User login
Pelvic Inflammatory Disease: How to Recognize and Treat Revised
IN THIS ARTICLE
- Diagnostic tests
- Complications of PID
- CDC treatment regimens
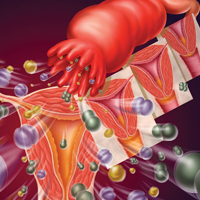
Pelvic inflammatory disease (PID) is an ascending polymicrobial infection of the female upper reproductive tract that primarily affects sexually active women ages 15 to 29. Around 5% of sexually active women in the United States were treated for PID from 2011-2013.1 The rates and severity of PID have declined in North America and Western Europe due to overall decrease in sexually transmitted infection (STI) rates, improved screening initiatives for Chlamydia trachomatis, better treatment compliance secondary to increased access to antibiotics, and diagnostic tests with higher sensitivity.2 Despite this rate reduction, PID remains a major public health concern given the significant long-term complications, which include infertility, ectopic pregnancy, and chronic pelvic pain.3
EPIDEMIOLOGY AND PATHOGENESIS
PID is caused by sexually transmitted bacteria or enteric organisms that have spread to internal reproductive organs. Historically, the two most common pathogens identified in cases of PID have been Chlamydia trachomatis and Neisseria gonorrhoeae; however, the decline in rates of gonorrhea has led to a diminished role for N gonorrhoeae (though it continues to be associated with more severe cases).4,5
More recent studies have suggested a shift in the causative organisms; less than half of women diagnosed with acute PID test positive for either N gonorrhoeae or C trachomatis.6 Emerging infectious agents associated with PID include Mycoplasma genitalium, Gardnerella vaginalis, and bacterial vaginosis–associated bacteria.5,7,8-10
RISK FACTORS
Women ages 15 to 25 are at an increased risk for PID. The high prevalence in this age group may be attributable to high-risk behaviors, including a high number of sexual partners, high frequency of new sexual partners, and engagement in sexual intercourse without condoms.11
Taking an accurate sexual history is imperative. Clinicians should maintain a high level of suspicion for PID in women with a history of the disease, as 25% will experience recurrence.12
Clinicians should not be deterred from screening for STIs and cervical cancer in women who report having sex with other women. In addition, transgender patients should be assessed for STIs and HIV-related risks based on current anatomy sexual practices.13
PHYSICAL EXAM
While some cases of PID are asymptomatic, the typical presentation includes bilateral abdominal pain and/or pelvic pain, with onset during or shortly after menses. The pain often worsens with movement and coitus. Associated signs and symptoms include abnormal uterine bleeding or vaginal discharge; dysuria; fever and chills; frequent urination; lower back pain; and nausea and/or vomiting.14,15
All females suspected of having PID should undergo both a bimanual exam and a speculum exam. On bimanual examination, adnexal tenderness has the highest sensitivity (93% to 95.5%) for ruling out acute PID, whereas on speculum exam, purulent endocervical discharge has the highest specificity (93%).16,17 Bimanual exam findings suggestive of PID include cervical motion tenderness, uterine tenderness, and/or adnexal tenderness. Suggestive speculum exam findings include abnormal discoloration or texture of the cervix and/or endocervical mucopurulent discharge.5,16,17
One cardinal rule that should not be overlooked is that all females of reproductive age who present with abdominal pain and/or pelvic pain should take a pregnancy test to rule out ectopic pregnancy and any other pregnancy-related complications.
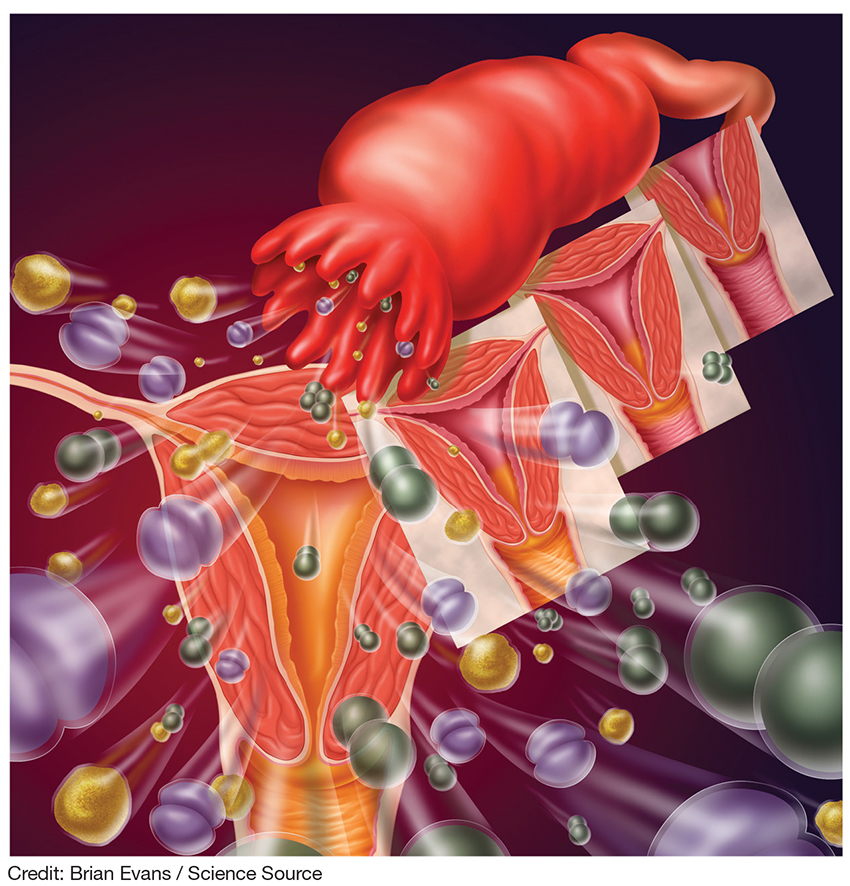
DIAGNOSIS
The diagnosis of PID relies on clinical judgement and a high index of suspicion.5,18 The CDC’s diagnostic criteria for acute PID include
- Sexually active female AND
- Pelvic or lower abdominal pain AND
- Cervical motion tenderness OR uterine tenderness OR adnexal tenderness.5
Additional findings that support the diagnosis include
- Abnormal cervical mucopurulent discharge or cervical friability
- Abundant white blood cells (WBCs) on saline microscopy of vaginal fluid
- Elevated C-reactive protein
- Elevated erythrocyte sedimentation rate
- Laboratory documentation of infection with C trachomatis or N gonorrhea
- Oral temperature > 101°F.5,18
The CDC notes that the first two findings (mucopurulent discharge and evidence of WBCs on microscopy) occur in most women with PID; in their absence, the diagnosis is unlikely and other sources of pain should be considered.5 The differential for PID includes acute appendicitis; adhesions; carcinoid tumor; cholecystitis; ectopic pregnancy; endometriosis; inflammatory bowel disease; and ovarian cyst.19
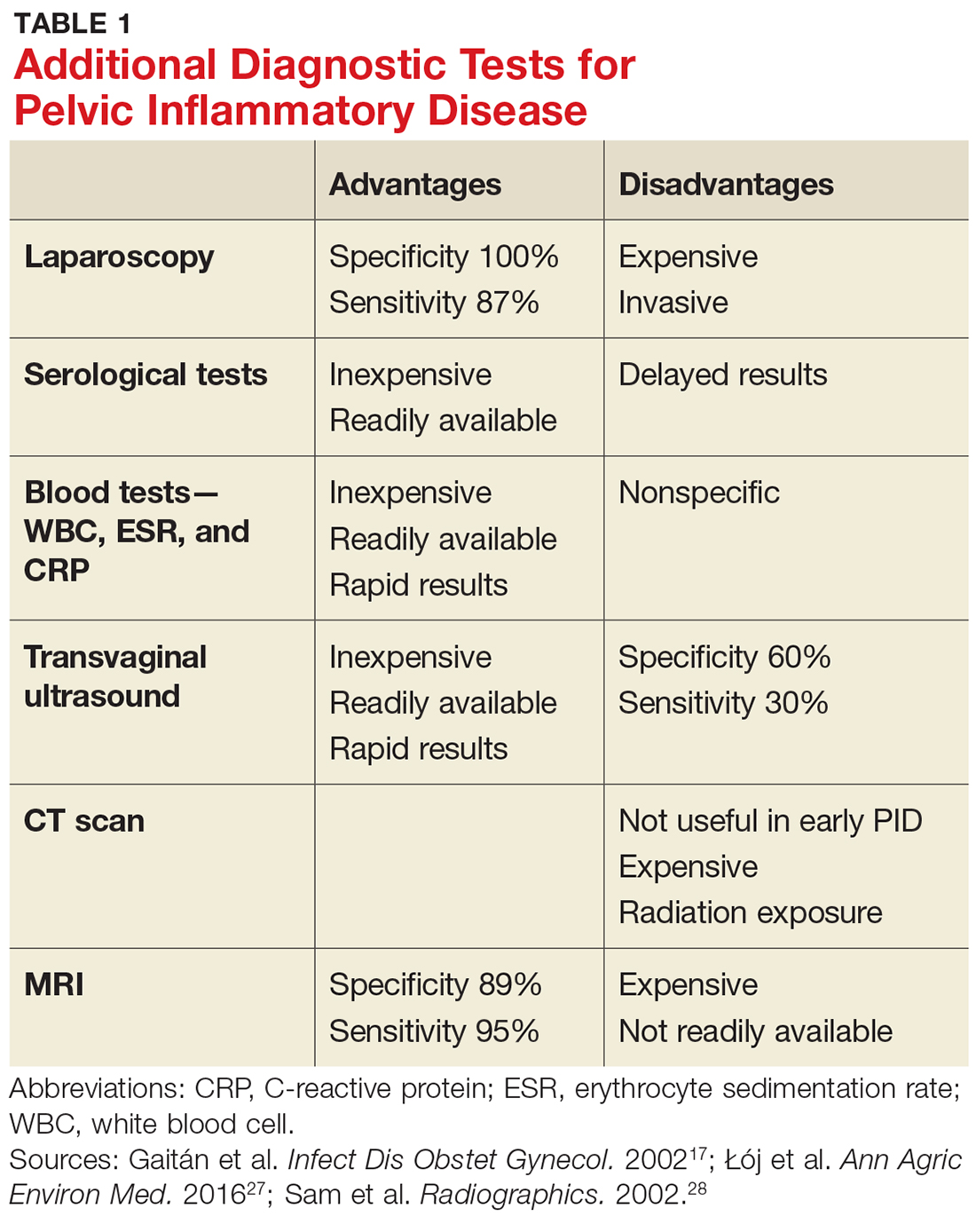
Given the variability in presentation, clinicians may find it useful to perform further diagnostic testing. There are additional laboratory tests that may be ordered for patients with a suspected diagnosis of PID (see Table 1).
TREATMENT
According to the CDC’s 2015 treatment guidelines for PID, a negative endocervical exam and negative microbial screening do not rule out an upper reproductive tract infection. Therefore, all sexually active women who present with lower abdominal pain and/or pelvic pain and have evidence of cervical motion, uterine, or adnexal tenderness on bimanual exam should be treated immediately.5
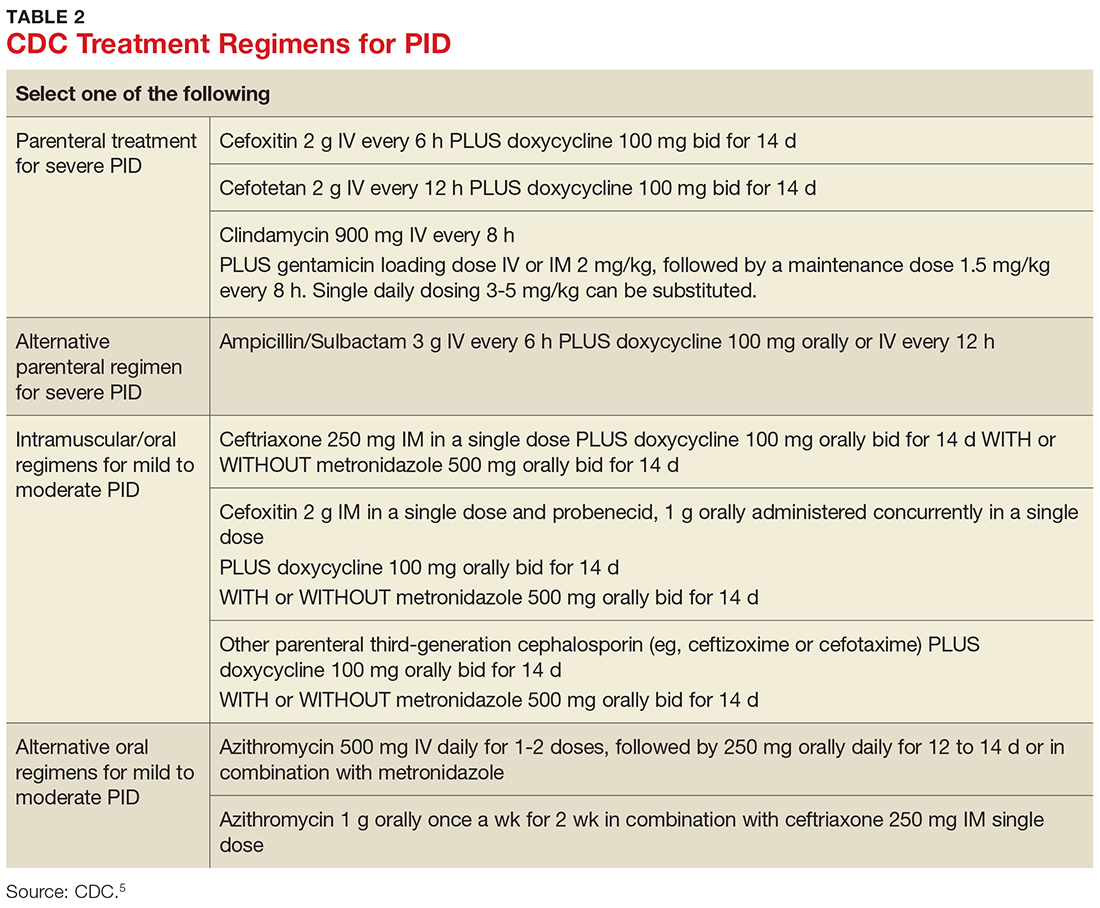
Treatment guidelines are outlined in Table 2. The polymicrobial nature of PID requires gram-negative antibiotic coverage, such as doxycycline plus a second/third-generation cephalosporin.5 Clinicians should note that cefoxitin, a second-generation cephalosporin, is recommended as firstline therapy for inpatients, as it has better anaerobic coverage than ceftriaxone.19 A targeted change in antibiotic coverage—such as inclusion of a macrolide and/or metronidazole—might be necessary if a causative organism is identified by culture.7
Treatment is indicated for all patients with a presumptive diagnosis of PID regardless of symptoms or exam findings, as PID may be asymptomatic and long-term sequelae (eg, infertility, ectopic pregnancy) are often irreversible. At-risk patients include sexually active adolescents, women with multiple sexual partners, women with a history of STI, those whose sexual partner has an STI, and women living in communities with a high prevalence of disease.20,21
Women being treated for PID should be advised to abstain from sexual intercourse until symptoms have resolved, treatment is completed, and any sexual partners have been treated as well. It is essential to emphasize to patients (and their partners) the importance of compliance to treatment regimens and the risk for PID co-infection and reinfection, as recurrence leads to an increase in long-term complications.5
Treatment of sexual partners. The CDC instructs that a woman’s most recent partner should be treated if she had sexual intercourse within 60 days of onset of symptoms or diagnosis. Furthermore, men who have had sexual contact with a woman who has PID in the 60 days prior to onset of her symptoms should be evaluated, tested, and treated for chlamydia and gonorrhea, regardless of the etiology of PID or the pathogens isolated from the woman.5
Admission criteria. Hospitalization should be based on provider judgment despite patient age. The suggested admission criteria include surgical emergency (eg, appendicitis), tubo-ovarian abscess, pregnancy, severe illness, nausea and vomiting, high fever, inability to follow or tolerate an outpatient oral regimen, and lack of clinical response to oral antimicrobial therapy.5
Follow-up care. Clinical improvement (ie, reduction in abdominal, uterine, adnexal, and cervical motion tenderness) should occur within 72 hours of antimicrobial therapy initiation. If it does not, hospital admission or adjustment in antimicrobial regimen should be considered, as well as additional diagnostic testing (eg, laparoscopy). In addition, all women with chlamydial- or gonococcal-related PID should return in three months for surveillance testing.22
COMPLICATIONS
Long-term complications—including infertility, chronic pelvic pain, and ectopic pregnancy—may occur, even when there has been a clinical response to adequate treatment. Data from the PID Evaluation and Clinical Health (PEACH) study were analyzed to assess long-term sequelae at seven years postdiagnosis and treatment. The researchers found that about 21% of women experienced recurrent PID, 19% developed infertility, and 42% reported chronic pelvic pain.3 Other research has also shown that repeat episodes of PID and delayed treatment increase the risk for long-term complications.23,24
SCREENING AND PREVENTION
Ten percent of women with an untreated STI will go on to develop PID.4 It is imperative to educate patients on the dangers and consequences of STIs when they become sexually active. Adolescents benefit the most from preventive education; this group is twice as likely as any other age group to be diagnosed with PID due to their inclination toward risky sexual behavior. Additionally, younger women tend to have a more friable cervix, increasing their risk for infection.25,26 Providers should promote safe sexual practices, such as condom use and less frequent partner exchange, in order to reduce STI exposure.
In 2015, the rate of reported cases of C trachomatis was 645.5 per 100,000 females, and of N gonorrheae, 107.2 per 100,000 females.23 The United States Preventive Services Task Force and the CDC recommend annual screening for chlamydia and gonorrhea in all sexually active women younger than 25, as well as sexually active women ages 25 and older who are considered at increased risk.5
CONCLUSION
PID is often difficult to diagnose, since patients may be asymptomatic or present with vague symptoms. Clinicians should maintain a high level of suspicion for PID in adolescent females due to the high incidence of STI exposure in this population. The best way to prevent long-term complications of PID is to prevent the first episode of PID and/or first exposure to STIs. Therefore, clinicians should be proactive in offering STI screenings to all sexually active patients younger than 25 who request care, regardless of their chief complaint, and educating patients on the potential long-term effects of PID and STIs.
1. Leichliter JS, Chandra A, Aral SO. Correlates of self-reported pelvic inflammatory disease treatment in sexually experienced reproductive-aged women in the United States, 1995 and 2006-2010. Sex Transm Dis. 2013;40(5):413-418.
2. Owusu-Edusei K Jr, Bohm MK, Chesson HW, Kent CK. Chlamydia screening and pelvic inflammatory disease: insights from exploratory time-series analyses. Am J Prev Med. 2010;38(6):652-657.
3. Trent M, Bass D, Ness RB, Haggerty C. Recurrent PID, subsequent STI, and reproductive health outcomes: findings from the PID evaluation and clinical health (PEACH) study. Sex Transm Dis. 2011;38(9):879-881.
4. Mitchell C, Prabhu M. Pelvic inflammatory disease: current concepts in pathogenesis, diagnosis and treatment. Infect Dis Clin North Am. 2013;27(4):793-809.
5. CDC. Pelvic inflammatory disease (PID). www.cdc.gov/std/tg2015/pid.htm. Accessed July 13, 2017.
6. Burnett AM, Anderson CP, Zwank MD. Laboratory-confirmed gonorrhea and/or chlamydia rates in clinically diagnosed pelvic inflammatory disease and cervicitis. Am J Emerg Med. 2012;30:1114–1117.
7. Bjartling C, Osser S, Persson K. The association between Mycoplasma genitalium and pelvic inflammatory disease after termination of pregnancy. BJOG. 2010;117(3):361-364.
8. Ness RB, Kip KE, Hillier SL, et al. A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am J Epidemiol. 2005;162(6):585-590.
9. Ness RB, Hillier SL, Kip KE, et al. Bacterial vaginosis and risk of pelvic inflammatory disease. Obstet Gynecol. 2004;104(4):761-769.
