User login
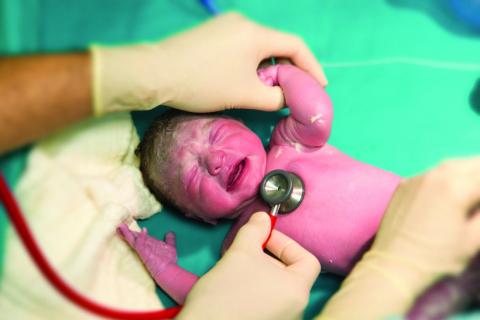
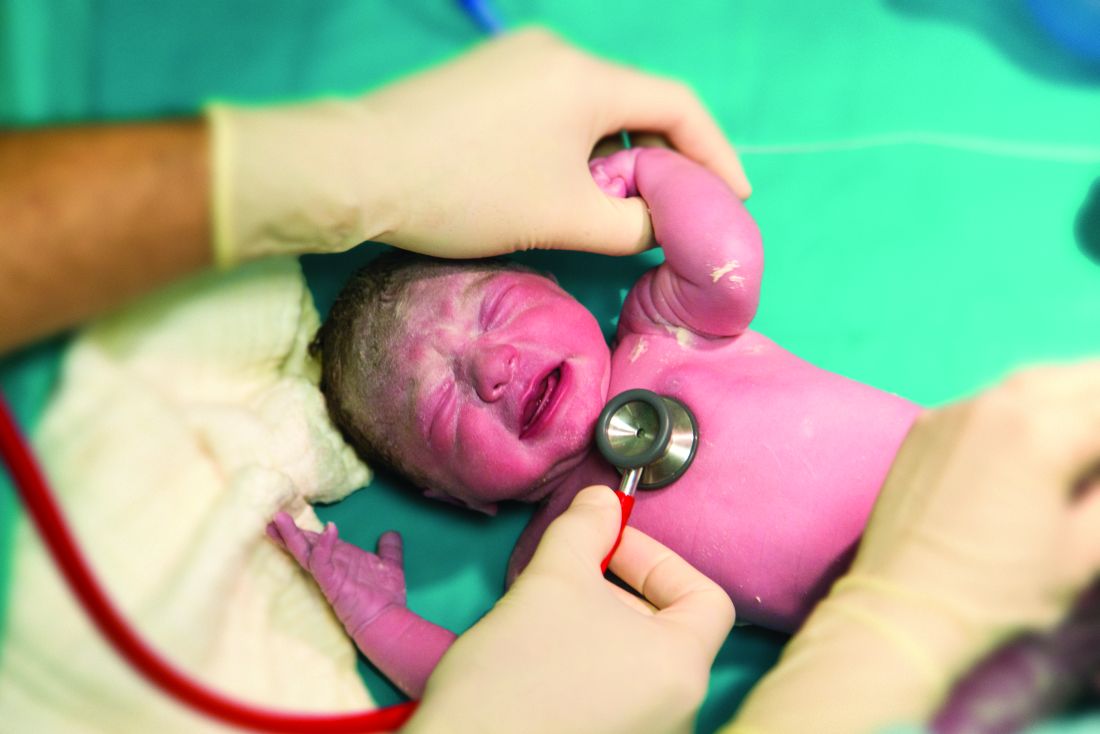
Inpatient Mobility Technicians: One Step Forward?
Prolonged bedrest with minimum mobility is associated with worse outcomes for hospitalized patients, particularly the elderly.1,2 Immobility accelerates loss of independent function and leads to complications such as deep vein thrombosis, pressure ulcers, and even death.3,4 Increasing activity and mobility early in hospitalization, even among critically ill patients, has proven safe.5 Patients with intravascular devices, urinary catheters, and even those requiring mechanical ventilation or extracorporeal membranous oxygenation can safely perform exercise and out-of-bed activities.5
Although the remedy for immobility and bedrest seems obvious, implementing workflows and strategies to increase inpatient mobility has proven challenging. Physical therapists—often the first solution considered to mobilize patients—are a limited resource and are often coordinating with other team members on care planning activities such as facilitating discharge, arranging for equipment, and educating patients and families, rather than assisting with routine mobility needs.6 Nurses share responsibility for patient activity, but they also have broad patient-care responsibilities competing for their time.7 Additionally, some nurses may feel they do not have the necessary training to safely mobilize patients.8,9
In this context, the work by Rothberg et al. is a welcome addition to the literature. In this single-blind randomized pilot trial, 102 inpatients aged 60 years and older were randomly assigned to either of two groups: intervention (ambulation protocol) or usual care. In the intervention arm, dedicated mobility technicians—ie, redeployed patient-care nursing assistants trained in safe patient-handling practices—were tasked to help patients walk three times daily. Patients in the intervention group took significantly more steps on average compared with those receiving usual care (994 versus 668). Additionally, patients with greater exposure to the mobility technicians (>2 days) had significantly higher step counts and were more likely to achieve >900 steps per day, below which patients are likely to experience functional decline.10 This study highlights the feasibility of using trained mobility technicians rather than more expensive providers (eg, physical therapists, occupational therapists, or nurses) to enhance inpatient ambulation.
The authors confirmed previously known findings that inpatient mobility, which was assessed in this study by accelerometers, predicts post-hospital patient disposition. Although consumer grade accelerometer devices (eg, Fitbit©), have limitations and may not count steps accurately for hospitalized patients who walk slowly or have gait abnormalities,11 Rothberg et al. still found that higher step count was associated with discharge home rather than to a facility. Discharge planning in the hospital is often delayed because clinicians fail to recognize impaired mobility until after resolution of acute medical/surgical issues.12 The use of routinely collected mobility measurements, such as step count, to inform decisions around care coordination and discharge planning may ultimately prove helpful for hospital throughput.
Despite the increased mobility observed in the intervention group, discharge disposition after hospitalization and hospital length of stay (LOS) did not differ between groups, whether analyzed according to per-protocol or intention-to-treat analysis. Although LOS and discharge disposition are known to be associated with patient functional status, they are also influenced by other factors, such as social support, health insurance, medical status, and patient or family preferences.13-16 Furthermore, illness severity may confound the association between step count and outcomes: sicker patients walk less, stay longer, and are more likely to need postacute rehabilitation. Thus, the effect size of a mobility intervention may be smaller than expected based on observational data, leading to underpowering. Another possibility is that the intervention did not affect these clinical outcomes because patients in the intervention group only received the intervention for an average of one-third of their hospitalization period and the mobility goal of three times per day was not consistently achieved. Mobility technician involvement was often delayed because the study required physical therapy evaluations to determine patient appropriateness before the mobility intervention was initiated. This aspect of study design belies a commonplace cultural practice to defer inpatient mobilization until a physical therapist has first evaluated the patient. Moreover, limiting mobility interventions to a single provider, such as a mobility technician, can mean that patients are less likely to be mobilized if that resource is not available. Establishing an interdisciplinary culture of mobility is more likely to be successful.17 One possible strategy is to start with nurse-performed systematic assessments of functional ability to set daily mobility goals that any appropriate provider, including a mobility technician, could help to implement.18,19
Although studies designed to increase hospital mobility have yielded mixed results,20 and larger high-quality clinical trials are needed to demonstrate clear and consistent benefits on patient-centered and operational outcomes, we applaud research and quality improvement efforts (including the current study) that promote inpatient mobility through strategies and measurements that do not require intensive physical therapist involvement. Mobility technicians may represent one step forward in enhancing a culture of mobility.
Disclosures
The authors certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated.
1. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. doi:10.1111/j.1532-5415.2009.02393.x PubMed
2. Greysen SR. Activating hospitalized older patients to confront the epidemic of low mobility. JAMA Intern Med. 2016;176(7):928. doi:10.1001/jamainternmed.2016.1874 PubMed
3. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “she was probably able to ambulate, but I’m not sure”. JAMA. 2011;306(16):1782-1793. doi:10.1001/jama.2011.1556 PubMed
4. Wu X, Li Z, Cao J, et al. The association between major complications of immobility during hospitalization and quality of life among bedridden patients: a 3 month prospective multi-center study. PLOS ONE. 2018;13(10):e0205729. doi:10.1371/journal.pone.0205729 PubMed
5. Nydahl P, Sricharoenchai T, Chandra S, et al. Safety of patient mobilization and rehabilitation in the intensive care unit: systematic review with meta-analysis. Ann Am Thorac Soc. 2017;14(5):766-777. doi:10.1513/AnnalsATS.201611-843SR PubMed
6. Masley PM, Havrilko C-L, Mahnensmith MR, Aubert M, Jette DU, Coffin-Zadai C. Physical Therapist practice in the acute care setting: a qualitative study. Phys Ther. 2011;91(6):906-922. doi:10.2522/ptj.20100296 PubMed
7. Young DL, Seltzer J, Glover M, et al. Identifying barriers to nurse-facilitated patient mobility in the intensive care unit. Am J Crit Care Off Publ Am Assoc Crit-Care Nurses. 2018;27(3):186-193. doi:10.4037/ajcc2018368 PubMed
8. Brown CJ, Williams BR, Woodby LL, Davis LL, Allman RM. Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J Hosp Med Off Publ Soc Hosp Med. 2007;2(5):305-313. doi:10.1002/jhm.209 PubMed
9. Hoyer EH, Brotman DJ, Chan KS, Needham DM. Barriers to early mobility of hospitalized general medicine patients: survey development and results. Am J Phys Med Rehabil. 2015;94(4):304-312. doi:10.1097/PHM.0000000000000185 PubMed
10. Agmon M, Zisberg A, Gil E, Rand D, Gur-Yaish N, Azriel M. Association Between 900 Steps a Day and Functional Decline in Older Hospitalized Patients. JAMA Intern Med. 2017;177(2):272. doi:10.1001/jamainternmed.2016.7266 PubMed
11. Anderson JL, Green AJ, Yoward LS, Hall HK. Validity and reliability of accelerometry in identification of lying, sitting, standing or purposeful activity in adult hospital inpatients recovering from acute or critical illness: a systematic review. Clin Rehabil. 2018;32(2):233-242. doi:10.1177/0269215517724850 PubMed
12. Roberts DE, Holloway RG, George BP. Post-acute care discharge delays for neurology inpatients: Opportunity to improve patient flow. Neurol Clin Pract. July 2018:8(4):302-310. doi:10.1212/CPJ.0000000000000492 PubMed
13. Hoyer EH, Friedman M, Lavezza A, et al. Promoting mobility and reducing length of stay in hospitalized general medicine patients: A quality-improvement project. J Hosp Med. 2016;11(5):341-34 7. doi:10.1002/jhm.2546 PubMed
14. Surkan MJ, Gibson W. Interventions to mobilize elderly patients and reduce length of hospital stay. Can J Cardiol. 2018;34(7):881-888. doi:10.1016/j.cjca.2018.04.033 PubMed
15. Ota H, Kawai H, Sato M, Ito K, Fujishima S, Suzuki H. Effect of early mobilization on discharge disposition of mechanically ventilated patients. J Phys Ther Sci. 2015;27(3):859-864. doi:10.1589/jpts.27.859 PubMed
16. Hoyer EH, Young DL, Friedman LA, et al. Routine inpatient mobility assessment and hospital discharge planning. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.5145 PubMed
17. Czaplijski T, Marshburn D, Hobbs T, Bankard S, Bennett W. Creating a culture of mobility: an interdisciplinary approach for hospitalized patients. Hosp Top. 2014;92(3):74-79. doi:10.1080/00185868.2014.937971 PubMed
18. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142.. doi:10.1093/ptj/pzx110 PubMed
19. Klein LM, Young D, Feng D, et al. Increasing patient mobility through an individualized goal-centered hospital mobility program: a quasi-experimental quality improvement project. Nurs Outlook. 2018;66(3):254-262. doi:10.1016/j.outlook.2018.02.006 PubMed
20. Kanach FA, Pastva AM, Hall KS, Pavon JM, Morey MC. Effects of structured exercise interventions for older adults hospitalized with acute medical illness: a systematic review. J Aging Phys Act. 2018;26(2):284-303. doi:10.1123/japa.2016-0372 PubMed
Prolonged bedrest with minimum mobility is associated with worse outcomes for hospitalized patients, particularly the elderly.1,2 Immobility accelerates loss of independent function and leads to complications such as deep vein thrombosis, pressure ulcers, and even death.3,4 Increasing activity and mobility early in hospitalization, even among critically ill patients, has proven safe.5 Patients with intravascular devices, urinary catheters, and even those requiring mechanical ventilation or extracorporeal membranous oxygenation can safely perform exercise and out-of-bed activities.5
Although the remedy for immobility and bedrest seems obvious, implementing workflows and strategies to increase inpatient mobility has proven challenging. Physical therapists—often the first solution considered to mobilize patients—are a limited resource and are often coordinating with other team members on care planning activities such as facilitating discharge, arranging for equipment, and educating patients and families, rather than assisting with routine mobility needs.6 Nurses share responsibility for patient activity, but they also have broad patient-care responsibilities competing for their time.7 Additionally, some nurses may feel they do not have the necessary training to safely mobilize patients.8,9
In this context, the work by Rothberg et al. is a welcome addition to the literature. In this single-blind randomized pilot trial, 102 inpatients aged 60 years and older were randomly assigned to either of two groups: intervention (ambulation protocol) or usual care. In the intervention arm, dedicated mobility technicians—ie, redeployed patient-care nursing assistants trained in safe patient-handling practices—were tasked to help patients walk three times daily. Patients in the intervention group took significantly more steps on average compared with those receiving usual care (994 versus 668). Additionally, patients with greater exposure to the mobility technicians (>2 days) had significantly higher step counts and were more likely to achieve >900 steps per day, below which patients are likely to experience functional decline.10 This study highlights the feasibility of using trained mobility technicians rather than more expensive providers (eg, physical therapists, occupational therapists, or nurses) to enhance inpatient ambulation.
The authors confirmed previously known findings that inpatient mobility, which was assessed in this study by accelerometers, predicts post-hospital patient disposition. Although consumer grade accelerometer devices (eg, Fitbit©), have limitations and may not count steps accurately for hospitalized patients who walk slowly or have gait abnormalities,11 Rothberg et al. still found that higher step count was associated with discharge home rather than to a facility. Discharge planning in the hospital is often delayed because clinicians fail to recognize impaired mobility until after resolution of acute medical/surgical issues.12 The use of routinely collected mobility measurements, such as step count, to inform decisions around care coordination and discharge planning may ultimately prove helpful for hospital throughput.
