User login
Rendered speechless
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 63-year-old man at an inpatient rehabilitation center was transferred to an academic tertiary care center for evaluation of slurred speech and episodic confusion. He was accompanied by his wife, who provided the history. Three weeks earlier, the patient had fallen, sustaining a right femur fracture. He underwent surgery and was discharged to rehabilitation on postoperative day 3. During the second week of rehabilitation, he developed a cough and low-grade fevers, which prompted treatment with cefpodoxime for 5 days for presumed pneumonia. The day after completing antimicrobial therapy, he became confused and began to slur his words.
Confusion is a nonspecific symptom that typically has a diffuse or multifocal localization within the cerebral hemispheres and is unlikely to be caused by a single lesion. Slurred speech may accompany global metabolic dysfunction. However, slurred speech typically localizes to the brainstem, the cerebellum in the posterior fossa, the nuclei, or the course of cranial nerves VII, X, or XII, including where these nerves pass through the subarachnoid space.
It seems this patient’s new neurologic symptoms have some relationship to his fall. Long-bone fractures and altered mental status (AMS) lead to consideration of fat emboli, but this syndrome typically presents in the acute period after the fracture. The patient is at risk for a number of complications, related to recent surgery and hospitalization, that could affect the central nervous system (CNS), including systemic infection (possibly with associated meningeal involvement) and venous thromboembolism with concomitant stroke by paradoxical emboli. The episodic nature of the confusion leads to consideration of seizures from structural lesions in the brain. Finally, the circumstances of the fall itself should be explored to determine whether an underlying neurologic dysfunction led to imbalance and gait difficulty.
Over the next 3 days at the inpatient rehabilitation center, the patient’s slurred speech became unintelligible, and he experienced intermittent disorientation to person, place, and time. There was no concomitant fever, dizziness, headache, neck pain, weakness, dyspnea, diarrhea, dysuria, or change in hearing or vision.
Progressive dysarthria argues for an expanding lesion in the posterior fossa, worsening metabolic disturbance, or a problem affecting the cranial nerves (eg, Guillain-Barré syndrome) or neuromuscular junctions (eg, myasthenia gravis). Lack of headache makes a CNS localization less likely, though disorientation must localize to the brain itself. The transient nature of the AMS could signal an ictal phenomenon or a fluctuating toxic or metabolic condition, such as hyperammonemia, drug reaction, or healthcare–acquired delirium.
His past medical history included end-stage liver disease secondary to nonalcoholic steatohepatitis status post transjugular intrahepatic portosystemic shunt (TIPS) procedure three years prior, hepatic encephalopathy, diabetes mellitus type 2, hypertension, previous melanoma excision on his back, and recurrent Clostridium difficile colitis. Two years prior to admission he had been started on an indefinite course of metronidazole 500 mg twice daily without any recurrence. The patient’s other medications were aspirin, furosemide, insulin, lactulose, mirtazapine, pantoprazole, propranolol, spironolactone, and zinc. At the rehabilitation center, he was prescribed oral oxycodone 5 mg as needed every 4 hours for pain. He denied use of tobacco, alcohol, and recreational drugs. He previously worked as a funeral home director and embalmer.
Hyperammonemia and hepatic encephalopathy can present with a fluctuating mental state that often correlates to dietary protein intake or the frequency of bowel movements; the previous TIPS history places the patient at further risk. Use of oxycodone or another narcotic commonly leads to confusion, , especially in patients who are older, have preexisting cognitive decline, or have concomitant medical comorbidities. Mirtazapine and propranolol have been associated more rarely with encephalopathy, and therefore a careful history of adherence, drug interactions, and appropriate dosing should be obtained. Metronidazole is most often associated neurologically with a peripheral neuropathy; however, it is increasingly recognized that some patients can develop a CNS syndrome that features an AMS, which can be severe and accompanied by ataxia, dysarthria, and characteristic brain magnetic resonance imaging (MRI) findings, including hyperintensity surrounding the fourth ventricle on T2-weighted images.
Embalming fluid has a high concentration of formaldehyde, and a recent epidemiologic study suggested a link between formaldehyde exposure and increased risk for amyotrophic lateral sclerosis (ALS). ALS uncommonly presents with isolated dysarthria, but its bulbar form can, usually over a much longer course than is demonstrated here. Finally, the patient’s history of melanoma places him at risk for stroke from hypercoagulability as well as potential brain metastases or carcinomatous meningitis.
Evaluation was initiated at the rehabilitation facility at the onset of the patient’s slurred speech and confusion. Physical examination were negative for focal neurologic deficits, asterixis, and jaundice. Ammonia level was 41 µmol/L (reference range, 11-35 µmol/L). Noncontrast computed tomography (CT) of the head showed no signs of acute infarct or hemorrhage. Symptoms were attributed to hepatic encephalopathy; lactulose was up-titrated to ensure 2 or 3 bowel movements per day, and rifaximin was started.
Hyperammonemia is a cause of non-inflammatory relapsing encephalopathy, but an elevated level is neither a sensitive nor specific indicator of hepatic encephalopathy. Levels of ammonia can fluctuate widely during the day based on the frequency of bowel movements as well as dietary protein intake. In addition, proper handling of samples with prompt delivery to the laboratory is essential to minimize errors.
The ammonia level of 41 µmol/L discovered here is only modestly elevated, but given the patient’s history of TIPS as well as the clinical picture, it is reasonable to aggressively treat hepatic encephalopathy with lactulose to reduce ammonia levels. If he does not improve, an MRI of the brain to exclude a structural lesion and spinal fluid examination looking for inflammatory or infectious conditions would be important next steps. Although CT excludes a large hemorrhage or mass, this screening examination does not visualize many of the findings of the metabolic etiology and the other etiologies under consideration here.
Despite 3 days of therapy for presumed hepatic encephalopathy, the patient’s slurred speech worsened, and he was transferred to an academic tertiary care center for further evaluation. On admission, his temperature was 36.9°C, heart rate was 80 beats per minute, blood pressure was 139/67 mm Hg, respiratory rate was 10 breaths per minute, and oxygen saturation was 99% on room air. He was alert, awake, and oriented to person, place, and time. He was not jaundiced. He exhibited a moderate dysarthria characterized by monotone speech, decreased volume, decreased breath support, and a hoarse vocal quality with intact language function. Motor control of the lips, tongue, and mandible were normal. Motor strength was 5/5 bilaterally in the upper and lower extremities with the exception of right hip flexion, which was 4/5. The patient exhibited mild bilateral dysmetria on finger-to-nose examination, consistent with appendicular ataxia of the upper extremities. Reflexes were depressed throughout, and there was no asterixis. He had 2+ pulses in all extremities and 1+ pitting edema of the right lower extremity to the mid leg. Pulmonary examination revealed inspiratory crackles at the left base. The rest of the examination findings were normal.
The patient’s altered mental state appears to have resolved, and the neurological examination is now mainly characterized by signs that point to the cerebellum. The description of monotone speech typically refers to loss of prosody, the variable stress or intonation of speech, which is characteristic of a cerebellar speech pattern. The hoarseness should be explored to determine if it is a feature of the patient’s speech or is a separate process. Hoarseness may involve the vocal cord and therefore, potentially, cranial nerve X or its nuclei in the brainstem. The appendicular ataxia of the limbs points definitively to the cerebellar hemispheres or their pathways through the brainstem.
Unilateral lower extremity edema, especially in the context of a recent fracture, raises the possibility of deep vein thrombosis. If this patient has a right-to-left intracardiac or intrapulmonary shunt, embolization could lead to an ischemic stroke of the brainstem or cerebellum, potentially causing dysarthria.
Laboratory evaluation revealed hemoglobin level of 10.9 g/dL, white blood cell count of 5.3 × 10 9 /L, platelet count of 169 × 10 9 /L, glucose level of 177 mg/dL, corrected calcium level of 9.0 mg/dL, sodium level of 135 mmol/L, bicarbonate level of 30 mmol/L, creatinine level of 0.9 mg/dL, total bilirubin level of 1.3 mg/dL, direct bilirubin level of 0.4 mg/dL, alkaline phosphatase level of 503 U/L, alanine aminotransferase level of 12 U/L, aspartate aminotransferase level of 33 U/L, ammonia level of 49 µmol/L (range, 0-30 µ mol/L), international normalized ratio of 1.2, and troponin level of <0.01 ng/mL. Electrocardiogram showed normal sinus rhythm.
Some patients with bacterial meningitis do not have a leukocytosis, but patients with meningitis caused by seeding from a systemic infection nearly always do. In this patient’s case, lack of a leukocytosis makes bacterial meningitis very unlikely. The elevated alkaline phosphatase level is expected, as this level peaks about 3 weeks after a long-bone fracture and returns to normal over a few months.
Non-contrast CT scan of the head performed on admission demonstrated no large vessel cortical-based infarct, intracranial hemorrhage, hydrocephalus, mass effect, midline shift, or extra-axial fluid. There was mild cortical atrophy as well as very mild periventricular white matter hypodensity.
The atrophy and mild white-matter hypodensities seen on repeat noncontrast CT are nonspecific for any particular entity in this patient’s age group. MRI is more effective in evaluating toxic encephalopathies, including metronidazole toxicity or Wernicke encephalopathy, and in characterizing small infarcts or inflammatory conditions of the brainstem and cerebellum, which are poorly evaluated by CT due to the bone surrounded space of the posterior fossa. An urgent lumbar puncture is not necessary due to the slow pace of illness, lack of fever, nuchal rigidity, or serum elevated white blood cell count. Rather, performing MRI should be prioritized. If MRI is nondiagnostic, then spinal fluid should be evaluated for evidence of an infectious, autoimmune, paraneoplastic, or neoplastic process.
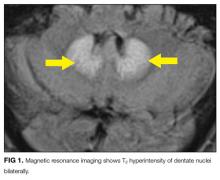
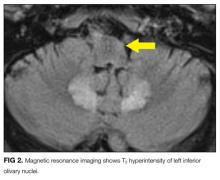
MRI was subsequently performed. It showed symmetric abnormal T2 hyperintensities involving dentate nuclei (Figure 1), left inferior olivary nuclei (Figure 2), restiform bodies, pontine tegmentum, superior cerebellar peduncles, oculomotor nuclei, and subthalamic nuclei. The most prominent hyperintensity was in the dentate nuclei.
The clinical and radiographic features confirm a diagnosis of metronidazole-associated CNS neurotoxicity. The reason for the predilection for edema in these specific areas of the brainstem and midline cerebellum is unclear but likely is related to selective neuronal vulnerability in these structures. The treatment is to stop metronidazole. In addition, the fluctuating mental status should be evaluated with electroencephalogram to ensure concomitant seizures are not occurring.
These MRI findings were consistent with metronidazole toxicity. Metronidazole was discontinued, and 2 days later the patient’s speech improved. Two weeks after medication discontinuation, his speech was normal. There were no more episodes of confusion.
DISCUSSION
Metronidazole was originally developed in France during the 1950s as an anti-parasitic medication to treat trichomonas infections. In 1962, its antibacterial properties were discovered after a patient with bacterial gingivitis improved while taking metronidazole for treatment of Trichomonas vaginalis.1 Since that time metronidazole has become a first-line treatment for anaerobic bacteria and is now recommended by the Infectious Diseases Society of America2 and the American College of Gastroenterology3 as a first-line therapy for mild and moderate C difficile infections.
Common side effects of metronidazole are nausea, vomiting, decreased appetite, diarrhea, headaches, peripheral neuropathy, and metallic taste; less common is CNS toxicity. Although the incidence of CNS toxicity is unknown, a systematic review of the literature found 64 cases reported between 1965 and 2011.4 CNS toxicity most often occurs between the fifth and sixth decades of life, and about two thirds of the people affected are men.4 CNS adverse effects characteristically fall into 4 categories: cerebellar dysfunction (eg, ataxia, dysarthria, dysmetria, nystagmus; 75%), AMS (33%), seizures (13%), and a combination of the first 3 categories.4
The exact mechanism of metronidazole CNS toxicity is unknown, but vasogenic or cytotoxic edema may be involved.5,6 Other potential etiologies are neural protein inhibition, reversible mitochondrial dysfunction, and modifications of the inhibitory neurotransmitter gamma-aminobutyric acid receptor in the cerebellum.7,8 There is no known genetic predisposition. Although the risk for CNS toxicity traditionally is thought to correlate with therapy duration and cumulative dose,7,9 in 2011 a systemic review found no significant correlation.4 In fact, 26% of patients with CNS toxicity were treated with metronidazole for less than 1 week at time of diagnosis.4
Brain CT is typically normal. On brain MRI, lesions most commonly appear as bilateral symmetric T2 hyperintensities, most often in the cerebellar dentate nuclei (85%) and less often in the midbrain (55%), the splenium of the corpus callosum (50%), the pons (35%), and the medulla (30%).4,10 Radiographic changes have been noted as early as 3 days after symptom onset. Based on damage severity and area affected (white or gray matter), vasogenic edema and cytotoxic edema may in combination be contributing to MRI abnormalities.6,10 Hyperintensities of the bilateral dentate nuclei can help in distinguishing metronidazole-induced encephalopathy from other potential disease processes, such as Wernicke encephalopathy.10
The prognosis for patients with metronidazole-induced neurotoxicity is favorable if metronidazole is discontinued. Approximately two-thirds of patients will have complete resolution of symptoms, which is more commonly observed when patients present with seizures or altered mental status. Approximately one-third will show partial improvement, particularly if the symptoms are due to cerebellar dysfunction. It is rare to experience permanent damage or death.4 Neurologic recovery usually begins within a week after medication discontinuation but may take months for complete recovery to occur.6,8,9,11 Follow-up imaging typically shows reversal of the original lesions, but this does not always correlate with symptom improvement.4,10
Despite its frequent use and long history, metronidazole can have potentially severe toxicity. When patients who are taking this medication present with new signs and symptoms of CNS dysfunction, hospitalists should include metronidazole CNS toxicity in the differential diagnosis and, if they suspect toxicity, have a brain MRI performed. Hospitalists often prescribe metronidazole because of the increasing number of patients being discharged from acute-care hospitals with a diagnosis of C difficile colitis.12 Brain MRI remains the imaging modality of choice for diagnosis. Discontinuation of metronidazole is usually salutary in reversing symptoms. Being keenly aware of this toxicity will help clinicians avoid being rendered speechless by a patient rendered speechless.
TEACHING POINTS
CNS toxicity is a rare but potentially devastating side effect of metronidazole exposure.
Metronidazole CNS adverse effects characteristically fall under 4 categories:
○ Cerebellar dysfunction, such as ataxia, dysarthria, dysmetria, or nystagmus (75%).
○ AMS (33%).
○ Seizures (13%).
○ A combination of the first 3 categories.
- Typically lesions indicating metronidazole toxicity on brain MRI are bilateral symmetric hyperintensities on T2-weighted imaging in the cerebellar dentate nuclei, corpus callosum, midbrain, pons, or medulla.
- Treatment of CNS toxicity is metronidazole discontinuation, which results in a high rate of symptom resolution.
Disclosure
Nothing to report.
1. Samuelson J. Why metronidazole is active against both bacteria and parasites. Antimicrob Agents Chemother. 1999;43(7):1533-1541. PubMed
2. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455. PubMed
3. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478-498. PubMed
4. Kuriyama A, Jackson JL, Doi A, Kamiya T. Metronidazole-induced central nervous system toxicity: a systemic review. Clin Neuropharmacol. 2011;34(6):241-247. PubMed
5. Graves TD, Condon M, Loucaidou M, Perry RJ. Reversible metronidazole-induced cerebellar toxicity in a multiple transplant recipient. J Neurol Sci. 2009;285(1-2):238-240. PubMed
6. Kim DW, Park JM, Yoon BW, Baek MJ, Kim JE, Kim S. Metronidazole-induced encephalopathy. J Neurol Sci. 2004;224(1-2):107-111. PubMed
7. Park KI, Chung JM, Kim JY. Metronidazole neurotoxicity: sequential neuroaxis involvement. Neurol India. 2011;59(1):104-107. PubMed
8. Patel K, Green-Hopkins I, Lu S, Tunkel AR. Cerebellar ataxia following prolonged use of metronidazole: case report and literature review. Int J Infect Dis. 2008;12(6):e111-e114. PubMed
9. Chandak S, Agarwal A, Shukla A, Joon P. A case report of metronidazole induced neurotoxicity in liver abscess patient and the usefulness of MRI for its diagnosis. J Clin Diagn Res. 2016;10(1):TD06-TD07. PubMed
10. Kim E, Na DG, Kim EY, Kim JH, Son KR, Chang KH. MR imaging of metronidazole-induced encephalopathy: lesion distribution and diffusion-weighted imaging findings. AJNR Am J Neuroradiol. 2007;28(9):1652-1658. PubMed
11. Chacko J, Pramod K, Sinha S, et al. Clinical, neuroimaging and pathological features of 5-nitroimidazole-induced encephalo-neuropathy in two patients: insights into possible pathogenesis. Neurol India. 2011;59(5):743-747. PubMed
12. Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179-1187.e1-e3. PubMed
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 63-year-old man at an inpatient rehabilitation center was transferred to an academic tertiary care center for evaluation of slurred speech and episodic confusion. He was accompanied by his wife, who provided the history. Three weeks earlier, the patient had fallen, sustaining a right femur fracture. He underwent surgery and was discharged to rehabilitation on postoperative day 3. During the second week of rehabilitation, he developed a cough and low-grade fevers, which prompted treatment with cefpodoxime for 5 days for presumed pneumonia. The day after completing antimicrobial therapy, he became confused and began to slur his words.
Confusion is a nonspecific symptom that typically has a diffuse or multifocal localization within the cerebral hemispheres and is unlikely to be caused by a single lesion. Slurred speech may accompany global metabolic dysfunction. However, slurred speech typically localizes to the brainstem, the cerebellum in the posterior fossa, the nuclei, or the course of cranial nerves VII, X, or XII, including where these nerves pass through the subarachnoid space.
It seems this patient’s new neurologic symptoms have some relationship to his fall. Long-bone fractures and altered mental status (AMS) lead to consideration of fat emboli, but this syndrome typically presents in the acute period after the fracture. The patient is at risk for a number of complications, related to recent surgery and hospitalization, that could affect the central nervous system (CNS), including systemic infection (possibly with associated meningeal involvement) and venous thromboembolism with concomitant stroke by paradoxical emboli. The episodic nature of the confusion leads to consideration of seizures from structural lesions in the brain. Finally, the circumstances of the fall itself should be explored to determine whether an underlying neurologic dysfunction led to imbalance and gait difficulty.
Over the next 3 days at the inpatient rehabilitation center, the patient’s slurred speech became unintelligible, and he experienced intermittent disorientation to person, place, and time. There was no concomitant fever, dizziness, headache, neck pain, weakness, dyspnea, diarrhea, dysuria, or change in hearing or vision.
Progressive dysarthria argues for an expanding lesion in the posterior fossa, worsening metabolic disturbance, or a problem affecting the cranial nerves (eg, Guillain-Barré syndrome) or neuromuscular junctions (eg, myasthenia gravis). Lack of headache makes a CNS localization less likely, though disorientation must localize to the brain itself. The transient nature of the AMS could signal an ictal phenomenon or a fluctuating toxic or metabolic condition, such as hyperammonemia, drug reaction, or healthcare–acquired delirium.
His past medical history included end-stage liver disease secondary to nonalcoholic steatohepatitis status post transjugular intrahepatic portosystemic shunt (TIPS) procedure three years prior, hepatic encephalopathy, diabetes mellitus type 2, hypertension, previous melanoma excision on his back, and recurrent Clostridium difficile colitis. Two years prior to admission he had been started on an indefinite course of metronidazole 500 mg twice daily without any recurrence. The patient’s other medications were aspirin, furosemide, insulin, lactulose, mirtazapine, pantoprazole, propranolol, spironolactone, and zinc. At the rehabilitation center, he was prescribed oral oxycodone 5 mg as needed every 4 hours for pain. He denied use of tobacco, alcohol, and recreational drugs. He previously worked as a funeral home director and embalmer.
Hyperammonemia and hepatic encephalopathy can present with a fluctuating mental state that often correlates to dietary protein intake or the frequency of bowel movements; the previous TIPS history places the patient at further risk. Use of oxycodone or another narcotic commonly leads to confusion, , especially in patients who are older, have preexisting cognitive decline, or have concomitant medical comorbidities. Mirtazapine and propranolol have been associated more rarely with encephalopathy, and therefore a careful history of adherence, drug interactions, and appropriate dosing should be obtained. Metronidazole is most often associated neurologically with a peripheral neuropathy; however, it is increasingly recognized that some patients can develop a CNS syndrome that features an AMS, which can be severe and accompanied by ataxia, dysarthria, and characteristic brain magnetic resonance imaging (MRI) findings, including hyperintensity surrounding the fourth ventricle on T2-weighted images.
Embalming fluid has a high concentration of formaldehyde, and a recent epidemiologic study suggested a link between formaldehyde exposure and increased risk for amyotrophic lateral sclerosis (ALS). ALS uncommonly presents with isolated dysarthria, but its bulbar form can, usually over a much longer course than is demonstrated here. Finally, the patient’s history of melanoma places him at risk for stroke from hypercoagulability as well as potential brain metastases or carcinomatous meningitis.
Evaluation was initiated at the rehabilitation facility at the onset of the patient’s slurred speech and confusion. Physical examination were negative for focal neurologic deficits, asterixis, and jaundice. Ammonia level was 41 µmol/L (reference range, 11-35 µmol/L). Noncontrast computed tomography (CT) of the head showed no signs of acute infarct or hemorrhage. Symptoms were attributed to hepatic encephalopathy; lactulose was up-titrated to ensure 2 or 3 bowel movements per day, and rifaximin was started.
Hyperammonemia is a cause of non-inflammatory relapsing encephalopathy, but an elevated level is neither a sensitive nor specific indicator of hepatic encephalopathy. Levels of ammonia can fluctuate widely during the day based on the frequency of bowel movements as well as dietary protein intake. In addition, proper handling of samples with prompt delivery to the laboratory is essential to minimize errors.
The ammonia level of 41 µmol/L discovered here is only modestly elevated, but given the patient’s history of TIPS as well as the clinical picture, it is reasonable to aggressively treat hepatic encephalopathy with lactulose to reduce ammonia levels. If he does not improve, an MRI of the brain to exclude a structural lesion and spinal fluid examination looking for inflammatory or infectious conditions would be important next steps. Although CT excludes a large hemorrhage or mass, this screening examination does not visualize many of the findings of the metabolic etiology and the other etiologies under consideration here.
Despite 3 days of therapy for presumed hepatic encephalopathy, the patient’s slurred speech worsened, and he was transferred to an academic tertiary care center for further evaluation. On admission, his temperature was 36.9°C, heart rate was 80 beats per minute, blood pressure was 139/67 mm Hg, respiratory rate was 10 breaths per minute, and oxygen saturation was 99% on room air. He was alert, awake, and oriented to person, place, and time. He was not jaundiced. He exhibited a moderate dysarthria characterized by monotone speech, decreased volume, decreased breath support, and a hoarse vocal quality with intact language function. Motor control of the lips, tongue, and mandible were normal. Motor strength was 5/5 bilaterally in the upper and lower extremities with the exception of right hip flexion, which was 4/5. The patient exhibited mild bilateral dysmetria on finger-to-nose examination, consistent with appendicular ataxia of the upper extremities. Reflexes were depressed throughout, and there was no asterixis. He had 2+ pulses in all extremities and 1+ pitting edema of the right lower extremity to the mid leg. Pulmonary examination revealed inspiratory crackles at the left base. The rest of the examination findings were normal.
The patient’s altered mental state appears to have resolved, and the neurological examination is now mainly characterized by signs that point to the cerebellum. The description of monotone speech typically refers to loss of prosody, the variable stress or intonation of speech, which is characteristic of a cerebellar speech pattern. The hoarseness should be explored to determine if it is a feature of the patient’s speech or is a separate process. Hoarseness may involve the vocal cord and therefore, potentially, cranial nerve X or its nuclei in the brainstem. The appendicular ataxia of the limbs points definitively to the cerebellar hemispheres or their pathways through the brainstem.
Unilateral lower extremity edema, especially in the context of a recent fracture, raises the possibility of deep vein thrombosis. If this patient has a right-to-left intracardiac or intrapulmonary shunt, embolization could lead to an ischemic stroke of the brainstem or cerebellum, potentially causing dysarthria.
Laboratory evaluation revealed hemoglobin level of 10.9 g/dL, white blood cell count of 5.3 × 10 9 /L, platelet count of 169 × 10 9 /L, glucose level of 177 mg/dL, corrected calcium level of 9.0 mg/dL, sodium level of 135 mmol/L, bicarbonate level of 30 mmol/L, creatinine level of 0.9 mg/dL, total bilirubin level of 1.3 mg/dL, direct bilirubin level of 0.4 mg/dL, alkaline phosphatase level of 503 U/L, alanine aminotransferase level of 12 U/L, aspartate aminotransferase level of 33 U/L, ammonia level of 49 µmol/L (range, 0-30 µ mol/L), international normalized ratio of 1.2, and troponin level of <0.01 ng/mL. Electrocardiogram showed normal sinus rhythm.
Some patients with bacterial meningitis do not have a leukocytosis, but patients with meningitis caused by seeding from a systemic infection nearly always do. In this patient’s case, lack of a leukocytosis makes bacterial meningitis very unlikely. The elevated alkaline phosphatase level is expected, as this level peaks about 3 weeks after a long-bone fracture and returns to normal over a few months.
Non-contrast CT scan of the head performed on admission demonstrated no large vessel cortical-based infarct, intracranial hemorrhage, hydrocephalus, mass effect, midline shift, or extra-axial fluid. There was mild cortical atrophy as well as very mild periventricular white matter hypodensity.
The atrophy and mild white-matter hypodensities seen on repeat noncontrast CT are nonspecific for any particular entity in this patient’s age group. MRI is more effective in evaluating toxic encephalopathies, including metronidazole toxicity or Wernicke encephalopathy, and in characterizing small infarcts or inflammatory conditions of the brainstem and cerebellum, which are poorly evaluated by CT due to the bone surrounded space of the posterior fossa. An urgent lumbar puncture is not necessary due to the slow pace of illness, lack of fever, nuchal rigidity, or serum elevated white blood cell count. Rather, performing MRI should be prioritized. If MRI is nondiagnostic, then spinal fluid should be evaluated for evidence of an infectious, autoimmune, paraneoplastic, or neoplastic process.
MRI was subsequently performed. It showed symmetric abnormal T2 hyperintensities involving dentate nuclei (Figure 1), left inferior olivary nuclei (Figure 2), restiform bodies, pontine tegmentum, superior cerebellar peduncles, oculomotor nuclei, and subthalamic nuclei. The most prominent hyperintensity was in the dentate nuclei.
The clinical and radiographic features confirm a diagnosis of metronidazole-associated CNS neurotoxicity. The reason for the predilection for edema in these specific areas of the brainstem and midline cerebellum is unclear but likely is related to selective neuronal vulnerability in these structures. The treatment is to stop metronidazole. In addition, the fluctuating mental status should be evaluated with electroencephalogram to ensure concomitant seizures are not occurring.
These MRI findings were consistent with metronidazole toxicity. Metronidazole was discontinued, and 2 days later the patient’s speech improved. Two weeks after medication discontinuation, his speech was normal. There were no more episodes of confusion.
DISCUSSION
Metronidazole was originally developed in France during the 1950s as an anti-parasitic medication to treat trichomonas infections. In 1962, its antibacterial properties were discovered after a patient with bacterial gingivitis improved while taking metronidazole for treatment of Trichomonas vaginalis.1 Since that time metronidazole has become a first-line treatment for anaerobic bacteria and is now recommended by the Infectious Diseases Society of America2 and the American College of Gastroenterology3 as a first-line therapy for mild and moderate C difficile infections.
Common side effects of metronidazole are nausea, vomiting, decreased appetite, diarrhea, headaches, peripheral neuropathy, and metallic taste; less common is CNS toxicity. Although the incidence of CNS toxicity is unknown, a systematic review of the literature found 64 cases reported between 1965 and 2011.4 CNS toxicity most often occurs between the fifth and sixth decades of life, and about two thirds of the people affected are men.4 CNS adverse effects characteristically fall into 4 categories: cerebellar dysfunction (eg, ataxia, dysarthria, dysmetria, nystagmus; 75%), AMS (33%), seizures (13%), and a combination of the first 3 categories.4
The exact mechanism of metronidazole CNS toxicity is unknown, but vasogenic or cytotoxic edema may be involved.5,6 Other potential etiologies are neural protein inhibition, reversible mitochondrial dysfunction, and modifications of the inhibitory neurotransmitter gamma-aminobutyric acid receptor in the cerebellum.7,8 There is no known genetic predisposition. Although the risk for CNS toxicity traditionally is thought to correlate with therapy duration and cumulative dose,7,9 in 2011 a systemic review found no significant correlation.4 In fact, 26% of patients with CNS toxicity were treated with metronidazole for less than 1 week at time of diagnosis.4
Brain CT is typically normal. On brain MRI, lesions most commonly appear as bilateral symmetric T2 hyperintensities, most often in the cerebellar dentate nuclei (85%) and less often in the midbrain (55%), the splenium of the corpus callosum (50%), the pons (35%), and the medulla (30%).4,10 Radiographic changes have been noted as early as 3 days after symptom onset. Based on damage severity and area affected (white or gray matter), vasogenic edema and cytotoxic edema may in combination be contributing to MRI abnormalities.6,10 Hyperintensities of the bilateral dentate nuclei can help in distinguishing metronidazole-induced encephalopathy from other potential disease processes, such as Wernicke encephalopathy.10
The prognosis for patients with metronidazole-induced neurotoxicity is favorable if metronidazole is discontinued. Approximately two-thirds of patients will have complete resolution of symptoms, which is more commonly observed when patients present with seizures or altered mental status. Approximately one-third will show partial improvement, particularly if the symptoms are due to cerebellar dysfunction. It is rare to experience permanent damage or death.4 Neurologic recovery usually begins within a week after medication discontinuation but may take months for complete recovery to occur.6,8,9,11 Follow-up imaging typically shows reversal of the original lesions, but this does not always correlate with symptom improvement.4,10
Despite its frequent use and long history, metronidazole can have potentially severe toxicity. When patients who are taking this medication present with new signs and symptoms of CNS dysfunction, hospitalists should include metronidazole CNS toxicity in the differential diagnosis and, if they suspect toxicity, have a brain MRI performed. Hospitalists often prescribe metronidazole because of the increasing number of patients being discharged from acute-care hospitals with a diagnosis of C difficile colitis.12 Brain MRI remains the imaging modality of choice for diagnosis. Discontinuation of metronidazole is usually salutary in reversing symptoms. Being keenly aware of this toxicity will help clinicians avoid being rendered speechless by a patient rendered speechless.
TEACHING POINTS
CNS toxicity is a rare but potentially devastating side effect of metronidazole exposure.
Metronidazole CNS adverse effects characteristically fall under 4 categories:
○ Cerebellar dysfunction, such as ataxia, dysarthria, dysmetria, or nystagmus (75%).
○ AMS (33%).
○ Seizures (13%).
○ A combination of the first 3 categories.
- Typically lesions indicating metronidazole toxicity on brain MRI are bilateral symmetric hyperintensities on T2-weighted imaging in the cerebellar dentate nuclei, corpus callosum, midbrain, pons, or medulla.
- Treatment of CNS toxicity is metronidazole discontinuation, which results in a high rate of symptom resolution.
Disclosure
Nothing to report.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient’s case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant. The bolded text represents the patient’s case. Each paragraph that follows represents the discussant’s thoughts.
A 63-year-old man at an inpatient rehabilitation center was transferred to an academic tertiary care center for evaluation of slurred speech and episodic confusion. He was accompanied by his wife, who provided the history. Three weeks earlier, the patient had fallen, sustaining a right femur fracture. He underwent surgery and was discharged to rehabilitation on postoperative day 3. During the second week of rehabilitation, he developed a cough and low-grade fevers, which prompted treatment with cefpodoxime for 5 days for presumed pneumonia. The day after completing antimicrobial therapy, he became confused and began to slur his words.
Confusion is a nonspecific symptom that typically has a diffuse or multifocal localization within the cerebral hemispheres and is unlikely to be caused by a single lesion. Slurred speech may accompany global metabolic dysfunction. However, slurred speech typically localizes to the brainstem, the cerebellum in the posterior fossa, the nuclei, or the course of cranial nerves VII, X, or XII, including where these nerves pass through the subarachnoid space.
It seems this patient’s new neurologic symptoms have some relationship to his fall. Long-bone fractures and altered mental status (AMS) lead to consideration of fat emboli, but this syndrome typically presents in the acute period after the fracture. The patient is at risk for a number of complications, related to recent surgery and hospitalization, that could affect the central nervous system (CNS), including systemic infection (possibly with associated meningeal involvement) and venous thromboembolism with concomitant stroke by paradoxical emboli. The episodic nature of the confusion leads to consideration of seizures from structural lesions in the brain. Finally, the circumstances of the fall itself should be explored to determine whether an underlying neurologic dysfunction led to imbalance and gait difficulty.
Over the next 3 days at the inpatient rehabilitation center, the patient’s slurred speech became unintelligible, and he experienced intermittent disorientation to person, place, and time. There was no concomitant fever, dizziness, headache, neck pain, weakness, dyspnea, diarrhea, dysuria, or change in hearing or vision.
Progressive dysarthria argues for an expanding lesion in the posterior fossa, worsening metabolic disturbance, or a problem affecting the cranial nerves (eg, Guillain-Barré syndrome) or neuromuscular junctions (eg, myasthenia gravis). Lack of headache makes a CNS localization less likely, though disorientation must localize to the brain itself. The transient nature of the AMS could signal an ictal phenomenon or a fluctuating toxic or metabolic condition, such as hyperammonemia, drug reaction, or healthcare–acquired delirium.
His past medical history included end-stage liver disease secondary to nonalcoholic steatohepatitis status post transjugular intrahepatic portosystemic shunt (TIPS) procedure three years prior, hepatic encephalopathy, diabetes mellitus type 2, hypertension, previous melanoma excision on his back, and recurrent Clostridium difficile colitis. Two years prior to admission he had been started on an indefinite course of metronidazole 500 mg twice daily without any recurrence. The patient’s other medications were aspirin, furosemide, insulin, lactulose, mirtazapine, pantoprazole, propranolol, spironolactone, and zinc. At the rehabilitation center, he was prescribed oral oxycodone 5 mg as needed every 4 hours for pain. He denied use of tobacco, alcohol, and recreational drugs. He previously worked as a funeral home director and embalmer.
Hyperammonemia and hepatic encephalopathy can present with a fluctuating mental state that often correlates to dietary protein intake or the frequency of bowel movements; the previous TIPS history places the patient at further risk. Use of oxycodone or another narcotic commonly leads to confusion, , especially in patients who are older, have preexisting cognitive decline, or have concomitant medical comorbidities. Mirtazapine and propranolol have been associated more rarely with encephalopathy, and therefore a careful history of adherence, drug interactions, and appropriate dosing should be obtained. Metronidazole is most often associated neurologically with a peripheral neuropathy; however, it is increasingly recognized that some patients can develop a CNS syndrome that features an AMS, which can be severe and accompanied by ataxia, dysarthria, and characteristic brain magnetic resonance imaging (MRI) findings, including hyperintensity surrounding the fourth ventricle on T2-weighted images.
Embalming fluid has a high concentration of formaldehyde, and a recent epidemiologic study suggested a link between formaldehyde exposure and increased risk for amyotrophic lateral sclerosis (ALS). ALS uncommonly presents with isolated dysarthria, but its bulbar form can, usually over a much longer course than is demonstrated here. Finally, the patient’s history of melanoma places him at risk for stroke from hypercoagulability as well as potential brain metastases or carcinomatous meningitis.
Evaluation was initiated at the rehabilitation facility at the onset of the patient’s slurred speech and confusion. Physical examination were negative for focal neurologic deficits, asterixis, and jaundice. Ammonia level was 41 µmol/L (reference range, 11-35 µmol/L). Noncontrast computed tomography (CT) of the head showed no signs of acute infarct or hemorrhage. Symptoms were attributed to hepatic encephalopathy; lactulose was up-titrated to ensure 2 or 3 bowel movements per day, and rifaximin was started.
Hyperammonemia is a cause of non-inflammatory relapsing encephalopathy, but an elevated level is neither a sensitive nor specific indicator of hepatic encephalopathy. Levels of ammonia can fluctuate widely during the day based on the frequency of bowel movements as well as dietary protein intake. In addition, proper handling of samples with prompt delivery to the laboratory is essential to minimize errors.
The ammonia level of 41 µmol/L discovered here is only modestly elevated, but given the patient’s history of TIPS as well as the clinical picture, it is reasonable to aggressively treat hepatic encephalopathy with lactulose to reduce ammonia levels. If he does not improve, an MRI of the brain to exclude a structural lesion and spinal fluid examination looking for inflammatory or infectious conditions would be important next steps. Although CT excludes a large hemorrhage or mass, this screening examination does not visualize many of the findings of the metabolic etiology and the other etiologies under consideration here.
Despite 3 days of therapy for presumed hepatic encephalopathy, the patient’s slurred speech worsened, and he was transferred to an academic tertiary care center for further evaluation. On admission, his temperature was 36.9°C, heart rate was 80 beats per minute, blood pressure was 139/67 mm Hg, respiratory rate was 10 breaths per minute, and oxygen saturation was 99% on room air. He was alert, awake, and oriented to person, place, and time. He was not jaundiced. He exhibited a moderate dysarthria characterized by monotone speech, decreased volume, decreased breath support, and a hoarse vocal quality with intact language function. Motor control of the lips, tongue, and mandible were normal. Motor strength was 5/5 bilaterally in the upper and lower extremities with the exception of right hip flexion, which was 4/5. The patient exhibited mild bilateral dysmetria on finger-to-nose examination, consistent with appendicular ataxia of the upper extremities. Reflexes were depressed throughout, and there was no asterixis. He had 2+ pulses in all extremities and 1+ pitting edema of the right lower extremity to the mid leg. Pulmonary examination revealed inspiratory crackles at the left base. The rest of the examination findings were normal.
The patient’s altered mental state appears to have resolved, and the neurological examination is now mainly characterized by signs that point to the cerebellum. The description of monotone speech typically refers to loss of prosody, the variable stress or intonation of speech, which is characteristic of a cerebellar speech pattern. The hoarseness should be explored to determine if it is a feature of the patient’s speech or is a separate process. Hoarseness may involve the vocal cord and therefore, potentially, cranial nerve X or its nuclei in the brainstem. The appendicular ataxia of the limbs points definitively to the cerebellar hemispheres or their pathways through the brainstem.
Unilateral lower extremity edema, especially in the context of a recent fracture, raises the possibility of deep vein thrombosis. If this patient has a right-to-left intracardiac or intrapulmonary shunt, embolization could lead to an ischemic stroke of the brainstem or cerebellum, potentially causing dysarthria.
Laboratory evaluation revealed hemoglobin level of 10.9 g/dL, white blood cell count of 5.3 × 10 9 /L, platelet count of 169 × 10 9 /L, glucose level of 177 mg/dL, corrected calcium level of 9.0 mg/dL, sodium level of 135 mmol/L, bicarbonate level of 30 mmol/L, creatinine level of 0.9 mg/dL, total bilirubin level of 1.3 mg/dL, direct bilirubin level of 0.4 mg/dL, alkaline phosphatase level of 503 U/L, alanine aminotransferase level of 12 U/L, aspartate aminotransferase level of 33 U/L, ammonia level of 49 µmol/L (range, 0-30 µ mol/L), international normalized ratio of 1.2, and troponin level of <0.01 ng/mL. Electrocardiogram showed normal sinus rhythm.
Some patients with bacterial meningitis do not have a leukocytosis, but patients with meningitis caused by seeding from a systemic infection nearly always do. In this patient’s case, lack of a leukocytosis makes bacterial meningitis very unlikely. The elevated alkaline phosphatase level is expected, as this level peaks about 3 weeks after a long-bone fracture and returns to normal over a few months.
Non-contrast CT scan of the head performed on admission demonstrated no large vessel cortical-based infarct, intracranial hemorrhage, hydrocephalus, mass effect, midline shift, or extra-axial fluid. There was mild cortical atrophy as well as very mild periventricular white matter hypodensity.
The atrophy and mild white-matter hypodensities seen on repeat noncontrast CT are nonspecific for any particular entity in this patient’s age group. MRI is more effective in evaluating toxic encephalopathies, including metronidazole toxicity or Wernicke encephalopathy, and in characterizing small infarcts or inflammatory conditions of the brainstem and cerebellum, which are poorly evaluated by CT due to the bone surrounded space of the posterior fossa. An urgent lumbar puncture is not necessary due to the slow pace of illness, lack of fever, nuchal rigidity, or serum elevated white blood cell count. Rather, performing MRI should be prioritized. If MRI is nondiagnostic, then spinal fluid should be evaluated for evidence of an infectious, autoimmune, paraneoplastic, or neoplastic process.
MRI was subsequently performed. It showed symmetric abnormal T2 hyperintensities involving dentate nuclei (Figure 1), left inferior olivary nuclei (Figure 2), restiform bodies, pontine tegmentum, superior cerebellar peduncles, oculomotor nuclei, and subthalamic nuclei. The most prominent hyperintensity was in the dentate nuclei.
The clinical and radiographic features confirm a diagnosis of metronidazole-associated CNS neurotoxicity. The reason for the predilection for edema in these specific areas of the brainstem and midline cerebellum is unclear but likely is related to selective neuronal vulnerability in these structures. The treatment is to stop metronidazole. In addition, the fluctuating mental status should be evaluated with electroencephalogram to ensure concomitant seizures are not occurring.
These MRI findings were consistent with metronidazole toxicity. Metronidazole was discontinued, and 2 days later the patient’s speech improved. Two weeks after medication discontinuation, his speech was normal. There were no more episodes of confusion.
DISCUSSION
Metronidazole was originally developed in France during the 1950s as an anti-parasitic medication to treat trichomonas infections. In 1962, its antibacterial properties were discovered after a patient with bacterial gingivitis improved while taking metronidazole for treatment of Trichomonas vaginalis.1 Since that time metronidazole has become a first-line treatment for anaerobic bacteria and is now recommended by the Infectious Diseases Society of America2 and the American College of Gastroenterology3 as a first-line therapy for mild and moderate C difficile infections.
Common side effects of metronidazole are nausea, vomiting, decreased appetite, diarrhea, headaches, peripheral neuropathy, and metallic taste; less common is CNS toxicity. Although the incidence of CNS toxicity is unknown, a systematic review of the literature found 64 cases reported between 1965 and 2011.4 CNS toxicity most often occurs between the fifth and sixth decades of life, and about two thirds of the people affected are men.4 CNS adverse effects characteristically fall into 4 categories: cerebellar dysfunction (eg, ataxia, dysarthria, dysmetria, nystagmus; 75%), AMS (33%), seizures (13%), and a combination of the first 3 categories.4
The exact mechanism of metronidazole CNS toxicity is unknown, but vasogenic or cytotoxic edema may be involved.5,6 Other potential etiologies are neural protein inhibition, reversible mitochondrial dysfunction, and modifications of the inhibitory neurotransmitter gamma-aminobutyric acid receptor in the cerebellum.7,8 There is no known genetic predisposition. Although the risk for CNS toxicity traditionally is thought to correlate with therapy duration and cumulative dose,7,9 in 2011 a systemic review found no significant correlation.4 In fact, 26% of patients with CNS toxicity were treated with metronidazole for less than 1 week at time of diagnosis.4
Brain CT is typically normal. On brain MRI, lesions most commonly appear as bilateral symmetric T2 hyperintensities, most often in the cerebellar dentate nuclei (85%) and less often in the midbrain (55%), the splenium of the corpus callosum (50%), the pons (35%), and the medulla (30%).4,10 Radiographic changes have been noted as early as 3 days after symptom onset. Based on damage severity and area affected (white or gray matter), vasogenic edema and cytotoxic edema may in combination be contributing to MRI abnormalities.6,10 Hyperintensities of the bilateral dentate nuclei can help in distinguishing metronidazole-induced encephalopathy from other potential disease processes, such as Wernicke encephalopathy.10
The prognosis for patients with metronidazole-induced neurotoxicity is favorable if metronidazole is discontinued. Approximately two-thirds of patients will have complete resolution of symptoms, which is more commonly observed when patients present with seizures or altered mental status. Approximately one-third will show partial improvement, particularly if the symptoms are due to cerebellar dysfunction. It is rare to experience permanent damage or death.4 Neurologic recovery usually begins within a week after medication discontinuation but may take months for complete recovery to occur.6,8,9,11 Follow-up imaging typically shows reversal of the original lesions, but this does not always correlate with symptom improvement.4,10
Despite its frequent use and long history, metronidazole can have potentially severe toxicity. When patients who are taking this medication present with new signs and symptoms of CNS dysfunction, hospitalists should include metronidazole CNS toxicity in the differential diagnosis and, if they suspect toxicity, have a brain MRI performed. Hospitalists often prescribe metronidazole because of the increasing number of patients being discharged from acute-care hospitals with a diagnosis of C difficile colitis.12 Brain MRI remains the imaging modality of choice for diagnosis. Discontinuation of metronidazole is usually salutary in reversing symptoms. Being keenly aware of this toxicity will help clinicians avoid being rendered speechless by a patient rendered speechless.
TEACHING POINTS
CNS toxicity is a rare but potentially devastating side effect of metronidazole exposure.
Metronidazole CNS adverse effects characteristically fall under 4 categories:
○ Cerebellar dysfunction, such as ataxia, dysarthria, dysmetria, or nystagmus (75%).
○ AMS (33%).
○ Seizures (13%).
○ A combination of the first 3 categories.
- Typically lesions indicating metronidazole toxicity on brain MRI are bilateral symmetric hyperintensities on T2-weighted imaging in the cerebellar dentate nuclei, corpus callosum, midbrain, pons, or medulla.
- Treatment of CNS toxicity is metronidazole discontinuation, which results in a high rate of symptom resolution.
Disclosure
Nothing to report.