10. Cherpes TL, Wiesenfeld HC, Melan MA, et al. The associations between pelvic inflammatory disease, Trichomonas vaginalis infection, and positive herpes simplex virus type 2 serology. Sex Transm Dis. 2006;33(12):747-752.
11. Simms I, Stephenson JM, Mallinson H, et al. Risk factors associated with pelvic inflammatory disease. Sex Transm Infect. 2006;82(6):452-457.
12. Schindlbeck C, Dziura D, Mylonas I. Diagnosis of pelvic inflammatory disease (PID): intra-operative findings and comparison of vaginal and intra-abdominal cultures. Arch Gynecol Obstet. 2014;289(6):1263-1269.
13. CDC. 2015 sexually transmitted diseases treatment guidelines. www.cdc.gov/std/tg2015/default.htm. Accessed September 6, 2017.
14. Korn AP, Hessol NA, Padian NS, et al. Risk factors for plasma cell endometritis among women with cervical Neisseria gonorrhoeae, cervical Chlamydia trachomatis, or bacterial vaginosis. Am J Obstet Gynecol. 1998;178(5):987-990.
15. Wiesenfeld HC, Sweet RL, Ness RB, et al. Comparison of acute and subclinical pelvic inflammatory disease. Sex Transm Dis. 2005;32(7):400-405.
16. Peipert JF, Ness RB, Blume J, et al. Clinical predictors of endometritis in women with symptoms and signs of pelvic inflammatory disease. Am J Obstet Gynecol. 2001;184(5):856-864.
17. Gaitán H, Angel E, Diaz R, et al. Accuracy of five different diagnostic techniques in mild-to-moderate pelvic inflammatory disease. Infect Dis Obstet Gynecol. 2002;10(4):171-180.
18. Tukeva TA, Aronen HJ, Karjalainen PT, et al. MR imaging in pelvic inflammatory disease: comparison with laparoscopy and US. Radiology. 1999;210(1):209-216.
19. Morino M, Pellegrino L, Castagna E, et al. Acute nonspecific abdominal pain. Ann Surg. 2006;244(6):881-888.
20. Woods JL, Scurlock AM, Hensel DJ. Pelvic inflammatory disease in the adolescent: understanding diagnosis and treatment as a health care provider. Pediatric Emergency Care. 2013;29(6):720-725.
21. LeFevre ML; U.S. Preventive Services Task Force. Screening for chlamydia and gonorrhea: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(12):902-910.
22. Hosenfeld CB, Workowski KA, Berman S, et al. Repeat infection with Chlamydia and gonorrhea among females: a systematic review of the literature. Sex Transm Dis. 2009;36(8):478-489.
23. CDC. Sexually transmitted disease surveillance. www.cdc.gov/std/stats15/STD-Surveillance-2015-print.pdf. Accessed July 13, 2017.
24. Hillis SD, Joesoef R, Marchbanks PA, et al. Delayed care of pelvic inflammatory disease as a risk factor for impaired fertility. Am J Obstet Gynecol. 1993;168(5):1503-1509.
25. Goyal M, Hersh A, Luan X, et al. National trends in pelvic inflammatory disease among adolescents in the emergency department. J Adolesc Health. 2013;53(2):249-252.
26. Gray-Swain MR, Peipert JF. Pelvic inflammatory disease in adolescents. Curr Opin Obstet Gynecol. 2006;18(5):503-510.
27. Łój B, Brodowska A, Ciecwiez S, et al. The role of serological testing for Chlamydia trachomatis in differential diagnosis of pelvic pain. Ann Agric Environ Med. 2016;23(3):506-510.
28. Sam JW, Jacobs JE, Birnbaum BA. Spectrum of CT findings in acute pyogenic pelvic inflammatory disease. Radiographics. 2002;22(6):1327-1 334.
IN THIS ARTICLE
- Diagnostic tests
- Complications of PID
- CDC treatment regimens
Pelvic inflammatory disease (PID) is an ascending polymicrobial infection of the female upper reproductive tract that primarily affects sexually active women ages 15 to 29. Around 5% of sexually active women in the United States were treated for PID from 2011-2013.1 The rates and severity of PID have declined in North America and Western Europe due to overall decrease in sexually transmitted infection (STI) rates, improved screening initiatives for Chlamydia trachomatis, better treatment compliance secondary to increased access to antibiotics, and diagnostic tests with higher sensitivity.2 Despite this rate reduction, PID remains a major public health concern given the significant long-term complications, which include infertility, ectopic pregnancy, and chronic pelvic pain.3
EPIDEMIOLOGY AND PATHOGENESIS
PID is caused by sexually transmitted bacteria or enteric organisms that have spread to internal reproductive organs. Historically, the two most common pathogens identified in cases of PID have been Chlamydia trachomatis and Neisseria gonorrhoeae; however, the decline in rates of gonorrhea has led to a diminished role for N gonorrhoeae (though it continues to be associated with more severe cases).4,5
More recent studies have suggested a shift in the causative organisms; less than half of women diagnosed with acute PID test positive for either N gonorrhoeae or C trachomatis.6 Emerging infectious agents associated with PID include Mycoplasma genitalium, Gardnerella vaginalis, and bacterial vaginosis–associated bacteria.5,7,8-10
RISK FACTORS
Women ages 15 to 25 are at an increased risk for PID. The high prevalence in this age group may be attributable to high-risk behaviors, including a high number of sexual partners, high frequency of new sexual partners, and engagement in sexual intercourse without condoms.11
Taking an accurate sexual history is imperative. Clinicians should maintain a high level of suspicion for PID in women with a history of the disease, as 25% will experience recurrence.12
Clinicians should not be deterred from screening for STIs and cervical cancer in women who report having sex with other women. In addition, transgender patients should be assessed for STIs and HIV-related risks based on current anatomy sexual practices.13
PHYSICAL EXAM
While some cases of PID are asymptomatic, the typical presentation includes bilateral abdominal pain and/or pelvic pain, with onset during or shortly after menses. The pain often worsens with movement and coitus. Associated signs and symptoms include abnormal uterine bleeding or vaginal discharge; dysuria; fever and chills; frequent urination; lower back pain; and nausea and/or vomiting.14,15
All females suspected of having PID should undergo both a bimanual exam and a speculum exam. On bimanual examination, adnexal tenderness has the highest sensitivity (93% to 95.5%) for ruling out acute PID, whereas on speculum exam, purulent endocervical discharge has the highest specificity (93%).16,17 Bimanual exam findings suggestive of PID include cervical motion tenderness, uterine tenderness, and/or adnexal tenderness. Suggestive speculum exam findings include abnormal discoloration or texture of the cervix and/or endocervical mucopurulent discharge.5,16,17
One cardinal rule that should not be overlooked is that all females of reproductive age who present with abdominal pain and/or pelvic pain should take a pregnancy test to rule out ectopic pregnancy and any other pregnancy-related complications.

DIAGNOSIS
The diagnosis of PID relies on clinical judgement and a high index of suspicion.5,18 The CDC’s diagnostic criteria for acute PID include
- Sexually active female AND
- Pelvic or lower abdominal pain AND
- Cervical motion tenderness OR uterine tenderness OR adnexal tenderness.5
Additional findings that support the diagnosis include
- Abnormal cervical mucopurulent discharge or cervical friability
- Abundant white blood cells (WBCs) on saline microscopy of vaginal fluid
- Elevated C-reactive protein
- Elevated erythrocyte sedimentation rate
- Laboratory documentation of infection with C trachomatis or N gonorrhea
- Oral temperature > 101°F.5,18
The CDC notes that the first two findings (mucopurulent discharge and evidence of WBCs on microscopy) occur in most women with PID; in their absence, the diagnosis is unlikely and other sources of pain should be considered.5 The differential for PID includes acute appendicitis; adhesions; carcinoid tumor; cholecystitis; ectopic pregnancy; endometriosis; inflammatory bowel disease; and ovarian cyst.19
Given the variability in presentation, clinicians may find it useful to perform further diagnostic testing. There are additional laboratory tests that may be ordered for patients with a suspected diagnosis of PID (see Table 1).

TREATMENT
According to the CDC’s 2015 treatment guidelines for PID, a negative endocervical exam and negative microbial screening do not rule out an upper reproductive tract infection. Therefore, all sexually active women who present with lower abdominal pain and/or pelvic pain and have evidence of cervical motion, uterine, or adnexal tenderness on bimanual exam should be treated immediately.5
Treatment guidelines are outlined in Table 2. The polymicrobial nature of PID requires gram-negative antibiotic coverage, such as doxycycline plus a second/third-generation cephalosporin.5 Clinicians should note that cefoxitin, a second-generation cephalosporin, is recommended as firstline therapy for inpatients, as it has better anaerobic coverage than ceftriaxone.19 A targeted change in antibiotic coverage—such as inclusion of a macrolide and/or metronidazole—might be necessary if a causative organism is identified by culture.7

Treatment is indicated for all patients with a presumptive diagnosis of PID regardless of symptoms or exam findings, as PID may be asymptomatic and long-term sequelae (eg, infertility, ectopic pregnancy) are often irreversible. At-risk patients include sexually active adolescents, women with multiple sexual partners, women with a history of STI, those whose sexual partner has an STI, and women living in communities with a high prevalence of disease.20,21
Women being treated for PID should be advised to abstain from sexual intercourse until symptoms have resolved, treatment is completed, and any sexual partners have been treated as well. It is essential to emphasize to patients (and their partners) the importance of compliance to treatment regimens and the risk for PID co-infection and reinfection, as recurrence leads to an increase in long-term complications.5
Treatment of sexual partners. The CDC instructs that a woman’s most recent partner should be treated if she had sexual intercourse within 60 days of onset of symptoms or diagnosis. Furthermore, men who have had sexual contact with a woman who has PID in the 60 days prior to onset of her symptoms should be evaluated, tested, and treated for chlamydia and gonorrhea, regardless of the etiology of PID or the pathogens isolated from the woman.5
Admission criteria. Hospitalization should be based on provider judgment despite patient age. The suggested admission criteria include surgical emergency (eg, appendicitis), tubo-ovarian abscess, pregnancy, severe illness, nausea and vomiting, high fever, inability to follow or tolerate an outpatient oral regimen, and lack of clinical response to oral antimicrobial therapy.5
Follow-up care. Clinical improvement (ie, reduction in abdominal, uterine, adnexal, and cervical motion tenderness) should occur within 72 hours of antimicrobial therapy initiation. If it does not, hospital admission or adjustment in antimicrobial regimen should be considered, as well as additional diagnostic testing (eg, laparoscopy). In addition, all women with chlamydial- or gonococcal-related PID should return in three months for surveillance testing.22
COMPLICATIONS
Long-term complications—including infertility, chronic pelvic pain, and ectopic pregnancy—may occur, even when there has been a clinical response to adequate treatment. Data from the PID Evaluation and Clinical Health (PEACH) study were analyzed to assess long-term sequelae at seven years postdiagnosis and treatment. The researchers found that about 21% of women experienced recurrent PID, 19% developed infertility, and 42% reported chronic pelvic pain.3 Other research has also shown that repeat episodes of PID and delayed treatment increase the risk for long-term complications.23,24
SCREENING AND PREVENTION
Ten percent of women with an untreated STI will go on to develop PID.4 It is imperative to educate patients on the dangers and consequences of STIs when they become sexually active. Adolescents benefit the most from preventive education; this group is twice as likely as any other age group to be diagnosed with PID due to their inclination toward risky sexual behavior. Additionally, younger women tend to have a more friable cervix, increasing their risk for infection.25,26 Providers should promote safe sexual practices, such as condom use and less frequent partner exchange, in order to reduce STI exposure.
In 2015, the rate of reported cases of C trachomatis was 645.5 per 100,000 females, and of N gonorrheae, 107.2 per 100,000 females.23 The United States Preventive Services Task Force and the CDC recommend annual screening for chlamydia and gonorrhea in all sexually active women younger than 25, as well as sexually active women ages 25 and older who are considered at increased risk.5
CONCLUSION
PID is often difficult to diagnose, since patients may be asymptomatic or present with vague symptoms. Clinicians should maintain a high level of suspicion for PID in adolescent females due to the high incidence of STI exposure in this population. The best way to prevent long-term complications of PID is to prevent the first episode of PID and/or first exposure to STIs. Therefore, clinicians should be proactive in offering STI screenings to all sexually active patients younger than 25 who request care, regardless of their chief complaint, and educating patients on the potential long-term effects of PID and STIs.
IN THIS ARTICLE
- Diagnostic tests
- Complications of PID
- CDC treatment regimens
Pelvic inflammatory disease (PID) is an ascending polymicrobial infection of the female upper reproductive tract that primarily affects sexually active women ages 15 to 29. Around 5% of sexually active women in the United States were treated for PID from 2011-2013.1 The rates and severity of PID have declined in North America and Western Europe due to overall decrease in sexually transmitted infection (STI) rates, improved screening initiatives for Chlamydia trachomatis, better treatment compliance secondary to increased access to antibiotics, and diagnostic tests with higher sensitivity.2 Despite this rate reduction, PID remains a major public health concern given the significant long-term complications, which include infertility, ectopic pregnancy, and chronic pelvic pain.3
EPIDEMIOLOGY AND PATHOGENESIS
PID is caused by sexually transmitted bacteria or enteric organisms that have spread to internal reproductive organs. Historically, the two most common pathogens identified in cases of PID have been Chlamydia trachomatis and Neisseria gonorrhoeae; however, the decline in rates of gonorrhea has led to a diminished role for N gonorrhoeae (though it continues to be associated with more severe cases).4,5
More recent studies have suggested a shift in the causative organisms; less than half of women diagnosed with acute PID test positive for either N gonorrhoeae or C trachomatis.6 Emerging infectious agents associated with PID include Mycoplasma genitalium, Gardnerella vaginalis, and bacterial vaginosis–associated bacteria.5,7,8-10
RISK FACTORS
Women ages 15 to 25 are at an increased risk for PID. The high prevalence in this age group may be attributable to high-risk behaviors, including a high number of sexual partners, high frequency of new sexual partners, and engagement in sexual intercourse without condoms.11
Taking an accurate sexual history is imperative. Clinicians should maintain a high level of suspicion for PID in women with a history of the disease, as 25% will experience recurrence.12
Clinicians should not be deterred from screening for STIs and cervical cancer in women who report having sex with other women. In addition, transgender patients should be assessed for STIs and HIV-related risks based on current anatomy sexual practices.13
PHYSICAL EXAM
While some cases of PID are asymptomatic, the typical presentation includes bilateral abdominal pain and/or pelvic pain, with onset during or shortly after menses. The pain often worsens with movement and coitus. Associated signs and symptoms include abnormal uterine bleeding or vaginal discharge; dysuria; fever and chills; frequent urination; lower back pain; and nausea and/or vomiting.14,15
All females suspected of having PID should undergo both a bimanual exam and a speculum exam. On bimanual examination, adnexal tenderness has the highest sensitivity (93% to 95.5%) for ruling out acute PID, whereas on speculum exam, purulent endocervical discharge has the highest specificity (93%).16,17 Bimanual exam findings suggestive of PID include cervical motion tenderness, uterine tenderness, and/or adnexal tenderness. Suggestive speculum exam findings include abnormal discoloration or texture of the cervix and/or endocervical mucopurulent discharge.5,16,17
One cardinal rule that should not be overlooked is that all females of reproductive age who present with abdominal pain and/or pelvic pain should take a pregnancy test to rule out ectopic pregnancy and any other pregnancy-related complications.

DIAGNOSIS
The diagnosis of PID relies on clinical judgement and a high index of suspicion.5,18 The CDC’s diagnostic criteria for acute PID include
- Sexually active female AND
- Pelvic or lower abdominal pain AND
- Cervical motion tenderness OR uterine tenderness OR adnexal tenderness.5
Additional findings that support the diagnosis include
- Abnormal cervical mucopurulent discharge or cervical friability
- Abundant white blood cells (WBCs) on saline microscopy of vaginal fluid
- Elevated C-reactive protein
- Elevated erythrocyte sedimentation rate
- Laboratory documentation of infection with C trachomatis or N gonorrhea
- Oral temperature > 101°F.5,18
The CDC notes that the first two findings (mucopurulent discharge and evidence of WBCs on microscopy) occur in most women with PID; in their absence, the diagnosis is unlikely and other sources of pain should be considered.5 The differential for PID includes acute appendicitis; adhesions; carcinoid tumor; cholecystitis; ectopic pregnancy; endometriosis; inflammatory bowel disease; and ovarian cyst.19
Given the variability in presentation, clinicians may find it useful to perform further diagnostic testing. There are additional laboratory tests that may be ordered for patients with a suspected diagnosis of PID (see Table 1).

TREATMENT
According to the CDC’s 2015 treatment guidelines for PID, a negative endocervical exam and negative microbial screening do not rule out an upper reproductive tract infection. Therefore, all sexually active women who present with lower abdominal pain and/or pelvic pain and have evidence of cervical motion, uterine, or adnexal tenderness on bimanual exam should be treated immediately.5
Treatment guidelines are outlined in Table 2. The polymicrobial nature of PID requires gram-negative antibiotic coverage, such as doxycycline plus a second/third-generation cephalosporin.5 Clinicians should note that cefoxitin, a second-generation cephalosporin, is recommended as firstline therapy for inpatients, as it has better anaerobic coverage than ceftriaxone.19 A targeted change in antibiotic coverage—such as inclusion of a macrolide and/or metronidazole—might be necessary if a causative organism is identified by culture.7

Treatment is indicated for all patients with a presumptive diagnosis of PID regardless of symptoms or exam findings, as PID may be asymptomatic and long-term sequelae (eg, infertility, ectopic pregnancy) are often irreversible. At-risk patients include sexually active adolescents, women with multiple sexual partners, women with a history of STI, those whose sexual partner has an STI, and women living in communities with a high prevalence of disease.20,21
Women being treated for PID should be advised to abstain from sexual intercourse until symptoms have resolved, treatment is completed, and any sexual partners have been treated as well. It is essential to emphasize to patients (and their partners) the importance of compliance to treatment regimens and the risk for PID co-infection and reinfection, as recurrence leads to an increase in long-term complications.5
Treatment of sexual partners. The CDC instructs that a woman’s most recent partner should be treated if she had sexual intercourse within 60 days of onset of symptoms or diagnosis. Furthermore, men who have had sexual contact with a woman who has PID in the 60 days prior to onset of her symptoms should be evaluated, tested, and treated for chlamydia and gonorrhea, regardless of the etiology of PID or the pathogens isolated from the woman.5
Admission criteria. Hospitalization should be based on provider judgment despite patient age. The suggested admission criteria include surgical emergency (eg, appendicitis), tubo-ovarian abscess, pregnancy, severe illness, nausea and vomiting, high fever, inability to follow or tolerate an outpatient oral regimen, and lack of clinical response to oral antimicrobial therapy.5
Follow-up care. Clinical improvement (ie, reduction in abdominal, uterine, adnexal, and cervical motion tenderness) should occur within 72 hours of antimicrobial therapy initiation. If it does not, hospital admission or adjustment in antimicrobial regimen should be considered, as well as additional diagnostic testing (eg, laparoscopy). In addition, all women with chlamydial- or gonococcal-related PID should return in three months for surveillance testing.22
COMPLICATIONS
Long-term complications—including infertility, chronic pelvic pain, and ectopic pregnancy—may occur, even when there has been a clinical response to adequate treatment. Data from the PID Evaluation and Clinical Health (PEACH) study were analyzed to assess long-term sequelae at seven years postdiagnosis and treatment. The researchers found that about 21% of women experienced recurrent PID, 19% developed infertility, and 42% reported chronic pelvic pain.3 Other research has also shown that repeat episodes of PID and delayed treatment increase the risk for long-term complications.23,24
SCREENING AND PREVENTION
Ten percent of women with an untreated STI will go on to develop PID.4 It is imperative to educate patients on the dangers and consequences of STIs when they become sexually active. Adolescents benefit the most from preventive education; this group is twice as likely as any other age group to be diagnosed with PID due to their inclination toward risky sexual behavior. Additionally, younger women tend to have a more friable cervix, increasing their risk for infection.25,26 Providers should promote safe sexual practices, such as condom use and less frequent partner exchange, in order to reduce STI exposure.
