User login
Anthony Fauci faces the ‘perpetual challenge’ of emerging infections
SAN DIEGO – Reflecting on his 33-year career as director of the National Institute of Allergy and Infectious Diseases, Anthony S. Fauci, MD, can say one thing for certain: Emerging and re-emerging infectious diseases in the continental United States are here to stay.
In an article that he and his colleagues published in the Lancet in 2008, they used the term “perpetual challenge” to describe emerging infections, a descriptor that resonates with him to this day.
Global examples of emerging and re-emerging infectious diseases he discussed include dengue, West Nile virus, chikungunya, and carbapenem-resistant Enterobacteriaceae, “which is becoming a progressively more serious problem in hospitalized patients,” said Dr. Fauci, who is also chief of the NIAID Laboratory of Immunoregulation.
“We had a serious challenge with that at our own clinical center in Bethesda just a few years ago,” he noted. Numerous cases of antimicrobial resistance in methicillin-resistant Staphylococcus aureus, Clostridium difficile, and Neisseria gonorrhoeae have been reported.
Dr. Fauci described the Ebola outbreak as “a globally important disease that had ripple effects in the United States that were unpredicted,” referring to the case of the infected man who traveled from Monrovia to Dallas on Sept. 19, 2014, and developed Ebola symptoms 5 days later. Between 2014 and 2016, there were 28,616 cases and 11,310 deaths combined in the countries of Guinea, Sierra Leone, and Liberia.
“There is virtually no health care system in those three countries,” he said. “There’s a distrust in authority, and anything we tried to do as a global health [effort] made things worse. What we’re trying to do now is built sustainable health care issues in countries that don’t have it.”
Of particular concern to public health officials worldwide is getting a lid on Zika virus, a mosquito-borne illness that can be passed from a pregnant woman to her fetus and cause an increased risk of microcephaly, particularly during the first trimester.
“Not only is there microcephaly, there’s a whole host of abnormalities that involve hearing loss, visual abnormalities, and a variety of other issues,” Dr. Fauci said. “There are about 50 countries in the Americas and the Caribbean that have Zika virus transmission.”
According to data from the Centers for Disease Control and Prevention, from Jan. 1, 2015, to March 29, 2017, there were 5,182 reported cases of Zika virus disease in the 50 states and the District of Columbia. The majority of those (4,886) were travel associated, 222 were locally acquired mosquito-borne, 45 were sexually transmitted, 27 congenital, 1 was laboratory acquired, and 1 was unknown.
At the same time, there have been 38,303 cases in the U.S. territories. Of those, 38,156 were locally acquired, and 147 were travel associated. “That’s why there’s such an intense effort to develop a Zika vaccine,” he said.
According to the CDC, as of March 15, 2017, there are 265 cases of locally transmitted cases in Florida: 216 by mosquito and the rest by sexual transmission. “Talk about surprises,” Dr. Fauci said. “Zika is the first mosquito-borne infection that can result in a congenital abnormality, the first mosquito-borne infection that can be sexually transmitted, and now we’re learning more about this problem, which is the reason why it’s very important for us to develop a vaccine.”
A phase I trial of a DNA vaccine developed by the NIH Vaccine Research Center has reached its enrollment goal of 80 patients age 18-35 years. Initial results are expected sometime in the first quarter of 2017. A phase II trial in the United States and Puerto Rico is expected to launch soon.
Dr. Fauci closed his presentation by sharing lessons learned from previous pandemics.
The first lesson is that global surveillance is required. “Namely, know what’s going on in real time,” he said. “That has to be linked to transparency and communication. So that if something happens in China, we don’t find out about it months later, but we know about it in real time.”
Infrastructure and capacity building are also important. “The lack of capacity in West Africa can ultimately have an indirect impact on us here in the United States,” he said.
“Finally, we need to coordinate and collaborate; we need adaptable platforms for vaccines,” Dr. Fauci cautioned. “Importantly, we need a stable funding mechanism such as a public health emergency fund so that we do not have to go scrambling before the Congress when we need emergency funds.”
SAN DIEGO – Reflecting on his 33-year career as director of the National Institute of Allergy and Infectious Diseases, Anthony S. Fauci, MD, can say one thing for certain: Emerging and re-emerging infectious diseases in the continental United States are here to stay.
In an article that he and his colleagues published in the Lancet in 2008, they used the term “perpetual challenge” to describe emerging infections, a descriptor that resonates with him to this day.
Global examples of emerging and re-emerging infectious diseases he discussed include dengue, West Nile virus, chikungunya, and carbapenem-resistant Enterobacteriaceae, “which is becoming a progressively more serious problem in hospitalized patients,” said Dr. Fauci, who is also chief of the NIAID Laboratory of Immunoregulation.
“We had a serious challenge with that at our own clinical center in Bethesda just a few years ago,” he noted. Numerous cases of antimicrobial resistance in methicillin-resistant Staphylococcus aureus, Clostridium difficile, and Neisseria gonorrhoeae have been reported.
Dr. Fauci described the Ebola outbreak as “a globally important disease that had ripple effects in the United States that were unpredicted,” referring to the case of the infected man who traveled from Monrovia to Dallas on Sept. 19, 2014, and developed Ebola symptoms 5 days later. Between 2014 and 2016, there were 28,616 cases and 11,310 deaths combined in the countries of Guinea, Sierra Leone, and Liberia.
“There is virtually no health care system in those three countries,” he said. “There’s a distrust in authority, and anything we tried to do as a global health [effort] made things worse. What we’re trying to do now is built sustainable health care issues in countries that don’t have it.”
Of particular concern to public health officials worldwide is getting a lid on Zika virus, a mosquito-borne illness that can be passed from a pregnant woman to her fetus and cause an increased risk of microcephaly, particularly during the first trimester.
“Not only is there microcephaly, there’s a whole host of abnormalities that involve hearing loss, visual abnormalities, and a variety of other issues,” Dr. Fauci said. “There are about 50 countries in the Americas and the Caribbean that have Zika virus transmission.”
According to data from the Centers for Disease Control and Prevention, from Jan. 1, 2015, to March 29, 2017, there were 5,182 reported cases of Zika virus disease in the 50 states and the District of Columbia. The majority of those (4,886) were travel associated, 222 were locally acquired mosquito-borne, 45 were sexually transmitted, 27 congenital, 1 was laboratory acquired, and 1 was unknown.
At the same time, there have been 38,303 cases in the U.S. territories. Of those, 38,156 were locally acquired, and 147 were travel associated. “That’s why there’s such an intense effort to develop a Zika vaccine,” he said.
According to the CDC, as of March 15, 2017, there are 265 cases of locally transmitted cases in Florida: 216 by mosquito and the rest by sexual transmission. “Talk about surprises,” Dr. Fauci said. “Zika is the first mosquito-borne infection that can result in a congenital abnormality, the first mosquito-borne infection that can be sexually transmitted, and now we’re learning more about this problem, which is the reason why it’s very important for us to develop a vaccine.”
A phase I trial of a DNA vaccine developed by the NIH Vaccine Research Center has reached its enrollment goal of 80 patients age 18-35 years. Initial results are expected sometime in the first quarter of 2017. A phase II trial in the United States and Puerto Rico is expected to launch soon.
Dr. Fauci closed his presentation by sharing lessons learned from previous pandemics.
The first lesson is that global surveillance is required. “Namely, know what’s going on in real time,” he said. “That has to be linked to transparency and communication. So that if something happens in China, we don’t find out about it months later, but we know about it in real time.”
Infrastructure and capacity building are also important. “The lack of capacity in West Africa can ultimately have an indirect impact on us here in the United States,” he said.
“Finally, we need to coordinate and collaborate; we need adaptable platforms for vaccines,” Dr. Fauci cautioned. “Importantly, we need a stable funding mechanism such as a public health emergency fund so that we do not have to go scrambling before the Congress when we need emergency funds.”
SAN DIEGO – Reflecting on his 33-year career as director of the National Institute of Allergy and Infectious Diseases, Anthony S. Fauci, MD, can say one thing for certain: Emerging and re-emerging infectious diseases in the continental United States are here to stay.
In an article that he and his colleagues published in the Lancet in 2008, they used the term “perpetual challenge” to describe emerging infections, a descriptor that resonates with him to this day.
Global examples of emerging and re-emerging infectious diseases he discussed include dengue, West Nile virus, chikungunya, and carbapenem-resistant Enterobacteriaceae, “which is becoming a progressively more serious problem in hospitalized patients,” said Dr. Fauci, who is also chief of the NIAID Laboratory of Immunoregulation.
“We had a serious challenge with that at our own clinical center in Bethesda just a few years ago,” he noted. Numerous cases of antimicrobial resistance in methicillin-resistant Staphylococcus aureus, Clostridium difficile, and Neisseria gonorrhoeae have been reported.
Dr. Fauci described the Ebola outbreak as “a globally important disease that had ripple effects in the United States that were unpredicted,” referring to the case of the infected man who traveled from Monrovia to Dallas on Sept. 19, 2014, and developed Ebola symptoms 5 days later. Between 2014 and 2016, there were 28,616 cases and 11,310 deaths combined in the countries of Guinea, Sierra Leone, and Liberia.
“There is virtually no health care system in those three countries,” he said. “There’s a distrust in authority, and anything we tried to do as a global health [effort] made things worse. What we’re trying to do now is built sustainable health care issues in countries that don’t have it.”
Of particular concern to public health officials worldwide is getting a lid on Zika virus, a mosquito-borne illness that can be passed from a pregnant woman to her fetus and cause an increased risk of microcephaly, particularly during the first trimester.
“Not only is there microcephaly, there’s a whole host of abnormalities that involve hearing loss, visual abnormalities, and a variety of other issues,” Dr. Fauci said. “There are about 50 countries in the Americas and the Caribbean that have Zika virus transmission.”
According to data from the Centers for Disease Control and Prevention, from Jan. 1, 2015, to March 29, 2017, there were 5,182 reported cases of Zika virus disease in the 50 states and the District of Columbia. The majority of those (4,886) were travel associated, 222 were locally acquired mosquito-borne, 45 were sexually transmitted, 27 congenital, 1 was laboratory acquired, and 1 was unknown.
At the same time, there have been 38,303 cases in the U.S. territories. Of those, 38,156 were locally acquired, and 147 were travel associated. “That’s why there’s such an intense effort to develop a Zika vaccine,” he said.
According to the CDC, as of March 15, 2017, there are 265 cases of locally transmitted cases in Florida: 216 by mosquito and the rest by sexual transmission. “Talk about surprises,” Dr. Fauci said. “Zika is the first mosquito-borne infection that can result in a congenital abnormality, the first mosquito-borne infection that can be sexually transmitted, and now we’re learning more about this problem, which is the reason why it’s very important for us to develop a vaccine.”
A phase I trial of a DNA vaccine developed by the NIH Vaccine Research Center has reached its enrollment goal of 80 patients age 18-35 years. Initial results are expected sometime in the first quarter of 2017. A phase II trial in the United States and Puerto Rico is expected to launch soon.
Dr. Fauci closed his presentation by sharing lessons learned from previous pandemics.
The first lesson is that global surveillance is required. “Namely, know what’s going on in real time,” he said. “That has to be linked to transparency and communication. So that if something happens in China, we don’t find out about it months later, but we know about it in real time.”
Infrastructure and capacity building are also important. “The lack of capacity in West Africa can ultimately have an indirect impact on us here in the United States,” he said.
“Finally, we need to coordinate and collaborate; we need adaptable platforms for vaccines,” Dr. Fauci cautioned. “Importantly, we need a stable funding mechanism such as a public health emergency fund so that we do not have to go scrambling before the Congress when we need emergency funds.”
EXPERT ANALYSIS FROM ACP INTERNAL MEDICINE
Fellow in Hospital Medicine designation symbolizes physician commitment to hospital medicine
Editor’s note: Each month, SHM puts the spotlight on some of our most active members who are making substantial contributions to hospital medicine. Log on to www.hospitalmedicine.org/getinvolved for more information on how you can lend your expertise to help SHM improve the care of hospitalized patients.
This month, The Hospitalist spotlights Umesh Sharma, MD, MBA, FHM, chair of the division of community hospital medicine at Mayo Clinic. Umesh became a Fellow in Hospital Medicine in 2016 and has found great value in attending the annual meeting each year.
What inspired you to join SHM, and what prompted you to apply for the Fellow in Hospital Medicine designation?
How did you use SHM resources to help you in your pathway to Fellowship in Hospital Medicine?
There are specific eligibility requirements for the Fellow in Hospital Medicine designation, including a minimum of 5 years as a practicing hospitalist and 3 years as an SHM member, endorsements from two active members, regular meeting attendance and more. SHM provides a checklist for Fellow applicants online and an FAQ page to make the application process as user-friendly as possible. A friend of mine, Dr. Deepak Pahuja, is a Fellow, and he mentored me throughout the process.
How else has SHM contributed to your professional growth and provided you with tools you need to lead hospitalists at Mayo Clinic?
There are many resources that SHM provides to help with professional growth both online and at in-person meetings. I referenced the Key Principles and Characteristics of an Effective Hospital Medicine Group, an online assessment guide, in my role as department chair in La Crosse, Wisc., to resurrect a hospital medicine group, secure resources, hire career hospitalists, and create a well-functioning, well-managed, efficient, effective group with zero turnover during a span of 4 years.
By focusing on the leadership track at annual meetings, I have been able to gain knowledge on proven leadership strategies and enhance my skills, which I have applied on many occasions in my practice. Being able to talk to multisite hospital medicine group colleagues in person helped me to learn best practices in how to successfully manage the integration of 14 hospital medicine community hospital sites across Mayo Midwest. I was able to get ideas on effectively understanding and managing challenges, like recruitment retention, staffing to workloads, and scope of practice, among others. SHM promotes peer-to-peer learning and has helped me share and learn best practices as it relates to the clinical and nonclinical aspect of the practice of hospital medicine.
