User login
2023 Update on contraception
More US women are using IUDs than ever before. With more use comes the potential for complications and more requests related to non-contraceptive benefits. New information provides contemporary insight into rare IUD complications and the use of hormonal IUDs for treatment of HMB.
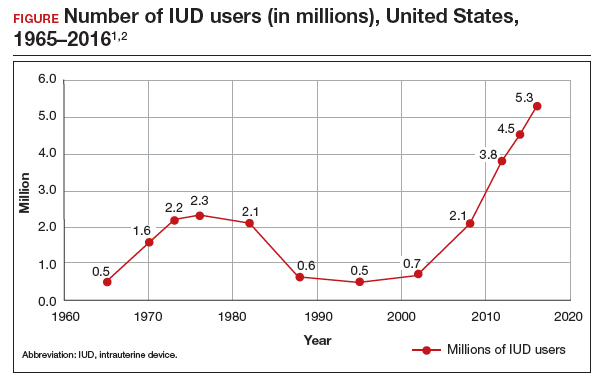
The first intrauterine device (IUD) to be approved in the United States, the Lippes Loop, became available in 1964. Sixty years later, more US women are using IUDs than ever before, and numbers are trending upward (FIGURE).1,2 Over the past year, contemporary information has become available to further inform IUD management when pregnancy occurs with an IUD in situ, as well as counseling about device breakage. Additionally, new data help clinicians expand which patients can use a levonorgestrel (LNG) 52-mg IUD for heavy menstrual bleeding (HMB) treatment.
As the total absolute number of IUD users increases, so do the absolute numbers of rare outcomes, such as pregnancy among IUD users. These highly effective contraceptives have a failure rate within the first year after placement ranging from 0.1% for the LNG 52-mg IUD to 0.8% for the copper 380-mm2 IUD.3 Although the possibility for extrauterine gestation is higher when pregnancy occurs while a patient is using an IUD as compared with most other contraceptive methods, most pregnancies that occur with an IUD in situ are intrauterine.4
The high contraceptive efficacy of IUDs make pregnancy with a retained IUD rare; therefore, it is difficult to perform a study with a large enough population to evaluate management of pregnancy complicated by an IUD in situ. Clinical management recommendations for these situations are 20 years old and are supported by limited data from case reports and series with fewer than 200 patients.5,6
Intrauterine device breakage is another rare event that is poorly understood due to the low absolute number of cases. Information about breakage has similarly been limited to case reports and case series.7,8 This past year, contemporary data were published to provide more insight into both intrauterine pregnancy with an IUD in situ and IUD breakage.
Beyond contraception, hormonal IUDs have become a popular and evidence-based treatment option for patients with HMB. The initial LNG 52-mg IUD (Mirena) regulatory approval studies for HMB treatment included data limited to parous patients and users with a body mass index (BMI) less than 35 kg/m2.9 Since that time, no studies have explored these populations. Although current practice has commonly extended use to include patients with these characteristics, we have lacked outcome data. New phase 3 data on the LNG 52-mg IUD (Liletta) included a broader range of participants and provide evidence to support this practice.
Removing retained copper 380-mm2 IUDs improves pregnancy outcomes
Panchal VR, Rau AR, Mandelbaum RS, et al. Pregnancy with retained intrauterine device: national-level assessment of characteristics and outcomes. Am J Obstet Gynecol MFM. 2023;5:101056. doi:10.1016/j.ajogmf.2023.101056
Karakuş SS, Karakuş R, Akalın EE, et al. Pregnancy outcomes with a copper 380 mm2 intrauterine device in place: a retrospective cohort study in Turkey, 2011-2021. Contraception. 2023;125:110090. doi:10.1016/j.contraception.2023.110090
To update our understanding of outcomes of pregnancy with an IUD in situ, Panchal and colleagues performed a cross-sectional study using the Healthcare Cost and Utilization Project’s National Inpatient Sample. This data set represents 85% of US hospital discharges. The population investigated included hospital deliveries from 2016 to 2020 with an ICD-10 (International Classification of Diseases, Tenth Revision) code of retained IUD. Those without the code were assigned to the comparison non-retained IUD group.
The primary outcome studied was the incidence rate of retained IUD, patient and pregnancy characteristics, and delivery outcomes including but not limited to gestational age at delivery, placental abnormalities, intrauterine fetal demise (IUFD), preterm premature rupture of membranes (PPROM), cesarean delivery, postpartum hemorrhage, and hysterectomy.
Outcomes were worse with retained IUD, regardless of IUD removal status
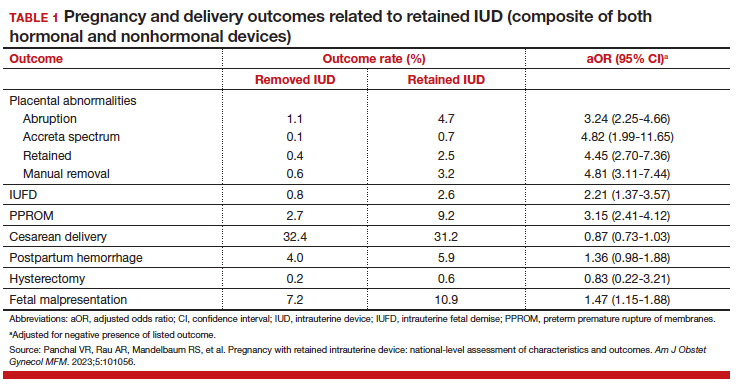
The authors found that an IUD in situ was reported in 1 out of 8,307 pregnancies and was associated with PPROM, fetal malpresentation, IUFD, placental abnormalities including abruption, accreta spectrum, retained placenta, and need for manual removal (TABLE 1). About three-quarters (76.3%) of patients had a term delivery (≥37 weeks).
Retained IUD was associated with previable loss, defined as less than 22 weeks’ gestation (adjusted odds ratio [aOR], 5.49; 95% confidence interval [CI], 3.30–9.15) and periviable delivery, defined as 22 to 25 weeks’ gestation (aOR, 2.81; 95% CI, 1.63–4.85). Retained IUD was not associated with preterm delivery beyond 26 weeks’ gestation, cesarean delivery, postpartum hemorrhage, or hysterectomy.
Important limitations of this study are the lack of information on IUD type (copper vs hormonal) and the timing of removal or attempted removal in relation to measured pregnancy outcomes.
Continue to: Removal of copper IUD improves, but does not eliminate, poor pregnancy outcomes...
Removal of copper IUD improves, but does not eliminate, poor pregnancy outcomes
Karakus and colleagues conducted a retrospective cohort study of 233 patients in Turkey with pregnancies that occurred during copper 380-mm2 IUD use from 2011 to 2021. The authors reported that, at the time of first contact with the health system and diagnosis of retained IUD, 18.9% of the pregnancies were ectopic, 13.2% were first trimester losses, and 67.5% were ongoing pregnancies.
The authors assessed outcomes in patients with ongoing pregnancies based on whether or not the IUD was removed or retained. Outcomes included gestational age at delivery and adverse pregnancy outcomes, assessed as a composite of preterm delivery, PPROM, chorioamnionitis, placental abruption, and postpartum hemorrhage.
Of those with ongoing pregnancies, 13.3% chose to have an abortion, leaving 137 (86.7%) with continuing pregnancy. The IUD was able to be removed in 39.4% of the sample, with an average gestational age of 7 weeks at the time of removal.
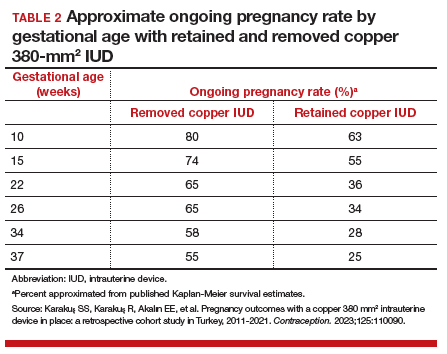
Compared with those with a retained IUD, patients in the removal group had a lower rate of pregnancy loss (33.3% vs 61.4%; P<.001) and a lower rate of the composite adverse pregnancy outcomes (53.1% vs 27.8%; P=.03). TABLE 2 shows the approximate rate of ongoing pregnancy by gestational age in patients with retained and removed copper 380-mm2 IUDs. Notably, the largest change occurred periviably, with the proportion of patients with an ongoing pregnancy after 26 weeks reducing to about half for patients with a retained IUD as compared with patients with a removed IUD; this proportion of ongoing pregnancies held through the remainder of gestation.
These studies confirm that a retained IUD is a rare outcome, occurring in about 1 in 8,000 pregnancies. Previous US national data from 2010 reported a similar incidence of 1 in 6,203 pregnancies (0.02%).10 Management and counseling depend on the patient’s desire to continue the pregnancy, gestational age, intrauterine IUD location, and ability to see the IUD strings. Contemporary data support management practices created from limited and outdated data, which include device removal (if able) and counseling those who desire to continue pregnancy about high-risk pregnancy complications. Those with a retained IUD should be counseled about increased risk of preterm or previable delivery, IUFD, and placental abnormalities (including accreta spectrum and retained placenta). Specifically, these contemporary data highlight that, beyond approximately 26 weeks’ gestation, the pregnancy loss rate is not different for those with a retained or removed IUD. Obstetric care providers should feel confident in using this more nuanced risk of extreme preterm delivery when counseling future patients. Implications for antepartum care and delivery timing with a retained IUD have not yet been defined.
Do national data reveal more breakage reports for copper 380-mm2 or LNG IUDs?
Latack KR, Nguyen BT. Trends in copper versus hormonal intrauterine device breakage reporting within the United States’ Food and Drug Administration Adverse Event Reporting System. Contraception. 2023;118:109909. doi:10.1016/j.contraception.2022.10.011
Latack and Nguyen reviewed postmarket surveillance data of IUD adverse events in the US Food and Drug Administration’s (FDA) Adverse Event Reporting System (FAERS) from 1998 to 2022. The FAERS is a voluntary, or passive, reporting system.
Study findings
Of the approximately 170,000 IUD-related adverse events reported to the agency during the 24-year timeframe, 25.4% were for copper IUDs and 74.6% were for hormonal IUDs. Slightly more than 4,000 reports were specific for device breakage, which the authors grouped into copper (copper 380-mm2)and hormonal (LNG 52 mg, 19.5 mg, and 13.5 mg) IUDs.
The copper 380-mm2 IUD was 6.19 times more likely to have a breakage report than hormonal IUDs (9.6% vs 1.7%; 95% CI, 5.87–6.53).
The overall proportion of IUD-related adverse events reported to the FDA was about 25% for copper and 75% for hormonal IUDs; this proportion is similar to sales figures, which show that about 15% of IUDs sold in the United States are copper and 85% are hormonal.11 However, the proportion of breakage events reported to the FDA is the inverse, with about 6 times more breakage reports with copper than with hormonal IUDs. Because these data come from a passive reporting system, the true incidence of IUD breakage cannot be assessed. However, these findings should remind clinicians to inform patients about this rare occurrence during counseling at the time of placement and, especially, when preparing for copper IUD removal. As the absolute number of IUD users increases, clinicians may be more likely to encounter this relatively rare event.
Management of IUD breakage is based on expert opinion, and recommendations are varied, ranging from observation to removal using an IUD hook, alligator forceps, manual vacuum aspiration, or hysteroscopy.7,10 Importantly, each individual patient situation will vary depending on the presence or absence of other symptoms and whether or not future pregnancy is desired.
Continue to: Data support the LNG 52-mg IUD for HMB in nulliparous and obese patients...
Data support the LNG 52-mg IUD for HMB in nulliparous and obese patients
Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi:10.1097AOG.0000000000005137
Creinin and colleagues conducted a study for US regulatory product approval of the LNG 52-mg IUD (Liletta) for HMB. This multicenter phase 3 open-label clinical trial recruited nonpregnant participants aged 18 to 50 years with HMB at 29 clinical sites in the United States. No BMI cutoff was used.
Baseline menstrual flow data were obtained over 2 to 3 screening cycles by collection of menstrual products and quantification of blood loss using alkaline hematin measurement. Patients with 2 cycles with a blood loss exceeding 80 mL had an IUD placement, with similar flow evaluations during the third and sixth postplacement cycles.
Treatment success was defined as a reduction in blood loss by more than 50% as compared with baseline (during screening) and measured blood loss of less than 80 mL. The enrolled population (n=105) included 28% nulliparous users, with 49% and 28% of participants having a BMI of 30 kg/m2 or higher and higher than 35 kg/m2, respectively.
Treatment highly successful in reducing blood loss
Participants in this trial had a 93% and a 98% reduction in blood loss at the third and sixth cycles of use, respectively. Additionally, during the sixth cycle of use, 19% of users had no bleeding. Treatment success occurred in about 80% of participants overall and occurred regardless of parity or BMI.
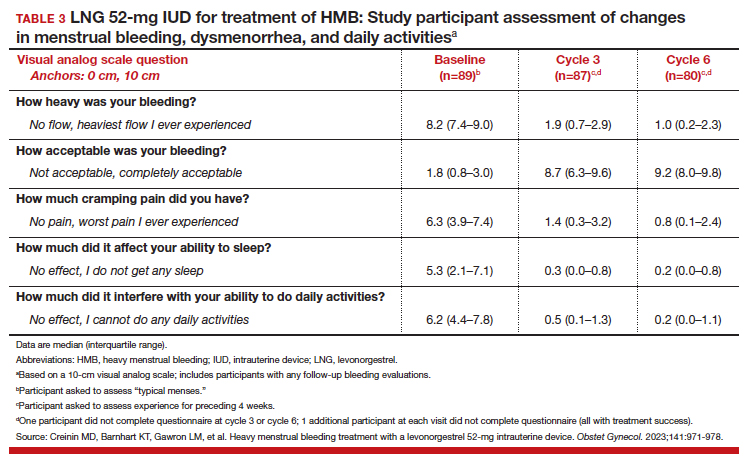
To assess a subjective measure of success, participants were asked to evaluate their menstrual bleeding and dysmenorrhea severity, acceptability, and overall impact on quality of life at 3 time points: during prior typical menses, cycle 3, and cycle 6. At cycle 6, all participants reported significantly improved acceptability of bleeding and uterine pain and, importantly, decreased overall menstrual interference with the ability to complete daily activities (TABLE 3).
IUD expulsion and replacement rates
Although bleeding greatly decreased in all participants, 13% (n=14) discontinued before cycle 6 due to expulsion or IUD-related symptoms, with the majority citing bleeding irregularities. Expulsion occurred in 9% (n=5) of users, with the majority (2/3) occurring in the first 3 months of use and more commonly in obese and/or parous users. About half of participants with expulsion had the IUD replaced during the study. ●
Interestingly, both LNG 52-mg IUDs have been approved in most countries throughout the world for HMB treatment, and only in the United States was one of the products (Liletta) not approved until this past year. The FDA required more stringent trials than had been previously performed for approval outside of the United States. However, a benefit for clinicians is that this phase 3 study provided data in a contemporary US population. Clinicians can feel confident in counseling and offering the LNG 52-mg IUD as a first-line treatment option for patients with HMB, including those who have never been pregnant or have a BMI greater than 35 kg/m2.
Importantly, though, clinicians should be realistic with all patients that this treatment, although highly effective, is not successful for about 20% of patients by about 6 months of use. For those in whom the treatment is beneficial, the quality-of-life improvement is dramatic. Additionally, this study reminds us that expulsion risk in a population primarily using the IUD for HMB, especially if also obese and/or parous, is higher in the first 6 months of use than patients using the method for contraception. Expulsion occurs in 1.6% of contraception users through 6 months of use.12 These data highlight that IUD expulsion risk is not a fixed number, but instead is modified by patient characteristics. Patients should be counseled regarding the appropriate expulsion risk and that the IUD can be safely replaced should expulsion occur.
- Hubacher D, Kavanaugh M. Historical record-setting trends in IUD use in the United States. Contraception. 2018;98:467470. doi:10.1016/j.contraception.2018.05.016
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93. doi:10.1016/j.xfre.2020.06.006
- Jensen JT, Creinin MD. Speroff & Darney’s Clinical Guide to Contraception. 6th ed. Lippincott Williams & Wilkins; 2020:15.
- Jensen JT, Creinin MD. Speroff & Darney’s Clinical Guide to Contraception. 6th ed. Lippincott Williams & Wilkins; 2020:185.
- Ozgu-Erdinc AS, Tasdemir UG, Uygur D, et al. Outcome of intrauterine pregnancies with intrauterine device in place and effects of device location on prognosis. Contraception. 2014;89:426-430. doi:10.1016/j.contraception.2014.01.002
- Brahmi D, Steenland MW, Renner RM, et al. Pregnancy outcomes with an IUD in situ: a systematic review. Contraception. 2012;85:131-139. doi:10.1016/j.contraception . 2011.06.010
- Wilson S, Tan G, Baylson M, et al. Controversies in family planning: how to manage a fractured IUD. Contraception. 2013;88:599-603. doi:10.1016/j.contraception.2013.07.007
- Fulkerson Schaeffer S, Gimovsky AC, Aly H, et al. Pregnancy and delivery with an intrauterine device in situ: outcomes in the National Inpatient Sample Database. J Matern Fetal Neonatal Med. 2019;32:798-803. doi:10.1080/14767058.2017.1 391783
- Mirena. Prescribing information. Bayer HealthCare Pharmaceuticals. Accessed August 22, 2023. https://www .mirena-us.com/pi
- Myo MG, Nguyen BT. Intrauterine device complications and their management. Curr Obstet Gynecol Rep. 2023;12:88-95. doi.org/10.1007/s13669-023-00357-8
- National Center for Health Statistics (NCHS). 2017-2019 National Survey of Family Growth. Public-Use Data File Documentation. CDC National Center for Health Statistics. Accessed August 28, 2023. https://www.cdc.gov/nchs/data /nsfg/NSFG-2017-2019-UG-MainText-508.pdf
- Gilliam ML, Jensen JT, Eisenberg DL, et al. Relationship of parity and prior cesarean delivery to levonorgestrel 52 mg intrauterine system expulsion over 6 years. Contraception. 2021;103:444-449. doi: 10.1016/j.contraception.2021.02.013
More US women are using IUDs than ever before. With more use comes the potential for complications and more requests related to non-contraceptive benefits. New information provides contemporary insight into rare IUD complications and the use of hormonal IUDs for treatment of HMB.
The first intrauterine device (IUD) to be approved in the United States, the Lippes Loop, became available in 1964. Sixty years later, more US women are using IUDs than ever before, and numbers are trending upward (FIGURE).1,2 Over the past year, contemporary information has become available to further inform IUD management when pregnancy occurs with an IUD in situ, as well as counseling about device breakage. Additionally, new data help clinicians expand which patients can use a levonorgestrel (LNG) 52-mg IUD for heavy menstrual bleeding (HMB) treatment.

As the total absolute number of IUD users increases, so do the absolute numbers of rare outcomes, such as pregnancy among IUD users. These highly effective contraceptives have a failure rate within the first year after placement ranging from 0.1% for the LNG 52-mg IUD to 0.8% for the copper 380-mm2 IUD.3 Although the possibility for extrauterine gestation is higher when pregnancy occurs while a patient is using an IUD as compared with most other contraceptive methods, most pregnancies that occur with an IUD in situ are intrauterine.4
The high contraceptive efficacy of IUDs make pregnancy with a retained IUD rare; therefore, it is difficult to perform a study with a large enough population to evaluate management of pregnancy complicated by an IUD in situ. Clinical management recommendations for these situations are 20 years old and are supported by limited data from case reports and series with fewer than 200 patients.5,6
Intrauterine device breakage is another rare event that is poorly understood due to the low absolute number of cases. Information about breakage has similarly been limited to case reports and case series.7,8 This past year, contemporary data were published to provide more insight into both intrauterine pregnancy with an IUD in situ and IUD breakage.
Beyond contraception, hormonal IUDs have become a popular and evidence-based treatment option for patients with HMB. The initial LNG 52-mg IUD (Mirena) regulatory approval studies for HMB treatment included data limited to parous patients and users with a body mass index (BMI) less than 35 kg/m2.9 Since that time, no studies have explored these populations. Although current practice has commonly extended use to include patients with these characteristics, we have lacked outcome data. New phase 3 data on the LNG 52-mg IUD (Liletta) included a broader range of participants and provide evidence to support this practice.
Removing retained copper 380-mm2 IUDs improves pregnancy outcomes
Panchal VR, Rau AR, Mandelbaum RS, et al. Pregnancy with retained intrauterine device: national-level assessment of characteristics and outcomes. Am J Obstet Gynecol MFM. 2023;5:101056. doi:10.1016/j.ajogmf.2023.101056
Karakuş SS, Karakuş R, Akalın EE, et al. Pregnancy outcomes with a copper 380 mm2 intrauterine device in place: a retrospective cohort study in Turkey, 2011-2021. Contraception. 2023;125:110090. doi:10.1016/j.contraception.2023.110090
To update our understanding of outcomes of pregnancy with an IUD in situ, Panchal and colleagues performed a cross-sectional study using the Healthcare Cost and Utilization Project’s National Inpatient Sample. This data set represents 85% of US hospital discharges. The population investigated included hospital deliveries from 2016 to 2020 with an ICD-10 (International Classification of Diseases, Tenth Revision) code of retained IUD. Those without the code were assigned to the comparison non-retained IUD group.
