User login
MDedge Daily News: Could gut bacteria trigger autoimmune diseases?
Vouchers might improve post–heart-attack drug compliance. How education affects depression risks for women. And why confusion reigns over the COPD-asthma overlap.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Vouchers might improve post–heart-attack drug compliance. How education affects depression risks for women. And why confusion reigns over the COPD-asthma overlap.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Vouchers might improve post–heart-attack drug compliance. How education affects depression risks for women. And why confusion reigns over the COPD-asthma overlap.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
When Your Neighborhood Is a CVD Risk Factor
Living in a neighborhood with poor access to food and poor walkability puts people at risk for cardiovascular disease (CVD)—and according to researchers from Morehouse School of Medicine in Atlanta, Georgia, African Americans seemed to have the highest risk.
In a CDC-funded study, the researchers examined the relationship between neighborhood-level food access and walkability on premature CVD mortality rates in Atlanta, using census tracts to map neighborhoods. Atlanta is in Fulton County, which has a food insecurity rating of nearly 20%. Food access was defined as the percentage of no-vehicle households living beyond a 0.9-mile radius of a food outlet in 2012. Walkability was measured in relationship to amenities, population density, and road metrics.
The researchers found no significant difference in walkability scores between high-poverty and low-poverty census tracts. However, they found significant racial differences: Census tracts with high concentrations of minority populations had higher levels of poor food access, poor walkability, and premature CVD mortality.
Of 124 census tracts, 87 contained 73% of the city’s population aged 35 to 64 years and accounted for 1,225 deaths between 2010-2014, with a premature CVD mortality rate of 11 per 1,000. Black premature CVD deaths accounted for nearly 85% of the premature CVD deaths in all census tracts—a “disproportionate number,” the researchers say, because blacks make up only 52% of the city’s total population aged 35 to 64 years.
The findings can be used to “calibrate” neighborhood interventions, based on racial/ethnic or other demographic characteristics, the researchers suggest. They add that the results highlight the need to examine racially stratified health outcomes.
Living in a neighborhood with poor access to food and poor walkability puts people at risk for cardiovascular disease (CVD)—and according to researchers from Morehouse School of Medicine in Atlanta, Georgia, African Americans seemed to have the highest risk.
In a CDC-funded study, the researchers examined the relationship between neighborhood-level food access and walkability on premature CVD mortality rates in Atlanta, using census tracts to map neighborhoods. Atlanta is in Fulton County, which has a food insecurity rating of nearly 20%. Food access was defined as the percentage of no-vehicle households living beyond a 0.9-mile radius of a food outlet in 2012. Walkability was measured in relationship to amenities, population density, and road metrics.
The researchers found no significant difference in walkability scores between high-poverty and low-poverty census tracts. However, they found significant racial differences: Census tracts with high concentrations of minority populations had higher levels of poor food access, poor walkability, and premature CVD mortality.
Of 124 census tracts, 87 contained 73% of the city’s population aged 35 to 64 years and accounted for 1,225 deaths between 2010-2014, with a premature CVD mortality rate of 11 per 1,000. Black premature CVD deaths accounted for nearly 85% of the premature CVD deaths in all census tracts—a “disproportionate number,” the researchers say, because blacks make up only 52% of the city’s total population aged 35 to 64 years.
The findings can be used to “calibrate” neighborhood interventions, based on racial/ethnic or other demographic characteristics, the researchers suggest. They add that the results highlight the need to examine racially stratified health outcomes.
Living in a neighborhood with poor access to food and poor walkability puts people at risk for cardiovascular disease (CVD)—and according to researchers from Morehouse School of Medicine in Atlanta, Georgia, African Americans seemed to have the highest risk.
In a CDC-funded study, the researchers examined the relationship between neighborhood-level food access and walkability on premature CVD mortality rates in Atlanta, using census tracts to map neighborhoods. Atlanta is in Fulton County, which has a food insecurity rating of nearly 20%. Food access was defined as the percentage of no-vehicle households living beyond a 0.9-mile radius of a food outlet in 2012. Walkability was measured in relationship to amenities, population density, and road metrics.
The researchers found no significant difference in walkability scores between high-poverty and low-poverty census tracts. However, they found significant racial differences: Census tracts with high concentrations of minority populations had higher levels of poor food access, poor walkability, and premature CVD mortality.
Of 124 census tracts, 87 contained 73% of the city’s population aged 35 to 64 years and accounted for 1,225 deaths between 2010-2014, with a premature CVD mortality rate of 11 per 1,000. Black premature CVD deaths accounted for nearly 85% of the premature CVD deaths in all census tracts—a “disproportionate number,” the researchers say, because blacks make up only 52% of the city’s total population aged 35 to 64 years.
The findings can be used to “calibrate” neighborhood interventions, based on racial/ethnic or other demographic characteristics, the researchers suggest. They add that the results highlight the need to examine racially stratified health outcomes.
Girl Faces Down Lesion
The lesion on this 8-year-old girl’s face was first noted about a year ago. Recently, however, it has started to grow, prompting her parents to consult the child’s primary care provider (PCP). The lesion is completely asymptomatic but nonetheless concerning to the parents and to the PCP, who has no idea what it could be. He therefore refers them to dermatology.
The child is otherwise healthy. There is no family history of chronic or inheritable disease.
EXAMINATION
The lesion is a 2-cm subcutaneous firm round mass located along the superior aspect of the right jawline. The only overlying skin change is a bluish discoloration. No surface punctum is seen. No other lesions are seen or felt on examination of the rest of the head and neck.
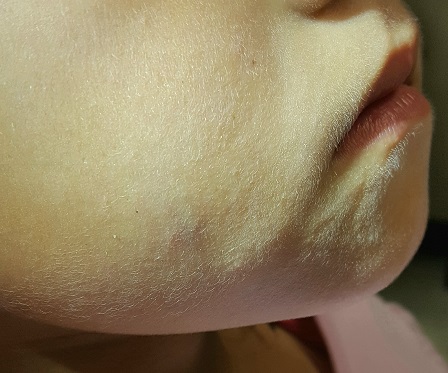
What is the diagnosis?
DISCUSSION
These findings are typical of pilomatricoma, a rather unusual lesion with multiple alternate names (among them: calcifying epithelioma of Malherbe and pilomatrixoma). These benign tumors arising from hair matrix cells are typically seen on the head, face, neck, and upper extremities, most often on children.
This patient’s lesion was typical in size, although they can vary from 3 mm (the smallest I’ve seen) to more than 20 cm. The firm feel, lack of a punctum (which would suggest an epidermal cyst), and bluish discoloration are all typical features.
These lesions generally merit little or no concern. However, in the rare instance when the patient has multiple lesions, the possibility of at least two conditions should be considered: Gardner disease and myotonic seizure.
In terms of treatment, pilomatricoma can be safely left alone; understandably, though, most parents will only be satisfied by excision. It is important to note that, unlike most true cysts, pilomatricomas have a very poorly defined cyst wall with contents that are equally odd—watery and full of tiny white flecks that represent calcified cells. All of this must be totally removed to prevent recurrence. In most cases, defects must be closed in two layers, to minimize “dead” space that might otherwise fill with blood.
One could argue that this child’s lesion should have been removed by a plastic surgeon—but the family had no insurance. Excision was therefore the treatment of choice; the most difficult aspect was persuading the patient to cooperate. (Sometimes, you have to wait years for the child to mature before you attempt it.) The outcome in this case proved to be quite acceptable. Of course, the lesion was sent to pathology, which confirmed the pre-op diagnosis.
The differential includes epidermal cysts (which are almost unknown in prepubertal children), and sweat gland cysts.
TAKE-HOME LEARNING POINTS
- Pilomatricomas are benign cysts that originate from hair matrix cells, usually appearing on the head, neck, face, and upper extremities of children.
- The cysts are typically firm and round and often display a faintly bluish tone (as seen in this case).
- As solitary lesions, they are of little or no significance; when seen in multiples, however, pilomatricomas suggest the possibility of Gardner syndrome or myotonic seizure.
- Excision is the best treatment, though these can be left alone long enough to allow the patient to mature sufficiently to cooperate with the surgical process.
The lesion on this 8-year-old girl’s face was first noted about a year ago. Recently, however, it has started to grow, prompting her parents to consult the child’s primary care provider (PCP). The lesion is completely asymptomatic but nonetheless concerning to the parents and to the PCP, who has no idea what it could be. He therefore refers them to dermatology.
The child is otherwise healthy. There is no family history of chronic or inheritable disease.
EXAMINATION
The lesion is a 2-cm subcutaneous firm round mass located along the superior aspect of the right jawline. The only overlying skin change is a bluish discoloration. No surface punctum is seen. No other lesions are seen or felt on examination of the rest of the head and neck.

What is the diagnosis?
DISCUSSION
These findings are typical of pilomatricoma, a rather unusual lesion with multiple alternate names (among them: calcifying epithelioma of Malherbe and pilomatrixoma). These benign tumors arising from hair matrix cells are typically seen on the head, face, neck, and upper extremities, most often on children.
This patient’s lesion was typical in size, although they can vary from 3 mm (the smallest I’ve seen) to more than 20 cm. The firm feel, lack of a punctum (which would suggest an epidermal cyst), and bluish discoloration are all typical features.
These lesions generally merit little or no concern. However, in the rare instance when the patient has multiple lesions, the possibility of at least two conditions should be considered: Gardner disease and myotonic seizure.
In terms of treatment, pilomatricoma can be safely left alone; understandably, though, most parents will only be satisfied by excision. It is important to note that, unlike most true cysts, pilomatricomas have a very poorly defined cyst wall with contents that are equally odd—watery and full of tiny white flecks that represent calcified cells. All of this must be totally removed to prevent recurrence. In most cases, defects must be closed in two layers, to minimize “dead” space that might otherwise fill with blood.
One could argue that this child’s lesion should have been removed by a plastic surgeon—but the family had no insurance. Excision was therefore the treatment of choice; the most difficult aspect was persuading the patient to cooperate. (Sometimes, you have to wait years for the child to mature before you attempt it.) The outcome in this case proved to be quite acceptable. Of course, the lesion was sent to pathology, which confirmed the pre-op diagnosis.
The differential includes epidermal cysts (which are almost unknown in prepubertal children), and sweat gland cysts.
TAKE-HOME LEARNING POINTS
- Pilomatricomas are benign cysts that originate from hair matrix cells, usually appearing on the head, neck, face, and upper extremities of children.
- The cysts are typically firm and round and often display a faintly bluish tone (as seen in this case).
- As solitary lesions, they are of little or no significance; when seen in multiples, however, pilomatricomas suggest the possibility of Gardner syndrome or myotonic seizure.
- Excision is the best treatment, though these can be left alone long enough to allow the patient to mature sufficiently to cooperate with the surgical process.
The lesion on this 8-year-old girl’s face was first noted about a year ago. Recently, however, it has started to grow, prompting her parents to consult the child’s primary care provider (PCP). The lesion is completely asymptomatic but nonetheless concerning to the parents and to the PCP, who has no idea what it could be. He therefore refers them to dermatology.
The child is otherwise healthy. There is no family history of chronic or inheritable disease.
EXAMINATION
The lesion is a 2-cm subcutaneous firm round mass located along the superior aspect of the right jawline. The only overlying skin change is a bluish discoloration. No surface punctum is seen. No other lesions are seen or felt on examination of the rest of the head and neck.

What is the diagnosis?
DISCUSSION
These findings are typical of pilomatricoma, a rather unusual lesion with multiple alternate names (among them: calcifying epithelioma of Malherbe and pilomatrixoma). These benign tumors arising from hair matrix cells are typically seen on the head, face, neck, and upper extremities, most often on children.
This patient’s lesion was typical in size, although they can vary from 3 mm (the smallest I’ve seen) to more than 20 cm. The firm feel, lack of a punctum (which would suggest an epidermal cyst), and bluish discoloration are all typical features.
These lesions generally merit little or no concern. However, in the rare instance when the patient has multiple lesions, the possibility of at least two conditions should be considered: Gardner disease and myotonic seizure.
In terms of treatment, pilomatricoma can be safely left alone; understandably, though, most parents will only be satisfied by excision. It is important to note that, unlike most true cysts, pilomatricomas have a very poorly defined cyst wall with contents that are equally odd—watery and full of tiny white flecks that represent calcified cells. All of this must be totally removed to prevent recurrence. In most cases, defects must be closed in two layers, to minimize “dead” space that might otherwise fill with blood.
One could argue that this child’s lesion should have been removed by a plastic surgeon—but the family had no insurance. Excision was therefore the treatment of choice; the most difficult aspect was persuading the patient to cooperate. (Sometimes, you have to wait years for the child to mature before you attempt it.) The outcome in this case proved to be quite acceptable. Of course, the lesion was sent to pathology, which confirmed the pre-op diagnosis.
