATM mutations,” he wrote. Currently, these two are “clumped in with ‘other genes.’ ... Most of the pretest discussion is currently focused on the implications of finding a BRCA1 or BRCA2 mutation.”
The new findings may lead to new risk management strategies, he suggested. “Most breast cancers that occur in women with a mutation in ATM or CHEK2 are estrogen receptor positive, so these women may be candidates for antiestrogen therapies such as tamoxifen, raloxifene, or aromatase inhibitors,” he wrote.
Dr. Narod observed that, for now, the management of most women with either mutation will consist of screening alone, starting with MRI at age 40 years.
The medical community is not ready yet to expand genetic screening to the general population, cautions Walton Taylor, MD, past president of the American Society of Breast Surgeons.
The ASBrS currently recommends that all patients with breast cancer as well as those at high risk for breast cancer be offered genetic testing. “All women at risk should be tested, and all patients with pathogenic variants need to be managed appropriately – it saves lives,” Dr. Taylor emphasized.
However, “unaffected people with no family history do not need genetic testing at this time,” he said in an interview.
As to what physicians might do to better manage patients with mutations that predispose to breast cancer, Dr. Taylor said, “It’s surprisingly easy.”
Every genetic testing company provides genetic counselors to guide patients through next steps, Dr. Taylor pointed out, and most cancer patients have nurse navigators who make sure patients get tested and followed appropriately.
Members of the ASBrS follow the National Comprehensive Cancer Network guidelines when they identify carriers of a pathogenic variant. Dr. Taylor said these are very useful guidelines for virtually all mutations identified thus far.
“This research is not necessarily new, but it is confirmatory for what we are doing, and that helps us make sure we are going down the right pathway,” Dr. Taylor said. “It confirms that what we think is right is right – and that matters,.”
CARRIERS consortium findings
The study led by Dr. Couch was carried out by the Cancer Risk Estimates Related to Susceptibility (CARRIERS) consortium. It involved analyzing data from 17 epidemiology studies that focused on women in the general population who develop breast cancer. For the studies, which were conducted in the United States, pathogenic variants in 28 cancer-predisposition genes were sequenced from 32,247 women with breast cancer (case patients) and 32,544 unaffected women (control persons).
In the overall CARRIERS analysis, the prevalence of pathogenic variants in 12 clinically actionable genes was 5.03% among case patients and 1.63% among control persons. The prevalence was similar in non-Hispanic White women, non-Hispanic Black women, and Hispanic case patients, as well as control persons, they added. The prevalence of pathogenic variants among Asian American case patients was lower, at only 1.64%.
Among patients who had breast cancer, the most common pathogenic variants included BRCA2, which occurred in 1.29% of case patients, followed by CHEK2, at a prevalence of 1.08%, and BRCA1, at a prevalence of 0.85%.
Mutations in BRCA1 increased the risk for breast cancer more than 7.5-fold; mutations in BRCA2 increased that risk more than fivefold, the investigators stated.
Mutations in PALB2 increased the risk of breast cancer approximately fourfold, they added.
Prevalence rates for both BRCA1 and BRCA2 among breast cancer patients declined rapidly after the age of 40. The decline in other variants, including ATM, CHEK2, and PALB2, was limited with increasing age.
Indeed, mutations in all five of these genes were associated with a lifetime absolute risk for breast cancer greater than 20% by the age of 85 years among non-Hispanic Whites.
Pathogenic variants in BRCA1 or BRCA2 yielded a lifetime risk for breast cancer of approximately 50%. Mutations in PALB2 yielded a lifetime breast cancer risk of approximately 32%.
The risk of having a mutation in specific genes varied depending on the type of breast cancer. For example, mutations in BARD1, RAD51C, and RAD51D increased the risk for estrogen receptor (ER)–negative breast cancer as well as triple-negative breast cancer, the authors noted, whereas mutations in ATM, CDH1, and CHEK2 increased the risk for ER-positive breast cancer.
“These refined estimates of the prevalences of pathogenic variants among women with breast cancer in the overall population, as opposed to selected high-risk patients, may inform ongoing discussions regarding testing in patients with breast cancer,” the CARRIERS authors observed.
“The risks of breast cancer associated with pathogenic variants in the genes evaluated in the population-based CARRIERS analysis also provide important information for risk assessment and counseling of women with breast cancer who do not meet high-risk selection criteria,” they suggested.
Similar findings in second study
The second study was conducted by the Breast Cancer Association Consortium under lead author Leila Dorling, PhD, University of Cambridge (England). This group sequenced 34 susceptibility genes from 60,466 women with breast cancer and 53,461 unaffected control persons.
“Protein-truncating variants in five genes (ATM, BRCA1, BRCA2, CHEK2, and PALB2) were associated with a significant risk of breast cancer overall (P < .0001),” the BCAC members reported. “For these genes, odds ratios ranged from 2.10 to 10.57.”
The association between overall breast cancer risk and mutations in seven other genes was more modest, conferring approximately twice the risk for breast cancer overall, although that risk was threefold higher for the TP53 mutation.
For the 12 genes the consortium singled out as being associated with either a significant or a more modest risk for breast cancer, the effect size did not vary significantly between European and Asian women, the authors noted. Again, the risk for ER-positive breast cancer was over two times greater for those who had either the ATM or the CHEK2 mutation. Having mutations in BARD1, BRCA1, BRCA1, PALB2, RAD51C, and RAD51D conferred a higher risk for ER-negative disease than for ER-positive disease.
There was also an association between rare missense variants in six genes – CHEK2, ATM, TP53, BRCA1, CDH1, and RECQL – and overall breast cancer risk, with the clearest evidence being for CHEK2.
“The absolute risk estimates place protein-truncating variants in BRCA1, BRCA2, and PALB2 in the high-risk category and place protein-truncating variants in ATM, BARD1, CHEK2, RAD51CC, and RAD51D in the moderate-risk category,” Dr. Dorling and colleagues reaffirmed.
“These results may guide screening as well as prevention with risk-reducing surgery or medication, in accordance with national guidelines,” the authors suggested.
The CARRIERS study was supported by the National Institutes of Health. The study by Dr. Dorling and colleagues was supported by the European Union Horizon 2020 research and innovation programs, among others. Dr. Narod disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The findings come from two large studies, both published on Jan. 20 in the New England Journal of Medicine.
The two articles are “extraordinary” for broadening and validating the genomic panel to help screen women at risk for breast cancer in the future, commented Eric Topol, MD, professor of molecular medicine, Scripps Research, La Jolla, Calif., and Medscape editor in chief.
“Traditionally, genetic testing of inherited breast cancer genes has focused on women at high risk who have a strong family history of breast cancer or those who were diagnosed at an early age, such as under 45 years,” commented the lead investigator of one of the studies, Fergus Couch, PhD, a pathologist at the Mayo Clinic, Rochester, Minn.
“[Although] the risk of developing breast cancer is generally lower for women without a family history of the disease ... when we looked at all women, we found that 30% of breast cancer mutations occurred in women who are not high risk,” he said.
In both studies, mutations or variants in eight genes – BRCA1, BRCA2, PALB2, BARD1, RAD51C, RAD51D, ATM, and CHEK2 – were found to be significantly associated with breast cancer risk.
However, the distribution of mutations among women with breast cancer differed from the distribution among unaffected women, noted Steven Narod, MD, from the Women’s College Research Institute, Toronto, in an accompanying editorial.
“What this means to clinicians, now that we are expanding the use of gene-panel testing to include unaffected women with a moderate risk of breast cancer in the family history, is that our time will increasingly be spent counseling women with CHEK2 and ATM mutations,” he wrote. Currently, these two are “clumped in with ‘other genes.’ ... Most of the pretest discussion is currently focused on the implications of finding a BRCA1 or BRCA2 mutation.”
The new findings may lead to new risk management strategies, he suggested. “Most breast cancers that occur in women with a mutation in ATM or CHEK2 are estrogen receptor positive, so these women may be candidates for antiestrogen therapies such as tamoxifen, raloxifene, or aromatase inhibitors,” he wrote.
Dr. Narod observed that, for now, the management of most women with either mutation will consist of screening alone, starting with MRI at age 40 years.
The medical community is not ready yet to expand genetic screening to the general population, cautions Walton Taylor, MD, past president of the American Society of Breast Surgeons.
The ASBrS currently recommends that all patients with breast cancer as well as those at high risk for breast cancer be offered genetic testing. “All women at risk should be tested, and all patients with pathogenic variants need to be managed appropriately – it saves lives,” Dr. Taylor emphasized.
However, “unaffected people with no family history do not need genetic testing at this time,” he said in an interview.
As to what physicians might do to better manage patients with mutations that predispose to breast cancer, Dr. Taylor said, “It’s surprisingly easy.”
Every genetic testing company provides genetic counselors to guide patients through next steps, Dr. Taylor pointed out, and most cancer patients have nurse navigators who make sure patients get tested and followed appropriately.
Members of the ASBrS follow the National Comprehensive Cancer Network guidelines when they identify carriers of a pathogenic variant. Dr. Taylor said these are very useful guidelines for virtually all mutations identified thus far.
“This research is not necessarily new, but it is confirmatory for what we are doing, and that helps us make sure we are going down the right pathway,” Dr. Taylor said. “It confirms that what we think is right is right – and that matters,.”
CARRIERS consortium findings
The study led by Dr. Couch was carried out by the Cancer Risk Estimates Related to Susceptibility (CARRIERS) consortium. It involved analyzing data from 17 epidemiology studies that focused on women in the general population who develop breast cancer. For the studies, which were conducted in the United States, pathogenic variants in 28 cancer-predisposition genes were sequenced from 32,247 women with breast cancer (case patients) and 32,544 unaffected women (control persons).
In the overall CARRIERS analysis, the prevalence of pathogenic variants in 12 clinically actionable genes was 5.03% among case patients and 1.63% among control persons. The prevalence was similar in non-Hispanic White women, non-Hispanic Black women, and Hispanic case patients, as well as control persons, they added. The prevalence of pathogenic variants among Asian American case patients was lower, at only 1.64%.
Among patients who had breast cancer, the most common pathogenic variants included BRCA2, which occurred in 1.29% of case patients, followed by CHEK2, at a prevalence of 1.08%, and BRCA1, at a prevalence of 0.85%.
Mutations in BRCA1 increased the risk for breast cancer more than 7.5-fold; mutations in BRCA2 increased that risk more than fivefold, the investigators stated.
Mutations in PALB2 increased the risk of breast cancer approximately fourfold, they added.
Prevalence rates for both BRCA1 and BRCA2 among breast cancer patients declined rapidly after the age of 40. The decline in other variants, including ATM, CHEK2, and PALB2, was limited with increasing age.
Indeed, mutations in all five of these genes were associated with a lifetime absolute risk for breast cancer greater than 20% by the age of 85 years among non-Hispanic Whites.
Pathogenic variants in BRCA1 or BRCA2 yielded a lifetime risk for breast cancer of approximately 50%. Mutations in PALB2 yielded a lifetime breast cancer risk of approximately 32%.
The risk of having a mutation in specific genes varied depending on the type of breast cancer. For example, mutations in BARD1, RAD51C, and RAD51D increased the risk for estrogen receptor (ER)–negative breast cancer as well as triple-negative breast cancer, the authors noted, whereas mutations in ATM, CDH1, and CHEK2 increased the risk for ER-positive breast cancer.
“These refined estimates of the prevalences of pathogenic variants among women with breast cancer in the overall population, as opposed to selected high-risk patients, may inform ongoing discussions regarding testing in patients with breast cancer,” the CARRIERS authors observed.
“The risks of breast cancer associated with pathogenic variants in the genes evaluated in the population-based CARRIERS analysis also provide important information for risk assessment and counseling of women with breast cancer who do not meet high-risk selection criteria,” they suggested.
Similar findings in second study
The second study was conducted by the Breast Cancer Association Consortium under lead author Leila Dorling, PhD, University of Cambridge (England). This group sequenced 34 susceptibility genes from 60,466 women with breast cancer and 53,461 unaffected control persons.
“Protein-truncating variants in five genes (ATM, BRCA1, BRCA2, CHEK2, and PALB2) were associated with a significant risk of breast cancer overall (P < .0001),” the BCAC members reported. “For these genes, odds ratios ranged from 2.10 to 10.57.”
The association between overall breast cancer risk and mutations in seven other genes was more modest, conferring approximately twice the risk for breast cancer overall, although that risk was threefold higher for the TP53 mutation.
For the 12 genes the consortium singled out as being associated with either a significant or a more modest risk for breast cancer, the effect size did not vary significantly between European and Asian women, the authors noted. Again, the risk for ER-positive breast cancer was over two times greater for those who had either the ATM or the CHEK2 mutation. Having mutations in BARD1, BRCA1, BRCA1, PALB2, RAD51C, and RAD51D conferred a higher risk for ER-negative disease than for ER-positive disease.
There was also an association between rare missense variants in six genes – CHEK2, ATM, TP53, BRCA1, CDH1, and RECQL – and overall breast cancer risk, with the clearest evidence being for CHEK2.
“The absolute risk estimates place protein-truncating variants in BRCA1, BRCA2, and PALB2 in the high-risk category and place protein-truncating variants in ATM, BARD1, CHEK2, RAD51CC, and RAD51D in the moderate-risk category,” Dr. Dorling and colleagues reaffirmed.
“These results may guide screening as well as prevention with risk-reducing surgery or medication, in accordance with national guidelines,” the authors suggested.
The CARRIERS study was supported by the National Institutes of Health. The study by Dr. Dorling and colleagues was supported by the European Union Horizon 2020 research and innovation programs, among others. Dr. Narod disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Advanced Imaging Study Reveals How COVID-19 Attacks the Brain
Researchers from the National Institute of Neurological Disorders and Stroke studying the brains of patients who died from COVID-19, “consistently” found microvascular damage—but no signs of COVID-19 infection. Of the 19 patients in the study, 14 had chronic illnesses, including diabetes mellitus and hypertension, and 11 had ben found dead or had died unexpectedly. Of the 16 with available medical histories, one had delirium and the others had respiratory or unknown symptoms. Two had pulmonary embolism.
Patients with COVID-19 often have neurological problems, such as headaches, delirium, and dizziness. Some have strokes. Several studies have shown that COVID-19 can cause inflammation and blood vessel damage, but the precise mode of action is still unclear. In this study, the researchers used a magnetic resonance imaging (MRI) scanner 4 to 10 times more sensitive than most MRI scanners to examine samples of the olfactory bulbs and brainstems from the samples.
In 9 patients, the MRI scan showed punctate hyperintensities (bright spots representing areas of microvascular injury and fibrinogen leakage) that often indicate inflammation. In 10 brains, they found punctate hypointensities (dark spots) that corresponded to congested blood vessels, with surrounding areas of fibrinogen leakage and relatively intact vasculature. Areas of linear hypointensities (dark spots) were interpreted as microhemorrhages.
Using the scans as a guide, the researchers examined the spots more closely under a microscope. They found that the bright spots contained blood vessels that were thinner than normal and sometimes leaked blood proteins into the brain. This, the researchers say, seemed to trigger an immune reaction. The spots were surrounded by T cells from the blood and the brain’s own immune cells. In contrast, the dark spots contained clotted and leaky blood vessels but no immune response.
Moreover, although they used several methods for detecting genetic material or proteins from SAS-CoV-2, they found none. It’s possible, the researchers say, that the virus was cleared by the time of death or that viral copy numbers were undetectable by their assays.
We were completely surprised,” said Avindra Nath, MD, NINDS clinical director. “Originally, we expected to see damage that is caused by a lack of oxygen. Instead, we saw multifocal areas of damage that is usually associated with strokes and neuroinflammatory diseases.”
In future, Nath says, they plan to study how COVID-19 harms the blood vessels and whether that produces some of the short- and long-term symptoms seen. “We hope these results will help doctors understand the full spectrum of problems patients may suffer so that we can come up with better treatments.”
Researchers from the National Institute of Neurological Disorders and Stroke studying the brains of patients who died from COVID-19, “consistently” found microvascular damage—but no signs of COVID-19 infection. Of the 19 patients in the study, 14 had chronic illnesses, including diabetes mellitus and hypertension, and 11 had ben found dead or had died unexpectedly. Of the 16 with available medical histories, one had delirium and the others had respiratory or unknown symptoms. Two had pulmonary embolism.
Patients with COVID-19 often have neurological problems, such as headaches, delirium, and dizziness. Some have strokes. Several studies have shown that COVID-19 can cause inflammation and blood vessel damage, but the precise mode of action is still unclear. In this study, the researchers used a magnetic resonance imaging (MRI) scanner 4 to 10 times more sensitive than most MRI scanners to examine samples of the olfactory bulbs and brainstems from the samples.
In 9 patients, the MRI scan showed punctate hyperintensities (bright spots representing areas of microvascular injury and fibrinogen leakage) that often indicate inflammation. In 10 brains, they found punctate hypointensities (dark spots) that corresponded to congested blood vessels, with surrounding areas of fibrinogen leakage and relatively intact vasculature. Areas of linear hypointensities (dark spots) were interpreted as microhemorrhages.
Using the scans as a guide, the researchers examined the spots more closely under a microscope. They found that the bright spots contained blood vessels that were thinner than normal and sometimes leaked blood proteins into the brain. This, the researchers say, seemed to trigger an immune reaction. The spots were surrounded by T cells from the blood and the brain’s own immune cells. In contrast, the dark spots contained clotted and leaky blood vessels but no immune response.
Moreover, although they used several methods for detecting genetic material or proteins from SAS-CoV-2, they found none. It’s possible, the researchers say, that the virus was cleared by the time of death or that viral copy numbers were undetectable by their assays.
We were completely surprised,” said Avindra Nath, MD, NINDS clinical director. “Originally, we expected to see damage that is caused by a lack of oxygen. Instead, we saw multifocal areas of damage that is usually associated with strokes and neuroinflammatory diseases.”
In future, Nath says, they plan to study how COVID-19 harms the blood vessels and whether that produces some of the short- and long-term symptoms seen. “We hope these results will help doctors understand the full spectrum of problems patients may suffer so that we can come up with better treatments.”
Researchers from the National Institute of Neurological Disorders and Stroke studying the brains of patients who died from COVID-19, “consistently” found microvascular damage—but no signs of COVID-19 infection. Of the 19 patients in the study, 14 had chronic illnesses, including diabetes mellitus and hypertension, and 11 had ben found dead or had died unexpectedly. Of the 16 with available medical histories, one had delirium and the others had respiratory or unknown symptoms. Two had pulmonary embolism.
Patients with COVID-19 often have neurological problems, such as headaches, delirium, and dizziness. Some have strokes. Several studies have shown that COVID-19 can cause inflammation and blood vessel damage, but the precise mode of action is still unclear. In this study, the researchers used a magnetic resonance imaging (MRI) scanner 4 to 10 times more sensitive than most MRI scanners to examine samples of the olfactory bulbs and brainstems from the samples.
In 9 patients, the MRI scan showed punctate hyperintensities (bright spots representing areas of microvascular injury and fibrinogen leakage) that often indicate inflammation. In 10 brains, they found punctate hypointensities (dark spots) that corresponded to congested blood vessels, with surrounding areas of fibrinogen leakage and relatively intact vasculature. Areas of linear hypointensities (dark spots) were interpreted as microhemorrhages.
