User login
A new way to expand HSCs for UCB transplant
Researchers say they have discovered a new approach to expand hematopoietic stem cells (HSCs) from umbilical cord blood (UCB).
The team identified a protein, YTHDF2, that affects multiple targets and pathways involved in HSC self-renewal.
Experiments showed that reducing the function of YTHDF2 allowed UCB HSCs to expand.
The researchers therefore believe this approach could be used to improve UCB transplants.
“If we can expand cord adult stem cells, that could potentially decrease the number of cords needed per treatment,” said Linheng Li, PhD, of Stowers Institute for Medical Research in Kansas City, Missouri. “That’s a huge advantage.”
Dr Li and his colleagues conducted this research and described the work in Cell Research.
Past studies suggested that N6-methyladenosine (m6A) modulates the expression of a group of mRNAs that are critical for stem cell fate determination.
As the m6A reader YTHDF2 promotes targeted mRNA decay, Dr Li and his colleagues decided to target YTHDF2.
The researchers knocked out YTHDF2 function in a mouse model and observed an increase in functional HSCs. However, impairing YTHDF2 function did not alter lineage differentiation or lead to an increase in hematologic malignancies.
The researchers also knocked down YTHDF2 function in human UCB hematopoietic stem and progenitor cells. After 7 days of ex vivo culture, there was a roughly 14-fold increase in both the frequency and absolute number of HSCs with YTHDF2 knockdown (KD) cells compared to control cells.
When human UCB cells were transplanted into mice, there was a 9-fold increase in hematopoietic cell engraftment with YTHDF2 KD cells compared to control cells. In addition, the HSC frequency was about 4-fold higher in YTHDF2 KD cells.
The researchers transplanted bone marrow from primary recipient mice into sublethally irradiated secondary mice and found that, 12 weeks after transplant, human hematopoietic cell chimerism in the bone marrow was higher in YTHDF2 KD mice than in controls.
There was an 8-fold increase in competitive repopulating units from YTHDF2 KD cells compared to control cells.
As for why targeting YTHDF2 results in HSC expansion, the researchers found that YTHDF2 regulates HSC self-renewal gene expression by m6A-mediated mRNA decay.
The team discovered that m6A was enriched in mRNAs encoding transcription factors that are critical for stem cell self-renewal (such as GATA2, ETV6, STAT5, and TAL1). YTHDF2 recognizes these mRNAs and promotes their degradation.
“This work represents a path forward by demonstrating the ability to reliably expand adult stem cells from umbilical cord blood in the laboratory without terminally differentiating the cells into more mature and relatively short-lived blood cells,” said Joseph McGuirk, MD, a professor at the University of Kansas Health System who was not directly involved with this study.
“These findings represent a major advance in the field and have significant potential to improve the outcomes of thousands of children and adults who undergo umbilical cord blood transplantation every year.”
Researchers say they have discovered a new approach to expand hematopoietic stem cells (HSCs) from umbilical cord blood (UCB).
The team identified a protein, YTHDF2, that affects multiple targets and pathways involved in HSC self-renewal.
Experiments showed that reducing the function of YTHDF2 allowed UCB HSCs to expand.
The researchers therefore believe this approach could be used to improve UCB transplants.
“If we can expand cord adult stem cells, that could potentially decrease the number of cords needed per treatment,” said Linheng Li, PhD, of Stowers Institute for Medical Research in Kansas City, Missouri. “That’s a huge advantage.”
Dr Li and his colleagues conducted this research and described the work in Cell Research.
Past studies suggested that N6-methyladenosine (m6A) modulates the expression of a group of mRNAs that are critical for stem cell fate determination.
As the m6A reader YTHDF2 promotes targeted mRNA decay, Dr Li and his colleagues decided to target YTHDF2.
The researchers knocked out YTHDF2 function in a mouse model and observed an increase in functional HSCs. However, impairing YTHDF2 function did not alter lineage differentiation or lead to an increase in hematologic malignancies.
The researchers also knocked down YTHDF2 function in human UCB hematopoietic stem and progenitor cells. After 7 days of ex vivo culture, there was a roughly 14-fold increase in both the frequency and absolute number of HSCs with YTHDF2 knockdown (KD) cells compared to control cells.
When human UCB cells were transplanted into mice, there was a 9-fold increase in hematopoietic cell engraftment with YTHDF2 KD cells compared to control cells. In addition, the HSC frequency was about 4-fold higher in YTHDF2 KD cells.
The researchers transplanted bone marrow from primary recipient mice into sublethally irradiated secondary mice and found that, 12 weeks after transplant, human hematopoietic cell chimerism in the bone marrow was higher in YTHDF2 KD mice than in controls.
There was an 8-fold increase in competitive repopulating units from YTHDF2 KD cells compared to control cells.
As for why targeting YTHDF2 results in HSC expansion, the researchers found that YTHDF2 regulates HSC self-renewal gene expression by m6A-mediated mRNA decay.
The team discovered that m6A was enriched in mRNAs encoding transcription factors that are critical for stem cell self-renewal (such as GATA2, ETV6, STAT5, and TAL1). YTHDF2 recognizes these mRNAs and promotes their degradation.
“This work represents a path forward by demonstrating the ability to reliably expand adult stem cells from umbilical cord blood in the laboratory without terminally differentiating the cells into more mature and relatively short-lived blood cells,” said Joseph McGuirk, MD, a professor at the University of Kansas Health System who was not directly involved with this study.
“These findings represent a major advance in the field and have significant potential to improve the outcomes of thousands of children and adults who undergo umbilical cord blood transplantation every year.”
Researchers say they have discovered a new approach to expand hematopoietic stem cells (HSCs) from umbilical cord blood (UCB).
The team identified a protein, YTHDF2, that affects multiple targets and pathways involved in HSC self-renewal.
Experiments showed that reducing the function of YTHDF2 allowed UCB HSCs to expand.
The researchers therefore believe this approach could be used to improve UCB transplants.
“If we can expand cord adult stem cells, that could potentially decrease the number of cords needed per treatment,” said Linheng Li, PhD, of Stowers Institute for Medical Research in Kansas City, Missouri. “That’s a huge advantage.”
Dr Li and his colleagues conducted this research and described the work in Cell Research.
Past studies suggested that N6-methyladenosine (m6A) modulates the expression of a group of mRNAs that are critical for stem cell fate determination.
As the m6A reader YTHDF2 promotes targeted mRNA decay, Dr Li and his colleagues decided to target YTHDF2.
The researchers knocked out YTHDF2 function in a mouse model and observed an increase in functional HSCs. However, impairing YTHDF2 function did not alter lineage differentiation or lead to an increase in hematologic malignancies.
The researchers also knocked down YTHDF2 function in human UCB hematopoietic stem and progenitor cells. After 7 days of ex vivo culture, there was a roughly 14-fold increase in both the frequency and absolute number of HSCs with YTHDF2 knockdown (KD) cells compared to control cells.
When human UCB cells were transplanted into mice, there was a 9-fold increase in hematopoietic cell engraftment with YTHDF2 KD cells compared to control cells. In addition, the HSC frequency was about 4-fold higher in YTHDF2 KD cells.
The researchers transplanted bone marrow from primary recipient mice into sublethally irradiated secondary mice and found that, 12 weeks after transplant, human hematopoietic cell chimerism in the bone marrow was higher in YTHDF2 KD mice than in controls.
There was an 8-fold increase in competitive repopulating units from YTHDF2 KD cells compared to control cells.
As for why targeting YTHDF2 results in HSC expansion, the researchers found that YTHDF2 regulates HSC self-renewal gene expression by m6A-mediated mRNA decay.
The team discovered that m6A was enriched in mRNAs encoding transcription factors that are critical for stem cell self-renewal (such as GATA2, ETV6, STAT5, and TAL1). YTHDF2 recognizes these mRNAs and promotes their degradation.
“This work represents a path forward by demonstrating the ability to reliably expand adult stem cells from umbilical cord blood in the laboratory without terminally differentiating the cells into more mature and relatively short-lived blood cells,” said Joseph McGuirk, MD, a professor at the University of Kansas Health System who was not directly involved with this study.