1. Samuelson J. Why metronidazole is active against both bacteria and parasites. Antimicrob Agents Chemother. 1999;43(7):1533-1541. PubMed
2. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455. PubMed
3. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478-498. PubMed
4. Kuriyama A, Jackson JL, Doi A, Kamiya T. Metronidazole-induced central nervous system toxicity: a systemic review. Clin Neuropharmacol. 2011;34(6):241-247. PubMed
5. Graves TD, Condon M, Loucaidou M, Perry RJ. Reversible metronidazole-induced cerebellar toxicity in a multiple transplant recipient. J Neurol Sci. 2009;285(1-2):238-240. PubMed
6. Kim DW, Park JM, Yoon BW, Baek MJ, Kim JE, Kim S. Metronidazole-induced encephalopathy. J Neurol Sci. 2004;224(1-2):107-111. PubMed
7. Park KI, Chung JM, Kim JY. Metronidazole neurotoxicity: sequential neuroaxis involvement. Neurol India. 2011;59(1):104-107. PubMed
8. Patel K, Green-Hopkins I, Lu S, Tunkel AR. Cerebellar ataxia following prolonged use of metronidazole: case report and literature review. Int J Infect Dis. 2008;12(6):e111-e114. PubMed
9. Chandak S, Agarwal A, Shukla A, Joon P. A case report of metronidazole induced neurotoxicity in liver abscess patient and the usefulness of MRI for its diagnosis. J Clin Diagn Res. 2016;10(1):TD06-TD07. PubMed
10. Kim E, Na DG, Kim EY, Kim JH, Son KR, Chang KH. MR imaging of metronidazole-induced encephalopathy: lesion distribution and diffusion-weighted imaging findings. AJNR Am J Neuroradiol. 2007;28(9):1652-1658. PubMed
11. Chacko J, Pramod K, Sinha S, et al. Clinical, neuroimaging and pathological features of 5-nitroimidazole-induced encephalo-neuropathy in two patients: insights into possible pathogenesis. Neurol India. 2011;59(5):743-747. PubMed
12. Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179-1187.e1-e3. PubMed
1. Samuelson J. Why metronidazole is active against both bacteria and parasites. Antimicrob Agents Chemother. 1999;43(7):1533-1541. PubMed
2. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455. PubMed
3. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478-498. PubMed
4. Kuriyama A, Jackson JL, Doi A, Kamiya T. Metronidazole-induced central nervous system toxicity: a systemic review. Clin Neuropharmacol. 2011;34(6):241-247. PubMed
5. Graves TD, Condon M, Loucaidou M, Perry RJ. Reversible metronidazole-induced cerebellar toxicity in a multiple transplant recipient. J Neurol Sci. 2009;285(1-2):238-240. PubMed
6. Kim DW, Park JM, Yoon BW, Baek MJ, Kim JE, Kim S. Metronidazole-induced encephalopathy. J Neurol Sci. 2004;224(1-2):107-111. PubMed
7. Park KI, Chung JM, Kim JY. Metronidazole neurotoxicity: sequential neuroaxis involvement. Neurol India. 2011;59(1):104-107. PubMed
8. Patel K, Green-Hopkins I, Lu S, Tunkel AR. Cerebellar ataxia following prolonged use of metronidazole: case report and literature review. Int J Infect Dis. 2008;12(6):e111-e114. PubMed
9. Chandak S, Agarwal A, Shukla A, Joon P. A case report of metronidazole induced neurotoxicity in liver abscess patient and the usefulness of MRI for its diagnosis. J Clin Diagn Res. 2016;10(1):TD06-TD07. PubMed
10. Kim E, Na DG, Kim EY, Kim JH, Son KR, Chang KH. MR imaging of metronidazole-induced encephalopathy: lesion distribution and diffusion-weighted imaging findings. AJNR Am J Neuroradiol. 2007;28(9):1652-1658. PubMed
11. Chacko J, Pramod K, Sinha S, et al. Clinical, neuroimaging and pathological features of 5-nitroimidazole-induced encephalo-neuropathy in two patients: insights into possible pathogenesis. Neurol India. 2011;59(5):743-747. PubMed
12. Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179-1187.e1-e3. PubMed
© 2017 Society of Hospital Medicine
Systematic review of interventions to reduce urinary tract infection in nursing home residents
Given the limited number of geriatricians in the U.S., hospitalists commonly manage nursing home residents admitted for post-acute care.1-4 Urinary tract infection (UTI) is one of the most common infections in nursing homes, often leading to sepsis and readmission to acute care.5 Inappropriate use of antibiotics to treat asymptomatic bacteriuria is both common and hazardous to nursing home residents.6 Up to 10% of nursing home residents will have an indwelling urinary catheter at some point during their stay.7-9 Residents with indwelling urinary catheters are at increased risk for catheter-associated urinary tract infection (CAUTI) and bacteriuria, with an estimated 50% of catheterized residents developing symptomatic CAUTI.5 While urinary catheter prevalence is lower in nursing homes than in the acute care setting, duration of use is often prolonged.7,10 In a setting where utilization is low, but use is prolonged, interventions designed to reduce UTI in acutely ill patients11 may not be as helpful for preventing infection in nursing home residents.
Our objective was to review the available evidence to prevent UTIs in nursing home residents to inform both bedside care and research efforts. Two types of literature review and summary were performed. First, we conducted a systematic review of individual studies reporting outcomes of UTI, CAUTI, bacteriuria, or urinary catheter use after interventions for reducing catheter use, improving insertion and maintenance of catheters, and/or general infection prevention strategies (eg, improving hand hygiene, infection surveillance, contact precautions, standardizing UTI diagnosis, and antibiotic use). Second, we performed a narrative review to generate an overview of evidence and published recommendations in both acute care and nursing home settings to prevent UTI in catheterized and non-catheterized older adults, which is provided as a comprehensive reference table for clinicians and researchers choosing and refining interventions to reduce UTIs.
METHODS
The systematic review was performed according to the criteria of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis recommendations. The protocol was registered at the PROSPERO International Prospective Register of Systematic Reviews, (CRD42013005787). The narrative review was performed using the articles obtained from the systematic search and a targeted literature review by topic for a comprehensive list of interventions, including other interventions summarized in published reviews and guidelines.
Eligibility Criteria Review
Study Design. To address the breadth and depth of literature available to inform interventions to prevent UTI in nursing homes, broad eligibility criteria were applied with the expectation of varied designs and outcomes. All included studies for the systematic review were published manuscripts reporting a comparison group. We included randomized controlled trials as well as nonrandomized trials (pretest/posttest, with or without concurrent or nonconcurrent controls), with any duration of postintervention follow-up. Observational and retrospective studies were excluded.
Participants. We were interested in interventions and outcomes reported for nursing homes, defined as facilities providing short-stay skilled nursing care and/or rehabilitation, as well as long-term care. We also included evidence derived from rehabilitation facilities and spinal cord injury programs focused on reducing CAUTI risk for chronically catheterized residents. We excluded long-term acute care hospitals, hospice, psychiatric/mental health facilities, pediatric, and community dwelling/outpatient settings.
Interventions. We included interventions involving urinary catheter use such as improving appropriate use, aseptic placement, maintenance care, and prompting removal of unnecessary catheters. We included infection prevention strategies with a particular interest in hand hygiene, barrier precautions, infection control strategies, infection surveillance, use of standardized infection definitions, and interventions to improve antibiotic use. We included single and multiple interventions.
Outcomes
1. Healthcare-associated urinary tract infection: UTI occurring after admission to a healthcare facility, not identified specifically as catheter-associated. We categorized UTI outcomes with as much detail as provided, such as whether the reported outcome included only noncatheter-associated UTIs, the time required after admission (eg, more than 2 days), and whether the UTIs were defined by only laboratory criteria, clinically diagnosed infections, symptomatic, or long-term care specific surveillance definitions.
2. Catheter-associated urinary tract infection: UTI occurring in patients during or immediately after use of a urinary catheter. We noted whether CAUTI was defined by laboratory criteria, clinical symptoms, provider diagnosis, or antimicrobial treatment for case identification. We were primarily interested in CAUTI developing after placing an indwelling urinary catheter, commonly known as a Foley, but also in CAUTI occurring with other catheter types such as intermittent straight catheters, external or “condom” catheters, and suprapubic catheters.
3. Bacteriuria: We included the laboratory-based definition of bacteriuria as an outcome to include studies that reduced asymptomatic bacteriuria.
4. Urinary catheter use measures: This includes measures such as urinary catheter utilization ratios (catheter-days/patient-days), prevalence of urinary catheter use, or percentage of catheters with an appropriate indication.
Study Characteristics for Inclusion. Our systematic search included published papers in the English language. We did not exclude studies based on the number of facilities included or eligible, residents/patients included (based on age, gender, catheter use or type, or antibiotic use), intervention details, study withdrawal, loss to follow-up, death, or duration of pre-intervention and postintervention phases.
Data Sources and Searches
The following data sources were searched: Ovid MEDLINE (1950 to June 22, 2015), Cochrane Library via Wiley (1960 to June 22, 2015), CINAHL (1981 to June 22, 2015), Web of Science (1926 to June 22, 2015), and Embase.com (1946 to June 22, 2015). Two major systematic search strategies were performed for this review (Figure). Systematic search 1 was designed broadly using all data sources described above to identify interventions aimed at reducing all UTI events (defined under “Outcomes” above) or urinary catheter use (all types), focusing on interventions evaluated in nursing homes. Systematic search 2 was conducted in Ovid MEDLINE to identify studies to reduce UTI events or urinary catheter use measures for patients with a history of long-term or chronic catheter use, including nursing homes and other post-acute care settings such as rehabilitation units or hospitals and spinal cord injury programs, which have large populations of patients with chronic catheter needs. To inform the completeness of the broader systematic searches, supplemental systematic search strategies were performed for specific topics including hydration (supplemental search 1), published work by nursing home researchers known to the authors (supplemental search 2), and contact precautions (supplemental search 3). Search 1 is available at http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42013005787. Full search strategies for search 2 and supplemental searches are available upon request.
Study Selection
One author performed an initial screen of all records retrieved by the systematic searches by title and abstract and applied the initial exclusions (eg, non-human, no outcomes of interest), identified duplicate records, and assigned potentially relevant studies into groups such as review articles, epidemiology, interventions, and articles requiring further text review before categorization (Figure). After initial screening, Dr. Meddings reviewed the records by title/abstract. Reference lists were reviewed for potential articles for inclusion. Full-text article review informed the selection of those for dual abstraction and quality scoring performed by 2 authors, with discrepancies resolved by a third author. We requested additional information from authors from whom our search had generated only an abstract or brief report, or when additional information such as pre-intervention data was needed.12-18
Data Extraction and Quality Assessment
Relevant data regarding study design, participants, inclusion/exclusion criteria, outcomes, and quality criteria were abstracted independently by 2 authors. Methodological quality scores were assigned using a modification of the Quality index checklist developed by Downs and Black appropriate for assessing both randomized and nonrandomized studies of healthcare interventions.19 We also reviewed study funding sources and other potential quality concerns.
Data Analysis
Due to large trial heterogeneity among these studies about interventions and outcomes reported, outcome data could not be combined into summary measures for meta-analysis to give overall estimates of treatment effects.
RESULTS
Systematic Search Results and Study Selection
As detailed in the study flow diagram (Figure), 5794 total records were retrieved by systematic search 1 (4697 studies), search 2 (909 studies), and supplemental searches (188 studies). Hand searching of reference lists of 41 reviews (including narrative and systematic reviews) yielded 77 additional studies for consideration. Twenty-nine records on interventions that were the focus of systematic reviews, including topics of cranberry use, catheter coatings, antimicrobial prophylaxis, washout/irrigation strategies, and sterile versus clean intermittent straight catheterization, were excluded from dual abstraction. Two records were excluded after team discussion of the dual-abstraction results, because 1 study did not meet criteria as an intervention study and 1 study’s setting was not applicable in nursing homes. A total of 20 records15,20-38 (in which 19 studies were described) were selected for final inclusion for detailed assessment and reporting for the systematic review.
Characteristics of Included Studies
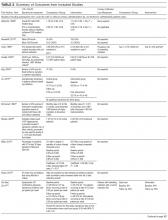
Table 1 describes the 19 intervention studies in terms of design, participants, setting, and whether the study included specific categories of interventions expected to decrease UTI or catheter use. These studies included 8 randomized controlled trials (4 with cluster-randomization at the facility or unit level), 10 pre-post nonrandomized interventions, and 1 nonrandomized intervention with concurrent controls. Twelve studies included participants with or without catheters (ie, not limited to catheterized patients only) in nursing homes.15,20-31 Seven32-38 studies included catheterized patients only or settings with high expected catheterization rates; settings for these studies included spinal cord units (n=3), nursing homes (n=2), rehabilitation ward (n=1) and VA hospital (n=1), including acute care, nursing home, and rehabilitation units. Total quality scores for the studies ranged from 8 to 25 (median, 15), detailed in Supplemental Table 1.
As detailed in Table 1 and Supplemental Table 2, 7 studies22,24,26,31,32,35,36 involved single interventions and 12 studies15,20,21,23,25,27-30,33,34,37,38 included multiple interventions. Interventions to impact catheter use and care were evaluated in 13 studies, including appropriateness of use,21,25,29,30 improving catheter maintenance care,15,20,29,30 securement,15,29,30,32 prompting removal of unnecessary catheters,21,25,29,30 improving incontinence care,15,21,23,25 bladder scanners,37,38 catheter changes,35and comparing alternatives (condom catheter or intermittent straight catheter) to use of an indwelling catheter.36,38 None focused on improving aseptic insertion. General infection control practices studied included improving hand hygiene,20-22,29-31,33,34 improving antibiotic use,15,20,21,28,34 initiation of infection control programs,20,21,28 interventions to improve identification of UTIs/CAUTIs using infection symptom/sign criteria,15,20,21,34 infection surveillance as an intervention,28-30,33,34 and barrier precautions,33,34 including preemptive precautions for catheterized patients.34 Hydration was assessed in 3 studies.24-26
Outcomes of Included Studies
Table 2 describes the studies’ outcomes reported for UTI, CAUTI, or bacteriuria.15,20-38 The outcome definitions of UTI and CAUTI varied widely. Only 2 studies22,39 reported UTI outcomes using definitions specific for nursing home settings such as McGeer’s criteria40 a detailed review and comparison of published CAUTI definitions used clinically and for surveillance in nursing homes is provided in Supplemental Table 3. Two studies reported symptomatic CAUTIs per 1000 catheter-days.32,34 Another study22 reported symptomatic CAUTIs per 1000 resident-days. Three reported symptomatic CAUTIs as counts.35,38 Saint et al36 reported CAUTIs as part of a combined outcome (ie, bacteriuria, CAUTI, or death).
The 19 studies (Table 2) reported 12 UTI outcomes,15,20,21,23,25-31,33 9 CAUTI outcomes,15,22,32,34,35,38 4 bacteriuria outcomes,24,36,38 and 5 catheter use outcomes.21,29,30,37,38 Five studies showed CAUTI reduction15,22,32,34,35 (1 significantly34); 9 studies showed UTI reduction13,18,19,21,23-25,27,28,31 (none significantly); 2 studies showed bacteriuria reduction (none significantly). One study36 reported 2 composite outcomes including bacteriuria or CAUTI or death, with statistically significant improvement reported for 1 composite measure. Four studies reported catheter use, with all showing reduced catheter use in the intervention group; however, only 1 achieved statistically significant reduction.37
Synthesis of Systematic Review Results
Overall, many studies reported decreases in UTI, CAUTI, and urinary catheter use measures but without statistical significance, with many studies likely underpowered for our outcomes of interest. Often, the outcomes of interest in this systematic review were not the main outcome for which the study was designed and originally powered. The interventions studied included several currently implemented as part of CAUTI bundles in the acute care setting, such as improving catheter use, and care and infection control strategies. Other included interventions target common challenges specific to the nursing home setting such as removing indwelling catheters upon admission to the nursing home from an acute-care facility21,25 and applying interventions to address incontinence by either general strategies21,23,25,30,38 or the use of an incontinence specialist23 to provide individual treatment plans. The only intervention that demonstrated a statistically significant reduction in CAUTI in chronically catheterized patients employed a comprehensive program to improve antimicrobial use, hand hygiene (including hand hygiene and gloves for catheter care), and preemptive precautions for patients with devices, along with promotion of standardized CAUTI definitions and active multidrug resistant organism surveillance.34
Narrative Review Results
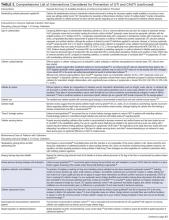
Table 3 includes a comprehensive list of potential interventions that have been considered for prevention of UTI or CAUTI (including those in acute care and nursing home settings), as summarized from this systematic review and prior narrative or systematic reviews.43-115
DISCUSSION
We performed a broad systematic review of strategies to decrease UTI, CAUTI, and urinary catheter use that may be helpful in nursing homes. While many studies reported decreased UTI, CAUTI, or urinary catheter use measures, few demonstrated statistically significant reductions perhaps because many were underpowered to assess statistical significance. Pooled analyses were not feasible to provide the expected impact of these interventions in the nursing home setting.
This review confirms that bundles of interventions for prevention of CAUTI have been implemented with some evidence of success in nursing home settings, with several components in common with those implemented in the acute care setting, such as hand hygiene and strategies to reduce and improve catheter use.41 Some studies focused on issues more common in nursing homes such as chronic catheterization and incontinence. A nursing home CAUTI bundle should be designed with the resources and challenges present in the nursing home environment in mind, and with recognition that, although the number of patients with catheters is less than in acute care, there will be more patients with chronic catheterization needs and incontinence.
Although catheter utilization in nursing homes is low, further reductions in catheter days and CAUTIs can be achieved. Catheter removal reminders and stop orders have demonstrated a greater than 50% reduction in CAUTIs in acute care settings;11 an example of a stop-order intervention in nursing homes is trial removal of indwelling catheters present at facility admission without clear urologic need present at the time of admission.25 Nursing home interventions to avoid catheter placement should include incontinence programs, discussion of alternatives to indwelling urinary catheters with patients, families, and frontline personnel, and urinary retention protocols. Programs to reduce CAUTI should include education to improve aseptic insertion, and to maintain awareness and proper care of catheters in place by regular assessment of catheter necessity, securement, hand hygiene, and preemptive barrier precautions for catheterized patients. Interventions that focus on improving appropriate use of urine tests and antibiotics to treat UTIs can also significantly affect the rates of reported symptomatic CAUTIs, with the potential to decrease unnecessary antibiotic use.20,21
The main limitation of this review is that many studies provided little information about their intervention and definition of outcomes. The strength of this review is the detailed and broad search strategy applied with generous inclusion of interventions and outcomes to highlight the available evidence and details of interventions that have been studied and implemented.
CONCLUSION
This review synthesizes the current state of evidence and proposes strategies to reduce UTIs in nursing homes. Interventions that motivate catheter avoidance and catheter removal to prevent CAUTI in acute care11 and nursing home settings are supported by the strongest available evidence, although the strength of that evidence is less in the nursing home setting. Limitations notwithstanding, interventions such as incontinence care planning and hydration programs can reduce UTI in this population and is important for overall wellbeing.
Acknowledgments
The authors appreciate the guidance that Vineet Chopra MD, MSc, provided regarding options for methodological quality assessment tools, and the assistance of Mary Rogers PhD, MS, in interpreting the published Downs and Black Quality Index items, which informed our modification of this tool for application in this study. The authors appreciate, also, the feedback provided by the Agency for Healthcare Research and Quality (AHRQ) Content and Materials Development Committee for the AHRQ Safety Program for Long-Term Care: Preventing CAUTI and other Healthcare-associated Infections.
Disclosures
Agency for Healthcare Research and Quality (AHRQ) contract #HHSA290201000025I provided funding for this study, which was developed in response to AHRQ Task Order #8 for ACTION II RFTO 26 CUSP for CAUTI in LTC. AHRQ developed the details of the task and provided comments on a draft report, which informed the report submitted to AHRQ in December 2013, used to inform the interventions for a national collaborative (http://www.hret.org/quality/projects/long-term-care-cauti.shtml). Dr. Meddings’s effort on this project was funded by concurrent effort from her AHRQ (K08 HS19767). Dr. Saint’s and Dr. Krein’s effort on this project was funded by concurrent effort from the Veterans Affairs National Center for Patient Safety, Ann Arbor Patient Safety Center of Inquiry. Dr. Meddings’s other research is funded by AHRQ (2R01HS018334-04), the NIH-LRP program, the VA National Center for Patient Safety, and the VA Ann Arbor Patient Safety Center of Inquiry. Dr. Krein’s other research is funded by a VA Health Services Research and Development Award (RCS 11-222). Dr. Mody’s other research is funded by VA Healthcare System Geriatric Research Clinical Care Center (GRECC), NIA-Pepper Center, NIA (R01AG032298, R01AG041780, K24AG050685-01). Dr. Saint has received fees for serving on advisory boards for Doximity and Jvion. All other authors report no financial conflicts of interest. The findings and conclusions in this report are those of the authors and do not necessarily represent those of the sponsor, the Agency for Healthcare Research and Quality, or the U.S. Department of Veterans Affairs. These analyses were presented in part as a poster presentation at the ID Week Annual Meeting on October 10, 2014 in Philadelphia, PA.
1. Beresford L. Post-acute patient care: new frontier for hospitalists. The Hospitalist.
July 2015. http://www.the-hospitalist.org/hospitalist/article/122330/post-acute-patient-
care-new-frontier-hospitalists. Accessed March 31, 2017.
2. Butterfield S. Hospital medicine matures: Hospitalists and hospitalist groups move into post-acute care. 2012. Available at http://www.acphospitalist.org/archives/2012/10/coverstory.htm. Accessed April 6, 2016.
3. Pittman D. SNFs: New Turf for Hospitalists? 2013; Available at http://www.medpagetoday.com/HospitalBasedMedicine/Hospitalists/39401. Accessed April 6, 2016.
4. Society of Hospital Medicine. SHM and IPC Healthcare Develop First SHM Primer for Hospitalists in Skilled Nursing Facilities. 2015; Available at http://www.hospitalmedicine.org/Web/Media_Center/Press_Release/2015/SHM_and_IPC_Healthcare_Develop_First_SHM_Primer_for_Hospitalists_in_Skilled_Nursing_Facilities.aspx. Accessed April 6, 2016.
5. Montoya A, Mody L. Common infections in nursing homes: a review of current issues and challenges. Aging Health. 2011;7(6):889-899. PubMed
6. Phillips CD, Adepoju O, Stone N, et al. Asymptomatic bacteriuria, antibiotic use, and suspected urinary tract infections in four nursing homes. BMC Geriatr. 2012;12:73. PubMed
7. Rogers M, Mody L, Kaufman S, Fries B, McMahon L, Saint S. Use of urinary collection devices in skilled nursing facilities in five states. J Amer Geriatr Soc. 2008;56:854-861. PubMed
8. Castle N, Engberg JB, Wagner LM, Handler S. Resident and facility factors associated with the incidence of urinary tract infections identified in the nursing home minimum data set. J Appl Gerontol. 2015:doi: 10.1177/0733464815584666. PubMed
9. Tsan L, Langberg R, Davis C, et al. Nursing home-associated infections in Department of Veterans Affairs community living centers. Am J Infect Control. 2010;38(6):461-466. PubMed
10. Kunin CM, Chin QF, Chambers S. Morbidity and mortality associated with indwelling urinary catheters in elderly patients in a nursing home--confounding due to the presence of associated diseases. J Am Geriatr Soc. 1987;35(11):1001-1006. PubMed
11. Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, Saint S. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf. 2013;23(4):277-289. PubMed
12. Abraham F, Abraham FP. A CAUTI bundle with a twist. Am J Infect Control. 2012;40(5):e79-e80.
13. Flynn ER, Zombolis K. Reducing hospital acquired indwelling urinary catheter-associated urinary tract infections through multidisciplinary team and shared governance practice model. Am J Infect Control. 2011;39(5):E28-E29.
14. Gokula MR, Gaspar P, Siram R. Implementation of an evidence based protocol to reduce use of indwelling urinary catheters in the long term care environment. J Am Med Dir Assoc. 2013;14(3):B23.
15. Brownhill K. Training in care homes to reduce avoidable harm. Nurs Times. 2013;109(43):20-22. PubMed
16. Galeon CP, Romero I. Implementing a performance improvement project in a multi-level teaching facility on reducing catheter associated urinary tract infections (CAUTI). Am J Infect Control. 2014:S130-S131.
17. Evans ME, Kralovic SM, Simbartl LA, et al. Nationwide reduction of health care-associated methicillin-resistant Staphylococcus aureus infections in Veterans Affairs long-term care facilities. Am J Infect Control. 2014;42(1):60-62. PubMed
18. Evans KA, Ligon R, Lipton C. Reduction of antibiotic starts for asymptomatic bacteriuria in skilled nursing facilities. J Am Geriatr Soc. 2015;63:S131.
19. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. PubMed
20. Ahlbrecht H, Shearen C, Degelau J, Guay DR. Team approach to infection prevention and control in the nursing home setting. Am J Infect Control. 1999;27(1):64-70. PubMed
21. Cools HJ, van der Meer JW. Infection control in a skilled nursing facility: a 6-year survey. J Hosp Infect. 1988;12(2):117-124. PubMed
22. Fendler EJ, Ali Y, Hammond BS, Lyons MK, Kelley MB, Vowell NA. The impact of alcohol hand sanitizer use on infection rates in an extended care facility. Am J Infect Control. 2002;30(4):226-233. PubMed
23. Klay M, Marfyak K. Use of a continence nurse specialist in an extended care facility. Urol Nurs. 2005;25(2):101-102. PubMed
24. Lin S. A pilot study: fluid intake and bacteriuria in nursing home residents in southern Taiwan. Nurs Res. 2013;62(1):66-72. PubMed
25. McConnell J. Preventing urinary tract infections. Geriatr Nurs. 1984;5(8):361-362. PubMed
26. Mentes JC, Culp K. Reducing hydration-linked events in nursing home residents. Clin Nurs Res. 2003;12(3):210-225; discussion 226-218. PubMed
27. Miller SC, Lepore M, Lima JC, Shield R, Tyler DA. Does the introduction of nursing home culture change practices improve quality? J Am Geriatr Soc. 2014;62(9):1675-1682. PubMed
28. Stuart RL, Orr E, Kotsanas D, Gillespie EE. A nurse-led antimicrobial stewardship intervention in two residential aged care facilities. Healthcare Infection. 2015;20(1):4-6.
29. van Gaal B, Schoonhoven L, Mintjes JAJ, Borm GF, Koopmans RTCM, van Achterberg T. The SAFE or SORRY? programme. Part II: Effect on preventive care. Int J Nurs Stud. 2011;48(9):1049-1057. PubMed
30. van Gaal BGI, Schoonhoven L, Mintjes JAJ, et al. Fewer adverse events as a result of the SAFE or SORRY? programme in hospitals and nursing homes. part I: primary outcome of a cluster randomised trial. Int J Nurs Stud. 2011;48(9):1040-1048. PubMed
31. Yeung WK, Wilson Tam WS, Wong TW. Clustered randomized controlled trial of a hand hygiene intervention involving pocket-sized containers of alcohol-based hand rub for the control of infections in long-term care facilities. Infect Control Hosp Epidemiol. 2011;32(1):67-76. PubMed
32. Darouiche RO, Goetz L, Kaldis T, Cerra-Stewart C, AlSharif A, Priebe M. Impact of StatLock securing device on symptomatic catheter-related urinary tract infection: a prospective, randomized, multicenter clinical trial. Am J Infect Control. 2006;34(9):555-560. PubMed
33. Evans ME, Kralovic SM, Simbartl LA, et al. Prevention of methicillin-resistant Staphylococcus aureus infections in spinal cord injury units. Am J Infect Control. 2013;41(5):422-426. PubMed
34. Mody L, Krein S, Saint S, et al. A targeted infection prevention intervention in nursing home residents with indwelling devices: a randomized clinical trial. JAMA Intern Med. 2015;175:714-723. PubMed
35. Priefer BA, Duthie Jr EH, Gambert SR. Frequency of urinary catheter change and clinical urinary tract infection. Study in hospital-based, skilled nursing home. Urology. 1982;20(2):141-142. PubMed
36. Saint S, Kaufman SR, Rogers MA, Baker PD, Ossenkop K, Lipsky BA. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54(7):1055-1061. PubMed
37. Suardi L, Cazzaniga M, Spinelli M, Tagliabue A. From intermittent catheterisation to time-volume dependent catheterisation in patients with spinal cord injuries, through the use of a portable, ultrasound instrument. Europa Medicophysica. 2001;37(2):111-114.
38. Tang MW, Kwok TC, Hui E, Woo J. Intermittent versus indwelling urinary catheterization in older female patients. Maturitas. 2006;53(3):274-281. PubMed
39. Cassel BG, Parkes V, Poon R, Rae H. Quality improvement best practices and long-term indwelling urinary catheters. Perspectives. 2008;32(1):13-17. PubMed
40. Stone ND, Ashraf MS, Calder J, et al. Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria. Infect Control Hosp Epidemiol. 2012;33(10):965-977. PubMed
41. Saint S, Greene MT, Krein SL, et al. A Program to Prevent Catheter-Associated Urinary Tract Infection in Acute Care. New England Journal of Medicine. 2016;374(22):2111-2119. PubMed
42. McGeer A, Campbell B, Emori TG, et al. Definitions of infection for surveillance in long-term care facilities. Am J Infect Control. 1991;19(1):1-7. PubMed
43. Nicolle LE. The chronic indwelling catheter and urinary infection in long-term-care facility residents. Infect Control Hosp Epidemiol. 2001;22(5):316-321. PubMed
44. Nicolle LE; SHEA Long-Term Care Committee. Urinary tract infections in long-term-care facilities. Infect Control Hosp Epidemiol. 2001;22(3):167-175. PubMed
45. Nicolle LE. Catheter-related urinary tract infection. Drug & Aging. 2005;22(8):627-639. PubMed
46. Cochran S. Care of the indwelling urinary catheter - Is it evidence based? J Wound Ostomy Cont Nurs. 2007;34(3):282-288. PubMed
47. Seiler WO, Stahelin HB. Practical management of catheter-associated UTIs. Geriatrics. 1988;43(8):43-50. PubMed
48. Stickler DJ, Chawla JC. The role of antiseptics in the management of patients with long-term indwelling bladder catheters. J Hosp Infect. 1987;10(3):219-228. PubMed
49. Gray M. Does the construction material affect outcomes in long-term catheterization? J Wound Ostomy Cont Nurs. 2006;33(2):116-121. PubMed
50. Trautner BW, Darouiche RO. Clinical review: prevention of urinary tract infection in patients with spinal cord injury. J Spinal Cord Med. 2002;2002(25):277-283. PubMed
51. Maloney C. Estrogen & recurrent UTI in postmenopausal women. Am J Nurs. 2002;102(8):44-52. PubMed
52. Raz R. Hormone replacement therapy or prophylaxis in postmenopausal women with recurrent urinary tract infection. J Infect Dis. 2001;183(suppl 1):S74-S76. PubMed
53. Godfrey H. Older people, continence care and catheters: dilemmas and resolutions. Br J Nurs. 2008;17(9):S4-S11. PubMed
54. Godfrey H, Evans A. Management of long-term urethral catheters: minimizing complications. Br J Nurs. 2000;9(2):74-76. PubMed
55. Kunin CM. Chemoprophylaxis and suppressive therapy in the management of urinary tract infections. J Antimicrob Chemother. 1994;33(suppl A):51-62. PubMed
56. Newman DK, Willson MM. Review of intermittent catheterization and current best practices. Urol Nurs. 2011;31(1):12-48. PubMed
57. Allan GM, Nicolle L. Cranberry for preventing urinary tract infection. Can Fam Physician. 2013;59(4):367. PubMed
58. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;10:CD001321. PubMed
59. Wang CH, Fang CC, Chen NC, et al. Cranberry-containing products for prevention of urinary tract infections in susceptible populations: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172(13):988-996. PubMed
60. Moore KN, Fader M, Getliffe K. Long-term bladder management by intermittent catheterisation in adults and children. Cochrane Database Syst Rev. 2007(4):CD006008. PubMed
61. Li L, Ye WQ, Ruan H, Yang BY, Zhang SQ. Impact of hydrophilic catheters on urinary tract infections in people with spinal cord injury: systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2013;94(4):782-787. PubMed
62. Jamison J, Maguire S, McCann J. Catheter policies for management of long term voiding problems in adults with neurogenic bladder disorders. Cochrane Database Syst Rev. 2011(12):CD004375. PubMed
63. Gray M. What nursing interventions reduce the risk of symptomatic urinary tract infections in the patient with an indwelling catheter? J Wound Ostomy Cont Nurs. 2004;31(1):3-13. PubMed
64. Marschall J, Carpenter C, Fowler S, Trautner B. Antibiotic prophylaxis for urinary tract infections after removal of urinary catheter: meta-analysis. BMJ. 2013;346:f3147. PubMed
65. Sinclair L, Hagen S, Cross S. Washout policies in long-term indwelling urinary catheterization in adults: a short version Cochrane review. Neurourol Urodyn. 2011;30(7):1208-1212. PubMed
66. Hunter KF, Bharmal A, Moore KN. Long-term bladder drainage: suprapubic catheter versus other methods: a scoping review. Neurourol Urodyn. 2013;32(7):944-951. PubMed
67. Morton SC, Shekelle PG, Adams JL, et al. Antimicrobial prophylaxis for urinary tract infection in persons with spinal cord dysfunction. Arch Phys Med Rehabil. 2002;83(1):129-138. PubMed
68. Niël-Weise BS, van den Broek PJ, da Silva EM, Silva LA. Urinary catheter policies for long-term bladder drainage. Cochrane Database Syst Rev. 2012(8). PubMed
69. Jepson R, Craig J. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008;10(CD001321). PubMed
70. Avorn J, Monane M, Gurwitz JH, Glynn RJ, Choodnovskiy I, Lipsitz LA. Reduction of bacteriuria and pyuria after ingestion of cranberry juice. JAMA. 1994;271(10):751-754. PubMed
71. Bianco L, Perrelli E, Towle V, Van Ness PH, Juthani-Mehta M. Pilot randomized controlled dosing study of cranberry capsules for reduction of bacteriuria plus pyuria in female nursing home residents. J Am Geriatr Soc. 2012;60(6):1180-1181. PubMed
72. Lin SC, Wang CC, Shih SC, Tjung JJ, Tsou MT, Lin CJ. Prevention of Asymptomatic Bacteriuria with Cranberries and Roselle Juice in Home-care Patients with Long-term Urinary Catheterization. Int J Gerontol. 2014;8(3):152-156.
73. Juthani-Mehta M, Perley L, Chen S, Dziura J, Gupta K. Feasibility of cranberry capsule administration and clean-catch urine collection in long-term care residents. J Am Geriatr Soc. 2010;58(10):2028-2030. PubMed
74. Tully CL, Bastone P, Vaughan J, Ballentine L. Urinary tract infection prophylaxis with cranberry extract in the nursing home setting. J Am Geriatr Soc. 2004;52(4):S206-S206.
75. Woodward N. Use of cranberry extract for the prevention of UTIs in an at-risk population. 41st Annual Wound, Ostomy and Continence Nurses Annual Conference, St. Louis, Missouri, June 6-10, 2009. J Wound Ostomy Continence Nurs. 2009;36(3S):S62-S62.
76. Linsenmeyer TA, Harrison B, Oakley A, Kirshblum S, Stock JA, Millis SR. Evaluation of cranberry supplement for reduction of urinary tract infections in individuals with neurogenic bladders secondary to spinal cord injury. A prospective, double-blinded, placebo-controlled, crossover study. J Spinal Cord Med. 2004;27(1):29-34. PubMed
77. Waites KB, Canupp KC, Armstrong S, DeVivo MJ. Effect of cranberry extract on bacteriuria and pyuria in persons with neurogenic bladder secondary to spinal cord injury. J Spinal Cord Med. 2004;27(1):35-40. PubMed
78. Caljouw MAA, Van Den Hout WB, Putter H, Achterberg WP, Cools HJM, Gussekloo J. Effectiveness of cranberry capsules to prevent urinary tract infections in vulnerable older persons. A double-blind randomized placebo-controlled trial in long-term care facilities. Eur Geriatr Med. 2013;4:S118-S119. PubMed
79. Hout WB, Caljouw MAA, Putter H, Cools HJM, Gussekloo J. Cost-effectiveness of cranberry capsules to prevent urinary tract infection in long-term care facilities: economic evaluation with a randomized controlled trial. J Am Geriatr Soc. 2014;62(1):111-116. PubMed
80. Liu BA, McGeer A, McArthur MA, et al. Effect of multivitamin and mineral supplementation on episodes of infection in nursing home residents: a randomized, placebo-controlled study. J Am Geriatr Soc. 2007;55(1):35-42. PubMed
81. Eriksen B. A randomized, open, parallel-group study on the preventive effect of an estradiol-releasing vaginal ring (Estring) on recurrent urinary tract infections in postmenopausal women. Am J Obstet Gynecol. 1999;180:1072-1079. PubMed
82. Maloney C. Hormone replacement therapy in female nursing home residents with recurrent urinary tract infection. Ann Long-Term Care. 1998;6(3):77-82.
83. Gokula RM, Smith MA, Hickner J. Emergency room staff education and use of a urinary catheter indication sheet improves appropriate use of foley catheters. Am J Infect Control. 2007;35(9):589-593. PubMed
84. Salamon L. Catheter-associated urinary tract infections: a nurse-sensitive indicator in an inpatient rehabilitation program. Rehabil Nurs. 2009;34(6):237-241. PubMed
85. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-326. PubMed
86. American Medical Directors Association (AMDA). Appropriate indications for use of a chronic indwelling catheter in the long-term care setting. Columbia, MD; excerpted from AMDA's Clinical Practice Guideline: Urinary Incontinence. 2005.
87. Rannikko S, Kyllastinen M, Granqvist B. Comparison of long-term indwelling catheters and bed-pads in the treatment of urinary incontinence in elderly patients. J Infect. 1986;12(3):221-227. PubMed
88. Carapeti E, Andrews S, Bentley P. Randomised study of sterile versus non-sterile urethral catheterization. Ann R. Coll Surg Engl. 1996;78(1):59-60. PubMed
89. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625-663. PubMed
90. Olsen-Scribner RJ, Hayes C, Pottinger P. Sustaining reduction of catheter-associated urinary tract infection (CAUTI)-outcomes after two educational methods in a regional university-affiliated medical center. Am J Infect Control. 2014;1:S22.
91. Duffy LM, Cleary J, Ahern S, et al. Clean intermittent catheterization: safe, cost-effective bladder management for male residents of VA nursing homes. J Am Geriatr Soc. 1995;43(8):865-870. PubMed
92. Joseph C, Jacobson C, Strausbaugh L, Maxwell M, French M, Colling J. Sterile vs clean urinary catheterization. J Am Geriatr Soc. 1991;39(10):1042-1043. PubMed
93. Moore KN, Burt J, Voaklander DC. Intermittent catheterization in the rehabilitation setting: a comparison of clean and sterile technique. Clin Rehabili. 2006;20(6):461-468. PubMed
94. Moore KN, Kelm M, Sinclair O, Cadrain G. Bacteriuria in intermittent catheterization users: the effect of sterile versus clean reused catheters. Rehabil Nurs J. 1993;18(5):306-309. PubMed
95. Niel-Weise BS, van den Broek PJ. Urinary catheter policies for short-term bladder drainage in adults. Cochrane Database Syst Rev. 2005(3):CD004203. PubMed
96. Ouslander JG, Greengold B, Chen S. External catheter use and urinary tract infections among incontinent male nursing home patients. J Am Geriatr Soc. 1987;35(12):1063-1070. PubMed
97. Wyndaele JJ, Brauner A, Geerlings SE, Bela K, Peter T, Bjerklund-Johanson TE. Clean intermittent catheterization and urinary tract infection: review and guide for future research. BJU Int. 2012;110(11 Pt C):E910-917. PubMed
98. Jahn P, Beutner K, Langer G. Types of indwelling urinary catheters for long-term bladder drainage in adults. Cochrane Database Syst Rev. 2012(10):CD004997. PubMed
99. Pickard R, Lam T, Maclennan G, et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: a multicentre randomised controlled trial. Lancet. 2012;380(9857):1927-1935. PubMed
100. Burke JP, Garibaldi RA, Britt MR, Jacobson JA, Conti M, Alling DW. Prevention of catheter-associated urinary tract infections. Efficacy of daily meatal care regimens. Am J Med. 1981;70(3):655-658. PubMed
101. Hagen S, Sinclair L, Cross S. Washout policies in long-term indwelling urinary catheterisation in adults. Cochrane Database Syst Rev. 2010(3). PubMed
102. Moore KN, Hunter KF, McGinnis R, et al. Do catheter washouts extend patency time in long-term indwelling urethral catheters? A randomized controlled trial of acidic washout solution, normal saline washout, or standard care. J Wound Ostomy Continence Nurs. 2009;36(1):82-90. PubMed
103. Muncie HL Jr, Hoopes JM, Damron DJ, Tenney JH, Warren JW. Once-daily irrigation of long-term urethral catheters with normal saline. Lack of benefit. Arch Intern Med. 1989;149(2):441- PubMed
104. Ruwaldt MM. Irrigation of indwelling urinary catheters. Urology. 1983;21(2):127-129. PubMed
105. Palka MA. Evidenced based review of recommendations addressing the frequency of changing long-term indwelling urinary catheters in older adults. Geriatr Nurs. 2014;35(5):357-363. PubMed
106. Warren JW. Catheter-associated urinary tract infections. Infect Dis Clin North Am. 1997;11(3):609-622. PubMed
107. Fryklund B, Haeggman S, Burman LG. Transmission of urinary bacterial strains between patients with indwelling catheters--nursing in the same room and in separate rooms compared. J Hosp Infect. 1997;36(2):147-153. PubMed
108. Anderson RU. Non-sterile intermittent catheterization with antibiotic prophylaxis in the acute spinal cord injured male patient. J Urol. 1980;124(3):392-394. PubMed
109. Anderson RU. Prophylaxis of bacteriuria during intermittent catheterization of the acute neurogenic bladder. J Urol. 1980;123(3):364-366. PubMed
110. Gribble MJ, Puterman ML. Prophylaxis of urinary tract infection in persons with recent spinal cord injury: a prospective, randomized, double-blind, placebo-controlled study of trimethoprim-sulfamethoxazole. Am J Med. 1993;95(2):141-152. PubMed
111. Rutschmann OT, Zwahlen A. Use of norfloxacin for prevention of symptomatic urinary tract infection in chronically catheterized patients. Eur J Clin Microbiol Infect Dis. 1995;14(5):441-444. PubMed
112. Jewes LA, Gillespie WA, Leadbetter A, et al. Bacteriuria and bacteraemia in patients with long-term indwelling catheters--a domiciliary study. J Med Microbiol. 1988;26(1):61-65. PubMed
113. Warren JW, Damron D, Tenney JH, Hoopes JM, Deforge B, Muncie HL, Jr. Fever, bacteremia, and death as complications of bacteriuria in women with long-term urethral catheters. J Infect Dis. 1987;155(6):1151-1158. PubMed
114. Prasad A, Cevallos ME, Riosa S, Darouiche RO, Trautner BW. A bacterial interference strategy for prevention of UTI in persons practicing intermittent catheterization. Spinal Cord. 2009;47(7):565-569. PubMed
Given the limited number of geriatricians in the U.S., hospitalists commonly manage nursing home residents admitted for post-acute care.1-4 Urinary tract infection (UTI) is one of the most common infections in nursing homes, often leading to sepsis and readmission to acute care.5 Inappropriate use of antibiotics to treat asymptomatic bacteriuria is both common and hazardous to nursing home residents.6 Up to 10% of nursing home residents will have an indwelling urinary catheter at some point during their stay.7-9 Residents with indwelling urinary catheters are at increased risk for catheter-associated urinary tract infection (CAUTI) and bacteriuria, with an estimated 50% of catheterized residents developing symptomatic CAUTI.5 While urinary catheter prevalence is lower in nursing homes than in the acute care setting, duration of use is often prolonged.7,10 In a setting where utilization is low, but use is prolonged, interventions designed to reduce UTI in acutely ill patients11 may not be as helpful for preventing infection in nursing home residents.
Our objective was to review the available evidence to prevent UTIs in nursing home residents to inform both bedside care and research efforts. Two types of literature review and summary were performed. First, we conducted a systematic review of individual studies reporting outcomes of UTI, CAUTI, bacteriuria, or urinary catheter use after interventions for reducing catheter use, improving insertion and maintenance of catheters, and/or general infection prevention strategies (eg, improving hand hygiene, infection surveillance, contact precautions, standardizing UTI diagnosis, and antibiotic use). Second, we performed a narrative review to generate an overview of evidence and published recommendations in both acute care and nursing home settings to prevent UTI in catheterized and non-catheterized older adults, which is provided as a comprehensive reference table for clinicians and researchers choosing and refining interventions to reduce UTIs.
METHODS
The systematic review was performed according to the criteria of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis recommendations. The protocol was registered at the PROSPERO International Prospective Register of Systematic Reviews, (CRD42013005787). The narrative review was performed using the articles obtained from the systematic search and a targeted literature review by topic for a comprehensive list of interventions, including other interventions summarized in published reviews and guidelines.
Eligibility Criteria Review
Study Design. To address the breadth and depth of literature available to inform interventions to prevent UTI in nursing homes, broad eligibility criteria were applied with the expectation of varied designs and outcomes. All included studies for the systematic review were published manuscripts reporting a comparison group. We included randomized controlled trials as well as nonrandomized trials (pretest/posttest, with or without concurrent or nonconcurrent controls), with any duration of postintervention follow-up. Observational and retrospective studies were excluded.
Participants. We were interested in interventions and outcomes reported for nursing homes, defined as facilities providing short-stay skilled nursing care and/or rehabilitation, as well as long-term care. We also included evidence derived from rehabilitation facilities and spinal cord injury programs focused on reducing CAUTI risk for chronically catheterized residents. We excluded long-term acute care hospitals, hospice, psychiatric/mental health facilities, pediatric, and community dwelling/outpatient settings.
Interventions. We included interventions involving urinary catheter use such as improving appropriate use, aseptic placement, maintenance care, and prompting removal of unnecessary catheters. We included infection prevention strategies with a particular interest in hand hygiene, barrier precautions, infection control strategies, infection surveillance, use of standardized infection definitions, and interventions to improve antibiotic use. We included single and multiple interventions.
Outcomes
1. Healthcare-associated urinary tract infection: UTI occurring after admission to a healthcare facility, not identified specifically as catheter-associated. We categorized UTI outcomes with as much detail as provided, such as whether the reported outcome included only noncatheter-associated UTIs, the time required after admission (eg, more than 2 days), and whether the UTIs were defined by only laboratory criteria, clinically diagnosed infections, symptomatic, or long-term care specific surveillance definitions.
2. Catheter-associated urinary tract infection: UTI occurring in patients during or immediately after use of a urinary catheter. We noted whether CAUTI was defined by laboratory criteria, clinical symptoms, provider diagnosis, or antimicrobial treatment for case identification. We were primarily interested in CAUTI developing after placing an indwelling urinary catheter, commonly known as a Foley, but also in CAUTI occurring with other catheter types such as intermittent straight catheters, external or “condom” catheters, and suprapubic catheters.
3. Bacteriuria: We included the laboratory-based definition of bacteriuria as an outcome to include studies that reduced asymptomatic bacteriuria.
4. Urinary catheter use measures: This includes measures such as urinary catheter utilization ratios (catheter-days/patient-days), prevalence of urinary catheter use, or percentage of catheters with an appropriate indication.
Study Characteristics for Inclusion. Our systematic search included published papers in the English language. We did not exclude studies based on the number of facilities included or eligible, residents/patients included (based on age, gender, catheter use or type, or antibiotic use), intervention details, study withdrawal, loss to follow-up, death, or duration of pre-intervention and postintervention phases.
