User login
A new protocol for RhD-negative pregnant women?
ILLUSTRATIVE CASE
A 30-year-old G1P0 woman presents to your office for routine obstetric care at 18 weeks’ gestation. Her pregnancy has been uncomplicated, but her prenatal lab evaluation is notable for blood type A-negative. She wants to know if she really needs the anti-D immune globulin injection.
Rhesus (Rh)D-negative women carrying an RhD-positive fetus are at risk of developing anti-D antibodies, placing the fetus at risk for HDFN (hemolytic disease of the fetus and newborn). If undiagnosed and/or untreated, HDFN carries significant risk of perinatal morbidity and mortality.2
With routine postnatal anti-D immunoglobulin prophylaxis of RhD-negative women who delivered an RhD-positive child (which began around 1970), the risk of maternal alloimmunization was reduced from 16% to 1.12%-1.3%.3-5 The risk was further reduced to approximately 0.28% with the addition of consistent prophylaxis at 28 weeks’ gestation.4 As a result, the current standard of care is to administer anti-D immunoglobulin at 28 weeks’ gestation, within 72 hours of delivery of an RhD-positive fetus, and after events with risk of fetal-to-maternal transfusion (eg, spontaneous, threatened, or induced abortion; invasive prenatal diagnostic procedures such as amniocentesis; blunt abdominal trauma; external cephalic version; second or third trimester antepartum bleeding).6
The problem of unnecessary Tx. However, under this current practice, many RhD-negative women are receiving anti-D immunoglobulin unnecessarily. This is because the fetus’s RhD status is not routinely known during the prenatal period.
Enter cell-free DNA testing. Cell-free DNA testing analyzes fragments of fetal DNA found in maternal blood. The use of cell-free DNA testing at 10 to 13 weeks’ gestation to screen for fetal chromosomal abnormalities is reliable (91%-99% sensitivity for trisomies 21, 18, and 137) and becoming increasingly more common.
A notable meta-analysis. A 2017 meta-analysis of 30 studies of cell-free DNA testing of RhD status in the first and second trimester calculated a sensitivity of 99.3% (95% confidence interval [CI], 98.2-99.7) and a specificity of 98.4% (95% CI, 96.4-99.3).7
This study evaluated the accuracy of using cell-free DNA testing at 27 weeks’ gestation to determine fetal RhD status compared with serologic typing of cord blood at delivery.
STUDY SUMMARY
Cell-free DNA test gets high marks in Netherlands trial
This large observational cohort trial from the Netherlands examined the accuracy of identifying RhD-positive fetuses using cell-free DNA isolates in maternal plasma. Over the 15-month study period, fetal RhD testing was conducted during Week 27 of gestation, and results were compared with those obtained using neonatal cord blood at birth. If the fetal RhD test was positive, providers administered 200 mcg anti-D immunoglobulin during the 30th week of gestation and within 48 hours of birth. If fetal RhD was negative, providers were told immunoglobulin was unnecessary.
More than 32,000 RhD-negative women were screened. The cell-free DNA test showed fetal RhD-positive results 62% of the time and RhD-negative results in the remainder. Cord blood samples were available for 25,789 pregnancies (80%).
Sensitivity, specificity. The sensitivity for identifying fetal RhD was 99% and the specificity was 98%. Both negative and positive predictive values were 99%. Overall, there were 225 false-positive results and 9 false-negative results. In the 9 false negatives, 6 were due to a lack of fetal DNA in the sample and 3 were due to technical error (defined as an operator ignoring a failure of the robot pipetting the plasma or other technical failures).
The false-negative rate (0.03%) was lower than the predetermined estimated false-negative rate of cord blood serology (0.25%). In 22 of the supposed false positives, follow-up serology or molecular testing found an RhD gene was actually present, meaning the results of the neonatal cord blood serology in these cases were falsely negative. If you recalculate with these data in mind, the false-negative rate for fetal DNA testing was actually less than half that of typical serologic determination.
WHAT’S NEW
An accurate test with the potential to reduce unnecessary Tx
Fetal RhD testing at 27 weeks’ gestation appears to be highly accurate and could reduce the unnecessary use of anti-D immunoglobulin when the fetal RhD is negative.
CAVEATS
Different results with different ethnicities?
Dutch participants are not necessarily reflective of the US population. Known variation in the rate of fetal RhD positivity among RhD-negative pregnant women by race and ethnicity could mean that the number of women able to forego anti-D-immunoglobulin prophylaxis would be different in the United States from that in other countries.
Also, in this study, polymerase chain reaction (PCR) for 2 RhD sequences was run in triplicate, and a computer-based algorithm was used to automatically score samples to provide results. For safe implementation, the cell-free fetal RhD DNA testing process would need to follow similar methods.
CHALLENGES TO IMPLEMENTATION
Test cost and availability are big unknowns
Cost and availability of the test may be barriers, but there is currently too little information on either subject in the United States to make a determination. A 2013 study indicated that the use of cell-free DNA testing to determine fetal RhD status was then approximately $682.10
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. de Haas M, Thurik FF, van der Ploeg CP, et al. Sensitivity of fetal RHD screening for safe guidance of targeted anti-D immunoglobulin prophylaxis: prospective cohort study of a nationwide programme in the Netherlands. BMJ. 2016;355:i5789.
2. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 75: Management of alloimmunization during pregnancy. Obstet Gynecol. 2006;108:457-464.
3. Urbaniak S, Greiss MA. RhD haemolytic disease of the fetus and the newborn. Blood Rev. 2000;14:44-61.
4. Mayne S, Parker JH, Harden TA, et al. Rate of RhD sensitisation before and after implementation of a community based antenatal prophylaxis programme. BMJ. 1997;315:1588-1588.
5. MacKenzie IZ, Bowell P, Gregory H, et al. Routine antenatal Rhesus D immunoglobulin prophylaxis: the results of a prospective 10 year study. Br J Obstet Gynecol: 1999;106:492-497.
6. Zolotor AJ, Carlough MC. Update on prenatal care. Am Fam Physician. 2014;89:199-208.
7. Mackie FL, Hemming K, Allen S, et al. The accuracy of cell-free fetal DNA-based non-invasive prenatal testing in singleton pregnancies: a systematic review and bivariate meta-analysis. BJOG. 2017;124:32-46.
8. Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 181: Prevention of Rh D Alloimmunization. Obstet Gynecol. 2017;130:e57-e70.
9. National Institute for Health and Care Excellence. High-throughput non-invasive prenatal testing for fetal RHD genotype 1: Recommendations. Available at: https://www.nice.org.uk/guidance/dg25/chapter/1-Recommendations. Accessed August 9, 2017.
10. Hawk AF, Chang EY, Shields SM, et al. Costs and clinical outcomes of noninvasive fetal RhD typing for targeted prophylaxis. Obstet Gynecol. 2013;122:579-585.
ILLUSTRATIVE CASE
A 30-year-old G1P0 woman presents to your office for routine obstetric care at 18 weeks’ gestation. Her pregnancy has been uncomplicated, but her prenatal lab evaluation is notable for blood type A-negative. She wants to know if she really needs the anti-D immune globulin injection.
Rhesus (Rh)D-negative women carrying an RhD-positive fetus are at risk of developing anti-D antibodies, placing the fetus at risk for HDFN (hemolytic disease of the fetus and newborn). If undiagnosed and/or untreated, HDFN carries significant risk of perinatal morbidity and mortality.2
With routine postnatal anti-D immunoglobulin prophylaxis of RhD-negative women who delivered an RhD-positive child (which began around 1970), the risk of maternal alloimmunization was reduced from 16% to 1.12%-1.3%.3-5 The risk was further reduced to approximately 0.28% with the addition of consistent prophylaxis at 28 weeks’ gestation.4 As a result, the current standard of care is to administer anti-D immunoglobulin at 28 weeks’ gestation, within 72 hours of delivery of an RhD-positive fetus, and after events with risk of fetal-to-maternal transfusion (eg, spontaneous, threatened, or induced abortion; invasive prenatal diagnostic procedures such as amniocentesis; blunt abdominal trauma; external cephalic version; second or third trimester antepartum bleeding).6
The problem of unnecessary Tx. However, under this current practice, many RhD-negative women are receiving anti-D immunoglobulin unnecessarily. This is because the fetus’s RhD status is not routinely known during the prenatal period.
Enter cell-free DNA testing. Cell-free DNA testing analyzes fragments of fetal DNA found in maternal blood. The use of cell-free DNA testing at 10 to 13 weeks’ gestation to screen for fetal chromosomal abnormalities is reliable (91%-99% sensitivity for trisomies 21, 18, and 137) and becoming increasingly more common.
A notable meta-analysis. A 2017 meta-analysis of 30 studies of cell-free DNA testing of RhD status in the first and second trimester calculated a sensitivity of 99.3% (95% confidence interval [CI], 98.2-99.7) and a specificity of 98.4% (95% CI, 96.4-99.3).7
This study evaluated the accuracy of using cell-free DNA testing at 27 weeks’ gestation to determine fetal RhD status compared with serologic typing of cord blood at delivery.
STUDY SUMMARY
Cell-free DNA test gets high marks in Netherlands trial
This large observational cohort trial from the Netherlands examined the accuracy of identifying RhD-positive fetuses using cell-free DNA isolates in maternal plasma. Over the 15-month study period, fetal RhD testing was conducted during Week 27 of gestation, and results were compared with those obtained using neonatal cord blood at birth. If the fetal RhD test was positive, providers administered 200 mcg anti-D immunoglobulin during the 30th week of gestation and within 48 hours of birth. If fetal RhD was negative, providers were told immunoglobulin was unnecessary.
More than 32,000 RhD-negative women were screened. The cell-free DNA test showed fetal RhD-positive results 62% of the time and RhD-negative results in the remainder. Cord blood samples were available for 25,789 pregnancies (80%).
Sensitivity, specificity. The sensitivity for identifying fetal RhD was 99% and the specificity was 98%. Both negative and positive predictive values were 99%. Overall, there were 225 false-positive results and 9 false-negative results. In the 9 false negatives, 6 were due to a lack of fetal DNA in the sample and 3 were due to technical error (defined as an operator ignoring a failure of the robot pipetting the plasma or other technical failures).
The false-negative rate (0.03%) was lower than the predetermined estimated false-negative rate of cord blood serology (0.25%). In 22 of the supposed false positives, follow-up serology or molecular testing found an RhD gene was actually present, meaning the results of the neonatal cord blood serology in these cases were falsely negative. If you recalculate with these data in mind, the false-negative rate for fetal DNA testing was actually less than half that of typical serologic determination.
WHAT’S NEW
An accurate test with the potential to reduce unnecessary Tx
Fetal RhD testing at 27 weeks’ gestation appears to be highly accurate and could reduce the unnecessary use of anti-D immunoglobulin when the fetal RhD is negative.
CAVEATS
Different results with different ethnicities?
Dutch participants are not necessarily reflective of the US population. Known variation in the rate of fetal RhD positivity among RhD-negative pregnant women by race and ethnicity could mean that the number of women able to forego anti-D-immunoglobulin prophylaxis would be different in the United States from that in other countries.
Also, in this study, polymerase chain reaction (PCR) for 2 RhD sequences was run in triplicate, and a computer-based algorithm was used to automatically score samples to provide results. For safe implementation, the cell-free fetal RhD DNA testing process would need to follow similar methods.
CHALLENGES TO IMPLEMENTATION
Test cost and availability are big unknowns
Cost and availability of the test may be barriers, but there is currently too little information on either subject in the United States to make a determination. A 2013 study indicated that the use of cell-free DNA testing to determine fetal RhD status was then approximately $682.10
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 30-year-old G1P0 woman presents to your office for routine obstetric care at 18 weeks’ gestation. Her pregnancy has been uncomplicated, but her prenatal lab evaluation is notable for blood type A-negative. She wants to know if she really needs the anti-D immune globulin injection.
Rhesus (Rh)D-negative women carrying an RhD-positive fetus are at risk of developing anti-D antibodies, placing the fetus at risk for HDFN (hemolytic disease of the fetus and newborn). If undiagnosed and/or untreated, HDFN carries significant risk of perinatal morbidity and mortality.2
With routine postnatal anti-D immunoglobulin prophylaxis of RhD-negative women who delivered an RhD-positive child (which began around 1970), the risk of maternal alloimmunization was reduced from 16% to 1.12%-1.3%.3-5 The risk was further reduced to approximately 0.28% with the addition of consistent prophylaxis at 28 weeks’ gestation.4 As a result, the current standard of care is to administer anti-D immunoglobulin at 28 weeks’ gestation, within 72 hours of delivery of an RhD-positive fetus, and after events with risk of fetal-to-maternal transfusion (eg, spontaneous, threatened, or induced abortion; invasive prenatal diagnostic procedures such as amniocentesis; blunt abdominal trauma; external cephalic version; second or third trimester antepartum bleeding).6
The problem of unnecessary Tx. However, under this current practice, many RhD-negative women are receiving anti-D immunoglobulin unnecessarily. This is because the fetus’s RhD status is not routinely known during the prenatal period.
Enter cell-free DNA testing. Cell-free DNA testing analyzes fragments of fetal DNA found in maternal blood. The use of cell-free DNA testing at 10 to 13 weeks’ gestation to screen for fetal chromosomal abnormalities is reliable (91%-99% sensitivity for trisomies 21, 18, and 137) and becoming increasingly more common.
A notable meta-analysis. A 2017 meta-analysis of 30 studies of cell-free DNA testing of RhD status in the first and second trimester calculated a sensitivity of 99.3% (95% confidence interval [CI], 98.2-99.7) and a specificity of 98.4% (95% CI, 96.4-99.3).7
This study evaluated the accuracy of using cell-free DNA testing at 27 weeks’ gestation to determine fetal RhD status compared with serologic typing of cord blood at delivery.
STUDY SUMMARY
Cell-free DNA test gets high marks in Netherlands trial
This large observational cohort trial from the Netherlands examined the accuracy of identifying RhD-positive fetuses using cell-free DNA isolates in maternal plasma. Over the 15-month study period, fetal RhD testing was conducted during Week 27 of gestation, and results were compared with those obtained using neonatal cord blood at birth. If the fetal RhD test was positive, providers administered 200 mcg anti-D immunoglobulin during the 30th week of gestation and within 48 hours of birth. If fetal RhD was negative, providers were told immunoglobulin was unnecessary.
More than 32,000 RhD-negative women were screened. The cell-free DNA test showed fetal RhD-positive results 62% of the time and RhD-negative results in the remainder. Cord blood samples were available for 25,789 pregnancies (80%).
Sensitivity, specificity. The sensitivity for identifying fetal RhD was 99% and the specificity was 98%. Both negative and positive predictive values were 99%. Overall, there were 225 false-positive results and 9 false-negative results. In the 9 false negatives, 6 were due to a lack of fetal DNA in the sample and 3 were due to technical error (defined as an operator ignoring a failure of the robot pipetting the plasma or other technical failures).
The false-negative rate (0.03%) was lower than the predetermined estimated false-negative rate of cord blood serology (0.25%). In 22 of the supposed false positives, follow-up serology or molecular testing found an RhD gene was actually present, meaning the results of the neonatal cord blood serology in these cases were falsely negative. If you recalculate with these data in mind, the false-negative rate for fetal DNA testing was actually less than half that of typical serologic determination.
WHAT’S NEW
An accurate test with the potential to reduce unnecessary Tx
Fetal RhD testing at 27 weeks’ gestation appears to be highly accurate and could reduce the unnecessary use of anti-D immunoglobulin when the fetal RhD is negative.
CAVEATS
Different results with different ethnicities?
Dutch participants are not necessarily reflective of the US population. Known variation in the rate of fetal RhD positivity among RhD-negative pregnant women by race and ethnicity could mean that the number of women able to forego anti-D-immunoglobulin prophylaxis would be different in the United States from that in other countries.
Also, in this study, polymerase chain reaction (PCR) for 2 RhD sequences was run in triplicate, and a computer-based algorithm was used to automatically score samples to provide results. For safe implementation, the cell-free fetal RhD DNA testing process would need to follow similar methods.
CHALLENGES TO IMPLEMENTATION
Test cost and availability are big unknowns
Cost and availability of the test may be barriers, but there is currently too little information on either subject in the United States to make a determination. A 2013 study indicated that the use of cell-free DNA testing to determine fetal RhD status was then approximately $682.10
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. de Haas M, Thurik FF, van der Ploeg CP, et al. Sensitivity of fetal RHD screening for safe guidance of targeted anti-D immunoglobulin prophylaxis: prospective cohort study of a nationwide programme in the Netherlands. BMJ. 2016;355:i5789.
2. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 75: Management of alloimmunization during pregnancy. Obstet Gynecol. 2006;108:457-464.
3. Urbaniak S, Greiss MA. RhD haemolytic disease of the fetus and the newborn. Blood Rev. 2000;14:44-61.
4. Mayne S, Parker JH, Harden TA, et al. Rate of RhD sensitisation before and after implementation of a community based antenatal prophylaxis programme. BMJ. 1997;315:1588-1588.
5. MacKenzie IZ, Bowell P, Gregory H, et al. Routine antenatal Rhesus D immunoglobulin prophylaxis: the results of a prospective 10 year study. Br J Obstet Gynecol: 1999;106:492-497.
6. Zolotor AJ, Carlough MC. Update on prenatal care. Am Fam Physician. 2014;89:199-208.
7. Mackie FL, Hemming K, Allen S, et al. The accuracy of cell-free fetal DNA-based non-invasive prenatal testing in singleton pregnancies: a systematic review and bivariate meta-analysis. BJOG. 2017;124:32-46.
8. Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 181: Prevention of Rh D Alloimmunization. Obstet Gynecol. 2017;130:e57-e70.
9. National Institute for Health and Care Excellence. High-throughput non-invasive prenatal testing for fetal RHD genotype 1: Recommendations. Available at: https://www.nice.org.uk/guidance/dg25/chapter/1-Recommendations. Accessed August 9, 2017.
10. Hawk AF, Chang EY, Shields SM, et al. Costs and clinical outcomes of noninvasive fetal RhD typing for targeted prophylaxis. Obstet Gynecol. 2013;122:579-585.
1. de Haas M, Thurik FF, van der Ploeg CP, et al. Sensitivity of fetal RHD screening for safe guidance of targeted anti-D immunoglobulin prophylaxis: prospective cohort study of a nationwide programme in the Netherlands. BMJ. 2016;355:i5789.
2. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 75: Management of alloimmunization during pregnancy. Obstet Gynecol. 2006;108:457-464.
3. Urbaniak S, Greiss MA. RhD haemolytic disease of the fetus and the newborn. Blood Rev. 2000;14:44-61.
4. Mayne S, Parker JH, Harden TA, et al. Rate of RhD sensitisation before and after implementation of a community based antenatal prophylaxis programme. BMJ. 1997;315:1588-1588.
5. MacKenzie IZ, Bowell P, Gregory H, et al. Routine antenatal Rhesus D immunoglobulin prophylaxis: the results of a prospective 10 year study. Br J Obstet Gynecol: 1999;106:492-497.
6. Zolotor AJ, Carlough MC. Update on prenatal care. Am Fam Physician. 2014;89:199-208.
7. Mackie FL, Hemming K, Allen S, et al. The accuracy of cell-free fetal DNA-based non-invasive prenatal testing in singleton pregnancies: a systematic review and bivariate meta-analysis. BJOG. 2017;124:32-46.
8. Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 181: Prevention of Rh D Alloimmunization. Obstet Gynecol. 2017;130:e57-e70.
9. National Institute for Health and Care Excellence. High-throughput non-invasive prenatal testing for fetal RHD genotype 1: Recommendations. Available at: https://www.nice.org.uk/guidance/dg25/chapter/1-Recommendations. Accessed August 9, 2017.
10. Hawk AF, Chang EY, Shields SM, et al. Costs and clinical outcomes of noninvasive fetal RhD typing for targeted prophylaxis. Obstet Gynecol. 2013;122:579-585.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Employ cell-free DNA testing at 27 weeks’ gestation in your RhD-negative obstetric patients to reduce unnecessary use of anti-D immunoglobulin.1
STRENGTH OF RECOMMENDATION
B: Based on a single, prospective, cohort study.
de Haas M, Thurik FF, van der Ploeg CP, et al. Sensitivity of fetal RHD screening for safe guidance of targeted anti-D immunoglobulin prophylaxis: prospective cohort study of a nationwide programme in the Netherlands. BMJ. 2016;355:i5789.
Critical anemia • light-headedness • bilateral leg swelling • Dx?
THE CASE
A 40-year-old man was referred to the emergency department (ED) with critical anemia after routine blood work at an outside clinic showed a hemoglobin level of 3.5 g/dL. On presentation, he reported symptoms of fatigue, shortness of breath, bilateral leg swelling, dizziness (characterized as light-headedness), and frequent heartburn. He said that the symptoms began 5 weeks earlier, after he was exposed to a relative with hand, foot, and mouth disease.
Additionally, the patient reported an intentional 14-lb weight loss over the 6 months prior to presentation. He denied fever, rash, chest pain, loss of consciousness, headache, abdominal pain, hematemesis, melena, and hematochezia. His medical history was significant for peptic ulcer disease (diagnosed and treated at age 8). He did not recall the specifics, and he denied any related chronic symptoms or complications. His family history (paternal) was significant for colon cancer.
The physical exam revealed conjunctival pallor, skin pallor, jaundice, +1 bilateral lower extremity edema, tachycardia, and tachypnea. Stool Hemoccult was negative. On repeat complete blood count (performed in the ED), hemoglobin was found to be 3.1 g/dL with a mean corpuscular volume of 47 fL.
THE DIAGNOSIS
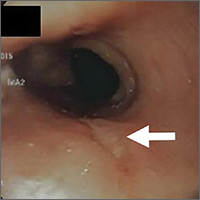
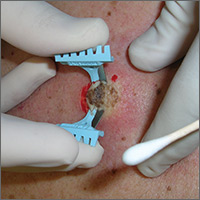
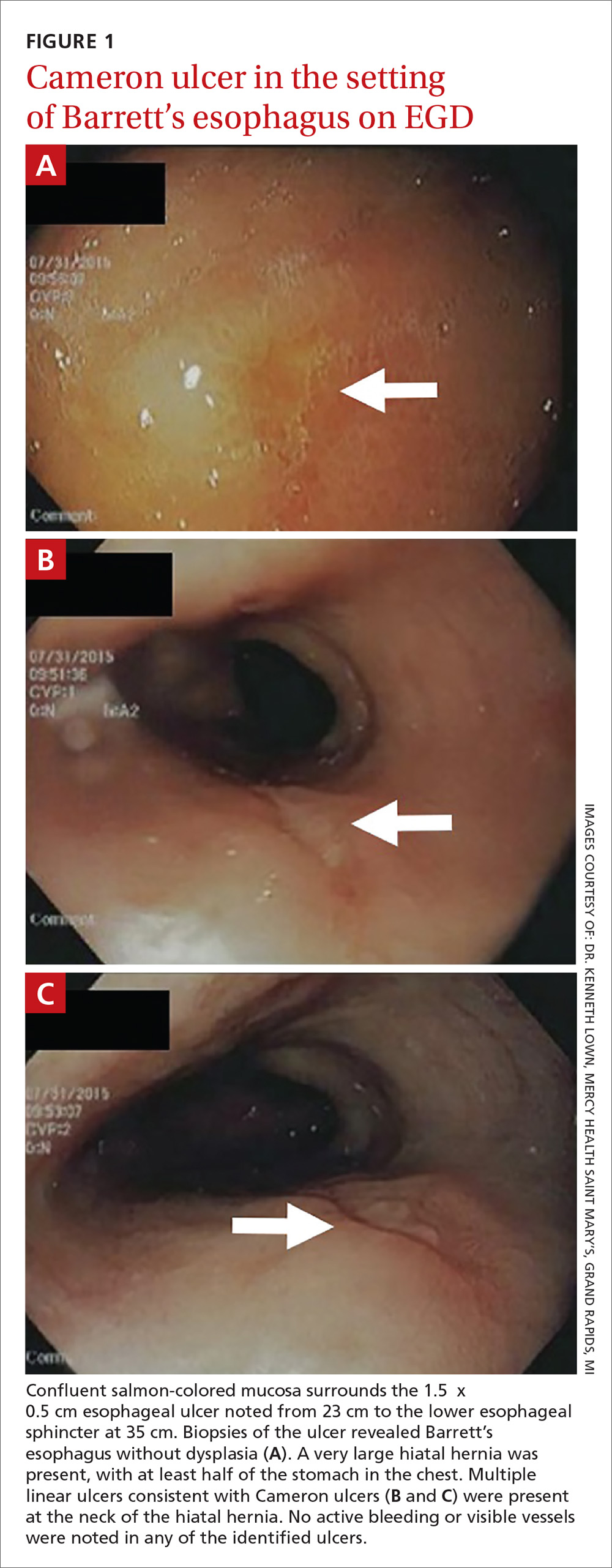
The patient was admitted to the family medicine service and received 4 units of packed red blood cells, which increased his hemoglobin to the target goal of >7 g/dL. A colonoscopy and an esophagogastroduodenoscopy (EGD) were performed (FIGURE 1A-1C), with results suggestive of diverticulosis, probable Barrett’s mucosa, esophageal ulcer, huge hiatal hernia (at least one-half of the stomach was in the chest), and Cameron ulcers. Esophageal biopsies showed cardiac mucosa with chronic inflammation. Esophageal ulcer biopsies revealed Barrett’s esophagus without dysplasia. Duodenal biopsies displayed normal mucosa.
DISCUSSION
Although rare, Cameron ulcers must be considered in the differential diagnosis of patients with chronic anemia of unknown origin. The potential for these lesions to result in chronic blood loss, which could over time manifest as severe anemia or hypovolemic shock, makes proper diagnosis and prompt treatment especially important.4,5
In our patient’s case, his severe anemia was likely the result of a combination of the esophageal and Cameron ulcers evidenced on EGD, rather than any single ulcer. In our review of the literature, we found no reports of any patients with anemia and Cameron ulcers who presented with hemoglobin levels as low as our patient had.
Treat with a PPI and iron supplementation
Multiple EGDs may be needed to properly diagnose Cameron ulcers, as they can be difficult to identify. Once a patient receives the diagnosis, he or she will typically be put on a daily proton pump inhibitor (PPI) regimen, such as omeprazole 20 mg bid. However, since many patients with Cameron ulcers also have acid-related problems (as was true in this case), a multifactorial acid suppression approach may be warranted.1 This may include recommending lifestyle modifications (eg, eating small meals, avoiding foods that provoke symptoms, or losing weight) and prescribing medications in addition to a PPI, such as an H2 blocker (eg, 300 mg qid, before meals and at bedtime).
In addition, iron sulfate (325 mg/d, in this case) and blood transfusions may be required to treat the anemia. In refractory cases, endoscopic or surgical interventions, such as hemoclipping, Nissen fundoplication, or laparoscopic gastropexy, may need to be performed.2
Our patient was given a prescription for ferrous sulfate 325 mg/d and omeprazole 20 mg bid. His symptoms improved with treatment, and he was discharged on Day 5; his hemoglobin remained >7 g/dL.
THE TAKEAWAY
The association between chronic iron deficiency anemia and Cameron ulcers has been established but is commonly overlooked in patients presenting with unexplained anemia or an undiagnosed hiatal hernia. This is likely due to their rarity as a cause of anemia, in general.
Furthermore, the lesions can be missed on EGD; multiple EGDs may be needed to make the diagnosis. Once diagnosed, Cameron ulcers typically respond well to twice daily PPI treatment. Patients with refractory, recurrent, or severe lesions, or large, symptomatic hiatal hernias should be referred for surgical assessment.
CORRESPONDENCE
Megan Yee, 801 Broadward Avenue NW, Grand Rapids, MI 49504; [email protected].
1. Maganty K, Smith RL. Cameron lesions: unusual cause of gastrointestinal bleeding and anemia. Digestion. 2008;77:214-217.
2. Camus M, Jensen DM, Ohning GV, et al. Severe upper gastrointestinal hemorrhage from linear gastric ulcers in large hiatal hernias: a large prospective case series of Cameron ulcers. Endoscopy. 2013;45:397-400.
3. Kimer N, Schmidt PN, Krag, A. Cameron lesions: an often overlooked cause of iron deficiency anaemia in patients with large hiatal hernias. BMJ Case Rep. 2010;2010.
4. Kapadia S, Jagroop S, Kumar, A. Cameron ulcers: an atypical source for a massive upper gastrointestinal bleed. World J Gastroenterol. 2012;18:4959-4961.
5. Gupta P, Suryadevara M, Das A, et al. Cameron ulcer causing severe anemia in a patient with diaphragmatic hernia. Am J Case Rep. 2015;16:733-736.
THE CASE
A 40-year-old man was referred to the emergency department (ED) with critical anemia after routine blood work at an outside clinic showed a hemoglobin level of 3.5 g/dL. On presentation, he reported symptoms of fatigue, shortness of breath, bilateral leg swelling, dizziness (characterized as light-headedness), and frequent heartburn. He said that the symptoms began 5 weeks earlier, after he was exposed to a relative with hand, foot, and mouth disease.
Additionally, the patient reported an intentional 14-lb weight loss over the 6 months prior to presentation. He denied fever, rash, chest pain, loss of consciousness, headache, abdominal pain, hematemesis, melena, and hematochezia. His medical history was significant for peptic ulcer disease (diagnosed and treated at age 8). He did not recall the specifics, and he denied any related chronic symptoms or complications. His family history (paternal) was significant for colon cancer.
The physical exam revealed conjunctival pallor, skin pallor, jaundice, +1 bilateral lower extremity edema, tachycardia, and tachypnea. Stool Hemoccult was negative. On repeat complete blood count (performed in the ED), hemoglobin was found to be 3.1 g/dL with a mean corpuscular volume of 47 fL.
THE DIAGNOSIS
The patient was admitted to the family medicine service and received 4 units of packed red blood cells, which increased his hemoglobin to the target goal of >7 g/dL. A colonoscopy and an esophagogastroduodenoscopy (EGD) were performed (FIGURE 1A-1C), with results suggestive of diverticulosis, probable Barrett’s mucosa, esophageal ulcer, huge hiatal hernia (at least one-half of the stomach was in the chest), and Cameron ulcers. Esophageal biopsies showed cardiac mucosa with chronic inflammation. Esophageal ulcer biopsies revealed Barrett’s esophagus without dysplasia. Duodenal biopsies displayed normal mucosa.

DISCUSSION
Although rare, Cameron ulcers must be considered in the differential diagnosis of patients with chronic anemia of unknown origin. The potential for these lesions to result in chronic blood loss, which could over time manifest as severe anemia or hypovolemic shock, makes proper diagnosis and prompt treatment especially important.4,5
In our patient’s case, his severe anemia was likely the result of a combination of the esophageal and Cameron ulcers evidenced on EGD, rather than any single ulcer. In our review of the literature, we found no reports of any patients with anemia and Cameron ulcers who presented with hemoglobin levels as low as our patient had.
Treat with a PPI and iron supplementation
Multiple EGDs may be needed to properly diagnose Cameron ulcers, as they can be difficult to identify. Once a patient receives the diagnosis, he or she will typically be put on a daily proton pump inhibitor (PPI) regimen, such as omeprazole 20 mg bid. However, since many patients with Cameron ulcers also have acid-related problems (as was true in this case), a multifactorial acid suppression approach may be warranted.1 This may include recommending lifestyle modifications (eg, eating small meals, avoiding foods that provoke symptoms, or losing weight) and prescribing medications in addition to a PPI, such as an H2 blocker (eg, 300 mg qid, before meals and at bedtime).
In addition, iron sulfate (325 mg/d, in this case) and blood transfusions may be required to treat the anemia. In refractory cases, endoscopic or surgical interventions, such as hemoclipping, Nissen fundoplication, or laparoscopic gastropexy, may need to be performed.2
Our patient was given a prescription for ferrous sulfate 325 mg/d and omeprazole 20 mg bid. His symptoms improved with treatment, and he was discharged on Day 5; his hemoglobin remained >7 g/dL.
THE TAKEAWAY
The association between chronic iron deficiency anemia and Cameron ulcers has been established but is commonly overlooked in patients presenting with unexplained anemia or an undiagnosed hiatal hernia. This is likely due to their rarity as a cause of anemia, in general.
Furthermore, the lesions can be missed on EGD; multiple EGDs may be needed to make the diagnosis. Once diagnosed, Cameron ulcers typically respond well to twice daily PPI treatment. Patients with refractory, recurrent, or severe lesions, or large, symptomatic hiatal hernias should be referred for surgical assessment.
CORRESPONDENCE
Megan Yee, 801 Broadward Avenue NW, Grand Rapids, MI 49504; [email protected].
THE CASE
A 40-year-old man was referred to the emergency department (ED) with critical anemia after routine blood work at an outside clinic showed a hemoglobin level of 3.5 g/dL. On presentation, he reported symptoms of fatigue, shortness of breath, bilateral leg swelling, dizziness (characterized as light-headedness), and frequent heartburn. He said that the symptoms began 5 weeks earlier, after he was exposed to a relative with hand, foot, and mouth disease.
Additionally, the patient reported an intentional 14-lb weight loss over the 6 months prior to presentation. He denied fever, rash, chest pain, loss of consciousness, headache, abdominal pain, hematemesis, melena, and hematochezia. His medical history was significant for peptic ulcer disease (diagnosed and treated at age 8). He did not recall the specifics, and he denied any related chronic symptoms or complications. His family history (paternal) was significant for colon cancer.
The physical exam revealed conjunctival pallor, skin pallor, jaundice, +1 bilateral lower extremity edema, tachycardia, and tachypnea. Stool Hemoccult was negative. On repeat complete blood count (performed in the ED), hemoglobin was found to be 3.1 g/dL with a mean corpuscular volume of 47 fL.
THE DIAGNOSIS
The patient was admitted to the family medicine service and received 4 units of packed red blood cells, which increased his hemoglobin to the target goal of >7 g/dL. A colonoscopy and an esophagogastroduodenoscopy (EGD) were performed (FIGURE 1A-1C), with results suggestive of diverticulosis, probable Barrett’s mucosa, esophageal ulcer, huge hiatal hernia (at least one-half of the stomach was in the chest), and Cameron ulcers. Esophageal biopsies showed cardiac mucosa with chronic inflammation. Esophageal ulcer biopsies revealed Barrett’s esophagus without dysplasia. Duodenal biopsies displayed normal mucosa.

DISCUSSION
Although rare, Cameron ulcers must be considered in the differential diagnosis of patients with chronic anemia of unknown origin. The potential for these lesions to result in chronic blood loss, which could over time manifest as severe anemia or hypovolemic shock, makes proper diagnosis and prompt treatment especially important.4,5
In our patient’s case, his severe anemia was likely the result of a combination of the esophageal and Cameron ulcers evidenced on EGD, rather than any single ulcer. In our review of the literature, we found no reports of any patients with anemia and Cameron ulcers who presented with hemoglobin levels as low as our patient had.
Treat with a PPI and iron supplementation
Multiple EGDs may be needed to properly diagnose Cameron ulcers, as they can be difficult to identify. Once a patient receives the diagnosis, he or she will typically be put on a daily proton pump inhibitor (PPI) regimen, such as omeprazole 20 mg bid. However, since many patients with Cameron ulcers also have acid-related problems (as was true in this case), a multifactorial acid suppression approach may be warranted.1 This may include recommending lifestyle modifications (eg, eating small meals, avoiding foods that provoke symptoms, or losing weight) and prescribing medications in addition to a PPI, such as an H2 blocker (eg, 300 mg qid, before meals and at bedtime).
In addition, iron sulfate (325 mg/d, in this case) and blood transfusions may be required to treat the anemia. In refractory cases, endoscopic or surgical interventions, such as hemoclipping, Nissen fundoplication, or laparoscopic gastropexy, may need to be performed.2
Our patient was given a prescription for ferrous sulfate 325 mg/d and omeprazole 20 mg bid. His symptoms improved with treatment, and he was discharged on Day 5; his hemoglobin remained >7 g/dL.
THE TAKEAWAY
The association between chronic iron deficiency anemia and Cameron ulcers has been established but is commonly overlooked in patients presenting with unexplained anemia or an undiagnosed hiatal hernia. This is likely due to their rarity as a cause of anemia, in general.
Furthermore, the lesions can be missed on EGD; multiple EGDs may be needed to make the diagnosis. Once diagnosed, Cameron ulcers typically respond well to twice daily PPI treatment. Patients with refractory, recurrent, or severe lesions, or large, symptomatic hiatal hernias should be referred for surgical assessment.
CORRESPONDENCE
Megan Yee, 801 Broadward Avenue NW, Grand Rapids, MI 49504; [email protected].
1. Maganty K, Smith RL. Cameron lesions: unusual cause of gastrointestinal bleeding and anemia. Digestion. 2008;77:214-217.
2. Camus M, Jensen DM, Ohning GV, et al. Severe upper gastrointestinal hemorrhage from linear gastric ulcers in large hiatal hernias: a large prospective case series of Cameron ulcers. Endoscopy. 2013;45:397-400.
3. Kimer N, Schmidt PN, Krag, A. Cameron lesions: an often overlooked cause of iron deficiency anaemia in patients with large hiatal hernias. BMJ Case Rep. 2010;2010.
4. Kapadia S, Jagroop S, Kumar, A. Cameron ulcers: an atypical source for a massive upper gastrointestinal bleed. World J Gastroenterol. 2012;18:4959-4961.
5. Gupta P, Suryadevara M, Das A, et al. Cameron ulcer causing severe anemia in a patient with diaphragmatic hernia. Am J Case Rep. 2015;16:733-736.
1. Maganty K, Smith RL. Cameron lesions: unusual cause of gastrointestinal bleeding and anemia. Digestion. 2008;77:214-217.
2. Camus M, Jensen DM, Ohning GV, et al. Severe upper gastrointestinal hemorrhage from linear gastric ulcers in large hiatal hernias: a large prospective case series of Cameron ulcers. Endoscopy. 2013;45:397-400.
3. Kimer N, Schmidt PN, Krag, A. Cameron lesions: an often overlooked cause of iron deficiency anaemia in patients with large hiatal hernias. BMJ Case Rep. 2010;2010.
4. Kapadia S, Jagroop S, Kumar, A. Cameron ulcers: an atypical source for a massive upper gastrointestinal bleed. World J Gastroenterol. 2012;18:4959-4961.
5. Gupta P, Suryadevara M, Das A, et al. Cameron ulcer causing severe anemia in a patient with diaphragmatic hernia. Am J Case Rep. 2015;16:733-736.
When the correct Dx is elusive
In this issue of JFP, Dr. Mendoza reminds us that “Parkinson’s disease can be a tough diagnosis to navigate.”1 Classically, Parkinson’s disease (PD) is associated with a resting tremor, but bradykinesia is actually the hallmark of the disease. PD can also present with subtle movement disorders, as well as depression and early dementia. It is, indeed, a difficult clinical diagnosis, and consultation with an expert to confirm or deny its presence can be quite helpful.
Other conundrums. PD, however, is not the only illness whose signs and symptoms can present a challenge. Chronic and intermittent shortness of breath, for example, can be very difficult to sort out. Is the shortness of breath due to congestive heart failure, chronic obstructive pulmonary disease, asthma, or a neurologic condition such as myasthenia gravis? Or is it the result of several causes?
When asthma isn’t asthma. Because it is a common illness, physicians often diagnose asthma in patients with shortness of breath or wheezing. But a recent study suggests that as many as 30% of primary care patients with a current diagnosis of asthma do not have asthma at all.2
In the study, Canadian researchers recruited 701 adults with physician-diagnosed asthma, all of whom were taking asthma medications regularly. The researchers did baseline pulmonary function testing (including methacholine challenge testing, if needed) and monitored symptoms frequently. Then they gradually withdrew asthma medications from those who did not appear to have a definitive diagnosis of asthma. They followed these patients for one year. One-third (203 of 613) of the patients with complete follow-up data were no longer taking asthma medications one year later and had no symptoms of asthma. Twelve patients had serious alternative diagnoses such as coronary artery disease and bronchiectasis.
Closer to home. In my practice, I found 2 patients with long-standing diagnoses of asthma who didn’t, in fact, have the condition at all. In both cases, my suspicion was raised by lung examination. In one case, fine bibasilar rales suggested pulmonary fibrosis, which was the correct diagnosis, and the patient is now on the lung transplant list. In the other case, a loud venous hum suggested an arteriovenous malformation. Surgery corrected the patient’s “asthma.”
I urge you to reevaluate your asthma patients to be sure they have the correct diagnosis and to keep PD in your differential for patients who present with atypical symptoms. Primary care clinicians must be expert diagnosticians, willing to question prior diagnoses.
1. Young J, Mendoza M. Parkinson’s disease: a treatment guide. J Fam Pract. 2018;67:276-286.
2. Aaron SD, Vandemheen KL, FitzGerald JM, et al for the Canadian Respiratory Research Network. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017:317:269-279.
In this issue of JFP, Dr. Mendoza reminds us that “Parkinson’s disease can be a tough diagnosis to navigate.”1 Classically, Parkinson’s disease (PD) is associated with a resting tremor, but bradykinesia is actually the hallmark of the disease. PD can also present with subtle movement disorders, as well as depression and early dementia. It is, indeed, a difficult clinical diagnosis, and consultation with an expert to confirm or deny its presence can be quite helpful.
Other conundrums. PD, however, is not the only illness whose signs and symptoms can present a challenge. Chronic and intermittent shortness of breath, for example, can be very difficult to sort out. Is the shortness of breath due to congestive heart failure, chronic obstructive pulmonary disease, asthma, or a neurologic condition such as myasthenia gravis? Or is it the result of several causes?
When asthma isn’t asthma. Because it is a common illness, physicians often diagnose asthma in patients with shortness of breath or wheezing. But a recent study suggests that as many as 30% of primary care patients with a current diagnosis of asthma do not have asthma at all.2
In the study, Canadian researchers recruited 701 adults with physician-diagnosed asthma, all of whom were taking asthma medications regularly. The researchers did baseline pulmonary function testing (including methacholine challenge testing, if needed) and monitored symptoms frequently. Then they gradually withdrew asthma medications from those who did not appear to have a definitive diagnosis of asthma. They followed these patients for one year. One-third (203 of 613) of the patients with complete follow-up data were no longer taking asthma medications one year later and had no symptoms of asthma. Twelve patients had serious alternative diagnoses such as coronary artery disease and bronchiectasis.
Closer to home. In my practice, I found 2 patients with long-standing diagnoses of asthma who didn’t, in fact, have the condition at all. In both cases, my suspicion was raised by lung examination. In one case, fine bibasilar rales suggested pulmonary fibrosis, which was the correct diagnosis, and the patient is now on the lung transplant list. In the other case, a loud venous hum suggested an arteriovenous malformation. Surgery corrected the patient’s “asthma.”
I urge you to reevaluate your asthma patients to be sure they have the correct diagnosis and to keep PD in your differential for patients who present with atypical symptoms. Primary care clinicians must be expert diagnosticians, willing to question prior diagnoses.
In this issue of JFP, Dr. Mendoza reminds us that “Parkinson’s disease can be a tough diagnosis to navigate.”1 Classically, Parkinson’s disease (PD) is associated with a resting tremor, but bradykinesia is actually the hallmark of the disease. PD can also present with subtle movement disorders, as well as depression and early dementia. It is, indeed, a difficult clinical diagnosis, and consultation with an expert to confirm or deny its presence can be quite helpful.
Other conundrums. PD, however, is not the only illness whose signs and symptoms can present a challenge. Chronic and intermittent shortness of breath, for example, can be very difficult to sort out. Is the shortness of breath due to congestive heart failure, chronic obstructive pulmonary disease, asthma, or a neurologic condition such as myasthenia gravis? Or is it the result of several causes?
When asthma isn’t asthma. Because it is a common illness, physicians often diagnose asthma in patients with shortness of breath or wheezing. But a recent study suggests that as many as 30% of primary care patients with a current diagnosis of asthma do not have asthma at all.2
In the study, Canadian researchers recruited 701 adults with physician-diagnosed asthma, all of whom were taking asthma medications regularly. The researchers did baseline pulmonary function testing (including methacholine challenge testing, if needed) and monitored symptoms frequently. Then they gradually withdrew asthma medications from those who did not appear to have a definitive diagnosis of asthma. They followed these patients for one year. One-third (203 of 613) of the patients with complete follow-up data were no longer taking asthma medications one year later and had no symptoms of asthma. Twelve patients had serious alternative diagnoses such as coronary artery disease and bronchiectasis.
Closer to home. In my practice, I found 2 patients with long-standing diagnoses of asthma who didn’t, in fact, have the condition at all. In both cases, my suspicion was raised by lung examination. In one case, fine bibasilar rales suggested pulmonary fibrosis, which was the correct diagnosis, and the patient is now on the lung transplant list. In the other case, a loud venous hum suggested an arteriovenous malformation. Surgery corrected the patient’s “asthma.”
I urge you to reevaluate your asthma patients to be sure they have the correct diagnosis and to keep PD in your differential for patients who present with atypical symptoms. Primary care clinicians must be expert diagnosticians, willing to question prior diagnoses.
1. Young J, Mendoza M. Parkinson’s disease: a treatment guide. J Fam Pract. 2018;67:276-286.
2. Aaron SD, Vandemheen KL, FitzGerald JM, et al for the Canadian Respiratory Research Network. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017:317:269-279.
1. Young J, Mendoza M. Parkinson’s disease: a treatment guide. J Fam Pract. 2018;67:276-286.
2. Aaron SD, Vandemheen KL, FitzGerald JM, et al for the Canadian Respiratory Research Network. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017:317:269-279.
Does niacin decrease cardiovascular morbidity and mortality in CVD patients?
EVIDENCE SUMMARY
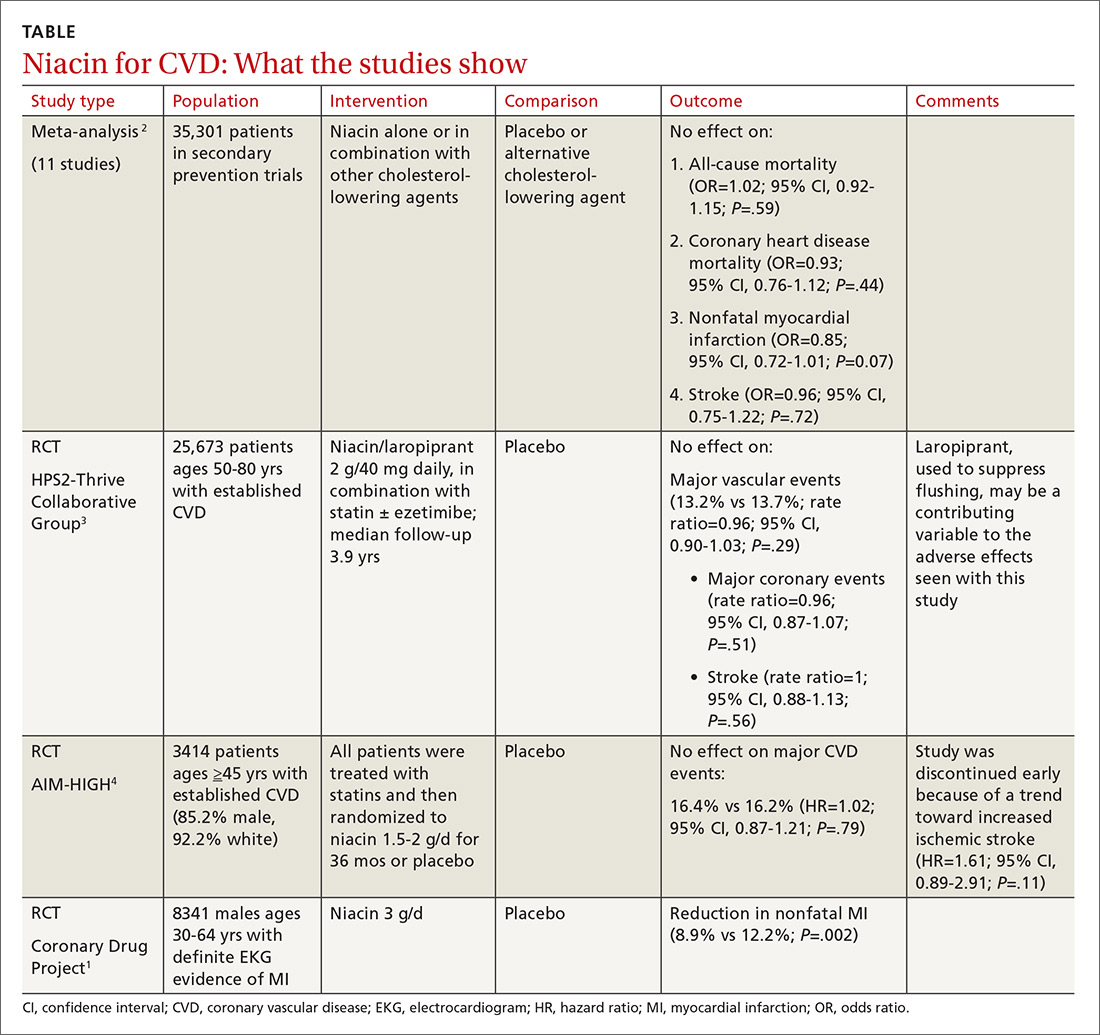
Before the statin era, the Coronary Drug Project RCT (8341 patients) showed that niacin monotherapy in patients with definite electrocardiographic evidence of previous myocardial infarction (MI) reduced nonfatal MI to 8.9% compared with 12.2% for placebo (P=.002).1 (See TABLE.1-4) It also decreased long-term mortality by 11% compared with placebo (P=.0004).5
Adverse effects such as flushing, hyperglycemia, gastrointestinal disturbance, and elevated liver enzymes interfered with adherence to niacin treatment (66.3% of patients were adherent to treatment with niacin vs 77.8% for placebo). The study was limited by the fact that flushing essentially unblinded participants and physicians.
But adding niacin to a statin has no effect
A 2014 meta-analysis driven by the power of the large HPS2-Thrive study evaluated data from 35,301 patients primarily in secondary prevention trials.2,3 It found that adding niacin to statins had no effect on all-cause mortality, coronary heart disease mortality, nonfatal MI, or stroke. The subset of 6 trials (N=4991) assessing niacin monotherapy did show a reduction in cardiovascular events (odds ratio [OR]=0.62; confidence interval [CI], 0.54-0.82), whereas the 5 studies (30,310 patients) involving niacin with a statin demonstrated no effect (OR=0.94; CI, 0.83-1.06).
No benefit from niacin/statin therapy despite an improved lipid profile
A 2011 RCT included 3414 patients with coronary heart disease on simvastatin who were randomized to niacin or placebo.4 All patients received simvastatin 40 to 80 mg ± ezetimibe 10 mg/d to achieve low-density lipoprotein (LDL) cholesterol levels of 40 to 80 mg/dL.
At 3 years, no benefit was seen in the composite CVD primary endpoint (hazard ratio=1.02; 95% CI, 0.87-1.21; P=.79) even though the niacin group had significantly increased median high-density lipoprotein (HDL) cholesterol compared with placebo and lower triglycerides and LDL cholesterol compared with baseline.
A nonsignificant trend toward increased stroke in the niacin group compared with placebo led to early termination of the study. However, multivariate analysis showed independent associations between ischemic stroke risk and age older than 65 years, history of stroke/transient ischemic attack/carotid artery disease, and elevated baseline cholesterol.6
Niacin combined with a statin increases the risk of adverse events
The largest RCT in the 2014 meta-analysis (HPS2-Thrive) evaluated 25,673 patients with established CVD receiving cholesterol-lowering therapy with simvastatin ± ezetimibe who were randomized to niacin or placebo for a median follow-up period of 3.9 years.3 A pre-randomization run-in phase established effective cholesterol-lowering therapy with simvastatin ± ezetimibe.
Niacin didn’t reduce the incidence of major vascular events even though, once again, it decreased LDL and increased HDL more than placebo. Niacin increased the risk of serious adverse events 56% vs 53% (risk ratio [RR]=6; 95% CI, 3-8; number needed to harm [NNH]=35; 95% CI, 25-60), such as new onset diabetes (5.7% vs 4.3%; P<.001; NNH=71) and gastrointestinal bleeding/ulceration and other gastrointestinal disorders (4.8% vs 3.8%; P<.001; NNH=100).
A subsequent 2014 study examined the adverse events recorded in the AIM-HIGH4 study and found that niacin caused more gastrointestinal disorders (7.4% vs 5.5%; P=.02; NNH=53) and infections and infestations (8.1% vs 5.8%; P=.008; NNH=43) than placebo.7 The overall observed rate of serious hemorrhagic adverse events was low, however, showing no significant difference between the 2 groups (3.4% vs 2.9%; P=.36).
RECOMMENDATIONS
As of November 2013, the Institute for Clinical Systems Improvement recommends against using niacin in combination with statins because of the increased risk of adverse events without a reduction in CVD outcomes. Niacin may be considered as monotherapy in patients who can’t tolerate statins or fibrates based on results of the Coronary Drug Project and other studies completed before the era of widespread statin use.8
Similarly, American College of Cardiology/American Heart Association guidelines state that patients who are completely statin intolerant may use nonstatin cholesterol-lowering drugs, including niacin, that have been shown to reduce CVD events in RCTs if the CVD risk-reduction benefits outweigh the potential for adverse effects.9
1. Coronary Drug Project Research Group. Colofibrate and niacin in coronary heart disease. JAMA. 1975;231:360-81.
2. Keene D, Price C, Shun-Shin MJ, et al. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients. BMJ. 2014;349:g4379.
3. HPS2-Thrive Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203-212.
4. AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
5. Canner PL, Berge KG, Wender NK, et al. Fifteen-year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8:1245-1255.
6. AIM-HIGH Investigators. Extended-release niacin therapy and risk of ischemic stroke in patients with cardiovascular disease: the Atherothrombosis Intervention in Metabolic Syndrome with low HDL/High Triglycerides: Impact on Global Health Outcome (AIM-HIGH) trial. Stroke. 2013;44:2688-2693.
7. AIM-HIGH Investigators. Safety profile of extended-release niacin in the AIM-HIGH trial. N Engl J Med. 2014;371:288-290.
8. Institute for Clinical Systems Improvement. Guideline summary: Lipid management in adults. National Guideline Clearinghouse. Rockville, MD: Agency for Healthcare Research and Quality. Available at: http://www.guideline.gov. Accessed July 20, 2015.
9. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;25 (suppl 2):S1-S45.
EVIDENCE SUMMARY
Before the statin era, the Coronary Drug Project RCT (8341 patients) showed that niacin monotherapy in patients with definite electrocardiographic evidence of previous myocardial infarction (MI) reduced nonfatal MI to 8.9% compared with 12.2% for placebo (P=.002).1 (See TABLE.1-4) It also decreased long-term mortality by 11% compared with placebo (P=.0004).5