“These findings represent a major advance in the field and have significant potential to improve the outcomes of thousands of children and adults who undergo umbilical cord blood transplantation every year.”
Treatment guidelines for CAR T-cell therapy
Researchers have developed treatment guidelines for pediatric patients receiving chimeric antigen receptor (CAR) T-cell therapy.
The guidelines include recommendations for patient selection and consent, treatment details, and advice on managing cytokine release syndrome (CRS) and other adverse events associated with CAR T-cell therapy.
The guidelines were published in Nature Reviews Clinical Oncology.
“CAR T-cell therapy has been associated with remarkable response rates for children and young adults with ALL [acute lymphoblastic leukemia], yet this innovative form of cellular immunotherapy has resulted in unique and severe toxicities which can lead to rapid cardiorespiratory and/or neurological deterioration,” said guidelines author Kris Mahadeo, MD, of The University of Texas MD Anderson Cancer Center in Houston.
“This novel therapy requires the medical vigilance of a diverse multi-disciplinary team and associated clinical infrastructure to ensure optimal patient outcomes.”
Pediatric patient selection and consent
The guidelines state that providers of CAR T-cell therapies should adhere to product information labels and guidance from risk evaluation and mitigation strategy programs (level of evidence: IV, grade: D).
In addition, patient selection should be based on the indications approved by the US Food and Drug Administration and criteria used in pivotal studies. However, this can change as new information becomes available (level of evidence: IV, grade: D).
Informed consent should include descriptions of the risks and benefits associated with leukapheresis, lymphodepletion, CRS, CAR T-cell-related encephalopathy syndrome (CRES), bridging chemotherapy, intensive care support, and anti-IL-6 therapy (level of evidence: IIA, grade: B).
Providers should obtain child assent when appropriate and may benefit from incorporating child life and psychological services in assent discussions (level of evidence: IV, grade: D).
Treatment specifics
The guidelines recommend cyclophosphamide–fludarabine regimens for lymphodepletion, although exceptions can be considered in cases of hemorrhagic cystitis and/or resistance to a prior cyclophosphamide-based regimen (level of evidence: IIA, grade: B).
Providers should consider inpatient admission for a minimum of 3 to 7 days after receipt of tisagenlecleucel. This was based on the experience in pediatric and young adult patients with CD19+ relapsed and/or refractory B-cell acute lymphoblastic leukemia (level of evidence: IIA, grade: B).
Patients should be closely monitored for hypotension, hypocalcemia, and catheter-related pain during leukapheresis (level of evidence: IIA, grade: B).
For patients receiving tocilizumab, those weighing <30 kg should receive 12 mg/kg, and those weighing ≥30 kg should receive 8 mg/kg (level of evidence: IIA, grade: B).
Adverse events
The guidelines say parent and/or caregiver concerns should be addressed as these individuals may be best equipped to recognize early signs or symptoms of CRS (level of evidence: III, grade: C).
When CAR T-cell therapy is administered in an outpatient setting, there should be a low threshold for patient admission upon the development of signs or symptoms suggestive of CRS and/or CRES (level of evidence: IIA, grade: B).
CRS grading should be performed at least once every 12 hours (level of evidence: IIA, grade: B). Detailed information on grading is provided in the guidelines.
Providers should suspect CRS if any of the following signs/symptoms are present within the first 2 weeks of CAR T-cell infusion:
- Fever ≥38 °C
- Hypotension
- Hypoxia with an arterial oxygen saturation of <90% on room air
- Evidence of organ toxicity as determined by the most recent CTCAE grading system and considerations detailed in the guidelines (level of evidence: IIA, grade: C).
The guidelines also recommend “high vigilance” for sinus tachycardia as an early sign of CRS (level of evidence: IIA, grade: B) as well as application of the PALICC (Pediatric Acute Lung Injury Consensus Conference) at-risk P-ARDS (pediatric acute respiratory distress syndrome) criteria for the CRS grading of hypoxia (level of evidence: IIA, grade: B).
Hemophagocytic lymphohistiocytosis and/or macrophage-activation syndrome can be treated with anti-IL-6 therapy and corticosteroids. However, refractory cases may require systemic and/or intrathecal therapy or use of the IL-1 receptor antagonist anakinra (level of evidence: IIA, grade: C).
The guidelines recommend that delirium screening be performed at least twice per 24-hour period among admitted patients and at least daily among outpatients during the high-risk periods for CRES (level of evidence: IIA, grade: C). Delirium screening should be performed with the CAPD (Cornell Assessment of Pediatric Delirium) tool or CARTOX-10 (CAR T-Cell Therapy-Associated Toxicity 10-point assessment scale) for patients age 12 and older who have sufficient cognitive abilities.
Acute kidney injury in children can be graded according to the CTCAE (Common Terminology Criteria for Adverse Events) using pRIFLE (Pediatric Risk, Injury, Failure, Loss, End-Stage Renal Disease) and KDIGO (Kidney Disease: Improving Global Outcomes) definitions of oliguria (level of evidence: IIA, grade: B).
Other considerations
The guidelines “strongly encourage” consideration of quality-adjusted life-years gained for pediatric patients who might achieve long-term remission from CAR T-cell therapy and encourage efforts to reduce the cost of care (level of evidence: IV, grade: D).
The guidelines also recommend that CAR T-cell programs seek FACT IEC (Foundation for the Accreditation of Cellular Therapy for Immune Effector Cells) accreditation to ensure adherence to quality standards (level of evidence: IV, grade: D).
Finally, the guidelines suggest the possibility of a prospective collaboration with intensive-care registries, which could allow accurate data entry of cell therapy variables into the CIBMTR registry with concurrent entry of intensive-care variables into an appropriate registry by pediatric critical care teams (level of evidence: IV, grade: D).
Researchers have developed treatment guidelines for pediatric patients receiving chimeric antigen receptor (CAR) T-cell therapy.
The guidelines include recommendations for patient selection and consent, treatment details, and advice on managing cytokine release syndrome (CRS) and other adverse events associated with CAR T-cell therapy.
The guidelines were published in Nature Reviews Clinical Oncology.
“CAR T-cell therapy has been associated with remarkable response rates for children and young adults with ALL [acute lymphoblastic leukemia], yet this innovative form of cellular immunotherapy has resulted in unique and severe toxicities which can lead to rapid cardiorespiratory and/or neurological deterioration,” said guidelines author Kris Mahadeo, MD, of The University of Texas MD Anderson Cancer Center in Houston.
“This novel therapy requires the medical vigilance of a diverse multi-disciplinary team and associated clinical infrastructure to ensure optimal patient outcomes.”
Pediatric patient selection and consent
The guidelines state that providers of CAR T-cell therapies should adhere to product information labels and guidance from risk evaluation and mitigation strategy programs (level of evidence: IV, grade: D).
In addition, patient selection should be based on the indications approved by the US Food and Drug Administration and criteria used in pivotal studies. However, this can change as new information becomes available (level of evidence: IV, grade: D).
Informed consent should include descriptions of the risks and benefits associated with leukapheresis, lymphodepletion, CRS, CAR T-cell-related encephalopathy syndrome (CRES), bridging chemotherapy, intensive care support, and anti-IL-6 therapy (level of evidence: IIA, grade: B).
Providers should obtain child assent when appropriate and may benefit from incorporating child life and psychological services in assent discussions (level of evidence: IV, grade: D).
Treatment specifics
The guidelines recommend cyclophosphamide–fludarabine regimens for lymphodepletion, although exceptions can be considered in cases of hemorrhagic cystitis and/or resistance to a prior cyclophosphamide-based regimen (level of evidence: IIA, grade: B).
Providers should consider inpatient admission for a minimum of 3 to 7 days after receipt of tisagenlecleucel. This was based on the experience in pediatric and young adult patients with CD19+ relapsed and/or refractory B-cell acute lymphoblastic leukemia (level of evidence: IIA, grade: B).
Patients should be closely monitored for hypotension, hypocalcemia, and catheter-related pain during leukapheresis (level of evidence: IIA, grade: B).
