User login
The path to becoming an esophagologist
Esophagology was a term coined in 1948 to describe a medical specialty devoted to the study of the anatomy, physiology, and pathology of the esophagus. The term was born out of increased interest and evolution in esophagology and supported by development in esophagoscopy.1 While still rooted in these basic tenets, the landscape of esophagology is dramatically different in 2020. The last decade alone has seen unprecedented technological advances in esophagology, from the transformation of line tracings to high-resolution esophageal pressure topography to more recent innovations such as the functional lumen imaging probe. Successful therapeutic developments have increased opportunities for effective and less invasive treatment approaches for achalasia and gastroesophageal reflux disease (GERD). With changing concepts in esophageal diseases such as eosinophilic esophagitis, successful management now incorporates findings from recent discoveries that have revolutionized care pathways. (see Figure 1).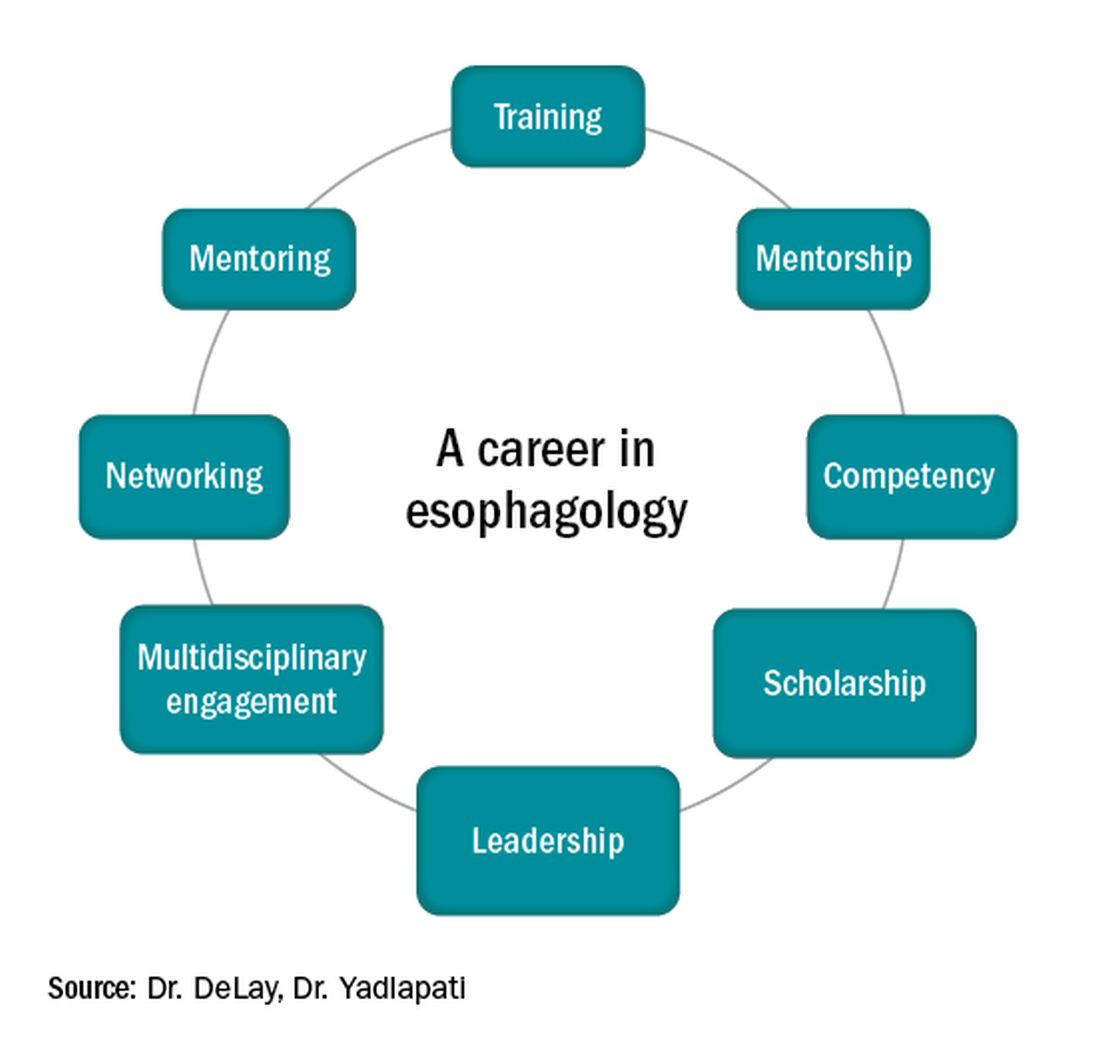
Figure 1
Optimizing esophagology training during fellowship
First, and most importantly, an esophagologist must have a foundation in the basic principles of esophageal anatomy, physiology, and pathology (see Figure 2). While newer digital learning resources exist, tried and true book-based resources – text books, chapters, and reviews – related to esophageal mechanics, the interplay between muscle function and neurogenics, and factors associated with nociception, remain the optimal learning strategy.
Once equipped with a foundation in esophageal physiology, one can readily engage with esophageal technologies, as there exists a vast array of testing to assess esophageal function. A comprehensive understanding of each, including device configuration, clinical protocol, and data storage, promotes a depth of knowledge every esophagologist should develop. Aspiring esophagologists should take time to observe and perform procedures in their motility labs, particularly esophageal high-resolution manometry and ambulatory reflux monitoring studies. If afforded the opportunity through a research study or a clinical indication, esophagologists should also undergo the tests themselves. Empathy regarding the discomfort and tolerability of motility tests, which are notoriously challenging for patients, can promote rapport and trust with patients, increase patient satisfaction, and enhance one’s own understanding of resource utilization and safety.
Perhaps most critical to becoming an esophagologist, is acquiring sufficient competency in interpretation of esophageal studies. Prior research highlights the limitations in achieving competency when trainees adhere to the minimum case volume of studies recommended by the GI core curriculum.2,3 With the bar set higher for the burgeoning esophagologist, one must not only practice with a higher case volume, but also engage in competency-based assessments and performance feedback.4 Trainees should start by reviewing tracings for their own patients. Preliminary interpretation of pending studies and review with a mentor before the final sign-off, participation in research that requires study, or even teaching co-trainees basic tenets of motility are other creative approaches to learning. Esophagologists will be expected to know how to navigate the software to access studies, manually review tracings, and generate reports. Trainees should refer to the multitude of societal guidelines and classification scheme recommendations available when developing competency in diagnostic impression.5
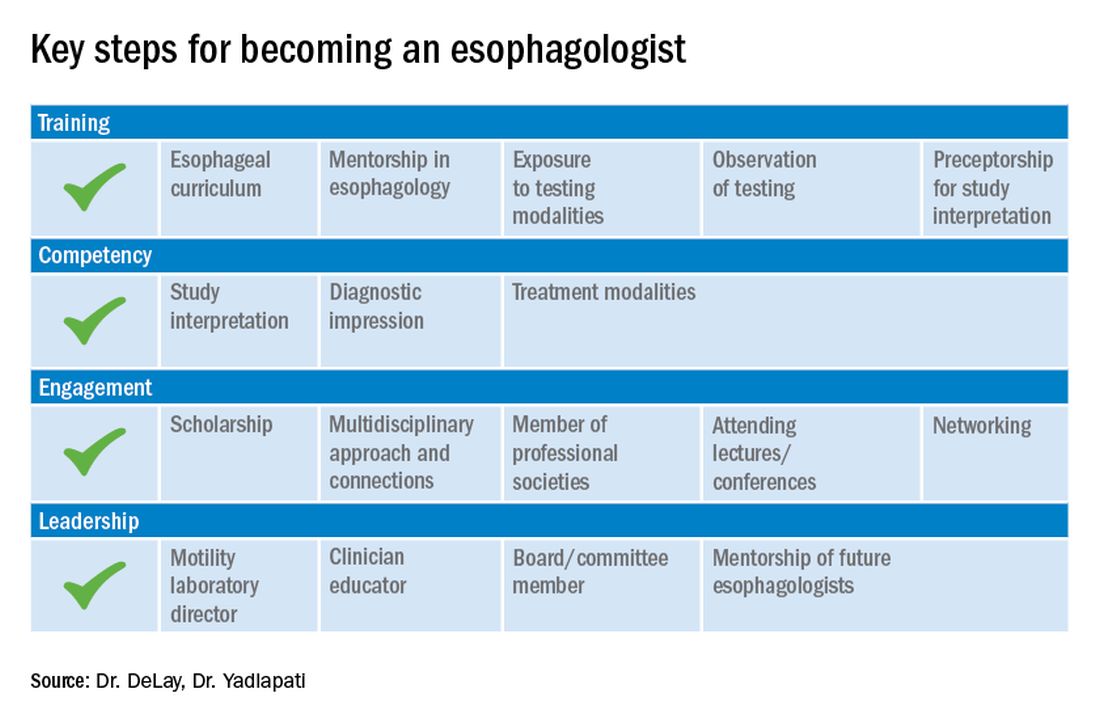
Figure 2
While esophagology is a medical specialty, it is imperative that the esophagologist has a robust understanding of therapeutic options and surgical interventions for esophageal pathology. Scrubbing into the operating room during foregut surgeries is an eye-opening experience. This includes thoracic and abdominal approaches, robotic, laparoscopic, and open techniques, and interventions for GERD, achalasia, diverticular disease, and bariatric management. Equally important is working alongside advanced endoscopy faculty to understand utilities of endoscopic ultrasound, ablative methods for Barrett’s esophagus, and advanced techniques such as peroral endoscopic myotomy and transoral incisionless fundoplication. This exposure is critical as the role of the esophagologist is to speak knowledgably of therapeutic options and the risks and benefits of alternative approaches. Further, the patient’s journey rarely ends with the intervention, and an esophagologist must understand how to evaluate symptoms and manage complications following therapy.
As with broader digestive health, the management of esophageal disorders is becoming increasingly integrated with psychological, lifestyle, and dietary interventions. Observing and understanding how other health care members interact with the patient and relay concepts of brain-gut interaction is helpful in one’s own practice and ability to speak to the value of focused interventions.
These key training aspects in esophagology can be acquired through different avenues (see Figure 3). Formal 1-year advanced esophageal or motility focused fellowships are available at leading esophageal centers. The American Neurogastroenterology and Motility Society (ANMS) offers a clinical training program for selected fellows to pursue apprenticeship-based training in gastrointestinal motility. A review of the benefits of additional training, available programs, and how to apply, can be found at The New Gastroenterologist. It may be possible to customize parts of the general clinical fellowship with a strong focus on esophagology. All budding esophagologists are strongly encouraged to attend and participate in subspecialty national meetings such as through the ANMS or the American Foregut Society.
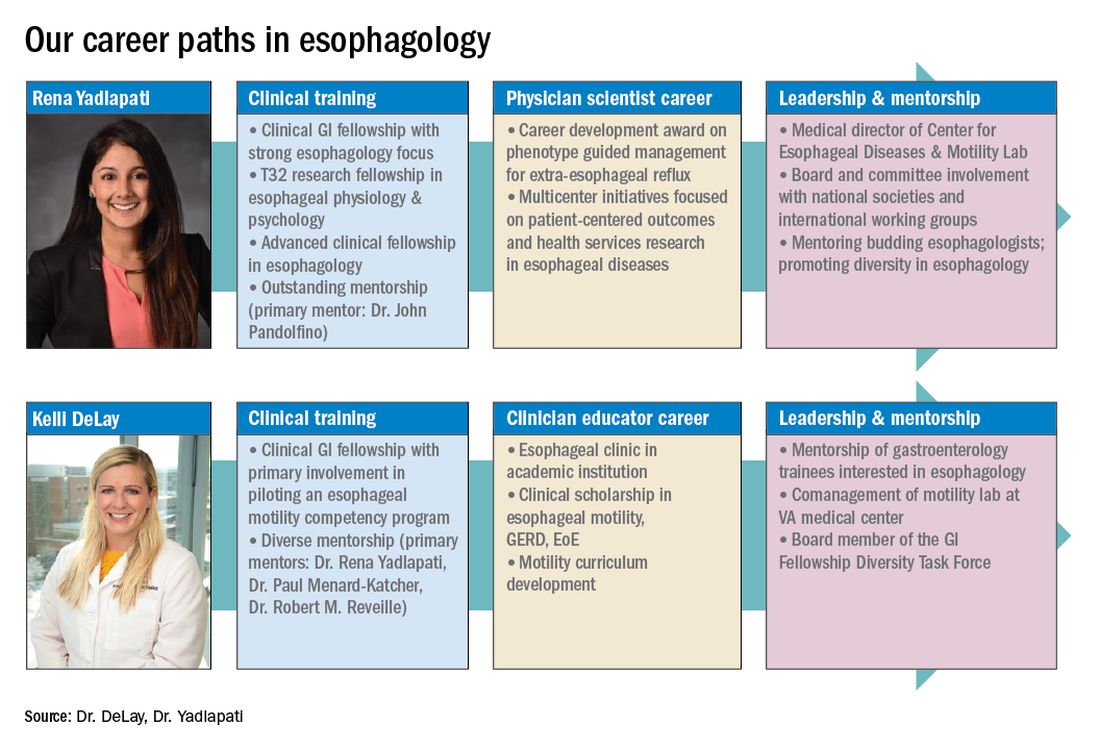
Figure 3
Steep learning curve post fellowship
Regardless of the robust nature of clinical esophagology training, early career esophagologists will face challenges and learn on the job.
Many esophagologists are directors of a motility lab early in their careers. This is often uncharted territory in terms of managing a team of nurses, technicians, and other providers. The director of a motility lab will be called upon to troubleshoot various arenas of diagnostic workup, from study acquisition and interpretation to technical barriers with equipment or software. Keys to maintaining a successful motility lab further include optimizing schedules and protocols, delineating roles and responsibilities of team members, ensuring adequate training across staff and providers, communicating expectations, and cultivating an open relationship with the motility lab supervisor. Crucial, yet often neglected during fellowship training, are the economic considerations of operating and expanding the motility lab, and the financial implications for one’s own practice.6 Participating in professional development workshops can be especially valuable in cultivating leadership skills.
The care an esophagologist provides relies heavily on collaborative relationships within the organization and peer mentorship, cooperation, and feedback. It is essential to cultivate multidisciplinary relationships with surgical (e.g., foregut surgery, laryngology), medical (e.g., pulmonology, allergy), radiology, and pathology colleagues, as well as with integrated health specialists including psychologists, dietitians, and speech language pathologists. It is also important to have open industry partnerships to ensure appropriate technical support and access to advancements.
Often organizations will have only one esophageal specialist within the group. Fortunately, the national and global community of esophagologists is highly collaborative and collegial. All esophagologists should have a network of mentors and colleagues within and outside of their organization to review complex cases, discuss challenges in the workplace, and foster research and innovation. Along these lines, both aspiring and practicing esophagologists should engage with professional societies as opportunities are abundant. Esophageal-focused societies include the ANMS, American Foregut Society, and International Society of Diseases of Esophagus, and the overarching GI societies also have a strong esophageal focus.
The path to becoming an esophagologist does not mirror the structure of the organ itself. Development is neither confined, unidirectional, nor set in length, but gradual, each step thoughtfully built on the last. Esophageal pathology is diverse, complex, and fascinating. With the appropriate training, mentorship, engagement, and leadership, esophagologists have the privilege of making a great impact on the lives of patients we meet, a fulfilling journey worth the time and effort it takes.
Dr. Delay is in the division of gastroenterology & hepatology, University of Colorado Anschutz Medical Campus, Aurora. Dr. Yadlapati is at the Center for Esophageal Diseases, division of gastroenterology, University of California San Diego, La Jolla. She is a consultant through institutional agreement to Medtronic, Ironwood Pharmaceuticals, and Diversatek; she has received research support from Ironwood Pharmaceuticals; and is on the advisory board of Phathom Pharmaceuticals.
References
1. Holinger PH. Arch Otolaryngol. 1948;47:119-26.
2. Yadlapati R et al. Clin Gastroenterol Hepatol. 2017;15:1708-14.e3.
3. Oversight Working Network et al. Gastrointest Endosc. 2014;80:16-27.
4. DeLay K et al. Am J Gastroenterol. 2020;115:1453-9.
5. Gyawali CP et al. Neurogastroenterol Motil. 2018;30(9):e13341.
6. Yadlapati R et al. Gastroenterology. 2020;158:1202-10.
Esophagology was a term coined in 1948 to describe a medical specialty devoted to the study of the anatomy, physiology, and pathology of the esophagus. The term was born out of increased interest and evolution in esophagology and supported by development in esophagoscopy.1 While still rooted in these basic tenets, the landscape of esophagology is dramatically different in 2020. The last decade alone has seen unprecedented technological advances in esophagology, from the transformation of line tracings to high-resolution esophageal pressure topography to more recent innovations such as the functional lumen imaging probe. Successful therapeutic developments have increased opportunities for effective and less invasive treatment approaches for achalasia and gastroesophageal reflux disease (GERD). With changing concepts in esophageal diseases such as eosinophilic esophagitis, successful management now incorporates findings from recent discoveries that have revolutionized care pathways. (see Figure 1).
Figure 1
Optimizing esophagology training during fellowship
First, and most importantly, an esophagologist must have a foundation in the basic principles of esophageal anatomy, physiology, and pathology (see Figure 2). While newer digital learning resources exist, tried and true book-based resources – text books, chapters, and reviews – related to esophageal mechanics, the interplay between muscle function and neurogenics, and factors associated with nociception, remain the optimal learning strategy.
Once equipped with a foundation in esophageal physiology, one can readily engage with esophageal technologies, as there exists a vast array of testing to assess esophageal function. A comprehensive understanding of each, including device configuration, clinical protocol, and data storage, promotes a depth of knowledge every esophagologist should develop. Aspiring esophagologists should take time to observe and perform procedures in their motility labs, particularly esophageal high-resolution manometry and ambulatory reflux monitoring studies. If afforded the opportunity through a research study or a clinical indication, esophagologists should also undergo the tests themselves. Empathy regarding the discomfort and tolerability of motility tests, which are notoriously challenging for patients, can promote rapport and trust with patients, increase patient satisfaction, and enhance one’s own understanding of resource utilization and safety.
Perhaps most critical to becoming an esophagologist, is acquiring sufficient competency in interpretation of esophageal studies. Prior research highlights the limitations in achieving competency when trainees adhere to the minimum case volume of studies recommended by the GI core curriculum.2,3 With the bar set higher for the burgeoning esophagologist, one must not only practice with a higher case volume, but also engage in competency-based assessments and performance feedback.4 Trainees should start by reviewing tracings for their own patients. Preliminary interpretation of pending studies and review with a mentor before the final sign-off, participation in research that requires study, or even teaching co-trainees basic tenets of motility are other creative approaches to learning. Esophagologists will be expected to know how to navigate the software to access studies, manually review tracings, and generate reports. Trainees should refer to the multitude of societal guidelines and classification scheme recommendations available when developing competency in diagnostic impression.5

Figure 2
While esophagology is a medical specialty, it is imperative that the esophagologist has a robust understanding of therapeutic options and surgical interventions for esophageal pathology. Scrubbing into the operating room during foregut surgeries is an eye-opening experience. This includes thoracic and abdominal approaches, robotic, laparoscopic, and open techniques, and interventions for GERD, achalasia, diverticular disease, and bariatric management. Equally important is working alongside advanced endoscopy faculty to understand utilities of endoscopic ultrasound, ablative methods for Barrett’s esophagus, and advanced techniques such as peroral endoscopic myotomy and transoral incisionless fundoplication. This exposure is critical as the role of the esophagologist is to speak knowledgably of therapeutic options and the risks and benefits of alternative approaches. Further, the patient’s journey rarely ends with the intervention, and an esophagologist must understand how to evaluate symptoms and manage complications following therapy.
As with broader digestive health, the management of esophageal disorders is becoming increasingly integrated with psychological, lifestyle, and dietary interventions. Observing and understanding how other health care members interact with the patient and relay concepts of brain-gut interaction is helpful in one’s own practice and ability to speak to the value of focused interventions.
These key training aspects in esophagology can be acquired through different avenues (see Figure 3). Formal 1-year advanced esophageal or motility focused fellowships are available at leading esophageal centers. The American Neurogastroenterology and Motility Society (ANMS) offers a clinical training program for selected fellows to pursue apprenticeship-based training in gastrointestinal motility. A review of the benefits of additional training, available programs, and how to apply, can be found at The New Gastroenterologist. It may be possible to customize parts of the general clinical fellowship with a strong focus on esophagology. All budding esophagologists are strongly encouraged to attend and participate in subspecialty national meetings such as through the ANMS or the American Foregut Society.

Figure 3
Steep learning curve post fellowship
Regardless of the robust nature of clinical esophagology training, early career esophagologists will face challenges and learn on the job.
Many esophagologists are directors of a motility lab early in their careers. This is often uncharted territory in terms of managing a team of nurses, technicians, and other providers. The director of a motility lab will be called upon to troubleshoot various arenas of diagnostic workup, from study acquisition and interpretation to technical barriers with equipment or software. Keys to maintaining a successful motility lab further include optimizing schedules and protocols, delineating roles and responsibilities of team members, ensuring adequate training across staff and providers, communicating expectations, and cultivating an open relationship with the motility lab supervisor. Crucial, yet often neglected during fellowship training, are the economic considerations of operating and expanding the motility lab, and the financial implications for one’s own practice.6 Participating in professional development workshops can be especially valuable in cultivating leadership skills.
The care an esophagologist provides relies heavily on collaborative relationships within the organization and peer mentorship, cooperation, and feedback. It is essential to cultivate multidisciplinary relationships with surgical (e.g., foregut surgery, laryngology), medical (e.g., pulmonology, allergy), radiology, and pathology colleagues, as well as with integrated health specialists including psychologists, dietitians, and speech language pathologists. It is also important to have open industry partnerships to ensure appropriate technical support and access to advancements.
Often organizations will have only one esophageal specialist within the group. Fortunately, the national and global community of esophagologists is highly collaborative and collegial. All esophagologists should have a network of mentors and colleagues within and outside of their organization to review complex cases, discuss challenges in the workplace, and foster research and innovation. Along these lines, both aspiring and practicing esophagologists should engage with professional societies as opportunities are abundant. Esophageal-focused societies include the ANMS, American Foregut Society, and International Society of Diseases of Esophagus, and the overarching GI societies also have a strong esophageal focus.
The path to becoming an esophagologist does not mirror the structure of the organ itself. Development is neither confined, unidirectional, nor set in length, but gradual, each step thoughtfully built on the last. Esophageal pathology is diverse, complex, and fascinating. With the appropriate training, mentorship, engagement, and leadership, esophagologists have the privilege of making a great impact on the lives of patients we meet, a fulfilling journey worth the time and effort it takes.
Dr. Delay is in the division of gastroenterology & hepatology, University of Colorado Anschutz Medical Campus, Aurora. Dr. Yadlapati is at the Center for Esophageal Diseases, division of gastroenterology, University of California San Diego, La Jolla. She is a consultant through institutional agreement to Medtronic, Ironwood Pharmaceuticals, and Diversatek; she has received research support from Ironwood Pharmaceuticals; and is on the advisory board of Phathom Pharmaceuticals.
References
1. Holinger PH. Arch Otolaryngol. 1948;47:119-26.
2. Yadlapati R et al. Clin Gastroenterol Hepatol. 2017;15:1708-14.e3.
3. Oversight Working Network et al. Gastrointest Endosc. 2014;80:16-27.
4. DeLay K et al. Am J Gastroenterol. 2020;115:1453-9.
5. Gyawali CP et al. Neurogastroenterol Motil. 2018;30(9):e13341.
6. Yadlapati R et al. Gastroenterology. 2020;158:1202-10.
Esophagology was a term coined in 1948 to describe a medical specialty devoted to the study of the anatomy, physiology, and pathology of the esophagus. The term was born out of increased interest and evolution in esophagology and supported by development in esophagoscopy.1 While still rooted in these basic tenets, the landscape of esophagology is dramatically different in 2020. The last decade alone has seen unprecedented technological advances in esophagology, from the transformation of line tracings to high-resolution esophageal pressure topography to more recent innovations such as the functional lumen imaging probe. Successful therapeutic developments have increased opportunities for effective and less invasive treatment approaches for achalasia and gastroesophageal reflux disease (GERD). With changing concepts in esophageal diseases such as eosinophilic esophagitis, successful management now incorporates findings from recent discoveries that have revolutionized care pathways. (see Figure 1).
Figure 1
Optimizing esophagology training during fellowship
First, and most importantly, an esophagologist must have a foundation in the basic principles of esophageal anatomy, physiology, and pathology (see Figure 2). While newer digital learning resources exist, tried and true book-based resources – text books, chapters, and reviews – related to esophageal mechanics, the interplay between muscle function and neurogenics, and factors associated with nociception, remain the optimal learning strategy.