Data Sources and Searches
The following data sources were searched: Ovid MEDLINE (1950 to June 22, 2015), Cochrane Library via Wiley (1960 to June 22, 2015), CINAHL (1981 to June 22, 2015), Web of Science (1926 to June 22, 2015), and Embase.com (1946 to June 22, 2015). Two major systematic search strategies were performed for this review (Figure). Systematic search 1 was designed broadly using all data sources described above to identify interventions aimed at reducing all UTI events (defined under “Outcomes” above) or urinary catheter use (all types), focusing on interventions evaluated in nursing homes. Systematic search 2 was conducted in Ovid MEDLINE to identify studies to reduce UTI events or urinary catheter use measures for patients with a history of long-term or chronic catheter use, including nursing homes and other post-acute care settings such as rehabilitation units or hospitals and spinal cord injury programs, which have large populations of patients with chronic catheter needs. To inform the completeness of the broader systematic searches, supplemental systematic search strategies were performed for specific topics including hydration (supplemental search 1), published work by nursing home researchers known to the authors (supplemental search 2), and contact precautions (supplemental search 3). Search 1 is available at http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42013005787. Full search strategies for search 2 and supplemental searches are available upon request.
Study Selection
One author performed an initial screen of all records retrieved by the systematic searches by title and abstract and applied the initial exclusions (eg, non-human, no outcomes of interest), identified duplicate records, and assigned potentially relevant studies into groups such as review articles, epidemiology, interventions, and articles requiring further text review before categorization (Figure). After initial screening, Dr. Meddings reviewed the records by title/abstract. Reference lists were reviewed for potential articles for inclusion. Full-text article review informed the selection of those for dual abstraction and quality scoring performed by 2 authors, with discrepancies resolved by a third author. We requested additional information from authors from whom our search had generated only an abstract or brief report, or when additional information such as pre-intervention data was needed.12-18
Data Extraction and Quality Assessment
Relevant data regarding study design, participants, inclusion/exclusion criteria, outcomes, and quality criteria were abstracted independently by 2 authors. Methodological quality scores were assigned using a modification of the Quality index checklist developed by Downs and Black appropriate for assessing both randomized and nonrandomized studies of healthcare interventions.19 We also reviewed study funding sources and other potential quality concerns.
Data Analysis
Due to large trial heterogeneity among these studies about interventions and outcomes reported, outcome data could not be combined into summary measures for meta-analysis to give overall estimates of treatment effects.
RESULTS
Systematic Search Results and Study Selection
As detailed in the study flow diagram (Figure), 5794 total records were retrieved by systematic search 1 (4697 studies), search 2 (909 studies), and supplemental searches (188 studies). Hand searching of reference lists of 41 reviews (including narrative and systematic reviews) yielded 77 additional studies for consideration. Twenty-nine records on interventions that were the focus of systematic reviews, including topics of cranberry use, catheter coatings, antimicrobial prophylaxis, washout/irrigation strategies, and sterile versus clean intermittent straight catheterization, were excluded from dual abstraction. Two records were excluded after team discussion of the dual-abstraction results, because 1 study did not meet criteria as an intervention study and 1 study’s setting was not applicable in nursing homes. A total of 20 records15,20-38 (in which 19 studies were described) were selected for final inclusion for detailed assessment and reporting for the systematic review.
Characteristics of Included Studies
Table 1 describes the 19 intervention studies in terms of design, participants, setting, and whether the study included specific categories of interventions expected to decrease UTI or catheter use. These studies included 8 randomized controlled trials (4 with cluster-randomization at the facility or unit level), 10 pre-post nonrandomized interventions, and 1 nonrandomized intervention with concurrent controls. Twelve studies included participants with or without catheters (ie, not limited to catheterized patients only) in nursing homes.15,20-31 Seven32-38 studies included catheterized patients only or settings with high expected catheterization rates; settings for these studies included spinal cord units (n=3), nursing homes (n=2), rehabilitation ward (n=1) and VA hospital (n=1), including acute care, nursing home, and rehabilitation units. Total quality scores for the studies ranged from 8 to 25 (median, 15), detailed in Supplemental Table 1.
As detailed in Table 1 and Supplemental Table 2, 7 studies22,24,26,31,32,35,36 involved single interventions and 12 studies15,20,21,23,25,27-30,33,34,37,38 included multiple interventions. Interventions to impact catheter use and care were evaluated in 13 studies, including appropriateness of use,21,25,29,30 improving catheter maintenance care,15,20,29,30 securement,15,29,30,32 prompting removal of unnecessary catheters,21,25,29,30 improving incontinence care,15,21,23,25 bladder scanners,37,38 catheter changes,35and comparing alternatives (condom catheter or intermittent straight catheter) to use of an indwelling catheter.36,38 None focused on improving aseptic insertion. General infection control practices studied included improving hand hygiene,20-22,29-31,33,34 improving antibiotic use,15,20,21,28,34 initiation of infection control programs,20,21,28 interventions to improve identification of UTIs/CAUTIs using infection symptom/sign criteria,15,20,21,34 infection surveillance as an intervention,28-30,33,34 and barrier precautions,33,34 including preemptive precautions for catheterized patients.34 Hydration was assessed in 3 studies.24-26
Outcomes of Included Studies
Table 2 describes the studies’ outcomes reported for UTI, CAUTI, or bacteriuria.15,20-38 The outcome definitions of UTI and CAUTI varied widely. Only 2 studies22,39 reported UTI outcomes using definitions specific for nursing home settings such as McGeer’s criteria40 a detailed review and comparison of published CAUTI definitions used clinically and for surveillance in nursing homes is provided in Supplemental Table 3. Two studies reported symptomatic CAUTIs per 1000 catheter-days.32,34 Another study22 reported symptomatic CAUTIs per 1000 resident-days. Three reported symptomatic CAUTIs as counts.35,38 Saint et al36 reported CAUTIs as part of a combined outcome (ie, bacteriuria, CAUTI, or death).
The 19 studies (Table 2) reported 12 UTI outcomes,15,20,21,23,25-31,33 9 CAUTI outcomes,15,22,32,34,35,38 4 bacteriuria outcomes,24,36,38 and 5 catheter use outcomes.21,29,30,37,38 Five studies showed CAUTI reduction15,22,32,34,35 (1 significantly34); 9 studies showed UTI reduction13,18,19,21,23-25,27,28,31 (none significantly); 2 studies showed bacteriuria reduction (none significantly). One study36 reported 2 composite outcomes including bacteriuria or CAUTI or death, with statistically significant improvement reported for 1 composite measure. Four studies reported catheter use, with all showing reduced catheter use in the intervention group; however, only 1 achieved statistically significant reduction.37
Synthesis of Systematic Review Results
Overall, many studies reported decreases in UTI, CAUTI, and urinary catheter use measures but without statistical significance, with many studies likely underpowered for our outcomes of interest. Often, the outcomes of interest in this systematic review were not the main outcome for which the study was designed and originally powered. The interventions studied included several currently implemented as part of CAUTI bundles in the acute care setting, such as improving catheter use, and care and infection control strategies. Other included interventions target common challenges specific to the nursing home setting such as removing indwelling catheters upon admission to the nursing home from an acute-care facility21,25 and applying interventions to address incontinence by either general strategies21,23,25,30,38 or the use of an incontinence specialist23 to provide individual treatment plans. The only intervention that demonstrated a statistically significant reduction in CAUTI in chronically catheterized patients employed a comprehensive program to improve antimicrobial use, hand hygiene (including hand hygiene and gloves for catheter care), and preemptive precautions for patients with devices, along with promotion of standardized CAUTI definitions and active multidrug resistant organism surveillance.34
Narrative Review Results
Table 3 includes a comprehensive list of potential interventions that have been considered for prevention of UTI or CAUTI (including those in acute care and nursing home settings), as summarized from this systematic review and prior narrative or systematic reviews.43-115
DISCUSSION
We performed a broad systematic review of strategies to decrease UTI, CAUTI, and urinary catheter use that may be helpful in nursing homes. While many studies reported decreased UTI, CAUTI, or urinary catheter use measures, few demonstrated statistically significant reductions perhaps because many were underpowered to assess statistical significance. Pooled analyses were not feasible to provide the expected impact of these interventions in the nursing home setting.
This review confirms that bundles of interventions for prevention of CAUTI have been implemented with some evidence of success in nursing home settings, with several components in common with those implemented in the acute care setting, such as hand hygiene and strategies to reduce and improve catheter use.41 Some studies focused on issues more common in nursing homes such as chronic catheterization and incontinence. A nursing home CAUTI bundle should be designed with the resources and challenges present in the nursing home environment in mind, and with recognition that, although the number of patients with catheters is less than in acute care, there will be more patients with chronic catheterization needs and incontinence.
Although catheter utilization in nursing homes is low, further reductions in catheter days and CAUTIs can be achieved. Catheter removal reminders and stop orders have demonstrated a greater than 50% reduction in CAUTIs in acute care settings;11 an example of a stop-order intervention in nursing homes is trial removal of indwelling catheters present at facility admission without clear urologic need present at the time of admission.25 Nursing home interventions to avoid catheter placement should include incontinence programs, discussion of alternatives to indwelling urinary catheters with patients, families, and frontline personnel, and urinary retention protocols. Programs to reduce CAUTI should include education to improve aseptic insertion, and to maintain awareness and proper care of catheters in place by regular assessment of catheter necessity, securement, hand hygiene, and preemptive barrier precautions for catheterized patients. Interventions that focus on improving appropriate use of urine tests and antibiotics to treat UTIs can also significantly affect the rates of reported symptomatic CAUTIs, with the potential to decrease unnecessary antibiotic use.20,21
The main limitation of this review is that many studies provided little information about their intervention and definition of outcomes. The strength of this review is the detailed and broad search strategy applied with generous inclusion of interventions and outcomes to highlight the available evidence and details of interventions that have been studied and implemented.
CONCLUSION
This review synthesizes the current state of evidence and proposes strategies to reduce UTIs in nursing homes. Interventions that motivate catheter avoidance and catheter removal to prevent CAUTI in acute care11 and nursing home settings are supported by the strongest available evidence, although the strength of that evidence is less in the nursing home setting. Limitations notwithstanding, interventions such as incontinence care planning and hydration programs can reduce UTI in this population and is important for overall wellbeing.
Acknowledgments
The authors appreciate the guidance that Vineet Chopra MD, MSc, provided regarding options for methodological quality assessment tools, and the assistance of Mary Rogers PhD, MS, in interpreting the published Downs and Black Quality Index items, which informed our modification of this tool for application in this study. The authors appreciate, also, the feedback provided by the Agency for Healthcare Research and Quality (AHRQ) Content and Materials Development Committee for the AHRQ Safety Program for Long-Term Care: Preventing CAUTI and other Healthcare-associated Infections.
Disclosures
Agency for Healthcare Research and Quality (AHRQ) contract #HHSA290201000025I provided funding for this study, which was developed in response to AHRQ Task Order #8 for ACTION II RFTO 26 CUSP for CAUTI in LTC. AHRQ developed the details of the task and provided comments on a draft report, which informed the report submitted to AHRQ in December 2013, used to inform the interventions for a national collaborative (http://www.hret.org/quality/projects/long-term-care-cauti.shtml). Dr. Meddings’s effort on this project was funded by concurrent effort from her AHRQ (K08 HS19767). Dr. Saint’s and Dr. Krein’s effort on this project was funded by concurrent effort from the Veterans Affairs National Center for Patient Safety, Ann Arbor Patient Safety Center of Inquiry. Dr. Meddings’s other research is funded by AHRQ (2R01HS018334-04), the NIH-LRP program, the VA National Center for Patient Safety, and the VA Ann Arbor Patient Safety Center of Inquiry. Dr. Krein’s other research is funded by a VA Health Services Research and Development Award (RCS 11-222). Dr. Mody’s other research is funded by VA Healthcare System Geriatric Research Clinical Care Center (GRECC), NIA-Pepper Center, NIA (R01AG032298, R01AG041780, K24AG050685-01). Dr. Saint has received fees for serving on advisory boards for Doximity and Jvion. All other authors report no financial conflicts of interest. The findings and conclusions in this report are those of the authors and do not necessarily represent those of the sponsor, the Agency for Healthcare Research and Quality, or the U.S. Department of Veterans Affairs. These analyses were presented in part as a poster presentation at the ID Week Annual Meeting on October 10, 2014 in Philadelphia, PA.
Given the limited number of geriatricians in the U.S., hospitalists commonly manage nursing home residents admitted for post-acute care.1-4 Urinary tract infection (UTI) is one of the most common infections in nursing homes, often leading to sepsis and readmission to acute care.5 Inappropriate use of antibiotics to treat asymptomatic bacteriuria is both common and hazardous to nursing home residents.6 Up to 10% of nursing home residents will have an indwelling urinary catheter at some point during their stay.7-9 Residents with indwelling urinary catheters are at increased risk for catheter-associated urinary tract infection (CAUTI) and bacteriuria, with an estimated 50% of catheterized residents developing symptomatic CAUTI.5 While urinary catheter prevalence is lower in nursing homes than in the acute care setting, duration of use is often prolonged.7,10 In a setting where utilization is low, but use is prolonged, interventions designed to reduce UTI in acutely ill patients11 may not be as helpful for preventing infection in nursing home residents.
Our objective was to review the available evidence to prevent UTIs in nursing home residents to inform both bedside care and research efforts. Two types of literature review and summary were performed. First, we conducted a systematic review of individual studies reporting outcomes of UTI, CAUTI, bacteriuria, or urinary catheter use after interventions for reducing catheter use, improving insertion and maintenance of catheters, and/or general infection prevention strategies (eg, improving hand hygiene, infection surveillance, contact precautions, standardizing UTI diagnosis, and antibiotic use). Second, we performed a narrative review to generate an overview of evidence and published recommendations in both acute care and nursing home settings to prevent UTI in catheterized and non-catheterized older adults, which is provided as a comprehensive reference table for clinicians and researchers choosing and refining interventions to reduce UTIs.
METHODS
The systematic review was performed according to the criteria of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis recommendations. The protocol was registered at the PROSPERO International Prospective Register of Systematic Reviews, (CRD42013005787). The narrative review was performed using the articles obtained from the systematic search and a targeted literature review by topic for a comprehensive list of interventions, including other interventions summarized in published reviews and guidelines.
Eligibility Criteria Review
Study Design. To address the breadth and depth of literature available to inform interventions to prevent UTI in nursing homes, broad eligibility criteria were applied with the expectation of varied designs and outcomes. All included studies for the systematic review were published manuscripts reporting a comparison group. We included randomized controlled trials as well as nonrandomized trials (pretest/posttest, with or without concurrent or nonconcurrent controls), with any duration of postintervention follow-up. Observational and retrospective studies were excluded.
Participants. We were interested in interventions and outcomes reported for nursing homes, defined as facilities providing short-stay skilled nursing care and/or rehabilitation, as well as long-term care. We also included evidence derived from rehabilitation facilities and spinal cord injury programs focused on reducing CAUTI risk for chronically catheterized residents. We excluded long-term acute care hospitals, hospice, psychiatric/mental health facilities, pediatric, and community dwelling/outpatient settings.
Interventions. We included interventions involving urinary catheter use such as improving appropriate use, aseptic placement, maintenance care, and prompting removal of unnecessary catheters. We included infection prevention strategies with a particular interest in hand hygiene, barrier precautions, infection control strategies, infection surveillance, use of standardized infection definitions, and interventions to improve antibiotic use. We included single and multiple interventions.
Outcomes
1. Healthcare-associated urinary tract infection: UTI occurring after admission to a healthcare facility, not identified specifically as catheter-associated. We categorized UTI outcomes with as much detail as provided, such as whether the reported outcome included only noncatheter-associated UTIs, the time required after admission (eg, more than 2 days), and whether the UTIs were defined by only laboratory criteria, clinically diagnosed infections, symptomatic, or long-term care specific surveillance definitions.
2. Catheter-associated urinary tract infection: UTI occurring in patients during or immediately after use of a urinary catheter. We noted whether CAUTI was defined by laboratory criteria, clinical symptoms, provider diagnosis, or antimicrobial treatment for case identification. We were primarily interested in CAUTI developing after placing an indwelling urinary catheter, commonly known as a Foley, but also in CAUTI occurring with other catheter types such as intermittent straight catheters, external or “condom” catheters, and suprapubic catheters.
3. Bacteriuria: We included the laboratory-based definition of bacteriuria as an outcome to include studies that reduced asymptomatic bacteriuria.
4. Urinary catheter use measures: This includes measures such as urinary catheter utilization ratios (catheter-days/patient-days), prevalence of urinary catheter use, or percentage of catheters with an appropriate indication.
Study Characteristics for Inclusion. Our systematic search included published papers in the English language. We did not exclude studies based on the number of facilities included or eligible, residents/patients included (based on age, gender, catheter use or type, or antibiotic use), intervention details, study withdrawal, loss to follow-up, death, or duration of pre-intervention and postintervention phases.
Data Sources and Searches
The following data sources were searched: Ovid MEDLINE (1950 to June 22, 2015), Cochrane Library via Wiley (1960 to June 22, 2015), CINAHL (1981 to June 22, 2015), Web of Science (1926 to June 22, 2015), and Embase.com (1946 to June 22, 2015). Two major systematic search strategies were performed for this review (Figure). Systematic search 1 was designed broadly using all data sources described above to identify interventions aimed at reducing all UTI events (defined under “Outcomes” above) or urinary catheter use (all types), focusing on interventions evaluated in nursing homes. Systematic search 2 was conducted in Ovid MEDLINE to identify studies to reduce UTI events or urinary catheter use measures for patients with a history of long-term or chronic catheter use, including nursing homes and other post-acute care settings such as rehabilitation units or hospitals and spinal cord injury programs, which have large populations of patients with chronic catheter needs. To inform the completeness of the broader systematic searches, supplemental systematic search strategies were performed for specific topics including hydration (supplemental search 1), published work by nursing home researchers known to the authors (supplemental search 2), and contact precautions (supplemental search 3). Search 1 is available at http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42013005787. Full search strategies for search 2 and supplemental searches are available upon request.
Study Selection
One author performed an initial screen of all records retrieved by the systematic searches by title and abstract and applied the initial exclusions (eg, non-human, no outcomes of interest), identified duplicate records, and assigned potentially relevant studies into groups such as review articles, epidemiology, interventions, and articles requiring further text review before categorization (Figure). After initial screening, Dr. Meddings reviewed the records by title/abstract. Reference lists were reviewed for potential articles for inclusion. Full-text article review informed the selection of those for dual abstraction and quality scoring performed by 2 authors, with discrepancies resolved by a third author. We requested additional information from authors from whom our search had generated only an abstract or brief report, or when additional information such as pre-intervention data was needed.12-18
Data Extraction and Quality Assessment
Relevant data regarding study design, participants, inclusion/exclusion criteria, outcomes, and quality criteria were abstracted independently by 2 authors. Methodological quality scores were assigned using a modification of the Quality index checklist developed by Downs and Black appropriate for assessing both randomized and nonrandomized studies of healthcare interventions.19 We also reviewed study funding sources and other potential quality concerns.
Data Analysis
Due to large trial heterogeneity among these studies about interventions and outcomes reported, outcome data could not be combined into summary measures for meta-analysis to give overall estimates of treatment effects.
RESULTS
Systematic Search Results and Study Selection
As detailed in the study flow diagram (Figure), 5794 total records were retrieved by systematic search 1 (4697 studies), search 2 (909 studies), and supplemental searches (188 studies). Hand searching of reference lists of 41 reviews (including narrative and systematic reviews) yielded 77 additional studies for consideration. Twenty-nine records on interventions that were the focus of systematic reviews, including topics of cranberry use, catheter coatings, antimicrobial prophylaxis, washout/irrigation strategies, and sterile versus clean intermittent straight catheterization, were excluded from dual abstraction. Two records were excluded after team discussion of the dual-abstraction results, because 1 study did not meet criteria as an intervention study and 1 study’s setting was not applicable in nursing homes. A total of 20 records15,20-38 (in which 19 studies were described) were selected for final inclusion for detailed assessment and reporting for the systematic review.
Characteristics of Included Studies
Table 1 describes the 19 intervention studies in terms of design, participants, setting, and whether the study included specific categories of interventions expected to decrease UTI or catheter use. These studies included 8 randomized controlled trials (4 with cluster-randomization at the facility or unit level), 10 pre-post nonrandomized interventions, and 1 nonrandomized intervention with concurrent controls. Twelve studies included participants with or without catheters (ie, not limited to catheterized patients only) in nursing homes.15,20-31 Seven32-38 studies included catheterized patients only or settings with high expected catheterization rates; settings for these studies included spinal cord units (n=3), nursing homes (n=2), rehabilitation ward (n=1) and VA hospital (n=1), including acute care, nursing home, and rehabilitation units. Total quality scores for the studies ranged from 8 to 25 (median, 15), detailed in Supplemental Table 1.
As detailed in Table 1 and Supplemental Table 2, 7 studies22,24,26,31,32,35,36 involved single interventions and 12 studies15,20,21,23,25,27-30,33,34,37,38 included multiple interventions. Interventions to impact catheter use and care were evaluated in 13 studies, including appropriateness of use,21,25,29,30 improving catheter maintenance care,15,20,29,30 securement,15,29,30,32 prompting removal of unnecessary catheters,21,25,29,30 improving incontinence care,15,21,23,25 bladder scanners,37,38 catheter changes,35and comparing alternatives (condom catheter or intermittent straight catheter) to use of an indwelling catheter.36,38 None focused on improving aseptic insertion. General infection control practices studied included improving hand hygiene,20-22,29-31,33,34 improving antibiotic use,15,20,21,28,34 initiation of infection control programs,20,21,28 interventions to improve identification of UTIs/CAUTIs using infection symptom/sign criteria,15,20,21,34 infection surveillance as an intervention,28-30,33,34 and barrier precautions,33,34 including preemptive precautions for catheterized patients.34 Hydration was assessed in 3 studies.24-26
Outcomes of Included Studies
Table 2 describes the studies’ outcomes reported for UTI, CAUTI, or bacteriuria.15,20-38 The outcome definitions of UTI and CAUTI varied widely. Only 2 studies22,39 reported UTI outcomes using definitions specific for nursing home settings such as McGeer’s criteria40 a detailed review and comparison of published CAUTI definitions used clinically and for surveillance in nursing homes is provided in Supplemental Table 3. Two studies reported symptomatic CAUTIs per 1000 catheter-days.32,34 Another study22 reported symptomatic CAUTIs per 1000 resident-days. Three reported symptomatic CAUTIs as counts.35,38 Saint et al36 reported CAUTIs as part of a combined outcome (ie, bacteriuria, CAUTI, or death).
The 19 studies (Table 2) reported 12 UTI outcomes,15,20,21,23,25-31,33 9 CAUTI outcomes,15,22,32,34,35,38 4 bacteriuria outcomes,24,36,38 and 5 catheter use outcomes.21,29,30,37,38 Five studies showed CAUTI reduction15,22,32,34,35 (1 significantly34); 9 studies showed UTI reduction13,18,19,21,23-25,27,28,31 (none significantly); 2 studies showed bacteriuria reduction (none significantly). One study36 reported 2 composite outcomes including bacteriuria or CAUTI or death, with statistically significant improvement reported for 1 composite measure. Four studies reported catheter use, with all showing reduced catheter use in the intervention group; however, only 1 achieved statistically significant reduction.37
Synthesis of Systematic Review Results
Overall, many studies reported decreases in UTI, CAUTI, and urinary catheter use measures but without statistical significance, with many studies likely underpowered for our outcomes of interest. Often, the outcomes of interest in this systematic review were not the main outcome for which the study was designed and originally powered. The interventions studied included several currently implemented as part of CAUTI bundles in the acute care setting, such as improving catheter use, and care and infection control strategies. Other included interventions target common challenges specific to the nursing home setting such as removing indwelling catheters upon admission to the nursing home from an acute-care facility21,25 and applying interventions to address incontinence by either general strategies21,23,25,30,38 or the use of an incontinence specialist23 to provide individual treatment plans. The only intervention that demonstrated a statistically significant reduction in CAUTI in chronically catheterized patients employed a comprehensive program to improve antimicrobial use, hand hygiene (including hand hygiene and gloves for catheter care), and preemptive precautions for patients with devices, along with promotion of standardized CAUTI definitions and active multidrug resistant organism surveillance.34
Narrative Review Results
Table 3 includes a comprehensive list of potential interventions that have been considered for prevention of UTI or CAUTI (including those in acute care and nursing home settings), as summarized from this systematic review and prior narrative or systematic reviews.43-115
DISCUSSION
We performed a broad systematic review of strategies to decrease UTI, CAUTI, and urinary catheter use that may be helpful in nursing homes. While many studies reported decreased UTI, CAUTI, or urinary catheter use measures, few demonstrated statistically significant reductions perhaps because many were underpowered to assess statistical significance. Pooled analyses were not feasible to provide the expected impact of these interventions in the nursing home setting.
This review confirms that bundles of interventions for prevention of CAUTI have been implemented with some evidence of success in nursing home settings, with several components in common with those implemented in the acute care setting, such as hand hygiene and strategies to reduce and improve catheter use.41 Some studies focused on issues more common in nursing homes such as chronic catheterization and incontinence. A nursing home CAUTI bundle should be designed with the resources and challenges present in the nursing home environment in mind, and with recognition that, although the number of patients with catheters is less than in acute care, there will be more patients with chronic catheterization needs and incontinence.
Although catheter utilization in nursing homes is low, further reductions in catheter days and CAUTIs can be achieved. Catheter removal reminders and stop orders have demonstrated a greater than 50% reduction in CAUTIs in acute care settings;11 an example of a stop-order intervention in nursing homes is trial removal of indwelling catheters present at facility admission without clear urologic need present at the time of admission.25 Nursing home interventions to avoid catheter placement should include incontinence programs, discussion of alternatives to indwelling urinary catheters with patients, families, and frontline personnel, and urinary retention protocols. Programs to reduce CAUTI should include education to improve aseptic insertion, and to maintain awareness and proper care of catheters in place by regular assessment of catheter necessity, securement, hand hygiene, and preemptive barrier precautions for catheterized patients. Interventions that focus on improving appropriate use of urine tests and antibiotics to treat UTIs can also significantly affect the rates of reported symptomatic CAUTIs, with the potential to decrease unnecessary antibiotic use.20,21
The main limitation of this review is that many studies provided little information about their intervention and definition of outcomes. The strength of this review is the detailed and broad search strategy applied with generous inclusion of interventions and outcomes to highlight the available evidence and details of interventions that have been studied and implemented.
CONCLUSION
This review synthesizes the current state of evidence and proposes strategies to reduce UTIs in nursing homes. Interventions that motivate catheter avoidance and catheter removal to prevent CAUTI in acute care11 and nursing home settings are supported by the strongest available evidence, although the strength of that evidence is less in the nursing home setting. Limitations notwithstanding, interventions such as incontinence care planning and hydration programs can reduce UTI in this population and is important for overall wellbeing.
Acknowledgments
The authors appreciate the guidance that Vineet Chopra MD, MSc, provided regarding options for methodological quality assessment tools, and the assistance of Mary Rogers PhD, MS, in interpreting the published Downs and Black Quality Index items, which informed our modification of this tool for application in this study. The authors appreciate, also, the feedback provided by the Agency for Healthcare Research and Quality (AHRQ) Content and Materials Development Committee for the AHRQ Safety Program for Long-Term Care: Preventing CAUTI and other Healthcare-associated Infections.
Disclosures
Agency for Healthcare Research and Quality (AHRQ) contract #HHSA290201000025I provided funding for this study, which was developed in response to AHRQ Task Order #8 for ACTION II RFTO 26 CUSP for CAUTI in LTC. AHRQ developed the details of the task and provided comments on a draft report, which informed the report submitted to AHRQ in December 2013, used to inform the interventions for a national collaborative (http://www.hret.org/quality/projects/long-term-care-cauti.shtml). Dr. Meddings’s effort on this project was funded by concurrent effort from her AHRQ (K08 HS19767). Dr. Saint’s and Dr. Krein’s effort on this project was funded by concurrent effort from the Veterans Affairs National Center for Patient Safety, Ann Arbor Patient Safety Center of Inquiry. Dr. Meddings’s other research is funded by AHRQ (2R01HS018334-04), the NIH-LRP program, the VA National Center for Patient Safety, and the VA Ann Arbor Patient Safety Center of Inquiry. Dr. Krein’s other research is funded by a VA Health Services Research and Development Award (RCS 11-222). Dr. Mody’s other research is funded by VA Healthcare System Geriatric Research Clinical Care Center (GRECC), NIA-Pepper Center, NIA (R01AG032298, R01AG041780, K24AG050685-01). Dr. Saint has received fees for serving on advisory boards for Doximity and Jvion. All other authors report no financial conflicts of interest. The findings and conclusions in this report are those of the authors and do not necessarily represent those of the sponsor, the Agency for Healthcare Research and Quality, or the U.S. Department of Veterans Affairs. These analyses were presented in part as a poster presentation at the ID Week Annual Meeting on October 10, 2014 in Philadelphia, PA.
1. Beresford L. Post-acute patient care: new frontier for hospitalists. The Hospitalist.
July 2015. http://www.the-hospitalist.org/hospitalist/article/122330/post-acute-patient-
care-new-frontier-hospitalists. Accessed March 31, 2017.
2. Butterfield S. Hospital medicine matures: Hospitalists and hospitalist groups move into post-acute care. 2012. Available at http://www.acphospitalist.org/archives/2012/10/coverstory.htm. Accessed April 6, 2016.
3. Pittman D. SNFs: New Turf for Hospitalists? 2013; Available at http://www.medpagetoday.com/HospitalBasedMedicine/Hospitalists/39401. Accessed April 6, 2016.
4. Society of Hospital Medicine. SHM and IPC Healthcare Develop First SHM Primer for Hospitalists in Skilled Nursing Facilities. 2015; Available at http://www.hospitalmedicine.org/Web/Media_Center/Press_Release/2015/SHM_and_IPC_Healthcare_Develop_First_SHM_Primer_for_Hospitalists_in_Skilled_Nursing_Facilities.aspx. Accessed April 6, 2016.
5. Montoya A, Mody L. Common infections in nursing homes: a review of current issues and challenges. Aging Health. 2011;7(6):889-899. PubMed
6. Phillips CD, Adepoju O, Stone N, et al. Asymptomatic bacteriuria, antibiotic use, and suspected urinary tract infections in four nursing homes. BMC Geriatr. 2012;12:73. PubMed
7. Rogers M, Mody L, Kaufman S, Fries B, McMahon L, Saint S. Use of urinary collection devices in skilled nursing facilities in five states. J Amer Geriatr Soc. 2008;56:854-861. PubMed
8. Castle N, Engberg JB, Wagner LM, Handler S. Resident and facility factors associated with the incidence of urinary tract infections identified in the nursing home minimum data set. J Appl Gerontol. 2015:doi: 10.1177/0733464815584666. PubMed
9. Tsan L, Langberg R, Davis C, et al. Nursing home-associated infections in Department of Veterans Affairs community living centers. Am J Infect Control. 2010;38(6):461-466. PubMed
10. Kunin CM, Chin QF, Chambers S. Morbidity and mortality associated with indwelling urinary catheters in elderly patients in a nursing home--confounding due to the presence of associated diseases. J Am Geriatr Soc. 1987;35(11):1001-1006. PubMed
11. Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, Saint S. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf. 2013;23(4):277-289. PubMed
12. Abraham F, Abraham FP. A CAUTI bundle with a twist. Am J Infect Control. 2012;40(5):e79-e80.
13. Flynn ER, Zombolis K. Reducing hospital acquired indwelling urinary catheter-associated urinary tract infections through multidisciplinary team and shared governance practice model. Am J Infect Control. 2011;39(5):E28-E29.
14. Gokula MR, Gaspar P, Siram R. Implementation of an evidence based protocol to reduce use of indwelling urinary catheters in the long term care environment. J Am Med Dir Assoc. 2013;14(3):B23.
15. Brownhill K. Training in care homes to reduce avoidable harm. Nurs Times. 2013;109(43):20-22. PubMed
16. Galeon CP, Romero I. Implementing a performance improvement project in a multi-level teaching facility on reducing catheter associated urinary tract infections (CAUTI). Am J Infect Control. 2014:S130-S131.
17. Evans ME, Kralovic SM, Simbartl LA, et al. Nationwide reduction of health care-associated methicillin-resistant Staphylococcus aureus infections in Veterans Affairs long-term care facilities. Am J Infect Control. 2014;42(1):60-62. PubMed
18. Evans KA, Ligon R, Lipton C. Reduction of antibiotic starts for asymptomatic bacteriuria in skilled nursing facilities. J Am Geriatr Soc. 2015;63:S131.
19. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. PubMed
20. Ahlbrecht H, Shearen C, Degelau J, Guay DR. Team approach to infection prevention and control in the nursing home setting. Am J Infect Control. 1999;27(1):64-70. PubMed
21. Cools HJ, van der Meer JW. Infection control in a skilled nursing facility: a 6-year survey. J Hosp Infect. 1988;12(2):117-124. PubMed
22. Fendler EJ, Ali Y, Hammond BS, Lyons MK, Kelley MB, Vowell NA. The impact of alcohol hand sanitizer use on infection rates in an extended care facility. Am J Infect Control. 2002;30(4):226-233. PubMed
23. Klay M, Marfyak K. Use of a continence nurse specialist in an extended care facility. Urol Nurs. 2005;25(2):101-102. PubMed
24. Lin S. A pilot study: fluid intake and bacteriuria in nursing home residents in southern Taiwan. Nurs Res. 2013;62(1):66-72. PubMed
25. McConnell J. Preventing urinary tract infections. Geriatr Nurs. 1984;5(8):361-362. PubMed
26. Mentes JC, Culp K. Reducing hydration-linked events in nursing home residents. Clin Nurs Res. 2003;12(3):210-225; discussion 226-218. PubMed
27. Miller SC, Lepore M, Lima JC, Shield R, Tyler DA. Does the introduction of nursing home culture change practices improve quality? J Am Geriatr Soc. 2014;62(9):1675-1682. PubMed
28. Stuart RL, Orr E, Kotsanas D, Gillespie EE. A nurse-led antimicrobial stewardship intervention in two residential aged care facilities. Healthcare Infection. 2015;20(1):4-6.
29. van Gaal B, Schoonhoven L, Mintjes JAJ, Borm GF, Koopmans RTCM, van Achterberg T. The SAFE or SORRY? programme. Part II: Effect on preventive care. Int J Nurs Stud. 2011;48(9):1049-1057. PubMed
30. van Gaal BGI, Schoonhoven L, Mintjes JAJ, et al. Fewer adverse events as a result of the SAFE or SORRY? programme in hospitals and nursing homes. part I: primary outcome of a cluster randomised trial. Int J Nurs Stud. 2011;48(9):1040-1048. PubMed
31. Yeung WK, Wilson Tam WS, Wong TW. Clustered randomized controlled trial of a hand hygiene intervention involving pocket-sized containers of alcohol-based hand rub for the control of infections in long-term care facilities. Infect Control Hosp Epidemiol. 2011;32(1):67-76. PubMed
32. Darouiche RO, Goetz L, Kaldis T, Cerra-Stewart C, AlSharif A, Priebe M. Impact of StatLock securing device on symptomatic catheter-related urinary tract infection: a prospective, randomized, multicenter clinical trial. Am J Infect Control. 2006;34(9):555-560. PubMed
33. Evans ME, Kralovic SM, Simbartl LA, et al. Prevention of methicillin-resistant Staphylococcus aureus infections in spinal cord injury units. Am J Infect Control. 2013;41(5):422-426. PubMed
34. Mody L, Krein S, Saint S, et al. A targeted infection prevention intervention in nursing home residents with indwelling devices: a randomized clinical trial. JAMA Intern Med. 2015;175:714-723. PubMed
35. Priefer BA, Duthie Jr EH, Gambert SR. Frequency of urinary catheter change and clinical urinary tract infection. Study in hospital-based, skilled nursing home. Urology. 1982;20(2):141-142. PubMed
36. Saint S, Kaufman SR, Rogers MA, Baker PD, Ossenkop K, Lipsky BA. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54(7):1055-1061. PubMed
37. Suardi L, Cazzaniga M, Spinelli M, Tagliabue A. From intermittent catheterisation to time-volume dependent catheterisation in patients with spinal cord injuries, through the use of a portable, ultrasound instrument. Europa Medicophysica. 2001;37(2):111-114.
38. Tang MW, Kwok TC, Hui E, Woo J. Intermittent versus indwelling urinary catheterization in older female patients. Maturitas. 2006;53(3):274-281. PubMed
39. Cassel BG, Parkes V, Poon R, Rae H. Quality improvement best practices and long-term indwelling urinary catheters. Perspectives. 2008;32(1):13-17. PubMed
40. Stone ND, Ashraf MS, Calder J, et al. Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria. Infect Control Hosp Epidemiol. 2012;33(10):965-977. PubMed
41. Saint S, Greene MT, Krein SL, et al. A Program to Prevent Catheter-Associated Urinary Tract Infection in Acute Care. New England Journal of Medicine. 2016;374(22):2111-2119. PubMed
42. McGeer A, Campbell B, Emori TG, et al. Definitions of infection for surveillance in long-term care facilities. Am J Infect Control. 1991;19(1):1-7. PubMed
43. Nicolle LE. The chronic indwelling catheter and urinary infection in long-term-care facility residents. Infect Control Hosp Epidemiol. 2001;22(5):316-321. PubMed
44. Nicolle LE; SHEA Long-Term Care Committee. Urinary tract infections in long-term-care facilities. Infect Control Hosp Epidemiol. 2001;22(3):167-175. PubMed
45. Nicolle LE. Catheter-related urinary tract infection. Drug & Aging. 2005;22(8):627-639. PubMed
46. Cochran S. Care of the indwelling urinary catheter - Is it evidence based? J Wound Ostomy Cont Nurs. 2007;34(3):282-288. PubMed
47. Seiler WO, Stahelin HB. Practical management of catheter-associated UTIs. Geriatrics. 1988;43(8):43-50. PubMed
48. Stickler DJ, Chawla JC. The role of antiseptics in the management of patients with long-term indwelling bladder catheters. J Hosp Infect. 1987;10(3):219-228. PubMed
49. Gray M. Does the construction material affect outcomes in long-term catheterization? J Wound Ostomy Cont Nurs. 2006;33(2):116-121. PubMed
50. Trautner BW, Darouiche RO. Clinical review: prevention of urinary tract infection in patients with spinal cord injury. J Spinal Cord Med. 2002;2002(25):277-283. PubMed
51. Maloney C. Estrogen & recurrent UTI in postmenopausal women. Am J Nurs. 2002;102(8):44-52. PubMed
52. Raz R. Hormone replacement therapy or prophylaxis in postmenopausal women with recurrent urinary tract infection. J Infect Dis. 2001;183(suppl 1):S74-S76. PubMed
53. Godfrey H. Older people, continence care and catheters: dilemmas and resolutions. Br J Nurs. 2008;17(9):S4-S11. PubMed
54. Godfrey H, Evans A. Management of long-term urethral catheters: minimizing complications. Br J Nurs. 2000;9(2):74-76. PubMed
55. Kunin CM. Chemoprophylaxis and suppressive therapy in the management of urinary tract infections. J Antimicrob Chemother. 1994;33(suppl A):51-62. PubMed
56. Newman DK, Willson MM. Review of intermittent catheterization and current best practices. Urol Nurs. 2011;31(1):12-48. PubMed
57. Allan GM, Nicolle L. Cranberry for preventing urinary tract infection. Can Fam Physician. 2013;59(4):367. PubMed
58. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;10:CD001321. PubMed
59. Wang CH, Fang CC, Chen NC, et al. Cranberry-containing products for prevention of urinary tract infections in susceptible populations: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172(13):988-996. PubMed
60. Moore KN, Fader M, Getliffe K. Long-term bladder management by intermittent catheterisation in adults and children. Cochrane Database Syst Rev. 2007(4):CD006008. PubMed
61. Li L, Ye WQ, Ruan H, Yang BY, Zhang SQ. Impact of hydrophilic catheters on urinary tract infections in people with spinal cord injury: systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2013;94(4):782-787. PubMed
62. Jamison J, Maguire S, McCann J. Catheter policies for management of long term voiding problems in adults with neurogenic bladder disorders. Cochrane Database Syst Rev. 2011(12):CD004375. PubMed
63. Gray M. What nursing interventions reduce the risk of symptomatic urinary tract infections in the patient with an indwelling catheter? J Wound Ostomy Cont Nurs. 2004;31(1):3-13. PubMed
64. Marschall J, Carpenter C, Fowler S, Trautner B. Antibiotic prophylaxis for urinary tract infections after removal of urinary catheter: meta-analysis. BMJ. 2013;346:f3147. PubMed
65. Sinclair L, Hagen S, Cross S. Washout policies in long-term indwelling urinary catheterization in adults: a short version Cochrane review. Neurourol Urodyn. 2011;30(7):1208-1212. PubMed
66. Hunter KF, Bharmal A, Moore KN. Long-term bladder drainage: suprapubic catheter versus other methods: a scoping review. Neurourol Urodyn. 2013;32(7):944-951. PubMed
67. Morton SC, Shekelle PG, Adams JL, et al. Antimicrobial prophylaxis for urinary tract infection in persons with spinal cord dysfunction. Arch Phys Med Rehabil. 2002;83(1):129-138. PubMed
68. Niël-Weise BS, van den Broek PJ, da Silva EM, Silva LA. Urinary catheter policies for long-term bladder drainage. Cochrane Database Syst Rev. 2012(8). PubMed
69. Jepson R, Craig J. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008;10(CD001321). PubMed
70. Avorn J, Monane M, Gurwitz JH, Glynn RJ, Choodnovskiy I, Lipsitz LA. Reduction of bacteriuria and pyuria after ingestion of cranberry juice. JAMA. 1994;271(10):751-754. PubMed
71. Bianco L, Perrelli E, Towle V, Van Ness PH, Juthani-Mehta M. Pilot randomized controlled dosing study of cranberry capsules for reduction of bacteriuria plus pyuria in female nursing home residents. J Am Geriatr Soc. 2012;60(6):1180-1181. PubMed
72. Lin SC, Wang CC, Shih SC, Tjung JJ, Tsou MT, Lin CJ. Prevention of Asymptomatic Bacteriuria with Cranberries and Roselle Juice in Home-care Patients with Long-term Urinary Catheterization. Int J Gerontol. 2014;8(3):152-156.
73. Juthani-Mehta M, Perley L, Chen S, Dziura J, Gupta K. Feasibility of cranberry capsule administration and clean-catch urine collection in long-term care residents. J Am Geriatr Soc. 2010;58(10):2028-2030. PubMed
74. Tully CL, Bastone P, Vaughan J, Ballentine L. Urinary tract infection prophylaxis with cranberry extract in the nursing home setting. J Am Geriatr Soc. 2004;52(4):S206-S206.
75. Woodward N. Use of cranberry extract for the prevention of UTIs in an at-risk population. 41st Annual Wound, Ostomy and Continence Nurses Annual Conference, St. Louis, Missouri, June 6-10, 2009. J Wound Ostomy Continence Nurs. 2009;36(3S):S62-S62.
76. Linsenmeyer TA, Harrison B, Oakley A, Kirshblum S, Stock JA, Millis SR. Evaluation of cranberry supplement for reduction of urinary tract infections in individuals with neurogenic bladders secondary to spinal cord injury. A prospective, double-blinded, placebo-controlled, crossover study. J Spinal Cord Med. 2004;27(1):29-34. PubMed
77. Waites KB, Canupp KC, Armstrong S, DeVivo MJ. Effect of cranberry extract on bacteriuria and pyuria in persons with neurogenic bladder secondary to spinal cord injury. J Spinal Cord Med. 2004;27(1):35-40. PubMed
78. Caljouw MAA, Van Den Hout WB, Putter H, Achterberg WP, Cools HJM, Gussekloo J. Effectiveness of cranberry capsules to prevent urinary tract infections in vulnerable older persons. A double-blind randomized placebo-controlled trial in long-term care facilities. Eur Geriatr Med. 2013;4:S118-S119. PubMed
79. Hout WB, Caljouw MAA, Putter H, Cools HJM, Gussekloo J. Cost-effectiveness of cranberry capsules to prevent urinary tract infection in long-term care facilities: economic evaluation with a randomized controlled trial. J Am Geriatr Soc. 2014;62(1):111-116. PubMed
80. Liu BA, McGeer A, McArthur MA, et al. Effect of multivitamin and mineral supplementation on episodes of infection in nursing home residents: a randomized, placebo-controlled study. J Am Geriatr Soc. 2007;55(1):35-42. PubMed
81. Eriksen B. A randomized, open, parallel-group study on the preventive effect of an estradiol-releasing vaginal ring (Estring) on recurrent urinary tract infections in postmenopausal women. Am J Obstet Gynecol. 1999;180:1072-1079. PubMed
82. Maloney C. Hormone replacement therapy in female nursing home residents with recurrent urinary tract infection. Ann Long-Term Care. 1998;6(3):77-82.
83. Gokula RM, Smith MA, Hickner J. Emergency room staff education and use of a urinary catheter indication sheet improves appropriate use of foley catheters. Am J Infect Control. 2007;35(9):589-593. PubMed
84. Salamon L. Catheter-associated urinary tract infections: a nurse-sensitive indicator in an inpatient rehabilitation program. Rehabil Nurs. 2009;34(6):237-241. PubMed
85. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-326. PubMed
86. American Medical Directors Association (AMDA). Appropriate indications for use of a chronic indwelling catheter in the long-term care setting. Columbia, MD; excerpted from AMDA's Clinical Practice Guideline: Urinary Incontinence. 2005.
87. Rannikko S, Kyllastinen M, Granqvist B. Comparison of long-term indwelling catheters and bed-pads in the treatment of urinary incontinence in elderly patients. J Infect. 1986;12(3):221-227. PubMed
88. Carapeti E, Andrews S, Bentley P. Randomised study of sterile versus non-sterile urethral catheterization. Ann R. Coll Surg Engl. 1996;78(1):59-60. PubMed
89. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625-663. PubMed
90. Olsen-Scribner RJ, Hayes C, Pottinger P. Sustaining reduction of catheter-associated urinary tract infection (CAUTI)-outcomes after two educational methods in a regional university-affiliated medical center. Am J Infect Control. 2014;1:S22.
