User login
MDedge Daily News: Why the barber’s chair can help hypertension
Why the barber’s chair can help hypertension, how family history affects colorectal cancer risk, a steroid shot could ease hip osteoarthritis pain, and why excessive daytime sleepiness could lead to dementia.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Why the barber’s chair can help hypertension, how family history affects colorectal cancer risk, a steroid shot could ease hip osteoarthritis pain, and why excessive daytime sleepiness could lead to dementia.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Why the barber’s chair can help hypertension, how family history affects colorectal cancer risk, a steroid shot could ease hip osteoarthritis pain, and why excessive daytime sleepiness could lead to dementia.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Incidence and Management of Asymptomatic Hypertensive Urgency at a VA Emergency Department
Hypertension affects more than 65 million individuals in the U.S., accounting for nearly 30% of the adult population.1 Less than 50% of those with hypertension are taking appropriate pharmacotherapy.2 Hypertension contributes to cardiovascular events, including cerebrovascular accident, transient ischemic attack, hypertensive retinopathy, renal failure, myocardial infarction, and heart failure.1 Chronic hypertension mainly is an asymptomatic condition, earning the nickname “the silent killer.”2 An acute, symptomatic elevation in blood pressure (BP) often is referred to as hypertensive emergency. Symptoms of end-organ damage can include headache, blurry vision, chest pain, shortness of breath, altered mental status, epistaxis, and oliguria.2 Although rare, hypertensive emergencies should be treated immediately. The Seventh Report of the Joint National Committee (JNC 7), and the more recent JNC 8, have published guidelines on managing chronic hypertension.3,4 However, neither report provides guidance on hypertensive emergency or the appropriate actions in cases of extremely elevated BP in an asymptomatic patient.3,4
Acute hypertensive episodes—often referred to as hypertensive crises—are responsible for nearly 8 million hospitalizations each year and 20 million visits to the emergency department (ED).5,6 Most of these visits are same-day “treat-and-release” events.5 There is no universally accepted BP value associated with a hypertensive crisis, but most resources state that a BP ≥ 180/110 mm Hg requires attention.2,7 Without other symptoms, elevated BP is not an emergency, yet ED referral for acute management is common.7
Three terms fall under the umbrella of hypertensive crises: hypertensive emergency, hypertensive urgency, and asymptomatic hypertension (AH).2 In a 2007 article, the American College of Chest Physicians defined hypertensive emergency as BP ≥ 180/110 mm Hg with evidence of end-organ damage.2 Symptoms are almost always present in true hypertensive emergencies, and immediate medical intervention is required to halt further organ damage. In the same article, hypertensive urgency is defined as BP ≥ 180/110 mm Hg without end-organ damage.2 The definition of hypertensive urgency could be further refined to include the presence of cardiovascular and renal risk factors, although this additional point is not consistent across the literature. Asymptomatic hypertension is similar to hypertensive urgency; however, there is an absence of signs or symptoms of end-organ damage.2 There is ambiguity in the literature concerning managing hypertensive urgency and AH, but both share a basic tenet: Immediate BP reduction is not essential. Gradual dosage adjustment(s) of oral medications, preferably by a primary care provider (PCP), and follow-up within 7 days are recommended.7
Limited evidence exists to guide ED providers in managing AH. Long-term outcomes and guidelines intended for the primary care setting should not be extrapolated to acute management in the ED. With limited treatment guidelines, providers might be more likely to refer patients with AH to the ED for evaluation. In 2013, the American College of Emergency Physicians (ACEP) created a clinical policy concerning AH in the ED. The ACEP concluded that screening for target organ injury and medical intervention in the ED does not reduce rates of adverse events (AEs) and could lead to overtreatment and acute hypoperfusion.7 More recently, Patel and colleagues published findings on hypertensive urgency in the ambulatory care setting, which similarly found that referral to the ED was associated with increased use of health care resources and no change in short-term major AEs.8 The ACEP recommends that patients presenting with AH be referred to primary care clinics where long-term monitoring and medication adjustments can be achieved more cost-effectively.7
The objective of this retrospective evaluation was to assess the incidence and management of AH within a VA ED. The authors aimed to provide insight into how these patients are managed and discuss alternatives to ED use.
Methods
This retrospective observational study was conducted within the North Florida/South Georgia Veterans Health System (NFSGVHS), which provides patient care at 2 medical centers in Gainesville and Lake City, Florida, as well as 11 outpatient clinics located throughout North Florida and South Georgia. The NFSGVHS serves rural and urban veteran populations. Study approval was granted by the NFSGVHS Institutional Review Board and Research and Development Committee.
Inclusion/Exclusion Criteria
Adult patients who were ordered at least 1 antihypertensive medication in the ED from July 1, 2011 to July 1, 2014, in addition to being asymptomatic with BP ≥ 180/110 mm Hg at ED triage were included. Based on clinical experience, the authors estimated that 3 years would provide a sample size of more than 100 patients. Patients were excluded if they presented with any acute symptoms or were hospitalized for further management.
Data Collection
Baseline demographics were collected for all participants. During the ED encounter, pre- and postintervention vital signs were recorded and prespecified laboratory data obtained. Interrater reliability was accounted for by performing random reviews of previously collected data to ensure consistency during the chart review process. Renal end-organ damage was defined using Acute Kidney Injury Network criteria, a serum creatinine 50% above baseline, or an absolute increase in baseline serum creatinine by 0.3 mg/dL.9 Additional laboratory markers of organ damage included cardiac troponin levels. Urinalysis results also were assessed to determine the presence of hematuria or proteinuria. Patient-reported nonadherence with medications was determined by reviewing ED provider and/or nurse documentation notes for the index ED encounter.
Investigators documented the route (IV or oral) and antihypertensive(s) medication selected for each patient. Adverse effects and any changes to patients’ outpatient medication regimens were noted. Investigators also assessed days to next medical contact after ED discharge to determine whether follow-up occurred according to the recommended standard of 7 days.9 Days to next medical contact was defined as any contact—in person or by telephone—that was documented in the electronic health record after the index ED visit.
Statistical Analysis
Descriptive statistics, including mean, median, and standard deviation, were used to analyze data.
Results
A total of 1,052 patients presented with BP ≥ 180/110 mm Hg and for whom antihypertensive medication was ordered but not necessarily given in the ED. Of the total, 724 patients were excluded because of hospital admission for other primary diagnoses; however, 6 of these patients were admitted for hypertensive urgency. The final analysis included 132 patients who presented with the primary condition of elevated BP without any accompanying symptoms. Among these patients, 2 had repeat ED visits for AH during the specified time frame.
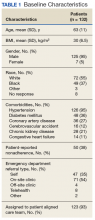
Most patients were male with an average age of 63 years and documented history of hypertension. Nearly all patients had established primary care within the NFSGVHS. The most common comorbidity was diabetes mellitus (36%), followed by coronary artery disease (27%) and chronic kidney disease (CKD) (21%) (Table 1). About one-third of patients presented to the ED on their own volition, and slightly more than half were referred to the ED by primary care or specialty clinics.
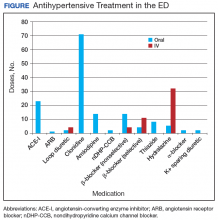
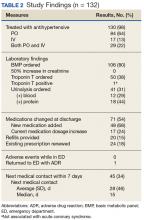
In the ED, 130 patients received BP treatment (Table 2). Medication was ordered for 2 patients who did not receive treatment. In total, 12 different medication classes were used for treating patients with AH in the ED (Figure).
Treatment in the ED resulted in an average BP and heart rate reduction of 27/20 mm Hg and 5 beats per minute, respectively. About 80% of patients had a basic metabolic panel drawn, and there were no instances of acute kidney injury. Of the patients in the study 38% had cardiac enzymes collected, and only 1 patient had a positive result, which was determined to be unrelated to acute coronary syndrome. Forty-one (31%) of patients had a urinalysis; 12 has positive results for hematuria, and 18 revealed proteinuria. Of note, the 6 patients who were hospitalized for hypertensive urgency had neither symptoms at presentation to the ED nor laboratory findings indicating end-organ damage. The reason these patients were admitted is unclear.
At discharge, ED providers made changes to 54% of patients’ outpatient antihypertensive regimens. These changes included adding a new medication (68%), increasing the dosage of an existing medication (24%), or multiple changes (8%). Refills were provided for 18% of prescriptions. Follow-up within 7 days from ED discharge was recorded for 34% of patients. One patient received follow-up outside the NFSGVHS and was not included in this analysis.
Discussion
The aim of this retrospective study was to determine the incidence of AH in a VA ED and describe how these patients were managed. Overall, the rate of patients presenting to the ED with AH during the study period was about 1 patient every 8 days or 45 patients per year. By comparison, more than 30,000 patients are seen at the NFSGVHS ED annually. Although AH seems to be an uncommon occurrence, study findings raise questions about the value of managing the condition in the ED.
This study found several management strategies as well as noteworthy trends. For example, laboratory tests were not ordered routinely for all patients, suggesting that some ED providers question their use for AH. There were no patients with acute elevations in serum creatinine that indicated acute kidney injury, and although hematuria and proteinuria were common findings, neither were specific for acute injury. However, there were findings typical of chronic hypertension, and urinalysis may provide little benefit when testing for acute kidney injury. Only 1 patient showed elevated cardiac enzymes, which was determined to be a result of CKD.
Although not included in the final analysis, the 6 patients who were hospitalized for hypertensive urgency were similar in that they had neither symptoms at presentation to the ED nor laboratory findings indicating end-organ damage. Collectively, these findings support existing literature that questions the utility of laboratory testing of patients with AH in the ED.10
Patients also were treated with a variety of antihypertensive agents in the ED. One explanation might be outpatient nonadherence with medications. In patients with AH, it is common to provide doses of chronic medications that the patient might have missed and should be taking on a regular basis. Therefore, assessing adherence with current medications before modifying chronic therapy is an important initial step when managing AH.
Although oral agents primarily were used, IV antihypertensives were administered to about one-third of patients. Preference for IV administration in the ED might be related to its ability to lower BP quickly. The practice of obtaining IV access for medication in a patient with AH is costly, unnecessary, and potentially harmful.7 The authors theorize that this practice is performed, in many cases, as an attempt to expedite ED discharge after an acceptable BP reading is documented.
Rapid reductions in BP can precipitate hypoperfusion inadvertently and are more likely to occur with IV agents than with oral ones. Therefore, the safety, convenience, and cost savings associated with oral administration make it the preferred route for managing AH.
Best Practices
Primary care clinics are best suited to manage AH because medication adjustments and long-term monitoring are easier to perform and at substantially lower costs when compared with that of the ED. Rather than immediately referring a patient to the ED, clinicians should consider factors that could elevate BP, such as medication nonadherence, anxiety, acute pain, recent tobacco or caffeine use, or white coat syndrome. Staff should be well educated on proper BP measurement and instructed to repeat the reading for confirmation. Before measuring BP, allow the patient to sit quietly for 5 minutes with the feet flat on the floor and arm supported.3 Ideally, the measurement used should be the average of 3 BP readings on an automated device.11 If BP readings are high, staff should ask the patient about medication adherence and missed medication(s) should be administered.
It also is reasonable to have the patient rest quietly for up to 30 minutes because rest has been shown to reduce BP in some patients.12 The drawback to the prolonged rest strategy is the potential to cause delays in care for other patients. However, it is important to remember that wait times in the ED often are measured in hours, which causes frustration for patients referred to the ED for AH management. Before completing the office visit, the provider should recheck BP using proper technique and confirm that the patient has antihypertensive medication(s) in his/her possession; a follow-up appointment should be scheduled for no later than 1 week.
Primary care providers might be concerned about taking on additional liability and could favor ED referral, but legislation makes it difficult for EDs to defer nonemergent issues to primary care clinics. The Emergency Medical Treatment and Labor Act states that hospitals are prohibited from denying a patient care during an emergency.13 Despite evidence that AH is not an emergency, many patients continue to be referred to the ED. One-third of patients presented to the ED on their own volition and more than one-half were referred by health care personnel. This strongly suggests that both patients and health care personnel consider AH an emergency medical condition requiring immediate attention. However, patients with AH rarely are found to have any acute end-organ damage; therefore, acute treatment and extensive laboratory or diagnostic testing in the ED provides little, if any, benefit.10 The authors believe the ACEP clinical policy should be adopted into mainstream practice to help reduce health care costs and preserve ED resources for patients with true emergencies.
Another pervasive issue that could contribute to inappropriate AH referrals to the ED is the shortage of PCPs and limited same-day appointments for nonemergent conditions. In a 2017 survey, the average wait time for a PCP appointment ranged between 12 and 109 days, depending on the metropolitan area. The national average wait time conducted by this survey was 29.3 days.14 When primary care appointments are unavailable, triage staff could recommend that patients seek care in the ED. Additionally, patients might choose to seek ED care rather than wait for the next available PCP appointment. Clinic proximity to an ED could influence referral rates. In other words, medical centers or health systems with primary care clinics and ED services under one roof could experience more frequent ED referrals.
A promising strategy to help overcome the challenges of addressing AH and avoiding ED referrals is increasing patient access to and use of qualified, nonphysician providers, such as clinical pharmacists and nurse practitioners. Large health systems such as the VA and Kaiser Permanente have employed clinical pharmacist providers to reduce follow-up times for patients in primary care settings.15 Furthermore, there is substantial evidence that supports the cost-effectiveness and clinical success of pharmacist-driven hypertension clinics.16-18 Nurse-driven efforts to improve hypertension control have been successfully implemented in health systems.19 Both clinical pharmacist and nurse-managed hypertension clinics are effective solutions to manage patients with AH who might otherwise use costly ED services.For example, the average cost of a single ED visit is $740 to $3,437.20 In comparison, a 2010 report from the Agency for Healthcare Research and Quality showed the average annual cost of managing hypertension in ambulatory care clinics was $442 per adult, a cost considerably lower than that of the ED.21
Limitations
The retrospective and observational design of this study are inherent limitations. This study was not designed to evaluate cardiovascular outcomes after ED encounters. The sample size could have been larger if patients with BP < 180/110 mm Hg at ED triage were included; however, the 180/110 mm Hg threshold was chosen because it was the most widely agreed on BP value in the literature. This study did not capture patients who presented with AH and did not receive any acute treatment in the ED.Prescribing patterns based on provider training (eg, emergency medicine, family medicine, or internal medicine) were not tracked and might have accounted for differences in selection of diagnostic tests, laboratory ordering, and route of drug administration preference.
A small subset of patients reported positive pain scores at triage but did not describe acute pain. Pain scores are highly subjective, and few primary literature sources link chronic pain with increased BP.22,23 Nevertheless, patients who reported acute pain and elevated BP were excluded in order to identify truly asymptomatic patients. VA hospitals are unique health systems and data obtained from this study might not be applicable to other public or private facilities. Last, the study did not take into account patients’ psychosocial circumstances that might have fostered a disproportionate reliance on the ED for health care.
Conclusion
Asymptomatic patients with elevated BP are treated in the ED despite no evidence supporting improved outcomes after acute BP lowering in this population. Follow-up after ED encounters for AH did not occur consistently within guideline-recommended 7 days, a trend that also occurs in non-VA systems.8 Clinics and health care systems could establish policies to prevent or minimize management of AH in the ED. Ideally, AH should be managed in a clinic setting by a PCP, but growing clinician workload might lead to increasing wait times and difficultly obtaining same-day appointments. Nurse-led clinics and clinical pharmacists operating under a scope of practice and working closely with a PCP are a cost-effective solution to ensure timely treatment and appropriate follow-up of patients with uncontrolled hypertension.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the North Florida South Georgia Veterans Health System in Gainesville, Florida.
1. Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief. 2013;(133):
1-8.
2. Marik PE, Varon J. Hypertensive crises: challenges and management. Chest. 2007;131(6):1949-1962.
3. Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206-1252.
4. James, PA, Oparil, S, Carter, BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520.
5. American Heart Association. High blood pressure ER visits jumped 25 percent in 2006-11. http://newsroom.heart.org/news/high-blood-pressure-er-visits-jumped-25-percent-in-2006-11. Published September 9, 2014. Accessed January 19, 2018.
6. Owens P, Mutter R. Statistical brief #100: emergency department visits for adults in community hospitals. Agency for Healthcare Research and Quality. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb100.pdf. Published November 2010. Accessed January 19, 2018.
7. Wolf SJ, Lo B, Shih RD, Smith MD, Fesmire FM; American College of Emergency Physicians Clinical Policies Committee. Clinical policy: critical issues in the evaluation and management of adult patients in the emergency department with asymptomatic elevated blood pressure. Ann Emerg Med. 2013;62(1):59-68.
8. Patel KK, Young L, Howell EH, et al. Characteristics and outcomes of patients presenting with hypertensive urgency in the office setting. JAMA Intern Med. 2016;176(7):981-988.
9. Acute Kidney Injury Network. AKIN studies. http://www.akinet.org/akinstudies.php. Updated 2017. Accessed January 19, 2018.
10. Karras DJ, Kruus LK, Cienki JJ, et al. Utility of routine testing for patients with asymptomatic severe blood pressure elevation in the emergency department. Ann Emerg Med. 2008;51(3):231-239.
11. The SPRINT Research Group. A Randomized trial of Intensive versus standard blood pressure control. N Engl J Med. 2015;373:2103-2116.
12. Grassi D, O’Flaherty M, Pellizzari M, et al; Group of Investigators of the REHASE Program. Hypertensive urgencies in the emergency department: evaluating blood pressure response to rest and to antihypertensive drugs with different profiles. J Clin Hypertens (Greenwich). 2008;10(9):662-667.