Once equipped with a foundation in esophageal physiology, one can readily engage with esophageal technologies, as there exists a vast array of testing to assess esophageal function. A comprehensive understanding of each, including device configuration, clinical protocol, and data storage, promotes a depth of knowledge every esophagologist should develop. Aspiring esophagologists should take time to observe and perform procedures in their motility labs, particularly esophageal high-resolution manometry and ambulatory reflux monitoring studies. If afforded the opportunity through a research study or a clinical indication, esophagologists should also undergo the tests themselves. Empathy regarding the discomfort and tolerability of motility tests, which are notoriously challenging for patients, can promote rapport and trust with patients, increase patient satisfaction, and enhance one’s own understanding of resource utilization and safety.
Perhaps most critical to becoming an esophagologist, is acquiring sufficient competency in interpretation of esophageal studies. Prior research highlights the limitations in achieving competency when trainees adhere to the minimum case volume of studies recommended by the GI core curriculum.2,3 With the bar set higher for the burgeoning esophagologist, one must not only practice with a higher case volume, but also engage in competency-based assessments and performance feedback.4 Trainees should start by reviewing tracings for their own patients. Preliminary interpretation of pending studies and review with a mentor before the final sign-off, participation in research that requires study, or even teaching co-trainees basic tenets of motility are other creative approaches to learning. Esophagologists will be expected to know how to navigate the software to access studies, manually review tracings, and generate reports. Trainees should refer to the multitude of societal guidelines and classification scheme recommendations available when developing competency in diagnostic impression.5

Figure 2
While esophagology is a medical specialty, it is imperative that the esophagologist has a robust understanding of therapeutic options and surgical interventions for esophageal pathology. Scrubbing into the operating room during foregut surgeries is an eye-opening experience. This includes thoracic and abdominal approaches, robotic, laparoscopic, and open techniques, and interventions for GERD, achalasia, diverticular disease, and bariatric management. Equally important is working alongside advanced endoscopy faculty to understand utilities of endoscopic ultrasound, ablative methods for Barrett’s esophagus, and advanced techniques such as peroral endoscopic myotomy and transoral incisionless fundoplication. This exposure is critical as the role of the esophagologist is to speak knowledgably of therapeutic options and the risks and benefits of alternative approaches. Further, the patient’s journey rarely ends with the intervention, and an esophagologist must understand how to evaluate symptoms and manage complications following therapy.
As with broader digestive health, the management of esophageal disorders is becoming increasingly integrated with psychological, lifestyle, and dietary interventions. Observing and understanding how other health care members interact with the patient and relay concepts of brain-gut interaction is helpful in one’s own practice and ability to speak to the value of focused interventions.
These key training aspects in esophagology can be acquired through different avenues (see Figure 3). Formal 1-year advanced esophageal or motility focused fellowships are available at leading esophageal centers. The American Neurogastroenterology and Motility Society (ANMS) offers a clinical training program for selected fellows to pursue apprenticeship-based training in gastrointestinal motility. A review of the benefits of additional training, available programs, and how to apply, can be found at The New Gastroenterologist. It may be possible to customize parts of the general clinical fellowship with a strong focus on esophagology. All budding esophagologists are strongly encouraged to attend and participate in subspecialty national meetings such as through the ANMS or the American Foregut Society.

Figure 3
Steep learning curve post fellowship
Regardless of the robust nature of clinical esophagology training, early career esophagologists will face challenges and learn on the job.
Many esophagologists are directors of a motility lab early in their careers. This is often uncharted territory in terms of managing a team of nurses, technicians, and other providers. The director of a motility lab will be called upon to troubleshoot various arenas of diagnostic workup, from study acquisition and interpretation to technical barriers with equipment or software. Keys to maintaining a successful motility lab further include optimizing schedules and protocols, delineating roles and responsibilities of team members, ensuring adequate training across staff and providers, communicating expectations, and cultivating an open relationship with the motility lab supervisor. Crucial, yet often neglected during fellowship training, are the economic considerations of operating and expanding the motility lab, and the financial implications for one’s own practice.6 Participating in professional development workshops can be especially valuable in cultivating leadership skills.
The care an esophagologist provides relies heavily on collaborative relationships within the organization and peer mentorship, cooperation, and feedback. It is essential to cultivate multidisciplinary relationships with surgical (e.g., foregut surgery, laryngology), medical (e.g., pulmonology, allergy), radiology, and pathology colleagues, as well as with integrated health specialists including psychologists, dietitians, and speech language pathologists. It is also important to have open industry partnerships to ensure appropriate technical support and access to advancements.
Often organizations will have only one esophageal specialist within the group. Fortunately, the national and global community of esophagologists is highly collaborative and collegial. All esophagologists should have a network of mentors and colleagues within and outside of their organization to review complex cases, discuss challenges in the workplace, and foster research and innovation. Along these lines, both aspiring and practicing esophagologists should engage with professional societies as opportunities are abundant. Esophageal-focused societies include the ANMS, American Foregut Society, and International Society of Diseases of Esophagus, and the overarching GI societies also have a strong esophageal focus.
The path to becoming an esophagologist does not mirror the structure of the organ itself. Development is neither confined, unidirectional, nor set in length, but gradual, each step thoughtfully built on the last. Esophageal pathology is diverse, complex, and fascinating. With the appropriate training, mentorship, engagement, and leadership, esophagologists have the privilege of making a great impact on the lives of patients we meet, a fulfilling journey worth the time and effort it takes.
Dr. Delay is in the division of gastroenterology & hepatology, University of Colorado Anschutz Medical Campus, Aurora. Dr. Yadlapati is at the Center for Esophageal Diseases, division of gastroenterology, University of California San Diego, La Jolla. She is a consultant through institutional agreement to Medtronic, Ironwood Pharmaceuticals, and Diversatek; she has received research support from Ironwood Pharmaceuticals; and is on the advisory board of Phathom Pharmaceuticals.
References
1. Holinger PH. Arch Otolaryngol. 1948;47:119-26.
2. Yadlapati R et al. Clin Gastroenterol Hepatol. 2017;15:1708-14.e3.
3. Oversight Working Network et al. Gastrointest Endosc. 2014;80:16-27.
4. DeLay K et al. Am J Gastroenterol. 2020;115:1453-9.
5. Gyawali CP et al. Neurogastroenterol Motil. 2018;30(9):e13341.
6. Yadlapati R et al. Gastroenterology. 2020;158:1202-10.
Photosensitivity diagnosis made simple
of the University of Southern California, Los Angeles.
“When a patient comes in who makes you suspect a photosensitivity, there will be two different presentations,” he said in a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
In some cases, the patient presents with a reaction they believe is sun related, although they don’t have a rash currently, he said. In other cases, “you as a good clinician suspect photosensitivity because the eruption is in a photo distribution,” although the patient may or may not relate it to sun exposure, he added.
Dr. DeLeo noted a few key points to include when taking the history in patients with likely photosensitivity, whether or not they present with a rash.
“I always ask patients when did the episode occur? Is it chronic?” Also ask about timing: Does the reaction occur in the sun, or later? Does it occur quickly and go away within hours, or occur within days or weeks of exposure?
“Always take a good drug history, as photosensitivity can often be related to drugs,” Dr. DeLeo noted. For example, approximately 50% of individuals on amiodarone will have some type of photosensitivity, he said.
Other drug-induced photosensitive conditions include drug-induced subacute cutaneous lupus and pseudoporphyria from NSAIDs, as well as hyperpigmentation from diltiazem, which most often occurs in Black women, he said.
“Photodrug reactions are usually related to UVA radiation, and that is important because you can develop it through the window while driving in your car”: The car windows do not protect against UVA, Dr. DeLeo said. If you have a patient who tells you about a photosensitivity or has a rash and they are on a photosensitizing drug, first rule out connective tissue disease, then discontinue the drug in collaboration with the patient’s internist and wait for the reaction to disappear, and it should, he said.
Some photosensitivity rashes have characteristic patterns, notably connective tissue disease patterns in lupus and dermatomyositis patients, bullous eruptions in cases of porphyria or phototoxic contact dermatitis, and eczematous eruptions, Dr. DeLeo noted.
Patients who present without a rash, but report a history of a reaction that they believe is related to sun exposure, fall into two categories: some had a rash that occurred while in the sun and disappeared quickly, and some had one that occurred hours or days after exposure and lasted a few days to weeks, said Dr. DeLeo.
The differential diagnosis in the patient with immediate photosensitivity is fairly clear: These patients usually have solar urticaria, he said. However, some lupus patients may report this reaction so it is important to rule out connective tissue disease. The diagnosis can be made with phototesting or do a simple test by having the patient sit out in the sunshine, he said.
For the patient who has a delayed reactivity after sun exposure, and doesn’t have the reaction when they come to the office, the differential diagnosis in a simply applied way is that, if the reaction spared the face, it is likely polymorphous light eruption (PMLE); but if the face is involved, the patient likely has photoallergic contact dermatitis, Dr. DeLeo explained. However, always consider the alternatives of connective tissue disease, drug reactions, and contact dermatitis that is not photoallergic, he noted.
PMLE “is the most common photosensitivity reaction that we see in the United States,” and it almost always occurs when people are away from home, usually on vacation, said Dr. DeLeo. The differential diagnosis for patients with recurrent or delayed rash involving the face could be photoallergic contact dermatitis, but rule out airborne contact dermatitis, personal care product contact dermatitis, and chronic actinic dermatitis, he said. A work-up for these patients could include a photo test, photopatch test, or patch test.
Dr. DeLeo disclosed serving as a consultant for Estee Lauder.
MedscapeLive and this news organization are owned by the same parent company.
of the University of Southern California, Los Angeles.
“When a patient comes in who makes you suspect a photosensitivity, there will be two different presentations,” he said in a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
In some cases, the patient presents with a reaction they believe is sun related, although they don’t have a rash currently, he said. In other cases, “you as a good clinician suspect photosensitivity because the eruption is in a photo distribution,” although the patient may or may not relate it to sun exposure, he added.
Dr. DeLeo noted a few key points to include when taking the history in patients with likely photosensitivity, whether or not they present with a rash.
“I always ask patients when did the episode occur? Is it chronic?” Also ask about timing: Does the reaction occur in the sun, or later? Does it occur quickly and go away within hours, or occur within days or weeks of exposure?
“Always take a good drug history, as photosensitivity can often be related to drugs,” Dr. DeLeo noted. For example, approximately 50% of individuals on amiodarone will have some type of photosensitivity, he said.
Other drug-induced photosensitive conditions include drug-induced subacute cutaneous lupus and pseudoporphyria from NSAIDs, as well as hyperpigmentation from diltiazem, which most often occurs in Black women, he said.
“Photodrug reactions are usually related to UVA radiation, and that is important because you can develop it through the window while driving in your car”: The car windows do not protect against UVA, Dr. DeLeo said. If you have a patient who tells you about a photosensitivity or has a rash and they are on a photosensitizing drug, first rule out connective tissue disease, then discontinue the drug in collaboration with the patient’s internist and wait for the reaction to disappear, and it should, he said.
Some photosensitivity rashes have characteristic patterns, notably connective tissue disease patterns in lupus and dermatomyositis patients, bullous eruptions in cases of porphyria or phototoxic contact dermatitis, and eczematous eruptions, Dr. DeLeo noted.
Patients who present without a rash, but report a history of a reaction that they believe is related to sun exposure, fall into two categories: some had a rash that occurred while in the sun and disappeared quickly, and some had one that occurred hours or days after exposure and lasted a few days to weeks, said Dr. DeLeo.
The differential diagnosis in the patient with immediate photosensitivity is fairly clear: These patients usually have solar urticaria, he said. However, some lupus patients may report this reaction so it is important to rule out connective tissue disease. The diagnosis can be made with phototesting or do a simple test by having the patient sit out in the sunshine, he said.
For the patient who has a delayed reactivity after sun exposure, and doesn’t have the reaction when they come to the office, the differential diagnosis in a simply applied way is that, if the reaction spared the face, it is likely polymorphous light eruption (PMLE); but if the face is involved, the patient likely has photoallergic contact dermatitis, Dr. DeLeo explained. However, always consider the alternatives of connective tissue disease, drug reactions, and contact dermatitis that is not photoallergic, he noted.
PMLE “is the most common photosensitivity reaction that we see in the United States,” and it almost always occurs when people are away from home, usually on vacation, said Dr. DeLeo. The differential diagnosis for patients with recurrent or delayed rash involving the face could be photoallergic contact dermatitis, but rule out airborne contact dermatitis, personal care product contact dermatitis, and chronic actinic dermatitis, he said. A work-up for these patients could include a photo test, photopatch test, or patch test.
Dr. DeLeo disclosed serving as a consultant for Estee Lauder.
MedscapeLive and this news organization are owned by the same parent company.
of the University of Southern California, Los Angeles.
“When a patient comes in who makes you suspect a photosensitivity, there will be two different presentations,” he said in a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
In some cases, the patient presents with a reaction they believe is sun related, although they don’t have a rash currently, he said. In other cases, “you as a good clinician suspect photosensitivity because the eruption is in a photo distribution,” although the patient may or may not relate it to sun exposure, he added.
Dr. DeLeo noted a few key points to include when taking the history in patients with likely photosensitivity, whether or not they present with a rash.
“I always ask patients when did the episode occur? Is it chronic?” Also ask about timing: Does the reaction occur in the sun, or later? Does it occur quickly and go away within hours, or occur within days or weeks of exposure?
“Always take a good drug history, as photosensitivity can often be related to drugs,” Dr. DeLeo noted. For example, approximately 50% of individuals on amiodarone will have some type of photosensitivity, he said.
Other drug-induced photosensitive conditions include drug-induced subacute cutaneous lupus and pseudoporphyria from NSAIDs, as well as hyperpigmentation from diltiazem, which most often occurs in Black women, he said.
“Photodrug reactions are usually related to UVA radiation, and that is important because you can develop it through the window while driving in your car”: The car windows do not protect against UVA, Dr. DeLeo said. If you have a patient who tells you about a photosensitivity or has a rash and they are on a photosensitizing drug, first rule out connective tissue disease, then discontinue the drug in collaboration with the patient’s internist and wait for the reaction to disappear, and it should, he said.
Some photosensitivity rashes have characteristic patterns, notably connective tissue disease patterns in lupus and dermatomyositis patients, bullous eruptions in cases of porphyria or phototoxic contact dermatitis, and eczematous eruptions, Dr. DeLeo noted.
Patients who present without a rash, but report a history of a reaction that they believe is related to sun exposure, fall into two categories: some had a rash that occurred while in the sun and disappeared quickly, and some had one that occurred hours or days after exposure and lasted a few days to weeks, said Dr. DeLeo.
The differential diagnosis in the patient with immediate photosensitivity is fairly clear: These patients usually have solar urticaria, he said. However, some lupus patients may report this reaction so it is important to rule out connective tissue disease. The diagnosis can be made with phototesting or do a simple test by having the patient sit out in the sunshine, he said.
For the patient who has a delayed reactivity after sun exposure, and doesn’t have the reaction when they come to the office, the differential diagnosis in a simply applied way is that, if the reaction spared the face, it is likely polymorphous light eruption (PMLE); but if the face is involved, the patient likely has photoallergic contact dermatitis, Dr. DeLeo explained. However, always consider the alternatives of connective tissue disease, drug reactions, and contact dermatitis that is not photoallergic, he noted.
PMLE “is the most common photosensitivity reaction that we see in the United States,” and it almost always occurs when people are away from home, usually on vacation, said Dr. DeLeo. The differential diagnosis for patients with recurrent or delayed rash involving the face could be photoallergic contact dermatitis, but rule out airborne contact dermatitis, personal care product contact dermatitis, and chronic actinic dermatitis, he said. A work-up for these patients could include a photo test, photopatch test, or patch test.
Dr. DeLeo disclosed serving as a consultant for Estee Lauder.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Sunscreen myths, controversies continue
, according to Steven Q. Wang, MD, director of dermatologic surgery and dermatology, Memorial Sloan-Kettering Cancer Center, Basking Ridge, N.J.
Although sunscreens are regulated as an OTC drug under the Food and Drug Administration, concerns persist about the safety of sunscreen active ingredients, including avobenzone, oxybenzone, and octocrylene, Dr. Wang said in a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
In 2019, the FDA proposed a rule that requested additional information on sunscreen ingredients. In response, researchers examined six active ingredients used in sunscreen products. The preliminary results were published in JAMA Dermatology in 2019, with a follow-up study published in 2020 . The studies examined the effect of sunscreen application on plasma concentration as a sign of absorption of sunscreen active ingredients.
High absorption
Overall, the maximum level of blood concentration went above the 0.5 ng/mL threshold for waiving nonclinical toxicology studies for all six ingredients. However, the studies had several key limitations, Dr. Wang pointed out. “The maximum usage condition applied in these studies was unrealistic,” he said. “Most people when they use a sunscreen don’t reapply and don’t use enough,” he said.
Also, just because an ingredient is absorbed into the bloodstream does not mean it is toxic or harmful to humans, he said. Sunscreens have been used for 5 or 6 decades with almost zero reports of systemic toxicity, he observed.
The conclusions from the studies were that the FDA wanted additional research, but “they do not indicate that individuals should refrain from using sunscreen as a way to protect themselves from skin cancer,” Dr. Wang emphasized.
Congress passed the CARES Act in March 2020 to provide financial relief for individuals affected by the novel coronavirus, COVID-19. “Within that act, there is a provision to reform modernized U.S. regulatory framework on OTC drug reviews,” which will add confusion to the development of a comprehensive monograph about sunscreen because the regulatory process will change, he said.
In the meantime, confusion will likely increase among patients, who may, among other strategies, attempt to make their own sunscreen products at home, as evidenced by videos of individuals making their own products that have had thousands of views, said Dr. Wang. However, these products have no UV protection, he said.
For current sunscreen products, manufacturers are likely to focus on titanium dioxide and zinc oxide products, which fall into the GRASE I category for active ingredients recognized as safe and effective. More research is needed on homosalate, avobenzone, octisalate, and octocrylene, which are currently in the GRASE III category, meaning the data are insufficient to make statements about safety, he said.
Vitamin D concerns
Another sunscreen concern is that use will block healthy vitamin D production, Dr. Wang said. Vitamin D enters the body in two ways, either through food or through the skin, and the latter requires UVB exposure, he explained. “If you started using a sunscreen with SPF 15 that blocks 93% of UVB, you can essentially shut down vitamin D production in the skin,” but that is in the laboratory setting, he said. What happens in reality is different, as people use much less than in a lab setting, and many people put on a small amount of sunscreen and then spend more time in the sun, thereby increasing exposure, Dr. Wang noted.
For example, a study published in 1988 showed that long-term sunscreen users had levels of vitamin D that were less than 50% of those seen in non–sunscreen users. However, another study published in 1995 showed that serum vitamin D levels were not significantly different between users of an SPF 17 sunscreen and a placebo over a 7-month period.
Is a higher SPF better?
Many patients believe that the difference between a sunscreen with an SPF of 30 and 60 is negligible. “People generally say that SPF 30 blocks 96.7% of UVB and SPF 60 blocks 98.3%, but that’s the wrong way of looking at it,” said Dr. Wang. Instead, consider “how much of the UV ray is able to pass through the sunscreen and reach your skin and do damage,” he said. If a product with SPF 30 allows a transmission of 3.3% and a product with SPF 60 allows a transmission of 1.7%, “the SPF 60 product has 194% better protection in preventing the UV reaching the skin,” he said.
Over a lifetime, individuals will build up more UV damage with consistent use of SPF 30, compared with SPF 60 products, so this myth is important to dispel, Dr. Wang emphasized. “It is the transmission we should focus on, not the blockage,” he said.
Also, consider that the inactive ingredients matter in sunscreens, such as water resistance and film-forming technology that helps promote full coverage, Dr. Wang said, but don’t discount features such as texture, aesthetics, smell, and color, all of which impact compliance.
“Sunscreen is very personal, and people do not want to use a product just because of the SPF value, they want to use a product based on how it makes them feel,” he said.
At the end of the day, “the best sunscreen is the one a patient will use regularly and actually enjoy using,” Dr. Wang concluded.
Dr. Wang had no relevant financial conflicts to disclose.
MedscapeLive and this news organization are owned by the same parent company.
, according to Steven Q. Wang, MD, director of dermatologic surgery and dermatology, Memorial Sloan-Kettering Cancer Center, Basking Ridge, N.J.
Although sunscreens are regulated as an OTC drug under the Food and Drug Administration, concerns persist about the safety of sunscreen active ingredients, including avobenzone, oxybenzone, and octocrylene, Dr. Wang said in a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
In 2019, the FDA proposed a rule that requested additional information on sunscreen ingredients. In response, researchers examined six active ingredients used in sunscreen products. The preliminary results were published in JAMA Dermatology in 2019, with a follow-up study published in 2020 . The studies examined the effect of sunscreen application on plasma concentration as a sign of absorption of sunscreen active ingredients.
High absorption
Overall, the maximum level of blood concentration went above the 0.5 ng/mL threshold for waiving nonclinical toxicology studies for all six ingredients. However, the studies had several key limitations, Dr. Wang pointed out. “The maximum usage condition applied in these studies was unrealistic,” he said. “Most people when they use a sunscreen don’t reapply and don’t use enough,” he said.
Also, just because an ingredient is absorbed into the bloodstream does not mean it is toxic or harmful to humans, he said. Sunscreens have been used for 5 or 6 decades with almost zero reports of systemic toxicity, he observed.
The conclusions from the studies were that the FDA wanted additional research, but “they do not indicate that individuals should refrain from using sunscreen as a way to protect themselves from skin cancer,” Dr. Wang emphasized.
Congress passed the CARES Act in March 2020 to provide financial relief for individuals affected by the novel coronavirus, COVID-19. “Within that act, there is a provision to reform modernized U.S. regulatory framework on OTC drug reviews,” which will add confusion to the development of a comprehensive monograph about sunscreen because the regulatory process will change, he said.
In the meantime, confusion will likely increase among patients, who may, among other strategies, attempt to make their own sunscreen products at home, as evidenced by videos of individuals making their own products that have had thousands of views, said Dr. Wang. However, these products have no UV protection, he said.
For current sunscreen products, manufacturers are likely to focus on titanium dioxide and zinc oxide products, which fall into the GRASE I category for active ingredients recognized as safe and effective. More research is needed on homosalate, avobenzone, octisalate, and octocrylene, which are currently in the GRASE III category, meaning the data are insufficient to make statements about safety, he said.
Vitamin D concerns
Another sunscreen concern is that use will block healthy vitamin D production, Dr. Wang said. Vitamin D enters the body in two ways, either through food or through the skin, and the latter requires UVB exposure, he explained. “If you started using a sunscreen with SPF 15 that blocks 93% of UVB, you can essentially shut down vitamin D production in the skin,” but that is in the laboratory setting, he said. What happens in reality is different, as people use much less than in a lab setting, and many people put on a small amount of sunscreen and then spend more time in the sun, thereby increasing exposure, Dr. Wang noted.
For example, a study published in 1988 showed that long-term sunscreen users had levels of vitamin D that were less than 50% of those seen in non–sunscreen users. However, another study published in 1995 showed that serum vitamin D levels were not significantly different between users of an SPF 17 sunscreen and a placebo over a 7-month period.
Is a higher SPF better?
Many patients believe that the difference between a sunscreen with an SPF of 30 and 60 is negligible. “People generally say that SPF 30 blocks 96.7% of UVB and SPF 60 blocks 98.3%, but that’s the wrong way of looking at it,” said Dr. Wang. Instead, consider “how much of the UV ray is able to pass through the sunscreen and reach your skin and do damage,” he said. If a product with SPF 30 allows a transmission of 3.3% and a product with SPF 60 allows a transmission of 1.7%, “the SPF 60 product has 194% better protection in preventing the UV reaching the skin,” he said.
Over a lifetime, individuals will build up more UV damage with consistent use of SPF 30, compared with SPF 60 products, so this myth is important to dispel, Dr. Wang emphasized. “It is the transmission we should focus on, not the blockage,” he said.
Also, consider that the inactive ingredients matter in sunscreens, such as water resistance and film-forming technology that helps promote full coverage, Dr. Wang said, but don’t discount features such as texture, aesthetics, smell, and color, all of which impact compliance.
“Sunscreen is very personal, and people do not want to use a product just because of the SPF value, they want to use a product based on how it makes them feel,” he said.
At the end of the day, “the best sunscreen is the one a patient will use regularly and actually enjoy using,” Dr. Wang concluded.
Dr. Wang had no relevant financial conflicts to disclose.
MedscapeLive and this news organization are owned by the same parent company.
, according to Steven Q. Wang, MD, director of dermatologic surgery and dermatology, Memorial Sloan-Kettering Cancer Center, Basking Ridge, N.J.
