User login
Quick Poll: Cosmetic Procedures
[polldaddy:9711250]
[polldaddy:9711250]
[polldaddy:9711250]
What happens when a baked egg oral challenge is negative?
ATLANTA – The majority of patients who had cooked egg exposure following a negative physician-supervised baked egg oral challenge are tolerating cooked egg, according to a retrospective study.
However, no correlation between results and development of tolerance was identified with skin prick testing or serum IgE testing.
To find out, Dr. Peng, a second-year fellow in the department of pediatrics at the University of California, Los Angeles, and her associates identified 22 patients who underwent negative physician-supervised baked egg oral challenges from July 2011 until June 2016. They reviewed medical charts to obtain data on age, clinical history, skin prick test results, and results of serum IgE testing to egg and its components. Next, the researchers contacted patients and their families and invited them to participate in a telephone survey about patterns of baked, cooked, and raw egg exposure and associated reactions, following their negative baked challenge. The patients ranged in age from 10 months to 44 years and their mean age was 7 years.
Dr. Peng presented results from 18 of the 22 patients who were successfully contacted. A mean of 26 months had passed since their baked egg oral challenge. Of these patients, 17 (94%) have had continued exposure to egg while 15 (83%) have shown tolerance to cooked egg. The researchers observed variable patterns of baked egg intake following the negative physician-supervised baked egg challenge. “Some patients are able to tolerate cooked egg rapidly but are not interested in continuing frequent consumption,” Dr. Peng said. “They may say, ‘My 3-year-old doesn’t like scrambled eggs, so I’m not going to keep pushing them.’ They’re not considering themselves egg allergic so their quality of life is much better. I understand that tolerating baked egg is a big deal, as an allergist I want to see them do more, such as tolerating cooked egg.”
When patients were asked about adverse reactions to egg consumption, three (17%) described gastrointestinal symptoms, five (28%) described cutaneous symptoms, and three (17%) described respiratory reactions. Dr. Peng noted that of the three patients who have not achieved tolerance to cooked egg, one patient reported mild reaction to baked egg 2 weeks after the baked egg challenge, while the other two continue to have baked egg exposure but have not yet introduced cooked egg into their diets. “I hope that most pediatricians and family practice physicians consider referral to an allergist if they’re not comfortable introducing a baked egg oral challenge.”
The researchers could not identify any correlation between skin prick or serum IgE test results and development of tolerance to cooked egg. “Data in the literature suggests that serum testing is predictive [of tolerance], but it’s not 100%,” Dr. Peng said.
She reported having no relevant financial disclosures.
ATLANTA – The majority of patients who had cooked egg exposure following a negative physician-supervised baked egg oral challenge are tolerating cooked egg, according to a retrospective study.
However, no correlation between results and development of tolerance was identified with skin prick testing or serum IgE testing.
To find out, Dr. Peng, a second-year fellow in the department of pediatrics at the University of California, Los Angeles, and her associates identified 22 patients who underwent negative physician-supervised baked egg oral challenges from July 2011 until June 2016. They reviewed medical charts to obtain data on age, clinical history, skin prick test results, and results of serum IgE testing to egg and its components. Next, the researchers contacted patients and their families and invited them to participate in a telephone survey about patterns of baked, cooked, and raw egg exposure and associated reactions, following their negative baked challenge. The patients ranged in age from 10 months to 44 years and their mean age was 7 years.
Dr. Peng presented results from 18 of the 22 patients who were successfully contacted. A mean of 26 months had passed since their baked egg oral challenge. Of these patients, 17 (94%) have had continued exposure to egg while 15 (83%) have shown tolerance to cooked egg. The researchers observed variable patterns of baked egg intake following the negative physician-supervised baked egg challenge. “Some patients are able to tolerate cooked egg rapidly but are not interested in continuing frequent consumption,” Dr. Peng said. “They may say, ‘My 3-year-old doesn’t like scrambled eggs, so I’m not going to keep pushing them.’ They’re not considering themselves egg allergic so their quality of life is much better. I understand that tolerating baked egg is a big deal, as an allergist I want to see them do more, such as tolerating cooked egg.”
When patients were asked about adverse reactions to egg consumption, three (17%) described gastrointestinal symptoms, five (28%) described cutaneous symptoms, and three (17%) described respiratory reactions. Dr. Peng noted that of the three patients who have not achieved tolerance to cooked egg, one patient reported mild reaction to baked egg 2 weeks after the baked egg challenge, while the other two continue to have baked egg exposure but have not yet introduced cooked egg into their diets. “I hope that most pediatricians and family practice physicians consider referral to an allergist if they’re not comfortable introducing a baked egg oral challenge.”
The researchers could not identify any correlation between skin prick or serum IgE test results and development of tolerance to cooked egg. “Data in the literature suggests that serum testing is predictive [of tolerance], but it’s not 100%,” Dr. Peng said.
She reported having no relevant financial disclosures.
ATLANTA – The majority of patients who had cooked egg exposure following a negative physician-supervised baked egg oral challenge are tolerating cooked egg, according to a retrospective study.
However, no correlation between results and development of tolerance was identified with skin prick testing or serum IgE testing.
To find out, Dr. Peng, a second-year fellow in the department of pediatrics at the University of California, Los Angeles, and her associates identified 22 patients who underwent negative physician-supervised baked egg oral challenges from July 2011 until June 2016. They reviewed medical charts to obtain data on age, clinical history, skin prick test results, and results of serum IgE testing to egg and its components. Next, the researchers contacted patients and their families and invited them to participate in a telephone survey about patterns of baked, cooked, and raw egg exposure and associated reactions, following their negative baked challenge. The patients ranged in age from 10 months to 44 years and their mean age was 7 years.
Dr. Peng presented results from 18 of the 22 patients who were successfully contacted. A mean of 26 months had passed since their baked egg oral challenge. Of these patients, 17 (94%) have had continued exposure to egg while 15 (83%) have shown tolerance to cooked egg. The researchers observed variable patterns of baked egg intake following the negative physician-supervised baked egg challenge. “Some patients are able to tolerate cooked egg rapidly but are not interested in continuing frequent consumption,” Dr. Peng said. “They may say, ‘My 3-year-old doesn’t like scrambled eggs, so I’m not going to keep pushing them.’ They’re not considering themselves egg allergic so their quality of life is much better. I understand that tolerating baked egg is a big deal, as an allergist I want to see them do more, such as tolerating cooked egg.”
When patients were asked about adverse reactions to egg consumption, three (17%) described gastrointestinal symptoms, five (28%) described cutaneous symptoms, and three (17%) described respiratory reactions. Dr. Peng noted that of the three patients who have not achieved tolerance to cooked egg, one patient reported mild reaction to baked egg 2 weeks after the baked egg challenge, while the other two continue to have baked egg exposure but have not yet introduced cooked egg into their diets. “I hope that most pediatricians and family practice physicians consider referral to an allergist if they’re not comfortable introducing a baked egg oral challenge.”
The researchers could not identify any correlation between skin prick or serum IgE test results and development of tolerance to cooked egg. “Data in the literature suggests that serum testing is predictive [of tolerance], but it’s not 100%,” Dr. Peng said.
She reported having no relevant financial disclosures.
AT THE 2017 AAAAI ANNUAL MEETING
Key clinical point:
Major finding: Following a negative physician-supervised baked egg oral challenge 94% of patients have had continued exposure to egg while 83% have shown tolerance to cooked egg.
Data source: A retrospective review of 22 patients who underwent a physician-supervised negative oral baked egg challenge.
Disclosures: Dr. Peng reported having no relevant financial disclosures.
Exposure to HPV vaccine in pregnancy not linked to birth defects
The quadrivalent human papillomavirus (HPV) vaccine was not associated with any adverse pregnancy outcomes when inadvertently given during pregnancy, according to the findings from a nationwide Danish study.
“Our results are consistent with other evidence that does not indicate that the vaccination of pregnant women with inactivated virus, bacterial, or toxoid vaccines generally confers a higher risk of adverse pregnancy outcomes than no such vaccination. Our results also confirm and considerably expand on results from previous studies of the quadrivalent HPV vaccine,” wrote Nikolai M. Scheller, MD, of the Statens Serum Institut, Copenhagen, and his colleagues.
Quadrivalent HPV vaccination was not associated with any increase in risk for major birth defects (prevalence odds ratio, 1.19), spontaneous abortion (hazard ratio, 0.71), preterm birth (prevalence OR, 1.15), small size for gestational age (prevalence OR, 0.86), or low birth weight (prevalence OR, 1.10). It also was not associated with increased risk for stillbirth, but this outcome occurred in only two case patients and four controls, making it “impossible to draw clinically meaningful conclusions” regarding the risk for stillbirth, Dr. Scheller and his colleagues reported (N Engl J Med. 2017;376[13]:1223-33).
“Because many [adverse] pregnancy outcomes are rare, our study did not have the statistical power to assess the risks of stillbirth and specific major birth defects associated with quadrivalent HPV vaccination. Larger studies would be needed to address these outcomes,” they added.
Novo Nordisk and the Danish Medical Research Council funded the study. Dr. Scheller reported having no relevant financial disclosures; two of his coauthors reported receiving research grants from the Novo Nordisk Foundation and the Danish Medical Research Council.
Although a large number of women (1,665) were immunized in the first trimester of pregnancy, when organogenesis occurs, their offspring did not have a significantly higher rate of major birth defects compared with offspring born to unvaccinated women. The numbers of spontaneous abortions, preterm births, infants with low birth weight, infants who were small for gestational age, and stillbirths were not higher in the vaccinated cohorts than in the unvaccinated cohorts, although the number of stillbirths was small.
These data are very encouraging and strongly support the safety of HPV vaccines if they are inadvertently given in pregnancy, a finding that complements previous safety reports of HPV vaccine in nonpregnant women. These data also show that adverse outcomes occur at a baseline rate in pregnancy and that when no control group is included in studies, these outcomes may be inappropriately attributed to the vaccine.