The differential includes epidermal cysts (which are almost unknown in prepubertal children), and sweat gland cysts.
TAKE-HOME LEARNING POINTS
- Pilomatricomas are benign cysts that originate from hair matrix cells, usually appearing on the head, neck, face, and upper extremities of children.
- The cysts are typically firm and round and often display a faintly bluish tone (as seen in this case).
- As solitary lesions, they are of little or no significance; when seen in multiples, however, pilomatricomas suggest the possibility of Gardner syndrome or myotonic seizure.
- Excision is the best treatment, though these can be left alone long enough to allow the patient to mature sufficiently to cooperate with the surgical process.
Aspirin appears comparable to rivaroxaban for VTE
Aspirin seems to be as effective as rivaroxaban for preventing venous thromboembolism (VTE) after total hip or knee replacement, according to research published in NEJM.
Researchers observed a similar incidence of VTE whether patients were randomized to receive aspirin or rivaroxaban prophylaxis starting 5 days after surgery, with all patients receiving rivaroxaban for the first 5 days after surgery.
There was no significant difference between the aspirin and rivaroxaban groups with regard to major bleeding or clinically important bleeding.
“We have always been very concerned about preventing blood clots in patients following major orthopedic surgery,” said study author David Zukor, MD, of Jewish General Hospital in Montreal, Quebec, Canada.
“It is the leading cause of preventable in-hospital death, so we always administer preventive therapy in conjunction with such surgeries. Rivaroxaban is known to be effective, but the great advantage of aspirin is that it is far less expensive, easily available, and has an excellent safety profile.”
Dr Zukor and his colleagues set out to compare aspirin and rivaroxaban in 3424 patients, 1804 who underwent hip replacement and 1620 who underwent knee replacement.
All patients took rivaroxaban (10 mg) for 5 days after surgery before being randomized to receive aspirin (81 mg daily, n=1707) or to continue with rivaroxaban (n=1717). The patients received this prophylaxis for an additional 9 days if they had knee replacement or for 30 days if they had hip replacement.
The researchers followed patients for 90 days, monitoring them for symptomatic VTE and major or clinically relevant nonmajor bleeding.
The rate of VTE was 0.64% (n=11) in the aspirin group and 0.70% (n=12) in the rivaroxaban group (difference, 0.06 percentage points; 95% confidence interval [CI], −0.55 to 0.66; P<0.001 for noninferiority, P=0.84 for superiority).
Major bleeding occurred in 0.47% (n=8) of patients in the aspirin group and in 0.29% (n=5) of those in the rivaroxaban group (difference, 0.18 percentage points; 95% CI, −0.65 to 0.29; P=0.42).
Clinically relevant nonmajor bleeding occurred in 1.29% (n=22) and 0.99% (n=17), respectively (difference, 0.30 percentage points; 95% CI, −1.07 to 0.47; P=0.43).
All bleeding events occurred at the surgical site, and most occurred within 10 days of randomization.
“These results are important,” said study author Susan Kahn, MD, of McGill University in Montreal.
“The protocols for preventing clots following major orthopedic surgery are well established. However, we are always interested in determining whether there are better options for treating our patients. We could well see aspirin emerge as a practical alternative to more expensive anticoagulants.”
Drs Kahn and Zukor both noted the need for additional trials that would include a randomized group of patients prescribed aspirin exclusively so as to test its efficacy directly against rivaroxaban.
Aspirin seems to be as effective as rivaroxaban for preventing venous thromboembolism (VTE) after total hip or knee replacement, according to research published in NEJM.
Researchers observed a similar incidence of VTE whether patients were randomized to receive aspirin or rivaroxaban prophylaxis starting 5 days after surgery, with all patients receiving rivaroxaban for the first 5 days after surgery.
There was no significant difference between the aspirin and rivaroxaban groups with regard to major bleeding or clinically important bleeding.
“We have always been very concerned about preventing blood clots in patients following major orthopedic surgery,” said study author David Zukor, MD, of Jewish General Hospital in Montreal, Quebec, Canada.
“It is the leading cause of preventable in-hospital death, so we always administer preventive therapy in conjunction with such surgeries. Rivaroxaban is known to be effective, but the great advantage of aspirin is that it is far less expensive, easily available, and has an excellent safety profile.”
Dr Zukor and his colleagues set out to compare aspirin and rivaroxaban in 3424 patients, 1804 who underwent hip replacement and 1620 who underwent knee replacement.
All patients took rivaroxaban (10 mg) for 5 days after surgery before being randomized to receive aspirin (81 mg daily, n=1707) or to continue with rivaroxaban (n=1717). The patients received this prophylaxis for an additional 9 days if they had knee replacement or for 30 days if they had hip replacement.
The researchers followed patients for 90 days, monitoring them for symptomatic VTE and major or clinically relevant nonmajor bleeding.
The rate of VTE was 0.64% (n=11) in the aspirin group and 0.70% (n=12) in the rivaroxaban group (difference, 0.06 percentage points; 95% confidence interval [CI], −0.55 to 0.66; P<0.001 for noninferiority, P=0.84 for superiority).
Major bleeding occurred in 0.47% (n=8) of patients in the aspirin group and in 0.29% (n=5) of those in the rivaroxaban group (difference, 0.18 percentage points; 95% CI, −0.65 to 0.29; P=0.42).
Clinically relevant nonmajor bleeding occurred in 1.29% (n=22) and 0.99% (n=17), respectively (difference, 0.30 percentage points; 95% CI, −1.07 to 0.47; P=0.43).
All bleeding events occurred at the surgical site, and most occurred within 10 days of randomization.
“These results are important,” said study author Susan Kahn, MD, of McGill University in Montreal.
“The protocols for preventing clots following major orthopedic surgery are well established. However, we are always interested in determining whether there are better options for treating our patients. We could well see aspirin emerge as a practical alternative to more expensive anticoagulants.”
Drs Kahn and Zukor both noted the need for additional trials that would include a randomized group of patients prescribed aspirin exclusively so as to test its efficacy directly against rivaroxaban.
Aspirin seems to be as effective as rivaroxaban for preventing venous thromboembolism (VTE) after total hip or knee replacement, according to research published in NEJM.
Researchers observed a similar incidence of VTE whether patients were randomized to receive aspirin or rivaroxaban prophylaxis starting 5 days after surgery, with all patients receiving rivaroxaban for the first 5 days after surgery.
There was no significant difference between the aspirin and rivaroxaban groups with regard to major bleeding or clinically important bleeding.
“We have always been very concerned about preventing blood clots in patients following major orthopedic surgery,” said study author David Zukor, MD, of Jewish General Hospital in Montreal, Quebec, Canada.
“It is the leading cause of preventable in-hospital death, so we always administer preventive therapy in conjunction with such surgeries. Rivaroxaban is known to be effective, but the great advantage of aspirin is that it is far less expensive, easily available, and has an excellent safety profile.”
Dr Zukor and his colleagues set out to compare aspirin and rivaroxaban in 3424 patients, 1804 who underwent hip replacement and 1620 who underwent knee replacement.
All patients took rivaroxaban (10 mg) for 5 days after surgery before being randomized to receive aspirin (81 mg daily, n=1707) or to continue with rivaroxaban (n=1717). The patients received this prophylaxis for an additional 9 days if they had knee replacement or for 30 days if they had hip replacement.
The researchers followed patients for 90 days, monitoring them for symptomatic VTE and major or clinically relevant nonmajor bleeding.
The rate of VTE was 0.64% (n=11) in the aspirin group and 0.70% (n=12) in the rivaroxaban group (difference, 0.06 percentage points; 95% confidence interval [CI], −0.55 to 0.66; P<0.001 for noninferiority, P=0.84 for superiority).
Major bleeding occurred in 0.47% (n=8) of patients in the aspirin group and in 0.29% (n=5) of those in the rivaroxaban group (difference, 0.18 percentage points; 95% CI, −0.65 to 0.29; P=0.42).
Clinically relevant nonmajor bleeding occurred in 1.29% (n=22) and 0.99% (n=17), respectively (difference, 0.30 percentage points; 95% CI, −1.07 to 0.47; P=0.43).
All bleeding events occurred at the surgical site, and most occurred within 10 days of randomization.
“These results are important,” said study author Susan Kahn, MD, of McGill University in Montreal.
“The protocols for preventing clots following major orthopedic surgery are well established. However, we are always interested in determining whether there are better options for treating our patients. We could well see aspirin emerge as a practical alternative to more expensive anticoagulants.”
Drs Kahn and Zukor both noted the need for additional trials that would include a randomized group of patients prescribed aspirin exclusively so as to test its efficacy directly against rivaroxaban.
Panic Disorder: Ensuring Prompt Recognition and Treatment
Lacey, 37, is seen by her primary care provider (PCP) as follow-up to a visit she made to the emergency department (ED). She has gone to the ED four times in the past year. Each time, she presents with tachycardia, dyspnea, nausea, numbness in her extremities, and a fear that she is having a heart attack. Despite negative workups at each visit (ECG, cardiac enzymes, complete blood count, toxicology screen, Holter monitoring), Lacey is terrified that the ED doctors are missing something. She is still “rattled” by the chest pain and shortness of breath she experiences. Mild symptoms are persisting, and she is worried that she will have a heart attack and die without the treatment she believes she needs. How do you proceed?
Panic disorder (PD) is characterized by the spontaneous and unexpected occurrence of panic attacks and by at least one month of persistent worry about having another attack or significant maladaptive behaviors related to the attack. Frequency of such attacks can vary from several a day to only a few per year. In a panic attack, an intense fear develops abruptly and peaks within 10 minutes of onset. At least four of the following 13 symptoms must accompany the attack, according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (DSM-5)
- Palpitations, pounding heart, or accelerated heart rate
- Sweating
- Trembling or shaking
- Sensations of shortness of breath or smothering
- Feeling of choking
- Chest pain or discomfort
- Feeling dizzy, unsteady, lightheaded, or faint
- Nausea or abdominal distress
- Derealization (feelings of unreality) or depersonalization (being detached from oneself)
- Fear of losing control or going crazy
- Fear of dying
- Paresthesia (numbness or tingling sensations)
- Chills or hot flushes.1
Lifetime incidence rates of PD are 1% to 3% for the general population.2 A closer look at patients presenting to the ED with chest pain reveals that 17% to 25% meet criteria for PD.3,4 And an estimated 6% of individuals experiencing a panic attack present to their primary care provider.5 Patients with PD tend to use health care resources at a disproportionately high rate.6
An international review of PD research suggests the average age of onset is 32 years.7 Triggers can vary widely, and no single stressor has been identified. The exact cause of PD is unknown, but a convergence of social and biological influences (including involvement of the amygdala) are implicated in its development.6 For individuals who have had a panic attack, 66.5% will have recurrent attacks.7 Lifetime prevalence of panic attacks is 13.2%.7
Differential goes far beyond myocardial infarction. Many medical conditions can mimic PD symptoms: cardiovascular, pulmonary, and neurologic diseases; endocrine diseases (eg, hyperthyroidism); drug intoxication (eg, stimulants [cocaine, amphetamines]); drug withdrawal (eg, benzodiazepines, alcohol, sedative-hypnotics); and ingestion of excessive quantities of caffeine. Common comorbid medical disorders include asthma, coronary artery disease, cancer, thyroid disease, hypertension, ulcer, and migraine headaches.8
When patients present with paniclike symptoms, suspect a possible medical condition when those symptoms include ataxia, altered mental status, or loss of bladder control, or when onset of panic symptoms occur later in life for a patient with no significant psychiatric history.