For patients receiving tocilizumab, those weighing <30 kg should receive 12 mg/kg, and those weighing ≥30 kg should receive 8 mg/kg (level of evidence: IIA, grade: B).
Adverse events
The guidelines say parent and/or caregiver concerns should be addressed as these individuals may be best equipped to recognize early signs or symptoms of CRS (level of evidence: III, grade: C).
When CAR T-cell therapy is administered in an outpatient setting, there should be a low threshold for patient admission upon the development of signs or symptoms suggestive of CRS and/or CRES (level of evidence: IIA, grade: B).
CRS grading should be performed at least once every 12 hours (level of evidence: IIA, grade: B). Detailed information on grading is provided in the guidelines.
Providers should suspect CRS if any of the following signs/symptoms are present within the first 2 weeks of CAR T-cell infusion:
- Fever ≥38 °C
- Hypotension
- Hypoxia with an arterial oxygen saturation of <90% on room air
- Evidence of organ toxicity as determined by the most recent CTCAE grading system and considerations detailed in the guidelines (level of evidence: IIA, grade: C).
The guidelines also recommend “high vigilance” for sinus tachycardia as an early sign of CRS (level of evidence: IIA, grade: B) as well as application of the PALICC (Pediatric Acute Lung Injury Consensus Conference) at-risk P-ARDS (pediatric acute respiratory distress syndrome) criteria for the CRS grading of hypoxia (level of evidence: IIA, grade: B).
Hemophagocytic lymphohistiocytosis and/or macrophage-activation syndrome can be treated with anti-IL-6 therapy and corticosteroids. However, refractory cases may require systemic and/or intrathecal therapy or use of the IL-1 receptor antagonist anakinra (level of evidence: IIA, grade: C).
The guidelines recommend that delirium screening be performed at least twice per 24-hour period among admitted patients and at least daily among outpatients during the high-risk periods for CRES (level of evidence: IIA, grade: C). Delirium screening should be performed with the CAPD (Cornell Assessment of Pediatric Delirium) tool or CARTOX-10 (CAR T-Cell Therapy-Associated Toxicity 10-point assessment scale) for patients age 12 and older who have sufficient cognitive abilities.
Acute kidney injury in children can be graded according to the CTCAE (Common Terminology Criteria for Adverse Events) using pRIFLE (Pediatric Risk, Injury, Failure, Loss, End-Stage Renal Disease) and KDIGO (Kidney Disease: Improving Global Outcomes) definitions of oliguria (level of evidence: IIA, grade: B).
Other considerations
The guidelines “strongly encourage” consideration of quality-adjusted life-years gained for pediatric patients who might achieve long-term remission from CAR T-cell therapy and encourage efforts to reduce the cost of care (level of evidence: IV, grade: D).
The guidelines also recommend that CAR T-cell programs seek FACT IEC (Foundation for the Accreditation of Cellular Therapy for Immune Effector Cells) accreditation to ensure adherence to quality standards (level of evidence: IV, grade: D).
Finally, the guidelines suggest the possibility of a prospective collaboration with intensive-care registries, which could allow accurate data entry of cell therapy variables into the CIBMTR registry with concurrent entry of intensive-care variables into an appropriate registry by pediatric critical care teams (level of evidence: IV, grade: D).
Researchers have developed treatment guidelines for pediatric patients receiving chimeric antigen receptor (CAR) T-cell therapy.
The guidelines include recommendations for patient selection and consent, treatment details, and advice on managing cytokine release syndrome (CRS) and other adverse events associated with CAR T-cell therapy.
The guidelines were published in Nature Reviews Clinical Oncology.
“CAR T-cell therapy has been associated with remarkable response rates for children and young adults with ALL [acute lymphoblastic leukemia], yet this innovative form of cellular immunotherapy has resulted in unique and severe toxicities which can lead to rapid cardiorespiratory and/or neurological deterioration,” said guidelines author Kris Mahadeo, MD, of The University of Texas MD Anderson Cancer Center in Houston.
“This novel therapy requires the medical vigilance of a diverse multi-disciplinary team and associated clinical infrastructure to ensure optimal patient outcomes.”
Pediatric patient selection and consent
The guidelines state that providers of CAR T-cell therapies should adhere to product information labels and guidance from risk evaluation and mitigation strategy programs (level of evidence: IV, grade: D).
In addition, patient selection should be based on the indications approved by the US Food and Drug Administration and criteria used in pivotal studies. However, this can change as new information becomes available (level of evidence: IV, grade: D).
Informed consent should include descriptions of the risks and benefits associated with leukapheresis, lymphodepletion, CRS, CAR T-cell-related encephalopathy syndrome (CRES), bridging chemotherapy, intensive care support, and anti-IL-6 therapy (level of evidence: IIA, grade: B).
Providers should obtain child assent when appropriate and may benefit from incorporating child life and psychological services in assent discussions (level of evidence: IV, grade: D).
Treatment specifics
The guidelines recommend cyclophosphamide–fludarabine regimens for lymphodepletion, although exceptions can be considered in cases of hemorrhagic cystitis and/or resistance to a prior cyclophosphamide-based regimen (level of evidence: IIA, grade: B).
Providers should consider inpatient admission for a minimum of 3 to 7 days after receipt of tisagenlecleucel. This was based on the experience in pediatric and young adult patients with CD19+ relapsed and/or refractory B-cell acute lymphoblastic leukemia (level of evidence: IIA, grade: B).
Patients should be closely monitored for hypotension, hypocalcemia, and catheter-related pain during leukapheresis (level of evidence: IIA, grade: B).
For patients receiving tocilizumab, those weighing <30 kg should receive 12 mg/kg, and those weighing ≥30 kg should receive 8 mg/kg (level of evidence: IIA, grade: B).
Adverse events
The guidelines say parent and/or caregiver concerns should be addressed as these individuals may be best equipped to recognize early signs or symptoms of CRS (level of evidence: III, grade: C).
When CAR T-cell therapy is administered in an outpatient setting, there should be a low threshold for patient admission upon the development of signs or symptoms suggestive of CRS and/or CRES (level of evidence: IIA, grade: B).
CRS grading should be performed at least once every 12 hours (level of evidence: IIA, grade: B). Detailed information on grading is provided in the guidelines.
Providers should suspect CRS if any of the following signs/symptoms are present within the first 2 weeks of CAR T-cell infusion:
- Fever ≥38 °C
- Hypotension
- Hypoxia with an arterial oxygen saturation of <90% on room air
- Evidence of organ toxicity as determined by the most recent CTCAE grading system and considerations detailed in the guidelines (level of evidence: IIA, grade: C).
The guidelines also recommend “high vigilance” for sinus tachycardia as an early sign of CRS (level of evidence: IIA, grade: B) as well as application of the PALICC (Pediatric Acute Lung Injury Consensus Conference) at-risk P-ARDS (pediatric acute respiratory distress syndrome) criteria for the CRS grading of hypoxia (level of evidence: IIA, grade: B).
Hemophagocytic lymphohistiocytosis and/or macrophage-activation syndrome can be treated with anti-IL-6 therapy and corticosteroids. However, refractory cases may require systemic and/or intrathecal therapy or use of the IL-1 receptor antagonist anakinra (level of evidence: IIA, grade: C).
The guidelines recommend that delirium screening be performed at least twice per 24-hour period among admitted patients and at least daily among outpatients during the high-risk periods for CRES (level of evidence: IIA, grade: C). Delirium screening should be performed with the CAPD (Cornell Assessment of Pediatric Delirium) tool or CARTOX-10 (CAR T-Cell Therapy-Associated Toxicity 10-point assessment scale) for patients age 12 and older who have sufficient cognitive abilities.
Acute kidney injury in children can be graded according to the CTCAE (Common Terminology Criteria for Adverse Events) using pRIFLE (Pediatric Risk, Injury, Failure, Loss, End-Stage Renal Disease) and KDIGO (Kidney Disease: Improving Global Outcomes) definitions of oliguria (level of evidence: IIA, grade: B).
