User login
VIDEO: Software predicts septic shock in hospitalized patients
WASHINGTON – Researchers have devised a program that can predict severe sepsis or septic shock about 12-30 hours in advance of its onset in hospitalized patients, a feat they hope will allow them to apply early interventions to stave off impending sepsis.
“We’d love to see a change in sepsis mortality. Will earlier recognition and implementation of the sepsis bundle – fluids, antibiotics, etc. – improve outcomes?” wondered Heather M. Giannini, MD, in a video interview at an international conference of the American Thoracic Society.
The computer program works by monitoring all the data that enter a patient’s electronic health record during hospitalization. Researchers developed it and designed it specifically for inpatients who are not in the intensive care unit or emergency department.
Results from initial testing during October-December 2015 in 10,448 patients hospitalized at one of three participating Philadelphia hospitals showed the program predicted subsequent severe sepsis or septic shock with a sensitivity of 26% and a specificity of 98%, reported Dr. Giannini, a researcher in the Center for Evidence-Based Practice at the University of Pennsylvania in Philadelphia. Analysis also showed a positive likelihood ratio of 13 for severe sepsis or septic shock actually occurring following an alert generated by the computer program, a level indicating a “very strong” ability to predict sepsis, she said.
Dr. Giannini and her associates developed the prediction program using a technique called “computational machine learning,” an alternative to standard logistic regression modeling that is better suited to analyzing large data sets and can better integrate outlier data points. They took EHR data for all non-ICU, non-ED inpatients at three Philadelphia hospitals during a 3-year period during 2011-2014 and had the program focus particularly on EHR data gleaned from the nearly 1,000 patients who developed severe sepsis or septic shock during the 12 hours preceding the start of these sepsis events. The analysis identified patients as having developed severe sepsis or shock if they had a blood draw positive for infection at the same time as having a blood lactate level above 2.2 mmol/L or a systolic blood pressure below 90 mm Hg.
To create the algorithm the machine-learning device compared the EHR entries for patients who developed severe sepsis or septic shock with EHR data from patients who did not, a process that involved hundred of thousands of data points, Dr. Giannini said. This identified 587 individual types of relevant EHR data entries and ranked them from most important to least important. Important, novel determinants of impending severe sepsis identified this way included anion gap, blood urea nitrogen, and platelet count. The development process also confirmed an important role for many classic markers of septic shock, such as respiration rate, heart rate, and temperature.
The researchers designed the algorithm to have a moderate level of sensitivity to avoid “alert fatigue” from generating too many alarms for impending severe sepsis. Their goal was for clinicians to receive no more than about 10 alerts per day for each hospital.
“We are satisfied with the sensitivity. We felt it was better to have too few alerts rather than overwhelm clinicians. About 10 alerts a day is reasonable,” Dr. Giannini explained. During initial 2015 testing, the system generated a daily average of 11 alerts.
[email protected]
On Twitter @mitchelzoler
WASHINGTON – Researchers have devised a program that can predict severe sepsis or septic shock about 12-30 hours in advance of its onset in hospitalized patients, a feat they hope will allow them to apply early interventions to stave off impending sepsis.
“We’d love to see a change in sepsis mortality. Will earlier recognition and implementation of the sepsis bundle – fluids, antibiotics, etc. – improve outcomes?” wondered Heather M. Giannini, MD, in a video interview at an international conference of the American Thoracic Society.
The computer program works by monitoring all the data that enter a patient’s electronic health record during hospitalization. Researchers developed it and designed it specifically for inpatients who are not in the intensive care unit or emergency department.
Results from initial testing during October-December 2015 in 10,448 patients hospitalized at one of three participating Philadelphia hospitals showed the program predicted subsequent severe sepsis or septic shock with a sensitivity of 26% and a specificity of 98%, reported Dr. Giannini, a researcher in the Center for Evidence-Based Practice at the University of Pennsylvania in Philadelphia. Analysis also showed a positive likelihood ratio of 13 for severe sepsis or septic shock actually occurring following an alert generated by the computer program, a level indicating a “very strong” ability to predict sepsis, she said.
Dr. Giannini and her associates developed the prediction program using a technique called “computational machine learning,” an alternative to standard logistic regression modeling that is better suited to analyzing large data sets and can better integrate outlier data points. They took EHR data for all non-ICU, non-ED inpatients at three Philadelphia hospitals during a 3-year period during 2011-2014 and had the program focus particularly on EHR data gleaned from the nearly 1,000 patients who developed severe sepsis or septic shock during the 12 hours preceding the start of these sepsis events. The analysis identified patients as having developed severe sepsis or shock if they had a blood draw positive for infection at the same time as having a blood lactate level above 2.2 mmol/L or a systolic blood pressure below 90 mm Hg.
To create the algorithm the machine-learning device compared the EHR entries for patients who developed severe sepsis or septic shock with EHR data from patients who did not, a process that involved hundred of thousands of data points, Dr. Giannini said. This identified 587 individual types of relevant EHR data entries and ranked them from most important to least important. Important, novel determinants of impending severe sepsis identified this way included anion gap, blood urea nitrogen, and platelet count. The development process also confirmed an important role for many classic markers of septic shock, such as respiration rate, heart rate, and temperature.
The researchers designed the algorithm to have a moderate level of sensitivity to avoid “alert fatigue” from generating too many alarms for impending severe sepsis. Their goal was for clinicians to receive no more than about 10 alerts per day for each hospital.
“We are satisfied with the sensitivity. We felt it was better to have too few alerts rather than overwhelm clinicians. About 10 alerts a day is reasonable,” Dr. Giannini explained. During initial 2015 testing, the system generated a daily average of 11 alerts.
[email protected]
On Twitter @mitchelzoler
WASHINGTON – Researchers have devised a program that can predict severe sepsis or septic shock about 12-30 hours in advance of its onset in hospitalized patients, a feat they hope will allow them to apply early interventions to stave off impending sepsis.
“We’d love to see a change in sepsis mortality. Will earlier recognition and implementation of the sepsis bundle – fluids, antibiotics, etc. – improve outcomes?” wondered Heather M. Giannini, MD, in a video interview at an international conference of the American Thoracic Society.
The computer program works by monitoring all the data that enter a patient’s electronic health record during hospitalization. Researchers developed it and designed it specifically for inpatients who are not in the intensive care unit or emergency department.
Results from initial testing during October-December 2015 in 10,448 patients hospitalized at one of three participating Philadelphia hospitals showed the program predicted subsequent severe sepsis or septic shock with a sensitivity of 26% and a specificity of 98%, reported Dr. Giannini, a researcher in the Center for Evidence-Based Practice at the University of Pennsylvania in Philadelphia. Analysis also showed a positive likelihood ratio of 13 for severe sepsis or septic shock actually occurring following an alert generated by the computer program, a level indicating a “very strong” ability to predict sepsis, she said.
Dr. Giannini and her associates developed the prediction program using a technique called “computational machine learning,” an alternative to standard logistic regression modeling that is better suited to analyzing large data sets and can better integrate outlier data points. They took EHR data for all non-ICU, non-ED inpatients at three Philadelphia hospitals during a 3-year period during 2011-2014 and had the program focus particularly on EHR data gleaned from the nearly 1,000 patients who developed severe sepsis or septic shock during the 12 hours preceding the start of these sepsis events. The analysis identified patients as having developed severe sepsis or shock if they had a blood draw positive for infection at the same time as having a blood lactate level above 2.2 mmol/L or a systolic blood pressure below 90 mm Hg.
To create the algorithm the machine-learning device compared the EHR entries for patients who developed severe sepsis or septic shock with EHR data from patients who did not, a process that involved hundred of thousands of data points, Dr. Giannini said. This identified 587 individual types of relevant EHR data entries and ranked them from most important to least important. Important, novel determinants of impending severe sepsis identified this way included anion gap, blood urea nitrogen, and platelet count. The development process also confirmed an important role for many classic markers of septic shock, such as respiration rate, heart rate, and temperature.
The researchers designed the algorithm to have a moderate level of sensitivity to avoid “alert fatigue” from generating too many alarms for impending severe sepsis. Their goal was for clinicians to receive no more than about 10 alerts per day for each hospital.
“We are satisfied with the sensitivity. We felt it was better to have too few alerts rather than overwhelm clinicians. About 10 alerts a day is reasonable,” Dr. Giannini explained. During initial 2015 testing, the system generated a daily average of 11 alerts.
[email protected]
On Twitter @mitchelzoler
AT ATS 2017
Key clinical point:
Major finding: The program predicted severe sepsis with a sensitivity of 26% and specificity of 98%.
Data source: A total of 10,448 inpatients at three Philadelphia hospitals during October-December 2015.
Disclosures: Dr. Giannini had no disclosures.
The role of NPs and PAs in hospital medicine programs
Background and growth
Hospitalist nurse practitioner (NP) and physician assistant (PA) providers have been a growing and evolving part of the inpatient medical workforce, seemingly since the inception of hospital medicine. Given the growth of these disciplines within hospital medicine, at this juncture it is helpful to look at this journey, to see what roles these providers have been serving, and to consider newer and novel trends in how NPs and PAs are being weaved into hospital medicine programs.
The drivers for growth in this provider population are not unlike those of physician hospitalists. The same milieu that provided inroads for physicians in hospital-based care have led the way for increased use of NP/PA providers. An aging physician workforce, residency work hour reforms, increasing complexity of patients and systems on the inpatient side, and the recognition that caring for inpatients is a specialty vastly different from the role of internist in primary care have all impacted the numbers of NPs and PAs in this arena.
• 2007 Today’s Hospitalist article: “Midlevels make a rocky entrance into hospital medicine”1
• 2009 ACP Hospitalist article: “When hiring midlevels, proceed with caution”2
These titles reflect the uncertainty at the time in how best to utilize NP/PA providers in hospital medicine (as well as an unfashionable vocabulary). The numbers at the time tell a similar story. In the Society of Hospital Medicine survey in 2007-2008, about 29% and 21% of hospital medicine practices utilized NPs and PAs, respectively. However, by 2014 about 50% of Veterans Affairs inpatient medical services deployed NP/PA providers, and most recent data from the Society of Hospital Medicine reveal that about 63% of groups use these advanced practice providers (APPs), with higher numbers in pediatric programs. Clearly there is evolving growth and enthusiasm for NP/PAs in hospital medicine.
Program models
Determining how best to use NP/PAs in hospital medicine programs has had a similar evolution. Reviewing past articles addressing these issues, one can see that there has been clear migration; initially NP/PAs were primarily hired to assist with late-afternoon admission surges, with about 60% of the APP workload being utilized to admit in 2007. Their role has continued to grow and change, much as hospitalist practices have; current program models consist of a few major types, with some novel models coming to the fore.
Another model is use of an NP/PA in an observation unit or with lower acuity observation patients. The majority of the management of the patients is completed and billed by the APP, with the physician available for backup. This hits the “sweet spot,” utilizing the right provider with the right skill set for the right patient. The program has to account for some reimbursement or compensation for the physician oversight time, but it is a very efficient use of APPs.
The third major deployment of APPs is with admissions. Many groups use APPs to admit into the late afternoon and evening, getting patients “tucked in,” including starting diagnostic work-ups and treatment plans. The physician hospitalist then evaluates the patient the next day and often bills for the admission. This model works in situations where the patient work-up is dependent on lab testing, imaging, or other diagnostic testing to understand and plan for the “arc” of the hospitalization; or in situations where the diagnosis is clear, but the patient needs time with treatment to determine response. The downside of this model is long-term job satisfaction for the APP (although some programs have them rotate through such a model at intervals).
Another area where APPs have made strong inroads is that of comanagement services. The NP or PA develops a long-term relationship with a surgical comanagement team, and is often highly engaged and extremely appreciated for managing chronic conditions such as hypertension and diabetes. This can be a very satisfying model for both teams. The NP/PA usually bills independently for these encounters.
APPS are also used in cross coverage and triage roles, allowing the day teams to focus on their primary patients. In a triage role, they can interface with the emergency department, providing a semi-neutral “mediator” for patient disposition.
On the more novel end of the spectrum, there is growth in more independent roles for APP hospitalists. Some groups are having success at using the paired rounding or dyad model, but having the physician see the patient every third day. This is most successful where there is strong onboarding and deep clarity for when to contact the backup physician. There are some data to support the effectiveness of this model, most recently in the Journal of Clinical Outcomes Management.3
Critical access hospitals are also having success in deploying APPs in a very independent role, staffing these hospitals at night. Smaller, rural hospitals with aging medical staff have learned to maximize the scope of practice of their APPs to remain viable and provide care for inpatients. This can be a very successful model for APPs working at the maximum scope of their practice. In addition, the use of telemedicine has been implemented to allow for remote physician backup. This may be a rapidly growing arm to hospital medicine practices in the future.
Ongoing barriers
There are many barriers to maximizing the scope of practice and efficiency of APPs in hospital medicine. They range from the “macro” to the “micro.”
On the larger stage, Medicare requires that home care orders be signed by an attending physician, which can be inefficient and difficult to accomplish. Other payers may have somewhat arcane statutes that limit billing practices, and state practice limitations vary widely. Although 22 states now allow for independent practice for NPs, other states may have a very restrictive practice environment that can impede creative care delivery models. But regardless of how liberal a practice the state allows, a hospital’s medical bylaws can still restrict the day-to-day practice of APPs. And those restrictive bylaws are emblematic of a more constant and corporeal barrier to APP practice, that of medical staff culture.
If there are physicians on the staff who fear that utilization of NP/PA providers will lead to a decay in the quality of care, or who feel threatened by the use of APPs, that can create a local stopgap to maximizing utilization of APPs. In addition, hospitalist physicians and leaders may lack knowledge or experience in APP practice. APPs take more time to successfully onboard than physicians; without clear expectations or road maps to accomplish this onboarding, leaders may feel that APP integration doesn’t work. And one bad experience can create long-term barriers for future practices.
Other barriers are the lack of standardized rigor and vigor in graduate education programs (in both educational and clinical experiences). This results in variation in the quality of NP/PA providers at graduation. Knowledge gaps may be perceived as incompetence, rather than just a lack of experience. There is a certificate for added qualification in hospital medicine for PA providers (which includes a specialty exam), and there is an acute care focus for NPs in training; however, there is no standardized licensure to ensure hospital medicine competency, creating a quagmire for hospitalist leaders who desire demonstrable competence of these providers.
Another barrier for some programs is financial; physicians may not want to give up their RVUs to an NP/PA provider. This can really inhibit a more independent role for the APP. It is important that financial incentives align with all members of the practice working at maximum scope.
Summary and future
In summary, the role of PA/NP in hospital medicine has continued to grow and evolve, to meet the needs of the industry. This includes an increase in the scope and independence of APPs, including the use of telehealth for required oversight. As a specialty, it is imperative that we continue to research APP model effectiveness, embrace innovative delivery models, and support effective onboarding and career development opportunities for our NP/PA providers.
Dr. Scheurer is a hospitalist and chief quality officer at the Medical University of South Carolina in Charleston. She is physician editor of The Hospitalist. Ms. Cardin is vice president, Advanced Practice Providers, at Sound Physicians, and is a member of SHM’s Board of Directors.
References
1. “Midlevels make a rocky entrance into hospital medicine,” by Bonnie Darves, Today’s Hospitalist, January 2007.
2. “When hiring midlevels, proceed with caution,” by Jessica Berthold, ACP Hospitalist, April 2009.
3. “A Comparison of Conventional and Expanded Physician Assistant Hospitalist Staffing Models at a Community Hospital,” J Clin Outcomes Manag. 2016 Oct 1;23[10]:455-61.
Background and growth
Hospitalist nurse practitioner (NP) and physician assistant (PA) providers have been a growing and evolving part of the inpatient medical workforce, seemingly since the inception of hospital medicine. Given the growth of these disciplines within hospital medicine, at this juncture it is helpful to look at this journey, to see what roles these providers have been serving, and to consider newer and novel trends in how NPs and PAs are being weaved into hospital medicine programs.
The drivers for growth in this provider population are not unlike those of physician hospitalists. The same milieu that provided inroads for physicians in hospital-based care have led the way for increased use of NP/PA providers. An aging physician workforce, residency work hour reforms, increasing complexity of patients and systems on the inpatient side, and the recognition that caring for inpatients is a specialty vastly different from the role of internist in primary care have all impacted the numbers of NPs and PAs in this arena.
• 2007 Today’s Hospitalist article: “Midlevels make a rocky entrance into hospital medicine”1
• 2009 ACP Hospitalist article: “When hiring midlevels, proceed with caution”2
These titles reflect the uncertainty at the time in how best to utilize NP/PA providers in hospital medicine (as well as an unfashionable vocabulary). The numbers at the time tell a similar story. In the Society of Hospital Medicine survey in 2007-2008, about 29% and 21% of hospital medicine practices utilized NPs and PAs, respectively. However, by 2014 about 50% of Veterans Affairs inpatient medical services deployed NP/PA providers, and most recent data from the Society of Hospital Medicine reveal that about 63% of groups use these advanced practice providers (APPs), with higher numbers in pediatric programs. Clearly there is evolving growth and enthusiasm for NP/PAs in hospital medicine.
Program models
Determining how best to use NP/PAs in hospital medicine programs has had a similar evolution. Reviewing past articles addressing these issues, one can see that there has been clear migration; initially NP/PAs were primarily hired to assist with late-afternoon admission surges, with about 60% of the APP workload being utilized to admit in 2007. Their role has continued to grow and change, much as hospitalist practices have; current program models consist of a few major types, with some novel models coming to the fore.
