User login
Avelumab active in recurrent or refractory ovarian cancer
Avelumab had antitumor activity and acceptable safety in heavily pretreated ovarian cancer patients enrolled in a large phase 1b trial, investigators have reported.
Treatment with the anti–programmed death-ligand 1 (anti–PD-L1) agent yielded an overall response rate of 9.6%, with a median duration of response exceeding 10 months and median overall survival greater than 11 months, according to investigators in the JAVELIN Solid Tumor trial.
There was no association between PD-L1 or BRCA status and response, which is a novel finding, investigators wrote in JAMA Oncology.
“Very few patients had tumors with high-level PD-L1 expression, which is associated with an increased probability of clinical benefit with anti–PD-1 or anti–PD-L1 treatment of non–small cell lung cancer,” said the investigators, led by Mary L. Disis, MD, of the Cancer Vaccine Institute at the University of Washington, Seattle.
The phase 1b, global, open-label study included 125 women with stage III or IV epithelial ovarian, fallopian tube, or peritoneal cancer who had recurrent or refractory disease and had received a median of three previous treatments for locally advanced or metastatic disease.
All patients received avelumab 10 mg/kg in a 1-hour intravenous infusion every 2 weeks until disease progression, unacceptable toxicity, or other protocol-defined criteria for study withdrawal.
Confirmed objective responses were seen in 12 patients, or 9.6%, including one complete response and 11 partial responses, the investigators reported. Another 53 patients, or 42.4%, had stable disease as their best response, for a combined disease control rate of 52.0%.
Median progression-free survival was 2.6 months, and median overall survival was 11.2 months, with a 12-month overall survival rate of 47.0%, investigators said.
Responses occurred irrespective of PD-L1 expression status, according to investigators, with no discernible trends in response looking at different PD-L1 expression cutoffs for tumor cells.
For example, at a PD-L1 expression cutoff of 5% or more, patients with positive tumors had an overall response rate of 12.5%, median progression-free survival of 2.7 months, and median overall survival of 10.6 months, they reported, while among negative patients, overall response rate was 9.8%, and median progression-free and overall survival were 2.2 and 11.9 months, respectively.
Treatment-related adverse events occurred in 86 patients, or 68.8%, of which infusion-related reactions and related symptoms were the most common, occurring in 25 patients (20%), investigators wrote. Immune-related adverse events were seen in 16.8% of patients, including three patients (2.4%) with grade 3, and zero with grade 4 or 5.
These findings track with results of other checkpoint inhibitor monotherapy trials in advanced, previously treated ovarian cancer, including smaller studies of pembrolizumab and nivolumab with overall response rates of 11.5% and 15.0%, respectively, according to investigators.
“Although response and survival findings with avelumab monotherapy in this study are encouraging, it would be of interest to determine whether efficacy can be increased through combination or sequential regimens involving chemotherapy or PARP [poly ADP-ribose polymerase] inhibitors,” said the investigators.
Combination studies are underway in women with ovarian cancer, including two global phase 3 trials evaluating avelumab plus chemotherapy in the first-line setting and in patients with platinum-resistant or platinum-refractory disease.
“Results from these ongoing studies will help to define an appropriate role for checkpoint inhibitors within the treatment of ovarian cancer,” study authors concluded.
The JAVELIN trial is sponsored by Merck KGaA as part of an arrangement between the company and Pfizer. Dr. Disis reported disclosures related to Celgene, EMD Serono, Epithany, Janssen, Pfizer, and Seattle Genetics. Coauthors reported disclosures with Merck, Blueprint, Bristol-Myers Squibb, Eisai, Loxo, Novartis, and others.
SOURCE: Disis ML et al. JAMA Oncol. 2019 Jan 24. doi: 10.1001/jamaoncol.2018.6258.
Avelumab had antitumor activity and acceptable safety in heavily pretreated ovarian cancer patients enrolled in a large phase 1b trial, investigators have reported.
Treatment with the anti–programmed death-ligand 1 (anti–PD-L1) agent yielded an overall response rate of 9.6%, with a median duration of response exceeding 10 months and median overall survival greater than 11 months, according to investigators in the JAVELIN Solid Tumor trial.
There was no association between PD-L1 or BRCA status and response, which is a novel finding, investigators wrote in JAMA Oncology.
“Very few patients had tumors with high-level PD-L1 expression, which is associated with an increased probability of clinical benefit with anti–PD-1 or anti–PD-L1 treatment of non–small cell lung cancer,” said the investigators, led by Mary L. Disis, MD, of the Cancer Vaccine Institute at the University of Washington, Seattle.
The phase 1b, global, open-label study included 125 women with stage III or IV epithelial ovarian, fallopian tube, or peritoneal cancer who had recurrent or refractory disease and had received a median of three previous treatments for locally advanced or metastatic disease.
All patients received avelumab 10 mg/kg in a 1-hour intravenous infusion every 2 weeks until disease progression, unacceptable toxicity, or other protocol-defined criteria for study withdrawal.
Confirmed objective responses were seen in 12 patients, or 9.6%, including one complete response and 11 partial responses, the investigators reported. Another 53 patients, or 42.4%, had stable disease as their best response, for a combined disease control rate of 52.0%.
Median progression-free survival was 2.6 months, and median overall survival was 11.2 months, with a 12-month overall survival rate of 47.0%, investigators said.
Responses occurred irrespective of PD-L1 expression status, according to investigators, with no discernible trends in response looking at different PD-L1 expression cutoffs for tumor cells.
For example, at a PD-L1 expression cutoff of 5% or more, patients with positive tumors had an overall response rate of 12.5%, median progression-free survival of 2.7 months, and median overall survival of 10.6 months, they reported, while among negative patients, overall response rate was 9.8%, and median progression-free and overall survival were 2.2 and 11.9 months, respectively.
Treatment-related adverse events occurred in 86 patients, or 68.8%, of which infusion-related reactions and related symptoms were the most common, occurring in 25 patients (20%), investigators wrote. Immune-related adverse events were seen in 16.8% of patients, including three patients (2.4%) with grade 3, and zero with grade 4 or 5.
These findings track with results of other checkpoint inhibitor monotherapy trials in advanced, previously treated ovarian cancer, including smaller studies of pembrolizumab and nivolumab with overall response rates of 11.5% and 15.0%, respectively, according to investigators.
“Although response and survival findings with avelumab monotherapy in this study are encouraging, it would be of interest to determine whether efficacy can be increased through combination or sequential regimens involving chemotherapy or PARP [poly ADP-ribose polymerase] inhibitors,” said the investigators.
Combination studies are underway in women with ovarian cancer, including two global phase 3 trials evaluating avelumab plus chemotherapy in the first-line setting and in patients with platinum-resistant or platinum-refractory disease.
“Results from these ongoing studies will help to define an appropriate role for checkpoint inhibitors within the treatment of ovarian cancer,” study authors concluded.
The JAVELIN trial is sponsored by Merck KGaA as part of an arrangement between the company and Pfizer. Dr. Disis reported disclosures related to Celgene, EMD Serono, Epithany, Janssen, Pfizer, and Seattle Genetics. Coauthors reported disclosures with Merck, Blueprint, Bristol-Myers Squibb, Eisai, Loxo, Novartis, and others.
SOURCE: Disis ML et al. JAMA Oncol. 2019 Jan 24. doi: 10.1001/jamaoncol.2018.6258.
Avelumab had antitumor activity and acceptable safety in heavily pretreated ovarian cancer patients enrolled in a large phase 1b trial, investigators have reported.
Treatment with the anti–programmed death-ligand 1 (anti–PD-L1) agent yielded an overall response rate of 9.6%, with a median duration of response exceeding 10 months and median overall survival greater than 11 months, according to investigators in the JAVELIN Solid Tumor trial.
There was no association between PD-L1 or BRCA status and response, which is a novel finding, investigators wrote in JAMA Oncology.
“Very few patients had tumors with high-level PD-L1 expression, which is associated with an increased probability of clinical benefit with anti–PD-1 or anti–PD-L1 treatment of non–small cell lung cancer,” said the investigators, led by Mary L. Disis, MD, of the Cancer Vaccine Institute at the University of Washington, Seattle.
The phase 1b, global, open-label study included 125 women with stage III or IV epithelial ovarian, fallopian tube, or peritoneal cancer who had recurrent or refractory disease and had received a median of three previous treatments for locally advanced or metastatic disease.
All patients received avelumab 10 mg/kg in a 1-hour intravenous infusion every 2 weeks until disease progression, unacceptable toxicity, or other protocol-defined criteria for study withdrawal.
Confirmed objective responses were seen in 12 patients, or 9.6%, including one complete response and 11 partial responses, the investigators reported. Another 53 patients, or 42.4%, had stable disease as their best response, for a combined disease control rate of 52.0%.
Median progression-free survival was 2.6 months, and median overall survival was 11.2 months, with a 12-month overall survival rate of 47.0%, investigators said.
Responses occurred irrespective of PD-L1 expression status, according to investigators, with no discernible trends in response looking at different PD-L1 expression cutoffs for tumor cells.
For example, at a PD-L1 expression cutoff of 5% or more, patients with positive tumors had an overall response rate of 12.5%, median progression-free survival of 2.7 months, and median overall survival of 10.6 months, they reported, while among negative patients, overall response rate was 9.8%, and median progression-free and overall survival were 2.2 and 11.9 months, respectively.
Treatment-related adverse events occurred in 86 patients, or 68.8%, of which infusion-related reactions and related symptoms were the most common, occurring in 25 patients (20%), investigators wrote. Immune-related adverse events were seen in 16.8% of patients, including three patients (2.4%) with grade 3, and zero with grade 4 or 5.
These findings track with results of other checkpoint inhibitor monotherapy trials in advanced, previously treated ovarian cancer, including smaller studies of pembrolizumab and nivolumab with overall response rates of 11.5% and 15.0%, respectively, according to investigators.
“Although response and survival findings with avelumab monotherapy in this study are encouraging, it would be of interest to determine whether efficacy can be increased through combination or sequential regimens involving chemotherapy or PARP [poly ADP-ribose polymerase] inhibitors,” said the investigators.
Combination studies are underway in women with ovarian cancer, including two global phase 3 trials evaluating avelumab plus chemotherapy in the first-line setting and in patients with platinum-resistant or platinum-refractory disease.
“Results from these ongoing studies will help to define an appropriate role for checkpoint inhibitors within the treatment of ovarian cancer,” study authors concluded.
The JAVELIN trial is sponsored by Merck KGaA as part of an arrangement between the company and Pfizer. Dr. Disis reported disclosures related to Celgene, EMD Serono, Epithany, Janssen, Pfizer, and Seattle Genetics. Coauthors reported disclosures with Merck, Blueprint, Bristol-Myers Squibb, Eisai, Loxo, Novartis, and others.
SOURCE: Disis ML et al. JAMA Oncol. 2019 Jan 24. doi: 10.1001/jamaoncol.2018.6258.
FROM JAMA ONCOLOGY
Key clinical point: Single-agent treatment with the anti–programmed death-ligand 1 (anti–PD-L1) agent avelumab had antitumor activity and acceptable safety in heavily pretreated ovarian cancer patients.
Major finding: Treatment yielded an overall response rate of 9.6%, with a median duration of response exceeding 10 months and median overall survival greater than 11 months.
Study details: Phase 1b results from the JAVELIN solid tumor trial, which included 125 women with recurrent or refractory ovarian cancer.
