User login
Consider PBC in diagnosing hepatobiliary disorders in UC patients
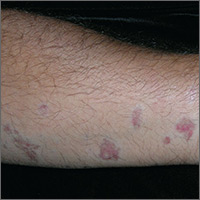
While primary sclerosing cholangitis (PSC) is the most common hepatobiliary disorder associated with ulcerative colitis, primary biliary cholangitis (PBC) should not be forgotten, according to Erietta Polychronopoulou, MD, and her associates.
In two case studies, a 67-year-old woman and a 71-year-old man presented with long-standing cases of asymptomatic elevation of cholestatic enzymes. Both patients had long histories of ulcerative colitis, but both were in remission. Both patients had previous clinical diagnoses of either small duct PSC or drug-induced liver injury. Both patients denied drug use, and imaging studies revealed nothing in either patient.
In testing for hepatobiliary disorders, both patients showed high titers of antimitochondrial antibodies, the hallmark of PBC. Despite the asymptomatic nature of the PBC, both patients were treated with 13 mg/kg per day ursodeoxycholic acid and have remained stable for 17 and 18 months, respectively.
“The relationship of PBC with UC [ulcerative colitis] remains obscure as there are few reported cases regarding the combined presentation of these diseases. Although the pathogenesis of either disease has not yet been completely clarified, environmental and genetic factors are considered important in the susceptibility to both diseases, suggesting that the two diseases may share common immunopathogenetic pathways,” the investigators noted.
Find the full report in BMJ Case Reports (2017 Sep 25. doi: 10.1136/bcr-2017-220824).
While primary sclerosing cholangitis (PSC) is the most common hepatobiliary disorder associated with ulcerative colitis, primary biliary cholangitis (PBC) should not be forgotten, according to Erietta Polychronopoulou, MD, and her associates.
In two case studies, a 67-year-old woman and a 71-year-old man presented with long-standing cases of asymptomatic elevation of cholestatic enzymes. Both patients had long histories of ulcerative colitis, but both were in remission. Both patients had previous clinical diagnoses of either small duct PSC or drug-induced liver injury. Both patients denied drug use, and imaging studies revealed nothing in either patient.
In testing for hepatobiliary disorders, both patients showed high titers of antimitochondrial antibodies, the hallmark of PBC. Despite the asymptomatic nature of the PBC, both patients were treated with 13 mg/kg per day ursodeoxycholic acid and have remained stable for 17 and 18 months, respectively.
“The relationship of PBC with UC [ulcerative colitis] remains obscure as there are few reported cases regarding the combined presentation of these diseases. Although the pathogenesis of either disease has not yet been completely clarified, environmental and genetic factors are considered important in the susceptibility to both diseases, suggesting that the two diseases may share common immunopathogenetic pathways,” the investigators noted.
Find the full report in BMJ Case Reports (2017 Sep 25. doi: 10.1136/bcr-2017-220824).
While primary sclerosing cholangitis (PSC) is the most common hepatobiliary disorder associated with ulcerative colitis, primary biliary cholangitis (PBC) should not be forgotten, according to Erietta Polychronopoulou, MD, and her associates.
In two case studies, a 67-year-old woman and a 71-year-old man presented with long-standing cases of asymptomatic elevation of cholestatic enzymes. Both patients had long histories of ulcerative colitis, but both were in remission. Both patients had previous clinical diagnoses of either small duct PSC or drug-induced liver injury. Both patients denied drug use, and imaging studies revealed nothing in either patient.
In testing for hepatobiliary disorders, both patients showed high titers of antimitochondrial antibodies, the hallmark of PBC. Despite the asymptomatic nature of the PBC, both patients were treated with 13 mg/kg per day ursodeoxycholic acid and have remained stable for 17 and 18 months, respectively.
“The relationship of PBC with UC [ulcerative colitis] remains obscure as there are few reported cases regarding the combined presentation of these diseases. Although the pathogenesis of either disease has not yet been completely clarified, environmental and genetic factors are considered important in the susceptibility to both diseases, suggesting that the two diseases may share common immunopathogenetic pathways,” the investigators noted.
Find the full report in BMJ Case Reports (2017 Sep 25. doi: 10.1136/bcr-2017-220824).
FROM BMJ CASE REPORTS
Cardio-oncology booms but awareness lags
Cardio-oncology is expanding, fed by a steadily increasing population of cancer survivors at elevated risk for a range of cardiovascular diseases and complications because of the anticancer treatments they received. Cardio-oncology’s quick growth has also been driven by the rapidly expanding universe of cancer treatments with direct or indirect adverse effects on a diverse range of cardiovascular functions.
During the past year, the field’s rapid evolution has featured the first formal diagnostic and care standards in two iterations: A position paper on the cardiovascular toxicities of cancer treatment from the European Society of Cardiology (ESC), released in August 2016 (Eur Heart J. 2016 Sept 21;37[36]:2766-801); and a guideline for preventing and monitoring cardiac dysfunction in adult cancer survivors, issued last December by the American Society of Clinical Oncology (ASCO) and endorsed by the American Heart Association (J Clin Oncol. 2017 March 10;35[8]:893-913), but notably not endorsed by the American College of Cardiology, despite having an ACC representative on the guideline panel. In 2015, the ACC started a Cardio-Oncology Section, one of 20 special-interest sections it maintains, and by mid-2017 the section had some 500 members.
“I’ve had recent conversations with cardiologists who said ‘I’m not sure what cardio-oncology is,’ ” said Tomas G. Neilan, MD, director of the cardio-oncology program at Massachusetts General Hospital in Boston.
More than just heart failure
A few decades ago, in the primordial days of cardio-oncology, the concept of cardiovascular damage during cancer therapy focused entirely on myocardial damage caused by anthracyclines and chest radiation, a concern that eventually expanded to include trastuzumab (Herceptin) and other agents that target the human epidermal growth factor receptor 2 (HER2). These treatments cause significantly reduced left ventricular ejection fractions and heart failure in a significant minority of treated patients. Patients who receive combined treatment with an anthracycline and trastuzumab are at the highest risk for developing heart failure with reduced ejection fraction, but even among patients treated with this combination, fewer than 5% develop outright heart failure.
While this parochial view of cardio-oncology has recently shifted, it remains true that myocardial damage from a relatively large cumulative anthracycline dose, or from radiation, causes some of the most extreme cases of cardiovascular adverse effects and remains an ongoing problem as these treatments stay front line for selected cancer patients.
But some of the recent burgeoning of cardio-oncology has followed the recognition that many other drugs and drug classes can cause a spectrum of adverse cardiovascular effects.
“There has been a significant focus on heart failure and cardiomyopathy due to anthracyclines and HER2-targeted therapies. I think the field will continue to evolve over the next 5 years to focus on other cardiovascular complications, including arrhythmias and vascular disease,” observed Michael Fradley, MD, director of cardio-oncology at Moffitt Cancer Center in Tampa. “In addition, there will be an increased focus on targeted drugs and immunotherapies,” agents that Dr. Fradley said “have many unique cardiovascular complications. We need additional guidelines regarding the management of a variety of cardiotoxicities as well as long-term monitoring strategies.”
In a review article Dr. Moslehi published toward the end of 2016, he fleshed out the wider scope of adverse cardiovascular effects from cancer therapies, noting that the vascular endothelial growth factor (VEGF) signaling pathway inhibitors, drugs such as bevacizumab (Avastin) and aflibercept (Zaltrap), have been documented to cause hypertension, arterial thromboembolic events, and cardiomyopathy; and that tyrosine kinase inhibitors have been shown to cause vascular events, QT interval prolongation, and cerebral and peripheral vascular events (N Engl J Med. 2016 Oct 13;375[15]:1457-67).
In his own recent review, Dr. Fradley highlighted adverse cardiovascular effects from additional anticancer drug classes, including proteasome inhibitors, which can trigger hypertension and cardiomyopathy; immunomodulators, implicated in causing both venous and arterial thromboembolism; and the immune checkpoint inhibitors, linked with myocarditis, arrhythmias, hypotension, and myocardial ischemia (Eur Heart J. 2016 Sept 21;37[36]:2740-2). A similarly broad spectrum of adverse cardiovascular effects linked with a wide range of anticancer treatments also appeared in the ESC 2016 position paper on cancer treatments.
But while the range of cancer treatments that can have some impact on the cardiovascular system is strikingly large, experts uniformly caution that far from every patient treated for cancer needs an immediate cardiology consult and work-up, especially when the cancers appear in young adults.
“We’re not quite at the point where every cancer patient needs to be seen by a cardiologist or cardio-oncologist,” Dr. Fradley noted in an interview.
“If a patient develops hypertension while on treatment I refer them to a PCP or cardiologist. I don’t treat hypertension myself. But if a patient is ‘normal’ they don’t need a cardiology assessment up front. It’s impossible to refer all patients, especially younger patients, with current resources. There are too many patients who receive cardiotoxic therapies to refer everyone. I involve the cardiologist once there is evidence of damage,”she explained.
Cardio-oncology centers or community practice?
The rise of cardio-oncology, especially over the last decade or so, has given rise to a new academic niche, the cardio-oncology clinic. Starting from almost no programs a few years ago, by 2016 one tally put the total number of U.S. self-designated cardio-oncology centers at about 40 (Heart Fail Clin. 2017 April;13[2]:347-55), and that number undoubtedly grew even more during the year since. While these programs promote and advance the nascent subspecialty of cardio-oncology, and provide a foundation for development of formalized training programs, many experts see a clear hierarchy of risk that distinguishes the patients who should ideally be managed at these focused, multidisciplinary programs from the lower-risk patients who probably do fine under the care of just their oncologist or their oncologist in collaboration with a community cardiologist or primary care physician.
“The cardio-oncology community recognizes that it is nice to have programs at academic centers but it’s more important to deliver this care in the community,” said Dr. Lenihan. “Many cancer patients have no prior history of cardiovascular disease. These low-risk patients don’t necessarily need a cardio-oncologist. They may need to have their blood pressure managed more effectively or receive other preventive care, but that can certainly be done locally. There are low-risk patients who don’t need to go to a major center.” Dr. Lenihan and other cardio-oncologists see the majority of cancer patients as low risk when it comes to cardiovascular complications.
But it’s different when patients receive an anthracycline or an anthracycline plus trastuzumab. “This high-risk population is best seen at a cardio-oncology center.” Dr. Lenihan also included in this high-risk subgroup patients treated with mediastinal radiation, an option often used during the 1980s-2000s.
“Any time a patient receives treatment with the potential to cause a cardiovascular effect, which is pretty much any drug that now comes out, you need an accurate baseline assessment. But that doesn’t mean you need do anything different; you still treat the patient’s cancer. A thorough baseline assessment is a necessity, but it does not need to be done at a cardio-oncology center,” Dr. Lenihan said in an interview.
“For the vast majority of patients, care can be at community hospitals, similar to the delivery of the vast majority of oncology care. Some patients need referral to tertiary cardiology centers for advanced heart failure or to undergo advanced procedures, but that is a very small percentage of patients,” said Ana Barac, MD, director of the cardio-oncology program at the MedStar Heart Institute in Washington, and chair of the ACC’s Cardio-Oncology Section.
“Patients receiving more novel or unusual therapies, and those participating in trials” are appropriate for centers, while community care by a cardiologist and oncologist should suffice for more routine patients, said Dr. Fradley.
“Cardio-oncology centers are good for patients with type I damage from anthracycline treatment, especially patients who already had underlying heart disease,” said Michael S. Ewer, MD, a cardiologist and professor of medicine at MD Anderson Cancer Center in Houston. Specialist centers are also for patients with cardiovascular risk factors: older age, diabetes, preexisting coronary artery disease, and patients who receive cardiotoxic type I therapy (J Clin Oncol. 2005 May;23[13]:2900-2). Also, patients with a significant, immediate cardiac reaction to treatment, and those with an unexpected cardiac reaction, Dr. Ewer said.