Another model is use of an NP/PA in an observation unit or with lower acuity observation patients. The majority of the management of the patients is completed and billed by the APP, with the physician available for backup. This hits the “sweet spot,” utilizing the right provider with the right skill set for the right patient. The program has to account for some reimbursement or compensation for the physician oversight time, but it is a very efficient use of APPs.
The third major deployment of APPs is with admissions. Many groups use APPs to admit into the late afternoon and evening, getting patients “tucked in,” including starting diagnostic work-ups and treatment plans. The physician hospitalist then evaluates the patient the next day and often bills for the admission. This model works in situations where the patient work-up is dependent on lab testing, imaging, or other diagnostic testing to understand and plan for the “arc” of the hospitalization; or in situations where the diagnosis is clear, but the patient needs time with treatment to determine response. The downside of this model is long-term job satisfaction for the APP (although some programs have them rotate through such a model at intervals).
Another area where APPs have made strong inroads is that of comanagement services. The NP or PA develops a long-term relationship with a surgical comanagement team, and is often highly engaged and extremely appreciated for managing chronic conditions such as hypertension and diabetes. This can be a very satisfying model for both teams. The NP/PA usually bills independently for these encounters.
APPS are also used in cross coverage and triage roles, allowing the day teams to focus on their primary patients. In a triage role, they can interface with the emergency department, providing a semi-neutral “mediator” for patient disposition.
On the more novel end of the spectrum, there is growth in more independent roles for APP hospitalists. Some groups are having success at using the paired rounding or dyad model, but having the physician see the patient every third day. This is most successful where there is strong onboarding and deep clarity for when to contact the backup physician. There are some data to support the effectiveness of this model, most recently in the Journal of Clinical Outcomes Management.3
Critical access hospitals are also having success in deploying APPs in a very independent role, staffing these hospitals at night. Smaller, rural hospitals with aging medical staff have learned to maximize the scope of practice of their APPs to remain viable and provide care for inpatients. This can be a very successful model for APPs working at the maximum scope of their practice. In addition, the use of telemedicine has been implemented to allow for remote physician backup. This may be a rapidly growing arm to hospital medicine practices in the future.
Ongoing barriers
There are many barriers to maximizing the scope of practice and efficiency of APPs in hospital medicine. They range from the “macro” to the “micro.”
On the larger stage, Medicare requires that home care orders be signed by an attending physician, which can be inefficient and difficult to accomplish. Other payers may have somewhat arcane statutes that limit billing practices, and state practice limitations vary widely. Although 22 states now allow for independent practice for NPs, other states may have a very restrictive practice environment that can impede creative care delivery models. But regardless of how liberal a practice the state allows, a hospital’s medical bylaws can still restrict the day-to-day practice of APPs. And those restrictive bylaws are emblematic of a more constant and corporeal barrier to APP practice, that of medical staff culture.
If there are physicians on the staff who fear that utilization of NP/PA providers will lead to a decay in the quality of care, or who feel threatened by the use of APPs, that can create a local stopgap to maximizing utilization of APPs. In addition, hospitalist physicians and leaders may lack knowledge or experience in APP practice. APPs take more time to successfully onboard than physicians; without clear expectations or road maps to accomplish this onboarding, leaders may feel that APP integration doesn’t work. And one bad experience can create long-term barriers for future practices.
Other barriers are the lack of standardized rigor and vigor in graduate education programs (in both educational and clinical experiences). This results in variation in the quality of NP/PA providers at graduation. Knowledge gaps may be perceived as incompetence, rather than just a lack of experience. There is a certificate for added qualification in hospital medicine for PA providers (which includes a specialty exam), and there is an acute care focus for NPs in training; however, there is no standardized licensure to ensure hospital medicine competency, creating a quagmire for hospitalist leaders who desire demonstrable competence of these providers.
Another barrier for some programs is financial; physicians may not want to give up their RVUs to an NP/PA provider. This can really inhibit a more independent role for the APP. It is important that financial incentives align with all members of the practice working at maximum scope.
Summary and future
In summary, the role of PA/NP in hospital medicine has continued to grow and evolve, to meet the needs of the industry. This includes an increase in the scope and independence of APPs, including the use of telehealth for required oversight. As a specialty, it is imperative that we continue to research APP model effectiveness, embrace innovative delivery models, and support effective onboarding and career development opportunities for our NP/PA providers.
Dr. Scheurer is a hospitalist and chief quality officer at the Medical University of South Carolina in Charleston. She is physician editor of The Hospitalist. Ms. Cardin is vice president, Advanced Practice Providers, at Sound Physicians, and is a member of SHM’s Board of Directors.
References
1. “Midlevels make a rocky entrance into hospital medicine,” by Bonnie Darves, Today’s Hospitalist, January 2007.
2. “When hiring midlevels, proceed with caution,” by Jessica Berthold, ACP Hospitalist, April 2009.
3. “A Comparison of Conventional and Expanded Physician Assistant Hospitalist Staffing Models at a Community Hospital,” J Clin Outcomes Manag. 2016 Oct 1;23[10]:455-61.
Background and growth
Hospitalist nurse practitioner (NP) and physician assistant (PA) providers have been a growing and evolving part of the inpatient medical workforce, seemingly since the inception of hospital medicine. Given the growth of these disciplines within hospital medicine, at this juncture it is helpful to look at this journey, to see what roles these providers have been serving, and to consider newer and novel trends in how NPs and PAs are being weaved into hospital medicine programs.
The drivers for growth in this provider population are not unlike those of physician hospitalists. The same milieu that provided inroads for physicians in hospital-based care have led the way for increased use of NP/PA providers. An aging physician workforce, residency work hour reforms, increasing complexity of patients and systems on the inpatient side, and the recognition that caring for inpatients is a specialty vastly different from the role of internist in primary care have all impacted the numbers of NPs and PAs in this arena.
• 2007 Today’s Hospitalist article: “Midlevels make a rocky entrance into hospital medicine”1
• 2009 ACP Hospitalist article: “When hiring midlevels, proceed with caution”2
These titles reflect the uncertainty at the time in how best to utilize NP/PA providers in hospital medicine (as well as an unfashionable vocabulary). The numbers at the time tell a similar story. In the Society of Hospital Medicine survey in 2007-2008, about 29% and 21% of hospital medicine practices utilized NPs and PAs, respectively. However, by 2014 about 50% of Veterans Affairs inpatient medical services deployed NP/PA providers, and most recent data from the Society of Hospital Medicine reveal that about 63% of groups use these advanced practice providers (APPs), with higher numbers in pediatric programs. Clearly there is evolving growth and enthusiasm for NP/PAs in hospital medicine.
Program models
Determining how best to use NP/PAs in hospital medicine programs has had a similar evolution. Reviewing past articles addressing these issues, one can see that there has been clear migration; initially NP/PAs were primarily hired to assist with late-afternoon admission surges, with about 60% of the APP workload being utilized to admit in 2007. Their role has continued to grow and change, much as hospitalist practices have; current program models consist of a few major types, with some novel models coming to the fore.
Another model is use of an NP/PA in an observation unit or with lower acuity observation patients. The majority of the management of the patients is completed and billed by the APP, with the physician available for backup. This hits the “sweet spot,” utilizing the right provider with the right skill set for the right patient. The program has to account for some reimbursement or compensation for the physician oversight time, but it is a very efficient use of APPs.
The third major deployment of APPs is with admissions. Many groups use APPs to admit into the late afternoon and evening, getting patients “tucked in,” including starting diagnostic work-ups and treatment plans. The physician hospitalist then evaluates the patient the next day and often bills for the admission. This model works in situations where the patient work-up is dependent on lab testing, imaging, or other diagnostic testing to understand and plan for the “arc” of the hospitalization; or in situations where the diagnosis is clear, but the patient needs time with treatment to determine response. The downside of this model is long-term job satisfaction for the APP (although some programs have them rotate through such a model at intervals).
Another area where APPs have made strong inroads is that of comanagement services. The NP or PA develops a long-term relationship with a surgical comanagement team, and is often highly engaged and extremely appreciated for managing chronic conditions such as hypertension and diabetes. This can be a very satisfying model for both teams. The NP/PA usually bills independently for these encounters.
APPS are also used in cross coverage and triage roles, allowing the day teams to focus on their primary patients. In a triage role, they can interface with the emergency department, providing a semi-neutral “mediator” for patient disposition.
On the more novel end of the spectrum, there is growth in more independent roles for APP hospitalists. Some groups are having success at using the paired rounding or dyad model, but having the physician see the patient every third day. This is most successful where there is strong onboarding and deep clarity for when to contact the backup physician. There are some data to support the effectiveness of this model, most recently in the Journal of Clinical Outcomes Management.3
Critical access hospitals are also having success in deploying APPs in a very independent role, staffing these hospitals at night. Smaller, rural hospitals with aging medical staff have learned to maximize the scope of practice of their APPs to remain viable and provide care for inpatients. This can be a very successful model for APPs working at the maximum scope of their practice. In addition, the use of telemedicine has been implemented to allow for remote physician backup. This may be a rapidly growing arm to hospital medicine practices in the future.
Ongoing barriers
There are many barriers to maximizing the scope of practice and efficiency of APPs in hospital medicine. They range from the “macro” to the “micro.”
On the larger stage, Medicare requires that home care orders be signed by an attending physician, which can be inefficient and difficult to accomplish. Other payers may have somewhat arcane statutes that limit billing practices, and state practice limitations vary widely. Although 22 states now allow for independent practice for NPs, other states may have a very restrictive practice environment that can impede creative care delivery models. But regardless of how liberal a practice the state allows, a hospital’s medical bylaws can still restrict the day-to-day practice of APPs. And those restrictive bylaws are emblematic of a more constant and corporeal barrier to APP practice, that of medical staff culture.
If there are physicians on the staff who fear that utilization of NP/PA providers will lead to a decay in the quality of care, or who feel threatened by the use of APPs, that can create a local stopgap to maximizing utilization of APPs. In addition, hospitalist physicians and leaders may lack knowledge or experience in APP practice. APPs take more time to successfully onboard than physicians; without clear expectations or road maps to accomplish this onboarding, leaders may feel that APP integration doesn’t work. And one bad experience can create long-term barriers for future practices.
Other barriers are the lack of standardized rigor and vigor in graduate education programs (in both educational and clinical experiences). This results in variation in the quality of NP/PA providers at graduation. Knowledge gaps may be perceived as incompetence, rather than just a lack of experience. There is a certificate for added qualification in hospital medicine for PA providers (which includes a specialty exam), and there is an acute care focus for NPs in training; however, there is no standardized licensure to ensure hospital medicine competency, creating a quagmire for hospitalist leaders who desire demonstrable competence of these providers.
Another barrier for some programs is financial; physicians may not want to give up their RVUs to an NP/PA provider. This can really inhibit a more independent role for the APP. It is important that financial incentives align with all members of the practice working at maximum scope.
Summary and future
In summary, the role of PA/NP in hospital medicine has continued to grow and evolve, to meet the needs of the industry. This includes an increase in the scope and independence of APPs, including the use of telehealth for required oversight. As a specialty, it is imperative that we continue to research APP model effectiveness, embrace innovative delivery models, and support effective onboarding and career development opportunities for our NP/PA providers.
Dr. Scheurer is a hospitalist and chief quality officer at the Medical University of South Carolina in Charleston. She is physician editor of The Hospitalist. Ms. Cardin is vice president, Advanced Practice Providers, at Sound Physicians, and is a member of SHM’s Board of Directors.
References
1. “Midlevels make a rocky entrance into hospital medicine,” by Bonnie Darves, Today’s Hospitalist, January 2007.
2. “When hiring midlevels, proceed with caution,” by Jessica Berthold, ACP Hospitalist, April 2009.
3. “A Comparison of Conventional and Expanded Physician Assistant Hospitalist Staffing Models at a Community Hospital,” J Clin Outcomes Manag. 2016 Oct 1;23[10]:455-61.
In Memoriam
CHEST has been informed of the following deaths.We extend our sincere condolences.
Henry J. Heimlich MD, FCCP (December 2016)
Sylvan Lee Weinberg, MD, FCCP (Past President-1983-84) (January 2017)
Clive Deutscher, MD, FCCP (January 2017)
Sandra Willsie, DO, FCCP (March 2017)
Arthur F. Reimann, MD, FCCP (March 2017)
Cynthia Ray, MD, FCCP (April 2017)
Brian J. Sproule, MD, MS, FCCP (April 2017)
Michael R. Bye, MD, FCCP (April 2017)
Paul J. Mathews, MD, FCCP (May 2017)
CHEST has been informed of the following deaths.We extend our sincere condolences.
Henry J. Heimlich MD, FCCP (December 2016)
Sylvan Lee Weinberg, MD, FCCP (Past President-1983-84) (January 2017)
Clive Deutscher, MD, FCCP (January 2017)
Sandra Willsie, DO, FCCP (March 2017)
Arthur F. Reimann, MD, FCCP (March 2017)
Cynthia Ray, MD, FCCP (April 2017)
Brian J. Sproule, MD, MS, FCCP (April 2017)
Michael R. Bye, MD, FCCP (April 2017)
Paul J. Mathews, MD, FCCP (May 2017)
CHEST has been informed of the following deaths.We extend our sincere condolences.
Henry J. Heimlich MD, FCCP (December 2016)
Sylvan Lee Weinberg, MD, FCCP (Past President-1983-84) (January 2017)
Clive Deutscher, MD, FCCP (January 2017)
Sandra Willsie, DO, FCCP (March 2017)
Arthur F. Reimann, MD, FCCP (March 2017)
Cynthia Ray, MD, FCCP (April 2017)
Brian J. Sproule, MD, MS, FCCP (April 2017)
Michael R. Bye, MD, FCCP (April 2017)
Paul J. Mathews, MD, FCCP (May 2017)
Expanding Disease Awareness Campaigns
In 2017, we’ve continued to push our disease awareness efforts in lung cancer, sarcoidosis, asthma, and COPD, trading our “awareness months” for longer, more sustainable campaigns.
Lung Cancer
Our lung cancer disease awareness campaign launched mid-May and goes through World Lung Cancer Day on August 1, 2017. The foundation is partnering with the Bonnie J. Addarrio Lung Cancer Foundation and LUNGevity to produce:
• Biopsy-specific infographics
• An animated biopsy video to show the importance of collecting core tissue
• Social media shareable postcards
• An updated lung cancer guide and infographic
• New lung cancer landing page and website
Sharing these resources through the CHEST social media channels, we have so far been able to reach more than 34.2K social media accounts and earn 512 social interactions, including likes/reactions, clicks, and shares/retweets from Twitter, Facebook, and LinkedIn.
We are also excited to participate in a Lung Cancer Living Room discussion with the Bonnie J. Addario Lung Cancer Foundation. This presentation will bring lung cancer specialists, physicians, patients, and the public together to discuss lung cancer in a relaxed and comfortable setting. Attendees will have the opportunity to ask questions, share their stories, and discuss issues surrounding lung cancer. The event will also be live-streamed and archived on the Bonnie J. Addario Lung Cancer Foundation’s website.
Asthma
We launched our asthma campaign at “The Air We Breathe” Summit with The Atlantic. We were able to reach more than 24.2K social accounts through live tweeting during the event and follow up posts on CHEST’s Twitter and Facebook accounts. The Atlantic was able to earn an impressive reach of 868K social accounts through their own social media promotion for the event. To read more about the event that focused on the quality of our air and the implications on our health, visit chestfoundation.org/summit.
We continue to partner with the Allergy & Asthma Network to create and distribute our materials and messaging, which focus on severe and difficult-to-control asthma. This campaign has already garnered over 53.4K social media impressions through CHEST channels, and over 1.3K social interactions. New components to the campaign include:
• Severity assessment tool
• Shared decision making tool
• Patient testimonial videos
Our campaign also included an 8-minute segment on the Access Health program, which aired two times in May on Lifetime NetWork, with an additional 200+ airings via syndication throughout 100 US markets.
Sarcoidosis
For the third consecutive year, we’ve partnered with the Foundation for Sarcoidosis Research to spread awareness on the disease. We also partnered with the American Osteopathic Association, the COPD Foundation, and The Society of Thoracic Surgeons to create
In CHEST member communications, our campaign reached more than 20,000 people, and our social media posts have reached more than 61.7K social accounts. CHEST’s press release on sarcoidosis has reached well over 11.6M clinicians and patients.
We are very grateful and proud of the work our partners have done to help us spread awareness on these diseases, so clinicians and patients will be able to use our resources to champion lung health.
In 2017, we’ve continued to push our disease awareness efforts in lung cancer, sarcoidosis, asthma, and COPD, trading our “awareness months” for longer, more sustainable campaigns.
Lung Cancer
Our lung cancer disease awareness campaign launched mid-May and goes through World Lung Cancer Day on August 1, 2017. The foundation is partnering with the Bonnie J. Addarrio Lung Cancer Foundation and LUNGevity to produce:
• Biopsy-specific infographics
• An animated biopsy video to show the importance of collecting core tissue
• Social media shareable postcards
• An updated lung cancer guide and infographic
• New lung cancer landing page and website
Sharing these resources through the CHEST social media channels, we have so far been able to reach more than 34.2K social media accounts and earn 512 social interactions, including likes/reactions, clicks, and shares/retweets from Twitter, Facebook, and LinkedIn.