Despite the increased mobility observed in the intervention group, discharge disposition after hospitalization and hospital length of stay (LOS) did not differ between groups, whether analyzed according to per-protocol or intention-to-treat analysis. Although LOS and discharge disposition are known to be associated with patient functional status, they are also influenced by other factors, such as social support, health insurance, medical status, and patient or family preferences.13-16 Furthermore, illness severity may confound the association between step count and outcomes: sicker patients walk less, stay longer, and are more likely to need postacute rehabilitation. Thus, the effect size of a mobility intervention may be smaller than expected based on observational data, leading to underpowering. Another possibility is that the intervention did not affect these clinical outcomes because patients in the intervention group only received the intervention for an average of one-third of their hospitalization period and the mobility goal of three times per day was not consistently achieved. Mobility technician involvement was often delayed because the study required physical therapy evaluations to determine patient appropriateness before the mobility intervention was initiated. This aspect of study design belies a commonplace cultural practice to defer inpatient mobilization until a physical therapist has first evaluated the patient. Moreover, limiting mobility interventions to a single provider, such as a mobility technician, can mean that patients are less likely to be mobilized if that resource is not available. Establishing an interdisciplinary culture of mobility is more likely to be successful.17 One possible strategy is to start with nurse-performed systematic assessments of functional ability to set daily mobility goals that any appropriate provider, including a mobility technician, could help to implement.18,19
Although studies designed to increase hospital mobility have yielded mixed results,20 and larger high-quality clinical trials are needed to demonstrate clear and consistent benefits on patient-centered and operational outcomes, we applaud research and quality improvement efforts (including the current study) that promote inpatient mobility through strategies and measurements that do not require intensive physical therapist involvement. Mobility technicians may represent one step forward in enhancing a culture of mobility.
Disclosures
The authors certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated.
Prolonged bedrest with minimum mobility is associated with worse outcomes for hospitalized patients, particularly the elderly.1,2 Immobility accelerates loss of independent function and leads to complications such as deep vein thrombosis, pressure ulcers, and even death.3,4 Increasing activity and mobility early in hospitalization, even among critically ill patients, has proven safe.5 Patients with intravascular devices, urinary catheters, and even those requiring mechanical ventilation or extracorporeal membranous oxygenation can safely perform exercise and out-of-bed activities.5
Although the remedy for immobility and bedrest seems obvious, implementing workflows and strategies to increase inpatient mobility has proven challenging. Physical therapists—often the first solution considered to mobilize patients—are a limited resource and are often coordinating with other team members on care planning activities such as facilitating discharge, arranging for equipment, and educating patients and families, rather than assisting with routine mobility needs.6 Nurses share responsibility for patient activity, but they also have broad patient-care responsibilities competing for their time.7 Additionally, some nurses may feel they do not have the necessary training to safely mobilize patients.8,9
In this context, the work by Rothberg et al. is a welcome addition to the literature. In this single-blind randomized pilot trial, 102 inpatients aged 60 years and older were randomly assigned to either of two groups: intervention (ambulation protocol) or usual care. In the intervention arm, dedicated mobility technicians—ie, redeployed patient-care nursing assistants trained in safe patient-handling practices—were tasked to help patients walk three times daily. Patients in the intervention group took significantly more steps on average compared with those receiving usual care (994 versus 668). Additionally, patients with greater exposure to the mobility technicians (>2 days) had significantly higher step counts and were more likely to achieve >900 steps per day, below which patients are likely to experience functional decline.10 This study highlights the feasibility of using trained mobility technicians rather than more expensive providers (eg, physical therapists, occupational therapists, or nurses) to enhance inpatient ambulation.
The authors confirmed previously known findings that inpatient mobility, which was assessed in this study by accelerometers, predicts post-hospital patient disposition. Although consumer grade accelerometer devices (eg, Fitbit©), have limitations and may not count steps accurately for hospitalized patients who walk slowly or have gait abnormalities,11 Rothberg et al. still found that higher step count was associated with discharge home rather than to a facility. Discharge planning in the hospital is often delayed because clinicians fail to recognize impaired mobility until after resolution of acute medical/surgical issues.12 The use of routinely collected mobility measurements, such as step count, to inform decisions around care coordination and discharge planning may ultimately prove helpful for hospital throughput.
Despite the increased mobility observed in the intervention group, discharge disposition after hospitalization and hospital length of stay (LOS) did not differ between groups, whether analyzed according to per-protocol or intention-to-treat analysis. Although LOS and discharge disposition are known to be associated with patient functional status, they are also influenced by other factors, such as social support, health insurance, medical status, and patient or family preferences.13-16 Furthermore, illness severity may confound the association between step count and outcomes: sicker patients walk less, stay longer, and are more likely to need postacute rehabilitation. Thus, the effect size of a mobility intervention may be smaller than expected based on observational data, leading to underpowering. Another possibility is that the intervention did not affect these clinical outcomes because patients in the intervention group only received the intervention for an average of one-third of their hospitalization period and the mobility goal of three times per day was not consistently achieved. Mobility technician involvement was often delayed because the study required physical therapy evaluations to determine patient appropriateness before the mobility intervention was initiated. This aspect of study design belies a commonplace cultural practice to defer inpatient mobilization until a physical therapist has first evaluated the patient. Moreover, limiting mobility interventions to a single provider, such as a mobility technician, can mean that patients are less likely to be mobilized if that resource is not available. Establishing an interdisciplinary culture of mobility is more likely to be successful.17 One possible strategy is to start with nurse-performed systematic assessments of functional ability to set daily mobility goals that any appropriate provider, including a mobility technician, could help to implement.18,19
Although studies designed to increase hospital mobility have yielded mixed results,20 and larger high-quality clinical trials are needed to demonstrate clear and consistent benefits on patient-centered and operational outcomes, we applaud research and quality improvement efforts (including the current study) that promote inpatient mobility through strategies and measurements that do not require intensive physical therapist involvement. Mobility technicians may represent one step forward in enhancing a culture of mobility.
Disclosures
The authors certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on us or on any organization with which we are associated.
1. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. doi:10.1111/j.1532-5415.2009.02393.x PubMed
2. Greysen SR. Activating hospitalized older patients to confront the epidemic of low mobility. JAMA Intern Med. 2016;176(7):928. doi:10.1001/jamainternmed.2016.1874 PubMed
3. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “she was probably able to ambulate, but I’m not sure”. JAMA. 2011;306(16):1782-1793. doi:10.1001/jama.2011.1556 PubMed
4. Wu X, Li Z, Cao J, et al. The association between major complications of immobility during hospitalization and quality of life among bedridden patients: a 3 month prospective multi-center study. PLOS ONE. 2018;13(10):e0205729. doi:10.1371/journal.pone.0205729 PubMed
5. Nydahl P, Sricharoenchai T, Chandra S, et al. Safety of patient mobilization and rehabilitation in the intensive care unit: systematic review with meta-analysis. Ann Am Thorac Soc. 2017;14(5):766-777. doi:10.1513/AnnalsATS.201611-843SR PubMed
6. Masley PM, Havrilko C-L, Mahnensmith MR, Aubert M, Jette DU, Coffin-Zadai C. Physical Therapist practice in the acute care setting: a qualitative study. Phys Ther. 2011;91(6):906-922. doi:10.2522/ptj.20100296 PubMed
7. Young DL, Seltzer J, Glover M, et al. Identifying barriers to nurse-facilitated patient mobility in the intensive care unit. Am J Crit Care Off Publ Am Assoc Crit-Care Nurses. 2018;27(3):186-193. doi:10.4037/ajcc2018368 PubMed
8. Brown CJ, Williams BR, Woodby LL, Davis LL, Allman RM. Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J Hosp Med Off Publ Soc Hosp Med. 2007;2(5):305-313. doi:10.1002/jhm.209 PubMed
9. Hoyer EH, Brotman DJ, Chan KS, Needham DM. Barriers to early mobility of hospitalized general medicine patients: survey development and results. Am J Phys Med Rehabil. 2015;94(4):304-312. doi:10.1097/PHM.0000000000000185 PubMed
10. Agmon M, Zisberg A, Gil E, Rand D, Gur-Yaish N, Azriel M. Association Between 900 Steps a Day and Functional Decline in Older Hospitalized Patients. JAMA Intern Med. 2017;177(2):272. doi:10.1001/jamainternmed.2016.7266 PubMed
11. Anderson JL, Green AJ, Yoward LS, Hall HK. Validity and reliability of accelerometry in identification of lying, sitting, standing or purposeful activity in adult hospital inpatients recovering from acute or critical illness: a systematic review. Clin Rehabil. 2018;32(2):233-242. doi:10.1177/0269215517724850 PubMed
12. Roberts DE, Holloway RG, George BP. Post-acute care discharge delays for neurology inpatients: Opportunity to improve patient flow. Neurol Clin Pract. July 2018:8(4):302-310. doi:10.1212/CPJ.0000000000000492 PubMed
13. Hoyer EH, Friedman M, Lavezza A, et al. Promoting mobility and reducing length of stay in hospitalized general medicine patients: A quality-improvement project. J Hosp Med. 2016;11(5):341-34 7. doi:10.1002/jhm.2546 PubMed
14. Surkan MJ, Gibson W. Interventions to mobilize elderly patients and reduce length of hospital stay. Can J Cardiol. 2018;34(7):881-888. doi:10.1016/j.cjca.2018.04.033 PubMed
15. Ota H, Kawai H, Sato M, Ito K, Fujishima S, Suzuki H. Effect of early mobilization on discharge disposition of mechanically ventilated patients. J Phys Ther Sci. 2015;27(3):859-864. doi:10.1589/jpts.27.859 PubMed
16. Hoyer EH, Young DL, Friedman LA, et al. Routine inpatient mobility assessment and hospital discharge planning. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.5145 PubMed
17. Czaplijski T, Marshburn D, Hobbs T, Bankard S, Bennett W. Creating a culture of mobility: an interdisciplinary approach for hospitalized patients. Hosp Top. 2014;92(3):74-79. doi:10.1080/00185868.2014.937971 PubMed
18. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142.. doi:10.1093/ptj/pzx110 PubMed
19. Klein LM, Young D, Feng D, et al. Increasing patient mobility through an individualized goal-centered hospital mobility program: a quasi-experimental quality improvement project. Nurs Outlook. 2018;66(3):254-262. doi:10.1016/j.outlook.2018.02.006 PubMed
20. Kanach FA, Pastva AM, Hall KS, Pavon JM, Morey MC. Effects of structured exercise interventions for older adults hospitalized with acute medical illness: a systematic review. J Aging Phys Act. 2018;26(2):284-303. doi:10.1123/japa.2016-0372 PubMed
1. Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660-1665. doi:10.1111/j.1532-5415.2009.02393.x PubMed
2. Greysen SR. Activating hospitalized older patients to confront the epidemic of low mobility. JAMA Intern Med. 2016;176(7):928. doi:10.1001/jamainternmed.2016.1874 PubMed
3. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “she was probably able to ambulate, but I’m not sure”. JAMA. 2011;306(16):1782-1793. doi:10.1001/jama.2011.1556 PubMed
4. Wu X, Li Z, Cao J, et al. The association between major complications of immobility during hospitalization and quality of life among bedridden patients: a 3 month prospective multi-center study. PLOS ONE. 2018;13(10):e0205729. doi:10.1371/journal.pone.0205729 PubMed
5. Nydahl P, Sricharoenchai T, Chandra S, et al. Safety of patient mobilization and rehabilitation in the intensive care unit: systematic review with meta-analysis. Ann Am Thorac Soc. 2017;14(5):766-777. doi:10.1513/AnnalsATS.201611-843SR PubMed
6. Masley PM, Havrilko C-L, Mahnensmith MR, Aubert M, Jette DU, Coffin-Zadai C. Physical Therapist practice in the acute care setting: a qualitative study. Phys Ther. 2011;91(6):906-922. doi:10.2522/ptj.20100296 PubMed
7. Young DL, Seltzer J, Glover M, et al. Identifying barriers to nurse-facilitated patient mobility in the intensive care unit. Am J Crit Care Off Publ Am Assoc Crit-Care Nurses. 2018;27(3):186-193. doi:10.4037/ajcc2018368 PubMed
8. Brown CJ, Williams BR, Woodby LL, Davis LL, Allman RM. Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J Hosp Med Off Publ Soc Hosp Med. 2007;2(5):305-313. doi:10.1002/jhm.209 PubMed
9. Hoyer EH, Brotman DJ, Chan KS, Needham DM. Barriers to early mobility of hospitalized general medicine patients: survey development and results. Am J Phys Med Rehabil. 2015;94(4):304-312. doi:10.1097/PHM.0000000000000185 PubMed
10. Agmon M, Zisberg A, Gil E, Rand D, Gur-Yaish N, Azriel M. Association Between 900 Steps a Day and Functional Decline in Older Hospitalized Patients. JAMA Intern Med. 2017;177(2):272. doi:10.1001/jamainternmed.2016.7266 PubMed
11. Anderson JL, Green AJ, Yoward LS, Hall HK. Validity and reliability of accelerometry in identification of lying, sitting, standing or purposeful activity in adult hospital inpatients recovering from acute or critical illness: a systematic review. Clin Rehabil. 2018;32(2):233-242. doi:10.1177/0269215517724850 PubMed
12. Roberts DE, Holloway RG, George BP. Post-acute care discharge delays for neurology inpatients: Opportunity to improve patient flow. Neurol Clin Pract. July 2018:8(4):302-310. doi:10.1212/CPJ.0000000000000492 PubMed
13. Hoyer EH, Friedman M, Lavezza A, et al. Promoting mobility and reducing length of stay in hospitalized general medicine patients: A quality-improvement project. J Hosp Med. 2016;11(5):341-34 7. doi:10.1002/jhm.2546 PubMed
14. Surkan MJ, Gibson W. Interventions to mobilize elderly patients and reduce length of hospital stay. Can J Cardiol. 2018;34(7):881-888. doi:10.1016/j.cjca.2018.04.033 PubMed
15. Ota H, Kawai H, Sato M, Ito K, Fujishima S, Suzuki H. Effect of early mobilization on discharge disposition of mechanically ventilated patients. J Phys Ther Sci. 2015;27(3):859-864. doi:10.1589/jpts.27.859 PubMed
16. Hoyer EH, Young DL, Friedman LA, et al. Routine inpatient mobility assessment and hospital discharge planning. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.5145 PubMed
17. Czaplijski T, Marshburn D, Hobbs T, Bankard S, Bennett W. Creating a culture of mobility: an interdisciplinary approach for hospitalized patients. Hosp Top. 2014;92(3):74-79. doi:10.1080/00185868.2014.937971 PubMed
18. Hoyer EH, Young DL, Klein LM, et al. Toward a common language for measuring patient mobility in the hospital: reliability and construct validity of interprofessional mobility measures. Phys Ther. 2018;98(2):133-142.. doi:10.1093/ptj/pzx110 PubMed
19. Klein LM, Young D, Feng D, et al. Increasing patient mobility through an individualized goal-centered hospital mobility program: a quasi-experimental quality improvement project. Nurs Outlook. 2018;66(3):254-262. doi:10.1016/j.outlook.2018.02.006 PubMed
20. Kanach FA, Pastva AM, Hall KS, Pavon JM, Morey MC. Effects of structured exercise interventions for older adults hospitalized with acute medical illness: a systematic review. J Aging Phys Act. 2018;26(2):284-303. doi:10.1123/japa.2016-0372 PubMed
©2019 Society of Hospital Medicine
A prescription for medicine’s gender inequality
A small study identifies skin microbiome changes after UV exposure. Lower prices drive OTC insulin sales at Walmart. And early intensive treatment of multiple sclerosis may benefit patients.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
A small study identifies skin microbiome changes after UV exposure. Lower prices drive OTC insulin sales at Walmart. And early intensive treatment of multiple sclerosis may benefit patients.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
A small study identifies skin microbiome changes after UV exposure. Lower prices drive OTC insulin sales at Walmart. And early intensive treatment of multiple sclerosis may benefit patients.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Crisis Communication for Multilingual Communities
Civil rights laws mandate that federally funded emergency response and recovery services must be accessible to all Americans. But at least 350 languages are spoken in the US, according to the US Census Bureau. And millions of people have hearing or vision problems or cannot read.