Using the scans as a guide, the researchers examined the spots more closely under a microscope. They found that the bright spots contained blood vessels that were thinner than normal and sometimes leaked blood proteins into the brain. This, the researchers say, seemed to trigger an immune reaction. The spots were surrounded by T cells from the blood and the brain’s own immune cells. In contrast, the dark spots contained clotted and leaky blood vessels but no immune response.
Moreover, although they used several methods for detecting genetic material or proteins from SAS-CoV-2, they found none. It’s possible, the researchers say, that the virus was cleared by the time of death or that viral copy numbers were undetectable by their assays.
We were completely surprised,” said Avindra Nath, MD, NINDS clinical director. “Originally, we expected to see damage that is caused by a lack of oxygen. Instead, we saw multifocal areas of damage that is usually associated with strokes and neuroinflammatory diseases.”
In future, Nath says, they plan to study how COVID-19 harms the blood vessels and whether that produces some of the short- and long-term symptoms seen. “We hope these results will help doctors understand the full spectrum of problems patients may suffer so that we can come up with better treatments.”
Influenza Plus COVID-19 Equals Greater Concern
Coinfection with COVID-19 and influenza was reported early in the pandemic. Although both infections on their own can cause severe complications and death, coinfection can double the odds of death when compared with COVID infection alone. Moreover, those odds can be raised by chronic medical conditions and environmental or occupational factors, such as congregate living settings, say physicians who report on the first 2 confirmed cases of COVID-19 and influenza coinfection among US Department of Defense personnel within the US Central Command area of responsibility.
In the first case, a 56-year-old contractor presented to a Role I clinic with anorexia, fever, chills, and headache, which had begun 3 days before. His initial vital signs were “unremarkable,” and he did not have symptoms of respiratory distress. An antigen test was positive for influenza type A. A COVID-19 test also was positive. He was placed on isolation and treated with oseltamivir, amlodipine, hydrochlorothiazide, and losartan. His condition did not warrant hospitalization. Of 3 close contacts, 1 tested positive and was isolated. Two remained asymptomatic during the 14-day quarantine. Ten days after onset, the patient returned to duty.
The second patient, a 34-year-old officer in the Army, was initially identified as a close contact of a confirmed COVID-19 case and placed in quarantine. He was asymptomatic but tested positive and was placed in isolation with precautions. As with the first patient, his vital signs were unremarkable. He continued to be asymptomatic, although he reported myalgias 2 days later. Since those are a classic sign of seasonal influenzas, he was tested and proved positive for type B influenza. He, too, was started on oseltamivir. By the end of the first week, he experienced loss of taste and smell, cough, and shortness of breath, but his vital signs remained normal. His symptoms improved through supportive care. All 6 of his close contacts remained asymptomatic. Ten days after his symptoms began, he also returned to duty.
Influenza-associated deaths among the US military have been relatively few, the authors say, most likely because of the good preexisting health status of the US military, prompt detection with rapid influenza diagnostic tests, several effective antiviral therapeutics, and a “robust, compulsory vaccination program.” Nonetheless, neither patient had received the 2020-2021 influenza vaccine, which underscores the importance of this intervention, the authors say.
Because both infections present with a wide variety of clinical manifestations and overlapping symptoms, providers should stay alert to the possibility of coinfection, especially among personnel who are higher risk. For instance, as a linguist who interacted daily with host nation partners, the civilian contractor had a high occupational exposure.
While the authors only discuss 2 cases, a Medical Surveillance Monthly Report editorial comment says their report “nevertheless supports the importance of implementing force health protection (FHP) measures to prevent, detect, and respond to the spread of both of these health threats.” It’s particularly important, the comment notes, in the current context of a drawdown in forces in many deployed locations, as further losses of personnel to illness may degrade the execution of critical missions.
Coinfection with COVID-19 and influenza was reported early in the pandemic. Although both infections on their own can cause severe complications and death, coinfection can double the odds of death when compared with COVID infection alone. Moreover, those odds can be raised by chronic medical conditions and environmental or occupational factors, such as congregate living settings, say physicians who report on the first 2 confirmed cases of COVID-19 and influenza coinfection among US Department of Defense personnel within the US Central Command area of responsibility.
In the first case, a 56-year-old contractor presented to a Role I clinic with anorexia, fever, chills, and headache, which had begun 3 days before. His initial vital signs were “unremarkable,” and he did not have symptoms of respiratory distress. An antigen test was positive for influenza type A. A COVID-19 test also was positive. He was placed on isolation and treated with oseltamivir, amlodipine, hydrochlorothiazide, and losartan. His condition did not warrant hospitalization. Of 3 close contacts, 1 tested positive and was isolated. Two remained asymptomatic during the 14-day quarantine. Ten days after onset, the patient returned to duty.
The second patient, a 34-year-old officer in the Army, was initially identified as a close contact of a confirmed COVID-19 case and placed in quarantine. He was asymptomatic but tested positive and was placed in isolation with precautions. As with the first patient, his vital signs were unremarkable. He continued to be asymptomatic, although he reported myalgias 2 days later. Since those are a classic sign of seasonal influenzas, he was tested and proved positive for type B influenza. He, too, was started on oseltamivir. By the end of the first week, he experienced loss of taste and smell, cough, and shortness of breath, but his vital signs remained normal. His symptoms improved through supportive care. All 6 of his close contacts remained asymptomatic. Ten days after his symptoms began, he also returned to duty.
Influenza-associated deaths among the US military have been relatively few, the authors say, most likely because of the good preexisting health status of the US military, prompt detection with rapid influenza diagnostic tests, several effective antiviral therapeutics, and a “robust, compulsory vaccination program.” Nonetheless, neither patient had received the 2020-2021 influenza vaccine, which underscores the importance of this intervention, the authors say.
Because both infections present with a wide variety of clinical manifestations and overlapping symptoms, providers should stay alert to the possibility of coinfection, especially among personnel who are higher risk. For instance, as a linguist who interacted daily with host nation partners, the civilian contractor had a high occupational exposure.
While the authors only discuss 2 cases, a Medical Surveillance Monthly Report editorial comment says their report “nevertheless supports the importance of implementing force health protection (FHP) measures to prevent, detect, and respond to the spread of both of these health threats.” It’s particularly important, the comment notes, in the current context of a drawdown in forces in many deployed locations, as further losses of personnel to illness may degrade the execution of critical missions.
Coinfection with COVID-19 and influenza was reported early in the pandemic. Although both infections on their own can cause severe complications and death, coinfection can double the odds of death when compared with COVID infection alone. Moreover, those odds can be raised by chronic medical conditions and environmental or occupational factors, such as congregate living settings, say physicians who report on the first 2 confirmed cases of COVID-19 and influenza coinfection among US Department of Defense personnel within the US Central Command area of responsibility.
In the first case, a 56-year-old contractor presented to a Role I clinic with anorexia, fever, chills, and headache, which had begun 3 days before. His initial vital signs were “unremarkable,” and he did not have symptoms of respiratory distress. An antigen test was positive for influenza type A. A COVID-19 test also was positive. He was placed on isolation and treated with oseltamivir, amlodipine, hydrochlorothiazide, and losartan. His condition did not warrant hospitalization. Of 3 close contacts, 1 tested positive and was isolated. Two remained asymptomatic during the 14-day quarantine. Ten days after onset, the patient returned to duty.
The second patient, a 34-year-old officer in the Army, was initially identified as a close contact of a confirmed COVID-19 case and placed in quarantine. He was asymptomatic but tested positive and was placed in isolation with precautions. As with the first patient, his vital signs were unremarkable. He continued to be asymptomatic, although he reported myalgias 2 days later. Since those are a classic sign of seasonal influenzas, he was tested and proved positive for type B influenza. He, too, was started on oseltamivir. By the end of the first week, he experienced loss of taste and smell, cough, and shortness of breath, but his vital signs remained normal. His symptoms improved through supportive care. All 6 of his close contacts remained asymptomatic. Ten days after his symptoms began, he also returned to duty.
Influenza-associated deaths among the US military have been relatively few, the authors say, most likely because of the good preexisting health status of the US military, prompt detection with rapid influenza diagnostic tests, several effective antiviral therapeutics, and a “robust, compulsory vaccination program.” Nonetheless, neither patient had received the 2020-2021 influenza vaccine, which underscores the importance of this intervention, the authors say.
Because both infections present with a wide variety of clinical manifestations and overlapping symptoms, providers should stay alert to the possibility of coinfection, especially among personnel who are higher risk. For instance, as a linguist who interacted daily with host nation partners, the civilian contractor had a high occupational exposure.
While the authors only discuss 2 cases, a Medical Surveillance Monthly Report editorial comment says their report “nevertheless supports the importance of implementing force health protection (FHP) measures to prevent, detect, and respond to the spread of both of these health threats.” It’s particularly important, the comment notes, in the current context of a drawdown in forces in many deployed locations, as further losses of personnel to illness may degrade the execution of critical missions.
Kids already coping with mental disorders spiral as pandemic topples vital support systems
A bag of Doritos, that’s all Princess wanted.
Her mom calls her Princess, but her real name is Lindsey. She’s 17 and lives with her mom, Sandra, a nurse, outside Atlanta. On May 17, 2020, a Sunday, Lindsey decided she didn’t want breakfast; she wanted Doritos. So she left home and walked to Family Dollar, taking her pants off on the way, while her mom followed on foot, talking to the police on her phone as they went.
Lindsey has autism. It can be hard for her to communicate and navigate social situations. She thrives on routine and gets special help at school. Or got help, before the coronavirus pandemic closed schools and forced tens of millions of children to stay home. Sandra said that’s when their living hell started.
“It’s like her brain was wired,” she said. “She’d just put on her jacket, and she’s out the door. And I’m chasing her.”
On May 17, Sandra chased her all the way to Family Dollar. Hours later, Lindsey was in jail, charged with assaulting her mom. (KHN and NPR are not using the family’s last name.)
Lindsey is 1 of almost 3 million children in the United States who have a serious emotional or behavioral health condition. When the pandemic forced schools and doctors’ offices to close last spring, it also cut children off from the trained teachers and therapists who understand their needs.
As a result, many, like Lindsey, spiraled into EDs and even police custody. Federal data shows a nationwide surge of children in mental health crisis during the pandemic – a surge that’s further taxing an already overstretched safety net.
‘Take her’
Even after schools closed, Lindsey continued to wake up early, get dressed and wait for the bus. When she realized it had stopped coming, Sandra said, her daughter just started walking out of the house, wandering, a few times a week.
In those situations, Sandra did what many families in crisis report they’ve had to do since the pandemic began: Race through the short list of places she could call for help.
First, her state’s mental health crisis hotline. But they often put Sandra on hold.
“This is ridiculous,” she said of the wait. “It’s supposed to be a crisis team. But I’m on hold for 40, 50 minutes. And by the time you get on the phone, [the crisis] is done!”
Then there’s the local hospital’s ED, but Sandra said she had taken Lindsey there for previous crises and been told there isn’t much they can do.
That’s why, on May 17, when Lindsey walked to Family Dollar in just a red T-shirt and underwear to get that bag of Doritos, Sandra called the last option on her list: the police.
Sandra arrived at the store before the police and paid for the chips. According to Sandra and police records, when an officer approached, Lindsey grew agitated and hit her mom on the back, hard.
Sandra said she explained to the officer: “‘She’s autistic. You know, I’m okay. I’m a nurse. I just need to take her home and give her her medication.’ ”
Lindsey takes a mood stabilizer, but because she left home before breakfast, she hadn’t taken it that morning. The officer asked if Sandra wanted to take her to the nearest hospital.
The hospital wouldn’t be able to help Lindsey, Sandra said. It hadn’t before. “They already told me: ‘Ma’am, there’s nothing we can do.’ They just check her labs, it’s fine, and they ship her back home. There’s nothing [the hospital] can do,” she recalled telling the officer.
Sandra asked if the police could drive her daughter home so the teen could take her medication, but the officer said no, they couldn’t. The only other thing they could do, the officer said, was take Lindsey to jail for hitting her mom.
“I’ve tried everything,” Sandra said, exasperated. She paced the parking lot, feeling hopeless, sad and out of options. Finally, in tears, she told the officers: “Take her.”
Lindsey does not like to be touched and fought back when authorities tried to handcuff her. Several officers wrestled her to the ground. At that point, Sandra protested and said an officer threatened to arrest her, too, if she didn’t back away. Lindsey was taken to jail, where she spent much of the night until Sandra was able to post bail.
Clayton County Solicitor-General Charles Brooks denied that Sandra was threatened with arrest and said that, while Lindsey’s case is still pending, his office “is working to ensure that the resolution in this matter involves a plan for medication compliance and not punitive action.”
Sandra isn’t alone in her experience. Multiple families interviewed for this story reported similar experiences of calling in the police when a child was in crisis because caretakers didn’t feel they had any other option.
‘The whole system is really grinding to a halt’
Roughly 6% of U.S. children ages 6-17 years are living with serious emotional or behavioral difficulties, including children with autism, severe anxiety, depression and trauma-related mental health conditions.
Many of these children depend on schools for access to vital therapies. When schools and doctors’ offices stopped providing in-person services last spring, kids were untethered from the people and supports they rely on.
“The lack of in-person services is really detrimental,” said Susan Duffy, MD,a pediatrician and professor of emergency medicine at Brown University, Providence, R.I.
Marjorie, a mother in Florida, said her 15-year-old son has suffered during these disruptions. He has ADHD and oppositional defiant disorder, a condition marked by frequent and persistent hostility. Little things – like being asked to do schoolwork – can send him into a rage, leading to holes punched in walls, broken doors and violent threats. (The family’s last name or her son’s first name are not used to protect her son’s privacy and future prospects.)
The pandemic has shifted both school and her son’s therapy sessions online. But Marjorie said virtual therapy isn’t working because her son doesn’t focus well during sessions and tries to watch television instead. Lately, she has simply been canceling them.
“I was paying for appointments and there was no therapeutic value,” Marjorie said.
The issues cut across socioeconomic lines – affecting families with private insurance, like Marjorie, as well as those who receive coverage through Medicaid, a federal-state program that provides health insurance to low-income people and those with disabilities.
In the first few months of the pandemic, between March and May, children on Medicaid received 44% fewer outpatient mental health services – including therapy and in-home support – compared with the same time period in 2019, according to the Centers for Medicare & Medicaid Services. That’s even after accounting for increased telehealth appointments.
And while the nation’s EDs have seen a decline in overall visits, there was a relative increase in mental health visits for kids in 2020, compared with 2019.
The Centers for Disease Control and Prevention found that, from April to October 2020, hospitals across the United States saw a 24% increase in the proportion of mental health emergency visits for children aged 5-11 years, and a 31% increase for children aged 12-17.
“Not only are we seeing more children, more children are being admitted” to inpatient care.
That’s because there are fewer outpatient services now available to children, she said, and because the conditions of the children showing up at EDs “are more serious.”
This crisis is not only making life harder for these kids and their families, but it’s also stressing the entire health care system.
Child and adolescent psychiatrists working in hospitals around the country said children are increasingly “boarding” in EDs for days, waiting for inpatient admission to a regular hospital or psychiatric hospital.
Before the pandemic, there was already a shortage of inpatient psychiatric beds for children, said Christopher Bellonci, MD, a child psychiatrist at Judge Baker Children’s Center in Boston. That shortage has only gotten worse as hospitals cut capacity to allow for more physical distancing within psychiatric units.
“The whole system is really grinding to a halt at a time when we have unprecedented need,” Dr. Bellonci said.
‘A signal that the rest of your system doesn’t work’
Psychiatrists on the front lines share the frustrations of parents struggling to find help for their children.
Part of the problem is there have never been enough psychiatrists and therapists trained to work with children, intervening in the early stages of their illness, said Jennifer Havens, MD, a child psychiatrist at New York University.
“Tons of people showing up in emergency rooms in bad shape is a signal that the rest of your system doesn’t work,” she said.
Too often, Dr. Havens said, services aren’t available until children are older – and in crisis. “Often for people who don’t have access to services, we wait until they’re too big to be managed.”
While the pandemic has made life harder for Marjorie and her son in Florida, she said it has always been difficult to find the support and care he needs. Last fall, he needed a psychiatric evaluation, but the nearest specialist who would accept her commercial insurance was 100 miles away, in Alabama.
“Even when you have the money or you have the insurance, it is still a travesty,” Marjorie said. “You cannot get help for these kids.”
Parents are frustrated, and so are psychiatrists on the front lines. C.J. Glawe, MD, who leads the psychiatric crisis department at Nationwide Children’s Hospital in Columbus, Ohio, said that once a child is stabilized after a crisis it can be hard to explain to parents that they may not be able to find follow-up care anywhere near their home.
“Especially when I can clearly tell you I know exactly what you need, I just can’t give it to you,” Dr. Glawe said. “It’s demoralizing.”
When states and communities fail to provide children the services they need to live at home, kids can deteriorate and even wind up in jail, like Lindsey. At that point, Dr. Glawe said, the cost and level of care required will be even higher, whether that’s hospitalization or long stays in residential treatment facilities.
That’s exactly the scenario Sandra, Lindsey’s mom, is hoping to avoid for her Princess.
“For me, as a nurse and as a provider, that will be the last thing for my daughter,” she said. “It’s like [state and local leaders] leave it to the school and the parent to deal with, and they don’t care. And that’s the problem. It’s sad because, if I’m not here...”
Her voice trailed off as tears welled.
“She didn’t ask to have autism.”
To help families like Sandra’s and Marjorie’s, advocates said, all levels of government need to invest in creating a mental health system that’s accessible to anyone who needs it.
But given that many states have seen their revenues drop because of the pandemic, there’s a concern services will instead be cut – at a time when the need has never been greater.
This story is part of a reporting partnership that includes NPR, Illinois Public Media and Kaiser Health News. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
A bag of Doritos, that’s all Princess wanted.
Her mom calls her Princess, but her real name is Lindsey. She’s 17 and lives with her mom, Sandra, a nurse, outside Atlanta. On May 17, 2020, a Sunday, Lindsey decided she didn’t want breakfast; she wanted Doritos. So she left home and walked to Family Dollar, taking her pants off on the way, while her mom followed on foot, talking to the police on her phone as they went.
Lindsey has autism. It can be hard for her to communicate and navigate social situations. She thrives on routine and gets special help at school. Or got help, before the coronavirus pandemic closed schools and forced tens of millions of children to stay home. Sandra said that’s when their living hell started.
“It’s like her brain was wired,” she said. “She’d just put on her jacket, and she’s out the door. And I’m chasing her.”
On May 17, Sandra chased her all the way to Family Dollar. Hours later, Lindsey was in jail, charged with assaulting her mom. (KHN and NPR are not using the family’s last name.)
Lindsey is 1 of almost 3 million children in the United States who have a serious emotional or behavioral health condition. When the pandemic forced schools and doctors’ offices to close last spring, it also cut children off from the trained teachers and therapists who understand their needs.
As a result, many, like Lindsey, spiraled into EDs and even police custody. Federal data shows a nationwide surge of children in mental health crisis during the pandemic – a surge that’s further taxing an already overstretched safety net.
‘Take her’
Even after schools closed, Lindsey continued to wake up early, get dressed and wait for the bus. When she realized it had stopped coming, Sandra said, her daughter just started walking out of the house, wandering, a few times a week.