RULE OUT ORGANIC CAUSES
In addition to obtaining a complete history and doing a physical exam on patients with paniclike symptoms, you’ll also need to ensure that the following are done: a neurologic examination, standard laboratory testing (thyroid function, complete blood cell count, chemistry panel), and possible additional testing (eg, urine toxicology screen and
If organic causes are ruled out, focus on a psychiatric assessment, including
- History of the present illness (onset, symptoms, frequency, predisposing/precipitating factors)
- Psychiatric history
- History of substance use
- Family history of psychiatric disorders (especially anxiety disorders)
- Social history (life events, including those preceding the onset of panic; history of child abuse)
- Medications
- Mental status examination
- Safety (PD is associated with higher risk for suicidal ideation).9
TREATMENT INCLUDES CBT AND MEDICATION
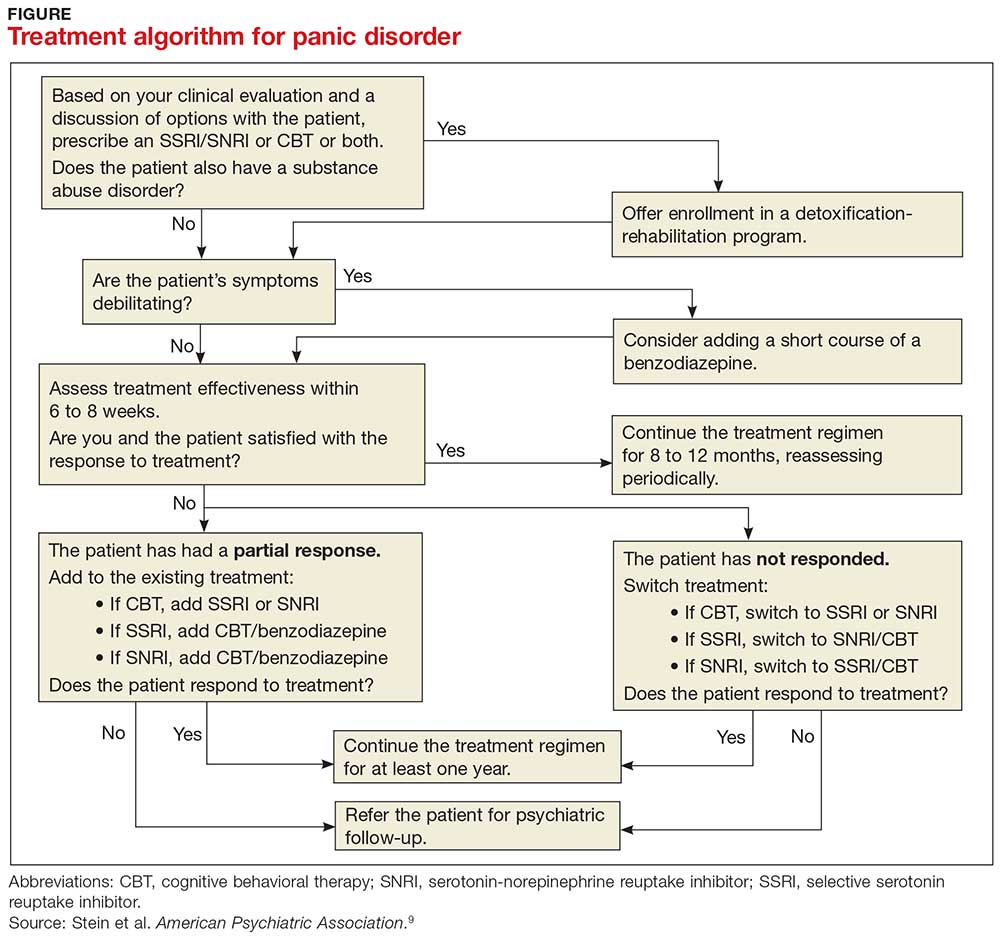
PD is a chronic disease with a variable course, but the long-term prognosis is good. PD is usually treated in an outpatient setting. Consider hospitalization if the patient is suicidal, if the potential for life-threatening withdrawal symptoms is high (as with alcohol or benzodiazepines), or if the symptoms are severely debilitating or attempted outpatient treatment is unsuccessful. Pharmacologic and psychotherapeutic interventions are used for PD (see Figure), although there is not enough evidence to recommend one versus the other or combination therapy versus monotherapy.9
All Lacey’s test results come back negative, and the psychiatric assessment reveals that she meets the DSM-5 criteria for PD. Counting on the strength of their relationship, her PCP talks to her about PD and discusses treatment options, which include counseling, medication, or both. Lacey agrees to a referral for cognitive behavioral therapy (CBT) with a psychologist embedded at her primary care clinic and to begin taking medication. Her PCP starts her on sertraline 25 mg/d.
In CBT, Lacey’s psychologist teaches her about “fight or flight” and explains that it is a normal physiologic response that can lead to panic. Lacey learns to approach her physical symptoms in a different way, and how to breathe in a way that slows her panic reaction.
Consider SSRIs and SNRIs
Firstline medication is a selective serotonin reuptake inhibitor (SSRI) or a serotonin-norepinephrine reuptake inhibitor (SNRI), due to the better tolerability and lower adverse effect profile of these classes compared with the tricyclic antidepressants or monoamine oxidase inhibitors (MAOIs). MAOIs are usually reserved for patients in whom multiple medication trials have failed.
Special considerations. American Psychiatric Association guidelines advise starting with a very low dose of an SSRI or SNRI, such as paroxetine 10 mg/d (although
Keep in mind that the onset of therapeutic effect is between two and four weeks, but that clinical response can take eight to 12 weeks. Continue pharmacotherapy for at least one year. When discontinuing the medication, taper it slowly, and monitor the patient for withdrawal symptoms and recurrence of PD.9
Consider adding a benzodiazepine if symptoms are debilitating.9 Keep in mind, though, that the potential for addiction with these medications is high and they are intended to be used for only four to 12 weeks.8 Onset of action is within the first week, and a scheduled dosing regimen is preferred to giving the medication as needed. The starting dose (eg, clonazepam 0.25 mg bid) may be increased three to five days following initiation.9
Evidence supports the use of CBT for PD
CBT is an evidenced-based treatment for PD.10-13 Up to 75% of patients treated with CBT are panic free within four months.10 Other techniques proven effective are progressive muscle relaxation training, breathing retraining, psychoeducation, exposure, and imagery.14
Treatment with medications and CBT, either combined or used individually, is effective in 80% to 90% of cases.15 CBT has been shown to decrease the likelihood of relapse in the year following treatment.15 Good premorbid functioning and a brief duration of symptoms increase the likelihood of a good prognosis.15
WHEN TO REFER TO A PSYCHIATRIST
Consider referral to a psychiatrist when patients have a comorbid psychiatric condition that complicates the clinical picture (eg, substance abuse disorder), if the diagnosis is uncertain, or if the patient does not respond to one or two adequate trials of medication and psychotherapy. Although psychiatric follow-up is sometimes difficult due to a lack of psychiatrist availability locally, it is a best-practice recommendation.
Ten days after Lacey starts the sertraline 25 mg/d, she calls the PCP to report daily diarrhea. She stopped the sertraline on her own and is asking for another medication. She also expresses her frustration with the severity of the symptoms. She is having three to five panic attacks daily and has been missing many days of work.
On the day of her follow-up PCP appointment, Lacey also sees the psychologist. She reports that she’s been practicing relaxation breathing, tracking her panic attacks, limiting her caffeine intake, and exercising regularly. But the attacks are still occurring.
The PCP switches her to paroxetine 10 mg/d and, due to the severity of the symptoms, prescribes clonazepam 0.5 mg bid. Two weeks later, Lacey reports that she is feeling a little better, has returned to work, and is hopeful that she will be her “normal self again.” The PCP plans to encourage continuation of CBT, titrate the paroxetine to 20 to 40 mg/d based on symptoms, and slowly taper the clonazepam toward discontinuation in the near future.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
2. Kumar S, Oakley-Browne M. Panic disorder. Clin Evid. 2006;15:1438-1452.
3. Yingling KW, Wulsin LR, Arnold LM, et al. Estimated prevalences of panic disorder and depression among consecutive patients seen in an emergency department with acute chest pain. J Gen Intern Med. 1993;8:231-235.
4. Fleet RP, Dupuis G, Marchand A, et al. Panic disorder in emergency department chest pain patients: prevalence, comorbidity, suicidal ideation, and physician recognition. Am J Med. 1996;101:371-380.
5. Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272:1749-1756.
6. Taylor CB. Panic disorder. BMJ. 2006;332:951-955.
7. de Jonge P, Roest AM, Lim CC, et al. Cross-national epidemiology of panic disorder and panic attacks in the world mental health surveys. Depress Anxiety. 2016;33: 1155-1177.
8. Sadock BJ, Sadock VA, Ruiz P. Panic disorder. In: Kaplan & Sadock’s Synopsis of Psychiatry. 11th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2015:392-397.
9. Stein MB, Goin MK, Pollack MH, et al. ractice Guideline for the Treatment of Patients with Panic Disorder. 2nd ed. Washington, DC: American Psychiatric Association; 2010. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Accessed February 14, 2018.
10. Westen D, Morrison K. A multidimensional meta-analysis of treatments for depression, panic, and generalized anxiety disorder: an empirical examination of the status of empirically supported therapies. J Consult Clin Psychol. 2001;69:875-899.
11. Gould RA, Otto MW, Pollack MH. A meta-analysis of treatment outcome for panic disorder. www.ncbi.nlm.nih.gov/books/NBK66380/. Accessed February 14, 2018.
12. Clum GA, Clum GA, Surls R. A meta-analysis of treatments for panic disorder. J Consult Clin Psychol. 1993; 61:317-326.
13. Shear MK, Houck P, Greeno C, et al. Emotion-focused psychotherapy for patients with panic disorder. Am J Psychiatry. 2001;158:1993-1998.
14. Stewart RE, Chambless DL. Cognitive-behavioral therapy for adult anxiety disorders in clinical practice: a meta-analysis of effectiveness studies. J Consult Clin Psychol. 2009;77:595-606.
15. Craske M. Psychotherapy for panic disorder in adults. Up to Date. 2017. www.uptodate.com/contents/psychotherapy-for-panic-disorder-with-or-without-agoraphobia-in-adults. Accessed February 14, 2018.
Lacey, 37, is seen by her primary care provider (PCP) as follow-up to a visit she made to the emergency department (ED). She has gone to the ED four times in the past year. Each time, she presents with tachycardia, dyspnea, nausea, numbness in her extremities, and a fear that she is having a heart attack. Despite negative workups at each visit (ECG, cardiac enzymes, complete blood count, toxicology screen, Holter monitoring), Lacey is terrified that the ED doctors are missing something. She is still “rattled” by the chest pain and shortness of breath she experiences. Mild symptoms are persisting, and she is worried that she will have a heart attack and die without the treatment she believes she needs. How do you proceed?
Panic disorder (PD) is characterized by the spontaneous and unexpected occurrence of panic attacks and by at least one month of persistent worry about having another attack or significant maladaptive behaviors related to the attack. Frequency of such attacks can vary from several a day to only a few per year. In a panic attack, an intense fear develops abruptly and peaks within 10 minutes of onset. At least four of the following 13 symptoms must accompany the attack, according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (DSM-5)
- Palpitations, pounding heart, or accelerated heart rate
- Sweating
- Trembling or shaking
- Sensations of shortness of breath or smothering
- Feeling of choking
- Chest pain or discomfort
- Feeling dizzy, unsteady, lightheaded, or faint
- Nausea or abdominal distress
- Derealization (feelings of unreality) or depersonalization (being detached from oneself)
- Fear of losing control or going crazy
- Fear of dying
- Paresthesia (numbness or tingling sensations)
- Chills or hot flushes.1
Lifetime incidence rates of PD are 1% to 3% for the general population.2 A closer look at patients presenting to the ED with chest pain reveals that 17% to 25% meet criteria for PD.3,4 And an estimated 6% of individuals experiencing a panic attack present to their primary care provider.5 Patients with PD tend to use health care resources at a disproportionately high rate.6
An international review of PD research suggests the average age of onset is 32 years.7 Triggers can vary widely, and no single stressor has been identified. The exact cause of PD is unknown, but a convergence of social and biological influences (including involvement of the amygdala) are implicated in its development.6 For individuals who have had a panic attack, 66.5% will have recurrent attacks.7 Lifetime prevalence of panic attacks is 13.2%.7
Differential goes far beyond myocardial infarction. Many medical conditions can mimic PD symptoms: cardiovascular, pulmonary, and neurologic diseases; endocrine diseases (eg, hyperthyroidism); drug intoxication (eg, stimulants [cocaine, amphetamines]); drug withdrawal (eg, benzodiazepines, alcohol, sedative-hypnotics); and ingestion of excessive quantities of caffeine. Common comorbid medical disorders include asthma, coronary artery disease, cancer, thyroid disease, hypertension, ulcer, and migraine headaches.8
When patients present with paniclike symptoms, suspect a possible medical condition when those symptoms include ataxia, altered mental status, or loss of bladder control, or when onset of panic symptoms occur later in life for a patient with no significant psychiatric history.