In 2015, the rate of reported cases of C trachomatis was 645.5 per 100,000 females, and of N gonorrheae, 107.2 per 100,000 females.23 The United States Preventive Services Task Force and the CDC recommend annual screening for chlamydia and gonorrhea in all sexually active women younger than 25, as well as sexually active women ages 25 and older who are considered at increased risk.5
CONCLUSION
PID is often difficult to diagnose, since patients may be asymptomatic or present with vague symptoms. Clinicians should maintain a high level of suspicion for PID in adolescent females due to the high incidence of STI exposure in this population. The best way to prevent long-term complications of PID is to prevent the first episode of PID and/or first exposure to STIs. Therefore, clinicians should be proactive in offering STI screenings to all sexually active patients younger than 25 who request care, regardless of their chief complaint, and educating patients on the potential long-term effects of PID and STIs.
1. Leichliter JS, Chandra A, Aral SO. Correlates of self-reported pelvic inflammatory disease treatment in sexually experienced reproductive-aged women in the United States, 1995 and 2006-2010. Sex Transm Dis. 2013;40(5):413-418.
2. Owusu-Edusei K Jr, Bohm MK, Chesson HW, Kent CK. Chlamydia screening and pelvic inflammatory disease: insights from exploratory time-series analyses. Am J Prev Med. 2010;38(6):652-657.
3. Trent M, Bass D, Ness RB, Haggerty C. Recurrent PID, subsequent STI, and reproductive health outcomes: findings from the PID evaluation and clinical health (PEACH) study. Sex Transm Dis. 2011;38(9):879-881.
4. Mitchell C, Prabhu M. Pelvic inflammatory disease: current concepts in pathogenesis, diagnosis and treatment. Infect Dis Clin North Am. 2013;27(4):793-809.
5. CDC. Pelvic inflammatory disease (PID). www.cdc.gov/std/tg2015/pid.htm. Accessed July 13, 2017.
6. Burnett AM, Anderson CP, Zwank MD. Laboratory-confirmed gonorrhea and/or chlamydia rates in clinically diagnosed pelvic inflammatory disease and cervicitis. Am J Emerg Med. 2012;30:1114–1117.
7. Bjartling C, Osser S, Persson K. The association between Mycoplasma genitalium and pelvic inflammatory disease after termination of pregnancy. BJOG. 2010;117(3):361-364.
8. Ness RB, Kip KE, Hillier SL, et al. A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am J Epidemiol. 2005;162(6):585-590.
9. Ness RB, Hillier SL, Kip KE, et al. Bacterial vaginosis and risk of pelvic inflammatory disease. Obstet Gynecol. 2004;104(4):761-769.
10. Cherpes TL, Wiesenfeld HC, Melan MA, et al. The associations between pelvic inflammatory disease, Trichomonas vaginalis infection, and positive herpes simplex virus type 2 serology. Sex Transm Dis. 2006;33(12):747-752.
11. Simms I, Stephenson JM, Mallinson H, et al. Risk factors associated with pelvic inflammatory disease. Sex Transm Infect. 2006;82(6):452-457.
12. Schindlbeck C, Dziura D, Mylonas I. Diagnosis of pelvic inflammatory disease (PID): intra-operative findings and comparison of vaginal and intra-abdominal cultures. Arch Gynecol Obstet. 2014;289(6):1263-1269.
13. CDC. 2015 sexually transmitted diseases treatment guidelines. www.cdc.gov/std/tg2015/default.htm. Accessed September 6, 2017.
14. Korn AP, Hessol NA, Padian NS, et al. Risk factors for plasma cell endometritis among women with cervical Neisseria gonorrhoeae, cervical Chlamydia trachomatis, or bacterial vaginosis. Am J Obstet Gynecol. 1998;178(5):987-990.
15. Wiesenfeld HC, Sweet RL, Ness RB, et al. Comparison of acute and subclinical pelvic inflammatory disease. Sex Transm Dis. 2005;32(7):400-405.
16. Peipert JF, Ness RB, Blume J, et al. Clinical predictors of endometritis in women with symptoms and signs of pelvic inflammatory disease. Am J Obstet Gynecol. 2001;184(5):856-864.
17. Gaitán H, Angel E, Diaz R, et al. Accuracy of five different diagnostic techniques in mild-to-moderate pelvic inflammatory disease. Infect Dis Obstet Gynecol. 2002;10(4):171-180.
18. Tukeva TA, Aronen HJ, Karjalainen PT, et al. MR imaging in pelvic inflammatory disease: comparison with laparoscopy and US. Radiology. 1999;210(1):209-216.
19. Morino M, Pellegrino L, Castagna E, et al. Acute nonspecific abdominal pain. Ann Surg. 2006;244(6):881-888.
20. Woods JL, Scurlock AM, Hensel DJ. Pelvic inflammatory disease in the adolescent: understanding diagnosis and treatment as a health care provider. Pediatric Emergency Care. 2013;29(6):720-725.
21. LeFevre ML; U.S. Preventive Services Task Force. Screening for chlamydia and gonorrhea: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(12):902-910.
22. Hosenfeld CB, Workowski KA, Berman S, et al. Repeat infection with Chlamydia and gonorrhea among females: a systematic review of the literature. Sex Transm Dis. 2009;36(8):478-489.
23. CDC. Sexually transmitted disease surveillance. www.cdc.gov/std/stats15/STD-Surveillance-2015-print.pdf. Accessed July 13, 2017.
24. Hillis SD, Joesoef R, Marchbanks PA, et al. Delayed care of pelvic inflammatory disease as a risk factor for impaired fertility. Am J Obstet Gynecol. 1993;168(5):1503-1509.
25. Goyal M, Hersh A, Luan X, et al. National trends in pelvic inflammatory disease among adolescents in the emergency department. J Adolesc Health. 2013;53(2):249-252.
26. Gray-Swain MR, Peipert JF. Pelvic inflammatory disease in adolescents. Curr Opin Obstet Gynecol. 2006;18(5):503-510.
27. Łój B, Brodowska A, Ciecwiez S, et al. The role of serological testing for Chlamydia trachomatis in differential diagnosis of pelvic pain. Ann Agric Environ Med. 2016;23(3):506-510.
28. Sam JW, Jacobs JE, Birnbaum BA. Spectrum of CT findings in acute pyogenic pelvic inflammatory disease. Radiographics. 2002;22(6):1327-1 334.
1. Leichliter JS, Chandra A, Aral SO. Correlates of self-reported pelvic inflammatory disease treatment in sexually experienced reproductive-aged women in the United States, 1995 and 2006-2010. Sex Transm Dis. 2013;40(5):413-418.
2. Owusu-Edusei K Jr, Bohm MK, Chesson HW, Kent CK. Chlamydia screening and pelvic inflammatory disease: insights from exploratory time-series analyses. Am J Prev Med. 2010;38(6):652-657.
3. Trent M, Bass D, Ness RB, Haggerty C. Recurrent PID, subsequent STI, and reproductive health outcomes: findings from the PID evaluation and clinical health (PEACH) study. Sex Transm Dis. 2011;38(9):879-881.
4. Mitchell C, Prabhu M. Pelvic inflammatory disease: current concepts in pathogenesis, diagnosis and treatment. Infect Dis Clin North Am. 2013;27(4):793-809.
5. CDC. Pelvic inflammatory disease (PID). www.cdc.gov/std/tg2015/pid.htm. Accessed July 13, 2017.
6. Burnett AM, Anderson CP, Zwank MD. Laboratory-confirmed gonorrhea and/or chlamydia rates in clinically diagnosed pelvic inflammatory disease and cervicitis. Am J Emerg Med. 2012;30:1114–1117.
7. Bjartling C, Osser S, Persson K. The association between Mycoplasma genitalium and pelvic inflammatory disease after termination of pregnancy. BJOG. 2010;117(3):361-364.
8. Ness RB, Kip KE, Hillier SL, et al. A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am J Epidemiol. 2005;162(6):585-590.
9. Ness RB, Hillier SL, Kip KE, et al. Bacterial vaginosis and risk of pelvic inflammatory disease. Obstet Gynecol. 2004;104(4):761-769.
10. Cherpes TL, Wiesenfeld HC, Melan MA, et al. The associations between pelvic inflammatory disease, Trichomonas vaginalis infection, and positive herpes simplex virus type 2 serology. Sex Transm Dis. 2006;33(12):747-752.
11. Simms I, Stephenson JM, Mallinson H, et al. Risk factors associated with pelvic inflammatory disease. Sex Transm Infect. 2006;82(6):452-457.
12. Schindlbeck C, Dziura D, Mylonas I. Diagnosis of pelvic inflammatory disease (PID): intra-operative findings and comparison of vaginal and intra-abdominal cultures. Arch Gynecol Obstet. 2014;289(6):1263-1269.
13. CDC. 2015 sexually transmitted diseases treatment guidelines. www.cdc.gov/std/tg2015/default.htm. Accessed September 6, 2017.
14. Korn AP, Hessol NA, Padian NS, et al. Risk factors for plasma cell endometritis among women with cervical Neisseria gonorrhoeae, cervical Chlamydia trachomatis, or bacterial vaginosis. Am J Obstet Gynecol. 1998;178(5):987-990.
15. Wiesenfeld HC, Sweet RL, Ness RB, et al. Comparison of acute and subclinical pelvic inflammatory disease. Sex Transm Dis. 2005;32(7):400-405.
16. Peipert JF, Ness RB, Blume J, et al. Clinical predictors of endometritis in women with symptoms and signs of pelvic inflammatory disease. Am J Obstet Gynecol. 2001;184(5):856-864.
17. Gaitán H, Angel E, Diaz R, et al. Accuracy of five different diagnostic techniques in mild-to-moderate pelvic inflammatory disease. Infect Dis Obstet Gynecol. 2002;10(4):171-180.
18. Tukeva TA, Aronen HJ, Karjalainen PT, et al. MR imaging in pelvic inflammatory disease: comparison with laparoscopy and US. Radiology. 1999;210(1):209-216.
19. Morino M, Pellegrino L, Castagna E, et al. Acute nonspecific abdominal pain. Ann Surg. 2006;244(6):881-888.
20. Woods JL, Scurlock AM, Hensel DJ. Pelvic inflammatory disease in the adolescent: understanding diagnosis and treatment as a health care provider. Pediatric Emergency Care. 2013;29(6):720-725.
21. LeFevre ML; U.S. Preventive Services Task Force. Screening for chlamydia and gonorrhea: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(12):902-910.
22. Hosenfeld CB, Workowski KA, Berman S, et al. Repeat infection with Chlamydia and gonorrhea among females: a systematic review of the literature. Sex Transm Dis. 2009;36(8):478-489.
23. CDC. Sexually transmitted disease surveillance. www.cdc.gov/std/stats15/STD-Surveillance-2015-print.pdf. Accessed July 13, 2017.
24. Hillis SD, Joesoef R, Marchbanks PA, et al. Delayed care of pelvic inflammatory disease as a risk factor for impaired fertility. Am J Obstet Gynecol. 1993;168(5):1503-1509.
25. Goyal M, Hersh A, Luan X, et al. National trends in pelvic inflammatory disease among adolescents in the emergency department. J Adolesc Health. 2013;53(2):249-252.
26. Gray-Swain MR, Peipert JF. Pelvic inflammatory disease in adolescents. Curr Opin Obstet Gynecol. 2006;18(5):503-510.
27. Łój B, Brodowska A, Ciecwiez S, et al. The role of serological testing for Chlamydia trachomatis in differential diagnosis of pelvic pain. Ann Agric Environ Med. 2016;23(3):506-510.
28. Sam JW, Jacobs JE, Birnbaum BA. Spectrum of CT findings in acute pyogenic pelvic inflammatory disease. Radiographics. 2002;22(6):1327-1 334.
Can we stop worrying about the age of blood?
Blood transfusions are common in critically ill patients; two in five adults admitted to an ICU receive at least one transfusion during their hospitalization (Corwin HL, et al. Crit Care Med. 2004;32[1]:39). Recently, there has been growing concern about the potential dangers involved with prolonged blood storage. Several provocative observational and retrospective studies found that prolonged storage time (ie, the age of the blood being transfused) negatively affects clinical outcomes (Wang D, et al. Transfusion. 2012;52[6]:1184). But now, some newly published trials on blood transfusion practice, including one published in late September 2017 (Cooper DJ, et al. N Engl J Med. Published online, September 27, 2017) seem to debunk much of this literature. Was all of the concern about age of blood overblown?
The appeal of “fresh” blood is intuitive. As consumers, we’re conditioned that the fresher the better. Fresh food tastes best. Carbonated beverages go “flat” over time. The newest iPhone® device is superior to your old one. So, of course, it follows that fresh blood is also better for your health than older blood.
But, in order to have a viable transfusion service, blood has to be stored. Blood is a scarce resource, and blood banks need to keep an adequate supply on hand for expected clinical necessities, as well as for emergencies. Donors can’t be on standby, waiting in the hospital to provide immediate whole blood transfusion. Also, blood needs to be tested for infections and for potential interactions with the patient, and whole blood must be broken down into individual components for transfusion. All of this requires time and storage.
In a randomized study of 100 critically ill adults supported by mechanical ventilation, 50 were randomized to receive “fresh” blood (median storage age 4 days, interquartile range 3-5 days) and 50 were randomized to receive “standard” blood (median storage age 26.5 days, interquartile range 21-36 days) (Kor DJ, et al. Am J Respir Crit Care Med. 2012;185[8]:842). The primary outcome was gas exchange, as prolonged storage of red blood cells could potentially lead to an increased inflammatory response in patients. However, the authors found no difference in gas exchange between the two groups, and there were no differences in immunologic function or coagulation status.
The ABLE (Age of Blood Evaluation) trial was a randomized, blinded trial of transfusion practices in critically ill patients (Lacroix J, et al. N Engl J Med. 2015;372:1410). In 64 centers in Canada and Europe, 2,430 critically ill adults were randomized to receive either “fresh” blood (mean storage age 6.1 ± 4.9 days) or “standard” blood (mean storage age 22.0 ± 8.4 days). The primary outcome was 90-day mortality, with a power of 90% to detect a 5% change in mortality between the two groups. The investigators found no statistically significant difference in 90-day mortality between the “fresh” and “standard” groups (37% vs 35.3%; hazard ratio 1.1; 95% CI 0.9 – 1.2). Additionally, there were no differences in secondary outcomes, including multiorgan system dysfunction, duration of supportive care, or development of nosocomial infections.
The INFORM (Informing Fresh versus Old Red Cell Management) trial was a randomized study of patients hospitalized in six centers in Canada, Australia, Israel, and the United States (Heddle NM, et al. N Engl J Med. 2016;375[2]:1937). A total of 24,736 patients received transfusions with either “fresh” blood (median storage age 11 days) or “standard” blood (median storage age 23 days). The primary outcome was in-hospital death, with a 90% power to detect a 15% lower relative risk. When comparing the 8,215 patients who received “fresh” blood and the 16,521 patients who received “standard” blood, the authors found no difference in mortality between the two groups (9.1% vs 8.8%; odds ratio 1.04; 95% CI 0.95 to 1.14). Furthermore, there were no differences in outcomes in the high-risk subgroups that included patients with cancer, patients in the ICU, and patients undergoing cardiovascular surgery.
A meta-analysis examined 12 trials of patients who received “fresh” blood compared with those who received “older” or “standard” blood (Alexander PE, et al. Blood. 2016;127[4]:400); 5,229 patients were included in these trials, in which “fresh” blood was defined as blood stored for 3 to 10 days and “older” blood was stored for longer durations. There was no difference in mortality between the two groups (relative risk 1.04; 95% CI 0.94 - 1.14), and no difference in adverse events (relative risk 1.02; 95% CI 0.91 - 1.14). However, perhaps surprisingly, “fresh” blood was associated with an increased risk of nosocomial infections (relative risk 1.09; 95% CI 1.00 - 1.18).
So, can we stop worrying about the age of the blood that we are about to transfuse? Probably. Taken together, these studies suggest that differences in the duration of red blood cell storage allowed within current US FDA standards aren’t clinically relevant, even in critically ill patients. At least, for now, the current practices for age of blood and duration of storage appear unrelated to adverse clinical outcomes.
Dr. Carroll is Professor of Pediatrics, University of Connecticut, Division of Critical Care, Connecticut Children’s Medical Center, Hartford, Connecticut.
Blood transfusions are common in critically ill patients; two in five adults admitted to an ICU receive at least one transfusion during their hospitalization (Corwin HL, et al. Crit Care Med. 2004;32[1]:39). Recently, there has been growing concern about the potential dangers involved with prolonged blood storage. Several provocative observational and retrospective studies found that prolonged storage time (ie, the age of the blood being transfused) negatively affects clinical outcomes (Wang D, et al. Transfusion. 2012;52[6]:1184). But now, some newly published trials on blood transfusion practice, including one published in late September 2017 (Cooper DJ, et al. N Engl J Med. Published online, September 27, 2017) seem to debunk much of this literature. Was all of the concern about age of blood overblown?
The appeal of “fresh” blood is intuitive. As consumers, we’re conditioned that the fresher the better. Fresh food tastes best. Carbonated beverages go “flat” over time. The newest iPhone® device is superior to your old one. So, of course, it follows that fresh blood is also better for your health than older blood.
But, in order to have a viable transfusion service, blood has to be stored. Blood is a scarce resource, and blood banks need to keep an adequate supply on hand for expected clinical necessities, as well as for emergencies. Donors can’t be on standby, waiting in the hospital to provide immediate whole blood transfusion. Also, blood needs to be tested for infections and for potential interactions with the patient, and whole blood must be broken down into individual components for transfusion. All of this requires time and storage.
In a randomized study of 100 critically ill adults supported by mechanical ventilation, 50 were randomized to receive “fresh” blood (median storage age 4 days, interquartile range 3-5 days) and 50 were randomized to receive “standard” blood (median storage age 26.5 days, interquartile range 21-36 days) (Kor DJ, et al. Am J Respir Crit Care Med. 2012;185[8]:842). The primary outcome was gas exchange, as prolonged storage of red blood cells could potentially lead to an increased inflammatory response in patients. However, the authors found no difference in gas exchange between the two groups, and there were no differences in immunologic function or coagulation status.
The ABLE (Age of Blood Evaluation) trial was a randomized, blinded trial of transfusion practices in critically ill patients (Lacroix J, et al. N Engl J Med. 2015;372:1410). In 64 centers in Canada and Europe, 2,430 critically ill adults were randomized to receive either “fresh” blood (mean storage age 6.1 ± 4.9 days) or “standard” blood (mean storage age 22.0 ± 8.4 days). The primary outcome was 90-day mortality, with a power of 90% to detect a 5% change in mortality between the two groups. The investigators found no statistically significant difference in 90-day mortality between the “fresh” and “standard” groups (37% vs 35.3%; hazard ratio 1.1; 95% CI 0.9 – 1.2). Additionally, there were no differences in secondary outcomes, including multiorgan system dysfunction, duration of supportive care, or development of nosocomial infections.
The INFORM (Informing Fresh versus Old Red Cell Management) trial was a randomized study of patients hospitalized in six centers in Canada, Australia, Israel, and the United States (Heddle NM, et al. N Engl J Med. 2016;375[2]:1937). A total of 24,736 patients received transfusions with either “fresh” blood (median storage age 11 days) or “standard” blood (median storage age 23 days). The primary outcome was in-hospital death, with a 90% power to detect a 15% lower relative risk. When comparing the 8,215 patients who received “fresh” blood and the 16,521 patients who received “standard” blood, the authors found no difference in mortality between the two groups (9.1% vs 8.8%; odds ratio 1.04; 95% CI 0.95 to 1.14). Furthermore, there were no differences in outcomes in the high-risk subgroups that included patients with cancer, patients in the ICU, and patients undergoing cardiovascular surgery.
A meta-analysis examined 12 trials of patients who received “fresh” blood compared with those who received “older” or “standard” blood (Alexander PE, et al. Blood. 2016;127[4]:400); 5,229 patients were included in these trials, in which “fresh” blood was defined as blood stored for 3 to 10 days and “older” blood was stored for longer durations. There was no difference in mortality between the two groups (relative risk 1.04; 95% CI 0.94 - 1.14), and no difference in adverse events (relative risk 1.02; 95% CI 0.91 - 1.14). However, perhaps surprisingly, “fresh” blood was associated with an increased risk of nosocomial infections (relative risk 1.09; 95% CI 1.00 - 1.18).
