User login
FDA proposes lower nicotine levels in cigarettes
Nicotine levels in cigarettes could see a significant reduction under regulatory options being considered by the Food and Drug Administration.
Cigarettes “are the only legal consumer product that, when used as intended, will kill half all long-term users,” FDA Commissioner Scott Gottlieb, MD, said in a statement announcing the effort.
The agency is seeking comment on a proposed regulation regarding “a potential maximum nicotine level that would be appropriate for the protection of public health, in light of scientific evidence about the addictive properties of nicotine in cigarettes.” An advance notice of proposed rule making was posted online March 15 and is scheduled for publication in the Federal Register on March 16.
The FDA also is seeking comments on a number of other areas to help inform potential regulatory action down the road, including whether a new standard for lower nicotine levels should be implemented at once or whether a phased-in approach should be taken; whether FDA should specify a method for manufacturers to use in order to detect nicotine levels in their products; and whether the proposed lower level is technically achievable.
The agency also is seeking comment on potential unintended effects of lowering the amount of nicotine in cigarettes, such as turning to other combustible tobacco products such as cigars in conjunction with or as a replacement for cigarette use; increasing the number of cigarettes smoked, or seeking comparable nicotine from noncombustible tobacco sources.
At this time, FDA is not suggesting what the target might be on a specific nicotine level. While the advanced notice asks specifically about the “merits of nicotine levels like 0.3, 0.4, and 0.5 mg nicotine/g of tobacco filler,” it is not suggesting that this is the range being considered.
[polldaddy:9960560]
“Not to prejudge any possible proposed rule that we would do or any possible level, that is the purpose of an advanced proposed rule making, but we share all the science that we are aware of, and we characterize the studies that have been done to date in trying to find out what that right level is,” Mitch Zeller, director of the FDA Center for Tobacco Products, said during a March 15 press call.
He said that the FDA aiming to make sure the level is low enough that it cannot be compensated for by smoking more or inhaling deeper and holding the breath in longer, much like how smokers compensated when they smoked “light” cigarettes in the unregulated market.
Mr. Zeller said that seeking comments on those levels is based on the scientific evidence that is laid out in the advanced notice, but it is not necessarily foreshadowing where the standard will be set.
Drastically reducing the amount of nicotine in cigarettes is expected to significantly lower not only the number of people addicted to cigarettes but also as the negative health effects of nicotine addiction, FDA experts wrote in a perspective piece published March 15 in the New England Journal of Medicine (doi: 10.1065/NEJMsr1714617).
“Our findings show that reducing the nicotine level in cigarettes has the potential to substantially reduce the enormous burden of smoking-related death and disease,” Benjamin J. Apelberg, PhD, director of the Division of Population Health Science, Office of Science, within the FDA Center for Tobacco Products, and his colleagues, wrote in the report.
Modeling for the implementation of a lower nicotine level policy suggests that smoking prevalence will decline from a median of 12.8% in baseline scenario to a median of 10.8% within a year of implementation, with the increase related to smoking cessation.
“We estimate that approximately 5 million additional smokers would quit smoking within a year after implementation of the hypothetical policy,” Dr. Apelberg and his colleagues wrote. “By 2060, smoking prevalence drops from 7.9% in the baseline scenario to 1.4% in the policy scenario.”
Their analysis is based on a nicotine level that is “so low that there would not be enough nicotine available in cigarette tobacco for smokers to sustain addiction,” they noted.
The FDA plans to release two more advanced notices of proposed rule making related to using regulatory levers to reduce cigarette smoking, including one that addresses flavoring in tobacco and one related to the regulation of premium cigars. Other than the 90-day comment period related to the advanced notices, the agency has not set any deadlines on when it will act.
“Legally, we are not going to prejudge how long this will take or what will happen,” Mr. Zeller said. “This is an early step in what could be a potential rule-making process using the product standard authority. We will take a long and hard look at all the comments that come in over the next 90 days and based upon our review of all the information, all the comments that come in, all the feedback that we have, we will then make a decision about taking the next step in the rule-making process.”
Nicotine levels in cigarettes could see a significant reduction under regulatory options being considered by the Food and Drug Administration.
Cigarettes “are the only legal consumer product that, when used as intended, will kill half all long-term users,” FDA Commissioner Scott Gottlieb, MD, said in a statement announcing the effort.
The agency is seeking comment on a proposed regulation regarding “a potential maximum nicotine level that would be appropriate for the protection of public health, in light of scientific evidence about the addictive properties of nicotine in cigarettes.” An advance notice of proposed rule making was posted online March 15 and is scheduled for publication in the Federal Register on March 16.
The FDA also is seeking comments on a number of other areas to help inform potential regulatory action down the road, including whether a new standard for lower nicotine levels should be implemented at once or whether a phased-in approach should be taken; whether FDA should specify a method for manufacturers to use in order to detect nicotine levels in their products; and whether the proposed lower level is technically achievable.
The agency also is seeking comment on potential unintended effects of lowering the amount of nicotine in cigarettes, such as turning to other combustible tobacco products such as cigars in conjunction with or as a replacement for cigarette use; increasing the number of cigarettes smoked, or seeking comparable nicotine from noncombustible tobacco sources.
At this time, FDA is not suggesting what the target might be on a specific nicotine level. While the advanced notice asks specifically about the “merits of nicotine levels like 0.3, 0.4, and 0.5 mg nicotine/g of tobacco filler,” it is not suggesting that this is the range being considered.
[polldaddy:9960560]
“Not to prejudge any possible proposed rule that we would do or any possible level, that is the purpose of an advanced proposed rule making, but we share all the science that we are aware of, and we characterize the studies that have been done to date in trying to find out what that right level is,” Mitch Zeller, director of the FDA Center for Tobacco Products, said during a March 15 press call.
He said that the FDA aiming to make sure the level is low enough that it cannot be compensated for by smoking more or inhaling deeper and holding the breath in longer, much like how smokers compensated when they smoked “light” cigarettes in the unregulated market.
Mr. Zeller said that seeking comments on those levels is based on the scientific evidence that is laid out in the advanced notice, but it is not necessarily foreshadowing where the standard will be set.
Drastically reducing the amount of nicotine in cigarettes is expected to significantly lower not only the number of people addicted to cigarettes but also as the negative health effects of nicotine addiction, FDA experts wrote in a perspective piece published March 15 in the New England Journal of Medicine (doi: 10.1065/NEJMsr1714617).
“Our findings show that reducing the nicotine level in cigarettes has the potential to substantially reduce the enormous burden of smoking-related death and disease,” Benjamin J. Apelberg, PhD, director of the Division of Population Health Science, Office of Science, within the FDA Center for Tobacco Products, and his colleagues, wrote in the report.
Modeling for the implementation of a lower nicotine level policy suggests that smoking prevalence will decline from a median of 12.8% in baseline scenario to a median of 10.8% within a year of implementation, with the increase related to smoking cessation.
“We estimate that approximately 5 million additional smokers would quit smoking within a year after implementation of the hypothetical policy,” Dr. Apelberg and his colleagues wrote. “By 2060, smoking prevalence drops from 7.9% in the baseline scenario to 1.4% in the policy scenario.”
Their analysis is based on a nicotine level that is “so low that there would not be enough nicotine available in cigarette tobacco for smokers to sustain addiction,” they noted.
The FDA plans to release two more advanced notices of proposed rule making related to using regulatory levers to reduce cigarette smoking, including one that addresses flavoring in tobacco and one related to the regulation of premium cigars. Other than the 90-day comment period related to the advanced notices, the agency has not set any deadlines on when it will act.
“Legally, we are not going to prejudge how long this will take or what will happen,” Mr. Zeller said. “This is an early step in what could be a potential rule-making process using the product standard authority. We will take a long and hard look at all the comments that come in over the next 90 days and based upon our review of all the information, all the comments that come in, all the feedback that we have, we will then make a decision about taking the next step in the rule-making process.”
Nicotine levels in cigarettes could see a significant reduction under regulatory options being considered by the Food and Drug Administration.
Cigarettes “are the only legal consumer product that, when used as intended, will kill half all long-term users,” FDA Commissioner Scott Gottlieb, MD, said in a statement announcing the effort.
The agency is seeking comment on a proposed regulation regarding “a potential maximum nicotine level that would be appropriate for the protection of public health, in light of scientific evidence about the addictive properties of nicotine in cigarettes.” An advance notice of proposed rule making was posted online March 15 and is scheduled for publication in the Federal Register on March 16.
The FDA also is seeking comments on a number of other areas to help inform potential regulatory action down the road, including whether a new standard for lower nicotine levels should be implemented at once or whether a phased-in approach should be taken; whether FDA should specify a method for manufacturers to use in order to detect nicotine levels in their products; and whether the proposed lower level is technically achievable.
The agency also is seeking comment on potential unintended effects of lowering the amount of nicotine in cigarettes, such as turning to other combustible tobacco products such as cigars in conjunction with or as a replacement for cigarette use; increasing the number of cigarettes smoked, or seeking comparable nicotine from noncombustible tobacco sources.
At this time, FDA is not suggesting what the target might be on a specific nicotine level. While the advanced notice asks specifically about the “merits of nicotine levels like 0.3, 0.4, and 0.5 mg nicotine/g of tobacco filler,” it is not suggesting that this is the range being considered.
[polldaddy:9960560]
“Not to prejudge any possible proposed rule that we would do or any possible level, that is the purpose of an advanced proposed rule making, but we share all the science that we are aware of, and we characterize the studies that have been done to date in trying to find out what that right level is,” Mitch Zeller, director of the FDA Center for Tobacco Products, said during a March 15 press call.
He said that the FDA aiming to make sure the level is low enough that it cannot be compensated for by smoking more or inhaling deeper and holding the breath in longer, much like how smokers compensated when they smoked “light” cigarettes in the unregulated market.
Mr. Zeller said that seeking comments on those levels is based on the scientific evidence that is laid out in the advanced notice, but it is not necessarily foreshadowing where the standard will be set.
Drastically reducing the amount of nicotine in cigarettes is expected to significantly lower not only the number of people addicted to cigarettes but also as the negative health effects of nicotine addiction, FDA experts wrote in a perspective piece published March 15 in the New England Journal of Medicine (doi: 10.1065/NEJMsr1714617).
“Our findings show that reducing the nicotine level in cigarettes has the potential to substantially reduce the enormous burden of smoking-related death and disease,” Benjamin J. Apelberg, PhD, director of the Division of Population Health Science, Office of Science, within the FDA Center for Tobacco Products, and his colleagues, wrote in the report.
Modeling for the implementation of a lower nicotine level policy suggests that smoking prevalence will decline from a median of 12.8% in baseline scenario to a median of 10.8% within a year of implementation, with the increase related to smoking cessation.
“We estimate that approximately 5 million additional smokers would quit smoking within a year after implementation of the hypothetical policy,” Dr. Apelberg and his colleagues wrote. “By 2060, smoking prevalence drops from 7.9% in the baseline scenario to 1.4% in the policy scenario.”
Their analysis is based on a nicotine level that is “so low that there would not be enough nicotine available in cigarette tobacco for smokers to sustain addiction,” they noted.
The FDA plans to release two more advanced notices of proposed rule making related to using regulatory levers to reduce cigarette smoking, including one that addresses flavoring in tobacco and one related to the regulation of premium cigars. Other than the 90-day comment period related to the advanced notices, the agency has not set any deadlines on when it will act.