In those situations, Sandra did what many families in crisis report they’ve had to do since the pandemic began: Race through the short list of places she could call for help.
First, her state’s mental health crisis hotline. But they often put Sandra on hold.
“This is ridiculous,” she said of the wait. “It’s supposed to be a crisis team. But I’m on hold for 40, 50 minutes. And by the time you get on the phone, [the crisis] is done!”
Then there’s the local hospital’s ED, but Sandra said she had taken Lindsey there for previous crises and been told there isn’t much they can do.
That’s why, on May 17, when Lindsey walked to Family Dollar in just a red T-shirt and underwear to get that bag of Doritos, Sandra called the last option on her list: the police.
Sandra arrived at the store before the police and paid for the chips. According to Sandra and police records, when an officer approached, Lindsey grew agitated and hit her mom on the back, hard.
Sandra said she explained to the officer: “‘She’s autistic. You know, I’m okay. I’m a nurse. I just need to take her home and give her her medication.’ ”
Lindsey takes a mood stabilizer, but because she left home before breakfast, she hadn’t taken it that morning. The officer asked if Sandra wanted to take her to the nearest hospital.
The hospital wouldn’t be able to help Lindsey, Sandra said. It hadn’t before. “They already told me: ‘Ma’am, there’s nothing we can do.’ They just check her labs, it’s fine, and they ship her back home. There’s nothing [the hospital] can do,” she recalled telling the officer.
Sandra asked if the police could drive her daughter home so the teen could take her medication, but the officer said no, they couldn’t. The only other thing they could do, the officer said, was take Lindsey to jail for hitting her mom.
“I’ve tried everything,” Sandra said, exasperated. She paced the parking lot, feeling hopeless, sad and out of options. Finally, in tears, she told the officers: “Take her.”
Lindsey does not like to be touched and fought back when authorities tried to handcuff her. Several officers wrestled her to the ground. At that point, Sandra protested and said an officer threatened to arrest her, too, if she didn’t back away. Lindsey was taken to jail, where she spent much of the night until Sandra was able to post bail.
Clayton County Solicitor-General Charles Brooks denied that Sandra was threatened with arrest and said that, while Lindsey’s case is still pending, his office “is working to ensure that the resolution in this matter involves a plan for medication compliance and not punitive action.”
Sandra isn’t alone in her experience. Multiple families interviewed for this story reported similar experiences of calling in the police when a child was in crisis because caretakers didn’t feel they had any other option.
‘The whole system is really grinding to a halt’
Roughly 6% of U.S. children ages 6-17 years are living with serious emotional or behavioral difficulties, including children with autism, severe anxiety, depression and trauma-related mental health conditions.
Many of these children depend on schools for access to vital therapies. When schools and doctors’ offices stopped providing in-person services last spring, kids were untethered from the people and supports they rely on.
“The lack of in-person services is really detrimental,” said Susan Duffy, MD,a pediatrician and professor of emergency medicine at Brown University, Providence, R.I.
Marjorie, a mother in Florida, said her 15-year-old son has suffered during these disruptions. He has ADHD and oppositional defiant disorder, a condition marked by frequent and persistent hostility. Little things – like being asked to do schoolwork – can send him into a rage, leading to holes punched in walls, broken doors and violent threats. (The family’s last name or her son’s first name are not used to protect her son’s privacy and future prospects.)
The pandemic has shifted both school and her son’s therapy sessions online. But Marjorie said virtual therapy isn’t working because her son doesn’t focus well during sessions and tries to watch television instead. Lately, she has simply been canceling them.
“I was paying for appointments and there was no therapeutic value,” Marjorie said.
The issues cut across socioeconomic lines – affecting families with private insurance, like Marjorie, as well as those who receive coverage through Medicaid, a federal-state program that provides health insurance to low-income people and those with disabilities.
In the first few months of the pandemic, between March and May, children on Medicaid received 44% fewer outpatient mental health services – including therapy and in-home support – compared with the same time period in 2019, according to the Centers for Medicare & Medicaid Services. That’s even after accounting for increased telehealth appointments.
And while the nation’s EDs have seen a decline in overall visits, there was a relative increase in mental health visits for kids in 2020, compared with 2019.
The Centers for Disease Control and Prevention found that, from April to October 2020, hospitals across the United States saw a 24% increase in the proportion of mental health emergency visits for children aged 5-11 years, and a 31% increase for children aged 12-17.
“Not only are we seeing more children, more children are being admitted” to inpatient care.
That’s because there are fewer outpatient services now available to children, she said, and because the conditions of the children showing up at EDs “are more serious.”
This crisis is not only making life harder for these kids and their families, but it’s also stressing the entire health care system.
Child and adolescent psychiatrists working in hospitals around the country said children are increasingly “boarding” in EDs for days, waiting for inpatient admission to a regular hospital or psychiatric hospital.
Before the pandemic, there was already a shortage of inpatient psychiatric beds for children, said Christopher Bellonci, MD, a child psychiatrist at Judge Baker Children’s Center in Boston. That shortage has only gotten worse as hospitals cut capacity to allow for more physical distancing within psychiatric units.
“The whole system is really grinding to a halt at a time when we have unprecedented need,” Dr. Bellonci said.
‘A signal that the rest of your system doesn’t work’
Psychiatrists on the front lines share the frustrations of parents struggling to find help for their children.
Part of the problem is there have never been enough psychiatrists and therapists trained to work with children, intervening in the early stages of their illness, said Jennifer Havens, MD, a child psychiatrist at New York University.
“Tons of people showing up in emergency rooms in bad shape is a signal that the rest of your system doesn’t work,” she said.
Too often, Dr. Havens said, services aren’t available until children are older – and in crisis. “Often for people who don’t have access to services, we wait until they’re too big to be managed.”
While the pandemic has made life harder for Marjorie and her son in Florida, she said it has always been difficult to find the support and care he needs. Last fall, he needed a psychiatric evaluation, but the nearest specialist who would accept her commercial insurance was 100 miles away, in Alabama.
“Even when you have the money or you have the insurance, it is still a travesty,” Marjorie said. “You cannot get help for these kids.”
Parents are frustrated, and so are psychiatrists on the front lines. C.J. Glawe, MD, who leads the psychiatric crisis department at Nationwide Children’s Hospital in Columbus, Ohio, said that once a child is stabilized after a crisis it can be hard to explain to parents that they may not be able to find follow-up care anywhere near their home.
“Especially when I can clearly tell you I know exactly what you need, I just can’t give it to you,” Dr. Glawe said. “It’s demoralizing.”
When states and communities fail to provide children the services they need to live at home, kids can deteriorate and even wind up in jail, like Lindsey. At that point, Dr. Glawe said, the cost and level of care required will be even higher, whether that’s hospitalization or long stays in residential treatment facilities.
That’s exactly the scenario Sandra, Lindsey’s mom, is hoping to avoid for her Princess.
“For me, as a nurse and as a provider, that will be the last thing for my daughter,” she said. “It’s like [state and local leaders] leave it to the school and the parent to deal with, and they don’t care. And that’s the problem. It’s sad because, if I’m not here...”
Her voice trailed off as tears welled.
“She didn’t ask to have autism.”
To help families like Sandra’s and Marjorie’s, advocates said, all levels of government need to invest in creating a mental health system that’s accessible to anyone who needs it.
But given that many states have seen their revenues drop because of the pandemic, there’s a concern services will instead be cut – at a time when the need has never been greater.
This story is part of a reporting partnership that includes NPR, Illinois Public Media and Kaiser Health News. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
A bag of Doritos, that’s all Princess wanted.
Her mom calls her Princess, but her real name is Lindsey. She’s 17 and lives with her mom, Sandra, a nurse, outside Atlanta. On May 17, 2020, a Sunday, Lindsey decided she didn’t want breakfast; she wanted Doritos. So she left home and walked to Family Dollar, taking her pants off on the way, while her mom followed on foot, talking to the police on her phone as they went.
Lindsey has autism. It can be hard for her to communicate and navigate social situations. She thrives on routine and gets special help at school. Or got help, before the coronavirus pandemic closed schools and forced tens of millions of children to stay home. Sandra said that’s when their living hell started.
“It’s like her brain was wired,” she said. “She’d just put on her jacket, and she’s out the door. And I’m chasing her.”
On May 17, Sandra chased her all the way to Family Dollar. Hours later, Lindsey was in jail, charged with assaulting her mom. (KHN and NPR are not using the family’s last name.)
Lindsey is 1 of almost 3 million children in the United States who have a serious emotional or behavioral health condition. When the pandemic forced schools and doctors’ offices to close last spring, it also cut children off from the trained teachers and therapists who understand their needs.
As a result, many, like Lindsey, spiraled into EDs and even police custody. Federal data shows a nationwide surge of children in mental health crisis during the pandemic – a surge that’s further taxing an already overstretched safety net.
‘Take her’
Even after schools closed, Lindsey continued to wake up early, get dressed and wait for the bus. When she realized it had stopped coming, Sandra said, her daughter just started walking out of the house, wandering, a few times a week.
In those situations, Sandra did what many families in crisis report they’ve had to do since the pandemic began: Race through the short list of places she could call for help.
First, her state’s mental health crisis hotline. But they often put Sandra on hold.
“This is ridiculous,” she said of the wait. “It’s supposed to be a crisis team. But I’m on hold for 40, 50 minutes. And by the time you get on the phone, [the crisis] is done!”
Then there’s the local hospital’s ED, but Sandra said she had taken Lindsey there for previous crises and been told there isn’t much they can do.
That’s why, on May 17, when Lindsey walked to Family Dollar in just a red T-shirt and underwear to get that bag of Doritos, Sandra called the last option on her list: the police.
Sandra arrived at the store before the police and paid for the chips. According to Sandra and police records, when an officer approached, Lindsey grew agitated and hit her mom on the back, hard.
Sandra said she explained to the officer: “‘She’s autistic. You know, I’m okay. I’m a nurse. I just need to take her home and give her her medication.’ ”
Lindsey takes a mood stabilizer, but because she left home before breakfast, she hadn’t taken it that morning. The officer asked if Sandra wanted to take her to the nearest hospital.
The hospital wouldn’t be able to help Lindsey, Sandra said. It hadn’t before. “They already told me: ‘Ma’am, there’s nothing we can do.’ They just check her labs, it’s fine, and they ship her back home. There’s nothing [the hospital] can do,” she recalled telling the officer.
Sandra asked if the police could drive her daughter home so the teen could take her medication, but the officer said no, they couldn’t. The only other thing they could do, the officer said, was take Lindsey to jail for hitting her mom.
“I’ve tried everything,” Sandra said, exasperated. She paced the parking lot, feeling hopeless, sad and out of options. Finally, in tears, she told the officers: “Take her.”
Lindsey does not like to be touched and fought back when authorities tried to handcuff her. Several officers wrestled her to the ground. At that point, Sandra protested and said an officer threatened to arrest her, too, if she didn’t back away. Lindsey was taken to jail, where she spent much of the night until Sandra was able to post bail.
Clayton County Solicitor-General Charles Brooks denied that Sandra was threatened with arrest and said that, while Lindsey’s case is still pending, his office “is working to ensure that the resolution in this matter involves a plan for medication compliance and not punitive action.”
Sandra isn’t alone in her experience. Multiple families interviewed for this story reported similar experiences of calling in the police when a child was in crisis because caretakers didn’t feel they had any other option.
‘The whole system is really grinding to a halt’
Roughly 6% of U.S. children ages 6-17 years are living with serious emotional or behavioral difficulties, including children with autism, severe anxiety, depression and trauma-related mental health conditions.
Many of these children depend on schools for access to vital therapies. When schools and doctors’ offices stopped providing in-person services last spring, kids were untethered from the people and supports they rely on.
“The lack of in-person services is really detrimental,” said Susan Duffy, MD,a pediatrician and professor of emergency medicine at Brown University, Providence, R.I.
Marjorie, a mother in Florida, said her 15-year-old son has suffered during these disruptions. He has ADHD and oppositional defiant disorder, a condition marked by frequent and persistent hostility. Little things – like being asked to do schoolwork – can send him into a rage, leading to holes punched in walls, broken doors and violent threats. (The family’s last name or her son’s first name are not used to protect her son’s privacy and future prospects.)
The pandemic has shifted both school and her son’s therapy sessions online. But Marjorie said virtual therapy isn’t working because her son doesn’t focus well during sessions and tries to watch television instead. Lately, she has simply been canceling them.
“I was paying for appointments and there was no therapeutic value,” Marjorie said.
The issues cut across socioeconomic lines – affecting families with private insurance, like Marjorie, as well as those who receive coverage through Medicaid, a federal-state program that provides health insurance to low-income people and those with disabilities.
In the first few months of the pandemic, between March and May, children on Medicaid received 44% fewer outpatient mental health services – including therapy and in-home support – compared with the same time period in 2019, according to the Centers for Medicare & Medicaid Services. That’s even after accounting for increased telehealth appointments.
And while the nation’s EDs have seen a decline in overall visits, there was a relative increase in mental health visits for kids in 2020, compared with 2019.
The Centers for Disease Control and Prevention found that, from April to October 2020, hospitals across the United States saw a 24% increase in the proportion of mental health emergency visits for children aged 5-11 years, and a 31% increase for children aged 12-17.
“Not only are we seeing more children, more children are being admitted” to inpatient care.
That’s because there are fewer outpatient services now available to children, she said, and because the conditions of the children showing up at EDs “are more serious.”
This crisis is not only making life harder for these kids and their families, but it’s also stressing the entire health care system.
Child and adolescent psychiatrists working in hospitals around the country said children are increasingly “boarding” in EDs for days, waiting for inpatient admission to a regular hospital or psychiatric hospital.
Before the pandemic, there was already a shortage of inpatient psychiatric beds for children, said Christopher Bellonci, MD, a child psychiatrist at Judge Baker Children’s Center in Boston. That shortage has only gotten worse as hospitals cut capacity to allow for more physical distancing within psychiatric units.
“The whole system is really grinding to a halt at a time when we have unprecedented need,” Dr. Bellonci said.
‘A signal that the rest of your system doesn’t work’
Psychiatrists on the front lines share the frustrations of parents struggling to find help for their children.
Part of the problem is there have never been enough psychiatrists and therapists trained to work with children, intervening in the early stages of their illness, said Jennifer Havens, MD, a child psychiatrist at New York University.
“Tons of people showing up in emergency rooms in bad shape is a signal that the rest of your system doesn’t work,” she said.
Too often, Dr. Havens said, services aren’t available until children are older – and in crisis. “Often for people who don’t have access to services, we wait until they’re too big to be managed.”
While the pandemic has made life harder for Marjorie and her son in Florida, she said it has always been difficult to find the support and care he needs. Last fall, he needed a psychiatric evaluation, but the nearest specialist who would accept her commercial insurance was 100 miles away, in Alabama.
“Even when you have the money or you have the insurance, it is still a travesty,” Marjorie said. “You cannot get help for these kids.”
Parents are frustrated, and so are psychiatrists on the front lines. C.J. Glawe, MD, who leads the psychiatric crisis department at Nationwide Children’s Hospital in Columbus, Ohio, said that once a child is stabilized after a crisis it can be hard to explain to parents that they may not be able to find follow-up care anywhere near their home.
“Especially when I can clearly tell you I know exactly what you need, I just can’t give it to you,” Dr. Glawe said. “It’s demoralizing.”
When states and communities fail to provide children the services they need to live at home, kids can deteriorate and even wind up in jail, like Lindsey. At that point, Dr. Glawe said, the cost and level of care required will be even higher, whether that’s hospitalization or long stays in residential treatment facilities.
That’s exactly the scenario Sandra, Lindsey’s mom, is hoping to avoid for her Princess.
“For me, as a nurse and as a provider, that will be the last thing for my daughter,” she said. “It’s like [state and local leaders] leave it to the school and the parent to deal with, and they don’t care. And that’s the problem. It’s sad because, if I’m not here...”
Her voice trailed off as tears welled.
“She didn’t ask to have autism.”
To help families like Sandra’s and Marjorie’s, advocates said, all levels of government need to invest in creating a mental health system that’s accessible to anyone who needs it.
But given that many states have seen their revenues drop because of the pandemic, there’s a concern services will instead be cut – at a time when the need has never been greater.
This story is part of a reporting partnership that includes NPR, Illinois Public Media and Kaiser Health News. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
JHM Twitter chat sparks connections
Monthly social media event links editors, authors, and readers
Once upon a time, physicians wrote letters to peers and colleagues around the world, sharing their medical discoveries, theories, case reports, and questions; conferring on problems; and then waiting for return mail to bring a reply. And the science of medicine advanced at a glacial pace.
Today, communication in multiple mediums flows much faster, almost instantaneously, between many more physicians, regardless of distance, addressing a much greater complexity of medical topics and treatments. And one of the chief mediums for this rapid electronic conversation among doctors is Twitter, according to Charlie Wray, DO, MS, a hospitalist at the University of California, San Francisco.
Dr. Wray, associate editor and digital media editor for the Journal of Hospital Medicine, is one of the moderators of #JHMchat, a monthly get-together on Twitter for interested hospitalists to link up virtually; respond to questions posed by JHM editors and other moderators; exchange perspectives, experiences, and tips with their peers; and build professional relationships and personal friendships. Relationship building has become particularly important in the age of COVID-19, when opportunities to connect in person at events such as SHM’s annual conferences have been curtailed.
The online #JHMchat community began in 2015, shortly after Dr. Wray completed a hospitalist research fellowship at the University of Chicago. His fellowship mentor, Vineet Arora, MD, MAPP, MHM, associate chief medical officer for the clinical learning environment at University of Chicago Medicine, had noticed how other online medical communities were engaging in discussions around different topics.
“She thought it could be a great way for hospitalists to meet online and talk about the articles published in JHM,” Dr. Wray explained. “We were all getting into social media and learning how to moderate interactive discussions such as Twitter.”
The chat’s founders approached JHM’s then-editor Andrew Auerbach, MD, MHM, a hospitalist at UCSF, who agreed that it was a great idea. They asked Christopher Moriates, MD, author of a recently published paper on the advisability of nebulized bronchodilators for obstructive pulmonary symptoms and assistant dean for health care value at the University of Texas, Austin, to come on the chat and talk about his paper. Visiting Dr. Arora at the time, he joined her in her living room for the first chat on Oct. 12, 2015. Seventy-five participants posted 431 tweets, with a total of 2 million Twitter impressions, suggesting that they had tapped a latent need.
How the chat works
To participate in the JHM chats on Twitter, one needs to open an account on the platform (it’s free) and follow the Journal’s Twitter feed (@jhospmedicine). But that’s pretty much it, Dr. Wray said. The group convenes on a Monday evening each month for an hour, starting at 9 p.m. Eastern time. Upcoming chats and topics are announced on Twitter and at SHM’s website.
The chats have grown and evolved since 2015, shifting in focus given recent social upheavals over the pandemic and heated discussions about diversity, equity, and racial justice in medicine, he said. “When COVID hit, the journal’s editor – Dr. Samir Shah – recognized that we were in a unique moment with the pandemic. The journal took advantage of the opportunity to publish a lot more personal perspectives and viewpoints around COVID, along with a special issue devoted to social justice.”