Adverse effects such as flushing, hyperglycemia, gastrointestinal disturbance, and elevated liver enzymes interfered with adherence to niacin treatment (66.3% of patients were adherent to treatment with niacin vs 77.8% for placebo). The study was limited by the fact that flushing essentially unblinded participants and physicians.
But adding niacin to a statin has no effect
A 2014 meta-analysis driven by the power of the large HPS2-Thrive study evaluated data from 35,301 patients primarily in secondary prevention trials.2,3 It found that adding niacin to statins had no effect on all-cause mortality, coronary heart disease mortality, nonfatal MI, or stroke. The subset of 6 trials (N=4991) assessing niacin monotherapy did show a reduction in cardiovascular events (odds ratio [OR]=0.62; confidence interval [CI], 0.54-0.82), whereas the 5 studies (30,310 patients) involving niacin with a statin demonstrated no effect (OR=0.94; CI, 0.83-1.06).
No benefit from niacin/statin therapy despite an improved lipid profile
A 2011 RCT included 3414 patients with coronary heart disease on simvastatin who were randomized to niacin or placebo.4 All patients received simvastatin 40 to 80 mg ± ezetimibe 10 mg/d to achieve low-density lipoprotein (LDL) cholesterol levels of 40 to 80 mg/dL.
At 3 years, no benefit was seen in the composite CVD primary endpoint (hazard ratio=1.02; 95% CI, 0.87-1.21; P=.79) even though the niacin group had significantly increased median high-density lipoprotein (HDL) cholesterol compared with placebo and lower triglycerides and LDL cholesterol compared with baseline.
A nonsignificant trend toward increased stroke in the niacin group compared with placebo led to early termination of the study. However, multivariate analysis showed independent associations between ischemic stroke risk and age older than 65 years, history of stroke/transient ischemic attack/carotid artery disease, and elevated baseline cholesterol.6
Niacin combined with a statin increases the risk of adverse events
The largest RCT in the 2014 meta-analysis (HPS2-Thrive) evaluated 25,673 patients with established CVD receiving cholesterol-lowering therapy with simvastatin ± ezetimibe who were randomized to niacin or placebo for a median follow-up period of 3.9 years.3 A pre-randomization run-in phase established effective cholesterol-lowering therapy with simvastatin ± ezetimibe.
Niacin didn’t reduce the incidence of major vascular events even though, once again, it decreased LDL and increased HDL more than placebo. Niacin increased the risk of serious adverse events 56% vs 53% (risk ratio [RR]=6; 95% CI, 3-8; number needed to harm [NNH]=35; 95% CI, 25-60), such as new onset diabetes (5.7% vs 4.3%; P<.001; NNH=71) and gastrointestinal bleeding/ulceration and other gastrointestinal disorders (4.8% vs 3.8%; P<.001; NNH=100).
A subsequent 2014 study examined the adverse events recorded in the AIM-HIGH4 study and found that niacin caused more gastrointestinal disorders (7.4% vs 5.5%; P=.02; NNH=53) and infections and infestations (8.1% vs 5.8%; P=.008; NNH=43) than placebo.7 The overall observed rate of serious hemorrhagic adverse events was low, however, showing no significant difference between the 2 groups (3.4% vs 2.9%; P=.36).
RECOMMENDATIONS
As of November 2013, the Institute for Clinical Systems Improvement recommends against using niacin in combination with statins because of the increased risk of adverse events without a reduction in CVD outcomes. Niacin may be considered as monotherapy in patients who can’t tolerate statins or fibrates based on results of the Coronary Drug Project and other studies completed before the era of widespread statin use.8
Similarly, American College of Cardiology/American Heart Association guidelines state that patients who are completely statin intolerant may use nonstatin cholesterol-lowering drugs, including niacin, that have been shown to reduce CVD events in RCTs if the CVD risk-reduction benefits outweigh the potential for adverse effects.9
EVIDENCE SUMMARY
Before the statin era, the Coronary Drug Project RCT (8341 patients) showed that niacin monotherapy in patients with definite electrocardiographic evidence of previous myocardial infarction (MI) reduced nonfatal MI to 8.9% compared with 12.2% for placebo (P=.002).1 (See TABLE.1-4) It also decreased long-term mortality by 11% compared with placebo (P=.0004).5

Adverse effects such as flushing, hyperglycemia, gastrointestinal disturbance, and elevated liver enzymes interfered with adherence to niacin treatment (66.3% of patients were adherent to treatment with niacin vs 77.8% for placebo). The study was limited by the fact that flushing essentially unblinded participants and physicians.
But adding niacin to a statin has no effect
A 2014 meta-analysis driven by the power of the large HPS2-Thrive study evaluated data from 35,301 patients primarily in secondary prevention trials.2,3 It found that adding niacin to statins had no effect on all-cause mortality, coronary heart disease mortality, nonfatal MI, or stroke. The subset of 6 trials (N=4991) assessing niacin monotherapy did show a reduction in cardiovascular events (odds ratio [OR]=0.62; confidence interval [CI], 0.54-0.82), whereas the 5 studies (30,310 patients) involving niacin with a statin demonstrated no effect (OR=0.94; CI, 0.83-1.06).
No benefit from niacin/statin therapy despite an improved lipid profile
A 2011 RCT included 3414 patients with coronary heart disease on simvastatin who were randomized to niacin or placebo.4 All patients received simvastatin 40 to 80 mg ± ezetimibe 10 mg/d to achieve low-density lipoprotein (LDL) cholesterol levels of 40 to 80 mg/dL.
At 3 years, no benefit was seen in the composite CVD primary endpoint (hazard ratio=1.02; 95% CI, 0.87-1.21; P=.79) even though the niacin group had significantly increased median high-density lipoprotein (HDL) cholesterol compared with placebo and lower triglycerides and LDL cholesterol compared with baseline.
A nonsignificant trend toward increased stroke in the niacin group compared with placebo led to early termination of the study. However, multivariate analysis showed independent associations between ischemic stroke risk and age older than 65 years, history of stroke/transient ischemic attack/carotid artery disease, and elevated baseline cholesterol.6
Niacin combined with a statin increases the risk of adverse events
The largest RCT in the 2014 meta-analysis (HPS2-Thrive) evaluated 25,673 patients with established CVD receiving cholesterol-lowering therapy with simvastatin ± ezetimibe who were randomized to niacin or placebo for a median follow-up period of 3.9 years.3 A pre-randomization run-in phase established effective cholesterol-lowering therapy with simvastatin ± ezetimibe.
Niacin didn’t reduce the incidence of major vascular events even though, once again, it decreased LDL and increased HDL more than placebo. Niacin increased the risk of serious adverse events 56% vs 53% (risk ratio [RR]=6; 95% CI, 3-8; number needed to harm [NNH]=35; 95% CI, 25-60), such as new onset diabetes (5.7% vs 4.3%; P<.001; NNH=71) and gastrointestinal bleeding/ulceration and other gastrointestinal disorders (4.8% vs 3.8%; P<.001; NNH=100).
A subsequent 2014 study examined the adverse events recorded in the AIM-HIGH4 study and found that niacin caused more gastrointestinal disorders (7.4% vs 5.5%; P=.02; NNH=53) and infections and infestations (8.1% vs 5.8%; P=.008; NNH=43) than placebo.7 The overall observed rate of serious hemorrhagic adverse events was low, however, showing no significant difference between the 2 groups (3.4% vs 2.9%; P=.36).
RECOMMENDATIONS
As of November 2013, the Institute for Clinical Systems Improvement recommends against using niacin in combination with statins because of the increased risk of adverse events without a reduction in CVD outcomes. Niacin may be considered as monotherapy in patients who can’t tolerate statins or fibrates based on results of the Coronary Drug Project and other studies completed before the era of widespread statin use.8
Similarly, American College of Cardiology/American Heart Association guidelines state that patients who are completely statin intolerant may use nonstatin cholesterol-lowering drugs, including niacin, that have been shown to reduce CVD events in RCTs if the CVD risk-reduction benefits outweigh the potential for adverse effects.9
1. Coronary Drug Project Research Group. Colofibrate and niacin in coronary heart disease. JAMA. 1975;231:360-81.
2. Keene D, Price C, Shun-Shin MJ, et al. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients. BMJ. 2014;349:g4379.
3. HPS2-Thrive Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203-212.
4. AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
5. Canner PL, Berge KG, Wender NK, et al. Fifteen-year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8:1245-1255.
6. AIM-HIGH Investigators. Extended-release niacin therapy and risk of ischemic stroke in patients with cardiovascular disease: the Atherothrombosis Intervention in Metabolic Syndrome with low HDL/High Triglycerides: Impact on Global Health Outcome (AIM-HIGH) trial. Stroke. 2013;44:2688-2693.
7. AIM-HIGH Investigators. Safety profile of extended-release niacin in the AIM-HIGH trial. N Engl J Med. 2014;371:288-290.
8. Institute for Clinical Systems Improvement. Guideline summary: Lipid management in adults. National Guideline Clearinghouse. Rockville, MD: Agency for Healthcare Research and Quality. Available at: http://www.guideline.gov. Accessed July 20, 2015.
9. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;25 (suppl 2):S1-S45.
1. Coronary Drug Project Research Group. Colofibrate and niacin in coronary heart disease. JAMA. 1975;231:360-81.
2. Keene D, Price C, Shun-Shin MJ, et al. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients. BMJ. 2014;349:g4379.
3. HPS2-Thrive Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203-212.
4. AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255-2267.
5. Canner PL, Berge KG, Wender NK, et al. Fifteen-year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8:1245-1255.
6. AIM-HIGH Investigators. Extended-release niacin therapy and risk of ischemic stroke in patients with cardiovascular disease: the Atherothrombosis Intervention in Metabolic Syndrome with low HDL/High Triglycerides: Impact on Global Health Outcome (AIM-HIGH) trial. Stroke. 2013;44:2688-2693.
7. AIM-HIGH Investigators. Safety profile of extended-release niacin in the AIM-HIGH trial. N Engl J Med. 2014;371:288-290.
8. Institute for Clinical Systems Improvement. Guideline summary: Lipid management in adults. National Guideline Clearinghouse. Rockville, MD: Agency for Healthcare Research and Quality. Available at: http://www.guideline.gov. Accessed July 20, 2015.
9. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;25 (suppl 2):S1-S45.
Evidence-based answers from the Family Physicians Inquiries Network
EVIDENCE BASED ANSWER:
No. Niacin doesn’t reduce cardiovascular disease (CVD) morbidity or mortality in patients with established disease (strength of recommendation [SOR]: A, meta-analyses of randomized controlled trials [RCTs] and subsequent large RCTs).
Niacin may be considered as monotherapy for patients intolerant of statins (SOR: B, one well-done RCT).
Hypothermia in adults: A strategy for detection and Tx
CASE
Patrick S, an 85-year-old man with multiple medical problems, was brought to his primary care provider after being found at home with altered mental status. His caretaker reported that Mr. S had been using extra blankets in bed and sleeping more, but he hadn’t had significant outdoor exposure. Measurement of his vital signs revealed tachycardia, tachypnea, hypotension, and a rectal temperature of 32°C (89.6°F).
How would you proceed with the care of this patient?
What is accidental hypothermia?
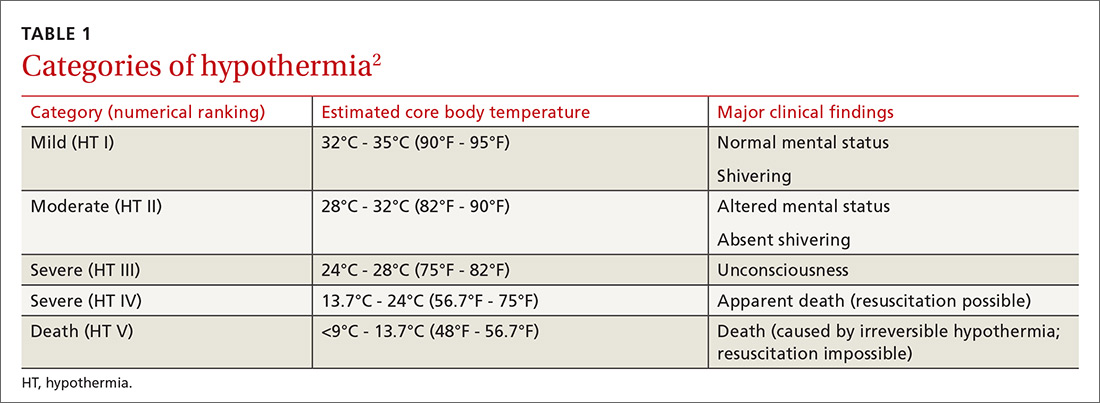
Accidental hypothermia is an unintentional drop in core body temperature to <35°C (<95°F). Mild hypothermia is defined as a core body temperature of 32°C to 35°C (90°F - 95°F); moderate hypothermia, 28°C to 32°C (82°F - 90°F); and severe hypothermia, <28°C (<82°F).1
The International Commission for Mountain Emergency Medicine divides hypothermia into 5 categories, emphasizing the clinical features of each stage as a guide to treatment (TABLE 1).2 These categories were adopted to help prehospital rescuers estimate the severity of hypothermia using physical symptoms. For example, most patients stop shivering at approximately 30°C (86°F)—the “moderate (HT II)” category of hypothermia—although this response varies widely from patient to patient. Notably, there are reports in the literature of survival in hypothermia with a temperature as low as 13.7°C (56.7°F) and with cardiac arrest for as long as 8 hours and 40 minutes, although these events are rare.3
Each year, approximately 700 deaths in the United States are the result of hypothermia.4 Between 1995 to 2004 in the United States, it is estimated that 15,574 visits were made to a health care provider or facility for hypothermia and other cold-related concerns.5 Based on reports in the international literature, the incidence of nonlethal hypothermia is much greater than the incidence of lethal hypothermia.5 Almost half of deaths from hypothermia are in people older than age 65 years; the male to female ratio is 2.5:1.1
Variables that predispose the body to temperature dysregulation include extremes of age, comorbid conditions, intoxication, chronic cold exposure, immersion accident, mental illness, impaired shivering, and lack of acclimatization.1 The most common causes of death associated with hypothermia are falls, drownings, and cardiovascular disease.4 In a 2008 study, hypothermia and other cold-related morbidity emergency department (ED) visits required more transfers of patients to a critical care unit than any other reason for visiting an ED (risk ratio, 6.73; 95% confidence interval, 1.8-25).5 Mortality among inpatients whose hypothermia is classified as moderate or severe reaches as high as 40%.3
More than just cold-weather exposure
Accidental hypothermia occurs when heat loss is superseded by the body’s ability to generate heat. It commonly happens in cold environments but can also occur at higher temperatures if the body’s thermoregulatory system malfunctions.
Environmental or iatrogenic factors (ie, primary hypothermia), such as wind, water immersion, wetness, aggressive fluid resuscitation, and heat stroke treatment can make people more susceptible to hypothermia. Medical conditions (ie, secondary hypothermia), such as burns, exfoliative dermatitis, severe psoriasis, hypoadrenalism, hypopituitarism, hypothyroidism, acute spinal cord transection, head trauma, stroke, tumor, pneumonia, Wernicke’s disease (encephalopathy), and sepsis can also predispose to hypothermia.1 Drugs, such as ethanol, phenothiazines, and sedative–hypnotics may decrease the hypothermia threshold.1 (For information on preventing hypothermia, see TABLE 2.6)

Pathophysiology: The role of the hypothalamus
Humans maintain body temperature by balancing heat production and heat loss to the environment. Heat is lost through the skin and lungs by 5 different mechanisms: radiation, conduction, convection, evaporation, and respiration. Convective heat loss to cold air and conductive heat loss to water are the most common mechanisms of accidental hypothermia.7
To maintain temperature homeostasis at 37°C (98.6°F) (±0.5°C [±0.9°F]), the hypothalamus receives input from central and peripheral thermal receptors and stimulates heat production through shivering, increasing the basal metabolic rate 2-fold to 5-fold.1 The hypothalamus also increases thyroid, catecholamine, and adrenal activity to increase the body’s production of heat and raise core temperature.
Heat conservation occurs by activation of sympathetically mediated vasoconstriction, reducing conduction to the skin, where cooling is greatest. After time, temperature regulation in the body becomes overwhelmed and catecholamine levels return to a pre-hypothermic state.
At 35°C (95°F), neurologic function begins to decline; at 32°C (89.6°F), metabolism, ventilation, and cardiac output decrease until shivering ceases. Changes in peripheral blood flow can create a false warming sensation, causing a person to remove clothing, a phenomenon referred to as paradoxical undressing. As hypothermia progresses, the neurologic, respiratory, and cardiac systems continue to slow until there is eventual cardiorespiratory failure.
Assessment and diagnosis
History and physical examination. A high index of suspicion for the diagnosis of hypothermia is essential, especially when caring for the elderly or patients presenting with unexplained illness. Often, symptoms of a primary condition may overshadow those reflecting hypothermia. In a multicenter survey that reviewed 428 cases of accidental hypothermia in the United States, 44% of patients had an underlying illness; 18%, coexisting infection; 19%, trauma; and 6%, overdose.3
There are no strict diagnostic criteria for hypothermia other than a core body temperature <35°C (<95°F). Standard thermometers often do not read below 34.4°C (93.2°F), so it is recommended that a rectal thermometer capable of reading low body temperatures be used for accurate measurement.
Hypothermic patients can exhibit a variety of symptoms, depending on the degree of decrease in core body temperature1:
- A mildly hypothermic patient might present with any combination of tachypnea, tachycardia, ataxia, impaired judgment, shivering, and vasoconstriction.
- Moderate hypothermia typically manifests as a decreased heart rate, decreased blood pressure, decreased level of consciousness, decreased respiratory effort, dilated pupils, extinction of shivering, and hyporeflexia. Cardiac abnormalities, such as atrial fibrillation and junctional bradycardia, may be seen in moderate hypothermia.
- Severe hypothermia presents with apnea, coma, nonreactive pupils, oliguria, areflexia, hypotension, bradycardia, and continued cardiac abnormalities, such as ventricular arrhythmias and asystole.
Laboratory evaluation. No specific laboratory tests are needed to diagnose hypothermia. General lab tests, however, may help determine whether hypothermia is the result, or the cause, of the clinical scenario. Recommended laboratory tests for making that determination include a complete blood count (CBC), chemistry panel, arterial blood gases, fingerstick glucose, and coagulation panel.
Results of lab tests may be abnormal because of the body’s decreased core body temperature. White blood cells and platelets in the CBC, for example, may be decreased due to splenic sequestration; these findings reverse with rewarming. With every 1°C (1.8°F) drop in core body temperature, hematocrit increases 2%.3 Sodium, chloride, and magnesium concentrations do not display consistent abnormalities with any core body temperature >25°C (77°F),3,8 but potassium levels may fluctuate because of acid-base changes that occur during rewarming.1 Creatinine and creatine kinase levels may be increased secondary to rhabdomyolysis or acute tubular necrosis.1
Arterial blood gases typically show metabolic acidosis or respiratory alkalosis, or both.8 Prothrombin time and partial thromboplastin time are typically elevated in vivo, secondary to temperature-dependent enzymes in the coagulation cascade, but are reported normal in a blood specimen that is heated to 37°C (98.6°F) prior to analysis.1,8
Both hyperglycemia and hypoglycemia can be associated with hypothermia. The lactate level can be elevated, due to hypoperfusion. Hepatic impairment may be seen secondary to decreased cardiac output. An increase in the lipase level may also occur.3
When a hypothermic patient fails to respond to rewarming, or there is no clear source of cold exposure, consider testing for other causes of the problem, including hypothyroidism and adrenal insufficiency (see “Differential diagnosis”). Hypothermia may also decrease thyroid function in people with preexisting disease.
Other laboratory studies that can be considered include fibrinogen, blood-alcohol level, urine toxicology screen, and blood and fluid cultures.3
Imaging. Imaging studies are not performed routinely in the setting of hypothermia; however:
- Chest radiography can be considered to assess for aspiration pneumonia, vascular congestion, and pulmonary edema.
- Computed tomography (CT) of the head is helpful in the setting of trauma or if mental status does not clear with rewarming.3
- Bedside ultrasonography can assess for cardiac activity, volume status, pulmonary edema, free fluid, and trauma. (See "Point-of-care ultrasound: Coming soon to primary care?" J Fam Pract. 2018;67:70-80.)
Electrocardiography. An electrocardiogram is essential to evaluate for arrhythmias. Findings associated with hypothermia are prolongation of PR, QRS, and QT intervals; ST-segment elevation, T-wave inversion; and Osborn waves (J waves), which represent a positive deflection at the termination of the QRS complex with associated J-point elevation.8 Osborn waves generally present when the core body temperature is <32°C (89.6°F) and become larger as the core body temperature drops further.3
Differential diagnosis. Hypothermia is most commonly caused by environmental exposure, but the differential diagnosis is broad: many medical conditions, as well as drug and alcohol intoxication, can contribute to hypothermia (TABLE 31).

Treatment: Usually unnecessary, sometimes crucial
Most patients with mild hypothermia recover completely with little intervention. These patients should be evaluated for cognitive irregularities and observed in the ED before discharge.9 Moderate and severe hypothermia patients should be assessed using pre-hospital protocols and given cardiopulmonary resuscitation (CPR) for cardiac arrest. Pre-hospital providers should rely more on symptoms in guiding their treatment response because core body temperature measurements can be difficult to obtain, and the response to a drop in core body temperature varies from patient to patient.10
Early considerations: Airway, breathing, circulation (ABC)
A first responder might have difficulty palpating the pulse of a hypothermic patient if that patient’s cardiopulmonary effort is diminished.9 This inability to palpate a pulse should not delay treatment unless the patient presents with lethal injury; the scene is unsafe; the chest is too stiff for CPR; do-not-resuscitate status is present; or the patient was buried in an avalanche for ≥35 minutes and the airway is filled with snow (FIGURE3,11,12). Pulse should be checked carefully for 60 seconds. If pulses are not present, CPR should be initiated.
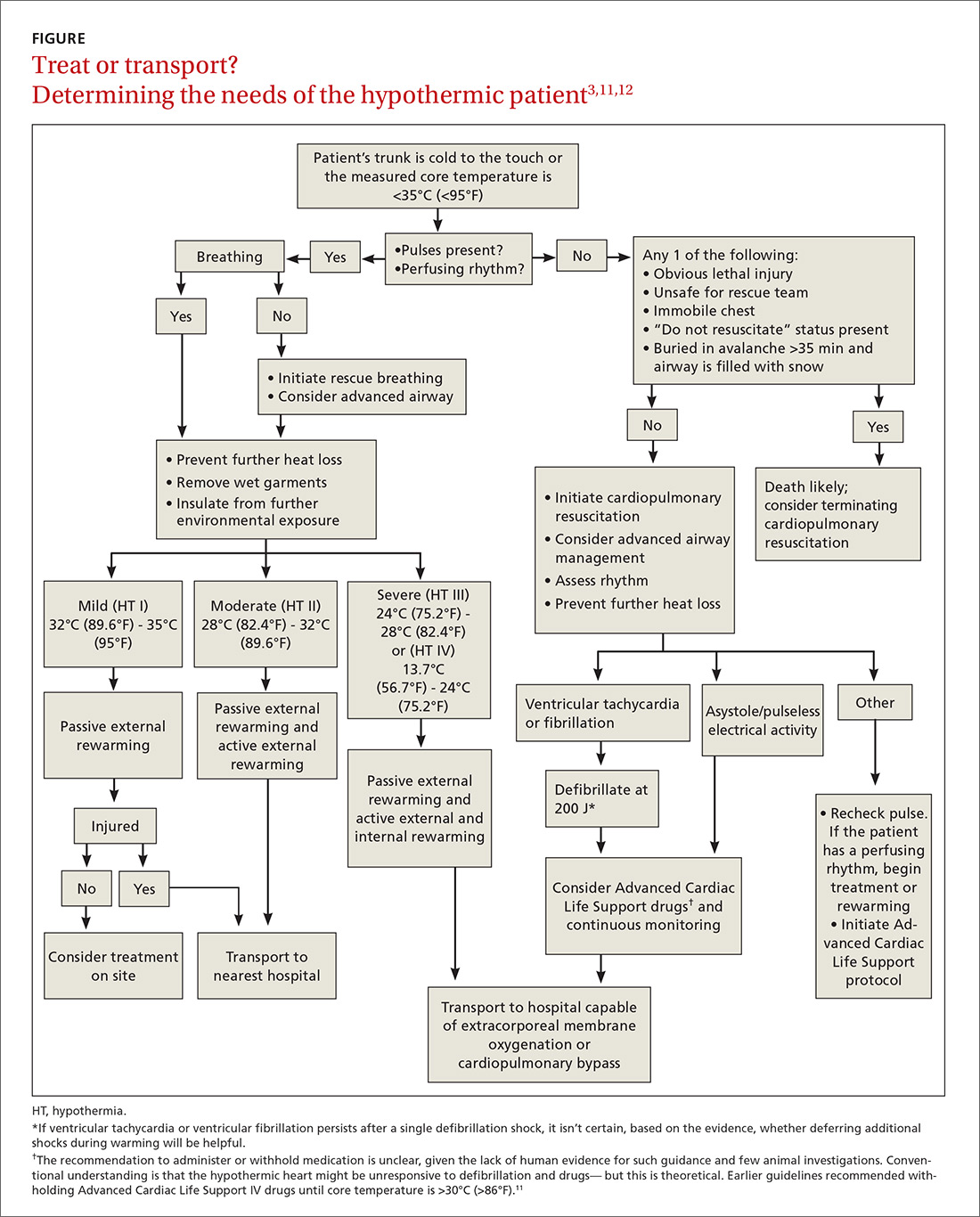
Prevention of further heat loss should begin promptly for hypothermic patients who retain a perfusing rhythm.11 Lifesaving interventions, such as airway management, vascular access for volume replenishment, and defibrillation for ventricular tachycardia or ventricular fibrillation should be carried out according to Advanced Cardiac Life Support protocols.11 Patients in respiratory distress or incapable of protecting their airway because of altered mental status should undergo endotracheal intubation. Fluid resuscitation with isotonic crystalloid fluids, warmed to 40°C (104°F) to 42°C (~107°F) and delivered through 2 large-bore, peripheral intravenous (IV) needles, can be considered.
Special care should be taken when moving a hypothermic patient. Excessive movement can lead to stimulation of the irritable hypothermic heart and cause an arrhythmia.
Medical therapy. Caution is advised because the reduced metabolism of a hypothermic patient can lead to potentially toxic accumulation of drugs peripherally. In fact, outcomes have not been positively influenced by routine use of medications, other than treatment of ventricular fibrillation with amiodarone.11 Any intravenous (IV) drug should be held until the patient’s core temperature is >30°C (>86°F).11
Vasopressors can be beneficial during rewarming for a patient in cardiac arrest and are a reasonable consideration.2 Nitroglycerin, in conjunction with active external rewarming, can increase the overall hourly temperature gain in a moderately hypothermic patient.13
Rewarming. The extent of rewarming required can be predicted by the severity of hypothermia (FIGURE3,11,12). Mildly hypothermic patients can generally be rewarmed using passive external measures. Patients with moderate hypothermia benefit from active rewarming in addition to passive measures. Intervention for severe hypothermia requires external rewarming and internal warming, with admission to the intensive care unit.
Treatment plans for severely hypothermic patients differ, depending on whether the person has a perfusing or nonperfusing cardiac rhythm. Patients who maintain a perfusing rhythm can be rewarmed using external methods (although core rewarming is used more often). Patients who do not have a perfusing rhythm require more invasive procedures.11 When using any rewarming method, afterdrop phenomenon can occur: ie, vasodilation, brought on by rewarming, causes a drop in core body temperature, as cooler peripheral blood returns to the central circulation. This effect may be reduced by focused rewarming of the trunk prior to rewarming the extremities.3
Rewarming for mild hypothermia patients begins with passive external techniques. First, the patient is moved away from the environment for protection from further exposure. Next, wet or damaged clothing is removed, blankets or foil insulators are applied, and room temperature is maintained at ≥28°C (82°F).3,11,13,14
If the patient’s temperature does not normalize, or if the patient presented with moderate or severe hypothermia, rewarming is continued with active external and internal measures. Active external rewarming can supplement passive measures using radiant heat from warmed blankets, air rewarming devices, and heating pads.3,13,14 Active internal rewarming techniques rely on invasive measures to raise the core temperature. Heated crystalloid IV fluids do not treat hypothermia, but do help reduce further heat loss and can be helpful in patients in need of volume resuscitation.3,13
Severely hypothermic patients might require more invasive active internal rewarming techniques, such as body-cavity lavage and extracorporeal methods. Body-cavity lavage can be facilitated with large volumes (10-120 L) of warm fluid at 40°C to 42°C, circulated through the thoracic or abdominal cavities to raise core body temperature 3°C to 6°C per hour.3,13
Extracorporeal rewarming can be achieved through hemodialysis, continuous arteriovenous rewarming (CAVR), continuous veno-venous rewarming (CVVR), or cardiopulmonary bypass.3,13 Research has shown cardiopulmonary bypass to be the most effective technique, with as high as a 7°C rise in core body temperature per hour; CVVR and CAVR are less invasive, however, and more readily available in hospitals.3,11,13
Rewarming interventions should continue until return of spontaneous circulation and core body temperature reaches 32°C (89.6°F) to 34°C (93.2°F).11 Overall, resuscitation efforts may take longer than normal due to the need for rewarming and should continue until the patient has achieved a normal temperature of 37°C (97.8°F).
Prognosis varies with severity, the health of the patient
In healthy, mildly hypothermic patients, full recovery is common if heat loss is minimized and the cause is treated. Moderately hypothermic patients who receive proper care can also have a favorable result. Outcomes for severe hypothermia vary with duration, comorbidities, and severity of core body temperature loss.15
Immediate initiation of rewarming by pre-hospital providers improves outcomes, and higher mortality has been demonstrated with hospital admission temperatures <35°C (95°F).15 Almost 100% of primary hypothermia patients with cardiac stability who were treated using active external and minimally invasive rewarming techniques survived with an intact neurologic system.12 Fifty percent of patients who endured cardiac arrest or who were treated with extracorporeal rewarming had an intact neurologic system. In cardiac arrest cases without significant underlying disease or trauma, and in which hypoxia did not precede hypothermia, full recovery is possible (and has been observed).12
CASE
Mr. S was given a diagnosis of mild to moderate hypothermia and transferred to the nearest ED for further treatment. His age had put him at increased risk of hypothermia. The work-up included laboratory testing (CBC, chemistry panel, thyroid-stimulating hormone, urinalysis, and blood cultures), electrocardiography, chest radiography, and CT of the head.
The chest radiograph showed pneumonia. Based on the results of blood culture, bacterial infection (pneumonia) was determined to be the underlying cause of hypothermia. Mr. S was started on antibiotics.
CORRESPONDENCE
Natasha J. Pyzocha, DO, Bldg 1058, 1856 Irwin Dr, Fort Carson, CO 80913; [email protected].
1. McCullough L, Arora S. Diagnosis and treatment of hypothermia. Am Fam Physician. 2004;70:2325-2332.
2. Durrer B, Brugger H, Syme D; International Commission for Mountain Emergency Medicine. The medical on-site treatment of hypothermia: ICAR-MEDCOM recommendation. High Alt Med Biol. 2003;4.
3. Rischall ML, Rowland-Fisher A. Evidence-based management of accidental hypothermia in the emergency department. Emerg Med Pract. 2016;18:1-18.
4. Study: Hypothermia-related deaths—United States, 2003-2004. Atlanta, GA: Centers for Disease Control and Prevention; 2005. Available at: www.cdc.gov/media/pressrel/fs050224.htm. Accessed March 1, 2018.
5. Baumgartner EA, Belson M, Rubin C, et al. Hypothermia and other cold-related morbidity emergency department visits: United States, 1995-2004. Wilderness Environ Med. 2008;19:233-237.
6. Centers for Disease Control and Prevention. Preventing injuries associated with extreme cold. Int J Trauma Nurs. 2001;7:26-30.
7. Jolly BT, Ghezzi KT. Accidental hypothermia. Emerg Med Clin North Am. 1992;10:311-327.
8. Mechem CC. Hypothermia and hyperthermia. In: Lanken PN, Manaker S, Hanson CW III, eds. The Intensive Care Unit Manual. Philadelphia: WB Saunders; 2000.
9. Weinberg AD. Hypothermia. Ann Emerg Med. 1993;22:370-377.
10. Zafren K, Giesbrecht GG, Danzl DF, et al. Wilderness Medical Society practice guidelines for the out-of-hospital evaluation and treatment of accidental hypothermia. Wilderness Environ Med. 2014;25:425-445.
11. Web-based integrated 2010 & 2015 guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Part 10: Special Circumstances of Resuscitation. Dallas, TX: American Heart Association; 2017. Available at: https://eccguidelines.heart.org/index.php/circulation/cpr-ecc-guidelines-2/part-10-special-circumstances-of-resuscitation. Accessed March 1, 2018.
12. Brown DJ, Brugger H, Boyd J, et al. Accidental hypothermia. N Engl J Med. 2012;367:1930-1938.
13. Petrone P, Asensio JA, Marini CP. Management of accidental hypothermia and cold injury. Curr Probl Surg. 2014;51:417-431.
14. Fudge J. Preventing and managing hypothermia and frostbite injury. Sports Health. 2016;8:133-139.
15. Martin RS, Kilgo PD, Miller PR, et al. Injury-associated hypothermia: an analysis of the 2004 National Trauma Data Bank. Shock. 2005;24:114-118.
CASE
Patrick S, an 85-year-old man with multiple medical problems, was brought to his primary care provider after being found at home with altered mental status. His caretaker reported that Mr. S had been using extra blankets in bed and sleeping more, but he hadn’t had significant outdoor exposure. Measurement of his vital signs revealed tachycardia, tachypnea, hypotension, and a rectal temperature of 32°C (89.6°F).
How would you proceed with the care of this patient?
What is accidental hypothermia?
Accidental hypothermia is an unintentional drop in core body temperature to <35°C (<95°F). Mild hypothermia is defined as a core body temperature of 32°C to 35°C (90°F - 95°F); moderate hypothermia, 28°C to 32°C (82°F - 90°F); and severe hypothermia, <28°C (<82°F).1
The International Commission for Mountain Emergency Medicine divides hypothermia into 5 categories, emphasizing the clinical features of each stage as a guide to treatment (TABLE 1).2 These categories were adopted to help prehospital rescuers estimate the severity of hypothermia using physical symptoms. For example, most patients stop shivering at approximately 30°C (86°F)—the “moderate (HT II)” category of hypothermia—although this response varies widely from patient to patient. Notably, there are reports in the literature of survival in hypothermia with a temperature as low as 13.7°C (56.7°F) and with cardiac arrest for as long as 8 hours and 40 minutes, although these events are rare.3

Each year, approximately 700 deaths in the United States are the result of hypothermia.4 Between 1995 to 2004 in the United States, it is estimated that 15,574 visits were made to a health care provider or facility for hypothermia and other cold-related concerns.5 Based on reports in the international literature, the incidence of nonlethal hypothermia is much greater than the incidence of lethal hypothermia.5 Almost half of deaths from hypothermia are in people older than age 65 years; the male to female ratio is 2.5:1.1
Variables that predispose the body to temperature dysregulation include extremes of age, comorbid conditions, intoxication, chronic cold exposure, immersion accident, mental illness, impaired shivering, and lack of acclimatization.1 The most common causes of death associated with hypothermia are falls, drownings, and cardiovascular disease.4 In a 2008 study, hypothermia and other cold-related morbidity emergency department (ED) visits required more transfers of patients to a critical care unit than any other reason for visiting an ED (risk ratio, 6.73; 95% confidence interval, 1.8-25).5 Mortality among inpatients whose hypothermia is classified as moderate or severe reaches as high as 40%.3
More than just cold-weather exposure
Accidental hypothermia occurs when heat loss is superseded by the body’s ability to generate heat. It commonly happens in cold environments but can also occur at higher temperatures if the body’s thermoregulatory system malfunctions.
Environmental or iatrogenic factors (ie, primary hypothermia), such as wind, water immersion, wetness, aggressive fluid resuscitation, and heat stroke treatment can make people more susceptible to hypothermia. Medical conditions (ie, secondary hypothermia), such as burns, exfoliative dermatitis, severe psoriasis, hypoadrenalism, hypopituitarism, hypothyroidism, acute spinal cord transection, head trauma, stroke, tumor, pneumonia, Wernicke’s disease (encephalopathy), and sepsis can also predispose to hypothermia.1 Drugs, such as ethanol, phenothiazines, and sedative–hypnotics may decrease the hypothermia threshold.1 (For information on preventing hypothermia, see TABLE 2.6)

Pathophysiology: The role of the hypothalamus
Humans maintain body temperature by balancing heat production and heat loss to the environment. Heat is lost through the skin and lungs by 5 different mechanisms: radiation, conduction, convection, evaporation, and respiration. Convective heat loss to cold air and conductive heat loss to water are the most common mechanisms of accidental hypothermia.7
To maintain temperature homeostasis at 37°C (98.6°F) (±0.5°C [±0.9°F]), the hypothalamus receives input from central and peripheral thermal receptors and stimulates heat production through shivering, increasing the basal metabolic rate 2-fold to 5-fold.1 The hypothalamus also increases thyroid, catecholamine, and adrenal activity to increase the body’s production of heat and raise core temperature.
Heat conservation occurs by activation of sympathetically mediated vasoconstriction, reducing conduction to the skin, where cooling is greatest. After time, temperature regulation in the body becomes overwhelmed and catecholamine levels return to a pre-hypothermic state.
At 35°C (95°F), neurologic function begins to decline; at 32°C (89.6°F), metabolism, ventilation, and cardiac output decrease until shivering ceases. Changes in peripheral blood flow can create a false warming sensation, causing a person to remove clothing, a phenomenon referred to as paradoxical undressing. As hypothermia progresses, the neurologic, respiratory, and cardiac systems continue to slow until there is eventual cardiorespiratory failure.
Assessment and diagnosis
History and physical examination. A high index of suspicion for the diagnosis of hypothermia is essential, especially when caring for the elderly or patients presenting with unexplained illness. Often, symptoms of a primary condition may overshadow those reflecting hypothermia. In a multicenter survey that reviewed 428 cases of accidental hypothermia in the United States, 44% of patients had an underlying illness; 18%, coexisting infection; 19%, trauma; and 6%, overdose.3
There are no strict diagnostic criteria for hypothermia other than a core body temperature <35°C (<95°F). Standard thermometers often do not read below 34.4°C (93.2°F), so it is recommended that a rectal thermometer capable of reading low body temperatures be used for accurate measurement.
Hypothermic patients can exhibit a variety of symptoms, depending on the degree of decrease in core body temperature1:
- A mildly hypothermic patient might present with any combination of tachypnea, tachycardia, ataxia, impaired judgment, shivering, and vasoconstriction.
- Moderate hypothermia typically manifests as a decreased heart rate, decreased blood pressure, decreased level of consciousness, decreased respiratory effort, dilated pupils, extinction of shivering, and hyporeflexia. Cardiac abnormalities, such as atrial fibrillation and junctional bradycardia, may be seen in moderate hypothermia.
- Severe hypothermia presents with apnea, coma, nonreactive pupils, oliguria, areflexia, hypotension, bradycardia, and continued cardiac abnormalities, such as ventricular arrhythmias and asystole.
Laboratory evaluation. No specific laboratory tests are needed to diagnose hypothermia. General lab tests, however, may help determine whether hypothermia is the result, or the cause, of the clinical scenario. Recommended laboratory tests for making that determination include a complete blood count (CBC), chemistry panel, arterial blood gases, fingerstick glucose, and coagulation panel.
Results of lab tests may be abnormal because of the body’s decreased core body temperature. White blood cells and platelets in the CBC, for example, may be decreased due to splenic sequestration; these findings reverse with rewarming. With every 1°C (1.8°F) drop in core body temperature, hematocrit increases 2%.3 Sodium, chloride, and magnesium concentrations do not display consistent abnormalities with any core body temperature >25°C (77°F),3,8 but potassium levels may fluctuate because of acid-base changes that occur during rewarming.1 Creatinine and creatine kinase levels may be increased secondary to rhabdomyolysis or acute tubular necrosis.1
Arterial blood gases typically show metabolic acidosis or respiratory alkalosis, or both.8 Prothrombin time and partial thromboplastin time are typically elevated in vivo, secondary to temperature-dependent enzymes in the coagulation cascade, but are reported normal in a blood specimen that is heated to 37°C (98.6°F) prior to analysis.1,8
Both hyperglycemia and hypoglycemia can be associated with hypothermia. The lactate level can be elevated, due to hypoperfusion. Hepatic impairment may be seen secondary to decreased cardiac output. An increase in the lipase level may also occur.3
When a hypothermic patient fails to respond to rewarming, or there is no clear source of cold exposure, consider testing for other causes of the problem, including hypothyroidism and adrenal insufficiency (see “Differential diagnosis”). Hypothermia may also decrease thyroid function in people with preexisting disease.
Other laboratory studies that can be considered include fibrinogen, blood-alcohol level, urine toxicology screen, and blood and fluid cultures.3
Imaging. Imaging studies are not performed routinely in the setting of hypothermia; however:
- Chest radiography can be considered to assess for aspiration pneumonia, vascular congestion, and pulmonary edema.
- Computed tomography (CT) of the head is helpful in the setting of trauma or if mental status does not clear with rewarming.3
- Bedside ultrasonography can assess for cardiac activity, volume status, pulmonary edema, free fluid, and trauma. (See "Point-of-care ultrasound: Coming soon to primary care?" J Fam Pract. 2018;67:70-80.)
Electrocardiography. An electrocardiogram is essential to evaluate for arrhythmias. Findings associated with hypothermia are prolongation of PR, QRS, and QT intervals; ST-segment elevation, T-wave inversion; and Osborn waves (J waves), which represent a positive deflection at the termination of the QRS complex with associated J-point elevation.8 Osborn waves generally present when the core body temperature is <32°C (89.6°F) and become larger as the core body temperature drops further.3
Differential diagnosis. Hypothermia is most commonly caused by environmental exposure, but the differential diagnosis is broad: many medical conditions, as well as drug and alcohol intoxication, can contribute to hypothermia (TABLE 31).

Treatment: Usually unnecessary, sometimes crucial
Most patients with mild hypothermia recover completely with little intervention. These patients should be evaluated for cognitive irregularities and observed in the ED before discharge.9 Moderate and severe hypothermia patients should be assessed using pre-hospital protocols and given cardiopulmonary resuscitation (CPR) for cardiac arrest. Pre-hospital providers should rely more on symptoms in guiding their treatment response because core body temperature measurements can be difficult to obtain, and the response to a drop in core body temperature varies from patient to patient.10
Early considerations: Airway, breathing, circulation (ABC)
A first responder might have difficulty palpating the pulse of a hypothermic patient if that patient’s cardiopulmonary effort is diminished.9 This inability to palpate a pulse should not delay treatment unless the patient presents with lethal injury; the scene is unsafe; the chest is too stiff for CPR; do-not-resuscitate status is present; or the patient was buried in an avalanche for ≥35 minutes and the airway is filled with snow (FIGURE3,11,12). Pulse should be checked carefully for 60 seconds. If pulses are not present, CPR should be initiated.

