User login
What’s Eating You? The South African Fattail Scorpion Revisited
Identification
The South African fattail scorpion (Parabuthus transvaalicus)(Figure) is one of the most poisonous scorpions in southern Africa.1 A member of the Buthidae scorpion family, it can grow as long as 15 cm and is dark brown-black with lighter red-brown pincers. Similar to other fattail scorpions, it has slender pincers (pedipalps) and a thick square tail (the telson). Parabuthus transvaalicus inhabits hot dry deserts, scrublands, and semiarid regions.1,2 It also is popular in exotic pet collections, the most common source of stings in the United States.

Stings and Envenomation
Scorpions with thicker tails generally have more potent venom than those with slender tails and thick pincers. Venom is injected by a stinger at the tip of the telson1; P transvaalicus also can spray venom as far as 3 m.1,2 Venom is not known to cause toxicity through skin contact but could represent a hazard if sprayed in the eye.
Scorpion toxins are a group of complex neurotoxins that act on sodium channels, either retarding inactivation (α toxin) or enhancing activation (β toxin), causing massive depolarization of excitable cells.1,3 The toxin causes neurons to fire repetitively.4 Neurotransmitters—noradrenaline, adrenaline, and acetylcholine—cause the observed sympathetic, parasympathetic, and skeletal muscle effects.1
Incidence
Worldwide, more than 1.2 million individuals are stung by a scorpion annually, causing more than 3250 deaths a year.5 Adults are stung more often, but children experience more severe envenomation, are more likely to develop severe illness requiring intensive supportive care, and have a higher mortality.4
As many as one-third of patients stung by a Parabuthus scorpion develop neuromuscular toxicity, which can be life-threatening.6 In a study of 277 envenomations by P transvaalicus, 10% of patients developed severe symptoms and 5 died. Children younger than 10 years and adults older than 50 years are at greatest risk for
Clinical Presentation
The clinical presentation of scorpion envenomation varies with the species involved, the amount of venom injected, and the victim’s weight and baseline health.1 Scorpion envenomation is divided into 4 grades based on the severity of a sting:
• Grade I: pain and paresthesia at the envenomation site; usually, no local inflammation
• Grade II: local symptoms as well as more remote pain and paresthesia; pain can radiate up the affected limb
• Grade III: cranial nerve or somatic skeletal neuromuscular dysfunction; either presentation can have associated autonomic dysfunction
• Grade IV: both cranial nerve and somatic skeletal neuromuscular dysfunction, with associated auto-nomic dysfunction
The initial symptom of a scorpion sting is intense burning pain. The sting site might be unimpressive, with only a mild local reaction. Symptoms usually progress to maximum severity within 5 hours.1 Muscle pain, cramps, and weakness are prominent. The patient might have difficulty walking and swallowing, with increased salivation and drooling, and visual disturbance with abnormal eye movements. Pulse, blood pressure, and temperature often are elevated. The patient might be hyperreflexic with clonus.1,6
Symptoms of increased sympathetic activity are hypertension, tachycardia, cardiac dysrhythmia, perspiration, hyperglycemia, and restlessness.1,2 Parasympathetic effects are increased salivation, hypotension, bradycardia, and gastric distension. Skeletal muscle effects include tremors and involuntary muscle movement, which can be severe. Cranial nerve dysfunction may manifest as dysphagia, drooling, abnormal eye movements, blurred vision, slurred speech, and tongue fasciculations. Subsequent development of muscle weakness, bulbar paralysis, and difficulty breathing may be caused by depletion of neurotransmitters after prolonged excessive neuronal activity.1
Distinctive Signs in Younger Patients
A child who is stung by a scorpion might have symptoms similar to those seen in an adult victim but can also experience an extreme form of restlessness that indicates severe envenomation characterized by inability to lay still, violent muscle twitching, and uncontrollable flailing of extremities. The child might have facial grimacing, with lip-smacking and chewing motions. In addition, bulbar paralysis and respiratory distress are more likely in children who have been stung than in adults.1,2
Management
Treatment of a P transvaalicus sting is directed at “scorpionism,” envenomation that is associated with systemic symptoms that can be life-threatening. Treatment comprises support of vital functions, symptomatic measures, and injection of antivenin.8
Support of Vital Functions
In adults, systemic symptoms can be delayed as long as 8 hours after the sting. However, most severe cases usually are evident within 60 minutes; infants can reach grade IV as quickly as 15 to 30 minutes.9,10 Loss of pharyngeal reflexes and development of respiratory distress are ominous warning signs requiring immediate respiratory support. Respiratory failure is the most common cause of death.1 An asymptomatic child should be admitted to a hospital for observation for a minimum of 12 hours if the species of scorpion was not identified.2
Pain Relief
Most patients cannot tolerate an ice pack because of severe hyperesthesia. Infiltration of the local sting site with an anesthetic generally is safe and can provide some local pain relief. Intravenous fentanyl has been used in closely monitored patients because the drug is not associated with histamine release. Medications that cause release of histamine, such as morphine, can exacerbate or confuse the clinical picture.
Antivenin
Scorpion antivenin contains purified IgG fragments; allergic reactions are now rare. The sooner antivenin is administered, the greater the benefit. When administered early, it can prevent many of the most serious complications.7 In a randomized, double-blind study of critically ill children with clinically significant signs of scorpion envenomation, intravenous administration of scorpion-specific fragment antigen-binding 2 (F[(ab’]2) antivenin resulted in resolution of clinical symptoms within 4 hours.11
When managing grade III or IV scorpion envenomation, all patients should be admitted to a medical facility equipped to provide intensive supportive care; consider consultation with a regional poison control center. The World Health Organization maintains an international poison control center (at https://www.who.int/ipcs/poisons/centre/en/) with regional telephone numbers; alternatively, in the United States, call the nationwide telephone number of the Poison Control Center (800-222-1222).
The World Health Organization has identified declining production of antivenin as a crisis.12
Resolution
Symptoms of envenomation typically resolve 9 to 30 hours after a sting in a patient with grade III or IV envenomation not treated with antivenin.4 However, pain and paresthesia occasionally last as long as 2 weeks. In rare cases, more long-term sequelae of burning paresthesia persist for months.4
Conclusion
It is important for dermatologists to be aware of the potential for life-threatening envenomation by certain scorpion species native to southern Africa. In the United States, stings of these species most often are seen in patients with a pet collection, but late sequelae also can be seen in travelers returning from an endemic region. The site of a sting often appears unimpressive initially, but severe hyperesthesia is common. Patients with cardiac, neurologic, or respiratory symptoms require intensive supportive care. Proper care can be lifesaving.
- Müller GJ, Modler H, Wium CA, et al. Scorpion sting in southern Africa: diagnosis and management. Continuing Medical Education. 2012;30:356-361.
- Müller GJ. Scorpionism in South Africa. a report of 42 serious scorpion envenomations. S Afr Med J. 1993;83:405-411.
- Quintero-Hernández V, Jiménez-Vargas JM, Gurrola GB, et al. Scorpion venom components that affect ion-channels function. Toxicon. 2013;76:328-342.
- LoVecchio F, McBride C. Scorpion envenomations in young children in central Arizona. J Toxicol Clin Toxicol. 2003;41:937-940.
- Chippaux JP, Goyffon M. Epidemiology of scorpionism: a global appraisal. Acta Trop. 2008;107:71-79.
- Bergman NJ. Clinical description of Parabuthus transvaalicus scorpionism in Zimbabwe. Toxicon. 1997;35:759-771.
- Chippaux JP. Emerging options for the management of scorpion stings. Drug Des Devel Ther. 2012;6:165-173.
- Santos MS, Silva CG, Neto BS, et al. Clinical and epidemiological aspects of scorpionism in the world: a systematic review. Wilderness Environ Med. 2016;27:504-518.
- Amaral CF, Rezende NA. Both cardiogenic and non-cardiogenic factors are involved in the pathogenesis of pulmonary oedema after scorpion envenoming. Toxicon. 1997;35:997-998.
- Bergman NJ. Scorpion sting in Zimbabwe. S Afr Med J. 1997;87:163-167.
- Boyer LV, Theodorou AA, Berg RA, et al; Arizona Envenomation Investigators. antivenom for critically ill children with neurotoxicity from scorpion stings. N Engl J Med. 2009;360:2090-2098.
- Theakston RD, Warrell DA, Griffiths E. Report of a WHO workshop on the standardization and control of antivenoms. Toxicon. 2003;41:541-557.
Identification
The South African fattail scorpion (Parabuthus transvaalicus)(Figure) is one of the most poisonous scorpions in southern Africa.1 A member of the Buthidae scorpion family, it can grow as long as 15 cm and is dark brown-black with lighter red-brown pincers. Similar to other fattail scorpions, it has slender pincers (pedipalps) and a thick square tail (the telson). Parabuthus transvaalicus inhabits hot dry deserts, scrublands, and semiarid regions.1,2 It also is popular in exotic pet collections, the most common source of stings in the United States.

Stings and Envenomation
Scorpions with thicker tails generally have more potent venom than those with slender tails and thick pincers. Venom is injected by a stinger at the tip of the telson1; P transvaalicus also can spray venom as far as 3 m.1,2 Venom is not known to cause toxicity through skin contact but could represent a hazard if sprayed in the eye.
Scorpion toxins are a group of complex neurotoxins that act on sodium channels, either retarding inactivation (α toxin) or enhancing activation (β toxin), causing massive depolarization of excitable cells.1,3 The toxin causes neurons to fire repetitively.4 Neurotransmitters—noradrenaline, adrenaline, and acetylcholine—cause the observed sympathetic, parasympathetic, and skeletal muscle effects.1
Incidence
Worldwide, more than 1.2 million individuals are stung by a scorpion annually, causing more than 3250 deaths a year.5 Adults are stung more often, but children experience more severe envenomation, are more likely to develop severe illness requiring intensive supportive care, and have a higher mortality.4
As many as one-third of patients stung by a Parabuthus scorpion develop neuromuscular toxicity, which can be life-threatening.6 In a study of 277 envenomations by P transvaalicus, 10% of patients developed severe symptoms and 5 died. Children younger than 10 years and adults older than 50 years are at greatest risk for
Clinical Presentation
The clinical presentation of scorpion envenomation varies with the species involved, the amount of venom injected, and the victim’s weight and baseline health.1 Scorpion envenomation is divided into 4 grades based on the severity of a sting:
• Grade I: pain and paresthesia at the envenomation site; usually, no local inflammation
• Grade II: local symptoms as well as more remote pain and paresthesia; pain can radiate up the affected limb
• Grade III: cranial nerve or somatic skeletal neuromuscular dysfunction; either presentation can have associated autonomic dysfunction
• Grade IV: both cranial nerve and somatic skeletal neuromuscular dysfunction, with associated auto-nomic dysfunction
The initial symptom of a scorpion sting is intense burning pain. The sting site might be unimpressive, with only a mild local reaction. Symptoms usually progress to maximum severity within 5 hours.1 Muscle pain, cramps, and weakness are prominent. The patient might have difficulty walking and swallowing, with increased salivation and drooling, and visual disturbance with abnormal eye movements. Pulse, blood pressure, and temperature often are elevated. The patient might be hyperreflexic with clonus.1,6
Symptoms of increased sympathetic activity are hypertension, tachycardia, cardiac dysrhythmia, perspiration, hyperglycemia, and restlessness.1,2 Parasympathetic effects are increased salivation, hypotension, bradycardia, and gastric distension. Skeletal muscle effects include tremors and involuntary muscle movement, which can be severe. Cranial nerve dysfunction may manifest as dysphagia, drooling, abnormal eye movements, blurred vision, slurred speech, and tongue fasciculations. Subsequent development of muscle weakness, bulbar paralysis, and difficulty breathing may be caused by depletion of neurotransmitters after prolonged excessive neuronal activity.1
Distinctive Signs in Younger Patients
A child who is stung by a scorpion might have symptoms similar to those seen in an adult victim but can also experience an extreme form of restlessness that indicates severe envenomation characterized by inability to lay still, violent muscle twitching, and uncontrollable flailing of extremities. The child might have facial grimacing, with lip-smacking and chewing motions. In addition, bulbar paralysis and respiratory distress are more likely in children who have been stung than in adults.1,2
Management
Treatment of a P transvaalicus sting is directed at “scorpionism,” envenomation that is associated with systemic symptoms that can be life-threatening. Treatment comprises support of vital functions, symptomatic measures, and injection of antivenin.8
Support of Vital Functions
In adults, systemic symptoms can be delayed as long as 8 hours after the sting. However, most severe cases usually are evident within 60 minutes; infants can reach grade IV as quickly as 15 to 30 minutes.9,10 Loss of pharyngeal reflexes and development of respiratory distress are ominous warning signs requiring immediate respiratory support. Respiratory failure is the most common cause of death.1 An asymptomatic child should be admitted to a hospital for observation for a minimum of 12 hours if the species of scorpion was not identified.2
Pain Relief
Most patients cannot tolerate an ice pack because of severe hyperesthesia. Infiltration of the local sting site with an anesthetic generally is safe and can provide some local pain relief. Intravenous fentanyl has been used in closely monitored patients because the drug is not associated with histamine release. Medications that cause release of histamine, such as morphine, can exacerbate or confuse the clinical picture.
Antivenin
Scorpion antivenin contains purified IgG fragments; allergic reactions are now rare. The sooner antivenin is administered, the greater the benefit. When administered early, it can prevent many of the most serious complications.7 In a randomized, double-blind study of critically ill children with clinically significant signs of scorpion envenomation, intravenous administration of scorpion-specific fragment antigen-binding 2 (F[(ab’]2) antivenin resulted in resolution of clinical symptoms within 4 hours.11
When managing grade III or IV scorpion envenomation, all patients should be admitted to a medical facility equipped to provide intensive supportive care; consider consultation with a regional poison control center. The World Health Organization maintains an international poison control center (at https://www.who.int/ipcs/poisons/centre/en/) with regional telephone numbers; alternatively, in the United States, call the nationwide telephone number of the Poison Control Center (800-222-1222).
The World Health Organization has identified declining production of antivenin as a crisis.12
Resolution
Symptoms of envenomation typically resolve 9 to 30 hours after a sting in a patient with grade III or IV envenomation not treated with antivenin.4 However, pain and paresthesia occasionally last as long as 2 weeks. In rare cases, more long-term sequelae of burning paresthesia persist for months.4
Conclusion
It is important for dermatologists to be aware of the potential for life-threatening envenomation by certain scorpion species native to southern Africa. In the United States, stings of these species most often are seen in patients with a pet collection, but late sequelae also can be seen in travelers returning from an endemic region. The site of a sting often appears unimpressive initially, but severe hyperesthesia is common. Patients with cardiac, neurologic, or respiratory symptoms require intensive supportive care. Proper care can be lifesaving.
Identification
The South African fattail scorpion (Parabuthus transvaalicus)(Figure) is one of the most poisonous scorpions in southern Africa.1 A member of the Buthidae scorpion family, it can grow as long as 15 cm and is dark brown-black with lighter red-brown pincers. Similar to other fattail scorpions, it has slender pincers (pedipalps) and a thick square tail (the telson). Parabuthus transvaalicus inhabits hot dry deserts, scrublands, and semiarid regions.1,2 It also is popular in exotic pet collections, the most common source of stings in the United States.

Stings and Envenomation
Scorpions with thicker tails generally have more potent venom than those with slender tails and thick pincers. Venom is injected by a stinger at the tip of the telson1; P transvaalicus also can spray venom as far as 3 m.1,2 Venom is not known to cause toxicity through skin contact but could represent a hazard if sprayed in the eye.
Scorpion toxins are a group of complex neurotoxins that act on sodium channels, either retarding inactivation (α toxin) or enhancing activation (β toxin), causing massive depolarization of excitable cells.1,3 The toxin causes neurons to fire repetitively.4 Neurotransmitters—noradrenaline, adrenaline, and acetylcholine—cause the observed sympathetic, parasympathetic, and skeletal muscle effects.1
Incidence
Worldwide, more than 1.2 million individuals are stung by a scorpion annually, causing more than 3250 deaths a year.5 Adults are stung more often, but children experience more severe envenomation, are more likely to develop severe illness requiring intensive supportive care, and have a higher mortality.4
As many as one-third of patients stung by a Parabuthus scorpion develop neuromuscular toxicity, which can be life-threatening.6 In a study of 277 envenomations by P transvaalicus, 10% of patients developed severe symptoms and 5 died. Children younger than 10 years and adults older than 50 years are at greatest risk for
Clinical Presentation
The clinical presentation of scorpion envenomation varies with the species involved, the amount of venom injected, and the victim’s weight and baseline health.1 Scorpion envenomation is divided into 4 grades based on the severity of a sting:
• Grade I: pain and paresthesia at the envenomation site; usually, no local inflammation
• Grade II: local symptoms as well as more remote pain and paresthesia; pain can radiate up the affected limb
• Grade III: cranial nerve or somatic skeletal neuromuscular dysfunction; either presentation can have associated autonomic dysfunction
• Grade IV: both cranial nerve and somatic skeletal neuromuscular dysfunction, with associated auto-nomic dysfunction
The initial symptom of a scorpion sting is intense burning pain. The sting site might be unimpressive, with only a mild local reaction. Symptoms usually progress to maximum severity within 5 hours.1 Muscle pain, cramps, and weakness are prominent. The patient might have difficulty walking and swallowing, with increased salivation and drooling, and visual disturbance with abnormal eye movements. Pulse, blood pressure, and temperature often are elevated. The patient might be hyperreflexic with clonus.1,6
Symptoms of increased sympathetic activity are hypertension, tachycardia, cardiac dysrhythmia, perspiration, hyperglycemia, and restlessness.1,2 Parasympathetic effects are increased salivation, hypotension, bradycardia, and gastric distension. Skeletal muscle effects include tremors and involuntary muscle movement, which can be severe. Cranial nerve dysfunction may manifest as dysphagia, drooling, abnormal eye movements, blurred vision, slurred speech, and tongue fasciculations. Subsequent development of muscle weakness, bulbar paralysis, and difficulty breathing may be caused by depletion of neurotransmitters after prolonged excessive neuronal activity.1
Distinctive Signs in Younger Patients
A child who is stung by a scorpion might have symptoms similar to those seen in an adult victim but can also experience an extreme form of restlessness that indicates severe envenomation characterized by inability to lay still, violent muscle twitching, and uncontrollable flailing of extremities. The child might have facial grimacing, with lip-smacking and chewing motions. In addition, bulbar paralysis and respiratory distress are more likely in children who have been stung than in adults.1,2
Management
Treatment of a P transvaalicus sting is directed at “scorpionism,” envenomation that is associated with systemic symptoms that can be life-threatening. Treatment comprises support of vital functions, symptomatic measures, and injection of antivenin.8
Support of Vital Functions
In adults, systemic symptoms can be delayed as long as 8 hours after the sting. However, most severe cases usually are evident within 60 minutes; infants can reach grade IV as quickly as 15 to 30 minutes.9,10 Loss of pharyngeal reflexes and development of respiratory distress are ominous warning signs requiring immediate respiratory support. Respiratory failure is the most common cause of death.1 An asymptomatic child should be admitted to a hospital for observation for a minimum of 12 hours if the species of scorpion was not identified.2
Pain Relief
Most patients cannot tolerate an ice pack because of severe hyperesthesia. Infiltration of the local sting site with an anesthetic generally is safe and can provide some local pain relief. Intravenous fentanyl has been used in closely monitored patients because the drug is not associated with histamine release. Medications that cause release of histamine, such as morphine, can exacerbate or confuse the clinical picture.
Antivenin
Scorpion antivenin contains purified IgG fragments; allergic reactions are now rare. The sooner antivenin is administered, the greater the benefit. When administered early, it can prevent many of the most serious complications.7 In a randomized, double-blind study of critically ill children with clinically significant signs of scorpion envenomation, intravenous administration of scorpion-specific fragment antigen-binding 2 (F[(ab’]2) antivenin resulted in resolution of clinical symptoms within 4 hours.11
When managing grade III or IV scorpion envenomation, all patients should be admitted to a medical facility equipped to provide intensive supportive care; consider consultation with a regional poison control center. The World Health Organization maintains an international poison control center (at https://www.who.int/ipcs/poisons/centre/en/) with regional telephone numbers; alternatively, in the United States, call the nationwide telephone number of the Poison Control Center (800-222-1222).
The World Health Organization has identified declining production of antivenin as a crisis.12
Resolution
Symptoms of envenomation typically resolve 9 to 30 hours after a sting in a patient with grade III or IV envenomation not treated with antivenin.4 However, pain and paresthesia occasionally last as long as 2 weeks. In rare cases, more long-term sequelae of burning paresthesia persist for months.4
Conclusion
It is important for dermatologists to be aware of the potential for life-threatening envenomation by certain scorpion species native to southern Africa. In the United States, stings of these species most often are seen in patients with a pet collection, but late sequelae also can be seen in travelers returning from an endemic region. The site of a sting often appears unimpressive initially, but severe hyperesthesia is common. Patients with cardiac, neurologic, or respiratory symptoms require intensive supportive care. Proper care can be lifesaving.
- Müller GJ, Modler H, Wium CA, et al. Scorpion sting in southern Africa: diagnosis and management. Continuing Medical Education. 2012;30:356-361.
- Müller GJ. Scorpionism in South Africa. a report of 42 serious scorpion envenomations. S Afr Med J. 1993;83:405-411.
- Quintero-Hernández V, Jiménez-Vargas JM, Gurrola GB, et al. Scorpion venom components that affect ion-channels function. Toxicon. 2013;76:328-342.
- LoVecchio F, McBride C. Scorpion envenomations in young children in central Arizona. J Toxicol Clin Toxicol. 2003;41:937-940.
- Chippaux JP, Goyffon M. Epidemiology of scorpionism: a global appraisal. Acta Trop. 2008;107:71-79.
- Bergman NJ. Clinical description of Parabuthus transvaalicus scorpionism in Zimbabwe. Toxicon. 1997;35:759-771.
- Chippaux JP. Emerging options for the management of scorpion stings. Drug Des Devel Ther. 2012;6:165-173.
- Santos MS, Silva CG, Neto BS, et al. Clinical and epidemiological aspects of scorpionism in the world: a systematic review. Wilderness Environ Med. 2016;27:504-518.
- Amaral CF, Rezende NA. Both cardiogenic and non-cardiogenic factors are involved in the pathogenesis of pulmonary oedema after scorpion envenoming. Toxicon. 1997;35:997-998.
- Bergman NJ. Scorpion sting in Zimbabwe. S Afr Med J. 1997;87:163-167.
- Boyer LV, Theodorou AA, Berg RA, et al; Arizona Envenomation Investigators. antivenom for critically ill children with neurotoxicity from scorpion stings. N Engl J Med. 2009;360:2090-2098.
- Theakston RD, Warrell DA, Griffiths E. Report of a WHO workshop on the standardization and control of antivenoms. Toxicon. 2003;41:541-557.
- Müller GJ, Modler H, Wium CA, et al. Scorpion sting in southern Africa: diagnosis and management. Continuing Medical Education. 2012;30:356-361.
- Müller GJ. Scorpionism in South Africa. a report of 42 serious scorpion envenomations. S Afr Med J. 1993;83:405-411.
- Quintero-Hernández V, Jiménez-Vargas JM, Gurrola GB, et al. Scorpion venom components that affect ion-channels function. Toxicon. 2013;76:328-342.
- LoVecchio F, McBride C. Scorpion envenomations in young children in central Arizona. J Toxicol Clin Toxicol. 2003;41:937-940.
- Chippaux JP, Goyffon M. Epidemiology of scorpionism: a global appraisal. Acta Trop. 2008;107:71-79.
- Bergman NJ. Clinical description of Parabuthus transvaalicus scorpionism in Zimbabwe. Toxicon. 1997;35:759-771.
- Chippaux JP. Emerging options for the management of scorpion stings. Drug Des Devel Ther. 2012;6:165-173.
- Santos MS, Silva CG, Neto BS, et al. Clinical and epidemiological aspects of scorpionism in the world: a systematic review. Wilderness Environ Med. 2016;27:504-518.
- Amaral CF, Rezende NA. Both cardiogenic and non-cardiogenic factors are involved in the pathogenesis of pulmonary oedema after scorpion envenoming. Toxicon. 1997;35:997-998.
- Bergman NJ. Scorpion sting in Zimbabwe. S Afr Med J. 1997;87:163-167.
- Boyer LV, Theodorou AA, Berg RA, et al; Arizona Envenomation Investigators. antivenom for critically ill children with neurotoxicity from scorpion stings. N Engl J Med. 2009;360:2090-2098.
- Theakston RD, Warrell DA, Griffiths E. Report of a WHO workshop on the standardization and control of antivenoms. Toxicon. 2003;41:541-557.
Practice Points
- Exotic and dangerous pets are becoming more popular. Scorpion stings cause potentially life-threatening neurotoxicity, with children particularly susceptible.
- Fattail scorpions are particularly dangerous and physicians should be aware that their stings may be encountered worldwide.
- Symptoms present 1 to 8 hours after envenomation, with severe cases showing hyperreflexia, clonus, difficulty swallowing, and respiratory distress. The sting site may be unimpressive.
Reflectance Confocal Microscopy to Facilitate Knifeless Skin Cancer Management
Practice Gap
Management of nonmelanoma skin cancer (NMSC) in elderly patients can cause morbidity because these patients frequently struggle to care for their biopsy sites and experience biopsy- and surgery-related complications. To minimize this treatment-related morbidity, we designed a knifeless treatment approach that employs reflectance confocal microscopy (RCM) in lieu of skin biopsy to establish the diagnosis of NMSC, then uses either intralesional or topical chemotherapy or immunotherapy (as appropriate, depending on depth of invasion) to cure the NMSC. With this approach, the patient is spared both biopsy- and surgery-related difficulties, though both intralesional and topical chemotherapy are accompanied by their own risks for adverse effects.
The Technique
Elderly patients, diabetic patients, and patients with lesions suspicious for NMSC on areas prone to poor wound healing or to notable treatment-related morbidity (eg, lower legs, genitals, the face of younger patients) are offered skin biopsy or RCM; the latter is performed during the appointment by an RC
When resolution is uncertain, RCM is repeated to assess for tumor clearance. Repeat RCM is performed at least 4 weeks after termination of treatment to avoid misinterpretation caused by treatment-related tissue inflammation. Patients who are not cured using this management approach are offered appropriate surgical management.
Practice Implications
Reflectance confocal microscopy has emerged as an effective modality for confirming the diagnosis of NMSC with high sensitivity and specificity.1,2 Emergence of this technology presents an opportunity for improving the way the NMSC is managed because RCM allows dermatologists to confirm the diagnosis of BCC and SCC by interpretation of RCM mosaics rather than by histopathologic examination of biopsied tissue. Our knifeless approach to skin cancer management is especially beneficial when biopsy and dermatologic surgery are likely to confer notable morbidity, such as managing NMSC on the face of a young adult, in the frail elderly population, or in diabetic patients, and when treating sites on the lower extremity prone to poor wound healing.
- Song E, Grant-Kels JM, Swede H, et al. Paired comparison of the sensitivity and specificity of multispectral digital skin lesion analysis and reflectance confocal microscopy in the detection of melanoma in vivo: a cross-sectional study. J Am Acad Dermatol. 2016;75:1187-1192.
- Ferrari B, Salgarelli AC, Mandel VD, et al. Non-melanoma skin cancer of the head and neck: the aid of reflectance confocal microscopy for the accurate diagnosis and management. G Ital Dermatol Venereol. 2017;152:169-177.
Practice Gap
Management of nonmelanoma skin cancer (NMSC) in elderly patients can cause morbidity because these patients frequently struggle to care for their biopsy sites and experience biopsy- and surgery-related complications. To minimize this treatment-related morbidity, we designed a knifeless treatment approach that employs reflectance confocal microscopy (RCM) in lieu of skin biopsy to establish the diagnosis of NMSC, then uses either intralesional or topical chemotherapy or immunotherapy (as appropriate, depending on depth of invasion) to cure the NMSC. With this approach, the patient is spared both biopsy- and surgery-related difficulties, though both intralesional and topical chemotherapy are accompanied by their own risks for adverse effects.
The Technique
Elderly patients, diabetic patients, and patients with lesions suspicious for NMSC on areas prone to poor wound healing or to notable treatment-related morbidity (eg, lower legs, genitals, the face of younger patients) are offered skin biopsy or RCM; the latter is performed during the appointment by an RC
When resolution is uncertain, RCM is repeated to assess for tumor clearance. Repeat RCM is performed at least 4 weeks after termination of treatment to avoid misinterpretation caused by treatment-related tissue inflammation. Patients who are not cured using this management approach are offered appropriate surgical management.
Practice Implications
Reflectance confocal microscopy has emerged as an effective modality for confirming the diagnosis of NMSC with high sensitivity and specificity.1,2 Emergence of this technology presents an opportunity for improving the way the NMSC is managed because RCM allows dermatologists to confirm the diagnosis of BCC and SCC by interpretation of RCM mosaics rather than by histopathologic examination of biopsied tissue. Our knifeless approach to skin cancer management is especially beneficial when biopsy and dermatologic surgery are likely to confer notable morbidity, such as managing NMSC on the face of a young adult, in the frail elderly population, or in diabetic patients, and when treating sites on the lower extremity prone to poor wound healing.
Practice Gap
Management of nonmelanoma skin cancer (NMSC) in elderly patients can cause morbidity because these patients frequently struggle to care for their biopsy sites and experience biopsy- and surgery-related complications. To minimize this treatment-related morbidity, we designed a knifeless treatment approach that employs reflectance confocal microscopy (RCM) in lieu of skin biopsy to establish the diagnosis of NMSC, then uses either intralesional or topical chemotherapy or immunotherapy (as appropriate, depending on depth of invasion) to cure the NMSC. With this approach, the patient is spared both biopsy- and surgery-related difficulties, though both intralesional and topical chemotherapy are accompanied by their own risks for adverse effects.
The Technique
Elderly patients, diabetic patients, and patients with lesions suspicious for NMSC on areas prone to poor wound healing or to notable treatment-related morbidity (eg, lower legs, genitals, the face of younger patients) are offered skin biopsy or RCM; the latter is performed during the appointment by an RC
When resolution is uncertain, RCM is repeated to assess for tumor clearance. Repeat RCM is performed at least 4 weeks after termination of treatment to avoid misinterpretation caused by treatment-related tissue inflammation. Patients who are not cured using this management approach are offered appropriate surgical management.
Practice Implications
Reflectance confocal microscopy has emerged as an effective modality for confirming the diagnosis of NMSC with high sensitivity and specificity.1,2 Emergence of this technology presents an opportunity for improving the way the NMSC is managed because RCM allows dermatologists to confirm the diagnosis of BCC and SCC by interpretation of RCM mosaics rather than by histopathologic examination of biopsied tissue. Our knifeless approach to skin cancer management is especially beneficial when biopsy and dermatologic surgery are likely to confer notable morbidity, such as managing NMSC on the face of a young adult, in the frail elderly population, or in diabetic patients, and when treating sites on the lower extremity prone to poor wound healing.
- Song E, Grant-Kels JM, Swede H, et al. Paired comparison of the sensitivity and specificity of multispectral digital skin lesion analysis and reflectance confocal microscopy in the detection of melanoma in vivo: a cross-sectional study. J Am Acad Dermatol. 2016;75:1187-1192.
- Ferrari B, Salgarelli AC, Mandel VD, et al. Non-melanoma skin cancer of the head and neck: the aid of reflectance confocal microscopy for the accurate diagnosis and management. G Ital Dermatol Venereol. 2017;152:169-177.
- Song E, Grant-Kels JM, Swede H, et al. Paired comparison of the sensitivity and specificity of multispectral digital skin lesion analysis and reflectance confocal microscopy in the detection of melanoma in vivo: a cross-sectional study. J Am Acad Dermatol. 2016;75:1187-1192.
- Ferrari B, Salgarelli AC, Mandel VD, et al. Non-melanoma skin cancer of the head and neck: the aid of reflectance confocal microscopy for the accurate diagnosis and management. G Ital Dermatol Venereol. 2017;152:169-177.
What Neglected Tropical Diseases Teach Us About Stigma
Neglected tropical diseases (NTDs) are a group of 20 diseases that typically are chronic and cause long-term disability, which negatively impacts work productivity, child survival, and school performance and attendance with adverse effect on future earnings.1 Data from the 2013 Global Burden of Disease study revealed that half of the world’s NTDs occur in poor populations living in wealthy countries.2 Neglected tropical diseases with skin manifestations include parasitic infections (eg, American trypanosomiasis, African trypanosomiasis, dracunculiasis, echinococcosis, foodborne trematodiases, leishmaniasis, lymphatic filariasis, onchocerciasis, scabies and other ectoparasites, schistosomiasis, soil-transmitted helminths, taeniasis/cysticercosis), bacterial infections (eg, Buruli ulcer, leprosy, yaws), fungal infections (eg, mycetoma, chromoblastomycosis, deep mycoses), and viral infections (eg, dengue, chikungunya). Rabies and snakebite envenomization involve the skin through inoculation. Within the larger group of NTDs, the World Health Organization has identified “skin NTDs” as a subgroup of NTDs that present primarily with changes in the skin.3 In the absence of early diagnosis and treatment of these diseases, chronic and lifelong disfigurement, disability, stigma, and socioeconomic losses ensue.