Disclosures: The JAVELIN trial is sponsored by Merck KGaA as part of an arrangement between the company and Pfizer. Study authors reported disclosures related to Pfizer, Merck, Celgene, EMD Serono, Epithany, Janssen, and Seattle Genetics, among others.
Source: Disis ML et al. JAMA Oncol. 2019 Jan 24. doi: 10.1001/jamaoncol.2018.6258.
Buprenorphine for NAS shows promise in reducing length of stay
In what is believed to be the first study of its kind to compare all available pharmacologic treatment options for relief of symptoms associated with neonatal abstinence syndrome (NAS), buprenorphine has the greatest probability of reducing duration of treatment and length of stay among newborns, reported Timothy Disher, PhD, of Dalhousie University School of Nursing, Halifax, N.S., and his associates.

It was noteworthy that the study also found morphine and phenobarbital monotherapies to be worst in overall effectiveness and ranking because these pharmacotherapies are the most frequently used treatments in the United States, according to the authors. Dr. Disher and his associates underscored the need for concern over the common rationale of treatment centers, especially in using phenobarbital, since the American Academy of Pediatrics “highlights that phenobarbital is most commonly used only as adjuvant therapy” and was not intended as a first-line treatment.
In their efforts to identify treatments that are most effective at easing the symptoms of NAS, Dr. Disher and his colleagues conducted a systematic review and network meta-analysis in June 2018, which included a search of the Cochrane Central Register of Controlled Trials, Ovid MEDLINE, Embase, and the Web of Science Core Collection. In addition, they referenced ClinicalTrials.gov to identify relevant ongoing trials. Studies ultimately included in the review were randomized clinical trials comparing at least two pharmacotherapies prescribed for NAS that had been published in peer-reviewed journals.
Eighteen studies examining treatment for NAS among 1,072 newborns, including 10 studies published since 2000, were identified; the remaining studies were published between 1977 and 1986. Altogether, eight treatment interventions were examined across 10 studies
Dr. Disher and his associates reported that, during 2004-2014, there was a fivefold increase in the number of babies presenting with NAS, from 1.5/1,000 live births to 8.0/1,000, which represented a sevenfold increase in treatment cost in the Medicaid population during the same period, from $65.4 million to $462 million.
Although Dr. Disher and his colleagues acknowledged that buprenorphine was identified as best treatment by median ranks, “the ranks for most treatments are imprecise,” they said. According to results of their analysis, buprenorphine was associated with a reduction in 2.19 days of treatment, compared with clonidine, and 12.75 days, compared with morphine. In terms of secondary outcomes, buprenorphine was associated with a reduction in length of stay of 5.35 days, compared with clonidine, and 11.43 days, compared with morphine.
Seven of the studies evaluated (n = 394) included infants requiring adjuvant treatment. Agthe et al. reported that no infants in the concomitant diluted tincture of opium (DTO) and clonidine arm needed adjuvant treatment compared with five infants in the DTO-only arm who did. Surran et al. reported 2 of 32 infants who failed attempts to wean in the concomitant morphine and clonidine group compared with none of the 34 who were in the morphine and phenobarbital group.
In terms of adverse events, one study reported a seizure that was unrelated to treatment (Kraft et al). Agthe et al. reported three infants experiencing seizure in the DTO-only group compared with no infants who received concomitant clonidine. In Surran et al., three infants receiving concomitant phenobarbital and morphine were reported to be oversedated.
In general, the rationale explaining differences in why pharmacologic therapies affect treatment length is underdeveloped, the authors said. Buprenorphine, in particular, is favored because of its ease of dosing schedule and the possible improved safety profile given its longer half-life and greater micro-opioid receptor activity. It has been further suggested that the prolonged half-life of buprenorphine may be responsible for preventing sudden withdrawal symptoms. The researchers found no significant adverse events associated with buprenorphine treatment.
Although there were differences across buprenorphine treatment protocols, Dr. Disher and his colleagues noted that they were “broadly similar.” The authors conceded, however, that there is reason to question “how much of the observed improvement in buprenorphine may be attributable to the differences in optimization of the treatment and weaning protocols.”
Based on findings in this review, the authors caution that it is unlikely “that the current evidence base is sufficient to recommend specific large-scale changes in treatment away from the current standard of care.”
Despite recent research, which proposes trying nonpharmacologic treatments first and incorporating shared rooms for families and infants to reduce length of stay when treatment is required, up to 70% of infants ultimately require pharmacologic treatment. When drug therapy is needed, the average length of stay and overall treatment costs double, 10.9 vs. 22 days and $20,708 vs. $44,720, respectively.
Since results of the analysis show benefit, however variable, in reducing the length of treatment, “continued efforts to identify the optimal pharmacological agents are justified,” urged Dr. Disher and his associates.
Ultimately, before buprenorphine can be considered as a universally accepted standard of care in the treatment of NAS, “a large multisite pragmatic trial that compares buprenorphine with other treatments” will be needed.
One of the researchers – Chris Cameron, PhD – is an employee and holds shares of the Cornerstone Research Group, which provides consultant services to various pharmaceutical and device companies. Dr. Disher is a subcontractor for the Cornerstone Research Group. There were no other disclosures to report.
SOURCE: Disher T et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2018.5044.
Most of the 50%-80% of newborns treated for NAS are treated pharmacologically in newborn ICUs at significant cost ($93,400 for mean stay of 23 days). To date, the wide variations in care, including pharmacologic options for treating NAS, leave clinicians with no consensus regarding which medication is best. The further absence of high-quality studies that depict effective management strategies for NAS offers “little guidance to inform best practice recommendations,” Elisha M. Wachman, MD, and Martha M. Werler, DSc, wrote in an editorial published with the study.
The network analysis approach followed by Disher et al. requires some assumptions, namely “minimal bias and homogeneity of methods,” the authors observed. Yet, some of the randomized, clinical trials included in their evaluation were “not blinded and thus carry high risk of bias.” In addition, given the varied methods employed across the studies cited, “the primary findings of this meta-analysis warrant further discussion.”
Disher et al. concede that the benefits afforded with buprenorphine treatment could be more pronounced because of the dosing and weaning methods rather than from the effect of the medicine alone. Given that some studies cited did experience a shorter absolute median length of treatment with morphine, it is possible that the shortened lengths of treatment and stay concerning buprenorphine treatment “may be overestimates,” suggested Dr. Wachman and Dr. Werler.
Because of the extent of variability across studies cited, “results of the network meta-analysis by Disher et al. should be interpreted with caution.” It is worth noting that most of the studies evaluated did not “examine long-term outcomes beyond the initial birth hospitalization.” The question is: Does shorter length of treatment lead to improved long-term outcomes, or “does it put the infant at risk for readmission and altered neurobehavior and development?”
Although the researchers provide evidence of buprenorphine’s effectiveness in significantly shortening length of treatment, compared with morphine, “results should be interpreted with caution given the small number of RCTs, small sample sizes, heterogeneous methods and study populations, and lack of long-term outcome data.”
Dr. Wachman is affiliated with the department of pediatrics, Boston Medical Center. Dr. Werler is chair of the department of epidemiology, Boston University School of Public Health. The authors were supported by a grant from the National Institute of Child Health and Human Development. Dr. Werler also is supported by a grant from the Centers for Disease Control and Prevention/Massachusetts Department of Public Health. This editorial accompanied the article by Disher et al. (JAMA Pediatrics. 2019. doi: 10. 1001/jamapediatric.2018.5029).
Most of the 50%-80% of newborns treated for NAS are treated pharmacologically in newborn ICUs at significant cost ($93,400 for mean stay of 23 days). To date, the wide variations in care, including pharmacologic options for treating NAS, leave clinicians with no consensus regarding which medication is best. The further absence of high-quality studies that depict effective management strategies for NAS offers “little guidance to inform best practice recommendations,” Elisha M. Wachman, MD, and Martha M. Werler, DSc, wrote in an editorial published with the study.
The network analysis approach followed by Disher et al. requires some assumptions, namely “minimal bias and homogeneity of methods,” the authors observed. Yet, some of the randomized, clinical trials included in their evaluation were “not blinded and thus carry high risk of bias.” In addition, given the varied methods employed across the studies cited, “the primary findings of this meta-analysis warrant further discussion.”
Disher et al. concede that the benefits afforded with buprenorphine treatment could be more pronounced because of the dosing and weaning methods rather than from the effect of the medicine alone. Given that some studies cited did experience a shorter absolute median length of treatment with morphine, it is possible that the shortened lengths of treatment and stay concerning buprenorphine treatment “may be overestimates,” suggested Dr. Wachman and Dr. Werler.
Because of the extent of variability across studies cited, “results of the network meta-analysis by Disher et al. should be interpreted with caution.” It is worth noting that most of the studies evaluated did not “examine long-term outcomes beyond the initial birth hospitalization.” The question is: Does shorter length of treatment lead to improved long-term outcomes, or “does it put the infant at risk for readmission and altered neurobehavior and development?”
Although the researchers provide evidence of buprenorphine’s effectiveness in significantly shortening length of treatment, compared with morphine, “results should be interpreted with caution given the small number of RCTs, small sample sizes, heterogeneous methods and study populations, and lack of long-term outcome data.”
Dr. Wachman is affiliated with the department of pediatrics, Boston Medical Center. Dr. Werler is chair of the department of epidemiology, Boston University School of Public Health. The authors were supported by a grant from the National Institute of Child Health and Human Development. Dr. Werler also is supported by a grant from the Centers for Disease Control and Prevention/Massachusetts Department of Public Health. This editorial accompanied the article by Disher et al. (JAMA Pediatrics. 2019. doi: 10. 1001/jamapediatric.2018.5029).
Most of the 50%-80% of newborns treated for NAS are treated pharmacologically in newborn ICUs at significant cost ($93,400 for mean stay of 23 days). To date, the wide variations in care, including pharmacologic options for treating NAS, leave clinicians with no consensus regarding which medication is best. The further absence of high-quality studies that depict effective management strategies for NAS offers “little guidance to inform best practice recommendations,” Elisha M. Wachman, MD, and Martha M. Werler, DSc, wrote in an editorial published with the study.
The network analysis approach followed by Disher et al. requires some assumptions, namely “minimal bias and homogeneity of methods,” the authors observed. Yet, some of the randomized, clinical trials included in their evaluation were “not blinded and thus carry high risk of bias.” In addition, given the varied methods employed across the studies cited, “the primary findings of this meta-analysis warrant further discussion.”
Disher et al. concede that the benefits afforded with buprenorphine treatment could be more pronounced because of the dosing and weaning methods rather than from the effect of the medicine alone. Given that some studies cited did experience a shorter absolute median length of treatment with morphine, it is possible that the shortened lengths of treatment and stay concerning buprenorphine treatment “may be overestimates,” suggested Dr. Wachman and Dr. Werler.
Because of the extent of variability across studies cited, “results of the network meta-analysis by Disher et al. should be interpreted with caution.” It is worth noting that most of the studies evaluated did not “examine long-term outcomes beyond the initial birth hospitalization.” The question is: Does shorter length of treatment lead to improved long-term outcomes, or “does it put the infant at risk for readmission and altered neurobehavior and development?”
Although the researchers provide evidence of buprenorphine’s effectiveness in significantly shortening length of treatment, compared with morphine, “results should be interpreted with caution given the small number of RCTs, small sample sizes, heterogeneous methods and study populations, and lack of long-term outcome data.”
