User login
VIDEO: About 1 in 20 ALS patients in Washington state chose assisted suicide
BOSTON – A new study estimates that 3.4%-6.7% of amyotrophic lateral sclerosis (ALS) patients in Washington state sought to commit physician-assisted suicide over a 5-year period.
The rate is many times higher than that among cancer patients in the state, researchers found. They also discovered that ALS patients were significantly more likely than were other terminally ill people to use the deadly medication after getting prescriptions for it.
The findings appear to reflect the unique hopelessness facing ALS patients. “They’re not afforded as much denial of decline and death as are patients with other terminal illnesses,” said Linda Ganzini, MD, MPH, a professor of psychiatry and medicine at Oregon Health & Science University, Portland, who has studied end of life in ALS patients.
“Many cancer patients, even in the final days of life, receive treatments that they hope will extend their lives,” she said in an interview after reviewing the study findings. “In contrast, treatments for ALS are minimally effective.”
Physician-assisted suicide is legal in California, Colorado, the District of Columbia, Montana, Oregon, Vermont, and Washington.
A team led by Leo H. Wang, MD, PhD, of the University of Washington, Seattle, examined the medical records of 39 ALS patients who sought medication to end their lives at three hospitals in Seattle from March 2009 to Dec. 31, 2014.
Washington’s Death with Dignity (DWD) law, which went into effect in 2009, allows physicians to prescribe lethal medication if the patient has a terminal illness and a prognosis of less than 6 months to live as judged by two physicians.
The researchers reported their findings, a follow-up to a previous study (Neurology. 2016 Nov 15;87[20]:2117-22), at the annual meeting of the American Academy of Neurology.
The median age of the ALS patients at symptom onset was 64 (range, 42-83), and a median of 712 days passed (range, 207-2,407) from the date of diagnosis to date of prescription for lethal medication.
The median time from prescription to death was 22 days, with at least one patient dying immediately (range, 0-386 days). All 39 patients had limb involvement, and 82%-92% had bulbar involvement, dysarthria, dysphagia, and/or dyspnea.
The researchers estimate that 3.4%-6.7% of 1,146 ALS patients in Washington who died over the time period of the study sought a physician-assisted death. The 3.4% figure assumes that the 39 patients at the three hospitals make up all the ALS patients who received medication prescriptions. The 6.7% figure assumes that all patients with neurodegenerative disease who sought DWD in the state over that period had ALS.
“Similarly, 5% (92 of 1,795) of Oregon ALS patient who died sought medication under DWD between 1998 and 2014,” Dr. Wang said. “This is slightly increased compared to the percentage during the first decade, following enactment of the Oregon law (1998-2007), when 2.7% (26 of 962) of ALS patients died using DWD medication.”
Using Washington state data, researchers also estimated that 0.6% of 73,319 cancer patients and 0.2% of 298,178 people in the state who died of all causes sought DWD over the study period.
A total of 30 (77%) ALS patients who received the deadly prescriptions chose to take them, compared with 67% of all-cause patients who took advantage of the DWD law and 60% of cancer patients.
All 30 patients died. The nine who chose to not take the prescribed medication died after a median of 76 days. The patients who did not take the medication were more likely to be married (88% vs. 69%), to be college educated (100% vs. 74%), and to use a motorized wheelchair (78% vs. 31%).
Those who chose to not take the prescribed medication were also less motivated by loss of dignity (63% vs. 93% among those who took the medication) and by being a burden on others (25% vs. 66%). They were more likely to identify themselves as religious (80% vs. 35%).
Multiple factors may explain why ALS patients made different choices regarding the deadly drugs, lead study author Dr. Wang said in an interview. “We thought that the loss of communication may have played a role based on our finding, as most patients who followed through had more substantial trouble speaking,” he said. “For the patients who ultimately did not choose to take the medication, we found more of them had stronger religious beliefs than those who did not.”
As for pain, he reported that it was not a major issue. “Only about 10% of ALS patients were worried about pain, as opposed to 30% of the general Death with Dignity patients,” he said.
Dr. Ganzini noted that some patients who seek the prescribed drugs “want reassurance that, if their quality of life becomes unbearable, they have the option of physician-assisted death. But, they continue to cope and find reasons to live. As such, they ultimately die of their disease without taking the medications. Others lose the ability to ingest the medications, often because of sudden worsening of their disease.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
No specific funding was reported. Dr. Ganzini and Dr. Wang had no disclosures.
BOSTON – A new study estimates that 3.4%-6.7% of amyotrophic lateral sclerosis (ALS) patients in Washington state sought to commit physician-assisted suicide over a 5-year period.
The rate is many times higher than that among cancer patients in the state, researchers found. They also discovered that ALS patients were significantly more likely than were other terminally ill people to use the deadly medication after getting prescriptions for it.
The findings appear to reflect the unique hopelessness facing ALS patients. “They’re not afforded as much denial of decline and death as are patients with other terminal illnesses,” said Linda Ganzini, MD, MPH, a professor of psychiatry and medicine at Oregon Health & Science University, Portland, who has studied end of life in ALS patients.
“Many cancer patients, even in the final days of life, receive treatments that they hope will extend their lives,” she said in an interview after reviewing the study findings. “In contrast, treatments for ALS are minimally effective.”
Physician-assisted suicide is legal in California, Colorado, the District of Columbia, Montana, Oregon, Vermont, and Washington.
A team led by Leo H. Wang, MD, PhD, of the University of Washington, Seattle, examined the medical records of 39 ALS patients who sought medication to end their lives at three hospitals in Seattle from March 2009 to Dec. 31, 2014.
Washington’s Death with Dignity (DWD) law, which went into effect in 2009, allows physicians to prescribe lethal medication if the patient has a terminal illness and a prognosis of less than 6 months to live as judged by two physicians.
The researchers reported their findings, a follow-up to a previous study (Neurology. 2016 Nov 15;87[20]:2117-22), at the annual meeting of the American Academy of Neurology.
The median age of the ALS patients at symptom onset was 64 (range, 42-83), and a median of 712 days passed (range, 207-2,407) from the date of diagnosis to date of prescription for lethal medication.
The median time from prescription to death was 22 days, with at least one patient dying immediately (range, 0-386 days). All 39 patients had limb involvement, and 82%-92% had bulbar involvement, dysarthria, dysphagia, and/or dyspnea.
The researchers estimate that 3.4%-6.7% of 1,146 ALS patients in Washington who died over the time period of the study sought a physician-assisted death. The 3.4% figure assumes that the 39 patients at the three hospitals make up all the ALS patients who received medication prescriptions. The 6.7% figure assumes that all patients with neurodegenerative disease who sought DWD in the state over that period had ALS.
“Similarly, 5% (92 of 1,795) of Oregon ALS patient who died sought medication under DWD between 1998 and 2014,” Dr. Wang said. “This is slightly increased compared to the percentage during the first decade, following enactment of the Oregon law (1998-2007), when 2.7% (26 of 962) of ALS patients died using DWD medication.”
Using Washington state data, researchers also estimated that 0.6% of 73,319 cancer patients and 0.2% of 298,178 people in the state who died of all causes sought DWD over the study period.
A total of 30 (77%) ALS patients who received the deadly prescriptions chose to take them, compared with 67% of all-cause patients who took advantage of the DWD law and 60% of cancer patients.
All 30 patients died. The nine who chose to not take the prescribed medication died after a median of 76 days. The patients who did not take the medication were more likely to be married (88% vs. 69%), to be college educated (100% vs. 74%), and to use a motorized wheelchair (78% vs. 31%).
Those who chose to not take the prescribed medication were also less motivated by loss of dignity (63% vs. 93% among those who took the medication) and by being a burden on others (25% vs. 66%). They were more likely to identify themselves as religious (80% vs. 35%).
Multiple factors may explain why ALS patients made different choices regarding the deadly drugs, lead study author Dr. Wang said in an interview. “We thought that the loss of communication may have played a role based on our finding, as most patients who followed through had more substantial trouble speaking,” he said. “For the patients who ultimately did not choose to take the medication, we found more of them had stronger religious beliefs than those who did not.”
As for pain, he reported that it was not a major issue. “Only about 10% of ALS patients were worried about pain, as opposed to 30% of the general Death with Dignity patients,” he said.
Dr. Ganzini noted that some patients who seek the prescribed drugs “want reassurance that, if their quality of life becomes unbearable, they have the option of physician-assisted death. But, they continue to cope and find reasons to live. As such, they ultimately die of their disease without taking the medications. Others lose the ability to ingest the medications, often because of sudden worsening of their disease.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
No specific funding was reported. Dr. Ganzini and Dr. Wang had no disclosures.
BOSTON – A new study estimates that 3.4%-6.7% of amyotrophic lateral sclerosis (ALS) patients in Washington state sought to commit physician-assisted suicide over a 5-year period.
The rate is many times higher than that among cancer patients in the state, researchers found. They also discovered that ALS patients were significantly more likely than were other terminally ill people to use the deadly medication after getting prescriptions for it.
The findings appear to reflect the unique hopelessness facing ALS patients. “They’re not afforded as much denial of decline and death as are patients with other terminal illnesses,” said Linda Ganzini, MD, MPH, a professor of psychiatry and medicine at Oregon Health & Science University, Portland, who has studied end of life in ALS patients.