Other considerations
The guidelines “strongly encourage” consideration of quality-adjusted life-years gained for pediatric patients who might achieve long-term remission from CAR T-cell therapy and encourage efforts to reduce the cost of care (level of evidence: IV, grade: D).
The guidelines also recommend that CAR T-cell programs seek FACT IEC (Foundation for the Accreditation of Cellular Therapy for Immune Effector Cells) accreditation to ensure adherence to quality standards (level of evidence: IV, grade: D).
Finally, the guidelines suggest the possibility of a prospective collaboration with intensive-care registries, which could allow accurate data entry of cell therapy variables into the CIBMTR registry with concurrent entry of intensive-care variables into an appropriate registry by pediatric critical care teams (level of evidence: IV, grade: D).
FDA advises against azithromycin use in allo-HSCT recipients
The US Food and Drug Administration (FDA) is warning against long-term use of azithromycin (Zithromax, Zmax) in patients who undergo allogeneic hematopoietic stem cell transplant (allo-HSCT).
Azithromycin has been used off-label as prophylaxis for bronchiolitis obliterans syndrome in these patients.
However, a trial published in JAMA last year suggested that long-term azithromycin use increases the risk of relapse and death in patients undergoing allo-HSCT as treatment for hematologic malignancies.
The FDA said it is reviewing additional data and will communicate its conclusions and recommendations when the review is complete.
In the meantime, the agency said healthcare professionals should not prescribe long-term azithromycin to allo-HSCT recipients for prophylaxis of bronchiolitis obliterans syndrome. However, patients should not stop taking azithromycin without first consulting their healthcare professionals.
Healthcare professionals and patients can report adverse events related to azithromycin to the FDA’s MedWatch program.
Pfizer, which markets Zithromax, has issued a Dear Healthcare Provider letter warning about the risks of relapse and death associated with long-term azithromycin use in allo-HSCT recipients.
The company said there is no evidence to suggest increased risks in other patient populations or when azithromycin is used for FDA-approved indications.
It isn’t clear why allo-HSCT recipients may have an increased risk of relapse/death with long-term azithromycin use. However, Pfizer said the available evidence raises questions about the safety of prophylactic azithromycin in this patient population, suggesting the risks outweigh the benefits.
The evidence comes from the ALLOZITHRO trial, which was published in JAMA in August 2017.
The trial included 480 patients who had undergone allo-HSCT for a hematologic malignancy. They were randomized to receive 250 mg of azithromycin (n=243) or placebo (n=237) 3 times a week for 2 years, beginning at the start of conditioning.
The trial was stopped about 13 months after enrollment was completed because there was an unexpected increase in the rate of relapse and death in patients taking azithromycin.
The 2-year cumulative incidence of relapse was 33.5% in the azithromycin group and 22.3% in the placebo group (unadjusted cause-specific hazard ratio [HR]=1.7, P=0.002).
The 2-year survival rate was 56.6% in the azithromycin group and 70.1% in the placebo group (adjusted HR=1.5, P=0.02).
The 2-year airflow decline-free survival rate was 32.8% in the azithromycin group and 41.3% in the placebo group (unadjusted HR=1.3, P=0.03).
And the incidence of bronchiolitis obliterans syndrome was 6% in the azithromycin group and 3% in the placebo group (P=0.08).
The US Food and Drug Administration (FDA) is warning against long-term use of azithromycin (Zithromax, Zmax) in patients who undergo allogeneic hematopoietic stem cell transplant (allo-HSCT).
Azithromycin has been used off-label as prophylaxis for bronchiolitis obliterans syndrome in these patients.
However, a trial published in JAMA last year suggested that long-term azithromycin use increases the risk of relapse and death in patients undergoing allo-HSCT as treatment for hematologic malignancies.
The FDA said it is reviewing additional data and will communicate its conclusions and recommendations when the review is complete.
In the meantime, the agency said healthcare professionals should not prescribe long-term azithromycin to allo-HSCT recipients for prophylaxis of bronchiolitis obliterans syndrome. However, patients should not stop taking azithromycin without first consulting their healthcare professionals.
Healthcare professionals and patients can report adverse events related to azithromycin to the FDA’s MedWatch program.
Pfizer, which markets Zithromax, has issued a Dear Healthcare Provider letter warning about the risks of relapse and death associated with long-term azithromycin use in allo-HSCT recipients.
The company said there is no evidence to suggest increased risks in other patient populations or when azithromycin is used for FDA-approved indications.
It isn’t clear why allo-HSCT recipients may have an increased risk of relapse/death with long-term azithromycin use. However, Pfizer said the available evidence raises questions about the safety of prophylactic azithromycin in this patient population, suggesting the risks outweigh the benefits.
The evidence comes from the ALLOZITHRO trial, which was published in JAMA in August 2017.
The trial included 480 patients who had undergone allo-HSCT for a hematologic malignancy. They were randomized to receive 250 mg of azithromycin (n=243) or placebo (n=237) 3 times a week for 2 years, beginning at the start of conditioning.
The trial was stopped about 13 months after enrollment was completed because there was an unexpected increase in the rate of relapse and death in patients taking azithromycin.
The 2-year cumulative incidence of relapse was 33.5% in the azithromycin group and 22.3% in the placebo group (unadjusted cause-specific hazard ratio [HR]=1.7, P=0.002).
The 2-year survival rate was 56.6% in the azithromycin group and 70.1% in the placebo group (adjusted HR=1.5, P=0.02).
The 2-year airflow decline-free survival rate was 32.8% in the azithromycin group and 41.3% in the placebo group (unadjusted HR=1.3, P=0.03).
And the incidence of bronchiolitis obliterans syndrome was 6% in the azithromycin group and 3% in the placebo group (P=0.08).
The US Food and Drug Administration (FDA) is warning against long-term use of azithromycin (Zithromax, Zmax) in patients who undergo allogeneic hematopoietic stem cell transplant (allo-HSCT).
Azithromycin has been used off-label as prophylaxis for bronchiolitis obliterans syndrome in these patients.
However, a trial published in JAMA last year suggested that long-term azithromycin use increases the risk of relapse and death in patients undergoing allo-HSCT as treatment for hematologic malignancies.
The FDA said it is reviewing additional data and will communicate its conclusions and recommendations when the review is complete.
In the meantime, the agency said healthcare professionals should not prescribe long-term azithromycin to allo-HSCT recipients for prophylaxis of bronchiolitis obliterans syndrome. However, patients should not stop taking azithromycin without first consulting their healthcare professionals.
Healthcare professionals and patients can report adverse events related to azithromycin to the FDA’s MedWatch program.
Pfizer, which markets Zithromax, has issued a Dear Healthcare Provider letter warning about the risks of relapse and death associated with long-term azithromycin use in allo-HSCT recipients.
The company said there is no evidence to suggest increased risks in other patient populations or when azithromycin is used for FDA-approved indications.
It isn’t clear why allo-HSCT recipients may have an increased risk of relapse/death with long-term azithromycin use. However, Pfizer said the available evidence raises questions about the safety of prophylactic azithromycin in this patient population, suggesting the risks outweigh the benefits.
The evidence comes from the ALLOZITHRO trial, which was published in JAMA in August 2017.
The trial included 480 patients who had undergone allo-HSCT for a hematologic malignancy. They were randomized to receive 250 mg of azithromycin (n=243) or placebo (n=237) 3 times a week for 2 years, beginning at the start of conditioning.
The trial was stopped about 13 months after enrollment was completed because there was an unexpected increase in the rate of relapse and death in patients taking azithromycin.
The 2-year cumulative incidence of relapse was 33.5% in the azithromycin group and 22.3% in the placebo group (unadjusted cause-specific hazard ratio [HR]=1.7, P=0.002).
The 2-year survival rate was 56.6% in the azithromycin group and 70.1% in the placebo group (adjusted HR=1.5, P=0.02).
The 2-year airflow decline-free survival rate was 32.8% in the azithromycin group and 41.3% in the placebo group (unadjusted HR=1.3, P=0.03).
And the incidence of bronchiolitis obliterans syndrome was 6% in the azithromycin group and 3% in the placebo group (P=0.08).