“Legally, we are not going to prejudge how long this will take or what will happen,” Mr. Zeller said. “This is an early step in what could be a potential rule-making process using the product standard authority. We will take a long and hard look at all the comments that come in over the next 90 days and based upon our review of all the information, all the comments that come in, all the feedback that we have, we will then make a decision about taking the next step in the rule-making process.”
A Message from the Executive Director: ACS continues to take on the issues of concern to surgeons and their patients
I am pleased to once again submit an annual report for publication in ACS Surgery News. The American College of Surgeons (ACS) had a productive year in 2017 and looks forward to seeing a range of new programs evolve in 2018.
Physician payment
A health policy issue of considerable concern to ACS Fellows is the Centers for Medicare & Medicaid Services’ efforts to implement the payment reforms in the Medicare Access and CHIP (Children’s Health Insurance Program) Reauthorization Act (MACRA) of 2015. Specifically, 2017 was the transition year for implementation of the Quality Payment Program’s (QPP’s) Merit-based Incentive Payment System (MIPS), and MIPS data collected in 2017 will be used to determine annual payment updates in 2019.
In 2018, the second year of MIPS, the penalty for nonparticipation has increased to 5 percent from 4 percent. Over time, the penalty for nonparticpation or poor performance will continue to rise. The College has created a variety of resources to assist Fellows in their efforts to comply with MIPS, which explain the purpose and structure of the MIPS program and help guide surgeons in choosing and achieving the goal that is right for their individual practice. These tools can be found on the ACS website at facs.org/qpp.
In addition to MIPS, the QPP calls for the establishment of Alternative Payment Models (APMs). The College has worked with thought leaders at Brandeis University, Waltham, MA, to develop the ACS-Brandeis Advanced APM. In 2017, the Secretary of the Department of Health and Human Services reviewed the proposal and made recommendations for improvement. Efforts to develop the model continue, and the ACS is working with private insurers and entities that may implement the APM model once available.
Education
The College is leading a significant effort to address the needs of surgeons who are looking to update their skills. The Steering Committee for Retraining and Retooling of Practicing Surgeons is working to define standards and establish a national infrastructure to achieve optimal outcomes. The ACS Accredited Education Institutes are at the core of this infrastructure.
At Clinical Congress 2017, we launched the ACS Academy of Master Surgeon Educators. The goals of the academy are to recognize master surgeon educators, advance the science and practice of leading-edge surgical education and training, foster innovation and collaboration, support faculty development and recognition, and underscore the importance of surgical education and training.
Also at Clinical Congress, the ACS Committee on Ethics unveiled Ethical Issues in Surgical Care, a landmark resource that defines a framework for the field of surgical ethics as it has evolved over the last decade. The book is organized into four sections that address the broad areas of general consideration, the surgeon-patient relationship, the surgeon and the surgical profession, and the surgeon and society.
Quality
The College released Optimal Resources for Surgical Quality and Safety, also known as the “red book,” in July 2017.This manual provides a guide for surgical quality leaders seeking to improve quality and safety in their institutions, departments, and practices. Efforts are under way to develop adjunctive or integrated resources/standards and to potentially establish a Surgical Quality Verification Program.
The red book was released at the 2017 ACS Quality and Safety Conference, formerly the ACS National Surgical Quality Improvement Program (ACS NSQIP®) Annual Conference, in New York, NY. The conference, which focused on a broad range of ACS Quality Programs, boasted a record-breaking attendance of more than 1,800 attendees.
The new Surgeon Specific Registry was the first ACS database to launch as part of the College’s integrated registry of the future, which ultimately will allow users to share relevant quality data across individual ACS Quality Programs, such as ACS NSQIP and the Trauma Quality Improvement Program (TQIP®).
Other new quality initiatives include the Agency for Healthcare Research and Quality Safety Program for Improving Surgical Care and Recovery (ISCR), which the ACS is conducting in collaboration with Johns Hopkins Medicine Armstrong Institute for Patient Safety and Quality, Baltimore, MD. This program supports hospitals in implementing perioperative evidence-based pathways to improve clinical outcomes, reduce hospital length of stay, and improve the patient experience.
The ACS also has become the new home of Strong for Surgery, originally developed by surgeons in Washington State. This program empowers hospitals and clinics to integrate checklists into the preoperative phase of care.
In addition, the ACS was awarded a three-year, multimillion dollar R01 grant from the National Institute on Minority Health and Health Disparities. ACS Past-President L.D. Britt, MD, MPH, DSc(Hon), FACS, FCCM, FRCSEng(Hon), FRCSEd(Hon), FWACS(Hon), FRCSI(Hon), FCS(SA)(Hon), FRCSGlasg(Hon), is the principal investigator on this award, which is aimed at eliminating variances in access to surgical care.
Trauma
The Committee on Trauma (COT), in collaboration with military partners and the National Highway Traffic Safety Administration (NHTSA), hosted a conference in April 2017 to advance the recommendations in the National Academies on Science, Engineering, and Medicine report, A National Trauma Care System: Integrating Military and Civilian Trauma Systems to Achieve Zero Preventable Deaths after Injury. The meeting brought together approximately 170 trauma care professionals with the goal of creating the framework for a National Trauma Care System Action Plan.
In light of recent tragedies and the ongoing public debate over how to stop the continuing violence at our nation’s schools, churches, and other public places, the COT Injury Prevention and Control Committee (IPCC) is advocating for a consensus-based, public health/trauma system approach to firearm injury prevention. Furthermore, at its February 2018 meeting the ACS Board of Regents unanimously approved a plan to expand the College’s focus from the successful Stop the Bleed® program to a broader prevention initiative focused on strategies that include research, advocacy, and strategic collaborations. An action plan was in development at press time.
ACS leadership
The ACS Board of Governors (B/G) continues to implement initiatives through its Pillars and Workgroups. Specific examples from this past year include the release of a white paper on out-of-network billing; production of the biannual e-newsletter, The Cutting Edge; conduct of the 2017 Board of Governors Annual Survey, which focuses on the Stop the Bleed campaign, the opioid crisis, work-related injuries/surgical ergonomics, and advanced practice providers in surgery; and development of a standardized letter of recommendation for applicants to surgery training programs.
The ACS Board of Regents approved and updated a number of statements in the last 12 months. New statements cover several topics of concern to the Fellowship, including gender salary equity, the use of anesthetics and sedation drugs in children and pregnant women, the opioid abuse epidemic, lithium batteries, opioids and motor vehicle crash prevention, maintaining surgical access with a locum tenens surgeon, social media, the Uniform Emergency Volunteer Health Practitioners Act, credentialing and privileging, and medical students and the electronic health record.
As these few examples demonstrate, the ACS is constantly moving forward to offer surgeons and the other members of the patient care team the tools, resources, and educational opportunities they need to succeed in practice and to provide optimal patient care. As always, you are encouraged to contact the ACS leadership, and let us know how we can best serve you.
Dr. Hoyt is the Executive Director of the ACS, Chicago, IL.
I am pleased to once again submit an annual report for publication in ACS Surgery News. The American College of Surgeons (ACS) had a productive year in 2017 and looks forward to seeing a range of new programs evolve in 2018.
Physician payment
A health policy issue of considerable concern to ACS Fellows is the Centers for Medicare & Medicaid Services’ efforts to implement the payment reforms in the Medicare Access and CHIP (Children’s Health Insurance Program) Reauthorization Act (MACRA) of 2015. Specifically, 2017 was the transition year for implementation of the Quality Payment Program’s (QPP’s) Merit-based Incentive Payment System (MIPS), and MIPS data collected in 2017 will be used to determine annual payment updates in 2019.
In 2018, the second year of MIPS, the penalty for nonparticipation has increased to 5 percent from 4 percent. Over time, the penalty for nonparticpation or poor performance will continue to rise. The College has created a variety of resources to assist Fellows in their efforts to comply with MIPS, which explain the purpose and structure of the MIPS program and help guide surgeons in choosing and achieving the goal that is right for their individual practice. These tools can be found on the ACS website at facs.org/qpp.
In addition to MIPS, the QPP calls for the establishment of Alternative Payment Models (APMs). The College has worked with thought leaders at Brandeis University, Waltham, MA, to develop the ACS-Brandeis Advanced APM. In 2017, the Secretary of the Department of Health and Human Services reviewed the proposal and made recommendations for improvement. Efforts to develop the model continue, and the ACS is working with private insurers and entities that may implement the APM model once available.
Education
The College is leading a significant effort to address the needs of surgeons who are looking to update their skills. The Steering Committee for Retraining and Retooling of Practicing Surgeons is working to define standards and establish a national infrastructure to achieve optimal outcomes. The ACS Accredited Education Institutes are at the core of this infrastructure.
At Clinical Congress 2017, we launched the ACS Academy of Master Surgeon Educators. The goals of the academy are to recognize master surgeon educators, advance the science and practice of leading-edge surgical education and training, foster innovation and collaboration, support faculty development and recognition, and underscore the importance of surgical education and training.
Also at Clinical Congress, the ACS Committee on Ethics unveiled Ethical Issues in Surgical Care, a landmark resource that defines a framework for the field of surgical ethics as it has evolved over the last decade. The book is organized into four sections that address the broad areas of general consideration, the surgeon-patient relationship, the surgeon and the surgical profession, and the surgeon and society.
Quality
The College released Optimal Resources for Surgical Quality and Safety, also known as the “red book,” in July 2017.This manual provides a guide for surgical quality leaders seeking to improve quality and safety in their institutions, departments, and practices. Efforts are under way to develop adjunctive or integrated resources/standards and to potentially establish a Surgical Quality Verification Program.
The red book was released at the 2017 ACS Quality and Safety Conference, formerly the ACS National Surgical Quality Improvement Program (ACS NSQIP®) Annual Conference, in New York, NY. The conference, which focused on a broad range of ACS Quality Programs, boasted a record-breaking attendance of more than 1,800 attendees.
The new Surgeon Specific Registry was the first ACS database to launch as part of the College’s integrated registry of the future, which ultimately will allow users to share relevant quality data across individual ACS Quality Programs, such as ACS NSQIP and the Trauma Quality Improvement Program (TQIP®).
Other new quality initiatives include the Agency for Healthcare Research and Quality Safety Program for Improving Surgical Care and Recovery (ISCR), which the ACS is conducting in collaboration with Johns Hopkins Medicine Armstrong Institute for Patient Safety and Quality, Baltimore, MD. This program supports hospitals in implementing perioperative evidence-based pathways to improve clinical outcomes, reduce hospital length of stay, and improve the patient experience.
The ACS also has become the new home of Strong for Surgery, originally developed by surgeons in Washington State. This program empowers hospitals and clinics to integrate checklists into the preoperative phase of care.
In addition, the ACS was awarded a three-year, multimillion dollar R01 grant from the National Institute on Minority Health and Health Disparities. ACS Past-President L.D. Britt, MD, MPH, DSc(Hon), FACS, FCCM, FRCSEng(Hon), FRCSEd(Hon), FWACS(Hon), FRCSI(Hon), FCS(SA)(Hon), FRCSGlasg(Hon), is the principal investigator on this award, which is aimed at eliminating variances in access to surgical care.
Trauma
The Committee on Trauma (COT), in collaboration with military partners and the National Highway Traffic Safety Administration (NHTSA), hosted a conference in April 2017 to advance the recommendations in the National Academies on Science, Engineering, and Medicine report, A National Trauma Care System: Integrating Military and Civilian Trauma Systems to Achieve Zero Preventable Deaths after Injury. The meeting brought together approximately 170 trauma care professionals with the goal of creating the framework for a National Trauma Care System Action Plan.