Kathryn M. Edwards, MD, is in the division of infectious diseases and the department of pediatrics at Vanderbilt University, Nashville, Tenn. She reported receiving grants from Novartis for research on group B strep vaccines in pregnancy. These comments are excerpted from an accompanying editorial (N Engl J Med. 2017;376[13]:1280-2).
Although a large number of women (1,665) were immunized in the first trimester of pregnancy, when organogenesis occurs, their offspring did not have a significantly higher rate of major birth defects compared with offspring born to unvaccinated women. The numbers of spontaneous abortions, preterm births, infants with low birth weight, infants who were small for gestational age, and stillbirths were not higher in the vaccinated cohorts than in the unvaccinated cohorts, although the number of stillbirths was small.
These data are very encouraging and strongly support the safety of HPV vaccines if they are inadvertently given in pregnancy, a finding that complements previous safety reports of HPV vaccine in nonpregnant women. These data also show that adverse outcomes occur at a baseline rate in pregnancy and that when no control group is included in studies, these outcomes may be inappropriately attributed to the vaccine.
Kathryn M. Edwards, MD, is in the division of infectious diseases and the department of pediatrics at Vanderbilt University, Nashville, Tenn. She reported receiving grants from Novartis for research on group B strep vaccines in pregnancy. These comments are excerpted from an accompanying editorial (N Engl J Med. 2017;376[13]:1280-2).
Although a large number of women (1,665) were immunized in the first trimester of pregnancy, when organogenesis occurs, their offspring did not have a significantly higher rate of major birth defects compared with offspring born to unvaccinated women. The numbers of spontaneous abortions, preterm births, infants with low birth weight, infants who were small for gestational age, and stillbirths were not higher in the vaccinated cohorts than in the unvaccinated cohorts, although the number of stillbirths was small.
These data are very encouraging and strongly support the safety of HPV vaccines if they are inadvertently given in pregnancy, a finding that complements previous safety reports of HPV vaccine in nonpregnant women. These data also show that adverse outcomes occur at a baseline rate in pregnancy and that when no control group is included in studies, these outcomes may be inappropriately attributed to the vaccine.
Kathryn M. Edwards, MD, is in the division of infectious diseases and the department of pediatrics at Vanderbilt University, Nashville, Tenn. She reported receiving grants from Novartis for research on group B strep vaccines in pregnancy. These comments are excerpted from an accompanying editorial (N Engl J Med. 2017;376[13]:1280-2).
The quadrivalent human papillomavirus (HPV) vaccine was not associated with any adverse pregnancy outcomes when inadvertently given during pregnancy, according to the findings from a nationwide Danish study.
“Our results are consistent with other evidence that does not indicate that the vaccination of pregnant women with inactivated virus, bacterial, or toxoid vaccines generally confers a higher risk of adverse pregnancy outcomes than no such vaccination. Our results also confirm and considerably expand on results from previous studies of the quadrivalent HPV vaccine,” wrote Nikolai M. Scheller, MD, of the Statens Serum Institut, Copenhagen, and his colleagues.
Quadrivalent HPV vaccination was not associated with any increase in risk for major birth defects (prevalence odds ratio, 1.19), spontaneous abortion (hazard ratio, 0.71), preterm birth (prevalence OR, 1.15), small size for gestational age (prevalence OR, 0.86), or low birth weight (prevalence OR, 1.10). It also was not associated with increased risk for stillbirth, but this outcome occurred in only two case patients and four controls, making it “impossible to draw clinically meaningful conclusions” regarding the risk for stillbirth, Dr. Scheller and his colleagues reported (N Engl J Med. 2017;376[13]:1223-33).
“Because many [adverse] pregnancy outcomes are rare, our study did not have the statistical power to assess the risks of stillbirth and specific major birth defects associated with quadrivalent HPV vaccination. Larger studies would be needed to address these outcomes,” they added.
Novo Nordisk and the Danish Medical Research Council funded the study. Dr. Scheller reported having no relevant financial disclosures; two of his coauthors reported receiving research grants from the Novo Nordisk Foundation and the Danish Medical Research Council.
The quadrivalent human papillomavirus (HPV) vaccine was not associated with any adverse pregnancy outcomes when inadvertently given during pregnancy, according to the findings from a nationwide Danish study.
“Our results are consistent with other evidence that does not indicate that the vaccination of pregnant women with inactivated virus, bacterial, or toxoid vaccines generally confers a higher risk of adverse pregnancy outcomes than no such vaccination. Our results also confirm and considerably expand on results from previous studies of the quadrivalent HPV vaccine,” wrote Nikolai M. Scheller, MD, of the Statens Serum Institut, Copenhagen, and his colleagues.
Quadrivalent HPV vaccination was not associated with any increase in risk for major birth defects (prevalence odds ratio, 1.19), spontaneous abortion (hazard ratio, 0.71), preterm birth (prevalence OR, 1.15), small size for gestational age (prevalence OR, 0.86), or low birth weight (prevalence OR, 1.10). It also was not associated with increased risk for stillbirth, but this outcome occurred in only two case patients and four controls, making it “impossible to draw clinically meaningful conclusions” regarding the risk for stillbirth, Dr. Scheller and his colleagues reported (N Engl J Med. 2017;376[13]:1223-33).
“Because many [adverse] pregnancy outcomes are rare, our study did not have the statistical power to assess the risks of stillbirth and specific major birth defects associated with quadrivalent HPV vaccination. Larger studies would be needed to address these outcomes,” they added.
Novo Nordisk and the Danish Medical Research Council funded the study. Dr. Scheller reported having no relevant financial disclosures; two of his coauthors reported receiving research grants from the Novo Nordisk Foundation and the Danish Medical Research Council.
Key clinical point:
Major finding: Quadrivalent HPV vaccination was not associated with any increase in risk for major birth defects (prevalence odds ratio, 1.19).
Data source: A population-based cohort study involving 581,550 pregnancies across Denmark during a 7-year period, including 1,665 in which the mother inadvertently received the HPV vaccine.
Disclosures: Novo Nordisk and the Danish Medical Research Council funded the study. Dr. Scheller reported having no relevant financial disclosures; two of his coauthors reported receiving research grants from the Novo Nordisk Foundation and the Danish Medical Research Council.
Aggressive HCC in males traced to higher serotonin
The greater frequency and aggressiveness of hepatocellular carcinoma (HCC) in men than in women might be attributable to greater synthesis and accumulation of serotonin in males, according to a report published online in Cellular and Molecular Gastroenterology and Hepatology (2017 May. doi: 10.1016/j.jcmgh.2017.01.002).
HCC is nearly five times more common in men than in women, and several molecular studies “have shown a more robust and active HCC tumor microenvironment” in men as well. For example, the density of infiltrating, tumor-associated macrophages is higher among males in a mouse model of the cancer, and human men have substantially higher amounts of intratumoral cluster-of-differentiation cells and neutrophils that indicate a poor prognosis, said Qiqi Yang, PhD, of the department of biological sciences at the National University of Singapore, and her associates.
The investigators developed several zebrafish models of HCC in which the cancer could be induced by transgenic expression of an oncogene in the animals’ hepatocytes. These models “allow the oncogene to be activated at a given and controlled timing in both sexes, providing an excellent platform to study the sex disparity in HCC initiation and progression,” they noted.
They also confirmed the zebrafish findings in human lab studies by analyzing tissue samples from 5 normal livers, 7 inflamed livers, 16 cirrhotic livers, and 30 livers affected with HCC.
The investigators found an increased level of serotonin production in male, compared with female, livers. They demonstrated that serotonin was necessary for the survival of hepatic stellate cells, which also are more abundant in males than in females and have recently been shown to promote tumorigenesis. Serotonin also was crucial for activating hepatic stellate cells during HCC carcinogenesis.
In addition, serotonin levels were significantly elevated in inflamed, cirrhotic, and cancerous livers, compared with normal livers, among men but not among women. “This is in line with the prevailing knowledge that men have a significantly higher rate of serotonin synthesis than do women,” Dr. Yang and her associates said.
Serotonin is a small molecule neurotransmitter with diverse functions such as modulation of mood, appetite, wound healing, gastrointestinal motility, and blood coagulation. It was shown that serotonin can promote liver regeneration in mice via a direct action on hepatocytes, the main building blocks of liver. However, other cell types such as liver stellate cells, the main liver fibrogenic cells, can also be influenced by serotonin. Serotonin action on liver stellate cells results in production of transforming growth factor–beta1 (TGF-beta1), a multifunctional cytokine. TGF-beta1 can then inhibit regeneration of hepatocytes and promote fibrosis. In a new study, scientists have shown that the same pathway is active during hepatic carcinogenesis and promotes development of cancer in a zebrafish model. They also discovered that hepatocytes can produce serotonin and increase TGF-beta1 synthesis in stellate cells. Interestingly, they uncovered a significant sexual dimorphism in both human and fish samples in components of this pathway (for example, more serotonin and TGF-beta1 in males). This study unravels underlying mechanisms of sex differences in liver cancer. Importantly, it can provide a therapeutic opportunity to treat human liver cancer by modulation of serotonin signaling. This approach is attractive since potent and selective pharmacologic agents for serotonin signaling are already available for other purposes such as modulation of gut motility or neurological disorders. Future studies using human cells or samples will pave the path toward clinical translation of these findings.
Mo Ebrahimkhani, MD, is an assistant professor in the school of biological and health systems engineering, Arizona State University, Tempe. He has no conflicts of interest.
Serotonin is a small molecule neurotransmitter with diverse functions such as modulation of mood, appetite, wound healing, gastrointestinal motility, and blood coagulation. It was shown that serotonin can promote liver regeneration in mice via a direct action on hepatocytes, the main building blocks of liver. However, other cell types such as liver stellate cells, the main liver fibrogenic cells, can also be influenced by serotonin. Serotonin action on liver stellate cells results in production of transforming growth factor–beta1 (TGF-beta1), a multifunctional cytokine. TGF-beta1 can then inhibit regeneration of hepatocytes and promote fibrosis. In a new study, scientists have shown that the same pathway is active during hepatic carcinogenesis and promotes development of cancer in a zebrafish model. They also discovered that hepatocytes can produce serotonin and increase TGF-beta1 synthesis in stellate cells. Interestingly, they uncovered a significant sexual dimorphism in both human and fish samples in components of this pathway (for example, more serotonin and TGF-beta1 in males). This study unravels underlying mechanisms of sex differences in liver cancer. Importantly, it can provide a therapeutic opportunity to treat human liver cancer by modulation of serotonin signaling. This approach is attractive since potent and selective pharmacologic agents for serotonin signaling are already available for other purposes such as modulation of gut motility or neurological disorders. Future studies using human cells or samples will pave the path toward clinical translation of these findings.