13. Canters for Medicare & Medicaid Services. Emergency medical treatment & labor act (EMTALA). https://www.cms.gov/Regulations-and-Guidance/Legislation/EMTALA/index.html. Updated March 26, 2012. Accessed January 19, 2018.
14. Merritt Hawkins. 2017 Survey of physician appointment wait times and Medicare and Medicaid acceptance rates. https://www.merritthawkins.com/uploadedFiles/Merritt-Hawkins/Pdf/mha2017waittimesurveyPDF.pdf. Published 2017. Accessed January 19, 2018.
15. Galewitz P. VA treats patients’ impatience with clinical pharmacists. USA Today. http://www.usatoday.com/story/news/2016/10/24/kaiser-va-treats-patients-impatience-clinical-pharmacists/92479132/. Published October 24, 2016. Accessed January 19, 2018.
16. Carter BL, Ardery G, Dawson JD, et al. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med. 2009;169(21):1996-2002.
17. Borenstein JE, Graber G, Saltiel E, et al. Physician-pharmacist comanagement of hypertension: a randomized comparative trial. Pharmacotherapy. 2003;23(2):209-216.
18. Okamoto MP, Nakahiro RK. Pharmacoeconomic evaluation of a pharmacist-managed hypertension clinic. Pharmacotherapy. 2001;21(11):1337-1344.
19. Brown VM. Managing patients with hypertension in nurse-led clinics. Nursing. 2017;47(4):16-19.
20. Caldwell N, Srebotnjak T, Wang T, Hsia R. “How Much Will I Get Charged for This?” Patient charges for top ten diagnoses in the emergency department. PLoS ONE. 2013;8(2): e55491.
21. Davis KE. Expenditures for hypertension among adults age 18 and older, 2010: estimates for the U.S. civilian noninstitutionalized population. Agency for Healthcare Research and Quality. https://meps.ahrq.gov/data_files/publications/st404/stat404.shtml. Published April 2013. Accessed January 19, 2018.
22. Marco CA, Plewa MC, Buderer N, et al. Self-reported pain scores in the emergency department: lack of association with vital signs. Acad Emerg Med. 2006;13(9):974-979.
23. Wood D, Goodnight S, Haig AJ, Nasari T. Body mass index, but not blood pressure is related to the level of pain in persons with chronic pain. J Back Musculoskelet Rehabil. 2011;24(2):
111-115.
Hypertension affects more than 65 million individuals in the U.S., accounting for nearly 30% of the adult population.1 Less than 50% of those with hypertension are taking appropriate pharmacotherapy.2 Hypertension contributes to cardiovascular events, including cerebrovascular accident, transient ischemic attack, hypertensive retinopathy, renal failure, myocardial infarction, and heart failure.1 Chronic hypertension mainly is an asymptomatic condition, earning the nickname “the silent killer.”2 An acute, symptomatic elevation in blood pressure (BP) often is referred to as hypertensive emergency. Symptoms of end-organ damage can include headache, blurry vision, chest pain, shortness of breath, altered mental status, epistaxis, and oliguria.2 Although rare, hypertensive emergencies should be treated immediately. The Seventh Report of the Joint National Committee (JNC 7), and the more recent JNC 8, have published guidelines on managing chronic hypertension.3,4 However, neither report provides guidance on hypertensive emergency or the appropriate actions in cases of extremely elevated BP in an asymptomatic patient.3,4
Acute hypertensive episodes—often referred to as hypertensive crises—are responsible for nearly 8 million hospitalizations each year and 20 million visits to the emergency department (ED).5,6 Most of these visits are same-day “treat-and-release” events.5 There is no universally accepted BP value associated with a hypertensive crisis, but most resources state that a BP ≥ 180/110 mm Hg requires attention.2,7 Without other symptoms, elevated BP is not an emergency, yet ED referral for acute management is common.7
Three terms fall under the umbrella of hypertensive crises: hypertensive emergency, hypertensive urgency, and asymptomatic hypertension (AH).2 In a 2007 article, the American College of Chest Physicians defined hypertensive emergency as BP ≥ 180/110 mm Hg with evidence of end-organ damage.2 Symptoms are almost always present in true hypertensive emergencies, and immediate medical intervention is required to halt further organ damage. In the same article, hypertensive urgency is defined as BP ≥ 180/110 mm Hg without end-organ damage.2 The definition of hypertensive urgency could be further refined to include the presence of cardiovascular and renal risk factors, although this additional point is not consistent across the literature. Asymptomatic hypertension is similar to hypertensive urgency; however, there is an absence of signs or symptoms of end-organ damage.2 There is ambiguity in the literature concerning managing hypertensive urgency and AH, but both share a basic tenet: Immediate BP reduction is not essential. Gradual dosage adjustment(s) of oral medications, preferably by a primary care provider (PCP), and follow-up within 7 days are recommended.7
Limited evidence exists to guide ED providers in managing AH. Long-term outcomes and guidelines intended for the primary care setting should not be extrapolated to acute management in the ED. With limited treatment guidelines, providers might be more likely to refer patients with AH to the ED for evaluation. In 2013, the American College of Emergency Physicians (ACEP) created a clinical policy concerning AH in the ED. The ACEP concluded that screening for target organ injury and medical intervention in the ED does not reduce rates of adverse events (AEs) and could lead to overtreatment and acute hypoperfusion.7 More recently, Patel and colleagues published findings on hypertensive urgency in the ambulatory care setting, which similarly found that referral to the ED was associated with increased use of health care resources and no change in short-term major AEs.8 The ACEP recommends that patients presenting with AH be referred to primary care clinics where long-term monitoring and medication adjustments can be achieved more cost-effectively.7
The objective of this retrospective evaluation was to assess the incidence and management of AH within a VA ED. The authors aimed to provide insight into how these patients are managed and discuss alternatives to ED use.
Methods
This retrospective observational study was conducted within the North Florida/South Georgia Veterans Health System (NFSGVHS), which provides patient care at 2 medical centers in Gainesville and Lake City, Florida, as well as 11 outpatient clinics located throughout North Florida and South Georgia. The NFSGVHS serves rural and urban veteran populations. Study approval was granted by the NFSGVHS Institutional Review Board and Research and Development Committee.
Inclusion/Exclusion Criteria
Adult patients who were ordered at least 1 antihypertensive medication in the ED from July 1, 2011 to July 1, 2014, in addition to being asymptomatic with BP ≥ 180/110 mm Hg at ED triage were included. Based on clinical experience, the authors estimated that 3 years would provide a sample size of more than 100 patients. Patients were excluded if they presented with any acute symptoms or were hospitalized for further management.
Data Collection
Baseline demographics were collected for all participants. During the ED encounter, pre- and postintervention vital signs were recorded and prespecified laboratory data obtained. Interrater reliability was accounted for by performing random reviews of previously collected data to ensure consistency during the chart review process. Renal end-organ damage was defined using Acute Kidney Injury Network criteria, a serum creatinine 50% above baseline, or an absolute increase in baseline serum creatinine by 0.3 mg/dL.9 Additional laboratory markers of organ damage included cardiac troponin levels. Urinalysis results also were assessed to determine the presence of hematuria or proteinuria. Patient-reported nonadherence with medications was determined by reviewing ED provider and/or nurse documentation notes for the index ED encounter.
Investigators documented the route (IV or oral) and antihypertensive(s) medication selected for each patient. Adverse effects and any changes to patients’ outpatient medication regimens were noted. Investigators also assessed days to next medical contact after ED discharge to determine whether follow-up occurred according to the recommended standard of 7 days.9 Days to next medical contact was defined as any contact—in person or by telephone—that was documented in the electronic health record after the index ED visit.
Statistical Analysis
Descriptive statistics, including mean, median, and standard deviation, were used to analyze data.
Results
A total of 1,052 patients presented with BP ≥ 180/110 mm Hg and for whom antihypertensive medication was ordered but not necessarily given in the ED. Of the total, 724 patients were excluded because of hospital admission for other primary diagnoses; however, 6 of these patients were admitted for hypertensive urgency. The final analysis included 132 patients who presented with the primary condition of elevated BP without any accompanying symptoms. Among these patients, 2 had repeat ED visits for AH during the specified time frame.
Most patients were male with an average age of 63 years and documented history of hypertension. Nearly all patients had established primary care within the NFSGVHS. The most common comorbidity was diabetes mellitus (36%), followed by coronary artery disease (27%) and chronic kidney disease (CKD) (21%) (Table 1). About one-third of patients presented to the ED on their own volition, and slightly more than half were referred to the ED by primary care or specialty clinics.
In the ED, 130 patients received BP treatment (Table 2). Medication was ordered for 2 patients who did not receive treatment. In total, 12 different medication classes were used for treating patients with AH in the ED (Figure).
Treatment in the ED resulted in an average BP and heart rate reduction of 27/20 mm Hg and 5 beats per minute, respectively. About 80% of patients had a basic metabolic panel drawn, and there were no instances of acute kidney injury. Of the patients in the study 38% had cardiac enzymes collected, and only 1 patient had a positive result, which was determined to be unrelated to acute coronary syndrome. Forty-one (31%) of patients had a urinalysis; 12 has positive results for hematuria, and 18 revealed proteinuria. Of note, the 6 patients who were hospitalized for hypertensive urgency had neither symptoms at presentation to the ED nor laboratory findings indicating end-organ damage. The reason these patients were admitted is unclear.
At discharge, ED providers made changes to 54% of patients’ outpatient antihypertensive regimens. These changes included adding a new medication (68%), increasing the dosage of an existing medication (24%), or multiple changes (8%). Refills were provided for 18% of prescriptions. Follow-up within 7 days from ED discharge was recorded for 34% of patients. One patient received follow-up outside the NFSGVHS and was not included in this analysis.
Discussion
The aim of this retrospective study was to determine the incidence of AH in a VA ED and describe how these patients were managed. Overall, the rate of patients presenting to the ED with AH during the study period was about 1 patient every 8 days or 45 patients per year. By comparison, more than 30,000 patients are seen at the NFSGVHS ED annually. Although AH seems to be an uncommon occurrence, study findings raise questions about the value of managing the condition in the ED.
This study found several management strategies as well as noteworthy trends. For example, laboratory tests were not ordered routinely for all patients, suggesting that some ED providers question their use for AH. There were no patients with acute elevations in serum creatinine that indicated acute kidney injury, and although hematuria and proteinuria were common findings, neither were specific for acute injury. However, there were findings typical of chronic hypertension, and urinalysis may provide little benefit when testing for acute kidney injury. Only 1 patient showed elevated cardiac enzymes, which was determined to be a result of CKD.
Although not included in the final analysis, the 6 patients who were hospitalized for hypertensive urgency were similar in that they had neither symptoms at presentation to the ED nor laboratory findings indicating end-organ damage. Collectively, these findings support existing literature that questions the utility of laboratory testing of patients with AH in the ED.10
Patients also were treated with a variety of antihypertensive agents in the ED. One explanation might be outpatient nonadherence with medications. In patients with AH, it is common to provide doses of chronic medications that the patient might have missed and should be taking on a regular basis. Therefore, assessing adherence with current medications before modifying chronic therapy is an important initial step when managing AH.
Although oral agents primarily were used, IV antihypertensives were administered to about one-third of patients. Preference for IV administration in the ED might be related to its ability to lower BP quickly. The practice of obtaining IV access for medication in a patient with AH is costly, unnecessary, and potentially harmful.7 The authors theorize that this practice is performed, in many cases, as an attempt to expedite ED discharge after an acceptable BP reading is documented.
Rapid reductions in BP can precipitate hypoperfusion inadvertently and are more likely to occur with IV agents than with oral ones. Therefore, the safety, convenience, and cost savings associated with oral administration make it the preferred route for managing AH.
Best Practices
Primary care clinics are best suited to manage AH because medication adjustments and long-term monitoring are easier to perform and at substantially lower costs when compared with that of the ED. Rather than immediately referring a patient to the ED, clinicians should consider factors that could elevate BP, such as medication nonadherence, anxiety, acute pain, recent tobacco or caffeine use, or white coat syndrome. Staff should be well educated on proper BP measurement and instructed to repeat the reading for confirmation. Before measuring BP, allow the patient to sit quietly for 5 minutes with the feet flat on the floor and arm supported.3 Ideally, the measurement used should be the average of 3 BP readings on an automated device.11 If BP readings are high, staff should ask the patient about medication adherence and missed medication(s) should be administered.
It also is reasonable to have the patient rest quietly for up to 30 minutes because rest has been shown to reduce BP in some patients.12 The drawback to the prolonged rest strategy is the potential to cause delays in care for other patients. However, it is important to remember that wait times in the ED often are measured in hours, which causes frustration for patients referred to the ED for AH management. Before completing the office visit, the provider should recheck BP using proper technique and confirm that the patient has antihypertensive medication(s) in his/her possession; a follow-up appointment should be scheduled for no later than 1 week.
Primary care providers might be concerned about taking on additional liability and could favor ED referral, but legislation makes it difficult for EDs to defer nonemergent issues to primary care clinics. The Emergency Medical Treatment and Labor Act states that hospitals are prohibited from denying a patient care during an emergency.13 Despite evidence that AH is not an emergency, many patients continue to be referred to the ED. One-third of patients presented to the ED on their own volition and more than one-half were referred by health care personnel. This strongly suggests that both patients and health care personnel consider AH an emergency medical condition requiring immediate attention. However, patients with AH rarely are found to have any acute end-organ damage; therefore, acute treatment and extensive laboratory or diagnostic testing in the ED provides little, if any, benefit.10 The authors believe the ACEP clinical policy should be adopted into mainstream practice to help reduce health care costs and preserve ED resources for patients with true emergencies.
Another pervasive issue that could contribute to inappropriate AH referrals to the ED is the shortage of PCPs and limited same-day appointments for nonemergent conditions. In a 2017 survey, the average wait time for a PCP appointment ranged between 12 and 109 days, depending on the metropolitan area. The national average wait time conducted by this survey was 29.3 days.14 When primary care appointments are unavailable, triage staff could recommend that patients seek care in the ED. Additionally, patients might choose to seek ED care rather than wait for the next available PCP appointment. Clinic proximity to an ED could influence referral rates. In other words, medical centers or health systems with primary care clinics and ED services under one roof could experience more frequent ED referrals.
A promising strategy to help overcome the challenges of addressing AH and avoiding ED referrals is increasing patient access to and use of qualified, nonphysician providers, such as clinical pharmacists and nurse practitioners. Large health systems such as the VA and Kaiser Permanente have employed clinical pharmacist providers to reduce follow-up times for patients in primary care settings.15 Furthermore, there is substantial evidence that supports the cost-effectiveness and clinical success of pharmacist-driven hypertension clinics.16-18 Nurse-driven efforts to improve hypertension control have been successfully implemented in health systems.19 Both clinical pharmacist and nurse-managed hypertension clinics are effective solutions to manage patients with AH who might otherwise use costly ED services.For example, the average cost of a single ED visit is $740 to $3,437.20 In comparison, a 2010 report from the Agency for Healthcare Research and Quality showed the average annual cost of managing hypertension in ambulatory care clinics was $442 per adult, a cost considerably lower than that of the ED.21
Limitations
The retrospective and observational design of this study are inherent limitations. This study was not designed to evaluate cardiovascular outcomes after ED encounters. The sample size could have been larger if patients with BP < 180/110 mm Hg at ED triage were included; however, the 180/110 mm Hg threshold was chosen because it was the most widely agreed on BP value in the literature. This study did not capture patients who presented with AH and did not receive any acute treatment in the ED.Prescribing patterns based on provider training (eg, emergency medicine, family medicine, or internal medicine) were not tracked and might have accounted for differences in selection of diagnostic tests, laboratory ordering, and route of drug administration preference.
A small subset of patients reported positive pain scores at triage but did not describe acute pain. Pain scores are highly subjective, and few primary literature sources link chronic pain with increased BP.22,23 Nevertheless, patients who reported acute pain and elevated BP were excluded in order to identify truly asymptomatic patients. VA hospitals are unique health systems and data obtained from this study might not be applicable to other public or private facilities. Last, the study did not take into account patients’ psychosocial circumstances that might have fostered a disproportionate reliance on the ED for health care.
Conclusion
Asymptomatic patients with elevated BP are treated in the ED despite no evidence supporting improved outcomes after acute BP lowering in this population. Follow-up after ED encounters for AH did not occur consistently within guideline-recommended 7 days, a trend that also occurs in non-VA systems.8 Clinics and health care systems could establish policies to prevent or minimize management of AH in the ED. Ideally, AH should be managed in a clinic setting by a PCP, but growing clinician workload might lead to increasing wait times and difficultly obtaining same-day appointments. Nurse-led clinics and clinical pharmacists operating under a scope of practice and working closely with a PCP are a cost-effective solution to ensure timely treatment and appropriate follow-up of patients with uncontrolled hypertension.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the North Florida South Georgia Veterans Health System in Gainesville, Florida.