In light of recent tragedies and the ongoing public debate over how to stop the continuing violence at our nation’s schools, churches, and other public places, the COT Injury Prevention and Control Committee (IPCC) is advocating for a consensus-based, public health/trauma system approach to firearm injury prevention. Furthermore, at its February 2018 meeting the ACS Board of Regents unanimously approved a plan to expand the College’s focus from the successful Stop the Bleed® program to a broader prevention initiative focused on strategies that include research, advocacy, and strategic collaborations. An action plan was in development at press time.
ACS leadership
The ACS Board of Governors (B/G) continues to implement initiatives through its Pillars and Workgroups. Specific examples from this past year include the release of a white paper on out-of-network billing; production of the biannual e-newsletter, The Cutting Edge; conduct of the 2017 Board of Governors Annual Survey, which focuses on the Stop the Bleed campaign, the opioid crisis, work-related injuries/surgical ergonomics, and advanced practice providers in surgery; and development of a standardized letter of recommendation for applicants to surgery training programs.
The ACS Board of Regents approved and updated a number of statements in the last 12 months. New statements cover several topics of concern to the Fellowship, including gender salary equity, the use of anesthetics and sedation drugs in children and pregnant women, the opioid abuse epidemic, lithium batteries, opioids and motor vehicle crash prevention, maintaining surgical access with a locum tenens surgeon, social media, the Uniform Emergency Volunteer Health Practitioners Act, credentialing and privileging, and medical students and the electronic health record.
As these few examples demonstrate, the ACS is constantly moving forward to offer surgeons and the other members of the patient care team the tools, resources, and educational opportunities they need to succeed in practice and to provide optimal patient care. As always, you are encouraged to contact the ACS leadership, and let us know how we can best serve you.
Dr. Hoyt is the Executive Director of the ACS, Chicago, IL.
I am pleased to once again submit an annual report for publication in ACS Surgery News. The American College of Surgeons (ACS) had a productive year in 2017 and looks forward to seeing a range of new programs evolve in 2018.
Physician payment
A health policy issue of considerable concern to ACS Fellows is the Centers for Medicare & Medicaid Services’ efforts to implement the payment reforms in the Medicare Access and CHIP (Children’s Health Insurance Program) Reauthorization Act (MACRA) of 2015. Specifically, 2017 was the transition year for implementation of the Quality Payment Program’s (QPP’s) Merit-based Incentive Payment System (MIPS), and MIPS data collected in 2017 will be used to determine annual payment updates in 2019.
In 2018, the second year of MIPS, the penalty for nonparticipation has increased to 5 percent from 4 percent. Over time, the penalty for nonparticpation or poor performance will continue to rise. The College has created a variety of resources to assist Fellows in their efforts to comply with MIPS, which explain the purpose and structure of the MIPS program and help guide surgeons in choosing and achieving the goal that is right for their individual practice. These tools can be found on the ACS website at facs.org/qpp.
In addition to MIPS, the QPP calls for the establishment of Alternative Payment Models (APMs). The College has worked with thought leaders at Brandeis University, Waltham, MA, to develop the ACS-Brandeis Advanced APM. In 2017, the Secretary of the Department of Health and Human Services reviewed the proposal and made recommendations for improvement. Efforts to develop the model continue, and the ACS is working with private insurers and entities that may implement the APM model once available.
Education
The College is leading a significant effort to address the needs of surgeons who are looking to update their skills. The Steering Committee for Retraining and Retooling of Practicing Surgeons is working to define standards and establish a national infrastructure to achieve optimal outcomes. The ACS Accredited Education Institutes are at the core of this infrastructure.
At Clinical Congress 2017, we launched the ACS Academy of Master Surgeon Educators. The goals of the academy are to recognize master surgeon educators, advance the science and practice of leading-edge surgical education and training, foster innovation and collaboration, support faculty development and recognition, and underscore the importance of surgical education and training.
Also at Clinical Congress, the ACS Committee on Ethics unveiled Ethical Issues in Surgical Care, a landmark resource that defines a framework for the field of surgical ethics as it has evolved over the last decade. The book is organized into four sections that address the broad areas of general consideration, the surgeon-patient relationship, the surgeon and the surgical profession, and the surgeon and society.
Quality
The College released Optimal Resources for Surgical Quality and Safety, also known as the “red book,” in July 2017.This manual provides a guide for surgical quality leaders seeking to improve quality and safety in their institutions, departments, and practices. Efforts are under way to develop adjunctive or integrated resources/standards and to potentially establish a Surgical Quality Verification Program.
The red book was released at the 2017 ACS Quality and Safety Conference, formerly the ACS National Surgical Quality Improvement Program (ACS NSQIP®) Annual Conference, in New York, NY. The conference, which focused on a broad range of ACS Quality Programs, boasted a record-breaking attendance of more than 1,800 attendees.
The new Surgeon Specific Registry was the first ACS database to launch as part of the College’s integrated registry of the future, which ultimately will allow users to share relevant quality data across individual ACS Quality Programs, such as ACS NSQIP and the Trauma Quality Improvement Program (TQIP®).
Other new quality initiatives include the Agency for Healthcare Research and Quality Safety Program for Improving Surgical Care and Recovery (ISCR), which the ACS is conducting in collaboration with Johns Hopkins Medicine Armstrong Institute for Patient Safety and Quality, Baltimore, MD. This program supports hospitals in implementing perioperative evidence-based pathways to improve clinical outcomes, reduce hospital length of stay, and improve the patient experience.
The ACS also has become the new home of Strong for Surgery, originally developed by surgeons in Washington State. This program empowers hospitals and clinics to integrate checklists into the preoperative phase of care.
In addition, the ACS was awarded a three-year, multimillion dollar R01 grant from the National Institute on Minority Health and Health Disparities. ACS Past-President L.D. Britt, MD, MPH, DSc(Hon), FACS, FCCM, FRCSEng(Hon), FRCSEd(Hon), FWACS(Hon), FRCSI(Hon), FCS(SA)(Hon), FRCSGlasg(Hon), is the principal investigator on this award, which is aimed at eliminating variances in access to surgical care.
Trauma
The Committee on Trauma (COT), in collaboration with military partners and the National Highway Traffic Safety Administration (NHTSA), hosted a conference in April 2017 to advance the recommendations in the National Academies on Science, Engineering, and Medicine report, A National Trauma Care System: Integrating Military and Civilian Trauma Systems to Achieve Zero Preventable Deaths after Injury. The meeting brought together approximately 170 trauma care professionals with the goal of creating the framework for a National Trauma Care System Action Plan.
In light of recent tragedies and the ongoing public debate over how to stop the continuing violence at our nation’s schools, churches, and other public places, the COT Injury Prevention and Control Committee (IPCC) is advocating for a consensus-based, public health/trauma system approach to firearm injury prevention. Furthermore, at its February 2018 meeting the ACS Board of Regents unanimously approved a plan to expand the College’s focus from the successful Stop the Bleed® program to a broader prevention initiative focused on strategies that include research, advocacy, and strategic collaborations. An action plan was in development at press time.
ACS leadership
The ACS Board of Governors (B/G) continues to implement initiatives through its Pillars and Workgroups. Specific examples from this past year include the release of a white paper on out-of-network billing; production of the biannual e-newsletter, The Cutting Edge; conduct of the 2017 Board of Governors Annual Survey, which focuses on the Stop the Bleed campaign, the opioid crisis, work-related injuries/surgical ergonomics, and advanced practice providers in surgery; and development of a standardized letter of recommendation for applicants to surgery training programs.
The ACS Board of Regents approved and updated a number of statements in the last 12 months. New statements cover several topics of concern to the Fellowship, including gender salary equity, the use of anesthetics and sedation drugs in children and pregnant women, the opioid abuse epidemic, lithium batteries, opioids and motor vehicle crash prevention, maintaining surgical access with a locum tenens surgeon, social media, the Uniform Emergency Volunteer Health Practitioners Act, credentialing and privileging, and medical students and the electronic health record.
As these few examples demonstrate, the ACS is constantly moving forward to offer surgeons and the other members of the patient care team the tools, resources, and educational opportunities they need to succeed in practice and to provide optimal patient care. As always, you are encouraged to contact the ACS leadership, and let us know how we can best serve you.
Dr. Hoyt is the Executive Director of the ACS, Chicago, IL.
Hospital Medicine: An international specialty
This past fall, I had the honor of being invited to speak at a hospitalist physician conference in Tokyo. The conference was hosted by the Japanese Society of Hospital General Medicine (JSHGM) and was attended by over 800 hospitalists, including some from other East Asian countries.
The JSHGM is 7 years old and has 1,400 members; its growth mirroring the growth of practicing hospitalists in Japan. They wanted me to speak about the evolution of the hospitalist model in the United States and to learn more about their efforts to grow the nascent specialty in Japan. We also jointly wanted to discuss the opportunity for the JSHGM and the Society of Hospital Medicine to work together to benefit the hospitalist model in both countries.
This emerging partnership of the two societies is only the latest of a growing series of efforts on the part of SHM to support the growth of the hospitalist specialty internationally. It started with Canada in 2001, when a contingent of Canadian hospitalists requested to form their own chapter of SHM. They wanted to become the first international chapter and to join a group that has now grown to 56 state and regional chapters. Within a few years, the Canadian Chapter evolved to become its own independent and flourishing Canadian Society of Hospital Medicine.
More recently, SHM has helped develop chapters in Brazil and the Middle East, with more chapters being planned. The International Special Interest Forum at Hospital Medicine 2018 in Orlando in April expects attendees from Holland, Germany, Spain, Chile, Taiwan, China and more.
So why all of this activity by hospitalists in countries whose health systems are so different from ours and from each other’s? What is it about our specialty that has captured the interest of physicians, health systems, and governments from around the globe?
Talking to hospitalists from abroad, the answer is very consistent and very simple. It is the desire to lower cost and improve the quality of care. It turns out that the American health care system is not the only one that struggles with these issues. It seems that health care costs are too high everywhere and that the quest for higher quality, lower cost health care is a universal struggle. As Scottish-born health care economist Ian Morrison jokes, “Every health care system sucks in its own way.”
Let’s look at Japan. They have the longest average life expectancy in the world at 84 years, with about a quarter of their population over 65 years of age. On any given day, almost 14% of the population has a physician visit. Historically, they also have long hospital length of stays with current average length of stay anywhere between 14 and 21 days. This, of course, is very costly. … and in their single-payer system, the entire cost of this care falls on the Japanese government. And as birth rates in Japan have decreased, there are fewer taxpayers to bear the financial burden of the progressively aging and sicker population.
Canada has a different challenge. Also a single-payer system, their largest issue for the acutely ill is the availability of an open hospital bed. Although the system varies somewhat from province to province, it is typical that hospitals are given a total annual budget that must cover all expenses for the year, independent of the volume of patients. Since most hospitals are perpetually full, the discharge of a patient results in another new admission, which in turn actually costs the hospital more money. This perverse incentive keeps hospital beds full as patients wait for one to open (especially for any elective procedure). Adding to the problem, physicians are paid fee for service and therefore also have no incentive to discharge patients.
As Canadian citizens clamor for more access to care, the government looks for ways to lower excessively long length of stays. Wait times for elective surgeries are unacceptable with some patients coming to the United States for surgery that they must then pay for themselves. The result is mounting pressure to move to models of care that are more efficient and less wasteful.
