User login
Developmental disabilities up significantly since 2014
In 2016, the prevalence of any diagnosed developmental disability in children aged 3-17 years was 6.99% – a statistically significant increase of 21% over the 5.76% recorded in 2014, the NCHS said in a recent Data Brief.
Autism spectrum disorder was up by a similar amount: 23% from 2014, when prevalence was 2.24%, to 2016, when the prevalence was 2.76% among children aged 3-17 years. Intellectual disability rose in 2015 but dropped in 2016, so the overall increase in prevalence was just 3.6%. The prevalence of other developmental delays, on the other hand, held steady from 2014 to 2015 and then took a big jump, 27.5%, in 2016, the NCHS investigators reported.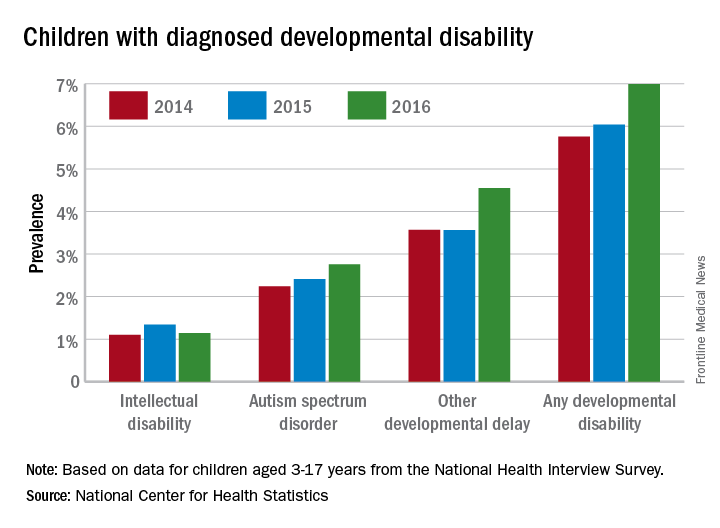
The estimates are based on reports by parents or guardians of ever receiving a diagnosis of each developmental disability from a physician or other medical professional.
In 2016, the prevalence of any diagnosed developmental disability in children aged 3-17 years was 6.99% – a statistically significant increase of 21% over the 5.76% recorded in 2014, the NCHS said in a recent Data Brief.
Autism spectrum disorder was up by a similar amount: 23% from 2014, when prevalence was 2.24%, to 2016, when the prevalence was 2.76% among children aged 3-17 years. Intellectual disability rose in 2015 but dropped in 2016, so the overall increase in prevalence was just 3.6%. The prevalence of other developmental delays, on the other hand, held steady from 2014 to 2015 and then took a big jump, 27.5%, in 2016, the NCHS investigators reported.
The estimates are based on reports by parents or guardians of ever receiving a diagnosis of each developmental disability from a physician or other medical professional.
In 2016, the prevalence of any diagnosed developmental disability in children aged 3-17 years was 6.99% – a statistically significant increase of 21% over the 5.76% recorded in 2014, the NCHS said in a recent Data Brief.
Autism spectrum disorder was up by a similar amount: 23% from 2014, when prevalence was 2.24%, to 2016, when the prevalence was 2.76% among children aged 3-17 years. Intellectual disability rose in 2015 but dropped in 2016, so the overall increase in prevalence was just 3.6%. The prevalence of other developmental delays, on the other hand, held steady from 2014 to 2015 and then took a big jump, 27.5%, in 2016, the NCHS investigators reported.
The estimates are based on reports by parents or guardians of ever receiving a diagnosis of each developmental disability from a physician or other medical professional.
Activating the Immune System to Treat Multiple Myeloma
Sagar Lonial, MD
Chief Medical Officer, Winship Cancer Institute of Emory University
Chair, Dept. of Hematology and Medical Oncology, Emory School of Medicine
Faculty/Faculty Disclosure
Dr. Lonial reports that he is a compensated consultant for Bristol-Myers Squibb; Celgene Corporation; Janssen Pharmaceuticals, Inc.; Merck & Co., Inc.; Millennium Pharmaceuticals, Inc.; Novartis Pharmaceuticals Corporation; and Onyx Pharmaceuticals, Inc.
Sagar Lonial, MD
Chief Medical Officer, Winship Cancer Institute of Emory University
Chair, Dept. of Hematology and Medical Oncology, Emory School of Medicine
Faculty/Faculty Disclosure
Dr. Lonial reports that he is a compensated consultant for Bristol-Myers Squibb; Celgene Corporation; Janssen Pharmaceuticals, Inc.; Merck & Co., Inc.; Millennium Pharmaceuticals, Inc.; Novartis Pharmaceuticals Corporation; and Onyx Pharmaceuticals, Inc.
Sagar Lonial, MD
Chief Medical Officer, Winship Cancer Institute of Emory University
Chair, Dept. of Hematology and Medical Oncology, Emory School of Medicine
Faculty/Faculty Disclosure
Dr. Lonial reports that he is a compensated consultant for Bristol-Myers Squibb; Celgene Corporation; Janssen Pharmaceuticals, Inc.; Merck & Co., Inc.; Millennium Pharmaceuticals, Inc.; Novartis Pharmaceuticals Corporation; and Onyx Pharmaceuticals, Inc.
MACRA Monday: BMI screening and follow-up
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #128: Preventive Care and Screening: Body Mass Index Screening and Follow-Up Plan
This measure is aimed at capturing the percentage of patients aged 18 years and older who have had their body mass index (BMI) calculated and documented in the last 6 months and a follow-up plan developed if the BMI was too high or too low.
What you need to do: Assess the patient’s BMI during the visit or document that it was done in the last 6 months. Patient-reported height and weight values cannot be used. If the BMI is outside of normal parameters (18.5 kg/m2 to 25 kg/m2), develop a follow-up plan or document that one was made in the last 6 months.
Eligible cases include patients who were aged 18 years or older on the date of the encounter and a patient encounter during the performance period. Applicable codes include (CPT or HCPCS): 90791, 90792, 90832, 90834, 90837, 96150, 96151, 96152, 97161, 97162, 97163, 97165, 97166, 97167, 97802, 97803, 98960, 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, D7140, D7210, G0101, G0108, G0270, G0271, G0402, G0438, G0439, G0447 without telehealth modifiers GQ or GT.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, G8420 indicates that BMI has been documented within normal parameters and no follow-up plan is required, while G8417 and G8418 indicate BMI above and below normal parameters, respectively, with a documented follow-up plan.
Use exclusion code G8938 if the BMI has been documented as being outside of normal limits, but a follow-up plan is not documented because the patient is not eligible. For example, patients are considered not eligible if they are 65 years or older and weight reduction or gain would complicate an underlying health condition.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #128: Preventive Care and Screening: Body Mass Index Screening and Follow-Up Plan
This measure is aimed at capturing the percentage of patients aged 18 years and older who have had their body mass index (BMI) calculated and documented in the last 6 months and a follow-up plan developed if the BMI was too high or too low.
What you need to do: Assess the patient’s BMI during the visit or document that it was done in the last 6 months. Patient-reported height and weight values cannot be used. If the BMI is outside of normal parameters (18.5 kg/m2 to 25 kg/m2), develop a follow-up plan or document that one was made in the last 6 months.
Eligible cases include patients who were aged 18 years or older on the date of the encounter and a patient encounter during the performance period. Applicable codes include (CPT or HCPCS): 90791, 90792, 90832, 90834, 90837, 96150, 96151, 96152, 97161, 97162, 97163, 97165, 97166, 97167, 97802, 97803, 98960, 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, D7140, D7210, G0101, G0108, G0270, G0271, G0402, G0438, G0439, G0447 without telehealth modifiers GQ or GT.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, G8420 indicates that BMI has been documented within normal parameters and no follow-up plan is required, while G8417 and G8418 indicate BMI above and below normal parameters, respectively, with a documented follow-up plan.
Use exclusion code G8938 if the BMI has been documented as being outside of normal limits, but a follow-up plan is not documented because the patient is not eligible. For example, patients are considered not eligible if they are 65 years or older and weight reduction or gain would complicate an underlying health condition.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #128: Preventive Care and Screening: Body Mass Index Screening and Follow-Up Plan
This measure is aimed at capturing the percentage of patients aged 18 years and older who have had their body mass index (BMI) calculated and documented in the last 6 months and a follow-up plan developed if the BMI was too high or too low.
What you need to do: Assess the patient’s BMI during the visit or document that it was done in the last 6 months. Patient-reported height and weight values cannot be used. If the BMI is outside of normal parameters (18.5 kg/m2 to 25 kg/m2), develop a follow-up plan or document that one was made in the last 6 months.
Eligible cases include patients who were aged 18 years or older on the date of the encounter and a patient encounter during the performance period. Applicable codes include (CPT or HCPCS): 90791, 90792, 90832, 90834, 90837, 96150, 96151, 96152, 97161, 97162, 97163, 97165, 97166, 97167, 97802, 97803, 98960, 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, D7140, D7210, G0101, G0108, G0270, G0271, G0402, G0438, G0439, G0447 without telehealth modifiers GQ or GT.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, G8420 indicates that BMI has been documented within normal parameters and no follow-up plan is required, while G8417 and G8418 indicate BMI above and below normal parameters, respectively, with a documented follow-up plan.
Use exclusion code G8938 if the BMI has been documented as being outside of normal limits, but a follow-up plan is not documented because the patient is not eligible. For example, patients are considered not eligible if they are 65 years or older and weight reduction or gain would complicate an underlying health condition.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).
A Medication Tracker That Patients Swallow
Making sure some patients are taking their medication correctly may be a little easier now. The FDA has approved Abilify MyCite (apiprazole), which has an ingestible sensor.
Abilify MyCite is approved for treatment of schizophrenia, acute treatment of manic and mixed episodes associated with bipolar 1 disorder, and as an add-on treatment for depression in adults.
The sensor, embedded in the pill, records when the medicine was taken, and sends a message to a wearable patch, which then transmits information to a mobile application. Patients can track the ingestion of the medication on their smartphone and can allow their caregivers and physicians to access information through a web-based portal.