What one piece of advice would you give fellow hospitalists during this transformational time in health care?
This is an exciting time in health care, especially for hospital medicine professionals, who are at the forefront of providing value-based care. Every change is an opportunity to improve and innovate; the best way to handle change is to embrace and lead it.
Ms. Steele is SHM’s communications coordinator.
To apply for the Fellow in Hospital Medicine designation, visit www.hospitalmedicine.org/fellows.
Editor’s note: Each month, SHM puts the spotlight on some of our most active members who are making substantial contributions to hospital medicine. Log on to www.hospitalmedicine.org/getinvolved for more information on how you can lend your expertise to help SHM improve the care of hospitalized patients.
This month, The Hospitalist spotlights Umesh Sharma, MD, MBA, FHM, chair of the division of community hospital medicine at Mayo Clinic. Umesh became a Fellow in Hospital Medicine in 2016 and has found great value in attending the annual meeting each year.
What inspired you to join SHM, and what prompted you to apply for the Fellow in Hospital Medicine designation?
How did you use SHM resources to help you in your pathway to Fellowship in Hospital Medicine?
There are specific eligibility requirements for the Fellow in Hospital Medicine designation, including a minimum of 5 years as a practicing hospitalist and 3 years as an SHM member, endorsements from two active members, regular meeting attendance and more. SHM provides a checklist for Fellow applicants online and an FAQ page to make the application process as user-friendly as possible. A friend of mine, Dr. Deepak Pahuja, is a Fellow, and he mentored me throughout the process.
How else has SHM contributed to your professional growth and provided you with tools you need to lead hospitalists at Mayo Clinic?
There are many resources that SHM provides to help with professional growth both online and at in-person meetings. I referenced the Key Principles and Characteristics of an Effective Hospital Medicine Group, an online assessment guide, in my role as department chair in La Crosse, Wisc., to resurrect a hospital medicine group, secure resources, hire career hospitalists, and create a well-functioning, well-managed, efficient, effective group with zero turnover during a span of 4 years.
By focusing on the leadership track at annual meetings, I have been able to gain knowledge on proven leadership strategies and enhance my skills, which I have applied on many occasions in my practice. Being able to talk to multisite hospital medicine group colleagues in person helped me to learn best practices in how to successfully manage the integration of 14 hospital medicine community hospital sites across Mayo Midwest. I was able to get ideas on effectively understanding and managing challenges, like recruitment retention, staffing to workloads, and scope of practice, among others. SHM promotes peer-to-peer learning and has helped me share and learn best practices as it relates to the clinical and nonclinical aspect of the practice of hospital medicine.
What one piece of advice would you give fellow hospitalists during this transformational time in health care?
This is an exciting time in health care, especially for hospital medicine professionals, who are at the forefront of providing value-based care. Every change is an opportunity to improve and innovate; the best way to handle change is to embrace and lead it.
Ms. Steele is SHM’s communications coordinator.
To apply for the Fellow in Hospital Medicine designation, visit www.hospitalmedicine.org/fellows.
Editor’s note: Each month, SHM puts the spotlight on some of our most active members who are making substantial contributions to hospital medicine. Log on to www.hospitalmedicine.org/getinvolved for more information on how you can lend your expertise to help SHM improve the care of hospitalized patients.
This month, The Hospitalist spotlights Umesh Sharma, MD, MBA, FHM, chair of the division of community hospital medicine at Mayo Clinic. Umesh became a Fellow in Hospital Medicine in 2016 and has found great value in attending the annual meeting each year.
What inspired you to join SHM, and what prompted you to apply for the Fellow in Hospital Medicine designation?
How did you use SHM resources to help you in your pathway to Fellowship in Hospital Medicine?
There are specific eligibility requirements for the Fellow in Hospital Medicine designation, including a minimum of 5 years as a practicing hospitalist and 3 years as an SHM member, endorsements from two active members, regular meeting attendance and more. SHM provides a checklist for Fellow applicants online and an FAQ page to make the application process as user-friendly as possible. A friend of mine, Dr. Deepak Pahuja, is a Fellow, and he mentored me throughout the process.
How else has SHM contributed to your professional growth and provided you with tools you need to lead hospitalists at Mayo Clinic?
There are many resources that SHM provides to help with professional growth both online and at in-person meetings. I referenced the Key Principles and Characteristics of an Effective Hospital Medicine Group, an online assessment guide, in my role as department chair in La Crosse, Wisc., to resurrect a hospital medicine group, secure resources, hire career hospitalists, and create a well-functioning, well-managed, efficient, effective group with zero turnover during a span of 4 years.
By focusing on the leadership track at annual meetings, I have been able to gain knowledge on proven leadership strategies and enhance my skills, which I have applied on many occasions in my practice. Being able to talk to multisite hospital medicine group colleagues in person helped me to learn best practices in how to successfully manage the integration of 14 hospital medicine community hospital sites across Mayo Midwest. I was able to get ideas on effectively understanding and managing challenges, like recruitment retention, staffing to workloads, and scope of practice, among others. SHM promotes peer-to-peer learning and has helped me share and learn best practices as it relates to the clinical and nonclinical aspect of the practice of hospital medicine.
What one piece of advice would you give fellow hospitalists during this transformational time in health care?
This is an exciting time in health care, especially for hospital medicine professionals, who are at the forefront of providing value-based care. Every change is an opportunity to improve and innovate; the best way to handle change is to embrace and lead it.
Ms. Steele is SHM’s communications coordinator.
To apply for the Fellow in Hospital Medicine designation, visit www.hospitalmedicine.org/fellows.
Immunotherapy exhibits antileukemic activity in high-risk patients
Blinatumomab has demonstrated activity in high-risk patients with Philadelphia chromosome-positive (Ph+) B-cell precursor acute lymphoblastic leukemia (BCP-ALL), according to research published in the Journal of Clinical Oncology.
This phase 2 trial enrolled patients with relapsed or refractory Ph+ BCP-ALL who had failed treatment with at least 1 tyrosine kinase inhibitor (TKI).
Blinatumomab produced a complete response (CR) in 31% of these patients, the median relapse-free survival was 6.7 months, and the median overall survival was 7.1 months.
The most common adverse events (AEs) were pyrexia, neurologic events, febrile neutropenia, and headache.
“Patients with Ph+ relapsed or refractory B-cell precursor ALL typically have lower remission rates, poor long-term prognosis, and shorter duration of remission than patients with Philadelphia chromosome-negative disease, and are especially in need of new treatment options beyond TKIs,” said study author Anthony Stein, MD, of City of Hope in Duarte, California.
“Results from this phase 2 study showed blinatumomab induced complete remission in these high-risk patients, regardless of prior TKI therapy or mutational status . . . .”
This study was supported by Amgen, the company developing and marketing blinatumomab.
The trial enrolled 45 patients with relapsed or refractory Ph+ BCP-ALL. Fifty-nine percent of patients had additional cytogenetic abnormalities. Forty-six percent had ABL1 kinase domain mutations, and 27% had the T315I mutation.
The patients’ median age was 55 (range, 23-78), and 55% were male. The median baseline bone marrow blast percentage was 80% (range, 6% to 98%).
Eighty-four percent of patients had received at least 2 prior TKIs. All patients were refractory to (56%), had relapsed on (33%), or progressed after (11%) TKI therapy. Forty-four percent of patients had undergone an allogeneic hematopoietic stem cell transplant (allo-HSCT).
The patients received blinatumomab in 28-day cycles by continuous intravenous infusion. The median number of cycles received was 2 (range, 1-5).
Efficacy
Thirty-six percent of patients (n=16) had a CR or CR with partial hematologic recovery (CRh) during the first 2 cycles of treatment. For 31% of patients (n=14), their best response was a CR.
Eighty-eight percent of patients who achieved a CR/CRh (n=14) achieved minimal residual disease (MRD) negativity.
Forty percent of patients with a T315I mutation had a CR/CRh (4/10), and all of these responders were MRD negative.
Seven responders (44%) went on to allo-HSCT, 6 of whom were transplant-naïve.
Eight of the 16 responders (50%) ultimately relapsed. Their median time to relapse was 6.7 months. Three patients relapsed during treatment, 2 relapsed without undergoing allo-HSCT, and 3 relapsed after allo-HSCT.
Seven responders (44%) were still alive and had not relapsed at last follow-up. The remaining responder died in CR after allo-HSCT.
The median relapse-free survival was 6.7 months, with or without censoring for allo-HSCT. And the median overall survival was 7.1 months, with or without censoring for allo-HSCT.
Safety
The most common AEs were pyrexia (58%), febrile neutropenia (40%), and headache (31%). Nearly half of patients (47%) had neurologic events.
Eighty-two percent of patients had grade 3 or higher treatment-emergent AEs. The most common were febrile neutropenia (27%), thrombocytopenia (22%), and anemia (16%).
Forty-four percent of patients had grade 3 or higher AEs that were considered possibly related to blinatumomab. The most common were febrile neutropenia and increased levels of alanine aminotransferase (11% each).
Five patients had fatal AEs—multiorgan failure, sepsis, septic shock, cerebral hemorrhage, and respiratory failure. The case of septic shock was considered related to treatment with blinatumomab.
Three patients developed cytokine release syndrome (all grade 1 or 2), but none of them had their treatment interrupted or discontinued as a result.
Three patients had grade 3 neurologic events, and 1 of these events (aphasia) required temporary treatment interruption. There were no grade 4 or 5 neurologic events. ![]()
Blinatumomab has demonstrated activity in high-risk patients with Philadelphia chromosome-positive (Ph+) B-cell precursor acute lymphoblastic leukemia (BCP-ALL), according to research published in the Journal of Clinical Oncology.
This phase 2 trial enrolled patients with relapsed or refractory Ph+ BCP-ALL who had failed treatment with at least 1 tyrosine kinase inhibitor (TKI).
Blinatumomab produced a complete response (CR) in 31% of these patients, the median relapse-free survival was 6.7 months, and the median overall survival was 7.1 months.
The most common adverse events (AEs) were pyrexia, neurologic events, febrile neutropenia, and headache.
“Patients with Ph+ relapsed or refractory B-cell precursor ALL typically have lower remission rates, poor long-term prognosis, and shorter duration of remission than patients with Philadelphia chromosome-negative disease, and are especially in need of new treatment options beyond TKIs,” said study author Anthony Stein, MD, of City of Hope in Duarte, California.
“Results from this phase 2 study showed blinatumomab induced complete remission in these high-risk patients, regardless of prior TKI therapy or mutational status . . . .”
This study was supported by Amgen, the company developing and marketing blinatumomab.
The trial enrolled 45 patients with relapsed or refractory Ph+ BCP-ALL. Fifty-nine percent of patients had additional cytogenetic abnormalities. Forty-six percent had ABL1 kinase domain mutations, and 27% had the T315I mutation.
The patients’ median age was 55 (range, 23-78), and 55% were male. The median baseline bone marrow blast percentage was 80% (range, 6% to 98%).
Eighty-four percent of patients had received at least 2 prior TKIs. All patients were refractory to (56%), had relapsed on (33%), or progressed after (11%) TKI therapy. Forty-four percent of patients had undergone an allogeneic hematopoietic stem cell transplant (allo-HSCT).
The patients received blinatumomab in 28-day cycles by continuous intravenous infusion. The median number of cycles received was 2 (range, 1-5).
Efficacy
Thirty-six percent of patients (n=16) had a CR or CR with partial hematologic recovery (CRh) during the first 2 cycles of treatment. For 31% of patients (n=14), their best response was a CR.
Eighty-eight percent of patients who achieved a CR/CRh (n=14) achieved minimal residual disease (MRD) negativity.
Forty percent of patients with a T315I mutation had a CR/CRh (4/10), and all of these responders were MRD negative.
Seven responders (44%) went on to allo-HSCT, 6 of whom were transplant-naïve.
Eight of the 16 responders (50%) ultimately relapsed. Their median time to relapse was 6.7 months. Three patients relapsed during treatment, 2 relapsed without undergoing allo-HSCT, and 3 relapsed after allo-HSCT.
Seven responders (44%) were still alive and had not relapsed at last follow-up. The remaining responder died in CR after allo-HSCT.
The median relapse-free survival was 6.7 months, with or without censoring for allo-HSCT. And the median overall survival was 7.1 months, with or without censoring for allo-HSCT.
Safety
The most common AEs were pyrexia (58%), febrile neutropenia (40%), and headache (31%). Nearly half of patients (47%) had neurologic events.
Eighty-two percent of patients had grade 3 or higher treatment-emergent AEs. The most common were febrile neutropenia (27%), thrombocytopenia (22%), and anemia (16%).
Forty-four percent of patients had grade 3 or higher AEs that were considered possibly related to blinatumomab. The most common were febrile neutropenia and increased levels of alanine aminotransferase (11% each).
Five patients had fatal AEs—multiorgan failure, sepsis, septic shock, cerebral hemorrhage, and respiratory failure. The case of septic shock was considered related to treatment with blinatumomab.
Three patients developed cytokine release syndrome (all grade 1 or 2), but none of them had their treatment interrupted or discontinued as a result.
Three patients had grade 3 neurologic events, and 1 of these events (aphasia) required temporary treatment interruption. There were no grade 4 or 5 neurologic events. ![]()
Blinatumomab has demonstrated activity in high-risk patients with Philadelphia chromosome-positive (Ph+) B-cell precursor acute lymphoblastic leukemia (BCP-ALL), according to research published in the Journal of Clinical Oncology.