It is no wonder that physicians and health care planners from Japan, Canada, and around the world have viewed with great interest what hospitalists have accomplished in the American health care system. They recognize the potential of this relatively new model to decrease hospital length of stays, lower health care costs, and improve outcomes. After all, this is what the hospitalist model was invented to accomplish – to create value not through high production, but by improving the efficiency of care delivery, overall quality of care, and contributing to improved hospital operations.
As unrelenting economic forces continue to put pressure on health care systems worldwide, it will be fascinating to follow and continue assessing the impact of the hospitalist model in nations where it is implemented. That includes, of course, in the United States, where the model is still very young and continually evolving.
In the meantime, SHM will continue to learn about and work with our international partners. This certainly will be the focus of a special “Hospital Medicine in Japan” session at Hospital Medicine 2018 along with the International Special Interest Forum. And for the first time, we will also have an International Lounge where our international members can meet with each other and our American members to share ideas and enthusiasm for the future of our specialty.
These are just the first steps to expanding the hospital medicine movement across the globe. SHM is optimistic about the future opportunities for international collaboration and is committed to supporting the growth of the specialty and its practitioners, not only in the United States, but worldwide.
Dr. Greeno is president of the Society of Hospital Medicine, and senior adviser for medical affairs, TeamHealth.
This past fall, I had the honor of being invited to speak at a hospitalist physician conference in Tokyo. The conference was hosted by the Japanese Society of Hospital General Medicine (JSHGM) and was attended by over 800 hospitalists, including some from other East Asian countries.
The JSHGM is 7 years old and has 1,400 members; its growth mirroring the growth of practicing hospitalists in Japan. They wanted me to speak about the evolution of the hospitalist model in the United States and to learn more about their efforts to grow the nascent specialty in Japan. We also jointly wanted to discuss the opportunity for the JSHGM and the Society of Hospital Medicine to work together to benefit the hospitalist model in both countries.
This emerging partnership of the two societies is only the latest of a growing series of efforts on the part of SHM to support the growth of the hospitalist specialty internationally. It started with Canada in 2001, when a contingent of Canadian hospitalists requested to form their own chapter of SHM. They wanted to become the first international chapter and to join a group that has now grown to 56 state and regional chapters. Within a few years, the Canadian Chapter evolved to become its own independent and flourishing Canadian Society of Hospital Medicine.
More recently, SHM has helped develop chapters in Brazil and the Middle East, with more chapters being planned. The International Special Interest Forum at Hospital Medicine 2018 in Orlando in April expects attendees from Holland, Germany, Spain, Chile, Taiwan, China and more.
So why all of this activity by hospitalists in countries whose health systems are so different from ours and from each other’s? What is it about our specialty that has captured the interest of physicians, health systems, and governments from around the globe?
Talking to hospitalists from abroad, the answer is very consistent and very simple. It is the desire to lower cost and improve the quality of care. It turns out that the American health care system is not the only one that struggles with these issues. It seems that health care costs are too high everywhere and that the quest for higher quality, lower cost health care is a universal struggle. As Scottish-born health care economist Ian Morrison jokes, “Every health care system sucks in its own way.”
Let’s look at Japan. They have the longest average life expectancy in the world at 84 years, with about a quarter of their population over 65 years of age. On any given day, almost 14% of the population has a physician visit. Historically, they also have long hospital length of stays with current average length of stay anywhere between 14 and 21 days. This, of course, is very costly. … and in their single-payer system, the entire cost of this care falls on the Japanese government. And as birth rates in Japan have decreased, there are fewer taxpayers to bear the financial burden of the progressively aging and sicker population.
Canada has a different challenge. Also a single-payer system, their largest issue for the acutely ill is the availability of an open hospital bed. Although the system varies somewhat from province to province, it is typical that hospitals are given a total annual budget that must cover all expenses for the year, independent of the volume of patients. Since most hospitals are perpetually full, the discharge of a patient results in another new admission, which in turn actually costs the hospital more money. This perverse incentive keeps hospital beds full as patients wait for one to open (especially for any elective procedure). Adding to the problem, physicians are paid fee for service and therefore also have no incentive to discharge patients.
As Canadian citizens clamor for more access to care, the government looks for ways to lower excessively long length of stays. Wait times for elective surgeries are unacceptable with some patients coming to the United States for surgery that they must then pay for themselves. The result is mounting pressure to move to models of care that are more efficient and less wasteful.
It is no wonder that physicians and health care planners from Japan, Canada, and around the world have viewed with great interest what hospitalists have accomplished in the American health care system. They recognize the potential of this relatively new model to decrease hospital length of stays, lower health care costs, and improve outcomes. After all, this is what the hospitalist model was invented to accomplish – to create value not through high production, but by improving the efficiency of care delivery, overall quality of care, and contributing to improved hospital operations.
As unrelenting economic forces continue to put pressure on health care systems worldwide, it will be fascinating to follow and continue assessing the impact of the hospitalist model in nations where it is implemented. That includes, of course, in the United States, where the model is still very young and continually evolving.
In the meantime, SHM will continue to learn about and work with our international partners. This certainly will be the focus of a special “Hospital Medicine in Japan” session at Hospital Medicine 2018 along with the International Special Interest Forum. And for the first time, we will also have an International Lounge where our international members can meet with each other and our American members to share ideas and enthusiasm for the future of our specialty.
These are just the first steps to expanding the hospital medicine movement across the globe. SHM is optimistic about the future opportunities for international collaboration and is committed to supporting the growth of the specialty and its practitioners, not only in the United States, but worldwide.
Dr. Greeno is president of the Society of Hospital Medicine, and senior adviser for medical affairs, TeamHealth.
This past fall, I had the honor of being invited to speak at a hospitalist physician conference in Tokyo. The conference was hosted by the Japanese Society of Hospital General Medicine (JSHGM) and was attended by over 800 hospitalists, including some from other East Asian countries.
The JSHGM is 7 years old and has 1,400 members; its growth mirroring the growth of practicing hospitalists in Japan. They wanted me to speak about the evolution of the hospitalist model in the United States and to learn more about their efforts to grow the nascent specialty in Japan. We also jointly wanted to discuss the opportunity for the JSHGM and the Society of Hospital Medicine to work together to benefit the hospitalist model in both countries.
This emerging partnership of the two societies is only the latest of a growing series of efforts on the part of SHM to support the growth of the hospitalist specialty internationally. It started with Canada in 2001, when a contingent of Canadian hospitalists requested to form their own chapter of SHM. They wanted to become the first international chapter and to join a group that has now grown to 56 state and regional chapters. Within a few years, the Canadian Chapter evolved to become its own independent and flourishing Canadian Society of Hospital Medicine.
More recently, SHM has helped develop chapters in Brazil and the Middle East, with more chapters being planned. The International Special Interest Forum at Hospital Medicine 2018 in Orlando in April expects attendees from Holland, Germany, Spain, Chile, Taiwan, China and more.
So why all of this activity by hospitalists in countries whose health systems are so different from ours and from each other’s? What is it about our specialty that has captured the interest of physicians, health systems, and governments from around the globe?
Talking to hospitalists from abroad, the answer is very consistent and very simple. It is the desire to lower cost and improve the quality of care. It turns out that the American health care system is not the only one that struggles with these issues. It seems that health care costs are too high everywhere and that the quest for higher quality, lower cost health care is a universal struggle. As Scottish-born health care economist Ian Morrison jokes, “Every health care system sucks in its own way.”
Let’s look at Japan. They have the longest average life expectancy in the world at 84 years, with about a quarter of their population over 65 years of age. On any given day, almost 14% of the population has a physician visit. Historically, they also have long hospital length of stays with current average length of stay anywhere between 14 and 21 days. This, of course, is very costly. … and in their single-payer system, the entire cost of this care falls on the Japanese government. And as birth rates in Japan have decreased, there are fewer taxpayers to bear the financial burden of the progressively aging and sicker population.
Canada has a different challenge. Also a single-payer system, their largest issue for the acutely ill is the availability of an open hospital bed. Although the system varies somewhat from province to province, it is typical that hospitals are given a total annual budget that must cover all expenses for the year, independent of the volume of patients. Since most hospitals are perpetually full, the discharge of a patient results in another new admission, which in turn actually costs the hospital more money. This perverse incentive keeps hospital beds full as patients wait for one to open (especially for any elective procedure). Adding to the problem, physicians are paid fee for service and therefore also have no incentive to discharge patients.
As Canadian citizens clamor for more access to care, the government looks for ways to lower excessively long length of stays. Wait times for elective surgeries are unacceptable with some patients coming to the United States for surgery that they must then pay for themselves. The result is mounting pressure to move to models of care that are more efficient and less wasteful.
It is no wonder that physicians and health care planners from Japan, Canada, and around the world have viewed with great interest what hospitalists have accomplished in the American health care system. They recognize the potential of this relatively new model to decrease hospital length of stays, lower health care costs, and improve outcomes. After all, this is what the hospitalist model was invented to accomplish – to create value not through high production, but by improving the efficiency of care delivery, overall quality of care, and contributing to improved hospital operations.
As unrelenting economic forces continue to put pressure on health care systems worldwide, it will be fascinating to follow and continue assessing the impact of the hospitalist model in nations where it is implemented. That includes, of course, in the United States, where the model is still very young and continually evolving.
In the meantime, SHM will continue to learn about and work with our international partners. This certainly will be the focus of a special “Hospital Medicine in Japan” session at Hospital Medicine 2018 along with the International Special Interest Forum. And for the first time, we will also have an International Lounge where our international members can meet with each other and our American members to share ideas and enthusiasm for the future of our specialty.
These are just the first steps to expanding the hospital medicine movement across the globe. SHM is optimistic about the future opportunities for international collaboration and is committed to supporting the growth of the specialty and its practitioners, not only in the United States, but worldwide.
Dr. Greeno is president of the Society of Hospital Medicine, and senior adviser for medical affairs, TeamHealth.
Bariatric surgery may adversely affect newborns
Bariatric surgery can offer a variety of benefits to mothers and improve neonatal outcomes but also offers substantial risk, according to a systematic literature review.
“Bariatric surgery, with patients matched for presurgery body mass index [BMI], resulted in a reduction in gestational diabetes mellitus, large-for-gestational-age infants, large babies (composite of large for gestational age and macrosomia), gestational hypertension, all hypertensive disorders, postpartum hemorrhage, and cesarean delivery rates,” wrote Wilson Kwong, MD, of the University of Toronto, and his colleagues. “However, there was an increase in small-for-gestational age infants, intrauterine growth restriction, small babies (composite of small for gestational age and intrauterine growth restriction), and preterm deliveries.”
Dr. Kwong and his research team developed this study to investigate the benefits and risks of bariatric surgery on neonatal outcomes. They designed a systematic review that involved a literature search of 2,616 abstracts using MEDLINE, Embase, Cochrane, Web of Science, and PubMed. They searched all from initiation of the databases to Dec. 12, 2016. Ultimately, this yielded 20 cohort studies and approximately 2.8 million subjects for review and meta-analysis. From this data, pooled odds ratios were estimated, as well as the number needed to benefit (NNTB) and the number need to harm (NNTH) to display the pooled absolute risk difference.