Recent devastating fires, hurricanes, and earthquakes have underscored the need for clear communication in disasters. Now the US Department of Health and Human Services (HHS) has unveiled a “plain language checklist” to help first responders make sure important information is shared.
The checklist, developed through the HHS Language Access Steering Committee, complements an emergency preparedness checklist released in 2016. It includes recommendations, action steps, and resources to help first responders provide on-the-ground language assistance. For example, a key recommendation is to not only identify languages and dialects spoken in the community, but specific types of sign language as well. The action steps include accessing state and local demographic data and identifying public spaces that serve people lacking English proficiency, such as libraries that offer language access resources.
The checklist also provides practical tips for working with interpreters, such as speaking directly in the first person to the individual (not the interpreter), avoiding idioms, acronyms, and double negatives. Red flags include interpreters who need repeated clarifications, who overuse English terms, and whose interpretations seem overly long or short compared with the statements being interpreted.
The HHS also recommends:
- Working with Centers for Independent Living and other groups who work with people with disabilities;
- Identifying local partners, such as hospitals, faith-based organizations, and legal services; and
- Coordinating with TV, print, radio, and online media to share plain-language, culturally appropriate emergency information.
The checklist is available at https://www.hhs.gov/about/news/2018/12/04/new-hhs-checklist-helps-first-responders-ensure-language-access-and-effective-communication-during-emergencies.html
Civil rights laws mandate that federally funded emergency response and recovery services must be accessible to all Americans. But at least 350 languages are spoken in the US, according to the US Census Bureau. And millions of people have hearing or vision problems or cannot read.
Recent devastating fires, hurricanes, and earthquakes have underscored the need for clear communication in disasters. Now the US Department of Health and Human Services (HHS) has unveiled a “plain language checklist” to help first responders make sure important information is shared.
The checklist, developed through the HHS Language Access Steering Committee, complements an emergency preparedness checklist released in 2016. It includes recommendations, action steps, and resources to help first responders provide on-the-ground language assistance. For example, a key recommendation is to not only identify languages and dialects spoken in the community, but specific types of sign language as well. The action steps include accessing state and local demographic data and identifying public spaces that serve people lacking English proficiency, such as libraries that offer language access resources.
The checklist also provides practical tips for working with interpreters, such as speaking directly in the first person to the individual (not the interpreter), avoiding idioms, acronyms, and double negatives. Red flags include interpreters who need repeated clarifications, who overuse English terms, and whose interpretations seem overly long or short compared with the statements being interpreted.
The HHS also recommends:
- Working with Centers for Independent Living and other groups who work with people with disabilities;
- Identifying local partners, such as hospitals, faith-based organizations, and legal services; and
- Coordinating with TV, print, radio, and online media to share plain-language, culturally appropriate emergency information.
The checklist is available at https://www.hhs.gov/about/news/2018/12/04/new-hhs-checklist-helps-first-responders-ensure-language-access-and-effective-communication-during-emergencies.html
Civil rights laws mandate that federally funded emergency response and recovery services must be accessible to all Americans. But at least 350 languages are spoken in the US, according to the US Census Bureau. And millions of people have hearing or vision problems or cannot read.
Recent devastating fires, hurricanes, and earthquakes have underscored the need for clear communication in disasters. Now the US Department of Health and Human Services (HHS) has unveiled a “plain language checklist” to help first responders make sure important information is shared.
The checklist, developed through the HHS Language Access Steering Committee, complements an emergency preparedness checklist released in 2016. It includes recommendations, action steps, and resources to help first responders provide on-the-ground language assistance. For example, a key recommendation is to not only identify languages and dialects spoken in the community, but specific types of sign language as well. The action steps include accessing state and local demographic data and identifying public spaces that serve people lacking English proficiency, such as libraries that offer language access resources.
The checklist also provides practical tips for working with interpreters, such as speaking directly in the first person to the individual (not the interpreter), avoiding idioms, acronyms, and double negatives. Red flags include interpreters who need repeated clarifications, who overuse English terms, and whose interpretations seem overly long or short compared with the statements being interpreted.
The HHS also recommends:
- Working with Centers for Independent Living and other groups who work with people with disabilities;
- Identifying local partners, such as hospitals, faith-based organizations, and legal services; and
- Coordinating with TV, print, radio, and online media to share plain-language, culturally appropriate emergency information.
The checklist is available at https://www.hhs.gov/about/news/2018/12/04/new-hhs-checklist-helps-first-responders-ensure-language-access-and-effective-communication-during-emergencies.html
Commentary: Physician burnout: It’s good to complain
Burnout among vascular surgeons and other physicians is a serious national epidemic that needs immediate attention by senior policy makers and health care leaders. Not only is maintaining an appropriate supply of fully qualified surgeons important to the medical demands of our country, the underlying causes of physician burnout clearly point to increased personal pain and suffering within the physician community.
While it is quite clear that a serious response to physician burnout requires immediate action, the most pressing and urgent question for senior leadership is exactly what can be done to best address the causes of this epidemic.
This commentary reflects an approach and strategy for building an effective response to physician burnout deeply rooted in the broad discipline of health care management theory and research. Our understanding of the problem starts with the simple and common observation that our thoughts about our job are deeply embedded in the conditions and “lived reality” of doing our job. We can see this link in everyday conversations when they quickly turn to detailed complaints about all things work related.
Listening to people complain about their jobs can sometimes sound like unfounded “whining.” But if we dig deeper into such complaints, we can start to see some common elements giving credence to such grievances. For example, if we step back a little from our current preoccupations and look at the history of work over the last 100 years or so, we can see the outline of a long and generally progressive arc of change aimed at improving the conditions for making a living.
This arc of change has allowed us to stop complaining so much about the risk of losing life and limb from industrial accidents because those complaints helped to create new laws that imposed strict regulations, making the conditions of working with big machines much safer. From the 40-hour week, paid vacations, and tenure to workplace discrimination, harassment, and abuse, there are many examples of how complaining about the conditions of one’s job has led to major changes in how people work together in an organization.
Coming back to the present, the big, clamoring machines that caused many to complain years ago have now been replaced by the clicking and hum of computers used by knowledge-based workers. But while the tools, physical environment, workforce, and other key characteristics of what people do for a living change over time, serious complaints about job conditions remain important sources of information about how to make those conditions job safer and healthier.
The importance of complaining
One of the primary goals of every health care organization should be to consciously create safe and healthy working conditions for physicians and everyone else involved in the daily production of health care services.
At present, there is considerable interest in developing new programs for addressing physician burnout by using therapeutic interventions. This approach is focused on mediating the severity of an unhealthy workplace by helping physicians better cope with personal frustrations and other psychological difficulties related to their job.
Personal counseling, yoga at noon, and other tools for building personal resilience can certainly improve coping skills but fundamentally miss the point for addressing the underlying causes for burnout.
The problem here is that a reliance on therapeutic interventions alone can mask and reflect the cause of the problem from their source in the conditions of the workplace back onto the physicians who must do their job under those conditions. This is roughly equivalent to providing therapeutic counseling to a factory worker who loses an arm to a machine in an industrial accident with no mention or effort to fix the dangerous machine that workers were loudly complaining about before the accident.
In order to develop an effective response to burnout, attention needs to be given to the specific content of what physicians are complaining about as existential threats to their personal health and safety in the environment in which they do their work as physicians.
A clear-eyed assessment of the real-life structures and processes that define how the work of physicians is routinely carried out every day is needed in every modern health care organization. Such an assessment is not a call for simply “whining” about everyday annoyances and bothers that are encountered as part of most people’s jobs. Rather, a thoughtful cataloging of what physicians are complaining about is required.
This examination needs to carefully listen to complaints to better understand two highly related factors. First: What do vascular surgeons and other physicians “want to do” in order to be personally “satisfied” with their job? And second: How does the organization (structure) and established “flow” (processes) of their given work environment encourage, help, hinder, or prevent them from being satisfied as a regular part of being a physician?
Such an assessment of complains will not be easy. Important methodological considerations will need to be made to make conceptual and measurable distinctions between complaints about major threats to physician health that are part of the current work environment and ongoing and rapid changes affecting the overall profession of medicine. For example, new and ongoing developments in medical technology, health informatics, generational shifts in the attributes of the workforce, evolution of state and federal policy, shifting patient and epidemiological profiles, and other major trends will continue to affect the workplace of physicians. Such changes are part of the current dynamics of the workplace of physicians and may be major components of the conditions of work that are generating complaints and contributing to burnout.
Viewing physician complaints as important tools for improving the working conditions of physician does not mean that such changes can be stopped. More directly, it means that physician complaints can become a critical part in the policy debate and management discussion about what changes in the physician workplace need to change to eliminate burnout.
From a health care management perspective, physicians should take the lead and keep complaining. It is an essential window for senior leadership to see exactly what needs to be done to create a safer and healthier workplace for physicians to be physicians.
Dr. Zimmerman is a professor of health care management at the University of New Orleans.
Burnout among vascular surgeons and other physicians is a serious national epidemic that needs immediate attention by senior policy makers and health care leaders. Not only is maintaining an appropriate supply of fully qualified surgeons important to the medical demands of our country, the underlying causes of physician burnout clearly point to increased personal pain and suffering within the physician community.
While it is quite clear that a serious response to physician burnout requires immediate action, the most pressing and urgent question for senior leadership is exactly what can be done to best address the causes of this epidemic.
This commentary reflects an approach and strategy for building an effective response to physician burnout deeply rooted in the broad discipline of health care management theory and research. Our understanding of the problem starts with the simple and common observation that our thoughts about our job are deeply embedded in the conditions and “lived reality” of doing our job. We can see this link in everyday conversations when they quickly turn to detailed complaints about all things work related.
Listening to people complain about their jobs can sometimes sound like unfounded “whining.” But if we dig deeper into such complaints, we can start to see some common elements giving credence to such grievances. For example, if we step back a little from our current preoccupations and look at the history of work over the last 100 years or so, we can see the outline of a long and generally progressive arc of change aimed at improving the conditions for making a living.
This arc of change has allowed us to stop complaining so much about the risk of losing life and limb from industrial accidents because those complaints helped to create new laws that imposed strict regulations, making the conditions of working with big machines much safer. From the 40-hour week, paid vacations, and tenure to workplace discrimination, harassment, and abuse, there are many examples of how complaining about the conditions of one’s job has led to major changes in how people work together in an organization.
Coming back to the present, the big, clamoring machines that caused many to complain years ago have now been replaced by the clicking and hum of computers used by knowledge-based workers. But while the tools, physical environment, workforce, and other key characteristics of what people do for a living change over time, serious complaints about job conditions remain important sources of information about how to make those conditions job safer and healthier.
The importance of complaining
One of the primary goals of every health care organization should be to consciously create safe and healthy working conditions for physicians and everyone else involved in the daily production of health care services.
At present, there is considerable interest in developing new programs for addressing physician burnout by using therapeutic interventions. This approach is focused on mediating the severity of an unhealthy workplace by helping physicians better cope with personal frustrations and other psychological difficulties related to their job.
Personal counseling, yoga at noon, and other tools for building personal resilience can certainly improve coping skills but fundamentally miss the point for addressing the underlying causes for burnout.
The problem here is that a reliance on therapeutic interventions alone can mask and reflect the cause of the problem from their source in the conditions of the workplace back onto the physicians who must do their job under those conditions. This is roughly equivalent to providing therapeutic counseling to a factory worker who loses an arm to a machine in an industrial accident with no mention or effort to fix the dangerous machine that workers were loudly complaining about before the accident.
In order to develop an effective response to burnout, attention needs to be given to the specific content of what physicians are complaining about as existential threats to their personal health and safety in the environment in which they do their work as physicians.
A clear-eyed assessment of the real-life structures and processes that define how the work of physicians is routinely carried out every day is needed in every modern health care organization. Such an assessment is not a call for simply “whining” about everyday annoyances and bothers that are encountered as part of most people’s jobs. Rather, a thoughtful cataloging of what physicians are complaining about is required.
This examination needs to carefully listen to complaints to better understand two highly related factors. First: What do vascular surgeons and other physicians “want to do” in order to be personally “satisfied” with their job? And second: How does the organization (structure) and established “flow” (processes) of their given work environment encourage, help, hinder, or prevent them from being satisfied as a regular part of being a physician?
Such an assessment of complains will not be easy. Important methodological considerations will need to be made to make conceptual and measurable distinctions between complaints about major threats to physician health that are part of the current work environment and ongoing and rapid changes affecting the overall profession of medicine. For example, new and ongoing developments in medical technology, health informatics, generational shifts in the attributes of the workforce, evolution of state and federal policy, shifting patient and epidemiological profiles, and other major trends will continue to affect the workplace of physicians. Such changes are part of the current dynamics of the workplace of physicians and may be major components of the conditions of work that are generating complaints and contributing to burnout.
Viewing physician complaints as important tools for improving the working conditions of physician does not mean that such changes can be stopped. More directly, it means that physician complaints can become a critical part in the policy debate and management discussion about what changes in the physician workplace need to change to eliminate burnout.
From a health care management perspective, physicians should take the lead and keep complaining. It is an essential window for senior leadership to see exactly what needs to be done to create a safer and healthier workplace for physicians to be physicians.
Dr. Zimmerman is a professor of health care management at the University of New Orleans.
Burnout among vascular surgeons and other physicians is a serious national epidemic that needs immediate attention by senior policy makers and health care leaders. Not only is maintaining an appropriate supply of fully qualified surgeons important to the medical demands of our country, the underlying causes of physician burnout clearly point to increased personal pain and suffering within the physician community.
While it is quite clear that a serious response to physician burnout requires immediate action, the most pressing and urgent question for senior leadership is exactly what can be done to best address the causes of this epidemic.
This commentary reflects an approach and strategy for building an effective response to physician burnout deeply rooted in the broad discipline of health care management theory and research. Our understanding of the problem starts with the simple and common observation that our thoughts about our job are deeply embedded in the conditions and “lived reality” of doing our job. We can see this link in everyday conversations when they quickly turn to detailed complaints about all things work related.
Listening to people complain about their jobs can sometimes sound like unfounded “whining.” But if we dig deeper into such complaints, we can start to see some common elements giving credence to such grievances. For example, if we step back a little from our current preoccupations and look at the history of work over the last 100 years or so, we can see the outline of a long and generally progressive arc of change aimed at improving the conditions for making a living.