91. Duffy LM, Cleary J, Ahern S, et al. Clean intermittent catheterization: safe, cost-effective bladder management for male residents of VA nursing homes. J Am Geriatr Soc. 1995;43(8):865-870. PubMed
92. Joseph C, Jacobson C, Strausbaugh L, Maxwell M, French M, Colling J. Sterile vs clean urinary catheterization. J Am Geriatr Soc. 1991;39(10):1042-1043. PubMed
93. Moore KN, Burt J, Voaklander DC. Intermittent catheterization in the rehabilitation setting: a comparison of clean and sterile technique. Clin Rehabili. 2006;20(6):461-468. PubMed
94. Moore KN, Kelm M, Sinclair O, Cadrain G. Bacteriuria in intermittent catheterization users: the effect of sterile versus clean reused catheters. Rehabil Nurs J. 1993;18(5):306-309. PubMed
95. Niel-Weise BS, van den Broek PJ. Urinary catheter policies for short-term bladder drainage in adults. Cochrane Database Syst Rev. 2005(3):CD004203. PubMed
96. Ouslander JG, Greengold B, Chen S. External catheter use and urinary tract infections among incontinent male nursing home patients. J Am Geriatr Soc. 1987;35(12):1063-1070. PubMed
97. Wyndaele JJ, Brauner A, Geerlings SE, Bela K, Peter T, Bjerklund-Johanson TE. Clean intermittent catheterization and urinary tract infection: review and guide for future research. BJU Int. 2012;110(11 Pt C):E910-917. PubMed
98. Jahn P, Beutner K, Langer G. Types of indwelling urinary catheters for long-term bladder drainage in adults. Cochrane Database Syst Rev. 2012(10):CD004997. PubMed
99. Pickard R, Lam T, Maclennan G, et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: a multicentre randomised controlled trial. Lancet. 2012;380(9857):1927-1935. PubMed
100. Burke JP, Garibaldi RA, Britt MR, Jacobson JA, Conti M, Alling DW. Prevention of catheter-associated urinary tract infections. Efficacy of daily meatal care regimens. Am J Med. 1981;70(3):655-658. PubMed
101. Hagen S, Sinclair L, Cross S. Washout policies in long-term indwelling urinary catheterisation in adults. Cochrane Database Syst Rev. 2010(3). PubMed
102. Moore KN, Hunter KF, McGinnis R, et al. Do catheter washouts extend patency time in long-term indwelling urethral catheters? A randomized controlled trial of acidic washout solution, normal saline washout, or standard care. J Wound Ostomy Continence Nurs. 2009;36(1):82-90. PubMed
103. Muncie HL Jr, Hoopes JM, Damron DJ, Tenney JH, Warren JW. Once-daily irrigation of long-term urethral catheters with normal saline. Lack of benefit. Arch Intern Med. 1989;149(2):441- PubMed
104. Ruwaldt MM. Irrigation of indwelling urinary catheters. Urology. 1983;21(2):127-129. PubMed
105. Palka MA. Evidenced based review of recommendations addressing the frequency of changing long-term indwelling urinary catheters in older adults. Geriatr Nurs. 2014;35(5):357-363. PubMed
106. Warren JW. Catheter-associated urinary tract infections. Infect Dis Clin North Am. 1997;11(3):609-622. PubMed
107. Fryklund B, Haeggman S, Burman LG. Transmission of urinary bacterial strains between patients with indwelling catheters--nursing in the same room and in separate rooms compared. J Hosp Infect. 1997;36(2):147-153. PubMed
108. Anderson RU. Non-sterile intermittent catheterization with antibiotic prophylaxis in the acute spinal cord injured male patient. J Urol. 1980;124(3):392-394. PubMed
109. Anderson RU. Prophylaxis of bacteriuria during intermittent catheterization of the acute neurogenic bladder. J Urol. 1980;123(3):364-366. PubMed
110. Gribble MJ, Puterman ML. Prophylaxis of urinary tract infection in persons with recent spinal cord injury: a prospective, randomized, double-blind, placebo-controlled study of trimethoprim-sulfamethoxazole. Am J Med. 1993;95(2):141-152. PubMed
111. Rutschmann OT, Zwahlen A. Use of norfloxacin for prevention of symptomatic urinary tract infection in chronically catheterized patients. Eur J Clin Microbiol Infect Dis. 1995;14(5):441-444. PubMed
112. Jewes LA, Gillespie WA, Leadbetter A, et al. Bacteriuria and bacteraemia in patients with long-term indwelling catheters--a domiciliary study. J Med Microbiol. 1988;26(1):61-65. PubMed
113. Warren JW, Damron D, Tenney JH, Hoopes JM, Deforge B, Muncie HL, Jr. Fever, bacteremia, and death as complications of bacteriuria in women with long-term urethral catheters. J Infect Dis. 1987;155(6):1151-1158. PubMed
114. Prasad A, Cevallos ME, Riosa S, Darouiche RO, Trautner BW. A bacterial interference strategy for prevention of UTI in persons practicing intermittent catheterization. Spinal Cord. 2009;47(7):565-569. PubMed
1. Beresford L. Post-acute patient care: new frontier for hospitalists. The Hospitalist.
July 2015. http://www.the-hospitalist.org/hospitalist/article/122330/post-acute-patient-
care-new-frontier-hospitalists. Accessed March 31, 2017.
2. Butterfield S. Hospital medicine matures: Hospitalists and hospitalist groups move into post-acute care. 2012. Available at http://www.acphospitalist.org/archives/2012/10/coverstory.htm. Accessed April 6, 2016.
3. Pittman D. SNFs: New Turf for Hospitalists? 2013; Available at http://www.medpagetoday.com/HospitalBasedMedicine/Hospitalists/39401. Accessed April 6, 2016.
4. Society of Hospital Medicine. SHM and IPC Healthcare Develop First SHM Primer for Hospitalists in Skilled Nursing Facilities. 2015; Available at http://www.hospitalmedicine.org/Web/Media_Center/Press_Release/2015/SHM_and_IPC_Healthcare_Develop_First_SHM_Primer_for_Hospitalists_in_Skilled_Nursing_Facilities.aspx. Accessed April 6, 2016.
5. Montoya A, Mody L. Common infections in nursing homes: a review of current issues and challenges. Aging Health. 2011;7(6):889-899. PubMed
6. Phillips CD, Adepoju O, Stone N, et al. Asymptomatic bacteriuria, antibiotic use, and suspected urinary tract infections in four nursing homes. BMC Geriatr. 2012;12:73. PubMed
7. Rogers M, Mody L, Kaufman S, Fries B, McMahon L, Saint S. Use of urinary collection devices in skilled nursing facilities in five states. J Amer Geriatr Soc. 2008;56:854-861. PubMed
8. Castle N, Engberg JB, Wagner LM, Handler S. Resident and facility factors associated with the incidence of urinary tract infections identified in the nursing home minimum data set. J Appl Gerontol. 2015:doi: 10.1177/0733464815584666. PubMed
9. Tsan L, Langberg R, Davis C, et al. Nursing home-associated infections in Department of Veterans Affairs community living centers. Am J Infect Control. 2010;38(6):461-466. PubMed
10. Kunin CM, Chin QF, Chambers S. Morbidity and mortality associated with indwelling urinary catheters in elderly patients in a nursing home--confounding due to the presence of associated diseases. J Am Geriatr Soc. 1987;35(11):1001-1006. PubMed
11. Meddings J, Rogers MA, Krein SL, Fakih MG, Olmsted RN, Saint S. Reducing unnecessary urinary catheter use and other strategies to prevent catheter-associated urinary tract infection: an integrative review. BMJ Qual Saf. 2013;23(4):277-289. PubMed
12. Abraham F, Abraham FP. A CAUTI bundle with a twist. Am J Infect Control. 2012;40(5):e79-e80.
13. Flynn ER, Zombolis K. Reducing hospital acquired indwelling urinary catheter-associated urinary tract infections through multidisciplinary team and shared governance practice model. Am J Infect Control. 2011;39(5):E28-E29.
14. Gokula MR, Gaspar P, Siram R. Implementation of an evidence based protocol to reduce use of indwelling urinary catheters in the long term care environment. J Am Med Dir Assoc. 2013;14(3):B23.
15. Brownhill K. Training in care homes to reduce avoidable harm. Nurs Times. 2013;109(43):20-22. PubMed
16. Galeon CP, Romero I. Implementing a performance improvement project in a multi-level teaching facility on reducing catheter associated urinary tract infections (CAUTI). Am J Infect Control. 2014:S130-S131.
17. Evans ME, Kralovic SM, Simbartl LA, et al. Nationwide reduction of health care-associated methicillin-resistant Staphylococcus aureus infections in Veterans Affairs long-term care facilities. Am J Infect Control. 2014;42(1):60-62. PubMed
18. Evans KA, Ligon R, Lipton C. Reduction of antibiotic starts for asymptomatic bacteriuria in skilled nursing facilities. J Am Geriatr Soc. 2015;63:S131.
19. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. PubMed
20. Ahlbrecht H, Shearen C, Degelau J, Guay DR. Team approach to infection prevention and control in the nursing home setting. Am J Infect Control. 1999;27(1):64-70. PubMed
21. Cools HJ, van der Meer JW. Infection control in a skilled nursing facility: a 6-year survey. J Hosp Infect. 1988;12(2):117-124. PubMed
22. Fendler EJ, Ali Y, Hammond BS, Lyons MK, Kelley MB, Vowell NA. The impact of alcohol hand sanitizer use on infection rates in an extended care facility. Am J Infect Control. 2002;30(4):226-233. PubMed
23. Klay M, Marfyak K. Use of a continence nurse specialist in an extended care facility. Urol Nurs. 2005;25(2):101-102. PubMed
24. Lin S. A pilot study: fluid intake and bacteriuria in nursing home residents in southern Taiwan. Nurs Res. 2013;62(1):66-72. PubMed
25. McConnell J. Preventing urinary tract infections. Geriatr Nurs. 1984;5(8):361-362. PubMed
26. Mentes JC, Culp K. Reducing hydration-linked events in nursing home residents. Clin Nurs Res. 2003;12(3):210-225; discussion 226-218. PubMed
27. Miller SC, Lepore M, Lima JC, Shield R, Tyler DA. Does the introduction of nursing home culture change practices improve quality? J Am Geriatr Soc. 2014;62(9):1675-1682. PubMed
28. Stuart RL, Orr E, Kotsanas D, Gillespie EE. A nurse-led antimicrobial stewardship intervention in two residential aged care facilities. Healthcare Infection. 2015;20(1):4-6.
29. van Gaal B, Schoonhoven L, Mintjes JAJ, Borm GF, Koopmans RTCM, van Achterberg T. The SAFE or SORRY? programme. Part II: Effect on preventive care. Int J Nurs Stud. 2011;48(9):1049-1057. PubMed
30. van Gaal BGI, Schoonhoven L, Mintjes JAJ, et al. Fewer adverse events as a result of the SAFE or SORRY? programme in hospitals and nursing homes. part I: primary outcome of a cluster randomised trial. Int J Nurs Stud. 2011;48(9):1040-1048. PubMed
31. Yeung WK, Wilson Tam WS, Wong TW. Clustered randomized controlled trial of a hand hygiene intervention involving pocket-sized containers of alcohol-based hand rub for the control of infections in long-term care facilities. Infect Control Hosp Epidemiol. 2011;32(1):67-76. PubMed
32. Darouiche RO, Goetz L, Kaldis T, Cerra-Stewart C, AlSharif A, Priebe M. Impact of StatLock securing device on symptomatic catheter-related urinary tract infection: a prospective, randomized, multicenter clinical trial. Am J Infect Control. 2006;34(9):555-560. PubMed
33. Evans ME, Kralovic SM, Simbartl LA, et al. Prevention of methicillin-resistant Staphylococcus aureus infections in spinal cord injury units. Am J Infect Control. 2013;41(5):422-426. PubMed
34. Mody L, Krein S, Saint S, et al. A targeted infection prevention intervention in nursing home residents with indwelling devices: a randomized clinical trial. JAMA Intern Med. 2015;175:714-723. PubMed
35. Priefer BA, Duthie Jr EH, Gambert SR. Frequency of urinary catheter change and clinical urinary tract infection. Study in hospital-based, skilled nursing home. Urology. 1982;20(2):141-142. PubMed
36. Saint S, Kaufman SR, Rogers MA, Baker PD, Ossenkop K, Lipsky BA. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54(7):1055-1061. PubMed
37. Suardi L, Cazzaniga M, Spinelli M, Tagliabue A. From intermittent catheterisation to time-volume dependent catheterisation in patients with spinal cord injuries, through the use of a portable, ultrasound instrument. Europa Medicophysica. 2001;37(2):111-114.
38. Tang MW, Kwok TC, Hui E, Woo J. Intermittent versus indwelling urinary catheterization in older female patients. Maturitas. 2006;53(3):274-281. PubMed
39. Cassel BG, Parkes V, Poon R, Rae H. Quality improvement best practices and long-term indwelling urinary catheters. Perspectives. 2008;32(1):13-17. PubMed
40. Stone ND, Ashraf MS, Calder J, et al. Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria. Infect Control Hosp Epidemiol. 2012;33(10):965-977. PubMed
41. Saint S, Greene MT, Krein SL, et al. A Program to Prevent Catheter-Associated Urinary Tract Infection in Acute Care. New England Journal of Medicine. 2016;374(22):2111-2119. PubMed
42. McGeer A, Campbell B, Emori TG, et al. Definitions of infection for surveillance in long-term care facilities. Am J Infect Control. 1991;19(1):1-7. PubMed
43. Nicolle LE. The chronic indwelling catheter and urinary infection in long-term-care facility residents. Infect Control Hosp Epidemiol. 2001;22(5):316-321. PubMed
44. Nicolle LE; SHEA Long-Term Care Committee. Urinary tract infections in long-term-care facilities. Infect Control Hosp Epidemiol. 2001;22(3):167-175. PubMed
45. Nicolle LE. Catheter-related urinary tract infection. Drug & Aging. 2005;22(8):627-639. PubMed
46. Cochran S. Care of the indwelling urinary catheter - Is it evidence based? J Wound Ostomy Cont Nurs. 2007;34(3):282-288. PubMed
47. Seiler WO, Stahelin HB. Practical management of catheter-associated UTIs. Geriatrics. 1988;43(8):43-50. PubMed
48. Stickler DJ, Chawla JC. The role of antiseptics in the management of patients with long-term indwelling bladder catheters. J Hosp Infect. 1987;10(3):219-228. PubMed
49. Gray M. Does the construction material affect outcomes in long-term catheterization? J Wound Ostomy Cont Nurs. 2006;33(2):116-121. PubMed
50. Trautner BW, Darouiche RO. Clinical review: prevention of urinary tract infection in patients with spinal cord injury. J Spinal Cord Med. 2002;2002(25):277-283. PubMed
51. Maloney C. Estrogen & recurrent UTI in postmenopausal women. Am J Nurs. 2002;102(8):44-52. PubMed
52. Raz R. Hormone replacement therapy or prophylaxis in postmenopausal women with recurrent urinary tract infection. J Infect Dis. 2001;183(suppl 1):S74-S76. PubMed
53. Godfrey H. Older people, continence care and catheters: dilemmas and resolutions. Br J Nurs. 2008;17(9):S4-S11. PubMed
54. Godfrey H, Evans A. Management of long-term urethral catheters: minimizing complications. Br J Nurs. 2000;9(2):74-76. PubMed
55. Kunin CM. Chemoprophylaxis and suppressive therapy in the management of urinary tract infections. J Antimicrob Chemother. 1994;33(suppl A):51-62. PubMed
56. Newman DK, Willson MM. Review of intermittent catheterization and current best practices. Urol Nurs. 2011;31(1):12-48. PubMed
57. Allan GM, Nicolle L. Cranberry for preventing urinary tract infection. Can Fam Physician. 2013;59(4):367. PubMed
58. Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012;10:CD001321. PubMed
59. Wang CH, Fang CC, Chen NC, et al. Cranberry-containing products for prevention of urinary tract infections in susceptible populations: a systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2012;172(13):988-996. PubMed
60. Moore KN, Fader M, Getliffe K. Long-term bladder management by intermittent catheterisation in adults and children. Cochrane Database Syst Rev. 2007(4):CD006008. PubMed
61. Li L, Ye WQ, Ruan H, Yang BY, Zhang SQ. Impact of hydrophilic catheters on urinary tract infections in people with spinal cord injury: systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 2013;94(4):782-787. PubMed
62. Jamison J, Maguire S, McCann J. Catheter policies for management of long term voiding problems in adults with neurogenic bladder disorders. Cochrane Database Syst Rev. 2011(12):CD004375. PubMed
63. Gray M. What nursing interventions reduce the risk of symptomatic urinary tract infections in the patient with an indwelling catheter? J Wound Ostomy Cont Nurs. 2004;31(1):3-13. PubMed
64. Marschall J, Carpenter C, Fowler S, Trautner B. Antibiotic prophylaxis for urinary tract infections after removal of urinary catheter: meta-analysis. BMJ. 2013;346:f3147. PubMed
65. Sinclair L, Hagen S, Cross S. Washout policies in long-term indwelling urinary catheterization in adults: a short version Cochrane review. Neurourol Urodyn. 2011;30(7):1208-1212. PubMed
66. Hunter KF, Bharmal A, Moore KN. Long-term bladder drainage: suprapubic catheter versus other methods: a scoping review. Neurourol Urodyn. 2013;32(7):944-951. PubMed
67. Morton SC, Shekelle PG, Adams JL, et al. Antimicrobial prophylaxis for urinary tract infection in persons with spinal cord dysfunction. Arch Phys Med Rehabil. 2002;83(1):129-138. PubMed
68. Niël-Weise BS, van den Broek PJ, da Silva EM, Silva LA. Urinary catheter policies for long-term bladder drainage. Cochrane Database Syst Rev. 2012(8). PubMed
69. Jepson R, Craig J. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008;10(CD001321). PubMed
70. Avorn J, Monane M, Gurwitz JH, Glynn RJ, Choodnovskiy I, Lipsitz LA. Reduction of bacteriuria and pyuria after ingestion of cranberry juice. JAMA. 1994;271(10):751-754. PubMed
71. Bianco L, Perrelli E, Towle V, Van Ness PH, Juthani-Mehta M. Pilot randomized controlled dosing study of cranberry capsules for reduction of bacteriuria plus pyuria in female nursing home residents. J Am Geriatr Soc. 2012;60(6):1180-1181. PubMed
72. Lin SC, Wang CC, Shih SC, Tjung JJ, Tsou MT, Lin CJ. Prevention of Asymptomatic Bacteriuria with Cranberries and Roselle Juice in Home-care Patients with Long-term Urinary Catheterization. Int J Gerontol. 2014;8(3):152-156.
73. Juthani-Mehta M, Perley L, Chen S, Dziura J, Gupta K. Feasibility of cranberry capsule administration and clean-catch urine collection in long-term care residents. J Am Geriatr Soc. 2010;58(10):2028-2030. PubMed
74. Tully CL, Bastone P, Vaughan J, Ballentine L. Urinary tract infection prophylaxis with cranberry extract in the nursing home setting. J Am Geriatr Soc. 2004;52(4):S206-S206.
75. Woodward N. Use of cranberry extract for the prevention of UTIs in an at-risk population. 41st Annual Wound, Ostomy and Continence Nurses Annual Conference, St. Louis, Missouri, June 6-10, 2009. J Wound Ostomy Continence Nurs. 2009;36(3S):S62-S62.
76. Linsenmeyer TA, Harrison B, Oakley A, Kirshblum S, Stock JA, Millis SR. Evaluation of cranberry supplement for reduction of urinary tract infections in individuals with neurogenic bladders secondary to spinal cord injury. A prospective, double-blinded, placebo-controlled, crossover study. J Spinal Cord Med. 2004;27(1):29-34. PubMed
77. Waites KB, Canupp KC, Armstrong S, DeVivo MJ. Effect of cranberry extract on bacteriuria and pyuria in persons with neurogenic bladder secondary to spinal cord injury. J Spinal Cord Med. 2004;27(1):35-40. PubMed
78. Caljouw MAA, Van Den Hout WB, Putter H, Achterberg WP, Cools HJM, Gussekloo J. Effectiveness of cranberry capsules to prevent urinary tract infections in vulnerable older persons. A double-blind randomized placebo-controlled trial in long-term care facilities. Eur Geriatr Med. 2013;4:S118-S119. PubMed
79. Hout WB, Caljouw MAA, Putter H, Cools HJM, Gussekloo J. Cost-effectiveness of cranberry capsules to prevent urinary tract infection in long-term care facilities: economic evaluation with a randomized controlled trial. J Am Geriatr Soc. 2014;62(1):111-116. PubMed
80. Liu BA, McGeer A, McArthur MA, et al. Effect of multivitamin and mineral supplementation on episodes of infection in nursing home residents: a randomized, placebo-controlled study. J Am Geriatr Soc. 2007;55(1):35-42. PubMed
81. Eriksen B. A randomized, open, parallel-group study on the preventive effect of an estradiol-releasing vaginal ring (Estring) on recurrent urinary tract infections in postmenopausal women. Am J Obstet Gynecol. 1999;180:1072-1079. PubMed
82. Maloney C. Hormone replacement therapy in female nursing home residents with recurrent urinary tract infection. Ann Long-Term Care. 1998;6(3):77-82.
83. Gokula RM, Smith MA, Hickner J. Emergency room staff education and use of a urinary catheter indication sheet improves appropriate use of foley catheters. Am J Infect Control. 2007;35(9):589-593. PubMed
84. Salamon L. Catheter-associated urinary tract infections: a nurse-sensitive indicator in an inpatient rehabilitation program. Rehabil Nurs. 2009;34(6):237-241. PubMed
85. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-326. PubMed
86. American Medical Directors Association (AMDA). Appropriate indications for use of a chronic indwelling catheter in the long-term care setting. Columbia, MD; excerpted from AMDA's Clinical Practice Guideline: Urinary Incontinence. 2005.
87. Rannikko S, Kyllastinen M, Granqvist B. Comparison of long-term indwelling catheters and bed-pads in the treatment of urinary incontinence in elderly patients. J Infect. 1986;12(3):221-227. PubMed
88. Carapeti E, Andrews S, Bentley P. Randomised study of sterile versus non-sterile urethral catheterization. Ann R. Coll Surg Engl. 1996;78(1):59-60. PubMed
89. Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(5):625-663. PubMed
90. Olsen-Scribner RJ, Hayes C, Pottinger P. Sustaining reduction of catheter-associated urinary tract infection (CAUTI)-outcomes after two educational methods in a regional university-affiliated medical center. Am J Infect Control. 2014;1:S22.
91. Duffy LM, Cleary J, Ahern S, et al. Clean intermittent catheterization: safe, cost-effective bladder management for male residents of VA nursing homes. J Am Geriatr Soc. 1995;43(8):865-870. PubMed
92. Joseph C, Jacobson C, Strausbaugh L, Maxwell M, French M, Colling J. Sterile vs clean urinary catheterization. J Am Geriatr Soc. 1991;39(10):1042-1043. PubMed
93. Moore KN, Burt J, Voaklander DC. Intermittent catheterization in the rehabilitation setting: a comparison of clean and sterile technique. Clin Rehabili. 2006;20(6):461-468. PubMed
94. Moore KN, Kelm M, Sinclair O, Cadrain G. Bacteriuria in intermittent catheterization users: the effect of sterile versus clean reused catheters. Rehabil Nurs J. 1993;18(5):306-309. PubMed
95. Niel-Weise BS, van den Broek PJ. Urinary catheter policies for short-term bladder drainage in adults. Cochrane Database Syst Rev. 2005(3):CD004203. PubMed
96. Ouslander JG, Greengold B, Chen S. External catheter use and urinary tract infections among incontinent male nursing home patients. J Am Geriatr Soc. 1987;35(12):1063-1070. PubMed
97. Wyndaele JJ, Brauner A, Geerlings SE, Bela K, Peter T, Bjerklund-Johanson TE. Clean intermittent catheterization and urinary tract infection: review and guide for future research. BJU Int. 2012;110(11 Pt C):E910-917. PubMed
98. Jahn P, Beutner K, Langer G. Types of indwelling urinary catheters for long-term bladder drainage in adults. Cochrane Database Syst Rev. 2012(10):CD004997. PubMed
99. Pickard R, Lam T, Maclennan G, et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: a multicentre randomised controlled trial. Lancet. 2012;380(9857):1927-1935. PubMed
100. Burke JP, Garibaldi RA, Britt MR, Jacobson JA, Conti M, Alling DW. Prevention of catheter-associated urinary tract infections. Efficacy of daily meatal care regimens. Am J Med. 1981;70(3):655-658. PubMed
101. Hagen S, Sinclair L, Cross S. Washout policies in long-term indwelling urinary catheterisation in adults. Cochrane Database Syst Rev. 2010(3). PubMed
102. Moore KN, Hunter KF, McGinnis R, et al. Do catheter washouts extend patency time in long-term indwelling urethral catheters? A randomized controlled trial of acidic washout solution, normal saline washout, or standard care. J Wound Ostomy Continence Nurs. 2009;36(1):82-90. PubMed
103. Muncie HL Jr, Hoopes JM, Damron DJ, Tenney JH, Warren JW. Once-daily irrigation of long-term urethral catheters with normal saline. Lack of benefit. Arch Intern Med. 1989;149(2):441- PubMed
104. Ruwaldt MM. Irrigation of indwelling urinary catheters. Urology. 1983;21(2):127-129. PubMed
105. Palka MA. Evidenced based review of recommendations addressing the frequency of changing long-term indwelling urinary catheters in older adults. Geriatr Nurs. 2014;35(5):357-363. PubMed
106. Warren JW. Catheter-associated urinary tract infections. Infect Dis Clin North Am. 1997;11(3):609-622. PubMed
107. Fryklund B, Haeggman S, Burman LG. Transmission of urinary bacterial strains between patients with indwelling catheters--nursing in the same room and in separate rooms compared. J Hosp Infect. 1997;36(2):147-153. PubMed
108. Anderson RU. Non-sterile intermittent catheterization with antibiotic prophylaxis in the acute spinal cord injured male patient. J Urol. 1980;124(3):392-394. PubMed
109. Anderson RU. Prophylaxis of bacteriuria during intermittent catheterization of the acute neurogenic bladder. J Urol. 1980;123(3):364-366. PubMed
110. Gribble MJ, Puterman ML. Prophylaxis of urinary tract infection in persons with recent spinal cord injury: a prospective, randomized, double-blind, placebo-controlled study of trimethoprim-sulfamethoxazole. Am J Med. 1993;95(2):141-152. PubMed
111. Rutschmann OT, Zwahlen A. Use of norfloxacin for prevention of symptomatic urinary tract infection in chronically catheterized patients. Eur J Clin Microbiol Infect Dis. 1995;14(5):441-444. PubMed
112. Jewes LA, Gillespie WA, Leadbetter A, et al. Bacteriuria and bacteraemia in patients with long-term indwelling catheters--a domiciliary study. J Med Microbiol. 1988;26(1):61-65. PubMed
113. Warren JW, Damron D, Tenney JH, Hoopes JM, Deforge B, Muncie HL, Jr. Fever, bacteremia, and death as complications of bacteriuria in women with long-term urethral catheters. J Infect Dis. 1987;155(6):1151-1158. PubMed
114. Prasad A, Cevallos ME, Riosa S, Darouiche RO, Trautner BW. A bacterial interference strategy for prevention of UTI in persons practicing intermittent catheterization. Spinal Cord. 2009;47(7):565-569. PubMed
© 2017 Society of Hospital Medicine
Inpatient management of opioid use disorder: A review for hospitalists
The United States is experiencing an epidemic of nonmedical opioid use. A concerted effort to better address pain increased the provision of prescription narcotics in the late 1990s and early 2000s.1 Since then, there has been significant growth of opioid use and acorresponding increase in overdose-related deaths.1-3 Public health officials have responded with initiatives to secure the opioid supply and improve outpatient treatment resources. However, the role of hospitalists in addressing opioid use disorder (OUD) is not well established. The inpatient needs for these individuals are complex and require a collaborative approach with input from outpatient clinicians, inpatient clinicians, addiction specialists, social workers, and case managers. Hospitals are often under-resourced to provide such comprehensive services. This frequently results in the hospitalist bearing significant responsibility for inpatient addiction management despite often insufficient addiction education or experience.4,5
Therefore, there is a need for hospitalists to become leaders in the inpatient management of OUD. In this review, we will discuss the hospitalist’s role in the inpatient management of individuals with OUD.
INPATIENT MANAGEMENT OF OPIOID USE DISORDER
Opioid use disorder is a medical illness resulting from neurobiological changes that cause drug tolerance, dependence, and cravings.6 It should be considered a treatable chronic medical condition, comparable to hypertension or diabetes,7 which requires a multifaceted treatment approach, including psychosocial, educational, and medical interventions.
Psychosocial Interventions
Individuals with OUD often have complicated social issues including stigmatization, involvement in the criminal justice system, unemployment, and homelessness,5,8-10 in addition to frequent comorbid mental health issues.11,12 Failure to address social or mental health barriers may lead to a lack of engagement in the treatment of OUD. The long-term management of OUD should involve outpatient psychotherapy and may include individual or group therapy, behavioral therapy, family counseling, or support groups.13 In the inpatient setting, hospitalists should use a collaborative approach to address psychosocial barriers. The authors recommend social work and case management consultations and consideration of psychiatric consultation when appropriate.
Management of Opioid Withdrawal
The prompt recognition and management of withdrawal is essential in hospitalized patients with OUD. The signs and symptoms of withdrawal can be evaluated by using the Clinical Opiate Withdrawal Scale or the Clinical Institute Narcotics Assessment, and may include lacrimation, rhinorrhea, diaphoresis, yawning, restlessness, insomnia, piloerection, myalgia, arthralgia, abdominal pain, nausea, vomiting, and diarrhea.4 Individuals using short-acting opioids, such as oxycodone or heroin, may develop withdrawal symptoms 8 to 12 hours after cessation of the opioid. Symptoms often peak on days 1 to 3 and can last for up to 10 days.14 Individuals taking long-acting opioids, such as methadone, may experience withdrawal symptoms for up to 21 days.14
While the goal of withdrawal treatment is to reduce the uncomfortable symptoms of withdrawal, there may be additional benefits. Around 16% of people who inject drugs will misuse drugs during their hospitalization, and 25% to 30% will be discharged against medical advice.15,16 In hospitalizations when patients are administered methadone for management of withdrawal, there is a significant reduction in discharges against medical advice.16 This may suggest that treatment of withdrawal has the added benefit of preventing discharges against medical advice, and the authors postulate that treatment may decrease surreptitious drug use during hospitalizations, although this has not been studied.
There are 2 approaches to treating opioid withdrawal—opioid substitution treatment and alpha2-adrenergic agonist treatment (Table 1).4,17-20 Of note, opioid substitution treatment, especially when using buprenorphine, should be started only when a patient has at least mild withdrawal symptoms.20
An important exception to the treatment approach listed in Table 1 occurs when a patient is already taking methadone or buprenorphine maintenance therapy. In this circumstance, the outpatient dose should be continued after confirmation of dose and timing of last administration with outpatient clinicians. It is important that clear communication with the patient’s addiction clinician occurs at admission and discharge to prevent an inadvertently duplicated, or missed, dose.
Factors to consider when selecting a withdrawal treatment regimen include comorbidities, anticipated length of stay, anticipated discharge setting, medications, interest in long-term addiction treatment, and other patient-specific factors. In general, treatment with methadone or buprenorphine is preferred, because they are better tolerated and may be more effective than clonidine.21-24 The selection of methadone or buprenorphine is often based on physician or patient preference, presence of contraindications, or formulary restrictions, as they have similar efficacy in the treatment of opioid withdrawal.23 In cases where opioid replacement therapy is contraindicated, such as in an individual who has received naltrexone, clonidine should be used.24
Methadone and buprenorphine are controlled substances that can be prescribed only in outpatients by certified clinicians. Therefore, hospitalists are prohibited from prescribing these medications at discharge for the treatment of OUD. However, inpatient clinicians are exempt from these regulations and may provide both medications for maintenance and withdrawal treatment in the inpatient setting.
As such, while a 10 to 14-day taper may be optimal in preventing relapse and minimizing withdrawal, patients are often medically ready to leave the hospital before their taper is completed. In these cases, a rapid taper over 3 to 5 days can be considered. The disadvantage of a rapid taper is the potential for recrudescence of withdrawal symptoms after discharge. Individuals who do not tolerate a rapid taper should be treated with a slower taper, or transitioned to a clonidine taper.
Many hospitals have protocols to help guide the inpatient management of withdrawal, and in many cases, subspecialist consultation is not necessary. However, the authors recommend involvement of an addiction specialist for patients in whom management of withdrawal may be complicated. Further, we strongly encourage hospitalists to be involved in creation and maintenance of withdrawal treatment protocols.
Medication-Assisted Treatment
It is important to recognize that treatment of withdrawal is not adequate to prevent long-term opioid misuse.25 The optimal long-term management of OUD includes the use of medication-assisted treatment (MAT). The initiation and titration of MAT should always be done in conjunction with an addiction specialist or buprenorphine-waivered physician who will ensure continuation of MAT as an outpatient. This means that, while hospitalists may be critical in facilitating linkage to MAT, in general, they will not have a significant role in the long-term management of OUD. However, hospitalists should be knowledgeable about MAT because it is relatively common and can complicate hospitalizations.
There are two types of MAT: opioid-agonist treatment (OAT) and opioid-antagonist treatment. Opioid-agonist treatment involves the use of methadone, a long-acting opioid agonist, or buprenorphine, a long-acting partial opioid agonist. These medications decrease the amount and severity of cravings and limit the euphoric effects of subsequent opioid use.17 Compared to abstinence-based treatment, OAT has been associated with increased retention in addiction treatment and employment, and reductions in incarceration, human immunodeficiency virus transmission, illicit drug use, opioid-overdose events, and mortality.26-32An alternative to OAT is naltrexone, an opioid antagonist. Naltrexone for OUD is administered as a monthly depot injection that prevents the user from experiencing opioid intoxication or dependence, and is associated with sustained abstinence.17,33,34 The authors strongly recommend that hospitalists discuss the benefits of MAT with hospitalized individuals with OUD. In addition, when appropriate, patients should receive consultation with, or referral to, an addiction specialist.
Adverse Effects of Methadone, Buprenorphine, and Naltrexone
The benefits of MAT are substantial, but there are adverse effects, potential drug-to-drug interactions, and patient-specific characteristics that may impact the inpatient management of individuals on MAT. Selected adverse effects of OAT are listed in Table 1. The adverse effects of naltrexone include nausea, vomiting, and transaminitis. It should also be noted that the initiation of buprenorphine and naltrexone may induce opioid withdrawal when administered to an opioid-dependent patient with recent opioid use. To avoid precipitating withdrawal, buprenorphine should be used only in individuals who have at least mild withdrawal symptoms or have completed detoxification,20 and naltrexone should be used only in patients who have abstained from opioids for at least 7 to 10 days.35
Opioid-agonist treatments are primarily metabolized by the cytochrome P450 3A4 isoenzyme system. Medications that inhibit cytochrome P450 3A4 metabolism such as fluconazole can result in OAT toxicity, while medications that induce cytochrome P450 3A4 metabolism such as dexamethasone can lead to withdrawal symptoms.18 If these interactions are unavoidable, the dose of methadone or buprenorphine should be adjusted to prevent toxicity or withdrawal symptoms. The major drug interaction with naltrexone is ineffective analgesia from opioids.
Another major concern with MAT is the risk of overdose-related deaths. As an opioid agonist, large doses of methadone can result in respiratory depression, while buprenorphine alone, due to its partial agonist effect, is unlikely to result in respiratory depression. When methadone or buprenorphine are taken with other substances that cause respiratory depression, such as benzodiazepines or alcohol, the risk of respiratory depression and overdose is significantly increased.36,37 Overdose-related death with naltrexone usually occurs after the medication has metabolized and results from a loss of opioid tolerance.38
Special Populations
Medication-assisted treatment in individuals with acute pain. Maintenance treatment with OAT does not provide sufficient analgesia to treat episodes of acute pain.39 In patients on methadone maintenance, the maintenance dose should be continued and adjunctive analgesia should be provided with nonopioid analgesics or short-acting opioids.39 The management of acute pain in individuals on buprenorphine maintenance is more complicated since buprenorphine is a partial opioid agonist with high affinity to the opioid receptor, which limits the impact of adjunctive opioids. The options for analgesia in buprenorphine maintenance treatment include 1) continuing daily dosing of buprenorphine and providing nonopioid or opioid analgesics, 2) dividing buprenorphine dosing into a 3 or 4 times a day medication, 3) discontinuing buprenorphine and treating with opioid analgesics, 4) discontinuing buprenorphine and starting methadone with nonopioid or opioid analgesics.39 In cases where buprenorphine is discontinued, it should be restarted before discharge upon resolution of the acute pain episode. An individual with acute pain on naltrexone may require nonopioid analgesia or regional blocks. In these patients, adequate pain control may be challenging and require the consultation of an acute pain specialist.
Pregnant or breastfeeding individuals. Opioid misuse puts the individual and fetus at risk of complications, and abrupt discontinuation can cause preterm labor, fetal distress, or fetal demise.40 The current standard is to initiate methadone in consultation with an addiction specialist.40 There is evidence that buprenorphine can be used during pregnancy; however, buprenorphine-naloxone is discouraged.18,40 Of note, use of OAT in pregnancy can result in neonatal abstinence syndrome, an expected complication that can be managed by a pediatrician.40
Methadone and buprenorphine can be found in low concentrations in breast milk.41 However, according to the Academy of Breastfeeding Medicine’s clinical guidelines, women on stable doses of methadone and buprenorphine should be encouraged to breastfeed.41 Naltrexone enters breast milk and has potential adverse effects for the newborn. Either the mother should discontinue naltrexone or should not breastfeed.35
Treatment of polysubstance misuse. Individuals with OUD may also misuse other substances. The concomitant use of opioids and other central nervous system depressants, such as alcohol and benzodiazepines, is especially worrisome as they can potentiate respiratory depression. The presence of polysubstance misuse does not preclude the use of MAT for the treatment of OUD. In those with comorbid alcohol use disorder, the use of naltrexone may be appealing as it can treat both alcohol use disorder and OUD. Given the complexities of managing polysubstance misuse, addiction specialists should be involved in the care of these patients.42 In addition, patients should be educated on the risks of polysubstance misuse, especially when it involves 2 central nervous system depressants.
Comorbid medical disease. In general, medical comorbidities do not significantly affect the treatment of OUD; however, dysfunction of certain organ systems may necessitate a dose reduction or discontinuation of MAT. Severe liver disease may result in decreased hepatic metabolism of OAT.35,42 Prolonged QTc, or history of arrhythmia, may preclude the use of methadone.17,35,42 In addition, chronic hypercapnic respiratory failure or severe asthma may be contraindications for the use of methadone in an unmonitored setting.35 Kidney failure is not known to be a contraindication to MAT, and there is no consensus on the need for dose reduction of MAT with decreasing glomerular filtration rate; however, some authors recommend a 25% to 50% dose reduction of methadone when the glomerular filtration rate is less than 10 milliliters per minute.43 There is no such recommendation with buprenorphine, although it has not been adequately studied in individuals with renal failure. Close monitoring for evidence of toxicity is prudent in individuals on MAT with acute or chronic renal failure.35
Rural or resource-limited areas. There is a significant shortage of addiction treatment options in many regions of the United States. As of 2012, there were an estimated 2.3 million individuals with OUD; however, more than 1 million of these individuals do not have access to treatment.44 As a result, many addiction treatment programs have wait lists that can last months or even years.45 These shortages are especially apparent in rural areas, where individuals with OUD are particularly reliant upon buprenorphine treatment because of prohibitive travel times to urban-based programs.46 To address this problem, new models of care delivery are being developed, including models incorporating telemedicine to support rural primary care management of OUD.47
The Future of Medication-Assisted Treatment
Currently, MAT is initiated and managed by outpatient addiction specialists. However, evidence supports initiation of MAT as an inpatient.48 A recent study compared inpatient buprenorphine detoxification to inpatient buprenorphine induction, dose stabilization, and postdischarge linkage-of-care to outpatient opioid treatment clinics. Patients who received inpatient buprenorphine initiation and linkage-of-care had improved buprenorphine treatment retention and reported less illicit opioid use.48 The development of partnerships between hospitals, inpatient clinicians, and outpatient addiction specialists is essential and could lead to significant advances in treating hospitalized patients with OUD.
The initiation of MAT in hospitalized patients with immediate linkage-of-care shows great promise; however, at this point, the initiation of MAT should be done only in conjunction with addiction specialists in patients with confirmed outpatient follow-up. In cases where inpatient MAT initiation is pursued, education of staff including nurses and pharmacists is essential.
Harm Reduction Interventions
Ideally, management of OUD results in abstinence from opioid misuse; however, some individuals are not ready for treatment or, despite MAT, have relapses of opioid misuse. Given this, a secondary goal in the management of OUD is the reduction of harm that can result from opioid misuse.
Many individuals inject opioids, which is associated with increased rates of viral and bacterial infections secondary to nonsterile injection practices.49 Educating patients on sterile injection methods (Table 2),50 including the importance of sterile-injecting equipment and water, and cleaning the skin prior to injection, may mitigate the risk of infections and should be provided for all hospitalized people who inject drugs.
Syringe-exchange programs provide sterile-injecting equipment in exchange for used needles, with a goal of increasing access to sterile supplies and removing contaminated syringes from circulation.51 While controversial, these programs may reduce the incidence of human immunodeficiency virus, hepatitis C virus, and hepatitis B virus.51
In addition, syringe-exchange programs often provide addiction treatment referrals, counseling, testing, and prevention education for human immunodeficiency virus, hepatitis C virus, and sexually transmitted infections.49 In the United States, there are 226 programs in 33 states (see https://nasen.org/directory for a list of programs and locations. Inpatient clinicians should provide a list of local resources including syringe-exchange programs at the time of discharge for any people who inject drugs. In addition, individuals with OUD are at increased risk for overdose, especially in the postdischarge setting due to decreased opioid tolerance.52 In 2014, there were 28,647 opioid overdose-related deaths in the United States.2 To address this troubling epidemic, opioid overdose education and naloxone distribution has been championed to educate patients at risk of opioid overdose and potential first responders on how to counteract an overdose by using naloxone, an opioid antagonist (see Table 2 for more information on opioid overdose education). The use of opioid overdose education and naloxone distribution has been observed to reduce opioid overdose-related death rates.53
Hospitalists should provide opioid overdose education and naloxone to all individuals at risk of opioid overdose (including those with OUD), as well as potential first responders where the law allows (more information including individual state laws can be found at http://prescribetoprevent.org).20
Considerations at Discharge
There are a number of considerations for the hospitalist at discharge (see Table 3 for a recommended discharge checklist). In addition, it is important to appreciate, and minimize, the ways that hospitalists contribute to the opioid epidemic. For instance, prescribing opioids at discharge in opioid-naïve patients increases the risk of chronic opioid use.54 It is also essential to recognize that increased doses of opioids are associated with increased rates of opioid overdose-related deaths.55 As such, hospitalists should maximize the use of nonopioid analgesics, prescribe opioids only when necessary, use the smallest effective dose of opioids, limit the number of opioid pills distributed to patients, and check prescription-monitoring programs for evidence of misuse.
CONCLUSION
Hospitalization serves as an important opportunity to address addiction in individuals with OUD. In addressing addiction, hospitalists should identify and intervene on psychosocial and mental health barriers, treat opioid withdrawal, and propagate harm reduction strategies. In addition, there is a growing role for hospitalists to be involved in the initiation of MAT and linkage-of-care to outpatient addiction treatment. If hospitalists become leaders in the inpatient management of OUD, they will significantly improve the care provided to this vulnerable patient population.
Disclosure
The authors report no financial conflicts of interest.
1. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300(22):2613-2620. PubMed
2. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382. PubMed
3. Jones CM, Logan J, Gladden RM, Bohm MK. Vital signs: demographic and substance use trends among heroin users – United States, 2002-2013. MMWR Morb Mortal Wkly Rep. 2015;64(26):719-725. PubMed
4. Haber PS, Demirkol A, Lange K, Murnion B. Management of injecting drug users admitted to hospital. Lancet. 2009;374(9697):1284-1293. PubMed
5. Miller NS, Sheppard LM, Colenda CC, Magen J. Why physicians are unprepared to treat patients who have alcohol- and drug-related disorders. Acad Med. 2001;76(5):410-418. PubMed
6. Cami J, Farré M. Drug addiction. N Engl J Med. 2003;349(10):975-986. PubMed
7. McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance and outcome evaluation. JAMA. 2000;284(13):1689-1695. PubMed
8. Reno RR, Aiken LS. Life activities and life quality of heroin addicts in and out of methadone treatment. Int J Addict. 1993;28(3):211-232. PubMed
9. Maddux JF, Desmond DP. Heroin addicts and nonaddicted brothers. Am J Drug Alcohol Abuse. 1984;10(2):237-248. PubMed
10. Galea S, Vlahov D. Social determinants and the health of drug users; socioeconomic status, homelessness, and incarceration. Public Health Rep. 2002;117(suppl 1):S135-S145. PubMed
11. Brooner RK, King VL, Kidorf M, Schmidt CW Jr, Bigelow GF. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54(1):71-80. PubMed
12.Darke S, Ross J. Polydrug dependence and psychiatric comorbidity among heroin injectors. Drug Alcohol Depend. 1997;48(2):135-141. PubMed
13. Treating opiate addiction, Part II: alternatives to maintenance. Harv Ment Health Lett. 2005;21(7):4-6. PubMed
14. Choo C. Medications used in opioid maintenance treatment. US Pharm. 2009;34:40-53.
15. Marks M, Pollock E, Armstrong M, et al. Needles and the damage done: reasons for admission and financial costs associated with injecting drug use in a Central London teaching hospital. J Infect. 2012;66(1):95-102. PubMed
16. Chan AC, Palepu A, Guh DP, et al. HIV-positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35(1):56-59. PubMed
17. Strain E. Pharmacotherapy for opioid use disorder. In: UpToDate, Herman R, ed. UpToDate, Waltham, MA. https://www.uptodate.com/contents/pharmacotherapy-for-opioid-use-disorderAccessed September 28, 2015.
18. Center for Substance Abuse Treatment. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction. Treatment Improvement Protocol (TIP) Series 40. DHHS Publication No. (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2004. PubMed
19. Weaver MF, Hopper JA. Medically supervised opioid withdrawal during treatment for addiction. In: UpToDate, Herman R, ed. UpToDate, Waltham, MA. https://www.uptodate.com/contents/medically-supervised-opioid-withdrawal-during-treatment-for-addiction Accessed on September 28, 2015.
20. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367. PubMed
21. NICE Clinical Guidelines and National Collaborating Centre for Mental Health. Drug Misuse: Opioid Detoxification. British Psychological Society. 2008. https://www.nice.org.uk/guidance/cg52/evidence/drug-misuse-opioid-detoxification-full-guideline-196515037. Accessed April 12, 2017.
