User login
HDL-P subfractions may be prognostic in heart failure
In heart failure, derangements in HDL cholesterol particle (HDL-P) subfractions have prognostic implications beyond those of conventional cardiovascular risk factors, according to investigators who analyzed plasma samples from more than 6,500 patients.
The study revealed derangements that were shared and more severe in heart failure with reduced ejection fraction (HFrEF) as compared to heart failure with preserved ejection fraction (HFpEF), according to the researchers, who said their study is the largest to date of HDL-P subfractions in heart failure.
Both total HDL-P and small HDL-P had a strong inverse association with adverse outcomes, consistent with the conclusions of previous studies, they said in a report on their study in the Journal of the American College of Cardiology.
“Altogether, our findings support total and small HDL-P as important markers of residual risk in both HFrEF and HFpEF,” said the investigators, led by Wynn G. Hunter, MD, of Duke University, Durham, N.C.
Dr. Hunter and colleagues used the CATHGEN (Catheterization Genetics) biorepository to identify plasma samples obtained at catheterization for 782 patients with HFrEF, 1,004 with HFpEF, and 4,742 with no heart failure.
Lipoprotein profiling of the samples revealed that mean HDL-P size was greater in HFrEF than in HFpEF, and in both of those cases, mean HDL-P size was greater than in patients with no heart failure (P less than .0001), investigators reported.
Concentrations of small HDL-P and total HDL-P were by contrast lower in HFrEF versus HFpEF, and again, the values for both HFrEF and HFpEF were lower than in patients without heart failure (P less than .0001), they added.
Small HDL-P and total HDL-P had an inverse association with time to adverse events and all-cause mortality for both the HFrEF and HFpEF groups, according to investigators, who said those links remained robust even after multivariate adjustment for 14 cardiovascular risk factors, including diabetes, LDL particle, and GlycA, a marker of inflammation.
For example, small HDL-P and total HDL-P were inversely associated with all-cause mortality risk, with adjusted hazard ratios of 0.69-0.79 (P less than .0001), they reported. Similarly, a greater mean HDL-P size was associated with increased risk of all-cause mortality, yielding adjusted hazard ratios of 1.23-1.46 (P less than .0001).
Further studies are needed to clarify the role of HDL-P in the pathophysiology of heart failure, and to identify treatments that might increase total and small HDL-P in heart failure patients, Dr. Hunter and coauthors concluded.
Dr. Hunter reported no disclosures related to the study. Coauthors provided disclosures related to Amgen, Ostuka, Roche Diagnostics, Novartis, Trevena, Singulex, Medtronic, AstraZeneca, Bristol-Myers Squibb, Janssen, Portola, Boston Scientific, Gilead, GlaxoSmithKline, Merck, Alnylam, Ikaria Pharmaceuticals, Pfizer, Philips, LipoScience, and Pfizer, among others.
SOURCE: Hunter WG et al. J Am Coll Cardiol. 2019 Jan 22;73(2):177-86.
Although the study by Dr. Hunter and colleagues confirms the role of HDL cholesterol and HDL-P subfractions in heart failure, the immediate clinical implications of their findings are uncertain.
However, clinical use as a biomarker remains a “distant vision,” in part because a useful biomarker must be proven to provide an incremental benefit in terms of reducing disease-associated morbidity or mortality.
Even so, the present study could begin to inform future therapeutic studies looking at increasing specific HDL-P subfractions, rather than increasing HDL cholesterol across the board.
“Perhaps, this study will possibly serve to spur investigation into therapies designed to reduce derangements of [HDL cholesterol] metabolism and primarily target HDL-P as a regulator molecule, a promise that may keep the HDL story alive into the near future of scientific excursion.”
These comments were taken from an accompanying editorial by Hector O. Ventura, MD, and Carl J. Lavie, MD, of the University of Queensland Ochsner Clinical School, Brisbane, Australia, and New Orleans; and Mandeep R. Mehra, MD, of the Center of Advanced Heart Disease, Harvard University, Boston (J Am Coll Cardiol 2019 Jan 22;73[2]:187-9). Dr. Mehra reported that he is a consultant for Abbott, Medtronic, nupulseCV, Portola, Bayer, and FineHeart.
Although the study by Dr. Hunter and colleagues confirms the role of HDL cholesterol and HDL-P subfractions in heart failure, the immediate clinical implications of their findings are uncertain.
However, clinical use as a biomarker remains a “distant vision,” in part because a useful biomarker must be proven to provide an incremental benefit in terms of reducing disease-associated morbidity or mortality.
Even so, the present study could begin to inform future therapeutic studies looking at increasing specific HDL-P subfractions, rather than increasing HDL cholesterol across the board.
“Perhaps, this study will possibly serve to spur investigation into therapies designed to reduce derangements of [HDL cholesterol] metabolism and primarily target HDL-P as a regulator molecule, a promise that may keep the HDL story alive into the near future of scientific excursion.”
These comments were taken from an accompanying editorial by Hector O. Ventura, MD, and Carl J. Lavie, MD, of the University of Queensland Ochsner Clinical School, Brisbane, Australia, and New Orleans; and Mandeep R. Mehra, MD, of the Center of Advanced Heart Disease, Harvard University, Boston (J Am Coll Cardiol 2019 Jan 22;73[2]:187-9). Dr. Mehra reported that he is a consultant for Abbott, Medtronic, nupulseCV, Portola, Bayer, and FineHeart.
Although the study by Dr. Hunter and colleagues confirms the role of HDL cholesterol and HDL-P subfractions in heart failure, the immediate clinical implications of their findings are uncertain.
However, clinical use as a biomarker remains a “distant vision,” in part because a useful biomarker must be proven to provide an incremental benefit in terms of reducing disease-associated morbidity or mortality.
Even so, the present study could begin to inform future therapeutic studies looking at increasing specific HDL-P subfractions, rather than increasing HDL cholesterol across the board.
“Perhaps, this study will possibly serve to spur investigation into therapies designed to reduce derangements of [HDL cholesterol] metabolism and primarily target HDL-P as a regulator molecule, a promise that may keep the HDL story alive into the near future of scientific excursion.”
These comments were taken from an accompanying editorial by Hector O. Ventura, MD, and Carl J. Lavie, MD, of the University of Queensland Ochsner Clinical School, Brisbane, Australia, and New Orleans; and Mandeep R. Mehra, MD, of the Center of Advanced Heart Disease, Harvard University, Boston (J Am Coll Cardiol 2019 Jan 22;73[2]:187-9). Dr. Mehra reported that he is a consultant for Abbott, Medtronic, nupulseCV, Portola, Bayer, and FineHeart.
In heart failure, derangements in HDL cholesterol particle (HDL-P) subfractions have prognostic implications beyond those of conventional cardiovascular risk factors, according to investigators who analyzed plasma samples from more than 6,500 patients.
The study revealed derangements that were shared and more severe in heart failure with reduced ejection fraction (HFrEF) as compared to heart failure with preserved ejection fraction (HFpEF), according to the researchers, who said their study is the largest to date of HDL-P subfractions in heart failure.
Both total HDL-P and small HDL-P had a strong inverse association with adverse outcomes, consistent with the conclusions of previous studies, they said in a report on their study in the Journal of the American College of Cardiology.
“Altogether, our findings support total and small HDL-P as important markers of residual risk in both HFrEF and HFpEF,” said the investigators, led by Wynn G. Hunter, MD, of Duke University, Durham, N.C.
Dr. Hunter and colleagues used the CATHGEN (Catheterization Genetics) biorepository to identify plasma samples obtained at catheterization for 782 patients with HFrEF, 1,004 with HFpEF, and 4,742 with no heart failure.
Lipoprotein profiling of the samples revealed that mean HDL-P size was greater in HFrEF than in HFpEF, and in both of those cases, mean HDL-P size was greater than in patients with no heart failure (P less than .0001), investigators reported.
Concentrations of small HDL-P and total HDL-P were by contrast lower in HFrEF versus HFpEF, and again, the values for both HFrEF and HFpEF were lower than in patients without heart failure (P less than .0001), they added.
Small HDL-P and total HDL-P had an inverse association with time to adverse events and all-cause mortality for both the HFrEF and HFpEF groups, according to investigators, who said those links remained robust even after multivariate adjustment for 14 cardiovascular risk factors, including diabetes, LDL particle, and GlycA, a marker of inflammation.
For example, small HDL-P and total HDL-P were inversely associated with all-cause mortality risk, with adjusted hazard ratios of 0.69-0.79 (P less than .0001), they reported. Similarly, a greater mean HDL-P size was associated with increased risk of all-cause mortality, yielding adjusted hazard ratios of 1.23-1.46 (P less than .0001).
Further studies are needed to clarify the role of HDL-P in the pathophysiology of heart failure, and to identify treatments that might increase total and small HDL-P in heart failure patients, Dr. Hunter and coauthors concluded.
Dr. Hunter reported no disclosures related to the study. Coauthors provided disclosures related to Amgen, Ostuka, Roche Diagnostics, Novartis, Trevena, Singulex, Medtronic, AstraZeneca, Bristol-Myers Squibb, Janssen, Portola, Boston Scientific, Gilead, GlaxoSmithKline, Merck, Alnylam, Ikaria Pharmaceuticals, Pfizer, Philips, LipoScience, and Pfizer, among others.
SOURCE: Hunter WG et al. J Am Coll Cardiol. 2019 Jan 22;73(2):177-86.
In heart failure, derangements in HDL cholesterol particle (HDL-P) subfractions have prognostic implications beyond those of conventional cardiovascular risk factors, according to investigators who analyzed plasma samples from more than 6,500 patients.
The study revealed derangements that were shared and more severe in heart failure with reduced ejection fraction (HFrEF) as compared to heart failure with preserved ejection fraction (HFpEF), according to the researchers, who said their study is the largest to date of HDL-P subfractions in heart failure.
Both total HDL-P and small HDL-P had a strong inverse association with adverse outcomes, consistent with the conclusions of previous studies, they said in a report on their study in the Journal of the American College of Cardiology.
“Altogether, our findings support total and small HDL-P as important markers of residual risk in both HFrEF and HFpEF,” said the investigators, led by Wynn G. Hunter, MD, of Duke University, Durham, N.C.
Dr. Hunter and colleagues used the CATHGEN (Catheterization Genetics) biorepository to identify plasma samples obtained at catheterization for 782 patients with HFrEF, 1,004 with HFpEF, and 4,742 with no heart failure.
Lipoprotein profiling of the samples revealed that mean HDL-P size was greater in HFrEF than in HFpEF, and in both of those cases, mean HDL-P size was greater than in patients with no heart failure (P less than .0001), investigators reported.
Concentrations of small HDL-P and total HDL-P were by contrast lower in HFrEF versus HFpEF, and again, the values for both HFrEF and HFpEF were lower than in patients without heart failure (P less than .0001), they added.
Small HDL-P and total HDL-P had an inverse association with time to adverse events and all-cause mortality for both the HFrEF and HFpEF groups, according to investigators, who said those links remained robust even after multivariate adjustment for 14 cardiovascular risk factors, including diabetes, LDL particle, and GlycA, a marker of inflammation.
For example, small HDL-P and total HDL-P were inversely associated with all-cause mortality risk, with adjusted hazard ratios of 0.69-0.79 (P less than .0001), they reported. Similarly, a greater mean HDL-P size was associated with increased risk of all-cause mortality, yielding adjusted hazard ratios of 1.23-1.46 (P less than .0001).
Further studies are needed to clarify the role of HDL-P in the pathophysiology of heart failure, and to identify treatments that might increase total and small HDL-P in heart failure patients, Dr. Hunter and coauthors concluded.
Dr. Hunter reported no disclosures related to the study. Coauthors provided disclosures related to Amgen, Ostuka, Roche Diagnostics, Novartis, Trevena, Singulex, Medtronic, AstraZeneca, Bristol-Myers Squibb, Janssen, Portola, Boston Scientific, Gilead, GlaxoSmithKline, Merck, Alnylam, Ikaria Pharmaceuticals, Pfizer, Philips, LipoScience, and Pfizer, among others.
SOURCE: Hunter WG et al. J Am Coll Cardiol. 2019 Jan 22;73(2):177-86.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: Derangements in HDL particle (HDL-P) subfractions may have prognostic implications in patients with heart failure with reduced or preserved ejection fraction.
Major finding: (P less than .0001).
Study details: Study based on lipid profiling of more than 6,500 plasma samples obtained at catheterization.
Disclosures: Study authors provided disclosures related to Amgen, Ostuka, Roche Diagnostics, Novartis, Medtronic, and others.
Source: Hunter WG et al. J Am Coll Cardiol. 2019 Jan 22;73(2):177-86.
Too much, too little sleep linked to atherosclerosis
in healthy middle-aged men and women in a Spanish investigation of bank employees.
“Overall, our findings support the potential role of healthy sleeping in protecting against atherosclerosis. Thus, recommending a good sleep hygiene” – 7-8 hours a night – “should be part of the lifestyle modifications provided in our daily clinical practice,” said investigators led by Fernando Domínguez, MD, PhD, of Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC), Madrid. The report is in the Journal of the American College of Cardiology.
Studies have linked sleep problems to increased cardiovascular risk before, but the investigations tended to focus on patients with obstructive sleep apnea (OSA) and other problems, and often relied on patient self-report. The investors wanted to see if the relationship held in healthy adults, using an objective measure.
The participants – all with no known cardiovascular disease – wore Acti Trainers accelerometers (Actigraph, Pensacola, Fla.) around their waists for 7 days to record sleep duration and quality. Subjects also had their plaque burdens assessed by 3-dimensional vascular ultrasound (VUS) at their carotid and femoral arteries bilaterally. Cardiac CT was used to assess coronary artery calcification as a surrogate for coronary artery atherosclerosis.