However, the FDA notes that Abilify MyCite’s prescribing information includes a caution that the product has not been shown to improve patient adherence with a treatment regimen. Moreover, Ability MyCite should not be used to track drug ingestion in real time or during an emergency, because detection may be delayed or may not occur. Before prescribing it for a patient, health care professionals should make sure the patient is capable and willing to use the drug, patch, and app.
Making sure some patients are taking their medication correctly may be a little easier now. The FDA has approved Abilify MyCite (apiprazole), which has an ingestible sensor.
Abilify MyCite is approved for treatment of schizophrenia, acute treatment of manic and mixed episodes associated with bipolar 1 disorder, and as an add-on treatment for depression in adults.
The sensor, embedded in the pill, records when the medicine was taken, and sends a message to a wearable patch, which then transmits information to a mobile application. Patients can track the ingestion of the medication on their smartphone and can allow their caregivers and physicians to access information through a web-based portal.
However, the FDA notes that Abilify MyCite’s prescribing information includes a caution that the product has not been shown to improve patient adherence with a treatment regimen. Moreover, Ability MyCite should not be used to track drug ingestion in real time or during an emergency, because detection may be delayed or may not occur. Before prescribing it for a patient, health care professionals should make sure the patient is capable and willing to use the drug, patch, and app.
Making sure some patients are taking their medication correctly may be a little easier now. The FDA has approved Abilify MyCite (apiprazole), which has an ingestible sensor.
Abilify MyCite is approved for treatment of schizophrenia, acute treatment of manic and mixed episodes associated with bipolar 1 disorder, and as an add-on treatment for depression in adults.
The sensor, embedded in the pill, records when the medicine was taken, and sends a message to a wearable patch, which then transmits information to a mobile application. Patients can track the ingestion of the medication on their smartphone and can allow their caregivers and physicians to access information through a web-based portal.
However, the FDA notes that Abilify MyCite’s prescribing information includes a caution that the product has not been shown to improve patient adherence with a treatment regimen. Moreover, Ability MyCite should not be used to track drug ingestion in real time or during an emergency, because detection may be delayed or may not occur. Before prescribing it for a patient, health care professionals should make sure the patient is capable and willing to use the drug, patch, and app.
When the Fix Fails
ANSWER
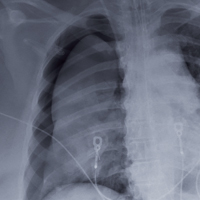
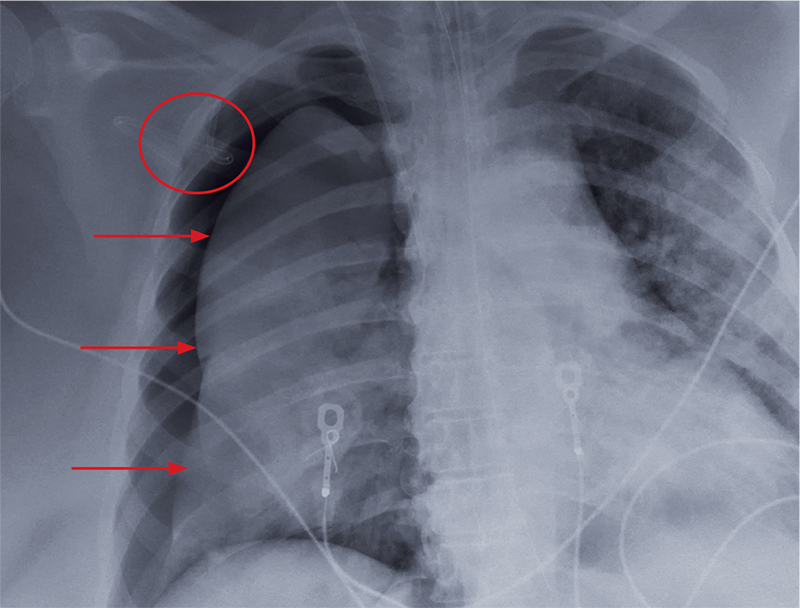
The radiograph shows an adequately positioned endotracheal tube. It also shows bilateral infiltrates, greater in the right than in the left lung.
A pigtail catheter is present, up high near the apex; despite this, a pneumothorax of moderate size remains on the right. The likely explanation is that the catheter is either not properly positioned or is kinked.
Prompt surgical consultation for new chest tube placement was obtained.

ANSWER
The radiograph shows an adequately positioned endotracheal tube. It also shows bilateral infiltrates, greater in the right than in the left lung.
A pigtail catheter is present, up high near the apex; despite this, a pneumothorax of moderate size remains on the right. The likely explanation is that the catheter is either not properly positioned or is kinked.
Prompt surgical consultation for new chest tube placement was obtained.

ANSWER
The radiograph shows an adequately positioned endotracheal tube. It also shows bilateral infiltrates, greater in the right than in the left lung.
A pigtail catheter is present, up high near the apex; despite this, a pneumothorax of moderate size remains on the right. The likely explanation is that the catheter is either not properly positioned or is kinked.
Prompt surgical consultation for new chest tube placement was obtained.
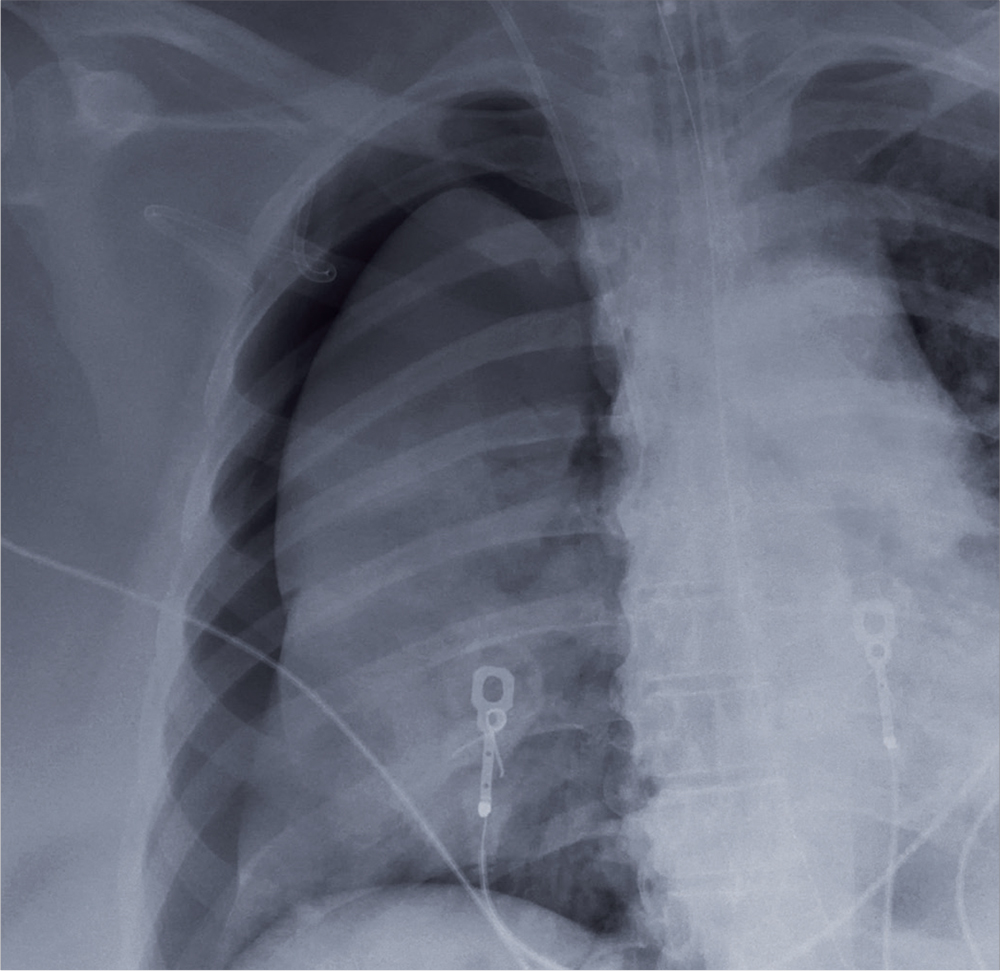
A 60-year-old woman is transferred to your facility from an outside hospital for tertiary care. She was reportedly at home with family when she suddenly collapsed and became unresponsive. She was taken to a nearby hospital, where she was resuscitated, stabilized, and urgently sent to your facility for possible cardiac intervention.
You assess the patient immediately upon arrival;with no family present, history is limited to the chart. You note an intubated female on mild sedation. Her vital signs include a temperature of 37.0°C; blood pressure, 130/80 mm Hg; heart rate, 70 beats/min; and O2 saturation, 98% on 100% FiO2.
The heart rate monitor shows sinus rhythm. A right chest tube is in place. Auscultation reveals bilateral rhonchi.
Portable chest radiograph is obtained (shown). What is your impression?
AHRQ Practice Toolbox: Health Literacy
This is the fifth in a series of articles from the National Center for Excellence in Primary Care Research (NCEPCR) in the Agency for Healthcare Research and Quality (AHRQ). This series introduces sets of tools and resources designed to help your practice.
Members of primary care teams know that to be successful, they have to communicate effectively with patients and family members. Communication skills, however, are not emphasized in health professional schools. Furthermore, clear communication is important not only for clinicians, but for all members of primary care practices. In order to help patients be successful, practices also need to help patients navigate what is often a confusing health system with demands that frequently exceed patients’ abilities.
Health literacy universal precautions are aimed at the following three things:
- Simplifying communication with and confirming comprehension for all patients.
- Making the office environment and health care system easier to navigate.
- Supporting patients’ efforts to improve their health.
The AHRQ Health Literacy Universal Precautions Toolkit is designed for the busy adult or pediatric primary care practice. Each of its 21 tools is only 3-5 pages long, and a practice can jump in wherever it likes. The tools specify concrete action steps and link to other resources. A Quick Start Guide lets you watch a 6-minute video, then pick one of three tools to implement: Conduct Brown Bag Medicine Reviews, Communicate Clearly, or Use the Teach-Back Method. Practices that want to start the journey toward becoming a health literate organization can start with the first two tools: Form a Team and Create a Health Literate Improvement Plan, which includes a practice self-assessment. Practices can also choose focus their efforts on one of the four health literacy domains:
- Spoken Communication
- Written Communication
- Self-Management and Empowerment
- Supportive Systems
In addition to the Toolkit, AHRQ has created a companion guide with concrete advice based on the implementation experiences of diverse primary care practices. At least one person – such as a practice facilitator, quality improvement specialist, or health literacy team leader – should read it before you get started.