Hypertension affects more than 65 million individuals in the U.S., accounting for nearly 30% of the adult population.1 Less than 50% of those with hypertension are taking appropriate pharmacotherapy.2 Hypertension contributes to cardiovascular events, including cerebrovascular accident, transient ischemic attack, hypertensive retinopathy, renal failure, myocardial infarction, and heart failure.1 Chronic hypertension mainly is an asymptomatic condition, earning the nickname “the silent killer.”2 An acute, symptomatic elevation in blood pressure (BP) often is referred to as hypertensive emergency. Symptoms of end-organ damage can include headache, blurry vision, chest pain, shortness of breath, altered mental status, epistaxis, and oliguria.2 Although rare, hypertensive emergencies should be treated immediately. The Seventh Report of the Joint National Committee (JNC 7), and the more recent JNC 8, have published guidelines on managing chronic hypertension.3,4 However, neither report provides guidance on hypertensive emergency or the appropriate actions in cases of extremely elevated BP in an asymptomatic patient.3,4
Acute hypertensive episodes—often referred to as hypertensive crises—are responsible for nearly 8 million hospitalizations each year and 20 million visits to the emergency department (ED).5,6 Most of these visits are same-day “treat-and-release” events.5 There is no universally accepted BP value associated with a hypertensive crisis, but most resources state that a BP ≥ 180/110 mm Hg requires attention.2,7 Without other symptoms, elevated BP is not an emergency, yet ED referral for acute management is common.7
Three terms fall under the umbrella of hypertensive crises: hypertensive emergency, hypertensive urgency, and asymptomatic hypertension (AH).2 In a 2007 article, the American College of Chest Physicians defined hypertensive emergency as BP ≥ 180/110 mm Hg with evidence of end-organ damage.2 Symptoms are almost always present in true hypertensive emergencies, and immediate medical intervention is required to halt further organ damage. In the same article, hypertensive urgency is defined as BP ≥ 180/110 mm Hg without end-organ damage.2 The definition of hypertensive urgency could be further refined to include the presence of cardiovascular and renal risk factors, although this additional point is not consistent across the literature. Asymptomatic hypertension is similar to hypertensive urgency; however, there is an absence of signs or symptoms of end-organ damage.2 There is ambiguity in the literature concerning managing hypertensive urgency and AH, but both share a basic tenet: Immediate BP reduction is not essential. Gradual dosage adjustment(s) of oral medications, preferably by a primary care provider (PCP), and follow-up within 7 days are recommended.7
Limited evidence exists to guide ED providers in managing AH. Long-term outcomes and guidelines intended for the primary care setting should not be extrapolated to acute management in the ED. With limited treatment guidelines, providers might be more likely to refer patients with AH to the ED for evaluation. In 2013, the American College of Emergency Physicians (ACEP) created a clinical policy concerning AH in the ED. The ACEP concluded that screening for target organ injury and medical intervention in the ED does not reduce rates of adverse events (AEs) and could lead to overtreatment and acute hypoperfusion.7 More recently, Patel and colleagues published findings on hypertensive urgency in the ambulatory care setting, which similarly found that referral to the ED was associated with increased use of health care resources and no change in short-term major AEs.8 The ACEP recommends that patients presenting with AH be referred to primary care clinics where long-term monitoring and medication adjustments can be achieved more cost-effectively.7
The objective of this retrospective evaluation was to assess the incidence and management of AH within a VA ED. The authors aimed to provide insight into how these patients are managed and discuss alternatives to ED use.
Methods
This retrospective observational study was conducted within the North Florida/South Georgia Veterans Health System (NFSGVHS), which provides patient care at 2 medical centers in Gainesville and Lake City, Florida, as well as 11 outpatient clinics located throughout North Florida and South Georgia. The NFSGVHS serves rural and urban veteran populations. Study approval was granted by the NFSGVHS Institutional Review Board and Research and Development Committee.
Inclusion/Exclusion Criteria
Adult patients who were ordered at least 1 antihypertensive medication in the ED from July 1, 2011 to July 1, 2014, in addition to being asymptomatic with BP ≥ 180/110 mm Hg at ED triage were included. Based on clinical experience, the authors estimated that 3 years would provide a sample size of more than 100 patients. Patients were excluded if they presented with any acute symptoms or were hospitalized for further management.
Data Collection
Baseline demographics were collected for all participants. During the ED encounter, pre- and postintervention vital signs were recorded and prespecified laboratory data obtained. Interrater reliability was accounted for by performing random reviews of previously collected data to ensure consistency during the chart review process. Renal end-organ damage was defined using Acute Kidney Injury Network criteria, a serum creatinine 50% above baseline, or an absolute increase in baseline serum creatinine by 0.3 mg/dL.9 Additional laboratory markers of organ damage included cardiac troponin levels. Urinalysis results also were assessed to determine the presence of hematuria or proteinuria. Patient-reported nonadherence with medications was determined by reviewing ED provider and/or nurse documentation notes for the index ED encounter.
Investigators documented the route (IV or oral) and antihypertensive(s) medication selected for each patient. Adverse effects and any changes to patients’ outpatient medication regimens were noted. Investigators also assessed days to next medical contact after ED discharge to determine whether follow-up occurred according to the recommended standard of 7 days.9 Days to next medical contact was defined as any contact—in person or by telephone—that was documented in the electronic health record after the index ED visit.
Statistical Analysis
Descriptive statistics, including mean, median, and standard deviation, were used to analyze data.
Results
A total of 1,052 patients presented with BP ≥ 180/110 mm Hg and for whom antihypertensive medication was ordered but not necessarily given in the ED. Of the total, 724 patients were excluded because of hospital admission for other primary diagnoses; however, 6 of these patients were admitted for hypertensive urgency. The final analysis included 132 patients who presented with the primary condition of elevated BP without any accompanying symptoms. Among these patients, 2 had repeat ED visits for AH during the specified time frame.
Most patients were male with an average age of 63 years and documented history of hypertension. Nearly all patients had established primary care within the NFSGVHS. The most common comorbidity was diabetes mellitus (36%), followed by coronary artery disease (27%) and chronic kidney disease (CKD) (21%) (Table 1). About one-third of patients presented to the ED on their own volition, and slightly more than half were referred to the ED by primary care or specialty clinics.
In the ED, 130 patients received BP treatment (Table 2). Medication was ordered for 2 patients who did not receive treatment. In total, 12 different medication classes were used for treating patients with AH in the ED (Figure).
Treatment in the ED resulted in an average BP and heart rate reduction of 27/20 mm Hg and 5 beats per minute, respectively. About 80% of patients had a basic metabolic panel drawn, and there were no instances of acute kidney injury. Of the patients in the study 38% had cardiac enzymes collected, and only 1 patient had a positive result, which was determined to be unrelated to acute coronary syndrome. Forty-one (31%) of patients had a urinalysis; 12 has positive results for hematuria, and 18 revealed proteinuria. Of note, the 6 patients who were hospitalized for hypertensive urgency had neither symptoms at presentation to the ED nor laboratory findings indicating end-organ damage. The reason these patients were admitted is unclear.
At discharge, ED providers made changes to 54% of patients’ outpatient antihypertensive regimens. These changes included adding a new medication (68%), increasing the dosage of an existing medication (24%), or multiple changes (8%). Refills were provided for 18% of prescriptions. Follow-up within 7 days from ED discharge was recorded for 34% of patients. One patient received follow-up outside the NFSGVHS and was not included in this analysis.
Discussion
The aim of this retrospective study was to determine the incidence of AH in a VA ED and describe how these patients were managed. Overall, the rate of patients presenting to the ED with AH during the study period was about 1 patient every 8 days or 45 patients per year. By comparison, more than 30,000 patients are seen at the NFSGVHS ED annually. Although AH seems to be an uncommon occurrence, study findings raise questions about the value of managing the condition in the ED.
This study found several management strategies as well as noteworthy trends. For example, laboratory tests were not ordered routinely for all patients, suggesting that some ED providers question their use for AH. There were no patients with acute elevations in serum creatinine that indicated acute kidney injury, and although hematuria and proteinuria were common findings, neither were specific for acute injury. However, there were findings typical of chronic hypertension, and urinalysis may provide little benefit when testing for acute kidney injury. Only 1 patient showed elevated cardiac enzymes, which was determined to be a result of CKD.
Although not included in the final analysis, the 6 patients who were hospitalized for hypertensive urgency were similar in that they had neither symptoms at presentation to the ED nor laboratory findings indicating end-organ damage. Collectively, these findings support existing literature that questions the utility of laboratory testing of patients with AH in the ED.10
Patients also were treated with a variety of antihypertensive agents in the ED. One explanation might be outpatient nonadherence with medications. In patients with AH, it is common to provide doses of chronic medications that the patient might have missed and should be taking on a regular basis. Therefore, assessing adherence with current medications before modifying chronic therapy is an important initial step when managing AH.
Although oral agents primarily were used, IV antihypertensives were administered to about one-third of patients. Preference for IV administration in the ED might be related to its ability to lower BP quickly. The practice of obtaining IV access for medication in a patient with AH is costly, unnecessary, and potentially harmful.7 The authors theorize that this practice is performed, in many cases, as an attempt to expedite ED discharge after an acceptable BP reading is documented.
Rapid reductions in BP can precipitate hypoperfusion inadvertently and are more likely to occur with IV agents than with oral ones. Therefore, the safety, convenience, and cost savings associated with oral administration make it the preferred route for managing AH.
Best Practices
Primary care clinics are best suited to manage AH because medication adjustments and long-term monitoring are easier to perform and at substantially lower costs when compared with that of the ED. Rather than immediately referring a patient to the ED, clinicians should consider factors that could elevate BP, such as medication nonadherence, anxiety, acute pain, recent tobacco or caffeine use, or white coat syndrome. Staff should be well educated on proper BP measurement and instructed to repeat the reading for confirmation. Before measuring BP, allow the patient to sit quietly for 5 minutes with the feet flat on the floor and arm supported.3 Ideally, the measurement used should be the average of 3 BP readings on an automated device.11 If BP readings are high, staff should ask the patient about medication adherence and missed medication(s) should be administered.
It also is reasonable to have the patient rest quietly for up to 30 minutes because rest has been shown to reduce BP in some patients.12 The drawback to the prolonged rest strategy is the potential to cause delays in care for other patients. However, it is important to remember that wait times in the ED often are measured in hours, which causes frustration for patients referred to the ED for AH management. Before completing the office visit, the provider should recheck BP using proper technique and confirm that the patient has antihypertensive medication(s) in his/her possession; a follow-up appointment should be scheduled for no later than 1 week.
Primary care providers might be concerned about taking on additional liability and could favor ED referral, but legislation makes it difficult for EDs to defer nonemergent issues to primary care clinics. The Emergency Medical Treatment and Labor Act states that hospitals are prohibited from denying a patient care during an emergency.13 Despite evidence that AH is not an emergency, many patients continue to be referred to the ED. One-third of patients presented to the ED on their own volition and more than one-half were referred by health care personnel. This strongly suggests that both patients and health care personnel consider AH an emergency medical condition requiring immediate attention. However, patients with AH rarely are found to have any acute end-organ damage; therefore, acute treatment and extensive laboratory or diagnostic testing in the ED provides little, if any, benefit.10 The authors believe the ACEP clinical policy should be adopted into mainstream practice to help reduce health care costs and preserve ED resources for patients with true emergencies.
Another pervasive issue that could contribute to inappropriate AH referrals to the ED is the shortage of PCPs and limited same-day appointments for nonemergent conditions. In a 2017 survey, the average wait time for a PCP appointment ranged between 12 and 109 days, depending on the metropolitan area. The national average wait time conducted by this survey was 29.3 days.14 When primary care appointments are unavailable, triage staff could recommend that patients seek care in the ED. Additionally, patients might choose to seek ED care rather than wait for the next available PCP appointment. Clinic proximity to an ED could influence referral rates. In other words, medical centers or health systems with primary care clinics and ED services under one roof could experience more frequent ED referrals.
A promising strategy to help overcome the challenges of addressing AH and avoiding ED referrals is increasing patient access to and use of qualified, nonphysician providers, such as clinical pharmacists and nurse practitioners. Large health systems such as the VA and Kaiser Permanente have employed clinical pharmacist providers to reduce follow-up times for patients in primary care settings.15 Furthermore, there is substantial evidence that supports the cost-effectiveness and clinical success of pharmacist-driven hypertension clinics.16-18 Nurse-driven efforts to improve hypertension control have been successfully implemented in health systems.19 Both clinical pharmacist and nurse-managed hypertension clinics are effective solutions to manage patients with AH who might otherwise use costly ED services.For example, the average cost of a single ED visit is $740 to $3,437.20 In comparison, a 2010 report from the Agency for Healthcare Research and Quality showed the average annual cost of managing hypertension in ambulatory care clinics was $442 per adult, a cost considerably lower than that of the ED.21
Limitations
The retrospective and observational design of this study are inherent limitations. This study was not designed to evaluate cardiovascular outcomes after ED encounters. The sample size could have been larger if patients with BP < 180/110 mm Hg at ED triage were included; however, the 180/110 mm Hg threshold was chosen because it was the most widely agreed on BP value in the literature. This study did not capture patients who presented with AH and did not receive any acute treatment in the ED.Prescribing patterns based on provider training (eg, emergency medicine, family medicine, or internal medicine) were not tracked and might have accounted for differences in selection of diagnostic tests, laboratory ordering, and route of drug administration preference.
A small subset of patients reported positive pain scores at triage but did not describe acute pain. Pain scores are highly subjective, and few primary literature sources link chronic pain with increased BP.22,23 Nevertheless, patients who reported acute pain and elevated BP were excluded in order to identify truly asymptomatic patients. VA hospitals are unique health systems and data obtained from this study might not be applicable to other public or private facilities. Last, the study did not take into account patients’ psychosocial circumstances that might have fostered a disproportionate reliance on the ED for health care.
Conclusion
Asymptomatic patients with elevated BP are treated in the ED despite no evidence supporting improved outcomes after acute BP lowering in this population. Follow-up after ED encounters for AH did not occur consistently within guideline-recommended 7 days, a trend that also occurs in non-VA systems.8 Clinics and health care systems could establish policies to prevent or minimize management of AH in the ED. Ideally, AH should be managed in a clinic setting by a PCP, but growing clinician workload might lead to increasing wait times and difficultly obtaining same-day appointments. Nurse-led clinics and clinical pharmacists operating under a scope of practice and working closely with a PCP are a cost-effective solution to ensure timely treatment and appropriate follow-up of patients with uncontrolled hypertension.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the North Florida South Georgia Veterans Health System in Gainesville, Florida.
1. Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief. 2013;(133):
1-8.
2. Marik PE, Varon J. Hypertensive crises: challenges and management. Chest. 2007;131(6):1949-1962.
3. Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206-1252.
4. James, PA, Oparil, S, Carter, BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520.
5. American Heart Association. High blood pressure ER visits jumped 25 percent in 2006-11. http://newsroom.heart.org/news/high-blood-pressure-er-visits-jumped-25-percent-in-2006-11. Published September 9, 2014. Accessed January 19, 2018.
6. Owens P, Mutter R. Statistical brief #100: emergency department visits for adults in community hospitals. Agency for Healthcare Research and Quality. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb100.pdf. Published November 2010. Accessed January 19, 2018.
7. Wolf SJ, Lo B, Shih RD, Smith MD, Fesmire FM; American College of Emergency Physicians Clinical Policies Committee. Clinical policy: critical issues in the evaluation and management of adult patients in the emergency department with asymptomatic elevated blood pressure. Ann Emerg Med. 2013;62(1):59-68.
8. Patel KK, Young L, Howell EH, et al. Characteristics and outcomes of patients presenting with hypertensive urgency in the office setting. JAMA Intern Med. 2016;176(7):981-988.
9. Acute Kidney Injury Network. AKIN studies. http://www.akinet.org/akinstudies.php. Updated 2017. Accessed January 19, 2018.
10. Karras DJ, Kruus LK, Cienki JJ, et al. Utility of routine testing for patients with asymptomatic severe blood pressure elevation in the emergency department. Ann Emerg Med. 2008;51(3):231-239.
11. The SPRINT Research Group. A Randomized trial of Intensive versus standard blood pressure control. N Engl J Med. 2015;373:2103-2116.
12. Grassi D, O’Flaherty M, Pellizzari M, et al; Group of Investigators of the REHASE Program. Hypertensive urgencies in the emergency department: evaluating blood pressure response to rest and to antihypertensive drugs with different profiles. J Clin Hypertens (Greenwich). 2008;10(9):662-667.
13. Canters for Medicare & Medicaid Services. Emergency medical treatment & labor act (EMTALA). https://www.cms.gov/Regulations-and-Guidance/Legislation/EMTALA/index.html. Updated March 26, 2012. Accessed January 19, 2018.
14. Merritt Hawkins. 2017 Survey of physician appointment wait times and Medicare and Medicaid acceptance rates. https://www.merritthawkins.com/uploadedFiles/Merritt-Hawkins/Pdf/mha2017waittimesurveyPDF.pdf. Published 2017. Accessed January 19, 2018.
15. Galewitz P. VA treats patients’ impatience with clinical pharmacists. USA Today. http://www.usatoday.com/story/news/2016/10/24/kaiser-va-treats-patients-impatience-clinical-pharmacists/92479132/. Published October 24, 2016. Accessed January 19, 2018.
16. Carter BL, Ardery G, Dawson JD, et al. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med. 2009;169(21):1996-2002.
17. Borenstein JE, Graber G, Saltiel E, et al. Physician-pharmacist comanagement of hypertension: a randomized comparative trial. Pharmacotherapy. 2003;23(2):209-216.
18. Okamoto MP, Nakahiro RK. Pharmacoeconomic evaluation of a pharmacist-managed hypertension clinic. Pharmacotherapy. 2001;21(11):1337-1344.
19. Brown VM. Managing patients with hypertension in nurse-led clinics. Nursing. 2017;47(4):16-19.
20. Caldwell N, Srebotnjak T, Wang T, Hsia R. “How Much Will I Get Charged for This?” Patient charges for top ten diagnoses in the emergency department. PLoS ONE. 2013;8(2): e55491.
21. Davis KE. Expenditures for hypertension among adults age 18 and older, 2010: estimates for the U.S. civilian noninstitutionalized population. Agency for Healthcare Research and Quality. https://meps.ahrq.gov/data_files/publications/st404/stat404.shtml. Published April 2013. Accessed January 19, 2018.
22. Marco CA, Plewa MC, Buderer N, et al. Self-reported pain scores in the emergency department: lack of association with vital signs. Acad Emerg Med. 2006;13(9):974-979.