The Department of Health of the Government of Western Australia stated:
Stigma is a mark of disgrace that sets a person apart from others. When a person is labeled by their illness they are no longer seen as an individual but as part of a stereotyped group. Negative attitudes and beliefs toward this group create prejudice which leads to negative actions and discrimination.4
Stigma associated with skin NTDs exemplifies how skin diseases can have enduring impact on individuals.5 For example, scarring from inactive cutaneous leishmaniasis carries heavy psychosocial burden. Young women reported that facial scarring from cutaneous leishmaniasis led to marriage rejections.6 Some even reported extreme suicidal ideations.7 Recently, major depressive disorder associated with scarring from inactive cutaneous leishmaniasis has been recognized as a notable contributor to disease burden from cutaneous leishmaniasis.8
Lymphatic filariasis is a major cause of leg and scrotal lymphedema worldwide. Even when the condition is treated, lymphedema often persists due to chronic irreversible lymphatic damage. A systematic review of 18 stigma studies in lymphatic filariasis found common themes related to the deleterious consequences of stigma on social relationships; work and education opportunities; health outcomes from reduced treatment-seeking behavior; and mental health, including anxiety, depression, and suicidal tendencies.9 In one subdistrict in India, implementation of a community-based lymphedema management program that consisted of teaching hygiene and limb care for more than 20,000 lymphedema patients and performing community outreach activities (eg, street plays, radio programs, informational brochures) to teach people about lymphatic filariasis and lymphedema care was associated with community members being accepting of patients and an improvement in their understanding of disease etiology.10
Skin involvement from onchocerciasis infection (onchocercal skin disease) is another condition associated with notable stigma.9 Through the African Programme for Onchocerciasis Control, annual mass drug administration of ivermectin in onchocerciasis-endemic communities has reduced the rate of onchocercal skin disease in these communities. In looking at perception of stigma in onchocercal skin diseases before community-directed ivermectin therapy and 7 to 10 years after, avoidance of people with onchocercal skin disease decreased from 32.7% to 4.3%. There also was an improvement in relationships between healthy people and those with onchocercal skin disease.11
One of the most stigmatizing conditions is leprosy, often referred to as Hansen disease to give credit to the person who discovered that leprosy was caused by Mycobacterium leprae and not from sin, being cursed, or genetic inheritance. Even with this knowledge, stigma persists that can lead to family abandonment and social isolation, which further impacts afflicted individuals’ willingness to seek care, thus leading to disease progression. More recently, there has been research looking at interventions to reduce the stigma that individuals afflicted with leprosy face. In a study from Indonesia where individuals with leprosy were randomized to counseling, socioeconomic development, or contact between community members and affected people, all interventions were associated with a reduction in stigma.12 A rights-based counseling module integrated individual, family, and group forms of counseling and consisted of 5 sessions that focused on medical knowledge of leprosy and rights of individuals with leprosy, along with elements of cognitive behavioral therapy. Socioeconomic development involved opportunities for business training, creation of community groups through which microfinance services were administered, and other assistance to improve livelihood. Informed by evidence from the field of human immunodeficiency virus and mental health that co
Although steps are being taken to address the psychosocial burden of skin NTDs, there is still much work to be done. From the public health lens that largely governs the policies and approaches toward addressing NTDs, the focus often is on interrupting and eliminating disease transmission. Morbidity management, including reduction in stigma and functional impairment, is not always the priority. It is in this space that dermatologists are uniquely positioned to advocate for management approaches that address the morbidity associated with skin NTDs. We have an intimate understanding of how impactful skin diseases can be, even if they are not commonly fatal. Globally, skin diseases are the fourth leading cause of nonfatal disease burden,14 yet dermatology lacks effective evidence-based interventions for reducing stigma in our patients with visible chronic diseases.15
Every day, we see firsthand how skin diseases affect not only our patients but also their families, friends, and caregivers. Although we may not see skin NTDs on a regular basis in our clinics, we can understand almost intuitively how devastating skin NTDs could be on individuals, families, and communities. For patients with skin NTDs, receiving medical therapy is only one component of treatment. In addition to optimizing early diagnosis and treatment, interventions taken to educate families and communities affected by skin NTDs are vitally important. Stigma reduction is possible, as we have seen from the aforementioned interventions used in communities with lymphatic filariasis, onchocerciasis, and leprosy. We call upon our fellow dermatologists to take interest in creating, evaluating, and promoting interventions that address stigma in skin NTDs; it is critical in achieving and maintaining health and well-being for our patients.
- Neglected tropical diseases. World Health Organization website. https://www.who.int/neglected_diseases/diseases/en/. Accessed September 10, 2019.
- Hotez PJ, Damania A, Naghavi M. Blue Marble Health and the Global Burden of Disease Study 2013. PLoS Negl Trop Dis. 2016;10:E0004744.
- Skin NTDs. World Health Organization website. https://www.who.int/neglected_diseases/skin-ntds/en/. Accessed September 10, 2019.
- Government of Western Australia Department of Health. Stigma, discrimination and mental illness. February 2009. http://www.health.wa.gov.au/docreg/Education/Population/Health_Problems/Mental_Illness/Mentalhealth_stigma_fact.pdf. Accessed September 10, 2019.
- Hotez PJ. Stigma: the stealth weapon of the NTD. PLoS Negl Trop Dis. 2008;2:E230.
- Bennis I, Belaid L, De Brouwere V, et al. “The mosquitoes that destroy your face.” social impact of cutaneous leishmaniasis in Southeastern Morocco, a qualitative study. PLoS One. 2017;12:E0189906.
- Bennis I, Thys S, Filali H, et al. Psychosocial impact of scars due to cutaneous leishmaniasis on high school students in Errachidia province, Morocco. Infect Dis Poverty. 2017;6:46.
- Bailey F, Mondragon-Shem K, Haines LR, et al. Cutaneous leishmaniasis and co-morbid major depressive disorder: a systematic review with burden estimates. PLoS Negl Trop Dis. 2019;13:E0007092.
- Hofstraat K, van Brakel WH. Social stigma towards neglected tropical diseases: a systematic review. Int Health. 2016;8(suppl 1):I53-I70.
- Cassidy T, Worrell CM, Little K, et al. Experiences of a community-based lymphedema management program for lymphatic filariasis in Odisha State, India: an analysis of focus group discussions with patients, families, community members and program volunteers. PLoS Negl Trop Dis. 2016;10:E0004424.
- Tchounkeu YF, Onyeneho NG, Wanji S, et al. Changes in stigma and discrimination of onchocerciasis in Africa. Trans R Soc Trop Med Hyg. 2012;106:340-347.
- Dadun D, Van Brakel WH, Peters RMH, et al. Impact of socio-economic development, contact and peer counselling on stigma against persons affected by leprosy in Cirebon, Indonesia—a randomised controlled trial. Lepr Rev. 2017;88:2-22.
- Kumar A, Lambert S, Lockwood DNJ. Picturing health: a new face for leprosy. Lancet. 2019;393:629-638.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Topp J, Andrees V, Weinberger NA, et al. Strategies to reduce stigma related to visible chronic skin diseases: a systematic review [published online June 8, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15734.
Neglected tropical diseases (NTDs) are a group of 20 diseases that typically are chronic and cause long-term disability, which negatively impacts work productivity, child survival, and school performance and attendance with adverse effect on future earnings.1 Data from the 2013 Global Burden of Disease study revealed that half of the world’s NTDs occur in poor populations living in wealthy countries.2 Neglected tropical diseases with skin manifestations include parasitic infections (eg, American trypanosomiasis, African trypanosomiasis, dracunculiasis, echinococcosis, foodborne trematodiases, leishmaniasis, lymphatic filariasis, onchocerciasis, scabies and other ectoparasites, schistosomiasis, soil-transmitted helminths, taeniasis/cysticercosis), bacterial infections (eg, Buruli ulcer, leprosy, yaws), fungal infections (eg, mycetoma, chromoblastomycosis, deep mycoses), and viral infections (eg, dengue, chikungunya). Rabies and snakebite envenomization involve the skin through inoculation. Within the larger group of NTDs, the World Health Organization has identified “skin NTDs” as a subgroup of NTDs that present primarily with changes in the skin.3 In the absence of early diagnosis and treatment of these diseases, chronic and lifelong disfigurement, disability, stigma, and socioeconomic losses ensue.
The Department of Health of the Government of Western Australia stated:
Stigma is a mark of disgrace that sets a person apart from others. When a person is labeled by their illness they are no longer seen as an individual but as part of a stereotyped group. Negative attitudes and beliefs toward this group create prejudice which leads to negative actions and discrimination.4
Stigma associated with skin NTDs exemplifies how skin diseases can have enduring impact on individuals.5 For example, scarring from inactive cutaneous leishmaniasis carries heavy psychosocial burden. Young women reported that facial scarring from cutaneous leishmaniasis led to marriage rejections.6 Some even reported extreme suicidal ideations.7 Recently, major depressive disorder associated with scarring from inactive cutaneous leishmaniasis has been recognized as a notable contributor to disease burden from cutaneous leishmaniasis.8
Lymphatic filariasis is a major cause of leg and scrotal lymphedema worldwide. Even when the condition is treated, lymphedema often persists due to chronic irreversible lymphatic damage. A systematic review of 18 stigma studies in lymphatic filariasis found common themes related to the deleterious consequences of stigma on social relationships; work and education opportunities; health outcomes from reduced treatment-seeking behavior; and mental health, including anxiety, depression, and suicidal tendencies.9 In one subdistrict in India, implementation of a community-based lymphedema management program that consisted of teaching hygiene and limb care for more than 20,000 lymphedema patients and performing community outreach activities (eg, street plays, radio programs, informational brochures) to teach people about lymphatic filariasis and lymphedema care was associated with community members being accepting of patients and an improvement in their understanding of disease etiology.10
Skin involvement from onchocerciasis infection (onchocercal skin disease) is another condition associated with notable stigma.9 Through the African Programme for Onchocerciasis Control, annual mass drug administration of ivermectin in onchocerciasis-endemic communities has reduced the rate of onchocercal skin disease in these communities. In looking at perception of stigma in onchocercal skin diseases before community-directed ivermectin therapy and 7 to 10 years after, avoidance of people with onchocercal skin disease decreased from 32.7% to 4.3%. There also was an improvement in relationships between healthy people and those with onchocercal skin disease.11
One of the most stigmatizing conditions is leprosy, often referred to as Hansen disease to give credit to the person who discovered that leprosy was caused by Mycobacterium leprae and not from sin, being cursed, or genetic inheritance. Even with this knowledge, stigma persists that can lead to family abandonment and social isolation, which further impacts afflicted individuals’ willingness to seek care, thus leading to disease progression. More recently, there has been research looking at interventions to reduce the stigma that individuals afflicted with leprosy face. In a study from Indonesia where individuals with leprosy were randomized to counseling, socioeconomic development, or contact between community members and affected people, all interventions were associated with a reduction in stigma.12 A rights-based counseling module integrated individual, family, and group forms of counseling and consisted of 5 sessions that focused on medical knowledge of leprosy and rights of individuals with leprosy, along with elements of cognitive behavioral therapy. Socioeconomic development involved opportunities for business training, creation of community groups through which microfinance services were administered, and other assistance to improve livelihood. Informed by evidence from the field of human immunodeficiency virus and mental health that co
Although steps are being taken to address the psychosocial burden of skin NTDs, there is still much work to be done. From the public health lens that largely governs the policies and approaches toward addressing NTDs, the focus often is on interrupting and eliminating disease transmission. Morbidity management, including reduction in stigma and functional impairment, is not always the priority. It is in this space that dermatologists are uniquely positioned to advocate for management approaches that address the morbidity associated with skin NTDs. We have an intimate understanding of how impactful skin diseases can be, even if they are not commonly fatal. Globally, skin diseases are the fourth leading cause of nonfatal disease burden,14 yet dermatology lacks effective evidence-based interventions for reducing stigma in our patients with visible chronic diseases.15
Every day, we see firsthand how skin diseases affect not only our patients but also their families, friends, and caregivers. Although we may not see skin NTDs on a regular basis in our clinics, we can understand almost intuitively how devastating skin NTDs could be on individuals, families, and communities. For patients with skin NTDs, receiving medical therapy is only one component of treatment. In addition to optimizing early diagnosis and treatment, interventions taken to educate families and communities affected by skin NTDs are vitally important. Stigma reduction is possible, as we have seen from the aforementioned interventions used in communities with lymphatic filariasis, onchocerciasis, and leprosy. We call upon our fellow dermatologists to take interest in creating, evaluating, and promoting interventions that address stigma in skin NTDs; it is critical in achieving and maintaining health and well-being for our patients.
Neglected tropical diseases (NTDs) are a group of 20 diseases that typically are chronic and cause long-term disability, which negatively impacts work productivity, child survival, and school performance and attendance with adverse effect on future earnings.1 Data from the 2013 Global Burden of Disease study revealed that half of the world’s NTDs occur in poor populations living in wealthy countries.2 Neglected tropical diseases with skin manifestations include parasitic infections (eg, American trypanosomiasis, African trypanosomiasis, dracunculiasis, echinococcosis, foodborne trematodiases, leishmaniasis, lymphatic filariasis, onchocerciasis, scabies and other ectoparasites, schistosomiasis, soil-transmitted helminths, taeniasis/cysticercosis), bacterial infections (eg, Buruli ulcer, leprosy, yaws), fungal infections (eg, mycetoma, chromoblastomycosis, deep mycoses), and viral infections (eg, dengue, chikungunya). Rabies and snakebite envenomization involve the skin through inoculation. Within the larger group of NTDs, the World Health Organization has identified “skin NTDs” as a subgroup of NTDs that present primarily with changes in the skin.3 In the absence of early diagnosis and treatment of these diseases, chronic and lifelong disfigurement, disability, stigma, and socioeconomic losses ensue.
The Department of Health of the Government of Western Australia stated:
Stigma is a mark of disgrace that sets a person apart from others. When a person is labeled by their illness they are no longer seen as an individual but as part of a stereotyped group. Negative attitudes and beliefs toward this group create prejudice which leads to negative actions and discrimination.4
Stigma associated with skin NTDs exemplifies how skin diseases can have enduring impact on individuals.5 For example, scarring from inactive cutaneous leishmaniasis carries heavy psychosocial burden. Young women reported that facial scarring from cutaneous leishmaniasis led to marriage rejections.6 Some even reported extreme suicidal ideations.7 Recently, major depressive disorder associated with scarring from inactive cutaneous leishmaniasis has been recognized as a notable contributor to disease burden from cutaneous leishmaniasis.8
Lymphatic filariasis is a major cause of leg and scrotal lymphedema worldwide. Even when the condition is treated, lymphedema often persists due to chronic irreversible lymphatic damage. A systematic review of 18 stigma studies in lymphatic filariasis found common themes related to the deleterious consequences of stigma on social relationships; work and education opportunities; health outcomes from reduced treatment-seeking behavior; and mental health, including anxiety, depression, and suicidal tendencies.9 In one subdistrict in India, implementation of a community-based lymphedema management program that consisted of teaching hygiene and limb care for more than 20,000 lymphedema patients and performing community outreach activities (eg, street plays, radio programs, informational brochures) to teach people about lymphatic filariasis and lymphedema care was associated with community members being accepting of patients and an improvement in their understanding of disease etiology.10
Skin involvement from onchocerciasis infection (onchocercal skin disease) is another condition associated with notable stigma.9 Through the African Programme for Onchocerciasis Control, annual mass drug administration of ivermectin in onchocerciasis-endemic communities has reduced the rate of onchocercal skin disease in these communities. In looking at perception of stigma in onchocercal skin diseases before community-directed ivermectin therapy and 7 to 10 years after, avoidance of people with onchocercal skin disease decreased from 32.7% to 4.3%. There also was an improvement in relationships between healthy people and those with onchocercal skin disease.11
One of the most stigmatizing conditions is leprosy, often referred to as Hansen disease to give credit to the person who discovered that leprosy was caused by Mycobacterium leprae and not from sin, being cursed, or genetic inheritance. Even with this knowledge, stigma persists that can lead to family abandonment and social isolation, which further impacts afflicted individuals’ willingness to seek care, thus leading to disease progression. More recently, there has been research looking at interventions to reduce the stigma that individuals afflicted with leprosy face. In a study from Indonesia where individuals with leprosy were randomized to counseling, socioeconomic development, or contact between community members and affected people, all interventions were associated with a reduction in stigma.12 A rights-based counseling module integrated individual, family, and group forms of counseling and consisted of 5 sessions that focused on medical knowledge of leprosy and rights of individuals with leprosy, along with elements of cognitive behavioral therapy. Socioeconomic development involved opportunities for business training, creation of community groups through which microfinance services were administered, and other assistance to improve livelihood. Informed by evidence from the field of human immunodeficiency virus and mental health that co
Although steps are being taken to address the psychosocial burden of skin NTDs, there is still much work to be done. From the public health lens that largely governs the policies and approaches toward addressing NTDs, the focus often is on interrupting and eliminating disease transmission. Morbidity management, including reduction in stigma and functional impairment, is not always the priority. It is in this space that dermatologists are uniquely positioned to advocate for management approaches that address the morbidity associated with skin NTDs. We have an intimate understanding of how impactful skin diseases can be, even if they are not commonly fatal. Globally, skin diseases are the fourth leading cause of nonfatal disease burden,14 yet dermatology lacks effective evidence-based interventions for reducing stigma in our patients with visible chronic diseases.15
Every day, we see firsthand how skin diseases affect not only our patients but also their families, friends, and caregivers. Although we may not see skin NTDs on a regular basis in our clinics, we can understand almost intuitively how devastating skin NTDs could be on individuals, families, and communities. For patients with skin NTDs, receiving medical therapy is only one component of treatment. In addition to optimizing early diagnosis and treatment, interventions taken to educate families and communities affected by skin NTDs are vitally important. Stigma reduction is possible, as we have seen from the aforementioned interventions used in communities with lymphatic filariasis, onchocerciasis, and leprosy. We call upon our fellow dermatologists to take interest in creating, evaluating, and promoting interventions that address stigma in skin NTDs; it is critical in achieving and maintaining health and well-being for our patients.
- Neglected tropical diseases. World Health Organization website. https://www.who.int/neglected_diseases/diseases/en/. Accessed September 10, 2019.
- Hotez PJ, Damania A, Naghavi M. Blue Marble Health and the Global Burden of Disease Study 2013. PLoS Negl Trop Dis. 2016;10:E0004744.
- Skin NTDs. World Health Organization website. https://www.who.int/neglected_diseases/skin-ntds/en/. Accessed September 10, 2019.
- Government of Western Australia Department of Health. Stigma, discrimination and mental illness. February 2009. http://www.health.wa.gov.au/docreg/Education/Population/Health_Problems/Mental_Illness/Mentalhealth_stigma_fact.pdf. Accessed September 10, 2019.
- Hotez PJ. Stigma: the stealth weapon of the NTD. PLoS Negl Trop Dis. 2008;2:E230.
- Bennis I, Belaid L, De Brouwere V, et al. “The mosquitoes that destroy your face.” social impact of cutaneous leishmaniasis in Southeastern Morocco, a qualitative study. PLoS One. 2017;12:E0189906.
- Bennis I, Thys S, Filali H, et al. Psychosocial impact of scars due to cutaneous leishmaniasis on high school students in Errachidia province, Morocco. Infect Dis Poverty. 2017;6:46.
- Bailey F, Mondragon-Shem K, Haines LR, et al. Cutaneous leishmaniasis and co-morbid major depressive disorder: a systematic review with burden estimates. PLoS Negl Trop Dis. 2019;13:E0007092.
- Hofstraat K, van Brakel WH. Social stigma towards neglected tropical diseases: a systematic review. Int Health. 2016;8(suppl 1):I53-I70.
- Cassidy T, Worrell CM, Little K, et al. Experiences of a community-based lymphedema management program for lymphatic filariasis in Odisha State, India: an analysis of focus group discussions with patients, families, community members and program volunteers. PLoS Negl Trop Dis. 2016;10:E0004424.
- Tchounkeu YF, Onyeneho NG, Wanji S, et al. Changes in stigma and discrimination of onchocerciasis in Africa. Trans R Soc Trop Med Hyg. 2012;106:340-347.
- Dadun D, Van Brakel WH, Peters RMH, et al. Impact of socio-economic development, contact and peer counselling on stigma against persons affected by leprosy in Cirebon, Indonesia—a randomised controlled trial. Lepr Rev. 2017;88:2-22.
- Kumar A, Lambert S, Lockwood DNJ. Picturing health: a new face for leprosy. Lancet. 2019;393:629-638.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Topp J, Andrees V, Weinberger NA, et al. Strategies to reduce stigma related to visible chronic skin diseases: a systematic review [published online June 8, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15734.
- Neglected tropical diseases. World Health Organization website. https://www.who.int/neglected_diseases/diseases/en/. Accessed September 10, 2019.
- Hotez PJ, Damania A, Naghavi M. Blue Marble Health and the Global Burden of Disease Study 2013. PLoS Negl Trop Dis. 2016;10:E0004744.
- Skin NTDs. World Health Organization website. https://www.who.int/neglected_diseases/skin-ntds/en/. Accessed September 10, 2019.
- Government of Western Australia Department of Health. Stigma, discrimination and mental illness. February 2009. http://www.health.wa.gov.au/docreg/Education/Population/Health_Problems/Mental_Illness/Mentalhealth_stigma_fact.pdf. Accessed September 10, 2019.
- Hotez PJ. Stigma: the stealth weapon of the NTD. PLoS Negl Trop Dis. 2008;2:E230.
- Bennis I, Belaid L, De Brouwere V, et al. “The mosquitoes that destroy your face.” social impact of cutaneous leishmaniasis in Southeastern Morocco, a qualitative study. PLoS One. 2017;12:E0189906.
- Bennis I, Thys S, Filali H, et al. Psychosocial impact of scars due to cutaneous leishmaniasis on high school students in Errachidia province, Morocco. Infect Dis Poverty. 2017;6:46.
- Bailey F, Mondragon-Shem K, Haines LR, et al. Cutaneous leishmaniasis and co-morbid major depressive disorder: a systematic review with burden estimates. PLoS Negl Trop Dis. 2019;13:E0007092.
- Hofstraat K, van Brakel WH. Social stigma towards neglected tropical diseases: a systematic review. Int Health. 2016;8(suppl 1):I53-I70.
- Cassidy T, Worrell CM, Little K, et al. Experiences of a community-based lymphedema management program for lymphatic filariasis in Odisha State, India: an analysis of focus group discussions with patients, families, community members and program volunteers. PLoS Negl Trop Dis. 2016;10:E0004424.
- Tchounkeu YF, Onyeneho NG, Wanji S, et al. Changes in stigma and discrimination of onchocerciasis in Africa. Trans R Soc Trop Med Hyg. 2012;106:340-347.
- Dadun D, Van Brakel WH, Peters RMH, et al. Impact of socio-economic development, contact and peer counselling on stigma against persons affected by leprosy in Cirebon, Indonesia—a randomised controlled trial. Lepr Rev. 2017;88:2-22.
- Kumar A, Lambert S, Lockwood DNJ. Picturing health: a new face for leprosy. Lancet. 2019;393:629-638.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Topp J, Andrees V, Weinberger NA, et al. Strategies to reduce stigma related to visible chronic skin diseases: a systematic review [published online June 8, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15734.
GRECC Connect: Geriatrics Telehealth to Empower Health Care Providers and Improve Management of Older Veterans in Rural Communities
Nearly 2.7 million veterans who rely on the Veterans Health Administration (VHA) for their health care live in rural communities.1 Of these, more than half are aged ≥ 65 years. Rural veterans have greater rates of service-related disability and chronic medical conditions than do their urban counterparts.1,2 Yet because of their rural location, they face unique challenges, including long travel times and distances to health care services, lack of public transportation options, and limited availability of specialized medical and social support services.
Compounding these geographic barriers is a more general lack of workforce infrastructure and a dearth of clinical health care providers (HCPs) skilled in geriatric medicine. The demand for geriatricians is projected to outpace supply and result in a national shortage of nearly 27 000 geriatricians by 2025.3 Moreover, the overwhelming majority (90%) of HCPs identifying as geriatric specialists reside in urban areas.4 This creates tremendous pressure on the health care system to provide remote care for older veterans contending with complex conditions, and ultimately these veterans may not receive the specialized care they need.
Telehealth modalities bridge these gaps by bringing health care to veterans in rural communities. They may also hold promise for strengthening community care in rural areas through workforce development and dissemination of educational resources. The VHA has been recognized as a leader in the field of telehealth since it began offering telehealth services to veterans in 19775-8 and served more than 677 000 Veterans via telehealth in fiscal year (FY) 2015.9 The VHA currently employs multiple modes of telehealth to increase veterans’ access to health care, including: (1) synchronous technology like clinical video telehealth (CVT), which provides live encounters between HCPs and patients using videoconferencing software; and (2) asynchronous technology, such as store-and-forward communication that offers remote transmission and clinical interpretation of veteran health data. The VHA has also strengthened its broad telehealth infrastructure by staffing VHA clinical sites with telehealth clinical technicians and providing telehealth hardware throughout.
The Department of Veterans Affairs (VA) Office of Geriatrics and Extended Care (GEC) and Office of Rural Health (ORH) established the Geriatric Research Education and Clinical Centers (GRECC) Connect project in 2014 to leverage the existing telehealth technologies at the VA to meet the health care needs of older veterans. GRECC Connect builds on the VHA network of geriatrics expertise in GRECCs by providing telehealth-based consultative support for rural primary care provider (PCP) teams, older veterans, and their families. This program profile describes this project’s mission, structure, and activities.
Program Overview
GRECC Connect leverages the clinical expertise and administrative infrastructure of participating GRECCs in order to reach clinicians and veterans in primarily rural communities.10 GRECCs are VA centers of excellence focused on aging and comprise a large network of interdisciplinary geriatrics expertise. All GRECCs have strong affiliations with local universities and are located in urban VA medical centers (VAMCs). GRECC Connect is based on a hub-and-spoke model in which urban GRECC hub sites are connected to community-based outpatient clinic (CBOC) and VAMC spokes that primarily serve veterans in other communities. CBOCs are stand-alone clinics that are geographically separate from a related VA medical center and provide outpatient primary care, mental health care services, and some specialty care services such as cardiology or neurology. They range in size from small, mainly telehealth clinics with 1 technician to large clinics with several specialty providers. Each GRECC hub site partners with an average of 6 CBOCs (range 3-16), each of which is an average distance of 92.8 miles from the related VA medical center (range 20-406 miles).
GRECC Connect was established under the umbrella of the VA Geriatric Scholars Program, which since 2008 integrates geriatrics into rural primary care practices through tailored education for continuing professional development.11 Through intensive courses in geriatrics and quality improvement methods and through participation in local quality improvement projects benefiting older veterans, the Geriatric Scholars Program trains rural PCPs so that they can more effectively and independently diagnose and manage common geriatric syndromes.12 The network of clinician scholars developed by the Geriatric Scholars Program, all rural frontline clinicians at VA clinics, has given the GRECC Connect project a well-prepared, geriatrics-trained workforce to act as project champions at rural CBOCs and VAMCs. The GRECC Connect project’s goals are to enhance access to geriatric specialty care among older veterans with complex medical problems, geriatric syndromes, and increased risk for institutionalization, and to provide geriatrics-focused educational support to rural HCP teams.
Geriatric Provider Consultations
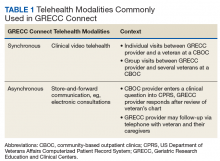
The first overarching goal of the GRECC Connect project is to improve access to geriatrics specialty care by facilitating linkages between GRECC hub sites and the CBOCs and VAMCs that primarily serve veterans in rural communities. GRECC hub sites offer consultative support from geriatrics specialty team members (eg, geriatricians, nurse practitioners, pharmacists, gero- or neuropsychologists, registered nurses [RNs], and social workers) to rural PCP in their catchment area. This support is offered through a variety of telehealth modalities readily available in the VA (Table 1). These include CVT, in which a veteran located at a rural CBOC is seen using videoconferencing software by a geriatrics specialty provider who is located at a GRECC hub site. At some GRECC hub sites, CVT has also been used to conduct group visits between a GRECC provider at the hub site and several veterans who participate from a rural CBOC. Electronic consultations, or e-consults, involve a rural provider entering a clinical question in the VA Computerized Patient Record System. The question is then triaged, and a geriatrics provider at a GRECC responds, based on review of that veteran’s chart. At some GRECC hub sites, the e-consults are more extensive and may include telephone contact with the veteran or their caregiver.
Consultations between GRECC-based teams and rural PCPs may cover any aspect of geriatrics care, ranging from broad concerns to subspecialty areas of geriatric medicine. For instance, general geriatrics consultation may address polypharmacy, during either care transitions or ongoing care. Consultation may also reflect the specific focus area of a particular GRECC, such as cognitive assessment (eg, Pittsburgh GRECC), management of osteoporosis to address falls (eg, Durham GRECC, Miami GRECC), and continence care (eg, Birmingham/Atlanta GRECC).13 Most consultations are initiated by a remote HCP who is seeking geriatrics expertise from the GRECC team.
Some GRECC hub sites, however, employ case finding strategies, or detailed chart reviews, in order to identify older veterans who may benefit from geriatrics consultation. For veterans identified through those mechanisms, the GRECC clinicians suggest that the rural HCP either request or allow an e-consult or evaluation via CVT for those veterans. The geriatric consultations may help identify additional care needs for older veterans and lead to recommendations, orders, or remote provision of a variety of other actions, including VA or non-VA services (eg, home-based primary care, home nursing service, respite service, social support services such as Meals on Wheels); neuropsychological testing; physical or occupational therapy; audiology or optometry referral; falls and fracture risk assessment and interventions to reduce falls (eg, home safety evaluation, physical therapy); osteoporosis risk assessments (eg, densitometry, recommendations for pharmacologic therapy) to reduce the risk of injury or nontraumatic fractures from falls; palliative care for incontinence and hospice; and counseling on geriatric issues such as dementia caregiving, advanced directives, and driving cessation.
More recently, the Miami GRECC has begun evaluating rural veterans at risk for hypoglycemia, providing patient education and counseling about hypoglycemia, and making recommendations to the veterans’ primary care teams.14 Consultations may also lead to the appropriate use or discontinuation of medications, related to polypharmacy. GRECC-based teams, for example, have helped rural HCPs modify medication doses, start appropriate medications for dementia and depression, and identify and stop potentially inappropriate medications (eg, those that increase fall risk or that have significant anticholinergic properties).15
GRECC Connect Geriatric Case Conference Series
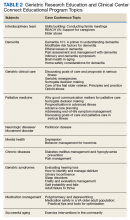
The second overarching goal of the GRECC Connect project is to provide geriatrics-focused educational support to equip PCPs to better serve their aging veteran patients. This is achieved through twice-monthly, case-based conferences supported by the VA Employee Education System (EES) and delivered through a webinar interface. Case conferences are targeted to members of the health care team who may provide care for rural older adults, including physicians, nurse practitioners, physician assistants, RNs, psychologists, social workers, physical and occupational therapists, and pharmacists. The format of these sessions includes a clinical case presentation, a didactic portion to enhance knowledge of participants, and an open question/answer period. The conferences focus on discussions of challenging clinical cases, addressing common problems (eg, driving concerns), and the assessment/management of geriatric syndromes (eg, cognitive decline, falls, polypharmacy). These conferences aim to improve the knowledge and skills of rural clinical teams in taking care of older veterans and to disseminate best practices in geriatric medicine, using case discussions to highlight practical applications of practices to clinical care. Recent GRECC Connect geriatric case conferences are listed in Table 2 and are recorded and archived to ensure that busy clinicians may access these trainings at the time of their choosing. These materials are catalogued and archived on the EES server.
Early Experience
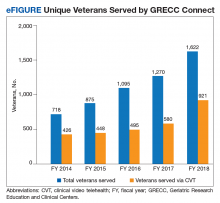
GRECC Connect tracks on an annual basis the number of unique veterans served, number of participating GRECC hub sites and CBOCs, mileage from veteran homes to teleconsultation sites, and number of clinicians and staff engaged in GRECC Connect education programs.16 Since its inception in 2014, the GRECC Connect project has provided direct clinical support to more than 4000 unique veterans (eFigure), of whom half were seen for a cognition-related issue. Consultations were made on behalf of 1,622 veterans in FY 2018, of whom 60% were from rural or highly rural communities and 56.8% were served by CVT visits. The number of GRECC hub sites has increased from 4 in FY 2014 to 12 (of 20 total GRECCs) in FY 2018. The locations of current GRECC hub sites can be found on the Geriatric Scholars website: www.gerischolars.org. Through this expansion, GRECC Connect provides geriatric consultative and educational support to > 70 rural VA clinics in 10 of the 18 Veterans Integrated Service Networks (VISNs).
To assess the reduction in commute times from teleconsultation, we calculated the difference between the mileage from veteran homes to teleconsultation sites (ie, rural clinics) and the mileage from veteran homes to VAMCs where geriatric teams are located. We estimate that the 1622 veterans served in FY 2018 saved a total of 179 121 miles in travel through GRECC Connect. Veterans traveled 106 fewer miles and on average saved $58 in out-of-pocket savings (based on US General Services Administration 2018 standard mileage reimbursement rate of $0.545 per mile). However, many of the veterans have reported anecdotally that the reduction in mileage traveled was less important than the elimination of stress involved in urban navigating, driving, and parking.
More difficult to measure, GRECC Connect seeks to enhance veteran safety by reducing driving distances for older veterans whose driving abilities may be influenced by many age-related health conditions (eg, visual changes, cognitive impairment). For these and other reasons, surveyed veterans overwhelmingly reported that they would be likely to recommend teleconsultation services to other veterans, and that they preferred telemedicine consultation over traveling long distances for in-person clinical consultations.16
Since its inception in 2014, GRECC Connect has provided case-based education to a total of 2335 unique clinicians and staff. Participants have included physicians, nurse practitioners, RNs, social workers, and pharmacists. This distribution reflects the interdisciplinary nature of geriatric care. A plurality of participants (39%) were RNs. Surveyed participants in the GRECC Connect geriatrics case conference series report high overall satisfaction with the learning activity, acquisition of new knowledge and skills, and intention to apply new knowledge and skills to improve job performance.10 In addition, participants agreed that the online training platform was effective for learning and that they would recommend the education series to other HCPs.10,16
Discussion
During its rapid 4-year scale up, GRECC Connect has established a national network and enhanced relationships between GRECC-based clinical teams and rural provider teams. In doing so, the program has begun to improve rural veterans’ access to geriatric specialty care. By providing continuing education to members of the interprofessional health care team, GRECC Connect develops rural providers’ clinical competency and promotes geriatrics skills and expertise. These activities are synergistic: Clinical support enables rural HCPs to become better at managing their own patients, while formal educational activities highlight the availability of specialized consultation available through GRECC Connect. Through ongoing creation of handbooks, workflows, and data analytic strategies, GRECC Connect aims to disseminate this model to additional GRECCs as well as other GEC programs to promote “anywhere to anywhere” VA health care.17
Barriers and Facilitators
GRECC Connect has had notable implementation challenges while new consultation relationships have been forged in order to provide geriatric expertise to rural areas where it is not otherwise available. Many GRECCs had already established connections with rural CBOCs. Among GRECCs that had previously established consultative relationships with rural clinics, the use of telehealth modalities to provide geriatric clinical resources has been a natural extension of these partnerships. GRECCs that lacked these connections, however, often had to obtain buy-in from multiple stakeholders, including rural HCPs and teams, administrative leads, and local telehealth coordinators, and they required VISN- and facility-level leadership to encourage and sustain rural team participation.
Depending on the distance of the GRECC hub-site to the CBOC, efforts to establish and sustain partnerships may require multiple contacts over time (eg, via face-to-face meetings, one-on-one outreach) and large-scale advertising of consultative services. Continuous engagement with CBOC-based teams also involves development of case finding strategies (eg, hospital discharge information, diagnoses, clinical criteria) to better identify veterans who may benefit from GRECC Connect consultation. Owing to the heterogeneity of technological resources, space, scheduling capacity, and staffing at CBOCs, GRECC sites continue to have variable engagement with their CBOC partners.
The inclusion of GRECC Connect within the Geriatric Scholars Program helps ensure that clinician scholars can serve as project champions at their respective rural sites. Rural HCPs with full-time clinical duties initially had difficulty carving out time to participate in GRECC Connect’s case-based conferences. However, the webinar platform has improved and sustained provider participation, and enduring recordings of the presentations allow clinicians to participate in the conferences at their convenience. Finally, the project experienced delays in taking certain administrative steps and hiring staff needed to support the establishment of telehealth modalities—even within a single health care system like the VA, each medical center and regional system has unique policies that complicate how telehealth modalities can be set up.