Adopting health literacy universal precautions can help you reach your practice’s goals, whether they are becoming a patient-centered medical home or preparing for value-based payment.
Links to health literacy resources:
AHRQ Health Literacy Universal Precautions Toolkit, 2nd edition: https://www.ahrq.gov/literacy
Guide to Implementing the Health Literacy Universal Precautions Toolkit: Practical Ideas for Primary Care Practices: https://www.ahrq.gov/professionals/quality-patient-safety/quality-resources/tools/literacy-toolkit/impguide/index.html
Crosswalk between the tools included in the Toolkit and the PCMH certification standards (as of 2014) of the National Committee for Quality Assurance (NCQA), The Joint Commission, and the Utilization Review Accreditation Committee (URAC): https://www.ahrq.gov/sites/default/files/wysiwyg/professionals/quality-patient-safety/quality-resources/tools/literacy-toolkit/pcmh-crosswalk.pdf
Index of AHRQ Health Literacy Resources: https://www.ahrq.gov/professionals/clinicians-providers/resources/health-literacy.html
Diplomates of the American Board of Pediatrics and the American Board of Family Medicine can take health literacy modules (both knowledge self-assessment and performance improvement modules) for maintenance of certification.
Ms. Brach is Senior Health Care Researcher at AHRQ.
This is the fifth in a series of articles from the National Center for Excellence in Primary Care Research (NCEPCR) in the Agency for Healthcare Research and Quality (AHRQ). This series introduces sets of tools and resources designed to help your practice.
Members of primary care teams know that to be successful, they have to communicate effectively with patients and family members. Communication skills, however, are not emphasized in health professional schools. Furthermore, clear communication is important not only for clinicians, but for all members of primary care practices. In order to help patients be successful, practices also need to help patients navigate what is often a confusing health system with demands that frequently exceed patients’ abilities.
Health literacy universal precautions are aimed at the following three things:
- Simplifying communication with and confirming comprehension for all patients.
- Making the office environment and health care system easier to navigate.
- Supporting patients’ efforts to improve their health.
The AHRQ Health Literacy Universal Precautions Toolkit is designed for the busy adult or pediatric primary care practice. Each of its 21 tools is only 3-5 pages long, and a practice can jump in wherever it likes. The tools specify concrete action steps and link to other resources. A Quick Start Guide lets you watch a 6-minute video, then pick one of three tools to implement: Conduct Brown Bag Medicine Reviews, Communicate Clearly, or Use the Teach-Back Method. Practices that want to start the journey toward becoming a health literate organization can start with the first two tools: Form a Team and Create a Health Literate Improvement Plan, which includes a practice self-assessment. Practices can also choose focus their efforts on one of the four health literacy domains:
- Spoken Communication
- Written Communication
- Self-Management and Empowerment
- Supportive Systems
In addition to the Toolkit, AHRQ has created a companion guide with concrete advice based on the implementation experiences of diverse primary care practices. At least one person – such as a practice facilitator, quality improvement specialist, or health literacy team leader – should read it before you get started.
Adopting health literacy universal precautions can help you reach your practice’s goals, whether they are becoming a patient-centered medical home or preparing for value-based payment.
Links to health literacy resources:
AHRQ Health Literacy Universal Precautions Toolkit, 2nd edition: https://www.ahrq.gov/literacy
Guide to Implementing the Health Literacy Universal Precautions Toolkit: Practical Ideas for Primary Care Practices: https://www.ahrq.gov/professionals/quality-patient-safety/quality-resources/tools/literacy-toolkit/impguide/index.html
Crosswalk between the tools included in the Toolkit and the PCMH certification standards (as of 2014) of the National Committee for Quality Assurance (NCQA), The Joint Commission, and the Utilization Review Accreditation Committee (URAC): https://www.ahrq.gov/sites/default/files/wysiwyg/professionals/quality-patient-safety/quality-resources/tools/literacy-toolkit/pcmh-crosswalk.pdf
Index of AHRQ Health Literacy Resources: https://www.ahrq.gov/professionals/clinicians-providers/resources/health-literacy.html
Diplomates of the American Board of Pediatrics and the American Board of Family Medicine can take health literacy modules (both knowledge self-assessment and performance improvement modules) for maintenance of certification.
Ms. Brach is Senior Health Care Researcher at AHRQ.
This is the fifth in a series of articles from the National Center for Excellence in Primary Care Research (NCEPCR) in the Agency for Healthcare Research and Quality (AHRQ). This series introduces sets of tools and resources designed to help your practice.
Members of primary care teams know that to be successful, they have to communicate effectively with patients and family members. Communication skills, however, are not emphasized in health professional schools. Furthermore, clear communication is important not only for clinicians, but for all members of primary care practices. In order to help patients be successful, practices also need to help patients navigate what is often a confusing health system with demands that frequently exceed patients’ abilities.
Health literacy universal precautions are aimed at the following three things:
- Simplifying communication with and confirming comprehension for all patients.
- Making the office environment and health care system easier to navigate.
- Supporting patients’ efforts to improve their health.
The AHRQ Health Literacy Universal Precautions Toolkit is designed for the busy adult or pediatric primary care practice. Each of its 21 tools is only 3-5 pages long, and a practice can jump in wherever it likes. The tools specify concrete action steps and link to other resources. A Quick Start Guide lets you watch a 6-minute video, then pick one of three tools to implement: Conduct Brown Bag Medicine Reviews, Communicate Clearly, or Use the Teach-Back Method. Practices that want to start the journey toward becoming a health literate organization can start with the first two tools: Form a Team and Create a Health Literate Improvement Plan, which includes a practice self-assessment. Practices can also choose focus their efforts on one of the four health literacy domains:
- Spoken Communication
- Written Communication
- Self-Management and Empowerment
- Supportive Systems
In addition to the Toolkit, AHRQ has created a companion guide with concrete advice based on the implementation experiences of diverse primary care practices. At least one person – such as a practice facilitator, quality improvement specialist, or health literacy team leader – should read it before you get started.
Adopting health literacy universal precautions can help you reach your practice’s goals, whether they are becoming a patient-centered medical home or preparing for value-based payment.
Links to health literacy resources:
AHRQ Health Literacy Universal Precautions Toolkit, 2nd edition: https://www.ahrq.gov/literacy
Guide to Implementing the Health Literacy Universal Precautions Toolkit: Practical Ideas for Primary Care Practices: https://www.ahrq.gov/professionals/quality-patient-safety/quality-resources/tools/literacy-toolkit/impguide/index.html
Crosswalk between the tools included in the Toolkit and the PCMH certification standards (as of 2014) of the National Committee for Quality Assurance (NCQA), The Joint Commission, and the Utilization Review Accreditation Committee (URAC): https://www.ahrq.gov/sites/default/files/wysiwyg/professionals/quality-patient-safety/quality-resources/tools/literacy-toolkit/pcmh-crosswalk.pdf
Index of AHRQ Health Literacy Resources: https://www.ahrq.gov/professionals/clinicians-providers/resources/health-literacy.html
Diplomates of the American Board of Pediatrics and the American Board of Family Medicine can take health literacy modules (both knowledge self-assessment and performance improvement modules) for maintenance of certification.
Ms. Brach is Senior Health Care Researcher at AHRQ.
MAVORIC: Mogamulizumab tops vorinostat in pretreated CTCL
ATLANTA – Intravenous treatment with mogamulizumab, an investigational antibody targeting CC chemokine receptor 4, more than doubled progression-free survival (PFS), compared with oral vorinostat in a phase 3 trial of 372 patients with heavily pretreated cutaneous T-cell lymphoma (CTCL).
After a median of three treatment cycles, median PFS with mogamulizumab was 7.7 months vs. 3.1 months with vorinostat (hazard ratio, 0.53; 95% confidence interval, 0.41-0.69; P less than .0001), Youn H. Kim, MD, reported at the annual meeting of the American Society of Hematology.
, said Dr. Kim, the Joanne and Peter Haas, Jr. Professor for Cutaneous Lymphoma Research at Stanford (Calif.) University. Adverse effects, such as infusion reactions, were expected and manageable, she added.
Mogamulizumab is approved in Japan for treating CTCL and received an FDA breakthrough therapy designation in August 2017.
Based on audits so far, the agency might green-light mogamulizumab for previously treated CTCL by early 2018 – its first approval in the United States, Dr. Kim said in an interview.
Cutaneous T-cell lymphoma responds poorly to treatments that work in other, more common types of non-Hodgkin lymphoma. Moreover, extensive disease can destroy quality of life.
“There’s a major psychosocial impact because if you’re infected, you smell bad,” Dr. Kim said during a press briefing. “Itch is very severe – patients often cannot sleep because of it.”
Mogamulizumab is a humanized monoclonal antibody that targets CC chemokine receptor 4 (CCR4), which facilitates trafficking of lymphocytes to skin and other organs. It is defucosylated, augmenting its toxicity against malignant T cells. In a prior phase 1/2 study in the United States, mogamulizumab showed a tolerable safety profile and a 37% overall response rate – “a good response, considering that other CTCL drugs are usually in the 30% range,” Dr. Kim said.
For the phase 3 study (MAVORIC), 372 patients with previously treated stage IB to stage IVB CTCL (mycosis fungoides or Sézary syndrome) without large-cell transformation received mogamulizumab (1.0 mg/kg IV weekly for 28 days; days 1 and 15 of subsequent 28-day cycles) or vorinostat (400 mg per oral daily). Treatment continued until disease progression or intolerable toxicity. Researchers evaluated PFS based on a global composite response score that covers the skin, blood, lymph nodes, and viscera, in accordance with international consensus guidelines (J Clin Oncol. 2011 Jun 20;29(18):2598-607; doi: 10.1200/JCO.2010.32.0630).