23. Wood D, Goodnight S, Haig AJ, Nasari T. Body mass index, but not blood pressure is related to the level of pain in persons with chronic pain. J Back Musculoskelet Rehabil. 2011;24(2):
111-115.
1. Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011-2012. NCHS Data Brief. 2013;(133):
1-8.
2. Marik PE, Varon J. Hypertensive crises: challenges and management. Chest. 2007;131(6):1949-1962.
3. Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206-1252.
4. James, PA, Oparil, S, Carter, BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520.
5. American Heart Association. High blood pressure ER visits jumped 25 percent in 2006-11. http://newsroom.heart.org/news/high-blood-pressure-er-visits-jumped-25-percent-in-2006-11. Published September 9, 2014. Accessed January 19, 2018.
6. Owens P, Mutter R. Statistical brief #100: emergency department visits for adults in community hospitals. Agency for Healthcare Research and Quality. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb100.pdf. Published November 2010. Accessed January 19, 2018.
7. Wolf SJ, Lo B, Shih RD, Smith MD, Fesmire FM; American College of Emergency Physicians Clinical Policies Committee. Clinical policy: critical issues in the evaluation and management of adult patients in the emergency department with asymptomatic elevated blood pressure. Ann Emerg Med. 2013;62(1):59-68.
8. Patel KK, Young L, Howell EH, et al. Characteristics and outcomes of patients presenting with hypertensive urgency in the office setting. JAMA Intern Med. 2016;176(7):981-988.
9. Acute Kidney Injury Network. AKIN studies. http://www.akinet.org/akinstudies.php. Updated 2017. Accessed January 19, 2018.
10. Karras DJ, Kruus LK, Cienki JJ, et al. Utility of routine testing for patients with asymptomatic severe blood pressure elevation in the emergency department. Ann Emerg Med. 2008;51(3):231-239.
11. The SPRINT Research Group. A Randomized trial of Intensive versus standard blood pressure control. N Engl J Med. 2015;373:2103-2116.
12. Grassi D, O’Flaherty M, Pellizzari M, et al; Group of Investigators of the REHASE Program. Hypertensive urgencies in the emergency department: evaluating blood pressure response to rest and to antihypertensive drugs with different profiles. J Clin Hypertens (Greenwich). 2008;10(9):662-667.
13. Canters for Medicare & Medicaid Services. Emergency medical treatment & labor act (EMTALA). https://www.cms.gov/Regulations-and-Guidance/Legislation/EMTALA/index.html. Updated March 26, 2012. Accessed January 19, 2018.
14. Merritt Hawkins. 2017 Survey of physician appointment wait times and Medicare and Medicaid acceptance rates. https://www.merritthawkins.com/uploadedFiles/Merritt-Hawkins/Pdf/mha2017waittimesurveyPDF.pdf. Published 2017. Accessed January 19, 2018.
15. Galewitz P. VA treats patients’ impatience with clinical pharmacists. USA Today. http://www.usatoday.com/story/news/2016/10/24/kaiser-va-treats-patients-impatience-clinical-pharmacists/92479132/. Published October 24, 2016. Accessed January 19, 2018.
16. Carter BL, Ardery G, Dawson JD, et al. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med. 2009;169(21):1996-2002.
17. Borenstein JE, Graber G, Saltiel E, et al. Physician-pharmacist comanagement of hypertension: a randomized comparative trial. Pharmacotherapy. 2003;23(2):209-216.
18. Okamoto MP, Nakahiro RK. Pharmacoeconomic evaluation of a pharmacist-managed hypertension clinic. Pharmacotherapy. 2001;21(11):1337-1344.
19. Brown VM. Managing patients with hypertension in nurse-led clinics. Nursing. 2017;47(4):16-19.
20. Caldwell N, Srebotnjak T, Wang T, Hsia R. “How Much Will I Get Charged for This?” Patient charges for top ten diagnoses in the emergency department. PLoS ONE. 2013;8(2): e55491.
21. Davis KE. Expenditures for hypertension among adults age 18 and older, 2010: estimates for the U.S. civilian noninstitutionalized population. Agency for Healthcare Research and Quality. https://meps.ahrq.gov/data_files/publications/st404/stat404.shtml. Published April 2013. Accessed January 19, 2018.
22. Marco CA, Plewa MC, Buderer N, et al. Self-reported pain scores in the emergency department: lack of association with vital signs. Acad Emerg Med. 2006;13(9):974-979.
23. Wood D, Goodnight S, Haig AJ, Nasari T. Body mass index, but not blood pressure is related to the level of pain in persons with chronic pain. J Back Musculoskelet Rehabil. 2011;24(2):
111-115.
How US healthcare compares to other countries
The US has similar healthcare utilization as other high-income countries but spends more and tends to have worse health outcomes, according to a new study.
In 2016, the US spent 17.8% of its gross domestic product on healthcare. For 10 other high-income countries, spending ranged from 9.6% to 12.4%.
However, sizes of physician and nursing workforces were comparable between the countries, numbers of hospital discharges were similar, and lengths of hospital stay were lower in the US than in most other countries.
Meanwhile, the US had the lowest life expectancy and the highest rate of infant mortality.
This research was published in JAMA.
“There’s been a lot of interest in international comparisons between America and other high-income countries, and there’s been a lot of vagueness about why exactly our [US] spending is so much higher and our health outcomes are not necessarily better and often worse,” said study author Ashish K. Jha, MD, of Harvard T. H. Chan School of Public Health in Boston, Massachusetts.
“This study really tries to fill in gaps, I think, across a wide range of issues, from structural capacity to utilization to prices to outcomes.”
For this study, Dr Jha and his colleagues analyzed recent healthcare data, primarily from 2013 to 2016. The team compared differences in healthcare spending, performance, and structural features between the US and 10 high-income countries—UK, Canada, Germany, Australia, Japan, Sweden, France, Netherlands, Switzerland, and Denmark.
Spending
In 2016, healthcare spending, as a percentage of gross domestic product, was as follows:
US—17.8%
Switzerland—12.4%
Sweden—11.9%
Germany—11.3%
France—11%
Japan—10.9%
Denmark—10.8%
Netherlands—10.5%
Canada—10.3%
UK—9.7%
Australia—9.6%.
“The big differences in spending really seem to be driven by prices,” Dr Jha said, noting that salaries for doctors and nurses, administrative expenditures, and pharmaceutical costs are “much higher” in the US.
The total spending on pharmaceuticals per capita was $1443 in the US but ranged from $466 (Netherlands) to $939 (Switzerland) in the other countries.
Administrative costs accounted for 8% of the total national health expenditure in the US but 1% (France, Japan) to 3% (Germany) in the other countries.
Outpatient care expenditures ranged from 22% (Netherlands) to 42% (US). Inpatient care expenditures ranged from 17% (Canada) to 32% (Netherlands), with 19% for the US. And expenditures for medical goods ranged from 10% (Denmark) to 20% (Germany), with the US at 14%.
Physicians’ and nurses’ salaries were higher in the US than other countries. For example, generalist physicians earned $218,173 in the US in 2016 but anywhere from $86,607 (Sweden) to $154,126 (Germany) in the other countries.
Utilization
Although US doctors and nurses earned more than their counterparts in comparator countries, there were no substantial between-country differences in the size of the physician and nursing workforces.
The number of working physicians for every 1000 people ranged from 2.1 (UK) to 4.3 (Switzerland), with the US at 2.6. The number of working nurses for every 1000 people ranged from 8.2 (UK) to 17.4 (Switzerland), with 11.1 for the US.
The US had comparable numbers of hospital beds as some of the other countries. The range was 2.5 (Sweden) to 13.2 (Japan) beds per 1000 people, with the US at 2.8.
The US ranked 6th when it came to hospital discharges for acute myocardial infarction (AMI, 192 per 100,000 people; range, 89 to 287), mental and behavioral issues (679 per 100,000 people; range, 119 to 1719), pneumonia (365 per 100,000 people; range, 187 to 567), and chronic obstructive pulmonary disease (230 per 100,000 people; range, 45 to 352).
The US had greater utilization of computed tomography than the other countries, with 245 examinations per 1000 people (range for other countries, 79 to 231). And US utilization of magnetic resonance imaging was higher than most countries, with 118 examinations per 1000 people (range, 41 to 131).
However, the US ranked on the lower end of the spectrum for length of hospital stay. The length of stay for a “normal delivery” childbirth ranged from a median of 1.5 days (UK) to 5.7 days (Japan), with the US clocking in at 2 days. The median length of stay for AMI ranged from a median of 3.9 days (Netherlands) to 10.3 days (Germany), with the US at 5.4 days.
“So much of the debate about healthcare these days is about over-utilization—that somehow our health system is uniquely bad at avoiding unnecessary services,” Dr Jha said. “I think these data really put that argument to rest. Except for a few pockets, utilization is not really different between us and these high-income Northern European countries, so maybe we need to spend a little less time focusing on that and a little bit more time focusing on prices of our healthcare system.”
Outcomes
The US ranked on the lower end of the spectrum for some mortality outcomes. Thirty-day stroke mortality per 1000 patients ranged from 4.2 in the US to 10 in Canada. Thirty-day AMI mortality per 1000 ranged from 4.1 (Australia) to 8.7 (Germany), with the US at 5.5.
However, infant mortality was highest in the US, at 5.8 deaths per 1000 live births (range for other countries, 2.1 to 5.1). And life expectancy was lowest in the US, at 78.8 years (range for other countries, 80.7 to 83.9).
The researchers noted that the US had the highest percentage of overweight or obese individuals age 15 and older, at 70.1% (range for other countries, 23.8% to 63.4%), but a low percentage of smokers (11.4%; range, 11.2% to 22.4%) and moderate alcohol consumption (8.8 L per capita; range, 7.2 to 11.9).
Limitations of this study include some differences in approaches to collecting and standardizing data across countries, as well as missing data for some countries.
The US has similar healthcare utilization as other high-income countries but spends more and tends to have worse health outcomes, according to a new study.
In 2016, the US spent 17.8% of its gross domestic product on healthcare. For 10 other high-income countries, spending ranged from 9.6% to 12.4%.
However, sizes of physician and nursing workforces were comparable between the countries, numbers of hospital discharges were similar, and lengths of hospital stay were lower in the US than in most other countries.
Meanwhile, the US had the lowest life expectancy and the highest rate of infant mortality.
This research was published in JAMA.
“There’s been a lot of interest in international comparisons between America and other high-income countries, and there’s been a lot of vagueness about why exactly our [US] spending is so much higher and our health outcomes are not necessarily better and often worse,” said study author Ashish K. Jha, MD, of Harvard T. H. Chan School of Public Health in Boston, Massachusetts.
“This study really tries to fill in gaps, I think, across a wide range of issues, from structural capacity to utilization to prices to outcomes.”
For this study, Dr Jha and his colleagues analyzed recent healthcare data, primarily from 2013 to 2016. The team compared differences in healthcare spending, performance, and structural features between the US and 10 high-income countries—UK, Canada, Germany, Australia, Japan, Sweden, France, Netherlands, Switzerland, and Denmark.
Spending
In 2016, healthcare spending, as a percentage of gross domestic product, was as follows:
US—17.8%
Switzerland—12.4%
Sweden—11.9%
Germany—11.3%
France—11%
Japan—10.9%
Denmark—10.8%
Netherlands—10.5%
Canada—10.3%
UK—9.7%
Australia—9.6%.
“The big differences in spending really seem to be driven by prices,” Dr Jha said, noting that salaries for doctors and nurses, administrative expenditures, and pharmaceutical costs are “much higher” in the US.
The total spending on pharmaceuticals per capita was $1443 in the US but ranged from $466 (Netherlands) to $939 (Switzerland) in the other countries.
Administrative costs accounted for 8% of the total national health expenditure in the US but 1% (France, Japan) to 3% (Germany) in the other countries.
Outpatient care expenditures ranged from 22% (Netherlands) to 42% (US). Inpatient care expenditures ranged from 17% (Canada) to 32% (Netherlands), with 19% for the US. And expenditures for medical goods ranged from 10% (Denmark) to 20% (Germany), with the US at 14%.
Physicians’ and nurses’ salaries were higher in the US than other countries. For example, generalist physicians earned $218,173 in the US in 2016 but anywhere from $86,607 (Sweden) to $154,126 (Germany) in the other countries.
Utilization
Although US doctors and nurses earned more than their counterparts in comparator countries, there were no substantial between-country differences in the size of the physician and nursing workforces.
The number of working physicians for every 1000 people ranged from 2.1 (UK) to 4.3 (Switzerland), with the US at 2.6. The number of working nurses for every 1000 people ranged from 8.2 (UK) to 17.4 (Switzerland), with 11.1 for the US.
The US had comparable numbers of hospital beds as some of the other countries. The range was 2.5 (Sweden) to 13.2 (Japan) beds per 1000 people, with the US at 2.8.
The US ranked 6th when it came to hospital discharges for acute myocardial infarction (AMI, 192 per 100,000 people; range, 89 to 287), mental and behavioral issues (679 per 100,000 people; range, 119 to 1719), pneumonia (365 per 100,000 people; range, 187 to 567), and chronic obstructive pulmonary disease (230 per 100,000 people; range, 45 to 352).
The US had greater utilization of computed tomography than the other countries, with 245 examinations per 1000 people (range for other countries, 79 to 231). And US utilization of magnetic resonance imaging was higher than most countries, with 118 examinations per 1000 people (range, 41 to 131).
However, the US ranked on the lower end of the spectrum for length of hospital stay. The length of stay for a “normal delivery” childbirth ranged from a median of 1.5 days (UK) to 5.7 days (Japan), with the US clocking in at 2 days. The median length of stay for AMI ranged from a median of 3.9 days (Netherlands) to 10.3 days (Germany), with the US at 5.4 days.
“So much of the debate about healthcare these days is about over-utilization—that somehow our health system is uniquely bad at avoiding unnecessary services,” Dr Jha said. “I think these data really put that argument to rest. Except for a few pockets, utilization is not really different between us and these high-income Northern European countries, so maybe we need to spend a little less time focusing on that and a little bit more time focusing on prices of our healthcare system.”
Outcomes
The US ranked on the lower end of the spectrum for some mortality outcomes. Thirty-day stroke mortality per 1000 patients ranged from 4.2 in the US to 10 in Canada. Thirty-day AMI mortality per 1000 ranged from 4.1 (Australia) to 8.7 (Germany), with the US at 5.5.
However, infant mortality was highest in the US, at 5.8 deaths per 1000 live births (range for other countries, 2.1 to 5.1). And life expectancy was lowest in the US, at 78.8 years (range for other countries, 80.7 to 83.9).
The researchers noted that the US had the highest percentage of overweight or obese individuals age 15 and older, at 70.1% (range for other countries, 23.8% to 63.4%), but a low percentage of smokers (11.4%; range, 11.2% to 22.4%) and moderate alcohol consumption (8.8 L per capita; range, 7.2 to 11.9).
Limitations of this study include some differences in approaches to collecting and standardizing data across countries, as well as missing data for some countries.
The US has similar healthcare utilization as other high-income countries but spends more and tends to have worse health outcomes, according to a new study.
In 2016, the US spent 17.8% of its gross domestic product on healthcare. For 10 other high-income countries, spending ranged from 9.6% to 12.4%.
However, sizes of physician and nursing workforces were comparable between the countries, numbers of hospital discharges were similar, and lengths of hospital stay were lower in the US than in most other countries.
Meanwhile, the US had the lowest life expectancy and the highest rate of infant mortality.
This research was published in JAMA.
“There’s been a lot of interest in international comparisons between America and other high-income countries, and there’s been a lot of vagueness about why exactly our [US] spending is so much higher and our health outcomes are not necessarily better and often worse,” said study author Ashish K. Jha, MD, of Harvard T. H. Chan School of Public Health in Boston, Massachusetts.
“This study really tries to fill in gaps, I think, across a wide range of issues, from structural capacity to utilization to prices to outcomes.”
For this study, Dr Jha and his colleagues analyzed recent healthcare data, primarily from 2013 to 2016. The team compared differences in healthcare spending, performance, and structural features between the US and 10 high-income countries—UK, Canada, Germany, Australia, Japan, Sweden, France, Netherlands, Switzerland, and Denmark.
Spending
In 2016, healthcare spending, as a percentage of gross domestic product, was as follows:
US—17.8%
Switzerland—12.4%
Sweden—11.9%
Germany—11.3%
France—11%
Japan—10.9%
Denmark—10.8%
Netherlands—10.5%
Canada—10.3%
UK—9.7%
Australia—9.6%.
“The big differences in spending really seem to be driven by prices,” Dr Jha said, noting that salaries for doctors and nurses, administrative expenditures, and pharmaceutical costs are “much higher” in the US.
The total spending on pharmaceuticals per capita was $1443 in the US but ranged from $466 (Netherlands) to $939 (Switzerland) in the other countries.
Administrative costs accounted for 8% of the total national health expenditure in the US but 1% (France, Japan) to 3% (Germany) in the other countries.
Outpatient care expenditures ranged from 22% (Netherlands) to 42% (US). Inpatient care expenditures ranged from 17% (Canada) to 32% (Netherlands), with 19% for the US. And expenditures for medical goods ranged from 10% (Denmark) to 20% (Germany), with the US at 14%.
Physicians’ and nurses’ salaries were higher in the US than other countries. For example, generalist physicians earned $218,173 in the US in 2016 but anywhere from $86,607 (Sweden) to $154,126 (Germany) in the other countries.
Utilization
Although US doctors and nurses earned more than their counterparts in comparator countries, there were no substantial between-country differences in the size of the physician and nursing workforces.
The number of working physicians for every 1000 people ranged from 2.1 (UK) to 4.3 (Switzerland), with the US at 2.6. The number of working nurses for every 1000 people ranged from 8.2 (UK) to 17.4 (Switzerland), with 11.1 for the US.