We are also excited to participate in a Lung Cancer Living Room discussion with the Bonnie J. Addario Lung Cancer Foundation. This presentation will bring lung cancer specialists, physicians, patients, and the public together to discuss lung cancer in a relaxed and comfortable setting. Attendees will have the opportunity to ask questions, share their stories, and discuss issues surrounding lung cancer. The event will also be live-streamed and archived on the Bonnie J. Addario Lung Cancer Foundation’s website.
Asthma
We launched our asthma campaign at “The Air We Breathe” Summit with The Atlantic. We were able to reach more than 24.2K social accounts through live tweeting during the event and follow up posts on CHEST’s Twitter and Facebook accounts. The Atlantic was able to earn an impressive reach of 868K social accounts through their own social media promotion for the event. To read more about the event that focused on the quality of our air and the implications on our health, visit chestfoundation.org/summit.
We continue to partner with the Allergy & Asthma Network to create and distribute our materials and messaging, which focus on severe and difficult-to-control asthma. This campaign has already garnered over 53.4K social media impressions through CHEST channels, and over 1.3K social interactions. New components to the campaign include:
• Severity assessment tool
• Shared decision making tool
• Patient testimonial videos
Our campaign also included an 8-minute segment on the Access Health program, which aired two times in May on Lifetime NetWork, with an additional 200+ airings via syndication throughout 100 US markets.
Sarcoidosis
For the third consecutive year, we’ve partnered with the Foundation for Sarcoidosis Research to spread awareness on the disease. We also partnered with the American Osteopathic Association, the COPD Foundation, and The Society of Thoracic Surgeons to create
In CHEST member communications, our campaign reached more than 20,000 people, and our social media posts have reached more than 61.7K social accounts. CHEST’s press release on sarcoidosis has reached well over 11.6M clinicians and patients.
We are very grateful and proud of the work our partners have done to help us spread awareness on these diseases, so clinicians and patients will be able to use our resources to champion lung health.
In 2017, we’ve continued to push our disease awareness efforts in lung cancer, sarcoidosis, asthma, and COPD, trading our “awareness months” for longer, more sustainable campaigns.
Lung Cancer
Our lung cancer disease awareness campaign launched mid-May and goes through World Lung Cancer Day on August 1, 2017. The foundation is partnering with the Bonnie J. Addarrio Lung Cancer Foundation and LUNGevity to produce:
• Biopsy-specific infographics
• An animated biopsy video to show the importance of collecting core tissue
• Social media shareable postcards
• An updated lung cancer guide and infographic
• New lung cancer landing page and website
Sharing these resources through the CHEST social media channels, we have so far been able to reach more than 34.2K social media accounts and earn 512 social interactions, including likes/reactions, clicks, and shares/retweets from Twitter, Facebook, and LinkedIn.
We are also excited to participate in a Lung Cancer Living Room discussion with the Bonnie J. Addario Lung Cancer Foundation. This presentation will bring lung cancer specialists, physicians, patients, and the public together to discuss lung cancer in a relaxed and comfortable setting. Attendees will have the opportunity to ask questions, share their stories, and discuss issues surrounding lung cancer. The event will also be live-streamed and archived on the Bonnie J. Addario Lung Cancer Foundation’s website.
Asthma
We launched our asthma campaign at “The Air We Breathe” Summit with The Atlantic. We were able to reach more than 24.2K social accounts through live tweeting during the event and follow up posts on CHEST’s Twitter and Facebook accounts. The Atlantic was able to earn an impressive reach of 868K social accounts through their own social media promotion for the event. To read more about the event that focused on the quality of our air and the implications on our health, visit chestfoundation.org/summit.
We continue to partner with the Allergy & Asthma Network to create and distribute our materials and messaging, which focus on severe and difficult-to-control asthma. This campaign has already garnered over 53.4K social media impressions through CHEST channels, and over 1.3K social interactions. New components to the campaign include:
• Severity assessment tool
• Shared decision making tool
• Patient testimonial videos
Our campaign also included an 8-minute segment on the Access Health program, which aired two times in May on Lifetime NetWork, with an additional 200+ airings via syndication throughout 100 US markets.
Sarcoidosis
For the third consecutive year, we’ve partnered with the Foundation for Sarcoidosis Research to spread awareness on the disease. We also partnered with the American Osteopathic Association, the COPD Foundation, and The Society of Thoracic Surgeons to create
In CHEST member communications, our campaign reached more than 20,000 people, and our social media posts have reached more than 61.7K social accounts. CHEST’s press release on sarcoidosis has reached well over 11.6M clinicians and patients.
We are very grateful and proud of the work our partners have done to help us spread awareness on these diseases, so clinicians and patients will be able to use our resources to champion lung health.
Evolute transcatheter valve, now FDA approved for intermediate-risk patients, impresses in real-world practice
PARIS – The Evolut R transcatheter aortic valve demonstrated excellent 30-day results in a real-world, mixed surgical risk population in the large Evolut R FORWARD study.
In this 1,038-patient observational study conducted at 53 sites in 20 countries, the Evolut R valve showed excellent forward hemodynamics and low 30-day rates of all-cause mortality and stroke that were unaffected by utilization of the device’s repositioning feature, Eberhard Grube, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The importance of the FORWARD study, Dr. Grube observed, is that it illustrates the clinical outcomes obtained in a large population drawn from routine clinical practice. Unlike in a randomized trial such as SURTAVI, the participating sites in the Evolut R Forward study weren’t all high-volume enrollment centers, and operators had widely varying degrees of experience with the valve.
Also, SURTAVI utilized the first generation of the self-expanding CoreValve, which lacked the repositioning feature introduced in the second-generation Evolut R. The FORWARD study is the first rigorous evaluation of Evolut R with centrally adjudicated outcomes.
The mean Society of Thoracic Surgeons predicted risk of mortality score in participants was 5.5%, and 47% had a low-risk score of less than 4%. However, the patients had a mean age of 82 years, one-third were deemed frail, 30% had diabetes, and 26% had chronic lung disease.
The primary study endpoint was 30-day all-cause mortality. The rate was 1.9%, compared with a predicted 5.5% rate based on STS score, for an impressive observed-to-expected ratio of 0.35.
Hemodynamically, the effective orifice area improved from 0.8 cm2 at baseline to 1.9 cm2 at 30 days, while the mean aortic valve gradient plunged from 41.7 to 8.5 mm Hg.
At baseline only 1.5% of patients were New York Heart Association functional class I and 26.5% were class II. At 30 days, 44.7% were class I and 43.4% were class II. The prevalence of NYHA class III status decreased from 63.8% to 11.3%.
There was no or only trace paravalvular leak at discharge in 67.2% of patients as adjudicated in a core laboratory, mild leak in 30.9%, moderate in 1.9%, and severe leak in just 0.1%.
The 30-day total stroke rate was 2.8%, including a 1.8% rate of disabling stroke. Major vascular complications occurred in 6.5% of patients, valve embolization in 0.7%, and life-threatening or disabling bleeding in 3.3%. There were no cases of coronary obstruction or annular rupture.
New pacemaker implantation was required within 30 days in 17.5% of patients. Three-quarters of the pacemakers were placed because of third-degree atrioventricular block.
The new valve ended up in proper anatomic position in 98.9% of patients.
The repositioning feature was utilized in 26% of participants. It had no impact on the rate of pacemaker implantation, mortality, stroke, or other safety endpoints.
“I think the ability to reposition this valve, which is a safety feature, is an important feature, particularly for centers that don’t have so much experience. If the valve is considered to be too high or too low, or you see, for example, a higher degree of paravalvular leak, you have the chance to correct that by using this feature. So it’s an important feature for the operator. It helps to get an optimal result. And the most important thing is there was no price in terms of safety that we paid for repositioning,” said Dr. Grube, professor of cardiology and head of the Center for Innovative Intervention in Cardiology at the University of Bonn in Siegberg, Germany.
Session cochair Alain Cribier, MD, famed for having performed the world’s first TAVR procedure, pronounced the FORWARD results “very impressive.”
“Less than 2% mortality, around a 2% disabling stroke rate, and the data on paravalvular leak are excellent as well. It’s very nice to see that what was a limited data set earlier, with a smaller number of patients, has now been replicated in 1,000 patients. So I think now we can confidently talk about the clinical outcomes – and they are excellent,” declared Dr. Cribier, professor of medicine at the University of Rouen (France) and chief of cardiology at Charles Nicolle Hospital.
The FORWARD study was sponsored by Medtronic. Dr. Grube reported serving as a consultant to that company as well as to Boston Scientific, Abbott, and Millipede Medical.
PARIS – The Evolut R transcatheter aortic valve demonstrated excellent 30-day results in a real-world, mixed surgical risk population in the large Evolut R FORWARD study.
In this 1,038-patient observational study conducted at 53 sites in 20 countries, the Evolut R valve showed excellent forward hemodynamics and low 30-day rates of all-cause mortality and stroke that were unaffected by utilization of the device’s repositioning feature, Eberhard Grube, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The importance of the FORWARD study, Dr. Grube observed, is that it illustrates the clinical outcomes obtained in a large population drawn from routine clinical practice. Unlike in a randomized trial such as SURTAVI, the participating sites in the Evolut R Forward study weren’t all high-volume enrollment centers, and operators had widely varying degrees of experience with the valve.
Also, SURTAVI utilized the first generation of the self-expanding CoreValve, which lacked the repositioning feature introduced in the second-generation Evolut R. The FORWARD study is the first rigorous evaluation of Evolut R with centrally adjudicated outcomes.
The mean Society of Thoracic Surgeons predicted risk of mortality score in participants was 5.5%, and 47% had a low-risk score of less than 4%. However, the patients had a mean age of 82 years, one-third were deemed frail, 30% had diabetes, and 26% had chronic lung disease.
The primary study endpoint was 30-day all-cause mortality. The rate was 1.9%, compared with a predicted 5.5% rate based on STS score, for an impressive observed-to-expected ratio of 0.35.
Hemodynamically, the effective orifice area improved from 0.8 cm2 at baseline to 1.9 cm2 at 30 days, while the mean aortic valve gradient plunged from 41.7 to 8.5 mm Hg.
At baseline only 1.5% of patients were New York Heart Association functional class I and 26.5% were class II. At 30 days, 44.7% were class I and 43.4% were class II. The prevalence of NYHA class III status decreased from 63.8% to 11.3%.
There was no or only trace paravalvular leak at discharge in 67.2% of patients as adjudicated in a core laboratory, mild leak in 30.9%, moderate in 1.9%, and severe leak in just 0.1%.
The 30-day total stroke rate was 2.8%, including a 1.8% rate of disabling stroke. Major vascular complications occurred in 6.5% of patients, valve embolization in 0.7%, and life-threatening or disabling bleeding in 3.3%. There were no cases of coronary obstruction or annular rupture.
New pacemaker implantation was required within 30 days in 17.5% of patients. Three-quarters of the pacemakers were placed because of third-degree atrioventricular block.
The new valve ended up in proper anatomic position in 98.9% of patients.
The repositioning feature was utilized in 26% of participants. It had no impact on the rate of pacemaker implantation, mortality, stroke, or other safety endpoints.
“I think the ability to reposition this valve, which is a safety feature, is an important feature, particularly for centers that don’t have so much experience. If the valve is considered to be too high or too low, or you see, for example, a higher degree of paravalvular leak, you have the chance to correct that by using this feature. So it’s an important feature for the operator. It helps to get an optimal result. And the most important thing is there was no price in terms of safety that we paid for repositioning,” said Dr. Grube, professor of cardiology and head of the Center for Innovative Intervention in Cardiology at the University of Bonn in Siegberg, Germany.
Session cochair Alain Cribier, MD, famed for having performed the world’s first TAVR procedure, pronounced the FORWARD results “very impressive.”
“Less than 2% mortality, around a 2% disabling stroke rate, and the data on paravalvular leak are excellent as well. It’s very nice to see that what was a limited data set earlier, with a smaller number of patients, has now been replicated in 1,000 patients. So I think now we can confidently talk about the clinical outcomes – and they are excellent,” declared Dr. Cribier, professor of medicine at the University of Rouen (France) and chief of cardiology at Charles Nicolle Hospital.
The FORWARD study was sponsored by Medtronic. Dr. Grube reported serving as a consultant to that company as well as to Boston Scientific, Abbott, and Millipede Medical.
PARIS – The Evolut R transcatheter aortic valve demonstrated excellent 30-day results in a real-world, mixed surgical risk population in the large Evolut R FORWARD study.
In this 1,038-patient observational study conducted at 53 sites in 20 countries, the Evolut R valve showed excellent forward hemodynamics and low 30-day rates of all-cause mortality and stroke that were unaffected by utilization of the device’s repositioning feature, Eberhard Grube, MD, reported at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
The importance of the FORWARD study, Dr. Grube observed, is that it illustrates the clinical outcomes obtained in a large population drawn from routine clinical practice. Unlike in a randomized trial such as SURTAVI, the participating sites in the Evolut R Forward study weren’t all high-volume enrollment centers, and operators had widely varying degrees of experience with the valve.
Also, SURTAVI utilized the first generation of the self-expanding CoreValve, which lacked the repositioning feature introduced in the second-generation Evolut R. The FORWARD study is the first rigorous evaluation of Evolut R with centrally adjudicated outcomes.
The mean Society of Thoracic Surgeons predicted risk of mortality score in participants was 5.5%, and 47% had a low-risk score of less than 4%. However, the patients had a mean age of 82 years, one-third were deemed frail, 30% had diabetes, and 26% had chronic lung disease.
The primary study endpoint was 30-day all-cause mortality. The rate was 1.9%, compared with a predicted 5.5% rate based on STS score, for an impressive observed-to-expected ratio of 0.35.
Hemodynamically, the effective orifice area improved from 0.8 cm2 at baseline to 1.9 cm2 at 30 days, while the mean aortic valve gradient plunged from 41.7 to 8.5 mm Hg.
At baseline only 1.5% of patients were New York Heart Association functional class I and 26.5% were class II. At 30 days, 44.7% were class I and 43.4% were class II. The prevalence of NYHA class III status decreased from 63.8% to 11.3%.
There was no or only trace paravalvular leak at discharge in 67.2% of patients as adjudicated in a core laboratory, mild leak in 30.9%, moderate in 1.9%, and severe leak in just 0.1%.
The 30-day total stroke rate was 2.8%, including a 1.8% rate of disabling stroke. Major vascular complications occurred in 6.5% of patients, valve embolization in 0.7%, and life-threatening or disabling bleeding in 3.3%. There were no cases of coronary obstruction or annular rupture.
New pacemaker implantation was required within 30 days in 17.5% of patients. Three-quarters of the pacemakers were placed because of third-degree atrioventricular block.
The new valve ended up in proper anatomic position in 98.9% of patients.
The repositioning feature was utilized in 26% of participants. It had no impact on the rate of pacemaker implantation, mortality, stroke, or other safety endpoints.
“I think the ability to reposition this valve, which is a safety feature, is an important feature, particularly for centers that don’t have so much experience. If the valve is considered to be too high or too low, or you see, for example, a higher degree of paravalvular leak, you have the chance to correct that by using this feature. So it’s an important feature for the operator. It helps to get an optimal result. And the most important thing is there was no price in terms of safety that we paid for repositioning,” said Dr. Grube, professor of cardiology and head of the Center for Innovative Intervention in Cardiology at the University of Bonn in Siegberg, Germany.
Session cochair Alain Cribier, MD, famed for having performed the world’s first TAVR procedure, pronounced the FORWARD results “very impressive.”
“Less than 2% mortality, around a 2% disabling stroke rate, and the data on paravalvular leak are excellent as well. It’s very nice to see that what was a limited data set earlier, with a smaller number of patients, has now been replicated in 1,000 patients. So I think now we can confidently talk about the clinical outcomes – and they are excellent,” declared Dr. Cribier, professor of medicine at the University of Rouen (France) and chief of cardiology at Charles Nicolle Hospital.
The FORWARD study was sponsored by Medtronic. Dr. Grube reported serving as a consultant to that company as well as to Boston Scientific, Abbott, and Millipede Medical.
AT EUROPCR
Key clinical point:
Major finding: Thirty-day all-cause mortality was 1.9% with a 2.8% stroke rate in a large, real-world study of TAVR with the repositionable self-expanding Evolut R transcatheter aortic valve.
Data source: The Evolut R FORWARD study of 1,038 recipients of the Evolut R transcatheter aortic valve at 53 sites in 20 countries.
Disclosures: The FORWARD study was sponsored by Medtronic. The presenter reported serving as a consultant to that company as well as to Boston Scientific, Abbott, and Millipede Medical.
Cerebral Venous Thrombosis
Cerebral venous thrombosis (CVT) is a rare cerebrovascular disease that affects about 5 in 1 million people each year and accounts for 0.5% of all strokes.1 Previously it was thought to be caused most commonly by infections (eg, mastoiditis, otitis, meningitis) affecting the superior sagittal sinus and often resulting in focal neurologic deficits, seizures, coma, and death. Although local and systemic infections are still prominent risk factors in its development, CVT is now primarily recognized as a nonseptic disease with a wide spectrum of clinical presentations.