So, can we stop worrying about the age of the blood that we are about to transfuse? Probably. Taken together, these studies suggest that differences in the duration of red blood cell storage allowed within current US FDA standards aren’t clinically relevant, even in critically ill patients. At least, for now, the current practices for age of blood and duration of storage appear unrelated to adverse clinical outcomes.
Dr. Carroll is Professor of Pediatrics, University of Connecticut, Division of Critical Care, Connecticut Children’s Medical Center, Hartford, Connecticut.
Blood transfusions are common in critically ill patients; two in five adults admitted to an ICU receive at least one transfusion during their hospitalization (Corwin HL, et al. Crit Care Med. 2004;32[1]:39). Recently, there has been growing concern about the potential dangers involved with prolonged blood storage. Several provocative observational and retrospective studies found that prolonged storage time (ie, the age of the blood being transfused) negatively affects clinical outcomes (Wang D, et al. Transfusion. 2012;52[6]:1184). But now, some newly published trials on blood transfusion practice, including one published in late September 2017 (Cooper DJ, et al. N Engl J Med. Published online, September 27, 2017) seem to debunk much of this literature. Was all of the concern about age of blood overblown?
The appeal of “fresh” blood is intuitive. As consumers, we’re conditioned that the fresher the better. Fresh food tastes best. Carbonated beverages go “flat” over time. The newest iPhone® device is superior to your old one. So, of course, it follows that fresh blood is also better for your health than older blood.
But, in order to have a viable transfusion service, blood has to be stored. Blood is a scarce resource, and blood banks need to keep an adequate supply on hand for expected clinical necessities, as well as for emergencies. Donors can’t be on standby, waiting in the hospital to provide immediate whole blood transfusion. Also, blood needs to be tested for infections and for potential interactions with the patient, and whole blood must be broken down into individual components for transfusion. All of this requires time and storage.
In a randomized study of 100 critically ill adults supported by mechanical ventilation, 50 were randomized to receive “fresh” blood (median storage age 4 days, interquartile range 3-5 days) and 50 were randomized to receive “standard” blood (median storage age 26.5 days, interquartile range 21-36 days) (Kor DJ, et al. Am J Respir Crit Care Med. 2012;185[8]:842). The primary outcome was gas exchange, as prolonged storage of red blood cells could potentially lead to an increased inflammatory response in patients. However, the authors found no difference in gas exchange between the two groups, and there were no differences in immunologic function or coagulation status.
The ABLE (Age of Blood Evaluation) trial was a randomized, blinded trial of transfusion practices in critically ill patients (Lacroix J, et al. N Engl J Med. 2015;372:1410). In 64 centers in Canada and Europe, 2,430 critically ill adults were randomized to receive either “fresh” blood (mean storage age 6.1 ± 4.9 days) or “standard” blood (mean storage age 22.0 ± 8.4 days). The primary outcome was 90-day mortality, with a power of 90% to detect a 5% change in mortality between the two groups. The investigators found no statistically significant difference in 90-day mortality between the “fresh” and “standard” groups (37% vs 35.3%; hazard ratio 1.1; 95% CI 0.9 – 1.2). Additionally, there were no differences in secondary outcomes, including multiorgan system dysfunction, duration of supportive care, or development of nosocomial infections.
The INFORM (Informing Fresh versus Old Red Cell Management) trial was a randomized study of patients hospitalized in six centers in Canada, Australia, Israel, and the United States (Heddle NM, et al. N Engl J Med. 2016;375[2]:1937). A total of 24,736 patients received transfusions with either “fresh” blood (median storage age 11 days) or “standard” blood (median storage age 23 days). The primary outcome was in-hospital death, with a 90% power to detect a 15% lower relative risk. When comparing the 8,215 patients who received “fresh” blood and the 16,521 patients who received “standard” blood, the authors found no difference in mortality between the two groups (9.1% vs 8.8%; odds ratio 1.04; 95% CI 0.95 to 1.14). Furthermore, there were no differences in outcomes in the high-risk subgroups that included patients with cancer, patients in the ICU, and patients undergoing cardiovascular surgery.
A meta-analysis examined 12 trials of patients who received “fresh” blood compared with those who received “older” or “standard” blood (Alexander PE, et al. Blood. 2016;127[4]:400); 5,229 patients were included in these trials, in which “fresh” blood was defined as blood stored for 3 to 10 days and “older” blood was stored for longer durations. There was no difference in mortality between the two groups (relative risk 1.04; 95% CI 0.94 - 1.14), and no difference in adverse events (relative risk 1.02; 95% CI 0.91 - 1.14). However, perhaps surprisingly, “fresh” blood was associated with an increased risk of nosocomial infections (relative risk 1.09; 95% CI 1.00 - 1.18).
So, can we stop worrying about the age of the blood that we are about to transfuse? Probably. Taken together, these studies suggest that differences in the duration of red blood cell storage allowed within current US FDA standards aren’t clinically relevant, even in critically ill patients. At least, for now, the current practices for age of blood and duration of storage appear unrelated to adverse clinical outcomes.
Dr. Carroll is Professor of Pediatrics, University of Connecticut, Division of Critical Care, Connecticut Children’s Medical Center, Hartford, Connecticut.
Ribaxamase reduced new CDI infection by 71%
SAN DIEGO – , results from a phase 2b study showed.
At an annual scientific meeting on infectious diseases, lead investigator John F. Kokai-Kun, PhD, said that the finding represents a paradigm shift in the use of intravenous beta-lactam antibiotics to prevent opportunistic infections. “We currently treat Clostridium difficile infection (CDI) with antibiotics, which attack the vegetative cells,” said Dr. Kokai-Kun, vice president of nonclinical affairs for Rockville, Md.–based Synthetic Biologics, which is developing ribaxamase. “Since C. diff. is primarily a toxin-mediated disease, certain products seem to neutralize the toxin. There’s also been work with probiotics and prebiotics to try to strengthen and repair the dysbiotic colon. Fecal replacement therapy has been shown to be fairly effective for treatment of recurrent C. diff. infection. What if we could simply block the initial insult that leads to this cascade? That’s the damage caused to the gut microbiome by the antibiotic that’s excreted to the intestine.”
That’s where ribaxamase comes in, he said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. Ribaxamase is an orally administered beta-lactamase designed to degrade penicillin and cephalosporins in the intestinal lumen. It’s formulated for release in the proximal small intestine and is expected to be given during or a short time after administration of IV beta-lactam antibiotics such as ceftriaxone. “This is expected to degrade the excess antibiotics that are excreted into the small intestine via the bile,” Dr. Kokai-Kun explained. “It’s designed to prevent disruption of the gut microbiome and thus protect from opportunistic GI infections like CDI.” Early-stage clinical studies demonstrated that ribaxamase was well tolerated and that it is not systemically absorbed, while phase 2 studies showed that ribaxamase degrades ceftriaxone in the intestine to below the level of detection while not affecting the pharmacokinetics of ceftriaxone in the plasma.
For the current study, 412 patients were enrolled at 84 multinational clinical sites. These patients were admitted to the hospital for treatment of a lower respiratory tract infection and were randomized 1:1 to receive ceftriaxone plus 150 mg ribaxamase or ceftriaxone plus placebo. Patients in both groups could also receive an oral macrolide at the discretion of the clinical investigator. The researchers also obtained fecal samples at screening, 72 hours post antibiotic treatment, and at the end of a 4-week follow-up visit, to determine colonization by opportunistic pathogens and to examine changes in the gut microbiome. Patients were monitored for 6 weeks for diarrhea and CDI. Diarrhea was defined as three or more loose or watery stools in a 24-hour period. “If that occurred, then we collected a sample, which was sent to the local lab to determine the presence of C. difficile toxins,” Dr. Kokai-Kun said.
The average age of study participants was 70 years, and about one-third in each arm received oral macrolides. The number of adverse events and serious adverse events were similar between active and placebo arms, and there was no trend associated with ribaxamase use. The lower respiratory tract infection cure rate to the ceftriaxone treatment was about 99% in both arms at 72 hours post treatment and at 2 weeks post treatment.
To analyze changes in the gut microbiome, the researchers conducted 16S rRNA sequencing of DNA extracted from fecal samples. In all, 652 samples were sequenced from 229 patients. Results from that analysis suggests that ribaxamase “appears to protect the gut microbiome from the onslaught of the ceftriaxone,” he said.
Ribaxamase reduced the incidence of new-onset CDI by 71%, compared with placebo (P = .045). “It apparently did this by protecting the integrity of the gut microbiome,” Dr. Kokai-Kun said. “There was also a significant reduction of new colonization by vancomycin-resistant enterococci at 72 hours and 4 weeks (P = .0001 and P = .0002, respectively) which is an opportunistic pathogen that is known to be able to inhabit gut microbiome when there is dysbiosis.”
The study was sponsored by Synthetic Biologics. Dr. Kokai-Kun is an employee of the company.
SAN DIEGO – , results from a phase 2b study showed.
At an annual scientific meeting on infectious diseases, lead investigator John F. Kokai-Kun, PhD, said that the finding represents a paradigm shift in the use of intravenous beta-lactam antibiotics to prevent opportunistic infections. “We currently treat Clostridium difficile infection (CDI) with antibiotics, which attack the vegetative cells,” said Dr. Kokai-Kun, vice president of nonclinical affairs for Rockville, Md.–based Synthetic Biologics, which is developing ribaxamase. “Since C. diff. is primarily a toxin-mediated disease, certain products seem to neutralize the toxin. There’s also been work with probiotics and prebiotics to try to strengthen and repair the dysbiotic colon. Fecal replacement therapy has been shown to be fairly effective for treatment of recurrent C. diff. infection. What if we could simply block the initial insult that leads to this cascade? That’s the damage caused to the gut microbiome by the antibiotic that’s excreted to the intestine.”
That’s where ribaxamase comes in, he said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. Ribaxamase is an orally administered beta-lactamase designed to degrade penicillin and cephalosporins in the intestinal lumen. It’s formulated for release in the proximal small intestine and is expected to be given during or a short time after administration of IV beta-lactam antibiotics such as ceftriaxone. “This is expected to degrade the excess antibiotics that are excreted into the small intestine via the bile,” Dr. Kokai-Kun explained. “It’s designed to prevent disruption of the gut microbiome and thus protect from opportunistic GI infections like CDI.” Early-stage clinical studies demonstrated that ribaxamase was well tolerated and that it is not systemically absorbed, while phase 2 studies showed that ribaxamase degrades ceftriaxone in the intestine to below the level of detection while not affecting the pharmacokinetics of ceftriaxone in the plasma.
For the current study, 412 patients were enrolled at 84 multinational clinical sites. These patients were admitted to the hospital for treatment of a lower respiratory tract infection and were randomized 1:1 to receive ceftriaxone plus 150 mg ribaxamase or ceftriaxone plus placebo. Patients in both groups could also receive an oral macrolide at the discretion of the clinical investigator. The researchers also obtained fecal samples at screening, 72 hours post antibiotic treatment, and at the end of a 4-week follow-up visit, to determine colonization by opportunistic pathogens and to examine changes in the gut microbiome. Patients were monitored for 6 weeks for diarrhea and CDI. Diarrhea was defined as three or more loose or watery stools in a 24-hour period. “If that occurred, then we collected a sample, which was sent to the local lab to determine the presence of C. difficile toxins,” Dr. Kokai-Kun said.
The average age of study participants was 70 years, and about one-third in each arm received oral macrolides. The number of adverse events and serious adverse events were similar between active and placebo arms, and there was no trend associated with ribaxamase use. The lower respiratory tract infection cure rate to the ceftriaxone treatment was about 99% in both arms at 72 hours post treatment and at 2 weeks post treatment.
To analyze changes in the gut microbiome, the researchers conducted 16S rRNA sequencing of DNA extracted from fecal samples. In all, 652 samples were sequenced from 229 patients. Results from that analysis suggests that ribaxamase “appears to protect the gut microbiome from the onslaught of the ceftriaxone,” he said.
Ribaxamase reduced the incidence of new-onset CDI by 71%, compared with placebo (P = .045). “It apparently did this by protecting the integrity of the gut microbiome,” Dr. Kokai-Kun said. “There was also a significant reduction of new colonization by vancomycin-resistant enterococci at 72 hours and 4 weeks (P = .0001 and P = .0002, respectively) which is an opportunistic pathogen that is known to be able to inhabit gut microbiome when there is dysbiosis.”
The study was sponsored by Synthetic Biologics. Dr. Kokai-Kun is an employee of the company.
SAN DIEGO – , results from a phase 2b study showed.
At an annual scientific meeting on infectious diseases, lead investigator John F. Kokai-Kun, PhD, said that the finding represents a paradigm shift in the use of intravenous beta-lactam antibiotics to prevent opportunistic infections. “We currently treat Clostridium difficile infection (CDI) with antibiotics, which attack the vegetative cells,” said Dr. Kokai-Kun, vice president of nonclinical affairs for Rockville, Md.–based Synthetic Biologics, which is developing ribaxamase. “Since C. diff. is primarily a toxin-mediated disease, certain products seem to neutralize the toxin. There’s also been work with probiotics and prebiotics to try to strengthen and repair the dysbiotic colon. Fecal replacement therapy has been shown to be fairly effective for treatment of recurrent C. diff. infection. What if we could simply block the initial insult that leads to this cascade? That’s the damage caused to the gut microbiome by the antibiotic that’s excreted to the intestine.”
That’s where ribaxamase comes in, he said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society. Ribaxamase is an orally administered beta-lactamase designed to degrade penicillin and cephalosporins in the intestinal lumen. It’s formulated for release in the proximal small intestine and is expected to be given during or a short time after administration of IV beta-lactam antibiotics such as ceftriaxone. “This is expected to degrade the excess antibiotics that are excreted into the small intestine via the bile,” Dr. Kokai-Kun explained. “It’s designed to prevent disruption of the gut microbiome and thus protect from opportunistic GI infections like CDI.” Early-stage clinical studies demonstrated that ribaxamase was well tolerated and that it is not systemically absorbed, while phase 2 studies showed that ribaxamase degrades ceftriaxone in the intestine to below the level of detection while not affecting the pharmacokinetics of ceftriaxone in the plasma.
For the current study, 412 patients were enrolled at 84 multinational clinical sites. These patients were admitted to the hospital for treatment of a lower respiratory tract infection and were randomized 1:1 to receive ceftriaxone plus 150 mg ribaxamase or ceftriaxone plus placebo. Patients in both groups could also receive an oral macrolide at the discretion of the clinical investigator. The researchers also obtained fecal samples at screening, 72 hours post antibiotic treatment, and at the end of a 4-week follow-up visit, to determine colonization by opportunistic pathogens and to examine changes in the gut microbiome. Patients were monitored for 6 weeks for diarrhea and CDI. Diarrhea was defined as three or more loose or watery stools in a 24-hour period. “If that occurred, then we collected a sample, which was sent to the local lab to determine the presence of C. difficile toxins,” Dr. Kokai-Kun said.
The average age of study participants was 70 years, and about one-third in each arm received oral macrolides. The number of adverse events and serious adverse events were similar between active and placebo arms, and there was no trend associated with ribaxamase use. The lower respiratory tract infection cure rate to the ceftriaxone treatment was about 99% in both arms at 72 hours post treatment and at 2 weeks post treatment.
To analyze changes in the gut microbiome, the researchers conducted 16S rRNA sequencing of DNA extracted from fecal samples. In all, 652 samples were sequenced from 229 patients. Results from that analysis suggests that ribaxamase “appears to protect the gut microbiome from the onslaught of the ceftriaxone,” he said.
Ribaxamase reduced the incidence of new-onset CDI by 71%, compared with placebo (P = .045). “It apparently did this by protecting the integrity of the gut microbiome,” Dr. Kokai-Kun said. “There was also a significant reduction of new colonization by vancomycin-resistant enterococci at 72 hours and 4 weeks (P = .0001 and P = .0002, respectively) which is an opportunistic pathogen that is known to be able to inhabit gut microbiome when there is dysbiosis.”
The study was sponsored by Synthetic Biologics. Dr. Kokai-Kun is an employee of the company.
REPORTING FROM ID WEEK 2017
Key clinical point: Ribaxamase reduced new colonization with C. diff. and vancomycin-resistant enterococci.
Major finding: Ribaxamase reduced the incidence of new onset CDI by 71%, compared with placebo (P = 0.045).
Study details: A trial of 412 patients admitted to the hospital for treatment of a lower respiratory tract infection who were randomized to receive ceftriaxone plus 150 mg ribaxamase or ceftriaxone plus placebo.
Disclosures: The study was sponsored by Synthetic Biologics. Dr. Kokai-Kun is an employee of the company.
New App Aims to Bring Personalized Headache Medicine One Step Closer
STANFORD, CA—Headache specialists have created a new app intended to support patients with headache pain. Developed by board-certified headache specialists in collaboration with a machine learning and artificial intelligence professional, the BonTriage Headache Compass may help patients with headache better manage and improve their condition. When used consistently over time, this app is intended to help patients reduce the frequency, severity, and duration of headaches, as well as the disability they cause.
“The BonTriage Headache Compass provides a diagnostic clinical impression and monitoring, which patients can take to their doctor or just use to gain insight into their condition,” said Robert Cowan, MD, Professor of Neurology and Chief of the Division of Headache Medicine at Stanford University and a co-creator of the new app.
Personalized Data
The BonTriage Headache Compass app is the second product developed by BonTriage. The first is a detailed web-based questionnaire, available at www.bontriage.com, that creates a detailed report about a patient’s headache. The questionnaire has been validated and its accuracy is greater than 90%. Once completed, the questionnaire can be printed and shared with the patient’s physician.
The app works together with the questionnaire, but can be used independently. “When you first download the app, it asks the questions that are necessary to make the diagnostic clinical impression,” Dr. Cowan said. After asking basic demographic questions, the app poses specific questions about the characteristics of typical and current headaches and other related conditions. This information, which takes about 10 minutes to enter, will populate the app and generate a clinical impression similar to one formed at an initial clinical presentation.
The BonTriage Headache Compass then prompts users daily for data on headaches, medications, triggers, and lifestyle factors. Based on these data, the app can predict headache frequency; report the effect of treatment strategies on headache frequency, severity, and duration in real time; and answer questions based on personalized data. Users can thus see potential connections between their headaches, triggers, and behaviors. Data entry requires a minute or two each day.
Individualized Advice
The app’s internal analytics analyze data to interpret the personalized information that it collects. This analysis is intended to help patients with headache avoid factors (behaviors, triggers, etc) that worsen their headaches and enhance behaviors that reduce their headaches. The app also provides feedback to indicate which management strategies are working. “One critical piece of information we want to feed back to patients is whether they are getting better or not, based on how they are treating their headaches,” said Dr. Cowan. The data that the BonTriage Headache Compass app collects can inform patients about their specific headache triggers, lifestyle choices, medication, or environmental factors, and about how they influence their headache. After a month of consistent data entry … the app can begin to predict headaches. With continued use, the app could, for example, send a text message advising the user that a storm is coming to their area and he orshe did not sleep well the night before, so he or she should skip wine with dinner because of an increased risk of headache on that particular night.
Users of the BonTriage Headache Compass can query the app for data on areas of personal concern. For example, it can compare headache severity or frequency between months or examine the relationship between exercise or sleep and headache duration or frequency. The Headache Compass tool can provide visual representations and headache indices (ie, scores) to compare a current headache with previous headaches.
The BonTriage Headache Compass is available for free in the iPhone App Store.
—Glenn S. Williams
STANFORD, CA—Headache specialists have created a new app intended to support patients with headache pain. Developed by board-certified headache specialists in collaboration with a machine learning and artificial intelligence professional, the BonTriage Headache Compass may help patients with headache better manage and improve their condition. When used consistently over time, this app is intended to help patients reduce the frequency, severity, and duration of headaches, as well as the disability they cause.