Everything’s Fine … Except His Spine
ANSWER
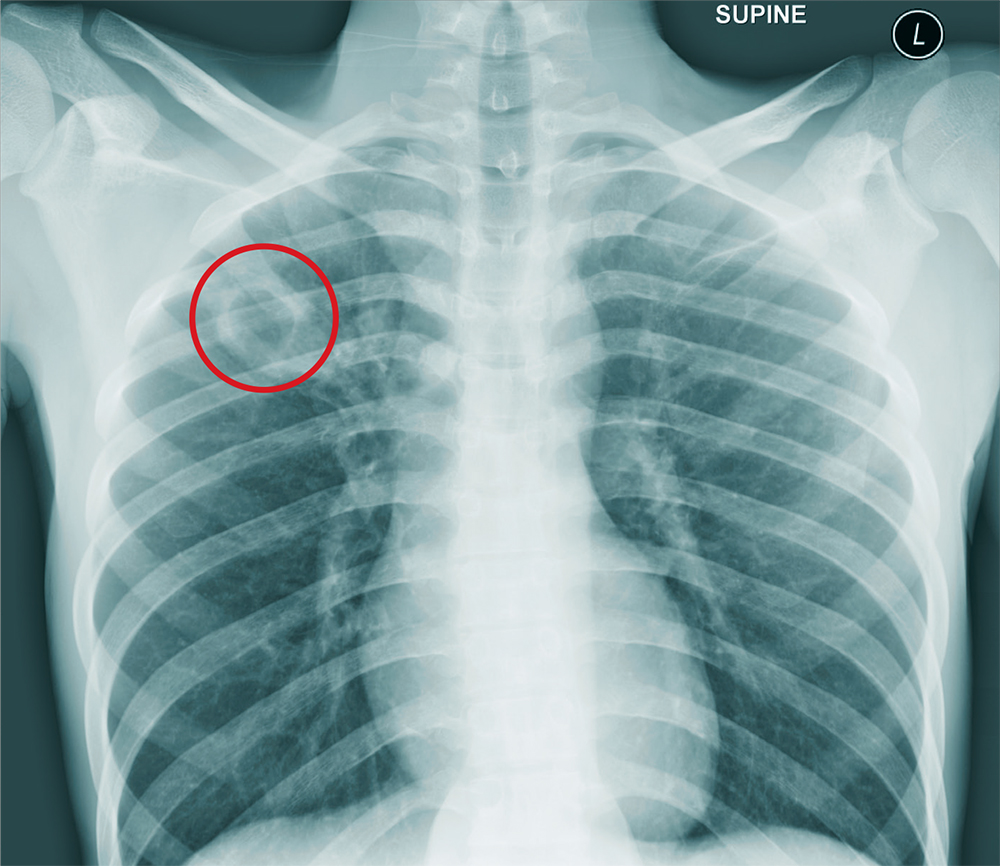
The chest radiograph shows an approximately 3-cm cavitary lesion in the right upper lobe. Such a lesion can indicate lung abscess, neoplasm, or tuberculosis.
Subsequent workup determined that he did, in fact, have tuberculosis, with involvement in his spine (known as Pott disease).

ANSWER
The chest radiograph shows an approximately 3-cm cavitary lesion in the right upper lobe. Such a lesion can indicate lung abscess, neoplasm, or tuberculosis.
Subsequent workup determined that he did, in fact, have tuberculosis, with involvement in his spine (known as Pott disease).

ANSWER
The chest radiograph shows an approximately 3-cm cavitary lesion in the right upper lobe. Such a lesion can indicate lung abscess, neoplasm, or tuberculosis.
Subsequent workup determined that he did, in fact, have tuberculosis, with involvement in his spine (known as Pott disease).
A 25-year-old man is admitted to your facility for a possible infection in his spine. He reports a two-week history of severe back pain with no history of injury or trauma. Imaging performed at an outside facility suggested compression and erosion of his vertebral bodies at the thoracolumbar junction, and the radiologist raised concern for possible osteomyelitis and diskitis.
The patient is otherwise healthy and denies any medical problems. He denies drug use of any form. Review of systems is significant for a three-month history of anorexia and night sweats but no fever.
Physical exam reveals a healthy-appearing male with normal vital signs. His heart and lung sounds are normal.
A chest radiograph is obtained (shown). What is your impression?
Treating immunotherapy-related AEs in the emergency department
In this interview, Dr David Henry and Dr Maura Sammon discuss some of the most common immunotherapy-related side effects – lung, gastrointestinal, rash, and endocrine-related problems – and Dr Sammon describes in detail how physicians in the ED would triage and treat the patient. However, the overarching takeaway is the importance of communication: first, between the oncologist and patient, so that the patient is aware of these nuances in advance of an emergency, and second, between the ED physician and the treating oncologist soon after the patient has presented and undergone an initial assessment.
Dr Henry is Editor-in-Chief of the JCSO, and Dr Sammon is at the Lewis Katz School of Medicine, Temple University, in Philadelphia, Pennsylvania
Listen here:
In this interview, Dr David Henry and Dr Maura Sammon discuss some of the most common immunotherapy-related side effects – lung, gastrointestinal, rash, and endocrine-related problems – and Dr Sammon describes in detail how physicians in the ED would triage and treat the patient. However, the overarching takeaway is the importance of communication: first, between the oncologist and patient, so that the patient is aware of these nuances in advance of an emergency, and second, between the ED physician and the treating oncologist soon after the patient has presented and undergone an initial assessment.
Dr Henry is Editor-in-Chief of the JCSO, and Dr Sammon is at the Lewis Katz School of Medicine, Temple University, in Philadelphia, Pennsylvania
Listen here:
In this interview, Dr David Henry and Dr Maura Sammon discuss some of the most common immunotherapy-related side effects – lung, gastrointestinal, rash, and endocrine-related problems – and Dr Sammon describes in detail how physicians in the ED would triage and treat the patient. However, the overarching takeaway is the importance of communication: first, between the oncologist and patient, so that the patient is aware of these nuances in advance of an emergency, and second, between the ED physician and the treating oncologist soon after the patient has presented and undergone an initial assessment.
Dr Henry is Editor-in-Chief of the JCSO, and Dr Sammon is at the Lewis Katz School of Medicine, Temple University, in Philadelphia, Pennsylvania
Listen here:
Low disease activity in SLE compares favorably with clinical remission as an acceptable goal
Canadian researchers report.
The findings endorse the endpoint used to define low disease activity in the study – Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) of 2 or less – as a meaningful treat-to-target outcome in SLE and also help to fill in missing information about the long-term longitudinal status of patients with recent-onset disease who enter either low disease activity or clinical remission status, first author Konstantinos Tselios, MD, PhD, and coauthors at the Centre for Prognosis Studies in the Rheumatic Diseases at the University Health Network’s Toronto Lupus Clinic, wrote in Arthritis Care & Research.
The concept of low disease activity (LDA) has emerged because it is rare for SLE patients to achieve sustained complete remission, the authors said. Existing studies on the outcomes of LDA and clinical remission have demonstrated comparable outcomes between the two in terms of damage accrual after 2 years, but they enrolled patients with prevalent disease, many of whom were in late stages.
“Given that disease duration has a significant impact on disease activity, the prevalence and characteristics of complete remission and LDA might have been affected,” they wrote.
The investigators therefore conducted the current study to assess damage accrual, medications used, flare rate, and mortality in inception patients who sustained complete remission and LDA status for 10 years.
The study involved 267 patients attending the University of Toronto Lupus Clinic who had at least 10 years of follow-up and no time interval between clinic visits exceeding 18 months. All patients fulfilled the revised American College of Rheumatology criteria for SLE classification or had three criteria and a supportive biopsy. The patients all had either LDA or clinical remission (SLEDAI-2K = 0, excluding serology) within the first 5 years since enrollment and maintained that status for 10 consecutive years, regardless of therapy.
A total of 10.1% of patients (n = 27) achieved prolonged clinical remission. Clinical manifestations at baseline included mucocutaneous disease in 70.4%, arthritis in 59.3%, cytopenias in 55.6%, nephritis in 25.9%, and central nervous involvement in 25.9%. Nephritis was class II in three patients, class III in one, and class IV in three.
