User login
A note from NORD
We extend our sincere thanks to busy health care professionals for taking time to engage with this issue, read the latest advances, and provide the best possible care for your patients. Your dedication is an inspiration, and we value the impact you make in the lives of others.
The year 2023 marks the 40th anniversary of the Orphan Drug Act (ODA), landmark legislation that incentivized drug companies to dedicate more resources towards the development of therapies for people with rare conditions. At the same time, we celebrate NORD’s 40th anniversary. The coalition of rare disease advocates who sparked rare disease advocacy and convinced lawmakers to pass the ODA in 1983 established NORD that same year to provide an ongoing, unified voice for the needs of the rare disease community. For 4 decades, NORD has worked tirelessly to drive supportive policies, advance medical research, and provide education and services for the now 30 million Americans with a rare disease, of which half are children.
In this issue of the Rare Neurological Disease Special Report, you will learn more about the history of the Orphan Drug Act and the founding of NORD. You will also find articles from rare disease specialists on specific diseases and some of the newest therapies approved under the ODA for conditions such as Friedreich ataxia, Fabry disease, Duchenne muscular dystrophy, and amyotrophic lateral sclerosis. Also in the issue are articles about stiff person syndrome and Guillain-Barré syndrome and COVID vaccination.
We invite you to visit NORD’s webpage (www.rarediseases.org) to access resources for yourself and the patients and families you serve. NORD’s Continuing Medical Education Video Library offers cost-free, for-credit, on-demand rare disease courses developed in collaboration with Platform Q Health. NORD’s Caring for Rare quarterly newsletter provides updates on educational opportunities, events, and issues important for the rare disease community. NORD’s Rare Disease Database provides expert-reviewed reports on rare diseases in patient-friendly language.
As we celebrate the incredible progress made over the past 40 years, we also recognize that more than 95% of rare conditions still lack effective therapies. Continued research, development of new orphan products, and advances in treatment and care are needed. NORD will remain steadfast in its commitment to driving progress, inspiring innovation, and providing services for the rare community. We are deeply appreciative of the support you provide to people living with neurological conditions and encourage you to contact NORD any time we can be helpful to you.
Edward Neilan, MD, PhD
NORD’s Chief Medical Officer
We extend our sincere thanks to busy health care professionals for taking time to engage with this issue, read the latest advances, and provide the best possible care for your patients. Your dedication is an inspiration, and we value the impact you make in the lives of others.
The year 2023 marks the 40th anniversary of the Orphan Drug Act (ODA), landmark legislation that incentivized drug companies to dedicate more resources towards the development of therapies for people with rare conditions. At the same time, we celebrate NORD’s 40th anniversary. The coalition of rare disease advocates who sparked rare disease advocacy and convinced lawmakers to pass the ODA in 1983 established NORD that same year to provide an ongoing, unified voice for the needs of the rare disease community. For 4 decades, NORD has worked tirelessly to drive supportive policies, advance medical research, and provide education and services for the now 30 million Americans with a rare disease, of which half are children.
In this issue of the Rare Neurological Disease Special Report, you will learn more about the history of the Orphan Drug Act and the founding of NORD. You will also find articles from rare disease specialists on specific diseases and some of the newest therapies approved under the ODA for conditions such as Friedreich ataxia, Fabry disease, Duchenne muscular dystrophy, and amyotrophic lateral sclerosis. Also in the issue are articles about stiff person syndrome and Guillain-Barré syndrome and COVID vaccination.
We invite you to visit NORD’s webpage (www.rarediseases.org) to access resources for yourself and the patients and families you serve. NORD’s Continuing Medical Education Video Library offers cost-free, for-credit, on-demand rare disease courses developed in collaboration with Platform Q Health. NORD’s Caring for Rare quarterly newsletter provides updates on educational opportunities, events, and issues important for the rare disease community. NORD’s Rare Disease Database provides expert-reviewed reports on rare diseases in patient-friendly language.
As we celebrate the incredible progress made over the past 40 years, we also recognize that more than 95% of rare conditions still lack effective therapies. Continued research, development of new orphan products, and advances in treatment and care are needed. NORD will remain steadfast in its commitment to driving progress, inspiring innovation, and providing services for the rare community. We are deeply appreciative of the support you provide to people living with neurological conditions and encourage you to contact NORD any time we can be helpful to you.
Edward Neilan, MD, PhD
NORD’s Chief Medical Officer
We extend our sincere thanks to busy health care professionals for taking time to engage with this issue, read the latest advances, and provide the best possible care for your patients. Your dedication is an inspiration, and we value the impact you make in the lives of others.
The year 2023 marks the 40th anniversary of the Orphan Drug Act (ODA), landmark legislation that incentivized drug companies to dedicate more resources towards the development of therapies for people with rare conditions. At the same time, we celebrate NORD’s 40th anniversary. The coalition of rare disease advocates who sparked rare disease advocacy and convinced lawmakers to pass the ODA in 1983 established NORD that same year to provide an ongoing, unified voice for the needs of the rare disease community. For 4 decades, NORD has worked tirelessly to drive supportive policies, advance medical research, and provide education and services for the now 30 million Americans with a rare disease, of which half are children.
In this issue of the Rare Neurological Disease Special Report, you will learn more about the history of the Orphan Drug Act and the founding of NORD. You will also find articles from rare disease specialists on specific diseases and some of the newest therapies approved under the ODA for conditions such as Friedreich ataxia, Fabry disease, Duchenne muscular dystrophy, and amyotrophic lateral sclerosis. Also in the issue are articles about stiff person syndrome and Guillain-Barré syndrome and COVID vaccination.
We invite you to visit NORD’s webpage (www.rarediseases.org) to access resources for yourself and the patients and families you serve. NORD’s Continuing Medical Education Video Library offers cost-free, for-credit, on-demand rare disease courses developed in collaboration with Platform Q Health. NORD’s Caring for Rare quarterly newsletter provides updates on educational opportunities, events, and issues important for the rare disease community. NORD’s Rare Disease Database provides expert-reviewed reports on rare diseases in patient-friendly language.
As we celebrate the incredible progress made over the past 40 years, we also recognize that more than 95% of rare conditions still lack effective therapies. Continued research, development of new orphan products, and advances in treatment and care are needed. NORD will remain steadfast in its commitment to driving progress, inspiring innovation, and providing services for the rare community. We are deeply appreciative of the support you provide to people living with neurological conditions and encourage you to contact NORD any time we can be helpful to you.
Edward Neilan, MD, PhD
NORD’s Chief Medical Officer
Editor’s note
2023 is indeed a noteworthy year. As you will read in this issue, it marks the 40th anniversary of the landmark Orphan Drug Act and the formation of the National Organization for Rare Disorders (NORD). 2023 also marks the 30th anniversary of Neurology Reviews, the parent publication of the Rare Neurological Disease Special Report. While Neurology Reviews covers rare disease news throughout the year (see our Rare Disease Roundup in this issue), it is in our annual supplement where our rare disease news coverage and our partnership with NORD truly shines.
In this issue we take pride in taking a deeper look at some of the rare neurological diseases that have made headlines as well as the therapeutic advances and research breakthroughs that continue to benefit patients and the rare disease community as a whole. While I would prefer to humbly serve the rare disease community through our news coverage and educational efforts, I would be remiss if I didn’t mention that our 2022 Rare Neurological Disease Special Report won a Silver Regional Award in the category of annual supplement in the American Society of Business Publication Editors (Azbee) yearly competition. With that moment of bragging aside, I invite you to read this year’s issue, and I thank you for the success that this supplement has enjoyed since it launched in 2015.
Glenn S. Williams,
2023 is indeed a noteworthy year. As you will read in this issue, it marks the 40th anniversary of the landmark Orphan Drug Act and the formation of the National Organization for Rare Disorders (NORD). 2023 also marks the 30th anniversary of Neurology Reviews, the parent publication of the Rare Neurological Disease Special Report. While Neurology Reviews covers rare disease news throughout the year (see our Rare Disease Roundup in this issue), it is in our annual supplement where our rare disease news coverage and our partnership with NORD truly shines.