Mo Ebrahimkhani, MD, is an assistant professor in the school of biological and health systems engineering, Arizona State University, Tempe. He has no conflicts of interest.
Serotonin is a small molecule neurotransmitter with diverse functions such as modulation of mood, appetite, wound healing, gastrointestinal motility, and blood coagulation. It was shown that serotonin can promote liver regeneration in mice via a direct action on hepatocytes, the main building blocks of liver. However, other cell types such as liver stellate cells, the main liver fibrogenic cells, can also be influenced by serotonin. Serotonin action on liver stellate cells results in production of transforming growth factor–beta1 (TGF-beta1), a multifunctional cytokine. TGF-beta1 can then inhibit regeneration of hepatocytes and promote fibrosis. In a new study, scientists have shown that the same pathway is active during hepatic carcinogenesis and promotes development of cancer in a zebrafish model. They also discovered that hepatocytes can produce serotonin and increase TGF-beta1 synthesis in stellate cells. Interestingly, they uncovered a significant sexual dimorphism in both human and fish samples in components of this pathway (for example, more serotonin and TGF-beta1 in males). This study unravels underlying mechanisms of sex differences in liver cancer. Importantly, it can provide a therapeutic opportunity to treat human liver cancer by modulation of serotonin signaling. This approach is attractive since potent and selective pharmacologic agents for serotonin signaling are already available for other purposes such as modulation of gut motility or neurological disorders. Future studies using human cells or samples will pave the path toward clinical translation of these findings.
Mo Ebrahimkhani, MD, is an assistant professor in the school of biological and health systems engineering, Arizona State University, Tempe. He has no conflicts of interest.
The greater frequency and aggressiveness of hepatocellular carcinoma (HCC) in men than in women might be attributable to greater synthesis and accumulation of serotonin in males, according to a report published online in Cellular and Molecular Gastroenterology and Hepatology (2017 May. doi: 10.1016/j.jcmgh.2017.01.002).
HCC is nearly five times more common in men than in women, and several molecular studies “have shown a more robust and active HCC tumor microenvironment” in men as well. For example, the density of infiltrating, tumor-associated macrophages is higher among males in a mouse model of the cancer, and human men have substantially higher amounts of intratumoral cluster-of-differentiation cells and neutrophils that indicate a poor prognosis, said Qiqi Yang, PhD, of the department of biological sciences at the National University of Singapore, and her associates.
The investigators developed several zebrafish models of HCC in which the cancer could be induced by transgenic expression of an oncogene in the animals’ hepatocytes. These models “allow the oncogene to be activated at a given and controlled timing in both sexes, providing an excellent platform to study the sex disparity in HCC initiation and progression,” they noted.
They also confirmed the zebrafish findings in human lab studies by analyzing tissue samples from 5 normal livers, 7 inflamed livers, 16 cirrhotic livers, and 30 livers affected with HCC.
The investigators found an increased level of serotonin production in male, compared with female, livers. They demonstrated that serotonin was necessary for the survival of hepatic stellate cells, which also are more abundant in males than in females and have recently been shown to promote tumorigenesis. Serotonin also was crucial for activating hepatic stellate cells during HCC carcinogenesis.
In addition, serotonin levels were significantly elevated in inflamed, cirrhotic, and cancerous livers, compared with normal livers, among men but not among women. “This is in line with the prevailing knowledge that men have a significantly higher rate of serotonin synthesis than do women,” Dr. Yang and her associates said.
The greater frequency and aggressiveness of hepatocellular carcinoma (HCC) in men than in women might be attributable to greater synthesis and accumulation of serotonin in males, according to a report published online in Cellular and Molecular Gastroenterology and Hepatology (2017 May. doi: 10.1016/j.jcmgh.2017.01.002).
HCC is nearly five times more common in men than in women, and several molecular studies “have shown a more robust and active HCC tumor microenvironment” in men as well. For example, the density of infiltrating, tumor-associated macrophages is higher among males in a mouse model of the cancer, and human men have substantially higher amounts of intratumoral cluster-of-differentiation cells and neutrophils that indicate a poor prognosis, said Qiqi Yang, PhD, of the department of biological sciences at the National University of Singapore, and her associates.
The investigators developed several zebrafish models of HCC in which the cancer could be induced by transgenic expression of an oncogene in the animals’ hepatocytes. These models “allow the oncogene to be activated at a given and controlled timing in both sexes, providing an excellent platform to study the sex disparity in HCC initiation and progression,” they noted.
They also confirmed the zebrafish findings in human lab studies by analyzing tissue samples from 5 normal livers, 7 inflamed livers, 16 cirrhotic livers, and 30 livers affected with HCC.
The investigators found an increased level of serotonin production in male, compared with female, livers. They demonstrated that serotonin was necessary for the survival of hepatic stellate cells, which also are more abundant in males than in females and have recently been shown to promote tumorigenesis. Serotonin also was crucial for activating hepatic stellate cells during HCC carcinogenesis.
In addition, serotonin levels were significantly elevated in inflamed, cirrhotic, and cancerous livers, compared with normal livers, among men but not among women. “This is in line with the prevailing knowledge that men have a significantly higher rate of serotonin synthesis than do women,” Dr. Yang and her associates said.
FROM CELLULAR & MOLECULAR GASTROENTEROLOGY & HEPATOLOGY
Key clinical point: The greater frequency and aggressiveness of hepatocellular carcinoma in men than in women might be attributable to greater synthesis and accumulation of serotonin in males.
Major finding: Serotonin levels were significantly elevated in 7 inflamed, 16 cirrhotic, and 30 cancerous livers, compared with 5 normal livers, among men but not among women.
Data source: Laboratory studies involving a zebrafish model of HCC and tissue samples from 60 human livers.
Disclosures: This study was supported by the National Medical Research Council and the Ministry of Education of Singapore. Dr. Yang and her associates reported no relevant financial disclosures.
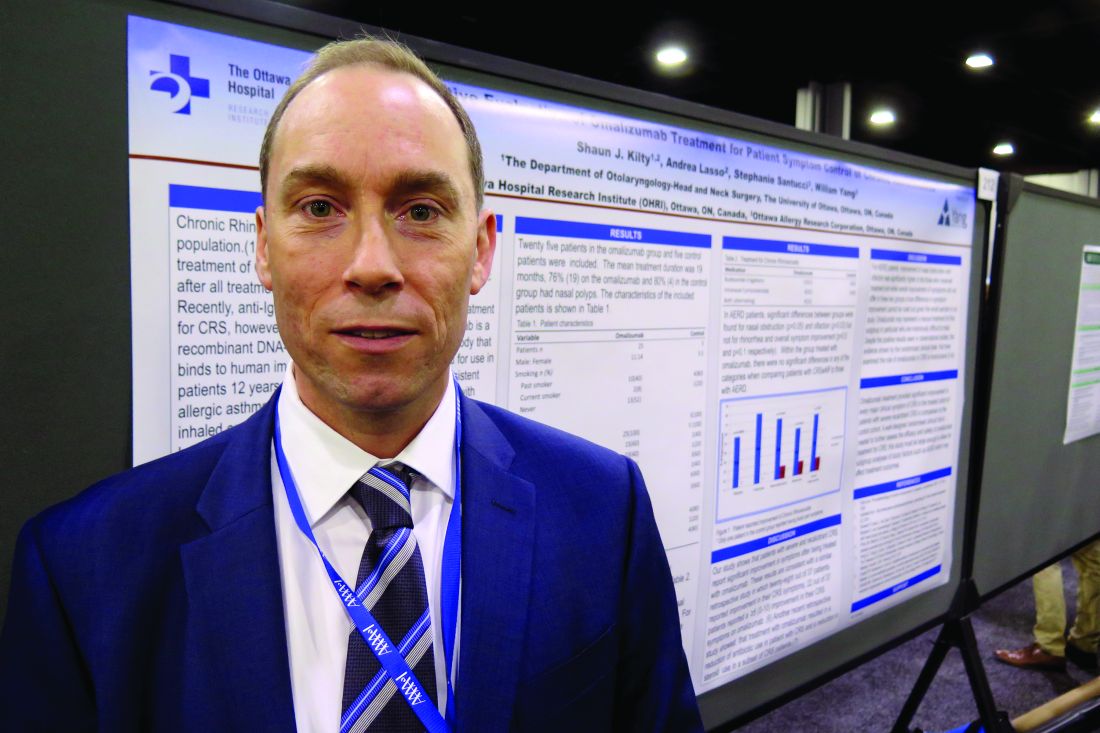
Omalizumab may help chronic rhinosinusitis symptoms
ATLANTA – Patients with severe and recalcitrant chronic rhinosinusitis who were treated with omalizumab reported improvement in symptoms, compared with controls, results from a small single-center study suggested.
A recombinant DNA-derived humanized monoclonal antibody that binds to human IgE, omalizumab is approved for use in patients 12 years or older with moderate to severe persistent asthma and inadequately controlled symptoms with inhaled corticosteroids. Two small randomized controlled trials (J Allergy Clin Immunol. 2013;131[1]:110-6 and Rhinology 2010;48[3]:318-24) have shown that omalizumab reduces polyp size and improves sinus inflammation, Shaun J. Kilty, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
For the current study, Dr. Kilty and his associates evaluated the clinical effect of omalizumab on sinus symptom and disease control in 25 patients with recalcitrant chronic rhinosinusitis (CRS) who were receiving anti-IgE therapy as part of their asthma treatment. Five control patients were included. The researchers used a visual analog scale to measure changes over time among study participants in overall CRS symptoms and major CRS symptoms, including facial pain, nasal obstruction, rhinorrhea, and olfaction. The mean age of the patients was 50 years and 49 years in the omalizumab and control groups, respectively. The mean duration of treatment was 19 months, and most had nasal polyps (76% in the omalizumab group and 80% in the control group).