Although sunscreens are regulated as an OTC drug under the Food and Drug Administration, concerns persist about the safety of sunscreen active ingredients, including avobenzone, oxybenzone, and octocrylene, Dr. Wang said in a virtual presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
In 2019, the FDA proposed a rule that requested additional information on sunscreen ingredients. In response, researchers examined six active ingredients used in sunscreen products. The preliminary results were published in JAMA Dermatology in 2019, with a follow-up study published in 2020 . The studies examined the effect of sunscreen application on plasma concentration as a sign of absorption of sunscreen active ingredients.
High absorption
Overall, the maximum level of blood concentration went above the 0.5 ng/mL threshold for waiving nonclinical toxicology studies for all six ingredients. However, the studies had several key limitations, Dr. Wang pointed out. “The maximum usage condition applied in these studies was unrealistic,” he said. “Most people when they use a sunscreen don’t reapply and don’t use enough,” he said.
Also, just because an ingredient is absorbed into the bloodstream does not mean it is toxic or harmful to humans, he said. Sunscreens have been used for 5 or 6 decades with almost zero reports of systemic toxicity, he observed.
The conclusions from the studies were that the FDA wanted additional research, but “they do not indicate that individuals should refrain from using sunscreen as a way to protect themselves from skin cancer,” Dr. Wang emphasized.
Congress passed the CARES Act in March 2020 to provide financial relief for individuals affected by the novel coronavirus, COVID-19. “Within that act, there is a provision to reform modernized U.S. regulatory framework on OTC drug reviews,” which will add confusion to the development of a comprehensive monograph about sunscreen because the regulatory process will change, he said.
In the meantime, confusion will likely increase among patients, who may, among other strategies, attempt to make their own sunscreen products at home, as evidenced by videos of individuals making their own products that have had thousands of views, said Dr. Wang. However, these products have no UV protection, he said.
For current sunscreen products, manufacturers are likely to focus on titanium dioxide and zinc oxide products, which fall into the GRASE I category for active ingredients recognized as safe and effective. More research is needed on homosalate, avobenzone, octisalate, and octocrylene, which are currently in the GRASE III category, meaning the data are insufficient to make statements about safety, he said.
Vitamin D concerns
Another sunscreen concern is that use will block healthy vitamin D production, Dr. Wang said. Vitamin D enters the body in two ways, either through food or through the skin, and the latter requires UVB exposure, he explained. “If you started using a sunscreen with SPF 15 that blocks 93% of UVB, you can essentially shut down vitamin D production in the skin,” but that is in the laboratory setting, he said. What happens in reality is different, as people use much less than in a lab setting, and many people put on a small amount of sunscreen and then spend more time in the sun, thereby increasing exposure, Dr. Wang noted.
For example, a study published in 1988 showed that long-term sunscreen users had levels of vitamin D that were less than 50% of those seen in non–sunscreen users. However, another study published in 1995 showed that serum vitamin D levels were not significantly different between users of an SPF 17 sunscreen and a placebo over a 7-month period.
Is a higher SPF better?
Many patients believe that the difference between a sunscreen with an SPF of 30 and 60 is negligible. “People generally say that SPF 30 blocks 96.7% of UVB and SPF 60 blocks 98.3%, but that’s the wrong way of looking at it,” said Dr. Wang. Instead, consider “how much of the UV ray is able to pass through the sunscreen and reach your skin and do damage,” he said. If a product with SPF 30 allows a transmission of 3.3% and a product with SPF 60 allows a transmission of 1.7%, “the SPF 60 product has 194% better protection in preventing the UV reaching the skin,” he said.
Over a lifetime, individuals will build up more UV damage with consistent use of SPF 30, compared with SPF 60 products, so this myth is important to dispel, Dr. Wang emphasized. “It is the transmission we should focus on, not the blockage,” he said.
Also, consider that the inactive ingredients matter in sunscreens, such as water resistance and film-forming technology that helps promote full coverage, Dr. Wang said, but don’t discount features such as texture, aesthetics, smell, and color, all of which impact compliance.
“Sunscreen is very personal, and people do not want to use a product just because of the SPF value, they want to use a product based on how it makes them feel,” he said.
At the end of the day, “the best sunscreen is the one a patient will use regularly and actually enjoy using,” Dr. Wang concluded.
Dr. Wang had no relevant financial conflicts to disclose.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Recurrent Cutaneous Exophiala Phaeohyphomycosis in an Immunosuppressed Patient
To the Editor:
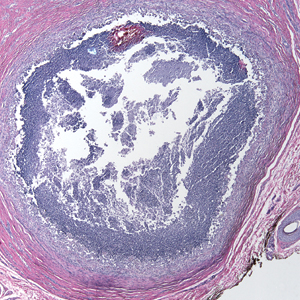
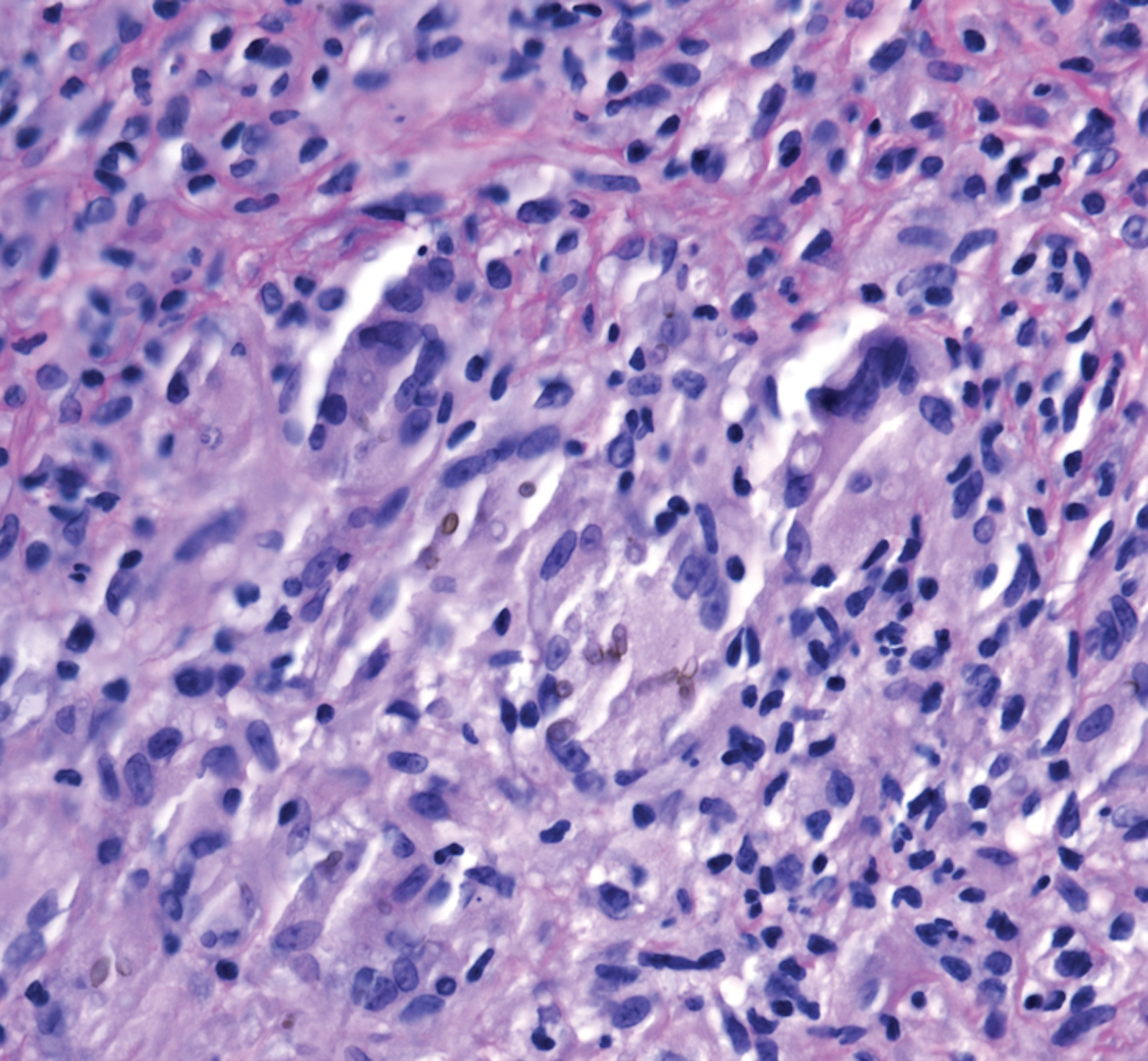
A 73-year-old man presented with a 2.5-cm, recurrent, fluctuant, multiloculated nodule on the left forearm. The lesion was nontender with occasional chalky, white to yellow discharge from multiple sinus tracts. He was otherwise well appearing without signs of systemic infection. He reported similar lesions in slightly different anatomic locations on the left forearm both 7 and 4 years prior to the current presentation. In both instances, the nodules were excised at an outside hospital without any additional treatment. Histopathology of the excised tissue from both prior occasions demonstrated brown septate hyphae surrounded by suppurative and granulomatous inflammation consistent with dematiaceous fungal infection of the dermis (Figures 1 and 2); the organisms were highlighted with periodic acid–Schiff stain.

The patient’s medical history was notable for advanced heart failure with an ejection fraction of 25% and autosomal-dominant polycystic kidney disease. He received an orthotopic kidney transplant 17 years prior to the current presentation. Medications included tacrolimus, mycophenolate mofetil, and prednisone. He denied any trauma or notable exposures to vegetation, and his travel history was unremarkable. A review of systems was negative.
At the current presentation, a sterile fungal culture was performed and found positive for Exophiala species, while bacterial and mycobacterial cultures were negative. A diagnosis of phaeohyphomycosis was made, and he was scheduled for re-excision. Out of concern for interactions with his immunosuppressive regimen, he chose to forgo any systemic antifungal therapy. He died from hospital-acquired pneumonia and volume overload unresponsive to diuretics or dialysis.
Phaeohyphomycosis is a rare fungal infection caused by several genera of dematiaceous fungi that are characterized by the presence of melaninlike cell wall pigments thought to locally hinder immune clearance by scavenging phagocyte-derived free radicals. These fungi are ubiquitous in soil and vegetation and usually penetrate the skin at sites of minor trauma.1 Phaeohyphomycosis typically affects immunosuppressed hosts, and its incidence among organ transplant recipients currently is 9%.2 The incidence in this population has been rising, however, as recent advances in immunosuppressive therapies have increased posttransplant survival.3
Subcutaneous phaeohyphomycosis can present with nodules, cysts, tumors, and/or verrucous plaques, and the diagnosis almost always requires clinicopathologic correlation.3 Rapid diagnosis can be made when septate brown hyphae and/or yeast forms are observed on hematoxylin and eosin stain. Rarely, patients present with disseminated infection, characterized by fungemia; central nervous system involvement; and/or infection of multiple deep structures including the eyes, lungs, bones, and sinuses.4 The risk for dissemination from the skin likely is related to the culprit organism’s genus; Lomentospora, Cladophialophora, and Verruconis often are associated with dissemination, while Alternaria, Exophiala, and Fonsecaea typically remain confined to the skin and subcutis.5 Due to this difference and its potential to impact management, obtaining a tissue fungal culture is advisable when phaeohyphomycosis is suspected.
There is no standard treatment of phaeohyphomycosis. Regimens typically consist of excision and prolonged courses of azole therapy, though excision alone with close follow-up may be a reasonable alternative.6 The latter is a particularly important consideration when managing phaeohyphomycosis in organ transplant recipients, as azoles are known cytochrome P450 3A4 inhibitors that can affect serum levels of common immunosuppressive medications including calcineurin inhibitors and mammalian target of rapamycin inhibitors.3 Local recurrence is common regardless of whether azole therapy is pursued,7 and dissemination of localized Exophiala infections is exceedingly rare.8 There is a strong argument to be made for our patient’s decision to forgo antifungal therapy.
This case underscores the difficulty inherent to eradicating local subcutaneous Exophiala phaeohyphomycosis while providing reassurance that with treatment, the risk of life-threatening complications is low. Obtaining tissue for both hematoxylin and eosin stain and sterile culture is crucial to ensuring prompt diagnosis and tailoring the optimal treatment and surveillance strategy to the culprit organism. To avoid delays in diagnosis and treatment, it is important for clinicians to consider phaeohyphomycosis in the differential diagnosis for recurrent nodulocystic lesions in immunosuppressed patients and to recognize that presentations may span many years.
- Bhardwaj S, Capoor MR, Kolte S, et al. Phaeohyphomycosis due to Exophiala jeanselmei: an emerging pathogen in India—case report and review. Mycopathologia. 2016;181:279-284.
- Isa-Isa R, Garcia C, Isa M, et al. Subcutaneous phaeohyphomycosis (mycotic cyst). Clin Dermatol. 2012;30:425-431.
- Tirico MCCP, Neto CF, Cruz LL, et al. Clinical spectrum of phaeohyphomycosis in solid organ transplant recipients. JAAD Case Rep. 2016;2:465-469.
- Revankar SG, Patterson JE, Sutton DA, et al. Disseminated phaeohyphomycosis: review of an emerging mycosis. Clin Infect Dis. 2002;34:467-476.
- Revankar SG, Baddley JW, Chen SC-A, et al. A mycoses study group international prospective study of phaeohyphomycosis: an analysis of 99 proven/probable cases. Open Forum Infect Dis. 2017;4:ofx200.
- Oberlin KE, Nichols AJ, Rosa R, et al. Phaeohyphomycosis due to Exophiala infections in solid organ transplant recipients: case report and literature review [published online June 26, 2017]. Transpl Infect Dis. 2017;19. doi:10.1111/tid.12723.
- Shirbur S, Telkar S, Goudar B, et al. Recurrent phaeohyphomycosis: a case report. J Clin Diagn Res. 2013;7:2015-2016.
- Li D-M, Li R-Y, de Hoog GS, et al. Fatal Exophiala infections in China, with a report of seven cases. Mycoses. 2011;54:E136-E142.
To the Editor:
A 73-year-old man presented with a 2.5-cm, recurrent, fluctuant, multiloculated nodule on the left forearm. The lesion was nontender with occasional chalky, white to yellow discharge from multiple sinus tracts. He was otherwise well appearing without signs of systemic infection. He reported similar lesions in slightly different anatomic locations on the left forearm both 7 and 4 years prior to the current presentation. In both instances, the nodules were excised at an outside hospital without any additional treatment. Histopathology of the excised tissue from both prior occasions demonstrated brown septate hyphae surrounded by suppurative and granulomatous inflammation consistent with dematiaceous fungal infection of the dermis (Figures 1 and 2); the organisms were highlighted with periodic acid–Schiff stain.


The patient’s medical history was notable for advanced heart failure with an ejection fraction of 25% and autosomal-dominant polycystic kidney disease. He received an orthotopic kidney transplant 17 years prior to the current presentation. Medications included tacrolimus, mycophenolate mofetil, and prednisone. He denied any trauma or notable exposures to vegetation, and his travel history was unremarkable. A review of systems was negative.
At the current presentation, a sterile fungal culture was performed and found positive for Exophiala species, while bacterial and mycobacterial cultures were negative. A diagnosis of phaeohyphomycosis was made, and he was scheduled for re-excision. Out of concern for interactions with his immunosuppressive regimen, he chose to forgo any systemic antifungal therapy. He died from hospital-acquired pneumonia and volume overload unresponsive to diuretics or dialysis.
Phaeohyphomycosis is a rare fungal infection caused by several genera of dematiaceous fungi that are characterized by the presence of melaninlike cell wall pigments thought to locally hinder immune clearance by scavenging phagocyte-derived free radicals. These fungi are ubiquitous in soil and vegetation and usually penetrate the skin at sites of minor trauma.1 Phaeohyphomycosis typically affects immunosuppressed hosts, and its incidence among organ transplant recipients currently is 9%.2 The incidence in this population has been rising, however, as recent advances in immunosuppressive therapies have increased posttransplant survival.3
Subcutaneous phaeohyphomycosis can present with nodules, cysts, tumors, and/or verrucous plaques, and the diagnosis almost always requires clinicopathologic correlation.3 Rapid diagnosis can be made when septate brown hyphae and/or yeast forms are observed on hematoxylin and eosin stain. Rarely, patients present with disseminated infection, characterized by fungemia; central nervous system involvement; and/or infection of multiple deep structures including the eyes, lungs, bones, and sinuses.4 The risk for dissemination from the skin likely is related to the culprit organism’s genus; Lomentospora, Cladophialophora, and Verruconis often are associated with dissemination, while Alternaria, Exophiala, and Fonsecaea typically remain confined to the skin and subcutis.5 Due to this difference and its potential to impact management, obtaining a tissue fungal culture is advisable when phaeohyphomycosis is suspected.
There is no standard treatment of phaeohyphomycosis. Regimens typically consist of excision and prolonged courses of azole therapy, though excision alone with close follow-up may be a reasonable alternative.6 The latter is a particularly important consideration when managing phaeohyphomycosis in organ transplant recipients, as azoles are known cytochrome P450 3A4 inhibitors that can affect serum levels of common immunosuppressive medications including calcineurin inhibitors and mammalian target of rapamycin inhibitors.3 Local recurrence is common regardless of whether azole therapy is pursued,7 and dissemination of localized Exophiala infections is exceedingly rare.8 There is a strong argument to be made for our patient’s decision to forgo antifungal therapy.
This case underscores the difficulty inherent to eradicating local subcutaneous Exophiala phaeohyphomycosis while providing reassurance that with treatment, the risk of life-threatening complications is low. Obtaining tissue for both hematoxylin and eosin stain and sterile culture is crucial to ensuring prompt diagnosis and tailoring the optimal treatment and surveillance strategy to the culprit organism. To avoid delays in diagnosis and treatment, it is important for clinicians to consider phaeohyphomycosis in the differential diagnosis for recurrent nodulocystic lesions in immunosuppressed patients and to recognize that presentations may span many years.
To the Editor:
A 73-year-old man presented with a 2.5-cm, recurrent, fluctuant, multiloculated nodule on the left forearm. The lesion was nontender with occasional chalky, white to yellow discharge from multiple sinus tracts. He was otherwise well appearing without signs of systemic infection. He reported similar lesions in slightly different anatomic locations on the left forearm both 7 and 4 years prior to the current presentation. In both instances, the nodules were excised at an outside hospital without any additional treatment. Histopathology of the excised tissue from both prior occasions demonstrated brown septate hyphae surrounded by suppurative and granulomatous inflammation consistent with dematiaceous fungal infection of the dermis (Figures 1 and 2); the organisms were highlighted with periodic acid–Schiff stain.


The patient’s medical history was notable for advanced heart failure with an ejection fraction of 25% and autosomal-dominant polycystic kidney disease. He received an orthotopic kidney transplant 17 years prior to the current presentation. Medications included tacrolimus, mycophenolate mofetil, and prednisone. He denied any trauma or notable exposures to vegetation, and his travel history was unremarkable. A review of systems was negative.
At the current presentation, a sterile fungal culture was performed and found positive for Exophiala species, while bacterial and mycobacterial cultures were negative. A diagnosis of phaeohyphomycosis was made, and he was scheduled for re-excision. Out of concern for interactions with his immunosuppressive regimen, he chose to forgo any systemic antifungal therapy. He died from hospital-acquired pneumonia and volume overload unresponsive to diuretics or dialysis.
Phaeohyphomycosis is a rare fungal infection caused by several genera of dematiaceous fungi that are characterized by the presence of melaninlike cell wall pigments thought to locally hinder immune clearance by scavenging phagocyte-derived free radicals. These fungi are ubiquitous in soil and vegetation and usually penetrate the skin at sites of minor trauma.1 Phaeohyphomycosis typically affects immunosuppressed hosts, and its incidence among organ transplant recipients currently is 9%.2 The incidence in this population has been rising, however, as recent advances in immunosuppressive therapies have increased posttransplant survival.3
Subcutaneous phaeohyphomycosis can present with nodules, cysts, tumors, and/or verrucous plaques, and the diagnosis almost always requires clinicopathologic correlation.3 Rapid diagnosis can be made when septate brown hyphae and/or yeast forms are observed on hematoxylin and eosin stain. Rarely, patients present with disseminated infection, characterized by fungemia; central nervous system involvement; and/or infection of multiple deep structures including the eyes, lungs, bones, and sinuses.4 The risk for dissemination from the skin likely is related to the culprit organism’s genus; Lomentospora, Cladophialophora, and Verruconis often are associated with dissemination, while Alternaria, Exophiala, and Fonsecaea typically remain confined to the skin and subcutis.5 Due to this difference and its potential to impact management, obtaining a tissue fungal culture is advisable when phaeohyphomycosis is suspected.
There is no standard treatment of phaeohyphomycosis. Regimens typically consist of excision and prolonged courses of azole therapy, though excision alone with close follow-up may be a reasonable alternative.6 The latter is a particularly important consideration when managing phaeohyphomycosis in organ transplant recipients, as azoles are known cytochrome P450 3A4 inhibitors that can affect serum levels of common immunosuppressive medications including calcineurin inhibitors and mammalian target of rapamycin inhibitors.3 Local recurrence is common regardless of whether azole therapy is pursued,7 and dissemination of localized Exophiala infections is exceedingly rare.8 There is a strong argument to be made for our patient’s decision to forgo antifungal therapy.
This case underscores the difficulty inherent to eradicating local subcutaneous Exophiala phaeohyphomycosis while providing reassurance that with treatment, the risk of life-threatening complications is low. Obtaining tissue for both hematoxylin and eosin stain and sterile culture is crucial to ensuring prompt diagnosis and tailoring the optimal treatment and surveillance strategy to the culprit organism. To avoid delays in diagnosis and treatment, it is important for clinicians to consider phaeohyphomycosis in the differential diagnosis for recurrent nodulocystic lesions in immunosuppressed patients and to recognize that presentations may span many years.
- Bhardwaj S, Capoor MR, Kolte S, et al. Phaeohyphomycosis due to Exophiala jeanselmei: an emerging pathogen in India—case report and review. Mycopathologia. 2016;181:279-284.
- Isa-Isa R, Garcia C, Isa M, et al. Subcutaneous phaeohyphomycosis (mycotic cyst). Clin Dermatol. 2012;30:425-431.
- Tirico MCCP, Neto CF, Cruz LL, et al. Clinical spectrum of phaeohyphomycosis in solid organ transplant recipients. JAAD Case Rep. 2016;2:465-469.
- Revankar SG, Patterson JE, Sutton DA, et al. Disseminated phaeohyphomycosis: review of an emerging mycosis. Clin Infect Dis. 2002;34:467-476.
- Revankar SG, Baddley JW, Chen SC-A, et al. A mycoses study group international prospective study of phaeohyphomycosis: an analysis of 99 proven/probable cases. Open Forum Infect Dis. 2017;4:ofx200.
- Oberlin KE, Nichols AJ, Rosa R, et al. Phaeohyphomycosis due to Exophiala infections in solid organ transplant recipients: case report and literature review [published online June 26, 2017]. Transpl Infect Dis. 2017;19. doi:10.1111/tid.12723.
- Shirbur S, Telkar S, Goudar B, et al. Recurrent phaeohyphomycosis: a case report. J Clin Diagn Res. 2013;7:2015-2016.
- Li D-M, Li R-Y, de Hoog GS, et al. Fatal Exophiala infections in China, with a report of seven cases. Mycoses. 2011;54:E136-E142.
- Bhardwaj S, Capoor MR, Kolte S, et al. Phaeohyphomycosis due to Exophiala jeanselmei: an emerging pathogen in India—case report and review. Mycopathologia. 2016;181:279-284.
- Isa-Isa R, Garcia C, Isa M, et al. Subcutaneous phaeohyphomycosis (mycotic cyst). Clin Dermatol. 2012;30:425-431.
- Tirico MCCP, Neto CF, Cruz LL, et al. Clinical spectrum of phaeohyphomycosis in solid organ transplant recipients. JAAD Case Rep. 2016;2:465-469.
- Revankar SG, Patterson JE, Sutton DA, et al. Disseminated phaeohyphomycosis: review of an emerging mycosis. Clin Infect Dis. 2002;34:467-476.
- Revankar SG, Baddley JW, Chen SC-A, et al. A mycoses study group international prospective study of phaeohyphomycosis: an analysis of 99 proven/probable cases. Open Forum Infect Dis. 2017;4:ofx200.
- Oberlin KE, Nichols AJ, Rosa R, et al. Phaeohyphomycosis due to Exophiala infections in solid organ transplant recipients: case report and literature review [published online June 26, 2017]. Transpl Infect Dis. 2017;19. doi:10.1111/tid.12723.
- Shirbur S, Telkar S, Goudar B, et al. Recurrent phaeohyphomycosis: a case report. J Clin Diagn Res. 2013;7:2015-2016.
- Li D-M, Li R-Y, de Hoog GS, et al. Fatal Exophiala infections in China, with a report of seven cases. Mycoses. 2011;54:E136-E142.