In this issue we take pride in taking a deeper look at some of the rare neurological diseases that have made headlines as well as the therapeutic advances and research breakthroughs that continue to benefit patients and the rare disease community as a whole. While I would prefer to humbly serve the rare disease community through our news coverage and educational efforts, I would be remiss if I didn’t mention that our 2022 Rare Neurological Disease Special Report won a Silver Regional Award in the category of annual supplement in the American Society of Business Publication Editors (Azbee) yearly competition. With that moment of bragging aside, I invite you to read this year’s issue, and I thank you for the success that this supplement has enjoyed since it launched in 2015.
Glenn S. Williams,
2023 is indeed a noteworthy year. As you will read in this issue, it marks the 40th anniversary of the landmark Orphan Drug Act and the formation of the National Organization for Rare Disorders (NORD). 2023 also marks the 30th anniversary of Neurology Reviews, the parent publication of the Rare Neurological Disease Special Report. While Neurology Reviews covers rare disease news throughout the year (see our Rare Disease Roundup in this issue), it is in our annual supplement where our rare disease news coverage and our partnership with NORD truly shines.
In this issue we take pride in taking a deeper look at some of the rare neurological diseases that have made headlines as well as the therapeutic advances and research breakthroughs that continue to benefit patients and the rare disease community as a whole. While I would prefer to humbly serve the rare disease community through our news coverage and educational efforts, I would be remiss if I didn’t mention that our 2022 Rare Neurological Disease Special Report won a Silver Regional Award in the category of annual supplement in the American Society of Business Publication Editors (Azbee) yearly competition. With that moment of bragging aside, I invite you to read this year’s issue, and I thank you for the success that this supplement has enjoyed since it launched in 2015.
Glenn S. Williams,
2023 Rare Neurological Disease Special Report
INTRODUCTIONS
Editor’s note
By Glenn S. Williams
2023 is indeed a noteworthy year. As you will read in this issue, it marks the 40th anniversary of the landmark Orphan Drug Act (ODA) and the formation of the National Organization for Rare Disorders. 2023 also marks the 30th anniversary of Neurology Reviews, the parent publication of the Rare Neurological Disease Special Report.
A note from NORD
By Edward Neilan, MD, PhD
The coalition of rare disease advocates who sparked rare disease advocacy and convinced lawmakers to pass the ODA in 1983 established NORD that same year to provide an ongoing, unified voice for the needs of the rare disease community.
Rare disease roundup
A look back at some of the 2023 rare disease headlines from Neurology Reviews.
CLINICAL REVIEWS
The Orphan Drug Act and NORD at their 40th anniversary: Dramatic achievements and ongoing innovation
By Batya Swift Yasgur, MA, MSW
The movement whose face is ODA and NORD continues to build its legacy. Next? Progress in rare disease care will require an all-in approach to solving a looming and massive public health challenge.
Emerging therapies in Duchenne and facioscapulohumeral muscular dystrophy
By Frieda Wiley, PharmD
Newly approved and investigational therapies, and enhanced diagnostics, are sparking optimism about treating MD – especially Duchenne and facioscapulohumeral types.
Has prompt diagnosis of amyotrophic lateral sclerosis become urgent?
By Ted Bosworth
Optimism is high about improving the survival and care of ALS patients. Neurologists who don’t specialize in ALS can add to the positivity by endorsing a role in speedier diagnostic pathways.
A new chapter for research on treating Huntington’s disease
By Jennie Smith
Setbacks in trials of protein-lowering therapies – mostly over their safety – mask a story of rapid advances and a more recently discovered treatment pathway that also offers promise for other diseases.
The dawning age of therapy for Friedreich ataxia
By Neil Osterweil
The first therapy to target the underlying pathology of Friedreich ataxia was approved in 2023. Other drug and genetic therapies are in the pipeline.
Stiff person syndrome: When a rare disorder hits the headlines
By Kate Johnson
Awareness of this disorder is increasing, but clinicians are challenged to apply the proper workup to avoid wrong turns in identifying affected patients.
Advances in testing and therapeutics are improving the lives of patients with Fabry disease
By Lorraine L. Janeczko, MPH
Thanks to robust research efforts, treatment options are expanding and patients are getting their diagnosis earlier – often, when they are presymptomatic and treatment has greater potential for enhancing quality of life.
Guillain-Barré syndrome: Honing treatment strategies
By John Jesitus
Classic subtypes of Guillain-Barré syndrome are varying manifestations of a shared disease process, novel insights into the disease indicate. This understanding is yielding new treatment strategies.
INTRODUCTIONS
Editor’s note
By Glenn S. Williams
2023 is indeed a noteworthy year. As you will read in this issue, it marks the 40th anniversary of the landmark Orphan Drug Act (ODA) and the formation of the National Organization for Rare Disorders. 2023 also marks the 30th anniversary of Neurology Reviews, the parent publication of the Rare Neurological Disease Special Report.
A note from NORD
By Edward Neilan, MD, PhD
The coalition of rare disease advocates who sparked rare disease advocacy and convinced lawmakers to pass the ODA in 1983 established NORD that same year to provide an ongoing, unified voice for the needs of the rare disease community.
Rare disease roundup
A look back at some of the 2023 rare disease headlines from Neurology Reviews.
CLINICAL REVIEWS
The Orphan Drug Act and NORD at their 40th anniversary: Dramatic achievements and ongoing innovation
By Batya Swift Yasgur, MA, MSW
The movement whose face is ODA and NORD continues to build its legacy. Next? Progress in rare disease care will require an all-in approach to solving a looming and massive public health challenge.
Emerging therapies in Duchenne and facioscapulohumeral muscular dystrophy
By Frieda Wiley, PharmD
Newly approved and investigational therapies, and enhanced diagnostics, are sparking optimism about treating MD – especially Duchenne and facioscapulohumeral types.
Has prompt diagnosis of amyotrophic lateral sclerosis become urgent?
By Ted Bosworth
Optimism is high about improving the survival and care of ALS patients. Neurologists who don’t specialize in ALS can add to the positivity by endorsing a role in speedier diagnostic pathways.
A new chapter for research on treating Huntington’s disease
By Jennie Smith
Setbacks in trials of protein-lowering therapies – mostly over their safety – mask a story of rapid advances and a more recently discovered treatment pathway that also offers promise for other diseases.
The dawning age of therapy for Friedreich ataxia
By Neil Osterweil
The first therapy to target the underlying pathology of Friedreich ataxia was approved in 2023. Other drug and genetic therapies are in the pipeline.
Stiff person syndrome: When a rare disorder hits the headlines
By Kate Johnson
Awareness of this disorder is increasing, but clinicians are challenged to apply the proper workup to avoid wrong turns in identifying affected patients.
Advances in testing and therapeutics are improving the lives of patients with Fabry disease
By Lorraine L. Janeczko, MPH
Thanks to robust research efforts, treatment options are expanding and patients are getting their diagnosis earlier – often, when they are presymptomatic and treatment has greater potential for enhancing quality of life.
Guillain-Barré syndrome: Honing treatment strategies
By John Jesitus
Classic subtypes of Guillain-Barré syndrome are varying manifestations of a shared disease process, novel insights into the disease indicate. This understanding is yielding new treatment strategies.
INTRODUCTIONS
Editor’s note
By Glenn S. Williams
2023 is indeed a noteworthy year. As you will read in this issue, it marks the 40th anniversary of the landmark Orphan Drug Act (ODA) and the formation of the National Organization for Rare Disorders. 2023 also marks the 30th anniversary of Neurology Reviews, the parent publication of the Rare Neurological Disease Special Report.
A note from NORD
By Edward Neilan, MD, PhD
The coalition of rare disease advocates who sparked rare disease advocacy and convinced lawmakers to pass the ODA in 1983 established NORD that same year to provide an ongoing, unified voice for the needs of the rare disease community.
Rare disease roundup
A look back at some of the 2023 rare disease headlines from Neurology Reviews.
CLINICAL REVIEWS
The Orphan Drug Act and NORD at their 40th anniversary: Dramatic achievements and ongoing innovation
By Batya Swift Yasgur, MA, MSW
The movement whose face is ODA and NORD continues to build its legacy. Next? Progress in rare disease care will require an all-in approach to solving a looming and massive public health challenge.