Conclusion and Future Directions
The GRECC Connect project aims to establish and support meaningful partnerships between urban geriatric specialists and rural HCPs to facilitate veterans’ increased access to geriatric specialty care. VA ORH has recognized it as a Rural Promising Practice, and GRECC Connect is currently being disseminated through an enterprise-wide initiative. Early evidence demonstrates that over 4 years, the expansion of GRECC Connect has helped meet critical aims of improving provider confidence and skills in geriatric management, and of increasing direct service provision. We have also used nationwide education platforms (eg, VA EES) to deliver geriatrics-focused education to health care teams.
Older rural veterans and their caregivers may benefit from this program through decreased travel-associated burden and report high satisfaction with these programs. Through a recently established collaboration with the GEC Data Analysis Center, we will use national data to refine our ability to identify at-risk, older rural veterans and to better evaluate their service needs and the GRECC Connect clinical impact. Because the VA is rapidly expanding use of telehealth and other virtual and digital methods to increase access to care, continued investments in telehealth are central to the VA 5-year strategic plan.18 In this spirit, GRECC Connect will continue to expand its program offerings and to leverage telehealth technologies to meet the needs of older veterans.
Acknowledgments
The authors wish to acknowledge Lisa Tenover, MD, PhD, (Palo Alto GRECC) for her contributions to this manuscript; the VA Rural Health Resource Center–Western Region; and GRECC Connect team members for their tireless work to ensure this project’s success. The GRECC Teams include Atlanta/Birmingham (Julia [Annette] Tedford, RN; Marquitta Cox, LMSW; Lisa Welch, LMSW; Mark Phillips; Lanie Walters, PharmD; Kroshona Tabb, PhD; Robert Langford, and Jason [Thomas] Sanders, HT, TCT); Bronx/NY Harbor (Ab Brody, RN; PhD, GNP-BC; Nick Koufacos, LMSW; and Shatice Jones); Canandaigua (Gary Kochersberger, MD; Suzanne Gillespie, MD; Gary Warner, PhD; Christie Hylwa, RPh CCP; Sharon Fell, LMSW; and Dorian Savino, MPA); Durham (Mamata Yanamadala, MBBS; Christy Knight, LCSW, MSW; and Julie Vognsen); Eastern Colorado (Larry Bourg, MD; Skotti Church, MD; Morgan Elmore, DO; Stephanie Hartz, LCSW; Carolyn Horney, MD; Steven Huart, AuD; Kathryn Nearing, PhD; Elizabeth O’Brien, PharmD; Laurence Robbins, MD; Robert Schwartz, MD; Karen Shea, MD; and Joleen Sussman, PhD); Little Rock (Prasad Padala, MD; and Tanya Taylor, RN); Madison (Ryan Bartkus, MD; Timothy Howell, MD; Lindsay Clark, PhD; Lauren Welch, PharmD, BCGP; Ellen Wanninger, MSW, CAPSW; Stacie Monson, RN, BSN; and Teresa Swader, MSW, LCSW); Miami (Carlos Gomez Orozo); New England (Malissa Kraft, PsyD); Palo Alto (Terri Huh, PhD, ABPP; Philip Choe, DO; Dawna Dougherty, LCSW; Ashley Scales, MPH); Pittsburgh (Stacey Shaffer, MD; Carol Dolbee, CRNP; Nancy Kovell, LCSW; Paul Bulgarelli, DO; Lauren Jost, PsyD; and Marcia Homer, RN-BC); and San Antonio (Becky Powers, MD; Che Kelly, RN, BSN; Cynthia Stewart, LCSW; Rebecca Rottman-Sagebiel, PharmD, BCPS, CGP; Melody Moris; Daniel MacCarthy; and Chen-pin Wang, PhD).
1. US Department of Veterans Affairs. Office of Rural Health Annual report: Thrive 2016. https://www.ruralhealth.va.gov/docs/ORH2016Thrive508_FINAL.pdf. Accessed September 10, 2019.
2. Holder KA. Veterans in Rural America: 2011–2015. US Census Bureau: Washington, DC; 2016. American Community Survey Reports, ACS-36.
3. US Department of Health and Human Services, Health Resources and Services Administration, Bureau of Health Workforce, National Center for Health Workforce Analysis.2017. National and regional projections of supply and demand for geriatricians: 2013-2025. https://bhw.hrsa.gov/sites/default/files/bhw/health-workforce-analysis/research/projections/GeriatricsReport51817.pdf. Published April 2017. Accessed September 10, 2019.
4. Peterson L, Bazemore A, Bragg E, Xierali I, Warshaw GA. Rural–urban distribution of the U.S. geriatrics physician workforce. J Am Geriatr Soc. 2011;59(4):699-703.
5. Lindeman D. Interview: lessons from a leader in telehealth diffusion: a conversation with Adam Darkins of the Veterans Health Administration. Ageing Int. 2010;36(1):146-154.
6. Darkins A, Foster L, Anderson C, Goldschmidt L, Selvin G. The design, implementation, and operational management of a comprehensive quality management program to support national telehealth networks. Telemed J E Health. 2013;19(7):557-564.
7. US Department of Veterans Affairs. Clinical video telehealth into the home (CVTHM)toolkit for providers. https://www.mirecc.va.gov/visn16//docs/CVTHM_Toolkit.pdf. Accessed September 10, 2019.
8. Darkins A. Telehealth services in the United States Department of Veterans Affairs (VA). https://myvitalz.com/wp-content/uploads/2016/07/Telehealth-Services-in-the-United-States.pdf. Published July 2016. Accessed September 10, 2019.
9. US Department of Veterans Affairs. VA announces telemental health clinical resource centers during telemedicine association gathering [press release]. https://www.va.gov/opa/pressrel/includes/viewPDF.cfm?id=2789. Published May 16, 2016. Accessed September 10, 2019.
10. Hung WW, Rossi M, Thielke S, et al. A multisite geriatric education program for rural providers in the Veteran Health Care System (GRECC Connect). Gerontol Geriatr Educ. 2014;35(1):23-40.
11. Kramer BJ. The VA geriatric scholars program. Fed Pract. 2015;32(5):46-48.
12. Kramer BJ, Creekmur B, Howe JL, et al. Veterans Affairs Geriatric Scholars Program: enhancing existing primary care clinician skills in caring for older veterans. J Am Geriatr Soc. 2016;64(11):2343-2348.
13. Powers BB, Homer MC, Morone N, Edmonds N, Rossi MI. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099.
14. Wright SM, Hedin SC, McConnell M, et al. Using shared decision-making to address possible overtreatment in patients at high risk for hypoglycemia: the Veterans Health Administration’s Choosing Wisely Hypoglycemia Safety Initiative. Clin Diabetes. 2018;36(2):120-127.
15. Chang W, Homer M, Rossi MI. Use of clinical video telehealth as a tool for optimizing medications for rural older veterans with dementia. Geriatrics (Basel). 2018;3(3):pii E44.
16. US Department of Veterans Affairs, Office of Rural Health. Rural promising practice issue brief: GRECC Connect Project: connecting rural providers with geriatric specialists through telemedicine. https://www.ruralhealth.va.gov/docs/promise/2017_02_01_Promising%20Practice_GRECC_Issue%20Brief.pdf. Published February 2017. Accessed September 10, 2019.
17. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA expands telehealth by allowing health care providers to treat patients across state lines [press release]. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=4054. Published May 11, 2018. Accessed September 10, 2019.
18. US Department of Veterans Affairs. Department of Veterans Affairs FY 2018 – 2024 strategic plan. https://www.va.gov/oei/docs/VA2018-2024strategicPlan.pdf. Updated May 31, 2019. Accessed September 10, 2019.
Nearly 2.7 million veterans who rely on the Veterans Health Administration (VHA) for their health care live in rural communities.1 Of these, more than half are aged ≥ 65 years. Rural veterans have greater rates of service-related disability and chronic medical conditions than do their urban counterparts.1,2 Yet because of their rural location, they face unique challenges, including long travel times and distances to health care services, lack of public transportation options, and limited availability of specialized medical and social support services.
Compounding these geographic barriers is a more general lack of workforce infrastructure and a dearth of clinical health care providers (HCPs) skilled in geriatric medicine. The demand for geriatricians is projected to outpace supply and result in a national shortage of nearly 27 000 geriatricians by 2025.3 Moreover, the overwhelming majority (90%) of HCPs identifying as geriatric specialists reside in urban areas.4 This creates tremendous pressure on the health care system to provide remote care for older veterans contending with complex conditions, and ultimately these veterans may not receive the specialized care they need.
Telehealth modalities bridge these gaps by bringing health care to veterans in rural communities. They may also hold promise for strengthening community care in rural areas through workforce development and dissemination of educational resources. The VHA has been recognized as a leader in the field of telehealth since it began offering telehealth services to veterans in 19775-8 and served more than 677 000 Veterans via telehealth in fiscal year (FY) 2015.9 The VHA currently employs multiple modes of telehealth to increase veterans’ access to health care, including: (1) synchronous technology like clinical video telehealth (CVT), which provides live encounters between HCPs and patients using videoconferencing software; and (2) asynchronous technology, such as store-and-forward communication that offers remote transmission and clinical interpretation of veteran health data. The VHA has also strengthened its broad telehealth infrastructure by staffing VHA clinical sites with telehealth clinical technicians and providing telehealth hardware throughout.
The Department of Veterans Affairs (VA) Office of Geriatrics and Extended Care (GEC) and Office of Rural Health (ORH) established the Geriatric Research Education and Clinical Centers (GRECC) Connect project in 2014 to leverage the existing telehealth technologies at the VA to meet the health care needs of older veterans. GRECC Connect builds on the VHA network of geriatrics expertise in GRECCs by providing telehealth-based consultative support for rural primary care provider (PCP) teams, older veterans, and their families. This program profile describes this project’s mission, structure, and activities.
Program Overview
GRECC Connect leverages the clinical expertise and administrative infrastructure of participating GRECCs in order to reach clinicians and veterans in primarily rural communities.10 GRECCs are VA centers of excellence focused on aging and comprise a large network of interdisciplinary geriatrics expertise. All GRECCs have strong affiliations with local universities and are located in urban VA medical centers (VAMCs). GRECC Connect is based on a hub-and-spoke model in which urban GRECC hub sites are connected to community-based outpatient clinic (CBOC) and VAMC spokes that primarily serve veterans in other communities. CBOCs are stand-alone clinics that are geographically separate from a related VA medical center and provide outpatient primary care, mental health care services, and some specialty care services such as cardiology or neurology. They range in size from small, mainly telehealth clinics with 1 technician to large clinics with several specialty providers. Each GRECC hub site partners with an average of 6 CBOCs (range 3-16), each of which is an average distance of 92.8 miles from the related VA medical center (range 20-406 miles).
GRECC Connect was established under the umbrella of the VA Geriatric Scholars Program, which since 2008 integrates geriatrics into rural primary care practices through tailored education for continuing professional development.11 Through intensive courses in geriatrics and quality improvement methods and through participation in local quality improvement projects benefiting older veterans, the Geriatric Scholars Program trains rural PCPs so that they can more effectively and independently diagnose and manage common geriatric syndromes.12 The network of clinician scholars developed by the Geriatric Scholars Program, all rural frontline clinicians at VA clinics, has given the GRECC Connect project a well-prepared, geriatrics-trained workforce to act as project champions at rural CBOCs and VAMCs. The GRECC Connect project’s goals are to enhance access to geriatric specialty care among older veterans with complex medical problems, geriatric syndromes, and increased risk for institutionalization, and to provide geriatrics-focused educational support to rural HCP teams.
Geriatric Provider Consultations
The first overarching goal of the GRECC Connect project is to improve access to geriatrics specialty care by facilitating linkages between GRECC hub sites and the CBOCs and VAMCs that primarily serve veterans in rural communities. GRECC hub sites offer consultative support from geriatrics specialty team members (eg, geriatricians, nurse practitioners, pharmacists, gero- or neuropsychologists, registered nurses [RNs], and social workers) to rural PCP in their catchment area. This support is offered through a variety of telehealth modalities readily available in the VA (Table 1). These include CVT, in which a veteran located at a rural CBOC is seen using videoconferencing software by a geriatrics specialty provider who is located at a GRECC hub site. At some GRECC hub sites, CVT has also been used to conduct group visits between a GRECC provider at the hub site and several veterans who participate from a rural CBOC. Electronic consultations, or e-consults, involve a rural provider entering a clinical question in the VA Computerized Patient Record System. The question is then triaged, and a geriatrics provider at a GRECC responds, based on review of that veteran’s chart. At some GRECC hub sites, the e-consults are more extensive and may include telephone contact with the veteran or their caregiver.
Consultations between GRECC-based teams and rural PCPs may cover any aspect of geriatrics care, ranging from broad concerns to subspecialty areas of geriatric medicine. For instance, general geriatrics consultation may address polypharmacy, during either care transitions or ongoing care. Consultation may also reflect the specific focus area of a particular GRECC, such as cognitive assessment (eg, Pittsburgh GRECC), management of osteoporosis to address falls (eg, Durham GRECC, Miami GRECC), and continence care (eg, Birmingham/Atlanta GRECC).13 Most consultations are initiated by a remote HCP who is seeking geriatrics expertise from the GRECC team.
Some GRECC hub sites, however, employ case finding strategies, or detailed chart reviews, in order to identify older veterans who may benefit from geriatrics consultation. For veterans identified through those mechanisms, the GRECC clinicians suggest that the rural HCP either request or allow an e-consult or evaluation via CVT for those veterans. The geriatric consultations may help identify additional care needs for older veterans and lead to recommendations, orders, or remote provision of a variety of other actions, including VA or non-VA services (eg, home-based primary care, home nursing service, respite service, social support services such as Meals on Wheels); neuropsychological testing; physical or occupational therapy; audiology or optometry referral; falls and fracture risk assessment and interventions to reduce falls (eg, home safety evaluation, physical therapy); osteoporosis risk assessments (eg, densitometry, recommendations for pharmacologic therapy) to reduce the risk of injury or nontraumatic fractures from falls; palliative care for incontinence and hospice; and counseling on geriatric issues such as dementia caregiving, advanced directives, and driving cessation.
More recently, the Miami GRECC has begun evaluating rural veterans at risk for hypoglycemia, providing patient education and counseling about hypoglycemia, and making recommendations to the veterans’ primary care teams.14 Consultations may also lead to the appropriate use or discontinuation of medications, related to polypharmacy. GRECC-based teams, for example, have helped rural HCPs modify medication doses, start appropriate medications for dementia and depression, and identify and stop potentially inappropriate medications (eg, those that increase fall risk or that have significant anticholinergic properties).15
GRECC Connect Geriatric Case Conference Series
The second overarching goal of the GRECC Connect project is to provide geriatrics-focused educational support to equip PCPs to better serve their aging veteran patients. This is achieved through twice-monthly, case-based conferences supported by the VA Employee Education System (EES) and delivered through a webinar interface. Case conferences are targeted to members of the health care team who may provide care for rural older adults, including physicians, nurse practitioners, physician assistants, RNs, psychologists, social workers, physical and occupational therapists, and pharmacists. The format of these sessions includes a clinical case presentation, a didactic portion to enhance knowledge of participants, and an open question/answer period. The conferences focus on discussions of challenging clinical cases, addressing common problems (eg, driving concerns), and the assessment/management of geriatric syndromes (eg, cognitive decline, falls, polypharmacy). These conferences aim to improve the knowledge and skills of rural clinical teams in taking care of older veterans and to disseminate best practices in geriatric medicine, using case discussions to highlight practical applications of practices to clinical care. Recent GRECC Connect geriatric case conferences are listed in Table 2 and are recorded and archived to ensure that busy clinicians may access these trainings at the time of their choosing. These materials are catalogued and archived on the EES server.
Early Experience
GRECC Connect tracks on an annual basis the number of unique veterans served, number of participating GRECC hub sites and CBOCs, mileage from veteran homes to teleconsultation sites, and number of clinicians and staff engaged in GRECC Connect education programs.16 Since its inception in 2014, the GRECC Connect project has provided direct clinical support to more than 4000 unique veterans (eFigure), of whom half were seen for a cognition-related issue. Consultations were made on behalf of 1,622 veterans in FY 2018, of whom 60% were from rural or highly rural communities and 56.8% were served by CVT visits. The number of GRECC hub sites has increased from 4 in FY 2014 to 12 (of 20 total GRECCs) in FY 2018. The locations of current GRECC hub sites can be found on the Geriatric Scholars website: www.gerischolars.org. Through this expansion, GRECC Connect provides geriatric consultative and educational support to > 70 rural VA clinics in 10 of the 18 Veterans Integrated Service Networks (VISNs).
To assess the reduction in commute times from teleconsultation, we calculated the difference between the mileage from veteran homes to teleconsultation sites (ie, rural clinics) and the mileage from veteran homes to VAMCs where geriatric teams are located. We estimate that the 1622 veterans served in FY 2018 saved a total of 179 121 miles in travel through GRECC Connect. Veterans traveled 106 fewer miles and on average saved $58 in out-of-pocket savings (based on US General Services Administration 2018 standard mileage reimbursement rate of $0.545 per mile). However, many of the veterans have reported anecdotally that the reduction in mileage traveled was less important than the elimination of stress involved in urban navigating, driving, and parking.
More difficult to measure, GRECC Connect seeks to enhance veteran safety by reducing driving distances for older veterans whose driving abilities may be influenced by many age-related health conditions (eg, visual changes, cognitive impairment). For these and other reasons, surveyed veterans overwhelmingly reported that they would be likely to recommend teleconsultation services to other veterans, and that they preferred telemedicine consultation over traveling long distances for in-person clinical consultations.16
Since its inception in 2014, GRECC Connect has provided case-based education to a total of 2335 unique clinicians and staff. Participants have included physicians, nurse practitioners, RNs, social workers, and pharmacists. This distribution reflects the interdisciplinary nature of geriatric care. A plurality of participants (39%) were RNs. Surveyed participants in the GRECC Connect geriatrics case conference series report high overall satisfaction with the learning activity, acquisition of new knowledge and skills, and intention to apply new knowledge and skills to improve job performance.10 In addition, participants agreed that the online training platform was effective for learning and that they would recommend the education series to other HCPs.10,16
Discussion
During its rapid 4-year scale up, GRECC Connect has established a national network and enhanced relationships between GRECC-based clinical teams and rural provider teams. In doing so, the program has begun to improve rural veterans’ access to geriatric specialty care. By providing continuing education to members of the interprofessional health care team, GRECC Connect develops rural providers’ clinical competency and promotes geriatrics skills and expertise. These activities are synergistic: Clinical support enables rural HCPs to become better at managing their own patients, while formal educational activities highlight the availability of specialized consultation available through GRECC Connect. Through ongoing creation of handbooks, workflows, and data analytic strategies, GRECC Connect aims to disseminate this model to additional GRECCs as well as other GEC programs to promote “anywhere to anywhere” VA health care.17
Barriers and Facilitators
GRECC Connect has had notable implementation challenges while new consultation relationships have been forged in order to provide geriatric expertise to rural areas where it is not otherwise available. Many GRECCs had already established connections with rural CBOCs. Among GRECCs that had previously established consultative relationships with rural clinics, the use of telehealth modalities to provide geriatric clinical resources has been a natural extension of these partnerships. GRECCs that lacked these connections, however, often had to obtain buy-in from multiple stakeholders, including rural HCPs and teams, administrative leads, and local telehealth coordinators, and they required VISN- and facility-level leadership to encourage and sustain rural team participation.
Depending on the distance of the GRECC hub-site to the CBOC, efforts to establish and sustain partnerships may require multiple contacts over time (eg, via face-to-face meetings, one-on-one outreach) and large-scale advertising of consultative services. Continuous engagement with CBOC-based teams also involves development of case finding strategies (eg, hospital discharge information, diagnoses, clinical criteria) to better identify veterans who may benefit from GRECC Connect consultation. Owing to the heterogeneity of technological resources, space, scheduling capacity, and staffing at CBOCs, GRECC sites continue to have variable engagement with their CBOC partners.
The inclusion of GRECC Connect within the Geriatric Scholars Program helps ensure that clinician scholars can serve as project champions at their respective rural sites. Rural HCPs with full-time clinical duties initially had difficulty carving out time to participate in GRECC Connect’s case-based conferences. However, the webinar platform has improved and sustained provider participation, and enduring recordings of the presentations allow clinicians to participate in the conferences at their convenience. Finally, the project experienced delays in taking certain administrative steps and hiring staff needed to support the establishment of telehealth modalities—even within a single health care system like the VA, each medical center and regional system has unique policies that complicate how telehealth modalities can be set up.
Conclusion and Future Directions
The GRECC Connect project aims to establish and support meaningful partnerships between urban geriatric specialists and rural HCPs to facilitate veterans’ increased access to geriatric specialty care. VA ORH has recognized it as a Rural Promising Practice, and GRECC Connect is currently being disseminated through an enterprise-wide initiative. Early evidence demonstrates that over 4 years, the expansion of GRECC Connect has helped meet critical aims of improving provider confidence and skills in geriatric management, and of increasing direct service provision. We have also used nationwide education platforms (eg, VA EES) to deliver geriatrics-focused education to health care teams.
Older rural veterans and their caregivers may benefit from this program through decreased travel-associated burden and report high satisfaction with these programs. Through a recently established collaboration with the GEC Data Analysis Center, we will use national data to refine our ability to identify at-risk, older rural veterans and to better evaluate their service needs and the GRECC Connect clinical impact. Because the VA is rapidly expanding use of telehealth and other virtual and digital methods to increase access to care, continued investments in telehealth are central to the VA 5-year strategic plan.18 In this spirit, GRECC Connect will continue to expand its program offerings and to leverage telehealth technologies to meet the needs of older veterans.
Acknowledgments
The authors wish to acknowledge Lisa Tenover, MD, PhD, (Palo Alto GRECC) for her contributions to this manuscript; the VA Rural Health Resource Center–Western Region; and GRECC Connect team members for their tireless work to ensure this project’s success. The GRECC Teams include Atlanta/Birmingham (Julia [Annette] Tedford, RN; Marquitta Cox, LMSW; Lisa Welch, LMSW; Mark Phillips; Lanie Walters, PharmD; Kroshona Tabb, PhD; Robert Langford, and Jason [Thomas] Sanders, HT, TCT); Bronx/NY Harbor (Ab Brody, RN; PhD, GNP-BC; Nick Koufacos, LMSW; and Shatice Jones); Canandaigua (Gary Kochersberger, MD; Suzanne Gillespie, MD; Gary Warner, PhD; Christie Hylwa, RPh CCP; Sharon Fell, LMSW; and Dorian Savino, MPA); Durham (Mamata Yanamadala, MBBS; Christy Knight, LCSW, MSW; and Julie Vognsen); Eastern Colorado (Larry Bourg, MD; Skotti Church, MD; Morgan Elmore, DO; Stephanie Hartz, LCSW; Carolyn Horney, MD; Steven Huart, AuD; Kathryn Nearing, PhD; Elizabeth O’Brien, PharmD; Laurence Robbins, MD; Robert Schwartz, MD; Karen Shea, MD; and Joleen Sussman, PhD); Little Rock (Prasad Padala, MD; and Tanya Taylor, RN); Madison (Ryan Bartkus, MD; Timothy Howell, MD; Lindsay Clark, PhD; Lauren Welch, PharmD, BCGP; Ellen Wanninger, MSW, CAPSW; Stacie Monson, RN, BSN; and Teresa Swader, MSW, LCSW); Miami (Carlos Gomez Orozo); New England (Malissa Kraft, PsyD); Palo Alto (Terri Huh, PhD, ABPP; Philip Choe, DO; Dawna Dougherty, LCSW; Ashley Scales, MPH); Pittsburgh (Stacey Shaffer, MD; Carol Dolbee, CRNP; Nancy Kovell, LCSW; Paul Bulgarelli, DO; Lauren Jost, PsyD; and Marcia Homer, RN-BC); and San Antonio (Becky Powers, MD; Che Kelly, RN, BSN; Cynthia Stewart, LCSW; Rebecca Rottman-Sagebiel, PharmD, BCPS, CGP; Melody Moris; Daniel MacCarthy; and Chen-pin Wang, PhD).
Nearly 2.7 million veterans who rely on the Veterans Health Administration (VHA) for their health care live in rural communities.1 Of these, more than half are aged ≥ 65 years. Rural veterans have greater rates of service-related disability and chronic medical conditions than do their urban counterparts.1,2 Yet because of their rural location, they face unique challenges, including long travel times and distances to health care services, lack of public transportation options, and limited availability of specialized medical and social support services.
Compounding these geographic barriers is a more general lack of workforce infrastructure and a dearth of clinical health care providers (HCPs) skilled in geriatric medicine. The demand for geriatricians is projected to outpace supply and result in a national shortage of nearly 27 000 geriatricians by 2025.3 Moreover, the overwhelming majority (90%) of HCPs identifying as geriatric specialists reside in urban areas.4 This creates tremendous pressure on the health care system to provide remote care for older veterans contending with complex conditions, and ultimately these veterans may not receive the specialized care they need.
Telehealth modalities bridge these gaps by bringing health care to veterans in rural communities. They may also hold promise for strengthening community care in rural areas through workforce development and dissemination of educational resources. The VHA has been recognized as a leader in the field of telehealth since it began offering telehealth services to veterans in 19775-8 and served more than 677 000 Veterans via telehealth in fiscal year (FY) 2015.9 The VHA currently employs multiple modes of telehealth to increase veterans’ access to health care, including: (1) synchronous technology like clinical video telehealth (CVT), which provides live encounters between HCPs and patients using videoconferencing software; and (2) asynchronous technology, such as store-and-forward communication that offers remote transmission and clinical interpretation of veteran health data. The VHA has also strengthened its broad telehealth infrastructure by staffing VHA clinical sites with telehealth clinical technicians and providing telehealth hardware throughout.
The Department of Veterans Affairs (VA) Office of Geriatrics and Extended Care (GEC) and Office of Rural Health (ORH) established the Geriatric Research Education and Clinical Centers (GRECC) Connect project in 2014 to leverage the existing telehealth technologies at the VA to meet the health care needs of older veterans. GRECC Connect builds on the VHA network of geriatrics expertise in GRECCs by providing telehealth-based consultative support for rural primary care provider (PCP) teams, older veterans, and their families. This program profile describes this project’s mission, structure, and activities.
Program Overview
GRECC Connect leverages the clinical expertise and administrative infrastructure of participating GRECCs in order to reach clinicians and veterans in primarily rural communities.10 GRECCs are VA centers of excellence focused on aging and comprise a large network of interdisciplinary geriatrics expertise. All GRECCs have strong affiliations with local universities and are located in urban VA medical centers (VAMCs). GRECC Connect is based on a hub-and-spoke model in which urban GRECC hub sites are connected to community-based outpatient clinic (CBOC) and VAMC spokes that primarily serve veterans in other communities. CBOCs are stand-alone clinics that are geographically separate from a related VA medical center and provide outpatient primary care, mental health care services, and some specialty care services such as cardiology or neurology. They range in size from small, mainly telehealth clinics with 1 technician to large clinics with several specialty providers. Each GRECC hub site partners with an average of 6 CBOCs (range 3-16), each of which is an average distance of 92.8 miles from the related VA medical center (range 20-406 miles).
GRECC Connect was established under the umbrella of the VA Geriatric Scholars Program, which since 2008 integrates geriatrics into rural primary care practices through tailored education for continuing professional development.11 Through intensive courses in geriatrics and quality improvement methods and through participation in local quality improvement projects benefiting older veterans, the Geriatric Scholars Program trains rural PCPs so that they can more effectively and independently diagnose and manage common geriatric syndromes.12 The network of clinician scholars developed by the Geriatric Scholars Program, all rural frontline clinicians at VA clinics, has given the GRECC Connect project a well-prepared, geriatrics-trained workforce to act as project champions at rural CBOCs and VAMCs. The GRECC Connect project’s goals are to enhance access to geriatric specialty care among older veterans with complex medical problems, geriatric syndromes, and increased risk for institutionalization, and to provide geriatrics-focused educational support to rural HCP teams.
Geriatric Provider Consultations
The first overarching goal of the GRECC Connect project is to improve access to geriatrics specialty care by facilitating linkages between GRECC hub sites and the CBOCs and VAMCs that primarily serve veterans in rural communities. GRECC hub sites offer consultative support from geriatrics specialty team members (eg, geriatricians, nurse practitioners, pharmacists, gero- or neuropsychologists, registered nurses [RNs], and social workers) to rural PCP in their catchment area. This support is offered through a variety of telehealth modalities readily available in the VA (Table 1). These include CVT, in which a veteran located at a rural CBOC is seen using videoconferencing software by a geriatrics specialty provider who is located at a GRECC hub site. At some GRECC hub sites, CVT has also been used to conduct group visits between a GRECC provider at the hub site and several veterans who participate from a rural CBOC. Electronic consultations, or e-consults, involve a rural provider entering a clinical question in the VA Computerized Patient Record System. The question is then triaged, and a geriatrics provider at a GRECC responds, based on review of that veteran’s chart. At some GRECC hub sites, the e-consults are more extensive and may include telephone contact with the veteran or their caregiver.
Consultations between GRECC-based teams and rural PCPs may cover any aspect of geriatrics care, ranging from broad concerns to subspecialty areas of geriatric medicine. For instance, general geriatrics consultation may address polypharmacy, during either care transitions or ongoing care. Consultation may also reflect the specific focus area of a particular GRECC, such as cognitive assessment (eg, Pittsburgh GRECC), management of osteoporosis to address falls (eg, Durham GRECC, Miami GRECC), and continence care (eg, Birmingham/Atlanta GRECC).13 Most consultations are initiated by a remote HCP who is seeking geriatrics expertise from the GRECC team.
Some GRECC hub sites, however, employ case finding strategies, or detailed chart reviews, in order to identify older veterans who may benefit from geriatrics consultation. For veterans identified through those mechanisms, the GRECC clinicians suggest that the rural HCP either request or allow an e-consult or evaluation via CVT for those veterans. The geriatric consultations may help identify additional care needs for older veterans and lead to recommendations, orders, or remote provision of a variety of other actions, including VA or non-VA services (eg, home-based primary care, home nursing service, respite service, social support services such as Meals on Wheels); neuropsychological testing; physical or occupational therapy; audiology or optometry referral; falls and fracture risk assessment and interventions to reduce falls (eg, home safety evaluation, physical therapy); osteoporosis risk assessments (eg, densitometry, recommendations for pharmacologic therapy) to reduce the risk of injury or nontraumatic fractures from falls; palliative care for incontinence and hospice; and counseling on geriatric issues such as dementia caregiving, advanced directives, and driving cessation.
More recently, the Miami GRECC has begun evaluating rural veterans at risk for hypoglycemia, providing patient education and counseling about hypoglycemia, and making recommendations to the veterans’ primary care teams.14 Consultations may also lead to the appropriate use or discontinuation of medications, related to polypharmacy. GRECC-based teams, for example, have helped rural HCPs modify medication doses, start appropriate medications for dementia and depression, and identify and stop potentially inappropriate medications (eg, those that increase fall risk or that have significant anticholinergic properties).15
GRECC Connect Geriatric Case Conference Series
The second overarching goal of the GRECC Connect project is to provide geriatrics-focused educational support to equip PCPs to better serve their aging veteran patients. This is achieved through twice-monthly, case-based conferences supported by the VA Employee Education System (EES) and delivered through a webinar interface. Case conferences are targeted to members of the health care team who may provide care for rural older adults, including physicians, nurse practitioners, physician assistants, RNs, psychologists, social workers, physical and occupational therapists, and pharmacists. The format of these sessions includes a clinical case presentation, a didactic portion to enhance knowledge of participants, and an open question/answer period. The conferences focus on discussions of challenging clinical cases, addressing common problems (eg, driving concerns), and the assessment/management of geriatric syndromes (eg, cognitive decline, falls, polypharmacy). These conferences aim to improve the knowledge and skills of rural clinical teams in taking care of older veterans and to disseminate best practices in geriatric medicine, using case discussions to highlight practical applications of practices to clinical care. Recent GRECC Connect geriatric case conferences are listed in Table 2 and are recorded and archived to ensure that busy clinicians may access these trainings at the time of their choosing. These materials are catalogued and archived on the EES server.
Early Experience
GRECC Connect tracks on an annual basis the number of unique veterans served, number of participating GRECC hub sites and CBOCs, mileage from veteran homes to teleconsultation sites, and number of clinicians and staff engaged in GRECC Connect education programs.16 Since its inception in 2014, the GRECC Connect project has provided direct clinical support to more than 4000 unique veterans (eFigure), of whom half were seen for a cognition-related issue. Consultations were made on behalf of 1,622 veterans in FY 2018, of whom 60% were from rural or highly rural communities and 56.8% were served by CVT visits. The number of GRECC hub sites has increased from 4 in FY 2014 to 12 (of 20 total GRECCs) in FY 2018. The locations of current GRECC hub sites can be found on the Geriatric Scholars website: www.gerischolars.org. Through this expansion, GRECC Connect provides geriatric consultative and educational support to > 70 rural VA clinics in 10 of the 18 Veterans Integrated Service Networks (VISNs).
To assess the reduction in commute times from teleconsultation, we calculated the difference between the mileage from veteran homes to teleconsultation sites (ie, rural clinics) and the mileage from veteran homes to VAMCs where geriatric teams are located. We estimate that the 1622 veterans served in FY 2018 saved a total of 179 121 miles in travel through GRECC Connect. Veterans traveled 106 fewer miles and on average saved $58 in out-of-pocket savings (based on US General Services Administration 2018 standard mileage reimbursement rate of $0.545 per mile). However, many of the veterans have reported anecdotally that the reduction in mileage traveled was less important than the elimination of stress involved in urban navigating, driving, and parking.
More difficult to measure, GRECC Connect seeks to enhance veteran safety by reducing driving distances for older veterans whose driving abilities may be influenced by many age-related health conditions (eg, visual changes, cognitive impairment). For these and other reasons, surveyed veterans overwhelmingly reported that they would be likely to recommend teleconsultation services to other veterans, and that they preferred telemedicine consultation over traveling long distances for in-person clinical consultations.16
Since its inception in 2014, GRECC Connect has provided case-based education to a total of 2335 unique clinicians and staff. Participants have included physicians, nurse practitioners, RNs, social workers, and pharmacists. This distribution reflects the interdisciplinary nature of geriatric care. A plurality of participants (39%) were RNs. Surveyed participants in the GRECC Connect geriatrics case conference series report high overall satisfaction with the learning activity, acquisition of new knowledge and skills, and intention to apply new knowledge and skills to improve job performance.10 In addition, participants agreed that the online training platform was effective for learning and that they would recommend the education series to other HCPs.10,16
Discussion
During its rapid 4-year scale up, GRECC Connect has established a national network and enhanced relationships between GRECC-based clinical teams and rural provider teams. In doing so, the program has begun to improve rural veterans’ access to geriatric specialty care. By providing continuing education to members of the interprofessional health care team, GRECC Connect develops rural providers’ clinical competency and promotes geriatrics skills and expertise. These activities are synergistic: Clinical support enables rural HCPs to become better at managing their own patients, while formal educational activities highlight the availability of specialized consultation available through GRECC Connect. Through ongoing creation of handbooks, workflows, and data analytic strategies, GRECC Connect aims to disseminate this model to additional GRECCs as well as other GEC programs to promote “anywhere to anywhere” VA health care.17
Barriers and Facilitators
GRECC Connect has had notable implementation challenges while new consultation relationships have been forged in order to provide geriatric expertise to rural areas where it is not otherwise available. Many GRECCs had already established connections with rural CBOCs. Among GRECCs that had previously established consultative relationships with rural clinics, the use of telehealth modalities to provide geriatric clinical resources has been a natural extension of these partnerships. GRECCs that lacked these connections, however, often had to obtain buy-in from multiple stakeholders, including rural HCPs and teams, administrative leads, and local telehealth coordinators, and they required VISN- and facility-level leadership to encourage and sustain rural team participation.