Cerebral venous thrombosis causes reduced outflow of blood and cerebrospinal fluid, which in half of affected patients leads to significant venous infarct. As opposed to arterial infarctions, CVT mainly affects children and young adults; it is an important cause of stroke in the younger population.2 There is significant overlap of the many risk factors for CVT and those for venous thromboembolism (VTE): cancer, obesity, genetic thrombophilia, trauma, infection, and prior neurosurgery. However, the most common acquired risk factors for CVT are oral contraceptive use and pregnancy, which explains why CVT is 3 times more likely to occur in young and middle-aged women.3
Cerebral venous thrombosis was first recognized by a French physician in the 19th century, a time when the condition was diagnosed at autopsy, which typically showed hemorrhagic lesions at the thrombus site.4 For many years heparin was contraindicated in the treatment of CVT, and only within the past 25 years did advances in neuroimaging allow for earlier diagnosis and change perspectives on the management of this disease.
Cerebral venous thrombosis occurs from formation of a thrombus within the cerebral venous sinuses, leading to elevated intracranial pressure and eventually ischemia or intracranial hemorrhage. Improved imaging techniques, notably magnetic resonance imaging (MRI) and computed tomography (CT) venography, allow physicians to identify thrombus formation earlier and begin anticoagulation therapy with heparin before infarction. A meta-analysis of studies found that heparin was safer and more efficacious in treating CVT compared with placebo.5 Furthermore, several small randomized trials found treatment with unfractionated heparin (UFH) or low-molecularweight heparin (LMWH) was not associated with higher risk of hemorrhagic stroke in these patients.6-8
Despite improvements in imaging modalities, in many cases diagnosis is delayed, as most patients with CVT have a wide spectrum of presentations with nonspecific symptoms, such as headache, seizure, focal sensorimotor deficits, and papilledema.9 Clinical presentations of CVT depend on a variety of factors, including age, time of onset, CVT location, and presence of parenchymal lesions. Isolated headache is the most common initial symptom, and in many cases is the only presenting manifestation of CVT.1 Encephalopathy with multifocal signs, delirium, or dysfunction in executive functions most commonly occurs in elderly populations.
Cavernous sinus thrombosis most commonly produces generalized headaches, orbital pain, proptosis, and diplopia, whereas cortical vein thrombosis often produces seizures and focal sensorimotor deficits. Aphasia may be present in patients with isolated left transverse sinus thrombosis. In the presence of deep cerebral venous occlusion, patients can present in coma or with severe cognitive deficits and widespread paresis.10 Thrombosis of different veins and sinuses results in a wide spectrum of diverse clinical pictures, posing a diagnostic challenge and affecting clinical outcomes.
Given the variable and often nonspecific clinical presentations of these cases, unenhanced CT typically is the first imaging study ordered. According to the literature, noncontrast CT is not sensitive (25%-56%) in detecting CVT, and findings are normal in up to 26% of patients, rarely providing a specific diagnosis.11 Furthermore, visualization on MRI can be difficult during the acute phase of CVT, as the thrombus initially appears isointense on T1-weighted images and gradually becomes hyperintense over the course of the disease process.12 These difficulties with the usual first-choice imaging examinations often result in a delay in diagnosing CVT. However, several points on close examination of these imaging studies may help physicians establish a high index of clinical suspicion and order the appropriate follow-up studies for CVT.
The authors report the case of a patient who presented with a 1-week history of confusion, headaches, and dizziness. His nonspecific presentation along with the absence of obvious signs of CVT on imaging prolonged his diagnosis and the initiation of an appropriate treatment plan.
Case Report
A 57-year-old white air-conditioning mechanic presented to the emergency department (ED) with a 1-week history of gradual-onset confusion, severe headaches, dizziness, light-headedness, poor memory, and increased sleep. Two days earlier, he presented with similar symptoms to an outside facility, where he was admitted and underwent a workup for stroke, hemorrhage, and cardiac abnormalities—including noncontrast CT of the head. With nothing of clinical significance found, the patient was discharged and was advised to follow up on an outpatient basis.
Persisting symptoms brought the patient to the ED 1 day later, and he was admitted. He described severe, progressive, generalized headaches that were more severe when he was lying down at night and waking in the morning. He did not report that the headaches worsened with coughing or straining, and he reported no history of trauma, neck stiffness, vision change, seizures, and migraines. His medical history was significant for hypertension, dyslipidemia, and in 2011, an unprovoked deep vein thrombosis (DVT) in the right leg. He reported no history of tobacco, alcohol, or illicit drug use. He had served in the U.S. Navy, working in electronics, intelligence, data systems, and satellite communications.
On initial physical examination, the patient was afebrile and lethargic, and his blood pressure was mildly elevated (144/83 mm Hg). Cardiopulmonary examination was normal. Neurologic examination revealed no severe focal deficits, and cranial nerves II to XII were intact. Funduscopic examination was normal, with no papilledema noted. Motor strength was 5/5 bilaterally in the deltoids, biceps, triceps, radial and ulnar wrist extensors, iliopsoas, quadriceps, hamstrings, tibialis anterior and posterior, fibulares, and gastrocnemius. Pinprick sensation and light-touch sensation were decreased within the lateral bicipital region of the left upper extremity. Pinprick sensation was intact bilaterally in 1-inch increments at all other distributions along the medial and lateral aspects of the upper and lower extremities. Muscle tone and bulk were normal in all extremities. Reflexes were 2+ bilaterally in the biceps, brachioradialis, triceps, quadriceps, and Achilles. The Babinski sign was absent bilaterally, the finger-tonose and heel-to-shin tests were normal, the Romberg sign was absent, and there was no evidence of pronator drift. Laboratory test results were normal except for slightly elevated hemoglobin (17.5 g/dL) and D-dimer (588 ng/mL) levels.
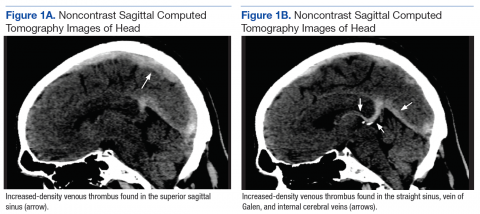
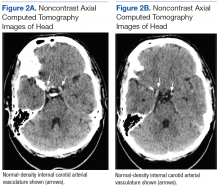
Although noncontrast CT of the head initially showed no acute intracranial abnormalities, retrospective close comparison with the arterial system revealed slightly increased attenuation in the superior sagittal sinus, straight sinus, vein of Galen, and internal cerebral veins (Figures 1A and 1B) relative to the arterial carotid anterior circulation (Figures 2A and 2B).
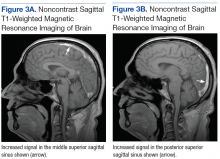
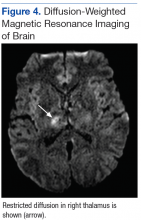
Subsequent brain MRI without contrast showed a hyperintense T1 signal involving the superior sagittal sinus (Figures 3A and 3B), extending into the straight sinus and the vein of Galen. Magnetic resonance imaging with contrast demonstrated a prominent filling defect primarily in the superior sagittal sinus, in the right transverse sinus, and in the vein of Galen. Diffusion-weighted brain MRI sequence showed restricted diffusion localized to the right thalamic area (Figure 4) and no evidence of hemorrhage.
Treatment
International guidelines recommend using heparin to achieve rapid anticoagulation, stop the thrombotic process, and prevent extension of the thrombus.13 Theoretically, more rapid recanalization may have been achieved by performing endovascular thrombectomy in the present case. However, severe bleeding complications, combined with higher cost and the limited availability of clinicians experienced in treating this rare disease, convince physicians to rely on heparin as first-line treatment for CVT.14 A small randomized clinical trial found LMWH safer and more efficacious than UFH in treating CVT.15 After stabilization, oral anticoagulation therapy is used to maintain an international normalized ratio (INR) between 2.0 and 3.0 for at least 3 months.14
Given these findings, the patient was initially treated with LMWH. Eventually he was switched to oral warfarin and showed signs of clinical improvement. A hypercoagulability state workup revealed that the patient was heterozygous for the prothrombin G20210A mutation, and he was discharged and instructed to continue the oral warfarin therapy.
On follow-up, the hematology and neurology team initiated indefinite treatment with warfarin for his genetic hypercoagulability state. Monitoring of the dose of anticoagulation therapy was started to maintain INR between 2.0 and 3.0. The patient began coming to the office for INR monitoring every 2 to 3 weeks, and his most recent INR, in May 2017, was 2.66. He is taking 7.5 mg of warfarin on Wednesdays and Sundays and 5 mg on all other days and currently does not report any progressive neurologic deficits.
Discussion
The clinical findings of CVT and the hypercoagulability state workup revealed that the patient was heterozygous for the prothrombin G20210A mutation. Prothrombin is the precursor to thrombin, which is a key regulator of the clotting cascade and a promoter of coagulation. Carriers of the mutation have elevated levels of blood plasma prothrombin and have been associated with a 4 times higher risk for VTE.16
Several large studies and systematic reviews have confirmed that the prothrombin G20210A mutation is associated with higher rates of VTE, leading to an increased risk for DVT of the leg or pulmonary embolism.17-19 More specifically, a metaanalysis of 15 case–control studies found strong associations between the mutation and CVT.20 Despite this significant association, studies are inconclusive about whether heterozygosity for the mutation is associated with increased rates of recurrent CVT or other VTE in the absence of other risk factors, such as oral contraceptive use, trauma, malignancy, and infection.21-23 Therefore, the optimal duration of anticoagulation therapy for CVT is not well established in patients with the mutation. However, the present patient was started on indefinite anticoagulation therapy because the underlying etiology of the CVT was not reversible or transient, and this CVT was his second episode of VTE, following a 2011 DVT in the right leg.
The case discussed here illustrates the clinical presentation and diagnostic complexities of CVT. Two days before coming to the ED, the patient presented to an outside facility and underwent a workup for nonspecific symptoms (eg, confusion, headaches). Due to the nonspecific presentation associated with CVT, a detailed history is imperative to distinguish symptoms suggesting increased intracranial pressure, such as headaches worse when lying down or present in the morning, with a high clinical suspicion of CVT. The ability to attain these specific details leads clinicians toward obtaining the necessary imaging studies for potential CVT patients, and may prevent delay in diagnosis and treatment. The thrombus in CVT initially consists of deoxyhemoglobin and appears on MRI as an isointense signal on T1-weighted images and a hypointense signal on T2-weighted images; over subsequent days, the thrombus changes to methemoglobin and appears as a slightly hyperintense signal on both T1- and T2-weighted images.24
During this phase, there are some false negatives, as the thrombus can be mistaken for imaging artifacts, hematocrit elevations, or low flow of normal venous blood. Given the clinical findings and imaging studies, it is essential to distinguish CVT from other benign etiologies. Earlier diagnosis and initiation of anticoagulation therapy may have precluded the small amount of localized ischemic changes in this patient’s right thalamus, thus preventing the mild sensory loss in the left upper extremity. With the variable and nonspecific clinical presentations and the difficulties in identifying CVT with first-line imaging, progression of thrombus formation may lead to severe focal neurologic deficits, coma, or death.
Using CT imaging studies to compare the blood in the draining cerebral sinuses with the blood in the arterial system can help distinguish CVT from other etiologies of hyperdense abnormalities, such as increased hematocrit or decreased flow. Retrospective close examination of the present patient’s noncontrast CT images of the head and brain revealed slight hyperdensity in the cerebral sinuses compared with the arterial blood, suggesting the presence of thrombus formation in the cerebral veins. As CT is often the first study used to evaluate the nonspecific clinical presentations of these patients, identifying subtle signaldensity differences between the arterial and venous systems could guide physicians in identifying CVT earlier.
The authors reiterate the importance of meticulous imaging interpretation in light of the entire clinical picture: In these patients, it is imperative to have a high index of clinical suspicion for CVT in order to prevent more serious complications, such as ischemic or hemorrhagic stroke.
1. Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007;6(2):162-170.
2. Coutinho JM. Cerebral venous thrombosis. J Thromb Haemost. 2015;13(suppl 1):S238-S244.
3. Coutinho JM, Ferro JM, Canhão P, et al. Cerebral venous and sinus thrombosis in women. Stroke. 2009;40(7):2356-2361.
4. Zuurbier SM, Coutinho JM. Cerebral venous thrombosis. Adv Exp Med Biol. 2017;906:183-193.
5. Einhäupl KM, Villringer A, Meister W, et al. Heparin treatment in sinus venous thrombosis. Lancet. 1991;338(8767):597-600.
6. de Bruijn SF, Stam J. Randomized, placebocontrolled trial of anticoagulant treatment with lowmolecular-weight heparin for cerebral sinus thrombosis. Stroke. 1999;30(3):484-488.
7. Nagaraja D, Haridas T, Taly AB, Veerendrakumar M, SubbuKrishna DK. Puerperal cerebral venous thrombosis: therapeutic benefit of low dose heparin. Neurol India. 1999;47(1):43-46.
8. Coutinho JM, de Bruijn SF, deVeber G, Stam J. Anticoagulation for cerebral venous sinus thrombosis. Stroke. 2012;43(4):e41-e42.
9. Sassi SB, Touati N, Baccouche H, Drissi C, Romdhane NB, Hentati F. Cerebral venous thrombosis. Clin Appl Thromb Hemost. 2016:1076029616665168. [Epub ahead of print.]
10. Ferro JM, Canhão P. Cerebral venous sinus thrombosis: update on diagnosis and management. Curr Cardiol Rep. 2014;16(9):523.
11. Albright KC, Freeman WD, Kruse BT. Cerebral venous thrombosis. J Emerg Med. 2010;38(2):238-239.
12. Lafitte F, Boukobza M, Guichard JP, et al. MRI and MRA for diagnosis and follow-up of cerebral venous thrombosis (CVT). Clin Radiol. 1997;52(9):672-679.
13. Saposnik G, Barinagarrementeria F, Brown RD Jr, et al; American Heart Association Stroke Council and Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158-1192.
14. Coutinho JM, Middeldorp S, Stam J. Advances in the treatment of cerebral venous thrombosis. Curr Treat Options Neurol. 2014;16(7):299.
15. Coutinho JM, Ferro JM, Canhão P, Barinagarrementeria F, Bousser MG, Stam J; ISCVT Investigators. Unfractionated or low-molecular weight heparin for the treatment of cerebral venous thrombosis. Stroke. 2010;41(11):2575-2580.
16. Rosendaal FR. Venous thrombosis: the role of genes, environment, and behavior. Hematology Am Soc Hematol Educ Program. 2005:1-12.
17. Dentali F, Crowther M, Ageno W. Thrombophilic abnormalities, oral contraceptives, and risk of cerebral vein thrombosis: a meta-analysis. Blood.2006;107(7):2766-2773.
18. Salomon O, Steinberg DM, Zivelin A, et al. Single and combined prothrombotic factors in patients with idiopathic venous thromboembolism: prevalence and risk assessment. Arterioscler Thromb Vasc Biol. 1999;19(3):511-518.
19. Emmerich J, Rosendaal FR, Cattaneo M, et al. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism— pooled analysis of 8 case–control studies including 2310 cases and 3204 controls. Study Group for Pooled-Analysis in Venous Thromboembolism. Thromb Haemost. 2001;86(3):809-816.
20. Lauw MN, Barco S, Coutinho JM, Middeldorp S. Cerebral venous thrombosis and thrombophilia: a systematic review and meta-analysis. Semin Thromb Hemost. 2013;39(8):913-927.
21. Dentali F, Poli D, Scoditti U, et al. Long-term outcomes of patients with cerebral vein thrombosis: a multicenter study. J Thromb Haemost. 2012;10(7):1297-1302.
22. Martinelli I, Bucciarelli P, Passamonti SM, Battaglioli T, Previtali E, Mannucci PM. Long-term evaluation of the risk of recurrence after cerebral sinus-venous thrombosis. Circulation. 2010;121(25):2740-2746.
23. Gosk-Bierska I, Wysokinski W, Brown RD Jr, et al. Cerebral venous sinus thrombosis: incidence of venous thrombosis recurrence and survival. Neurology. 2006;67(5):814-819.
24. Galidie G, Le Gall R, Cordoliani YS, Pharaboz C, Le Marec E, Cosnard G. Thrombosis of the cerebral veins. X-ray computed tomography and MRI imaging. 11 cases [in French]. J Radiol. 1992;73(3):175-190
Cerebral venous thrombosis (CVT) is a rare cerebrovascular disease that affects about 5 in 1 million people each year and accounts for 0.5% of all strokes.1 Previously it was thought to be caused most commonly by infections (eg, mastoiditis, otitis, meningitis) affecting the superior sagittal sinus and often resulting in focal neurologic deficits, seizures, coma, and death. Although local and systemic infections are still prominent risk factors in its development, CVT is now primarily recognized as a nonseptic disease with a wide spectrum of clinical presentations.
Cerebral venous thrombosis causes reduced outflow of blood and cerebrospinal fluid, which in half of affected patients leads to significant venous infarct. As opposed to arterial infarctions, CVT mainly affects children and young adults; it is an important cause of stroke in the younger population.2 There is significant overlap of the many risk factors for CVT and those for venous thromboembolism (VTE): cancer, obesity, genetic thrombophilia, trauma, infection, and prior neurosurgery. However, the most common acquired risk factors for CVT are oral contraceptive use and pregnancy, which explains why CVT is 3 times more likely to occur in young and middle-aged women.3
Cerebral venous thrombosis was first recognized by a French physician in the 19th century, a time when the condition was diagnosed at autopsy, which typically showed hemorrhagic lesions at the thrombus site.4 For many years heparin was contraindicated in the treatment of CVT, and only within the past 25 years did advances in neuroimaging allow for earlier diagnosis and change perspectives on the management of this disease.