This phase 2 trial enrolled patients with relapsed or refractory Ph+ BCP-ALL who had failed treatment with at least 1 tyrosine kinase inhibitor (TKI).
Blinatumomab produced a complete response (CR) in 31% of these patients, the median relapse-free survival was 6.7 months, and the median overall survival was 7.1 months.
The most common adverse events (AEs) were pyrexia, neurologic events, febrile neutropenia, and headache.
“Patients with Ph+ relapsed or refractory B-cell precursor ALL typically have lower remission rates, poor long-term prognosis, and shorter duration of remission than patients with Philadelphia chromosome-negative disease, and are especially in need of new treatment options beyond TKIs,” said study author Anthony Stein, MD, of City of Hope in Duarte, California.
“Results from this phase 2 study showed blinatumomab induced complete remission in these high-risk patients, regardless of prior TKI therapy or mutational status . . . .”
This study was supported by Amgen, the company developing and marketing blinatumomab.
The trial enrolled 45 patients with relapsed or refractory Ph+ BCP-ALL. Fifty-nine percent of patients had additional cytogenetic abnormalities. Forty-six percent had ABL1 kinase domain mutations, and 27% had the T315I mutation.
The patients’ median age was 55 (range, 23-78), and 55% were male. The median baseline bone marrow blast percentage was 80% (range, 6% to 98%).
Eighty-four percent of patients had received at least 2 prior TKIs. All patients were refractory to (56%), had relapsed on (33%), or progressed after (11%) TKI therapy. Forty-four percent of patients had undergone an allogeneic hematopoietic stem cell transplant (allo-HSCT).
The patients received blinatumomab in 28-day cycles by continuous intravenous infusion. The median number of cycles received was 2 (range, 1-5).
Efficacy
Thirty-six percent of patients (n=16) had a CR or CR with partial hematologic recovery (CRh) during the first 2 cycles of treatment. For 31% of patients (n=14), their best response was a CR.
Eighty-eight percent of patients who achieved a CR/CRh (n=14) achieved minimal residual disease (MRD) negativity.
Forty percent of patients with a T315I mutation had a CR/CRh (4/10), and all of these responders were MRD negative.
Seven responders (44%) went on to allo-HSCT, 6 of whom were transplant-naïve.
Eight of the 16 responders (50%) ultimately relapsed. Their median time to relapse was 6.7 months. Three patients relapsed during treatment, 2 relapsed without undergoing allo-HSCT, and 3 relapsed after allo-HSCT.
Seven responders (44%) were still alive and had not relapsed at last follow-up. The remaining responder died in CR after allo-HSCT.
The median relapse-free survival was 6.7 months, with or without censoring for allo-HSCT. And the median overall survival was 7.1 months, with or without censoring for allo-HSCT.
Safety
The most common AEs were pyrexia (58%), febrile neutropenia (40%), and headache (31%). Nearly half of patients (47%) had neurologic events.
Eighty-two percent of patients had grade 3 or higher treatment-emergent AEs. The most common were febrile neutropenia (27%), thrombocytopenia (22%), and anemia (16%).
Forty-four percent of patients had grade 3 or higher AEs that were considered possibly related to blinatumomab. The most common were febrile neutropenia and increased levels of alanine aminotransferase (11% each).
Five patients had fatal AEs—multiorgan failure, sepsis, septic shock, cerebral hemorrhage, and respiratory failure. The case of septic shock was considered related to treatment with blinatumomab.
Three patients developed cytokine release syndrome (all grade 1 or 2), but none of them had their treatment interrupted or discontinued as a result.
Three patients had grade 3 neurologic events, and 1 of these events (aphasia) required temporary treatment interruption. There were no grade 4 or 5 neurologic events. ![]()
FDA lifts partial clinical hold for some selinexor trials
The US Food and Drug Administration (FDA) has lifted the partial clinical hold on trials of selinexor (KPT-330) in patients with hematologic malignancies.
The partial hold, which was announced on March 10, was placed on all trials of the drug, including those in patients with solid tumor malignancies.
The hold meant that no new patients could be enrolled in selinexor trials.
Patients who were already enrolled and had stable disease or better could remain on selinexor therapy.
Now, the FDA has lifted the hold on trials of patients with hematologic malignancies, so new patients can be enrolled in these trials and begin receiving selinexor.
The FDA had placed the hold due to a lack of information in the investigator’s brochure, including an incomplete list of serious adverse events associated with selinexor.
Karyopharm Therapeutics Inc., the company developing selinexor, noted that the hold was not the result of patient deaths or any new information regarding the safety profile of selinexor.
In response to the hold, Karyopharm amended the investigator’s brochure, updated informed consent documents, and submitted the documents to the FDA.
“The Karyopharm team worked diligently to update and submit the required documents to the FDA, which allowed the hematology division to expeditiously remove the partial clinical hold,” said Michael G. Kauffman, MD, PhD, chief executive officer of Karyopharm.
“We anticipate that the solid tumor divisions will follow suit shortly. Patient enrollment is again underway in our hematologic oncology studies. Our previously disclosed enrollment rates and timelines for both ongoing and planned trials are not expected to be materially impacted.”
About selinexor
Selinexor is a first-in-class, oral, selective inhibitor of nuclear export compound. The drug functions by inhibiting the nuclear export protein XPO1 (also called CRM1).
This leads to the accumulation of tumor suppressor proteins in the cell nucleus, which subsequently reinitiates and amplifies their tumor suppressor function. This is thought to prompt apoptosis in cancer cells while largely sparing normal cells.
To date, more than 1900 patients have been treated with selinexor.
The drug is currently being evaluated in clinical trials across multiple cancer indications, including in acute myeloid leukemia (SOPRA), in multiple myeloma in combination with low-dose dexamethasone (STORM) and backbone therapies (STOMP), as well as in diffuse large B-cell lymphoma (SADAL).
The US Food and Drug Administration (FDA) has lifted the partial clinical hold on trials of selinexor (KPT-330) in patients with hematologic malignancies.
The partial hold, which was announced on March 10, was placed on all trials of the drug, including those in patients with solid tumor malignancies.
The hold meant that no new patients could be enrolled in selinexor trials.
Patients who were already enrolled and had stable disease or better could remain on selinexor therapy.
Now, the FDA has lifted the hold on trials of patients with hematologic malignancies, so new patients can be enrolled in these trials and begin receiving selinexor.
The FDA had placed the hold due to a lack of information in the investigator’s brochure, including an incomplete list of serious adverse events associated with selinexor.
Karyopharm Therapeutics Inc., the company developing selinexor, noted that the hold was not the result of patient deaths or any new information regarding the safety profile of selinexor.
In response to the hold, Karyopharm amended the investigator’s brochure, updated informed consent documents, and submitted the documents to the FDA.
“The Karyopharm team worked diligently to update and submit the required documents to the FDA, which allowed the hematology division to expeditiously remove the partial clinical hold,” said Michael G. Kauffman, MD, PhD, chief executive officer of Karyopharm.
“We anticipate that the solid tumor divisions will follow suit shortly. Patient enrollment is again underway in our hematologic oncology studies. Our previously disclosed enrollment rates and timelines for both ongoing and planned trials are not expected to be materially impacted.”
About selinexor
Selinexor is a first-in-class, oral, selective inhibitor of nuclear export compound. The drug functions by inhibiting the nuclear export protein XPO1 (also called CRM1).
This leads to the accumulation of tumor suppressor proteins in the cell nucleus, which subsequently reinitiates and amplifies their tumor suppressor function. This is thought to prompt apoptosis in cancer cells while largely sparing normal cells.
To date, more than 1900 patients have been treated with selinexor.
The drug is currently being evaluated in clinical trials across multiple cancer indications, including in acute myeloid leukemia (SOPRA), in multiple myeloma in combination with low-dose dexamethasone (STORM) and backbone therapies (STOMP), as well as in diffuse large B-cell lymphoma (SADAL).
The US Food and Drug Administration (FDA) has lifted the partial clinical hold on trials of selinexor (KPT-330) in patients with hematologic malignancies.
The partial hold, which was announced on March 10, was placed on all trials of the drug, including those in patients with solid tumor malignancies.
The hold meant that no new patients could be enrolled in selinexor trials.
Patients who were already enrolled and had stable disease or better could remain on selinexor therapy.
Now, the FDA has lifted the hold on trials of patients with hematologic malignancies, so new patients can be enrolled in these trials and begin receiving selinexor.
The FDA had placed the hold due to a lack of information in the investigator’s brochure, including an incomplete list of serious adverse events associated with selinexor.
Karyopharm Therapeutics Inc., the company developing selinexor, noted that the hold was not the result of patient deaths or any new information regarding the safety profile of selinexor.
In response to the hold, Karyopharm amended the investigator’s brochure, updated informed consent documents, and submitted the documents to the FDA.
“The Karyopharm team worked diligently to update and submit the required documents to the FDA, which allowed the hematology division to expeditiously remove the partial clinical hold,” said Michael G. Kauffman, MD, PhD, chief executive officer of Karyopharm.
“We anticipate that the solid tumor divisions will follow suit shortly. Patient enrollment is again underway in our hematologic oncology studies. Our previously disclosed enrollment rates and timelines for both ongoing and planned trials are not expected to be materially impacted.”
About selinexor
Selinexor is a first-in-class, oral, selective inhibitor of nuclear export compound. The drug functions by inhibiting the nuclear export protein XPO1 (also called CRM1).
This leads to the accumulation of tumor suppressor proteins in the cell nucleus, which subsequently reinitiates and amplifies their tumor suppressor function. This is thought to prompt apoptosis in cancer cells while largely sparing normal cells.
To date, more than 1900 patients have been treated with selinexor.
The drug is currently being evaluated in clinical trials across multiple cancer indications, including in acute myeloid leukemia (SOPRA), in multiple myeloma in combination with low-dose dexamethasone (STORM) and backbone therapies (STOMP), as well as in diffuse large B-cell lymphoma (SADAL).
Phase 2 study of daratumumab in NHL won’t proceed
The phase 2 CARINA study of daratumumab in non-Hodgkin lymphoma (NHL) will not proceed to stage 2, according to Genmab A/S and Janssen Biotech, Inc.
In this study, researchers have been investigating daratumumab monotherapy in patients with relapsed or refractory follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL), or mantle cell lymphoma (MCL).
Researchers planned to enroll up to 210 patients in this trial in 2 stages. Stage 1 was designed to provide a preliminary assessment of activity.
The goal of stage 2 was to further evaluate the safety and efficacy of daratumumab in the 3 patient groups.
Stage 2 will not proceed because a data review showed the FL and DLBCL cohorts did not reach the predefined futility thresholds, which were overall response rates of 50% and 30%, respectively. In the MCL cohort, the overall response rate was not evaluable due to slow recruitment.
The decision regarding this study has no impact on other ongoing or planned studies with daratumumab.
“While we hoped that daratumumab as a monotherapy could potentially provide a new treatment option in NHL patients with a high unmet medical need, the preliminary activity profile seen was not sufficient for the study to continue,” said Jan van de Winkel, PhD, chief executive officer of Genmab.
“Daratumumab is still being investigated in a number of indications, including multiple myeloma and other hematological cancers, such as NK/T-cell lymphoma and myelodysplastic syndrome, as well as in solid tumors.”
About daratumumab
Daratumumab is a human IgG1k monoclonal antibody that binds to the CD38 molecule.
In the US, daratumumab is approved for use in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, for the treatment of patients with multiple myeloma who have received at least 1 prior therapy.
Daratumumab monotherapy is approved in the US for patients with multiple myeloma who have received at least 3 prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent, or who are double-refractory to a proteasome inhibitor and an immunomodulatory agent.
Daratumumab is being developed by Janssen Biotech, Inc. under an exclusive worldwide license from Genmab. ![]()
The phase 2 CARINA study of daratumumab in non-Hodgkin lymphoma (NHL) will not proceed to stage 2, according to Genmab A/S and Janssen Biotech, Inc.
In this study, researchers have been investigating daratumumab monotherapy in patients with relapsed or refractory follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL), or mantle cell lymphoma (MCL).
Researchers planned to enroll up to 210 patients in this trial in 2 stages. Stage 1 was designed to provide a preliminary assessment of activity.
The goal of stage 2 was to further evaluate the safety and efficacy of daratumumab in the 3 patient groups.
Stage 2 will not proceed because a data review showed the FL and DLBCL cohorts did not reach the predefined futility thresholds, which were overall response rates of 50% and 30%, respectively. In the MCL cohort, the overall response rate was not evaluable due to slow recruitment.
The decision regarding this study has no impact on other ongoing or planned studies with daratumumab.
“While we hoped that daratumumab as a monotherapy could potentially provide a new treatment option in NHL patients with a high unmet medical need, the preliminary activity profile seen was not sufficient for the study to continue,” said Jan van de Winkel, PhD, chief executive officer of Genmab.
“Daratumumab is still being investigated in a number of indications, including multiple myeloma and other hematological cancers, such as NK/T-cell lymphoma and myelodysplastic syndrome, as well as in solid tumors.”
About daratumumab
Daratumumab is a human IgG1k monoclonal antibody that binds to the CD38 molecule.
In the US, daratumumab is approved for use in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, for the treatment of patients with multiple myeloma who have received at least 1 prior therapy.
Daratumumab monotherapy is approved in the US for patients with multiple myeloma who have received at least 3 prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent, or who are double-refractory to a proteasome inhibitor and an immunomodulatory agent.
Daratumumab is being developed by Janssen Biotech, Inc. under an exclusive worldwide license from Genmab. ![]()
The phase 2 CARINA study of daratumumab in non-Hodgkin lymphoma (NHL) will not proceed to stage 2, according to Genmab A/S and Janssen Biotech, Inc.