The primary outcome studied was the incidence rate of retained IUD, patient and pregnancy characteristics, and delivery outcomes including but not limited to gestational age at delivery, placental abnormalities, intrauterine fetal demise (IUFD), preterm premature rupture of membranes (PPROM), cesarean delivery, postpartum hemorrhage, and hysterectomy.
Outcomes were worse with retained IUD, regardless of IUD removal status
The authors found that an IUD in situ was reported in 1 out of 8,307 pregnancies and was associated with PPROM, fetal malpresentation, IUFD, placental abnormalities including abruption, accreta spectrum, retained placenta, and need for manual removal (TABLE 1). About three-quarters (76.3%) of patients had a term delivery (≥37 weeks).

Retained IUD was associated with previable loss, defined as less than 22 weeks’ gestation (adjusted odds ratio [aOR], 5.49; 95% confidence interval [CI], 3.30–9.15) and periviable delivery, defined as 22 to 25 weeks’ gestation (aOR, 2.81; 95% CI, 1.63–4.85). Retained IUD was not associated with preterm delivery beyond 26 weeks’ gestation, cesarean delivery, postpartum hemorrhage, or hysterectomy.
Important limitations of this study are the lack of information on IUD type (copper vs hormonal) and the timing of removal or attempted removal in relation to measured pregnancy outcomes.
Continue to: Removal of copper IUD improves, but does not eliminate, poor pregnancy outcomes...
Removal of copper IUD improves, but does not eliminate, poor pregnancy outcomes
Karakus and colleagues conducted a retrospective cohort study of 233 patients in Turkey with pregnancies that occurred during copper 380-mm2 IUD use from 2011 to 2021. The authors reported that, at the time of first contact with the health system and diagnosis of retained IUD, 18.9% of the pregnancies were ectopic, 13.2% were first trimester losses, and 67.5% were ongoing pregnancies.
The authors assessed outcomes in patients with ongoing pregnancies based on whether or not the IUD was removed or retained. Outcomes included gestational age at delivery and adverse pregnancy outcomes, assessed as a composite of preterm delivery, PPROM, chorioamnionitis, placental abruption, and postpartum hemorrhage.
Of those with ongoing pregnancies, 13.3% chose to have an abortion, leaving 137 (86.7%) with continuing pregnancy. The IUD was able to be removed in 39.4% of the sample, with an average gestational age of 7 weeks at the time of removal.
Compared with those with a retained IUD, patients in the removal group had a lower rate of pregnancy loss (33.3% vs 61.4%; P<.001) and a lower rate of the composite adverse pregnancy outcomes (53.1% vs 27.8%; P=.03). TABLE 2 shows the approximate rate of ongoing pregnancy by gestational age in patients with retained and removed copper 380-mm2 IUDs. Notably, the largest change occurred periviably, with the proportion of patients with an ongoing pregnancy after 26 weeks reducing to about half for patients with a retained IUD as compared with patients with a removed IUD; this proportion of ongoing pregnancies held through the remainder of gestation.

These studies confirm that a retained IUD is a rare outcome, occurring in about 1 in 8,000 pregnancies. Previous US national data from 2010 reported a similar incidence of 1 in 6,203 pregnancies (0.02%).10 Management and counseling depend on the patient’s desire to continue the pregnancy, gestational age, intrauterine IUD location, and ability to see the IUD strings. Contemporary data support management practices created from limited and outdated data, which include device removal (if able) and counseling those who desire to continue pregnancy about high-risk pregnancy complications. Those with a retained IUD should be counseled about increased risk of preterm or previable delivery, IUFD, and placental abnormalities (including accreta spectrum and retained placenta). Specifically, these contemporary data highlight that, beyond approximately 26 weeks’ gestation, the pregnancy loss rate is not different for those with a retained or removed IUD. Obstetric care providers should feel confident in using this more nuanced risk of extreme preterm delivery when counseling future patients. Implications for antepartum care and delivery timing with a retained IUD have not yet been defined.
Do national data reveal more breakage reports for copper 380-mm2 or LNG IUDs?
Latack KR, Nguyen BT. Trends in copper versus hormonal intrauterine device breakage reporting within the United States’ Food and Drug Administration Adverse Event Reporting System. Contraception. 2023;118:109909. doi:10.1016/j.contraception.2022.10.011
Latack and Nguyen reviewed postmarket surveillance data of IUD adverse events in the US Food and Drug Administration’s (FDA) Adverse Event Reporting System (FAERS) from 1998 to 2022. The FAERS is a voluntary, or passive, reporting system.
Study findings
Of the approximately 170,000 IUD-related adverse events reported to the agency during the 24-year timeframe, 25.4% were for copper IUDs and 74.6% were for hormonal IUDs. Slightly more than 4,000 reports were specific for device breakage, which the authors grouped into copper (copper 380-mm2)and hormonal (LNG 52 mg, 19.5 mg, and 13.5 mg) IUDs.
The copper 380-mm2 IUD was 6.19 times more likely to have a breakage report than hormonal IUDs (9.6% vs 1.7%; 95% CI, 5.87–6.53).
The overall proportion of IUD-related adverse events reported to the FDA was about 25% for copper and 75% for hormonal IUDs; this proportion is similar to sales figures, which show that about 15% of IUDs sold in the United States are copper and 85% are hormonal.11 However, the proportion of breakage events reported to the FDA is the inverse, with about 6 times more breakage reports with copper than with hormonal IUDs. Because these data come from a passive reporting system, the true incidence of IUD breakage cannot be assessed. However, these findings should remind clinicians to inform patients about this rare occurrence during counseling at the time of placement and, especially, when preparing for copper IUD removal. As the absolute number of IUD users increases, clinicians may be more likely to encounter this relatively rare event.
Management of IUD breakage is based on expert opinion, and recommendations are varied, ranging from observation to removal using an IUD hook, alligator forceps, manual vacuum aspiration, or hysteroscopy.7,10 Importantly, each individual patient situation will vary depending on the presence or absence of other symptoms and whether or not future pregnancy is desired.
Continue to: Data support the LNG 52-mg IUD for HMB in nulliparous and obese patients...
Data support the LNG 52-mg IUD for HMB in nulliparous and obese patients
Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi:10.1097AOG.0000000000005137
Creinin and colleagues conducted a study for US regulatory product approval of the LNG 52-mg IUD (Liletta) for HMB. This multicenter phase 3 open-label clinical trial recruited nonpregnant participants aged 18 to 50 years with HMB at 29 clinical sites in the United States. No BMI cutoff was used.
Baseline menstrual flow data were obtained over 2 to 3 screening cycles by collection of menstrual products and quantification of blood loss using alkaline hematin measurement. Patients with 2 cycles with a blood loss exceeding 80 mL had an IUD placement, with similar flow evaluations during the third and sixth postplacement cycles.
Treatment success was defined as a reduction in blood loss by more than 50% as compared with baseline (during screening) and measured blood loss of less than 80 mL. The enrolled population (n=105) included 28% nulliparous users, with 49% and 28% of participants having a BMI of 30 kg/m2 or higher and higher than 35 kg/m2, respectively.
Treatment highly successful in reducing blood loss
Participants in this trial had a 93% and a 98% reduction in blood loss at the third and sixth cycles of use, respectively. Additionally, during the sixth cycle of use, 19% of users had no bleeding. Treatment success occurred in about 80% of participants overall and occurred regardless of parity or BMI.
To assess a subjective measure of success, participants were asked to evaluate their menstrual bleeding and dysmenorrhea severity, acceptability, and overall impact on quality of life at 3 time points: during prior typical menses, cycle 3, and cycle 6. At cycle 6, all participants reported significantly improved acceptability of bleeding and uterine pain and, importantly, decreased overall menstrual interference with the ability to complete daily activities (TABLE 3).

IUD expulsion and replacement rates
Although bleeding greatly decreased in all participants, 13% (n=14) discontinued before cycle 6 due to expulsion or IUD-related symptoms, with the majority citing bleeding irregularities. Expulsion occurred in 9% (n=5) of users, with the majority (2/3) occurring in the first 3 months of use and more commonly in obese and/or parous users. About half of participants with expulsion had the IUD replaced during the study. ●
Interestingly, both LNG 52-mg IUDs have been approved in most countries throughout the world for HMB treatment, and only in the United States was one of the products (Liletta) not approved until this past year. The FDA required more stringent trials than had been previously performed for approval outside of the United States. However, a benefit for clinicians is that this phase 3 study provided data in a contemporary US population. Clinicians can feel confident in counseling and offering the LNG 52-mg IUD as a first-line treatment option for patients with HMB, including those who have never been pregnant or have a BMI greater than 35 kg/m2.
Importantly, though, clinicians should be realistic with all patients that this treatment, although highly effective, is not successful for about 20% of patients by about 6 months of use. For those in whom the treatment is beneficial, the quality-of-life improvement is dramatic. Additionally, this study reminds us that expulsion risk in a population primarily using the IUD for HMB, especially if also obese and/or parous, is higher in the first 6 months of use than patients using the method for contraception. Expulsion occurs in 1.6% of contraception users through 6 months of use.12 These data highlight that IUD expulsion risk is not a fixed number, but instead is modified by patient characteristics. Patients should be counseled regarding the appropriate expulsion risk and that the IUD can be safely replaced should expulsion occur.
More US women are using IUDs than ever before. With more use comes the potential for complications and more requests related to non-contraceptive benefits. New information provides contemporary insight into rare IUD complications and the use of hormonal IUDs for treatment of HMB.
The first intrauterine device (IUD) to be approved in the United States, the Lippes Loop, became available in 1964. Sixty years later, more US women are using IUDs than ever before, and numbers are trending upward (FIGURE).1,2 Over the past year, contemporary information has become available to further inform IUD management when pregnancy occurs with an IUD in situ, as well as counseling about device breakage. Additionally, new data help clinicians expand which patients can use a levonorgestrel (LNG) 52-mg IUD for heavy menstrual bleeding (HMB) treatment.

As the total absolute number of IUD users increases, so do the absolute numbers of rare outcomes, such as pregnancy among IUD users. These highly effective contraceptives have a failure rate within the first year after placement ranging from 0.1% for the LNG 52-mg IUD to 0.8% for the copper 380-mm2 IUD.3 Although the possibility for extrauterine gestation is higher when pregnancy occurs while a patient is using an IUD as compared with most other contraceptive methods, most pregnancies that occur with an IUD in situ are intrauterine.4
The high contraceptive efficacy of IUDs make pregnancy with a retained IUD rare; therefore, it is difficult to perform a study with a large enough population to evaluate management of pregnancy complicated by an IUD in situ. Clinical management recommendations for these situations are 20 years old and are supported by limited data from case reports and series with fewer than 200 patients.5,6
Intrauterine device breakage is another rare event that is poorly understood due to the low absolute number of cases. Information about breakage has similarly been limited to case reports and case series.7,8 This past year, contemporary data were published to provide more insight into both intrauterine pregnancy with an IUD in situ and IUD breakage.
Beyond contraception, hormonal IUDs have become a popular and evidence-based treatment option for patients with HMB. The initial LNG 52-mg IUD (Mirena) regulatory approval studies for HMB treatment included data limited to parous patients and users with a body mass index (BMI) less than 35 kg/m2.9 Since that time, no studies have explored these populations. Although current practice has commonly extended use to include patients with these characteristics, we have lacked outcome data. New phase 3 data on the LNG 52-mg IUD (Liletta) included a broader range of participants and provide evidence to support this practice.
Removing retained copper 380-mm2 IUDs improves pregnancy outcomes
Panchal VR, Rau AR, Mandelbaum RS, et al. Pregnancy with retained intrauterine device: national-level assessment of characteristics and outcomes. Am J Obstet Gynecol MFM. 2023;5:101056. doi:10.1016/j.ajogmf.2023.101056
Karakuş SS, Karakuş R, Akalın EE, et al. Pregnancy outcomes with a copper 380 mm2 intrauterine device in place: a retrospective cohort study in Turkey, 2011-2021. Contraception. 2023;125:110090. doi:10.1016/j.contraception.2023.110090
To update our understanding of outcomes of pregnancy with an IUD in situ, Panchal and colleagues performed a cross-sectional study using the Healthcare Cost and Utilization Project’s National Inpatient Sample. This data set represents 85% of US hospital discharges. The population investigated included hospital deliveries from 2016 to 2020 with an ICD-10 (International Classification of Diseases, Tenth Revision) code of retained IUD. Those without the code were assigned to the comparison non-retained IUD group.
The primary outcome studied was the incidence rate of retained IUD, patient and pregnancy characteristics, and delivery outcomes including but not limited to gestational age at delivery, placental abnormalities, intrauterine fetal demise (IUFD), preterm premature rupture of membranes (PPROM), cesarean delivery, postpartum hemorrhage, and hysterectomy.
Outcomes were worse with retained IUD, regardless of IUD removal status
The authors found that an IUD in situ was reported in 1 out of 8,307 pregnancies and was associated with PPROM, fetal malpresentation, IUFD, placental abnormalities including abruption, accreta spectrum, retained placenta, and need for manual removal (TABLE 1). About three-quarters (76.3%) of patients had a term delivery (≥37 weeks).

Retained IUD was associated with previable loss, defined as less than 22 weeks’ gestation (adjusted odds ratio [aOR], 5.49; 95% confidence interval [CI], 3.30–9.15) and periviable delivery, defined as 22 to 25 weeks’ gestation (aOR, 2.81; 95% CI, 1.63–4.85). Retained IUD was not associated with preterm delivery beyond 26 weeks’ gestation, cesarean delivery, postpartum hemorrhage, or hysterectomy.
Important limitations of this study are the lack of information on IUD type (copper vs hormonal) and the timing of removal or attempted removal in relation to measured pregnancy outcomes.
Continue to: Removal of copper IUD improves, but does not eliminate, poor pregnancy outcomes...
Removal of copper IUD improves, but does not eliminate, poor pregnancy outcomes
Karakus and colleagues conducted a retrospective cohort study of 233 patients in Turkey with pregnancies that occurred during copper 380-mm2 IUD use from 2011 to 2021. The authors reported that, at the time of first contact with the health system and diagnosis of retained IUD, 18.9% of the pregnancies were ectopic, 13.2% were first trimester losses, and 67.5% were ongoing pregnancies.
The authors assessed outcomes in patients with ongoing pregnancies based on whether or not the IUD was removed or retained. Outcomes included gestational age at delivery and adverse pregnancy outcomes, assessed as a composite of preterm delivery, PPROM, chorioamnionitis, placental abruption, and postpartum hemorrhage.
Of those with ongoing pregnancies, 13.3% chose to have an abortion, leaving 137 (86.7%) with continuing pregnancy. The IUD was able to be removed in 39.4% of the sample, with an average gestational age of 7 weeks at the time of removal.
Compared with those with a retained IUD, patients in the removal group had a lower rate of pregnancy loss (33.3% vs 61.4%; P<.001) and a lower rate of the composite adverse pregnancy outcomes (53.1% vs 27.8%; P=.03). TABLE 2 shows the approximate rate of ongoing pregnancy by gestational age in patients with retained and removed copper 380-mm2 IUDs. Notably, the largest change occurred periviably, with the proportion of patients with an ongoing pregnancy after 26 weeks reducing to about half for patients with a retained IUD as compared with patients with a removed IUD; this proportion of ongoing pregnancies held through the remainder of gestation.

These studies confirm that a retained IUD is a rare outcome, occurring in about 1 in 8,000 pregnancies. Previous US national data from 2010 reported a similar incidence of 1 in 6,203 pregnancies (0.02%).10 Management and counseling depend on the patient’s desire to continue the pregnancy, gestational age, intrauterine IUD location, and ability to see the IUD strings. Contemporary data support management practices created from limited and outdated data, which include device removal (if able) and counseling those who desire to continue pregnancy about high-risk pregnancy complications. Those with a retained IUD should be counseled about increased risk of preterm or previable delivery, IUFD, and placental abnormalities (including accreta spectrum and retained placenta). Specifically, these contemporary data highlight that, beyond approximately 26 weeks’ gestation, the pregnancy loss rate is not different for those with a retained or removed IUD. Obstetric care providers should feel confident in using this more nuanced risk of extreme preterm delivery when counseling future patients. Implications for antepartum care and delivery timing with a retained IUD have not yet been defined.
Do national data reveal more breakage reports for copper 380-mm2 or LNG IUDs?
Latack KR, Nguyen BT. Trends in copper versus hormonal intrauterine device breakage reporting within the United States’ Food and Drug Administration Adverse Event Reporting System. Contraception. 2023;118:109909. doi:10.1016/j.contraception.2022.10.011
Latack and Nguyen reviewed postmarket surveillance data of IUD adverse events in the US Food and Drug Administration’s (FDA) Adverse Event Reporting System (FAERS) from 1998 to 2022. The FAERS is a voluntary, or passive, reporting system.
Study findings
Of the approximately 170,000 IUD-related adverse events reported to the agency during the 24-year timeframe, 25.4% were for copper IUDs and 74.6% were for hormonal IUDs. Slightly more than 4,000 reports were specific for device breakage, which the authors grouped into copper (copper 380-mm2)and hormonal (LNG 52 mg, 19.5 mg, and 13.5 mg) IUDs.
The copper 380-mm2 IUD was 6.19 times more likely to have a breakage report than hormonal IUDs (9.6% vs 1.7%; 95% CI, 5.87–6.53).
The overall proportion of IUD-related adverse events reported to the FDA was about 25% for copper and 75% for hormonal IUDs; this proportion is similar to sales figures, which show that about 15% of IUDs sold in the United States are copper and 85% are hormonal.11 However, the proportion of breakage events reported to the FDA is the inverse, with about 6 times more breakage reports with copper than with hormonal IUDs. Because these data come from a passive reporting system, the true incidence of IUD breakage cannot be assessed. However, these findings should remind clinicians to inform patients about this rare occurrence during counseling at the time of placement and, especially, when preparing for copper IUD removal. As the absolute number of IUD users increases, clinicians may be more likely to encounter this relatively rare event.
Management of IUD breakage is based on expert opinion, and recommendations are varied, ranging from observation to removal using an IUD hook, alligator forceps, manual vacuum aspiration, or hysteroscopy.7,10 Importantly, each individual patient situation will vary depending on the presence or absence of other symptoms and whether or not future pregnancy is desired.
Continue to: Data support the LNG 52-mg IUD for HMB in nulliparous and obese patients...
Data support the LNG 52-mg IUD for HMB in nulliparous and obese patients
Creinin MD, Barnhart KT, Gawron LM, et al. Heavy menstrual bleeding treatment with a levonorgestrel 52-mg intrauterine device. Obstet Gynecol. 2023;141:971-978. doi:10.1097AOG.0000000000005137
Creinin and colleagues conducted a study for US regulatory product approval of the LNG 52-mg IUD (Liletta) for HMB. This multicenter phase 3 open-label clinical trial recruited nonpregnant participants aged 18 to 50 years with HMB at 29 clinical sites in the United States. No BMI cutoff was used.
Baseline menstrual flow data were obtained over 2 to 3 screening cycles by collection of menstrual products and quantification of blood loss using alkaline hematin measurement. Patients with 2 cycles with a blood loss exceeding 80 mL had an IUD placement, with similar flow evaluations during the third and sixth postplacement cycles.
Treatment success was defined as a reduction in blood loss by more than 50% as compared with baseline (during screening) and measured blood loss of less than 80 mL. The enrolled population (n=105) included 28% nulliparous users, with 49% and 28% of participants having a BMI of 30 kg/m2 or higher and higher than 35 kg/m2, respectively.
Treatment highly successful in reducing blood loss
Participants in this trial had a 93% and a 98% reduction in blood loss at the third and sixth cycles of use, respectively. Additionally, during the sixth cycle of use, 19% of users had no bleeding. Treatment success occurred in about 80% of participants overall and occurred regardless of parity or BMI.
To assess a subjective measure of success, participants were asked to evaluate their menstrual bleeding and dysmenorrhea severity, acceptability, and overall impact on quality of life at 3 time points: during prior typical menses, cycle 3, and cycle 6. At cycle 6, all participants reported significantly improved acceptability of bleeding and uterine pain and, importantly, decreased overall menstrual interference with the ability to complete daily activities (TABLE 3).