Cerebral venous thrombosis occurs from formation of a thrombus within the cerebral venous sinuses, leading to elevated intracranial pressure and eventually ischemia or intracranial hemorrhage. Improved imaging techniques, notably magnetic resonance imaging (MRI) and computed tomography (CT) venography, allow physicians to identify thrombus formation earlier and begin anticoagulation therapy with heparin before infarction. A meta-analysis of studies found that heparin was safer and more efficacious in treating CVT compared with placebo.5 Furthermore, several small randomized trials found treatment with unfractionated heparin (UFH) or low-molecularweight heparin (LMWH) was not associated with higher risk of hemorrhagic stroke in these patients.6-8
Despite improvements in imaging modalities, in many cases diagnosis is delayed, as most patients with CVT have a wide spectrum of presentations with nonspecific symptoms, such as headache, seizure, focal sensorimotor deficits, and papilledema.9 Clinical presentations of CVT depend on a variety of factors, including age, time of onset, CVT location, and presence of parenchymal lesions. Isolated headache is the most common initial symptom, and in many cases is the only presenting manifestation of CVT.1 Encephalopathy with multifocal signs, delirium, or dysfunction in executive functions most commonly occurs in elderly populations.
Cavernous sinus thrombosis most commonly produces generalized headaches, orbital pain, proptosis, and diplopia, whereas cortical vein thrombosis often produces seizures and focal sensorimotor deficits. Aphasia may be present in patients with isolated left transverse sinus thrombosis. In the presence of deep cerebral venous occlusion, patients can present in coma or with severe cognitive deficits and widespread paresis.10 Thrombosis of different veins and sinuses results in a wide spectrum of diverse clinical pictures, posing a diagnostic challenge and affecting clinical outcomes.
Given the variable and often nonspecific clinical presentations of these cases, unenhanced CT typically is the first imaging study ordered. According to the literature, noncontrast CT is not sensitive (25%-56%) in detecting CVT, and findings are normal in up to 26% of patients, rarely providing a specific diagnosis.11 Furthermore, visualization on MRI can be difficult during the acute phase of CVT, as the thrombus initially appears isointense on T1-weighted images and gradually becomes hyperintense over the course of the disease process.12 These difficulties with the usual first-choice imaging examinations often result in a delay in diagnosing CVT. However, several points on close examination of these imaging studies may help physicians establish a high index of clinical suspicion and order the appropriate follow-up studies for CVT.
The authors report the case of a patient who presented with a 1-week history of confusion, headaches, and dizziness. His nonspecific presentation along with the absence of obvious signs of CVT on imaging prolonged his diagnosis and the initiation of an appropriate treatment plan.
Case Report
A 57-year-old white air-conditioning mechanic presented to the emergency department (ED) with a 1-week history of gradual-onset confusion, severe headaches, dizziness, light-headedness, poor memory, and increased sleep. Two days earlier, he presented with similar symptoms to an outside facility, where he was admitted and underwent a workup for stroke, hemorrhage, and cardiac abnormalities—including noncontrast CT of the head. With nothing of clinical significance found, the patient was discharged and was advised to follow up on an outpatient basis.
Persisting symptoms brought the patient to the ED 1 day later, and he was admitted. He described severe, progressive, generalized headaches that were more severe when he was lying down at night and waking in the morning. He did not report that the headaches worsened with coughing or straining, and he reported no history of trauma, neck stiffness, vision change, seizures, and migraines. His medical history was significant for hypertension, dyslipidemia, and in 2011, an unprovoked deep vein thrombosis (DVT) in the right leg. He reported no history of tobacco, alcohol, or illicit drug use. He had served in the U.S. Navy, working in electronics, intelligence, data systems, and satellite communications.
On initial physical examination, the patient was afebrile and lethargic, and his blood pressure was mildly elevated (144/83 mm Hg). Cardiopulmonary examination was normal. Neurologic examination revealed no severe focal deficits, and cranial nerves II to XII were intact. Funduscopic examination was normal, with no papilledema noted. Motor strength was 5/5 bilaterally in the deltoids, biceps, triceps, radial and ulnar wrist extensors, iliopsoas, quadriceps, hamstrings, tibialis anterior and posterior, fibulares, and gastrocnemius. Pinprick sensation and light-touch sensation were decreased within the lateral bicipital region of the left upper extremity. Pinprick sensation was intact bilaterally in 1-inch increments at all other distributions along the medial and lateral aspects of the upper and lower extremities. Muscle tone and bulk were normal in all extremities. Reflexes were 2+ bilaterally in the biceps, brachioradialis, triceps, quadriceps, and Achilles. The Babinski sign was absent bilaterally, the finger-tonose and heel-to-shin tests were normal, the Romberg sign was absent, and there was no evidence of pronator drift. Laboratory test results were normal except for slightly elevated hemoglobin (17.5 g/dL) and D-dimer (588 ng/mL) levels.
Although noncontrast CT of the head initially showed no acute intracranial abnormalities, retrospective close comparison with the arterial system revealed slightly increased attenuation in the superior sagittal sinus, straight sinus, vein of Galen, and internal cerebral veins (Figures 1A and 1B) relative to the arterial carotid anterior circulation (Figures 2A and 2B).
Subsequent brain MRI without contrast showed a hyperintense T1 signal involving the superior sagittal sinus (Figures 3A and 3B), extending into the straight sinus and the vein of Galen. Magnetic resonance imaging with contrast demonstrated a prominent filling defect primarily in the superior sagittal sinus, in the right transverse sinus, and in the vein of Galen. Diffusion-weighted brain MRI sequence showed restricted diffusion localized to the right thalamic area (Figure 4) and no evidence of hemorrhage.
Treatment
International guidelines recommend using heparin to achieve rapid anticoagulation, stop the thrombotic process, and prevent extension of the thrombus.13 Theoretically, more rapid recanalization may have been achieved by performing endovascular thrombectomy in the present case. However, severe bleeding complications, combined with higher cost and the limited availability of clinicians experienced in treating this rare disease, convince physicians to rely on heparin as first-line treatment for CVT.14 A small randomized clinical trial found LMWH safer and more efficacious than UFH in treating CVT.15 After stabilization, oral anticoagulation therapy is used to maintain an international normalized ratio (INR) between 2.0 and 3.0 for at least 3 months.14
Given these findings, the patient was initially treated with LMWH. Eventually he was switched to oral warfarin and showed signs of clinical improvement. A hypercoagulability state workup revealed that the patient was heterozygous for the prothrombin G20210A mutation, and he was discharged and instructed to continue the oral warfarin therapy.
On follow-up, the hematology and neurology team initiated indefinite treatment with warfarin for his genetic hypercoagulability state. Monitoring of the dose of anticoagulation therapy was started to maintain INR between 2.0 and 3.0. The patient began coming to the office for INR monitoring every 2 to 3 weeks, and his most recent INR, in May 2017, was 2.66. He is taking 7.5 mg of warfarin on Wednesdays and Sundays and 5 mg on all other days and currently does not report any progressive neurologic deficits.
Discussion
The clinical findings of CVT and the hypercoagulability state workup revealed that the patient was heterozygous for the prothrombin G20210A mutation. Prothrombin is the precursor to thrombin, which is a key regulator of the clotting cascade and a promoter of coagulation. Carriers of the mutation have elevated levels of blood plasma prothrombin and have been associated with a 4 times higher risk for VTE.16
Several large studies and systematic reviews have confirmed that the prothrombin G20210A mutation is associated with higher rates of VTE, leading to an increased risk for DVT of the leg or pulmonary embolism.17-19 More specifically, a metaanalysis of 15 case–control studies found strong associations between the mutation and CVT.20 Despite this significant association, studies are inconclusive about whether heterozygosity for the mutation is associated with increased rates of recurrent CVT or other VTE in the absence of other risk factors, such as oral contraceptive use, trauma, malignancy, and infection.21-23 Therefore, the optimal duration of anticoagulation therapy for CVT is not well established in patients with the mutation. However, the present patient was started on indefinite anticoagulation therapy because the underlying etiology of the CVT was not reversible or transient, and this CVT was his second episode of VTE, following a 2011 DVT in the right leg.
The case discussed here illustrates the clinical presentation and diagnostic complexities of CVT. Two days before coming to the ED, the patient presented to an outside facility and underwent a workup for nonspecific symptoms (eg, confusion, headaches). Due to the nonspecific presentation associated with CVT, a detailed history is imperative to distinguish symptoms suggesting increased intracranial pressure, such as headaches worse when lying down or present in the morning, with a high clinical suspicion of CVT. The ability to attain these specific details leads clinicians toward obtaining the necessary imaging studies for potential CVT patients, and may prevent delay in diagnosis and treatment. The thrombus in CVT initially consists of deoxyhemoglobin and appears on MRI as an isointense signal on T1-weighted images and a hypointense signal on T2-weighted images; over subsequent days, the thrombus changes to methemoglobin and appears as a slightly hyperintense signal on both T1- and T2-weighted images.24
During this phase, there are some false negatives, as the thrombus can be mistaken for imaging artifacts, hematocrit elevations, or low flow of normal venous blood. Given the clinical findings and imaging studies, it is essential to distinguish CVT from other benign etiologies. Earlier diagnosis and initiation of anticoagulation therapy may have precluded the small amount of localized ischemic changes in this patient’s right thalamus, thus preventing the mild sensory loss in the left upper extremity. With the variable and nonspecific clinical presentations and the difficulties in identifying CVT with first-line imaging, progression of thrombus formation may lead to severe focal neurologic deficits, coma, or death.
Using CT imaging studies to compare the blood in the draining cerebral sinuses with the blood in the arterial system can help distinguish CVT from other etiologies of hyperdense abnormalities, such as increased hematocrit or decreased flow. Retrospective close examination of the present patient’s noncontrast CT images of the head and brain revealed slight hyperdensity in the cerebral sinuses compared with the arterial blood, suggesting the presence of thrombus formation in the cerebral veins. As CT is often the first study used to evaluate the nonspecific clinical presentations of these patients, identifying subtle signaldensity differences between the arterial and venous systems could guide physicians in identifying CVT earlier.
The authors reiterate the importance of meticulous imaging interpretation in light of the entire clinical picture: In these patients, it is imperative to have a high index of clinical suspicion for CVT in order to prevent more serious complications, such as ischemic or hemorrhagic stroke.
Cerebral venous thrombosis (CVT) is a rare cerebrovascular disease that affects about 5 in 1 million people each year and accounts for 0.5% of all strokes.1 Previously it was thought to be caused most commonly by infections (eg, mastoiditis, otitis, meningitis) affecting the superior sagittal sinus and often resulting in focal neurologic deficits, seizures, coma, and death. Although local and systemic infections are still prominent risk factors in its development, CVT is now primarily recognized as a nonseptic disease with a wide spectrum of clinical presentations.
Cerebral venous thrombosis causes reduced outflow of blood and cerebrospinal fluid, which in half of affected patients leads to significant venous infarct. As opposed to arterial infarctions, CVT mainly affects children and young adults; it is an important cause of stroke in the younger population.2 There is significant overlap of the many risk factors for CVT and those for venous thromboembolism (VTE): cancer, obesity, genetic thrombophilia, trauma, infection, and prior neurosurgery. However, the most common acquired risk factors for CVT are oral contraceptive use and pregnancy, which explains why CVT is 3 times more likely to occur in young and middle-aged women.3
Cerebral venous thrombosis was first recognized by a French physician in the 19th century, a time when the condition was diagnosed at autopsy, which typically showed hemorrhagic lesions at the thrombus site.4 For many years heparin was contraindicated in the treatment of CVT, and only within the past 25 years did advances in neuroimaging allow for earlier diagnosis and change perspectives on the management of this disease.
Cerebral venous thrombosis occurs from formation of a thrombus within the cerebral venous sinuses, leading to elevated intracranial pressure and eventually ischemia or intracranial hemorrhage. Improved imaging techniques, notably magnetic resonance imaging (MRI) and computed tomography (CT) venography, allow physicians to identify thrombus formation earlier and begin anticoagulation therapy with heparin before infarction. A meta-analysis of studies found that heparin was safer and more efficacious in treating CVT compared with placebo.5 Furthermore, several small randomized trials found treatment with unfractionated heparin (UFH) or low-molecularweight heparin (LMWH) was not associated with higher risk of hemorrhagic stroke in these patients.6-8
Despite improvements in imaging modalities, in many cases diagnosis is delayed, as most patients with CVT have a wide spectrum of presentations with nonspecific symptoms, such as headache, seizure, focal sensorimotor deficits, and papilledema.9 Clinical presentations of CVT depend on a variety of factors, including age, time of onset, CVT location, and presence of parenchymal lesions. Isolated headache is the most common initial symptom, and in many cases is the only presenting manifestation of CVT.1 Encephalopathy with multifocal signs, delirium, or dysfunction in executive functions most commonly occurs in elderly populations.
Cavernous sinus thrombosis most commonly produces generalized headaches, orbital pain, proptosis, and diplopia, whereas cortical vein thrombosis often produces seizures and focal sensorimotor deficits. Aphasia may be present in patients with isolated left transverse sinus thrombosis. In the presence of deep cerebral venous occlusion, patients can present in coma or with severe cognitive deficits and widespread paresis.10 Thrombosis of different veins and sinuses results in a wide spectrum of diverse clinical pictures, posing a diagnostic challenge and affecting clinical outcomes.
Given the variable and often nonspecific clinical presentations of these cases, unenhanced CT typically is the first imaging study ordered. According to the literature, noncontrast CT is not sensitive (25%-56%) in detecting CVT, and findings are normal in up to 26% of patients, rarely providing a specific diagnosis.11 Furthermore, visualization on MRI can be difficult during the acute phase of CVT, as the thrombus initially appears isointense on T1-weighted images and gradually becomes hyperintense over the course of the disease process.12 These difficulties with the usual first-choice imaging examinations often result in a delay in diagnosing CVT. However, several points on close examination of these imaging studies may help physicians establish a high index of clinical suspicion and order the appropriate follow-up studies for CVT.
The authors report the case of a patient who presented with a 1-week history of confusion, headaches, and dizziness. His nonspecific presentation along with the absence of obvious signs of CVT on imaging prolonged his diagnosis and the initiation of an appropriate treatment plan.
Case Report
A 57-year-old white air-conditioning mechanic presented to the emergency department (ED) with a 1-week history of gradual-onset confusion, severe headaches, dizziness, light-headedness, poor memory, and increased sleep. Two days earlier, he presented with similar symptoms to an outside facility, where he was admitted and underwent a workup for stroke, hemorrhage, and cardiac abnormalities—including noncontrast CT of the head. With nothing of clinical significance found, the patient was discharged and was advised to follow up on an outpatient basis.
Persisting symptoms brought the patient to the ED 1 day later, and he was admitted. He described severe, progressive, generalized headaches that were more severe when he was lying down at night and waking in the morning. He did not report that the headaches worsened with coughing or straining, and he reported no history of trauma, neck stiffness, vision change, seizures, and migraines. His medical history was significant for hypertension, dyslipidemia, and in 2011, an unprovoked deep vein thrombosis (DVT) in the right leg. He reported no history of tobacco, alcohol, or illicit drug use. He had served in the U.S. Navy, working in electronics, intelligence, data systems, and satellite communications.
On initial physical examination, the patient was afebrile and lethargic, and his blood pressure was mildly elevated (144/83 mm Hg). Cardiopulmonary examination was normal. Neurologic examination revealed no severe focal deficits, and cranial nerves II to XII were intact. Funduscopic examination was normal, with no papilledema noted. Motor strength was 5/5 bilaterally in the deltoids, biceps, triceps, radial and ulnar wrist extensors, iliopsoas, quadriceps, hamstrings, tibialis anterior and posterior, fibulares, and gastrocnemius. Pinprick sensation and light-touch sensation were decreased within the lateral bicipital region of the left upper extremity. Pinprick sensation was intact bilaterally in 1-inch increments at all other distributions along the medial and lateral aspects of the upper and lower extremities. Muscle tone and bulk were normal in all extremities. Reflexes were 2+ bilaterally in the biceps, brachioradialis, triceps, quadriceps, and Achilles. The Babinski sign was absent bilaterally, the finger-tonose and heel-to-shin tests were normal, the Romberg sign was absent, and there was no evidence of pronator drift. Laboratory test results were normal except for slightly elevated hemoglobin (17.5 g/dL) and D-dimer (588 ng/mL) levels.
Although noncontrast CT of the head initially showed no acute intracranial abnormalities, retrospective close comparison with the arterial system revealed slightly increased attenuation in the superior sagittal sinus, straight sinus, vein of Galen, and internal cerebral veins (Figures 1A and 1B) relative to the arterial carotid anterior circulation (Figures 2A and 2B).
Subsequent brain MRI without contrast showed a hyperintense T1 signal involving the superior sagittal sinus (Figures 3A and 3B), extending into the straight sinus and the vein of Galen. Magnetic resonance imaging with contrast demonstrated a prominent filling defect primarily in the superior sagittal sinus, in the right transverse sinus, and in the vein of Galen. Diffusion-weighted brain MRI sequence showed restricted diffusion localized to the right thalamic area (Figure 4) and no evidence of hemorrhage.