Moderators for the chat typically choose three or four questions based on recently published articles or other relevant topics, such as racial inequities in health care or how to apply military principles to hospital medicine leadership. “We reach out to authors and tell them they can explain their articles to interested readers through the chat. I can’t think of a single one who said no. They see the opportunity to highlight their work and engage with readers who want to ask them questions,” Dr. Wray said.
The moderators’ questions are posed to stimulate participation, but another goal is to use that hour for networking. “It’s a powerful tool to allow SHM members to engage with each other,” he said. “Sometimes the chat has the feeling of trying to drink water from a fire hose – with the messages flashing past so quickly. But the key is not to try to respond to everything but rather to follow those threads that particularly interest you. We encourage you to engage, but it’s totally fine if you just sit back and observe. One thing we have done to make it a little more formal is to offer CME credits for participants.”
The chat welcomes hospitalists and nonhospitalists, nurse practitioners, physician assistants, academics, and nonacademics. “No matter how engaged you are with Twitter, if you have 10,000 followers or 10, we’ll amplify your voice,” he said. “We also have medical students participating and consider their perspectives valuable, too.”
Dr. Wray identified three main types of participants in the monthly chats. The first are regulars who come every month, rain or shine. Like the character Norm in the old television comedy “Cheers,” everybody knows their name. They become friends, sharing and reveling in each other’s accomplishments. “These are people who have multiple connections, personally and professionally, at a lot of different levels. I probably know a hundred or more people who I’ve primarily gotten to know online.”
A second and larger group might be drawn in because of an interest in a specific topic or article, but they’re also welcome to participate in the chat. And the third group may lurk in the background, following along but not commenting. The size of that third group is unknown, but metrics from SHM show a total of 796 participants posting 4,088 tweets during chats in 2020 (for an average of 132 participants and 681 tweets per chat). This adds up to a total of 34 million impressions across the platform for #JHMchat tweets for the year.
Creating community online
“Why do we do it? It’s difficult to read all of the relevant published articles and keep up to date,” said Dr. Arora, a medical educator whose job at Chicago Medicine is to improve the clinical learning environment for trainees and staff by aligning learning with the health system’s institutional quality, safety and value missions.
“Our idea was to bring together a kind of virtual journal club and have discussions around topics such as: how do you create a shared vision on rounds? How do you integrate that into clinical practice? How do we preserve work/life balance or address structural racism?” she said. Other topics have included work flow concerns, burnout, difficult conversations with patients, and career planning.
“The people we’re trying to reach are hospitalists – and they’re busy at the front lines of care. We also thought this was an interesting way to raise the journal’s profile and spark broader interest in the articles it publishes. But it’s really about creating community, with people who look forward to talking and connecting with each other each month through the chats,” Dr. Arora said. If they miss a chat, they feel they’ve missed important interactions.
“Many times when people log onto the chat, they give a status report on where they are at, such as ‘I’m home putting my kids to bed,’ or ‘I’m on call tonight,’ ” she added. “People are willing to engage with the medium because it’s easy to engage with. We can forget that physicians are like everyone else. They like to learn, but they want that learning to be fun.”
On Dec. 14, 2020, at 9 p.m. Eastern time, the first question for the monthly #JHMchat was posted: How will caring for COVID-19 patients this winter differ from caring for patients in the first wave? Given that another surge of hospitalized COVID patients is looming, participants posted that they feel familiar and more confident with effective clinical strategies for hospitalized COVID patients, having learned so much more about the virus. But they’re facing greater numbers of patients than in prior surges. “In March, we were in crisis, now we’re in complexity,” one noted.
Joining the moderators was the Pediatric Overflow Planning Contingency Response Network (POPCoRN), a group formed earlier this year to help mobilize pediatric medical capacity for COVID patients during pandemic surges (see “POPCoRN network mobilizes pediatric capacity during pandemic,” The Hospitalist, April 30, 2020). One of its questions involved the redeployment of physicians in response to COVID demands and what, for example, pediatric hospitalists need as resources and tools when they are reassigned to adult patients or to new roles in unfamiliar settings. A variety of educational resources were cited from POPCoRN, SHM, and ImproveDX, among others.
Defining medical communication
Another chat moderator is Angela Castellanos, MD, a pediatric hospitalist at Tufts Medical Center in Boston. Dr. Castellanos did a 1-year, full-time fellowship right after residency at the New England Journal of Medicine, participating hands-on as a member of the editorial team for the print and online editions of the venerable journal. She is now doing a digital media fellowship with JHM, a part-time commitment while holding down a full-time job as a hospitalist. She also puts together a Spanish language podcast covering primary care pediatric issues for parents and families.
“I’m interested in medical communication generally, as I try to figure out what that means,” she said. “I have continued to look for ways to be part of the social media community and to be more creative about it. The JHM fellowship came at a perfect time for me to learn to do more in digital media.”
COVID has created new opportunities for more immediate dialogue with colleagues – what are they seeing and what’s working in the absence of clinical trials, she explained. “That’s how we communicate, as a way to get information out fast, such as when hospitals began proning COVID patients to make it easier for them to breathe.”
Dr. Castellanos said she grew up with text messaging and social media and wants to continue to grow her skills in this area. “I think I developed some skills at NEJM, but the opportunity to see how they do things at another journal with a different mission was also valuable. I get to share the space with people in academic settings and leaders in my field. I tweet at them; they tweet at me. These two fellowships have given me unique insights and mentorships. I know I want to continue doing pediatric hospital medicine and to engage academically and learn how to do research.”
Twitter sometimes gets a bad reputation for hostile or incendiary posts, Dr. Wray noted. “If you look at social media writ large, it can sometimes seem like a dumpster fire.” But what has happened in the medical community and in most medical Twitter encounters is a more cordial approach to conversations. “People who work in medicine converse with each other, with room for respectful disagreements. We’re extra supportive of each other,” he said.
“I think if hospitalists are looking for a community of peers, to engage with them and network and to find colleagues in similar circumstances, the JHM chat is such a fantastic place,” Dr. Wray concluded. “Don’t just come once, come several times, meet people along the way. For me, one of the most beneficial ways to advance my career has been by connecting with people through the chat. It allows me to share my work and success with the hospitalist community, as well as highlighting my trainees’ and colleagues’ successes, and it has created opportunities I never would have expected for getting involved in other projects.”
Monthly social media event links editors, authors, and readers
Monthly social media event links editors, authors, and readers
Once upon a time, physicians wrote letters to peers and colleagues around the world, sharing their medical discoveries, theories, case reports, and questions; conferring on problems; and then waiting for return mail to bring a reply. And the science of medicine advanced at a glacial pace.
Today, communication in multiple mediums flows much faster, almost instantaneously, between many more physicians, regardless of distance, addressing a much greater complexity of medical topics and treatments. And one of the chief mediums for this rapid electronic conversation among doctors is Twitter, according to Charlie Wray, DO, MS, a hospitalist at the University of California, San Francisco.
Dr. Wray, associate editor and digital media editor for the Journal of Hospital Medicine, is one of the moderators of #JHMchat, a monthly get-together on Twitter for interested hospitalists to link up virtually; respond to questions posed by JHM editors and other moderators; exchange perspectives, experiences, and tips with their peers; and build professional relationships and personal friendships. Relationship building has become particularly important in the age of COVID-19, when opportunities to connect in person at events such as SHM’s annual conferences have been curtailed.
The online #JHMchat community began in 2015, shortly after Dr. Wray completed a hospitalist research fellowship at the University of Chicago. His fellowship mentor, Vineet Arora, MD, MAPP, MHM, associate chief medical officer for the clinical learning environment at University of Chicago Medicine, had noticed how other online medical communities were engaging in discussions around different topics.
“She thought it could be a great way for hospitalists to meet online and talk about the articles published in JHM,” Dr. Wray explained. “We were all getting into social media and learning how to moderate interactive discussions such as Twitter.”
The chat’s founders approached JHM’s then-editor Andrew Auerbach, MD, MHM, a hospitalist at UCSF, who agreed that it was a great idea. They asked Christopher Moriates, MD, author of a recently published paper on the advisability of nebulized bronchodilators for obstructive pulmonary symptoms and assistant dean for health care value at the University of Texas, Austin, to come on the chat and talk about his paper. Visiting Dr. Arora at the time, he joined her in her living room for the first chat on Oct. 12, 2015. Seventy-five participants posted 431 tweets, with a total of 2 million Twitter impressions, suggesting that they had tapped a latent need.
How the chat works
To participate in the JHM chats on Twitter, one needs to open an account on the platform (it’s free) and follow the Journal’s Twitter feed (@jhospmedicine). But that’s pretty much it, Dr. Wray said. The group convenes on a Monday evening each month for an hour, starting at 9 p.m. Eastern time. Upcoming chats and topics are announced on Twitter and at SHM’s website.
The chats have grown and evolved since 2015, shifting in focus given recent social upheavals over the pandemic and heated discussions about diversity, equity, and racial justice in medicine, he said. “When COVID hit, the journal’s editor – Dr. Samir Shah – recognized that we were in a unique moment with the pandemic. The journal took advantage of the opportunity to publish a lot more personal perspectives and viewpoints around COVID, along with a special issue devoted to social justice.”
Moderators for the chat typically choose three or four questions based on recently published articles or other relevant topics, such as racial inequities in health care or how to apply military principles to hospital medicine leadership. “We reach out to authors and tell them they can explain their articles to interested readers through the chat. I can’t think of a single one who said no. They see the opportunity to highlight their work and engage with readers who want to ask them questions,” Dr. Wray said.
The moderators’ questions are posed to stimulate participation, but another goal is to use that hour for networking. “It’s a powerful tool to allow SHM members to engage with each other,” he said. “Sometimes the chat has the feeling of trying to drink water from a fire hose – with the messages flashing past so quickly. But the key is not to try to respond to everything but rather to follow those threads that particularly interest you. We encourage you to engage, but it’s totally fine if you just sit back and observe. One thing we have done to make it a little more formal is to offer CME credits for participants.”
The chat welcomes hospitalists and nonhospitalists, nurse practitioners, physician assistants, academics, and nonacademics. “No matter how engaged you are with Twitter, if you have 10,000 followers or 10, we’ll amplify your voice,” he said. “We also have medical students participating and consider their perspectives valuable, too.”
Dr. Wray identified three main types of participants in the monthly chats. The first are regulars who come every month, rain or shine. Like the character Norm in the old television comedy “Cheers,” everybody knows their name. They become friends, sharing and reveling in each other’s accomplishments. “These are people who have multiple connections, personally and professionally, at a lot of different levels. I probably know a hundred or more people who I’ve primarily gotten to know online.”
A second and larger group might be drawn in because of an interest in a specific topic or article, but they’re also welcome to participate in the chat. And the third group may lurk in the background, following along but not commenting. The size of that third group is unknown, but metrics from SHM show a total of 796 participants posting 4,088 tweets during chats in 2020 (for an average of 132 participants and 681 tweets per chat). This adds up to a total of 34 million impressions across the platform for #JHMchat tweets for the year.
Creating community online
“Why do we do it? It’s difficult to read all of the relevant published articles and keep up to date,” said Dr. Arora, a medical educator whose job at Chicago Medicine is to improve the clinical learning environment for trainees and staff by aligning learning with the health system’s institutional quality, safety and value missions.
“Our idea was to bring together a kind of virtual journal club and have discussions around topics such as: how do you create a shared vision on rounds? How do you integrate that into clinical practice? How do we preserve work/life balance or address structural racism?” she said. Other topics have included work flow concerns, burnout, difficult conversations with patients, and career planning.
“The people we’re trying to reach are hospitalists – and they’re busy at the front lines of care. We also thought this was an interesting way to raise the journal’s profile and spark broader interest in the articles it publishes. But it’s really about creating community, with people who look forward to talking and connecting with each other each month through the chats,” Dr. Arora said. If they miss a chat, they feel they’ve missed important interactions.
“Many times when people log onto the chat, they give a status report on where they are at, such as ‘I’m home putting my kids to bed,’ or ‘I’m on call tonight,’ ” she added. “People are willing to engage with the medium because it’s easy to engage with. We can forget that physicians are like everyone else. They like to learn, but they want that learning to be fun.”
On Dec. 14, 2020, at 9 p.m. Eastern time, the first question for the monthly #JHMchat was posted: How will caring for COVID-19 patients this winter differ from caring for patients in the first wave? Given that another surge of hospitalized COVID patients is looming, participants posted that they feel familiar and more confident with effective clinical strategies for hospitalized COVID patients, having learned so much more about the virus. But they’re facing greater numbers of patients than in prior surges. “In March, we were in crisis, now we’re in complexity,” one noted.
Joining the moderators was the Pediatric Overflow Planning Contingency Response Network (POPCoRN), a group formed earlier this year to help mobilize pediatric medical capacity for COVID patients during pandemic surges (see “POPCoRN network mobilizes pediatric capacity during pandemic,” The Hospitalist, April 30, 2020). One of its questions involved the redeployment of physicians in response to COVID demands and what, for example, pediatric hospitalists need as resources and tools when they are reassigned to adult patients or to new roles in unfamiliar settings. A variety of educational resources were cited from POPCoRN, SHM, and ImproveDX, among others.
Defining medical communication
Another chat moderator is Angela Castellanos, MD, a pediatric hospitalist at Tufts Medical Center in Boston. Dr. Castellanos did a 1-year, full-time fellowship right after residency at the New England Journal of Medicine, participating hands-on as a member of the editorial team for the print and online editions of the venerable journal. She is now doing a digital media fellowship with JHM, a part-time commitment while holding down a full-time job as a hospitalist. She also puts together a Spanish language podcast covering primary care pediatric issues for parents and families.
“I’m interested in medical communication generally, as I try to figure out what that means,” she said. “I have continued to look for ways to be part of the social media community and to be more creative about it. The JHM fellowship came at a perfect time for me to learn to do more in digital media.”
COVID has created new opportunities for more immediate dialogue with colleagues – what are they seeing and what’s working in the absence of clinical trials, she explained. “That’s how we communicate, as a way to get information out fast, such as when hospitals began proning COVID patients to make it easier for them to breathe.”
Dr. Castellanos said she grew up with text messaging and social media and wants to continue to grow her skills in this area. “I think I developed some skills at NEJM, but the opportunity to see how they do things at another journal with a different mission was also valuable. I get to share the space with people in academic settings and leaders in my field. I tweet at them; they tweet at me. These two fellowships have given me unique insights and mentorships. I know I want to continue doing pediatric hospital medicine and to engage academically and learn how to do research.”
Twitter sometimes gets a bad reputation for hostile or incendiary posts, Dr. Wray noted. “If you look at social media writ large, it can sometimes seem like a dumpster fire.” But what has happened in the medical community and in most medical Twitter encounters is a more cordial approach to conversations. “People who work in medicine converse with each other, with room for respectful disagreements. We’re extra supportive of each other,” he said.
“I think if hospitalists are looking for a community of peers, to engage with them and network and to find colleagues in similar circumstances, the JHM chat is such a fantastic place,” Dr. Wray concluded. “Don’t just come once, come several times, meet people along the way. For me, one of the most beneficial ways to advance my career has been by connecting with people through the chat. It allows me to share my work and success with the hospitalist community, as well as highlighting my trainees’ and colleagues’ successes, and it has created opportunities I never would have expected for getting involved in other projects.”
Once upon a time, physicians wrote letters to peers and colleagues around the world, sharing their medical discoveries, theories, case reports, and questions; conferring on problems; and then waiting for return mail to bring a reply. And the science of medicine advanced at a glacial pace.
Today, communication in multiple mediums flows much faster, almost instantaneously, between many more physicians, regardless of distance, addressing a much greater complexity of medical topics and treatments. And one of the chief mediums for this rapid electronic conversation among doctors is Twitter, according to Charlie Wray, DO, MS, a hospitalist at the University of California, San Francisco.
Dr. Wray, associate editor and digital media editor for the Journal of Hospital Medicine, is one of the moderators of #JHMchat, a monthly get-together on Twitter for interested hospitalists to link up virtually; respond to questions posed by JHM editors and other moderators; exchange perspectives, experiences, and tips with their peers; and build professional relationships and personal friendships. Relationship building has become particularly important in the age of COVID-19, when opportunities to connect in person at events such as SHM’s annual conferences have been curtailed.
The online #JHMchat community began in 2015, shortly after Dr. Wray completed a hospitalist research fellowship at the University of Chicago. His fellowship mentor, Vineet Arora, MD, MAPP, MHM, associate chief medical officer for the clinical learning environment at University of Chicago Medicine, had noticed how other online medical communities were engaging in discussions around different topics.
“She thought it could be a great way for hospitalists to meet online and talk about the articles published in JHM,” Dr. Wray explained. “We were all getting into social media and learning how to moderate interactive discussions such as Twitter.”
The chat’s founders approached JHM’s then-editor Andrew Auerbach, MD, MHM, a hospitalist at UCSF, who agreed that it was a great idea. They asked Christopher Moriates, MD, author of a recently published paper on the advisability of nebulized bronchodilators for obstructive pulmonary symptoms and assistant dean for health care value at the University of Texas, Austin, to come on the chat and talk about his paper. Visiting Dr. Arora at the time, he joined her in her living room for the first chat on Oct. 12, 2015. Seventy-five participants posted 431 tweets, with a total of 2 million Twitter impressions, suggesting that they had tapped a latent need.
How the chat works
To participate in the JHM chats on Twitter, one needs to open an account on the platform (it’s free) and follow the Journal’s Twitter feed (@jhospmedicine). But that’s pretty much it, Dr. Wray said. The group convenes on a Monday evening each month for an hour, starting at 9 p.m. Eastern time. Upcoming chats and topics are announced on Twitter and at SHM’s website.
The chats have grown and evolved since 2015, shifting in focus given recent social upheavals over the pandemic and heated discussions about diversity, equity, and racial justice in medicine, he said. “When COVID hit, the journal’s editor – Dr. Samir Shah – recognized that we were in a unique moment with the pandemic. The journal took advantage of the opportunity to publish a lot more personal perspectives and viewpoints around COVID, along with a special issue devoted to social justice.”
Moderators for the chat typically choose three or four questions based on recently published articles or other relevant topics, such as racial inequities in health care or how to apply military principles to hospital medicine leadership. “We reach out to authors and tell them they can explain their articles to interested readers through the chat. I can’t think of a single one who said no. They see the opportunity to highlight their work and engage with readers who want to ask them questions,” Dr. Wray said.
The moderators’ questions are posed to stimulate participation, but another goal is to use that hour for networking. “It’s a powerful tool to allow SHM members to engage with each other,” he said. “Sometimes the chat has the feeling of trying to drink water from a fire hose – with the messages flashing past so quickly. But the key is not to try to respond to everything but rather to follow those threads that particularly interest you. We encourage you to engage, but it’s totally fine if you just sit back and observe. One thing we have done to make it a little more formal is to offer CME credits for participants.”
The chat welcomes hospitalists and nonhospitalists, nurse practitioners, physician assistants, academics, and nonacademics. “No matter how engaged you are with Twitter, if you have 10,000 followers or 10, we’ll amplify your voice,” he said. “We also have medical students participating and consider their perspectives valuable, too.”
Dr. Wray identified three main types of participants in the monthly chats. The first are regulars who come every month, rain or shine. Like the character Norm in the old television comedy “Cheers,” everybody knows their name. They become friends, sharing and reveling in each other’s accomplishments. “These are people who have multiple connections, personally and professionally, at a lot of different levels. I probably know a hundred or more people who I’ve primarily gotten to know online.”