“Many cancer patients, even in the final days of life, receive treatments that they hope will extend their lives,” she said in an interview after reviewing the study findings. “In contrast, treatments for ALS are minimally effective.”
Physician-assisted suicide is legal in California, Colorado, the District of Columbia, Montana, Oregon, Vermont, and Washington.
A team led by Leo H. Wang, MD, PhD, of the University of Washington, Seattle, examined the medical records of 39 ALS patients who sought medication to end their lives at three hospitals in Seattle from March 2009 to Dec. 31, 2014.
Washington’s Death with Dignity (DWD) law, which went into effect in 2009, allows physicians to prescribe lethal medication if the patient has a terminal illness and a prognosis of less than 6 months to live as judged by two physicians.
The researchers reported their findings, a follow-up to a previous study (Neurology. 2016 Nov 15;87[20]:2117-22), at the annual meeting of the American Academy of Neurology.
The median age of the ALS patients at symptom onset was 64 (range, 42-83), and a median of 712 days passed (range, 207-2,407) from the date of diagnosis to date of prescription for lethal medication.
The median time from prescription to death was 22 days, with at least one patient dying immediately (range, 0-386 days). All 39 patients had limb involvement, and 82%-92% had bulbar involvement, dysarthria, dysphagia, and/or dyspnea.
The researchers estimate that 3.4%-6.7% of 1,146 ALS patients in Washington who died over the time period of the study sought a physician-assisted death. The 3.4% figure assumes that the 39 patients at the three hospitals make up all the ALS patients who received medication prescriptions. The 6.7% figure assumes that all patients with neurodegenerative disease who sought DWD in the state over that period had ALS.
“Similarly, 5% (92 of 1,795) of Oregon ALS patient who died sought medication under DWD between 1998 and 2014,” Dr. Wang said. “This is slightly increased compared to the percentage during the first decade, following enactment of the Oregon law (1998-2007), when 2.7% (26 of 962) of ALS patients died using DWD medication.”
Using Washington state data, researchers also estimated that 0.6% of 73,319 cancer patients and 0.2% of 298,178 people in the state who died of all causes sought DWD over the study period.
A total of 30 (77%) ALS patients who received the deadly prescriptions chose to take them, compared with 67% of all-cause patients who took advantage of the DWD law and 60% of cancer patients.
All 30 patients died. The nine who chose to not take the prescribed medication died after a median of 76 days. The patients who did not take the medication were more likely to be married (88% vs. 69%), to be college educated (100% vs. 74%), and to use a motorized wheelchair (78% vs. 31%).
Those who chose to not take the prescribed medication were also less motivated by loss of dignity (63% vs. 93% among those who took the medication) and by being a burden on others (25% vs. 66%). They were more likely to identify themselves as religious (80% vs. 35%).
Multiple factors may explain why ALS patients made different choices regarding the deadly drugs, lead study author Dr. Wang said in an interview. “We thought that the loss of communication may have played a role based on our finding, as most patients who followed through had more substantial trouble speaking,” he said. “For the patients who ultimately did not choose to take the medication, we found more of them had stronger religious beliefs than those who did not.”
As for pain, he reported that it was not a major issue. “Only about 10% of ALS patients were worried about pain, as opposed to 30% of the general Death with Dignity patients,” he said.
Dr. Ganzini noted that some patients who seek the prescribed drugs “want reassurance that, if their quality of life becomes unbearable, they have the option of physician-assisted death. But, they continue to cope and find reasons to live. As such, they ultimately die of their disease without taking the medications. Others lose the ability to ingest the medications, often because of sudden worsening of their disease.”
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
No specific funding was reported. Dr. Ganzini and Dr. Wang had no disclosures.
At AAN 2017
Key clinical point:
Major finding: An estimated 3.4%-6.7% of ALS patients in Washington state sought physician-assisted death, and 77% took the prescribed deadly medication, a higher rate than all-cause (67%) and cancer patients (60%).
Data source: Analysis of 39 ALS patients who sought deadly medication from three Seattle hospitals from March 2009 to Dec. 31, 2014.
Disclosures: No specific funding was reported, and Dr. Wang had no disclosures.
Start your day with meditation
Take a breather from conferencing and join your colleagues for a meditation session on Tuesday, May 2, 7–8 a.m., and Thursday, May 4, 7–7:40 a.m.
Physician facilitators with training in mindfulness practices will lead you in exercises that will attune you with the five senses, the language of your mind and body, and the healing power of your breath.
The session will sustain you throughout the day.
Tuesday, May 2 7–8 a.m.
Mandalay Bay K
Facilitators:
Elizabeth Harry, MD, instructor of medicine, Harvard Medical School, Brigham and Women’s Hospital, and Christie Masters, MD, assistant clinical professor, UCLA Hospitalist Service
Thursday, May 4 7–7:40 a.m.
Mandalay Bay K
Facilitator:
Aditi Dave, MD, Carolina East Medical Center, Wake Forest University
Take a breather from conferencing and join your colleagues for a meditation session on Tuesday, May 2, 7–8 a.m., and Thursday, May 4, 7–7:40 a.m.
Physician facilitators with training in mindfulness practices will lead you in exercises that will attune you with the five senses, the language of your mind and body, and the healing power of your breath.
The session will sustain you throughout the day.
Tuesday, May 2 7–8 a.m.
Mandalay Bay K
Facilitators:
Elizabeth Harry, MD, instructor of medicine, Harvard Medical School, Brigham and Women’s Hospital, and Christie Masters, MD, assistant clinical professor, UCLA Hospitalist Service
Thursday, May 4 7–7:40 a.m.
Mandalay Bay K
Facilitator:
Aditi Dave, MD, Carolina East Medical Center, Wake Forest University
Take a breather from conferencing and join your colleagues for a meditation session on Tuesday, May 2, 7–8 a.m., and Thursday, May 4, 7–7:40 a.m.
Physician facilitators with training in mindfulness practices will lead you in exercises that will attune you with the five senses, the language of your mind and body, and the healing power of your breath.
The session will sustain you throughout the day.
Tuesday, May 2 7–8 a.m.
Mandalay Bay K
Facilitators:
Elizabeth Harry, MD, instructor of medicine, Harvard Medical School, Brigham and Women’s Hospital, and Christie Masters, MD, assistant clinical professor, UCLA Hospitalist Service
Thursday, May 4 7–7:40 a.m.
Mandalay Bay K
Facilitator:
Aditi Dave, MD, Carolina East Medical Center, Wake Forest University
Enjoy Las Vegas and HM17
Welcome to HM17 and Las Vegas! We invite you to network and get to know more than 4,000 of your closest colleagues over the next 3 days. Please have fun taking advantage of the many unique learning opportunities we have developed for this year’s meeting. We hope you will be pleased with the offerings that our Annual Meeting Committee has produced on your behalf. You will see committee members wearing buttons that identify them as members of the Annual Meeting Committee. Please take the time to give them your feedback about the meeting and, if you feel so inclined, thank them for the time and energy they committed to create this year’s meeting.
We think you will find this meeting and the precourses have something (many things!) for everyone. Whether you are a community or an academic hospitalist, a newly minted hospitalist or a seasoned veteran, a clinician who takes care of the young or the old (and everyone in between), an advanced practice clinician or hospital medicine administrator, a researcher or educator or clinician (or any combination of the three), we had you in mind as we developed the content for HM17.
We have added medical education and health policy tracks and are bringing back favorites like the young-hospitalist, academic, pediatric, practice management, and quality tracks. Don’t forget to attend our interactive workshops. More than 150 workshop ideas were submitted, and we are proud to feature 18 of the best.
Finally, your HM17 experience will not be complete until you attend the much-anticipated Updates in Hospital Medicine talks and plenary sessions; network with your colleagues at the Research, Innovations, and Clinical Vignettes (RIV) Poster Competition; roam the Exhibit Hall; and join in a Special Interest Forum.
This meeting would not be possible without the tireless effort of the SHM staff and leadership, conference faculty, and committee members. Most importantly, we sincerely thank all of you for attending HM17. We have created this meeting for you, and we hope it is your most valuable educational and networking opportunity of 2017.
Enjoy Las Vegas and HM17, and we will see you in 2018 in Orlando!
Dr. Feldman is a hospitalist at Johns Hopkins in Baltimore and course director for HM17.
Welcome to HM17 and Las Vegas! We invite you to network and get to know more than 4,000 of your closest colleagues over the next 3 days. Please have fun taking advantage of the many unique learning opportunities we have developed for this year’s meeting. We hope you will be pleased with the offerings that our Annual Meeting Committee has produced on your behalf. You will see committee members wearing buttons that identify them as members of the Annual Meeting Committee. Please take the time to give them your feedback about the meeting and, if you feel so inclined, thank them for the time and energy they committed to create this year’s meeting.
We think you will find this meeting and the precourses have something (many things!) for everyone. Whether you are a community or an academic hospitalist, a newly minted hospitalist or a seasoned veteran, a clinician who takes care of the young or the old (and everyone in between), an advanced practice clinician or hospital medicine administrator, a researcher or educator or clinician (or any combination of the three), we had you in mind as we developed the content for HM17.
We have added medical education and health policy tracks and are bringing back favorites like the young-hospitalist, academic, pediatric, practice management, and quality tracks. Don’t forget to attend our interactive workshops. More than 150 workshop ideas were submitted, and we are proud to feature 18 of the best.