The 3,974 participants had a mean age of 46 years, and a third were women; they had a low prevalence of both hypertension and diabetes. OSA patients were excluded from the study. Overall, 27% had very short sleep duration (VSSD), less than 6 hours a night; 38% had short sleep duration (SSD), 31% slept from 7 to 8 hours per night, and served as the reference group for healthy sleep habits; and 4% had long sleep duration (LSD), greater than 8 hours.
After adjustment for a wide range of cardiovascular risk factors, including body mass index, hypertension, and smoking, VSSD was independently associated with a higher atherosclerotic burden, compared to the reference group (odds ratio, 1.27; 95% confidence interval, 1.06-1.52; P = 0.008). Participants in the highest quintile of sleep fragmentation were more likely to have plaques at multiple sites (OR, 1.34; 95% CI, 1.09-1.64; P = 0.006). The Framingham risk score at both 10 and 30 years was significantly higher in participants with VSSD or SSD, and in the highest quintiles of sleep fragmentation.
LSD was also associated with a higher plaque burden, which reached statistical significance in women. “Too-long sleep duration may not be healthy either ... Recommendations should be restricted to 7 to 8 hours,” the investigators said.
Sleep duration and quality were not associated with inflammation markers or coronary artery calcification. The investigators noted that CT for coronary artery calcification might not be as sensitive as VUS for picking up subclinical atherosclerosis.
Short sleepers tended to have higher intakes of alcohol and caffeine than did those in the 7- to 8-hour group.
The work was funded by CNIC and Banco Santander, among others. Dr. Domínguez had no disclosures. Investigator Hector Bueno, MD, PhD, reported research funding and fees from a number of companies, including AstraZeneca and Novartis. The second author, Valentín Fuster, MD, PhD, is the editor of the Journal of the American College of Cardiology, which published the report.
SOURCE: Domínguez F et al. J Am Coll Cardiol 2019;73:134-44.
This study extends the published reports on sleep duration and vascular disease to an early middle-aged cohort by using an objective measure of sleep duration and sensitive measures of atherosclerosis in multiple vascular territories.
Ultimately, studies of sleep extension are needed to determine whether modification of sleep behaviors will improve vascular health outcomes. The potentially enormous impact of sleep deprivation and disruption on population health, reinforced by the present study, is ample justification for such trials, which are needed to place sleep with confidence alongside diet and exercise as a key pillar of a healthy lifestyle.
However, both hypertension and diabetes were more common in the group sleeping fewer than 6 hours per night, but neither blood pressure nor glucose metabolism was assessed with sufficiently comprehensive measures to explore these factors as potential effect mediators.
More importantly, the causes of short sleep duration and sleep fragmentation in this cohort are unknown. It is unclear to what extent short sleep duration in this cohort reflects voluntary behaviors that limit time available for sleep versus insomnia. Insomnia is itself associated with increased risk of vascular disease.
Deepak Bhatt , MD, professor of cardiovascular medicine, and Daniel Gottlieb , MD, an associate professor of medicine at Harvard Medical School, Boston, made these comments in an accompanying editorial ( J Am Coll Cardiol. 2019 Jan 14;73[2]:145-7 ). Dr. Gottlieb is also the director of the Boston Veterans Affairs Sleep Disorders Center. Dr. Bhatt reported research funding and income from a number of companies, including Abbott, Boehringer Ingelheim, and Medtronic.
This study extends the published reports on sleep duration and vascular disease to an early middle-aged cohort by using an objective measure of sleep duration and sensitive measures of atherosclerosis in multiple vascular territories.
Ultimately, studies of sleep extension are needed to determine whether modification of sleep behaviors will improve vascular health outcomes. The potentially enormous impact of sleep deprivation and disruption on population health, reinforced by the present study, is ample justification for such trials, which are needed to place sleep with confidence alongside diet and exercise as a key pillar of a healthy lifestyle.
However, both hypertension and diabetes were more common in the group sleeping fewer than 6 hours per night, but neither blood pressure nor glucose metabolism was assessed with sufficiently comprehensive measures to explore these factors as potential effect mediators.
More importantly, the causes of short sleep duration and sleep fragmentation in this cohort are unknown. It is unclear to what extent short sleep duration in this cohort reflects voluntary behaviors that limit time available for sleep versus insomnia. Insomnia is itself associated with increased risk of vascular disease.
Deepak Bhatt , MD, professor of cardiovascular medicine, and Daniel Gottlieb , MD, an associate professor of medicine at Harvard Medical School, Boston, made these comments in an accompanying editorial ( J Am Coll Cardiol. 2019 Jan 14;73[2]:145-7 ). Dr. Gottlieb is also the director of the Boston Veterans Affairs Sleep Disorders Center. Dr. Bhatt reported research funding and income from a number of companies, including Abbott, Boehringer Ingelheim, and Medtronic.
This study extends the published reports on sleep duration and vascular disease to an early middle-aged cohort by using an objective measure of sleep duration and sensitive measures of atherosclerosis in multiple vascular territories.
Ultimately, studies of sleep extension are needed to determine whether modification of sleep behaviors will improve vascular health outcomes. The potentially enormous impact of sleep deprivation and disruption on population health, reinforced by the present study, is ample justification for such trials, which are needed to place sleep with confidence alongside diet and exercise as a key pillar of a healthy lifestyle.
However, both hypertension and diabetes were more common in the group sleeping fewer than 6 hours per night, but neither blood pressure nor glucose metabolism was assessed with sufficiently comprehensive measures to explore these factors as potential effect mediators.
More importantly, the causes of short sleep duration and sleep fragmentation in this cohort are unknown. It is unclear to what extent short sleep duration in this cohort reflects voluntary behaviors that limit time available for sleep versus insomnia. Insomnia is itself associated with increased risk of vascular disease.
Deepak Bhatt , MD, professor of cardiovascular medicine, and Daniel Gottlieb , MD, an associate professor of medicine at Harvard Medical School, Boston, made these comments in an accompanying editorial ( J Am Coll Cardiol. 2019 Jan 14;73[2]:145-7 ). Dr. Gottlieb is also the director of the Boston Veterans Affairs Sleep Disorders Center. Dr. Bhatt reported research funding and income from a number of companies, including Abbott, Boehringer Ingelheim, and Medtronic.
in healthy middle-aged men and women in a Spanish investigation of bank employees.
“Overall, our findings support the potential role of healthy sleeping in protecting against atherosclerosis. Thus, recommending a good sleep hygiene” – 7-8 hours a night – “should be part of the lifestyle modifications provided in our daily clinical practice,” said investigators led by Fernando Domínguez, MD, PhD, of Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC), Madrid. The report is in the Journal of the American College of Cardiology.
Studies have linked sleep problems to increased cardiovascular risk before, but the investigations tended to focus on patients with obstructive sleep apnea (OSA) and other problems, and often relied on patient self-report. The investors wanted to see if the relationship held in healthy adults, using an objective measure.
The participants – all with no known cardiovascular disease – wore Acti Trainers accelerometers (Actigraph, Pensacola, Fla.) around their waists for 7 days to record sleep duration and quality. Subjects also had their plaque burdens assessed by 3-dimensional vascular ultrasound (VUS) at their carotid and femoral arteries bilaterally. Cardiac CT was used to assess coronary artery calcification as a surrogate for coronary artery atherosclerosis.
The 3,974 participants had a mean age of 46 years, and a third were women; they had a low prevalence of both hypertension and diabetes. OSA patients were excluded from the study. Overall, 27% had very short sleep duration (VSSD), less than 6 hours a night; 38% had short sleep duration (SSD), 31% slept from 7 to 8 hours per night, and served as the reference group for healthy sleep habits; and 4% had long sleep duration (LSD), greater than 8 hours.
After adjustment for a wide range of cardiovascular risk factors, including body mass index, hypertension, and smoking, VSSD was independently associated with a higher atherosclerotic burden, compared to the reference group (odds ratio, 1.27; 95% confidence interval, 1.06-1.52; P = 0.008). Participants in the highest quintile of sleep fragmentation were more likely to have plaques at multiple sites (OR, 1.34; 95% CI, 1.09-1.64; P = 0.006). The Framingham risk score at both 10 and 30 years was significantly higher in participants with VSSD or SSD, and in the highest quintiles of sleep fragmentation.
LSD was also associated with a higher plaque burden, which reached statistical significance in women. “Too-long sleep duration may not be healthy either ... Recommendations should be restricted to 7 to 8 hours,” the investigators said.
Sleep duration and quality were not associated with inflammation markers or coronary artery calcification. The investigators noted that CT for coronary artery calcification might not be as sensitive as VUS for picking up subclinical atherosclerosis.
Short sleepers tended to have higher intakes of alcohol and caffeine than did those in the 7- to 8-hour group.
The work was funded by CNIC and Banco Santander, among others. Dr. Domínguez had no disclosures. Investigator Hector Bueno, MD, PhD, reported research funding and fees from a number of companies, including AstraZeneca and Novartis. The second author, Valentín Fuster, MD, PhD, is the editor of the Journal of the American College of Cardiology, which published the report.
SOURCE: Domínguez F et al. J Am Coll Cardiol 2019;73:134-44.
in healthy middle-aged men and women in a Spanish investigation of bank employees.
“Overall, our findings support the potential role of healthy sleeping in protecting against atherosclerosis. Thus, recommending a good sleep hygiene” – 7-8 hours a night – “should be part of the lifestyle modifications provided in our daily clinical practice,” said investigators led by Fernando Domínguez, MD, PhD, of Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC), Madrid. The report is in the Journal of the American College of Cardiology.
Studies have linked sleep problems to increased cardiovascular risk before, but the investigations tended to focus on patients with obstructive sleep apnea (OSA) and other problems, and often relied on patient self-report. The investors wanted to see if the relationship held in healthy adults, using an objective measure.
The participants – all with no known cardiovascular disease – wore Acti Trainers accelerometers (Actigraph, Pensacola, Fla.) around their waists for 7 days to record sleep duration and quality. Subjects also had their plaque burdens assessed by 3-dimensional vascular ultrasound (VUS) at their carotid and femoral arteries bilaterally. Cardiac CT was used to assess coronary artery calcification as a surrogate for coronary artery atherosclerosis.
The 3,974 participants had a mean age of 46 years, and a third were women; they had a low prevalence of both hypertension and diabetes. OSA patients were excluded from the study. Overall, 27% had very short sleep duration (VSSD), less than 6 hours a night; 38% had short sleep duration (SSD), 31% slept from 7 to 8 hours per night, and served as the reference group for healthy sleep habits; and 4% had long sleep duration (LSD), greater than 8 hours.
After adjustment for a wide range of cardiovascular risk factors, including body mass index, hypertension, and smoking, VSSD was independently associated with a higher atherosclerotic burden, compared to the reference group (odds ratio, 1.27; 95% confidence interval, 1.06-1.52; P = 0.008). Participants in the highest quintile of sleep fragmentation were more likely to have plaques at multiple sites (OR, 1.34; 95% CI, 1.09-1.64; P = 0.006). The Framingham risk score at both 10 and 30 years was significantly higher in participants with VSSD or SSD, and in the highest quintiles of sleep fragmentation.
LSD was also associated with a higher plaque burden, which reached statistical significance in women. “Too-long sleep duration may not be healthy either ... Recommendations should be restricted to 7 to 8 hours,” the investigators said.
Sleep duration and quality were not associated with inflammation markers or coronary artery calcification. The investigators noted that CT for coronary artery calcification might not be as sensitive as VUS for picking up subclinical atherosclerosis.
Short sleepers tended to have higher intakes of alcohol and caffeine than did those in the 7- to 8-hour group.
The work was funded by CNIC and Banco Santander, among others. Dr. Domínguez had no disclosures. Investigator Hector Bueno, MD, PhD, reported research funding and fees from a number of companies, including AstraZeneca and Novartis. The second author, Valentín Fuster, MD, PhD, is the editor of the Journal of the American College of Cardiology, which published the report.
SOURCE: Domínguez F et al. J Am Coll Cardiol 2019;73:134-44.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: Even among healthy men and women, both too much and too little sleep are associated with atherosclerosis.
Major finding: Sleeping less than 6 hours a night was independently associated with a higher noncardiac atherosclerotic burden (OR ,1.27; 95% CI, 1.06-1.52; P = .008)
Study details: Spanish study of 3,974 bank employees.
Disclosures: The work was funded by CNIC and Banco Santander, among others. The study lead had no disclosures. Investigator Hector Bueno, MD, PhD, reported research funding and fees from a number of companies, including AstraZeneca and Novartis. The second author, Valentín Fuster, MD, PhD, is the editor of the Journal of the American College of Cardiology, which published the report.
Source: Domínguez F et al. J Am Coll Cardiol. 2019;73:134-44.
No drop in gout prevalence, but no increase either
according to data from an ongoing, nationally representative survey.
Findings from the National Health and Nutrition Examination Survey (NHANES) also show that only about one-third of gout patients were using urate-lowering therapies, Michael Chen-Xu, MBChB, MPH, of the Harvard T.H. Chan School of Public Health, Boston, and his associates wrote in Arthritis & Rheumatology.
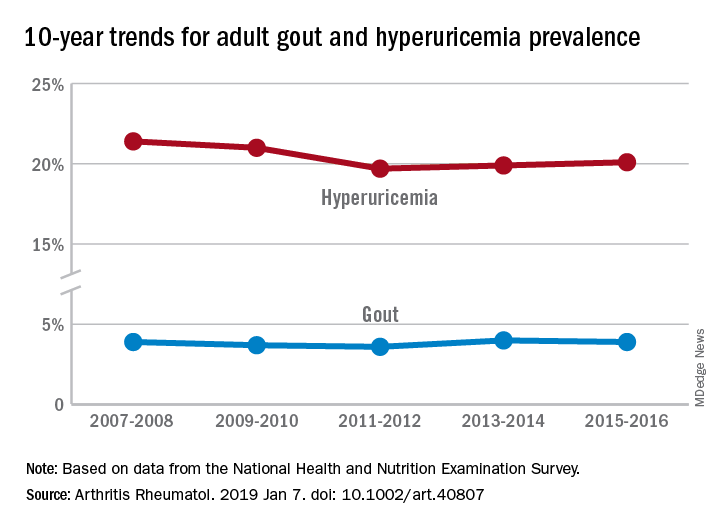
“The prevalence of gout and hyperuricemia in the United States more than doubled between the 1960s and the 1990s and continued to increase steadily afterwards,” they wrote. By 2007-2008, the earliest 2-year NHANES cycle in the study, gout prevalence among adults stood at 3.9%, but after a slight dip and a rise it was 3.9% again in 2015-2016, the last cycle in the study, the investigators reported.