The results of the primary analysis, in which BMIs were similar between control subjects and the presurgery BMIs of women receiving treatment, yielded positives for mothers who underwent bariatric surgery and their newborns. As stated by Dr. Kwong and his research team, newborns were less likely to be large-for-gestational-age babies or deal with macrosomia (odds ratio, 0.36; 95% confidence interval, 0.20-0.66; NNTB, 7) and mothers were less likely to experience hypertensive disorders (OR, 0.38; 95% CI, 0.27-0.53, NNTB, 8) and postpartum hemorrhage (OR, 0.32; 95% CI, 0.08-1.37; NNTB, 9).
Despite the positives, the risk for harm was higher in a number of outcomes. Babies were more likely to be small for gestational age, have intrauterine growth restriction (OR, 2.16; 95% CI, 1.34-3.48; NNTH, 21) ,and be delivered preterm (OR, 1.35; 95% CI, 1.02-1.79; NNTH, 35).
A secondary analysis revealed that malabsorptive surgeries resulted in a significantly greater decrease (P = .012) in large babies (OR, 0.28; 95% CI, 0.22-0.36), compared with restrictive surgeries (OR, 0.50; 95% CI, 0.35-0.73). This analysis also revealed that malabsorptive bariatric surgeries corresponded to an increase in the number of small babies (OR, 2.39; 95% CI, 1.94-2.94; P = .023).
The increased risk of smaller babies may be caused by micronutrient deficiencies in pregnancy, according to Dr. Kwong. Nutritional deficiencies are reported in up to 80% of bariatric surgery patients, malabsorptive patients in particular.
“Common nutrient deficiencies after bariatric surgery include protein, B vitamins, fat-soluble vitamins, essential fatty acids, and minerals (zinc and copper), which may persist throughout pregnancy,” wrote Dr. Kwong and his colleagues.
Similar nutrient deficiencies have been identified in a case study of a woman who underwent a duodenal switch published in the Journal of Obstetrics & Gynaecology Canada. After several failed pregnancies from complications from malnutrition, the patient presented herself to physicians 10 weeks into her next pregnancy and was found to be deficient in vitamins A, D, E, and K, and minerals iron and zinc. After supplementation with a daily nutritional drink containing vitamins and protein, her vitamin levels were near normal at week 24. She eventually delivered a healthy newborn with normal-appearing features. The researchers from this study recommended that patients seeking bariatric surgery should avoid malabsorptive surgeries and defer to less extreme surgical methods.
A strength of the systematic review was the matching of studies based on presurgery and prepregnancy BMI. This was a departure from previous studies that combined all studies. Additionally, this study compared the outcomes based on the type of data source that was used. Despite these strengths, the design and results of the studies in the analysis limited the results of the review. None of the included studies were randomized; instead, they consisted of observational cohort studies, which are prone for confounding by indication.
“In counseling patients of childbearing potential who are interested in pursuing bariatric surgery, a discussion of both possible benefits and risks on pregnancy outcomes must take place,” cautioned Dr. Kwong and his associates. “Future studies should explore the causes of these potential adverse pregnancy outcomes and develop strategies that may prevent them.”
All authors affiliated with the study had no relevant financial conflicts of interest to report.
SOURCE: Kwong W et al. Am J Obstet Gynecol. 2018 Feb 15. doi: 10.1016/j.ajog.2018.02.003.
Bariatric surgery can offer a variety of benefits to mothers and improve neonatal outcomes but also offers substantial risk, according to a systematic literature review.
“Bariatric surgery, with patients matched for presurgery body mass index [BMI], resulted in a reduction in gestational diabetes mellitus, large-for-gestational-age infants, large babies (composite of large for gestational age and macrosomia), gestational hypertension, all hypertensive disorders, postpartum hemorrhage, and cesarean delivery rates,” wrote Wilson Kwong, MD, of the University of Toronto, and his colleagues. “However, there was an increase in small-for-gestational age infants, intrauterine growth restriction, small babies (composite of small for gestational age and intrauterine growth restriction), and preterm deliveries.”
Dr. Kwong and his research team developed this study to investigate the benefits and risks of bariatric surgery on neonatal outcomes. They designed a systematic review that involved a literature search of 2,616 abstracts using MEDLINE, Embase, Cochrane, Web of Science, and PubMed. They searched all from initiation of the databases to Dec. 12, 2016. Ultimately, this yielded 20 cohort studies and approximately 2.8 million subjects for review and meta-analysis. From this data, pooled odds ratios were estimated, as well as the number needed to benefit (NNTB) and the number need to harm (NNTH) to display the pooled absolute risk difference.
The results of the primary analysis, in which BMIs were similar between control subjects and the presurgery BMIs of women receiving treatment, yielded positives for mothers who underwent bariatric surgery and their newborns. As stated by Dr. Kwong and his research team, newborns were less likely to be large-for-gestational-age babies or deal with macrosomia (odds ratio, 0.36; 95% confidence interval, 0.20-0.66; NNTB, 7) and mothers were less likely to experience hypertensive disorders (OR, 0.38; 95% CI, 0.27-0.53, NNTB, 8) and postpartum hemorrhage (OR, 0.32; 95% CI, 0.08-1.37; NNTB, 9).
Despite the positives, the risk for harm was higher in a number of outcomes. Babies were more likely to be small for gestational age, have intrauterine growth restriction (OR, 2.16; 95% CI, 1.34-3.48; NNTH, 21) ,and be delivered preterm (OR, 1.35; 95% CI, 1.02-1.79; NNTH, 35).
A secondary analysis revealed that malabsorptive surgeries resulted in a significantly greater decrease (P = .012) in large babies (OR, 0.28; 95% CI, 0.22-0.36), compared with restrictive surgeries (OR, 0.50; 95% CI, 0.35-0.73). This analysis also revealed that malabsorptive bariatric surgeries corresponded to an increase in the number of small babies (OR, 2.39; 95% CI, 1.94-2.94; P = .023).
The increased risk of smaller babies may be caused by micronutrient deficiencies in pregnancy, according to Dr. Kwong. Nutritional deficiencies are reported in up to 80% of bariatric surgery patients, malabsorptive patients in particular.
“Common nutrient deficiencies after bariatric surgery include protein, B vitamins, fat-soluble vitamins, essential fatty acids, and minerals (zinc and copper), which may persist throughout pregnancy,” wrote Dr. Kwong and his colleagues.
Similar nutrient deficiencies have been identified in a case study of a woman who underwent a duodenal switch published in the Journal of Obstetrics & Gynaecology Canada. After several failed pregnancies from complications from malnutrition, the patient presented herself to physicians 10 weeks into her next pregnancy and was found to be deficient in vitamins A, D, E, and K, and minerals iron and zinc. After supplementation with a daily nutritional drink containing vitamins and protein, her vitamin levels were near normal at week 24. She eventually delivered a healthy newborn with normal-appearing features. The researchers from this study recommended that patients seeking bariatric surgery should avoid malabsorptive surgeries and defer to less extreme surgical methods.
A strength of the systematic review was the matching of studies based on presurgery and prepregnancy BMI. This was a departure from previous studies that combined all studies. Additionally, this study compared the outcomes based on the type of data source that was used. Despite these strengths, the design and results of the studies in the analysis limited the results of the review. None of the included studies were randomized; instead, they consisted of observational cohort studies, which are prone for confounding by indication.
“In counseling patients of childbearing potential who are interested in pursuing bariatric surgery, a discussion of both possible benefits and risks on pregnancy outcomes must take place,” cautioned Dr. Kwong and his associates. “Future studies should explore the causes of these potential adverse pregnancy outcomes and develop strategies that may prevent them.”
All authors affiliated with the study had no relevant financial conflicts of interest to report.
SOURCE: Kwong W et al. Am J Obstet Gynecol. 2018 Feb 15. doi: 10.1016/j.ajog.2018.02.003.
Bariatric surgery can offer a variety of benefits to mothers and improve neonatal outcomes but also offers substantial risk, according to a systematic literature review.
“Bariatric surgery, with patients matched for presurgery body mass index [BMI], resulted in a reduction in gestational diabetes mellitus, large-for-gestational-age infants, large babies (composite of large for gestational age and macrosomia), gestational hypertension, all hypertensive disorders, postpartum hemorrhage, and cesarean delivery rates,” wrote Wilson Kwong, MD, of the University of Toronto, and his colleagues. “However, there was an increase in small-for-gestational age infants, intrauterine growth restriction, small babies (composite of small for gestational age and intrauterine growth restriction), and preterm deliveries.”
Dr. Kwong and his research team developed this study to investigate the benefits and risks of bariatric surgery on neonatal outcomes. They designed a systematic review that involved a literature search of 2,616 abstracts using MEDLINE, Embase, Cochrane, Web of Science, and PubMed. They searched all from initiation of the databases to Dec. 12, 2016. Ultimately, this yielded 20 cohort studies and approximately 2.8 million subjects for review and meta-analysis. From this data, pooled odds ratios were estimated, as well as the number needed to benefit (NNTB) and the number need to harm (NNTH) to display the pooled absolute risk difference.
The results of the primary analysis, in which BMIs were similar between control subjects and the presurgery BMIs of women receiving treatment, yielded positives for mothers who underwent bariatric surgery and their newborns. As stated by Dr. Kwong and his research team, newborns were less likely to be large-for-gestational-age babies or deal with macrosomia (odds ratio, 0.36; 95% confidence interval, 0.20-0.66; NNTB, 7) and mothers were less likely to experience hypertensive disorders (OR, 0.38; 95% CI, 0.27-0.53, NNTB, 8) and postpartum hemorrhage (OR, 0.32; 95% CI, 0.08-1.37; NNTB, 9).
Despite the positives, the risk for harm was higher in a number of outcomes. Babies were more likely to be small for gestational age, have intrauterine growth restriction (OR, 2.16; 95% CI, 1.34-3.48; NNTH, 21) ,and be delivered preterm (OR, 1.35; 95% CI, 1.02-1.79; NNTH, 35).
A secondary analysis revealed that malabsorptive surgeries resulted in a significantly greater decrease (P = .012) in large babies (OR, 0.28; 95% CI, 0.22-0.36), compared with restrictive surgeries (OR, 0.50; 95% CI, 0.35-0.73). This analysis also revealed that malabsorptive bariatric surgeries corresponded to an increase in the number of small babies (OR, 2.39; 95% CI, 1.94-2.94; P = .023).
The increased risk of smaller babies may be caused by micronutrient deficiencies in pregnancy, according to Dr. Kwong. Nutritional deficiencies are reported in up to 80% of bariatric surgery patients, malabsorptive patients in particular.
“Common nutrient deficiencies after bariatric surgery include protein, B vitamins, fat-soluble vitamins, essential fatty acids, and minerals (zinc and copper), which may persist throughout pregnancy,” wrote Dr. Kwong and his colleagues.
Similar nutrient deficiencies have been identified in a case study of a woman who underwent a duodenal switch published in the Journal of Obstetrics & Gynaecology Canada. After several failed pregnancies from complications from malnutrition, the patient presented herself to physicians 10 weeks into her next pregnancy and was found to be deficient in vitamins A, D, E, and K, and minerals iron and zinc. After supplementation with a daily nutritional drink containing vitamins and protein, her vitamin levels were near normal at week 24. She eventually delivered a healthy newborn with normal-appearing features. The researchers from this study recommended that patients seeking bariatric surgery should avoid malabsorptive surgeries and defer to less extreme surgical methods.