RULE OUT ORGANIC CAUSES
In addition to obtaining a complete history and doing a physical exam on patients with paniclike symptoms, you’ll also need to ensure that the following are done: a neurologic examination, standard laboratory testing (thyroid function, complete blood cell count, chemistry panel), and possible additional testing (eg, urine toxicology screen and
If organic causes are ruled out, focus on a psychiatric assessment, including
- History of the present illness (onset, symptoms, frequency, predisposing/precipitating factors)
- Psychiatric history
- History of substance use
- Family history of psychiatric disorders (especially anxiety disorders)
- Social history (life events, including those preceding the onset of panic; history of child abuse)
- Medications
- Mental status examination
- Safety (PD is associated with higher risk for suicidal ideation).9
TREATMENT INCLUDES CBT AND MEDICATION
PD is a chronic disease with a variable course, but the long-term prognosis is good. PD is usually treated in an outpatient setting. Consider hospitalization if the patient is suicidal, if the potential for life-threatening withdrawal symptoms is high (as with alcohol or benzodiazepines), or if the symptoms are severely debilitating or attempted outpatient treatment is unsuccessful. Pharmacologic and psychotherapeutic interventions are used for PD (see Figure), although there is not enough evidence to recommend one versus the other or combination therapy versus monotherapy.9

All Lacey’s test results come back negative, and the psychiatric assessment reveals that she meets the DSM-5 criteria for PD. Counting on the strength of their relationship, her PCP talks to her about PD and discusses treatment options, which include counseling, medication, or both. Lacey agrees to a referral for cognitive behavioral therapy (CBT) with a psychologist embedded at her primary care clinic and to begin taking medication. Her PCP starts her on sertraline 25 mg/d.
In CBT, Lacey’s psychologist teaches her about “fight or flight” and explains that it is a normal physiologic response that can lead to panic. Lacey learns to approach her physical symptoms in a different way, and how to breathe in a way that slows her panic reaction.
Consider SSRIs and SNRIs
Firstline medication is a selective serotonin reuptake inhibitor (SSRI) or a serotonin-norepinephrine reuptake inhibitor (SNRI), due to the better tolerability and lower adverse effect profile of these classes compared with the tricyclic antidepressants or monoamine oxidase inhibitors (MAOIs). MAOIs are usually reserved for patients in whom multiple medication trials have failed.
Special considerations. American Psychiatric Association guidelines advise starting with a very low dose of an SSRI or SNRI, such as paroxetine 10 mg/d (although
Keep in mind that the onset of therapeutic effect is between two and four weeks, but that clinical response can take eight to 12 weeks. Continue pharmacotherapy for at least one year. When discontinuing the medication, taper it slowly, and monitor the patient for withdrawal symptoms and recurrence of PD.9
Consider adding a benzodiazepine if symptoms are debilitating.9 Keep in mind, though, that the potential for addiction with these medications is high and they are intended to be used for only four to 12 weeks.8 Onset of action is within the first week, and a scheduled dosing regimen is preferred to giving the medication as needed. The starting dose (eg, clonazepam 0.25 mg bid) may be increased three to five days following initiation.9
Evidence supports the use of CBT for PD
CBT is an evidenced-based treatment for PD.10-13 Up to 75% of patients treated with CBT are panic free within four months.10 Other techniques proven effective are progressive muscle relaxation training, breathing retraining, psychoeducation, exposure, and imagery.14
Treatment with medications and CBT, either combined or used individually, is effective in 80% to 90% of cases.15 CBT has been shown to decrease the likelihood of relapse in the year following treatment.15 Good premorbid functioning and a brief duration of symptoms increase the likelihood of a good prognosis.15
WHEN TO REFER TO A PSYCHIATRIST
Consider referral to a psychiatrist when patients have a comorbid psychiatric condition that complicates the clinical picture (eg, substance abuse disorder), if the diagnosis is uncertain, or if the patient does not respond to one or two adequate trials of medication and psychotherapy. Although psychiatric follow-up is sometimes difficult due to a lack of psychiatrist availability locally, it is a best-practice recommendation.
Ten days after Lacey starts the sertraline 25 mg/d, she calls the PCP to report daily diarrhea. She stopped the sertraline on her own and is asking for another medication. She also expresses her frustration with the severity of the symptoms. She is having three to five panic attacks daily and has been missing many days of work.
On the day of her follow-up PCP appointment, Lacey also sees the psychologist. She reports that she’s been practicing relaxation breathing, tracking her panic attacks, limiting her caffeine intake, and exercising regularly. But the attacks are still occurring.
The PCP switches her to paroxetine 10 mg/d and, due to the severity of the symptoms, prescribes clonazepam 0.5 mg bid. Two weeks later, Lacey reports that she is feeling a little better, has returned to work, and is hopeful that she will be her “normal self again.” The PCP plans to encourage continuation of CBT, titrate the paroxetine to 20 to 40 mg/d based on symptoms, and slowly taper the clonazepam toward discontinuation in the near future.
Lacey, 37, is seen by her primary care provider (PCP) as follow-up to a visit she made to the emergency department (ED). She has gone to the ED four times in the past year. Each time, she presents with tachycardia, dyspnea, nausea, numbness in her extremities, and a fear that she is having a heart attack. Despite negative workups at each visit (ECG, cardiac enzymes, complete blood count, toxicology screen, Holter monitoring), Lacey is terrified that the ED doctors are missing something. She is still “rattled” by the chest pain and shortness of breath she experiences. Mild symptoms are persisting, and she is worried that she will have a heart attack and die without the treatment she believes she needs. How do you proceed?
Panic disorder (PD) is characterized by the spontaneous and unexpected occurrence of panic attacks and by at least one month of persistent worry about having another attack or significant maladaptive behaviors related to the attack. Frequency of such attacks can vary from several a day to only a few per year. In a panic attack, an intense fear develops abruptly and peaks within 10 minutes of onset. At least four of the following 13 symptoms must accompany the attack, according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (DSM-5)
- Palpitations, pounding heart, or accelerated heart rate
- Sweating
- Trembling or shaking
- Sensations of shortness of breath or smothering
- Feeling of choking
- Chest pain or discomfort
- Feeling dizzy, unsteady, lightheaded, or faint
- Nausea or abdominal distress
- Derealization (feelings of unreality) or depersonalization (being detached from oneself)
- Fear of losing control or going crazy
- Fear of dying
- Paresthesia (numbness or tingling sensations)
- Chills or hot flushes.1
Lifetime incidence rates of PD are 1% to 3% for the general population.2 A closer look at patients presenting to the ED with chest pain reveals that 17% to 25% meet criteria for PD.3,4 And an estimated 6% of individuals experiencing a panic attack present to their primary care provider.5 Patients with PD tend to use health care resources at a disproportionately high rate.6
An international review of PD research suggests the average age of onset is 32 years.7 Triggers can vary widely, and no single stressor has been identified. The exact cause of PD is unknown, but a convergence of social and biological influences (including involvement of the amygdala) are implicated in its development.6 For individuals who have had a panic attack, 66.5% will have recurrent attacks.7 Lifetime prevalence of panic attacks is 13.2%.7
Differential goes far beyond myocardial infarction. Many medical conditions can mimic PD symptoms: cardiovascular, pulmonary, and neurologic diseases; endocrine diseases (eg, hyperthyroidism); drug intoxication (eg, stimulants [cocaine, amphetamines]); drug withdrawal (eg, benzodiazepines, alcohol, sedative-hypnotics); and ingestion of excessive quantities of caffeine. Common comorbid medical disorders include asthma, coronary artery disease, cancer, thyroid disease, hypertension, ulcer, and migraine headaches.8
When patients present with paniclike symptoms, suspect a possible medical condition when those symptoms include ataxia, altered mental status, or loss of bladder control, or when onset of panic symptoms occur later in life for a patient with no significant psychiatric history.
RULE OUT ORGANIC CAUSES
In addition to obtaining a complete history and doing a physical exam on patients with paniclike symptoms, you’ll also need to ensure that the following are done: a neurologic examination, standard laboratory testing (thyroid function, complete blood cell count, chemistry panel), and possible additional testing (eg, urine toxicology screen and
If organic causes are ruled out, focus on a psychiatric assessment, including
- History of the present illness (onset, symptoms, frequency, predisposing/precipitating factors)
- Psychiatric history
- History of substance use
- Family history of psychiatric disorders (especially anxiety disorders)
- Social history (life events, including those preceding the onset of panic; history of child abuse)
- Medications
- Mental status examination
- Safety (PD is associated with higher risk for suicidal ideation).9
TREATMENT INCLUDES CBT AND MEDICATION
PD is a chronic disease with a variable course, but the long-term prognosis is good. PD is usually treated in an outpatient setting. Consider hospitalization if the patient is suicidal, if the potential for life-threatening withdrawal symptoms is high (as with alcohol or benzodiazepines), or if the symptoms are severely debilitating or attempted outpatient treatment is unsuccessful. Pharmacologic and psychotherapeutic interventions are used for PD (see Figure), although there is not enough evidence to recommend one versus the other or combination therapy versus monotherapy.9

All Lacey’s test results come back negative, and the psychiatric assessment reveals that she meets the DSM-5 criteria for PD. Counting on the strength of their relationship, her PCP talks to her about PD and discusses treatment options, which include counseling, medication, or both. Lacey agrees to a referral for cognitive behavioral therapy (CBT) with a psychologist embedded at her primary care clinic and to begin taking medication. Her PCP starts her on sertraline 25 mg/d.
In CBT, Lacey’s psychologist teaches her about “fight or flight” and explains that it is a normal physiologic response that can lead to panic. Lacey learns to approach her physical symptoms in a different way, and how to breathe in a way that slows her panic reaction.
Consider SSRIs and SNRIs
Firstline medication is a selective serotonin reuptake inhibitor (SSRI) or a serotonin-norepinephrine reuptake inhibitor (SNRI), due to the better tolerability and lower adverse effect profile of these classes compared with the tricyclic antidepressants or monoamine oxidase inhibitors (MAOIs). MAOIs are usually reserved for patients in whom multiple medication trials have failed.
Special considerations. American Psychiatric Association guidelines advise starting with a very low dose of an SSRI or SNRI, such as paroxetine 10 mg/d (although
Keep in mind that the onset of therapeutic effect is between two and four weeks, but that clinical response can take eight to 12 weeks. Continue pharmacotherapy for at least one year. When discontinuing the medication, taper it slowly, and monitor the patient for withdrawal symptoms and recurrence of PD.9
Consider adding a benzodiazepine if symptoms are debilitating.9 Keep in mind, though, that the potential for addiction with these medications is high and they are intended to be used for only four to 12 weeks.8 Onset of action is within the first week, and a scheduled dosing regimen is preferred to giving the medication as needed. The starting dose (eg, clonazepam 0.25 mg bid) may be increased three to five days following initiation.9
Evidence supports the use of CBT for PD
CBT is an evidenced-based treatment for PD.10-13 Up to 75% of patients treated with CBT are panic free within four months.10 Other techniques proven effective are progressive muscle relaxation training, breathing retraining, psychoeducation, exposure, and imagery.14
Treatment with medications and CBT, either combined or used individually, is effective in 80% to 90% of cases.15 CBT has been shown to decrease the likelihood of relapse in the year following treatment.15 Good premorbid functioning and a brief duration of symptoms increase the likelihood of a good prognosis.15
WHEN TO REFER TO A PSYCHIATRIST
Consider referral to a psychiatrist when patients have a comorbid psychiatric condition that complicates the clinical picture (eg, substance abuse disorder), if the diagnosis is uncertain, or if the patient does not respond to one or two adequate trials of medication and psychotherapy. Although psychiatric follow-up is sometimes difficult due to a lack of psychiatrist availability locally, it is a best-practice recommendation.
Ten days after Lacey starts the sertraline 25 mg/d, she calls the PCP to report daily diarrhea. She stopped the sertraline on her own and is asking for another medication. She also expresses her frustration with the severity of the symptoms. She is having three to five panic attacks daily and has been missing many days of work.
On the day of her follow-up PCP appointment, Lacey also sees the psychologist. She reports that she’s been practicing relaxation breathing, tracking her panic attacks, limiting her caffeine intake, and exercising regularly. But the attacks are still occurring.
The PCP switches her to paroxetine 10 mg/d and, due to the severity of the symptoms, prescribes clonazepam 0.5 mg bid. Two weeks later, Lacey reports that she is feeling a little better, has returned to work, and is hopeful that she will be her “normal self again.” The PCP plans to encourage continuation of CBT, titrate the paroxetine to 20 to 40 mg/d based on symptoms, and slowly taper the clonazepam toward discontinuation in the near future.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
2. Kumar S, Oakley-Browne M. Panic disorder. Clin Evid. 2006;15:1438-1452.
3. Yingling KW, Wulsin LR, Arnold LM, et al. Estimated prevalences of panic disorder and depression among consecutive patients seen in an emergency department with acute chest pain. J Gen Intern Med. 1993;8:231-235.
4. Fleet RP, Dupuis G, Marchand A, et al. Panic disorder in emergency department chest pain patients: prevalence, comorbidity, suicidal ideation, and physician recognition. Am J Med. 1996;101:371-380.
5. Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272:1749-1756.