A somewhat more expansive view of the typical cardio-oncology patient came from Dr. Neilan, based on the patients he sees at his program in Boston. Dr. Neilan estimated that roughly 60%-70% of his patients first present while they undergo active cancer treatment, with another 20% coming to the program as cancer survivors, and a small percentage of patients showing up for cardiology assessments and treatments without a cancer history. Among those with a cancer history, he guessed that perhaps 10%-20% were treated with an anthracycline, at least 10% received trastuzumab, and about 10% received radiation treatment. “I also see a lot of patients with complications from treatment” with tyrosine kinase inhibitors, VEGF inhibitors, and immunotherapies. “I don’t see a lot of patients for cardiovascular disease assessment before they start cancer therapy,” Dr. Neilan added.
Cardio-oncology heads toward a new cardiology subspecialty
These views of how cardio-oncology is practiced in the real world raise a question about the role of the growing roster of U.S. cardio-oncology programs. If most cancer patients can have their cardiology needs taken care of in the community, how do all the academic programs fit in? The answer seems to be that they model successful oncology and cardiology collaborations, provide a training ground for physicians from both specialties to learn how to collaborate, and serve as the home for research that broadens the field’s evidence base and moves knowledge forward.
“Education and partnerships with oncology teams is the key,” said Dr. Barac. “Our traditional subspecialty training focused on ‘treating cancer’ and ‘treating cardiovascular disease.’ Learning about and seeing effective partnerships during training” is the best model to foster cardiology and oncology partnerships among early-career physicians, she suggested.
“What is the spectrum of knowledge required to be proficient in cardio-oncology, and how do we enhance training at the resident or fellowship level? How do we get [all cardiology] trainees exposed to this knowledge?” wondered Dr. Lenihan, who viewed cardio-oncology programs as a way to meet these needs. “Cardio-oncology is not an established subspecialty. A goal is to establish training requirements and expand training opportunities. And the whole field needs to contribute to clinical research. We need cardio-oncologists to share their experience and improve our level of research.”
ASCO’s cardiac dysfunction practice guideline, first released last December and formally published in March, is likely helping to further entrench cardio-oncology as a new subspecialty. The guideline was “a remarkable step forward,” said Dr. Barac. In addition to establishing a U.S. standard of care for preventing and monitoring cardiac dysfunction in cancer patients, “I use it as a guide for creation of referral pathways with my oncology colleagues, as well as in education of cardiovascular and oncology trainees,” she said in an interview.
Though produced primarily through ASCO’s leadership, the target audience for the guideline seems to be as much cardiologists as it is oncologists. Dissemination of the guideline to cardiologists snagged when it failed to appear in the cardiology literature. That wasn’t the original plan, said guideline participants.
“Before we started, it was agreed that both ASCO and the ACC would publish it. We had a [letter] signed by the president of the ACC saying the ACC would publish it,” recalled Dr. Lenihan, a guideline coauthor. “After all the details were settled, the ACC bailed. They said that they had changed their organizational structure and that they wouldn’t publish the guideline even though they had agreed to.” Not having the guideline appear simultaneously in the cardiology literature “hinders getting the message to the cardiology community,” he said, a sentiment echoed by other cardio-oncologists.
“I served as the ACC representative on the guideline, and the lack of ACC endorsement was the unfortunate consequence of approval and publication timing that coincided with restructuring of the ACC committees,” said Dr. Barac. “It absolutely does not reflect a lack of interest from the ACC.” As an example of the College’s commitment example, she cited an ACC 1.5-day educational course on cardiovascular care of oncology patients held for the first time in February 2017 and scheduled for a second edition next February.
Publication of the guideline in a cardiology journal “would indeed help dissemination among U.S. cardiologists,” agreed Pamela S. Douglas, MD, professor of medicine at Duke University in Durham, N.C., and another of the several cardiologists who served on the ASCO guideline’s panel.
Further advancing awareness of patients with cardio-oncology issues, what Dr. Moslehi has called “an emerging epidemic,” seems the most fundamental of the goals currently advanced by many active in this field.
One step to grow the subspecialty that he and his associates at Vanderbilt have taken is to start this year a formally recognized fellowship program in cardio-oncology; an initial class of three cardiologists started in the program this summer. The Vanderbilt group also plans to launch a website before the end of 2017 that will include an oncology-drug database that compiles all available information on each agent’s cardiovascular effects. The planned website will aggregate links to all existing cardio-oncology programs.
“We will absolutely see the field grow,” said Dr. Swain. “It has only sprung up in the past 10 or so years. It is now getting recognition, people are being trained in cardio-oncology, and it will grow as a subspecialty. It’s very exciting, and it’s better for patients.”
“A cardiologist with no cancer patients or survivors in their practice is unheard of; many cardiologists just don’t realize that,” Dr. Lenihan said. At least 10%-15% of the U.S. population in their 60s or older has a cancer history, he noted. The common mindset among cardiologists has been that cancer patients and survivors are not among their patients.
“It’s unlikely that a busy cardiology practice has no cancer survivors or active cancer patients,” Dr. Douglas suggested. When this happens, a likely explanations is that the cardiologist simply failed to elicit a completely comprehensive history from the practice’s patient roster. And even a cardiology practice today that includes no cancer patients or survivors will likely see some turning up soon, she predicted, because so many are receiving cardiovascular-toxic therapies and then surviving longer than ever before.
“What oncologists and cardiologists want to do is to optimize oncologic outcomes but with an acceptable adverse event profile. The cardio-oncologist helps push that envelope. The goal is not to eliminate cardiac events at the expense of oncologic outcomes, but to shift the balance to fewer and less severe cardiac events without unduly compromising oncologic outcomes,” explained Dr. Ewer. Cardio-oncology grapples with one of the core challenges of medicine, how to balance the potential risks from treatment against its potential benefits, he observed.
Dr. Neilan has been a consultant to Ariad and Takeda. Dr. Lenihan has been a consultant to Janssen and Roche and has received research funding from Takeda. Dr. Moslehi has been a consultant to Acceleron, Ariad, Bristol-Myers Squibb, Incyte, Pfizer, Takeda/Millennium, Verastem and Vertex. Dr. Ewer, Dr. Fradley, and Dr. Barac had no relevant disclosures. Dr. Swain has been a consultant to Genentech and Roche. Dr. Douglas has been a consultant to CardioDx, Interleukin Genetics, and Omicia, and has an ownership interest in CardioDx.
[email protected]
On Twitter @mitchelzoler
Cardio-oncology is expanding, fed by a steadily increasing population of cancer survivors at elevated risk for a range of cardiovascular diseases and complications because of the anticancer treatments they received. Cardio-oncology’s quick growth has also been driven by the rapidly expanding universe of cancer treatments with direct or indirect adverse effects on a diverse range of cardiovascular functions.
During the past year, the field’s rapid evolution has featured the first formal diagnostic and care standards in two iterations: A position paper on the cardiovascular toxicities of cancer treatment from the European Society of Cardiology (ESC), released in August 2016 (Eur Heart J. 2016 Sept 21;37[36]:2766-801); and a guideline for preventing and monitoring cardiac dysfunction in adult cancer survivors, issued last December by the American Society of Clinical Oncology (ASCO) and endorsed by the American Heart Association (J Clin Oncol. 2017 March 10;35[8]:893-913), but notably not endorsed by the American College of Cardiology, despite having an ACC representative on the guideline panel. In 2015, the ACC started a Cardio-Oncology Section, one of 20 special-interest sections it maintains, and by mid-2017 the section had some 500 members.
“I’ve had recent conversations with cardiologists who said ‘I’m not sure what cardio-oncology is,’ ” said Tomas G. Neilan, MD, director of the cardio-oncology program at Massachusetts General Hospital in Boston.
More than just heart failure
A few decades ago, in the primordial days of cardio-oncology, the concept of cardiovascular damage during cancer therapy focused entirely on myocardial damage caused by anthracyclines and chest radiation, a concern that eventually expanded to include trastuzumab (Herceptin) and other agents that target the human epidermal growth factor receptor 2 (HER2). These treatments cause significantly reduced left ventricular ejection fractions and heart failure in a significant minority of treated patients. Patients who receive combined treatment with an anthracycline and trastuzumab are at the highest risk for developing heart failure with reduced ejection fraction, but even among patients treated with this combination, fewer than 5% develop outright heart failure.
While this parochial view of cardio-oncology has recently shifted, it remains true that myocardial damage from a relatively large cumulative anthracycline dose, or from radiation, causes some of the most extreme cases of cardiovascular adverse effects and remains an ongoing problem as these treatments stay front line for selected cancer patients.
But some of the recent burgeoning of cardio-oncology has followed the recognition that many other drugs and drug classes can cause a spectrum of adverse cardiovascular effects.
“There has been a significant focus on heart failure and cardiomyopathy due to anthracyclines and HER2-targeted therapies. I think the field will continue to evolve over the next 5 years to focus on other cardiovascular complications, including arrhythmias and vascular disease,” observed Michael Fradley, MD, director of cardio-oncology at Moffitt Cancer Center in Tampa. “In addition, there will be an increased focus on targeted drugs and immunotherapies,” agents that Dr. Fradley said “have many unique cardiovascular complications. We need additional guidelines regarding the management of a variety of cardiotoxicities as well as long-term monitoring strategies.”
In a review article Dr. Moslehi published toward the end of 2016, he fleshed out the wider scope of adverse cardiovascular effects from cancer therapies, noting that the vascular endothelial growth factor (VEGF) signaling pathway inhibitors, drugs such as bevacizumab (Avastin) and aflibercept (Zaltrap), have been documented to cause hypertension, arterial thromboembolic events, and cardiomyopathy; and that tyrosine kinase inhibitors have been shown to cause vascular events, QT interval prolongation, and cerebral and peripheral vascular events (N Engl J Med. 2016 Oct 13;375[15]:1457-67).
In his own recent review, Dr. Fradley highlighted adverse cardiovascular effects from additional anticancer drug classes, including proteasome inhibitors, which can trigger hypertension and cardiomyopathy; immunomodulators, implicated in causing both venous and arterial thromboembolism; and the immune checkpoint inhibitors, linked with myocarditis, arrhythmias, hypotension, and myocardial ischemia (Eur Heart J. 2016 Sept 21;37[36]:2740-2). A similarly broad spectrum of adverse cardiovascular effects linked with a wide range of anticancer treatments also appeared in the ESC 2016 position paper on cancer treatments.
But while the range of cancer treatments that can have some impact on the cardiovascular system is strikingly large, experts uniformly caution that far from every patient treated for cancer needs an immediate cardiology consult and work-up, especially when the cancers appear in young adults.
“We’re not quite at the point where every cancer patient needs to be seen by a cardiologist or cardio-oncologist,” Dr. Fradley noted in an interview.
“If a patient develops hypertension while on treatment I refer them to a PCP or cardiologist. I don’t treat hypertension myself. But if a patient is ‘normal’ they don’t need a cardiology assessment up front. It’s impossible to refer all patients, especially younger patients, with current resources. There are too many patients who receive cardiotoxic therapies to refer everyone. I involve the cardiologist once there is evidence of damage,”she explained.
Cardio-oncology centers or community practice?
The rise of cardio-oncology, especially over the last decade or so, has given rise to a new academic niche, the cardio-oncology clinic. Starting from almost no programs a few years ago, by 2016 one tally put the total number of U.S. self-designated cardio-oncology centers at about 40 (Heart Fail Clin. 2017 April;13[2]:347-55), and that number undoubtedly grew even more during the year since. While these programs promote and advance the nascent subspecialty of cardio-oncology, and provide a foundation for development of formalized training programs, many experts see a clear hierarchy of risk that distinguishes the patients who should ideally be managed at these focused, multidisciplinary programs from the lower-risk patients who probably do fine under the care of just their oncologist or their oncologist in collaboration with a community cardiologist or primary care physician.