For the 18% of patients (n = 48) who achieved sustained LDA, clinical manifestations included mucocutaneous disease in 81.3%, cytopenias in 66.7%, arthritis in 60.4%, serositis in 29.2%, nephritis in 22.9%, and central nervous system disease in 8.3%. The nephritis was class II in two patients, class III in four, class IV in two, and class VI in one. Patients in both groups were similar at baseline, but mean prednisone dose was higher in the remission group and more patients in the LDA group were taking antimalarials.
Time to remission and time to LDA were 1.2 years in both groups. After 10 years of follow-up, the mean SLEDAI was 1.2 in the remission group and 1.6 in the LDA group (P = .31) and 3.7 among patients not achieving either goal (n = 192; P less than .001).
Moreover, serology (complement C3/C4 and anti-double stranded DNA positivity) and antiextractable nuclear antigen antibody profile were similar in both groups.
No differences in flare rate or mortality emerged. Disease flares were observed in 25.9% of the remission group and 31.2% of the LDA group (P = .63) and flare rates were 0.038/patient-year and 0.065/patient-year, respectively (P = .23). Mortality after 10 years was 11.1% in the remission group and 10.4% in the LDA group (P = .93).
“Maintenance of this state could be an acceptable target for the long-term management of SLE. The concept of LDA is relatively new in SLE. Since complete remission has not been strictly defined yet and is rare, a more realistic target, such as LDA, may be of value in daily clinical practice as well as in clinical trials,” the investigators wrote.
The authors noted that the study was limited by the relatively low number of patients.
The University of Toronto Lupus Research Program is supported by the University Health Network, Lou & Marissa Rocca, and the Lupus Foundation of Ontario. The authors declared no potential conflicts of interests.
SOURCE: Tselios K et al. Arthritis Care Res. 2018 Jul 28. doi: 10.1002/acr.23720
Canadian researchers report.
The findings endorse the endpoint used to define low disease activity in the study – Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) of 2 or less – as a meaningful treat-to-target outcome in SLE and also help to fill in missing information about the long-term longitudinal status of patients with recent-onset disease who enter either low disease activity or clinical remission status, first author Konstantinos Tselios, MD, PhD, and coauthors at the Centre for Prognosis Studies in the Rheumatic Diseases at the University Health Network’s Toronto Lupus Clinic, wrote in Arthritis Care & Research.
The concept of low disease activity (LDA) has emerged because it is rare for SLE patients to achieve sustained complete remission, the authors said. Existing studies on the outcomes of LDA and clinical remission have demonstrated comparable outcomes between the two in terms of damage accrual after 2 years, but they enrolled patients with prevalent disease, many of whom were in late stages.
“Given that disease duration has a significant impact on disease activity, the prevalence and characteristics of complete remission and LDA might have been affected,” they wrote.
The investigators therefore conducted the current study to assess damage accrual, medications used, flare rate, and mortality in inception patients who sustained complete remission and LDA status for 10 years.
The study involved 267 patients attending the University of Toronto Lupus Clinic who had at least 10 years of follow-up and no time interval between clinic visits exceeding 18 months. All patients fulfilled the revised American College of Rheumatology criteria for SLE classification or had three criteria and a supportive biopsy. The patients all had either LDA or clinical remission (SLEDAI-2K = 0, excluding serology) within the first 5 years since enrollment and maintained that status for 10 consecutive years, regardless of therapy.
A total of 10.1% of patients (n = 27) achieved prolonged clinical remission. Clinical manifestations at baseline included mucocutaneous disease in 70.4%, arthritis in 59.3%, cytopenias in 55.6%, nephritis in 25.9%, and central nervous involvement in 25.9%. Nephritis was class II in three patients, class III in one, and class IV in three.
For the 18% of patients (n = 48) who achieved sustained LDA, clinical manifestations included mucocutaneous disease in 81.3%, cytopenias in 66.7%, arthritis in 60.4%, serositis in 29.2%, nephritis in 22.9%, and central nervous system disease in 8.3%. The nephritis was class II in two patients, class III in four, class IV in two, and class VI in one. Patients in both groups were similar at baseline, but mean prednisone dose was higher in the remission group and more patients in the LDA group were taking antimalarials.
Time to remission and time to LDA were 1.2 years in both groups. After 10 years of follow-up, the mean SLEDAI was 1.2 in the remission group and 1.6 in the LDA group (P = .31) and 3.7 among patients not achieving either goal (n = 192; P less than .001).
Moreover, serology (complement C3/C4 and anti-double stranded DNA positivity) and antiextractable nuclear antigen antibody profile were similar in both groups.
No differences in flare rate or mortality emerged. Disease flares were observed in 25.9% of the remission group and 31.2% of the LDA group (P = .63) and flare rates were 0.038/patient-year and 0.065/patient-year, respectively (P = .23). Mortality after 10 years was 11.1% in the remission group and 10.4% in the LDA group (P = .93).
“Maintenance of this state could be an acceptable target for the long-term management of SLE. The concept of LDA is relatively new in SLE. Since complete remission has not been strictly defined yet and is rare, a more realistic target, such as LDA, may be of value in daily clinical practice as well as in clinical trials,” the investigators wrote.
The authors noted that the study was limited by the relatively low number of patients.
The University of Toronto Lupus Research Program is supported by the University Health Network, Lou & Marissa Rocca, and the Lupus Foundation of Ontario. The authors declared no potential conflicts of interests.
SOURCE: Tselios K et al. Arthritis Care Res. 2018 Jul 28. doi: 10.1002/acr.23720
Canadian researchers report.
The findings endorse the endpoint used to define low disease activity in the study – Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) of 2 or less – as a meaningful treat-to-target outcome in SLE and also help to fill in missing information about the long-term longitudinal status of patients with recent-onset disease who enter either low disease activity or clinical remission status, first author Konstantinos Tselios, MD, PhD, and coauthors at the Centre for Prognosis Studies in the Rheumatic Diseases at the University Health Network’s Toronto Lupus Clinic, wrote in Arthritis Care & Research.
The concept of low disease activity (LDA) has emerged because it is rare for SLE patients to achieve sustained complete remission, the authors said. Existing studies on the outcomes of LDA and clinical remission have demonstrated comparable outcomes between the two in terms of damage accrual after 2 years, but they enrolled patients with prevalent disease, many of whom were in late stages.
“Given that disease duration has a significant impact on disease activity, the prevalence and characteristics of complete remission and LDA might have been affected,” they wrote.
The investigators therefore conducted the current study to assess damage accrual, medications used, flare rate, and mortality in inception patients who sustained complete remission and LDA status for 10 years.
The study involved 267 patients attending the University of Toronto Lupus Clinic who had at least 10 years of follow-up and no time interval between clinic visits exceeding 18 months. All patients fulfilled the revised American College of Rheumatology criteria for SLE classification or had three criteria and a supportive biopsy. The patients all had either LDA or clinical remission (SLEDAI-2K = 0, excluding serology) within the first 5 years since enrollment and maintained that status for 10 consecutive years, regardless of therapy.
A total of 10.1% of patients (n = 27) achieved prolonged clinical remission. Clinical manifestations at baseline included mucocutaneous disease in 70.4%, arthritis in 59.3%, cytopenias in 55.6%, nephritis in 25.9%, and central nervous involvement in 25.9%. Nephritis was class II in three patients, class III in one, and class IV in three.
For the 18% of patients (n = 48) who achieved sustained LDA, clinical manifestations included mucocutaneous disease in 81.3%, cytopenias in 66.7%, arthritis in 60.4%, serositis in 29.2%, nephritis in 22.9%, and central nervous system disease in 8.3%. The nephritis was class II in two patients, class III in four, class IV in two, and class VI in one. Patients in both groups were similar at baseline, but mean prednisone dose was higher in the remission group and more patients in the LDA group were taking antimalarials.