6. Taylor CB. Panic disorder. BMJ. 2006;332:951-955.
7. de Jonge P, Roest AM, Lim CC, et al. Cross-national epidemiology of panic disorder and panic attacks in the world mental health surveys. Depress Anxiety. 2016;33: 1155-1177.
8. Sadock BJ, Sadock VA, Ruiz P. Panic disorder. In: Kaplan & Sadock’s Synopsis of Psychiatry. 11th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2015:392-397.
9. Stein MB, Goin MK, Pollack MH, et al. ractice Guideline for the Treatment of Patients with Panic Disorder. 2nd ed. Washington, DC: American Psychiatric Association; 2010. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Accessed February 14, 2018.
10. Westen D, Morrison K. A multidimensional meta-analysis of treatments for depression, panic, and generalized anxiety disorder: an empirical examination of the status of empirically supported therapies. J Consult Clin Psychol. 2001;69:875-899.
11. Gould RA, Otto MW, Pollack MH. A meta-analysis of treatment outcome for panic disorder. www.ncbi.nlm.nih.gov/books/NBK66380/. Accessed February 14, 2018.
12. Clum GA, Clum GA, Surls R. A meta-analysis of treatments for panic disorder. J Consult Clin Psychol. 1993; 61:317-326.
13. Shear MK, Houck P, Greeno C, et al. Emotion-focused psychotherapy for patients with panic disorder. Am J Psychiatry. 2001;158:1993-1998.
14. Stewart RE, Chambless DL. Cognitive-behavioral therapy for adult anxiety disorders in clinical practice: a meta-analysis of effectiveness studies. J Consult Clin Psychol. 2009;77:595-606.
15. Craske M. Psychotherapy for panic disorder in adults. Up to Date. 2017. www.uptodate.com/contents/psychotherapy-for-panic-disorder-with-or-without-agoraphobia-in-adults. Accessed February 14, 2018.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
2. Kumar S, Oakley-Browne M. Panic disorder. Clin Evid. 2006;15:1438-1452.
3. Yingling KW, Wulsin LR, Arnold LM, et al. Estimated prevalences of panic disorder and depression among consecutive patients seen in an emergency department with acute chest pain. J Gen Intern Med. 1993;8:231-235.
4. Fleet RP, Dupuis G, Marchand A, et al. Panic disorder in emergency department chest pain patients: prevalence, comorbidity, suicidal ideation, and physician recognition. Am J Med. 1996;101:371-380.
5. Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272:1749-1756.
6. Taylor CB. Panic disorder. BMJ. 2006;332:951-955.
7. de Jonge P, Roest AM, Lim CC, et al. Cross-national epidemiology of panic disorder and panic attacks in the world mental health surveys. Depress Anxiety. 2016;33: 1155-1177.
8. Sadock BJ, Sadock VA, Ruiz P. Panic disorder. In: Kaplan & Sadock’s Synopsis of Psychiatry. 11th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2015:392-397.
9. Stein MB, Goin MK, Pollack MH, et al. ractice Guideline for the Treatment of Patients with Panic Disorder. 2nd ed. Washington, DC: American Psychiatric Association; 2010. http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Accessed February 14, 2018.
10. Westen D, Morrison K. A multidimensional meta-analysis of treatments for depression, panic, and generalized anxiety disorder: an empirical examination of the status of empirically supported therapies. J Consult Clin Psychol. 2001;69:875-899.
11. Gould RA, Otto MW, Pollack MH. A meta-analysis of treatment outcome for panic disorder. www.ncbi.nlm.nih.gov/books/NBK66380/. Accessed February 14, 2018.
12. Clum GA, Clum GA, Surls R. A meta-analysis of treatments for panic disorder. J Consult Clin Psychol. 1993; 61:317-326.
13. Shear MK, Houck P, Greeno C, et al. Emotion-focused psychotherapy for patients with panic disorder. Am J Psychiatry. 2001;158:1993-1998.
14. Stewart RE, Chambless DL. Cognitive-behavioral therapy for adult anxiety disorders in clinical practice: a meta-analysis of effectiveness studies. J Consult Clin Psychol. 2009;77:595-606.
15. Craske M. Psychotherapy for panic disorder in adults. Up to Date. 2017. www.uptodate.com/contents/psychotherapy-for-panic-disorder-with-or-without-agoraphobia-in-adults. Accessed February 14, 2018.
From the Washington Office: Gratifying success for ACS legislative advocacy efforts
On the morning of February 9, 2018, President Trump signed into law the Bipartisan Budget Act of 2018. The law included legislative priorities that were championed by the ACS and for which staff of the DC office and engaged Fellows of the College have advocated, in some cases, for a number of years.
ACS worked particularly hard in the week leading up to the passage of the Bipartisan Budget Act of 2018 with the goal of ensuring that certain items were included, and certain other items were excluded, in the Continuing Resolution (CR) under consideration by Congress to continue funding the government. The original version of the CR considered and debated by the House of Representatives early in the week included both positive and negative items. The ACS was successful in its efforts to get the Senate to consider a much-improved version of the CR – eliminating a major impediment in the House version. Ultimately, it was the Senate version that was signed into law by President Trump.
The provisions in the Bipartisan Budget Act of 2018 include:
• Flexibility for the Merit-based Incentive Payment System (MIPS) related to how much weight will be ascribed to the Cost component in an individual physician’s MIPS score as well as flexibility in setting the level at which physicians will either receive a positive or negative payment update. Without this flexibility, there was significant concern that Fellows would have significantly greater challenges in avoiding a cut under the MIPS. This language, and the effort to include it, was spearheaded and long championed by the ACS including the drafting of model legislation remedying the problem which was then provided to the leadership and staff of committees of jurisdiction.
• Easing meaningful use (MU) requirements by removing an outdated requirement directing the Secretary of Health and Human Services (HHS) to continue to make meaningful use standards increasingly stringent over time. The ACS has long advocated against increasingly stringent MU requirements that do not lead to improvements in patient care, and feels they are unnecessary and unfair to both patients and providers. Further, easing MU requirements has long been supported by ACS Fellows.
• An additional 4 years of funding for the Children’s Health Insurance Program (CHIP), bringing the reauthorization period to a total of 10 years. ACS has consistently advocated and aggressively pursued reauthorization of CHIP every time reauthorization was necessary. During the most recent negotiations, following expiration of funding in September 2017, the ACS advocated for the longest possible period of reauthorization of funds.
• Full repeal of the Independent Payment Advisory Board (IPAB), included as part of the Affordable Care Act. Though members of the board were never appointed, the ACS has fought to eliminate this advisory board of unelected bureaucrats who had the power to cut physician payment since 2010.
• Additional funding to address both the opioid epidemic and to support the work of the National Institutes of Health (NIH). The ACS has long supported funding to fight cancer and has been proactive in its response to the national crisis of opioid abuse and misuse.
• Lastly, as mentioned above, the ACS strongly opposed language in the version of the bill passed by the House that would have allowed the use of the “Misvalued Codes” as part of the “pay-for” or offset for the legislation. The ACS anticipated that this language would have unfairly resulted in significant cuts to surgeons and we were pleased that this language was not included in the version ultimately agreed to and signed into law by President Trump. We sincerely hope that this ends the use of this flawed policy.
To have this many policy priorities enacted through one legislative package is a rare occurrence for any organization and accordingly is most gratifying. The emails and phone calls delivered by Fellows during the week of February 5, in combination with the work of staff here in Washington DC, no doubt played a significant role in securing these priorities. However, the work is not done and the ACS will continue to fight for improvements to issues facing surgeons and surgical patients.
We urge all Fellows to continue participating in these efforts.
Until next month ….
On the morning of February 9, 2018, President Trump signed into law the Bipartisan Budget Act of 2018. The law included legislative priorities that were championed by the ACS and for which staff of the DC office and engaged Fellows of the College have advocated, in some cases, for a number of years.
ACS worked particularly hard in the week leading up to the passage of the Bipartisan Budget Act of 2018 with the goal of ensuring that certain items were included, and certain other items were excluded, in the Continuing Resolution (CR) under consideration by Congress to continue funding the government. The original version of the CR considered and debated by the House of Representatives early in the week included both positive and negative items. The ACS was successful in its efforts to get the Senate to consider a much-improved version of the CR – eliminating a major impediment in the House version. Ultimately, it was the Senate version that was signed into law by President Trump.
The provisions in the Bipartisan Budget Act of 2018 include:
• Flexibility for the Merit-based Incentive Payment System (MIPS) related to how much weight will be ascribed to the Cost component in an individual physician’s MIPS score as well as flexibility in setting the level at which physicians will either receive a positive or negative payment update. Without this flexibility, there was significant concern that Fellows would have significantly greater challenges in avoiding a cut under the MIPS. This language, and the effort to include it, was spearheaded and long championed by the ACS including the drafting of model legislation remedying the problem which was then provided to the leadership and staff of committees of jurisdiction.
• Easing meaningful use (MU) requirements by removing an outdated requirement directing the Secretary of Health and Human Services (HHS) to continue to make meaningful use standards increasingly stringent over time. The ACS has long advocated against increasingly stringent MU requirements that do not lead to improvements in patient care, and feels they are unnecessary and unfair to both patients and providers. Further, easing MU requirements has long been supported by ACS Fellows.
• An additional 4 years of funding for the Children’s Health Insurance Program (CHIP), bringing the reauthorization period to a total of 10 years. ACS has consistently advocated and aggressively pursued reauthorization of CHIP every time reauthorization was necessary. During the most recent negotiations, following expiration of funding in September 2017, the ACS advocated for the longest possible period of reauthorization of funds.
• Full repeal of the Independent Payment Advisory Board (IPAB), included as part of the Affordable Care Act. Though members of the board were never appointed, the ACS has fought to eliminate this advisory board of unelected bureaucrats who had the power to cut physician payment since 2010.
• Additional funding to address both the opioid epidemic and to support the work of the National Institutes of Health (NIH). The ACS has long supported funding to fight cancer and has been proactive in its response to the national crisis of opioid abuse and misuse.
• Lastly, as mentioned above, the ACS strongly opposed language in the version of the bill passed by the House that would have allowed the use of the “Misvalued Codes” as part of the “pay-for” or offset for the legislation. The ACS anticipated that this language would have unfairly resulted in significant cuts to surgeons and we were pleased that this language was not included in the version ultimately agreed to and signed into law by President Trump. We sincerely hope that this ends the use of this flawed policy.
To have this many policy priorities enacted through one legislative package is a rare occurrence for any organization and accordingly is most gratifying. The emails and phone calls delivered by Fellows during the week of February 5, in combination with the work of staff here in Washington DC, no doubt played a significant role in securing these priorities. However, the work is not done and the ACS will continue to fight for improvements to issues facing surgeons and surgical patients.
We urge all Fellows to continue participating in these efforts.
Until next month ….
On the morning of February 9, 2018, President Trump signed into law the Bipartisan Budget Act of 2018. The law included legislative priorities that were championed by the ACS and for which staff of the DC office and engaged Fellows of the College have advocated, in some cases, for a number of years.
ACS worked particularly hard in the week leading up to the passage of the Bipartisan Budget Act of 2018 with the goal of ensuring that certain items were included, and certain other items were excluded, in the Continuing Resolution (CR) under consideration by Congress to continue funding the government. The original version of the CR considered and debated by the House of Representatives early in the week included both positive and negative items. The ACS was successful in its efforts to get the Senate to consider a much-improved version of the CR – eliminating a major impediment in the House version. Ultimately, it was the Senate version that was signed into law by President Trump.
The provisions in the Bipartisan Budget Act of 2018 include:
• Flexibility for the Merit-based Incentive Payment System (MIPS) related to how much weight will be ascribed to the Cost component in an individual physician’s MIPS score as well as flexibility in setting the level at which physicians will either receive a positive or negative payment update. Without this flexibility, there was significant concern that Fellows would have significantly greater challenges in avoiding a cut under the MIPS. This language, and the effort to include it, was spearheaded and long championed by the ACS including the drafting of model legislation remedying the problem which was then provided to the leadership and staff of committees of jurisdiction.
• Easing meaningful use (MU) requirements by removing an outdated requirement directing the Secretary of Health and Human Services (HHS) to continue to make meaningful use standards increasingly stringent over time. The ACS has long advocated against increasingly stringent MU requirements that do not lead to improvements in patient care, and feels they are unnecessary and unfair to both patients and providers. Further, easing MU requirements has long been supported by ACS Fellows.
• An additional 4 years of funding for the Children’s Health Insurance Program (CHIP), bringing the reauthorization period to a total of 10 years. ACS has consistently advocated and aggressively pursued reauthorization of CHIP every time reauthorization was necessary. During the most recent negotiations, following expiration of funding in September 2017, the ACS advocated for the longest possible period of reauthorization of funds.