Finally, your HM17 experience will not be complete until you attend the much-anticipated Updates in Hospital Medicine talks and plenary sessions; network with your colleagues at the Research, Innovations, and Clinical Vignettes (RIV) Poster Competition; roam the Exhibit Hall; and join in a Special Interest Forum.
This meeting would not be possible without the tireless effort of the SHM staff and leadership, conference faculty, and committee members. Most importantly, we sincerely thank all of you for attending HM17. We have created this meeting for you, and we hope it is your most valuable educational and networking opportunity of 2017.
Enjoy Las Vegas and HM17, and we will see you in 2018 in Orlando!
Dr. Feldman is a hospitalist at Johns Hopkins in Baltimore and course director for HM17.
Welcome to HM17 and Las Vegas! We invite you to network and get to know more than 4,000 of your closest colleagues over the next 3 days. Please have fun taking advantage of the many unique learning opportunities we have developed for this year’s meeting. We hope you will be pleased with the offerings that our Annual Meeting Committee has produced on your behalf. You will see committee members wearing buttons that identify them as members of the Annual Meeting Committee. Please take the time to give them your feedback about the meeting and, if you feel so inclined, thank them for the time and energy they committed to create this year’s meeting.
We think you will find this meeting and the precourses have something (many things!) for everyone. Whether you are a community or an academic hospitalist, a newly minted hospitalist or a seasoned veteran, a clinician who takes care of the young or the old (and everyone in between), an advanced practice clinician or hospital medicine administrator, a researcher or educator or clinician (or any combination of the three), we had you in mind as we developed the content for HM17.
We have added medical education and health policy tracks and are bringing back favorites like the young-hospitalist, academic, pediatric, practice management, and quality tracks. Don’t forget to attend our interactive workshops. More than 150 workshop ideas were submitted, and we are proud to feature 18 of the best.
Finally, your HM17 experience will not be complete until you attend the much-anticipated Updates in Hospital Medicine talks and plenary sessions; network with your colleagues at the Research, Innovations, and Clinical Vignettes (RIV) Poster Competition; roam the Exhibit Hall; and join in a Special Interest Forum.
This meeting would not be possible without the tireless effort of the SHM staff and leadership, conference faculty, and committee members. Most importantly, we sincerely thank all of you for attending HM17. We have created this meeting for you, and we hope it is your most valuable educational and networking opportunity of 2017.
Enjoy Las Vegas and HM17, and we will see you in 2018 in Orlando!
Dr. Feldman is a hospitalist at Johns Hopkins in Baltimore and course director for HM17.
Pembrolizumab could change treatment paradigm, team says
The anti-PD-1 therapy pembrolizumab could change the treatment paradigm of relapsed or refractory classic Hodgkin lymphoma (cHL), according to researchers.
In a phase 2 trial, pembrolizumab produced an overall response rate (ORR) of 69% and a complete response (CR) rate of 22% in adults with cHL who had failed treatment with brentuximab vedotin (BV), autologous hematopoietic stem cell transplant (auto-HSCT), or both.
The researchers said the safety profile of pembrolizumab was “acceptable” and largely consistent with safety in previous studies of the drug.
Twelve percent of patients temporarily stopped taking pembrolizumab due to treatment-related adverse events (AEs), and 4% stopped taking the drug entirely as a result of AEs.
Craig H. Moskowitz, MD, of Memorial Sloan Kettering Cancer Center in New York, New York, and his colleagues reported these results, from the KEYNOTE-087 trial, in the Journal of Clinical Oncology.
The trial was sponsored by Merck, the company that markets pembrolizumab as Keytruda.
In KEYNOTE-087, the researchers evaluated pembrolizumab (a 200 mg fixed dose every 3 weeks) in patients with relapsed or refractory cHL across 3 cohorts:
Cohort 1: Patients who progressed after auto-HSCT and subsequent treatment with BV
Cohort 2: Patients who failed salvage chemotherapy, were ineligible for a transplant, and progressed after BV
Cohort 3: Patients who progressed after auto-HSCT and did not receive BV after transplant.
Efficacy
Across all 210 enrolled patients, the ORR was 69.0%, and the CR rate was 22.4%.
In Cohort 1 (n=69), the ORR was 73.9%. The CR rate was 21.7%, the partial response (PR) rate was 52.2%, 15.9% of patients had stable disease (SD), and 7.2% progressed. In 82.2% of responders, the response lasted 6 months or more.
In Cohort 2 (n=81), the ORR was 64.2%. The CR rate was 24.7%, the PR rate was 39.5%, 12.3% of patients had SD, and 21.0% progressed. In 70.0% of responders, the response lasted 6 months or more.
In Cohort 3 (n=60), the ORR was 70.0%. Twenty percent of patients had a CR, 50.0% had a PR, 16.7% had SD, and 13.3% progressed. In 75.6% of responders, the response lasted 6 months or more.
Results also included an analysis of patients with primary refractory disease (n=73), which was defined as failure to achieve CR or PR with first-line treatment. In this patient population, the ORR was 79.5%.
An ORR of 67.8% was reported in patients who relapsed after 3 or more lines of prior therapy (99/146).
For the entire study cohort, the median duration of response was not reached, and the median overall survival was not reached. At 9 months, the overall survival rate was 97.5%, and the progression-free survival rate was 63.4%.
Safety
The most common AEs related to pembrolizumab were hypothyroidism (12.4%), pyrexia (10.5%), fatigue (9.0%), rash (7.6%), diarrhea (7.1%), headache (6.2%), nausea (5.7%), cough (5.7%), and neutropenia (5.2%).
The most common grade 3/4 treatment-related AEs were neutropenia (2.4%), diarrhea (1.0%), and dyspnea (1.0%). Immune-mediated AEs included pneumonitis (2.9%), hyperthyroidism (2.9%), colitis (1.0%), and myositis (1.0%).
Nine patients (4.3%) stopped taking pembrolizumab due to treatment-related AEs, including myocarditis, myelitis, myositis, pneumonitis, infusion-related reactions, and cytokine release syndrome.
Twenty-six patients (12.4%) had treatment interruptions due to pembrolizumab-related AEs.
Two patients died during follow-up, but neither death was considered related to pembrolizumab. One patient died of septic shock and the other of acute graft-versus-host disease. ![]()
The anti-PD-1 therapy pembrolizumab could change the treatment paradigm of relapsed or refractory classic Hodgkin lymphoma (cHL), according to researchers.
In a phase 2 trial, pembrolizumab produced an overall response rate (ORR) of 69% and a complete response (CR) rate of 22% in adults with cHL who had failed treatment with brentuximab vedotin (BV), autologous hematopoietic stem cell transplant (auto-HSCT), or both.
The researchers said the safety profile of pembrolizumab was “acceptable” and largely consistent with safety in previous studies of the drug.
Twelve percent of patients temporarily stopped taking pembrolizumab due to treatment-related adverse events (AEs), and 4% stopped taking the drug entirely as a result of AEs.
Craig H. Moskowitz, MD, of Memorial Sloan Kettering Cancer Center in New York, New York, and his colleagues reported these results, from the KEYNOTE-087 trial, in the Journal of Clinical Oncology.
The trial was sponsored by Merck, the company that markets pembrolizumab as Keytruda.
In KEYNOTE-087, the researchers evaluated pembrolizumab (a 200 mg fixed dose every 3 weeks) in patients with relapsed or refractory cHL across 3 cohorts:
Cohort 1: Patients who progressed after auto-HSCT and subsequent treatment with BV
Cohort 2: Patients who failed salvage chemotherapy, were ineligible for a transplant, and progressed after BV
Cohort 3: Patients who progressed after auto-HSCT and did not receive BV after transplant.
Efficacy
Across all 210 enrolled patients, the ORR was 69.0%, and the CR rate was 22.4%.
In Cohort 1 (n=69), the ORR was 73.9%. The CR rate was 21.7%, the partial response (PR) rate was 52.2%, 15.9% of patients had stable disease (SD), and 7.2% progressed. In 82.2% of responders, the response lasted 6 months or more.
In Cohort 2 (n=81), the ORR was 64.2%. The CR rate was 24.7%, the PR rate was 39.5%, 12.3% of patients had SD, and 21.0% progressed. In 70.0% of responders, the response lasted 6 months or more.
In Cohort 3 (n=60), the ORR was 70.0%. Twenty percent of patients had a CR, 50.0% had a PR, 16.7% had SD, and 13.3% progressed. In 75.6% of responders, the response lasted 6 months or more.
Results also included an analysis of patients with primary refractory disease (n=73), which was defined as failure to achieve CR or PR with first-line treatment. In this patient population, the ORR was 79.5%.
An ORR of 67.8% was reported in patients who relapsed after 3 or more lines of prior therapy (99/146).
For the entire study cohort, the median duration of response was not reached, and the median overall survival was not reached. At 9 months, the overall survival rate was 97.5%, and the progression-free survival rate was 63.4%.
Safety
The most common AEs related to pembrolizumab were hypothyroidism (12.4%), pyrexia (10.5%), fatigue (9.0%), rash (7.6%), diarrhea (7.1%), headache (6.2%), nausea (5.7%), cough (5.7%), and neutropenia (5.2%).