“The BonTriage Headache Compass provides a diagnostic clinical impression and monitoring, which patients can take to their doctor or just use to gain insight into their condition,” said Robert Cowan, MD, Professor of Neurology and Chief of the Division of Headache Medicine at Stanford University and a co-creator of the new app.
Personalized Data
The BonTriage Headache Compass app is the second product developed by BonTriage. The first is a detailed web-based questionnaire, available at www.bontriage.com, that creates a detailed report about a patient’s headache. The questionnaire has been validated and its accuracy is greater than 90%. Once completed, the questionnaire can be printed and shared with the patient’s physician.
The app works together with the questionnaire, but can be used independently. “When you first download the app, it asks the questions that are necessary to make the diagnostic clinical impression,” Dr. Cowan said. After asking basic demographic questions, the app poses specific questions about the characteristics of typical and current headaches and other related conditions. This information, which takes about 10 minutes to enter, will populate the app and generate a clinical impression similar to one formed at an initial clinical presentation.
The BonTriage Headache Compass then prompts users daily for data on headaches, medications, triggers, and lifestyle factors. Based on these data, the app can predict headache frequency; report the effect of treatment strategies on headache frequency, severity, and duration in real time; and answer questions based on personalized data. Users can thus see potential connections between their headaches, triggers, and behaviors. Data entry requires a minute or two each day.
Individualized Advice
The app’s internal analytics analyze data to interpret the personalized information that it collects. This analysis is intended to help patients with headache avoid factors (behaviors, triggers, etc) that worsen their headaches and enhance behaviors that reduce their headaches. The app also provides feedback to indicate which management strategies are working. “One critical piece of information we want to feed back to patients is whether they are getting better or not, based on how they are treating their headaches,” said Dr. Cowan. The data that the BonTriage Headache Compass app collects can inform patients about their specific headache triggers, lifestyle choices, medication, or environmental factors, and about how they influence their headache. After a month of consistent data entry … the app can begin to predict headaches. With continued use, the app could, for example, send a text message advising the user that a storm is coming to their area and he orshe did not sleep well the night before, so he or she should skip wine with dinner because of an increased risk of headache on that particular night.
Users of the BonTriage Headache Compass can query the app for data on areas of personal concern. For example, it can compare headache severity or frequency between months or examine the relationship between exercise or sleep and headache duration or frequency. The Headache Compass tool can provide visual representations and headache indices (ie, scores) to compare a current headache with previous headaches.
The BonTriage Headache Compass is available for free in the iPhone App Store.
—Glenn S. Williams
STANFORD, CA—Headache specialists have created a new app intended to support patients with headache pain. Developed by board-certified headache specialists in collaboration with a machine learning and artificial intelligence professional, the BonTriage Headache Compass may help patients with headache better manage and improve their condition. When used consistently over time, this app is intended to help patients reduce the frequency, severity, and duration of headaches, as well as the disability they cause.
“The BonTriage Headache Compass provides a diagnostic clinical impression and monitoring, which patients can take to their doctor or just use to gain insight into their condition,” said Robert Cowan, MD, Professor of Neurology and Chief of the Division of Headache Medicine at Stanford University and a co-creator of the new app.
Personalized Data
The BonTriage Headache Compass app is the second product developed by BonTriage. The first is a detailed web-based questionnaire, available at www.bontriage.com, that creates a detailed report about a patient’s headache. The questionnaire has been validated and its accuracy is greater than 90%. Once completed, the questionnaire can be printed and shared with the patient’s physician.
The app works together with the questionnaire, but can be used independently. “When you first download the app, it asks the questions that are necessary to make the diagnostic clinical impression,” Dr. Cowan said. After asking basic demographic questions, the app poses specific questions about the characteristics of typical and current headaches and other related conditions. This information, which takes about 10 minutes to enter, will populate the app and generate a clinical impression similar to one formed at an initial clinical presentation.
The BonTriage Headache Compass then prompts users daily for data on headaches, medications, triggers, and lifestyle factors. Based on these data, the app can predict headache frequency; report the effect of treatment strategies on headache frequency, severity, and duration in real time; and answer questions based on personalized data. Users can thus see potential connections between their headaches, triggers, and behaviors. Data entry requires a minute or two each day.
Individualized Advice
The app’s internal analytics analyze data to interpret the personalized information that it collects. This analysis is intended to help patients with headache avoid factors (behaviors, triggers, etc) that worsen their headaches and enhance behaviors that reduce their headaches. The app also provides feedback to indicate which management strategies are working. “One critical piece of information we want to feed back to patients is whether they are getting better or not, based on how they are treating their headaches,” said Dr. Cowan. The data that the BonTriage Headache Compass app collects can inform patients about their specific headache triggers, lifestyle choices, medication, or environmental factors, and about how they influence their headache. After a month of consistent data entry … the app can begin to predict headaches. With continued use, the app could, for example, send a text message advising the user that a storm is coming to their area and he orshe did not sleep well the night before, so he or she should skip wine with dinner because of an increased risk of headache on that particular night.
Users of the BonTriage Headache Compass can query the app for data on areas of personal concern. For example, it can compare headache severity or frequency between months or examine the relationship between exercise or sleep and headache duration or frequency. The Headache Compass tool can provide visual representations and headache indices (ie, scores) to compare a current headache with previous headaches.
The BonTriage Headache Compass is available for free in the iPhone App Store.
—Glenn S. Williams
Transbronchial cryobiopsy, updated guidelines for chronic cough in children, PD-1 inhibition
Interventional Chest/Diagnostic Procedures
Cryobiopsy for ILD: Careful stewardship needed
Interest in transbronchial cryobiopsy has accelerated rapidly in recent years. This procedure is performed by advancing a cryoprobe into the peripheral lung via flexible bronchoscopy, where lung tissue freezes and adheres to the probe and is subsequently extracted as a cryobiopsy. The number of cryobiopsy-related publications has increased exponentially since it was described in 2009 (Babiak A, et al. Respiration. 2009;78[2]:203). This interest stems from reports of high diagnostic yields in patients with interstitial lung disease (ILD) while maintaining complication rates similar to that of conventional bronchoscopic biopsy.
Traditional bronchoscopic biopsies are notoriously insensitive; a specific diagnosis can be established in fewer than a third of cases (Sheth JS, et al. Chest. 2017;151[2]:389). As such, surgical lung biopsy continues to be recommended but is associated with significant mortality (2%) and morbidity (30%) in patients with ILD (Hutchinson JP, et al. ARJCCM. 2016;193[10]:1161). Cryobiopsy, which appears to rival surgical lung biopsy in terms of ability to contribute to a specific diagnosis, is, therefore, a highly promising alternative (Tomassetti S, et al. AJRCCM. 2016;193[7]:745).
As cryobiopsy is increasingly adopted around the world, however, troubling reports of serious complications have surfaced. Most notable is the recently reported experience of the initial 25 cases performed at the University of Pennsylvania, in which almost one in four patients suffered serious complications (DiBardino DM, et al. Ann Am Thorac Soc. 2017;14[6]:851). The authors pointed to lack of a predefined procedural protocol, as well as several choices relating to the specific technique used, including inconsistent use of fluoroscopy, lack of prophylactic bronchial blocker placement, and predominant use of laryngeal mask airways as potential contributing factors. Indeed, many variations of the basic cryobiopsy procedure have been described (Lentz RJ, et al. J Thoracic Dis. 2017;9[7]:2186), with no formal guidance or training available to inform advanced bronchoscopists interested in this procedure.
It is incumbent on the interventional pulmonology and ILD specialist communities to be responsible stewards of this promising procedure. Implementation of three parallel efforts to standardize and rigorously study this procedure should be considered as soon as possible: creation of expert consensus guidelines establishing best-practices for safe and effective biopsy technique; a training requirement before independent performance of the procedure; and creation of an international cryobiopsy registry to facilitate higher-quality research into optimal technique and outcomes. We owe this to our patients.
Robert J. Lentz, MD
NetWork Member
Fabien Maldonado, MD, FCCP
NetWork Member
Pediatric Chest Medicine
Chronic cough in children: New guidelines
A chronic cough is a common complaint among children whose parents seek medical evaluation. Chronic wet cough can indicate an underlying illness; therefore, an early diagnosis can lead to prevention of complications of the disease and improvement in quality of life.
CHEST is a leading resource in evidence and consensus-based guidelines on important topics affecting children. The most recent guidelines entitled Management of Children with Chronic Wet Cough and Protracted Bacterial Bronchitis (Chest. 2017;151(4):884-890) and Use of Management Pathways or Algorithms in Children with Chronic Cough (Chest. 2017;151(4):875-873) are updates from the 2006 CHEST guidelines on chronic cough in children.
The present updates utilized the CHEST methodological guidelines with chronic wet or productive cough and Grading of Recommendations Assessment, Development, and Evaluation framework and also performed a systematic review addressing key questions concerning the management of childhood disease for children 14 years and younger.
Guidance provided by the expert panel focused on recommendations to answer six key questions concerning the management of children 14 years and younger with a chronic wet cough unrelated to established chronic lung disease. The recommendations are:
1. Chronic cough is defined as the presence of a cough 4 weeks or longer in duration.
2. Assessment of the effect of the cough on the child and the family be undertaken as part of clinical consultation.
3. Evaluation of a chronic cough should be done with a systematic approach with pediatric-specific cough management protocols or algorithms.
4. Chest radiograph and, when age appropriate, spirometry with bronchodilator be undertaken as evaluation; tests for pertussis infection only to be performed if clinically suspected.
5. Chronic wet cough with no specific clinical features should receive antibiotics for 2 weeks targeted for common respiratory bacteria (Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis).
6. When cough persists despite 2 weeks of appropriate antibiotics, it is recommended to continue for an additional 2 weeks.
7. Additional tests (eg skin prick test, Mantoux, bronchoscopy, chest CT scan) should be individualized in accordance with the clinical setting and child’s clinical symptoms and signs.
The panel recognizes the need for prospective studies to assess current algorithms outcomes of children with chronic cough. Both articles can be found on the guidelines section of the CHEST site.
John Bishara, DO
Fellow-in-Training Member
Pulmonary Physiology, Function, and Rehabilitation
Functional imaging of the lung
Quantifying heterogeneity of ventilation and gas exchange in lung diseases remains a clinical challenge. Conventional pulmonary function test is insensitive to regional changes. The multiple inert gas elimination technique can quantify ventilation-perfusion distribution, but it requires invasive instrumentation (eg, pulmonary artery catheterization) and is not practical for clinical use. Computed tomography (CT) scans delineate spatial changes in lung structures but do not directly measure changes in ventilation and gas exchange. With its radiation, it is difficult to apply CT scanning repeatedly in patients. More recently, MR imaging techniques have been developed to directly “visualize” and quantify regional lung function (Kruger SJ, et al. J Magn Reson Imaging. 2016;43(2):295; Roos JE, et al. Magn Reson Imaging Clin N Am. 2015;23(2):217). These techniques employ inhalation of gases, such as oxygen, perfluorinated gases, and hyperpolarized 3He and 129Xe. Hyperpolarized 3He has been studied the most; however, the dwindling supply of 3He gas and its rising cost have prevented its further development. 129Xe has abundant supply and has emerged to be the inert gas of choice for MR imaging. Hyperpolarized 129Xe can measure ventilation, like hyperpolarized 3He. In addition, Xe diffuses into alveolar barrier (interstitium and plasma) and red blood cells, where it exhibits distinct resonant frequency shifts that can be captured by MR. Therefore, in one test, information on pulmonary ventilation and gas transfer can be obtained. To date, the results from MR imaging studies have provided new insights into the pathophysiology of obstructive and restrictive lung diseases. With continuous development, MR imaging of the lung could become a clinically useful tool in the near future.
Yuh-Chin T. Huang, MD, MHS, FCCP
Steering Committee Member
Thoracic Oncology
Immune-mediated pneumonitis and PD-1 inhibition
Inhibitors of the programmed cell death 1 receptor (PD-1) have shown significant promise in the treatment of advanced stage malignancy. With the recent expansion of indications for use of these agents, the number of patients treated will continue to grow. Clinicians must be aware of their potential for serious adverse side effects, including dermatitis, colitis, and potentially life-threatening pneumonitis.
The development of pneumonitis secondary to PD-1 inhibitions is reported to occur in 2% to 5% of patients and can present at any time during therapy, with 1% of patients developing grade 3 or higher pneumonitis.1,2 The most common symptoms are dyspnea and cough, though one-third of patients are asymptomatic at presentation.2 Radiographic and pathologic features vary greatly and include organizing pneumonia, interstitial pneumonitis, hypersensitivity pneumonitis, or diffuse alveolar damage.3 While pneumonitis due to PD-1 inhibition is reportedly uncommon, the increasing number of patients expected to receive these medications will predictably result in increasing overall frequency of pneumonitis cases. In addition, the lack of large prospective randomized trials and reliance on radiographic rather than pathologic data in diagnosing immune-mediated pneumonitis gives one pause. Given the variability of presentation, lack of routine pathologic data, and increasing use of dual agents (eg, PD-1 and CTLA-4), chest physicians and medical oncologists should have a high index of suspicion yet practice equipoise in patients receiving immunotherapy who develop unexplained pulmonary symptoms or infiltrates. More research is needed to help improve the multidisciplinary diagnosis and treatment of this potentially serious complication.
David Maurice Chambers, MD
Fellow-in-Training Member
Jason Atticus Akulian, MD, MPH
Steering Committee Member
References
1. Nishino M, et al. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncology. 2016;2(12):1607.
2. Naidoo J, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35(7):709.
3. Nishino M, et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res. 2016;22(24):6051.
Pulmonary Vascular Disease
Pulmonary Arterial Hypertension Associated With SLE
While pulmonary arterial hypertension (PAH) commonly complicates scleroderma (SSc), it is a rare complication of other connective tissue diseases (CTD), such as systemic lupus erythematosus (SLE). In the few prospective studies that utilize right-sided heart catheterization (RHC), the estimated prevalence of PAH in SLE is about 4%. However, since the prevalence of SLE is 10 to 15 times greater than SSc in the United States, the true prevalence of SLE-PAH may be higher than previously thought, and, thus, clinically relevant. Despite this, little is known about SLE-PAH.
A recent retrospective study from the French Pulmonary Hypertension Registry has added significantly to our understanding of this complication of SLE. Hachulla and colleagues studied 51 patients with RHC-proven SLE-PAH compared with 101 SLE control subjects without PAH. While the authors did not find any relevant differences in the demographics between groups, they did find a significantly higher prevalence of SSA and SSB antibodies in SLE-PAH. Interestingly, the presence of anti-U1 RNP antibody appeared to be less common in SLE-PAH patients; this lack of association is in contrast to prior studies in mixed CTD patients with anti-U1 RNP antibodies in which the prevalence of PAH can be as high as 60%. Further, none of the SLE-PAH patients demonstrated an acute response to vasodilator challenge during RHC, emphasizing that this maneuver does not need to be performed in SLE patients at risk of PAH. Trends toward improved survival in SLE-PAH patients treated with hydroxychloroquine are preliminary and hypothesis-generating but require confirmation in larger clinical studies.
Stephen Mathai, MD, FCCP
Chair
Leena Palwar, MD
Fellow-in-Training Member
References
Hachulla E, Jais X, Cinquetti G, et al. Pulmonary arterial hypertension associated with SLE: Results from the French pulmonary hypertension registry. Chest. 2017 Aug 26. pii: S0012-3692(17)31430-7. doi: 10.1016/j.chest.2017.08.014. [Epub ahead of print]
Chung L, Liu J, Parsons L, et al. Characterization of connective tissue disease-associated pulmonary arterial hypertension from REVEAL: identifying systemic sclerosis as a unique phenotype. Chest. 2010;138:1383-1394.
Shirai Y, Yasuoka H, Okano Y, Takeuchi T, Satoh T, Kuwana M. Clinical characteristics and survival of Japanese patients with connective tissue disease and pulmonary arterial hypertension: a singlecentre cohort. Rheumatology. 2012;51:1846-1854.
Hao YJ, Jiang X, Zhou W, et al. Connective tissue disease-associated pulmonary arterial hypertension in Chinese patients. Eur Respir J. 2014;44: 963-972.
Huang C, Li M, Liu Y, et al. Baseline characteristics and risk factors of pulmonary arterial hypertension in systemic lupus erythematosus patients. Medicine. 2016;95:e2761.
Pérez-Peñate GM, Rúa-Figueroa I, Juliá- Serdá G, et al. Pulmonary arterial hypertension in systemic lupus erythematosus: prevalence and predictors. J Rheumatol. 2016;43:323-329.
Alpert MA, Goldberg SH, Sindem BH, et al. Cardiovascular manifestations of mixed connective tissue disease in adults. Circulation. 1983;63:1182-1193.
Interventional Chest/Diagnostic Procedures
Cryobiopsy for ILD: Careful stewardship needed
Interest in transbronchial cryobiopsy has accelerated rapidly in recent years. This procedure is performed by advancing a cryoprobe into the peripheral lung via flexible bronchoscopy, where lung tissue freezes and adheres to the probe and is subsequently extracted as a cryobiopsy. The number of cryobiopsy-related publications has increased exponentially since it was described in 2009 (Babiak A, et al. Respiration. 2009;78[2]:203). This interest stems from reports of high diagnostic yields in patients with interstitial lung disease (ILD) while maintaining complication rates similar to that of conventional bronchoscopic biopsy.
Traditional bronchoscopic biopsies are notoriously insensitive; a specific diagnosis can be established in fewer than a third of cases (Sheth JS, et al. Chest. 2017;151[2]:389). As such, surgical lung biopsy continues to be recommended but is associated with significant mortality (2%) and morbidity (30%) in patients with ILD (Hutchinson JP, et al. ARJCCM. 2016;193[10]:1161). Cryobiopsy, which appears to rival surgical lung biopsy in terms of ability to contribute to a specific diagnosis, is, therefore, a highly promising alternative (Tomassetti S, et al. AJRCCM. 2016;193[7]:745).
As cryobiopsy is increasingly adopted around the world, however, troubling reports of serious complications have surfaced. Most notable is the recently reported experience of the initial 25 cases performed at the University of Pennsylvania, in which almost one in four patients suffered serious complications (DiBardino DM, et al. Ann Am Thorac Soc. 2017;14[6]:851). The authors pointed to lack of a predefined procedural protocol, as well as several choices relating to the specific technique used, including inconsistent use of fluoroscopy, lack of prophylactic bronchial blocker placement, and predominant use of laryngeal mask airways as potential contributing factors. Indeed, many variations of the basic cryobiopsy procedure have been described (Lentz RJ, et al. J Thoracic Dis. 2017;9[7]:2186), with no formal guidance or training available to inform advanced bronchoscopists interested in this procedure.
It is incumbent on the interventional pulmonology and ILD specialist communities to be responsible stewards of this promising procedure. Implementation of three parallel efforts to standardize and rigorously study this procedure should be considered as soon as possible: creation of expert consensus guidelines establishing best-practices for safe and effective biopsy technique; a training requirement before independent performance of the procedure; and creation of an international cryobiopsy registry to facilitate higher-quality research into optimal technique and outcomes. We owe this to our patients.
Robert J. Lentz, MD
NetWork Member
Fabien Maldonado, MD, FCCP
NetWork Member
Pediatric Chest Medicine
Chronic cough in children: New guidelines
A chronic cough is a common complaint among children whose parents seek medical evaluation. Chronic wet cough can indicate an underlying illness; therefore, an early diagnosis can lead to prevention of complications of the disease and improvement in quality of life.
CHEST is a leading resource in evidence and consensus-based guidelines on important topics affecting children. The most recent guidelines entitled Management of Children with Chronic Wet Cough and Protracted Bacterial Bronchitis (Chest. 2017;151(4):884-890) and Use of Management Pathways or Algorithms in Children with Chronic Cough (Chest. 2017;151(4):875-873) are updates from the 2006 CHEST guidelines on chronic cough in children.
The present updates utilized the CHEST methodological guidelines with chronic wet or productive cough and Grading of Recommendations Assessment, Development, and Evaluation framework and also performed a systematic review addressing key questions concerning the management of childhood disease for children 14 years and younger.