Time to remission and time to LDA were 1.2 years in both groups. After 10 years of follow-up, the mean SLEDAI was 1.2 in the remission group and 1.6 in the LDA group (P = .31) and 3.7 among patients not achieving either goal (n = 192; P less than .001).
Moreover, serology (complement C3/C4 and anti-double stranded DNA positivity) and antiextractable nuclear antigen antibody profile were similar in both groups.
No differences in flare rate or mortality emerged. Disease flares were observed in 25.9% of the remission group and 31.2% of the LDA group (P = .63) and flare rates were 0.038/patient-year and 0.065/patient-year, respectively (P = .23). Mortality after 10 years was 11.1% in the remission group and 10.4% in the LDA group (P = .93).
“Maintenance of this state could be an acceptable target for the long-term management of SLE. The concept of LDA is relatively new in SLE. Since complete remission has not been strictly defined yet and is rare, a more realistic target, such as LDA, may be of value in daily clinical practice as well as in clinical trials,” the investigators wrote.
The authors noted that the study was limited by the relatively low number of patients.
The University of Toronto Lupus Research Program is supported by the University Health Network, Lou & Marissa Rocca, and the Lupus Foundation of Ontario. The authors declared no potential conflicts of interests.
SOURCE: Tselios K et al. Arthritis Care Res. 2018 Jul 28. doi: 10.1002/acr.23720
FROM ARTHRITIS CARE & RESEARCH
Key clinical point: Low disease activity, as defined by a SLE Disease Activity Index 2000 score of 2 or less, appears to be a meaningful treat-to-target outcome in SLE.
Major finding: SLE patients with either prolonged clinical remission or prolonged low disease activity had comparable damage accrual, flare rate, and mortality in a 10-year period after enrollment.
Study details: 267 patients attending the University of Toronto Lupus Clinic who had at least 10 years of follow-up.
Disclosures: The University of Toronto Lupus Research Program is supported by the University Health Network, Lou & Marissa Rocca, and the Lupus Foundation of Ontario. The authors declared no potential conflicts of interests.
Source: Tselios K et al. Arthritis Care Res. 2018 Jul 28. doi: 10.1002/acr.23720
Children, adolescents of divorcing parents want more say
Parents who are in the midst of a divorce might want to protect their children from details tied to the breakup, such as parenting arrangements. But they might be doing more harm than good.
A survey of 61 children and adolescents who recently had gone through their parents’ divorce suggested that many want a say in how the divorce will affect them, according to a report broadcast by the Australian Broadcasting Corporation (ABC).
The parents’ actions are benevolent in their intention, but they “may be associated with the experience of harm on the part of children and young people where their agency and capacity to participate in decision making that affects them isn’t acknowledged and accommodated,” said study lead Rachel Carson, PhD, of the Australian Institute of Family Studies, in an interview.
To conduct the survey, the research team conducted interviews with the children and adolescents, aged 10-17 years. They also interviewed 47 parents of those children.
Most of the children and adolescents interviewed said they felt isolated from their parents’ divorce, with little or no influence on the process. The ABC report included several quotations from the interviewees, including “Phoebe,” who was 15 years old at the time of the survey.
“When a kid goes through a divorce, a lot of the time the adults become very immature, so the children grow up a lot quicker,” Phoebe said.
The survey concluded that a “child-inclusive approach be adopted incorporating the features of effective professional practice. ...A commitment to this approach would importantly be a step towards meeting the loud and clear calls from participating children and young people to ‘give children a bigger voice, more of the time.’ ”
Click here to listen to the ABC story.
Parents who are in the midst of a divorce might want to protect their children from details tied to the breakup, such as parenting arrangements. But they might be doing more harm than good.
A survey of 61 children and adolescents who recently had gone through their parents’ divorce suggested that many want a say in how the divorce will affect them, according to a report broadcast by the Australian Broadcasting Corporation (ABC).
The parents’ actions are benevolent in their intention, but they “may be associated with the experience of harm on the part of children and young people where their agency and capacity to participate in decision making that affects them isn’t acknowledged and accommodated,” said study lead Rachel Carson, PhD, of the Australian Institute of Family Studies, in an interview.
To conduct the survey, the research team conducted interviews with the children and adolescents, aged 10-17 years. They also interviewed 47 parents of those children.
Most of the children and adolescents interviewed said they felt isolated from their parents’ divorce, with little or no influence on the process. The ABC report included several quotations from the interviewees, including “Phoebe,” who was 15 years old at the time of the survey.
“When a kid goes through a divorce, a lot of the time the adults become very immature, so the children grow up a lot quicker,” Phoebe said.
The survey concluded that a “child-inclusive approach be adopted incorporating the features of effective professional practice. ...A commitment to this approach would importantly be a step towards meeting the loud and clear calls from participating children and young people to ‘give children a bigger voice, more of the time.’ ”
Click here to listen to the ABC story.
Parents who are in the midst of a divorce might want to protect their children from details tied to the breakup, such as parenting arrangements. But they might be doing more harm than good.
A survey of 61 children and adolescents who recently had gone through their parents’ divorce suggested that many want a say in how the divorce will affect them, according to a report broadcast by the Australian Broadcasting Corporation (ABC).
The parents’ actions are benevolent in their intention, but they “may be associated with the experience of harm on the part of children and young people where their agency and capacity to participate in decision making that affects them isn’t acknowledged and accommodated,” said study lead Rachel Carson, PhD, of the Australian Institute of Family Studies, in an interview.
To conduct the survey, the research team conducted interviews with the children and adolescents, aged 10-17 years. They also interviewed 47 parents of those children.
Most of the children and adolescents interviewed said they felt isolated from their parents’ divorce, with little or no influence on the process. The ABC report included several quotations from the interviewees, including “Phoebe,” who was 15 years old at the time of the survey.
“When a kid goes through a divorce, a lot of the time the adults become very immature, so the children grow up a lot quicker,” Phoebe said.
The survey concluded that a “child-inclusive approach be adopted incorporating the features of effective professional practice. ...A commitment to this approach would importantly be a step towards meeting the loud and clear calls from participating children and young people to ‘give children a bigger voice, more of the time.’ ”
Click here to listen to the ABC story.
Company is giving drug users a second chance
An electric wire plant in Richmond, Ind., is bucking the practice of rejecting job applicants who fail drug tests.
Instead, the century-old Belden, a St. Louis-based company that also makes cable products, Internet routers, and other high-tech equipment, is offering Richmond plant applicants a job – if they complete a free 1- to 4-month drug treatment program.
The pilot program – called Pathways to Employment – aims to address two problems: the opioid crisis gripping the country and the worker shortage faced by many U.S. companies. Belden works with several local partners. The initiative was conceived with Mitchell S. Rosenthal, MD, a psychiatrist who serves as president of the Rosenthal Center for Addiction Studies and was founder of Phoenix House, the nonprofit drug treatment group. The program is thought to be the first of its kind.
in hiring the folks we needed,” said Doug Brenneke, Belden’s vice president of research and development, in an interview with National Public Radio.
Belden also is offering the program to the broader community – not just people who are part of the plant’s workforce. “We think part of the solution is offering basically a path out,” Mr. Brenneke told NPR. “It’s not a silver bullet, but it is part of an overall solution, we believe, to the epidemic.”
Click here to hear the NPR story.
An electric wire plant in Richmond, Ind., is bucking the practice of rejecting job applicants who fail drug tests.
Instead, the century-old Belden, a St. Louis-based company that also makes cable products, Internet routers, and other high-tech equipment, is offering Richmond plant applicants a job – if they complete a free 1- to 4-month drug treatment program.
The pilot program – called Pathways to Employment – aims to address two problems: the opioid crisis gripping the country and the worker shortage faced by many U.S. companies. Belden works with several local partners. The initiative was conceived with Mitchell S. Rosenthal, MD, a psychiatrist who serves as president of the Rosenthal Center for Addiction Studies and was founder of Phoenix House, the nonprofit drug treatment group. The program is thought to be the first of its kind.
in hiring the folks we needed,” said Doug Brenneke, Belden’s vice president of research and development, in an interview with National Public Radio.