In this study, researchers have been investigating daratumumab monotherapy in patients with relapsed or refractory follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL), or mantle cell lymphoma (MCL).
Researchers planned to enroll up to 210 patients in this trial in 2 stages. Stage 1 was designed to provide a preliminary assessment of activity.
The goal of stage 2 was to further evaluate the safety and efficacy of daratumumab in the 3 patient groups.
Stage 2 will not proceed because a data review showed the FL and DLBCL cohorts did not reach the predefined futility thresholds, which were overall response rates of 50% and 30%, respectively. In the MCL cohort, the overall response rate was not evaluable due to slow recruitment.
The decision regarding this study has no impact on other ongoing or planned studies with daratumumab.
“While we hoped that daratumumab as a monotherapy could potentially provide a new treatment option in NHL patients with a high unmet medical need, the preliminary activity profile seen was not sufficient for the study to continue,” said Jan van de Winkel, PhD, chief executive officer of Genmab.
“Daratumumab is still being investigated in a number of indications, including multiple myeloma and other hematological cancers, such as NK/T-cell lymphoma and myelodysplastic syndrome, as well as in solid tumors.”
About daratumumab
Daratumumab is a human IgG1k monoclonal antibody that binds to the CD38 molecule.
In the US, daratumumab is approved for use in combination with lenalidomide and dexamethasone, or bortezomib and dexamethasone, for the treatment of patients with multiple myeloma who have received at least 1 prior therapy.
Daratumumab monotherapy is approved in the US for patients with multiple myeloma who have received at least 3 prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent, or who are double-refractory to a proteasome inhibitor and an immunomodulatory agent.
Daratumumab is being developed by Janssen Biotech, Inc. under an exclusive worldwide license from Genmab. ![]()
FDA grants priority review to sBLA for blinatumomab
The US Food and Drug Administration (FDA) has accepted for priority review the supplemental biologics license application (sBLA) for blinatumomab (Blincyto®).
The aim of this application is to expand the indication for blinatumomab to include all patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (BCP-ALL) and to convert blinatumomab’s current accelerated approval to a full approval.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The Prescription Drug User Fee Act target action date for the blinatumomab sBLA is August 14, 2017.
About blinatumomab
Blinatumomab is a bispecific, CD19-directed, CD3 T-cell engager (BiTE®) antibody construct that binds specifically to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of T cells.
Blinatumomab currently has accelerated approval in the US as a treatment for adult and pediatric patients with Philadelphia chromosome-negative (Ph-) relapsed or refractory BCP-ALL.
The FDA-approved prescribing information for blinatumomab includes a boxed warning detailing the risk of cytokine release syndrome and neurologic toxicities.
Blinatumomab is also under a Risk Evaluation and Mitigation Strategy program in the US intended to inform healthcare providers about these risks.
Blinatumomab is being developed and marketed by Amgen.
About the sBLA
With this sBLA, Amgen is seeking to make blinatumomab available as a treatment for patients with Philadelphia chromosome-positive (Ph+) relapsed/refractory BCP-ALL (as well as Ph-).
To this end, the application includes data from the ALCANTARA study, which were just published in the Journal of Clinical Oncology.
In this trial, researchers evaluated blinatumomab in adults with Ph+ relapsed/refractory BCP-ALL who had failed treatment with at least 1 tyrosine kinase inhibitor.
Thirty-six percent of patients achieved a complete response or complete response with partial hematologic recovery within the first 2 cycles of blinatumomab treatment. Of these patients, 88% were minimal residual disease negative.
The most frequent adverse events (AEs) in this trial were pyrexia (58%), neurologic events (47%), febrile neutropenia (40%), and headache (31%). Three patients had grade 1/2 cytokine release syndrome, and 3 patients had grade 3 neurologic AEs.
The sBLA also includes overall survival (OS) data from the phase 3 TOWER trial, which is intended to support the conversion of blinatumomab’s accelerated approval to a full approval.
Results from the TOWER trial were recently published in NEJM.
In this study, researchers compared blinatumomab to standard of care (SOC) chemotherapy (4 different regimens) in adults with Ph- relapsed/refractory BCP-ALL.
Blinatumomab produced higher response rates and nearly doubled OS compared to SOC. The median OS was 7.7 months in the blinatumomab arm and 4 months in the SOC arm. The hazard ratio for death was 0.71 (P=0.012).
The incidence of grade 3 or higher AEs was higher in the SOC arm, but the incidence of serious AEs was higher in the blinatumomab arm. ![]()
The US Food and Drug Administration (FDA) has accepted for priority review the supplemental biologics license application (sBLA) for blinatumomab (Blincyto®).
The aim of this application is to expand the indication for blinatumomab to include all patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (BCP-ALL) and to convert blinatumomab’s current accelerated approval to a full approval.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The Prescription Drug User Fee Act target action date for the blinatumomab sBLA is August 14, 2017.
About blinatumomab
Blinatumomab is a bispecific, CD19-directed, CD3 T-cell engager (BiTE®) antibody construct that binds specifically to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of T cells.
Blinatumomab currently has accelerated approval in the US as a treatment for adult and pediatric patients with Philadelphia chromosome-negative (Ph-) relapsed or refractory BCP-ALL.
The FDA-approved prescribing information for blinatumomab includes a boxed warning detailing the risk of cytokine release syndrome and neurologic toxicities.
Blinatumomab is also under a Risk Evaluation and Mitigation Strategy program in the US intended to inform healthcare providers about these risks.
Blinatumomab is being developed and marketed by Amgen.
About the sBLA
With this sBLA, Amgen is seeking to make blinatumomab available as a treatment for patients with Philadelphia chromosome-positive (Ph+) relapsed/refractory BCP-ALL (as well as Ph-).
To this end, the application includes data from the ALCANTARA study, which were just published in the Journal of Clinical Oncology.
In this trial, researchers evaluated blinatumomab in adults with Ph+ relapsed/refractory BCP-ALL who had failed treatment with at least 1 tyrosine kinase inhibitor.
Thirty-six percent of patients achieved a complete response or complete response with partial hematologic recovery within the first 2 cycles of blinatumomab treatment. Of these patients, 88% were minimal residual disease negative.
The most frequent adverse events (AEs) in this trial were pyrexia (58%), neurologic events (47%), febrile neutropenia (40%), and headache (31%). Three patients had grade 1/2 cytokine release syndrome, and 3 patients had grade 3 neurologic AEs.
The sBLA also includes overall survival (OS) data from the phase 3 TOWER trial, which is intended to support the conversion of blinatumomab’s accelerated approval to a full approval.
Results from the TOWER trial were recently published in NEJM.
In this study, researchers compared blinatumomab to standard of care (SOC) chemotherapy (4 different regimens) in adults with Ph- relapsed/refractory BCP-ALL.
Blinatumomab produced higher response rates and nearly doubled OS compared to SOC. The median OS was 7.7 months in the blinatumomab arm and 4 months in the SOC arm. The hazard ratio for death was 0.71 (P=0.012).
The incidence of grade 3 or higher AEs was higher in the SOC arm, but the incidence of serious AEs was higher in the blinatumomab arm. ![]()
The US Food and Drug Administration (FDA) has accepted for priority review the supplemental biologics license application (sBLA) for blinatumomab (Blincyto®).
The aim of this application is to expand the indication for blinatumomab to include all patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (BCP-ALL) and to convert blinatumomab’s current accelerated approval to a full approval.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The Prescription Drug User Fee Act target action date for the blinatumomab sBLA is August 14, 2017.
About blinatumomab
Blinatumomab is a bispecific, CD19-directed, CD3 T-cell engager (BiTE®) antibody construct that binds specifically to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of T cells.
Blinatumomab currently has accelerated approval in the US as a treatment for adult and pediatric patients with Philadelphia chromosome-negative (Ph-) relapsed or refractory BCP-ALL.
The FDA-approved prescribing information for blinatumomab includes a boxed warning detailing the risk of cytokine release syndrome and neurologic toxicities.
Blinatumomab is also under a Risk Evaluation and Mitigation Strategy program in the US intended to inform healthcare providers about these risks.
Blinatumomab is being developed and marketed by Amgen.
About the sBLA
With this sBLA, Amgen is seeking to make blinatumomab available as a treatment for patients with Philadelphia chromosome-positive (Ph+) relapsed/refractory BCP-ALL (as well as Ph-).
To this end, the application includes data from the ALCANTARA study, which were just published in the Journal of Clinical Oncology.
In this trial, researchers evaluated blinatumomab in adults with Ph+ relapsed/refractory BCP-ALL who had failed treatment with at least 1 tyrosine kinase inhibitor.
Thirty-six percent of patients achieved a complete response or complete response with partial hematologic recovery within the first 2 cycles of blinatumomab treatment. Of these patients, 88% were minimal residual disease negative.
The most frequent adverse events (AEs) in this trial were pyrexia (58%), neurologic events (47%), febrile neutropenia (40%), and headache (31%). Three patients had grade 1/2 cytokine release syndrome, and 3 patients had grade 3 neurologic AEs.
The sBLA also includes overall survival (OS) data from the phase 3 TOWER trial, which is intended to support the conversion of blinatumomab’s accelerated approval to a full approval.
Results from the TOWER trial were recently published in NEJM.
In this study, researchers compared blinatumomab to standard of care (SOC) chemotherapy (4 different regimens) in adults with Ph- relapsed/refractory BCP-ALL.
Blinatumomab produced higher response rates and nearly doubled OS compared to SOC. The median OS was 7.7 months in the blinatumomab arm and 4 months in the SOC arm. The hazard ratio for death was 0.71 (P=0.012).
The incidence of grade 3 or higher AEs was higher in the SOC arm, but the incidence of serious AEs was higher in the blinatumomab arm. ![]()
Can Phone Coaching Motivate Veterans to Try Preventive Care?
The VHA offers several preventive care programs to veterans who are at high risk for various chronic illnesses: For instance, 20% of veterans smoke, and > 70% of VHA patients are overweight.
Although those programs are well supported and have strong evidence for effectiveness, they’re underused, say researchers from Durham VAMC and Duke University in North Carolina, VA Ann Arbor Healthcare System in Michigan, and VA Salt Lake City Center for Informatics Decision Enhancement and Surveillance and University of Utah. The VHA’s MOVE! Program produced significant weight loss among participants—the only problem was that < 10% of eligible veterans actually joined.
The researchers conducted the ACTIVATE trial, which involved a web-based health risk assessment (HRA) coupled with a health coaching intervention to link veterans to a local prevention program. In the study, veterans completed an online HRA. The researchers then tested whether 2 telephone-based coaching sessions were more effective in getting the veterans to enroll in prevention programs than did completing the HRA.
The coaching was not designed to change behavior but specifically aimed at helping veterans set a “first step” goal by choosing a program to enroll in that aligned with their values and preferences as well as risk factors highlighted by their HRA surveys.
The results aren’t in, but the researchers expect their findings to help the VHA implement its plan to engage veterans in preventive health care. Their “robustly designed trial,” they say, “will add valuable knowledge at a critical time when VHA and other health systems are working to understand how to effectively incorporate HRA findings into the busy clinic flow of primary care.”
The VHA offers several preventive care programs to veterans who are at high risk for various chronic illnesses: For instance, 20% of veterans smoke, and > 70% of VHA patients are overweight.
Although those programs are well supported and have strong evidence for effectiveness, they’re underused, say researchers from Durham VAMC and Duke University in North Carolina, VA Ann Arbor Healthcare System in Michigan, and VA Salt Lake City Center for Informatics Decision Enhancement and Surveillance and University of Utah. The VHA’s MOVE! Program produced significant weight loss among participants—the only problem was that < 10% of eligible veterans actually joined.
The researchers conducted the ACTIVATE trial, which involved a web-based health risk assessment (HRA) coupled with a health coaching intervention to link veterans to a local prevention program. In the study, veterans completed an online HRA. The researchers then tested whether 2 telephone-based coaching sessions were more effective in getting the veterans to enroll in prevention programs than did completing the HRA.
The coaching was not designed to change behavior but specifically aimed at helping veterans set a “first step” goal by choosing a program to enroll in that aligned with their values and preferences as well as risk factors highlighted by their HRA surveys.
The results aren’t in, but the researchers expect their findings to help the VHA implement its plan to engage veterans in preventive health care. Their “robustly designed trial,” they say, “will add valuable knowledge at a critical time when VHA and other health systems are working to understand how to effectively incorporate HRA findings into the busy clinic flow of primary care.”
The VHA offers several preventive care programs to veterans who are at high risk for various chronic illnesses: For instance, 20% of veterans smoke, and > 70% of VHA patients are overweight.
Although those programs are well supported and have strong evidence for effectiveness, they’re underused, say researchers from Durham VAMC and Duke University in North Carolina, VA Ann Arbor Healthcare System in Michigan, and VA Salt Lake City Center for Informatics Decision Enhancement and Surveillance and University of Utah. The VHA’s MOVE! Program produced significant weight loss among participants—the only problem was that < 10% of eligible veterans actually joined.
The researchers conducted the ACTIVATE trial, which involved a web-based health risk assessment (HRA) coupled with a health coaching intervention to link veterans to a local prevention program. In the study, veterans completed an online HRA. The researchers then tested whether 2 telephone-based coaching sessions were more effective in getting the veterans to enroll in prevention programs than did completing the HRA.
The coaching was not designed to change behavior but specifically aimed at helping veterans set a “first step” goal by choosing a program to enroll in that aligned with their values and preferences as well as risk factors highlighted by their HRA surveys.
The results aren’t in, but the researchers expect their findings to help the VHA implement its plan to engage veterans in preventive health care. Their “robustly designed trial,” they say, “will add valuable knowledge at a critical time when VHA and other health systems are working to understand how to effectively incorporate HRA findings into the busy clinic flow of primary care.”