“The cardio-oncology community recognizes that it is nice to have programs at academic centers but it’s more important to deliver this care in the community,” said Dr. Lenihan. “Many cancer patients have no prior history of cardiovascular disease. These low-risk patients don’t necessarily need a cardio-oncologist. They may need to have their blood pressure managed more effectively or receive other preventive care, but that can certainly be done locally. There are low-risk patients who don’t need to go to a major center.” Dr. Lenihan and other cardio-oncologists see the majority of cancer patients as low risk when it comes to cardiovascular complications.
But it’s different when patients receive an anthracycline or an anthracycline plus trastuzumab. “This high-risk population is best seen at a cardio-oncology center.” Dr. Lenihan also included in this high-risk subgroup patients treated with mediastinal radiation, an option often used during the 1980s-2000s.
“Any time a patient receives treatment with the potential to cause a cardiovascular effect, which is pretty much any drug that now comes out, you need an accurate baseline assessment. But that doesn’t mean you need do anything different; you still treat the patient’s cancer. A thorough baseline assessment is a necessity, but it does not need to be done at a cardio-oncology center,” Dr. Lenihan said in an interview.
“For the vast majority of patients, care can be at community hospitals, similar to the delivery of the vast majority of oncology care. Some patients need referral to tertiary cardiology centers for advanced heart failure or to undergo advanced procedures, but that is a very small percentage of patients,” said Ana Barac, MD, director of the cardio-oncology program at the MedStar Heart Institute in Washington, and chair of the ACC’s Cardio-Oncology Section.
“Patients receiving more novel or unusual therapies, and those participating in trials” are appropriate for centers, while community care by a cardiologist and oncologist should suffice for more routine patients, said Dr. Fradley.
“Cardio-oncology centers are good for patients with type I damage from anthracycline treatment, especially patients who already had underlying heart disease,” said Michael S. Ewer, MD, a cardiologist and professor of medicine at MD Anderson Cancer Center in Houston. Specialist centers are also for patients with cardiovascular risk factors: older age, diabetes, preexisting coronary artery disease, and patients who receive cardiotoxic type I therapy (J Clin Oncol. 2005 May;23[13]:2900-2). Also, patients with a significant, immediate cardiac reaction to treatment, and those with an unexpected cardiac reaction, Dr. Ewer said.
A somewhat more expansive view of the typical cardio-oncology patient came from Dr. Neilan, based on the patients he sees at his program in Boston. Dr. Neilan estimated that roughly 60%-70% of his patients first present while they undergo active cancer treatment, with another 20% coming to the program as cancer survivors, and a small percentage of patients showing up for cardiology assessments and treatments without a cancer history. Among those with a cancer history, he guessed that perhaps 10%-20% were treated with an anthracycline, at least 10% received trastuzumab, and about 10% received radiation treatment. “I also see a lot of patients with complications from treatment” with tyrosine kinase inhibitors, VEGF inhibitors, and immunotherapies. “I don’t see a lot of patients for cardiovascular disease assessment before they start cancer therapy,” Dr. Neilan added.
Cardio-oncology heads toward a new cardiology subspecialty
These views of how cardio-oncology is practiced in the real world raise a question about the role of the growing roster of U.S. cardio-oncology programs. If most cancer patients can have their cardiology needs taken care of in the community, how do all the academic programs fit in? The answer seems to be that they model successful oncology and cardiology collaborations, provide a training ground for physicians from both specialties to learn how to collaborate, and serve as the home for research that broadens the field’s evidence base and moves knowledge forward.
“Education and partnerships with oncology teams is the key,” said Dr. Barac. “Our traditional subspecialty training focused on ‘treating cancer’ and ‘treating cardiovascular disease.’ Learning about and seeing effective partnerships during training” is the best model to foster cardiology and oncology partnerships among early-career physicians, she suggested.
“What is the spectrum of knowledge required to be proficient in cardio-oncology, and how do we enhance training at the resident or fellowship level? How do we get [all cardiology] trainees exposed to this knowledge?” wondered Dr. Lenihan, who viewed cardio-oncology programs as a way to meet these needs. “Cardio-oncology is not an established subspecialty. A goal is to establish training requirements and expand training opportunities. And the whole field needs to contribute to clinical research. We need cardio-oncologists to share their experience and improve our level of research.”
ASCO’s cardiac dysfunction practice guideline, first released last December and formally published in March, is likely helping to further entrench cardio-oncology as a new subspecialty. The guideline was “a remarkable step forward,” said Dr. Barac. In addition to establishing a U.S. standard of care for preventing and monitoring cardiac dysfunction in cancer patients, “I use it as a guide for creation of referral pathways with my oncology colleagues, as well as in education of cardiovascular and oncology trainees,” she said in an interview.
Though produced primarily through ASCO’s leadership, the target audience for the guideline seems to be as much cardiologists as it is oncologists. Dissemination of the guideline to cardiologists snagged when it failed to appear in the cardiology literature. That wasn’t the original plan, said guideline participants.
“Before we started, it was agreed that both ASCO and the ACC would publish it. We had a [letter] signed by the president of the ACC saying the ACC would publish it,” recalled Dr. Lenihan, a guideline coauthor. “After all the details were settled, the ACC bailed. They said that they had changed their organizational structure and that they wouldn’t publish the guideline even though they had agreed to.” Not having the guideline appear simultaneously in the cardiology literature “hinders getting the message to the cardiology community,” he said, a sentiment echoed by other cardio-oncologists.
“I served as the ACC representative on the guideline, and the lack of ACC endorsement was the unfortunate consequence of approval and publication timing that coincided with restructuring of the ACC committees,” said Dr. Barac. “It absolutely does not reflect a lack of interest from the ACC.” As an example of the College’s commitment example, she cited an ACC 1.5-day educational course on cardiovascular care of oncology patients held for the first time in February 2017 and scheduled for a second edition next February.
Publication of the guideline in a cardiology journal “would indeed help dissemination among U.S. cardiologists,” agreed Pamela S. Douglas, MD, professor of medicine at Duke University in Durham, N.C., and another of the several cardiologists who served on the ASCO guideline’s panel.
Further advancing awareness of patients with cardio-oncology issues, what Dr. Moslehi has called “an emerging epidemic,” seems the most fundamental of the goals currently advanced by many active in this field.
One step to grow the subspecialty that he and his associates at Vanderbilt have taken is to start this year a formally recognized fellowship program in cardio-oncology; an initial class of three cardiologists started in the program this summer. The Vanderbilt group also plans to launch a website before the end of 2017 that will include an oncology-drug database that compiles all available information on each agent’s cardiovascular effects. The planned website will aggregate links to all existing cardio-oncology programs.
“We will absolutely see the field grow,” said Dr. Swain. “It has only sprung up in the past 10 or so years. It is now getting recognition, people are being trained in cardio-oncology, and it will grow as a subspecialty. It’s very exciting, and it’s better for patients.”
“A cardiologist with no cancer patients or survivors in their practice is unheard of; many cardiologists just don’t realize that,” Dr. Lenihan said. At least 10%-15% of the U.S. population in their 60s or older has a cancer history, he noted. The common mindset among cardiologists has been that cancer patients and survivors are not among their patients.
“It’s unlikely that a busy cardiology practice has no cancer survivors or active cancer patients,” Dr. Douglas suggested. When this happens, a likely explanations is that the cardiologist simply failed to elicit a completely comprehensive history from the practice’s patient roster. And even a cardiology practice today that includes no cancer patients or survivors will likely see some turning up soon, she predicted, because so many are receiving cardiovascular-toxic therapies and then surviving longer than ever before.
“What oncologists and cardiologists want to do is to optimize oncologic outcomes but with an acceptable adverse event profile. The cardio-oncologist helps push that envelope. The goal is not to eliminate cardiac events at the expense of oncologic outcomes, but to shift the balance to fewer and less severe cardiac events without unduly compromising oncologic outcomes,” explained Dr. Ewer. Cardio-oncology grapples with one of the core challenges of medicine, how to balance the potential risks from treatment against its potential benefits, he observed.
Dr. Neilan has been a consultant to Ariad and Takeda. Dr. Lenihan has been a consultant to Janssen and Roche and has received research funding from Takeda. Dr. Moslehi has been a consultant to Acceleron, Ariad, Bristol-Myers Squibb, Incyte, Pfizer, Takeda/Millennium, Verastem and Vertex. Dr. Ewer, Dr. Fradley, and Dr. Barac had no relevant disclosures. Dr. Swain has been a consultant to Genentech and Roche. Dr. Douglas has been a consultant to CardioDx, Interleukin Genetics, and Omicia, and has an ownership interest in CardioDx.
[email protected]
On Twitter @mitchelzoler
Cardio-oncology is expanding, fed by a steadily increasing population of cancer survivors at elevated risk for a range of cardiovascular diseases and complications because of the anticancer treatments they received. Cardio-oncology’s quick growth has also been driven by the rapidly expanding universe of cancer treatments with direct or indirect adverse effects on a diverse range of cardiovascular functions.
During the past year, the field’s rapid evolution has featured the first formal diagnostic and care standards in two iterations: A position paper on the cardiovascular toxicities of cancer treatment from the European Society of Cardiology (ESC), released in August 2016 (Eur Heart J. 2016 Sept 21;37[36]:2766-801); and a guideline for preventing and monitoring cardiac dysfunction in adult cancer survivors, issued last December by the American Society of Clinical Oncology (ASCO) and endorsed by the American Heart Association (J Clin Oncol. 2017 March 10;35[8]:893-913), but notably not endorsed by the American College of Cardiology, despite having an ACC representative on the guideline panel. In 2015, the ACC started a Cardio-Oncology Section, one of 20 special-interest sections it maintains, and by mid-2017 the section had some 500 members.
“I’ve had recent conversations with cardiologists who said ‘I’m not sure what cardio-oncology is,’ ” said Tomas G. Neilan, MD, director of the cardio-oncology program at Massachusetts General Hospital in Boston.
More than just heart failure
A few decades ago, in the primordial days of cardio-oncology, the concept of cardiovascular damage during cancer therapy focused entirely on myocardial damage caused by anthracyclines and chest radiation, a concern that eventually expanded to include trastuzumab (Herceptin) and other agents that target the human epidermal growth factor receptor 2 (HER2). These treatments cause significantly reduced left ventricular ejection fractions and heart failure in a significant minority of treated patients. Patients who receive combined treatment with an anthracycline and trastuzumab are at the highest risk for developing heart failure with reduced ejection fraction, but even among patients treated with this combination, fewer than 5% develop outright heart failure.
While this parochial view of cardio-oncology has recently shifted, it remains true that myocardial damage from a relatively large cumulative anthracycline dose, or from radiation, causes some of the most extreme cases of cardiovascular adverse effects and remains an ongoing problem as these treatments stay front line for selected cancer patients.
But some of the recent burgeoning of cardio-oncology has followed the recognition that many other drugs and drug classes can cause a spectrum of adverse cardiovascular effects.
“There has been a significant focus on heart failure and cardiomyopathy due to anthracyclines and HER2-targeted therapies. I think the field will continue to evolve over the next 5 years to focus on other cardiovascular complications, including arrhythmias and vascular disease,” observed Michael Fradley, MD, director of cardio-oncology at Moffitt Cancer Center in Tampa. “In addition, there will be an increased focus on targeted drugs and immunotherapies,” agents that Dr. Fradley said “have many unique cardiovascular complications. We need additional guidelines regarding the management of a variety of cardiotoxicities as well as long-term monitoring strategies.”
In a review article Dr. Moslehi published toward the end of 2016, he fleshed out the wider scope of adverse cardiovascular effects from cancer therapies, noting that the vascular endothelial growth factor (VEGF) signaling pathway inhibitors, drugs such as bevacizumab (Avastin) and aflibercept (Zaltrap), have been documented to cause hypertension, arterial thromboembolic events, and cardiomyopathy; and that tyrosine kinase inhibitors have been shown to cause vascular events, QT interval prolongation, and cerebral and peripheral vascular events (N Engl J Med. 2016 Oct 13;375[15]:1457-67).