The prevalence of hyperuricemia – defined as a serum urate level of more than 7.0 mg/dL in males and more than 5.7 mg/dL in females – did drop from 21.4% in 2007-2008 to 20.1% in 2015-16, but the change was not significant, Dr. Chen-Xu and his associates said.
Overall use of urate-lowering therapy (ULT) among patients with gout was 32.8% over the study period, with use showing a nonsignificant increase from 33.0% in 2007-2008 to 35.5% in 2013-2014, with a dip down to 29.4% in 2011-2012. (Data on ULT use were not available for the 2015-2016 NHANES cycle.) Current ULT use among male gout patients was 35.5% in 2013-2014 and 15.5% among women, and nearly all ULT use (95.3%) consisted of allopurinol, they said.
“Although we did not find a significant change in the trends of gout or hyperuricemia prevalence from 2007 to 2016,” Dr. Chen-Xu and his associates wrote, “10 years may not be long enough to detect what might actually be a significant trend(s) over a longer period.”
The study was supported by Ironwood and Horizon. Dr. Chen-Xu had no conflicts to disclose. One of his associates has served on advisory boards for Takeda, Ironwood, Horizon, Kowa, and Selecta. Another has served on advisory boards for Pfizer, Horizon, SOBI, and Ironwood and as a study site investigator for Takeda.
SOURCE: Chen-Xu M et al. Arthritis Rheumatol. 2019 Jan 7. doi: 10.1002/art.40807.
according to data from an ongoing, nationally representative survey.

Findings from the National Health and Nutrition Examination Survey (NHANES) also show that only about one-third of gout patients were using urate-lowering therapies, Michael Chen-Xu, MBChB, MPH, of the Harvard T.H. Chan School of Public Health, Boston, and his associates wrote in Arthritis & Rheumatology.
“The prevalence of gout and hyperuricemia in the United States more than doubled between the 1960s and the 1990s and continued to increase steadily afterwards,” they wrote. By 2007-2008, the earliest 2-year NHANES cycle in the study, gout prevalence among adults stood at 3.9%, but after a slight dip and a rise it was 3.9% again in 2015-2016, the last cycle in the study, the investigators reported.
The prevalence of hyperuricemia – defined as a serum urate level of more than 7.0 mg/dL in males and more than 5.7 mg/dL in females – did drop from 21.4% in 2007-2008 to 20.1% in 2015-16, but the change was not significant, Dr. Chen-Xu and his associates said.
Overall use of urate-lowering therapy (ULT) among patients with gout was 32.8% over the study period, with use showing a nonsignificant increase from 33.0% in 2007-2008 to 35.5% in 2013-2014, with a dip down to 29.4% in 2011-2012. (Data on ULT use were not available for the 2015-2016 NHANES cycle.) Current ULT use among male gout patients was 35.5% in 2013-2014 and 15.5% among women, and nearly all ULT use (95.3%) consisted of allopurinol, they said.
“Although we did not find a significant change in the trends of gout or hyperuricemia prevalence from 2007 to 2016,” Dr. Chen-Xu and his associates wrote, “10 years may not be long enough to detect what might actually be a significant trend(s) over a longer period.”
The study was supported by Ironwood and Horizon. Dr. Chen-Xu had no conflicts to disclose. One of his associates has served on advisory boards for Takeda, Ironwood, Horizon, Kowa, and Selecta. Another has served on advisory boards for Pfizer, Horizon, SOBI, and Ironwood and as a study site investigator for Takeda.
SOURCE: Chen-Xu M et al. Arthritis Rheumatol. 2019 Jan 7. doi: 10.1002/art.40807.
according to data from an ongoing, nationally representative survey.

Findings from the National Health and Nutrition Examination Survey (NHANES) also show that only about one-third of gout patients were using urate-lowering therapies, Michael Chen-Xu, MBChB, MPH, of the Harvard T.H. Chan School of Public Health, Boston, and his associates wrote in Arthritis & Rheumatology.
“The prevalence of gout and hyperuricemia in the United States more than doubled between the 1960s and the 1990s and continued to increase steadily afterwards,” they wrote. By 2007-2008, the earliest 2-year NHANES cycle in the study, gout prevalence among adults stood at 3.9%, but after a slight dip and a rise it was 3.9% again in 2015-2016, the last cycle in the study, the investigators reported.
The prevalence of hyperuricemia – defined as a serum urate level of more than 7.0 mg/dL in males and more than 5.7 mg/dL in females – did drop from 21.4% in 2007-2008 to 20.1% in 2015-16, but the change was not significant, Dr. Chen-Xu and his associates said.
Overall use of urate-lowering therapy (ULT) among patients with gout was 32.8% over the study period, with use showing a nonsignificant increase from 33.0% in 2007-2008 to 35.5% in 2013-2014, with a dip down to 29.4% in 2011-2012. (Data on ULT use were not available for the 2015-2016 NHANES cycle.) Current ULT use among male gout patients was 35.5% in 2013-2014 and 15.5% among women, and nearly all ULT use (95.3%) consisted of allopurinol, they said.
“Although we did not find a significant change in the trends of gout or hyperuricemia prevalence from 2007 to 2016,” Dr. Chen-Xu and his associates wrote, “10 years may not be long enough to detect what might actually be a significant trend(s) over a longer period.”
The study was supported by Ironwood and Horizon. Dr. Chen-Xu had no conflicts to disclose. One of his associates has served on advisory boards for Takeda, Ironwood, Horizon, Kowa, and Selecta. Another has served on advisory boards for Pfizer, Horizon, SOBI, and Ironwood and as a study site investigator for Takeda.
SOURCE: Chen-Xu M et al. Arthritis Rheumatol. 2019 Jan 7. doi: 10.1002/art.40807.
FROM ARTHRITIS & RHEUMATOLOGY
Baselga moves to industry; MD Anderson mourns
Jose Baselga, MD, PhD, the former chief medical officer at Memorial Sloan Kettering Cancer Center in New York, made headlines recently by accepting a position with the drugmaker AstraZeneca.
Dr. Baselga had stepped down from his position at Memorial Sloan Kettering in September 2018 after a ProPublica investigation revealed that he had failed to disclose industry funding in dozens of instances when publishing in top medical journals. In his new role, Dr. Baselga, who acknowledged the missing disclosures but said they were unintentional, will head up the research and development unit for oncology, AstraZeneca said in a press release.
The American Society of Clinical Oncology (ASCO) has elected new leadership. Lori J. Pierce, MD, a radiation oncologist and leader in breast cancer research, is the newly elected president-elect. She will serve in that role beginning June 2019 and will end her 1-year term as president of ASCO in June 2020. Dr. Pierce is a professor and vice provost for academic and faculty affairs at the University of Michigan, Ann Arbor, and the director of the Michigan Radiation Oncology Quality Consortium.
Three physicians were also elected to the ASCO board of directors. Michael A. Thompson, MD, PhD, of Advocate Aurora Health in Milwaukee, was elected to the community oncologist seat. Elizabeth A. Mittendorf, MD, PhD, of Brigham and Women’s Hospital and the Dana-Farber Cancer Institute in Boston, was elected to the surgical oncologist seat. Ethan Basch, MD, of the University of North Carolina, was elected to the undesignated specialty seat. They will all begin 4-year terms on the board of directors starting in June 2019.
A radiation oncologist is moving up the ranks at the Centers for Medicare & Medicaid Services. Anand Shah, MD, has been named senior medical advisor for innovation at the Center for Medicare & Medicaid Innovation (CMMI), part of CMS. He had previously served as the CMMI’s chief medical officer. Dr. Shah is getting congratulations from ASCO and the American Society of Radiation Oncology (ASTRO), both of whom have a lot to say about CMMI policies such as attempts to revive the Competitive Acquisition Program for Part B drugs and alternative payment models for oncology.
Julian Schink, MD, has been appointed chief medical officer at Cancer Treatment Centers of America (CTCA). Dr. Schink, a gynecologic oncologist, joined CTCA in 2017 as chief of gynecologic oncology. Before that, he had worked at the University of Wisconsin–Madison and the Northwestern University, Chicago.
In sad news, John Mendelsohn, MD, the president emeritus of the University of Texas MD Anderson Cancer Center, died on Jan. 7 at the age of 82 years. He had been diagnosed with glioblastoma 15 months earlier. Before working in medical leadership, Dr. Mendelsohn had worked with his colleagues at the University of California, San Diego, on research to block epidermal growth factor receptors. That work led to the development of the drug cetuximab, which was approved by the Food and Drug Administration to treat advanced colorectal cancer and later head and neck cancer.
The MD Anderson community also mourned the passing of Waun Ki Hong, MD, a physician-scientist who led the institution’s division of cancer medicine during 2001-2014. He died at age 76 years. Dr. Hong, who was also a past president of the American Association for Cancer Research, was well known for advancing the fields of targeted therapy and chemoprevention.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at [email protected], and you could be featured in Movers in Medicine.
Jose Baselga, MD, PhD, the former chief medical officer at Memorial Sloan Kettering Cancer Center in New York, made headlines recently by accepting a position with the drugmaker AstraZeneca.
Dr. Baselga had stepped down from his position at Memorial Sloan Kettering in September 2018 after a ProPublica investigation revealed that he had failed to disclose industry funding in dozens of instances when publishing in top medical journals. In his new role, Dr. Baselga, who acknowledged the missing disclosures but said they were unintentional, will head up the research and development unit for oncology, AstraZeneca said in a press release.
The American Society of Clinical Oncology (ASCO) has elected new leadership. Lori J. Pierce, MD, a radiation oncologist and leader in breast cancer research, is the newly elected president-elect. She will serve in that role beginning June 2019 and will end her 1-year term as president of ASCO in June 2020. Dr. Pierce is a professor and vice provost for academic and faculty affairs at the University of Michigan, Ann Arbor, and the director of the Michigan Radiation Oncology Quality Consortium.
Three physicians were also elected to the ASCO board of directors. Michael A. Thompson, MD, PhD, of Advocate Aurora Health in Milwaukee, was elected to the community oncologist seat. Elizabeth A. Mittendorf, MD, PhD, of Brigham and Women’s Hospital and the Dana-Farber Cancer Institute in Boston, was elected to the surgical oncologist seat. Ethan Basch, MD, of the University of North Carolina, was elected to the undesignated specialty seat. They will all begin 4-year terms on the board of directors starting in June 2019.
A radiation oncologist is moving up the ranks at the Centers for Medicare & Medicaid Services. Anand Shah, MD, has been named senior medical advisor for innovation at the Center for Medicare & Medicaid Innovation (CMMI), part of CMS. He had previously served as the CMMI’s chief medical officer. Dr. Shah is getting congratulations from ASCO and the American Society of Radiation Oncology (ASTRO), both of whom have a lot to say about CMMI policies such as attempts to revive the Competitive Acquisition Program for Part B drugs and alternative payment models for oncology.
Julian Schink, MD, has been appointed chief medical officer at Cancer Treatment Centers of America (CTCA). Dr. Schink, a gynecologic oncologist, joined CTCA in 2017 as chief of gynecologic oncology. Before that, he had worked at the University of Wisconsin–Madison and the Northwestern University, Chicago.
In sad news, John Mendelsohn, MD, the president emeritus of the University of Texas MD Anderson Cancer Center, died on Jan. 7 at the age of 82 years. He had been diagnosed with glioblastoma 15 months earlier. Before working in medical leadership, Dr. Mendelsohn had worked with his colleagues at the University of California, San Diego, on research to block epidermal growth factor receptors. That work led to the development of the drug cetuximab, which was approved by the Food and Drug Administration to treat advanced colorectal cancer and later head and neck cancer.
The MD Anderson community also mourned the passing of Waun Ki Hong, MD, a physician-scientist who led the institution’s division of cancer medicine during 2001-2014. He died at age 76 years. Dr. Hong, who was also a past president of the American Association for Cancer Research, was well known for advancing the fields of targeted therapy and chemoprevention.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at [email protected], and you could be featured in Movers in Medicine.
Jose Baselga, MD, PhD, the former chief medical officer at Memorial Sloan Kettering Cancer Center in New York, made headlines recently by accepting a position with the drugmaker AstraZeneca.
Dr. Baselga had stepped down from his position at Memorial Sloan Kettering in September 2018 after a ProPublica investigation revealed that he had failed to disclose industry funding in dozens of instances when publishing in top medical journals. In his new role, Dr. Baselga, who acknowledged the missing disclosures but said they were unintentional, will head up the research and development unit for oncology, AstraZeneca said in a press release.
The American Society of Clinical Oncology (ASCO) has elected new leadership. Lori J. Pierce, MD, a radiation oncologist and leader in breast cancer research, is the newly elected president-elect. She will serve in that role beginning June 2019 and will end her 1-year term as president of ASCO in June 2020. Dr. Pierce is a professor and vice provost for academic and faculty affairs at the University of Michigan, Ann Arbor, and the director of the Michigan Radiation Oncology Quality Consortium.
Three physicians were also elected to the ASCO board of directors. Michael A. Thompson, MD, PhD, of Advocate Aurora Health in Milwaukee, was elected to the community oncologist seat. Elizabeth A. Mittendorf, MD, PhD, of Brigham and Women’s Hospital and the Dana-Farber Cancer Institute in Boston, was elected to the surgical oncologist seat. Ethan Basch, MD, of the University of North Carolina, was elected to the undesignated specialty seat. They will all begin 4-year terms on the board of directors starting in June 2019.