Practice Points
- Phaeohyphomycosis is an infection with dematiaceous fungi that most commonly affects immunosuppressed patients.
- Subcutaneous phaeohyphomycosis may present with nodulocystic lesions that recur over the course of years.
- Tissue fungal culture should be obtained when the diagnosis is suspected, as the risk for dissemination is related to the culprit organism.
- Surgical excision with close follow-up may be an appropriate management strategy for patients on immunosuppressive medications to avoid interactions with azole therapy.
Scalp Arteriovenous Fistula With Intracranial Communication
To the Editor:
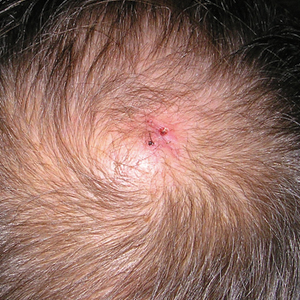
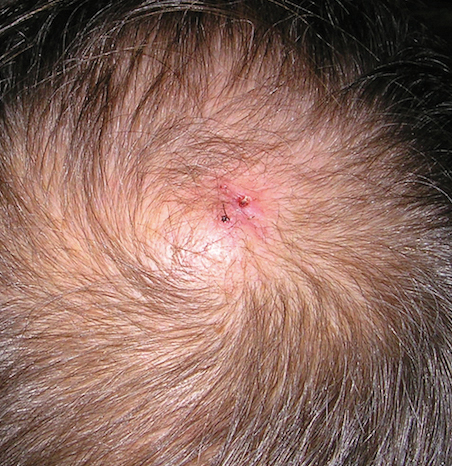
A 71-year-old man presented with a nodule on the vertex of the scalp of 1 year’s duration. The lesion had become soft and tender during the week prior to presentation. He noted that he was experiencing headaches and a buzzing sound in his head. He denied all other neurologic symptoms. The patient was given amoxicillin from a primary care physician and was referred to our institution for evaluation of a presumed inflamed cyst.
The patient’s medical history included an intracranial arteriovenous fistula (AVF) treated with endovascular embolization 1 year prior to presentation, 2 substantial falls in childhood with head trauma and loss of consciousness, essential hypertension, and an aortic aneurysm. His medications included amlodipine, lisinopril, amoxicillin, a multivitamin, and grape seed extract.
Physical examination revealed a 2-cm, pink, somewhat rubbery, subcutaneous, nonmobile nodule on the vertex of the scalp (Figure 1). The lesion was not consistent with a common pilar cyst, and an excisional biopsy was performed to exclude malignancy. Upon superficial incision, the lesion bled moderately, and the procedure was immediately discontinued. Hemostasis was obtained, and the patient was sent for ultrasonography of the lesion.
Ultrasonography demonstrated a small hypoechoic nodule measuring up to 0.5 cm containing a tangle of vessels in the subcutaneous soft tissue corresponding to the palpable abnormality. A cerebral angiogram demonstrated a dural AVF of the superior sagittal sinus with multifocal supply that connected with this scalp nodule (Figure 2). The patient was treated by interventional neuroradiology with endovascular embolization, which resulted in complete resolution of the scalp nodule.
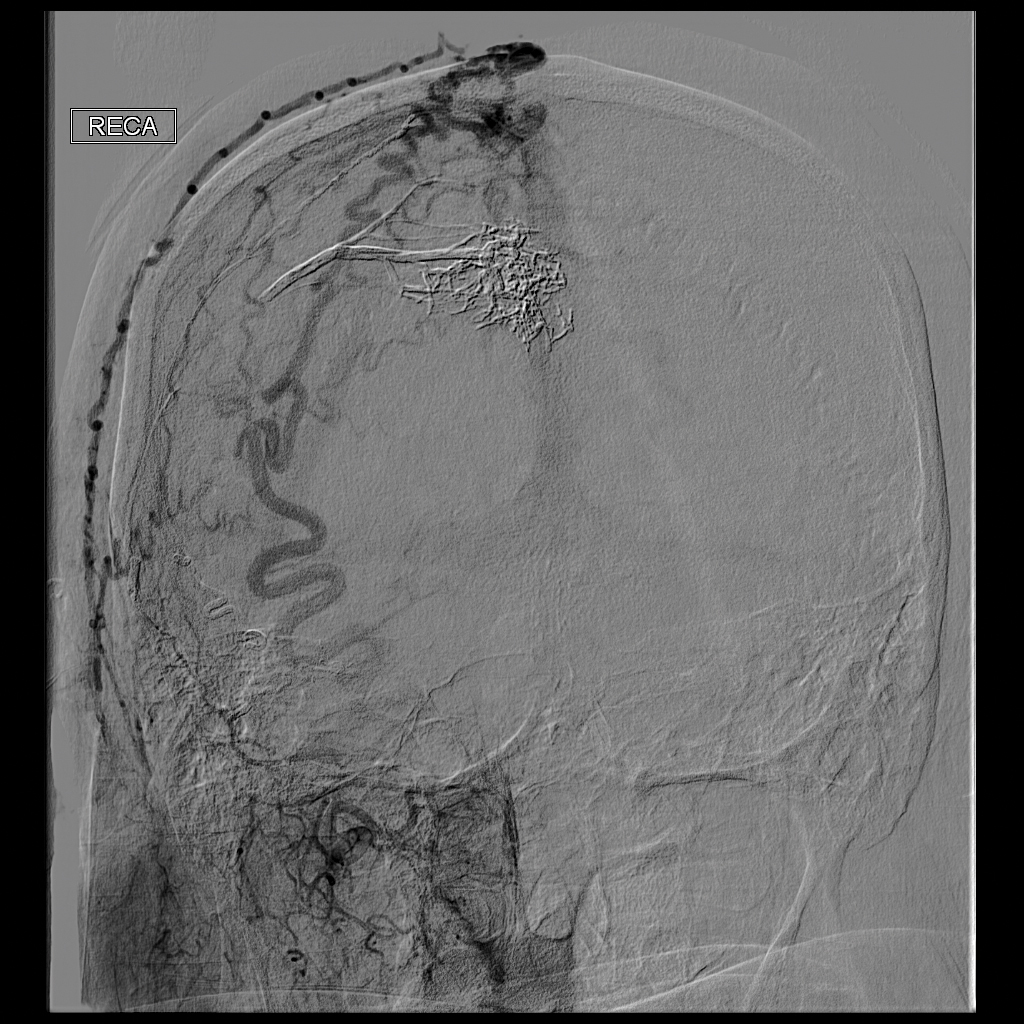
Scalp arteriovenous fistulas (S-AVFs) are characterized by abnormal connections between supplying arteries and draining veins in the subcutaneous plane of the scalp.1,2 The veins of an S-AVF undergo progressive aneurysmal dilatation from abnormal hemodynamics.1-3 Scalp arteriovenous fistulas are rare and may present as either an innocuous-looking scalp nodule or a progressively enlarging pulsatile mass on the scalp.2-4 Associated symptoms often include headache, local pain, bruits, tinnitus, and thrill.1,3,4 Recurrent hemorrhage, scalp necrosis, congestive heart failure, epilepsy, mental retardation, and intracranial ischemia also may occur.4
Scalp AVFs may occur with or without intracranial communication.4 Spontaneous S-AVFs with intracranial communication are uncommon, and their etiology is unclear. They may form as congenital malformations or may be idiopathic. Factors increasing circulation through the S-AVF such as trauma, pregnancy, hormonal changes, and inflammation prompt the development of symptoms.4 Scalp AVFs also may be caused by trauma.3 Scalp AVFs without intracranial communication have been reported following hair transplantation.1 Scalp AVFs with intracranial communication have been reported months to years after skull fracture or craniotomy.2 True spontaneous S-AVFs are difficult to differentiate from traumatic S-AVFs other than by history alone.2
Increased venous pressure has been shown to generate AVFs in rats.5 It has been suggested that S-AVFs can become enlarged by capturing subcutaneous or intracranial feeder vessels and that the consequent hemodynamic stress may induce de novo aneurysms in S-AVFs. Additionally, intracranial AVFs may alter the intracranial hemodynamics, leading to increased venous pressure in the superior sagittal sinus and the formation of communicating S-AVFs.5 Interestingly, our patient had an intracranial AVF treated with endovascular embolization 1 year prior to the formation of the S-AVF. An angiogram at the time of this embolization procedure did not demonstrate any S-AVFs. Furthermore, our patient has a history of 2 substantial falls in childhood with head trauma and loss of consciousness. Perhaps these traumas initiated a channel through the cranium where an S-AVF with intracranial communication was able to form and may have only become clinically or radiographically detectable once it enlarged due to the altered hemodynamics caused by the intracranial AVF 1 year prior.
The diagnosis of an S-AVF is confirmed with imaging studies. Doppler ultrasonography initially will help to detect that a lesion is vascular in nature. Intra-arterial digital subtraction angiography is the gold-standard imaging technique and is necessary to delineate the feeding arteries and the draining channels as well as possible communication with intracranial vasculature.1,2 There is controversy regarding the appropriate treatment of S-AVFs.2 Each S-AVF possesses unique anatomic features that dictate appropriate management. The prognosis for an S-AVF is extremely variable, and the decision to treat is based on the patient’s symptoms and risk for exsanguinating hemorrhage.2,4 Neurosurgical approaches include ligation of the feeding arteries, surgical resection, electrothrombosis, direct intralesional injection of sclerosing agents, and endovascular embolization. Endovascular intervention increasingly is utilized as a primary treatment or as a preoperative adjunct to surgery.2,4 Large S-AVFs have a high risk for recurrence after treatment with endovascular embolization alone. In cases with intracranial communication, the intracranial component is treated first.2
This case emphasizes the importance of including S-AVFs on the dermatologic differential diagnosis of a scalp nodule, especially in patients with any history of intracranial AVF. A thorough history, detailed intake of potential signs and symptoms of AVF, and palpation for bruits is recommended as part of the surgical evaluation of a scalp nodule. Imaging of scalp nodules also should be considered for patients with any history of intracranial AVF; S-AVFs should be referred to neurosurgery or interventional neuroradiology for evaluation and possible treatment.
- Bernstein J, Podnos S, Leavitt M. Arteriovenous fistula following hair transplantation. Dermatol Surg. 2011;37:873-875.
- Kumar R, Sharma G, Sharma BS. Management of scalp arterio-venous malformation: case series and review of literature. Br J Neurosurg. 2012;26:371-377.
- Gurkanlar D, Gonul M, Solmaz I, et al. Cirsoid aneurysms of the scalp. Neurosurg Rev. 2006;29:208-212.
- Senoglu M, Yasim A, Gokce M, et al. Nontraumatic scalp arteriovenous fistula in an adult: technical report on an illustrative case. Surg Neurol. 2008;70:194-197.
- Lanzino G, Passacantilli E, Lemole G, et al. Scalp arteriovenous malformation draining into the superior sagittal sinus associated with an intracranial arteriovenous malformation: just a coincidence? case report. Neurosurgery. 2003;52:440-443.
To the Editor:
A 71-year-old man presented with a nodule on the vertex of the scalp of 1 year’s duration. The lesion had become soft and tender during the week prior to presentation. He noted that he was experiencing headaches and a buzzing sound in his head. He denied all other neurologic symptoms. The patient was given amoxicillin from a primary care physician and was referred to our institution for evaluation of a presumed inflamed cyst.
The patient’s medical history included an intracranial arteriovenous fistula (AVF) treated with endovascular embolization 1 year prior to presentation, 2 substantial falls in childhood with head trauma and loss of consciousness, essential hypertension, and an aortic aneurysm. His medications included amlodipine, lisinopril, amoxicillin, a multivitamin, and grape seed extract.
Physical examination revealed a 2-cm, pink, somewhat rubbery, subcutaneous, nonmobile nodule on the vertex of the scalp (Figure 1). The lesion was not consistent with a common pilar cyst, and an excisional biopsy was performed to exclude malignancy. Upon superficial incision, the lesion bled moderately, and the procedure was immediately discontinued. Hemostasis was obtained, and the patient was sent for ultrasonography of the lesion.

Ultrasonography demonstrated a small hypoechoic nodule measuring up to 0.5 cm containing a tangle of vessels in the subcutaneous soft tissue corresponding to the palpable abnormality. A cerebral angiogram demonstrated a dural AVF of the superior sagittal sinus with multifocal supply that connected with this scalp nodule (Figure 2). The patient was treated by interventional neuroradiology with endovascular embolization, which resulted in complete resolution of the scalp nodule.

Scalp arteriovenous fistulas (S-AVFs) are characterized by abnormal connections between supplying arteries and draining veins in the subcutaneous plane of the scalp.1,2 The veins of an S-AVF undergo progressive aneurysmal dilatation from abnormal hemodynamics.1-3 Scalp arteriovenous fistulas are rare and may present as either an innocuous-looking scalp nodule or a progressively enlarging pulsatile mass on the scalp.2-4 Associated symptoms often include headache, local pain, bruits, tinnitus, and thrill.1,3,4 Recurrent hemorrhage, scalp necrosis, congestive heart failure, epilepsy, mental retardation, and intracranial ischemia also may occur.4
Scalp AVFs may occur with or without intracranial communication.4 Spontaneous S-AVFs with intracranial communication are uncommon, and their etiology is unclear. They may form as congenital malformations or may be idiopathic. Factors increasing circulation through the S-AVF such as trauma, pregnancy, hormonal changes, and inflammation prompt the development of symptoms.4 Scalp AVFs also may be caused by trauma.3 Scalp AVFs without intracranial communication have been reported following hair transplantation.1 Scalp AVFs with intracranial communication have been reported months to years after skull fracture or craniotomy.2 True spontaneous S-AVFs are difficult to differentiate from traumatic S-AVFs other than by history alone.2
Increased venous pressure has been shown to generate AVFs in rats.5 It has been suggested that S-AVFs can become enlarged by capturing subcutaneous or intracranial feeder vessels and that the consequent hemodynamic stress may induce de novo aneurysms in S-AVFs. Additionally, intracranial AVFs may alter the intracranial hemodynamics, leading to increased venous pressure in the superior sagittal sinus and the formation of communicating S-AVFs.5 Interestingly, our patient had an intracranial AVF treated with endovascular embolization 1 year prior to the formation of the S-AVF. An angiogram at the time of this embolization procedure did not demonstrate any S-AVFs. Furthermore, our patient has a history of 2 substantial falls in childhood with head trauma and loss of consciousness. Perhaps these traumas initiated a channel through the cranium where an S-AVF with intracranial communication was able to form and may have only become clinically or radiographically detectable once it enlarged due to the altered hemodynamics caused by the intracranial AVF 1 year prior.
The diagnosis of an S-AVF is confirmed with imaging studies. Doppler ultrasonography initially will help to detect that a lesion is vascular in nature. Intra-arterial digital subtraction angiography is the gold-standard imaging technique and is necessary to delineate the feeding arteries and the draining channels as well as possible communication with intracranial vasculature.1,2 There is controversy regarding the appropriate treatment of S-AVFs.2 Each S-AVF possesses unique anatomic features that dictate appropriate management. The prognosis for an S-AVF is extremely variable, and the decision to treat is based on the patient’s symptoms and risk for exsanguinating hemorrhage.2,4 Neurosurgical approaches include ligation of the feeding arteries, surgical resection, electrothrombosis, direct intralesional injection of sclerosing agents, and endovascular embolization. Endovascular intervention increasingly is utilized as a primary treatment or as a preoperative adjunct to surgery.2,4 Large S-AVFs have a high risk for recurrence after treatment with endovascular embolization alone. In cases with intracranial communication, the intracranial component is treated first.2
This case emphasizes the importance of including S-AVFs on the dermatologic differential diagnosis of a scalp nodule, especially in patients with any history of intracranial AVF. A thorough history, detailed intake of potential signs and symptoms of AVF, and palpation for bruits is recommended as part of the surgical evaluation of a scalp nodule. Imaging of scalp nodules also should be considered for patients with any history of intracranial AVF; S-AVFs should be referred to neurosurgery or interventional neuroradiology for evaluation and possible treatment.
To the Editor:
A 71-year-old man presented with a nodule on the vertex of the scalp of 1 year’s duration. The lesion had become soft and tender during the week prior to presentation. He noted that he was experiencing headaches and a buzzing sound in his head. He denied all other neurologic symptoms. The patient was given amoxicillin from a primary care physician and was referred to our institution for evaluation of a presumed inflamed cyst.
The patient’s medical history included an intracranial arteriovenous fistula (AVF) treated with endovascular embolization 1 year prior to presentation, 2 substantial falls in childhood with head trauma and loss of consciousness, essential hypertension, and an aortic aneurysm. His medications included amlodipine, lisinopril, amoxicillin, a multivitamin, and grape seed extract.
Physical examination revealed a 2-cm, pink, somewhat rubbery, subcutaneous, nonmobile nodule on the vertex of the scalp (Figure 1). The lesion was not consistent with a common pilar cyst, and an excisional biopsy was performed to exclude malignancy. Upon superficial incision, the lesion bled moderately, and the procedure was immediately discontinued. Hemostasis was obtained, and the patient was sent for ultrasonography of the lesion.

Ultrasonography demonstrated a small hypoechoic nodule measuring up to 0.5 cm containing a tangle of vessels in the subcutaneous soft tissue corresponding to the palpable abnormality. A cerebral angiogram demonstrated a dural AVF of the superior sagittal sinus with multifocal supply that connected with this scalp nodule (Figure 2). The patient was treated by interventional neuroradiology with endovascular embolization, which resulted in complete resolution of the scalp nodule.

Scalp arteriovenous fistulas (S-AVFs) are characterized by abnormal connections between supplying arteries and draining veins in the subcutaneous plane of the scalp.1,2 The veins of an S-AVF undergo progressive aneurysmal dilatation from abnormal hemodynamics.1-3 Scalp arteriovenous fistulas are rare and may present as either an innocuous-looking scalp nodule or a progressively enlarging pulsatile mass on the scalp.2-4 Associated symptoms often include headache, local pain, bruits, tinnitus, and thrill.1,3,4 Recurrent hemorrhage, scalp necrosis, congestive heart failure, epilepsy, mental retardation, and intracranial ischemia also may occur.4
Scalp AVFs may occur with or without intracranial communication.4 Spontaneous S-AVFs with intracranial communication are uncommon, and their etiology is unclear. They may form as congenital malformations or may be idiopathic. Factors increasing circulation through the S-AVF such as trauma, pregnancy, hormonal changes, and inflammation prompt the development of symptoms.4 Scalp AVFs also may be caused by trauma.3 Scalp AVFs without intracranial communication have been reported following hair transplantation.1 Scalp AVFs with intracranial communication have been reported months to years after skull fracture or craniotomy.2 True spontaneous S-AVFs are difficult to differentiate from traumatic S-AVFs other than by history alone.2
Increased venous pressure has been shown to generate AVFs in rats.5 It has been suggested that S-AVFs can become enlarged by capturing subcutaneous or intracranial feeder vessels and that the consequent hemodynamic stress may induce de novo aneurysms in S-AVFs. Additionally, intracranial AVFs may alter the intracranial hemodynamics, leading to increased venous pressure in the superior sagittal sinus and the formation of communicating S-AVFs.5 Interestingly, our patient had an intracranial AVF treated with endovascular embolization 1 year prior to the formation of the S-AVF. An angiogram at the time of this embolization procedure did not demonstrate any S-AVFs. Furthermore, our patient has a history of 2 substantial falls in childhood with head trauma and loss of consciousness. Perhaps these traumas initiated a channel through the cranium where an S-AVF with intracranial communication was able to form and may have only become clinically or radiographically detectable once it enlarged due to the altered hemodynamics caused by the intracranial AVF 1 year prior.
The diagnosis of an S-AVF is confirmed with imaging studies. Doppler ultrasonography initially will help to detect that a lesion is vascular in nature. Intra-arterial digital subtraction angiography is the gold-standard imaging technique and is necessary to delineate the feeding arteries and the draining channels as well as possible communication with intracranial vasculature.1,2 There is controversy regarding the appropriate treatment of S-AVFs.2 Each S-AVF possesses unique anatomic features that dictate appropriate management. The prognosis for an S-AVF is extremely variable, and the decision to treat is based on the patient’s symptoms and risk for exsanguinating hemorrhage.2,4 Neurosurgical approaches include ligation of the feeding arteries, surgical resection, electrothrombosis, direct intralesional injection of sclerosing agents, and endovascular embolization. Endovascular intervention increasingly is utilized as a primary treatment or as a preoperative adjunct to surgery.2,4 Large S-AVFs have a high risk for recurrence after treatment with endovascular embolization alone. In cases with intracranial communication, the intracranial component is treated first.2
This case emphasizes the importance of including S-AVFs on the dermatologic differential diagnosis of a scalp nodule, especially in patients with any history of intracranial AVF. A thorough history, detailed intake of potential signs and symptoms of AVF, and palpation for bruits is recommended as part of the surgical evaluation of a scalp nodule. Imaging of scalp nodules also should be considered for patients with any history of intracranial AVF; S-AVFs should be referred to neurosurgery or interventional neuroradiology for evaluation and possible treatment.
- Bernstein J, Podnos S, Leavitt M. Arteriovenous fistula following hair transplantation. Dermatol Surg. 2011;37:873-875.
- Kumar R, Sharma G, Sharma BS. Management of scalp arterio-venous malformation: case series and review of literature. Br J Neurosurg. 2012;26:371-377.
- Gurkanlar D, Gonul M, Solmaz I, et al. Cirsoid aneurysms of the scalp. Neurosurg Rev. 2006;29:208-212.
- Senoglu M, Yasim A, Gokce M, et al. Nontraumatic scalp arteriovenous fistula in an adult: technical report on an illustrative case. Surg Neurol. 2008;70:194-197.
- Lanzino G, Passacantilli E, Lemole G, et al. Scalp arteriovenous malformation draining into the superior sagittal sinus associated with an intracranial arteriovenous malformation: just a coincidence? case report. Neurosurgery. 2003;52:440-443.
- Bernstein J, Podnos S, Leavitt M. Arteriovenous fistula following hair transplantation. Dermatol Surg. 2011;37:873-875.
- Kumar R, Sharma G, Sharma BS. Management of scalp arterio-venous malformation: case series and review of literature. Br J Neurosurg. 2012;26:371-377.
- Gurkanlar D, Gonul M, Solmaz I, et al. Cirsoid aneurysms of the scalp. Neurosurg Rev. 2006;29:208-212.
- Senoglu M, Yasim A, Gokce M, et al. Nontraumatic scalp arteriovenous fistula in an adult: technical report on an illustrative case. Surg Neurol. 2008;70:194-197.
- Lanzino G, Passacantilli E, Lemole G, et al. Scalp arteriovenous malformation draining into the superior sagittal sinus associated with an intracranial arteriovenous malformation: just a coincidence? case report. Neurosurgery. 2003;52:440-443.
Practice Points
- Scalp arteriovenous fistulas may be traumatic or spontaneous and present as either an innocuous-looking scalp nodule or as a progressively enlarging pulsatile mass on the scalp.
- Clinical detection followed by appropriate imaging and referral to neurosurgery or interventional neuroradiology is vital to patient safety.
Would it be smart to sell your medical practice now?
The COVID-19 pandemic has decimated the bottom lines of many private practices, prompting physician-owners to seriously contemplate selling.
Physician-owners have had to sell at lower prices, reflecting lower cash flow under COVID-19. But sales prices may rebound following news on Nov. 9 that a COVID-19 vaccine candidate produced by Pfizer and its German partner, BioNTech, may be ready for initial distribution before the end of the year.
“There are a lot of ifs still, but if things go according to expectations, we may see an increase in the value of practices,” said Mark O. Dietrich, a CPA in Framingham, Mass., who deals mostly with valuations of physician practices.
“Practice valuations have been lower because many patients have kept away and cash flow has been reduced,” Mr. Dietrich said. “But once patients feel safe, that barrier would be removed, and cash flow, which sales prices are generally based on, could rise. However, this may take a while. One major hurdle would be getting people to take the vaccine.”
Many doctors have been contemplating closing
The nation is currently undergoing a significant spike in COVID-19 hospitalizations, which could prompt another COVID-19–related downturn in practice volume, as occurred earlier in the year. That downturn forced many private practitioners to contemplate selling their practices.
In a survey released this summer by McKinsey & Company, 53% of independent physicians reported that they were worried about their practices surviving. Although many physicians have now reopened their offices, patient volume is reduced, and physicians are earning far less than before.
“In many cases, physicians who had been considering retirement in the next few years have moved their planning up and want to sell as soon as possible,” said John D. Fanburg, an attorney at Brach Eichler, a law firm in Roseland, N.J., who specializes in medical practice sales and mergers.
“For physicians over age 65, it’s not just worries about finances; it’s also worries about the health risks of staying open,” Mr. Fanburg added.
Mid-career physicians are also selling their practices. Many of them become employees of the hospital, large practice, or private-equity firm that bought the practice – receiving a level of compensation set by the sales agreement.