A second and larger group might be drawn in because of an interest in a specific topic or article, but they’re also welcome to participate in the chat. And the third group may lurk in the background, following along but not commenting. The size of that third group is unknown, but metrics from SHM show a total of 796 participants posting 4,088 tweets during chats in 2020 (for an average of 132 participants and 681 tweets per chat). This adds up to a total of 34 million impressions across the platform for #JHMchat tweets for the year.
Creating community online
“Why do we do it? It’s difficult to read all of the relevant published articles and keep up to date,” said Dr. Arora, a medical educator whose job at Chicago Medicine is to improve the clinical learning environment for trainees and staff by aligning learning with the health system’s institutional quality, safety and value missions.
“Our idea was to bring together a kind of virtual journal club and have discussions around topics such as: how do you create a shared vision on rounds? How do you integrate that into clinical practice? How do we preserve work/life balance or address structural racism?” she said. Other topics have included work flow concerns, burnout, difficult conversations with patients, and career planning.
“The people we’re trying to reach are hospitalists – and they’re busy at the front lines of care. We also thought this was an interesting way to raise the journal’s profile and spark broader interest in the articles it publishes. But it’s really about creating community, with people who look forward to talking and connecting with each other each month through the chats,” Dr. Arora said. If they miss a chat, they feel they’ve missed important interactions.
“Many times when people log onto the chat, they give a status report on where they are at, such as ‘I’m home putting my kids to bed,’ or ‘I’m on call tonight,’ ” she added. “People are willing to engage with the medium because it’s easy to engage with. We can forget that physicians are like everyone else. They like to learn, but they want that learning to be fun.”
On Dec. 14, 2020, at 9 p.m. Eastern time, the first question for the monthly #JHMchat was posted: How will caring for COVID-19 patients this winter differ from caring for patients in the first wave? Given that another surge of hospitalized COVID patients is looming, participants posted that they feel familiar and more confident with effective clinical strategies for hospitalized COVID patients, having learned so much more about the virus. But they’re facing greater numbers of patients than in prior surges. “In March, we were in crisis, now we’re in complexity,” one noted.
Joining the moderators was the Pediatric Overflow Planning Contingency Response Network (POPCoRN), a group formed earlier this year to help mobilize pediatric medical capacity for COVID patients during pandemic surges (see “POPCoRN network mobilizes pediatric capacity during pandemic,” The Hospitalist, April 30, 2020). One of its questions involved the redeployment of physicians in response to COVID demands and what, for example, pediatric hospitalists need as resources and tools when they are reassigned to adult patients or to new roles in unfamiliar settings. A variety of educational resources were cited from POPCoRN, SHM, and ImproveDX, among others.
Defining medical communication
Another chat moderator is Angela Castellanos, MD, a pediatric hospitalist at Tufts Medical Center in Boston. Dr. Castellanos did a 1-year, full-time fellowship right after residency at the New England Journal of Medicine, participating hands-on as a member of the editorial team for the print and online editions of the venerable journal. She is now doing a digital media fellowship with JHM, a part-time commitment while holding down a full-time job as a hospitalist. She also puts together a Spanish language podcast covering primary care pediatric issues for parents and families.
“I’m interested in medical communication generally, as I try to figure out what that means,” she said. “I have continued to look for ways to be part of the social media community and to be more creative about it. The JHM fellowship came at a perfect time for me to learn to do more in digital media.”
COVID has created new opportunities for more immediate dialogue with colleagues – what are they seeing and what’s working in the absence of clinical trials, she explained. “That’s how we communicate, as a way to get information out fast, such as when hospitals began proning COVID patients to make it easier for them to breathe.”
Dr. Castellanos said she grew up with text messaging and social media and wants to continue to grow her skills in this area. “I think I developed some skills at NEJM, but the opportunity to see how they do things at another journal with a different mission was also valuable. I get to share the space with people in academic settings and leaders in my field. I tweet at them; they tweet at me. These two fellowships have given me unique insights and mentorships. I know I want to continue doing pediatric hospital medicine and to engage academically and learn how to do research.”
Twitter sometimes gets a bad reputation for hostile or incendiary posts, Dr. Wray noted. “If you look at social media writ large, it can sometimes seem like a dumpster fire.” But what has happened in the medical community and in most medical Twitter encounters is a more cordial approach to conversations. “People who work in medicine converse with each other, with room for respectful disagreements. We’re extra supportive of each other,” he said.
“I think if hospitalists are looking for a community of peers, to engage with them and network and to find colleagues in similar circumstances, the JHM chat is such a fantastic place,” Dr. Wray concluded. “Don’t just come once, come several times, meet people along the way. For me, one of the most beneficial ways to advance my career has been by connecting with people through the chat. It allows me to share my work and success with the hospitalist community, as well as highlighting my trainees’ and colleagues’ successes, and it has created opportunities I never would have expected for getting involved in other projects.”
‘Alarming finding’ in schizophrenia patients with COVID-19
Schizophrenia spectrum disorder is associated with a significantly increased risk of dying from COVID-19, new research shows.
After adjusting for demographic and medical risk factors, the investigators found that patients who had been diagnosed with schizophrenia were two to three times more likely to die of COVID-19 if they contracted the disease.
“ and efforts should be taken to reduce risk of infection [social distancing, masks, etc.], particularly in people with schizophrenia who live in congregate living situations [hospitals and group residences],” Donald Goff, MD, department of psychiatry, New York University Langone Medical Center, said in an interview.
The study was published online Jan. 27 in JAMA Psychiatry.
The study included 7,348 adults with laboratory-confirmed SARS-CoV-2 infection from the NYU Langone Health System; 75 (1.0%) had a history of schizophrenia spectrum disorder, 564 (7.7%) had a history of a mood disorder, and 360 (4.9%) had a history of an anxiety disorder.
Overall, 864 patients (11.8%) died or were discharged to hospice within 45 days of a positive SARS-CoV-2 test.
In the fully adjusted model, a premorbid diagnosis of schizophrenia spectrum disorder, but not mood or anxiety disorder, was significantly associated with an increased risk of dying from COVID-19 within 45 days.
”A higher risk with schizophrenia spectrum diagnoses was expected based on previous studies of all-cause mortality, but the magnitude of the increase after adjusting for comorbid medical risk factors was unexpected,” the researchers wrote in the study, first authored by Katlyn Nemani, MD, research assistant professor of psychiatry at NYU Langone.
‘Alarming finding’
In an interview, Luming Li, MD, Yale New Haven (Conn.) Psychiatric Hospital, noted that, although the number patients with schizophrenia spectrum disorders in the sample is “fairly low,” she was not surprised by the increased risk for death from COVID-19.
“Schizophrenia falls into the serious mental illness category, and these patients are more often predisposed to homelessness, comorbid medical and substance use, living in congregate settings, lower socioeconomic status, etc,” Dr. Li noted.
Dr. Li’s advice for clinicians who treat patients who have schizophrenia during the COVID-19 pandemic is to minimize their risk in various care settings through the use of personal protective equipment and other infection prevention techniques.
“If a patient does contract COVID-19, make sure patient’s care is escalated appropriately, given the higher risk for mortality in patients with schizophrenia spectrum disorders,” she said.
Tom Pollak, PhD, MRCPsych, King’s College London, said that it has been known for some time that patients with serious mental illness have poorer physical health outcomes. More recently, it has been shown that those who have been diagnosed with psychiatric disorders appear to be at greater risk for poor COVID-19 outcomes.
“This study is the first to specifically highlight schizophrenia spectrum disorders as being particularly at risk. This is an alarming finding. These patients are already amongst the most vulnerable members of society and are probably underserved by most health care systems worldwide,” Dr. Pollak said in a statement.
“Although these findings need urgent replication in larger samples, there are clear reasons for policymakers to take notice now, including giving immediate consideration for prioritization of patients with serious mental illness in nationwide COVID-19 vaccination programs,” he added.
Matthew Hotopf, PhD, FRCPsych, FMedSci, also with King’s College London, said that the New York group has identified people with severe mental disorders as “a high-risk group, and this has immediate public health implications regarding vaccination – that’s the important message of the paper.
“Schizophrenia and other severe psychiatric disorders are risk factors for mortality in the general population before COVID. This is a group with a 10- to 20-year reduction in life expectancy – more than for many diseases we associated with early death,” said Dr. Hotopf.
“The reasons for this are multifactorial, including social deprivation, lifestyle factors (people with schizophrenia smoke more and have high rates of obesity), harms associated with some medications used to treat psychosis, and differential access to health care,” he noted.
“In COVID, we know that deprivation is associated with a much higher mortality, so we would therefore expect that people with severe mental illness will be particularly disadvantaged,” he said.
The study had no specific funding. Dr. Goff has received research support and travel reimbursement from Avanir Pharmaceuticals and Takeda. Dr. Nemani, Dr. Li, Dr. Pollak, and Dr. Hotopf disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Schizophrenia spectrum disorder is associated with a significantly increased risk of dying from COVID-19, new research shows.
After adjusting for demographic and medical risk factors, the investigators found that patients who had been diagnosed with schizophrenia were two to three times more likely to die of COVID-19 if they contracted the disease.
“ and efforts should be taken to reduce risk of infection [social distancing, masks, etc.], particularly in people with schizophrenia who live in congregate living situations [hospitals and group residences],” Donald Goff, MD, department of psychiatry, New York University Langone Medical Center, said in an interview.
The study was published online Jan. 27 in JAMA Psychiatry.
The study included 7,348 adults with laboratory-confirmed SARS-CoV-2 infection from the NYU Langone Health System; 75 (1.0%) had a history of schizophrenia spectrum disorder, 564 (7.7%) had a history of a mood disorder, and 360 (4.9%) had a history of an anxiety disorder.
Overall, 864 patients (11.8%) died or were discharged to hospice within 45 days of a positive SARS-CoV-2 test.
In the fully adjusted model, a premorbid diagnosis of schizophrenia spectrum disorder, but not mood or anxiety disorder, was significantly associated with an increased risk of dying from COVID-19 within 45 days.
”A higher risk with schizophrenia spectrum diagnoses was expected based on previous studies of all-cause mortality, but the magnitude of the increase after adjusting for comorbid medical risk factors was unexpected,” the researchers wrote in the study, first authored by Katlyn Nemani, MD, research assistant professor of psychiatry at NYU Langone.
‘Alarming finding’
In an interview, Luming Li, MD, Yale New Haven (Conn.) Psychiatric Hospital, noted that, although the number patients with schizophrenia spectrum disorders in the sample is “fairly low,” she was not surprised by the increased risk for death from COVID-19.
“Schizophrenia falls into the serious mental illness category, and these patients are more often predisposed to homelessness, comorbid medical and substance use, living in congregate settings, lower socioeconomic status, etc,” Dr. Li noted.
Dr. Li’s advice for clinicians who treat patients who have schizophrenia during the COVID-19 pandemic is to minimize their risk in various care settings through the use of personal protective equipment and other infection prevention techniques.
“If a patient does contract COVID-19, make sure patient’s care is escalated appropriately, given the higher risk for mortality in patients with schizophrenia spectrum disorders,” she said.
Tom Pollak, PhD, MRCPsych, King’s College London, said that it has been known for some time that patients with serious mental illness have poorer physical health outcomes. More recently, it has been shown that those who have been diagnosed with psychiatric disorders appear to be at greater risk for poor COVID-19 outcomes.
“This study is the first to specifically highlight schizophrenia spectrum disorders as being particularly at risk. This is an alarming finding. These patients are already amongst the most vulnerable members of society and are probably underserved by most health care systems worldwide,” Dr. Pollak said in a statement.
“Although these findings need urgent replication in larger samples, there are clear reasons for policymakers to take notice now, including giving immediate consideration for prioritization of patients with serious mental illness in nationwide COVID-19 vaccination programs,” he added.
Matthew Hotopf, PhD, FRCPsych, FMedSci, also with King’s College London, said that the New York group has identified people with severe mental disorders as “a high-risk group, and this has immediate public health implications regarding vaccination – that’s the important message of the paper.
“Schizophrenia and other severe psychiatric disorders are risk factors for mortality in the general population before COVID. This is a group with a 10- to 20-year reduction in life expectancy – more than for many diseases we associated with early death,” said Dr. Hotopf.
“The reasons for this are multifactorial, including social deprivation, lifestyle factors (people with schizophrenia smoke more and have high rates of obesity), harms associated with some medications used to treat psychosis, and differential access to health care,” he noted.
“In COVID, we know that deprivation is associated with a much higher mortality, so we would therefore expect that people with severe mental illness will be particularly disadvantaged,” he said.
The study had no specific funding. Dr. Goff has received research support and travel reimbursement from Avanir Pharmaceuticals and Takeda. Dr. Nemani, Dr. Li, Dr. Pollak, and Dr. Hotopf disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Schizophrenia spectrum disorder is associated with a significantly increased risk of dying from COVID-19, new research shows.
After adjusting for demographic and medical risk factors, the investigators found that patients who had been diagnosed with schizophrenia were two to three times more likely to die of COVID-19 if they contracted the disease.
“ and efforts should be taken to reduce risk of infection [social distancing, masks, etc.], particularly in people with schizophrenia who live in congregate living situations [hospitals and group residences],” Donald Goff, MD, department of psychiatry, New York University Langone Medical Center, said in an interview.
The study was published online Jan. 27 in JAMA Psychiatry.
The study included 7,348 adults with laboratory-confirmed SARS-CoV-2 infection from the NYU Langone Health System; 75 (1.0%) had a history of schizophrenia spectrum disorder, 564 (7.7%) had a history of a mood disorder, and 360 (4.9%) had a history of an anxiety disorder.
Overall, 864 patients (11.8%) died or were discharged to hospice within 45 days of a positive SARS-CoV-2 test.
In the fully adjusted model, a premorbid diagnosis of schizophrenia spectrum disorder, but not mood or anxiety disorder, was significantly associated with an increased risk of dying from COVID-19 within 45 days.
”A higher risk with schizophrenia spectrum diagnoses was expected based on previous studies of all-cause mortality, but the magnitude of the increase after adjusting for comorbid medical risk factors was unexpected,” the researchers wrote in the study, first authored by Katlyn Nemani, MD, research assistant professor of psychiatry at NYU Langone.
‘Alarming finding’
In an interview, Luming Li, MD, Yale New Haven (Conn.) Psychiatric Hospital, noted that, although the number patients with schizophrenia spectrum disorders in the sample is “fairly low,” she was not surprised by the increased risk for death from COVID-19.
“Schizophrenia falls into the serious mental illness category, and these patients are more often predisposed to homelessness, comorbid medical and substance use, living in congregate settings, lower socioeconomic status, etc,” Dr. Li noted.
Dr. Li’s advice for clinicians who treat patients who have schizophrenia during the COVID-19 pandemic is to minimize their risk in various care settings through the use of personal protective equipment and other infection prevention techniques.
“If a patient does contract COVID-19, make sure patient’s care is escalated appropriately, given the higher risk for mortality in patients with schizophrenia spectrum disorders,” she said.
Tom Pollak, PhD, MRCPsych, King’s College London, said that it has been known for some time that patients with serious mental illness have poorer physical health outcomes. More recently, it has been shown that those who have been diagnosed with psychiatric disorders appear to be at greater risk for poor COVID-19 outcomes.
“This study is the first to specifically highlight schizophrenia spectrum disorders as being particularly at risk. This is an alarming finding. These patients are already amongst the most vulnerable members of society and are probably underserved by most health care systems worldwide,” Dr. Pollak said in a statement.
“Although these findings need urgent replication in larger samples, there are clear reasons for policymakers to take notice now, including giving immediate consideration for prioritization of patients with serious mental illness in nationwide COVID-19 vaccination programs,” he added.
Matthew Hotopf, PhD, FRCPsych, FMedSci, also with King’s College London, said that the New York group has identified people with severe mental disorders as “a high-risk group, and this has immediate public health implications regarding vaccination – that’s the important message of the paper.
“Schizophrenia and other severe psychiatric disorders are risk factors for mortality in the general population before COVID. This is a group with a 10- to 20-year reduction in life expectancy – more than for many diseases we associated with early death,” said Dr. Hotopf.
“The reasons for this are multifactorial, including social deprivation, lifestyle factors (people with schizophrenia smoke more and have high rates of obesity), harms associated with some medications used to treat psychosis, and differential access to health care,” he noted.
“In COVID, we know that deprivation is associated with a much higher mortality, so we would therefore expect that people with severe mental illness will be particularly disadvantaged,” he said.
The study had no specific funding. Dr. Goff has received research support and travel reimbursement from Avanir Pharmaceuticals and Takeda. Dr. Nemani, Dr. Li, Dr. Pollak, and Dr. Hotopf disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pitted Depressions on the Hands and Elbows
The Diagnosis: Bazex‐Dupré‐Christol Syndrome
Bazex‐Dupré‐Christol syndrome (BDCS) is a rare X-linked dominant genodermatosis characterized by a triad of hypotrichosis, follicular atrophoderma, and multiple basal cell carcinomas (BCCs). Since first being described in 1964,1 there have been fewer than 200 reported cases of BDCS.2 Although a causative gene has not yet been identified, the mutation has been mapped to an 11.4-Mb interval in the Xq25-27.1 region of the X chromosome.3
Classically, congenital hypotrichosis is the first observed symptom and can present shortly after birth.4 It typically is widespread, though sometimes it may be confined to the eyebrows, eyelashes, and scalp. Follicular atrophoderma, which occurs due to a laxation and deepening of the follicular ostia, is seen in 80% of cases and typically presents in early childhood as depressions lacking hair.2 It commonly is found on the face, extensor surfaces of the elbows and knees, and dorsal aspects of the hands and feet. Physical examination of our patient revealed follicular atrophoderma on both the dorsal surfaces of the hands and the extensor surfaces of the elbows. Hair shaft anomalies including pili torti, pili bifurcati, and trichorrhexis nodosa are infrequently observed symptoms of BDCS.2
Basal cell carcinoma often manifests in the second or third decades of life, though there are reports of BCC developing in BDCS patients as young as 3 years. Basal cell carcinoma typically arises on sun-exposed areas, especially the face, neck, and chest. These lesions can be pigmented or nonpigmented and range from 2 to 20 mm in diameter.4 Our patient presented with a BCC on the forehead (Figure 1). Histopathologic evaluation showed a proliferation of basaloid cells with peripheral palisading (Figure 2), confirming the diagnosis of BCC.