Depending on the distance of the GRECC hub-site to the CBOC, efforts to establish and sustain partnerships may require multiple contacts over time (eg, via face-to-face meetings, one-on-one outreach) and large-scale advertising of consultative services. Continuous engagement with CBOC-based teams also involves development of case finding strategies (eg, hospital discharge information, diagnoses, clinical criteria) to better identify veterans who may benefit from GRECC Connect consultation. Owing to the heterogeneity of technological resources, space, scheduling capacity, and staffing at CBOCs, GRECC sites continue to have variable engagement with their CBOC partners.
The inclusion of GRECC Connect within the Geriatric Scholars Program helps ensure that clinician scholars can serve as project champions at their respective rural sites. Rural HCPs with full-time clinical duties initially had difficulty carving out time to participate in GRECC Connect’s case-based conferences. However, the webinar platform has improved and sustained provider participation, and enduring recordings of the presentations allow clinicians to participate in the conferences at their convenience. Finally, the project experienced delays in taking certain administrative steps and hiring staff needed to support the establishment of telehealth modalities—even within a single health care system like the VA, each medical center and regional system has unique policies that complicate how telehealth modalities can be set up.
Conclusion and Future Directions
The GRECC Connect project aims to establish and support meaningful partnerships between urban geriatric specialists and rural HCPs to facilitate veterans’ increased access to geriatric specialty care. VA ORH has recognized it as a Rural Promising Practice, and GRECC Connect is currently being disseminated through an enterprise-wide initiative. Early evidence demonstrates that over 4 years, the expansion of GRECC Connect has helped meet critical aims of improving provider confidence and skills in geriatric management, and of increasing direct service provision. We have also used nationwide education platforms (eg, VA EES) to deliver geriatrics-focused education to health care teams.
Older rural veterans and their caregivers may benefit from this program through decreased travel-associated burden and report high satisfaction with these programs. Through a recently established collaboration with the GEC Data Analysis Center, we will use national data to refine our ability to identify at-risk, older rural veterans and to better evaluate their service needs and the GRECC Connect clinical impact. Because the VA is rapidly expanding use of telehealth and other virtual and digital methods to increase access to care, continued investments in telehealth are central to the VA 5-year strategic plan.18 In this spirit, GRECC Connect will continue to expand its program offerings and to leverage telehealth technologies to meet the needs of older veterans.
Acknowledgments
The authors wish to acknowledge Lisa Tenover, MD, PhD, (Palo Alto GRECC) for her contributions to this manuscript; the VA Rural Health Resource Center–Western Region; and GRECC Connect team members for their tireless work to ensure this project’s success. The GRECC Teams include Atlanta/Birmingham (Julia [Annette] Tedford, RN; Marquitta Cox, LMSW; Lisa Welch, LMSW; Mark Phillips; Lanie Walters, PharmD; Kroshona Tabb, PhD; Robert Langford, and Jason [Thomas] Sanders, HT, TCT); Bronx/NY Harbor (Ab Brody, RN; PhD, GNP-BC; Nick Koufacos, LMSW; and Shatice Jones); Canandaigua (Gary Kochersberger, MD; Suzanne Gillespie, MD; Gary Warner, PhD; Christie Hylwa, RPh CCP; Sharon Fell, LMSW; and Dorian Savino, MPA); Durham (Mamata Yanamadala, MBBS; Christy Knight, LCSW, MSW; and Julie Vognsen); Eastern Colorado (Larry Bourg, MD; Skotti Church, MD; Morgan Elmore, DO; Stephanie Hartz, LCSW; Carolyn Horney, MD; Steven Huart, AuD; Kathryn Nearing, PhD; Elizabeth O’Brien, PharmD; Laurence Robbins, MD; Robert Schwartz, MD; Karen Shea, MD; and Joleen Sussman, PhD); Little Rock (Prasad Padala, MD; and Tanya Taylor, RN); Madison (Ryan Bartkus, MD; Timothy Howell, MD; Lindsay Clark, PhD; Lauren Welch, PharmD, BCGP; Ellen Wanninger, MSW, CAPSW; Stacie Monson, RN, BSN; and Teresa Swader, MSW, LCSW); Miami (Carlos Gomez Orozo); New England (Malissa Kraft, PsyD); Palo Alto (Terri Huh, PhD, ABPP; Philip Choe, DO; Dawna Dougherty, LCSW; Ashley Scales, MPH); Pittsburgh (Stacey Shaffer, MD; Carol Dolbee, CRNP; Nancy Kovell, LCSW; Paul Bulgarelli, DO; Lauren Jost, PsyD; and Marcia Homer, RN-BC); and San Antonio (Becky Powers, MD; Che Kelly, RN, BSN; Cynthia Stewart, LCSW; Rebecca Rottman-Sagebiel, PharmD, BCPS, CGP; Melody Moris; Daniel MacCarthy; and Chen-pin Wang, PhD).
1. US Department of Veterans Affairs. Office of Rural Health Annual report: Thrive 2016. https://www.ruralhealth.va.gov/docs/ORH2016Thrive508_FINAL.pdf. Accessed September 10, 2019.
2. Holder KA. Veterans in Rural America: 2011–2015. US Census Bureau: Washington, DC; 2016. American Community Survey Reports, ACS-36.
3. US Department of Health and Human Services, Health Resources and Services Administration, Bureau of Health Workforce, National Center for Health Workforce Analysis.2017. National and regional projections of supply and demand for geriatricians: 2013-2025. https://bhw.hrsa.gov/sites/default/files/bhw/health-workforce-analysis/research/projections/GeriatricsReport51817.pdf. Published April 2017. Accessed September 10, 2019.
4. Peterson L, Bazemore A, Bragg E, Xierali I, Warshaw GA. Rural–urban distribution of the U.S. geriatrics physician workforce. J Am Geriatr Soc. 2011;59(4):699-703.
5. Lindeman D. Interview: lessons from a leader in telehealth diffusion: a conversation with Adam Darkins of the Veterans Health Administration. Ageing Int. 2010;36(1):146-154.
6. Darkins A, Foster L, Anderson C, Goldschmidt L, Selvin G. The design, implementation, and operational management of a comprehensive quality management program to support national telehealth networks. Telemed J E Health. 2013;19(7):557-564.
7. US Department of Veterans Affairs. Clinical video telehealth into the home (CVTHM)toolkit for providers. https://www.mirecc.va.gov/visn16//docs/CVTHM_Toolkit.pdf. Accessed September 10, 2019.
8. Darkins A. Telehealth services in the United States Department of Veterans Affairs (VA). https://myvitalz.com/wp-content/uploads/2016/07/Telehealth-Services-in-the-United-States.pdf. Published July 2016. Accessed September 10, 2019.
9. US Department of Veterans Affairs. VA announces telemental health clinical resource centers during telemedicine association gathering [press release]. https://www.va.gov/opa/pressrel/includes/viewPDF.cfm?id=2789. Published May 16, 2016. Accessed September 10, 2019.
10. Hung WW, Rossi M, Thielke S, et al. A multisite geriatric education program for rural providers in the Veteran Health Care System (GRECC Connect). Gerontol Geriatr Educ. 2014;35(1):23-40.
11. Kramer BJ. The VA geriatric scholars program. Fed Pract. 2015;32(5):46-48.
12. Kramer BJ, Creekmur B, Howe JL, et al. Veterans Affairs Geriatric Scholars Program: enhancing existing primary care clinician skills in caring for older veterans. J Am Geriatr Soc. 2016;64(11):2343-2348.
13. Powers BB, Homer MC, Morone N, Edmonds N, Rossi MI. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099.
14. Wright SM, Hedin SC, McConnell M, et al. Using shared decision-making to address possible overtreatment in patients at high risk for hypoglycemia: the Veterans Health Administration’s Choosing Wisely Hypoglycemia Safety Initiative. Clin Diabetes. 2018;36(2):120-127.
15. Chang W, Homer M, Rossi MI. Use of clinical video telehealth as a tool for optimizing medications for rural older veterans with dementia. Geriatrics (Basel). 2018;3(3):pii E44.
16. US Department of Veterans Affairs, Office of Rural Health. Rural promising practice issue brief: GRECC Connect Project: connecting rural providers with geriatric specialists through telemedicine. https://www.ruralhealth.va.gov/docs/promise/2017_02_01_Promising%20Practice_GRECC_Issue%20Brief.pdf. Published February 2017. Accessed September 10, 2019.
17. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA expands telehealth by allowing health care providers to treat patients across state lines [press release]. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=4054. Published May 11, 2018. Accessed September 10, 2019.
18. US Department of Veterans Affairs. Department of Veterans Affairs FY 2018 – 2024 strategic plan. https://www.va.gov/oei/docs/VA2018-2024strategicPlan.pdf. Updated May 31, 2019. Accessed September 10, 2019.
1. US Department of Veterans Affairs. Office of Rural Health Annual report: Thrive 2016. https://www.ruralhealth.va.gov/docs/ORH2016Thrive508_FINAL.pdf. Accessed September 10, 2019.
2. Holder KA. Veterans in Rural America: 2011–2015. US Census Bureau: Washington, DC; 2016. American Community Survey Reports, ACS-36.
3. US Department of Health and Human Services, Health Resources and Services Administration, Bureau of Health Workforce, National Center for Health Workforce Analysis.2017. National and regional projections of supply and demand for geriatricians: 2013-2025. https://bhw.hrsa.gov/sites/default/files/bhw/health-workforce-analysis/research/projections/GeriatricsReport51817.pdf. Published April 2017. Accessed September 10, 2019.
4. Peterson L, Bazemore A, Bragg E, Xierali I, Warshaw GA. Rural–urban distribution of the U.S. geriatrics physician workforce. J Am Geriatr Soc. 2011;59(4):699-703.
5. Lindeman D. Interview: lessons from a leader in telehealth diffusion: a conversation with Adam Darkins of the Veterans Health Administration. Ageing Int. 2010;36(1):146-154.
6. Darkins A, Foster L, Anderson C, Goldschmidt L, Selvin G. The design, implementation, and operational management of a comprehensive quality management program to support national telehealth networks. Telemed J E Health. 2013;19(7):557-564.
7. US Department of Veterans Affairs. Clinical video telehealth into the home (CVTHM)toolkit for providers. https://www.mirecc.va.gov/visn16//docs/CVTHM_Toolkit.pdf. Accessed September 10, 2019.
8. Darkins A. Telehealth services in the United States Department of Veterans Affairs (VA). https://myvitalz.com/wp-content/uploads/2016/07/Telehealth-Services-in-the-United-States.pdf. Published July 2016. Accessed September 10, 2019.
9. US Department of Veterans Affairs. VA announces telemental health clinical resource centers during telemedicine association gathering [press release]. https://www.va.gov/opa/pressrel/includes/viewPDF.cfm?id=2789. Published May 16, 2016. Accessed September 10, 2019.
10. Hung WW, Rossi M, Thielke S, et al. A multisite geriatric education program for rural providers in the Veteran Health Care System (GRECC Connect). Gerontol Geriatr Educ. 2014;35(1):23-40.
11. Kramer BJ. The VA geriatric scholars program. Fed Pract. 2015;32(5):46-48.
12. Kramer BJ, Creekmur B, Howe JL, et al. Veterans Affairs Geriatric Scholars Program: enhancing existing primary care clinician skills in caring for older veterans. J Am Geriatr Soc. 2016;64(11):2343-2348.
13. Powers BB, Homer MC, Morone N, Edmonds N, Rossi MI. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099.
14. Wright SM, Hedin SC, McConnell M, et al. Using shared decision-making to address possible overtreatment in patients at high risk for hypoglycemia: the Veterans Health Administration’s Choosing Wisely Hypoglycemia Safety Initiative. Clin Diabetes. 2018;36(2):120-127.
15. Chang W, Homer M, Rossi MI. Use of clinical video telehealth as a tool for optimizing medications for rural older veterans with dementia. Geriatrics (Basel). 2018;3(3):pii E44.
16. US Department of Veterans Affairs, Office of Rural Health. Rural promising practice issue brief: GRECC Connect Project: connecting rural providers with geriatric specialists through telemedicine. https://www.ruralhealth.va.gov/docs/promise/2017_02_01_Promising%20Practice_GRECC_Issue%20Brief.pdf. Published February 2017. Accessed September 10, 2019.
17. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA expands telehealth by allowing health care providers to treat patients across state lines [press release]. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=4054. Published May 11, 2018. Accessed September 10, 2019.
18. US Department of Veterans Affairs. Department of Veterans Affairs FY 2018 – 2024 strategic plan. https://www.va.gov/oei/docs/VA2018-2024strategicPlan.pdf. Updated May 31, 2019. Accessed September 10, 2019.
Psoriatic Arthritis Pathophysiology & Etiology
Refractory Status Asthmaticus: Treatment With Sevoflurane
Asthma attacks account for 1.8 million emergency department (ED) visits each year in the US and for 10 deaths daily.1 Management of asthma attacks includes administration of inhaled ß2 adrenergic agonists, inhaled anticholinergic agents, IV magnesium sulfate, and corticosteroids.2 Status asthmaticus is an intense acute exacerbation of asthma that does not respond to repeated treatments of bronchodilators and corticosteroids.3 It is a medical emergency requiring immediate recognition and treatment. The decision to intubate a patient with status asthmaticus is a clinical decision based on work of breathing, respiratory acidosis, and failure to respond to medical interventions.
In refractory cases of status asthmaticus, intubation and mechanical ventilation are undertaken to provide oxygenation and ventilation until the bronchospasm resolves. However, mechanical ventilation is associated with significant risks, including high end-inspiratory pressures, barotrauma, and volutrauma.4 Rescue therapies include muscle relaxation, infusion of ketamine (central acting nonopioid analgesic with bronchodilatory properties), heliox, and general anesthesia.2,4 We report a case of a patient with life-threatening asthma and status asthmaticus treated with sevoflurane general anesthesia.
Case Presentation
A 55-year-old woman whose medical history was notable for asthma, psoriasis, hypothyroidism, tobacco, and alcohol abuse, and posttraumatic stress disorder (PTSD) presented to the ED. The patient had rarely sought medical attention and had no prior ED visits or hospitalizations in the electronic health record. Her home regimen included an albuterol inhaler used as needed. Her family reported that they had found her in distress in bed in a tripod position, unable to speak and struggling to breath.
Emergency medical services found the patient cyanotic, apneic, and pulseless. She received cardiopulmonary resuscitation for 30 seconds and 1-mg IV epinephrine, and spontaneous circulation returned. The patient arrived in the ED with an oral airway in place receiving bag valve mask ventilation. The patient expelled the oral airway. She was unable to speak due to dyspnea, exhibited persistent cyanosis, fatigue due to work of breathing, and failed to respond to nebulized albuterol/ipratropium bromide, IV methylprednisolone, and magnesium sulfate. The patient met criteria for acute severe asthma, or status asthmaticus. Thus, the patient received rapid sequence induction with rocuronium and ketamine and was intubated.
According to her family, the patient had no previous intensive care unit (ICU) admissions or prior intubations. Her only asthma medication was an albuterol inhaler as needed. The patient worked as a supervisor at a window blind manufacturing company. She lived alone, smoked 2 packs of cigarettes a day for more than 30 years, had no pets, drank unknown quantities of beer, wine, and hard liquor daily, and had smoked marijuana for several years.
The patient’s physical examination was notable for diffuse expiratory wheezes. Laboratory analysis revealed white blood cell count of 13.7 k/mcL, sodium 140 mmol/L, potassium 4.9 mmol/L, chloride 105 mmol/L, CO2 17 mmol/L, creatinine 0.98 mg/dL, troponin 0.03 ng/mL, lactate 7.2 mmol/L. Her chest X-ray showed hyperinflation but no focal opacities, pneumothorax, or pulmonary edema. Her endotracheal tube was in good position (Figure 1). A computed tomography pulmonary angiogram showed no pulmonary embolus or emphysema. There were atelectatic changes in the dependent portion of the right lower lobe, central bronchial wall thickening, and no stigmata of air trapping (Figure 2). An echocardiogram revealed a left ventricular ejection fraction of 45%, normal right ventricle and right ventricular size and function with an estimated right ventricular systolic pressure of 40 mm Hg.
The patient was admitted to the ICU and started on continuous infusion cisatracurium for paralysis and deep sedation to improve ventilatory synchrony and decrease auto positive end-expiratory pressure (PEEP). Mechanical ventilation was initiated with volume-cycled assist control ventilation, 6 mL/kg/ideal body weight (IBW) at 5-cm H2O PEEP, and 1 minute ventilation of 10 liters. The patient had severe air trapping and high airway pressures. The dynamic PEEP was 22-cm H2O (normal PEEP of 5-cm H2O), peak airway pressure (PAP) 41-cm H2O, and plateau pressure 31-cm H2O. In addition, the arterial blood gas (ABG) showed severe hypercapnic respiratory acidosis without significant hypoxemia with pH 7.15, PaCO2 90 mm Hg, and PaO2 150 mm Hg.
Pressure controlled ventilation was attempted unsuccessfully due to high airway resistance. Ultimately, the patient was set on volume control with low tidal volume, 6 mL/kg/IBW, high flow 90 L/min, PEEP 0 cm of H2O, and a low respiratory rate of 10 to achieve an inspiratory to expiratory (I:E) ratio of 1:7. Managing the ventilator to avoid dynamic hyperinflation and auto-PEEP, she remained relatively stable and improved.
By day 4 the patient’s ventilator was set to volume assist control with respiratory rate of 16, tidal volume, 6 mL/kg/IBW, PEEP 5-cm H2O with auto PEEP of 3-cm H2O, and fraction of inspired ABG O2 (FiO2) 0.35 with PAP of 46-cm H2O and plateau pressure of 17-cm H2O. The ABG was pH 7.32, PaCO2 65 mm Hg, and PaO2 74 mm Hg. However, on hospital day 5, she developed worsening PAP 60 to 77-cm H2O, plateau pressures 17-cm H2O, and a dynamic PEEP 16-cm H2O and was unresponsive to ventilator maneuvers to lower airway pressures and improve ventilation.
The patient had been receiving continuous albuterol and ipratropium nebulizer treatments. Ketamine infusion was considered fraught with potential for a dissociative reaction due to the patient’s significant PTSD. The patient’s family requested avoidance of ketamine infusion since the patient was paralyzed and psychiatric effects could not be monitored. Heliox 80/20 mixture was considered; however, it is incompatible with the ventilator that was being used since it could not account for the density of the helium gas flow in the tidal volumes. Extracorporeal membrane oxygenation (ECMO) was not available at our facility, and the patient was not a candidate for the regional ECMO center.
On hospital day 8, the patient developed worsening respiratory acidosis. The patient’s PAP increased to > 77-cm H2O, and her ABG revealed pH 7.22, PaCO2 90 mm Hg, and PaO2 77 mm Hg with FiO2 0.4. A chest X-ray demonstrated a new left lower lobe infiltrate. Fiber optic bronchoscopy was notable for scattered thick secretions throughout both lungs without obstructing mucus plug. Removal of airway secretions did not improve airway pressures or dynamic hyperinflation.
After consultation and discussion with the chief of anesthesia, the patient was placed on an anesthesia ventilator and started on sevoflurane 1.5% in the ICU. Anesthesiology was available 24 hours a day, and the anesthesiologist rounded with the intensivist frequently for this patient. The anesthesia technician worked closely with respiratory therapy regarding ventilator setting and changing the anesthesia gas scavenging charcoal canister. Within 4 hours, her gas exchange normalized (Table). The patient’s ABG was pH 7.44, PaCO2 52 mm Hg, and PaO2 69 mm Hg on FiO2 0.4. On volume cycled ventilation with a rate of 12, flow rate of 40 L/min, and tidal volume 6 mL/kg/IBW, the PAP decreased to 41-cm H2O.
Within 24 hours bronchospasm improved as evidenced by decreased airway pressures, resolution of wheezing, and decreased CO2 retention. The sevoflurane was easily weaned over the next 48 hours by decreasing the dose by 25% every 12-hour shift without rebound bronchospasm. Airway pressures and ABGs were frequently monitored during the weaning process. The patient resumed conventional mechanical ventilation, cisatracurium was discontinued, and she underwent a percutaneous tracheostomy for critical illness polymyopathy. Her respiratory muscle strength recovered more robustly than anticipated. Prior to discharge to a skilled nursing facility for continued rehabilitation, she was removed from mechanical ventilation and decannulated.
Discussion
This case illustrates the successful treatment of a patient with extreme status asthmaticus given inhalational anesthesia as supportive care while the bronchospasm and status asthmaticus abated. This is an unusual treatment in an ominous situation. Inhalational anesthetics are potent bronchodilators and have been successfully used in the management of status asthmaticus refractory to conventional therapy.4 Inhalational anesthetics have been shown to decrease airway resistance, dynamic hyperinflation, and intrinsic PEEP.5 These agents result in rapid bronchodilation by relaxing the smooth muscle and are associated with early liberation from mechanical ventilation.5,6 Although there are no guidelines regarding which inhalational agent is best, specific dosing, duration, or titration, case reports in the literature regarding the successful use of inhalational agents in life-threatening status asthmaticus exist.2,5,7
Caveats regarding the use of inhalational anesthetics in status asthmaticus include proarrhythmias, severe hepatic and renal toxicity. Although isoflurane is less likely to cause arrhythmia, both isoflurane and sevoflurane can cause dose-dependent hypotension by peripheral vasodilatation.7 Ourpatient did not manifest any adverse effects.
Additional challenges regarding the use of inhalational anesthetics for status asthmaticus include differences in ventilators and occupational hazards.8 Anesthesia or operating room ventilators differ from ICU ventilators in flow and pressure capabilities.7 The anesthesia ventilator is not capable of generating inspiratory pressures sufficient to ventilate patients with severely elevated airway resistance. Thus, the decrease inspiratory flow that occurs with increasing airway pressure limits the tidal volume delivered and consequently the minute volume. Although newer anesthesia ventilators have increased flow capabilities, they require a fully trained staff.8
Potential occupational exposure to these volatile anesthetic gases occurs as patients being treated may exhale considerable amounts of volatile anesthetics.8 An anesthesia gas scavenging device, such as a charcoal canister, must be attached to the ventilator to capture the exhaled anesthetic gases and should be changed every 12 hours.8 Finally, there is a potential for rebound bronchospasm as the anesthetic agent is tapered.6,7,9-11
Conclusion
Inhalational anesthetics are an option as rescue therapy for severe life-threatening asthma when all other therapies have failed. Use of inhalational anesthetics in status asthmaticus consists of case reports of which half are in children.2,5,7 Our patient contributes to the literature of case reports regarding using sevoflurane in refractory status asthmaticus. A decision to choose them must be a collaborative team approach with anesthesiology, pulmonary/critical care medicine, respiratory therapy, and ICU nurses, and the risks and benefits should be discussed with decision-making family members. Since there are no specific guidelines for the use of inhalational agents in status asthmaticus, close attention to inspiratory flows, gas scavenging devices, and clinical response is required. Additionally, the team must be comfortable with the plan to use an anesthesia ventilator and trained on its limitations.
1. Centers for Disease Control and Prevention. Most recent national asthma data. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Updated May 2019. Accessed September 5, 2019.
2. Lazarus SC. Emergency treatment of asthma. N Engl J Med. 2010;363(8):755-764.
3. Shah R, Saltoun CA. Acute severe asthma (status asthmaticus). Allergy Asthma Proc. 2012;33(suppl 1):47-50.
4. Mutlu GM, Factor P, Schwartz DE, Snajder JI. Severe status asthmaticus: management with permissive hypercapnia and inhalation anesthesia Crit Care Med. 2002;30(2):477-480.
5. Maltais, F, Sovilj M, Goldber P, Gottfried SB. Respiratory mechanism in status asthmaticus. Effects of inhalational anesthesia. Chest. 1994;106(5):1401-1406.
6. Parnass SM, Feld JM, Chamberlin WH, Segil LJ. Status asthmaticus treated with isoflurane and enflurane. Anesth Analg. 1987;66(2):193-195.
7. Johnston RG, Noseworthy TW, Friesen EG, Yule HA, Shustack A. Isoflurane therapy for status asthmaticus in children and adults. Chest. 1990;97(3):698-701.
8. Meiser A, Laubenthal H. Inhalational anesthetics in the ICU: theory and practice of inhalational sedation in the ICU economics, risk-benefit. Best Pract Res Clin Anesthesiol. 2005;19(3):523-538.
9. Miller RD. Miller’s Anesthesia. 7th ed. Philadelphia, PA: Churchill Livingstone; 2010.
10. Nakao S, Hatano K, Sumi C, et al. Sevoflurane causes greater QTc interval prolongation in elderly patients than in younger patients. Anesth Analg. 2010;110(3):775-779.
11. Stachnik J. Inhaled anesthetic agents. Am J Health-Syst Pharm. 2006;63(7):623-634.
Asthma attacks account for 1.8 million emergency department (ED) visits each year in the US and for 10 deaths daily.1 Management of asthma attacks includes administration of inhaled ß2 adrenergic agonists, inhaled anticholinergic agents, IV magnesium sulfate, and corticosteroids.2 Status asthmaticus is an intense acute exacerbation of asthma that does not respond to repeated treatments of bronchodilators and corticosteroids.3 It is a medical emergency requiring immediate recognition and treatment. The decision to intubate a patient with status asthmaticus is a clinical decision based on work of breathing, respiratory acidosis, and failure to respond to medical interventions.
In refractory cases of status asthmaticus, intubation and mechanical ventilation are undertaken to provide oxygenation and ventilation until the bronchospasm resolves. However, mechanical ventilation is associated with significant risks, including high end-inspiratory pressures, barotrauma, and volutrauma.4 Rescue therapies include muscle relaxation, infusion of ketamine (central acting nonopioid analgesic with bronchodilatory properties), heliox, and general anesthesia.2,4 We report a case of a patient with life-threatening asthma and status asthmaticus treated with sevoflurane general anesthesia.
Case Presentation
A 55-year-old woman whose medical history was notable for asthma, psoriasis, hypothyroidism, tobacco, and alcohol abuse, and posttraumatic stress disorder (PTSD) presented to the ED. The patient had rarely sought medical attention and had no prior ED visits or hospitalizations in the electronic health record. Her home regimen included an albuterol inhaler used as needed. Her family reported that they had found her in distress in bed in a tripod position, unable to speak and struggling to breath.
Emergency medical services found the patient cyanotic, apneic, and pulseless. She received cardiopulmonary resuscitation for 30 seconds and 1-mg IV epinephrine, and spontaneous circulation returned. The patient arrived in the ED with an oral airway in place receiving bag valve mask ventilation. The patient expelled the oral airway. She was unable to speak due to dyspnea, exhibited persistent cyanosis, fatigue due to work of breathing, and failed to respond to nebulized albuterol/ipratropium bromide, IV methylprednisolone, and magnesium sulfate. The patient met criteria for acute severe asthma, or status asthmaticus. Thus, the patient received rapid sequence induction with rocuronium and ketamine and was intubated.
According to her family, the patient had no previous intensive care unit (ICU) admissions or prior intubations. Her only asthma medication was an albuterol inhaler as needed. The patient worked as a supervisor at a window blind manufacturing company. She lived alone, smoked 2 packs of cigarettes a day for more than 30 years, had no pets, drank unknown quantities of beer, wine, and hard liquor daily, and had smoked marijuana for several years.
The patient’s physical examination was notable for diffuse expiratory wheezes. Laboratory analysis revealed white blood cell count of 13.7 k/mcL, sodium 140 mmol/L, potassium 4.9 mmol/L, chloride 105 mmol/L, CO2 17 mmol/L, creatinine 0.98 mg/dL, troponin 0.03 ng/mL, lactate 7.2 mmol/L. Her chest X-ray showed hyperinflation but no focal opacities, pneumothorax, or pulmonary edema. Her endotracheal tube was in good position (Figure 1). A computed tomography pulmonary angiogram showed no pulmonary embolus or emphysema. There were atelectatic changes in the dependent portion of the right lower lobe, central bronchial wall thickening, and no stigmata of air trapping (Figure 2). An echocardiogram revealed a left ventricular ejection fraction of 45%, normal right ventricle and right ventricular size and function with an estimated right ventricular systolic pressure of 40 mm Hg.
The patient was admitted to the ICU and started on continuous infusion cisatracurium for paralysis and deep sedation to improve ventilatory synchrony and decrease auto positive end-expiratory pressure (PEEP). Mechanical ventilation was initiated with volume-cycled assist control ventilation, 6 mL/kg/ideal body weight (IBW) at 5-cm H2O PEEP, and 1 minute ventilation of 10 liters. The patient had severe air trapping and high airway pressures. The dynamic PEEP was 22-cm H2O (normal PEEP of 5-cm H2O), peak airway pressure (PAP) 41-cm H2O, and plateau pressure 31-cm H2O. In addition, the arterial blood gas (ABG) showed severe hypercapnic respiratory acidosis without significant hypoxemia with pH 7.15, PaCO2 90 mm Hg, and PaO2 150 mm Hg.
Pressure controlled ventilation was attempted unsuccessfully due to high airway resistance. Ultimately, the patient was set on volume control with low tidal volume, 6 mL/kg/IBW, high flow 90 L/min, PEEP 0 cm of H2O, and a low respiratory rate of 10 to achieve an inspiratory to expiratory (I:E) ratio of 1:7. Managing the ventilator to avoid dynamic hyperinflation and auto-PEEP, she remained relatively stable and improved.
By day 4 the patient’s ventilator was set to volume assist control with respiratory rate of 16, tidal volume, 6 mL/kg/IBW, PEEP 5-cm H2O with auto PEEP of 3-cm H2O, and fraction of inspired ABG O2 (FiO2) 0.35 with PAP of 46-cm H2O and plateau pressure of 17-cm H2O. The ABG was pH 7.32, PaCO2 65 mm Hg, and PaO2 74 mm Hg. However, on hospital day 5, she developed worsening PAP 60 to 77-cm H2O, plateau pressures 17-cm H2O, and a dynamic PEEP 16-cm H2O and was unresponsive to ventilator maneuvers to lower airway pressures and improve ventilation.
The patient had been receiving continuous albuterol and ipratropium nebulizer treatments. Ketamine infusion was considered fraught with potential for a dissociative reaction due to the patient’s significant PTSD. The patient’s family requested avoidance of ketamine infusion since the patient was paralyzed and psychiatric effects could not be monitored. Heliox 80/20 mixture was considered; however, it is incompatible with the ventilator that was being used since it could not account for the density of the helium gas flow in the tidal volumes. Extracorporeal membrane oxygenation (ECMO) was not available at our facility, and the patient was not a candidate for the regional ECMO center.
On hospital day 8, the patient developed worsening respiratory acidosis. The patient’s PAP increased to > 77-cm H2O, and her ABG revealed pH 7.22, PaCO2 90 mm Hg, and PaO2 77 mm Hg with FiO2 0.4. A chest X-ray demonstrated a new left lower lobe infiltrate. Fiber optic bronchoscopy was notable for scattered thick secretions throughout both lungs without obstructing mucus plug. Removal of airway secretions did not improve airway pressures or dynamic hyperinflation.
After consultation and discussion with the chief of anesthesia, the patient was placed on an anesthesia ventilator and started on sevoflurane 1.5% in the ICU. Anesthesiology was available 24 hours a day, and the anesthesiologist rounded with the intensivist frequently for this patient. The anesthesia technician worked closely with respiratory therapy regarding ventilator setting and changing the anesthesia gas scavenging charcoal canister. Within 4 hours, her gas exchange normalized (Table). The patient’s ABG was pH 7.44, PaCO2 52 mm Hg, and PaO2 69 mm Hg on FiO2 0.4. On volume cycled ventilation with a rate of 12, flow rate of 40 L/min, and tidal volume 6 mL/kg/IBW, the PAP decreased to 41-cm H2O.
Within 24 hours bronchospasm improved as evidenced by decreased airway pressures, resolution of wheezing, and decreased CO2 retention. The sevoflurane was easily weaned over the next 48 hours by decreasing the dose by 25% every 12-hour shift without rebound bronchospasm. Airway pressures and ABGs were frequently monitored during the weaning process. The patient resumed conventional mechanical ventilation, cisatracurium was discontinued, and she underwent a percutaneous tracheostomy for critical illness polymyopathy. Her respiratory muscle strength recovered more robustly than anticipated. Prior to discharge to a skilled nursing facility for continued rehabilitation, she was removed from mechanical ventilation and decannulated.
Discussion
This case illustrates the successful treatment of a patient with extreme status asthmaticus given inhalational anesthesia as supportive care while the bronchospasm and status asthmaticus abated. This is an unusual treatment in an ominous situation. Inhalational anesthetics are potent bronchodilators and have been successfully used in the management of status asthmaticus refractory to conventional therapy.4 Inhalational anesthetics have been shown to decrease airway resistance, dynamic hyperinflation, and intrinsic PEEP.5 These agents result in rapid bronchodilation by relaxing the smooth muscle and are associated with early liberation from mechanical ventilation.5,6 Although there are no guidelines regarding which inhalational agent is best, specific dosing, duration, or titration, case reports in the literature regarding the successful use of inhalational agents in life-threatening status asthmaticus exist.2,5,7
Caveats regarding the use of inhalational anesthetics in status asthmaticus include proarrhythmias, severe hepatic and renal toxicity. Although isoflurane is less likely to cause arrhythmia, both isoflurane and sevoflurane can cause dose-dependent hypotension by peripheral vasodilatation.7 Ourpatient did not manifest any adverse effects.
Additional challenges regarding the use of inhalational anesthetics for status asthmaticus include differences in ventilators and occupational hazards.8 Anesthesia or operating room ventilators differ from ICU ventilators in flow and pressure capabilities.7 The anesthesia ventilator is not capable of generating inspiratory pressures sufficient to ventilate patients with severely elevated airway resistance. Thus, the decrease inspiratory flow that occurs with increasing airway pressure limits the tidal volume delivered and consequently the minute volume. Although newer anesthesia ventilators have increased flow capabilities, they require a fully trained staff.8
Potential occupational exposure to these volatile anesthetic gases occurs as patients being treated may exhale considerable amounts of volatile anesthetics.8 An anesthesia gas scavenging device, such as a charcoal canister, must be attached to the ventilator to capture the exhaled anesthetic gases and should be changed every 12 hours.8 Finally, there is a potential for rebound bronchospasm as the anesthetic agent is tapered.6,7,9-11
Conclusion
Inhalational anesthetics are an option as rescue therapy for severe life-threatening asthma when all other therapies have failed. Use of inhalational anesthetics in status asthmaticus consists of case reports of which half are in children.2,5,7 Our patient contributes to the literature of case reports regarding using sevoflurane in refractory status asthmaticus. A decision to choose them must be a collaborative team approach with anesthesiology, pulmonary/critical care medicine, respiratory therapy, and ICU nurses, and the risks and benefits should be discussed with decision-making family members. Since there are no specific guidelines for the use of inhalational agents in status asthmaticus, close attention to inspiratory flows, gas scavenging devices, and clinical response is required. Additionally, the team must be comfortable with the plan to use an anesthesia ventilator and trained on its limitations.