Will your practice be hard to sell?
With so many physicians ready to sell, are there enough potential buyers to acquire them all? Probably not, said Mr. Dietrich.
“Many hospitals may not need new practices right now,” he said. “In the depths of the pandemic, they furloughed many of their existing doctors and may not have brought all of them back yet.”
In fact, because of the pandemic, some buyers have delayed sales that were already in progress, said Monica H. Kaden, director of business valuations at Sobel Valuations, based in Livingston, N.J.
“Buyers are not only worried about their own cash flow but also about the possibility of lower revenues of the selling practices due to COVID-19,” she said, citing a very large multispecialty group that has put its purchase of a another large multispecialty group on hold.
Practice values have (temporarily) fallen
Many potential buyers are still looking, though. One thing that drives them is the possibility of discounted sales because of COVID-19. “The sense I get is that a lot of hospitals see this as an opportunity to pick up practices on the cheap,” Mr. Dietrich said.
COVID-19 has been reducing practice values somewhat, said Reed Tinsley, a CPA in Houston who performs medical practice valuations and runs a practice brokerage firm. “Practice revenues and net income are lower under COVID-19, so prices are lower.”
Ms. Kadan advised physicians to hold off selling if they can afford to wait. “It’s always best to sell when the practice volume looks the best, because then the practice is worth more. But there are doctors who can’t wait because revenues are really falling and they are running out of money. They may have no choice but to sell.”
Even in the best of times, not all practices can be sold, said Sean Tinsley, a broker and licensed financial adviser at Tinsley Medical Practice Brokers in Austin, Tex., which he runs with his father, Reed Tinsley.
“We turn down about as many deals to sell practices as we accept,” he said. “Brokers have to be very selective because we don’t get paid until the practice gets sold. Generally, we won’t take practices in rural areas or practices that still only have a fraction of their pre–COVID-19 volume.”
How long will it take to sell your practice?
Some practices find a buyer within weeks, but in other cases, it can take as long as a year, he said. Once the buyer is located, preparing the paperwork for the sale can take 45-60 days.
Doctors can sell their practices on their own, but a broker can help them find potential buyers and select the right price. Business brokers generally receive a greater percentage of the sales price than residential brokers. They have greater command of business and finance, and the sale is more complex than a residential sale.
The broker may also help with selling the building where the practice is located, which is usually a separate sale, said Bruce E. Wood, an attorney at CCB Law in Syracuse, N.Y., who deals with practice sales. “A hospital buying your practice may not want to buy the building, so it has to be sold separately. You can always sell the space to a different buyer.”
What’s the right price for your practice?
For small practices, brokers often set a price by establishing a multiple, such as two times net earnings, Sean Tinsley said. In many cases, practices haven’t retained net earnings, so the broker uses gross annual revenue and sets the price at 50%-55% of that figure.
An alternative that is widely used in the business world and for many large practices is to base the price on earnings before interest, taxes, depreciation, and amortization (EBITDA). To determine a price, the EBITDA is then multiplied by a particular multiple, which depends on the perceived value of the practice.
Higher multiples go to practices that have a qualified management team, have documented financial policies and procedures, or have had significant past growth. Generally, the multiple of EBITDA at smaller practices is 1 or 2; larger practices have a multiple of 5-7 times EBITDA, Sean Tinsley said.
COVID-19 has had the effect of reducing the multiple somewhat. “As market forces shift from a seller’s market to a buyer’s market, multiples will likely remain below pre–COVID-19 levels for the remainder of 2020 and the first half of 2021,” one report stated.
Certified valuators like Reed Tinsley have more complex ways to establish the value of a practice, but as a broker, Sean Tinsley tends to use the multiples approach. He asserted that the prices derived from this method are on the mark. “Almost all the time we sell at the asking price.”
Using valuations to set the price
A more complex and expensive way to set a price for a practice is to order a valuation of the practice. The valuator issues a report that runs dozens of pages and costs thousands of dollars.
Mr. Fanburg said that very few physicians selling practices order valuation reports, owing to the cost and complexity. As a result, “they don’t have a clear idea what their practices are worth.”
A comprehensive report is called a conclusion of value. The amount it finds – expressed as a range – is called “fair market value.” The report can be used in the courts for legal disputes as well as for deriving a sales price.
Practices that don’t want to pay for a conclusion of value can ask a valuator to assemble a less extensive report, called an opinion of calculated value. Also known as a calculation engagement or engagement letter, it still costs several thousand dollars.
This report has limited validity and can’t be used in the courts, according to Jarrod Barraza, a certified valuator in the Nashville, Tenn., office of Horne, a health care business valuator. “When I issue an engagement letter, I am not talking as an appraiser but as a valuation consultant, and I don’t call the result fair market value; it’s only estimating,” he said.
For all of the precision of formal reports, however, valuations of a practice can vary widely, according to Reed Tinsley. “Two valuations using the same methodology can differ by $300,000.”
Also, the valuation can be well above a reasonable asking price, said Sean Tinsley. “The market dictates the price. A traditional valuation almost invariably quotes a higher return than the market is willing to pay.”
Buyers’ valuations
Physicians who decide not to get a valuation still have to deal with valuations ordered by buyers. Hospitals and large practices often order valuations of the practices they want to buy, and private-equity firms use methods much like a valuation for the practices they are interested in.
Buyers rarely share the valuation report with the seller, so the seller has to accept the buyer’s price without being able to review the thought process behind it, Mr. Fanburg said. “Relying on the buyer to tell you what you’re worth means you may sell your practice well below its true value.”
When the buyer orders a valuation, the valuator interviews managers of the practice and asks for a great deal of information, says G. Don Barbo, managing director at VMG Health, a health care valuation firm based in Dallas.
Mr. Barbo said these documents include financial statements for the practice, usually going back 3-5 years; productivity reports for doctors and other providers; accounts receivables; reports of fixed assets; a roster of employees; employment agreements and management services agreements; reports on payer mix; facility leases and equipment lease agreements; budgets and projections; and tax returns.
Mr. Dietrich said valuators hone in on the practice’s current procedural terminology codes. “If the practice is coding too high, this would artificially increase the profit and purported value of the practice. For example, coding at 99214 rather than 99213 for an established patient means that the practice is being paid 45% more for each visit.” The valuator then reduces the value of the practice on the basis of the extent of the improper up-coding.
Mr. Barbo said some sellers don’t want all the scrutiny of the buyer’s valuation and just sell the practice’s tangible assets – furnishings, fixtures, and equipment – which do not require a great deal of documentation but yield a much lower price.
A primer on valuations
As a valuator, “my job is to project into the future,” Mr. Barraza said. “I am trying to see how the practice will fare going forward.”
Mr. Dietrich agreed, with one caveat: “As Yogi Berra said: ‘It’s difficult to make predictions, especially about the future.’ ”
The formal valuation assesses the practice in three ways: measuring income, assets, and what other practices sell for, called the market approach.
With the income approach, the most used measurement for practices, one tries to determine future income, which is what buyers are most interested in, Mr. Dietrich said. The income equals revenue (total collections) minus operating expenses and overhead.
“You are then left with all the money the physician is paid,” he said. “The issue is, how much is attributed to the physician’s own labor and how much to his or her ownership of the practice? This second category helps determine the value of the practice.”
The market approach is often used as a way to double-check the accuracy of the income approach. The appraiser looks for the prices of similar practices that have already been sold and then adjusts the price on the basis of differences with the practice up for sale.
The asset approach may be used when the practice has no positive cash flow. It establishes a price for tangible assets, which are often much lower in value than the values that the other approaches come up with. The asset approach can be a lower-priced alternative for practices that can’t be measured under the income or market approach.
“Equipment appraisers can do an inventory of your equipment,” Mr. Wood said. “Generally, equipment that is more than 3 years old, such as computers, is not that valuable, but an ultrasound machine probably has some resale value.”
Will the buyer pay for goodwill?
Many practice owners hope they can get money for the “goodwill” of their practice when they sell. Goodwill basically represents the reputation of the practice, which is difficult to pinpoint, and Mr. Wood said buyers often don’t want to pay for it.
“The goodwill is a wild card,” Mr. Wood said. “It can range from zero to crazy numbers. There is a Goodwill Registry – a list of the goodwill in other practice sales – that you can consult.”
One simple way to calculate the goodwill, he said, is to take the value of the practice based on examining income and remove the value of tangible assets. What is left is considered the goodwill.
Another form of intangible asset that is sometimes lumped together with goodwill is the value of the practice’s trained staff. “Some buyers agree to pay for the staff in place, because they plan to use that staff,” Ms. Kadan said. In one large deal she was involved with, the buyer agreed to pay something for the selling practice’s staff of 180 people.
Another item that buyers also do not typically pay for is the practice’s accounts receivable. They may also not pay for any liabilities the practice holds, such as the facility lease, equipment lease, and maintenance contracts, Mr. Barbo said. “The buyer then often stipulates that all liabilities are left to the practice, or stipulates any specific liabilities that it may assume.”
Selling to other doctors
Doctors can sell practices or shares in practices to other doctors. A retiring physician, for example, can sell his or her share to the other partners. A valuator may be brought in to establish the value of the doctor’s equity interest in the practice.
“Generally, practice buyouts aren’t lucrative for selling physician,” Mr. Wood said. “There are exceptions, of course, such as specialty practices in some cases.”
A practice can also be sold to a new doctor or to a previously employed physician who wants to be an owner. These physicians usually need to get a bank loan to buy the practice.
The bank assesses the finances of the selling practice to determine whether the buying physician will earn enough money to pay back the loan. “Banks don’t want lend more than the gross annual revenue of the practice, and some banks will only lend at 65% of gross annual revenue,” Sean Tinsley said.
COVID-19 has seriously affected banks’ lending decisions. Banks stopped lending to practice buyers at the beginning of the pandemic, and when they started lending again, they were more cautious, Sean Tinsley said. “Generally, banks want to see the practice at 85%-90% of pre–COVID-19 numbers before they make a loan.”
He added that, if a buyer can’t get a bank loan, the selling doctor may decide to finance the sale. The buyer agrees to a payment schedule to pay off the full price over several years.
Selling to or merging with other practices
The usual buyer is another practice, Reed Tinsley said. “You can sell to a group, but prices are low because, with COVID-19, buyers don’t want to incur a lot of money up front. Or you can merge with the practice, which means the selling doctor usually doesn’t get any money, but he does get a share in the larger practice. In that case, the partnership is the object of value, and it can be cashed out when the physician leaves the practice.”
Mergers can get very complicated. Mr. Fanburg said he has been working with seven groups that are merging into one. “The merger was scheduled to go live last January, but it was slowed down over negotiations about new managed care contracts and putting together a management structure, plus the groups were a little wary of each other. Now the deal is scheduled to go live next January.”
One advantage to selling to a larger entity, such as a big group practice or a hospital, is that the selling physician benefits from the higher reimbursement rates that large providers usually command. “If the buyer has more favorable reimbursement rates with insurers, it could pay the selling doctor much more than he is making now,” Mr. Barraza said.
Hospitals as buyers
Because of COVID-19, currently many hospitals don’t have money to buy more practices. However, this is most likely a temporary situation.
Hospitals typically offer less money than other buyers, according to Sean Tinsley. “We have never sold to a hospital, because hospitals generally don’t pay for goodwill. They pay for the practice assets and offer a dollar amount for each chart.”
Hospitals have to be careful not to pay physicians more than the usual amount for their practices, because the extra amount could be seen as a kickback for referrals, which would violate the federal Stark law and Anti-Kickback Statute. Not-for-profit hospitals also have to comply with regulations at the Internal Revenue Service.
Hospitals usually require that the selling physician continue to work in the practice after it is sold. The selling physician’s presence helps ensure that the practice’s output will not decline after sale. Although the sales price may be low, the hospital may make up for it by paying a higher compensation, Sean Tinsley said.
Selling to private-equity firms
Private-equity purchases are financed by investors who essentially want to “flip” practices – that is, they want to make them more profitable and then sell them to someone else. The private-equity firm starts by buying a “platform” practice, which forms the core of the venture. It then buys smaller practices that will be managed by the platform practice.
The number of private-equity deals increased continually through 2019, then plummeted in March because of COVID-19, but by the summer, activity began to rise again.
Physicians are very intrigued about selling to private-equity firms because they are known to pay the most for practices. But private-equity buyers focus on a fairly narrow group of specialties.
Generally, Sean Tinsley said, private-equity firms only look for pain, dermatology, and ophthalmology practices, but they have been starting to branch out to specialties such as gastroenterology. In 2018, there were only two private-equity deals for gastroenterology practices, but in 2019, there were 16, according to one assessment.
Private-equity firms buy very few of the practices they initially review, according to Mr. Fanburg. “Private equity negotiates with dozens or even hundreds of physician practices at a time, with only 1%-5% of those practices actually being acquired.”
The private-equity firm’s upfront payment to selling physicians is quite high, but then the physicians become employees of the new group and earn much less in compensation than they earned on this own.
“In order for the venture to get any value out of the acquisition, the doctors have to make less going forward than they did historically,” Mr. Dietrich said. That freed-up money boosts the value of the venture.
When the platform practice is sold – usually after 5 years or so – “chances are the management team will be replaced,” Mr. Fanburg said. “There could be new policies and objectives, which could mean a bumpy ride for physicians.”
Do you really want to sell?
“When a group of physicians comes to me asking for help selling their practice, my first question is, Why are you doing this?” Mr. Fanburg said. “You need a better reason for selling than just the money.
“Once you make the leap, there is a certain amount of autonomy you lose,” he continued. “The sale gives you an economic boost, but it may not be enough for the long haul. If you stay on with the buyer, your compensation is often lower. That makes sense if you’re retiring, but not if you’re a younger physician with many years of practice in the years ahead.
“When physicians say they see no other way out except to sell,” Mr. Fanburg said, “I tell them that their buyer will see a path to future growth for your practice. If you think reimbursements are getting worse, why are the buyers pressing ahead?”
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic has decimated the bottom lines of many private practices, prompting physician-owners to seriously contemplate selling.
Physician-owners have had to sell at lower prices, reflecting lower cash flow under COVID-19. But sales prices may rebound following news on Nov. 9 that a COVID-19 vaccine candidate produced by Pfizer and its German partner, BioNTech, may be ready for initial distribution before the end of the year.
“There are a lot of ifs still, but if things go according to expectations, we may see an increase in the value of practices,” said Mark O. Dietrich, a CPA in Framingham, Mass., who deals mostly with valuations of physician practices.
“Practice valuations have been lower because many patients have kept away and cash flow has been reduced,” Mr. Dietrich said. “But once patients feel safe, that barrier would be removed, and cash flow, which sales prices are generally based on, could rise. However, this may take a while. One major hurdle would be getting people to take the vaccine.”
Many doctors have been contemplating closing
The nation is currently undergoing a significant spike in COVID-19 hospitalizations, which could prompt another COVID-19–related downturn in practice volume, as occurred earlier in the year. That downturn forced many private practitioners to contemplate selling their practices.
In a survey released this summer by McKinsey & Company, 53% of independent physicians reported that they were worried about their practices surviving. Although many physicians have now reopened their offices, patient volume is reduced, and physicians are earning far less than before.
“In many cases, physicians who had been considering retirement in the next few years have moved their planning up and want to sell as soon as possible,” said John D. Fanburg, an attorney at Brach Eichler, a law firm in Roseland, N.J., who specializes in medical practice sales and mergers.
“For physicians over age 65, it’s not just worries about finances; it’s also worries about the health risks of staying open,” Mr. Fanburg added.
Mid-career physicians are also selling their practices. Many of them become employees of the hospital, large practice, or private-equity firm that bought the practice – receiving a level of compensation set by the sales agreement.
Will your practice be hard to sell?
With so many physicians ready to sell, are there enough potential buyers to acquire them all? Probably not, said Mr. Dietrich.
“Many hospitals may not need new practices right now,” he said. “In the depths of the pandemic, they furloughed many of their existing doctors and may not have brought all of them back yet.”
In fact, because of the pandemic, some buyers have delayed sales that were already in progress, said Monica H. Kaden, director of business valuations at Sobel Valuations, based in Livingston, N.J.
“Buyers are not only worried about their own cash flow but also about the possibility of lower revenues of the selling practices due to COVID-19,” she said, citing a very large multispecialty group that has put its purchase of a another large multispecialty group on hold.
Practice values have (temporarily) fallen
Many potential buyers are still looking, though. One thing that drives them is the possibility of discounted sales because of COVID-19. “The sense I get is that a lot of hospitals see this as an opportunity to pick up practices on the cheap,” Mr. Dietrich said.
COVID-19 has been reducing practice values somewhat, said Reed Tinsley, a CPA in Houston who performs medical practice valuations and runs a practice brokerage firm. “Practice revenues and net income are lower under COVID-19, so prices are lower.”
Ms. Kadan advised physicians to hold off selling if they can afford to wait. “It’s always best to sell when the practice volume looks the best, because then the practice is worth more. But there are doctors who can’t wait because revenues are really falling and they are running out of money. They may have no choice but to sell.”
Even in the best of times, not all practices can be sold, said Sean Tinsley, a broker and licensed financial adviser at Tinsley Medical Practice Brokers in Austin, Tex., which he runs with his father, Reed Tinsley.
“We turn down about as many deals to sell practices as we accept,” he said. “Brokers have to be very selective because we don’t get paid until the practice gets sold. Generally, we won’t take practices in rural areas or practices that still only have a fraction of their pre–COVID-19 volume.”
How long will it take to sell your practice?
Some practices find a buyer within weeks, but in other cases, it can take as long as a year, he said. Once the buyer is located, preparing the paperwork for the sale can take 45-60 days.
Doctors can sell their practices on their own, but a broker can help them find potential buyers and select the right price. Business brokers generally receive a greater percentage of the sales price than residential brokers. They have greater command of business and finance, and the sale is more complex than a residential sale.
The broker may also help with selling the building where the practice is located, which is usually a separate sale, said Bruce E. Wood, an attorney at CCB Law in Syracuse, N.Y., who deals with practice sales. “A hospital buying your practice may not want to buy the building, so it has to be sold separately. You can always sell the space to a different buyer.”
What’s the right price for your practice?
For small practices, brokers often set a price by establishing a multiple, such as two times net earnings, Sean Tinsley said. In many cases, practices haven’t retained net earnings, so the broker uses gross annual revenue and sets the price at 50%-55% of that figure.
An alternative that is widely used in the business world and for many large practices is to base the price on earnings before interest, taxes, depreciation, and amortization (EBITDA). To determine a price, the EBITDA is then multiplied by a particular multiple, which depends on the perceived value of the practice.
Higher multiples go to practices that have a qualified management team, have documented financial policies and procedures, or have had significant past growth. Generally, the multiple of EBITDA at smaller practices is 1 or 2; larger practices have a multiple of 5-7 times EBITDA, Sean Tinsley said.
COVID-19 has had the effect of reducing the multiple somewhat. “As market forces shift from a seller’s market to a buyer’s market, multiples will likely remain below pre–COVID-19 levels for the remainder of 2020 and the first half of 2021,” one report stated.
Certified valuators like Reed Tinsley have more complex ways to establish the value of a practice, but as a broker, Sean Tinsley tends to use the multiples approach. He asserted that the prices derived from this method are on the mark. “Almost all the time we sell at the asking price.”
Using valuations to set the price
A more complex and expensive way to set a price for a practice is to order a valuation of the practice. The valuator issues a report that runs dozens of pages and costs thousands of dollars.
Mr. Fanburg said that very few physicians selling practices order valuation reports, owing to the cost and complexity. As a result, “they don’t have a clear idea what their practices are worth.”
A comprehensive report is called a conclusion of value. The amount it finds – expressed as a range – is called “fair market value.” The report can be used in the courts for legal disputes as well as for deriving a sales price.
Practices that don’t want to pay for a conclusion of value can ask a valuator to assemble a less extensive report, called an opinion of calculated value. Also known as a calculation engagement or engagement letter, it still costs several thousand dollars.
This report has limited validity and can’t be used in the courts, according to Jarrod Barraza, a certified valuator in the Nashville, Tenn., office of Horne, a health care business valuator. “When I issue an engagement letter, I am not talking as an appraiser but as a valuation consultant, and I don’t call the result fair market value; it’s only estimating,” he said.
For all of the precision of formal reports, however, valuations of a practice can vary widely, according to Reed Tinsley. “Two valuations using the same methodology can differ by $300,000.”
Also, the valuation can be well above a reasonable asking price, said Sean Tinsley. “The market dictates the price. A traditional valuation almost invariably quotes a higher return than the market is willing to pay.”
Buyers’ valuations
Physicians who decide not to get a valuation still have to deal with valuations ordered by buyers. Hospitals and large practices often order valuations of the practices they want to buy, and private-equity firms use methods much like a valuation for the practices they are interested in.
Buyers rarely share the valuation report with the seller, so the seller has to accept the buyer’s price without being able to review the thought process behind it, Mr. Fanburg said. “Relying on the buyer to tell you what you’re worth means you may sell your practice well below its true value.”
When the buyer orders a valuation, the valuator interviews managers of the practice and asks for a great deal of information, says G. Don Barbo, managing director at VMG Health, a health care valuation firm based in Dallas.
Mr. Barbo said these documents include financial statements for the practice, usually going back 3-5 years; productivity reports for doctors and other providers; accounts receivables; reports of fixed assets; a roster of employees; employment agreements and management services agreements; reports on payer mix; facility leases and equipment lease agreements; budgets and projections; and tax returns.
Mr. Dietrich said valuators hone in on the practice’s current procedural terminology codes. “If the practice is coding too high, this would artificially increase the profit and purported value of the practice. For example, coding at 99214 rather than 99213 for an established patient means that the practice is being paid 45% more for each visit.” The valuator then reduces the value of the practice on the basis of the extent of the improper up-coding.
Mr. Barbo said some sellers don’t want all the scrutiny of the buyer’s valuation and just sell the practice’s tangible assets – furnishings, fixtures, and equipment – which do not require a great deal of documentation but yield a much lower price.
A primer on valuations
As a valuator, “my job is to project into the future,” Mr. Barraza said. “I am trying to see how the practice will fare going forward.”
Mr. Dietrich agreed, with one caveat: “As Yogi Berra said: ‘It’s difficult to make predictions, especially about the future.’ ”
The formal valuation assesses the practice in three ways: measuring income, assets, and what other practices sell for, called the market approach.
With the income approach, the most used measurement for practices, one tries to determine future income, which is what buyers are most interested in, Mr. Dietrich said. The income equals revenue (total collections) minus operating expenses and overhead.
“You are then left with all the money the physician is paid,” he said. “The issue is, how much is attributed to the physician’s own labor and how much to his or her ownership of the practice? This second category helps determine the value of the practice.”
The market approach is often used as a way to double-check the accuracy of the income approach. The appraiser looks for the prices of similar practices that have already been sold and then adjusts the price on the basis of differences with the practice up for sale.
The asset approach may be used when the practice has no positive cash flow. It establishes a price for tangible assets, which are often much lower in value than the values that the other approaches come up with. The asset approach can be a lower-priced alternative for practices that can’t be measured under the income or market approach.
“Equipment appraisers can do an inventory of your equipment,” Mr. Wood said. “Generally, equipment that is more than 3 years old, such as computers, is not that valuable, but an ultrasound machine probably has some resale value.”
Will the buyer pay for goodwill?
Many practice owners hope they can get money for the “goodwill” of their practice when they sell. Goodwill basically represents the reputation of the practice, which is difficult to pinpoint, and Mr. Wood said buyers often don’t want to pay for it.
“The goodwill is a wild card,” Mr. Wood said. “It can range from zero to crazy numbers. There is a Goodwill Registry – a list of the goodwill in other practice sales – that you can consult.”
One simple way to calculate the goodwill, he said, is to take the value of the practice based on examining income and remove the value of tangible assets. What is left is considered the goodwill.
Another form of intangible asset that is sometimes lumped together with goodwill is the value of the practice’s trained staff. “Some buyers agree to pay for the staff in place, because they plan to use that staff,” Ms. Kadan said. In one large deal she was involved with, the buyer agreed to pay something for the selling practice’s staff of 180 people.
Another item that buyers also do not typically pay for is the practice’s accounts receivable. They may also not pay for any liabilities the practice holds, such as the facility lease, equipment lease, and maintenance contracts, Mr. Barbo said. “The buyer then often stipulates that all liabilities are left to the practice, or stipulates any specific liabilities that it may assume.”
Selling to other doctors
Doctors can sell practices or shares in practices to other doctors. A retiring physician, for example, can sell his or her share to the other partners. A valuator may be brought in to establish the value of the doctor’s equity interest in the practice.
“Generally, practice buyouts aren’t lucrative for selling physician,” Mr. Wood said. “There are exceptions, of course, such as specialty practices in some cases.”
A practice can also be sold to a new doctor or to a previously employed physician who wants to be an owner. These physicians usually need to get a bank loan to buy the practice.