Dr. Kilty reported that among patients in the omalizumab treatment group, overall symptoms improved by 70%. The individual symptom that improved the most was facial pain (79%), followed by nasal obstruction (70%), rhinorrhea (56%), and olfaction (56%). Among the control group, overall symptoms improved by 17%. Rhinorrhea improved by 16% and nasal obstruction by 15%, but no improvements in facial pain or in olfaction were observed. Symptom improvement was significantly higher for omalizumab-treated patients in every category (P less than .05).
A subset analysis of eight patients in the treatment group and three in the control group who had aspirin-exacerbated respiratory disease (AERD) revealed significant differences between groups for nasal obstruction (P = .05) and olfaction (P = .03), but not for rhinorrhea and overall symptom improvement (P = .5 and P = .1, respectively). “Within the group treated with omalizumab, there were no significant differences in any of the categories when comparing patients with CRS with nasal polyps to those with AERD,” the researchers wrote in their abstract.
“I think there’s a strong enough signal to indicate that this biologic therapy does work in patients with chronic sinusitis with polyps,” Dr. Kilty said. “I think it needs to be better determined exactly what patient cohort is best to use it, and similarly better-structured randomized trials are needed to evaluate the effect of this biologic.”
He reported having no relevant financial disclosures.
ATLANTA – Patients with severe and recalcitrant chronic rhinosinusitis who were treated with omalizumab reported improvement in symptoms, compared with controls, results from a small single-center study suggested.
A recombinant DNA-derived humanized monoclonal antibody that binds to human IgE, omalizumab is approved for use in patients 12 years or older with moderate to severe persistent asthma and inadequately controlled symptoms with inhaled corticosteroids. Two small randomized controlled trials (J Allergy Clin Immunol. 2013;131[1]:110-6 and Rhinology 2010;48[3]:318-24) have shown that omalizumab reduces polyp size and improves sinus inflammation, Shaun J. Kilty, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
For the current study, Dr. Kilty and his associates evaluated the clinical effect of omalizumab on sinus symptom and disease control in 25 patients with recalcitrant chronic rhinosinusitis (CRS) who were receiving anti-IgE therapy as part of their asthma treatment. Five control patients were included. The researchers used a visual analog scale to measure changes over time among study participants in overall CRS symptoms and major CRS symptoms, including facial pain, nasal obstruction, rhinorrhea, and olfaction. The mean age of the patients was 50 years and 49 years in the omalizumab and control groups, respectively. The mean duration of treatment was 19 months, and most had nasal polyps (76% in the omalizumab group and 80% in the control group).
Dr. Kilty reported that among patients in the omalizumab treatment group, overall symptoms improved by 70%. The individual symptom that improved the most was facial pain (79%), followed by nasal obstruction (70%), rhinorrhea (56%), and olfaction (56%). Among the control group, overall symptoms improved by 17%. Rhinorrhea improved by 16% and nasal obstruction by 15%, but no improvements in facial pain or in olfaction were observed. Symptom improvement was significantly higher for omalizumab-treated patients in every category (P less than .05).
A subset analysis of eight patients in the treatment group and three in the control group who had aspirin-exacerbated respiratory disease (AERD) revealed significant differences between groups for nasal obstruction (P = .05) and olfaction (P = .03), but not for rhinorrhea and overall symptom improvement (P = .5 and P = .1, respectively). “Within the group treated with omalizumab, there were no significant differences in any of the categories when comparing patients with CRS with nasal polyps to those with AERD,” the researchers wrote in their abstract.
“I think there’s a strong enough signal to indicate that this biologic therapy does work in patients with chronic sinusitis with polyps,” Dr. Kilty said. “I think it needs to be better determined exactly what patient cohort is best to use it, and similarly better-structured randomized trials are needed to evaluate the effect of this biologic.”
He reported having no relevant financial disclosures.
ATLANTA – Patients with severe and recalcitrant chronic rhinosinusitis who were treated with omalizumab reported improvement in symptoms, compared with controls, results from a small single-center study suggested.
A recombinant DNA-derived humanized monoclonal antibody that binds to human IgE, omalizumab is approved for use in patients 12 years or older with moderate to severe persistent asthma and inadequately controlled symptoms with inhaled corticosteroids. Two small randomized controlled trials (J Allergy Clin Immunol. 2013;131[1]:110-6 and Rhinology 2010;48[3]:318-24) have shown that omalizumab reduces polyp size and improves sinus inflammation, Shaun J. Kilty, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
For the current study, Dr. Kilty and his associates evaluated the clinical effect of omalizumab on sinus symptom and disease control in 25 patients with recalcitrant chronic rhinosinusitis (CRS) who were receiving anti-IgE therapy as part of their asthma treatment. Five control patients were included. The researchers used a visual analog scale to measure changes over time among study participants in overall CRS symptoms and major CRS symptoms, including facial pain, nasal obstruction, rhinorrhea, and olfaction. The mean age of the patients was 50 years and 49 years in the omalizumab and control groups, respectively. The mean duration of treatment was 19 months, and most had nasal polyps (76% in the omalizumab group and 80% in the control group).
Dr. Kilty reported that among patients in the omalizumab treatment group, overall symptoms improved by 70%. The individual symptom that improved the most was facial pain (79%), followed by nasal obstruction (70%), rhinorrhea (56%), and olfaction (56%). Among the control group, overall symptoms improved by 17%. Rhinorrhea improved by 16% and nasal obstruction by 15%, but no improvements in facial pain or in olfaction were observed. Symptom improvement was significantly higher for omalizumab-treated patients in every category (P less than .05).
A subset analysis of eight patients in the treatment group and three in the control group who had aspirin-exacerbated respiratory disease (AERD) revealed significant differences between groups for nasal obstruction (P = .05) and olfaction (P = .03), but not for rhinorrhea and overall symptom improvement (P = .5 and P = .1, respectively). “Within the group treated with omalizumab, there were no significant differences in any of the categories when comparing patients with CRS with nasal polyps to those with AERD,” the researchers wrote in their abstract.
“I think there’s a strong enough signal to indicate that this biologic therapy does work in patients with chronic sinusitis with polyps,” Dr. Kilty said. “I think it needs to be better determined exactly what patient cohort is best to use it, and similarly better-structured randomized trials are needed to evaluate the effect of this biologic.”
He reported having no relevant financial disclosures.
AT 2017 AAAAI ANNUAL MEETING
Key clinical point: In patients with severe chronic rhinosinusitis, omalizumab treatment provided significant improvement in every major clinical symptom of the condition.
Major finding: Among patients in the omalizumab treatment group, overall symptoms improved by 70%.
Data source: A study that evaluated the clinical effect of omalizumab in 25 patients with recalcitrant chronic rhinosinusitis who were receiving anti-IgE therapy as part of their asthma treatment and 5 control patients.
Disclosures: Dr. Kilty reported having no relevant financial disclosures.
Can VNS Improve Arm Function After Stroke?
HOUSTON—Compared with intensive physical therapy alone, vagus nerve stimulation (VNS) plus intensive physical therapy may improve upper-limb motor function in the long term among people with ischemic stroke, according to data described at the International Stroke Conference 2017. The current results are consistent with those of a previous study, and a larger pivotal study of the treatment is scheduled to begin this summer.
Research in animal models of stroke indicates that VNS in addition to physical therapy promotes task-specific neuroplasticity and increases recovery. In a study of 20 patients, Dr. Dawson and colleagues found that the combination of neurostimulation and physical therapy was safe and feasible. They also observed functional improvement in patients who received VNS plus physical therapy, compared with controls.
A Blinded Trial of VNS
To further assess the combination of VNS and physical therapy, Dr. Dawson and colleagues performed a blinded, randomized clinical trial. They enrolled participants with chronic moderate-to-severe upper-limb hemiparesis after ischemic stroke. Stroke had occurred between four months and five years before study enrollment. All participants received implantation with a VNS device and were randomized to VNS (0.8 mA) plus rehabilitation or control VNS (0.0 mA) plus rehabilitation.
All participants underwent six weeks of intensive and task-specific rehabilitation that included three two-hour sessions per week. A half second of stimulation was given with each movement in the active group. After six weeks of in-clinic therapy, participants did not receive a specific therapy for 30 days. Participants began exercising at home at day 30 of the follow-up period. This exercise included 30 min/day of self-administered VNS or sham stimulation.
Blinded assessors evaluated participants’ outcomes on days 1, 30, and 90 after completion of the six-week in-clinic rehabilitation period. Therapists and participants also remained blinded to therapy assignment throughout the study. The primary efficacy outcome was change in Upper Extremity Fugl-Meyer score. An increase of at least 6 points from baseline was considered clinically meaningful.
At the end of the main phase of the study, the controls received active VNS during six weeks of in-clinic therapy.
Significant Difference at 90 Days
In all, 17 patients were implanted and randomized. Eight patients received VNS, and nine patients received sham stimulation. At baseline, participants’ mean age was 60, and mean time since stroke onset was 1.5 years. The average Upper Extremity Fugl-Meyer score was 29.5 in the VNS group and 36.4 in the control group. Right-sided stroke predominated in the active group.
The investigators recorded three adverse events related to surgery; all occurred in the control group. One patient had an infection that was resolved, a second patient had dysphagia and shortness of breath that were resolved, and a third patient had vocal cord paralysis that later improved. Stimulation was not associated with any adverse events.
At day 1 after therapy, mean change in Upper Extremity Fugl-Meyer score was 2.3 points higher in the active group (ie, improvement of 7.6 points vs 5.3 points), but the difference was not statistically significant. The responder rate was 75% in the VNS group, compared with 33% in controls, but this difference also was not statistically significant. At day 90 after therapy, mean change in Upper Extremity Fugl-Meyer score was 9.5 in the active group and 3.8 in controls, and the difference was statistically significant. The responder rate at day 90 was 88% in the active group and 33% among controls, and this difference also was statistically significant. Controls who crossed over to receive six weeks of VNS therapy after the main phase of the study had improvements comparable to those of patients initially assigned to the active group.