A radiation oncologist is moving up the ranks at the Centers for Medicare & Medicaid Services. Anand Shah, MD, has been named senior medical advisor for innovation at the Center for Medicare & Medicaid Innovation (CMMI), part of CMS. He had previously served as the CMMI’s chief medical officer. Dr. Shah is getting congratulations from ASCO and the American Society of Radiation Oncology (ASTRO), both of whom have a lot to say about CMMI policies such as attempts to revive the Competitive Acquisition Program for Part B drugs and alternative payment models for oncology.
Julian Schink, MD, has been appointed chief medical officer at Cancer Treatment Centers of America (CTCA). Dr. Schink, a gynecologic oncologist, joined CTCA in 2017 as chief of gynecologic oncology. Before that, he had worked at the University of Wisconsin–Madison and the Northwestern University, Chicago.
In sad news, John Mendelsohn, MD, the president emeritus of the University of Texas MD Anderson Cancer Center, died on Jan. 7 at the age of 82 years. He had been diagnosed with glioblastoma 15 months earlier. Before working in medical leadership, Dr. Mendelsohn had worked with his colleagues at the University of California, San Diego, on research to block epidermal growth factor receptors. That work led to the development of the drug cetuximab, which was approved by the Food and Drug Administration to treat advanced colorectal cancer and later head and neck cancer.
The MD Anderson community also mourned the passing of Waun Ki Hong, MD, a physician-scientist who led the institution’s division of cancer medicine during 2001-2014. He died at age 76 years. Dr. Hong, who was also a past president of the American Association for Cancer Research, was well known for advancing the fields of targeted therapy and chemoprevention.
Movers in Medicine highlights career moves and personal achievements by hematologists and oncologists. Did you switch jobs, take on a new role, climb a mountain? Tell us all about it at [email protected], and you could be featured in Movers in Medicine.
Platinum-etoposide, taxanes best for small cell transformed EGFR-mutant lung cancer
Epidermal growth factor receptor (EGFR)–mutant non–small cell lung tumors transformed to small cell disease an average of 17.8 months after diagnosis, and this shift often involved Rb1, TP53, and PIK3CA mutations, according to the findings of a multicenter retrospective study of 67 patients.
After transformation, platinum-etoposide, paclitaxel, and nab-paclitaxel each yielded clinical response rates of 71%, while patients did not respond to programmed death-1 or programmed death-ligand 1 checkpoint inhibition, reported Nicolas Marcoux, MD, of Massachusetts General Hospital in Boston and his associates. “Indeed, none of the 17 patients [who received a checkpoint inhibitor] even seemed to derive clinical benefit from these therapies, as the longest time to progression was only 9 weeks,” the researchers wrote in the Journal of Clinical Oncology.
Interestingly, docetaxel produced no responses among six treated patients. Transformation often led to central nervous system metastases and patients survived a median of 10.7 months after transformation (95% confidence interval, 8.0-13.7 months).
Repeat biopsies showed that 3%-10% of EGFR-mutant non–small cell lung cancers transformed to small cell lung cancers. However, the subsequent clinical course has not been well characterized. Patients in this study were treated at eight cancer centers, had a history of EGFR-mutant small cell lung cancer, and most (87%) had non–small cell histology at diagnosis and received at least one EGFR tyrosine kinase inhibitor. The other nine patients had de novo small cell lung cancer or mixed histology.
All 59 patients with tissue genotyping at first evidence of small cell lung cancer retained their founder EGFR mutations, Dr. Marcoux and his associates reported. Among 19 patients with a history of EGFR T790M positivity, 15 patients were T790 wild-type at transformation. “Other recurrent mutations included TP53, Rb1, and PIK3CA,” they wrote.
The study supports the first-line use of platinum-etoposide for EGFR-mutant lung cancers that transform to small cell lung cancer, the researchers concluded. “Conversely, these tumors do not respond well to checkpoint inhibitors and the use of these therapies outside of a clinical trial should currently be discouraged.”
Funders included the National Institutes of Health, LungStrong, Targeting a Cure for Lung Cancer, Be a Piece of the Solution, the Susanne E. Coyne Memorial Fund, and a STOP Cancer Carrie Scott Grant. Dr. Marcoux disclosed honoraria from Bristol-Myers Squibb.
SOURCE: Marcoux N et al. J Clin Oncol. 2018 Dec 14. doi: 10.1200/JCO.18.01585.
Epidermal growth factor receptor (EGFR)–mutant non–small cell lung tumors transformed to small cell disease an average of 17.8 months after diagnosis, and this shift often involved Rb1, TP53, and PIK3CA mutations, according to the findings of a multicenter retrospective study of 67 patients.
After transformation, platinum-etoposide, paclitaxel, and nab-paclitaxel each yielded clinical response rates of 71%, while patients did not respond to programmed death-1 or programmed death-ligand 1 checkpoint inhibition, reported Nicolas Marcoux, MD, of Massachusetts General Hospital in Boston and his associates. “Indeed, none of the 17 patients [who received a checkpoint inhibitor] even seemed to derive clinical benefit from these therapies, as the longest time to progression was only 9 weeks,” the researchers wrote in the Journal of Clinical Oncology.
Interestingly, docetaxel produced no responses among six treated patients. Transformation often led to central nervous system metastases and patients survived a median of 10.7 months after transformation (95% confidence interval, 8.0-13.7 months).
Repeat biopsies showed that 3%-10% of EGFR-mutant non–small cell lung cancers transformed to small cell lung cancers. However, the subsequent clinical course has not been well characterized. Patients in this study were treated at eight cancer centers, had a history of EGFR-mutant small cell lung cancer, and most (87%) had non–small cell histology at diagnosis and received at least one EGFR tyrosine kinase inhibitor. The other nine patients had de novo small cell lung cancer or mixed histology.
All 59 patients with tissue genotyping at first evidence of small cell lung cancer retained their founder EGFR mutations, Dr. Marcoux and his associates reported. Among 19 patients with a history of EGFR T790M positivity, 15 patients were T790 wild-type at transformation. “Other recurrent mutations included TP53, Rb1, and PIK3CA,” they wrote.
The study supports the first-line use of platinum-etoposide for EGFR-mutant lung cancers that transform to small cell lung cancer, the researchers concluded. “Conversely, these tumors do not respond well to checkpoint inhibitors and the use of these therapies outside of a clinical trial should currently be discouraged.”
Funders included the National Institutes of Health, LungStrong, Targeting a Cure for Lung Cancer, Be a Piece of the Solution, the Susanne E. Coyne Memorial Fund, and a STOP Cancer Carrie Scott Grant. Dr. Marcoux disclosed honoraria from Bristol-Myers Squibb.
SOURCE: Marcoux N et al. J Clin Oncol. 2018 Dec 14. doi: 10.1200/JCO.18.01585.
Epidermal growth factor receptor (EGFR)–mutant non–small cell lung tumors transformed to small cell disease an average of 17.8 months after diagnosis, and this shift often involved Rb1, TP53, and PIK3CA mutations, according to the findings of a multicenter retrospective study of 67 patients.
After transformation, platinum-etoposide, paclitaxel, and nab-paclitaxel each yielded clinical response rates of 71%, while patients did not respond to programmed death-1 or programmed death-ligand 1 checkpoint inhibition, reported Nicolas Marcoux, MD, of Massachusetts General Hospital in Boston and his associates. “Indeed, none of the 17 patients [who received a checkpoint inhibitor] even seemed to derive clinical benefit from these therapies, as the longest time to progression was only 9 weeks,” the researchers wrote in the Journal of Clinical Oncology.
Interestingly, docetaxel produced no responses among six treated patients. Transformation often led to central nervous system metastases and patients survived a median of 10.7 months after transformation (95% confidence interval, 8.0-13.7 months).
Repeat biopsies showed that 3%-10% of EGFR-mutant non–small cell lung cancers transformed to small cell lung cancers. However, the subsequent clinical course has not been well characterized. Patients in this study were treated at eight cancer centers, had a history of EGFR-mutant small cell lung cancer, and most (87%) had non–small cell histology at diagnosis and received at least one EGFR tyrosine kinase inhibitor. The other nine patients had de novo small cell lung cancer or mixed histology.
All 59 patients with tissue genotyping at first evidence of small cell lung cancer retained their founder EGFR mutations, Dr. Marcoux and his associates reported. Among 19 patients with a history of EGFR T790M positivity, 15 patients were T790 wild-type at transformation. “Other recurrent mutations included TP53, Rb1, and PIK3CA,” they wrote.
The study supports the first-line use of platinum-etoposide for EGFR-mutant lung cancers that transform to small cell lung cancer, the researchers concluded. “Conversely, these tumors do not respond well to checkpoint inhibitors and the use of these therapies outside of a clinical trial should currently be discouraged.”
Funders included the National Institutes of Health, LungStrong, Targeting a Cure for Lung Cancer, Be a Piece of the Solution, the Susanne E. Coyne Memorial Fund, and a STOP Cancer Carrie Scott Grant. Dr. Marcoux disclosed honoraria from Bristol-Myers Squibb.
SOURCE: Marcoux N et al. J Clin Oncol. 2018 Dec 14. doi: 10.1200/JCO.18.01585.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Platinum-etoposide and taxane therapies elicited high response rates after transformation of EGFR-mutant non–small cell lung cancer.
Major finding: Median time to transformation was 17.8 months (95% CI, 14.3-26.2 months). Both platinum-etoposide and taxane therapies produced high response rates (71% each), but patients did not respond to checkpoint inhibitor therapy.
Data source: Multicenter retrospective study of 67 patients with EGFR-mutant small cell lung cancer.
Disclosures: Funders included the National Institutes of Health, LungStrong, Targeting a Cure for Lung Cancer, Be a Piece of the Solution, the Susanne E. Coyne Memorial Fund, and a STOP Cancer Carrie Scott Grant. Dr. Marcoux disclosed honoraria from Bristol-Myers Squibb.
Source: Marcoux N et al. J Clin Oncol. 2018 Dec 14. doi: 10.1200/JCO.18.01585.
Looking into the future and making history
Emergence of population health management
For the first time ever, on March 7, 2019, tens of thousands of hospitalists across the United States and around the world will celebrate their day, National Hospitalist Day.
On this day, we will honor the hard work and dedication of hospitalists in the care of millions of hospitalized patients. With more than 62,000 hospitalists across the United States, hospital medicine has been the fastest growing medical specialty and among the largest of all specialties in medicine. Hospitalists now lead clinical care in over 75% of U.S. hospitals, caring for patients in their communities. We educate the future providers of health care by serving as teachers and mentors. We push the boundaries of science in hospital care through innovative research that defines the evidence-based practices for our field. Hospitalists, proudly celebrate all that we have accomplished together on March 7, and moving forward, every first Thursday in March annually.
The Society for Hospital Medicine’s celebration of National Hospitalist Day will include spotlights on hospitalists, a social medical campaign, downloadable customizable posters, and much more. Stay tuned for details!
Attend the only meeting designed just for you
Be among the thousands of hospitalists who will celebrate hospital medicine in person at Hospital Medicine 2019 (HM19), March 24-27 in National Harbor, Md.
While at HM19, check out more than 20 educational tracks, including clinical updates, diagnostic reasoning, and health policy. New this year are two mini tracks: “Between the Guidelines” and “Clinical Mastery”. Between the Guidelines explores how we can address some of the most challenging cases we encounter in hospital medicine, where clear guidelines don’t exist. Clinical Mastery is designed to enhance our bedside diagnostic skills, from ECGs to ultrasounds.
Get ready to vote in HM19’s “The Great Debate” – pairing two talented clinicians who will debate opposing sides of challenging clinical decisions that we encounter on the front lines of health care delivery. Attendees have the opportunity to hear the two sides and then vote on who they believe has the right approach. There are six precourses planned for HM19, with a new offering in Palliative Care and Pain Management. This year, the annual conference also features additional sessions for our NP/PA attendees. They include specific workshops as well as a track that includes 4 didactic sessions. Lastly, HM19 will offer CME, MOC, AOS, AAFP, and Pharmacology credits to address the needs of our attendees.
Looking into the future
While hospitalists are a vital part of U.S. health care, our delivery systems are in transition with greater focus on value-based care. To ensure hospital medicine continues to thrive in today’s dynamic scene, SHM’s Board of Directors held a strategic meeting in October 2018 to focus on the role of hospitalists and hospital medicine in population health management.
There are many hospitalists across the nation who are currently involved in population health management. These range from medical directors to vice presidents of accountable care organizations, population health management, or value-based care. Hospitalists are seeking communities focused on population health management to share best practices and learn from each other. To address this, SHM’s Advocacy and Public Policy HMX community has served as a meeting point to discuss issues related to value-based care. To join the discussion, visit the community by logging in at hospitalmedicine.org/hmx. Furthermore, at HM19, hospitalists will have the opportunity to meet face to face regarding these issues in the Advocacy Special Interest Forum.
Key points: Population health management
- Source of truth
SHM has served as the source of reliable and trusted information about hospital medicine. We will continue to develop content and resources specific to population health management on our website so hospitalists can easily access this information. To increase our awareness about population health management, presenters at HM19 will integrate a slide about the implications of population health management on their clinical topic. These slides will illustrate the clinical and nonclinical services that are necessary to enhance the patient’s quality of care and life. In addition to best practice care, these slides will highlight topics like the role of style modification and prevention, risk stratification, chronic disease management, and care coordination throughout the continuum of care.
- Advocating for us
In addition to providing a home for hospitalists to collaborate regarding population health management, SHM will advance this agenda from a regulatory perspective. The Public Policy and Performance Measurement & Reporting Committees are actively evaluating and leading the transition from value to volume. SHM is also working with potential key partners and organizations in the areas of primary care, skilled nursing facilities, and accountable care organizations that will help improve the effectiveness of delivering population health management.