The bank assesses the finances of the selling practice to determine whether the buying physician will earn enough money to pay back the loan. “Banks don’t want lend more than the gross annual revenue of the practice, and some banks will only lend at 65% of gross annual revenue,” Sean Tinsley said.
COVID-19 has seriously affected banks’ lending decisions. Banks stopped lending to practice buyers at the beginning of the pandemic, and when they started lending again, they were more cautious, Sean Tinsley said. “Generally, banks want to see the practice at 85%-90% of pre–COVID-19 numbers before they make a loan.”
He added that, if a buyer can’t get a bank loan, the selling doctor may decide to finance the sale. The buyer agrees to a payment schedule to pay off the full price over several years.
Selling to or merging with other practices
The usual buyer is another practice, Reed Tinsley said. “You can sell to a group, but prices are low because, with COVID-19, buyers don’t want to incur a lot of money up front. Or you can merge with the practice, which means the selling doctor usually doesn’t get any money, but he does get a share in the larger practice. In that case, the partnership is the object of value, and it can be cashed out when the physician leaves the practice.”
Mergers can get very complicated. Mr. Fanburg said he has been working with seven groups that are merging into one. “The merger was scheduled to go live last January, but it was slowed down over negotiations about new managed care contracts and putting together a management structure, plus the groups were a little wary of each other. Now the deal is scheduled to go live next January.”
One advantage to selling to a larger entity, such as a big group practice or a hospital, is that the selling physician benefits from the higher reimbursement rates that large providers usually command. “If the buyer has more favorable reimbursement rates with insurers, it could pay the selling doctor much more than he is making now,” Mr. Barraza said.
Hospitals as buyers
Because of COVID-19, currently many hospitals don’t have money to buy more practices. However, this is most likely a temporary situation.
Hospitals typically offer less money than other buyers, according to Sean Tinsley. “We have never sold to a hospital, because hospitals generally don’t pay for goodwill. They pay for the practice assets and offer a dollar amount for each chart.”
Hospitals have to be careful not to pay physicians more than the usual amount for their practices, because the extra amount could be seen as a kickback for referrals, which would violate the federal Stark law and Anti-Kickback Statute. Not-for-profit hospitals also have to comply with regulations at the Internal Revenue Service.
Hospitals usually require that the selling physician continue to work in the practice after it is sold. The selling physician’s presence helps ensure that the practice’s output will not decline after sale. Although the sales price may be low, the hospital may make up for it by paying a higher compensation, Sean Tinsley said.
Selling to private-equity firms
Private-equity purchases are financed by investors who essentially want to “flip” practices – that is, they want to make them more profitable and then sell them to someone else. The private-equity firm starts by buying a “platform” practice, which forms the core of the venture. It then buys smaller practices that will be managed by the platform practice.
The number of private-equity deals increased continually through 2019, then plummeted in March because of COVID-19, but by the summer, activity began to rise again.
Physicians are very intrigued about selling to private-equity firms because they are known to pay the most for practices. But private-equity buyers focus on a fairly narrow group of specialties.
Generally, Sean Tinsley said, private-equity firms only look for pain, dermatology, and ophthalmology practices, but they have been starting to branch out to specialties such as gastroenterology. In 2018, there were only two private-equity deals for gastroenterology practices, but in 2019, there were 16, according to one assessment.
Private-equity firms buy very few of the practices they initially review, according to Mr. Fanburg. “Private equity negotiates with dozens or even hundreds of physician practices at a time, with only 1%-5% of those practices actually being acquired.”
The private-equity firm’s upfront payment to selling physicians is quite high, but then the physicians become employees of the new group and earn much less in compensation than they earned on this own.
“In order for the venture to get any value out of the acquisition, the doctors have to make less going forward than they did historically,” Mr. Dietrich said. That freed-up money boosts the value of the venture.
When the platform practice is sold – usually after 5 years or so – “chances are the management team will be replaced,” Mr. Fanburg said. “There could be new policies and objectives, which could mean a bumpy ride for physicians.”
Do you really want to sell?
“When a group of physicians comes to me asking for help selling their practice, my first question is, Why are you doing this?” Mr. Fanburg said. “You need a better reason for selling than just the money.
“Once you make the leap, there is a certain amount of autonomy you lose,” he continued. “The sale gives you an economic boost, but it may not be enough for the long haul. If you stay on with the buyer, your compensation is often lower. That makes sense if you’re retiring, but not if you’re a younger physician with many years of practice in the years ahead.
“When physicians say they see no other way out except to sell,” Mr. Fanburg said, “I tell them that their buyer will see a path to future growth for your practice. If you think reimbursements are getting worse, why are the buyers pressing ahead?”
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic has decimated the bottom lines of many private practices, prompting physician-owners to seriously contemplate selling.
Physician-owners have had to sell at lower prices, reflecting lower cash flow under COVID-19. But sales prices may rebound following news on Nov. 9 that a COVID-19 vaccine candidate produced by Pfizer and its German partner, BioNTech, may be ready for initial distribution before the end of the year.
“There are a lot of ifs still, but if things go according to expectations, we may see an increase in the value of practices,” said Mark O. Dietrich, a CPA in Framingham, Mass., who deals mostly with valuations of physician practices.
“Practice valuations have been lower because many patients have kept away and cash flow has been reduced,” Mr. Dietrich said. “But once patients feel safe, that barrier would be removed, and cash flow, which sales prices are generally based on, could rise. However, this may take a while. One major hurdle would be getting people to take the vaccine.”
Many doctors have been contemplating closing
The nation is currently undergoing a significant spike in COVID-19 hospitalizations, which could prompt another COVID-19–related downturn in practice volume, as occurred earlier in the year. That downturn forced many private practitioners to contemplate selling their practices.
In a survey released this summer by McKinsey & Company, 53% of independent physicians reported that they were worried about their practices surviving. Although many physicians have now reopened their offices, patient volume is reduced, and physicians are earning far less than before.
“In many cases, physicians who had been considering retirement in the next few years have moved their planning up and want to sell as soon as possible,” said John D. Fanburg, an attorney at Brach Eichler, a law firm in Roseland, N.J., who specializes in medical practice sales and mergers.
“For physicians over age 65, it’s not just worries about finances; it’s also worries about the health risks of staying open,” Mr. Fanburg added.
Mid-career physicians are also selling their practices. Many of them become employees of the hospital, large practice, or private-equity firm that bought the practice – receiving a level of compensation set by the sales agreement.
Will your practice be hard to sell?
With so many physicians ready to sell, are there enough potential buyers to acquire them all? Probably not, said Mr. Dietrich.
“Many hospitals may not need new practices right now,” he said. “In the depths of the pandemic, they furloughed many of their existing doctors and may not have brought all of them back yet.”
In fact, because of the pandemic, some buyers have delayed sales that were already in progress, said Monica H. Kaden, director of business valuations at Sobel Valuations, based in Livingston, N.J.
“Buyers are not only worried about their own cash flow but also about the possibility of lower revenues of the selling practices due to COVID-19,” she said, citing a very large multispecialty group that has put its purchase of a another large multispecialty group on hold.
Practice values have (temporarily) fallen
Many potential buyers are still looking, though. One thing that drives them is the possibility of discounted sales because of COVID-19. “The sense I get is that a lot of hospitals see this as an opportunity to pick up practices on the cheap,” Mr. Dietrich said.
COVID-19 has been reducing practice values somewhat, said Reed Tinsley, a CPA in Houston who performs medical practice valuations and runs a practice brokerage firm. “Practice revenues and net income are lower under COVID-19, so prices are lower.”
Ms. Kadan advised physicians to hold off selling if they can afford to wait. “It’s always best to sell when the practice volume looks the best, because then the practice is worth more. But there are doctors who can’t wait because revenues are really falling and they are running out of money. They may have no choice but to sell.”
Even in the best of times, not all practices can be sold, said Sean Tinsley, a broker and licensed financial adviser at Tinsley Medical Practice Brokers in Austin, Tex., which he runs with his father, Reed Tinsley.
“We turn down about as many deals to sell practices as we accept,” he said. “Brokers have to be very selective because we don’t get paid until the practice gets sold. Generally, we won’t take practices in rural areas or practices that still only have a fraction of their pre–COVID-19 volume.”
How long will it take to sell your practice?
Some practices find a buyer within weeks, but in other cases, it can take as long as a year, he said. Once the buyer is located, preparing the paperwork for the sale can take 45-60 days.
Doctors can sell their practices on their own, but a broker can help them find potential buyers and select the right price. Business brokers generally receive a greater percentage of the sales price than residential brokers. They have greater command of business and finance, and the sale is more complex than a residential sale.
The broker may also help with selling the building where the practice is located, which is usually a separate sale, said Bruce E. Wood, an attorney at CCB Law in Syracuse, N.Y., who deals with practice sales. “A hospital buying your practice may not want to buy the building, so it has to be sold separately. You can always sell the space to a different buyer.”
What’s the right price for your practice?
For small practices, brokers often set a price by establishing a multiple, such as two times net earnings, Sean Tinsley said. In many cases, practices haven’t retained net earnings, so the broker uses gross annual revenue and sets the price at 50%-55% of that figure.
An alternative that is widely used in the business world and for many large practices is to base the price on earnings before interest, taxes, depreciation, and amortization (EBITDA). To determine a price, the EBITDA is then multiplied by a particular multiple, which depends on the perceived value of the practice.
Higher multiples go to practices that have a qualified management team, have documented financial policies and procedures, or have had significant past growth. Generally, the multiple of EBITDA at smaller practices is 1 or 2; larger practices have a multiple of 5-7 times EBITDA, Sean Tinsley said.
COVID-19 has had the effect of reducing the multiple somewhat. “As market forces shift from a seller’s market to a buyer’s market, multiples will likely remain below pre–COVID-19 levels for the remainder of 2020 and the first half of 2021,” one report stated.
Certified valuators like Reed Tinsley have more complex ways to establish the value of a practice, but as a broker, Sean Tinsley tends to use the multiples approach. He asserted that the prices derived from this method are on the mark. “Almost all the time we sell at the asking price.”
Using valuations to set the price
A more complex and expensive way to set a price for a practice is to order a valuation of the practice. The valuator issues a report that runs dozens of pages and costs thousands of dollars.
Mr. Fanburg said that very few physicians selling practices order valuation reports, owing to the cost and complexity. As a result, “they don’t have a clear idea what their practices are worth.”
A comprehensive report is called a conclusion of value. The amount it finds – expressed as a range – is called “fair market value.” The report can be used in the courts for legal disputes as well as for deriving a sales price.
Practices that don’t want to pay for a conclusion of value can ask a valuator to assemble a less extensive report, called an opinion of calculated value. Also known as a calculation engagement or engagement letter, it still costs several thousand dollars.
This report has limited validity and can’t be used in the courts, according to Jarrod Barraza, a certified valuator in the Nashville, Tenn., office of Horne, a health care business valuator. “When I issue an engagement letter, I am not talking as an appraiser but as a valuation consultant, and I don’t call the result fair market value; it’s only estimating,” he said.
For all of the precision of formal reports, however, valuations of a practice can vary widely, according to Reed Tinsley. “Two valuations using the same methodology can differ by $300,000.”
Also, the valuation can be well above a reasonable asking price, said Sean Tinsley. “The market dictates the price. A traditional valuation almost invariably quotes a higher return than the market is willing to pay.”
Buyers’ valuations
Physicians who decide not to get a valuation still have to deal with valuations ordered by buyers. Hospitals and large practices often order valuations of the practices they want to buy, and private-equity firms use methods much like a valuation for the practices they are interested in.
Buyers rarely share the valuation report with the seller, so the seller has to accept the buyer’s price without being able to review the thought process behind it, Mr. Fanburg said. “Relying on the buyer to tell you what you’re worth means you may sell your practice well below its true value.”
When the buyer orders a valuation, the valuator interviews managers of the practice and asks for a great deal of information, says G. Don Barbo, managing director at VMG Health, a health care valuation firm based in Dallas.
Mr. Barbo said these documents include financial statements for the practice, usually going back 3-5 years; productivity reports for doctors and other providers; accounts receivables; reports of fixed assets; a roster of employees; employment agreements and management services agreements; reports on payer mix; facility leases and equipment lease agreements; budgets and projections; and tax returns.
Mr. Dietrich said valuators hone in on the practice’s current procedural terminology codes. “If the practice is coding too high, this would artificially increase the profit and purported value of the practice. For example, coding at 99214 rather than 99213 for an established patient means that the practice is being paid 45% more for each visit.” The valuator then reduces the value of the practice on the basis of the extent of the improper up-coding.
Mr. Barbo said some sellers don’t want all the scrutiny of the buyer’s valuation and just sell the practice’s tangible assets – furnishings, fixtures, and equipment – which do not require a great deal of documentation but yield a much lower price.
A primer on valuations
As a valuator, “my job is to project into the future,” Mr. Barraza said. “I am trying to see how the practice will fare going forward.”
Mr. Dietrich agreed, with one caveat: “As Yogi Berra said: ‘It’s difficult to make predictions, especially about the future.’ ”
The formal valuation assesses the practice in three ways: measuring income, assets, and what other practices sell for, called the market approach.
With the income approach, the most used measurement for practices, one tries to determine future income, which is what buyers are most interested in, Mr. Dietrich said. The income equals revenue (total collections) minus operating expenses and overhead.
“You are then left with all the money the physician is paid,” he said. “The issue is, how much is attributed to the physician’s own labor and how much to his or her ownership of the practice? This second category helps determine the value of the practice.”
The market approach is often used as a way to double-check the accuracy of the income approach. The appraiser looks for the prices of similar practices that have already been sold and then adjusts the price on the basis of differences with the practice up for sale.
The asset approach may be used when the practice has no positive cash flow. It establishes a price for tangible assets, which are often much lower in value than the values that the other approaches come up with. The asset approach can be a lower-priced alternative for practices that can’t be measured under the income or market approach.
“Equipment appraisers can do an inventory of your equipment,” Mr. Wood said. “Generally, equipment that is more than 3 years old, such as computers, is not that valuable, but an ultrasound machine probably has some resale value.”
Will the buyer pay for goodwill?
Many practice owners hope they can get money for the “goodwill” of their practice when they sell. Goodwill basically represents the reputation of the practice, which is difficult to pinpoint, and Mr. Wood said buyers often don’t want to pay for it.
“The goodwill is a wild card,” Mr. Wood said. “It can range from zero to crazy numbers. There is a Goodwill Registry – a list of the goodwill in other practice sales – that you can consult.”
One simple way to calculate the goodwill, he said, is to take the value of the practice based on examining income and remove the value of tangible assets. What is left is considered the goodwill.
Another form of intangible asset that is sometimes lumped together with goodwill is the value of the practice’s trained staff. “Some buyers agree to pay for the staff in place, because they plan to use that staff,” Ms. Kadan said. In one large deal she was involved with, the buyer agreed to pay something for the selling practice’s staff of 180 people.
Another item that buyers also do not typically pay for is the practice’s accounts receivable. They may also not pay for any liabilities the practice holds, such as the facility lease, equipment lease, and maintenance contracts, Mr. Barbo said. “The buyer then often stipulates that all liabilities are left to the practice, or stipulates any specific liabilities that it may assume.”
Selling to other doctors
Doctors can sell practices or shares in practices to other doctors. A retiring physician, for example, can sell his or her share to the other partners. A valuator may be brought in to establish the value of the doctor’s equity interest in the practice.
“Generally, practice buyouts aren’t lucrative for selling physician,” Mr. Wood said. “There are exceptions, of course, such as specialty practices in some cases.”
A practice can also be sold to a new doctor or to a previously employed physician who wants to be an owner. These physicians usually need to get a bank loan to buy the practice.
The bank assesses the finances of the selling practice to determine whether the buying physician will earn enough money to pay back the loan. “Banks don’t want lend more than the gross annual revenue of the practice, and some banks will only lend at 65% of gross annual revenue,” Sean Tinsley said.
COVID-19 has seriously affected banks’ lending decisions. Banks stopped lending to practice buyers at the beginning of the pandemic, and when they started lending again, they were more cautious, Sean Tinsley said. “Generally, banks want to see the practice at 85%-90% of pre–COVID-19 numbers before they make a loan.”
He added that, if a buyer can’t get a bank loan, the selling doctor may decide to finance the sale. The buyer agrees to a payment schedule to pay off the full price over several years.
Selling to or merging with other practices
The usual buyer is another practice, Reed Tinsley said. “You can sell to a group, but prices are low because, with COVID-19, buyers don’t want to incur a lot of money up front. Or you can merge with the practice, which means the selling doctor usually doesn’t get any money, but he does get a share in the larger practice. In that case, the partnership is the object of value, and it can be cashed out when the physician leaves the practice.”
Mergers can get very complicated. Mr. Fanburg said he has been working with seven groups that are merging into one. “The merger was scheduled to go live last January, but it was slowed down over negotiations about new managed care contracts and putting together a management structure, plus the groups were a little wary of each other. Now the deal is scheduled to go live next January.”
One advantage to selling to a larger entity, such as a big group practice or a hospital, is that the selling physician benefits from the higher reimbursement rates that large providers usually command. “If the buyer has more favorable reimbursement rates with insurers, it could pay the selling doctor much more than he is making now,” Mr. Barraza said.
Hospitals as buyers
Because of COVID-19, currently many hospitals don’t have money to buy more practices. However, this is most likely a temporary situation.
Hospitals typically offer less money than other buyers, according to Sean Tinsley. “We have never sold to a hospital, because hospitals generally don’t pay for goodwill. They pay for the practice assets and offer a dollar amount for each chart.”
Hospitals have to be careful not to pay physicians more than the usual amount for their practices, because the extra amount could be seen as a kickback for referrals, which would violate the federal Stark law and Anti-Kickback Statute. Not-for-profit hospitals also have to comply with regulations at the Internal Revenue Service.
Hospitals usually require that the selling physician continue to work in the practice after it is sold. The selling physician’s presence helps ensure that the practice’s output will not decline after sale. Although the sales price may be low, the hospital may make up for it by paying a higher compensation, Sean Tinsley said.
Selling to private-equity firms
Private-equity purchases are financed by investors who essentially want to “flip” practices – that is, they want to make them more profitable and then sell them to someone else. The private-equity firm starts by buying a “platform” practice, which forms the core of the venture. It then buys smaller practices that will be managed by the platform practice.
The number of private-equity deals increased continually through 2019, then plummeted in March because of COVID-19, but by the summer, activity began to rise again.
Physicians are very intrigued about selling to private-equity firms because they are known to pay the most for practices. But private-equity buyers focus on a fairly narrow group of specialties.
Generally, Sean Tinsley said, private-equity firms only look for pain, dermatology, and ophthalmology practices, but they have been starting to branch out to specialties such as gastroenterology. In 2018, there were only two private-equity deals for gastroenterology practices, but in 2019, there were 16, according to one assessment.
Private-equity firms buy very few of the practices they initially review, according to Mr. Fanburg. “Private equity negotiates with dozens or even hundreds of physician practices at a time, with only 1%-5% of those practices actually being acquired.”
The private-equity firm’s upfront payment to selling physicians is quite high, but then the physicians become employees of the new group and earn much less in compensation than they earned on this own.
“In order for the venture to get any value out of the acquisition, the doctors have to make less going forward than they did historically,” Mr. Dietrich said. That freed-up money boosts the value of the venture.
When the platform practice is sold – usually after 5 years or so – “chances are the management team will be replaced,” Mr. Fanburg said. “There could be new policies and objectives, which could mean a bumpy ride for physicians.”
Do you really want to sell?
“When a group of physicians comes to me asking for help selling their practice, my first question is, Why are you doing this?” Mr. Fanburg said. “You need a better reason for selling than just the money.
“Once you make the leap, there is a certain amount of autonomy you lose,” he continued. “The sale gives you an economic boost, but it may not be enough for the long haul. If you stay on with the buyer, your compensation is often lower. That makes sense if you’re retiring, but not if you’re a younger physician with many years of practice in the years ahead.
“When physicians say they see no other way out except to sell,” Mr. Fanburg said, “I tell them that their buyer will see a path to future growth for your practice. If you think reimbursements are getting worse, why are the buyers pressing ahead?”
A version of this article originally appeared on Medscape.com.
Immunotherapy could fill unmet need in leptomeningeal metastases
Results from the trial were reported at the Society for Immunotherapy of Cancer’s 35th Anniversary Annual Meeting.
“Unfortunately, when patients present with leptomeningeal disease, they usually have a poor prognosis. Their median survival is measured at 6-24 weeks,” commented lead study author Jarushka Naidoo, MBBCh, an adjunct assistant professor of oncology at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, and a consultant medical oncologist at Beaumont Hospital in Dublin.
“While there may be some standard approaches for how we treat leptomeningeal disease, there are no universal standard therapies that are efficacious across solid tumor types,” Dr. Naidoo added.
With this in mind, Dr. Naidoo and colleagues tested systemic pembrolizumab in a trial of patients with leptomeningeal metastases from solid tumors.
The trial closed early because of poor accrual, after enrolling 13 patients: 5 with breast carcinoma, 3 with high-grade glioma, 3 with non–small cell lung cancer, 1 with squamous cell carcinoma of the skin, and 1 with head and neck squamous carcinoma. Nine patients (69%) had received at least two prior lines of systemic therapy.
Response, safety, and biomarkers
Overall, five patients (38%) had a central nervous system response, as ascertained from radiologic response on MRI, cytologic response in cerebrospinal fluid (CSF), and/or clinical response in neurologic symptoms, Dr. Naidoo reported.
Two patients had a complete CNS response: a patient with squamous cell carcinoma of the skin, who was still alive at 3 years, and a patient with non–small cell lung cancer, who survived 9 months but succumbed to metastases elsewhere.
For the entire cohort, median CNS progression-free survival was 2.9 months, and median overall survival was 4.9 months.
“This is consistent with published prospective studies of systemic agents for leptomeningeal disease,” Dr. Naidoo pointed out. “Notably, even though numbers are small, we do see the tail-on-the-curve phenomenon in both of these survival curves, which is consistent with immune checkpoint blockade prospective studies.”
The rate of grade 3 or higher treatment-related adverse events was 15.4%, and there were no grade 3 or higher immune-related adverse events.
The number of patients was too small for formal correlational testing, but both patients who achieved a complete response developed immune-related adverse events.
The trial’s biomarker analyses showed that an aneuploidy assay using CSF tumor-derived DNA performed well at detecting leptomeningeal metastases, with sensitivity of 84.6%, compared with just 53.8% for CSF cytopathology (the current preferred method).
A multiplex assay of CSF cytokines identified similar baseline profiles for patients who went on to have responses and showed similar changes in profile (notably a reduction in proinflammatory cytokines) for the two patients who had complete responses.
Given the trial’s 38% CNS response rate, pembrolizumab “needs to be studied in larger populations of patients to confirm this result, but it could be used as a potential treatment option for patients with leptomeningeal disease from solid tumors,” Dr. Naidoo concluded. “Reassuringly, pembrolizumab was well tolerated, and this is extremely important in a patient population that is traditionally quite frail and in which other standard therapies that are used, such as high-dose methotrexate or intrathecal chemotherapy, are associated with far higher rates of toxicity.”
An unmet need
“Leptomeningeal metastasis is a strong unmet need, although its occurrence is fortunately quite rare,” commented Kim Margolin, MD, a clinical professor and medical oncologist at City of Hope National Medical Center in Duarte, Calif., who was not involved in this study.
The trial is noteworthy for showing activity of programmed death–1 (PD-1) blockade given only systemically and not with additional intrathecal therapy (as has been done in a concurrent study at MD Anderson Cancer Center) and for providing insight into various biomarkers, Dr. Margolin said in an interview.
“I cannot take a stand on author conclusions other than to agree it warrants further evaluation in carefully selected patients, and it would be great to compare something like peripheral PD-1 blockade alone versus in combination with intrathecal therapy versus a combination such as CTLA4 blockade plus PD-1 blockade such as our group and others have shown to have increased activity in CNS metastases over PD-1 block alone,” Dr. Margolin said.
“The drugs in this class are already approved, so there is no reason not to try them,” she noted.
However, patients with leptomeningeal metastases of melanoma, for example, are likely to have already received anti-PD-1 immunotherapy.
“So the settings in which off-the-shelf PD-1 blockade would be useful are extremely limited,” she concluded.
The current trial was funded by Merck, the National Institutes of Health, the Lung Cancer Foundation of America, the International Association for the Study of Lung Cancer, and Johns Hopkins University Seed Grants. Dr. Naidoo disclosed relationships with AstraZeneca, Merck, Bristol Myers Squibb, and Roche/Genentech. Dr. Margolin disclosed no relevant conflicts of interest.
SOURCE: Naidoo J et al. SITC 2020, Abstract 788.
Results from the trial were reported at the Society for Immunotherapy of Cancer’s 35th Anniversary Annual Meeting.