22. Amato L, Davoli M, Minozzi S, Ferroni E, Ali R, Ferri M. Methadone at tapered doses for the management of opioid withdrawal. Cochrane Database Syst Rev. 2013;2:CD003409. PubMed
23. Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2009;3:CD002025. PubMed
24. Gowing L, Farrell M, Ali R, White JM. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2016;5:CD002024. PubMed
25. Gossop M, Stewart D, Brown N, Marsden J. Factors associated with abstinence, lapse or relapse to heroin use after residential treatment: protective effect of coping responses. Addiction. 2002;97(10):1259-1267. PubMed
26. Farrell M, Ward J, Mattick R, et al. Methadone maintenance treatment in opiate dependence: a review. BMJ. 1994;309(6960):997-1001. PubMed
27. Connock M, Juarez Garcia A, Jowett S, et al. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11(9):1–171. PubMed
28. Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;3:CD002209. PubMed
29. Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;2:CD002207. PubMed
30. Gowing LR, Farrell M, Bornemann R, Sullivan LE, Ali RL. Brief report: methadone treatment of injecting opioid users for prevention of HIV infection. J Gen Intern Med. 2006;21(2):193-195. PubMed
31. Nurco DN, Ball JC, Shaffer JW, Hanlon TE. The criminality of narcotic addicts. J Nerv Ment Dis. 1985;173(2):94-102. PubMed
32. Gibson A, Degenhardt L, Mattick RP, Ali R, White J, O’Brien S. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103(3):462-468. PubMed
33. Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev. 2011;4:CD001333. PubMed
34. Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled trial. Lancet. 2011;377(9776):1506-1513. PubMed
35. Substance Abuse and Mental Health Services Administration. Clinical Use of Extended-Release Injectable Naltrexone in the Treatment of Opioid Use Disorder: A Brief Guide. HHS Publication No. 14-4892R. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2015.
36. Caplehorn JR, Drummer OH. Fatal methadone toxicity: signs and circumstances, and the role of benzodiazepines. Aust N Z J Public Health. 2002;26(4):358-362. PubMed
37. Tracqui A, Kintz P, Ludes B. Buprenorphine-related deaths among drug addicts in France: a report on 20 fatalities. J Anal Toxicol. 1998;22(6):430-434. PubMed
38. Kelty E, Hulse G. Examination of mortality rates in a retrospective cohort of patients treated with oral or implant naltrexone for problematic opiate use. Addiction. 2012;107(1):1817-1824. PubMed
39. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144(2):127-134. PubMed
40. ACOG Committee on Health Care for Underserved Women: American Society of Addiction Medicine. ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy. Obstet Gynecol. 2012;119(5):1070-1076. PubMed
41. Reece-Stremtan S, Marinelli KA. ABM clinical protocol #21: guidelines for breastfeeding and substance use or substance use disorder, revised 2015. Breastfeed Med. 2015;10(3):135-141. PubMed
42. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol (TIP) Series 43. HHS Publication No. 12-4214. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2005.
43. Brier ME, Aronoff GR (eds). Drug Prescribing in Renal Failure. 5thedition. Philadelphia, PA: American College of Physicians; 2007.
44. Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health. 2015;105(8):e55-E63. PubMed
45. Sigmon SC. Access to treatment for opioid dependence in rural America: challenges and future directions. JAMA Psychiatry. 2014;71(4):359-360. PubMed
46. Rosenblatt RA, Andrilla CH, Catlin M, Larson EH. Geographic and specialty distribution of US physicians trained to treat opioid use disorder. Ann Fam Med. 2015;13(1):23-26. PubMed
47. Komaromy M, Duhigg D, Metcalf A, et al. Project ECHO (Extension for Community Healthcare Outcomes): A new model for educating primary care providers about treatment of substance use disorders. Subst Abus. 2016;37(1):20-24. PubMed
48. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. PubMed
49. Centers for Disease Control and Prevention (CDC). Syringe exchange programs – United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;59(45):1488-1491. PubMed
50. Harm Reduction Coalition. Getting off right: A safety manual for injection drug users. New York, NY: Harm Reduction Coalition; 1998.
51. Vlahov D, Junge B. The role of needle exchange programs in HIV prevention. Public Health Rep. 1998.113(suppl 1):75-80. PubMed
52. Strang J, McCambridge J, Best D, et al. Loss of tolerance and overdose mortality after inpatient opiate detoxification: follow up study. BMJ. 2003;326(7396):959-960. PubMed
53. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174. PubMed
54. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswnager IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2016;31(5):478-485. PubMed
55. Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92. PubMed
The United States is experiencing an epidemic of nonmedical opioid use. A concerted effort to better address pain increased the provision of prescription narcotics in the late 1990s and early 2000s.1 Since then, there has been significant growth of opioid use and acorresponding increase in overdose-related deaths.1-3 Public health officials have responded with initiatives to secure the opioid supply and improve outpatient treatment resources. However, the role of hospitalists in addressing opioid use disorder (OUD) is not well established. The inpatient needs for these individuals are complex and require a collaborative approach with input from outpatient clinicians, inpatient clinicians, addiction specialists, social workers, and case managers. Hospitals are often under-resourced to provide such comprehensive services. This frequently results in the hospitalist bearing significant responsibility for inpatient addiction management despite often insufficient addiction education or experience.4,5
Therefore, there is a need for hospitalists to become leaders in the inpatient management of OUD. In this review, we will discuss the hospitalist’s role in the inpatient management of individuals with OUD.
INPATIENT MANAGEMENT OF OPIOID USE DISORDER
Opioid use disorder is a medical illness resulting from neurobiological changes that cause drug tolerance, dependence, and cravings.6 It should be considered a treatable chronic medical condition, comparable to hypertension or diabetes,7 which requires a multifaceted treatment approach, including psychosocial, educational, and medical interventions.
Psychosocial Interventions
Individuals with OUD often have complicated social issues including stigmatization, involvement in the criminal justice system, unemployment, and homelessness,5,8-10 in addition to frequent comorbid mental health issues.11,12 Failure to address social or mental health barriers may lead to a lack of engagement in the treatment of OUD. The long-term management of OUD should involve outpatient psychotherapy and may include individual or group therapy, behavioral therapy, family counseling, or support groups.13 In the inpatient setting, hospitalists should use a collaborative approach to address psychosocial barriers. The authors recommend social work and case management consultations and consideration of psychiatric consultation when appropriate.
Management of Opioid Withdrawal
The prompt recognition and management of withdrawal is essential in hospitalized patients with OUD. The signs and symptoms of withdrawal can be evaluated by using the Clinical Opiate Withdrawal Scale or the Clinical Institute Narcotics Assessment, and may include lacrimation, rhinorrhea, diaphoresis, yawning, restlessness, insomnia, piloerection, myalgia, arthralgia, abdominal pain, nausea, vomiting, and diarrhea.4 Individuals using short-acting opioids, such as oxycodone or heroin, may develop withdrawal symptoms 8 to 12 hours after cessation of the opioid. Symptoms often peak on days 1 to 3 and can last for up to 10 days.14 Individuals taking long-acting opioids, such as methadone, may experience withdrawal symptoms for up to 21 days.14
While the goal of withdrawal treatment is to reduce the uncomfortable symptoms of withdrawal, there may be additional benefits. Around 16% of people who inject drugs will misuse drugs during their hospitalization, and 25% to 30% will be discharged against medical advice.15,16 In hospitalizations when patients are administered methadone for management of withdrawal, there is a significant reduction in discharges against medical advice.16 This may suggest that treatment of withdrawal has the added benefit of preventing discharges against medical advice, and the authors postulate that treatment may decrease surreptitious drug use during hospitalizations, although this has not been studied.
There are 2 approaches to treating opioid withdrawal—opioid substitution treatment and alpha2-adrenergic agonist treatment (Table 1).4,17-20 Of note, opioid substitution treatment, especially when using buprenorphine, should be started only when a patient has at least mild withdrawal symptoms.20
An important exception to the treatment approach listed in Table 1 occurs when a patient is already taking methadone or buprenorphine maintenance therapy. In this circumstance, the outpatient dose should be continued after confirmation of dose and timing of last administration with outpatient clinicians. It is important that clear communication with the patient’s addiction clinician occurs at admission and discharge to prevent an inadvertently duplicated, or missed, dose.
Factors to consider when selecting a withdrawal treatment regimen include comorbidities, anticipated length of stay, anticipated discharge setting, medications, interest in long-term addiction treatment, and other patient-specific factors. In general, treatment with methadone or buprenorphine is preferred, because they are better tolerated and may be more effective than clonidine.21-24 The selection of methadone or buprenorphine is often based on physician or patient preference, presence of contraindications, or formulary restrictions, as they have similar efficacy in the treatment of opioid withdrawal.23 In cases where opioid replacement therapy is contraindicated, such as in an individual who has received naltrexone, clonidine should be used.24
Methadone and buprenorphine are controlled substances that can be prescribed only in outpatients by certified clinicians. Therefore, hospitalists are prohibited from prescribing these medications at discharge for the treatment of OUD. However, inpatient clinicians are exempt from these regulations and may provide both medications for maintenance and withdrawal treatment in the inpatient setting.
As such, while a 10 to 14-day taper may be optimal in preventing relapse and minimizing withdrawal, patients are often medically ready to leave the hospital before their taper is completed. In these cases, a rapid taper over 3 to 5 days can be considered. The disadvantage of a rapid taper is the potential for recrudescence of withdrawal symptoms after discharge. Individuals who do not tolerate a rapid taper should be treated with a slower taper, or transitioned to a clonidine taper.
Many hospitals have protocols to help guide the inpatient management of withdrawal, and in many cases, subspecialist consultation is not necessary. However, the authors recommend involvement of an addiction specialist for patients in whom management of withdrawal may be complicated. Further, we strongly encourage hospitalists to be involved in creation and maintenance of withdrawal treatment protocols.
Medication-Assisted Treatment
It is important to recognize that treatment of withdrawal is not adequate to prevent long-term opioid misuse.25 The optimal long-term management of OUD includes the use of medication-assisted treatment (MAT). The initiation and titration of MAT should always be done in conjunction with an addiction specialist or buprenorphine-waivered physician who will ensure continuation of MAT as an outpatient. This means that, while hospitalists may be critical in facilitating linkage to MAT, in general, they will not have a significant role in the long-term management of OUD. However, hospitalists should be knowledgeable about MAT because it is relatively common and can complicate hospitalizations.
There are two types of MAT: opioid-agonist treatment (OAT) and opioid-antagonist treatment. Opioid-agonist treatment involves the use of methadone, a long-acting opioid agonist, or buprenorphine, a long-acting partial opioid agonist. These medications decrease the amount and severity of cravings and limit the euphoric effects of subsequent opioid use.17 Compared to abstinence-based treatment, OAT has been associated with increased retention in addiction treatment and employment, and reductions in incarceration, human immunodeficiency virus transmission, illicit drug use, opioid-overdose events, and mortality.26-32An alternative to OAT is naltrexone, an opioid antagonist. Naltrexone for OUD is administered as a monthly depot injection that prevents the user from experiencing opioid intoxication or dependence, and is associated with sustained abstinence.17,33,34 The authors strongly recommend that hospitalists discuss the benefits of MAT with hospitalized individuals with OUD. In addition, when appropriate, patients should receive consultation with, or referral to, an addiction specialist.
Adverse Effects of Methadone, Buprenorphine, and Naltrexone
The benefits of MAT are substantial, but there are adverse effects, potential drug-to-drug interactions, and patient-specific characteristics that may impact the inpatient management of individuals on MAT. Selected adverse effects of OAT are listed in Table 1. The adverse effects of naltrexone include nausea, vomiting, and transaminitis. It should also be noted that the initiation of buprenorphine and naltrexone may induce opioid withdrawal when administered to an opioid-dependent patient with recent opioid use. To avoid precipitating withdrawal, buprenorphine should be used only in individuals who have at least mild withdrawal symptoms or have completed detoxification,20 and naltrexone should be used only in patients who have abstained from opioids for at least 7 to 10 days.35
Opioid-agonist treatments are primarily metabolized by the cytochrome P450 3A4 isoenzyme system. Medications that inhibit cytochrome P450 3A4 metabolism such as fluconazole can result in OAT toxicity, while medications that induce cytochrome P450 3A4 metabolism such as dexamethasone can lead to withdrawal symptoms.18 If these interactions are unavoidable, the dose of methadone or buprenorphine should be adjusted to prevent toxicity or withdrawal symptoms. The major drug interaction with naltrexone is ineffective analgesia from opioids.
Another major concern with MAT is the risk of overdose-related deaths. As an opioid agonist, large doses of methadone can result in respiratory depression, while buprenorphine alone, due to its partial agonist effect, is unlikely to result in respiratory depression. When methadone or buprenorphine are taken with other substances that cause respiratory depression, such as benzodiazepines or alcohol, the risk of respiratory depression and overdose is significantly increased.36,37 Overdose-related death with naltrexone usually occurs after the medication has metabolized and results from a loss of opioid tolerance.38
Special Populations
Medication-assisted treatment in individuals with acute pain. Maintenance treatment with OAT does not provide sufficient analgesia to treat episodes of acute pain.39 In patients on methadone maintenance, the maintenance dose should be continued and adjunctive analgesia should be provided with nonopioid analgesics or short-acting opioids.39 The management of acute pain in individuals on buprenorphine maintenance is more complicated since buprenorphine is a partial opioid agonist with high affinity to the opioid receptor, which limits the impact of adjunctive opioids. The options for analgesia in buprenorphine maintenance treatment include 1) continuing daily dosing of buprenorphine and providing nonopioid or opioid analgesics, 2) dividing buprenorphine dosing into a 3 or 4 times a day medication, 3) discontinuing buprenorphine and treating with opioid analgesics, 4) discontinuing buprenorphine and starting methadone with nonopioid or opioid analgesics.39 In cases where buprenorphine is discontinued, it should be restarted before discharge upon resolution of the acute pain episode. An individual with acute pain on naltrexone may require nonopioid analgesia or regional blocks. In these patients, adequate pain control may be challenging and require the consultation of an acute pain specialist.
Pregnant or breastfeeding individuals. Opioid misuse puts the individual and fetus at risk of complications, and abrupt discontinuation can cause preterm labor, fetal distress, or fetal demise.40 The current standard is to initiate methadone in consultation with an addiction specialist.40 There is evidence that buprenorphine can be used during pregnancy; however, buprenorphine-naloxone is discouraged.18,40 Of note, use of OAT in pregnancy can result in neonatal abstinence syndrome, an expected complication that can be managed by a pediatrician.40
Methadone and buprenorphine can be found in low concentrations in breast milk.41 However, according to the Academy of Breastfeeding Medicine’s clinical guidelines, women on stable doses of methadone and buprenorphine should be encouraged to breastfeed.41 Naltrexone enters breast milk and has potential adverse effects for the newborn. Either the mother should discontinue naltrexone or should not breastfeed.35
Treatment of polysubstance misuse. Individuals with OUD may also misuse other substances. The concomitant use of opioids and other central nervous system depressants, such as alcohol and benzodiazepines, is especially worrisome as they can potentiate respiratory depression. The presence of polysubstance misuse does not preclude the use of MAT for the treatment of OUD. In those with comorbid alcohol use disorder, the use of naltrexone may be appealing as it can treat both alcohol use disorder and OUD. Given the complexities of managing polysubstance misuse, addiction specialists should be involved in the care of these patients.42 In addition, patients should be educated on the risks of polysubstance misuse, especially when it involves 2 central nervous system depressants.
Comorbid medical disease. In general, medical comorbidities do not significantly affect the treatment of OUD; however, dysfunction of certain organ systems may necessitate a dose reduction or discontinuation of MAT. Severe liver disease may result in decreased hepatic metabolism of OAT.35,42 Prolonged QTc, or history of arrhythmia, may preclude the use of methadone.17,35,42 In addition, chronic hypercapnic respiratory failure or severe asthma may be contraindications for the use of methadone in an unmonitored setting.35 Kidney failure is not known to be a contraindication to MAT, and there is no consensus on the need for dose reduction of MAT with decreasing glomerular filtration rate; however, some authors recommend a 25% to 50% dose reduction of methadone when the glomerular filtration rate is less than 10 milliliters per minute.43 There is no such recommendation with buprenorphine, although it has not been adequately studied in individuals with renal failure. Close monitoring for evidence of toxicity is prudent in individuals on MAT with acute or chronic renal failure.35
Rural or resource-limited areas. There is a significant shortage of addiction treatment options in many regions of the United States. As of 2012, there were an estimated 2.3 million individuals with OUD; however, more than 1 million of these individuals do not have access to treatment.44 As a result, many addiction treatment programs have wait lists that can last months or even years.45 These shortages are especially apparent in rural areas, where individuals with OUD are particularly reliant upon buprenorphine treatment because of prohibitive travel times to urban-based programs.46 To address this problem, new models of care delivery are being developed, including models incorporating telemedicine to support rural primary care management of OUD.47
The Future of Medication-Assisted Treatment
Currently, MAT is initiated and managed by outpatient addiction specialists. However, evidence supports initiation of MAT as an inpatient.48 A recent study compared inpatient buprenorphine detoxification to inpatient buprenorphine induction, dose stabilization, and postdischarge linkage-of-care to outpatient opioid treatment clinics. Patients who received inpatient buprenorphine initiation and linkage-of-care had improved buprenorphine treatment retention and reported less illicit opioid use.48 The development of partnerships between hospitals, inpatient clinicians, and outpatient addiction specialists is essential and could lead to significant advances in treating hospitalized patients with OUD.
The initiation of MAT in hospitalized patients with immediate linkage-of-care shows great promise; however, at this point, the initiation of MAT should be done only in conjunction with addiction specialists in patients with confirmed outpatient follow-up. In cases where inpatient MAT initiation is pursued, education of staff including nurses and pharmacists is essential.
Harm Reduction Interventions
Ideally, management of OUD results in abstinence from opioid misuse; however, some individuals are not ready for treatment or, despite MAT, have relapses of opioid misuse. Given this, a secondary goal in the management of OUD is the reduction of harm that can result from opioid misuse.
Many individuals inject opioids, which is associated with increased rates of viral and bacterial infections secondary to nonsterile injection practices.49 Educating patients on sterile injection methods (Table 2),50 including the importance of sterile-injecting equipment and water, and cleaning the skin prior to injection, may mitigate the risk of infections and should be provided for all hospitalized people who inject drugs.
Syringe-exchange programs provide sterile-injecting equipment in exchange for used needles, with a goal of increasing access to sterile supplies and removing contaminated syringes from circulation.51 While controversial, these programs may reduce the incidence of human immunodeficiency virus, hepatitis C virus, and hepatitis B virus.51
In addition, syringe-exchange programs often provide addiction treatment referrals, counseling, testing, and prevention education for human immunodeficiency virus, hepatitis C virus, and sexually transmitted infections.49 In the United States, there are 226 programs in 33 states (see https://nasen.org/directory for a list of programs and locations. Inpatient clinicians should provide a list of local resources including syringe-exchange programs at the time of discharge for any people who inject drugs. In addition, individuals with OUD are at increased risk for overdose, especially in the postdischarge setting due to decreased opioid tolerance.52 In 2014, there were 28,647 opioid overdose-related deaths in the United States.2 To address this troubling epidemic, opioid overdose education and naloxone distribution has been championed to educate patients at risk of opioid overdose and potential first responders on how to counteract an overdose by using naloxone, an opioid antagonist (see Table 2 for more information on opioid overdose education). The use of opioid overdose education and naloxone distribution has been observed to reduce opioid overdose-related death rates.53
Hospitalists should provide opioid overdose education and naloxone to all individuals at risk of opioid overdose (including those with OUD), as well as potential first responders where the law allows (more information including individual state laws can be found at http://prescribetoprevent.org).20
Considerations at Discharge
There are a number of considerations for the hospitalist at discharge (see Table 3 for a recommended discharge checklist). In addition, it is important to appreciate, and minimize, the ways that hospitalists contribute to the opioid epidemic. For instance, prescribing opioids at discharge in opioid-naïve patients increases the risk of chronic opioid use.54 It is also essential to recognize that increased doses of opioids are associated with increased rates of opioid overdose-related deaths.55 As such, hospitalists should maximize the use of nonopioid analgesics, prescribe opioids only when necessary, use the smallest effective dose of opioids, limit the number of opioid pills distributed to patients, and check prescription-monitoring programs for evidence of misuse.
CONCLUSION
Hospitalization serves as an important opportunity to address addiction in individuals with OUD. In addressing addiction, hospitalists should identify and intervene on psychosocial and mental health barriers, treat opioid withdrawal, and propagate harm reduction strategies. In addition, there is a growing role for hospitalists to be involved in the initiation of MAT and linkage-of-care to outpatient addiction treatment. If hospitalists become leaders in the inpatient management of OUD, they will significantly improve the care provided to this vulnerable patient population.
Disclosure
The authors report no financial conflicts of interest.
The United States is experiencing an epidemic of nonmedical opioid use. A concerted effort to better address pain increased the provision of prescription narcotics in the late 1990s and early 2000s.1 Since then, there has been significant growth of opioid use and acorresponding increase in overdose-related deaths.1-3 Public health officials have responded with initiatives to secure the opioid supply and improve outpatient treatment resources. However, the role of hospitalists in addressing opioid use disorder (OUD) is not well established. The inpatient needs for these individuals are complex and require a collaborative approach with input from outpatient clinicians, inpatient clinicians, addiction specialists, social workers, and case managers. Hospitals are often under-resourced to provide such comprehensive services. This frequently results in the hospitalist bearing significant responsibility for inpatient addiction management despite often insufficient addiction education or experience.4,5
Therefore, there is a need for hospitalists to become leaders in the inpatient management of OUD. In this review, we will discuss the hospitalist’s role in the inpatient management of individuals with OUD.
INPATIENT MANAGEMENT OF OPIOID USE DISORDER
Opioid use disorder is a medical illness resulting from neurobiological changes that cause drug tolerance, dependence, and cravings.6 It should be considered a treatable chronic medical condition, comparable to hypertension or diabetes,7 which requires a multifaceted treatment approach, including psychosocial, educational, and medical interventions.
Psychosocial Interventions
Individuals with OUD often have complicated social issues including stigmatization, involvement in the criminal justice system, unemployment, and homelessness,5,8-10 in addition to frequent comorbid mental health issues.11,12 Failure to address social or mental health barriers may lead to a lack of engagement in the treatment of OUD. The long-term management of OUD should involve outpatient psychotherapy and may include individual or group therapy, behavioral therapy, family counseling, or support groups.13 In the inpatient setting, hospitalists should use a collaborative approach to address psychosocial barriers. The authors recommend social work and case management consultations and consideration of psychiatric consultation when appropriate.
Management of Opioid Withdrawal
The prompt recognition and management of withdrawal is essential in hospitalized patients with OUD. The signs and symptoms of withdrawal can be evaluated by using the Clinical Opiate Withdrawal Scale or the Clinical Institute Narcotics Assessment, and may include lacrimation, rhinorrhea, diaphoresis, yawning, restlessness, insomnia, piloerection, myalgia, arthralgia, abdominal pain, nausea, vomiting, and diarrhea.4 Individuals using short-acting opioids, such as oxycodone or heroin, may develop withdrawal symptoms 8 to 12 hours after cessation of the opioid. Symptoms often peak on days 1 to 3 and can last for up to 10 days.14 Individuals taking long-acting opioids, such as methadone, may experience withdrawal symptoms for up to 21 days.14
While the goal of withdrawal treatment is to reduce the uncomfortable symptoms of withdrawal, there may be additional benefits. Around 16% of people who inject drugs will misuse drugs during their hospitalization, and 25% to 30% will be discharged against medical advice.15,16 In hospitalizations when patients are administered methadone for management of withdrawal, there is a significant reduction in discharges against medical advice.16 This may suggest that treatment of withdrawal has the added benefit of preventing discharges against medical advice, and the authors postulate that treatment may decrease surreptitious drug use during hospitalizations, although this has not been studied.
There are 2 approaches to treating opioid withdrawal—opioid substitution treatment and alpha2-adrenergic agonist treatment (Table 1).4,17-20 Of note, opioid substitution treatment, especially when using buprenorphine, should be started only when a patient has at least mild withdrawal symptoms.20
An important exception to the treatment approach listed in Table 1 occurs when a patient is already taking methadone or buprenorphine maintenance therapy. In this circumstance, the outpatient dose should be continued after confirmation of dose and timing of last administration with outpatient clinicians. It is important that clear communication with the patient’s addiction clinician occurs at admission and discharge to prevent an inadvertently duplicated, or missed, dose.
Factors to consider when selecting a withdrawal treatment regimen include comorbidities, anticipated length of stay, anticipated discharge setting, medications, interest in long-term addiction treatment, and other patient-specific factors. In general, treatment with methadone or buprenorphine is preferred, because they are better tolerated and may be more effective than clonidine.21-24 The selection of methadone or buprenorphine is often based on physician or patient preference, presence of contraindications, or formulary restrictions, as they have similar efficacy in the treatment of opioid withdrawal.23 In cases where opioid replacement therapy is contraindicated, such as in an individual who has received naltrexone, clonidine should be used.24
Methadone and buprenorphine are controlled substances that can be prescribed only in outpatients by certified clinicians. Therefore, hospitalists are prohibited from prescribing these medications at discharge for the treatment of OUD. However, inpatient clinicians are exempt from these regulations and may provide both medications for maintenance and withdrawal treatment in the inpatient setting.
As such, while a 10 to 14-day taper may be optimal in preventing relapse and minimizing withdrawal, patients are often medically ready to leave the hospital before their taper is completed. In these cases, a rapid taper over 3 to 5 days can be considered. The disadvantage of a rapid taper is the potential for recrudescence of withdrawal symptoms after discharge. Individuals who do not tolerate a rapid taper should be treated with a slower taper, or transitioned to a clonidine taper.
Many hospitals have protocols to help guide the inpatient management of withdrawal, and in many cases, subspecialist consultation is not necessary. However, the authors recommend involvement of an addiction specialist for patients in whom management of withdrawal may be complicated. Further, we strongly encourage hospitalists to be involved in creation and maintenance of withdrawal treatment protocols.
Medication-Assisted Treatment
It is important to recognize that treatment of withdrawal is not adequate to prevent long-term opioid misuse.25 The optimal long-term management of OUD includes the use of medication-assisted treatment (MAT). The initiation and titration of MAT should always be done in conjunction with an addiction specialist or buprenorphine-waivered physician who will ensure continuation of MAT as an outpatient. This means that, while hospitalists may be critical in facilitating linkage to MAT, in general, they will not have a significant role in the long-term management of OUD. However, hospitalists should be knowledgeable about MAT because it is relatively common and can complicate hospitalizations.
There are two types of MAT: opioid-agonist treatment (OAT) and opioid-antagonist treatment. Opioid-agonist treatment involves the use of methadone, a long-acting opioid agonist, or buprenorphine, a long-acting partial opioid agonist. These medications decrease the amount and severity of cravings and limit the euphoric effects of subsequent opioid use.17 Compared to abstinence-based treatment, OAT has been associated with increased retention in addiction treatment and employment, and reductions in incarceration, human immunodeficiency virus transmission, illicit drug use, opioid-overdose events, and mortality.26-32An alternative to OAT is naltrexone, an opioid antagonist. Naltrexone for OUD is administered as a monthly depot injection that prevents the user from experiencing opioid intoxication or dependence, and is associated with sustained abstinence.17,33,34 The authors strongly recommend that hospitalists discuss the benefits of MAT with hospitalized individuals with OUD. In addition, when appropriate, patients should receive consultation with, or referral to, an addiction specialist.
Adverse Effects of Methadone, Buprenorphine, and Naltrexone
The benefits of MAT are substantial, but there are adverse effects, potential drug-to-drug interactions, and patient-specific characteristics that may impact the inpatient management of individuals on MAT. Selected adverse effects of OAT are listed in Table 1. The adverse effects of naltrexone include nausea, vomiting, and transaminitis. It should also be noted that the initiation of buprenorphine and naltrexone may induce opioid withdrawal when administered to an opioid-dependent patient with recent opioid use. To avoid precipitating withdrawal, buprenorphine should be used only in individuals who have at least mild withdrawal symptoms or have completed detoxification,20 and naltrexone should be used only in patients who have abstained from opioids for at least 7 to 10 days.35
Opioid-agonist treatments are primarily metabolized by the cytochrome P450 3A4 isoenzyme system. Medications that inhibit cytochrome P450 3A4 metabolism such as fluconazole can result in OAT toxicity, while medications that induce cytochrome P450 3A4 metabolism such as dexamethasone can lead to withdrawal symptoms.18 If these interactions are unavoidable, the dose of methadone or buprenorphine should be adjusted to prevent toxicity or withdrawal symptoms. The major drug interaction with naltrexone is ineffective analgesia from opioids.
Another major concern with MAT is the risk of overdose-related deaths. As an opioid agonist, large doses of methadone can result in respiratory depression, while buprenorphine alone, due to its partial agonist effect, is unlikely to result in respiratory depression. When methadone or buprenorphine are taken with other substances that cause respiratory depression, such as benzodiazepines or alcohol, the risk of respiratory depression and overdose is significantly increased.36,37 Overdose-related death with naltrexone usually occurs after the medication has metabolized and results from a loss of opioid tolerance.38
Special Populations
Medication-assisted treatment in individuals with acute pain. Maintenance treatment with OAT does not provide sufficient analgesia to treat episodes of acute pain.39 In patients on methadone maintenance, the maintenance dose should be continued and adjunctive analgesia should be provided with nonopioid analgesics or short-acting opioids.39 The management of acute pain in individuals on buprenorphine maintenance is more complicated since buprenorphine is a partial opioid agonist with high affinity to the opioid receptor, which limits the impact of adjunctive opioids. The options for analgesia in buprenorphine maintenance treatment include 1) continuing daily dosing of buprenorphine and providing nonopioid or opioid analgesics, 2) dividing buprenorphine dosing into a 3 or 4 times a day medication, 3) discontinuing buprenorphine and treating with opioid analgesics, 4) discontinuing buprenorphine and starting methadone with nonopioid or opioid analgesics.39 In cases where buprenorphine is discontinued, it should be restarted before discharge upon resolution of the acute pain episode. An individual with acute pain on naltrexone may require nonopioid analgesia or regional blocks. In these patients, adequate pain control may be challenging and require the consultation of an acute pain specialist.
Pregnant or breastfeeding individuals. Opioid misuse puts the individual and fetus at risk of complications, and abrupt discontinuation can cause preterm labor, fetal distress, or fetal demise.40 The current standard is to initiate methadone in consultation with an addiction specialist.40 There is evidence that buprenorphine can be used during pregnancy; however, buprenorphine-naloxone is discouraged.18,40 Of note, use of OAT in pregnancy can result in neonatal abstinence syndrome, an expected complication that can be managed by a pediatrician.40
Methadone and buprenorphine can be found in low concentrations in breast milk.41 However, according to the Academy of Breastfeeding Medicine’s clinical guidelines, women on stable doses of methadone and buprenorphine should be encouraged to breastfeed.41 Naltrexone enters breast milk and has potential adverse effects for the newborn. Either the mother should discontinue naltrexone or should not breastfeed.35
Treatment of polysubstance misuse. Individuals with OUD may also misuse other substances. The concomitant use of opioids and other central nervous system depressants, such as alcohol and benzodiazepines, is especially worrisome as they can potentiate respiratory depression. The presence of polysubstance misuse does not preclude the use of MAT for the treatment of OUD. In those with comorbid alcohol use disorder, the use of naltrexone may be appealing as it can treat both alcohol use disorder and OUD. Given the complexities of managing polysubstance misuse, addiction specialists should be involved in the care of these patients.42 In addition, patients should be educated on the risks of polysubstance misuse, especially when it involves 2 central nervous system depressants.
Comorbid medical disease. In general, medical comorbidities do not significantly affect the treatment of OUD; however, dysfunction of certain organ systems may necessitate a dose reduction or discontinuation of MAT. Severe liver disease may result in decreased hepatic metabolism of OAT.35,42 Prolonged QTc, or history of arrhythmia, may preclude the use of methadone.17,35,42 In addition, chronic hypercapnic respiratory failure or severe asthma may be contraindications for the use of methadone in an unmonitored setting.35 Kidney failure is not known to be a contraindication to MAT, and there is no consensus on the need for dose reduction of MAT with decreasing glomerular filtration rate; however, some authors recommend a 25% to 50% dose reduction of methadone when the glomerular filtration rate is less than 10 milliliters per minute.43 There is no such recommendation with buprenorphine, although it has not been adequately studied in individuals with renal failure. Close monitoring for evidence of toxicity is prudent in individuals on MAT with acute or chronic renal failure.35
Rural or resource-limited areas. There is a significant shortage of addiction treatment options in many regions of the United States. As of 2012, there were an estimated 2.3 million individuals with OUD; however, more than 1 million of these individuals do not have access to treatment.44 As a result, many addiction treatment programs have wait lists that can last months or even years.45 These shortages are especially apparent in rural areas, where individuals with OUD are particularly reliant upon buprenorphine treatment because of prohibitive travel times to urban-based programs.46 To address this problem, new models of care delivery are being developed, including models incorporating telemedicine to support rural primary care management of OUD.47
The Future of Medication-Assisted Treatment
Currently, MAT is initiated and managed by outpatient addiction specialists. However, evidence supports initiation of MAT as an inpatient.48 A recent study compared inpatient buprenorphine detoxification to inpatient buprenorphine induction, dose stabilization, and postdischarge linkage-of-care to outpatient opioid treatment clinics. Patients who received inpatient buprenorphine initiation and linkage-of-care had improved buprenorphine treatment retention and reported less illicit opioid use.48 The development of partnerships between hospitals, inpatient clinicians, and outpatient addiction specialists is essential and could lead to significant advances in treating hospitalized patients with OUD.
The initiation of MAT in hospitalized patients with immediate linkage-of-care shows great promise; however, at this point, the initiation of MAT should be done only in conjunction with addiction specialists in patients with confirmed outpatient follow-up. In cases where inpatient MAT initiation is pursued, education of staff including nurses and pharmacists is essential.
Harm Reduction Interventions
Ideally, management of OUD results in abstinence from opioid misuse; however, some individuals are not ready for treatment or, despite MAT, have relapses of opioid misuse. Given this, a secondary goal in the management of OUD is the reduction of harm that can result from opioid misuse.
Many individuals inject opioids, which is associated with increased rates of viral and bacterial infections secondary to nonsterile injection practices.49 Educating patients on sterile injection methods (Table 2),50 including the importance of sterile-injecting equipment and water, and cleaning the skin prior to injection, may mitigate the risk of infections and should be provided for all hospitalized people who inject drugs.
Syringe-exchange programs provide sterile-injecting equipment in exchange for used needles, with a goal of increasing access to sterile supplies and removing contaminated syringes from circulation.51 While controversial, these programs may reduce the incidence of human immunodeficiency virus, hepatitis C virus, and hepatitis B virus.51
In addition, syringe-exchange programs often provide addiction treatment referrals, counseling, testing, and prevention education for human immunodeficiency virus, hepatitis C virus, and sexually transmitted infections.49 In the United States, there are 226 programs in 33 states (see https://nasen.org/directory for a list of programs and locations. Inpatient clinicians should provide a list of local resources including syringe-exchange programs at the time of discharge for any people who inject drugs. In addition, individuals with OUD are at increased risk for overdose, especially in the postdischarge setting due to decreased opioid tolerance.52 In 2014, there were 28,647 opioid overdose-related deaths in the United States.2 To address this troubling epidemic, opioid overdose education and naloxone distribution has been championed to educate patients at risk of opioid overdose and potential first responders on how to counteract an overdose by using naloxone, an opioid antagonist (see Table 2 for more information on opioid overdose education). The use of opioid overdose education and naloxone distribution has been observed to reduce opioid overdose-related death rates.53
Hospitalists should provide opioid overdose education and naloxone to all individuals at risk of opioid overdose (including those with OUD), as well as potential first responders where the law allows (more information including individual state laws can be found at http://prescribetoprevent.org).20
Considerations at Discharge
There are a number of considerations for the hospitalist at discharge (see Table 3 for a recommended discharge checklist). In addition, it is important to appreciate, and minimize, the ways that hospitalists contribute to the opioid epidemic. For instance, prescribing opioids at discharge in opioid-naïve patients increases the risk of chronic opioid use.54 It is also essential to recognize that increased doses of opioids are associated with increased rates of opioid overdose-related deaths.55 As such, hospitalists should maximize the use of nonopioid analgesics, prescribe opioids only when necessary, use the smallest effective dose of opioids, limit the number of opioid pills distributed to patients, and check prescription-monitoring programs for evidence of misuse.
CONCLUSION
Hospitalization serves as an important opportunity to address addiction in individuals with OUD. In addressing addiction, hospitalists should identify and intervene on psychosocial and mental health barriers, treat opioid withdrawal, and propagate harm reduction strategies. In addition, there is a growing role for hospitalists to be involved in the initiation of MAT and linkage-of-care to outpatient addiction treatment. If hospitalists become leaders in the inpatient management of OUD, they will significantly improve the care provided to this vulnerable patient population.
Disclosure
The authors report no financial conflicts of interest.
1. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300(22):2613-2620. PubMed
2. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382. PubMed
3. Jones CM, Logan J, Gladden RM, Bohm MK. Vital signs: demographic and substance use trends among heroin users – United States, 2002-2013. MMWR Morb Mortal Wkly Rep. 2015;64(26):719-725. PubMed
4. Haber PS, Demirkol A, Lange K, Murnion B. Management of injecting drug users admitted to hospital. Lancet. 2009;374(9697):1284-1293. PubMed
5. Miller NS, Sheppard LM, Colenda CC, Magen J. Why physicians are unprepared to treat patients who have alcohol- and drug-related disorders. Acad Med. 2001;76(5):410-418. PubMed
6. Cami J, Farré M. Drug addiction. N Engl J Med. 2003;349(10):975-986. PubMed
7. McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance and outcome evaluation. JAMA. 2000;284(13):1689-1695. PubMed
8. Reno RR, Aiken LS. Life activities and life quality of heroin addicts in and out of methadone treatment. Int J Addict. 1993;28(3):211-232. PubMed
9. Maddux JF, Desmond DP. Heroin addicts and nonaddicted brothers. Am J Drug Alcohol Abuse. 1984;10(2):237-248. PubMed
10. Galea S, Vlahov D. Social determinants and the health of drug users; socioeconomic status, homelessness, and incarceration. Public Health Rep. 2002;117(suppl 1):S135-S145. PubMed
11. Brooner RK, King VL, Kidorf M, Schmidt CW Jr, Bigelow GF. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54(1):71-80. PubMed
12.Darke S, Ross J. Polydrug dependence and psychiatric comorbidity among heroin injectors. Drug Alcohol Depend. 1997;48(2):135-141. PubMed
13. Treating opiate addiction, Part II: alternatives to maintenance. Harv Ment Health Lett. 2005;21(7):4-6. PubMed
14. Choo C. Medications used in opioid maintenance treatment. US Pharm. 2009;34:40-53.
15. Marks M, Pollock E, Armstrong M, et al. Needles and the damage done: reasons for admission and financial costs associated with injecting drug use in a Central London teaching hospital. J Infect. 2012;66(1):95-102. PubMed
16. Chan AC, Palepu A, Guh DP, et al. HIV-positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35(1):56-59. PubMed
17. Strain E. Pharmacotherapy for opioid use disorder. In: UpToDate, Herman R, ed. UpToDate, Waltham, MA. https://www.uptodate.com/contents/pharmacotherapy-for-opioid-use-disorderAccessed September 28, 2015.
18. Center for Substance Abuse Treatment. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction. Treatment Improvement Protocol (TIP) Series 40. DHHS Publication No. (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2004. PubMed
19. Weaver MF, Hopper JA. Medically supervised opioid withdrawal during treatment for addiction. In: UpToDate, Herman R, ed. UpToDate, Waltham, MA. https://www.uptodate.com/contents/medically-supervised-opioid-withdrawal-during-treatment-for-addiction Accessed on September 28, 2015.
20. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367. PubMed
21. NICE Clinical Guidelines and National Collaborating Centre for Mental Health. Drug Misuse: Opioid Detoxification. British Psychological Society. 2008. https://www.nice.org.uk/guidance/cg52/evidence/drug-misuse-opioid-detoxification-full-guideline-196515037. Accessed April 12, 2017.
22. Amato L, Davoli M, Minozzi S, Ferroni E, Ali R, Ferri M. Methadone at tapered doses for the management of opioid withdrawal. Cochrane Database Syst Rev. 2013;2:CD003409. PubMed
23. Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2009;3:CD002025. PubMed
24. Gowing L, Farrell M, Ali R, White JM. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2016;5:CD002024. PubMed
25. Gossop M, Stewart D, Brown N, Marsden J. Factors associated with abstinence, lapse or relapse to heroin use after residential treatment: protective effect of coping responses. Addiction. 2002;97(10):1259-1267. PubMed
26. Farrell M, Ward J, Mattick R, et al. Methadone maintenance treatment in opiate dependence: a review. BMJ. 1994;309(6960):997-1001. PubMed
27. Connock M, Juarez Garcia A, Jowett S, et al. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11(9):1–171. PubMed
28. Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;3:CD002209. PubMed
29. Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;2:CD002207. PubMed
30. Gowing LR, Farrell M, Bornemann R, Sullivan LE, Ali RL. Brief report: methadone treatment of injecting opioid users for prevention of HIV infection. J Gen Intern Med. 2006;21(2):193-195. PubMed
31. Nurco DN, Ball JC, Shaffer JW, Hanlon TE. The criminality of narcotic addicts. J Nerv Ment Dis. 1985;173(2):94-102. PubMed
32. Gibson A, Degenhardt L, Mattick RP, Ali R, White J, O’Brien S. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103(3):462-468. PubMed
33. Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev. 2011;4:CD001333. PubMed
34. Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled trial. Lancet. 2011;377(9776):1506-1513. PubMed
35. Substance Abuse and Mental Health Services Administration. Clinical Use of Extended-Release Injectable Naltrexone in the Treatment of Opioid Use Disorder: A Brief Guide. HHS Publication No. 14-4892R. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2015.
36. Caplehorn JR, Drummer OH. Fatal methadone toxicity: signs and circumstances, and the role of benzodiazepines. Aust N Z J Public Health. 2002;26(4):358-362. PubMed
37. Tracqui A, Kintz P, Ludes B. Buprenorphine-related deaths among drug addicts in France: a report on 20 fatalities. J Anal Toxicol. 1998;22(6):430-434. PubMed
38. Kelty E, Hulse G. Examination of mortality rates in a retrospective cohort of patients treated with oral or implant naltrexone for problematic opiate use. Addiction. 2012;107(1):1817-1824. PubMed
39. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144(2):127-134. PubMed
40. ACOG Committee on Health Care for Underserved Women: American Society of Addiction Medicine. ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy. Obstet Gynecol. 2012;119(5):1070-1076. PubMed
41. Reece-Stremtan S, Marinelli KA. ABM clinical protocol #21: guidelines for breastfeeding and substance use or substance use disorder, revised 2015. Breastfeed Med. 2015;10(3):135-141. PubMed
42. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol (TIP) Series 43. HHS Publication No. 12-4214. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2005.
43. Brier ME, Aronoff GR (eds). Drug Prescribing in Renal Failure. 5thedition. Philadelphia, PA: American College of Physicians; 2007.
44. Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health. 2015;105(8):e55-E63. PubMed
45. Sigmon SC. Access to treatment for opioid dependence in rural America: challenges and future directions. JAMA Psychiatry. 2014;71(4):359-360. PubMed
46. Rosenblatt RA, Andrilla CH, Catlin M, Larson EH. Geographic and specialty distribution of US physicians trained to treat opioid use disorder. Ann Fam Med. 2015;13(1):23-26. PubMed
47. Komaromy M, Duhigg D, Metcalf A, et al. Project ECHO (Extension for Community Healthcare Outcomes): A new model for educating primary care providers about treatment of substance use disorders. Subst Abus. 2016;37(1):20-24. PubMed
48. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. PubMed
49. Centers for Disease Control and Prevention (CDC). Syringe exchange programs – United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;59(45):1488-1491. PubMed
50. Harm Reduction Coalition. Getting off right: A safety manual for injection drug users. New York, NY: Harm Reduction Coalition; 1998.
51. Vlahov D, Junge B. The role of needle exchange programs in HIV prevention. Public Health Rep. 1998.113(suppl 1):75-80. PubMed
52. Strang J, McCambridge J, Best D, et al. Loss of tolerance and overdose mortality after inpatient opiate detoxification: follow up study. BMJ. 2003;326(7396):959-960. PubMed
53. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174. PubMed
54. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswnager IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2016;31(5):478-485. PubMed
55. Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92. PubMed
1. Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300(22):2613-2620. PubMed
2. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382. PubMed
3. Jones CM, Logan J, Gladden RM, Bohm MK. Vital signs: demographic and substance use trends among heroin users – United States, 2002-2013. MMWR Morb Mortal Wkly Rep. 2015;64(26):719-725. PubMed
4. Haber PS, Demirkol A, Lange K, Murnion B. Management of injecting drug users admitted to hospital. Lancet. 2009;374(9697):1284-1293. PubMed
5. Miller NS, Sheppard LM, Colenda CC, Magen J. Why physicians are unprepared to treat patients who have alcohol- and drug-related disorders. Acad Med. 2001;76(5):410-418. PubMed
6. Cami J, Farré M. Drug addiction. N Engl J Med. 2003;349(10):975-986. PubMed
7. McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance and outcome evaluation. JAMA. 2000;284(13):1689-1695. PubMed
8. Reno RR, Aiken LS. Life activities and life quality of heroin addicts in and out of methadone treatment. Int J Addict. 1993;28(3):211-232. PubMed
9. Maddux JF, Desmond DP. Heroin addicts and nonaddicted brothers. Am J Drug Alcohol Abuse. 1984;10(2):237-248. PubMed
10. Galea S, Vlahov D. Social determinants and the health of drug users; socioeconomic status, homelessness, and incarceration. Public Health Rep. 2002;117(suppl 1):S135-S145. PubMed
11. Brooner RK, King VL, Kidorf M, Schmidt CW Jr, Bigelow GF. Psychiatric and substance use comorbidity among treatment-seeking opioid abusers. Arch Gen Psychiatry. 1997;54(1):71-80. PubMed
12.Darke S, Ross J. Polydrug dependence and psychiatric comorbidity among heroin injectors. Drug Alcohol Depend. 1997;48(2):135-141. PubMed
13. Treating opiate addiction, Part II: alternatives to maintenance. Harv Ment Health Lett. 2005;21(7):4-6. PubMed
14. Choo C. Medications used in opioid maintenance treatment. US Pharm. 2009;34:40-53.
15. Marks M, Pollock E, Armstrong M, et al. Needles and the damage done: reasons for admission and financial costs associated with injecting drug use in a Central London teaching hospital. J Infect. 2012;66(1):95-102. PubMed
16. Chan AC, Palepu A, Guh DP, et al. HIV-positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35(1):56-59. PubMed
17. Strain E. Pharmacotherapy for opioid use disorder. In: UpToDate, Herman R, ed. UpToDate, Waltham, MA. https://www.uptodate.com/contents/pharmacotherapy-for-opioid-use-disorderAccessed September 28, 2015.