Emerging therapies in Duchenne and facioscapulohumeral muscular dystrophy
By Frieda Wiley, PharmD
Newly approved and investigational therapies, and enhanced diagnostics, are sparking optimism about treating MD – especially Duchenne and facioscapulohumeral types.
Has prompt diagnosis of amyotrophic lateral sclerosis become urgent?
By Ted Bosworth
Optimism is high about improving the survival and care of ALS patients. Neurologists who don’t specialize in ALS can add to the positivity by endorsing a role in speedier diagnostic pathways.
A new chapter for research on treating Huntington’s disease
By Jennie Smith
Setbacks in trials of protein-lowering therapies – mostly over their safety – mask a story of rapid advances and a more recently discovered treatment pathway that also offers promise for other diseases.
The dawning age of therapy for Friedreich ataxia
By Neil Osterweil
The first therapy to target the underlying pathology of Friedreich ataxia was approved in 2023. Other drug and genetic therapies are in the pipeline.
Stiff person syndrome: When a rare disorder hits the headlines
By Kate Johnson
Awareness of this disorder is increasing, but clinicians are challenged to apply the proper workup to avoid wrong turns in identifying affected patients.
Advances in testing and therapeutics are improving the lives of patients with Fabry disease
By Lorraine L. Janeczko, MPH
Thanks to robust research efforts, treatment options are expanding and patients are getting their diagnosis earlier – often, when they are presymptomatic and treatment has greater potential for enhancing quality of life.
Guillain-Barré syndrome: Honing treatment strategies
By John Jesitus
Classic subtypes of Guillain-Barré syndrome are varying manifestations of a shared disease process, novel insights into the disease indicate. This understanding is yielding new treatment strategies.
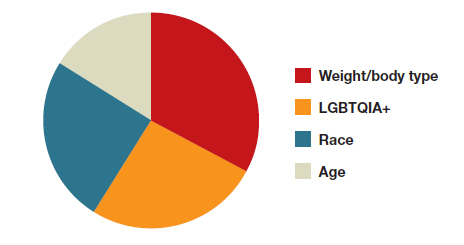
Has prompt diagnosis of amyotrophic lateral sclerosis become urgent?
Amyotrophic lateral sclerosis (ALS) falls easily into the Food and Drug Administration definition of “rare disease.” With an estimated prevalence in the United States of fewer than 20,000 cases,1 ALS sits comfortably below the cutoff of 200,000 cases that serves to define a disease as “rare.”
After a recent steep climb, there are something on the order of 50 therapies, across more than 10 drug classes, in clinical trials for the treatment of ALS.2 This bounty represents exciting progress toward the development of targeted therapies for a characteristically fatal disease.
That headway is coupled with a sobering limitation, however: Relatively few ALS patients are being enrolled.
The knotty problem with therapeutic trials for ALS
“Trials are generally designed for patients with adequate functional reserve and predicted survival, to ensure that a signal of benefit can be seen,” said Nicholas John Maragakis, MD, director of the ALS Clinical Trials Unit at Johns Hopkins University, Baltimore. “Many of my patients are too severely affected at presentation.”
Dr. Maragakis hasn’t calculated the precise percentage of patients he is enrolling in one of the many available trials available at the Johns Hopkins center. He estimates that it is less than 20%, however.
That percentage is comparable to what is reported by Stephen Scelsa, MD, and Daniel J. Macgowan, MD, who share much of the ALS caseload in a dedicated, comprehensive ALS center at Mount Sinai Beth Israel, New York. Both are on the faculty at the Icahn School of Medicine at Mount Sinai.
“The considerable delay in the diagnosis of ALS remains a challenge,” Dr. Scelsa acknowledges. Like Dr. Maragakis, he reports that, by the time patients develop symptoms that make referral to a comprehensive ALS center like Mount Sinai Beth Israel appropriate, many no longer meet eligibility criteria for most experimental treatments.
Some therapeutic targets in clinical trials, such as neuroinflammation, offer potential benefit even in advancing disease, but it is prevention that is usually the goal of experimental ALS therapies. This approach is associated with far more promise than attempting to reverse existing neurologic damage, which might not be possible, according to both Dr. Scelsa and Dr. Macgowan.
“The clinical trials are typically looking for patients with less than 2 years since the onset of symptoms and at least 60% of predicted respiratory function,” Dr. Macgowan said.
Because of these or other similarly restrictive criteria, coupled with common delays before patients arrive at a center where trials are available, “the window for clinical research closes very quickly,” Dr. Macgowan added, and “the band of patients who are eligible is relatively narrow.”
At Hennepin Healthcare in Minneapolis, which, like Johns Hopkins and Mount Sinai, offers an advanced multidisciplinary approach to ALS care in a dedicated clinic, the problem of late referrals is no different. Samuel Maiser, MD, chair of neurology, does attempt to counter this delay by moving quickly.
“I almost always offer a therapeutic trial to a patient with early-stage ALS,” he said. He does so earlier, rather than later, and explains: “I do not want to delay that conversation, because any delay might reduce the chance for getting into a trial.”
The generalist can make a difference in therapeutic success
The proliferation of clinical trials has made early diagnosis of ALS urgent. However, the experts interviewed for this article agreed: Accelerating the time to diagnosis is more dependent on the general neurologist or primary care physician than on the ALS specialist. ALS is a diagnosis of exclusion, but there is now very little delay in reaching a probable diagnosis at a dedicated center.
Yet neurodegenerative complaints in early-stage ALS are often nonspecific and mild; confidence in making a potential diagnosis of ALS is limited among primary care clinicians and general neurologists, who almost always see these patients first. Usually, the problem is not failure to include ALS in the differential diagnosis but hesitation in being candid when there is still doubt.
General neurologists, in particular, Dr. Maragakis said, “are often highly suspicious of a diagnosis of ALS very early on but are concerned about using this term until the clinical signs are more compelling.”
This is understandable. There is reluctance to deliver bad news when confidence in the diagnosis is limited. But the experts agreed: Delayed diagnosis is not in the patient’s interest now that there is at least the potential for entering a trial supported by a scientific rationale for benefit.
“Waiting for 100% certainty – this could actually harm our patients,” Dr. Maiser said. The tendency to avoid delivering bad news, he said, “is human nature, and it is not easy to tell people that ALS is the potential cause, but it’s important for early treatment.”
Some evidence suggests that the incidence of ALS is increasing3 but this is not necessarily evident at the clinical level. “It is not my impression that the incidence of ALS is increasing,” Dr. Macgowan said, “so much as I think we are getting better at making the diagnosis.”
Where we stand: Pathophysiology, diagnosis, treatment
Pathophysiology. ALS is characterized by muscle denervation.4 In the great majority of cases, the disease represents a proteinopathy involving loss of the TDP-43 protein from nuclei. However, pathological heterogeneity means that other pathophysiological mechanisms – mediated by oxidative stress, mitochondrial dysfunction, and neurotoxicity related to excessive stimulation of postsynaptic glutamate receptors – can participate.2,5,6
Approximately 10% of patients have a known gene associated with ALS.7 The rest have what is considered sporadic ALS, although some experts estimate that heritability will eventually be confirmed in 50% or more of cases that have been given the “sporadic” label.8,9 More than 30 genes have been linked to ALS in genomewide association studies. Among patients whose disease carries a known familial link, four genes – SOD1, TARDBP, FUS, and C9orf72 – account for approximately 70% of cases.2
Diagnosis. Genetic testing in patients with suspected or confirmed ALS is the standard of care at most, if not all, comprehensive ALS treatment centers, according to the four experts interviewed by Neurology Reviews 2023 Rare Neurological Disease Special Report for this article. Such testing was routine for years because of its potential for helping researchers to understand subtypes of disease; today, testing has assumed even greater practical value with recent approval of the first ALS gene therapy: Tofersen (Qalsody, Biogen), licensed in 2023, is an antisense oligonucleotide therapy that targets SOD1 mRNA to reduce production of the SOD1 protein, a mediator of disease progression.
“Genetic testing has been useful for telling us something about the disease and its prognosis,” Dr. Maragakis said, “but an approved gene therapy means it can have a direct effect on treatment.”
ALS therapeutics. Other gene therapies are in development. Gene signatures are likely to provide even more opportunities for clinical trials in the future.