“Unfortunately, when patients present with leptomeningeal disease, they usually have a poor prognosis. Their median survival is measured at 6-24 weeks,” commented lead study author Jarushka Naidoo, MBBCh, an adjunct assistant professor of oncology at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, and a consultant medical oncologist at Beaumont Hospital in Dublin.
“While there may be some standard approaches for how we treat leptomeningeal disease, there are no universal standard therapies that are efficacious across solid tumor types,” Dr. Naidoo added.
With this in mind, Dr. Naidoo and colleagues tested systemic pembrolizumab in a trial of patients with leptomeningeal metastases from solid tumors.
The trial closed early because of poor accrual, after enrolling 13 patients: 5 with breast carcinoma, 3 with high-grade glioma, 3 with non–small cell lung cancer, 1 with squamous cell carcinoma of the skin, and 1 with head and neck squamous carcinoma. Nine patients (69%) had received at least two prior lines of systemic therapy.
Response, safety, and biomarkers
Overall, five patients (38%) had a central nervous system response, as ascertained from radiologic response on MRI, cytologic response in cerebrospinal fluid (CSF), and/or clinical response in neurologic symptoms, Dr. Naidoo reported.
Two patients had a complete CNS response: a patient with squamous cell carcinoma of the skin, who was still alive at 3 years, and a patient with non–small cell lung cancer, who survived 9 months but succumbed to metastases elsewhere.
For the entire cohort, median CNS progression-free survival was 2.9 months, and median overall survival was 4.9 months.
“This is consistent with published prospective studies of systemic agents for leptomeningeal disease,” Dr. Naidoo pointed out. “Notably, even though numbers are small, we do see the tail-on-the-curve phenomenon in both of these survival curves, which is consistent with immune checkpoint blockade prospective studies.”
The rate of grade 3 or higher treatment-related adverse events was 15.4%, and there were no grade 3 or higher immune-related adverse events.
The number of patients was too small for formal correlational testing, but both patients who achieved a complete response developed immune-related adverse events.
The trial’s biomarker analyses showed that an aneuploidy assay using CSF tumor-derived DNA performed well at detecting leptomeningeal metastases, with sensitivity of 84.6%, compared with just 53.8% for CSF cytopathology (the current preferred method).
A multiplex assay of CSF cytokines identified similar baseline profiles for patients who went on to have responses and showed similar changes in profile (notably a reduction in proinflammatory cytokines) for the two patients who had complete responses.
Given the trial’s 38% CNS response rate, pembrolizumab “needs to be studied in larger populations of patients to confirm this result, but it could be used as a potential treatment option for patients with leptomeningeal disease from solid tumors,” Dr. Naidoo concluded. “Reassuringly, pembrolizumab was well tolerated, and this is extremely important in a patient population that is traditionally quite frail and in which other standard therapies that are used, such as high-dose methotrexate or intrathecal chemotherapy, are associated with far higher rates of toxicity.”
An unmet need
“Leptomeningeal metastasis is a strong unmet need, although its occurrence is fortunately quite rare,” commented Kim Margolin, MD, a clinical professor and medical oncologist at City of Hope National Medical Center in Duarte, Calif., who was not involved in this study.
The trial is noteworthy for showing activity of programmed death–1 (PD-1) blockade given only systemically and not with additional intrathecal therapy (as has been done in a concurrent study at MD Anderson Cancer Center) and for providing insight into various biomarkers, Dr. Margolin said in an interview.
“I cannot take a stand on author conclusions other than to agree it warrants further evaluation in carefully selected patients, and it would be great to compare something like peripheral PD-1 blockade alone versus in combination with intrathecal therapy versus a combination such as CTLA4 blockade plus PD-1 blockade such as our group and others have shown to have increased activity in CNS metastases over PD-1 block alone,” Dr. Margolin said.
“The drugs in this class are already approved, so there is no reason not to try them,” she noted.
However, patients with leptomeningeal metastases of melanoma, for example, are likely to have already received anti-PD-1 immunotherapy.
“So the settings in which off-the-shelf PD-1 blockade would be useful are extremely limited,” she concluded.
The current trial was funded by Merck, the National Institutes of Health, the Lung Cancer Foundation of America, the International Association for the Study of Lung Cancer, and Johns Hopkins University Seed Grants. Dr. Naidoo disclosed relationships with AstraZeneca, Merck, Bristol Myers Squibb, and Roche/Genentech. Dr. Margolin disclosed no relevant conflicts of interest.
SOURCE: Naidoo J et al. SITC 2020, Abstract 788.
Results from the trial were reported at the Society for Immunotherapy of Cancer’s 35th Anniversary Annual Meeting.
“Unfortunately, when patients present with leptomeningeal disease, they usually have a poor prognosis. Their median survival is measured at 6-24 weeks,” commented lead study author Jarushka Naidoo, MBBCh, an adjunct assistant professor of oncology at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, and a consultant medical oncologist at Beaumont Hospital in Dublin.
“While there may be some standard approaches for how we treat leptomeningeal disease, there are no universal standard therapies that are efficacious across solid tumor types,” Dr. Naidoo added.
With this in mind, Dr. Naidoo and colleagues tested systemic pembrolizumab in a trial of patients with leptomeningeal metastases from solid tumors.
The trial closed early because of poor accrual, after enrolling 13 patients: 5 with breast carcinoma, 3 with high-grade glioma, 3 with non–small cell lung cancer, 1 with squamous cell carcinoma of the skin, and 1 with head and neck squamous carcinoma. Nine patients (69%) had received at least two prior lines of systemic therapy.
Response, safety, and biomarkers
Overall, five patients (38%) had a central nervous system response, as ascertained from radiologic response on MRI, cytologic response in cerebrospinal fluid (CSF), and/or clinical response in neurologic symptoms, Dr. Naidoo reported.
Two patients had a complete CNS response: a patient with squamous cell carcinoma of the skin, who was still alive at 3 years, and a patient with non–small cell lung cancer, who survived 9 months but succumbed to metastases elsewhere.
For the entire cohort, median CNS progression-free survival was 2.9 months, and median overall survival was 4.9 months.
“This is consistent with published prospective studies of systemic agents for leptomeningeal disease,” Dr. Naidoo pointed out. “Notably, even though numbers are small, we do see the tail-on-the-curve phenomenon in both of these survival curves, which is consistent with immune checkpoint blockade prospective studies.”
The rate of grade 3 or higher treatment-related adverse events was 15.4%, and there were no grade 3 or higher immune-related adverse events.
The number of patients was too small for formal correlational testing, but both patients who achieved a complete response developed immune-related adverse events.
The trial’s biomarker analyses showed that an aneuploidy assay using CSF tumor-derived DNA performed well at detecting leptomeningeal metastases, with sensitivity of 84.6%, compared with just 53.8% for CSF cytopathology (the current preferred method).
A multiplex assay of CSF cytokines identified similar baseline profiles for patients who went on to have responses and showed similar changes in profile (notably a reduction in proinflammatory cytokines) for the two patients who had complete responses.
Given the trial’s 38% CNS response rate, pembrolizumab “needs to be studied in larger populations of patients to confirm this result, but it could be used as a potential treatment option for patients with leptomeningeal disease from solid tumors,” Dr. Naidoo concluded. “Reassuringly, pembrolizumab was well tolerated, and this is extremely important in a patient population that is traditionally quite frail and in which other standard therapies that are used, such as high-dose methotrexate or intrathecal chemotherapy, are associated with far higher rates of toxicity.”
An unmet need
“Leptomeningeal metastasis is a strong unmet need, although its occurrence is fortunately quite rare,” commented Kim Margolin, MD, a clinical professor and medical oncologist at City of Hope National Medical Center in Duarte, Calif., who was not involved in this study.
The trial is noteworthy for showing activity of programmed death–1 (PD-1) blockade given only systemically and not with additional intrathecal therapy (as has been done in a concurrent study at MD Anderson Cancer Center) and for providing insight into various biomarkers, Dr. Margolin said in an interview.
“I cannot take a stand on author conclusions other than to agree it warrants further evaluation in carefully selected patients, and it would be great to compare something like peripheral PD-1 blockade alone versus in combination with intrathecal therapy versus a combination such as CTLA4 blockade plus PD-1 blockade such as our group and others have shown to have increased activity in CNS metastases over PD-1 block alone,” Dr. Margolin said.
“The drugs in this class are already approved, so there is no reason not to try them,” she noted.
However, patients with leptomeningeal metastases of melanoma, for example, are likely to have already received anti-PD-1 immunotherapy.
“So the settings in which off-the-shelf PD-1 blockade would be useful are extremely limited,” she concluded.
The current trial was funded by Merck, the National Institutes of Health, the Lung Cancer Foundation of America, the International Association for the Study of Lung Cancer, and Johns Hopkins University Seed Grants. Dr. Naidoo disclosed relationships with AstraZeneca, Merck, Bristol Myers Squibb, and Roche/Genentech. Dr. Margolin disclosed no relevant conflicts of interest.
SOURCE: Naidoo J et al. SITC 2020, Abstract 788.
FROM SITC 2020
Expert shares key facts about keloid therapy
although few understand what this process entails, according to Hilary E. Baldwin, MD, of Rutgers Robert Wood Johnson Medical School, New Brunswick, N.J.
A key point to keep in mind about keloids is that, while they result from trauma, however slight, trauma alone does not cause them, Dr. Baldwin said in a presentation at the virtual MedscapeLive’s annual Las Vegas Dermatology Seminar.
In general, people with darker skin form keloids more easily and consistently than those with lighter skin, but keloids in people with darker skin are often easier to treat, Dr. Baldwin added. Also worth noting is the fact that earlobe keloids recur less frequently, she said.
Most patients with keloids are not surgical candidates, and they need convincing to pursue alternative options, Dr. Baldwin said.
However, successful management of keloids starts with sorting out what the patient wants. Some want “eradication with normal skin,” which is not realistic, versus simply flattening, lightening, or eradication of the keloid and leaving a scar, she noted. “That skin is never going to look normal,” she said. “Very often, they don’t need the whole thing gone, they just want to be better, and not itch or cause them to think about it all the time.”
Quality clinical research on the management of keloids is limited, Dr. Baldwin continued. “If you are holding out for a good randomized, placebo-controlled, double-blind study with a healthy ‘N,’ adequate follow-up rational conclusions, don’t hold your breath,” she said. The few literature reviews on keloids in recent decades concluded that modalities used to treat keloids are based on anecdotal evidence rather than rigorous research, she noted.
Size (and shape) matters
The decision to cut a keloid depends on several factors, including lesion size, shape, age, and location, but especially patient commitment to follow up and postsurgery care, said Dr. Baldwin.
She noted that larger keloids are no more difficult to remove than smaller ones, and patients tend to be more satisfied with the outcome with larger keloids. In terms of shape, pedunculated lesions are most amenable to surgery because of their small footprint. “Often the base does not contain keloidal tissue, and the patient gets the maximum benefit for the least risk,” she said. In addition, the residue from the removal of large keloids is often more acceptable.
Options for adjunctive therapy when excising keloids include corticosteroids, radiation, interferon, pressure dressings, dextran hydrogel scaffolding, and possibly botulinum toxin A, Dr. Baldwin said.
Adjunctive treatment alternatives
Intralesional corticosteroids can prevent the recurrence of keloids, and Dr. Baldwin recommends a 40 mg/cc injection into the base and walls of the excision site immediately postop, with repeat injections every 2 weeks for 2 months regardless of the patient’s clinical appearance. However, appearance determines the dose and concentration during 6 months of monthly follow-up, she said.
Radiation therapy, while not an effective monotherapy for keloids, can be used as an adjunct. A short radiation treatment plan may improve compliance, and no local malignancies linked to radiation therapy for keloids have been reported, she said. Dr. Baldwin also shared details of using an in-office superficial radiation therapy with the SRT-100 device, which she said has shown some ability to reduce recurrence of keloids.
Interferon, which can reduce production of collagen and increase collagenase can be used in an amount of 1.5 million units per linear cm around the base and walls of a keloid excision (maximum is 5 million units a day). Be aware that patients can develop flulike symptoms within a day or so, and warn patients to take it easy and monitor for symptoms, she said.
Studies of imiquimod for keloid recurrence have yielded mixed results, and a 2020 literature review concluded that it is not recommended as a treatment option for keloids, said Dr. Baldwin. Pressure dressings also have not shown effectiveness on existing lesions.
Botulinum toxin A has been studied as a way to prevent hypertrophic scars and keloids and potentially for preventing recurrence by injecting at the wound edges, she said. A meta-analysis showed that botulinum toxin was superior to corticosteroids for treating keloids, but “there were a lot of problems with the studies,” she said.
One other option for postexcision keloid treatment is dextran hydrogel scaffolding, which involves a triple-stranded collagen denatured by heat, with the addition of dextran to form a scaffold for fibroblasts, Dr. Baldwin said. This product, when injected prior to the final closure of surgical excision of keloids, may improve outcomes in certain areas, such as the earlobe, she said.
Dr. Baldwin concluded with comments about preventing other keloids from getting out of hand, which is extraordinarily challenging. However, treatment with dupilumab might provide an answer, although data are limited and more research is needed. She cited a case study of a male patient who had severe atopic dermatitis, with two keloids that improved after 7 months on dupilumab. The Th2 cytokines interleukin (IL)–4 and IL-13 have been implicated as key mediators in the pathogenesis of fibroproliferative disorders, which may respond to dupilumab, which targets Th2, she noted.
Dr. Baldwin had no relevant financial conflicts to disclose.
MedscapeLive and this news organization are owned by the same parent company.
although few understand what this process entails, according to Hilary E. Baldwin, MD, of Rutgers Robert Wood Johnson Medical School, New Brunswick, N.J.
A key point to keep in mind about keloids is that, while they result from trauma, however slight, trauma alone does not cause them, Dr. Baldwin said in a presentation at the virtual MedscapeLive’s annual Las Vegas Dermatology Seminar.
In general, people with darker skin form keloids more easily and consistently than those with lighter skin, but keloids in people with darker skin are often easier to treat, Dr. Baldwin added. Also worth noting is the fact that earlobe keloids recur less frequently, she said.
Most patients with keloids are not surgical candidates, and they need convincing to pursue alternative options, Dr. Baldwin said.
However, successful management of keloids starts with sorting out what the patient wants. Some want “eradication with normal skin,” which is not realistic, versus simply flattening, lightening, or eradication of the keloid and leaving a scar, she noted. “That skin is never going to look normal,” she said. “Very often, they don’t need the whole thing gone, they just want to be better, and not itch or cause them to think about it all the time.”
Quality clinical research on the management of keloids is limited, Dr. Baldwin continued. “If you are holding out for a good randomized, placebo-controlled, double-blind study with a healthy ‘N,’ adequate follow-up rational conclusions, don’t hold your breath,” she said. The few literature reviews on keloids in recent decades concluded that modalities used to treat keloids are based on anecdotal evidence rather than rigorous research, she noted.
Size (and shape) matters
The decision to cut a keloid depends on several factors, including lesion size, shape, age, and location, but especially patient commitment to follow up and postsurgery care, said Dr. Baldwin.
She noted that larger keloids are no more difficult to remove than smaller ones, and patients tend to be more satisfied with the outcome with larger keloids. In terms of shape, pedunculated lesions are most amenable to surgery because of their small footprint. “Often the base does not contain keloidal tissue, and the patient gets the maximum benefit for the least risk,” she said. In addition, the residue from the removal of large keloids is often more acceptable.
Options for adjunctive therapy when excising keloids include corticosteroids, radiation, interferon, pressure dressings, dextran hydrogel scaffolding, and possibly botulinum toxin A, Dr. Baldwin said.
Adjunctive treatment alternatives
Intralesional corticosteroids can prevent the recurrence of keloids, and Dr. Baldwin recommends a 40 mg/cc injection into the base and walls of the excision site immediately postop, with repeat injections every 2 weeks for 2 months regardless of the patient’s clinical appearance. However, appearance determines the dose and concentration during 6 months of monthly follow-up, she said.
Radiation therapy, while not an effective monotherapy for keloids, can be used as an adjunct. A short radiation treatment plan may improve compliance, and no local malignancies linked to radiation therapy for keloids have been reported, she said. Dr. Baldwin also shared details of using an in-office superficial radiation therapy with the SRT-100 device, which she said has shown some ability to reduce recurrence of keloids.
Interferon, which can reduce production of collagen and increase collagenase can be used in an amount of 1.5 million units per linear cm around the base and walls of a keloid excision (maximum is 5 million units a day). Be aware that patients can develop flulike symptoms within a day or so, and warn patients to take it easy and monitor for symptoms, she said.
Studies of imiquimod for keloid recurrence have yielded mixed results, and a 2020 literature review concluded that it is not recommended as a treatment option for keloids, said Dr. Baldwin. Pressure dressings also have not shown effectiveness on existing lesions.
Botulinum toxin A has been studied as a way to prevent hypertrophic scars and keloids and potentially for preventing recurrence by injecting at the wound edges, she said. A meta-analysis showed that botulinum toxin was superior to corticosteroids for treating keloids, but “there were a lot of problems with the studies,” she said.
One other option for postexcision keloid treatment is dextran hydrogel scaffolding, which involves a triple-stranded collagen denatured by heat, with the addition of dextran to form a scaffold for fibroblasts, Dr. Baldwin said. This product, when injected prior to the final closure of surgical excision of keloids, may improve outcomes in certain areas, such as the earlobe, she said.
Dr. Baldwin concluded with comments about preventing other keloids from getting out of hand, which is extraordinarily challenging. However, treatment with dupilumab might provide an answer, although data are limited and more research is needed. She cited a case study of a male patient who had severe atopic dermatitis, with two keloids that improved after 7 months on dupilumab. The Th2 cytokines interleukin (IL)–4 and IL-13 have been implicated as key mediators in the pathogenesis of fibroproliferative disorders, which may respond to dupilumab, which targets Th2, she noted.
Dr. Baldwin had no relevant financial conflicts to disclose.
MedscapeLive and this news organization are owned by the same parent company.
although few understand what this process entails, according to Hilary E. Baldwin, MD, of Rutgers Robert Wood Johnson Medical School, New Brunswick, N.J.
A key point to keep in mind about keloids is that, while they result from trauma, however slight, trauma alone does not cause them, Dr. Baldwin said in a presentation at the virtual MedscapeLive’s annual Las Vegas Dermatology Seminar.
In general, people with darker skin form keloids more easily and consistently than those with lighter skin, but keloids in people with darker skin are often easier to treat, Dr. Baldwin added. Also worth noting is the fact that earlobe keloids recur less frequently, she said.
Most patients with keloids are not surgical candidates, and they need convincing to pursue alternative options, Dr. Baldwin said.
However, successful management of keloids starts with sorting out what the patient wants. Some want “eradication with normal skin,” which is not realistic, versus simply flattening, lightening, or eradication of the keloid and leaving a scar, she noted. “That skin is never going to look normal,” she said. “Very often, they don’t need the whole thing gone, they just want to be better, and not itch or cause them to think about it all the time.”
Quality clinical research on the management of keloids is limited, Dr. Baldwin continued. “If you are holding out for a good randomized, placebo-controlled, double-blind study with a healthy ‘N,’ adequate follow-up rational conclusions, don’t hold your breath,” she said. The few literature reviews on keloids in recent decades concluded that modalities used to treat keloids are based on anecdotal evidence rather than rigorous research, she noted.
Size (and shape) matters
The decision to cut a keloid depends on several factors, including lesion size, shape, age, and location, but especially patient commitment to follow up and postsurgery care, said Dr. Baldwin.
She noted that larger keloids are no more difficult to remove than smaller ones, and patients tend to be more satisfied with the outcome with larger keloids. In terms of shape, pedunculated lesions are most amenable to surgery because of their small footprint. “Often the base does not contain keloidal tissue, and the patient gets the maximum benefit for the least risk,” she said. In addition, the residue from the removal of large keloids is often more acceptable.
Options for adjunctive therapy when excising keloids include corticosteroids, radiation, interferon, pressure dressings, dextran hydrogel scaffolding, and possibly botulinum toxin A, Dr. Baldwin said.
Adjunctive treatment alternatives
Intralesional corticosteroids can prevent the recurrence of keloids, and Dr. Baldwin recommends a 40 mg/cc injection into the base and walls of the excision site immediately postop, with repeat injections every 2 weeks for 2 months regardless of the patient’s clinical appearance. However, appearance determines the dose and concentration during 6 months of monthly follow-up, she said.
Radiation therapy, while not an effective monotherapy for keloids, can be used as an adjunct. A short radiation treatment plan may improve compliance, and no local malignancies linked to radiation therapy for keloids have been reported, she said. Dr. Baldwin also shared details of using an in-office superficial radiation therapy with the SRT-100 device, which she said has shown some ability to reduce recurrence of keloids.
Interferon, which can reduce production of collagen and increase collagenase can be used in an amount of 1.5 million units per linear cm around the base and walls of a keloid excision (maximum is 5 million units a day). Be aware that patients can develop flulike symptoms within a day or so, and warn patients to take it easy and monitor for symptoms, she said.
Studies of imiquimod for keloid recurrence have yielded mixed results, and a 2020 literature review concluded that it is not recommended as a treatment option for keloids, said Dr. Baldwin. Pressure dressings also have not shown effectiveness on existing lesions.
Botulinum toxin A has been studied as a way to prevent hypertrophic scars and keloids and potentially for preventing recurrence by injecting at the wound edges, she said. A meta-analysis showed that botulinum toxin was superior to corticosteroids for treating keloids, but “there were a lot of problems with the studies,” she said.
One other option for postexcision keloid treatment is dextran hydrogel scaffolding, which involves a triple-stranded collagen denatured by heat, with the addition of dextran to form a scaffold for fibroblasts, Dr. Baldwin said. This product, when injected prior to the final closure of surgical excision of keloids, may improve outcomes in certain areas, such as the earlobe, she said.
Dr. Baldwin concluded with comments about preventing other keloids from getting out of hand, which is extraordinarily challenging. However, treatment with dupilumab might provide an answer, although data are limited and more research is needed. She cited a case study of a male patient who had severe atopic dermatitis, with two keloids that improved after 7 months on dupilumab. The Th2 cytokines interleukin (IL)–4 and IL-13 have been implicated as key mediators in the pathogenesis of fibroproliferative disorders, which may respond to dupilumab, which targets Th2, she noted.
Dr. Baldwin had no relevant financial conflicts to disclose.
MedscapeLive and this news organization are owned by the same parent company.
FROM MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Food preservative for early psychosis: Final word?
Adjunctive use of sodium benzoate (BZ), a common food preservative that has previously shown promise in the treatment of chronic refractory psychosis, appears to be ineffective in the early stages of the disorder, new research suggests.
Results of a randomized controlled trial show the agent was no more effective than placebo in reducing early psychosis symptoms, although it was safe and well tolerated.
“Both groups of patients improved over the 12 weeks of the study, [suggesting] that most people with early psychosis will get well with antipsychotic medication and psychosocial interventions and adding sodium benzoate to their treatment does not add any additional benefits,” the study’s lead author, James Scott, MBBS, PhD, head of mental health research, QIMR Berghofer Medical Research Institute, Herston, Australia, told Medscape Medical News.
The paper was published online November 10 in JAMA Network Open.
Positive outcomes in chronic disease
Despite treatment with antipsychotics, many patients with psychosis experience persistent impairment, the investigators note.
Most antipsychotics are dopaminergic in action, but it is now recognized that the pathophysiology underlying psychosis extends beyond dopaminergic dysregulation with hypofunction of the N-methyl-D-aspartate (NMDA) receptors also implicated but not addressed by standard antipsychotics, they add.
NMDA receptors consist of two main subunits – the glutamate and glycine-binding sites. D-amino acids (DAAs) are agonists of the glycine subunit and have shown promise as adjunctive therapies for the treatment of schizophrenia, the investigators note.
DAAs are subject to oxidation by the flavoenzyme D-amino acid oxidase (DAAO). The oxidation limits their bioavailability and can cause nephrotoxic side effects. The food preservative BZ, which is not related to the benzodiazepine class of medications, inhibits DAAO and therefore may make DAAs safer and more effective.
Scott noted that two previous trials of BZ – a 2013 study and a 2017 investigation – in chronic, treatment-refractory schizophrenia have “reported excellent outcomes with significant improvement in clinical symptoms.”
“We saw that sodium benzoate was a safe and well-tolerated agent, and we thought it was important to conduct a trial of this medication in people in the early stages of psychotic illness,” he said.
To investigate, the researchers randomly assigned 100 individuals who were experiencing early psychosis, which was defined as illness onset within the last 2 years, to receive either 500 mg of BZ twice daily or placebo for 12 weeks.
Participants (mean [SD] age 21.4 [4.1] years, 73% male) were required to be taking antipsychotic medications for at least 1 continuous month during the previous 2 years and to be free of comorbid physical illnesses requiring additional treatment or hospitalization.