Quality-of-Life Data Would Be Informative
“We have shown that we can deliver a partly home-based brain stimulation technique that appears to have promise,” said Dr. Dawson. The upcoming phase III pivotal trial of the technique will include 120 patients.
The study results are “spectacular,” said Philip B. Gorelick, MD, MPH, Medical Director of the Hauenstein Neuroscience Center at Saint Mary’s Health Care in Grand Rapids, Michigan. The fact that therapy does not need to begin immediately after stroke is especially promising, he added. Dr. Gorelick did not participate in the research.
Neurologists need to understand the way the brain reorganizes itself during therapy, and fMRI data for the patients would be informative, Dr. Gorelick continued. Furthermore, data about the technique’s effect on quality of life could provide a more complete picture of the benefits that it provides. “Obviously, the study is
The trial was funded by MicroTransponder, the company that manufactures the VNS device.
—Erik Greb
Suggested Reading
Dawson J, Pierce D, Dixit A, et al. Safety, feasibility, and efficacy of vagus nerve stimulation paired with upper-limb rehabilitation after ischemic stroke. Stroke. 2016;47(1):143-150.
Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009; 8(8):741-754.
HOUSTON—Compared with intensive physical therapy alone, vagus nerve stimulation (VNS) plus intensive physical therapy may improve upper-limb motor function in the long term among people with ischemic stroke, according to data described at the International Stroke Conference 2017. The current results are consistent with those of a previous study, and a larger pivotal study of the treatment is scheduled to begin this summer.
Research in animal models of stroke indicates that VNS in addition to physical therapy promotes task-specific neuroplasticity and increases recovery. In a study of 20 patients, Dr. Dawson and colleagues found that the combination of neurostimulation and physical therapy was safe and feasible. They also observed functional improvement in patients who received VNS plus physical therapy, compared with controls.
A Blinded Trial of VNS
To further assess the combination of VNS and physical therapy, Dr. Dawson and colleagues performed a blinded, randomized clinical trial. They enrolled participants with chronic moderate-to-severe upper-limb hemiparesis after ischemic stroke. Stroke had occurred between four months and five years before study enrollment. All participants received implantation with a VNS device and were randomized to VNS (0.8 mA) plus rehabilitation or control VNS (0.0 mA) plus rehabilitation.
All participants underwent six weeks of intensive and task-specific rehabilitation that included three two-hour sessions per week. A half second of stimulation was given with each movement in the active group. After six weeks of in-clinic therapy, participants did not receive a specific therapy for 30 days. Participants began exercising at home at day 30 of the follow-up period. This exercise included 30 min/day of self-administered VNS or sham stimulation.
Blinded assessors evaluated participants’ outcomes on days 1, 30, and 90 after completion of the six-week in-clinic rehabilitation period. Therapists and participants also remained blinded to therapy assignment throughout the study. The primary efficacy outcome was change in Upper Extremity Fugl-Meyer score. An increase of at least 6 points from baseline was considered clinically meaningful.
At the end of the main phase of the study, the controls received active VNS during six weeks of in-clinic therapy.
Significant Difference at 90 Days
In all, 17 patients were implanted and randomized. Eight patients received VNS, and nine patients received sham stimulation. At baseline, participants’ mean age was 60, and mean time since stroke onset was 1.5 years. The average Upper Extremity Fugl-Meyer score was 29.5 in the VNS group and 36.4 in the control group. Right-sided stroke predominated in the active group.
The investigators recorded three adverse events related to surgery; all occurred in the control group. One patient had an infection that was resolved, a second patient had dysphagia and shortness of breath that were resolved, and a third patient had vocal cord paralysis that later improved. Stimulation was not associated with any adverse events.
At day 1 after therapy, mean change in Upper Extremity Fugl-Meyer score was 2.3 points higher in the active group (ie, improvement of 7.6 points vs 5.3 points), but the difference was not statistically significant. The responder rate was 75% in the VNS group, compared with 33% in controls, but this difference also was not statistically significant. At day 90 after therapy, mean change in Upper Extremity Fugl-Meyer score was 9.5 in the active group and 3.8 in controls, and the difference was statistically significant. The responder rate at day 90 was 88% in the active group and 33% among controls, and this difference also was statistically significant. Controls who crossed over to receive six weeks of VNS therapy after the main phase of the study had improvements comparable to those of patients initially assigned to the active group.
Quality-of-Life Data Would Be Informative
“We have shown that we can deliver a partly home-based brain stimulation technique that appears to have promise,” said Dr. Dawson. The upcoming phase III pivotal trial of the technique will include 120 patients.
The study results are “spectacular,” said Philip B. Gorelick, MD, MPH, Medical Director of the Hauenstein Neuroscience Center at Saint Mary’s Health Care in Grand Rapids, Michigan. The fact that therapy does not need to begin immediately after stroke is especially promising, he added. Dr. Gorelick did not participate in the research.
Neurologists need to understand the way the brain reorganizes itself during therapy, and fMRI data for the patients would be informative, Dr. Gorelick continued. Furthermore, data about the technique’s effect on quality of life could provide a more complete picture of the benefits that it provides. “Obviously, the study is
The trial was funded by MicroTransponder, the company that manufactures the VNS device.
—Erik Greb
Suggested Reading
Dawson J, Pierce D, Dixit A, et al. Safety, feasibility, and efficacy of vagus nerve stimulation paired with upper-limb rehabilitation after ischemic stroke. Stroke. 2016;47(1):143-150.
Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009; 8(8):741-754.
HOUSTON—Compared with intensive physical therapy alone, vagus nerve stimulation (VNS) plus intensive physical therapy may improve upper-limb motor function in the long term among people with ischemic stroke, according to data described at the International Stroke Conference 2017. The current results are consistent with those of a previous study, and a larger pivotal study of the treatment is scheduled to begin this summer.
Research in animal models of stroke indicates that VNS in addition to physical therapy promotes task-specific neuroplasticity and increases recovery. In a study of 20 patients, Dr. Dawson and colleagues found that the combination of neurostimulation and physical therapy was safe and feasible. They also observed functional improvement in patients who received VNS plus physical therapy, compared with controls.
A Blinded Trial of VNS
To further assess the combination of VNS and physical therapy, Dr. Dawson and colleagues performed a blinded, randomized clinical trial. They enrolled participants with chronic moderate-to-severe upper-limb hemiparesis after ischemic stroke. Stroke had occurred between four months and five years before study enrollment. All participants received implantation with a VNS device and were randomized to VNS (0.8 mA) plus rehabilitation or control VNS (0.0 mA) plus rehabilitation.
All participants underwent six weeks of intensive and task-specific rehabilitation that included three two-hour sessions per week. A half second of stimulation was given with each movement in the active group. After six weeks of in-clinic therapy, participants did not receive a specific therapy for 30 days. Participants began exercising at home at day 30 of the follow-up period. This exercise included 30 min/day of self-administered VNS or sham stimulation.
Blinded assessors evaluated participants’ outcomes on days 1, 30, and 90 after completion of the six-week in-clinic rehabilitation period. Therapists and participants also remained blinded to therapy assignment throughout the study. The primary efficacy outcome was change in Upper Extremity Fugl-Meyer score. An increase of at least 6 points from baseline was considered clinically meaningful.
At the end of the main phase of the study, the controls received active VNS during six weeks of in-clinic therapy.
Significant Difference at 90 Days
In all, 17 patients were implanted and randomized. Eight patients received VNS, and nine patients received sham stimulation. At baseline, participants’ mean age was 60, and mean time since stroke onset was 1.5 years. The average Upper Extremity Fugl-Meyer score was 29.5 in the VNS group and 36.4 in the control group. Right-sided stroke predominated in the active group.
The investigators recorded three adverse events related to surgery; all occurred in the control group. One patient had an infection that was resolved, a second patient had dysphagia and shortness of breath that were resolved, and a third patient had vocal cord paralysis that later improved. Stimulation was not associated with any adverse events.
At day 1 after therapy, mean change in Upper Extremity Fugl-Meyer score was 2.3 points higher in the active group (ie, improvement of 7.6 points vs 5.3 points), but the difference was not statistically significant. The responder rate was 75% in the VNS group, compared with 33% in controls, but this difference also was not statistically significant. At day 90 after therapy, mean change in Upper Extremity Fugl-Meyer score was 9.5 in the active group and 3.8 in controls, and the difference was statistically significant. The responder rate at day 90 was 88% in the active group and 33% among controls, and this difference also was statistically significant. Controls who crossed over to receive six weeks of VNS therapy after the main phase of the study had improvements comparable to those of patients initially assigned to the active group.
Quality-of-Life Data Would Be Informative
“We have shown that we can deliver a partly home-based brain stimulation technique that appears to have promise,” said Dr. Dawson. The upcoming phase III pivotal trial of the technique will include 120 patients.
The study results are “spectacular,” said Philip B. Gorelick, MD, MPH, Medical Director of the Hauenstein Neuroscience Center at Saint Mary’s Health Care in Grand Rapids, Michigan. The fact that therapy does not need to begin immediately after stroke is especially promising, he added. Dr. Gorelick did not participate in the research.
Neurologists need to understand the way the brain reorganizes itself during therapy, and fMRI data for the patients would be informative, Dr. Gorelick continued. Furthermore, data about the technique’s effect on quality of life could provide a more complete picture of the benefits that it provides. “Obviously, the study is
The trial was funded by MicroTransponder, the company that manufactures the VNS device.
—Erik Greb
Suggested Reading
Dawson J, Pierce D, Dixit A, et al. Safety, feasibility, and efficacy of vagus nerve stimulation paired with upper-limb rehabilitation after ischemic stroke. Stroke. 2016;47(1):143-150.
Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009; 8(8):741-754.
Benefits of DBS for Dystonia May Be Sustained Over 10 Years
MIAMI—Deep brain stimulation (DBS) for dystonia may substantially improve function and quality of life for over a decade post implantation, according to patient survey results presented at the First Pan American Parkinson's Disease and Movement Disorders Congress.