Following three loading doses of tofersen at 14-day intervals, the maintenance regimen, administered intrathecally by lumbar puncture, is every 28 days. In the phase 3 trial, tofersen reduced levels of SOD1 protein and neurofilament light chain, a biomarker of axonal injury.10 Tofersen is appropriate only in patients with SOD1-associated ALS; the drug’s favorable clinical impact, including a positive effect, if any, on survival has not been demonstrated. Extension studies are underway.
Tofersen joins three other FDA-approved ALS therapies:
• Riluzole, an oral drug available since 1995 that slows disease progression by blocking glutamate.
• Edaravone, an antioxidant approved in 2017, administered orally or intravenously.
• An orally administered combination of sodium phenylbutyrate and taurursodiol marketed as Relyvrio and formerly known as AMX0035, that was introduced in 2022.
“We offer riluzole, which is safe in combination with other therapies, to most patients,” said Dr. Scelsa, who noted that treatment trials often test experimental drugs on top of riluzole. He moves to edaravone or Relyvrio, which are far more expensive, selectively. Tofersen, which is also expensive, is reserved for patients with SOD1-associated disease; however, not all eligible patients opt for this therapy after reviewing its benefits and risks.
“There is not yet a guarantee that tofersen will improve outcomes, and it requires intrathecal injections for life,”
Dr. Maiser said. “Some patients, particularly my older patients, have said, ‘No thank you,’ based on the available data.”
Dr. Macgowan pointed out that lumbar puncture repeated indefinitely can be “challenging.” He, too, discusses all available treatment options with every patient, including riluzole, which he agreed is associated with a meaningful benefit, particularly when started early.
Because of the safety of riluzole, Dr. Maragakis takes early treatment a step further. For neurologists who have a high level of suspicion of ALS in a given patient, “my advice would be to treat aggressively from the get-go. Even if not 100% certain of the diagnosis, I would start them on riluzole while waiting for confirmation.” Like the other experts interviewed here, he acknowledged that referral to a busy comprehensive ALS center often takes time, making it reasonable to initiate treatment when suspicion is high.
On the front lines, “the neurologist can tell the patient that ALS is just one of several potential explanations for symptoms but there is concern,” said Dr. Maragakis, proposing a strategy to introduce the possibility of ALS and start treatment that might slow disease while waiting for confirmation of the diagnosis. “My biggest concern is that no one is making that call,” he said, trying to address at least one reason for the current delay in making referrals.
Comprehensive care at specialty centers
Whenever possible, ALS is a disease best managed at a center that offers comprehensive management, including multidisciplinary care. On this point, the four experts agreed.
“Tertiary-care centers for ALS serve a critical purpose,”
Dr. Maiser said. For a disease that affects nearly every aspect of life, the skills of a multidisciplinary support staff offer an “opportunity to stay in front of the disease” for as long as possible. Teamwork often leads to “outside-of-the-box thinking” for helping patients and families cope with the range of disabilities that undermine the patient’s quality of life.
Details of ALS management matter. At Mount Sinai and Hennepin Healthcare, and at Johns Hopkins, where demand recently led to the opening of a second ALS clinic, the ALS center is set up to address the full spectrum of needs. Staff members have multiple skills so that they can work together to make patients comfortable and prepare them for what is inevitably progression – even if the rate of that progression varies.
All these centers incorporate a rational, thorough discussion of end-of-life options in a palliative care approach that targets optimized quality of life. One goal is to prepare patients to consider and be prepared to make decisions when it is time for tracheostomy, percutaneous endoscopic gastrostomy, and other life support options that are not always well tolerated. The goal? Avoiding unnecessary anguish during end-stage disease when impaired respiratory function – the primary cause of ALS-related death – no longer sustains unassisted survival.
“I am concerned for the many ALS patients without access to this type of comprehensive care,” Dr. Macgowan said.
Like the other experts here, he emphasized that the demands of ALS care can be “overwhelming” outside a comprehensive care setting – for the patient, their family, and individual providers.
Looking ahead
There are many reasons to be optimistic about improving the survival and care of patients with ALS. Besides therapies in clinical trials, Dr. Scelsa explained, there is the potential role for monitoring neurofilament light changes, a biomarker of neurodegeneration, in patients who are at risk of ALS.
Dr. Maragakis offered an analogy to the gene therapy onasemnogene abeparvovec, which can prevent the associated neurodegeneration of spinal muscular atrophy if initiated before symptoms appear. He said that, in ALS, neurofilament light changes or other biomarkers might offer an opportunity to halt the progression of disease before it starts – if one or more therapies in development prove workable.
In the meantime, neurologists who do not specialize in ALS should be thinking about how they can participate in speedier diagnostic pathways.
“There are a number of therapies that look promising,” Dr. Maiser told Rare Neurological Disease Special Report. He singled out strategies to degrade TDP-43 or prevent it from forming. If these treatments are found effective, it’s expected that they would be of value in sporadic ALS, the most common form. Again, though, “the challenge is getting patients on this therapy at the earliest stages of disease.”
Dr. Maragakis discloses equity ownership/stock options with Braintrust Bio and Akava; he is a patent holder with Johns Hopkins [ALS] and has received grant/research/clinical trial support from Apellis Pharma, Biogen Idec, Cytokinetics, Helixmith, Calico, Sanofi, Department of Defense ALSRP, Maryland Stem Cell Research Fund, Massachusetts General Hospital, Medicinova, and NINDS. He serves as consultant or advisory board member for Amylyx; Cytokinetics, Roche, Healey Center, Nura Bio, Northeast ALS Consortium, Akava, Inflammx, and Secretome. Dr. Scelsa did not report any conflicts of interest. Dr. Macgowan and Dr. Maiser have no relevant conflicts of interest to disclose.
References
1. Mehta P et al. Prevalence of amyotrophic lateral sclerosis in the United States using established and novel methodologies, 2017. Amyotroph Lateral Scler Frontotemporal Degener. 2023;24(1-2):108-16. doi: 10.1080/21678421.2022.2059380.
2. Mead RJ et al. Amyotrophic lateral sclerosis: A neurodegenerative disorder poised for successful therapeutic translation. Nat Rev Drug Discov. 2023;22(3):185-212. doi: 10.1038/s41573-022-00612-2.
3. Longinetti E and Fang F. Epidemiology of amyotrophic lateral sclerosis: An update of recent literature. Curr Opin Neurol. 2019;32(5):771-6. doi: 10.1097/WCO.0000000000000730.
4. van den Bos MAJ et al. Pathophysiology and diagnosis of ALS: Insights from advances in neurophysiological techniques. Int J Mol Sci. 2019;20(11):2818. doi: 10.3390/ijms20112818.
5. Neumann M et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130-3. doi: 10.1126/science.1134108.
6. Ling S-C et al. Converging mechanisms in ALS and FTD: Disrupted RNA and protein homeostasis. Neuron. 2013;79(3):416-38. doi: 10.1016/j.neuron.2013.07.033.
7. Ranganathan R et al. Multifaceted genes in amyotrophic lateral sclerosis-frontotemporal dementia. Front Neurosci. 2020;14:684. doi: 10.3389/fnins.2020.00684.
8. Ryan M et al. Lifetime risk and heritability of amyotrophic lateral sclerosis. JAMA Neurol. 2019;76(11):1367-74. doi: 10.1001/jamaneurol.2019.2044.
9. van Rheenen W et al. Common and rare variant association analyses in amyotrophic lateral sclerosis identify 15 risk loci with distinct genetic architectures and neuron-specific biology. Nat Genet. 2021;53(12):1636-48. doi: 10.1038/s41588-021-00973-1.
10. Miller TM et al; VALOR and OLE Working Group. Trial of antisense oligonucleotide tofersen for SOD1 ALS. N Engl J Med. 2022;387(12):1099-110. doi: 10.1056/NEJMoa2204705.
Amyotrophic lateral sclerosis (ALS) falls easily into the Food and Drug Administration definition of “rare disease.” With an estimated prevalence in the United States of fewer than 20,000 cases,1 ALS sits comfortably below the cutoff of 200,000 cases that serves to define a disease as “rare.”
After a recent steep climb, there are something on the order of 50 therapies, across more than 10 drug classes, in clinical trials for the treatment of ALS.2 This bounty represents exciting progress toward the development of targeted therapies for a characteristically fatal disease.