In his own recent review, Dr. Fradley highlighted adverse cardiovascular effects from additional anticancer drug classes, including proteasome inhibitors, which can trigger hypertension and cardiomyopathy; immunomodulators, implicated in causing both venous and arterial thromboembolism; and the immune checkpoint inhibitors, linked with myocarditis, arrhythmias, hypotension, and myocardial ischemia (Eur Heart J. 2016 Sept 21;37[36]:2740-2). A similarly broad spectrum of adverse cardiovascular effects linked with a wide range of anticancer treatments also appeared in the ESC 2016 position paper on cancer treatments.
But while the range of cancer treatments that can have some impact on the cardiovascular system is strikingly large, experts uniformly caution that far from every patient treated for cancer needs an immediate cardiology consult and work-up, especially when the cancers appear in young adults.
“We’re not quite at the point where every cancer patient needs to be seen by a cardiologist or cardio-oncologist,” Dr. Fradley noted in an interview.
“If a patient develops hypertension while on treatment I refer them to a PCP or cardiologist. I don’t treat hypertension myself. But if a patient is ‘normal’ they don’t need a cardiology assessment up front. It’s impossible to refer all patients, especially younger patients, with current resources. There are too many patients who receive cardiotoxic therapies to refer everyone. I involve the cardiologist once there is evidence of damage,”she explained.
Cardio-oncology centers or community practice?
The rise of cardio-oncology, especially over the last decade or so, has given rise to a new academic niche, the cardio-oncology clinic. Starting from almost no programs a few years ago, by 2016 one tally put the total number of U.S. self-designated cardio-oncology centers at about 40 (Heart Fail Clin. 2017 April;13[2]:347-55), and that number undoubtedly grew even more during the year since. While these programs promote and advance the nascent subspecialty of cardio-oncology, and provide a foundation for development of formalized training programs, many experts see a clear hierarchy of risk that distinguishes the patients who should ideally be managed at these focused, multidisciplinary programs from the lower-risk patients who probably do fine under the care of just their oncologist or their oncologist in collaboration with a community cardiologist or primary care physician.
“The cardio-oncology community recognizes that it is nice to have programs at academic centers but it’s more important to deliver this care in the community,” said Dr. Lenihan. “Many cancer patients have no prior history of cardiovascular disease. These low-risk patients don’t necessarily need a cardio-oncologist. They may need to have their blood pressure managed more effectively or receive other preventive care, but that can certainly be done locally. There are low-risk patients who don’t need to go to a major center.” Dr. Lenihan and other cardio-oncologists see the majority of cancer patients as low risk when it comes to cardiovascular complications.
But it’s different when patients receive an anthracycline or an anthracycline plus trastuzumab. “This high-risk population is best seen at a cardio-oncology center.” Dr. Lenihan also included in this high-risk subgroup patients treated with mediastinal radiation, an option often used during the 1980s-2000s.
“Any time a patient receives treatment with the potential to cause a cardiovascular effect, which is pretty much any drug that now comes out, you need an accurate baseline assessment. But that doesn’t mean you need do anything different; you still treat the patient’s cancer. A thorough baseline assessment is a necessity, but it does not need to be done at a cardio-oncology center,” Dr. Lenihan said in an interview.
“For the vast majority of patients, care can be at community hospitals, similar to the delivery of the vast majority of oncology care. Some patients need referral to tertiary cardiology centers for advanced heart failure or to undergo advanced procedures, but that is a very small percentage of patients,” said Ana Barac, MD, director of the cardio-oncology program at the MedStar Heart Institute in Washington, and chair of the ACC’s Cardio-Oncology Section.
“Patients receiving more novel or unusual therapies, and those participating in trials” are appropriate for centers, while community care by a cardiologist and oncologist should suffice for more routine patients, said Dr. Fradley.
“Cardio-oncology centers are good for patients with type I damage from anthracycline treatment, especially patients who already had underlying heart disease,” said Michael S. Ewer, MD, a cardiologist and professor of medicine at MD Anderson Cancer Center in Houston. Specialist centers are also for patients with cardiovascular risk factors: older age, diabetes, preexisting coronary artery disease, and patients who receive cardiotoxic type I therapy (J Clin Oncol. 2005 May;23[13]:2900-2). Also, patients with a significant, immediate cardiac reaction to treatment, and those with an unexpected cardiac reaction, Dr. Ewer said.
A somewhat more expansive view of the typical cardio-oncology patient came from Dr. Neilan, based on the patients he sees at his program in Boston. Dr. Neilan estimated that roughly 60%-70% of his patients first present while they undergo active cancer treatment, with another 20% coming to the program as cancer survivors, and a small percentage of patients showing up for cardiology assessments and treatments without a cancer history. Among those with a cancer history, he guessed that perhaps 10%-20% were treated with an anthracycline, at least 10% received trastuzumab, and about 10% received radiation treatment. “I also see a lot of patients with complications from treatment” with tyrosine kinase inhibitors, VEGF inhibitors, and immunotherapies. “I don’t see a lot of patients for cardiovascular disease assessment before they start cancer therapy,” Dr. Neilan added.
Cardio-oncology heads toward a new cardiology subspecialty
These views of how cardio-oncology is practiced in the real world raise a question about the role of the growing roster of U.S. cardio-oncology programs. If most cancer patients can have their cardiology needs taken care of in the community, how do all the academic programs fit in? The answer seems to be that they model successful oncology and cardiology collaborations, provide a training ground for physicians from both specialties to learn how to collaborate, and serve as the home for research that broadens the field’s evidence base and moves knowledge forward.
“Education and partnerships with oncology teams is the key,” said Dr. Barac. “Our traditional subspecialty training focused on ‘treating cancer’ and ‘treating cardiovascular disease.’ Learning about and seeing effective partnerships during training” is the best model to foster cardiology and oncology partnerships among early-career physicians, she suggested.
“What is the spectrum of knowledge required to be proficient in cardio-oncology, and how do we enhance training at the resident or fellowship level? How do we get [all cardiology] trainees exposed to this knowledge?” wondered Dr. Lenihan, who viewed cardio-oncology programs as a way to meet these needs. “Cardio-oncology is not an established subspecialty. A goal is to establish training requirements and expand training opportunities. And the whole field needs to contribute to clinical research. We need cardio-oncologists to share their experience and improve our level of research.”
ASCO’s cardiac dysfunction practice guideline, first released last December and formally published in March, is likely helping to further entrench cardio-oncology as a new subspecialty. The guideline was “a remarkable step forward,” said Dr. Barac. In addition to establishing a U.S. standard of care for preventing and monitoring cardiac dysfunction in cancer patients, “I use it as a guide for creation of referral pathways with my oncology colleagues, as well as in education of cardiovascular and oncology trainees,” she said in an interview.
Though produced primarily through ASCO’s leadership, the target audience for the guideline seems to be as much cardiologists as it is oncologists. Dissemination of the guideline to cardiologists snagged when it failed to appear in the cardiology literature. That wasn’t the original plan, said guideline participants.
“Before we started, it was agreed that both ASCO and the ACC would publish it. We had a [letter] signed by the president of the ACC saying the ACC would publish it,” recalled Dr. Lenihan, a guideline coauthor. “After all the details were settled, the ACC bailed. They said that they had changed their organizational structure and that they wouldn’t publish the guideline even though they had agreed to.” Not having the guideline appear simultaneously in the cardiology literature “hinders getting the message to the cardiology community,” he said, a sentiment echoed by other cardio-oncologists.
“I served as the ACC representative on the guideline, and the lack of ACC endorsement was the unfortunate consequence of approval and publication timing that coincided with restructuring of the ACC committees,” said Dr. Barac. “It absolutely does not reflect a lack of interest from the ACC.” As an example of the College’s commitment example, she cited an ACC 1.5-day educational course on cardiovascular care of oncology patients held for the first time in February 2017 and scheduled for a second edition next February.
Publication of the guideline in a cardiology journal “would indeed help dissemination among U.S. cardiologists,” agreed Pamela S. Douglas, MD, professor of medicine at Duke University in Durham, N.C., and another of the several cardiologists who served on the ASCO guideline’s panel.
Further advancing awareness of patients with cardio-oncology issues, what Dr. Moslehi has called “an emerging epidemic,” seems the most fundamental of the goals currently advanced by many active in this field.
One step to grow the subspecialty that he and his associates at Vanderbilt have taken is to start this year a formally recognized fellowship program in cardio-oncology; an initial class of three cardiologists started in the program this summer. The Vanderbilt group also plans to launch a website before the end of 2017 that will include an oncology-drug database that compiles all available information on each agent’s cardiovascular effects. The planned website will aggregate links to all existing cardio-oncology programs.
“We will absolutely see the field grow,” said Dr. Swain. “It has only sprung up in the past 10 or so years. It is now getting recognition, people are being trained in cardio-oncology, and it will grow as a subspecialty. It’s very exciting, and it’s better for patients.”
“A cardiologist with no cancer patients or survivors in their practice is unheard of; many cardiologists just don’t realize that,” Dr. Lenihan said. At least 10%-15% of the U.S. population in their 60s or older has a cancer history, he noted. The common mindset among cardiologists has been that cancer patients and survivors are not among their patients.
“It’s unlikely that a busy cardiology practice has no cancer survivors or active cancer patients,” Dr. Douglas suggested. When this happens, a likely explanations is that the cardiologist simply failed to elicit a completely comprehensive history from the practice’s patient roster. And even a cardiology practice today that includes no cancer patients or survivors will likely see some turning up soon, she predicted, because so many are receiving cardiovascular-toxic therapies and then surviving longer than ever before.
“What oncologists and cardiologists want to do is to optimize oncologic outcomes but with an acceptable adverse event profile. The cardio-oncologist helps push that envelope. The goal is not to eliminate cardiac events at the expense of oncologic outcomes, but to shift the balance to fewer and less severe cardiac events without unduly compromising oncologic outcomes,” explained Dr. Ewer. Cardio-oncology grapples with one of the core challenges of medicine, how to balance the potential risks from treatment against its potential benefits, he observed.
Dr. Neilan has been a consultant to Ariad and Takeda. Dr. Lenihan has been a consultant to Janssen and Roche and has received research funding from Takeda. Dr. Moslehi has been a consultant to Acceleron, Ariad, Bristol-Myers Squibb, Incyte, Pfizer, Takeda/Millennium, Verastem and Vertex. Dr. Ewer, Dr. Fradley, and Dr. Barac had no relevant disclosures. Dr. Swain has been a consultant to Genentech and Roche. Dr. Douglas has been a consultant to CardioDx, Interleukin Genetics, and Omicia, and has an ownership interest in CardioDx.
[email protected]
On Twitter @mitchelzoler
A Synergistic Antitumor Delivery System
Oncolytic viruses (OVs) are proving to be a promising modality in anticancer therapy. They selectively grow, replicate within, and kill tumor cells while leaving alone untransformed cells. But OVs, such as the respiratory enteric orphan viruses (or reoviruses), have been hampered by neutralizing antireoviral antibodies, which block the viruses from binding to cellular surface receptors, inhibiting viral infection and replication. Researchers from Guizhou Medical University in China and Stanford University in California may have found a solution: a novel strategy using cytokine-induced killer (CIK) cells—which also have antitumor activity—as the delivery system.
Related: Advances in Targeted Therapy for Breast Cancer
Reoviruses have been shown to infect and use peripheral blood mononuclear cells (PBMCs) to reach tumors. Combining cytokine-induced killer cells (developed from PBMCs) with a reovirus could be the way in, the researchers theorized. Their strategy relies on cell carriage—the capacity of some OVs to be “carried” by immune cells to the tumor, where they selectively infect and kill tumor cells.
The researchers found that CIK cells provided cell carriage to the reovirus, which exerted an oncolytic effect on tumor cells but not CIK cells. Moreover, the CIK cells promoted reovirus infection of tumor cells in the presence of neutralizing antibodies. At the same time, the reovirus infection boosted the power of the CIK cells.
Related: Potential New Targeted Treatment for Chondrosarcoma
The researchers’ findings support the idea that reoviruseas and CIK cells are both potent antitumor agents and are “superior as a combination strategy.”