A strength of the systematic review was the matching of studies based on presurgery and prepregnancy BMI. This was a departure from previous studies that combined all studies. Additionally, this study compared the outcomes based on the type of data source that was used. Despite these strengths, the design and results of the studies in the analysis limited the results of the review. None of the included studies were randomized; instead, they consisted of observational cohort studies, which are prone for confounding by indication.
“In counseling patients of childbearing potential who are interested in pursuing bariatric surgery, a discussion of both possible benefits and risks on pregnancy outcomes must take place,” cautioned Dr. Kwong and his associates. “Future studies should explore the causes of these potential adverse pregnancy outcomes and develop strategies that may prevent them.”
All authors affiliated with the study had no relevant financial conflicts of interest to report.
SOURCE: Kwong W et al. Am J Obstet Gynecol. 2018 Feb 15. doi: 10.1016/j.ajog.2018.02.003.
FROM THE AMERICAN JOURNAL OF OBSTETRICS & GYNECOLOGY
Key clinical point: Bariatric surgery can offer a variety of benefits to mothers and improve neonatal outcomes but also offers substantial risk.
Major finding: Babies were more likely to be small (OR, 2.16; 95% CI, 1.34-3.48; number needed to harm, 21) and be delivered preterm (OR, 1.35; 95% CI, 1.02-1.79; NNTH, 35).
Study details: A systematic literature search of 20 cohort studies and approximately 2.8 million subjects using MEDLINE, Embase, Cochrane, Web of Science, and PubMed from the date of the databases’ inception to Dec. 12, 2016.
Disclosures: All authors affiliated with the study had no relevant financial conflicts of interest to report.
Source: Kwong W et al. Am J Obstet Gynecol. 2018 Feb 15. doi: 10.1016/j.ajog.2018.02.003.
Impact of sleep loss on metabolism is highly individualistic
Shift work – and the various light exposures that go with it – can place some people at a greater risk of weight gain and obesity. But the impact of various light exposures inherent in shift work appear to affect the metabolism of each person differently, reported Edward L. Melanson, PhD, and his coinvestigators.
“Such individual differences were not explained by sex, age, weight, fat mass, or fat free mass,” Dr. Melanson and his coinvestigators wrote. “Thus, understanding mechanisms underlying such individual differences in waking and sleep energy metabolism and how they may or may not contribute to health outcomes ... requires additional research.”
The investigators’ conclusions are based on the results of two studies. Both studies used whole-room, indirect calorimetry to measure energy expenditure. The participants, all of whom were free of medications and illicit drugs, maintained a consistent 8-hour sleep schedule before the study took place, and consumed a specified diet throughout the study. .
The first study, comprising 15 healthy young adults, looked for changes in energy expenditure and glucose metabolism in response to different lighting conditions, such as full-spectrum bright light or blue-enriched bright light. In that study, no effects were found on patients’ metabolism. The other study, comprising 14 healthy young adults, used a simulated shift work protocol and found a decrease in 24-hour energy expenditure in certain individuals in response to circadian misalignment. “This finding may help identify individuals who may be at a higher risk of unwanted weight gain and obesity during shift work,” the investigators wrote.
Read the full report in Neurobiology of Sleep and Circadian Rhythms.
SOURCE: Melanson EL et al. Neurobiol Sleep Circadian Rhythms. 2017 Dec 29. doi: 10.1016/j.nbscr.2017.12.002.
Shift work – and the various light exposures that go with it – can place some people at a greater risk of weight gain and obesity. But the impact of various light exposures inherent in shift work appear to affect the metabolism of each person differently, reported Edward L. Melanson, PhD, and his coinvestigators.
“Such individual differences were not explained by sex, age, weight, fat mass, or fat free mass,” Dr. Melanson and his coinvestigators wrote. “Thus, understanding mechanisms underlying such individual differences in waking and sleep energy metabolism and how they may or may not contribute to health outcomes ... requires additional research.”
The investigators’ conclusions are based on the results of two studies. Both studies used whole-room, indirect calorimetry to measure energy expenditure. The participants, all of whom were free of medications and illicit drugs, maintained a consistent 8-hour sleep schedule before the study took place, and consumed a specified diet throughout the study. .
The first study, comprising 15 healthy young adults, looked for changes in energy expenditure and glucose metabolism in response to different lighting conditions, such as full-spectrum bright light or blue-enriched bright light. In that study, no effects were found on patients’ metabolism. The other study, comprising 14 healthy young adults, used a simulated shift work protocol and found a decrease in 24-hour energy expenditure in certain individuals in response to circadian misalignment. “This finding may help identify individuals who may be at a higher risk of unwanted weight gain and obesity during shift work,” the investigators wrote.
Read the full report in Neurobiology of Sleep and Circadian Rhythms.
SOURCE: Melanson EL et al. Neurobiol Sleep Circadian Rhythms. 2017 Dec 29. doi: 10.1016/j.nbscr.2017.12.002.
Shift work – and the various light exposures that go with it – can place some people at a greater risk of weight gain and obesity. But the impact of various light exposures inherent in shift work appear to affect the metabolism of each person differently, reported Edward L. Melanson, PhD, and his coinvestigators.
“Such individual differences were not explained by sex, age, weight, fat mass, or fat free mass,” Dr. Melanson and his coinvestigators wrote. “Thus, understanding mechanisms underlying such individual differences in waking and sleep energy metabolism and how they may or may not contribute to health outcomes ... requires additional research.”
The investigators’ conclusions are based on the results of two studies. Both studies used whole-room, indirect calorimetry to measure energy expenditure. The participants, all of whom were free of medications and illicit drugs, maintained a consistent 8-hour sleep schedule before the study took place, and consumed a specified diet throughout the study. .
The first study, comprising 15 healthy young adults, looked for changes in energy expenditure and glucose metabolism in response to different lighting conditions, such as full-spectrum bright light or blue-enriched bright light. In that study, no effects were found on patients’ metabolism. The other study, comprising 14 healthy young adults, used a simulated shift work protocol and found a decrease in 24-hour energy expenditure in certain individuals in response to circadian misalignment. “This finding may help identify individuals who may be at a higher risk of unwanted weight gain and obesity during shift work,” the investigators wrote.
Read the full report in Neurobiology of Sleep and Circadian Rhythms.
SOURCE: Melanson EL et al. Neurobiol Sleep Circadian Rhythms. 2017 Dec 29. doi: 10.1016/j.nbscr.2017.12.002.
FROM NEUROBIOLOGY OF SLEEP AND CIRCADIAN RHYTHMS
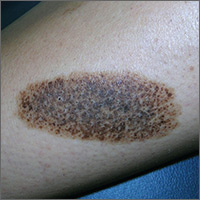
Dark patch on leg
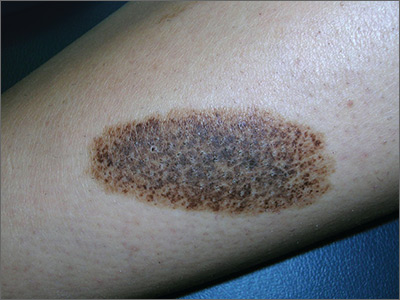
The FP recognized this to be a nevus spilus (speckled nevus).
He explained that this was a benign nevus and that no treatment was indicated—especially because the only effective treatment would be deep excision down to the subcutaneous fat layer (requiring a graft for closure). The patient probably wouldn’t like the cosmetic result and the risks of this surgery (pain, infection, graft not taking) are not acceptable for this condition. Also, the procedure would not be covered by insurance, as this would be considered cosmetic surgery.
The physician explained that in the unlikely event of significant changes in the nevus, she should seek further evaluation. The patient understood the reality of the situation and thanked the doctor for explaining the diagnosis to her. She was reassured that this was benign.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M, Usatine R. Benign nevi. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:945-952.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP recognized this to be a nevus spilus (speckled nevus).
He explained that this was a benign nevus and that no treatment was indicated—especially because the only effective treatment would be deep excision down to the subcutaneous fat layer (requiring a graft for closure). The patient probably wouldn’t like the cosmetic result and the risks of this surgery (pain, infection, graft not taking) are not acceptable for this condition. Also, the procedure would not be covered by insurance, as this would be considered cosmetic surgery.
The physician explained that in the unlikely event of significant changes in the nevus, she should seek further evaluation. The patient understood the reality of the situation and thanked the doctor for explaining the diagnosis to her. She was reassured that this was benign.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M, Usatine R. Benign nevi. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:945-952.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.

The FP recognized this to be a nevus spilus (speckled nevus).
He explained that this was a benign nevus and that no treatment was indicated—especially because the only effective treatment would be deep excision down to the subcutaneous fat layer (requiring a graft for closure). The patient probably wouldn’t like the cosmetic result and the risks of this surgery (pain, infection, graft not taking) are not acceptable for this condition. Also, the procedure would not be covered by insurance, as this would be considered cosmetic surgery.
The physician explained that in the unlikely event of significant changes in the nevus, she should seek further evaluation. The patient understood the reality of the situation and thanked the doctor for explaining the diagnosis to her. She was reassured that this was benign.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Smith M, Usatine R. Benign nevi. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013:945-952.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/.
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com.
cSCC staging systems poorly determine metastasis risk
Current staging systems for cutaneous squamous cell carcinoma (cSCC) poorly discerned between patients with and without metastases, according to researchers.
In a population-based case-control study of 6,721 cSCC patients, the American Joint Committee on Cancer, 7th edition (AJCC 7) staging system had the lowest rate of correctly classified cases (61.8%), followed by the AJCC 8 (68.2%), the Brigham and Women’s Hospital (BWH) system (72.3%), and the Breuninger system (76.2%). The Breuninger system performed best, with sensitivity of 77.3%, specificity of 75%, correctly classified tumor rate of 76.2%, and concordance index (C-index) of 0.81, reported Ingrid Roscher, MD, of the department of dermatology at Oslo University Hospital, and her coauthors. The report was published in JAMA Dermatology.
Investigators used data from the Cancer Registry of Norway to identify 6,721 patients with a first cSCC diagnosis between Jan. 1, 2000, and Dec. 31, 2004. Metastasis status was split into one of two categories: no metastases (local disease only) and metastasis (regional lymph node or distant metastasis).
Within 5 years follow-up, 112 patients had developed metastasis, and 112 patients without metastasis were selected at random as controls. Tumor tissue was collected for all 224 patients and checked by a pathologist. Tumors were classified under all four staging systems, and a chi-squared test was performed to compare patients with and without metastasis. Relative risk of metastasis was calculated via logistic regression analyses.
Sensitivity, specificity, and correctly identified cases were used to evaluate performance of the staging systems, and C-index was used to measure discriminatory ability, wrote Dr. Roscher and her colleagues.
AJCC 7 had a sensitivity of 85.6%, specificity of 33.3%, and C-index value of 0.59; compared with AJCC scores of 67.1%, 69.6%, and 0.70 for sensitivity, specificity, and C-index, respectively. BWH had a sensitivity of 68.9%, specificity of 76.5%, and C-index of 0.75.
Within the AJCC 7 system, 85.6% of patients with metastasis and 66.7% without metastasis fell into the T2 category, and no patients were grouped into T3 or T4 (P = .003). Under the BWH system, patients without metastasis fell mostly into the T1 category, while those with metastasis were about equally distributed among T1, T2a, and T2b (P less than .001). With the Breuninger system, more patients with metastasis than without metastasis fell into the high-risk categories for tumor diameter and depth (P less than .001). Lastly, under AJCC 8, 10% of all patients fell into the T2 category, while less than 20% of patients without metastasis and more than 50% of patients with metastasis fell into the T3 category (P less than .001).