The most common grade 3/4 treatment-related AEs were neutropenia (2.4%), diarrhea (1.0%), and dyspnea (1.0%). Immune-mediated AEs included pneumonitis (2.9%), hyperthyroidism (2.9%), colitis (1.0%), and myositis (1.0%).
Nine patients (4.3%) stopped taking pembrolizumab due to treatment-related AEs, including myocarditis, myelitis, myositis, pneumonitis, infusion-related reactions, and cytokine release syndrome.
Twenty-six patients (12.4%) had treatment interruptions due to pembrolizumab-related AEs.
Two patients died during follow-up, but neither death was considered related to pembrolizumab. One patient died of septic shock and the other of acute graft-versus-host disease. ![]()
The anti-PD-1 therapy pembrolizumab could change the treatment paradigm of relapsed or refractory classic Hodgkin lymphoma (cHL), according to researchers.
In a phase 2 trial, pembrolizumab produced an overall response rate (ORR) of 69% and a complete response (CR) rate of 22% in adults with cHL who had failed treatment with brentuximab vedotin (BV), autologous hematopoietic stem cell transplant (auto-HSCT), or both.
The researchers said the safety profile of pembrolizumab was “acceptable” and largely consistent with safety in previous studies of the drug.
Twelve percent of patients temporarily stopped taking pembrolizumab due to treatment-related adverse events (AEs), and 4% stopped taking the drug entirely as a result of AEs.
Craig H. Moskowitz, MD, of Memorial Sloan Kettering Cancer Center in New York, New York, and his colleagues reported these results, from the KEYNOTE-087 trial, in the Journal of Clinical Oncology.
The trial was sponsored by Merck, the company that markets pembrolizumab as Keytruda.
In KEYNOTE-087, the researchers evaluated pembrolizumab (a 200 mg fixed dose every 3 weeks) in patients with relapsed or refractory cHL across 3 cohorts:
Cohort 1: Patients who progressed after auto-HSCT and subsequent treatment with BV
Cohort 2: Patients who failed salvage chemotherapy, were ineligible for a transplant, and progressed after BV
Cohort 3: Patients who progressed after auto-HSCT and did not receive BV after transplant.
Efficacy
Across all 210 enrolled patients, the ORR was 69.0%, and the CR rate was 22.4%.
In Cohort 1 (n=69), the ORR was 73.9%. The CR rate was 21.7%, the partial response (PR) rate was 52.2%, 15.9% of patients had stable disease (SD), and 7.2% progressed. In 82.2% of responders, the response lasted 6 months or more.
In Cohort 2 (n=81), the ORR was 64.2%. The CR rate was 24.7%, the PR rate was 39.5%, 12.3% of patients had SD, and 21.0% progressed. In 70.0% of responders, the response lasted 6 months or more.
In Cohort 3 (n=60), the ORR was 70.0%. Twenty percent of patients had a CR, 50.0% had a PR, 16.7% had SD, and 13.3% progressed. In 75.6% of responders, the response lasted 6 months or more.
Results also included an analysis of patients with primary refractory disease (n=73), which was defined as failure to achieve CR or PR with first-line treatment. In this patient population, the ORR was 79.5%.
An ORR of 67.8% was reported in patients who relapsed after 3 or more lines of prior therapy (99/146).
For the entire study cohort, the median duration of response was not reached, and the median overall survival was not reached. At 9 months, the overall survival rate was 97.5%, and the progression-free survival rate was 63.4%.
Safety
The most common AEs related to pembrolizumab were hypothyroidism (12.4%), pyrexia (10.5%), fatigue (9.0%), rash (7.6%), diarrhea (7.1%), headache (6.2%), nausea (5.7%), cough (5.7%), and neutropenia (5.2%).
The most common grade 3/4 treatment-related AEs were neutropenia (2.4%), diarrhea (1.0%), and dyspnea (1.0%). Immune-mediated AEs included pneumonitis (2.9%), hyperthyroidism (2.9%), colitis (1.0%), and myositis (1.0%).
Nine patients (4.3%) stopped taking pembrolizumab due to treatment-related AEs, including myocarditis, myelitis, myositis, pneumonitis, infusion-related reactions, and cytokine release syndrome.
Twenty-six patients (12.4%) had treatment interruptions due to pembrolizumab-related AEs.
Two patients died during follow-up, but neither death was considered related to pembrolizumab. One patient died of septic shock and the other of acute graft-versus-host disease. ![]()
Medicaid acceptance up among cardiologists
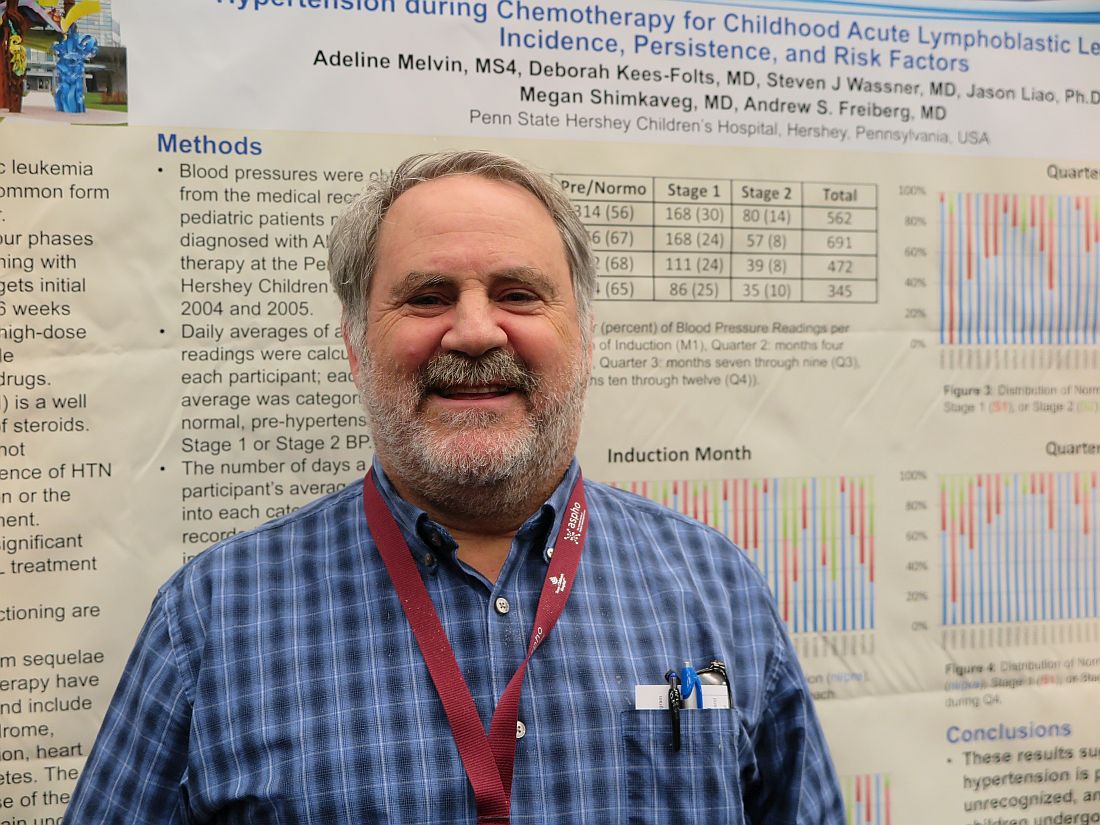
Medicaid acceptance was 77% among cardiologists in the 2017 edition of an ongoing survey conducted in 15 large cities by physician recruitment firm Merritt Hawkins.
That was up from 61% in the previous survey, conducted in 2014, and higher than the average of 72% for cardiologists in 15 midsized cities that were included for the first time in 2017, the company reported.
There were four large cities with Medicaid acceptance rates of 100% – Boston, Detroit, Minneapolis (up from just 7% in 2014), and Portland, Ore. – along with four midsized cities – Cedar Rapids, Iowa; Evansville, Ind.; Fargo, N.D.; and Yakima, Wash. The lowest rate among the large cities was in Dallas (15%), with the midsized basement occupied by Lafayette, La., at 13%, Merritt Hawkins said.
Investigators called 259 randomly selected cardiologists in the large cities and 87 cardiologists in the midsized cities in January and February. It was the fourth such survey the company has conducted since 2004.
The survey also included four other specialties – dermatology, family medicine, ob.gyn., and orthopedic surgery. The Medicaid acceptance rate for all 1,414 physicians in all five specialties in the 15 large cities was 53%, and the average rate for all specialties in the midsized cities was 60% for the 494 offices surveyed, the company said. Cardiology had the highest rates by specialty and dermatology the lowest in both the large and midsized cities.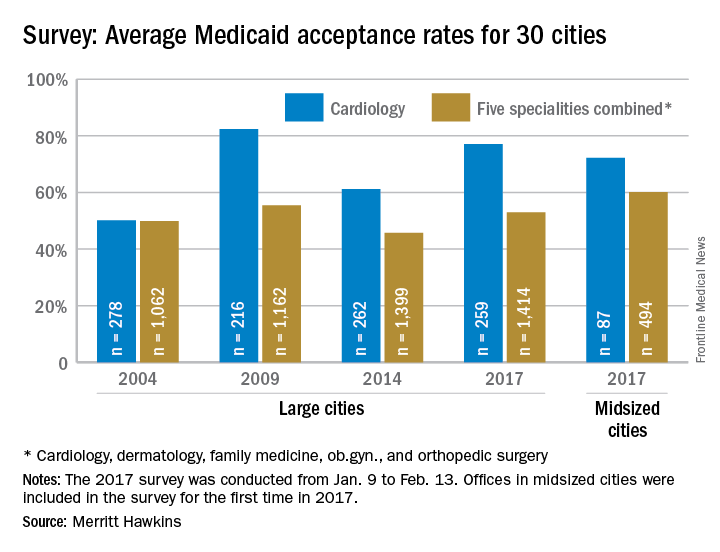
Medicaid acceptance was 77% among cardiologists in the 2017 edition of an ongoing survey conducted in 15 large cities by physician recruitment firm Merritt Hawkins.