• Full repeal of the Independent Payment Advisory Board (IPAB), included as part of the Affordable Care Act. Though members of the board were never appointed, the ACS has fought to eliminate this advisory board of unelected bureaucrats who had the power to cut physician payment since 2010.
• Additional funding to address both the opioid epidemic and to support the work of the National Institutes of Health (NIH). The ACS has long supported funding to fight cancer and has been proactive in its response to the national crisis of opioid abuse and misuse.
• Lastly, as mentioned above, the ACS strongly opposed language in the version of the bill passed by the House that would have allowed the use of the “Misvalued Codes” as part of the “pay-for” or offset for the legislation. The ACS anticipated that this language would have unfairly resulted in significant cuts to surgeons and we were pleased that this language was not included in the version ultimately agreed to and signed into law by President Trump. We sincerely hope that this ends the use of this flawed policy.
To have this many policy priorities enacted through one legislative package is a rare occurrence for any organization and accordingly is most gratifying. The emails and phone calls delivered by Fellows during the week of February 5, in combination with the work of staff here in Washington DC, no doubt played a significant role in securing these priorities. However, the work is not done and the ACS will continue to fight for improvements to issues facing surgeons and surgical patients.
We urge all Fellows to continue participating in these efforts.
Until next month ….
From the Editors: Unexpected benefits
I am a member of several surgical societies, and more than once I’ve extolled the importance of going to meetings as part of my personal definition of a “good” surgeon.
While I mostly enjoy meetings nowadays, it hasn’t always been easy. My first experiences at surgical society meetings were painful. I was 32 years old, just out of training, and didn’t know anyone at the meetings. Receptions were the worst. My only friend sometimes was either my host or my wife. I was surrounded by these old guys, most of whom were famous.
Among the highlights of my life was being invited to join the Western Surgical Association. The year I was elected, J. David Richardson was the President. He gave such a rousing speech that at the President’s Dinner I rallied the courage to speak to the great man. When I called him “Dr. Richardson,” he waved that off and insisted I call him “Dave.” Little did I know that Dave would become one of my personal heroes and play an important part in the path that led to my being in a position to write a column such as this.
I never thought that being at a meeting might save my own life. As a devoted member of the Western Surgical Association, I breezed into Phoenix last year for the annual meeting. But I wasn’t feeling so well. Typical of a surgeon, I’d been denying that the symptoms in my abdomen and back were anything other than arthritis. Also, like most surgeons, I’d been pushing the accelerator pedal of life to the floorboard for some months. I arrived at the hotel at one in the morning with a stomach ache that clearly was becoming serious.
I lay in bed wondering what to do. Surgeons don’t get sick! Calling 911 seemed like an invitation to a 6-hour ED experience during which I would be demoted from surgeon to “the patient in Room 9” in a town where I didn’t know many people. As a surgeon, I knew that belly pain of this magnitude was not something I wanted to entrust to a nonsurgeon who was just trying to get through another long shift. This was personal.
Here’s where being a member of a surgical society became more than an academic exercise. I was in a hotel with many of the finest surgeons in the world – and I knew many of them! Among the attendees was my very good friend Margo Shoup, a first-rate cancer surgeon from Chicago. I knew she had come in that day because we were to have dinner together the next night. Rather than call her at 0300, I waited until 6. She came right over and examined me. We decided on a plan. As is always the case at a surgical meeting, one of the surgeon members is the local arrangement person. In this instance, it was none other than James Madura, MD, Chief of MIS GI surgery at Mayo Clinic, Scottsdale. We called James and when my abdomen decided to go nuclear with pain, he drove me to his hospital in his own car and got me in their ED.
The rest of the story is pretty mundane. My diagnosis turned out to be temporarily serious but benign. It was serious enough that without good people treating me, I could have done poorly. James and his fine team took superb care of me. Almost all of us go into surgery with the intent to help others. We take that extraseriously when it’s one of “us.” I can imagine the increased pressure on James when he was treating a colleague and the entire rest of the Western Surgical Association knew it. He never even broke a sweat. I am so glad he was my surgeon. His chief resident Ryan Day, MD, spent extra time with me. I saw a bunch of other residents as well. They reminded me of my fellow residents many years ago – eager, bright, and hyperdedicated. Several members of the Western came by the hospital to see me and even reviewed my images and diagnosis with me. I felt like I had a whole family of physicians who were both my friends and my support system a long way from home. I cannot thank James, Margo, and the rest enough for all they did. It’s great to feel back to normal again as well.
So, I would suggest you join and participate in the surgical societies that you think are a good fit for you. Develop those important professional relationships. Such activity will do your patients a world of good – and just might save your own life.
I am a member of several surgical societies, and more than once I’ve extolled the importance of going to meetings as part of my personal definition of a “good” surgeon.
While I mostly enjoy meetings nowadays, it hasn’t always been easy. My first experiences at surgical society meetings were painful. I was 32 years old, just out of training, and didn’t know anyone at the meetings. Receptions were the worst. My only friend sometimes was either my host or my wife. I was surrounded by these old guys, most of whom were famous.
Among the highlights of my life was being invited to join the Western Surgical Association. The year I was elected, J. David Richardson was the President. He gave such a rousing speech that at the President’s Dinner I rallied the courage to speak to the great man. When I called him “Dr. Richardson,” he waved that off and insisted I call him “Dave.” Little did I know that Dave would become one of my personal heroes and play an important part in the path that led to my being in a position to write a column such as this.
I never thought that being at a meeting might save my own life. As a devoted member of the Western Surgical Association, I breezed into Phoenix last year for the annual meeting. But I wasn’t feeling so well. Typical of a surgeon, I’d been denying that the symptoms in my abdomen and back were anything other than arthritis. Also, like most surgeons, I’d been pushing the accelerator pedal of life to the floorboard for some months. I arrived at the hotel at one in the morning with a stomach ache that clearly was becoming serious.
I lay in bed wondering what to do. Surgeons don’t get sick! Calling 911 seemed like an invitation to a 6-hour ED experience during which I would be demoted from surgeon to “the patient in Room 9” in a town where I didn’t know many people. As a surgeon, I knew that belly pain of this magnitude was not something I wanted to entrust to a nonsurgeon who was just trying to get through another long shift. This was personal.
Here’s where being a member of a surgical society became more than an academic exercise. I was in a hotel with many of the finest surgeons in the world – and I knew many of them! Among the attendees was my very good friend Margo Shoup, a first-rate cancer surgeon from Chicago. I knew she had come in that day because we were to have dinner together the next night. Rather than call her at 0300, I waited until 6. She came right over and examined me. We decided on a plan. As is always the case at a surgical meeting, one of the surgeon members is the local arrangement person. In this instance, it was none other than James Madura, MD, Chief of MIS GI surgery at Mayo Clinic, Scottsdale. We called James and when my abdomen decided to go nuclear with pain, he drove me to his hospital in his own car and got me in their ED.
The rest of the story is pretty mundane. My diagnosis turned out to be temporarily serious but benign. It was serious enough that without good people treating me, I could have done poorly. James and his fine team took superb care of me. Almost all of us go into surgery with the intent to help others. We take that extraseriously when it’s one of “us.” I can imagine the increased pressure on James when he was treating a colleague and the entire rest of the Western Surgical Association knew it. He never even broke a sweat. I am so glad he was my surgeon. His chief resident Ryan Day, MD, spent extra time with me. I saw a bunch of other residents as well. They reminded me of my fellow residents many years ago – eager, bright, and hyperdedicated. Several members of the Western came by the hospital to see me and even reviewed my images and diagnosis with me. I felt like I had a whole family of physicians who were both my friends and my support system a long way from home. I cannot thank James, Margo, and the rest enough for all they did. It’s great to feel back to normal again as well.
So, I would suggest you join and participate in the surgical societies that you think are a good fit for you. Develop those important professional relationships. Such activity will do your patients a world of good – and just might save your own life.
I am a member of several surgical societies, and more than once I’ve extolled the importance of going to meetings as part of my personal definition of a “good” surgeon.
While I mostly enjoy meetings nowadays, it hasn’t always been easy. My first experiences at surgical society meetings were painful. I was 32 years old, just out of training, and didn’t know anyone at the meetings. Receptions were the worst. My only friend sometimes was either my host or my wife. I was surrounded by these old guys, most of whom were famous.
Among the highlights of my life was being invited to join the Western Surgical Association. The year I was elected, J. David Richardson was the President. He gave such a rousing speech that at the President’s Dinner I rallied the courage to speak to the great man. When I called him “Dr. Richardson,” he waved that off and insisted I call him “Dave.” Little did I know that Dave would become one of my personal heroes and play an important part in the path that led to my being in a position to write a column such as this.
I never thought that being at a meeting might save my own life. As a devoted member of the Western Surgical Association, I breezed into Phoenix last year for the annual meeting. But I wasn’t feeling so well. Typical of a surgeon, I’d been denying that the symptoms in my abdomen and back were anything other than arthritis. Also, like most surgeons, I’d been pushing the accelerator pedal of life to the floorboard for some months. I arrived at the hotel at one in the morning with a stomach ache that clearly was becoming serious.
I lay in bed wondering what to do. Surgeons don’t get sick! Calling 911 seemed like an invitation to a 6-hour ED experience during which I would be demoted from surgeon to “the patient in Room 9” in a town where I didn’t know many people. As a surgeon, I knew that belly pain of this magnitude was not something I wanted to entrust to a nonsurgeon who was just trying to get through another long shift. This was personal.
Here’s where being a member of a surgical society became more than an academic exercise. I was in a hotel with many of the finest surgeons in the world – and I knew many of them! Among the attendees was my very good friend Margo Shoup, a first-rate cancer surgeon from Chicago. I knew she had come in that day because we were to have dinner together the next night. Rather than call her at 0300, I waited until 6. She came right over and examined me. We decided on a plan. As is always the case at a surgical meeting, one of the surgeon members is the local arrangement person. In this instance, it was none other than James Madura, MD, Chief of MIS GI surgery at Mayo Clinic, Scottsdale. We called James and when my abdomen decided to go nuclear with pain, he drove me to his hospital in his own car and got me in their ED.
The rest of the story is pretty mundane. My diagnosis turned out to be temporarily serious but benign. It was serious enough that without good people treating me, I could have done poorly. James and his fine team took superb care of me. Almost all of us go into surgery with the intent to help others. We take that extraseriously when it’s one of “us.” I can imagine the increased pressure on James when he was treating a colleague and the entire rest of the Western Surgical Association knew it. He never even broke a sweat. I am so glad he was my surgeon. His chief resident Ryan Day, MD, spent extra time with me. I saw a bunch of other residents as well. They reminded me of my fellow residents many years ago – eager, bright, and hyperdedicated. Several members of the Western came by the hospital to see me and even reviewed my images and diagnosis with me. I felt like I had a whole family of physicians who were both my friends and my support system a long way from home. I cannot thank James, Margo, and the rest enough for all they did. It’s great to feel back to normal again as well.
So, I would suggest you join and participate in the surgical societies that you think are a good fit for you. Develop those important professional relationships. Such activity will do your patients a world of good – and just might save your own life.
Eileen Metzger Bulger, MD, FACS, is new Chair of the ACS Committee on Trauma
Eileen Metzger Bulger, MD, FACS, chief of trauma and trauma medical director for adults and pediatrics, Harborview Medical Center, Seattle, WA, begins serving as the new Chair of the American College of Surgeons (ACS) Committee on Trauma (COT) this month. Dr. Bulger was appointed as the next COT Chair in October 2017 by the ACS Board of Regents. She is the 20th Chair of the COT, succeeding Ronald M. Stewart, MD, FACS, of San Antonio, TX.
“We look forward to Dr. Bulger’s exceptional vision and leadership as she directs the COT into its 96th year of working to improve the care of the injured patient. She is the perfect person to lead the COT into its next century of transforming care and reducing injuries across the globe,” Dr. Stewart said.
A diplomate of the American Board of Surgery, Dr. Bulger also is board certified in surgical critical care. She earned a medical doctorate at Cornell University Medical College, New York, NY (1992). She completed a residency in general surgery at the University of Washington (UW), Seattle (1992–1999), where she concurrently completed a two-year National Institutes of Health Trauma Research Fellowship during her years of residency training (1995–1997), and then went on to complete a surgical critical care fellowship at UW in 2000.
Recognized leadership
Throughout her career, Dr. Bulger has mentored many surgical residents in paper and scholarship competitions. For nearly two decades, she has served as the co-principal or principal investigator of a variety of innovative, grant-funded research projects related to trauma care, some of which focus on improving outcomes for crash injury victims, pediatric patients, and older adults.