The internal globus pallidus (GPI) has emerged as the optimal surgical target for the treatment of dystonia, and quality of life improvements have been documented for as long as five years on average, said Elliot Hogg, MD, of the Department of Neurology at Cedars-Sinai Medical Center in Los Angeles, and colleagues.
"No large series have consistently followed patients treated with DBS beyond five years from implantation. Whereas some studies have shown that the efficacy of stimulation improves with time, others have suggested that there may be late recurrence of dystonia," the researchers said. "Given that DBS is often performed in young patients who can be expected to have DBS for many years, and possibly decades, sustained benefit is an important clinical concern."
To study the long-term effects of GPI DBS on the quality of life of people with dystonia, Dr. Hogg and colleagues mailed the 36-Item Short Form survey (SF-36) to people with dystonia who had undergone DBS surgery at least five years prior to the study. Subjects were instructed to use the survey to compare their quality of life in the period immediately preceding DBS implantation with that of the current day. The researchers compared the scores using a matched pair t-test analysis.
Twenty-seven patients participated in the study (mean age, 38.9; 15 female). Eighteen subjects were diagnosed with a DYT1 mutation, six had other primary generalized dystonias, two had secondary dystonias, and one had cervical dystonia.
Average time from implantation was 9.5 years (range, five to 15 years). Average total SF-36 scores were 36.9 before DBS and 72.1 at current day.
Patients reported improvement across all subcategories of the SF-36, with the greatest relative improvement in social function. Patients who had had DBS for longer than 10 years (n = 10) reported similar overall improvements, compared with the population as a whole. Subjects younger than 30 (n = 11) reported higher total SF-36 improvements (ie, from 33.0 to 81.2), compared with the entire sample.
"Our study provides the longest follow-up data on DBS for dystonia at an average of 10 years since implantation," the researchers concluded. "Despite the limitations of self-reporting and possible recall bias, the results show a substantial benefit in all areas of function and quality of life, which were sustained over a decade post implantation."
Because studies have suggested that stimulation-induced parkinsonism may complicate bilateral DBS of the GPI, Dr. Hogg and colleagues also asked 27 patients who had had at least five years of DBS to complete a nine-item survey on parkinsonian symptoms. Patients reported 1.33 parkinsonian symptoms on average. The most commonly reported symptoms were hypophonia (33% of subjects) and micrographia (26%).
Both of Dr. Hogg's studies were supported in part by the Dystonia Medical Research Foundation.
—Jake Remaly
Suggested Reading
Baizabal-Carvallo JF, Jankovic J. Movement disorders induced by deep brain stimulation. Parkinsonism Relat Disord. 2016;25:1-9.
Loher TJ, Capelle HH, Kaelin-Lang A, et al. Deep brain stimulation for dystonia: outcome at long-term follow-up. J Neurol. 2008;255(6):881-884.
MIAMI—Deep brain stimulation (DBS) for dystonia may substantially improve function and quality of life for over a decade post implantation, according to patient survey results presented at the First Pan American Parkinson's Disease and Movement Disorders Congress.
The internal globus pallidus (GPI) has emerged as the optimal surgical target for the treatment of dystonia, and quality of life improvements have been documented for as long as five years on average, said Elliot Hogg, MD, of the Department of Neurology at Cedars-Sinai Medical Center in Los Angeles, and colleagues.
"No large series have consistently followed patients treated with DBS beyond five years from implantation. Whereas some studies have shown that the efficacy of stimulation improves with time, others have suggested that there may be late recurrence of dystonia," the researchers said. "Given that DBS is often performed in young patients who can be expected to have DBS for many years, and possibly decades, sustained benefit is an important clinical concern."
To study the long-term effects of GPI DBS on the quality of life of people with dystonia, Dr. Hogg and colleagues mailed the 36-Item Short Form survey (SF-36) to people with dystonia who had undergone DBS surgery at least five years prior to the study. Subjects were instructed to use the survey to compare their quality of life in the period immediately preceding DBS implantation with that of the current day. The researchers compared the scores using a matched pair t-test analysis.
Twenty-seven patients participated in the study (mean age, 38.9; 15 female). Eighteen subjects were diagnosed with a DYT1 mutation, six had other primary generalized dystonias, two had secondary dystonias, and one had cervical dystonia.
Average time from implantation was 9.5 years (range, five to 15 years). Average total SF-36 scores were 36.9 before DBS and 72.1 at current day.
Patients reported improvement across all subcategories of the SF-36, with the greatest relative improvement in social function. Patients who had had DBS for longer than 10 years (n = 10) reported similar overall improvements, compared with the population as a whole. Subjects younger than 30 (n = 11) reported higher total SF-36 improvements (ie, from 33.0 to 81.2), compared with the entire sample.
"Our study provides the longest follow-up data on DBS for dystonia at an average of 10 years since implantation," the researchers concluded. "Despite the limitations of self-reporting and possible recall bias, the results show a substantial benefit in all areas of function and quality of life, which were sustained over a decade post implantation."
Because studies have suggested that stimulation-induced parkinsonism may complicate bilateral DBS of the GPI, Dr. Hogg and colleagues also asked 27 patients who had had at least five years of DBS to complete a nine-item survey on parkinsonian symptoms. Patients reported 1.33 parkinsonian symptoms on average. The most commonly reported symptoms were hypophonia (33% of subjects) and micrographia (26%).
Both of Dr. Hogg's studies were supported in part by the Dystonia Medical Research Foundation.
—Jake Remaly
Suggested Reading
Baizabal-Carvallo JF, Jankovic J. Movement disorders induced by deep brain stimulation. Parkinsonism Relat Disord. 2016;25:1-9.
Loher TJ, Capelle HH, Kaelin-Lang A, et al. Deep brain stimulation for dystonia: outcome at long-term follow-up. J Neurol. 2008;255(6):881-884.
MIAMI—Deep brain stimulation (DBS) for dystonia may substantially improve function and quality of life for over a decade post implantation, according to patient survey results presented at the First Pan American Parkinson's Disease and Movement Disorders Congress.
The internal globus pallidus (GPI) has emerged as the optimal surgical target for the treatment of dystonia, and quality of life improvements have been documented for as long as five years on average, said Elliot Hogg, MD, of the Department of Neurology at Cedars-Sinai Medical Center in Los Angeles, and colleagues.
"No large series have consistently followed patients treated with DBS beyond five years from implantation. Whereas some studies have shown that the efficacy of stimulation improves with time, others have suggested that there may be late recurrence of dystonia," the researchers said. "Given that DBS is often performed in young patients who can be expected to have DBS for many years, and possibly decades, sustained benefit is an important clinical concern."
To study the long-term effects of GPI DBS on the quality of life of people with dystonia, Dr. Hogg and colleagues mailed the 36-Item Short Form survey (SF-36) to people with dystonia who had undergone DBS surgery at least five years prior to the study. Subjects were instructed to use the survey to compare their quality of life in the period immediately preceding DBS implantation with that of the current day. The researchers compared the scores using a matched pair t-test analysis.
Twenty-seven patients participated in the study (mean age, 38.9; 15 female). Eighteen subjects were diagnosed with a DYT1 mutation, six had other primary generalized dystonias, two had secondary dystonias, and one had cervical dystonia.
Average time from implantation was 9.5 years (range, five to 15 years). Average total SF-36 scores were 36.9 before DBS and 72.1 at current day.
Patients reported improvement across all subcategories of the SF-36, with the greatest relative improvement in social function. Patients who had had DBS for longer than 10 years (n = 10) reported similar overall improvements, compared with the population as a whole. Subjects younger than 30 (n = 11) reported higher total SF-36 improvements (ie, from 33.0 to 81.2), compared with the entire sample.
"Our study provides the longest follow-up data on DBS for dystonia at an average of 10 years since implantation," the researchers concluded. "Despite the limitations of self-reporting and possible recall bias, the results show a substantial benefit in all areas of function and quality of life, which were sustained over a decade post implantation."
Because studies have suggested that stimulation-induced parkinsonism may complicate bilateral DBS of the GPI, Dr. Hogg and colleagues also asked 27 patients who had had at least five years of DBS to complete a nine-item survey on parkinsonian symptoms. Patients reported 1.33 parkinsonian symptoms on average. The most commonly reported symptoms were hypophonia (33% of subjects) and micrographia (26%).
Both of Dr. Hogg's studies were supported in part by the Dystonia Medical Research Foundation.
—Jake Remaly
Suggested Reading
Baizabal-Carvallo JF, Jankovic J. Movement disorders induced by deep brain stimulation. Parkinsonism Relat Disord. 2016;25:1-9.
Loher TJ, Capelle HH, Kaelin-Lang A, et al. Deep brain stimulation for dystonia: outcome at long-term follow-up. J Neurol. 2008;255(6):881-884.
Polypharmacy for Parkinson’s Disease Is More Common in Younger Patients
MIAMI—Patients younger than 65 have a higher polypharmacy burden in the management of Parkinson’s disease symptoms, compared with patients age 65 and older, according to research presented at the First Pan American Parkinson’s Disease and Movement Disorders Congress.
A claims database analysis found that a majority of patients were on levodopa-based therapy, and patients on levodopa-based therapy remained on their current regimens longer, compared with patients on non–levodopa-based regimens, “suggesting that levodopa remains the cornerstone of care across all observed therapies,” the researchers said.
To assess patterns of antiparkinson drug use and treatment changes in the United States, Alexander Niyazov, PharmD, MPH, Associate Director of Health Economics and Outcomes Research at Acorda Therapeutics in Ardsley, New York, and colleagues analyzed data from the Truven Health MarketScan retrospective claims database from the first quarter of 2010 to the fourth quarter of 2014.
The study population included people who were diagnosed with Parkinson’s disease and who had filled at least two prescriptions for antiparkinson drugs, with the second fill occurring within 30 days after the previous supply’s end. Patients were included in the study until their insurance coverage ended or until their data cutoff.