18. Center for Substance Abuse Treatment. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction. Treatment Improvement Protocol (TIP) Series 40. DHHS Publication No. (SMA) 04-3939. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2004. PubMed
19. Weaver MF, Hopper JA. Medically supervised opioid withdrawal during treatment for addiction. In: UpToDate, Herman R, ed. UpToDate, Waltham, MA. https://www.uptodate.com/contents/medically-supervised-opioid-withdrawal-during-treatment-for-addiction Accessed on September 28, 2015.
20. Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) national practice guideline for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367. PubMed
21. NICE Clinical Guidelines and National Collaborating Centre for Mental Health. Drug Misuse: Opioid Detoxification. British Psychological Society. 2008. https://www.nice.org.uk/guidance/cg52/evidence/drug-misuse-opioid-detoxification-full-guideline-196515037. Accessed April 12, 2017.
22. Amato L, Davoli M, Minozzi S, Ferroni E, Ali R, Ferri M. Methadone at tapered doses for the management of opioid withdrawal. Cochrane Database Syst Rev. 2013;2:CD003409. PubMed
23. Gowing L, Ali R, White J. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2009;3:CD002025. PubMed
24. Gowing L, Farrell M, Ali R, White JM. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev. 2016;5:CD002024. PubMed
25. Gossop M, Stewart D, Brown N, Marsden J. Factors associated with abstinence, lapse or relapse to heroin use after residential treatment: protective effect of coping responses. Addiction. 2002;97(10):1259-1267. PubMed
26. Farrell M, Ward J, Mattick R, et al. Methadone maintenance treatment in opiate dependence: a review. BMJ. 1994;309(6960):997-1001. PubMed
27. Connock M, Juarez Garcia A, Jowett S, et al. Methadone and buprenorphine for the management of opioid dependence: a systematic review and economic evaluation. Health Technol Assess. 2007;11(9):1–171. PubMed
28. Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;3:CD002209. PubMed
29. Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;2:CD002207. PubMed
30. Gowing LR, Farrell M, Bornemann R, Sullivan LE, Ali RL. Brief report: methadone treatment of injecting opioid users for prevention of HIV infection. J Gen Intern Med. 2006;21(2):193-195. PubMed
31. Nurco DN, Ball JC, Shaffer JW, Hanlon TE. The criminality of narcotic addicts. J Nerv Ment Dis. 1985;173(2):94-102. PubMed
32. Gibson A, Degenhardt L, Mattick RP, Ali R, White J, O’Brien S. Exposure to opioid maintenance treatment reduces long-term mortality. Addiction. 2008;103(3):462-468. PubMed
33. Minozzi S, Amato L, Vecchi S, Davoli M, Kirchmayer U, Verster A. Oral naltrexone maintenance treatment for opioid dependence. Cochrane Database Syst Rev. 2011;4:CD001333. PubMed
34. Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled trial. Lancet. 2011;377(9776):1506-1513. PubMed
35. Substance Abuse and Mental Health Services Administration. Clinical Use of Extended-Release Injectable Naltrexone in the Treatment of Opioid Use Disorder: A Brief Guide. HHS Publication No. 14-4892R. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2015.
36. Caplehorn JR, Drummer OH. Fatal methadone toxicity: signs and circumstances, and the role of benzodiazepines. Aust N Z J Public Health. 2002;26(4):358-362. PubMed
37. Tracqui A, Kintz P, Ludes B. Buprenorphine-related deaths among drug addicts in France: a report on 20 fatalities. J Anal Toxicol. 1998;22(6):430-434. PubMed
38. Kelty E, Hulse G. Examination of mortality rates in a retrospective cohort of patients treated with oral or implant naltrexone for problematic opiate use. Addiction. 2012;107(1):1817-1824. PubMed
39. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144(2):127-134. PubMed
40. ACOG Committee on Health Care for Underserved Women: American Society of Addiction Medicine. ACOG Committee Opinion No. 524: Opioid abuse, dependence, and addiction in pregnancy. Obstet Gynecol. 2012;119(5):1070-1076. PubMed
41. Reece-Stremtan S, Marinelli KA. ABM clinical protocol #21: guidelines for breastfeeding and substance use or substance use disorder, revised 2015. Breastfeed Med. 2015;10(3):135-141. PubMed
42. Center for Substance Abuse Treatment. Medication-Assisted Treatment for Opioid Addiction in Opioid Treatment Programs. Treatment Improvement Protocol (TIP) Series 43. HHS Publication No. 12-4214. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2005.
43. Brier ME, Aronoff GR (eds). Drug Prescribing in Renal Failure. 5thedition. Philadelphia, PA: American College of Physicians; 2007.
44. Jones CM, Campopiano M, Baldwin G, McCance-Katz E. National and state treatment need and capacity for opioid agonist medication-assisted treatment. Am J Public Health. 2015;105(8):e55-E63. PubMed
45. Sigmon SC. Access to treatment for opioid dependence in rural America: challenges and future directions. JAMA Psychiatry. 2014;71(4):359-360. PubMed
46. Rosenblatt RA, Andrilla CH, Catlin M, Larson EH. Geographic and specialty distribution of US physicians trained to treat opioid use disorder. Ann Fam Med. 2015;13(1):23-26. PubMed
47. Komaromy M, Duhigg D, Metcalf A, et al. Project ECHO (Extension for Community Healthcare Outcomes): A new model for educating primary care providers about treatment of substance use disorders. Subst Abus. 2016;37(1):20-24. PubMed
48. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med. 2014;174(8):1369-1376. PubMed
49. Centers for Disease Control and Prevention (CDC). Syringe exchange programs – United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;59(45):1488-1491. PubMed
50. Harm Reduction Coalition. Getting off right: A safety manual for injection drug users. New York, NY: Harm Reduction Coalition; 1998.
51. Vlahov D, Junge B. The role of needle exchange programs in HIV prevention. Public Health Rep. 1998.113(suppl 1):75-80. PubMed
52. Strang J, McCambridge J, Best D, et al. Loss of tolerance and overdose mortality after inpatient opiate detoxification: follow up study. BMJ. 2003;326(7396):959-960. PubMed
53. Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174. PubMed
54. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswnager IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. 2016;31(5):478-485. PubMed
55. Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85-92. PubMed
© 2017 Society of Hospital Medicine
Acute pain management in hospitalized adult patients with opioid dependence: a narrative review and guide for clinicians
Up to 40% of Americans experience chronic pain of some kind.1 In the United States, opioid analgesics are the most prescribed class of medications,2 with 245 million prescriptions filled in 2014 alone. Thirty-five percent of these prescriptions were for long-term therapy.3 It is now apparent that opioid pain medication use presents serious risks. In 2014, 10.3 million persons reported using prescription opioids for nonmedical reasons.4 Between 1999 and 2014, more than 165,000 people in the United States died of overdose related to opioid medication.5 In addition, heroin use in the United States has increased over the past decade.6 Opioid agonist maintenance therapy is also increasingly used to treat patients with opioid use disorder.
Given the prevalence of opioid use in the United States, it is important for hospitalists to be able to appropriately and safely manage acute pain in patients who have been exposed long-term to opioids, whether it is therapeutic or non-medical in origin. Although nonopioid medications and nondrug treatments are essential components of managing all acute pain, opioids continue to be the mainstay of treatment for severe acute pain in both opioid-naïve and opioid-dependent patients.
Given the paucity of published trials meeting the typical criteria, we did not perform a structured meta-analysis but, instead, a case-based narrative review of the relevant published literature. Our goal in performing this review is to guide hospitalists in the appropriate and safe use of opioid analgesics in treating acute pain in hospitalized patients who are opioid-dependent.
DEFINITIONS
When managing acute pain in patients with opioid dependence it is important to have a clear understanding of the definitions related to opioid use. Addiction, physical dependence and tolerance have been defined by a joint consensus statement of the American Society of Addiction Medicine, American Academy of Pain Medicine, and American Pain Society7: Addiction is a primary, chronic, biological disease, with genetic, psychosocial and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving.
Physical Dependence is a state of adaptation that is manifested by a drug class specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist.
Tolerance is the state of adaptation in which exposure to a drug induces changes that result in a diminution of one or more of the drug’s effects over time.
Opioid use disorder (OUD) is defined as a problematic pattern of opioid use leading to clinically significant impairment or distress with symptoms including a strong desire for opioids, inability to control or reduce use of opioids, continued use despite adverse consequences, and development of tolerance and withdrawal symptoms.8
PATHOPHYSIOLOGY
Physical dependence and tolerance are common consequences of long-term opioid use. In contrast, OUD has been reported to affect only 2% to 6% of individuals exposed to opioids.9 The underlying mechanisms that lead an individual to abuse or become addicted to opioids largely due to the effects opioids have on endogenous μ-opioid receptors. As analgesics, opioids exert their effects by binding primarily to these μ-opioid receptors, with a large concentration in the brain regions regulating pain perception.10,11 There is also a large concentration of μ-opioid receptors in the brain reward regions, leading to perceptions of pleasure and euphoria. Repeated administration of opioids conditions the brain to a learned association between receiving the opiate and euphoria.12,13 This association becomes stronger as the frequency and duration of administration increases over time, ultimately leading to the desire or craving of the opioid’s effect.
The effect of tolerance also contributes to the pathophysiology of opioid abuse as it leads to a decrease in opioid potency with repeated administration.14-16 To achieve analgesia as well as the reward effect, opioid dosage and/or frequency must be increased, strengthening the association between receipt of opioid and reward. Tolerance to the reward effect occurs quickly, whereas tolerance to respiratory depression occurs much more slowly.17 This mismatch in tolerance of effect may lead to increase in opioid doses to maintain analgesia or euphoria, and also places patients at a higher risk of overdose.18
ACUTE PAIN MANAGEMENT
Clinical Example: Heroin User
A 47-year-old man is admitted with fever, chills, and severe mid-back pain and receives a diagnosis of sepsis. The patient admits to using intravenous heroin 500 mg (five 100 mg “bags”) on a daily basis. He is admitted, fluid resuscitated and started on broad spectrum antibiotics. Blood cultures quickly grow Staphylococcus aureus. Magnetic resonance imaging of the spine shows cervical vertebral osteomyelitis. On examination, the patient is diaphoretic and complains of diffuse myalgias and diarrhea. The patient’s back pain is so severe that he cannot ambulate. What is the best way to manage this patient’s acute pain and communicate with him about his pain management?
Managing acute pain in a patient using heroin can be challenging for many reasons. First, both physicians and pharmacists report a lack of confidence in their ability to prescribe opioids safely or to treat patients with a history of opioid abuse.19 Second, there is a paucity of evidence in treating acute pain in heroin users. Finally, due to the clandestine manufacturing of illicit drugs, the actual purity of the drug is often unknown making it difficult to assess the dose of opioids in heroin users. Drug Enforcement Agency seizure data indicate a wide range of heroin purity: 30% to 70%.20
In the hospital setting, acute pain is often undertreated in patients with a history of active opioid abuse. This may be due to providers’ misconceptions regarding pain and behavior in opioid addicts, including worrying that the patient’s pain is exaggerated in order to obtain drugs, thinking that a regular opioid habit eliminates pain, believing that opioid therapy is not effective in drug addicts, or worrying that prescribing opioids will exacerbate drug addiction.21 Data demonstrates that the presence of opioid addiction seems to worsen the experience of acute pain.22 These patients also often have a higher tolerance and thus require higher dosages and more frequent dosing of opioids to adequately treat their pain.23
Converting daily heroin use to morphine equivalents is necessary to establish a baseline analgesic requirement and to prevent withdrawal. It is challenging to convert illicit heroin to morphine equivalents however, as one must take into account the wide variation in purity and understand that the stated use of heroin (e.g. 500 mg daily) reflects weight and not dosage of heroin.20
In these patients, treatment of acute pain should be individualized according to presenting illness and comorbidities. Previous data and an average purity of 40% suggest that the parenteral morphine equivalent to a bag of heroin (100 mg) is 15 to 30 mg.20,24,25 Common equianalgesic doses of opioid medications are listed in Table 1. Because of increased tolerance, the frequency of administration should be shortened, from every 4 hours to every 2 or 3 hours. Except for a shorter onset of action, there has not been a difference shown in superiority between oral and parenteral routes of administration. Finally, patients should receive both long-acting basal and short-acting as-needed analgesics based on their daily use of opioids.23
In our clinical example, IV heroin 500 mg daily converts to parenteral morphine 75 to 150 mg every 24 hours. We recommend initiating IV morphine 10 mg every 3 hours as needed for pain and withdrawal symptoms, with early reassessment regarding need for a higher dose or a shorter frequency based on symptoms. Nonopioid analgesics should also be administered with the goal of decreasing the opioid requirement. As soon as possible, the patient should be changed to oral basal and short-acting opioids as needed for breakthrough pain. The appropriate dose of long acting basal analgesia can be determined the following day based on the patient’s total daily dose (TDD) of opioids. An example of converting from intravenous PRN morphine to oral basal and short acting opioids is shown in Table 2.
In communicating with a patient with opioid-use disorder with acute pain, it is best to outline the pain management plan at admission including: the plan to effectively treat the patient’s acute pain, prevent opioid withdrawal symptoms, change to oral opioid analgesics as soon as possible, discussion of non-opioid and non-drug treatments, reinforcement that opioids will be tapered as the acute pain episode resolves, and a detailed plan for discharge Later in this article, we describe discharge planning.
Clinical Example: Patient on Chronic Opioid Therapy for Chronic Pain
A 64 year-old man was involved in a motorcycle accident and suffered a right distal tibia-fibula fracture and several broken ribs with a secondary pneumothorax. The patient’s past medical history is significant for chronic low back pain for which he states he takes morphine sustained release 30 mg orally every 8 hours and morphine immediate release 15 mg orally four times daily for breakthrough pain. The patient states his pain is much worse than prior to the accident. Trauma surgery requests recommendations on appropriate pain management. What is the best way to manage this patient’s acute pain and communicate with him about his pain management?
When treating acute pain in patients with chronic pain on opioid therapy, it is vital to establish the patient’s baseline pain level and to accurately reconcile the patient’s outpatient daily opioid use. The patient’s prescription record should be verified in the state’s prescription drug monitoring program. On admission, a urine drug test should be obtained to assess for use of other potential illicit substances (eg, cocaine). Patients who test positive for illicit substances are at high risk for a substance use disorder. Management and discharge plans should be as outlined in the above case. It is important to know that the first-tier immunoassay urine toxicology screens used by hospitals test for natural opioids (morphine, codeine, heroin). Semi-synthetic (example, oxycodone) or synthetic (example, fentanyl) opioids are unlikely to be detected and thus the urine drug screen may not be helpful to determine adherence to certain prescription opioids. Gas chromatography/mass spectrometry is the most sensitive and specific type of urine screen and can be ordered to confirm a prescribed opioid if needed.26
Pain management should begin with calculating the TDD of oral opioids that the patient was taking prior to admission, and converting to morphine equivalents. For moderate acute pain, TDD can be increased by 25% to 50%. The revised TDD can then be prescribed as a long-acting opioid every 8 to 12 hours to provide basal analgesia. The dose of additional immediate-release medication available throughout the day to manage breakthrough pain is determined by dividing the new TDD into every 3 to 4 hours as-needed dosing (Table 2).
If severe pain is anticipated, patient controlled analgesia (PCA) is an effective alternative to deliver opioids. The use of PCA allows self-titration, on demand analgesia, and minimizes the likelihood of under-dosing the patient.27 The revised TDD is a useful starting point when calculating the PCA dosage regimen. Ideally, the revised TDD should be prescribed as a long acting oral opioid medication every 8 to 12 hours for basal analgesia, with PCA administered as an as-needed bolus. If a patient cannot tolerate oral medications, PCA can provide continuous infusion of medication to provide basal analgesia, though the risk of oversedation and respiratory depression is increased.28
For our clinical example, we recommend increasing the preadmission TDD of opioids (180 mg morphine equivalents) by 25% (225 mg) and administering as morphine 75 mg sustained-release every 8 hours to provide baseline analgesia and prevent withdrawal symptoms. The acute pain can be managed by initiating morphine PCA without continuous infusion at 0.5 mg bolus every 8 minutes as needed for breakthrough pain or oral morphine 30 mg immediate-release tablets every 3 hours as needed for pain. The patient should be assessed frequently, and naloxone kept readily available. In addition, nonopioid and nondrug treatments should be optimized.
When communicating with patients with underlying chronic pain on chronic opioid therapy, it is important to discuss the treatment plan early, including addressing that they will likely not be pain free during their hospitalization, but rather goals of pain relief and improved function should be established. The plan to change to oral opioid analgesics as soon as possible and importance of multi-modal treatment should be emphasized. The patient should be informed that medication changes are for the short-term only and that the underlying chronic pain will likely remain unchanged.
Clinical Example: Patient on Medication-Assisted Therapy
A 42-year-old woman presents with acute epigastric pain and receives a diagnosis of acute gallstone pancreatitis. She states that her pain is very severe and appears uncomfortable. Her past medical history is significant for heroin addiction, but she has been successfully treated for opioid-use disorder with buprenorphine 16 mg daily for the past three years. What is the best way to manage this patient’s acute pain and communicate with her about her pain management?
Medication-assisted therapies (MATs) for treatment of opioid abuse, which include methadone and buprenorphine (Table 3), have been shown to be effective in helping patients recover in opioid-use disorder, are cost-effective and reduce the risk of opioid overdose.29 However, treatment for acute pain in patients who are receiving methadone or buprenorphine MAT is a challenge because of pharmacokinetic changes that occur with prolonged use. It is important to know that patients receiving opioid agonist MAT are usually treated with 1 dose every 24 to 48 hours and do not receive sustained analgesia.30
In the case of patients on methadone as MAT, the methadone should be continued at the prescribed daily dose and additional short-acting opioid analgesics given to provide appropriate pain relief.27,31 Because of opioid tolerance, patients receiving MAT often require increased and more frequent doses of short-acting opioid analgesics to achieve adequate pain control.
Buprenorphine is a mu-opioid receptor partial agonist. The partial agonist properties of buprenorphine result in a “ceiling effect” that limits maximal analgesic and euphoric potential. Buprenorphine’s high affinity for the mu receptor also may result in competition with full opioid agonist analgesics, creating a challenge in treating acute pain. Because of the erratic dissociation of buprenorphine from the mu receptor, naloxone should be available and patients should be frequently monitored when the two agents are administered together. Recommendations regarding acute pain management in patients being treated with buprenorphine are largely based on expert opinion. Treatment options include32-34:
- Continue maintenance therapy with buprenorphine and treat acute pain with short acting opioid agonists. Higher doses of opioid agonists and more frequent dosing may be needed to provide adequate pain relief since they compete with buprenorphine at the mu receptor. Opioids with higher affinity for the mu receptor (morphine, hydromorphone, fentanyl) may be more efficacious.
- Discontinue buprenorphine and treat the patient with scheduled full opioid analgesics, titrating the dose initially to try to avoid withdrawal and then to provide pain relief. The partial agonism of the mu-receptor from buprenorphine and the blockade of other opioids can persist for as long as 72 hours. During this period, close monitoring and keeping naloxone available are important. When acute pain resolves, discontinue full opioid agonist therapy and resume buprenorphine using an induction protocol.
For our clinical example, we recommend continuing buprenorphine at 16 mg daily, optimizing nonopioid treatment strategies, and using a higher dose parenteral full opioid agonist every 3 hours as needed to achieve adequate analgesia. The patient should be frequently monitored for adverse effects, and naloxone kept available. Full opioid analgesics should be tapered and discontinued as the acute pain resolves. The patient should be reassured that there is no evidence that using opioids to treat acute pain episodes increases the risk of relapse and that untreated acute pain is a more likely trigger for relapse. The patient’s buprenorphine provider should be contacted at admission to verify dose as well as at discharge.
DISCHARGE PLANNING AND MANAGEMENT
Early discharge planning is essential for appropriate and safe management of acute pain in hospitalized patients with opioid dependence. The major goals are to treat acute pain effectively, improve function, and return care to the patient’s usual treating physician or methadone clinic. Patients on chronic opioid therapy often have a written opioid treatment agreement specifying only 1 prescriber. Therefore, verbal communication with the patient’s authorized prescriber at admission and at discharge is essential, particularly given that the discharge summary may not be available at follow-up. Additional or higher doses of opioids should not be prescribed at discharge unless discussed with the patient’s authorized prescriber. If it is believed necessary to provide opioid medication at discharge it should only be provided for a short period: 3 to 7 days.35 Patients with OUD should be referred for addiction treatment, including MAT, and should be educated on harm-reduction strategies, including safe injecting, obtaining clean needles, and recognizing, avoiding, and treating opioid overdose. Prescribing intranasal naloxone should be strongly considered for patients with OUD and for patients who are taking more than 50 mg oral morphine equivalents for chronic pain.34
CONCLUSION
Management of acute pain in opioid-dependent patients is a complex and increasingly common problem encountered by hospitalists. In addition, given the OUD epidemic in the United States, safe opioid prescribing has become a paramount goal for all physicians. Although acute pain management will be individualized and will encompass clinical judgment, this review provides an evidence-based guide to effective and safe acute pain management and optimal opioid prescribing for hospitalized opioid-dependent patients.
Disclosure
Nothing to report.
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education and Research. Washington, DC: National Academies Press; 2011. PubMed
2. Centers for Disease Control and Prevention. FastStats. Therapeutic drug use. 2014. http://www.cdc.gov/nchs/faststats/drug-use-therapeutic.htm. Accessed August 23, 2016.
3. National Institute on Drug Abuse. The Latest Prescription Trends for Controlled Prescription Drugs. http://www.drugabuse.gov/news-events/meetings-events/2015/09/latest-prescription-trends-controlled-prescription-drugs. Published September 1, 2015. Accessed August 23, 2016.
4. Center for Behavioral Health Statistics and Quality. 2014 National Survey on Drug Use and Health: Detailed Tables. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
5. Centers for Disease Control and Prevention. Multiple cause of death data. https://wonder.cdc.gov/mcd.html. Accessed September 9, 2016.
6. Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374(2):154-163. PubMed
7. American Academy of Pain Medicine, American Pain Society, American Society of Addiction Medicine. https://www.naabt.org/documents/APS_consenus_document.pdf. Published 2001. Accessed August 23, 2016.
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
9. Christie MJ. Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol. 2008;154(2):384-396. PubMed
10. McNicol E, Carr DB. Pharmacological treatment of pain. In: McCarberg B, Passik SD, eds. Expert Guide to Pain Management. Philadelphia, PA: American College of Physicians; 2005:145-178.
11. Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker, JM. Endogenous opioids: biology and function. Annu Rev Neurosci. 1984;7:223-255. PubMed
12. Miguez G, Laborda MA, Miller RR. Classical conditioning and pain: conditioned analgesia and hyperalgesia. Acta Psychol (Amst). 2014;145:10-20. PubMed
13. Ewan EE, Martin TJ. Analgesics as reinforcers with chronic pain: evidence from operant studies. Neurosci Lett. 2013;557(pt A):60-64. PubMed
14. Mehta V, Langford R. Acute pain management in opioid dependent patients. Rev Pain. 2009;3(2):10-14. PubMed
15. Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med. 2016;374(13):1253-1263. PubMed
16. Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81(1):299-343. PubMed
17. Ling GS, Paul D, Simantov R, Pasternak GW. Differential development of acute tolerance to analgesia, respiratory depression, gastrointestinal transit and hormone release in a morphine infusion model. Life Sci. 1989;45(18):1627-1636. PubMed
18. Pattinson KT. Opioids and the control of respiration. Br J Anaesth. 2008;100(6):747-758. PubMed
19. Hagemeier NE, Gray JA, Pack RP. Prescription drug abuse: a comparison of prescriber and pharmacist perspectives. Subst Use Misuse. 2013;48(9):761-768. PubMed
20. Drug Enforcement Administration, US Department of Justice. National Heroin Threat Assessment Summary. Washington, DC: Drug Enforcement Administration, US Dept of Justice; 2015. DEA intelligence report DEA-DCT-DIR-039-15.
21. Laroche F, Rostaing S, Aubrun F, Perrot S. Pain management in heroin and cocaine users. Joint Bone Spine. 2012;79(5):446-450. PubMed
22. Savage SR, Schofferman J. Pharmacological therapies of pain in drug and alcohol addictions. In: Miller N, Gold M, eds. Pharmacological Therapies for Drug and Alcohol Addictions. New York, NY: Dekker; 1995:373-409.
23. Vadivelu N, Lumermann L, Zhu R, Kodumudi G, Elhassan AO, Kaye AD. Pain control in the presence of drug addiction. Curr Pain Headache Rep. 2016;20(5):35. PubMed
24. Johns AR, Gossop M. Prescribing methadone for the opiate addict: a problem of dosage conversion. Drug Alcohol Depend. 1985;16(1):61-66. PubMed
25. Halbsguth U, Rentsch KM, Eich-Höchli D, Diterich I, Fattinger K. Oral diacetylmorphine (heroin) yields greater morphine bioavailability than oral morphine: bioavailability related to dosage and prior opioid exposure. Br J Clin Pharmacol. 2008;66(6):781-791. PubMed
26. Milone MC. Laboratory testing for prescription opioids. J Med Toxicol. 2012;8(4):408-416. PubMed
27. Huxtable CA, Roberts LJ, Somogyi AA, MacIntyre PE. Acute pain management in opioid-tolerant patients: a growing challenge. Anaesth Intensive Care. 2011;39(5):804-823. PubMed
28. George JA, Lin EE, Hanna MN, et al. The effect of intravenous opioid patient-controlled analgesia with and without background infusion on respiratory depression: a meta-analysis. J Opioid Manag. 2010;6(1):47-54. PubMed
29. Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063-2066. PubMed
30. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144(2):127-134. PubMed
31. Mehta V, Langford RM. Acute pain management for opioid dependent patients. Anaesthesia. 2006;61(3):269-276. PubMed
32. Sen S, Arulkumar S, Cornett EM, et al. New pain management options for the surgical patient on methadone and buprenorphine. Curr Pain Headache Rep. 2016;20(3):16. PubMed
33. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645. PubMed
34. Fanucchi L, Lofwall MR. Putting parity into practice—integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;375(9):811-813. PubMed
35. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49. PubMed
Up to 40% of Americans experience chronic pain of some kind.1 In the United States, opioid analgesics are the most prescribed class of medications,2 with 245 million prescriptions filled in 2014 alone. Thirty-five percent of these prescriptions were for long-term therapy.3 It is now apparent that opioid pain medication use presents serious risks. In 2014, 10.3 million persons reported using prescription opioids for nonmedical reasons.4 Between 1999 and 2014, more than 165,000 people in the United States died of overdose related to opioid medication.5 In addition, heroin use in the United States has increased over the past decade.6 Opioid agonist maintenance therapy is also increasingly used to treat patients with opioid use disorder.
Given the prevalence of opioid use in the United States, it is important for hospitalists to be able to appropriately and safely manage acute pain in patients who have been exposed long-term to opioids, whether it is therapeutic or non-medical in origin. Although nonopioid medications and nondrug treatments are essential components of managing all acute pain, opioids continue to be the mainstay of treatment for severe acute pain in both opioid-naïve and opioid-dependent patients.
Given the paucity of published trials meeting the typical criteria, we did not perform a structured meta-analysis but, instead, a case-based narrative review of the relevant published literature. Our goal in performing this review is to guide hospitalists in the appropriate and safe use of opioid analgesics in treating acute pain in hospitalized patients who are opioid-dependent.
DEFINITIONS
When managing acute pain in patients with opioid dependence it is important to have a clear understanding of the definitions related to opioid use. Addiction, physical dependence and tolerance have been defined by a joint consensus statement of the American Society of Addiction Medicine, American Academy of Pain Medicine, and American Pain Society7: Addiction is a primary, chronic, biological disease, with genetic, psychosocial and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving.
Physical Dependence is a state of adaptation that is manifested by a drug class specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist.
Tolerance is the state of adaptation in which exposure to a drug induces changes that result in a diminution of one or more of the drug’s effects over time.
Opioid use disorder (OUD) is defined as a problematic pattern of opioid use leading to clinically significant impairment or distress with symptoms including a strong desire for opioids, inability to control or reduce use of opioids, continued use despite adverse consequences, and development of tolerance and withdrawal symptoms.8
PATHOPHYSIOLOGY
Physical dependence and tolerance are common consequences of long-term opioid use. In contrast, OUD has been reported to affect only 2% to 6% of individuals exposed to opioids.9 The underlying mechanisms that lead an individual to abuse or become addicted to opioids largely due to the effects opioids have on endogenous μ-opioid receptors. As analgesics, opioids exert their effects by binding primarily to these μ-opioid receptors, with a large concentration in the brain regions regulating pain perception.10,11 There is also a large concentration of μ-opioid receptors in the brain reward regions, leading to perceptions of pleasure and euphoria. Repeated administration of opioids conditions the brain to a learned association between receiving the opiate and euphoria.12,13 This association becomes stronger as the frequency and duration of administration increases over time, ultimately leading to the desire or craving of the opioid’s effect.
The effect of tolerance also contributes to the pathophysiology of opioid abuse as it leads to a decrease in opioid potency with repeated administration.14-16 To achieve analgesia as well as the reward effect, opioid dosage and/or frequency must be increased, strengthening the association between receipt of opioid and reward. Tolerance to the reward effect occurs quickly, whereas tolerance to respiratory depression occurs much more slowly.17 This mismatch in tolerance of effect may lead to increase in opioid doses to maintain analgesia or euphoria, and also places patients at a higher risk of overdose.18
ACUTE PAIN MANAGEMENT
Clinical Example: Heroin User
A 47-year-old man is admitted with fever, chills, and severe mid-back pain and receives a diagnosis of sepsis. The patient admits to using intravenous heroin 500 mg (five 100 mg “bags”) on a daily basis. He is admitted, fluid resuscitated and started on broad spectrum antibiotics. Blood cultures quickly grow Staphylococcus aureus. Magnetic resonance imaging of the spine shows cervical vertebral osteomyelitis. On examination, the patient is diaphoretic and complains of diffuse myalgias and diarrhea. The patient’s back pain is so severe that he cannot ambulate. What is the best way to manage this patient’s acute pain and communicate with him about his pain management?
Managing acute pain in a patient using heroin can be challenging for many reasons. First, both physicians and pharmacists report a lack of confidence in their ability to prescribe opioids safely or to treat patients with a history of opioid abuse.19 Second, there is a paucity of evidence in treating acute pain in heroin users. Finally, due to the clandestine manufacturing of illicit drugs, the actual purity of the drug is often unknown making it difficult to assess the dose of opioids in heroin users. Drug Enforcement Agency seizure data indicate a wide range of heroin purity: 30% to 70%.20
In the hospital setting, acute pain is often undertreated in patients with a history of active opioid abuse. This may be due to providers’ misconceptions regarding pain and behavior in opioid addicts, including worrying that the patient’s pain is exaggerated in order to obtain drugs, thinking that a regular opioid habit eliminates pain, believing that opioid therapy is not effective in drug addicts, or worrying that prescribing opioids will exacerbate drug addiction.21 Data demonstrates that the presence of opioid addiction seems to worsen the experience of acute pain.22 These patients also often have a higher tolerance and thus require higher dosages and more frequent dosing of opioids to adequately treat their pain.23
Converting daily heroin use to morphine equivalents is necessary to establish a baseline analgesic requirement and to prevent withdrawal. It is challenging to convert illicit heroin to morphine equivalents however, as one must take into account the wide variation in purity and understand that the stated use of heroin (e.g. 500 mg daily) reflects weight and not dosage of heroin.20
In these patients, treatment of acute pain should be individualized according to presenting illness and comorbidities. Previous data and an average purity of 40% suggest that the parenteral morphine equivalent to a bag of heroin (100 mg) is 15 to 30 mg.20,24,25 Common equianalgesic doses of opioid medications are listed in Table 1. Because of increased tolerance, the frequency of administration should be shortened, from every 4 hours to every 2 or 3 hours. Except for a shorter onset of action, there has not been a difference shown in superiority between oral and parenteral routes of administration. Finally, patients should receive both long-acting basal and short-acting as-needed analgesics based on their daily use of opioids.23
In our clinical example, IV heroin 500 mg daily converts to parenteral morphine 75 to 150 mg every 24 hours. We recommend initiating IV morphine 10 mg every 3 hours as needed for pain and withdrawal symptoms, with early reassessment regarding need for a higher dose or a shorter frequency based on symptoms. Nonopioid analgesics should also be administered with the goal of decreasing the opioid requirement. As soon as possible, the patient should be changed to oral basal and short-acting opioids as needed for breakthrough pain. The appropriate dose of long acting basal analgesia can be determined the following day based on the patient’s total daily dose (TDD) of opioids. An example of converting from intravenous PRN morphine to oral basal and short acting opioids is shown in Table 2.
In communicating with a patient with opioid-use disorder with acute pain, it is best to outline the pain management plan at admission including: the plan to effectively treat the patient’s acute pain, prevent opioid withdrawal symptoms, change to oral opioid analgesics as soon as possible, discussion of non-opioid and non-drug treatments, reinforcement that opioids will be tapered as the acute pain episode resolves, and a detailed plan for discharge Later in this article, we describe discharge planning.
Clinical Example: Patient on Chronic Opioid Therapy for Chronic Pain
A 64 year-old man was involved in a motorcycle accident and suffered a right distal tibia-fibula fracture and several broken ribs with a secondary pneumothorax. The patient’s past medical history is significant for chronic low back pain for which he states he takes morphine sustained release 30 mg orally every 8 hours and morphine immediate release 15 mg orally four times daily for breakthrough pain. The patient states his pain is much worse than prior to the accident. Trauma surgery requests recommendations on appropriate pain management. What is the best way to manage this patient’s acute pain and communicate with him about his pain management?
When treating acute pain in patients with chronic pain on opioid therapy, it is vital to establish the patient’s baseline pain level and to accurately reconcile the patient’s outpatient daily opioid use. The patient’s prescription record should be verified in the state’s prescription drug monitoring program. On admission, a urine drug test should be obtained to assess for use of other potential illicit substances (eg, cocaine). Patients who test positive for illicit substances are at high risk for a substance use disorder. Management and discharge plans should be as outlined in the above case. It is important to know that the first-tier immunoassay urine toxicology screens used by hospitals test for natural opioids (morphine, codeine, heroin). Semi-synthetic (example, oxycodone) or synthetic (example, fentanyl) opioids are unlikely to be detected and thus the urine drug screen may not be helpful to determine adherence to certain prescription opioids. Gas chromatography/mass spectrometry is the most sensitive and specific type of urine screen and can be ordered to confirm a prescribed opioid if needed.26
Pain management should begin with calculating the TDD of oral opioids that the patient was taking prior to admission, and converting to morphine equivalents. For moderate acute pain, TDD can be increased by 25% to 50%. The revised TDD can then be prescribed as a long-acting opioid every 8 to 12 hours to provide basal analgesia. The dose of additional immediate-release medication available throughout the day to manage breakthrough pain is determined by dividing the new TDD into every 3 to 4 hours as-needed dosing (Table 2).
If severe pain is anticipated, patient controlled analgesia (PCA) is an effective alternative to deliver opioids. The use of PCA allows self-titration, on demand analgesia, and minimizes the likelihood of under-dosing the patient.27 The revised TDD is a useful starting point when calculating the PCA dosage regimen. Ideally, the revised TDD should be prescribed as a long acting oral opioid medication every 8 to 12 hours for basal analgesia, with PCA administered as an as-needed bolus. If a patient cannot tolerate oral medications, PCA can provide continuous infusion of medication to provide basal analgesia, though the risk of oversedation and respiratory depression is increased.28
For our clinical example, we recommend increasing the preadmission TDD of opioids (180 mg morphine equivalents) by 25% (225 mg) and administering as morphine 75 mg sustained-release every 8 hours to provide baseline analgesia and prevent withdrawal symptoms. The acute pain can be managed by initiating morphine PCA without continuous infusion at 0.5 mg bolus every 8 minutes as needed for breakthrough pain or oral morphine 30 mg immediate-release tablets every 3 hours as needed for pain. The patient should be assessed frequently, and naloxone kept readily available. In addition, nonopioid and nondrug treatments should be optimized.
When communicating with patients with underlying chronic pain on chronic opioid therapy, it is important to discuss the treatment plan early, including addressing that they will likely not be pain free during their hospitalization, but rather goals of pain relief and improved function should be established. The plan to change to oral opioid analgesics as soon as possible and importance of multi-modal treatment should be emphasized. The patient should be informed that medication changes are for the short-term only and that the underlying chronic pain will likely remain unchanged.
Clinical Example: Patient on Medication-Assisted Therapy
A 42-year-old woman presents with acute epigastric pain and receives a diagnosis of acute gallstone pancreatitis. She states that her pain is very severe and appears uncomfortable. Her past medical history is significant for heroin addiction, but she has been successfully treated for opioid-use disorder with buprenorphine 16 mg daily for the past three years. What is the best way to manage this patient’s acute pain and communicate with her about her pain management?
Medication-assisted therapies (MATs) for treatment of opioid abuse, which include methadone and buprenorphine (Table 3), have been shown to be effective in helping patients recover in opioid-use disorder, are cost-effective and reduce the risk of opioid overdose.29 However, treatment for acute pain in patients who are receiving methadone or buprenorphine MAT is a challenge because of pharmacokinetic changes that occur with prolonged use. It is important to know that patients receiving opioid agonist MAT are usually treated with 1 dose every 24 to 48 hours and do not receive sustained analgesia.30
In the case of patients on methadone as MAT, the methadone should be continued at the prescribed daily dose and additional short-acting opioid analgesics given to provide appropriate pain relief.27,31 Because of opioid tolerance, patients receiving MAT often require increased and more frequent doses of short-acting opioid analgesics to achieve adequate pain control.
Buprenorphine is a mu-opioid receptor partial agonist. The partial agonist properties of buprenorphine result in a “ceiling effect” that limits maximal analgesic and euphoric potential. Buprenorphine’s high affinity for the mu receptor also may result in competition with full opioid agonist analgesics, creating a challenge in treating acute pain. Because of the erratic dissociation of buprenorphine from the mu receptor, naloxone should be available and patients should be frequently monitored when the two agents are administered together. Recommendations regarding acute pain management in patients being treated with buprenorphine are largely based on expert opinion. Treatment options include32-34:
- Continue maintenance therapy with buprenorphine and treat acute pain with short acting opioid agonists. Higher doses of opioid agonists and more frequent dosing may be needed to provide adequate pain relief since they compete with buprenorphine at the mu receptor. Opioids with higher affinity for the mu receptor (morphine, hydromorphone, fentanyl) may be more efficacious.
- Discontinue buprenorphine and treat the patient with scheduled full opioid analgesics, titrating the dose initially to try to avoid withdrawal and then to provide pain relief. The partial agonism of the mu-receptor from buprenorphine and the blockade of other opioids can persist for as long as 72 hours. During this period, close monitoring and keeping naloxone available are important. When acute pain resolves, discontinue full opioid agonist therapy and resume buprenorphine using an induction protocol.
For our clinical example, we recommend continuing buprenorphine at 16 mg daily, optimizing nonopioid treatment strategies, and using a higher dose parenteral full opioid agonist every 3 hours as needed to achieve adequate analgesia. The patient should be frequently monitored for adverse effects, and naloxone kept available. Full opioid analgesics should be tapered and discontinued as the acute pain resolves. The patient should be reassured that there is no evidence that using opioids to treat acute pain episodes increases the risk of relapse and that untreated acute pain is a more likely trigger for relapse. The patient’s buprenorphine provider should be contacted at admission to verify dose as well as at discharge.
DISCHARGE PLANNING AND MANAGEMENT
Early discharge planning is essential for appropriate and safe management of acute pain in hospitalized patients with opioid dependence. The major goals are to treat acute pain effectively, improve function, and return care to the patient’s usual treating physician or methadone clinic. Patients on chronic opioid therapy often have a written opioid treatment agreement specifying only 1 prescriber. Therefore, verbal communication with the patient’s authorized prescriber at admission and at discharge is essential, particularly given that the discharge summary may not be available at follow-up. Additional or higher doses of opioids should not be prescribed at discharge unless discussed with the patient’s authorized prescriber. If it is believed necessary to provide opioid medication at discharge it should only be provided for a short period: 3 to 7 days.35 Patients with OUD should be referred for addiction treatment, including MAT, and should be educated on harm-reduction strategies, including safe injecting, obtaining clean needles, and recognizing, avoiding, and treating opioid overdose. Prescribing intranasal naloxone should be strongly considered for patients with OUD and for patients who are taking more than 50 mg oral morphine equivalents for chronic pain.34
CONCLUSION
Management of acute pain in opioid-dependent patients is a complex and increasingly common problem encountered by hospitalists. In addition, given the OUD epidemic in the United States, safe opioid prescribing has become a paramount goal for all physicians. Although acute pain management will be individualized and will encompass clinical judgment, this review provides an evidence-based guide to effective and safe acute pain management and optimal opioid prescribing for hospitalized opioid-dependent patients.
Disclosure
Nothing to report.
Up to 40% of Americans experience chronic pain of some kind.1 In the United States, opioid analgesics are the most prescribed class of medications,2 with 245 million prescriptions filled in 2014 alone. Thirty-five percent of these prescriptions were for long-term therapy.3 It is now apparent that opioid pain medication use presents serious risks. In 2014, 10.3 million persons reported using prescription opioids for nonmedical reasons.4 Between 1999 and 2014, more than 165,000 people in the United States died of overdose related to opioid medication.5 In addition, heroin use in the United States has increased over the past decade.6 Opioid agonist maintenance therapy is also increasingly used to treat patients with opioid use disorder.
Given the prevalence of opioid use in the United States, it is important for hospitalists to be able to appropriately and safely manage acute pain in patients who have been exposed long-term to opioids, whether it is therapeutic or non-medical in origin. Although nonopioid medications and nondrug treatments are essential components of managing all acute pain, opioids continue to be the mainstay of treatment for severe acute pain in both opioid-naïve and opioid-dependent patients.
Given the paucity of published trials meeting the typical criteria, we did not perform a structured meta-analysis but, instead, a case-based narrative review of the relevant published literature. Our goal in performing this review is to guide hospitalists in the appropriate and safe use of opioid analgesics in treating acute pain in hospitalized patients who are opioid-dependent.
DEFINITIONS
When managing acute pain in patients with opioid dependence it is important to have a clear understanding of the definitions related to opioid use. Addiction, physical dependence and tolerance have been defined by a joint consensus statement of the American Society of Addiction Medicine, American Academy of Pain Medicine, and American Pain Society7: Addiction is a primary, chronic, biological disease, with genetic, psychosocial and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving.
Physical Dependence is a state of adaptation that is manifested by a drug class specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist.
Tolerance is the state of adaptation in which exposure to a drug induces changes that result in a diminution of one or more of the drug’s effects over time.
Opioid use disorder (OUD) is defined as a problematic pattern of opioid use leading to clinically significant impairment or distress with symptoms including a strong desire for opioids, inability to control or reduce use of opioids, continued use despite adverse consequences, and development of tolerance and withdrawal symptoms.8
PATHOPHYSIOLOGY
Physical dependence and tolerance are common consequences of long-term opioid use. In contrast, OUD has been reported to affect only 2% to 6% of individuals exposed to opioids.9 The underlying mechanisms that lead an individual to abuse or become addicted to opioids largely due to the effects opioids have on endogenous μ-opioid receptors. As analgesics, opioids exert their effects by binding primarily to these μ-opioid receptors, with a large concentration in the brain regions regulating pain perception.10,11 There is also a large concentration of μ-opioid receptors in the brain reward regions, leading to perceptions of pleasure and euphoria. Repeated administration of opioids conditions the brain to a learned association between receiving the opiate and euphoria.12,13 This association becomes stronger as the frequency and duration of administration increases over time, ultimately leading to the desire or craving of the opioid’s effect.
The effect of tolerance also contributes to the pathophysiology of opioid abuse as it leads to a decrease in opioid potency with repeated administration.14-16 To achieve analgesia as well as the reward effect, opioid dosage and/or frequency must be increased, strengthening the association between receipt of opioid and reward. Tolerance to the reward effect occurs quickly, whereas tolerance to respiratory depression occurs much more slowly.17 This mismatch in tolerance of effect may lead to increase in opioid doses to maintain analgesia or euphoria, and also places patients at a higher risk of overdose.18
ACUTE PAIN MANAGEMENT
Clinical Example: Heroin User
A 47-year-old man is admitted with fever, chills, and severe mid-back pain and receives a diagnosis of sepsis. The patient admits to using intravenous heroin 500 mg (five 100 mg “bags”) on a daily basis. He is admitted, fluid resuscitated and started on broad spectrum antibiotics. Blood cultures quickly grow Staphylococcus aureus. Magnetic resonance imaging of the spine shows cervical vertebral osteomyelitis. On examination, the patient is diaphoretic and complains of diffuse myalgias and diarrhea. The patient’s back pain is so severe that he cannot ambulate. What is the best way to manage this patient’s acute pain and communicate with him about his pain management?
Managing acute pain in a patient using heroin can be challenging for many reasons. First, both physicians and pharmacists report a lack of confidence in their ability to prescribe opioids safely or to treat patients with a history of opioid abuse.19 Second, there is a paucity of evidence in treating acute pain in heroin users. Finally, due to the clandestine manufacturing of illicit drugs, the actual purity of the drug is often unknown making it difficult to assess the dose of opioids in heroin users. Drug Enforcement Agency seizure data indicate a wide range of heroin purity: 30% to 70%.20
In the hospital setting, acute pain is often undertreated in patients with a history of active opioid abuse. This may be due to providers’ misconceptions regarding pain and behavior in opioid addicts, including worrying that the patient’s pain is exaggerated in order to obtain drugs, thinking that a regular opioid habit eliminates pain, believing that opioid therapy is not effective in drug addicts, or worrying that prescribing opioids will exacerbate drug addiction.21 Data demonstrates that the presence of opioid addiction seems to worsen the experience of acute pain.22 These patients also often have a higher tolerance and thus require higher dosages and more frequent dosing of opioids to adequately treat their pain.23
Converting daily heroin use to morphine equivalents is necessary to establish a baseline analgesic requirement and to prevent withdrawal. It is challenging to convert illicit heroin to morphine equivalents however, as one must take into account the wide variation in purity and understand that the stated use of heroin (e.g. 500 mg daily) reflects weight and not dosage of heroin.20
In these patients, treatment of acute pain should be individualized according to presenting illness and comorbidities. Previous data and an average purity of 40% suggest that the parenteral morphine equivalent to a bag of heroin (100 mg) is 15 to 30 mg.20,24,25 Common equianalgesic doses of opioid medications are listed in Table 1. Because of increased tolerance, the frequency of administration should be shortened, from every 4 hours to every 2 or 3 hours. Except for a shorter onset of action, there has not been a difference shown in superiority between oral and parenteral routes of administration. Finally, patients should receive both long-acting basal and short-acting as-needed analgesics based on their daily use of opioids.23
In our clinical example, IV heroin 500 mg daily converts to parenteral morphine 75 to 150 mg every 24 hours. We recommend initiating IV morphine 10 mg every 3 hours as needed for pain and withdrawal symptoms, with early reassessment regarding need for a higher dose or a shorter frequency based on symptoms. Nonopioid analgesics should also be administered with the goal of decreasing the opioid requirement. As soon as possible, the patient should be changed to oral basal and short-acting opioids as needed for breakthrough pain. The appropriate dose of long acting basal analgesia can be determined the following day based on the patient’s total daily dose (TDD) of opioids. An example of converting from intravenous PRN morphine to oral basal and short acting opioids is shown in Table 2.