That headway is coupled with a sobering limitation, however: Relatively few ALS patients are being enrolled.
The knotty problem with therapeutic trials for ALS
“Trials are generally designed for patients with adequate functional reserve and predicted survival, to ensure that a signal of benefit can be seen,” said Nicholas John Maragakis, MD, director of the ALS Clinical Trials Unit at Johns Hopkins University, Baltimore. “Many of my patients are too severely affected at presentation.”
Dr. Maragakis hasn’t calculated the precise percentage of patients he is enrolling in one of the many available trials available at the Johns Hopkins center. He estimates that it is less than 20%, however.
That percentage is comparable to what is reported by Stephen Scelsa, MD, and Daniel J. Macgowan, MD, who share much of the ALS caseload in a dedicated, comprehensive ALS center at Mount Sinai Beth Israel, New York. Both are on the faculty at the Icahn School of Medicine at Mount Sinai.
“The considerable delay in the diagnosis of ALS remains a challenge,” Dr. Scelsa acknowledges. Like Dr. Maragakis, he reports that, by the time patients develop symptoms that make referral to a comprehensive ALS center like Mount Sinai Beth Israel appropriate, many no longer meet eligibility criteria for most experimental treatments.
Some therapeutic targets in clinical trials, such as neuroinflammation, offer potential benefit even in advancing disease, but it is prevention that is usually the goal of experimental ALS therapies. This approach is associated with far more promise than attempting to reverse existing neurologic damage, which might not be possible, according to both Dr. Scelsa and Dr. Macgowan.
“The clinical trials are typically looking for patients with less than 2 years since the onset of symptoms and at least 60% of predicted respiratory function,” Dr. Macgowan said.
Because of these or other similarly restrictive criteria, coupled with common delays before patients arrive at a center where trials are available, “the window for clinical research closes very quickly,” Dr. Macgowan added, and “the band of patients who are eligible is relatively narrow.”
At Hennepin Healthcare in Minneapolis, which, like Johns Hopkins and Mount Sinai, offers an advanced multidisciplinary approach to ALS care in a dedicated clinic, the problem of late referrals is no different. Samuel Maiser, MD, chair of neurology, does attempt to counter this delay by moving quickly.
“I almost always offer a therapeutic trial to a patient with early-stage ALS,” he said. He does so earlier, rather than later, and explains: “I do not want to delay that conversation, because any delay might reduce the chance for getting into a trial.”
The generalist can make a difference in therapeutic success
The proliferation of clinical trials has made early diagnosis of ALS urgent. However, the experts interviewed for this article agreed: Accelerating the time to diagnosis is more dependent on the general neurologist or primary care physician than on the ALS specialist. ALS is a diagnosis of exclusion, but there is now very little delay in reaching a probable diagnosis at a dedicated center.
Yet neurodegenerative complaints in early-stage ALS are often nonspecific and mild; confidence in making a potential diagnosis of ALS is limited among primary care clinicians and general neurologists, who almost always see these patients first. Usually, the problem is not failure to include ALS in the differential diagnosis but hesitation in being candid when there is still doubt.
General neurologists, in particular, Dr. Maragakis said, “are often highly suspicious of a diagnosis of ALS very early on but are concerned about using this term until the clinical signs are more compelling.”
This is understandable. There is reluctance to deliver bad news when confidence in the diagnosis is limited. But the experts agreed: Delayed diagnosis is not in the patient’s interest now that there is at least the potential for entering a trial supported by a scientific rationale for benefit.
“Waiting for 100% certainty – this could actually harm our patients,” Dr. Maiser said. The tendency to avoid delivering bad news, he said, “is human nature, and it is not easy to tell people that ALS is the potential cause, but it’s important for early treatment.”
Some evidence suggests that the incidence of ALS is increasing3 but this is not necessarily evident at the clinical level. “It is not my impression that the incidence of ALS is increasing,” Dr. Macgowan said, “so much as I think we are getting better at making the diagnosis.”
Where we stand: Pathophysiology, diagnosis, treatment
Pathophysiology. ALS is characterized by muscle denervation.4 In the great majority of cases, the disease represents a proteinopathy involving loss of the TDP-43 protein from nuclei. However, pathological heterogeneity means that other pathophysiological mechanisms – mediated by oxidative stress, mitochondrial dysfunction, and neurotoxicity related to excessive stimulation of postsynaptic glutamate receptors – can participate.2,5,6
Approximately 10% of patients have a known gene associated with ALS.7 The rest have what is considered sporadic ALS, although some experts estimate that heritability will eventually be confirmed in 50% or more of cases that have been given the “sporadic” label.8,9 More than 30 genes have been linked to ALS in genomewide association studies. Among patients whose disease carries a known familial link, four genes – SOD1, TARDBP, FUS, and C9orf72 – account for approximately 70% of cases.2
Diagnosis. Genetic testing in patients with suspected or confirmed ALS is the standard of care at most, if not all, comprehensive ALS treatment centers, according to the four experts interviewed by Neurology Reviews 2023 Rare Neurological Disease Special Report for this article. Such testing was routine for years because of its potential for helping researchers to understand subtypes of disease; today, testing has assumed even greater practical value with recent approval of the first ALS gene therapy: Tofersen (Qalsody, Biogen), licensed in 2023, is an antisense oligonucleotide therapy that targets SOD1 mRNA to reduce production of the SOD1 protein, a mediator of disease progression.
“Genetic testing has been useful for telling us something about the disease and its prognosis,” Dr. Maragakis said, “but an approved gene therapy means it can have a direct effect on treatment.”
ALS therapeutics. Other gene therapies are in development. Gene signatures are likely to provide even more opportunities for clinical trials in the future.
Following three loading doses of tofersen at 14-day intervals, the maintenance regimen, administered intrathecally by lumbar puncture, is every 28 days. In the phase 3 trial, tofersen reduced levels of SOD1 protein and neurofilament light chain, a biomarker of axonal injury.10 Tofersen is appropriate only in patients with SOD1-associated ALS; the drug’s favorable clinical impact, including a positive effect, if any, on survival has not been demonstrated. Extension studies are underway.
Tofersen joins three other FDA-approved ALS therapies:
• Riluzole, an oral drug available since 1995 that slows disease progression by blocking glutamate.
• Edaravone, an antioxidant approved in 2017, administered orally or intravenously.
• An orally administered combination of sodium phenylbutyrate and taurursodiol marketed as Relyvrio and formerly known as AMX0035, that was introduced in 2022.
“We offer riluzole, which is safe in combination with other therapies, to most patients,” said Dr. Scelsa, who noted that treatment trials often test experimental drugs on top of riluzole. He moves to edaravone or Relyvrio, which are far more expensive, selectively. Tofersen, which is also expensive, is reserved for patients with SOD1-associated disease; however, not all eligible patients opt for this therapy after reviewing its benefits and risks.
“There is not yet a guarantee that tofersen will improve outcomes, and it requires intrathecal injections for life,”
Dr. Maiser said. “Some patients, particularly my older patients, have said, ‘No thank you,’ based on the available data.”
Dr. Macgowan pointed out that lumbar puncture repeated indefinitely can be “challenging.” He, too, discusses all available treatment options with every patient, including riluzole, which he agreed is associated with a meaningful benefit, particularly when started early.
Because of the safety of riluzole, Dr. Maragakis takes early treatment a step further. For neurologists who have a high level of suspicion of ALS in a given patient, “my advice would be to treat aggressively from the get-go. Even if not 100% certain of the diagnosis, I would start them on riluzole while waiting for confirmation.” Like the other experts interviewed here, he acknowledged that referral to a busy comprehensive ALS center often takes time, making it reasonable to initiate treatment when suspicion is high.
On the front lines, “the neurologist can tell the patient that ALS is just one of several potential explanations for symptoms but there is concern,” said Dr. Maragakis, proposing a strategy to introduce the possibility of ALS and start treatment that might slow disease while waiting for confirmation of the diagnosis. “My biggest concern is that no one is making that call,” he said, trying to address at least one reason for the current delay in making referrals.
Comprehensive care at specialty centers
Whenever possible, ALS is a disease best managed at a center that offers comprehensive management, including multidisciplinary care. On this point, the four experts agreed.