Psychosis in BPD
Health law changes under new administration
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
A veteran who is suicidal while sleeping
CASE Suicidal while asleep
Mr. R, age 28, an Iraq and Afghanistan veteran with major depressive disorder and posttraumatic stress disorder (PTSD), is awoken by his wife to check on their daughter approximately 30 minutes after he takes his nightly regimen of zolpidem, 10 mg, melatonin, 6 mg, and hydroxyzine, 20 mg. When Mr. R returns to the bedroom, he appears to be confused. Mr. R grabs an unloaded gun from under the mattress, puts it in his mouth, and pulls the trigger. Then Mr. R holds the gun to his head and pulls the trigger while saying that his wife and children will be better off without him. His wife takes the gun away, but he grabs another gun from his gun box and loads it. His wife convinces him to remove the ammunition; however, Mr. R gets the other unloaded gun and pulls the trigger on himself again. After his wife takes this gun away, he tries cutting himself with a pocketknife, causing superficial cuts. Eventually, Mr. R goes back to bed. He does not remember these events in the morning.
What increased the likelihood of parasomnia in Mr. R?
a) high zolpidem dosage
b) concomitant use of other sedating agents
c) sleep deprivation
d) dehydration
[polldaddy:9712545]
The authors’ observations
Parasomnias are sleep-wake transition disorders classified by the sleep stage from which they arise, either NREM or rapid eye movement (REM). NREM parasomnias could result from incomplete awakening from NREM sleep, typically in Stage N3 (slow-wave) sleep.1 DSM-5 describes NREM parasomnias as arousal disorders in which the disturbance is not attributable to the physiological effects of substance; substance/medication-induced sleep disorder, parasomnia type, is when the disturbance can be attributed to a substance.2 The latter also can occur during REM sleep.
NREM parasomnias are characterized by abnormal behaviors during sleep with significant harm potential.3 Somnambulism or sleepwalking and sleep terrors are the 2 types of NREM parasomnias in DSM-5. Sleepwalking could involve complex behaviors, including:
- eating
- talking
- cooking
- shopping
- driving
- sexual activity.
Zolpidem, a benzodiazepine receptor agonist, is a preferred hypnotic agent for insomnia because of its low risk for abuse and daytime sedation.4 However, the drug has been associated with NREM parasomnias, namely somnambulism or sleepwalking, and its variants including sleep-driving, sleep-related eating disorder, and rarely sexsomnia (sleep-sex), with anterograde amnesia for the event.5 Suicidal behavior that occurs while the patient is asleep with next-day amnesia is another variant of somnambulism. There are several reports of suicidal behavior during sleep,6,7 but to our knowledge, there are only 2 previous cases implicating zolpidem as the cause:
- Gibson et al8 described a 49-year-old man who sustained a self-inflicted gunshot wound to his head while asleep. He just had started taking zolpidem, and in the weeks before the incident he had several episodes of sleepwalking and sleep-eating. He had consumed alcohol the night of the self-inflicted gunshot wound, but had no other psychiatric history.
- Chopra et al4 described a 37-year-old man, with no prior episodes of sleepwalking or associated complex behaviors, who was taking zolpidem, 10 mg/d, for chronic insomnia. He shot a gun in the basement of his home, and then held the loaded gun to his neck while asleep. The authors attributed the event to zolpidem in combination with other predisposing factors, including dehydration after intense exercise and alcohol use. The authors categorized this type of event as “para-suicidal amnestic behavior,” although “sleep-related pseudo-suicidal behavior” might be a better term for this type of parasomnia because of its occurrence during sleep and non-deliberate nature.
In another case report, a 27-year-old man took additional zolpidem after he did not experience desired sedative effects from an initial 20 mg.9 Because the patient remembered the suicidal thoughts, the authors believed that the patient attempted suicide while under the influence of zolpidem. The authors did not believe the incident to be sleep-related suicidal behavior, because it was uncertain if he attempted suicide while asleep.
Mr. R does not remember the events his wife witnessed while he was asleep. To our knowledge, Mr. R’s case is the first sleep-related pseudo-suicidal behavior case resulting from zolpidem, 10 mg/d, without concurrent alcohol use in an adult male veteran with PTSD and no suicidal ideation while awake.
HISTORY Further details revealed
Mr. R says that in the days leading to the incident he was not sleep-deprived and was getting at least 6 hours of restful sleep every night. He had been taking zolpidem every night. He has no childhood or family history of NREM parasomnias. He says he did not engage in intense exercise that evening or have a fever the night of the incident and has abstained from alcohol for 2 years.
His wife says that after he took zolpidem, when he was woken up, “He was not there; his eyes were glazed and glossy, and it’s like he was in another world,” and his speech and behavior were bizarre. She also reports that his eyes were open when he engaged in this behavior that appeared suicidal.
Three months before the incident, Mr. R had reported nightmares with dream enactment behaviors, hypervigilance on awakening and during the daytime, irritability, and anxious and depressed mood with neurovegetative symptoms, and was referred to our clinic for medication management. He also reported no prior or current manic or psychotic symptoms, denied suicidal thoughts, and had no history of suicide attempts. Mr. R’s medication regimen included tramadol, 400 mg/d, for chronic knee pain; fluoxetine, 60 mg/d, for depression and PTSD; and propranolol ER, 60 mg/d, and propranolol, 10 mg/d as needed, for anxiety. He was started on prazosin, 2 mg/d, titrated to 4 mg/d, for medication management of nightmares.
Mr. R also was referred to the sleep laboratory for a polysomnogram (PSG) because of reported loud snoring and witnessed apneas, especially because sleep apnea can cause nightmares and dream enactment behaviors. The PSG was negative for sleep apnea or excessive periodic limb movements of sleep, but showed increased electromyographic (EMG) activity during REM sleep, which was consistent with his report of dream enactment behaviors. Two months later, he reported improvement in nightmares and depression, but not in dream enactment behaviors. Because of prominent anxiety and irritability, he was started on gabapentin, 300 mg, 3 times a day.
What factor increases the risk of NREM parasomnias with zolpidem compared with benzodiazepines?
a) greater preservation of Stage N3 sleep
b) lesser degree of muscle relaxation
c) both a and b
d) none of the above
[polldaddy:9712556]
The authors’ observations
Factors that increase the likelihood of parasomnias include:
- zolpidem >10 mg at bedtime
- concomitant use of other CNS depressants, including sedative hypnotic agents and alcohol
- female sex
- not falling asleep immediately after taking zolpidem
- personal or family history of parasomnias
- living alone
- poor pill management
- presence of sleep disruptors such as sleep apnea and periodic limb movements of sleep.1,4,5,10
Higher dosages of zolpidem (>10 mg/d) have been identified as the predictive risk factor.5 In the Chopra et al4 case report on sleep-related suicidal behavior related to zolpidem, 10 mg at bedtime, concomitant dehydration and alcohol use were implicated as facilitating factors. Dehydration could increase serum levels of zolpidem resulting in greater CNS effects. Alcohol use was implicated in the Gibson et al8 case report as well, and the patient had multiple episodes of sleepwalking and sleep-related eating.However, Mr. R was not dehydrated or using alcohol.
An interesting feature of Mr. R’s case is that he was taking fluoxetine. Cytochrome P450 (CYP) 3A4 is involved in metabolizing zolpidem, and norfluoxetine, a metabolite of fluoxetine, inhibits CYP3A4. Although studies have not found pharmacokinetic interactions between fluoxetine and zolpidem, these studies did not investigate fluoxetine dosages >20 mg/d.11 The inhibition of CYP enzymes by fluoxetine likely is dose-dependent,12 and therefore concomitant administration of high-dosage fluoxetine (>20 mg/d) with zolpidem might result in higher serum levels of zolpidem.
Mr. R also was taking several sedating agents (gabapentin, hydroxyzine, melatonin, and tramadol). The concomitant use of these sedative-hypnotic agents could have increased his risk of parasomnia. A review of the literature did not reveal any reports of gabapentin, hydroxyzine, melatonin, or tramadol causing parasomnias. This observation, as well as the well-known role of zolpidem5 in etiopathogenesis of parasomnias, indicates that the pseudo-suicidal behavior Mr. R displayed while asleep likely was a direct result of zolpidem use in presence of other facilitating factors. Gabapentin, which is known to increase the depth of sleep, was added to his regimen 1 month before his parasomnia episode. Therefore, gabapentin could have triggered parasomnia with zolpidem therapy.1,13
Conditions that provoke repeated cortical arousals (eg, periodic limb movement disorder [PLMD] and sleep apnea) or increase depth or pressure of sleep (intense exercise in the evening, fever, sleep deprivation) are thought to be associated with NREM parasomnias.1-4 However, Mr. R underwent in-laboratory PSG and tested negative for major cortical arousal-inducing conditions, such as PLMD and sleep apnea.
Some other sleep disruptors likely were involved in Mr. R’s case. Auditory and tactile stimuli are known to cause cortical arousals, with additive effect seen when these 2 stimuli are combined.3,14 Additionally, these exogenous stimuli are known to trigger sleep-related violent parasomnias.15 Mr. R displayed this behavior after his wife woke him up. The auditory stimulus of his wife’s voice and/or tactile stimulus involved in the act of waking Mr. R likely played a role in the suicidal and violent nature of his NREM parasomnia.
[polldaddy:9712581]
The authors’ observations
In general, the mechanisms by which zolpidem causes NREM parasomnias are not completely understood. The sedation-related amnestic properties of zolpidem might explain some of these behaviors. Patients could perform these behaviors after waking and have subsequent amnesia.4 There is greater preservation of Stage N3 sleep with zolpidem compared with benzodiazepines. Benzodiazepines also cause muscle relaxation while the motor system remains relatively more active during sleep with zolpidem because of its selectivity for α-1 subunit of gamma-aminobutyric acid A receptor. These factors might increase the likelihood of NREM parasomnias with zolpidem compared with benzodiazepines.4
Types of parasomnias
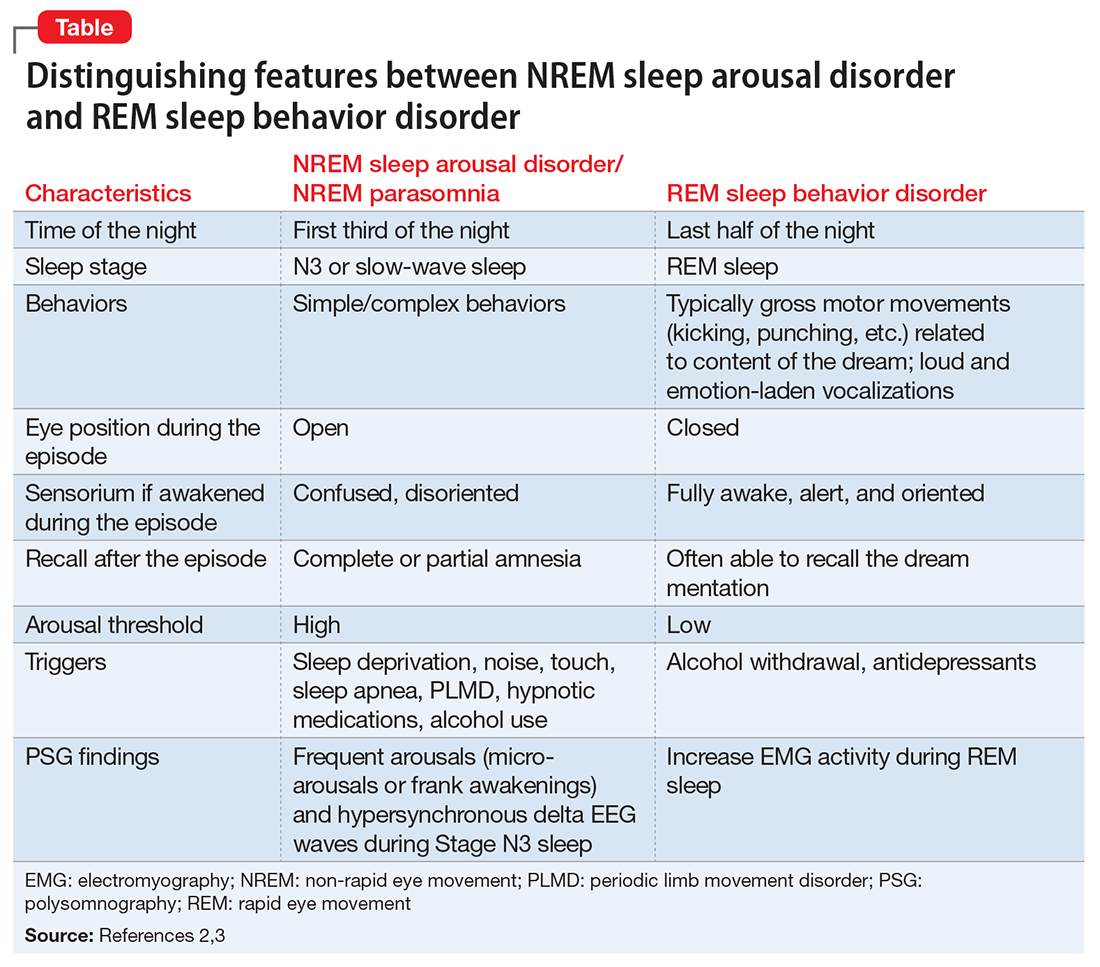
According to DSM-5, there are 2 categories of parasomnias based on the sleep stage from which a parasomnia emerges.2 REM sleep behavior disorder (RBD) refers to complex motor and/or vocalizations during REM sleep, accompanied by increased EMG activity during REM sleep (Table).2,3
The pseudo-suicidal behavior Mr. R displayed likely was NREM parasomnia because it occurred in the first third of the night with his eyes open and impaired recall after the event. Interestingly, Mr. R had RBD in addition to the NREM parasomnia likely caused by zolpidem. This is evident from Mr. R’s frequent dream enactment behaviors, such as kicking, thrashing, and punching during sleep, along with increased EMG activity during REM sleep as recorded on the PSG.10 The presence of RBD could be explained by selective serotonin reuptake inhibitor (fluoxetine) use, and comorbidity with PTSD.2,16
Management of parasomnias
Initial management of parasomnias involves decreasing the risk of parasomnia-related injury. Suggested safety measures include:
- sleeping away from windows
- sleeping in a sleeping bag
- sleeping on a lower floor
- locking windows and doors
- removing potentially dangerous objects from the bedroom
- putting gates across stairwells
- installing bells or alarms on door knobs.15
Removing access to firearms or other weapons such as knives is of utmost importance especially with patients who have easy access during wakefulness. If removing weapons is not feasible, consider disarming, securing, or locking them.15 These considerations are relevant to veterans with PTSD because of the high prevalence of symptoms, including depression, insomnia, and pain, which require sedating medications.17 A review of parasomnias among a large sample of psychiatric outpatients revealed that a variety of sedating medications, including antidepressants, can lead to NREM parasomnias.18 Therefore, exercise caution when prescribing sedating medications, especially in patients vulnerable to developing dangerous parasomnias, such as a veteran with PTSD and easy access to guns.19
TREATMENT Zolpidem stopped
Mr. R immediately stops taking zolpidem because he is aware of its association with abnormal behaviors during sleep, and his wife removes his access to firearms and knives at night. Because of his history of clinical benefit and no history of parasomnias with mirtazapine, Mr. R is started on mirtazapine for insomnia that previously was treated with zolpidem, and residual depression. Six months after discontinuing zolpidem, he does not experience NREM parasomnias, and there are no changes in his dream enactment behaviors.