Risk of metastasis for T2 patients was greater than for T1 patients under the AJCC 7 system (odds ratio = 2.96; 95% confidence interval, 1.43-6.15). Under BWH, OR for metastasis were 4.6 (95% CI, 2.23-9.49) for T2a patients and 21.31 (95% CI, 6.07-74.88) for T2b patients. Under the Breuninger system, tumor diameter greater than 2 cm (OR = 5.92; 95% CI, 2.18-16.07) and depth of invasion greater than 6 mm (OR = 9.00; 95% CI, 3.51-32.31) increased risk of metastasis. The AJCC 8 system showed increased metastasis risk for T2 (OR = 2.00; 95% CI, 0.62-6.44) and T3 (OR = 6.14; 95% CI, 0.41-1.09) patients, the authors reported.
The results suggest that the current staging systems “distinguished poorly to moderately between patients who developed metastases and those who did not,” Dr. Roscher and her coauthors wrote. Moreover, “the poorest results were found for the AJCC 7 system, which is most widely used,” they added.
“Our findings indicate a need for a more reliable, easy-to-perform, and clinically useful staging system than those presently available,” they concluded.
Oslo University Hospital and the Cancer Registry of Norway funded the study. No other disclosures were reported.
SOURCE: Roscher et al. JAMA Dermatol. 2018 March 7 doi: 10.1001/jamadermatol.2017.6428.
Current staging systems for cutaneous squamous cell carcinoma (cSCC) poorly discerned between patients with and without metastases, according to researchers.
In a population-based case-control study of 6,721 cSCC patients, the American Joint Committee on Cancer, 7th edition (AJCC 7) staging system had the lowest rate of correctly classified cases (61.8%), followed by the AJCC 8 (68.2%), the Brigham and Women’s Hospital (BWH) system (72.3%), and the Breuninger system (76.2%). The Breuninger system performed best, with sensitivity of 77.3%, specificity of 75%, correctly classified tumor rate of 76.2%, and concordance index (C-index) of 0.81, reported Ingrid Roscher, MD, of the department of dermatology at Oslo University Hospital, and her coauthors. The report was published in JAMA Dermatology.
Investigators used data from the Cancer Registry of Norway to identify 6,721 patients with a first cSCC diagnosis between Jan. 1, 2000, and Dec. 31, 2004. Metastasis status was split into one of two categories: no metastases (local disease only) and metastasis (regional lymph node or distant metastasis).
Within 5 years follow-up, 112 patients had developed metastasis, and 112 patients without metastasis were selected at random as controls. Tumor tissue was collected for all 224 patients and checked by a pathologist. Tumors were classified under all four staging systems, and a chi-squared test was performed to compare patients with and without metastasis. Relative risk of metastasis was calculated via logistic regression analyses.
Sensitivity, specificity, and correctly identified cases were used to evaluate performance of the staging systems, and C-index was used to measure discriminatory ability, wrote Dr. Roscher and her colleagues.
AJCC 7 had a sensitivity of 85.6%, specificity of 33.3%, and C-index value of 0.59; compared with AJCC scores of 67.1%, 69.6%, and 0.70 for sensitivity, specificity, and C-index, respectively. BWH had a sensitivity of 68.9%, specificity of 76.5%, and C-index of 0.75.
Within the AJCC 7 system, 85.6% of patients with metastasis and 66.7% without metastasis fell into the T2 category, and no patients were grouped into T3 or T4 (P = .003). Under the BWH system, patients without metastasis fell mostly into the T1 category, while those with metastasis were about equally distributed among T1, T2a, and T2b (P less than .001). With the Breuninger system, more patients with metastasis than without metastasis fell into the high-risk categories for tumor diameter and depth (P less than .001). Lastly, under AJCC 8, 10% of all patients fell into the T2 category, while less than 20% of patients without metastasis and more than 50% of patients with metastasis fell into the T3 category (P less than .001).
Risk of metastasis for T2 patients was greater than for T1 patients under the AJCC 7 system (odds ratio = 2.96; 95% confidence interval, 1.43-6.15). Under BWH, OR for metastasis were 4.6 (95% CI, 2.23-9.49) for T2a patients and 21.31 (95% CI, 6.07-74.88) for T2b patients. Under the Breuninger system, tumor diameter greater than 2 cm (OR = 5.92; 95% CI, 2.18-16.07) and depth of invasion greater than 6 mm (OR = 9.00; 95% CI, 3.51-32.31) increased risk of metastasis. The AJCC 8 system showed increased metastasis risk for T2 (OR = 2.00; 95% CI, 0.62-6.44) and T3 (OR = 6.14; 95% CI, 0.41-1.09) patients, the authors reported.
The results suggest that the current staging systems “distinguished poorly to moderately between patients who developed metastases and those who did not,” Dr. Roscher and her coauthors wrote. Moreover, “the poorest results were found for the AJCC 7 system, which is most widely used,” they added.
“Our findings indicate a need for a more reliable, easy-to-perform, and clinically useful staging system than those presently available,” they concluded.
Oslo University Hospital and the Cancer Registry of Norway funded the study. No other disclosures were reported.
SOURCE: Roscher et al. JAMA Dermatol. 2018 March 7 doi: 10.1001/jamadermatol.2017.6428.
Current staging systems for cutaneous squamous cell carcinoma (cSCC) poorly discerned between patients with and without metastases, according to researchers.
In a population-based case-control study of 6,721 cSCC patients, the American Joint Committee on Cancer, 7th edition (AJCC 7) staging system had the lowest rate of correctly classified cases (61.8%), followed by the AJCC 8 (68.2%), the Brigham and Women’s Hospital (BWH) system (72.3%), and the Breuninger system (76.2%). The Breuninger system performed best, with sensitivity of 77.3%, specificity of 75%, correctly classified tumor rate of 76.2%, and concordance index (C-index) of 0.81, reported Ingrid Roscher, MD, of the department of dermatology at Oslo University Hospital, and her coauthors. The report was published in JAMA Dermatology.
Investigators used data from the Cancer Registry of Norway to identify 6,721 patients with a first cSCC diagnosis between Jan. 1, 2000, and Dec. 31, 2004. Metastasis status was split into one of two categories: no metastases (local disease only) and metastasis (regional lymph node or distant metastasis).
Within 5 years follow-up, 112 patients had developed metastasis, and 112 patients without metastasis were selected at random as controls. Tumor tissue was collected for all 224 patients and checked by a pathologist. Tumors were classified under all four staging systems, and a chi-squared test was performed to compare patients with and without metastasis. Relative risk of metastasis was calculated via logistic regression analyses.
Sensitivity, specificity, and correctly identified cases were used to evaluate performance of the staging systems, and C-index was used to measure discriminatory ability, wrote Dr. Roscher and her colleagues.
AJCC 7 had a sensitivity of 85.6%, specificity of 33.3%, and C-index value of 0.59; compared with AJCC scores of 67.1%, 69.6%, and 0.70 for sensitivity, specificity, and C-index, respectively. BWH had a sensitivity of 68.9%, specificity of 76.5%, and C-index of 0.75.
Within the AJCC 7 system, 85.6% of patients with metastasis and 66.7% without metastasis fell into the T2 category, and no patients were grouped into T3 or T4 (P = .003). Under the BWH system, patients without metastasis fell mostly into the T1 category, while those with metastasis were about equally distributed among T1, T2a, and T2b (P less than .001). With the Breuninger system, more patients with metastasis than without metastasis fell into the high-risk categories for tumor diameter and depth (P less than .001). Lastly, under AJCC 8, 10% of all patients fell into the T2 category, while less than 20% of patients without metastasis and more than 50% of patients with metastasis fell into the T3 category (P less than .001).
Risk of metastasis for T2 patients was greater than for T1 patients under the AJCC 7 system (odds ratio = 2.96; 95% confidence interval, 1.43-6.15). Under BWH, OR for metastasis were 4.6 (95% CI, 2.23-9.49) for T2a patients and 21.31 (95% CI, 6.07-74.88) for T2b patients. Under the Breuninger system, tumor diameter greater than 2 cm (OR = 5.92; 95% CI, 2.18-16.07) and depth of invasion greater than 6 mm (OR = 9.00; 95% CI, 3.51-32.31) increased risk of metastasis. The AJCC 8 system showed increased metastasis risk for T2 (OR = 2.00; 95% CI, 0.62-6.44) and T3 (OR = 6.14; 95% CI, 0.41-1.09) patients, the authors reported.
The results suggest that the current staging systems “distinguished poorly to moderately between patients who developed metastases and those who did not,” Dr. Roscher and her coauthors wrote. Moreover, “the poorest results were found for the AJCC 7 system, which is most widely used,” they added.
“Our findings indicate a need for a more reliable, easy-to-perform, and clinically useful staging system than those presently available,” they concluded.
Oslo University Hospital and the Cancer Registry of Norway funded the study. No other disclosures were reported.
SOURCE: Roscher et al. JAMA Dermatol. 2018 March 7 doi: 10.1001/jamadermatol.2017.6428.
FROM JAMA DERMATOLOGY
Key clinical point: The four cSCC staging systems, especially the AJCC 7, poorly identified metastasis risk.
Major finding: The AJCC 7 staging system had the lowest rate of correctly classified cases (61.8%), followed by the AJCC 8 (68.2%), the Brigham and Women’s Hospital (BWH) system (72.3%), and the Breuninger system (76.2%).
Data source: A population-based case-control study of 6,721 patients with cSCC.
Disclosures: Oslo University Hospital and the Cancer Registry of Norway funded the study.
Source: Roscher et al. JAMA Dermatol. 2018 Mar 7. doi: 10.1001/jamadermatol.2017.6428.
Follow-up care for pediatric concussions not heeded in Ontario
reported Liraz Fridman, PhD, of York University, Toronto, and associates.
In a 10-year, retrospective, population-based study, 126,654 children aged 5-18 years presented with concussions to emergency departments and physician offices in Ontario between April 1, 2003, and March 31, 2014. In 2003, 7,126 children were evaluated for a concussion, compared with 21,681 children by 2013.
Limitations to this study include that children treated by athletic therapists or chiropractors would have been missed, and that these data may not generalize across Canada or the United States, they said.
“In Ontario, the rate of follow-up care for concussions nearly tripled in both emergency departments and physician’s offices. Despite this significant improvement over time, more than two-thirds of all child and youth concussion patients still do not receive the minimum standard of care, according to accepted guidelines,” the researchers wrote, adding that the study’s findings suggest that better instructions for health care providers on management of concussion are needed.
SOURCE: Fridman L et al. J Pediatr. 2018;192:184-8
reported Liraz Fridman, PhD, of York University, Toronto, and associates.
In a 10-year, retrospective, population-based study, 126,654 children aged 5-18 years presented with concussions to emergency departments and physician offices in Ontario between April 1, 2003, and March 31, 2014. In 2003, 7,126 children were evaluated for a concussion, compared with 21,681 children by 2013.
Limitations to this study include that children treated by athletic therapists or chiropractors would have been missed, and that these data may not generalize across Canada or the United States, they said.
“In Ontario, the rate of follow-up care for concussions nearly tripled in both emergency departments and physician’s offices. Despite this significant improvement over time, more than two-thirds of all child and youth concussion patients still do not receive the minimum standard of care, according to accepted guidelines,” the researchers wrote, adding that the study’s findings suggest that better instructions for health care providers on management of concussion are needed.