That was up from 61% in the previous survey, conducted in 2014, and higher than the average of 72% for cardiologists in 15 midsized cities that were included for the first time in 2017, the company reported.
There were four large cities with Medicaid acceptance rates of 100% – Boston, Detroit, Minneapolis (up from just 7% in 2014), and Portland, Ore. – along with four midsized cities – Cedar Rapids, Iowa; Evansville, Ind.; Fargo, N.D.; and Yakima, Wash. The lowest rate among the large cities was in Dallas (15%), with the midsized basement occupied by Lafayette, La., at 13%, Merritt Hawkins said.
Investigators called 259 randomly selected cardiologists in the large cities and 87 cardiologists in the midsized cities in January and February. It was the fourth such survey the company has conducted since 2004.
The survey also included four other specialties – dermatology, family medicine, ob.gyn., and orthopedic surgery. The Medicaid acceptance rate for all 1,414 physicians in all five specialties in the 15 large cities was 53%, and the average rate for all specialties in the midsized cities was 60% for the 494 offices surveyed, the company said. Cardiology had the highest rates by specialty and dermatology the lowest in both the large and midsized cities.
Medicaid acceptance was 77% among cardiologists in the 2017 edition of an ongoing survey conducted in 15 large cities by physician recruitment firm Merritt Hawkins.
That was up from 61% in the previous survey, conducted in 2014, and higher than the average of 72% for cardiologists in 15 midsized cities that were included for the first time in 2017, the company reported.
There were four large cities with Medicaid acceptance rates of 100% – Boston, Detroit, Minneapolis (up from just 7% in 2014), and Portland, Ore. – along with four midsized cities – Cedar Rapids, Iowa; Evansville, Ind.; Fargo, N.D.; and Yakima, Wash. The lowest rate among the large cities was in Dallas (15%), with the midsized basement occupied by Lafayette, La., at 13%, Merritt Hawkins said.
Investigators called 259 randomly selected cardiologists in the large cities and 87 cardiologists in the midsized cities in January and February. It was the fourth such survey the company has conducted since 2004.
The survey also included four other specialties – dermatology, family medicine, ob.gyn., and orthopedic surgery. The Medicaid acceptance rate for all 1,414 physicians in all five specialties in the 15 large cities was 53%, and the average rate for all specialties in the midsized cities was 60% for the 494 offices surveyed, the company said. Cardiology had the highest rates by specialty and dermatology the lowest in both the large and midsized cities.
VIDEO: Surgery use declines for non–small cell lung cancer
BOSTON – The use of surgical therapy for early stage lung cancer in the United States has declined as other nonsurgical treatment options have become available, according to a study reported at the annual meeting of the American Association for Thoracic Surgery.
Most notably, the study finds that surgery for early stage non–small cell lung cancer decreased by 12% from 2004 to 2013.
In a video interview, Keith Naunheim, MD, a professor of surgery at Saint Louis University, discusses the study findings and the potential reasons behind declining surgery use for lung cancer. Dr. Naunheim also addresses why physicians should keep an open mind about alternative therapy options for lung cancer, while ensuring that the treatments are safe and effective for patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @legal_med
BOSTON – The use of surgical therapy for early stage lung cancer in the United States has declined as other nonsurgical treatment options have become available, according to a study reported at the annual meeting of the American Association for Thoracic Surgery.
Most notably, the study finds that surgery for early stage non–small cell lung cancer decreased by 12% from 2004 to 2013.
In a video interview, Keith Naunheim, MD, a professor of surgery at Saint Louis University, discusses the study findings and the potential reasons behind declining surgery use for lung cancer. Dr. Naunheim also addresses why physicians should keep an open mind about alternative therapy options for lung cancer, while ensuring that the treatments are safe and effective for patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @legal_med
BOSTON – The use of surgical therapy for early stage lung cancer in the United States has declined as other nonsurgical treatment options have become available, according to a study reported at the annual meeting of the American Association for Thoracic Surgery.
Most notably, the study finds that surgery for early stage non–small cell lung cancer decreased by 12% from 2004 to 2013.
In a video interview, Keith Naunheim, MD, a professor of surgery at Saint Louis University, discusses the study findings and the potential reasons behind declining surgery use for lung cancer. Dr. Naunheim also addresses why physicians should keep an open mind about alternative therapy options for lung cancer, while ensuring that the treatments are safe and effective for patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @legal_med
AT THE AATS ANNUAL MEETING
Preeclampsia/eclampsia rate highest in black women
The rate of preeclampsia and eclampsia for black women is 61% higher than it is for white women and 50% higher than for women overall, according to the Agency for Healthcare Research and Quality.
In 2014, black women experienced preeclampsia/eclampsia in 69.8 of every 1,000 deliveries, compared with 43.3 per 1,000 deliveries in white women and 46.6 per 1,000 for all women. Hispanic women were just above the overall rate at 46.8 per 1,000 deliveries, and Asian/Pacific Islander women were 38% lower than the overall rate at 28.8 per 1,000 deliveries, AHRQ said in its report. The overall rate was up 21% over the 38.4 per 1,000 reported in 2005.
Looking at degree of severity, 1.7% of all preeclampsia/eclampsia births in black women were eclampsia, compared with 1.4% for white and Hispanic women and 0.9% for Asian/Pacific Islanders. Severe preeclampsia was most common in Asian/Pacific Islanders – 40.4% of those diagnosed – with Hispanics at 40.3%, blacks at 38.5%, and whites at 34.3%. Mild or unspecified preeclampsia was diagnosed in 52% of preeclamptic/eclamptic white women, 46% of Hispanic women, 45% of Asians/Pacific Islanders, and 37% of black women, the AHRQ said in its analysis of data from the National Inpatient Sample.
Altogether, there were almost 177,000 delivery hospitalizations with preeclampsia/eclampsia in 2014, representing 4.7% of all deliveries and making it the most common hypertension-related diagnosis, followed by gestational hypertension (3.8%) and preexisting hypertension (1.7%). Compared with hospital stays for all other deliveries, those complicated by preeclampsia/eclampsia were 70% longer (mean, 4.4 vs. 2.6 days) and 70% more expensive (mean, $7,500 vs. $4,400), according to the report. 
The rate of preeclampsia and eclampsia for black women is 61% higher than it is for white women and 50% higher than for women overall, according to the Agency for Healthcare Research and Quality.
In 2014, black women experienced preeclampsia/eclampsia in 69.8 of every 1,000 deliveries, compared with 43.3 per 1,000 deliveries in white women and 46.6 per 1,000 for all women. Hispanic women were just above the overall rate at 46.8 per 1,000 deliveries, and Asian/Pacific Islander women were 38% lower than the overall rate at 28.8 per 1,000 deliveries, AHRQ said in its report. The overall rate was up 21% over the 38.4 per 1,000 reported in 2005.
Looking at degree of severity, 1.7% of all preeclampsia/eclampsia births in black women were eclampsia, compared with 1.4% for white and Hispanic women and 0.9% for Asian/Pacific Islanders. Severe preeclampsia was most common in Asian/Pacific Islanders – 40.4% of those diagnosed – with Hispanics at 40.3%, blacks at 38.5%, and whites at 34.3%. Mild or unspecified preeclampsia was diagnosed in 52% of preeclamptic/eclamptic white women, 46% of Hispanic women, 45% of Asians/Pacific Islanders, and 37% of black women, the AHRQ said in its analysis of data from the National Inpatient Sample.
Altogether, there were almost 177,000 delivery hospitalizations with preeclampsia/eclampsia in 2014, representing 4.7% of all deliveries and making it the most common hypertension-related diagnosis, followed by gestational hypertension (3.8%) and preexisting hypertension (1.7%). Compared with hospital stays for all other deliveries, those complicated by preeclampsia/eclampsia were 70% longer (mean, 4.4 vs. 2.6 days) and 70% more expensive (mean, $7,500 vs. $4,400), according to the report. 
The rate of preeclampsia and eclampsia for black women is 61% higher than it is for white women and 50% higher than for women overall, according to the Agency for Healthcare Research and Quality.
In 2014, black women experienced preeclampsia/eclampsia in 69.8 of every 1,000 deliveries, compared with 43.3 per 1,000 deliveries in white women and 46.6 per 1,000 for all women. Hispanic women were just above the overall rate at 46.8 per 1,000 deliveries, and Asian/Pacific Islander women were 38% lower than the overall rate at 28.8 per 1,000 deliveries, AHRQ said in its report. The overall rate was up 21% over the 38.4 per 1,000 reported in 2005.