Since her initial involvement with the COT in 2002, Dr. Bulger has contributed to many COT activities, often serving in a leadership role. She is a Course Instructor for the internationally recognized Advanced Traum
The COT is dedicated to all phases of injury care, from prevention to rehabilitation. The committee is supported by a network of 65 state and provincial committees, 11 international committees, and five military committees, and the majority of members are ACS Fellows.
Eileen Metzger Bulger, MD, FACS, chief of trauma and trauma medical director for adults and pediatrics, Harborview Medical Center, Seattle, WA, begins serving as the new Chair of the American College of Surgeons (ACS) Committee on Trauma (COT) this month. Dr. Bulger was appointed as the next COT Chair in October 2017 by the ACS Board of Regents. She is the 20th Chair of the COT, succeeding Ronald M. Stewart, MD, FACS, of San Antonio, TX.
“We look forward to Dr. Bulger’s exceptional vision and leadership as she directs the COT into its 96th year of working to improve the care of the injured patient. She is the perfect person to lead the COT into its next century of transforming care and reducing injuries across the globe,” Dr. Stewart said.
A diplomate of the American Board of Surgery, Dr. Bulger also is board certified in surgical critical care. She earned a medical doctorate at Cornell University Medical College, New York, NY (1992). She completed a residency in general surgery at the University of Washington (UW), Seattle (1992–1999), where she concurrently completed a two-year National Institutes of Health Trauma Research Fellowship during her years of residency training (1995–1997), and then went on to complete a surgical critical care fellowship at UW in 2000.
Recognized leadership
Throughout her career, Dr. Bulger has mentored many surgical residents in paper and scholarship competitions. For nearly two decades, she has served as the co-principal or principal investigator of a variety of innovative, grant-funded research projects related to trauma care, some of which focus on improving outcomes for crash injury victims, pediatric patients, and older adults.
Since her initial involvement with the COT in 2002, Dr. Bulger has contributed to many COT activities, often serving in a leadership role. She is a Course Instructor for the internationally recognized Advanced Traum
The COT is dedicated to all phases of injury care, from prevention to rehabilitation. The committee is supported by a network of 65 state and provincial committees, 11 international committees, and five military committees, and the majority of members are ACS Fellows.
Eileen Metzger Bulger, MD, FACS, chief of trauma and trauma medical director for adults and pediatrics, Harborview Medical Center, Seattle, WA, begins serving as the new Chair of the American College of Surgeons (ACS) Committee on Trauma (COT) this month. Dr. Bulger was appointed as the next COT Chair in October 2017 by the ACS Board of Regents. She is the 20th Chair of the COT, succeeding Ronald M. Stewart, MD, FACS, of San Antonio, TX.
“We look forward to Dr. Bulger’s exceptional vision and leadership as she directs the COT into its 96th year of working to improve the care of the injured patient. She is the perfect person to lead the COT into its next century of transforming care and reducing injuries across the globe,” Dr. Stewart said.
A diplomate of the American Board of Surgery, Dr. Bulger also is board certified in surgical critical care. She earned a medical doctorate at Cornell University Medical College, New York, NY (1992). She completed a residency in general surgery at the University of Washington (UW), Seattle (1992–1999), where she concurrently completed a two-year National Institutes of Health Trauma Research Fellowship during her years of residency training (1995–1997), and then went on to complete a surgical critical care fellowship at UW in 2000.
Recognized leadership
Throughout her career, Dr. Bulger has mentored many surgical residents in paper and scholarship competitions. For nearly two decades, she has served as the co-principal or principal investigator of a variety of innovative, grant-funded research projects related to trauma care, some of which focus on improving outcomes for crash injury victims, pediatric patients, and older adults.
Since her initial involvement with the COT in 2002, Dr. Bulger has contributed to many COT activities, often serving in a leadership role. She is a Course Instructor for the internationally recognized Advanced Traum
The COT is dedicated to all phases of injury care, from prevention to rehabilitation. The committee is supported by a network of 65 state and provincial committees, 11 international committees, and five military committees, and the majority of members are ACS Fellows.
Bipartisan Budget Act of 2018 addresses ACS priorities
On February 9, Congress passed and President Trump signed into law the Bipartisan Budget Act of 2018. The law addresses many key physician and patient issues, including important technical corrections to the Merit-based Incentive Payment System (MIPS) that the American College of Surgeons (ACS) strongly favors.
The law addresses several other ACS priorities, including:
The addition of a long-term funding extension (10 years) for the Children’s Health Insurance Program (CHIP), ensuring that children continue to have access to surgical care
The inclusion of language that eases electronic health record system meaningful use requirements, alleviating some of the burdens imposed on physicians and their practices
Additional funding to address the opioid epidemic and to support the work of the National Institutes of Health Repeal of the Independent Payment Advisory Board
For more information, contact Mark Lukaszewski, ACS Congressional Lobbyist, at [email protected].
On February 9, Congress passed and President Trump signed into law the Bipartisan Budget Act of 2018. The law addresses many key physician and patient issues, including important technical corrections to the Merit-based Incentive Payment System (MIPS) that the American College of Surgeons (ACS) strongly favors.
The law addresses several other ACS priorities, including:
The addition of a long-term funding extension (10 years) for the Children’s Health Insurance Program (CHIP), ensuring that children continue to have access to surgical care
The inclusion of language that eases electronic health record system meaningful use requirements, alleviating some of the burdens imposed on physicians and their practices
Additional funding to address the opioid epidemic and to support the work of the National Institutes of Health Repeal of the Independent Payment Advisory Board
For more information, contact Mark Lukaszewski, ACS Congressional Lobbyist, at [email protected].
On February 9, Congress passed and President Trump signed into law the Bipartisan Budget Act of 2018. The law addresses many key physician and patient issues, including important technical corrections to the Merit-based Incentive Payment System (MIPS) that the American College of Surgeons (ACS) strongly favors.
The law addresses several other ACS priorities, including:
The addition of a long-term funding extension (10 years) for the Children’s Health Insurance Program (CHIP), ensuring that children continue to have access to surgical care
The inclusion of language that eases electronic health record system meaningful use requirements, alleviating some of the burdens imposed on physicians and their practices
Additional funding to address the opioid epidemic and to support the work of the National Institutes of Health Repeal of the Independent Payment Advisory Board
For more information, contact Mark Lukaszewski, ACS Congressional Lobbyist, at [email protected].
ACS delegation shapes policy at AMA HOD meeting
The American Medical Association (AMA) Interim Meeting of the House of Delegates (HOD) took place November 11–14, 2017, in Honolulu, HI. A total of 532 delegates were in attendance to debate the policy implications of 36 reports and 99 resolutions.
The American College of Surgeons (ACS) sent a six-member delegation to the meeting. The ACS also participates in AMA activities in other capacities, including in the AMA Young Physician Section Assembly, the AMA Resident and Fellow Section Assembly, and the AMA Council on Medical Education. These three groups met in conjunction with the HOD meeting. See the sidebar on page 74 for the list of ACS delegates and their other AMA roles.
ACS cosponsored issues
The AMA HOD brings together a variety of perspectives in medicine, and the job of the ACS delegation is to shape AMA policy consistent with College priorities. One way the ACS achieves this objective is by cosponsoring resolutions that have been submitted by other delegations and that are relevant to the College Fellowship. The ACS delegation cosponsored the following three resolutions at the November meeting—two on scope-of-practice issues and one on physician payment—all of which were adopted.
Resolution 214, Advanced Practice Registered Nurse (APRN) Compact, was initiated by the American Society of Anesthesiologists and strengthened with amendments. AMA policy opposes enactment of the Advanced APRN Multistate Compact because of its potential to supersede state laws that require APRNs to practice under physician supervision, as well as legislation that authorizes the independent practice of medicine by any individual who has not completed the state’s requirement for licensure to practice medicine. The AMA will convene an in-person meeting of relevant physician stakeholders to create a consistent national strategy to prevent fulfillment of the APRN Compact.
Resolution 230, Oppose Physician Assistant Independent Practice, with support from a spectrum of state medical and national specialty societies, continued the theme of opposition to legislation or regulation that allows physician extenders—in this case physician assistants—to practice independently. Another resolution addressed the emerging advanced physician assistant degree known as doctor of medical science. The AMA opposes holders of this degree from being recognized as a new category of health care practitioners licensed for the independent practice of medicine.
Resolution 808, Opposition to Reduced Payment for the 25-Modifier, was offered by the American Academy of Dermatology. The resolution was a response to private insurers discounting evaluation and management (E/M) codes by 50 percent when linked through the 25-modifier to a procedure on the same day. This resolution passed as simplified by amendment to have AMA aggressively and immediately advocate, through any legal means possible (such as direct payor negotiations, regulations, legislation, or litigation), for non-reduced allowable payment of appropriately reported 25-modifier E/M codes when linked with procedures.
Other HOD-adopted resolutions of interest
BOT (Board of Trustees) Report 5, Effective Peer Review, amended the AMA Physician and Medical Staff Member Bill of Rights to add “protection from any retaliatory actions” to the list of immunity rights when physicians participate in good faith peer-review activities. In testimony at the reference committee, the delegation highlighted the value of the new ACS “red book,” Optimal Resources for Surgical Quality and Safety, for establishing peer-review standards in surgical care.
Council on Science and Public Health Report 2, Targeted Education to Increase Organ Donation, amended the AMA policy, Methods to Increase the U.S. Organ Donor Pool. As a result, the AMA supports studies that evaluate the effectiveness of mandated choice and presumed consent models for increasing organ donation and urges development of effective methods to inform populations with historically low participation rates about donating.
Resolution 953, Fees for Taking Maintenance of Certification (MOC) Examination, amended AMA MOC policy to assert that the MOC process should reflect the cost of development and administration of the MOC components, ensure a fair fee structure, and not hinder patient care. The AMA will advocate that value in MOC includes cost-effectiveness with full financial transparency, respect for physicians’ time and patient care commitments, alignment of MOC requirements with other regulator and payor requirements, and adherence to an evidence basis for both MOC content and processes.
Not every item was viewed favorably at the AMA meeting. Council on Ethical and Judicial Affairs (CEJA) Report 1, Competence, Self-Assessment and Self-Awareness, sought to provide guidance for physicians in determining their own competence when practicing medicine. The council observed, “As an ethical responsibility, competence encompasses more than medical knowledge and skill. It requires physicians to understand that as a practical matter in the care of actual patients, competence is fluid and dependent on context.” Considerable testimony emphasized a lack of reliable tools and available resources to assist physicians in self-assessment. Thus, the report was referred back to CEJA for more work.
Surgical caucus
In addition to facilitating an agenda review and business meeting for surgeons, anesthesiologists, and emergency physicians, the caucus sponsored a popular education session, Hazards of the Deep: Trauma in Paradise. Michael Hayashi, MD, FACS, Chair of the Hawaii Committee on Trauma, discussed system challenges in caring for injured patients from geographically remote and less populated areas. Lieutenant Matthew Brown, MC, USN, an undersea/diving medical officer stationed at Pearl Harbor, HI, shared insights about injuries and medical conditions experienced by scuba divers, swimmers, surfers, and other beach enthusiasts.
Leadership transition
After extended service on the delegation, including eight years as Chair, Dr. Armstrong bid “aloha” to the HOD as a retiring delegate. Dr. Turner has accepted the role as Chair, maintaining continued College leadership in the HOD.
Next meeting
The next meeting of the AMA HOD is scheduled for June 9–13 in Chicago, IL. In addition to debate on numerous issues, elections for AMA officers, trustees, and councils will be held at the meeting. Surgeons with suggestions for potential resolutions or questions about ACS activities at the AMA HOD should e-mail [email protected]
ACS Delegation at the AMA HOD
John H. Armstrong, MD, FACS (Delegation Chair), acute care surgery, Tampa, FL
Brian J. Gavitt, MD, MPH (also Young Physicians Section delegate), general surgery, Cincinnati, OH
Jacob Moalem, MD, FACS, general surgery, Rochester, NY
Leigh A. Neumayer, MD, FACS, general surgery, Tucson, AZ; Chair, ACS Board of Regents
Naveen F. Sangji, MD (also Resident and Fellow Section delegate), general surgery resident, Boston, MA
Patricia L. Turner, MD, FACS, general surgery, Chicago, IL; Director, ACS Division of Member Services; member and immediate past-chair, AMA Council on Medical EducationDr. Armstrong is affiliate associate professor of surgery, University of South Florida Morsani College of Medicine, Tampa, and former Florida Surgeon General and Secretary of Health (2012–2016). He is a member, ACS Health Policy and Advocacy Group, and Past-Chair, ACS Professional Association political action committee (ACSPA-SurgeonsPAC).
Mr. Sutton is Manager, State Affairs, ACS Division of Advocacy and Health Policy.
The American Medical Association (AMA) Interim Meeting of the House of Delegates (HOD) took place November 11–14, 2017, in Honolulu, HI. A total of 532 delegates were in attendance to debate the policy implications of 36 reports and 99 resolutions.