IUD expulsion and replacement rates
Although bleeding greatly decreased in all participants, 13% (n=14) discontinued before cycle 6 due to expulsion or IUD-related symptoms, with the majority citing bleeding irregularities. Expulsion occurred in 9% (n=5) of users, with the majority (2/3) occurring in the first 3 months of use and more commonly in obese and/or parous users. About half of participants with expulsion had the IUD replaced during the study. ●
Interestingly, both LNG 52-mg IUDs have been approved in most countries throughout the world for HMB treatment, and only in the United States was one of the products (Liletta) not approved until this past year. The FDA required more stringent trials than had been previously performed for approval outside of the United States. However, a benefit for clinicians is that this phase 3 study provided data in a contemporary US population. Clinicians can feel confident in counseling and offering the LNG 52-mg IUD as a first-line treatment option for patients with HMB, including those who have never been pregnant or have a BMI greater than 35 kg/m2.
Importantly, though, clinicians should be realistic with all patients that this treatment, although highly effective, is not successful for about 20% of patients by about 6 months of use. For those in whom the treatment is beneficial, the quality-of-life improvement is dramatic. Additionally, this study reminds us that expulsion risk in a population primarily using the IUD for HMB, especially if also obese and/or parous, is higher in the first 6 months of use than patients using the method for contraception. Expulsion occurs in 1.6% of contraception users through 6 months of use.12 These data highlight that IUD expulsion risk is not a fixed number, but instead is modified by patient characteristics. Patients should be counseled regarding the appropriate expulsion risk and that the IUD can be safely replaced should expulsion occur.
- Hubacher D, Kavanaugh M. Historical record-setting trends in IUD use in the United States. Contraception. 2018;98:467470. doi:10.1016/j.contraception.2018.05.016
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93. doi:10.1016/j.xfre.2020.06.006
- Jensen JT, Creinin MD. Speroff & Darney’s Clinical Guide to Contraception. 6th ed. Lippincott Williams & Wilkins; 2020:15.
- Jensen JT, Creinin MD. Speroff & Darney’s Clinical Guide to Contraception. 6th ed. Lippincott Williams & Wilkins; 2020:185.
- Ozgu-Erdinc AS, Tasdemir UG, Uygur D, et al. Outcome of intrauterine pregnancies with intrauterine device in place and effects of device location on prognosis. Contraception. 2014;89:426-430. doi:10.1016/j.contraception.2014.01.002
- Brahmi D, Steenland MW, Renner RM, et al. Pregnancy outcomes with an IUD in situ: a systematic review. Contraception. 2012;85:131-139. doi:10.1016/j.contraception . 2011.06.010
- Wilson S, Tan G, Baylson M, et al. Controversies in family planning: how to manage a fractured IUD. Contraception. 2013;88:599-603. doi:10.1016/j.contraception.2013.07.007
- Fulkerson Schaeffer S, Gimovsky AC, Aly H, et al. Pregnancy and delivery with an intrauterine device in situ: outcomes in the National Inpatient Sample Database. J Matern Fetal Neonatal Med. 2019;32:798-803. doi:10.1080/14767058.2017.1 391783
- Mirena. Prescribing information. Bayer HealthCare Pharmaceuticals. Accessed August 22, 2023. https://www .mirena-us.com/pi
- Myo MG, Nguyen BT. Intrauterine device complications and their management. Curr Obstet Gynecol Rep. 2023;12:88-95. doi.org/10.1007/s13669-023-00357-8
- National Center for Health Statistics (NCHS). 2017-2019 National Survey of Family Growth. Public-Use Data File Documentation. CDC National Center for Health Statistics. Accessed August 28, 2023. https://www.cdc.gov/nchs/data /nsfg/NSFG-2017-2019-UG-MainText-508.pdf
- Gilliam ML, Jensen JT, Eisenberg DL, et al. Relationship of parity and prior cesarean delivery to levonorgestrel 52 mg intrauterine system expulsion over 6 years. Contraception. 2021;103:444-449. doi: 10.1016/j.contraception.2021.02.013
- Hubacher D, Kavanaugh M. Historical record-setting trends in IUD use in the United States. Contraception. 2018;98:467470. doi:10.1016/j.contraception.2018.05.016
- Kavanaugh ML, Pliskin E. Use of contraception among reproductive-aged women in the United States, 2014 and 2016. F S Rep. 2020;1:83-93. doi:10.1016/j.xfre.2020.06.006
- Jensen JT, Creinin MD. Speroff & Darney’s Clinical Guide to Contraception. 6th ed. Lippincott Williams & Wilkins; 2020:15.
- Jensen JT, Creinin MD. Speroff & Darney’s Clinical Guide to Contraception. 6th ed. Lippincott Williams & Wilkins; 2020:185.
- Ozgu-Erdinc AS, Tasdemir UG, Uygur D, et al. Outcome of intrauterine pregnancies with intrauterine device in place and effects of device location on prognosis. Contraception. 2014;89:426-430. doi:10.1016/j.contraception.2014.01.002
- Brahmi D, Steenland MW, Renner RM, et al. Pregnancy outcomes with an IUD in situ: a systematic review. Contraception. 2012;85:131-139. doi:10.1016/j.contraception . 2011.06.010
- Wilson S, Tan G, Baylson M, et al. Controversies in family planning: how to manage a fractured IUD. Contraception. 2013;88:599-603. doi:10.1016/j.contraception.2013.07.007
- Fulkerson Schaeffer S, Gimovsky AC, Aly H, et al. Pregnancy and delivery with an intrauterine device in situ: outcomes in the National Inpatient Sample Database. J Matern Fetal Neonatal Med. 2019;32:798-803. doi:10.1080/14767058.2017.1 391783
- Mirena. Prescribing information. Bayer HealthCare Pharmaceuticals. Accessed August 22, 2023. https://www .mirena-us.com/pi
- Myo MG, Nguyen BT. Intrauterine device complications and their management. Curr Obstet Gynecol Rep. 2023;12:88-95. doi.org/10.1007/s13669-023-00357-8
- National Center for Health Statistics (NCHS). 2017-2019 National Survey of Family Growth. Public-Use Data File Documentation. CDC National Center for Health Statistics. Accessed August 28, 2023. https://www.cdc.gov/nchs/data /nsfg/NSFG-2017-2019-UG-MainText-508.pdf
- Gilliam ML, Jensen JT, Eisenberg DL, et al. Relationship of parity and prior cesarean delivery to levonorgestrel 52 mg intrauterine system expulsion over 6 years. Contraception. 2021;103:444-449. doi: 10.1016/j.contraception.2021.02.013
Next up in MS trials: More insight into progressive disease
MILAN – , neurologist Jeremy Chataway, MD, PhD, of University College London and Queen Square Multiple Sclerosis Center told colleagues at the 9th Joint ECTRIMS-ACTRIMS meeting.
“They’re all very different, and I think that’s exciting,” he said. “It’s a rich trial environment.”
The problem: At a median of almost 3 years in treatment for primary progressive MS, “we know that about a third of patients will progress despite on being on anti-inflammatory treatment. The same is true for secondary progressive MS. That is the hard core of what we have to think about. We want to improve the efficacy gap between control and active.”
First, Dr. Chataway highlighted the MS-STAT2 trial of simvastatin (Zocor), an inexpensive statin used to lower cholesterol. He is one of the leaders of the 3-year, multicenter, double-blind, randomized, placebo-controlled study, which is testing whether 80-mg daily doses of simvastatin will slow MS progression.
As Dr. Chataway noted, an earlier study – MS-STAT1 – found less brain atrophy in patients who took a high dose of the drug, which was “well tolerated and safe.”
Vascular morbidity drives disability and mortality in MS. “This is low-hanging fruit because we have the tools to do something about it,” he said. “There’s an opportunity here to add into our treatment paradigms across people with MS by actively treating their vascular comorbidity. It will have an effect.”
Recruitment for a trial of this approach is complete, and study results are expected in 2024 and 2025, Dr. Chataway said.
Another new study is exploring the possible effects of the antioxidant lipoic acid, also known as alpha-lipoic acid. As Dr. Chataway noted, a 2017 single-center, randomized, double-blind pilot study of daily oral 1,200 mg lipoic acid versus placebo linked the intervention to a dramatic lowering of brain atrophy – by about 50%.
The new LAPMS study, sponsored by the Veterans Administration, will explore whether lipoic acid affects walking ability, clinical outcome, and brain atrophy, Dr. Chataway said. Results from phase 2 are expected in a year or two, he said.
Dr. Chataway also highlighted one of his own trials, the OCTOPUS study, a multiarm, multistage study that will examine multiple drugs to treat progressive MS. It’s starting with metformin and will look at lipoic acid too, he said.
He also noted the phase 2 CALLIPER trial, which has completed enrollment and expects to provide top-line data in 2025. The multicenter, randomized, double-blind, placebo-controlled will test vidofludimus calcium in patients with progressive MS.
Finally, Dr. Chataway highlighted the randomized, double-blind, placebo-controlled, add-on phase 2 NACPMS trial of n-acetyl cysteine and the phase 1 randomized, double-blind, placebo-controlled trial of SAR443820, a central nervous system penetrant oral RIPK1 inhibitor.
Dr. Chataway discloses grants (UK Multiple Sclerosis Society, National Multiple Sclerosis Society, Efficacy and Mechanism Evaluation Board, Health Technology Assessment, Multiple Sclerosis Trials Collaboration, and Rosetrees Trust), advisory board service (Azadyne, Biogen, Lucid, Janssen, Merck, NervGen, Novartis, and Roche), other support (National Institute of Health Research Support, University College London Hospitals Biomedical Research Centers funding scheme), and serving as an trial investigator (Canadian MS Society, Ionis, Novartis, and Roche).
MILAN – , neurologist Jeremy Chataway, MD, PhD, of University College London and Queen Square Multiple Sclerosis Center told colleagues at the 9th Joint ECTRIMS-ACTRIMS meeting.
“They’re all very different, and I think that’s exciting,” he said. “It’s a rich trial environment.”
The problem: At a median of almost 3 years in treatment for primary progressive MS, “we know that about a third of patients will progress despite on being on anti-inflammatory treatment. The same is true for secondary progressive MS. That is the hard core of what we have to think about. We want to improve the efficacy gap between control and active.”
First, Dr. Chataway highlighted the MS-STAT2 trial of simvastatin (Zocor), an inexpensive statin used to lower cholesterol. He is one of the leaders of the 3-year, multicenter, double-blind, randomized, placebo-controlled study, which is testing whether 80-mg daily doses of simvastatin will slow MS progression.
As Dr. Chataway noted, an earlier study – MS-STAT1 – found less brain atrophy in patients who took a high dose of the drug, which was “well tolerated and safe.”
Vascular morbidity drives disability and mortality in MS. “This is low-hanging fruit because we have the tools to do something about it,” he said. “There’s an opportunity here to add into our treatment paradigms across people with MS by actively treating their vascular comorbidity. It will have an effect.”
Recruitment for a trial of this approach is complete, and study results are expected in 2024 and 2025, Dr. Chataway said.
Another new study is exploring the possible effects of the antioxidant lipoic acid, also known as alpha-lipoic acid. As Dr. Chataway noted, a 2017 single-center, randomized, double-blind pilot study of daily oral 1,200 mg lipoic acid versus placebo linked the intervention to a dramatic lowering of brain atrophy – by about 50%.
The new LAPMS study, sponsored by the Veterans Administration, will explore whether lipoic acid affects walking ability, clinical outcome, and brain atrophy, Dr. Chataway said. Results from phase 2 are expected in a year or two, he said.
Dr. Chataway also highlighted one of his own trials, the OCTOPUS study, a multiarm, multistage study that will examine multiple drugs to treat progressive MS. It’s starting with metformin and will look at lipoic acid too, he said.
He also noted the phase 2 CALLIPER trial, which has completed enrollment and expects to provide top-line data in 2025. The multicenter, randomized, double-blind, placebo-controlled will test vidofludimus calcium in patients with progressive MS.
Finally, Dr. Chataway highlighted the randomized, double-blind, placebo-controlled, add-on phase 2 NACPMS trial of n-acetyl cysteine and the phase 1 randomized, double-blind, placebo-controlled trial of SAR443820, a central nervous system penetrant oral RIPK1 inhibitor.
Dr. Chataway discloses grants (UK Multiple Sclerosis Society, National Multiple Sclerosis Society, Efficacy and Mechanism Evaluation Board, Health Technology Assessment, Multiple Sclerosis Trials Collaboration, and Rosetrees Trust), advisory board service (Azadyne, Biogen, Lucid, Janssen, Merck, NervGen, Novartis, and Roche), other support (National Institute of Health Research Support, University College London Hospitals Biomedical Research Centers funding scheme), and serving as an trial investigator (Canadian MS Society, Ionis, Novartis, and Roche).
MILAN – , neurologist Jeremy Chataway, MD, PhD, of University College London and Queen Square Multiple Sclerosis Center told colleagues at the 9th Joint ECTRIMS-ACTRIMS meeting.
“They’re all very different, and I think that’s exciting,” he said. “It’s a rich trial environment.”
The problem: At a median of almost 3 years in treatment for primary progressive MS, “we know that about a third of patients will progress despite on being on anti-inflammatory treatment. The same is true for secondary progressive MS. That is the hard core of what we have to think about. We want to improve the efficacy gap between control and active.”
First, Dr. Chataway highlighted the MS-STAT2 trial of simvastatin (Zocor), an inexpensive statin used to lower cholesterol. He is one of the leaders of the 3-year, multicenter, double-blind, randomized, placebo-controlled study, which is testing whether 80-mg daily doses of simvastatin will slow MS progression.
As Dr. Chataway noted, an earlier study – MS-STAT1 – found less brain atrophy in patients who took a high dose of the drug, which was “well tolerated and safe.”
Vascular morbidity drives disability and mortality in MS. “This is low-hanging fruit because we have the tools to do something about it,” he said. “There’s an opportunity here to add into our treatment paradigms across people with MS by actively treating their vascular comorbidity. It will have an effect.”
Recruitment for a trial of this approach is complete, and study results are expected in 2024 and 2025, Dr. Chataway said.
Another new study is exploring the possible effects of the antioxidant lipoic acid, also known as alpha-lipoic acid. As Dr. Chataway noted, a 2017 single-center, randomized, double-blind pilot study of daily oral 1,200 mg lipoic acid versus placebo linked the intervention to a dramatic lowering of brain atrophy – by about 50%.
The new LAPMS study, sponsored by the Veterans Administration, will explore whether lipoic acid affects walking ability, clinical outcome, and brain atrophy, Dr. Chataway said. Results from phase 2 are expected in a year or two, he said.
Dr. Chataway also highlighted one of his own trials, the OCTOPUS study, a multiarm, multistage study that will examine multiple drugs to treat progressive MS. It’s starting with metformin and will look at lipoic acid too, he said.
He also noted the phase 2 CALLIPER trial, which has completed enrollment and expects to provide top-line data in 2025. The multicenter, randomized, double-blind, placebo-controlled will test vidofludimus calcium in patients with progressive MS.
Finally, Dr. Chataway highlighted the randomized, double-blind, placebo-controlled, add-on phase 2 NACPMS trial of n-acetyl cysteine and the phase 1 randomized, double-blind, placebo-controlled trial of SAR443820, a central nervous system penetrant oral RIPK1 inhibitor.
Dr. Chataway discloses grants (UK Multiple Sclerosis Society, National Multiple Sclerosis Society, Efficacy and Mechanism Evaluation Board, Health Technology Assessment, Multiple Sclerosis Trials Collaboration, and Rosetrees Trust), advisory board service (Azadyne, Biogen, Lucid, Janssen, Merck, NervGen, Novartis, and Roche), other support (National Institute of Health Research Support, University College London Hospitals Biomedical Research Centers funding scheme), and serving as an trial investigator (Canadian MS Society, Ionis, Novartis, and Roche).
AT ECTRIMS 2023
Topical botanical drug coacillium curbs childhood alopecia
Considerable hair regrowth can be achieved in children with alopecia areata with the use of a novel plant-based drug, according to research presented during the first late-breaking news session at the annual congress of the European Academy of Dermatology and Venereology.
(–8.0%), with a significant 31% overall difference (P < .0001).
“Coacillium cutaneous solution was used for the first time for treatment of alopecia areata and also for the first time used in a pediatric population,” the presenting investigator Ulrike Blume-Peytavi, MD, said at the meeting.
“It’s well tolerated, and in fact what is interesting is, it has a durable response, even after treatment discontinuation,” added Dr. Blume-Peytavi, who is the deputy head of the department of dermatology, venereology and allergology at Charité-Universitätsmedizin Berlin.
Backing the botanical?
Paola Pasquali, MD, a dermatologist at Pius Hospital de Valls in Spain, who cochaired the session where the findings were presented, commented, “Thank you for showing that chocolate is great! I knew it. It is fantastic to see how chocolate is used.”
Dr. Pasquali was referring to the coacillium ingredient Theobroma cacao extract. The seeds of T. cacao, or the cocoa tree, are used to make various types of chocolate products. Theobroma cacao is one of four plant extracts that make up coacillium, the others being Allium cepa (onion), Citrus limon (lemon), and Paullinia cupana (guaraná, a source of caffeine).
The four plant extracts are classified as “generally regarded as safe” (GRAS), Dr. Blume-Peytavi observed, noting that the development of coacillium fell under the category of a prescription botanical drug as set out by the U.S. Food and Drug Administration or a herbal medicinal product as set out by the European Medicines Agency.
But how does it work?
The botanical’s mode of action of acting positively on hair follicle cycling and endothelial cell activation was called into question, however, by Emma Guttman-Yassky, MD, PhD, who was in the audience.
She asked, “So how do you explain that, after three large studies with topical JAK inhibitors that did not work actually in alopecia areata because it’s very hard to penetrate the scalp for a topical [drug], this one works?”
Dr. Guttman-Yassky, professor of dermatology and immunology at the Icahn School of Medicine at Mount Sinai, New York, added: “Looking at the ingredients, to me, it seems that it’s more like a DPCP [diphenylcyclopropenone]-like reaction.”
DPCP, which has been used to treat alopecia, purportedly works by stimulating the immune response to target the skin surface – causing an allergic reaction – rather than the hair follicle.
It’s an interesting question as to how a molecule penetrates the hair follicle, and it depends on the size of the molecule, Dr. Blume-Peytavi responded.
“We have done a lot of studies on follicular penetration, and we are quite aware that you need a certain size of the molecule,” she said. Between 14 and 200 nanometers appears to produce “the best penetrators,” she observed.
Dr. Blume-Peytavi commented that even after topical JAK inhibitors are applied, the molecules that penetrate do not remain in the local area for very long, yet still produce an inhibitory signaling effect.
No scalp irritation was seen in the trial, which suggests that coacillium is not working in the same way as DPCP, Dr. Blume-Peytavi countered.
Evaluating efficacy and safety: The RAAINBOW study
Dr. Blume-Peytavi acknowledged that JAK inhibitors were “a tremendous advance in treating severe and very severe alopecia areata,” but because of their benefit-to-risk ratio, there was still an unmet need for new treatments, particularly in children, in whom drug safety is of critical importance.
Having a drug that could be given safely and also have an effect early on in the disease, while it is still at a mild to moderate stage, would be of considerable value, Dr. Blume-Peytavi maintained.
The RAAINBOW study was a randomized, double-blind, phase 2/3 trial conducted at 12 sites in Germany and three other countries between March 2018 and March 2022 to evaluate the efficacy and safety of coacillium in the treatment of children and adolescents with moderate to severe alopecia areata.
In all, 62 children aged 2-18 years (mean age, 11 years) participated; 42 were treated twice daily with coacillium cutaneous solution 22.5% and 20 received placebo for 24 weeks. Treatment was then stopped, and participants followed for another 24 weeks off treatment to check for disease relapse, bringing the total study duration up to 48 weeks.
Baseline characteristics were “relatively comparable for severity,” Dr. Blume-Peytavi said. Most of the children had severe alopecia areata (57% for coacillium and 65% for placebo); the remainder had moderate disease (43% vs. 35%, respectively).
The average SALT scores at the start of treatment were 56 in the coacillium group and 62 in the placebo group, and a respective 44 and 61 at the end of 24 weeks’ treatment.
Perhaps the most important results, Dr. Blume-Peytavi said, was that at 48 weeks of follow-up, which was 24 weeks after treatment had been discontinued, the mean SALT scores were 29 for coacillium and 56 for placebo (P < .0001).
“You can see the improvement in the treated group is continuing even without treatment. However, the placebo group stays relatively about the same range,” she said.
Overall, 82% of patients treated with coacillium and 37% of those who received placebo experienced hair growth after treatment had stopped, and by week 48, a respective 46.7% vs. 9.1% had a SALT score of 20 or less, and 30.0% vs. 0% had a SALT score of 10 or less.
No safety concerns were raised, with no serious treatment-related reactions, no immunosuppressant-like reactions, and no steroidlike side effects.
Beyond the RAAINBOW
Larger studies are needed, Dr. Blume-Peytavi said. According to developer Legacy Healthcare’s website, coacillium cutaneous solution is not being developed just for childhood alopecia areata. It is also under investigation as a treatment for persistent chemotherapy-induced alopecia, atopic dermatitis, and psoriasis. In addition, an oral solution is being tested for cancer-related fatigue.