Treatment groups resembled each other at baseline. Most had received three systemic therapies for CTCL, and some had received as many as 18. Median duration of response was 14 months in the mogamulizumab arm and 9 months in the vorinostat arm. Patients tended to respond to mogamulizumab 2 months sooner than to vorinostat (3.3 vs. 5.1 months), Dr. Kim said.
Mogamulizumab also significantly improved quality of life on the Skindex-29 Symptoms (P less than .05), Skindex-29 Function (P less than 05), and FACT-G Functional Well-Being (P less than .05) quality of life scales, which is part of what earned it a breakthrough therapy designation, Dr. Kim said.
MAVORIC is the largest randomized study to compare systemic therapies in CTCL and the first to use PFS as the primary endpoint, Dr. Kim noted. Patients’ level of CCR4 expression was not a criterion for enrollment because CCR4 is consistently and highly expressed in this disease, she noted. Thus, using mogamulizumab to treat CTCL in the United States would not require CCR4 testing.
Joseph M. Connors, MD, who specializes in lymphoid cancers at the BC Cancer Agency, a division of the British Columbia Provincial Health Services Authority, and who was not involved in the study, agreed that these data represent real headway in treating CTCL.
“I can state unequivocally that we just haven’t had effective therapy for CTCL,” he said at the press briefing. “We’ve had treatments that might help patients feel somewhat better, but we’ve had no consensus on a treatment that is right for this disease. These data provide an opportunity to have that consensus. They could create a platform for making further progress.”
Kyowa Kirin Pharmaceutical Development provided funding. Dr. Kim disclosed research and advisory relationships with Kyowa Kirin and ties to Millennium Pharmaceuticals, Seattle Genetics, Soligenix, and other companies.
SOURCE: Kim YH et al. ASH 2017 Abstract 817.
ATLANTA – Intravenous treatment with mogamulizumab, an investigational antibody targeting CC chemokine receptor 4, more than doubled progression-free survival (PFS), compared with oral vorinostat in a phase 3 trial of 372 patients with heavily pretreated cutaneous T-cell lymphoma (CTCL).
After a median of three treatment cycles, median PFS with mogamulizumab was 7.7 months vs. 3.1 months with vorinostat (hazard ratio, 0.53; 95% confidence interval, 0.41-0.69; P less than .0001), Youn H. Kim, MD, reported at the annual meeting of the American Society of Hematology.
, said Dr. Kim, the Joanne and Peter Haas, Jr. Professor for Cutaneous Lymphoma Research at Stanford (Calif.) University. Adverse effects, such as infusion reactions, were expected and manageable, she added.
Mogamulizumab is approved in Japan for treating CTCL and received an FDA breakthrough therapy designation in August 2017.
Based on audits so far, the agency might green-light mogamulizumab for previously treated CTCL by early 2018 – its first approval in the United States, Dr. Kim said in an interview.
Cutaneous T-cell lymphoma responds poorly to treatments that work in other, more common types of non-Hodgkin lymphoma. Moreover, extensive disease can destroy quality of life.
“There’s a major psychosocial impact because if you’re infected, you smell bad,” Dr. Kim said during a press briefing. “Itch is very severe – patients often cannot sleep because of it.”
Mogamulizumab is a humanized monoclonal antibody that targets CC chemokine receptor 4 (CCR4), which facilitates trafficking of lymphocytes to skin and other organs. It is defucosylated, augmenting its toxicity against malignant T cells. In a prior phase 1/2 study in the United States, mogamulizumab showed a tolerable safety profile and a 37% overall response rate – “a good response, considering that other CTCL drugs are usually in the 30% range,” Dr. Kim said.
For the phase 3 study (MAVORIC), 372 patients with previously treated stage IB to stage IVB CTCL (mycosis fungoides or Sézary syndrome) without large-cell transformation received mogamulizumab (1.0 mg/kg IV weekly for 28 days; days 1 and 15 of subsequent 28-day cycles) or vorinostat (400 mg per oral daily). Treatment continued until disease progression or intolerable toxicity. Researchers evaluated PFS based on a global composite response score that covers the skin, blood, lymph nodes, and viscera, in accordance with international consensus guidelines (J Clin Oncol. 2011 Jun 20;29(18):2598-607; doi: 10.1200/JCO.2010.32.0630).
Treatment groups resembled each other at baseline. Most had received three systemic therapies for CTCL, and some had received as many as 18. Median duration of response was 14 months in the mogamulizumab arm and 9 months in the vorinostat arm. Patients tended to respond to mogamulizumab 2 months sooner than to vorinostat (3.3 vs. 5.1 months), Dr. Kim said.
Mogamulizumab also significantly improved quality of life on the Skindex-29 Symptoms (P less than .05), Skindex-29 Function (P less than 05), and FACT-G Functional Well-Being (P less than .05) quality of life scales, which is part of what earned it a breakthrough therapy designation, Dr. Kim said.
MAVORIC is the largest randomized study to compare systemic therapies in CTCL and the first to use PFS as the primary endpoint, Dr. Kim noted. Patients’ level of CCR4 expression was not a criterion for enrollment because CCR4 is consistently and highly expressed in this disease, she noted. Thus, using mogamulizumab to treat CTCL in the United States would not require CCR4 testing.
Joseph M. Connors, MD, who specializes in lymphoid cancers at the BC Cancer Agency, a division of the British Columbia Provincial Health Services Authority, and who was not involved in the study, agreed that these data represent real headway in treating CTCL.
“I can state unequivocally that we just haven’t had effective therapy for CTCL,” he said at the press briefing. “We’ve had treatments that might help patients feel somewhat better, but we’ve had no consensus on a treatment that is right for this disease. These data provide an opportunity to have that consensus. They could create a platform for making further progress.”
Kyowa Kirin Pharmaceutical Development provided funding. Dr. Kim disclosed research and advisory relationships with Kyowa Kirin and ties to Millennium Pharmaceuticals, Seattle Genetics, Soligenix, and other companies.
SOURCE: Kim YH et al. ASH 2017 Abstract 817.
ATLANTA – Intravenous treatment with mogamulizumab, an investigational antibody targeting CC chemokine receptor 4, more than doubled progression-free survival (PFS), compared with oral vorinostat in a phase 3 trial of 372 patients with heavily pretreated cutaneous T-cell lymphoma (CTCL).
After a median of three treatment cycles, median PFS with mogamulizumab was 7.7 months vs. 3.1 months with vorinostat (hazard ratio, 0.53; 95% confidence interval, 0.41-0.69; P less than .0001), Youn H. Kim, MD, reported at the annual meeting of the American Society of Hematology.
, said Dr. Kim, the Joanne and Peter Haas, Jr. Professor for Cutaneous Lymphoma Research at Stanford (Calif.) University. Adverse effects, such as infusion reactions, were expected and manageable, she added.
Mogamulizumab is approved in Japan for treating CTCL and received an FDA breakthrough therapy designation in August 2017.
Based on audits so far, the agency might green-light mogamulizumab for previously treated CTCL by early 2018 – its first approval in the United States, Dr. Kim said in an interview.
Cutaneous T-cell lymphoma responds poorly to treatments that work in other, more common types of non-Hodgkin lymphoma. Moreover, extensive disease can destroy quality of life.
“There’s a major psychosocial impact because if you’re infected, you smell bad,” Dr. Kim said during a press briefing. “Itch is very severe – patients often cannot sleep because of it.”
Mogamulizumab is a humanized monoclonal antibody that targets CC chemokine receptor 4 (CCR4), which facilitates trafficking of lymphocytes to skin and other organs. It is defucosylated, augmenting its toxicity against malignant T cells. In a prior phase 1/2 study in the United States, mogamulizumab showed a tolerable safety profile and a 37% overall response rate – “a good response, considering that other CTCL drugs are usually in the 30% range,” Dr. Kim said.
For the phase 3 study (MAVORIC), 372 patients with previously treated stage IB to stage IVB CTCL (mycosis fungoides or Sézary syndrome) without large-cell transformation received mogamulizumab (1.0 mg/kg IV weekly for 28 days; days 1 and 15 of subsequent 28-day cycles) or vorinostat (400 mg per oral daily). Treatment continued until disease progression or intolerable toxicity. Researchers evaluated PFS based on a global composite response score that covers the skin, blood, lymph nodes, and viscera, in accordance with international consensus guidelines (J Clin Oncol. 2011 Jun 20;29(18):2598-607; doi: 10.1200/JCO.2010.32.0630).
Treatment groups resembled each other at baseline. Most had received three systemic therapies for CTCL, and some had received as many as 18. Median duration of response was 14 months in the mogamulizumab arm and 9 months in the vorinostat arm. Patients tended to respond to mogamulizumab 2 months sooner than to vorinostat (3.3 vs. 5.1 months), Dr. Kim said.
Mogamulizumab also significantly improved quality of life on the Skindex-29 Symptoms (P less than .05), Skindex-29 Function (P less than 05), and FACT-G Functional Well-Being (P less than .05) quality of life scales, which is part of what earned it a breakthrough therapy designation, Dr. Kim said.
MAVORIC is the largest randomized study to compare systemic therapies in CTCL and the first to use PFS as the primary endpoint, Dr. Kim noted. Patients’ level of CCR4 expression was not a criterion for enrollment because CCR4 is consistently and highly expressed in this disease, she noted. Thus, using mogamulizumab to treat CTCL in the United States would not require CCR4 testing.
Joseph M. Connors, MD, who specializes in lymphoid cancers at the BC Cancer Agency, a division of the British Columbia Provincial Health Services Authority, and who was not involved in the study, agreed that these data represent real headway in treating CTCL.
“I can state unequivocally that we just haven’t had effective therapy for CTCL,” he said at the press briefing. “We’ve had treatments that might help patients feel somewhat better, but we’ve had no consensus on a treatment that is right for this disease. These data provide an opportunity to have that consensus. They could create a platform for making further progress.”
Kyowa Kirin Pharmaceutical Development provided funding. Dr. Kim disclosed research and advisory relationships with Kyowa Kirin and ties to Millennium Pharmaceuticals, Seattle Genetics, Soligenix, and other companies.
SOURCE: Kim YH et al. ASH 2017 Abstract 817.