Dr. Wachman is affiliated with the department of pediatrics, Boston Medical Center. Dr. Werler is chair of the department of epidemiology, Boston University School of Public Health. The authors were supported by a grant from the National Institute of Child Health and Human Development. Dr. Werler also is supported by a grant from the Centers for Disease Control and Prevention/Massachusetts Department of Public Health. This editorial accompanied the article by Disher et al. (JAMA Pediatrics. 2019. doi: 10. 1001/jamapediatric.2018.5029).
In what is believed to be the first study of its kind to compare all available pharmacologic treatment options for relief of symptoms associated with neonatal abstinence syndrome (NAS), buprenorphine has the greatest probability of reducing duration of treatment and length of stay among newborns, reported Timothy Disher, PhD, of Dalhousie University School of Nursing, Halifax, N.S., and his associates.

It was noteworthy that the study also found morphine and phenobarbital monotherapies to be worst in overall effectiveness and ranking because these pharmacotherapies are the most frequently used treatments in the United States, according to the authors. Dr. Disher and his associates underscored the need for concern over the common rationale of treatment centers, especially in using phenobarbital, since the American Academy of Pediatrics “highlights that phenobarbital is most commonly used only as adjuvant therapy” and was not intended as a first-line treatment.
In their efforts to identify treatments that are most effective at easing the symptoms of NAS, Dr. Disher and his colleagues conducted a systematic review and network meta-analysis in June 2018, which included a search of the Cochrane Central Register of Controlled Trials, Ovid MEDLINE, Embase, and the Web of Science Core Collection. In addition, they referenced ClinicalTrials.gov to identify relevant ongoing trials. Studies ultimately included in the review were randomized clinical trials comparing at least two pharmacotherapies prescribed for NAS that had been published in peer-reviewed journals.
Eighteen studies examining treatment for NAS among 1,072 newborns, including 10 studies published since 2000, were identified; the remaining studies were published between 1977 and 1986. Altogether, eight treatment interventions were examined across 10 studies
Dr. Disher and his associates reported that, during 2004-2014, there was a fivefold increase in the number of babies presenting with NAS, from 1.5/1,000 live births to 8.0/1,000, which represented a sevenfold increase in treatment cost in the Medicaid population during the same period, from $65.4 million to $462 million.
Although Dr. Disher and his colleagues acknowledged that buprenorphine was identified as best treatment by median ranks, “the ranks for most treatments are imprecise,” they said. According to results of their analysis, buprenorphine was associated with a reduction in 2.19 days of treatment, compared with clonidine, and 12.75 days, compared with morphine. In terms of secondary outcomes, buprenorphine was associated with a reduction in length of stay of 5.35 days, compared with clonidine, and 11.43 days, compared with morphine.
Seven of the studies evaluated (n = 394) included infants requiring adjuvant treatment. Agthe et al. reported that no infants in the concomitant diluted tincture of opium (DTO) and clonidine arm needed adjuvant treatment compared with five infants in the DTO-only arm who did. Surran et al. reported 2 of 32 infants who failed attempts to wean in the concomitant morphine and clonidine group compared with none of the 34 who were in the morphine and phenobarbital group.
In terms of adverse events, one study reported a seizure that was unrelated to treatment (Kraft et al). Agthe et al. reported three infants experiencing seizure in the DTO-only group compared with no infants who received concomitant clonidine. In Surran et al., three infants receiving concomitant phenobarbital and morphine were reported to be oversedated.
In general, the rationale explaining differences in why pharmacologic therapies affect treatment length is underdeveloped, the authors said. Buprenorphine, in particular, is favored because of its ease of dosing schedule and the possible improved safety profile given its longer half-life and greater micro-opioid receptor activity. It has been further suggested that the prolonged half-life of buprenorphine may be responsible for preventing sudden withdrawal symptoms. The researchers found no significant adverse events associated with buprenorphine treatment.
Although there were differences across buprenorphine treatment protocols, Dr. Disher and his colleagues noted that they were “broadly similar.” The authors conceded, however, that there is reason to question “how much of the observed improvement in buprenorphine may be attributable to the differences in optimization of the treatment and weaning protocols.”
Based on findings in this review, the authors caution that it is unlikely “that the current evidence base is sufficient to recommend specific large-scale changes in treatment away from the current standard of care.”
Despite recent research, which proposes trying nonpharmacologic treatments first and incorporating shared rooms for families and infants to reduce length of stay when treatment is required, up to 70% of infants ultimately require pharmacologic treatment. When drug therapy is needed, the average length of stay and overall treatment costs double, 10.9 vs. 22 days and $20,708 vs. $44,720, respectively.
Since results of the analysis show benefit, however variable, in reducing the length of treatment, “continued efforts to identify the optimal pharmacological agents are justified,” urged Dr. Disher and his associates.
Ultimately, before buprenorphine can be considered as a universally accepted standard of care in the treatment of NAS, “a large multisite pragmatic trial that compares buprenorphine with other treatments” will be needed.
One of the researchers – Chris Cameron, PhD – is an employee and holds shares of the Cornerstone Research Group, which provides consultant services to various pharmaceutical and device companies. Dr. Disher is a subcontractor for the Cornerstone Research Group. There were no other disclosures to report.
SOURCE: Disher T et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2018.5044.
In what is believed to be the first study of its kind to compare all available pharmacologic treatment options for relief of symptoms associated with neonatal abstinence syndrome (NAS), buprenorphine has the greatest probability of reducing duration of treatment and length of stay among newborns, reported Timothy Disher, PhD, of Dalhousie University School of Nursing, Halifax, N.S., and his associates.

It was noteworthy that the study also found morphine and phenobarbital monotherapies to be worst in overall effectiveness and ranking because these pharmacotherapies are the most frequently used treatments in the United States, according to the authors. Dr. Disher and his associates underscored the need for concern over the common rationale of treatment centers, especially in using phenobarbital, since the American Academy of Pediatrics “highlights that phenobarbital is most commonly used only as adjuvant therapy” and was not intended as a first-line treatment.
In their efforts to identify treatments that are most effective at easing the symptoms of NAS, Dr. Disher and his colleagues conducted a systematic review and network meta-analysis in June 2018, which included a search of the Cochrane Central Register of Controlled Trials, Ovid MEDLINE, Embase, and the Web of Science Core Collection. In addition, they referenced ClinicalTrials.gov to identify relevant ongoing trials. Studies ultimately included in the review were randomized clinical trials comparing at least two pharmacotherapies prescribed for NAS that had been published in peer-reviewed journals.
Eighteen studies examining treatment for NAS among 1,072 newborns, including 10 studies published since 2000, were identified; the remaining studies were published between 1977 and 1986. Altogether, eight treatment interventions were examined across 10 studies
Dr. Disher and his associates reported that, during 2004-2014, there was a fivefold increase in the number of babies presenting with NAS, from 1.5/1,000 live births to 8.0/1,000, which represented a sevenfold increase in treatment cost in the Medicaid population during the same period, from $65.4 million to $462 million.
Although Dr. Disher and his colleagues acknowledged that buprenorphine was identified as best treatment by median ranks, “the ranks for most treatments are imprecise,” they said. According to results of their analysis, buprenorphine was associated with a reduction in 2.19 days of treatment, compared with clonidine, and 12.75 days, compared with morphine. In terms of secondary outcomes, buprenorphine was associated with a reduction in length of stay of 5.35 days, compared with clonidine, and 11.43 days, compared with morphine.
Seven of the studies evaluated (n = 394) included infants requiring adjuvant treatment. Agthe et al. reported that no infants in the concomitant diluted tincture of opium (DTO) and clonidine arm needed adjuvant treatment compared with five infants in the DTO-only arm who did. Surran et al. reported 2 of 32 infants who failed attempts to wean in the concomitant morphine and clonidine group compared with none of the 34 who were in the morphine and phenobarbital group.
In terms of adverse events, one study reported a seizure that was unrelated to treatment (Kraft et al). Agthe et al. reported three infants experiencing seizure in the DTO-only group compared with no infants who received concomitant clonidine. In Surran et al., three infants receiving concomitant phenobarbital and morphine were reported to be oversedated.
In general, the rationale explaining differences in why pharmacologic therapies affect treatment length is underdeveloped, the authors said. Buprenorphine, in particular, is favored because of its ease of dosing schedule and the possible improved safety profile given its longer half-life and greater micro-opioid receptor activity. It has been further suggested that the prolonged half-life of buprenorphine may be responsible for preventing sudden withdrawal symptoms. The researchers found no significant adverse events associated with buprenorphine treatment.
Although there were differences across buprenorphine treatment protocols, Dr. Disher and his colleagues noted that they were “broadly similar.” The authors conceded, however, that there is reason to question “how much of the observed improvement in buprenorphine may be attributable to the differences in optimization of the treatment and weaning protocols.”
Based on findings in this review, the authors caution that it is unlikely “that the current evidence base is sufficient to recommend specific large-scale changes in treatment away from the current standard of care.”
Despite recent research, which proposes trying nonpharmacologic treatments first and incorporating shared rooms for families and infants to reduce length of stay when treatment is required, up to 70% of infants ultimately require pharmacologic treatment. When drug therapy is needed, the average length of stay and overall treatment costs double, 10.9 vs. 22 days and $20,708 vs. $44,720, respectively.
Since results of the analysis show benefit, however variable, in reducing the length of treatment, “continued efforts to identify the optimal pharmacological agents are justified,” urged Dr. Disher and his associates.
Ultimately, before buprenorphine can be considered as a universally accepted standard of care in the treatment of NAS, “a large multisite pragmatic trial that compares buprenorphine with other treatments” will be needed.
One of the researchers – Chris Cameron, PhD – is an employee and holds shares of the Cornerstone Research Group, which provides consultant services to various pharmaceutical and device companies. Dr. Disher is a subcontractor for the Cornerstone Research Group. There were no other disclosures to report.
SOURCE: Disher T et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2018.5044.
FROM JAMA PEDIATRICS
Key clinical point: A larger study comparing buprenorphine and morphine is needed to confirm study findings.
Major finding: Although morphine and phenobarbital are prescribed most frequently in the United States, they were found to be the least effective treatments available.
Study details: Systematic review and network meta-analysis.
Disclosures: The authors had no financial relationships relevant to this article to disclose.
Source: Disher T et al. JAMA Pediatr. 2019. doi: 10.1001/jamapediatrics.2018.5044.