The study was funded by Legacy Healthcare. Dr. Blume-Peytavi has received research funding and acts as an advisor to the company, among others; four of the study’s coauthors are employees of the company. Dr. Pasquali and Dr. Guttman-Yassky were not involved in the study and had no relevant financial ties to disclose.
A version of this article first appeared on Medscape.com.
Considerable hair regrowth can be achieved in children with alopecia areata with the use of a novel plant-based drug, according to research presented during the first late-breaking news session at the annual congress of the European Academy of Dermatology and Venereology.
(–8.0%), with a significant 31% overall difference (P < .0001).
“Coacillium cutaneous solution was used for the first time for treatment of alopecia areata and also for the first time used in a pediatric population,” the presenting investigator Ulrike Blume-Peytavi, MD, said at the meeting.
“It’s well tolerated, and in fact what is interesting is, it has a durable response, even after treatment discontinuation,” added Dr. Blume-Peytavi, who is the deputy head of the department of dermatology, venereology and allergology at Charité-Universitätsmedizin Berlin.
Backing the botanical?
Paola Pasquali, MD, a dermatologist at Pius Hospital de Valls in Spain, who cochaired the session where the findings were presented, commented, “Thank you for showing that chocolate is great! I knew it. It is fantastic to see how chocolate is used.”
Dr. Pasquali was referring to the coacillium ingredient Theobroma cacao extract. The seeds of T. cacao, or the cocoa tree, are used to make various types of chocolate products. Theobroma cacao is one of four plant extracts that make up coacillium, the others being Allium cepa (onion), Citrus limon (lemon), and Paullinia cupana (guaraná, a source of caffeine).
The four plant extracts are classified as “generally regarded as safe” (GRAS), Dr. Blume-Peytavi observed, noting that the development of coacillium fell under the category of a prescription botanical drug as set out by the U.S. Food and Drug Administration or a herbal medicinal product as set out by the European Medicines Agency.
But how does it work?
The botanical’s mode of action of acting positively on hair follicle cycling and endothelial cell activation was called into question, however, by Emma Guttman-Yassky, MD, PhD, who was in the audience.
She asked, “So how do you explain that, after three large studies with topical JAK inhibitors that did not work actually in alopecia areata because it’s very hard to penetrate the scalp for a topical [drug], this one works?”
Dr. Guttman-Yassky, professor of dermatology and immunology at the Icahn School of Medicine at Mount Sinai, New York, added: “Looking at the ingredients, to me, it seems that it’s more like a DPCP [diphenylcyclopropenone]-like reaction.”
DPCP, which has been used to treat alopecia, purportedly works by stimulating the immune response to target the skin surface – causing an allergic reaction – rather than the hair follicle.
It’s an interesting question as to how a molecule penetrates the hair follicle, and it depends on the size of the molecule, Dr. Blume-Peytavi responded.
“We have done a lot of studies on follicular penetration, and we are quite aware that you need a certain size of the molecule,” she said. Between 14 and 200 nanometers appears to produce “the best penetrators,” she observed.
Dr. Blume-Peytavi commented that even after topical JAK inhibitors are applied, the molecules that penetrate do not remain in the local area for very long, yet still produce an inhibitory signaling effect.
No scalp irritation was seen in the trial, which suggests that coacillium is not working in the same way as DPCP, Dr. Blume-Peytavi countered.
Evaluating efficacy and safety: The RAAINBOW study
Dr. Blume-Peytavi acknowledged that JAK inhibitors were “a tremendous advance in treating severe and very severe alopecia areata,” but because of their benefit-to-risk ratio, there was still an unmet need for new treatments, particularly in children, in whom drug safety is of critical importance.
Having a drug that could be given safely and also have an effect early on in the disease, while it is still at a mild to moderate stage, would be of considerable value, Dr. Blume-Peytavi maintained.
The RAAINBOW study was a randomized, double-blind, phase 2/3 trial conducted at 12 sites in Germany and three other countries between March 2018 and March 2022 to evaluate the efficacy and safety of coacillium in the treatment of children and adolescents with moderate to severe alopecia areata.
In all, 62 children aged 2-18 years (mean age, 11 years) participated; 42 were treated twice daily with coacillium cutaneous solution 22.5% and 20 received placebo for 24 weeks. Treatment was then stopped, and participants followed for another 24 weeks off treatment to check for disease relapse, bringing the total study duration up to 48 weeks.
Baseline characteristics were “relatively comparable for severity,” Dr. Blume-Peytavi said. Most of the children had severe alopecia areata (57% for coacillium and 65% for placebo); the remainder had moderate disease (43% vs. 35%, respectively).
The average SALT scores at the start of treatment were 56 in the coacillium group and 62 in the placebo group, and a respective 44 and 61 at the end of 24 weeks’ treatment.
Perhaps the most important results, Dr. Blume-Peytavi said, was that at 48 weeks of follow-up, which was 24 weeks after treatment had been discontinued, the mean SALT scores were 29 for coacillium and 56 for placebo (P < .0001).
“You can see the improvement in the treated group is continuing even without treatment. However, the placebo group stays relatively about the same range,” she said.
Overall, 82% of patients treated with coacillium and 37% of those who received placebo experienced hair growth after treatment had stopped, and by week 48, a respective 46.7% vs. 9.1% had a SALT score of 20 or less, and 30.0% vs. 0% had a SALT score of 10 or less.
No safety concerns were raised, with no serious treatment-related reactions, no immunosuppressant-like reactions, and no steroidlike side effects.
Beyond the RAAINBOW
Larger studies are needed, Dr. Blume-Peytavi said. According to developer Legacy Healthcare’s website, coacillium cutaneous solution is not being developed just for childhood alopecia areata. It is also under investigation as a treatment for persistent chemotherapy-induced alopecia, atopic dermatitis, and psoriasis. In addition, an oral solution is being tested for cancer-related fatigue.
The study was funded by Legacy Healthcare. Dr. Blume-Peytavi has received research funding and acts as an advisor to the company, among others; four of the study’s coauthors are employees of the company. Dr. Pasquali and Dr. Guttman-Yassky were not involved in the study and had no relevant financial ties to disclose.
A version of this article first appeared on Medscape.com.
Considerable hair regrowth can be achieved in children with alopecia areata with the use of a novel plant-based drug, according to research presented during the first late-breaking news session at the annual congress of the European Academy of Dermatology and Venereology.
(–8.0%), with a significant 31% overall difference (P < .0001).
“Coacillium cutaneous solution was used for the first time for treatment of alopecia areata and also for the first time used in a pediatric population,” the presenting investigator Ulrike Blume-Peytavi, MD, said at the meeting.
“It’s well tolerated, and in fact what is interesting is, it has a durable response, even after treatment discontinuation,” added Dr. Blume-Peytavi, who is the deputy head of the department of dermatology, venereology and allergology at Charité-Universitätsmedizin Berlin.
Backing the botanical?
Paola Pasquali, MD, a dermatologist at Pius Hospital de Valls in Spain, who cochaired the session where the findings were presented, commented, “Thank you for showing that chocolate is great! I knew it. It is fantastic to see how chocolate is used.”
Dr. Pasquali was referring to the coacillium ingredient Theobroma cacao extract. The seeds of T. cacao, or the cocoa tree, are used to make various types of chocolate products. Theobroma cacao is one of four plant extracts that make up coacillium, the others being Allium cepa (onion), Citrus limon (lemon), and Paullinia cupana (guaraná, a source of caffeine).
The four plant extracts are classified as “generally regarded as safe” (GRAS), Dr. Blume-Peytavi observed, noting that the development of coacillium fell under the category of a prescription botanical drug as set out by the U.S. Food and Drug Administration or a herbal medicinal product as set out by the European Medicines Agency.
But how does it work?
The botanical’s mode of action of acting positively on hair follicle cycling and endothelial cell activation was called into question, however, by Emma Guttman-Yassky, MD, PhD, who was in the audience.
She asked, “So how do you explain that, after three large studies with topical JAK inhibitors that did not work actually in alopecia areata because it’s very hard to penetrate the scalp for a topical [drug], this one works?”
Dr. Guttman-Yassky, professor of dermatology and immunology at the Icahn School of Medicine at Mount Sinai, New York, added: “Looking at the ingredients, to me, it seems that it’s more like a DPCP [diphenylcyclopropenone]-like reaction.”
DPCP, which has been used to treat alopecia, purportedly works by stimulating the immune response to target the skin surface – causing an allergic reaction – rather than the hair follicle.
It’s an interesting question as to how a molecule penetrates the hair follicle, and it depends on the size of the molecule, Dr. Blume-Peytavi responded.
“We have done a lot of studies on follicular penetration, and we are quite aware that you need a certain size of the molecule,” she said. Between 14 and 200 nanometers appears to produce “the best penetrators,” she observed.
Dr. Blume-Peytavi commented that even after topical JAK inhibitors are applied, the molecules that penetrate do not remain in the local area for very long, yet still produce an inhibitory signaling effect.
No scalp irritation was seen in the trial, which suggests that coacillium is not working in the same way as DPCP, Dr. Blume-Peytavi countered.
Evaluating efficacy and safety: The RAAINBOW study
Dr. Blume-Peytavi acknowledged that JAK inhibitors were “a tremendous advance in treating severe and very severe alopecia areata,” but because of their benefit-to-risk ratio, there was still an unmet need for new treatments, particularly in children, in whom drug safety is of critical importance.
Having a drug that could be given safely and also have an effect early on in the disease, while it is still at a mild to moderate stage, would be of considerable value, Dr. Blume-Peytavi maintained.
The RAAINBOW study was a randomized, double-blind, phase 2/3 trial conducted at 12 sites in Germany and three other countries between March 2018 and March 2022 to evaluate the efficacy and safety of coacillium in the treatment of children and adolescents with moderate to severe alopecia areata.
In all, 62 children aged 2-18 years (mean age, 11 years) participated; 42 were treated twice daily with coacillium cutaneous solution 22.5% and 20 received placebo for 24 weeks. Treatment was then stopped, and participants followed for another 24 weeks off treatment to check for disease relapse, bringing the total study duration up to 48 weeks.
Baseline characteristics were “relatively comparable for severity,” Dr. Blume-Peytavi said. Most of the children had severe alopecia areata (57% for coacillium and 65% for placebo); the remainder had moderate disease (43% vs. 35%, respectively).
The average SALT scores at the start of treatment were 56 in the coacillium group and 62 in the placebo group, and a respective 44 and 61 at the end of 24 weeks’ treatment.
Perhaps the most important results, Dr. Blume-Peytavi said, was that at 48 weeks of follow-up, which was 24 weeks after treatment had been discontinued, the mean SALT scores were 29 for coacillium and 56 for placebo (P < .0001).
“You can see the improvement in the treated group is continuing even without treatment. However, the placebo group stays relatively about the same range,” she said.
Overall, 82% of patients treated with coacillium and 37% of those who received placebo experienced hair growth after treatment had stopped, and by week 48, a respective 46.7% vs. 9.1% had a SALT score of 20 or less, and 30.0% vs. 0% had a SALT score of 10 or less.
No safety concerns were raised, with no serious treatment-related reactions, no immunosuppressant-like reactions, and no steroidlike side effects.
Beyond the RAAINBOW
Larger studies are needed, Dr. Blume-Peytavi said. According to developer Legacy Healthcare’s website, coacillium cutaneous solution is not being developed just for childhood alopecia areata. It is also under investigation as a treatment for persistent chemotherapy-induced alopecia, atopic dermatitis, and psoriasis. In addition, an oral solution is being tested for cancer-related fatigue.
The study was funded by Legacy Healthcare. Dr. Blume-Peytavi has received research funding and acts as an advisor to the company, among others; four of the study’s coauthors are employees of the company. Dr. Pasquali and Dr. Guttman-Yassky were not involved in the study and had no relevant financial ties to disclose.
A version of this article first appeared on Medscape.com.
AT THE EADV CONGRESS
Cannabis use growing among menopausal women
PHILADELPHIA – presented at the annual meeting of the Menopause Society (formerly The North American Menopause Society).
Though most women reported using cannabis for recreational reasons, 13% used it only for medical reasons, most often for chronic pain, anxiety, sleep, and stress.
“These findings highlight the importance of recognizing and discussing cannabis use in the health care setting, and the need for additional research to evaluate the potential harms and/or benefits of use in this vulnerable population,” Carolyn J. Gibson, PhD, MPH, a staff psychologist in women’s health at the San Francisco VA Health Care System and an assistant professor of psychiatry and behavioral sciences at the University of California, San Francisco, told attendees.
As cannabis has become more accessible, with its use legalized in 38 states and Washington, D.C., the proportion of U.S. adults using it has doubled over about a decade, from 6% in 2007 to 12% in 2019, Dr. Gibson said. Further, women aged 50 and older are among the fastest-growing groups of users of cannabis, and it’s being increasingly used – and marketed – for treating menopause-related and aging-related symptoms, including insomnia, anxiety, and chronic pain, she said.
“With these decisions to use cannabis, medically or for these other purposes, there’s this perception that it’s harmless,” Dr. Gibson said. Yet potential health risks associated with cannabis include the usual health effects associated with any kind of smoking as well as dependence in those who use it more frequently and/or develop a tolerance for it. She noted that average THC potency has increased over time, and acute risks for using cannabis with high levels of THC – at least 15% or at least 10 mg – can include anxiety/panic, confusion, disturbing/intrusive thoughts, psychosis, and effects on coordination and cognition. She also acknowledged, however, that most of the data available on risks come from studies of men and younger adults rather than older women.
Given the growing normalization of cannabis use, Dr. Gibson’s team sought to better understand prevalence of use as well as types of use and reasons for use in perimenopasual and postmenopausal women. They analyzed data from a cross-sectional survey of women and gender-diverse members, aged 45-64, of Ipsos KnowledgePanel, an online panel with more than 60,000 participating members in the United States.
All the respondents identified themselves as female at birth and had not used gender-affirming therapy or undergone gender-affirming surgery. The survey included questions on sociodemographics, menopause status, frequency of cannabis use, types of cannabis used, reasons for using cannabis, and use of cannabis in the previous 30 days. The 5,174 respondents were an average 55 years old and predominantly non-Hispanic white (63%), with 13% non-Hispanic Black and 16% Hispanic. Two-thirds of the women reported working full- or part-time (67%) and two-thirds were postmenopausal (68%), with 64% reporting experiencing menopause symptoms.
About two in five respondents (42%) had ever used cannabis in any form, most often smoking it (83%) or consuming edibles (51%). Among those who had ever used it, 30% reported having smoked it daily or nearly daily for at least a year at some point.
Ten percent of respondents had used cannabis in the past month, again primarily smoking (56%) or edibles (52%), though 39% said they used it in more than one form, including vaping, dabbing, or topical use. Nearly half (46%) of the respondents who smoked cannabis recently did not know the THC potency of what they consumed, and just over 20% of those consuming edibles didn’t know the THC potency of what they used. However, about a third of those taking edibles used cannabis with less than 10 mg of THC, and a little over a quarter used edibles with 10 mg of THC.
Within the 10% who had used cannabis in the past month, nearly a third (31%) of respondents – or around 3.1% of the total sample – reported smoking cannabis daily or almost daily, and 19% (or 1.9% of the overall sample) consumed cannabis edibles daily or almost daily.
Most of the respondents who used cannabis said it was for recreational use (62%), but a quarter (25%) reported using it for both recreational and medical reasons, and 13% used it only for medical reasons. The most common reason women used cannabis was to treat chronic pain (28%), followed by nearly as many women reporting cannabis use for anxiety (24%), sleep (22%), and stress (22%). Six percent of women used cannabis specifically for menopause-related sleep and mood problems.
Given the growing use of cannabis in this population and the dearth of data on its effects in older women, Dr. Gibson highlighted the need for research examining the potential benefits and harms of cannabis for menopausal women.
Not risk-free
Susan D. Reed, MD, MPH, MSCP, a professor emeritus of ob.gyn. at the University of Washington, Seattle, and president of the Menopause Society, found the study well-executed and was not surprised by how many respondents had ever used cannabis.
“What did surprise me was that nearly a third reported daily use for at least 1 year and that 38% were medical marijuana users, not just recreational,” Dr. Reed said in an interview. The proportions of women using cannabis for menopausal symptoms or using it daily are concerning, she added.
“These individuals are at risk for dependence and health risks related to marijuana use,” Dr. Reed said. “Providers should always ask patients about OTC products, herbals, supplements, cannabis use, and alternative management of menopausal symptoms to better understand patient preferences for menopausal symptom therapies, so that treatment plans can be discussed with individual patient preferences in mind. We need to start with where the patient is coming from.”
Data presented throughout the conference has shown how people are “disillusioned with the care they are receiving for menopause,” Dr. Reed added. “It is so difficult to distinguish truth from myths based on information gained through social media, family, and friends, and that often is where most people are getting their information.”
Physicians often have not received adequate training on how to provide people with accurate information about menopause and managing menopausal symptoms, so she advises patients and physicians to visit reliable sites such as the Menopause Society, the Swan Study, and My Menoplan.
The research was funded by the Tobacco-Related Disease Research Program and the Veterans Administration. Dr. Gibson has provided unpaid consultation to Astellas Pharmaceuticals. Dr. Reed has received research support from Bayer and receives royalties from UpToDate.
PHILADELPHIA – presented at the annual meeting of the Menopause Society (formerly The North American Menopause Society).
Though most women reported using cannabis for recreational reasons, 13% used it only for medical reasons, most often for chronic pain, anxiety, sleep, and stress.
“These findings highlight the importance of recognizing and discussing cannabis use in the health care setting, and the need for additional research to evaluate the potential harms and/or benefits of use in this vulnerable population,” Carolyn J. Gibson, PhD, MPH, a staff psychologist in women’s health at the San Francisco VA Health Care System and an assistant professor of psychiatry and behavioral sciences at the University of California, San Francisco, told attendees.
As cannabis has become more accessible, with its use legalized in 38 states and Washington, D.C., the proportion of U.S. adults using it has doubled over about a decade, from 6% in 2007 to 12% in 2019, Dr. Gibson said. Further, women aged 50 and older are among the fastest-growing groups of users of cannabis, and it’s being increasingly used – and marketed – for treating menopause-related and aging-related symptoms, including insomnia, anxiety, and chronic pain, she said.
“With these decisions to use cannabis, medically or for these other purposes, there’s this perception that it’s harmless,” Dr. Gibson said. Yet potential health risks associated with cannabis include the usual health effects associated with any kind of smoking as well as dependence in those who use it more frequently and/or develop a tolerance for it. She noted that average THC potency has increased over time, and acute risks for using cannabis with high levels of THC – at least 15% or at least 10 mg – can include anxiety/panic, confusion, disturbing/intrusive thoughts, psychosis, and effects on coordination and cognition. She also acknowledged, however, that most of the data available on risks come from studies of men and younger adults rather than older women.
Given the growing normalization of cannabis use, Dr. Gibson’s team sought to better understand prevalence of use as well as types of use and reasons for use in perimenopasual and postmenopausal women. They analyzed data from a cross-sectional survey of women and gender-diverse members, aged 45-64, of Ipsos KnowledgePanel, an online panel with more than 60,000 participating members in the United States.
All the respondents identified themselves as female at birth and had not used gender-affirming therapy or undergone gender-affirming surgery. The survey included questions on sociodemographics, menopause status, frequency of cannabis use, types of cannabis used, reasons for using cannabis, and use of cannabis in the previous 30 days. The 5,174 respondents were an average 55 years old and predominantly non-Hispanic white (63%), with 13% non-Hispanic Black and 16% Hispanic. Two-thirds of the women reported working full- or part-time (67%) and two-thirds were postmenopausal (68%), with 64% reporting experiencing menopause symptoms.
About two in five respondents (42%) had ever used cannabis in any form, most often smoking it (83%) or consuming edibles (51%). Among those who had ever used it, 30% reported having smoked it daily or nearly daily for at least a year at some point.
Ten percent of respondents had used cannabis in the past month, again primarily smoking (56%) or edibles (52%), though 39% said they used it in more than one form, including vaping, dabbing, or topical use. Nearly half (46%) of the respondents who smoked cannabis recently did not know the THC potency of what they consumed, and just over 20% of those consuming edibles didn’t know the THC potency of what they used. However, about a third of those taking edibles used cannabis with less than 10 mg of THC, and a little over a quarter used edibles with 10 mg of THC.
Within the 10% who had used cannabis in the past month, nearly a third (31%) of respondents – or around 3.1% of the total sample – reported smoking cannabis daily or almost daily, and 19% (or 1.9% of the overall sample) consumed cannabis edibles daily or almost daily.