The American College of Surgeons (ACS) sent a six-member delegation to the meeting. The ACS also participates in AMA activities in other capacities, including in the AMA Young Physician Section Assembly, the AMA Resident and Fellow Section Assembly, and the AMA Council on Medical Education. These three groups met in conjunction with the HOD meeting. See the sidebar on page 74 for the list of ACS delegates and their other AMA roles.
ACS cosponsored issues
The AMA HOD brings together a variety of perspectives in medicine, and the job of the ACS delegation is to shape AMA policy consistent with College priorities. One way the ACS achieves this objective is by cosponsoring resolutions that have been submitted by other delegations and that are relevant to the College Fellowship. The ACS delegation cosponsored the following three resolutions at the November meeting—two on scope-of-practice issues and one on physician payment—all of which were adopted.
Resolution 214, Advanced Practice Registered Nurse (APRN) Compact, was initiated by the American Society of Anesthesiologists and strengthened with amendments. AMA policy opposes enactment of the Advanced APRN Multistate Compact because of its potential to supersede state laws that require APRNs to practice under physician supervision, as well as legislation that authorizes the independent practice of medicine by any individual who has not completed the state’s requirement for licensure to practice medicine. The AMA will convene an in-person meeting of relevant physician stakeholders to create a consistent national strategy to prevent fulfillment of the APRN Compact.
Resolution 230, Oppose Physician Assistant Independent Practice, with support from a spectrum of state medical and national specialty societies, continued the theme of opposition to legislation or regulation that allows physician extenders—in this case physician assistants—to practice independently. Another resolution addressed the emerging advanced physician assistant degree known as doctor of medical science. The AMA opposes holders of this degree from being recognized as a new category of health care practitioners licensed for the independent practice of medicine.
Resolution 808, Opposition to Reduced Payment for the 25-Modifier, was offered by the American Academy of Dermatology. The resolution was a response to private insurers discounting evaluation and management (E/M) codes by 50 percent when linked through the 25-modifier to a procedure on the same day. This resolution passed as simplified by amendment to have AMA aggressively and immediately advocate, through any legal means possible (such as direct payor negotiations, regulations, legislation, or litigation), for non-reduced allowable payment of appropriately reported 25-modifier E/M codes when linked with procedures.
Other HOD-adopted resolutions of interest
BOT (Board of Trustees) Report 5, Effective Peer Review, amended the AMA Physician and Medical Staff Member Bill of Rights to add “protection from any retaliatory actions” to the list of immunity rights when physicians participate in good faith peer-review activities. In testimony at the reference committee, the delegation highlighted the value of the new ACS “red book,” Optimal Resources for Surgical Quality and Safety, for establishing peer-review standards in surgical care.
Council on Science and Public Health Report 2, Targeted Education to Increase Organ Donation, amended the AMA policy, Methods to Increase the U.S. Organ Donor Pool. As a result, the AMA supports studies that evaluate the effectiveness of mandated choice and presumed consent models for increasing organ donation and urges development of effective methods to inform populations with historically low participation rates about donating.
Resolution 953, Fees for Taking Maintenance of Certification (MOC) Examination, amended AMA MOC policy to assert that the MOC process should reflect the cost of development and administration of the MOC components, ensure a fair fee structure, and not hinder patient care. The AMA will advocate that value in MOC includes cost-effectiveness with full financial transparency, respect for physicians’ time and patient care commitments, alignment of MOC requirements with other regulator and payor requirements, and adherence to an evidence basis for both MOC content and processes.
Not every item was viewed favorably at the AMA meeting. Council on Ethical and Judicial Affairs (CEJA) Report 1, Competence, Self-Assessment and Self-Awareness, sought to provide guidance for physicians in determining their own competence when practicing medicine. The council observed, “As an ethical responsibility, competence encompasses more than medical knowledge and skill. It requires physicians to understand that as a practical matter in the care of actual patients, competence is fluid and dependent on context.” Considerable testimony emphasized a lack of reliable tools and available resources to assist physicians in self-assessment. Thus, the report was referred back to CEJA for more work.
Surgical caucus
In addition to facilitating an agenda review and business meeting for surgeons, anesthesiologists, and emergency physicians, the caucus sponsored a popular education session, Hazards of the Deep: Trauma in Paradise. Michael Hayashi, MD, FACS, Chair of the Hawaii Committee on Trauma, discussed system challenges in caring for injured patients from geographically remote and less populated areas. Lieutenant Matthew Brown, MC, USN, an undersea/diving medical officer stationed at Pearl Harbor, HI, shared insights about injuries and medical conditions experienced by scuba divers, swimmers, surfers, and other beach enthusiasts.
Leadership transition
After extended service on the delegation, including eight years as Chair, Dr. Armstrong bid “aloha” to the HOD as a retiring delegate. Dr. Turner has accepted the role as Chair, maintaining continued College leadership in the HOD.
Next meeting
The next meeting of the AMA HOD is scheduled for June 9–13 in Chicago, IL. In addition to debate on numerous issues, elections for AMA officers, trustees, and councils will be held at the meeting. Surgeons with suggestions for potential resolutions or questions about ACS activities at the AMA HOD should e-mail [email protected]
ACS Delegation at the AMA HOD
John H. Armstrong, MD, FACS (Delegation Chair), acute care surgery, Tampa, FL
Brian J. Gavitt, MD, MPH (also Young Physicians Section delegate), general surgery, Cincinnati, OH
Jacob Moalem, MD, FACS, general surgery, Rochester, NY
Leigh A. Neumayer, MD, FACS, general surgery, Tucson, AZ; Chair, ACS Board of Regents
Naveen F. Sangji, MD (also Resident and Fellow Section delegate), general surgery resident, Boston, MA
Patricia L. Turner, MD, FACS, general surgery, Chicago, IL; Director, ACS Division of Member Services; member and immediate past-chair, AMA Council on Medical EducationDr. Armstrong is affiliate associate professor of surgery, University of South Florida Morsani College of Medicine, Tampa, and former Florida Surgeon General and Secretary of Health (2012–2016). He is a member, ACS Health Policy and Advocacy Group, and Past-Chair, ACS Professional Association political action committee (ACSPA-SurgeonsPAC).
Mr. Sutton is Manager, State Affairs, ACS Division of Advocacy and Health Policy.
The American Medical Association (AMA) Interim Meeting of the House of Delegates (HOD) took place November 11–14, 2017, in Honolulu, HI. A total of 532 delegates were in attendance to debate the policy implications of 36 reports and 99 resolutions.
The American College of Surgeons (ACS) sent a six-member delegation to the meeting. The ACS also participates in AMA activities in other capacities, including in the AMA Young Physician Section Assembly, the AMA Resident and Fellow Section Assembly, and the AMA Council on Medical Education. These three groups met in conjunction with the HOD meeting. See the sidebar on page 74 for the list of ACS delegates and their other AMA roles.
ACS cosponsored issues
The AMA HOD brings together a variety of perspectives in medicine, and the job of the ACS delegation is to shape AMA policy consistent with College priorities. One way the ACS achieves this objective is by cosponsoring resolutions that have been submitted by other delegations and that are relevant to the College Fellowship. The ACS delegation cosponsored the following three resolutions at the November meeting—two on scope-of-practice issues and one on physician payment—all of which were adopted.
Resolution 214, Advanced Practice Registered Nurse (APRN) Compact, was initiated by the American Society of Anesthesiologists and strengthened with amendments. AMA policy opposes enactment of the Advanced APRN Multistate Compact because of its potential to supersede state laws that require APRNs to practice under physician supervision, as well as legislation that authorizes the independent practice of medicine by any individual who has not completed the state’s requirement for licensure to practice medicine. The AMA will convene an in-person meeting of relevant physician stakeholders to create a consistent national strategy to prevent fulfillment of the APRN Compact.
Resolution 230, Oppose Physician Assistant Independent Practice, with support from a spectrum of state medical and national specialty societies, continued the theme of opposition to legislation or regulation that allows physician extenders—in this case physician assistants—to practice independently. Another resolution addressed the emerging advanced physician assistant degree known as doctor of medical science. The AMA opposes holders of this degree from being recognized as a new category of health care practitioners licensed for the independent practice of medicine.
Resolution 808, Opposition to Reduced Payment for the 25-Modifier, was offered by the American Academy of Dermatology. The resolution was a response to private insurers discounting evaluation and management (E/M) codes by 50 percent when linked through the 25-modifier to a procedure on the same day. This resolution passed as simplified by amendment to have AMA aggressively and immediately advocate, through any legal means possible (such as direct payor negotiations, regulations, legislation, or litigation), for non-reduced allowable payment of appropriately reported 25-modifier E/M codes when linked with procedures.
Other HOD-adopted resolutions of interest
BOT (Board of Trustees) Report 5, Effective Peer Review, amended the AMA Physician and Medical Staff Member Bill of Rights to add “protection from any retaliatory actions” to the list of immunity rights when physicians participate in good faith peer-review activities. In testimony at the reference committee, the delegation highlighted the value of the new ACS “red book,” Optimal Resources for Surgical Quality and Safety, for establishing peer-review standards in surgical care.
Council on Science and Public Health Report 2, Targeted Education to Increase Organ Donation, amended the AMA policy, Methods to Increase the U.S. Organ Donor Pool. As a result, the AMA supports studies that evaluate the effectiveness of mandated choice and presumed consent models for increasing organ donation and urges development of effective methods to inform populations with historically low participation rates about donating.
Resolution 953, Fees for Taking Maintenance of Certification (MOC) Examination, amended AMA MOC policy to assert that the MOC process should reflect the cost of development and administration of the MOC components, ensure a fair fee structure, and not hinder patient care. The AMA will advocate that value in MOC includes cost-effectiveness with full financial transparency, respect for physicians’ time and patient care commitments, alignment of MOC requirements with other regulator and payor requirements, and adherence to an evidence basis for both MOC content and processes.
Not every item was viewed favorably at the AMA meeting. Council on Ethical and Judicial Affairs (CEJA) Report 1, Competence, Self-Assessment and Self-Awareness, sought to provide guidance for physicians in determining their own competence when practicing medicine. The council observed, “As an ethical responsibility, competence encompasses more than medical knowledge and skill. It requires physicians to understand that as a practical matter in the care of actual patients, competence is fluid and dependent on context.” Considerable testimony emphasized a lack of reliable tools and available resources to assist physicians in self-assessment. Thus, the report was referred back to CEJA for more work.
Surgical caucus
In addition to facilitating an agenda review and business meeting for surgeons, anesthesiologists, and emergency physicians, the caucus sponsored a popular education session, Hazards of the Deep: Trauma in Paradise. Michael Hayashi, MD, FACS, Chair of the Hawaii Committee on Trauma, discussed system challenges in caring for injured patients from geographically remote and less populated areas. Lieutenant Matthew Brown, MC, USN, an undersea/diving medical officer stationed at Pearl Harbor, HI, shared insights about injuries and medical conditions experienced by scuba divers, swimmers, surfers, and other beach enthusiasts.
Leadership transition
After extended service on the delegation, including eight years as Chair, Dr. Armstrong bid “aloha” to the HOD as a retiring delegate. Dr. Turner has accepted the role as Chair, maintaining continued College leadership in the HOD.
Next meeting
The next meeting of the AMA HOD is scheduled for June 9–13 in Chicago, IL. In addition to debate on numerous issues, elections for AMA officers, trustees, and councils will be held at the meeting. Surgeons with suggestions for potential resolutions or questions about ACS activities at the AMA HOD should e-mail [email protected]
ACS Delegation at the AMA HOD
John H. Armstrong, MD, FACS (Delegation Chair), acute care surgery, Tampa, FL
Brian J. Gavitt, MD, MPH (also Young Physicians Section delegate), general surgery, Cincinnati, OH
Jacob Moalem, MD, FACS, general surgery, Rochester, NY
Leigh A. Neumayer, MD, FACS, general surgery, Tucson, AZ; Chair, ACS Board of Regents
Naveen F. Sangji, MD (also Resident and Fellow Section delegate), general surgery resident, Boston, MA
Patricia L. Turner, MD, FACS, general surgery, Chicago, IL; Director, ACS Division of Member Services; member and immediate past-chair, AMA Council on Medical EducationDr. Armstrong is affiliate associate professor of surgery, University of South Florida Morsani College of Medicine, Tampa, and former Florida Surgeon General and Secretary of Health (2012–2016). He is a member, ACS Health Policy and Advocacy Group, and Past-Chair, ACS Professional Association political action committee (ACSPA-SurgeonsPAC).
Mr. Sutton is Manager, State Affairs, ACS Division of Advocacy and Health Policy.