The US had comparable numbers of hospital beds as some of the other countries. The range was 2.5 (Sweden) to 13.2 (Japan) beds per 1000 people, with the US at 2.8.
The US ranked 6th when it came to hospital discharges for acute myocardial infarction (AMI, 192 per 100,000 people; range, 89 to 287), mental and behavioral issues (679 per 100,000 people; range, 119 to 1719), pneumonia (365 per 100,000 people; range, 187 to 567), and chronic obstructive pulmonary disease (230 per 100,000 people; range, 45 to 352).
The US had greater utilization of computed tomography than the other countries, with 245 examinations per 1000 people (range for other countries, 79 to 231). And US utilization of magnetic resonance imaging was higher than most countries, with 118 examinations per 1000 people (range, 41 to 131).
However, the US ranked on the lower end of the spectrum for length of hospital stay. The length of stay for a “normal delivery” childbirth ranged from a median of 1.5 days (UK) to 5.7 days (Japan), with the US clocking in at 2 days. The median length of stay for AMI ranged from a median of 3.9 days (Netherlands) to 10.3 days (Germany), with the US at 5.4 days.
“So much of the debate about healthcare these days is about over-utilization—that somehow our health system is uniquely bad at avoiding unnecessary services,” Dr Jha said. “I think these data really put that argument to rest. Except for a few pockets, utilization is not really different between us and these high-income Northern European countries, so maybe we need to spend a little less time focusing on that and a little bit more time focusing on prices of our healthcare system.”
Outcomes
The US ranked on the lower end of the spectrum for some mortality outcomes. Thirty-day stroke mortality per 1000 patients ranged from 4.2 in the US to 10 in Canada. Thirty-day AMI mortality per 1000 ranged from 4.1 (Australia) to 8.7 (Germany), with the US at 5.5.
However, infant mortality was highest in the US, at 5.8 deaths per 1000 live births (range for other countries, 2.1 to 5.1). And life expectancy was lowest in the US, at 78.8 years (range for other countries, 80.7 to 83.9).
The researchers noted that the US had the highest percentage of overweight or obese individuals age 15 and older, at 70.1% (range for other countries, 23.8% to 63.4%), but a low percentage of smokers (11.4%; range, 11.2% to 22.4%) and moderate alcohol consumption (8.8 L per capita; range, 7.2 to 11.9).
Limitations of this study include some differences in approaches to collecting and standardizing data across countries, as well as missing data for some countries.
DSCs ‘promising’ for severe acute GVHD
Placenta-derived decidua stromal cells (DSCs) can treat severe acute graft-versus-host disease (GVHD), according to a small study.
When given in the optimal way, DSCs produced GVHD responses in all patients, and the 1-year survival rate was 76%.
Steroid-refractory (SR) patients who received DSCs had superior 1-year survival rates compared to SR historical controls and SR patients who received mesenchymal stem cells (MSCs).
Olle Ringdén, MD, PhD, of Karolinska Institutet in Huddinge, Sweden, and his colleagues reported these findings in STEM CELLS Translational Medicine.
“There were a couple of things that led us to be curious about [DSCs as a treatment for GVHD],” Dr Ringdén said. “First, placenta plays an important role in helping the mother’s body tolerate the developing fetus.”
“[S]econd, placenta has been used in Africa for 100 years to successfully treat burn injuries. This speaks somewhat to its effectiveness and safety. We also found that placenta-derived DSCs are immunosuppressive in vitro and in vivo, which led us to wonder if they might cure severe acute GVHD.”
To test their theory, the team conducted a study of 38 patients with severe acute GVHD, including 25 SR patients.
The patients received DSCs in 1 of 2 groups. In group 1 (n=17), DSCs were infused in buffer supplemented with 10% AB plasma. In group 2 (n=21), the buffer was supplemented with 5% albumin.
Group 1 received significantly fewer infusions than group 2—1 (range, 1-5) and 2 (range, 1-6), respectively (P=0.002). But group 1 had a significantly higher median cell dose—2.0 x 106 DSCs/kg (range, 0.9-2.8) vs 1.2 x 106 DSCs/kg (range, 0.9-2.9; P<0.001).
Cell passage was significantly lower in group 1 than group 2—2 (range, 2-4) and 4 (range, 2-4), respectively (P<0.001). And cell viability was significantly lower in group 1 than 2—90% (range, 70-97) and 95% (range, 69-100), respectively (P<0.001).
Patients
There were no significant differences in baseline characteristics between groups 1 and 2. The median ages were 54.5 (range, 0.9-65.6) and 48.9 (range, 1.6-72.4), respectively.
Most patients were male (9 in group 1 and 16 in group 2), and most had malignant disease (14 and 17, respectively).
The most common graft source was peripheral blood stem cells (14 in group 1 and 16 in group 2), and most patients had a matched, unrelated donor (10 and 14, respectively).
Most patients received reduced-intensity conditioning (9 in group 1 and 17 in group 2) and GVHD prophylaxis with cyclosporine/methotrexate (13 in both groups).
All cases of GVHD were localized to the gut. Fifteen patients in each group had grade 3 GVHD. Two patients in group 1 and 6 in group 2 had grade 2 GVHD.
Results
Responses and survival rates were superior in group 2, but there was no significant difference in relapse or chronic GVHD between the groups.
In group 1, 7 patients did not respond, 5 had a partial response, and 5 had a complete response. In group 2, all patients responded, 10 with a partial response and 11 with a complete response (between-group difference, P=0.01).
The 1-year survival rate was 47% in group 1 and 76% in group 2 (P=0.016). The rate of GVHD-related mortality was 41% and 5%, respectively (P=0.003).
The cumulative incidence of chronic GVHD at 1.5 years was 36% in group 1 and 31% in group 2. The relapse rate was 29% and 18%, respectively.
The researchers compared SR patients in groups 1 (n=13) and 2 (n=11) to SR patients treated with bone marrow-derived MSCs (n=15) and SR historical controls (n=32).
The 1-year survival rate was 73% in SR group 2, which was significantly higher than the 31% survival rate in SR group 1 (P=0.02), the 20% rate in SR MSC recipients (P=0.0015), and the 3% rate in SR historical controls (P<0.001).
Severe adverse events in the DSC recipients included relapse (n=8), pneumonia (n=5), proven or probable invasive fungal infection (n=6), bacterial infection (n=2), graft failure (n=3), multiple organ failure (n=1), viral infection (n=2), central nervous system complications (n=2), septicemia (n=2), skin squamous cell carcinoma (n=2), and acute pancreatitis (n=1).
Causes of death in DSC recipients included acute GVHD (n=9), relapse (n=2), bacterial infection (n=2), invasive fungal infection (n=1), liver failure (n=1), hemorrhage (n=1), and secondary malignancy (n=1).
“Collectively, we think these data demonstrate that DSCs are a promising treatment for severe acute GVHD,” Dr Ringdén said. “But it was a small patient group, so, to further assess safety and efficacy, a larger, prospective trial will be necessary.”
“If an effective therapy for severe acute GVHD is indeed found and validated, it will increase the usefulness of stem cell transplantation with a possible broadening of indications.”
Placenta-derived decidua stromal cells (DSCs) can treat severe acute graft-versus-host disease (GVHD), according to a small study.
When given in the optimal way, DSCs produced GVHD responses in all patients, and the 1-year survival rate was 76%.
Steroid-refractory (SR) patients who received DSCs had superior 1-year survival rates compared to SR historical controls and SR patients who received mesenchymal stem cells (MSCs).
Olle Ringdén, MD, PhD, of Karolinska Institutet in Huddinge, Sweden, and his colleagues reported these findings in STEM CELLS Translational Medicine.
“There were a couple of things that led us to be curious about [DSCs as a treatment for GVHD],” Dr Ringdén said. “First, placenta plays an important role in helping the mother’s body tolerate the developing fetus.”
“[S]econd, placenta has been used in Africa for 100 years to successfully treat burn injuries. This speaks somewhat to its effectiveness and safety. We also found that placenta-derived DSCs are immunosuppressive in vitro and in vivo, which led us to wonder if they might cure severe acute GVHD.”
To test their theory, the team conducted a study of 38 patients with severe acute GVHD, including 25 SR patients.
The patients received DSCs in 1 of 2 groups. In group 1 (n=17), DSCs were infused in buffer supplemented with 10% AB plasma. In group 2 (n=21), the buffer was supplemented with 5% albumin.
Group 1 received significantly fewer infusions than group 2—1 (range, 1-5) and 2 (range, 1-6), respectively (P=0.002). But group 1 had a significantly higher median cell dose—2.0 x 106 DSCs/kg (range, 0.9-2.8) vs 1.2 x 106 DSCs/kg (range, 0.9-2.9; P<0.001).
Cell passage was significantly lower in group 1 than group 2—2 (range, 2-4) and 4 (range, 2-4), respectively (P<0.001). And cell viability was significantly lower in group 1 than 2—90% (range, 70-97) and 95% (range, 69-100), respectively (P<0.001).
Patients
There were no significant differences in baseline characteristics between groups 1 and 2. The median ages were 54.5 (range, 0.9-65.6) and 48.9 (range, 1.6-72.4), respectively.
Most patients were male (9 in group 1 and 16 in group 2), and most had malignant disease (14 and 17, respectively).
The most common graft source was peripheral blood stem cells (14 in group 1 and 16 in group 2), and most patients had a matched, unrelated donor (10 and 14, respectively).
Most patients received reduced-intensity conditioning (9 in group 1 and 17 in group 2) and GVHD prophylaxis with cyclosporine/methotrexate (13 in both groups).
All cases of GVHD were localized to the gut. Fifteen patients in each group had grade 3 GVHD. Two patients in group 1 and 6 in group 2 had grade 2 GVHD.
Results
Responses and survival rates were superior in group 2, but there was no significant difference in relapse or chronic GVHD between the groups.
In group 1, 7 patients did not respond, 5 had a partial response, and 5 had a complete response. In group 2, all patients responded, 10 with a partial response and 11 with a complete response (between-group difference, P=0.01).
The 1-year survival rate was 47% in group 1 and 76% in group 2 (P=0.016). The rate of GVHD-related mortality was 41% and 5%, respectively (P=0.003).
The cumulative incidence of chronic GVHD at 1.5 years was 36% in group 1 and 31% in group 2. The relapse rate was 29% and 18%, respectively.
The researchers compared SR patients in groups 1 (n=13) and 2 (n=11) to SR patients treated with bone marrow-derived MSCs (n=15) and SR historical controls (n=32).
The 1-year survival rate was 73% in SR group 2, which was significantly higher than the 31% survival rate in SR group 1 (P=0.02), the 20% rate in SR MSC recipients (P=0.0015), and the 3% rate in SR historical controls (P<0.001).
Severe adverse events in the DSC recipients included relapse (n=8), pneumonia (n=5), proven or probable invasive fungal infection (n=6), bacterial infection (n=2), graft failure (n=3), multiple organ failure (n=1), viral infection (n=2), central nervous system complications (n=2), septicemia (n=2), skin squamous cell carcinoma (n=2), and acute pancreatitis (n=1).
Causes of death in DSC recipients included acute GVHD (n=9), relapse (n=2), bacterial infection (n=2), invasive fungal infection (n=1), liver failure (n=1), hemorrhage (n=1), and secondary malignancy (n=1).
“Collectively, we think these data demonstrate that DSCs are a promising treatment for severe acute GVHD,” Dr Ringdén said. “But it was a small patient group, so, to further assess safety and efficacy, a larger, prospective trial will be necessary.”
“If an effective therapy for severe acute GVHD is indeed found and validated, it will increase the usefulness of stem cell transplantation with a possible broadening of indications.”
Placenta-derived decidua stromal cells (DSCs) can treat severe acute graft-versus-host disease (GVHD), according to a small study.
When given in the optimal way, DSCs produced GVHD responses in all patients, and the 1-year survival rate was 76%.
Steroid-refractory (SR) patients who received DSCs had superior 1-year survival rates compared to SR historical controls and SR patients who received mesenchymal stem cells (MSCs).
Olle Ringdén, MD, PhD, of Karolinska Institutet in Huddinge, Sweden, and his colleagues reported these findings in STEM CELLS Translational Medicine.
“There were a couple of things that led us to be curious about [DSCs as a treatment for GVHD],” Dr Ringdén said. “First, placenta plays an important role in helping the mother’s body tolerate the developing fetus.”
“[S]econd, placenta has been used in Africa for 100 years to successfully treat burn injuries. This speaks somewhat to its effectiveness and safety. We also found that placenta-derived DSCs are immunosuppressive in vitro and in vivo, which led us to wonder if they might cure severe acute GVHD.”
To test their theory, the team conducted a study of 38 patients with severe acute GVHD, including 25 SR patients.
The patients received DSCs in 1 of 2 groups. In group 1 (n=17), DSCs were infused in buffer supplemented with 10% AB plasma. In group 2 (n=21), the buffer was supplemented with 5% albumin.
Group 1 received significantly fewer infusions than group 2—1 (range, 1-5) and 2 (range, 1-6), respectively (P=0.002). But group 1 had a significantly higher median cell dose—2.0 x 106 DSCs/kg (range, 0.9-2.8) vs 1.2 x 106 DSCs/kg (range, 0.9-2.9; P<0.001).
Cell passage was significantly lower in group 1 than group 2—2 (range, 2-4) and 4 (range, 2-4), respectively (P<0.001). And cell viability was significantly lower in group 1 than 2—90% (range, 70-97) and 95% (range, 69-100), respectively (P<0.001).
Patients
There were no significant differences in baseline characteristics between groups 1 and 2. The median ages were 54.5 (range, 0.9-65.6) and 48.9 (range, 1.6-72.4), respectively.
Most patients were male (9 in group 1 and 16 in group 2), and most had malignant disease (14 and 17, respectively).
The most common graft source was peripheral blood stem cells (14 in group 1 and 16 in group 2), and most patients had a matched, unrelated donor (10 and 14, respectively).
Most patients received reduced-intensity conditioning (9 in group 1 and 17 in group 2) and GVHD prophylaxis with cyclosporine/methotrexate (13 in both groups).
All cases of GVHD were localized to the gut. Fifteen patients in each group had grade 3 GVHD. Two patients in group 1 and 6 in group 2 had grade 2 GVHD.
Results
Responses and survival rates were superior in group 2, but there was no significant difference in relapse or chronic GVHD between the groups.
In group 1, 7 patients did not respond, 5 had a partial response, and 5 had a complete response. In group 2, all patients responded, 10 with a partial response and 11 with a complete response (between-group difference, P=0.01).
The 1-year survival rate was 47% in group 1 and 76% in group 2 (P=0.016). The rate of GVHD-related mortality was 41% and 5%, respectively (P=0.003).
The cumulative incidence of chronic GVHD at 1.5 years was 36% in group 1 and 31% in group 2. The relapse rate was 29% and 18%, respectively.
The researchers compared SR patients in groups 1 (n=13) and 2 (n=11) to SR patients treated with bone marrow-derived MSCs (n=15) and SR historical controls (n=32).
The 1-year survival rate was 73% in SR group 2, which was significantly higher than the 31% survival rate in SR group 1 (P=0.02), the 20% rate in SR MSC recipients (P=0.0015), and the 3% rate in SR historical controls (P<0.001).
Severe adverse events in the DSC recipients included relapse (n=8), pneumonia (n=5), proven or probable invasive fungal infection (n=6), bacterial infection (n=2), graft failure (n=3), multiple organ failure (n=1), viral infection (n=2), central nervous system complications (n=2), septicemia (n=2), skin squamous cell carcinoma (n=2), and acute pancreatitis (n=1).
Causes of death in DSC recipients included acute GVHD (n=9), relapse (n=2), bacterial infection (n=2), invasive fungal infection (n=1), liver failure (n=1), hemorrhage (n=1), and secondary malignancy (n=1).
“Collectively, we think these data demonstrate that DSCs are a promising treatment for severe acute GVHD,” Dr Ringdén said. “But it was a small patient group, so, to further assess safety and efficacy, a larger, prospective trial will be necessary.”
“If an effective therapy for severe acute GVHD is indeed found and validated, it will increase the usefulness of stem cell transplantation with a possible broadening of indications.”
Clopidogrel proves noninferior to Plavix
Generic clopidogrel is noninferior to the brand-name antiplatelet drug Plavix, according to research published in Circulation: Cardiovascular Quality and Outcomes.
When a Canadian health system switched from prescribing Plavix to generic clopidogrel, patients with acute coronary syndrome (ACS) were no more likely to die or experience ACS recurrence within a year.
In addition, there were no significant differences between the Plavix and clopidogrel groups in the percentage of patients who were hospitalized for any reason, had a stroke or transient ischemic attack, or experienced bleeding.
“People can safely use generic clopidogrel,” said study author Dennis T. Ko, MD, of the Institute for Clinical Evaluative Sciences in Toronto, Ontario, Canada.
“This large and real-world study should be reassuring to physicians and healthcare organizations who have been concerned about changing what is prescribed.”
Dr Ko and his colleagues compared outcomes in patients who were prescribed clopidogrel after hospitalization for ACS in Ontario, Canada, where the Ministry of Health began to automatically substitute generic clopidogrel for Plavix once the brand name drug’s patent expired in 2012.
Between 2009 and 2014, 12,643 patients were prescribed Plavix, and 11,887 were prescribed generic clopidogrel.
“There are quite a few different generic brands,” said study author Cynthia Jackevicius, PharmD, of Western University of Health Sciences in Pomona, California.
“In this study, we considered them as a group but later found no differences in outcome when we compared between different generics.”