Most of the respondents who used cannabis said it was for recreational use (62%), but a quarter (25%) reported using it for both recreational and medical reasons, and 13% used it only for medical reasons. The most common reason women used cannabis was to treat chronic pain (28%), followed by nearly as many women reporting cannabis use for anxiety (24%), sleep (22%), and stress (22%). Six percent of women used cannabis specifically for menopause-related sleep and mood problems.
Given the growing use of cannabis in this population and the dearth of data on its effects in older women, Dr. Gibson highlighted the need for research examining the potential benefits and harms of cannabis for menopausal women.
Not risk-free
Susan D. Reed, MD, MPH, MSCP, a professor emeritus of ob.gyn. at the University of Washington, Seattle, and president of the Menopause Society, found the study well-executed and was not surprised by how many respondents had ever used cannabis.
“What did surprise me was that nearly a third reported daily use for at least 1 year and that 38% were medical marijuana users, not just recreational,” Dr. Reed said in an interview. The proportions of women using cannabis for menopausal symptoms or using it daily are concerning, she added.
“These individuals are at risk for dependence and health risks related to marijuana use,” Dr. Reed said. “Providers should always ask patients about OTC products, herbals, supplements, cannabis use, and alternative management of menopausal symptoms to better understand patient preferences for menopausal symptom therapies, so that treatment plans can be discussed with individual patient preferences in mind. We need to start with where the patient is coming from.”
Data presented throughout the conference has shown how people are “disillusioned with the care they are receiving for menopause,” Dr. Reed added. “It is so difficult to distinguish truth from myths based on information gained through social media, family, and friends, and that often is where most people are getting their information.”
Physicians often have not received adequate training on how to provide people with accurate information about menopause and managing menopausal symptoms, so she advises patients and physicians to visit reliable sites such as the Menopause Society, the Swan Study, and My Menoplan.
The research was funded by the Tobacco-Related Disease Research Program and the Veterans Administration. Dr. Gibson has provided unpaid consultation to Astellas Pharmaceuticals. Dr. Reed has received research support from Bayer and receives royalties from UpToDate.
PHILADELPHIA – presented at the annual meeting of the Menopause Society (formerly The North American Menopause Society).
Though most women reported using cannabis for recreational reasons, 13% used it only for medical reasons, most often for chronic pain, anxiety, sleep, and stress.
“These findings highlight the importance of recognizing and discussing cannabis use in the health care setting, and the need for additional research to evaluate the potential harms and/or benefits of use in this vulnerable population,” Carolyn J. Gibson, PhD, MPH, a staff psychologist in women’s health at the San Francisco VA Health Care System and an assistant professor of psychiatry and behavioral sciences at the University of California, San Francisco, told attendees.
As cannabis has become more accessible, with its use legalized in 38 states and Washington, D.C., the proportion of U.S. adults using it has doubled over about a decade, from 6% in 2007 to 12% in 2019, Dr. Gibson said. Further, women aged 50 and older are among the fastest-growing groups of users of cannabis, and it’s being increasingly used – and marketed – for treating menopause-related and aging-related symptoms, including insomnia, anxiety, and chronic pain, she said.
“With these decisions to use cannabis, medically or for these other purposes, there’s this perception that it’s harmless,” Dr. Gibson said. Yet potential health risks associated with cannabis include the usual health effects associated with any kind of smoking as well as dependence in those who use it more frequently and/or develop a tolerance for it. She noted that average THC potency has increased over time, and acute risks for using cannabis with high levels of THC – at least 15% or at least 10 mg – can include anxiety/panic, confusion, disturbing/intrusive thoughts, psychosis, and effects on coordination and cognition. She also acknowledged, however, that most of the data available on risks come from studies of men and younger adults rather than older women.
Given the growing normalization of cannabis use, Dr. Gibson’s team sought to better understand prevalence of use as well as types of use and reasons for use in perimenopasual and postmenopausal women. They analyzed data from a cross-sectional survey of women and gender-diverse members, aged 45-64, of Ipsos KnowledgePanel, an online panel with more than 60,000 participating members in the United States.
All the respondents identified themselves as female at birth and had not used gender-affirming therapy or undergone gender-affirming surgery. The survey included questions on sociodemographics, menopause status, frequency of cannabis use, types of cannabis used, reasons for using cannabis, and use of cannabis in the previous 30 days. The 5,174 respondents were an average 55 years old and predominantly non-Hispanic white (63%), with 13% non-Hispanic Black and 16% Hispanic. Two-thirds of the women reported working full- or part-time (67%) and two-thirds were postmenopausal (68%), with 64% reporting experiencing menopause symptoms.
About two in five respondents (42%) had ever used cannabis in any form, most often smoking it (83%) or consuming edibles (51%). Among those who had ever used it, 30% reported having smoked it daily or nearly daily for at least a year at some point.
Ten percent of respondents had used cannabis in the past month, again primarily smoking (56%) or edibles (52%), though 39% said they used it in more than one form, including vaping, dabbing, or topical use. Nearly half (46%) of the respondents who smoked cannabis recently did not know the THC potency of what they consumed, and just over 20% of those consuming edibles didn’t know the THC potency of what they used. However, about a third of those taking edibles used cannabis with less than 10 mg of THC, and a little over a quarter used edibles with 10 mg of THC.
Within the 10% who had used cannabis in the past month, nearly a third (31%) of respondents – or around 3.1% of the total sample – reported smoking cannabis daily or almost daily, and 19% (or 1.9% of the overall sample) consumed cannabis edibles daily or almost daily.
Most of the respondents who used cannabis said it was for recreational use (62%), but a quarter (25%) reported using it for both recreational and medical reasons, and 13% used it only for medical reasons. The most common reason women used cannabis was to treat chronic pain (28%), followed by nearly as many women reporting cannabis use for anxiety (24%), sleep (22%), and stress (22%). Six percent of women used cannabis specifically for menopause-related sleep and mood problems.
Given the growing use of cannabis in this population and the dearth of data on its effects in older women, Dr. Gibson highlighted the need for research examining the potential benefits and harms of cannabis for menopausal women.
Not risk-free
Susan D. Reed, MD, MPH, MSCP, a professor emeritus of ob.gyn. at the University of Washington, Seattle, and president of the Menopause Society, found the study well-executed and was not surprised by how many respondents had ever used cannabis.
“What did surprise me was that nearly a third reported daily use for at least 1 year and that 38% were medical marijuana users, not just recreational,” Dr. Reed said in an interview. The proportions of women using cannabis for menopausal symptoms or using it daily are concerning, she added.
“These individuals are at risk for dependence and health risks related to marijuana use,” Dr. Reed said. “Providers should always ask patients about OTC products, herbals, supplements, cannabis use, and alternative management of menopausal symptoms to better understand patient preferences for menopausal symptom therapies, so that treatment plans can be discussed with individual patient preferences in mind. We need to start with where the patient is coming from.”
Data presented throughout the conference has shown how people are “disillusioned with the care they are receiving for menopause,” Dr. Reed added. “It is so difficult to distinguish truth from myths based on information gained through social media, family, and friends, and that often is where most people are getting their information.”
Physicians often have not received adequate training on how to provide people with accurate information about menopause and managing menopausal symptoms, so she advises patients and physicians to visit reliable sites such as the Menopause Society, the Swan Study, and My Menoplan.
The research was funded by the Tobacco-Related Disease Research Program and the Veterans Administration. Dr. Gibson has provided unpaid consultation to Astellas Pharmaceuticals. Dr. Reed has received research support from Bayer and receives royalties from UpToDate.
AT NAMS 2023
Bone degradation measure can sway osteoporosis diagnosis
Assessing a key aspect of bone architecture, for which clinicians can now be reimbursed under Medicare, can significantly improve the ability to predict a patient’s risk for bone fracture.
Although bone mineral density (BMD) is traditionally used to identify patients with osteoporosis or low bone mass, some physicians have begun incorporating the trabecular bone score (TBS) into their exams.
At the Cleveland Clinic Center for Specialized Women’s Health, factoring in the TBS changed the diagnosis for 16% of 432 patients, according to Holly Thacker, MD, the center’s director.
“Importantly, 11% got worse diagnoses, and I use that in terms of prioritizing treatment,” Dr. Thacker said in an interview. The ability to determine how degraded the bone microarchitecture is through a software system “is a huge advance.”
Dr. Thacker described her center’s experience with the technology at the annual meeting of the Menopause Society (formerly The North American Menopause Society).
While BMD captures the amount of minerals like calcium in the skeleton, TBS assesses the underlying microarchitecture by looking at the distribution of shades of gray on dual-energy x-ray absorptiometry (DXA) scans.
Based on the TBS, patients’ bones are classified as normal, partially degraded, or degraded. Among the 432 patients who received a TBS analysis in 2022, 3% shifted from a normal diagnosis to osteopenia, 8% worsened from osteopenia to osteoporosis, 4% went from osteopenia to normal, and 1.6% downgraded from osteoporosis to osteopenia, Dr. Thacker reported.
The new test may also provide some reassurance for female patients who have thinner bones, which may raise alarms based on BMD. TBS, however, may show that the structure of the bone looks normal.
“When you know that the microarchitecture is normal, you’re a lot less concerned that they actually have a bone disease of osteoporosis,” Dr. Thacker said.
Conversely, unexpectedly degraded bone raises questions.
“That makes you go back and say [to the patient]: ‘Have you been on steroids? Were you malnourished? Is there some other metabolic problem? Have you had some calcium disorder?’ ” Dr. Thacker said.
Dr. Thacker leverages the TBS to help patients obtain effective therapy, typically an anabolic agent followed by antiresorptive medication.
“When I see a patient who not only has osteoporosis on bone density but has completely degraded bone architecture, it’s a lot easier for me to make the argument to the insurance company that this patient is at grave risk for a low trauma fracture and bad outcome without the best treatment,” Dr. Thacker said.
10-year-old tech, recently covered
The Food and Drug Administration approved TBS software in 2012, but Medicare only recently started paying for it.
Medimaps Group, a company that markets imaging software to calculate TBS, announced in 2022 that reimbursement from the Centers for Medicare & Medicaid Services was available, at $41.53 on the Physician Fee Schedule and $82.61 on the Hospital Outpatient Prospective Payment Schedule.
“Reimbursement through CMS is an important endorsement of the clinical value of TBS for clinicians and their patients,” Didier Hans, PhD, MBA, the CEO of Medimaps, said in a statement at the time. He noted that more than 600,000 TBS procedures were being performed in the United States each year.
Nevertheless, the initial investment in purchasing the software may be a barrier for health systems.
“We are the first and only site in our health system to offer TBS, as this is an extra expense and not uniformly reimbursed by insurers,” Dr. Thacker reported at the meeting.
Potential drawbacks
The TBS software used in Dr. Thacker’s study has been validated only in Asian and White patients between certain ages and weights, meaning the system is not designed to be used for other populations. Other researchers have highlighted a need for trabecular bone scoring to be validated more broadly. The authors of a recent analysis, however, suggest that TBS can be used the same way no matter a patient’s race.
TBS “is going to be most helpful in those with osteopenia who are right near the threshold for treatment,” said Marcella Donovan Walker, MD, MS, in a presentation on bone quality at the meeting.
Many studies have shown that TBS “provides additive information to bone density,” said Dr. Walker, a professor of medicine in the division of endocrinology at Columbia University, New York. For example, a large study of women in Manitoba found that, regardless of whether their bone density was normal, osteopenic, or osteoporotic, those with a low TBS had a much higher risk for fracture.
‘Opportunistic screening’ with CT?
TBS relies on the same DXA scans that are used to calculate bone mineral density, so obtaining the score does not add time or radiation to the scanning process, Dr. Thacker said.
But many patients who should receive DXA scans do not, which adds to the promise of “opportunistic screening” for osteoporosis, Dr. Walker said. With this approach, physicians would analyze a CT scan that a patient received for another purpose, such as to investigate abdominal pain or chest pain.
“In these images is information about the bone,” Dr. Walker said.
Researchers have used high-resolution peripheral quantitative CT to perform finite element analysis, where a computer program simulates compression of the bone to create a measure of bone stiffness and determine the load required for a break.
One study found that including those elements predicted fractures better than bone mineral density or the Fracture Risk Assessment Tool alone, Dr. Walker noted.
Other aspects of bone quality include how many cracks are in the bone, the amount of adipose in the marrow space, and the rate at which bone is broken down and rebuilt. But Dr. Walker suggested that the longstanding focus on bone mineral density in clinical practice makes sense.
“By far, bone mass is the most important bone quality,” Dr. Walker said.
Dr. Thacker is the executive director of the nonprofit Speaking of Women’s Health. Dr. Walker reported receiving funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and Amgen.
A version of this article first appeared on Medscape.com.
Assessing a key aspect of bone architecture, for which clinicians can now be reimbursed under Medicare, can significantly improve the ability to predict a patient’s risk for bone fracture.
Although bone mineral density (BMD) is traditionally used to identify patients with osteoporosis or low bone mass, some physicians have begun incorporating the trabecular bone score (TBS) into their exams.
At the Cleveland Clinic Center for Specialized Women’s Health, factoring in the TBS changed the diagnosis for 16% of 432 patients, according to Holly Thacker, MD, the center’s director.
“Importantly, 11% got worse diagnoses, and I use that in terms of prioritizing treatment,” Dr. Thacker said in an interview. The ability to determine how degraded the bone microarchitecture is through a software system “is a huge advance.”
Dr. Thacker described her center’s experience with the technology at the annual meeting of the Menopause Society (formerly The North American Menopause Society).
While BMD captures the amount of minerals like calcium in the skeleton, TBS assesses the underlying microarchitecture by looking at the distribution of shades of gray on dual-energy x-ray absorptiometry (DXA) scans.
Based on the TBS, patients’ bones are classified as normal, partially degraded, or degraded. Among the 432 patients who received a TBS analysis in 2022, 3% shifted from a normal diagnosis to osteopenia, 8% worsened from osteopenia to osteoporosis, 4% went from osteopenia to normal, and 1.6% downgraded from osteoporosis to osteopenia, Dr. Thacker reported.
The new test may also provide some reassurance for female patients who have thinner bones, which may raise alarms based on BMD. TBS, however, may show that the structure of the bone looks normal.
“When you know that the microarchitecture is normal, you’re a lot less concerned that they actually have a bone disease of osteoporosis,” Dr. Thacker said.
Conversely, unexpectedly degraded bone raises questions.
“That makes you go back and say [to the patient]: ‘Have you been on steroids? Were you malnourished? Is there some other metabolic problem? Have you had some calcium disorder?’ ” Dr. Thacker said.
Dr. Thacker leverages the TBS to help patients obtain effective therapy, typically an anabolic agent followed by antiresorptive medication.
“When I see a patient who not only has osteoporosis on bone density but has completely degraded bone architecture, it’s a lot easier for me to make the argument to the insurance company that this patient is at grave risk for a low trauma fracture and bad outcome without the best treatment,” Dr. Thacker said.
10-year-old tech, recently covered
The Food and Drug Administration approved TBS software in 2012, but Medicare only recently started paying for it.
Medimaps Group, a company that markets imaging software to calculate TBS, announced in 2022 that reimbursement from the Centers for Medicare & Medicaid Services was available, at $41.53 on the Physician Fee Schedule and $82.61 on the Hospital Outpatient Prospective Payment Schedule.
“Reimbursement through CMS is an important endorsement of the clinical value of TBS for clinicians and their patients,” Didier Hans, PhD, MBA, the CEO of Medimaps, said in a statement at the time. He noted that more than 600,000 TBS procedures were being performed in the United States each year.
Nevertheless, the initial investment in purchasing the software may be a barrier for health systems.
“We are the first and only site in our health system to offer TBS, as this is an extra expense and not uniformly reimbursed by insurers,” Dr. Thacker reported at the meeting.
Potential drawbacks
The TBS software used in Dr. Thacker’s study has been validated only in Asian and White patients between certain ages and weights, meaning the system is not designed to be used for other populations. Other researchers have highlighted a need for trabecular bone scoring to be validated more broadly. The authors of a recent analysis, however, suggest that TBS can be used the same way no matter a patient’s race.
TBS “is going to be most helpful in those with osteopenia who are right near the threshold for treatment,” said Marcella Donovan Walker, MD, MS, in a presentation on bone quality at the meeting.
Many studies have shown that TBS “provides additive information to bone density,” said Dr. Walker, a professor of medicine in the division of endocrinology at Columbia University, New York. For example, a large study of women in Manitoba found that, regardless of whether their bone density was normal, osteopenic, or osteoporotic, those with a low TBS had a much higher risk for fracture.
‘Opportunistic screening’ with CT?
TBS relies on the same DXA scans that are used to calculate bone mineral density, so obtaining the score does not add time or radiation to the scanning process, Dr. Thacker said.
But many patients who should receive DXA scans do not, which adds to the promise of “opportunistic screening” for osteoporosis, Dr. Walker said. With this approach, physicians would analyze a CT scan that a patient received for another purpose, such as to investigate abdominal pain or chest pain.
“In these images is information about the bone,” Dr. Walker said.
Researchers have used high-resolution peripheral quantitative CT to perform finite element analysis, where a computer program simulates compression of the bone to create a measure of bone stiffness and determine the load required for a break.
One study found that including those elements predicted fractures better than bone mineral density or the Fracture Risk Assessment Tool alone, Dr. Walker noted.
Other aspects of bone quality include how many cracks are in the bone, the amount of adipose in the marrow space, and the rate at which bone is broken down and rebuilt. But Dr. Walker suggested that the longstanding focus on bone mineral density in clinical practice makes sense.
“By far, bone mass is the most important bone quality,” Dr. Walker said.
Dr. Thacker is the executive director of the nonprofit Speaking of Women’s Health. Dr. Walker reported receiving funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and Amgen.
A version of this article first appeared on Medscape.com.
Assessing a key aspect of bone architecture, for which clinicians can now be reimbursed under Medicare, can significantly improve the ability to predict a patient’s risk for bone fracture.
Although bone mineral density (BMD) is traditionally used to identify patients with osteoporosis or low bone mass, some physicians have begun incorporating the trabecular bone score (TBS) into their exams.
At the Cleveland Clinic Center for Specialized Women’s Health, factoring in the TBS changed the diagnosis for 16% of 432 patients, according to Holly Thacker, MD, the center’s director.
“Importantly, 11% got worse diagnoses, and I use that in terms of prioritizing treatment,” Dr. Thacker said in an interview. The ability to determine how degraded the bone microarchitecture is through a software system “is a huge advance.”
Dr. Thacker described her center’s experience with the technology at the annual meeting of the Menopause Society (formerly The North American Menopause Society).
While BMD captures the amount of minerals like calcium in the skeleton, TBS assesses the underlying microarchitecture by looking at the distribution of shades of gray on dual-energy x-ray absorptiometry (DXA) scans.
Based on the TBS, patients’ bones are classified as normal, partially degraded, or degraded. Among the 432 patients who received a TBS analysis in 2022, 3% shifted from a normal diagnosis to osteopenia, 8% worsened from osteopenia to osteoporosis, 4% went from osteopenia to normal, and 1.6% downgraded from osteoporosis to osteopenia, Dr. Thacker reported.
The new test may also provide some reassurance for female patients who have thinner bones, which may raise alarms based on BMD. TBS, however, may show that the structure of the bone looks normal.
“When you know that the microarchitecture is normal, you’re a lot less concerned that they actually have a bone disease of osteoporosis,” Dr. Thacker said.
Conversely, unexpectedly degraded bone raises questions.
“That makes you go back and say [to the patient]: ‘Have you been on steroids? Were you malnourished? Is there some other metabolic problem? Have you had some calcium disorder?’ ” Dr. Thacker said.
Dr. Thacker leverages the TBS to help patients obtain effective therapy, typically an anabolic agent followed by antiresorptive medication.
“When I see a patient who not only has osteoporosis on bone density but has completely degraded bone architecture, it’s a lot easier for me to make the argument to the insurance company that this patient is at grave risk for a low trauma fracture and bad outcome without the best treatment,” Dr. Thacker said.
10-year-old tech, recently covered
The Food and Drug Administration approved TBS software in 2012, but Medicare only recently started paying for it.
Medimaps Group, a company that markets imaging software to calculate TBS, announced in 2022 that reimbursement from the Centers for Medicare & Medicaid Services was available, at $41.53 on the Physician Fee Schedule and $82.61 on the Hospital Outpatient Prospective Payment Schedule.
“Reimbursement through CMS is an important endorsement of the clinical value of TBS for clinicians and their patients,” Didier Hans, PhD, MBA, the CEO of Medimaps, said in a statement at the time. He noted that more than 600,000 TBS procedures were being performed in the United States each year.
Nevertheless, the initial investment in purchasing the software may be a barrier for health systems.
“We are the first and only site in our health system to offer TBS, as this is an extra expense and not uniformly reimbursed by insurers,” Dr. Thacker reported at the meeting.
Potential drawbacks
The TBS software used in Dr. Thacker’s study has been validated only in Asian and White patients between certain ages and weights, meaning the system is not designed to be used for other populations. Other researchers have highlighted a need for trabecular bone scoring to be validated more broadly. The authors of a recent analysis, however, suggest that TBS can be used the same way no matter a patient’s race.