Source:
Zhao X, Ouyang W, Chester C, Long S, Wang N, He Z. PLoS One. 2017;12(9):e0184816.
doi: 10.1371/journal.pone.0184816.
Oncolytic viruses (OVs) are proving to be a promising modality in anticancer therapy. They selectively grow, replicate within, and kill tumor cells while leaving alone untransformed cells. But OVs, such as the respiratory enteric orphan viruses (or reoviruses), have been hampered by neutralizing antireoviral antibodies, which block the viruses from binding to cellular surface receptors, inhibiting viral infection and replication. Researchers from Guizhou Medical University in China and Stanford University in California may have found a solution: a novel strategy using cytokine-induced killer (CIK) cells—which also have antitumor activity—as the delivery system.
Related: Advances in Targeted Therapy for Breast Cancer
Reoviruses have been shown to infect and use peripheral blood mononuclear cells (PBMCs) to reach tumors. Combining cytokine-induced killer cells (developed from PBMCs) with a reovirus could be the way in, the researchers theorized. Their strategy relies on cell carriage—the capacity of some OVs to be “carried” by immune cells to the tumor, where they selectively infect and kill tumor cells.
The researchers found that CIK cells provided cell carriage to the reovirus, which exerted an oncolytic effect on tumor cells but not CIK cells. Moreover, the CIK cells promoted reovirus infection of tumor cells in the presence of neutralizing antibodies. At the same time, the reovirus infection boosted the power of the CIK cells.
Related: Potential New Targeted Treatment for Chondrosarcoma
The researchers’ findings support the idea that reoviruseas and CIK cells are both potent antitumor agents and are “superior as a combination strategy.”
Source:
Zhao X, Ouyang W, Chester C, Long S, Wang N, He Z. PLoS One. 2017;12(9):e0184816.
doi: 10.1371/journal.pone.0184816.
Oncolytic viruses (OVs) are proving to be a promising modality in anticancer therapy. They selectively grow, replicate within, and kill tumor cells while leaving alone untransformed cells. But OVs, such as the respiratory enteric orphan viruses (or reoviruses), have been hampered by neutralizing antireoviral antibodies, which block the viruses from binding to cellular surface receptors, inhibiting viral infection and replication. Researchers from Guizhou Medical University in China and Stanford University in California may have found a solution: a novel strategy using cytokine-induced killer (CIK) cells—which also have antitumor activity—as the delivery system.
Related: Advances in Targeted Therapy for Breast Cancer
Reoviruses have been shown to infect and use peripheral blood mononuclear cells (PBMCs) to reach tumors. Combining cytokine-induced killer cells (developed from PBMCs) with a reovirus could be the way in, the researchers theorized. Their strategy relies on cell carriage—the capacity of some OVs to be “carried” by immune cells to the tumor, where they selectively infect and kill tumor cells.
The researchers found that CIK cells provided cell carriage to the reovirus, which exerted an oncolytic effect on tumor cells but not CIK cells. Moreover, the CIK cells promoted reovirus infection of tumor cells in the presence of neutralizing antibodies. At the same time, the reovirus infection boosted the power of the CIK cells.
Related: Potential New Targeted Treatment for Chondrosarcoma
The researchers’ findings support the idea that reoviruseas and CIK cells are both potent antitumor agents and are “superior as a combination strategy.”
Source:
Zhao X, Ouyang W, Chester C, Long S, Wang N, He Z. PLoS One. 2017;12(9):e0184816.
doi: 10.1371/journal.pone.0184816.
Reminder calls to patients improve fecal test sample response rates
Automated and live phone calls were shown to improve patient return of fecal test samples for both English and Spanish speakers, based on the results of a pilot study.
Colorectal cancer (CRC) is the second deadliest cancer in the United States. In 2017 alone, 50,000 people died from the disease. Screening has been shown to be a very effective tool in decreasing the mortality and incidence of CRC, but screening rates are low with 63% of adults adhering to recommended screening schedules. This problem has been addressed by direct-mail fecal immunochemical testing (FIT) kits with associated reminders, but few studies have evaluated effectiveness of follow-up techniques on FIT return rates until this pilot study.
“While many direct-mail fecal testing programs have delivered patient reminders, ours is the first study to rigorously test the effectiveness of these reminders in a community health center population, and among patients with differing language preferences,” wrote Gloria Coronado, PhD, of the Center for Health Research at Kaiser Permanente and her colleagues. “We observed important variations in return rates based on reminder mode used, and both the overall return rate and reminder mode effectiveness differed according to patients’ language preferences.”
The trial had two groups, one randomized and the other nonrandomized. Nonrandomized patients had active patient portals and received email reminders through the portal. The randomized group was sorted into seven intervention groups: Four of the groups used a unimodal contact method and three groups used a multimodal contact method. The unimodal contact methods were letter reminders, automated call reminders, text reminders, and live call reminders. The multimodal contact methods were a reminder letter plus live call reminders, automated calls plus live call reminders, and text message reminders plus live call reminders. All written materials to contact patients were developed in English and later translated into Spanish and Russian. Phone call scripts were also developed in English and later translated into Spanish but not Russian due to a lack of Russian speaking outreach workers.
After combining early-return FIT samples, those in the nonrandomized patient portal group, and the randomized samples, the overall return rate was 32.7%.
The method of contact for patients strongly influenced return rates for patients. Patients who received live phone calls were 50% more likely to return their FIT kit, compared with those who simply received a reminder email. Both English and Spanish speakers were much more likely to return their FIT kits if they were contacted with live or automated phone calls with odds ratios of 2.17 and 3.45, respectively. All other reminder techniques that did not include a phone call had similar completion rates to that of a reminder letter.
“Automated phone calls and text messages are the least costly options to implement, yet live reminders may allow staff to address or triage other patient health care needs,” they wrote.
Dr. Gloria Coronado was a coinvestigator for a study funded by Epigenomics. All other authors had no conflicts of interest to report.
Automated and live phone calls were shown to improve patient return of fecal test samples for both English and Spanish speakers, based on the results of a pilot study.
Colorectal cancer (CRC) is the second deadliest cancer in the United States. In 2017 alone, 50,000 people died from the disease. Screening has been shown to be a very effective tool in decreasing the mortality and incidence of CRC, but screening rates are low with 63% of adults adhering to recommended screening schedules. This problem has been addressed by direct-mail fecal immunochemical testing (FIT) kits with associated reminders, but few studies have evaluated effectiveness of follow-up techniques on FIT return rates until this pilot study.
“While many direct-mail fecal testing programs have delivered patient reminders, ours is the first study to rigorously test the effectiveness of these reminders in a community health center population, and among patients with differing language preferences,” wrote Gloria Coronado, PhD, of the Center for Health Research at Kaiser Permanente and her colleagues. “We observed important variations in return rates based on reminder mode used, and both the overall return rate and reminder mode effectiveness differed according to patients’ language preferences.”
The trial had two groups, one randomized and the other nonrandomized. Nonrandomized patients had active patient portals and received email reminders through the portal. The randomized group was sorted into seven intervention groups: Four of the groups used a unimodal contact method and three groups used a multimodal contact method. The unimodal contact methods were letter reminders, automated call reminders, text reminders, and live call reminders. The multimodal contact methods were a reminder letter plus live call reminders, automated calls plus live call reminders, and text message reminders plus live call reminders. All written materials to contact patients were developed in English and later translated into Spanish and Russian. Phone call scripts were also developed in English and later translated into Spanish but not Russian due to a lack of Russian speaking outreach workers.
After combining early-return FIT samples, those in the nonrandomized patient portal group, and the randomized samples, the overall return rate was 32.7%.
The method of contact for patients strongly influenced return rates for patients. Patients who received live phone calls were 50% more likely to return their FIT kit, compared with those who simply received a reminder email. Both English and Spanish speakers were much more likely to return their FIT kits if they were contacted with live or automated phone calls with odds ratios of 2.17 and 3.45, respectively. All other reminder techniques that did not include a phone call had similar completion rates to that of a reminder letter.
“Automated phone calls and text messages are the least costly options to implement, yet live reminders may allow staff to address or triage other patient health care needs,” they wrote.
Dr. Gloria Coronado was a coinvestigator for a study funded by Epigenomics. All other authors had no conflicts of interest to report.
Automated and live phone calls were shown to improve patient return of fecal test samples for both English and Spanish speakers, based on the results of a pilot study.
Colorectal cancer (CRC) is the second deadliest cancer in the United States. In 2017 alone, 50,000 people died from the disease. Screening has been shown to be a very effective tool in decreasing the mortality and incidence of CRC, but screening rates are low with 63% of adults adhering to recommended screening schedules. This problem has been addressed by direct-mail fecal immunochemical testing (FIT) kits with associated reminders, but few studies have evaluated effectiveness of follow-up techniques on FIT return rates until this pilot study.
“While many direct-mail fecal testing programs have delivered patient reminders, ours is the first study to rigorously test the effectiveness of these reminders in a community health center population, and among patients with differing language preferences,” wrote Gloria Coronado, PhD, of the Center for Health Research at Kaiser Permanente and her colleagues. “We observed important variations in return rates based on reminder mode used, and both the overall return rate and reminder mode effectiveness differed according to patients’ language preferences.”
The trial had two groups, one randomized and the other nonrandomized. Nonrandomized patients had active patient portals and received email reminders through the portal. The randomized group was sorted into seven intervention groups: Four of the groups used a unimodal contact method and three groups used a multimodal contact method. The unimodal contact methods were letter reminders, automated call reminders, text reminders, and live call reminders. The multimodal contact methods were a reminder letter plus live call reminders, automated calls plus live call reminders, and text message reminders plus live call reminders. All written materials to contact patients were developed in English and later translated into Spanish and Russian. Phone call scripts were also developed in English and later translated into Spanish but not Russian due to a lack of Russian speaking outreach workers.
After combining early-return FIT samples, those in the nonrandomized patient portal group, and the randomized samples, the overall return rate was 32.7%.
The method of contact for patients strongly influenced return rates for patients. Patients who received live phone calls were 50% more likely to return their FIT kit, compared with those who simply received a reminder email. Both English and Spanish speakers were much more likely to return their FIT kits if they were contacted with live or automated phone calls with odds ratios of 2.17 and 3.45, respectively. All other reminder techniques that did not include a phone call had similar completion rates to that of a reminder letter.
“Automated phone calls and text messages are the least costly options to implement, yet live reminders may allow staff to address or triage other patient health care needs,” they wrote.
Dr. Gloria Coronado was a coinvestigator for a study funded by Epigenomics. All other authors had no conflicts of interest to report.
FROM THE JOURNAL OF GENERAL INTERNAL MEDICINE
Key clinical point:
Major finding: 32.7% of patients returned their fecal testing kit after receiving a reminder.
Data source: Patient-randomized control trial of 2,478 adults who were not up to date on their colorectal cancer screening.
Disclosures: Dr. Gloria Coronado was a coinvestigator for a study funded by Epigenomics. All other authors had no conflicts of interest to report.
VA Proposes New Telehealth Rule
Not willing to wait for a pending legislative fix, the VA has proposed a new rule that would allow expanding telehealth offerings across state lines. The Authority of Health Care Providers to Practice Telehealth rule was proposed on October 2, 2017 and comments will remain open through November 1, 2017.
“VA has developed a telehealth program as a modern, beneficiary- and family-centered health care delivery model that leverages information and telecommunication technologies to connect beneficiaries with health care providers, irrespective of the State or location within a State where the health care provider or the beneficiary is physically located at the time the health care is provided,” the rule states. “Telehealth increases the accessibility of VA health care, bringing VA medical services to locations convenient for beneficiaries, including clinics in remote communities and beneficiaries' homes. By providing health care services by telehealth from one State to a beneficiary located in another State or within the same State, whether that beneficiary is located at a VA medical facility or in his or her own home, VA can use its limited health care resources most efficiently.”