REPORTING FROM ASH 2017
Key clinical point: Mogamulizumab more than doubled median progression-free survival, compared with vorinostat in patients with previously treated cutaneous T-cell lymphoma.
Major finding: Median progression-free survival was 7.7 months vs. 3.1 months (HR, 0.53; 95% CI, 0.41 to 0.69; P less than .0001).
Data source: An open-label phase 3 trial of 372 patients with previously treated cutaneous T-cell lymphoma (MAVORIC).
Disclosures: Kyowa Kirin Pharmaceutical Development provided funding. Dr. Kim disclosed research and advisory relationships with Kyowa Kirin and ties to Millennium Pharmaceuticals, Seattle Genetics, Soligenix, and other companies.
Source: Kim YH et al. ASH 2017 Abstract 817.
CLARITY: Ibrutinib/venetoclax combo results look promising for relapsed/refractory CLL
ATLANTA – Combination therapy with ibrutinib and venetoclax is well tolerated and shows promise for the treatment of relapsed/refractory chronic lymphocytic leukemia (CLL), according to initial results from the CLARITY feasibility trial.
Of 38 patients who received at least 6 months of treatment with combination ibrutinib (Imbruvica)/venetoclax (Venclexta) and reached month 8 – and therefore had computed tomography, clinical data, and peripheral blood and marrow assessments available – 15 (37%) achieved peripheral blood minimal residual disease (MRD) negativity, and 12 (32%) achieved bone marrow MRD negativity, Peter Hillmen, MBChB, PhD, reported during a press briefing at the annual meeting of the American Society of Hematology.
The rates of MRD negativity in the blood and marrow, and of normal trephine biopsy, were similar in subsets of patients who relapsed within 36 months of prior treatment with fludarabine/cyclophosphamide/rituximab (FCR) or bendamustine/rituximab (BR), and with prior idelalisib exposure, he noted.
“In terms of [International Workshop on Chronic Lymphocytic Leukemia] response criteria, which is a secondary endpoint, 47% of patients achieved a [complete remission or complete remission with incomplete hematologic recovery] and every patient has had an overall response, which for this group of patients is impressive,” he said.
Again, the findings were similar in those who were refractory to prior FCR/BR or to previous idelalisib, he noted.
Both ibrutinib and venetoclax are approved as single agents for the treatment of CLL. Ibrutinib is a Bruton’s tyrosine kinase inhibitor that has had a major effect on patient outcomes, showing overall survival advantages in numerous trials, Dr. Hillmen said.
“However, ibrutinib does not eradicate disease, and patients remain on treatment indefinitely or until progression,” he said.
Venetoclax is a highly selective B cell lymphoma–2 inhibitor approved for refractory CLL in patients with 17p deletion. It has a rapid effect, which can lead to tumor lysis syndrome, but also leads to eradication of MRD in some patients, which can lead to prolonged survival, he said.
The CLARITY trial was designed to investigate the safety and efficacy of the two in combination in relapsed/refractory CLL patients.
The primary endpoint of the study is MRD eradication in the marrow after 12 months of treatment. The current analysis looks at a key secondary endpoint of the study – MRD eradication in the marrow after 6 months of treatment.
The study enrolled 54 patients, including 37 men and 17 women with a median age of 64 years; 20% have 17p deletion, and the population was heavily pretreated, with 81% having prior FCR or BR (44% with relapse within 3 years of treatment), and 20% with previous idelalisib exposure. Patients were excluded if they had prior exposure to ibrutinib or venetoclax.
Treatment involves ibrutinib monotherapy at a dose of 420 mg/day for 2 months to debulk the disease, after which venetoclax is added at a dose escalating from 20 mg to 400 mg/day over 2 months to reduce the risk of tumor lysis syndrome.
Bone marrow biopsies are performed at 6, 12, and 24 months. Treatment is discontinued at 12 months in those who achieve MRD negativity at 6 months, and is discontinued at 24 months in those who achieve MRD negativity at 12 months.
The combination treatment was well tolerated in the first 38 patients. Bruising (mainly grade 1) occurred in 33 patients, and neutropenia (including 16 grade 3 cases and 6 grade 4 cases) occurred in 25, and some GI toxicity occurred, but was largely grade 1 or 2, Dr. Hillmen said.
“There really was otherwise very acceptable toxicity,” he added, noting that a single case of tumor lysis syndrome occurred, but was managed successfully by delaying venetoclax.
“That patient re-escalated back onto treatment and is doing well,” he said.
No patients stopped treatment, and only seven had treatment interruption, and then only for a few days, he noted.
The findings are encouraging, and suggest a potent synergy between ibrutinib and venetoclax, said Dr. Hillmen.
“We’re seeing, even at this very early stage, over 30% of patients achieving MRD negative remission, which was our target at the 12-month bone marrow stage with this combination,” he said.
In light of these results, the ongoing phase 3 FLAIR trial, which is actively recruiting, has been modified to include combination ibrutinib and venetoclax in front-line CLL, he said.
Dr. Hillmen reported financial relationships with AbbVie and several other pharmaceutical companies. The CLARITY trial is supported by AbbVie, Bloodwise, Experimental Cancer Medicine Centre, Janssen-Cilag, the National Institute for Health Research Clinical Research Network: Cancer, and the University of Birmingham (England).
[email protected]
SOURCE: Hillmen P et al., ASH abstract 428.
ATLANTA – Combination therapy with ibrutinib and venetoclax is well tolerated and shows promise for the treatment of relapsed/refractory chronic lymphocytic leukemia (CLL), according to initial results from the CLARITY feasibility trial.
Of 38 patients who received at least 6 months of treatment with combination ibrutinib (Imbruvica)/venetoclax (Venclexta) and reached month 8 – and therefore had computed tomography, clinical data, and peripheral blood and marrow assessments available – 15 (37%) achieved peripheral blood minimal residual disease (MRD) negativity, and 12 (32%) achieved bone marrow MRD negativity, Peter Hillmen, MBChB, PhD, reported during a press briefing at the annual meeting of the American Society of Hematology.
The rates of MRD negativity in the blood and marrow, and of normal trephine biopsy, were similar in subsets of patients who relapsed within 36 months of prior treatment with fludarabine/cyclophosphamide/rituximab (FCR) or bendamustine/rituximab (BR), and with prior idelalisib exposure, he noted.
“In terms of [International Workshop on Chronic Lymphocytic Leukemia] response criteria, which is a secondary endpoint, 47% of patients achieved a [complete remission or complete remission with incomplete hematologic recovery] and every patient has had an overall response, which for this group of patients is impressive,” he said.
Again, the findings were similar in those who were refractory to prior FCR/BR or to previous idelalisib, he noted.
Both ibrutinib and venetoclax are approved as single agents for the treatment of CLL. Ibrutinib is a Bruton’s tyrosine kinase inhibitor that has had a major effect on patient outcomes, showing overall survival advantages in numerous trials, Dr. Hillmen said.
“However, ibrutinib does not eradicate disease, and patients remain on treatment indefinitely or until progression,” he said.
Venetoclax is a highly selective B cell lymphoma–2 inhibitor approved for refractory CLL in patients with 17p deletion. It has a rapid effect, which can lead to tumor lysis syndrome, but also leads to eradication of MRD in some patients, which can lead to prolonged survival, he said.
The CLARITY trial was designed to investigate the safety and efficacy of the two in combination in relapsed/refractory CLL patients.
The primary endpoint of the study is MRD eradication in the marrow after 12 months of treatment. The current analysis looks at a key secondary endpoint of the study – MRD eradication in the marrow after 6 months of treatment.
The study enrolled 54 patients, including 37 men and 17 women with a median age of 64 years; 20% have 17p deletion, and the population was heavily pretreated, with 81% having prior FCR or BR (44% with relapse within 3 years of treatment), and 20% with previous idelalisib exposure. Patients were excluded if they had prior exposure to ibrutinib or venetoclax.
Treatment involves ibrutinib monotherapy at a dose of 420 mg/day for 2 months to debulk the disease, after which venetoclax is added at a dose escalating from 20 mg to 400 mg/day over 2 months to reduce the risk of tumor lysis syndrome.
Bone marrow biopsies are performed at 6, 12, and 24 months. Treatment is discontinued at 12 months in those who achieve MRD negativity at 6 months, and is discontinued at 24 months in those who achieve MRD negativity at 12 months.
The combination treatment was well tolerated in the first 38 patients. Bruising (mainly grade 1) occurred in 33 patients, and neutropenia (including 16 grade 3 cases and 6 grade 4 cases) occurred in 25, and some GI toxicity occurred, but was largely grade 1 or 2, Dr. Hillmen said.
“There really was otherwise very acceptable toxicity,” he added, noting that a single case of tumor lysis syndrome occurred, but was managed successfully by delaying venetoclax.
“That patient re-escalated back onto treatment and is doing well,” he said.
No patients stopped treatment, and only seven had treatment interruption, and then only for a few days, he noted.
The findings are encouraging, and suggest a potent synergy between ibrutinib and venetoclax, said Dr. Hillmen.
“We’re seeing, even at this very early stage, over 30% of patients achieving MRD negative remission, which was our target at the 12-month bone marrow stage with this combination,” he said.
In light of these results, the ongoing phase 3 FLAIR trial, which is actively recruiting, has been modified to include combination ibrutinib and venetoclax in front-line CLL, he said.
Dr. Hillmen reported financial relationships with AbbVie and several other pharmaceutical companies. The CLARITY trial is supported by AbbVie, Bloodwise, Experimental Cancer Medicine Centre, Janssen-Cilag, the National Institute for Health Research Clinical Research Network: Cancer, and the University of Birmingham (England).
[email protected]
SOURCE: Hillmen P et al., ASH abstract 428.
ATLANTA – Combination therapy with ibrutinib and venetoclax is well tolerated and shows promise for the treatment of relapsed/refractory chronic lymphocytic leukemia (CLL), according to initial results from the CLARITY feasibility trial.
Of 38 patients who received at least 6 months of treatment with combination ibrutinib (Imbruvica)/venetoclax (Venclexta) and reached month 8 – and therefore had computed tomography, clinical data, and peripheral blood and marrow assessments available – 15 (37%) achieved peripheral blood minimal residual disease (MRD) negativity, and 12 (32%) achieved bone marrow MRD negativity, Peter Hillmen, MBChB, PhD, reported during a press briefing at the annual meeting of the American Society of Hematology.