This arc of change has allowed us to stop complaining so much about the risk of losing life and limb from industrial accidents because those complaints helped to create new laws that imposed strict regulations, making the conditions of working with big machines much safer. From the 40-hour week, paid vacations, and tenure to workplace discrimination, harassment, and abuse, there are many examples of how complaining about the conditions of one’s job has led to major changes in how people work together in an organization.
Coming back to the present, the big, clamoring machines that caused many to complain years ago have now been replaced by the clicking and hum of computers used by knowledge-based workers. But while the tools, physical environment, workforce, and other key characteristics of what people do for a living change over time, serious complaints about job conditions remain important sources of information about how to make those conditions job safer and healthier.
The importance of complaining
One of the primary goals of every health care organization should be to consciously create safe and healthy working conditions for physicians and everyone else involved in the daily production of health care services.
At present, there is considerable interest in developing new programs for addressing physician burnout by using therapeutic interventions. This approach is focused on mediating the severity of an unhealthy workplace by helping physicians better cope with personal frustrations and other psychological difficulties related to their job.
Personal counseling, yoga at noon, and other tools for building personal resilience can certainly improve coping skills but fundamentally miss the point for addressing the underlying causes for burnout.
The problem here is that a reliance on therapeutic interventions alone can mask and reflect the cause of the problem from their source in the conditions of the workplace back onto the physicians who must do their job under those conditions. This is roughly equivalent to providing therapeutic counseling to a factory worker who loses an arm to a machine in an industrial accident with no mention or effort to fix the dangerous machine that workers were loudly complaining about before the accident.
In order to develop an effective response to burnout, attention needs to be given to the specific content of what physicians are complaining about as existential threats to their personal health and safety in the environment in which they do their work as physicians.
A clear-eyed assessment of the real-life structures and processes that define how the work of physicians is routinely carried out every day is needed in every modern health care organization. Such an assessment is not a call for simply “whining” about everyday annoyances and bothers that are encountered as part of most people’s jobs. Rather, a thoughtful cataloging of what physicians are complaining about is required.
This examination needs to carefully listen to complaints to better understand two highly related factors. First: What do vascular surgeons and other physicians “want to do” in order to be personally “satisfied” with their job? And second: How does the organization (structure) and established “flow” (processes) of their given work environment encourage, help, hinder, or prevent them from being satisfied as a regular part of being a physician?
Such an assessment of complains will not be easy. Important methodological considerations will need to be made to make conceptual and measurable distinctions between complaints about major threats to physician health that are part of the current work environment and ongoing and rapid changes affecting the overall profession of medicine. For example, new and ongoing developments in medical technology, health informatics, generational shifts in the attributes of the workforce, evolution of state and federal policy, shifting patient and epidemiological profiles, and other major trends will continue to affect the workplace of physicians. Such changes are part of the current dynamics of the workplace of physicians and may be major components of the conditions of work that are generating complaints and contributing to burnout.
Viewing physician complaints as important tools for improving the working conditions of physician does not mean that such changes can be stopped. More directly, it means that physician complaints can become a critical part in the policy debate and management discussion about what changes in the physician workplace need to change to eliminate burnout.
From a health care management perspective, physicians should take the lead and keep complaining. It is an essential window for senior leadership to see exactly what needs to be done to create a safer and healthier workplace for physicians to be physicians.
Dr. Zimmerman is a professor of health care management at the University of New Orleans.
Disruptive behavior on the job linked to depression, burnout
SAN DIEGO – Hospitals pay a price for bad behavior by staff in the workplace, results of a large multicenter study suggest.
A work culture in which disruptive behavior is tolerated can have consequences. Research on this topic has linked disruptive behavior by staff in the health care setting to increased frequency of medical errors and lower quality of care (Am J Med Qual. 2011 Sep-Oct;26(5):372-9; J Caring Sci. 2016 Sep 1;5(3):241-9). This new study, based on a workplace culture survey of 7,923 health care workers and 325 work settings at 16 hospitals in a large West Coast health care system, found , researchers found. The paper was presented by study lead Allison Hadley, MD, of Duke Children’s Hospital, Durham, N.C., at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The investigators developed a novel survey scale for evaluating disruptive behaviors in the health care setting. The objective was to look at the associations between disruptive behavior, teamwork, safety culture, burnout, and depression. Disruptive behaviors included turning backs or hanging up the phone before a conversation is over, bullying or trying to publicly humiliate other staff, making inappropriate comments (with sexual, racial, religious, or ethnic slurs), and physical aggression (such as throwing, hitting, and pushing).
San Francisco internist Alan H. Rosenstein, MD, who studies disruptive behavior in medicine, said in an interview that the findings confirm anecdotal experience of medical staff. “One of the downsides of disruptive behavior is very unsatisfied and unhappy people,” he said
The investigators used a t-test analysis to study the strength of the association between disruptive behavior and work culture in health care work settings. They found a statistically significant association between less disruptive behavior and lower levels of burnout and depression among staff (t = 6.4 and t = 4.1, respectively, P less than .001) and higher levels of teamwork, safety culture, and work-life balance (t = 10.2, t = 9.5 and t = 5.8, respectively, P less than .001). Settings in which disruptive behaviors were more common were more likely to have poor teamwork culture (P less than .001) and safety climate (P less than .001), and higher rates of depression (P less than .001). Settings in which disruptive behaviors were more common were more likely to have poor teamwork culture (P less than .001) and safety climate (P less than .001), and higher rates of depression (P less than .001).
Bullying was reported at about 40% of workplaces with low teamwork levels, compared with nearly 20% in those with high teamwork levels.
Physical aggression was reported in nearly 20% of those workplaces with low teamwork levels, compared with 5% in workplaces with high teamwork levels (P less than .001).
Researchers also found that disruptive behaviors were least common during day shifts and more common among health care workers who care for both adults and children than among those who care for only adults. “Teamwork, safety culture, and work-life balance were highest in those [hospital] units with the least disruptive behaviors,” said Dr. Hadley.
Overall, the highest positive correlation was found between higher levels of teamwork and lower levels of disruptive behavior, Dr. Hadley said. If a hospital department is trying to address one issue to improve disruptive behavior, she’d suggest it “focus on teamwork first. I hope that would have the greatest impact.”
Dr. Rosenstein, who has conducted several studies on disruptive behavior, said the key to improving the workplace is to “build a culture based on the mission of providing patient care. It’s not to save a dollar, to make a dollar. The mission is patient care.”
What’s next? Dr. Hadley said her team is continuing to work on developing a scale to measure disruptive behavior in the workplace.
No study funding was reported. Dr. Hadley and Dr. Rosenstein reported no relevant disclosures.
SOURCE: Hadley A et al. CCC48, Abstract 114.
SAN DIEGO – Hospitals pay a price for bad behavior by staff in the workplace, results of a large multicenter study suggest.
A work culture in which disruptive behavior is tolerated can have consequences. Research on this topic has linked disruptive behavior by staff in the health care setting to increased frequency of medical errors and lower quality of care (Am J Med Qual. 2011 Sep-Oct;26(5):372-9; J Caring Sci. 2016 Sep 1;5(3):241-9). This new study, based on a workplace culture survey of 7,923 health care workers and 325 work settings at 16 hospitals in a large West Coast health care system, found , researchers found. The paper was presented by study lead Allison Hadley, MD, of Duke Children’s Hospital, Durham, N.C., at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The investigators developed a novel survey scale for evaluating disruptive behaviors in the health care setting. The objective was to look at the associations between disruptive behavior, teamwork, safety culture, burnout, and depression. Disruptive behaviors included turning backs or hanging up the phone before a conversation is over, bullying or trying to publicly humiliate other staff, making inappropriate comments (with sexual, racial, religious, or ethnic slurs), and physical aggression (such as throwing, hitting, and pushing).
San Francisco internist Alan H. Rosenstein, MD, who studies disruptive behavior in medicine, said in an interview that the findings confirm anecdotal experience of medical staff. “One of the downsides of disruptive behavior is very unsatisfied and unhappy people,” he said
The investigators used a t-test analysis to study the strength of the association between disruptive behavior and work culture in health care work settings. They found a statistically significant association between less disruptive behavior and lower levels of burnout and depression among staff (t = 6.4 and t = 4.1, respectively, P less than .001) and higher levels of teamwork, safety culture, and work-life balance (t = 10.2, t = 9.5 and t = 5.8, respectively, P less than .001). Settings in which disruptive behaviors were more common were more likely to have poor teamwork culture (P less than .001) and safety climate (P less than .001), and higher rates of depression (P less than .001). Settings in which disruptive behaviors were more common were more likely to have poor teamwork culture (P less than .001) and safety climate (P less than .001), and higher rates of depression (P less than .001).
Bullying was reported at about 40% of workplaces with low teamwork levels, compared with nearly 20% in those with high teamwork levels.
Physical aggression was reported in nearly 20% of those workplaces with low teamwork levels, compared with 5% in workplaces with high teamwork levels (P less than .001).
Researchers also found that disruptive behaviors were least common during day shifts and more common among health care workers who care for both adults and children than among those who care for only adults. “Teamwork, safety culture, and work-life balance were highest in those [hospital] units with the least disruptive behaviors,” said Dr. Hadley.
Overall, the highest positive correlation was found between higher levels of teamwork and lower levels of disruptive behavior, Dr. Hadley said. If a hospital department is trying to address one issue to improve disruptive behavior, she’d suggest it “focus on teamwork first. I hope that would have the greatest impact.”
Dr. Rosenstein, who has conducted several studies on disruptive behavior, said the key to improving the workplace is to “build a culture based on the mission of providing patient care. It’s not to save a dollar, to make a dollar. The mission is patient care.”
What’s next? Dr. Hadley said her team is continuing to work on developing a scale to measure disruptive behavior in the workplace.
No study funding was reported. Dr. Hadley and Dr. Rosenstein reported no relevant disclosures.
SOURCE: Hadley A et al. CCC48, Abstract 114.
SAN DIEGO – Hospitals pay a price for bad behavior by staff in the workplace, results of a large multicenter study suggest.
A work culture in which disruptive behavior is tolerated can have consequences. Research on this topic has linked disruptive behavior by staff in the health care setting to increased frequency of medical errors and lower quality of care (Am J Med Qual. 2011 Sep-Oct;26(5):372-9; J Caring Sci. 2016 Sep 1;5(3):241-9). This new study, based on a workplace culture survey of 7,923 health care workers and 325 work settings at 16 hospitals in a large West Coast health care system, found , researchers found. The paper was presented by study lead Allison Hadley, MD, of Duke Children’s Hospital, Durham, N.C., at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The investigators developed a novel survey scale for evaluating disruptive behaviors in the health care setting. The objective was to look at the associations between disruptive behavior, teamwork, safety culture, burnout, and depression. Disruptive behaviors included turning backs or hanging up the phone before a conversation is over, bullying or trying to publicly humiliate other staff, making inappropriate comments (with sexual, racial, religious, or ethnic slurs), and physical aggression (such as throwing, hitting, and pushing).
San Francisco internist Alan H. Rosenstein, MD, who studies disruptive behavior in medicine, said in an interview that the findings confirm anecdotal experience of medical staff. “One of the downsides of disruptive behavior is very unsatisfied and unhappy people,” he said
The investigators used a t-test analysis to study the strength of the association between disruptive behavior and work culture in health care work settings. They found a statistically significant association between less disruptive behavior and lower levels of burnout and depression among staff (t = 6.4 and t = 4.1, respectively, P less than .001) and higher levels of teamwork, safety culture, and work-life balance (t = 10.2, t = 9.5 and t = 5.8, respectively, P less than .001). Settings in which disruptive behaviors were more common were more likely to have poor teamwork culture (P less than .001) and safety climate (P less than .001), and higher rates of depression (P less than .001). Settings in which disruptive behaviors were more common were more likely to have poor teamwork culture (P less than .001) and safety climate (P less than .001), and higher rates of depression (P less than .001).
Bullying was reported at about 40% of workplaces with low teamwork levels, compared with nearly 20% in those with high teamwork levels.
Physical aggression was reported in nearly 20% of those workplaces with low teamwork levels, compared with 5% in workplaces with high teamwork levels (P less than .001).
Researchers also found that disruptive behaviors were least common during day shifts and more common among health care workers who care for both adults and children than among those who care for only adults. “Teamwork, safety culture, and work-life balance were highest in those [hospital] units with the least disruptive behaviors,” said Dr. Hadley.
Overall, the highest positive correlation was found between higher levels of teamwork and lower levels of disruptive behavior, Dr. Hadley said. If a hospital department is trying to address one issue to improve disruptive behavior, she’d suggest it “focus on teamwork first. I hope that would have the greatest impact.”
Dr. Rosenstein, who has conducted several studies on disruptive behavior, said the key to improving the workplace is to “build a culture based on the mission of providing patient care. It’s not to save a dollar, to make a dollar. The mission is patient care.”
What’s next? Dr. Hadley said her team is continuing to work on developing a scale to measure disruptive behavior in the workplace.
No study funding was reported. Dr. Hadley and Dr. Rosenstein reported no relevant disclosures.
SOURCE: Hadley A et al. CCC48, Abstract 114.
REPORTING FROM CCC48
AACE/ACE algorithm provides practical clinical guidance on managing diabetes
Leading endocrinology societies have copublished an algorithm offering updated, specific clinical guidance on lifestyle therapy, management of hypertension and dyslipidemia, and glucose control in patients with type 2 diabetes.
This update from the American Association of Clinical Endocrinologists and the American College of Endocrinology, published in Endocrine Practice, also highlights obesity and prediabetes as underlying risk factors for development of diabetes.
The algorithm, based on new and “comprehensive clinical data” on type 2 diabetes management, is designed as a supplement 2015 AACE/ACE clinical practice guidelines, according to Alan J. Garber, MD, PhD, chair of the Diabetes Management Algorithm Task Force.
“It’s intended to provide clinicians with a practical guide that prompts them to look for factors or influences in the patient’s lifestyle or health that may be a factor in identifying the best treatment approach or medication,” he said in a statement.
Lifestyle medication is critical for all patients with diabetes, according to Dr. Garber and the algorithm coauthors, who recommended a “primarily plant-based meal plan” that limits intake of saturated fatty acids and avoids trans fats. They said overweight patients should restrict caloric intake with a goal of reducing body weight by up to 10%.
Physical activity should include at least 150 minutes per week of activities such as brisk walking or weight training, they said, adding that patients should be advised to sleep 7 hours per night, on average.
Weight loss medications might be needed along with lifestyle modification for patients with body mass index (BMI) over 27 kg/m2 with complications, and for all patients with BMI over 30 regardless of whether they have complications, according to the AACE/ACE committee members who drafted the report.
Bariatric surgery might be considered in patients with BMI over 35 and comorbidities, particularly if patients fail to achieve weight loss goals using other means, they added.