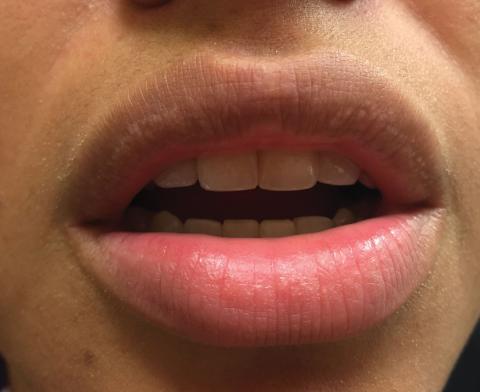
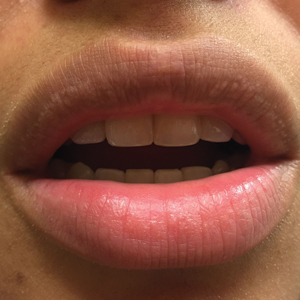
Small White Spots on the Lips
The Diagnosis: Fordyce Granules
Fordyce granules are prevalent benign anatomic variations that occur in approximately 80% of the population.1 The spots usually present as multiple (usually >10) 1- to 2-mm, painless, yellow-white papules in a symmetric bilateral distribution. They are normal superficial sebaceous glands seen on mucosal surfaces including the oral mucosa, lips, and genitalia. The papules are asymptomatic, and patients often are unaware of their presence. They can appear at any age and can last for months to years. No treatment is indicated, and patients need only reassurance.1
There are several differential diagnoses.2 Granular cell tumors present as solitary, yellowish or pink, slightly indurated, nonmobile, firm masses that usually measure less than 2 cm in diameter and can be associated with local paresthesia. The oral cavity is the second most common site after the skin and usually involves the dorsum of the tongue; however, granular cell tumors also may develop in the substance of the buccal mucosa, lips, or floor of the mouth. On histopathology, the neoplasm is composed of cells with granular cytoplasm that is of neural origin. Granular cell tumors are slow growing and may be present for months. The mean age of onset is in the fourth decade, and females are more likely to be affected. Excisional biopsy is diagnostic and curative.2
Mucoceles of the mouth are solitary, bluish clear, fluctuant, dome-shaped, well-demarcated nodules that usually appear on the lower lip.3 They are caused by rupture of a salivary gland duct due to minor trauma. Mucin is excreted into the surrounding soft tissues, leading to abrupt nontender swelling over the next several weeks. If they originate deeper within the lip they may appear normal in color. Most range from 1 to 2 mm in diameter but can grow to up to several centimeters in size. Other affected sites may include the ventral tongue, posterior buccal mucosa, or soft palate. Excisional biopsy and conservative surgical excision are recommended for diagnosis and management, respectively.3
Oral leukoplakia is a sharply demarcated, white, mucosal plaque that represents either epithelial dysplasia, carcinoma in situ, invasive carcinoma, or hyperkeratosis of unknown etiology. It is a clinical diagnosis of exclusion. The patient may present with a hoarse voice and history of tobacco use. The risk for malignant transformation to squamous cell carcinoma varies from 0% to 20% over the course of 30
years.4 The lesions occur on any mucosal surface, cannot be rubbed off, and usually are asymptomatic.5 The ventral tongue, floor of the mouth, and soft palate are associated with epithelial dysplasia and invasive carcinoma more often than other mucosal sites. There are 2 main types of leukoplakia: localized (unilateral plaque) and proliferative. Because of the risk for cancer, biopsy always is indicated and should be taken from different areas of the lesion (ie, red, verrucous, or nodular areas) if the lesion is nonhomogeneous. Treatment involves excision in the setting of dysplasia or invasive carcinoma. Photodynamic therapy has been shown to reduce the size of oral leukoplakia lesions and is being studied as an alternative therapy.5
Herpes simplex virus type 1 is a common infection of the oral mucosa that classically causes multiple vesicular lesions with an inflammatory erythematous base.6 The lesions are painful and may last for 10 to 14 days. Patients also may develop systemic symptoms such as fever and malaise. Once primary infection with herpes simplex virus has occurred, the virus lives in a latent state in ganglion neurons and can reactivate.6
- Massmanian A, Sorni Valls G, Vera Sempere FJ. Fordyce spots on the glans penis. Br J Dermatol. 1995;133:498-500.
- Lerman M, Freedman PD. Nonneural granular cell tumor of the oral cavity: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:382-384.
- Oka M, Nishioka E, Miyachi R, et al. Case of superficial mucocele of the lower lip. J Dermatol. 2007;34:754-756.
- Lodi G, Sardella A, Bez C, et al. Interventions for treating oral leukoplakia. Cochrane Database Syst Rev. 2006:CD001829.
- Selvam NP, Sadaksharam J, Singaravelu G, et al. Treatment of oral leukoplakia with photodynamic therapy: a pilot study. J Cancer Res Ther. 2015;11:464-467.
- Klein RS. Clinical manifestations and diagnosis of herpes simplex virus type 1 infection. UpToDate website. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-herpes-simplex-virus-type-1-infection.
The Diagnosis: Fordyce Granules
Fordyce granules are prevalent benign anatomic variations that occur in approximately 80% of the population.1 The spots usually present as multiple (usually >10) 1- to 2-mm, painless, yellow-white papules in a symmetric bilateral distribution. They are normal superficial sebaceous glands seen on mucosal surfaces including the oral mucosa, lips, and genitalia. The papules are asymptomatic, and patients often are unaware of their presence. They can appear at any age and can last for months to years. No treatment is indicated, and patients need only reassurance.1
There are several differential diagnoses.2 Granular cell tumors present as solitary, yellowish or pink, slightly indurated, nonmobile, firm masses that usually measure less than 2 cm in diameter and can be associated with local paresthesia. The oral cavity is the second most common site after the skin and usually involves the dorsum of the tongue; however, granular cell tumors also may develop in the substance of the buccal mucosa, lips, or floor of the mouth. On histopathology, the neoplasm is composed of cells with granular cytoplasm that is of neural origin. Granular cell tumors are slow growing and may be present for months. The mean age of onset is in the fourth decade, and females are more likely to be affected. Excisional biopsy is diagnostic and curative.2
Mucoceles of the mouth are solitary, bluish clear, fluctuant, dome-shaped, well-demarcated nodules that usually appear on the lower lip.3 They are caused by rupture of a salivary gland duct due to minor trauma. Mucin is excreted into the surrounding soft tissues, leading to abrupt nontender swelling over the next several weeks. If they originate deeper within the lip they may appear normal in color. Most range from 1 to 2 mm in diameter but can grow to up to several centimeters in size. Other affected sites may include the ventral tongue, posterior buccal mucosa, or soft palate. Excisional biopsy and conservative surgical excision are recommended for diagnosis and management, respectively.3
Oral leukoplakia is a sharply demarcated, white, mucosal plaque that represents either epithelial dysplasia, carcinoma in situ, invasive carcinoma, or hyperkeratosis of unknown etiology. It is a clinical diagnosis of exclusion. The patient may present with a hoarse voice and history of tobacco use. The risk for malignant transformation to squamous cell carcinoma varies from 0% to 20% over the course of 30
years.4 The lesions occur on any mucosal surface, cannot be rubbed off, and usually are asymptomatic.5 The ventral tongue, floor of the mouth, and soft palate are associated with epithelial dysplasia and invasive carcinoma more often than other mucosal sites. There are 2 main types of leukoplakia: localized (unilateral plaque) and proliferative. Because of the risk for cancer, biopsy always is indicated and should be taken from different areas of the lesion (ie, red, verrucous, or nodular areas) if the lesion is nonhomogeneous. Treatment involves excision in the setting of dysplasia or invasive carcinoma. Photodynamic therapy has been shown to reduce the size of oral leukoplakia lesions and is being studied as an alternative therapy.5
Herpes simplex virus type 1 is a common infection of the oral mucosa that classically causes multiple vesicular lesions with an inflammatory erythematous base.6 The lesions are painful and may last for 10 to 14 days. Patients also may develop systemic symptoms such as fever and malaise. Once primary infection with herpes simplex virus has occurred, the virus lives in a latent state in ganglion neurons and can reactivate.6
The Diagnosis: Fordyce Granules
Fordyce granules are prevalent benign anatomic variations that occur in approximately 80% of the population.1 The spots usually present as multiple (usually >10) 1- to 2-mm, painless, yellow-white papules in a symmetric bilateral distribution. They are normal superficial sebaceous glands seen on mucosal surfaces including the oral mucosa, lips, and genitalia. The papules are asymptomatic, and patients often are unaware of their presence. They can appear at any age and can last for months to years. No treatment is indicated, and patients need only reassurance.1
There are several differential diagnoses.2 Granular cell tumors present as solitary, yellowish or pink, slightly indurated, nonmobile, firm masses that usually measure less than 2 cm in diameter and can be associated with local paresthesia. The oral cavity is the second most common site after the skin and usually involves the dorsum of the tongue; however, granular cell tumors also may develop in the substance of the buccal mucosa, lips, or floor of the mouth. On histopathology, the neoplasm is composed of cells with granular cytoplasm that is of neural origin. Granular cell tumors are slow growing and may be present for months. The mean age of onset is in the fourth decade, and females are more likely to be affected. Excisional biopsy is diagnostic and curative.2
Mucoceles of the mouth are solitary, bluish clear, fluctuant, dome-shaped, well-demarcated nodules that usually appear on the lower lip.3 They are caused by rupture of a salivary gland duct due to minor trauma. Mucin is excreted into the surrounding soft tissues, leading to abrupt nontender swelling over the next several weeks. If they originate deeper within the lip they may appear normal in color. Most range from 1 to 2 mm in diameter but can grow to up to several centimeters in size. Other affected sites may include the ventral tongue, posterior buccal mucosa, or soft palate. Excisional biopsy and conservative surgical excision are recommended for diagnosis and management, respectively.3
Oral leukoplakia is a sharply demarcated, white, mucosal plaque that represents either epithelial dysplasia, carcinoma in situ, invasive carcinoma, or hyperkeratosis of unknown etiology. It is a clinical diagnosis of exclusion. The patient may present with a hoarse voice and history of tobacco use. The risk for malignant transformation to squamous cell carcinoma varies from 0% to 20% over the course of 30
years.4 The lesions occur on any mucosal surface, cannot be rubbed off, and usually are asymptomatic.5 The ventral tongue, floor of the mouth, and soft palate are associated with epithelial dysplasia and invasive carcinoma more often than other mucosal sites. There are 2 main types of leukoplakia: localized (unilateral plaque) and proliferative. Because of the risk for cancer, biopsy always is indicated and should be taken from different areas of the lesion (ie, red, verrucous, or nodular areas) if the lesion is nonhomogeneous. Treatment involves excision in the setting of dysplasia or invasive carcinoma. Photodynamic therapy has been shown to reduce the size of oral leukoplakia lesions and is being studied as an alternative therapy.5
Herpes simplex virus type 1 is a common infection of the oral mucosa that classically causes multiple vesicular lesions with an inflammatory erythematous base.6 The lesions are painful and may last for 10 to 14 days. Patients also may develop systemic symptoms such as fever and malaise. Once primary infection with herpes simplex virus has occurred, the virus lives in a latent state in ganglion neurons and can reactivate.6
- Massmanian A, Sorni Valls G, Vera Sempere FJ. Fordyce spots on the glans penis. Br J Dermatol. 1995;133:498-500.
- Lerman M, Freedman PD. Nonneural granular cell tumor of the oral cavity: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:382-384.
- Oka M, Nishioka E, Miyachi R, et al. Case of superficial mucocele of the lower lip. J Dermatol. 2007;34:754-756.
- Lodi G, Sardella A, Bez C, et al. Interventions for treating oral leukoplakia. Cochrane Database Syst Rev. 2006:CD001829.
- Selvam NP, Sadaksharam J, Singaravelu G, et al. Treatment of oral leukoplakia with photodynamic therapy: a pilot study. J Cancer Res Ther. 2015;11:464-467.
- Klein RS. Clinical manifestations and diagnosis of herpes simplex virus type 1 infection. UpToDate website. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-herpes-simplex-virus-type-1-infection.
- Massmanian A, Sorni Valls G, Vera Sempere FJ. Fordyce spots on the glans penis. Br J Dermatol. 1995;133:498-500.
- Lerman M, Freedman PD. Nonneural granular cell tumor of the oral cavity: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:382-384.
- Oka M, Nishioka E, Miyachi R, et al. Case of superficial mucocele of the lower lip. J Dermatol. 2007;34:754-756.
- Lodi G, Sardella A, Bez C, et al. Interventions for treating oral leukoplakia. Cochrane Database Syst Rev. 2006:CD001829.