Looking at degree of severity, 1.7% of all preeclampsia/eclampsia births in black women were eclampsia, compared with 1.4% for white and Hispanic women and 0.9% for Asian/Pacific Islanders. Severe preeclampsia was most common in Asian/Pacific Islanders – 40.4% of those diagnosed – with Hispanics at 40.3%, blacks at 38.5%, and whites at 34.3%. Mild or unspecified preeclampsia was diagnosed in 52% of preeclamptic/eclamptic white women, 46% of Hispanic women, 45% of Asians/Pacific Islanders, and 37% of black women, the AHRQ said in its analysis of data from the National Inpatient Sample.
Altogether, there were almost 177,000 delivery hospitalizations with preeclampsia/eclampsia in 2014, representing 4.7% of all deliveries and making it the most common hypertension-related diagnosis, followed by gestational hypertension (3.8%) and preexisting hypertension (1.7%). Compared with hospital stays for all other deliveries, those complicated by preeclampsia/eclampsia were 70% longer (mean, 4.4 vs. 2.6 days) and 70% more expensive (mean, $7,500 vs. $4,400), according to the report. 
VIDEO: Setbacks of serelaxin, ularitide prompt rethinking acute heart failure strategies
PARIS – Serelaxin’s failure to meet its primary endpoints in an acute heart failure trial with more than 6,500 patients, coupled with a similar failure by ularitide in the same patient population in pivotal trial results first reported in November 2016, led some experts to rethink their conception of potential interventions for patients hospitalized for acute heart failure decompensations.
“We learned in TRUE-AHF that giving a drug very early [in acute heart failure] does not prevent [long-term] death. It means that early is not early enough,” Alexandre Mebazaa, MD, said in a video interview at a meeting held by the Heart Failure Association of the European Society of Cardiology.

In terms of finding new management strategies for patients who develop acute decompensations, “we need to better understand acute heart failure and the best subset of patients who might benefit” from existing or new drugs, he said.
The RELAX-AHF-2 trial enrolled and analyzed 6,545 patients hospitalized with an acute heart failure decompensation at more than 500 sites in 34 countries. The study compared the impact of a 48-hour IV infusion of serelaxin with placebo when begun within 16 hours of hospitalization for acute heart failure and added to standard treatment.
These findings closely matched the performance of ularitide in a similar study design, TRUE-AHF (New Engl J Med. 2017 Apr 12. doi: 10.1056/NEJMoa1601895).
At the 2016 meeting of the Heart Failure Association of the ESC, the organization released revised guidelines for diagnosing and managing heart failure that stressed the importance of rapid response to acute heart failure, including possible treatment with vasodilator drugs. The guidelines acknowledged that while “Vasodilators are the second most often used agents in acute heart failure for symptomatic relief; however, there is no robust evidence confirming their beneficial effects” (Eur Heart J. 2016 Jul 14;37[27]:2129-200).
Both ularitide and serelaxin are potent IV vasodilators, and their failure to meet their efficacy endpoints in these two trials put vasodilation and rapid decongestion into question as strategies to improve midterm prognosis in heart failure patients following acute decompensation episodes.
Serelaxin has been developed by Novartis, and ularitide has been developed by Cardiorentis. Dr. Mebazaa has received honoraria from Novartis and Cardiorentis, as well as from several other companies. Dr. Metra has been a consultant to Novartis. She has also served as consultant or spokesperson for Abbott Vascular, Amgen, AstraZeneca, Fresenius, Relypsa, and Servier.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
PARIS – Serelaxin’s failure to meet its primary endpoints in an acute heart failure trial with more than 6,500 patients, coupled with a similar failure by ularitide in the same patient population in pivotal trial results first reported in November 2016, led some experts to rethink their conception of potential interventions for patients hospitalized for acute heart failure decompensations.
“We learned in TRUE-AHF that giving a drug very early [in acute heart failure] does not prevent [long-term] death. It means that early is not early enough,” Alexandre Mebazaa, MD, said in a video interview at a meeting held by the Heart Failure Association of the European Society of Cardiology.

In terms of finding new management strategies for patients who develop acute decompensations, “we need to better understand acute heart failure and the best subset of patients who might benefit” from existing or new drugs, he said.
The RELAX-AHF-2 trial enrolled and analyzed 6,545 patients hospitalized with an acute heart failure decompensation at more than 500 sites in 34 countries. The study compared the impact of a 48-hour IV infusion of serelaxin with placebo when begun within 16 hours of hospitalization for acute heart failure and added to standard treatment.
These findings closely matched the performance of ularitide in a similar study design, TRUE-AHF (New Engl J Med. 2017 Apr 12. doi: 10.1056/NEJMoa1601895).
At the 2016 meeting of the Heart Failure Association of the ESC, the organization released revised guidelines for diagnosing and managing heart failure that stressed the importance of rapid response to acute heart failure, including possible treatment with vasodilator drugs. The guidelines acknowledged that while “Vasodilators are the second most often used agents in acute heart failure for symptomatic relief; however, there is no robust evidence confirming their beneficial effects” (Eur Heart J. 2016 Jul 14;37[27]:2129-200).
Both ularitide and serelaxin are potent IV vasodilators, and their failure to meet their efficacy endpoints in these two trials put vasodilation and rapid decongestion into question as strategies to improve midterm prognosis in heart failure patients following acute decompensation episodes.
Serelaxin has been developed by Novartis, and ularitide has been developed by Cardiorentis. Dr. Mebazaa has received honoraria from Novartis and Cardiorentis, as well as from several other companies. Dr. Metra has been a consultant to Novartis. She has also served as consultant or spokesperson for Abbott Vascular, Amgen, AstraZeneca, Fresenius, Relypsa, and Servier.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
PARIS – Serelaxin’s failure to meet its primary endpoints in an acute heart failure trial with more than 6,500 patients, coupled with a similar failure by ularitide in the same patient population in pivotal trial results first reported in November 2016, led some experts to rethink their conception of potential interventions for patients hospitalized for acute heart failure decompensations.
“We learned in TRUE-AHF that giving a drug very early [in acute heart failure] does not prevent [long-term] death. It means that early is not early enough,” Alexandre Mebazaa, MD, said in a video interview at a meeting held by the Heart Failure Association of the European Society of Cardiology.

In terms of finding new management strategies for patients who develop acute decompensations, “we need to better understand acute heart failure and the best subset of patients who might benefit” from existing or new drugs, he said.
The RELAX-AHF-2 trial enrolled and analyzed 6,545 patients hospitalized with an acute heart failure decompensation at more than 500 sites in 34 countries. The study compared the impact of a 48-hour IV infusion of serelaxin with placebo when begun within 16 hours of hospitalization for acute heart failure and added to standard treatment.
These findings closely matched the performance of ularitide in a similar study design, TRUE-AHF (New Engl J Med. 2017 Apr 12. doi: 10.1056/NEJMoa1601895).
At the 2016 meeting of the Heart Failure Association of the ESC, the organization released revised guidelines for diagnosing and managing heart failure that stressed the importance of rapid response to acute heart failure, including possible treatment with vasodilator drugs. The guidelines acknowledged that while “Vasodilators are the second most often used agents in acute heart failure for symptomatic relief; however, there is no robust evidence confirming their beneficial effects” (Eur Heart J. 2016 Jul 14;37[27]:2129-200).
Both ularitide and serelaxin are potent IV vasodilators, and their failure to meet their efficacy endpoints in these two trials put vasodilation and rapid decongestion into question as strategies to improve midterm prognosis in heart failure patients following acute decompensation episodes.
Serelaxin has been developed by Novartis, and ularitide has been developed by Cardiorentis. Dr. Mebazaa has received honoraria from Novartis and Cardiorentis, as well as from several other companies. Dr. Metra has been a consultant to Novartis. She has also served as consultant or spokesperson for Abbott Vascular, Amgen, AstraZeneca, Fresenius, Relypsa, and Servier.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM HEART FAILURE 2017
Acne, rosacea prescriptions cost more when prescribed by dermatologists
PORTLAND, ORE. – Dermatologists have plenty of room to improve when it comes to choosing cost-effective medications for acne or rosacea, based on the results of a large retrospective analysis of Medicare claims data.
Patients with acne or rosacea consistently paid more for topical retinoids, topical antibiotics, and oral tetracyclines when the prescriber was a dermatologist instead of a family or internal medicine physician, Myron Zhang reported at the annual meeting of the Society for Investigative Dermatology.
“There is a large potential for reducing expenditures on health care for patients with acne and rosacea by choosing more generics and less expensive options within drug classes,” said Mr. Zhang, a medical student at the Ohio State University, Columbus, who conducted the study with colleagues there and at Northwestern University in Chicago.
Treating acne and rosacea falls under the purview of both primary and specialty outpatient care, but, researchers had not broken down costs of prescriptions for these conditions by provider type. To help fill that gap, Mr. Zhang and his colleagues retrospectively analyzed all Medicare drug claims for topical retinoids, topical antibiotics, isotretinoin, and oral tetracycline-class antibiotics used to treat acne and rosacea in the United States in 2008 and 2010.
Medicare claims for these prescriptions added up to $65 million in 2008 and $74 million in 2004, Mr. Zhang said. Although most generics either dropped in cost or rose by small amounts in that 2-year span, many brand name prescriptions rose by between 30% and 70%. “Specialist prescriptions were associated with higher brand name usage, a greater variety of medications, and higher costs,” Mr. Zhang said.