The rates of MRD negativity in the blood and marrow, and of normal trephine biopsy, were similar in subsets of patients who relapsed within 36 months of prior treatment with fludarabine/cyclophosphamide/rituximab (FCR) or bendamustine/rituximab (BR), and with prior idelalisib exposure, he noted.
“In terms of [International Workshop on Chronic Lymphocytic Leukemia] response criteria, which is a secondary endpoint, 47% of patients achieved a [complete remission or complete remission with incomplete hematologic recovery] and every patient has had an overall response, which for this group of patients is impressive,” he said.
Again, the findings were similar in those who were refractory to prior FCR/BR or to previous idelalisib, he noted.
Both ibrutinib and venetoclax are approved as single agents for the treatment of CLL. Ibrutinib is a Bruton’s tyrosine kinase inhibitor that has had a major effect on patient outcomes, showing overall survival advantages in numerous trials, Dr. Hillmen said.
“However, ibrutinib does not eradicate disease, and patients remain on treatment indefinitely or until progression,” he said.
Venetoclax is a highly selective B cell lymphoma–2 inhibitor approved for refractory CLL in patients with 17p deletion. It has a rapid effect, which can lead to tumor lysis syndrome, but also leads to eradication of MRD in some patients, which can lead to prolonged survival, he said.
The CLARITY trial was designed to investigate the safety and efficacy of the two in combination in relapsed/refractory CLL patients.
The primary endpoint of the study is MRD eradication in the marrow after 12 months of treatment. The current analysis looks at a key secondary endpoint of the study – MRD eradication in the marrow after 6 months of treatment.
The study enrolled 54 patients, including 37 men and 17 women with a median age of 64 years; 20% have 17p deletion, and the population was heavily pretreated, with 81% having prior FCR or BR (44% with relapse within 3 years of treatment), and 20% with previous idelalisib exposure. Patients were excluded if they had prior exposure to ibrutinib or venetoclax.
Treatment involves ibrutinib monotherapy at a dose of 420 mg/day for 2 months to debulk the disease, after which venetoclax is added at a dose escalating from 20 mg to 400 mg/day over 2 months to reduce the risk of tumor lysis syndrome.
Bone marrow biopsies are performed at 6, 12, and 24 months. Treatment is discontinued at 12 months in those who achieve MRD negativity at 6 months, and is discontinued at 24 months in those who achieve MRD negativity at 12 months.
The combination treatment was well tolerated in the first 38 patients. Bruising (mainly grade 1) occurred in 33 patients, and neutropenia (including 16 grade 3 cases and 6 grade 4 cases) occurred in 25, and some GI toxicity occurred, but was largely grade 1 or 2, Dr. Hillmen said.
“There really was otherwise very acceptable toxicity,” he added, noting that a single case of tumor lysis syndrome occurred, but was managed successfully by delaying venetoclax.
“That patient re-escalated back onto treatment and is doing well,” he said.
No patients stopped treatment, and only seven had treatment interruption, and then only for a few days, he noted.
The findings are encouraging, and suggest a potent synergy between ibrutinib and venetoclax, said Dr. Hillmen.
“We’re seeing, even at this very early stage, over 30% of patients achieving MRD negative remission, which was our target at the 12-month bone marrow stage with this combination,” he said.
In light of these results, the ongoing phase 3 FLAIR trial, which is actively recruiting, has been modified to include combination ibrutinib and venetoclax in front-line CLL, he said.
Dr. Hillmen reported financial relationships with AbbVie and several other pharmaceutical companies. The CLARITY trial is supported by AbbVie, Bloodwise, Experimental Cancer Medicine Centre, Janssen-Cilag, the National Institute for Health Research Clinical Research Network: Cancer, and the University of Birmingham (England).
[email protected]
SOURCE: Hillmen P et al., ASH abstract 428.
REPORTING FROM ASH 2017
Key clinical point:
Major finding: 37% and 32% of patients achieved peripheral blood and marrow MRD negativity, respectively.
Study details: Initial results from 38 patients in the CLARITY feasibility trial.
Disclosures: Dr. Hillmen reported financial relationships with AbbVie and several other pharmaceutical companies. The CLARITY trial is supported by AbbVie, Bloodwise, Experimental Cancer Medicine Centre, Janssen-Cilag, the National Institute for Health Research Clinical Research Network: Cancer, and the University of Birmingham.
Source: Hillmen P et al. ASH Abstract 428.
Updated ZUMA-1 data show durable CAR-T responses in B-cell lymphomas
ATLANTA – More than one-third of patients with refractory large B-cell lymphomas treated with the chimeric antigen receptor (CAR) T-cell product axicabtagene ciloleucel (Yescarta), often called axi-cel, had durable responses, with some patients having complete responses lasting more than 1 year after a single infusion, according to investigators in the ZUMA-1 trial.
Updated combined phase 1 and phase 2 results in 108 patients with diffuse large B-cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma (PMBCL), or transformed follicular lymphoma (TFL) showed an objective response rate (ORR) of 82%, including 58% complete responses, after a median follow-up of 15.4 months, reported Sattva S. Neelapu, MD, from the University of Texas MD Anderson Cancer Center in Houston.
“Axi-cel is highly effective in patients with large B-cell lymphoma who otherwise have no curative treatment options,” he said in a briefing at the annual meeting of the American Society of Hematology, prior to his presentation of the data in an oral session.
The trial results were also published simultaneously in the New England Journal of Medicine.As previously reported, in the multicenter phase 2 ZUMA-1 trial, 111 patients with treatment refractory DLBCL, PMBCL, or TFL were enrolled and treated with axi-cel at a target dose of 2 x 106 cells/kg, following a conditioning regimen with low-dose cyclophosphamide and fludarabine.
The median patient age was 58 years. Patients had stage III or IV disease, 48% had International Prognostic Index scores of 3-4, 76% had disease that was refractory to third-line therapies or beyond, and 21% had disease that relapsed within 12 months of an autologous bone marrow transplant
Axi-cel was successfully manufactured with sufficient cells for transfusion in all but one of the 111 patients, and 101 patients eventually received infusions in phase 2 (modified intention-to-treat population). The average turnaround time from apheresis to the clinical site was 17 days.
Dr. Neelapu also presented data on seven patients enrolled in phase 1; the data were combined with the phase 2 results for an updated analysis of those patients who had at least 1 year of follow-up.
The phase 2 trial met its primary endpoint at the time of the primary analysis, with an 82% ORR, consisting of 54% complete responses and 28% partial responses at a median follow-up of 8.7 months.
In the updated analysis, the ORR and respective remission rates were 82%, 58%, and 34%, at a median of 15.4 months follow-up.
The median duration of response in the updated analysis was 11.1 months. The median duration of complete responses had not been reached at the time of data cutoff in August 2017. The median duration of partial responses was 1.9 months.
At the 15.4-month mark, 42% of patients remained free of disease progression, and 56% were alive, with the median overall survival not yet reached.
The treatment had generally acceptable toxicities, with only 13% of patients in phase 2 experiencing grade 3 or greater cytokine release syndrome (CRS), although one patient with CRS died from hemophagocytic lymphohistiocytosis, and one with CRS died from cardiac arrest. Grade 3 or greater neurologic events occurred in 28% of patients, and included encephalopathy, confusional state, aphasia, and somnolence.
The events were generally reversible, and the rates of each declined over time. The use of tocilizumab or steroids to control adverse events did not have a negative effect on responses.
Since the primary analysis with at least 6 months of follow-up, there have been no new axi-cel–related cases of CRS, neurologic events, or deaths.
Dr. Neelapu also presented safety data on serious adverse events occurring more than 6 months after therapy in 10 patients who developed symptoms after the data cutoff.
Grade 3 events in these patients included lung infection, recurrent upper respiratory viral infection, and rotavirus infection, pneumonias, atrial fibrillation with rapid ventricular response, lung infection, febrile neutropenia, and influenza B infection. One patient had grade 4 sepsis.
In an editorial accompanying the study in the New England Journal of Medicine, Eric Tran, PhD, and Walter J. Urba, MD, PhD, from the Earle A. Chiles Research Institute and the Providence Portland (Ore.) Medical Center, and Dan L. Longo, MD, deputy editor of the journal, praised ZUMA-1 as “a landmark study because it involved 22 institutions and showed that a personalized gene-engineered T-cell product could be rapidly generated at a centralized cell-manufacturing facility and safely administered to patients at transplantation-capable medical centers.”
They noted, however, that about half of all patients with relapsed or refractory large B-cell lymphomas will not have durable responses to CAR T-cell therapy directed against CD19, and that new strategies will be needed to improve responses (N Engl J Med. 2017 Dec 10; doi: 10.1056/NEJMe1714680).
In the question and answer session at the end of the briefing, Dr. Neelapu said the preliminary observations of mechanisms of relapse or disease progression in some patients may be related to the loss of the CD19 antigen, which occurs in about one-third of patients who experience relapse, and to high expression of the programmed death ligand-1, which can potentially inhibit CAR-T cell function. A clinical trial is currently underway to evaluate potential strategies for improving response rates to CAR-T therapies, he said.
ZUMA-1 is supported by Kite Pharma and the Leukemia and Lymphoma Society Therapy Acceleration Program. Dr. Neelapu reported receiving advisory board fees from the company. Myriad coauthors also reported financial relationship with multiple companies.
SOURCE: Neelapu S et al. ASH 2017 Abstract 578.
ATLANTA – More than one-third of patients with refractory large B-cell lymphomas treated with the chimeric antigen receptor (CAR) T-cell product axicabtagene ciloleucel (Yescarta), often called axi-cel, had durable responses, with some patients having complete responses lasting more than 1 year after a single infusion, according to investigators in the ZUMA-1 trial.
Updated combined phase 1 and phase 2 results in 108 patients with diffuse large B-cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma (PMBCL), or transformed follicular lymphoma (TFL) showed an objective response rate (ORR) of 82%, including 58% complete responses, after a median follow-up of 15.4 months, reported Sattva S. Neelapu, MD, from the University of Texas MD Anderson Cancer Center in Houston.