- Selvam NP, Sadaksharam J, Singaravelu G, et al. Treatment of oral leukoplakia with photodynamic therapy: a pilot study. J Cancer Res Ther. 2015;11:464-467.
- Klein RS. Clinical manifestations and diagnosis of herpes simplex virus type 1 infection. UpToDate website. https://www.uptodate.com/contents/clinical-manifestations-and-diagnosis-of-herpes-simplex-virus-type-1-infection.
Isotretinoin treatment reorganizes dermal microbiome in acne patients
GRAND CAYMAN, CAYMAN ISLANDS – Isotretinoin, the go-to guy for severe acne, may not be so much a local cop as a community organizer, Kenneth B. Gordon, MD, said at the meeting provided by Global Academy for Medical Education.
“It now appears that with and that the microbial community is replenished with the types associated with healthy skin,” said Dr. Gordon, professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee. When these new bacteria move in, they push pathogenic species out of the neighborhood “and create a new skin microbial community. Maybe this is the real reason our patients tend to stay better, once we get them better with isotretinoin.”
Dr. Gordon discussed new data published last October in the Journal of Investigative Dermatology (J Invest Dermatol. 2018 Oct 24. doi: 10.1016/j.jid.2018.09.023). In a letter to the editor, William H. McCoy, IV, MD, PhD, of Washington University, St. Louis, and his associates suggest that isotretinoin induces a “sebaceous drought,” which shifts the skin microbiome from pathogenic to normophysiological.
Isotretinoin is the gold standard treatment for severe acne, but its method of action has never been fully elucidated, Dr. Gordon said. It clearly targets the sebaceous gland – decreasing sebocyte proliferation and suppressing sebum production – but an emerging body of research suggests that the drug also markedly affects dermal microbial colonization.
The entire concept of a skin microbiome is nearly as new as this new concept of isotretinoin’s effect upon it. Only in the last few years have researchers begun to characterize the complex microbial film that keeps skin healthy and resistant to infection. Dermal dysbiosis has now been associated with acne, psoriasis and psoriatic arthritis, and atopic dermatitis.
The 2-year pilot study compared the dermal microbiome of isotretinoin-treated acne patients with that of patients with untreated acne and normal skin. Skin samples underwent genomic analysis before isotretinoin treatment, at several periods during treatment, and about 5 months after treatment stopped. Untreated controls were evaluated at baseline and at 2, 5, and 10 months.
Not surprisingly, before treatment the microbiome was similar in both acne groups, but markedly different from that seen in normal skin. As isotretinoin’s “oil drought” dragged on, levels of Cutibacterium acnes (the new appellation for P. acnes) declined. Staphylococcus species initially increased, but then declined as well. Simultaneously, four new taxa (Rothia, Flavobacterium, Enterobacter, and Micrococcus) increased. Most patients had a restructuring of their Propionibacterium community, populated largely by the less-pathogenic strains found on normal skin.
“We suggest that isotretinoin creates a Propionibacterium ‘population bottleneck’ that selects for ‘healthy’ Propionibacterium communities and other sebaceous skin taxa that persist after treatment, resulting in long-term acne remission [i.e., normal skin],” the investigators wrote.
This is a new and very exciting finding, Dr. Gordon commented. “It appears that the reason our isotretinoin patients stay better once they get better is not from targeting the sebaceous gland itself, but by repairing the skin’s microbiome and getting it back to normal.”
Dr. Gordon reported financial relationships with numerous pharmaceutical companies. Global Academy and this news organization are owned by the same parent company.
This article was updated 2/1/19.
GRAND CAYMAN, CAYMAN ISLANDS – Isotretinoin, the go-to guy for severe acne, may not be so much a local cop as a community organizer, Kenneth B. Gordon, MD, said at the meeting provided by Global Academy for Medical Education.
“It now appears that with and that the microbial community is replenished with the types associated with healthy skin,” said Dr. Gordon, professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee. When these new bacteria move in, they push pathogenic species out of the neighborhood “and create a new skin microbial community. Maybe this is the real reason our patients tend to stay better, once we get them better with isotretinoin.”
Dr. Gordon discussed new data published last October in the Journal of Investigative Dermatology (J Invest Dermatol. 2018 Oct 24. doi: 10.1016/j.jid.2018.09.023). In a letter to the editor, William H. McCoy, IV, MD, PhD, of Washington University, St. Louis, and his associates suggest that isotretinoin induces a “sebaceous drought,” which shifts the skin microbiome from pathogenic to normophysiological.
Isotretinoin is the gold standard treatment for severe acne, but its method of action has never been fully elucidated, Dr. Gordon said. It clearly targets the sebaceous gland – decreasing sebocyte proliferation and suppressing sebum production – but an emerging body of research suggests that the drug also markedly affects dermal microbial colonization.
The entire concept of a skin microbiome is nearly as new as this new concept of isotretinoin’s effect upon it. Only in the last few years have researchers begun to characterize the complex microbial film that keeps skin healthy and resistant to infection. Dermal dysbiosis has now been associated with acne, psoriasis and psoriatic arthritis, and atopic dermatitis.
The 2-year pilot study compared the dermal microbiome of isotretinoin-treated acne patients with that of patients with untreated acne and normal skin. Skin samples underwent genomic analysis before isotretinoin treatment, at several periods during treatment, and about 5 months after treatment stopped. Untreated controls were evaluated at baseline and at 2, 5, and 10 months.
Not surprisingly, before treatment the microbiome was similar in both acne groups, but markedly different from that seen in normal skin. As isotretinoin’s “oil drought” dragged on, levels of Cutibacterium acnes (the new appellation for P. acnes) declined. Staphylococcus species initially increased, but then declined as well. Simultaneously, four new taxa (Rothia, Flavobacterium, Enterobacter, and Micrococcus) increased. Most patients had a restructuring of their Propionibacterium community, populated largely by the less-pathogenic strains found on normal skin.
“We suggest that isotretinoin creates a Propionibacterium ‘population bottleneck’ that selects for ‘healthy’ Propionibacterium communities and other sebaceous skin taxa that persist after treatment, resulting in long-term acne remission [i.e., normal skin],” the investigators wrote.
This is a new and very exciting finding, Dr. Gordon commented. “It appears that the reason our isotretinoin patients stay better once they get better is not from targeting the sebaceous gland itself, but by repairing the skin’s microbiome and getting it back to normal.”
Dr. Gordon reported financial relationships with numerous pharmaceutical companies. Global Academy and this news organization are owned by the same parent company.
This article was updated 2/1/19.
GRAND CAYMAN, CAYMAN ISLANDS – Isotretinoin, the go-to guy for severe acne, may not be so much a local cop as a community organizer, Kenneth B. Gordon, MD, said at the meeting provided by Global Academy for Medical Education.
“It now appears that with and that the microbial community is replenished with the types associated with healthy skin,” said Dr. Gordon, professor and chair of dermatology at the Medical College of Wisconsin, Milwaukee. When these new bacteria move in, they push pathogenic species out of the neighborhood “and create a new skin microbial community. Maybe this is the real reason our patients tend to stay better, once we get them better with isotretinoin.”
Dr. Gordon discussed new data published last October in the Journal of Investigative Dermatology (J Invest Dermatol. 2018 Oct 24. doi: 10.1016/j.jid.2018.09.023). In a letter to the editor, William H. McCoy, IV, MD, PhD, of Washington University, St. Louis, and his associates suggest that isotretinoin induces a “sebaceous drought,” which shifts the skin microbiome from pathogenic to normophysiological.
Isotretinoin is the gold standard treatment for severe acne, but its method of action has never been fully elucidated, Dr. Gordon said. It clearly targets the sebaceous gland – decreasing sebocyte proliferation and suppressing sebum production – but an emerging body of research suggests that the drug also markedly affects dermal microbial colonization.
The entire concept of a skin microbiome is nearly as new as this new concept of isotretinoin’s effect upon it. Only in the last few years have researchers begun to characterize the complex microbial film that keeps skin healthy and resistant to infection. Dermal dysbiosis has now been associated with acne, psoriasis and psoriatic arthritis, and atopic dermatitis.
The 2-year pilot study compared the dermal microbiome of isotretinoin-treated acne patients with that of patients with untreated acne and normal skin. Skin samples underwent genomic analysis before isotretinoin treatment, at several periods during treatment, and about 5 months after treatment stopped. Untreated controls were evaluated at baseline and at 2, 5, and 10 months.
Not surprisingly, before treatment the microbiome was similar in both acne groups, but markedly different from that seen in normal skin. As isotretinoin’s “oil drought” dragged on, levels of Cutibacterium acnes (the new appellation for P. acnes) declined. Staphylococcus species initially increased, but then declined as well. Simultaneously, four new taxa (Rothia, Flavobacterium, Enterobacter, and Micrococcus) increased. Most patients had a restructuring of their Propionibacterium community, populated largely by the less-pathogenic strains found on normal skin.
“We suggest that isotretinoin creates a Propionibacterium ‘population bottleneck’ that selects for ‘healthy’ Propionibacterium communities and other sebaceous skin taxa that persist after treatment, resulting in long-term acne remission [i.e., normal skin],” the investigators wrote.
This is a new and very exciting finding, Dr. Gordon commented. “It appears that the reason our isotretinoin patients stay better once they get better is not from targeting the sebaceous gland itself, but by repairing the skin’s microbiome and getting it back to normal.”
Dr. Gordon reported financial relationships with numerous pharmaceutical companies. Global Academy and this news organization are owned by the same parent company.
This article was updated 2/1/19.
REPORTING FROM THE CARIBBEAN DERMATOLOGY SYMPOSIUM
Suicide trends among youth on Medicaid
The risk for acute myeloid leukemia and myeloma is higher for breast cancer survivors, matched transplant improves stroke risk indicator in sickle cell anemia, and a diet low in free sugars shows some promise for adolescent non-alcoholic fatty liver disease.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
The risk for acute myeloid leukemia and myeloma is higher for breast cancer survivors, matched transplant improves stroke risk indicator in sickle cell anemia, and a diet low in free sugars shows some promise for adolescent non-alcoholic fatty liver disease.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
The risk for acute myeloid leukemia and myeloma is higher for breast cancer survivors, matched transplant improves stroke risk indicator in sickle cell anemia, and a diet low in free sugars shows some promise for adolescent non-alcoholic fatty liver disease.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
A Safe Treatment Switch for Patients With Prostate Cancer
Degarelix was developed as a novel gonadotropin-releasing hormone (GnRH) antagonist, with the aim of counteracting the testosterone surge often experienced with GnRH agonists. Degarelix blocks the GnRH receptors in the pituitary gland, rapidly reducing testosterone production, but the effects last only for a month. It is common practice to switch from degarelix to a GnRH agonist once the testosterone levels are down and stable. Degarelix is safe and effective for the short term, but what about the long term?
Researchers from Osaka City University and Bell Land General Hospital in Japan evaluated 5-year survival and time to castration-resistant prostate cancer (CRPC) in 108 patients with prostate cancer treated with degarelix. In the study, 57 patients were switched from degarelix to a GnRH agonist; 51 continued on degarelix.
Overall 5-year survival was high (89%) but statistically superior in the changed group (97% vs 74%). The 5-year cancer-specific survival also was longer in the changed group (100% vs 85%). Average time to CRCP was comparable in both groups (43 months in the changed group, 35 months in the continued group). The CRPC conversion did not reach the median at the time of data analysis. The median percentage decrease in prostate-specific antigen level for all patients treated with degarelix was 99.7%. The lowered levels were maintained even after switching to GnRH agonists.