In communicating with a patient with opioid-use disorder with acute pain, it is best to outline the pain management plan at admission including: the plan to effectively treat the patient’s acute pain, prevent opioid withdrawal symptoms, change to oral opioid analgesics as soon as possible, discussion of non-opioid and non-drug treatments, reinforcement that opioids will be tapered as the acute pain episode resolves, and a detailed plan for discharge Later in this article, we describe discharge planning.
Clinical Example: Patient on Chronic Opioid Therapy for Chronic Pain
A 64 year-old man was involved in a motorcycle accident and suffered a right distal tibia-fibula fracture and several broken ribs with a secondary pneumothorax. The patient’s past medical history is significant for chronic low back pain for which he states he takes morphine sustained release 30 mg orally every 8 hours and morphine immediate release 15 mg orally four times daily for breakthrough pain. The patient states his pain is much worse than prior to the accident. Trauma surgery requests recommendations on appropriate pain management. What is the best way to manage this patient’s acute pain and communicate with him about his pain management?
When treating acute pain in patients with chronic pain on opioid therapy, it is vital to establish the patient’s baseline pain level and to accurately reconcile the patient’s outpatient daily opioid use. The patient’s prescription record should be verified in the state’s prescription drug monitoring program. On admission, a urine drug test should be obtained to assess for use of other potential illicit substances (eg, cocaine). Patients who test positive for illicit substances are at high risk for a substance use disorder. Management and discharge plans should be as outlined in the above case. It is important to know that the first-tier immunoassay urine toxicology screens used by hospitals test for natural opioids (morphine, codeine, heroin). Semi-synthetic (example, oxycodone) or synthetic (example, fentanyl) opioids are unlikely to be detected and thus the urine drug screen may not be helpful to determine adherence to certain prescription opioids. Gas chromatography/mass spectrometry is the most sensitive and specific type of urine screen and can be ordered to confirm a prescribed opioid if needed.26
Pain management should begin with calculating the TDD of oral opioids that the patient was taking prior to admission, and converting to morphine equivalents. For moderate acute pain, TDD can be increased by 25% to 50%. The revised TDD can then be prescribed as a long-acting opioid every 8 to 12 hours to provide basal analgesia. The dose of additional immediate-release medication available throughout the day to manage breakthrough pain is determined by dividing the new TDD into every 3 to 4 hours as-needed dosing (Table 2).
If severe pain is anticipated, patient controlled analgesia (PCA) is an effective alternative to deliver opioids. The use of PCA allows self-titration, on demand analgesia, and minimizes the likelihood of under-dosing the patient.27 The revised TDD is a useful starting point when calculating the PCA dosage regimen. Ideally, the revised TDD should be prescribed as a long acting oral opioid medication every 8 to 12 hours for basal analgesia, with PCA administered as an as-needed bolus. If a patient cannot tolerate oral medications, PCA can provide continuous infusion of medication to provide basal analgesia, though the risk of oversedation and respiratory depression is increased.28
For our clinical example, we recommend increasing the preadmission TDD of opioids (180 mg morphine equivalents) by 25% (225 mg) and administering as morphine 75 mg sustained-release every 8 hours to provide baseline analgesia and prevent withdrawal symptoms. The acute pain can be managed by initiating morphine PCA without continuous infusion at 0.5 mg bolus every 8 minutes as needed for breakthrough pain or oral morphine 30 mg immediate-release tablets every 3 hours as needed for pain. The patient should be assessed frequently, and naloxone kept readily available. In addition, nonopioid and nondrug treatments should be optimized.
When communicating with patients with underlying chronic pain on chronic opioid therapy, it is important to discuss the treatment plan early, including addressing that they will likely not be pain free during their hospitalization, but rather goals of pain relief and improved function should be established. The plan to change to oral opioid analgesics as soon as possible and importance of multi-modal treatment should be emphasized. The patient should be informed that medication changes are for the short-term only and that the underlying chronic pain will likely remain unchanged.
Clinical Example: Patient on Medication-Assisted Therapy
A 42-year-old woman presents with acute epigastric pain and receives a diagnosis of acute gallstone pancreatitis. She states that her pain is very severe and appears uncomfortable. Her past medical history is significant for heroin addiction, but she has been successfully treated for opioid-use disorder with buprenorphine 16 mg daily for the past three years. What is the best way to manage this patient’s acute pain and communicate with her about her pain management?
Medication-assisted therapies (MATs) for treatment of opioid abuse, which include methadone and buprenorphine (Table 3), have been shown to be effective in helping patients recover in opioid-use disorder, are cost-effective and reduce the risk of opioid overdose.29 However, treatment for acute pain in patients who are receiving methadone or buprenorphine MAT is a challenge because of pharmacokinetic changes that occur with prolonged use. It is important to know that patients receiving opioid agonist MAT are usually treated with 1 dose every 24 to 48 hours and do not receive sustained analgesia.30
In the case of patients on methadone as MAT, the methadone should be continued at the prescribed daily dose and additional short-acting opioid analgesics given to provide appropriate pain relief.27,31 Because of opioid tolerance, patients receiving MAT often require increased and more frequent doses of short-acting opioid analgesics to achieve adequate pain control.
Buprenorphine is a mu-opioid receptor partial agonist. The partial agonist properties of buprenorphine result in a “ceiling effect” that limits maximal analgesic and euphoric potential. Buprenorphine’s high affinity for the mu receptor also may result in competition with full opioid agonist analgesics, creating a challenge in treating acute pain. Because of the erratic dissociation of buprenorphine from the mu receptor, naloxone should be available and patients should be frequently monitored when the two agents are administered together. Recommendations regarding acute pain management in patients being treated with buprenorphine are largely based on expert opinion. Treatment options include32-34:
- Continue maintenance therapy with buprenorphine and treat acute pain with short acting opioid agonists. Higher doses of opioid agonists and more frequent dosing may be needed to provide adequate pain relief since they compete with buprenorphine at the mu receptor. Opioids with higher affinity for the mu receptor (morphine, hydromorphone, fentanyl) may be more efficacious.
- Discontinue buprenorphine and treat the patient with scheduled full opioid analgesics, titrating the dose initially to try to avoid withdrawal and then to provide pain relief. The partial agonism of the mu-receptor from buprenorphine and the blockade of other opioids can persist for as long as 72 hours. During this period, close monitoring and keeping naloxone available are important. When acute pain resolves, discontinue full opioid agonist therapy and resume buprenorphine using an induction protocol.
For our clinical example, we recommend continuing buprenorphine at 16 mg daily, optimizing nonopioid treatment strategies, and using a higher dose parenteral full opioid agonist every 3 hours as needed to achieve adequate analgesia. The patient should be frequently monitored for adverse effects, and naloxone kept available. Full opioid analgesics should be tapered and discontinued as the acute pain resolves. The patient should be reassured that there is no evidence that using opioids to treat acute pain episodes increases the risk of relapse and that untreated acute pain is a more likely trigger for relapse. The patient’s buprenorphine provider should be contacted at admission to verify dose as well as at discharge.
DISCHARGE PLANNING AND MANAGEMENT
Early discharge planning is essential for appropriate and safe management of acute pain in hospitalized patients with opioid dependence. The major goals are to treat acute pain effectively, improve function, and return care to the patient’s usual treating physician or methadone clinic. Patients on chronic opioid therapy often have a written opioid treatment agreement specifying only 1 prescriber. Therefore, verbal communication with the patient’s authorized prescriber at admission and at discharge is essential, particularly given that the discharge summary may not be available at follow-up. Additional or higher doses of opioids should not be prescribed at discharge unless discussed with the patient’s authorized prescriber. If it is believed necessary to provide opioid medication at discharge it should only be provided for a short period: 3 to 7 days.35 Patients with OUD should be referred for addiction treatment, including MAT, and should be educated on harm-reduction strategies, including safe injecting, obtaining clean needles, and recognizing, avoiding, and treating opioid overdose. Prescribing intranasal naloxone should be strongly considered for patients with OUD and for patients who are taking more than 50 mg oral morphine equivalents for chronic pain.34
CONCLUSION
Management of acute pain in opioid-dependent patients is a complex and increasingly common problem encountered by hospitalists. In addition, given the OUD epidemic in the United States, safe opioid prescribing has become a paramount goal for all physicians. Although acute pain management will be individualized and will encompass clinical judgment, this review provides an evidence-based guide to effective and safe acute pain management and optimal opioid prescribing for hospitalized opioid-dependent patients.
Disclosure
Nothing to report.
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education and Research. Washington, DC: National Academies Press; 2011. PubMed
2. Centers for Disease Control and Prevention. FastStats. Therapeutic drug use. 2014. http://www.cdc.gov/nchs/faststats/drug-use-therapeutic.htm. Accessed August 23, 2016.
3. National Institute on Drug Abuse. The Latest Prescription Trends for Controlled Prescription Drugs. http://www.drugabuse.gov/news-events/meetings-events/2015/09/latest-prescription-trends-controlled-prescription-drugs. Published September 1, 2015. Accessed August 23, 2016.
4. Center for Behavioral Health Statistics and Quality. 2014 National Survey on Drug Use and Health: Detailed Tables. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
5. Centers for Disease Control and Prevention. Multiple cause of death data. https://wonder.cdc.gov/mcd.html. Accessed September 9, 2016.
6. Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374(2):154-163. PubMed
7. American Academy of Pain Medicine, American Pain Society, American Society of Addiction Medicine. https://www.naabt.org/documents/APS_consenus_document.pdf. Published 2001. Accessed August 23, 2016.
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
9. Christie MJ. Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol. 2008;154(2):384-396. PubMed
10. McNicol E, Carr DB. Pharmacological treatment of pain. In: McCarberg B, Passik SD, eds. Expert Guide to Pain Management. Philadelphia, PA: American College of Physicians; 2005:145-178.
11. Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker, JM. Endogenous opioids: biology and function. Annu Rev Neurosci. 1984;7:223-255. PubMed
12. Miguez G, Laborda MA, Miller RR. Classical conditioning and pain: conditioned analgesia and hyperalgesia. Acta Psychol (Amst). 2014;145:10-20. PubMed
13. Ewan EE, Martin TJ. Analgesics as reinforcers with chronic pain: evidence from operant studies. Neurosci Lett. 2013;557(pt A):60-64. PubMed
14. Mehta V, Langford R. Acute pain management in opioid dependent patients. Rev Pain. 2009;3(2):10-14. PubMed
15. Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med. 2016;374(13):1253-1263. PubMed
16. Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81(1):299-343. PubMed
17. Ling GS, Paul D, Simantov R, Pasternak GW. Differential development of acute tolerance to analgesia, respiratory depression, gastrointestinal transit and hormone release in a morphine infusion model. Life Sci. 1989;45(18):1627-1636. PubMed
18. Pattinson KT. Opioids and the control of respiration. Br J Anaesth. 2008;100(6):747-758. PubMed
19. Hagemeier NE, Gray JA, Pack RP. Prescription drug abuse: a comparison of prescriber and pharmacist perspectives. Subst Use Misuse. 2013;48(9):761-768. PubMed
20. Drug Enforcement Administration, US Department of Justice. National Heroin Threat Assessment Summary. Washington, DC: Drug Enforcement Administration, US Dept of Justice; 2015. DEA intelligence report DEA-DCT-DIR-039-15.
21. Laroche F, Rostaing S, Aubrun F, Perrot S. Pain management in heroin and cocaine users. Joint Bone Spine. 2012;79(5):446-450. PubMed
22. Savage SR, Schofferman J. Pharmacological therapies of pain in drug and alcohol addictions. In: Miller N, Gold M, eds. Pharmacological Therapies for Drug and Alcohol Addictions. New York, NY: Dekker; 1995:373-409.
23. Vadivelu N, Lumermann L, Zhu R, Kodumudi G, Elhassan AO, Kaye AD. Pain control in the presence of drug addiction. Curr Pain Headache Rep. 2016;20(5):35. PubMed
24. Johns AR, Gossop M. Prescribing methadone for the opiate addict: a problem of dosage conversion. Drug Alcohol Depend. 1985;16(1):61-66. PubMed
25. Halbsguth U, Rentsch KM, Eich-Höchli D, Diterich I, Fattinger K. Oral diacetylmorphine (heroin) yields greater morphine bioavailability than oral morphine: bioavailability related to dosage and prior opioid exposure. Br J Clin Pharmacol. 2008;66(6):781-791. PubMed
26. Milone MC. Laboratory testing for prescription opioids. J Med Toxicol. 2012;8(4):408-416. PubMed
27. Huxtable CA, Roberts LJ, Somogyi AA, MacIntyre PE. Acute pain management in opioid-tolerant patients: a growing challenge. Anaesth Intensive Care. 2011;39(5):804-823. PubMed
28. George JA, Lin EE, Hanna MN, et al. The effect of intravenous opioid patient-controlled analgesia with and without background infusion on respiratory depression: a meta-analysis. J Opioid Manag. 2010;6(1):47-54. PubMed
29. Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063-2066. PubMed
30. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144(2):127-134. PubMed
31. Mehta V, Langford RM. Acute pain management for opioid dependent patients. Anaesthesia. 2006;61(3):269-276. PubMed
32. Sen S, Arulkumar S, Cornett EM, et al. New pain management options for the surgical patient on methadone and buprenorphine. Curr Pain Headache Rep. 2016;20(3):16. PubMed
33. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645. PubMed
34. Fanucchi L, Lofwall MR. Putting parity into practice—integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;375(9):811-813. PubMed
35. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49. PubMed
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education and Research. Washington, DC: National Academies Press; 2011. PubMed
2. Centers for Disease Control and Prevention. FastStats. Therapeutic drug use. 2014. http://www.cdc.gov/nchs/faststats/drug-use-therapeutic.htm. Accessed August 23, 2016.
3. National Institute on Drug Abuse. The Latest Prescription Trends for Controlled Prescription Drugs. http://www.drugabuse.gov/news-events/meetings-events/2015/09/latest-prescription-trends-controlled-prescription-drugs. Published September 1, 2015. Accessed August 23, 2016.
4. Center for Behavioral Health Statistics and Quality. 2014 National Survey on Drug Use and Health: Detailed Tables. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
5. Centers for Disease Control and Prevention. Multiple cause of death data. https://wonder.cdc.gov/mcd.html. Accessed September 9, 2016.
6. Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374(2):154-163. PubMed
7. American Academy of Pain Medicine, American Pain Society, American Society of Addiction Medicine. https://www.naabt.org/documents/APS_consenus_document.pdf. Published 2001. Accessed August 23, 2016.
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
9. Christie MJ. Cellular neuroadaptations to chronic opioids: tolerance, withdrawal and addiction. Br J Pharmacol. 2008;154(2):384-396. PubMed
10. McNicol E, Carr DB. Pharmacological treatment of pain. In: McCarberg B, Passik SD, eds. Expert Guide to Pain Management. Philadelphia, PA: American College of Physicians; 2005:145-178.
11. Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker, JM. Endogenous opioids: biology and function. Annu Rev Neurosci. 1984;7:223-255. PubMed
12. Miguez G, Laborda MA, Miller RR. Classical conditioning and pain: conditioned analgesia and hyperalgesia. Acta Psychol (Amst). 2014;145:10-20. PubMed
13. Ewan EE, Martin TJ. Analgesics as reinforcers with chronic pain: evidence from operant studies. Neurosci Lett. 2013;557(pt A):60-64. PubMed
14. Mehta V, Langford R. Acute pain management in opioid dependent patients. Rev Pain. 2009;3(2):10-14. PubMed
15. Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med. 2016;374(13):1253-1263. PubMed
16. Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81(1):299-343. PubMed
17. Ling GS, Paul D, Simantov R, Pasternak GW. Differential development of acute tolerance to analgesia, respiratory depression, gastrointestinal transit and hormone release in a morphine infusion model. Life Sci. 1989;45(18):1627-1636. PubMed
18. Pattinson KT. Opioids and the control of respiration. Br J Anaesth. 2008;100(6):747-758. PubMed
19. Hagemeier NE, Gray JA, Pack RP. Prescription drug abuse: a comparison of prescriber and pharmacist perspectives. Subst Use Misuse. 2013;48(9):761-768. PubMed
20. Drug Enforcement Administration, US Department of Justice. National Heroin Threat Assessment Summary. Washington, DC: Drug Enforcement Administration, US Dept of Justice; 2015. DEA intelligence report DEA-DCT-DIR-039-15.
21. Laroche F, Rostaing S, Aubrun F, Perrot S. Pain management in heroin and cocaine users. Joint Bone Spine. 2012;79(5):446-450. PubMed
22. Savage SR, Schofferman J. Pharmacological therapies of pain in drug and alcohol addictions. In: Miller N, Gold M, eds. Pharmacological Therapies for Drug and Alcohol Addictions. New York, NY: Dekker; 1995:373-409.
23. Vadivelu N, Lumermann L, Zhu R, Kodumudi G, Elhassan AO, Kaye AD. Pain control in the presence of drug addiction. Curr Pain Headache Rep. 2016;20(5):35. PubMed
24. Johns AR, Gossop M. Prescribing methadone for the opiate addict: a problem of dosage conversion. Drug Alcohol Depend. 1985;16(1):61-66. PubMed
25. Halbsguth U, Rentsch KM, Eich-Höchli D, Diterich I, Fattinger K. Oral diacetylmorphine (heroin) yields greater morphine bioavailability than oral morphine: bioavailability related to dosage and prior opioid exposure. Br J Clin Pharmacol. 2008;66(6):781-791. PubMed
26. Milone MC. Laboratory testing for prescription opioids. J Med Toxicol. 2012;8(4):408-416. PubMed
27. Huxtable CA, Roberts LJ, Somogyi AA, MacIntyre PE. Acute pain management in opioid-tolerant patients: a growing challenge. Anaesth Intensive Care. 2011;39(5):804-823. PubMed
28. George JA, Lin EE, Hanna MN, et al. The effect of intravenous opioid patient-controlled analgesia with and without background infusion on respiratory depression: a meta-analysis. J Opioid Manag. 2010;6(1):47-54. PubMed
29. Volkow ND, Frieden TR, Hyde PS, Cha SS. Medication-assisted therapies—tackling the opioid-overdose epidemic. N Engl J Med. 2014;370(22):2063-2066. PubMed
30. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144(2):127-134. PubMed
31. Mehta V, Langford RM. Acute pain management for opioid dependent patients. Anaesthesia. 2006;61(3):269-276. PubMed
32. Sen S, Arulkumar S, Cornett EM, et al. New pain management options for the surgical patient on methadone and buprenorphine. Curr Pain Headache Rep. 2016;20(3):16. PubMed
33. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645. PubMed
34. Fanucchi L, Lofwall MR. Putting parity into practice—integrating opioid-use disorder treatment into the hospital setting. N Engl J Med. 2016;375(9):811-813. PubMed
35. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49. PubMed
© 2017 Society of Hospital Medicine
Diagnostic testing in AKI: Let’s move the field forward
In this issue of the Journal of Hospital Medicine, Lusica et al.1 discuss the utility of urine eosinophils (UEs) in evaluating for acute interstitial nephritis (AIN) in patients with acute kidney injury (AKI), an important and oft-confused concern in medicine. I can’t think of a more appropriate topic for the “Things We Do for No Reason” (TWDFNR) series. Numerous tests are ordered in the evaluation of AKI.2 Many, such as batteries of serological tests, are unnecessary and add little diagnostic information. Some, such as UEs and fractional excretion of sodium (FENa), provide misinformation. And others, such as contrast-enhanced computed tomography scans, are potentially harmful.2 In a previous TWDFNR article, the limitations of FENa in the evaluation of AKI were reviewed.3 There are common threads linking the shortcomings of UEs and FENa and even new diagnostic tests. What are the lessons from these studies, and how might clinicians best apply them in their practice?
As reviewed in this issue, UE testing is employed in AKI to evaluate for hospital-acquired AIN. Small initial studies led to widespread use of this test, despite methodological flaws.4 A later, definitive study involving 566 patients who had both UEs and kidney biopsies performed within the same week demonstrated that UEs offered no diagnostic value in AKI.5 The same pattern occurred in the increased use of FENa to distinguish prerenal azotemia from acute tubular necrosis in AKI patients.3 Small studies in highly select patients supported its use for this purpose.6 Subsequently, larger studies in more diverse populations noted that FENa was associated with many false positive and negative results,6 likely due to more widespread use of this test in disease states such as cirrhosis, congestive heart failure, chronic kidney disease, and diabetes, which were not included in initial studies.
It is apparent that clinicians have been led astray by small, flawed positive studies employed in highly selected populations. These initial positive studies based on excessively large effect size estimates were subsequently shown to be negative in larger studies with more plausible effect sizes. Examples of this error are seen in publications involving prophylactic measures to reduce contrast nephrotoxicity.7 Early studies on N-acetylcysteine administration prior to radiocontrast exposure showed positive results. Examination of these studies, however, demonstrates 2 key problems: 1) inclusion of small numbers of patients due to power calculations based on excessively large effect sizes, and 2) use of clinically unimportant endpoints such as serum creatinine changes.7 The same issue complicates studies evaluating isotonic sodium bicarbonate vs. normal saline for contrast prophylaxis.7
The past 10-plus years have seen a proliferation of studies evaluating the utility of novel biomarkers for early diagnosis and prognosis in AKI. Have we fallen down the same rabbit hole in evaluating these new diagnostic tests for AKI? There is reason for concern if we examine published studies of novel biomarkers in other areas of medicine. To this point, many highly cited novel biomarker studies used for various diagnostic purposes (eg, cancer, infection, cardiovascular disease) employed excessively large effect size estimates for postulated associations that resulted in small, underpowered studies with initially positive results.8 Subsequent large studies and meta-analyses reported negative or modestly positive test results when examining these same associations.8 But we may be moving in the right direction. An early urine biomarker publication from a small, single center study9 revealed overly optimistic results (area under the curve [AUC], 0.998; sensitivity, 100%; specificity, 98%) for AKI prediction. Subsequent large, multicenter biomarker studies showed only modest improvement in their discriminative value when compared with traditional clinical models.10 These results precluded U.S. Food and Drug Administration (FDA) approval of most novel biomarkers for clinical practice and they were not adopted. In 2014, the FDA approved the point-of-care urinary biomarker TIMP-2/IGFBP7 (NephroCheck®) for predicting risk of AKI based on fairly rigorous testing using larger numbers of patients, heterogeneous populations, and important clinical endpoints.11 In a 522-patient discovery cohort, this biomarker had an AUC of 0.80 for AKI prediction, which was validated in a 722-patient cohort and subsequently followed by a 420-patient multicenter cohort study revealing similar test characteristics (AUC, 0.82; sensitivity, 92%; specificity, 46%).11 A study involving 382 critically ill AKI patients noted that this biomarker had a hazard ratio of 2.16 (95% confidence interval [CI] 1.32 to 3.53) for predict
In summary, clinicians should be aware of the strengths and limitations of diagnostic tests ordered in AKI patients, as seen with the overly optimistic results in small, flawed UE and FENa studies. While we have taken a step in the right direction with diagnostic and prognostic biomarkers for AKI, we must apply rigorous study design to diagnostic tests under evaluation before adopting them into clinical practice. Only then can we move the field forward and improve patient care.
Disclosure
Nothing to report.
1. Lusica M, Rondon-Berrios H, Feldman L. Urine eosinophils for acute interstitial nephritis. J Hosp Med. 2017;12(5):343-345. PubMed
2. Leaf DE, Srivastava A, Zeng X, et al. Excessive diagnostic testing in acute kidney injury. BMC Nephrol. 2016;17:9. PubMed
3. Pahwa AK, Sperati CJ. Urinary fractional excretion indices in the evaluation of acute kidney injury. J Hosp Med. 2016;11(1):77-80. PubMed
4. Perazella MA, Bomback AS. Urinary eosinophils in AIN: farewell to an old biomarker? Clin J Am Soc Nephrol. 2013;8(11):1841-1843. PubMed
5. Muriithi AK, Nasr SH, Leung N. Utility of urine eosinophils in the diagnosis of acute interstitial nephritis. Clin J Am Soc Nephrol. 2013;8(11):1857-1862. PubMed
6. Perazella MA, Coca SG. Traditional urinary biomarkers in the assessment of hospital-acquired AKI. Clin J Am Soc Nephrol. 2012;7(1):167-174. PubMed
7. Weisbord SD, Palevsky PM. Strategies for the prevention of contrast-induced acute kidney injury. Curr Opin Nephrol Hypertens. 2010;19(6):539-549. PubMed
8. Ioannidis JP, Panagiotou OA. Comparison of effect sizes associated with biomarkers reported in highly cited individual articles and in subsequent meta-analyses. JAMA. 2011;305(21):2200-2210. PubMed
9. Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231-1238. PubMed
10. Schaub JA, Parikh CR. Biomarkers of acute kidney injury and associations with short- and long-term outcomes. F1000Res. 2016;5(F1000 Faculty Rev.):986. PubMed
11. McMahon BA, Koyner JL. Risk stratification for acute kidney injury: Are biomarkers enough? Adv Chronic Kidney Dis. 2016;23(3):167-178. PubMed
In this issue of the Journal of Hospital Medicine, Lusica et al.1 discuss the utility of urine eosinophils (UEs) in evaluating for acute interstitial nephritis (AIN) in patients with acute kidney injury (AKI), an important and oft-confused concern in medicine. I can’t think of a more appropriate topic for the “Things We Do for No Reason” (TWDFNR) series. Numerous tests are ordered in the evaluation of AKI.2 Many, such as batteries of serological tests, are unnecessary and add little diagnostic information. Some, such as UEs and fractional excretion of sodium (FENa), provide misinformation. And others, such as contrast-enhanced computed tomography scans, are potentially harmful.2 In a previous TWDFNR article, the limitations of FENa in the evaluation of AKI were reviewed.3 There are common threads linking the shortcomings of UEs and FENa and even new diagnostic tests. What are the lessons from these studies, and how might clinicians best apply them in their practice?
As reviewed in this issue, UE testing is employed in AKI to evaluate for hospital-acquired AIN. Small initial studies led to widespread use of this test, despite methodological flaws.4 A later, definitive study involving 566 patients who had both UEs and kidney biopsies performed within the same week demonstrated that UEs offered no diagnostic value in AKI.5 The same pattern occurred in the increased use of FENa to distinguish prerenal azotemia from acute tubular necrosis in AKI patients.3 Small studies in highly select patients supported its use for this purpose.6 Subsequently, larger studies in more diverse populations noted that FENa was associated with many false positive and negative results,6 likely due to more widespread use of this test in disease states such as cirrhosis, congestive heart failure, chronic kidney disease, and diabetes, which were not included in initial studies.
It is apparent that clinicians have been led astray by small, flawed positive studies employed in highly selected populations. These initial positive studies based on excessively large effect size estimates were subsequently shown to be negative in larger studies with more plausible effect sizes. Examples of this error are seen in publications involving prophylactic measures to reduce contrast nephrotoxicity.7 Early studies on N-acetylcysteine administration prior to radiocontrast exposure showed positive results. Examination of these studies, however, demonstrates 2 key problems: 1) inclusion of small numbers of patients due to power calculations based on excessively large effect sizes, and 2) use of clinically unimportant endpoints such as serum creatinine changes.7 The same issue complicates studies evaluating isotonic sodium bicarbonate vs. normal saline for contrast prophylaxis.7
The past 10-plus years have seen a proliferation of studies evaluating the utility of novel biomarkers for early diagnosis and prognosis in AKI. Have we fallen down the same rabbit hole in evaluating these new diagnostic tests for AKI? There is reason for concern if we examine published studies of novel biomarkers in other areas of medicine. To this point, many highly cited novel biomarker studies used for various diagnostic purposes (eg, cancer, infection, cardiovascular disease) employed excessively large effect size estimates for postulated associations that resulted in small, underpowered studies with initially positive results.8 Subsequent large studies and meta-analyses reported negative or modestly positive test results when examining these same associations.8 But we may be moving in the right direction. An early urine biomarker publication from a small, single center study9 revealed overly optimistic results (area under the curve [AUC], 0.998; sensitivity, 100%; specificity, 98%) for AKI prediction. Subsequent large, multicenter biomarker studies showed only modest improvement in their discriminative value when compared with traditional clinical models.10 These results precluded U.S. Food and Drug Administration (FDA) approval of most novel biomarkers for clinical practice and they were not adopted. In 2014, the FDA approved the point-of-care urinary biomarker TIMP-2/IGFBP7 (NephroCheck®) for predicting risk of AKI based on fairly rigorous testing using larger numbers of patients, heterogeneous populations, and important clinical endpoints.11 In a 522-patient discovery cohort, this biomarker had an AUC of 0.80 for AKI prediction, which was validated in a 722-patient cohort and subsequently followed by a 420-patient multicenter cohort study revealing similar test characteristics (AUC, 0.82; sensitivity, 92%; specificity, 46%).11 A study involving 382 critically ill AKI patients noted that this biomarker had a hazard ratio of 2.16 (95% confidence interval [CI] 1.32 to 3.53) for predict
In summary, clinicians should be aware of the strengths and limitations of diagnostic tests ordered in AKI patients, as seen with the overly optimistic results in small, flawed UE and FENa studies. While we have taken a step in the right direction with diagnostic and prognostic biomarkers for AKI, we must apply rigorous study design to diagnostic tests under evaluation before adopting them into clinical practice. Only then can we move the field forward and improve patient care.
Disclosure
Nothing to report.
In this issue of the Journal of Hospital Medicine, Lusica et al.1 discuss the utility of urine eosinophils (UEs) in evaluating for acute interstitial nephritis (AIN) in patients with acute kidney injury (AKI), an important and oft-confused concern in medicine. I can’t think of a more appropriate topic for the “Things We Do for No Reason” (TWDFNR) series. Numerous tests are ordered in the evaluation of AKI.2 Many, such as batteries of serological tests, are unnecessary and add little diagnostic information. Some, such as UEs and fractional excretion of sodium (FENa), provide misinformation. And others, such as contrast-enhanced computed tomography scans, are potentially harmful.2 In a previous TWDFNR article, the limitations of FENa in the evaluation of AKI were reviewed.3 There are common threads linking the shortcomings of UEs and FENa and even new diagnostic tests. What are the lessons from these studies, and how might clinicians best apply them in their practice?
As reviewed in this issue, UE testing is employed in AKI to evaluate for hospital-acquired AIN. Small initial studies led to widespread use of this test, despite methodological flaws.4 A later, definitive study involving 566 patients who had both UEs and kidney biopsies performed within the same week demonstrated that UEs offered no diagnostic value in AKI.5 The same pattern occurred in the increased use of FENa to distinguish prerenal azotemia from acute tubular necrosis in AKI patients.3 Small studies in highly select patients supported its use for this purpose.6 Subsequently, larger studies in more diverse populations noted that FENa was associated with many false positive and negative results,6 likely due to more widespread use of this test in disease states such as cirrhosis, congestive heart failure, chronic kidney disease, and diabetes, which were not included in initial studies.
It is apparent that clinicians have been led astray by small, flawed positive studies employed in highly selected populations. These initial positive studies based on excessively large effect size estimates were subsequently shown to be negative in larger studies with more plausible effect sizes. Examples of this error are seen in publications involving prophylactic measures to reduce contrast nephrotoxicity.7 Early studies on N-acetylcysteine administration prior to radiocontrast exposure showed positive results. Examination of these studies, however, demonstrates 2 key problems: 1) inclusion of small numbers of patients due to power calculations based on excessively large effect sizes, and 2) use of clinically unimportant endpoints such as serum creatinine changes.7 The same issue complicates studies evaluating isotonic sodium bicarbonate vs. normal saline for contrast prophylaxis.7
The past 10-plus years have seen a proliferation of studies evaluating the utility of novel biomarkers for early diagnosis and prognosis in AKI. Have we fallen down the same rabbit hole in evaluating these new diagnostic tests for AKI? There is reason for concern if we examine published studies of novel biomarkers in other areas of medicine. To this point, many highly cited novel biomarker studies used for various diagnostic purposes (eg, cancer, infection, cardiovascular disease) employed excessively large effect size estimates for postulated associations that resulted in small, underpowered studies with initially positive results.8 Subsequent large studies and meta-analyses reported negative or modestly positive test results when examining these same associations.8 But we may be moving in the right direction. An early urine biomarker publication from a small, single center study9 revealed overly optimistic results (area under the curve [AUC], 0.998; sensitivity, 100%; specificity, 98%) for AKI prediction. Subsequent large, multicenter biomarker studies showed only modest improvement in their discriminative value when compared with traditional clinical models.10 These results precluded U.S. Food and Drug Administration (FDA) approval of most novel biomarkers for clinical practice and they were not adopted. In 2014, the FDA approved the point-of-care urinary biomarker TIMP-2/IGFBP7 (NephroCheck®) for predicting risk of AKI based on fairly rigorous testing using larger numbers of patients, heterogeneous populations, and important clinical endpoints.11 In a 522-patient discovery cohort, this biomarker had an AUC of 0.80 for AKI prediction, which was validated in a 722-patient cohort and subsequently followed by a 420-patient multicenter cohort study revealing similar test characteristics (AUC, 0.82; sensitivity, 92%; specificity, 46%).11 A study involving 382 critically ill AKI patients noted that this biomarker had a hazard ratio of 2.16 (95% confidence interval [CI] 1.32 to 3.53) for predict
In summary, clinicians should be aware of the strengths and limitations of diagnostic tests ordered in AKI patients, as seen with the overly optimistic results in small, flawed UE and FENa studies. While we have taken a step in the right direction with diagnostic and prognostic biomarkers for AKI, we must apply rigorous study design to diagnostic tests under evaluation before adopting them into clinical practice. Only then can we move the field forward and improve patient care.
Disclosure
Nothing to report.
1. Lusica M, Rondon-Berrios H, Feldman L. Urine eosinophils for acute interstitial nephritis. J Hosp Med. 2017;12(5):343-345. PubMed
2. Leaf DE, Srivastava A, Zeng X, et al. Excessive diagnostic testing in acute kidney injury. BMC Nephrol. 2016;17:9. PubMed
3. Pahwa AK, Sperati CJ. Urinary fractional excretion indices in the evaluation of acute kidney injury. J Hosp Med. 2016;11(1):77-80. PubMed
4. Perazella MA, Bomback AS. Urinary eosinophils in AIN: farewell to an old biomarker? Clin J Am Soc Nephrol. 2013;8(11):1841-1843. PubMed
5. Muriithi AK, Nasr SH, Leung N. Utility of urine eosinophils in the diagnosis of acute interstitial nephritis. Clin J Am Soc Nephrol. 2013;8(11):1857-1862. PubMed
6. Perazella MA, Coca SG. Traditional urinary biomarkers in the assessment of hospital-acquired AKI. Clin J Am Soc Nephrol. 2012;7(1):167-174. PubMed
7. Weisbord SD, Palevsky PM. Strategies for the prevention of contrast-induced acute kidney injury. Curr Opin Nephrol Hypertens. 2010;19(6):539-549. PubMed
8. Ioannidis JP, Panagiotou OA. Comparison of effect sizes associated with biomarkers reported in highly cited individual articles and in subsequent meta-analyses. JAMA. 2011;305(21):2200-2210. PubMed
9. Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231-1238. PubMed
10. Schaub JA, Parikh CR. Biomarkers of acute kidney injury and associations with short- and long-term outcomes. F1000Res. 2016;5(F1000 Faculty Rev.):986. PubMed
11. McMahon BA, Koyner JL. Risk stratification for acute kidney injury: Are biomarkers enough? Adv Chronic Kidney Dis. 2016;23(3):167-178. PubMed
1. Lusica M, Rondon-Berrios H, Feldman L. Urine eosinophils for acute interstitial nephritis. J Hosp Med. 2017;12(5):343-345. PubMed
2. Leaf DE, Srivastava A, Zeng X, et al. Excessive diagnostic testing in acute kidney injury. BMC Nephrol. 2016;17:9. PubMed
3. Pahwa AK, Sperati CJ. Urinary fractional excretion indices in the evaluation of acute kidney injury. J Hosp Med. 2016;11(1):77-80. PubMed
4. Perazella MA, Bomback AS. Urinary eosinophils in AIN: farewell to an old biomarker? Clin J Am Soc Nephrol. 2013;8(11):1841-1843. PubMed
5. Muriithi AK, Nasr SH, Leung N. Utility of urine eosinophils in the diagnosis of acute interstitial nephritis. Clin J Am Soc Nephrol. 2013;8(11):1857-1862. PubMed
6. Perazella MA, Coca SG. Traditional urinary biomarkers in the assessment of hospital-acquired AKI. Clin J Am Soc Nephrol. 2012;7(1):167-174. PubMed
7. Weisbord SD, Palevsky PM. Strategies for the prevention of contrast-induced acute kidney injury. Curr Opin Nephrol Hypertens. 2010;19(6):539-549. PubMed
8. Ioannidis JP, Panagiotou OA. Comparison of effect sizes associated with biomarkers reported in highly cited individual articles and in subsequent meta-analyses. JAMA. 2011;305(21):2200-2210. PubMed
9. Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365(9466):1231-1238. PubMed
10. Schaub JA, Parikh CR. Biomarkers of acute kidney injury and associations with short- and long-term outcomes. F1000Res. 2016;5(F1000 Faculty Rev.):986. PubMed
11. McMahon BA, Koyner JL. Risk stratification for acute kidney injury: Are biomarkers enough? Adv Chronic Kidney Dis. 2016;23(3):167-178. PubMed
© 2017 Society of Hospital Medicine
Moving antibiotic stewardship from theory to practice
We both attend on the Infectious Disease consult team in Veterans Affairs (VA) Hospitals, and predictably the conversation on afternoon rounds often revolves around antibiotics. When we have those discussions, our focus is not on a need to “preserve antibiotics” so they might be available to some unknown patient in the future. Rather, we are working with the primary team to provide the very best treatment for the patient entrusted to our care in the bed right in front of us. We believe it is in this context—providing optimal patient care—that the current efforts in the United States to improve antibiotic use should be viewed.
The growing challenges posed by antibiotic-resistant infections and the related threat of Clostridium difficile infection combine to sicken more than 2 million people each year and contribute to the deaths of more than 25,000 patients.1 Improving antibiotic use through antibiotic stewardship is often proposed to hospitalists as an important part of stemming this tide. While this is true, even as infectious disease specialists with strong interests in antimicrobial stewardship we do not find that pitch compelling when we are on clinical service.
What motivates us to optimize antibiotic use for our patients is the evidence that doing so will have direct and immediate benefits to the patients under our care. Improving antibiotic use has been proven to decrease a patient’s risk of acquiring C. difficile infection or an antibiotic-resistant infection not at some ill-defined time in the future, but during their current hospital stay.2,3 Even more important, support from antibiotic stewardship programs has been proven to improve infection cure rates and reduce the risk of treatment failure for hospitalized patients.4 The bottom line of antibiotic stewardship is better patient care. Sometimes that means narrowing or stopping antibiotics to reduce the risks of adverse events. In other cases, like in the treatment of suspected sepsis, it means ensuring patients get broad spectrum antibiotics quickly.
The patient care benefits of improving antibiotic use led the Centers for Disease Control and Prevention (CDC) to issue a call in 2014 for all hospitals to have antibiotic stewardship programs, and to the development of The Core Elements of Hospital Antibiotic Stewardship Programs to support that effort. As of January 1, 2017, antibiotic stewardship programs that incorporate all the CDC core elements became an accreditation requirement of The Joint Commission, and the Centers for Medicare and Medicaid Services has proposed making the same requirement of all hospitals that participate in their payment programs.
STEWARDSHIP IN PRACTICE: PNEUMONIA
The literature on treatment of pneumonia is increasingly demonstrating that shorter use of antibiotics is often better.7 Even though current guidelines recommend 5 to 7 days of antibiotics for uncomplicated community-acquired pneumonia, average durations of therapy are often longer.8 Previous work published in the Journal of Hospital Medicine focused on improving antimicrobial documentation as well as access to local clinical guidelines and implementing a 72-hour antimicrobial “time out” by hospitalists.9 When these multimodal interventions tailored for hospitalists were in place, utilization of antibiotics improved. Graber et al.5 also found that facility educational programs for prudent antimicrobial use and frequency of de-escalation review were associated with decreased overall antimicrobial use. Providing vague recommendations on antibiotic course, or none at all, at discharge or sign-out can lead to unnecessary antibiotics or an extended course of them. Pneumonia-specific interventions could target duration by outlining antibiotic course in hospitalist progress notes and at hand-off.
STEWARDSHIP IN PRACTICE: UTI
Misuse of antibiotics in UTI often stems from overtreatment of asymptomatic bacteriuria or unneeded diagnostic testing. Often, the pivotal step in avoiding unnecessary treatment lies in the ordering of the urine culture.10 Graber et al.5 showed that order sets were associated with decreased antimicrobial use. In the case of UTI, hospitalists could work with the stewardship team to design order sets that guide providers to appropriate reasons for ordering a urine culture. Order sets could also help providers recognize important patient-specific risks for certain antibiotics, such as the risk of C. difficile with fluoroquinolones in an elderly patient. Targeting different steps in overutilization of antibiotics would encompass more prescribers and could lead to reducing other unnecessary testing, which is a current focus for many hospitalists.
STEWARDSHIP IN PRACTICE: SSTI
Skin and soft tissue infections (SSTI) also offer a specific disease state to use order sets and education to improve duration of antibiotics, decrease overuse of broad spectrum antibiotics, and reduce unnecessary diagnostic studies. For example, gram negative and/or anaerobic coverage are rarely indicated in treating SSTIs but are often used. SSTI-specific order sets and guidelines have already been shown to improve both diagnostic work-up and antibiotic treatment.11 As the providers who manage most of these infections in hospitals, hospitalists are ideally positioned to inform the development of SSTI order sets and pathways. The work by Graber et al.5 provides some important insights into how we can effectively implement interventions to improve antibiotic use. These insights have never been more important as more hospitals move toward starting or expanding antibiotic stewardship programs. As leaders in patient safety and quality, and as the most important antibiotic prescribers in hospitals, hospitalists must play a central role in stewardship if we are to make meaningful progress.
Disclosure
Nothing to report.
1. Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed April 12, 2017.
2. Feazel LM, Malhotra A, Perencevich EN, Kaboli P, Diekema DJ, Schweizer ML. Effect of antibiotic stewardship programmes on Clostridium difficile incidence: a systematic review and meta-analysis. J Antimicrob Chemother. 2014;69(7):1748-1754. PubMed
3. Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162(2 Pt 1):505-511. PubMed
4. Fishman N. Antimicrobial stewardship. Am J Med. 2006;119(6 Suppl 1):S53-S61; discussion S62-S70. PubMed
5. Graber CJ, Jones MM, Chou AF, et al. Association of inpatient antimicrobial utilization measures with antimicrobial stewardship activities and facility characteristics of Veterans Affairs medical centers. J Hosp Med. 2017;12:301-309. PubMed
6. Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312(14):1438-1446. PubMed
7. Viasus D, Vecino-Moreno M, De La Hoz JM, Carratala J. Antibiotic stewardship in community-acquired pneumonia. Expert Rev Anti Infect Ther. 2016:1-2019. PubMed
8. Avdic E, Cushinotto LA, Hughes AH, et al. Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community-acquired pneumonia. Clin Infect Dis. 2012;54(11):1581-1587. PubMed
9. Mack MR, Rohde JM, Jacobsen D, et al. Engaging hospitalists in antimicrobial stewardship: Lessons from a multihospital collaborative. J Hosp Med. 2016;11(8):576-580. PubMed
10. Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an Antimicrobial Stewardship Approach for Urinary Catheter-Associated Asymptomatic Bacteriuria. JAMA Intern Med. 2015;175(7):1120-1127. PubMed
11. Jenkins TC, Knepper BC, Sabel AL, et al. Decreased antibiotic utilization after implementation of a guideline for inpatient cellulitis and cutaneous abscess. Arch Intern Med. 2011;171(12):1072-1079. PubMed
We both attend on the Infectious Disease consult team in Veterans Affairs (VA) Hospitals, and predictably the conversation on afternoon rounds often revolves around antibiotics. When we have those discussions, our focus is not on a need to “preserve antibiotics” so they might be available to some unknown patient in the future. Rather, we are working with the primary team to provide the very best treatment for the patient entrusted to our care in the bed right in front of us. We believe it is in this context—providing optimal patient care—that the current efforts in the United States to improve antibiotic use should be viewed.
The growing challenges posed by antibiotic-resistant infections and the related threat of Clostridium difficile infection combine to sicken more than 2 million people each year and contribute to the deaths of more than 25,000 patients.1 Improving antibiotic use through antibiotic stewardship is often proposed to hospitalists as an important part of stemming this tide. While this is true, even as infectious disease specialists with strong interests in antimicrobial stewardship we do not find that pitch compelling when we are on clinical service.
What motivates us to optimize antibiotic use for our patients is the evidence that doing so will have direct and immediate benefits to the patients under our care. Improving antibiotic use has been proven to decrease a patient’s risk of acquiring C. difficile infection or an antibiotic-resistant infection not at some ill-defined time in the future, but during their current hospital stay.2,3 Even more important, support from antibiotic stewardship programs has been proven to improve infection cure rates and reduce the risk of treatment failure for hospitalized patients.4 The bottom line of antibiotic stewardship is better patient care. Sometimes that means narrowing or stopping antibiotics to reduce the risks of adverse events. In other cases, like in the treatment of suspected sepsis, it means ensuring patients get broad spectrum antibiotics quickly.
The patient care benefits of improving antibiotic use led the Centers for Disease Control and Prevention (CDC) to issue a call in 2014 for all hospitals to have antibiotic stewardship programs, and to the development of The Core Elements of Hospital Antibiotic Stewardship Programs to support that effort. As of January 1, 2017, antibiotic stewardship programs that incorporate all the CDC core elements became an accreditation requirement of The Joint Commission, and the Centers for Medicare and Medicaid Services has proposed making the same requirement of all hospitals that participate in their payment programs.
STEWARDSHIP IN PRACTICE: PNEUMONIA
The literature on treatment of pneumonia is increasingly demonstrating that shorter use of antibiotics is often better.7 Even though current guidelines recommend 5 to 7 days of antibiotics for uncomplicated community-acquired pneumonia, average durations of therapy are often longer.8 Previous work published in the Journal of Hospital Medicine focused on improving antimicrobial documentation as well as access to local clinical guidelines and implementing a 72-hour antimicrobial “time out” by hospitalists.9 When these multimodal interventions tailored for hospitalists were in place, utilization of antibiotics improved. Graber et al.5 also found that facility educational programs for prudent antimicrobial use and frequency of de-escalation review were associated with decreased overall antimicrobial use. Providing vague recommendations on antibiotic course, or none at all, at discharge or sign-out can lead to unnecessary antibiotics or an extended course of them. Pneumonia-specific interventions could target duration by outlining antibiotic course in hospitalist progress notes and at hand-off.