Patients paid an average of about $2-$3 more for a topical retinoid, $3-$4 more for oral tetracycline, and $1 more for a topical antibiotic prescribed by a specialist instead of a primary care physician.
This finding might indicate that dermatologists are more comfortable prescribing a greater variety of medications for acne and rosacea, such as tazarotene, azelaic acid, and sulfacetamide, while primary care physicians might stick to a narrower range of medicines, Mr. Zhang said. However, the prescribing behavior of dermatologists could also reflect “external factors,” such as increased contact with representatives from pharmaceutical companies, he added.
“As always, prescription costs need to be weighed against patient preference, compliance, and quality of care,” he said. “More outcomes research is needed to understand the comparative efficacy of generic and brand name treatments.”
The study included both rosacea and acne so that the researchers could capture the total costs of medications such as tretinoin, which is used to treat both conditions. Approximately 80% of topical retinoid prescriptions were for tretinoin, about 50% of topical antibiotic prescriptions were for metronidazole, while about 27% were for clindamycin. About half of oral antibiotic prescriptions were for tetracycline, while about 40% were for minocycline.
Mr. Zhang reported having no conflicts of interest.
PORTLAND, ORE. – Dermatologists have plenty of room to improve when it comes to choosing cost-effective medications for acne or rosacea, based on the results of a large retrospective analysis of Medicare claims data.
Patients with acne or rosacea consistently paid more for topical retinoids, topical antibiotics, and oral tetracyclines when the prescriber was a dermatologist instead of a family or internal medicine physician, Myron Zhang reported at the annual meeting of the Society for Investigative Dermatology.
“There is a large potential for reducing expenditures on health care for patients with acne and rosacea by choosing more generics and less expensive options within drug classes,” said Mr. Zhang, a medical student at the Ohio State University, Columbus, who conducted the study with colleagues there and at Northwestern University in Chicago.
Treating acne and rosacea falls under the purview of both primary and specialty outpatient care, but, researchers had not broken down costs of prescriptions for these conditions by provider type. To help fill that gap, Mr. Zhang and his colleagues retrospectively analyzed all Medicare drug claims for topical retinoids, topical antibiotics, isotretinoin, and oral tetracycline-class antibiotics used to treat acne and rosacea in the United States in 2008 and 2010.
Medicare claims for these prescriptions added up to $65 million in 2008 and $74 million in 2004, Mr. Zhang said. Although most generics either dropped in cost or rose by small amounts in that 2-year span, many brand name prescriptions rose by between 30% and 70%. “Specialist prescriptions were associated with higher brand name usage, a greater variety of medications, and higher costs,” Mr. Zhang said.
Patients paid an average of about $2-$3 more for a topical retinoid, $3-$4 more for oral tetracycline, and $1 more for a topical antibiotic prescribed by a specialist instead of a primary care physician.
This finding might indicate that dermatologists are more comfortable prescribing a greater variety of medications for acne and rosacea, such as tazarotene, azelaic acid, and sulfacetamide, while primary care physicians might stick to a narrower range of medicines, Mr. Zhang said. However, the prescribing behavior of dermatologists could also reflect “external factors,” such as increased contact with representatives from pharmaceutical companies, he added.
“As always, prescription costs need to be weighed against patient preference, compliance, and quality of care,” he said. “More outcomes research is needed to understand the comparative efficacy of generic and brand name treatments.”
The study included both rosacea and acne so that the researchers could capture the total costs of medications such as tretinoin, which is used to treat both conditions. Approximately 80% of topical retinoid prescriptions were for tretinoin, about 50% of topical antibiotic prescriptions were for metronidazole, while about 27% were for clindamycin. About half of oral antibiotic prescriptions were for tetracycline, while about 40% were for minocycline.
Mr. Zhang reported having no conflicts of interest.
PORTLAND, ORE. – Dermatologists have plenty of room to improve when it comes to choosing cost-effective medications for acne or rosacea, based on the results of a large retrospective analysis of Medicare claims data.
Patients with acne or rosacea consistently paid more for topical retinoids, topical antibiotics, and oral tetracyclines when the prescriber was a dermatologist instead of a family or internal medicine physician, Myron Zhang reported at the annual meeting of the Society for Investigative Dermatology.
“There is a large potential for reducing expenditures on health care for patients with acne and rosacea by choosing more generics and less expensive options within drug classes,” said Mr. Zhang, a medical student at the Ohio State University, Columbus, who conducted the study with colleagues there and at Northwestern University in Chicago.
Treating acne and rosacea falls under the purview of both primary and specialty outpatient care, but, researchers had not broken down costs of prescriptions for these conditions by provider type. To help fill that gap, Mr. Zhang and his colleagues retrospectively analyzed all Medicare drug claims for topical retinoids, topical antibiotics, isotretinoin, and oral tetracycline-class antibiotics used to treat acne and rosacea in the United States in 2008 and 2010.
Medicare claims for these prescriptions added up to $65 million in 2008 and $74 million in 2004, Mr. Zhang said. Although most generics either dropped in cost or rose by small amounts in that 2-year span, many brand name prescriptions rose by between 30% and 70%. “Specialist prescriptions were associated with higher brand name usage, a greater variety of medications, and higher costs,” Mr. Zhang said.
Patients paid an average of about $2-$3 more for a topical retinoid, $3-$4 more for oral tetracycline, and $1 more for a topical antibiotic prescribed by a specialist instead of a primary care physician.
This finding might indicate that dermatologists are more comfortable prescribing a greater variety of medications for acne and rosacea, such as tazarotene, azelaic acid, and sulfacetamide, while primary care physicians might stick to a narrower range of medicines, Mr. Zhang said. However, the prescribing behavior of dermatologists could also reflect “external factors,” such as increased contact with representatives from pharmaceutical companies, he added.
“As always, prescription costs need to be weighed against patient preference, compliance, and quality of care,” he said. “More outcomes research is needed to understand the comparative efficacy of generic and brand name treatments.”
The study included both rosacea and acne so that the researchers could capture the total costs of medications such as tretinoin, which is used to treat both conditions. Approximately 80% of topical retinoid prescriptions were for tretinoin, about 50% of topical antibiotic prescriptions were for metronidazole, while about 27% were for clindamycin. About half of oral antibiotic prescriptions were for tetracycline, while about 40% were for minocycline.
Mr. Zhang reported having no conflicts of interest.
At SID 2017
Key clinical point:
Major finding: Patients paid an average of $2-$3 more for a topical retinoid, $3-$4 more for an oral tetracycline-class antibiotic, and $1 more for a topical antibiotic prescribed by a dermatologist, compared with prescriptions from a primary care physician.
Data source: A retrospective analysis of Medicare prescription claims for oral antibiotics, topical antibiotics, and topical retinoids for acne and rosacea in 2008 and 2010.
Disclosures: Mr. Zhang reported having no conflicts of interest.
Treatment-related hypertension, kidney injury are undertreated in kids with leukemia
MONTREAL – Hypertension is a frequent, but underrecognized and undertreated, complication of chemotherapy for acute lymphoblastic leukemia (ALL), the most common childhood malignancy, investigators in a single-center U.S. study reported.
Additionally, there is a “concerningly high” incidence of acute kidney injury among children and young adults who undergo multiagent chemotherapy for acute myeloid leukemia (AML), said the authors of a study conducted in Canada.
Both studies were reported in a scientific poster session at the annual meeting of the American Society for Pediatric Hematology/Oncology.
Hypertension in ALL
Although standard induction regimens for childhood ALL contain high-dose steroids, which are known to be associated with increased risk for hypertension, the incidence of hypertension throughout induction, consolidation, and maintenance for ALL in children has not been adequately evaluated, according to Andrew S. Freiberg, MD, and his colleagues at Penn State Children’s Hospital in Hershey, Penn.
“The incidence of hypertension was much higher than the under 5% expected for a healthy pediatric population,” the investigators found.
Of 562 total readings taken during induction, 56% were in the normo- or prehypertensive range, but 30% were classified as stage 1 and 14% as stage II hypertension.
The combined percentage of stage 1 and 2 readings declined slightly over the year from 44% during induction to 35% during Q4 “but remained well above expected for the pediatric population,” the investigators reported.
Despite the high incidence, “I was surprised at how few of the patients with hypertension we actually treated,” Dr. Freiberg said in an interview.
Just 3 of the 36 patients studied received treatment for hypertension, he said, possibly because clinicians assumed that the effect was steroid related and transient.
“Now that we’re paying attention, however, we’re treating more of these patients,” Dr. Freiberg said.
The electronic record system used at his institution now alerts clinicians to hypertensive episodes during treatment, he added.
Kidney injury in AML
Like Dr. Freiberg and his colleagues, Liezl du Plessis, MBChB, from the British Columbia Children’s Hospital in Vancouver, Canada, and her colleagues were similarly taken aback when they looked into the incidence of acute kidney injury (AKI) in children and adolescents undergoing multidrug chemotherapy for AML.
“Chemotherapy agents that are used in acute myeloid leukemia are not considered to be nephrotoxic, so it was quite alarming to us to see that there is such a high rate of kidney injury in these patients,” Dr. du Plessis said in an interview.