“Tertiary-care centers for ALS serve a critical purpose,”
Dr. Maiser said. For a disease that affects nearly every aspect of life, the skills of a multidisciplinary support staff offer an “opportunity to stay in front of the disease” for as long as possible. Teamwork often leads to “outside-of-the-box thinking” for helping patients and families cope with the range of disabilities that undermine the patient’s quality of life.
Details of ALS management matter. At Mount Sinai and Hennepin Healthcare, and at Johns Hopkins, where demand recently led to the opening of a second ALS clinic, the ALS center is set up to address the full spectrum of needs. Staff members have multiple skills so that they can work together to make patients comfortable and prepare them for what is inevitably progression – even if the rate of that progression varies.
All these centers incorporate a rational, thorough discussion of end-of-life options in a palliative care approach that targets optimized quality of life. One goal is to prepare patients to consider and be prepared to make decisions when it is time for tracheostomy, percutaneous endoscopic gastrostomy, and other life support options that are not always well tolerated. The goal? Avoiding unnecessary anguish during end-stage disease when impaired respiratory function – the primary cause of ALS-related death – no longer sustains unassisted survival.
“I am concerned for the many ALS patients without access to this type of comprehensive care,” Dr. Macgowan said.
Like the other experts here, he emphasized that the demands of ALS care can be “overwhelming” outside a comprehensive care setting – for the patient, their family, and individual providers.
Looking ahead
There are many reasons to be optimistic about improving the survival and care of patients with ALS. Besides therapies in clinical trials, Dr. Scelsa explained, there is the potential role for monitoring neurofilament light changes, a biomarker of neurodegeneration, in patients who are at risk of ALS.
Dr. Maragakis offered an analogy to the gene therapy onasemnogene abeparvovec, which can prevent the associated neurodegeneration of spinal muscular atrophy if initiated before symptoms appear. He said that, in ALS, neurofilament light changes or other biomarkers might offer an opportunity to halt the progression of disease before it starts – if one or more therapies in development prove workable.
In the meantime, neurologists who do not specialize in ALS should be thinking about how they can participate in speedier diagnostic pathways.
“There are a number of therapies that look promising,” Dr. Maiser told Rare Neurological Disease Special Report. He singled out strategies to degrade TDP-43 or prevent it from forming. If these treatments are found effective, it’s expected that they would be of value in sporadic ALS, the most common form. Again, though, “the challenge is getting patients on this therapy at the earliest stages of disease.”
Dr. Maragakis discloses equity ownership/stock options with Braintrust Bio and Akava; he is a patent holder with Johns Hopkins [ALS] and has received grant/research/clinical trial support from Apellis Pharma, Biogen Idec, Cytokinetics, Helixmith, Calico, Sanofi, Department of Defense ALSRP, Maryland Stem Cell Research Fund, Massachusetts General Hospital, Medicinova, and NINDS. He serves as consultant or advisory board member for Amylyx; Cytokinetics, Roche, Healey Center, Nura Bio, Northeast ALS Consortium, Akava, Inflammx, and Secretome. Dr. Scelsa did not report any conflicts of interest. Dr. Macgowan and Dr. Maiser have no relevant conflicts of interest to disclose.
References
1. Mehta P et al. Prevalence of amyotrophic lateral sclerosis in the United States using established and novel methodologies, 2017. Amyotroph Lateral Scler Frontotemporal Degener. 2023;24(1-2):108-16. doi: 10.1080/21678421.2022.2059380.
2. Mead RJ et al. Amyotrophic lateral sclerosis: A neurodegenerative disorder poised for successful therapeutic translation. Nat Rev Drug Discov. 2023;22(3):185-212. doi: 10.1038/s41573-022-00612-2.
3. Longinetti E and Fang F. Epidemiology of amyotrophic lateral sclerosis: An update of recent literature. Curr Opin Neurol. 2019;32(5):771-6. doi: 10.1097/WCO.0000000000000730.
4. van den Bos MAJ et al. Pathophysiology and diagnosis of ALS: Insights from advances in neurophysiological techniques. Int J Mol Sci. 2019;20(11):2818. doi: 10.3390/ijms20112818.
5. Neumann M et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130-3. doi: 10.1126/science.1134108.
6. Ling S-C et al. Converging mechanisms in ALS and FTD: Disrupted RNA and protein homeostasis. Neuron. 2013;79(3):416-38. doi: 10.1016/j.neuron.2013.07.033.
7. Ranganathan R et al. Multifaceted genes in amyotrophic lateral sclerosis-frontotemporal dementia. Front Neurosci. 2020;14:684. doi: 10.3389/fnins.2020.00684.
8. Ryan M et al. Lifetime risk and heritability of amyotrophic lateral sclerosis. JAMA Neurol. 2019;76(11):1367-74. doi: 10.1001/jamaneurol.2019.2044.
9. van Rheenen W et al. Common and rare variant association analyses in amyotrophic lateral sclerosis identify 15 risk loci with distinct genetic architectures and neuron-specific biology. Nat Genet. 2021;53(12):1636-48. doi: 10.1038/s41588-021-00973-1.
10. Miller TM et al; VALOR and OLE Working Group. Trial of antisense oligonucleotide tofersen for SOD1 ALS. N Engl J Med. 2022;387(12):1099-110. doi: 10.1056/NEJMoa2204705.
Amyotrophic lateral sclerosis (ALS) falls easily into the Food and Drug Administration definition of “rare disease.” With an estimated prevalence in the United States of fewer than 20,000 cases,1 ALS sits comfortably below the cutoff of 200,000 cases that serves to define a disease as “rare.”
After a recent steep climb, there are something on the order of 50 therapies, across more than 10 drug classes, in clinical trials for the treatment of ALS.2 This bounty represents exciting progress toward the development of targeted therapies for a characteristically fatal disease.
That headway is coupled with a sobering limitation, however: Relatively few ALS patients are being enrolled.
The knotty problem with therapeutic trials for ALS
“Trials are generally designed for patients with adequate functional reserve and predicted survival, to ensure that a signal of benefit can be seen,” said Nicholas John Maragakis, MD, director of the ALS Clinical Trials Unit at Johns Hopkins University, Baltimore. “Many of my patients are too severely affected at presentation.”
Dr. Maragakis hasn’t calculated the precise percentage of patients he is enrolling in one of the many available trials available at the Johns Hopkins center. He estimates that it is less than 20%, however.
That percentage is comparable to what is reported by Stephen Scelsa, MD, and Daniel J. Macgowan, MD, who share much of the ALS caseload in a dedicated, comprehensive ALS center at Mount Sinai Beth Israel, New York. Both are on the faculty at the Icahn School of Medicine at Mount Sinai.
“The considerable delay in the diagnosis of ALS remains a challenge,” Dr. Scelsa acknowledges. Like Dr. Maragakis, he reports that, by the time patients develop symptoms that make referral to a comprehensive ALS center like Mount Sinai Beth Israel appropriate, many no longer meet eligibility criteria for most experimental treatments.
Some therapeutic targets in clinical trials, such as neuroinflammation, offer potential benefit even in advancing disease, but it is prevention that is usually the goal of experimental ALS therapies. This approach is associated with far more promise than attempting to reverse existing neurologic damage, which might not be possible, according to both Dr. Scelsa and Dr. Macgowan.
“The clinical trials are typically looking for patients with less than 2 years since the onset of symptoms and at least 60% of predicted respiratory function,” Dr. Macgowan said.
Because of these or other similarly restrictive criteria, coupled with common delays before patients arrive at a center where trials are available, “the window for clinical research closes very quickly,” Dr. Macgowan added, and “the band of patients who are eligible is relatively narrow.”
At Hennepin Healthcare in Minneapolis, which, like Johns Hopkins and Mount Sinai, offers an advanced multidisciplinary approach to ALS care in a dedicated clinic, the problem of late referrals is no different. Samuel Maiser, MD, chair of neurology, does attempt to counter this delay by moving quickly.
“I almost always offer a therapeutic trial to a patient with early-stage ALS,” he said. He does so earlier, rather than later, and explains: “I do not want to delay that conversation, because any delay might reduce the chance for getting into a trial.”