“Axi-cel is highly effective in patients with large B-cell lymphoma who otherwise have no curative treatment options,” he said in a briefing at the annual meeting of the American Society of Hematology, prior to his presentation of the data in an oral session.
The trial results were also published simultaneously in the New England Journal of Medicine.As previously reported, in the multicenter phase 2 ZUMA-1 trial, 111 patients with treatment refractory DLBCL, PMBCL, or TFL were enrolled and treated with axi-cel at a target dose of 2 x 106 cells/kg, following a conditioning regimen with low-dose cyclophosphamide and fludarabine.
The median patient age was 58 years. Patients had stage III or IV disease, 48% had International Prognostic Index scores of 3-4, 76% had disease that was refractory to third-line therapies or beyond, and 21% had disease that relapsed within 12 months of an autologous bone marrow transplant
Axi-cel was successfully manufactured with sufficient cells for transfusion in all but one of the 111 patients, and 101 patients eventually received infusions in phase 2 (modified intention-to-treat population). The average turnaround time from apheresis to the clinical site was 17 days.
Dr. Neelapu also presented data on seven patients enrolled in phase 1; the data were combined with the phase 2 results for an updated analysis of those patients who had at least 1 year of follow-up.
The phase 2 trial met its primary endpoint at the time of the primary analysis, with an 82% ORR, consisting of 54% complete responses and 28% partial responses at a median follow-up of 8.7 months.
In the updated analysis, the ORR and respective remission rates were 82%, 58%, and 34%, at a median of 15.4 months follow-up.
The median duration of response in the updated analysis was 11.1 months. The median duration of complete responses had not been reached at the time of data cutoff in August 2017. The median duration of partial responses was 1.9 months.
At the 15.4-month mark, 42% of patients remained free of disease progression, and 56% were alive, with the median overall survival not yet reached.
The treatment had generally acceptable toxicities, with only 13% of patients in phase 2 experiencing grade 3 or greater cytokine release syndrome (CRS), although one patient with CRS died from hemophagocytic lymphohistiocytosis, and one with CRS died from cardiac arrest. Grade 3 or greater neurologic events occurred in 28% of patients, and included encephalopathy, confusional state, aphasia, and somnolence.
The events were generally reversible, and the rates of each declined over time. The use of tocilizumab or steroids to control adverse events did not have a negative effect on responses.
Since the primary analysis with at least 6 months of follow-up, there have been no new axi-cel–related cases of CRS, neurologic events, or deaths.
Dr. Neelapu also presented safety data on serious adverse events occurring more than 6 months after therapy in 10 patients who developed symptoms after the data cutoff.
Grade 3 events in these patients included lung infection, recurrent upper respiratory viral infection, and rotavirus infection, pneumonias, atrial fibrillation with rapid ventricular response, lung infection, febrile neutropenia, and influenza B infection. One patient had grade 4 sepsis.
In an editorial accompanying the study in the New England Journal of Medicine, Eric Tran, PhD, and Walter J. Urba, MD, PhD, from the Earle A. Chiles Research Institute and the Providence Portland (Ore.) Medical Center, and Dan L. Longo, MD, deputy editor of the journal, praised ZUMA-1 as “a landmark study because it involved 22 institutions and showed that a personalized gene-engineered T-cell product could be rapidly generated at a centralized cell-manufacturing facility and safely administered to patients at transplantation-capable medical centers.”
They noted, however, that about half of all patients with relapsed or refractory large B-cell lymphomas will not have durable responses to CAR T-cell therapy directed against CD19, and that new strategies will be needed to improve responses (N Engl J Med. 2017 Dec 10; doi: 10.1056/NEJMe1714680).
In the question and answer session at the end of the briefing, Dr. Neelapu said the preliminary observations of mechanisms of relapse or disease progression in some patients may be related to the loss of the CD19 antigen, which occurs in about one-third of patients who experience relapse, and to high expression of the programmed death ligand-1, which can potentially inhibit CAR-T cell function. A clinical trial is currently underway to evaluate potential strategies for improving response rates to CAR-T therapies, he said.
ZUMA-1 is supported by Kite Pharma and the Leukemia and Lymphoma Society Therapy Acceleration Program. Dr. Neelapu reported receiving advisory board fees from the company. Myriad coauthors also reported financial relationship with multiple companies.
SOURCE: Neelapu S et al. ASH 2017 Abstract 578.
ATLANTA – More than one-third of patients with refractory large B-cell lymphomas treated with the chimeric antigen receptor (CAR) T-cell product axicabtagene ciloleucel (Yescarta), often called axi-cel, had durable responses, with some patients having complete responses lasting more than 1 year after a single infusion, according to investigators in the ZUMA-1 trial.
Updated combined phase 1 and phase 2 results in 108 patients with diffuse large B-cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma (PMBCL), or transformed follicular lymphoma (TFL) showed an objective response rate (ORR) of 82%, including 58% complete responses, after a median follow-up of 15.4 months, reported Sattva S. Neelapu, MD, from the University of Texas MD Anderson Cancer Center in Houston.
“Axi-cel is highly effective in patients with large B-cell lymphoma who otherwise have no curative treatment options,” he said in a briefing at the annual meeting of the American Society of Hematology, prior to his presentation of the data in an oral session.
The trial results were also published simultaneously in the New England Journal of Medicine.As previously reported, in the multicenter phase 2 ZUMA-1 trial, 111 patients with treatment refractory DLBCL, PMBCL, or TFL were enrolled and treated with axi-cel at a target dose of 2 x 106 cells/kg, following a conditioning regimen with low-dose cyclophosphamide and fludarabine.
The median patient age was 58 years. Patients had stage III or IV disease, 48% had International Prognostic Index scores of 3-4, 76% had disease that was refractory to third-line therapies or beyond, and 21% had disease that relapsed within 12 months of an autologous bone marrow transplant
Axi-cel was successfully manufactured with sufficient cells for transfusion in all but one of the 111 patients, and 101 patients eventually received infusions in phase 2 (modified intention-to-treat population). The average turnaround time from apheresis to the clinical site was 17 days.
Dr. Neelapu also presented data on seven patients enrolled in phase 1; the data were combined with the phase 2 results for an updated analysis of those patients who had at least 1 year of follow-up.
The phase 2 trial met its primary endpoint at the time of the primary analysis, with an 82% ORR, consisting of 54% complete responses and 28% partial responses at a median follow-up of 8.7 months.
In the updated analysis, the ORR and respective remission rates were 82%, 58%, and 34%, at a median of 15.4 months follow-up.
The median duration of response in the updated analysis was 11.1 months. The median duration of complete responses had not been reached at the time of data cutoff in August 2017. The median duration of partial responses was 1.9 months.
At the 15.4-month mark, 42% of patients remained free of disease progression, and 56% were alive, with the median overall survival not yet reached.
The treatment had generally acceptable toxicities, with only 13% of patients in phase 2 experiencing grade 3 or greater cytokine release syndrome (CRS), although one patient with CRS died from hemophagocytic lymphohistiocytosis, and one with CRS died from cardiac arrest. Grade 3 or greater neurologic events occurred in 28% of patients, and included encephalopathy, confusional state, aphasia, and somnolence.
The events were generally reversible, and the rates of each declined over time. The use of tocilizumab or steroids to control adverse events did not have a negative effect on responses.
Since the primary analysis with at least 6 months of follow-up, there have been no new axi-cel–related cases of CRS, neurologic events, or deaths.
Dr. Neelapu also presented safety data on serious adverse events occurring more than 6 months after therapy in 10 patients who developed symptoms after the data cutoff.
Grade 3 events in these patients included lung infection, recurrent upper respiratory viral infection, and rotavirus infection, pneumonias, atrial fibrillation with rapid ventricular response, lung infection, febrile neutropenia, and influenza B infection. One patient had grade 4 sepsis.
In an editorial accompanying the study in the New England Journal of Medicine, Eric Tran, PhD, and Walter J. Urba, MD, PhD, from the Earle A. Chiles Research Institute and the Providence Portland (Ore.) Medical Center, and Dan L. Longo, MD, deputy editor of the journal, praised ZUMA-1 as “a landmark study because it involved 22 institutions and showed that a personalized gene-engineered T-cell product could be rapidly generated at a centralized cell-manufacturing facility and safely administered to patients at transplantation-capable medical centers.”
They noted, however, that about half of all patients with relapsed or refractory large B-cell lymphomas will not have durable responses to CAR T-cell therapy directed against CD19, and that new strategies will be needed to improve responses (N Engl J Med. 2017 Dec 10; doi: 10.1056/NEJMe1714680).
In the question and answer session at the end of the briefing, Dr. Neelapu said the preliminary observations of mechanisms of relapse or disease progression in some patients may be related to the loss of the CD19 antigen, which occurs in about one-third of patients who experience relapse, and to high expression of the programmed death ligand-1, which can potentially inhibit CAR-T cell function. A clinical trial is currently underway to evaluate potential strategies for improving response rates to CAR-T therapies, he said.
ZUMA-1 is supported by Kite Pharma and the Leukemia and Lymphoma Society Therapy Acceleration Program. Dr. Neelapu reported receiving advisory board fees from the company. Myriad coauthors also reported financial relationship with multiple companies.
SOURCE: Neelapu S et al. ASH 2017 Abstract 578.
REPORTING FROM ASH 2017
Key clinical point:.
Major finding: The objective response rate was 82%, including 58% complete responses at a median of 15.4 months of follow-up.
Data source: Update analysis of phase 1 and 2 data from the ZUMA-1 trial in 108 patients with large B-cell lymphomas.
Disclosures: ZUMA-1 is supported by Kite Pharma and the Leukemia and Lymphoma Society Therapy Acceleration Program. Dr. Neelapu reported receiving advisory board fees from the company. Myriad coauthors also reported financial relationship with multiple companies.
Source: Neelapu S et al. ASH 2017 Abstract 578
Guideline preview: ASH to recommend against VTE prophylaxis for lower-risk cancer patients
ATLANTA – An expert panel convened by the American Society of Hematology will recommend against routine venous thromboembolism (VTE) prophylaxis with low-molecular-weight heparin, except in patients deemed high risk, the panel chair told attendees at the group’s annual meeting.