Summing up
Zolpidem therapy could be associated with unusual variants of NREM parasomnia, sleepwalking type; sleep-related pseudo-suicidal behavior is one such variant. Several factors could play a role in increasing the likelihood of NREM parasomnia with zolpidem therapy. In Mr. R’s case, the pharmacokinetic drug interactions between fluoxetine and zolpidem, as well as concomitant use of several sedating agents could have played a role in increasing the likelihood of NREM parasomnia, with audio-tactile stimuli contributing to the violent and suicidal nature of the parasomnia. Exercise caution when using CYP enzyme inhibitors, such as fluoxetine and paroxetine, in combination with zolpidem. Knowledge of the potential interaction between zolpidem and fluoxetine is important because antidepressants and hypnotics are commonly co-prescribed because insomnia often is comorbid with other psychiatric disorders.
In veterans with PTSD who do not have suicidal ideations while awake, life-threatening non-intentional behavior is a risk because of easy access to guns or other weapons. Sedative-hypnotic medications commonly are prescribed to patients with PTSD. Exercise caution when using hypnotic agents such as zolpidem, and consider sleep aids with a lower risk of parasomnias (based on the author’s experience, trazodone, mirtazapine, melatonin, and gabapentin) when possible. Non-pharmacologic treatments of insomnia, such as sleep hygiene education and, more importantly, cognitive-behavioral therapy for insomnia, are preferred. If a patient is already taking zolpidem, nightly dosage should not be >10 mg. Polypharmacy with other sedating medications should be avoided when possible and both exogenous (noise, pets) and endogenous sleep disruptors (sleep apnea, PLMD) should be addressed. Advise the patient to avoid alcohol and remove firearms and other potential weapons. Discontinue zolpidem if the patient develops sleep-related abnormal behavior because of its potential to take on violent forms.
1. Howell MJ. Parasomnias: an updated review. Neurotherapeutics. 2012;9(4):753-775.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Zadra A, Desautels A, Petit D, et al. Somnambulism: clinical aspects and pathophysiological hypotheses. Lancet Neurol. 2013;12(3):285-294.
4. Chopra A, Selim B, Silber MH, et al. Para-suicidal amnestic behavior associated with chronic zolpidem use: implications for patient safety. Psychosomatics. 2013;54(5):498-501.
5. Hwang TJ, Ni HC, Chen HC, et al. Risk predictors for hypnosedative-related complex sleep behaviors: a retrospective, cross-sectional pilot study. J Clin Psychiatry. 2010;71(10):1331-1335.
6. Shatkin JP, Feinfield K, Strober M. The misinterpretation of a non-REM sleep parasomnia as suicidal behavior in an adolescent. Sleep Breath. 2002;6(4):175-179.
7. Mahowald MW, Schenck CH, Goldner M, et al. Parasomnia pseudo-suicide. J Forensic Sci. 2003;48(5):1158-1162.
8. Gibson CE, Caplan JP. Zolpidem-associated parasomnia with serious self-injury: a shot in the dark. Psychosomatics. 2011;52(1):88-91.
9. Mortaz Hejri S, Faizi M, Babaeian M. Zolpidem-induced suicide attempt: a case report. Daru. 2013;20;21(1):77.
10. Poceta JS. Zolpidem ingestion, automatisms, and sleep driving: a clinical and legal case series. J Clin Sleep Med. 2011;7(6):632-638.
11. Hesse LM, von Moltke LL, Greenblatt DJ. Clinically important drug interactions with zopiclone, zolpidem and zaleplon. CNS Drugs. 2003;17(7):513-532.
12. Catterson ML, Preskorn SH. Pharmacokinetics of selective serotonin reuptake inhibitors: clinical relevance. Pharmacol Toxicol. 1996;78(4):203-208.
13. Rosenberg RP, Hull SG, Lankford DA, et al. A randomized, double-blind, single-dose, placebo-controlled, multicenter, polysomnographic study of gabapentin in transient insomnia induced by sleep phase advance. J Clin Sleep Med. 2014;10(10):1093-1100.
14. Kato T, Montplaisir JY, Lavigne GJ. Experimentally induced arousals during sleep: a cross-modality matching paradigm. J Sleep Res. 2004;13(3):229-238.
15. Siclari F, Khatami R, Urbaniok F, et al. Violence in sleep. Brain. 2010;133(pt 12):3494-3509.
16. Husain AM, Miller PP, Carwile ST. Rem sleep behavior disorder: potential relationship to post-traumatic stress disorder. J Clin Neurophysiol. 2001;18(2):148-157.
17. Bernardy NC, Lund BC, Alexander B, et al. Increased polysedative use in veterans with posttraumatic stress disorder. Pain Med. 2014;15(7):1083-1090.
18. Lam SP, Fong SY, Ho CK, et al. Parasomnia among psychiatric outpatients: a clinical, epidemiologic, cross-sectional study. J Clin Psychiatry. 2008;69(9):1374-1382.
19. Freeman TW, Roca V, Kimbrell T. A survey of gun collection and use among three groups of veteran patients admitted to veterans affairs hospital treatment programs. South Med J. 2003;96(3):240-243.
CASE Suicidal while asleep
Mr. R, age 28, an Iraq and Afghanistan veteran with major depressive disorder and posttraumatic stress disorder (PTSD), is awoken by his wife to check on their daughter approximately 30 minutes after he takes his nightly regimen of zolpidem, 10 mg, melatonin, 6 mg, and hydroxyzine, 20 mg. When Mr. R returns to the bedroom, he appears to be confused. Mr. R grabs an unloaded gun from under the mattress, puts it in his mouth, and pulls the trigger. Then Mr. R holds the gun to his head and pulls the trigger while saying that his wife and children will be better off without him. His wife takes the gun away, but he grabs another gun from his gun box and loads it. His wife convinces him to remove the ammunition; however, Mr. R gets the other unloaded gun and pulls the trigger on himself again. After his wife takes this gun away, he tries cutting himself with a pocketknife, causing superficial cuts. Eventually, Mr. R goes back to bed. He does not remember these events in the morning.
What increased the likelihood of parasomnia in Mr. R?
a) high zolpidem dosage
b) concomitant use of other sedating agents
c) sleep deprivation
d) dehydration
[polldaddy:9712545]
The authors’ observations
Parasomnias are sleep-wake transition disorders classified by the sleep stage from which they arise, either NREM or rapid eye movement (REM). NREM parasomnias could result from incomplete awakening from NREM sleep, typically in Stage N3 (slow-wave) sleep.1 DSM-5 describes NREM parasomnias as arousal disorders in which the disturbance is not attributable to the physiological effects of substance; substance/medication-induced sleep disorder, parasomnia type, is when the disturbance can be attributed to a substance.2 The latter also can occur during REM sleep.
NREM parasomnias are characterized by abnormal behaviors during sleep with significant harm potential.3 Somnambulism or sleepwalking and sleep terrors are the 2 types of NREM parasomnias in DSM-5. Sleepwalking could involve complex behaviors, including:
- eating
- talking
- cooking
- shopping
- driving
- sexual activity.
Zolpidem, a benzodiazepine receptor agonist, is a preferred hypnotic agent for insomnia because of its low risk for abuse and daytime sedation.4 However, the drug has been associated with NREM parasomnias, namely somnambulism or sleepwalking, and its variants including sleep-driving, sleep-related eating disorder, and rarely sexsomnia (sleep-sex), with anterograde amnesia for the event.5 Suicidal behavior that occurs while the patient is asleep with next-day amnesia is another variant of somnambulism. There are several reports of suicidal behavior during sleep,6,7 but to our knowledge, there are only 2 previous cases implicating zolpidem as the cause:
- Gibson et al8 described a 49-year-old man who sustained a self-inflicted gunshot wound to his head while asleep. He just had started taking zolpidem, and in the weeks before the incident he had several episodes of sleepwalking and sleep-eating. He had consumed alcohol the night of the self-inflicted gunshot wound, but had no other psychiatric history.
- Chopra et al4 described a 37-year-old man, with no prior episodes of sleepwalking or associated complex behaviors, who was taking zolpidem, 10 mg/d, for chronic insomnia. He shot a gun in the basement of his home, and then held the loaded gun to his neck while asleep. The authors attributed the event to zolpidem in combination with other predisposing factors, including dehydration after intense exercise and alcohol use. The authors categorized this type of event as “para-suicidal amnestic behavior,” although “sleep-related pseudo-suicidal behavior” might be a better term for this type of parasomnia because of its occurrence during sleep and non-deliberate nature.
In another case report, a 27-year-old man took additional zolpidem after he did not experience desired sedative effects from an initial 20 mg.9 Because the patient remembered the suicidal thoughts, the authors believed that the patient attempted suicide while under the influence of zolpidem. The authors did not believe the incident to be sleep-related suicidal behavior, because it was uncertain if he attempted suicide while asleep.
Mr. R does not remember the events his wife witnessed while he was asleep. To our knowledge, Mr. R’s case is the first sleep-related pseudo-suicidal behavior case resulting from zolpidem, 10 mg/d, without concurrent alcohol use in an adult male veteran with PTSD and no suicidal ideation while awake.
HISTORY Further details revealed
Mr. R says that in the days leading to the incident he was not sleep-deprived and was getting at least 6 hours of restful sleep every night. He had been taking zolpidem every night. He has no childhood or family history of NREM parasomnias. He says he did not engage in intense exercise that evening or have a fever the night of the incident and has abstained from alcohol for 2 years.
His wife says that after he took zolpidem, when he was woken up, “He was not there; his eyes were glazed and glossy, and it’s like he was in another world,” and his speech and behavior were bizarre. She also reports that his eyes were open when he engaged in this behavior that appeared suicidal.
Three months before the incident, Mr. R had reported nightmares with dream enactment behaviors, hypervigilance on awakening and during the daytime, irritability, and anxious and depressed mood with neurovegetative symptoms, and was referred to our clinic for medication management. He also reported no prior or current manic or psychotic symptoms, denied suicidal thoughts, and had no history of suicide attempts. Mr. R’s medication regimen included tramadol, 400 mg/d, for chronic knee pain; fluoxetine, 60 mg/d, for depression and PTSD; and propranolol ER, 60 mg/d, and propranolol, 10 mg/d as needed, for anxiety. He was started on prazosin, 2 mg/d, titrated to 4 mg/d, for medication management of nightmares.
Mr. R also was referred to the sleep laboratory for a polysomnogram (PSG) because of reported loud snoring and witnessed apneas, especially because sleep apnea can cause nightmares and dream enactment behaviors. The PSG was negative for sleep apnea or excessive periodic limb movements of sleep, but showed increased electromyographic (EMG) activity during REM sleep, which was consistent with his report of dream enactment behaviors. Two months later, he reported improvement in nightmares and depression, but not in dream enactment behaviors. Because of prominent anxiety and irritability, he was started on gabapentin, 300 mg, 3 times a day.
What factor increases the risk of NREM parasomnias with zolpidem compared with benzodiazepines?
a) greater preservation of Stage N3 sleep
b) lesser degree of muscle relaxation
c) both a and b
d) none of the above
[polldaddy:9712556]
The authors’ observations
Factors that increase the likelihood of parasomnias include:
- zolpidem >10 mg at bedtime
- concomitant use of other CNS depressants, including sedative hypnotic agents and alcohol
- female sex
- not falling asleep immediately after taking zolpidem
- personal or family history of parasomnias
- living alone
- poor pill management
- presence of sleep disruptors such as sleep apnea and periodic limb movements of sleep.1,4,5,10
Higher dosages of zolpidem (>10 mg/d) have been identified as the predictive risk factor.5 In the Chopra et al4 case report on sleep-related suicidal behavior related to zolpidem, 10 mg at bedtime, concomitant dehydration and alcohol use were implicated as facilitating factors. Dehydration could increase serum levels of zolpidem resulting in greater CNS effects. Alcohol use was implicated in the Gibson et al8 case report as well, and the patient had multiple episodes of sleepwalking and sleep-related eating.However, Mr. R was not dehydrated or using alcohol.