Results
The study’s primary outcome—a composite of death and hospitalization for ACS at 1 year—was met by 17.6% of patients prescribed Plavix and 17.9% of patients prescribed clopidogrel. The hazard ratio (HR) was 1.02 (95% confidence interval [CI], 0.96–1.08; P=0.005 for noninferiority, P=0.619 for superiority).
The 1-year mortality rate was 10.5% in the Plavix group and 11.2% in the clopidogrel group (HR=1.07; 95% CI, 0.99–1.15, P=0.210 for noninferiority, P=0.114 for superiority).
And hospitalization for ACS occurred in 9.7% and 9.2%, respectively (HR=0.94; 95% CI, 0.87–1.03, P<0.001 for noninferiority, P=0.190 for superiority).
Hospitalization for any reason occurred in 39.4% of patients in the Plavix group and 39.8% of those in the clopidogrel group (HR=1.02; 95% CI, 0.97–1.06, P<0.001 for noninferiority, P=0.482 for superiority).
Hospitalization for stroke or transient ischemic attack occurred in 1.5% and 1.4%, respectively (HR=0.92; 95% CI, 0.74–1.15, P=0.056 for noninferiority, P=0.455 for superiority).
And hospitalization for bleeding occurred in 2.3% and 2.7%, respectively (HR=1.17; 95% CI, 0.99–1.39, P=0.772 for noninferiority, P=0.063 for superiority).
The researchers noted that, in 2010, Plavix cost about $2.58 Canadian dollars per pill. It was projected to cost the Ontario Drug Benefit Program $72.8 million by 2012.
By switching to generic clopidogrel, which costs $0.39 per pill in 2018, the expense was $19 million Canadian dollars.
“Plavix was one of the most commonly used drugs in cardiology,” Dr Ko said, “so switching to generics can reduce a lot of cost for individuals and health systems.”
Generic clopidogrel is noninferior to the brand-name antiplatelet drug Plavix, according to research published in Circulation: Cardiovascular Quality and Outcomes.
When a Canadian health system switched from prescribing Plavix to generic clopidogrel, patients with acute coronary syndrome (ACS) were no more likely to die or experience ACS recurrence within a year.
In addition, there were no significant differences between the Plavix and clopidogrel groups in the percentage of patients who were hospitalized for any reason, had a stroke or transient ischemic attack, or experienced bleeding.
“People can safely use generic clopidogrel,” said study author Dennis T. Ko, MD, of the Institute for Clinical Evaluative Sciences in Toronto, Ontario, Canada.
“This large and real-world study should be reassuring to physicians and healthcare organizations who have been concerned about changing what is prescribed.”
Dr Ko and his colleagues compared outcomes in patients who were prescribed clopidogrel after hospitalization for ACS in Ontario, Canada, where the Ministry of Health began to automatically substitute generic clopidogrel for Plavix once the brand name drug’s patent expired in 2012.
Between 2009 and 2014, 12,643 patients were prescribed Plavix, and 11,887 were prescribed generic clopidogrel.
“There are quite a few different generic brands,” said study author Cynthia Jackevicius, PharmD, of Western University of Health Sciences in Pomona, California.
“In this study, we considered them as a group but later found no differences in outcome when we compared between different generics.”
Results
The study’s primary outcome—a composite of death and hospitalization for ACS at 1 year—was met by 17.6% of patients prescribed Plavix and 17.9% of patients prescribed clopidogrel. The hazard ratio (HR) was 1.02 (95% confidence interval [CI], 0.96–1.08; P=0.005 for noninferiority, P=0.619 for superiority).
The 1-year mortality rate was 10.5% in the Plavix group and 11.2% in the clopidogrel group (HR=1.07; 95% CI, 0.99–1.15, P=0.210 for noninferiority, P=0.114 for superiority).
And hospitalization for ACS occurred in 9.7% and 9.2%, respectively (HR=0.94; 95% CI, 0.87–1.03, P<0.001 for noninferiority, P=0.190 for superiority).
Hospitalization for any reason occurred in 39.4% of patients in the Plavix group and 39.8% of those in the clopidogrel group (HR=1.02; 95% CI, 0.97–1.06, P<0.001 for noninferiority, P=0.482 for superiority).
Hospitalization for stroke or transient ischemic attack occurred in 1.5% and 1.4%, respectively (HR=0.92; 95% CI, 0.74–1.15, P=0.056 for noninferiority, P=0.455 for superiority).
And hospitalization for bleeding occurred in 2.3% and 2.7%, respectively (HR=1.17; 95% CI, 0.99–1.39, P=0.772 for noninferiority, P=0.063 for superiority).
The researchers noted that, in 2010, Plavix cost about $2.58 Canadian dollars per pill. It was projected to cost the Ontario Drug Benefit Program $72.8 million by 2012.
By switching to generic clopidogrel, which costs $0.39 per pill in 2018, the expense was $19 million Canadian dollars.
“Plavix was one of the most commonly used drugs in cardiology,” Dr Ko said, “so switching to generics can reduce a lot of cost for individuals and health systems.”
Generic clopidogrel is noninferior to the brand-name antiplatelet drug Plavix, according to research published in Circulation: Cardiovascular Quality and Outcomes.
When a Canadian health system switched from prescribing Plavix to generic clopidogrel, patients with acute coronary syndrome (ACS) were no more likely to die or experience ACS recurrence within a year.
In addition, there were no significant differences between the Plavix and clopidogrel groups in the percentage of patients who were hospitalized for any reason, had a stroke or transient ischemic attack, or experienced bleeding.
“People can safely use generic clopidogrel,” said study author Dennis T. Ko, MD, of the Institute for Clinical Evaluative Sciences in Toronto, Ontario, Canada.
“This large and real-world study should be reassuring to physicians and healthcare organizations who have been concerned about changing what is prescribed.”
Dr Ko and his colleagues compared outcomes in patients who were prescribed clopidogrel after hospitalization for ACS in Ontario, Canada, where the Ministry of Health began to automatically substitute generic clopidogrel for Plavix once the brand name drug’s patent expired in 2012.
Between 2009 and 2014, 12,643 patients were prescribed Plavix, and 11,887 were prescribed generic clopidogrel.
“There are quite a few different generic brands,” said study author Cynthia Jackevicius, PharmD, of Western University of Health Sciences in Pomona, California.
“In this study, we considered them as a group but later found no differences in outcome when we compared between different generics.”
Results
The study’s primary outcome—a composite of death and hospitalization for ACS at 1 year—was met by 17.6% of patients prescribed Plavix and 17.9% of patients prescribed clopidogrel. The hazard ratio (HR) was 1.02 (95% confidence interval [CI], 0.96–1.08; P=0.005 for noninferiority, P=0.619 for superiority).
The 1-year mortality rate was 10.5% in the Plavix group and 11.2% in the clopidogrel group (HR=1.07; 95% CI, 0.99–1.15, P=0.210 for noninferiority, P=0.114 for superiority).
And hospitalization for ACS occurred in 9.7% and 9.2%, respectively (HR=0.94; 95% CI, 0.87–1.03, P<0.001 for noninferiority, P=0.190 for superiority).
Hospitalization for any reason occurred in 39.4% of patients in the Plavix group and 39.8% of those in the clopidogrel group (HR=1.02; 95% CI, 0.97–1.06, P<0.001 for noninferiority, P=0.482 for superiority).
Hospitalization for stroke or transient ischemic attack occurred in 1.5% and 1.4%, respectively (HR=0.92; 95% CI, 0.74–1.15, P=0.056 for noninferiority, P=0.455 for superiority).
And hospitalization for bleeding occurred in 2.3% and 2.7%, respectively (HR=1.17; 95% CI, 0.99–1.39, P=0.772 for noninferiority, P=0.063 for superiority).
The researchers noted that, in 2010, Plavix cost about $2.58 Canadian dollars per pill. It was projected to cost the Ontario Drug Benefit Program $72.8 million by 2012.
By switching to generic clopidogrel, which costs $0.39 per pill in 2018, the expense was $19 million Canadian dollars.
“Plavix was one of the most commonly used drugs in cardiology,” Dr Ko said, “so switching to generics can reduce a lot of cost for individuals and health systems.”
Does Fish Oil During Pregnancy Help Prevent Asthma in Kids?

A 24-year-old G2P1 at 24 weeks’ gestation presents to your clinic for a routine prenatal visit. Her older daughter has asthma, and she wants to know if there is anything she can do to reduce her second child’s risk for it. What do you recommend?
Asthma is the most common chronic disease in children in resource-rich countries such as the United States.2 According to the CDC, 8.4% of children were diagnosed with asthma in 2015.3
Omega-3 fatty acids, found naturally in fish oil, are thought to confer anti-inflammatory properties that offer protection against asthma. Clinical trials have shown that fish oil supplementation in pregnancy results in higher levels of omega-3 fatty acids, along with anti-inflammatory changes, in offspring.4 Previous epidemiologic studies have also found that consumption of omega-3 fatty acids decreases the risk for atopy and asthma in offspring.5,6
A Cochrane review published in 2015, however, concluded that omega-3 supplementation during pregnancy had no benefit on wheeze or asthma in offspring.7 Five RCTs were included in the analysis. The largest trial, by Palmer et al, which included 706 women, showed no benefit for supplementation.8 The second largest, by Olsen et al, which included 533 women, did show a benefit (hazard ratio [HR], 0.37; number needed to treat [NNT], 19.6).9
These results, however, were limited by heterogeneity in the amount of fish oil supplemented and duration of follow-up. For example, the children in the Palmer study were followed only until age 3, which is around the time that asthma can be formally diagnosed—potentially leading to underreporting.8 In addition, the diagnosis of asthma was based on parent report of three episodes of wheezing, use of daily asthma medication, or use of a national registry—all of which can underestimate the incidence of asthma. The reported rate of childhood asthma with IgE-sensitization (rate without sensitization was not reported) was 1.8% in both study groups—much lower than the CDC’s rate of 8.4%, suggesting underdiagnosis.3,8 Due to these biases and other potential confounders, no firm conclusions can be drawn from the Cochrane review.
STUDY SUMMARY
Maternal fish oil supplementation reduces asthma in children
This single-center, double-blind RCT of 736 pregnant women evaluated the effect of 2.4 g/d of n-3 long-chain polyunsaturated fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) or placebo (olive oil), starting at an estimated gestational age of 24 to 26 weeks, on wheeze or asthma incidence in their offspring.1
Eligible women were between 22 and 26 weeks’ pregnant at the time of recruitment. Exclusion criteria included supplementation of 600 IU/d or more of vitamin D, or having any endocrine, cardiac, or renal disorders. The investigators randomized the women in a 1:1 ratio to either fish oil or placebo. Maternal EPA and DHA blood levels were tested at the time of randomization and one week after birth.
The primary outcome was persistent wheeze or asthma (after age 3, persistent wheeze was termed asthma), determined based on daily diary recordings of five episodes of troublesome lung symptoms within the past six months (each lasting for at least three consecutive days); rescue use of inhaled ß2-agonists; and/or relapse after a three-month course of inhaled glucocorticoids. Secondary outcomes included reduced incidence of respiratory tract infections, asthma exacerbations, eczema, and allergic sensitization.
In total, 695 offspring were included in the study, with 95.5% follow-up at three years and 93.1% at five. The children had scheduled pediatric visits at 1 week; at one, three, six, 12, 18, 24, 30, and 36 months; and at 4 and 5 years. They also had acute visits for any pulmonary, allergic, or dermatologic symptoms that arose.
Results. The investigators found that the children of mothers who took fish oil had a lower risk for persistent wheeze or asthma at ages 3 to 5, compared to those who received placebo (16.9% vs 23.7%; HR, 0.69; NNT, 14.7). But this effect was significant only in the children whose mothers had baseline EPA and DHA levels in the lowest third (17.5% vs 34.1%; HR, 0.46; NNT, 5.6). Similarly, fish oil supplementation had a greater benefit in children whose mothers had consumed the least EPA and DHA before the start of the study (18.5% vs 32.4%; HR, 0.55; NNT, 7.2).
As for the secondary outcomes, only a reduction in lower respiratory infections was associated with fish oil supplementation compared with placebo (38.8% vs 45.5%; HR, 0.77; NNT, 14.9). There was no reduction in asthma exacerbations, eczema, or risk for sensitization in the fish oil group.
WHAT’S NEW?
Study adds fuel to the fire
This study strengthens the case for fish oil supplementation during pregnancy to reduce the risk for asthma in offspring, despite the recent Cochrane review that showed no benefit.1,7 The Palmer study used a much lower amount of omega-3s (900 mg/d fish oil vs 2,400 mg/d in the current trial).1,8 Olsen et al supplemented with a greater amount of omega-3s (2,700 mg/d) and did find a benefit.9 The NNT from the Olsen study (19.6) is consistent with that of the current investigation, suggesting that a higher dosage may be necessary to prevent the onset of asthma.
Additionally, this study followed children for a longer period than did the Palmer study, which may have led to more accurate diagnoses of asthma.1,8 Lastly, the diagnosis of asthma in the Palmer study was based on parent survey data and use of daily asthma medicine rather than on daily diary cards, which are often more accurate.
Consider fish consumption. Both this study and the Olsen trial were performed in Denmark.1,9 While Denmark and the United States have had a relatively similar level of fish consumption since the 1990s, women in Denmark may eat a higher proportion of oily fish than women in the United States, given the more common inclusion of mackerel and herring in their diet.10 Thus, the effect of supplementation may be more pronounced in women in the US.
CAVEATS
Ideal dose? Which women to treat?
The FDA currently recommends 8 to 12 oz of fish per week for pregnant women, but there are no guidelines on the ideal amount of fish oil to be consumed.11 The Palmer study, using 900 mg/d of fish oil, did not show a benefit, whereas there did appear to be a benefit in this study (2,400 mg/d) and the Olsen study (2,700 mg/d).1,8,9 Further research is needed to determine the optimal dosage.
The decreased risk for persistent wheeze or asthma was seen only in the children of women whose EPA and DHA blood levels were in the lowest third of the study population. Thus, only women whose blood levels are low to begin with will likely benefit from this intervention. Currently, EPA and DHA levels are not routinely checked, but there may be some benefit to doing so.
One proxy for blood levels is maternal intake of fish at baseline. The investigators found that there was an association between dietary intake of fish and blood levels of EPA and DHA (r, 0.32).1 Therefore, additional screening questions to gauge fish consumption would be useful to identify women most likely to benefit from supplementation.
CHALLENGES TO IMPLEMENTATION
Multiple pills, additional cost
Since omega-3 fatty acids are relatively safe and the NNT in the general population is low, it may be worth supplementing all pregnant women, even without a commercially available blood test for EPA or DHA. Nevertheless, some women may find it challenging to take up to four additional pills per day for 13 or more weeks. Also, there is an associated cost with these supplements, although it is low.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[2]: 100-102).
1. Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375(26):2530-2539.
2. Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy. 2004;59(5):469-478.
3. CDC . Asthma. www.cdc.gov/asthma/most_recent_data.htm. Accessed February 1, 2018.
4. Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int. 2015;64(1):27-34.
5. Salam MT, Li YF, Langholz B, et al. Maternal fish consumption during pregnancy and risk of early childhood asthma. J Asthma. 2005;42(6):513-518.
6. Calvani M, Alessandri C, Sopo SM, et al. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr Allergy Immunol. 2006;17(2):94-102.
7. Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. 2015;22(7): CD010085.
8. Palmer D, Sullivan T, Gold M, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy. 2013;68:1370-1376.
9. Olsen SF, Østerdal ML, Salvig JD, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. 2008;88(1): 167-175.
10. Helgi Library. Fish consumption per capita by country. www.helgilibrary.com/indicators/fish-consumption-per-capita/. Accessed February 1, 2018.
11. FDA Advice About Eating Fish, From the Environmental Protection Agency and Food and Drug Administration; Revised Fish Advice; Availability. Fed Regist. 2017;82:6571-6574.

A 24-year-old G2P1 at 24 weeks’ gestation presents to your clinic for a routine prenatal visit. Her older daughter has asthma, and she wants to know if there is anything she can do to reduce her second child’s risk for it. What do you recommend?
Asthma is the most common chronic disease in children in resource-rich countries such as the United States.2 According to the CDC, 8.4% of children were diagnosed with asthma in 2015.3
Omega-3 fatty acids, found naturally in fish oil, are thought to confer anti-inflammatory properties that offer protection against asthma. Clinical trials have shown that fish oil supplementation in pregnancy results in higher levels of omega-3 fatty acids, along with anti-inflammatory changes, in offspring.4 Previous epidemiologic studies have also found that consumption of omega-3 fatty acids decreases the risk for atopy and asthma in offspring.5,6
A Cochrane review published in 2015, however, concluded that omega-3 supplementation during pregnancy had no benefit on wheeze or asthma in offspring.7 Five RCTs were included in the analysis. The largest trial, by Palmer et al, which included 706 women, showed no benefit for supplementation.8 The second largest, by Olsen et al, which included 533 women, did show a benefit (hazard ratio [HR], 0.37; number needed to treat [NNT], 19.6).9
These results, however, were limited by heterogeneity in the amount of fish oil supplemented and duration of follow-up. For example, the children in the Palmer study were followed only until age 3, which is around the time that asthma can be formally diagnosed—potentially leading to underreporting.8 In addition, the diagnosis of asthma was based on parent report of three episodes of wheezing, use of daily asthma medication, or use of a national registry—all of which can underestimate the incidence of asthma. The reported rate of childhood asthma with IgE-sensitization (rate without sensitization was not reported) was 1.8% in both study groups—much lower than the CDC’s rate of 8.4%, suggesting underdiagnosis.3,8 Due to these biases and other potential confounders, no firm conclusions can be drawn from the Cochrane review.