TBS “is going to be most helpful in those with osteopenia who are right near the threshold for treatment,” said Marcella Donovan Walker, MD, MS, in a presentation on bone quality at the meeting.
Many studies have shown that TBS “provides additive information to bone density,” said Dr. Walker, a professor of medicine in the division of endocrinology at Columbia University, New York. For example, a large study of women in Manitoba found that, regardless of whether their bone density was normal, osteopenic, or osteoporotic, those with a low TBS had a much higher risk for fracture.
‘Opportunistic screening’ with CT?
TBS relies on the same DXA scans that are used to calculate bone mineral density, so obtaining the score does not add time or radiation to the scanning process, Dr. Thacker said.
But many patients who should receive DXA scans do not, which adds to the promise of “opportunistic screening” for osteoporosis, Dr. Walker said. With this approach, physicians would analyze a CT scan that a patient received for another purpose, such as to investigate abdominal pain or chest pain.
“In these images is information about the bone,” Dr. Walker said.
Researchers have used high-resolution peripheral quantitative CT to perform finite element analysis, where a computer program simulates compression of the bone to create a measure of bone stiffness and determine the load required for a break.
One study found that including those elements predicted fractures better than bone mineral density or the Fracture Risk Assessment Tool alone, Dr. Walker noted.
Other aspects of bone quality include how many cracks are in the bone, the amount of adipose in the marrow space, and the rate at which bone is broken down and rebuilt. But Dr. Walker suggested that the longstanding focus on bone mineral density in clinical practice makes sense.
“By far, bone mass is the most important bone quality,” Dr. Walker said.
Dr. Thacker is the executive director of the nonprofit Speaking of Women’s Health. Dr. Walker reported receiving funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and Amgen.
A version of this article first appeared on Medscape.com.
FROM NAMS 2023
When to treat DLBCL with radiotherapy?
SAN DIEGO –
For example, radiation may not be needed for advanced-stage patients who’ve received at least four cycles of R-CHOP chemotherapy (cyclophosphamide, doxorubicin, vincristine, and prednisolone plus rituximab), and whose PET scans show no sign of disease at interim or end-of treatment phases, said Joanna Yang, MD, MPH, of Washington University in St. Louis, in a presentation at the annual meeting of the American Society for Radiation Oncology.
These patients “may be able to omit radiotherapy without sacrificing good outcomes,” Dr. Yang said. In contrast, those whose PET scans show signs of disease at interim and end-of-treatment points may benefit from radiotherapy to selected sites, she said.
Dr. Yang highlighted a 2021 study in Blood that tracked 723 patients with advanced-stage DLBCL who were diagnosed from 2005 to 2017. All were treated with R-CHOP, and some of those who were PET-positive – that is, showing signs of malignant disease – were treated with radiotherapy.
Over a mean follow-up of 4.3 years, the study reported “time to progression and overall survival at 3 years were 83% vs. 56% and 87% vs. 64% in patients with PET-NEG and PET-POS scans, respectively.”
These findings aren’t surprising, Dr. Yang said. But “the PET-positive patients who got radiation actually had outcomes that came close to the outcomes that the PET-negative patients were able to achieve.” Their 3-year overall survival was 80% vs. 87% in the PET-negative, no-radiation group vs. 44% in the PET-positive, no-radiation group.
Dr. Yang cautioned, however, that withholding radiation in PET-negative patients isn’t right for everyone: “This doesn’t mean this should be the approach for every single patient.”
What about early-stage DLBCL? In patients without risk factors, Dr. Yang recommends PET scans after four treatments with R-CHOP. “Getting that end-of-treatment PET is going to be super-critical because that’s going to help guide you in terms of the patients who you may feel comfortable omitting radiation versus the patients who remain PET-positive at the end of chemotherapy. Many places will also add an interim PET as well.”
According to her, radiotherapy is appropriate in patients who are PET-positive, based on the findings of the FLYER and LYSA-GOELAMS 02-03 trials.
In early-stage patients who have risk factors such as advanced age or bulky or extra-nodal disease, Dr. Yang suggests examining interim PET scans after three treatments with R-CHOP. If they are negative, another R-CHOP treatment is appropriate – with or without radiotherapy.
“There’s a lot that goes into that decision. The first thing I think about in patients who have risk factors is: What salvage options are available for my patient? Can they tolerate these salvage option? If they’re older, they might not be eligible for auto [autologous hematopoietic cell transplantation]. If they’re frail, they might not be eligible for auto or CAR T cells. If they have bulk, it’s certainly an area of concern. It seems like radiation does help control disease in areas of bulk for patients with DLBCL.”
If these patients are PET-positive, go directly to radiotherapy, Dr. Yang advised. Trials that support this approach include S1001, LYSA-GOELAMS 02-03, and RICOVER-noRTH, she said.
What about double-hit and triple-hit lymphomas, which are especially aggressive due to genetic variations? Research suggests that “even if double hit/triple hit is not responding to chemo, it still responds to radiation,” Dr. Yang said.
In regard to advanced-stage disease, “if patients are receiving full-dose chemo for least six cycles, I use that end-of-treatment PET to help guide me. And then I make an individualized decision based on how bulky that disease is, where the location is, how morbid a relapse would be. If they’re older or receiving reduced-dose chemotherapy, then I’ll more seriously consider radiation just because there are limited options for these patients. And we know that DLCBL is most commonly a disease of the elderly.”
In an adjoining presentation at ASTRO, Andrea Ng, MD, MPH, of Harvard Medical School/Dana-Farber Brigham Cancer Center, Boston, discussed which patients with incomplete response or refractory/relapsed DLCBL can benefit from radiotherapy.
She highlighted patients with good partial response and end-of-treatment PET-positive with evidence of residual 18F-fluorodeoxyglucose activity via PET scan (Deauville 4/5) – a group that “we’re increasingly seeing.” In these patients, “radiation can be quite effective” at doses of 36-45 Gy. She highlighted a study from 2011 that linked consolidation radiotherapy to 5-year event-free survival in 65% of patients.
As for relapsed/refractory disease in patients who aren’t candidates for further systemic therapy – the “frail without good options” – Dr. Ng said data about salvage radiotherapy is limited. However, a 2015 study tracked 65 patients who were treated with a median dose of 40 Gy with “curative” intent. Local control was “not great” at 72% at 2 years, Dr. Ng said, while overall survival was 60% and progress-free survival was 46%.
Dr. Ng, who was one of this study’s authors, said several groups did better: Those with refractory vs. relapsed disease and those who were responsive to chemotherapy vs. those who were not.
She also highlighted a similar 2019 study of 32 patients with refractory/relapsed disease treated with salvage radiotherapy (median dose of 42.7 Gy) found that 61.8% reached progress-free survival at 5 years – a better outcome.
Dr. Yang has no disclosures. Dr. Ng discloses royalties from UpToDate and Elsevier.
SAN DIEGO –
For example, radiation may not be needed for advanced-stage patients who’ve received at least four cycles of R-CHOP chemotherapy (cyclophosphamide, doxorubicin, vincristine, and prednisolone plus rituximab), and whose PET scans show no sign of disease at interim or end-of treatment phases, said Joanna Yang, MD, MPH, of Washington University in St. Louis, in a presentation at the annual meeting of the American Society for Radiation Oncology.
These patients “may be able to omit radiotherapy without sacrificing good outcomes,” Dr. Yang said. In contrast, those whose PET scans show signs of disease at interim and end-of-treatment points may benefit from radiotherapy to selected sites, she said.
Dr. Yang highlighted a 2021 study in Blood that tracked 723 patients with advanced-stage DLBCL who were diagnosed from 2005 to 2017. All were treated with R-CHOP, and some of those who were PET-positive – that is, showing signs of malignant disease – were treated with radiotherapy.
Over a mean follow-up of 4.3 years, the study reported “time to progression and overall survival at 3 years were 83% vs. 56% and 87% vs. 64% in patients with PET-NEG and PET-POS scans, respectively.”
These findings aren’t surprising, Dr. Yang said. But “the PET-positive patients who got radiation actually had outcomes that came close to the outcomes that the PET-negative patients were able to achieve.” Their 3-year overall survival was 80% vs. 87% in the PET-negative, no-radiation group vs. 44% in the PET-positive, no-radiation group.
Dr. Yang cautioned, however, that withholding radiation in PET-negative patients isn’t right for everyone: “This doesn’t mean this should be the approach for every single patient.”
What about early-stage DLBCL? In patients without risk factors, Dr. Yang recommends PET scans after four treatments with R-CHOP. “Getting that end-of-treatment PET is going to be super-critical because that’s going to help guide you in terms of the patients who you may feel comfortable omitting radiation versus the patients who remain PET-positive at the end of chemotherapy. Many places will also add an interim PET as well.”
According to her, radiotherapy is appropriate in patients who are PET-positive, based on the findings of the FLYER and LYSA-GOELAMS 02-03 trials.
In early-stage patients who have risk factors such as advanced age or bulky or extra-nodal disease, Dr. Yang suggests examining interim PET scans after three treatments with R-CHOP. If they are negative, another R-CHOP treatment is appropriate – with or without radiotherapy.
“There’s a lot that goes into that decision. The first thing I think about in patients who have risk factors is: What salvage options are available for my patient? Can they tolerate these salvage option? If they’re older, they might not be eligible for auto [autologous hematopoietic cell transplantation]. If they’re frail, they might not be eligible for auto or CAR T cells. If they have bulk, it’s certainly an area of concern. It seems like radiation does help control disease in areas of bulk for patients with DLBCL.”
If these patients are PET-positive, go directly to radiotherapy, Dr. Yang advised. Trials that support this approach include S1001, LYSA-GOELAMS 02-03, and RICOVER-noRTH, she said.
What about double-hit and triple-hit lymphomas, which are especially aggressive due to genetic variations? Research suggests that “even if double hit/triple hit is not responding to chemo, it still responds to radiation,” Dr. Yang said.
In regard to advanced-stage disease, “if patients are receiving full-dose chemo for least six cycles, I use that end-of-treatment PET to help guide me. And then I make an individualized decision based on how bulky that disease is, where the location is, how morbid a relapse would be. If they’re older or receiving reduced-dose chemotherapy, then I’ll more seriously consider radiation just because there are limited options for these patients. And we know that DLCBL is most commonly a disease of the elderly.”
In an adjoining presentation at ASTRO, Andrea Ng, MD, MPH, of Harvard Medical School/Dana-Farber Brigham Cancer Center, Boston, discussed which patients with incomplete response or refractory/relapsed DLCBL can benefit from radiotherapy.
She highlighted patients with good partial response and end-of-treatment PET-positive with evidence of residual 18F-fluorodeoxyglucose activity via PET scan (Deauville 4/5) – a group that “we’re increasingly seeing.” In these patients, “radiation can be quite effective” at doses of 36-45 Gy. She highlighted a study from 2011 that linked consolidation radiotherapy to 5-year event-free survival in 65% of patients.
As for relapsed/refractory disease in patients who aren’t candidates for further systemic therapy – the “frail without good options” – Dr. Ng said data about salvage radiotherapy is limited. However, a 2015 study tracked 65 patients who were treated with a median dose of 40 Gy with “curative” intent. Local control was “not great” at 72% at 2 years, Dr. Ng said, while overall survival was 60% and progress-free survival was 46%.
Dr. Ng, who was one of this study’s authors, said several groups did better: Those with refractory vs. relapsed disease and those who were responsive to chemotherapy vs. those who were not.
She also highlighted a similar 2019 study of 32 patients with refractory/relapsed disease treated with salvage radiotherapy (median dose of 42.7 Gy) found that 61.8% reached progress-free survival at 5 years – a better outcome.
Dr. Yang has no disclosures. Dr. Ng discloses royalties from UpToDate and Elsevier.
SAN DIEGO –
For example, radiation may not be needed for advanced-stage patients who’ve received at least four cycles of R-CHOP chemotherapy (cyclophosphamide, doxorubicin, vincristine, and prednisolone plus rituximab), and whose PET scans show no sign of disease at interim or end-of treatment phases, said Joanna Yang, MD, MPH, of Washington University in St. Louis, in a presentation at the annual meeting of the American Society for Radiation Oncology.
These patients “may be able to omit radiotherapy without sacrificing good outcomes,” Dr. Yang said. In contrast, those whose PET scans show signs of disease at interim and end-of-treatment points may benefit from radiotherapy to selected sites, she said.
Dr. Yang highlighted a 2021 study in Blood that tracked 723 patients with advanced-stage DLBCL who were diagnosed from 2005 to 2017. All were treated with R-CHOP, and some of those who were PET-positive – that is, showing signs of malignant disease – were treated with radiotherapy.
Over a mean follow-up of 4.3 years, the study reported “time to progression and overall survival at 3 years were 83% vs. 56% and 87% vs. 64% in patients with PET-NEG and PET-POS scans, respectively.”
These findings aren’t surprising, Dr. Yang said. But “the PET-positive patients who got radiation actually had outcomes that came close to the outcomes that the PET-negative patients were able to achieve.” Their 3-year overall survival was 80% vs. 87% in the PET-negative, no-radiation group vs. 44% in the PET-positive, no-radiation group.
Dr. Yang cautioned, however, that withholding radiation in PET-negative patients isn’t right for everyone: “This doesn’t mean this should be the approach for every single patient.”
What about early-stage DLBCL? In patients without risk factors, Dr. Yang recommends PET scans after four treatments with R-CHOP. “Getting that end-of-treatment PET is going to be super-critical because that’s going to help guide you in terms of the patients who you may feel comfortable omitting radiation versus the patients who remain PET-positive at the end of chemotherapy. Many places will also add an interim PET as well.”
According to her, radiotherapy is appropriate in patients who are PET-positive, based on the findings of the FLYER and LYSA-GOELAMS 02-03 trials.
In early-stage patients who have risk factors such as advanced age or bulky or extra-nodal disease, Dr. Yang suggests examining interim PET scans after three treatments with R-CHOP. If they are negative, another R-CHOP treatment is appropriate – with or without radiotherapy.
“There’s a lot that goes into that decision. The first thing I think about in patients who have risk factors is: What salvage options are available for my patient? Can they tolerate these salvage option? If they’re older, they might not be eligible for auto [autologous hematopoietic cell transplantation]. If they’re frail, they might not be eligible for auto or CAR T cells. If they have bulk, it’s certainly an area of concern. It seems like radiation does help control disease in areas of bulk for patients with DLBCL.”
If these patients are PET-positive, go directly to radiotherapy, Dr. Yang advised. Trials that support this approach include S1001, LYSA-GOELAMS 02-03, and RICOVER-noRTH, she said.
What about double-hit and triple-hit lymphomas, which are especially aggressive due to genetic variations? Research suggests that “even if double hit/triple hit is not responding to chemo, it still responds to radiation,” Dr. Yang said.
In regard to advanced-stage disease, “if patients are receiving full-dose chemo for least six cycles, I use that end-of-treatment PET to help guide me. And then I make an individualized decision based on how bulky that disease is, where the location is, how morbid a relapse would be. If they’re older or receiving reduced-dose chemotherapy, then I’ll more seriously consider radiation just because there are limited options for these patients. And we know that DLCBL is most commonly a disease of the elderly.”
In an adjoining presentation at ASTRO, Andrea Ng, MD, MPH, of Harvard Medical School/Dana-Farber Brigham Cancer Center, Boston, discussed which patients with incomplete response or refractory/relapsed DLCBL can benefit from radiotherapy.
She highlighted patients with good partial response and end-of-treatment PET-positive with evidence of residual 18F-fluorodeoxyglucose activity via PET scan (Deauville 4/5) – a group that “we’re increasingly seeing.” In these patients, “radiation can be quite effective” at doses of 36-45 Gy. She highlighted a study from 2011 that linked consolidation radiotherapy to 5-year event-free survival in 65% of patients.
As for relapsed/refractory disease in patients who aren’t candidates for further systemic therapy – the “frail without good options” – Dr. Ng said data about salvage radiotherapy is limited. However, a 2015 study tracked 65 patients who were treated with a median dose of 40 Gy with “curative” intent. Local control was “not great” at 72% at 2 years, Dr. Ng said, while overall survival was 60% and progress-free survival was 46%.
Dr. Ng, who was one of this study’s authors, said several groups did better: Those with refractory vs. relapsed disease and those who were responsive to chemotherapy vs. those who were not.
She also highlighted a similar 2019 study of 32 patients with refractory/relapsed disease treated with salvage radiotherapy (median dose of 42.7 Gy) found that 61.8% reached progress-free survival at 5 years – a better outcome.
Dr. Yang has no disclosures. Dr. Ng discloses royalties from UpToDate and Elsevier.
FROM ASTRO 2023
AI tool reveals MS drug interactions, offers safer options
MILAN – , German researchers reported.
The team fed the medication plans of almost 630 patients into a deep neural network, which identified drug-drug interactions in more than 80% of cases, in particular when switching from one medication to another, alongside potential food interactions.
The tool was able to identify specific interactions that could be avoided if a drug was replaced with one with a similar pharmacologic profile, but a lower risk of adverse effects.
“Potential drug-drug interactions are a major safety concern in patients with MS,” said study presenter Michael Hecker, PhD, department of neurology, Rostock (Germany) University Medical Center.
Such deep learning–based methods are “useful” in screening for potential interactions both between drugs and with foods, they concluded.
The findings were presented at the 9th Joint ECTRIMS-ACTRIMS Meeting.
Unknown interactions
During his presentation, Dr. Hecker noted that most patients with MS take two or more drugs “to treat their disease and to mitigate their symptoms and comorbidities.” He pointed out, however, that patients who take multiple medications are at an increased risk for side effects, as one drug may affect the pharmacokinetic or pharmacodynamic properties of another.
“For instance, it may change its metabolism,” Dr. Hecker said, and therefore affect its mechanism of action and the response to the drug, with medications potentially having synergistic, antagonistic, or additive effects.
He explained that the online DrugBank database “provides a huge collection” of known drug-drug interactions for compounds that have a track record. “However, for other drugs, and especially those that are tested only in clinical trials, there’s no information about drug-drug and drug-food interactions,” Dr. Hecker said.
“Moreover, it is quite time-consuming to search a database for individual drug-drug interactions,” he added.
34 million parameters
Consequently, there is increasing interest in the use of deep neural networks to study drug-drug interactions, Dr. Hecker said. DeepDDI is the “state-of-the-art deep learning framework” for predicting interactions. It takes drug-drug or drug-food pairs and compares their structures to determine their similarity. This information is fed into a deep neural network with almost 34 million trainable parameters.
The framework then provides a prediction of any interactions in the same terms as the DrugBank, suggesting, for example, that Drug A may decrease the antihypertensive activities of Drug B.
For the current study, the researchers trained the deep neural network on the most recent release of the DrugBank database, finding it was able to replicate the drug-drug interactions in the database at an accuracy of 92.2% in the validation set and 92.1% in the testing set. They then put the medication plans of 627 patients with MS into the deep neural network.
The patients had a mean age of 48.6 years, 70.3% were women, and the median disease duration was 10 years. They were taking an average of 5.3 medications, and 62% were using disease-modifying therapies (DMT).
The team compared the structures of the drugs they were taking with those of 367 drugs used for the treatment of MS, as well as with structural data for 1,673 food compounds from the FooDB database.
Swapping drugs could reduce interactions
The overall prevalence of potential drug-drug interactions among the patients included in the study was 81.2%.
The researchers then determined the proportion of patients who would be at risk of additional drug-drug interaction if they switched from one DMT to another, or to a Bruton tyrosine kinase inhibitor, given all their other medications.
They found, for example, that more than 40% of patients who switched to the immunomodulator fingolimod (Gilenya) would be at increased risk for bradycardia.
Just under 40% of patients who changed their DMT to the purine analogue cladribine (Mavenclad) would have an increased risk, or worsening, of bleeding, as would approximately 25% of those who switched to the anthracenedione antineoplastic agent mitoxantrone (Novantrone).
Dr. Hecker also showed the deep neural network could make suggestions as to how critical drug-drug interactions could be avoided by replacing interacting drugs with alternatives that have similar pharmacological effects.
For example, carbamazepine (Tegretol, Equetro) could be replaced with topiramate (several brand names) to avoid hepatotoxicity in patients also taking acetaminophen, while liothyronine (Cytomel, Triostat) could replace levothyroxine in patients also taking teriflunomide (Aubagio).
Finally, Dr. Hecker reported there was a subset of 6,860 potential drug-food interactions with the patients’ medications, resulting in reduced or increased concentrations of the drugs, particularly with fish or mushroom consumption.
He conceded, however, there were several limitations to their study, including that it included only small-molecule drugs, and that they did not ask patients about their diet or if they had observed any undesired drug effects.
Furthermore, “only a small number” of the potential drug-drug or drug-food interactions they identified would be “clinically relevant.”