An interesting feature of Mr. R’s case is that he was taking fluoxetine. Cytochrome P450 (CYP) 3A4 is involved in metabolizing zolpidem, and norfluoxetine, a metabolite of fluoxetine, inhibits CYP3A4. Although studies have not found pharmacokinetic interactions between fluoxetine and zolpidem, these studies did not investigate fluoxetine dosages >20 mg/d.11 The inhibition of CYP enzymes by fluoxetine likely is dose-dependent,12 and therefore concomitant administration of high-dosage fluoxetine (>20 mg/d) with zolpidem might result in higher serum levels of zolpidem.
Mr. R also was taking several sedating agents (gabapentin, hydroxyzine, melatonin, and tramadol). The concomitant use of these sedative-hypnotic agents could have increased his risk of parasomnia. A review of the literature did not reveal any reports of gabapentin, hydroxyzine, melatonin, or tramadol causing parasomnias. This observation, as well as the well-known role of zolpidem5 in etiopathogenesis of parasomnias, indicates that the pseudo-suicidal behavior Mr. R displayed while asleep likely was a direct result of zolpidem use in presence of other facilitating factors. Gabapentin, which is known to increase the depth of sleep, was added to his regimen 1 month before his parasomnia episode. Therefore, gabapentin could have triggered parasomnia with zolpidem therapy.1,13
Conditions that provoke repeated cortical arousals (eg, periodic limb movement disorder [PLMD] and sleep apnea) or increase depth or pressure of sleep (intense exercise in the evening, fever, sleep deprivation) are thought to be associated with NREM parasomnias.1-4 However, Mr. R underwent in-laboratory PSG and tested negative for major cortical arousal-inducing conditions, such as PLMD and sleep apnea.
Some other sleep disruptors likely were involved in Mr. R’s case. Auditory and tactile stimuli are known to cause cortical arousals, with additive effect seen when these 2 stimuli are combined.3,14 Additionally, these exogenous stimuli are known to trigger sleep-related violent parasomnias.15 Mr. R displayed this behavior after his wife woke him up. The auditory stimulus of his wife’s voice and/or tactile stimulus involved in the act of waking Mr. R likely played a role in the suicidal and violent nature of his NREM parasomnia.
[polldaddy:9712581]
The authors’ observations
In general, the mechanisms by which zolpidem causes NREM parasomnias are not completely understood. The sedation-related amnestic properties of zolpidem might explain some of these behaviors. Patients could perform these behaviors after waking and have subsequent amnesia.4 There is greater preservation of Stage N3 sleep with zolpidem compared with benzodiazepines. Benzodiazepines also cause muscle relaxation while the motor system remains relatively more active during sleep with zolpidem because of its selectivity for α-1 subunit of gamma-aminobutyric acid A receptor. These factors might increase the likelihood of NREM parasomnias with zolpidem compared with benzodiazepines.4
Types of parasomnias
According to DSM-5, there are 2 categories of parasomnias based on the sleep stage from which a parasomnia emerges.2 REM sleep behavior disorder (RBD) refers to complex motor and/or vocalizations during REM sleep, accompanied by increased EMG activity during REM sleep (Table).2,3
The pseudo-suicidal behavior Mr. R displayed likely was NREM parasomnia because it occurred in the first third of the night with his eyes open and impaired recall after the event. Interestingly, Mr. R had RBD in addition to the NREM parasomnia likely caused by zolpidem. This is evident from Mr. R’s frequent dream enactment behaviors, such as kicking, thrashing, and punching during sleep, along with increased EMG activity during REM sleep as recorded on the PSG.10 The presence of RBD could be explained by selective serotonin reuptake inhibitor (fluoxetine) use, and comorbidity with PTSD.2,16
Management of parasomnias
Initial management of parasomnias involves decreasing the risk of parasomnia-related injury. Suggested safety measures include:
- sleeping away from windows
- sleeping in a sleeping bag
- sleeping on a lower floor
- locking windows and doors
- removing potentially dangerous objects from the bedroom
- putting gates across stairwells
- installing bells or alarms on door knobs.15
Removing access to firearms or other weapons such as knives is of utmost importance especially with patients who have easy access during wakefulness. If removing weapons is not feasible, consider disarming, securing, or locking them.15 These considerations are relevant to veterans with PTSD because of the high prevalence of symptoms, including depression, insomnia, and pain, which require sedating medications.17 A review of parasomnias among a large sample of psychiatric outpatients revealed that a variety of sedating medications, including antidepressants, can lead to NREM parasomnias.18 Therefore, exercise caution when prescribing sedating medications, especially in patients vulnerable to developing dangerous parasomnias, such as a veteran with PTSD and easy access to guns.19
TREATMENT Zolpidem stopped
Mr. R immediately stops taking zolpidem because he is aware of its association with abnormal behaviors during sleep, and his wife removes his access to firearms and knives at night. Because of his history of clinical benefit and no history of parasomnias with mirtazapine, Mr. R is started on mirtazapine for insomnia that previously was treated with zolpidem, and residual depression. Six months after discontinuing zolpidem, he does not experience NREM parasomnias, and there are no changes in his dream enactment behaviors.
Summing up
Zolpidem therapy could be associated with unusual variants of NREM parasomnia, sleepwalking type; sleep-related pseudo-suicidal behavior is one such variant. Several factors could play a role in increasing the likelihood of NREM parasomnia with zolpidem therapy. In Mr. R’s case, the pharmacokinetic drug interactions between fluoxetine and zolpidem, as well as concomitant use of several sedating agents could have played a role in increasing the likelihood of NREM parasomnia, with audio-tactile stimuli contributing to the violent and suicidal nature of the parasomnia. Exercise caution when using CYP enzyme inhibitors, such as fluoxetine and paroxetine, in combination with zolpidem. Knowledge of the potential interaction between zolpidem and fluoxetine is important because antidepressants and hypnotics are commonly co-prescribed because insomnia often is comorbid with other psychiatric disorders.
In veterans with PTSD who do not have suicidal ideations while awake, life-threatening non-intentional behavior is a risk because of easy access to guns or other weapons. Sedative-hypnotic medications commonly are prescribed to patients with PTSD. Exercise caution when using hypnotic agents such as zolpidem, and consider sleep aids with a lower risk of parasomnias (based on the author’s experience, trazodone, mirtazapine, melatonin, and gabapentin) when possible. Non-pharmacologic treatments of insomnia, such as sleep hygiene education and, more importantly, cognitive-behavioral therapy for insomnia, are preferred. If a patient is already taking zolpidem, nightly dosage should not be >10 mg. Polypharmacy with other sedating medications should be avoided when possible and both exogenous (noise, pets) and endogenous sleep disruptors (sleep apnea, PLMD) should be addressed. Advise the patient to avoid alcohol and remove firearms and other potential weapons. Discontinue zolpidem if the patient develops sleep-related abnormal behavior because of its potential to take on violent forms.
CASE Suicidal while asleep
Mr. R, age 28, an Iraq and Afghanistan veteran with major depressive disorder and posttraumatic stress disorder (PTSD), is awoken by his wife to check on their daughter approximately 30 minutes after he takes his nightly regimen of zolpidem, 10 mg, melatonin, 6 mg, and hydroxyzine, 20 mg. When Mr. R returns to the bedroom, he appears to be confused. Mr. R grabs an unloaded gun from under the mattress, puts it in his mouth, and pulls the trigger. Then Mr. R holds the gun to his head and pulls the trigger while saying that his wife and children will be better off without him. His wife takes the gun away, but he grabs another gun from his gun box and loads it. His wife convinces him to remove the ammunition; however, Mr. R gets the other unloaded gun and pulls the trigger on himself again. After his wife takes this gun away, he tries cutting himself with a pocketknife, causing superficial cuts. Eventually, Mr. R goes back to bed. He does not remember these events in the morning.
What increased the likelihood of parasomnia in Mr. R?
a) high zolpidem dosage
b) concomitant use of other sedating agents
c) sleep deprivation
d) dehydration
[polldaddy:9712545]
The authors’ observations
Parasomnias are sleep-wake transition disorders classified by the sleep stage from which they arise, either NREM or rapid eye movement (REM). NREM parasomnias could result from incomplete awakening from NREM sleep, typically in Stage N3 (slow-wave) sleep.1 DSM-5 describes NREM parasomnias as arousal disorders in which the disturbance is not attributable to the physiological effects of substance; substance/medication-induced sleep disorder, parasomnia type, is when the disturbance can be attributed to a substance.2 The latter also can occur during REM sleep.
NREM parasomnias are characterized by abnormal behaviors during sleep with significant harm potential.3 Somnambulism or sleepwalking and sleep terrors are the 2 types of NREM parasomnias in DSM-5. Sleepwalking could involve complex behaviors, including:
- eating
- talking
- cooking
- shopping
- driving
- sexual activity.
Zolpidem, a benzodiazepine receptor agonist, is a preferred hypnotic agent for insomnia because of its low risk for abuse and daytime sedation.4 However, the drug has been associated with NREM parasomnias, namely somnambulism or sleepwalking, and its variants including sleep-driving, sleep-related eating disorder, and rarely sexsomnia (sleep-sex), with anterograde amnesia for the event.5 Suicidal behavior that occurs while the patient is asleep with next-day amnesia is another variant of somnambulism. There are several reports of suicidal behavior during sleep,6,7 but to our knowledge, there are only 2 previous cases implicating zolpidem as the cause:
- Gibson et al8 described a 49-year-old man who sustained a self-inflicted gunshot wound to his head while asleep. He just had started taking zolpidem, and in the weeks before the incident he had several episodes of sleepwalking and sleep-eating. He had consumed alcohol the night of the self-inflicted gunshot wound, but had no other psychiatric history.
- Chopra et al4 described a 37-year-old man, with no prior episodes of sleepwalking or associated complex behaviors, who was taking zolpidem, 10 mg/d, for chronic insomnia. He shot a gun in the basement of his home, and then held the loaded gun to his neck while asleep. The authors attributed the event to zolpidem in combination with other predisposing factors, including dehydration after intense exercise and alcohol use. The authors categorized this type of event as “para-suicidal amnestic behavior,” although “sleep-related pseudo-suicidal behavior” might be a better term for this type of parasomnia because of its occurrence during sleep and non-deliberate nature.
In another case report, a 27-year-old man took additional zolpidem after he did not experience desired sedative effects from an initial 20 mg.9 Because the patient remembered the suicidal thoughts, the authors believed that the patient attempted suicide while under the influence of zolpidem. The authors did not believe the incident to be sleep-related suicidal behavior, because it was uncertain if he attempted suicide while asleep.
Mr. R does not remember the events his wife witnessed while he was asleep. To our knowledge, Mr. R’s case is the first sleep-related pseudo-suicidal behavior case resulting from zolpidem, 10 mg/d, without concurrent alcohol use in an adult male veteran with PTSD and no suicidal ideation while awake.
HISTORY Further details revealed
Mr. R says that in the days leading to the incident he was not sleep-deprived and was getting at least 6 hours of restful sleep every night. He had been taking zolpidem every night. He has no childhood or family history of NREM parasomnias. He says he did not engage in intense exercise that evening or have a fever the night of the incident and has abstained from alcohol for 2 years.
His wife says that after he took zolpidem, when he was woken up, “He was not there; his eyes were glazed and glossy, and it’s like he was in another world,” and his speech and behavior were bizarre. She also reports that his eyes were open when he engaged in this behavior that appeared suicidal.
Three months before the incident, Mr. R had reported nightmares with dream enactment behaviors, hypervigilance on awakening and during the daytime, irritability, and anxious and depressed mood with neurovegetative symptoms, and was referred to our clinic for medication management. He also reported no prior or current manic or psychotic symptoms, denied suicidal thoughts, and had no history of suicide attempts. Mr. R’s medication regimen included tramadol, 400 mg/d, for chronic knee pain; fluoxetine, 60 mg/d, for depression and PTSD; and propranolol ER, 60 mg/d, and propranolol, 10 mg/d as needed, for anxiety. He was started on prazosin, 2 mg/d, titrated to 4 mg/d, for medication management of nightmares.
Mr. R also was referred to the sleep laboratory for a polysomnogram (PSG) because of reported loud snoring and witnessed apneas, especially because sleep apnea can cause nightmares and dream enactment behaviors. The PSG was negative for sleep apnea or excessive periodic limb movements of sleep, but showed increased electromyographic (EMG) activity during REM sleep, which was consistent with his report of dream enactment behaviors. Two months later, he reported improvement in nightmares and depression, but not in dream enactment behaviors. Because of prominent anxiety and irritability, he was started on gabapentin, 300 mg, 3 times a day.
What factor increases the risk of NREM parasomnias with zolpidem compared with benzodiazepines?
a) greater preservation of Stage N3 sleep
b) lesser degree of muscle relaxation
c) both a and b
d) none of the above
[polldaddy:9712556]
The authors’ observations
Factors that increase the likelihood of parasomnias include:
- zolpidem >10 mg at bedtime
- concomitant use of other CNS depressants, including sedative hypnotic agents and alcohol
- female sex
- not falling asleep immediately after taking zolpidem
- personal or family history of parasomnias
- living alone
- poor pill management
- presence of sleep disruptors such as sleep apnea and periodic limb movements of sleep.1,4,5,10
Higher dosages of zolpidem (>10 mg/d) have been identified as the predictive risk factor.5 In the Chopra et al4 case report on sleep-related suicidal behavior related to zolpidem, 10 mg at bedtime, concomitant dehydration and alcohol use were implicated as facilitating factors. Dehydration could increase serum levels of zolpidem resulting in greater CNS effects. Alcohol use was implicated in the Gibson et al8 case report as well, and the patient had multiple episodes of sleepwalking and sleep-related eating.However, Mr. R was not dehydrated or using alcohol.