Guidance provided by the expert panel focused on recommendations to answer six key questions concerning the management of children 14 years and younger with a chronic wet cough unrelated to established chronic lung disease. The recommendations are:
1. Chronic cough is defined as the presence of a cough 4 weeks or longer in duration.
2. Assessment of the effect of the cough on the child and the family be undertaken as part of clinical consultation.
3. Evaluation of a chronic cough should be done with a systematic approach with pediatric-specific cough management protocols or algorithms.
4. Chest radiograph and, when age appropriate, spirometry with bronchodilator be undertaken as evaluation; tests for pertussis infection only to be performed if clinically suspected.
5. Chronic wet cough with no specific clinical features should receive antibiotics for 2 weeks targeted for common respiratory bacteria (Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis).
6. When cough persists despite 2 weeks of appropriate antibiotics, it is recommended to continue for an additional 2 weeks.
7. Additional tests (eg skin prick test, Mantoux, bronchoscopy, chest CT scan) should be individualized in accordance with the clinical setting and child’s clinical symptoms and signs.
The panel recognizes the need for prospective studies to assess current algorithms outcomes of children with chronic cough. Both articles can be found on the guidelines section of the CHEST site.
John Bishara, DO
Fellow-in-Training Member
Pulmonary Physiology, Function, and Rehabilitation
Functional imaging of the lung
Quantifying heterogeneity of ventilation and gas exchange in lung diseases remains a clinical challenge. Conventional pulmonary function test is insensitive to regional changes. The multiple inert gas elimination technique can quantify ventilation-perfusion distribution, but it requires invasive instrumentation (eg, pulmonary artery catheterization) and is not practical for clinical use. Computed tomography (CT) scans delineate spatial changes in lung structures but do not directly measure changes in ventilation and gas exchange. With its radiation, it is difficult to apply CT scanning repeatedly in patients. More recently, MR imaging techniques have been developed to directly “visualize” and quantify regional lung function (Kruger SJ, et al. J Magn Reson Imaging. 2016;43(2):295; Roos JE, et al. Magn Reson Imaging Clin N Am. 2015;23(2):217). These techniques employ inhalation of gases, such as oxygen, perfluorinated gases, and hyperpolarized 3He and 129Xe. Hyperpolarized 3He has been studied the most; however, the dwindling supply of 3He gas and its rising cost have prevented its further development. 129Xe has abundant supply and has emerged to be the inert gas of choice for MR imaging. Hyperpolarized 129Xe can measure ventilation, like hyperpolarized 3He. In addition, Xe diffuses into alveolar barrier (interstitium and plasma) and red blood cells, where it exhibits distinct resonant frequency shifts that can be captured by MR. Therefore, in one test, information on pulmonary ventilation and gas transfer can be obtained. To date, the results from MR imaging studies have provided new insights into the pathophysiology of obstructive and restrictive lung diseases. With continuous development, MR imaging of the lung could become a clinically useful tool in the near future.
Yuh-Chin T. Huang, MD, MHS, FCCP
Steering Committee Member
Thoracic Oncology
Immune-mediated pneumonitis and PD-1 inhibition
Inhibitors of the programmed cell death 1 receptor (PD-1) have shown significant promise in the treatment of advanced stage malignancy. With the recent expansion of indications for use of these agents, the number of patients treated will continue to grow. Clinicians must be aware of their potential for serious adverse side effects, including dermatitis, colitis, and potentially life-threatening pneumonitis.
The development of pneumonitis secondary to PD-1 inhibitions is reported to occur in 2% to 5% of patients and can present at any time during therapy, with 1% of patients developing grade 3 or higher pneumonitis.1,2 The most common symptoms are dyspnea and cough, though one-third of patients are asymptomatic at presentation.2 Radiographic and pathologic features vary greatly and include organizing pneumonia, interstitial pneumonitis, hypersensitivity pneumonitis, or diffuse alveolar damage.3 While pneumonitis due to PD-1 inhibition is reportedly uncommon, the increasing number of patients expected to receive these medications will predictably result in increasing overall frequency of pneumonitis cases. In addition, the lack of large prospective randomized trials and reliance on radiographic rather than pathologic data in diagnosing immune-mediated pneumonitis gives one pause. Given the variability of presentation, lack of routine pathologic data, and increasing use of dual agents (eg, PD-1 and CTLA-4), chest physicians and medical oncologists should have a high index of suspicion yet practice equipoise in patients receiving immunotherapy who develop unexplained pulmonary symptoms or infiltrates. More research is needed to help improve the multidisciplinary diagnosis and treatment of this potentially serious complication.
David Maurice Chambers, MD
Fellow-in-Training Member
Jason Atticus Akulian, MD, MPH
Steering Committee Member
References
1. Nishino M, et al. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncology. 2016;2(12):1607.
2. Naidoo J, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35(7):709.
3. Nishino M, et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res. 2016;22(24):6051.
Pulmonary Vascular Disease
Pulmonary Arterial Hypertension Associated With SLE
While pulmonary arterial hypertension (PAH) commonly complicates scleroderma (SSc), it is a rare complication of other connective tissue diseases (CTD), such as systemic lupus erythematosus (SLE). In the few prospective studies that utilize right-sided heart catheterization (RHC), the estimated prevalence of PAH in SLE is about 4%. However, since the prevalence of SLE is 10 to 15 times greater than SSc in the United States, the true prevalence of SLE-PAH may be higher than previously thought, and, thus, clinically relevant. Despite this, little is known about SLE-PAH.
A recent retrospective study from the French Pulmonary Hypertension Registry has added significantly to our understanding of this complication of SLE. Hachulla and colleagues studied 51 patients with RHC-proven SLE-PAH compared with 101 SLE control subjects without PAH. While the authors did not find any relevant differences in the demographics between groups, they did find a significantly higher prevalence of SSA and SSB antibodies in SLE-PAH. Interestingly, the presence of anti-U1 RNP antibody appeared to be less common in SLE-PAH patients; this lack of association is in contrast to prior studies in mixed CTD patients with anti-U1 RNP antibodies in which the prevalence of PAH can be as high as 60%. Further, none of the SLE-PAH patients demonstrated an acute response to vasodilator challenge during RHC, emphasizing that this maneuver does not need to be performed in SLE patients at risk of PAH. Trends toward improved survival in SLE-PAH patients treated with hydroxychloroquine are preliminary and hypothesis-generating but require confirmation in larger clinical studies.
Stephen Mathai, MD, FCCP
Chair
Leena Palwar, MD
Fellow-in-Training Member
References
Hachulla E, Jais X, Cinquetti G, et al. Pulmonary arterial hypertension associated with SLE: Results from the French pulmonary hypertension registry. Chest. 2017 Aug 26. pii: S0012-3692(17)31430-7. doi: 10.1016/j.chest.2017.08.014. [Epub ahead of print]
Chung L, Liu J, Parsons L, et al. Characterization of connective tissue disease-associated pulmonary arterial hypertension from REVEAL: identifying systemic sclerosis as a unique phenotype. Chest. 2010;138:1383-1394.
Shirai Y, Yasuoka H, Okano Y, Takeuchi T, Satoh T, Kuwana M. Clinical characteristics and survival of Japanese patients with connective tissue disease and pulmonary arterial hypertension: a singlecentre cohort. Rheumatology. 2012;51:1846-1854.
Hao YJ, Jiang X, Zhou W, et al. Connective tissue disease-associated pulmonary arterial hypertension in Chinese patients. Eur Respir J. 2014;44: 963-972.
Huang C, Li M, Liu Y, et al. Baseline characteristics and risk factors of pulmonary arterial hypertension in systemic lupus erythematosus patients. Medicine. 2016;95:e2761.
Pérez-Peñate GM, Rúa-Figueroa I, Juliá- Serdá G, et al. Pulmonary arterial hypertension in systemic lupus erythematosus: prevalence and predictors. J Rheumatol. 2016;43:323-329.
Alpert MA, Goldberg SH, Sindem BH, et al. Cardiovascular manifestations of mixed connective tissue disease in adults. Circulation. 1983;63:1182-1193.
Interventional Chest/Diagnostic Procedures
Cryobiopsy for ILD: Careful stewardship needed
Interest in transbronchial cryobiopsy has accelerated rapidly in recent years. This procedure is performed by advancing a cryoprobe into the peripheral lung via flexible bronchoscopy, where lung tissue freezes and adheres to the probe and is subsequently extracted as a cryobiopsy. The number of cryobiopsy-related publications has increased exponentially since it was described in 2009 (Babiak A, et al. Respiration. 2009;78[2]:203). This interest stems from reports of high diagnostic yields in patients with interstitial lung disease (ILD) while maintaining complication rates similar to that of conventional bronchoscopic biopsy.
Traditional bronchoscopic biopsies are notoriously insensitive; a specific diagnosis can be established in fewer than a third of cases (Sheth JS, et al. Chest. 2017;151[2]:389). As such, surgical lung biopsy continues to be recommended but is associated with significant mortality (2%) and morbidity (30%) in patients with ILD (Hutchinson JP, et al. ARJCCM. 2016;193[10]:1161). Cryobiopsy, which appears to rival surgical lung biopsy in terms of ability to contribute to a specific diagnosis, is, therefore, a highly promising alternative (Tomassetti S, et al. AJRCCM. 2016;193[7]:745).
As cryobiopsy is increasingly adopted around the world, however, troubling reports of serious complications have surfaced. Most notable is the recently reported experience of the initial 25 cases performed at the University of Pennsylvania, in which almost one in four patients suffered serious complications (DiBardino DM, et al. Ann Am Thorac Soc. 2017;14[6]:851). The authors pointed to lack of a predefined procedural protocol, as well as several choices relating to the specific technique used, including inconsistent use of fluoroscopy, lack of prophylactic bronchial blocker placement, and predominant use of laryngeal mask airways as potential contributing factors. Indeed, many variations of the basic cryobiopsy procedure have been described (Lentz RJ, et al. J Thoracic Dis. 2017;9[7]:2186), with no formal guidance or training available to inform advanced bronchoscopists interested in this procedure.
It is incumbent on the interventional pulmonology and ILD specialist communities to be responsible stewards of this promising procedure. Implementation of three parallel efforts to standardize and rigorously study this procedure should be considered as soon as possible: creation of expert consensus guidelines establishing best-practices for safe and effective biopsy technique; a training requirement before independent performance of the procedure; and creation of an international cryobiopsy registry to facilitate higher-quality research into optimal technique and outcomes. We owe this to our patients.
Robert J. Lentz, MD
NetWork Member
Fabien Maldonado, MD, FCCP
NetWork Member
Pediatric Chest Medicine
Chronic cough in children: New guidelines
A chronic cough is a common complaint among children whose parents seek medical evaluation. Chronic wet cough can indicate an underlying illness; therefore, an early diagnosis can lead to prevention of complications of the disease and improvement in quality of life.
CHEST is a leading resource in evidence and consensus-based guidelines on important topics affecting children. The most recent guidelines entitled Management of Children with Chronic Wet Cough and Protracted Bacterial Bronchitis (Chest. 2017;151(4):884-890) and Use of Management Pathways or Algorithms in Children with Chronic Cough (Chest. 2017;151(4):875-873) are updates from the 2006 CHEST guidelines on chronic cough in children.
The present updates utilized the CHEST methodological guidelines with chronic wet or productive cough and Grading of Recommendations Assessment, Development, and Evaluation framework and also performed a systematic review addressing key questions concerning the management of childhood disease for children 14 years and younger.
Guidance provided by the expert panel focused on recommendations to answer six key questions concerning the management of children 14 years and younger with a chronic wet cough unrelated to established chronic lung disease. The recommendations are:
1. Chronic cough is defined as the presence of a cough 4 weeks or longer in duration.
2. Assessment of the effect of the cough on the child and the family be undertaken as part of clinical consultation.
3. Evaluation of a chronic cough should be done with a systematic approach with pediatric-specific cough management protocols or algorithms.
4. Chest radiograph and, when age appropriate, spirometry with bronchodilator be undertaken as evaluation; tests for pertussis infection only to be performed if clinically suspected.
5. Chronic wet cough with no specific clinical features should receive antibiotics for 2 weeks targeted for common respiratory bacteria (Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis).
6. When cough persists despite 2 weeks of appropriate antibiotics, it is recommended to continue for an additional 2 weeks.
7. Additional tests (eg skin prick test, Mantoux, bronchoscopy, chest CT scan) should be individualized in accordance with the clinical setting and child’s clinical symptoms and signs.
The panel recognizes the need for prospective studies to assess current algorithms outcomes of children with chronic cough. Both articles can be found on the guidelines section of the CHEST site.
John Bishara, DO
Fellow-in-Training Member
Pulmonary Physiology, Function, and Rehabilitation
Functional imaging of the lung
Quantifying heterogeneity of ventilation and gas exchange in lung diseases remains a clinical challenge. Conventional pulmonary function test is insensitive to regional changes. The multiple inert gas elimination technique can quantify ventilation-perfusion distribution, but it requires invasive instrumentation (eg, pulmonary artery catheterization) and is not practical for clinical use. Computed tomography (CT) scans delineate spatial changes in lung structures but do not directly measure changes in ventilation and gas exchange. With its radiation, it is difficult to apply CT scanning repeatedly in patients. More recently, MR imaging techniques have been developed to directly “visualize” and quantify regional lung function (Kruger SJ, et al. J Magn Reson Imaging. 2016;43(2):295; Roos JE, et al. Magn Reson Imaging Clin N Am. 2015;23(2):217). These techniques employ inhalation of gases, such as oxygen, perfluorinated gases, and hyperpolarized 3He and 129Xe. Hyperpolarized 3He has been studied the most; however, the dwindling supply of 3He gas and its rising cost have prevented its further development. 129Xe has abundant supply and has emerged to be the inert gas of choice for MR imaging. Hyperpolarized 129Xe can measure ventilation, like hyperpolarized 3He. In addition, Xe diffuses into alveolar barrier (interstitium and plasma) and red blood cells, where it exhibits distinct resonant frequency shifts that can be captured by MR. Therefore, in one test, information on pulmonary ventilation and gas transfer can be obtained. To date, the results from MR imaging studies have provided new insights into the pathophysiology of obstructive and restrictive lung diseases. With continuous development, MR imaging of the lung could become a clinically useful tool in the near future.
Yuh-Chin T. Huang, MD, MHS, FCCP
Steering Committee Member
Thoracic Oncology
Immune-mediated pneumonitis and PD-1 inhibition
Inhibitors of the programmed cell death 1 receptor (PD-1) have shown significant promise in the treatment of advanced stage malignancy. With the recent expansion of indications for use of these agents, the number of patients treated will continue to grow. Clinicians must be aware of their potential for serious adverse side effects, including dermatitis, colitis, and potentially life-threatening pneumonitis.
The development of pneumonitis secondary to PD-1 inhibitions is reported to occur in 2% to 5% of patients and can present at any time during therapy, with 1% of patients developing grade 3 or higher pneumonitis.1,2 The most common symptoms are dyspnea and cough, though one-third of patients are asymptomatic at presentation.2 Radiographic and pathologic features vary greatly and include organizing pneumonia, interstitial pneumonitis, hypersensitivity pneumonitis, or diffuse alveolar damage.3 While pneumonitis due to PD-1 inhibition is reportedly uncommon, the increasing number of patients expected to receive these medications will predictably result in increasing overall frequency of pneumonitis cases. In addition, the lack of large prospective randomized trials and reliance on radiographic rather than pathologic data in diagnosing immune-mediated pneumonitis gives one pause. Given the variability of presentation, lack of routine pathologic data, and increasing use of dual agents (eg, PD-1 and CTLA-4), chest physicians and medical oncologists should have a high index of suspicion yet practice equipoise in patients receiving immunotherapy who develop unexplained pulmonary symptoms or infiltrates. More research is needed to help improve the multidisciplinary diagnosis and treatment of this potentially serious complication.
David Maurice Chambers, MD
Fellow-in-Training Member
Jason Atticus Akulian, MD, MPH
Steering Committee Member
References
1. Nishino M, et al. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncology. 2016;2(12):1607.
2. Naidoo J, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35(7):709.
3. Nishino M, et al. PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res. 2016;22(24):6051.
Pulmonary Vascular Disease
Pulmonary Arterial Hypertension Associated With SLE
While pulmonary arterial hypertension (PAH) commonly complicates scleroderma (SSc), it is a rare complication of other connective tissue diseases (CTD), such as systemic lupus erythematosus (SLE). In the few prospective studies that utilize right-sided heart catheterization (RHC), the estimated prevalence of PAH in SLE is about 4%. However, since the prevalence of SLE is 10 to 15 times greater than SSc in the United States, the true prevalence of SLE-PAH may be higher than previously thought, and, thus, clinically relevant. Despite this, little is known about SLE-PAH.
A recent retrospective study from the French Pulmonary Hypertension Registry has added significantly to our understanding of this complication of SLE. Hachulla and colleagues studied 51 patients with RHC-proven SLE-PAH compared with 101 SLE control subjects without PAH. While the authors did not find any relevant differences in the demographics between groups, they did find a significantly higher prevalence of SSA and SSB antibodies in SLE-PAH. Interestingly, the presence of anti-U1 RNP antibody appeared to be less common in SLE-PAH patients; this lack of association is in contrast to prior studies in mixed CTD patients with anti-U1 RNP antibodies in which the prevalence of PAH can be as high as 60%. Further, none of the SLE-PAH patients demonstrated an acute response to vasodilator challenge during RHC, emphasizing that this maneuver does not need to be performed in SLE patients at risk of PAH. Trends toward improved survival in SLE-PAH patients treated with hydroxychloroquine are preliminary and hypothesis-generating but require confirmation in larger clinical studies.
Stephen Mathai, MD, FCCP
Chair
Leena Palwar, MD
Fellow-in-Training Member
References
Hachulla E, Jais X, Cinquetti G, et al. Pulmonary arterial hypertension associated with SLE: Results from the French pulmonary hypertension registry. Chest. 2017 Aug 26. pii: S0012-3692(17)31430-7. doi: 10.1016/j.chest.2017.08.014. [Epub ahead of print]
Chung L, Liu J, Parsons L, et al. Characterization of connective tissue disease-associated pulmonary arterial hypertension from REVEAL: identifying systemic sclerosis as a unique phenotype. Chest. 2010;138:1383-1394.
Shirai Y, Yasuoka H, Okano Y, Takeuchi T, Satoh T, Kuwana M. Clinical characteristics and survival of Japanese patients with connective tissue disease and pulmonary arterial hypertension: a singlecentre cohort. Rheumatology. 2012;51:1846-1854.
Hao YJ, Jiang X, Zhou W, et al. Connective tissue disease-associated pulmonary arterial hypertension in Chinese patients. Eur Respir J. 2014;44: 963-972.
Huang C, Li M, Liu Y, et al. Baseline characteristics and risk factors of pulmonary arterial hypertension in systemic lupus erythematosus patients. Medicine. 2016;95:e2761.
Pérez-Peñate GM, Rúa-Figueroa I, Juliá- Serdá G, et al. Pulmonary arterial hypertension in systemic lupus erythematosus: prevalence and predictors. J Rheumatol. 2016;43:323-329.
Alpert MA, Goldberg SH, Sindem BH, et al. Cardiovascular manifestations of mixed connective tissue disease in adults. Circulation. 1983;63:1182-1193.
CDC: Forty percent of cancers linked to overweight or obesity
Being overweight or obese significantly increased the risk of developing at least 13 types of cancer, according to a report by the Centers for Disease Control and Prevention.
Now that a larger proportion of the American population is overweight or obese, the rates of obesity-related cancers have increased. Between 2005 and 2014, the rate of obesity-related cancers, excluding colorectal cancer, increased by 7%. Over the same period, non–obesity-related cancers declined, according to C. Brooke Steele, DO, of the CDC’s Division of Cancer Prevention and Control, and her associates (MMWR Morb Mortal Wkly Rep. 2017 Oct 3;66[39]:1052-8).
They found that 631,604 people were diagnosed with an overweight- or obesity-related cancer, 40% of nearly 1.6 million of all cancer diagnoses in 2014. The effect was more pronounced in older people (age at least 50 years), compared with younger people, with two-thirds of cases occurring in the 50- to 74-year-old age range.