Prevention of further heat loss should begin promptly for hypothermic patients who retain a perfusing rhythm.11 Lifesaving interventions, such as airway management, vascular access for volume replenishment, and defibrillation for ventricular tachycardia or ventricular fibrillation should be carried out according to Advanced Cardiac Life Support protocols.11 Patients in respiratory distress or incapable of protecting their airway because of altered mental status should undergo endotracheal intubation. Fluid resuscitation with isotonic crystalloid fluids, warmed to 40°C (104°F) to 42°C (~107°F) and delivered through 2 large-bore, peripheral intravenous (IV) needles, can be considered.
Special care should be taken when moving a hypothermic patient. Excessive movement can lead to stimulation of the irritable hypothermic heart and cause an arrhythmia.
Medical therapy. Caution is advised because the reduced metabolism of a hypothermic patient can lead to potentially toxic accumulation of drugs peripherally. In fact, outcomes have not been positively influenced by routine use of medications, other than treatment of ventricular fibrillation with amiodarone.11 Any intravenous (IV) drug should be held until the patient’s core temperature is >30°C (>86°F).11
Vasopressors can be beneficial during rewarming for a patient in cardiac arrest and are a reasonable consideration.2 Nitroglycerin, in conjunction with active external rewarming, can increase the overall hourly temperature gain in a moderately hypothermic patient.13
Rewarming. The extent of rewarming required can be predicted by the severity of hypothermia (FIGURE3,11,12). Mildly hypothermic patients can generally be rewarmed using passive external measures. Patients with moderate hypothermia benefit from active rewarming in addition to passive measures. Intervention for severe hypothermia requires external rewarming and internal warming, with admission to the intensive care unit.
Treatment plans for severely hypothermic patients differ, depending on whether the person has a perfusing or nonperfusing cardiac rhythm. Patients who maintain a perfusing rhythm can be rewarmed using external methods (although core rewarming is used more often). Patients who do not have a perfusing rhythm require more invasive procedures.11 When using any rewarming method, afterdrop phenomenon can occur: ie, vasodilation, brought on by rewarming, causes a drop in core body temperature, as cooler peripheral blood returns to the central circulation. This effect may be reduced by focused rewarming of the trunk prior to rewarming the extremities.3
Rewarming for mild hypothermia patients begins with passive external techniques. First, the patient is moved away from the environment for protection from further exposure. Next, wet or damaged clothing is removed, blankets or foil insulators are applied, and room temperature is maintained at ≥28°C (82°F).3,11,13,14
If the patient’s temperature does not normalize, or if the patient presented with moderate or severe hypothermia, rewarming is continued with active external and internal measures. Active external rewarming can supplement passive measures using radiant heat from warmed blankets, air rewarming devices, and heating pads.3,13,14 Active internal rewarming techniques rely on invasive measures to raise the core temperature. Heated crystalloid IV fluids do not treat hypothermia, but do help reduce further heat loss and can be helpful in patients in need of volume resuscitation.3,13
Severely hypothermic patients might require more invasive active internal rewarming techniques, such as body-cavity lavage and extracorporeal methods. Body-cavity lavage can be facilitated with large volumes (10-120 L) of warm fluid at 40°C to 42°C, circulated through the thoracic or abdominal cavities to raise core body temperature 3°C to 6°C per hour.3,13
Extracorporeal rewarming can be achieved through hemodialysis, continuous arteriovenous rewarming (CAVR), continuous veno-venous rewarming (CVVR), or cardiopulmonary bypass.3,13 Research has shown cardiopulmonary bypass to be the most effective technique, with as high as a 7°C rise in core body temperature per hour; CVVR and CAVR are less invasive, however, and more readily available in hospitals.3,11,13
Rewarming interventions should continue until return of spontaneous circulation and core body temperature reaches 32°C (89.6°F) to 34°C (93.2°F).11 Overall, resuscitation efforts may take longer than normal due to the need for rewarming and should continue until the patient has achieved a normal temperature of 37°C (97.8°F).
Prognosis varies with severity, the health of the patient
In healthy, mildly hypothermic patients, full recovery is common if heat loss is minimized and the cause is treated. Moderately hypothermic patients who receive proper care can also have a favorable result. Outcomes for severe hypothermia vary with duration, comorbidities, and severity of core body temperature loss.15
Immediate initiation of rewarming by pre-hospital providers improves outcomes, and higher mortality has been demonstrated with hospital admission temperatures <35°C (95°F).15 Almost 100% of primary hypothermia patients with cardiac stability who were treated using active external and minimally invasive rewarming techniques survived with an intact neurologic system.12 Fifty percent of patients who endured cardiac arrest or who were treated with extracorporeal rewarming had an intact neurologic system. In cardiac arrest cases without significant underlying disease or trauma, and in which hypoxia did not precede hypothermia, full recovery is possible (and has been observed).12
CASE
Mr. S was given a diagnosis of mild to moderate hypothermia and transferred to the nearest ED for further treatment. His age had put him at increased risk of hypothermia. The work-up included laboratory testing (CBC, chemistry panel, thyroid-stimulating hormone, urinalysis, and blood cultures), electrocardiography, chest radiography, and CT of the head.
The chest radiograph showed pneumonia. Based on the results of blood culture, bacterial infection (pneumonia) was determined to be the underlying cause of hypothermia. Mr. S was started on antibiotics.
CORRESPONDENCE
Natasha J. Pyzocha, DO, Bldg 1058, 1856 Irwin Dr, Fort Carson, CO 80913; [email protected].
CASE
Patrick S, an 85-year-old man with multiple medical problems, was brought to his primary care provider after being found at home with altered mental status. His caretaker reported that Mr. S had been using extra blankets in bed and sleeping more, but he hadn’t had significant outdoor exposure. Measurement of his vital signs revealed tachycardia, tachypnea, hypotension, and a rectal temperature of 32°C (89.6°F).
How would you proceed with the care of this patient?
What is accidental hypothermia?
Accidental hypothermia is an unintentional drop in core body temperature to <35°C (<95°F). Mild hypothermia is defined as a core body temperature of 32°C to 35°C (90°F - 95°F); moderate hypothermia, 28°C to 32°C (82°F - 90°F); and severe hypothermia, <28°C (<82°F).1
The International Commission for Mountain Emergency Medicine divides hypothermia into 5 categories, emphasizing the clinical features of each stage as a guide to treatment (TABLE 1).2 These categories were adopted to help prehospital rescuers estimate the severity of hypothermia using physical symptoms. For example, most patients stop shivering at approximately 30°C (86°F)—the “moderate (HT II)” category of hypothermia—although this response varies widely from patient to patient. Notably, there are reports in the literature of survival in hypothermia with a temperature as low as 13.7°C (56.7°F) and with cardiac arrest for as long as 8 hours and 40 minutes, although these events are rare.3

Each year, approximately 700 deaths in the United States are the result of hypothermia.4 Between 1995 to 2004 in the United States, it is estimated that 15,574 visits were made to a health care provider or facility for hypothermia and other cold-related concerns.5 Based on reports in the international literature, the incidence of nonlethal hypothermia is much greater than the incidence of lethal hypothermia.5 Almost half of deaths from hypothermia are in people older than age 65 years; the male to female ratio is 2.5:1.1
Variables that predispose the body to temperature dysregulation include extremes of age, comorbid conditions, intoxication, chronic cold exposure, immersion accident, mental illness, impaired shivering, and lack of acclimatization.1 The most common causes of death associated with hypothermia are falls, drownings, and cardiovascular disease.4 In a 2008 study, hypothermia and other cold-related morbidity emergency department (ED) visits required more transfers of patients to a critical care unit than any other reason for visiting an ED (risk ratio, 6.73; 95% confidence interval, 1.8-25).5 Mortality among inpatients whose hypothermia is classified as moderate or severe reaches as high as 40%.3
More than just cold-weather exposure
Accidental hypothermia occurs when heat loss is superseded by the body’s ability to generate heat. It commonly happens in cold environments but can also occur at higher temperatures if the body’s thermoregulatory system malfunctions.
Environmental or iatrogenic factors (ie, primary hypothermia), such as wind, water immersion, wetness, aggressive fluid resuscitation, and heat stroke treatment can make people more susceptible to hypothermia. Medical conditions (ie, secondary hypothermia), such as burns, exfoliative dermatitis, severe psoriasis, hypoadrenalism, hypopituitarism, hypothyroidism, acute spinal cord transection, head trauma, stroke, tumor, pneumonia, Wernicke’s disease (encephalopathy), and sepsis can also predispose to hypothermia.1 Drugs, such as ethanol, phenothiazines, and sedative–hypnotics may decrease the hypothermia threshold.1 (For information on preventing hypothermia, see TABLE 2.6)

Pathophysiology: The role of the hypothalamus
Humans maintain body temperature by balancing heat production and heat loss to the environment. Heat is lost through the skin and lungs by 5 different mechanisms: radiation, conduction, convection, evaporation, and respiration. Convective heat loss to cold air and conductive heat loss to water are the most common mechanisms of accidental hypothermia.7
To maintain temperature homeostasis at 37°C (98.6°F) (±0.5°C [±0.9°F]), the hypothalamus receives input from central and peripheral thermal receptors and stimulates heat production through shivering, increasing the basal metabolic rate 2-fold to 5-fold.1 The hypothalamus also increases thyroid, catecholamine, and adrenal activity to increase the body’s production of heat and raise core temperature.
Heat conservation occurs by activation of sympathetically mediated vasoconstriction, reducing conduction to the skin, where cooling is greatest. After time, temperature regulation in the body becomes overwhelmed and catecholamine levels return to a pre-hypothermic state.
At 35°C (95°F), neurologic function begins to decline; at 32°C (89.6°F), metabolism, ventilation, and cardiac output decrease until shivering ceases. Changes in peripheral blood flow can create a false warming sensation, causing a person to remove clothing, a phenomenon referred to as paradoxical undressing. As hypothermia progresses, the neurologic, respiratory, and cardiac systems continue to slow until there is eventual cardiorespiratory failure.
Assessment and diagnosis
History and physical examination. A high index of suspicion for the diagnosis of hypothermia is essential, especially when caring for the elderly or patients presenting with unexplained illness. Often, symptoms of a primary condition may overshadow those reflecting hypothermia. In a multicenter survey that reviewed 428 cases of accidental hypothermia in the United States, 44% of patients had an underlying illness; 18%, coexisting infection; 19%, trauma; and 6%, overdose.3
There are no strict diagnostic criteria for hypothermia other than a core body temperature <35°C (<95°F). Standard thermometers often do not read below 34.4°C (93.2°F), so it is recommended that a rectal thermometer capable of reading low body temperatures be used for accurate measurement.
Hypothermic patients can exhibit a variety of symptoms, depending on the degree of decrease in core body temperature1:
- A mildly hypothermic patient might present with any combination of tachypnea, tachycardia, ataxia, impaired judgment, shivering, and vasoconstriction.
- Moderate hypothermia typically manifests as a decreased heart rate, decreased blood pressure, decreased level of consciousness, decreased respiratory effort, dilated pupils, extinction of shivering, and hyporeflexia. Cardiac abnormalities, such as atrial fibrillation and junctional bradycardia, may be seen in moderate hypothermia.
- Severe hypothermia presents with apnea, coma, nonreactive pupils, oliguria, areflexia, hypotension, bradycardia, and continued cardiac abnormalities, such as ventricular arrhythmias and asystole.
Laboratory evaluation. No specific laboratory tests are needed to diagnose hypothermia. General lab tests, however, may help determine whether hypothermia is the result, or the cause, of the clinical scenario. Recommended laboratory tests for making that determination include a complete blood count (CBC), chemistry panel, arterial blood gases, fingerstick glucose, and coagulation panel.
Results of lab tests may be abnormal because of the body’s decreased core body temperature. White blood cells and platelets in the CBC, for example, may be decreased due to splenic sequestration; these findings reverse with rewarming. With every 1°C (1.8°F) drop in core body temperature, hematocrit increases 2%.3 Sodium, chloride, and magnesium concentrations do not display consistent abnormalities with any core body temperature >25°C (77°F),3,8 but potassium levels may fluctuate because of acid-base changes that occur during rewarming.1 Creatinine and creatine kinase levels may be increased secondary to rhabdomyolysis or acute tubular necrosis.1
Arterial blood gases typically show metabolic acidosis or respiratory alkalosis, or both.8 Prothrombin time and partial thromboplastin time are typically elevated in vivo, secondary to temperature-dependent enzymes in the coagulation cascade, but are reported normal in a blood specimen that is heated to 37°C (98.6°F) prior to analysis.1,8
Both hyperglycemia and hypoglycemia can be associated with hypothermia. The lactate level can be elevated, due to hypoperfusion. Hepatic impairment may be seen secondary to decreased cardiac output. An increase in the lipase level may also occur.3
When a hypothermic patient fails to respond to rewarming, or there is no clear source of cold exposure, consider testing for other causes of the problem, including hypothyroidism and adrenal insufficiency (see “Differential diagnosis”). Hypothermia may also decrease thyroid function in people with preexisting disease.
Other laboratory studies that can be considered include fibrinogen, blood-alcohol level, urine toxicology screen, and blood and fluid cultures.3
Imaging. Imaging studies are not performed routinely in the setting of hypothermia; however:
- Chest radiography can be considered to assess for aspiration pneumonia, vascular congestion, and pulmonary edema.
- Computed tomography (CT) of the head is helpful in the setting of trauma or if mental status does not clear with rewarming.3
- Bedside ultrasonography can assess for cardiac activity, volume status, pulmonary edema, free fluid, and trauma. (See "Point-of-care ultrasound: Coming soon to primary care?" J Fam Pract. 2018;67:70-80.)
Electrocardiography. An electrocardiogram is essential to evaluate for arrhythmias. Findings associated with hypothermia are prolongation of PR, QRS, and QT intervals; ST-segment elevation, T-wave inversion; and Osborn waves (J waves), which represent a positive deflection at the termination of the QRS complex with associated J-point elevation.8 Osborn waves generally present when the core body temperature is <32°C (89.6°F) and become larger as the core body temperature drops further.3
Differential diagnosis. Hypothermia is most commonly caused by environmental exposure, but the differential diagnosis is broad: many medical conditions, as well as drug and alcohol intoxication, can contribute to hypothermia (TABLE 31).

Treatment: Usually unnecessary, sometimes crucial
Most patients with mild hypothermia recover completely with little intervention. These patients should be evaluated for cognitive irregularities and observed in the ED before discharge.9 Moderate and severe hypothermia patients should be assessed using pre-hospital protocols and given cardiopulmonary resuscitation (CPR) for cardiac arrest. Pre-hospital providers should rely more on symptoms in guiding their treatment response because core body temperature measurements can be difficult to obtain, and the response to a drop in core body temperature varies from patient to patient.10
Early considerations: Airway, breathing, circulation (ABC)
A first responder might have difficulty palpating the pulse of a hypothermic patient if that patient’s cardiopulmonary effort is diminished.9 This inability to palpate a pulse should not delay treatment unless the patient presents with lethal injury; the scene is unsafe; the chest is too stiff for CPR; do-not-resuscitate status is present; or the patient was buried in an avalanche for ≥35 minutes and the airway is filled with snow (FIGURE3,11,12). Pulse should be checked carefully for 60 seconds. If pulses are not present, CPR should be initiated.

Prevention of further heat loss should begin promptly for hypothermic patients who retain a perfusing rhythm.11 Lifesaving interventions, such as airway management, vascular access for volume replenishment, and defibrillation for ventricular tachycardia or ventricular fibrillation should be carried out according to Advanced Cardiac Life Support protocols.11 Patients in respiratory distress or incapable of protecting their airway because of altered mental status should undergo endotracheal intubation. Fluid resuscitation with isotonic crystalloid fluids, warmed to 40°C (104°F) to 42°C (~107°F) and delivered through 2 large-bore, peripheral intravenous (IV) needles, can be considered.
Special care should be taken when moving a hypothermic patient. Excessive movement can lead to stimulation of the irritable hypothermic heart and cause an arrhythmia.
Medical therapy. Caution is advised because the reduced metabolism of a hypothermic patient can lead to potentially toxic accumulation of drugs peripherally. In fact, outcomes have not been positively influenced by routine use of medications, other than treatment of ventricular fibrillation with amiodarone.11 Any intravenous (IV) drug should be held until the patient’s core temperature is >30°C (>86°F).11
Vasopressors can be beneficial during rewarming for a patient in cardiac arrest and are a reasonable consideration.2 Nitroglycerin, in conjunction with active external rewarming, can increase the overall hourly temperature gain in a moderately hypothermic patient.13
Rewarming. The extent of rewarming required can be predicted by the severity of hypothermia (FIGURE3,11,12). Mildly hypothermic patients can generally be rewarmed using passive external measures. Patients with moderate hypothermia benefit from active rewarming in addition to passive measures. Intervention for severe hypothermia requires external rewarming and internal warming, with admission to the intensive care unit.
Treatment plans for severely hypothermic patients differ, depending on whether the person has a perfusing or nonperfusing cardiac rhythm. Patients who maintain a perfusing rhythm can be rewarmed using external methods (although core rewarming is used more often). Patients who do not have a perfusing rhythm require more invasive procedures.11 When using any rewarming method, afterdrop phenomenon can occur: ie, vasodilation, brought on by rewarming, causes a drop in core body temperature, as cooler peripheral blood returns to the central circulation. This effect may be reduced by focused rewarming of the trunk prior to rewarming the extremities.3
Rewarming for mild hypothermia patients begins with passive external techniques. First, the patient is moved away from the environment for protection from further exposure. Next, wet or damaged clothing is removed, blankets or foil insulators are applied, and room temperature is maintained at ≥28°C (82°F).3,11,13,14
If the patient’s temperature does not normalize, or if the patient presented with moderate or severe hypothermia, rewarming is continued with active external and internal measures. Active external rewarming can supplement passive measures using radiant heat from warmed blankets, air rewarming devices, and heating pads.3,13,14 Active internal rewarming techniques rely on invasive measures to raise the core temperature. Heated crystalloid IV fluids do not treat hypothermia, but do help reduce further heat loss and can be helpful in patients in need of volume resuscitation.3,13
Severely hypothermic patients might require more invasive active internal rewarming techniques, such as body-cavity lavage and extracorporeal methods. Body-cavity lavage can be facilitated with large volumes (10-120 L) of warm fluid at 40°C to 42°C, circulated through the thoracic or abdominal cavities to raise core body temperature 3°C to 6°C per hour.3,13
Extracorporeal rewarming can be achieved through hemodialysis, continuous arteriovenous rewarming (CAVR), continuous veno-venous rewarming (CVVR), or cardiopulmonary bypass.3,13 Research has shown cardiopulmonary bypass to be the most effective technique, with as high as a 7°C rise in core body temperature per hour; CVVR and CAVR are less invasive, however, and more readily available in hospitals.3,11,13
Rewarming interventions should continue until return of spontaneous circulation and core body temperature reaches 32°C (89.6°F) to 34°C (93.2°F).11 Overall, resuscitation efforts may take longer than normal due to the need for rewarming and should continue until the patient has achieved a normal temperature of 37°C (97.8°F).
Prognosis varies with severity, the health of the patient
In healthy, mildly hypothermic patients, full recovery is common if heat loss is minimized and the cause is treated. Moderately hypothermic patients who receive proper care can also have a favorable result. Outcomes for severe hypothermia vary with duration, comorbidities, and severity of core body temperature loss.15
Immediate initiation of rewarming by pre-hospital providers improves outcomes, and higher mortality has been demonstrated with hospital admission temperatures <35°C (95°F).15 Almost 100% of primary hypothermia patients with cardiac stability who were treated using active external and minimally invasive rewarming techniques survived with an intact neurologic system.12 Fifty percent of patients who endured cardiac arrest or who were treated with extracorporeal rewarming had an intact neurologic system. In cardiac arrest cases without significant underlying disease or trauma, and in which hypoxia did not precede hypothermia, full recovery is possible (and has been observed).12
CASE
Mr. S was given a diagnosis of mild to moderate hypothermia and transferred to the nearest ED for further treatment. His age had put him at increased risk of hypothermia. The work-up included laboratory testing (CBC, chemistry panel, thyroid-stimulating hormone, urinalysis, and blood cultures), electrocardiography, chest radiography, and CT of the head.
The chest radiograph showed pneumonia. Based on the results of blood culture, bacterial infection (pneumonia) was determined to be the underlying cause of hypothermia. Mr. S was started on antibiotics.
CORRESPONDENCE
Natasha J. Pyzocha, DO, Bldg 1058, 1856 Irwin Dr, Fort Carson, CO 80913; [email protected].
1. McCullough L, Arora S. Diagnosis and treatment of hypothermia. Am Fam Physician. 2004;70:2325-2332.
2. Durrer B, Brugger H, Syme D; International Commission for Mountain Emergency Medicine. The medical on-site treatment of hypothermia: ICAR-MEDCOM recommendation. High Alt Med Biol. 2003;4.
3. Rischall ML, Rowland-Fisher A. Evidence-based management of accidental hypothermia in the emergency department. Emerg Med Pract. 2016;18:1-18.
4. Study: Hypothermia-related deaths—United States, 2003-2004. Atlanta, GA: Centers for Disease Control and Prevention; 2005. Available at: www.cdc.gov/media/pressrel/fs050224.htm. Accessed March 1, 2018.
5. Baumgartner EA, Belson M, Rubin C, et al. Hypothermia and other cold-related morbidity emergency department visits: United States, 1995-2004. Wilderness Environ Med. 2008;19:233-237.
6. Centers for Disease Control and Prevention. Preventing injuries associated with extreme cold. Int J Trauma Nurs. 2001;7:26-30.
7. Jolly BT, Ghezzi KT. Accidental hypothermia. Emerg Med Clin North Am. 1992;10:311-327.
8. Mechem CC. Hypothermia and hyperthermia. In: Lanken PN, Manaker S, Hanson CW III, eds. The Intensive Care Unit Manual. Philadelphia: WB Saunders; 2000.
9. Weinberg AD. Hypothermia. Ann Emerg Med. 1993;22:370-377.
10. Zafren K, Giesbrecht GG, Danzl DF, et al. Wilderness Medical Society practice guidelines for the out-of-hospital evaluation and treatment of accidental hypothermia. Wilderness Environ Med. 2014;25:425-445.
11. Web-based integrated 2010 & 2015 guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Part 10: Special Circumstances of Resuscitation. Dallas, TX: American Heart Association; 2017. Available at: https://eccguidelines.heart.org/index.php/circulation/cpr-ecc-guidelines-2/part-10-special-circumstances-of-resuscitation. Accessed March 1, 2018.
12. Brown DJ, Brugger H, Boyd J, et al. Accidental hypothermia. N Engl J Med. 2012;367:1930-1938.
13. Petrone P, Asensio JA, Marini CP. Management of accidental hypothermia and cold injury. Curr Probl Surg. 2014;51:417-431.
14. Fudge J. Preventing and managing hypothermia and frostbite injury. Sports Health. 2016;8:133-139.
15. Martin RS, Kilgo PD, Miller PR, et al. Injury-associated hypothermia: an analysis of the 2004 National Trauma Data Bank. Shock. 2005;24:114-118.
1. McCullough L, Arora S. Diagnosis and treatment of hypothermia. Am Fam Physician. 2004;70:2325-2332.
2. Durrer B, Brugger H, Syme D; International Commission for Mountain Emergency Medicine. The medical on-site treatment of hypothermia: ICAR-MEDCOM recommendation. High Alt Med Biol. 2003;4.
3. Rischall ML, Rowland-Fisher A. Evidence-based management of accidental hypothermia in the emergency department. Emerg Med Pract. 2016;18:1-18.
4. Study: Hypothermia-related deaths—United States, 2003-2004. Atlanta, GA: Centers for Disease Control and Prevention; 2005. Available at: www.cdc.gov/media/pressrel/fs050224.htm. Accessed March 1, 2018.
5. Baumgartner EA, Belson M, Rubin C, et al. Hypothermia and other cold-related morbidity emergency department visits: United States, 1995-2004. Wilderness Environ Med. 2008;19:233-237.
6. Centers for Disease Control and Prevention. Preventing injuries associated with extreme cold. Int J Trauma Nurs. 2001;7:26-30.
7. Jolly BT, Ghezzi KT. Accidental hypothermia. Emerg Med Clin North Am. 1992;10:311-327.
8. Mechem CC. Hypothermia and hyperthermia. In: Lanken PN, Manaker S, Hanson CW III, eds. The Intensive Care Unit Manual. Philadelphia: WB Saunders; 2000.
9. Weinberg AD. Hypothermia. Ann Emerg Med. 1993;22:370-377.
10. Zafren K, Giesbrecht GG, Danzl DF, et al. Wilderness Medical Society practice guidelines for the out-of-hospital evaluation and treatment of accidental hypothermia. Wilderness Environ Med. 2014;25:425-445.
11. Web-based integrated 2010 & 2015 guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Part 10: Special Circumstances of Resuscitation. Dallas, TX: American Heart Association; 2017. Available at: https://eccguidelines.heart.org/index.php/circulation/cpr-ecc-guidelines-2/part-10-special-circumstances-of-resuscitation. Accessed March 1, 2018.
12. Brown DJ, Brugger H, Boyd J, et al. Accidental hypothermia. N Engl J Med. 2012;367:1930-1938.
13. Petrone P, Asensio JA, Marini CP. Management of accidental hypothermia and cold injury. Curr Probl Surg. 2014;51:417-431.
14. Fudge J. Preventing and managing hypothermia and frostbite injury. Sports Health. 2016;8:133-139.
15. Martin RS, Kilgo PD, Miller PR, et al. Injury-associated hypothermia: an analysis of the 2004 National Trauma Data Bank. Shock. 2005;24:114-118.
PRACTICE RECOMMENDATIONS
› Measure the patient's temperature with a rectal thermometer capable of reading a temperature <35°C (<95°F) when hypothermia is suspected. C
› Begin prevention of further heat loss promptly for hypothermic patients who retain a perfusing rhythm. C
› Do not consider a patient dead until body temperature has normalized. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
USPSTF update: New and revised recommendations
Over the past year the US Preventive Services Task Force made 14 recommendations on 12 conditions (TABLE 11-12). One of these pronouncements was the unusual reversal of a previous “D” recommendation against screening for scoliosis in adolescents, changing it to an “I” statement (insufficient evidence).
Affirmative recommendations
Four interventions were given an “A” or “B” recommendation this past year. Both grades signify a recommendation to perform the service, with “A” reflecting a higher level of certainty or a higher level of net benefit than “B.”
Recommend folic acid to prevent neural tube defects (A)
The evidence is very strong that folic acid intake prevents neural tube defects. In 2009 the Task Force recommended folic acid supplementation for women of childbearing age. In 2017 this recommendation was updated and slightly reworded to advise that all women who are planning a pregnancy or capable of becoming pregnant take a daily supplement containing 0.4 mg to 0.8 mg (400-800 mcg) of folic acid.
In the United States many grain products have been fortified with folic acid since 1996. This step has reduced the prevalence of neural tube defects from 10.7 cases per 10,000 live births to 7 cases per 10,000 live births in 2011.1 However, in spite of food fortification, most women in the United States do not consume the recommended daily amount of 0.4 mg (400 mcg) of folic acid. This supplementation is most important from one month before conception through the first 3 months of pregnancy.
Screen for obesity in children and adolescents (B)
Nearly 17% of children and adolescents ages 2 to 19 years in the United States are obese, and almost 32% are overweight or obese.2 Obesity is defined as a body mass index (BMI) ≥95th percentile, based on year-2000 growth charts published by the Centers for Disease Control and Prevention. Overweight is defined as a BMI between the 85th and 94th percentiles.
Obesity in children and adolescents is associated with many physical problems, including obstructive sleep apnea, orthopedic problems, high blood pressure, hyperlipidemia, and diabetes, as well as psychological harms from being teased and bullied. Obesity that continues into adulthood is associated with diabetes, cardiovascular disease, and orthopedic problems.
The Task Force found that intensive behavioral interventions for obesity in children ≥6 years of age and in adolescents can lead to moderate improvements in weight status for up to 12 months. Intensive behavioral interventions need to include at least 26 contact hours over 2 to 12 months. The recommendation statement includes a more detailed description of the types of programs that have evidence to support them.2
The Task Force did not recommend the use of either metformin or orlistat because of inadequate evidence on the harmful effects of metformin and because of sound evidence that orlistat causes moderate harms, such as abdominal pain, cramping, incontinence, and flatus.
Screen for preeclampsia (B), but dipstick testing is unreliable
Preeclampsia occurs in a little more than 3% of pregnancies in the United States.13 For the mother, this condition can lead to stroke, eclampsia, organ failure, and death; for the fetus, intrauterine growth retardation, preterm birth, low birth weight, and still birth. Preeclampsia is a leading cause of maternal mortality worldwide. Adverse health outcomes can be prevented by early detection of preeclampsia and by managing it appropriately.3
In 1996 the Task Force recommended screening for preeclampsia during pregnancy, and it reaffirmed that recommendation last year. The Task Force recommends taking blood pressure measurements at every prenatal visit, but does not recommend testing for urine protein with a dipstick because of the technique’s low accuracy.
Since 2014 the Task Force has also recommended using low-dose aspirin after Week 12 of pregnancy to prevent preeclampsia in women who are at high risk.14
Conduct vision screening in all children ages 3 to 5 years (B)
One of the more nuanced recommendations involves vision screening in children. The Task Force recently reaffirmed its 2011 recommendation to perform vision screening at least once in all children ages 3 to 5 years to detect amblyopia or its risk factors. But it found insufficient evidence to test children <3 years of age.
Amblyopia is a “functional reduction in visual acuity characterized by abnormal processing of visual images; [it is] established by the brain during a critical period of vision development.”4 Risk factors associated with the development of amblyopia include strabismus (ocular misalignment); vision loss caused by cataracts; refractive errors such as near and far sightedness, astigmatism (“blurred vision at any distance due to abnormal curvature of the cornea or lens”); and anisometropia (“asymmetric refractive error between the … eyes that causes image suppression in the eye with the larger error”). 4
Physical exam- and machine-based screening tests are available in the primary care setting (TABLE 2).4
At first glance it appears that the Task Force recommends screening only for amblyopia, but the addition of “risk factors” implies a more comprehensive vision evaluation that would include visual acuity. This interpretation more closely aligns the Task Force recommendation with that of a joint report by the American Academy of Pediatrics, American Association for Pediatric Ophthalmology and Strabismus, American Academy of Certified Orthoptists, and American Academy of Ophthalmology, which recommends testing for a variety of vision problems in children.15 Nevertheless, the Task Force maintains that the evidence of benefit in testing more extensively before age 3 is insufficient, while the other organizations recommend starting testing at age 6 months.
Negative “D” recommendations
Equally as important as affirmative recommendations for effective interventions are the “D” recommendations advising against interventions that are ineffective or cause more harm than benefits. This past year, the Task Force recommended against 4 interventions. Two pertain to the use of estrogen or combined estrogen and progestin for the primary prevention of chronic conditions in postmenopausal women.5 This topic has been discussed in a recent JFP audiocast. Also receiving “D” recommendations were screening for ovarian cancer in asymptomatic women,6 discussed in another JFP audiocast, and screening for thyroid cancer in asymptomatic adults.7
The “D” recommendation for thyroid cancer screening was based on the low incidence of thyroid cancer, the evidence showing no change in mortality after the introduction of population-based screening, and the likelihood of overdiagnosis and overtreatment that would result from screening. The screening tests considered by the Task Force included neck palpation and ultrasound.7
Insufficient evidence
In addition to the previously mentioned “I” statement on vision screening for children <3 years of age,4 4 other interventions lacked sufficient evidence that the Task Force could use in determining relative levels of harms and benefits. These interventions were screening for obstructive sleep apnea in asymptomatic adults,8 screening for celiac disease in asymptomatic patients of all ages,9 screening with a pelvic examination in asymptomatic women,10 and screening for adolescent idiopathic scoliosis in children and adolescents ages 10 to 18 years.11
The lack of evidence regarding the value of a routine pelvic exam for asymptomatic women is surprising given how often this procedure is performed. The Task Force defined a pelvic exam as an “assessment of the external genitalia, internal speculum examination, bimanual palpation, and rectovaginal examination.”10 The Task Force found very little evidence on the accuracy and effectiveness of this exam for a range of gynecologic conditions other than cervical cancer, gonorrhea, and chlamydia, for which screening is recommended.10
The “I” statement on screening for adolescent idiopathic scoliosis in children and adolescents is an unusual revision of a “D” recommendation from 2004. At that time, the Task Force found that treatment of adolescent idiopathic scoliosis leads to health benefits in only a small proportion of individuals and that there are harms of treatment such as unnecessary bracing and referral to specialty care. For the most recent evidence report, the Task Force used a new methodology to assess treatment harms and concluded that the evidence is now inadequate. That finding, along with new evidence that “suggests that brace treatment can interrupt or slow scoliosis progression” led the Task Force to move away from a “D” recommendation.11
The enigmatic “C” recommendation
Perhaps the most difficult recommendation category to understand and implement is the “C” recommendation. With a “C” intervention, there is moderate certainty that the net benefit of universal implementation would be very small; but there are some individuals who might benefit from it, and physicians should offer it selectively.
The Task Force made one “C” recommendation over the past year: for adults who are not obese and who do not have other cardiovascular disease (CVD) risks, the net gain in referring them to behavioral counseling to promote a healthful diet and physical activity is small. However, the harms from such referrals are also small. Counseling interventions can result in healthier habits and in small improvements in intermediate outcomes, such as blood pressure, cholesterol levels, and weight. The effect on overall CVD mortality, though, has been minimal.12 The Task Force concluded that “[those] who are interested and ready to make behavioral changes may be most likely to benefit from behavioral counseling.”
1. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication. Accessed March 22, 2018.
2. USPSTF. Obesity in children and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/obesity-in-children-and-adolescents-screening1. Accessed March 22, 2018.
3. USPSTF. Preeclampsia: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/preeclampsia-screening1. Accessed March 22, 2018.
4. USPSTF. Vision in children ages 6 months to 5 years: Screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/vision-in-children-ages-6-months-to-5-years-screening. Accessed March 22, 2018.
5. USPSTF. Hormone therapy in postmenopausal women: primary prevention of chronic conditions. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/menopausal-hormone-therapy-preventive-medication1. Accessed March 24, 2018.
6. USPSTF. Ovarian cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/ovarian-cancer-screening1. Accessed March 24, 2018.
7. USPSTF. Thyroid cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/thyroid-cancer-screening1. Accessed March 22, 2018.
8. USPSTF. Obstructive sleep apnea in adults: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/obstructive-sleep-apnea-in-adults-screening. Accessed March 22, 2018.
9. USPSTF. Celiac disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/celiac-disease-screening. Accessed March 24, 2018.
10. USPSTF. Gynecological conditions: periodic screening with the pelvic examination. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/gynecological-conditions-screening-with-the-pelvic-examination. Accessed March 22, 2018.
11. USPSTF. Adolescent idiopathic scoliosis: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 22, 2018.
12. USPSTF. Healthful diet and physical activity for cardiovascular disease prevention in adults without known risk factors: behavioral counseling. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/healthful-diet-and-physical-activity-for-cardiovascular-disease-prevention-in-adults-without-known-risk-factors-behavioral-counseling. Accessed March 22, 2018.
13. Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ. 2013;347:f6564.
14. USPSTF. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication. Accessed March 22, 2018.
15. Donahue SP, Baker CN; Committee on Practice and Ambulatory Medicine, American Academy of Pediatrics; Section on Ophthalmology, American Academy of Pediatrics; American Association of Certified Orthoptists; American Association for Pediatric Ophthalmology and Strabismus; American Academy of Ophthalmology. Procedures for the evaluation of the visual system by pediatricians. Pediatrics. 2016;137.2015-3597.
Over the past year the US Preventive Services Task Force made 14 recommendations on 12 conditions (TABLE 11-12). One of these pronouncements was the unusual reversal of a previous “D” recommendation against screening for scoliosis in adolescents, changing it to an “I” statement (insufficient evidence).

Affirmative recommendations
Four interventions were given an “A” or “B” recommendation this past year. Both grades signify a recommendation to perform the service, with “A” reflecting a higher level of certainty or a higher level of net benefit than “B.”
Recommend folic acid to prevent neural tube defects (A)
The evidence is very strong that folic acid intake prevents neural tube defects. In 2009 the Task Force recommended folic acid supplementation for women of childbearing age. In 2017 this recommendation was updated and slightly reworded to advise that all women who are planning a pregnancy or capable of becoming pregnant take a daily supplement containing 0.4 mg to 0.8 mg (400-800 mcg) of folic acid.
In the United States many grain products have been fortified with folic acid since 1996. This step has reduced the prevalence of neural tube defects from 10.7 cases per 10,000 live births to 7 cases per 10,000 live births in 2011.1 However, in spite of food fortification, most women in the United States do not consume the recommended daily amount of 0.4 mg (400 mcg) of folic acid. This supplementation is most important from one month before conception through the first 3 months of pregnancy.
Screen for obesity in children and adolescents (B)
Nearly 17% of children and adolescents ages 2 to 19 years in the United States are obese, and almost 32% are overweight or obese.2 Obesity is defined as a body mass index (BMI) ≥95th percentile, based on year-2000 growth charts published by the Centers for Disease Control and Prevention. Overweight is defined as a BMI between the 85th and 94th percentiles.
Obesity in children and adolescents is associated with many physical problems, including obstructive sleep apnea, orthopedic problems, high blood pressure, hyperlipidemia, and diabetes, as well as psychological harms from being teased and bullied. Obesity that continues into adulthood is associated with diabetes, cardiovascular disease, and orthopedic problems.
The Task Force found that intensive behavioral interventions for obesity in children ≥6 years of age and in adolescents can lead to moderate improvements in weight status for up to 12 months. Intensive behavioral interventions need to include at least 26 contact hours over 2 to 12 months. The recommendation statement includes a more detailed description of the types of programs that have evidence to support them.2
The Task Force did not recommend the use of either metformin or orlistat because of inadequate evidence on the harmful effects of metformin and because of sound evidence that orlistat causes moderate harms, such as abdominal pain, cramping, incontinence, and flatus.
Screen for preeclampsia (B), but dipstick testing is unreliable
Preeclampsia occurs in a little more than 3% of pregnancies in the United States.13 For the mother, this condition can lead to stroke, eclampsia, organ failure, and death; for the fetus, intrauterine growth retardation, preterm birth, low birth weight, and still birth. Preeclampsia is a leading cause of maternal mortality worldwide. Adverse health outcomes can be prevented by early detection of preeclampsia and by managing it appropriately.3
In 1996 the Task Force recommended screening for preeclampsia during pregnancy, and it reaffirmed that recommendation last year. The Task Force recommends taking blood pressure measurements at every prenatal visit, but does not recommend testing for urine protein with a dipstick because of the technique’s low accuracy.
Since 2014 the Task Force has also recommended using low-dose aspirin after Week 12 of pregnancy to prevent preeclampsia in women who are at high risk.14
Conduct vision screening in all children ages 3 to 5 years (B)
One of the more nuanced recommendations involves vision screening in children. The Task Force recently reaffirmed its 2011 recommendation to perform vision screening at least once in all children ages 3 to 5 years to detect amblyopia or its risk factors. But it found insufficient evidence to test children <3 years of age.
Amblyopia is a “functional reduction in visual acuity characterized by abnormal processing of visual images; [it is] established by the brain during a critical period of vision development.”4 Risk factors associated with the development of amblyopia include strabismus (ocular misalignment); vision loss caused by cataracts; refractive errors such as near and far sightedness, astigmatism (“blurred vision at any distance due to abnormal curvature of the cornea or lens”); and anisometropia (“asymmetric refractive error between the … eyes that causes image suppression in the eye with the larger error”). 4
Physical exam- and machine-based screening tests are available in the primary care setting (TABLE 2).4

At first glance it appears that the Task Force recommends screening only for amblyopia, but the addition of “risk factors” implies a more comprehensive vision evaluation that would include visual acuity. This interpretation more closely aligns the Task Force recommendation with that of a joint report by the American Academy of Pediatrics, American Association for Pediatric Ophthalmology and Strabismus, American Academy of Certified Orthoptists, and American Academy of Ophthalmology, which recommends testing for a variety of vision problems in children.15 Nevertheless, the Task Force maintains that the evidence of benefit in testing more extensively before age 3 is insufficient, while the other organizations recommend starting testing at age 6 months.
Negative “D” recommendations
Equally as important as affirmative recommendations for effective interventions are the “D” recommendations advising against interventions that are ineffective or cause more harm than benefits. This past year, the Task Force recommended against 4 interventions. Two pertain to the use of estrogen or combined estrogen and progestin for the primary prevention of chronic conditions in postmenopausal women.5 This topic has been discussed in a recent JFP audiocast. Also receiving “D” recommendations were screening for ovarian cancer in asymptomatic women,6 discussed in another JFP audiocast, and screening for thyroid cancer in asymptomatic adults.7
The “D” recommendation for thyroid cancer screening was based on the low incidence of thyroid cancer, the evidence showing no change in mortality after the introduction of population-based screening, and the likelihood of overdiagnosis and overtreatment that would result from screening. The screening tests considered by the Task Force included neck palpation and ultrasound.7
Insufficient evidence
In addition to the previously mentioned “I” statement on vision screening for children <3 years of age,4 4 other interventions lacked sufficient evidence that the Task Force could use in determining relative levels of harms and benefits. These interventions were screening for obstructive sleep apnea in asymptomatic adults,8 screening for celiac disease in asymptomatic patients of all ages,9 screening with a pelvic examination in asymptomatic women,10 and screening for adolescent idiopathic scoliosis in children and adolescents ages 10 to 18 years.11
The lack of evidence regarding the value of a routine pelvic exam for asymptomatic women is surprising given how often this procedure is performed. The Task Force defined a pelvic exam as an “assessment of the external genitalia, internal speculum examination, bimanual palpation, and rectovaginal examination.”10 The Task Force found very little evidence on the accuracy and effectiveness of this exam for a range of gynecologic conditions other than cervical cancer, gonorrhea, and chlamydia, for which screening is recommended.10
The “I” statement on screening for adolescent idiopathic scoliosis in children and adolescents is an unusual revision of a “D” recommendation from 2004. At that time, the Task Force found that treatment of adolescent idiopathic scoliosis leads to health benefits in only a small proportion of individuals and that there are harms of treatment such as unnecessary bracing and referral to specialty care. For the most recent evidence report, the Task Force used a new methodology to assess treatment harms and concluded that the evidence is now inadequate. That finding, along with new evidence that “suggests that brace treatment can interrupt or slow scoliosis progression” led the Task Force to move away from a “D” recommendation.11
The enigmatic “C” recommendation
Perhaps the most difficult recommendation category to understand and implement is the “C” recommendation. With a “C” intervention, there is moderate certainty that the net benefit of universal implementation would be very small; but there are some individuals who might benefit from it, and physicians should offer it selectively.
The Task Force made one “C” recommendation over the past year: for adults who are not obese and who do not have other cardiovascular disease (CVD) risks, the net gain in referring them to behavioral counseling to promote a healthful diet and physical activity is small. However, the harms from such referrals are also small. Counseling interventions can result in healthier habits and in small improvements in intermediate outcomes, such as blood pressure, cholesterol levels, and weight. The effect on overall CVD mortality, though, has been minimal.12 The Task Force concluded that “[those] who are interested and ready to make behavioral changes may be most likely to benefit from behavioral counseling.”
Over the past year the US Preventive Services Task Force made 14 recommendations on 12 conditions (TABLE 11-12). One of these pronouncements was the unusual reversal of a previous “D” recommendation against screening for scoliosis in adolescents, changing it to an “I” statement (insufficient evidence).

Affirmative recommendations
Four interventions were given an “A” or “B” recommendation this past year. Both grades signify a recommendation to perform the service, with “A” reflecting a higher level of certainty or a higher level of net benefit than “B.”
Recommend folic acid to prevent neural tube defects (A)
The evidence is very strong that folic acid intake prevents neural tube defects. In 2009 the Task Force recommended folic acid supplementation for women of childbearing age. In 2017 this recommendation was updated and slightly reworded to advise that all women who are planning a pregnancy or capable of becoming pregnant take a daily supplement containing 0.4 mg to 0.8 mg (400-800 mcg) of folic acid.
In the United States many grain products have been fortified with folic acid since 1996. This step has reduced the prevalence of neural tube defects from 10.7 cases per 10,000 live births to 7 cases per 10,000 live births in 2011.1 However, in spite of food fortification, most women in the United States do not consume the recommended daily amount of 0.4 mg (400 mcg) of folic acid. This supplementation is most important from one month before conception through the first 3 months of pregnancy.
Screen for obesity in children and adolescents (B)
Nearly 17% of children and adolescents ages 2 to 19 years in the United States are obese, and almost 32% are overweight or obese.2 Obesity is defined as a body mass index (BMI) ≥95th percentile, based on year-2000 growth charts published by the Centers for Disease Control and Prevention. Overweight is defined as a BMI between the 85th and 94th percentiles.
Obesity in children and adolescents is associated with many physical problems, including obstructive sleep apnea, orthopedic problems, high blood pressure, hyperlipidemia, and diabetes, as well as psychological harms from being teased and bullied. Obesity that continues into adulthood is associated with diabetes, cardiovascular disease, and orthopedic problems.
The Task Force found that intensive behavioral interventions for obesity in children ≥6 years of age and in adolescents can lead to moderate improvements in weight status for up to 12 months. Intensive behavioral interventions need to include at least 26 contact hours over 2 to 12 months. The recommendation statement includes a more detailed description of the types of programs that have evidence to support them.2
The Task Force did not recommend the use of either metformin or orlistat because of inadequate evidence on the harmful effects of metformin and because of sound evidence that orlistat causes moderate harms, such as abdominal pain, cramping, incontinence, and flatus.
Screen for preeclampsia (B), but dipstick testing is unreliable
Preeclampsia occurs in a little more than 3% of pregnancies in the United States.13 For the mother, this condition can lead to stroke, eclampsia, organ failure, and death; for the fetus, intrauterine growth retardation, preterm birth, low birth weight, and still birth. Preeclampsia is a leading cause of maternal mortality worldwide. Adverse health outcomes can be prevented by early detection of preeclampsia and by managing it appropriately.3
In 1996 the Task Force recommended screening for preeclampsia during pregnancy, and it reaffirmed that recommendation last year. The Task Force recommends taking blood pressure measurements at every prenatal visit, but does not recommend testing for urine protein with a dipstick because of the technique’s low accuracy.
Since 2014 the Task Force has also recommended using low-dose aspirin after Week 12 of pregnancy to prevent preeclampsia in women who are at high risk.14
Conduct vision screening in all children ages 3 to 5 years (B)
One of the more nuanced recommendations involves vision screening in children. The Task Force recently reaffirmed its 2011 recommendation to perform vision screening at least once in all children ages 3 to 5 years to detect amblyopia or its risk factors. But it found insufficient evidence to test children <3 years of age.
Amblyopia is a “functional reduction in visual acuity characterized by abnormal processing of visual images; [it is] established by the brain during a critical period of vision development.”4 Risk factors associated with the development of amblyopia include strabismus (ocular misalignment); vision loss caused by cataracts; refractive errors such as near and far sightedness, astigmatism (“blurred vision at any distance due to abnormal curvature of the cornea or lens”); and anisometropia (“asymmetric refractive error between the … eyes that causes image suppression in the eye with the larger error”). 4
Physical exam- and machine-based screening tests are available in the primary care setting (TABLE 2).4