The VA has long recognized the importance of telehealth for delivering health care to rural veterans who live far from VAMCs and community-based outpatient clinics. Providing specialty care can be especially challenging, given the dearth of specialists in many rural locations. In addition, many veterans live in a different state than the closest VAMC, creating health care delivery challenges.
The legal standing of telehealth is unclear. Currently, VA requires that health care providers (HCPs) are licensed in the same state as the patient location at time of health care delivery. VA Secretary David J. Shulkin, MD, has called for new legislation clarifying that VA HCPs do have authority to care for patients located in a different state. However, the new rule cites previously granted legislative authority. “In an effort to furnish care to all beneficiaries and use its resources most efficiently,” the rule states, “VA needs to operate its telehealth program with health care providers who will provide services via telehealth to beneficiaries in States in which they are not licensed.”
To date, the rule has received relatively few comments, and none dispute the VA interpretation of its legal authority to change telehealth rules. A few commenters have raised concerns over the quality use of telehealth care, especially for mental health care. Most comments, however, supported the expansion of telehealth to increase access to care for veterans.
House Resolution 2123, the VETS Act of 2017, also contains similar provisions that would streamline interstate telehealth. However, that bill has yet to receive a vote in the House Veterans Affairs committee.
Not willing to wait for a pending legislative fix, the VA has proposed a new rule that would allow expanding telehealth offerings across state lines. The Authority of Health Care Providers to Practice Telehealth rule was proposed on October 2, 2017 and comments will remain open through November 1, 2017.
“VA has developed a telehealth program as a modern, beneficiary- and family-centered health care delivery model that leverages information and telecommunication technologies to connect beneficiaries with health care providers, irrespective of the State or location within a State where the health care provider or the beneficiary is physically located at the time the health care is provided,” the rule states. “Telehealth increases the accessibility of VA health care, bringing VA medical services to locations convenient for beneficiaries, including clinics in remote communities and beneficiaries' homes. By providing health care services by telehealth from one State to a beneficiary located in another State or within the same State, whether that beneficiary is located at a VA medical facility or in his or her own home, VA can use its limited health care resources most efficiently.”
The VA has long recognized the importance of telehealth for delivering health care to rural veterans who live far from VAMCs and community-based outpatient clinics. Providing specialty care can be especially challenging, given the dearth of specialists in many rural locations. In addition, many veterans live in a different state than the closest VAMC, creating health care delivery challenges.
The legal standing of telehealth is unclear. Currently, VA requires that health care providers (HCPs) are licensed in the same state as the patient location at time of health care delivery. VA Secretary David J. Shulkin, MD, has called for new legislation clarifying that VA HCPs do have authority to care for patients located in a different state. However, the new rule cites previously granted legislative authority. “In an effort to furnish care to all beneficiaries and use its resources most efficiently,” the rule states, “VA needs to operate its telehealth program with health care providers who will provide services via telehealth to beneficiaries in States in which they are not licensed.”
To date, the rule has received relatively few comments, and none dispute the VA interpretation of its legal authority to change telehealth rules. A few commenters have raised concerns over the quality use of telehealth care, especially for mental health care. Most comments, however, supported the expansion of telehealth to increase access to care for veterans.
House Resolution 2123, the VETS Act of 2017, also contains similar provisions that would streamline interstate telehealth. However, that bill has yet to receive a vote in the House Veterans Affairs committee.
Not willing to wait for a pending legislative fix, the VA has proposed a new rule that would allow expanding telehealth offerings across state lines. The Authority of Health Care Providers to Practice Telehealth rule was proposed on October 2, 2017 and comments will remain open through November 1, 2017.
“VA has developed a telehealth program as a modern, beneficiary- and family-centered health care delivery model that leverages information and telecommunication technologies to connect beneficiaries with health care providers, irrespective of the State or location within a State where the health care provider or the beneficiary is physically located at the time the health care is provided,” the rule states. “Telehealth increases the accessibility of VA health care, bringing VA medical services to locations convenient for beneficiaries, including clinics in remote communities and beneficiaries' homes. By providing health care services by telehealth from one State to a beneficiary located in another State or within the same State, whether that beneficiary is located at a VA medical facility or in his or her own home, VA can use its limited health care resources most efficiently.”
The VA has long recognized the importance of telehealth for delivering health care to rural veterans who live far from VAMCs and community-based outpatient clinics. Providing specialty care can be especially challenging, given the dearth of specialists in many rural locations. In addition, many veterans live in a different state than the closest VAMC, creating health care delivery challenges.
The legal standing of telehealth is unclear. Currently, VA requires that health care providers (HCPs) are licensed in the same state as the patient location at time of health care delivery. VA Secretary David J. Shulkin, MD, has called for new legislation clarifying that VA HCPs do have authority to care for patients located in a different state. However, the new rule cites previously granted legislative authority. “In an effort to furnish care to all beneficiaries and use its resources most efficiently,” the rule states, “VA needs to operate its telehealth program with health care providers who will provide services via telehealth to beneficiaries in States in which they are not licensed.”
To date, the rule has received relatively few comments, and none dispute the VA interpretation of its legal authority to change telehealth rules. A few commenters have raised concerns over the quality use of telehealth care, especially for mental health care. Most comments, however, supported the expansion of telehealth to increase access to care for veterans.
House Resolution 2123, the VETS Act of 2017, also contains similar provisions that would streamline interstate telehealth. However, that bill has yet to receive a vote in the House Veterans Affairs committee.
Red and brown lesions
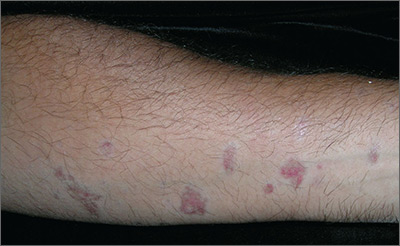
Biopsy results revealed that this was a case of atrophic lichen planus, one of the rarer types of lichen planus that is defined by nonpalpable lesions. The FP examined areas of the patient where lichen planus is commonly found (including the wrist, ankles, and back) and discovered a few similar lesions around the patient’s ankles.
The FP explained the diagnosis to the patient and offered a mid-potency topical corticosteroid—0.1% triamcinolone cream. She advised the patient that if the lesions became more atrophic, they could try to get a topical vitamin D medication approved as an alternative. There is evidence that topical vitamin D may be beneficial, with no risk of skin atrophy. However, these are high-cost medications—despite the fact that some are now generic. A prior authorization for one of these vitamin D medications is more likely to be approved if there has been a failure, or adverse effects, from a first-line topical steroid.
Other treatment options for atrophic lichen planus include oral metronidazole and oral prednisone. The mechanism for the effectiveness of oral metronidazole is unknown, but it is a relatively safe option if topical treatments fail. Oral prednisone taken for 20 to 30 days is more likely to be effective, but is also more likely to cause adverse effects.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Kraft RL, Usatine R. Lichen planus. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 901-909.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

Biopsy results revealed that this was a case of atrophic lichen planus, one of the rarer types of lichen planus that is defined by nonpalpable lesions. The FP examined areas of the patient where lichen planus is commonly found (including the wrist, ankles, and back) and discovered a few similar lesions around the patient’s ankles.
The FP explained the diagnosis to the patient and offered a mid-potency topical corticosteroid—0.1% triamcinolone cream. She advised the patient that if the lesions became more atrophic, they could try to get a topical vitamin D medication approved as an alternative. There is evidence that topical vitamin D may be beneficial, with no risk of skin atrophy. However, these are high-cost medications—despite the fact that some are now generic. A prior authorization for one of these vitamin D medications is more likely to be approved if there has been a failure, or adverse effects, from a first-line topical steroid.
Other treatment options for atrophic lichen planus include oral metronidazole and oral prednisone. The mechanism for the effectiveness of oral metronidazole is unknown, but it is a relatively safe option if topical treatments fail. Oral prednisone taken for 20 to 30 days is more likely to be effective, but is also more likely to cause adverse effects.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Kraft RL, Usatine R. Lichen planus. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 901-909.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com

Biopsy results revealed that this was a case of atrophic lichen planus, one of the rarer types of lichen planus that is defined by nonpalpable lesions. The FP examined areas of the patient where lichen planus is commonly found (including the wrist, ankles, and back) and discovered a few similar lesions around the patient’s ankles.
The FP explained the diagnosis to the patient and offered a mid-potency topical corticosteroid—0.1% triamcinolone cream. She advised the patient that if the lesions became more atrophic, they could try to get a topical vitamin D medication approved as an alternative. There is evidence that topical vitamin D may be beneficial, with no risk of skin atrophy. However, these are high-cost medications—despite the fact that some are now generic. A prior authorization for one of these vitamin D medications is more likely to be approved if there has been a failure, or adverse effects, from a first-line topical steroid.
Other treatment options for atrophic lichen planus include oral metronidazole and oral prednisone. The mechanism for the effectiveness of oral metronidazole is unknown, but it is a relatively safe option if topical treatments fail. Oral prednisone taken for 20 to 30 days is more likely to be effective, but is also more likely to cause adverse effects.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Kraft RL, Usatine R. Lichen planus. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill; 2013: 901-909.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
Can’t Quite Put My Finger On It …
ANSWER
The correct answer is perform a shave biopsy (choice “c”). It is a bedrock principle in dermatology that there is no substitute for a correct diagnosis, because correct diagnosis dictates proper treatment. When practical, biopsy is an excellent way to establish the true nature of a lesion and rule out other possibilities; it cuts through all conjecture.
DISCUSSION
The report showed intraepidermal squamous cell carcinoma, also known as Bowen disease. In this case, overexposure to the sun was the probable cause; however, Bowen disease can also develop from non–UV-related triggers, including human papillomavirus, arsenic (usually in contaminated ground water), and radiation treatment.
The differential includes psoriasis (which waxes and wanes), fungal infection (unlikely to last 10 years with so little growth), and superficial basal cell carcinoma.
Treatment success can be achieved by electrodessication and curettage or by the application of 5-fluorouracil or imiquimod cream for a month or two. Rarely, Bowen lesions can become invasive and metastasize if left untreated.
This patient’s prognosis, however, is excellent—at least, as far as this lesion is concerned. His history of sun exposure and numerous skin cancers
ANSWER
The correct answer is perform a shave biopsy (choice “c”). It is a bedrock principle in dermatology that there is no substitute for a correct diagnosis, because correct diagnosis dictates proper treatment. When practical, biopsy is an excellent way to establish the true nature of a lesion and rule out other possibilities; it cuts through all conjecture.
DISCUSSION
The report showed intraepidermal squamous cell carcinoma, also known as Bowen disease. In this case, overexposure to the sun was the probable cause; however, Bowen disease can also develop from non–UV-related triggers, including human papillomavirus, arsenic (usually in contaminated ground water), and radiation treatment.
The differential includes psoriasis (which waxes and wanes), fungal infection (unlikely to last 10 years with so little growth), and superficial basal cell carcinoma.
Treatment success can be achieved by electrodessication and curettage or by the application of 5-fluorouracil or imiquimod cream for a month or two. Rarely, Bowen lesions can become invasive and metastasize if left untreated.
This patient’s prognosis, however, is excellent—at least, as far as this lesion is concerned. His history of sun exposure and numerous skin cancers
ANSWER
The correct answer is perform a shave biopsy (choice “c”). It is a bedrock principle in dermatology that there is no substitute for a correct diagnosis, because correct diagnosis dictates proper treatment. When practical, biopsy is an excellent way to establish the true nature of a lesion and rule out other possibilities; it cuts through all conjecture.
DISCUSSION
The report showed intraepidermal squamous cell carcinoma, also known as Bowen disease. In this case, overexposure to the sun was the probable cause; however, Bowen disease can also develop from non–UV-related triggers, including human papillomavirus, arsenic (usually in contaminated ground water), and radiation treatment.
The differential includes psoriasis (which waxes and wanes), fungal infection (unlikely to last 10 years with so little growth), and superficial basal cell carcinoma.
Treatment success can be achieved by electrodessication and curettage or by the application of 5-fluorouracil or imiquimod cream for a month or two. Rarely, Bowen lesions can become invasive and metastasize if left untreated.