Treatment
International guidelines recommend using heparin to achieve rapid anticoagulation, stop the thrombotic process, and prevent extension of the thrombus.13 Theoretically, more rapid recanalization may have been achieved by performing endovascular thrombectomy in the present case. However, severe bleeding complications, combined with higher cost and the limited availability of clinicians experienced in treating this rare disease, convince physicians to rely on heparin as first-line treatment for CVT.14 A small randomized clinical trial found LMWH safer and more efficacious than UFH in treating CVT.15 After stabilization, oral anticoagulation therapy is used to maintain an international normalized ratio (INR) between 2.0 and 3.0 for at least 3 months.14
Given these findings, the patient was initially treated with LMWH. Eventually he was switched to oral warfarin and showed signs of clinical improvement. A hypercoagulability state workup revealed that the patient was heterozygous for the prothrombin G20210A mutation, and he was discharged and instructed to continue the oral warfarin therapy.
On follow-up, the hematology and neurology team initiated indefinite treatment with warfarin for his genetic hypercoagulability state. Monitoring of the dose of anticoagulation therapy was started to maintain INR between 2.0 and 3.0. The patient began coming to the office for INR monitoring every 2 to 3 weeks, and his most recent INR, in May 2017, was 2.66. He is taking 7.5 mg of warfarin on Wednesdays and Sundays and 5 mg on all other days and currently does not report any progressive neurologic deficits.
Discussion
The clinical findings of CVT and the hypercoagulability state workup revealed that the patient was heterozygous for the prothrombin G20210A mutation. Prothrombin is the precursor to thrombin, which is a key regulator of the clotting cascade and a promoter of coagulation. Carriers of the mutation have elevated levels of blood plasma prothrombin and have been associated with a 4 times higher risk for VTE.16
Several large studies and systematic reviews have confirmed that the prothrombin G20210A mutation is associated with higher rates of VTE, leading to an increased risk for DVT of the leg or pulmonary embolism.17-19 More specifically, a metaanalysis of 15 case–control studies found strong associations between the mutation and CVT.20 Despite this significant association, studies are inconclusive about whether heterozygosity for the mutation is associated with increased rates of recurrent CVT or other VTE in the absence of other risk factors, such as oral contraceptive use, trauma, malignancy, and infection.21-23 Therefore, the optimal duration of anticoagulation therapy for CVT is not well established in patients with the mutation. However, the present patient was started on indefinite anticoagulation therapy because the underlying etiology of the CVT was not reversible or transient, and this CVT was his second episode of VTE, following a 2011 DVT in the right leg.
The case discussed here illustrates the clinical presentation and diagnostic complexities of CVT. Two days before coming to the ED, the patient presented to an outside facility and underwent a workup for nonspecific symptoms (eg, confusion, headaches). Due to the nonspecific presentation associated with CVT, a detailed history is imperative to distinguish symptoms suggesting increased intracranial pressure, such as headaches worse when lying down or present in the morning, with a high clinical suspicion of CVT. The ability to attain these specific details leads clinicians toward obtaining the necessary imaging studies for potential CVT patients, and may prevent delay in diagnosis and treatment. The thrombus in CVT initially consists of deoxyhemoglobin and appears on MRI as an isointense signal on T1-weighted images and a hypointense signal on T2-weighted images; over subsequent days, the thrombus changes to methemoglobin and appears as a slightly hyperintense signal on both T1- and T2-weighted images.24
During this phase, there are some false negatives, as the thrombus can be mistaken for imaging artifacts, hematocrit elevations, or low flow of normal venous blood. Given the clinical findings and imaging studies, it is essential to distinguish CVT from other benign etiologies. Earlier diagnosis and initiation of anticoagulation therapy may have precluded the small amount of localized ischemic changes in this patient’s right thalamus, thus preventing the mild sensory loss in the left upper extremity. With the variable and nonspecific clinical presentations and the difficulties in identifying CVT with first-line imaging, progression of thrombus formation may lead to severe focal neurologic deficits, coma, or death.
Using CT imaging studies to compare the blood in the draining cerebral sinuses with the blood in the arterial system can help distinguish CVT from other etiologies of hyperdense abnormalities, such as increased hematocrit or decreased flow. Retrospective close examination of the present patient’s noncontrast CT images of the head and brain revealed slight hyperdensity in the cerebral sinuses compared with the arterial blood, suggesting the presence of thrombus formation in the cerebral veins. As CT is often the first study used to evaluate the nonspecific clinical presentations of these patients, identifying subtle signaldensity differences between the arterial and venous systems could guide physicians in identifying CVT earlier.
The authors reiterate the importance of meticulous imaging interpretation in light of the entire clinical picture: In these patients, it is imperative to have a high index of clinical suspicion for CVT in order to prevent more serious complications, such as ischemic or hemorrhagic stroke.
1. Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007;6(2):162-170.
2. Coutinho JM. Cerebral venous thrombosis. J Thromb Haemost. 2015;13(suppl 1):S238-S244.
3. Coutinho JM, Ferro JM, Canhão P, et al. Cerebral venous and sinus thrombosis in women. Stroke. 2009;40(7):2356-2361.
4. Zuurbier SM, Coutinho JM. Cerebral venous thrombosis. Adv Exp Med Biol. 2017;906:183-193.
5. Einhäupl KM, Villringer A, Meister W, et al. Heparin treatment in sinus venous thrombosis. Lancet. 1991;338(8767):597-600.
6. de Bruijn SF, Stam J. Randomized, placebocontrolled trial of anticoagulant treatment with lowmolecular-weight heparin for cerebral sinus thrombosis. Stroke. 1999;30(3):484-488.
7. Nagaraja D, Haridas T, Taly AB, Veerendrakumar M, SubbuKrishna DK. Puerperal cerebral venous thrombosis: therapeutic benefit of low dose heparin. Neurol India. 1999;47(1):43-46.
8. Coutinho JM, de Bruijn SF, deVeber G, Stam J. Anticoagulation for cerebral venous sinus thrombosis. Stroke. 2012;43(4):e41-e42.
9. Sassi SB, Touati N, Baccouche H, Drissi C, Romdhane NB, Hentati F. Cerebral venous thrombosis. Clin Appl Thromb Hemost. 2016:1076029616665168. [Epub ahead of print.]
10. Ferro JM, Canhão P. Cerebral venous sinus thrombosis: update on diagnosis and management. Curr Cardiol Rep. 2014;16(9):523.
11. Albright KC, Freeman WD, Kruse BT. Cerebral venous thrombosis. J Emerg Med. 2010;38(2):238-239.
12. Lafitte F, Boukobza M, Guichard JP, et al. MRI and MRA for diagnosis and follow-up of cerebral venous thrombosis (CVT). Clin Radiol. 1997;52(9):672-679.
13. Saposnik G, Barinagarrementeria F, Brown RD Jr, et al; American Heart Association Stroke Council and Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158-1192.
14. Coutinho JM, Middeldorp S, Stam J. Advances in the treatment of cerebral venous thrombosis. Curr Treat Options Neurol. 2014;16(7):299.
15. Coutinho JM, Ferro JM, Canhão P, Barinagarrementeria F, Bousser MG, Stam J; ISCVT Investigators. Unfractionated or low-molecular weight heparin for the treatment of cerebral venous thrombosis. Stroke. 2010;41(11):2575-2580.
16. Rosendaal FR. Venous thrombosis: the role of genes, environment, and behavior. Hematology Am Soc Hematol Educ Program. 2005:1-12.
17. Dentali F, Crowther M, Ageno W. Thrombophilic abnormalities, oral contraceptives, and risk of cerebral vein thrombosis: a meta-analysis. Blood.2006;107(7):2766-2773.
18. Salomon O, Steinberg DM, Zivelin A, et al. Single and combined prothrombotic factors in patients with idiopathic venous thromboembolism: prevalence and risk assessment. Arterioscler Thromb Vasc Biol. 1999;19(3):511-518.
19. Emmerich J, Rosendaal FR, Cattaneo M, et al. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism— pooled analysis of 8 case–control studies including 2310 cases and 3204 controls. Study Group for Pooled-Analysis in Venous Thromboembolism. Thromb Haemost. 2001;86(3):809-816.
20. Lauw MN, Barco S, Coutinho JM, Middeldorp S. Cerebral venous thrombosis and thrombophilia: a systematic review and meta-analysis. Semin Thromb Hemost. 2013;39(8):913-927.
21. Dentali F, Poli D, Scoditti U, et al. Long-term outcomes of patients with cerebral vein thrombosis: a multicenter study. J Thromb Haemost. 2012;10(7):1297-1302.
22. Martinelli I, Bucciarelli P, Passamonti SM, Battaglioli T, Previtali E, Mannucci PM. Long-term evaluation of the risk of recurrence after cerebral sinus-venous thrombosis. Circulation. 2010;121(25):2740-2746.
23. Gosk-Bierska I, Wysokinski W, Brown RD Jr, et al. Cerebral venous sinus thrombosis: incidence of venous thrombosis recurrence and survival. Neurology. 2006;67(5):814-819.
24. Galidie G, Le Gall R, Cordoliani YS, Pharaboz C, Le Marec E, Cosnard G. Thrombosis of the cerebral veins. X-ray computed tomography and MRI imaging. 11 cases [in French]. J Radiol. 1992;73(3):175-190
1. Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007;6(2):162-170.
2. Coutinho JM. Cerebral venous thrombosis. J Thromb Haemost. 2015;13(suppl 1):S238-S244.
3. Coutinho JM, Ferro JM, Canhão P, et al. Cerebral venous and sinus thrombosis in women. Stroke. 2009;40(7):2356-2361.
4. Zuurbier SM, Coutinho JM. Cerebral venous thrombosis. Adv Exp Med Biol. 2017;906:183-193.
5. Einhäupl KM, Villringer A, Meister W, et al. Heparin treatment in sinus venous thrombosis. Lancet. 1991;338(8767):597-600.
6. de Bruijn SF, Stam J. Randomized, placebocontrolled trial of anticoagulant treatment with lowmolecular-weight heparin for cerebral sinus thrombosis. Stroke. 1999;30(3):484-488.
7. Nagaraja D, Haridas T, Taly AB, Veerendrakumar M, SubbuKrishna DK. Puerperal cerebral venous thrombosis: therapeutic benefit of low dose heparin. Neurol India. 1999;47(1):43-46.
8. Coutinho JM, de Bruijn SF, deVeber G, Stam J. Anticoagulation for cerebral venous sinus thrombosis. Stroke. 2012;43(4):e41-e42.
9. Sassi SB, Touati N, Baccouche H, Drissi C, Romdhane NB, Hentati F. Cerebral venous thrombosis. Clin Appl Thromb Hemost. 2016:1076029616665168. [Epub ahead of print.]
10. Ferro JM, Canhão P. Cerebral venous sinus thrombosis: update on diagnosis and management. Curr Cardiol Rep. 2014;16(9):523.
11. Albright KC, Freeman WD, Kruse BT. Cerebral venous thrombosis. J Emerg Med. 2010;38(2):238-239.
12. Lafitte F, Boukobza M, Guichard JP, et al. MRI and MRA for diagnosis and follow-up of cerebral venous thrombosis (CVT). Clin Radiol. 1997;52(9):672-679.
13. Saposnik G, Barinagarrementeria F, Brown RD Jr, et al; American Heart Association Stroke Council and Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158-1192.
14. Coutinho JM, Middeldorp S, Stam J. Advances in the treatment of cerebral venous thrombosis. Curr Treat Options Neurol. 2014;16(7):299.
15. Coutinho JM, Ferro JM, Canhão P, Barinagarrementeria F, Bousser MG, Stam J; ISCVT Investigators. Unfractionated or low-molecular weight heparin for the treatment of cerebral venous thrombosis. Stroke. 2010;41(11):2575-2580.
16. Rosendaal FR. Venous thrombosis: the role of genes, environment, and behavior. Hematology Am Soc Hematol Educ Program. 2005:1-12.
17. Dentali F, Crowther M, Ageno W. Thrombophilic abnormalities, oral contraceptives, and risk of cerebral vein thrombosis: a meta-analysis. Blood.2006;107(7):2766-2773.
18. Salomon O, Steinberg DM, Zivelin A, et al. Single and combined prothrombotic factors in patients with idiopathic venous thromboembolism: prevalence and risk assessment. Arterioscler Thromb Vasc Biol. 1999;19(3):511-518.
19. Emmerich J, Rosendaal FR, Cattaneo M, et al. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism— pooled analysis of 8 case–control studies including 2310 cases and 3204 controls. Study Group for Pooled-Analysis in Venous Thromboembolism. Thromb Haemost. 2001;86(3):809-816.
20. Lauw MN, Barco S, Coutinho JM, Middeldorp S. Cerebral venous thrombosis and thrombophilia: a systematic review and meta-analysis. Semin Thromb Hemost. 2013;39(8):913-927.
21. Dentali F, Poli D, Scoditti U, et al. Long-term outcomes of patients with cerebral vein thrombosis: a multicenter study. J Thromb Haemost. 2012;10(7):1297-1302.
22. Martinelli I, Bucciarelli P, Passamonti SM, Battaglioli T, Previtali E, Mannucci PM. Long-term evaluation of the risk of recurrence after cerebral sinus-venous thrombosis. Circulation. 2010;121(25):2740-2746.
23. Gosk-Bierska I, Wysokinski W, Brown RD Jr, et al. Cerebral venous sinus thrombosis: incidence of venous thrombosis recurrence and survival. Neurology. 2006;67(5):814-819.
24. Galidie G, Le Gall R, Cordoliani YS, Pharaboz C, Le Marec E, Cosnard G. Thrombosis of the cerebral veins. X-ray computed tomography and MRI imaging. 11 cases [in French]. J Radiol. 1992;73(3):175-190
Acne & Rosacea: July 2017
A supplement to Dermatology News
Articles include:
- Expert shares new insights on the pathophysiology of rosacea
- Acne and diet: Still no solid basis for dietary recommendations
- Rosacea research reveals advances, promising therapies
- Study identifies link between rosacea and several GI disorders
- Caution is key when prescribing spironolactone for adult acne
- Commentary by Dr. Julie C. Harper
- And more …
Faculty:
Julie C. Harper, M.D.
Dr. Harper is a dermatologist in private practice in Alabama at the Dermatology and Skin Care Center of Birmingham. She was a member of the American Academy of Dermatology work group that developed the AAD’s updated guidelines for the management of acne vulgaris, published in February 2016, and is the current president of the American Acne & Rosacea Society. She is also a clinical associate professor at the University of Alabama, Birmingham. Dr. Harper has been a speaker for Allergan, Bayer, Galderma, La Roche-Posay, SkinCeuticals, and Valeant Pharmaceuticals. She is also an investigator for Bayer and an advisory board member for Allergan, Bayer, BioPharmX, Galderma, and Valeant.
Click here to view the supplement
©Copyright 2017, by Frontline Medical Communications Inc. All rights reserved.
A supplement to Dermatology News
Articles include:
- Expert shares new insights on the pathophysiology of rosacea
- Acne and diet: Still no solid basis for dietary recommendations
- Rosacea research reveals advances, promising therapies
- Study identifies link between rosacea and several GI disorders
- Caution is key when prescribing spironolactone for adult acne
- Commentary by Dr. Julie C. Harper
- And more …
Faculty:
Julie C. Harper, M.D.
Dr. Harper is a dermatologist in private practice in Alabama at the Dermatology and Skin Care Center of Birmingham. She was a member of the American Academy of Dermatology work group that developed the AAD’s updated guidelines for the management of acne vulgaris, published in February 2016, and is the current president of the American Acne & Rosacea Society. She is also a clinical associate professor at the University of Alabama, Birmingham. Dr. Harper has been a speaker for Allergan, Bayer, Galderma, La Roche-Posay, SkinCeuticals, and Valeant Pharmaceuticals. She is also an investigator for Bayer and an advisory board member for Allergan, Bayer, BioPharmX, Galderma, and Valeant.
Click here to view the supplement
©Copyright 2017, by Frontline Medical Communications Inc. All rights reserved.
A supplement to Dermatology News
Articles include:
- Expert shares new insights on the pathophysiology of rosacea
- Acne and diet: Still no solid basis for dietary recommendations
- Rosacea research reveals advances, promising therapies
- Study identifies link between rosacea and several GI disorders
- Caution is key when prescribing spironolactone for adult acne
- Commentary by Dr. Julie C. Harper
- And more …
Faculty:
Julie C. Harper, M.D.
Dr. Harper is a dermatologist in private practice in Alabama at the Dermatology and Skin Care Center of Birmingham. She was a member of the American Academy of Dermatology work group that developed the AAD’s updated guidelines for the management of acne vulgaris, published in February 2016, and is the current president of the American Acne & Rosacea Society. She is also a clinical associate professor at the University of Alabama, Birmingham. Dr. Harper has been a speaker for Allergan, Bayer, Galderma, La Roche-Posay, SkinCeuticals, and Valeant Pharmaceuticals. She is also an investigator for Bayer and an advisory board member for Allergan, Bayer, BioPharmX, Galderma, and Valeant.
Click here to view the supplement
©Copyright 2017, by Frontline Medical Communications Inc. All rights reserved.
Gene therapy maintains normal FVIII levels long-term
BERLIN—New research suggests the investigational gene therapy BMN 270 can allow patients with severe hemophilia A to maintain normal factor VIII (FVIII) levels for over a year.
In a phase 1/2 study, patients who received the highest dose of BMN 270 had median and mean FVIII levels that were consistently within the normal range from week 20 to week 52 and beyond.
The highest dose of BMN 270 reduced the mean annual FVIII infusions by 94% and the mean annualized bleed rate (ABR) by 97%, when compared to prophylaxis.
BMN 270 was also considered well tolerated, and none of the patients developed FVIII inhibitors.