SOURCE: Fridman L et al. J Pediatr. 2018;192:184-8
reported Liraz Fridman, PhD, of York University, Toronto, and associates.
In a 10-year, retrospective, population-based study, 126,654 children aged 5-18 years presented with concussions to emergency departments and physician offices in Ontario between April 1, 2003, and March 31, 2014. In 2003, 7,126 children were evaluated for a concussion, compared with 21,681 children by 2013.
Limitations to this study include that children treated by athletic therapists or chiropractors would have been missed, and that these data may not generalize across Canada or the United States, they said.
“In Ontario, the rate of follow-up care for concussions nearly tripled in both emergency departments and physician’s offices. Despite this significant improvement over time, more than two-thirds of all child and youth concussion patients still do not receive the minimum standard of care, according to accepted guidelines,” the researchers wrote, adding that the study’s findings suggest that better instructions for health care providers on management of concussion are needed.
SOURCE: Fridman L et al. J Pediatr. 2018;192:184-8
FROM THE JOURNAL OF PEDIATRICS
Mission to Mars: Endocrinologists take on populating deep space
by studying the effects of space radiation and other factors on male and female fertility, according to Teresa K. Woodruff, PhD, the Thomas J. Watkins Memorial Professor of Obstetrics & Gynecology, the vice chair of research for the department of obstetrics and gynecology, and the chief of the division of reproductive science and medicine at Northwestern University, Chicago, who is chairing a session on March 17 at the 2018 Endocrine Society annual meeting entitled: “Mission to Mars.”
The project was developed based on the anticipation that “we will continue to explore space as an inhabitable frontier.” The talks in this session will examine endocrine-based reproduction such as sperm function and biologic fertilization that has occurred in space, noted Dr. Woodruff, who is also professor of molecular biosciences in the Weinberg College of Arts and Sciences and professor of biomedical engineering in the McCormick School of Engineering, both at Northwestern University.
Dr. Woodruff currently has a grant under review by the National Aeronautics and Space Administration that is relevant to the mission of populating space. “If we are going to populate space, which we will need to do based on the length of time we will be there, we are going to have to get gametes into space,” she said. There will be more and more research effort focused on the needs of the humans who are “non-Earth dwelling,” she said.
The next step might be to study embryo development in that environment. Such research would take place at the International Space Station.
“I am interested in endocrine disruptors. What we learn from the study of the challenges to reproduction in space we will be able to apply on earth,” Dr. Woodruff said.
Also on the panel will be Joseph S. Tash, PhD, of Kansas University, Kansas City, whose grant on Space Flight Impacts on Gonadal and Gamete Function already has NASA funding, according to Dr. Woodruff.
by studying the effects of space radiation and other factors on male and female fertility, according to Teresa K. Woodruff, PhD, the Thomas J. Watkins Memorial Professor of Obstetrics & Gynecology, the vice chair of research for the department of obstetrics and gynecology, and the chief of the division of reproductive science and medicine at Northwestern University, Chicago, who is chairing a session on March 17 at the 2018 Endocrine Society annual meeting entitled: “Mission to Mars.”
The project was developed based on the anticipation that “we will continue to explore space as an inhabitable frontier.” The talks in this session will examine endocrine-based reproduction such as sperm function and biologic fertilization that has occurred in space, noted Dr. Woodruff, who is also professor of molecular biosciences in the Weinberg College of Arts and Sciences and professor of biomedical engineering in the McCormick School of Engineering, both at Northwestern University.
Dr. Woodruff currently has a grant under review by the National Aeronautics and Space Administration that is relevant to the mission of populating space. “If we are going to populate space, which we will need to do based on the length of time we will be there, we are going to have to get gametes into space,” she said. There will be more and more research effort focused on the needs of the humans who are “non-Earth dwelling,” she said.
The next step might be to study embryo development in that environment. Such research would take place at the International Space Station.
“I am interested in endocrine disruptors. What we learn from the study of the challenges to reproduction in space we will be able to apply on earth,” Dr. Woodruff said.
Also on the panel will be Joseph S. Tash, PhD, of Kansas University, Kansas City, whose grant on Space Flight Impacts on Gonadal and Gamete Function already has NASA funding, according to Dr. Woodruff.
by studying the effects of space radiation and other factors on male and female fertility, according to Teresa K. Woodruff, PhD, the Thomas J. Watkins Memorial Professor of Obstetrics & Gynecology, the vice chair of research for the department of obstetrics and gynecology, and the chief of the division of reproductive science and medicine at Northwestern University, Chicago, who is chairing a session on March 17 at the 2018 Endocrine Society annual meeting entitled: “Mission to Mars.”
The project was developed based on the anticipation that “we will continue to explore space as an inhabitable frontier.” The talks in this session will examine endocrine-based reproduction such as sperm function and biologic fertilization that has occurred in space, noted Dr. Woodruff, who is also professor of molecular biosciences in the Weinberg College of Arts and Sciences and professor of biomedical engineering in the McCormick School of Engineering, both at Northwestern University.
Dr. Woodruff currently has a grant under review by the National Aeronautics and Space Administration that is relevant to the mission of populating space. “If we are going to populate space, which we will need to do based on the length of time we will be there, we are going to have to get gametes into space,” she said. There will be more and more research effort focused on the needs of the humans who are “non-Earth dwelling,” she said.
The next step might be to study embryo development in that environment. Such research would take place at the International Space Station.
“I am interested in endocrine disruptors. What we learn from the study of the challenges to reproduction in space we will be able to apply on earth,” Dr. Woodruff said.
Also on the panel will be Joseph S. Tash, PhD, of Kansas University, Kansas City, whose grant on Space Flight Impacts on Gonadal and Gamete Function already has NASA funding, according to Dr. Woodruff.
Laparoscopic hysterectomy safest even for markedly enlarged uteri
ORLANDO – according to findings from a nationwide cohort of more than 27,000 women.
After adjusting for numerous potential confounding factors, including medical risk factors, procedure-related variables, and patient demographics, increasing uterine weight was significantly associated with increasing odds of complications – particularly after hysterectomy for uteri over 500 g, Michelle Louie, MD, reported during an oral poster session at the annual scientific meeting of the Society of Gynecologic Surgeons.
The same was true for uteri of 250-500 g (adjusted OR, 0.99, 1.73, and 1.06, respectively), she noted, adding that “abdominal hysterectomy always has the highest rate of a complication, except at above 850 g, when a vaginal hysterectomy is associated with a greater odds of complications.”
This secondary analysis was performed using prospectively collected quality improvement data abstracted from the American College of Surgeons National Surgical Quality Improvement Program database, which includes patient information and 30-day outcomes from more than 500 U.S. hospitals. Patients included in the analysis were 27,167 women who underwent a hysterectomy for benign conditions during 2014-2015 for whom uterine size was reported. Complications assessed included infection, vascular complications, reoperation, and readmission.
“Our study suggests that uterine weight is not an appropriate indication for abdominal hysterectomy – that we can, and should, offer a laparoscopic approach even for a markedly enlarged uterus,” she said. “We believe, therefore, that patients may benefit from referral to specialty surgeons who are able to offer a laparoscopic approach, even for a very large uterus.”
In response to a question from the audience about the role of physician experience in the findings, Dr. Louie said that it was not a covariate for which information was available, thus it was not included in the analysis.
“However, I think all of us realize that surgeon volume and surgeon experience is an important factor for patient safety,” she said.
Dr. Louie has received consulting fees from Teleflex.
SOURCE: Louie M et al. SGS 2018, Oral Poster 06.
ORLANDO – according to findings from a nationwide cohort of more than 27,000 women.
After adjusting for numerous potential confounding factors, including medical risk factors, procedure-related variables, and patient demographics, increasing uterine weight was significantly associated with increasing odds of complications – particularly after hysterectomy for uteri over 500 g, Michelle Louie, MD, reported during an oral poster session at the annual scientific meeting of the Society of Gynecologic Surgeons.
The same was true for uteri of 250-500 g (adjusted OR, 0.99, 1.73, and 1.06, respectively), she noted, adding that “abdominal hysterectomy always has the highest rate of a complication, except at above 850 g, when a vaginal hysterectomy is associated with a greater odds of complications.”
This secondary analysis was performed using prospectively collected quality improvement data abstracted from the American College of Surgeons National Surgical Quality Improvement Program database, which includes patient information and 30-day outcomes from more than 500 U.S. hospitals. Patients included in the analysis were 27,167 women who underwent a hysterectomy for benign conditions during 2014-2015 for whom uterine size was reported. Complications assessed included infection, vascular complications, reoperation, and readmission.
“Our study suggests that uterine weight is not an appropriate indication for abdominal hysterectomy – that we can, and should, offer a laparoscopic approach even for a markedly enlarged uterus,” she said. “We believe, therefore, that patients may benefit from referral to specialty surgeons who are able to offer a laparoscopic approach, even for a very large uterus.”
In response to a question from the audience about the role of physician experience in the findings, Dr. Louie said that it was not a covariate for which information was available, thus it was not included in the analysis.
“However, I think all of us realize that surgeon volume and surgeon experience is an important factor for patient safety,” she said.
Dr. Louie has received consulting fees from Teleflex.
SOURCE: Louie M et al. SGS 2018, Oral Poster 06.
ORLANDO – according to findings from a nationwide cohort of more than 27,000 women.
After adjusting for numerous potential confounding factors, including medical risk factors, procedure-related variables, and patient demographics, increasing uterine weight was significantly associated with increasing odds of complications – particularly after hysterectomy for uteri over 500 g, Michelle Louie, MD, reported during an oral poster session at the annual scientific meeting of the Society of Gynecologic Surgeons.
The same was true for uteri of 250-500 g (adjusted OR, 0.99, 1.73, and 1.06, respectively), she noted, adding that “abdominal hysterectomy always has the highest rate of a complication, except at above 850 g, when a vaginal hysterectomy is associated with a greater odds of complications.”
This secondary analysis was performed using prospectively collected quality improvement data abstracted from the American College of Surgeons National Surgical Quality Improvement Program database, which includes patient information and 30-day outcomes from more than 500 U.S. hospitals. Patients included in the analysis were 27,167 women who underwent a hysterectomy for benign conditions during 2014-2015 for whom uterine size was reported. Complications assessed included infection, vascular complications, reoperation, and readmission.
“Our study suggests that uterine weight is not an appropriate indication for abdominal hysterectomy – that we can, and should, offer a laparoscopic approach even for a markedly enlarged uterus,” she said. “We believe, therefore, that patients may benefit from referral to specialty surgeons who are able to offer a laparoscopic approach, even for a very large uterus.”
In response to a question from the audience about the role of physician experience in the findings, Dr. Louie said that it was not a covariate for which information was available, thus it was not included in the analysis.
“However, I think all of us realize that surgeon volume and surgeon experience is an important factor for patient safety,” she said.
Dr. Louie has received consulting fees from Teleflex.
SOURCE: Louie M et al. SGS 2018, Oral Poster 06.
REPORTING FROM SGS 2018
Key clinical point: Laparoscopic hysterectomy can and should be offered to women with uteri over 500 g.
Major finding: The odds ratios for complications after laparoscopic, abdominal, and vaginal hysterectomy for uteri over 500 g were 1.61, 2.16, and 2.57, respectively.
Study details: A secondary analysis of a nationwide cohort of 27,167 women.
Disclosures: Dr. Louie has received consulting fees from Teleflex.
Source: Louie M et al. SGS 2018, Oral Poster 06.