The primary goal of prediabetes management is weight loss, wrote the authors. While there are no Food and Drug Administration–approved agents for prediabetes management, they said, antihyperglycemic agents such as metformin and acarbose have been shown to reduce risk of diabetes by 25%-30% in patients with prediabetes.
While pressure control needs to be individualized, but a goal of less than 130/80 mm Hg is warranted for most patients with diabetes, according to the authors, who note that most patients will require medication to reach their goal.
“Because angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers can slow progression of nephropathy and retinopathy, they are preferred for patients with type 2 diabetes,” said Dr. Garber and his coauthors in the executive summary accompanying the algorithm.
Early and intensive management of dyslipidemia is important to reduce the significant risk of atherosclerotic cardiovascular disease in patients with diabetes, according to the authors, who say diabetes patients should be classified as high risk, very high risk, or extreme risk. They recommended LDL cholesterol targets of less than 100 mg/dL for high-risk patients, less than 70 mg/dL for very-high-risk patients, and less than 55 mg/dL for the extreme-risk group.
Statins should be considered first-line treatment for lowering cholesterol, unless contraindicated, with other lipid-modifying agents added as needed to reach lipid targets.
Inhibitors of proprotein convertase subtilisin/kexin type 9 serine protease (PCSK9) address a “large unmet need” for more aggressive lipid lowering in patients with clinical atherosclerotic disease and diabetes, the authors noted.
Added to maximal statin therapy, PCSK9 inhibitors reduce LDL cholesterol by about 50% while also raising HDL cholesterol and having positive effects on other lipids, according to the authors.
Pharmacotherapy for type 2 diabetes requires a “nuanced approach” that takes into account factors such as age, comorbidities, and risk of hypoglycemia, the authors wrote, noting that the AACE supports a hemoglobin A1c target of 6.5% or less for most patients.
The algorithm for glycemic control lists glucose-lowering agents in order of recommended usage. For example, in patients with an entry HbA1c less than 7.5%, the strongest recommendations for were monotherapy with metformin, followed by GLP1 receptor agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors.
If insulin becomes necessary, the recommended approach is to add a single daily dose of basal insulin, and if a basal insulin regimen fails to control glucose, it may help to add a GLP1 receptor agonist or dipeptidyl peptidase 4 (DPP4) inhibitor, according to the algorithm.
Avoiding hypoglycemia is important, and one possible “safety measure” to prevent that is using a continuous glucose monitoring device that provides real-time glucose data. “Significant advances have been made in accuracy and availability of CGM devices,” the authors wrote.
Current expert consensus is that clinical CGM devices should be considered if patients have not achieved their glycemic target after 3 months or if they need a treatment that puts them at risk for hypoglycemia, according to Dr. Garber and his colleagues.
Dr. Garber reported that he had no financial relationships relevant to the consensus statement and algorithm. Coauthors of the report provided disclosures related to Novo Nordisk, Eli Lilly, Janssen Pharmaceuticals, Abbott, Sanofi-Aventis, and other pharmaceutical companies.
SOURCE: Garber AJ et al. Endocr Pract. 2019 Jan;25(1):91-120.
Leading endocrinology societies have copublished an algorithm offering updated, specific clinical guidance on lifestyle therapy, management of hypertension and dyslipidemia, and glucose control in patients with type 2 diabetes.
This update from the American Association of Clinical Endocrinologists and the American College of Endocrinology, published in Endocrine Practice, also highlights obesity and prediabetes as underlying risk factors for development of diabetes.
The algorithm, based on new and “comprehensive clinical data” on type 2 diabetes management, is designed as a supplement 2015 AACE/ACE clinical practice guidelines, according to Alan J. Garber, MD, PhD, chair of the Diabetes Management Algorithm Task Force.
“It’s intended to provide clinicians with a practical guide that prompts them to look for factors or influences in the patient’s lifestyle or health that may be a factor in identifying the best treatment approach or medication,” he said in a statement.
Lifestyle medication is critical for all patients with diabetes, according to Dr. Garber and the algorithm coauthors, who recommended a “primarily plant-based meal plan” that limits intake of saturated fatty acids and avoids trans fats. They said overweight patients should restrict caloric intake with a goal of reducing body weight by up to 10%.
Physical activity should include at least 150 minutes per week of activities such as brisk walking or weight training, they said, adding that patients should be advised to sleep 7 hours per night, on average.
Weight loss medications might be needed along with lifestyle modification for patients with body mass index (BMI) over 27 kg/m2 with complications, and for all patients with BMI over 30 regardless of whether they have complications, according to the AACE/ACE committee members who drafted the report.
Bariatric surgery might be considered in patients with BMI over 35 and comorbidities, particularly if patients fail to achieve weight loss goals using other means, they added.
The primary goal of prediabetes management is weight loss, wrote the authors. While there are no Food and Drug Administration–approved agents for prediabetes management, they said, antihyperglycemic agents such as metformin and acarbose have been shown to reduce risk of diabetes by 25%-30% in patients with prediabetes.
While pressure control needs to be individualized, but a goal of less than 130/80 mm Hg is warranted for most patients with diabetes, according to the authors, who note that most patients will require medication to reach their goal.
“Because angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers can slow progression of nephropathy and retinopathy, they are preferred for patients with type 2 diabetes,” said Dr. Garber and his coauthors in the executive summary accompanying the algorithm.
Early and intensive management of dyslipidemia is important to reduce the significant risk of atherosclerotic cardiovascular disease in patients with diabetes, according to the authors, who say diabetes patients should be classified as high risk, very high risk, or extreme risk. They recommended LDL cholesterol targets of less than 100 mg/dL for high-risk patients, less than 70 mg/dL for very-high-risk patients, and less than 55 mg/dL for the extreme-risk group.
Statins should be considered first-line treatment for lowering cholesterol, unless contraindicated, with other lipid-modifying agents added as needed to reach lipid targets.
Inhibitors of proprotein convertase subtilisin/kexin type 9 serine protease (PCSK9) address a “large unmet need” for more aggressive lipid lowering in patients with clinical atherosclerotic disease and diabetes, the authors noted.
Added to maximal statin therapy, PCSK9 inhibitors reduce LDL cholesterol by about 50% while also raising HDL cholesterol and having positive effects on other lipids, according to the authors.
Pharmacotherapy for type 2 diabetes requires a “nuanced approach” that takes into account factors such as age, comorbidities, and risk of hypoglycemia, the authors wrote, noting that the AACE supports a hemoglobin A1c target of 6.5% or less for most patients.
The algorithm for glycemic control lists glucose-lowering agents in order of recommended usage. For example, in patients with an entry HbA1c less than 7.5%, the strongest recommendations for were monotherapy with metformin, followed by GLP1 receptor agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors.
If insulin becomes necessary, the recommended approach is to add a single daily dose of basal insulin, and if a basal insulin regimen fails to control glucose, it may help to add a GLP1 receptor agonist or dipeptidyl peptidase 4 (DPP4) inhibitor, according to the algorithm.
Avoiding hypoglycemia is important, and one possible “safety measure” to prevent that is using a continuous glucose monitoring device that provides real-time glucose data. “Significant advances have been made in accuracy and availability of CGM devices,” the authors wrote.
Current expert consensus is that clinical CGM devices should be considered if patients have not achieved their glycemic target after 3 months or if they need a treatment that puts them at risk for hypoglycemia, according to Dr. Garber and his colleagues.
Dr. Garber reported that he had no financial relationships relevant to the consensus statement and algorithm. Coauthors of the report provided disclosures related to Novo Nordisk, Eli Lilly, Janssen Pharmaceuticals, Abbott, Sanofi-Aventis, and other pharmaceutical companies.
SOURCE: Garber AJ et al. Endocr Pract. 2019 Jan;25(1):91-120.
Leading endocrinology societies have copublished an algorithm offering updated, specific clinical guidance on lifestyle therapy, management of hypertension and dyslipidemia, and glucose control in patients with type 2 diabetes.
This update from the American Association of Clinical Endocrinologists and the American College of Endocrinology, published in Endocrine Practice, also highlights obesity and prediabetes as underlying risk factors for development of diabetes.
The algorithm, based on new and “comprehensive clinical data” on type 2 diabetes management, is designed as a supplement 2015 AACE/ACE clinical practice guidelines, according to Alan J. Garber, MD, PhD, chair of the Diabetes Management Algorithm Task Force.
“It’s intended to provide clinicians with a practical guide that prompts them to look for factors or influences in the patient’s lifestyle or health that may be a factor in identifying the best treatment approach or medication,” he said in a statement.
Lifestyle medication is critical for all patients with diabetes, according to Dr. Garber and the algorithm coauthors, who recommended a “primarily plant-based meal plan” that limits intake of saturated fatty acids and avoids trans fats. They said overweight patients should restrict caloric intake with a goal of reducing body weight by up to 10%.
Physical activity should include at least 150 minutes per week of activities such as brisk walking or weight training, they said, adding that patients should be advised to sleep 7 hours per night, on average.
Weight loss medications might be needed along with lifestyle modification for patients with body mass index (BMI) over 27 kg/m2 with complications, and for all patients with BMI over 30 regardless of whether they have complications, according to the AACE/ACE committee members who drafted the report.
Bariatric surgery might be considered in patients with BMI over 35 and comorbidities, particularly if patients fail to achieve weight loss goals using other means, they added.
The primary goal of prediabetes management is weight loss, wrote the authors. While there are no Food and Drug Administration–approved agents for prediabetes management, they said, antihyperglycemic agents such as metformin and acarbose have been shown to reduce risk of diabetes by 25%-30% in patients with prediabetes.
While pressure control needs to be individualized, but a goal of less than 130/80 mm Hg is warranted for most patients with diabetes, according to the authors, who note that most patients will require medication to reach their goal.
“Because angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers can slow progression of nephropathy and retinopathy, they are preferred for patients with type 2 diabetes,” said Dr. Garber and his coauthors in the executive summary accompanying the algorithm.
Early and intensive management of dyslipidemia is important to reduce the significant risk of atherosclerotic cardiovascular disease in patients with diabetes, according to the authors, who say diabetes patients should be classified as high risk, very high risk, or extreme risk. They recommended LDL cholesterol targets of less than 100 mg/dL for high-risk patients, less than 70 mg/dL for very-high-risk patients, and less than 55 mg/dL for the extreme-risk group.
Statins should be considered first-line treatment for lowering cholesterol, unless contraindicated, with other lipid-modifying agents added as needed to reach lipid targets.
Inhibitors of proprotein convertase subtilisin/kexin type 9 serine protease (PCSK9) address a “large unmet need” for more aggressive lipid lowering in patients with clinical atherosclerotic disease and diabetes, the authors noted.
Added to maximal statin therapy, PCSK9 inhibitors reduce LDL cholesterol by about 50% while also raising HDL cholesterol and having positive effects on other lipids, according to the authors.
Pharmacotherapy for type 2 diabetes requires a “nuanced approach” that takes into account factors such as age, comorbidities, and risk of hypoglycemia, the authors wrote, noting that the AACE supports a hemoglobin A1c target of 6.5% or less for most patients.
The algorithm for glycemic control lists glucose-lowering agents in order of recommended usage. For example, in patients with an entry HbA1c less than 7.5%, the strongest recommendations for were monotherapy with metformin, followed by GLP1 receptor agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors.
If insulin becomes necessary, the recommended approach is to add a single daily dose of basal insulin, and if a basal insulin regimen fails to control glucose, it may help to add a GLP1 receptor agonist or dipeptidyl peptidase 4 (DPP4) inhibitor, according to the algorithm.
Avoiding hypoglycemia is important, and one possible “safety measure” to prevent that is using a continuous glucose monitoring device that provides real-time glucose data. “Significant advances have been made in accuracy and availability of CGM devices,” the authors wrote.
Current expert consensus is that clinical CGM devices should be considered if patients have not achieved their glycemic target after 3 months or if they need a treatment that puts them at risk for hypoglycemia, according to Dr. Garber and his colleagues.
Dr. Garber reported that he had no financial relationships relevant to the consensus statement and algorithm. Coauthors of the report provided disclosures related to Novo Nordisk, Eli Lilly, Janssen Pharmaceuticals, Abbott, Sanofi-Aventis, and other pharmaceutical companies.
SOURCE: Garber AJ et al. Endocr Pract. 2019 Jan;25(1):91-120.
FROM ENDOCRINE PRACTICE
FDA approves pembrolizumab for completely resected melanoma
The Food and Drug Administration has approved pembrolizumab (Keytruda) for the adjuvant treatment of patients with melanoma with lymph node involvement following resection.
FDA approval is based on results from the randomized, double-blind, placebo-controlled EORTC1325/KEYNOTE‑054 trial, in which 1,019 patients with completely resected stage III melanoma received either a placebo or 200 mg of pembrolizumab every 3 weeks for up to 1 year until disease recurrence or unacceptable toxicity.
Recurrence-free survival was significantly better in the pembrolizumab group than in the placebo group (hazard ratio, 0.57; 95% confidence interval, 0.46-0.70; P less than .001). The median recurrence-free survival time was 20.4 months in the placebo group and was not reached in the pembrolizumab group, the FDA said in a press release.
About three-quarters of patients received pembrolizumab for at least 6 months, while 14% of patients had to stop pembrolizumab treatment because of adverse events. The most common adverse events in pembrolizumab-treated patients included diarrhea, pruritus, nausea, arthralgia, hypothyroidism, cough, rash, asthenia, influenzalike illness, weight loss, and hyperthyroidism.
“The recommended pembrolizumab dose and schedule for the adjuvant treatment of melanoma is 200 mg administered as an IV infusion over 30 minutes every 3 weeks until disease recurrence or unacceptable toxicity, for a maximum of 1 year,” the FDA said in the press release.
The Food and Drug Administration has approved pembrolizumab (Keytruda) for the adjuvant treatment of patients with melanoma with lymph node involvement following resection.
FDA approval is based on results from the randomized, double-blind, placebo-controlled EORTC1325/KEYNOTE‑054 trial, in which 1,019 patients with completely resected stage III melanoma received either a placebo or 200 mg of pembrolizumab every 3 weeks for up to 1 year until disease recurrence or unacceptable toxicity.
Recurrence-free survival was significantly better in the pembrolizumab group than in the placebo group (hazard ratio, 0.57; 95% confidence interval, 0.46-0.70; P less than .001). The median recurrence-free survival time was 20.4 months in the placebo group and was not reached in the pembrolizumab group, the FDA said in a press release.
About three-quarters of patients received pembrolizumab for at least 6 months, while 14% of patients had to stop pembrolizumab treatment because of adverse events. The most common adverse events in pembrolizumab-treated patients included diarrhea, pruritus, nausea, arthralgia, hypothyroidism, cough, rash, asthenia, influenzalike illness, weight loss, and hyperthyroidism.
“The recommended pembrolizumab dose and schedule for the adjuvant treatment of melanoma is 200 mg administered as an IV infusion over 30 minutes every 3 weeks until disease recurrence or unacceptable toxicity, for a maximum of 1 year,” the FDA said in the press release.