The recommendations will be posted for public comment in early 2018 and are expected to be finalized and published later in the year.
The panel found strong evidence to recommend against prophylaxis in cancer patients at low risk of VTE, said Gary H. Lyman, MD, of the Fred Hutchinson Cancer Research Center and the University of Washington, both in Seattle.
For cancer patients at high risk of VTE, the panel will make a conditional recommendation – based on weaker evidence – in favor of low-molecular-weight thromboprophylaxis (LMWH) “with a caveat that patients should have no major bleeding risk or other contraindication to anticoagulation,” Dr. Lyman said.
The panel also found weaker evidence to support a conditional recommendation against routine prophylaxis in patients at intermediate risk of VTE, based on results from a systematic review.
These recommendations, which were described during a special education session at the annual meeting of the American Society of Hematology, are just one piece of a larger effort by ASH to develop evidence-based guidelines on VTE management. More than 100 thrombosis experts have reviewed evidence and drafted more than 200 recommendations on diagnosis, prevention, and treatment of VTE, according to ASH.
The cancer-specific recommendations described by Dr. Lyman are based in part on a meta-analysis of 17 randomized controlled trials of cancer patients receiving LMWH thromboprophylaxis that were published between 2008 and 2017. Across cancer types and settings, LMWH prophylaxis in these trials was associated with a reduced risk of VTE and an increased risk of bleeding, Dr. Lyman said.
For all cancer types, relative risk (RR) of VTE was 0.55 (95% confidence interval, 0.46-0.65), with an absolute risk reduction of just 2.7% “because of the low baseline risk of VTE in this setting,” Dr. Lyman said.
However, the baseline risk increased for certain cancer types, including lung cancer, which had a similar relative risk of 0.53 (95% CI, 0.41-0.69) but an absolute risk reduction of 4.3%, according to data Dr. Lyman presented. Likewise, pancreatic cancer was associated with a relative risk of 0.43 (95% CI, 0.25-0.73) but an absolute risk reduction of 10.1%.
Major bleeding across all trials was significantly increased (RR 1.40; 95% CI, 1.01-1.92), but the presented data show that, in absolute terms, the increase was just 0.7% overall and similarly, 0.7% and 0.9% for lung and pancreatic cancer, respectively.
However, VTE risk depends on more than the site of the cancer, Dr. Lyman noted. In their deliberations, the panel considered the validated Khorana Risk Score, which incorporates factors such as body mass index, platelet count, leukocyte count, and hemoglobin level.
Specific analysis of high-risk patients suggested a pronounced risk reduction associated with LMWH prophylaxis, according to Dr. Lyman, who noted that a patient-level analysis including nearly 8,000 patients from 13 randomized, controlled trials showed a relative risk of 0.58, with VTE events seen in 4.0% patients in the LMWH group versus 7.0% of controls.
The forthcoming ASH guidelines also will address other aspects of cancer-associated VTE, according to Dr. Lyman, including initial treatment, secondary prophylaxis, perioperative prophylaxis, and prophylaxis or treatment in patients with a central venous catheter.
“The risk of venous thromboembolism in patients with cancer is well recognized, and that risk is especially notable in patients receiving cancer treatment,” Dr. Lyman said.
Dr. Lyman reported having no relevant financial disclosures.
ATLANTA – An expert panel convened by the American Society of Hematology will recommend against routine venous thromboembolism (VTE) prophylaxis with low-molecular-weight heparin, except in patients deemed high risk, the panel chair told attendees at the group’s annual meeting.
The recommendations will be posted for public comment in early 2018 and are expected to be finalized and published later in the year.
The panel found strong evidence to recommend against prophylaxis in cancer patients at low risk of VTE, said Gary H. Lyman, MD, of the Fred Hutchinson Cancer Research Center and the University of Washington, both in Seattle.
For cancer patients at high risk of VTE, the panel will make a conditional recommendation – based on weaker evidence – in favor of low-molecular-weight thromboprophylaxis (LMWH) “with a caveat that patients should have no major bleeding risk or other contraindication to anticoagulation,” Dr. Lyman said.
The panel also found weaker evidence to support a conditional recommendation against routine prophylaxis in patients at intermediate risk of VTE, based on results from a systematic review.
These recommendations, which were described during a special education session at the annual meeting of the American Society of Hematology, are just one piece of a larger effort by ASH to develop evidence-based guidelines on VTE management. More than 100 thrombosis experts have reviewed evidence and drafted more than 200 recommendations on diagnosis, prevention, and treatment of VTE, according to ASH.
The cancer-specific recommendations described by Dr. Lyman are based in part on a meta-analysis of 17 randomized controlled trials of cancer patients receiving LMWH thromboprophylaxis that were published between 2008 and 2017. Across cancer types and settings, LMWH prophylaxis in these trials was associated with a reduced risk of VTE and an increased risk of bleeding, Dr. Lyman said.
For all cancer types, relative risk (RR) of VTE was 0.55 (95% confidence interval, 0.46-0.65), with an absolute risk reduction of just 2.7% “because of the low baseline risk of VTE in this setting,” Dr. Lyman said.
However, the baseline risk increased for certain cancer types, including lung cancer, which had a similar relative risk of 0.53 (95% CI, 0.41-0.69) but an absolute risk reduction of 4.3%, according to data Dr. Lyman presented. Likewise, pancreatic cancer was associated with a relative risk of 0.43 (95% CI, 0.25-0.73) but an absolute risk reduction of 10.1%.
Major bleeding across all trials was significantly increased (RR 1.40; 95% CI, 1.01-1.92), but the presented data show that, in absolute terms, the increase was just 0.7% overall and similarly, 0.7% and 0.9% for lung and pancreatic cancer, respectively.
However, VTE risk depends on more than the site of the cancer, Dr. Lyman noted. In their deliberations, the panel considered the validated Khorana Risk Score, which incorporates factors such as body mass index, platelet count, leukocyte count, and hemoglobin level.
Specific analysis of high-risk patients suggested a pronounced risk reduction associated with LMWH prophylaxis, according to Dr. Lyman, who noted that a patient-level analysis including nearly 8,000 patients from 13 randomized, controlled trials showed a relative risk of 0.58, with VTE events seen in 4.0% patients in the LMWH group versus 7.0% of controls.
The forthcoming ASH guidelines also will address other aspects of cancer-associated VTE, according to Dr. Lyman, including initial treatment, secondary prophylaxis, perioperative prophylaxis, and prophylaxis or treatment in patients with a central venous catheter.
“The risk of venous thromboembolism in patients with cancer is well recognized, and that risk is especially notable in patients receiving cancer treatment,” Dr. Lyman said.
Dr. Lyman reported having no relevant financial disclosures.
ATLANTA – An expert panel convened by the American Society of Hematology will recommend against routine venous thromboembolism (VTE) prophylaxis with low-molecular-weight heparin, except in patients deemed high risk, the panel chair told attendees at the group’s annual meeting.
The recommendations will be posted for public comment in early 2018 and are expected to be finalized and published later in the year.
The panel found strong evidence to recommend against prophylaxis in cancer patients at low risk of VTE, said Gary H. Lyman, MD, of the Fred Hutchinson Cancer Research Center and the University of Washington, both in Seattle.
For cancer patients at high risk of VTE, the panel will make a conditional recommendation – based on weaker evidence – in favor of low-molecular-weight thromboprophylaxis (LMWH) “with a caveat that patients should have no major bleeding risk or other contraindication to anticoagulation,” Dr. Lyman said.
The panel also found weaker evidence to support a conditional recommendation against routine prophylaxis in patients at intermediate risk of VTE, based on results from a systematic review.
These recommendations, which were described during a special education session at the annual meeting of the American Society of Hematology, are just one piece of a larger effort by ASH to develop evidence-based guidelines on VTE management. More than 100 thrombosis experts have reviewed evidence and drafted more than 200 recommendations on diagnosis, prevention, and treatment of VTE, according to ASH.
The cancer-specific recommendations described by Dr. Lyman are based in part on a meta-analysis of 17 randomized controlled trials of cancer patients receiving LMWH thromboprophylaxis that were published between 2008 and 2017. Across cancer types and settings, LMWH prophylaxis in these trials was associated with a reduced risk of VTE and an increased risk of bleeding, Dr. Lyman said.
For all cancer types, relative risk (RR) of VTE was 0.55 (95% confidence interval, 0.46-0.65), with an absolute risk reduction of just 2.7% “because of the low baseline risk of VTE in this setting,” Dr. Lyman said.
However, the baseline risk increased for certain cancer types, including lung cancer, which had a similar relative risk of 0.53 (95% CI, 0.41-0.69) but an absolute risk reduction of 4.3%, according to data Dr. Lyman presented. Likewise, pancreatic cancer was associated with a relative risk of 0.43 (95% CI, 0.25-0.73) but an absolute risk reduction of 10.1%.
Major bleeding across all trials was significantly increased (RR 1.40; 95% CI, 1.01-1.92), but the presented data show that, in absolute terms, the increase was just 0.7% overall and similarly, 0.7% and 0.9% for lung and pancreatic cancer, respectively.
However, VTE risk depends on more than the site of the cancer, Dr. Lyman noted. In their deliberations, the panel considered the validated Khorana Risk Score, which incorporates factors such as body mass index, platelet count, leukocyte count, and hemoglobin level.
Specific analysis of high-risk patients suggested a pronounced risk reduction associated with LMWH prophylaxis, according to Dr. Lyman, who noted that a patient-level analysis including nearly 8,000 patients from 13 randomized, controlled trials showed a relative risk of 0.58, with VTE events seen in 4.0% patients in the LMWH group versus 7.0% of controls.
The forthcoming ASH guidelines also will address other aspects of cancer-associated VTE, according to Dr. Lyman, including initial treatment, secondary prophylaxis, perioperative prophylaxis, and prophylaxis or treatment in patients with a central venous catheter.
“The risk of venous thromboembolism in patients with cancer is well recognized, and that risk is especially notable in patients receiving cancer treatment,” Dr. Lyman said.
Dr. Lyman reported having no relevant financial disclosures.
REPORTING FROM ASH 2017