At first glance it appears that the Task Force recommends screening only for amblyopia, but the addition of “risk factors” implies a more comprehensive vision evaluation that would include visual acuity. This interpretation more closely aligns the Task Force recommendation with that of a joint report by the American Academy of Pediatrics, American Association for Pediatric Ophthalmology and Strabismus, American Academy of Certified Orthoptists, and American Academy of Ophthalmology, which recommends testing for a variety of vision problems in children.15 Nevertheless, the Task Force maintains that the evidence of benefit in testing more extensively before age 3 is insufficient, while the other organizations recommend starting testing at age 6 months.
Negative “D” recommendations
Equally as important as affirmative recommendations for effective interventions are the “D” recommendations advising against interventions that are ineffective or cause more harm than benefits. This past year, the Task Force recommended against 4 interventions. Two pertain to the use of estrogen or combined estrogen and progestin for the primary prevention of chronic conditions in postmenopausal women.5 This topic has been discussed in a recent JFP audiocast. Also receiving “D” recommendations were screening for ovarian cancer in asymptomatic women,6 discussed in another JFP audiocast, and screening for thyroid cancer in asymptomatic adults.7
The “D” recommendation for thyroid cancer screening was based on the low incidence of thyroid cancer, the evidence showing no change in mortality after the introduction of population-based screening, and the likelihood of overdiagnosis and overtreatment that would result from screening. The screening tests considered by the Task Force included neck palpation and ultrasound.7
Insufficient evidence
In addition to the previously mentioned “I” statement on vision screening for children <3 years of age,4 4 other interventions lacked sufficient evidence that the Task Force could use in determining relative levels of harms and benefits. These interventions were screening for obstructive sleep apnea in asymptomatic adults,8 screening for celiac disease in asymptomatic patients of all ages,9 screening with a pelvic examination in asymptomatic women,10 and screening for adolescent idiopathic scoliosis in children and adolescents ages 10 to 18 years.11
The lack of evidence regarding the value of a routine pelvic exam for asymptomatic women is surprising given how often this procedure is performed. The Task Force defined a pelvic exam as an “assessment of the external genitalia, internal speculum examination, bimanual palpation, and rectovaginal examination.”10 The Task Force found very little evidence on the accuracy and effectiveness of this exam for a range of gynecologic conditions other than cervical cancer, gonorrhea, and chlamydia, for which screening is recommended.10
The “I” statement on screening for adolescent idiopathic scoliosis in children and adolescents is an unusual revision of a “D” recommendation from 2004. At that time, the Task Force found that treatment of adolescent idiopathic scoliosis leads to health benefits in only a small proportion of individuals and that there are harms of treatment such as unnecessary bracing and referral to specialty care. For the most recent evidence report, the Task Force used a new methodology to assess treatment harms and concluded that the evidence is now inadequate. That finding, along with new evidence that “suggests that brace treatment can interrupt or slow scoliosis progression” led the Task Force to move away from a “D” recommendation.11
The enigmatic “C” recommendation
Perhaps the most difficult recommendation category to understand and implement is the “C” recommendation. With a “C” intervention, there is moderate certainty that the net benefit of universal implementation would be very small; but there are some individuals who might benefit from it, and physicians should offer it selectively.
The Task Force made one “C” recommendation over the past year: for adults who are not obese and who do not have other cardiovascular disease (CVD) risks, the net gain in referring them to behavioral counseling to promote a healthful diet and physical activity is small. However, the harms from such referrals are also small. Counseling interventions can result in healthier habits and in small improvements in intermediate outcomes, such as blood pressure, cholesterol levels, and weight. The effect on overall CVD mortality, though, has been minimal.12 The Task Force concluded that “[those] who are interested and ready to make behavioral changes may be most likely to benefit from behavioral counseling.”
1. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication. Accessed March 22, 2018.
2. USPSTF. Obesity in children and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/obesity-in-children-and-adolescents-screening1. Accessed March 22, 2018.
3. USPSTF. Preeclampsia: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/preeclampsia-screening1. Accessed March 22, 2018.
4. USPSTF. Vision in children ages 6 months to 5 years: Screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/vision-in-children-ages-6-months-to-5-years-screening. Accessed March 22, 2018.
5. USPSTF. Hormone therapy in postmenopausal women: primary prevention of chronic conditions. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/menopausal-hormone-therapy-preventive-medication1. Accessed March 24, 2018.
6. USPSTF. Ovarian cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/ovarian-cancer-screening1. Accessed March 24, 2018.
7. USPSTF. Thyroid cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/thyroid-cancer-screening1. Accessed March 22, 2018.
8. USPSTF. Obstructive sleep apnea in adults: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/obstructive-sleep-apnea-in-adults-screening. Accessed March 22, 2018.
9. USPSTF. Celiac disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/celiac-disease-screening. Accessed March 24, 2018.
10. USPSTF. Gynecological conditions: periodic screening with the pelvic examination. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/gynecological-conditions-screening-with-the-pelvic-examination. Accessed March 22, 2018.
11. USPSTF. Adolescent idiopathic scoliosis: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 22, 2018.
12. USPSTF. Healthful diet and physical activity for cardiovascular disease prevention in adults without known risk factors: behavioral counseling. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/healthful-diet-and-physical-activity-for-cardiovascular-disease-prevention-in-adults-without-known-risk-factors-behavioral-counseling. Accessed March 22, 2018.
13. Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ. 2013;347:f6564.
14. USPSTF. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication. Accessed March 22, 2018.
15. Donahue SP, Baker CN; Committee on Practice and Ambulatory Medicine, American Academy of Pediatrics; Section on Ophthalmology, American Academy of Pediatrics; American Association of Certified Orthoptists; American Association for Pediatric Ophthalmology and Strabismus; American Academy of Ophthalmology. Procedures for the evaluation of the visual system by pediatricians. Pediatrics. 2016;137.2015-3597.
1. USPSTF. Folic acid for the prevention of neural tube defects: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication. Accessed March 22, 2018.
2. USPSTF. Obesity in children and adolescents: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/obesity-in-children-and-adolescents-screening1. Accessed March 22, 2018.
3. USPSTF. Preeclampsia: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/preeclampsia-screening1. Accessed March 22, 2018.
4. USPSTF. Vision in children ages 6 months to 5 years: Screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/vision-in-children-ages-6-months-to-5-years-screening. Accessed March 22, 2018.
5. USPSTF. Hormone therapy in postmenopausal women: primary prevention of chronic conditions. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/menopausal-hormone-therapy-preventive-medication1. Accessed March 24, 2018.
6. USPSTF. Ovarian cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/ovarian-cancer-screening1. Accessed March 24, 2018.
7. USPSTF. Thyroid cancer: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/thyroid-cancer-screening1. Accessed March 22, 2018.
8. USPSTF. Obstructive sleep apnea in adults: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/obstructive-sleep-apnea-in-adults-screening. Accessed March 22, 2018.
9. USPSTF. Celiac disease: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/celiac-disease-screening. Accessed March 24, 2018.
10. USPSTF. Gynecological conditions: periodic screening with the pelvic examination. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/gynecological-conditions-screening-with-the-pelvic-examination. Accessed March 22, 2018.
11. USPSTF. Adolescent idiopathic scoliosis: screening. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/adolescent-idiopathic-scoliosis-screening1. Accessed March 22, 2018.
12. USPSTF. Healthful diet and physical activity for cardiovascular disease prevention in adults without known risk factors: behavioral counseling. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/healthful-diet-and-physical-activity-for-cardiovascular-disease-prevention-in-adults-without-known-risk-factors-behavioral-counseling. Accessed March 22, 2018.
13. Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ. 2013;347:f6564.
14. USPSTF. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: preventive medication. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/low-dose-aspirin-use-for-the-prevention-of-morbidity-and-mortality-from-preeclampsia-preventive-medication. Accessed March 22, 2018.
15. Donahue SP, Baker CN; Committee on Practice and Ambulatory Medicine, American Academy of Pediatrics; Section on Ophthalmology, American Academy of Pediatrics; American Association of Certified Orthoptists; American Association for Pediatric Ophthalmology and Strabismus; American Academy of Ophthalmology. Procedures for the evaluation of the visual system by pediatricians. Pediatrics. 2016;137.2015-3597.
From The Journal of Family Practice | 2018;67(5):294-296,298-299.
Biopsies for skin cancer detection: Dispelling the myths
Once it’s determined that a growth requires a biopsy, there is often uncertainty about which type of biopsy to perform. Insufficient knowledge of, and/or experience with, the various biopsy modalities may deter FPs from performing skin biopsies when they are indicated. To help fill the knowledge gaps and better position FPs to tackle skin cancer in its earliest stages, this article identifies and dispels 5 of the most common myths surrounding skin biopsies for the detection of basal and squamous cell carcinoma and melanoma.
MYTH #1
A punch biopsy is always preferred for suspected melanoma because it gets full depth.
A deep shave biopsy (saucerization)—not a punch biopsy—is usually the procedure of choice when biopsying a lesion suspected to be melanoma.2 The National Comprehensive Cancer Network (NCCN) "Melanoma Clinical Practice Guidelines in Oncology" state that an excisional biopsy (elliptical, punch, or saucerization) with a 1- to 3-mm margin is the preferred method of biopsy for suspected melanoma.3 However, a punch biopsy should be performed only if a 1- to 3-mm margin all around a suspected melanoma can be obtained. Otherwise, a saucerization or elliptical excision is preferred.3
The saucerization technique generally permits optimal sampling in terms of both the breadth and depth of the growth, providing the pathologist with sufficient tissue from both the epidermis and dermis (FIGURE 1).
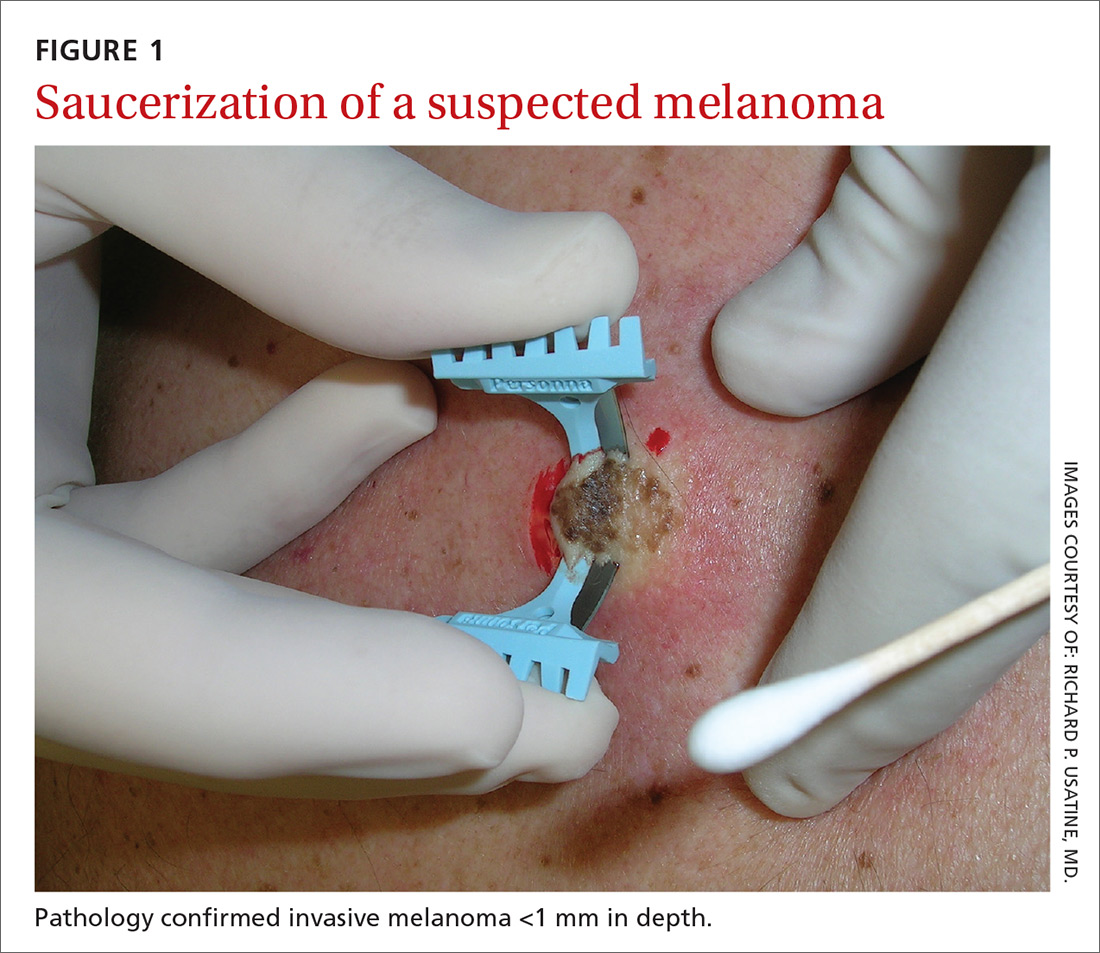
Why are breadth/depth important? Breadth is important because showing the pathologist the epidermis (especially the edge) of a suspected melanocytic tumor allows for detection of pagetoid spread (upward movement through the epidermis) of melanocytes and of single melanocytes at the edge of a tumor. Single melanocytes at the edge of a tumor and pagetoid spread are histologic features of melanoma that help to distinguish these lesions from nevi, which tend to have nested melanocytes.2
Depth is important because it predicts prognosis and impacts management. For tumors 0.8 mm to 1 mm deep, a sentinel lymph node biopsy (SLNB) should be considered.3,4 Although the tumor depth threshold for a SLNB is still debated, most skin cancer experts in the United States agree that a melanoma thicker than 1 mm qualifies for this procedure. Some melanomas with high-risk features (such as ulceration) qualify for an SNLB even if they are <1 mm in depth.5 An SLNB provides prognostic information, and a positive SLNB directly affects staging.
[polldaddy:9990508]
Avoid partial biopsies. For tumors that have been partially biopsied with a punch or shallow shave biopsy, evaluation of the remaining neoplasm after subsequent excision leads to tumor upstaging in 21% of patients, with 10% qualifying for an SLNB.6 Thus, the goal should always be to obtain the entire depth of the tumor with the initial biopsy.
In addition, surgical margins are determined by primary tumor depth. To ensure a depth greater than 1 mm, aim to obtain a tissue specimen that is at least as thick as a dime (1.3 mm).
Because the goal is to avoid partial sampling, a challenge exists when the suspicious growth is large. Many melanomas are broader than a centimeter. And while punch biopsies ensure a depth of 1 mm or more, they risk missing the thickest portion of the tumor.7
Partial sampling of large melanocytic tumors with punch biopsies can lead to sampling error.8 Ng et al9 found there was a significant increase in histopathologic misdiagnosis with a punch biopsy of part of a melanoma (odds ratio [OR]=16.6; 95% confidence interval [CI], 10-27; P<.001) and with shallow shave biopsy (OR=2.6; 95% CI, 1.2-5.7; P=.02) compared with excisional biopsy (including saucerization).9 Punch biopsy of part of a melanoma was also associated with increased odds of misdiagnosis with an adverse outcome (OR=20; 95% CI, 10-41; P<.001).
Punch biopsies do, however, offer a reasonable alternative when the melanoma is too broad for a complete saucerization. In these cases, consider multiple 4- to 6-mm punch biopsies to reduce the risk of sampling error.
Avoid performing punch biopsies <4 mm, as the breadth of tissue is inadequate. For example, even with dermoscopy, facial lentigo maligna melanoma is often difficult to differentiate from pigmented actinic keratosis and solar lentigines. (See JFP’s Watch and Learn Video on dermoscopy.) A broad shave biopsy is the preferred method of biopsy for lentigo maligna melanoma in situ according to the NCCN.3 And there have been several reports showing that the results of shave biopsies of melanocytic lesions are cosmetically acceptable to patients.10,11
If the biopsy confirms malignancy, a larger surgery with suturing will be needed. The most important issue to keep in mind is that if partial sampling leads to a benign diagnosis of a suspicious lesion, then the remainder of the lesion must be excised and sent for pathology.
Saucerization is also the preferred biopsy type for basal cell and squamous cell carcinomas (SCCs). Studies have shown tumor depth is the most important factor in predicting metastasis of SCC, as well as tumor relapse rate, making accurate identification of the depth of the tumor important for both management and prognosis.12,13 Determining the thickness of the SCC is important for guiding management. SCC in situ is more amenable than invasive SCC to topical therapy or electrodesiccation and curettage.
What you’ll need. FPs can perform saucerization quickly and easily in the office during a standard 15-minute visit. Of course, it is essential to have all the necessary materials available. The key materials needed are lidocaine and epinephrine, a sharp razor blade such as a DermaBlade, and something for hemostasis (aluminum chloride and/or an electrosurgical instrument). Cotton-tipped applicators to apply the aluminum chloride and needles and syringes to administer the local anesthetic are also needed. (See JFP’s Watch and Learn Video on shave biopsy.) A quick saucerization eliminates the need for the patient to return for an elliptical excision and prevents a delayed diagnosis that can occur as a result of a long wait to see a dermatologist.
As a final note, the pathology order form should be completed with information on biopsy type, clinical presentation, differential diagnosis, and whether or not the full lesion was excised.
MYTH #2
A wide excisional biopsy is required for a suspected melanoma.
While complete excision of the entire tumor does allow the pathologist to evaluate the entire growth, wide (>3 mm) margins on the initial biopsy are not necessary. In fact, there are potential disadvantages to full excisional biopsy.
For example, seborrheic keratoses and other benign growths can mimic melanoma. Neither the physician nor the patient wants to learn that a large elliptical wound was created for a growth that turned out to be a benign seborrheic keratosis. Saucerization provides the pathologist with the entire lesion, and the resulting shallow wound heals as a round scar that is most often acceptable to patients.10,11,14 In addition, excisional biopsies carry a higher risk of infection than does saucerization.
Even when the index of suspicion is high for melanoma, a wide margin is not indicated. NCCN guidelines suggest that the margins around a suspected melanoma on initial biopsy not exceed 3 mm to avoid disrupting the accuracy of an SLNB (FIGURE 2).3
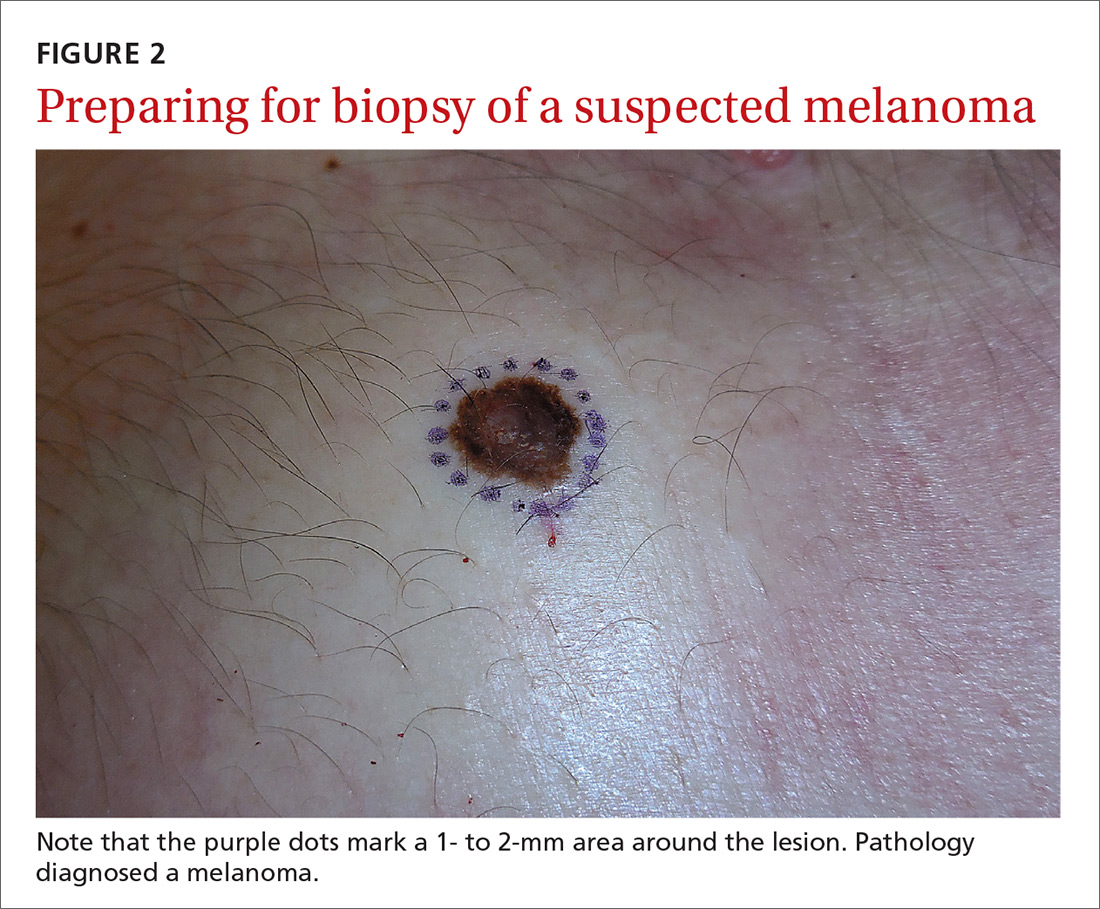
In addition, time constraints (elliptical excisional biopsies can take up to one hour, especially when a layered closure is performed) and a lack of surgical training may prohibit FPs from performing excisions.
One study found that while dermatologists prefer shave biopsies (80.5%), surgeons prefer excisional biopsies (46.3%) and primary care physicians prefer punch biopsies (44%) for biopsy of a growth suspicious for melanoma.7 In fact, of the biopsies FPs perform, only 29% are of the shave variety.7
However, deep shave biopsies can be performed quickly, with the whole process taking less than 5 minutes. We advocate performing them at the time of presentation, as the evidence shows that deep shave biopsies of suspected melanoma are reliable and accurate in 97% of cases.15
MYTH #3
A partial biopsy can make the cancer spread.
There is no evidence to support that a partial biopsy has any effect on the local recurrence or metastatic potential of malignant melanoma.16 In fact, a biopsy elicits an inflammatory response that activates the patient’s immune system and often causes tumor lysis. Some tumors may even resolve after biopsy. In our clinical practice, we have had several cases of basal cell carcinoma resolve after a biopsy without additional treatment.
MYTH #4
If after performing a deep shave biopsy, tumor or pigment remains, you must leave it because a second biopsy specimen can’t be added to the first.
If pigment is visible after an initial shave or punch biopsy, it is reasonable to obtain additional tissue from the base of the biopsy site. While the deeper tissue cannot be added to the initial specimen for the purposes of Breslow’s depth, it is still helpful for the pathologist to have the sample so that he or she can analyze the tumor cells in the dermis. (Melanoma tumor depth is measured as the maximum distance between malignant cells and the top of the granular layer.17) In these situations, be sure to let the pathologist know that there are 2 specimens in the container.
In general, it is valuable to get as much of the tissue as possible at the time of the initial biopsy. One way to avoid leaving tumor at the base of the biopsy is to look at
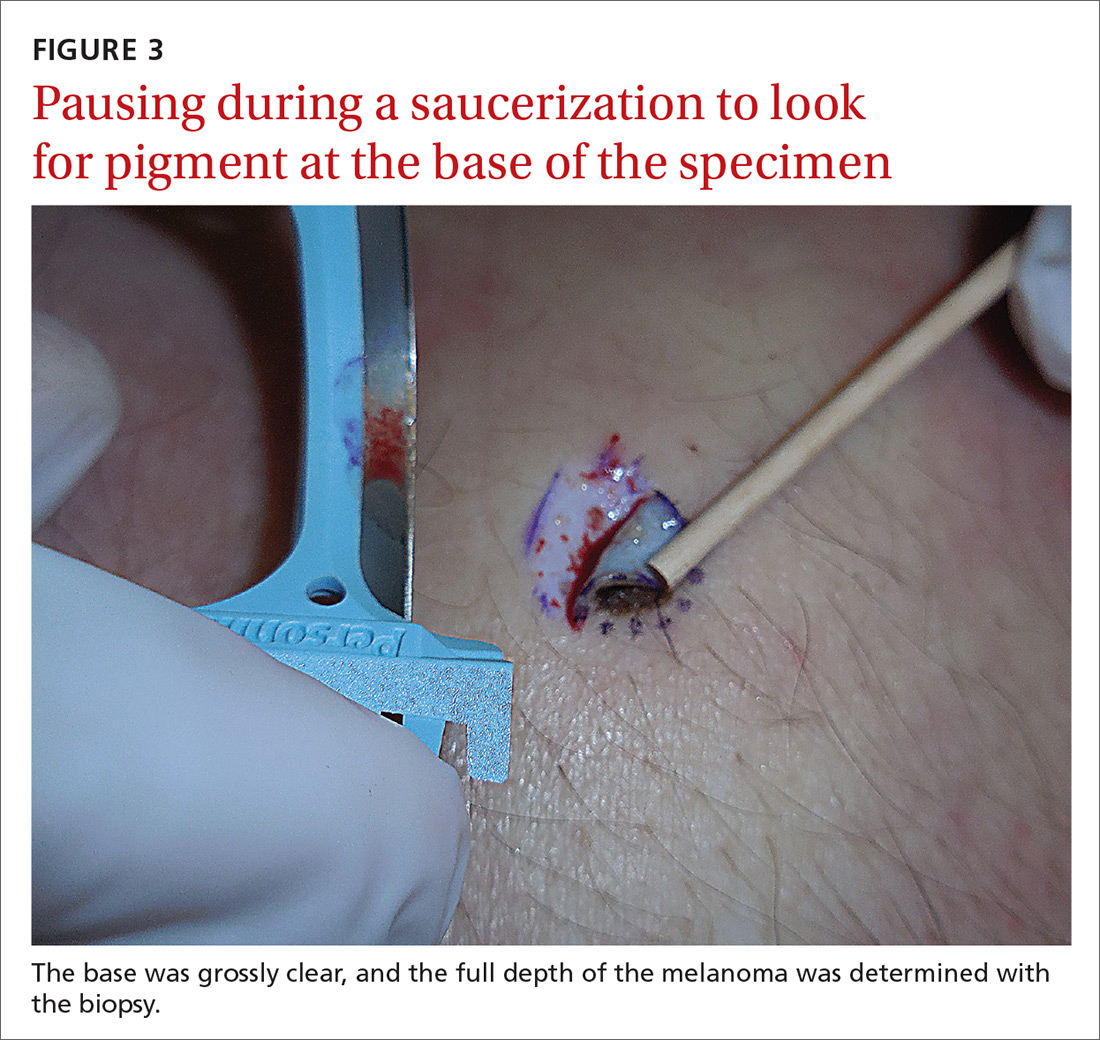
MYTH #5
Epinephrine cannot be used for biopsies on the fingers, toes, nose, or penis.
Lidocaine with epinephrine is safe to use in areas with end-arteries, such as the fingers, toes, nose (FIGURE 4), and penis. There is no evidence to support the notion that local anesthesia with vasoconstriction can cause necrosis in these areas, and no case of necrosis has been reported since the introduction of commercial lidocaine with epinephrine in 1948.18
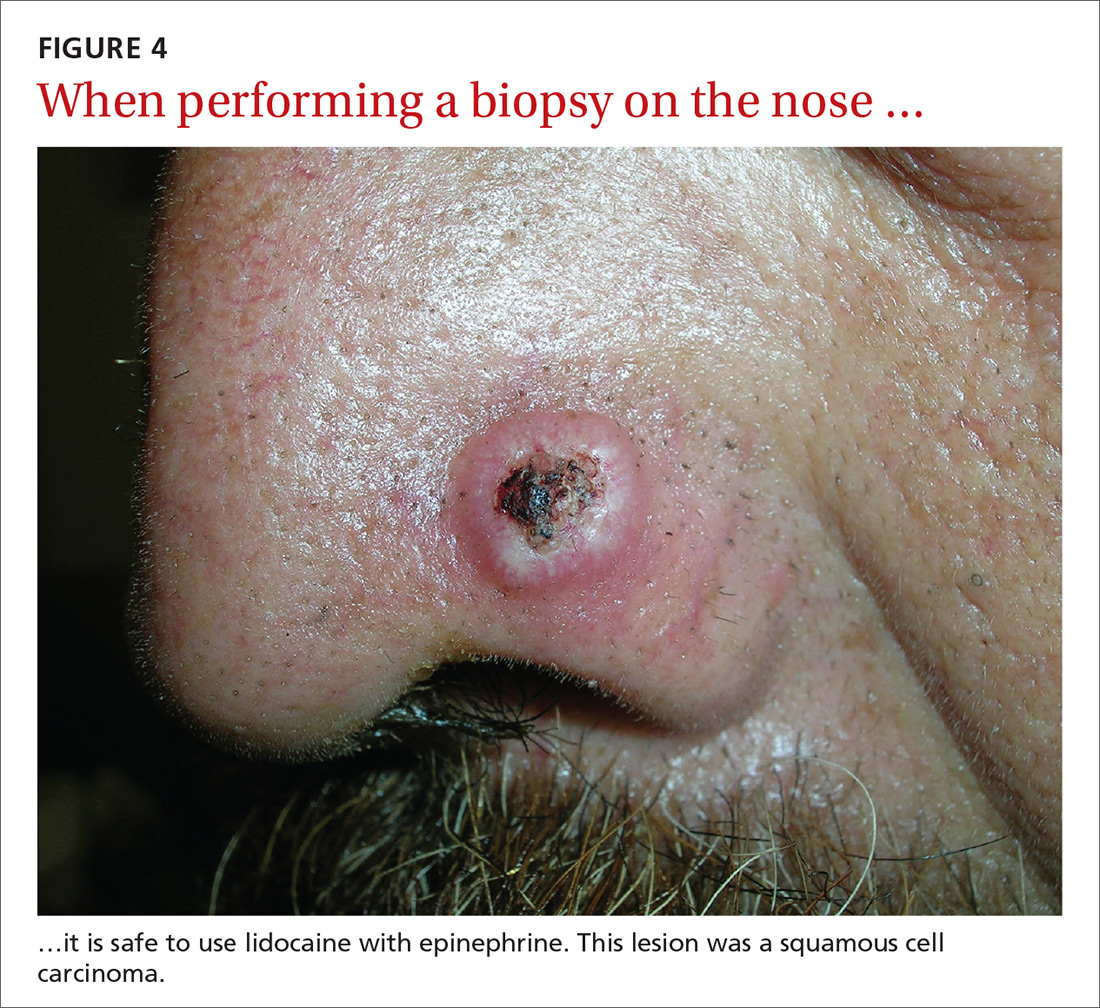
In addition to an absence of complications, epinephrine supplementation results in a relatively bloodless operating field and longer effectiveness of local anesthesia, as a study of more than 10,000 ear and nose surgeries using epinephrine-supplemented local anesthetics showed.19 The relative absence of blood in the operating field significantly reduces the duration of surgery and increases the healing rate because less electrocautery is needed.19
Similarly, the addition of epinephrine in digital blocks minimizes the need for tourniquets and large volumes of anesthetic and provides better and longer pain control during procedures.20 This topic was addressed by Prabhakar et al in a Cochrane Review in 2015.21 While digital surgeries are common, there were only 4 randomized controlled studies addressing the use of epinephrine in digital blocks. In these studies, there were no reports of adverse events, such as ischemia distal to the injection site. Evidence suggests that epinephrine in digital blocks can even be used safely in patients with vascular disease.22
And while the use of epinephrine with lidocaine in sites with end-arteries is beneficial for hemostasis and does not seem to pose a risk of ischemia, it is prudent to use the smallest volume of epinephrine (with lidocaine) needed to achieve anesthesia for the site.
CORRESPONDENCE
Elizabeth V. Seiverling, MD, 300 Southborough Drive, Suite 201, South Portland, ME 04106; [email protected].
1. Kerr OA, Tidman MJ, Walker JJ, et al. The profile of dermatological problems in primary care. Clin Exp Dermatol. 2010;35:380-383.
2. Hosler GA, Patterson JW. Lentigines, nevi, and melanomas. In: Patterson JW, ed. Weedon’s Skin Pathology. Elsevier; 2015:32,837-901.
3. Coit DG, Andtbacka R, Bichakjian CK, et al. Melanoma. J Natl Compr Canc Netw. 2009;7:250-275.
4. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the skin. In: Amin MB, Edge S, Greene F, et al, eds. AJCC Cancer Staging Manual. Springer International Publishing; 2017;8:563-585.
5. American Joint Committee on Cancer. Implementation of AJCC 8th Edition Cancer Staging System. Available at: https://cancerstaging.org/About/news/Pages/Implementation-of-AJCC-8th-Edition-Cancer-Staging-System.aspx. Accessed April 2, 2018.
6. Karimipour DJ, Schwartz JL, Wang TS, et al. Microstaging accuracy after subtotal incisional biopsy of cutaneous melanoma. J Am Acad Dermatol. 2005;52:798-802.
7. Kaiser S, Vassell R, Pinckney RG, et al. Clinical impact of biopsy method on the quality of surgical management in melanoma. J Surg Oncol. 2014;109:775-779.
8. Montgomery BD, Sadler GM. Punch biopsy of pigmented lesions is potentially hazardous. Can Fam Physician. 2009;55:24.
9. Ng JC, Swain S, Dowling JP, et al. The impact of partial biopsy on histopathologic diagnosis of cutaneous melanoma: experience of an Australian tertiary referral service. Arch Dermatol. 2010;146:234-239.
10. Gambichler T, Senger E, Rapp S, et al. Deep shave excision of macular melanocytic nevi with the razor blade biopsy technique. Dermatol Surg. 2000;26:662-666.
11. Ferrandiz L, Moreno-Ramirez D, Camacho FM. Shave excision of common acquired melanocytic nevi: cosmetic outcome, recurrences, and complications. Dermatol Surg. 2005;31(9 Pt 1):1112-1115.
12. D'souza G, Carey TE, William WN Jr, et al. Epidemiology of head and neck squamous cell cancer among HIV-infected patients. J Acquir Immune Defic Syndr. 2014;65:603-610.
13. Edge SB, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Handbook. 7th ed. New York, NY: Springer; 2010.
14. Elston DM, Stratman EJ, Miller SJ, et al. Skin biopsy. J Am Acad Dermatol. 2016;74:1-16.
15. Zager JS, Hochwald SN, Marzban SS, et al. Shave biopsy is a safe and accurate method for the initial evaluation of melanoma. J Am Coll Surg. 2011;212:454-460.
16. Chanda JJ, Callen JP. Adverse effect of melanoma incision. J Am Acad Dermatol. 1985;13:519-522.
17. Noroozi N, Zakerolhosseini A. Computerized measurement of melanocytic tumor depth in skin histopathological images. Micron. 2015;77:44-56.
18. Nielsen LJ, Lumholt P, Hölmich LR. [Local anaesthesia with vasoconstrictor is safe to use in areas with end-arteries in fingers, toes, noses and ears]. Ugeskr Laeger. 2014;176(44).
19. Häfner HM, Röcken M, Breuninger H. Epinephrine-supplemented local anesthetics for ear and nose surgery: clinical use without complications in more than 10,000 surgical procedures. J Dtsch Dermatol Ges. 2005;3:195-199.
20. Krunic AL, Wang LC, Soltani K, et al. Digital anesthesia with epinephrine: an old myth revisited. J Am Acad Dermatol. 2004;51:755-759.
21. Prabhakar H, Rath S, Kalaivani M, et al. Adrenaline with lidocaine for digital nerve blocks. Cochrane Database Syst Rev. 2015;(3):CD010645.
22. Ilicki J. Safety of epinephrine in digital nerve blocks: a literature review. J Emerg Med. 2015;49:799-809.
Once it’s determined that a growth requires a biopsy, there is often uncertainty about which type of biopsy to perform. Insufficient knowledge of, and/or experience with, the various biopsy modalities may deter FPs from performing skin biopsies when they are indicated. To help fill the knowledge gaps and better position FPs to tackle skin cancer in its earliest stages, this article identifies and dispels 5 of the most common myths surrounding skin biopsies for the detection of basal and squamous cell carcinoma and melanoma.
MYTH #1
A punch biopsy is always preferred for suspected melanoma because it gets full depth.
A deep shave biopsy (saucerization)—not a punch biopsy—is usually the procedure of choice when biopsying a lesion suspected to be melanoma.2 The National Comprehensive Cancer Network (NCCN) "Melanoma Clinical Practice Guidelines in Oncology" state that an excisional biopsy (elliptical, punch, or saucerization) with a 1- to 3-mm margin is the preferred method of biopsy for suspected melanoma.3 However, a punch biopsy should be performed only if a 1- to 3-mm margin all around a suspected melanoma can be obtained. Otherwise, a saucerization or elliptical excision is preferred.3
The saucerization technique generally permits optimal sampling in terms of both the breadth and depth of the growth, providing the pathologist with sufficient tissue from both the epidermis and dermis (FIGURE 1).

Why are breadth/depth important? Breadth is important because showing the pathologist the epidermis (especially the edge) of a suspected melanocytic tumor allows for detection of pagetoid spread (upward movement through the epidermis) of melanocytes and of single melanocytes at the edge of a tumor. Single melanocytes at the edge of a tumor and pagetoid spread are histologic features of melanoma that help to distinguish these lesions from nevi, which tend to have nested melanocytes.2
Depth is important because it predicts prognosis and impacts management. For tumors 0.8 mm to 1 mm deep, a sentinel lymph node biopsy (SLNB) should be considered.3,4 Although the tumor depth threshold for a SLNB is still debated, most skin cancer experts in the United States agree that a melanoma thicker than 1 mm qualifies for this procedure. Some melanomas with high-risk features (such as ulceration) qualify for an SNLB even if they are <1 mm in depth.5 An SLNB provides prognostic information, and a positive SLNB directly affects staging.
[polldaddy:9990508]
Avoid partial biopsies. For tumors that have been partially biopsied with a punch or shallow shave biopsy, evaluation of the remaining neoplasm after subsequent excision leads to tumor upstaging in 21% of patients, with 10% qualifying for an SLNB.6 Thus, the goal should always be to obtain the entire depth of the tumor with the initial biopsy.
In addition, surgical margins are determined by primary tumor depth. To ensure a depth greater than 1 mm, aim to obtain a tissue specimen that is at least as thick as a dime (1.3 mm).
Because the goal is to avoid partial sampling, a challenge exists when the suspicious growth is large. Many melanomas are broader than a centimeter. And while punch biopsies ensure a depth of 1 mm or more, they risk missing the thickest portion of the tumor.7
Partial sampling of large melanocytic tumors with punch biopsies can lead to sampling error.8 Ng et al9 found there was a significant increase in histopathologic misdiagnosis with a punch biopsy of part of a melanoma (odds ratio [OR]=16.6; 95% confidence interval [CI], 10-27; P<.001) and with shallow shave biopsy (OR=2.6; 95% CI, 1.2-5.7; P=.02) compared with excisional biopsy (including saucerization).9 Punch biopsy of part of a melanoma was also associated with increased odds of misdiagnosis with an adverse outcome (OR=20; 95% CI, 10-41; P<.001).
Punch biopsies do, however, offer a reasonable alternative when the melanoma is too broad for a complete saucerization. In these cases, consider multiple 4- to 6-mm punch biopsies to reduce the risk of sampling error.
Avoid performing punch biopsies <4 mm, as the breadth of tissue is inadequate. For example, even with dermoscopy, facial lentigo maligna melanoma is often difficult to differentiate from pigmented actinic keratosis and solar lentigines. (See JFP’s Watch and Learn Video on dermoscopy.) A broad shave biopsy is the preferred method of biopsy for lentigo maligna melanoma in situ according to the NCCN.3 And there have been several reports showing that the results of shave biopsies of melanocytic lesions are cosmetically acceptable to patients.10,11
If the biopsy confirms malignancy, a larger surgery with suturing will be needed. The most important issue to keep in mind is that if partial sampling leads to a benign diagnosis of a suspicious lesion, then the remainder of the lesion must be excised and sent for pathology.
Saucerization is also the preferred biopsy type for basal cell and squamous cell carcinomas (SCCs). Studies have shown tumor depth is the most important factor in predicting metastasis of SCC, as well as tumor relapse rate, making accurate identification of the depth of the tumor important for both management and prognosis.12,13 Determining the thickness of the SCC is important for guiding management. SCC in situ is more amenable than invasive SCC to topical therapy or electrodesiccation and curettage.
What you’ll need. FPs can perform saucerization quickly and easily in the office during a standard 15-minute visit. Of course, it is essential to have all the necessary materials available. The key materials needed are lidocaine and epinephrine, a sharp razor blade such as a DermaBlade, and something for hemostasis (aluminum chloride and/or an electrosurgical instrument). Cotton-tipped applicators to apply the aluminum chloride and needles and syringes to administer the local anesthetic are also needed. (See JFP’s Watch and Learn Video on shave biopsy.) A quick saucerization eliminates the need for the patient to return for an elliptical excision and prevents a delayed diagnosis that can occur as a result of a long wait to see a dermatologist.
As a final note, the pathology order form should be completed with information on biopsy type, clinical presentation, differential diagnosis, and whether or not the full lesion was excised.
MYTH #2
A wide excisional biopsy is required for a suspected melanoma.
While complete excision of the entire tumor does allow the pathologist to evaluate the entire growth, wide (>3 mm) margins on the initial biopsy are not necessary. In fact, there are potential disadvantages to full excisional biopsy.
For example, seborrheic keratoses and other benign growths can mimic melanoma. Neither the physician nor the patient wants to learn that a large elliptical wound was created for a growth that turned out to be a benign seborrheic keratosis. Saucerization provides the pathologist with the entire lesion, and the resulting shallow wound heals as a round scar that is most often acceptable to patients.10,11,14 In addition, excisional biopsies carry a higher risk of infection than does saucerization.
Even when the index of suspicion is high for melanoma, a wide margin is not indicated. NCCN guidelines suggest that the margins around a suspected melanoma on initial biopsy not exceed 3 mm to avoid disrupting the accuracy of an SLNB (FIGURE 2).3

In addition, time constraints (elliptical excisional biopsies can take up to one hour, especially when a layered closure is performed) and a lack of surgical training may prohibit FPs from performing excisions.
One study found that while dermatologists prefer shave biopsies (80.5%), surgeons prefer excisional biopsies (46.3%) and primary care physicians prefer punch biopsies (44%) for biopsy of a growth suspicious for melanoma.7 In fact, of the biopsies FPs perform, only 29% are of the shave variety.7
However, deep shave biopsies can be performed quickly, with the whole process taking less than 5 minutes. We advocate performing them at the time of presentation, as the evidence shows that deep shave biopsies of suspected melanoma are reliable and accurate in 97% of cases.15
MYTH #3
A partial biopsy can make the cancer spread.
There is no evidence to support that a partial biopsy has any effect on the local recurrence or metastatic potential of malignant melanoma.16 In fact, a biopsy elicits an inflammatory response that activates the patient’s immune system and often causes tumor lysis. Some tumors may even resolve after biopsy. In our clinical practice, we have had several cases of basal cell carcinoma resolve after a biopsy without additional treatment.
MYTH #4
If after performing a deep shave biopsy, tumor or pigment remains, you must leave it because a second biopsy specimen can’t be added to the first.
If pigment is visible after an initial shave or punch biopsy, it is reasonable to obtain additional tissue from the base of the biopsy site. While the deeper tissue cannot be added to the initial specimen for the purposes of Breslow’s depth, it is still helpful for the pathologist to have the sample so that he or she can analyze the tumor cells in the dermis. (Melanoma tumor depth is measured as the maximum distance between malignant cells and the top of the granular layer.17) In these situations, be sure to let the pathologist know that there are 2 specimens in the container.
In general, it is valuable to get as much of the tissue as possible at the time of the initial biopsy. One way to avoid leaving tumor at the base of the biopsy is to look at

MYTH #5
Epinephrine cannot be used for biopsies on the fingers, toes, nose, or penis.
Lidocaine with epinephrine is safe to use in areas with end-arteries, such as the fingers, toes, nose (FIGURE 4), and penis. There is no evidence to support the notion that local anesthesia with vasoconstriction can cause necrosis in these areas, and no case of necrosis has been reported since the introduction of commercial lidocaine with epinephrine in 1948.18

In addition to an absence of complications, epinephrine supplementation results in a relatively bloodless operating field and longer effectiveness of local anesthesia, as a study of more than 10,000 ear and nose surgeries using epinephrine-supplemented local anesthetics showed.19 The relative absence of blood in the operating field significantly reduces the duration of surgery and increases the healing rate because less electrocautery is needed.19
Similarly, the addition of epinephrine in digital blocks minimizes the need for tourniquets and large volumes of anesthetic and provides better and longer pain control during procedures.20 This topic was addressed by Prabhakar et al in a Cochrane Review in 2015.21 While digital surgeries are common, there were only 4 randomized controlled studies addressing the use of epinephrine in digital blocks. In these studies, there were no reports of adverse events, such as ischemia distal to the injection site. Evidence suggests that epinephrine in digital blocks can even be used safely in patients with vascular disease.22
And while the use of epinephrine with lidocaine in sites with end-arteries is beneficial for hemostasis and does not seem to pose a risk of ischemia, it is prudent to use the smallest volume of epinephrine (with lidocaine) needed to achieve anesthesia for the site.
CORRESPONDENCE
Elizabeth V. Seiverling, MD, 300 Southborough Drive, Suite 201, South Portland, ME 04106; [email protected].
Once it’s determined that a growth requires a biopsy, there is often uncertainty about which type of biopsy to perform. Insufficient knowledge of, and/or experience with, the various biopsy modalities may deter FPs from performing skin biopsies when they are indicated. To help fill the knowledge gaps and better position FPs to tackle skin cancer in its earliest stages, this article identifies and dispels 5 of the most common myths surrounding skin biopsies for the detection of basal and squamous cell carcinoma and melanoma.
MYTH #1
A punch biopsy is always preferred for suspected melanoma because it gets full depth.
A deep shave biopsy (saucerization)—not a punch biopsy—is usually the procedure of choice when biopsying a lesion suspected to be melanoma.2 The National Comprehensive Cancer Network (NCCN) "Melanoma Clinical Practice Guidelines in Oncology" state that an excisional biopsy (elliptical, punch, or saucerization) with a 1- to 3-mm margin is the preferred method of biopsy for suspected melanoma.3 However, a punch biopsy should be performed only if a 1- to 3-mm margin all around a suspected melanoma can be obtained. Otherwise, a saucerization or elliptical excision is preferred.3
The saucerization technique generally permits optimal sampling in terms of both the breadth and depth of the growth, providing the pathologist with sufficient tissue from both the epidermis and dermis (FIGURE 1).

Why are breadth/depth important? Breadth is important because showing the pathologist the epidermis (especially the edge) of a suspected melanocytic tumor allows for detection of pagetoid spread (upward movement through the epidermis) of melanocytes and of single melanocytes at the edge of a tumor. Single melanocytes at the edge of a tumor and pagetoid spread are histologic features of melanoma that help to distinguish these lesions from nevi, which tend to have nested melanocytes.2
Depth is important because it predicts prognosis and impacts management. For tumors 0.8 mm to 1 mm deep, a sentinel lymph node biopsy (SLNB) should be considered.3,4 Although the tumor depth threshold for a SLNB is still debated, most skin cancer experts in the United States agree that a melanoma thicker than 1 mm qualifies for this procedure. Some melanomas with high-risk features (such as ulceration) qualify for an SNLB even if they are <1 mm in depth.5 An SLNB provides prognostic information, and a positive SLNB directly affects staging.
[polldaddy:9990508]
Avoid partial biopsies. For tumors that have been partially biopsied with a punch or shallow shave biopsy, evaluation of the remaining neoplasm after subsequent excision leads to tumor upstaging in 21% of patients, with 10% qualifying for an SLNB.6 Thus, the goal should always be to obtain the entire depth of the tumor with the initial biopsy.
In addition, surgical margins are determined by primary tumor depth. To ensure a depth greater than 1 mm, aim to obtain a tissue specimen that is at least as thick as a dime (1.3 mm).
Because the goal is to avoid partial sampling, a challenge exists when the suspicious growth is large. Many melanomas are broader than a centimeter. And while punch biopsies ensure a depth of 1 mm or more, they risk missing the thickest portion of the tumor.7
Partial sampling of large melanocytic tumors with punch biopsies can lead to sampling error.8 Ng et al9 found there was a significant increase in histopathologic misdiagnosis with a punch biopsy of part of a melanoma (odds ratio [OR]=16.6; 95% confidence interval [CI], 10-27; P<.001) and with shallow shave biopsy (OR=2.6; 95% CI, 1.2-5.7; P=.02) compared with excisional biopsy (including saucerization).9 Punch biopsy of part of a melanoma was also associated with increased odds of misdiagnosis with an adverse outcome (OR=20; 95% CI, 10-41; P<.001).
Punch biopsies do, however, offer a reasonable alternative when the melanoma is too broad for a complete saucerization. In these cases, consider multiple 4- to 6-mm punch biopsies to reduce the risk of sampling error.
Avoid performing punch biopsies <4 mm, as the breadth of tissue is inadequate. For example, even with dermoscopy, facial lentigo maligna melanoma is often difficult to differentiate from pigmented actinic keratosis and solar lentigines. (See JFP’s Watch and Learn Video on dermoscopy.) A broad shave biopsy is the preferred method of biopsy for lentigo maligna melanoma in situ according to the NCCN.3 And there have been several reports showing that the results of shave biopsies of melanocytic lesions are cosmetically acceptable to patients.10,11
If the biopsy confirms malignancy, a larger surgery with suturing will be needed. The most important issue to keep in mind is that if partial sampling leads to a benign diagnosis of a suspicious lesion, then the remainder of the lesion must be excised and sent for pathology.
Saucerization is also the preferred biopsy type for basal cell and squamous cell carcinomas (SCCs). Studies have shown tumor depth is the most important factor in predicting metastasis of SCC, as well as tumor relapse rate, making accurate identification of the depth of the tumor important for both management and prognosis.12,13 Determining the thickness of the SCC is important for guiding management. SCC in situ is more amenable than invasive SCC to topical therapy or electrodesiccation and curettage.
What you’ll need. FPs can perform saucerization quickly and easily in the office during a standard 15-minute visit. Of course, it is essential to have all the necessary materials available. The key materials needed are lidocaine and epinephrine, a sharp razor blade such as a DermaBlade, and something for hemostasis (aluminum chloride and/or an electrosurgical instrument). Cotton-tipped applicators to apply the aluminum chloride and needles and syringes to administer the local anesthetic are also needed. (See JFP’s Watch and Learn Video on shave biopsy.) A quick saucerization eliminates the need for the patient to return for an elliptical excision and prevents a delayed diagnosis that can occur as a result of a long wait to see a dermatologist.
As a final note, the pathology order form should be completed with information on biopsy type, clinical presentation, differential diagnosis, and whether or not the full lesion was excised.
MYTH #2
A wide excisional biopsy is required for a suspected melanoma.
While complete excision of the entire tumor does allow the pathologist to evaluate the entire growth, wide (>3 mm) margins on the initial biopsy are not necessary. In fact, there are potential disadvantages to full excisional biopsy.
For example, seborrheic keratoses and other benign growths can mimic melanoma. Neither the physician nor the patient wants to learn that a large elliptical wound was created for a growth that turned out to be a benign seborrheic keratosis. Saucerization provides the pathologist with the entire lesion, and the resulting shallow wound heals as a round scar that is most often acceptable to patients.10,11,14 In addition, excisional biopsies carry a higher risk of infection than does saucerization.
Even when the index of suspicion is high for melanoma, a wide margin is not indicated. NCCN guidelines suggest that the margins around a suspected melanoma on initial biopsy not exceed 3 mm to avoid disrupting the accuracy of an SLNB (FIGURE 2).3

In addition, time constraints (elliptical excisional biopsies can take up to one hour, especially when a layered closure is performed) and a lack of surgical training may prohibit FPs from performing excisions.
One study found that while dermatologists prefer shave biopsies (80.5%), surgeons prefer excisional biopsies (46.3%) and primary care physicians prefer punch biopsies (44%) for biopsy of a growth suspicious for melanoma.7 In fact, of the biopsies FPs perform, only 29% are of the shave variety.7
However, deep shave biopsies can be performed quickly, with the whole process taking less than 5 minutes. We advocate performing them at the time of presentation, as the evidence shows that deep shave biopsies of suspected melanoma are reliable and accurate in 97% of cases.15
MYTH #3
A partial biopsy can make the cancer spread.
There is no evidence to support that a partial biopsy has any effect on the local recurrence or metastatic potential of malignant melanoma.16 In fact, a biopsy elicits an inflammatory response that activates the patient’s immune system and often causes tumor lysis. Some tumors may even resolve after biopsy. In our clinical practice, we have had several cases of basal cell carcinoma resolve after a biopsy without additional treatment.
MYTH #4
If after performing a deep shave biopsy, tumor or pigment remains, you must leave it because a second biopsy specimen can’t be added to the first.
If pigment is visible after an initial shave or punch biopsy, it is reasonable to obtain additional tissue from the base of the biopsy site. While the deeper tissue cannot be added to the initial specimen for the purposes of Breslow’s depth, it is still helpful for the pathologist to have the sample so that he or she can analyze the tumor cells in the dermis. (Melanoma tumor depth is measured as the maximum distance between malignant cells and the top of the granular layer.17) In these situations, be sure to let the pathologist know that there are 2 specimens in the container.
In general, it is valuable to get as much of the tissue as possible at the time of the initial biopsy. One way to avoid leaving tumor at the base of the biopsy is to look at

MYTH #5
Epinephrine cannot be used for biopsies on the fingers, toes, nose, or penis.
Lidocaine with epinephrine is safe to use in areas with end-arteries, such as the fingers, toes, nose (FIGURE 4), and penis. There is no evidence to support the notion that local anesthesia with vasoconstriction can cause necrosis in these areas, and no case of necrosis has been reported since the introduction of commercial lidocaine with epinephrine in 1948.18