The researchers say theirs is the first report of long-term data on degarelix and the first study to report that changing the treatment from a GnRH antagonist to a GnRH agonist did not affect the oncologic outcomes in patients with hormone-sensitive prostate cancer.
Source:
Asakawa J, Iguchi T, Tamada S, et al. Basic Clin Androl. 2018;28:9.
doi: 10.1186/s12610-018-0074-2.
Degarelix was developed as a novel gonadotropin-releasing hormone (GnRH) antagonist, with the aim of counteracting the testosterone surge often experienced with GnRH agonists. Degarelix blocks the GnRH receptors in the pituitary gland, rapidly reducing testosterone production, but the effects last only for a month. It is common practice to switch from degarelix to a GnRH agonist once the testosterone levels are down and stable. Degarelix is safe and effective for the short term, but what about the long term?
Researchers from Osaka City University and Bell Land General Hospital in Japan evaluated 5-year survival and time to castration-resistant prostate cancer (CRPC) in 108 patients with prostate cancer treated with degarelix. In the study, 57 patients were switched from degarelix to a GnRH agonist; 51 continued on degarelix.
Overall 5-year survival was high (89%) but statistically superior in the changed group (97% vs 74%). The 5-year cancer-specific survival also was longer in the changed group (100% vs 85%). Average time to CRCP was comparable in both groups (43 months in the changed group, 35 months in the continued group). The CRPC conversion did not reach the median at the time of data analysis. The median percentage decrease in prostate-specific antigen level for all patients treated with degarelix was 99.7%. The lowered levels were maintained even after switching to GnRH agonists.
The researchers say theirs is the first report of long-term data on degarelix and the first study to report that changing the treatment from a GnRH antagonist to a GnRH agonist did not affect the oncologic outcomes in patients with hormone-sensitive prostate cancer.
Source:
Asakawa J, Iguchi T, Tamada S, et al. Basic Clin Androl. 2018;28:9.
doi: 10.1186/s12610-018-0074-2.
Degarelix was developed as a novel gonadotropin-releasing hormone (GnRH) antagonist, with the aim of counteracting the testosterone surge often experienced with GnRH agonists. Degarelix blocks the GnRH receptors in the pituitary gland, rapidly reducing testosterone production, but the effects last only for a month. It is common practice to switch from degarelix to a GnRH agonist once the testosterone levels are down and stable. Degarelix is safe and effective for the short term, but what about the long term?
Researchers from Osaka City University and Bell Land General Hospital in Japan evaluated 5-year survival and time to castration-resistant prostate cancer (CRPC) in 108 patients with prostate cancer treated with degarelix. In the study, 57 patients were switched from degarelix to a GnRH agonist; 51 continued on degarelix.
Overall 5-year survival was high (89%) but statistically superior in the changed group (97% vs 74%). The 5-year cancer-specific survival also was longer in the changed group (100% vs 85%). Average time to CRCP was comparable in both groups (43 months in the changed group, 35 months in the continued group). The CRPC conversion did not reach the median at the time of data analysis. The median percentage decrease in prostate-specific antigen level for all patients treated with degarelix was 99.7%. The lowered levels were maintained even after switching to GnRH agonists.
The researchers say theirs is the first report of long-term data on degarelix and the first study to report that changing the treatment from a GnRH antagonist to a GnRH agonist did not affect the oncologic outcomes in patients with hormone-sensitive prostate cancer.
Source:
Asakawa J, Iguchi T, Tamada S, et al. Basic Clin Androl. 2018;28:9.
doi: 10.1186/s12610-018-0074-2.
Potential antidepressant overprescribing found in 24% of elderly cohort
Almost a quarter of an elderly U.S. population who were prescribed an antidepressant potentially received an overprescription, according to William V. Bobo, MD, MPH, of the Mayo Clinic in Jacksonville, Fla., and his associates.
In a study published in Pharmacology Research & Perspectives, the authors drew data from the Rochester Epidemiology Project and included 3,199 incident antidepressant prescriptions from adults aged at least 65 years who lived in Olmsted County, Minn., from 2005 to 2012. Selective serotonin reuptake inhibitors (SSRIs) were the most commonly prescribed medication (40%), followed by trazodone/nefazodone (20%), tricyclic antidepressants (16%), and mirtazapine (12%). , 22% were for nonspecific symptoms, and 21% were for general medical diagnoses, Dr. Bobo and his associates reported.
Potential antidepressant overprescribing occurred in 24% of all prescriptions; SSRIs were most commonly overprescribed, accounting for 74% of all overprescriptions, followed by mirtazapine (19%). Overprescription was most common when antidepressants were prescribed for nonspecific psychiatric symptoms (18%), compared with specific psychiatric indications (3.5%) and general medical diagnoses (2.5%).
Other factors associated with antidepressant overprescription included living in a nursing home, having a higher number of comorbid medical conditions and outpatient prescribers, taking more concomitant medications, more commonly using urgent or acute care in the year prior to index prescription, and being prescribed antidepressants via telephone, email, or patient portal.
“Potential antidepressant overprescribing in a large cohort of elderly patients mainly involved the use of newer antidepressants for nonspecific psychiatric symptoms and indications,” the investigators wrote. “However, the majority of incident antidepressant starts did not represent potential overprescribing. When overprescribing occurred, it was associated with factors representing higher multimorbidity, clinical complexity, and severity – and with antidepressant prescribing that did not involve face-to-face interaction of patients with prescribers.”
The authors reported no conflicts of interest.
SOURCE: Bobo WV et al. Pharmacol Res Perspect. 2019 Jan 24. doi: 10.1002/prp2.461.
Almost a quarter of an elderly U.S. population who were prescribed an antidepressant potentially received an overprescription, according to William V. Bobo, MD, MPH, of the Mayo Clinic in Jacksonville, Fla., and his associates.
In a study published in Pharmacology Research & Perspectives, the authors drew data from the Rochester Epidemiology Project and included 3,199 incident antidepressant prescriptions from adults aged at least 65 years who lived in Olmsted County, Minn., from 2005 to 2012. Selective serotonin reuptake inhibitors (SSRIs) were the most commonly prescribed medication (40%), followed by trazodone/nefazodone (20%), tricyclic antidepressants (16%), and mirtazapine (12%). , 22% were for nonspecific symptoms, and 21% were for general medical diagnoses, Dr. Bobo and his associates reported.
Potential antidepressant overprescribing occurred in 24% of all prescriptions; SSRIs were most commonly overprescribed, accounting for 74% of all overprescriptions, followed by mirtazapine (19%). Overprescription was most common when antidepressants were prescribed for nonspecific psychiatric symptoms (18%), compared with specific psychiatric indications (3.5%) and general medical diagnoses (2.5%).
Other factors associated with antidepressant overprescription included living in a nursing home, having a higher number of comorbid medical conditions and outpatient prescribers, taking more concomitant medications, more commonly using urgent or acute care in the year prior to index prescription, and being prescribed antidepressants via telephone, email, or patient portal.
“Potential antidepressant overprescribing in a large cohort of elderly patients mainly involved the use of newer antidepressants for nonspecific psychiatric symptoms and indications,” the investigators wrote. “However, the majority of incident antidepressant starts did not represent potential overprescribing. When overprescribing occurred, it was associated with factors representing higher multimorbidity, clinical complexity, and severity – and with antidepressant prescribing that did not involve face-to-face interaction of patients with prescribers.”
The authors reported no conflicts of interest.
SOURCE: Bobo WV et al. Pharmacol Res Perspect. 2019 Jan 24. doi: 10.1002/prp2.461.
Almost a quarter of an elderly U.S. population who were prescribed an antidepressant potentially received an overprescription, according to William V. Bobo, MD, MPH, of the Mayo Clinic in Jacksonville, Fla., and his associates.
In a study published in Pharmacology Research & Perspectives, the authors drew data from the Rochester Epidemiology Project and included 3,199 incident antidepressant prescriptions from adults aged at least 65 years who lived in Olmsted County, Minn., from 2005 to 2012. Selective serotonin reuptake inhibitors (SSRIs) were the most commonly prescribed medication (40%), followed by trazodone/nefazodone (20%), tricyclic antidepressants (16%), and mirtazapine (12%). , 22% were for nonspecific symptoms, and 21% were for general medical diagnoses, Dr. Bobo and his associates reported.
Potential antidepressant overprescribing occurred in 24% of all prescriptions; SSRIs were most commonly overprescribed, accounting for 74% of all overprescriptions, followed by mirtazapine (19%). Overprescription was most common when antidepressants were prescribed for nonspecific psychiatric symptoms (18%), compared with specific psychiatric indications (3.5%) and general medical diagnoses (2.5%).
Other factors associated with antidepressant overprescription included living in a nursing home, having a higher number of comorbid medical conditions and outpatient prescribers, taking more concomitant medications, more commonly using urgent or acute care in the year prior to index prescription, and being prescribed antidepressants via telephone, email, or patient portal.
“Potential antidepressant overprescribing in a large cohort of elderly patients mainly involved the use of newer antidepressants for nonspecific psychiatric symptoms and indications,” the investigators wrote. “However, the majority of incident antidepressant starts did not represent potential overprescribing. When overprescribing occurred, it was associated with factors representing higher multimorbidity, clinical complexity, and severity – and with antidepressant prescribing that did not involve face-to-face interaction of patients with prescribers.”
The authors reported no conflicts of interest.
SOURCE: Bobo WV et al. Pharmacol Res Perspect. 2019 Jan 24. doi: 10.1002/prp2.461.
FROM PHARMACOLOGY RESEARCH & PERSPECTIVES
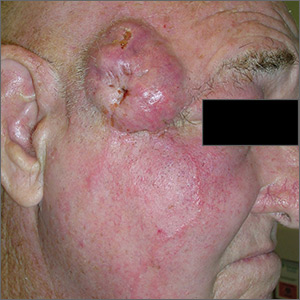
Growing cyst on face
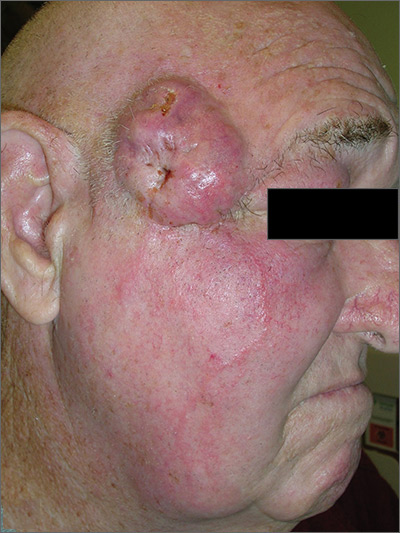
The FP suspected this was more than just a simple cyst, and his differential diagnosis included basal cell carcinoma and squamous cell carcinoma (SCC). The FP advised the patient that a punch biopsy was needed to determine the diagnosis and that it was not possible to just remove the cyst. The patient consented to the biopsy, and the FP performed a 4-mm punch biopsy. (See the Watch & Learn video on “Punch biopsy.”)
The pathology showed an invasive SCC. Note that cutaneous SCCs can appear cystic on presentation. Due to the location and size of the SCC, the patient was referred to Head and Neck Surgery for resection of the tumor and flap repair. The temporal branch of the facial nerve was spared. And, while it appeared that the red lines radiating down the cheek from the tumor were lymphangitic spread, the pathology at the time of the tumor resection did not show this. The surgery achieved clear margins, and the patient recovered well.