Most participants (84%) had schizophrenia and the remainder had affective psychoses. Most participants (88%) lived independently.
The BZ and the placebo groups were similar with respect to baseline characteristics, except that the mean waist circumference was higher in the placebo group than in the BZ group.
The majority of patients were being treated with antipsychotics alone (83%), followed by antipsychotics in combination with mood stabilizers (13%) and a small number were taking mood stabilizers alone (4%). The most commonly used antipsychotics were olanzapine and aripiprazole.
Not recommended
Psychosis was confirmed using the Positive and Negative Syndrome Scale (PANSS), and the inclusion criteria was a baseline score of ≥ 55. Secondary outcomes were scores on the Hamilton Depression Rating Scale, the clinician-rated Global Assessment of Function, and the Assessment of Quality of Life Scale.
The researchers also measured concentrations of the amino acids oxidized by DAAO (D-alanine and L-alanine, D-serine and L-serine).
Although both groups experienced a reduction in total PANSS scores during the study, there were no significant differences in PANSS total score between the BZ and the placebo groups at 12 weeks (endpoint least-square mean difference [SE] −1.2 [2.4] t = −0.49, P = .63).
There were also no significant differences between the groups on all PANSS subscales as well as any of the secondary clinical measures (P < .007).
A total of 122 adverse events (AEs) overall were reported by 66 participants, but rates of AEs were comparable between the BZ and placebo groups at 55% vs. 46% respectively. There were 11 serious AEs reported by 10 participants. Only one of these was related to the study drug.
There were no statistically significant changes in amino acid concentrations between the two groups.
The authors note several limitations of the study, including the possibility that protective agents may need longer times than 12 weeks – possibly as long as 6 to 12 months – to show efficacy. Moreover, the dose of BZ needed to produce a response remains “uncertain.”
“ and should further investigate whether benzoate acts by altering amino acid levels or by reducing oxidative stress in people with schizophrenia,” the authors suggest.
Scott added that further research in patients with treatment-refractory schizophrenia is needed to determine whether BZ “has a role in this patient population.”
The authors conclude that at present, “the routine use of this agent as an adjunctive treatment for early psychosis is not recommended.”
No surprise?
Commenting on the study for Medscape Medical News, Kenji Hashimoto, PhD, Division of Clinical Neuroscience, Chiba University Center for Forensic Mental Health, Chiba, Japan, said that, although previous studies of BZ showed benefit in stable patients with chronic schizophrenia, “it is unlikely that [sodium] benzoate may have beneficial effects in the acute phase of psychosis.”
Hashimoto, who was not involved with the study, noted that BZ is a “weak DAAO inhibitor and that DAAO expression in the frontal cortex of human beings is very low.”
This project was supported by a John Cade Fellowship from the National Health and Medical Research Council and support from the Queensland Centre for Mental Health Research, which receives funding from the Queensland Health Department. Scott is supported by an NHMRC Practitioner Fellowship. The other authors’ disclosures are listed on the original article. Hashimoto has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Adjunctive use of sodium benzoate (BZ), a common food preservative that has previously shown promise in the treatment of chronic refractory psychosis, appears to be ineffective in the early stages of the disorder, new research suggests.
Results of a randomized controlled trial show the agent was no more effective than placebo in reducing early psychosis symptoms, although it was safe and well tolerated.
“Both groups of patients improved over the 12 weeks of the study, [suggesting] that most people with early psychosis will get well with antipsychotic medication and psychosocial interventions and adding sodium benzoate to their treatment does not add any additional benefits,” the study’s lead author, James Scott, MBBS, PhD, head of mental health research, QIMR Berghofer Medical Research Institute, Herston, Australia, told Medscape Medical News.
The paper was published online November 10 in JAMA Network Open.
Positive outcomes in chronic disease
Despite treatment with antipsychotics, many patients with psychosis experience persistent impairment, the investigators note.
Most antipsychotics are dopaminergic in action, but it is now recognized that the pathophysiology underlying psychosis extends beyond dopaminergic dysregulation with hypofunction of the N-methyl-D-aspartate (NMDA) receptors also implicated but not addressed by standard antipsychotics, they add.
NMDA receptors consist of two main subunits – the glutamate and glycine-binding sites. D-amino acids (DAAs) are agonists of the glycine subunit and have shown promise as adjunctive therapies for the treatment of schizophrenia, the investigators note.
DAAs are subject to oxidation by the flavoenzyme D-amino acid oxidase (DAAO). The oxidation limits their bioavailability and can cause nephrotoxic side effects. The food preservative BZ, which is not related to the benzodiazepine class of medications, inhibits DAAO and therefore may make DAAs safer and more effective.
Scott noted that two previous trials of BZ – a 2013 study and a 2017 investigation – in chronic, treatment-refractory schizophrenia have “reported excellent outcomes with significant improvement in clinical symptoms.”
“We saw that sodium benzoate was a safe and well-tolerated agent, and we thought it was important to conduct a trial of this medication in people in the early stages of psychotic illness,” he said.
To investigate, the researchers randomly assigned 100 individuals who were experiencing early psychosis, which was defined as illness onset within the last 2 years, to receive either 500 mg of BZ twice daily or placebo for 12 weeks.
Participants (mean [SD] age 21.4 [4.1] years, 73% male) were required to be taking antipsychotic medications for at least 1 continuous month during the previous 2 years and to be free of comorbid physical illnesses requiring additional treatment or hospitalization.
Most participants (84%) had schizophrenia and the remainder had affective psychoses. Most participants (88%) lived independently.
The BZ and the placebo groups were similar with respect to baseline characteristics, except that the mean waist circumference was higher in the placebo group than in the BZ group.
The majority of patients were being treated with antipsychotics alone (83%), followed by antipsychotics in combination with mood stabilizers (13%) and a small number were taking mood stabilizers alone (4%). The most commonly used antipsychotics were olanzapine and aripiprazole.
Not recommended
Psychosis was confirmed using the Positive and Negative Syndrome Scale (PANSS), and the inclusion criteria was a baseline score of ≥ 55. Secondary outcomes were scores on the Hamilton Depression Rating Scale, the clinician-rated Global Assessment of Function, and the Assessment of Quality of Life Scale.
The researchers also measured concentrations of the amino acids oxidized by DAAO (D-alanine and L-alanine, D-serine and L-serine).
Although both groups experienced a reduction in total PANSS scores during the study, there were no significant differences in PANSS total score between the BZ and the placebo groups at 12 weeks (endpoint least-square mean difference [SE] −1.2 [2.4] t = −0.49, P = .63).
There were also no significant differences between the groups on all PANSS subscales as well as any of the secondary clinical measures (P < .007).
A total of 122 adverse events (AEs) overall were reported by 66 participants, but rates of AEs were comparable between the BZ and placebo groups at 55% vs. 46% respectively. There were 11 serious AEs reported by 10 participants. Only one of these was related to the study drug.
There were no statistically significant changes in amino acid concentrations between the two groups.
The authors note several limitations of the study, including the possibility that protective agents may need longer times than 12 weeks – possibly as long as 6 to 12 months – to show efficacy. Moreover, the dose of BZ needed to produce a response remains “uncertain.”
“ and should further investigate whether benzoate acts by altering amino acid levels or by reducing oxidative stress in people with schizophrenia,” the authors suggest.
Scott added that further research in patients with treatment-refractory schizophrenia is needed to determine whether BZ “has a role in this patient population.”
The authors conclude that at present, “the routine use of this agent as an adjunctive treatment for early psychosis is not recommended.”
No surprise?
Commenting on the study for Medscape Medical News, Kenji Hashimoto, PhD, Division of Clinical Neuroscience, Chiba University Center for Forensic Mental Health, Chiba, Japan, said that, although previous studies of BZ showed benefit in stable patients with chronic schizophrenia, “it is unlikely that [sodium] benzoate may have beneficial effects in the acute phase of psychosis.”
Hashimoto, who was not involved with the study, noted that BZ is a “weak DAAO inhibitor and that DAAO expression in the frontal cortex of human beings is very low.”
This project was supported by a John Cade Fellowship from the National Health and Medical Research Council and support from the Queensland Centre for Mental Health Research, which receives funding from the Queensland Health Department. Scott is supported by an NHMRC Practitioner Fellowship. The other authors’ disclosures are listed on the original article. Hashimoto has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Adjunctive use of sodium benzoate (BZ), a common food preservative that has previously shown promise in the treatment of chronic refractory psychosis, appears to be ineffective in the early stages of the disorder, new research suggests.
Results of a randomized controlled trial show the agent was no more effective than placebo in reducing early psychosis symptoms, although it was safe and well tolerated.
“Both groups of patients improved over the 12 weeks of the study, [suggesting] that most people with early psychosis will get well with antipsychotic medication and psychosocial interventions and adding sodium benzoate to their treatment does not add any additional benefits,” the study’s lead author, James Scott, MBBS, PhD, head of mental health research, QIMR Berghofer Medical Research Institute, Herston, Australia, told Medscape Medical News.
The paper was published online November 10 in JAMA Network Open.
Positive outcomes in chronic disease
Despite treatment with antipsychotics, many patients with psychosis experience persistent impairment, the investigators note.
Most antipsychotics are dopaminergic in action, but it is now recognized that the pathophysiology underlying psychosis extends beyond dopaminergic dysregulation with hypofunction of the N-methyl-D-aspartate (NMDA) receptors also implicated but not addressed by standard antipsychotics, they add.
NMDA receptors consist of two main subunits – the glutamate and glycine-binding sites. D-amino acids (DAAs) are agonists of the glycine subunit and have shown promise as adjunctive therapies for the treatment of schizophrenia, the investigators note.
DAAs are subject to oxidation by the flavoenzyme D-amino acid oxidase (DAAO). The oxidation limits their bioavailability and can cause nephrotoxic side effects. The food preservative BZ, which is not related to the benzodiazepine class of medications, inhibits DAAO and therefore may make DAAs safer and more effective.
Scott noted that two previous trials of BZ – a 2013 study and a 2017 investigation – in chronic, treatment-refractory schizophrenia have “reported excellent outcomes with significant improvement in clinical symptoms.”
“We saw that sodium benzoate was a safe and well-tolerated agent, and we thought it was important to conduct a trial of this medication in people in the early stages of psychotic illness,” he said.
To investigate, the researchers randomly assigned 100 individuals who were experiencing early psychosis, which was defined as illness onset within the last 2 years, to receive either 500 mg of BZ twice daily or placebo for 12 weeks.
Participants (mean [SD] age 21.4 [4.1] years, 73% male) were required to be taking antipsychotic medications for at least 1 continuous month during the previous 2 years and to be free of comorbid physical illnesses requiring additional treatment or hospitalization.
Most participants (84%) had schizophrenia and the remainder had affective psychoses. Most participants (88%) lived independently.
The BZ and the placebo groups were similar with respect to baseline characteristics, except that the mean waist circumference was higher in the placebo group than in the BZ group.
The majority of patients were being treated with antipsychotics alone (83%), followed by antipsychotics in combination with mood stabilizers (13%) and a small number were taking mood stabilizers alone (4%). The most commonly used antipsychotics were olanzapine and aripiprazole.
Not recommended
Psychosis was confirmed using the Positive and Negative Syndrome Scale (PANSS), and the inclusion criteria was a baseline score of ≥ 55. Secondary outcomes were scores on the Hamilton Depression Rating Scale, the clinician-rated Global Assessment of Function, and the Assessment of Quality of Life Scale.
The researchers also measured concentrations of the amino acids oxidized by DAAO (D-alanine and L-alanine, D-serine and L-serine).
Although both groups experienced a reduction in total PANSS scores during the study, there were no significant differences in PANSS total score between the BZ and the placebo groups at 12 weeks (endpoint least-square mean difference [SE] −1.2 [2.4] t = −0.49, P = .63).
There were also no significant differences between the groups on all PANSS subscales as well as any of the secondary clinical measures (P < .007).
A total of 122 adverse events (AEs) overall were reported by 66 participants, but rates of AEs were comparable between the BZ and placebo groups at 55% vs. 46% respectively. There were 11 serious AEs reported by 10 participants. Only one of these was related to the study drug.
There were no statistically significant changes in amino acid concentrations between the two groups.
The authors note several limitations of the study, including the possibility that protective agents may need longer times than 12 weeks – possibly as long as 6 to 12 months – to show efficacy. Moreover, the dose of BZ needed to produce a response remains “uncertain.”
“ and should further investigate whether benzoate acts by altering amino acid levels or by reducing oxidative stress in people with schizophrenia,” the authors suggest.
Scott added that further research in patients with treatment-refractory schizophrenia is needed to determine whether BZ “has a role in this patient population.”
The authors conclude that at present, “the routine use of this agent as an adjunctive treatment for early psychosis is not recommended.”
No surprise?
Commenting on the study for Medscape Medical News, Kenji Hashimoto, PhD, Division of Clinical Neuroscience, Chiba University Center for Forensic Mental Health, Chiba, Japan, said that, although previous studies of BZ showed benefit in stable patients with chronic schizophrenia, “it is unlikely that [sodium] benzoate may have beneficial effects in the acute phase of psychosis.”
Hashimoto, who was not involved with the study, noted that BZ is a “weak DAAO inhibitor and that DAAO expression in the frontal cortex of human beings is very low.”
This project was supported by a John Cade Fellowship from the National Health and Medical Research Council and support from the Queensland Centre for Mental Health Research, which receives funding from the Queensland Health Department. Scott is supported by an NHMRC Practitioner Fellowship. The other authors’ disclosures are listed on the original article. Hashimoto has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Pfizer files for FDA emergency use authorization of COVID vaccine
Pfizer and its German partner BioNTech have filed an application with the US Food and Drug Administration (FDA) for an emergency use authorization of its vaccine against COVID-19, the disease caused by SARS-CoV-2, according to a company news release.
It is the latest step in what has been an extraordinarily fast-paced development and testing process, with the companies having reported interim results of phase 3 trials on November 9 and final results this past Wednesday, as reported by Medscape Medical News. The vaccine, BNT162b2, which uses a messenger RNA-based platform, was ultimately found to have 95% efficacy and more than 94% efficacy in individuals over age 65.
“The process of the speed did not compromise at all safety, nor did it compromise scientific integrity,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases at a White House press briefing yesterday.
“We need to put to rest any concept that this was rushed in an inappropriate way,” he said. “This is really solid.”
Pfizer and BioNTech said they believe they have met the FDA’s safety data requirements for emergency use authorization (EUA). The agency in October outlined its expectations for safety and efficacy to secure an EUA.
“Filing in the US represents a critical milestone in our journey to deliver a COVID-19 vaccine to the world, and we now have a more complete picture of both the efficacy and safety profile of our vaccine, giving us confidence in its potential,” said Albert Bourla, MD, Pfizer’s chairman and CEO, in its release.
The FDA is expected to hold a meeting of its Vaccines and Related Biological Products Advisory Committee sometime in December to review the safety and efficacy data in the companies’ application. The committee will review:
- Efficacy data from a total 170 confirmed cases of COVID-19 in the phase 3 study.
- Safety data from a randomly assigned subset of 8000 participants 18 years and older.
- Data on 19,000 enrollees who have been followed for a median of 2 months after the second and final dose.
- Data on the manufacturing processes.
According to Pfizer, the companies plan to submit the efficacy and safety data to a peer-reviewed journal once they have completed their analysis.
Vaccine logistics
The companies — which funded their own trials — signed an agreement with the US government’s Operation Warp Speed program in July to provide 100 million doses of its vaccine following FDA authorization or approval in exchange for $1.95 billion. The US government has the option to acquire up to 500 million more doses.
Pfizer and BioNTech said they will be able to supply 50 million doses globally in 2020 and up to 1.3 billion doses by the end of 2021. The vaccine must be given in two doses, spaced 21 days apart. Pfizer expects to be ready to distribute the vaccine within hours after FDA authorization.
The US government is still on track to deliver the Pfizer vaccine within 24 hours of an FDA authorization, said Operation Warp Speed’s Chief Operating Officer Gen. Gustave F. Perna at yesterday’s White House briefing.
Vice President Mike Pence emphasized that point at the briefing: “The moment that the FDA concludes that that vaccine is safe and effective, we have a system in place to begin within 24 hours shipping that vaccine to hospitals, healthcare facilities and, 24 hours after that, literally injecting that vaccine into Americans,” he said.
The vaccine will be pushed out through 64 jurisdictions already part of the Centers for Disease Control and Prevention’s vaccines for children distribution program, and will likely be divided up according to population, said Perna.
Pfizer’s vaccine must be shipped and stored at –70°C (–94°F), which has presented logistical and storage issues. The company is testing out delivery methods, including a pilot delivery program in New Mexico, Rhode Island, Tennessee, and Texas that will be active after an FDA authorization. States, hospitals, and pharmacy chains are also buying special freezers.
The National Academies of Sciences, Engineering, and Medicine issued recommendations in October that healthcare workers, first responders, older Americans living in congregate settings (eg, nursing homes), and people with underlying health conditions be the first to receive a coronavirus vaccine. The CDC’s Advisory Committee on Immunization Practices will also be issuing recommendations as soon as the FDA authorizes a vaccine.
Pfizer and BioNTech are also seeking approval for the vaccine with several regulatory agencies around the world, including the European Medicines Agency and the Medicines & Healthcare Products Regulatory Agency (MHRA) in the United Kingdom.
This article first appeared on Medscape.com.
Pfizer and its German partner BioNTech have filed an application with the US Food and Drug Administration (FDA) for an emergency use authorization of its vaccine against COVID-19, the disease caused by SARS-CoV-2, according to a company news release.
It is the latest step in what has been an extraordinarily fast-paced development and testing process, with the companies having reported interim results of phase 3 trials on November 9 and final results this past Wednesday, as reported by Medscape Medical News. The vaccine, BNT162b2, which uses a messenger RNA-based platform, was ultimately found to have 95% efficacy and more than 94% efficacy in individuals over age 65.
“The process of the speed did not compromise at all safety, nor did it compromise scientific integrity,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases at a White House press briefing yesterday.
“We need to put to rest any concept that this was rushed in an inappropriate way,” he said. “This is really solid.”
Pfizer and BioNTech said they believe they have met the FDA’s safety data requirements for emergency use authorization (EUA). The agency in October outlined its expectations for safety and efficacy to secure an EUA.
“Filing in the US represents a critical milestone in our journey to deliver a COVID-19 vaccine to the world, and we now have a more complete picture of both the efficacy and safety profile of our vaccine, giving us confidence in its potential,” said Albert Bourla, MD, Pfizer’s chairman and CEO, in its release.
The FDA is expected to hold a meeting of its Vaccines and Related Biological Products Advisory Committee sometime in December to review the safety and efficacy data in the companies’ application. The committee will review:
- Efficacy data from a total 170 confirmed cases of COVID-19 in the phase 3 study.
- Safety data from a randomly assigned subset of 8000 participants 18 years and older.
- Data on 19,000 enrollees who have been followed for a median of 2 months after the second and final dose.
- Data on the manufacturing processes.
According to Pfizer, the companies plan to submit the efficacy and safety data to a peer-reviewed journal once they have completed their analysis.
Vaccine logistics
The companies — which funded their own trials — signed an agreement with the US government’s Operation Warp Speed program in July to provide 100 million doses of its vaccine following FDA authorization or approval in exchange for $1.95 billion. The US government has the option to acquire up to 500 million more doses.
Pfizer and BioNTech said they will be able to supply 50 million doses globally in 2020 and up to 1.3 billion doses by the end of 2021. The vaccine must be given in two doses, spaced 21 days apart. Pfizer expects to be ready to distribute the vaccine within hours after FDA authorization.
The US government is still on track to deliver the Pfizer vaccine within 24 hours of an FDA authorization, said Operation Warp Speed’s Chief Operating Officer Gen. Gustave F. Perna at yesterday’s White House briefing.
Vice President Mike Pence emphasized that point at the briefing: “The moment that the FDA concludes that that vaccine is safe and effective, we have a system in place to begin within 24 hours shipping that vaccine to hospitals, healthcare facilities and, 24 hours after that, literally injecting that vaccine into Americans,” he said.
The vaccine will be pushed out through 64 jurisdictions already part of the Centers for Disease Control and Prevention’s vaccines for children distribution program, and will likely be divided up according to population, said Perna.
Pfizer’s vaccine must be shipped and stored at –70°C (–94°F), which has presented logistical and storage issues. The company is testing out delivery methods, including a pilot delivery program in New Mexico, Rhode Island, Tennessee, and Texas that will be active after an FDA authorization. States, hospitals, and pharmacy chains are also buying special freezers.
The National Academies of Sciences, Engineering, and Medicine issued recommendations in October that healthcare workers, first responders, older Americans living in congregate settings (eg, nursing homes), and people with underlying health conditions be the first to receive a coronavirus vaccine. The CDC’s Advisory Committee on Immunization Practices will also be issuing recommendations as soon as the FDA authorizes a vaccine.
Pfizer and BioNTech are also seeking approval for the vaccine with several regulatory agencies around the world, including the European Medicines Agency and the Medicines & Healthcare Products Regulatory Agency (MHRA) in the United Kingdom.
This article first appeared on Medscape.com.
Pfizer and its German partner BioNTech have filed an application with the US Food and Drug Administration (FDA) for an emergency use authorization of its vaccine against COVID-19, the disease caused by SARS-CoV-2, according to a company news release.
It is the latest step in what has been an extraordinarily fast-paced development and testing process, with the companies having reported interim results of phase 3 trials on November 9 and final results this past Wednesday, as reported by Medscape Medical News. The vaccine, BNT162b2, which uses a messenger RNA-based platform, was ultimately found to have 95% efficacy and more than 94% efficacy in individuals over age 65.
“The process of the speed did not compromise at all safety, nor did it compromise scientific integrity,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases at a White House press briefing yesterday.
“We need to put to rest any concept that this was rushed in an inappropriate way,” he said. “This is really solid.”
Pfizer and BioNTech said they believe they have met the FDA’s safety data requirements for emergency use authorization (EUA). The agency in October outlined its expectations for safety and efficacy to secure an EUA.
“Filing in the US represents a critical milestone in our journey to deliver a COVID-19 vaccine to the world, and we now have a more complete picture of both the efficacy and safety profile of our vaccine, giving us confidence in its potential,” said Albert Bourla, MD, Pfizer’s chairman and CEO, in its release.
The FDA is expected to hold a meeting of its Vaccines and Related Biological Products Advisory Committee sometime in December to review the safety and efficacy data in the companies’ application. The committee will review:
- Efficacy data from a total 170 confirmed cases of COVID-19 in the phase 3 study.
- Safety data from a randomly assigned subset of 8000 participants 18 years and older.
- Data on 19,000 enrollees who have been followed for a median of 2 months after the second and final dose.
- Data on the manufacturing processes.
According to Pfizer, the companies plan to submit the efficacy and safety data to a peer-reviewed journal once they have completed their analysis.
Vaccine logistics
The companies — which funded their own trials — signed an agreement with the US government’s Operation Warp Speed program in July to provide 100 million doses of its vaccine following FDA authorization or approval in exchange for $1.95 billion. The US government has the option to acquire up to 500 million more doses.
Pfizer and BioNTech said they will be able to supply 50 million doses globally in 2020 and up to 1.3 billion doses by the end of 2021. The vaccine must be given in two doses, spaced 21 days apart. Pfizer expects to be ready to distribute the vaccine within hours after FDA authorization.
The US government is still on track to deliver the Pfizer vaccine within 24 hours of an FDA authorization, said Operation Warp Speed’s Chief Operating Officer Gen. Gustave F. Perna at yesterday’s White House briefing.
Vice President Mike Pence emphasized that point at the briefing: “The moment that the FDA concludes that that vaccine is safe and effective, we have a system in place to begin within 24 hours shipping that vaccine to hospitals, healthcare facilities and, 24 hours after that, literally injecting that vaccine into Americans,” he said.
The vaccine will be pushed out through 64 jurisdictions already part of the Centers for Disease Control and Prevention’s vaccines for children distribution program, and will likely be divided up according to population, said Perna.
Pfizer’s vaccine must be shipped and stored at –70°C (–94°F), which has presented logistical and storage issues. The company is testing out delivery methods, including a pilot delivery program in New Mexico, Rhode Island, Tennessee, and Texas that will be active after an FDA authorization. States, hospitals, and pharmacy chains are also buying special freezers.
The National Academies of Sciences, Engineering, and Medicine issued recommendations in October that healthcare workers, first responders, older Americans living in congregate settings (eg, nursing homes), and people with underlying health conditions be the first to receive a coronavirus vaccine. The CDC’s Advisory Committee on Immunization Practices will also be issuing recommendations as soon as the FDA authorizes a vaccine.
Pfizer and BioNTech are also seeking approval for the vaccine with several regulatory agencies around the world, including the European Medicines Agency and the Medicines & Healthcare Products Regulatory Agency (MHRA) in the United Kingdom.
This article first appeared on Medscape.com.