STUDY SUMMARY
Maternal fish oil supplementation reduces asthma in children
This single-center, double-blind RCT of 736 pregnant women evaluated the effect of 2.4 g/d of n-3 long-chain polyunsaturated fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) or placebo (olive oil), starting at an estimated gestational age of 24 to 26 weeks, on wheeze or asthma incidence in their offspring.1
Eligible women were between 22 and 26 weeks’ pregnant at the time of recruitment. Exclusion criteria included supplementation of 600 IU/d or more of vitamin D, or having any endocrine, cardiac, or renal disorders. The investigators randomized the women in a 1:1 ratio to either fish oil or placebo. Maternal EPA and DHA blood levels were tested at the time of randomization and one week after birth.
The primary outcome was persistent wheeze or asthma (after age 3, persistent wheeze was termed asthma), determined based on daily diary recordings of five episodes of troublesome lung symptoms within the past six months (each lasting for at least three consecutive days); rescue use of inhaled ß2-agonists; and/or relapse after a three-month course of inhaled glucocorticoids. Secondary outcomes included reduced incidence of respiratory tract infections, asthma exacerbations, eczema, and allergic sensitization.
In total, 695 offspring were included in the study, with 95.5% follow-up at three years and 93.1% at five. The children had scheduled pediatric visits at 1 week; at one, three, six, 12, 18, 24, 30, and 36 months; and at 4 and 5 years. They also had acute visits for any pulmonary, allergic, or dermatologic symptoms that arose.
Results. The investigators found that the children of mothers who took fish oil had a lower risk for persistent wheeze or asthma at ages 3 to 5, compared to those who received placebo (16.9% vs 23.7%; HR, 0.69; NNT, 14.7). But this effect was significant only in the children whose mothers had baseline EPA and DHA levels in the lowest third (17.5% vs 34.1%; HR, 0.46; NNT, 5.6). Similarly, fish oil supplementation had a greater benefit in children whose mothers had consumed the least EPA and DHA before the start of the study (18.5% vs 32.4%; HR, 0.55; NNT, 7.2).
As for the secondary outcomes, only a reduction in lower respiratory infections was associated with fish oil supplementation compared with placebo (38.8% vs 45.5%; HR, 0.77; NNT, 14.9). There was no reduction in asthma exacerbations, eczema, or risk for sensitization in the fish oil group.
WHAT’S NEW?
Study adds fuel to the fire
This study strengthens the case for fish oil supplementation during pregnancy to reduce the risk for asthma in offspring, despite the recent Cochrane review that showed no benefit.1,7 The Palmer study used a much lower amount of omega-3s (900 mg/d fish oil vs 2,400 mg/d in the current trial).1,8 Olsen et al supplemented with a greater amount of omega-3s (2,700 mg/d) and did find a benefit.9 The NNT from the Olsen study (19.6) is consistent with that of the current investigation, suggesting that a higher dosage may be necessary to prevent the onset of asthma.
Additionally, this study followed children for a longer period than did the Palmer study, which may have led to more accurate diagnoses of asthma.1,8 Lastly, the diagnosis of asthma in the Palmer study was based on parent survey data and use of daily asthma medicine rather than on daily diary cards, which are often more accurate.
Consider fish consumption. Both this study and the Olsen trial were performed in Denmark.1,9 While Denmark and the United States have had a relatively similar level of fish consumption since the 1990s, women in Denmark may eat a higher proportion of oily fish than women in the United States, given the more common inclusion of mackerel and herring in their diet.10 Thus, the effect of supplementation may be more pronounced in women in the US.
CAVEATS
Ideal dose? Which women to treat?
The FDA currently recommends 8 to 12 oz of fish per week for pregnant women, but there are no guidelines on the ideal amount of fish oil to be consumed.11 The Palmer study, using 900 mg/d of fish oil, did not show a benefit, whereas there did appear to be a benefit in this study (2,400 mg/d) and the Olsen study (2,700 mg/d).1,8,9 Further research is needed to determine the optimal dosage.
The decreased risk for persistent wheeze or asthma was seen only in the children of women whose EPA and DHA blood levels were in the lowest third of the study population. Thus, only women whose blood levels are low to begin with will likely benefit from this intervention. Currently, EPA and DHA levels are not routinely checked, but there may be some benefit to doing so.
One proxy for blood levels is maternal intake of fish at baseline. The investigators found that there was an association between dietary intake of fish and blood levels of EPA and DHA (r, 0.32).1 Therefore, additional screening questions to gauge fish consumption would be useful to identify women most likely to benefit from supplementation.
CHALLENGES TO IMPLEMENTATION
Multiple pills, additional cost
Since omega-3 fatty acids are relatively safe and the NNT in the general population is low, it may be worth supplementing all pregnant women, even without a commercially available blood test for EPA or DHA. Nevertheless, some women may find it challenging to take up to four additional pills per day for 13 or more weeks. Also, there is an associated cost with these supplements, although it is low.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[2]: 100-102).

A 24-year-old G2P1 at 24 weeks’ gestation presents to your clinic for a routine prenatal visit. Her older daughter has asthma, and she wants to know if there is anything she can do to reduce her second child’s risk for it. What do you recommend?
Asthma is the most common chronic disease in children in resource-rich countries such as the United States.2 According to the CDC, 8.4% of children were diagnosed with asthma in 2015.3
Omega-3 fatty acids, found naturally in fish oil, are thought to confer anti-inflammatory properties that offer protection against asthma. Clinical trials have shown that fish oil supplementation in pregnancy results in higher levels of omega-3 fatty acids, along with anti-inflammatory changes, in offspring.4 Previous epidemiologic studies have also found that consumption of omega-3 fatty acids decreases the risk for atopy and asthma in offspring.5,6
A Cochrane review published in 2015, however, concluded that omega-3 supplementation during pregnancy had no benefit on wheeze or asthma in offspring.7 Five RCTs were included in the analysis. The largest trial, by Palmer et al, which included 706 women, showed no benefit for supplementation.8 The second largest, by Olsen et al, which included 533 women, did show a benefit (hazard ratio [HR], 0.37; number needed to treat [NNT], 19.6).9
These results, however, were limited by heterogeneity in the amount of fish oil supplemented and duration of follow-up. For example, the children in the Palmer study were followed only until age 3, which is around the time that asthma can be formally diagnosed—potentially leading to underreporting.8 In addition, the diagnosis of asthma was based on parent report of three episodes of wheezing, use of daily asthma medication, or use of a national registry—all of which can underestimate the incidence of asthma. The reported rate of childhood asthma with IgE-sensitization (rate without sensitization was not reported) was 1.8% in both study groups—much lower than the CDC’s rate of 8.4%, suggesting underdiagnosis.3,8 Due to these biases and other potential confounders, no firm conclusions can be drawn from the Cochrane review.
STUDY SUMMARY
Maternal fish oil supplementation reduces asthma in children
This single-center, double-blind RCT of 736 pregnant women evaluated the effect of 2.4 g/d of n-3 long-chain polyunsaturated fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) or placebo (olive oil), starting at an estimated gestational age of 24 to 26 weeks, on wheeze or asthma incidence in their offspring.1
Eligible women were between 22 and 26 weeks’ pregnant at the time of recruitment. Exclusion criteria included supplementation of 600 IU/d or more of vitamin D, or having any endocrine, cardiac, or renal disorders. The investigators randomized the women in a 1:1 ratio to either fish oil or placebo. Maternal EPA and DHA blood levels were tested at the time of randomization and one week after birth.
The primary outcome was persistent wheeze or asthma (after age 3, persistent wheeze was termed asthma), determined based on daily diary recordings of five episodes of troublesome lung symptoms within the past six months (each lasting for at least three consecutive days); rescue use of inhaled ß2-agonists; and/or relapse after a three-month course of inhaled glucocorticoids. Secondary outcomes included reduced incidence of respiratory tract infections, asthma exacerbations, eczema, and allergic sensitization.
In total, 695 offspring were included in the study, with 95.5% follow-up at three years and 93.1% at five. The children had scheduled pediatric visits at 1 week; at one, three, six, 12, 18, 24, 30, and 36 months; and at 4 and 5 years. They also had acute visits for any pulmonary, allergic, or dermatologic symptoms that arose.
Results. The investigators found that the children of mothers who took fish oil had a lower risk for persistent wheeze or asthma at ages 3 to 5, compared to those who received placebo (16.9% vs 23.7%; HR, 0.69; NNT, 14.7). But this effect was significant only in the children whose mothers had baseline EPA and DHA levels in the lowest third (17.5% vs 34.1%; HR, 0.46; NNT, 5.6). Similarly, fish oil supplementation had a greater benefit in children whose mothers had consumed the least EPA and DHA before the start of the study (18.5% vs 32.4%; HR, 0.55; NNT, 7.2).
As for the secondary outcomes, only a reduction in lower respiratory infections was associated with fish oil supplementation compared with placebo (38.8% vs 45.5%; HR, 0.77; NNT, 14.9). There was no reduction in asthma exacerbations, eczema, or risk for sensitization in the fish oil group.
WHAT’S NEW?
Study adds fuel to the fire
This study strengthens the case for fish oil supplementation during pregnancy to reduce the risk for asthma in offspring, despite the recent Cochrane review that showed no benefit.1,7 The Palmer study used a much lower amount of omega-3s (900 mg/d fish oil vs 2,400 mg/d in the current trial).1,8 Olsen et al supplemented with a greater amount of omega-3s (2,700 mg/d) and did find a benefit.9 The NNT from the Olsen study (19.6) is consistent with that of the current investigation, suggesting that a higher dosage may be necessary to prevent the onset of asthma.
Additionally, this study followed children for a longer period than did the Palmer study, which may have led to more accurate diagnoses of asthma.1,8 Lastly, the diagnosis of asthma in the Palmer study was based on parent survey data and use of daily asthma medicine rather than on daily diary cards, which are often more accurate.
Consider fish consumption. Both this study and the Olsen trial were performed in Denmark.1,9 While Denmark and the United States have had a relatively similar level of fish consumption since the 1990s, women in Denmark may eat a higher proportion of oily fish than women in the United States, given the more common inclusion of mackerel and herring in their diet.10 Thus, the effect of supplementation may be more pronounced in women in the US.
CAVEATS
Ideal dose? Which women to treat?
The FDA currently recommends 8 to 12 oz of fish per week for pregnant women, but there are no guidelines on the ideal amount of fish oil to be consumed.11 The Palmer study, using 900 mg/d of fish oil, did not show a benefit, whereas there did appear to be a benefit in this study (2,400 mg/d) and the Olsen study (2,700 mg/d).1,8,9 Further research is needed to determine the optimal dosage.
The decreased risk for persistent wheeze or asthma was seen only in the children of women whose EPA and DHA blood levels were in the lowest third of the study population. Thus, only women whose blood levels are low to begin with will likely benefit from this intervention. Currently, EPA and DHA levels are not routinely checked, but there may be some benefit to doing so.
One proxy for blood levels is maternal intake of fish at baseline. The investigators found that there was an association between dietary intake of fish and blood levels of EPA and DHA (r, 0.32).1 Therefore, additional screening questions to gauge fish consumption would be useful to identify women most likely to benefit from supplementation.
CHALLENGES TO IMPLEMENTATION
Multiple pills, additional cost
Since omega-3 fatty acids are relatively safe and the NNT in the general population is low, it may be worth supplementing all pregnant women, even without a commercially available blood test for EPA or DHA. Nevertheless, some women may find it challenging to take up to four additional pills per day for 13 or more weeks. Also, there is an associated cost with these supplements, although it is low.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[2]: 100-102).
1. Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375(26):2530-2539.
2. Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy. 2004;59(5):469-478.
3. CDC . Asthma. www.cdc.gov/asthma/most_recent_data.htm. Accessed February 1, 2018.
4. Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int. 2015;64(1):27-34.
5. Salam MT, Li YF, Langholz B, et al. Maternal fish consumption during pregnancy and risk of early childhood asthma. J Asthma. 2005;42(6):513-518.
6. Calvani M, Alessandri C, Sopo SM, et al. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr Allergy Immunol. 2006;17(2):94-102.
7. Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. 2015;22(7): CD010085.
8. Palmer D, Sullivan T, Gold M, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy. 2013;68:1370-1376.
9. Olsen SF, Østerdal ML, Salvig JD, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. 2008;88(1): 167-175.
10. Helgi Library. Fish consumption per capita by country. www.helgilibrary.com/indicators/fish-consumption-per-capita/. Accessed February 1, 2018.
11. FDA Advice About Eating Fish, From the Environmental Protection Agency and Food and Drug Administration; Revised Fish Advice; Availability. Fed Regist. 2017;82:6571-6574.
1. Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375(26):2530-2539.
2. Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy. 2004;59(5):469-478.
3. CDC . Asthma. www.cdc.gov/asthma/most_recent_data.htm. Accessed February 1, 2018.
4. Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int. 2015;64(1):27-34.
5. Salam MT, Li YF, Langholz B, et al. Maternal fish consumption during pregnancy and risk of early childhood asthma. J Asthma. 2005;42(6):513-518.
6. Calvani M, Alessandri C, Sopo SM, et al. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr Allergy Immunol. 2006;17(2):94-102.
7. Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. 2015;22(7): CD010085.
8. Palmer D, Sullivan T, Gold M, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy. 2013;68:1370-1376.
9. Olsen SF, Østerdal ML, Salvig JD, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. 2008;88(1): 167-175.
10. Helgi Library. Fish consumption per capita by country. www.helgilibrary.com/indicators/fish-consumption-per-capita/. Accessed February 1, 2018.
11. FDA Advice About Eating Fish, From the Environmental Protection Agency and Food and Drug Administration; Revised Fish Advice; Availability. Fed Regist. 2017;82:6571-6574.
Quick Byte: U.S. health care can still innovate
“The United States health care system has many problems, but it also promotes more innovation than its counterparts in other nations. … It has more clinical trials than any other country. It has the most Nobel laureates in physiology or medicine. It has won more patents. At least one publication ranks it No. 1 in overall scientific innovation. … The nation’s innovation advantage arises from a first-class research university system, along with robust intellectual property laws and significant public and private investment in research and development. Perhaps most important, this country offers a large market in which patients, organizations, and government spend a lot on health and companies are able to profit greatly from health care innovation.”
Reference
1. Carroll AE et al. “Can the U.S. repair its health care while keeping its innovation edge?” The New York Times. Oct 9, 2017.
. Accessed Oct 10, 2017.
“The United States health care system has many problems, but it also promotes more innovation than its counterparts in other nations. … It has more clinical trials than any other country. It has the most Nobel laureates in physiology or medicine. It has won more patents. At least one publication ranks it No. 1 in overall scientific innovation. … The nation’s innovation advantage arises from a first-class research university system, along with robust intellectual property laws and significant public and private investment in research and development. Perhaps most important, this country offers a large market in which patients, organizations, and government spend a lot on health and companies are able to profit greatly from health care innovation.”
Reference
1. Carroll AE et al. “Can the U.S. repair its health care while keeping its innovation edge?” The New York Times. Oct 9, 2017.
. Accessed Oct 10, 2017.
“The United States health care system has many problems, but it also promotes more innovation than its counterparts in other nations. … It has more clinical trials than any other country. It has the most Nobel laureates in physiology or medicine. It has won more patents. At least one publication ranks it No. 1 in overall scientific innovation. … The nation’s innovation advantage arises from a first-class research university system, along with robust intellectual property laws and significant public and private investment in research and development. Perhaps most important, this country offers a large market in which patients, organizations, and government spend a lot on health and companies are able to profit greatly from health care innovation.”
Reference
1. Carroll AE et al. “Can the U.S. repair its health care while keeping its innovation edge?” The New York Times. Oct 9, 2017.
. Accessed Oct 10, 2017.
KD025 shows promise for steroid-dependent cGVHD
SALT LAKE CITY – KD025, an orally available Rho-associated coiled-coil kinase 2–selective inhibitor, is demonstrating encouraging activity and safety in patients with steroid-dependent or refractory chronic graft-versus-host disease (cGVHD) in a phase 2a clinical trial.
Initial results from the ongoing open-label trial known as KD025-208 showed that 11 of 17 patients (65%) and 11 of 16 patients (69%) enrolled in 200-mg daily and 200-mg twice-daily dose cohorts, respectively, had a clinical response with no reported treatment-related serious adverse events at any evaluation time point, Aleksandr Lazaryan, MD, PhD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The median duration of treatment in the 200-mg daily group (cohort 1) was 37 weeks, and in the 200-mg twice-daily group (cohort 2) was 28 weeks. At last follow-up, eight patients remained active in each cohort, and these patients had a median treatment duration of 53 and 38 weeks, respectively, he said.
In cohort 1, four patients went off the study because of cGVHD progression, and five withdrew, including two who experienced recurrence of their underlying hematologic malignancy. In cohort 2, 7 of the 16 patients experienced progression of cGVHD, he noted.
Patients in cohorts 1 and 2 had a median age of 52 years and had received at least 2 months of steroid treatment and no more than 3 prior lines of therapy. They were comparable with respect to baseline characteristics, including median time to and duration of GVHD, time from diagnosis to enrollment, median prednisone dose, and median number of prior therapies. They had involvement of various – and often multiple – organ systems: 58% had four or more systems affected at the time of enrollment, and 21% had five or more systems affected.
“This, in a way, reflects a real-life mix of the cGVHD population of patients, with some of those patients having advanced cGVHD,” said Dr. Lazaryan.
Responses were observed across all affected organ systems, with complete responses documented in the upper and lower gastrointestinal tracts. About 75% of patients in cohort 1 who had multiple organ systems involved at enrollment demonstrated responses in at least four organ systems.
Furthermore, the responses were rapid: 68% of responses occurred in the first 8 weeks of treatment and appeared durable, Dr. Lazaryan said, noting that 7 of the 17 patients in cohort 1 had sustained responses for more than 20 weeks, and 3 patients had sustained responses for more than 32 weeks.