This patient’s prognosis, however, is excellent—at least, as far as this lesion is concerned. His history of sun exposure and numerous skin cancers
For at least 10 years, a now 70-year-old man has had a lesion on his left third finger. It is asymptomatic but gradually growing larger. Various primary care providers have offered diagnoses, the most recent being “fungal infection,” but treatments including nystatin cream have had no good effect.
The oval, pink, scaly lesion is located on the medial aspect of the proximal phalanx of the patient’s left hand; it measures 2.3 cm with well-defined borders. It is barely palpable and is not at all tender. No nodes can be felt in the arm or axilla.
The patient is otherwise healthy and is not immunosuppressed. His skin elsewhere shows evidence of sun damage—including actinic keratosis, solar lentigines, and telangiectasias—and removal of several basal cell carcinomas from his face and arms. His elbows, knees, scalp, and nails are free of any notable skin changes.
Stroke Deaths: Reversing a Healthy Trend?
After decades of decline, progress has slowed in preventing stroke deaths, according to a CDC Vital Signs report. The report is a “wake-up call,” CDC Director Brenda Fitzgerald says.
About 3 in every 4 states showed stalled rates of decline between 2000 and 2015. In some states, the trend of declining stroke deaths has actually reversed. It is a “disturbing” finding, the researchers say—particularly because 80% of strokes are preventable.
Every 40 seconds, someone in the U.S. has a stroke. Each year, > 140,000 die. Blacks continue to be hardest hit by stroke but stroke deaths are on the rise among Hispanics (by 6% each year between 2013 and 2015) and people living in the South.
Death rates continued to drop steadily between the years 2000 and 2015 among adults aged ≥ 35 years. However, people are dying of stroke at younger ages. Over the past 15 years, stroke hospitalizations have increased among adults aged 18 to 54 years. But the researchers note that risk factors, such as high blood pressure, high cholesterol, obesity, and diabetes are also appearing in younger people. Moreover, those risk factors may not be recognized and treated in middle-aged adults aged 35 to 64 years.
The study categorizes stroke deaths in the U.S. from 2000 to 2015, by age, sex, race/ethnicity, and geographic area. It does not, however, address causes for the slowdown, although it cites other studies that point to obesity and diabetes as contributors. High blood pressure is the single “most important preventable and treatable risk factor for stroke,” the CDC says.
After decades of decline, progress has slowed in preventing stroke deaths, according to a CDC Vital Signs report. The report is a “wake-up call,” CDC Director Brenda Fitzgerald says.
About 3 in every 4 states showed stalled rates of decline between 2000 and 2015. In some states, the trend of declining stroke deaths has actually reversed. It is a “disturbing” finding, the researchers say—particularly because 80% of strokes are preventable.
Every 40 seconds, someone in the U.S. has a stroke. Each year, > 140,000 die. Blacks continue to be hardest hit by stroke but stroke deaths are on the rise among Hispanics (by 6% each year between 2013 and 2015) and people living in the South.
Death rates continued to drop steadily between the years 2000 and 2015 among adults aged ≥ 35 years. However, people are dying of stroke at younger ages. Over the past 15 years, stroke hospitalizations have increased among adults aged 18 to 54 years. But the researchers note that risk factors, such as high blood pressure, high cholesterol, obesity, and diabetes are also appearing in younger people. Moreover, those risk factors may not be recognized and treated in middle-aged adults aged 35 to 64 years.
The study categorizes stroke deaths in the U.S. from 2000 to 2015, by age, sex, race/ethnicity, and geographic area. It does not, however, address causes for the slowdown, although it cites other studies that point to obesity and diabetes as contributors. High blood pressure is the single “most important preventable and treatable risk factor for stroke,” the CDC says.
After decades of decline, progress has slowed in preventing stroke deaths, according to a CDC Vital Signs report. The report is a “wake-up call,” CDC Director Brenda Fitzgerald says.
About 3 in every 4 states showed stalled rates of decline between 2000 and 2015. In some states, the trend of declining stroke deaths has actually reversed. It is a “disturbing” finding, the researchers say—particularly because 80% of strokes are preventable.
Every 40 seconds, someone in the U.S. has a stroke. Each year, > 140,000 die. Blacks continue to be hardest hit by stroke but stroke deaths are on the rise among Hispanics (by 6% each year between 2013 and 2015) and people living in the South.
Death rates continued to drop steadily between the years 2000 and 2015 among adults aged ≥ 35 years. However, people are dying of stroke at younger ages. Over the past 15 years, stroke hospitalizations have increased among adults aged 18 to 54 years. But the researchers note that risk factors, such as high blood pressure, high cholesterol, obesity, and diabetes are also appearing in younger people. Moreover, those risk factors may not be recognized and treated in middle-aged adults aged 35 to 64 years.
The study categorizes stroke deaths in the U.S. from 2000 to 2015, by age, sex, race/ethnicity, and geographic area. It does not, however, address causes for the slowdown, although it cites other studies that point to obesity and diabetes as contributors. High blood pressure is the single “most important preventable and treatable risk factor for stroke,” the CDC says.
Predicting neurotoxicity after CAR T-cell therapy
Researchers say they have identified potential biomarkers that may be used to help identify patients at an increased risk of neurotoxicity after chimeric antigen receptor (CAR) T-cell therapy.
The team also created an algorithm intended to identify patients whose symptoms were most likely to be life-threatening.
The researchers discovered the biomarkers and developed the algorithm based on data from a trial of JCAR014, an anti-CD19 CAR T-cell therapy, in patients with B-cell malignancies.
Cameron J. Turtle, MBBS, PhD, of Fred Hutchinson Cancer Research Center in Seattle, Washington, and his colleagues described this research in Cancer Discovery.
“It’s essential that we understand the potential side effects of CAR T therapies” Dr Turtle said. “While use of these cell therapies is likely to dramatically increase because they’ve been so effective in patients with resistant or refractory B-cell malignancies, there is still much to learn.”
Dr Turtle and his colleagues sought to provide a detailed clinical, radiological, and pathological characterization of neurotoxicity arising from anti-CD19 CAR T-cell therapy.
So the team analyzed data from a phase 1/2 trial of 133 adults with relapsed and/or refractory CD19+ B-cell acute lymphoblastic leukemia, non-Hodgkin lymphoma, or chronic lymphocytic leukemia.
The patients received lymphodepleting chemotherapy followed by an infusion of JCAR014.
Neurotoxicity
Within 28 days of treatment, 53 patients (40%) developed grade 1 or higher neurologic adverse events (AEs), 28 patients (21%) had grade 3 or higher neurotoxicity, and 4 patients (3%) developed fatal neurotoxicity.
Of the 53 patients with any neurologic AE, 48 (91%) also had cytokine release syndrome (CRS). All neurologic AEs in the 5 patients who did not have CRS were mild (grade 1) and transient.
Neurologic AEs included delirium with preserved alertness (66%), headache (55%), language disturbance (34%), decreased level of consciousness (25%), seizures (8%), and macroscopic intracranial hemorrhage (2%).
For most patients, neurotoxicity resolved by day 28 after CAR T-cell infusion. The exceptions were 1 patient in whom a grade 1 neurologic AE resolved 2 months after CAR T-cell infusion and the 4 patients who died of neurotoxicity.
The 4 neurotoxicity-related deaths were due to:
- Acute cerebral edema (n=2)
- Multifocal brainstem hemorrhage and edema associated with disseminated intravascular coagulation (n=1)
- Cortical laminar necrosis with a persistent minimally conscious state until death (n=1).
Potential biomarkers
In a univariate analysis, neurotoxicity was significantly more frequent in patients who:
- Had CRS (P<0.0001)
- Received a high CAR T-cell dose (P<0.0001)
- Had pre-existing neurologic comorbidities at baseline (P=0.0059).
In a multivariable analysis (which did not include CRS as a variable), patients had an increased risk of neurotoxicity if they:
- Had pre-existing neurologic comorbidities (P=0.0023)
- Received cyclophosphamide and fludarabine lymphodepletion (P=0.0259)
- Received a higher CAR T-cell dose (P=0.0009)
- Had a higher burden of malignant CD19+ B cells in the bone marrow (P=0.0165).
The researchers noted that patients who developed grade 3 or higher neurotoxicity had more severe CRS (P<0.0001).
“It appears that cytokine release syndrome is probably necessary for most cases of severe neurotoxicity, but, in terms of what triggers a person with cytokine release syndrome to get neurotoxicity, that’s something we need to investigate further,” said study author Kevin Hay, MD, of Fred Hutchinson Cancer Research Center.
Dr Hay and his colleagues also found that patients with severe neurotoxicity exhibited evidence of endothelial activation, which could contribute to manifestations such as capillary leak, disseminated intravascular coagulation, and disruption of the blood-brain barrier.
Algorithm
The researchers developed a predictive classification tree algorithm to identify patients who have an increased risk of severe neurotoxicity.
The algorithm suggests patients who meet the following criteria in the first 36 hours after CAR T-cell infusion have a high risk of grade 4-5 neurotoxicity:
- Fever of 38.9°C or greater
- Serum levels of IL6 at 16 pg/mL or higher
- Serum levels of MCP1 at 1343.5 pg/mL or higher.
This algorithm predicted severe neurotoxicity with 100% sensitivity and 94% specificity. Eight patients were misclassified, 1 of whom did not subsequently develop grade 2-3 neurotoxicity and/or grade 2 or higher CRS.
Funding
This research was funded by Juno Therapeutics Inc. (the company developing JCAR014), the National Cancer Institute, Life Science Discovery Fund, the Bezos family, the University of British Columbia Clinical Investigator Program, and via institutional funds from Bloodworks Northwest.
Dr Turtle receives research funding from Juno Therapeutics, holds patents licensed by Juno, and has pending patent applications that could be licensed by nonprofit institutions and for-profit companies, including Juno.
The Fred Hutchinson Cancer Research Center has a financial interest in Juno and receives licensing and other payments from the company. ![]()
Researchers say they have identified potential biomarkers that may be used to help identify patients at an increased risk of neurotoxicity after chimeric antigen receptor (CAR) T-cell therapy.
The team also created an algorithm intended to identify patients whose symptoms were most likely to be life-threatening.
The researchers discovered the biomarkers and developed the algorithm based on data from a trial of JCAR014, an anti-CD19 CAR T-cell therapy, in patients with B-cell malignancies.
Cameron J. Turtle, MBBS, PhD, of Fred Hutchinson Cancer Research Center in Seattle, Washington, and his colleagues described this research in Cancer Discovery.
“It’s essential that we understand the potential side effects of CAR T therapies” Dr Turtle said. “While use of these cell therapies is likely to dramatically increase because they’ve been so effective in patients with resistant or refractory B-cell malignancies, there is still much to learn.”
Dr Turtle and his colleagues sought to provide a detailed clinical, radiological, and pathological characterization of neurotoxicity arising from anti-CD19 CAR T-cell therapy.
So the team analyzed data from a phase 1/2 trial of 133 adults with relapsed and/or refractory CD19+ B-cell acute lymphoblastic leukemia, non-Hodgkin lymphoma, or chronic lymphocytic leukemia.
The patients received lymphodepleting chemotherapy followed by an infusion of JCAR014.
Neurotoxicity
Within 28 days of treatment, 53 patients (40%) developed grade 1 or higher neurologic adverse events (AEs), 28 patients (21%) had grade 3 or higher neurotoxicity, and 4 patients (3%) developed fatal neurotoxicity.
Of the 53 patients with any neurologic AE, 48 (91%) also had cytokine release syndrome (CRS). All neurologic AEs in the 5 patients who did not have CRS were mild (grade 1) and transient.
Neurologic AEs included delirium with preserved alertness (66%), headache (55%), language disturbance (34%), decreased level of consciousness (25%), seizures (8%), and macroscopic intracranial hemorrhage (2%).
For most patients, neurotoxicity resolved by day 28 after CAR T-cell infusion. The exceptions were 1 patient in whom a grade 1 neurologic AE resolved 2 months after CAR T-cell infusion and the 4 patients who died of neurotoxicity.