The investigators included 76,602 patients in the study, with an average study period of 25.3 months. At the index date, patients’ mean age was 71.5, 30.7% were younger than 65, 59.4% were male, and 40.7% were treated by a neurologist.
Overall, 57.9% of patients remained on their first observed therapy, while 42.1% proceeded to a second therapy. Of those with a second therapy, 37.6% of patients proceeded to a third therapy. “The high rate of therapy changes highlights unmet needs and opportunities for improving the management of Parkinson’s disease,” the researchers said.
Initially, 72.7% of patients received monotherapy, and levodopa was the most common monotherapy (51.3%). Combination therapies were more common among patients who were younger than 65 and who were being treated by a neurologist.
Patients treated with a levodopa-based therapy remained on their current regimens for a median of 11.1 months, whereas patients receiving non–levodopa-based therapies remained on their current regimens for a median of 6.0 months.
Limitations of the study include potential inaccuracies and incompleteness of claims coding, and the fact that filled prescriptions may not include all drugs prescribed or represent the patient’s drug utilization. The study was supported by Acorda Therapeutics.
—Jake Remaly
MIAMI—Patients younger than 65 have a higher polypharmacy burden in the management of Parkinson’s disease symptoms, compared with patients age 65 and older, according to research presented at the First Pan American Parkinson’s Disease and Movement Disorders Congress.
A claims database analysis found that a majority of patients were on levodopa-based therapy, and patients on levodopa-based therapy remained on their current regimens longer, compared with patients on non–levodopa-based regimens, “suggesting that levodopa remains the cornerstone of care across all observed therapies,” the researchers said.
To assess patterns of antiparkinson drug use and treatment changes in the United States, Alexander Niyazov, PharmD, MPH, Associate Director of Health Economics and Outcomes Research at Acorda Therapeutics in Ardsley, New York, and colleagues analyzed data from the Truven Health MarketScan retrospective claims database from the first quarter of 2010 to the fourth quarter of 2014.
The study population included people who were diagnosed with Parkinson’s disease and who had filled at least two prescriptions for antiparkinson drugs, with the second fill occurring within 30 days after the previous supply’s end. Patients were included in the study until their insurance coverage ended or until their data cutoff.
The investigators included 76,602 patients in the study, with an average study period of 25.3 months. At the index date, patients’ mean age was 71.5, 30.7% were younger than 65, 59.4% were male, and 40.7% were treated by a neurologist.
Overall, 57.9% of patients remained on their first observed therapy, while 42.1% proceeded to a second therapy. Of those with a second therapy, 37.6% of patients proceeded to a third therapy. “The high rate of therapy changes highlights unmet needs and opportunities for improving the management of Parkinson’s disease,” the researchers said.
Initially, 72.7% of patients received monotherapy, and levodopa was the most common monotherapy (51.3%). Combination therapies were more common among patients who were younger than 65 and who were being treated by a neurologist.
Patients treated with a levodopa-based therapy remained on their current regimens for a median of 11.1 months, whereas patients receiving non–levodopa-based therapies remained on their current regimens for a median of 6.0 months.
Limitations of the study include potential inaccuracies and incompleteness of claims coding, and the fact that filled prescriptions may not include all drugs prescribed or represent the patient’s drug utilization. The study was supported by Acorda Therapeutics.
—Jake Remaly
MIAMI—Patients younger than 65 have a higher polypharmacy burden in the management of Parkinson’s disease symptoms, compared with patients age 65 and older, according to research presented at the First Pan American Parkinson’s Disease and Movement Disorders Congress.
A claims database analysis found that a majority of patients were on levodopa-based therapy, and patients on levodopa-based therapy remained on their current regimens longer, compared with patients on non–levodopa-based regimens, “suggesting that levodopa remains the cornerstone of care across all observed therapies,” the researchers said.
To assess patterns of antiparkinson drug use and treatment changes in the United States, Alexander Niyazov, PharmD, MPH, Associate Director of Health Economics and Outcomes Research at Acorda Therapeutics in Ardsley, New York, and colleagues analyzed data from the Truven Health MarketScan retrospective claims database from the first quarter of 2010 to the fourth quarter of 2014.
The study population included people who were diagnosed with Parkinson’s disease and who had filled at least two prescriptions for antiparkinson drugs, with the second fill occurring within 30 days after the previous supply’s end. Patients were included in the study until their insurance coverage ended or until their data cutoff.
The investigators included 76,602 patients in the study, with an average study period of 25.3 months. At the index date, patients’ mean age was 71.5, 30.7% were younger than 65, 59.4% were male, and 40.7% were treated by a neurologist.
Overall, 57.9% of patients remained on their first observed therapy, while 42.1% proceeded to a second therapy. Of those with a second therapy, 37.6% of patients proceeded to a third therapy. “The high rate of therapy changes highlights unmet needs and opportunities for improving the management of Parkinson’s disease,” the researchers said.
Initially, 72.7% of patients received monotherapy, and levodopa was the most common monotherapy (51.3%). Combination therapies were more common among patients who were younger than 65 and who were being treated by a neurologist.
Patients treated with a levodopa-based therapy remained on their current regimens for a median of 11.1 months, whereas patients receiving non–levodopa-based therapies remained on their current regimens for a median of 6.0 months.
Limitations of the study include potential inaccuracies and incompleteness of claims coding, and the fact that filled prescriptions may not include all drugs prescribed or represent the patient’s drug utilization. The study was supported by Acorda Therapeutics.
—Jake Remaly
FDA grants breakthrough therapy status to rituximab for pemphigus vulgaris
The Food and Drug Administration has granted breakthrough therapy status to rituximab (Rituxan) for treating pemphigus vulgaris, according to the manufacturer.
Rituximab, a CD20-directed cytolytic antibody approved in 1997, is currently in a phase III study evaluating its efficacy for the pemphigus indication. It is approved in the United States for treating non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis (with methotrexate), granulomatosis with polyangiitis (Wegener’s granulomatosis), and microscopic polyangiitis (with glucocorticoids).
The patients, who were experiencing their first episode of pemphigus vulgaris, were randomized to daily oral prednisone, tapered over a 12- to 18-month period, or rituximab administered intravenously (at days 0 and 14, and months 12 and 18), plus daily oral prednisone, tapered over 3 or 6 months. At 2 years, when they were no longer on therapy, 89% of those treated with rituximab and prednisone were in complete remission, compared with 34% of those treated with prednisone alone (P less than .0001).
The breakthrough therapy process is “designed to expedite the development and review of drugs that are intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint(s),” according to the FDA.
The study was supported by the French Ministry of Health, the French Society of Dermatology, and Roche, which owns Genentech. Genentech markets rituximab in the United States with Biogen and is conducting the phase III study.
The Food and Drug Administration has granted breakthrough therapy status to rituximab (Rituxan) for treating pemphigus vulgaris, according to the manufacturer.
Rituximab, a CD20-directed cytolytic antibody approved in 1997, is currently in a phase III study evaluating its efficacy for the pemphigus indication. It is approved in the United States for treating non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis (with methotrexate), granulomatosis with polyangiitis (Wegener’s granulomatosis), and microscopic polyangiitis (with glucocorticoids).
The patients, who were experiencing their first episode of pemphigus vulgaris, were randomized to daily oral prednisone, tapered over a 12- to 18-month period, or rituximab administered intravenously (at days 0 and 14, and months 12 and 18), plus daily oral prednisone, tapered over 3 or 6 months. At 2 years, when they were no longer on therapy, 89% of those treated with rituximab and prednisone were in complete remission, compared with 34% of those treated with prednisone alone (P less than .0001).
The breakthrough therapy process is “designed to expedite the development and review of drugs that are intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint(s),” according to the FDA.
The study was supported by the French Ministry of Health, the French Society of Dermatology, and Roche, which owns Genentech. Genentech markets rituximab in the United States with Biogen and is conducting the phase III study.
The Food and Drug Administration has granted breakthrough therapy status to rituximab (Rituxan) for treating pemphigus vulgaris, according to the manufacturer.
Rituximab, a CD20-directed cytolytic antibody approved in 1997, is currently in a phase III study evaluating its efficacy for the pemphigus indication. It is approved in the United States for treating non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis (with methotrexate), granulomatosis with polyangiitis (Wegener’s granulomatosis), and microscopic polyangiitis (with glucocorticoids).
The patients, who were experiencing their first episode of pemphigus vulgaris, were randomized to daily oral prednisone, tapered over a 12- to 18-month period, or rituximab administered intravenously (at days 0 and 14, and months 12 and 18), plus daily oral prednisone, tapered over 3 or 6 months. At 2 years, when they were no longer on therapy, 89% of those treated with rituximab and prednisone were in complete remission, compared with 34% of those treated with prednisone alone (P less than .0001).
The breakthrough therapy process is “designed to expedite the development and review of drugs that are intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint(s),” according to the FDA.
The study was supported by the French Ministry of Health, the French Society of Dermatology, and Roche, which owns Genentech. Genentech markets rituximab in the United States with Biogen and is conducting the phase III study.
A history of asthma exacerbations predicts future exacerbations
ATLANTA – Asthma exacerbations are common, severe events that can be life-threatening and can accelerate loss of lung function. That’s why it’s important to flag high-risk patients in your clinical practice, Nizar N. Jarjour, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Each year in the United States there are 15 million clinic visits, 2 million ED visits, and 500,000 hospitalizations for severe asthma exacerbations. “These exacerbations cause a high cost on the health care system, they lead to loss of work or school, and they’re a burden to patients and certainly to their families,” said Dr. Jarjour, professor of medicine and division head of allergy, pulmonary and critical care at the University of Wisconsin, Madison.
Factors implicated in asthma exacerbation include air pollution, cigarette smoke, occupational exposure, stress, and allergen exposure, but 50%-85% of cases are related to viral upper respiratory infections. “Any respiratory pathogen can precipitate attacks, but rhinoviruses are the most common,” he said. “Seasonal viral [upper respiratory infections] correlate with hospital admissions for asthma, and peak in spring and fall.”