The generalist can make a difference in therapeutic success
The proliferation of clinical trials has made early diagnosis of ALS urgent. However, the experts interviewed for this article agreed: Accelerating the time to diagnosis is more dependent on the general neurologist or primary care physician than on the ALS specialist. ALS is a diagnosis of exclusion, but there is now very little delay in reaching a probable diagnosis at a dedicated center.
Yet neurodegenerative complaints in early-stage ALS are often nonspecific and mild; confidence in making a potential diagnosis of ALS is limited among primary care clinicians and general neurologists, who almost always see these patients first. Usually, the problem is not failure to include ALS in the differential diagnosis but hesitation in being candid when there is still doubt.
General neurologists, in particular, Dr. Maragakis said, “are often highly suspicious of a diagnosis of ALS very early on but are concerned about using this term until the clinical signs are more compelling.”
This is understandable. There is reluctance to deliver bad news when confidence in the diagnosis is limited. But the experts agreed: Delayed diagnosis is not in the patient’s interest now that there is at least the potential for entering a trial supported by a scientific rationale for benefit.
“Waiting for 100% certainty – this could actually harm our patients,” Dr. Maiser said. The tendency to avoid delivering bad news, he said, “is human nature, and it is not easy to tell people that ALS is the potential cause, but it’s important for early treatment.”
Some evidence suggests that the incidence of ALS is increasing3 but this is not necessarily evident at the clinical level. “It is not my impression that the incidence of ALS is increasing,” Dr. Macgowan said, “so much as I think we are getting better at making the diagnosis.”
Where we stand: Pathophysiology, diagnosis, treatment
Pathophysiology. ALS is characterized by muscle denervation.4 In the great majority of cases, the disease represents a proteinopathy involving loss of the TDP-43 protein from nuclei. However, pathological heterogeneity means that other pathophysiological mechanisms – mediated by oxidative stress, mitochondrial dysfunction, and neurotoxicity related to excessive stimulation of postsynaptic glutamate receptors – can participate.2,5,6
Approximately 10% of patients have a known gene associated with ALS.7 The rest have what is considered sporadic ALS, although some experts estimate that heritability will eventually be confirmed in 50% or more of cases that have been given the “sporadic” label.8,9 More than 30 genes have been linked to ALS in genomewide association studies. Among patients whose disease carries a known familial link, four genes – SOD1, TARDBP, FUS, and C9orf72 – account for approximately 70% of cases.2
Diagnosis. Genetic testing in patients with suspected or confirmed ALS is the standard of care at most, if not all, comprehensive ALS treatment centers, according to the four experts interviewed by Neurology Reviews 2023 Rare Neurological Disease Special Report for this article. Such testing was routine for years because of its potential for helping researchers to understand subtypes of disease; today, testing has assumed even greater practical value with recent approval of the first ALS gene therapy: Tofersen (Qalsody, Biogen), licensed in 2023, is an antisense oligonucleotide therapy that targets SOD1 mRNA to reduce production of the SOD1 protein, a mediator of disease progression.
“Genetic testing has been useful for telling us something about the disease and its prognosis,” Dr. Maragakis said, “but an approved gene therapy means it can have a direct effect on treatment.”
ALS therapeutics. Other gene therapies are in development. Gene signatures are likely to provide even more opportunities for clinical trials in the future.
Following three loading doses of tofersen at 14-day intervals, the maintenance regimen, administered intrathecally by lumbar puncture, is every 28 days. In the phase 3 trial, tofersen reduced levels of SOD1 protein and neurofilament light chain, a biomarker of axonal injury.10 Tofersen is appropriate only in patients with SOD1-associated ALS; the drug’s favorable clinical impact, including a positive effect, if any, on survival has not been demonstrated. Extension studies are underway.
Tofersen joins three other FDA-approved ALS therapies:
• Riluzole, an oral drug available since 1995 that slows disease progression by blocking glutamate.
• Edaravone, an antioxidant approved in 2017, administered orally or intravenously.
• An orally administered combination of sodium phenylbutyrate and taurursodiol marketed as Relyvrio and formerly known as AMX0035, that was introduced in 2022.
“We offer riluzole, which is safe in combination with other therapies, to most patients,” said Dr. Scelsa, who noted that treatment trials often test experimental drugs on top of riluzole. He moves to edaravone or Relyvrio, which are far more expensive, selectively. Tofersen, which is also expensive, is reserved for patients with SOD1-associated disease; however, not all eligible patients opt for this therapy after reviewing its benefits and risks.
“There is not yet a guarantee that tofersen will improve outcomes, and it requires intrathecal injections for life,”
Dr. Maiser said. “Some patients, particularly my older patients, have said, ‘No thank you,’ based on the available data.”
Dr. Macgowan pointed out that lumbar puncture repeated indefinitely can be “challenging.” He, too, discusses all available treatment options with every patient, including riluzole, which he agreed is associated with a meaningful benefit, particularly when started early.
Because of the safety of riluzole, Dr. Maragakis takes early treatment a step further. For neurologists who have a high level of suspicion of ALS in a given patient, “my advice would be to treat aggressively from the get-go. Even if not 100% certain of the diagnosis, I would start them on riluzole while waiting for confirmation.” Like the other experts interviewed here, he acknowledged that referral to a busy comprehensive ALS center often takes time, making it reasonable to initiate treatment when suspicion is high.
On the front lines, “the neurologist can tell the patient that ALS is just one of several potential explanations for symptoms but there is concern,” said Dr. Maragakis, proposing a strategy to introduce the possibility of ALS and start treatment that might slow disease while waiting for confirmation of the diagnosis. “My biggest concern is that no one is making that call,” he said, trying to address at least one reason for the current delay in making referrals.
Comprehensive care at specialty centers
Whenever possible, ALS is a disease best managed at a center that offers comprehensive management, including multidisciplinary care. On this point, the four experts agreed.
“Tertiary-care centers for ALS serve a critical purpose,”
Dr. Maiser said. For a disease that affects nearly every aspect of life, the skills of a multidisciplinary support staff offer an “opportunity to stay in front of the disease” for as long as possible. Teamwork often leads to “outside-of-the-box thinking” for helping patients and families cope with the range of disabilities that undermine the patient’s quality of life.
Details of ALS management matter. At Mount Sinai and Hennepin Healthcare, and at Johns Hopkins, where demand recently led to the opening of a second ALS clinic, the ALS center is set up to address the full spectrum of needs. Staff members have multiple skills so that they can work together to make patients comfortable and prepare them for what is inevitably progression – even if the rate of that progression varies.
All these centers incorporate a rational, thorough discussion of end-of-life options in a palliative care approach that targets optimized quality of life. One goal is to prepare patients to consider and be prepared to make decisions when it is time for tracheostomy, percutaneous endoscopic gastrostomy, and other life support options that are not always well tolerated. The goal? Avoiding unnecessary anguish during end-stage disease when impaired respiratory function – the primary cause of ALS-related death – no longer sustains unassisted survival.
“I am concerned for the many ALS patients without access to this type of comprehensive care,” Dr. Macgowan said.
Like the other experts here, he emphasized that the demands of ALS care can be “overwhelming” outside a comprehensive care setting – for the patient, their family, and individual providers.
Looking ahead
There are many reasons to be optimistic about improving the survival and care of patients with ALS. Besides therapies in clinical trials, Dr. Scelsa explained, there is the potential role for monitoring neurofilament light changes, a biomarker of neurodegeneration, in patients who are at risk of ALS.
Dr. Maragakis offered an analogy to the gene therapy onasemnogene abeparvovec, which can prevent the associated neurodegeneration of spinal muscular atrophy if initiated before symptoms appear. He said that, in ALS, neurofilament light changes or other biomarkers might offer an opportunity to halt the progression of disease before it starts – if one or more therapies in development prove workable.
In the meantime, neurologists who do not specialize in ALS should be thinking about how they can participate in speedier diagnostic pathways.
“There are a number of therapies that look promising,” Dr. Maiser told Rare Neurological Disease Special Report. He singled out strategies to degrade TDP-43 or prevent it from forming. If these treatments are found effective, it’s expected that they would be of value in sporadic ALS, the most common form. Again, though, “the challenge is getting patients on this therapy at the earliest stages of disease.”