An interesting feature of Mr. R’s case is that he was taking fluoxetine. Cytochrome P450 (CYP) 3A4 is involved in metabolizing zolpidem, and norfluoxetine, a metabolite of fluoxetine, inhibits CYP3A4. Although studies have not found pharmacokinetic interactions between fluoxetine and zolpidem, these studies did not investigate fluoxetine dosages >20 mg/d.11 The inhibition of CYP enzymes by fluoxetine likely is dose-dependent,12 and therefore concomitant administration of high-dosage fluoxetine (>20 mg/d) with zolpidem might result in higher serum levels of zolpidem.
Mr. R also was taking several sedating agents (gabapentin, hydroxyzine, melatonin, and tramadol). The concomitant use of these sedative-hypnotic agents could have increased his risk of parasomnia. A review of the literature did not reveal any reports of gabapentin, hydroxyzine, melatonin, or tramadol causing parasomnias. This observation, as well as the well-known role of zolpidem5 in etiopathogenesis of parasomnias, indicates that the pseudo-suicidal behavior Mr. R displayed while asleep likely was a direct result of zolpidem use in presence of other facilitating factors. Gabapentin, which is known to increase the depth of sleep, was added to his regimen 1 month before his parasomnia episode. Therefore, gabapentin could have triggered parasomnia with zolpidem therapy.1,13
Conditions that provoke repeated cortical arousals (eg, periodic limb movement disorder [PLMD] and sleep apnea) or increase depth or pressure of sleep (intense exercise in the evening, fever, sleep deprivation) are thought to be associated with NREM parasomnias.1-4 However, Mr. R underwent in-laboratory PSG and tested negative for major cortical arousal-inducing conditions, such as PLMD and sleep apnea.
Some other sleep disruptors likely were involved in Mr. R’s case. Auditory and tactile stimuli are known to cause cortical arousals, with additive effect seen when these 2 stimuli are combined.3,14 Additionally, these exogenous stimuli are known to trigger sleep-related violent parasomnias.15 Mr. R displayed this behavior after his wife woke him up. The auditory stimulus of his wife’s voice and/or tactile stimulus involved in the act of waking Mr. R likely played a role in the suicidal and violent nature of his NREM parasomnia.
[polldaddy:9712581]
The authors’ observations
In general, the mechanisms by which zolpidem causes NREM parasomnias are not completely understood. The sedation-related amnestic properties of zolpidem might explain some of these behaviors. Patients could perform these behaviors after waking and have subsequent amnesia.4 There is greater preservation of Stage N3 sleep with zolpidem compared with benzodiazepines. Benzodiazepines also cause muscle relaxation while the motor system remains relatively more active during sleep with zolpidem because of its selectivity for α-1 subunit of gamma-aminobutyric acid A receptor. These factors might increase the likelihood of NREM parasomnias with zolpidem compared with benzodiazepines.4
Types of parasomnias
According to DSM-5, there are 2 categories of parasomnias based on the sleep stage from which a parasomnia emerges.2 REM sleep behavior disorder (RBD) refers to complex motor and/or vocalizations during REM sleep, accompanied by increased EMG activity during REM sleep (Table).2,3
The pseudo-suicidal behavior Mr. R displayed likely was NREM parasomnia because it occurred in the first third of the night with his eyes open and impaired recall after the event. Interestingly, Mr. R had RBD in addition to the NREM parasomnia likely caused by zolpidem. This is evident from Mr. R’s frequent dream enactment behaviors, such as kicking, thrashing, and punching during sleep, along with increased EMG activity during REM sleep as recorded on the PSG.10 The presence of RBD could be explained by selective serotonin reuptake inhibitor (fluoxetine) use, and comorbidity with PTSD.2,16
Management of parasomnias
Initial management of parasomnias involves decreasing the risk of parasomnia-related injury. Suggested safety measures include:
- sleeping away from windows
- sleeping in a sleeping bag
- sleeping on a lower floor
- locking windows and doors
- removing potentially dangerous objects from the bedroom
- putting gates across stairwells
- installing bells or alarms on door knobs.15
Removing access to firearms or other weapons such as knives is of utmost importance especially with patients who have easy access during wakefulness. If removing weapons is not feasible, consider disarming, securing, or locking them.15 These considerations are relevant to veterans with PTSD because of the high prevalence of symptoms, including depression, insomnia, and pain, which require sedating medications.17 A review of parasomnias among a large sample of psychiatric outpatients revealed that a variety of sedating medications, including antidepressants, can lead to NREM parasomnias.18 Therefore, exercise caution when prescribing sedating medications, especially in patients vulnerable to developing dangerous parasomnias, such as a veteran with PTSD and easy access to guns.19
TREATMENT Zolpidem stopped
Mr. R immediately stops taking zolpidem because he is aware of its association with abnormal behaviors during sleep, and his wife removes his access to firearms and knives at night. Because of his history of clinical benefit and no history of parasomnias with mirtazapine, Mr. R is started on mirtazapine for insomnia that previously was treated with zolpidem, and residual depression. Six months after discontinuing zolpidem, he does not experience NREM parasomnias, and there are no changes in his dream enactment behaviors.
Summing up
Zolpidem therapy could be associated with unusual variants of NREM parasomnia, sleepwalking type; sleep-related pseudo-suicidal behavior is one such variant. Several factors could play a role in increasing the likelihood of NREM parasomnia with zolpidem therapy. In Mr. R’s case, the pharmacokinetic drug interactions between fluoxetine and zolpidem, as well as concomitant use of several sedating agents could have played a role in increasing the likelihood of NREM parasomnia, with audio-tactile stimuli contributing to the violent and suicidal nature of the parasomnia. Exercise caution when using CYP enzyme inhibitors, such as fluoxetine and paroxetine, in combination with zolpidem. Knowledge of the potential interaction between zolpidem and fluoxetine is important because antidepressants and hypnotics are commonly co-prescribed because insomnia often is comorbid with other psychiatric disorders.
In veterans with PTSD who do not have suicidal ideations while awake, life-threatening non-intentional behavior is a risk because of easy access to guns or other weapons. Sedative-hypnotic medications commonly are prescribed to patients with PTSD. Exercise caution when using hypnotic agents such as zolpidem, and consider sleep aids with a lower risk of parasomnias (based on the author’s experience, trazodone, mirtazapine, melatonin, and gabapentin) when possible. Non-pharmacologic treatments of insomnia, such as sleep hygiene education and, more importantly, cognitive-behavioral therapy for insomnia, are preferred. If a patient is already taking zolpidem, nightly dosage should not be >10 mg. Polypharmacy with other sedating medications should be avoided when possible and both exogenous (noise, pets) and endogenous sleep disruptors (sleep apnea, PLMD) should be addressed. Advise the patient to avoid alcohol and remove firearms and other potential weapons. Discontinue zolpidem if the patient develops sleep-related abnormal behavior because of its potential to take on violent forms.
1. Howell MJ. Parasomnias: an updated review. Neurotherapeutics. 2012;9(4):753-775.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Zadra A, Desautels A, Petit D, et al. Somnambulism: clinical aspects and pathophysiological hypotheses. Lancet Neurol. 2013;12(3):285-294.
4. Chopra A, Selim B, Silber MH, et al. Para-suicidal amnestic behavior associated with chronic zolpidem use: implications for patient safety. Psychosomatics. 2013;54(5):498-501.
5. Hwang TJ, Ni HC, Chen HC, et al. Risk predictors for hypnosedative-related complex sleep behaviors: a retrospective, cross-sectional pilot study. J Clin Psychiatry. 2010;71(10):1331-1335.
6. Shatkin JP, Feinfield K, Strober M. The misinterpretation of a non-REM sleep parasomnia as suicidal behavior in an adolescent. Sleep Breath. 2002;6(4):175-179.
7. Mahowald MW, Schenck CH, Goldner M, et al. Parasomnia pseudo-suicide. J Forensic Sci. 2003;48(5):1158-1162.
8. Gibson CE, Caplan JP. Zolpidem-associated parasomnia with serious self-injury: a shot in the dark. Psychosomatics. 2011;52(1):88-91.
9. Mortaz Hejri S, Faizi M, Babaeian M. Zolpidem-induced suicide attempt: a case report. Daru. 2013;20;21(1):77.
10. Poceta JS. Zolpidem ingestion, automatisms, and sleep driving: a clinical and legal case series. J Clin Sleep Med. 2011;7(6):632-638.
11. Hesse LM, von Moltke LL, Greenblatt DJ. Clinically important drug interactions with zopiclone, zolpidem and zaleplon. CNS Drugs. 2003;17(7):513-532.
12. Catterson ML, Preskorn SH. Pharmacokinetics of selective serotonin reuptake inhibitors: clinical relevance. Pharmacol Toxicol. 1996;78(4):203-208.
13. Rosenberg RP, Hull SG, Lankford DA, et al. A randomized, double-blind, single-dose, placebo-controlled, multicenter, polysomnographic study of gabapentin in transient insomnia induced by sleep phase advance. J Clin Sleep Med. 2014;10(10):1093-1100.
14. Kato T, Montplaisir JY, Lavigne GJ. Experimentally induced arousals during sleep: a cross-modality matching paradigm. J Sleep Res. 2004;13(3):229-238.
15. Siclari F, Khatami R, Urbaniok F, et al. Violence in sleep. Brain. 2010;133(pt 12):3494-3509.
16. Husain AM, Miller PP, Carwile ST. Rem sleep behavior disorder: potential relationship to post-traumatic stress disorder. J Clin Neurophysiol. 2001;18(2):148-157.
17. Bernardy NC, Lund BC, Alexander B, et al. Increased polysedative use in veterans with posttraumatic stress disorder. Pain Med. 2014;15(7):1083-1090.
18. Lam SP, Fong SY, Ho CK, et al. Parasomnia among psychiatric outpatients: a clinical, epidemiologic, cross-sectional study. J Clin Psychiatry. 2008;69(9):1374-1382.
19. Freeman TW, Roca V, Kimbrell T. A survey of gun collection and use among three groups of veteran patients admitted to veterans affairs hospital treatment programs. South Med J. 2003;96(3):240-243.
1. Howell MJ. Parasomnias: an updated review. Neurotherapeutics. 2012;9(4):753-775.
2. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
3. Zadra A, Desautels A, Petit D, et al. Somnambulism: clinical aspects and pathophysiological hypotheses. Lancet Neurol. 2013;12(3):285-294.
4. Chopra A, Selim B, Silber MH, et al. Para-suicidal amnestic behavior associated with chronic zolpidem use: implications for patient safety. Psychosomatics. 2013;54(5):498-501.
5. Hwang TJ, Ni HC, Chen HC, et al. Risk predictors for hypnosedative-related complex sleep behaviors: a retrospective, cross-sectional pilot study. J Clin Psychiatry. 2010;71(10):1331-1335.
6. Shatkin JP, Feinfield K, Strober M. The misinterpretation of a non-REM sleep parasomnia as suicidal behavior in an adolescent. Sleep Breath. 2002;6(4):175-179.
7. Mahowald MW, Schenck CH, Goldner M, et al. Parasomnia pseudo-suicide. J Forensic Sci. 2003;48(5):1158-1162.
8. Gibson CE, Caplan JP. Zolpidem-associated parasomnia with serious self-injury: a shot in the dark. Psychosomatics. 2011;52(1):88-91.
9. Mortaz Hejri S, Faizi M, Babaeian M. Zolpidem-induced suicide attempt: a case report. Daru. 2013;20;21(1):77.
10. Poceta JS. Zolpidem ingestion, automatisms, and sleep driving: a clinical and legal case series. J Clin Sleep Med. 2011;7(6):632-638.
11. Hesse LM, von Moltke LL, Greenblatt DJ. Clinically important drug interactions with zopiclone, zolpidem and zaleplon. CNS Drugs. 2003;17(7):513-532.
12. Catterson ML, Preskorn SH. Pharmacokinetics of selective serotonin reuptake inhibitors: clinical relevance. Pharmacol Toxicol. 1996;78(4):203-208.
13. Rosenberg RP, Hull SG, Lankford DA, et al. A randomized, double-blind, single-dose, placebo-controlled, multicenter, polysomnographic study of gabapentin in transient insomnia induced by sleep phase advance. J Clin Sleep Med. 2014;10(10):1093-1100.
14. Kato T, Montplaisir JY, Lavigne GJ. Experimentally induced arousals during sleep: a cross-modality matching paradigm. J Sleep Res. 2004;13(3):229-238.
15. Siclari F, Khatami R, Urbaniok F, et al. Violence in sleep. Brain. 2010;133(pt 12):3494-3509.
16. Husain AM, Miller PP, Carwile ST. Rem sleep behavior disorder: potential relationship to post-traumatic stress disorder. J Clin Neurophysiol. 2001;18(2):148-157.
17. Bernardy NC, Lund BC, Alexander B, et al. Increased polysedative use in veterans with posttraumatic stress disorder. Pain Med. 2014;15(7):1083-1090.
18. Lam SP, Fong SY, Ho CK, et al. Parasomnia among psychiatric outpatients: a clinical, epidemiologic, cross-sectional study. J Clin Psychiatry. 2008;69(9):1374-1382.
19. Freeman TW, Roca V, Kimbrell T. A survey of gun collection and use among three groups of veteran patients admitted to veterans affairs hospital treatment programs. South Med J. 2003;96(3):240-243.