The most common adverse event (AE) in this study was alanine aminotransferase (ALT) elevations. In fact, the increase in ALT levels exceeded a pre-specified threshold, which resulted in the study being placed on hold last year.
However, the hold was ultimately lifted. All cases of ALT increase were non-serious, and all patients with ALT increases have either stopped receiving corticosteroids or are being tapered off of them.
John Pasi, PhD, of Barts and the London School of Medicine and Dentistry in London, UK, presented these results as a late-breaking abstract at the International Society on Thrombosis and Haemostasis (ISTH) 2017 Congress. The research was sponsored by BioMarin.
In this phase 1/2 study, 15 patients with severe hemophilia A (FVIII activity levels less than 1%) received a single dose of BMN 270.
Seven patients received a dose of 6e13 vg/kg, 6 received 4e13 vg/kg, and 2 were treated at lower doses as part of dose-escalation and did not achieve therapeutic efficacy.
Safety
Overall, BMN 270 was considered well tolerated across all doses. None of the patients developed inhibitors to FVIII, and none of them withdrew from the study.
The most common AEs across all dose cohorts were ALT elevations (n=10, 67%), arthralgia (n=7, 47%), and back pain, fatigue, and headache (n=5, 33% each).
All ALT increases were non-serious, of limited duration (lasting 0.4 to 7 weeks), and grade 1 in severity. All 7 patients at the 6e13 vg/kg dose remain off of corticosteroids, with no lasting significant impact on FVIII expression and normal ALT levels.
Three of the 6 patients in the 4e13 vg/kg dose cohort received corticosteroids for ALT increases. One has successfully tapered off corticosteroids, and 2 are still tapering off of them.
Two patients reported serious AEs during the study.
One patient was hospitalized for observation after developing grade 2 pyrexia with myalgia and headache within 24 hours of receiving BMN 270 (4e13 vg/kg dose). The event resolved within 48 hours following treatment with paracetamol. The event was considered related to BMN 270.
The other serious AE—planned total knee replacement for chronic arthropathy—was not related to BMN 270.
FVIII levels: 6e13 vg/kg
For patients in the 6e13 vg/kg dose cohort, median and mean FVIII levels from week 20 through 52 have been consistently within the normal levels post-treatment. At 1 year after dosing, the median and mean FVIII levels continue to be above 50%.
Table 1: FVIII levels (%) of 6e13 vg/kg dose patients by visit (n=7)
| Week*
|
20 | 24 | 28 | 32 | 36 | 40 | 44 | 48 | 52 |
| 6e13 vg/kg dose | |||||||||
| N** | 7 | 7 | 7 | 6 | 7 | 7 | 7 | 7 | 7 |
| Median
FVIII level*** (%) |
97 | 101 | 122 | 99 | 99 | 111 | 105 | 105 | 89 |
| Mean
FVIII level*** (%) |
118 | 129 | 123 | 122 | 116 | 124 | 122 | 106 | 104 |
| Range
(low, high) |
(12, 254) | (12, 227) | (15, 257) | (26, 316) | (31, 273) | (17, 264) | (20,242) | (23,196) | (20, 218) |
* Weeks were windowed by +/- 2 weeks
** For week 32, one patient had no FVIII reading
***Bolded numbers are in the normal range of FVIII as defined by the World Federation of Hemophilia
FVIII levels: 4e13 vg/kg
For the 4e13 vg/kg dose cohort, the median and mean FVIII levels from week 8 to 24 are in the mild level. Three of these subjects, who have been observed for 24 weeks, are at the upper end of mild.
Table 2: FVIII levels (%) of 4e13 vg/kg dose patients* by visit (n=6)
| Week*
|
4 | 8 | 12 | 16 | 20 | 24 |
|
4e13 vg/kg dose |
||||||
| N | 6 | 6 | 6 | 3 | 3 | 3 |
| Median
FVIII level** (%) |
4 | 15 | 21 | 35 | 37 | 33 |
| Mean
FVIII level** (%) |
5 | 13 | 19 | 33 | 38 | 33 |
| Range
(low, high) |
(2,10) | (3,21) | (6,32) | (28,38) | (31,45) | (24,41) |
*Weeks were windowed by +/- 2 weeks
** Bolded numbers are in the mild range of FVIII as defined by the World Federation of Hemophilia
ABR and FVIII infusions
Six of the 7 patients in the 6e13 vg/kg cohort were on pre-study prophylaxis.
After these patients received a single dose BMN270 and reached a FVIII level above 5%, the mean ABR was reduced by 97%, from 16.3 to 0.5. The median ABR for these patients was reduced from 16.5 to 0.
The mean annualized FVIII infusions were reduced by 94%, from 136.7 to 8.5. And the median annualized FVIII infusions were reduced from 138.5 to 0.
All 6 patients in the 4e13 vg/kg dose cohort were on pre-study prophylaxis.
The mean ABR was reduced from 12.2 pre-study to 0 after they reached an FVIII level above 5%. The median ABR for these patients was reduced from 8.0 to 0.
The mean annualized FVIII infusions were reduced from 144.2 to 0. The median annualized FVIII infusions were reduced from 155.5 to 0.
“Patients at the 2 highest doses have stopped prophylactic treatment and, to date, bleeds have effectively been eliminated,” Dr Pasi noted.
Based on these results, BioMarin is planning a phase 3 registrational study of BMN 270. The study will likely include fewer than 100 patients and collect data for no longer than a year after a single dose of BMN 270, with subsequent long-term follow-up.
The company is moving the 6e13 vg/kg dose forward but will consider doing an additional study with the 4e13 vg/kg dose at a later date. ![]()
BERLIN—New research suggests the investigational gene therapy BMN 270 can allow patients with severe hemophilia A to maintain normal factor VIII (FVIII) levels for over a year.
In a phase 1/2 study, patients who received the highest dose of BMN 270 had median and mean FVIII levels that were consistently within the normal range from week 20 to week 52 and beyond.
The highest dose of BMN 270 reduced the mean annual FVIII infusions by 94% and the mean annualized bleed rate (ABR) by 97%, when compared to prophylaxis.
BMN 270 was also considered well tolerated, and none of the patients developed FVIII inhibitors.
The most common adverse event (AE) in this study was alanine aminotransferase (ALT) elevations. In fact, the increase in ALT levels exceeded a pre-specified threshold, which resulted in the study being placed on hold last year.
However, the hold was ultimately lifted. All cases of ALT increase were non-serious, and all patients with ALT increases have either stopped receiving corticosteroids or are being tapered off of them.
John Pasi, PhD, of Barts and the London School of Medicine and Dentistry in London, UK, presented these results as a late-breaking abstract at the International Society on Thrombosis and Haemostasis (ISTH) 2017 Congress. The research was sponsored by BioMarin.
In this phase 1/2 study, 15 patients with severe hemophilia A (FVIII activity levels less than 1%) received a single dose of BMN 270.
Seven patients received a dose of 6e13 vg/kg, 6 received 4e13 vg/kg, and 2 were treated at lower doses as part of dose-escalation and did not achieve therapeutic efficacy.
Safety
Overall, BMN 270 was considered well tolerated across all doses. None of the patients developed inhibitors to FVIII, and none of them withdrew from the study.
The most common AEs across all dose cohorts were ALT elevations (n=10, 67%), arthralgia (n=7, 47%), and back pain, fatigue, and headache (n=5, 33% each).
All ALT increases were non-serious, of limited duration (lasting 0.4 to 7 weeks), and grade 1 in severity. All 7 patients at the 6e13 vg/kg dose remain off of corticosteroids, with no lasting significant impact on FVIII expression and normal ALT levels.
Three of the 6 patients in the 4e13 vg/kg dose cohort received corticosteroids for ALT increases. One has successfully tapered off corticosteroids, and 2 are still tapering off of them.
Two patients reported serious AEs during the study.
One patient was hospitalized for observation after developing grade 2 pyrexia with myalgia and headache within 24 hours of receiving BMN 270 (4e13 vg/kg dose). The event resolved within 48 hours following treatment with paracetamol. The event was considered related to BMN 270.
The other serious AE—planned total knee replacement for chronic arthropathy—was not related to BMN 270.
FVIII levels: 6e13 vg/kg
For patients in the 6e13 vg/kg dose cohort, median and mean FVIII levels from week 20 through 52 have been consistently within the normal levels post-treatment. At 1 year after dosing, the median and mean FVIII levels continue to be above 50%.
Table 1: FVIII levels (%) of 6e13 vg/kg dose patients by visit (n=7)
| Week*
|
20 | 24 | 28 | 32 | 36 | 40 | 44 | 48 | 52 |
| 6e13 vg/kg dose | |||||||||
| N** | 7 | 7 | 7 | 6 | 7 | 7 | 7 | 7 | 7 |
| Median
FVIII level*** (%) |
97 | 101 | 122 | 99 | 99 | 111 | 105 | 105 | 89 |
| Mean
FVIII level*** (%) |
118 | 129 | 123 | 122 | 116 | 124 | 122 | 106 | 104 |
| Range
(low, high) |
(12, 254) | (12, 227) | (15, 257) | (26, 316) | (31, 273) | (17, 264) | (20,242) | (23,196) | (20, 218) |
* Weeks were windowed by +/- 2 weeks
** For week 32, one patient had no FVIII reading
***Bolded numbers are in the normal range of FVIII as defined by the World Federation of Hemophilia
FVIII levels: 4e13 vg/kg
For the 4e13 vg/kg dose cohort, the median and mean FVIII levels from week 8 to 24 are in the mild level. Three of these subjects, who have been observed for 24 weeks, are at the upper end of mild.
Table 2: FVIII levels (%) of 4e13 vg/kg dose patients* by visit (n=6)
| Week*
|
4 | 8 | 12 | 16 | 20 | 24 |
|
4e13 vg/kg dose |
||||||
| N | 6 | 6 | 6 | 3 | 3 | 3 |
| Median
FVIII level** (%) |
4 | 15 | 21 | 35 | 37 | 33 |
| Mean
FVIII level** (%) |
5 | 13 | 19 | 33 | 38 | 33 |
| Range
(low, high) |
(2,10) | (3,21) | (6,32) | (28,38) | (31,45) | (24,41) |
*Weeks were windowed by +/- 2 weeks
** Bolded numbers are in the mild range of FVIII as defined by the World Federation of Hemophilia
ABR and FVIII infusions
Six of the 7 patients in the 6e13 vg/kg cohort were on pre-study prophylaxis.
After these patients received a single dose BMN270 and reached a FVIII level above 5%, the mean ABR was reduced by 97%, from 16.3 to 0.5. The median ABR for these patients was reduced from 16.5 to 0.
The mean annualized FVIII infusions were reduced by 94%, from 136.7 to 8.5. And the median annualized FVIII infusions were reduced from 138.5 to 0.
All 6 patients in the 4e13 vg/kg dose cohort were on pre-study prophylaxis.
The mean ABR was reduced from 12.2 pre-study to 0 after they reached an FVIII level above 5%. The median ABR for these patients was reduced from 8.0 to 0.
The mean annualized FVIII infusions were reduced from 144.2 to 0. The median annualized FVIII infusions were reduced from 155.5 to 0.
“Patients at the 2 highest doses have stopped prophylactic treatment and, to date, bleeds have effectively been eliminated,” Dr Pasi noted.
Based on these results, BioMarin is planning a phase 3 registrational study of BMN 270. The study will likely include fewer than 100 patients and collect data for no longer than a year after a single dose of BMN 270, with subsequent long-term follow-up.
The company is moving the 6e13 vg/kg dose forward but will consider doing an additional study with the 4e13 vg/kg dose at a later date. ![]()
BERLIN—New research suggests the investigational gene therapy BMN 270 can allow patients with severe hemophilia A to maintain normal factor VIII (FVIII) levels for over a year.
In a phase 1/2 study, patients who received the highest dose of BMN 270 had median and mean FVIII levels that were consistently within the normal range from week 20 to week 52 and beyond.
The highest dose of BMN 270 reduced the mean annual FVIII infusions by 94% and the mean annualized bleed rate (ABR) by 97%, when compared to prophylaxis.
BMN 270 was also considered well tolerated, and none of the patients developed FVIII inhibitors.
The most common adverse event (AE) in this study was alanine aminotransferase (ALT) elevations. In fact, the increase in ALT levels exceeded a pre-specified threshold, which resulted in the study being placed on hold last year.
However, the hold was ultimately lifted. All cases of ALT increase were non-serious, and all patients with ALT increases have either stopped receiving corticosteroids or are being tapered off of them.
John Pasi, PhD, of Barts and the London School of Medicine and Dentistry in London, UK, presented these results as a late-breaking abstract at the International Society on Thrombosis and Haemostasis (ISTH) 2017 Congress. The research was sponsored by BioMarin.
In this phase 1/2 study, 15 patients with severe hemophilia A (FVIII activity levels less than 1%) received a single dose of BMN 270.
Seven patients received a dose of 6e13 vg/kg, 6 received 4e13 vg/kg, and 2 were treated at lower doses as part of dose-escalation and did not achieve therapeutic efficacy.
Safety
Overall, BMN 270 was considered well tolerated across all doses. None of the patients developed inhibitors to FVIII, and none of them withdrew from the study.
The most common AEs across all dose cohorts were ALT elevations (n=10, 67%), arthralgia (n=7, 47%), and back pain, fatigue, and headache (n=5, 33% each).
All ALT increases were non-serious, of limited duration (lasting 0.4 to 7 weeks), and grade 1 in severity. All 7 patients at the 6e13 vg/kg dose remain off of corticosteroids, with no lasting significant impact on FVIII expression and normal ALT levels.
Three of the 6 patients in the 4e13 vg/kg dose cohort received corticosteroids for ALT increases. One has successfully tapered off corticosteroids, and 2 are still tapering off of them.
Two patients reported serious AEs during the study.
One patient was hospitalized for observation after developing grade 2 pyrexia with myalgia and headache within 24 hours of receiving BMN 270 (4e13 vg/kg dose). The event resolved within 48 hours following treatment with paracetamol. The event was considered related to BMN 270.
The other serious AE—planned total knee replacement for chronic arthropathy—was not related to BMN 270.
FVIII levels: 6e13 vg/kg
For patients in the 6e13 vg/kg dose cohort, median and mean FVIII levels from week 20 through 52 have been consistently within the normal levels post-treatment. At 1 year after dosing, the median and mean FVIII levels continue to be above 50%.
Table 1: FVIII levels (%) of 6e13 vg/kg dose patients by visit (n=7)
| Week*
|
20 | 24 | 28 | 32 | 36 | 40 | 44 | 48 | 52 |
| 6e13 vg/kg dose | |||||||||
| N** | 7 | 7 | 7 | 6 | 7 | 7 | 7 | 7 | 7 |
| Median
FVIII level*** (%) |
97 | 101 | 122 | 99 | 99 | 111 | 105 | 105 | 89 |
| Mean
FVIII level*** (%) |
118 | 129 | 123 | 122 | 116 | 124 | 122 | 106 | 104 |
| Range
(low, high) |
(12, 254) | (12, 227) | (15, 257) | (26, 316) | (31, 273) | (17, 264) | (20,242) | (23,196) | (20, 218) |
* Weeks were windowed by +/- 2 weeks
** For week 32, one patient had no FVIII reading
***Bolded numbers are in the normal range of FVIII as defined by the World Federation of Hemophilia
FVIII levels: 4e13 vg/kg
For the 4e13 vg/kg dose cohort, the median and mean FVIII levels from week 8 to 24 are in the mild level. Three of these subjects, who have been observed for 24 weeks, are at the upper end of mild.
Table 2: FVIII levels (%) of 4e13 vg/kg dose patients* by visit (n=6)
| Week*
|
4 | 8 | 12 | 16 | 20 | 24 |
|
4e13 vg/kg dose |
||||||
| N | 6 | 6 | 6 | 3 | 3 | 3 |
| Median
FVIII level** (%) |
4 | 15 | 21 | 35 | 37 | 33 |
| Mean
FVIII level** (%) |
5 | 13 | 19 | 33 | 38 | 33 |
| Range
(low, high) |
(2,10) | (3,21) | (6,32) | (28,38) | (31,45) | (24,41) |
*Weeks were windowed by +/- 2 weeks
** Bolded numbers are in the mild range of FVIII as defined by the World Federation of Hemophilia
ABR and FVIII infusions
Six of the 7 patients in the 6e13 vg/kg cohort were on pre-study prophylaxis.
After these patients received a single dose BMN270 and reached a FVIII level above 5%, the mean ABR was reduced by 97%, from 16.3 to 0.5. The median ABR for these patients was reduced from 16.5 to 0.
The mean annualized FVIII infusions were reduced by 94%, from 136.7 to 8.5. And the median annualized FVIII infusions were reduced from 138.5 to 0.
All 6 patients in the 4e13 vg/kg dose cohort were on pre-study prophylaxis.
The mean ABR was reduced from 12.2 pre-study to 0 after they reached an FVIII level above 5%. The median ABR for these patients was reduced from 8.0 to 0.
The mean annualized FVIII infusions were reduced from 144.2 to 0. The median annualized FVIII infusions were reduced from 155.5 to 0.
“Patients at the 2 highest doses have stopped prophylactic treatment and, to date, bleeds have effectively been eliminated,” Dr Pasi noted.
Based on these results, BioMarin is planning a phase 3 registrational study of BMN 270. The study will likely include fewer than 100 patients and collect data for no longer than a year after a single dose of BMN 270, with subsequent long-term follow-up.