On a follow-up visit, the FP performed a total body skin exam to look for other skin cancers and found none. He also counseled the patient on sun avoidance, the consistent use of a hat outdoors, and the use of sunscreen when exposed to the sun.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP suspected this was more than just a simple cyst, and his differential diagnosis included basal cell carcinoma and squamous cell carcinoma (SCC). The FP advised the patient that a punch biopsy was needed to determine the diagnosis and that it was not possible to just remove the cyst. The patient consented to the biopsy, and the FP performed a 4-mm punch biopsy. (See the Watch & Learn video on “Punch biopsy.”)
The pathology showed an invasive SCC. Note that cutaneous SCCs can appear cystic on presentation. Due to the location and size of the SCC, the patient was referred to Head and Neck Surgery for resection of the tumor and flap repair. The temporal branch of the facial nerve was spared. And, while it appeared that the red lines radiating down the cheek from the tumor were lymphangitic spread, the pathology at the time of the tumor resection did not show this. The surgery achieved clear margins, and the patient recovered well.
On a follow-up visit, the FP performed a total body skin exam to look for other skin cancers and found none. He also counseled the patient on sun avoidance, the consistent use of a hat outdoors, and the use of sunscreen when exposed to the sun.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP suspected this was more than just a simple cyst, and his differential diagnosis included basal cell carcinoma and squamous cell carcinoma (SCC). The FP advised the patient that a punch biopsy was needed to determine the diagnosis and that it was not possible to just remove the cyst. The patient consented to the biopsy, and the FP performed a 4-mm punch biopsy. (See the Watch & Learn video on “Punch biopsy.”)
The pathology showed an invasive SCC. Note that cutaneous SCCs can appear cystic on presentation. Due to the location and size of the SCC, the patient was referred to Head and Neck Surgery for resection of the tumor and flap repair. The temporal branch of the facial nerve was spared. And, while it appeared that the red lines radiating down the cheek from the tumor were lymphangitic spread, the pathology at the time of the tumor resection did not show this. The surgery achieved clear margins, and the patient recovered well.
On a follow-up visit, the FP performed a total body skin exam to look for other skin cancers and found none. He also counseled the patient on sun avoidance, the consistent use of a hat outdoors, and the use of sunscreen when exposed to the sun.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Squamous cell carcinoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:999-1007.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com
Stigma against gay fathers still common, especially in low-equality states
Gay men who become fathers still commonly experience barriers and stigma, but those living in states that offer legal protections experienced less stigma and fewer barriers, according to Ellen C. Perrin, MD, of Tufts Medical Center in Boston and her associates.
A total of 732 fathers living in 47 states, with 1,316 children (average age, 13 years), responded to a survey, they wrote in Pediatrics. More than 80% had a male partner, 64% had earned a bachelor degree or higher, and 81% were white and non-Hispanic.
In 35% of cases, children entered a family through adoption and/or foster care, 14% through the assistance of a pregnancy carrier or surrogate, and 39% through a heterosexual relationship. Families in states with fewer legal protections were more likely to have been formed through heterosexual relationships (odds ratio, 1.42; 95% confidence interval, 1.11-1.81), while families in states with a greater equality rating were more likely to have been formed through a pregnancy surrogate (OR, 1.41; 95% CI, 1.08-1.84). A total of 41% of fathers reported facing barriers to adoption, and 33% reported having difficulty arranging custody of children born in a heterosexual relationship.
Active stigma experienced by the fathers was most commonly experienced in a religious setting, reported by 35%, with other common sources including neighbors (28%), service providers (26%), family members (24%), gay friends (24%), the child’s school (18%), the workplace (16%), and in health care (11%). Children most often experienced active stigma by their friends (33%), followed by a religious setting (17%), school (16%), neighbors (15%), family (11%), and in health care settings (4%).
Active and avoidant stigma was more likely in states with a low equality rating, especially in religious settings and among family members and neighbors, the investigators noted.
“Given their important role as leaders in the community’s support for all families, pediatricians caring for children and their gay fathers should recognize the likelihood that stigma may be a part of the family’s experience and help both families and communities to counteract it. Pediatricians also have the opportunity to be leaders in opposing discrimination in religious and other community institutions,” Dr. Perrin and her associates wrote.
The study received funding from the Gil Foundation, the Arcus Foundation, and private donations. The study authors reported no relevant financial disclosures or conflicts of interest.
SOURCE: Perrin EC et al. Pediatrics. 2019 Jan 14. doi: 10.1542/peds.2018-0683.
Gay men who become fathers still commonly experience barriers and stigma, but those living in states that offer legal protections experienced less stigma and fewer barriers, according to Ellen C. Perrin, MD, of Tufts Medical Center in Boston and her associates.
A total of 732 fathers living in 47 states, with 1,316 children (average age, 13 years), responded to a survey, they wrote in Pediatrics. More than 80% had a male partner, 64% had earned a bachelor degree or higher, and 81% were white and non-Hispanic.
In 35% of cases, children entered a family through adoption and/or foster care, 14% through the assistance of a pregnancy carrier or surrogate, and 39% through a heterosexual relationship. Families in states with fewer legal protections were more likely to have been formed through heterosexual relationships (odds ratio, 1.42; 95% confidence interval, 1.11-1.81), while families in states with a greater equality rating were more likely to have been formed through a pregnancy surrogate (OR, 1.41; 95% CI, 1.08-1.84). A total of 41% of fathers reported facing barriers to adoption, and 33% reported having difficulty arranging custody of children born in a heterosexual relationship.
Active stigma experienced by the fathers was most commonly experienced in a religious setting, reported by 35%, with other common sources including neighbors (28%), service providers (26%), family members (24%), gay friends (24%), the child’s school (18%), the workplace (16%), and in health care (11%). Children most often experienced active stigma by their friends (33%), followed by a religious setting (17%), school (16%), neighbors (15%), family (11%), and in health care settings (4%).
Active and avoidant stigma was more likely in states with a low equality rating, especially in religious settings and among family members and neighbors, the investigators noted.
“Given their important role as leaders in the community’s support for all families, pediatricians caring for children and their gay fathers should recognize the likelihood that stigma may be a part of the family’s experience and help both families and communities to counteract it. Pediatricians also have the opportunity to be leaders in opposing discrimination in religious and other community institutions,” Dr. Perrin and her associates wrote.
The study received funding from the Gil Foundation, the Arcus Foundation, and private donations. The study authors reported no relevant financial disclosures or conflicts of interest.
SOURCE: Perrin EC et al. Pediatrics. 2019 Jan 14. doi: 10.1542/peds.2018-0683.
Gay men who become fathers still commonly experience barriers and stigma, but those living in states that offer legal protections experienced less stigma and fewer barriers, according to Ellen C. Perrin, MD, of Tufts Medical Center in Boston and her associates.
A total of 732 fathers living in 47 states, with 1,316 children (average age, 13 years), responded to a survey, they wrote in Pediatrics. More than 80% had a male partner, 64% had earned a bachelor degree or higher, and 81% were white and non-Hispanic.
In 35% of cases, children entered a family through adoption and/or foster care, 14% through the assistance of a pregnancy carrier or surrogate, and 39% through a heterosexual relationship. Families in states with fewer legal protections were more likely to have been formed through heterosexual relationships (odds ratio, 1.42; 95% confidence interval, 1.11-1.81), while families in states with a greater equality rating were more likely to have been formed through a pregnancy surrogate (OR, 1.41; 95% CI, 1.08-1.84). A total of 41% of fathers reported facing barriers to adoption, and 33% reported having difficulty arranging custody of children born in a heterosexual relationship.
Active stigma experienced by the fathers was most commonly experienced in a religious setting, reported by 35%, with other common sources including neighbors (28%), service providers (26%), family members (24%), gay friends (24%), the child’s school (18%), the workplace (16%), and in health care (11%). Children most often experienced active stigma by their friends (33%), followed by a religious setting (17%), school (16%), neighbors (15%), family (11%), and in health care settings (4%).
Active and avoidant stigma was more likely in states with a low equality rating, especially in religious settings and among family members and neighbors, the investigators noted.
“Given their important role as leaders in the community’s support for all families, pediatricians caring for children and their gay fathers should recognize the likelihood that stigma may be a part of the family’s experience and help both families and communities to counteract it. Pediatricians also have the opportunity to be leaders in opposing discrimination in religious and other community institutions,” Dr. Perrin and her associates wrote.
The study received funding from the Gil Foundation, the Arcus Foundation, and private donations. The study authors reported no relevant financial disclosures or conflicts of interest.
SOURCE: Perrin EC et al. Pediatrics. 2019 Jan 14. doi: 10.1542/peds.2018-0683.
FROM PEDIATRICS
Products being developed for AKs therapy may appeal to patients
GRAND CAYMAN, CAYMAN ISLANDS – It’s unanimous: Patients with actinic keratoses (AKs) want them to go away quickly, painlessly, and pretty much invisibly. In fact, they’d rather risk developing cancer than deal with weeks of painful, red, oozing crusts.
But unless Ronco comes up with the AK-Away Wand, dermatologists and patients have to face facts, Theodore Rosen, MD, said at the Caribbean Dermatology Symposium, provided by Global Academy for Medical Education.
“Some AKs are going to just go away, and some are going to just sit there unchanging. Not all AKs are going to turn into squamous cell cancer. But you can’t tell which ones will, and because you can’t predict, they should all be treated. It’s our job to make patients care about this.”
That job starts with the very first conversation, said Dr. Rosen, professor of dermatology, Baylor University, Houston. “The way you frame the information at the very beginning is so important. You have to get the word ‘cancer’ in there.”
Most patients don’t fully grasp the serious threat that a transformed AK can pose, as illustrated by a survey of patients at the Milton S. Hershey Medical Center in Hershey, Pa.. The survey also highlighted the importance of the first discussion with the physician. Almost 550 dermatology clinic patients completed the survey, which presented five AK treatment decision scenarios, asking patients how likely they would be to pursue treatment in each situation (JAMA Dermatol. 2017;153[5]:421-6). Each scenario was factual, but the emphasis on facts varied. The first four questions characterized the lesions as sun damage and stressed the low incidence of malignant transformation (0.5%), and the large percentage that remain unchanged (75%) and spontaneously disappear (25%).
The last question was much simpler and more direct: “Actinic keratoses are precancers. Based on this statement, how likely are you to want treatment?”
“When AK was presented without the word ‘cancer’ in the description, there were lower proportions of individuals who said they would want to receive treatment [about 60%],” Dr. Rosen said. “Presenting AK as a precancer had the highest proportion of patients saying they would prefer treatment – about 92%.”
But current treatments aren’t ideal, at least from the standpoint of patients who prefer fast results with a minimum of erythema, oozing, crusting, and pain. Dr. Rosen looked into his crystal ball and saw a few encouraging treatment options coming down the drug development pike. To make it past regulatory hurdles, though, any new treatment has to hit the sweet spot of approximately 80% lesion clearance, with less than 40% recurrence at 1 year. Whether these investigational protocols can complete that journey remains to be seen.
VDA-1102
VDA-1102, in an ointment formulation, is based on a stress response chemical found in the jasmine plant. It contains a synthetic derivative of methyl jasmonate, a plant stress hormone found in jasmine. According to the patent record for VDA-1102, jasmonates are released in extreme ultraviolet radiation, osmotic shock, heat shock, and pathogen attack to initiate injury response and repair cascades.