In addition to an absence of complications, epinephrine supplementation results in a relatively bloodless operating field and longer effectiveness of local anesthesia, as a study of more than 10,000 ear and nose surgeries using epinephrine-supplemented local anesthetics showed.19 The relative absence of blood in the operating field significantly reduces the duration of surgery and increases the healing rate because less electrocautery is needed.19
Similarly, the addition of epinephrine in digital blocks minimizes the need for tourniquets and large volumes of anesthetic and provides better and longer pain control during procedures.20 This topic was addressed by Prabhakar et al in a Cochrane Review in 2015.21 While digital surgeries are common, there were only 4 randomized controlled studies addressing the use of epinephrine in digital blocks. In these studies, there were no reports of adverse events, such as ischemia distal to the injection site. Evidence suggests that epinephrine in digital blocks can even be used safely in patients with vascular disease.22
And while the use of epinephrine with lidocaine in sites with end-arteries is beneficial for hemostasis and does not seem to pose a risk of ischemia, it is prudent to use the smallest volume of epinephrine (with lidocaine) needed to achieve anesthesia for the site.
CORRESPONDENCE
Elizabeth V. Seiverling, MD, 300 Southborough Drive, Suite 201, South Portland, ME 04106; [email protected].
1. Kerr OA, Tidman MJ, Walker JJ, et al. The profile of dermatological problems in primary care. Clin Exp Dermatol. 2010;35:380-383.
2. Hosler GA, Patterson JW. Lentigines, nevi, and melanomas. In: Patterson JW, ed. Weedon’s Skin Pathology. Elsevier; 2015:32,837-901.
3. Coit DG, Andtbacka R, Bichakjian CK, et al. Melanoma. J Natl Compr Canc Netw. 2009;7:250-275.
4. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the skin. In: Amin MB, Edge S, Greene F, et al, eds. AJCC Cancer Staging Manual. Springer International Publishing; 2017;8:563-585.
5. American Joint Committee on Cancer. Implementation of AJCC 8th Edition Cancer Staging System. Available at: https://cancerstaging.org/About/news/Pages/Implementation-of-AJCC-8th-Edition-Cancer-Staging-System.aspx. Accessed April 2, 2018.
6. Karimipour DJ, Schwartz JL, Wang TS, et al. Microstaging accuracy after subtotal incisional biopsy of cutaneous melanoma. J Am Acad Dermatol. 2005;52:798-802.
7. Kaiser S, Vassell R, Pinckney RG, et al. Clinical impact of biopsy method on the quality of surgical management in melanoma. J Surg Oncol. 2014;109:775-779.
8. Montgomery BD, Sadler GM. Punch biopsy of pigmented lesions is potentially hazardous. Can Fam Physician. 2009;55:24.
9. Ng JC, Swain S, Dowling JP, et al. The impact of partial biopsy on histopathologic diagnosis of cutaneous melanoma: experience of an Australian tertiary referral service. Arch Dermatol. 2010;146:234-239.
10. Gambichler T, Senger E, Rapp S, et al. Deep shave excision of macular melanocytic nevi with the razor blade biopsy technique. Dermatol Surg. 2000;26:662-666.
11. Ferrandiz L, Moreno-Ramirez D, Camacho FM. Shave excision of common acquired melanocytic nevi: cosmetic outcome, recurrences, and complications. Dermatol Surg. 2005;31(9 Pt 1):1112-1115.
12. D'souza G, Carey TE, William WN Jr, et al. Epidemiology of head and neck squamous cell cancer among HIV-infected patients. J Acquir Immune Defic Syndr. 2014;65:603-610.
13. Edge SB, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Handbook. 7th ed. New York, NY: Springer; 2010.
14. Elston DM, Stratman EJ, Miller SJ, et al. Skin biopsy. J Am Acad Dermatol. 2016;74:1-16.
15. Zager JS, Hochwald SN, Marzban SS, et al. Shave biopsy is a safe and accurate method for the initial evaluation of melanoma. J Am Coll Surg. 2011;212:454-460.
16. Chanda JJ, Callen JP. Adverse effect of melanoma incision. J Am Acad Dermatol. 1985;13:519-522.
17. Noroozi N, Zakerolhosseini A. Computerized measurement of melanocytic tumor depth in skin histopathological images. Micron. 2015;77:44-56.
18. Nielsen LJ, Lumholt P, Hölmich LR. [Local anaesthesia with vasoconstrictor is safe to use in areas with end-arteries in fingers, toes, noses and ears]. Ugeskr Laeger. 2014;176(44).
19. Häfner HM, Röcken M, Breuninger H. Epinephrine-supplemented local anesthetics for ear and nose surgery: clinical use without complications in more than 10,000 surgical procedures. J Dtsch Dermatol Ges. 2005;3:195-199.
20. Krunic AL, Wang LC, Soltani K, et al. Digital anesthesia with epinephrine: an old myth revisited. J Am Acad Dermatol. 2004;51:755-759.
21. Prabhakar H, Rath S, Kalaivani M, et al. Adrenaline with lidocaine for digital nerve blocks. Cochrane Database Syst Rev. 2015;(3):CD010645.
22. Ilicki J. Safety of epinephrine in digital nerve blocks: a literature review. J Emerg Med. 2015;49:799-809.
1. Kerr OA, Tidman MJ, Walker JJ, et al. The profile of dermatological problems in primary care. Clin Exp Dermatol. 2010;35:380-383.
2. Hosler GA, Patterson JW. Lentigines, nevi, and melanomas. In: Patterson JW, ed. Weedon’s Skin Pathology. Elsevier; 2015:32,837-901.
3. Coit DG, Andtbacka R, Bichakjian CK, et al. Melanoma. J Natl Compr Canc Netw. 2009;7:250-275.
4. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma of the skin. In: Amin MB, Edge S, Greene F, et al, eds. AJCC Cancer Staging Manual. Springer International Publishing; 2017;8:563-585.
5. American Joint Committee on Cancer. Implementation of AJCC 8th Edition Cancer Staging System. Available at: https://cancerstaging.org/About/news/Pages/Implementation-of-AJCC-8th-Edition-Cancer-Staging-System.aspx. Accessed April 2, 2018.
6. Karimipour DJ, Schwartz JL, Wang TS, et al. Microstaging accuracy after subtotal incisional biopsy of cutaneous melanoma. J Am Acad Dermatol. 2005;52:798-802.
7. Kaiser S, Vassell R, Pinckney RG, et al. Clinical impact of biopsy method on the quality of surgical management in melanoma. J Surg Oncol. 2014;109:775-779.
8. Montgomery BD, Sadler GM. Punch biopsy of pigmented lesions is potentially hazardous. Can Fam Physician. 2009;55:24.
9. Ng JC, Swain S, Dowling JP, et al. The impact of partial biopsy on histopathologic diagnosis of cutaneous melanoma: experience of an Australian tertiary referral service. Arch Dermatol. 2010;146:234-239.
10. Gambichler T, Senger E, Rapp S, et al. Deep shave excision of macular melanocytic nevi with the razor blade biopsy technique. Dermatol Surg. 2000;26:662-666.
11. Ferrandiz L, Moreno-Ramirez D, Camacho FM. Shave excision of common acquired melanocytic nevi: cosmetic outcome, recurrences, and complications. Dermatol Surg. 2005;31(9 Pt 1):1112-1115.
12. D'souza G, Carey TE, William WN Jr, et al. Epidemiology of head and neck squamous cell cancer among HIV-infected patients. J Acquir Immune Defic Syndr. 2014;65:603-610.
13. Edge SB, Byrd DR, Compton CC, et al, eds. AJCC Cancer Staging Handbook. 7th ed. New York, NY: Springer; 2010.
14. Elston DM, Stratman EJ, Miller SJ, et al. Skin biopsy. J Am Acad Dermatol. 2016;74:1-16.
15. Zager JS, Hochwald SN, Marzban SS, et al. Shave biopsy is a safe and accurate method for the initial evaluation of melanoma. J Am Coll Surg. 2011;212:454-460.
16. Chanda JJ, Callen JP. Adverse effect of melanoma incision. J Am Acad Dermatol. 1985;13:519-522.
17. Noroozi N, Zakerolhosseini A. Computerized measurement of melanocytic tumor depth in skin histopathological images. Micron. 2015;77:44-56.
18. Nielsen LJ, Lumholt P, Hölmich LR. [Local anaesthesia with vasoconstrictor is safe to use in areas with end-arteries in fingers, toes, noses and ears]. Ugeskr Laeger. 2014;176(44).
19. Häfner HM, Röcken M, Breuninger H. Epinephrine-supplemented local anesthetics for ear and nose surgery: clinical use without complications in more than 10,000 surgical procedures. J Dtsch Dermatol Ges. 2005;3:195-199.
20. Krunic AL, Wang LC, Soltani K, et al. Digital anesthesia with epinephrine: an old myth revisited. J Am Acad Dermatol. 2004;51:755-759.
21. Prabhakar H, Rath S, Kalaivani M, et al. Adrenaline with lidocaine for digital nerve blocks. Cochrane Database Syst Rev. 2015;(3):CD010645.
22. Ilicki J. Safety of epinephrine in digital nerve blocks: a literature review. J Emerg Med. 2015;49:799-809.
From The Journal of Family Practice | 2018;67(5):270-274.
The naloxone option
More than 64,000 people in the United States died of drug overdoses in 2016.1 Of those overdose deaths, more than 34,000 were related to the use of natural (eg, codeine, morphine); synthetic (eg, fentanyl); and semisynthetic (eg, oxycodone, hydrocodone) opioids.1 The number of drug-overdose fatalities (driven largely by opioids) has increased so dramatically in recent years that drug overdose is now the leading cause of intentional and unintentional injury-related death in the United States.2 Furthermore, opioid use is increasing among college students, with many injecting these agents.3 Those injecting (as opposed to other routes of delivery) have the highest death rate.4
The Department of Health and Human Services has identified 3 important issues to address with regard to the opioid epidemic: prescriber education, community naloxone access, and better interventions (such as naloxone overdose-reversal take-home kits) for people with opioid use disorders and/or a history of overdoses.5 (For more on overdose reversal kits, see “What FPs need to know about naloxone kits,” a 3-in-3 video.) With these goals in mind, we provide the following review of naloxone dosing and postoverdose treatment.
Steps FPs can take to reverse the overdose
Opioids act on delta, kappa, and mu receptors in the brain to produce analgesic effects,6 but, in large quantities, their mu receptor activity can cause fatal respiratory depression.7 Some of the most commonly abused opioids are heroin and the prescription opioids fentanyl, oxycodone, and hydrocodone.8
People who have overdosed on opioids generally present with evidence of obtundation, miosis, and difficulty breathing. Respiratory failure is the most common cause of death.9 Hypothermia, compartment syndrome, rhabdomyolysis, renal failure, and acute pulmonary edema are less common complications. Overdoses and these medical issues can potentially be reversed and/or mitigated by naloxone administration.10,11
Naloxone and its routes of administration. Naloxone is the agent of choice in overdose situations.12 It works as an antagonist of the delta, kappa, and mu receptors,6,13 has a rapid onset of action, and is associated with minimal adverse effects.14
Naloxone can be administered via the intravenous (IV), intranasal, intramuscular, subcutaneous, intraosseous, or endotracheal routes.6 Although IV administration has been the most common and is still generally preferred in the hospital setting, the intranasal route has gained favor, partly because it can be difficult to establish an IV in IV drug users and partly because it is easier for nonmedical people to administer.6
In addition, the nasal mucosa has an abundant blood supply resulting in rapid absorption. The drug reaches the systemic circulation quickly and avoids first-pass hepatic metabolism.6 Intranasal route absorption is enhanced by deep inhalation and patient cooperation, but it can still be effective in an unconscious patient. Response time is nearly the same as that with IV administration (both act within 1-2 mins).6
Naloxone has a short duration of action (shorter than that of some opiates), and its duration of action is influenced by the pharmacology and toxicity of the overdose drug.15 The serum half-life in adults ranges from 30 to 81 mins, and clinical impact varies from minutes to an hour.15 Thus, even if a patient initially improves after administration, close observation is mandatory due to the frequent need for repeat naloxone dosing.
Adverse effects. Naloxone is considered safe, with relatively few adverse effects and doesn’t have any effects on someone who isn’t experiencing an opioid overdose or currently on opioids.15 The only downside is that naloxone administration to an opioid-dependent person often precipitates an acute withdrawal event, characterized by global pain, agitation, generalized distress, and gastrointestinal complaints, including vomiting and diarrhea. Although withdrawal is not life-threatening, it can cause great discomfort.
Getting a handle on naloxone dosing
The starting dose of naloxone used to be 0.04 mg, but this was later increased to 0.4 mg. The advent and high overdose lethality of more potent drugs like fentanyl and carfentanil has made low-dose naloxone less effective.12
Currently, 1 mg is often the initial recommendation, but doses of 2 to 4 mg are not uncommon, and multiple administrations or continuous IV administration are frequently needed to reverse severe toxicities, such as those involving fentanyl or longer-action opioids like methadone. Anyone exhibiting difficulty breathing mandates a starting naloxone dose of at least 1 to 2 mg.12,16 In addition to breathing, additional doses are indicated clinically by medical parameters such as vital signs, ocular pupil diameter, and/or alertness.6
Intranasal administration often utilizes up to 4 mg of naloxone in one nostril, followed by a titrated additional administration in the other nostril. In life-threatening circumstances, especially those in which a patient is exhibiting respiratory depression, a much larger quantity of naloxone—up to 10 mg—may be administered by trained medical personnel.12,16 In the end, all dosing varies and must be individualized to the patient’s signs and symptoms. Those who have overdosed require prolonged monitoring to treat potential complications.
Emergency assistance and transport. Because of the dangers that can result from opioid toxicities, any hint or evidence of physiologic compromise merits a 911 call for emergency medical assistance and transport to a hospital emergency department (ED). Hospitalization is at the physician’s discretion.
Expanding the availability of naloxone in the community
The availability of naloxone overdose-reversal kits is growing among hospitals, other types of health care facilities, first responders, medical offices, and the general public. Distributing the kits to opioid users and their families has wide support but remains controversial (more on this in a bit).
Support even includes that from the current US Surgeon General, Jerome Adams, MD, MPH, who noted in a statement on April 5, 2018, the lifesaving success of opioid-overdose reversal naloxone kits by medical personnel, first responders, and other people. As a result, he formally recommended that more Americans keep such kits available in order to be able to quickly diminish opioid toxicities.17,18 His advice was especially directed toward people at risk for an opioid overdose or anyone associated with opioid drug users.
Prehospital management of overdoses is ideally managed by emergency medical service (EMS) personnel,10 but even nonmedical people can safely administer naloxone. About 10,000 overdose cases were documented to have been reversed by nonmedical providers between 1996 and 2010.10 Many states have laws limiting the civil and criminal liability for naloxone administrators. New Mexico was the first state to legally allow naloxone administration by individuals without a prescription.7 Pharmacists often participate in efforts to counter opioid drug overdose deaths by offering naloxone administration kits, along with training about techniques of use, to people filling opioid prescriptions and to household members and/or other individuals in the social support network of an opioid user.6 Some physicians co-prescribe naloxone to patients along with opioid therapies during long-term pain management. Such dual prescribing is encouraged by many clinics.19 This method has decreased opioid overdose deaths in North Carolina,20 in its army base at Fort Bragg,19 and in California.21
The issue of “risk compensation”
To those who say that having naloxone available to users of opioids or those in their social network promotes even riskier behavior resulting in increased overdoses, research points to just the opposite. A nonrandomized study that examined co-prescribing naloxone to patients on chronic opioid therapy for non-cancer-related pain, documented fewer opioid-related ED visits following use by prescribers and patients at community health centers.22 Other research has demonstrated a reduced number of community-level opioid overdose deaths once opioid overdose education and community naloxone distribution were implemented.23,24
After the overdose: Getting patients into treatment
After reversing initial toxicities, a protracted period of assessment is required to assure patient safety. Beyond prolonged observation after an overdose, it is critical to recommend and provide long-term substance abuse therapies. Simply reversing the overdose is not medically sufficient, even if postoverdose patients refuse such treatment referrals. The fact that many of these people subsequently die is evidence of the importance of adhering to a formal, long-term chemical dependence intervention program.
Persistent diligence is usually needed to convince a patient who has recovered from an acute drug overdose event to accept a treatment referral. Some EDs institute special teams to facilitate such referrals, using a multidisciplinary approach, including substance abuse counselors and social workers. Referral agencies are also sometimes included to aid patient acceptance and retention in drug abuse treatment interventions. (See "Resources" below for more information.)
SIDEBAR
Resources
- The Centers for Disease Control and Prevention’s Guideline for Prescribing Opioids for Chronic Pain. Available at: https://www.cdc.gov/drugoverdose/prescribing/guideline.html.
- National Institute on Drug Abuse. Available at: https://www.drugabuse.gov.
- Substance Abuse and Mental Health Services Administration. Available at: https://www.samhsa.gov/find-help/national-helpline.
- Your state’s prescription drug monitoring program. Available at: https://www.cdc.gov/drugoverdose/pdmp/states.html.
CORRESPONDENCE
Steven Lippmann, MD, 401 East Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
1. National Institute on Drug Abuse. Overdose death rates. Revised September 2017. Available at: https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Accessed April 11, 2018.
2. Xu J, Murphy SL, Kochanek KD, et al. Deaths: final data for 2013. Nat Vital Stat Syst. 2016;64:1-119.
3. McCabe SE, West BT, Teter CJ, et al. Trends in medical use, diversion, and nonmedical use of prescription medications among college students from 2003 to 2013: connecting the dots. Addict Behav. 2014;39:1176-1182.
4. Green TC, Heimer R, Grau LE. Distinguishing signs of opioid overdose and indication for naloxone: an evaluation of six overdose training and naloxone distribution programs in the United States. Addiction. 2008;103:979-989.
5. US Department of Health and Human Services. HHS takes strong steps to address opioid-drug related overdose, death and dependence. March 26, 2015. Available at: http://wayback.archive-it.org/3926/20170127185704/https://www.hhs.gov/about/news/2015/03/26/hhs-takes-strong-steps-to-address-opioid-drug-related-overdose-death-and-dependence.html. Accessed April 16, 2018.
6. Robinson A, Wermeling DP. Intranasal naloxone administration for treatment of opioid overdose. Am J Health Syst Pharm. 2014;71:2129-2135.
7. Doyon S, Aks SE, Schaeffer S. Expanding access to naloxone in the United States. J Med Toxicol. 2014;10:431-434.
8. National Institute on Drug Abuse. Which classes of prescription drugs are commonly misused? Available at: https://www.drugabuse.gov/publications/research-reports/misuse-prescription-drugs/which-classes-prescription-drugs-are-commonly-misused. Accessed April 16, 2018.
9. Boom M, Niesters M, Sarton E, et al. Non-analgesic effects of opioids: opioid-induced respiratory depression. Curr Pharm Des. 2012;18:5994-6004.
10. Weaver L, Palombi L, Bastianelli KMS. Naloxone administration for opioid overdose reversal in the prehospital setting: implications for pharmacists. J Pharm Pract. 2018;31:91-98.
11. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367:146-155.
12. Jordan MR, Morrisonponce D. Naloxone. StatPearls. Available at: https://www.ncbi.nlm.nih.gov/books/NBK441910/. Accessed September 1, 2017.
13. Wilkerson RG, Kim HK, Windsor TA, et al. The opioid epidemic in the United States. Emerg Med Clin North Am. 2016;34:e1-e23.
14. Jeffery RM, Dickinson L, Ng ND, et al. Naloxone administration for suspected opioid overdose: an expanded scope of practice by a basic life support collegiate-based emergency medical services agency. J Am Coll Health. 2017;65:212-216.
15. Drugs.com. Naloxone. Available at: https://www.drugs.com/pro/naloxone.html. Accessed April 16, 2018.
16. Prabhu A, Abaid B, Naik S, et al. Naloxone for opioid overdoses. Internet and Psychiatry 2017. Available at: https://www.internetandpsychiatry.com/wp/editorials/naloxone-for-opioid-overdoses/. Accessed September 19, 2017.
17. HHS.gov. Surgeon General releases advisory on naloxone, an opioid overdose-reversing drug. Available at: https://www.hhs.gov/about/news/2018/04/05/surgeon-general-releases-advisory-on-naloxone-an-opioid-overdose-reversing-drug.html. Accessed April 16, 2018.
18. US Department of Health and Human Services. Surgeongeneral.gov. Surgeon General’s advisory on naloxone and opioid overdose. Available at: https://www.surgeongeneral.gov/priorities/opioid-overdose-prevention/naloxone-advisory.html. Accessed April 16, 2018.
19. Behar E, Rowe C, Santos GM, et al. Acceptability of naloxone co-prescription among primary care providers treating patients on long-term opioid therapy for pain. J Gen Intern Med. 2017;32:291-295.
20. Albert S, Brason FW 2nd, Sanford CK, et al. Project Lazarus: community‐based overdose prevention in rural North Carolina. Pain Med. 2011;12:S77-S85.
21. Rowe C, Santos GM, Vittinghoff E, et al. Predictors of participant engagement and naloxone utilization in a community‐based naloxone distribution program. Addiction. 2015;110:1301-1310.
22. Coffin PO, Behar E, Rowe C, et al. Nonrandomized intervention study of naloxone coprescription for primary care patients receiving long-term opioid therapy for pain. Ann Intern Med. 2016;165:245-252.
23. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174.
24. Bird SM, McAuley A, Perry S, et al. Effectiveness of Scotland’s National Naloxone Programme for reducing opioid-related deaths: a before (2006-10) versus after (2011-13) comparison. Addiction. 2016;111:883-891.
More than 64,000 people in the United States died of drug overdoses in 2016.1 Of those overdose deaths, more than 34,000 were related to the use of natural (eg, codeine, morphine); synthetic (eg, fentanyl); and semisynthetic (eg, oxycodone, hydrocodone) opioids.1 The number of drug-overdose fatalities (driven largely by opioids) has increased so dramatically in recent years that drug overdose is now the leading cause of intentional and unintentional injury-related death in the United States.2 Furthermore, opioid use is increasing among college students, with many injecting these agents.3 Those injecting (as opposed to other routes of delivery) have the highest death rate.4
The Department of Health and Human Services has identified 3 important issues to address with regard to the opioid epidemic: prescriber education, community naloxone access, and better interventions (such as naloxone overdose-reversal take-home kits) for people with opioid use disorders and/or a history of overdoses.5 (For more on overdose reversal kits, see “What FPs need to know about naloxone kits,” a 3-in-3 video.) With these goals in mind, we provide the following review of naloxone dosing and postoverdose treatment.
Steps FPs can take to reverse the overdose
Opioids act on delta, kappa, and mu receptors in the brain to produce analgesic effects,6 but, in large quantities, their mu receptor activity can cause fatal respiratory depression.7 Some of the most commonly abused opioids are heroin and the prescription opioids fentanyl, oxycodone, and hydrocodone.8
People who have overdosed on opioids generally present with evidence of obtundation, miosis, and difficulty breathing. Respiratory failure is the most common cause of death.9 Hypothermia, compartment syndrome, rhabdomyolysis, renal failure, and acute pulmonary edema are less common complications. Overdoses and these medical issues can potentially be reversed and/or mitigated by naloxone administration.10,11
Naloxone and its routes of administration. Naloxone is the agent of choice in overdose situations.12 It works as an antagonist of the delta, kappa, and mu receptors,6,13 has a rapid onset of action, and is associated with minimal adverse effects.14
Naloxone can be administered via the intravenous (IV), intranasal, intramuscular, subcutaneous, intraosseous, or endotracheal routes.6 Although IV administration has been the most common and is still generally preferred in the hospital setting, the intranasal route has gained favor, partly because it can be difficult to establish an IV in IV drug users and partly because it is easier for nonmedical people to administer.6
In addition, the nasal mucosa has an abundant blood supply resulting in rapid absorption. The drug reaches the systemic circulation quickly and avoids first-pass hepatic metabolism.6 Intranasal route absorption is enhanced by deep inhalation and patient cooperation, but it can still be effective in an unconscious patient. Response time is nearly the same as that with IV administration (both act within 1-2 mins).6
Naloxone has a short duration of action (shorter than that of some opiates), and its duration of action is influenced by the pharmacology and toxicity of the overdose drug.15 The serum half-life in adults ranges from 30 to 81 mins, and clinical impact varies from minutes to an hour.15 Thus, even if a patient initially improves after administration, close observation is mandatory due to the frequent need for repeat naloxone dosing.
Adverse effects. Naloxone is considered safe, with relatively few adverse effects and doesn’t have any effects on someone who isn’t experiencing an opioid overdose or currently on opioids.15 The only downside is that naloxone administration to an opioid-dependent person often precipitates an acute withdrawal event, characterized by global pain, agitation, generalized distress, and gastrointestinal complaints, including vomiting and diarrhea. Although withdrawal is not life-threatening, it can cause great discomfort.
Getting a handle on naloxone dosing
The starting dose of naloxone used to be 0.04 mg, but this was later increased to 0.4 mg. The advent and high overdose lethality of more potent drugs like fentanyl and carfentanil has made low-dose naloxone less effective.12
Currently, 1 mg is often the initial recommendation, but doses of 2 to 4 mg are not uncommon, and multiple administrations or continuous IV administration are frequently needed to reverse severe toxicities, such as those involving fentanyl or longer-action opioids like methadone. Anyone exhibiting difficulty breathing mandates a starting naloxone dose of at least 1 to 2 mg.12,16 In addition to breathing, additional doses are indicated clinically by medical parameters such as vital signs, ocular pupil diameter, and/or alertness.6
Intranasal administration often utilizes up to 4 mg of naloxone in one nostril, followed by a titrated additional administration in the other nostril. In life-threatening circumstances, especially those in which a patient is exhibiting respiratory depression, a much larger quantity of naloxone—up to 10 mg—may be administered by trained medical personnel.12,16 In the end, all dosing varies and must be individualized to the patient’s signs and symptoms. Those who have overdosed require prolonged monitoring to treat potential complications.
Emergency assistance and transport. Because of the dangers that can result from opioid toxicities, any hint or evidence of physiologic compromise merits a 911 call for emergency medical assistance and transport to a hospital emergency department (ED). Hospitalization is at the physician’s discretion.
Expanding the availability of naloxone in the community
The availability of naloxone overdose-reversal kits is growing among hospitals, other types of health care facilities, first responders, medical offices, and the general public. Distributing the kits to opioid users and their families has wide support but remains controversial (more on this in a bit).
Support even includes that from the current US Surgeon General, Jerome Adams, MD, MPH, who noted in a statement on April 5, 2018, the lifesaving success of opioid-overdose reversal naloxone kits by medical personnel, first responders, and other people. As a result, he formally recommended that more Americans keep such kits available in order to be able to quickly diminish opioid toxicities.17,18 His advice was especially directed toward people at risk for an opioid overdose or anyone associated with opioid drug users.
Prehospital management of overdoses is ideally managed by emergency medical service (EMS) personnel,10 but even nonmedical people can safely administer naloxone. About 10,000 overdose cases were documented to have been reversed by nonmedical providers between 1996 and 2010.10 Many states have laws limiting the civil and criminal liability for naloxone administrators. New Mexico was the first state to legally allow naloxone administration by individuals without a prescription.7 Pharmacists often participate in efforts to counter opioid drug overdose deaths by offering naloxone administration kits, along with training about techniques of use, to people filling opioid prescriptions and to household members and/or other individuals in the social support network of an opioid user.6 Some physicians co-prescribe naloxone to patients along with opioid therapies during long-term pain management. Such dual prescribing is encouraged by many clinics.19 This method has decreased opioid overdose deaths in North Carolina,20 in its army base at Fort Bragg,19 and in California.21
The issue of “risk compensation”
To those who say that having naloxone available to users of opioids or those in their social network promotes even riskier behavior resulting in increased overdoses, research points to just the opposite. A nonrandomized study that examined co-prescribing naloxone to patients on chronic opioid therapy for non-cancer-related pain, documented fewer opioid-related ED visits following use by prescribers and patients at community health centers.22 Other research has demonstrated a reduced number of community-level opioid overdose deaths once opioid overdose education and community naloxone distribution were implemented.23,24
After the overdose: Getting patients into treatment
After reversing initial toxicities, a protracted period of assessment is required to assure patient safety. Beyond prolonged observation after an overdose, it is critical to recommend and provide long-term substance abuse therapies. Simply reversing the overdose is not medically sufficient, even if postoverdose patients refuse such treatment referrals. The fact that many of these people subsequently die is evidence of the importance of adhering to a formal, long-term chemical dependence intervention program.
Persistent diligence is usually needed to convince a patient who has recovered from an acute drug overdose event to accept a treatment referral. Some EDs institute special teams to facilitate such referrals, using a multidisciplinary approach, including substance abuse counselors and social workers. Referral agencies are also sometimes included to aid patient acceptance and retention in drug abuse treatment interventions. (See "Resources" below for more information.)
SIDEBAR
Resources
- The Centers for Disease Control and Prevention’s Guideline for Prescribing Opioids for Chronic Pain. Available at: https://www.cdc.gov/drugoverdose/prescribing/guideline.html.
- National Institute on Drug Abuse. Available at: https://www.drugabuse.gov.
- Substance Abuse and Mental Health Services Administration. Available at: https://www.samhsa.gov/find-help/national-helpline.
- Your state’s prescription drug monitoring program. Available at: https://www.cdc.gov/drugoverdose/pdmp/states.html.
CORRESPONDENCE
Steven Lippmann, MD, 401 East Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
More than 64,000 people in the United States died of drug overdoses in 2016.1 Of those overdose deaths, more than 34,000 were related to the use of natural (eg, codeine, morphine); synthetic (eg, fentanyl); and semisynthetic (eg, oxycodone, hydrocodone) opioids.1 The number of drug-overdose fatalities (driven largely by opioids) has increased so dramatically in recent years that drug overdose is now the leading cause of intentional and unintentional injury-related death in the United States.2 Furthermore, opioid use is increasing among college students, with many injecting these agents.3 Those injecting (as opposed to other routes of delivery) have the highest death rate.4
The Department of Health and Human Services has identified 3 important issues to address with regard to the opioid epidemic: prescriber education, community naloxone access, and better interventions (such as naloxone overdose-reversal take-home kits) for people with opioid use disorders and/or a history of overdoses.5 (For more on overdose reversal kits, see “What FPs need to know about naloxone kits,” a 3-in-3 video.) With these goals in mind, we provide the following review of naloxone dosing and postoverdose treatment.
Steps FPs can take to reverse the overdose
Opioids act on delta, kappa, and mu receptors in the brain to produce analgesic effects,6 but, in large quantities, their mu receptor activity can cause fatal respiratory depression.7 Some of the most commonly abused opioids are heroin and the prescription opioids fentanyl, oxycodone, and hydrocodone.8
People who have overdosed on opioids generally present with evidence of obtundation, miosis, and difficulty breathing. Respiratory failure is the most common cause of death.9 Hypothermia, compartment syndrome, rhabdomyolysis, renal failure, and acute pulmonary edema are less common complications. Overdoses and these medical issues can potentially be reversed and/or mitigated by naloxone administration.10,11
Naloxone and its routes of administration. Naloxone is the agent of choice in overdose situations.12 It works as an antagonist of the delta, kappa, and mu receptors,6,13 has a rapid onset of action, and is associated with minimal adverse effects.14
Naloxone can be administered via the intravenous (IV), intranasal, intramuscular, subcutaneous, intraosseous, or endotracheal routes.6 Although IV administration has been the most common and is still generally preferred in the hospital setting, the intranasal route has gained favor, partly because it can be difficult to establish an IV in IV drug users and partly because it is easier for nonmedical people to administer.6
In addition, the nasal mucosa has an abundant blood supply resulting in rapid absorption. The drug reaches the systemic circulation quickly and avoids first-pass hepatic metabolism.6 Intranasal route absorption is enhanced by deep inhalation and patient cooperation, but it can still be effective in an unconscious patient. Response time is nearly the same as that with IV administration (both act within 1-2 mins).6
Naloxone has a short duration of action (shorter than that of some opiates), and its duration of action is influenced by the pharmacology and toxicity of the overdose drug.15 The serum half-life in adults ranges from 30 to 81 mins, and clinical impact varies from minutes to an hour.15 Thus, even if a patient initially improves after administration, close observation is mandatory due to the frequent need for repeat naloxone dosing.
Adverse effects. Naloxone is considered safe, with relatively few adverse effects and doesn’t have any effects on someone who isn’t experiencing an opioid overdose or currently on opioids.15 The only downside is that naloxone administration to an opioid-dependent person often precipitates an acute withdrawal event, characterized by global pain, agitation, generalized distress, and gastrointestinal complaints, including vomiting and diarrhea. Although withdrawal is not life-threatening, it can cause great discomfort.
Getting a handle on naloxone dosing
The starting dose of naloxone used to be 0.04 mg, but this was later increased to 0.4 mg. The advent and high overdose lethality of more potent drugs like fentanyl and carfentanil has made low-dose naloxone less effective.12
Currently, 1 mg is often the initial recommendation, but doses of 2 to 4 mg are not uncommon, and multiple administrations or continuous IV administration are frequently needed to reverse severe toxicities, such as those involving fentanyl or longer-action opioids like methadone. Anyone exhibiting difficulty breathing mandates a starting naloxone dose of at least 1 to 2 mg.12,16 In addition to breathing, additional doses are indicated clinically by medical parameters such as vital signs, ocular pupil diameter, and/or alertness.6
Intranasal administration often utilizes up to 4 mg of naloxone in one nostril, followed by a titrated additional administration in the other nostril. In life-threatening circumstances, especially those in which a patient is exhibiting respiratory depression, a much larger quantity of naloxone—up to 10 mg—may be administered by trained medical personnel.12,16 In the end, all dosing varies and must be individualized to the patient’s signs and symptoms. Those who have overdosed require prolonged monitoring to treat potential complications.
Emergency assistance and transport. Because of the dangers that can result from opioid toxicities, any hint or evidence of physiologic compromise merits a 911 call for emergency medical assistance and transport to a hospital emergency department (ED). Hospitalization is at the physician’s discretion.
Expanding the availability of naloxone in the community
The availability of naloxone overdose-reversal kits is growing among hospitals, other types of health care facilities, first responders, medical offices, and the general public. Distributing the kits to opioid users and their families has wide support but remains controversial (more on this in a bit).
Support even includes that from the current US Surgeon General, Jerome Adams, MD, MPH, who noted in a statement on April 5, 2018, the lifesaving success of opioid-overdose reversal naloxone kits by medical personnel, first responders, and other people. As a result, he formally recommended that more Americans keep such kits available in order to be able to quickly diminish opioid toxicities.17,18 His advice was especially directed toward people at risk for an opioid overdose or anyone associated with opioid drug users.
Prehospital management of overdoses is ideally managed by emergency medical service (EMS) personnel,10 but even nonmedical people can safely administer naloxone. About 10,000 overdose cases were documented to have been reversed by nonmedical providers between 1996 and 2010.10 Many states have laws limiting the civil and criminal liability for naloxone administrators. New Mexico was the first state to legally allow naloxone administration by individuals without a prescription.7 Pharmacists often participate in efforts to counter opioid drug overdose deaths by offering naloxone administration kits, along with training about techniques of use, to people filling opioid prescriptions and to household members and/or other individuals in the social support network of an opioid user.6 Some physicians co-prescribe naloxone to patients along with opioid therapies during long-term pain management. Such dual prescribing is encouraged by many clinics.19 This method has decreased opioid overdose deaths in North Carolina,20 in its army base at Fort Bragg,19 and in California.21
The issue of “risk compensation”
To those who say that having naloxone available to users of opioids or those in their social network promotes even riskier behavior resulting in increased overdoses, research points to just the opposite. A nonrandomized study that examined co-prescribing naloxone to patients on chronic opioid therapy for non-cancer-related pain, documented fewer opioid-related ED visits following use by prescribers and patients at community health centers.22 Other research has demonstrated a reduced number of community-level opioid overdose deaths once opioid overdose education and community naloxone distribution were implemented.23,24
After the overdose: Getting patients into treatment
After reversing initial toxicities, a protracted period of assessment is required to assure patient safety. Beyond prolonged observation after an overdose, it is critical to recommend and provide long-term substance abuse therapies. Simply reversing the overdose is not medically sufficient, even if postoverdose patients refuse such treatment referrals. The fact that many of these people subsequently die is evidence of the importance of adhering to a formal, long-term chemical dependence intervention program.
Persistent diligence is usually needed to convince a patient who has recovered from an acute drug overdose event to accept a treatment referral. Some EDs institute special teams to facilitate such referrals, using a multidisciplinary approach, including substance abuse counselors and social workers. Referral agencies are also sometimes included to aid patient acceptance and retention in drug abuse treatment interventions. (See "Resources" below for more information.)
SIDEBAR
Resources
- The Centers for Disease Control and Prevention’s Guideline for Prescribing Opioids for Chronic Pain. Available at: https://www.cdc.gov/drugoverdose/prescribing/guideline.html.
- National Institute on Drug Abuse. Available at: https://www.drugabuse.gov.
- Substance Abuse and Mental Health Services Administration. Available at: https://www.samhsa.gov/find-help/national-helpline.
- Your state’s prescription drug monitoring program. Available at: https://www.cdc.gov/drugoverdose/pdmp/states.html.
CORRESPONDENCE
Steven Lippmann, MD, 401 East Chestnut Street, Suite 610, Louisville, KY 40202; [email protected].
1. National Institute on Drug Abuse. Overdose death rates. Revised September 2017. Available at: https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Accessed April 11, 2018.
2. Xu J, Murphy SL, Kochanek KD, et al. Deaths: final data for 2013. Nat Vital Stat Syst. 2016;64:1-119.
3. McCabe SE, West BT, Teter CJ, et al. Trends in medical use, diversion, and nonmedical use of prescription medications among college students from 2003 to 2013: connecting the dots. Addict Behav. 2014;39:1176-1182.
4. Green TC, Heimer R, Grau LE. Distinguishing signs of opioid overdose and indication for naloxone: an evaluation of six overdose training and naloxone distribution programs in the United States. Addiction. 2008;103:979-989.
5. US Department of Health and Human Services. HHS takes strong steps to address opioid-drug related overdose, death and dependence. March 26, 2015. Available at: http://wayback.archive-it.org/3926/20170127185704/https://www.hhs.gov/about/news/2015/03/26/hhs-takes-strong-steps-to-address-opioid-drug-related-overdose-death-and-dependence.html. Accessed April 16, 2018.
6. Robinson A, Wermeling DP. Intranasal naloxone administration for treatment of opioid overdose. Am J Health Syst Pharm. 2014;71:2129-2135.
7. Doyon S, Aks SE, Schaeffer S. Expanding access to naloxone in the United States. J Med Toxicol. 2014;10:431-434.
8. National Institute on Drug Abuse. Which classes of prescription drugs are commonly misused? Available at: https://www.drugabuse.gov/publications/research-reports/misuse-prescription-drugs/which-classes-prescription-drugs-are-commonly-misused. Accessed April 16, 2018.
9. Boom M, Niesters M, Sarton E, et al. Non-analgesic effects of opioids: opioid-induced respiratory depression. Curr Pharm Des. 2012;18:5994-6004.
10. Weaver L, Palombi L, Bastianelli KMS. Naloxone administration for opioid overdose reversal in the prehospital setting: implications for pharmacists. J Pharm Pract. 2018;31:91-98.
11. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367:146-155.
12. Jordan MR, Morrisonponce D. Naloxone. StatPearls. Available at: https://www.ncbi.nlm.nih.gov/books/NBK441910/. Accessed September 1, 2017.
13. Wilkerson RG, Kim HK, Windsor TA, et al. The opioid epidemic in the United States. Emerg Med Clin North Am. 2016;34:e1-e23.
14. Jeffery RM, Dickinson L, Ng ND, et al. Naloxone administration for suspected opioid overdose: an expanded scope of practice by a basic life support collegiate-based emergency medical services agency. J Am Coll Health. 2017;65:212-216.
15. Drugs.com. Naloxone. Available at: https://www.drugs.com/pro/naloxone.html. Accessed April 16, 2018.
16. Prabhu A, Abaid B, Naik S, et al. Naloxone for opioid overdoses. Internet and Psychiatry 2017. Available at: https://www.internetandpsychiatry.com/wp/editorials/naloxone-for-opioid-overdoses/. Accessed September 19, 2017.
17. HHS.gov. Surgeon General releases advisory on naloxone, an opioid overdose-reversing drug. Available at: https://www.hhs.gov/about/news/2018/04/05/surgeon-general-releases-advisory-on-naloxone-an-opioid-overdose-reversing-drug.html. Accessed April 16, 2018.
18. US Department of Health and Human Services. Surgeongeneral.gov. Surgeon General’s advisory on naloxone and opioid overdose. Available at: https://www.surgeongeneral.gov/priorities/opioid-overdose-prevention/naloxone-advisory.html. Accessed April 16, 2018.
19. Behar E, Rowe C, Santos GM, et al. Acceptability of naloxone co-prescription among primary care providers treating patients on long-term opioid therapy for pain. J Gen Intern Med. 2017;32:291-295.
20. Albert S, Brason FW 2nd, Sanford CK, et al. Project Lazarus: community‐based overdose prevention in rural North Carolina. Pain Med. 2011;12:S77-S85.
21. Rowe C, Santos GM, Vittinghoff E, et al. Predictors of participant engagement and naloxone utilization in a community‐based naloxone distribution program. Addiction. 2015;110:1301-1310.
22. Coffin PO, Behar E, Rowe C, et al. Nonrandomized intervention study of naloxone coprescription for primary care patients receiving long-term opioid therapy for pain. Ann Intern Med. 2016;165:245-252.
23. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174.
24. Bird SM, McAuley A, Perry S, et al. Effectiveness of Scotland’s National Naloxone Programme for reducing opioid-related deaths: a before (2006-10) versus after (2011-13) comparison. Addiction. 2016;111:883-891.
1. National Institute on Drug Abuse. Overdose death rates. Revised September 2017. Available at: https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Accessed April 11, 2018.
2. Xu J, Murphy SL, Kochanek KD, et al. Deaths: final data for 2013. Nat Vital Stat Syst. 2016;64:1-119.
3. McCabe SE, West BT, Teter CJ, et al. Trends in medical use, diversion, and nonmedical use of prescription medications among college students from 2003 to 2013: connecting the dots. Addict Behav. 2014;39:1176-1182.
4. Green TC, Heimer R, Grau LE. Distinguishing signs of opioid overdose and indication for naloxone: an evaluation of six overdose training and naloxone distribution programs in the United States. Addiction. 2008;103:979-989.
5. US Department of Health and Human Services. HHS takes strong steps to address opioid-drug related overdose, death and dependence. March 26, 2015. Available at: http://wayback.archive-it.org/3926/20170127185704/https://www.hhs.gov/about/news/2015/03/26/hhs-takes-strong-steps-to-address-opioid-drug-related-overdose-death-and-dependence.html. Accessed April 16, 2018.
6. Robinson A, Wermeling DP. Intranasal naloxone administration for treatment of opioid overdose. Am J Health Syst Pharm. 2014;71:2129-2135.
7. Doyon S, Aks SE, Schaeffer S. Expanding access to naloxone in the United States. J Med Toxicol. 2014;10:431-434.
8. National Institute on Drug Abuse. Which classes of prescription drugs are commonly misused? Available at: https://www.drugabuse.gov/publications/research-reports/misuse-prescription-drugs/which-classes-prescription-drugs-are-commonly-misused. Accessed April 16, 2018.
9. Boom M, Niesters M, Sarton E, et al. Non-analgesic effects of opioids: opioid-induced respiratory depression. Curr Pharm Des. 2012;18:5994-6004.
10. Weaver L, Palombi L, Bastianelli KMS. Naloxone administration for opioid overdose reversal in the prehospital setting: implications for pharmacists. J Pharm Pract. 2018;31:91-98.
11. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367:146-155.
12. Jordan MR, Morrisonponce D. Naloxone. StatPearls. Available at: https://www.ncbi.nlm.nih.gov/books/NBK441910/. Accessed September 1, 2017.
13. Wilkerson RG, Kim HK, Windsor TA, et al. The opioid epidemic in the United States. Emerg Med Clin North Am. 2016;34:e1-e23.
14. Jeffery RM, Dickinson L, Ng ND, et al. Naloxone administration for suspected opioid overdose: an expanded scope of practice by a basic life support collegiate-based emergency medical services agency. J Am Coll Health. 2017;65:212-216.
15. Drugs.com. Naloxone. Available at: https://www.drugs.com/pro/naloxone.html. Accessed April 16, 2018.
16. Prabhu A, Abaid B, Naik S, et al. Naloxone for opioid overdoses. Internet and Psychiatry 2017. Available at: https://www.internetandpsychiatry.com/wp/editorials/naloxone-for-opioid-overdoses/. Accessed September 19, 2017.
17. HHS.gov. Surgeon General releases advisory on naloxone, an opioid overdose-reversing drug. Available at: https://www.hhs.gov/about/news/2018/04/05/surgeon-general-releases-advisory-on-naloxone-an-opioid-overdose-reversing-drug.html. Accessed April 16, 2018.
18. US Department of Health and Human Services. Surgeongeneral.gov. Surgeon General’s advisory on naloxone and opioid overdose. Available at: https://www.surgeongeneral.gov/priorities/opioid-overdose-prevention/naloxone-advisory.html. Accessed April 16, 2018.
19. Behar E, Rowe C, Santos GM, et al. Acceptability of naloxone co-prescription among primary care providers treating patients on long-term opioid therapy for pain. J Gen Intern Med. 2017;32:291-295.
20. Albert S, Brason FW 2nd, Sanford CK, et al. Project Lazarus: community‐based overdose prevention in rural North Carolina. Pain Med. 2011;12:S77-S85.
21. Rowe C, Santos GM, Vittinghoff E, et al. Predictors of participant engagement and naloxone utilization in a community‐based naloxone distribution program. Addiction. 2015;110:1301-1310.
22. Coffin PO, Behar E, Rowe C, et al. Nonrandomized intervention study of naloxone coprescription for primary care patients receiving long-term opioid therapy for pain. Ann Intern Med. 2016;165:245-252.
23. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174.
24. Bird SM, McAuley A, Perry S, et al. Effectiveness of Scotland’s National Naloxone Programme for reducing opioid-related deaths: a before (2006-10) versus after (2011-13) comparison. Addiction. 2016;111:883-891.
From The Journal of Family Practice | 2018;67(5):288-290,292.
Heart Failure: A Dynamic Approach to Classification and Management
CE/CME No: CR-1805
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Develop an understanding of the classification of heart failure and its associated signs and symptoms.
• Describe clinical findings of heart failure from the physical exam.
• Understand the utility of laboratory and imaging studies for diagnosis of heart failure and its underlying causes.
• Demonstrate knowledge of nonpharmacotherapeutic and pharmacotherapeutic options for managing heart failure.
FACULTY
Michael Roscoe is Chair/Program Director and Associate Professor, Andrew Lampkins is Associate Professor, Sean Harper is Assistant Professor, and Gina Niemeier is Associate Program Director and Assistant Professor, in the PA Department at the University of Evansville in Indiana.
The authors have no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through April 30, 2019.
Article begins on next page >>
Heart failure is a complex syndrome with a spectrum of signs and symptoms that range from asymptomatic to terminal. This variability of presentation, paired with the irreversibility of the process, make it both difficult and critical to identify this syndrome early to prevent progression. Here is an overview of the classification and common presentations of heart failure, as well as a guide to diagnostic modalities and treatment options.
Heart failure (HF) is a complex syndrome, not a specific disease; it is always associated with an underlying cause. Hypertension and coronary artery disease are the two most common causes in all age groups, but a number of other conditions—valvular disease, unrecognized obstructive sleep apnea, obesity, chronic kidney disease, anemia, hyperlipidemia, diabetes, and atrial fibrillation—have been identified as secondary causes.1-3
Often, however, clinicians do not identify HF until the syndrome reaches an advanced stage—at which point, the damage is irreversible and pharmacotherapeutic management is limited to control of signs and symptoms. The ramifications are concerning, since HF has achieved near-epidemic scope in the United States. An estimated 5.7 million Americans ages 20 and older have HF; prevalence is projected to increase by 46% between 2012 and 2030—resulting in more than 8 million affected individuals (ages 18 and older).4,5 More than 1 million patients are discharged from the hospital annually with a primary diagnosis of HF.4 And in 2013, one in nine death certificates in the US mentioned HF.4
Most cases of HF are managed in primary care. Established, evidence-based therapies should be implemented in the outpatient setting when possible, as early in the course as possible. Referral to a cardiologist is needed when the underlying cause of HF remains undetermined, or when specialized treatment is required.
CLASSIFICATION OF HF
The two most widely recognized classification systems for HF are those of the American College of Cardiology and the American Heart Association (ACC/AHA) and of the New York Heart Association (NYHA). Both focus on one-year mortality. The stages of the ACC/AHA system (A to D) are based on worsening of both structural heart disease and clinical symptoms of HF. The NYHA designations (Class I to IV) are based on the functional capability associated with physical activity. Both systems are outlined in Table 1.3,6,7