The Food and Drug Administration has approved pembrolizumab (Keytruda) for the adjuvant treatment of patients with melanoma with lymph node involvement following resection.
FDA approval is based on results from the randomized, double-blind, placebo-controlled EORTC1325/KEYNOTE‑054 trial, in which 1,019 patients with completely resected stage III melanoma received either a placebo or 200 mg of pembrolizumab every 3 weeks for up to 1 year until disease recurrence or unacceptable toxicity.
Recurrence-free survival was significantly better in the pembrolizumab group than in the placebo group (hazard ratio, 0.57; 95% confidence interval, 0.46-0.70; P less than .001). The median recurrence-free survival time was 20.4 months in the placebo group and was not reached in the pembrolizumab group, the FDA said in a press release.
About three-quarters of patients received pembrolizumab for at least 6 months, while 14% of patients had to stop pembrolizumab treatment because of adverse events. The most common adverse events in pembrolizumab-treated patients included diarrhea, pruritus, nausea, arthralgia, hypothyroidism, cough, rash, asthenia, influenzalike illness, weight loss, and hyperthyroidism.
“The recommended pembrolizumab dose and schedule for the adjuvant treatment of melanoma is 200 mg administered as an IV infusion over 30 minutes every 3 weeks until disease recurrence or unacceptable toxicity, for a maximum of 1 year,” the FDA said in the press release.
Sildenafil associated with persistent pulmonary hypertension in neonates with early IUGR
LAS VEGAS – Increased rates of persistent neonatal pulmonary hypertension in neonates put the brakes on STRIDER, an international placebo-controlled study looking at sildenafil as a treatment for early-onset intrauterine growth restriction (IUGR).
The study’s independent data safety monitoring board halted STRIDER (Sildenafil Therapy in Dismal Prognosis Early-Onset Fetal Growth Restriction) last July, after an interim safety analysis identified possible fetal harm and no signal of benefit over placebo, Dr. Anouk Pels reported at the annual meeting of the Society for Maternal-Fetal Medicine. The late-breaking presentation at the meeting revealed the first outcome data details.
The board had “serious concerns that sildenafil may cause harm to newborn children. … Given the results , it is extremely unlikely that any benefit could be shown on the primary endpoint if the trial is continued to its completion,” said Dr. Pels of the University of Amsterdam. “Our recommendation is not to use sildenafil for this indication in pregnant women.”
Although the link remains as-yet unproven, pulmonary hypertension among sildenafil-exposed neonates is biologically plausible, she said. It could have been a symptomatic rebound response to the discontinuation of constant intrauterine sildenafil exposure – or it could have been a hint of something more profound. Like the genital vasculature, pulmonary vasculature is a target of the drug. Intrauterine exposure to sildenafil could theoretically alter its development.
“It’s possible that sildenafil may be causing structural changes in the pulmonary vasculature of fetuses. This needs to be explored further, and we will do so by performing additional analyses on autopsy data and placental histology.”
STRIDER involved 261 pregnant women diagnosed with severe early-onset fetal growth restriction. They were randomized to sildenafil 25 mg or placebo three times daily until delivery or 32 weeks’ gestation. A safety analysis was conducted after every 50 patients were enrolled. The preplanned interim analysis was conducted after half of the cohort had been enrolled and received at least one dose of the study medication.
The primary outcome was a composite measure of neonatal mortality or major neonatal morbidity at hospital discharge.
Gestational age at baseline was about 24.6 weeks, and the estimated fetal weight by ultrasound, 465 g. About 45% of pregnancies had evidence of a notching in the uterine artery. In about 42%, the pulsatility index of the umbilical artery was above the 95th percentile; the pulsatility index of the middle cerebral artery was below the 5% percentile in about 70% of cases.
About a quarter of the women had a diagnosis of pregnancy-induced hypertension, and another quarter, preeclampsia. Women used the study medication for a mean of 22 days.
There were no significant between-group differences in maternal outcomes. Median gestational age at delivery was 28 weeks in both groups. About 10% in each group experienced new-onset pregnancy-induced hypertension; About a quarter of each group developed new-onset preeclampsia or HELLP (hemolysis, elevated liver enzymes, low platelet count). There were no between-group differences in the number of maternal antihypertensives prescribed.
The primary combined outcome of neonatal mortality or major neonatal morbidity occurred in 66 of the sildenafil-exposed neonates and 58 of the placebo-exposed infants (61% vs. 54%) – not a significant difference. Fetal death occurred in 23 and 29 pregnancies, respectively (21% and 27%); neonatal death occurred in 21 and 11, respectively (19% and 10%). Overall, fetal/neonatal mortality was similar between the sildenafil and placebo groups (41% and 37%, respectively).
Of the 64 sildenafil-exposed neonates who survived to hospital discharge, 22 exhibited clinically relevant morbidity. Of the 67 in the placebo-treated group who survived to hospital discharge, 18 had clinically relevant morbidity. Overall, 42 in the sildenafil group and 49 of the placebo group survived to hospital discharge without relevant morbidity.
There were a number of secondary outcomes, none exhibiting any significant between-group differences. These included the median weights of those who experienced intrauterine death (425 g and 350 g), median live birth weight (725 g and 783 g), intraventricular hemorrhage of grade II or IV (3% and 2%), periventricular hemorrhage grade II or higher (0%, both groups), necrotizing enterocolitis grade II or higher (7% and 8%), and at least one culture-proven or clinical infection (41% and 33%).
The significantly higher rates of pulmonary hypertension in the sildenafil-exposed neonates was the showstopper. Almost half of the 21 who died had proven pulmonary hypertension (10), as did 6 of the 64 who survived – an excess of 16 cases. Of proven cases, 11 were persistent pulmonary hypertension. There were also two cases of sepsis-associated pulmonary hypertension and four cases of bronchopulmonary dysplasia associated with the disorder. In the placebo-exposed group, pulmonary hypertension occurred in 3 of the 11 deaths and 1 of the 67 who survived. Of proven cases, two were persistent pulmonary hypertension. There was no sepsis-associated pulmonary hypertension, but there were three cases of bronchopulmonary dysplasia associated with the disorder. Some children had both persistent pulmonary hypertension and bronchopulmonary dysplasia.
“While there was no difference in the primary outcomes or in overall mortality, there were more cases of pulmonary hypertension in the sildenafil group,” Dr. Pels said. “We can speculate on the cause, whether it was related to sildenafil and why, or whether it was simply chance. This is the reason we need to conduct more in-depth analyses of these data.”
Funding came from federal health agencies and universities in the countries where STRIDER was conducted, including New Zealand, Australia, the United Kingdom, Ireland, and the Netherlands. Dr. Pels had no relevant financial disclosures.
SOURCE: Pels A et al. The Pregnancy Meeting, Late-Breaker 2.
LAS VEGAS – Increased rates of persistent neonatal pulmonary hypertension in neonates put the brakes on STRIDER, an international placebo-controlled study looking at sildenafil as a treatment for early-onset intrauterine growth restriction (IUGR).
The study’s independent data safety monitoring board halted STRIDER (Sildenafil Therapy in Dismal Prognosis Early-Onset Fetal Growth Restriction) last July, after an interim safety analysis identified possible fetal harm and no signal of benefit over placebo, Dr. Anouk Pels reported at the annual meeting of the Society for Maternal-Fetal Medicine. The late-breaking presentation at the meeting revealed the first outcome data details.
The board had “serious concerns that sildenafil may cause harm to newborn children. … Given the results , it is extremely unlikely that any benefit could be shown on the primary endpoint if the trial is continued to its completion,” said Dr. Pels of the University of Amsterdam. “Our recommendation is not to use sildenafil for this indication in pregnant women.”
Although the link remains as-yet unproven, pulmonary hypertension among sildenafil-exposed neonates is biologically plausible, she said. It could have been a symptomatic rebound response to the discontinuation of constant intrauterine sildenafil exposure – or it could have been a hint of something more profound. Like the genital vasculature, pulmonary vasculature is a target of the drug. Intrauterine exposure to sildenafil could theoretically alter its development.
“It’s possible that sildenafil may be causing structural changes in the pulmonary vasculature of fetuses. This needs to be explored further, and we will do so by performing additional analyses on autopsy data and placental histology.”
STRIDER involved 261 pregnant women diagnosed with severe early-onset fetal growth restriction. They were randomized to sildenafil 25 mg or placebo three times daily until delivery or 32 weeks’ gestation. A safety analysis was conducted after every 50 patients were enrolled. The preplanned interim analysis was conducted after half of the cohort had been enrolled and received at least one dose of the study medication.
The primary outcome was a composite measure of neonatal mortality or major neonatal morbidity at hospital discharge.
Gestational age at baseline was about 24.6 weeks, and the estimated fetal weight by ultrasound, 465 g. About 45% of pregnancies had evidence of a notching in the uterine artery. In about 42%, the pulsatility index of the umbilical artery was above the 95th percentile; the pulsatility index of the middle cerebral artery was below the 5% percentile in about 70% of cases.
About a quarter of the women had a diagnosis of pregnancy-induced hypertension, and another quarter, preeclampsia. Women used the study medication for a mean of 22 days.
There were no significant between-group differences in maternal outcomes. Median gestational age at delivery was 28 weeks in both groups. About 10% in each group experienced new-onset pregnancy-induced hypertension; About a quarter of each group developed new-onset preeclampsia or HELLP (hemolysis, elevated liver enzymes, low platelet count). There were no between-group differences in the number of maternal antihypertensives prescribed.
The primary combined outcome of neonatal mortality or major neonatal morbidity occurred in 66 of the sildenafil-exposed neonates and 58 of the placebo-exposed infants (61% vs. 54%) – not a significant difference. Fetal death occurred in 23 and 29 pregnancies, respectively (21% and 27%); neonatal death occurred in 21 and 11, respectively (19% and 10%). Overall, fetal/neonatal mortality was similar between the sildenafil and placebo groups (41% and 37%, respectively).
Of the 64 sildenafil-exposed neonates who survived to hospital discharge, 22 exhibited clinically relevant morbidity. Of the 67 in the placebo-treated group who survived to hospital discharge, 18 had clinically relevant morbidity. Overall, 42 in the sildenafil group and 49 of the placebo group survived to hospital discharge without relevant morbidity.
There were a number of secondary outcomes, none exhibiting any significant between-group differences. These included the median weights of those who experienced intrauterine death (425 g and 350 g), median live birth weight (725 g and 783 g), intraventricular hemorrhage of grade II or IV (3% and 2%), periventricular hemorrhage grade II or higher (0%, both groups), necrotizing enterocolitis grade II or higher (7% and 8%), and at least one culture-proven or clinical infection (41% and 33%).
The significantly higher rates of pulmonary hypertension in the sildenafil-exposed neonates was the showstopper. Almost half of the 21 who died had proven pulmonary hypertension (10), as did 6 of the 64 who survived – an excess of 16 cases. Of proven cases, 11 were persistent pulmonary hypertension. There were also two cases of sepsis-associated pulmonary hypertension and four cases of bronchopulmonary dysplasia associated with the disorder. In the placebo-exposed group, pulmonary hypertension occurred in 3 of the 11 deaths and 1 of the 67 who survived. Of proven cases, two were persistent pulmonary hypertension. There was no sepsis-associated pulmonary hypertension, but there were three cases of bronchopulmonary dysplasia associated with the disorder. Some children had both persistent pulmonary hypertension and bronchopulmonary dysplasia.
“While there was no difference in the primary outcomes or in overall mortality, there were more cases of pulmonary hypertension in the sildenafil group,” Dr. Pels said. “We can speculate on the cause, whether it was related to sildenafil and why, or whether it was simply chance. This is the reason we need to conduct more in-depth analyses of these data.”
Funding came from federal health agencies and universities in the countries where STRIDER was conducted, including New Zealand, Australia, the United Kingdom, Ireland, and the Netherlands. Dr. Pels had no relevant financial disclosures.
SOURCE: Pels A et al. The Pregnancy Meeting, Late-Breaker 2.
LAS VEGAS – Increased rates of persistent neonatal pulmonary hypertension in neonates put the brakes on STRIDER, an international placebo-controlled study looking at sildenafil as a treatment for early-onset intrauterine growth restriction (IUGR).
The study’s independent data safety monitoring board halted STRIDER (Sildenafil Therapy in Dismal Prognosis Early-Onset Fetal Growth Restriction) last July, after an interim safety analysis identified possible fetal harm and no signal of benefit over placebo, Dr. Anouk Pels reported at the annual meeting of the Society for Maternal-Fetal Medicine. The late-breaking presentation at the meeting revealed the first outcome data details.
The board had “serious concerns that sildenafil may cause harm to newborn children. … Given the results , it is extremely unlikely that any benefit could be shown on the primary endpoint if the trial is continued to its completion,” said Dr. Pels of the University of Amsterdam. “Our recommendation is not to use sildenafil for this indication in pregnant women.”
Although the link remains as-yet unproven, pulmonary hypertension among sildenafil-exposed neonates is biologically plausible, she said. It could have been a symptomatic rebound response to the discontinuation of constant intrauterine sildenafil exposure – or it could have been a hint of something more profound. Like the genital vasculature, pulmonary vasculature is a target of the drug. Intrauterine exposure to sildenafil could theoretically alter its development.
“It’s possible that sildenafil may be causing structural changes in the pulmonary vasculature of fetuses. This needs to be explored further, and we will do so by performing additional analyses on autopsy data and placental histology.”
STRIDER involved 261 pregnant women diagnosed with severe early-onset fetal growth restriction. They were randomized to sildenafil 25 mg or placebo three times daily until delivery or 32 weeks’ gestation. A safety analysis was conducted after every 50 patients were enrolled. The preplanned interim analysis was conducted after half of the cohort had been enrolled and received at least one dose of the study medication.
The primary outcome was a composite measure of neonatal mortality or major neonatal morbidity at hospital discharge.
Gestational age at baseline was about 24.6 weeks, and the estimated fetal weight by ultrasound, 465 g. About 45% of pregnancies had evidence of a notching in the uterine artery. In about 42%, the pulsatility index of the umbilical artery was above the 95th percentile; the pulsatility index of the middle cerebral artery was below the 5% percentile in about 70% of cases.
About a quarter of the women had a diagnosis of pregnancy-induced hypertension, and another quarter, preeclampsia. Women used the study medication for a mean of 22 days.
There were no significant between-group differences in maternal outcomes. Median gestational age at delivery was 28 weeks in both groups. About 10% in each group experienced new-onset pregnancy-induced hypertension; About a quarter of each group developed new-onset preeclampsia or HELLP (hemolysis, elevated liver enzymes, low platelet count). There were no between-group differences in the number of maternal antihypertensives prescribed.
The primary combined outcome of neonatal mortality or major neonatal morbidity occurred in 66 of the sildenafil-exposed neonates and 58 of the placebo-exposed infants (61% vs. 54%) – not a significant difference. Fetal death occurred in 23 and 29 pregnancies, respectively (21% and 27%); neonatal death occurred in 21 and 11, respectively (19% and 10%). Overall, fetal/neonatal mortality was similar between the sildenafil and placebo groups (41% and 37%, respectively).