The drug stops tumor growth by inhibiting glycolysis; it removes hexokinase 2 (HK2) from mitochondria. HK2 is found only in malignant cells; normal cells have the hexokinase 1 variant. Hexokinase is a key modulator of the transformation of adenosine triphosphate to adenosine diphosphate. As an HK2 modulator, VDA-1102 should, therefore, only induce apoptosis in the malignant cells, Dr. Rosen said.
“In preclinical studies in a hairless mouse model, they were approaching that 80% mark with lesion regression.” But the drug doesn’t induce necrosis or inflammation – a huge plus for patients. “There’s almost nothing in terms of redness, scaling, inflammation, or pain. This could be a really attractive addition to the AK toolkit. Improved aesthetics during treatment translates into improved patient willingness to undergo recurrent treatments. It may also be useful for treating large fields of AK, and in immunosuppressed patients.”
An Israeli company, Vidac Pharma, is conducting a phase 2b study of 150 patients with AK. The big question? Duration of effect – something that can’t be determined in the 21-week study. The company is aiming to launch a phase 3 trial next year.
KX-01
KX-01 (formerly KX2-391), being developed by Athenex, is a dual-action anticancer agent compounded into a 1% ointment. It inhibits both Src kinase and tubulin polymerization. Src regulates several signaling pathways in tumor cells, including proliferation, survival, migration, invasion, and angiogenesis. Tubulin formation is critical for cell replication: Without tubulin polymerization, mitotic spindles can’t form.
The drug passed two phase 3 studies (NCT03285477 and NCT03285490) with flying colors last year, clearing 100% of AK lesions by day 57 when used as field therapy on the head and neck. The studies comprised 702 subjects who applied the active ointment or vehicle once daily for 5 days.
“Local skin reactions were very low and resolved very quickly,” Dr. Rosen said. “But we don’t have any longterm data yet ... we need the 1-year clearance rate to see if it falls in that 40% sweet spot.”
Dr. Rosen disclosed being a consultant for Valeant (Ortho) and Cutanea Life Sciences.
Global Academy and this news organization are owned by the same parent company.
GRAND CAYMAN, CAYMAN ISLANDS – It’s unanimous: Patients with actinic keratoses (AKs) want them to go away quickly, painlessly, and pretty much invisibly. In fact, they’d rather risk developing cancer than deal with weeks of painful, red, oozing crusts.
But unless Ronco comes up with the AK-Away Wand, dermatologists and patients have to face facts, Theodore Rosen, MD, said at the Caribbean Dermatology Symposium, provided by Global Academy for Medical Education.
“Some AKs are going to just go away, and some are going to just sit there unchanging. Not all AKs are going to turn into squamous cell cancer. But you can’t tell which ones will, and because you can’t predict, they should all be treated. It’s our job to make patients care about this.”
That job starts with the very first conversation, said Dr. Rosen, professor of dermatology, Baylor University, Houston. “The way you frame the information at the very beginning is so important. You have to get the word ‘cancer’ in there.”
Most patients don’t fully grasp the serious threat that a transformed AK can pose, as illustrated by a survey of patients at the Milton S. Hershey Medical Center in Hershey, Pa.. The survey also highlighted the importance of the first discussion with the physician. Almost 550 dermatology clinic patients completed the survey, which presented five AK treatment decision scenarios, asking patients how likely they would be to pursue treatment in each situation (JAMA Dermatol. 2017;153[5]:421-6). Each scenario was factual, but the emphasis on facts varied. The first four questions characterized the lesions as sun damage and stressed the low incidence of malignant transformation (0.5%), and the large percentage that remain unchanged (75%) and spontaneously disappear (25%).
The last question was much simpler and more direct: “Actinic keratoses are precancers. Based on this statement, how likely are you to want treatment?”
“When AK was presented without the word ‘cancer’ in the description, there were lower proportions of individuals who said they would want to receive treatment [about 60%],” Dr. Rosen said. “Presenting AK as a precancer had the highest proportion of patients saying they would prefer treatment – about 92%.”
But current treatments aren’t ideal, at least from the standpoint of patients who prefer fast results with a minimum of erythema, oozing, crusting, and pain. Dr. Rosen looked into his crystal ball and saw a few encouraging treatment options coming down the drug development pike. To make it past regulatory hurdles, though, any new treatment has to hit the sweet spot of approximately 80% lesion clearance, with less than 40% recurrence at 1 year. Whether these investigational protocols can complete that journey remains to be seen.
VDA-1102
VDA-1102, in an ointment formulation, is based on a stress response chemical found in the jasmine plant. It contains a synthetic derivative of methyl jasmonate, a plant stress hormone found in jasmine. According to the patent record for VDA-1102, jasmonates are released in extreme ultraviolet radiation, osmotic shock, heat shock, and pathogen attack to initiate injury response and repair cascades.
The drug stops tumor growth by inhibiting glycolysis; it removes hexokinase 2 (HK2) from mitochondria. HK2 is found only in malignant cells; normal cells have the hexokinase 1 variant. Hexokinase is a key modulator of the transformation of adenosine triphosphate to adenosine diphosphate. As an HK2 modulator, VDA-1102 should, therefore, only induce apoptosis in the malignant cells, Dr. Rosen said.
“In preclinical studies in a hairless mouse model, they were approaching that 80% mark with lesion regression.” But the drug doesn’t induce necrosis or inflammation – a huge plus for patients. “There’s almost nothing in terms of redness, scaling, inflammation, or pain. This could be a really attractive addition to the AK toolkit. Improved aesthetics during treatment translates into improved patient willingness to undergo recurrent treatments. It may also be useful for treating large fields of AK, and in immunosuppressed patients.”
An Israeli company, Vidac Pharma, is conducting a phase 2b study of 150 patients with AK. The big question? Duration of effect – something that can’t be determined in the 21-week study. The company is aiming to launch a phase 3 trial next year.
KX-01
KX-01 (formerly KX2-391), being developed by Athenex, is a dual-action anticancer agent compounded into a 1% ointment. It inhibits both Src kinase and tubulin polymerization. Src regulates several signaling pathways in tumor cells, including proliferation, survival, migration, invasion, and angiogenesis. Tubulin formation is critical for cell replication: Without tubulin polymerization, mitotic spindles can’t form.
The drug passed two phase 3 studies (NCT03285477 and NCT03285490) with flying colors last year, clearing 100% of AK lesions by day 57 when used as field therapy on the head and neck. The studies comprised 702 subjects who applied the active ointment or vehicle once daily for 5 days.
“Local skin reactions were very low and resolved very quickly,” Dr. Rosen said. “But we don’t have any longterm data yet ... we need the 1-year clearance rate to see if it falls in that 40% sweet spot.”
Dr. Rosen disclosed being a consultant for Valeant (Ortho) and Cutanea Life Sciences.
Global Academy and this news organization are owned by the same parent company.
GRAND CAYMAN, CAYMAN ISLANDS – It’s unanimous: Patients with actinic keratoses (AKs) want them to go away quickly, painlessly, and pretty much invisibly. In fact, they’d rather risk developing cancer than deal with weeks of painful, red, oozing crusts.
But unless Ronco comes up with the AK-Away Wand, dermatologists and patients have to face facts, Theodore Rosen, MD, said at the Caribbean Dermatology Symposium, provided by Global Academy for Medical Education.
“Some AKs are going to just go away, and some are going to just sit there unchanging. Not all AKs are going to turn into squamous cell cancer. But you can’t tell which ones will, and because you can’t predict, they should all be treated. It’s our job to make patients care about this.”
That job starts with the very first conversation, said Dr. Rosen, professor of dermatology, Baylor University, Houston. “The way you frame the information at the very beginning is so important. You have to get the word ‘cancer’ in there.”
Most patients don’t fully grasp the serious threat that a transformed AK can pose, as illustrated by a survey of patients at the Milton S. Hershey Medical Center in Hershey, Pa.. The survey also highlighted the importance of the first discussion with the physician. Almost 550 dermatology clinic patients completed the survey, which presented five AK treatment decision scenarios, asking patients how likely they would be to pursue treatment in each situation (JAMA Dermatol. 2017;153[5]:421-6). Each scenario was factual, but the emphasis on facts varied. The first four questions characterized the lesions as sun damage and stressed the low incidence of malignant transformation (0.5%), and the large percentage that remain unchanged (75%) and spontaneously disappear (25%).
The last question was much simpler and more direct: “Actinic keratoses are precancers. Based on this statement, how likely are you to want treatment?”
“When AK was presented without the word ‘cancer’ in the description, there were lower proportions of individuals who said they would want to receive treatment [about 60%],” Dr. Rosen said. “Presenting AK as a precancer had the highest proportion of patients saying they would prefer treatment – about 92%.”
But current treatments aren’t ideal, at least from the standpoint of patients who prefer fast results with a minimum of erythema, oozing, crusting, and pain. Dr. Rosen looked into his crystal ball and saw a few encouraging treatment options coming down the drug development pike. To make it past regulatory hurdles, though, any new treatment has to hit the sweet spot of approximately 80% lesion clearance, with less than 40% recurrence at 1 year. Whether these investigational protocols can complete that journey remains to be seen.
VDA-1102
VDA-1102, in an ointment formulation, is based on a stress response chemical found in the jasmine plant. It contains a synthetic derivative of methyl jasmonate, a plant stress hormone found in jasmine. According to the patent record for VDA-1102, jasmonates are released in extreme ultraviolet radiation, osmotic shock, heat shock, and pathogen attack to initiate injury response and repair cascades.
The drug stops tumor growth by inhibiting glycolysis; it removes hexokinase 2 (HK2) from mitochondria. HK2 is found only in malignant cells; normal cells have the hexokinase 1 variant. Hexokinase is a key modulator of the transformation of adenosine triphosphate to adenosine diphosphate. As an HK2 modulator, VDA-1102 should, therefore, only induce apoptosis in the malignant cells, Dr. Rosen said.
“In preclinical studies in a hairless mouse model, they were approaching that 80% mark with lesion regression.” But the drug doesn’t induce necrosis or inflammation – a huge plus for patients. “There’s almost nothing in terms of redness, scaling, inflammation, or pain. This could be a really attractive addition to the AK toolkit. Improved aesthetics during treatment translates into improved patient willingness to undergo recurrent treatments. It may also be useful for treating large fields of AK, and in immunosuppressed patients.”
An Israeli company, Vidac Pharma, is conducting a phase 2b study of 150 patients with AK. The big question? Duration of effect – something that can’t be determined in the 21-week study. The company is aiming to launch a phase 3 trial next year.
KX-01
KX-01 (formerly KX2-391), being developed by Athenex, is a dual-action anticancer agent compounded into a 1% ointment. It inhibits both Src kinase and tubulin polymerization. Src regulates several signaling pathways in tumor cells, including proliferation, survival, migration, invasion, and angiogenesis. Tubulin formation is critical for cell replication: Without tubulin polymerization, mitotic spindles can’t form.
The drug passed two phase 3 studies (NCT03285477 and NCT03285490) with flying colors last year, clearing 100% of AK lesions by day 57 when used as field therapy on the head and neck. The studies comprised 702 subjects who applied the active ointment or vehicle once daily for 5 days.
“Local skin reactions were very low and resolved very quickly,” Dr. Rosen said. “But we don’t have any longterm data yet ... we need the 1-year clearance rate to see if it falls in that 40% sweet spot.”
Dr. Rosen disclosed being a consultant for Valeant (Ortho) and Cutanea Life Sciences.
Global Academy and this news organization are owned by the same parent company.
REPORTING FROM CARIBBEAN DERMATOLOGY SYMPOSIUM