The 4 neurotoxicity-related deaths were due to:
- Acute cerebral edema (n=2)
- Multifocal brainstem hemorrhage and edema associated with disseminated intravascular coagulation (n=1)
- Cortical laminar necrosis with a persistent minimally conscious state until death (n=1).
Potential biomarkers
In a univariate analysis, neurotoxicity was significantly more frequent in patients who:
- Had CRS (P<0.0001)
- Received a high CAR T-cell dose (P<0.0001)
- Had pre-existing neurologic comorbidities at baseline (P=0.0059).
In a multivariable analysis (which did not include CRS as a variable), patients had an increased risk of neurotoxicity if they:
- Had pre-existing neurologic comorbidities (P=0.0023)
- Received cyclophosphamide and fludarabine lymphodepletion (P=0.0259)
- Received a higher CAR T-cell dose (P=0.0009)
- Had a higher burden of malignant CD19+ B cells in the bone marrow (P=0.0165).
The researchers noted that patients who developed grade 3 or higher neurotoxicity had more severe CRS (P<0.0001).
“It appears that cytokine release syndrome is probably necessary for most cases of severe neurotoxicity, but, in terms of what triggers a person with cytokine release syndrome to get neurotoxicity, that’s something we need to investigate further,” said study author Kevin Hay, MD, of Fred Hutchinson Cancer Research Center.
Dr Hay and his colleagues also found that patients with severe neurotoxicity exhibited evidence of endothelial activation, which could contribute to manifestations such as capillary leak, disseminated intravascular coagulation, and disruption of the blood-brain barrier.
Algorithm
The researchers developed a predictive classification tree algorithm to identify patients who have an increased risk of severe neurotoxicity.
The algorithm suggests patients who meet the following criteria in the first 36 hours after CAR T-cell infusion have a high risk of grade 4-5 neurotoxicity:
- Fever of 38.9°C or greater
- Serum levels of IL6 at 16 pg/mL or higher
- Serum levels of MCP1 at 1343.5 pg/mL or higher.
This algorithm predicted severe neurotoxicity with 100% sensitivity and 94% specificity. Eight patients were misclassified, 1 of whom did not subsequently develop grade 2-3 neurotoxicity and/or grade 2 or higher CRS.
Funding
This research was funded by Juno Therapeutics Inc. (the company developing JCAR014), the National Cancer Institute, Life Science Discovery Fund, the Bezos family, the University of British Columbia Clinical Investigator Program, and via institutional funds from Bloodworks Northwest.
Dr Turtle receives research funding from Juno Therapeutics, holds patents licensed by Juno, and has pending patent applications that could be licensed by nonprofit institutions and for-profit companies, including Juno.
The Fred Hutchinson Cancer Research Center has a financial interest in Juno and receives licensing and other payments from the company. ![]()
Researchers say they have identified potential biomarkers that may be used to help identify patients at an increased risk of neurotoxicity after chimeric antigen receptor (CAR) T-cell therapy.
The team also created an algorithm intended to identify patients whose symptoms were most likely to be life-threatening.
The researchers discovered the biomarkers and developed the algorithm based on data from a trial of JCAR014, an anti-CD19 CAR T-cell therapy, in patients with B-cell malignancies.
Cameron J. Turtle, MBBS, PhD, of Fred Hutchinson Cancer Research Center in Seattle, Washington, and his colleagues described this research in Cancer Discovery.
“It’s essential that we understand the potential side effects of CAR T therapies” Dr Turtle said. “While use of these cell therapies is likely to dramatically increase because they’ve been so effective in patients with resistant or refractory B-cell malignancies, there is still much to learn.”
Dr Turtle and his colleagues sought to provide a detailed clinical, radiological, and pathological characterization of neurotoxicity arising from anti-CD19 CAR T-cell therapy.
So the team analyzed data from a phase 1/2 trial of 133 adults with relapsed and/or refractory CD19+ B-cell acute lymphoblastic leukemia, non-Hodgkin lymphoma, or chronic lymphocytic leukemia.
The patients received lymphodepleting chemotherapy followed by an infusion of JCAR014.
Neurotoxicity
Within 28 days of treatment, 53 patients (40%) developed grade 1 or higher neurologic adverse events (AEs), 28 patients (21%) had grade 3 or higher neurotoxicity, and 4 patients (3%) developed fatal neurotoxicity.
Of the 53 patients with any neurologic AE, 48 (91%) also had cytokine release syndrome (CRS). All neurologic AEs in the 5 patients who did not have CRS were mild (grade 1) and transient.
Neurologic AEs included delirium with preserved alertness (66%), headache (55%), language disturbance (34%), decreased level of consciousness (25%), seizures (8%), and macroscopic intracranial hemorrhage (2%).
For most patients, neurotoxicity resolved by day 28 after CAR T-cell infusion. The exceptions were 1 patient in whom a grade 1 neurologic AE resolved 2 months after CAR T-cell infusion and the 4 patients who died of neurotoxicity.
The 4 neurotoxicity-related deaths were due to:
- Acute cerebral edema (n=2)
- Multifocal brainstem hemorrhage and edema associated with disseminated intravascular coagulation (n=1)
- Cortical laminar necrosis with a persistent minimally conscious state until death (n=1).
Potential biomarkers
In a univariate analysis, neurotoxicity was significantly more frequent in patients who:
- Had CRS (P<0.0001)
- Received a high CAR T-cell dose (P<0.0001)
- Had pre-existing neurologic comorbidities at baseline (P=0.0059).
In a multivariable analysis (which did not include CRS as a variable), patients had an increased risk of neurotoxicity if they:
- Had pre-existing neurologic comorbidities (P=0.0023)
- Received cyclophosphamide and fludarabine lymphodepletion (P=0.0259)
- Received a higher CAR T-cell dose (P=0.0009)
- Had a higher burden of malignant CD19+ B cells in the bone marrow (P=0.0165).
The researchers noted that patients who developed grade 3 or higher neurotoxicity had more severe CRS (P<0.0001).
“It appears that cytokine release syndrome is probably necessary for most cases of severe neurotoxicity, but, in terms of what triggers a person with cytokine release syndrome to get neurotoxicity, that’s something we need to investigate further,” said study author Kevin Hay, MD, of Fred Hutchinson Cancer Research Center.
Dr Hay and his colleagues also found that patients with severe neurotoxicity exhibited evidence of endothelial activation, which could contribute to manifestations such as capillary leak, disseminated intravascular coagulation, and disruption of the blood-brain barrier.
Algorithm
The researchers developed a predictive classification tree algorithm to identify patients who have an increased risk of severe neurotoxicity.
The algorithm suggests patients who meet the following criteria in the first 36 hours after CAR T-cell infusion have a high risk of grade 4-5 neurotoxicity:
- Fever of 38.9°C or greater
- Serum levels of IL6 at 16 pg/mL or higher
- Serum levels of MCP1 at 1343.5 pg/mL or higher.
This algorithm predicted severe neurotoxicity with 100% sensitivity and 94% specificity. Eight patients were misclassified, 1 of whom did not subsequently develop grade 2-3 neurotoxicity and/or grade 2 or higher CRS.
Funding
This research was funded by Juno Therapeutics Inc. (the company developing JCAR014), the National Cancer Institute, Life Science Discovery Fund, the Bezos family, the University of British Columbia Clinical Investigator Program, and via institutional funds from Bloodworks Northwest.
Dr Turtle receives research funding from Juno Therapeutics, holds patents licensed by Juno, and has pending patent applications that could be licensed by nonprofit institutions and for-profit companies, including Juno.
The Fred Hutchinson Cancer Research Center has a financial interest in Juno and receives licensing and other payments from the company. ![]()
FDA grants drug fast track designation for rel/ref AML
The US Food and Drug Administration (FDA) has granted fast track designation to gilteritinib for the treatment of adults with FLT3 mutation-positive relapsed or refractory acute myeloid leukemia (AML).
Gilteritinib is a compound that has demonstrated inhibitory activity against FLT3 internal tandem duplication (ITD) and FLT3 tyrosine kinase domain, 2 mutations that are seen in approximately one-third of patients with AML.
Gilteritinib has also demonstrated inhibition of AXL, which is reported to be associated with therapeutic resistance.
Astellas Pharma Inc. is currently investigating gilteritinib in phase 3 trials of AML patients.
Results from a phase 1/2 study of gilteritinib in AML were presented at the 2017 ASCO Annual Meeting.
The goal of the study was to determine the tolerability and antileukemic activity of once-daily gilteritinib in a FLT3-ITD-enriched, relapsed/refractory AML population.
Researchers said the drug exhibited potent FLT3 inhibition at doses greater than 80 mg/day. In patients who received such doses, the greatest overall response rate was 52%, and the longest median overall survival was 31 weeks.
The maximum tolerated dose of gilteritinib was 300 mg/day. Dose-limiting toxicities included diarrhea and liver function abnormalities.
About fast track designation
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the new drug application or biologics license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
About orphan designation
Gilteritinib also has orphan drug designation for the treatment of AML.
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted fast track designation to gilteritinib for the treatment of adults with FLT3 mutation-positive relapsed or refractory acute myeloid leukemia (AML).
Gilteritinib is a compound that has demonstrated inhibitory activity against FLT3 internal tandem duplication (ITD) and FLT3 tyrosine kinase domain, 2 mutations that are seen in approximately one-third of patients with AML.
Gilteritinib has also demonstrated inhibition of AXL, which is reported to be associated with therapeutic resistance.
Astellas Pharma Inc. is currently investigating gilteritinib in phase 3 trials of AML patients.
Results from a phase 1/2 study of gilteritinib in AML were presented at the 2017 ASCO Annual Meeting.
The goal of the study was to determine the tolerability and antileukemic activity of once-daily gilteritinib in a FLT3-ITD-enriched, relapsed/refractory AML population.
Researchers said the drug exhibited potent FLT3 inhibition at doses greater than 80 mg/day. In patients who received such doses, the greatest overall response rate was 52%, and the longest median overall survival was 31 weeks.
The maximum tolerated dose of gilteritinib was 300 mg/day. Dose-limiting toxicities included diarrhea and liver function abnormalities.
About fast track designation
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the new drug application or biologics license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
About orphan designation
Gilteritinib also has orphan drug designation for the treatment of AML.
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
The US Food and Drug Administration (FDA) has granted fast track designation to gilteritinib for the treatment of adults with FLT3 mutation-positive relapsed or refractory acute myeloid leukemia (AML).
Gilteritinib is a compound that has demonstrated inhibitory activity against FLT3 internal tandem duplication (ITD) and FLT3 tyrosine kinase domain, 2 mutations that are seen in approximately one-third of patients with AML.
Gilteritinib has also demonstrated inhibition of AXL, which is reported to be associated with therapeutic resistance.
Astellas Pharma Inc. is currently investigating gilteritinib in phase 3 trials of AML patients.
Results from a phase 1/2 study of gilteritinib in AML were presented at the 2017 ASCO Annual Meeting.
The goal of the study was to determine the tolerability and antileukemic activity of once-daily gilteritinib in a FLT3-ITD-enriched, relapsed/refractory AML population.
Researchers said the drug exhibited potent FLT3 inhibition at doses greater than 80 mg/day. In patients who received such doses, the greatest overall response rate was 52%, and the longest median overall survival was 31 weeks.
The maximum tolerated dose of gilteritinib was 300 mg/day. Dose-limiting toxicities included diarrhea and liver function abnormalities.
About fast track designation
The FDA’s fast track program is designed to facilitate the development and expedite the review of products intended to treat or prevent serious or life-threatening conditions and address unmet medical need.
Through the fast track program, a product may be eligible for priority review. In addition, the company developing the product may be allowed to submit sections of the new drug application or biologics license application on a rolling basis as data become available.
Fast track designation also provides the company with opportunities for more frequent meetings and written communications with the FDA.
About orphan designation
Gilteritinib also has orphan drug designation for the treatment of AML.
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()