Of the 64 sildenafil-exposed neonates who survived to hospital discharge, 22 exhibited clinically relevant morbidity. Of the 67 in the placebo-treated group who survived to hospital discharge, 18 had clinically relevant morbidity. Overall, 42 in the sildenafil group and 49 of the placebo group survived to hospital discharge without relevant morbidity.
There were a number of secondary outcomes, none exhibiting any significant between-group differences. These included the median weights of those who experienced intrauterine death (425 g and 350 g), median live birth weight (725 g and 783 g), intraventricular hemorrhage of grade II or IV (3% and 2%), periventricular hemorrhage grade II or higher (0%, both groups), necrotizing enterocolitis grade II or higher (7% and 8%), and at least one culture-proven or clinical infection (41% and 33%).
The significantly higher rates of pulmonary hypertension in the sildenafil-exposed neonates was the showstopper. Almost half of the 21 who died had proven pulmonary hypertension (10), as did 6 of the 64 who survived – an excess of 16 cases. Of proven cases, 11 were persistent pulmonary hypertension. There were also two cases of sepsis-associated pulmonary hypertension and four cases of bronchopulmonary dysplasia associated with the disorder. In the placebo-exposed group, pulmonary hypertension occurred in 3 of the 11 deaths and 1 of the 67 who survived. Of proven cases, two were persistent pulmonary hypertension. There was no sepsis-associated pulmonary hypertension, but there were three cases of bronchopulmonary dysplasia associated with the disorder. Some children had both persistent pulmonary hypertension and bronchopulmonary dysplasia.
“While there was no difference in the primary outcomes or in overall mortality, there were more cases of pulmonary hypertension in the sildenafil group,” Dr. Pels said. “We can speculate on the cause, whether it was related to sildenafil and why, or whether it was simply chance. This is the reason we need to conduct more in-depth analyses of these data.”
Funding came from federal health agencies and universities in the countries where STRIDER was conducted, including New Zealand, Australia, the United Kingdom, Ireland, and the Netherlands. Dr. Pels had no relevant financial disclosures.
SOURCE: Pels A et al. The Pregnancy Meeting, Late-Breaker 2.
REPORTING FROM THE PREGNANCY MEETING
Key clinical point:
Major finding: There were 16 cases of persistent pulmonary hypertension in the treated group and four in the placebo group.
Study details: The randomized study involved 261 pregnant women treated with sildenafil for early-onset intrauterine growth restriction.
Disclosures: Funding agencies and universities in the countries involved in the trial contributed to funding. Dr. Pels had no relevant financial disclosures.
Source: Pels A et al. The Pregnancy Meeting, Late-Breaker 2.
When to “Undiagnose” Asthma

Two years ago, a now 45-year-old woman was diagnosed with asthma based on her history and physical exam findings; she was prescribed an inhaled corticosteroid and a bronchodilator rescue inhaler. She has had no exacerbations since. Should you consider weaning her off the inhalers?
Asthma is a prevalent problem; 8% of adults ages 18 to 64 have the chronic lung disease.2 Diagnosis can be challenging, partially because it requires measurement of transient airway resistance, and treatment entails significant costs and possible adverse effects. Without pulmonary function measurement or trials off medication, there is no clinical way to differentiate patients with well-controlled asthma from those who are being treated unnecessarily. Not surprisingly, studies have shown that ruling out active asthma and reducing medication use are cost effective.3,4 This study followed a cohort of patients to see how many could be weaned off their asthma medications.
STUDY SUMMARY
About one-third of adults with asthma are “undiagnosed” within 5 years
The researchers recruited participants from the general population of the 10 largest cities and surrounding areas in Canada by randomly dialing cellular and landline phone numbers and asking about adult household members with asthma.1 The researchers focused on those with a recent (<5 years) asthma diagnosis to represent contemporary diagnostic practice and make it easier to collect medical records. Participants lived within 90 minutes of 10 medical centers. Patients were excluded if they were using long-term oral steroids, were pregnant or breastfeeding, were unable to tolerate spirometry or methacholine challenges, or had a smoking history of >10 pack-years.
Of the 701 patients enrolled, 613 (87.4%) completed all study assessments. Patients progressed through a series of spirometry tests and were then tapered off their asthma-controlling medications.
The initial spirometry test confirmed asthma if bronchodilators caused a significant improvement in forced expiratory volume in one second (FEV1). Patients who showed no improvement took a methacholine challenge 1 week later; if they did well, their maintenance medications were reduced by half. About 1 month later, another methacholine challenge was
Asthma was confirmed at any methacholine challenge if there was a 20% decrease in FEV1 from baseline at a methacholine concentration of ≤8 mg/mL; these patients were restarted on appropriate medications. If current asthma was ruled out, follow-up bronchial challenges were repeated at 6 and 12 months.
Results. Among the patients with clinician-diagnosed asthma, 33.1% no longer met criteria for an asthma diagnosis. Of those who no longer had asthma, 44% had previously undergone objective testing of airflow limitation. Another 12 patients (2%) had other serious cardiorespiratory conditions instead of asthma (eg, ischemic heart disease, subglottic stenosis, and bronchiectasis).
Continue to: During the 1-year follow-up period...
During the 1-year follow-up period, 22 (10.8%) of the 203 patients who were initially judged to no longer have asthma had a positive bronchial challenge test; 16 had no symptoms and continued to do well without any asthma medications. Six (3%) presented with respiratory symptoms and resumed treatment with asthma medications, but only 1 (0.5%) required oral corticosteroid therapy.
WHAT’S NEW?
Asthma meds of no benefit for one-third of patients taking them
This study found that one-third of patients with asthma diagnosed in the past 5 years no longer had symptoms or spirometry results consistent with asthma and did well in the subsequent year. For those patients, asthma medications appear to have no benefit. The Global Institute for Asthma recommends stepping down treatment in adults with asthma that is well controlled for 3 months or more.5 Patients with objectively confirmed asthma diagnoses were more likely to still have asthma in this study—but more than 40% of patients who no longer had asthma had been objectively proven to have the disease at the time of diagnosis.
CAVEATS
High level of rigor; no randomized trial
This study used a very structured protocol for tapering patients off their medications, including multiple spirometry tests (most including methacholine challenges) and oversight by pulmonologists. It is unclear whether this level of rigor is necessary for weaning in other clinical settings.
Also, this study was not a randomized trial, which is the gold standard for withdrawal of therapy. However, a cohort study is adequate to assess diagnostic testing, and this could be considered a trial of “undiagnosing” asthma in adults. These results are consistent with those of another study of asthma disappearance in patients with and without obesity; in that study, about 30% of patients in either group no longer had a diagnosis of asthma.6
Using random dialing is likely to have broadened the pool of patients this study drew upon. Also, there is a possibility that the patients who were lost to follow-up in this study represented those who had worsening symptoms. Some patients with mild asthma may have a waxing and waning course; it is possible that the study period was not long enough to capture this. In this study, only
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
“Undiagnosis” is unusual
Using objective testing may provide some logistical or financial challenges for patients. Furthermore, “undiagnosing” a chronic disease like asthma is not a clinician’s typical work, and it may take some time and effort to educate and monitor patients throughout the process.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[11]:704,706-707).
1. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
2. QuickStats: percentage of adults aged 18-64 years with current asthma, by state—National Health Interview Survey, 2014-2016. MMWR Morb Mortal Wkly Rep. 2018; 67:590.
3. Pakhale S, Sumner A, Coyle D, et al. (Correcting) misdiagnoses of asthma: a cost effectiveness analysis. BMC Pulm Med. 2011;11:27.
4. Rank MA, Liesinger JT, Branda ME, et al. Comparative safety and costs of stepping down asthma medications in patients with controlled asthma. J Allergy Clin Immunol. 2016;137:1373-1379.
5. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. https://ginasthma.org/gina-reports. Accessed February 6, 2019.
6. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.

Two years ago, a now 45-year-old woman was diagnosed with asthma based on her history and physical exam findings; she was prescribed an inhaled corticosteroid and a bronchodilator rescue inhaler. She has had no exacerbations since. Should you consider weaning her off the inhalers?
Asthma is a prevalent problem; 8% of adults ages 18 to 64 have the chronic lung disease.2 Diagnosis can be challenging, partially because it requires measurement of transient airway resistance, and treatment entails significant costs and possible adverse effects. Without pulmonary function measurement or trials off medication, there is no clinical way to differentiate patients with well-controlled asthma from those who are being treated unnecessarily. Not surprisingly, studies have shown that ruling out active asthma and reducing medication use are cost effective.3,4 This study followed a cohort of patients to see how many could be weaned off their asthma medications.
STUDY SUMMARY
About one-third of adults with asthma are “undiagnosed” within 5 years
The researchers recruited participants from the general population of the 10 largest cities and surrounding areas in Canada by randomly dialing cellular and landline phone numbers and asking about adult household members with asthma.1 The researchers focused on those with a recent (<5 years) asthma diagnosis to represent contemporary diagnostic practice and make it easier to collect medical records. Participants lived within 90 minutes of 10 medical centers. Patients were excluded if they were using long-term oral steroids, were pregnant or breastfeeding, were unable to tolerate spirometry or methacholine challenges, or had a smoking history of >10 pack-years.
Of the 701 patients enrolled, 613 (87.4%) completed all study assessments. Patients progressed through a series of spirometry tests and were then tapered off their asthma-controlling medications.
The initial spirometry test confirmed asthma if bronchodilators caused a significant improvement in forced expiratory volume in one second (FEV1). Patients who showed no improvement took a methacholine challenge 1 week later; if they did well, their maintenance medications were reduced by half. About 1 month later, another methacholine challenge was
Asthma was confirmed at any methacholine challenge if there was a 20% decrease in FEV1 from baseline at a methacholine concentration of ≤8 mg/mL; these patients were restarted on appropriate medications. If current asthma was ruled out, follow-up bronchial challenges were repeated at 6 and 12 months.
Results. Among the patients with clinician-diagnosed asthma, 33.1% no longer met criteria for an asthma diagnosis. Of those who no longer had asthma, 44% had previously undergone objective testing of airflow limitation. Another 12 patients (2%) had other serious cardiorespiratory conditions instead of asthma (eg, ischemic heart disease, subglottic stenosis, and bronchiectasis).
Continue to: During the 1-year follow-up period...
During the 1-year follow-up period, 22 (10.8%) of the 203 patients who were initially judged to no longer have asthma had a positive bronchial challenge test; 16 had no symptoms and continued to do well without any asthma medications. Six (3%) presented with respiratory symptoms and resumed treatment with asthma medications, but only 1 (0.5%) required oral corticosteroid therapy.
WHAT’S NEW?
Asthma meds of no benefit for one-third of patients taking them
This study found that one-third of patients with asthma diagnosed in the past 5 years no longer had symptoms or spirometry results consistent with asthma and did well in the subsequent year. For those patients, asthma medications appear to have no benefit. The Global Institute for Asthma recommends stepping down treatment in adults with asthma that is well controlled for 3 months or more.5 Patients with objectively confirmed asthma diagnoses were more likely to still have asthma in this study—but more than 40% of patients who no longer had asthma had been objectively proven to have the disease at the time of diagnosis.
CAVEATS
High level of rigor; no randomized trial
This study used a very structured protocol for tapering patients off their medications, including multiple spirometry tests (most including methacholine challenges) and oversight by pulmonologists. It is unclear whether this level of rigor is necessary for weaning in other clinical settings.
Also, this study was not a randomized trial, which is the gold standard for withdrawal of therapy. However, a cohort study is adequate to assess diagnostic testing, and this could be considered a trial of “undiagnosing” asthma in adults. These results are consistent with those of another study of asthma disappearance in patients with and without obesity; in that study, about 30% of patients in either group no longer had a diagnosis of asthma.6
Using random dialing is likely to have broadened the pool of patients this study drew upon. Also, there is a possibility that the patients who were lost to follow-up in this study represented those who had worsening symptoms. Some patients with mild asthma may have a waxing and waning course; it is possible that the study period was not long enough to capture this. In this study, only
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
“Undiagnosis” is unusual
Using objective testing may provide some logistical or financial challenges for patients. Furthermore, “undiagnosing” a chronic disease like asthma is not a clinician’s typical work, and it may take some time and effort to educate and monitor patients throughout the process.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[11]:704,706-707).

Two years ago, a now 45-year-old woman was diagnosed with asthma based on her history and physical exam findings; she was prescribed an inhaled corticosteroid and a bronchodilator rescue inhaler. She has had no exacerbations since. Should you consider weaning her off the inhalers?
Asthma is a prevalent problem; 8% of adults ages 18 to 64 have the chronic lung disease.2 Diagnosis can be challenging, partially because it requires measurement of transient airway resistance, and treatment entails significant costs and possible adverse effects. Without pulmonary function measurement or trials off medication, there is no clinical way to differentiate patients with well-controlled asthma from those who are being treated unnecessarily. Not surprisingly, studies have shown that ruling out active asthma and reducing medication use are cost effective.3,4 This study followed a cohort of patients to see how many could be weaned off their asthma medications.
STUDY SUMMARY
About one-third of adults with asthma are “undiagnosed” within 5 years
The researchers recruited participants from the general population of the 10 largest cities and surrounding areas in Canada by randomly dialing cellular and landline phone numbers and asking about adult household members with asthma.1 The researchers focused on those with a recent (<5 years) asthma diagnosis to represent contemporary diagnostic practice and make it easier to collect medical records. Participants lived within 90 minutes of 10 medical centers. Patients were excluded if they were using long-term oral steroids, were pregnant or breastfeeding, were unable to tolerate spirometry or methacholine challenges, or had a smoking history of >10 pack-years.
Of the 701 patients enrolled, 613 (87.4%) completed all study assessments. Patients progressed through a series of spirometry tests and were then tapered off their asthma-controlling medications.
The initial spirometry test confirmed asthma if bronchodilators caused a significant improvement in forced expiratory volume in one second (FEV1). Patients who showed no improvement took a methacholine challenge 1 week later; if they did well, their maintenance medications were reduced by half. About 1 month later, another methacholine challenge was
Asthma was confirmed at any methacholine challenge if there was a 20% decrease in FEV1 from baseline at a methacholine concentration of ≤8 mg/mL; these patients were restarted on appropriate medications. If current asthma was ruled out, follow-up bronchial challenges were repeated at 6 and 12 months.
Results. Among the patients with clinician-diagnosed asthma, 33.1% no longer met criteria for an asthma diagnosis. Of those who no longer had asthma, 44% had previously undergone objective testing of airflow limitation. Another 12 patients (2%) had other serious cardiorespiratory conditions instead of asthma (eg, ischemic heart disease, subglottic stenosis, and bronchiectasis).
Continue to: During the 1-year follow-up period...
During the 1-year follow-up period, 22 (10.8%) of the 203 patients who were initially judged to no longer have asthma had a positive bronchial challenge test; 16 had no symptoms and continued to do well without any asthma medications. Six (3%) presented with respiratory symptoms and resumed treatment with asthma medications, but only 1 (0.5%) required oral corticosteroid therapy.