Dr. Hecker also pointed out that each drug has one record, but it is used for different indications, with different dosages, and has different side effects, depending how it is used. “The model does not distinguish this,” he said, and so some of the interactions it highlights could be related to other doses than the one used in MS, for example.
Promise for the future
Pavan Bhargava, MBBS, MD, associate professor of neurology, Johns Hopkins Precision Medicine Center of Excellence for Multiple Sclerosis, Baltimore, commented that, as with all AI tools, “it’s only as good as what we’re putting into it.”
Dr. Bhargava, who cochaired the session, said that “there’s limitations on the information in the databases” that are being fed into the deep neural network.
He also highlighted that, “at this point, it didn’t seem like it was coming up with much clinically useful information,” but noted that, “we may get to that point.”
“Right now, there’s promise,” Dr. Bhargava said, but “it’s still not quite there.”
No funding was declared. Dr. Hecker declares relationships with Bayer HealthCare, Biogen, Merck Healthcare, Novartis, and Teva. Several other coauthors also declared financial relationships with industry.
A version of this article first appeared on Medscape.com.
MILAN – , German researchers reported.
The team fed the medication plans of almost 630 patients into a deep neural network, which identified drug-drug interactions in more than 80% of cases, in particular when switching from one medication to another, alongside potential food interactions.
The tool was able to identify specific interactions that could be avoided if a drug was replaced with one with a similar pharmacologic profile, but a lower risk of adverse effects.
“Potential drug-drug interactions are a major safety concern in patients with MS,” said study presenter Michael Hecker, PhD, department of neurology, Rostock (Germany) University Medical Center.
Such deep learning–based methods are “useful” in screening for potential interactions both between drugs and with foods, they concluded.
The findings were presented at the 9th Joint ECTRIMS-ACTRIMS Meeting.
Unknown interactions
During his presentation, Dr. Hecker noted that most patients with MS take two or more drugs “to treat their disease and to mitigate their symptoms and comorbidities.” He pointed out, however, that patients who take multiple medications are at an increased risk for side effects, as one drug may affect the pharmacokinetic or pharmacodynamic properties of another.
“For instance, it may change its metabolism,” Dr. Hecker said, and therefore affect its mechanism of action and the response to the drug, with medications potentially having synergistic, antagonistic, or additive effects.
He explained that the online DrugBank database “provides a huge collection” of known drug-drug interactions for compounds that have a track record. “However, for other drugs, and especially those that are tested only in clinical trials, there’s no information about drug-drug and drug-food interactions,” Dr. Hecker said.
“Moreover, it is quite time-consuming to search a database for individual drug-drug interactions,” he added.
34 million parameters
Consequently, there is increasing interest in the use of deep neural networks to study drug-drug interactions, Dr. Hecker said. DeepDDI is the “state-of-the-art deep learning framework” for predicting interactions. It takes drug-drug or drug-food pairs and compares their structures to determine their similarity. This information is fed into a deep neural network with almost 34 million trainable parameters.
The framework then provides a prediction of any interactions in the same terms as the DrugBank, suggesting, for example, that Drug A may decrease the antihypertensive activities of Drug B.
For the current study, the researchers trained the deep neural network on the most recent release of the DrugBank database, finding it was able to replicate the drug-drug interactions in the database at an accuracy of 92.2% in the validation set and 92.1% in the testing set. They then put the medication plans of 627 patients with MS into the deep neural network.
The patients had a mean age of 48.6 years, 70.3% were women, and the median disease duration was 10 years. They were taking an average of 5.3 medications, and 62% were using disease-modifying therapies (DMT).
The team compared the structures of the drugs they were taking with those of 367 drugs used for the treatment of MS, as well as with structural data for 1,673 food compounds from the FooDB database.
Swapping drugs could reduce interactions
The overall prevalence of potential drug-drug interactions among the patients included in the study was 81.2%.
The researchers then determined the proportion of patients who would be at risk of additional drug-drug interaction if they switched from one DMT to another, or to a Bruton tyrosine kinase inhibitor, given all their other medications.
They found, for example, that more than 40% of patients who switched to the immunomodulator fingolimod (Gilenya) would be at increased risk for bradycardia.
Just under 40% of patients who changed their DMT to the purine analogue cladribine (Mavenclad) would have an increased risk, or worsening, of bleeding, as would approximately 25% of those who switched to the anthracenedione antineoplastic agent mitoxantrone (Novantrone).
Dr. Hecker also showed the deep neural network could make suggestions as to how critical drug-drug interactions could be avoided by replacing interacting drugs with alternatives that have similar pharmacological effects.
For example, carbamazepine (Tegretol, Equetro) could be replaced with topiramate (several brand names) to avoid hepatotoxicity in patients also taking acetaminophen, while liothyronine (Cytomel, Triostat) could replace levothyroxine in patients also taking teriflunomide (Aubagio).
Finally, Dr. Hecker reported there was a subset of 6,860 potential drug-food interactions with the patients’ medications, resulting in reduced or increased concentrations of the drugs, particularly with fish or mushroom consumption.
He conceded, however, there were several limitations to their study, including that it included only small-molecule drugs, and that they did not ask patients about their diet or if they had observed any undesired drug effects.
Furthermore, “only a small number” of the potential drug-drug or drug-food interactions they identified would be “clinically relevant.”
Dr. Hecker also pointed out that each drug has one record, but it is used for different indications, with different dosages, and has different side effects, depending how it is used. “The model does not distinguish this,” he said, and so some of the interactions it highlights could be related to other doses than the one used in MS, for example.
Promise for the future
Pavan Bhargava, MBBS, MD, associate professor of neurology, Johns Hopkins Precision Medicine Center of Excellence for Multiple Sclerosis, Baltimore, commented that, as with all AI tools, “it’s only as good as what we’re putting into it.”
Dr. Bhargava, who cochaired the session, said that “there’s limitations on the information in the databases” that are being fed into the deep neural network.
He also highlighted that, “at this point, it didn’t seem like it was coming up with much clinically useful information,” but noted that, “we may get to that point.”
“Right now, there’s promise,” Dr. Bhargava said, but “it’s still not quite there.”
No funding was declared. Dr. Hecker declares relationships with Bayer HealthCare, Biogen, Merck Healthcare, Novartis, and Teva. Several other coauthors also declared financial relationships with industry.
A version of this article first appeared on Medscape.com.
MILAN – , German researchers reported.
The team fed the medication plans of almost 630 patients into a deep neural network, which identified drug-drug interactions in more than 80% of cases, in particular when switching from one medication to another, alongside potential food interactions.
The tool was able to identify specific interactions that could be avoided if a drug was replaced with one with a similar pharmacologic profile, but a lower risk of adverse effects.
“Potential drug-drug interactions are a major safety concern in patients with MS,” said study presenter Michael Hecker, PhD, department of neurology, Rostock (Germany) University Medical Center.
Such deep learning–based methods are “useful” in screening for potential interactions both between drugs and with foods, they concluded.
The findings were presented at the 9th Joint ECTRIMS-ACTRIMS Meeting.
Unknown interactions
During his presentation, Dr. Hecker noted that most patients with MS take two or more drugs “to treat their disease and to mitigate their symptoms and comorbidities.” He pointed out, however, that patients who take multiple medications are at an increased risk for side effects, as one drug may affect the pharmacokinetic or pharmacodynamic properties of another.
“For instance, it may change its metabolism,” Dr. Hecker said, and therefore affect its mechanism of action and the response to the drug, with medications potentially having synergistic, antagonistic, or additive effects.
He explained that the online DrugBank database “provides a huge collection” of known drug-drug interactions for compounds that have a track record. “However, for other drugs, and especially those that are tested only in clinical trials, there’s no information about drug-drug and drug-food interactions,” Dr. Hecker said.
“Moreover, it is quite time-consuming to search a database for individual drug-drug interactions,” he added.
34 million parameters
Consequently, there is increasing interest in the use of deep neural networks to study drug-drug interactions, Dr. Hecker said. DeepDDI is the “state-of-the-art deep learning framework” for predicting interactions. It takes drug-drug or drug-food pairs and compares their structures to determine their similarity. This information is fed into a deep neural network with almost 34 million trainable parameters.
The framework then provides a prediction of any interactions in the same terms as the DrugBank, suggesting, for example, that Drug A may decrease the antihypertensive activities of Drug B.
For the current study, the researchers trained the deep neural network on the most recent release of the DrugBank database, finding it was able to replicate the drug-drug interactions in the database at an accuracy of 92.2% in the validation set and 92.1% in the testing set. They then put the medication plans of 627 patients with MS into the deep neural network.
The patients had a mean age of 48.6 years, 70.3% were women, and the median disease duration was 10 years. They were taking an average of 5.3 medications, and 62% were using disease-modifying therapies (DMT).
The team compared the structures of the drugs they were taking with those of 367 drugs used for the treatment of MS, as well as with structural data for 1,673 food compounds from the FooDB database.
Swapping drugs could reduce interactions
The overall prevalence of potential drug-drug interactions among the patients included in the study was 81.2%.
The researchers then determined the proportion of patients who would be at risk of additional drug-drug interaction if they switched from one DMT to another, or to a Bruton tyrosine kinase inhibitor, given all their other medications.
They found, for example, that more than 40% of patients who switched to the immunomodulator fingolimod (Gilenya) would be at increased risk for bradycardia.
Just under 40% of patients who changed their DMT to the purine analogue cladribine (Mavenclad) would have an increased risk, or worsening, of bleeding, as would approximately 25% of those who switched to the anthracenedione antineoplastic agent mitoxantrone (Novantrone).
Dr. Hecker also showed the deep neural network could make suggestions as to how critical drug-drug interactions could be avoided by replacing interacting drugs with alternatives that have similar pharmacological effects.
For example, carbamazepine (Tegretol, Equetro) could be replaced with topiramate (several brand names) to avoid hepatotoxicity in patients also taking acetaminophen, while liothyronine (Cytomel, Triostat) could replace levothyroxine in patients also taking teriflunomide (Aubagio).
Finally, Dr. Hecker reported there was a subset of 6,860 potential drug-food interactions with the patients’ medications, resulting in reduced or increased concentrations of the drugs, particularly with fish or mushroom consumption.
He conceded, however, there were several limitations to their study, including that it included only small-molecule drugs, and that they did not ask patients about their diet or if they had observed any undesired drug effects.
Furthermore, “only a small number” of the potential drug-drug or drug-food interactions they identified would be “clinically relevant.”
Dr. Hecker also pointed out that each drug has one record, but it is used for different indications, with different dosages, and has different side effects, depending how it is used. “The model does not distinguish this,” he said, and so some of the interactions it highlights could be related to other doses than the one used in MS, for example.
Promise for the future
Pavan Bhargava, MBBS, MD, associate professor of neurology, Johns Hopkins Precision Medicine Center of Excellence for Multiple Sclerosis, Baltimore, commented that, as with all AI tools, “it’s only as good as what we’re putting into it.”
Dr. Bhargava, who cochaired the session, said that “there’s limitations on the information in the databases” that are being fed into the deep neural network.
He also highlighted that, “at this point, it didn’t seem like it was coming up with much clinically useful information,” but noted that, “we may get to that point.”
“Right now, there’s promise,” Dr. Bhargava said, but “it’s still not quite there.”
No funding was declared. Dr. Hecker declares relationships with Bayer HealthCare, Biogen, Merck Healthcare, Novartis, and Teva. Several other coauthors also declared financial relationships with industry.
A version of this article first appeared on Medscape.com.
FROM ECTRIMS 2023
Mixed CRC screening messaging. Confusing? Some docs think so
Recently updated colorectal cancer (CRC) screening guidance from the American College of Physicians is raising concerns among some specialists.
The ACP’s clinical guidance, published in Annals of Internal Medicine, called for CRC screenings to start at age 50 in average-risk individuals who are asymptomatic. This recommendation, however, conflicts with guidelines from the American Cancer Society and the U.S. Preventive Services Task Force, which, in 2021, officially lowered the recommended initial age of screening to 45.
Following the ACP’s announcement, several professional organizations, such as the American College of Radiology, criticized the new guidelines, calling them “a step backward” and warning they may hinder recent gains against CRC.
Some physicians believe the discordance will confuse patients and lead to varying referral practices among primary care physicians. And while insurers will likely continue to pay for screening procedures based on the USPSTF guidelines, which dictate insurance coverage, some physicians worry that insurers could create additional roadblocks for CRC screening coverage, such as requiring prior authorization.
“We’re in a conflicted space on this issue as a country,” said John L. Marshall, MD, a GI oncologist and director of The Ruesch Center for the Cure of GI Cancers at Georgetown University, Washington.
Ultimately, the physician community wants an inexpensive screening test that’s effective at preventing cancer and deaths, but the evidence thus far doesn’t necessarily support colonoscopy as that test, said Dr. Marshall, also chief medical officer for Lombardi Comprehensive Cancer Center.
Although colonoscopy can prevent CRC by removing precancerous polyps and can reduce deaths from cancer, it has not been shown to lower all-cause mortality, Dr. Marshall explained. A recent meta-analysis, for example, found that, aside from sigmoidoscopy for colon cancer screening, no other cancer screening modalities meaningfully changed life expectancy.
“That’s why we’re struggling,” Dr. Marshall said. “We’re emotionally invested in having screening available to younger people because we’re seeing colon cancer in younger people. So, we want it to move earlier, but it’s expensive and it’s invasive.”
Docs debate differing guidance
The new ACP guidance, based on a critical review of existing guidelines, evidence, and modeling studies, argues that the potential harms of screening average-risk individuals under age 50 may outweigh the potential benefits.
The benefits of screening, of course, include identifying and removing precancerous lesions or localized cancer, while the potential harms include false positives that may lead to unnecessary additional tests, treatments, and costs. More invasive screening procedures, such as colonoscopy, can also come with their own risks, including serious bleeding and perforation.
For colonoscopy, for instance, the ACP team determined that starting screening at age 45 vs. 50 could prevent three additional CRC cases per 1,000 individuals screened (58 vs. 61) and one CRC death (27 vs. 28) over the recommended screening time frame. On the flip side, screening starting at age 45 could increase the incidence of gastrointestinal or cardiovascular events (14 vs. 16).
“Even if we assumed the modeling study had no limitations and accepted the results at face value, we would conclude that the small estimated benefits and harms roughly balance each other out, resulting in an inadequate net benefit to warrant CRC screening in average-risk adults aged 45 to 49 years,” Amir Qaseem, MD, PhD, and ACP coauthors write.
Family physician Kenny Lin, MD, MPH, believes the updated ACP guidelines are reasonable, and points out the ACP is not the first group to disagree with the USPSTF’s recommendations.
“I think the [ACP] guidelines make a lot of sense,” said Dr. Lin, who practices in Lancaster, Pa. The American Academy of Family Physicians “also did not endorse the recommendations to start screenings at 45.” In its 2021 updated guidance, the AAFP recommended screening for CRC starting at age 50, concluding there was “insufficient evidence to assess the benefits and harms of screening” in the 45 to 49 population.
However, Jason R. Woloski, MD, a family physician based in Wilkes-Barre, Pa., expressed concern that the differing guidelines will confuse patients as well as present challenges for primary care physicians.
“I feel like we took the last couple of years convincing people that earlier is better,” said Dr. Woloski, an associate professor of family medicine at Geisinger Commonwealth School of Medicine, Scranton, Pa. “It can send a mixed message to a patient after we’ve been stressing the importance of earlier [screening], and then saying, ‘Maybe we got it wrong; maybe we were okay the first time.’ ”
Mark A. Lewis, MD, a GI oncologist, had a similar initial reaction upon hearing about the updated guidelines: “The lack of synchronization across groups is going to create confusion among patients.”
Although he could not say definitively whether the recommendations will affect GI oncologists, because he only sees patients with advanced CRC, he does see the demands in primary care and gastroenterology shifting.
“I think the much bigger impact will be on primary care physicians and gastroenterologists,” said Dr. Lewis, director of gastrointestinal oncology at Intermountain Healthcare in Murray, Utah. “My best guess is that the procedural burden on the latter will be mitigated by more stool testing ordered by primary care physicians. Patients may understandably prefer the convenience and lack of invasiveness of home-based fecal testing, but a positive FIT [fecal immunochemical test] without a follow-up scope is an incomplete screening.”
Dr. Marshall, however, had a different take. He does not envision the updated guidelines having much of a practical impact on physician practice. Most of the country is already not receiving proper colon cancer screenings, he said. Research shows more than 40% of Americans skip standard CRC screenings. Even anecdotally, he noted, friends in their 60s come to him and admit they haven’t had a colonoscopy yet.
Potential impact on patient outcomes, costs
Beyond mixed messaging, some experts worry that pushing CRC screening later could mean cancers are caught later, when they’re more advanced.
Finding cancers earlier, when they are easier and less expensive to treat, make earlier CRC screenings worthwhile, Dr. Woloski explained.
Dr. Lewis sees earlier screening as a way to stop a tumor from progressing before it can really pick up steam.
“To me the biggest advantage of colonoscopy is the interruption of the adenoma-to-carcinoma sequence, whereby a polyp that is completely removed cannot become an invasive adenocarcinoma,” Dr. Lewis said. “We’ve also had evidence for well over a decade that flexible sigmoidoscopy, which doesn’t come close to visualizing the entire colon, can confer a survival benefit.”
Another concern is the potential effect on insurance coverage.
Medicare and other insurers use USPSTF guidelines to make coverage decisions. However, because of this mixed message, Dr. Woloski questioned whether there would be more challenges regarding insurance coverage. “Does it mean primary care doctors are going to have to preauthorize a lot of these screenings even if you have shared decision-making with the patient?” he asked.
When it comes to screening referrals, Douglas A. Corley, MD, PhD, a gastroenterologist at Kaiser Permanente in northern California, said it’s critical for primary care physicians to educate patients about the differing views on screening benefits and harms as well as the different screening options.
“Given the different opinions, it is important to let people in this age group know that screening is an option recommended by some groups,” Dr. Corley said. “Colorectal cancer screening is very effective for decreasing the risk for death from colorectal cancer, which is the second leading cause of cancer death in the United States. Making sure all eligible people know this is an option provides the best way for patients to have an informed choice.”
Dr. Lin has already begun talking with patients about the differing recommendations. He said it’s helpful to simplify the issue and focus the conversation on what patients value most. For more assertive patients whose priority is finding every possible cancer early, starting screenings at age 45 may be reasonable, he said, whereas other patients may not find the process or possible side effects worth it.
“And then you have the middle group that decides, ‘Yes, I want to start at 45, but I want the fecal test. I don’t want to just jump into colonoscopy.’ ” Dr. Lin said. “That would be kind of a compromise where you’d be starting screening earlier, but not subjecting yourself to something that has more potential for harms.”
Dr. Woloski said he plans to continue making referrals based on the USPSTF recommendations.
“With every screening, it is about informed decision-making with the patient, but I think for now, since USPSTF still supports the earlier screening, I will probably stick with offering it earlier,” he said.
But when deciding on the appropriate timing for evaluating CRC, the most important distinction is between screening and diagnosis, Dr. Lewis added.
“The former is only appropriate in patients who are truly asymptomatic and who are truly average-risk,” he said. “The latter is critical in any patient with symptoms. I cannot count the number of times I have seen blood in the stool discounted as hemorrhoids without even an exam, digital rectal, or scope, to demonstrate that hemorrhoids are present and the culprit for blood loss.”
A version of this article first appeared on Medscape.com.
Recently updated colorectal cancer (CRC) screening guidance from the American College of Physicians is raising concerns among some specialists.
The ACP’s clinical guidance, published in Annals of Internal Medicine, called for CRC screenings to start at age 50 in average-risk individuals who are asymptomatic. This recommendation, however, conflicts with guidelines from the American Cancer Society and the U.S. Preventive Services Task Force, which, in 2021, officially lowered the recommended initial age of screening to 45.
Following the ACP’s announcement, several professional organizations, such as the American College of Radiology, criticized the new guidelines, calling them “a step backward” and warning they may hinder recent gains against CRC.
Some physicians believe the discordance will confuse patients and lead to varying referral practices among primary care physicians. And while insurers will likely continue to pay for screening procedures based on the USPSTF guidelines, which dictate insurance coverage, some physicians worry that insurers could create additional roadblocks for CRC screening coverage, such as requiring prior authorization.
“We’re in a conflicted space on this issue as a country,” said John L. Marshall, MD, a GI oncologist and director of The Ruesch Center for the Cure of GI Cancers at Georgetown University, Washington.
Ultimately, the physician community wants an inexpensive screening test that’s effective at preventing cancer and deaths, but the evidence thus far doesn’t necessarily support colonoscopy as that test, said Dr. Marshall, also chief medical officer for Lombardi Comprehensive Cancer Center.
Although colonoscopy can prevent CRC by removing precancerous polyps and can reduce deaths from cancer, it has not been shown to lower all-cause mortality, Dr. Marshall explained. A recent meta-analysis, for example, found that, aside from sigmoidoscopy for colon cancer screening, no other cancer screening modalities meaningfully changed life expectancy.