- Creating expertise
SHM will lead best practice development for tools and skills that are necessary for hospitalists to lead population health management. Telemedicine is an increasingly critical tool as we help manage our patients in other facilities, inpatient or skilled nursing facilities, as well as at home. SHM has developed a white paper about telemedicine in hospital medicine that highlights modalities, offerings, implementation of programs, and work flows necessary for success. You can find it under “Resources” at hospitalmedicine.org/telemedicine.
SHM will continue to actively develop tools that appropriately address the challenges we’re facing. From National Hospitalist Day to population health management, this is an exciting time in hospital medicine – I hope to see you at HM19 to celebrate our specialty and our bright future.
Dr. Afsar is president of the Society of Hospital Medicine, and chief ambulatory officer and chief medical officer for accountable care organizations at UC Irvine Health.
Emergence of population health management
Emergence of population health management
For the first time ever, on March 7, 2019, tens of thousands of hospitalists across the United States and around the world will celebrate their day, National Hospitalist Day.
On this day, we will honor the hard work and dedication of hospitalists in the care of millions of hospitalized patients. With more than 62,000 hospitalists across the United States, hospital medicine has been the fastest growing medical specialty and among the largest of all specialties in medicine. Hospitalists now lead clinical care in over 75% of U.S. hospitals, caring for patients in their communities. We educate the future providers of health care by serving as teachers and mentors. We push the boundaries of science in hospital care through innovative research that defines the evidence-based practices for our field. Hospitalists, proudly celebrate all that we have accomplished together on March 7, and moving forward, every first Thursday in March annually.
The Society for Hospital Medicine’s celebration of National Hospitalist Day will include spotlights on hospitalists, a social medical campaign, downloadable customizable posters, and much more. Stay tuned for details!
Attend the only meeting designed just for you
Be among the thousands of hospitalists who will celebrate hospital medicine in person at Hospital Medicine 2019 (HM19), March 24-27 in National Harbor, Md.
While at HM19, check out more than 20 educational tracks, including clinical updates, diagnostic reasoning, and health policy. New this year are two mini tracks: “Between the Guidelines” and “Clinical Mastery”. Between the Guidelines explores how we can address some of the most challenging cases we encounter in hospital medicine, where clear guidelines don’t exist. Clinical Mastery is designed to enhance our bedside diagnostic skills, from ECGs to ultrasounds.
Get ready to vote in HM19’s “The Great Debate” – pairing two talented clinicians who will debate opposing sides of challenging clinical decisions that we encounter on the front lines of health care delivery. Attendees have the opportunity to hear the two sides and then vote on who they believe has the right approach. There are six precourses planned for HM19, with a new offering in Palliative Care and Pain Management. This year, the annual conference also features additional sessions for our NP/PA attendees. They include specific workshops as well as a track that includes 4 didactic sessions. Lastly, HM19 will offer CME, MOC, AOS, AAFP, and Pharmacology credits to address the needs of our attendees.
Looking into the future
While hospitalists are a vital part of U.S. health care, our delivery systems are in transition with greater focus on value-based care. To ensure hospital medicine continues to thrive in today’s dynamic scene, SHM’s Board of Directors held a strategic meeting in October 2018 to focus on the role of hospitalists and hospital medicine in population health management.
There are many hospitalists across the nation who are currently involved in population health management. These range from medical directors to vice presidents of accountable care organizations, population health management, or value-based care. Hospitalists are seeking communities focused on population health management to share best practices and learn from each other. To address this, SHM’s Advocacy and Public Policy HMX community has served as a meeting point to discuss issues related to value-based care. To join the discussion, visit the community by logging in at hospitalmedicine.org/hmx. Furthermore, at HM19, hospitalists will have the opportunity to meet face to face regarding these issues in the Advocacy Special Interest Forum.
Key points: Population health management
- Source of truth
SHM has served as the source of reliable and trusted information about hospital medicine. We will continue to develop content and resources specific to population health management on our website so hospitalists can easily access this information. To increase our awareness about population health management, presenters at HM19 will integrate a slide about the implications of population health management on their clinical topic. These slides will illustrate the clinical and nonclinical services that are necessary to enhance the patient’s quality of care and life. In addition to best practice care, these slides will highlight topics like the role of style modification and prevention, risk stratification, chronic disease management, and care coordination throughout the continuum of care.
- Advocating for us
In addition to providing a home for hospitalists to collaborate regarding population health management, SHM will advance this agenda from a regulatory perspective. The Public Policy and Performance Measurement & Reporting Committees are actively evaluating and leading the transition from value to volume. SHM is also working with potential key partners and organizations in the areas of primary care, skilled nursing facilities, and accountable care organizations that will help improve the effectiveness of delivering population health management.
- Creating expertise
SHM will lead best practice development for tools and skills that are necessary for hospitalists to lead population health management. Telemedicine is an increasingly critical tool as we help manage our patients in other facilities, inpatient or skilled nursing facilities, as well as at home. SHM has developed a white paper about telemedicine in hospital medicine that highlights modalities, offerings, implementation of programs, and work flows necessary for success. You can find it under “Resources” at hospitalmedicine.org/telemedicine.
SHM will continue to actively develop tools that appropriately address the challenges we’re facing. From National Hospitalist Day to population health management, this is an exciting time in hospital medicine – I hope to see you at HM19 to celebrate our specialty and our bright future.
Dr. Afsar is president of the Society of Hospital Medicine, and chief ambulatory officer and chief medical officer for accountable care organizations at UC Irvine Health.
For the first time ever, on March 7, 2019, tens of thousands of hospitalists across the United States and around the world will celebrate their day, National Hospitalist Day.
On this day, we will honor the hard work and dedication of hospitalists in the care of millions of hospitalized patients. With more than 62,000 hospitalists across the United States, hospital medicine has been the fastest growing medical specialty and among the largest of all specialties in medicine. Hospitalists now lead clinical care in over 75% of U.S. hospitals, caring for patients in their communities. We educate the future providers of health care by serving as teachers and mentors. We push the boundaries of science in hospital care through innovative research that defines the evidence-based practices for our field. Hospitalists, proudly celebrate all that we have accomplished together on March 7, and moving forward, every first Thursday in March annually.
The Society for Hospital Medicine’s celebration of National Hospitalist Day will include spotlights on hospitalists, a social medical campaign, downloadable customizable posters, and much more. Stay tuned for details!
Attend the only meeting designed just for you
Be among the thousands of hospitalists who will celebrate hospital medicine in person at Hospital Medicine 2019 (HM19), March 24-27 in National Harbor, Md.
While at HM19, check out more than 20 educational tracks, including clinical updates, diagnostic reasoning, and health policy. New this year are two mini tracks: “Between the Guidelines” and “Clinical Mastery”. Between the Guidelines explores how we can address some of the most challenging cases we encounter in hospital medicine, where clear guidelines don’t exist. Clinical Mastery is designed to enhance our bedside diagnostic skills, from ECGs to ultrasounds.
Get ready to vote in HM19’s “The Great Debate” – pairing two talented clinicians who will debate opposing sides of challenging clinical decisions that we encounter on the front lines of health care delivery. Attendees have the opportunity to hear the two sides and then vote on who they believe has the right approach. There are six precourses planned for HM19, with a new offering in Palliative Care and Pain Management. This year, the annual conference also features additional sessions for our NP/PA attendees. They include specific workshops as well as a track that includes 4 didactic sessions. Lastly, HM19 will offer CME, MOC, AOS, AAFP, and Pharmacology credits to address the needs of our attendees.
Looking into the future
While hospitalists are a vital part of U.S. health care, our delivery systems are in transition with greater focus on value-based care. To ensure hospital medicine continues to thrive in today’s dynamic scene, SHM’s Board of Directors held a strategic meeting in October 2018 to focus on the role of hospitalists and hospital medicine in population health management.
There are many hospitalists across the nation who are currently involved in population health management. These range from medical directors to vice presidents of accountable care organizations, population health management, or value-based care. Hospitalists are seeking communities focused on population health management to share best practices and learn from each other. To address this, SHM’s Advocacy and Public Policy HMX community has served as a meeting point to discuss issues related to value-based care. To join the discussion, visit the community by logging in at hospitalmedicine.org/hmx. Furthermore, at HM19, hospitalists will have the opportunity to meet face to face regarding these issues in the Advocacy Special Interest Forum.
Key points: Population health management
- Source of truth
SHM has served as the source of reliable and trusted information about hospital medicine. We will continue to develop content and resources specific to population health management on our website so hospitalists can easily access this information. To increase our awareness about population health management, presenters at HM19 will integrate a slide about the implications of population health management on their clinical topic. These slides will illustrate the clinical and nonclinical services that are necessary to enhance the patient’s quality of care and life. In addition to best practice care, these slides will highlight topics like the role of style modification and prevention, risk stratification, chronic disease management, and care coordination throughout the continuum of care.
- Advocating for us
In addition to providing a home for hospitalists to collaborate regarding population health management, SHM will advance this agenda from a regulatory perspective. The Public Policy and Performance Measurement & Reporting Committees are actively evaluating and leading the transition from value to volume. SHM is also working with potential key partners and organizations in the areas of primary care, skilled nursing facilities, and accountable care organizations that will help improve the effectiveness of delivering population health management.
- Creating expertise
SHM will lead best practice development for tools and skills that are necessary for hospitalists to lead population health management. Telemedicine is an increasingly critical tool as we help manage our patients in other facilities, inpatient or skilled nursing facilities, as well as at home. SHM has developed a white paper about telemedicine in hospital medicine that highlights modalities, offerings, implementation of programs, and work flows necessary for success. You can find it under “Resources” at hospitalmedicine.org/telemedicine.
SHM will continue to actively develop tools that appropriately address the challenges we’re facing. From National Hospitalist Day to population health management, this is an exciting time in hospital medicine – I hope to see you at HM19 to celebrate our specialty and our bright future.
Dr. Afsar is president of the Society of Hospital Medicine, and chief ambulatory officer and chief medical officer for accountable care organizations at UC Irvine Health.
Back pain persists in one in five patients
according to data from a population-based study of more than 12,000 adults in Canada.
“Given that back pain [BP] is often recurrent, it is important to understand the course of back pain over time as this can provide additional insights on risk factors for nonfavorable outcomes,” wrote Mayilee Canizares, PhD, and her colleagues at the University Health Network’s Krembil Research Institute in Toronto.
In a longitudinal study published in Arthritis Care & Research, the investigators followed 12,782 adults from 1994 to 2011. The study population was a representative sample of the Canadian population via the National Population Health Survey, which collected data every 2 years for a total of nine cycles of data. They included people aged 15 years or older in 1994-1995 who had at least three cycles of data from baseline onward.
Over the 16-year study period, 46% of the participants reported at least one episode of back pain. Of these, 18% were identified as persistent, 28% as developing, 21% as recovering, and 33% as occasional.
“A major finding from this study is the negative impact of persistent BP on a range of health-related outcomes, including health care use, after adjustments for sociodemographic, behavior-related factors, and comorbidities,” the researchers wrote.
They examined several sociodemographic variables, including age, gender, educational level, and household income, as well as behavior-related variables including physical activity, work activity, smoking status, and obesity. The average age of the participants at baseline was 39 years; 51% were female.
Individuals who reported any back pain were more likely than those with no back pain to be overweight or obese, to smoke, to engage in moderate to heavy physical activity each day, and to have chronic conditions, including arthritis, depression, high blood pressure, and migraine.
Overall, individuals with persistent or developing BP had more pain, disability, health care visits, and medication use, compared with those in the recovery and occasional BP groups. However, individuals in the recovery group showed increased use of opioids and antidepressants over time as well, suggesting a need for long-term monitoring of back pain patients.
The trend in general disability was greatest for individuals in the persistent group followed by the developing group, recovery group, and occasional BP group.
The study findings were limited by several factors, including the use of self-reports, potential selection bias, and the inability to differentiate the specific types of back pain, the researchers noted. However, the results support and extend data from previous studies and provide clinical implications for understanding back pain.
The researchers concluded that “the different trajectory patterns potentially represent subgroups in the population that may require different interventions. In light of the trend of marked worsening outcomes, particularly for the persistent and developing groups, studies are needed to determine the nature of these groups.”
The authors reported no relevant financial conflicts.
SOURCE: Canizares M et al. Arthritis Care Res. 2019 Jan 14. doi: 10.1002/acr.23811.
according to data from a population-based study of more than 12,000 adults in Canada.
“Given that back pain [BP] is often recurrent, it is important to understand the course of back pain over time as this can provide additional insights on risk factors for nonfavorable outcomes,” wrote Mayilee Canizares, PhD, and her colleagues at the University Health Network’s Krembil Research Institute in Toronto.
In a longitudinal study published in Arthritis Care & Research, the investigators followed 12,782 adults from 1994 to 2011. The study population was a representative sample of the Canadian population via the National Population Health Survey, which collected data every 2 years for a total of nine cycles of data. They included people aged 15 years or older in 1994-1995 who had at least three cycles of data from baseline onward.
Over the 16-year study period, 46% of the participants reported at least one episode of back pain. Of these, 18% were identified as persistent, 28% as developing, 21% as recovering, and 33% as occasional.
“A major finding from this study is the negative impact of persistent BP on a range of health-related outcomes, including health care use, after adjustments for sociodemographic, behavior-related factors, and comorbidities,” the researchers wrote.
They examined several sociodemographic variables, including age, gender, educational level, and household income, as well as behavior-related variables including physical activity, work activity, smoking status, and obesity. The average age of the participants at baseline was 39 years; 51% were female.
Individuals who reported any back pain were more likely than those with no back pain to be overweight or obese, to smoke, to engage in moderate to heavy physical activity each day, and to have chronic conditions, including arthritis, depression, high blood pressure, and migraine.