WHAT’S NEW?
Asthma meds of no benefit for one-third of patients taking them
This study found that one-third of patients with asthma diagnosed in the past 5 years no longer had symptoms or spirometry results consistent with asthma and did well in the subsequent year. For those patients, asthma medications appear to have no benefit. The Global Institute for Asthma recommends stepping down treatment in adults with asthma that is well controlled for 3 months or more.5 Patients with objectively confirmed asthma diagnoses were more likely to still have asthma in this study—but more than 40% of patients who no longer had asthma had been objectively proven to have the disease at the time of diagnosis.
CAVEATS
High level of rigor; no randomized trial
This study used a very structured protocol for tapering patients off their medications, including multiple spirometry tests (most including methacholine challenges) and oversight by pulmonologists. It is unclear whether this level of rigor is necessary for weaning in other clinical settings.
Also, this study was not a randomized trial, which is the gold standard for withdrawal of therapy. However, a cohort study is adequate to assess diagnostic testing, and this could be considered a trial of “undiagnosing” asthma in adults. These results are consistent with those of another study of asthma disappearance in patients with and without obesity; in that study, about 30% of patients in either group no longer had a diagnosis of asthma.6
Using random dialing is likely to have broadened the pool of patients this study drew upon. Also, there is a possibility that the patients who were lost to follow-up in this study represented those who had worsening symptoms. Some patients with mild asthma may have a waxing and waning course; it is possible that the study period was not long enough to capture this. In this study, only
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
“Undiagnosis” is unusual
Using objective testing may provide some logistical or financial challenges for patients. Furthermore, “undiagnosing” a chronic disease like asthma is not a clinician’s typical work, and it may take some time and effort to educate and monitor patients throughout the process.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[11]:704,706-707).
1. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
2. QuickStats: percentage of adults aged 18-64 years with current asthma, by state—National Health Interview Survey, 2014-2016. MMWR Morb Mortal Wkly Rep. 2018; 67:590.
3. Pakhale S, Sumner A, Coyle D, et al. (Correcting) misdiagnoses of asthma: a cost effectiveness analysis. BMC Pulm Med. 2011;11:27.
4. Rank MA, Liesinger JT, Branda ME, et al. Comparative safety and costs of stepping down asthma medications in patients with controlled asthma. J Allergy Clin Immunol. 2016;137:1373-1379.
5. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. https://ginasthma.org/gina-reports. Accessed February 6, 2019.
6. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
1. Aaron SD, Vandemheen KL, FitzGerald JM, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269-279.
2. QuickStats: percentage of adults aged 18-64 years with current asthma, by state—National Health Interview Survey, 2014-2016. MMWR Morb Mortal Wkly Rep. 2018; 67:590.
3. Pakhale S, Sumner A, Coyle D, et al. (Correcting) misdiagnoses of asthma: a cost effectiveness analysis. BMC Pulm Med. 2011;11:27.
4. Rank MA, Liesinger JT, Branda ME, et al. Comparative safety and costs of stepping down asthma medications in patients with controlled asthma. J Allergy Clin Immunol. 2016;137:1373-1379.
5. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2018. https://ginasthma.org/gina-reports. Accessed February 6, 2019.
6. Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179:1121-1131.
Tropical travelers’ top dermatologic infestations
WAIKOLOA, HAWAII – The Caribbean islands and Central and South America are among the most popular travel destinations for Americans. And some of these visitors will come home harboring unwelcome guests: Infestations that will eventually bring them to a dermatologist’s attention.
“I always tell the residents that if a patient’s country of travel starts with a B – Barbados, Belize, Bolivia, Brazil – it’s going to be something fun,” Natasha A. Mesinkovska, MD, PhD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
According to surveillance conducted by the Centers for Disease Control and Prevention and the International Society for Travel Medicine,
Cutaneous larva migrans is the easiest to diagnosis because it’s a creeping eruption that often migrates at a rate of 1-2 cm per day. Patients with the other disorders often present with a complaint of a common skin condition – described as a pimple, a wart, a patch of sunburn – that just doesn’t go away, according to Dr. Mesinkovska, director of clinical research in the department of dermatology at the University of California, Irvine.
Tungiasis
Tungiasis is caused by the female sand flea, Tunga penetrans, which burrows into the skin, where it lays hundreds of eggs within a matter of a few days. The sand flea is harbored by dogs, cats, pigs, cows, and rats. It’s rare to encounter tungiasis in travelers who’ve spent their time in fancy resorts, ecolodges, or yoga retreats, even if they’ve been parading around with lots of exposed skin. This is a disease of impoverished neighborhoods; hence, affected Americans often have been doing mission work abroad. In tropical areas, tungiasis is a debilitating, mutilating disorder marked by repeated infections, persistent inflammation, fissures, and ulcers.
Treatment involves a topical antiparasitic agent such as ivermectin, metrifonate, or thiabendazole and removal of the flea with sterile forceps or needles. But there is a promising new treatment concept: topical dimethicone, or polydimethylsiloxane. Studies have shown that following application of dimethicone, roughly 80%-90% of sand fleas are dead within 7 days.
“It’s nontoxic and has a purely physical mechanism of action, so resistance is unlikely ... I think it’s going to change the way this condition gets controlled,” Dr. Mesinkovska said.
Myiasis
The differential diagnosis of myiasis includes impetigo, a furuncle, an infected cyst, or a retained foreign body. Myiasis is a cutaneous infestation of the larva of certain flies, among the most notorious of which are the botfly, blowfly, and screwfly. The female fly lays her eggs in hot, humid, shady areas in soil contaminated by feces or urine. The larva can invade unbroken skin instantaneously and painlessly. Then it begins burrowing in. An air hole is always present in the skin so the organism can breathe. Ophthalmomyiasis is common, as are nasal and aural infections, the latter often accompanied by complaints of a crawling sensation inside the ear along with a buzzing noise. To avoid infection, in endemic areas it’s important not to go barefoot or to dry clothes on bushes or on the ground. Treatment entails elimination of the larva. Covering the air hole with petroleum jelly will force it to the surface. There is just one larva per furuncle, so no need for further extensive exploration once that critter has been extracted.
Leishmaniasis
The vector for this protozoan infection is the sandfly, which feeds from dusk to dawn noiselessly and painlessly. Because cutaneous and mucocutaneous leishmaniasis are understudied orphan diseases for which current treatments are less than satisfactory, prevention is the watchword. In endemic areas it’s important to close the windows and make use of air conditioning and ceiling fans when available. When in doubt, it’s advisable to sleep using a bed net treated with permethrin.
Cutaneous larva migrans
This skin eruption is caused by parasitic hookworms, the most common of which in the Americas is Ancylostoma braziliense. The eggs are transmitted through dog and cat feces deposited on soil or sand.
“Avoid laying or sitting on dry sand, even on a towel. And wear shoes,” Dr. Mesinkovska advised.
Among the CDC’s treatment recommendations for cutaneous larva migrans are several agents with poor efficacy and/or considerable side effects. But there is one standout therapy.
“Really, I would say nowadays the easiest thing is one 12-mg oral dose of ivermectin. It’s almost 100% effective,” she said.
Dr. Mesinkovska reported having no financial interests relevant to her talk.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The Caribbean islands and Central and South America are among the most popular travel destinations for Americans. And some of these visitors will come home harboring unwelcome guests: Infestations that will eventually bring them to a dermatologist’s attention.
“I always tell the residents that if a patient’s country of travel starts with a B – Barbados, Belize, Bolivia, Brazil – it’s going to be something fun,” Natasha A. Mesinkovska, MD, PhD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
According to surveillance conducted by the Centers for Disease Control and Prevention and the International Society for Travel Medicine,
Cutaneous larva migrans is the easiest to diagnosis because it’s a creeping eruption that often migrates at a rate of 1-2 cm per day. Patients with the other disorders often present with a complaint of a common skin condition – described as a pimple, a wart, a patch of sunburn – that just doesn’t go away, according to Dr. Mesinkovska, director of clinical research in the department of dermatology at the University of California, Irvine.
Tungiasis
Tungiasis is caused by the female sand flea, Tunga penetrans, which burrows into the skin, where it lays hundreds of eggs within a matter of a few days. The sand flea is harbored by dogs, cats, pigs, cows, and rats. It’s rare to encounter tungiasis in travelers who’ve spent their time in fancy resorts, ecolodges, or yoga retreats, even if they’ve been parading around with lots of exposed skin. This is a disease of impoverished neighborhoods; hence, affected Americans often have been doing mission work abroad. In tropical areas, tungiasis is a debilitating, mutilating disorder marked by repeated infections, persistent inflammation, fissures, and ulcers.
Treatment involves a topical antiparasitic agent such as ivermectin, metrifonate, or thiabendazole and removal of the flea with sterile forceps or needles. But there is a promising new treatment concept: topical dimethicone, or polydimethylsiloxane. Studies have shown that following application of dimethicone, roughly 80%-90% of sand fleas are dead within 7 days.
“It’s nontoxic and has a purely physical mechanism of action, so resistance is unlikely ... I think it’s going to change the way this condition gets controlled,” Dr. Mesinkovska said.
Myiasis
The differential diagnosis of myiasis includes impetigo, a furuncle, an infected cyst, or a retained foreign body. Myiasis is a cutaneous infestation of the larva of certain flies, among the most notorious of which are the botfly, blowfly, and screwfly. The female fly lays her eggs in hot, humid, shady areas in soil contaminated by feces or urine. The larva can invade unbroken skin instantaneously and painlessly. Then it begins burrowing in. An air hole is always present in the skin so the organism can breathe. Ophthalmomyiasis is common, as are nasal and aural infections, the latter often accompanied by complaints of a crawling sensation inside the ear along with a buzzing noise. To avoid infection, in endemic areas it’s important not to go barefoot or to dry clothes on bushes or on the ground. Treatment entails elimination of the larva. Covering the air hole with petroleum jelly will force it to the surface. There is just one larva per furuncle, so no need for further extensive exploration once that critter has been extracted.
Leishmaniasis
The vector for this protozoan infection is the sandfly, which feeds from dusk to dawn noiselessly and painlessly. Because cutaneous and mucocutaneous leishmaniasis are understudied orphan diseases for which current treatments are less than satisfactory, prevention is the watchword. In endemic areas it’s important to close the windows and make use of air conditioning and ceiling fans when available. When in doubt, it’s advisable to sleep using a bed net treated with permethrin.
Cutaneous larva migrans

This skin eruption is caused by parasitic hookworms, the most common of which in the Americas is Ancylostoma braziliense. The eggs are transmitted through dog and cat feces deposited on soil or sand.
“Avoid laying or sitting on dry sand, even on a towel. And wear shoes,” Dr. Mesinkovska advised.
Among the CDC’s treatment recommendations for cutaneous larva migrans are several agents with poor efficacy and/or considerable side effects. But there is one standout therapy.
“Really, I would say nowadays the easiest thing is one 12-mg oral dose of ivermectin. It’s almost 100% effective,” she said.
Dr. Mesinkovska reported having no financial interests relevant to her talk.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The Caribbean islands and Central and South America are among the most popular travel destinations for Americans. And some of these visitors will come home harboring unwelcome guests: Infestations that will eventually bring them to a dermatologist’s attention.
“I always tell the residents that if a patient’s country of travel starts with a B – Barbados, Belize, Bolivia, Brazil – it’s going to be something fun,” Natasha A. Mesinkovska, MD, PhD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
According to surveillance conducted by the Centers for Disease Control and Prevention and the International Society for Travel Medicine,
Cutaneous larva migrans is the easiest to diagnosis because it’s a creeping eruption that often migrates at a rate of 1-2 cm per day. Patients with the other disorders often present with a complaint of a common skin condition – described as a pimple, a wart, a patch of sunburn – that just doesn’t go away, according to Dr. Mesinkovska, director of clinical research in the department of dermatology at the University of California, Irvine.
Tungiasis
Tungiasis is caused by the female sand flea, Tunga penetrans, which burrows into the skin, where it lays hundreds of eggs within a matter of a few days. The sand flea is harbored by dogs, cats, pigs, cows, and rats. It’s rare to encounter tungiasis in travelers who’ve spent their time in fancy resorts, ecolodges, or yoga retreats, even if they’ve been parading around with lots of exposed skin. This is a disease of impoverished neighborhoods; hence, affected Americans often have been doing mission work abroad. In tropical areas, tungiasis is a debilitating, mutilating disorder marked by repeated infections, persistent inflammation, fissures, and ulcers.
Treatment involves a topical antiparasitic agent such as ivermectin, metrifonate, or thiabendazole and removal of the flea with sterile forceps or needles. But there is a promising new treatment concept: topical dimethicone, or polydimethylsiloxane. Studies have shown that following application of dimethicone, roughly 80%-90% of sand fleas are dead within 7 days.
“It’s nontoxic and has a purely physical mechanism of action, so resistance is unlikely ... I think it’s going to change the way this condition gets controlled,” Dr. Mesinkovska said.
Myiasis
The differential diagnosis of myiasis includes impetigo, a furuncle, an infected cyst, or a retained foreign body. Myiasis is a cutaneous infestation of the larva of certain flies, among the most notorious of which are the botfly, blowfly, and screwfly. The female fly lays her eggs in hot, humid, shady areas in soil contaminated by feces or urine. The larva can invade unbroken skin instantaneously and painlessly. Then it begins burrowing in. An air hole is always present in the skin so the organism can breathe. Ophthalmomyiasis is common, as are nasal and aural infections, the latter often accompanied by complaints of a crawling sensation inside the ear along with a buzzing noise. To avoid infection, in endemic areas it’s important not to go barefoot or to dry clothes on bushes or on the ground. Treatment entails elimination of the larva. Covering the air hole with petroleum jelly will force it to the surface. There is just one larva per furuncle, so no need for further extensive exploration once that critter has been extracted.
Leishmaniasis
The vector for this protozoan infection is the sandfly, which feeds from dusk to dawn noiselessly and painlessly. Because cutaneous and mucocutaneous leishmaniasis are understudied orphan diseases for which current treatments are less than satisfactory, prevention is the watchword. In endemic areas it’s important to close the windows and make use of air conditioning and ceiling fans when available. When in doubt, it’s advisable to sleep using a bed net treated with permethrin.
Cutaneous larva migrans

This skin eruption is caused by parasitic hookworms, the most common of which in the Americas is Ancylostoma braziliense. The eggs are transmitted through dog and cat feces deposited on soil or sand.
“Avoid laying or sitting on dry sand, even on a towel. And wear shoes,” Dr. Mesinkovska advised.
Among the CDC’s treatment recommendations for cutaneous larva migrans are several agents with poor efficacy and/or considerable side effects. But there is one standout therapy.
“Really, I would say nowadays the easiest thing is one 12-mg oral dose of ivermectin. It’s almost 100% effective,” she said.
Dr. Mesinkovska reported having no financial interests relevant to her talk.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM THE SDEF HAWAII DERMATOLOGY SEMINAR