Asthma attacks account for 1.8 million emergency department (ED) visits each year in the US and for 10 deaths daily.1 Management of asthma attacks includes administration of inhaled ß2 adrenergic agonists, inhaled anticholinergic agents, IV magnesium sulfate, and corticosteroids.2 Status asthmaticus is an intense acute exacerbation of asthma that does not respond to repeated treatments of bronchodilators and corticosteroids.3 It is a medical emergency requiring immediate recognition and treatment. The decision to intubate a patient with status asthmaticus is a clinical decision based on work of breathing, respiratory acidosis, and failure to respond to medical interventions.
In refractory cases of status asthmaticus, intubation and mechanical ventilation are undertaken to provide oxygenation and ventilation until the bronchospasm resolves. However, mechanical ventilation is associated with significant risks, including high end-inspiratory pressures, barotrauma, and volutrauma.4 Rescue therapies include muscle relaxation, infusion of ketamine (central acting nonopioid analgesic with bronchodilatory properties), heliox, and general anesthesia.2,4 We report a case of a patient with life-threatening asthma and status asthmaticus treated with sevoflurane general anesthesia.
Case Presentation
A 55-year-old woman whose medical history was notable for asthma, psoriasis, hypothyroidism, tobacco, and alcohol abuse, and posttraumatic stress disorder (PTSD) presented to the ED. The patient had rarely sought medical attention and had no prior ED visits or hospitalizations in the electronic health record. Her home regimen included an albuterol inhaler used as needed. Her family reported that they had found her in distress in bed in a tripod position, unable to speak and struggling to breath.
Emergency medical services found the patient cyanotic, apneic, and pulseless. She received cardiopulmonary resuscitation for 30 seconds and 1-mg IV epinephrine, and spontaneous circulation returned. The patient arrived in the ED with an oral airway in place receiving bag valve mask ventilation. The patient expelled the oral airway. She was unable to speak due to dyspnea, exhibited persistent cyanosis, fatigue due to work of breathing, and failed to respond to nebulized albuterol/ipratropium bromide, IV methylprednisolone, and magnesium sulfate. The patient met criteria for acute severe asthma, or status asthmaticus. Thus, the patient received rapid sequence induction with rocuronium and ketamine and was intubated.
According to her family, the patient had no previous intensive care unit (ICU) admissions or prior intubations. Her only asthma medication was an albuterol inhaler as needed. The patient worked as a supervisor at a window blind manufacturing company. She lived alone, smoked 2 packs of cigarettes a day for more than 30 years, had no pets, drank unknown quantities of beer, wine, and hard liquor daily, and had smoked marijuana for several years.
The patient’s physical examination was notable for diffuse expiratory wheezes. Laboratory analysis revealed white blood cell count of 13.7 k/mcL, sodium 140 mmol/L, potassium 4.9 mmol/L, chloride 105 mmol/L, CO2 17 mmol/L, creatinine 0.98 mg/dL, troponin 0.03 ng/mL, lactate 7.2 mmol/L. Her chest X-ray showed hyperinflation but no focal opacities, pneumothorax, or pulmonary edema. Her endotracheal tube was in good position (Figure 1). A computed tomography pulmonary angiogram showed no pulmonary embolus or emphysema. There were atelectatic changes in the dependent portion of the right lower lobe, central bronchial wall thickening, and no stigmata of air trapping (Figure 2). An echocardiogram revealed a left ventricular ejection fraction of 45%, normal right ventricle and right ventricular size and function with an estimated right ventricular systolic pressure of 40 mm Hg.
The patient was admitted to the ICU and started on continuous infusion cisatracurium for paralysis and deep sedation to improve ventilatory synchrony and decrease auto positive end-expiratory pressure (PEEP). Mechanical ventilation was initiated with volume-cycled assist control ventilation, 6 mL/kg/ideal body weight (IBW) at 5-cm H2O PEEP, and 1 minute ventilation of 10 liters. The patient had severe air trapping and high airway pressures. The dynamic PEEP was 22-cm H2O (normal PEEP of 5-cm H2O), peak airway pressure (PAP) 41-cm H2O, and plateau pressure 31-cm H2O. In addition, the arterial blood gas (ABG) showed severe hypercapnic respiratory acidosis without significant hypoxemia with pH 7.15, PaCO2 90 mm Hg, and PaO2 150 mm Hg.
Pressure controlled ventilation was attempted unsuccessfully due to high airway resistance. Ultimately, the patient was set on volume control with low tidal volume, 6 mL/kg/IBW, high flow 90 L/min, PEEP 0 cm of H2O, and a low respiratory rate of 10 to achieve an inspiratory to expiratory (I:E) ratio of 1:7. Managing the ventilator to avoid dynamic hyperinflation and auto-PEEP, she remained relatively stable and improved.
By day 4 the patient’s ventilator was set to volume assist control with respiratory rate of 16, tidal volume, 6 mL/kg/IBW, PEEP 5-cm H2O with auto PEEP of 3-cm H2O, and fraction of inspired ABG O2 (FiO2) 0.35 with PAP of 46-cm H2O and plateau pressure of 17-cm H2O. The ABG was pH 7.32, PaCO2 65 mm Hg, and PaO2 74 mm Hg. However, on hospital day 5, she developed worsening PAP 60 to 77-cm H2O, plateau pressures 17-cm H2O, and a dynamic PEEP 16-cm H2O and was unresponsive to ventilator maneuvers to lower airway pressures and improve ventilation.
The patient had been receiving continuous albuterol and ipratropium nebulizer treatments. Ketamine infusion was considered fraught with potential for a dissociative reaction due to the patient’s significant PTSD. The patient’s family requested avoidance of ketamine infusion since the patient was paralyzed and psychiatric effects could not be monitored. Heliox 80/20 mixture was considered; however, it is incompatible with the ventilator that was being used since it could not account for the density of the helium gas flow in the tidal volumes. Extracorporeal membrane oxygenation (ECMO) was not available at our facility, and the patient was not a candidate for the regional ECMO center.
On hospital day 8, the patient developed worsening respiratory acidosis. The patient’s PAP increased to > 77-cm H2O, and her ABG revealed pH 7.22, PaCO2 90 mm Hg, and PaO2 77 mm Hg with FiO2 0.4. A chest X-ray demonstrated a new left lower lobe infiltrate. Fiber optic bronchoscopy was notable for scattered thick secretions throughout both lungs without obstructing mucus plug. Removal of airway secretions did not improve airway pressures or dynamic hyperinflation.
After consultation and discussion with the chief of anesthesia, the patient was placed on an anesthesia ventilator and started on sevoflurane 1.5% in the ICU. Anesthesiology was available 24 hours a day, and the anesthesiologist rounded with the intensivist frequently for this patient. The anesthesia technician worked closely with respiratory therapy regarding ventilator setting and changing the anesthesia gas scavenging charcoal canister. Within 4 hours, her gas exchange normalized (Table). The patient’s ABG was pH 7.44, PaCO2 52 mm Hg, and PaO2 69 mm Hg on FiO2 0.4. On volume cycled ventilation with a rate of 12, flow rate of 40 L/min, and tidal volume 6 mL/kg/IBW, the PAP decreased to 41-cm H2O.
Within 24 hours bronchospasm improved as evidenced by decreased airway pressures, resolution of wheezing, and decreased CO2 retention. The sevoflurane was easily weaned over the next 48 hours by decreasing the dose by 25% every 12-hour shift without rebound bronchospasm. Airway pressures and ABGs were frequently monitored during the weaning process. The patient resumed conventional mechanical ventilation, cisatracurium was discontinued, and she underwent a percutaneous tracheostomy for critical illness polymyopathy. Her respiratory muscle strength recovered more robustly than anticipated. Prior to discharge to a skilled nursing facility for continued rehabilitation, she was removed from mechanical ventilation and decannulated.
Discussion
This case illustrates the successful treatment of a patient with extreme status asthmaticus given inhalational anesthesia as supportive care while the bronchospasm and status asthmaticus abated. This is an unusual treatment in an ominous situation. Inhalational anesthetics are potent bronchodilators and have been successfully used in the management of status asthmaticus refractory to conventional therapy.4 Inhalational anesthetics have been shown to decrease airway resistance, dynamic hyperinflation, and intrinsic PEEP.5 These agents result in rapid bronchodilation by relaxing the smooth muscle and are associated with early liberation from mechanical ventilation.5,6 Although there are no guidelines regarding which inhalational agent is best, specific dosing, duration, or titration, case reports in the literature regarding the successful use of inhalational agents in life-threatening status asthmaticus exist.2,5,7
Caveats regarding the use of inhalational anesthetics in status asthmaticus include proarrhythmias, severe hepatic and renal toxicity. Although isoflurane is less likely to cause arrhythmia, both isoflurane and sevoflurane can cause dose-dependent hypotension by peripheral vasodilatation.7 Ourpatient did not manifest any adverse effects.
Additional challenges regarding the use of inhalational anesthetics for status asthmaticus include differences in ventilators and occupational hazards.8 Anesthesia or operating room ventilators differ from ICU ventilators in flow and pressure capabilities.7 The anesthesia ventilator is not capable of generating inspiratory pressures sufficient to ventilate patients with severely elevated airway resistance. Thus, the decrease inspiratory flow that occurs with increasing airway pressure limits the tidal volume delivered and consequently the minute volume. Although newer anesthesia ventilators have increased flow capabilities, they require a fully trained staff.8
Potential occupational exposure to these volatile anesthetic gases occurs as patients being treated may exhale considerable amounts of volatile anesthetics.8 An anesthesia gas scavenging device, such as a charcoal canister, must be attached to the ventilator to capture the exhaled anesthetic gases and should be changed every 12 hours.8 Finally, there is a potential for rebound bronchospasm as the anesthetic agent is tapered.6,7,9-11
Conclusion
Inhalational anesthetics are an option as rescue therapy for severe life-threatening asthma when all other therapies have failed. Use of inhalational anesthetics in status asthmaticus consists of case reports of which half are in children.2,5,7 Our patient contributes to the literature of case reports regarding using sevoflurane in refractory status asthmaticus. A decision to choose them must be a collaborative team approach with anesthesiology, pulmonary/critical care medicine, respiratory therapy, and ICU nurses, and the risks and benefits should be discussed with decision-making family members. Since there are no specific guidelines for the use of inhalational agents in status asthmaticus, close attention to inspiratory flows, gas scavenging devices, and clinical response is required. Additionally, the team must be comfortable with the plan to use an anesthesia ventilator and trained on its limitations.
1. Centers for Disease Control and Prevention. Most recent national asthma data. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Updated May 2019. Accessed September 5, 2019.
2. Lazarus SC. Emergency treatment of asthma. N Engl J Med. 2010;363(8):755-764.
3. Shah R, Saltoun CA. Acute severe asthma (status asthmaticus). Allergy Asthma Proc. 2012;33(suppl 1):47-50.
4. Mutlu GM, Factor P, Schwartz DE, Snajder JI. Severe status asthmaticus: management with permissive hypercapnia and inhalation anesthesia Crit Care Med. 2002;30(2):477-480.
5. Maltais, F, Sovilj M, Goldber P, Gottfried SB. Respiratory mechanism in status asthmaticus. Effects of inhalational anesthesia. Chest. 1994;106(5):1401-1406.
6. Parnass SM, Feld JM, Chamberlin WH, Segil LJ. Status asthmaticus treated with isoflurane and enflurane. Anesth Analg. 1987;66(2):193-195.
7. Johnston RG, Noseworthy TW, Friesen EG, Yule HA, Shustack A. Isoflurane therapy for status asthmaticus in children and adults. Chest. 1990;97(3):698-701.
8. Meiser A, Laubenthal H. Inhalational anesthetics in the ICU: theory and practice of inhalational sedation in the ICU economics, risk-benefit. Best Pract Res Clin Anesthesiol. 2005;19(3):523-538.
9. Miller RD. Miller’s Anesthesia. 7th ed. Philadelphia, PA: Churchill Livingstone; 2010.
10. Nakao S, Hatano K, Sumi C, et al. Sevoflurane causes greater QTc interval prolongation in elderly patients than in younger patients. Anesth Analg. 2010;110(3):775-779.
11. Stachnik J. Inhaled anesthetic agents. Am J Health-Syst Pharm. 2006;63(7):623-634.
1. Centers for Disease Control and Prevention. Most recent national asthma data. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Updated May 2019. Accessed September 5, 2019.
2. Lazarus SC. Emergency treatment of asthma. N Engl J Med. 2010;363(8):755-764.
3. Shah R, Saltoun CA. Acute severe asthma (status asthmaticus). Allergy Asthma Proc. 2012;33(suppl 1):47-50.
4. Mutlu GM, Factor P, Schwartz DE, Snajder JI. Severe status asthmaticus: management with permissive hypercapnia and inhalation anesthesia Crit Care Med. 2002;30(2):477-480.
5. Maltais, F, Sovilj M, Goldber P, Gottfried SB. Respiratory mechanism in status asthmaticus. Effects of inhalational anesthesia. Chest. 1994;106(5):1401-1406.
6. Parnass SM, Feld JM, Chamberlin WH, Segil LJ. Status asthmaticus treated with isoflurane and enflurane. Anesth Analg. 1987;66(2):193-195.
7. Johnston RG, Noseworthy TW, Friesen EG, Yule HA, Shustack A. Isoflurane therapy for status asthmaticus in children and adults. Chest. 1990;97(3):698-701.
8. Meiser A, Laubenthal H. Inhalational anesthetics in the ICU: theory and practice of inhalational sedation in the ICU economics, risk-benefit. Best Pract Res Clin Anesthesiol. 2005;19(3):523-538.
9. Miller RD. Miller’s Anesthesia. 7th ed. Philadelphia, PA: Churchill Livingstone; 2010.
10. Nakao S, Hatano K, Sumi C, et al. Sevoflurane causes greater QTc interval prolongation in elderly patients than in younger patients. Anesth Analg. 2010;110(3):775-779.
11. Stachnik J. Inhaled anesthetic agents. Am J Health-Syst Pharm. 2006;63(7):623-634.
Advancing Order Set Design
In the current health care environment, hospitals are constantly challenged to improve quality metrics and deliver better health care outcomes. One means to achieving quality improvement is through the use of order sets, groups of related orders that a health care provider (HCP) can place with either a few keystrokes or mouse clicks.1
Historically, design of order sets has largely focused on clicking checkboxes containing evidence-based practices. According to Bates and colleagues and the Institute for Safe Medication Practices, incorporating evidence-based medicine (EBM) into order sets is not by itself sufficient.2,3Execution of proper design coupled with simplicity and provider efficiency is paramount to HCP buy-in, increased likelihood of order set adherence, and to potentially better outcomes.
In this article, we outline advancements in order set design. These improvements increase provider efficiency and ease of use; incorporate human factors engineering (HFE); apply failure mode and effects analysis; and include EBM.
Methods
An inpatient nicotine replacement therapy (NRT) order was developed as part of a multifaceted solution to improve tobacco cessation care at the James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, a complexity level 1a facility. This NRT order set used the 4-step order set design framework the authors’ developed (for additional information about the NRT order set, contact the authors). We distinguish order set design technique between 2 different inpatient NRT order sets. The first order set in the comparison (Figure 1) is an inpatient NRT order set of unknown origin—it is common for US Department of Veterans Affairs (VA) medical facilities to share order sets and other resources. The second order set (Figure 2) is an inpatient NRT order set we designed using our 4-step process for comparison in this article. No institutional review board approval was required as this work met criteria for operational improvement activities exempt from ethics review.
Justin Iannello, DO, MBA, was the team leader and developer of the 4-step order set design technique. The intervention team consisted of 4 internal medicine physicians with expertise in quality improvement and patient safety: 1 certified professional in patient safety and certified as a Lean Six Sigma Black Belt; 2 physicians certified as Lean Six Sigma Black Belts; and 1 physician certified as a Lean Six Sigma Green Belt. Two inpatient clinical pharmacists and 1 quality management specialist also were involved in its development.
Development of a new NRT order set was felt to be an integral part of the tobacco cessation care delivery process. An NRT order set perceived by users as value-added required a solution that merged EBM with standardization and applied quality improvement principles. The result was an approach to order set design that focused on 4 key questions: Is the order set efficient and easy to use/navigate? Is human factors engineering incorporated? Is failure mode and effects analysis applied? Are evidence-based practices included?
Ease of Use and Navigation
Implementing an order set that is efficient and easy to use or navigate seems straightforward but can be difficult to execute. Figure 1 shows many detailed options consisting of different combinations of nicotine patches, lozenges, and gum. Also included are oral tobacco cessation options (bupropion and varenicline). Although more options may seem better, confusion about appropriate medication selection can occur.
According to Heath and Heath, too many options can result in lack of action.4 For example, Heath and Heath discuss a food store that offered 6 free samples of different jams on one day and 24 jams the following day. The customers who sampled 6 different types of jam were 10 times more likely to buy jam. The authors concluded that the more options available, the more difficulty a potential buyer has in deciding on a course of action.4
In clinical situations where a HCP is using an order set, the number of options can mean the difference between use vs avoidance if the choices are overwhelming. HCPs process layers of detail every day when creating differential diagnoses and treatment plans. While that level of detail is necessary clinically, that same level of detail included in orders sets can create challenges for HCPs.
Figure 2 advances the order set in Figure 1 by providing a simpler and cleaner design, so HCPs can more easily review and process the information. This order set design minimizes the number of options available to help users make the right decision, focusing on value for the appropriate setting and audience. In other words, order sets should not be a “one size fits all” approach.
Order sets should be tailored to the appropriate clinical setting (eg, inpatient acute care, outpatient clinic setting, etc) and HCP (eg, hospitalist, tobacco cessation specialist, etc). We are comparing NRT order sets designed for HCPs who do not routinely prescribe oral tobacco cessation products in the inpatient setting. When possible, autogenerated bundle orders should also be used according to evidence-based recommendations (such as nicotine patch tapers) for ease of use and further simplification of order sets.
Finally, usability testing known as “evaluating a product or service by testing it with representative users” helps further refine an order set.5Usability testing should be applied during all phases of order set development with end user(s) as it helps identify problems with order set design prior to implementation. By applying usability testing, the order set becomes more meaningful and valued by the user.
Human Factors Engineering
HFE is “the study of all the factors that make it easier to do the work in the right way.”6 HFE seeks to identify, align, and apply processes for people and the world within which they live and work to promote safe and efficient practices, especially in relation to the technology and physical design features in their work environment.6
The average American adult makes about 35,000 decisions per day.7 Thus, there is potential for error at any moment. Design that does not take HFE into account can be dangerous. For example, when tube feed and IV line connectors look similar and are compatible, patients may inadvertently receive food administered directly into their bloodstream.8
HFE can and should be applied to order sets. Everything from the look, feel, and verbiage of an order set affects potential outcomes. For example, consider the impact even seemingly minor modifications can have on outcomes simply by guiding users in a different way: Figure 1 provides NRT options based on cigarette use per day, whereas Figure 2 conveys pack use per day in relation to the equivalent number of cigarettes used daily. These differences may seem small; however, it helps guide users to the right choice when considering that health care providers have been historically trained on social history gathering that emphasizes packs per day and pack-years.
Failure Mode and Effects Analysis
Failure mode and effects analysis (FMEA) is “a structured way to identify and address potential problems, or failures and their resulting effects on the system or process before an adverse event occurs.”9 The benefit of an order set must be weighed against the risk during development. FMEA should be applied during order set design to assess and limit risk just as with any other clinical care process.
FMEA examines both level of risk and frequency of risk occurrence associated with a new proposed process. For example, let’s evaluate an order set designed for pain control after surgery that consists of multiple high-risk opioids along with antihistamine medications for as-needed itch relief (a non-life-threatening adverse event (AE) of opioids well known by the medical community). An interdisciplinary FMEA team consisting of subject matter experts may examine how the process should flow in step-by-step detail and then discuss the benefit of a process and risk for potential error. A FMEA team would then analyze what could go wrong with each part of the process and assign a level of risk and risk frequency for various steps in the process, and then decide that certain steps should be modified or eliminated. Perhaps after FMEA, a facility might conclude that the risk of serious complications is high when you combine opioid use with antihistamine medications. The facility could decide to remove antihistamine medications from an order set if it is determined that risks outweigh benefits. While a root cause analysis might identify the cause of an AE after order set use, these situations can be prevented with FMEA.
When applying FMEA to Figure 1, while bupropion is known as an evidence-based oral tobacco cessation option, there is the possibility that bupropion could be inadvertently prescribed from the order set in a hospitalized patient with alcohol withdrawal and withdrawal seizure history. These potentially dangerous situations can be avoided with FMEA. Thus, although bupropion may be evidence-based for NRT, decisions regarding order set design using EBM alone are insufficient.
The practitioner must consider possible unintended consequences within order sets and target treatment options to the appropriate setting and audience. Although Figure 1 may appear to be more inclusive, the interdisciplinary committee designing the inpatient NRT order set felt there was heightened risk with introducing bupropion in Figure 1 and decided the risk would be lowered by removing bupropion from the redesigned NRT order set (Figure 2). In addition to the goal of balancing availability of NRT options with acceptable risk, Figure 2 also focused on building an NRT order set most applicable to the inpatient setting.
Including Evidence-Based Practices
EBM has become a routine part of clinical decision making. Therefore, including EBM in order set design is vital. EBM for NRT has demonstrated that combination therapy is more effective than is monotherapy to help tobacco users quit. Incremental doses of NRT are recommended for patients who use tobacco more frequently.10
As shown in Figures 1 and 2, both order set designs incorporate EBM for NRT. Although the importance of implementing EBM is evident, critical factors, such as HFE and FMEA make a difference with well-designed order sets.
Results
The 4-step order set design technique was used during development of an inpatient NRT order set at the JAHVH. Results for the inpatient Joint Commission Tobacco Treatment Measures were obtained from the Veterans Health Administration quality metric reporting system known as Strategic Analytics for Improvement and Learning (SAIL). SAIL performance measure outcomes, which include the inpatient Joint Commission Tobacco Treatment Measures, are derived from chart reviews conducted by the External Peer Review Program. Outcomes demonstrated that TOB-2 and TOB-3 (2 inpatient Joint Commission Tobacco Treatment Measures) known as tob20 and tob40, respectively, within SAIL improved by more than 300% after development of an NRT order set using the 4-step order set design framework along with implementation of a multifaceted tobacco cessation care delivery system at JAHVH.
Discussion
While the overall tobacco cessation care delivery system contributed to improved outcomes with the inpatient Joint Commission Tobacco Treatment Measures at JAHVH, the NRT order set was a cornerstone of the design. Although using our order set design technique does not necessarily guarantee successful outcomes, we believe using the 4-step order set design process increases the value of order sets and has potential to improve quality outcomes.
Limitations
Although improved outcomes following implementation of our NRT order set suggest correlation, causation cannot be proven. Also while the NRT order set is believed to have helped tremendously with outcomes, the entire tobacco cessation care delivery system at JAHVH contributed to the results. In addition, the inpatient Joint Commission Tobacco Treatment Measures help improve processes for tobacco cessation care. However, we are uncertain whether the results of our improvement efforts helped patients stop tobacco use. Further studies are needed to determine impact on population health. Finally, our results were based on improvement work done at a single center. Further studies are necessary to see whether results are reproducible.
Conclusion
There was significant improvement with the inpatient Joint Commission Tobacco Treatment Measures outcomes following development of a tobacco cessation care delivery system that included design of an inpatient NRT order set using a 4-step process we developed. This 4-step structure includes emphasis on efficiency and ease of use; human factors engineering; failure mode and effects analysis; and incorporation of evidence-based medicine (Box.) Postimplementation results showed improvement of the inpatient Joint Commission Tobacco Treatment Measures by greater than 3-fold at a single hospital.
The next steps for this initiative include testing the 4-step order set design process in multiple clinical settings to determine the effectiveness of this approach in other areas of clinical care.
1. Order set. http://clinfowiki.org/wiki/index.php/Order_set. Updated October 15, 2015. Accessed August 30, 2019.
2. Bates DW, Kuperman GJ, Wang S, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc. 2003;10(6):523-530.
3. Institute for Safe Medication Practices. Guidelines for standard order sets. https://www.ismp.org/tools/guidelines/standardordersets.pdf. Published January 12, 2010. Accessed August 30, 2019.
4. Heath C, Heath D. Switch: How to Change Things When Change Is Hard. New York, NY: Crown Business; 2010:50-51.
5. US Department of Health and Human Services. Usability testing. https://www.usability.gov/how-to-and-tools/methods/usability-testing.html. Accessed August 30, 2019.
6. World Health Organization. What is human factors and why is it important to patient safety? www.who.int/patientsafety/education/curriculum/who_mc_topic-2.pdf. Accessed August 30, 2019.
7. Sollisch J. The cure for decision fatigue. Wall Street Journal. June 10, 2016. https://www.wsj.com/articles/the-cure-for-decision-fatigue-1465596928. Accessed August 30, 2019.
8. ECRI Institute. Implementing the ENFit initiative for preventing enteral tubing misconnections. https://www.ecri.org/components/HDJournal/Pages/ENFit-for-Preventing-Enteral-Tubing-Misconnections.aspx. Published March 29, 2017. Accessed August 30, 2019.
9. Guidance for performing failure mode and effects analysis with performance improvement projects. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/QAPI/downloads/GuidanceForFMEA.pdf. Accessed August 30, 2019.
10. Diefanbach LJ, Smith PO, Nashelsky JM, Lindbloom E. What is the most effective nicotine replacement therapy? J Fam Pract. 2003;52(6):492-497.
In the current health care environment, hospitals are constantly challenged to improve quality metrics and deliver better health care outcomes. One means to achieving quality improvement is through the use of order sets, groups of related orders that a health care provider (HCP) can place with either a few keystrokes or mouse clicks.1
Historically, design of order sets has largely focused on clicking checkboxes containing evidence-based practices. According to Bates and colleagues and the Institute for Safe Medication Practices, incorporating evidence-based medicine (EBM) into order sets is not by itself sufficient.2,3Execution of proper design coupled with simplicity and provider efficiency is paramount to HCP buy-in, increased likelihood of order set adherence, and to potentially better outcomes.
In this article, we outline advancements in order set design. These improvements increase provider efficiency and ease of use; incorporate human factors engineering (HFE); apply failure mode and effects analysis; and include EBM.
Methods
An inpatient nicotine replacement therapy (NRT) order was developed as part of a multifaceted solution to improve tobacco cessation care at the James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, a complexity level 1a facility. This NRT order set used the 4-step order set design framework the authors’ developed (for additional information about the NRT order set, contact the authors). We distinguish order set design technique between 2 different inpatient NRT order sets. The first order set in the comparison (Figure 1) is an inpatient NRT order set of unknown origin—it is common for US Department of Veterans Affairs (VA) medical facilities to share order sets and other resources. The second order set (Figure 2) is an inpatient NRT order set we designed using our 4-step process for comparison in this article. No institutional review board approval was required as this work met criteria for operational improvement activities exempt from ethics review.
Justin Iannello, DO, MBA, was the team leader and developer of the 4-step order set design technique. The intervention team consisted of 4 internal medicine physicians with expertise in quality improvement and patient safety: 1 certified professional in patient safety and certified as a Lean Six Sigma Black Belt; 2 physicians certified as Lean Six Sigma Black Belts; and 1 physician certified as a Lean Six Sigma Green Belt. Two inpatient clinical pharmacists and 1 quality management specialist also were involved in its development.
Development of a new NRT order set was felt to be an integral part of the tobacco cessation care delivery process. An NRT order set perceived by users as value-added required a solution that merged EBM with standardization and applied quality improvement principles. The result was an approach to order set design that focused on 4 key questions: Is the order set efficient and easy to use/navigate? Is human factors engineering incorporated? Is failure mode and effects analysis applied? Are evidence-based practices included?
Ease of Use and Navigation
Implementing an order set that is efficient and easy to use or navigate seems straightforward but can be difficult to execute. Figure 1 shows many detailed options consisting of different combinations of nicotine patches, lozenges, and gum. Also included are oral tobacco cessation options (bupropion and varenicline). Although more options may seem better, confusion about appropriate medication selection can occur.
According to Heath and Heath, too many options can result in lack of action.4 For example, Heath and Heath discuss a food store that offered 6 free samples of different jams on one day and 24 jams the following day. The customers who sampled 6 different types of jam were 10 times more likely to buy jam. The authors concluded that the more options available, the more difficulty a potential buyer has in deciding on a course of action.4
In clinical situations where a HCP is using an order set, the number of options can mean the difference between use vs avoidance if the choices are overwhelming. HCPs process layers of detail every day when creating differential diagnoses and treatment plans. While that level of detail is necessary clinically, that same level of detail included in orders sets can create challenges for HCPs.
Figure 2 advances the order set in Figure 1 by providing a simpler and cleaner design, so HCPs can more easily review and process the information. This order set design minimizes the number of options available to help users make the right decision, focusing on value for the appropriate setting and audience. In other words, order sets should not be a “one size fits all” approach.
Order sets should be tailored to the appropriate clinical setting (eg, inpatient acute care, outpatient clinic setting, etc) and HCP (eg, hospitalist, tobacco cessation specialist, etc). We are comparing NRT order sets designed for HCPs who do not routinely prescribe oral tobacco cessation products in the inpatient setting. When possible, autogenerated bundle orders should also be used according to evidence-based recommendations (such as nicotine patch tapers) for ease of use and further simplification of order sets.
Finally, usability testing known as “evaluating a product or service by testing it with representative users” helps further refine an order set.5Usability testing should be applied during all phases of order set development with end user(s) as it helps identify problems with order set design prior to implementation. By applying usability testing, the order set becomes more meaningful and valued by the user.
Human Factors Engineering
HFE is “the study of all the factors that make it easier to do the work in the right way.”6 HFE seeks to identify, align, and apply processes for people and the world within which they live and work to promote safe and efficient practices, especially in relation to the technology and physical design features in their work environment.6
The average American adult makes about 35,000 decisions per day.7 Thus, there is potential for error at any moment. Design that does not take HFE into account can be dangerous. For example, when tube feed and IV line connectors look similar and are compatible, patients may inadvertently receive food administered directly into their bloodstream.8
HFE can and should be applied to order sets. Everything from the look, feel, and verbiage of an order set affects potential outcomes. For example, consider the impact even seemingly minor modifications can have on outcomes simply by guiding users in a different way: Figure 1 provides NRT options based on cigarette use per day, whereas Figure 2 conveys pack use per day in relation to the equivalent number of cigarettes used daily. These differences may seem small; however, it helps guide users to the right choice when considering that health care providers have been historically trained on social history gathering that emphasizes packs per day and pack-years.
Failure Mode and Effects Analysis
Failure mode and effects analysis (FMEA) is “a structured way to identify and address potential problems, or failures and their resulting effects on the system or process before an adverse event occurs.”9 The benefit of an order set must be weighed against the risk during development. FMEA should be applied during order set design to assess and limit risk just as with any other clinical care process.
FMEA examines both level of risk and frequency of risk occurrence associated with a new proposed process. For example, let’s evaluate an order set designed for pain control after surgery that consists of multiple high-risk opioids along with antihistamine medications for as-needed itch relief (a non-life-threatening adverse event (AE) of opioids well known by the medical community). An interdisciplinary FMEA team consisting of subject matter experts may examine how the process should flow in step-by-step detail and then discuss the benefit of a process and risk for potential error. A FMEA team would then analyze what could go wrong with each part of the process and assign a level of risk and risk frequency for various steps in the process, and then decide that certain steps should be modified or eliminated. Perhaps after FMEA, a facility might conclude that the risk of serious complications is high when you combine opioid use with antihistamine medications. The facility could decide to remove antihistamine medications from an order set if it is determined that risks outweigh benefits. While a root cause analysis might identify the cause of an AE after order set use, these situations can be prevented with FMEA.
When applying FMEA to Figure 1, while bupropion is known as an evidence-based oral tobacco cessation option, there is the possibility that bupropion could be inadvertently prescribed from the order set in a hospitalized patient with alcohol withdrawal and withdrawal seizure history. These potentially dangerous situations can be avoided with FMEA. Thus, although bupropion may be evidence-based for NRT, decisions regarding order set design using EBM alone are insufficient.
The practitioner must consider possible unintended consequences within order sets and target treatment options to the appropriate setting and audience. Although Figure 1 may appear to be more inclusive, the interdisciplinary committee designing the inpatient NRT order set felt there was heightened risk with introducing bupropion in Figure 1 and decided the risk would be lowered by removing bupropion from the redesigned NRT order set (Figure 2). In addition to the goal of balancing availability of NRT options with acceptable risk, Figure 2 also focused on building an NRT order set most applicable to the inpatient setting.
Including Evidence-Based Practices
EBM has become a routine part of clinical decision making. Therefore, including EBM in order set design is vital. EBM for NRT has demonstrated that combination therapy is more effective than is monotherapy to help tobacco users quit. Incremental doses of NRT are recommended for patients who use tobacco more frequently.10
As shown in Figures 1 and 2, both order set designs incorporate EBM for NRT. Although the importance of implementing EBM is evident, critical factors, such as HFE and FMEA make a difference with well-designed order sets.
Results
The 4-step order set design technique was used during development of an inpatient NRT order set at the JAHVH. Results for the inpatient Joint Commission Tobacco Treatment Measures were obtained from the Veterans Health Administration quality metric reporting system known as Strategic Analytics for Improvement and Learning (SAIL). SAIL performance measure outcomes, which include the inpatient Joint Commission Tobacco Treatment Measures, are derived from chart reviews conducted by the External Peer Review Program. Outcomes demonstrated that TOB-2 and TOB-3 (2 inpatient Joint Commission Tobacco Treatment Measures) known as tob20 and tob40, respectively, within SAIL improved by more than 300% after development of an NRT order set using the 4-step order set design framework along with implementation of a multifaceted tobacco cessation care delivery system at JAHVH.
Discussion
While the overall tobacco cessation care delivery system contributed to improved outcomes with the inpatient Joint Commission Tobacco Treatment Measures at JAHVH, the NRT order set was a cornerstone of the design. Although using our order set design technique does not necessarily guarantee successful outcomes, we believe using the 4-step order set design process increases the value of order sets and has potential to improve quality outcomes.