STEWARDSHIP IN PRACTICE: UTI
Misuse of antibiotics in UTI often stems from overtreatment of asymptomatic bacteriuria or unneeded diagnostic testing. Often, the pivotal step in avoiding unnecessary treatment lies in the ordering of the urine culture.10 Graber et al.5 showed that order sets were associated with decreased antimicrobial use. In the case of UTI, hospitalists could work with the stewardship team to design order sets that guide providers to appropriate reasons for ordering a urine culture. Order sets could also help providers recognize important patient-specific risks for certain antibiotics, such as the risk of C. difficile with fluoroquinolones in an elderly patient. Targeting different steps in overutilization of antibiotics would encompass more prescribers and could lead to reducing other unnecessary testing, which is a current focus for many hospitalists.
STEWARDSHIP IN PRACTICE: SSTI
Skin and soft tissue infections (SSTI) also offer a specific disease state to use order sets and education to improve duration of antibiotics, decrease overuse of broad spectrum antibiotics, and reduce unnecessary diagnostic studies. For example, gram negative and/or anaerobic coverage are rarely indicated in treating SSTIs but are often used. SSTI-specific order sets and guidelines have already been shown to improve both diagnostic work-up and antibiotic treatment.11 As the providers who manage most of these infections in hospitals, hospitalists are ideally positioned to inform the development of SSTI order sets and pathways. The work by Graber et al.5 provides some important insights into how we can effectively implement interventions to improve antibiotic use. These insights have never been more important as more hospitals move toward starting or expanding antibiotic stewardship programs. As leaders in patient safety and quality, and as the most important antibiotic prescribers in hospitals, hospitalists must play a central role in stewardship if we are to make meaningful progress.
Disclosure
Nothing to report.
We both attend on the Infectious Disease consult team in Veterans Affairs (VA) Hospitals, and predictably the conversation on afternoon rounds often revolves around antibiotics. When we have those discussions, our focus is not on a need to “preserve antibiotics” so they might be available to some unknown patient in the future. Rather, we are working with the primary team to provide the very best treatment for the patient entrusted to our care in the bed right in front of us. We believe it is in this context—providing optimal patient care—that the current efforts in the United States to improve antibiotic use should be viewed.
The growing challenges posed by antibiotic-resistant infections and the related threat of Clostridium difficile infection combine to sicken more than 2 million people each year and contribute to the deaths of more than 25,000 patients.1 Improving antibiotic use through antibiotic stewardship is often proposed to hospitalists as an important part of stemming this tide. While this is true, even as infectious disease specialists with strong interests in antimicrobial stewardship we do not find that pitch compelling when we are on clinical service.
What motivates us to optimize antibiotic use for our patients is the evidence that doing so will have direct and immediate benefits to the patients under our care. Improving antibiotic use has been proven to decrease a patient’s risk of acquiring C. difficile infection or an antibiotic-resistant infection not at some ill-defined time in the future, but during their current hospital stay.2,3 Even more important, support from antibiotic stewardship programs has been proven to improve infection cure rates and reduce the risk of treatment failure for hospitalized patients.4 The bottom line of antibiotic stewardship is better patient care. Sometimes that means narrowing or stopping antibiotics to reduce the risks of adverse events. In other cases, like in the treatment of suspected sepsis, it means ensuring patients get broad spectrum antibiotics quickly.
The patient care benefits of improving antibiotic use led the Centers for Disease Control and Prevention (CDC) to issue a call in 2014 for all hospitals to have antibiotic stewardship programs, and to the development of The Core Elements of Hospital Antibiotic Stewardship Programs to support that effort. As of January 1, 2017, antibiotic stewardship programs that incorporate all the CDC core elements became an accreditation requirement of The Joint Commission, and the Centers for Medicare and Medicaid Services has proposed making the same requirement of all hospitals that participate in their payment programs.
STEWARDSHIP IN PRACTICE: PNEUMONIA
The literature on treatment of pneumonia is increasingly demonstrating that shorter use of antibiotics is often better.7 Even though current guidelines recommend 5 to 7 days of antibiotics for uncomplicated community-acquired pneumonia, average durations of therapy are often longer.8 Previous work published in the Journal of Hospital Medicine focused on improving antimicrobial documentation as well as access to local clinical guidelines and implementing a 72-hour antimicrobial “time out” by hospitalists.9 When these multimodal interventions tailored for hospitalists were in place, utilization of antibiotics improved. Graber et al.5 also found that facility educational programs for prudent antimicrobial use and frequency of de-escalation review were associated with decreased overall antimicrobial use. Providing vague recommendations on antibiotic course, or none at all, at discharge or sign-out can lead to unnecessary antibiotics or an extended course of them. Pneumonia-specific interventions could target duration by outlining antibiotic course in hospitalist progress notes and at hand-off.
STEWARDSHIP IN PRACTICE: UTI
Misuse of antibiotics in UTI often stems from overtreatment of asymptomatic bacteriuria or unneeded diagnostic testing. Often, the pivotal step in avoiding unnecessary treatment lies in the ordering of the urine culture.10 Graber et al.5 showed that order sets were associated with decreased antimicrobial use. In the case of UTI, hospitalists could work with the stewardship team to design order sets that guide providers to appropriate reasons for ordering a urine culture. Order sets could also help providers recognize important patient-specific risks for certain antibiotics, such as the risk of C. difficile with fluoroquinolones in an elderly patient. Targeting different steps in overutilization of antibiotics would encompass more prescribers and could lead to reducing other unnecessary testing, which is a current focus for many hospitalists.
STEWARDSHIP IN PRACTICE: SSTI
Skin and soft tissue infections (SSTI) also offer a specific disease state to use order sets and education to improve duration of antibiotics, decrease overuse of broad spectrum antibiotics, and reduce unnecessary diagnostic studies. For example, gram negative and/or anaerobic coverage are rarely indicated in treating SSTIs but are often used. SSTI-specific order sets and guidelines have already been shown to improve both diagnostic work-up and antibiotic treatment.11 As the providers who manage most of these infections in hospitals, hospitalists are ideally positioned to inform the development of SSTI order sets and pathways. The work by Graber et al.5 provides some important insights into how we can effectively implement interventions to improve antibiotic use. These insights have never been more important as more hospitals move toward starting or expanding antibiotic stewardship programs. As leaders in patient safety and quality, and as the most important antibiotic prescribers in hospitals, hospitalists must play a central role in stewardship if we are to make meaningful progress.
Disclosure
Nothing to report.
1. Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed April 12, 2017.
2. Feazel LM, Malhotra A, Perencevich EN, Kaboli P, Diekema DJ, Schweizer ML. Effect of antibiotic stewardship programmes on Clostridium difficile incidence: a systematic review and meta-analysis. J Antimicrob Chemother. 2014;69(7):1748-1754. PubMed
3. Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162(2 Pt 1):505-511. PubMed
4. Fishman N. Antimicrobial stewardship. Am J Med. 2006;119(6 Suppl 1):S53-S61; discussion S62-S70. PubMed
5. Graber CJ, Jones MM, Chou AF, et al. Association of inpatient antimicrobial utilization measures with antimicrobial stewardship activities and facility characteristics of Veterans Affairs medical centers. J Hosp Med. 2017;12:301-309. PubMed
6. Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312(14):1438-1446. PubMed
7. Viasus D, Vecino-Moreno M, De La Hoz JM, Carratala J. Antibiotic stewardship in community-acquired pneumonia. Expert Rev Anti Infect Ther. 2016:1-2019. PubMed
8. Avdic E, Cushinotto LA, Hughes AH, et al. Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community-acquired pneumonia. Clin Infect Dis. 2012;54(11):1581-1587. PubMed
9. Mack MR, Rohde JM, Jacobsen D, et al. Engaging hospitalists in antimicrobial stewardship: Lessons from a multihospital collaborative. J Hosp Med. 2016;11(8):576-580. PubMed
10. Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an Antimicrobial Stewardship Approach for Urinary Catheter-Associated Asymptomatic Bacteriuria. JAMA Intern Med. 2015;175(7):1120-1127. PubMed
11. Jenkins TC, Knepper BC, Sabel AL, et al. Decreased antibiotic utilization after implementation of a guideline for inpatient cellulitis and cutaneous abscess. Arch Intern Med. 2011;171(12):1072-1079. PubMed
1. Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed April 12, 2017.
2. Feazel LM, Malhotra A, Perencevich EN, Kaboli P, Diekema DJ, Schweizer ML. Effect of antibiotic stewardship programmes on Clostridium difficile incidence: a systematic review and meta-analysis. J Antimicrob Chemother. 2014;69(7):1748-1754. PubMed
3. Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162(2 Pt 1):505-511. PubMed
4. Fishman N. Antimicrobial stewardship. Am J Med. 2006;119(6 Suppl 1):S53-S61; discussion S62-S70. PubMed
5. Graber CJ, Jones MM, Chou AF, et al. Association of inpatient antimicrobial utilization measures with antimicrobial stewardship activities and facility characteristics of Veterans Affairs medical centers. J Hosp Med. 2017;12:301-309. PubMed
6. Magill SS, Edwards JR, Beldavs ZG, et al. Prevalence of antimicrobial use in US acute care hospitals, May-September 2011. JAMA. 2014;312(14):1438-1446. PubMed
7. Viasus D, Vecino-Moreno M, De La Hoz JM, Carratala J. Antibiotic stewardship in community-acquired pneumonia. Expert Rev Anti Infect Ther. 2016:1-2019. PubMed
8. Avdic E, Cushinotto LA, Hughes AH, et al. Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community-acquired pneumonia. Clin Infect Dis. 2012;54(11):1581-1587. PubMed
9. Mack MR, Rohde JM, Jacobsen D, et al. Engaging hospitalists in antimicrobial stewardship: Lessons from a multihospital collaborative. J Hosp Med. 2016;11(8):576-580. PubMed
10. Trautner BW, Grigoryan L, Petersen NJ, et al. Effectiveness of an Antimicrobial Stewardship Approach for Urinary Catheter-Associated Asymptomatic Bacteriuria. JAMA Intern Med. 2015;175(7):1120-1127. PubMed
11. Jenkins TC, Knepper BC, Sabel AL, et al. Decreased antibiotic utilization after implementation of a guideline for inpatient cellulitis and cutaneous abscess. Arch Intern Med. 2011;171(12):1072-1079. PubMed
© 2017 Society of Hospital Medicine
Hospitalist movers and shakers
Andrew Auerbach, MD, MPH, SFHM, and Vineet Arora, MD, MPP, MHM, recently were elected to the new member class of American Society for Clinical Investigation (ASCI) for 2017. Members must have “accomplished meritorious, original, creative, and independent investigations in the clinical or allied sciences of medicine and enjoy an unimpeachable moral standing in the medical profession.”
Dr. Auerbach and Dr. Arora are just the third and fourth hospitalists to become ASCI members. Dr. Auerbach is the professor of medicine in residence and director of the research division of hospital medicine at the University of California, San Francisco. Dr. Aurora is associate professor of medicine, assistant dean for scholarship and discovery, and director of graduate medical education’s clinical learning environment innovation at the University of Chicago.
Mark V. Williams, MD, FACP, MHM, director of the University of Kentucky’s Center for Health Services Research (CHSR), recently presented at the International Conference of Hospital Medicine held in Taiwan.
Olevia M. Pitts, MD, SFHM, made history at Research Medical Center in Kansas City, becoming the first woman and the first person of color to be named the facility’s chief medical officer. Dr. Pitts assumed her role at the 131-year-old RMC on January 30.
Greta Boynton, MD, SFHM, was promoted to the role of associate chief medical officer of Sound Physicians’ northeast region. She was elevated from her position as regional medical director for Sound Physicians, a health care organization that serves as a provider practice in 225 hospitals in 38 states.
Dr. Boynton will be charged with overseeing clinical operation of 13 programs, 120 providers, and a team of regional medical directors. She joined Sound Physicians in 2013 as chief hospitalist and divisional chief at Baystate Medical Center in Springfield, Mass. She was, previously, chief of hospital medicine for Eastern Connecticut Health Network, Manchester, from 2008-2013.
Business Moves
Sound Physicians, Tacoma, Wash., added to its list of partners on March 1, when Eagle Hospital Medicine Practices, Atlanta, joined the Sound group’s organization. Eagle’s 150 providers in 16 hospitals across the United States raises Sound’s resume to more than 2,500 providers.
Eagle will continue to run its own Locum Connections and Telemedicine divisions.
The Society of Hospital Medicine’s Center for Quality Improvement recently was recognized and honored by the Centers for Medicare & Medicaid Services (CMS) for its patient-safety partnership with CMS. The two entities have maintained a relationship since August 2016.
SHM’s Center for QI has participated in weekly CMS webinars to generate strategies intended to limit opioid use, including SHM’s pilot RADEO – Reducing Adverse Drug Events Related to Opioids – program. In January 2017, CMS contacted SHM to provide best practices for patients receiving opioids and better use data to monitor those patients.
University of Iowa Health Care, Iowa City, and Van Buren County Hospital, Keosauqua, Iowa, have created a partnership, allowing patients at VBCH access to UI hospitalists through a telemedicine connection. The relationship will allow VBCH patients to remain at their local hospital – located 90 minutes from Iowa City – while getting care and treatment advice from UI hospitalists through videoconferencing and a shared electronic health record.
With their VBCH provider bedside, patients meet face-to–virtual face with the UI hospitalist during twice-daily virtual rounding.
Unity Medical Center, Manchester, Tenn., recently partnered with physician-owned and -operated Concord Medical Group, Knoxville, Tenn., to provide hospitalist services at its facility in Manchester. Unity now will have hospitalists on duty 24 hours per day thanks to the relationship with Concord, a hospital management and staffing specialist group.
Andrew Auerbach, MD, MPH, SFHM, and Vineet Arora, MD, MPP, MHM, recently were elected to the new member class of American Society for Clinical Investigation (ASCI) for 2017. Members must have “accomplished meritorious, original, creative, and independent investigations in the clinical or allied sciences of medicine and enjoy an unimpeachable moral standing in the medical profession.”
Dr. Auerbach and Dr. Arora are just the third and fourth hospitalists to become ASCI members. Dr. Auerbach is the professor of medicine in residence and director of the research division of hospital medicine at the University of California, San Francisco. Dr. Aurora is associate professor of medicine, assistant dean for scholarship and discovery, and director of graduate medical education’s clinical learning environment innovation at the University of Chicago.
Mark V. Williams, MD, FACP, MHM, director of the University of Kentucky’s Center for Health Services Research (CHSR), recently presented at the International Conference of Hospital Medicine held in Taiwan.
Olevia M. Pitts, MD, SFHM, made history at Research Medical Center in Kansas City, becoming the first woman and the first person of color to be named the facility’s chief medical officer. Dr. Pitts assumed her role at the 131-year-old RMC on January 30.
Greta Boynton, MD, SFHM, was promoted to the role of associate chief medical officer of Sound Physicians’ northeast region. She was elevated from her position as regional medical director for Sound Physicians, a health care organization that serves as a provider practice in 225 hospitals in 38 states.
Dr. Boynton will be charged with overseeing clinical operation of 13 programs, 120 providers, and a team of regional medical directors. She joined Sound Physicians in 2013 as chief hospitalist and divisional chief at Baystate Medical Center in Springfield, Mass. She was, previously, chief of hospital medicine for Eastern Connecticut Health Network, Manchester, from 2008-2013.
Business Moves
Sound Physicians, Tacoma, Wash., added to its list of partners on March 1, when Eagle Hospital Medicine Practices, Atlanta, joined the Sound group’s organization. Eagle’s 150 providers in 16 hospitals across the United States raises Sound’s resume to more than 2,500 providers.
Eagle will continue to run its own Locum Connections and Telemedicine divisions.
The Society of Hospital Medicine’s Center for Quality Improvement recently was recognized and honored by the Centers for Medicare & Medicaid Services (CMS) for its patient-safety partnership with CMS. The two entities have maintained a relationship since August 2016.
SHM’s Center for QI has participated in weekly CMS webinars to generate strategies intended to limit opioid use, including SHM’s pilot RADEO – Reducing Adverse Drug Events Related to Opioids – program. In January 2017, CMS contacted SHM to provide best practices for patients receiving opioids and better use data to monitor those patients.
University of Iowa Health Care, Iowa City, and Van Buren County Hospital, Keosauqua, Iowa, have created a partnership, allowing patients at VBCH access to UI hospitalists through a telemedicine connection. The relationship will allow VBCH patients to remain at their local hospital – located 90 minutes from Iowa City – while getting care and treatment advice from UI hospitalists through videoconferencing and a shared electronic health record.
With their VBCH provider bedside, patients meet face-to–virtual face with the UI hospitalist during twice-daily virtual rounding.
Unity Medical Center, Manchester, Tenn., recently partnered with physician-owned and -operated Concord Medical Group, Knoxville, Tenn., to provide hospitalist services at its facility in Manchester. Unity now will have hospitalists on duty 24 hours per day thanks to the relationship with Concord, a hospital management and staffing specialist group.
Andrew Auerbach, MD, MPH, SFHM, and Vineet Arora, MD, MPP, MHM, recently were elected to the new member class of American Society for Clinical Investigation (ASCI) for 2017. Members must have “accomplished meritorious, original, creative, and independent investigations in the clinical or allied sciences of medicine and enjoy an unimpeachable moral standing in the medical profession.”
Dr. Auerbach and Dr. Arora are just the third and fourth hospitalists to become ASCI members. Dr. Auerbach is the professor of medicine in residence and director of the research division of hospital medicine at the University of California, San Francisco. Dr. Aurora is associate professor of medicine, assistant dean for scholarship and discovery, and director of graduate medical education’s clinical learning environment innovation at the University of Chicago.
Mark V. Williams, MD, FACP, MHM, director of the University of Kentucky’s Center for Health Services Research (CHSR), recently presented at the International Conference of Hospital Medicine held in Taiwan.
Olevia M. Pitts, MD, SFHM, made history at Research Medical Center in Kansas City, becoming the first woman and the first person of color to be named the facility’s chief medical officer. Dr. Pitts assumed her role at the 131-year-old RMC on January 30.
Greta Boynton, MD, SFHM, was promoted to the role of associate chief medical officer of Sound Physicians’ northeast region. She was elevated from her position as regional medical director for Sound Physicians, a health care organization that serves as a provider practice in 225 hospitals in 38 states.
Dr. Boynton will be charged with overseeing clinical operation of 13 programs, 120 providers, and a team of regional medical directors. She joined Sound Physicians in 2013 as chief hospitalist and divisional chief at Baystate Medical Center in Springfield, Mass. She was, previously, chief of hospital medicine for Eastern Connecticut Health Network, Manchester, from 2008-2013.
Business Moves
Sound Physicians, Tacoma, Wash., added to its list of partners on March 1, when Eagle Hospital Medicine Practices, Atlanta, joined the Sound group’s organization. Eagle’s 150 providers in 16 hospitals across the United States raises Sound’s resume to more than 2,500 providers.
Eagle will continue to run its own Locum Connections and Telemedicine divisions.
The Society of Hospital Medicine’s Center for Quality Improvement recently was recognized and honored by the Centers for Medicare & Medicaid Services (CMS) for its patient-safety partnership with CMS. The two entities have maintained a relationship since August 2016.
SHM’s Center for QI has participated in weekly CMS webinars to generate strategies intended to limit opioid use, including SHM’s pilot RADEO – Reducing Adverse Drug Events Related to Opioids – program. In January 2017, CMS contacted SHM to provide best practices for patients receiving opioids and better use data to monitor those patients.
University of Iowa Health Care, Iowa City, and Van Buren County Hospital, Keosauqua, Iowa, have created a partnership, allowing patients at VBCH access to UI hospitalists through a telemedicine connection. The relationship will allow VBCH patients to remain at their local hospital – located 90 minutes from Iowa City – while getting care and treatment advice from UI hospitalists through videoconferencing and a shared electronic health record.
With their VBCH provider bedside, patients meet face-to–virtual face with the UI hospitalist during twice-daily virtual rounding.
Unity Medical Center, Manchester, Tenn., recently partnered with physician-owned and -operated Concord Medical Group, Knoxville, Tenn., to provide hospitalist services at its facility in Manchester. Unity now will have hospitalists on duty 24 hours per day thanks to the relationship with Concord, a hospital management and staffing specialist group.
Digoxin and heart failure mortality: The Swedes weigh in
WASHINGTON – The use of digoxin by Swedish Heart Failure Registry participants with heart failure with reduced ejection fraction was associated with significantly increased risk of all-cause mortality if they had concomitant paroxysmal atrial fibrillation or were in normal sinus rhythm, Gianluigi Savarese, MD, reported at the annual meeting of the American College of Cardiology.
In contrast, digoxin in Swedish patients with heart failure with reduced ejection fraction (HFrEF) and permanent atrial fibrillation (AF) was associated with a reduced risk of heart failure hospitalization but had no impact on mortality, added Dr. Savarese of the Karolinska Institute in Stockholm.
The Swedish Heart Failure Registry includes the majority of heart failure patients in that country. Data on 80 variables gets collected for each participant.
Dr. Savarese reported on 23,708 Swedes with HFrEF, 18% of whom were on digoxin. In a multivariate Cox regression analysis adjusted for numerous potential confounders, the use of digoxin was associated with an 8% increased risk of all-cause mortality and a 10% lower risk of heart failure hospitalizations during up to 11 years of follow-up.
In the 12,162 patients with HFrEF and comorbid AF, 30% of whom were on digoxin, the drug was associated with a 12% reduction in heart failure hospitalizations and had no effect on all-cause mortality.
In contrast, among patients with HFrEF without AF, 5% of whom were taking digoxin, use of the drug was associated with an adjusted 31% increase in mortality risk. But digoxin didn’t affect the risk of heart failure hospitalization one way or the other in this group.
Stratifying subjects by their type of AF, the use of digoxin in patients with HFrEF and permanent AF was associated with a 16% reduction in risk of heart failure hospitalization with no impact on mortality. In contrast, among the 2,723 patients with HFrEF and paroxysmal AF, digoxin was associated with a 29% increase in the risk of mortality and no effect on hospitalization.
Current ACC/American Heart Association heart failure guidelines give digoxin a strong Class IIa recommendation for reducing heart failure hospitalizations in patients with HFrEF. European Society of Cardiology guidelines provide a Class IIb recommendation for digoxin to reduce the risk of hospitalization in patients with symptomatic HFrEF in normal sinus rhythm.
Dr. Savarese said he and his coinvestigators decided to examine the impact of digoxin in the Swedish Heart Failure Registry because despite the guideline support for the drug’s use, recent years have brought conflicting data regarding digoxin’s impact on mortality. For example, a meta-analysis of nine studies in more than 235,000 AF patients, seven studies in patients with heart failure, and three in patients with both disorders showed that digoxin was associated with a 29% increased mortality risk in AF patients and a 14% increase in those with heart failure (Eur Heart J. 2015 Jul 21;36[28]:1831-8).
Moreover, at a late-breaking clinical trial session elsewhere at ACC 17, a secondary analysis of the roughly 18,000-patient ARISTOTLE trial came down emphatically on the side of avoiding the venerable drug in patients with AF, where it was found to be associated with a fourfold increased risk of sudden death.
Session comoderator Lee R. Goldberg, MD, medical director of the University of Pennsylvania Heart Failure and Transplantation Program in Philadelphia, observed that the use of digoxin has become quite controversial. He posed a question to Dr. Savarese: “Every few months someone writes the last paper on digoxin as they look at thousands of patients, and then there’s always a new paper. If you were to rewrite the guidelines now, what would you recommend for digoxin?”
Dr. Savarese replied that the current guidelines rely heavily upon the results of a 20-year-old randomized, double-blind, placebo-controlled trial of digoxin in heart failure (N Engl J Med. 1997 Feb 20;336[8]:525-33). Those study participants look nothing at all like the heart failure patients physicians see today in clinical practice. Hardly any of them were on what today is guideline-directed medical therapy with a beta-blocker or mineralocorticoid receptor antagonist. So the trial’s applicability is dubious.
“Our Swedish data are observational. They are hypothesis-generating. They should drive trialists to design a new trial of digoxin. But I think we all know that’s not going to happen. So actually I don’t think there is still space for a IIb or IIa recommendation for digoxin in the guidelines,” Dr. Savarese said.
He reported having no financial conflicts.
WASHINGTON – The use of digoxin by Swedish Heart Failure Registry participants with heart failure with reduced ejection fraction was associated with significantly increased risk of all-cause mortality if they had concomitant paroxysmal atrial fibrillation or were in normal sinus rhythm, Gianluigi Savarese, MD, reported at the annual meeting of the American College of Cardiology.
In contrast, digoxin in Swedish patients with heart failure with reduced ejection fraction (HFrEF) and permanent atrial fibrillation (AF) was associated with a reduced risk of heart failure hospitalization but had no impact on mortality, added Dr. Savarese of the Karolinska Institute in Stockholm.
The Swedish Heart Failure Registry includes the majority of heart failure patients in that country. Data on 80 variables gets collected for each participant.
Dr. Savarese reported on 23,708 Swedes with HFrEF, 18% of whom were on digoxin. In a multivariate Cox regression analysis adjusted for numerous potential confounders, the use of digoxin was associated with an 8% increased risk of all-cause mortality and a 10% lower risk of heart failure hospitalizations during up to 11 years of follow-up.
In the 12,162 patients with HFrEF and comorbid AF, 30% of whom were on digoxin, the drug was associated with a 12% reduction in heart failure hospitalizations and had no effect on all-cause mortality.
In contrast, among patients with HFrEF without AF, 5% of whom were taking digoxin, use of the drug was associated with an adjusted 31% increase in mortality risk. But digoxin didn’t affect the risk of heart failure hospitalization one way or the other in this group.
Stratifying subjects by their type of AF, the use of digoxin in patients with HFrEF and permanent AF was associated with a 16% reduction in risk of heart failure hospitalization with no impact on mortality. In contrast, among the 2,723 patients with HFrEF and paroxysmal AF, digoxin was associated with a 29% increase in the risk of mortality and no effect on hospitalization.
Current ACC/American Heart Association heart failure guidelines give digoxin a strong Class IIa recommendation for reducing heart failure hospitalizations in patients with HFrEF. European Society of Cardiology guidelines provide a Class IIb recommendation for digoxin to reduce the risk of hospitalization in patients with symptomatic HFrEF in normal sinus rhythm.
Dr. Savarese said he and his coinvestigators decided to examine the impact of digoxin in the Swedish Heart Failure Registry because despite the guideline support for the drug’s use, recent years have brought conflicting data regarding digoxin’s impact on mortality. For example, a meta-analysis of nine studies in more than 235,000 AF patients, seven studies in patients with heart failure, and three in patients with both disorders showed that digoxin was associated with a 29% increased mortality risk in AF patients and a 14% increase in those with heart failure (Eur Heart J. 2015 Jul 21;36[28]:1831-8).
Moreover, at a late-breaking clinical trial session elsewhere at ACC 17, a secondary analysis of the roughly 18,000-patient ARISTOTLE trial came down emphatically on the side of avoiding the venerable drug in patients with AF, where it was found to be associated with a fourfold increased risk of sudden death.
Session comoderator Lee R. Goldberg, MD, medical director of the University of Pennsylvania Heart Failure and Transplantation Program in Philadelphia, observed that the use of digoxin has become quite controversial. He posed a question to Dr. Savarese: “Every few months someone writes the last paper on digoxin as they look at thousands of patients, and then there’s always a new paper. If you were to rewrite the guidelines now, what would you recommend for digoxin?”
Dr. Savarese replied that the current guidelines rely heavily upon the results of a 20-year-old randomized, double-blind, placebo-controlled trial of digoxin in heart failure (N Engl J Med. 1997 Feb 20;336[8]:525-33). Those study participants look nothing at all like the heart failure patients physicians see today in clinical practice. Hardly any of them were on what today is guideline-directed medical therapy with a beta-blocker or mineralocorticoid receptor antagonist. So the trial’s applicability is dubious.
“Our Swedish data are observational. They are hypothesis-generating. They should drive trialists to design a new trial of digoxin. But I think we all know that’s not going to happen. So actually I don’t think there is still space for a IIb or IIa recommendation for digoxin in the guidelines,” Dr. Savarese said.
He reported having no financial conflicts.
WASHINGTON – The use of digoxin by Swedish Heart Failure Registry participants with heart failure with reduced ejection fraction was associated with significantly increased risk of all-cause mortality if they had concomitant paroxysmal atrial fibrillation or were in normal sinus rhythm, Gianluigi Savarese, MD, reported at the annual meeting of the American College of Cardiology.
In contrast, digoxin in Swedish patients with heart failure with reduced ejection fraction (HFrEF) and permanent atrial fibrillation (AF) was associated with a reduced risk of heart failure hospitalization but had no impact on mortality, added Dr. Savarese of the Karolinska Institute in Stockholm.
The Swedish Heart Failure Registry includes the majority of heart failure patients in that country. Data on 80 variables gets collected for each participant.
Dr. Savarese reported on 23,708 Swedes with HFrEF, 18% of whom were on digoxin. In a multivariate Cox regression analysis adjusted for numerous potential confounders, the use of digoxin was associated with an 8% increased risk of all-cause mortality and a 10% lower risk of heart failure hospitalizations during up to 11 years of follow-up.
In the 12,162 patients with HFrEF and comorbid AF, 30% of whom were on digoxin, the drug was associated with a 12% reduction in heart failure hospitalizations and had no effect on all-cause mortality.
In contrast, among patients with HFrEF without AF, 5% of whom were taking digoxin, use of the drug was associated with an adjusted 31% increase in mortality risk. But digoxin didn’t affect the risk of heart failure hospitalization one way or the other in this group.
Stratifying subjects by their type of AF, the use of digoxin in patients with HFrEF and permanent AF was associated with a 16% reduction in risk of heart failure hospitalization with no impact on mortality. In contrast, among the 2,723 patients with HFrEF and paroxysmal AF, digoxin was associated with a 29% increase in the risk of mortality and no effect on hospitalization.
Current ACC/American Heart Association heart failure guidelines give digoxin a strong Class IIa recommendation for reducing heart failure hospitalizations in patients with HFrEF. European Society of Cardiology guidelines provide a Class IIb recommendation for digoxin to reduce the risk of hospitalization in patients with symptomatic HFrEF in normal sinus rhythm.
Dr. Savarese said he and his coinvestigators decided to examine the impact of digoxin in the Swedish Heart Failure Registry because despite the guideline support for the drug’s use, recent years have brought conflicting data regarding digoxin’s impact on mortality. For example, a meta-analysis of nine studies in more than 235,000 AF patients, seven studies in patients with heart failure, and three in patients with both disorders showed that digoxin was associated with a 29% increased mortality risk in AF patients and a 14% increase in those with heart failure (Eur Heart J. 2015 Jul 21;36[28]:1831-8).
Moreover, at a late-breaking clinical trial session elsewhere at ACC 17, a secondary analysis of the roughly 18,000-patient ARISTOTLE trial came down emphatically on the side of avoiding the venerable drug in patients with AF, where it was found to be associated with a fourfold increased risk of sudden death.
Session comoderator Lee R. Goldberg, MD, medical director of the University of Pennsylvania Heart Failure and Transplantation Program in Philadelphia, observed that the use of digoxin has become quite controversial. He posed a question to Dr. Savarese: “Every few months someone writes the last paper on digoxin as they look at thousands of patients, and then there’s always a new paper. If you were to rewrite the guidelines now, what would you recommend for digoxin?”
Dr. Savarese replied that the current guidelines rely heavily upon the results of a 20-year-old randomized, double-blind, placebo-controlled trial of digoxin in heart failure (N Engl J Med. 1997 Feb 20;336[8]:525-33). Those study participants look nothing at all like the heart failure patients physicians see today in clinical practice. Hardly any of them were on what today is guideline-directed medical therapy with a beta-blocker or mineralocorticoid receptor antagonist. So the trial’s applicability is dubious.
“Our Swedish data are observational. They are hypothesis-generating. They should drive trialists to design a new trial of digoxin. But I think we all know that’s not going to happen. So actually I don’t think there is still space for a IIb or IIa recommendation for digoxin in the guidelines,” Dr. Savarese said.
He reported having no financial conflicts.
AT ACC 17
Key clinical point:
Major finding: The use of digoxin in patients with heart failure with reduced ejection fraction was associated with significantly increased risk of all-cause mortality if they had concomitant paroxysmal atrial fibrillation or were in normal sinus rhythm.
Data source: An observational study of nearly 24,000 patients enrolled in the Swedish Heart Failure Registry, 18% of whom were on digoxin.
Disclosures: The study presenter reported having no financial conflicts.
Just over half of FPs accept Medicaid
Medicaid acceptance was 55% among family physicians in the 2017 edition of an ongoing survey conducted in 15 large cities by physician recruitment firm Merritt Hawkins.
That was up from almost 52% in the previous survey, conducted in 2014, but lower than the average of 64% for FPs in 15 midsized cities that were included for the first time in 2017, the company reported.
There was one large city with a Medicaid acceptance rate of 100% – Minneapolis (up from 35% in 2014) – along with three midsized cities – Billings, Mt.; Dayton, Ohio; and Fargo, N.D. The lowest rate among the large cities was in Denver (20%), with the midsized basement occupied by Lafayette, La., at 20%, Merritt Hawkins reported.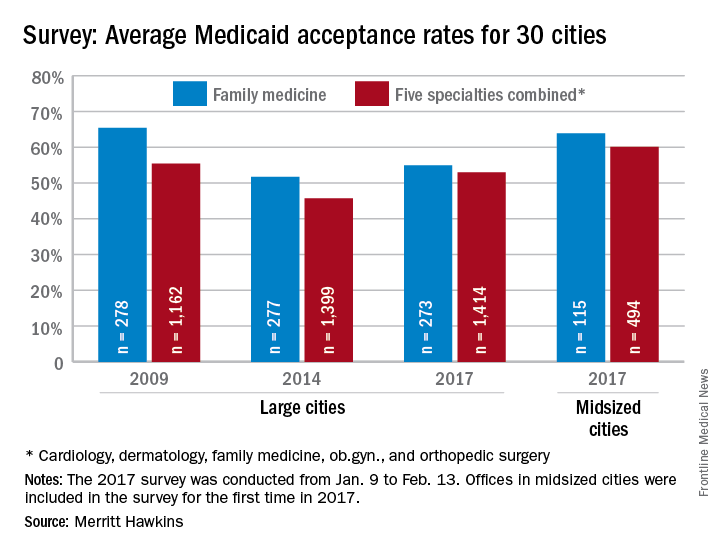
Investigators called 273 randomly selected family physicians in the large cities and 115 FPs in the midsized cities in January and February. It was the fourth such survey the company has conducted since 2004.
The survey included four other specialties – cardiology, dermatology, ob.gyn., and orthopedic surgery. The Medicaid acceptance rate for all 1,414 physicians in all five specialties in the 15 large cities was 53%, and the average rate for all specialties in the midsized cities was 60% for the 494 offices surveyed, the company noted.
Cardiology had the highest rates by specialty and dermatology the lowest in both the large and midsized cities. For all five specialties combined, Minneapolis (97%) and Fargo (100%) had the highest acceptance rates, with the lowest rates coming from Dallas (17%) for large cities and Lafayette (11%) for midsized cities, the Merritt Hawkins data show.
Medicaid acceptance was 55% among family physicians in the 2017 edition of an ongoing survey conducted in 15 large cities by physician recruitment firm Merritt Hawkins.
That was up from almost 52% in the previous survey, conducted in 2014, but lower than the average of 64% for FPs in 15 midsized cities that were included for the first time in 2017, the company reported.
There was one large city with a Medicaid acceptance rate of 100% – Minneapolis (up from 35% in 2014) – along with three midsized cities – Billings, Mt.; Dayton, Ohio; and Fargo, N.D. The lowest rate among the large cities was in Denver (20%), with the midsized basement occupied by Lafayette, La., at 20%, Merritt Hawkins reported.
Investigators called 273 randomly selected family physicians in the large cities and 115 FPs in the midsized cities in January and February. It was the fourth such survey the company has conducted since 2004.
The survey included four other specialties – cardiology, dermatology, ob.gyn., and orthopedic surgery. The Medicaid acceptance rate for all 1,414 physicians in all five specialties in the 15 large cities was 53%, and the average rate for all specialties in the midsized cities was 60% for the 494 offices surveyed, the company noted.
Cardiology had the highest rates by specialty and dermatology the lowest in both the large and midsized cities. For all five specialties combined, Minneapolis (97%) and Fargo (100%) had the highest acceptance rates, with the lowest rates coming from Dallas (17%) for large cities and Lafayette (11%) for midsized cities, the Merritt Hawkins data show.
Medicaid acceptance was 55% among family physicians in the 2017 edition of an ongoing survey conducted in 15 large cities by physician recruitment firm Merritt Hawkins.
That was up from almost 52% in the previous survey, conducted in 2014, but lower than the average of 64% for FPs in 15 midsized cities that were included for the first time in 2017, the company reported.
There was one large city with a Medicaid acceptance rate of 100% – Minneapolis (up from 35% in 2014) – along with three midsized cities – Billings, Mt.; Dayton, Ohio; and Fargo, N.D. The lowest rate among the large cities was in Denver (20%), with the midsized basement occupied by Lafayette, La., at 20%, Merritt Hawkins reported.
Investigators called 273 randomly selected family physicians in the large cities and 115 FPs in the midsized cities in January and February. It was the fourth such survey the company has conducted since 2004.
The survey included four other specialties – cardiology, dermatology, ob.gyn., and orthopedic surgery. The Medicaid acceptance rate for all 1,414 physicians in all five specialties in the 15 large cities was 53%, and the average rate for all specialties in the midsized cities was 60% for the 494 offices surveyed, the company noted.
Cardiology had the highest rates by specialty and dermatology the lowest in both the large and midsized cities. For all five specialties combined, Minneapolis (97%) and Fargo (100%) had the highest acceptance rates, with the lowest rates coming from Dallas (17%) for large cities and Lafayette (11%) for midsized cities, the Merritt Hawkins data show.
Address procrastination, disorganization in hoarding disorder
SAN FRANCISCO – Procrastination, disorganization, indecisiveness, and perfectionism each are significant independent predictors of hoarding severity, even though none of these associated factors is included in the DSM-5 diagnostic criteria for hoarding disorder, according to Sanjaya Saxena, MD.
Of these four associated factors, disorganization and procrastination had the strongest correlation with hoarding severity in his study. Patients meeting the DSM-5 criteria for hoarding disorder scored significantly higher on measures of disorganization and procrastination than did patients with nonhoarding obsessive-compulsive disorder or anxiety disorders, said Dr. Saxena, professor of psychiatry and director of the obsessive-compulsive disorders program at the University of California, San Diego.
The DSM-5 lists as the core symptoms of hoarding disorder difficulty in discarding possessions; perceived need to save items; excessive acquisition, clutter, and resultant distress; and impaired functioning. But while procrastination, disorganization, perfectionism, and indecisiveness aren’t included in the diagnostic criteria, Dr. Saxena said he and some other experts have considered those features to be characteristic of affected individuals. So he decided to formally test the strength of the associations.
He reported on 21 patients with hoarding disorder and 13 controls with nonhoarding OCD or an anxiety disorder. All subjects completed a battery of assessment tools, including the Beck Depression Inventory, the Beck Anxiety Inventory, the Frost Multidimensional Perfectionism Scale, the Frost Indecisiveness Scale, the Adult Inventory of Procrastination Scale, and three different measures of hoarding severity. Participants also completed a disorganization index based on their answers to three questions drawn from the Swanson, Nolan, and Pelham (SNAP-IV) Rating Scale: How often did you have difficulty organizing tasks and activities as a child? How disorganized are you in your thinking, planning, and time management? And how disorganized are your belongings at home?
Neither disorganization, procrastination, perfectionism, nor indecisiveness turned out to be associated with severity of nonhoarding OCD or anxiety disorder symptoms. Surprisingly, no significant differences were found between hoarding disorder patients and controls on the measures of indecisiveness or perfectionism, Dr. Saxena said. And the two groups did not differ in their levels of anxiety and depression.
However, levels of procrastination and disorganization were strongly correlated with hoarding severity as assessed via the Saving Inventory – Revised, the UCLA Hoarding Severity Scale, and the Hoarding Rating Scale. The hoarding disorder group’s average score on the disorganization index was 5.67, more than twice that of the 2.67 in the control group. And patients with hoarding disorder had an average Adult Inventory of Procrastination score of 50.9 out of a possible maximum of 75 points, compared with 41 in controls.
In a multivariate regression analysis, age and level of depression collectively explained 23.5% of the variance in hoarding severity scores in the study population. Disorganization independently explained an additional 29.9% of the variance, and procrastination accounted for another 19.1%, Dr. Saxena reported.
He reported having no financial conflicts regarding his study, which was funded by the university’s department of psychiatry.
SAN FRANCISCO – Procrastination, disorganization, indecisiveness, and perfectionism each are significant independent predictors of hoarding severity, even though none of these associated factors is included in the DSM-5 diagnostic criteria for hoarding disorder, according to Sanjaya Saxena, MD.
Of these four associated factors, disorganization and procrastination had the strongest correlation with hoarding severity in his study. Patients meeting the DSM-5 criteria for hoarding disorder scored significantly higher on measures of disorganization and procrastination than did patients with nonhoarding obsessive-compulsive disorder or anxiety disorders, said Dr. Saxena, professor of psychiatry and director of the obsessive-compulsive disorders program at the University of California, San Diego.
The DSM-5 lists as the core symptoms of hoarding disorder difficulty in discarding possessions; perceived need to save items; excessive acquisition, clutter, and resultant distress; and impaired functioning. But while procrastination, disorganization, perfectionism, and indecisiveness aren’t included in the diagnostic criteria, Dr. Saxena said he and some other experts have considered those features to be characteristic of affected individuals. So he decided to formally test the strength of the associations.
He reported on 21 patients with hoarding disorder and 13 controls with nonhoarding OCD or an anxiety disorder. All subjects completed a battery of assessment tools, including the Beck Depression Inventory, the Beck Anxiety Inventory, the Frost Multidimensional Perfectionism Scale, the Frost Indecisiveness Scale, the Adult Inventory of Procrastination Scale, and three different measures of hoarding severity. Participants also completed a disorganization index based on their answers to three questions drawn from the Swanson, Nolan, and Pelham (SNAP-IV) Rating Scale: How often did you have difficulty organizing tasks and activities as a child? How disorganized are you in your thinking, planning, and time management? And how disorganized are your belongings at home?
Neither disorganization, procrastination, perfectionism, nor indecisiveness turned out to be associated with severity of nonhoarding OCD or anxiety disorder symptoms. Surprisingly, no significant differences were found between hoarding disorder patients and controls on the measures of indecisiveness or perfectionism, Dr. Saxena said. And the two groups did not differ in their levels of anxiety and depression.
However, levels of procrastination and disorganization were strongly correlated with hoarding severity as assessed via the Saving Inventory – Revised, the UCLA Hoarding Severity Scale, and the Hoarding Rating Scale. The hoarding disorder group’s average score on the disorganization index was 5.67, more than twice that of the 2.67 in the control group. And patients with hoarding disorder had an average Adult Inventory of Procrastination score of 50.9 out of a possible maximum of 75 points, compared with 41 in controls.
In a multivariate regression analysis, age and level of depression collectively explained 23.5% of the variance in hoarding severity scores in the study population. Disorganization independently explained an additional 29.9% of the variance, and procrastination accounted for another 19.1%, Dr. Saxena reported.
He reported having no financial conflicts regarding his study, which was funded by the university’s department of psychiatry.
SAN FRANCISCO – Procrastination, disorganization, indecisiveness, and perfectionism each are significant independent predictors of hoarding severity, even though none of these associated factors is included in the DSM-5 diagnostic criteria for hoarding disorder, according to Sanjaya Saxena, MD.
Of these four associated factors, disorganization and procrastination had the strongest correlation with hoarding severity in his study. Patients meeting the DSM-5 criteria for hoarding disorder scored significantly higher on measures of disorganization and procrastination than did patients with nonhoarding obsessive-compulsive disorder or anxiety disorders, said Dr. Saxena, professor of psychiatry and director of the obsessive-compulsive disorders program at the University of California, San Diego.
The DSM-5 lists as the core symptoms of hoarding disorder difficulty in discarding possessions; perceived need to save items; excessive acquisition, clutter, and resultant distress; and impaired functioning. But while procrastination, disorganization, perfectionism, and indecisiveness aren’t included in the diagnostic criteria, Dr. Saxena said he and some other experts have considered those features to be characteristic of affected individuals. So he decided to formally test the strength of the associations.
He reported on 21 patients with hoarding disorder and 13 controls with nonhoarding OCD or an anxiety disorder. All subjects completed a battery of assessment tools, including the Beck Depression Inventory, the Beck Anxiety Inventory, the Frost Multidimensional Perfectionism Scale, the Frost Indecisiveness Scale, the Adult Inventory of Procrastination Scale, and three different measures of hoarding severity. Participants also completed a disorganization index based on their answers to three questions drawn from the Swanson, Nolan, and Pelham (SNAP-IV) Rating Scale: How often did you have difficulty organizing tasks and activities as a child? How disorganized are you in your thinking, planning, and time management? And how disorganized are your belongings at home?
Neither disorganization, procrastination, perfectionism, nor indecisiveness turned out to be associated with severity of nonhoarding OCD or anxiety disorder symptoms. Surprisingly, no significant differences were found between hoarding disorder patients and controls on the measures of indecisiveness or perfectionism, Dr. Saxena said. And the two groups did not differ in their levels of anxiety and depression.
However, levels of procrastination and disorganization were strongly correlated with hoarding severity as assessed via the Saving Inventory – Revised, the UCLA Hoarding Severity Scale, and the Hoarding Rating Scale. The hoarding disorder group’s average score on the disorganization index was 5.67, more than twice that of the 2.67 in the control group. And patients with hoarding disorder had an average Adult Inventory of Procrastination score of 50.9 out of a possible maximum of 75 points, compared with 41 in controls.
In a multivariate regression analysis, age and level of depression collectively explained 23.5% of the variance in hoarding severity scores in the study population. Disorganization independently explained an additional 29.9% of the variance, and procrastination accounted for another 19.1%, Dr. Saxena reported.
He reported having no financial conflicts regarding his study, which was funded by the university’s department of psychiatry.
AT THE ANXIETY AND DEPRESSION CONFERENCE 2017
Key clinical point:
Major finding: Severity of procrastination explained 30% of the variance in hoarding severity scores between patients with hoarding disorder and controls with nonhoarding obsessive-compulsive disorder or an anxiety disorder.
Data source: A cross-sectional study involving 21 patients with hoarding disorder and 13 controls, all of whom completed a battery of tests assessing anxiety, depression, hoarding severity, disorganization, procrastination, indecisiveness, and perfectionism.
Disclosures: The presenter reported having no financial conflicts regarding the study, which was funded by a university psychiatry department.