Overall, individuals with persistent or developing BP had more pain, disability, health care visits, and medication use, compared with those in the recovery and occasional BP groups. However, individuals in the recovery group showed increased use of opioids and antidepressants over time as well, suggesting a need for long-term monitoring of back pain patients.
The trend in general disability was greatest for individuals in the persistent group followed by the developing group, recovery group, and occasional BP group.
The study findings were limited by several factors, including the use of self-reports, potential selection bias, and the inability to differentiate the specific types of back pain, the researchers noted. However, the results support and extend data from previous studies and provide clinical implications for understanding back pain.
The researchers concluded that “the different trajectory patterns potentially represent subgroups in the population that may require different interventions. In light of the trend of marked worsening outcomes, particularly for the persistent and developing groups, studies are needed to determine the nature of these groups.”
The authors reported no relevant financial conflicts.
SOURCE: Canizares M et al. Arthritis Care Res. 2019 Jan 14. doi: 10.1002/acr.23811.
according to data from a population-based study of more than 12,000 adults in Canada.
“Given that back pain [BP] is often recurrent, it is important to understand the course of back pain over time as this can provide additional insights on risk factors for nonfavorable outcomes,” wrote Mayilee Canizares, PhD, and her colleagues at the University Health Network’s Krembil Research Institute in Toronto.
In a longitudinal study published in Arthritis Care & Research, the investigators followed 12,782 adults from 1994 to 2011. The study population was a representative sample of the Canadian population via the National Population Health Survey, which collected data every 2 years for a total of nine cycles of data. They included people aged 15 years or older in 1994-1995 who had at least three cycles of data from baseline onward.
Over the 16-year study period, 46% of the participants reported at least one episode of back pain. Of these, 18% were identified as persistent, 28% as developing, 21% as recovering, and 33% as occasional.
“A major finding from this study is the negative impact of persistent BP on a range of health-related outcomes, including health care use, after adjustments for sociodemographic, behavior-related factors, and comorbidities,” the researchers wrote.
They examined several sociodemographic variables, including age, gender, educational level, and household income, as well as behavior-related variables including physical activity, work activity, smoking status, and obesity. The average age of the participants at baseline was 39 years; 51% were female.
Individuals who reported any back pain were more likely than those with no back pain to be overweight or obese, to smoke, to engage in moderate to heavy physical activity each day, and to have chronic conditions, including arthritis, depression, high blood pressure, and migraine.
Overall, individuals with persistent or developing BP had more pain, disability, health care visits, and medication use, compared with those in the recovery and occasional BP groups. However, individuals in the recovery group showed increased use of opioids and antidepressants over time as well, suggesting a need for long-term monitoring of back pain patients.
The trend in general disability was greatest for individuals in the persistent group followed by the developing group, recovery group, and occasional BP group.
The study findings were limited by several factors, including the use of self-reports, potential selection bias, and the inability to differentiate the specific types of back pain, the researchers noted. However, the results support and extend data from previous studies and provide clinical implications for understanding back pain.
The researchers concluded that “the different trajectory patterns potentially represent subgroups in the population that may require different interventions. In light of the trend of marked worsening outcomes, particularly for the persistent and developing groups, studies are needed to determine the nature of these groups.”
The authors reported no relevant financial conflicts.
SOURCE: Canizares M et al. Arthritis Care Res. 2019 Jan 14. doi: 10.1002/acr.23811.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: Back pain is a common health problem, and one in five Canadian adults reported persistent back pain.
Major finding: Approximately half (46%) of Canadian adults reported some type of back pain over a 16-year period.
Study details: The data come from a population-based study of 12,782 adults followed from 1994 to 2011.
Disclosures: The authors reported no relevant financial conflicts.
Source: Canizares M et al. Arthritis Care Res. 2019 Jan 14. doi: 10.1002/acr.23811.
Opioid crisis offers poignant lessons for public health
Populations and circumstances matter
As a medical student in New York City in the mid-1980s, I did several of my clinical clerkships at Memorial Sloan Kettering Cancer Center. One night during my general surgery rotation, there was a young woman with anal cancer who complained of pain.
My resident did not want to give her more medication and I asked why. After all, this was a cancer center with progressive ideas about pain management, this patient was suffering, and she was ill enough to be hospitalized.
The resident responded to my inquiry: “She doesn’t have a terminal condition and she has an addictive personality.” It seemed to me a draconian (and perhaps sexist) response in a hospital where patient-controlled analgesia was becoming routine and, as an aspiring psychiatrist, I didn’t quite trust the surgical resident’s evaluation of the patient’s personality or his ability to predict if she might become addicted to opiates.
This encounter happened about 6 years after Jane Porter and Hershel Jink, MD, had published a letter titled, “Addiction Rare in Patients Treated with Narcotics” in the New England Journal of Medicine (1980 Jan 10;302:123) with the following finding: “... of 11,800 patients given narcotic painkillers while in hospital, only four developed an addiction to those drugs.” This fragment of a sentence, published as a one-paragraph letter and not as a full, peer-reviewed study, was in the process of changing how all of American medicine responded to pain.
In his book, “Dreamland: The True Tale of America’s Opiate Epidemic” (Bloomsbury Press, 2015), journalist Sam Quinones was quick to point out that these findings were made at a time when doctors, like my surgical resident, were hesitant to use opiates for fear of addiction. Their use was limited to cancer patients, postoperative patients, and those suffering from an acute injury. This finding that prescribed opiates did not cause addiction was true in these hospitalized patients, at a time when pills were doled out with caution for short-term use, and their use for chronic pain had not yet been tested.
Nearly 40 years later, we know that the answer to that national experiment did not work out so well: A proportion of patients given long-term, sometimes high-dose, opiates for chronic pain do sometimes become addicted. Some chronic pain patients received narcotics at “pill mills,” and some went on to use heroin obtained illegally. Furthermore, the widespread use of these medicines made them more readily available to those looking for something besides pain relief.
I would like to suggest that the opioid epidemic is not solely the fault of the medical community: We had drug addiction long before we had the Porter and Jink paragraph and not all addiction starts with a prescription pad. Still, the lesson for public health is a poignant one: Populations and circumstances matter. Be careful with generalizations.
Still, we see these generalizations all the time. I am sometimes surprised at how many people have “the answer.” Whether it’s more widespread availability of Narcan, medication-assisted treatment (MAT), safe injection sites, 12-step programs, or “Just Say No,” every method has its proponents. I always wonder when I see public health officials propose safe injection sites as something that would surely save thousands of lives, citing data out of cities such as Vancouver, as well as in Europe, and Australia, if results in those places would transfer to my city – Baltimore – where drug addiction, violence, and poverty are rampant. Perhaps they would, and I would love to see Baltimore try anything that might work. But I hope cities that do set up such sites will follow the numbers and halt any program that does not offer robust results.
I wonder, as well, why, with the clear success of MAT strategies in reducing mortality, we don’t experiment with ways of making these methods more accessible. Might Suboxone work if doctors could prescribe it as easily as they can prescribe oxycodone, with no 8-hour course or DEA waiver? Might methadone both work and be more acceptable to patients if given in a way that didn’t require daily travel to a clinic for administration? With such a deadly pervasive epidemic, I wonder also about our focus on treating addiction, when it seems we should have a parallel focus on understanding and addressing the factors that cause addiction. Medical prescribing is but one avenue to addiction, yet we have no understanding as to why some people become addicted when others do not. Shouldn’t we be able to prevent addiction? From Richard Nixon’s “war on drugs” to Donald Trump’s physical border wall, there are many answers, but few solutions.
There are other public health issues that suffer from the same generalizations. In psychiatry, advocacy groups tout involuntary outpatient treatment as a successful way of getting treatment to vulnerable individuals who will not willingly negotiate their own care. While a pilot study at Bellevue showed no benefit to mandated care, a follow-up study showed that mandated treatment was effective at reducing hospital days. While outpatient commitment studies look at rates of hospitalization, incarceration, and quality-of-life measures, mandated treatment is often cited as a means to prevent all forms of violence, including mass shootings, while there is no evidence to support these ideas. Still, 47 states and the District of Columbia now have outpatient commitment laws.
Does involuntary care benefit those with substance use disorders? In Massachusetts, Section 35 allows for civil commitment for drug treatment, and many of the treatment facilities are run by the Department of Corrections. It would be good to know if these measures worked. So far, it looks like opioid deaths in Massachusetts have stabilized, while the overdose death rate continues to rise in other states. Whether this is a result of Section 35 or other measures is unknown.
I’m not against innovation, and desperate situations call for creative responses. We need to be careful that our responses are measured and these experiments are contained while ascertaining what really does work and what does not cause unintended harms. Will a concrete wall stem the flow of illegal heroin? I imagine a new world of drones making drug drops.
Sometimes our innovative best guesses don’t work, and sometimes they do. Despite easy access to antidepressant medications, a national suicide hotline, increased numbers of mental health professionals, and anti-stigma/awareness campaigns, suicide rates continue to rise. Efforts to end smoking, however, have been quite successful, as have measures to get Americans to buckle their seat belts, and these measures have decreased mortality rates. The recommendation for healthy women to take hormone therapy is a good example: It was an innovative recommendation to help cardiac and orthopedic outcomes, yet studies that were run alongside these recommendations were quick to show an unintended increased risk of breast and uterine cancer.
I don’t know what happened to the young woman on my surgical rotation. If the decision were mine, I would have given her more pain medication, even now, but I don’t know if that would have been the right thing to do., and to look carefully at our outcomes in a variety of populations and circumstances.
Dr. Miller is the coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice in Baltimore.
Populations and circumstances matter
Populations and circumstances matter
As a medical student in New York City in the mid-1980s, I did several of my clinical clerkships at Memorial Sloan Kettering Cancer Center. One night during my general surgery rotation, there was a young woman with anal cancer who complained of pain.
My resident did not want to give her more medication and I asked why. After all, this was a cancer center with progressive ideas about pain management, this patient was suffering, and she was ill enough to be hospitalized.
The resident responded to my inquiry: “She doesn’t have a terminal condition and she has an addictive personality.” It seemed to me a draconian (and perhaps sexist) response in a hospital where patient-controlled analgesia was becoming routine and, as an aspiring psychiatrist, I didn’t quite trust the surgical resident’s evaluation of the patient’s personality or his ability to predict if she might become addicted to opiates.
This encounter happened about 6 years after Jane Porter and Hershel Jink, MD, had published a letter titled, “Addiction Rare in Patients Treated with Narcotics” in the New England Journal of Medicine (1980 Jan 10;302:123) with the following finding: “... of 11,800 patients given narcotic painkillers while in hospital, only four developed an addiction to those drugs.” This fragment of a sentence, published as a one-paragraph letter and not as a full, peer-reviewed study, was in the process of changing how all of American medicine responded to pain.
In his book, “Dreamland: The True Tale of America’s Opiate Epidemic” (Bloomsbury Press, 2015), journalist Sam Quinones was quick to point out that these findings were made at a time when doctors, like my surgical resident, were hesitant to use opiates for fear of addiction. Their use was limited to cancer patients, postoperative patients, and those suffering from an acute injury. This finding that prescribed opiates did not cause addiction was true in these hospitalized patients, at a time when pills were doled out with caution for short-term use, and their use for chronic pain had not yet been tested.
Nearly 40 years later, we know that the answer to that national experiment did not work out so well: A proportion of patients given long-term, sometimes high-dose, opiates for chronic pain do sometimes become addicted. Some chronic pain patients received narcotics at “pill mills,” and some went on to use heroin obtained illegally. Furthermore, the widespread use of these medicines made them more readily available to those looking for something besides pain relief.
I would like to suggest that the opioid epidemic is not solely the fault of the medical community: We had drug addiction long before we had the Porter and Jink paragraph and not all addiction starts with a prescription pad. Still, the lesson for public health is a poignant one: Populations and circumstances matter. Be careful with generalizations.
Still, we see these generalizations all the time. I am sometimes surprised at how many people have “the answer.” Whether it’s more widespread availability of Narcan, medication-assisted treatment (MAT), safe injection sites, 12-step programs, or “Just Say No,” every method has its proponents. I always wonder when I see public health officials propose safe injection sites as something that would surely save thousands of lives, citing data out of cities such as Vancouver, as well as in Europe, and Australia, if results in those places would transfer to my city – Baltimore – where drug addiction, violence, and poverty are rampant. Perhaps they would, and I would love to see Baltimore try anything that might work. But I hope cities that do set up such sites will follow the numbers and halt any program that does not offer robust results.
I wonder, as well, why, with the clear success of MAT strategies in reducing mortality, we don’t experiment with ways of making these methods more accessible. Might Suboxone work if doctors could prescribe it as easily as they can prescribe oxycodone, with no 8-hour course or DEA waiver? Might methadone both work and be more acceptable to patients if given in a way that didn’t require daily travel to a clinic for administration? With such a deadly pervasive epidemic, I wonder also about our focus on treating addiction, when it seems we should have a parallel focus on understanding and addressing the factors that cause addiction. Medical prescribing is but one avenue to addiction, yet we have no understanding as to why some people become addicted when others do not. Shouldn’t we be able to prevent addiction? From Richard Nixon’s “war on drugs” to Donald Trump’s physical border wall, there are many answers, but few solutions.
There are other public health issues that suffer from the same generalizations. In psychiatry, advocacy groups tout involuntary outpatient treatment as a successful way of getting treatment to vulnerable individuals who will not willingly negotiate their own care. While a pilot study at Bellevue showed no benefit to mandated care, a follow-up study showed that mandated treatment was effective at reducing hospital days. While outpatient commitment studies look at rates of hospitalization, incarceration, and quality-of-life measures, mandated treatment is often cited as a means to prevent all forms of violence, including mass shootings, while there is no evidence to support these ideas. Still, 47 states and the District of Columbia now have outpatient commitment laws.