Belden also is offering the program to the broader community – not just people who are part of the plant’s workforce. “We think part of the solution is offering basically a path out,” Mr. Brenneke told NPR. “It’s not a silver bullet, but it is part of an overall solution, we believe, to the epidemic.”
Click here to hear the NPR story.
An electric wire plant in Richmond, Ind., is bucking the practice of rejecting job applicants who fail drug tests.
Instead, the century-old Belden, a St. Louis-based company that also makes cable products, Internet routers, and other high-tech equipment, is offering Richmond plant applicants a job – if they complete a free 1- to 4-month drug treatment program.
The pilot program – called Pathways to Employment – aims to address two problems: the opioid crisis gripping the country and the worker shortage faced by many U.S. companies. Belden works with several local partners. The initiative was conceived with Mitchell S. Rosenthal, MD, a psychiatrist who serves as president of the Rosenthal Center for Addiction Studies and was founder of Phoenix House, the nonprofit drug treatment group. The program is thought to be the first of its kind.
in hiring the folks we needed,” said Doug Brenneke, Belden’s vice president of research and development, in an interview with National Public Radio.
Belden also is offering the program to the broader community – not just people who are part of the plant’s workforce. “We think part of the solution is offering basically a path out,” Mr. Brenneke told NPR. “It’s not a silver bullet, but it is part of an overall solution, we believe, to the epidemic.”
Click here to hear the NPR story.
Mothers’ parenting decisions and social norms
Leaving children to fend for themselves used to be viewed as a positive aspect of parenting. Now, in an age of fear, a parent, particularly a mother, can be judged as negligent – to the point of criminal prosecution – for actions like leaving a child to play in a park.
In the New York Times, writer Kim Brooks recounted her own story of leaving her 4-year-old son locked in a car on what she calls a “cool March day” so that she could run a quick errand. That fateful decision led to a series of encounters between Ms. Brooks and the criminal justice system, including a charge of “contributing to the delinquency of a minor.”
Ms. Brooks examined the challenges that mothers face in a world quick to pass judgment on their parenting decisions. She called those moral judgments “mother-shaming” and said that the judgments and risk assessments are intertwined.
“People don’t only think that leaving children alone is dangerous and therefore immoral. They also think it is immoral and therefore dangerous. said Barbara W. Sarnecka, PhD, a cognitive science professor at the University of California, Irvine, told Ms. Brooks. Dr. Sarnecka went on to say that, although children do not have the same rights as adults, “they have some rights, and not just to safety. They have the right to some freedom, to some independence, and to a little bit of danger.”
Click here to read the Time article.
Leaving children to fend for themselves used to be viewed as a positive aspect of parenting. Now, in an age of fear, a parent, particularly a mother, can be judged as negligent – to the point of criminal prosecution – for actions like leaving a child to play in a park.
In the New York Times, writer Kim Brooks recounted her own story of leaving her 4-year-old son locked in a car on what she calls a “cool March day” so that she could run a quick errand. That fateful decision led to a series of encounters between Ms. Brooks and the criminal justice system, including a charge of “contributing to the delinquency of a minor.”
Ms. Brooks examined the challenges that mothers face in a world quick to pass judgment on their parenting decisions. She called those moral judgments “mother-shaming” and said that the judgments and risk assessments are intertwined.
“People don’t only think that leaving children alone is dangerous and therefore immoral. They also think it is immoral and therefore dangerous. said Barbara W. Sarnecka, PhD, a cognitive science professor at the University of California, Irvine, told Ms. Brooks. Dr. Sarnecka went on to say that, although children do not have the same rights as adults, “they have some rights, and not just to safety. They have the right to some freedom, to some independence, and to a little bit of danger.”
Click here to read the Time article.
Leaving children to fend for themselves used to be viewed as a positive aspect of parenting. Now, in an age of fear, a parent, particularly a mother, can be judged as negligent – to the point of criminal prosecution – for actions like leaving a child to play in a park.
In the New York Times, writer Kim Brooks recounted her own story of leaving her 4-year-old son locked in a car on what she calls a “cool March day” so that she could run a quick errand. That fateful decision led to a series of encounters between Ms. Brooks and the criminal justice system, including a charge of “contributing to the delinquency of a minor.”
Ms. Brooks examined the challenges that mothers face in a world quick to pass judgment on their parenting decisions. She called those moral judgments “mother-shaming” and said that the judgments and risk assessments are intertwined.
“People don’t only think that leaving children alone is dangerous and therefore immoral. They also think it is immoral and therefore dangerous. said Barbara W. Sarnecka, PhD, a cognitive science professor at the University of California, Irvine, told Ms. Brooks. Dr. Sarnecka went on to say that, although children do not have the same rights as adults, “they have some rights, and not just to safety. They have the right to some freedom, to some independence, and to a little bit of danger.”
Click here to read the Time article.
Native American writer explores identity
The debut novel “There There” by Tommy Orange explores what it means to be Native American in contemporary urban America.
In an interview with the PBS Newshour, Mr. Orange, a member of the Cheyenne and Arapaho tribes who grew up in Oakland, Calif., describes the ways in which living in an urban environment in an ethnically diverse American city can be isolating and lonely for Native Americans.
“We know the sound of the freeway better than we do rivers, the howl of distant trains better than wolf howls,” Mr. Orange said, reading from his book. “We know the smell of gas and freshly wet concrete and burned rubber better than we do the smell of cedar or sage or even frybread. We ride buses, trains, and cars across, over, and under concrete plains. Being Indian has never been about returning to the land. The land is everywhere or nowhere.”
For Mr. Orange, whose mother is white and father is Native American, identity in Oakland has been a fluid concept. “If I’m in the Fruitvale [area of Oakland], people will speak Spanish to me first,” he said in the interview.
Establishing identity can be a difficult task for Native Americans, Mr. Orange said, because parents often do not share stories about history – “sometimes because of pain,” he said.
To watch the PBS Newshour interview with Mr. Orange, click here.
The debut novel “There There” by Tommy Orange explores what it means to be Native American in contemporary urban America.
In an interview with the PBS Newshour, Mr. Orange, a member of the Cheyenne and Arapaho tribes who grew up in Oakland, Calif., describes the ways in which living in an urban environment in an ethnically diverse American city can be isolating and lonely for Native Americans.
“We know the sound of the freeway better than we do rivers, the howl of distant trains better than wolf howls,” Mr. Orange said, reading from his book. “We know the smell of gas and freshly wet concrete and burned rubber better than we do the smell of cedar or sage or even frybread. We ride buses, trains, and cars across, over, and under concrete plains. Being Indian has never been about returning to the land. The land is everywhere or nowhere.”
For Mr. Orange, whose mother is white and father is Native American, identity in Oakland has been a fluid concept. “If I’m in the Fruitvale [area of Oakland], people will speak Spanish to me first,” he said in the interview.
Establishing identity can be a difficult task for Native Americans, Mr. Orange said, because parents often do not share stories about history – “sometimes because of pain,” he said.
To watch the PBS Newshour interview with Mr. Orange, click here.
The debut novel “There There” by Tommy Orange explores what it means to be Native American in contemporary urban America.
In an interview with the PBS Newshour, Mr. Orange, a member of the Cheyenne and Arapaho tribes who grew up in Oakland, Calif., describes the ways in which living in an urban environment in an ethnically diverse American city can be isolating and lonely for Native Americans.
“We know the sound of the freeway better than we do rivers, the howl of distant trains better than wolf howls,” Mr. Orange said, reading from his book. “We know the smell of gas and freshly wet concrete and burned rubber better than we do the smell of cedar or sage or even frybread. We ride buses, trains, and cars across, over, and under concrete plains. Being Indian has never been about returning to the land. The land is everywhere or nowhere.”
For Mr. Orange, whose mother is white and father is Native American, identity in Oakland has been a fluid concept. “If I’m in the Fruitvale [area of Oakland], people will speak Spanish to me first,” he said in the interview.
Establishing identity can be a difficult task for Native Americans, Mr. Orange said, because parents often do not share stories about history – “sometimes because of pain,” he said.
To watch the PBS Newshour interview with Mr. Orange, click here.