Purported ways that a viral upper respiratory infection can lead to asthma exacerbation include enhanced airway responsiveness, increased eosinophilic airway inflammation in response to antigen, enhanced lower airway neutrophilic inflammation, and direct infection of the lower airway. “There are is an accentuated eosinophilic inflammation in response to allergen challenge when somebody has a cold,” Dr. Jarjour explained. “But the viral infection can actually directly lead to eosinophilic inflammation, perhaps through TSLP [thymic stromal lymphopoietin], interleukin (IL)-25, and IL-33 simulating the ILC2 (type 2 innate lymphoid cells). So there are both accentuating response to allergens as well as direct enhancement of eosinophilic inflammation following viral infection.”
One study that examined airway lavage following experimental infection found that patients had increased numbers of neutrophils in their airway sample (J Allergy Clin Immunol. 2000;105:1169-77). This means that asthma exacerbation triggers neutrophilic inflammation, which relates to the induction of cytokines and chemokines in the upper airway. “We have also demonstrated increased circulation of G-CSF [granulocyte–colony stimulating factor] and nasal IL-8 in the upper airway related to increased neutrophil recruitment,” Dr. Jarjour said.
Host factors associated with asthma exacerbations include altered innate immune response in the form of a defect in production of antiviral cytokines in response to viral infection, and a greater T helper (Th)2/Th1 ratio. Other factors include eosinophilic inflammation and greater levels of specific IgE to dust mite. Baseline data from 709 patients enrolled in the National Heart, Lung, and Blood Institute Severe Asthma Research Program III (SARP-3) showed that three top risk factors for asthma exacerbations are gastroesophageal reflux disease (relative risk (RR) 1.6), greater blood eosinophil count (RR 1.6), and obesity (RR 1.2) (Am J Resp Care Med. 2017; 195:302-13).
Risk factors for exacerbation noted in various epidemiological studies include African American and Hispanic races, poor access to medical care, inadequate chronic control, smoking, allergen sensitivity to cats and dogs, gastroesophageal reflux disease, high body mass index, sinusitis, and uncontrolled eosinophilic inflammation. Key findings from a follow-up study of SARP-3 patients include the fact that the absence of exacerbations on a 12-month recall predicted future stability.
“More importantly, participants with severe disease and two or more exacerbations at baseline had a 75% chance of having an exacerbation in the following year,” Dr. Jarjour said. “This is a call for us to pay more close attention to these patients and is an important fact to keep in mind when designing clinical trials. A history of exacerbations is the best predictor of future exacerbation.”
Dr. Jarjour disclosed that he has received research funding from the National Heart, Lung, and Blood Institute and consulting fees from AstraZeneca and Teva Pharmaceutical. He is a member of the American Board of Internal Medicine–Pulmonary Exam Subcommittee.
ATLANTA – Asthma exacerbations are common, severe events that can be life-threatening and can accelerate loss of lung function. That’s why it’s important to flag high-risk patients in your clinical practice, Nizar N. Jarjour, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Each year in the United States there are 15 million clinic visits, 2 million ED visits, and 500,000 hospitalizations for severe asthma exacerbations. “These exacerbations cause a high cost on the health care system, they lead to loss of work or school, and they’re a burden to patients and certainly to their families,” said Dr. Jarjour, professor of medicine and division head of allergy, pulmonary and critical care at the University of Wisconsin, Madison.
Factors implicated in asthma exacerbation include air pollution, cigarette smoke, occupational exposure, stress, and allergen exposure, but 50%-85% of cases are related to viral upper respiratory infections. “Any respiratory pathogen can precipitate attacks, but rhinoviruses are the most common,” he said. “Seasonal viral [upper respiratory infections] correlate with hospital admissions for asthma, and peak in spring and fall.”
Purported ways that a viral upper respiratory infection can lead to asthma exacerbation include enhanced airway responsiveness, increased eosinophilic airway inflammation in response to antigen, enhanced lower airway neutrophilic inflammation, and direct infection of the lower airway. “There are is an accentuated eosinophilic inflammation in response to allergen challenge when somebody has a cold,” Dr. Jarjour explained. “But the viral infection can actually directly lead to eosinophilic inflammation, perhaps through TSLP [thymic stromal lymphopoietin], interleukin (IL)-25, and IL-33 simulating the ILC2 (type 2 innate lymphoid cells). So there are both accentuating response to allergens as well as direct enhancement of eosinophilic inflammation following viral infection.”
One study that examined airway lavage following experimental infection found that patients had increased numbers of neutrophils in their airway sample (J Allergy Clin Immunol. 2000;105:1169-77). This means that asthma exacerbation triggers neutrophilic inflammation, which relates to the induction of cytokines and chemokines in the upper airway. “We have also demonstrated increased circulation of G-CSF [granulocyte–colony stimulating factor] and nasal IL-8 in the upper airway related to increased neutrophil recruitment,” Dr. Jarjour said.
Host factors associated with asthma exacerbations include altered innate immune response in the form of a defect in production of antiviral cytokines in response to viral infection, and a greater T helper (Th)2/Th1 ratio. Other factors include eosinophilic inflammation and greater levels of specific IgE to dust mite. Baseline data from 709 patients enrolled in the National Heart, Lung, and Blood Institute Severe Asthma Research Program III (SARP-3) showed that three top risk factors for asthma exacerbations are gastroesophageal reflux disease (relative risk (RR) 1.6), greater blood eosinophil count (RR 1.6), and obesity (RR 1.2) (Am J Resp Care Med. 2017; 195:302-13).
Risk factors for exacerbation noted in various epidemiological studies include African American and Hispanic races, poor access to medical care, inadequate chronic control, smoking, allergen sensitivity to cats and dogs, gastroesophageal reflux disease, high body mass index, sinusitis, and uncontrolled eosinophilic inflammation. Key findings from a follow-up study of SARP-3 patients include the fact that the absence of exacerbations on a 12-month recall predicted future stability.
“More importantly, participants with severe disease and two or more exacerbations at baseline had a 75% chance of having an exacerbation in the following year,” Dr. Jarjour said. “This is a call for us to pay more close attention to these patients and is an important fact to keep in mind when designing clinical trials. A history of exacerbations is the best predictor of future exacerbation.”
Dr. Jarjour disclosed that he has received research funding from the National Heart, Lung, and Blood Institute and consulting fees from AstraZeneca and Teva Pharmaceutical. He is a member of the American Board of Internal Medicine–Pulmonary Exam Subcommittee.
ATLANTA – Asthma exacerbations are common, severe events that can be life-threatening and can accelerate loss of lung function. That’s why it’s important to flag high-risk patients in your clinical practice, Nizar N. Jarjour, MD, said at the annual meeting of the American Academy of Allergy, Asthma, and Immunology.
Each year in the United States there are 15 million clinic visits, 2 million ED visits, and 500,000 hospitalizations for severe asthma exacerbations. “These exacerbations cause a high cost on the health care system, they lead to loss of work or school, and they’re a burden to patients and certainly to their families,” said Dr. Jarjour, professor of medicine and division head of allergy, pulmonary and critical care at the University of Wisconsin, Madison.
Factors implicated in asthma exacerbation include air pollution, cigarette smoke, occupational exposure, stress, and allergen exposure, but 50%-85% of cases are related to viral upper respiratory infections. “Any respiratory pathogen can precipitate attacks, but rhinoviruses are the most common,” he said. “Seasonal viral [upper respiratory infections] correlate with hospital admissions for asthma, and peak in spring and fall.”
Purported ways that a viral upper respiratory infection can lead to asthma exacerbation include enhanced airway responsiveness, increased eosinophilic airway inflammation in response to antigen, enhanced lower airway neutrophilic inflammation, and direct infection of the lower airway. “There are is an accentuated eosinophilic inflammation in response to allergen challenge when somebody has a cold,” Dr. Jarjour explained. “But the viral infection can actually directly lead to eosinophilic inflammation, perhaps through TSLP [thymic stromal lymphopoietin], interleukin (IL)-25, and IL-33 simulating the ILC2 (type 2 innate lymphoid cells). So there are both accentuating response to allergens as well as direct enhancement of eosinophilic inflammation following viral infection.”
One study that examined airway lavage following experimental infection found that patients had increased numbers of neutrophils in their airway sample (J Allergy Clin Immunol. 2000;105:1169-77). This means that asthma exacerbation triggers neutrophilic inflammation, which relates to the induction of cytokines and chemokines in the upper airway. “We have also demonstrated increased circulation of G-CSF [granulocyte–colony stimulating factor] and nasal IL-8 in the upper airway related to increased neutrophil recruitment,” Dr. Jarjour said.
Host factors associated with asthma exacerbations include altered innate immune response in the form of a defect in production of antiviral cytokines in response to viral infection, and a greater T helper (Th)2/Th1 ratio. Other factors include eosinophilic inflammation and greater levels of specific IgE to dust mite. Baseline data from 709 patients enrolled in the National Heart, Lung, and Blood Institute Severe Asthma Research Program III (SARP-3) showed that three top risk factors for asthma exacerbations are gastroesophageal reflux disease (relative risk (RR) 1.6), greater blood eosinophil count (RR 1.6), and obesity (RR 1.2) (Am J Resp Care Med. 2017; 195:302-13).
Risk factors for exacerbation noted in various epidemiological studies include African American and Hispanic races, poor access to medical care, inadequate chronic control, smoking, allergen sensitivity to cats and dogs, gastroesophageal reflux disease, high body mass index, sinusitis, and uncontrolled eosinophilic inflammation. Key findings from a follow-up study of SARP-3 patients include the fact that the absence of exacerbations on a 12-month recall predicted future stability.
“More importantly, participants with severe disease and two or more exacerbations at baseline had a 75% chance of having an exacerbation in the following year,” Dr. Jarjour said. “This is a call for us to pay more close attention to these patients and is an important fact to keep in mind when designing clinical trials. A history of exacerbations is the best predictor of future exacerbation.”
Dr. Jarjour disclosed that he has received research funding from the National Heart, Lung, and Blood Institute and consulting fees from AstraZeneca and Teva Pharmaceutical. He is a member of the American Board of Internal Medicine–Pulmonary Exam Subcommittee.