Limitations
Although improved outcomes following implementation of our NRT order set suggest correlation, causation cannot be proven. Also while the NRT order set is believed to have helped tremendously with outcomes, the entire tobacco cessation care delivery system at JAHVH contributed to the results. In addition, the inpatient Joint Commission Tobacco Treatment Measures help improve processes for tobacco cessation care. However, we are uncertain whether the results of our improvement efforts helped patients stop tobacco use. Further studies are needed to determine impact on population health. Finally, our results were based on improvement work done at a single center. Further studies are necessary to see whether results are reproducible.
Conclusion
There was significant improvement with the inpatient Joint Commission Tobacco Treatment Measures outcomes following development of a tobacco cessation care delivery system that included design of an inpatient NRT order set using a 4-step process we developed. This 4-step structure includes emphasis on efficiency and ease of use; human factors engineering; failure mode and effects analysis; and incorporation of evidence-based medicine (Box.) Postimplementation results showed improvement of the inpatient Joint Commission Tobacco Treatment Measures by greater than 3-fold at a single hospital.
The next steps for this initiative include testing the 4-step order set design process in multiple clinical settings to determine the effectiveness of this approach in other areas of clinical care.
In the current health care environment, hospitals are constantly challenged to improve quality metrics and deliver better health care outcomes. One means to achieving quality improvement is through the use of order sets, groups of related orders that a health care provider (HCP) can place with either a few keystrokes or mouse clicks.1
Historically, design of order sets has largely focused on clicking checkboxes containing evidence-based practices. According to Bates and colleagues and the Institute for Safe Medication Practices, incorporating evidence-based medicine (EBM) into order sets is not by itself sufficient.2,3Execution of proper design coupled with simplicity and provider efficiency is paramount to HCP buy-in, increased likelihood of order set adherence, and to potentially better outcomes.
In this article, we outline advancements in order set design. These improvements increase provider efficiency and ease of use; incorporate human factors engineering (HFE); apply failure mode and effects analysis; and include EBM.
Methods
An inpatient nicotine replacement therapy (NRT) order was developed as part of a multifaceted solution to improve tobacco cessation care at the James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, a complexity level 1a facility. This NRT order set used the 4-step order set design framework the authors’ developed (for additional information about the NRT order set, contact the authors). We distinguish order set design technique between 2 different inpatient NRT order sets. The first order set in the comparison (Figure 1) is an inpatient NRT order set of unknown origin—it is common for US Department of Veterans Affairs (VA) medical facilities to share order sets and other resources. The second order set (Figure 2) is an inpatient NRT order set we designed using our 4-step process for comparison in this article. No institutional review board approval was required as this work met criteria for operational improvement activities exempt from ethics review.
Justin Iannello, DO, MBA, was the team leader and developer of the 4-step order set design technique. The intervention team consisted of 4 internal medicine physicians with expertise in quality improvement and patient safety: 1 certified professional in patient safety and certified as a Lean Six Sigma Black Belt; 2 physicians certified as Lean Six Sigma Black Belts; and 1 physician certified as a Lean Six Sigma Green Belt. Two inpatient clinical pharmacists and 1 quality management specialist also were involved in its development.
Development of a new NRT order set was felt to be an integral part of the tobacco cessation care delivery process. An NRT order set perceived by users as value-added required a solution that merged EBM with standardization and applied quality improvement principles. The result was an approach to order set design that focused on 4 key questions: Is the order set efficient and easy to use/navigate? Is human factors engineering incorporated? Is failure mode and effects analysis applied? Are evidence-based practices included?
Ease of Use and Navigation
Implementing an order set that is efficient and easy to use or navigate seems straightforward but can be difficult to execute. Figure 1 shows many detailed options consisting of different combinations of nicotine patches, lozenges, and gum. Also included are oral tobacco cessation options (bupropion and varenicline). Although more options may seem better, confusion about appropriate medication selection can occur.
According to Heath and Heath, too many options can result in lack of action.4 For example, Heath and Heath discuss a food store that offered 6 free samples of different jams on one day and 24 jams the following day. The customers who sampled 6 different types of jam were 10 times more likely to buy jam. The authors concluded that the more options available, the more difficulty a potential buyer has in deciding on a course of action.4
In clinical situations where a HCP is using an order set, the number of options can mean the difference between use vs avoidance if the choices are overwhelming. HCPs process layers of detail every day when creating differential diagnoses and treatment plans. While that level of detail is necessary clinically, that same level of detail included in orders sets can create challenges for HCPs.
Figure 2 advances the order set in Figure 1 by providing a simpler and cleaner design, so HCPs can more easily review and process the information. This order set design minimizes the number of options available to help users make the right decision, focusing on value for the appropriate setting and audience. In other words, order sets should not be a “one size fits all” approach.
Order sets should be tailored to the appropriate clinical setting (eg, inpatient acute care, outpatient clinic setting, etc) and HCP (eg, hospitalist, tobacco cessation specialist, etc). We are comparing NRT order sets designed for HCPs who do not routinely prescribe oral tobacco cessation products in the inpatient setting. When possible, autogenerated bundle orders should also be used according to evidence-based recommendations (such as nicotine patch tapers) for ease of use and further simplification of order sets.
Finally, usability testing known as “evaluating a product or service by testing it with representative users” helps further refine an order set.5Usability testing should be applied during all phases of order set development with end user(s) as it helps identify problems with order set design prior to implementation. By applying usability testing, the order set becomes more meaningful and valued by the user.
Human Factors Engineering
HFE is “the study of all the factors that make it easier to do the work in the right way.”6 HFE seeks to identify, align, and apply processes for people and the world within which they live and work to promote safe and efficient practices, especially in relation to the technology and physical design features in their work environment.6
The average American adult makes about 35,000 decisions per day.7 Thus, there is potential for error at any moment. Design that does not take HFE into account can be dangerous. For example, when tube feed and IV line connectors look similar and are compatible, patients may inadvertently receive food administered directly into their bloodstream.8
HFE can and should be applied to order sets. Everything from the look, feel, and verbiage of an order set affects potential outcomes. For example, consider the impact even seemingly minor modifications can have on outcomes simply by guiding users in a different way: Figure 1 provides NRT options based on cigarette use per day, whereas Figure 2 conveys pack use per day in relation to the equivalent number of cigarettes used daily. These differences may seem small; however, it helps guide users to the right choice when considering that health care providers have been historically trained on social history gathering that emphasizes packs per day and pack-years.
Failure Mode and Effects Analysis
Failure mode and effects analysis (FMEA) is “a structured way to identify and address potential problems, or failures and their resulting effects on the system or process before an adverse event occurs.”9 The benefit of an order set must be weighed against the risk during development. FMEA should be applied during order set design to assess and limit risk just as with any other clinical care process.
FMEA examines both level of risk and frequency of risk occurrence associated with a new proposed process. For example, let’s evaluate an order set designed for pain control after surgery that consists of multiple high-risk opioids along with antihistamine medications for as-needed itch relief (a non-life-threatening adverse event (AE) of opioids well known by the medical community). An interdisciplinary FMEA team consisting of subject matter experts may examine how the process should flow in step-by-step detail and then discuss the benefit of a process and risk for potential error. A FMEA team would then analyze what could go wrong with each part of the process and assign a level of risk and risk frequency for various steps in the process, and then decide that certain steps should be modified or eliminated. Perhaps after FMEA, a facility might conclude that the risk of serious complications is high when you combine opioid use with antihistamine medications. The facility could decide to remove antihistamine medications from an order set if it is determined that risks outweigh benefits. While a root cause analysis might identify the cause of an AE after order set use, these situations can be prevented with FMEA.
When applying FMEA to Figure 1, while bupropion is known as an evidence-based oral tobacco cessation option, there is the possibility that bupropion could be inadvertently prescribed from the order set in a hospitalized patient with alcohol withdrawal and withdrawal seizure history. These potentially dangerous situations can be avoided with FMEA. Thus, although bupropion may be evidence-based for NRT, decisions regarding order set design using EBM alone are insufficient.
The practitioner must consider possible unintended consequences within order sets and target treatment options to the appropriate setting and audience. Although Figure 1 may appear to be more inclusive, the interdisciplinary committee designing the inpatient NRT order set felt there was heightened risk with introducing bupropion in Figure 1 and decided the risk would be lowered by removing bupropion from the redesigned NRT order set (Figure 2). In addition to the goal of balancing availability of NRT options with acceptable risk, Figure 2 also focused on building an NRT order set most applicable to the inpatient setting.
Including Evidence-Based Practices
EBM has become a routine part of clinical decision making. Therefore, including EBM in order set design is vital. EBM for NRT has demonstrated that combination therapy is more effective than is monotherapy to help tobacco users quit. Incremental doses of NRT are recommended for patients who use tobacco more frequently.10
As shown in Figures 1 and 2, both order set designs incorporate EBM for NRT. Although the importance of implementing EBM is evident, critical factors, such as HFE and FMEA make a difference with well-designed order sets.
Results
The 4-step order set design technique was used during development of an inpatient NRT order set at the JAHVH. Results for the inpatient Joint Commission Tobacco Treatment Measures were obtained from the Veterans Health Administration quality metric reporting system known as Strategic Analytics for Improvement and Learning (SAIL). SAIL performance measure outcomes, which include the inpatient Joint Commission Tobacco Treatment Measures, are derived from chart reviews conducted by the External Peer Review Program. Outcomes demonstrated that TOB-2 and TOB-3 (2 inpatient Joint Commission Tobacco Treatment Measures) known as tob20 and tob40, respectively, within SAIL improved by more than 300% after development of an NRT order set using the 4-step order set design framework along with implementation of a multifaceted tobacco cessation care delivery system at JAHVH.
Discussion
While the overall tobacco cessation care delivery system contributed to improved outcomes with the inpatient Joint Commission Tobacco Treatment Measures at JAHVH, the NRT order set was a cornerstone of the design. Although using our order set design technique does not necessarily guarantee successful outcomes, we believe using the 4-step order set design process increases the value of order sets and has potential to improve quality outcomes.
Limitations
Although improved outcomes following implementation of our NRT order set suggest correlation, causation cannot be proven. Also while the NRT order set is believed to have helped tremendously with outcomes, the entire tobacco cessation care delivery system at JAHVH contributed to the results. In addition, the inpatient Joint Commission Tobacco Treatment Measures help improve processes for tobacco cessation care. However, we are uncertain whether the results of our improvement efforts helped patients stop tobacco use. Further studies are needed to determine impact on population health. Finally, our results were based on improvement work done at a single center. Further studies are necessary to see whether results are reproducible.
Conclusion
There was significant improvement with the inpatient Joint Commission Tobacco Treatment Measures outcomes following development of a tobacco cessation care delivery system that included design of an inpatient NRT order set using a 4-step process we developed. This 4-step structure includes emphasis on efficiency and ease of use; human factors engineering; failure mode and effects analysis; and incorporation of evidence-based medicine (Box.) Postimplementation results showed improvement of the inpatient Joint Commission Tobacco Treatment Measures by greater than 3-fold at a single hospital.
The next steps for this initiative include testing the 4-step order set design process in multiple clinical settings to determine the effectiveness of this approach in other areas of clinical care.
1. Order set. http://clinfowiki.org/wiki/index.php/Order_set. Updated October 15, 2015. Accessed August 30, 2019.
2. Bates DW, Kuperman GJ, Wang S, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc. 2003;10(6):523-530.
3. Institute for Safe Medication Practices. Guidelines for standard order sets. https://www.ismp.org/tools/guidelines/standardordersets.pdf. Published January 12, 2010. Accessed August 30, 2019.
4. Heath C, Heath D. Switch: How to Change Things When Change Is Hard. New York, NY: Crown Business; 2010:50-51.
5. US Department of Health and Human Services. Usability testing. https://www.usability.gov/how-to-and-tools/methods/usability-testing.html. Accessed August 30, 2019.
6. World Health Organization. What is human factors and why is it important to patient safety? www.who.int/patientsafety/education/curriculum/who_mc_topic-2.pdf. Accessed August 30, 2019.
7. Sollisch J. The cure for decision fatigue. Wall Street Journal. June 10, 2016. https://www.wsj.com/articles/the-cure-for-decision-fatigue-1465596928. Accessed August 30, 2019.
8. ECRI Institute. Implementing the ENFit initiative for preventing enteral tubing misconnections. https://www.ecri.org/components/HDJournal/Pages/ENFit-for-Preventing-Enteral-Tubing-Misconnections.aspx. Published March 29, 2017. Accessed August 30, 2019.
9. Guidance for performing failure mode and effects analysis with performance improvement projects. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/QAPI/downloads/GuidanceForFMEA.pdf. Accessed August 30, 2019.
10. Diefanbach LJ, Smith PO, Nashelsky JM, Lindbloom E. What is the most effective nicotine replacement therapy? J Fam Pract. 2003;52(6):492-497.
1. Order set. http://clinfowiki.org/wiki/index.php/Order_set. Updated October 15, 2015. Accessed August 30, 2019.
2. Bates DW, Kuperman GJ, Wang S, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc. 2003;10(6):523-530.
3. Institute for Safe Medication Practices. Guidelines for standard order sets. https://www.ismp.org/tools/guidelines/standardordersets.pdf. Published January 12, 2010. Accessed August 30, 2019.
4. Heath C, Heath D. Switch: How to Change Things When Change Is Hard. New York, NY: Crown Business; 2010:50-51.
5. US Department of Health and Human Services. Usability testing. https://www.usability.gov/how-to-and-tools/methods/usability-testing.html. Accessed August 30, 2019.
6. World Health Organization. What is human factors and why is it important to patient safety? www.who.int/patientsafety/education/curriculum/who_mc_topic-2.pdf. Accessed August 30, 2019.
7. Sollisch J. The cure for decision fatigue. Wall Street Journal. June 10, 2016. https://www.wsj.com/articles/the-cure-for-decision-fatigue-1465596928. Accessed August 30, 2019.
8. ECRI Institute. Implementing the ENFit initiative for preventing enteral tubing misconnections. https://www.ecri.org/components/HDJournal/Pages/ENFit-for-Preventing-Enteral-Tubing-Misconnections.aspx. Published March 29, 2017. Accessed August 30, 2019.
9. Guidance for performing failure mode and effects analysis with performance improvement projects. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/QAPI/downloads/GuidanceForFMEA.pdf. Accessed August 30, 2019.
10. Diefanbach LJ, Smith PO, Nashelsky JM, Lindbloom E. What is the most effective nicotine replacement therapy? J Fam Pract. 2003;52(6):492-497.
The VA Ketamine Controversies
"Extreme remedies are very appropriate for extreme diseases"
- Hippocrates Aphorisms
On March 5, 2019, the US Food and Drug Administration (FDA) approved a nasal spray formulation of the drug ketamine, an old anesthetic that has been put to a new use over the past 10 years as therapy for treatment-resistant severe depression. Ketamine, known on the street as Special K, has long been known to cause dissociation, hallucinations, and other hallucinogenic effects. In many randomized controlled trials, subanesthetic doses administered intravenously have demonstrated rapid and often dramatic relief of depressive symptoms.
Neuroscientists have heralded ketamine as the paradigm
When the FDA approved Spravato (esketamine), a nasal administration of ketamine, many people hoped that researchers had succeeded in overcoming these barriers. The risks of serious adverse events (AEs) as well as the potential for abuse and diversion led the FDA to limit prescriptions under a Risk Evaluation and Mitigation Strategy (REMS).3 Patients self-administer the nasal spray but only in a certified medical facility under the observation of a health care practitioner. Patients also must agree to remain on site for 2 hours after administration of the drug to ensure their safety. The FDA recommends the drug be given twice a week for 4 weeks along with a conventional monoamine-acting antidepressant.When the US Department of Veterans Affairs (VA) cleared the way for use of esketamine, less than 2 weeks after the FDA approval, it also launched a series of controversies over how to use the drug in its massive health care system, which is the subject of this editorial. On March 19, 2019, the VA announced that VA practitioners would be able to prescribe the nasal spray for patients who were determined to have treatment-resistant depression but only after appropriate clinical assessment and in accordance with their patients’ preferences.
A number of controversies have emerged surrounding the VA adoption of esketamine, including its cost/benefit/risk ratio and who should be able to access the medication. Each of these issues has onion layers of political, regulatory, and ethical concerns that can only be superficially noted here and warrant fuller unpeeling. In June The New York Times featured a story alleging that in response to the tragic tide of ever-increasing veteran suicides, the VA sanctioned esketamine prescribing despite its cost and the serious questions experts raised about the data the FDA cited to establish its safety and efficacy. Although the cost to the VA of Spravato is unclear, it is much higher than generic IV ketamine.4
The access controversy is almost the ethical inverse of the first. In June 2019, a Veterans Health Administration advisory panel voted against allowing general use of esketamine, limiting it to individual cases of patients who are preapproved and have failed 2 antidepressant trials. Esketamine will not be on the VA formulary for widespread use. Congressional and public advocacy groups have noted that the formulary decision came in the wake of ongoing attention to the role of the pharmaceutical industry in the VA’s rapid adoption of the drug.5,6 For the thousands of veterans for whom the data show conventional antidepressants even in combination with other psychotropic medications and evidence-based psychotherapies resulted in AEs or only partial remission of depression symptoms, the VA’s restriction will likely seem unfair and even uncaring.7
As a practicing VA psychiatrist, I know firsthand how desperately we need new, more effective, and better-tolerated treatments for severe unipolar and bipolar depression. Although I have not prescribed ketamine or esketamine, several of my most respected colleagues do. I have seen patients with chronic, severe, depression respond and even recover in ways that seem just a little short of miraculous when compared with other therapies. Yet as a longtime student of the history of psychiatry, I have also seen that often the treatments that initially seem so auspicious, in time, turn out to have a dark side. Families, communities, the country, VA, and the US Department of Defense and its practitioners in and out of mental health cannot in any moral universe abide by the fact that 20 plus men and women who served take their lives every day.8
As the epigraph to this column notes, we must often try radical therapies for grave cases in drastic crises. Yet we must also in making serious public health decisions fraught with unseen consequences take all due and considered diligence that we do not violate the even more fundamental dictum of the Hippocratic School, “at least do not harm.” That means trying to balance safety and availability while VA conducts its own research in a precarious way that leaves almost no stakeholder completely happy.
1. Lener MS, Kadriu B, Zarate CA Jr. Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77(4):381-401.
2. Thielking M. “Is the Ketamine Boon Getting out of Hand?” STAT. September 24, 2018. https://www.statnews.com/2018/09/24/ketamine-clinics-severe-depression-treatment. Accessed September 17, 2019.
3. US Food and Drug Administration. FDA approves new nasal spray medication for treatment-resistant depression: available only at a certified doctor’s office or clinic [press release]. https://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified. Published March 5, 2019. Accessed September 17, 2019.
4. Carey B, Steinhauser J. Veterans agency to offer new depression drug, despite safety and efficacy concerns. The New York Times. June 21, 2019. https://www.nytimes.com/2019/06/21/health/ketamine-depression-veterans.html. Accessed September 17, 2019.
5. US House of Representatives, Committee on Veterans Affairs. Chairman Takano statement following reports that VA fast-tracked controversial drug Spravato to treat veterans [press release]. https://veterans.house.gov/news/press-releases/chairman-takano-statement-following-reports-that-va-fast-tracked-controversial-drug-spravato-to-treat-veterans. Published June 18, 2019. Accessed September 17, 2019.
6. Cary P. Trump’s praise put drug for vets on fast track, but experts are not sure it works. https://publicintegrity.org/federal-politics/trumps-raves-put-drug-for-vets-on-fast-track-but-experts-arent-sure-it-works. Published June 18, 2019. Accessed September 17, 2019.
7. Zisook S, Tal I, Weingart K, et al. Characteristics of U.S. veteran patients with major depressive disorder who require ‘next-step’ treatments: A VAST-D report. J Affect Disord. 2016;206:232-240.
8. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. VA National Suicide Data Report 2005-2016. https://www.mentalhealth.va.gov/docs/data-sheets/OMHSP_National_Suicide_Data_Report_2005-2016_508.pdf. Updated 2018. Accessed September 17, 2019.
"Extreme remedies are very appropriate for extreme diseases"
- Hippocrates Aphorisms
On March 5, 2019, the US Food and Drug Administration (FDA) approved a nasal spray formulation of the drug ketamine, an old anesthetic that has been put to a new use over the past 10 years as therapy for treatment-resistant severe depression. Ketamine, known on the street as Special K, has long been known to cause dissociation, hallucinations, and other hallucinogenic effects. In many randomized controlled trials, subanesthetic doses administered intravenously have demonstrated rapid and often dramatic relief of depressive symptoms.
Neuroscientists have heralded ketamine as the paradigm
When the FDA approved Spravato (esketamine), a nasal administration of ketamine, many people hoped that researchers had succeeded in overcoming these barriers. The risks of serious adverse events (AEs) as well as the potential for abuse and diversion led the FDA to limit prescriptions under a Risk Evaluation and Mitigation Strategy (REMS).3 Patients self-administer the nasal spray but only in a certified medical facility under the observation of a health care practitioner. Patients also must agree to remain on site for 2 hours after administration of the drug to ensure their safety. The FDA recommends the drug be given twice a week for 4 weeks along with a conventional monoamine-acting antidepressant.When the US Department of Veterans Affairs (VA) cleared the way for use of esketamine, less than 2 weeks after the FDA approval, it also launched a series of controversies over how to use the drug in its massive health care system, which is the subject of this editorial. On March 19, 2019, the VA announced that VA practitioners would be able to prescribe the nasal spray for patients who were determined to have treatment-resistant depression but only after appropriate clinical assessment and in accordance with their patients’ preferences.
A number of controversies have emerged surrounding the VA adoption of esketamine, including its cost/benefit/risk ratio and who should be able to access the medication. Each of these issues has onion layers of political, regulatory, and ethical concerns that can only be superficially noted here and warrant fuller unpeeling. In June The New York Times featured a story alleging that in response to the tragic tide of ever-increasing veteran suicides, the VA sanctioned esketamine prescribing despite its cost and the serious questions experts raised about the data the FDA cited to establish its safety and efficacy. Although the cost to the VA of Spravato is unclear, it is much higher than generic IV ketamine.4
The access controversy is almost the ethical inverse of the first. In June 2019, a Veterans Health Administration advisory panel voted against allowing general use of esketamine, limiting it to individual cases of patients who are preapproved and have failed 2 antidepressant trials. Esketamine will not be on the VA formulary for widespread use. Congressional and public advocacy groups have noted that the formulary decision came in the wake of ongoing attention to the role of the pharmaceutical industry in the VA’s rapid adoption of the drug.5,6 For the thousands of veterans for whom the data show conventional antidepressants even in combination with other psychotropic medications and evidence-based psychotherapies resulted in AEs or only partial remission of depression symptoms, the VA’s restriction will likely seem unfair and even uncaring.7
As a practicing VA psychiatrist, I know firsthand how desperately we need new, more effective, and better-tolerated treatments for severe unipolar and bipolar depression. Although I have not prescribed ketamine or esketamine, several of my most respected colleagues do. I have seen patients with chronic, severe, depression respond and even recover in ways that seem just a little short of miraculous when compared with other therapies. Yet as a longtime student of the history of psychiatry, I have also seen that often the treatments that initially seem so auspicious, in time, turn out to have a dark side. Families, communities, the country, VA, and the US Department of Defense and its practitioners in and out of mental health cannot in any moral universe abide by the fact that 20 plus men and women who served take their lives every day.8
As the epigraph to this column notes, we must often try radical therapies for grave cases in drastic crises. Yet we must also in making serious public health decisions fraught with unseen consequences take all due and considered diligence that we do not violate the even more fundamental dictum of the Hippocratic School, “at least do not harm.” That means trying to balance safety and availability while VA conducts its own research in a precarious way that leaves almost no stakeholder completely happy.
"Extreme remedies are very appropriate for extreme diseases"
- Hippocrates Aphorisms
On March 5, 2019, the US Food and Drug Administration (FDA) approved a nasal spray formulation of the drug ketamine, an old anesthetic that has been put to a new use over the past 10 years as therapy for treatment-resistant severe depression. Ketamine, known on the street as Special K, has long been known to cause dissociation, hallucinations, and other hallucinogenic effects. In many randomized controlled trials, subanesthetic doses administered intravenously have demonstrated rapid and often dramatic relief of depressive symptoms.
Neuroscientists have heralded ketamine as the paradigm
When the FDA approved Spravato (esketamine), a nasal administration of ketamine, many people hoped that researchers had succeeded in overcoming these barriers. The risks of serious adverse events (AEs) as well as the potential for abuse and diversion led the FDA to limit prescriptions under a Risk Evaluation and Mitigation Strategy (REMS).3 Patients self-administer the nasal spray but only in a certified medical facility under the observation of a health care practitioner. Patients also must agree to remain on site for 2 hours after administration of the drug to ensure their safety. The FDA recommends the drug be given twice a week for 4 weeks along with a conventional monoamine-acting antidepressant.When the US Department of Veterans Affairs (VA) cleared the way for use of esketamine, less than 2 weeks after the FDA approval, it also launched a series of controversies over how to use the drug in its massive health care system, which is the subject of this editorial. On March 19, 2019, the VA announced that VA practitioners would be able to prescribe the nasal spray for patients who were determined to have treatment-resistant depression but only after appropriate clinical assessment and in accordance with their patients’ preferences.
A number of controversies have emerged surrounding the VA adoption of esketamine, including its cost/benefit/risk ratio and who should be able to access the medication. Each of these issues has onion layers of political, regulatory, and ethical concerns that can only be superficially noted here and warrant fuller unpeeling. In June The New York Times featured a story alleging that in response to the tragic tide of ever-increasing veteran suicides, the VA sanctioned esketamine prescribing despite its cost and the serious questions experts raised about the data the FDA cited to establish its safety and efficacy. Although the cost to the VA of Spravato is unclear, it is much higher than generic IV ketamine.4
The access controversy is almost the ethical inverse of the first. In June 2019, a Veterans Health Administration advisory panel voted against allowing general use of esketamine, limiting it to individual cases of patients who are preapproved and have failed 2 antidepressant trials. Esketamine will not be on the VA formulary for widespread use. Congressional and public advocacy groups have noted that the formulary decision came in the wake of ongoing attention to the role of the pharmaceutical industry in the VA’s rapid adoption of the drug.5,6 For the thousands of veterans for whom the data show conventional antidepressants even in combination with other psychotropic medications and evidence-based psychotherapies resulted in AEs or only partial remission of depression symptoms, the VA’s restriction will likely seem unfair and even uncaring.7
As a practicing VA psychiatrist, I know firsthand how desperately we need new, more effective, and better-tolerated treatments for severe unipolar and bipolar depression. Although I have not prescribed ketamine or esketamine, several of my most respected colleagues do. I have seen patients with chronic, severe, depression respond and even recover in ways that seem just a little short of miraculous when compared with other therapies. Yet as a longtime student of the history of psychiatry, I have also seen that often the treatments that initially seem so auspicious, in time, turn out to have a dark side. Families, communities, the country, VA, and the US Department of Defense and its practitioners in and out of mental health cannot in any moral universe abide by the fact that 20 plus men and women who served take their lives every day.8
As the epigraph to this column notes, we must often try radical therapies for grave cases in drastic crises. Yet we must also in making serious public health decisions fraught with unseen consequences take all due and considered diligence that we do not violate the even more fundamental dictum of the Hippocratic School, “at least do not harm.” That means trying to balance safety and availability while VA conducts its own research in a precarious way that leaves almost no stakeholder completely happy.
1. Lener MS, Kadriu B, Zarate CA Jr. Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77(4):381-401.
2. Thielking M. “Is the Ketamine Boon Getting out of Hand?” STAT. September 24, 2018. https://www.statnews.com/2018/09/24/ketamine-clinics-severe-depression-treatment. Accessed September 17, 2019.
3. US Food and Drug Administration. FDA approves new nasal spray medication for treatment-resistant depression: available only at a certified doctor’s office or clinic [press release]. https://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified. Published March 5, 2019. Accessed September 17, 2019.
4. Carey B, Steinhauser J. Veterans agency to offer new depression drug, despite safety and efficacy concerns. The New York Times. June 21, 2019. https://www.nytimes.com/2019/06/21/health/ketamine-depression-veterans.html. Accessed September 17, 2019.
5. US House of Representatives, Committee on Veterans Affairs. Chairman Takano statement following reports that VA fast-tracked controversial drug Spravato to treat veterans [press release]. https://veterans.house.gov/news/press-releases/chairman-takano-statement-following-reports-that-va-fast-tracked-controversial-drug-spravato-to-treat-veterans. Published June 18, 2019. Accessed September 17, 2019.
6. Cary P. Trump’s praise put drug for vets on fast track, but experts are not sure it works. https://publicintegrity.org/federal-politics/trumps-raves-put-drug-for-vets-on-fast-track-but-experts-arent-sure-it-works. Published June 18, 2019. Accessed September 17, 2019.
7. Zisook S, Tal I, Weingart K, et al. Characteristics of U.S. veteran patients with major depressive disorder who require ‘next-step’ treatments: A VAST-D report. J Affect Disord. 2016;206:232-240.
8. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. VA National Suicide Data Report 2005-2016. https://www.mentalhealth.va.gov/docs/data-sheets/OMHSP_National_Suicide_Data_Report_2005-2016_508.pdf. Updated 2018. Accessed September 17, 2019.
1. Lener MS, Kadriu B, Zarate CA Jr. Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77(4):381-401.
2. Thielking M. “Is the Ketamine Boon Getting out of Hand?” STAT. September 24, 2018. https://www.statnews.com/2018/09/24/ketamine-clinics-severe-depression-treatment. Accessed September 17, 2019.
3. US Food and Drug Administration. FDA approves new nasal spray medication for treatment-resistant depression: available only at a certified doctor’s office or clinic [press release]. https://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified. Published March 5, 2019. Accessed September 17, 2019.
4. Carey B, Steinhauser J. Veterans agency to offer new depression drug, despite safety and efficacy concerns. The New York Times. June 21, 2019. https://www.nytimes.com/2019/06/21/health/ketamine-depression-veterans.html. Accessed September 17, 2019.
5. US House of Representatives, Committee on Veterans Affairs. Chairman Takano statement following reports that VA fast-tracked controversial drug Spravato to treat veterans [press release]. https://veterans.house.gov/news/press-releases/chairman-takano-statement-following-reports-that-va-fast-tracked-controversial-drug-spravato-to-treat-veterans. Published June 18, 2019. Accessed September 17, 2019.
6. Cary P. Trump’s praise put drug for vets on fast track, but experts are not sure it works. https://publicintegrity.org/federal-politics/trumps-raves-put-drug-for-vets-on-fast-track-but-experts-arent-sure-it-works. Published June 18, 2019. Accessed September 17, 2019.
7. Zisook S, Tal I, Weingart K, et al. Characteristics of U.S. veteran patients with major depressive disorder who require ‘next-step’ treatments: A VAST-D report. J Affect Disord. 2016;206:232-240.
8. US Department of Veterans Affairs, Office of Mental Health and Suicide Prevention. VA National Suicide Data Report 2005-2016. https://www.mentalhealth.va.gov/docs/data-sheets/OMHSP_National_Suicide_Data_Report_2005-2016_508.pdf. Updated 2018. Accessed September 17, 2019.
Comparing Artificial Intelligence Platforms for Histopathologic Cancer Diagnosis
Artificial intelligence (AI), first described in 1956, encompasses the field of computer science in which machines are trained to learn from experience. The term was popularized by the 1956 Dartmouth College Summer Research Project on Artificial Intelligence.1 The field of AI is rapidly growing and has the potential to affect many aspects of our lives. The emerging importance of AI is demonstrated by a February 2019 executive order that launched the American AI Initiative, allocating resources and funding for AI development.2 The executive order stresses the potential impact of AI in the health care field, including its potential utility to diagnose disease. Federal agencies were directed to invest in AI research and development to promote rapid breakthroughs in AI technology that may impact multiple areas of society.
Machine learning (ML), a subset of AI, was defined in 1959 by Arthur Samuel and is achieved by employing mathematic models to compute sample data sets.3 Originating from statistical linear models, neural networks were conceived to accomplish these tasks.4 These pioneering scientific achievements led to recent developments of deep neural networks. These models are developed to recognize patterns and achieve complex computational tasks within a matter of minutes, often far exceeding human ability.5 ML can increase efficiency with decreased computation time, high precision, and recall when compared with that of human decision making.6
ML has the potential for numerous applications in the health care field.7-9 One promising application is in the field of anatomic pathology. ML allows representative images to be used to train a computer to recognize patterns from labeled photographs. Based on a set of images selected to represent a specific tissue or disease process, the computer can be trained to evaluate and recognize new and unique images from patients and render a diagnosis.10 Prior to modern ML models, users would have to import many thousands of training images to produce algorithms that could recognize patterns with high accuracy. Modern ML algorithms allow for a model known as transfer learning, such that far fewer images are required for training.11-13
Two novel ML platforms available for public use are offered through Google (Mountain View, CA) and Apple (Cupertino, CA).14,15 They each offer a user-friendly interface with minimal experience required in computer science. Google AutoML uses ML via cloud services to store and retrieve data with ease. No coding knowledge is required. The Apple Create ML Module provides computer-based ML, requiring only a few lines of code.
The Veterans Health Administration (VHA) is the largest single health care system in the US, and nearly 50 000 cancer cases are diagnosed at the VHA annually.16 Cancers of the lung and colon are among the most common sources of invasive cancer and are the 2 most common causes of cancer deaths in America.16 We have previously reported using Apple ML in detecting non-small cell lung cancers (NSCLCs), including adenocarcinomas and squamous cell carcinomas (SCCs); and colon cancers with accuracy.17,18 In the present study, we expand on these findings by comparing Apple and Google ML platforms in a variety of common pathologic scenarios in veteran patients. Using limited training data, both programs are compared for precision and recall in differentiating conditions involving lung and colon pathology.
In the first 4 experiments, we evaluated the ability of the platforms to differentiate normal lung tissue from cancerous lung tissue, to distinguish lung adenocarcinoma from SCC, and to differentiate colon adenocarcinoma from normal colon tissue. Next, cases of colon adenocarcinoma were assessed to determine whether the presence or absence of the KRAS proto-oncogene could be determined histologically using the AI platforms. KRAS is found in a variety of cancers, including about 40% of colon adenocarcinomas.19 For colon cancers, the presence or absence of the mutation in KRAS has important implications for patients as it determines whether the tumor will respond to specific chemotherapy agents.20 The presence of the KRAS gene is currently determined by complex molecular testing of tumor tissue.21 However, we assessed the potential of ML to determine whether the mutation is present by computerized morphologic analysis alone. Our last experiment examined the ability of the Apple and Google platforms to differentiate between adenocarcinomas of lung origin vs colon origin. This has potential utility in determining the site of origin of metastatic carcinoma.22
Methods
Fifty cases of lung SCC, 50 cases of lung adenocarcinoma, and 50 cases of colon adenocarcinoma were randomly retrieved from our molecular database. Twenty-five colon adenocarcinoma cases were positive for mutation in KRAS, while 25 cases were negative for mutation in KRAS. Seven hundred fifty total images of lung tissue (250 benign lung tissue, 250 lung adenocarcinomas, and 250 lung SCCs) and 500 total images of colon tissue (250 benign colon tissue and 250 colon adenocarcinoma) were obtained using a Leica Microscope MC190 HD Camera (Wetzlar, Germany) connected to an Olympus BX41 microscope (Center Valley, PA) and the Leica Acquire 9072 software for Apple computers. All the images were captured at a resolution of 1024 x 768 pixels using a 60x dry objective. Lung tissue images were captured and saved on a 2012 Apple MacBook Pro computer, and colon images were captured and saved on a 2011 Apple iMac computer. Both computers were running macOS v10.13.