They found that 34 of the 53 patients (64%) had AKI, with 11 patients having stage 1 (rise in serum creatinine of 1.5 or more times the baseline level), 11 having stage 2 (SCr 2 or more times baseline), and 12 having stage 3 AKI (SCr 3 or more times baseline or the need for dialysis).
Creatinine changes were counted only if they occurred within 7 days from nadir to peak.
AKI occurred in all chemotherapy cycles, with severe injury having the highest frequency in cycle 1.
In a logistical regression model, factors significantly associated with risk for AKI were male sex (odds ratio, 0.2; P = .03) and age 10 years or older (OR, 17.3; P less than .01). Neither sepsis nor aminoglycoside or vancomycin use for more than 3 days was significantly associated with risk for AKI, however.
“I think people need to realize that many of these injuries are happening on the oncology ward, and some of these kids may not even look acutely unwell, so people need to take note.” Dr. du Plessis said.
She recommended curtailing use of nephrotoxic agents whenever possible, and emphasized that clinicians need to document AKI in the medical record.
“A big portion of our AML population goes on to bone marrow transplant, and that is known to be a high risk for further kidney injury. So, I think it would be important to know that before your patient goes for his transplant that he already has certain toxicities and organ injury, so that you can limit things like fluids and get the nephrology team on board to help with the management of these patients,” she said.
The study by Dr. Freiberg was supported by the Four Diamonds Fund. The study by Dr. du Plessis was internally funded. The investigators for each study reported having no relevant financial disclosures.
MONTREAL – Hypertension is a frequent, but underrecognized and undertreated, complication of chemotherapy for acute lymphoblastic leukemia (ALL), the most common childhood malignancy, investigators in a single-center U.S. study reported.
Additionally, there is a “concerningly high” incidence of acute kidney injury among children and young adults who undergo multiagent chemotherapy for acute myeloid leukemia (AML), said the authors of a study conducted in Canada.
Both studies were reported in a scientific poster session at the annual meeting of the American Society for Pediatric Hematology/Oncology.
Hypertension in ALL
Although standard induction regimens for childhood ALL contain high-dose steroids, which are known to be associated with increased risk for hypertension, the incidence of hypertension throughout induction, consolidation, and maintenance for ALL in children has not been adequately evaluated, according to Andrew S. Freiberg, MD, and his colleagues at Penn State Children’s Hospital in Hershey, Penn.
“The incidence of hypertension was much higher than the under 5% expected for a healthy pediatric population,” the investigators found.
Of 562 total readings taken during induction, 56% were in the normo- or prehypertensive range, but 30% were classified as stage 1 and 14% as stage II hypertension.
The combined percentage of stage 1 and 2 readings declined slightly over the year from 44% during induction to 35% during Q4 “but remained well above expected for the pediatric population,” the investigators reported.
Despite the high incidence, “I was surprised at how few of the patients with hypertension we actually treated,” Dr. Freiberg said in an interview.
Just 3 of the 36 patients studied received treatment for hypertension, he said, possibly because clinicians assumed that the effect was steroid related and transient.
“Now that we’re paying attention, however, we’re treating more of these patients,” Dr. Freiberg said.
The electronic record system used at his institution now alerts clinicians to hypertensive episodes during treatment, he added.
Kidney injury in AML
Like Dr. Freiberg and his colleagues, Liezl du Plessis, MBChB, from the British Columbia Children’s Hospital in Vancouver, Canada, and her colleagues were similarly taken aback when they looked into the incidence of acute kidney injury (AKI) in children and adolescents undergoing multidrug chemotherapy for AML.
“Chemotherapy agents that are used in acute myeloid leukemia are not considered to be nephrotoxic, so it was quite alarming to us to see that there is such a high rate of kidney injury in these patients,” Dr. du Plessis said in an interview.
They found that 34 of the 53 patients (64%) had AKI, with 11 patients having stage 1 (rise in serum creatinine of 1.5 or more times the baseline level), 11 having stage 2 (SCr 2 or more times baseline), and 12 having stage 3 AKI (SCr 3 or more times baseline or the need for dialysis).
Creatinine changes were counted only if they occurred within 7 days from nadir to peak.
AKI occurred in all chemotherapy cycles, with severe injury having the highest frequency in cycle 1.
In a logistical regression model, factors significantly associated with risk for AKI were male sex (odds ratio, 0.2; P = .03) and age 10 years or older (OR, 17.3; P less than .01). Neither sepsis nor aminoglycoside or vancomycin use for more than 3 days was significantly associated with risk for AKI, however.
“I think people need to realize that many of these injuries are happening on the oncology ward, and some of these kids may not even look acutely unwell, so people need to take note.” Dr. du Plessis said.
She recommended curtailing use of nephrotoxic agents whenever possible, and emphasized that clinicians need to document AKI in the medical record.
“A big portion of our AML population goes on to bone marrow transplant, and that is known to be a high risk for further kidney injury. So, I think it would be important to know that before your patient goes for his transplant that he already has certain toxicities and organ injury, so that you can limit things like fluids and get the nephrology team on board to help with the management of these patients,” she said.
The study by Dr. Freiberg was supported by the Four Diamonds Fund. The study by Dr. du Plessis was internally funded. The investigators for each study reported having no relevant financial disclosures.
MONTREAL – Hypertension is a frequent, but underrecognized and undertreated, complication of chemotherapy for acute lymphoblastic leukemia (ALL), the most common childhood malignancy, investigators in a single-center U.S. study reported.
Additionally, there is a “concerningly high” incidence of acute kidney injury among children and young adults who undergo multiagent chemotherapy for acute myeloid leukemia (AML), said the authors of a study conducted in Canada.
Both studies were reported in a scientific poster session at the annual meeting of the American Society for Pediatric Hematology/Oncology.
Hypertension in ALL
Although standard induction regimens for childhood ALL contain high-dose steroids, which are known to be associated with increased risk for hypertension, the incidence of hypertension throughout induction, consolidation, and maintenance for ALL in children has not been adequately evaluated, according to Andrew S. Freiberg, MD, and his colleagues at Penn State Children’s Hospital in Hershey, Penn.
“The incidence of hypertension was much higher than the under 5% expected for a healthy pediatric population,” the investigators found.
Of 562 total readings taken during induction, 56% were in the normo- or prehypertensive range, but 30% were classified as stage 1 and 14% as stage II hypertension.
The combined percentage of stage 1 and 2 readings declined slightly over the year from 44% during induction to 35% during Q4 “but remained well above expected for the pediatric population,” the investigators reported.
Despite the high incidence, “I was surprised at how few of the patients with hypertension we actually treated,” Dr. Freiberg said in an interview.
Just 3 of the 36 patients studied received treatment for hypertension, he said, possibly because clinicians assumed that the effect was steroid related and transient.
“Now that we’re paying attention, however, we’re treating more of these patients,” Dr. Freiberg said.
The electronic record system used at his institution now alerts clinicians to hypertensive episodes during treatment, he added.
Kidney injury in AML
Like Dr. Freiberg and his colleagues, Liezl du Plessis, MBChB, from the British Columbia Children’s Hospital in Vancouver, Canada, and her colleagues were similarly taken aback when they looked into the incidence of acute kidney injury (AKI) in children and adolescents undergoing multidrug chemotherapy for AML.
“Chemotherapy agents that are used in acute myeloid leukemia are not considered to be nephrotoxic, so it was quite alarming to us to see that there is such a high rate of kidney injury in these patients,” Dr. du Plessis said in an interview.
They found that 34 of the 53 patients (64%) had AKI, with 11 patients having stage 1 (rise in serum creatinine of 1.5 or more times the baseline level), 11 having stage 2 (SCr 2 or more times baseline), and 12 having stage 3 AKI (SCr 3 or more times baseline or the need for dialysis).
Creatinine changes were counted only if they occurred within 7 days from nadir to peak.
AKI occurred in all chemotherapy cycles, with severe injury having the highest frequency in cycle 1.
In a logistical regression model, factors significantly associated with risk for AKI were male sex (odds ratio, 0.2; P = .03) and age 10 years or older (OR, 17.3; P less than .01). Neither sepsis nor aminoglycoside or vancomycin use for more than 3 days was significantly associated with risk for AKI, however.
“I think people need to realize that many of these injuries are happening on the oncology ward, and some of these kids may not even look acutely unwell, so people need to take note.” Dr. du Plessis said.
She recommended curtailing use of nephrotoxic agents whenever possible, and emphasized that clinicians need to document AKI in the medical record.
“A big portion of our AML population goes on to bone marrow transplant, and that is known to be a high risk for further kidney injury. So, I think it would be important to know that before your patient goes for his transplant that he already has certain toxicities and organ injury, so that you can limit things like fluids and get the nephrology team on board to help with the management of these patients,” she said.
The study by Dr. Freiberg was supported by the Four Diamonds Fund. The study by Dr. du Plessis was internally funded. The investigators for each study reported having no relevant financial disclosures.
At ASPHO 2017
Key clinical point:
Major finding: The combined incidence of stage 1 or 2 hypertension in children during induction therapy for acute lymphoblastic leukemia was 44%.
Data source: Two retrospective institutional studies.
Disclosures: The study by Dr. Freiberg was supported by the Four Diamonds Fund. The study by Dr. du Plessis was internally funded. The investigators for each study reported having no relevant financial disclosures.