The company is moving the 6e13 vg/kg dose forward but will consider doing an additional study with the 4e13 vg/kg dose at a later date. ![]()
CTL019 produces responses in rel/ref DLBCL
The chimeric antigen receptor (CAR) T-cell therapy CTL019 produced a high response rate in a phase 2 trial of adults with relapsed/refractory diffuse large B-cell lymphoma (DLBCL), according to researchers.
An interim analysis of the JULIET trial revealed a 3-month overall response rate (ORR) of 45%.
Thirty-seven percent of patients had a complete response (CR), and all of the patients who were in CR at 3 months were still in CR at the data cutoff point.
This confirms the high response rates and durable responses observed in a previous single-center trial*, said JULIET investigator Gilles Salles, MD, PhD, of Hospices Civils de Lyon in Lyon, France.
Dr Salles presented results from JULIET at the 22nd Congress of the European Hematology Association (EHA) in Madrid, Spain (abstract LB2604).
The results were also presented at the 14th International Conference on Malignant Lymphoma (ICML) in Lugano, Switzerland (abstract 007).
The study was sponsored by Novartis.
Patients
JULIET enrolled 141 patients age 18 and older with relapsed/refractory DLBCL.
Forty-three patients discontinued the study before receiving CTL019—28 due to progressive disease (including 16 deaths), 9 due to an inability to manufacture CTL019, 2 due to adverse events, 1 due to investigator decision, 1 due to study withdrawal, and 1 due to protocol deviation.
Eighty-five patients were infused. The remaining 13 patients were pending infusion at the data cutoff—December 20, 2016.
The median age of the 85 infused patients was 56 (range, 24-75). Ninety-six percent of patients had DLBCL not otherwise specified. Fifty-one percent had germinal center B-cell type DLBCL.
Fifty-four percent of patients had an ECOG performance status of 0, and 46% had a status of 1. Fifty-three percent of patients had stage IV disease, and 8% had bone marrow involvement.
Forty percent of patients had 2 prior lines of antineoplastic therapy, 29% had 3, and 31% had 4 to 7 prior lines.
Forty-one percent of patients were refractory to their last therapy, and 59% had relapsed after their last therapy. Fifty-one percent of patients had prior autologous stem cell transplant.
Treatment
Seventy-six patients (89%) received bridging chemotherapy to prevent disease progression during CTL019 manufacturing. This treatment was completed 2 to 14 days prior to CTL019 infusion.
The median CTL019 cell dose was 3.1 x 108 (range, 0.1-6.0 x 108).
Response
Fifty-one patients were evaluated for response. The median time from infusion to data cutoff was 3.7 months.
The best ORR was 59%, with a CR rate of 43% and a partial response rate of 16%. Twelve percent of patients had stable disease, and 24% progressed.
At 3 months, the ORR was 45%. The CR rate was 37%, and the partial response rate was 8%.
“All patients in CR at 3 months remained in CR at follow-up, and the median duration of response was not reached,” Dr Salles noted.
None of the responders went on to receive a transplant.
Safety
All 85 patients were evaluated for safety. Adverse events of special interest, occurring within 8 weeks of CTL019 infusion, were:
- Cytokine release syndrome—57% all grades, 17% grade 3, 9% grade 4
- Infections—27% all grades, 12% grade 3, 1% grade 4
- Cytopenias not resolved by day 28—26% all grades, 13% grade 3, 8% grade 4
- Neurologic events—21% all grades, 9% grade 3, 4% grade 4
- Febrile neutropenia—14% all grades, 13% grade 3, 1% grade 4
- Tumor lysis syndrome—1% grade 3.
“Adverse events were reversible and effectively managed at the different centers by appropriately trained study personnel,” Dr Salles noted. “There were no CTL019-related deaths and no cerebral edema.” ![]()
The chimeric antigen receptor (CAR) T-cell therapy CTL019 produced a high response rate in a phase 2 trial of adults with relapsed/refractory diffuse large B-cell lymphoma (DLBCL), according to researchers.
An interim analysis of the JULIET trial revealed a 3-month overall response rate (ORR) of 45%.
Thirty-seven percent of patients had a complete response (CR), and all of the patients who were in CR at 3 months were still in CR at the data cutoff point.
This confirms the high response rates and durable responses observed in a previous single-center trial*, said JULIET investigator Gilles Salles, MD, PhD, of Hospices Civils de Lyon in Lyon, France.
Dr Salles presented results from JULIET at the 22nd Congress of the European Hematology Association (EHA) in Madrid, Spain (abstract LB2604).
The results were also presented at the 14th International Conference on Malignant Lymphoma (ICML) in Lugano, Switzerland (abstract 007).
The study was sponsored by Novartis.
Patients
JULIET enrolled 141 patients age 18 and older with relapsed/refractory DLBCL.
Forty-three patients discontinued the study before receiving CTL019—28 due to progressive disease (including 16 deaths), 9 due to an inability to manufacture CTL019, 2 due to adverse events, 1 due to investigator decision, 1 due to study withdrawal, and 1 due to protocol deviation.
Eighty-five patients were infused. The remaining 13 patients were pending infusion at the data cutoff—December 20, 2016.
The median age of the 85 infused patients was 56 (range, 24-75). Ninety-six percent of patients had DLBCL not otherwise specified. Fifty-one percent had germinal center B-cell type DLBCL.
Fifty-four percent of patients had an ECOG performance status of 0, and 46% had a status of 1. Fifty-three percent of patients had stage IV disease, and 8% had bone marrow involvement.
Forty percent of patients had 2 prior lines of antineoplastic therapy, 29% had 3, and 31% had 4 to 7 prior lines.
Forty-one percent of patients were refractory to their last therapy, and 59% had relapsed after their last therapy. Fifty-one percent of patients had prior autologous stem cell transplant.
Treatment
Seventy-six patients (89%) received bridging chemotherapy to prevent disease progression during CTL019 manufacturing. This treatment was completed 2 to 14 days prior to CTL019 infusion.
The median CTL019 cell dose was 3.1 x 108 (range, 0.1-6.0 x 108).
Response
Fifty-one patients were evaluated for response. The median time from infusion to data cutoff was 3.7 months.
The best ORR was 59%, with a CR rate of 43% and a partial response rate of 16%. Twelve percent of patients had stable disease, and 24% progressed.
At 3 months, the ORR was 45%. The CR rate was 37%, and the partial response rate was 8%.
“All patients in CR at 3 months remained in CR at follow-up, and the median duration of response was not reached,” Dr Salles noted.
None of the responders went on to receive a transplant.
Safety
All 85 patients were evaluated for safety. Adverse events of special interest, occurring within 8 weeks of CTL019 infusion, were:
- Cytokine release syndrome—57% all grades, 17% grade 3, 9% grade 4
- Infections—27% all grades, 12% grade 3, 1% grade 4
- Cytopenias not resolved by day 28—26% all grades, 13% grade 3, 8% grade 4
- Neurologic events—21% all grades, 9% grade 3, 4% grade 4
- Febrile neutropenia—14% all grades, 13% grade 3, 1% grade 4
- Tumor lysis syndrome—1% grade 3.
“Adverse events were reversible and effectively managed at the different centers by appropriately trained study personnel,” Dr Salles noted. “There were no CTL019-related deaths and no cerebral edema.” ![]()
The chimeric antigen receptor (CAR) T-cell therapy CTL019 produced a high response rate in a phase 2 trial of adults with relapsed/refractory diffuse large B-cell lymphoma (DLBCL), according to researchers.
An interim analysis of the JULIET trial revealed a 3-month overall response rate (ORR) of 45%.
Thirty-seven percent of patients had a complete response (CR), and all of the patients who were in CR at 3 months were still in CR at the data cutoff point.
This confirms the high response rates and durable responses observed in a previous single-center trial*, said JULIET investigator Gilles Salles, MD, PhD, of Hospices Civils de Lyon in Lyon, France.
Dr Salles presented results from JULIET at the 22nd Congress of the European Hematology Association (EHA) in Madrid, Spain (abstract LB2604).
The results were also presented at the 14th International Conference on Malignant Lymphoma (ICML) in Lugano, Switzerland (abstract 007).
The study was sponsored by Novartis.
Patients
JULIET enrolled 141 patients age 18 and older with relapsed/refractory DLBCL.
Forty-three patients discontinued the study before receiving CTL019—28 due to progressive disease (including 16 deaths), 9 due to an inability to manufacture CTL019, 2 due to adverse events, 1 due to investigator decision, 1 due to study withdrawal, and 1 due to protocol deviation.
Eighty-five patients were infused. The remaining 13 patients were pending infusion at the data cutoff—December 20, 2016.
The median age of the 85 infused patients was 56 (range, 24-75). Ninety-six percent of patients had DLBCL not otherwise specified. Fifty-one percent had germinal center B-cell type DLBCL.
Fifty-four percent of patients had an ECOG performance status of 0, and 46% had a status of 1. Fifty-three percent of patients had stage IV disease, and 8% had bone marrow involvement.
Forty percent of patients had 2 prior lines of antineoplastic therapy, 29% had 3, and 31% had 4 to 7 prior lines.
Forty-one percent of patients were refractory to their last therapy, and 59% had relapsed after their last therapy. Fifty-one percent of patients had prior autologous stem cell transplant.
Treatment
Seventy-six patients (89%) received bridging chemotherapy to prevent disease progression during CTL019 manufacturing. This treatment was completed 2 to 14 days prior to CTL019 infusion.
The median CTL019 cell dose was 3.1 x 108 (range, 0.1-6.0 x 108).
Response
Fifty-one patients were evaluated for response. The median time from infusion to data cutoff was 3.7 months.
The best ORR was 59%, with a CR rate of 43% and a partial response rate of 16%. Twelve percent of patients had stable disease, and 24% progressed.
At 3 months, the ORR was 45%. The CR rate was 37%, and the partial response rate was 8%.
“All patients in CR at 3 months remained in CR at follow-up, and the median duration of response was not reached,” Dr Salles noted.
None of the responders went on to receive a transplant.
Safety
All 85 patients were evaluated for safety. Adverse events of special interest, occurring within 8 weeks of CTL019 infusion, were:
- Cytokine release syndrome—57% all grades, 17% grade 3, 9% grade 4
- Infections—27% all grades, 12% grade 3, 1% grade 4
- Cytopenias not resolved by day 28—26% all grades, 13% grade 3, 8% grade 4
- Neurologic events—21% all grades, 9% grade 3, 4% grade 4
- Febrile neutropenia—14% all grades, 13% grade 3, 1% grade 4
- Tumor lysis syndrome—1% grade 3.
“Adverse events were reversible and effectively managed at the different centers by appropriately trained study personnel,” Dr Salles noted. “There were no CTL019-related deaths and no cerebral edema.” ![]()
De-escalation may improve success of TKI cessation
MADRID—Results of the DESTINY trial suggest that chronic myeloid leukemia (CML) patients may improve their chances of successfully stopping treatment with tyrosine kinase inhibitors (TKIs) by first reducing the dose they receive.
CML patients in deep molecular response (MR4) at study entry had a low rate of recurrence when they first de-escalated their TKI dose—receiving half the standard dose—for a year and then completely stopped receiving TKI treatment for a year.
The 2-year recurrence-free survival (RFS) rate was 77%, which is better than the RFS in any comparable study to date, according to Richard Clark, MD, of the University of Liverpool in Liverpool, UK, and his colleagues.
Dr Clark presented results from DESTINY at the 22nd Congress of the European Hematology Association (EHA) as abstract S423.
DESTINY included 174 CML patients (98 male, 76 female) in stable major molecular response (MMR).
At study entry, patients had received imatinib (n=148), nilotinib (n=16), or dasatinib (n=10) for a median duration of 6.8 years.
For the first 12 months of the study, patients had their TKI dose reduced to half the standard dose. So they received imatinib at 200 mg daily, dasatinib at 50 mg daily, or nilotinib at 200 mg twice daily. After that, patients stopped treatment completely.
After the first 12 months, molecular recurrence was lower in patients with stable MR4 at study entry than in patients who were not in MR4 (but still in MMR)—2.4% (3/125) and 18.4% (9/49), respectively (P<0.001).
During the following 12 months, in which patients had completely stopped TKI treatment, there were 26 recurrences and 4 withdrawals among the remaining 117 patients who were in MR4 at baseline, as well as 20 recurrences and 4 withdrawals among the 36 patients not in MR4.
So the RFS was 77% among patients in MR4 at baseline and 39% among the patients not in MR4 (P<0.001).
The researchers said the probability of RFS was unrelated to patients’ age, gender, performance status, or the prior TKI they received (imatinib vs second-generation TKI).
All patients with recurrence ultimately returned to deep remissions when they resumed their TKI treatment.
“TKI de-escalation is safe for most CML patients with stable and excellent responses to TKI therapy after some years of treatment and is associated with improvement in symptoms,” Dr Clark said.
“Overall, our findings are better than any other studies worldwide and imply that our unique, gradual withdrawal of treatment might be important. We don’t yet understand why our results are so good, but this is a happy problem to have.” ![]()
MADRID—Results of the DESTINY trial suggest that chronic myeloid leukemia (CML) patients may improve their chances of successfully stopping treatment with tyrosine kinase inhibitors (TKIs) by first reducing the dose they receive.
CML patients in deep molecular response (MR4) at study entry had a low rate of recurrence when they first de-escalated their TKI dose—receiving half the standard dose—for a year and then completely stopped receiving TKI treatment for a year.
The 2-year recurrence-free survival (RFS) rate was 77%, which is better than the RFS in any comparable study to date, according to Richard Clark, MD, of the University of Liverpool in Liverpool, UK, and his colleagues.
Dr Clark presented results from DESTINY at the 22nd Congress of the European Hematology Association (EHA) as abstract S423.
DESTINY included 174 CML patients (98 male, 76 female) in stable major molecular response (MMR).
At study entry, patients had received imatinib (n=148), nilotinib (n=16), or dasatinib (n=10) for a median duration of 6.8 years.
For the first 12 months of the study, patients had their TKI dose reduced to half the standard dose. So they received imatinib at 200 mg daily, dasatinib at 50 mg daily, or nilotinib at 200 mg twice daily. After that, patients stopped treatment completely.
After the first 12 months, molecular recurrence was lower in patients with stable MR4 at study entry than in patients who were not in MR4 (but still in MMR)—2.4% (3/125) and 18.4% (9/49), respectively (P<0.001).
During the following 12 months, in which patients had completely stopped TKI treatment, there were 26 recurrences and 4 withdrawals among the remaining 117 patients who were in MR4 at baseline, as well as 20 recurrences and 4 withdrawals among the 36 patients not in MR4.
So the RFS was 77% among patients in MR4 at baseline and 39% among the patients not in MR4 (P<0.001).
The researchers said the probability of RFS was unrelated to patients’ age, gender, performance status, or the prior TKI they received (imatinib vs second-generation TKI).
All patients with recurrence ultimately returned to deep remissions when they resumed their TKI treatment.
“TKI de-escalation is safe for most CML patients with stable and excellent responses to TKI therapy after some years of treatment and is associated with improvement in symptoms,” Dr Clark said.
“Overall, our findings are better than any other studies worldwide and imply that our unique, gradual withdrawal of treatment might be important. We don’t yet understand why our results are so good, but this is a happy problem to have.” ![]()
MADRID—Results of the DESTINY trial suggest that chronic myeloid leukemia (CML) patients may improve their chances of successfully stopping treatment with tyrosine kinase inhibitors (TKIs) by first reducing the dose they receive.
CML patients in deep molecular response (MR4) at study entry had a low rate of recurrence when they first de-escalated their TKI dose—receiving half the standard dose—for a year and then completely stopped receiving TKI treatment for a year.
The 2-year recurrence-free survival (RFS) rate was 77%, which is better than the RFS in any comparable study to date, according to Richard Clark, MD, of the University of Liverpool in Liverpool, UK, and his colleagues.
Dr Clark presented results from DESTINY at the 22nd Congress of the European Hematology Association (EHA) as abstract S423.
DESTINY included 174 CML patients (98 male, 76 female) in stable major molecular response (MMR).
At study entry, patients had received imatinib (n=148), nilotinib (n=16), or dasatinib (n=10) for a median duration of 6.8 years.
For the first 12 months of the study, patients had their TKI dose reduced to half the standard dose. So they received imatinib at 200 mg daily, dasatinib at 50 mg daily, or nilotinib at 200 mg twice daily. After that, patients stopped treatment completely.
After the first 12 months, molecular recurrence was lower in patients with stable MR4 at study entry than in patients who were not in MR4 (but still in MMR)—2.4% (3/125) and 18.4% (9/49), respectively (P<0.001).
During the following 12 months, in which patients had completely stopped TKI treatment, there were 26 recurrences and 4 withdrawals among the remaining 117 patients who were in MR4 at baseline, as well as 20 recurrences and 4 withdrawals among the 36 patients not in MR4.
So the RFS was 77% among patients in MR4 at baseline and 39% among the patients not in MR4 (P<0.001).
The researchers said the probability of RFS was unrelated to patients’ age, gender, performance status, or the prior TKI they received (imatinib vs second-generation TKI).
All patients with recurrence ultimately returned to deep remissions when they resumed their TKI treatment.
“TKI de-escalation is safe for most CML patients with stable and excellent responses to TKI therapy after some years of treatment and is associated with improvement in symptoms,” Dr Clark said.
“Overall, our findings are better than any other studies worldwide and imply that our unique, gradual withdrawal of treatment might be important. We don’t yet understand why our results are so good, but this is a happy problem to have.” ![]()