While these systems are used to “stage” HF, there are several ways the syndrome is classified in the medical literature. For example, HF can be described by
- Anatomy (left- or right-sided)
- Physiology (dilated and hypertrophic)
- Course (chronic or acute heart failure [cardiogenic shock])
- Output (high- or low-output failure)
- Ejection fraction (reduced or preserved)
- Pressure phase (systolic and diastolic).
All these classifications have merit; however, this article will attempt to simplify the approach to patients with HF and focus on systolic HF, defined as a reduced left ventricular ejection fraction (LVEF), and diastolic HF, defined as a preserved LVEF. Although the common perception of HF among clinicians—and thus the traditional diagnostic focus—is reduced LVEF (systolic HF), preserved LVEF (diastolic HF) in fact represents approximately 50% of cases.1 Diastolic HF is estimated to be increasing in prevalence and is expected to become the more common phenotype.8
Continue to: SIGNS AND SYMPTOMS
SIGNS AND SYMPTOMS
Heart failure is characterized by a constellation of signs and symptoms of pulmonary and/or systemic venous congestion caused by impaired ability of the heart to fill with or eject blood in proportion to the metabolic needs of the body.1 Manifestations include fatigue, dyspnea, fluid retention, and cachexia, and patients can present anywhere on the spectrum—from asymptomatic at rest to severely symptomatic.
Symptoms
In both reduced LVEF and preserved LVEF HF, common early symptoms include dyspnea and fatigue on exertion, with or without some degree of lower-extremity swelling.2 Lack of treatment and disease progression increase symptom severity—to the extent that dyspnea and fatigue start to occur at rest. Reviewing the anatomic classifications/findings of HF facilitates understanding of the clinical symptoms.
Right HF. Right ventricular dysfunction is rarely found in isolation; when symptoms are present, further evaluation of the left ventricle and the pulmonary system (to look for cor pulmonale) is warranted. Right HF is associated with an inability to manage venous return and move volume into the pulmonary circuit. This produces the predominant symptom of fluid retention. Peripheral edema is a cardinal symptom of right HF; edema can also present systemically, mostly as hepatic congestion or general gastrointestinal complaints resulting from impaired gastrointestinal perfusion.9
Left HF. Left ventricular dysfunction is the more common anatomic finding in HF and is often generalized to represent all cases. The dysfunction can be found in isolation and is actually the leading cause of right HF. In left HF (regardless of etiology), the heart does not produce enough “forward” pressure (ie, cannot pump or fill with enough blood) for the cardiovascular system to remain in balance. Thus, “back” pressure into the pulmonary circuit develops.
The combination of insufficient systemic perfusion capability with a dysfunctional left pump means that the most common symptom in all cases of left HF is shortness of breath—specifically, exertional dyspnea. Dyspnea can progress to orthopnea, paroxysmal nocturnal dyspnea, and, finally, dyspnea at rest. Patients often present with a cough that is worse while lying down and that can be nonproductive or productive, depending on volume status.9,10
Signs
Vital signs range from normal to indicative of shock. Increased sympathetic nervous system activity is common; this may manifest as coldness of the extremities and diaphoresis. Keep in mind that HF is a clinical diagnosis: The physical examination, focusing on peripheral signs, is key in all HF patients for both diagnosis and management.
PHYSICAL EXAMINATION
The physical exam includes the heart, neck, lungs, abdomen, and extremities. The cardiac exam includes evaluation for hypertrophy, by palpating for the point of maximum impulse to assess for lifts, heaves, valvular disease, and S3 and S4 sounds. Respiratory, abdominal, and extremity exams all focus on the evaluation of fluid status and edema.
A key, often underutilized measurement is jugular venous pressure (JVP). Elevated JVP has been identified as the most specific sign of fluid overload in HF and the most important physical finding in the initial and subsequent examinations of a patient with HF.11 (For good reason, clinicians who treat patients with HF must be able to recognize volume overload and hypovolemia; euvolemia allows patients to remain symptom-free and makes it possible to initiate life-prolonging therapy, which will be discussed in the Treatment section.2) JVP is an indirect measure of pressure within the right side of the heart (central venous pressure).
Most texts on performing the physical exam recommend measuring JVP using the right internal jugular vein. However, use of the internal jugular vein is limiting in HF patients, because it is covered by the sternocleidomastoid muscle for most of its course in the neck and visible only in a small triangle between the two heads in the root of the neck (see Figure). Conversely, the external jugular veins are subcutaneous along their entire course and pulsations are easily visible—but superficial and prone to external pressure and internal occlusion. A nonpulsatile, distended jugular vein should not be used to estimate venous pressure.2
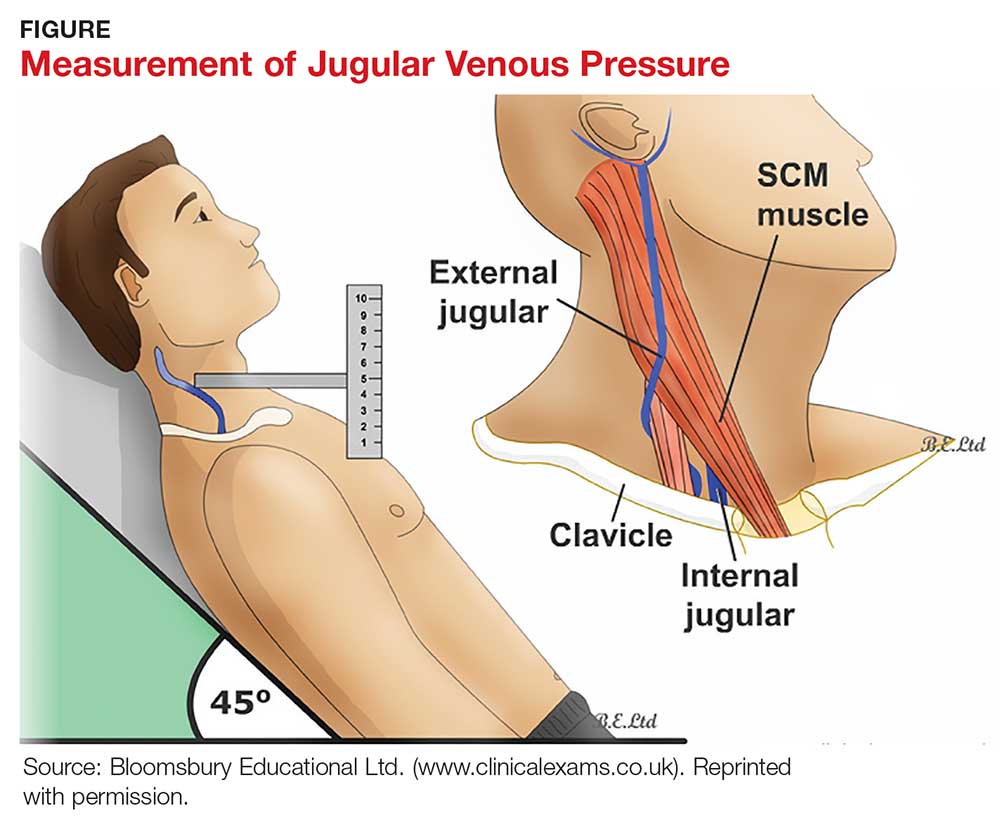
What, then, is the best method? Vinayak et al evaluated the comparative effectiveness of the internal and external jugular veins for detection of central venous pressure. They found that the external jugular vein is easier to visualize and has excellent reliability for determining low and high venous pressures.12
The process for estimating JVP is the same regardless of which jugular vein (internal or external) is used. The technique is described by Bickley in Bates’ Guide to Physical Examination and History Taking:
The patient lies supine and at an angle between 30° and 45°. Turn the head slightly, and with tangential lighting, identify the external and internal jugular veins. Identify the highest point of pulsation in the right jugular vein and extend a long rectangular object or card horizontally from this point and a centimeter ruler vertically from the sternal angle, making an exact right angle. Measure the vertical distance (in centimeters) above the sternal angle where the horizontal object crosses the ruler and add this distance to 4 cm. Measurements of > 3-4 cm above the sternal angle or > 8 cm in total distance are considered elevated.13
Continue to: DIAGNOSTIC AND SCREENING TESTS
DIAGNOSTIC AND SCREENING TESTS
Several diagnostic studies can identify HF or elucidate underlying causes. However, not all tests yield sufficient information for diagnosis—and commonly held “maxims” about findings that definitively rule in or out the diagnosis have been confounded by the available evidence.
Laboratory tests. The lab parameter most associated with HF is brain natriuretic peptide (BNP). Natriuretic peptides are produced primarily within the heart and released into the circulation in response to increased wall tension.14 In contrast to atrial natriuretic peptide (ANP), BNP is secreted not only from the atria but also from the ventricles, especially in patients with HF.15
Circulating concentrations of several cardiac natriuretic peptides—including ANP, BNP, and the two peptides’ N-terminal pro-hormones (N-terminal pro-atrial natriuretic peptide and N-terminal pro-brain natriuretic peptide, respectively) are elevated in both symptomatic and asymptomatic patients with left ventricular dysfunction.16 These levels are generally elevated for both systolic and diastolic HF. However, in one study, as many as 30% of diastolic HF patients had a low BNP level, despite signs and symptoms of HF and significantly elevated left ventricular filling pressures, as determined by invasive hemodynamic monitoring.17 Comorbid obesity is associated with low levels of natriuretic peptides.18
Additional lab tests can provide information about underlying causes of HF or reveal contraindications to certain treatment options (to be discussed in the Treatment section). A complete blood count (CBC) might reveal anemia, which can cause or aggravate HF, and which is an important consideration in management because of its association with decreased renal function, hemodilution, and proinflammatory cytokines. Leukocytosis can signal underlying infection. A troponin assay is helpful for ruling out acute MI as a cause of worsening HF in acute cases. Thyroid function tests and iron studies can be considered to rule out secondary causes of HF.
A serum electrolyte screen should be ordered; results are usually within normal ranges. Hyponatremia is an indicator of activation of the renin–angiotensin–aldosterone system (RAAS) and may be seen in the context of prolonged salt restriction and diuretic therapy. Hyperkalemia and hypokalemia are also prevalent in HF; both are limiting factors for some treatment options. A low sodium level is often the result of increased congestion and release of vasopressin; a level of ≤ 135 mEq/L predicts a poorer outcome.
Kidney function tests can determine whether HF is associated with impaired kidney function, secondary to poor renal perfusion. Poor renal function may limit treatment options. Patients with severe HF, particularly those receiving a high dosage of a diuretic for a long period, may have elevated levels of blood urea nitrogen and creatinine, indicating renal insufficiency.
Electrocardiography and chest radiography. ECGs and chest radiographs are noninvasive tests that have been used widely in the diagnosis of HF. They might indicate an underlying cause (eg, acute MI, ischemia, secondary arrhythmia) but are often nonspecific—and thus may be unhelpful in the diagnosis and treatment of HF.
The most common ECG findings in HF are nonspecific ST-T wave abnormalities.19 Other findings often consist of low-voltage left ventricular hypertrophy, conduction defects, and repolarization changes. With chest radiography, primary findings in HF include pulmonary edema—seen as perivascular edema, peribronchial cuffing, perihilar haze, interstitial edema (Kerley B, or septal, lines), and alveolar fluid—and pleural effusion.19,20
For both these modalities, however, there are commonly held conceptions that particular findings rule out HF—which has been disproven by Fonseca and colleagues.19 Because most patients with HF have an abnormal ECG, some studies have proposed that a normal ECG virtually rules out left ventricular systolic dysfunction.20 Evaluating the value of ECG in HF diagnosis, Fonseca et al found that about 85% of patients with an abnormal ECG had HF—but so did 30% of patients with a normal ECG. They concluded that, if used alone, ECG could have missed as many as 25% of patients with HF.19
Likewise, it has been suggested that a patient cannot have HF if heart size is normal on a chest radiograph.20 Fonseca et al found that cardiac enlargement, while the most informative radiologic measurement in HF, was present in only half of patients with HF.19 About 57% of patients who had an abnormal chest radiograph had HF, but so did about 40% of those who had a normal chest radiograph.19 Therefore, abnormal chest radiograph for identification of HF had an estimated sensitivity of 57%, a specificity of 78%, positive predictive value of 50%, and negative predictive value of 83%.19 The conclusion: Caution should be taken regarding the use of chest radiography in isolation to make a diagnosis of HF.
Echocardiography. The most useful test in evaluating HF is the echocardiogram, because it can distinguish between HF with and without preserved left ventricular systolic function. This is critical: The most clinically relevant classification of HF differentiates systolic and diastolic HF, based on LVEF.2 This determination has both prognostic and therapeutic implications.21
Echocardiography is widely available, safe, and noninvasive. The “echo” can identify the size of the atria and ventricles, valve function and dysfunction, and any associated shunting. Pericardial effusions and heart wall-motion abnormalities (for example, an old MI) are also easily identified.9
A normal ejection fraction does not rule out HF. Therefore, assessment of LVEF should not be considered until after a clinical diagnosis has been made, because more than half of HF patients have a normal LVEF—evidence that can confound the diagnostic process.2
Continue to: TREATMENT OF HEART FAILURE WITH REDUCED LVEF
TREATMENT OF HEART FAILURE WITH REDUCED LVEF
The body’s neurohormonal system, including the RAAS and the sympathetic nervous system, is activated to compensate for the insufficient cardiac performance found in HF. However, activation of these systems contributes to worsening HF, deterioration of quality of life, and poor outcomes.22 Therefore, therapies that suppress these responses can reduce the progression of HF.
Treatment of HF is generally divided into symptom-relieving treatment and disease-modifying/life-prolonging treatment.1 Symptom relief is similar in both systolic and diastolic HF. However, most evidence-based, disease-modifying treatment focuses on systolic HF; guidelines for disease-modifying treatment of diastolic HF are minimal.1
Treatment of reversible causes
Since HF is caused by something else, the primary focus of management is addressing underlying causes. The primary goal is to relieve symptoms while improving functional status—which should lead to a decrease in hospitalization and premature death.
The first step is to evaluate patients’ use of medications that can contribute to a worsening of HF.9 The most common offending medications are calcium-channel blockers with negative inotropy (non-dihydropyridine calcium-channel blockers, eg, verapamil and diltiazem); some antiarrhythmic drugs (eg, amiodarone); thiazolidinediones (glitazones); and NSAIDs.9 If identified as a possible contributor to HF symptoms, these agents should be stopped (if possible) or replaced.
Nonpharmacotherapy
Effective counseling and education of patients with HF may help with long-term adherence to treatment plans. Patients can be taught to monitor their weight at home and to adjust the dosage of diuretics as advised: A sudden increase in weight (> 2 kg in one to three d), for example, should alert a patient to alter treatment or seek advice.23
Diet modification is a multifactorial recommendation. Proper nutrition is critical because HF patients are at increased risk for malnutrition due to poor appetite, malabsorption, and increased nutritional requirements.23 Weight reduction in obese patients helps reduce cardiac workload. Patients should be placed on salt restriction (2 to 2.6 g/d of sodium).9,23
Exercise has been shown to relieve symptoms, provide a greater sense of well-being, and improve functional capacity. It does not, however, result in obvious improvement in cardiac function.22
Alcohol consumption should be restricted because of the myocardial depressant properties of alcohol and its direct toxic effect on the myocardium.22 Smoking should be discouraged because it has a direct effect on coronary artery disease.
Influenza and pneumococcal vaccination should be considered in all patients with HF.23 Heart failure predisposes patients to, and can be exacerbated by, pulmonary infection and exacerbation of chronic obstructive pulmonary disease.
Evaluation and management of obstructive sleep apnea should be performed. Sleep-disordered breathing, an umbrella term that covers obstructive and central sleep apneas, has been found to increase the risk for poor prognosis in HF.24 All patients with HF should be tested for obstructive sleep apnea because, often, only the patient’s bed partner is aware of disordered sleep. For unknown reasons, patients with HF do not report subjective sleepiness.25
Continue to: Pharmacotherapy
Pharmacotherapy
Diuretics have not been found to have benefit for reducing early mortality but are the most common agents used for symptomatic relief of sodium and water retention.26 In fact, few patients with signs and symptoms of fluid retention can be managed without a diuretic.9 Caution must be observed, however, because excessive diuresis can lead to electrolyte imbalance and neurohormonal activation.
Treating mild fluid retention with a thiazide diuretic (hydrochlorothiazide, metolazone, or chlorthalidone) is often sufficient (see Table 2 for dosing and other information on these and other drugs for treating HF).9 Thiazide diuretics are dependent on the glomerular filtration rate and are ineffective when it falls below 30-40 mL/min. Adverse reactions to diuretics include hypokalemia, dehydration (intravascular volume depletion) with prerenal azotemia, skin rash, neutropenia, thrombocytopenia, hyperglycemia, hyperuricemia, and hepatic dysfunction.
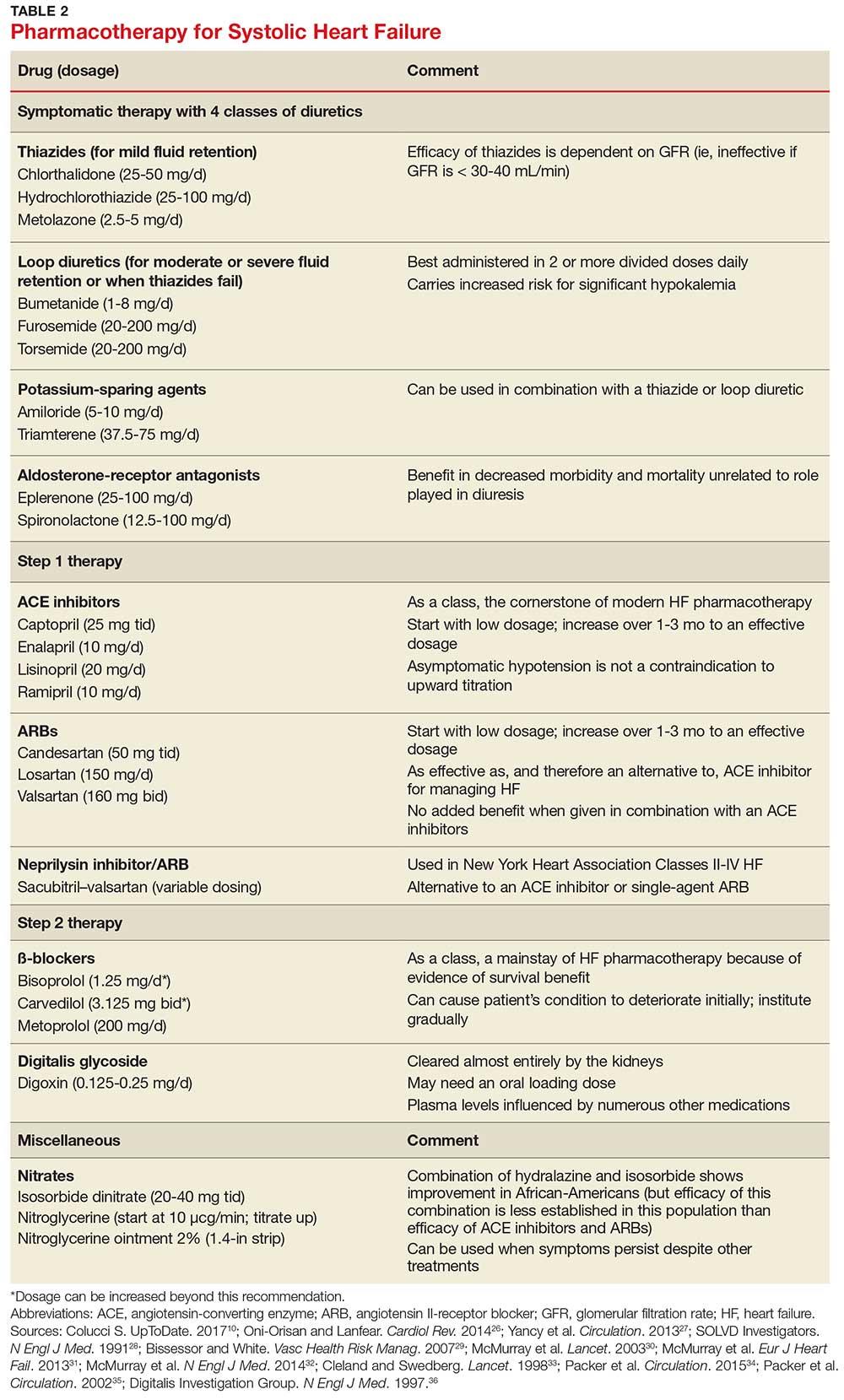
In cases of moderate or severe HF, or failure of thiazide diuretics to relieve mild symptoms, an oral loop diuretic (furosemide, bumetanide, or torsemide; see Table 2 for dosing) can be used if kidney function is preserved.9 These agents are best administered in two or more divided doses. Major adverse reactions are similar to those of thiazide diuretics, plus ototoxicity. Special caution must be used when a loop diuretic is co-administered with digitalis because the combination can cause significant hypokalemia.9
A potassium-sparing diuretic (triamterene or amiloride; see Table 2) can be used in combination with thiazide and loop diuretics.9 The location of their action is at the distal tubule, but diuretic potency is mild. Potassium-sparing agents can minimize hypokalemia induced by other diuretics. Adverse effects include hyperkalemia, kidney dysfunction, and gastrointestinal symptoms.
The aldosterone-receptor antagonists spironolactone and eplerenone are specific inhibitors of aldosterone, an effect that has been shown to improve clinical outcomes.9 Aldosterone-receptor antagonists are indicated for patients with NYHA class II-IV HF who have LVEF ≤ 35% or those with a history of acute MI, LVEF < 40%, and symptoms of HF.27 In these patients, a reduction in mortality and relative risk has been demonstrated with the use of aldosterone-receptor antagonists, unrelated to their role in diuresis. The adverse effect profile of spironolactone includes gynecomastia.
Continue to: Inhibitors of the RAAS
Inhibitors of the RAAS. As noted, the RAAS plays a key role during the development and worsening of HF.22
Angiotensin-converting enzyme inhibitors (ACE inhibitors) constitute the cornerstone of modern HF pharmacotherapy and have compelling evidence of survival benefit.28 These agents (captopril, enalapril, lisinopril, and ramipril; see Table 2 for dosing) have been shown to be effective therapy for HF and post-MI left ventricular dysfunction.29 They reduce early mortality by approximately 20% in symptomatic HF and can prevent hospitalization, increase exercise tolerance, and reduce adverse events (symptoms).9
Because of these benefits, ACE inhibitors should be firstline therapy in all HF patients with left ventricular dysfunction. They are usually used in combination with a diuretic. In addition, ACE inhibitors should be used in patients with reduced LVEF, even if they are asymptomatic, because these agents prevent progression to clinical HF.
Since ACE inhibitors carry the risk for severe hypotension, caution must be used, especially during treatment initiation. Patients whose systolic blood pressure is < 100 mm Hg, or who are hypovolemic, should be started at a low dosage (captopril, 6.25 mg tid; enalapril, 2.5 mg/d; or other equivalent ACE inhibitor dose); for other patients, these dosages can be doubled at initiation.9 Within days of initiation, but no longer than two weeks later, patients should be screened for hypotension and have both kidney function and potassium levels monitored. The dosage of ACE inhibitors should be increased over one to three months to an effective dosage (eg, captopril, 25 mg tid; enalapril, 10 mg bid; ramipril, 10 mg/d; and lisinopril, 20 mg/d, or other equivalent ACE inhibitor dose).9
Asymptomatic hypotension is not a contraindication to continuation or uptitration of the dosage of ACE inhibitors. Patients may also experience an increase in the serum creatinine or potassium level; likewise, this should not prompt a change in dosage if the elevated level stabilizes. The most common adverse effects of ACE inhibitors are dizziness and cough.9
Angiotensin II-receptor blockers (ARBs) have been shown to be as effective as ACE inhibitors for the management of hypertension, congestive HF, and chronic renal failure.30 ARBs decrease the adverse effects of angiotensin II, but do not have the same effects that ACE inhibitors do on other pathways found in HF (specifically, on bradykinin, prostaglandins, and nitric oxide).29 Candesartan and valsartan have been shown to have benefits in HF and are an equivalent alternative to ACE inhibitors; they are often used when a patient cannot tolerate an ACE inhibitor. Because ARBs and ACE inhibitors affect the RAAS at different points in the pathway, there is a theoretical basis for ARB and ACE inhibitor combination therapy; in clinical studies to date, however, the combination has shown no beneficial effect and rather was associated with more adverse effects.29
Neprilysin inhibitor. Recently, the combination of the neprilysin inhibitor sacubitril and an ARB, valsartan, has surfaced as an alternative to ACE inhibitor or single-agent ARB therapy.31,32 Inhibition of the enzyme neprilysin results in an increase in levels of endogenous vasoactive peptides (eg, natriuretic peptides and bradykinin), which may benefit hemodynamics in patients with HF. However, use of an ARB in combination with sacubitril is necessary to counteract the increase in angiotensin II levels that also results from inhibition of neprilysin.33
Sacubitril–valsartan is typically reserved for patients with mild-to-moderate HF who have either an elevated BNP (≥ 50 pg/mL) or an HF-related hospitalization within the past year. Upon transitioning from an ACE inhibitor (or single-agent ARB), a 36-hour washout period must be observed before starting sacubitril–valsartan to minimize the risk for angioedema. Because therapy with sacubitril–valsartan leads directly to an elevation in the BNP level, using the level of N-terminal pro-brain natriuretic peptide—which is not degraded by neprilysin—to monitor disease progression is recommended.34
ß-
Initiation of ß-blockers in stable patients can cause general deterioration; initiation must therefore be done gradually:
- Carvedilol, initiated at 3.125 mg bid, can be increased to 6.25 mg, then 12.5 mg, and then 25 mg, all bid, at intervals of approximately two weeks
- Sustained-release metoprolol can be started at 12.5 mg/d or 25 mg/d and doubled at two weeks to a target of 200 mg/d
- Bisoprolol, initiated at 1.25 mg/d, can be increased incrementally to 2.5 mg/d, 3.75 mg/d, 5 mg/d, 7.5 mg/d, or 10 mg/d at one-to-four-week intervals.9
Patients taking ß-blockers need to monitor their weight at home as an indicator of fluid retention. If HF becomes worse, an increase in the dosage of the accompanying diuretic, a delay in the increase of the ß-blocker, or downward adjustment of the ß-blocker is usually sufficient.
Continue to: Digitalis glycoside
Digitalis glycoside. Digoxin has been shown to relieve the symptoms of HF, decrease the risk for hospitalization, and increase exercise tolerance; however, it has not been shown to offer a mortality benefit.36 Digoxin should be considered for patients who remain symptomatic when taking a diuretic and an ACE inhibitor and for patients who are also in atrial fibrillation and need rate control.
Digoxin is cleared almost entirely by the kidneys and therefore must be used with care in patients with renal dysfunction. Patients usually can be started on the expected maintenance dosage (0.125-0.25 mg/d). An oral loading dose of 0.75-1.25 mg over 24 to 48 hours can be used if an early effect is needed.9
Digoxin can induce ventricular arrhythmias (especially in a setting of hypokalemia and ischemia) and is sensitive to other medications that can drastically increase its level—most notably, amiodarone, quinidine, propafenone, and verapamil.9 The digoxin blood level should be measured every seven to 14 days until a maintenance dosage is established, and again whenever there is a change in medication or kidney function. The optimum serum level is 0.7-1.2 ng/mL; toxicity is not usually seen at < 1.8 ng/mL.9
Hydralazine and nitrates. The combination of hydralazine and isosorbide dinitrate has been shown to improve outcomes in African-American patients with HF, but efficacy is less well-established in this population than for ACE inhibitor and ARB therapy.9 This combination can be considered in patients who are unable to tolerate ACE inhibitor or ARB therapy. It can also be considered in those who have persistent symptoms despite treatment with a ß-blocker, ACE inhibitor, or aldosterone antagonist.9
Intravenous nitrates are used primarily for acute HF, especially when accompanied by hypertension or myocardial ischemia. The starting dosage for nitroglycerin is approximately 10 μcg/min, titrated upward by 10-20 μcg/min to a maximum of 200 μcg/min. Isosorbide dinitrate (20-40 mg tid) and nitroglycerine ointment 2% (1.4 in applied every six to eight hours [although generally reserved for inpatient therapy]) are equally effective.9 Adverse effects that may limit the use of these agents are headache and hypotension. Patients also develop tolerance for nitrates, which can be mitigated if a daily 8-to-12–hour nitrate-free period is instituted.9
TREATMENT OF HEART FAILURE WITH PRESERVED LVEF
Treatment options for patients with preserved LVEF (diastolic HF) are not as clear as those for patients with reduced LVEF (systolic HF). No traditional therapies (ACE inhibitors, ARBs, ß-blockers, digoxin) have been shown to improve survival in this population, although a recent study on the effects of spironolactone in diastolic HF did show some improvement of diastolic dysfunction without adverse effects.17,37 In the absense of clear evidence-based therapies, treatment focuses on managing comorbidities, addressing reversible causes, and alleviating fluid overload with a diuretic.9,17
Diuretic therapy is critical to control fluid overload; regimens are similar to those for HF with reduced LVEF. ACE inhibitors and ARBs have not been shown to improve outcomes in this population but can be used to manage comorbid hypertension. Spironolactone has also not been shown to improve outcomes in patients with diastolic HF.9
The principal conditions that can lead to HF with preserved LVEF are hypertension, pericardial disease, and atrial tachycardia. Tachycardia is associated with overall shorter diastolic filling time; controlling an accelerated heart rate, therefore, is theorized to be an important therapeutic goal.9 Other disease states, including diabetes mellitus, sleep-disordered breathing, obesity, and chronic kidney disease, can all lead to HF with preserved LVEF.
CONCLUSION
Managing HF today requires an “upstream” model of care, by which providers consider the diagnosis/syndrome of HF in the asymptomatic patient with risk factors (Stage A/Class I and Stage B/Class II). Perhaps a better way to state this is that providers must change their approach from ruling-in to ruling-out HF. Any person in whom a decrease in activity level, mild shortness of breath, or edema are observed, and who has known risk factors, should be considered to have HF until proven otherwise.
Because of the wide variability in the underlying causes of HF, providers must be dynamic in both their clinical approach and their treatment plans to optimize care for the individual patient. Finally, providers must also take note of the increasing prevalence of diastolic HF and, again, focus on early diagnosis.
1. Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13(1):18-28.
2. Ahmed A. DEFEAT - Heart failure: a guide to management of geriatric heart failure by generalist physicians. Minerva Med. 2009;100(1):39-50.
3. Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1995 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol. 2001; 38(7):2101-2113.
4. Mozaffarian D, Benjamin EJ, Go AS, et al; Writing Group Members, American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2016 Update: A report from the American Heart Association. Circulation. 2016;26: 133(4):e38-e360.
5. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619.
6. American Heart Association. Classes of heart failure (2017). www.heart.org/HEARTORG/Conditions/HeartFailure/AboutHeartFailure/Classes-of-Heart-Failure_UCM_306328_Article.jsp#.V8cFhfkrLRY. Accessed March 7, 2018.
7. New York Heart Association Criteria Committee. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed. Boston, MA: Lippincott Williams and Wilkins; 1994.
8. Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251-259.
9. Bashore T, Granger C, Hranitzy P, Patel M. Heart diseases. In: Papadakis MA, McPhee SJ, Rabow MW, eds. Current Medical Diagnosis and Treatment. 55th ed. New York, NY: McGraw-Hill Education; 2016:402.
10. Colucci S. Patient education: heart failure (beyond the basics). Waltham, MA: UpToDate; 2017. www.uptodate.com/contents/heart-failure-beyond-the-basics?view=print. Accessed March 7, 2018.
11. Butman S, Ewy GA, Standen J, et al. Bedside cardiovascular examination in patients with severe chronic heart failure: importance of rest or inducible jugular venous distention. J Am Coll Cardiol. 1993;22(4):968-974.
12. Vinayak A, Levitt J, Gehlbach B, et al. Usefulness of the external jugular vein examination in detecting abnormal central venous pressure in critically ill patients. Arch Intern Med. 2006;166(19):2132-2137.
13. Bickley LA. Bates’ Guide to Physical Examination and History Taking. 11th ed. Alphen aan den Rijn, The Netherlands: Wolters Kluwer; 2012:364-365.
14. Kinnunen P, Vuolteenaho O, Ruskoaho H. Mechanisms of atrial and brain natriuretic peptide release from rat ventricular myocardium: effect of stretching. Endocrinology. 1993;132(5):1961-1970.
15. Yasue H, Yoshimura M, Sumida H, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90(1):195-204.
16. Cowie M, Struthers A, Wood D, et al. Value of natriuretic peptides in assessment of patients with possible new heart failure in primary care. Lancet. 1997;350(9088):1349-1353.
17. Oktay AA, Shah SJ. Diagnosis and management of heart failure with preserved ejection fraction: 10 key lessons. Curr Cardiol Rev. 2015;11(1):42-52.
18. Horwich TB, Hamilton MA, Fonarow GC. B-type natriuretic peptide levels in obese patients with advanced heart failure. J Am Coll Cardiol. 2006;47(1):85-90.
19. Fonseca C, Mota T, Morais H; EPICA Investigators. The value of the electrocardiogram and chest X-ray for confirming or refuting a suspected diagnosis of heart failure in the community. Eur J Heart Fail. 2006;6(6):807-812.
20. Rihal CS, Davis KB, Kennedy JW, Gersch BJ. The utility of clinical, electrocardiographic, and roentgenographic variables in the prediction of left ventricular function. Am J Cardiol. 1995;75(4):220-223.
21. Joint Commission on Accreditation of Healthcare Organizations. A Comprehensive Review of Development and Testing for National Implementation of Hospital Core Measures. 2002. www.jointcommission.org/assets/1/18/A_Comprehensive_Review_of_Development_for_Core_Measures.pdf. Accessed March 6, 2018.
22. Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovasc Pathol. 2012;21(5):365-371.
23. Gibbs CR, Jackson G, Lip GY. ABC of heart failure: Nondrug management. BMJ. 2000;320(7231):365-369.
24. Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49(15):1625-1631.
25. Arzt M, Young T, Finn L, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166(16):1716-1722.
26. Oni-Orisan A, Lanfear DE. Pharmacogenomics in heart failure: where are we now and how can we reach clinical application? Cardiol Rev. 2014;22(5):193-198.
27. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810-1852.
28. SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293-302.
29. Bissessor N, White H. Valsartan in the treatment of heart failure or left ventricular dysfunction after myocardial infarction. Vasc Health Risk Manag. 2007;3(4):425-430.
30. McMurray JJ, Ostegren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362(9386):767-771.
31. McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Committees and Investigators. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail. 2013;15(9):1062-1073.
32. McMurray J, Packer M, Desai A, et al; PARADIGM-HF Committees and Investigators. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004.
33. Cleland JG, Swedberg K. Lack of efficacy of neutral endopeptidase inhibitor ecadotril in heart failure. The International Ecadotril Multi-centre Dose-ranging Study Investigators. Lancet. 1998;351(9116):1657-1658.
34. Packer M, McMurray JJ, Desai AS, et al; PARADIGM-HF Committees and Investigators. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131(1):54-61.
35. Packer M, Fowler MB, Roecker EB, et al; Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106(17):2194-2199.
36. Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336(8):525-533.
37. Edelmann F, Wachter R, Schmidt AG, et al; Aldo-DHF Investigators. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309(8):781-791.
CE/CME No: CR-1805
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Develop an understanding of the classification of heart failure and its associated signs and symptoms.
• Describe clinical findings of heart failure from the physical exam.
• Understand the utility of laboratory and imaging studies for diagnosis of heart failure and its underlying causes.
• Demonstrate knowledge of nonpharmacotherapeutic and pharmacotherapeutic options for managing heart failure.
FACULTY
Michael Roscoe is Chair/Program Director and Associate Professor, Andrew Lampkins is Associate Professor, Sean Harper is Assistant Professor, and Gina Niemeier is Associate Program Director and Assistant Professor, in the PA Department at the University of Evansville in Indiana.
The authors have no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through April 30, 2019.
Article begins on next page >>
Heart failure is a complex syndrome with a spectrum of signs and symptoms that range from asymptomatic to terminal. This variability of presentation, paired with the irreversibility of the process, make it both difficult and critical to identify this syndrome early to prevent progression. Here is an overview of the classification and common presentations of heart failure, as well as a guide to diagnostic modalities and treatment options.
Heart failure (HF) is a complex syndrome, not a specific disease; it is always associated with an underlying cause. Hypertension and coronary artery disease are the two most common causes in all age groups, but a number of other conditions—valvular disease, unrecognized obstructive sleep apnea, obesity, chronic kidney disease, anemia, hyperlipidemia, diabetes, and atrial fibrillation—have been identified as secondary causes.1-3
Often, however, clinicians do not identify HF until the syndrome reaches an advanced stage—at which point, the damage is irreversible and pharmacotherapeutic management is limited to control of signs and symptoms. The ramifications are concerning, since HF has achieved near-epidemic scope in the United States. An estimated 5.7 million Americans ages 20 and older have HF; prevalence is projected to increase by 46% between 2012 and 2030—resulting in more than 8 million affected individuals (ages 18 and older).4,5 More than 1 million patients are discharged from the hospital annually with a primary diagnosis of HF.4 And in 2013, one in nine death certificates in the US mentioned HF.4
Most cases of HF are managed in primary care. Established, evidence-based therapies should be implemented in the outpatient setting when possible, as early in the course as possible. Referral to a cardiologist is needed when the underlying cause of HF remains undetermined, or when specialized treatment is required.
CLASSIFICATION OF HF
The two most widely recognized classification systems for HF are those of the American College of Cardiology and the American Heart Association (ACC/AHA) and of the New York Heart Association (NYHA). Both focus on one-year mortality. The stages of the ACC/AHA system (A to D) are based on worsening of both structural heart disease and clinical symptoms of HF. The NYHA designations (Class I to IV) are based on the functional capability associated with physical activity. Both systems are outlined in Table 1.3,6,7

While these systems are used to “stage” HF, there are several ways the syndrome is classified in the medical literature. For example, HF can be described by
- Anatomy (left- or right-sided)
- Physiology (dilated and hypertrophic)
- Course (chronic or acute heart failure [cardiogenic shock])
- Output (high- or low-output failure)
- Ejection fraction (reduced or preserved)
- Pressure phase (systolic and diastolic).
All these classifications have merit; however, this article will attempt to simplify the approach to patients with HF and focus on systolic HF, defined as a reduced left ventricular ejection fraction (LVEF), and diastolic HF, defined as a preserved LVEF. Although the common perception of HF among clinicians—and thus the traditional diagnostic focus—is reduced LVEF (systolic HF), preserved LVEF (diastolic HF) in fact represents approximately 50% of cases.1 Diastolic HF is estimated to be increasing in prevalence and is expected to become the more common phenotype.8
Continue to: SIGNS AND SYMPTOMS
SIGNS AND SYMPTOMS
Heart failure is characterized by a constellation of signs and symptoms of pulmonary and/or systemic venous congestion caused by impaired ability of the heart to fill with or eject blood in proportion to the metabolic needs of the body.1 Manifestations include fatigue, dyspnea, fluid retention, and cachexia, and patients can present anywhere on the spectrum—from asymptomatic at rest to severely symptomatic.
Symptoms
In both reduced LVEF and preserved LVEF HF, common early symptoms include dyspnea and fatigue on exertion, with or without some degree of lower-extremity swelling.2 Lack of treatment and disease progression increase symptom severity—to the extent that dyspnea and fatigue start to occur at rest. Reviewing the anatomic classifications/findings of HF facilitates understanding of the clinical symptoms.
Right HF. Right ventricular dysfunction is rarely found in isolation; when symptoms are present, further evaluation of the left ventricle and the pulmonary system (to look for cor pulmonale) is warranted. Right HF is associated with an inability to manage venous return and move volume into the pulmonary circuit. This produces the predominant symptom of fluid retention. Peripheral edema is a cardinal symptom of right HF; edema can also present systemically, mostly as hepatic congestion or general gastrointestinal complaints resulting from impaired gastrointestinal perfusion.9
Left HF. Left ventricular dysfunction is the more common anatomic finding in HF and is often generalized to represent all cases. The dysfunction can be found in isolation and is actually the leading cause of right HF. In left HF (regardless of etiology), the heart does not produce enough “forward” pressure (ie, cannot pump or fill with enough blood) for the cardiovascular system to remain in balance. Thus, “back” pressure into the pulmonary circuit develops.
The combination of insufficient systemic perfusion capability with a dysfunctional left pump means that the most common symptom in all cases of left HF is shortness of breath—specifically, exertional dyspnea. Dyspnea can progress to orthopnea, paroxysmal nocturnal dyspnea, and, finally, dyspnea at rest. Patients often present with a cough that is worse while lying down and that can be nonproductive or productive, depending on volume status.9,10
Signs
Vital signs range from normal to indicative of shock. Increased sympathetic nervous system activity is common; this may manifest as coldness of the extremities and diaphoresis. Keep in mind that HF is a clinical diagnosis: The physical examination, focusing on peripheral signs, is key in all HF patients for both diagnosis and management.
PHYSICAL EXAMINATION
The physical exam includes the heart, neck, lungs, abdomen, and extremities. The cardiac exam includes evaluation for hypertrophy, by palpating for the point of maximum impulse to assess for lifts, heaves, valvular disease, and S3 and S4 sounds. Respiratory, abdominal, and extremity exams all focus on the evaluation of fluid status and edema.
A key, often underutilized measurement is jugular venous pressure (JVP). Elevated JVP has been identified as the most specific sign of fluid overload in HF and the most important physical finding in the initial and subsequent examinations of a patient with HF.11 (For good reason, clinicians who treat patients with HF must be able to recognize volume overload and hypovolemia; euvolemia allows patients to remain symptom-free and makes it possible to initiate life-prolonging therapy, which will be discussed in the Treatment section.2) JVP is an indirect measure of pressure within the right side of the heart (central venous pressure).
Most texts on performing the physical exam recommend measuring JVP using the right internal jugular vein. However, use of the internal jugular vein is limiting in HF patients, because it is covered by the sternocleidomastoid muscle for most of its course in the neck and visible only in a small triangle between the two heads in the root of the neck (see Figure). Conversely, the external jugular veins are subcutaneous along their entire course and pulsations are easily visible—but superficial and prone to external pressure and internal occlusion. A nonpulsatile, distended jugular vein should not be used to estimate venous pressure.2

What, then, is the best method? Vinayak et al evaluated the comparative effectiveness of the internal and external jugular veins for detection of central venous pressure. They found that the external jugular vein is easier to visualize and has excellent reliability for determining low and high venous pressures.12
The process for estimating JVP is the same regardless of which jugular vein (internal or external) is used. The technique is described by Bickley in Bates’ Guide to Physical Examination and History Taking:
The patient lies supine and at an angle between 30° and 45°. Turn the head slightly, and with tangential lighting, identify the external and internal jugular veins. Identify the highest point of pulsation in the right jugular vein and extend a long rectangular object or card horizontally from this point and a centimeter ruler vertically from the sternal angle, making an exact right angle. Measure the vertical distance (in centimeters) above the sternal angle where the horizontal object crosses the ruler and add this distance to 4 cm. Measurements of > 3-4 cm above the sternal angle or > 8 cm in total distance are considered elevated.13
Continue to: DIAGNOSTIC AND SCREENING TESTS
DIAGNOSTIC AND SCREENING TESTS
Several diagnostic studies can identify HF or elucidate underlying causes. However, not all tests yield sufficient information for diagnosis—and commonly held “maxims” about findings that definitively rule in or out the diagnosis have been confounded by the available evidence.
Laboratory tests. The lab parameter most associated with HF is brain natriuretic peptide (BNP). Natriuretic peptides are produced primarily within the heart and released into the circulation in response to increased wall tension.14 In contrast to atrial natriuretic peptide (ANP), BNP is secreted not only from the atria but also from the ventricles, especially in patients with HF.15
Circulating concentrations of several cardiac natriuretic peptides—including ANP, BNP, and the two peptides’ N-terminal pro-hormones (N-terminal pro-atrial natriuretic peptide and N-terminal pro-brain natriuretic peptide, respectively) are elevated in both symptomatic and asymptomatic patients with left ventricular dysfunction.16 These levels are generally elevated for both systolic and diastolic HF. However, in one study, as many as 30% of diastolic HF patients had a low BNP level, despite signs and symptoms of HF and significantly elevated left ventricular filling pressures, as determined by invasive hemodynamic monitoring.17 Comorbid obesity is associated with low levels of natriuretic peptides.18
Additional lab tests can provide information about underlying causes of HF or reveal contraindications to certain treatment options (to be discussed in the Treatment section). A complete blood count (CBC) might reveal anemia, which can cause or aggravate HF, and which is an important consideration in management because of its association with decreased renal function, hemodilution, and proinflammatory cytokines. Leukocytosis can signal underlying infection. A troponin assay is helpful for ruling out acute MI as a cause of worsening HF in acute cases. Thyroid function tests and iron studies can be considered to rule out secondary causes of HF.
A serum electrolyte screen should be ordered; results are usually within normal ranges. Hyponatremia is an indicator of activation of the renin–angiotensin–aldosterone system (RAAS) and may be seen in the context of prolonged salt restriction and diuretic therapy. Hyperkalemia and hypokalemia are also prevalent in HF; both are limiting factors for some treatment options. A low sodium level is often the result of increased congestion and release of vasopressin; a level of ≤ 135 mEq/L predicts a poorer outcome.
Kidney function tests can determine whether HF is associated with impaired kidney function, secondary to poor renal perfusion. Poor renal function may limit treatment options. Patients with severe HF, particularly those receiving a high dosage of a diuretic for a long period, may have elevated levels of blood urea nitrogen and creatinine, indicating renal insufficiency.
Electrocardiography and chest radiography. ECGs and chest radiographs are noninvasive tests that have been used widely in the diagnosis of HF. They might indicate an underlying cause (eg, acute MI, ischemia, secondary arrhythmia) but are often nonspecific—and thus may be unhelpful in the diagnosis and treatment of HF.
The most common ECG findings in HF are nonspecific ST-T wave abnormalities.19 Other findings often consist of low-voltage left ventricular hypertrophy, conduction defects, and repolarization changes. With chest radiography, primary findings in HF include pulmonary edema—seen as perivascular edema, peribronchial cuffing, perihilar haze, interstitial edema (Kerley B, or septal, lines), and alveolar fluid—and pleural effusion.19,20
For both these modalities, however, there are commonly held conceptions that particular findings rule out HF—which has been disproven by Fonseca and colleagues.19 Because most patients with HF have an abnormal ECG, some studies have proposed that a normal ECG virtually rules out left ventricular systolic dysfunction.20 Evaluating the value of ECG in HF diagnosis, Fonseca et al found that about 85% of patients with an abnormal ECG had HF—but so did 30% of patients with a normal ECG. They concluded that, if used alone, ECG could have missed as many as 25% of patients with HF.19
Likewise, it has been suggested that a patient cannot have HF if heart size is normal on a chest radiograph.20 Fonseca et al found that cardiac enlargement, while the most informative radiologic measurement in HF, was present in only half of patients with HF.19 About 57% of patients who had an abnormal chest radiograph had HF, but so did about 40% of those who had a normal chest radiograph.19 Therefore, abnormal chest radiograph for identification of HF had an estimated sensitivity of 57%, a specificity of 78%, positive predictive value of 50%, and negative predictive value of 83%.19 The conclusion: Caution should be taken regarding the use of chest radiography in isolation to make a diagnosis of HF.
Echocardiography. The most useful test in evaluating HF is the echocardiogram, because it can distinguish between HF with and without preserved left ventricular systolic function. This is critical: The most clinically relevant classification of HF differentiates systolic and diastolic HF, based on LVEF.2 This determination has both prognostic and therapeutic implications.21
Echocardiography is widely available, safe, and noninvasive. The “echo” can identify the size of the atria and ventricles, valve function and dysfunction, and any associated shunting. Pericardial effusions and heart wall-motion abnormalities (for example, an old MI) are also easily identified.9
A normal ejection fraction does not rule out HF. Therefore, assessment of LVEF should not be considered until after a clinical diagnosis has been made, because more than half of HF patients have a normal LVEF—evidence that can confound the diagnostic process.2
Continue to: TREATMENT OF HEART FAILURE WITH REDUCED LVEF
TREATMENT OF HEART FAILURE WITH REDUCED LVEF
The body’s neurohormonal system, including the RAAS and the sympathetic nervous system, is activated to compensate for the insufficient cardiac performance found in HF. However, activation of these systems contributes to worsening HF, deterioration of quality of life, and poor outcomes.22 Therefore, therapies that suppress these responses can reduce the progression of HF.
Treatment of HF is generally divided into symptom-relieving treatment and disease-modifying/life-prolonging treatment.1 Symptom relief is similar in both systolic and diastolic HF. However, most evidence-based, disease-modifying treatment focuses on systolic HF; guidelines for disease-modifying treatment of diastolic HF are minimal.1
Treatment of reversible causes
Since HF is caused by something else, the primary focus of management is addressing underlying causes. The primary goal is to relieve symptoms while improving functional status—which should lead to a decrease in hospitalization and premature death.
The first step is to evaluate patients’ use of medications that can contribute to a worsening of HF.9 The most common offending medications are calcium-channel blockers with negative inotropy (non-dihydropyridine calcium-channel blockers, eg, verapamil and diltiazem); some antiarrhythmic drugs (eg, amiodarone); thiazolidinediones (glitazones); and NSAIDs.9 If identified as a possible contributor to HF symptoms, these agents should be stopped (if possible) or replaced.
Nonpharmacotherapy
Effective counseling and education of patients with HF may help with long-term adherence to treatment plans. Patients can be taught to monitor their weight at home and to adjust the dosage of diuretics as advised: A sudden increase in weight (> 2 kg in one to three d), for example, should alert a patient to alter treatment or seek advice.23
Diet modification is a multifactorial recommendation. Proper nutrition is critical because HF patients are at increased risk for malnutrition due to poor appetite, malabsorption, and increased nutritional requirements.23 Weight reduction in obese patients helps reduce cardiac workload. Patients should be placed on salt restriction (2 to 2.6 g/d of sodium).9,23
Exercise has been shown to relieve symptoms, provide a greater sense of well-being, and improve functional capacity. It does not, however, result in obvious improvement in cardiac function.22
Alcohol consumption should be restricted because of the myocardial depressant properties of alcohol and its direct toxic effect on the myocardium.22 Smoking should be discouraged because it has a direct effect on coronary artery disease.
Influenza and pneumococcal vaccination should be considered in all patients with HF.23 Heart failure predisposes patients to, and can be exacerbated by, pulmonary infection and exacerbation of chronic obstructive pulmonary disease.
Evaluation and management of obstructive sleep apnea should be performed. Sleep-disordered breathing, an umbrella term that covers obstructive and central sleep apneas, has been found to increase the risk for poor prognosis in HF.24 All patients with HF should be tested for obstructive sleep apnea because, often, only the patient’s bed partner is aware of disordered sleep. For unknown reasons, patients with HF do not report subjective sleepiness.25
Continue to: Pharmacotherapy
Pharmacotherapy
Diuretics have not been found to have benefit for reducing early mortality but are the most common agents used for symptomatic relief of sodium and water retention.26 In fact, few patients with signs and symptoms of fluid retention can be managed without a diuretic.9 Caution must be observed, however, because excessive diuresis can lead to electrolyte imbalance and neurohormonal activation.
Treating mild fluid retention with a thiazide diuretic (hydrochlorothiazide, metolazone, or chlorthalidone) is often sufficient (see Table 2 for dosing and other information on these and other drugs for treating HF).9 Thiazide diuretics are dependent on the glomerular filtration rate and are ineffective when it falls below 30-40 mL/min. Adverse reactions to diuretics include hypokalemia, dehydration (intravascular volume depletion) with prerenal azotemia, skin rash, neutropenia, thrombocytopenia, hyperglycemia, hyperuricemia, and hepatic dysfunction.

In cases of moderate or severe HF, or failure of thiazide diuretics to relieve mild symptoms, an oral loop diuretic (furosemide, bumetanide, or torsemide; see Table 2 for dosing) can be used if kidney function is preserved.9 These agents are best administered in two or more divided doses. Major adverse reactions are similar to those of thiazide diuretics, plus ototoxicity. Special caution must be used when a loop diuretic is co-administered with digitalis because the combination can cause significant hypokalemia.9
A potassium-sparing diuretic (triamterene or amiloride; see Table 2) can be used in combination with thiazide and loop diuretics.9 The location of their action is at the distal tubule, but diuretic potency is mild. Potassium-sparing agents can minimize hypokalemia induced by other diuretics. Adverse effects include hyperkalemia, kidney dysfunction, and gastrointestinal symptoms.
The aldosterone-receptor antagonists spironolactone and eplerenone are specific inhibitors of aldosterone, an effect that has been shown to improve clinical outcomes.9 Aldosterone-receptor antagonists are indicated for patients with NYHA class II-IV HF who have LVEF ≤ 35% or those with a history of acute MI, LVEF < 40%, and symptoms of HF.27 In these patients, a reduction in mortality and relative risk has been demonstrated with the use of aldosterone-receptor antagonists, unrelated to their role in diuresis. The adverse effect profile of spironolactone includes gynecomastia.
Continue to: Inhibitors of the RAAS
Inhibitors of the RAAS. As noted, the RAAS plays a key role during the development and worsening of HF.22
Angiotensin-converting enzyme inhibitors (ACE inhibitors) constitute the cornerstone of modern HF pharmacotherapy and have compelling evidence of survival benefit.28 These agents (captopril, enalapril, lisinopril, and ramipril; see Table 2 for dosing) have been shown to be effective therapy for HF and post-MI left ventricular dysfunction.29 They reduce early mortality by approximately 20% in symptomatic HF and can prevent hospitalization, increase exercise tolerance, and reduce adverse events (symptoms).9
Because of these benefits, ACE inhibitors should be firstline therapy in all HF patients with left ventricular dysfunction. They are usually used in combination with a diuretic. In addition, ACE inhibitors should be used in patients with reduced LVEF, even if they are asymptomatic, because these agents prevent progression to clinical HF.
Since ACE inhibitors carry the risk for severe hypotension, caution must be used, especially during treatment initiation. Patients whose systolic blood pressure is < 100 mm Hg, or who are hypovolemic, should be started at a low dosage (captopril, 6.25 mg tid; enalapril, 2.5 mg/d; or other equivalent ACE inhibitor dose); for other patients, these dosages can be doubled at initiation.9 Within days of initiation, but no longer than two weeks later, patients should be screened for hypotension and have both kidney function and potassium levels monitored. The dosage of ACE inhibitors should be increased over one to three months to an effective dosage (eg, captopril, 25 mg tid; enalapril, 10 mg bid; ramipril, 10 mg/d; and lisinopril, 20 mg/d, or other equivalent ACE inhibitor dose).9
Asymptomatic hypotension is not a contraindication to continuation or uptitration of the dosage of ACE inhibitors. Patients may also experience an increase in the serum creatinine or potassium level; likewise, this should not prompt a change in dosage if the elevated level stabilizes. The most common adverse effects of ACE inhibitors are dizziness and cough.9
Angiotensin II-receptor blockers (ARBs) have been shown to be as effective as ACE inhibitors for the management of hypertension, congestive HF, and chronic renal failure.30 ARBs decrease the adverse effects of angiotensin II, but do not have the same effects that ACE inhibitors do on other pathways found in HF (specifically, on bradykinin, prostaglandins, and nitric oxide).29 Candesartan and valsartan have been shown to have benefits in HF and are an equivalent alternative to ACE inhibitors; they are often used when a patient cannot tolerate an ACE inhibitor. Because ARBs and ACE inhibitors affect the RAAS at different points in the pathway, there is a theoretical basis for ARB and ACE inhibitor combination therapy; in clinical studies to date, however, the combination has shown no beneficial effect and rather was associated with more adverse effects.29
Neprilysin inhibitor. Recently, the combination of the neprilysin inhibitor sacubitril and an ARB, valsartan, has surfaced as an alternative to ACE inhibitor or single-agent ARB therapy.31,32 Inhibition of the enzyme neprilysin results in an increase in levels of endogenous vasoactive peptides (eg, natriuretic peptides and bradykinin), which may benefit hemodynamics in patients with HF. However, use of an ARB in combination with sacubitril is necessary to counteract the increase in angiotensin II levels that also results from inhibition of neprilysin.33
Sacubitril–valsartan is typically reserved for patients with mild-to-moderate HF who have either an elevated BNP (≥ 50 pg/mL) or an HF-related hospitalization within the past year. Upon transitioning from an ACE inhibitor (or single-agent ARB), a 36-hour washout period must be observed before starting sacubitril–valsartan to minimize the risk for angioedema. Because therapy with sacubitril–valsartan leads directly to an elevation in the BNP level, using the level of N-terminal pro-brain natriuretic peptide—which is not degraded by neprilysin—to monitor disease progression is recommended.34
ß-
Initiation of ß-blockers in stable patients can cause general deterioration; initiation must therefore be done gradually:
- Carvedilol, initiated at 3.125 mg bid, can be increased to 6.25 mg, then 12.5 mg, and then 25 mg, all bid, at intervals of approximately two weeks
- Sustained-release metoprolol can be started at 12.5 mg/d or 25 mg/d and doubled at two weeks to a target of 200 mg/d
- Bisoprolol, initiated at 1.25 mg/d, can be increased incrementally to 2.5 mg/d, 3.75 mg/d, 5 mg/d, 7.5 mg/d, or 10 mg/d at one-to-four-week intervals.9
Patients taking ß-blockers need to monitor their weight at home as an indicator of fluid retention. If HF becomes worse, an increase in the dosage of the accompanying diuretic, a delay in the increase of the ß-blocker, or downward adjustment of the ß-blocker is usually sufficient.
Continue to: Digitalis glycoside
Digitalis glycoside. Digoxin has been shown to relieve the symptoms of HF, decrease the risk for hospitalization, and increase exercise tolerance; however, it has not been shown to offer a mortality benefit.36 Digoxin should be considered for patients who remain symptomatic when taking a diuretic and an ACE inhibitor and for patients who are also in atrial fibrillation and need rate control.
Digoxin is cleared almost entirely by the kidneys and therefore must be used with care in patients with renal dysfunction. Patients usually can be started on the expected maintenance dosage (0.125-0.25 mg/d). An oral loading dose of 0.75-1.25 mg over 24 to 48 hours can be used if an early effect is needed.9
Digoxin can induce ventricular arrhythmias (especially in a setting of hypokalemia and ischemia) and is sensitive to other medications that can drastically increase its level—most notably, amiodarone, quinidine, propafenone, and verapamil.9 The digoxin blood level should be measured every seven to 14 days until a maintenance dosage is established, and again whenever there is a change in medication or kidney function. The optimum serum level is 0.7-1.2 ng/mL; toxicity is not usually seen at < 1.8 ng/mL.9
Hydralazine and nitrates. The combination of hydralazine and isosorbide dinitrate has been shown to improve outcomes in African-American patients with HF, but efficacy is less well-established in this population than for ACE inhibitor and ARB therapy.9 This combination can be considered in patients who are unable to tolerate ACE inhibitor or ARB therapy. It can also be considered in those who have persistent symptoms despite treatment with a ß-blocker, ACE inhibitor, or aldosterone antagonist.9
Intravenous nitrates are used primarily for acute HF, especially when accompanied by hypertension or myocardial ischemia. The starting dosage for nitroglycerin is approximately 10 μcg/min, titrated upward by 10-20 μcg/min to a maximum of 200 μcg/min. Isosorbide dinitrate (20-40 mg tid) and nitroglycerine ointment 2% (1.4 in applied every six to eight hours [although generally reserved for inpatient therapy]) are equally effective.9 Adverse effects that may limit the use of these agents are headache and hypotension. Patients also develop tolerance for nitrates, which can be mitigated if a daily 8-to-12–hour nitrate-free period is instituted.9
TREATMENT OF HEART FAILURE WITH PRESERVED LVEF
Treatment options for patients with preserved LVEF (diastolic HF) are not as clear as those for patients with reduced LVEF (systolic HF). No traditional therapies (ACE inhibitors, ARBs, ß-blockers, digoxin) have been shown to improve survival in this population, although a recent study on the effects of spironolactone in diastolic HF did show some improvement of diastolic dysfunction without adverse effects.17,37 In the absense of clear evidence-based therapies, treatment focuses on managing comorbidities, addressing reversible causes, and alleviating fluid overload with a diuretic.9,17
Diuretic therapy is critical to control fluid overload; regimens are similar to those for HF with reduced LVEF. ACE inhibitors and ARBs have not been shown to improve outcomes in this population but can be used to manage comorbid hypertension. Spironolactone has also not been shown to improve outcomes in patients with diastolic HF.9
The principal conditions that can lead to HF with preserved LVEF are hypertension, pericardial disease, and atrial tachycardia. Tachycardia is associated with overall shorter diastolic filling time; controlling an accelerated heart rate, therefore, is theorized to be an important therapeutic goal.9 Other disease states, including diabetes mellitus, sleep-disordered breathing, obesity, and chronic kidney disease, can all lead to HF with preserved LVEF.
CONCLUSION
Managing HF today requires an “upstream” model of care, by which providers consider the diagnosis/syndrome of HF in the asymptomatic patient with risk factors (Stage A/Class I and Stage B/Class II). Perhaps a better way to state this is that providers must change their approach from ruling-in to ruling-out HF. Any person in whom a decrease in activity level, mild shortness of breath, or edema are observed, and who has known risk factors, should be considered to have HF until proven otherwise.
Because of the wide variability in the underlying causes of HF, providers must be dynamic in both their clinical approach and their treatment plans to optimize care for the individual patient. Finally, providers must also take note of the increasing prevalence of diastolic HF and, again, focus on early diagnosis.
CE/CME No: CR-1805
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Develop an understanding of the classification of heart failure and its associated signs and symptoms.
• Describe clinical findings of heart failure from the physical exam.
• Understand the utility of laboratory and imaging studies for diagnosis of heart failure and its underlying causes.
• Demonstrate knowledge of nonpharmacotherapeutic and pharmacotherapeutic options for managing heart failure.
FACULTY
Michael Roscoe is Chair/Program Director and Associate Professor, Andrew Lampkins is Associate Professor, Sean Harper is Assistant Professor, and Gina Niemeier is Associate Program Director and Assistant Professor, in the PA Department at the University of Evansville in Indiana.
The authors have no financial relationships to disclose.

ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through April 30, 2019.
Article begins on next page >>
Heart failure is a complex syndrome with a spectrum of signs and symptoms that range from asymptomatic to terminal. This variability of presentation, paired with the irreversibility of the process, make it both difficult and critical to identify this syndrome early to prevent progression. Here is an overview of the classification and common presentations of heart failure, as well as a guide to diagnostic modalities and treatment options.
Heart failure (HF) is a complex syndrome, not a specific disease; it is always associated with an underlying cause. Hypertension and coronary artery disease are the two most common causes in all age groups, but a number of other conditions—valvular disease, unrecognized obstructive sleep apnea, obesity, chronic kidney disease, anemia, hyperlipidemia, diabetes, and atrial fibrillation—have been identified as secondary causes.1-3
Often, however, clinicians do not identify HF until the syndrome reaches an advanced stage—at which point, the damage is irreversible and pharmacotherapeutic management is limited to control of signs and symptoms. The ramifications are concerning, since HF has achieved near-epidemic scope in the United States. An estimated 5.7 million Americans ages 20 and older have HF; prevalence is projected to increase by 46% between 2012 and 2030—resulting in more than 8 million affected individuals (ages 18 and older).4,5 More than 1 million patients are discharged from the hospital annually with a primary diagnosis of HF.4 And in 2013, one in nine death certificates in the US mentioned HF.4
Most cases of HF are managed in primary care. Established, evidence-based therapies should be implemented in the outpatient setting when possible, as early in the course as possible. Referral to a cardiologist is needed when the underlying cause of HF remains undetermined, or when specialized treatment is required.
CLASSIFICATION OF HF
The two most widely recognized classification systems for HF are those of the American College of Cardiology and the American Heart Association (ACC/AHA) and of the New York Heart Association (NYHA). Both focus on one-year mortality. The stages of the ACC/AHA system (A to D) are based on worsening of both structural heart disease and clinical symptoms of HF. The NYHA designations (Class I to IV) are based on the functional capability associated with physical activity. Both systems are outlined in Table 1.3,6,7

While these systems are used to “stage” HF, there are several ways the syndrome is classified in the medical literature. For example, HF can be described by
- Anatomy (left- or right-sided)
- Physiology (dilated and hypertrophic)
- Course (chronic or acute heart failure [cardiogenic shock])
- Output (high- or low-output failure)
- Ejection fraction (reduced or preserved)
- Pressure phase (systolic and diastolic).
All these classifications have merit; however, this article will attempt to simplify the approach to patients with HF and focus on systolic HF, defined as a reduced left ventricular ejection fraction (LVEF), and diastolic HF, defined as a preserved LVEF. Although the common perception of HF among clinicians—and thus the traditional diagnostic focus—is reduced LVEF (systolic HF), preserved LVEF (diastolic HF) in fact represents approximately 50% of cases.1 Diastolic HF is estimated to be increasing in prevalence and is expected to become the more common phenotype.8
Continue to: SIGNS AND SYMPTOMS
SIGNS AND SYMPTOMS
Heart failure is characterized by a constellation of signs and symptoms of pulmonary and/or systemic venous congestion caused by impaired ability of the heart to fill with or eject blood in proportion to the metabolic needs of the body.1 Manifestations include fatigue, dyspnea, fluid retention, and cachexia, and patients can present anywhere on the spectrum—from asymptomatic at rest to severely symptomatic.
Symptoms
In both reduced LVEF and preserved LVEF HF, common early symptoms include dyspnea and fatigue on exertion, with or without some degree of lower-extremity swelling.2 Lack of treatment and disease progression increase symptom severity—to the extent that dyspnea and fatigue start to occur at rest. Reviewing the anatomic classifications/findings of HF facilitates understanding of the clinical symptoms.
Right HF. Right ventricular dysfunction is rarely found in isolation; when symptoms are present, further evaluation of the left ventricle and the pulmonary system (to look for cor pulmonale) is warranted. Right HF is associated with an inability to manage venous return and move volume into the pulmonary circuit. This produces the predominant symptom of fluid retention. Peripheral edema is a cardinal symptom of right HF; edema can also present systemically, mostly as hepatic congestion or general gastrointestinal complaints resulting from impaired gastrointestinal perfusion.9
Left HF. Left ventricular dysfunction is the more common anatomic finding in HF and is often generalized to represent all cases. The dysfunction can be found in isolation and is actually the leading cause of right HF. In left HF (regardless of etiology), the heart does not produce enough “forward” pressure (ie, cannot pump or fill with enough blood) for the cardiovascular system to remain in balance. Thus, “back” pressure into the pulmonary circuit develops.
The combination of insufficient systemic perfusion capability with a dysfunctional left pump means that the most common symptom in all cases of left HF is shortness of breath—specifically, exertional dyspnea. Dyspnea can progress to orthopnea, paroxysmal nocturnal dyspnea, and, finally, dyspnea at rest. Patients often present with a cough that is worse while lying down and that can be nonproductive or productive, depending on volume status.9,10
Signs
Vital signs range from normal to indicative of shock. Increased sympathetic nervous system activity is common; this may manifest as coldness of the extremities and diaphoresis. Keep in mind that HF is a clinical diagnosis: The physical examination, focusing on peripheral signs, is key in all HF patients for both diagnosis and management.
PHYSICAL EXAMINATION
The physical exam includes the heart, neck, lungs, abdomen, and extremities. The cardiac exam includes evaluation for hypertrophy, by palpating for the point of maximum impulse to assess for lifts, heaves, valvular disease, and S3 and S4 sounds. Respiratory, abdominal, and extremity exams all focus on the evaluation of fluid status and edema.
A key, often underutilized measurement is jugular venous pressure (JVP). Elevated JVP has been identified as the most specific sign of fluid overload in HF and the most important physical finding in the initial and subsequent examinations of a patient with HF.11 (For good reason, clinicians who treat patients with HF must be able to recognize volume overload and hypovolemia; euvolemia allows patients to remain symptom-free and makes it possible to initiate life-prolonging therapy, which will be discussed in the Treatment section.2) JVP is an indirect measure of pressure within the right side of the heart (central venous pressure).
Most texts on performing the physical exam recommend measuring JVP using the right internal jugular vein. However, use of the internal jugular vein is limiting in HF patients, because it is covered by the sternocleidomastoid muscle for most of its course in the neck and visible only in a small triangle between the two heads in the root of the neck (see Figure). Conversely, the external jugular veins are subcutaneous along their entire course and pulsations are easily visible—but superficial and prone to external pressure and internal occlusion. A nonpulsatile, distended jugular vein should not be used to estimate venous pressure.2

What, then, is the best method? Vinayak et al evaluated the comparative effectiveness of the internal and external jugular veins for detection of central venous pressure. They found that the external jugular vein is easier to visualize and has excellent reliability for determining low and high venous pressures.12
The process for estimating JVP is the same regardless of which jugular vein (internal or external) is used. The technique is described by Bickley in Bates’ Guide to Physical Examination and History Taking:
The patient lies supine and at an angle between 30° and 45°. Turn the head slightly, and with tangential lighting, identify the external and internal jugular veins. Identify the highest point of pulsation in the right jugular vein and extend a long rectangular object or card horizontally from this point and a centimeter ruler vertically from the sternal angle, making an exact right angle. Measure the vertical distance (in centimeters) above the sternal angle where the horizontal object crosses the ruler and add this distance to 4 cm. Measurements of > 3-4 cm above the sternal angle or > 8 cm in total distance are considered elevated.13
Continue to: DIAGNOSTIC AND SCREENING TESTS
DIAGNOSTIC AND SCREENING TESTS
Several diagnostic studies can identify HF or elucidate underlying causes. However, not all tests yield sufficient information for diagnosis—and commonly held “maxims” about findings that definitively rule in or out the diagnosis have been confounded by the available evidence.
Laboratory tests. The lab parameter most associated with HF is brain natriuretic peptide (BNP). Natriuretic peptides are produced primarily within the heart and released into the circulation in response to increased wall tension.14 In contrast to atrial natriuretic peptide (ANP), BNP is secreted not only from the atria but also from the ventricles, especially in patients with HF.15
Circulating concentrations of several cardiac natriuretic peptides—including ANP, BNP, and the two peptides’ N-terminal pro-hormones (N-terminal pro-atrial natriuretic peptide and N-terminal pro-brain natriuretic peptide, respectively) are elevated in both symptomatic and asymptomatic patients with left ventricular dysfunction.16 These levels are generally elevated for both systolic and diastolic HF. However, in one study, as many as 30% of diastolic HF patients had a low BNP level, despite signs and symptoms of HF and significantly elevated left ventricular filling pressures, as determined by invasive hemodynamic monitoring.17 Comorbid obesity is associated with low levels of natriuretic peptides.18
Additional lab tests can provide information about underlying causes of HF or reveal contraindications to certain treatment options (to be discussed in the Treatment section). A complete blood count (CBC) might reveal anemia, which can cause or aggravate HF, and which is an important consideration in management because of its association with decreased renal function, hemodilution, and proinflammatory cytokines. Leukocytosis can signal underlying infection. A troponin assay is helpful for ruling out acute MI as a cause of worsening HF in acute cases. Thyroid function tests and iron studies can be considered to rule out secondary causes of HF.
A serum electrolyte screen should be ordered; results are usually within normal ranges. Hyponatremia is an indicator of activation of the renin–angiotensin–aldosterone system (RAAS) and may be seen in the context of prolonged salt restriction and diuretic therapy. Hyperkalemia and hypokalemia are also prevalent in HF; both are limiting factors for some treatment options. A low sodium level is often the result of increased congestion and release of vasopressin; a level of ≤ 135 mEq/L predicts a poorer outcome.
Kidney function tests can determine whether HF is associated with impaired kidney function, secondary to poor renal perfusion. Poor renal function may limit treatment options. Patients with severe HF, particularly those receiving a high dosage of a diuretic for a long period, may have elevated levels of blood urea nitrogen and creatinine, indicating renal insufficiency.
Electrocardiography and chest radiography. ECGs and chest radiographs are noninvasive tests that have been used widely in the diagnosis of HF. They might indicate an underlying cause (eg, acute MI, ischemia, secondary arrhythmia) but are often nonspecific—and thus may be unhelpful in the diagnosis and treatment of HF.
The most common ECG findings in HF are nonspecific ST-T wave abnormalities.19 Other findings often consist of low-voltage left ventricular hypertrophy, conduction defects, and repolarization changes. With chest radiography, primary findings in HF include pulmonary edema—seen as perivascular edema, peribronchial cuffing, perihilar haze, interstitial edema (Kerley B, or septal, lines), and alveolar fluid—and pleural effusion.19,20
For both these modalities, however, there are commonly held conceptions that particular findings rule out HF—which has been disproven by Fonseca and colleagues.19 Because most patients with HF have an abnormal ECG, some studies have proposed that a normal ECG virtually rules out left ventricular systolic dysfunction.20 Evaluating the value of ECG in HF diagnosis, Fonseca et al found that about 85% of patients with an abnormal ECG had HF—but so did 30% of patients with a normal ECG. They concluded that, if used alone, ECG could have missed as many as 25% of patients with HF.19
Likewise, it has been suggested that a patient cannot have HF if heart size is normal on a chest radiograph.20 Fonseca et al found that cardiac enlargement, while the most informative radiologic measurement in HF, was present in only half of patients with HF.19 About 57% of patients who had an abnormal chest radiograph had HF, but so did about 40% of those who had a normal chest radiograph.19 Therefore, abnormal chest radiograph for identification of HF had an estimated sensitivity of 57%, a specificity of 78%, positive predictive value of 50%, and negative predictive value of 83%.19 The conclusion: Caution should be taken regarding the use of chest radiography in isolation to make a diagnosis of HF.
Echocardiography. The most useful test in evaluating HF is the echocardiogram, because it can distinguish between HF with and without preserved left ventricular systolic function. This is critical: The most clinically relevant classification of HF differentiates systolic and diastolic HF, based on LVEF.2 This determination has both prognostic and therapeutic implications.21
Echocardiography is widely available, safe, and noninvasive. The “echo” can identify the size of the atria and ventricles, valve function and dysfunction, and any associated shunting. Pericardial effusions and heart wall-motion abnormalities (for example, an old MI) are also easily identified.9
A normal ejection fraction does not rule out HF. Therefore, assessment of LVEF should not be considered until after a clinical diagnosis has been made, because more than half of HF patients have a normal LVEF—evidence that can confound the diagnostic process.2
Continue to: TREATMENT OF HEART FAILURE WITH REDUCED LVEF
TREATMENT OF HEART FAILURE WITH REDUCED LVEF
The body’s neurohormonal system, including the RAAS and the sympathetic nervous system, is activated to compensate for the insufficient cardiac performance found in HF. However, activation of these systems contributes to worsening HF, deterioration of quality of life, and poor outcomes.22 Therefore, therapies that suppress these responses can reduce the progression of HF.
Treatment of HF is generally divided into symptom-relieving treatment and disease-modifying/life-prolonging treatment.1 Symptom relief is similar in both systolic and diastolic HF. However, most evidence-based, disease-modifying treatment focuses on systolic HF; guidelines for disease-modifying treatment of diastolic HF are minimal.1
Treatment of reversible causes
Since HF is caused by something else, the primary focus of management is addressing underlying causes. The primary goal is to relieve symptoms while improving functional status—which should lead to a decrease in hospitalization and premature death.
The first step is to evaluate patients’ use of medications that can contribute to a worsening of HF.9 The most common offending medications are calcium-channel blockers with negative inotropy (non-dihydropyridine calcium-channel blockers, eg, verapamil and diltiazem); some antiarrhythmic drugs (eg, amiodarone); thiazolidinediones (glitazones); and NSAIDs.9 If identified as a possible contributor to HF symptoms, these agents should be stopped (if possible) or replaced.
Nonpharmacotherapy
Effective counseling and education of patients with HF may help with long-term adherence to treatment plans. Patients can be taught to monitor their weight at home and to adjust the dosage of diuretics as advised: A sudden increase in weight (> 2 kg in one to three d), for example, should alert a patient to alter treatment or seek advice.23
Diet modification is a multifactorial recommendation. Proper nutrition is critical because HF patients are at increased risk for malnutrition due to poor appetite, malabsorption, and increased nutritional requirements.23 Weight reduction in obese patients helps reduce cardiac workload. Patients should be placed on salt restriction (2 to 2.6 g/d of sodium).9,23
Exercise has been shown to relieve symptoms, provide a greater sense of well-being, and improve functional capacity. It does not, however, result in obvious improvement in cardiac function.22
Alcohol consumption should be restricted because of the myocardial depressant properties of alcohol and its direct toxic effect on the myocardium.22 Smoking should be discouraged because it has a direct effect on coronary artery disease.
Influenza and pneumococcal vaccination should be considered in all patients with HF.23 Heart failure predisposes patients to, and can be exacerbated by, pulmonary infection and exacerbation of chronic obstructive pulmonary disease.
Evaluation and management of obstructive sleep apnea should be performed. Sleep-disordered breathing, an umbrella term that covers obstructive and central sleep apneas, has been found to increase the risk for poor prognosis in HF.24 All patients with HF should be tested for obstructive sleep apnea because, often, only the patient’s bed partner is aware of disordered sleep. For unknown reasons, patients with HF do not report subjective sleepiness.25
Continue to: Pharmacotherapy
Pharmacotherapy
Diuretics have not been found to have benefit for reducing early mortality but are the most common agents used for symptomatic relief of sodium and water retention.26 In fact, few patients with signs and symptoms of fluid retention can be managed without a diuretic.9 Caution must be observed, however, because excessive diuresis can lead to electrolyte imbalance and neurohormonal activation.
Treating mild fluid retention with a thiazide diuretic (hydrochlorothiazide, metolazone, or chlorthalidone) is often sufficient (see Table 2 for dosing and other information on these and other drugs for treating HF).9 Thiazide diuretics are dependent on the glomerular filtration rate and are ineffective when it falls below 30-40 mL/min. Adverse reactions to diuretics include hypokalemia, dehydration (intravascular volume depletion) with prerenal azotemia, skin rash, neutropenia, thrombocytopenia, hyperglycemia, hyperuricemia, and hepatic dysfunction.

In cases of moderate or severe HF, or failure of thiazide diuretics to relieve mild symptoms, an oral loop diuretic (furosemide, bumetanide, or torsemide; see Table 2 for dosing) can be used if kidney function is preserved.9 These agents are best administered in two or more divided doses. Major adverse reactions are similar to those of thiazide diuretics, plus ototoxicity. Special caution must be used when a loop diuretic is co-administered with digitalis because the combination can cause significant hypokalemia.9
A potassium-sparing diuretic (triamterene or amiloride; see Table 2) can be used in combination with thiazide and loop diuretics.9 The location of their action is at the distal tubule, but diuretic potency is mild. Potassium-sparing agents can minimize hypokalemia induced by other diuretics. Adverse effects include hyperkalemia, kidney dysfunction, and gastrointestinal symptoms.
The aldosterone-receptor antagonists spironolactone and eplerenone are specific inhibitors of aldosterone, an effect that has been shown to improve clinical outcomes.9 Aldosterone-receptor antagonists are indicated for patients with NYHA class II-IV HF who have LVEF ≤ 35% or those with a history of acute MI, LVEF < 40%, and symptoms of HF.27 In these patients, a reduction in mortality and relative risk has been demonstrated with the use of aldosterone-receptor antagonists, unrelated to their role in diuresis. The adverse effect profile of spironolactone includes gynecomastia.
Continue to: Inhibitors of the RAAS
Inhibitors of the RAAS. As noted, the RAAS plays a key role during the development and worsening of HF.22
Angiotensin-converting enzyme inhibitors (ACE inhibitors) constitute the cornerstone of modern HF pharmacotherapy and have compelling evidence of survival benefit.28 These agents (captopril, enalapril, lisinopril, and ramipril; see Table 2 for dosing) have been shown to be effective therapy for HF and post-MI left ventricular dysfunction.29 They reduce early mortality by approximately 20% in symptomatic HF and can prevent hospitalization, increase exercise tolerance, and reduce adverse events (symptoms).9
Because of these benefits, ACE inhibitors should be firstline therapy in all HF patients with left ventricular dysfunction. They are usually used in combination with a diuretic. In addition, ACE inhibitors should be used in patients with reduced LVEF, even if they are asymptomatic, because these agents prevent progression to clinical HF.
Since ACE inhibitors carry the risk for severe hypotension, caution must be used, especially during treatment initiation. Patients whose systolic blood pressure is < 100 mm Hg, or who are hypovolemic, should be started at a low dosage (captopril, 6.25 mg tid; enalapril, 2.5 mg/d; or other equivalent ACE inhibitor dose); for other patients, these dosages can be doubled at initiation.9 Within days of initiation, but no longer than two weeks later, patients should be screened for hypotension and have both kidney function and potassium levels monitored. The dosage of ACE inhibitors should be increased over one to three months to an effective dosage (eg, captopril, 25 mg tid; enalapril, 10 mg bid; ramipril, 10 mg/d; and lisinopril, 20 mg/d, or other equivalent ACE inhibitor dose).9
Asymptomatic hypotension is not a contraindication to continuation or uptitration of the dosage of ACE inhibitors. Patients may also experience an increase in the serum creatinine or potassium level; likewise, this should not prompt a change in dosage if the elevated level stabilizes. The most common adverse effects of ACE inhibitors are dizziness and cough.9
Angiotensin II-receptor blockers (ARBs) have been shown to be as effective as ACE inhibitors for the management of hypertension, congestive HF, and chronic renal failure.30 ARBs decrease the adverse effects of angiotensin II, but do not have the same effects that ACE inhibitors do on other pathways found in HF (specifically, on bradykinin, prostaglandins, and nitric oxide).29 Candesartan and valsartan have been shown to have benefits in HF and are an equivalent alternative to ACE inhibitors; they are often used when a patient cannot tolerate an ACE inhibitor. Because ARBs and ACE inhibitors affect the RAAS at different points in the pathway, there is a theoretical basis for ARB and ACE inhibitor combination therapy; in clinical studies to date, however, the combination has shown no beneficial effect and rather was associated with more adverse effects.29
Neprilysin inhibitor. Recently, the combination of the neprilysin inhibitor sacubitril and an ARB, valsartan, has surfaced as an alternative to ACE inhibitor or single-agent ARB therapy.31,32 Inhibition of the enzyme neprilysin results in an increase in levels of endogenous vasoactive peptides (eg, natriuretic peptides and bradykinin), which may benefit hemodynamics in patients with HF. However, use of an ARB in combination with sacubitril is necessary to counteract the increase in angiotensin II levels that also results from inhibition of neprilysin.33
Sacubitril–valsartan is typically reserved for patients with mild-to-moderate HF who have either an elevated BNP (≥ 50 pg/mL) or an HF-related hospitalization within the past year. Upon transitioning from an ACE inhibitor (or single-agent ARB), a 36-hour washout period must be observed before starting sacubitril–valsartan to minimize the risk for angioedema. Because therapy with sacubitril–valsartan leads directly to an elevation in the BNP level, using the level of N-terminal pro-brain natriuretic peptide—which is not degraded by neprilysin—to monitor disease progression is recommended.34
ß-
Initiation of ß-blockers in stable patients can cause general deterioration; initiation must therefore be done gradually:
- Carvedilol, initiated at 3.125 mg bid, can be increased to 6.25 mg, then 12.5 mg, and then 25 mg, all bid, at intervals of approximately two weeks
- Sustained-release metoprolol can be started at 12.5 mg/d or 25 mg/d and doubled at two weeks to a target of 200 mg/d
- Bisoprolol, initiated at 1.25 mg/d, can be increased incrementally to 2.5 mg/d, 3.75 mg/d, 5 mg/d, 7.5 mg/d, or 10 mg/d at one-to-four-week intervals.9
Patients taking ß-blockers need to monitor their weight at home as an indicator of fluid retention. If HF becomes worse, an increase in the dosage of the accompanying diuretic, a delay in the increase of the ß-blocker, or downward adjustment of the ß-blocker is usually sufficient.
Continue to: Digitalis glycoside
Digitalis glycoside. Digoxin has been shown to relieve the symptoms of HF, decrease the risk for hospitalization, and increase exercise tolerance; however, it has not been shown to offer a mortality benefit.36 Digoxin should be considered for patients who remain symptomatic when taking a diuretic and an ACE inhibitor and for patients who are also in atrial fibrillation and need rate control.
Digoxin is cleared almost entirely by the kidneys and therefore must be used with care in patients with renal dysfunction. Patients usually can be started on the expected maintenance dosage (0.125-0.25 mg/d). An oral loading dose of 0.75-1.25 mg over 24 to 48 hours can be used if an early effect is needed.9
Digoxin can induce ventricular arrhythmias (especially in a setting of hypokalemia and ischemia) and is sensitive to other medications that can drastically increase its level—most notably, amiodarone, quinidine, propafenone, and verapamil.9 The digoxin blood level should be measured every seven to 14 days until a maintenance dosage is established, and again whenever there is a change in medication or kidney function. The optimum serum level is 0.7-1.2 ng/mL; toxicity is not usually seen at < 1.8 ng/mL.9
Hydralazine and nitrates. The combination of hydralazine and isosorbide dinitrate has been shown to improve outcomes in African-American patients with HF, but efficacy is less well-established in this population than for ACE inhibitor and ARB therapy.9 This combination can be considered in patients who are unable to tolerate ACE inhibitor or ARB therapy. It can also be considered in those who have persistent symptoms despite treatment with a ß-blocker, ACE inhibitor, or aldosterone antagonist.9
Intravenous nitrates are used primarily for acute HF, especially when accompanied by hypertension or myocardial ischemia. The starting dosage for nitroglycerin is approximately 10 μcg/min, titrated upward by 10-20 μcg/min to a maximum of 200 μcg/min. Isosorbide dinitrate (20-40 mg tid) and nitroglycerine ointment 2% (1.4 in applied every six to eight hours [although generally reserved for inpatient therapy]) are equally effective.9 Adverse effects that may limit the use of these agents are headache and hypotension. Patients also develop tolerance for nitrates, which can be mitigated if a daily 8-to-12–hour nitrate-free period is instituted.9
TREATMENT OF HEART FAILURE WITH PRESERVED LVEF
Treatment options for patients with preserved LVEF (diastolic HF) are not as clear as those for patients with reduced LVEF (systolic HF). No traditional therapies (ACE inhibitors, ARBs, ß-blockers, digoxin) have been shown to improve survival in this population, although a recent study on the effects of spironolactone in diastolic HF did show some improvement of diastolic dysfunction without adverse effects.17,37 In the absense of clear evidence-based therapies, treatment focuses on managing comorbidities, addressing reversible causes, and alleviating fluid overload with a diuretic.9,17
Diuretic therapy is critical to control fluid overload; regimens are similar to those for HF with reduced LVEF. ACE inhibitors and ARBs have not been shown to improve outcomes in this population but can be used to manage comorbid hypertension. Spironolactone has also not been shown to improve outcomes in patients with diastolic HF.9
The principal conditions that can lead to HF with preserved LVEF are hypertension, pericardial disease, and atrial tachycardia. Tachycardia is associated with overall shorter diastolic filling time; controlling an accelerated heart rate, therefore, is theorized to be an important therapeutic goal.9 Other disease states, including diabetes mellitus, sleep-disordered breathing, obesity, and chronic kidney disease, can all lead to HF with preserved LVEF.
CONCLUSION
Managing HF today requires an “upstream” model of care, by which providers consider the diagnosis/syndrome of HF in the asymptomatic patient with risk factors (Stage A/Class I and Stage B/Class II). Perhaps a better way to state this is that providers must change their approach from ruling-in to ruling-out HF. Any person in whom a decrease in activity level, mild shortness of breath, or edema are observed, and who has known risk factors, should be considered to have HF until proven otherwise.
Because of the wide variability in the underlying causes of HF, providers must be dynamic in both their clinical approach and their treatment plans to optimize care for the individual patient. Finally, providers must also take note of the increasing prevalence of diastolic HF and, again, focus on early diagnosis.
1. Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13(1):18-28.
2. Ahmed A. DEFEAT - Heart failure: a guide to management of geriatric heart failure by generalist physicians. Minerva Med. 2009;100(1):39-50.
3. Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1995 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol. 2001; 38(7):2101-2113.
4. Mozaffarian D, Benjamin EJ, Go AS, et al; Writing Group Members, American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2016 Update: A report from the American Heart Association. Circulation. 2016;26: 133(4):e38-e360.
5. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619.
6. American Heart Association. Classes of heart failure (2017). www.heart.org/HEARTORG/Conditions/HeartFailure/AboutHeartFailure/Classes-of-Heart-Failure_UCM_306328_Article.jsp#.V8cFhfkrLRY. Accessed March 7, 2018.
7. New York Heart Association Criteria Committee. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed. Boston, MA: Lippincott Williams and Wilkins; 1994.
8. Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251-259.
9. Bashore T, Granger C, Hranitzy P, Patel M. Heart diseases. In: Papadakis MA, McPhee SJ, Rabow MW, eds. Current Medical Diagnosis and Treatment. 55th ed. New York, NY: McGraw-Hill Education; 2016:402.
10. Colucci S. Patient education: heart failure (beyond the basics). Waltham, MA: UpToDate; 2017. www.uptodate.com/contents/heart-failure-beyond-the-basics?view=print. Accessed March 7, 2018.
11. Butman S, Ewy GA, Standen J, et al. Bedside cardiovascular examination in patients with severe chronic heart failure: importance of rest or inducible jugular venous distention. J Am Coll Cardiol. 1993;22(4):968-974.
12. Vinayak A, Levitt J, Gehlbach B, et al. Usefulness of the external jugular vein examination in detecting abnormal central venous pressure in critically ill patients. Arch Intern Med. 2006;166(19):2132-2137.
13. Bickley LA. Bates’ Guide to Physical Examination and History Taking. 11th ed. Alphen aan den Rijn, The Netherlands: Wolters Kluwer; 2012:364-365.
14. Kinnunen P, Vuolteenaho O, Ruskoaho H. Mechanisms of atrial and brain natriuretic peptide release from rat ventricular myocardium: effect of stretching. Endocrinology. 1993;132(5):1961-1970.
15. Yasue H, Yoshimura M, Sumida H, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90(1):195-204.
16. Cowie M, Struthers A, Wood D, et al. Value of natriuretic peptides in assessment of patients with possible new heart failure in primary care. Lancet. 1997;350(9088):1349-1353.
17. Oktay AA, Shah SJ. Diagnosis and management of heart failure with preserved ejection fraction: 10 key lessons. Curr Cardiol Rev. 2015;11(1):42-52.
18. Horwich TB, Hamilton MA, Fonarow GC. B-type natriuretic peptide levels in obese patients with advanced heart failure. J Am Coll Cardiol. 2006;47(1):85-90.
19. Fonseca C, Mota T, Morais H; EPICA Investigators. The value of the electrocardiogram and chest X-ray for confirming or refuting a suspected diagnosis of heart failure in the community. Eur J Heart Fail. 2006;6(6):807-812.
20. Rihal CS, Davis KB, Kennedy JW, Gersch BJ. The utility of clinical, electrocardiographic, and roentgenographic variables in the prediction of left ventricular function. Am J Cardiol. 1995;75(4):220-223.
21. Joint Commission on Accreditation of Healthcare Organizations. A Comprehensive Review of Development and Testing for National Implementation of Hospital Core Measures. 2002. www.jointcommission.org/assets/1/18/A_Comprehensive_Review_of_Development_for_Core_Measures.pdf. Accessed March 6, 2018.
22. Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovasc Pathol. 2012;21(5):365-371.
23. Gibbs CR, Jackson G, Lip GY. ABC of heart failure: Nondrug management. BMJ. 2000;320(7231):365-369.
24. Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49(15):1625-1631.
25. Arzt M, Young T, Finn L, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166(16):1716-1722.
26. Oni-Orisan A, Lanfear DE. Pharmacogenomics in heart failure: where are we now and how can we reach clinical application? Cardiol Rev. 2014;22(5):193-198.
27. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810-1852.
28. SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293-302.
29. Bissessor N, White H. Valsartan in the treatment of heart failure or left ventricular dysfunction after myocardial infarction. Vasc Health Risk Manag. 2007;3(4):425-430.
30. McMurray JJ, Ostegren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362(9386):767-771.
31. McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Committees and Investigators. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail. 2013;15(9):1062-1073.
32. McMurray J, Packer M, Desai A, et al; PARADIGM-HF Committees and Investigators. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004.
33. Cleland JG, Swedberg K. Lack of efficacy of neutral endopeptidase inhibitor ecadotril in heart failure. The International Ecadotril Multi-centre Dose-ranging Study Investigators. Lancet. 1998;351(9116):1657-1658.
34. Packer M, McMurray JJ, Desai AS, et al; PARADIGM-HF Committees and Investigators. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131(1):54-61.
35. Packer M, Fowler MB, Roecker EB, et al; Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106(17):2194-2199.
36. Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336(8):525-533.
37. Edelmann F, Wachter R, Schmidt AG, et al; Aldo-DHF Investigators. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309(8):781-791.
1. Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13(1):18-28.
2. Ahmed A. DEFEAT - Heart failure: a guide to management of geriatric heart failure by generalist physicians. Minerva Med. 2009;100(1):39-50.
3. Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1995 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol. 2001; 38(7):2101-2113.
4. Mozaffarian D, Benjamin EJ, Go AS, et al; Writing Group Members, American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2016 Update: A report from the American Heart Association. Circulation. 2016;26: 133(4):e38-e360.
5. Heidenreich PA, Albert NM, Allen LA, et al; American Heart Association Advocacy Coordinating Committee; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Stroke Council. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606-619.
6. American Heart Association. Classes of heart failure (2017). www.heart.org/HEARTORG/Conditions/HeartFailure/AboutHeartFailure/Classes-of-Heart-Failure_UCM_306328_Article.jsp#.V8cFhfkrLRY. Accessed March 7, 2018.
7. New York Heart Association Criteria Committee. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed. Boston, MA: Lippincott Williams and Wilkins; 1994.
8. Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251-259.
9. Bashore T, Granger C, Hranitzy P, Patel M. Heart diseases. In: Papadakis MA, McPhee SJ, Rabow MW, eds. Current Medical Diagnosis and Treatment. 55th ed. New York, NY: McGraw-Hill Education; 2016:402.
10. Colucci S. Patient education: heart failure (beyond the basics). Waltham, MA: UpToDate; 2017. www.uptodate.com/contents/heart-failure-beyond-the-basics?view=print. Accessed March 7, 2018.
11. Butman S, Ewy GA, Standen J, et al. Bedside cardiovascular examination in patients with severe chronic heart failure: importance of rest or inducible jugular venous distention. J Am Coll Cardiol. 1993;22(4):968-974.
12. Vinayak A, Levitt J, Gehlbach B, et al. Usefulness of the external jugular vein examination in detecting abnormal central venous pressure in critically ill patients. Arch Intern Med. 2006;166(19):2132-2137.
13. Bickley LA. Bates’ Guide to Physical Examination and History Taking. 11th ed. Alphen aan den Rijn, The Netherlands: Wolters Kluwer; 2012:364-365.
14. Kinnunen P, Vuolteenaho O, Ruskoaho H. Mechanisms of atrial and brain natriuretic peptide release from rat ventricular myocardium: effect of stretching. Endocrinology. 1993;132(5):1961-1970.
15. Yasue H, Yoshimura M, Sumida H, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90(1):195-204.
16. Cowie M, Struthers A, Wood D, et al. Value of natriuretic peptides in assessment of patients with possible new heart failure in primary care. Lancet. 1997;350(9088):1349-1353.
17. Oktay AA, Shah SJ. Diagnosis and management of heart failure with preserved ejection fraction: 10 key lessons. Curr Cardiol Rev. 2015;11(1):42-52.
18. Horwich TB, Hamilton MA, Fonarow GC. B-type natriuretic peptide levels in obese patients with advanced heart failure. J Am Coll Cardiol. 2006;47(1):85-90.
19. Fonseca C, Mota T, Morais H; EPICA Investigators. The value of the electrocardiogram and chest X-ray for confirming or refuting a suspected diagnosis of heart failure in the community. Eur J Heart Fail. 2006;6(6):807-812.
20. Rihal CS, Davis KB, Kennedy JW, Gersch BJ. The utility of clinical, electrocardiographic, and roentgenographic variables in the prediction of left ventricular function. Am J Cardiol. 1995;75(4):220-223.
21. Joint Commission on Accreditation of Healthcare Organizations. A Comprehensive Review of Development and Testing for National Implementation of Hospital Core Measures. 2002. www.jointcommission.org/assets/1/18/A_Comprehensive_Review_of_Development_for_Core_Measures.pdf. Accessed March 6, 2018.
22. Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovasc Pathol. 2012;21(5):365-371.
23. Gibbs CR, Jackson G, Lip GY. ABC of heart failure: Nondrug management. BMJ. 2000;320(7231):365-369.
24. Wang H, Parker JD, Newton GE, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. 2007;49(15):1625-1631.
25. Arzt M, Young T, Finn L, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166(16):1716-1722.
26. Oni-Orisan A, Lanfear DE. Pharmacogenomics in heart failure: where are we now and how can we reach clinical application? Cardiol Rev. 2014;22(5):193-198.
27. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810-1852.
28. SOLVD Investigators, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293-302.
29. Bissessor N, White H. Valsartan in the treatment of heart failure or left ventricular dysfunction after myocardial infarction. Vasc Health Risk Manag. 2007;3(4):425-430.
30. McMurray JJ, Ostegren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362(9386):767-771.
31. McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Committees and Investigators. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail. 2013;15(9):1062-1073.
32. McMurray J, Packer M, Desai A, et al; PARADIGM-HF Committees and Investigators. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004.
33. Cleland JG, Swedberg K. Lack of efficacy of neutral endopeptidase inhibitor ecadotril in heart failure. The International Ecadotril Multi-centre Dose-ranging Study Investigators. Lancet. 1998;351(9116):1657-1658.
34. Packer M, McMurray JJ, Desai AS, et al; PARADIGM-HF Committees and Investigators. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131(1):54-61.
35. Packer M, Fowler MB, Roecker EB, et al; Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106(17):2194-2199.
36. Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336(8):525-533.
37. Edelmann F, Wachter R, Schmidt AG, et al; Aldo-DHF Investigators. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309(8):781-791.
Underserved populations and colorectal cancer screening: Patient perceptions of barriers to care and effective interventions
Editor's Note:
Importantly, these barriers often vary between specific population subsets. In this month’s In Focus article, brought to you by The New Gastroenterologist, the members of the AGA Institute Diversity Committee provide an enlightening overview of the barriers affecting underserved populations as well as strategies that can be employed to overcome these impediments. Better understanding of patient-specific barriers will, I hope, allow us to more effectively redress them and ultimately increase colorectal cancer screening rates in all populations.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Despite the positive public health effects of colorectal cancer (CRC) screening, there remains differential uptake of CRC screening in the United States. Minority populations born in the United States and immigrant populations are among those with the lowest rates of CRC screening, and both socioeconomic status and ethnicity are strongly associated with stage of CRC at diagnosis.1,2 Thus, recognizing the economic, social, and cultural factors that result in low rates of CRC screening in underserved populations is important in order to devise targeted interventions to increase CRC uptake and reduce morbidity and mortality in these populations.