“That’s why we’re struggling,” Dr. Marshall said. “We’re emotionally invested in having screening available to younger people because we’re seeing colon cancer in younger people. So, we want it to move earlier, but it’s expensive and it’s invasive.”
Docs debate differing guidance
The new ACP guidance, based on a critical review of existing guidelines, evidence, and modeling studies, argues that the potential harms of screening average-risk individuals under age 50 may outweigh the potential benefits.
The benefits of screening, of course, include identifying and removing precancerous lesions or localized cancer, while the potential harms include false positives that may lead to unnecessary additional tests, treatments, and costs. More invasive screening procedures, such as colonoscopy, can also come with their own risks, including serious bleeding and perforation.
For colonoscopy, for instance, the ACP team determined that starting screening at age 45 vs. 50 could prevent three additional CRC cases per 1,000 individuals screened (58 vs. 61) and one CRC death (27 vs. 28) over the recommended screening time frame. On the flip side, screening starting at age 45 could increase the incidence of gastrointestinal or cardiovascular events (14 vs. 16).
“Even if we assumed the modeling study had no limitations and accepted the results at face value, we would conclude that the small estimated benefits and harms roughly balance each other out, resulting in an inadequate net benefit to warrant CRC screening in average-risk adults aged 45 to 49 years,” Amir Qaseem, MD, PhD, and ACP coauthors write.
Family physician Kenny Lin, MD, MPH, believes the updated ACP guidelines are reasonable, and points out the ACP is not the first group to disagree with the USPSTF’s recommendations.
“I think the [ACP] guidelines make a lot of sense,” said Dr. Lin, who practices in Lancaster, Pa. The American Academy of Family Physicians “also did not endorse the recommendations to start screenings at 45.” In its 2021 updated guidance, the AAFP recommended screening for CRC starting at age 50, concluding there was “insufficient evidence to assess the benefits and harms of screening” in the 45 to 49 population.
However, Jason R. Woloski, MD, a family physician based in Wilkes-Barre, Pa., expressed concern that the differing guidelines will confuse patients as well as present challenges for primary care physicians.
“I feel like we took the last couple of years convincing people that earlier is better,” said Dr. Woloski, an associate professor of family medicine at Geisinger Commonwealth School of Medicine, Scranton, Pa. “It can send a mixed message to a patient after we’ve been stressing the importance of earlier [screening], and then saying, ‘Maybe we got it wrong; maybe we were okay the first time.’ ”
Mark A. Lewis, MD, a GI oncologist, had a similar initial reaction upon hearing about the updated guidelines: “The lack of synchronization across groups is going to create confusion among patients.”
Although he could not say definitively whether the recommendations will affect GI oncologists, because he only sees patients with advanced CRC, he does see the demands in primary care and gastroenterology shifting.
“I think the much bigger impact will be on primary care physicians and gastroenterologists,” said Dr. Lewis, director of gastrointestinal oncology at Intermountain Healthcare in Murray, Utah. “My best guess is that the procedural burden on the latter will be mitigated by more stool testing ordered by primary care physicians. Patients may understandably prefer the convenience and lack of invasiveness of home-based fecal testing, but a positive FIT [fecal immunochemical test] without a follow-up scope is an incomplete screening.”
Dr. Marshall, however, had a different take. He does not envision the updated guidelines having much of a practical impact on physician practice. Most of the country is already not receiving proper colon cancer screenings, he said. Research shows more than 40% of Americans skip standard CRC screenings. Even anecdotally, he noted, friends in their 60s come to him and admit they haven’t had a colonoscopy yet.
Potential impact on patient outcomes, costs
Beyond mixed messaging, some experts worry that pushing CRC screening later could mean cancers are caught later, when they’re more advanced.
Finding cancers earlier, when they are easier and less expensive to treat, make earlier CRC screenings worthwhile, Dr. Woloski explained.
Dr. Lewis sees earlier screening as a way to stop a tumor from progressing before it can really pick up steam.
“To me the biggest advantage of colonoscopy is the interruption of the adenoma-to-carcinoma sequence, whereby a polyp that is completely removed cannot become an invasive adenocarcinoma,” Dr. Lewis said. “We’ve also had evidence for well over a decade that flexible sigmoidoscopy, which doesn’t come close to visualizing the entire colon, can confer a survival benefit.”
Another concern is the potential effect on insurance coverage.
Medicare and other insurers use USPSTF guidelines to make coverage decisions. However, because of this mixed message, Dr. Woloski questioned whether there would be more challenges regarding insurance coverage. “Does it mean primary care doctors are going to have to preauthorize a lot of these screenings even if you have shared decision-making with the patient?” he asked.
When it comes to screening referrals, Douglas A. Corley, MD, PhD, a gastroenterologist at Kaiser Permanente in northern California, said it’s critical for primary care physicians to educate patients about the differing views on screening benefits and harms as well as the different screening options.
“Given the different opinions, it is important to let people in this age group know that screening is an option recommended by some groups,” Dr. Corley said. “Colorectal cancer screening is very effective for decreasing the risk for death from colorectal cancer, which is the second leading cause of cancer death in the United States. Making sure all eligible people know this is an option provides the best way for patients to have an informed choice.”
Dr. Lin has already begun talking with patients about the differing recommendations. He said it’s helpful to simplify the issue and focus the conversation on what patients value most. For more assertive patients whose priority is finding every possible cancer early, starting screenings at age 45 may be reasonable, he said, whereas other patients may not find the process or possible side effects worth it.
“And then you have the middle group that decides, ‘Yes, I want to start at 45, but I want the fecal test. I don’t want to just jump into colonoscopy.’ ” Dr. Lin said. “That would be kind of a compromise where you’d be starting screening earlier, but not subjecting yourself to something that has more potential for harms.”
Dr. Woloski said he plans to continue making referrals based on the USPSTF recommendations.
“With every screening, it is about informed decision-making with the patient, but I think for now, since USPSTF still supports the earlier screening, I will probably stick with offering it earlier,” he said.
But when deciding on the appropriate timing for evaluating CRC, the most important distinction is between screening and diagnosis, Dr. Lewis added.
“The former is only appropriate in patients who are truly asymptomatic and who are truly average-risk,” he said. “The latter is critical in any patient with symptoms. I cannot count the number of times I have seen blood in the stool discounted as hemorrhoids without even an exam, digital rectal, or scope, to demonstrate that hemorrhoids are present and the culprit for blood loss.”
A version of this article first appeared on Medscape.com.
Recently updated colorectal cancer (CRC) screening guidance from the American College of Physicians is raising concerns among some specialists.
The ACP’s clinical guidance, published in Annals of Internal Medicine, called for CRC screenings to start at age 50 in average-risk individuals who are asymptomatic. This recommendation, however, conflicts with guidelines from the American Cancer Society and the U.S. Preventive Services Task Force, which, in 2021, officially lowered the recommended initial age of screening to 45.
Following the ACP’s announcement, several professional organizations, such as the American College of Radiology, criticized the new guidelines, calling them “a step backward” and warning they may hinder recent gains against CRC.
Some physicians believe the discordance will confuse patients and lead to varying referral practices among primary care physicians. And while insurers will likely continue to pay for screening procedures based on the USPSTF guidelines, which dictate insurance coverage, some physicians worry that insurers could create additional roadblocks for CRC screening coverage, such as requiring prior authorization.
“We’re in a conflicted space on this issue as a country,” said John L. Marshall, MD, a GI oncologist and director of The Ruesch Center for the Cure of GI Cancers at Georgetown University, Washington.
Ultimately, the physician community wants an inexpensive screening test that’s effective at preventing cancer and deaths, but the evidence thus far doesn’t necessarily support colonoscopy as that test, said Dr. Marshall, also chief medical officer for Lombardi Comprehensive Cancer Center.
Although colonoscopy can prevent CRC by removing precancerous polyps and can reduce deaths from cancer, it has not been shown to lower all-cause mortality, Dr. Marshall explained. A recent meta-analysis, for example, found that, aside from sigmoidoscopy for colon cancer screening, no other cancer screening modalities meaningfully changed life expectancy.
“That’s why we’re struggling,” Dr. Marshall said. “We’re emotionally invested in having screening available to younger people because we’re seeing colon cancer in younger people. So, we want it to move earlier, but it’s expensive and it’s invasive.”
Docs debate differing guidance
The new ACP guidance, based on a critical review of existing guidelines, evidence, and modeling studies, argues that the potential harms of screening average-risk individuals under age 50 may outweigh the potential benefits.
The benefits of screening, of course, include identifying and removing precancerous lesions or localized cancer, while the potential harms include false positives that may lead to unnecessary additional tests, treatments, and costs. More invasive screening procedures, such as colonoscopy, can also come with their own risks, including serious bleeding and perforation.
For colonoscopy, for instance, the ACP team determined that starting screening at age 45 vs. 50 could prevent three additional CRC cases per 1,000 individuals screened (58 vs. 61) and one CRC death (27 vs. 28) over the recommended screening time frame. On the flip side, screening starting at age 45 could increase the incidence of gastrointestinal or cardiovascular events (14 vs. 16).
“Even if we assumed the modeling study had no limitations and accepted the results at face value, we would conclude that the small estimated benefits and harms roughly balance each other out, resulting in an inadequate net benefit to warrant CRC screening in average-risk adults aged 45 to 49 years,” Amir Qaseem, MD, PhD, and ACP coauthors write.
Family physician Kenny Lin, MD, MPH, believes the updated ACP guidelines are reasonable, and points out the ACP is not the first group to disagree with the USPSTF’s recommendations.
“I think the [ACP] guidelines make a lot of sense,” said Dr. Lin, who practices in Lancaster, Pa. The American Academy of Family Physicians “also did not endorse the recommendations to start screenings at 45.” In its 2021 updated guidance, the AAFP recommended screening for CRC starting at age 50, concluding there was “insufficient evidence to assess the benefits and harms of screening” in the 45 to 49 population.
However, Jason R. Woloski, MD, a family physician based in Wilkes-Barre, Pa., expressed concern that the differing guidelines will confuse patients as well as present challenges for primary care physicians.
“I feel like we took the last couple of years convincing people that earlier is better,” said Dr. Woloski, an associate professor of family medicine at Geisinger Commonwealth School of Medicine, Scranton, Pa. “It can send a mixed message to a patient after we’ve been stressing the importance of earlier [screening], and then saying, ‘Maybe we got it wrong; maybe we were okay the first time.’ ”
Mark A. Lewis, MD, a GI oncologist, had a similar initial reaction upon hearing about the updated guidelines: “The lack of synchronization across groups is going to create confusion among patients.”
Although he could not say definitively whether the recommendations will affect GI oncologists, because he only sees patients with advanced CRC, he does see the demands in primary care and gastroenterology shifting.
“I think the much bigger impact will be on primary care physicians and gastroenterologists,” said Dr. Lewis, director of gastrointestinal oncology at Intermountain Healthcare in Murray, Utah. “My best guess is that the procedural burden on the latter will be mitigated by more stool testing ordered by primary care physicians. Patients may understandably prefer the convenience and lack of invasiveness of home-based fecal testing, but a positive FIT [fecal immunochemical test] without a follow-up scope is an incomplete screening.”
Dr. Marshall, however, had a different take. He does not envision the updated guidelines having much of a practical impact on physician practice. Most of the country is already not receiving proper colon cancer screenings, he said. Research shows more than 40% of Americans skip standard CRC screenings. Even anecdotally, he noted, friends in their 60s come to him and admit they haven’t had a colonoscopy yet.
Potential impact on patient outcomes, costs
Beyond mixed messaging, some experts worry that pushing CRC screening later could mean cancers are caught later, when they’re more advanced.
Finding cancers earlier, when they are easier and less expensive to treat, make earlier CRC screenings worthwhile, Dr. Woloski explained.
Dr. Lewis sees earlier screening as a way to stop a tumor from progressing before it can really pick up steam.
“To me the biggest advantage of colonoscopy is the interruption of the adenoma-to-carcinoma sequence, whereby a polyp that is completely removed cannot become an invasive adenocarcinoma,” Dr. Lewis said. “We’ve also had evidence for well over a decade that flexible sigmoidoscopy, which doesn’t come close to visualizing the entire colon, can confer a survival benefit.”
Another concern is the potential effect on insurance coverage.
Medicare and other insurers use USPSTF guidelines to make coverage decisions. However, because of this mixed message, Dr. Woloski questioned whether there would be more challenges regarding insurance coverage. “Does it mean primary care doctors are going to have to preauthorize a lot of these screenings even if you have shared decision-making with the patient?” he asked.
When it comes to screening referrals, Douglas A. Corley, MD, PhD, a gastroenterologist at Kaiser Permanente in northern California, said it’s critical for primary care physicians to educate patients about the differing views on screening benefits and harms as well as the different screening options.
“Given the different opinions, it is important to let people in this age group know that screening is an option recommended by some groups,” Dr. Corley said. “Colorectal cancer screening is very effective for decreasing the risk for death from colorectal cancer, which is the second leading cause of cancer death in the United States. Making sure all eligible people know this is an option provides the best way for patients to have an informed choice.”
Dr. Lin has already begun talking with patients about the differing recommendations. He said it’s helpful to simplify the issue and focus the conversation on what patients value most. For more assertive patients whose priority is finding every possible cancer early, starting screenings at age 45 may be reasonable, he said, whereas other patients may not find the process or possible side effects worth it.
“And then you have the middle group that decides, ‘Yes, I want to start at 45, but I want the fecal test. I don’t want to just jump into colonoscopy.’ ” Dr. Lin said. “That would be kind of a compromise where you’d be starting screening earlier, but not subjecting yourself to something that has more potential for harms.”
Dr. Woloski said he plans to continue making referrals based on the USPSTF recommendations.
“With every screening, it is about informed decision-making with the patient, but I think for now, since USPSTF still supports the earlier screening, I will probably stick with offering it earlier,” he said.
But when deciding on the appropriate timing for evaluating CRC, the most important distinction is between screening and diagnosis, Dr. Lewis added.
“The former is only appropriate in patients who are truly asymptomatic and who are truly average-risk,” he said. “The latter is critical in any patient with symptoms. I cannot count the number of times I have seen blood in the stool discounted as hemorrhoids without even an exam, digital rectal, or scope, to demonstrate that hemorrhoids are present and the culprit for blood loss.”
A version of this article first appeared on Medscape.com.
FDA approves new drug for ulcerative colitis
Pfizer announced on Oct. 13.
Etrasimod is an oral sphingosine-1-phosphate (S1P) receptor that binds with high affinity to receptors 1, 4, and 5. The approved recommended dose is 2 mg once daily.
Etrasimod is the second agent in the S1P class approved for UC in the United States. The other agent, ozanimod (Zeposia, Bristol-Myers Squibb), received FDA approval for moderately to severely active UC in May 2021.
The approval of etrasimod was based on safety and efficacy data from two randomized, double-blind, placebo-controlled phase 3 trials: ELEVATE UC 52 trial and the ELEVATE UC 12 trial. The Lancet published full results from the two trials in March.
Both trials enrolled patients with UC who had previously failed or were intolerant of at least one conventional, biologic, or Janus kinase inhibitor therapy.
In ELEVATE UC 52, clinical remission at 12 weeks occurred in 27% of patients taking etrasimod versus 7% of patients taking a placebo (20% difference; P < .001). At week 52, remission rates were 32% with active treatment verus 7% with placebo (26% difference; P < .001).
In ELEVATE UC 12, clinical remission was achieved among 26% of patients who received etrasimod versus 15.0% of patients who received placebo (11% difference; P < .05).
Statistically significant improvements were also observed with etrasimod (vs. placebo) on all key secondary endpoints, including endoscopic improvement and mucosal healing at weeks 12 and 52, and corticosteroid-free remission and sustained clinical remission at week 52.
The most common side effects of etrasimod were found to be headache, elevated values on liver tests, worsening of UC, SARS-CoV-2 infection, dizziness, pyrexia, arthralgia, abdominal pain, and nausea. Full prescribing information is available online.
Etrasimod is “a proven advanced treatment with a favorable benefit-risk profile,” Michael Chiorean, MD, codirector of the IBD Center at Swedish Medical Center, Seattle, who is an investigator in the ELEVATE studies, said in a Pfizer news release.
“UC can affect patients differently and many people living with this disease struggle with ongoing symptoms. The introduction of a new treatment for UC could increase options for patients, and we look forward to seeing the impact of Velsipity for patients across the U.S.,” added Michael Osso, president and CEO of the Crohn’s & Colitis Foundation.
A version of this article first appeared on Medscape.com.
Pfizer announced on Oct. 13.
Etrasimod is an oral sphingosine-1-phosphate (S1P) receptor that binds with high affinity to receptors 1, 4, and 5. The approved recommended dose is 2 mg once daily.
Etrasimod is the second agent in the S1P class approved for UC in the United States. The other agent, ozanimod (Zeposia, Bristol-Myers Squibb), received FDA approval for moderately to severely active UC in May 2021.
The approval of etrasimod was based on safety and efficacy data from two randomized, double-blind, placebo-controlled phase 3 trials: ELEVATE UC 52 trial and the ELEVATE UC 12 trial. The Lancet published full results from the two trials in March.
Both trials enrolled patients with UC who had previously failed or were intolerant of at least one conventional, biologic, or Janus kinase inhibitor therapy.
In ELEVATE UC 52, clinical remission at 12 weeks occurred in 27% of patients taking etrasimod versus 7% of patients taking a placebo (20% difference; P < .001). At week 52, remission rates were 32% with active treatment verus 7% with placebo (26% difference; P < .001).
In ELEVATE UC 12, clinical remission was achieved among 26% of patients who received etrasimod versus 15.0% of patients who received placebo (11% difference; P < .05).
Statistically significant improvements were also observed with etrasimod (vs. placebo) on all key secondary endpoints, including endoscopic improvement and mucosal healing at weeks 12 and 52, and corticosteroid-free remission and sustained clinical remission at week 52.
The most common side effects of etrasimod were found to be headache, elevated values on liver tests, worsening of UC, SARS-CoV-2 infection, dizziness, pyrexia, arthralgia, abdominal pain, and nausea. Full prescribing information is available online.
Etrasimod is “a proven advanced treatment with a favorable benefit-risk profile,” Michael Chiorean, MD, codirector of the IBD Center at Swedish Medical Center, Seattle, who is an investigator in the ELEVATE studies, said in a Pfizer news release.
“UC can affect patients differently and many people living with this disease struggle with ongoing symptoms. The introduction of a new treatment for UC could increase options for patients, and we look forward to seeing the impact of Velsipity for patients across the U.S.,” added Michael Osso, president and CEO of the Crohn’s & Colitis Foundation.
A version of this article first appeared on Medscape.com.
Pfizer announced on Oct. 13.
Etrasimod is an oral sphingosine-1-phosphate (S1P) receptor that binds with high affinity to receptors 1, 4, and 5. The approved recommended dose is 2 mg once daily.
Etrasimod is the second agent in the S1P class approved for UC in the United States. The other agent, ozanimod (Zeposia, Bristol-Myers Squibb), received FDA approval for moderately to severely active UC in May 2021.
The approval of etrasimod was based on safety and efficacy data from two randomized, double-blind, placebo-controlled phase 3 trials: ELEVATE UC 52 trial and the ELEVATE UC 12 trial. The Lancet published full results from the two trials in March.
Both trials enrolled patients with UC who had previously failed or were intolerant of at least one conventional, biologic, or Janus kinase inhibitor therapy.
In ELEVATE UC 52, clinical remission at 12 weeks occurred in 27% of patients taking etrasimod versus 7% of patients taking a placebo (20% difference; P < .001). At week 52, remission rates were 32% with active treatment verus 7% with placebo (26% difference; P < .001).
In ELEVATE UC 12, clinical remission was achieved among 26% of patients who received etrasimod versus 15.0% of patients who received placebo (11% difference; P < .05).
Statistically significant improvements were also observed with etrasimod (vs. placebo) on all key secondary endpoints, including endoscopic improvement and mucosal healing at weeks 12 and 52, and corticosteroid-free remission and sustained clinical remission at week 52.
The most common side effects of etrasimod were found to be headache, elevated values on liver tests, worsening of UC, SARS-CoV-2 infection, dizziness, pyrexia, arthralgia, abdominal pain, and nausea. Full prescribing information is available online.
Etrasimod is “a proven advanced treatment with a favorable benefit-risk profile,” Michael Chiorean, MD, codirector of the IBD Center at Swedish Medical Center, Seattle, who is an investigator in the ELEVATE studies, said in a Pfizer news release.
“UC can affect patients differently and many people living with this disease struggle with ongoing symptoms. The introduction of a new treatment for UC could increase options for patients, and we look forward to seeing the impact of Velsipity for patients across the U.S.,” added Michael Osso, president and CEO of the Crohn’s & Colitis Foundation.
A version of this article first appeared on Medscape.com.