Creating Image Classifier Models Using Apple Create ML
Apple Create ML is a suite of products that use various tools to create and train custom ML models on Apple computers.15 The suite contains many features, including image classification to train a ML model to classify images, natural language processing to classify natural language text, and tabular data to train models that deal with labeling information or estimating new quantities. We used Create ML Image Classification to create image classifier models for our project (Appendix A).
Creating ML Modules Using Google Cloud AutoML Vision Beta
Google Cloud AutoML is a suite of machine learning products, including AutoML Vision, AutoML Natural Language and AutoML Translation.14 All Cloud AutoML machine learning products were in beta version at the time of experimentation. We used Cloud AutoML Vision beta to create ML modules for our project. Unlike Apple Create ML, which is run on a local Apple computer, the Google Cloud AutoML is run online using a Google Cloud account. There are no minimum specifications requirements for the local computer since it is using the cloud-based architecture (Appendix B).
Experiment 1
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to detect and subclassify NSCLC based on the histopathologic images. We created 3 classes of images (250 images each): benign lung tissue, lung adenocarcinoma, and lung SCC.
Experiment 2
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to differentiate between normal lung tissue and NSCLC histopathologic images with 50/50 mixture of lung adenocarcinoma and lung SCC. We created 2 classes of images (250 images each): benign lung tissue and lung NSCLC.
Experiment 3
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to differentiate between lung adenocarcinoma and lung SCC histopathologic images. We created 2 classes of images (250 images each): adenocarcinoma and SCC.
Experiment 4
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to detect colon cancer histopathologic images regardless of mutation in KRAS status. We created 2 classes of images (250 images each): benign colon tissue and colon adenocarcinoma.
Experiment 5
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to differentiate between colon adenocarcinoma with mutations in KRAS and colon adenocarcinoma without the mutation in KRAS histopathologic images. We created 2 classes of images (125 images each): colon adenocarcinoma cases with mutation in KRAS and colon adenocarcinoma cases without the mutation in KRAS.
Experiment 6
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to differentiate between lung adenocarcinoma and colon adenocarcinoma histopathologic images. We created 2 classes of images (250 images each): colon adenocarcinoma lung adenocarcinoma.
Results
Twelve machine learning models were created in 6 experiments using the Apple Create ML and the Google AutoML (Table). To investigate recall and precision differences between the Apple and the Google ML algorithms, we performed 2-tailed distribution, paired t tests. No statistically significant differences were found (P = .52 for recall and .60 for precision).
Overall, each model performed well in distinguishing between normal and neoplastic tissue for both lung and colon cancers. In subclassifying NSCLC into adenocarcinoma and SCC, the models were shown to have high levels of precision and recall. The models also were successful in distinguishing between lung and colonic origin of adenocarcinoma (Figures 1-4). However, both systems had trouble discerning colon adenocarcinoma with mutations in KRAS from adenocarcinoma without mutations in KRAS.
Discussion
Image classifier models using ML algorithms hold a promising future to revolutionize the health care field. ML products, such as those modules offered by Apple and Google, are easy to use and have a simple graphic user interface to allow individuals to train models to perform humanlike tasks in real time. In our experiments, we compared multiple algorithms to determine their ability to differentiate and subclassify histopathologic images with high precision and recall using common scenarios in treating veteran patients.
Analysis of the results revealed high precision and recall values illustrating the models’ ability to differentiate and detect benign lung tissue from lung SCC and lung adenocarcinoma in ML model 1, benign lung from NSCLC carcinoma in ML model 2, and benign colon from colonic adenocarcinoma in ML model 4. In ML model 3 and 6, both ML algorithms performed at a high level to differentiate lung SCC from lung adenocarcinoma and lung adenocarcinoma from colonic adenocarcinoma, respectively. Of note, ML model 5 had the lowest precision and recall values across both algorithms demonstrating the models’ limited utility in predicting molecular profiles, such as mutations in KRAS as tested here. This is not surprising as pathologists currently require complex molecular tests to detect mutations in KRAS reliably in colon cancer.
Both modules require minimal programming experience and are easy to use. In our comparison, we demonstrated critical distinguishing characteristics that differentiate the 2 products.
Apple Create ML image classifier is available for use on local Mac computers that use Xcode version 10 and macOS 10.14 or later, with just 3 lines of code required to perform computations. Although this product is limited to Apple computers, it is free to use, and images are stored on the computer hard drive. Of unique significance on the Apple system platform, images can be augmented to alter their appearance to enhance model training. For example, imported images can be cropped, rotated, blurred, and flipped, in order to optimize the model’s training abilities to recognize test images and perform pattern recognition. This feature is not as readily available on the Google platform. Apple Create ML Image classifier’s default training set consists of 75% of total imported images with 5% of the total images being randomly used as a validation set. The remaining 20% of images comprise the testing set. The module’s computational analysis to train the model is achieved in about 2 minutes on average. The score threshold is set at 50% and cannot be manipulated for each image class as in Google AutoML Vision.
Google AutoML Vision is open and can be accessed from many devices. It stores images on remote Google servers but requires computing fees after a $300 credit for 12 months. On AutoML Vision, random 80% of the total images are used in the training set, 10% are used in the validation set, and 10% are used in the testing set. It is important to highlight the different percentages used in the default settings on the respective modules. The time to train the Google AutoML Vision with default computational power is longer on average than Apple Create ML, with about 8 minutes required to train the machine learning module. However, it is possible to choose more computational power for an additional fee and decrease module training time. The user will receive e-mail alerts when the computer time begins and is completed. The computation time is calculated by subtracting the time of the initial e-mail from the final e-mail.
Based on our calculations, we determined there was no significant difference between the 2 machine learning algorithms tested at the default settings with recall and precision values obtained. These findings demonstrate the promise of using a ML algorithm to assist in the performance of human tasks and behaviors, specifically the diagnosis of histopathologic images. These results have numerous potential uses in clinical medicine. ML algorithms have been successfully applied to diagnostic and prognostic endeavors in pathology,23-28 dermatology,29-31 ophthalmology,32 cardiology,33 and radiology.34-36
Pathologists often use additional tests, such as special staining of tissues or molecular tests, to assist with accurate classification of tumors. ML platforms offer the potential of an additional tool for pathologists to use along with human microscopic interpretation.37,38 In addition, the number of pathologists in the US is dramatically decreasing, and many other countries have marked physician shortages, especially in fields of specialized training such as pathology.39-42 These models could readily assist physicians in underserved countries and impact shortages of pathologists elsewhere by providing more specific diagnoses in an expedited manner.43
Finally, although we have explored the application of these platforms in common cancer scenarios, great potential exists to use similar techniques in the detection of other conditions. These include the potential for classification and risk assessment of precancerous lesions, infectious processes in tissue (eg, detection of tuberculosis or malaria),24,44 inflammatory conditions (eg, arthritis subtypes, gout),45 blood disorders (eg, abnormal blood cell morphology),46 and many others. The potential of these technologies to improve health care delivery to veteran patients seems to be limited only by the imagination of the user.47
Regarding the limited effectiveness in determining the presence or absence of mutations in KRAS for colon adenocarcinoma, it is mentioned that currently pathologists rely on complex molecular tests to detect the mutations at the DNA level.21 It is possible that the use of more extensive training data sets may improve recall and precision in cases such as these and warrants further study. Our experiments were limited to the stipulations placed by the free trial software agreements; no costs were expended to use the algorithms, though an Apple computer was required.
Conclusion
We have demonstrated the successful application of 2 readily available ML platforms in providing diagnostic guidance in differentiation between common cancer conditions in veteran patient populations. Although both platforms performed very well with no statistically significant differences in results, some distinctions are worth noting. Apple Create ML can be used on local computers but is limited to an Apple operating system. Google AutoML is not platform-specific but runs only via Google Cloud with associated computational fees. Using these readily available models, we demonstrated the vast potential of AI in diagnostic pathology. The application of AI to clinical medicine remains in the very early stages. The VA is uniquely poised to provide leadership as AI technologies will continue to dramatically change the future of health care, both in veteran and nonveteran patients nationwide.
Acknowledgments
The authors thank Paul Borkowski for his constructive criticism and proofreading of this manuscript. This material is the result of work supported with resources and the use of facilities at the James A. Haley Veterans’ Hospital.
1. Moor J. The Dartmouth College artificial intelligence conference: the next fifty years. AI Mag. 2006;27(4):87-91.
2. Trump D. Accelerating America’s leadership in artificial intelligence. https://www.whitehouse.gov/articles/accelerating-americas-leadership-in-artificial-intelligence. Published February 11, 2019. Accessed September 4, 2019.
3. Samuel AL. Some studies in machine learning using the game of checkers. IBM J Res Dev. 1959;3(3):210-229.
4. SAS Users Group International. Neural networks and statistical models. In: Sarle WS. Proceedings of the Nineteenth Annual SAS Users Group International Conference. SAS Institute: Cary, North Carolina; 1994:1538-1550. http://www.sascommunity.org/sugi/SUGI94/Sugi-94-255%20Sarle.pdf. Accessed September 16, 2019.
5. Schmidhuber J. Deep learning in neural networks: an overview. Neural Networks. 2015;61:85-117.
6. LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436-444.
7. Jiang F, Jiang Y, Li H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230-243.
8. Erickson BJ, Korfiatis P, Akkus Z, Kline TL. Machine learning for medical imaging. Radiographics. 2017;37(2):505-515.
9. Deo RC. Machine learning in medicine. Circulation. 2015;132(20):1920-1930.
10. Janowczyk A, Madabhushi A. Deep learning for digital pathology image analysis: a comprehensive tutorial with selected use cases. J Pathol Inform. 2016;7(1):29.
11. Oquab M, Bottou L, Laptev I, Sivic J. Learning and transferring mid-level image representations using convolutional neural networks. Presented at: IEEE Conference on Computer Vision and Pattern Recognition, 2014. http://openaccess.thecvf.com/content_cvpr_2014/html/Oquab_Learning_and_Transferring_2014_CVPR_paper.html. Accessed September 4, 2019.
12. Shin HC, Roth HR, Gao M, et al. Deep convolutional neural networks for computer-aided detection: CNN architectures, dataset characteristics and transfer learning. IEEE Trans Med Imaging. 2016;35(5):1285-1298.
13. Tajbakhsh N, Shin JY, Gurudu SR, et al. Convolutional neural networks for medical image analysis: full training or fine tuning? IEEE Trans Med Imaging. 2016;35(5):1299-1312.
14. Cloud AutoML. https://cloud.google.com/automl. Accessed September 4, 2019.
15. Create ML. https://developer.apple.com/documentation/createml. Accessed September 4, 2019.
16. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 Update. Mil Med. 2017;182(7):e1883-e1891. 17. Borkowski AA, Wilson CP, Borkowski SA, Deland LA, Mastorides SM. Using Apple machine learning algorithms to detect and subclassify non-small cell lung cancer. https://arxiv.org/ftp/arxiv/papers/1808/1808.08230.pdf. Accessed September 4, 2019.
18. Borkowski AA, Wilson CP, Borkowski SA, Thomas LB, Deland LA, Mastorides SM. Apple machine learning algorithms successfully detect colon cancer but fail to predict KRAS mutation status. http://arxiv.org/abs/1812.04660. Revised January 15,2019. Accessed September 4, 2019.
19. Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointest Cancer Res. 2012;5(1):19-27.
20. Herzig DO, Tsikitis VL. Molecular markers for colon diagnosis, prognosis and targeted therapy. J Surg Oncol. 2015;111(1):96-102.
21. Ma W, Brodie S, Agersborg S, Funari VA, Albitar M. Significant improvement in detecting BRAF, KRAS, and EGFR mutations using next-generation sequencing as compared with FDA-cleared kits. Mol Diagn Ther. 2017;21(5):571-579.
22. Greco FA. Molecular diagnosis of the tissue of origin in cancer of unknown primary site: useful in patient management. Curr Treat Options Oncol. 2013;14(4):634-642.
23. Bejnordi BE, Veta M, van Diest PJ, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA. 2017;318(22):2199-2210.
24. Xiong Y, Ba X, Hou A, Zhang K, Chen L, Li T. Automatic detection of mycobacterium tuberculosis using artificial intelligence. J Thorac Dis. 2018;10(3):1936-1940.
25. Cruz-Roa A, Gilmore H, Basavanhally A, et al. Accurate and reproducible invasive breast cancer detection in whole-slide images: a deep learning approach for quantifying tumor extent. Sci Rep. 2017;7:46450.
26. Coudray N, Ocampo PS, Sakellaropoulos T, et al. Classification and mutation prediction from non–small cell lung cancer histopathology images using deep learning. Nat Med. 2018;24(10):1559-1567.
27. Ertosun MG, Rubin DL. Automated grading of gliomas using deep learning in digital pathology images: a modular approach with ensemble of convolutional neural networks. AMIA Annu Symp Proc. 2015;2015:1899-1908.
28. Wahab N, Khan A, Lee YS. Two-phase deep convolutional neural network for reducing class skewness in histopathological images based breast cancer detection. Comput Biol Med. 2017;85:86-97.
29. Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542(7639):115-118.
30. Han SS, Park GH, Lim W, et al. Deep neural networks show an equivalent and often superior performance to dermatologists in onychomycosis diagnosis: automatic construction of onychomycosis datasets by region-based convolutional deep neural network. PLoS One. 2018;13(1):e0191493.
31. Fujisawa Y, Otomo Y, Ogata Y, et al. Deep-learning-based, computer-aided classifier developed with a small dataset of clinical images surpasses board-certified dermatologists in skin tumour diagnosis. Br J Dermatol. 2019;180(2):373-381.
32. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402-2010.
33. Weng SF, Reps J, Kai J, Garibaldi JM, Qureshi N. Can machine-learning improve cardiovascular risk prediction using routine clinical data? PLoS One. 2017;12(4):e0174944.
34. Cheng J-Z, Ni D, Chou Y-H, et al. Computer-aided diagnosis with deep learning architecture: applications to breast lesions in US images and pulmonary nodules in CT scans. Sci Rep. 2016;6(1):24454.
35. Wang X, Yang W, Weinreb J, et al. Searching for prostate cancer by fully automated magnetic resonance imaging classification: deep learning versus non-deep learning. Sci Rep. 2017;7(1):15415.
36. Lakhani P, Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284(2):574-582.
37. Bardou D, Zhang K, Ahmad SM. Classification of breast cancer based on histology images using convolutional neural networks. IEEE Access. 2018;6(6):24680-24693.
38. Sheikhzadeh F, Ward RK, van Niekerk D, Guillaud M. Automatic labeling of molecular biomarkers of immunohistochemistry images using fully convolutional networks. PLoS One. 2018;13(1):e0190783.
39. Metter DM, Colgan TJ, Leung ST, Timmons CF, Park JY. Trends in the US and Canadian pathologist workforces from 2007 to 2017. JAMA Netw Open. 2019;2(5):e194337.
40. Benediktsson, H, Whitelaw J, Roy I. Pathology services in developing countries: a challenge. Arch Pathol Lab Med. 2007;131(11):1636-1639.
41. Graves D. The impact of the pathology workforce crisis on acute health care. Aust Health Rev. 2007;31(suppl 1):S28-S30.
42. NHS pathology shortages cause cancer diagnosis delays. https://www.gmjournal.co.uk/nhs-pathology-shortages-are-causing-cancer-diagnosis-delays. Published September 18, 2018. Accessed September 4, 2019.
43. Abbott LM, Smith SD. Smartphone apps for skin cancer diagnosis: Implications for patients and practitioners. Australas J Dermatol. 2018;59(3):168-170.
44. Poostchi M, Silamut K, Maude RJ, Jaeger S, Thoma G. Image analysis and machine learning for detecting malaria. Transl Res. 2018;194:36-55.
45. Orange DE, Agius P, DiCarlo EF, et al. Identification of three rheumatoid arthritis disease subtypes by machine learning integration of synovial histologic features and RNA sequencing data. Arthritis Rheumatol. 2018;70(5):690-701.
46. Rodellar J, Alférez S, Acevedo A, Molina A, Merino A. Image processing and machine learning in the morphological analysis of blood cells. Int J Lab Hematol. 2018;40(suppl 1):46-53.
47. Litjens G, Kooi T, Bejnordi BE, et al. A survey on deep learning in medical image analysis. Med Image Anal. 2017;42:60-88.
Artificial intelligence (AI), first described in 1956, encompasses the field of computer science in which machines are trained to learn from experience. The term was popularized by the 1956 Dartmouth College Summer Research Project on Artificial Intelligence.1 The field of AI is rapidly growing and has the potential to affect many aspects of our lives. The emerging importance of AI is demonstrated by a February 2019 executive order that launched the American AI Initiative, allocating resources and funding for AI development.2 The executive order stresses the potential impact of AI in the health care field, including its potential utility to diagnose disease. Federal agencies were directed to invest in AI research and development to promote rapid breakthroughs in AI technology that may impact multiple areas of society.
Machine learning (ML), a subset of AI, was defined in 1959 by Arthur Samuel and is achieved by employing mathematic models to compute sample data sets.3 Originating from statistical linear models, neural networks were conceived to accomplish these tasks.4 These pioneering scientific achievements led to recent developments of deep neural networks. These models are developed to recognize patterns and achieve complex computational tasks within a matter of minutes, often far exceeding human ability.5 ML can increase efficiency with decreased computation time, high precision, and recall when compared with that of human decision making.6
ML has the potential for numerous applications in the health care field.7-9 One promising application is in the field of anatomic pathology. ML allows representative images to be used to train a computer to recognize patterns from labeled photographs. Based on a set of images selected to represent a specific tissue or disease process, the computer can be trained to evaluate and recognize new and unique images from patients and render a diagnosis.10 Prior to modern ML models, users would have to import many thousands of training images to produce algorithms that could recognize patterns with high accuracy. Modern ML algorithms allow for a model known as transfer learning, such that far fewer images are required for training.11-13
Two novel ML platforms available for public use are offered through Google (Mountain View, CA) and Apple (Cupertino, CA).14,15 They each offer a user-friendly interface with minimal experience required in computer science. Google AutoML uses ML via cloud services to store and retrieve data with ease. No coding knowledge is required. The Apple Create ML Module provides computer-based ML, requiring only a few lines of code.
The Veterans Health Administration (VHA) is the largest single health care system in the US, and nearly 50 000 cancer cases are diagnosed at the VHA annually.16 Cancers of the lung and colon are among the most common sources of invasive cancer and are the 2 most common causes of cancer deaths in America.16 We have previously reported using Apple ML in detecting non-small cell lung cancers (NSCLCs), including adenocarcinomas and squamous cell carcinomas (SCCs); and colon cancers with accuracy.17,18 In the present study, we expand on these findings by comparing Apple and Google ML platforms in a variety of common pathologic scenarios in veteran patients. Using limited training data, both programs are compared for precision and recall in differentiating conditions involving lung and colon pathology.
In the first 4 experiments, we evaluated the ability of the platforms to differentiate normal lung tissue from cancerous lung tissue, to distinguish lung adenocarcinoma from SCC, and to differentiate colon adenocarcinoma from normal colon tissue. Next, cases of colon adenocarcinoma were assessed to determine whether the presence or absence of the KRAS proto-oncogene could be determined histologically using the AI platforms. KRAS is found in a variety of cancers, including about 40% of colon adenocarcinomas.19 For colon cancers, the presence or absence of the mutation in KRAS has important implications for patients as it determines whether the tumor will respond to specific chemotherapy agents.20 The presence of the KRAS gene is currently determined by complex molecular testing of tumor tissue.21 However, we assessed the potential of ML to determine whether the mutation is present by computerized morphologic analysis alone. Our last experiment examined the ability of the Apple and Google platforms to differentiate between adenocarcinomas of lung origin vs colon origin. This has potential utility in determining the site of origin of metastatic carcinoma.22
Methods
Fifty cases of lung SCC, 50 cases of lung adenocarcinoma, and 50 cases of colon adenocarcinoma were randomly retrieved from our molecular database. Twenty-five colon adenocarcinoma cases were positive for mutation in KRAS, while 25 cases were negative for mutation in KRAS. Seven hundred fifty total images of lung tissue (250 benign lung tissue, 250 lung adenocarcinomas, and 250 lung SCCs) and 500 total images of colon tissue (250 benign colon tissue and 250 colon adenocarcinoma) were obtained using a Leica Microscope MC190 HD Camera (Wetzlar, Germany) connected to an Olympus BX41 microscope (Center Valley, PA) and the Leica Acquire 9072 software for Apple computers. All the images were captured at a resolution of 1024 x 768 pixels using a 60x dry objective. Lung tissue images were captured and saved on a 2012 Apple MacBook Pro computer, and colon images were captured and saved on a 2011 Apple iMac computer. Both computers were running macOS v10.13.
Creating Image Classifier Models Using Apple Create ML
Apple Create ML is a suite of products that use various tools to create and train custom ML models on Apple computers.15 The suite contains many features, including image classification to train a ML model to classify images, natural language processing to classify natural language text, and tabular data to train models that deal with labeling information or estimating new quantities. We used Create ML Image Classification to create image classifier models for our project (Appendix A).
Creating ML Modules Using Google Cloud AutoML Vision Beta
Google Cloud AutoML is a suite of machine learning products, including AutoML Vision, AutoML Natural Language and AutoML Translation.14 All Cloud AutoML machine learning products were in beta version at the time of experimentation. We used Cloud AutoML Vision beta to create ML modules for our project. Unlike Apple Create ML, which is run on a local Apple computer, the Google Cloud AutoML is run online using a Google Cloud account. There are no minimum specifications requirements for the local computer since it is using the cloud-based architecture (Appendix B).
Experiment 1
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to detect and subclassify NSCLC based on the histopathologic images. We created 3 classes of images (250 images each): benign lung tissue, lung adenocarcinoma, and lung SCC.
Experiment 2
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to differentiate between normal lung tissue and NSCLC histopathologic images with 50/50 mixture of lung adenocarcinoma and lung SCC. We created 2 classes of images (250 images each): benign lung tissue and lung NSCLC.
Experiment 3
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to differentiate between lung adenocarcinoma and lung SCC histopathologic images. We created 2 classes of images (250 images each): adenocarcinoma and SCC.
Experiment 4
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to detect colon cancer histopathologic images regardless of mutation in KRAS status. We created 2 classes of images (250 images each): benign colon tissue and colon adenocarcinoma.
Experiment 5
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to differentiate between colon adenocarcinoma with mutations in KRAS and colon adenocarcinoma without the mutation in KRAS histopathologic images. We created 2 classes of images (125 images each): colon adenocarcinoma cases with mutation in KRAS and colon adenocarcinoma cases without the mutation in KRAS.
Experiment 6
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to differentiate between lung adenocarcinoma and colon adenocarcinoma histopathologic images. We created 2 classes of images (250 images each): colon adenocarcinoma lung adenocarcinoma.
Results
Twelve machine learning models were created in 6 experiments using the Apple Create ML and the Google AutoML (Table). To investigate recall and precision differences between the Apple and the Google ML algorithms, we performed 2-tailed distribution, paired t tests. No statistically significant differences were found (P = .52 for recall and .60 for precision).
Overall, each model performed well in distinguishing between normal and neoplastic tissue for both lung and colon cancers. In subclassifying NSCLC into adenocarcinoma and SCC, the models were shown to have high levels of precision and recall. The models also were successful in distinguishing between lung and colonic origin of adenocarcinoma (Figures 1-4). However, both systems had trouble discerning colon adenocarcinoma with mutations in KRAS from adenocarcinoma without mutations in KRAS.
Discussion
Image classifier models using ML algorithms hold a promising future to revolutionize the health care field. ML products, such as those modules offered by Apple and Google, are easy to use and have a simple graphic user interface to allow individuals to train models to perform humanlike tasks in real time. In our experiments, we compared multiple algorithms to determine their ability to differentiate and subclassify histopathologic images with high precision and recall using common scenarios in treating veteran patients.
Analysis of the results revealed high precision and recall values illustrating the models’ ability to differentiate and detect benign lung tissue from lung SCC and lung adenocarcinoma in ML model 1, benign lung from NSCLC carcinoma in ML model 2, and benign colon from colonic adenocarcinoma in ML model 4. In ML model 3 and 6, both ML algorithms performed at a high level to differentiate lung SCC from lung adenocarcinoma and lung adenocarcinoma from colonic adenocarcinoma, respectively. Of note, ML model 5 had the lowest precision and recall values across both algorithms demonstrating the models’ limited utility in predicting molecular profiles, such as mutations in KRAS as tested here. This is not surprising as pathologists currently require complex molecular tests to detect mutations in KRAS reliably in colon cancer.
Both modules require minimal programming experience and are easy to use. In our comparison, we demonstrated critical distinguishing characteristics that differentiate the 2 products.
Apple Create ML image classifier is available for use on local Mac computers that use Xcode version 10 and macOS 10.14 or later, with just 3 lines of code required to perform computations. Although this product is limited to Apple computers, it is free to use, and images are stored on the computer hard drive. Of unique significance on the Apple system platform, images can be augmented to alter their appearance to enhance model training. For example, imported images can be cropped, rotated, blurred, and flipped, in order to optimize the model’s training abilities to recognize test images and perform pattern recognition. This feature is not as readily available on the Google platform. Apple Create ML Image classifier’s default training set consists of 75% of total imported images with 5% of the total images being randomly used as a validation set. The remaining 20% of images comprise the testing set. The module’s computational analysis to train the model is achieved in about 2 minutes on average. The score threshold is set at 50% and cannot be manipulated for each image class as in Google AutoML Vision.
Google AutoML Vision is open and can be accessed from many devices. It stores images on remote Google servers but requires computing fees after a $300 credit for 12 months. On AutoML Vision, random 80% of the total images are used in the training set, 10% are used in the validation set, and 10% are used in the testing set. It is important to highlight the different percentages used in the default settings on the respective modules. The time to train the Google AutoML Vision with default computational power is longer on average than Apple Create ML, with about 8 minutes required to train the machine learning module. However, it is possible to choose more computational power for an additional fee and decrease module training time. The user will receive e-mail alerts when the computer time begins and is completed. The computation time is calculated by subtracting the time of the initial e-mail from the final e-mail.
Based on our calculations, we determined there was no significant difference between the 2 machine learning algorithms tested at the default settings with recall and precision values obtained. These findings demonstrate the promise of using a ML algorithm to assist in the performance of human tasks and behaviors, specifically the diagnosis of histopathologic images. These results have numerous potential uses in clinical medicine. ML algorithms have been successfully applied to diagnostic and prognostic endeavors in pathology,23-28 dermatology,29-31 ophthalmology,32 cardiology,33 and radiology.34-36
Pathologists often use additional tests, such as special staining of tissues or molecular tests, to assist with accurate classification of tumors. ML platforms offer the potential of an additional tool for pathologists to use along with human microscopic interpretation.37,38 In addition, the number of pathologists in the US is dramatically decreasing, and many other countries have marked physician shortages, especially in fields of specialized training such as pathology.39-42 These models could readily assist physicians in underserved countries and impact shortages of pathologists elsewhere by providing more specific diagnoses in an expedited manner.43
Finally, although we have explored the application of these platforms in common cancer scenarios, great potential exists to use similar techniques in the detection of other conditions. These include the potential for classification and risk assessment of precancerous lesions, infectious processes in tissue (eg, detection of tuberculosis or malaria),24,44 inflammatory conditions (eg, arthritis subtypes, gout),45 blood disorders (eg, abnormal blood cell morphology),46 and many others. The potential of these technologies to improve health care delivery to veteran patients seems to be limited only by the imagination of the user.47
Regarding the limited effectiveness in determining the presence or absence of mutations in KRAS for colon adenocarcinoma, it is mentioned that currently pathologists rely on complex molecular tests to detect the mutations at the DNA level.21 It is possible that the use of more extensive training data sets may improve recall and precision in cases such as these and warrants further study. Our experiments were limited to the stipulations placed by the free trial software agreements; no costs were expended to use the algorithms, though an Apple computer was required.
Conclusion
We have demonstrated the successful application of 2 readily available ML platforms in providing diagnostic guidance in differentiation between common cancer conditions in veteran patient populations. Although both platforms performed very well with no statistically significant differences in results, some distinctions are worth noting. Apple Create ML can be used on local computers but is limited to an Apple operating system. Google AutoML is not platform-specific but runs only via Google Cloud with associated computational fees. Using these readily available models, we demonstrated the vast potential of AI in diagnostic pathology. The application of AI to clinical medicine remains in the very early stages. The VA is uniquely poised to provide leadership as AI technologies will continue to dramatically change the future of health care, both in veteran and nonveteran patients nationwide.
Acknowledgments
The authors thank Paul Borkowski for his constructive criticism and proofreading of this manuscript. This material is the result of work supported with resources and the use of facilities at the James A. Haley Veterans’ Hospital.
Artificial intelligence (AI), first described in 1956, encompasses the field of computer science in which machines are trained to learn from experience. The term was popularized by the 1956 Dartmouth College Summer Research Project on Artificial Intelligence.1 The field of AI is rapidly growing and has the potential to affect many aspects of our lives. The emerging importance of AI is demonstrated by a February 2019 executive order that launched the American AI Initiative, allocating resources and funding for AI development.2 The executive order stresses the potential impact of AI in the health care field, including its potential utility to diagnose disease. Federal agencies were directed to invest in AI research and development to promote rapid breakthroughs in AI technology that may impact multiple areas of society.
Machine learning (ML), a subset of AI, was defined in 1959 by Arthur Samuel and is achieved by employing mathematic models to compute sample data sets.3 Originating from statistical linear models, neural networks were conceived to accomplish these tasks.4 These pioneering scientific achievements led to recent developments of deep neural networks. These models are developed to recognize patterns and achieve complex computational tasks within a matter of minutes, often far exceeding human ability.5 ML can increase efficiency with decreased computation time, high precision, and recall when compared with that of human decision making.6
ML has the potential for numerous applications in the health care field.7-9 One promising application is in the field of anatomic pathology. ML allows representative images to be used to train a computer to recognize patterns from labeled photographs. Based on a set of images selected to represent a specific tissue or disease process, the computer can be trained to evaluate and recognize new and unique images from patients and render a diagnosis.10 Prior to modern ML models, users would have to import many thousands of training images to produce algorithms that could recognize patterns with high accuracy. Modern ML algorithms allow for a model known as transfer learning, such that far fewer images are required for training.11-13
Two novel ML platforms available for public use are offered through Google (Mountain View, CA) and Apple (Cupertino, CA).14,15 They each offer a user-friendly interface with minimal experience required in computer science. Google AutoML uses ML via cloud services to store and retrieve data with ease. No coding knowledge is required. The Apple Create ML Module provides computer-based ML, requiring only a few lines of code.
The Veterans Health Administration (VHA) is the largest single health care system in the US, and nearly 50 000 cancer cases are diagnosed at the VHA annually.16 Cancers of the lung and colon are among the most common sources of invasive cancer and are the 2 most common causes of cancer deaths in America.16 We have previously reported using Apple ML in detecting non-small cell lung cancers (NSCLCs), including adenocarcinomas and squamous cell carcinomas (SCCs); and colon cancers with accuracy.17,18 In the present study, we expand on these findings by comparing Apple and Google ML platforms in a variety of common pathologic scenarios in veteran patients. Using limited training data, both programs are compared for precision and recall in differentiating conditions involving lung and colon pathology.
In the first 4 experiments, we evaluated the ability of the platforms to differentiate normal lung tissue from cancerous lung tissue, to distinguish lung adenocarcinoma from SCC, and to differentiate colon adenocarcinoma from normal colon tissue. Next, cases of colon adenocarcinoma were assessed to determine whether the presence or absence of the KRAS proto-oncogene could be determined histologically using the AI platforms. KRAS is found in a variety of cancers, including about 40% of colon adenocarcinomas.19 For colon cancers, the presence or absence of the mutation in KRAS has important implications for patients as it determines whether the tumor will respond to specific chemotherapy agents.20 The presence of the KRAS gene is currently determined by complex molecular testing of tumor tissue.21 However, we assessed the potential of ML to determine whether the mutation is present by computerized morphologic analysis alone. Our last experiment examined the ability of the Apple and Google platforms to differentiate between adenocarcinomas of lung origin vs colon origin. This has potential utility in determining the site of origin of metastatic carcinoma.22
Methods
Fifty cases of lung SCC, 50 cases of lung adenocarcinoma, and 50 cases of colon adenocarcinoma were randomly retrieved from our molecular database. Twenty-five colon adenocarcinoma cases were positive for mutation in KRAS, while 25 cases were negative for mutation in KRAS. Seven hundred fifty total images of lung tissue (250 benign lung tissue, 250 lung adenocarcinomas, and 250 lung SCCs) and 500 total images of colon tissue (250 benign colon tissue and 250 colon adenocarcinoma) were obtained using a Leica Microscope MC190 HD Camera (Wetzlar, Germany) connected to an Olympus BX41 microscope (Center Valley, PA) and the Leica Acquire 9072 software for Apple computers. All the images were captured at a resolution of 1024 x 768 pixels using a 60x dry objective. Lung tissue images were captured and saved on a 2012 Apple MacBook Pro computer, and colon images were captured and saved on a 2011 Apple iMac computer. Both computers were running macOS v10.13.
Creating Image Classifier Models Using Apple Create ML
Apple Create ML is a suite of products that use various tools to create and train custom ML models on Apple computers.15 The suite contains many features, including image classification to train a ML model to classify images, natural language processing to classify natural language text, and tabular data to train models that deal with labeling information or estimating new quantities. We used Create ML Image Classification to create image classifier models for our project (Appendix A).
Creating ML Modules Using Google Cloud AutoML Vision Beta
Google Cloud AutoML is a suite of machine learning products, including AutoML Vision, AutoML Natural Language and AutoML Translation.14 All Cloud AutoML machine learning products were in beta version at the time of experimentation. We used Cloud AutoML Vision beta to create ML modules for our project. Unlike Apple Create ML, which is run on a local Apple computer, the Google Cloud AutoML is run online using a Google Cloud account. There are no minimum specifications requirements for the local computer since it is using the cloud-based architecture (Appendix B).
Experiment 1
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to detect and subclassify NSCLC based on the histopathologic images. We created 3 classes of images (250 images each): benign lung tissue, lung adenocarcinoma, and lung SCC.
Experiment 2
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to differentiate between normal lung tissue and NSCLC histopathologic images with 50/50 mixture of lung adenocarcinoma and lung SCC. We created 2 classes of images (250 images each): benign lung tissue and lung NSCLC.
Experiment 3
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to differentiate between lung adenocarcinoma and lung SCC histopathologic images. We created 2 classes of images (250 images each): adenocarcinoma and SCC.