“The durability data continue to mature in this trial,” he added.
The adverse events that occurred were consistent with what would be expected for the cGVHD patient population treated with steroids, he said, reporting that no patients discontinued treatment because of infection, no opportunistic or fungal infections have been reported to date, and no treatment-related serious adverse events were reported.
Steroid dose reductions were experienced by 40% and 26% of patients in cohorts 1 and 2, respectively. The dose reductions were achieved in both KD025 responders and nonresponders, he noted.
Overall, four patients (12%) were able to discontinue steroids, and 80% in both cohorts experienced reductions in background tacrolimus.
In addition, up to 65% of patients in cohort 1 achieved a greater than seven point reduction on the Lee cGVHD Symptom Scale, with both responders and nonresponders experiencing improvement on this endpoint.
Chronic GVHD remains a leading cause of post-transplant morbidity and mortality. KD025, which is currently in phase 2 development for inflammatory fibrotic disease, has been shown in preclinical models to down-regulate T helper 17 cells and T follicular helper cells while up-regulating anti-inflammatory regulatory T cells, thereby potentially correcting the immunological imbalance seen in cGVHD, Dr. Lazaryan said.
Analysis is ongoing in this study, including in a third cohort of patients treated with 200 mg of KD025 four times daily, which recently completed accrual. An expansion cohort, at a dose yet to be determined, will include approximately 40 patients, he noted.
The trial is sponsored by Kadmon. Dr. Lazaryan reported advisory board membership and consultancy for GLyPharma Therapeutic.
SOURCE: Lazaryan A et al. 2018 BMT Tandem Meetings, Abstract 38.
SALT LAKE CITY – KD025, an orally available Rho-associated coiled-coil kinase 2–selective inhibitor, is demonstrating encouraging activity and safety in patients with steroid-dependent or refractory chronic graft-versus-host disease (cGVHD) in a phase 2a clinical trial.
Initial results from the ongoing open-label trial known as KD025-208 showed that 11 of 17 patients (65%) and 11 of 16 patients (69%) enrolled in 200-mg daily and 200-mg twice-daily dose cohorts, respectively, had a clinical response with no reported treatment-related serious adverse events at any evaluation time point, Aleksandr Lazaryan, MD, PhD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The median duration of treatment in the 200-mg daily group (cohort 1) was 37 weeks, and in the 200-mg twice-daily group (cohort 2) was 28 weeks. At last follow-up, eight patients remained active in each cohort, and these patients had a median treatment duration of 53 and 38 weeks, respectively, he said.
In cohort 1, four patients went off the study because of cGVHD progression, and five withdrew, including two who experienced recurrence of their underlying hematologic malignancy. In cohort 2, 7 of the 16 patients experienced progression of cGVHD, he noted.
Patients in cohorts 1 and 2 had a median age of 52 years and had received at least 2 months of steroid treatment and no more than 3 prior lines of therapy. They were comparable with respect to baseline characteristics, including median time to and duration of GVHD, time from diagnosis to enrollment, median prednisone dose, and median number of prior therapies. They had involvement of various – and often multiple – organ systems: 58% had four or more systems affected at the time of enrollment, and 21% had five or more systems affected.
“This, in a way, reflects a real-life mix of the cGVHD population of patients, with some of those patients having advanced cGVHD,” said Dr. Lazaryan.
Responses were observed across all affected organ systems, with complete responses documented in the upper and lower gastrointestinal tracts. About 75% of patients in cohort 1 who had multiple organ systems involved at enrollment demonstrated responses in at least four organ systems.
Furthermore, the responses were rapid: 68% of responses occurred in the first 8 weeks of treatment and appeared durable, Dr. Lazaryan said, noting that 7 of the 17 patients in cohort 1 had sustained responses for more than 20 weeks, and 3 patients had sustained responses for more than 32 weeks.
“The durability data continue to mature in this trial,” he added.
The adverse events that occurred were consistent with what would be expected for the cGVHD patient population treated with steroids, he said, reporting that no patients discontinued treatment because of infection, no opportunistic or fungal infections have been reported to date, and no treatment-related serious adverse events were reported.
Steroid dose reductions were experienced by 40% and 26% of patients in cohorts 1 and 2, respectively. The dose reductions were achieved in both KD025 responders and nonresponders, he noted.
Overall, four patients (12%) were able to discontinue steroids, and 80% in both cohorts experienced reductions in background tacrolimus.
In addition, up to 65% of patients in cohort 1 achieved a greater than seven point reduction on the Lee cGVHD Symptom Scale, with both responders and nonresponders experiencing improvement on this endpoint.
Chronic GVHD remains a leading cause of post-transplant morbidity and mortality. KD025, which is currently in phase 2 development for inflammatory fibrotic disease, has been shown in preclinical models to down-regulate T helper 17 cells and T follicular helper cells while up-regulating anti-inflammatory regulatory T cells, thereby potentially correcting the immunological imbalance seen in cGVHD, Dr. Lazaryan said.
Analysis is ongoing in this study, including in a third cohort of patients treated with 200 mg of KD025 four times daily, which recently completed accrual. An expansion cohort, at a dose yet to be determined, will include approximately 40 patients, he noted.
The trial is sponsored by Kadmon. Dr. Lazaryan reported advisory board membership and consultancy for GLyPharma Therapeutic.
SOURCE: Lazaryan A et al. 2018 BMT Tandem Meetings, Abstract 38.
SALT LAKE CITY – KD025, an orally available Rho-associated coiled-coil kinase 2–selective inhibitor, is demonstrating encouraging activity and safety in patients with steroid-dependent or refractory chronic graft-versus-host disease (cGVHD) in a phase 2a clinical trial.
Initial results from the ongoing open-label trial known as KD025-208 showed that 11 of 17 patients (65%) and 11 of 16 patients (69%) enrolled in 200-mg daily and 200-mg twice-daily dose cohorts, respectively, had a clinical response with no reported treatment-related serious adverse events at any evaluation time point, Aleksandr Lazaryan, MD, PhD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The median duration of treatment in the 200-mg daily group (cohort 1) was 37 weeks, and in the 200-mg twice-daily group (cohort 2) was 28 weeks. At last follow-up, eight patients remained active in each cohort, and these patients had a median treatment duration of 53 and 38 weeks, respectively, he said.
In cohort 1, four patients went off the study because of cGVHD progression, and five withdrew, including two who experienced recurrence of their underlying hematologic malignancy. In cohort 2, 7 of the 16 patients experienced progression of cGVHD, he noted.
Patients in cohorts 1 and 2 had a median age of 52 years and had received at least 2 months of steroid treatment and no more than 3 prior lines of therapy. They were comparable with respect to baseline characteristics, including median time to and duration of GVHD, time from diagnosis to enrollment, median prednisone dose, and median number of prior therapies. They had involvement of various – and often multiple – organ systems: 58% had four or more systems affected at the time of enrollment, and 21% had five or more systems affected.
“This, in a way, reflects a real-life mix of the cGVHD population of patients, with some of those patients having advanced cGVHD,” said Dr. Lazaryan.
Responses were observed across all affected organ systems, with complete responses documented in the upper and lower gastrointestinal tracts. About 75% of patients in cohort 1 who had multiple organ systems involved at enrollment demonstrated responses in at least four organ systems.
Furthermore, the responses were rapid: 68% of responses occurred in the first 8 weeks of treatment and appeared durable, Dr. Lazaryan said, noting that 7 of the 17 patients in cohort 1 had sustained responses for more than 20 weeks, and 3 patients had sustained responses for more than 32 weeks.
“The durability data continue to mature in this trial,” he added.
The adverse events that occurred were consistent with what would be expected for the cGVHD patient population treated with steroids, he said, reporting that no patients discontinued treatment because of infection, no opportunistic or fungal infections have been reported to date, and no treatment-related serious adverse events were reported.
Steroid dose reductions were experienced by 40% and 26% of patients in cohorts 1 and 2, respectively. The dose reductions were achieved in both KD025 responders and nonresponders, he noted.
Overall, four patients (12%) were able to discontinue steroids, and 80% in both cohorts experienced reductions in background tacrolimus.
In addition, up to 65% of patients in cohort 1 achieved a greater than seven point reduction on the Lee cGVHD Symptom Scale, with both responders and nonresponders experiencing improvement on this endpoint.
Chronic GVHD remains a leading cause of post-transplant morbidity and mortality. KD025, which is currently in phase 2 development for inflammatory fibrotic disease, has been shown in preclinical models to down-regulate T helper 17 cells and T follicular helper cells while up-regulating anti-inflammatory regulatory T cells, thereby potentially correcting the immunological imbalance seen in cGVHD, Dr. Lazaryan said.
Analysis is ongoing in this study, including in a third cohort of patients treated with 200 mg of KD025 four times daily, which recently completed accrual. An expansion cohort, at a dose yet to be determined, will include approximately 40 patients, he noted.
The trial is sponsored by Kadmon. Dr. Lazaryan reported advisory board membership and consultancy for GLyPharma Therapeutic.
SOURCE: Lazaryan A et al. 2018 BMT Tandem Meetings, Abstract 38.
REPORTING FROM THE 2018 BMT TANDEM MEETINGS
Key clinical point:
Major finding: Clinical response rates in cohorts 1 and 2 were 65% and 69%, respectively.
Study details: Preliminary findings in 33 patients from a phase 2a trial.
Disclosures: The trial is sponsored by Kadmon. Dr. Lazaryan reported advisory board membership and consultancy for GLyPharma Therapeutic.
Source: Lazaryan A et al. 2018 BMT Tandem Meetings, Abstract 38.
Minor differences with electric and manual aspiration of molar pregnancy
Manual vacuum aspiration of molar pregnancy achieves similar outcomes to electric vacuum aspiration, although it may lead to a lower incidence of uterine synechia, according to a paper published in the April edition of Obstetrics & Gynecology.
While electric vacuum aspiration of molar pregnancy is the dominant technique in North America, in other parts of the world, such as Brazil, manual vacuum aspiration is far more commonly used.
In a retrospective cohort study, researchers looked at outcomes for 1,727 patients with molar pregnancy; 1,206 of these patients underwent electric vacuum aspiration, and 521 underwent manual vacuum aspiration.
Patients who underwent electric vacuum aspiration had significantly shorter operative times (25.3 minutes vs. 34.2 minutes; P less than .001) and showed a greater drop in hemoglobin levels after evacuation (–0.3 g/dL vs. –0.19 g/dL; P less than .001), compared with those who underwent manual vacuum aspiration.
The electric procedure was also associated with a significantly higher risk of intrauterine adhesions after the procedure, compared with the manual vacuum aspiration (5.2% vs. 1.2%; P less than .001).
Lilian Padrón, MD, of the Trophoblastic Disease Center at the Rio de Janeiro Federal University and coauthors commented that the vacuum pressure is about 100 mm Hg higher in the electric technique than it is in the manual technique, which may be responsible for the greater risk of synechia.
However, there were no significant differences seen between the two groups in the risk of developing postmolar gestational trophoblastic neoplasia (14.2% with electric vs. 17.3% with manual; P = .074) nor in the presence of metastatic disease (19.9% vs. 17.8%; P = .082) or the need for multiagent chemotherapy.
Around 13% of patients had incomplete uterine evacuation, but the risk was similar between electric and manual vacuum aspiration.
“In our sample, formed exclusively by patients with molar pregnancy, the rate of complete uterine emptying did not reach 90% with either technique,” the authors wrote. “This may reflect not only the greater amount of molar trophoblastic tissue, compared with an abortion, but also the invasiveness of molar trophoblastic cells into the maternal decidua.”
There were nine cases of uterine perforation in the electric vacuum aspiration group (0.7%), and none in the manual group, although the difference was not statistically significant.
“Although differences in rates of uterine perforation as well as prolonged length of stay were not statistically different between the groups,” the authors wrote, “both of these were rare events, and we lacked sufficient power to detect differences in rare outcomes.”
No conflicts of interest were declared.
SOURCE: Padrón L et al. Obstet Gynecol. 2018;131:652-9.
Manual vacuum aspiration of molar pregnancy achieves similar outcomes to electric vacuum aspiration, although it may lead to a lower incidence of uterine synechia, according to a paper published in the April edition of Obstetrics & Gynecology.
While electric vacuum aspiration of molar pregnancy is the dominant technique in North America, in other parts of the world, such as Brazil, manual vacuum aspiration is far more commonly used.
In a retrospective cohort study, researchers looked at outcomes for 1,727 patients with molar pregnancy; 1,206 of these patients underwent electric vacuum aspiration, and 521 underwent manual vacuum aspiration.
Patients who underwent electric vacuum aspiration had significantly shorter operative times (25.3 minutes vs. 34.2 minutes; P less than .001) and showed a greater drop in hemoglobin levels after evacuation (–0.3 g/dL vs. –0.19 g/dL; P less than .001), compared with those who underwent manual vacuum aspiration.
The electric procedure was also associated with a significantly higher risk of intrauterine adhesions after the procedure, compared with the manual vacuum aspiration (5.2% vs. 1.2%; P less than .001).
Lilian Padrón, MD, of the Trophoblastic Disease Center at the Rio de Janeiro Federal University and coauthors commented that the vacuum pressure is about 100 mm Hg higher in the electric technique than it is in the manual technique, which may be responsible for the greater risk of synechia.
However, there were no significant differences seen between the two groups in the risk of developing postmolar gestational trophoblastic neoplasia (14.2% with electric vs. 17.3% with manual; P = .074) nor in the presence of metastatic disease (19.9% vs. 17.8%; P = .082) or the need for multiagent chemotherapy.
Around 13% of patients had incomplete uterine evacuation, but the risk was similar between electric and manual vacuum aspiration.
“In our sample, formed exclusively by patients with molar pregnancy, the rate of complete uterine emptying did not reach 90% with either technique,” the authors wrote. “This may reflect not only the greater amount of molar trophoblastic tissue, compared with an abortion, but also the invasiveness of molar trophoblastic cells into the maternal decidua.”
There were nine cases of uterine perforation in the electric vacuum aspiration group (0.7%), and none in the manual group, although the difference was not statistically significant.
“Although differences in rates of uterine perforation as well as prolonged length of stay were not statistically different between the groups,” the authors wrote, “both of these were rare events, and we lacked sufficient power to detect differences in rare outcomes.”
No conflicts of interest were declared.
SOURCE: Padrón L et al. Obstet Gynecol. 2018;131:652-9.
Manual vacuum aspiration of molar pregnancy achieves similar outcomes to electric vacuum aspiration, although it may lead to a lower incidence of uterine synechia, according to a paper published in the April edition of Obstetrics & Gynecology.
While electric vacuum aspiration of molar pregnancy is the dominant technique in North America, in other parts of the world, such as Brazil, manual vacuum aspiration is far more commonly used.
In a retrospective cohort study, researchers looked at outcomes for 1,727 patients with molar pregnancy; 1,206 of these patients underwent electric vacuum aspiration, and 521 underwent manual vacuum aspiration.
Patients who underwent electric vacuum aspiration had significantly shorter operative times (25.3 minutes vs. 34.2 minutes; P less than .001) and showed a greater drop in hemoglobin levels after evacuation (–0.3 g/dL vs. –0.19 g/dL; P less than .001), compared with those who underwent manual vacuum aspiration.
The electric procedure was also associated with a significantly higher risk of intrauterine adhesions after the procedure, compared with the manual vacuum aspiration (5.2% vs. 1.2%; P less than .001).
Lilian Padrón, MD, of the Trophoblastic Disease Center at the Rio de Janeiro Federal University and coauthors commented that the vacuum pressure is about 100 mm Hg higher in the electric technique than it is in the manual technique, which may be responsible for the greater risk of synechia.
However, there were no significant differences seen between the two groups in the risk of developing postmolar gestational trophoblastic neoplasia (14.2% with electric vs. 17.3% with manual; P = .074) nor in the presence of metastatic disease (19.9% vs. 17.8%; P = .082) or the need for multiagent chemotherapy.
Around 13% of patients had incomplete uterine evacuation, but the risk was similar between electric and manual vacuum aspiration.
“In our sample, formed exclusively by patients with molar pregnancy, the rate of complete uterine emptying did not reach 90% with either technique,” the authors wrote. “This may reflect not only the greater amount of molar trophoblastic tissue, compared with an abortion, but also the invasiveness of molar trophoblastic cells into the maternal decidua.”
There were nine cases of uterine perforation in the electric vacuum aspiration group (0.7%), and none in the manual group, although the difference was not statistically significant.
“Although differences in rates of uterine perforation as well as prolonged length of stay were not statistically different between the groups,” the authors wrote, “both of these were rare events, and we lacked sufficient power to detect differences in rare outcomes.”
No conflicts of interest were declared.
SOURCE: Padrón L et al. Obstet Gynecol. 2018;131:652-9.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Manual vacuum aspiration of molar pregnancy achieves similar outcomes to electric vacuum aspiration, although it may lead to a lower incidence of uterine synechia.
Major finding: Electric vacuum aspiration of molar pregnancy is associated with a higher risk of synechia than manual vacuum aspiration.
Data source: A retrospective cohort study in 1,727 patients with molar pregnancy.
Disclosures: No conflicts of interest were declared.
Source: Padrón L et al. Obstet Gynecol. 2018;131:652-9.
FDA meeting on medical devices for sleep apnea scheduled
In a statement sent to members, CHEST invited all to attend this open meeting, which will be held at the FDA White Oak Campus in Silver Spring, Md.
In a statement sent to members, CHEST invited all to attend this open meeting, which will be held at the FDA White Oak Campus in Silver Spring, Md.
In a statement sent to members, CHEST invited all to attend this open meeting, which will be held at the FDA White Oak Campus in Silver Spring, Md.