Does involuntary care benefit those with substance use disorders? In Massachusetts, Section 35 allows for civil commitment for drug treatment, and many of the treatment facilities are run by the Department of Corrections. It would be good to know if these measures worked. So far, it looks like opioid deaths in Massachusetts have stabilized, while the overdose death rate continues to rise in other states. Whether this is a result of Section 35 or other measures is unknown.
I’m not against innovation, and desperate situations call for creative responses. We need to be careful that our responses are measured and these experiments are contained while ascertaining what really does work and what does not cause unintended harms. Will a concrete wall stem the flow of illegal heroin? I imagine a new world of drones making drug drops.
Sometimes our innovative best guesses don’t work, and sometimes they do. Despite easy access to antidepressant medications, a national suicide hotline, increased numbers of mental health professionals, and anti-stigma/awareness campaigns, suicide rates continue to rise. Efforts to end smoking, however, have been quite successful, as have measures to get Americans to buckle their seat belts, and these measures have decreased mortality rates. The recommendation for healthy women to take hormone therapy is a good example: It was an innovative recommendation to help cardiac and orthopedic outcomes, yet studies that were run alongside these recommendations were quick to show an unintended increased risk of breast and uterine cancer.
I don’t know what happened to the young woman on my surgical rotation. If the decision were mine, I would have given her more pain medication, even now, but I don’t know if that would have been the right thing to do., and to look carefully at our outcomes in a variety of populations and circumstances.
Dr. Miller is the coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice in Baltimore.
As a medical student in New York City in the mid-1980s, I did several of my clinical clerkships at Memorial Sloan Kettering Cancer Center. One night during my general surgery rotation, there was a young woman with anal cancer who complained of pain.
My resident did not want to give her more medication and I asked why. After all, this was a cancer center with progressive ideas about pain management, this patient was suffering, and she was ill enough to be hospitalized.
The resident responded to my inquiry: “She doesn’t have a terminal condition and she has an addictive personality.” It seemed to me a draconian (and perhaps sexist) response in a hospital where patient-controlled analgesia was becoming routine and, as an aspiring psychiatrist, I didn’t quite trust the surgical resident’s evaluation of the patient’s personality or his ability to predict if she might become addicted to opiates.
This encounter happened about 6 years after Jane Porter and Hershel Jink, MD, had published a letter titled, “Addiction Rare in Patients Treated with Narcotics” in the New England Journal of Medicine (1980 Jan 10;302:123) with the following finding: “... of 11,800 patients given narcotic painkillers while in hospital, only four developed an addiction to those drugs.” This fragment of a sentence, published as a one-paragraph letter and not as a full, peer-reviewed study, was in the process of changing how all of American medicine responded to pain.
In his book, “Dreamland: The True Tale of America’s Opiate Epidemic” (Bloomsbury Press, 2015), journalist Sam Quinones was quick to point out that these findings were made at a time when doctors, like my surgical resident, were hesitant to use opiates for fear of addiction. Their use was limited to cancer patients, postoperative patients, and those suffering from an acute injury. This finding that prescribed opiates did not cause addiction was true in these hospitalized patients, at a time when pills were doled out with caution for short-term use, and their use for chronic pain had not yet been tested.
Nearly 40 years later, we know that the answer to that national experiment did not work out so well: A proportion of patients given long-term, sometimes high-dose, opiates for chronic pain do sometimes become addicted. Some chronic pain patients received narcotics at “pill mills,” and some went on to use heroin obtained illegally. Furthermore, the widespread use of these medicines made them more readily available to those looking for something besides pain relief.
I would like to suggest that the opioid epidemic is not solely the fault of the medical community: We had drug addiction long before we had the Porter and Jink paragraph and not all addiction starts with a prescription pad. Still, the lesson for public health is a poignant one: Populations and circumstances matter. Be careful with generalizations.
Still, we see these generalizations all the time. I am sometimes surprised at how many people have “the answer.” Whether it’s more widespread availability of Narcan, medication-assisted treatment (MAT), safe injection sites, 12-step programs, or “Just Say No,” every method has its proponents. I always wonder when I see public health officials propose safe injection sites as something that would surely save thousands of lives, citing data out of cities such as Vancouver, as well as in Europe, and Australia, if results in those places would transfer to my city – Baltimore – where drug addiction, violence, and poverty are rampant. Perhaps they would, and I would love to see Baltimore try anything that might work. But I hope cities that do set up such sites will follow the numbers and halt any program that does not offer robust results.
I wonder, as well, why, with the clear success of MAT strategies in reducing mortality, we don’t experiment with ways of making these methods more accessible. Might Suboxone work if doctors could prescribe it as easily as they can prescribe oxycodone, with no 8-hour course or DEA waiver? Might methadone both work and be more acceptable to patients if given in a way that didn’t require daily travel to a clinic for administration? With such a deadly pervasive epidemic, I wonder also about our focus on treating addiction, when it seems we should have a parallel focus on understanding and addressing the factors that cause addiction. Medical prescribing is but one avenue to addiction, yet we have no understanding as to why some people become addicted when others do not. Shouldn’t we be able to prevent addiction? From Richard Nixon’s “war on drugs” to Donald Trump’s physical border wall, there are many answers, but few solutions.
There are other public health issues that suffer from the same generalizations. In psychiatry, advocacy groups tout involuntary outpatient treatment as a successful way of getting treatment to vulnerable individuals who will not willingly negotiate their own care. While a pilot study at Bellevue showed no benefit to mandated care, a follow-up study showed that mandated treatment was effective at reducing hospital days. While outpatient commitment studies look at rates of hospitalization, incarceration, and quality-of-life measures, mandated treatment is often cited as a means to prevent all forms of violence, including mass shootings, while there is no evidence to support these ideas. Still, 47 states and the District of Columbia now have outpatient commitment laws.
Does involuntary care benefit those with substance use disorders? In Massachusetts, Section 35 allows for civil commitment for drug treatment, and many of the treatment facilities are run by the Department of Corrections. It would be good to know if these measures worked. So far, it looks like opioid deaths in Massachusetts have stabilized, while the overdose death rate continues to rise in other states. Whether this is a result of Section 35 or other measures is unknown.
I’m not against innovation, and desperate situations call for creative responses. We need to be careful that our responses are measured and these experiments are contained while ascertaining what really does work and what does not cause unintended harms. Will a concrete wall stem the flow of illegal heroin? I imagine a new world of drones making drug drops.
Sometimes our innovative best guesses don’t work, and sometimes they do. Despite easy access to antidepressant medications, a national suicide hotline, increased numbers of mental health professionals, and anti-stigma/awareness campaigns, suicide rates continue to rise. Efforts to end smoking, however, have been quite successful, as have measures to get Americans to buckle their seat belts, and these measures have decreased mortality rates. The recommendation for healthy women to take hormone therapy is a good example: It was an innovative recommendation to help cardiac and orthopedic outcomes, yet studies that were run alongside these recommendations were quick to show an unintended increased risk of breast and uterine cancer.
I don’t know what happened to the young woman on my surgical rotation. If the decision were mine, I would have given her more pain medication, even now, but I don’t know if that would have been the right thing to do., and to look carefully at our outcomes in a variety of populations and circumstances.
Dr. Miller is the coauthor with Annette Hanson, MD, of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice in Baltimore.
Tic disorders are associated with obesity and diabetes
The movement disorders are associated with cardiometabolic problems “even after taking into account a number of covariates and shared familial confounders and excluding relevant psychiatric comorbidities,” the researchers wrote. “The results highlight the importance of carefully monitoring cardiometabolic health in patients with Tourette syndrome or chronic tic disorder across the lifespan, particularly in those with comorbid attention-deficit/hyperactivity disorder (ADHD).”
Gustaf Brander, a researcher in the department of clinical neuroscience at Karolinska Institutet in Stockholm, and his colleagues conducted a longitudinal population-based cohort study of individuals living in Sweden between Jan. 1, 1973, and Dec. 31, 2013. The researchers assessed outcomes for patients with previously validated diagnoses of Tourette syndrome or chronic tic disorder in the Swedish National Patient Register. Main outcomes included obesity, dyslipidemia, hypertension, T2DM, and cardiovascular diseases, including ischemic heart diseases, arrhythmia, cerebrovascular diseases, transient ischemic attack, and arteriosclerosis. In addition, the researchers identified families with full siblings discordant for Tourette syndrome or chronic tic disorder.
Of the more than 14 million individuals in the cohort, 7,804 (76.4% male; median age at first diagnosis, 13.3 years) had a diagnosis of Tourette syndrome or chronic tic disorder in specialist care. Furthermore, the cohort included 5,141 families with full siblings who were discordant for these disorders.
Individuals with Tourette syndrome or chronic tic disorder had a higher risk for any metabolic or cardiovascular disorder, compared with the general population (hazard ratio adjusted by sex and birth year [aHR], 1.99) and sibling controls (aHR, 1.37). Specifically, individuals with Tourette syndrome or chronic tic disorder had higher risks for obesity (aHR, 2.76), T2DM(aHR, 1.67), and circulatory system diseases (aHR, 1.76).
The increased risk of any cardiometabolic disorder was significantly greater for males than it was for females (aHRs, 2.13 vs. 1.79), as was the risk of obesity (aHRs, 3.24 vs. 1.97).
The increased risk for cardiometabolic disorders in this patient population was evident by age 8 years. Exclusion of those patients with comorbid ADHD reduced but did not eliminate the risk (aHR, 1.52). The exclusion of other comorbidities did not significantly affect the results. Among patients with Tourette syndrome or chronic tic disorder, those who had received antipsychotic treatment for more than 1 year were significantly less likely to have metabolic and cardiovascular disorders, compared with patients not taking antipsychotic medication. This association may be related to “greater medical vigilance” and “should not be taken as evidence that antipsychotics are free from cardiometabolic adverse effects,” the authors noted.
The study was supported by a research grant from Tourettes Action. In addition, authors reported support from the Swedish Research Council and a Karolinska Institutet PhD stipend. Two authors disclosed personal fees from publishers, and one author disclosed grants and other funding from Shire.
SOURCE: Brander G et al. JAMA Neurol. 2019 Jan 14. doi: 10.1001/jamaneurol.2018.4279.
The movement disorders are associated with cardiometabolic problems “even after taking into account a number of covariates and shared familial confounders and excluding relevant psychiatric comorbidities,” the researchers wrote. “The results highlight the importance of carefully monitoring cardiometabolic health in patients with Tourette syndrome or chronic tic disorder across the lifespan, particularly in those with comorbid attention-deficit/hyperactivity disorder (ADHD).”
Gustaf Brander, a researcher in the department of clinical neuroscience at Karolinska Institutet in Stockholm, and his colleagues conducted a longitudinal population-based cohort study of individuals living in Sweden between Jan. 1, 1973, and Dec. 31, 2013. The researchers assessed outcomes for patients with previously validated diagnoses of Tourette syndrome or chronic tic disorder in the Swedish National Patient Register. Main outcomes included obesity, dyslipidemia, hypertension, T2DM, and cardiovascular diseases, including ischemic heart diseases, arrhythmia, cerebrovascular diseases, transient ischemic attack, and arteriosclerosis. In addition, the researchers identified families with full siblings discordant for Tourette syndrome or chronic tic disorder.
Of the more than 14 million individuals in the cohort, 7,804 (76.4% male; median age at first diagnosis, 13.3 years) had a diagnosis of Tourette syndrome or chronic tic disorder in specialist care. Furthermore, the cohort included 5,141 families with full siblings who were discordant for these disorders.
Individuals with Tourette syndrome or chronic tic disorder had a higher risk for any metabolic or cardiovascular disorder, compared with the general population (hazard ratio adjusted by sex and birth year [aHR], 1.99) and sibling controls (aHR, 1.37). Specifically, individuals with Tourette syndrome or chronic tic disorder had higher risks for obesity (aHR, 2.76), T2DM(aHR, 1.67), and circulatory system diseases (aHR, 1.76).
The increased risk of any cardiometabolic disorder was significantly greater for males than it was for females (aHRs, 2.13 vs. 1.79), as was the risk of obesity (aHRs, 3.24 vs. 1.97).
The increased risk for cardiometabolic disorders in this patient population was evident by age 8 years. Exclusion of those patients with comorbid ADHD reduced but did not eliminate the risk (aHR, 1.52). The exclusion of other comorbidities did not significantly affect the results. Among patients with Tourette syndrome or chronic tic disorder, those who had received antipsychotic treatment for more than 1 year were significantly less likely to have metabolic and cardiovascular disorders, compared with patients not taking antipsychotic medication. This association may be related to “greater medical vigilance” and “should not be taken as evidence that antipsychotics are free from cardiometabolic adverse effects,” the authors noted.
The study was supported by a research grant from Tourettes Action. In addition, authors reported support from the Swedish Research Council and a Karolinska Institutet PhD stipend. Two authors disclosed personal fees from publishers, and one author disclosed grants and other funding from Shire.
SOURCE: Brander G et al. JAMA Neurol. 2019 Jan 14. doi: 10.1001/jamaneurol.2018.4279.
The movement disorders are associated with cardiometabolic problems “even after taking into account a number of covariates and shared familial confounders and excluding relevant psychiatric comorbidities,” the researchers wrote. “The results highlight the importance of carefully monitoring cardiometabolic health in patients with Tourette syndrome or chronic tic disorder across the lifespan, particularly in those with comorbid attention-deficit/hyperactivity disorder (ADHD).”
Gustaf Brander, a researcher in the department of clinical neuroscience at Karolinska Institutet in Stockholm, and his colleagues conducted a longitudinal population-based cohort study of individuals living in Sweden between Jan. 1, 1973, and Dec. 31, 2013. The researchers assessed outcomes for patients with previously validated diagnoses of Tourette syndrome or chronic tic disorder in the Swedish National Patient Register. Main outcomes included obesity, dyslipidemia, hypertension, T2DM, and cardiovascular diseases, including ischemic heart diseases, arrhythmia, cerebrovascular diseases, transient ischemic attack, and arteriosclerosis. In addition, the researchers identified families with full siblings discordant for Tourette syndrome or chronic tic disorder.