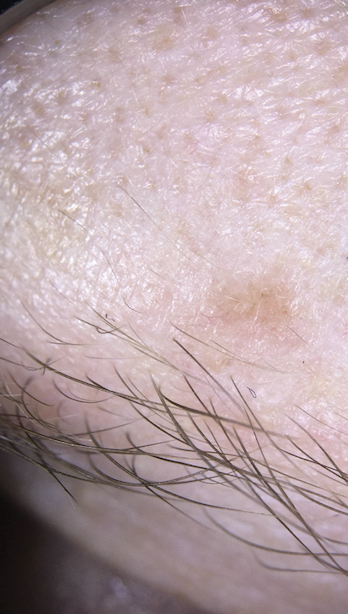
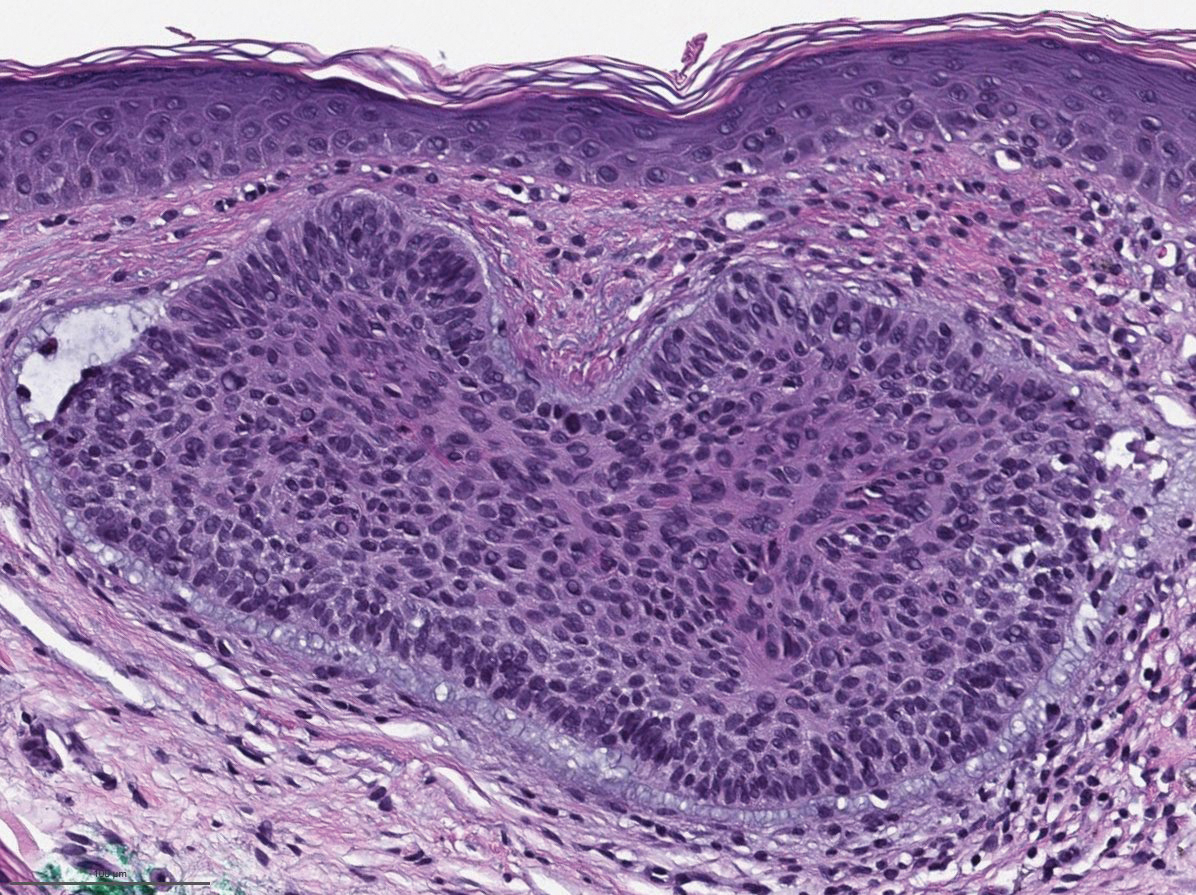
Milia, which are not considered part of the classic BDCS triad, are seen in 70% of cases.2 They commonly are found on the face and often diminish with age. Milia may precede the formation of follicular atrophoderma and BCC. Hypohidrosis most commonly occurs on the forehead but can be widespread.2 Other less commonly observed features include epidermal cysts, hyperpigmentation of the face, and trichoepitheliomas.4 The management of BDCS involves frequent clinical examinations, BCC treatment, genetic counseling, and photoprotection.2,4
Nevoid BCC syndrome (NBCCS), also known as Gorlin-Goltz syndrome, is an autosomal-dominant disease characterized by multiple nevoid BCCs, macrocephaly with a large forehead, cleft lip or palate, jaw keratocysts, palmar and plantar pits, and calcification of the falx cerebri.5 Nevoid BCC syndrome is caused by a mutation in the PTCH1 gene in the hedgehog signaling pathway.6 The absence of common symptoms of NBCCS including macrocephaly, palmar or plantar pits, and cleft lip or palate, as well as negative genetic testing, suggested that our patient did not have NBCCS.
Rombo syndrome shares features with BDCS. Similar to BDCS, symptoms of Rombo syndrome include follicular atrophy, milialike papules, and BCC. Patients with Rombo syndrome typically present with atrophoderma vermiculatum on the cheeks and forehead in childhood.7 This atrophoderma presents with a pitted atrophic appearance in a reticular pattern on sun-exposed areas. Other distinguishing features from BDCS include cyanotic redness of sun-exposed skin and telangiectatic vessels.8
Multiple hereditary infundibulocystic BCC is another rare genodermatosis that is characterized by the presence of multiple infundibulocystic BCCs on the face and genitals. Infundibulocystic BCC is a well-differentiated subtype of BCC characterized by buds and cords of basaloid cells with scant stroma. Multiple hereditary infundibulocystic BCC is inherited in an autosomal-dominant fashion and has been linked to SUFU mutation in the sonic hedgehog pathway.9
Rothmund-Thomson syndrome is an autosomalrecessive disorder characterized by sparse hair, skeletal and dental abnormalities, and a high risk for developing keratinocyte carcinomas. It is differentiated from BDCS clinically by the presence of erythema, edema, and blistering, resulting in poikiloderma, plantar hyperkeratotic lesions, and bone defects.10
- Bazex A. Génodermatose complexe de type indéterminé associant une hypotrichose, un état atrophodermique généralisé et des dégénérescences cutanées multiples (épitheliomas baso-cellulaires). Bull Soc Fr Derm Syphiligr. 1964;71:206.
- Al Sabbagh MM, Baqi MA. Bazex-Dupre-Christol syndrome: review of clinical and molecular aspects. Int J Dermatol. 2018;57:1102-1106.
- Parren LJ, Abuzahra F, Wagenvoort T, et al. Linkage refinement of Bazex-Dupre-Christol syndrome to an 11.4-Mb interval on chromosome Xq25-27.1. Br J Dermatol. 2011;165:201-203.
- Abuzahra F, Parren LJ, Frank J. Multiple familial and pigmented basal cell carcinomas in early childhood--Bazex-Dupre-Christol syndrome. J Eur Acad Dermatol Venereol. 2012;26:117-121.
- Shevchenko A, Durkin JR, Moon AT. Generalized basaloid follicular hamartoma syndrome versus Gorlin syndrome: a diagnostic challenge. Pediatr Dermatol. 2018;35:E396-E397.
- Fujii K, Miyashita T. Gorlin syndrome (nevoid basal cell carcinoma syndrome): update and literature review. Pediatr Int. 2014;56:667-674.
- van Steensel MA, Jaspers NG, Steijlen PM. A case of Rombo syndrome. Br J Dermatol. 2001;144:1215-1218.
- Lee YC, Son SJ, Han TY, et al. A case of atrophoderma vermiculatum showing a good response to topical tretinoin. Ann Dermatol. 2018;30:116-118.
- Schulman JM, Oh DH, Sanborn JZ, et al. Multiple hereditary infundibulocystic basal cell carcinoma syndrome associated with a germline SUFU mutation. JAMA Dermatol. 2016;152:323-327.
- Larizza L, Roversi G, Volpi L. Rothmund-Thomson syndrome. Orphanet J Rare Dis. 2010;5:2.
The Diagnosis: Bazex‐Dupré‐Christol Syndrome
Bazex‐Dupré‐Christol syndrome (BDCS) is a rare X-linked dominant genodermatosis characterized by a triad of hypotrichosis, follicular atrophoderma, and multiple basal cell carcinomas (BCCs). Since first being described in 1964,1 there have been fewer than 200 reported cases of BDCS.2 Although a causative gene has not yet been identified, the mutation has been mapped to an 11.4-Mb interval in the Xq25-27.1 region of the X chromosome.3
Classically, congenital hypotrichosis is the first observed symptom and can present shortly after birth.4 It typically is widespread, though sometimes it may be confined to the eyebrows, eyelashes, and scalp. Follicular atrophoderma, which occurs due to a laxation and deepening of the follicular ostia, is seen in 80% of cases and typically presents in early childhood as depressions lacking hair.2 It commonly is found on the face, extensor surfaces of the elbows and knees, and dorsal aspects of the hands and feet. Physical examination of our patient revealed follicular atrophoderma on both the dorsal surfaces of the hands and the extensor surfaces of the elbows. Hair shaft anomalies including pili torti, pili bifurcati, and trichorrhexis nodosa are infrequently observed symptoms of BDCS.2
Basal cell carcinoma often manifests in the second or third decades of life, though there are reports of BCC developing in BDCS patients as young as 3 years. Basal cell carcinoma typically arises on sun-exposed areas, especially the face, neck, and chest. These lesions can be pigmented or nonpigmented and range from 2 to 20 mm in diameter.4 Our patient presented with a BCC on the forehead (Figure 1). Histopathologic evaluation showed a proliferation of basaloid cells with peripheral palisading (Figure 2), confirming the diagnosis of BCC.


Milia, which are not considered part of the classic BDCS triad, are seen in 70% of cases.2 They commonly are found on the face and often diminish with age. Milia may precede the formation of follicular atrophoderma and BCC. Hypohidrosis most commonly occurs on the forehead but can be widespread.2 Other less commonly observed features include epidermal cysts, hyperpigmentation of the face, and trichoepitheliomas.4 The management of BDCS involves frequent clinical examinations, BCC treatment, genetic counseling, and photoprotection.2,4
Nevoid BCC syndrome (NBCCS), also known as Gorlin-Goltz syndrome, is an autosomal-dominant disease characterized by multiple nevoid BCCs, macrocephaly with a large forehead, cleft lip or palate, jaw keratocysts, palmar and plantar pits, and calcification of the falx cerebri.5 Nevoid BCC syndrome is caused by a mutation in the PTCH1 gene in the hedgehog signaling pathway.6 The absence of common symptoms of NBCCS including macrocephaly, palmar or plantar pits, and cleft lip or palate, as well as negative genetic testing, suggested that our patient did not have NBCCS.
Rombo syndrome shares features with BDCS. Similar to BDCS, symptoms of Rombo syndrome include follicular atrophy, milialike papules, and BCC. Patients with Rombo syndrome typically present with atrophoderma vermiculatum on the cheeks and forehead in childhood.7 This atrophoderma presents with a pitted atrophic appearance in a reticular pattern on sun-exposed areas. Other distinguishing features from BDCS include cyanotic redness of sun-exposed skin and telangiectatic vessels.8
Multiple hereditary infundibulocystic BCC is another rare genodermatosis that is characterized by the presence of multiple infundibulocystic BCCs on the face and genitals. Infundibulocystic BCC is a well-differentiated subtype of BCC characterized by buds and cords of basaloid cells with scant stroma. Multiple hereditary infundibulocystic BCC is inherited in an autosomal-dominant fashion and has been linked to SUFU mutation in the sonic hedgehog pathway.9
Rothmund-Thomson syndrome is an autosomalrecessive disorder characterized by sparse hair, skeletal and dental abnormalities, and a high risk for developing keratinocyte carcinomas. It is differentiated from BDCS clinically by the presence of erythema, edema, and blistering, resulting in poikiloderma, plantar hyperkeratotic lesions, and bone defects.10
The Diagnosis: Bazex‐Dupré‐Christol Syndrome
Bazex‐Dupré‐Christol syndrome (BDCS) is a rare X-linked dominant genodermatosis characterized by a triad of hypotrichosis, follicular atrophoderma, and multiple basal cell carcinomas (BCCs). Since first being described in 1964,1 there have been fewer than 200 reported cases of BDCS.2 Although a causative gene has not yet been identified, the mutation has been mapped to an 11.4-Mb interval in the Xq25-27.1 region of the X chromosome.3
Classically, congenital hypotrichosis is the first observed symptom and can present shortly after birth.4 It typically is widespread, though sometimes it may be confined to the eyebrows, eyelashes, and scalp. Follicular atrophoderma, which occurs due to a laxation and deepening of the follicular ostia, is seen in 80% of cases and typically presents in early childhood as depressions lacking hair.2 It commonly is found on the face, extensor surfaces of the elbows and knees, and dorsal aspects of the hands and feet. Physical examination of our patient revealed follicular atrophoderma on both the dorsal surfaces of the hands and the extensor surfaces of the elbows. Hair shaft anomalies including pili torti, pili bifurcati, and trichorrhexis nodosa are infrequently observed symptoms of BDCS.2
Basal cell carcinoma often manifests in the second or third decades of life, though there are reports of BCC developing in BDCS patients as young as 3 years. Basal cell carcinoma typically arises on sun-exposed areas, especially the face, neck, and chest. These lesions can be pigmented or nonpigmented and range from 2 to 20 mm in diameter.4 Our patient presented with a BCC on the forehead (Figure 1). Histopathologic evaluation showed a proliferation of basaloid cells with peripheral palisading (Figure 2), confirming the diagnosis of BCC.


Milia, which are not considered part of the classic BDCS triad, are seen in 70% of cases.2 They commonly are found on the face and often diminish with age. Milia may precede the formation of follicular atrophoderma and BCC. Hypohidrosis most commonly occurs on the forehead but can be widespread.2 Other less commonly observed features include epidermal cysts, hyperpigmentation of the face, and trichoepitheliomas.4 The management of BDCS involves frequent clinical examinations, BCC treatment, genetic counseling, and photoprotection.2,4
Nevoid BCC syndrome (NBCCS), also known as Gorlin-Goltz syndrome, is an autosomal-dominant disease characterized by multiple nevoid BCCs, macrocephaly with a large forehead, cleft lip or palate, jaw keratocysts, palmar and plantar pits, and calcification of the falx cerebri.5 Nevoid BCC syndrome is caused by a mutation in the PTCH1 gene in the hedgehog signaling pathway.6 The absence of common symptoms of NBCCS including macrocephaly, palmar or plantar pits, and cleft lip or palate, as well as negative genetic testing, suggested that our patient did not have NBCCS.
Rombo syndrome shares features with BDCS. Similar to BDCS, symptoms of Rombo syndrome include follicular atrophy, milialike papules, and BCC. Patients with Rombo syndrome typically present with atrophoderma vermiculatum on the cheeks and forehead in childhood.7 This atrophoderma presents with a pitted atrophic appearance in a reticular pattern on sun-exposed areas. Other distinguishing features from BDCS include cyanotic redness of sun-exposed skin and telangiectatic vessels.8
Multiple hereditary infundibulocystic BCC is another rare genodermatosis that is characterized by the presence of multiple infundibulocystic BCCs on the face and genitals. Infundibulocystic BCC is a well-differentiated subtype of BCC characterized by buds and cords of basaloid cells with scant stroma. Multiple hereditary infundibulocystic BCC is inherited in an autosomal-dominant fashion and has been linked to SUFU mutation in the sonic hedgehog pathway.9
Rothmund-Thomson syndrome is an autosomalrecessive disorder characterized by sparse hair, skeletal and dental abnormalities, and a high risk for developing keratinocyte carcinomas. It is differentiated from BDCS clinically by the presence of erythema, edema, and blistering, resulting in poikiloderma, plantar hyperkeratotic lesions, and bone defects.10
- Bazex A. Génodermatose complexe de type indéterminé associant une hypotrichose, un état atrophodermique généralisé et des dégénérescences cutanées multiples (épitheliomas baso-cellulaires). Bull Soc Fr Derm Syphiligr. 1964;71:206.
- Al Sabbagh MM, Baqi MA. Bazex-Dupre-Christol syndrome: review of clinical and molecular aspects. Int J Dermatol. 2018;57:1102-1106.
- Parren LJ, Abuzahra F, Wagenvoort T, et al. Linkage refinement of Bazex-Dupre-Christol syndrome to an 11.4-Mb interval on chromosome Xq25-27.1. Br J Dermatol. 2011;165:201-203.
- Abuzahra F, Parren LJ, Frank J. Multiple familial and pigmented basal cell carcinomas in early childhood--Bazex-Dupre-Christol syndrome. J Eur Acad Dermatol Venereol. 2012;26:117-121.
- Shevchenko A, Durkin JR, Moon AT. Generalized basaloid follicular hamartoma syndrome versus Gorlin syndrome: a diagnostic challenge. Pediatr Dermatol. 2018;35:E396-E397.
- Fujii K, Miyashita T. Gorlin syndrome (nevoid basal cell carcinoma syndrome): update and literature review. Pediatr Int. 2014;56:667-674.
- van Steensel MA, Jaspers NG, Steijlen PM. A case of Rombo syndrome. Br J Dermatol. 2001;144:1215-1218.
- Lee YC, Son SJ, Han TY, et al. A case of atrophoderma vermiculatum showing a good response to topical tretinoin. Ann Dermatol. 2018;30:116-118.
- Schulman JM, Oh DH, Sanborn JZ, et al. Multiple hereditary infundibulocystic basal cell carcinoma syndrome associated with a germline SUFU mutation. JAMA Dermatol. 2016;152:323-327.
- Larizza L, Roversi G, Volpi L. Rothmund-Thomson syndrome. Orphanet J Rare Dis. 2010;5:2.
- Bazex A. Génodermatose complexe de type indéterminé associant une hypotrichose, un état atrophodermique généralisé et des dégénérescences cutanées multiples (épitheliomas baso-cellulaires). Bull Soc Fr Derm Syphiligr. 1964;71:206.
- Al Sabbagh MM, Baqi MA. Bazex-Dupre-Christol syndrome: review of clinical and molecular aspects. Int J Dermatol. 2018;57:1102-1106.
- Parren LJ, Abuzahra F, Wagenvoort T, et al. Linkage refinement of Bazex-Dupre-Christol syndrome to an 11.4-Mb interval on chromosome Xq25-27.1. Br J Dermatol. 2011;165:201-203.
- Abuzahra F, Parren LJ, Frank J. Multiple familial and pigmented basal cell carcinomas in early childhood--Bazex-Dupre-Christol syndrome. J Eur Acad Dermatol Venereol. 2012;26:117-121.
- Shevchenko A, Durkin JR, Moon AT. Generalized basaloid follicular hamartoma syndrome versus Gorlin syndrome: a diagnostic challenge. Pediatr Dermatol. 2018;35:E396-E397.
- Fujii K, Miyashita T. Gorlin syndrome (nevoid basal cell carcinoma syndrome): update and literature review. Pediatr Int. 2014;56:667-674.
- van Steensel MA, Jaspers NG, Steijlen PM. A case of Rombo syndrome. Br J Dermatol. 2001;144:1215-1218.
- Lee YC, Son SJ, Han TY, et al. A case of atrophoderma vermiculatum showing a good response to topical tretinoin. Ann Dermatol. 2018;30:116-118.
- Schulman JM, Oh DH, Sanborn JZ, et al. Multiple hereditary infundibulocystic basal cell carcinoma syndrome associated with a germline SUFU mutation. JAMA Dermatol. 2016;152:323-327.
- Larizza L, Roversi G, Volpi L. Rothmund-Thomson syndrome. Orphanet J Rare Dis. 2010;5:2.