Women were much more likely to have overweight- and obesity-related cancers, with higher incidence rates (218.1 per 100,000 population) than those of men (115.0 per 100,000). A contributing factor for this difference between men and women was female-specific cancers such as endometrial, ovarian, and postmenopausal breast cancers, which accounted for 42% (268,091) of overweight- and obesity-related cancers.
Researchers found that, between 2005 and 2014, the overall incidence of overweight- and obesity-related cancers (including colorectal cancer) decreased by 2%, colorectal cancer decreased by 23%, and cancers unrelated to body weight decreased by 13%. A contributing factor to the decrease in colorectal cancer was most likely cancer screening tests, which can detect and lead to the removal of precancerous polyps.
“A majority of American adults weigh more than recommended – and being overweight or obese puts people at higher risk for a number of cancers – so these findings are a cause for concern,” CDC Director Brenda Fitzgerald, MD, said in a statement. “By getting to and keeping a healthy weight, we all can play a role in cancer prevention.”
The researchers had no conflicts of interest to report.
Being overweight or obese significantly increased the risk of developing at least 13 types of cancer, according to a report by the Centers for Disease Control and Prevention.
Now that a larger proportion of the American population is overweight or obese, the rates of obesity-related cancers have increased. Between 2005 and 2014, the rate of obesity-related cancers, excluding colorectal cancer, increased by 7%. Over the same period, non–obesity-related cancers declined, according to C. Brooke Steele, DO, of the CDC’s Division of Cancer Prevention and Control, and her associates (MMWR Morb Mortal Wkly Rep. 2017 Oct 3;66[39]:1052-8).
They found that 631,604 people were diagnosed with an overweight- or obesity-related cancer, 40% of nearly 1.6 million of all cancer diagnoses in 2014. The effect was more pronounced in older people (age at least 50 years), compared with younger people, with two-thirds of cases occurring in the 50- to 74-year-old age range.
Women were much more likely to have overweight- and obesity-related cancers, with higher incidence rates (218.1 per 100,000 population) than those of men (115.0 per 100,000). A contributing factor for this difference between men and women was female-specific cancers such as endometrial, ovarian, and postmenopausal breast cancers, which accounted for 42% (268,091) of overweight- and obesity-related cancers.
Researchers found that, between 2005 and 2014, the overall incidence of overweight- and obesity-related cancers (including colorectal cancer) decreased by 2%, colorectal cancer decreased by 23%, and cancers unrelated to body weight decreased by 13%. A contributing factor to the decrease in colorectal cancer was most likely cancer screening tests, which can detect and lead to the removal of precancerous polyps.
“A majority of American adults weigh more than recommended – and being overweight or obese puts people at higher risk for a number of cancers – so these findings are a cause for concern,” CDC Director Brenda Fitzgerald, MD, said in a statement. “By getting to and keeping a healthy weight, we all can play a role in cancer prevention.”
The researchers had no conflicts of interest to report.
Being overweight or obese significantly increased the risk of developing at least 13 types of cancer, according to a report by the Centers for Disease Control and Prevention.
Now that a larger proportion of the American population is overweight or obese, the rates of obesity-related cancers have increased. Between 2005 and 2014, the rate of obesity-related cancers, excluding colorectal cancer, increased by 7%. Over the same period, non–obesity-related cancers declined, according to C. Brooke Steele, DO, of the CDC’s Division of Cancer Prevention and Control, and her associates (MMWR Morb Mortal Wkly Rep. 2017 Oct 3;66[39]:1052-8).
They found that 631,604 people were diagnosed with an overweight- or obesity-related cancer, 40% of nearly 1.6 million of all cancer diagnoses in 2014. The effect was more pronounced in older people (age at least 50 years), compared with younger people, with two-thirds of cases occurring in the 50- to 74-year-old age range.
Women were much more likely to have overweight- and obesity-related cancers, with higher incidence rates (218.1 per 100,000 population) than those of men (115.0 per 100,000). A contributing factor for this difference between men and women was female-specific cancers such as endometrial, ovarian, and postmenopausal breast cancers, which accounted for 42% (268,091) of overweight- and obesity-related cancers.
Researchers found that, between 2005 and 2014, the overall incidence of overweight- and obesity-related cancers (including colorectal cancer) decreased by 2%, colorectal cancer decreased by 23%, and cancers unrelated to body weight decreased by 13%. A contributing factor to the decrease in colorectal cancer was most likely cancer screening tests, which can detect and lead to the removal of precancerous polyps.
“A majority of American adults weigh more than recommended – and being overweight or obese puts people at higher risk for a number of cancers – so these findings are a cause for concern,” CDC Director Brenda Fitzgerald, MD, said in a statement. “By getting to and keeping a healthy weight, we all can play a role in cancer prevention.”
The researchers had no conflicts of interest to report.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Key clinical point:
Major finding: In the United States, more than 631,000 patients received cancer diagnoses related to overweight or obesity, representing 40% of nearly 1.6 million cancer diagnoses in 2014.
Data source: An analysis of United States Cancer Statistics data from 2005 to 2014.
Disclosures: The researchers had no conflicts of interest to report.
Don't just blame Facebook for fake news: It's us, too
Critics such as Zeynep Tufekci are quite right to take Facebook and Twitter to task for allowing nefarious and hostile actors, likely including the Russian state, to hold sway on social media (“Facebook’s Ad Scandal Isn’t a ‘Fail,’ It’s a Feature,” New York Times, Sept. 23, 2017).
These actors must be reined in online, for the very simple reason that so many of us are immersed in our news feeds and Twitter streams, and thus susceptible to whatever toxins are allowed to proliferate there.
Some have called apps, including social media, “the race to the bottom of the brainstem.” The most primitive parts of our brain, responsible for fight-flight and survival, are always on the lookout for perceived threats. In our restless, clicking and scrolling state, we are often naturally attracted to and activated by tantalizing, titillating, and emotionally charged posts that are lacking in reason, but are inflammatory and viscerally captivating. Viral news, click bait, gossip, rumors, and scandals get a disproportionate share of our attention. And since we become what we do, our reason is overwhelmed by reaction. In fact, a recent 60 Minutes segment demonstrated how President Trump’s political operatives optimized Facebook ads for reaction, not thought. As we engage in social media, we may become less reasonable, and prone to reacting out of our most base and primal fears.
The late neurologist and psychiatrist Viktor E. Frankl pointed out our freedom lies in our ability to put space between stimulus and response. In this space lies our freedom to choose, he wrote. Online, this inner life can shrink. Our wandering minds look for cheap thrills and anything that confirms our biases of threat. We reduce the world to simplistic one-size-fits-all narratives, and heap blame on our usual suspects. I call the Internet the angernet. Anger is the most viral and contagious emotion on social networks, and as we use social media, we deepen the groove of anger – making our anger more likely and the most readily shared.
But we’ve been racing to the bottom of the brainstem, where fear, anger, hatred, and reactivity reside, for decades in this country. Again, we can’t simply blame social media. Trust has been eroding for decades, for many reasons. No matter the generation, only a minority of us trust each other, according to the Pew Research Center’s surveys. Millennials are the least trusting of all: Only 19% of them agree that “generally speaking, people are to be trusted.” Any scroll of the news feed quickly confirms this dangerous bias to mistrust, as we are besieged by a near cataclysmic barrage of traumatic events. We are also more polarized than ever, with a great percentage of partisans believing that the other side aims at the destruction of all they hold dear in America. As we feel more vulnerable, we are more likely to mistrust and become further polarized, and the cycle continues.
More importantly, as we flee real-world relationships and interaction for the ease and seeming reach of the online world, we lose subtle cues and important connections that would normally soothe our reactivity. Humanity has evolved and progressed precisely because of our social connections. We are who happens to us, and what we make of the happening. When we connect in more superficial ways, we endanger our very identities. As a nation, we are having an identity crisis: We have not worked out who we are in relation to one another, and to ourselves. This is expressed politically, and in our confused and fearful opinions and appraisals of each other and government. Without the good graces that come out of shared presence in real-world relationships, we are more likely to think badly of each other. We are more likely to retreat into narrow and tribal identities where we think safety resides, and deemphasize communal identity. In this space of narrow identity, ideological self-righteousness and dogma prevail, and we are less likely to listen and cultivate curiosity, understanding, and empathy for others. One example lies in how representatives and senators spend less time socializing with each other than in years past, and are thus less likely to forge compromise. Online, we are all refugees from the real world, and we fall to building our own personal walls and exiling those with whom we disagree – who then become those we hate, fear, blame, and fight.
We have met the enemy, and it is us.
The antidote to mistrust is in cultivating the top of our nervous systems, where long-term planning and positive emotions reside. We can only do this through real-world relationships. Social media will not ultimately take us to a feeling of society. It might provide a glimmer of connection, but its inherent vectors of disconnection and division are proving deadly, from Internet radicalization, to the increased levels of depression, anxiety, loneliness, and suicidality noted with increasing Internet and social media use.
We each have incredible human possibilities, but to reach them, we have to transcend the biases and reactivity of our habitually and usually unconsciously self-centered egos. We can start with awareness of what the Internet siren is doing to us and steer our ships in a more fruitful direction. Toward home, toward our hearts, and toward each other.
Dr. Chandra is a psychiatrist and writer in San Francisco. He is the author of Facebuddha: Transcendence in the Age of Social Networks (Pacific Heart Books, 2017).
Critics such as Zeynep Tufekci are quite right to take Facebook and Twitter to task for allowing nefarious and hostile actors, likely including the Russian state, to hold sway on social media (“Facebook’s Ad Scandal Isn’t a ‘Fail,’ It’s a Feature,” New York Times, Sept. 23, 2017).
These actors must be reined in online, for the very simple reason that so many of us are immersed in our news feeds and Twitter streams, and thus susceptible to whatever toxins are allowed to proliferate there.
Some have called apps, including social media, “the race to the bottom of the brainstem.” The most primitive parts of our brain, responsible for fight-flight and survival, are always on the lookout for perceived threats. In our restless, clicking and scrolling state, we are often naturally attracted to and activated by tantalizing, titillating, and emotionally charged posts that are lacking in reason, but are inflammatory and viscerally captivating. Viral news, click bait, gossip, rumors, and scandals get a disproportionate share of our attention. And since we become what we do, our reason is overwhelmed by reaction. In fact, a recent 60 Minutes segment demonstrated how President Trump’s political operatives optimized Facebook ads for reaction, not thought. As we engage in social media, we may become less reasonable, and prone to reacting out of our most base and primal fears.
The late neurologist and psychiatrist Viktor E. Frankl pointed out our freedom lies in our ability to put space between stimulus and response. In this space lies our freedom to choose, he wrote. Online, this inner life can shrink. Our wandering minds look for cheap thrills and anything that confirms our biases of threat. We reduce the world to simplistic one-size-fits-all narratives, and heap blame on our usual suspects. I call the Internet the angernet. Anger is the most viral and contagious emotion on social networks, and as we use social media, we deepen the groove of anger – making our anger more likely and the most readily shared.
But we’ve been racing to the bottom of the brainstem, where fear, anger, hatred, and reactivity reside, for decades in this country. Again, we can’t simply blame social media. Trust has been eroding for decades, for many reasons. No matter the generation, only a minority of us trust each other, according to the Pew Research Center’s surveys. Millennials are the least trusting of all: Only 19% of them agree that “generally speaking, people are to be trusted.” Any scroll of the news feed quickly confirms this dangerous bias to mistrust, as we are besieged by a near cataclysmic barrage of traumatic events. We are also more polarized than ever, with a great percentage of partisans believing that the other side aims at the destruction of all they hold dear in America. As we feel more vulnerable, we are more likely to mistrust and become further polarized, and the cycle continues.
More importantly, as we flee real-world relationships and interaction for the ease and seeming reach of the online world, we lose subtle cues and important connections that would normally soothe our reactivity. Humanity has evolved and progressed precisely because of our social connections. We are who happens to us, and what we make of the happening. When we connect in more superficial ways, we endanger our very identities. As a nation, we are having an identity crisis: We have not worked out who we are in relation to one another, and to ourselves. This is expressed politically, and in our confused and fearful opinions and appraisals of each other and government. Without the good graces that come out of shared presence in real-world relationships, we are more likely to think badly of each other. We are more likely to retreat into narrow and tribal identities where we think safety resides, and deemphasize communal identity. In this space of narrow identity, ideological self-righteousness and dogma prevail, and we are less likely to listen and cultivate curiosity, understanding, and empathy for others. One example lies in how representatives and senators spend less time socializing with each other than in years past, and are thus less likely to forge compromise. Online, we are all refugees from the real world, and we fall to building our own personal walls and exiling those with whom we disagree – who then become those we hate, fear, blame, and fight.
We have met the enemy, and it is us.
The antidote to mistrust is in cultivating the top of our nervous systems, where long-term planning and positive emotions reside. We can only do this through real-world relationships. Social media will not ultimately take us to a feeling of society. It might provide a glimmer of connection, but its inherent vectors of disconnection and division are proving deadly, from Internet radicalization, to the increased levels of depression, anxiety, loneliness, and suicidality noted with increasing Internet and social media use.
We each have incredible human possibilities, but to reach them, we have to transcend the biases and reactivity of our habitually and usually unconsciously self-centered egos. We can start with awareness of what the Internet siren is doing to us and steer our ships in a more fruitful direction. Toward home, toward our hearts, and toward each other.
Dr. Chandra is a psychiatrist and writer in San Francisco. He is the author of Facebuddha: Transcendence in the Age of Social Networks (Pacific Heart Books, 2017).
Critics such as Zeynep Tufekci are quite right to take Facebook and Twitter to task for allowing nefarious and hostile actors, likely including the Russian state, to hold sway on social media (“Facebook’s Ad Scandal Isn’t a ‘Fail,’ It’s a Feature,” New York Times, Sept. 23, 2017).
These actors must be reined in online, for the very simple reason that so many of us are immersed in our news feeds and Twitter streams, and thus susceptible to whatever toxins are allowed to proliferate there.
Some have called apps, including social media, “the race to the bottom of the brainstem.” The most primitive parts of our brain, responsible for fight-flight and survival, are always on the lookout for perceived threats. In our restless, clicking and scrolling state, we are often naturally attracted to and activated by tantalizing, titillating, and emotionally charged posts that are lacking in reason, but are inflammatory and viscerally captivating. Viral news, click bait, gossip, rumors, and scandals get a disproportionate share of our attention. And since we become what we do, our reason is overwhelmed by reaction. In fact, a recent 60 Minutes segment demonstrated how President Trump’s political operatives optimized Facebook ads for reaction, not thought. As we engage in social media, we may become less reasonable, and prone to reacting out of our most base and primal fears.
The late neurologist and psychiatrist Viktor E. Frankl pointed out our freedom lies in our ability to put space between stimulus and response. In this space lies our freedom to choose, he wrote. Online, this inner life can shrink. Our wandering minds look for cheap thrills and anything that confirms our biases of threat. We reduce the world to simplistic one-size-fits-all narratives, and heap blame on our usual suspects. I call the Internet the angernet. Anger is the most viral and contagious emotion on social networks, and as we use social media, we deepen the groove of anger – making our anger more likely and the most readily shared.
But we’ve been racing to the bottom of the brainstem, where fear, anger, hatred, and reactivity reside, for decades in this country. Again, we can’t simply blame social media. Trust has been eroding for decades, for many reasons. No matter the generation, only a minority of us trust each other, according to the Pew Research Center’s surveys. Millennials are the least trusting of all: Only 19% of them agree that “generally speaking, people are to be trusted.” Any scroll of the news feed quickly confirms this dangerous bias to mistrust, as we are besieged by a near cataclysmic barrage of traumatic events. We are also more polarized than ever, with a great percentage of partisans believing that the other side aims at the destruction of all they hold dear in America. As we feel more vulnerable, we are more likely to mistrust and become further polarized, and the cycle continues.
More importantly, as we flee real-world relationships and interaction for the ease and seeming reach of the online world, we lose subtle cues and important connections that would normally soothe our reactivity. Humanity has evolved and progressed precisely because of our social connections. We are who happens to us, and what we make of the happening. When we connect in more superficial ways, we endanger our very identities. As a nation, we are having an identity crisis: We have not worked out who we are in relation to one another, and to ourselves. This is expressed politically, and in our confused and fearful opinions and appraisals of each other and government. Without the good graces that come out of shared presence in real-world relationships, we are more likely to think badly of each other. We are more likely to retreat into narrow and tribal identities where we think safety resides, and deemphasize communal identity. In this space of narrow identity, ideological self-righteousness and dogma prevail, and we are less likely to listen and cultivate curiosity, understanding, and empathy for others. One example lies in how representatives and senators spend less time socializing with each other than in years past, and are thus less likely to forge compromise. Online, we are all refugees from the real world, and we fall to building our own personal walls and exiling those with whom we disagree – who then become those we hate, fear, blame, and fight.
We have met the enemy, and it is us.
The antidote to mistrust is in cultivating the top of our nervous systems, where long-term planning and positive emotions reside. We can only do this through real-world relationships. Social media will not ultimately take us to a feeling of society. It might provide a glimmer of connection, but its inherent vectors of disconnection and division are proving deadly, from Internet radicalization, to the increased levels of depression, anxiety, loneliness, and suicidality noted with increasing Internet and social media use.
We each have incredible human possibilities, but to reach them, we have to transcend the biases and reactivity of our habitually and usually unconsciously self-centered egos. We can start with awareness of what the Internet siren is doing to us and steer our ships in a more fruitful direction. Toward home, toward our hearts, and toward each other.
Dr. Chandra is a psychiatrist and writer in San Francisco. He is the author of Facebuddha: Transcendence in the Age of Social Networks (Pacific Heart Books, 2017).
This Month in CHEST Editor’s Picks
Changes to CPT® codes coming January 2018
There will be a number of changes to Current Procedural Terminology (CPT®) codes of interest to pulmonary/critical care providers in January 2018. A thorough understanding of these changes is important for appropriate coding and reimbursement for the services described by these codes.
There are two changes in the CPT codes for bronchoscopy involving 31645 and 31646. CPT code 31645 describes a therapeutic bronchoscopy, eg, removal of viscous, copious or tenacious secretions from the airway. It had previously included wording that suggested it was used for abscess drainage, and this has been removed. If a therapeutic bronchoscopy procedure is repeated during the same hospital stay, then CPT code 31646 should be utilized. If a therapeutic bronchoscopy procedure is performed in the non-hospital setting and later repeated, then CPT code 31645 would be used for both procedures.
CPT code 94620 Pulmonary stress testing; simple (eg, 6-minute walk test, prolonged exercise test for bronchospasm with pre- and post-spirometry and oximetry) has been deleted and replaced by two new codes. CPT code 94617 Exercise test for bronchospasm, including pre- and postspirometry, electrocardiographic recording(s), and pulse oximetry describes the procedure used to assess for exercise-induced bronchospasm. CPT code 94618 Pulmonary stress testing (eg, 6-minute walk test), including measurement of heart rate, oximetry, and oxygen titration, when performed, describes the typical simple pulmonary stress test. After January 1, 2018, if CPT code 94620 is used, the claim will be denied. CPT code 94621 Cardiopulmonary exercise testing, including measurements of minute ventilation, CO2 production, O2 uptake, and electrocardiographic recordings has been reworded to better describe the procedure of cardiopulmonary exercise testing. Additionally, there are numerous parentheticals appended that list the CPT codes that may not be used in conjunction with 94617, 94618, and 94621. Please refer to the 2018 CPT manual for further information on these exclusions.
There will be a number of changes to Current Procedural Terminology (CPT®) codes of interest to pulmonary/critical care providers in January 2018. A thorough understanding of these changes is important for appropriate coding and reimbursement for the services described by these codes.
There are two changes in the CPT codes for bronchoscopy involving 31645 and 31646. CPT code 31645 describes a therapeutic bronchoscopy, eg, removal of viscous, copious or tenacious secretions from the airway. It had previously included wording that suggested it was used for abscess drainage, and this has been removed. If a therapeutic bronchoscopy procedure is repeated during the same hospital stay, then CPT code 31646 should be utilized. If a therapeutic bronchoscopy procedure is performed in the non-hospital setting and later repeated, then CPT code 31645 would be used for both procedures.