Of the more than 14 million individuals in the cohort, 7,804 (76.4% male; median age at first diagnosis, 13.3 years) had a diagnosis of Tourette syndrome or chronic tic disorder in specialist care. Furthermore, the cohort included 5,141 families with full siblings who were discordant for these disorders.
Individuals with Tourette syndrome or chronic tic disorder had a higher risk for any metabolic or cardiovascular disorder, compared with the general population (hazard ratio adjusted by sex and birth year [aHR], 1.99) and sibling controls (aHR, 1.37). Specifically, individuals with Tourette syndrome or chronic tic disorder had higher risks for obesity (aHR, 2.76), T2DM(aHR, 1.67), and circulatory system diseases (aHR, 1.76).
The increased risk of any cardiometabolic disorder was significantly greater for males than it was for females (aHRs, 2.13 vs. 1.79), as was the risk of obesity (aHRs, 3.24 vs. 1.97).
The increased risk for cardiometabolic disorders in this patient population was evident by age 8 years. Exclusion of those patients with comorbid ADHD reduced but did not eliminate the risk (aHR, 1.52). The exclusion of other comorbidities did not significantly affect the results. Among patients with Tourette syndrome or chronic tic disorder, those who had received antipsychotic treatment for more than 1 year were significantly less likely to have metabolic and cardiovascular disorders, compared with patients not taking antipsychotic medication. This association may be related to “greater medical vigilance” and “should not be taken as evidence that antipsychotics are free from cardiometabolic adverse effects,” the authors noted.
The study was supported by a research grant from Tourettes Action. In addition, authors reported support from the Swedish Research Council and a Karolinska Institutet PhD stipend. Two authors disclosed personal fees from publishers, and one author disclosed grants and other funding from Shire.
SOURCE: Brander G et al. JAMA Neurol. 2019 Jan 14. doi: 10.1001/jamaneurol.2018.4279.
FROM JAMA NEUROLOGY
Key clinical point: Monitor cardiometabolic health in patients with Tourette syndrome or chronic tic disorder.
Major finding: Patients with Tourette syndrome or chronic tic disorder have a higher risk of metabolic or cardiovascular disorders, compared with the general population (adjusted hazard ratio, 1.99) and sibling controls (adjusted hazard ratio, 1.37).
Study details: A Swedish longitudinal, population-based cohort study of 7,804 individuals with Tourette syndrome or chronic tic disorder.
Disclosures: The study was supported by a research grant from Tourettes Action. Authors reported support from the Swedish Research Council and a Karolinska Institutet PhD stipend. Two authors disclosed personal fees from publishers, and one author disclosed grants and other funding from Shire.
Source: Brander G et al. JAMA Neurol. 2019 Jan 14. doi: 10.1001/jamaneurol.2018.4279.
Lung screening complications costly, more prevalent than in trial setting
The real-world rate of is substantially higher than in clinical trials, a study suggests.
Those complications related to low-dose computed tomography (LDCT) screening are potentially costly, according to the analysis of commercial and Medicare claims data for nearly 350,000 individuals.
While tentative, these results emphasize the need to discuss the risk of adverse events and their costs as part of the shared decision-making process between physicians and patients, researchers said in a report on their study in JAMA Internal Medicine.
“As the number of individuals seeking lung cancer screening with LDCT increases, so too will the number of individuals undergoing invasive diagnostic procedures as a results of abnormal findings,” said Jinhai Huo, MD, PhD, of the department of health services research, management, and policy at the University of Florida, Gainesville.
The retrospective cohort study included 174,702 individuals who underwent an invasive diagnostic procedure related to lung cancer screening and 169,808 control subjects.
All individuals studied were between 55 and 77 years old, the targeted age range for lung cancer screening specified by the Centers for Medicare & Medicaid Services.
Complication rates were about twice as high as they were in the landmark National Lung Screening Trial (NLST), both for a younger cohort of individuals aged 55-64 years, and an older Medicare age group of individuals aged 65 to 77 years, Dr. Huo and coinvestigators reported.
The estimated rate of complications was 22.0% (95% confidence interval, 21.7%-22.7%) in the younger age group, and even higher in the older age group, at 23.8% (95% CI, 23.0%-24.6%), according to investigators. By contrast, complication rates in the NLST were 9.8% and 8.5% for younger and older age cohorts, respectively.
The cost of managing postprocedural complications was higher than the cost of the diagnostic procedures, investigators said.
Mean costs ranged from $6,320 for minor complications to $56,845 for major complications, they reported.
The most common invasive diagnostic procedure in the study cohort was cytology test or biopsy in 26.1%, followed by bronchoscopy in 25.6%, according to study data. Another 5.4% underwent thoracic surgery.
In a previous Medicare advisory committee meeting, some experts expressed concern that complication rates in settings outside of the NLST would be higher than what was reported in that study, Dr. Huo and coauthors noted in their report.
“Our findings echoed this concern,” they said in a discussion of their results.
Dr. Huo and coauthors reported no conflicts of interest related to the research, which was supported in part by grants or fellowships from the University of Texas MD Anderson Cancer Center, the University of Florida, the National Cancer Institute, and the National Institutes of Health.
SOURCE: Huo J et al. JAMA Intern Med. 2019 Jan 14.
“The conversations that are occurring about lung cancer screening are woefully inadequate and do not discuss harms,” wrote Rita F. Redberg, MD, in an editorial note. Shared decision-making visits were made mandatory prior to lung cancer screening by the Centers for Medicare & Medicaid Services. That decision was made because of an evidence review suggesting a “low likelihood” that benefits of lung cancer screening would exceed harms in the Medicare population, Dr. Redberg wrote. Despite that, most Medicare beneficiaries are not having the required visit for shared decision making before they undergo the CT scan.
“It is likely that patients’ decisions not to undergo low-dose computed tomography for lung cancer screening are driven by the high false-positive rate, high chance of incidental findings, and subsequent need for invasive procedures, and small chance of benefit,” she said in her comment.
Shared decision-making visits are also rarely happening in the privately insured population, as shown in previous research, Dr. Redberg noted.
She reported no conflicts of interest related to her Editor’s Note, which appears in JAMA Internal Medicine (2019 Jan 14).
Dr. Redberg is with the department of medicine, University of California, San Francisco.
“The conversations that are occurring about lung cancer screening are woefully inadequate and do not discuss harms,” wrote Rita F. Redberg, MD, in an editorial note. Shared decision-making visits were made mandatory prior to lung cancer screening by the Centers for Medicare & Medicaid Services. That decision was made because of an evidence review suggesting a “low likelihood” that benefits of lung cancer screening would exceed harms in the Medicare population, Dr. Redberg wrote. Despite that, most Medicare beneficiaries are not having the required visit for shared decision making before they undergo the CT scan.
“It is likely that patients’ decisions not to undergo low-dose computed tomography for lung cancer screening are driven by the high false-positive rate, high chance of incidental findings, and subsequent need for invasive procedures, and small chance of benefit,” she said in her comment.
Shared decision-making visits are also rarely happening in the privately insured population, as shown in previous research, Dr. Redberg noted.
She reported no conflicts of interest related to her Editor’s Note, which appears in JAMA Internal Medicine (2019 Jan 14).
Dr. Redberg is with the department of medicine, University of California, San Francisco.
“The conversations that are occurring about lung cancer screening are woefully inadequate and do not discuss harms,” wrote Rita F. Redberg, MD, in an editorial note. Shared decision-making visits were made mandatory prior to lung cancer screening by the Centers for Medicare & Medicaid Services. That decision was made because of an evidence review suggesting a “low likelihood” that benefits of lung cancer screening would exceed harms in the Medicare population, Dr. Redberg wrote. Despite that, most Medicare beneficiaries are not having the required visit for shared decision making before they undergo the CT scan.
“It is likely that patients’ decisions not to undergo low-dose computed tomography for lung cancer screening are driven by the high false-positive rate, high chance of incidental findings, and subsequent need for invasive procedures, and small chance of benefit,” she said in her comment.
Shared decision-making visits are also rarely happening in the privately insured population, as shown in previous research, Dr. Redberg noted.
She reported no conflicts of interest related to her Editor’s Note, which appears in JAMA Internal Medicine (2019 Jan 14).
Dr. Redberg is with the department of medicine, University of California, San Francisco.
The real-world rate of is substantially higher than in clinical trials, a study suggests.
Those complications related to low-dose computed tomography (LDCT) screening are potentially costly, according to the analysis of commercial and Medicare claims data for nearly 350,000 individuals.
While tentative, these results emphasize the need to discuss the risk of adverse events and their costs as part of the shared decision-making process between physicians and patients, researchers said in a report on their study in JAMA Internal Medicine.
“As the number of individuals seeking lung cancer screening with LDCT increases, so too will the number of individuals undergoing invasive diagnostic procedures as a results of abnormal findings,” said Jinhai Huo, MD, PhD, of the department of health services research, management, and policy at the University of Florida, Gainesville.
The retrospective cohort study included 174,702 individuals who underwent an invasive diagnostic procedure related to lung cancer screening and 169,808 control subjects.
All individuals studied were between 55 and 77 years old, the targeted age range for lung cancer screening specified by the Centers for Medicare & Medicaid Services.
Complication rates were about twice as high as they were in the landmark National Lung Screening Trial (NLST), both for a younger cohort of individuals aged 55-64 years, and an older Medicare age group of individuals aged 65 to 77 years, Dr. Huo and coinvestigators reported.
The estimated rate of complications was 22.0% (95% confidence interval, 21.7%-22.7%) in the younger age group, and even higher in the older age group, at 23.8% (95% CI, 23.0%-24.6%), according to investigators. By contrast, complication rates in the NLST were 9.8% and 8.5% for younger and older age cohorts, respectively.
The cost of managing postprocedural complications was higher than the cost of the diagnostic procedures, investigators said.
Mean costs ranged from $6,320 for minor complications to $56,845 for major complications, they reported.
The most common invasive diagnostic procedure in the study cohort was cytology test or biopsy in 26.1%, followed by bronchoscopy in 25.6%, according to study data. Another 5.4% underwent thoracic surgery.
In a previous Medicare advisory committee meeting, some experts expressed concern that complication rates in settings outside of the NLST would be higher than what was reported in that study, Dr. Huo and coauthors noted in their report.
“Our findings echoed this concern,” they said in a discussion of their results.
Dr. Huo and coauthors reported no conflicts of interest related to the research, which was supported in part by grants or fellowships from the University of Texas MD Anderson Cancer Center, the University of Florida, the National Cancer Institute, and the National Institutes of Health.
SOURCE: Huo J et al. JAMA Intern Med. 2019 Jan 14.
The real-world rate of is substantially higher than in clinical trials, a study suggests.
Those complications related to low-dose computed tomography (LDCT) screening are potentially costly, according to the analysis of commercial and Medicare claims data for nearly 350,000 individuals.
While tentative, these results emphasize the need to discuss the risk of adverse events and their costs as part of the shared decision-making process between physicians and patients, researchers said in a report on their study in JAMA Internal Medicine.
“As the number of individuals seeking lung cancer screening with LDCT increases, so too will the number of individuals undergoing invasive diagnostic procedures as a results of abnormal findings,” said Jinhai Huo, MD, PhD, of the department of health services research, management, and policy at the University of Florida, Gainesville.
The retrospective cohort study included 174,702 individuals who underwent an invasive diagnostic procedure related to lung cancer screening and 169,808 control subjects.
All individuals studied were between 55 and 77 years old, the targeted age range for lung cancer screening specified by the Centers for Medicare & Medicaid Services.
Complication rates were about twice as high as they were in the landmark National Lung Screening Trial (NLST), both for a younger cohort of individuals aged 55-64 years, and an older Medicare age group of individuals aged 65 to 77 years, Dr. Huo and coinvestigators reported.
The estimated rate of complications was 22.0% (95% confidence interval, 21.7%-22.7%) in the younger age group, and even higher in the older age group, at 23.8% (95% CI, 23.0%-24.6%), according to investigators. By contrast, complication rates in the NLST were 9.8% and 8.5% for younger and older age cohorts, respectively.
The cost of managing postprocedural complications was higher than the cost of the diagnostic procedures, investigators said.
Mean costs ranged from $6,320 for minor complications to $56,845 for major complications, they reported.
The most common invasive diagnostic procedure in the study cohort was cytology test or biopsy in 26.1%, followed by bronchoscopy in 25.6%, according to study data. Another 5.4% underwent thoracic surgery.
In a previous Medicare advisory committee meeting, some experts expressed concern that complication rates in settings outside of the NLST would be higher than what was reported in that study, Dr. Huo and coauthors noted in their report.
“Our findings echoed this concern,” they said in a discussion of their results.
Dr. Huo and coauthors reported no conflicts of interest related to the research, which was supported in part by grants or fellowships from the University of Texas MD Anderson Cancer Center, the University of Florida, the National Cancer Institute, and the National Institutes of Health.
SOURCE: Huo J et al. JAMA Intern Med. 2019 Jan 14.
FROM JAMA INTERNAL MEDICINE
Key clinical point: The risk of complications following lung cancer screening is higher in a real-world setting as compared with the landmark National Lung Screening Trial (NLST).
Major finding: Estimated complication rates were 22.0%-23.8%, compared with 8.5%-9.8% in the NLST.
Study details: A retrospective cohort study including 174,702 individuals who underwent an invasive diagnostic procedure related to lung cancer screening and 169,808 controls.
Disclosures: Authors reported no conflicts of interest. The research was supported in part by grants or fellowships from the University of Texas MD Anderson Cancer Center, the University of Florida, the National Cancer Institute, and the National Institutes of Health.
Source: Huo J et al. JAMA Intern Med. 2019 Jan 14.