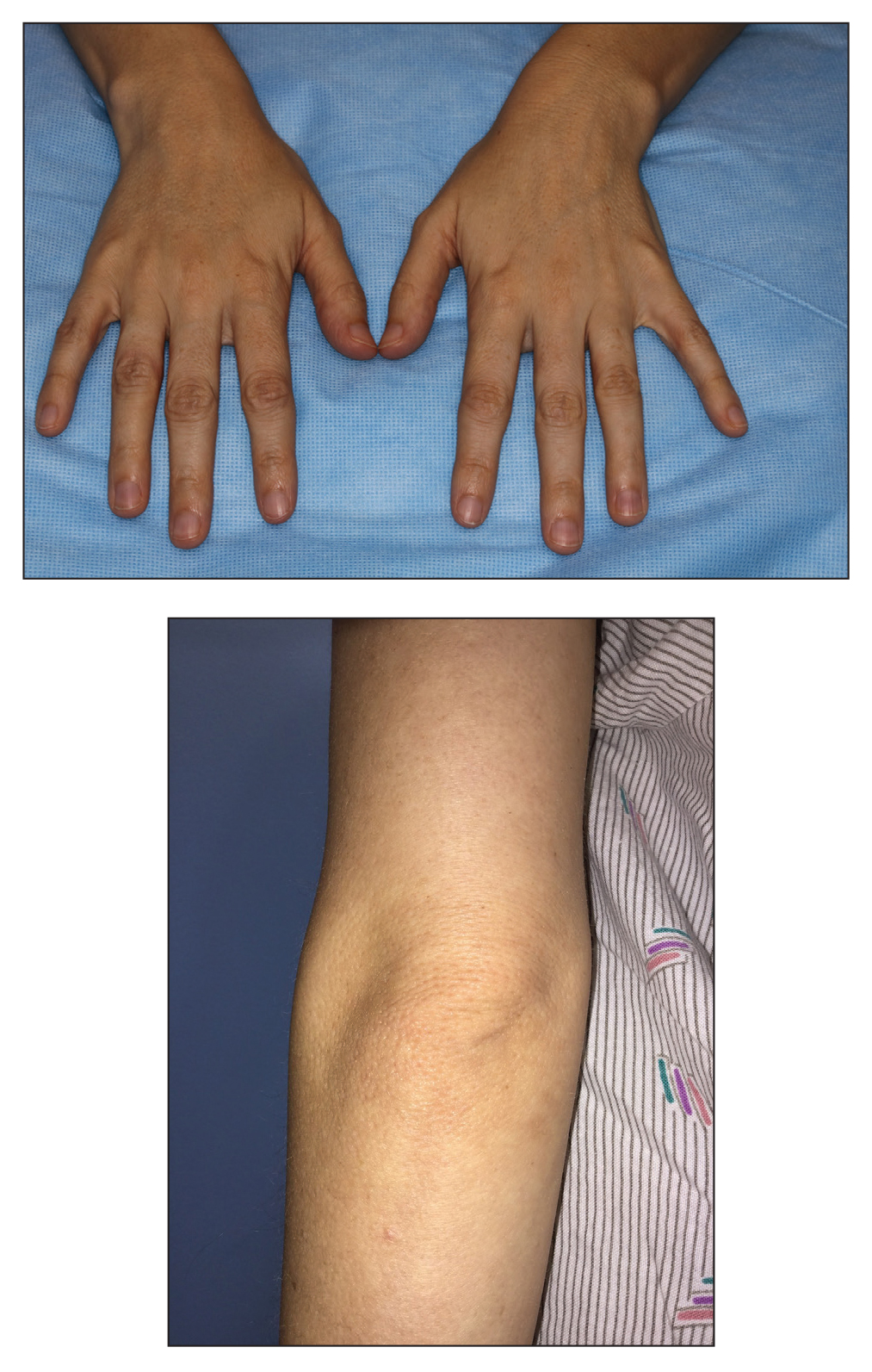
A 28-year-old woman presented for evaluation of a pearly papule on the forehead of several months’ duration that was concerning for basal cell carcinoma (BCC). She had a history of numerous BCCs starting at the age of 17 years. She denied radiation or other carcinogenic exposures and had no other notable medical history. The patient’s mother and grandmother also had numerous BCCs. Physical examination revealed hypotrichosis; numerous 3- to 5-mm white cystic papules on the face, chest, and upper arms; and 1- to 5-mm pitted depressions on the dorsal aspects of the hands (top) and extensor surfaces of the elbows (bottom). A proliferation of basaloid cells with peripheral palisading was seen on histopathologic evaluation. Genetic testing revealed no protein patched homolog 1, PTCH1, or suppressor of fused homolog, SUFU, gene mutations.
RAP device cleared for short-term improvement in appearance of cellulite
As described in a press release issued by Soliton, the RAP device emits rapid acoustic pulses (shock waves) that are transmitted through the skin at a rate of up to 100 pulses per second to rupture or “shear” the fibrotic septa. This causes the release of septa, which results in a smoothening of skin dimples. The procedure takes 40-60 minutes to perform.
“This is a novel, noninvasive treatment for cellulite that appears to be safe, with little pain and little downtime,” Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said in an interview. “Further study and experience will determine the efficacy of this device and the optimization of its parameters going forward.”
In clinical trials that were part of the FDA’s 510(k) application process, patients underwent a single, noninvasive treatment that required no anesthesia and caused no unexpected or serious adverse events. The procedure also received strong patient satisfaction ratings, and clinical trial participants rated their average pain score as 2.4 out of 10.
Soliton plans to begin selling the device for both tattoo removal and cellulite treatment in the first half of 2021. “While the technology is broadly the same, the replaceable treatment cartridges [for tattoo removal and cellulite treatment] differ in significant ways,” Dr. Avram said.
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton. He also reported having ownership and/or shareholder interest in Cytrellis.
As described in a press release issued by Soliton, the RAP device emits rapid acoustic pulses (shock waves) that are transmitted through the skin at a rate of up to 100 pulses per second to rupture or “shear” the fibrotic septa. This causes the release of septa, which results in a smoothening of skin dimples. The procedure takes 40-60 minutes to perform.
“This is a novel, noninvasive treatment for cellulite that appears to be safe, with little pain and little downtime,” Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said in an interview. “Further study and experience will determine the efficacy of this device and the optimization of its parameters going forward.”
In clinical trials that were part of the FDA’s 510(k) application process, patients underwent a single, noninvasive treatment that required no anesthesia and caused no unexpected or serious adverse events. The procedure also received strong patient satisfaction ratings, and clinical trial participants rated their average pain score as 2.4 out of 10.
Soliton plans to begin selling the device for both tattoo removal and cellulite treatment in the first half of 2021. “While the technology is broadly the same, the replaceable treatment cartridges [for tattoo removal and cellulite treatment] differ in significant ways,” Dr. Avram said.
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton. He also reported having ownership and/or shareholder interest in Cytrellis.
As described in a press release issued by Soliton, the RAP device emits rapid acoustic pulses (shock waves) that are transmitted through the skin at a rate of up to 100 pulses per second to rupture or “shear” the fibrotic septa. This causes the release of septa, which results in a smoothening of skin dimples. The procedure takes 40-60 minutes to perform.
“This is a novel, noninvasive treatment for cellulite that appears to be safe, with little pain and little downtime,” Mathew M. Avram, MD, JD, director of laser, cosmetics, and dermatologic surgery at Massachusetts General Hospital, Boston, said in an interview. “Further study and experience will determine the efficacy of this device and the optimization of its parameters going forward.”
In clinical trials that were part of the FDA’s 510(k) application process, patients underwent a single, noninvasive treatment that required no anesthesia and caused no unexpected or serious adverse events. The procedure also received strong patient satisfaction ratings, and clinical trial participants rated their average pain score as 2.4 out of 10.
Soliton plans to begin selling the device for both tattoo removal and cellulite treatment in the first half of 2021. “While the technology is broadly the same, the replaceable treatment cartridges [for tattoo removal and cellulite treatment] differ in significant ways,” Dr. Avram said.
Dr. Avram disclosed that he has received consulting fees from Allergan, Merz, Sciton, and Soliton. He also reported having ownership and/or shareholder interest in Cytrellis.
Algorithm trims time to treatment of acute hypertension in pregnancy
Use of a semiautonomous algorithm to initiate treatment for hypertension emergencies in pregnancy significantly increased the number of individuals treated promptly, based on data from 959 obstetric patients.
Data show poor compliance with the current American College of Obstetricians and Gynecologists recommendations for treatment of acute severe hypertension with no more than 30-60 minutes’ delay; low compliance may be caused by “multiple factors including lack of intravenous access, inadequate health care practitioner or nursing availability, and implicit racial biases,” wrote Courtney Martin, DO, of Loma Linda (Calif.) University School of Medicine and colleagues.
Semiautomated treatment algorithms have been used to improve timely treatment of conditions including myocardial infarction, heart failure, acute stroke, and asthma, but their use in obstetrics to date has been limited, the researchers noted.
In a retrospective cohort study published in Obstetrics & Gynecology, the researchers identified pregnant and postpartum women treated for severe hypertension at a single center between January 2017 and March 2020. A semiautonomous treatment algorithm was implemented between May 2018 and March 2019. The algorithm included vital sign monitoring, blood pressure thresholds for diagnosis of severe hypertension, and automated order sets for recommended first-line antihypertensive therapy. The primary outcomes were treatment with antihypertensive therapy within 15, 30, and 60 minutes of diagnosis. “Severe hypertension was defined as systolic blood pressure 160 mm Hg or higher or diastolic blood pressure 110 mm Hg or higher,” the researchers said.
The study population was divided into three groups; a preimplementation group (373 patients) managed between January 2017 and April 2018, a during-implementation group (334 patients) managed between May 2018 and March 2019, and a postimplementation group (252 patients) managed between April 2019 and March 2020. Patient demographics were similar among all three groups.
Timely treatment improves with algorithm
Overall, treatment of severe hypertension within 15 minutes of diagnosis was 36.5% preimplementation, 45.8% during implementation, and 55.6% postimplementation. Severe hypertension treatment within 30 minutes of diagnosis was 65.9% preimplementation, 77.8% during implementation, and 79.0% post implementation. Differences were significant between pre- and post implementation for 15 minutes and 30 minutes, but no significant differences occurred in the patients treated within 60 minutes before and after implementation of the algorithm.
The study findings were limited by several factors, including the inability to separate peer-to-peer education and other training from the impact of the algorithm, as well as a lack of data on the effect of the algorithm on maternal or neonatal outcomes, the researchers noted.
However, the results support the potential of a semiautonomous algorithm to significantly improve adherence to the recommended treatment guidelines for severe hypertension in pregnancy and post partum, they said. Given the expected increase in hypertensive disorders in pregnancy because of the trends in older age and higher obesity rates in pregnant women, “Integration of semiautonomous treatment algorithms similar to ours into routine obstetric practices could help reduce the health care burden and improve clinical outcomes, especially in areas with limited health care resources,” they concluded.
Algorithm may reduce disparities
The overall rise in maternal mortality in the United States remains a concern, but “Even more concerning are the disturbing racial disparities that persist across socioeconomic strata,” wrote Alisse Hauspurg, MD, of the University of Pittsburgh in an accompanying editorial. “There is clear evidence that expeditious treatment of obstetric hypertensive emergency reduces the risk of severe morbidities including stroke, eclampsia, and maternal death,” she emphasized, but compliance with the ACOG recommendations to treat severe hypertension within 30-60 minutes of confirmation remains low, she said.
In this study, not only did use of the algorithm reduce time to antihypertensive therapy, but more than 50% of patients were treated for severe hypertension within 15 minutes, and more than 90% within 60 minutes, “which was sustained after the implementation phase,” and aligns with the ACOG recommendations, Dr. Hauspurg said. “Although Martin et al.’s algorithm was limited to the initial management of obstetric hypertensive emergency, it could readily be expanded to follow the full ACOG algorithm for management of hypertension in pregnancy,” she noted.
In addition, Black women are more frequently diagnosed with hypertensive disorders of pregnancy, including severe hypertension, and the algorithm might improve disparities, she said.
“It is plausible that widespread implementation of such a semiautonomous algorithm at hospitals across the country could reduce delays in treatment and prevent hypertension-related morbidities,” said Dr. Hauspurg. “The use of innovative approaches to management of severe hypertension and other obstetric emergencies has the potential to allow provision of more equitable care by overcoming health care practitioner and system biases, which could meaningfully reduce disparities in care and change the trajectory of maternal morbidity and mortality in the United States,” she emphasized.
Need to create culture of safety
“Maternal mortality in the United States is the highest among developed nations, and shocking disparities exist in outcomes for non-Hispanic Black and American Indian/Alaskan Native women,” said Lisa Hollier, MD, of Texas Children’s Health Plan in Bellaire. “In a California review of maternal deaths, the greatest quality improvement opportunities were missed diagnosis and ineffective treatment of preeclampsia and related diseases, which occurred in 65% of the cases where women died of preeclampsia/eclampsia,” she said.
The current study “is very timely as more and more states across the nation are participating in the AIM (Alliance for Innovation on Maternal Health) programs to prevent pregnancy-related mortality,” Dr. Hollier noted.
“This study demonstrated a significant association between implementation of the algorithm and an increased percentage of treatment of severe hypertension within 30 minutes,” Dr. Hollier said. “With the implementation of a comprehensive program that included treatment algorithms, the Illinois Perinatal Quality Collaborative improved timely treatment for women with severe high blood pressure, increasing the percentage of patients treated within 60 minutes from 41% at baseline to 79% in the first year of the project.”
The take-home message is that “implementation of the semiautonomous treatment algorithm can address important clinical variation, including delays in appropriate treatment of severe hypertension,” said Dr. Hollier. However, “One of the potential barriers [to use of an algorithm] is the need for accurate, real-time clinical assessment. Resources must be available to ensure appropriate monitoring,” Dr. Hollier noted. “Collaboration and support of implementation of these treatment algorithms must extend through the nursing staff, the physicians, and advanced-practice providers. Medical staff and administrative leaders are essential in creating a culture of safety and continuous process improvement,” she said.
In addition, “long-term follow-up on the implementation of broader quality improvement programs is essential,” Dr. Hollier said. “While implementation of an algorithm can, and did, result in process improvements, assessment of broader implementation of evidence-based bundles, combined with a systematic approach to redesign of multiple related processes needs to occur and include outcomes of severe maternal morbidity and mortality,” she explained.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Neither Dr. Hauspurg nor Dr. Hollier had financial conflicts to disclose.
Use of a semiautonomous algorithm to initiate treatment for hypertension emergencies in pregnancy significantly increased the number of individuals treated promptly, based on data from 959 obstetric patients.
Data show poor compliance with the current American College of Obstetricians and Gynecologists recommendations for treatment of acute severe hypertension with no more than 30-60 minutes’ delay; low compliance may be caused by “multiple factors including lack of intravenous access, inadequate health care practitioner or nursing availability, and implicit racial biases,” wrote Courtney Martin, DO, of Loma Linda (Calif.) University School of Medicine and colleagues.
Semiautomated treatment algorithms have been used to improve timely treatment of conditions including myocardial infarction, heart failure, acute stroke, and asthma, but their use in obstetrics to date has been limited, the researchers noted.
In a retrospective cohort study published in Obstetrics & Gynecology, the researchers identified pregnant and postpartum women treated for severe hypertension at a single center between January 2017 and March 2020. A semiautonomous treatment algorithm was implemented between May 2018 and March 2019. The algorithm included vital sign monitoring, blood pressure thresholds for diagnosis of severe hypertension, and automated order sets for recommended first-line antihypertensive therapy. The primary outcomes were treatment with antihypertensive therapy within 15, 30, and 60 minutes of diagnosis. “Severe hypertension was defined as systolic blood pressure 160 mm Hg or higher or diastolic blood pressure 110 mm Hg or higher,” the researchers said.
The study population was divided into three groups; a preimplementation group (373 patients) managed between January 2017 and April 2018, a during-implementation group (334 patients) managed between May 2018 and March 2019, and a postimplementation group (252 patients) managed between April 2019 and March 2020. Patient demographics were similar among all three groups.
Timely treatment improves with algorithm
Overall, treatment of severe hypertension within 15 minutes of diagnosis was 36.5% preimplementation, 45.8% during implementation, and 55.6% postimplementation. Severe hypertension treatment within 30 minutes of diagnosis was 65.9% preimplementation, 77.8% during implementation, and 79.0% post implementation. Differences were significant between pre- and post implementation for 15 minutes and 30 minutes, but no significant differences occurred in the patients treated within 60 minutes before and after implementation of the algorithm.
The study findings were limited by several factors, including the inability to separate peer-to-peer education and other training from the impact of the algorithm, as well as a lack of data on the effect of the algorithm on maternal or neonatal outcomes, the researchers noted.
However, the results support the potential of a semiautonomous algorithm to significantly improve adherence to the recommended treatment guidelines for severe hypertension in pregnancy and post partum, they said. Given the expected increase in hypertensive disorders in pregnancy because of the trends in older age and higher obesity rates in pregnant women, “Integration of semiautonomous treatment algorithms similar to ours into routine obstetric practices could help reduce the health care burden and improve clinical outcomes, especially in areas with limited health care resources,” they concluded.
Algorithm may reduce disparities
The overall rise in maternal mortality in the United States remains a concern, but “Even more concerning are the disturbing racial disparities that persist across socioeconomic strata,” wrote Alisse Hauspurg, MD, of the University of Pittsburgh in an accompanying editorial. “There is clear evidence that expeditious treatment of obstetric hypertensive emergency reduces the risk of severe morbidities including stroke, eclampsia, and maternal death,” she emphasized, but compliance with the ACOG recommendations to treat severe hypertension within 30-60 minutes of confirmation remains low, she said.
In this study, not only did use of the algorithm reduce time to antihypertensive therapy, but more than 50% of patients were treated for severe hypertension within 15 minutes, and more than 90% within 60 minutes, “which was sustained after the implementation phase,” and aligns with the ACOG recommendations, Dr. Hauspurg said. “Although Martin et al.’s algorithm was limited to the initial management of obstetric hypertensive emergency, it could readily be expanded to follow the full ACOG algorithm for management of hypertension in pregnancy,” she noted.
In addition, Black women are more frequently diagnosed with hypertensive disorders of pregnancy, including severe hypertension, and the algorithm might improve disparities, she said.
“It is plausible that widespread implementation of such a semiautonomous algorithm at hospitals across the country could reduce delays in treatment and prevent hypertension-related morbidities,” said Dr. Hauspurg. “The use of innovative approaches to management of severe hypertension and other obstetric emergencies has the potential to allow provision of more equitable care by overcoming health care practitioner and system biases, which could meaningfully reduce disparities in care and change the trajectory of maternal morbidity and mortality in the United States,” she emphasized.
Need to create culture of safety
“Maternal mortality in the United States is the highest among developed nations, and shocking disparities exist in outcomes for non-Hispanic Black and American Indian/Alaskan Native women,” said Lisa Hollier, MD, of Texas Children’s Health Plan in Bellaire. “In a California review of maternal deaths, the greatest quality improvement opportunities were missed diagnosis and ineffective treatment of preeclampsia and related diseases, which occurred in 65% of the cases where women died of preeclampsia/eclampsia,” she said.
The current study “is very timely as more and more states across the nation are participating in the AIM (Alliance for Innovation on Maternal Health) programs to prevent pregnancy-related mortality,” Dr. Hollier noted.