Experiment 4
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to detect colon cancer histopathologic images regardless of mutation in KRAS status. We created 2 classes of images (250 images each): benign colon tissue and colon adenocarcinoma.
Experiment 5
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to differentiate between colon adenocarcinoma with mutations in KRAS and colon adenocarcinoma without the mutation in KRAS histopathologic images. We created 2 classes of images (125 images each): colon adenocarcinoma cases with mutation in KRAS and colon adenocarcinoma cases without the mutation in KRAS.
Experiment 6
We compared Apple Create ML Image Classifier and Google AutoML Vision in their ability to differentiate between lung adenocarcinoma and colon adenocarcinoma histopathologic images. We created 2 classes of images (250 images each): colon adenocarcinoma lung adenocarcinoma.
Results
Twelve machine learning models were created in 6 experiments using the Apple Create ML and the Google AutoML (Table). To investigate recall and precision differences between the Apple and the Google ML algorithms, we performed 2-tailed distribution, paired t tests. No statistically significant differences were found (P = .52 for recall and .60 for precision).
Overall, each model performed well in distinguishing between normal and neoplastic tissue for both lung and colon cancers. In subclassifying NSCLC into adenocarcinoma and SCC, the models were shown to have high levels of precision and recall. The models also were successful in distinguishing between lung and colonic origin of adenocarcinoma (Figures 1-4). However, both systems had trouble discerning colon adenocarcinoma with mutations in KRAS from adenocarcinoma without mutations in KRAS.
Discussion
Image classifier models using ML algorithms hold a promising future to revolutionize the health care field. ML products, such as those modules offered by Apple and Google, are easy to use and have a simple graphic user interface to allow individuals to train models to perform humanlike tasks in real time. In our experiments, we compared multiple algorithms to determine their ability to differentiate and subclassify histopathologic images with high precision and recall using common scenarios in treating veteran patients.
Analysis of the results revealed high precision and recall values illustrating the models’ ability to differentiate and detect benign lung tissue from lung SCC and lung adenocarcinoma in ML model 1, benign lung from NSCLC carcinoma in ML model 2, and benign colon from colonic adenocarcinoma in ML model 4. In ML model 3 and 6, both ML algorithms performed at a high level to differentiate lung SCC from lung adenocarcinoma and lung adenocarcinoma from colonic adenocarcinoma, respectively. Of note, ML model 5 had the lowest precision and recall values across both algorithms demonstrating the models’ limited utility in predicting molecular profiles, such as mutations in KRAS as tested here. This is not surprising as pathologists currently require complex molecular tests to detect mutations in KRAS reliably in colon cancer.
Both modules require minimal programming experience and are easy to use. In our comparison, we demonstrated critical distinguishing characteristics that differentiate the 2 products.
Apple Create ML image classifier is available for use on local Mac computers that use Xcode version 10 and macOS 10.14 or later, with just 3 lines of code required to perform computations. Although this product is limited to Apple computers, it is free to use, and images are stored on the computer hard drive. Of unique significance on the Apple system platform, images can be augmented to alter their appearance to enhance model training. For example, imported images can be cropped, rotated, blurred, and flipped, in order to optimize the model’s training abilities to recognize test images and perform pattern recognition. This feature is not as readily available on the Google platform. Apple Create ML Image classifier’s default training set consists of 75% of total imported images with 5% of the total images being randomly used as a validation set. The remaining 20% of images comprise the testing set. The module’s computational analysis to train the model is achieved in about 2 minutes on average. The score threshold is set at 50% and cannot be manipulated for each image class as in Google AutoML Vision.
Google AutoML Vision is open and can be accessed from many devices. It stores images on remote Google servers but requires computing fees after a $300 credit for 12 months. On AutoML Vision, random 80% of the total images are used in the training set, 10% are used in the validation set, and 10% are used in the testing set. It is important to highlight the different percentages used in the default settings on the respective modules. The time to train the Google AutoML Vision with default computational power is longer on average than Apple Create ML, with about 8 minutes required to train the machine learning module. However, it is possible to choose more computational power for an additional fee and decrease module training time. The user will receive e-mail alerts when the computer time begins and is completed. The computation time is calculated by subtracting the time of the initial e-mail from the final e-mail.
Based on our calculations, we determined there was no significant difference between the 2 machine learning algorithms tested at the default settings with recall and precision values obtained. These findings demonstrate the promise of using a ML algorithm to assist in the performance of human tasks and behaviors, specifically the diagnosis of histopathologic images. These results have numerous potential uses in clinical medicine. ML algorithms have been successfully applied to diagnostic and prognostic endeavors in pathology,23-28 dermatology,29-31 ophthalmology,32 cardiology,33 and radiology.34-36
Pathologists often use additional tests, such as special staining of tissues or molecular tests, to assist with accurate classification of tumors. ML platforms offer the potential of an additional tool for pathologists to use along with human microscopic interpretation.37,38 In addition, the number of pathologists in the US is dramatically decreasing, and many other countries have marked physician shortages, especially in fields of specialized training such as pathology.39-42 These models could readily assist physicians in underserved countries and impact shortages of pathologists elsewhere by providing more specific diagnoses in an expedited manner.43
Finally, although we have explored the application of these platforms in common cancer scenarios, great potential exists to use similar techniques in the detection of other conditions. These include the potential for classification and risk assessment of precancerous lesions, infectious processes in tissue (eg, detection of tuberculosis or malaria),24,44 inflammatory conditions (eg, arthritis subtypes, gout),45 blood disorders (eg, abnormal blood cell morphology),46 and many others. The potential of these technologies to improve health care delivery to veteran patients seems to be limited only by the imagination of the user.47
Regarding the limited effectiveness in determining the presence or absence of mutations in KRAS for colon adenocarcinoma, it is mentioned that currently pathologists rely on complex molecular tests to detect the mutations at the DNA level.21 It is possible that the use of more extensive training data sets may improve recall and precision in cases such as these and warrants further study. Our experiments were limited to the stipulations placed by the free trial software agreements; no costs were expended to use the algorithms, though an Apple computer was required.
Conclusion
We have demonstrated the successful application of 2 readily available ML platforms in providing diagnostic guidance in differentiation between common cancer conditions in veteran patient populations. Although both platforms performed very well with no statistically significant differences in results, some distinctions are worth noting. Apple Create ML can be used on local computers but is limited to an Apple operating system. Google AutoML is not platform-specific but runs only via Google Cloud with associated computational fees. Using these readily available models, we demonstrated the vast potential of AI in diagnostic pathology. The application of AI to clinical medicine remains in the very early stages. The VA is uniquely poised to provide leadership as AI technologies will continue to dramatically change the future of health care, both in veteran and nonveteran patients nationwide.
Acknowledgments
The authors thank Paul Borkowski for his constructive criticism and proofreading of this manuscript. This material is the result of work supported with resources and the use of facilities at the James A. Haley Veterans’ Hospital.
1. Moor J. The Dartmouth College artificial intelligence conference: the next fifty years. AI Mag. 2006;27(4):87-91.
2. Trump D. Accelerating America’s leadership in artificial intelligence. https://www.whitehouse.gov/articles/accelerating-americas-leadership-in-artificial-intelligence. Published February 11, 2019. Accessed September 4, 2019.
3. Samuel AL. Some studies in machine learning using the game of checkers. IBM J Res Dev. 1959;3(3):210-229.
4. SAS Users Group International. Neural networks and statistical models. In: Sarle WS. Proceedings of the Nineteenth Annual SAS Users Group International Conference. SAS Institute: Cary, North Carolina; 1994:1538-1550. http://www.sascommunity.org/sugi/SUGI94/Sugi-94-255%20Sarle.pdf. Accessed September 16, 2019.
5. Schmidhuber J. Deep learning in neural networks: an overview. Neural Networks. 2015;61:85-117.
6. LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436-444.
7. Jiang F, Jiang Y, Li H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230-243.
8. Erickson BJ, Korfiatis P, Akkus Z, Kline TL. Machine learning for medical imaging. Radiographics. 2017;37(2):505-515.
9. Deo RC. Machine learning in medicine. Circulation. 2015;132(20):1920-1930.
10. Janowczyk A, Madabhushi A. Deep learning for digital pathology image analysis: a comprehensive tutorial with selected use cases. J Pathol Inform. 2016;7(1):29.
11. Oquab M, Bottou L, Laptev I, Sivic J. Learning and transferring mid-level image representations using convolutional neural networks. Presented at: IEEE Conference on Computer Vision and Pattern Recognition, 2014. http://openaccess.thecvf.com/content_cvpr_2014/html/Oquab_Learning_and_Transferring_2014_CVPR_paper.html. Accessed September 4, 2019.
12. Shin HC, Roth HR, Gao M, et al. Deep convolutional neural networks for computer-aided detection: CNN architectures, dataset characteristics and transfer learning. IEEE Trans Med Imaging. 2016;35(5):1285-1298.
13. Tajbakhsh N, Shin JY, Gurudu SR, et al. Convolutional neural networks for medical image analysis: full training or fine tuning? IEEE Trans Med Imaging. 2016;35(5):1299-1312.
14. Cloud AutoML. https://cloud.google.com/automl. Accessed September 4, 2019.
15. Create ML. https://developer.apple.com/documentation/createml. Accessed September 4, 2019.
16. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 Update. Mil Med. 2017;182(7):e1883-e1891. 17. Borkowski AA, Wilson CP, Borkowski SA, Deland LA, Mastorides SM. Using Apple machine learning algorithms to detect and subclassify non-small cell lung cancer. https://arxiv.org/ftp/arxiv/papers/1808/1808.08230.pdf. Accessed September 4, 2019.
18. Borkowski AA, Wilson CP, Borkowski SA, Thomas LB, Deland LA, Mastorides SM. Apple machine learning algorithms successfully detect colon cancer but fail to predict KRAS mutation status. http://arxiv.org/abs/1812.04660. Revised January 15,2019. Accessed September 4, 2019.
19. Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointest Cancer Res. 2012;5(1):19-27.
20. Herzig DO, Tsikitis VL. Molecular markers for colon diagnosis, prognosis and targeted therapy. J Surg Oncol. 2015;111(1):96-102.
21. Ma W, Brodie S, Agersborg S, Funari VA, Albitar M. Significant improvement in detecting BRAF, KRAS, and EGFR mutations using next-generation sequencing as compared with FDA-cleared kits. Mol Diagn Ther. 2017;21(5):571-579.
22. Greco FA. Molecular diagnosis of the tissue of origin in cancer of unknown primary site: useful in patient management. Curr Treat Options Oncol. 2013;14(4):634-642.
23. Bejnordi BE, Veta M, van Diest PJ, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA. 2017;318(22):2199-2210.
24. Xiong Y, Ba X, Hou A, Zhang K, Chen L, Li T. Automatic detection of mycobacterium tuberculosis using artificial intelligence. J Thorac Dis. 2018;10(3):1936-1940.
25. Cruz-Roa A, Gilmore H, Basavanhally A, et al. Accurate and reproducible invasive breast cancer detection in whole-slide images: a deep learning approach for quantifying tumor extent. Sci Rep. 2017;7:46450.
26. Coudray N, Ocampo PS, Sakellaropoulos T, et al. Classification and mutation prediction from non–small cell lung cancer histopathology images using deep learning. Nat Med. 2018;24(10):1559-1567.
27. Ertosun MG, Rubin DL. Automated grading of gliomas using deep learning in digital pathology images: a modular approach with ensemble of convolutional neural networks. AMIA Annu Symp Proc. 2015;2015:1899-1908.
28. Wahab N, Khan A, Lee YS. Two-phase deep convolutional neural network for reducing class skewness in histopathological images based breast cancer detection. Comput Biol Med. 2017;85:86-97.
29. Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542(7639):115-118.
30. Han SS, Park GH, Lim W, et al. Deep neural networks show an equivalent and often superior performance to dermatologists in onychomycosis diagnosis: automatic construction of onychomycosis datasets by region-based convolutional deep neural network. PLoS One. 2018;13(1):e0191493.
31. Fujisawa Y, Otomo Y, Ogata Y, et al. Deep-learning-based, computer-aided classifier developed with a small dataset of clinical images surpasses board-certified dermatologists in skin tumour diagnosis. Br J Dermatol. 2019;180(2):373-381.
32. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402-2010.
33. Weng SF, Reps J, Kai J, Garibaldi JM, Qureshi N. Can machine-learning improve cardiovascular risk prediction using routine clinical data? PLoS One. 2017;12(4):e0174944.
34. Cheng J-Z, Ni D, Chou Y-H, et al. Computer-aided diagnosis with deep learning architecture: applications to breast lesions in US images and pulmonary nodules in CT scans. Sci Rep. 2016;6(1):24454.
35. Wang X, Yang W, Weinreb J, et al. Searching for prostate cancer by fully automated magnetic resonance imaging classification: deep learning versus non-deep learning. Sci Rep. 2017;7(1):15415.
36. Lakhani P, Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284(2):574-582.
37. Bardou D, Zhang K, Ahmad SM. Classification of breast cancer based on histology images using convolutional neural networks. IEEE Access. 2018;6(6):24680-24693.
38. Sheikhzadeh F, Ward RK, van Niekerk D, Guillaud M. Automatic labeling of molecular biomarkers of immunohistochemistry images using fully convolutional networks. PLoS One. 2018;13(1):e0190783.
39. Metter DM, Colgan TJ, Leung ST, Timmons CF, Park JY. Trends in the US and Canadian pathologist workforces from 2007 to 2017. JAMA Netw Open. 2019;2(5):e194337.
40. Benediktsson, H, Whitelaw J, Roy I. Pathology services in developing countries: a challenge. Arch Pathol Lab Med. 2007;131(11):1636-1639.
41. Graves D. The impact of the pathology workforce crisis on acute health care. Aust Health Rev. 2007;31(suppl 1):S28-S30.
42. NHS pathology shortages cause cancer diagnosis delays. https://www.gmjournal.co.uk/nhs-pathology-shortages-are-causing-cancer-diagnosis-delays. Published September 18, 2018. Accessed September 4, 2019.
43. Abbott LM, Smith SD. Smartphone apps for skin cancer diagnosis: Implications for patients and practitioners. Australas J Dermatol. 2018;59(3):168-170.
44. Poostchi M, Silamut K, Maude RJ, Jaeger S, Thoma G. Image analysis and machine learning for detecting malaria. Transl Res. 2018;194:36-55.
45. Orange DE, Agius P, DiCarlo EF, et al. Identification of three rheumatoid arthritis disease subtypes by machine learning integration of synovial histologic features and RNA sequencing data. Arthritis Rheumatol. 2018;70(5):690-701.
46. Rodellar J, Alférez S, Acevedo A, Molina A, Merino A. Image processing and machine learning in the morphological analysis of blood cells. Int J Lab Hematol. 2018;40(suppl 1):46-53.
47. Litjens G, Kooi T, Bejnordi BE, et al. A survey on deep learning in medical image analysis. Med Image Anal. 2017;42:60-88.
1. Moor J. The Dartmouth College artificial intelligence conference: the next fifty years. AI Mag. 2006;27(4):87-91.
2. Trump D. Accelerating America’s leadership in artificial intelligence. https://www.whitehouse.gov/articles/accelerating-americas-leadership-in-artificial-intelligence. Published February 11, 2019. Accessed September 4, 2019.
3. Samuel AL. Some studies in machine learning using the game of checkers. IBM J Res Dev. 1959;3(3):210-229.
4. SAS Users Group International. Neural networks and statistical models. In: Sarle WS. Proceedings of the Nineteenth Annual SAS Users Group International Conference. SAS Institute: Cary, North Carolina; 1994:1538-1550. http://www.sascommunity.org/sugi/SUGI94/Sugi-94-255%20Sarle.pdf. Accessed September 16, 2019.
5. Schmidhuber J. Deep learning in neural networks: an overview. Neural Networks. 2015;61:85-117.
6. LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436-444.
7. Jiang F, Jiang Y, Li H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230-243.
8. Erickson BJ, Korfiatis P, Akkus Z, Kline TL. Machine learning for medical imaging. Radiographics. 2017;37(2):505-515.
9. Deo RC. Machine learning in medicine. Circulation. 2015;132(20):1920-1930.
10. Janowczyk A, Madabhushi A. Deep learning for digital pathology image analysis: a comprehensive tutorial with selected use cases. J Pathol Inform. 2016;7(1):29.
11. Oquab M, Bottou L, Laptev I, Sivic J. Learning and transferring mid-level image representations using convolutional neural networks. Presented at: IEEE Conference on Computer Vision and Pattern Recognition, 2014. http://openaccess.thecvf.com/content_cvpr_2014/html/Oquab_Learning_and_Transferring_2014_CVPR_paper.html. Accessed September 4, 2019.
12. Shin HC, Roth HR, Gao M, et al. Deep convolutional neural networks for computer-aided detection: CNN architectures, dataset characteristics and transfer learning. IEEE Trans Med Imaging. 2016;35(5):1285-1298.
13. Tajbakhsh N, Shin JY, Gurudu SR, et al. Convolutional neural networks for medical image analysis: full training or fine tuning? IEEE Trans Med Imaging. 2016;35(5):1299-1312.
14. Cloud AutoML. https://cloud.google.com/automl. Accessed September 4, 2019.
15. Create ML. https://developer.apple.com/documentation/createml. Accessed September 4, 2019.
16. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 Update. Mil Med. 2017;182(7):e1883-e1891. 17. Borkowski AA, Wilson CP, Borkowski SA, Deland LA, Mastorides SM. Using Apple machine learning algorithms to detect and subclassify non-small cell lung cancer. https://arxiv.org/ftp/arxiv/papers/1808/1808.08230.pdf. Accessed September 4, 2019.
18. Borkowski AA, Wilson CP, Borkowski SA, Thomas LB, Deland LA, Mastorides SM. Apple machine learning algorithms successfully detect colon cancer but fail to predict KRAS mutation status. http://arxiv.org/abs/1812.04660. Revised January 15,2019. Accessed September 4, 2019.
19. Armaghany T, Wilson JD, Chu Q, Mills G. Genetic alterations in colorectal cancer. Gastrointest Cancer Res. 2012;5(1):19-27.
20. Herzig DO, Tsikitis VL. Molecular markers for colon diagnosis, prognosis and targeted therapy. J Surg Oncol. 2015;111(1):96-102.
21. Ma W, Brodie S, Agersborg S, Funari VA, Albitar M. Significant improvement in detecting BRAF, KRAS, and EGFR mutations using next-generation sequencing as compared with FDA-cleared kits. Mol Diagn Ther. 2017;21(5):571-579.
22. Greco FA. Molecular diagnosis of the tissue of origin in cancer of unknown primary site: useful in patient management. Curr Treat Options Oncol. 2013;14(4):634-642.
23. Bejnordi BE, Veta M, van Diest PJ, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA. 2017;318(22):2199-2210.
24. Xiong Y, Ba X, Hou A, Zhang K, Chen L, Li T. Automatic detection of mycobacterium tuberculosis using artificial intelligence. J Thorac Dis. 2018;10(3):1936-1940.
25. Cruz-Roa A, Gilmore H, Basavanhally A, et al. Accurate and reproducible invasive breast cancer detection in whole-slide images: a deep learning approach for quantifying tumor extent. Sci Rep. 2017;7:46450.
26. Coudray N, Ocampo PS, Sakellaropoulos T, et al. Classification and mutation prediction from non–small cell lung cancer histopathology images using deep learning. Nat Med. 2018;24(10):1559-1567.
27. Ertosun MG, Rubin DL. Automated grading of gliomas using deep learning in digital pathology images: a modular approach with ensemble of convolutional neural networks. AMIA Annu Symp Proc. 2015;2015:1899-1908.
28. Wahab N, Khan A, Lee YS. Two-phase deep convolutional neural network for reducing class skewness in histopathological images based breast cancer detection. Comput Biol Med. 2017;85:86-97.
29. Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542(7639):115-118.
30. Han SS, Park GH, Lim W, et al. Deep neural networks show an equivalent and often superior performance to dermatologists in onychomycosis diagnosis: automatic construction of onychomycosis datasets by region-based convolutional deep neural network. PLoS One. 2018;13(1):e0191493.
31. Fujisawa Y, Otomo Y, Ogata Y, et al. Deep-learning-based, computer-aided classifier developed with a small dataset of clinical images surpasses board-certified dermatologists in skin tumour diagnosis. Br J Dermatol. 2019;180(2):373-381.
32. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. 2016;316(22):2402-2010.
33. Weng SF, Reps J, Kai J, Garibaldi JM, Qureshi N. Can machine-learning improve cardiovascular risk prediction using routine clinical data? PLoS One. 2017;12(4):e0174944.
34. Cheng J-Z, Ni D, Chou Y-H, et al. Computer-aided diagnosis with deep learning architecture: applications to breast lesions in US images and pulmonary nodules in CT scans. Sci Rep. 2016;6(1):24454.
35. Wang X, Yang W, Weinreb J, et al. Searching for prostate cancer by fully automated magnetic resonance imaging classification: deep learning versus non-deep learning. Sci Rep. 2017;7(1):15415.
36. Lakhani P, Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284(2):574-582.
37. Bardou D, Zhang K, Ahmad SM. Classification of breast cancer based on histology images using convolutional neural networks. IEEE Access. 2018;6(6):24680-24693.
38. Sheikhzadeh F, Ward RK, van Niekerk D, Guillaud M. Automatic labeling of molecular biomarkers of immunohistochemistry images using fully convolutional networks. PLoS One. 2018;13(1):e0190783.
39. Metter DM, Colgan TJ, Leung ST, Timmons CF, Park JY. Trends in the US and Canadian pathologist workforces from 2007 to 2017. JAMA Netw Open. 2019;2(5):e194337.
40. Benediktsson, H, Whitelaw J, Roy I. Pathology services in developing countries: a challenge. Arch Pathol Lab Med. 2007;131(11):1636-1639.
41. Graves D. The impact of the pathology workforce crisis on acute health care. Aust Health Rev. 2007;31(suppl 1):S28-S30.
42. NHS pathology shortages cause cancer diagnosis delays. https://www.gmjournal.co.uk/nhs-pathology-shortages-are-causing-cancer-diagnosis-delays. Published September 18, 2018. Accessed September 4, 2019.
43. Abbott LM, Smith SD. Smartphone apps for skin cancer diagnosis: Implications for patients and practitioners. Australas J Dermatol. 2018;59(3):168-170.
44. Poostchi M, Silamut K, Maude RJ, Jaeger S, Thoma G. Image analysis and machine learning for detecting malaria. Transl Res. 2018;194:36-55.
45. Orange DE, Agius P, DiCarlo EF, et al. Identification of three rheumatoid arthritis disease subtypes by machine learning integration of synovial histologic features and RNA sequencing data. Arthritis Rheumatol. 2018;70(5):690-701.
46. Rodellar J, Alférez S, Acevedo A, Molina A, Merino A. Image processing and machine learning in the morphological analysis of blood cells. Int J Lab Hematol. 2018;40(suppl 1):46-53.
47. Litjens G, Kooi T, Bejnordi BE, et al. A survey on deep learning in medical image analysis. Med Image Anal. 2017;42:60-88.
Six factors predicted benefit from asthma triple therapy
MADRID – Two newly published but previously reported phase 3 trials associated triple therapy in a single inhaler with a 23% reduction (P = .008) in asthma exacerbations relative to a two-drug inhaler, but fresh data from a prespecified analysis presented at the annual congress of the European Respiratory Society has identified those patients most likely to benefit.
“Six easily identifiable factors appear to be associated with the most prominent response to treatment and may help in the treatment step-up decision at the point of care,” reported Dave Singh, MD, professor in the division of infection, immunity, and respiratory medicine, University of Manchester (England).
The primary results of these trials were presented several months ago at the 2019 American Thoracic Society (ATS) meeting, but the full data were published on the day that Dr. Singh spoke at the ERS.
To identify predictors of response, the pooled analysis of TRIMARIN and TRIGGER was prespecified. Both of these trials, which were similarly designed, compared a single inhaler of inhaled corticosteroids (ICS), long-acting beta agonist (LABA), and long-acting muscarinic antagonist (LAMA) to a single ICS/LABA inhaler.
On the basis of risk for severe exacerbations, greater protection from triple therapy relative to a conventional ICS/LABA inhaler was identified for those with a high degree of reversibility (defined as greater than 400 mL) relative to those with a lower degree (RR, 0.729; P = .024), those with a body mass index less than 25 kg/m2 relative to a higher BMI (RR, 0.570; P = .005), those with only one exacerbation in the previous 12 months relative to those with more (RR, 0.731; P = .009), never-smokers relative to those with smoking history (RR, 0.764; P = .013), those younger than age 65 years relative to older (RR, 0.770; P = .17), and males relative to females (RR, 0.651; P = .009).
“This gives us six factors to consider when you are thinking about stepping up to triple therapy and are trying to determine which patients would benefit the most,” Dr. Singh said.
Both the TRIMARIN and the TRIGGER trials were double blind and placebo controlled. In both, the experimental arm was a single inhaler triple therapy of the ICS beclomethasone, the LABA formoterol, and the LAMA glycopyrronium. The control arm was a single inhaler combination of beclomethasone and formoterol. All inhalers were used twice daily.
TRIMARIN, with 171 participating sites in 16 countries, randomized 1,155 patients to the triple-drug inhaler with a moderate dose of ICS (100 mcg) or to the ICS/LABA inhaler. In TRIGGER, with 221 sites in 17 countries, 1,437 patients were randomized to one of three arms. Both the triple-drug inhaler arm and the ICS/LABA arm contained a higher dose of ICS (200 mcg) than in TRIMARIN. In an open-label third arm, patients also received the higher dose of ICS plus LABA and a second inhaler with tiotropium. The formoterol dose in all arms of both studies was 6 mcg.
As reported at the ATS and now published in the Lancet, the reduction in exacerbations on single inhaler triple therapy relative to ICS/LABA was significant when the data were pooled (even though the reduction in the TRIGGER study fell short of statistical significance). The median improvement in lung function for single inhaler triple therapy relative to ICS/LABA was significant in both TRIMARIN (57 mL; P = .008) and TRIGGER (73 mL; P = .0025).
In discussing the new pooled analysis of response predictors in TRIMARIN/TRIGGER, the ERS-invited discussant, Celeste M. Porsbjerg, MD, Bispebjerg Hospital, Copenhagen, expressed particular interest in reversibility. A positive reversibility test to salbutamol was an entry criterion for both trials, but Dr. Porsbjerg pointed out that a greater response in those with the highest reversibility suggests these patients have a phenotype in which bronchodilation is a more important driver of disease than is inflammation.
While conceding that this was possible, Dr. Singh cautioned that he considers these predictors of response to be “exploratory.” He believes that the TRIMARIN/TRIGGER studies were not designed to tease out the relative importance of mechanisms of asthma in response to the assigned therapies. However, he believes the response predictor analysis is a step in this direction, which might be valuable for better individualizing therapy.
The studies were funded by Chiesi Farmaceutici. Dr. Singh reports no potential conflicts of interest.
Virchow JC et al. Lancet. 2019 Sep 30. doi. org/10.1016/S0140-6736(19)32215-9.
MADRID – Two newly published but previously reported phase 3 trials associated triple therapy in a single inhaler with a 23% reduction (P = .008) in asthma exacerbations relative to a two-drug inhaler, but fresh data from a prespecified analysis presented at the annual congress of the European Respiratory Society has identified those patients most likely to benefit.
“Six easily identifiable factors appear to be associated with the most prominent response to treatment and may help in the treatment step-up decision at the point of care,” reported Dave Singh, MD, professor in the division of infection, immunity, and respiratory medicine, University of Manchester (England).
The primary results of these trials were presented several months ago at the 2019 American Thoracic Society (ATS) meeting, but the full data were published on the day that Dr. Singh spoke at the ERS.
To identify predictors of response, the pooled analysis of TRIMARIN and TRIGGER was prespecified. Both of these trials, which were similarly designed, compared a single inhaler of inhaled corticosteroids (ICS), long-acting beta agonist (LABA), and long-acting muscarinic antagonist (LAMA) to a single ICS/LABA inhaler.
On the basis of risk for severe exacerbations, greater protection from triple therapy relative to a conventional ICS/LABA inhaler was identified for those with a high degree of reversibility (defined as greater than 400 mL) relative to those with a lower degree (RR, 0.729; P = .024), those with a body mass index less than 25 kg/m2 relative to a higher BMI (RR, 0.570; P = .005), those with only one exacerbation in the previous 12 months relative to those with more (RR, 0.731; P = .009), never-smokers relative to those with smoking history (RR, 0.764; P = .013), those younger than age 65 years relative to older (RR, 0.770; P = .17), and males relative to females (RR, 0.651; P = .009).
“This gives us six factors to consider when you are thinking about stepping up to triple therapy and are trying to determine which patients would benefit the most,” Dr. Singh said.
Both the TRIMARIN and the TRIGGER trials were double blind and placebo controlled. In both, the experimental arm was a single inhaler triple therapy of the ICS beclomethasone, the LABA formoterol, and the LAMA glycopyrronium. The control arm was a single inhaler combination of beclomethasone and formoterol. All inhalers were used twice daily.
TRIMARIN, with 171 participating sites in 16 countries, randomized 1,155 patients to the triple-drug inhaler with a moderate dose of ICS (100 mcg) or to the ICS/LABA inhaler. In TRIGGER, with 221 sites in 17 countries, 1,437 patients were randomized to one of three arms. Both the triple-drug inhaler arm and the ICS/LABA arm contained a higher dose of ICS (200 mcg) than in TRIMARIN. In an open-label third arm, patients also received the higher dose of ICS plus LABA and a second inhaler with tiotropium. The formoterol dose in all arms of both studies was 6 mcg.
As reported at the ATS and now published in the Lancet, the reduction in exacerbations on single inhaler triple therapy relative to ICS/LABA was significant when the data were pooled (even though the reduction in the TRIGGER study fell short of statistical significance). The median improvement in lung function for single inhaler triple therapy relative to ICS/LABA was significant in both TRIMARIN (57 mL; P = .008) and TRIGGER (73 mL; P = .0025).
In discussing the new pooled analysis of response predictors in TRIMARIN/TRIGGER, the ERS-invited discussant, Celeste M. Porsbjerg, MD, Bispebjerg Hospital, Copenhagen, expressed particular interest in reversibility. A positive reversibility test to salbutamol was an entry criterion for both trials, but Dr. Porsbjerg pointed out that a greater response in those with the highest reversibility suggests these patients have a phenotype in which bronchodilation is a more important driver of disease than is inflammation.
While conceding that this was possible, Dr. Singh cautioned that he considers these predictors of response to be “exploratory.” He believes that the TRIMARIN/TRIGGER studies were not designed to tease out the relative importance of mechanisms of asthma in response to the assigned therapies. However, he believes the response predictor analysis is a step in this direction, which might be valuable for better individualizing therapy.
The studies were funded by Chiesi Farmaceutici. Dr. Singh reports no potential conflicts of interest.
Virchow JC et al. Lancet. 2019 Sep 30. doi. org/10.1016/S0140-6736(19)32215-9.
MADRID – Two newly published but previously reported phase 3 trials associated triple therapy in a single inhaler with a 23% reduction (P = .008) in asthma exacerbations relative to a two-drug inhaler, but fresh data from a prespecified analysis presented at the annual congress of the European Respiratory Society has identified those patients most likely to benefit.
“Six easily identifiable factors appear to be associated with the most prominent response to treatment and may help in the treatment step-up decision at the point of care,” reported Dave Singh, MD, professor in the division of infection, immunity, and respiratory medicine, University of Manchester (England).
The primary results of these trials were presented several months ago at the 2019 American Thoracic Society (ATS) meeting, but the full data were published on the day that Dr. Singh spoke at the ERS.
To identify predictors of response, the pooled analysis of TRIMARIN and TRIGGER was prespecified. Both of these trials, which were similarly designed, compared a single inhaler of inhaled corticosteroids (ICS), long-acting beta agonist (LABA), and long-acting muscarinic antagonist (LAMA) to a single ICS/LABA inhaler.
On the basis of risk for severe exacerbations, greater protection from triple therapy relative to a conventional ICS/LABA inhaler was identified for those with a high degree of reversibility (defined as greater than 400 mL) relative to those with a lower degree (RR, 0.729; P = .024), those with a body mass index less than 25 kg/m2 relative to a higher BMI (RR, 0.570; P = .005), those with only one exacerbation in the previous 12 months relative to those with more (RR, 0.731; P = .009), never-smokers relative to those with smoking history (RR, 0.764; P = .013), those younger than age 65 years relative to older (RR, 0.770; P = .17), and males relative to females (RR, 0.651; P = .009).
“This gives us six factors to consider when you are thinking about stepping up to triple therapy and are trying to determine which patients would benefit the most,” Dr. Singh said.
Both the TRIMARIN and the TRIGGER trials were double blind and placebo controlled. In both, the experimental arm was a single inhaler triple therapy of the ICS beclomethasone, the LABA formoterol, and the LAMA glycopyrronium. The control arm was a single inhaler combination of beclomethasone and formoterol. All inhalers were used twice daily.
TRIMARIN, with 171 participating sites in 16 countries, randomized 1,155 patients to the triple-drug inhaler with a moderate dose of ICS (100 mcg) or to the ICS/LABA inhaler. In TRIGGER, with 221 sites in 17 countries, 1,437 patients were randomized to one of three arms. Both the triple-drug inhaler arm and the ICS/LABA arm contained a higher dose of ICS (200 mcg) than in TRIMARIN. In an open-label third arm, patients also received the higher dose of ICS plus LABA and a second inhaler with tiotropium. The formoterol dose in all arms of both studies was 6 mcg.
As reported at the ATS and now published in the Lancet, the reduction in exacerbations on single inhaler triple therapy relative to ICS/LABA was significant when the data were pooled (even though the reduction in the TRIGGER study fell short of statistical significance). The median improvement in lung function for single inhaler triple therapy relative to ICS/LABA was significant in both TRIMARIN (57 mL; P = .008) and TRIGGER (73 mL; P = .0025).
In discussing the new pooled analysis of response predictors in TRIMARIN/TRIGGER, the ERS-invited discussant, Celeste M. Porsbjerg, MD, Bispebjerg Hospital, Copenhagen, expressed particular interest in reversibility. A positive reversibility test to salbutamol was an entry criterion for both trials, but Dr. Porsbjerg pointed out that a greater response in those with the highest reversibility suggests these patients have a phenotype in which bronchodilation is a more important driver of disease than is inflammation.
While conceding that this was possible, Dr. Singh cautioned that he considers these predictors of response to be “exploratory.” He believes that the TRIMARIN/TRIGGER studies were not designed to tease out the relative importance of mechanisms of asthma in response to the assigned therapies. However, he believes the response predictor analysis is a step in this direction, which might be valuable for better individualizing therapy.
The studies were funded by Chiesi Farmaceutici. Dr. Singh reports no potential conflicts of interest.
Virchow JC et al. Lancet. 2019 Sep 30. doi. org/10.1016/S0140-6736(19)32215-9.
REPORTING FROM ERS 2019