What are the facts and figures?
The overall rate of screening colonoscopies has increased in all ethnic groups in the past 10 years but still falls below the goal of 71% established by the Healthy People project (www.healthypeople.gov) for the year 2020.3 According to the Centers for Disease Control and Prevention ethnicity-specific data for U.S.-born populations, 60% of whites, 55% of African Americans (AA), 50% of American Indian/Alaskan natives (AI/AN), 46% of Latino Americans, and 47% of Asians undergo CRC screening (Figure 1A).4 While CRC incidence in non-Hispanic whites age 50 years and older has dropped by 32% since 2000 because of screening, this trend has not been observed in AAs.5,6
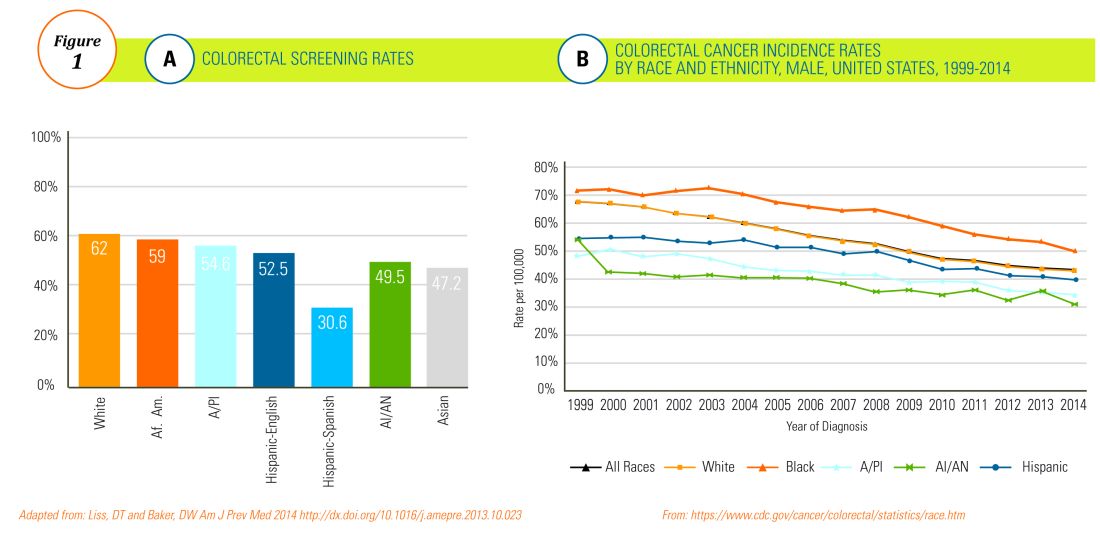
The incidence of CRC in AAs is estimated at 49/10,000, one of the highest amongst U.S. populations and is the second and third most common cancer in AA women and men, respectively (Figure 1B).
Similar to AAs, AI/AN patients present with more advanced CRC disease and at younger ages and have lower survival rates, compared with other racial groups, a trend that has not changed in the last decade.7 CRC screening data in this population vary according to sex, geographic location, and health care utilization, with as few as 4.0% of asymptomatic, average-risk AI/ANs who receive medical care in the Indian Health Services being screened for CRC.8
The low rate of CRC screening among Latinos also poses a significant obstacle to the Healthy People project since it is expected that by 2060 Latinos will constitute 30% of the U.S. population. Therefore, strategies to improve CRC screening in this population are needed to continue the gains made in overall CRC mortality rates.
The percentage of immigrants in the U.S. population increased from 4.7% in 1970 to 13.5% in 2015. Immigrants, regardless of their ethnicity, represent a very vulnerable population, and CRC screening data in this population are not as robust as for U.S.-born groups. In general, immigrants have substantially lower CRC screening rates, compared with U.S.-born populations (21% vs. 60%),9 and it is suspected that additional, significant barriers to CRC screening and care exist for undocumented immigrants.
Another often overlooked group, are individuals with physical or cognitive disabilities. In this group, screening rates range from 49% to 65%.10
Finally, while information is available for many health care conditions and disparities faced by various ethnic groups, there are few CRC screening data for the LGBTQ community. Perhaps amplifying this problem is the existence of conflicting data in this population, with some studies suggesting there is no difference in CRC risk across groups in the LGBTQ community and others suggesting an increased risk.11,12 Notably, sexual orientation has been identified as a positive predictor of CRC screening in gay and bisexual men – CRC screening rates are higher in these groups, compared with heterosexual men.13 In contrast, no such difference has been found between homosexual and heterosexual women.14
What are the barriers?
Several common themes contribute to disparities in CRC screening among minority groups, including psychosocial/cultural, socioeconomic, provider-specific, and insurance-related factors. Some patient-related barriers include issues of illiteracy, having poor health literacy or English proficiency, having only grade school education,15,16 cultural misconceptions, transportation issues, difficulties affording copayments or deductibles, and a lack of follow-up for scheduled appointments and exams.17-20 Poor health literacy has a profound effect on exam perceptions, fear of test results, and compliance with scheduling tests and bowel preparation instructions21-25; it also affects one’s understanding of the importance of CRC screening, the recommended screening age, and the available choice of screening tests.
Even when some apparent barriers are mitigated, disparities in CRC screening remain. For example, even among the insured and among Medicare beneficiaries, screening rates and adequate follow-up rates after abnormal findings remain lower among AAs and those of low socioeconomic status than they are among whites.26-28 At least part of this paradox results from the presence of unmeasured cultural/belief systems that affect CRC screening uptake. Some of these factors include fear and/or denial of CRC diagnoses, mistrust of the health care system, and reluctance to undergo medical treatment and surgery.16,29 AAs are also less likely to be aware of a family history of CRC and to discuss personal and/or family history of CRC or polyps, which can thereby hinder the identification of high-risk individuals who would benefit from early screening.15,30
The deeply rooted sense of fatalism also plays a crucial role and has been cited for many minority and immigrant populations. Fatalism leads patients to view a diagnosis of cancer as a matter of “fate” or “God’s will,” and therefore, it is to be endured.23,31 Similarly, in a qualitative study of 44 Somali men living in St. Paul and Minneapolis, believing cancer was more common in whites, believing they were protected from cancer by God, fearing a cancer diagnosis, and fearing ostracism from their community were reported as barriers to cancer screening.32
Perceptions about CRC screening methods in Latino populations also have a tremendous influence and can include fear, stigma of sexual prejudice, embarrassment of being exposed during the exam, worries about humiliation in a male sense of masculinity, a lack of trust in the medical professionals, a sense of being a “guinea pig” for physicians, concerns about health care racism, and expectations of pain.33-37 Studies have reported that immigrants are afraid to seek health care because of the increasingly hostile environment associated with immigration enforcement.38 In addition, the impending dissolution of the Deferred Action for Childhood Arrivals act is likely to augment the barriers to care for Latino groups.39
In addition, provider-specific barriers to care also exist. Racial and ethnic minorities are less likely than whites to receive recommendations for screening by their physician. In fact, this factor alone has been demonstrated to be the main reason for lack of screening among AAs in a Californian cohort.40 In addition, patients from rural areas or those from AI/AN communities are at especially increased risk for lack of access to care because of a scarcity of providers along with patient perceptions regarding their primary care provider’s ability to connect them to subspecialists.41-43 Other cited examples include misconceptions about and poor treatment of the LGBTQ population by health care providers/systems.44
How can we intervene successfully?
Characterization of barriers is important because it promotes the development of targeted interventions. Intervention models include community engagement programs, incorporation of fecal occult testing, and patient navigator programs.45-47 In response to the alarming disparity in CRC screening rates in Latino communities, several interventions have been set in motion in different clinical scenarios, which include patient navigation and a focus on patient education.
Randomized trials have shown that outreach efforts and patient navigation increase CRC screening rates in AAs.48,54,55 Studies evaluating the effects of print-based educational materials on improving screening showed improvement in screening rates, decreases in cancer-related fatalistic attitudes, and patients had a better understanding of the benefits of screening as compared with the cost associated with screening and the cost of advanced disease.56 Similarly, the use of touch-screen computers that tailor informational messages to decisional stage and screening barriers increased participation in CRC screening.57 Including patient navigators along with printed education material was even more effective at increasing the proportion of patients getting colonoscopy screening than providing printed material alone, with more-intensive navigation needed for individuals with low literacy.58 Grubbs et al.reported the success of their patient navigation program, which included wider comprehensive screening and coverage for colonoscopy screening.59 In AAs, they estimated an annual reduction of CRC incidence and mortality of 4,200 and 2,700 patients, respectively.
Among immigrants, there is an increased likelihood of CRC screening in those immigrants with a higher number of primary care visits.60 The intersection of culture, race, socioeconomic status, housing enclaves, limited English proficiency, low health literacy, and immigration policy all play a role in immigrant health and access to health care.61
Therefore, different strategies may be needed for each immigrant group to improve CRC screening. For this group of patients, efforts aimed at mitigating the adverse effects of national immigration policies on immigrant populations may have the additional consequence of improving health care access and CRC screening for these patients.
Data gaps still exist in our understanding of patient perceptions, perspectives, and barriers that present opportunities for further study to develop long-lasting interventions that will improve health care of underserved populations. By raising awareness of the barriers, physicians can enhance their own self-awareness to keenly be attuned to these challenges as patients cross their clinic threshold for medical care.
Additional resources link: www.cdc.gov/cancer/colorectal/
References
1. Klabunde CN et al. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011 Aug;20(8):1611-21.
2. Parikh-Patel A et al. Colorectal cancer stage at diagnosis by socioeconomic and urban/rural status in California, 1988-2000. Cancer. 2006 Sep;107(5 Suppl):1189-95.
3. Promotion OoDPaH. Healthy People 2020. Cancer. Volume 2017.
4. Centers for Disease Control and Prevention. Cancer screening – United States, 2010. MMWR Morb Mortal Wkly Rep. 2012 Jan 27;61(3):41-5.
5. Doubeni CA et al. Racial and ethnic trends of colorectal cancer screening among Medicare enrollees. Am J Prev Med. 2010 Feb;38(2):184-91.
6. Kupfer SS et al. Reducing colorectal cancer risk among African Americans. Gastroenterology. 2015 Nov;149(6):1302-4.
7. Espey DK et al. Annual report to the nation on the status of cancer, 1975-2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007 Nov;110(10):2119-52.
8. Day LW et al. Screening prevalence and incidence of colorectal cancer among American Indian/Alaskan natives in the Indian Health Service. Dig Dis Sci. 2011 Jul;56(7):2104-13.
9. Gupta S et al. Challenges and possible solutions to colorectal cancer screening for the underserved. J Natl Cancer Inst. 2014 Apr;106(4):dju032.
10. Steele CB et al. Colorectal cancer incidence and screening – United States, 2008 and 2010. MMWR Suppl. 2013 Nov 22;62(3):53-60.
11. Boehmer U et al. Cancer survivorship and sexual orientation. Cancer 2011 Aug 15;117(16):3796-804.
12. Austin SB, Pazaris MJ, Wei EK, et al. Application of the Rosner-Wei risk-prediction model to estimate sexual orientation patterns in colon cancer risk in a prospective cohort of US women. Cancer Causes Control. 2014 Aug;25(8):999-1006.
13. Heslin KC et al. Sexual orientation and testing for prostate and colorectal cancers among men in California. Med Care. 2008 Dec;46(12):1240-8.
14. McElroy JA et al. Advancing Health Care for Lesbian, Gay, Bisexual, and Transgender Patients in Missouri. Mo Med. 2015 Jul-Aug;112(4):262-5.
15. Greiner KA et al. Knowledge and perceptions of colorectal cancer screening among urban African Americans. J Gen Intern Med. 2005 Nov;20(11):977-83.
16. Green PM, Kelly BA. Colorectal cancer knowledge, perceptions, and behaviors in African Americans. Cancer Nurs. 2004 May-Jun;27(3):206-15; quiz 216-7.
17. Berkowitz Z et al. Beliefs, risk perceptions, and gaps in knowledge as barriers to colorectal cancer screening in older adults. J Am Geriatr Soc. 2008 Feb;56(2):307-14.
18. Dolan NC et al. Colorectal cancer screening knowledge, attitudes, and beliefs among veterans: Does literacy make a difference? J Clin Oncol. 2004 Jul;22(13):2617-22.
19. Peterson NB et al. The influence of health literacy on colorectal cancer screening knowledge, beliefs and behavior. J Natl Med Assoc. 2007 Oct;99(10):1105-12.
20. Haddock MG et al. Intraoperative irradiation for locally recurrent colorectal cancer in previously irradiated patients. Int J Radiat Oncol Biol Phys. 2001 Apr 1;49(5):1267-74.
21. Jones RM et al. Patient-reported barriers to colorectal cancer screening: a mixed-methods analysis. Am J Prev Med. 2010 May;38(5):508-16.
22. Basch CH et al. Screening colonoscopy bowel preparation: experience in an urban minority population. Therap Adv Gastroenterol. 2013 Nov;6(6):442-6.
23. Davis JL et al. Sociodemographic differences in fears and mistrust contributing to unwillingness to participate in cancer screenings. J Health Care Poor Underserved. 2012 Nov;23(4 Suppl):67-76.
24. Robinson CM et al. Barriers to colorectal cancer screening among publicly insured urban women: no knowledge of tests and no clinician recommendation. J Natl Med Assoc. 2011 Aug;103(8):746-53.
25. Goldman RE et al. Perspectives of colorectal cancer risk and screening among Dominicans and Puerto Ricans: Stigma and misperceptions. Qual Health Res. 2009 Nov;19(11):1559-68.
26. Laiyemo AO et al. Race and colorectal cancer disparities: Health-care utilization vs different cancer susceptibilities. J Natl Cancer Inst. 2010 Apr 21;102(8):538-46.
27. White A et al. Racial disparities and treatment trends in a large cohort of elderly African Americans and Caucasians with colorectal cancer, 1991 to 2002. Cancer. 2008 Dec 15;113(12):3400-9.
28. Doubeni CA et al. Neighborhood socioeconomic status and use of colonoscopy in an insured population – A retrospective cohort study. PLoS One. 2012;7(5):e36392.
29. Tammana VS, Laiyemo AO. Colorectal cancer disparities: Issues, controversies and solutions. World J Gastroenterol. 2014 Jan 28;20(4):869-76.
30. Carethers JM. Screening for colorectal cancer in African Americans: determinants and rationale for an earlier age to commence screening. Dig Dis Sci. 2015 Mar;60(3):711-21.
31. Miranda-Diaz C et al. Barriers for Compliance to Breast, Colorectal, and Cervical Screening Cancer Tests among Hispanic Patients. Int J Environ Res Public Health. 2015 Dec 22;13(1):ijerph13010021.
32. Sewali B et al. Understanding cancer screening service utilization by Somali men in Minnesota. J Immigr Minor Health. 2015 Jun;17(3):773-80.
33. Walsh JM et al. Barriers to colorectal cancer screening in Latino and Vietnamese Americans. Compared with non-Latino white Americans. J Gen Intern Med. 2004 Feb;19(2):156-66.
34. Perez-Stable EJ et al. Self-reported use of cancer screening tests among Latinos and Anglos in a prepaid health plan. Arch Intern Med. 1994 May 23;154(10):1073-81.
35. Shariff-Marco S et al. Racial/ethnic differences in self-reported racism and its association with cancer-related health behaviors. Am J Public Health. 2010 Feb;100(2):364-74.
36. Powe BD et al. Comparing knowledge of colorectal and prostate cancer among African American and Hispanic men. Cancer Nurs. 2009 Sep-Oct;32(5):412-7.
37. Jun J, Oh KM. Asian and Hispanic Americans’ cancer fatalism and colon cancer screening. Am J Health Behav. 2013 Mar;37(2):145-54.
38. Hacker K et al. The impact of Immigration and Customs Enforcement on immigrant health: Perceptions of immigrants in Everett, Massachusetts, USA. Soc Sci Med. 2011 Aug;73(4):586-94.
39. Firger J. Rescinding DACA could spur a public health crisis, from lost services to higher rates of depression, substance abuse. Newsweek.
40. May FP et al. Racial minorities are more likely than whites to report lack of provider recommendation for colon cancer screening. Am J Gastroenterol. 2015 Oct;110(10):1388-94.
41. Levy BT et al. Why hasn’t this patient been screened for colon cancer? An Iowa Research Network study. J Am Board Fam Med. 2007 Sep-Oct;20(5):458-68.
42. Rosenblatt RA. A view from the periphery – health care in rural America. N Engl J Med. 2004 Sep 9;351(11):1049-51.
43. Young WF et al. Predictors of colorectal screening in rural Colorado: testing to prevent colon cancer in the high plains research network. J Rural Health. 2007 Summer;23(3):238-45.
44. Kates J et al. Health and Access to Care and Coverage for Lesbian, Gay, Bisexual, and Transgender (LGBT) Individuals in the U.S. In: Foundation KF, ed. Disparities Policy Issue Brief. Volume 2017; Aug 30, 2017.
45. Katz ML et al. Improving colorectal cancer screening by using community volunteers: results of the Carolinas cancer education and screening (CARES) project. Cancer. 2007 Oct 1;110(7):1602-10.
46. Jean-Jacques M et al. Program to improve colorectal cancer screening in a low-income, racially diverse population: A randomized controlled trial. Ann Fam Med. 2012 Sep-Oct;10(5):412-7.
47. Reuland DS et al. Effect of combined patient decision aid and patient navigation vs usual care for colorectal cancer screening in a vulnerable patient population: A randomized clinical trial. JAMA Intern Med. 2017 Jul 1;177(7):967-74.
48. Percac-Lima S et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. J Gen Intern Med. 2009 Feb;24(2):211-7.
49. Nash D et al. Evaluation of an intervention to increase screening colonoscopy in an urban public hospital setting. J Urban Health. 2006 Mar;83(2):231-43.
50. Lebwohl B et al. Effect of a patient navigator program on the volume and quality of colonoscopy. J Clin Gastroenterol. 2011 May-Jun;45(5):e47-53.
51. Khankari K et al. Improving colorectal cancer screening among the medically underserved: A pilot study within a federally qualified health center. J Gen Intern Med. 2007 Oct;22(10):1410-4.
52. Wang JH et al. Recruiting Chinese Americans into cancer screening intervention trials: Strategies and outcomes. Clin Trials. 2014 Apr;11(2):167-77.
53. Katz ML et al. Patient activation increases colorectal cancer screening rates: a randomized trial among low-income minority patients. Cancer Epidemiol Biomarkers Prev. 2012 Jan;21(1):45-52.
54. Ford ME et al. Enhancing adherence among older African American men enrolled in a longitudinal cancer screening trial. Gerontologist. 2006 Aug;46(4):545-50.
55. Christie J et al. A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. J Natl Med Assoc. 2008 Mar;100(3):278-84.
56. Philip EJ et al. Evaluating the impact of an educational intervention to increase CRC screening rates in the African American community: A preliminary study. Cancer Causes Control. 2010 Oct;21(10):1685-91.
57. Greiner KA et al. Implementation intentions and colorectal screening: A randomized trial in safety-net clinics. Am J Prev Med. 2014 Dec;47(6):703-14.
58. Horne HN et al. Effect of patient navigation on colorectal cancer screening in a community-based randomized controlled trial of urban African American adults. Cancer Causes Control. 2015 Feb;26(2):239-46.
59. Grubbs SS et al. Eliminating racial disparities in colorectal cancer in the real world: It took a village. J Clin Oncol. 2013 Jun 1;31(16):1928-30.
60. Jung MY et al. The Chinese and Korean American immigrant experience: a mixed-methods examination of facilitators and barriers of colorectal cancer screening. Ethn Health. 2017 Feb 25:1-20.
61. Viruell-Fuentes EA et al. More than culture: structural racism, intersectionality theory, and immigrant health. Soc Sci Med. 2012 Dec;75(12):2099-106.
Dr. Oduyebo is a third-year fellow at the Mayo Clinic, Rochester, Minn.; Dr. Malespin is an assistant professor in the department of medicine and the medical director of hepatology at the University of Florida Health, Jacksonville; Dr. Mendoza Ladd is an assistant professor of medicine at Texas Tech University, El Paso; Dr. Day is an associate professor of medicine at the University of California, San Francisco; Dr. Charabaty is an associate professor of medicine and the director of the IBD Center in the division of gastroenterology at Medstar-Georgetown University Center, Washington; Dr. Chen is an associate professor of medicine, the director of patient safety and quality, and the director of the small-bowel endoscopy program in division of gastroenterology at Washington University, St. Louis; Dr. Carr is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia; Dr. Quezada is an assistant dean for admissions, an assistant dean for academic and multicultural affairs, and an assistant professor of medicine in the division of gastroenterology and hepatology at the University of Maryland, Baltimore; and Dr. Lamousé-Smith is a director of translational medicine, immunology, and early development at Janssen Pharmaceuticals Research and Development, Spring House, Penn.
Editor's Note:
Importantly, these barriers often vary between specific population subsets. In this month’s In Focus article, brought to you by The New Gastroenterologist, the members of the AGA Institute Diversity Committee provide an enlightening overview of the barriers affecting underserved populations as well as strategies that can be employed to overcome these impediments. Better understanding of patient-specific barriers will, I hope, allow us to more effectively redress them and ultimately increase colorectal cancer screening rates in all populations.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Despite the positive public health effects of colorectal cancer (CRC) screening, there remains differential uptake of CRC screening in the United States. Minority populations born in the United States and immigrant populations are among those with the lowest rates of CRC screening, and both socioeconomic status and ethnicity are strongly associated with stage of CRC at diagnosis.1,2 Thus, recognizing the economic, social, and cultural factors that result in low rates of CRC screening in underserved populations is important in order to devise targeted interventions to increase CRC uptake and reduce morbidity and mortality in these populations.

What are the facts and figures?
The overall rate of screening colonoscopies has increased in all ethnic groups in the past 10 years but still falls below the goal of 71% established by the Healthy People project (www.healthypeople.gov) for the year 2020.3 According to the Centers for Disease Control and Prevention ethnicity-specific data for U.S.-born populations, 60% of whites, 55% of African Americans (AA), 50% of American Indian/Alaskan natives (AI/AN), 46% of Latino Americans, and 47% of Asians undergo CRC screening (Figure 1A).4 While CRC incidence in non-Hispanic whites age 50 years and older has dropped by 32% since 2000 because of screening, this trend has not been observed in AAs.5,6

The incidence of CRC in AAs is estimated at 49/10,000, one of the highest amongst U.S. populations and is the second and third most common cancer in AA women and men, respectively (Figure 1B).
Similar to AAs, AI/AN patients present with more advanced CRC disease and at younger ages and have lower survival rates, compared with other racial groups, a trend that has not changed in the last decade.7 CRC screening data in this population vary according to sex, geographic location, and health care utilization, with as few as 4.0% of asymptomatic, average-risk AI/ANs who receive medical care in the Indian Health Services being screened for CRC.8
The low rate of CRC screening among Latinos also poses a significant obstacle to the Healthy People project since it is expected that by 2060 Latinos will constitute 30% of the U.S. population. Therefore, strategies to improve CRC screening in this population are needed to continue the gains made in overall CRC mortality rates.
The percentage of immigrants in the U.S. population increased from 4.7% in 1970 to 13.5% in 2015. Immigrants, regardless of their ethnicity, represent a very vulnerable population, and CRC screening data in this population are not as robust as for U.S.-born groups. In general, immigrants have substantially lower CRC screening rates, compared with U.S.-born populations (21% vs. 60%),9 and it is suspected that additional, significant barriers to CRC screening and care exist for undocumented immigrants.
Another often overlooked group, are individuals with physical or cognitive disabilities. In this group, screening rates range from 49% to 65%.10
Finally, while information is available for many health care conditions and disparities faced by various ethnic groups, there are few CRC screening data for the LGBTQ community. Perhaps amplifying this problem is the existence of conflicting data in this population, with some studies suggesting there is no difference in CRC risk across groups in the LGBTQ community and others suggesting an increased risk.11,12 Notably, sexual orientation has been identified as a positive predictor of CRC screening in gay and bisexual men – CRC screening rates are higher in these groups, compared with heterosexual men.13 In contrast, no such difference has been found between homosexual and heterosexual women.14
What are the barriers?
Several common themes contribute to disparities in CRC screening among minority groups, including psychosocial/cultural, socioeconomic, provider-specific, and insurance-related factors. Some patient-related barriers include issues of illiteracy, having poor health literacy or English proficiency, having only grade school education,15,16 cultural misconceptions, transportation issues, difficulties affording copayments or deductibles, and a lack of follow-up for scheduled appointments and exams.17-20 Poor health literacy has a profound effect on exam perceptions, fear of test results, and compliance with scheduling tests and bowel preparation instructions21-25; it also affects one’s understanding of the importance of CRC screening, the recommended screening age, and the available choice of screening tests.
Even when some apparent barriers are mitigated, disparities in CRC screening remain. For example, even among the insured and among Medicare beneficiaries, screening rates and adequate follow-up rates after abnormal findings remain lower among AAs and those of low socioeconomic status than they are among whites.26-28 At least part of this paradox results from the presence of unmeasured cultural/belief systems that affect CRC screening uptake. Some of these factors include fear and/or denial of CRC diagnoses, mistrust of the health care system, and reluctance to undergo medical treatment and surgery.16,29 AAs are also less likely to be aware of a family history of CRC and to discuss personal and/or family history of CRC or polyps, which can thereby hinder the identification of high-risk individuals who would benefit from early screening.15,30
The deeply rooted sense of fatalism also plays a crucial role and has been cited for many minority and immigrant populations. Fatalism leads patients to view a diagnosis of cancer as a matter of “fate” or “God’s will,” and therefore, it is to be endured.23,31 Similarly, in a qualitative study of 44 Somali men living in St. Paul and Minneapolis, believing cancer was more common in whites, believing they were protected from cancer by God, fearing a cancer diagnosis, and fearing ostracism from their community were reported as barriers to cancer screening.32
Perceptions about CRC screening methods in Latino populations also have a tremendous influence and can include fear, stigma of sexual prejudice, embarrassment of being exposed during the exam, worries about humiliation in a male sense of masculinity, a lack of trust in the medical professionals, a sense of being a “guinea pig” for physicians, concerns about health care racism, and expectations of pain.33-37 Studies have reported that immigrants are afraid to seek health care because of the increasingly hostile environment associated with immigration enforcement.38 In addition, the impending dissolution of the Deferred Action for Childhood Arrivals act is likely to augment the barriers to care for Latino groups.39
In addition, provider-specific barriers to care also exist. Racial and ethnic minorities are less likely than whites to receive recommendations for screening by their physician. In fact, this factor alone has been demonstrated to be the main reason for lack of screening among AAs in a Californian cohort.40 In addition, patients from rural areas or those from AI/AN communities are at especially increased risk for lack of access to care because of a scarcity of providers along with patient perceptions regarding their primary care provider’s ability to connect them to subspecialists.41-43 Other cited examples include misconceptions about and poor treatment of the LGBTQ population by health care providers/systems.44
How can we intervene successfully?
Characterization of barriers is important because it promotes the development of targeted interventions. Intervention models include community engagement programs, incorporation of fecal occult testing, and patient navigator programs.45-47 In response to the alarming disparity in CRC screening rates in Latino communities, several interventions have been set in motion in different clinical scenarios, which include patient navigation and a focus on patient education.

Randomized trials have shown that outreach efforts and patient navigation increase CRC screening rates in AAs.48,54,55 Studies evaluating the effects of print-based educational materials on improving screening showed improvement in screening rates, decreases in cancer-related fatalistic attitudes, and patients had a better understanding of the benefits of screening as compared with the cost associated with screening and the cost of advanced disease.56 Similarly, the use of touch-screen computers that tailor informational messages to decisional stage and screening barriers increased participation in CRC screening.57 Including patient navigators along with printed education material was even more effective at increasing the proportion of patients getting colonoscopy screening than providing printed material alone, with more-intensive navigation needed for individuals with low literacy.58 Grubbs et al.reported the success of their patient navigation program, which included wider comprehensive screening and coverage for colonoscopy screening.59 In AAs, they estimated an annual reduction of CRC incidence and mortality of 4,200 and 2,700 patients, respectively.
Among immigrants, there is an increased likelihood of CRC screening in those immigrants with a higher number of primary care visits.60 The intersection of culture, race, socioeconomic status, housing enclaves, limited English proficiency, low health literacy, and immigration policy all play a role in immigrant health and access to health care.61
Therefore, different strategies may be needed for each immigrant group to improve CRC screening. For this group of patients, efforts aimed at mitigating the adverse effects of national immigration policies on immigrant populations may have the additional consequence of improving health care access and CRC screening for these patients.
Data gaps still exist in our understanding of patient perceptions, perspectives, and barriers that present opportunities for further study to develop long-lasting interventions that will improve health care of underserved populations. By raising awareness of the barriers, physicians can enhance their own self-awareness to keenly be attuned to these challenges as patients cross their clinic threshold for medical care.
Additional resources link: www.cdc.gov/cancer/colorectal/
References
1. Klabunde CN et al. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011 Aug;20(8):1611-21.
2. Parikh-Patel A et al. Colorectal cancer stage at diagnosis by socioeconomic and urban/rural status in California, 1988-2000. Cancer. 2006 Sep;107(5 Suppl):1189-95.
3. Promotion OoDPaH. Healthy People 2020. Cancer. Volume 2017.
4. Centers for Disease Control and Prevention. Cancer screening – United States, 2010. MMWR Morb Mortal Wkly Rep. 2012 Jan 27;61(3):41-5.
5. Doubeni CA et al. Racial and ethnic trends of colorectal cancer screening among Medicare enrollees. Am J Prev Med. 2010 Feb;38(2):184-91.
6. Kupfer SS et al. Reducing colorectal cancer risk among African Americans. Gastroenterology. 2015 Nov;149(6):1302-4.
7. Espey DK et al. Annual report to the nation on the status of cancer, 1975-2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007 Nov;110(10):2119-52.
8. Day LW et al. Screening prevalence and incidence of colorectal cancer among American Indian/Alaskan natives in the Indian Health Service. Dig Dis Sci. 2011 Jul;56(7):2104-13.
9. Gupta S et al. Challenges and possible solutions to colorectal cancer screening for the underserved. J Natl Cancer Inst. 2014 Apr;106(4):dju032.
10. Steele CB et al. Colorectal cancer incidence and screening – United States, 2008 and 2010. MMWR Suppl. 2013 Nov 22;62(3):53-60.
11. Boehmer U et al. Cancer survivorship and sexual orientation. Cancer 2011 Aug 15;117(16):3796-804.
12. Austin SB, Pazaris MJ, Wei EK, et al. Application of the Rosner-Wei risk-prediction model to estimate sexual orientation patterns in colon cancer risk in a prospective cohort of US women. Cancer Causes Control. 2014 Aug;25(8):999-1006.
13. Heslin KC et al. Sexual orientation and testing for prostate and colorectal cancers among men in California. Med Care. 2008 Dec;46(12):1240-8.
14. McElroy JA et al. Advancing Health Care for Lesbian, Gay, Bisexual, and Transgender Patients in Missouri. Mo Med. 2015 Jul-Aug;112(4):262-5.
15. Greiner KA et al. Knowledge and perceptions of colorectal cancer screening among urban African Americans. J Gen Intern Med. 2005 Nov;20(11):977-83.
16. Green PM, Kelly BA. Colorectal cancer knowledge, perceptions, and behaviors in African Americans. Cancer Nurs. 2004 May-Jun;27(3):206-15; quiz 216-7.
17. Berkowitz Z et al. Beliefs, risk perceptions, and gaps in knowledge as barriers to colorectal cancer screening in older adults. J Am Geriatr Soc. 2008 Feb;56(2):307-14.
18. Dolan NC et al. Colorectal cancer screening knowledge, attitudes, and beliefs among veterans: Does literacy make a difference? J Clin Oncol. 2004 Jul;22(13):2617-22.
19. Peterson NB et al. The influence of health literacy on colorectal cancer screening knowledge, beliefs and behavior. J Natl Med Assoc. 2007 Oct;99(10):1105-12.
20. Haddock MG et al. Intraoperative irradiation for locally recurrent colorectal cancer in previously irradiated patients. Int J Radiat Oncol Biol Phys. 2001 Apr 1;49(5):1267-74.
21. Jones RM et al. Patient-reported barriers to colorectal cancer screening: a mixed-methods analysis. Am J Prev Med. 2010 May;38(5):508-16.
22. Basch CH et al. Screening colonoscopy bowel preparation: experience in an urban minority population. Therap Adv Gastroenterol. 2013 Nov;6(6):442-6.
23. Davis JL et al. Sociodemographic differences in fears and mistrust contributing to unwillingness to participate in cancer screenings. J Health Care Poor Underserved. 2012 Nov;23(4 Suppl):67-76.
24. Robinson CM et al. Barriers to colorectal cancer screening among publicly insured urban women: no knowledge of tests and no clinician recommendation. J Natl Med Assoc. 2011 Aug;103(8):746-53.
25. Goldman RE et al. Perspectives of colorectal cancer risk and screening among Dominicans and Puerto Ricans: Stigma and misperceptions. Qual Health Res. 2009 Nov;19(11):1559-68.
26. Laiyemo AO et al. Race and colorectal cancer disparities: Health-care utilization vs different cancer susceptibilities. J Natl Cancer Inst. 2010 Apr 21;102(8):538-46.
27. White A et al. Racial disparities and treatment trends in a large cohort of elderly African Americans and Caucasians with colorectal cancer, 1991 to 2002. Cancer. 2008 Dec 15;113(12):3400-9.
28. Doubeni CA et al. Neighborhood socioeconomic status and use of colonoscopy in an insured population – A retrospective cohort study. PLoS One. 2012;7(5):e36392.
29. Tammana VS, Laiyemo AO. Colorectal cancer disparities: Issues, controversies and solutions. World J Gastroenterol. 2014 Jan 28;20(4):869-76.
30. Carethers JM. Screening for colorectal cancer in African Americans: determinants and rationale for an earlier age to commence screening. Dig Dis Sci. 2015 Mar;60(3):711-21.
31. Miranda-Diaz C et al. Barriers for Compliance to Breast, Colorectal, and Cervical Screening Cancer Tests among Hispanic Patients. Int J Environ Res Public Health. 2015 Dec 22;13(1):ijerph13010021.
32. Sewali B et al. Understanding cancer screening service utilization by Somali men in Minnesota. J Immigr Minor Health. 2015 Jun;17(3):773-80.
33. Walsh JM et al. Barriers to colorectal cancer screening in Latino and Vietnamese Americans. Compared with non-Latino white Americans. J Gen Intern Med. 2004 Feb;19(2):156-66.
34. Perez-Stable EJ et al. Self-reported use of cancer screening tests among Latinos and Anglos in a prepaid health plan. Arch Intern Med. 1994 May 23;154(10):1073-81.
35. Shariff-Marco S et al. Racial/ethnic differences in self-reported racism and its association with cancer-related health behaviors. Am J Public Health. 2010 Feb;100(2):364-74.
36. Powe BD et al. Comparing knowledge of colorectal and prostate cancer among African American and Hispanic men. Cancer Nurs. 2009 Sep-Oct;32(5):412-7.
37. Jun J, Oh KM. Asian and Hispanic Americans’ cancer fatalism and colon cancer screening. Am J Health Behav. 2013 Mar;37(2):145-54.
38. Hacker K et al. The impact of Immigration and Customs Enforcement on immigrant health: Perceptions of immigrants in Everett, Massachusetts, USA. Soc Sci Med. 2011 Aug;73(4):586-94.
39. Firger J. Rescinding DACA could spur a public health crisis, from lost services to higher rates of depression, substance abuse. Newsweek.
40. May FP et al. Racial minorities are more likely than whites to report lack of provider recommendation for colon cancer screening. Am J Gastroenterol. 2015 Oct;110(10):1388-94.
41. Levy BT et al. Why hasn’t this patient been screened for colon cancer? An Iowa Research Network study. J Am Board Fam Med. 2007 Sep-Oct;20(5):458-68.
42. Rosenblatt RA. A view from the periphery – health care in rural America. N Engl J Med. 2004 Sep 9;351(11):1049-51.
43. Young WF et al. Predictors of colorectal screening in rural Colorado: testing to prevent colon cancer in the high plains research network. J Rural Health. 2007 Summer;23(3):238-45.
44. Kates J et al. Health and Access to Care and Coverage for Lesbian, Gay, Bisexual, and Transgender (LGBT) Individuals in the U.S. In: Foundation KF, ed. Disparities Policy Issue Brief. Volume 2017; Aug 30, 2017.
45. Katz ML et al. Improving colorectal cancer screening by using community volunteers: results of the Carolinas cancer education and screening (CARES) project. Cancer. 2007 Oct 1;110(7):1602-10.
46. Jean-Jacques M et al. Program to improve colorectal cancer screening in a low-income, racially diverse population: A randomized controlled trial. Ann Fam Med. 2012 Sep-Oct;10(5):412-7.
47. Reuland DS et al. Effect of combined patient decision aid and patient navigation vs usual care for colorectal cancer screening in a vulnerable patient population: A randomized clinical trial. JAMA Intern Med. 2017 Jul 1;177(7):967-74.
48. Percac-Lima S et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. J Gen Intern Med. 2009 Feb;24(2):211-7.
49. Nash D et al. Evaluation of an intervention to increase screening colonoscopy in an urban public hospital setting. J Urban Health. 2006 Mar;83(2):231-43.
50. Lebwohl B et al. Effect of a patient navigator program on the volume and quality of colonoscopy. J Clin Gastroenterol. 2011 May-Jun;45(5):e47-53.
51. Khankari K et al. Improving colorectal cancer screening among the medically underserved: A pilot study within a federally qualified health center. J Gen Intern Med. 2007 Oct;22(10):1410-4.
52. Wang JH et al. Recruiting Chinese Americans into cancer screening intervention trials: Strategies and outcomes. Clin Trials. 2014 Apr;11(2):167-77.
53. Katz ML et al. Patient activation increases colorectal cancer screening rates: a randomized trial among low-income minority patients. Cancer Epidemiol Biomarkers Prev. 2012 Jan;21(1):45-52.
54. Ford ME et al. Enhancing adherence among older African American men enrolled in a longitudinal cancer screening trial. Gerontologist. 2006 Aug;46(4):545-50.
55. Christie J et al. A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. J Natl Med Assoc. 2008 Mar;100(3):278-84.
56. Philip EJ et al. Evaluating the impact of an educational intervention to increase CRC screening rates in the African American community: A preliminary study. Cancer Causes Control. 2010 Oct;21(10):1685-91.
57. Greiner KA et al. Implementation intentions and colorectal screening: A randomized trial in safety-net clinics. Am J Prev Med. 2014 Dec;47(6):703-14.
58. Horne HN et al. Effect of patient navigation on colorectal cancer screening in a community-based randomized controlled trial of urban African American adults. Cancer Causes Control. 2015 Feb;26(2):239-46.
59. Grubbs SS et al. Eliminating racial disparities in colorectal cancer in the real world: It took a village. J Clin Oncol. 2013 Jun 1;31(16):1928-30.
60. Jung MY et al. The Chinese and Korean American immigrant experience: a mixed-methods examination of facilitators and barriers of colorectal cancer screening. Ethn Health. 2017 Feb 25:1-20.
61. Viruell-Fuentes EA et al. More than culture: structural racism, intersectionality theory, and immigrant health. Soc Sci Med. 2012 Dec;75(12):2099-106.
Dr. Oduyebo is a third-year fellow at the Mayo Clinic, Rochester, Minn.; Dr. Malespin is an assistant professor in the department of medicine and the medical director of hepatology at the University of Florida Health, Jacksonville; Dr. Mendoza Ladd is an assistant professor of medicine at Texas Tech University, El Paso; Dr. Day is an associate professor of medicine at the University of California, San Francisco; Dr. Charabaty is an associate professor of medicine and the director of the IBD Center in the division of gastroenterology at Medstar-Georgetown University Center, Washington; Dr. Chen is an associate professor of medicine, the director of patient safety and quality, and the director of the small-bowel endoscopy program in division of gastroenterology at Washington University, St. Louis; Dr. Carr is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia; Dr. Quezada is an assistant dean for admissions, an assistant dean for academic and multicultural affairs, and an assistant professor of medicine in the division of gastroenterology and hepatology at the University of Maryland, Baltimore; and Dr. Lamousé-Smith is a director of translational medicine, immunology, and early development at Janssen Pharmaceuticals Research and Development, Spring House, Penn.
Editor's Note:
Importantly, these barriers often vary between specific population subsets. In this month’s In Focus article, brought to you by The New Gastroenterologist, the members of the AGA Institute Diversity Committee provide an enlightening overview of the barriers affecting underserved populations as well as strategies that can be employed to overcome these impediments. Better understanding of patient-specific barriers will, I hope, allow us to more effectively redress them and ultimately increase colorectal cancer screening rates in all populations.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Despite the positive public health effects of colorectal cancer (CRC) screening, there remains differential uptake of CRC screening in the United States. Minority populations born in the United States and immigrant populations are among those with the lowest rates of CRC screening, and both socioeconomic status and ethnicity are strongly associated with stage of CRC at diagnosis.1,2 Thus, recognizing the economic, social, and cultural factors that result in low rates of CRC screening in underserved populations is important in order to devise targeted interventions to increase CRC uptake and reduce morbidity and mortality in these populations.

What are the facts and figures?
The overall rate of screening colonoscopies has increased in all ethnic groups in the past 10 years but still falls below the goal of 71% established by the Healthy People project (www.healthypeople.gov) for the year 2020.3 According to the Centers for Disease Control and Prevention ethnicity-specific data for U.S.-born populations, 60% of whites, 55% of African Americans (AA), 50% of American Indian/Alaskan natives (AI/AN), 46% of Latino Americans, and 47% of Asians undergo CRC screening (Figure 1A).4 While CRC incidence in non-Hispanic whites age 50 years and older has dropped by 32% since 2000 because of screening, this trend has not been observed in AAs.5,6

The incidence of CRC in AAs is estimated at 49/10,000, one of the highest amongst U.S. populations and is the second and third most common cancer in AA women and men, respectively (Figure 1B).
Similar to AAs, AI/AN patients present with more advanced CRC disease and at younger ages and have lower survival rates, compared with other racial groups, a trend that has not changed in the last decade.7 CRC screening data in this population vary according to sex, geographic location, and health care utilization, with as few as 4.0% of asymptomatic, average-risk AI/ANs who receive medical care in the Indian Health Services being screened for CRC.8
The low rate of CRC screening among Latinos also poses a significant obstacle to the Healthy People project since it is expected that by 2060 Latinos will constitute 30% of the U.S. population. Therefore, strategies to improve CRC screening in this population are needed to continue the gains made in overall CRC mortality rates.
The percentage of immigrants in the U.S. population increased from 4.7% in 1970 to 13.5% in 2015. Immigrants, regardless of their ethnicity, represent a very vulnerable population, and CRC screening data in this population are not as robust as for U.S.-born groups. In general, immigrants have substantially lower CRC screening rates, compared with U.S.-born populations (21% vs. 60%),9 and it is suspected that additional, significant barriers to CRC screening and care exist for undocumented immigrants.
Another often overlooked group, are individuals with physical or cognitive disabilities. In this group, screening rates range from 49% to 65%.10
Finally, while information is available for many health care conditions and disparities faced by various ethnic groups, there are few CRC screening data for the LGBTQ community. Perhaps amplifying this problem is the existence of conflicting data in this population, with some studies suggesting there is no difference in CRC risk across groups in the LGBTQ community and others suggesting an increased risk.11,12 Notably, sexual orientation has been identified as a positive predictor of CRC screening in gay and bisexual men – CRC screening rates are higher in these groups, compared with heterosexual men.13 In contrast, no such difference has been found between homosexual and heterosexual women.14
What are the barriers?
Several common themes contribute to disparities in CRC screening among minority groups, including psychosocial/cultural, socioeconomic, provider-specific, and insurance-related factors. Some patient-related barriers include issues of illiteracy, having poor health literacy or English proficiency, having only grade school education,15,16 cultural misconceptions, transportation issues, difficulties affording copayments or deductibles, and a lack of follow-up for scheduled appointments and exams.17-20 Poor health literacy has a profound effect on exam perceptions, fear of test results, and compliance with scheduling tests and bowel preparation instructions21-25; it also affects one’s understanding of the importance of CRC screening, the recommended screening age, and the available choice of screening tests.
Even when some apparent barriers are mitigated, disparities in CRC screening remain. For example, even among the insured and among Medicare beneficiaries, screening rates and adequate follow-up rates after abnormal findings remain lower among AAs and those of low socioeconomic status than they are among whites.26-28 At least part of this paradox results from the presence of unmeasured cultural/belief systems that affect CRC screening uptake. Some of these factors include fear and/or denial of CRC diagnoses, mistrust of the health care system, and reluctance to undergo medical treatment and surgery.16,29 AAs are also less likely to be aware of a family history of CRC and to discuss personal and/or family history of CRC or polyps, which can thereby hinder the identification of high-risk individuals who would benefit from early screening.15,30
The deeply rooted sense of fatalism also plays a crucial role and has been cited for many minority and immigrant populations. Fatalism leads patients to view a diagnosis of cancer as a matter of “fate” or “God’s will,” and therefore, it is to be endured.23,31 Similarly, in a qualitative study of 44 Somali men living in St. Paul and Minneapolis, believing cancer was more common in whites, believing they were protected from cancer by God, fearing a cancer diagnosis, and fearing ostracism from their community were reported as barriers to cancer screening.32
Perceptions about CRC screening methods in Latino populations also have a tremendous influence and can include fear, stigma of sexual prejudice, embarrassment of being exposed during the exam, worries about humiliation in a male sense of masculinity, a lack of trust in the medical professionals, a sense of being a “guinea pig” for physicians, concerns about health care racism, and expectations of pain.33-37 Studies have reported that immigrants are afraid to seek health care because of the increasingly hostile environment associated with immigration enforcement.38 In addition, the impending dissolution of the Deferred Action for Childhood Arrivals act is likely to augment the barriers to care for Latino groups.39
In addition, provider-specific barriers to care also exist. Racial and ethnic minorities are less likely than whites to receive recommendations for screening by their physician. In fact, this factor alone has been demonstrated to be the main reason for lack of screening among AAs in a Californian cohort.40 In addition, patients from rural areas or those from AI/AN communities are at especially increased risk for lack of access to care because of a scarcity of providers along with patient perceptions regarding their primary care provider’s ability to connect them to subspecialists.41-43 Other cited examples include misconceptions about and poor treatment of the LGBTQ population by health care providers/systems.44
How can we intervene successfully?
Characterization of barriers is important because it promotes the development of targeted interventions. Intervention models include community engagement programs, incorporation of fecal occult testing, and patient navigator programs.45-47 In response to the alarming disparity in CRC screening rates in Latino communities, several interventions have been set in motion in different clinical scenarios, which include patient navigation and a focus on patient education.

Randomized trials have shown that outreach efforts and patient navigation increase CRC screening rates in AAs.48,54,55 Studies evaluating the effects of print-based educational materials on improving screening showed improvement in screening rates, decreases in cancer-related fatalistic attitudes, and patients had a better understanding of the benefits of screening as compared with the cost associated with screening and the cost of advanced disease.56 Similarly, the use of touch-screen computers that tailor informational messages to decisional stage and screening barriers increased participation in CRC screening.57 Including patient navigators along with printed education material was even more effective at increasing the proportion of patients getting colonoscopy screening than providing printed material alone, with more-intensive navigation needed for individuals with low literacy.58 Grubbs et al.reported the success of their patient navigation program, which included wider comprehensive screening and coverage for colonoscopy screening.59 In AAs, they estimated an annual reduction of CRC incidence and mortality of 4,200 and 2,700 patients, respectively.
Among immigrants, there is an increased likelihood of CRC screening in those immigrants with a higher number of primary care visits.60 The intersection of culture, race, socioeconomic status, housing enclaves, limited English proficiency, low health literacy, and immigration policy all play a role in immigrant health and access to health care.61
Therefore, different strategies may be needed for each immigrant group to improve CRC screening. For this group of patients, efforts aimed at mitigating the adverse effects of national immigration policies on immigrant populations may have the additional consequence of improving health care access and CRC screening for these patients.
Data gaps still exist in our understanding of patient perceptions, perspectives, and barriers that present opportunities for further study to develop long-lasting interventions that will improve health care of underserved populations. By raising awareness of the barriers, physicians can enhance their own self-awareness to keenly be attuned to these challenges as patients cross their clinic threshold for medical care.
Additional resources link: www.cdc.gov/cancer/colorectal/
References
1. Klabunde CN et al. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011 Aug;20(8):1611-21.
2. Parikh-Patel A et al. Colorectal cancer stage at diagnosis by socioeconomic and urban/rural status in California, 1988-2000. Cancer. 2006 Sep;107(5 Suppl):1189-95.
3. Promotion OoDPaH. Healthy People 2020. Cancer. Volume 2017.
4. Centers for Disease Control and Prevention. Cancer screening – United States, 2010. MMWR Morb Mortal Wkly Rep. 2012 Jan 27;61(3):41-5.
5. Doubeni CA et al. Racial and ethnic trends of colorectal cancer screening among Medicare enrollees. Am J Prev Med. 2010 Feb;38(2):184-91.
6. Kupfer SS et al. Reducing colorectal cancer risk among African Americans. Gastroenterology. 2015 Nov;149(6):1302-4.
7. Espey DK et al. Annual report to the nation on the status of cancer, 1975-2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007 Nov;110(10):2119-52.
8. Day LW et al. Screening prevalence and incidence of colorectal cancer among American Indian/Alaskan natives in the Indian Health Service. Dig Dis Sci. 2011 Jul;56(7):2104-13.
9. Gupta S et al. Challenges and possible solutions to colorectal cancer screening for the underserved. J Natl Cancer Inst. 2014 Apr;106(4):dju032.
10. Steele CB et al. Colorectal cancer incidence and screening – United States, 2008 and 2010. MMWR Suppl. 2013 Nov 22;62(3):53-60.
11. Boehmer U et al. Cancer survivorship and sexual orientation. Cancer 2011 Aug 15;117(16):3796-804.
12. Austin SB, Pazaris MJ, Wei EK, et al. Application of the Rosner-Wei risk-prediction model to estimate sexual orientation patterns in colon cancer risk in a prospective cohort of US women. Cancer Causes Control. 2014 Aug;25(8):999-1006.
13. Heslin KC et al. Sexual orientation and testing for prostate and colorectal cancers among men in California. Med Care. 2008 Dec;46(12):1240-8.
14. McElroy JA et al. Advancing Health Care for Lesbian, Gay, Bisexual, and Transgender Patients in Missouri. Mo Med. 2015 Jul-Aug;112(4):262-5.
15. Greiner KA et al. Knowledge and perceptions of colorectal cancer screening among urban African Americans. J Gen Intern Med. 2005 Nov;20(11):977-83.
16. Green PM, Kelly BA. Colorectal cancer knowledge, perceptions, and behaviors in African Americans. Cancer Nurs. 2004 May-Jun;27(3):206-15; quiz 216-7.
17. Berkowitz Z et al. Beliefs, risk perceptions, and gaps in knowledge as barriers to colorectal cancer screening in older adults. J Am Geriatr Soc. 2008 Feb;56(2):307-14.
18. Dolan NC et al. Colorectal cancer screening knowledge, attitudes, and beliefs among veterans: Does literacy make a difference? J Clin Oncol. 2004 Jul;22(13):2617-22.
19. Peterson NB et al. The influence of health literacy on colorectal cancer screening knowledge, beliefs and behavior. J Natl Med Assoc. 2007 Oct;99(10):1105-12.
20. Haddock MG et al. Intraoperative irradiation for locally recurrent colorectal cancer in previously irradiated patients. Int J Radiat Oncol Biol Phys. 2001 Apr 1;49(5):1267-74.
21. Jones RM et al. Patient-reported barriers to colorectal cancer screening: a mixed-methods analysis. Am J Prev Med. 2010 May;38(5):508-16.
22. Basch CH et al. Screening colonoscopy bowel preparation: experience in an urban minority population. Therap Adv Gastroenterol. 2013 Nov;6(6):442-6.
23. Davis JL et al. Sociodemographic differences in fears and mistrust contributing to unwillingness to participate in cancer screenings. J Health Care Poor Underserved. 2012 Nov;23(4 Suppl):67-76.
24. Robinson CM et al. Barriers to colorectal cancer screening among publicly insured urban women: no knowledge of tests and no clinician recommendation. J Natl Med Assoc. 2011 Aug;103(8):746-53.
25. Goldman RE et al. Perspectives of colorectal cancer risk and screening among Dominicans and Puerto Ricans: Stigma and misperceptions. Qual Health Res. 2009 Nov;19(11):1559-68.
26. Laiyemo AO et al. Race and colorectal cancer disparities: Health-care utilization vs different cancer susceptibilities. J Natl Cancer Inst. 2010 Apr 21;102(8):538-46.
27. White A et al. Racial disparities and treatment trends in a large cohort of elderly African Americans and Caucasians with colorectal cancer, 1991 to 2002. Cancer. 2008 Dec 15;113(12):3400-9.
28. Doubeni CA et al. Neighborhood socioeconomic status and use of colonoscopy in an insured population – A retrospective cohort study. PLoS One. 2012;7(5):e36392.
29. Tammana VS, Laiyemo AO. Colorectal cancer disparities: Issues, controversies and solutions. World J Gastroenterol. 2014 Jan 28;20(4):869-76.
30. Carethers JM. Screening for colorectal cancer in African Americans: determinants and rationale for an earlier age to commence screening. Dig Dis Sci. 2015 Mar;60(3):711-21.
31. Miranda-Diaz C et al. Barriers for Compliance to Breast, Colorectal, and Cervical Screening Cancer Tests among Hispanic Patients. Int J Environ Res Public Health. 2015 Dec 22;13(1):ijerph13010021.
32. Sewali B et al. Understanding cancer screening service utilization by Somali men in Minnesota. J Immigr Minor Health. 2015 Jun;17(3):773-80.
33. Walsh JM et al. Barriers to colorectal cancer screening in Latino and Vietnamese Americans. Compared with non-Latino white Americans. J Gen Intern Med. 2004 Feb;19(2):156-66.
34. Perez-Stable EJ et al. Self-reported use of cancer screening tests among Latinos and Anglos in a prepaid health plan. Arch Intern Med. 1994 May 23;154(10):1073-81.
35. Shariff-Marco S et al. Racial/ethnic differences in self-reported racism and its association with cancer-related health behaviors. Am J Public Health. 2010 Feb;100(2):364-74.
36. Powe BD et al. Comparing knowledge of colorectal and prostate cancer among African American and Hispanic men. Cancer Nurs. 2009 Sep-Oct;32(5):412-7.
37. Jun J, Oh KM. Asian and Hispanic Americans’ cancer fatalism and colon cancer screening. Am J Health Behav. 2013 Mar;37(2):145-54.
38. Hacker K et al. The impact of Immigration and Customs Enforcement on immigrant health: Perceptions of immigrants in Everett, Massachusetts, USA. Soc Sci Med. 2011 Aug;73(4):586-94.
39. Firger J. Rescinding DACA could spur a public health crisis, from lost services to higher rates of depression, substance abuse. Newsweek.
40. May FP et al. Racial minorities are more likely than whites to report lack of provider recommendation for colon cancer screening. Am J Gastroenterol. 2015 Oct;110(10):1388-94.
41. Levy BT et al. Why hasn’t this patient been screened for colon cancer? An Iowa Research Network study. J Am Board Fam Med. 2007 Sep-Oct;20(5):458-68.
42. Rosenblatt RA. A view from the periphery – health care in rural America. N Engl J Med. 2004 Sep 9;351(11):1049-51.
43. Young WF et al. Predictors of colorectal screening in rural Colorado: testing to prevent colon cancer in the high plains research network. J Rural Health. 2007 Summer;23(3):238-45.
44. Kates J et al. Health and Access to Care and Coverage for Lesbian, Gay, Bisexual, and Transgender (LGBT) Individuals in the U.S. In: Foundation KF, ed. Disparities Policy Issue Brief. Volume 2017; Aug 30, 2017.
45. Katz ML et al. Improving colorectal cancer screening by using community volunteers: results of the Carolinas cancer education and screening (CARES) project. Cancer. 2007 Oct 1;110(7):1602-10.
46. Jean-Jacques M et al. Program to improve colorectal cancer screening in a low-income, racially diverse population: A randomized controlled trial. Ann Fam Med. 2012 Sep-Oct;10(5):412-7.
47. Reuland DS et al. Effect of combined patient decision aid and patient navigation vs usual care for colorectal cancer screening in a vulnerable patient population: A randomized clinical trial. JAMA Intern Med. 2017 Jul 1;177(7):967-74.
48. Percac-Lima S et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. J Gen Intern Med. 2009 Feb;24(2):211-7.
49. Nash D et al. Evaluation of an intervention to increase screening colonoscopy in an urban public hospital setting. J Urban Health. 2006 Mar;83(2):231-43.
50. Lebwohl B et al. Effect of a patient navigator program on the volume and quality of colonoscopy. J Clin Gastroenterol. 2011 May-Jun;45(5):e47-53.
51. Khankari K et al. Improving colorectal cancer screening among the medically underserved: A pilot study within a federally qualified health center. J Gen Intern Med. 2007 Oct;22(10):1410-4.
52. Wang JH et al. Recruiting Chinese Americans into cancer screening intervention trials: Strategies and outcomes. Clin Trials. 2014 Apr;11(2):167-77.
53. Katz ML et al. Patient activation increases colorectal cancer screening rates: a randomized trial among low-income minority patients. Cancer Epidemiol Biomarkers Prev. 2012 Jan;21(1):45-52.
54. Ford ME et al. Enhancing adherence among older African American men enrolled in a longitudinal cancer screening trial. Gerontologist. 2006 Aug;46(4):545-50.
55. Christie J et al. A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. J Natl Med Assoc. 2008 Mar;100(3):278-84.
56. Philip EJ et al. Evaluating the impact of an educational intervention to increase CRC screening rates in the African American community: A preliminary study. Cancer Causes Control. 2010 Oct;21(10):1685-91.
57. Greiner KA et al. Implementation intentions and colorectal screening: A randomized trial in safety-net clinics. Am J Prev Med. 2014 Dec;47(6):703-14.
58. Horne HN et al. Effect of patient navigation on colorectal cancer screening in a community-based randomized controlled trial of urban African American adults. Cancer Causes Control. 2015 Feb;26(2):239-46.
59. Grubbs SS et al. Eliminating racial disparities in colorectal cancer in the real world: It took a village. J Clin Oncol. 2013 Jun 1;31(16):1928-30.
60. Jung MY et al. The Chinese and Korean American immigrant experience: a mixed-methods examination of facilitators and barriers of colorectal cancer screening. Ethn Health. 2017 Feb 25:1-20.
61. Viruell-Fuentes EA et al. More than culture: structural racism, intersectionality theory, and immigrant health. Soc Sci Med. 2012 Dec;75(12):2099-106.
Dr. Oduyebo is a third-year fellow at the Mayo Clinic, Rochester, Minn.; Dr. Malespin is an assistant professor in the department of medicine and the medical director of hepatology at the University of Florida Health, Jacksonville; Dr. Mendoza Ladd is an assistant professor of medicine at Texas Tech University, El Paso; Dr. Day is an associate professor of medicine at the University of California, San Francisco; Dr. Charabaty is an associate professor of medicine and the director of the IBD Center in the division of gastroenterology at Medstar-Georgetown University Center, Washington; Dr. Chen is an associate professor of medicine, the director of patient safety and quality, and the director of the small-bowel endoscopy program in division of gastroenterology at Washington University, St. Louis; Dr. Carr is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia; Dr. Quezada is an assistant dean for admissions, an assistant dean for academic and multicultural affairs, and an assistant professor of medicine in the division of gastroenterology and hepatology at the University of Maryland, Baltimore; and Dr. Lamousé-Smith is a director of translational medicine, immunology, and early development at Janssen Pharmaceuticals Research and Development, Spring House, Penn.