“This study demonstrated a significant association between implementation of the algorithm and an increased percentage of treatment of severe hypertension within 30 minutes,” Dr. Hollier said. “With the implementation of a comprehensive program that included treatment algorithms, the Illinois Perinatal Quality Collaborative improved timely treatment for women with severe high blood pressure, increasing the percentage of patients treated within 60 minutes from 41% at baseline to 79% in the first year of the project.”
The take-home message is that “implementation of the semiautonomous treatment algorithm can address important clinical variation, including delays in appropriate treatment of severe hypertension,” said Dr. Hollier. However, “One of the potential barriers [to use of an algorithm] is the need for accurate, real-time clinical assessment. Resources must be available to ensure appropriate monitoring,” Dr. Hollier noted. “Collaboration and support of implementation of these treatment algorithms must extend through the nursing staff, the physicians, and advanced-practice providers. Medical staff and administrative leaders are essential in creating a culture of safety and continuous process improvement,” she said.
In addition, “long-term follow-up on the implementation of broader quality improvement programs is essential,” Dr. Hollier said. “While implementation of an algorithm can, and did, result in process improvements, assessment of broader implementation of evidence-based bundles, combined with a systematic approach to redesign of multiple related processes needs to occur and include outcomes of severe maternal morbidity and mortality,” she explained.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Neither Dr. Hauspurg nor Dr. Hollier had financial conflicts to disclose.
Use of a semiautonomous algorithm to initiate treatment for hypertension emergencies in pregnancy significantly increased the number of individuals treated promptly, based on data from 959 obstetric patients.
Data show poor compliance with the current American College of Obstetricians and Gynecologists recommendations for treatment of acute severe hypertension with no more than 30-60 minutes’ delay; low compliance may be caused by “multiple factors including lack of intravenous access, inadequate health care practitioner or nursing availability, and implicit racial biases,” wrote Courtney Martin, DO, of Loma Linda (Calif.) University School of Medicine and colleagues.
Semiautomated treatment algorithms have been used to improve timely treatment of conditions including myocardial infarction, heart failure, acute stroke, and asthma, but their use in obstetrics to date has been limited, the researchers noted.
In a retrospective cohort study published in Obstetrics & Gynecology, the researchers identified pregnant and postpartum women treated for severe hypertension at a single center between January 2017 and March 2020. A semiautonomous treatment algorithm was implemented between May 2018 and March 2019. The algorithm included vital sign monitoring, blood pressure thresholds for diagnosis of severe hypertension, and automated order sets for recommended first-line antihypertensive therapy. The primary outcomes were treatment with antihypertensive therapy within 15, 30, and 60 minutes of diagnosis. “Severe hypertension was defined as systolic blood pressure 160 mm Hg or higher or diastolic blood pressure 110 mm Hg or higher,” the researchers said.
The study population was divided into three groups; a preimplementation group (373 patients) managed between January 2017 and April 2018, a during-implementation group (334 patients) managed between May 2018 and March 2019, and a postimplementation group (252 patients) managed between April 2019 and March 2020. Patient demographics were similar among all three groups.
Timely treatment improves with algorithm
Overall, treatment of severe hypertension within 15 minutes of diagnosis was 36.5% preimplementation, 45.8% during implementation, and 55.6% postimplementation. Severe hypertension treatment within 30 minutes of diagnosis was 65.9% preimplementation, 77.8% during implementation, and 79.0% post implementation. Differences were significant between pre- and post implementation for 15 minutes and 30 minutes, but no significant differences occurred in the patients treated within 60 minutes before and after implementation of the algorithm.
The study findings were limited by several factors, including the inability to separate peer-to-peer education and other training from the impact of the algorithm, as well as a lack of data on the effect of the algorithm on maternal or neonatal outcomes, the researchers noted.
However, the results support the potential of a semiautonomous algorithm to significantly improve adherence to the recommended treatment guidelines for severe hypertension in pregnancy and post partum, they said. Given the expected increase in hypertensive disorders in pregnancy because of the trends in older age and higher obesity rates in pregnant women, “Integration of semiautonomous treatment algorithms similar to ours into routine obstetric practices could help reduce the health care burden and improve clinical outcomes, especially in areas with limited health care resources,” they concluded.
Algorithm may reduce disparities
The overall rise in maternal mortality in the United States remains a concern, but “Even more concerning are the disturbing racial disparities that persist across socioeconomic strata,” wrote Alisse Hauspurg, MD, of the University of Pittsburgh in an accompanying editorial. “There is clear evidence that expeditious treatment of obstetric hypertensive emergency reduces the risk of severe morbidities including stroke, eclampsia, and maternal death,” she emphasized, but compliance with the ACOG recommendations to treat severe hypertension within 30-60 minutes of confirmation remains low, she said.
In this study, not only did use of the algorithm reduce time to antihypertensive therapy, but more than 50% of patients were treated for severe hypertension within 15 minutes, and more than 90% within 60 minutes, “which was sustained after the implementation phase,” and aligns with the ACOG recommendations, Dr. Hauspurg said. “Although Martin et al.’s algorithm was limited to the initial management of obstetric hypertensive emergency, it could readily be expanded to follow the full ACOG algorithm for management of hypertension in pregnancy,” she noted.
In addition, Black women are more frequently diagnosed with hypertensive disorders of pregnancy, including severe hypertension, and the algorithm might improve disparities, she said.
“It is plausible that widespread implementation of such a semiautonomous algorithm at hospitals across the country could reduce delays in treatment and prevent hypertension-related morbidities,” said Dr. Hauspurg. “The use of innovative approaches to management of severe hypertension and other obstetric emergencies has the potential to allow provision of more equitable care by overcoming health care practitioner and system biases, which could meaningfully reduce disparities in care and change the trajectory of maternal morbidity and mortality in the United States,” she emphasized.
Need to create culture of safety
“Maternal mortality in the United States is the highest among developed nations, and shocking disparities exist in outcomes for non-Hispanic Black and American Indian/Alaskan Native women,” said Lisa Hollier, MD, of Texas Children’s Health Plan in Bellaire. “In a California review of maternal deaths, the greatest quality improvement opportunities were missed diagnosis and ineffective treatment of preeclampsia and related diseases, which occurred in 65% of the cases where women died of preeclampsia/eclampsia,” she said.
The current study “is very timely as more and more states across the nation are participating in the AIM (Alliance for Innovation on Maternal Health) programs to prevent pregnancy-related mortality,” Dr. Hollier noted.
“This study demonstrated a significant association between implementation of the algorithm and an increased percentage of treatment of severe hypertension within 30 minutes,” Dr. Hollier said. “With the implementation of a comprehensive program that included treatment algorithms, the Illinois Perinatal Quality Collaborative improved timely treatment for women with severe high blood pressure, increasing the percentage of patients treated within 60 minutes from 41% at baseline to 79% in the first year of the project.”
The take-home message is that “implementation of the semiautonomous treatment algorithm can address important clinical variation, including delays in appropriate treatment of severe hypertension,” said Dr. Hollier. However, “One of the potential barriers [to use of an algorithm] is the need for accurate, real-time clinical assessment. Resources must be available to ensure appropriate monitoring,” Dr. Hollier noted. “Collaboration and support of implementation of these treatment algorithms must extend through the nursing staff, the physicians, and advanced-practice providers. Medical staff and administrative leaders are essential in creating a culture of safety and continuous process improvement,” she said.
In addition, “long-term follow-up on the implementation of broader quality improvement programs is essential,” Dr. Hollier said. “While implementation of an algorithm can, and did, result in process improvements, assessment of broader implementation of evidence-based bundles, combined with a systematic approach to redesign of multiple related processes needs to occur and include outcomes of severe maternal morbidity and mortality,” she explained.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Neither Dr. Hauspurg nor Dr. Hollier had financial conflicts to disclose.
FROM OBSTETRICS & GYNECOLOGY
J&J vaccine 85% efficacious against severe COVID globally
The Janssen/Johnson & Johnson single-dose adenovirus vaccine provides 85% efficacy globally against severe COVID-19 illness, according to the highly anticipated interim phase 3 results announced this morning.
The efficacy against severe disease provided by the Janssen/J&J vaccine held true regardless of age, race/ethnicity, absence or presence of comorbidities, and geography. The 44,000-participant ENSEMBLE study was conducted in the United States, South America, and South Africa.
“The team is very diligently monitoring all the variants that come up, and there are literally thousands of these. We are acting in anticipation of a variant being a potential problem. The South African variant we too acted on right away. So we too are preparing that antigen for testing.
“With data today, we do see that not a single South African, after 28 days post vaccination, ended up needing to go to the hospital, no South African died who was vaccinated.
“We do see that 85%-plus protection in South African against severe disease. That is one of the most exciting results in the dataset today,” said Mathai Mammen, MD, PhD, global head of Janssen Research & Development.
The overall efficacy was 66% globally, 72% in the United States, 66% in Latin America, and 57% in South Africa against moderate to severe SARS-CoV-2 28 days post vaccination, officials from the National Institutes of Health and Janssen reported during a media briefing.
But the J&J vaccine has potential advantages over the existing two-dose Pfizer/BioNTech and Moderna mRNA vaccines because it’s single dose and has less stringent storage requirements – only regular refrigeration is needed versus a need to freeze the two-dose Pfizer/BioNTech and Moderna COVID-19 vaccines. The J&J vaccine can be refrigerated for up to 3 months at 36°-46° F (2°-8° C).
But the difference between these just-released efficacy figures and the 94%-95% efficacy provided by the existing Pfizer/BioNTech and Moderna mRNA vaccines generated many questions during the briefing.
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said the focus should not just be on the overall numbers. “The most important thing from a public health standpoint domestically is to keep people out of the hospital and prevent them from getting severe illness,” he said. “Many in the general public might look at a number and want to know if they get symptomatic disease or not.”
“More important than preventing someone from getting some aches and a sore throat is to prevent people – particularly people who have underlying conditions and the elderly, the ones most susceptible to a severe outcome – [from getting] severe disease,” Dr. Fauci added. Prevention of severe outcomes in a high percentage of individuals “will alleviate so much of the stress, human suffering, and death.”
Dr. Fauci acknowledged that many people will naturally focus on the distinction between 72% efficacy and 94%-95% efficacy. “This could be a messaging challenge [but] you have to make sure people understand the implications.”
It is more complex, he added, than just asking people: “If you go to the door on the left, you get 94% or 95%. If you go to the door to the right, you get 72%. What door do you want to go to?”
Instead, the messaging should be that “this and the other vaccines we have are actually preventing severe disease to a very substantial degree.”
Company defends numbers
Janssen defended their efficacy findings, pointing out that it is not a fair comparison.
“The vaccine programs that went a couple of months ago, they ran their studies during different times, when the pandemic was less complex. There were not these variants, and there was not the same level of incidence, which puts pressure on vaccine efficacy,” said Mathai Mammen, MD, PhD, global head of research and development for Janssen.
“So the numbers cannot really be compared, and that does pose a messaging challenge,” he said. “But the reality is, if one was to run the same studies [for the Pfizer and Moderna vaccines] today you would likely see different results.”
Asked if the efficacy figures could affect vaccine hesitancy, National Institutes of Health Director Francis Collins, MD, PhD, said at the announcement that most reluctance among people to get vaccinated against SARS-CoV-2 stems from concerns about safety. “The safety record is extremely good for this vaccine, as it is for the others that have received emergency use authorization.”
Janssen/J&J plans to submit for emergency use authorization from the U.S. Food and Drug Administration next week, at which point the company plans to release more information on side effects, deaths, and patient subpopulation efficacy, and more from the ENSEMBLE trial.
Janssen is aiming to provide 1 billion doses by the end of this year.
A version of this article first appeared on Medscape.com.
The Janssen/Johnson & Johnson single-dose adenovirus vaccine provides 85% efficacy globally against severe COVID-19 illness, according to the highly anticipated interim phase 3 results announced this morning.
The efficacy against severe disease provided by the Janssen/J&J vaccine held true regardless of age, race/ethnicity, absence or presence of comorbidities, and geography. The 44,000-participant ENSEMBLE study was conducted in the United States, South America, and South Africa.
“The team is very diligently monitoring all the variants that come up, and there are literally thousands of these. We are acting in anticipation of a variant being a potential problem. The South African variant we too acted on right away. So we too are preparing that antigen for testing.
“With data today, we do see that not a single South African, after 28 days post vaccination, ended up needing to go to the hospital, no South African died who was vaccinated.
“We do see that 85%-plus protection in South African against severe disease. That is one of the most exciting results in the dataset today,” said Mathai Mammen, MD, PhD, global head of Janssen Research & Development.
The overall efficacy was 66% globally, 72% in the United States, 66% in Latin America, and 57% in South Africa against moderate to severe SARS-CoV-2 28 days post vaccination, officials from the National Institutes of Health and Janssen reported during a media briefing.
But the J&J vaccine has potential advantages over the existing two-dose Pfizer/BioNTech and Moderna mRNA vaccines because it’s single dose and has less stringent storage requirements – only regular refrigeration is needed versus a need to freeze the two-dose Pfizer/BioNTech and Moderna COVID-19 vaccines. The J&J vaccine can be refrigerated for up to 3 months at 36°-46° F (2°-8° C).
But the difference between these just-released efficacy figures and the 94%-95% efficacy provided by the existing Pfizer/BioNTech and Moderna mRNA vaccines generated many questions during the briefing.
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said the focus should not just be on the overall numbers. “The most important thing from a public health standpoint domestically is to keep people out of the hospital and prevent them from getting severe illness,” he said. “Many in the general public might look at a number and want to know if they get symptomatic disease or not.”
“More important than preventing someone from getting some aches and a sore throat is to prevent people – particularly people who have underlying conditions and the elderly, the ones most susceptible to a severe outcome – [from getting] severe disease,” Dr. Fauci added. Prevention of severe outcomes in a high percentage of individuals “will alleviate so much of the stress, human suffering, and death.”
Dr. Fauci acknowledged that many people will naturally focus on the distinction between 72% efficacy and 94%-95% efficacy. “This could be a messaging challenge [but] you have to make sure people understand the implications.”
It is more complex, he added, than just asking people: “If you go to the door on the left, you get 94% or 95%. If you go to the door to the right, you get 72%. What door do you want to go to?”
Instead, the messaging should be that “this and the other vaccines we have are actually preventing severe disease to a very substantial degree.”
Company defends numbers
Janssen defended their efficacy findings, pointing out that it is not a fair comparison.
“The vaccine programs that went a couple of months ago, they ran their studies during different times, when the pandemic was less complex. There were not these variants, and there was not the same level of incidence, which puts pressure on vaccine efficacy,” said Mathai Mammen, MD, PhD, global head of research and development for Janssen.
“So the numbers cannot really be compared, and that does pose a messaging challenge,” he said. “But the reality is, if one was to run the same studies [for the Pfizer and Moderna vaccines] today you would likely see different results.”
Asked if the efficacy figures could affect vaccine hesitancy, National Institutes of Health Director Francis Collins, MD, PhD, said at the announcement that most reluctance among people to get vaccinated against SARS-CoV-2 stems from concerns about safety. “The safety record is extremely good for this vaccine, as it is for the others that have received emergency use authorization.”
Janssen/J&J plans to submit for emergency use authorization from the U.S. Food and Drug Administration next week, at which point the company plans to release more information on side effects, deaths, and patient subpopulation efficacy, and more from the ENSEMBLE trial.
Janssen is aiming to provide 1 billion doses by the end of this year.
A version of this article first appeared on Medscape.com.
The Janssen/Johnson & Johnson single-dose adenovirus vaccine provides 85% efficacy globally against severe COVID-19 illness, according to the highly anticipated interim phase 3 results announced this morning.
The efficacy against severe disease provided by the Janssen/J&J vaccine held true regardless of age, race/ethnicity, absence or presence of comorbidities, and geography. The 44,000-participant ENSEMBLE study was conducted in the United States, South America, and South Africa.
“The team is very diligently monitoring all the variants that come up, and there are literally thousands of these. We are acting in anticipation of a variant being a potential problem. The South African variant we too acted on right away. So we too are preparing that antigen for testing.
“With data today, we do see that not a single South African, after 28 days post vaccination, ended up needing to go to the hospital, no South African died who was vaccinated.
“We do see that 85%-plus protection in South African against severe disease. That is one of the most exciting results in the dataset today,” said Mathai Mammen, MD, PhD, global head of Janssen Research & Development.
The overall efficacy was 66% globally, 72% in the United States, 66% in Latin America, and 57% in South Africa against moderate to severe SARS-CoV-2 28 days post vaccination, officials from the National Institutes of Health and Janssen reported during a media briefing.
But the J&J vaccine has potential advantages over the existing two-dose Pfizer/BioNTech and Moderna mRNA vaccines because it’s single dose and has less stringent storage requirements – only regular refrigeration is needed versus a need to freeze the two-dose Pfizer/BioNTech and Moderna COVID-19 vaccines. The J&J vaccine can be refrigerated for up to 3 months at 36°-46° F (2°-8° C).
But the difference between these just-released efficacy figures and the 94%-95% efficacy provided by the existing Pfizer/BioNTech and Moderna mRNA vaccines generated many questions during the briefing.
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said the focus should not just be on the overall numbers. “The most important thing from a public health standpoint domestically is to keep people out of the hospital and prevent them from getting severe illness,” he said. “Many in the general public might look at a number and want to know if they get symptomatic disease or not.”
“More important than preventing someone from getting some aches and a sore throat is to prevent people – particularly people who have underlying conditions and the elderly, the ones most susceptible to a severe outcome – [from getting] severe disease,” Dr. Fauci added. Prevention of severe outcomes in a high percentage of individuals “will alleviate so much of the stress, human suffering, and death.”
Dr. Fauci acknowledged that many people will naturally focus on the distinction between 72% efficacy and 94%-95% efficacy. “This could be a messaging challenge [but] you have to make sure people understand the implications.”
It is more complex, he added, than just asking people: “If you go to the door on the left, you get 94% or 95%. If you go to the door to the right, you get 72%. What door do you want to go to?”
Instead, the messaging should be that “this and the other vaccines we have are actually preventing severe disease to a very substantial degree.”
Company defends numbers
Janssen defended their efficacy findings, pointing out that it is not a fair comparison.
“The vaccine programs that went a couple of months ago, they ran their studies during different times, when the pandemic was less complex. There were not these variants, and there was not the same level of incidence, which puts pressure on vaccine efficacy,” said Mathai Mammen, MD, PhD, global head of research and development for Janssen.
“So the numbers cannot really be compared, and that does pose a messaging challenge,” he said. “But the reality is, if one was to run the same studies [for the Pfizer and Moderna vaccines] today you would likely see different results.”
Asked if the efficacy figures could affect vaccine hesitancy, National Institutes of Health Director Francis Collins, MD, PhD, said at the announcement that most reluctance among people to get vaccinated against SARS-CoV-2 stems from concerns about safety. “The safety record is extremely good for this vaccine, as it is for the others that have received emergency use authorization.”
Janssen/J&J plans to submit for emergency use authorization from the U.S. Food and Drug Administration next week, at which point the company plans to release more information on side effects, deaths, and patient subpopulation efficacy, and more from the ENSEMBLE trial.
Janssen is aiming to provide 1 billion doses by the end of this year.
A version of this article first appeared on Medscape.com.