CPT code 94620 Pulmonary stress testing; simple (eg, 6-minute walk test, prolonged exercise test for bronchospasm with pre- and post-spirometry and oximetry) has been deleted and replaced by two new codes. CPT code 94617 Exercise test for bronchospasm, including pre- and postspirometry, electrocardiographic recording(s), and pulse oximetry describes the procedure used to assess for exercise-induced bronchospasm. CPT code 94618 Pulmonary stress testing (eg, 6-minute walk test), including measurement of heart rate, oximetry, and oxygen titration, when performed, describes the typical simple pulmonary stress test. After January 1, 2018, if CPT code 94620 is used, the claim will be denied. CPT code 94621 Cardiopulmonary exercise testing, including measurements of minute ventilation, CO2 production, O2 uptake, and electrocardiographic recordings has been reworded to better describe the procedure of cardiopulmonary exercise testing. Additionally, there are numerous parentheticals appended that list the CPT codes that may not be used in conjunction with 94617, 94618, and 94621. Please refer to the 2018 CPT manual for further information on these exclusions.
There will be a number of changes to Current Procedural Terminology (CPT®) codes of interest to pulmonary/critical care providers in January 2018. A thorough understanding of these changes is important for appropriate coding and reimbursement for the services described by these codes.
There are two changes in the CPT codes for bronchoscopy involving 31645 and 31646. CPT code 31645 describes a therapeutic bronchoscopy, eg, removal of viscous, copious or tenacious secretions from the airway. It had previously included wording that suggested it was used for abscess drainage, and this has been removed. If a therapeutic bronchoscopy procedure is repeated during the same hospital stay, then CPT code 31646 should be utilized. If a therapeutic bronchoscopy procedure is performed in the non-hospital setting and later repeated, then CPT code 31645 would be used for both procedures.
CPT code 94620 Pulmonary stress testing; simple (eg, 6-minute walk test, prolonged exercise test for bronchospasm with pre- and post-spirometry and oximetry) has been deleted and replaced by two new codes. CPT code 94617 Exercise test for bronchospasm, including pre- and postspirometry, electrocardiographic recording(s), and pulse oximetry describes the procedure used to assess for exercise-induced bronchospasm. CPT code 94618 Pulmonary stress testing (eg, 6-minute walk test), including measurement of heart rate, oximetry, and oxygen titration, when performed, describes the typical simple pulmonary stress test. After January 1, 2018, if CPT code 94620 is used, the claim will be denied. CPT code 94621 Cardiopulmonary exercise testing, including measurements of minute ventilation, CO2 production, O2 uptake, and electrocardiographic recordings has been reworded to better describe the procedure of cardiopulmonary exercise testing. Additionally, there are numerous parentheticals appended that list the CPT codes that may not be used in conjunction with 94617, 94618, and 94621. Please refer to the 2018 CPT manual for further information on these exclusions.
Evidence is mixed on probiotics in pediatric patients
CHICAGO – Outside of that, things are less clear.
“In terms of diarrhea, the evidence is positive, but probiotics only provide about 25 hours of benefit. And treatment of antibiotic-associated diarrhea is really dependent on patient adherence,” said Michael D. Cabana, MD. When it comes to treating colic, there is a particular probiotic that looks promising, he added, but the research so far demonstrating effectiveness is limited to breastfed babies. Also, the probiotic therapy appears to work best when started relatively early.
You are very likely to be asked your take on probiotics for a wide range of conditions, Dr. Cabana said, Overall, however, skepticism is warranted. Advise patients and families to be aware of advertising that promotes many different products as “probiotic,” especially around claims of improved “gut health” or “balanced microbiota.” He emphasized: “Make sure what your patients are using has some evidence behind it.”
Knowing the particular probiotic strain is essential to researching the evidence around its use, said Dr. Cabana, professor of pediatrics at the University of California, San Francisco. “I used the Canis familiaris example. All dogs are C. familiaris. But there are different breeds. You want to make sure you match the right breed to the task. If you were in an avalanche in the Swiss Alps, you would want a St. Bernard to rescue you, not a Chihuahua,” he said. “Similarly, when you are using probiotics you want to make sure you have the right strain, not just the genus and species.” For example, if a product label states it contains Bifidobacterium breve C50, the “C50” is the strain.
Another tip is to look for labeling that lists probiotic concentrations in colony-forming units or CFUs, Dr. Cabana said. He’s seen concentrations listed in mg, a red flag that a product is not legitimate.
Families also might ask if it’s better to take a probiotic supplement or choose food that contains probiotics. “Food products offer additional nutritional benefits, but you can give a relatively higher dose with supplements with a much lower volume ingested,” Dr. Cabana said. “And supplements theoretically provide a more consistent dose.” Speaking of dose, it’s difficult to counsel patients on dosing and frequency in general because probiotics really vary by the indication and formulation.
“As a pediatrician, I also get this question: Should kids get a lower dose of probiotic?” Dr. Cabana said. There are no known reports of toxicity associated with probiotic use in either adults or children, he said. “Unless a dose modification has been documented in a clinical trial, it is not clear that this is necessary. You’re just giving less of the probiotic.”
Treating diarrhea and antibiotic-associated diarrhea
When it comes to probiotics for treating acute diarrhea in children, “the literature is actually fairly good here,” Dr. Cabana said. More than 60 studies with an excess of 8,000 participants, the majority with rotavirus infection, suggests probiotics are not associated with any adverse effects and generally shorten duration of diarrhea.
In fact, Dr. Cabana added, multiple meta-analyses support a shorter course of diarrhea. He added, “Look at the units here – it’s hours, not days. You can treat, but on average it’s only 25 hours.” He added that a day less of diarrhea can be significant for patients and parents, however.
In another meta-analysis probiotics, particularly Lactobacillus strains, were analyzed for prevention of antibiotic-associated diarrhea (JAMA. 2012 May 9;307[18]:1959-69). Researchers assessed 63 randomized controlled trials with nearly 12,000 participants. The pooled results showed a statistically significant positive reduction in antibiotic-associated diarrhea (relative risk, 0.58; P less than .001). “Note the number needed to treat to see the effect is 13, so it won’t work in every patient,” Dr. Cabana said.
“So prevention of antibiotic-associated diarrhea is well documented. However, it’s also highly dependent on patent adherence,” he emphasized.
The clinical evidence on colic
For treating babies with colic, the best evidence is behind use of Lactobacilus reuteri DSM 17938, Dr. Cabana said. It tends to work best in breastfed infants, babies not on any gastrointestinal meds, and babies that start therapy early in the course of symptoms. “Use in formula-fed infants is unknown, because there are not enough data so far,” he said.
In some cases, during a prenatal visit, soon-to-be-parents will ask if they should start a probiotic to prevent colic. Dr. Cabana has seen only one prophylaxis study for this indication (JAMA Pediatr. 2014 Mar;168[3]:228-33). In the study, 589 infants were randomly allocated to take L. reuteri DSM 17938 or placebo daily for 90 days. At 3 months of age, the researchers discovered a significantly shorter mean duration of daily crying in the probiotic group (38 vs. 71 minutes; P less than .01).
What’s known about efficacy for eczema
The evidence for treating a child who presents with eczema with probiotics does not support efficacy in general, Dr. Cabana said. And the evidence on prevention of atopic eczema is mixed.
For example, in a randomized, controlled study from Finland, investigators randomized mothers to receive Lactobacillus GG or placebo during the prenatal period (Lancet. 2001;357:1076-9). Of 132 of the children, 35% were later diagnosed with atopic eczema, and the rate in the probiotic group, 23%, was half the 46% rate in the placebo group.
In contrast, researchers found no benefit regarding prevention of atopic dermatitis when 105 pregnant women were randomized to Lactobacillus GG or placebo. At the age of 2 years, atopic dermatitis was diagnosed in 28% of the 50 children in the probiotic group and 27.3% of the 44 in the placebo group (Pediatrics. 2008;121:e850-6).
The region of Germany where the study was conducted was rural/agricultural, so the diet could be different, Dr. Cabana said. Also, the median duration of breastfeeding differed between the Finnish and German study population, 6.8 months versus 9.2 months, respectively. “So that could potentially explain it, or there are just differences that cannot be explained.”
For more information, Dr. Cabana recommended information provided by the International Scientific Association of Prebiotics & Probiotics (https://isappscience.org/infographics/). The association’s website has easy to understand infographics including: What are probiotics and what can they do for you?; What’s so special about fermented foods?; and How do you read a probiotic label?
Dr. Cabana reported he receives research support from the National Institutes of Health, Wyeth Nutrition, and Nestle; is on the speakers bureau for Merck; owns stocks or bonds in Abbot and AbbVie; and is a consultant for Mead Johnson, Abbott, Genentech, Biogaia, General Mills, and Nestle.
CHICAGO – Outside of that, things are less clear.
“In terms of diarrhea, the evidence is positive, but probiotics only provide about 25 hours of benefit. And treatment of antibiotic-associated diarrhea is really dependent on patient adherence,” said Michael D. Cabana, MD. When it comes to treating colic, there is a particular probiotic that looks promising, he added, but the research so far demonstrating effectiveness is limited to breastfed babies. Also, the probiotic therapy appears to work best when started relatively early.
You are very likely to be asked your take on probiotics for a wide range of conditions, Dr. Cabana said, Overall, however, skepticism is warranted. Advise patients and families to be aware of advertising that promotes many different products as “probiotic,” especially around claims of improved “gut health” or “balanced microbiota.” He emphasized: “Make sure what your patients are using has some evidence behind it.”
Knowing the particular probiotic strain is essential to researching the evidence around its use, said Dr. Cabana, professor of pediatrics at the University of California, San Francisco. “I used the Canis familiaris example. All dogs are C. familiaris. But there are different breeds. You want to make sure you match the right breed to the task. If you were in an avalanche in the Swiss Alps, you would want a St. Bernard to rescue you, not a Chihuahua,” he said. “Similarly, when you are using probiotics you want to make sure you have the right strain, not just the genus and species.” For example, if a product label states it contains Bifidobacterium breve C50, the “C50” is the strain.
Another tip is to look for labeling that lists probiotic concentrations in colony-forming units or CFUs, Dr. Cabana said. He’s seen concentrations listed in mg, a red flag that a product is not legitimate.
Families also might ask if it’s better to take a probiotic supplement or choose food that contains probiotics. “Food products offer additional nutritional benefits, but you can give a relatively higher dose with supplements with a much lower volume ingested,” Dr. Cabana said. “And supplements theoretically provide a more consistent dose.” Speaking of dose, it’s difficult to counsel patients on dosing and frequency in general because probiotics really vary by the indication and formulation.
“As a pediatrician, I also get this question: Should kids get a lower dose of probiotic?” Dr. Cabana said. There are no known reports of toxicity associated with probiotic use in either adults or children, he said. “Unless a dose modification has been documented in a clinical trial, it is not clear that this is necessary. You’re just giving less of the probiotic.”
Treating diarrhea and antibiotic-associated diarrhea
When it comes to probiotics for treating acute diarrhea in children, “the literature is actually fairly good here,” Dr. Cabana said. More than 60 studies with an excess of 8,000 participants, the majority with rotavirus infection, suggests probiotics are not associated with any adverse effects and generally shorten duration of diarrhea.
In fact, Dr. Cabana added, multiple meta-analyses support a shorter course of diarrhea. He added, “Look at the units here – it’s hours, not days. You can treat, but on average it’s only 25 hours.” He added that a day less of diarrhea can be significant for patients and parents, however.
In another meta-analysis probiotics, particularly Lactobacillus strains, were analyzed for prevention of antibiotic-associated diarrhea (JAMA. 2012 May 9;307[18]:1959-69). Researchers assessed 63 randomized controlled trials with nearly 12,000 participants. The pooled results showed a statistically significant positive reduction in antibiotic-associated diarrhea (relative risk, 0.58; P less than .001). “Note the number needed to treat to see the effect is 13, so it won’t work in every patient,” Dr. Cabana said.
“So prevention of antibiotic-associated diarrhea is well documented. However, it’s also highly dependent on patent adherence,” he emphasized.
The clinical evidence on colic
For treating babies with colic, the best evidence is behind use of Lactobacilus reuteri DSM 17938, Dr. Cabana said. It tends to work best in breastfed infants, babies not on any gastrointestinal meds, and babies that start therapy early in the course of symptoms. “Use in formula-fed infants is unknown, because there are not enough data so far,” he said.
In some cases, during a prenatal visit, soon-to-be-parents will ask if they should start a probiotic to prevent colic. Dr. Cabana has seen only one prophylaxis study for this indication (JAMA Pediatr. 2014 Mar;168[3]:228-33). In the study, 589 infants were randomly allocated to take L. reuteri DSM 17938 or placebo daily for 90 days. At 3 months of age, the researchers discovered a significantly shorter mean duration of daily crying in the probiotic group (38 vs. 71 minutes; P less than .01).
What’s known about efficacy for eczema
The evidence for treating a child who presents with eczema with probiotics does not support efficacy in general, Dr. Cabana said. And the evidence on prevention of atopic eczema is mixed.
For example, in a randomized, controlled study from Finland, investigators randomized mothers to receive Lactobacillus GG or placebo during the prenatal period (Lancet. 2001;357:1076-9). Of 132 of the children, 35% were later diagnosed with atopic eczema, and the rate in the probiotic group, 23%, was half the 46% rate in the placebo group.
In contrast, researchers found no benefit regarding prevention of atopic dermatitis when 105 pregnant women were randomized to Lactobacillus GG or placebo. At the age of 2 years, atopic dermatitis was diagnosed in 28% of the 50 children in the probiotic group and 27.3% of the 44 in the placebo group (Pediatrics. 2008;121:e850-6).
The region of Germany where the study was conducted was rural/agricultural, so the diet could be different, Dr. Cabana said. Also, the median duration of breastfeeding differed between the Finnish and German study population, 6.8 months versus 9.2 months, respectively. “So that could potentially explain it, or there are just differences that cannot be explained.”
For more information, Dr. Cabana recommended information provided by the International Scientific Association of Prebiotics & Probiotics (https://isappscience.org/infographics/). The association’s website has easy to understand infographics including: What are probiotics and what can they do for you?; What’s so special about fermented foods?; and How do you read a probiotic label?
Dr. Cabana reported he receives research support from the National Institutes of Health, Wyeth Nutrition, and Nestle; is on the speakers bureau for Merck; owns stocks or bonds in Abbot and AbbVie; and is a consultant for Mead Johnson, Abbott, Genentech, Biogaia, General Mills, and Nestle.
CHICAGO – Outside of that, things are less clear.
“In terms of diarrhea, the evidence is positive, but probiotics only provide about 25 hours of benefit. And treatment of antibiotic-associated diarrhea is really dependent on patient adherence,” said Michael D. Cabana, MD. When it comes to treating colic, there is a particular probiotic that looks promising, he added, but the research so far demonstrating effectiveness is limited to breastfed babies. Also, the probiotic therapy appears to work best when started relatively early.
You are very likely to be asked your take on probiotics for a wide range of conditions, Dr. Cabana said, Overall, however, skepticism is warranted. Advise patients and families to be aware of advertising that promotes many different products as “probiotic,” especially around claims of improved “gut health” or “balanced microbiota.” He emphasized: “Make sure what your patients are using has some evidence behind it.”
Knowing the particular probiotic strain is essential to researching the evidence around its use, said Dr. Cabana, professor of pediatrics at the University of California, San Francisco. “I used the Canis familiaris example. All dogs are C. familiaris. But there are different breeds. You want to make sure you match the right breed to the task. If you were in an avalanche in the Swiss Alps, you would want a St. Bernard to rescue you, not a Chihuahua,” he said. “Similarly, when you are using probiotics you want to make sure you have the right strain, not just the genus and species.” For example, if a product label states it contains Bifidobacterium breve C50, the “C50” is the strain.
Another tip is to look for labeling that lists probiotic concentrations in colony-forming units or CFUs, Dr. Cabana said. He’s seen concentrations listed in mg, a red flag that a product is not legitimate.
Families also might ask if it’s better to take a probiotic supplement or choose food that contains probiotics. “Food products offer additional nutritional benefits, but you can give a relatively higher dose with supplements with a much lower volume ingested,” Dr. Cabana said. “And supplements theoretically provide a more consistent dose.” Speaking of dose, it’s difficult to counsel patients on dosing and frequency in general because probiotics really vary by the indication and formulation.
“As a pediatrician, I also get this question: Should kids get a lower dose of probiotic?” Dr. Cabana said. There are no known reports of toxicity associated with probiotic use in either adults or children, he said. “Unless a dose modification has been documented in a clinical trial, it is not clear that this is necessary. You’re just giving less of the probiotic.”
Treating diarrhea and antibiotic-associated diarrhea
When it comes to probiotics for treating acute diarrhea in children, “the literature is actually fairly good here,” Dr. Cabana said. More than 60 studies with an excess of 8,000 participants, the majority with rotavirus infection, suggests probiotics are not associated with any adverse effects and generally shorten duration of diarrhea.
In fact, Dr. Cabana added, multiple meta-analyses support a shorter course of diarrhea. He added, “Look at the units here – it’s hours, not days. You can treat, but on average it’s only 25 hours.” He added that a day less of diarrhea can be significant for patients and parents, however.
In another meta-analysis probiotics, particularly Lactobacillus strains, were analyzed for prevention of antibiotic-associated diarrhea (JAMA. 2012 May 9;307[18]:1959-69). Researchers assessed 63 randomized controlled trials with nearly 12,000 participants. The pooled results showed a statistically significant positive reduction in antibiotic-associated diarrhea (relative risk, 0.58; P less than .001). “Note the number needed to treat to see the effect is 13, so it won’t work in every patient,” Dr. Cabana said.
“So prevention of antibiotic-associated diarrhea is well documented. However, it’s also highly dependent on patent adherence,” he emphasized.
The clinical evidence on colic
For treating babies with colic, the best evidence is behind use of Lactobacilus reuteri DSM 17938, Dr. Cabana said. It tends to work best in breastfed infants, babies not on any gastrointestinal meds, and babies that start therapy early in the course of symptoms. “Use in formula-fed infants is unknown, because there are not enough data so far,” he said.
In some cases, during a prenatal visit, soon-to-be-parents will ask if they should start a probiotic to prevent colic. Dr. Cabana has seen only one prophylaxis study for this indication (JAMA Pediatr. 2014 Mar;168[3]:228-33). In the study, 589 infants were randomly allocated to take L. reuteri DSM 17938 or placebo daily for 90 days. At 3 months of age, the researchers discovered a significantly shorter mean duration of daily crying in the probiotic group (38 vs. 71 minutes; P less than .01).
What’s known about efficacy for eczema
The evidence for treating a child who presents with eczema with probiotics does not support efficacy in general, Dr. Cabana said. And the evidence on prevention of atopic eczema is mixed.
For example, in a randomized, controlled study from Finland, investigators randomized mothers to receive Lactobacillus GG or placebo during the prenatal period (Lancet. 2001;357:1076-9). Of 132 of the children, 35% were later diagnosed with atopic eczema, and the rate in the probiotic group, 23%, was half the 46% rate in the placebo group.
In contrast, researchers found no benefit regarding prevention of atopic dermatitis when 105 pregnant women were randomized to Lactobacillus GG or placebo. At the age of 2 years, atopic dermatitis was diagnosed in 28% of the 50 children in the probiotic group and 27.3% of the 44 in the placebo group (Pediatrics. 2008;121:e850-6).
The region of Germany where the study was conducted was rural/agricultural, so the diet could be different, Dr. Cabana said. Also, the median duration of breastfeeding differed between the Finnish and German study population, 6.8 months versus 9.2 months, respectively. “So that could potentially explain it, or there are just differences that cannot be explained.”
For more information, Dr. Cabana recommended information provided by the International Scientific Association of Prebiotics & Probiotics (https://isappscience.org/infographics/). The association’s website has easy to understand infographics including: What are probiotics and what can they do for you?; What’s so special about fermented foods?; and How do you read a probiotic label?
Dr. Cabana reported he receives research support from the National Institutes of Health, Wyeth Nutrition, and Nestle; is on the speakers bureau for Merck; owns stocks or bonds in Abbot and AbbVie; and is a consultant for Mead Johnson, Abbott, Genentech, Biogaia, General Mills, and Nestle.
EXPERT ANALYSIS FROM AAP 2017