Dr. Maragakis discloses equity ownership/stock options with Braintrust Bio and Akava; he is a patent holder with Johns Hopkins [ALS] and has received grant/research/clinical trial support from Apellis Pharma, Biogen Idec, Cytokinetics, Helixmith, Calico, Sanofi, Department of Defense ALSRP, Maryland Stem Cell Research Fund, Massachusetts General Hospital, Medicinova, and NINDS. He serves as consultant or advisory board member for Amylyx; Cytokinetics, Roche, Healey Center, Nura Bio, Northeast ALS Consortium, Akava, Inflammx, and Secretome. Dr. Scelsa did not report any conflicts of interest. Dr. Macgowan and Dr. Maiser have no relevant conflicts of interest to disclose.
References
1. Mehta P et al. Prevalence of amyotrophic lateral sclerosis in the United States using established and novel methodologies, 2017. Amyotroph Lateral Scler Frontotemporal Degener. 2023;24(1-2):108-16. doi: 10.1080/21678421.2022.2059380.
2. Mead RJ et al. Amyotrophic lateral sclerosis: A neurodegenerative disorder poised for successful therapeutic translation. Nat Rev Drug Discov. 2023;22(3):185-212. doi: 10.1038/s41573-022-00612-2.
3. Longinetti E and Fang F. Epidemiology of amyotrophic lateral sclerosis: An update of recent literature. Curr Opin Neurol. 2019;32(5):771-6. doi: 10.1097/WCO.0000000000000730.
4. van den Bos MAJ et al. Pathophysiology and diagnosis of ALS: Insights from advances in neurophysiological techniques. Int J Mol Sci. 2019;20(11):2818. doi: 10.3390/ijms20112818.
5. Neumann M et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130-3. doi: 10.1126/science.1134108.
6. Ling S-C et al. Converging mechanisms in ALS and FTD: Disrupted RNA and protein homeostasis. Neuron. 2013;79(3):416-38. doi: 10.1016/j.neuron.2013.07.033.
7. Ranganathan R et al. Multifaceted genes in amyotrophic lateral sclerosis-frontotemporal dementia. Front Neurosci. 2020;14:684. doi: 10.3389/fnins.2020.00684.
8. Ryan M et al. Lifetime risk and heritability of amyotrophic lateral sclerosis. JAMA Neurol. 2019;76(11):1367-74. doi: 10.1001/jamaneurol.2019.2044.
9. van Rheenen W et al. Common and rare variant association analyses in amyotrophic lateral sclerosis identify 15 risk loci with distinct genetic architectures and neuron-specific biology. Nat Genet. 2021;53(12):1636-48. doi: 10.1038/s41588-021-00973-1.
10. Miller TM et al; VALOR and OLE Working Group. Trial of antisense oligonucleotide tofersen for SOD1 ALS. N Engl J Med. 2022;387(12):1099-110. doi: 10.1056/NEJMoa2204705.
Emerging therapies in Duchenne and facioscapulohumeral muscular dystrophy
“There have been so many breakthroughs recently on the side of genetically targeted treatment [for muscular dystrophy] that supports muscle better,” said John F. Brandsema, MD, a child neurologist and section head at Children’s Hospital of Philadelphia, in an interview with Neurology Reviews 2023 Rare Neurological Disease Special Report. “We’re starting to see clinical response to some things that have been in trials – after decades of banging our heads on the wall trying new therapies, only to see them fail. I think it’s about reframing Duchenne muscular dystrophy [DMD] and facioscapulohumeral muscular dystrophy [FSHD] as treatable by target therapy because previously, they were treated with supportive care.”
DMD: Current and emerging therapies
DMD is caused by a mutation in the dystrophin gene on the X chromosome that inhibits production of dystrophin, a protein that shields muscles from injury during contraction. Dystrophin deficiency prevents muscle recovery, resulting in muscle-cell death and, ultimately, loss of function due to muscle degeneration.
FDA-approved exon-skipping therapies. Treatment modalities for what has historically been an incurable, lifespan-shortening disease involved supportive care that addresses symptoms, not the underlying cause. Consequently, many patients with DMD live only into their 20s and 30s. The tide began to turn in 2016, however, when the U.S. Food and Drug Administration granted accelerated approval for eteplirsen, an exon 51–skipping treatment that was the first RNA-based therapy for DMD to target the underlying cause. Additional exon-skipping therapies followed, including casimersen, which skips exon 45, and golodirsen and viltolarsen, which skip exon 53.
AOC 1044: Novel exon-skipping. In April 2023, the FDA granted orphan-drug designation to the experimental drug antibody oligonucleotide conjugate (AOC) 1044 that skips exon 44. A small interfering RNA (siRNA), AOC 1044 works in patients who have a mutation amenable to exon 44 skipping (a disease type known as DMD44) by delivering phosphorodiamidate morpholino to skeletal muscle and heart tissue that skips exon 44. The process allows for dystrophin production, thereby preventing degradation of muscle tissue.
The orphan drug status of AOC 1044 made it available to the population of patients enrolled in the EXPLORE44 Phase 1/2 trial. However, studies demonstrating effectiveness of the drug – with the hope of, ultimately, providing widespread access to AOC 1044 – are still underway. In one of those studies, investigators expect to enroll approximately 40 healthy volunteers and 24 DMD44 patients 7-27 years of age.3 The study will evaluate the effects of exon skipping and dystrophin protein levels in participants who have DMD44.
Delandistrogene moxeparvovec. Oct. 27, 2021, marked the inception of the phase 3 Multinational, Randomized, Double-Blind, Placebo-Controlled Systemic Gene Delivery Study to Evaluate the Safety and Efficacy of SRP-9001 in Subjects With Duchenne Muscular Dystrophy (EMBARK). The trial is evaluating the safety and efficacy of the gene-therapy agent delandistrogene moxeparvovec in ambulatory boys who were 4 to less than 8 years of age at randomization. The 126 boys enrolled in the trial met the criteria of (1) a diagnosis of DMD confirmed by documented clinical findings and previous genetic testing and (2) a pathogenic frameshift mutation stop codon located between exons 18 and 79 (inclusive), except for a mutation fully contained within exon 45.
Additional inclusion criteria were (1) the ability to cooperate with motor-assessment testing and (2) receiving a steady daily dose of oral corticosteroid for 12 weeks or longer prior to screening, and (3) the expectation of maintaining the study dosage throughout screening. Boys who had previously received gene therapy, investigational medication, or any treatment that could have amplified dystrophin expression within the time limit specified by the protocol were ineligible to participate. Boys were excluded from the study if they presented with any other illness, medical condition, or need for chronic drug treatment.
Exon-skipping therapies in trials. Various biotech and pharmaceutical companies have initiated clinical trials to explore the potential of additional exon-skipping therapies for the DMD population:
ENTR-601-44 is another exon 44–skipping therapy in the pipeline.
On Aug. 22, 2023, the FDA approved delandistrogene moxeparvovec-rokl, a recombinant gene therapy utilizing an adenovirus vector. The product is indicated for ambulatory patients with DMD 4-5 years of age who have a confirmed mutation of the dystrophin gene.
Dyne Therapeutics is actively recruiting participants to investigate Dyne 251, its exon 51–skipping therapy.
Trials are in the works by BioMarin Pharmaceutical for its next-generation peptide-conjugated phosphorodiamidate morpholino oligomers (PPMO) for skipping exon 51.
Despite the prospects of such therapy, therapeutic targeting of exon 44 addresses only patients with DMD44, who account for approximately 10% of the DMD population. Disease involving the most prevalent site of a dystrophin gene mutation, exon 51, affects 13% of the DMD population. This leaves the majority of patients with DMD without gene therapy. Yet Dr. Brandsema is optimistic nevertheless.
“We were just failing over and over again with DMD treatment, but there is some hope now,” Dr. Brandsema said. “Also, FSHD is right on the cusp of having new therapies approaching.”
FSHD: Emerging therapies
The third more common type of muscular dystrophy is not a life-threatening condition. FSHD affects approximately 4 of every 100,000 people.1 An autosomal-dominant condition, FSHD is ultimately caused by inappropriate expression of the DUX4 protein product – a consequence of a complex genetic activity involving DUX4, its chromosomal locus, and the number of repeats of a microsatellite called D4Z4.4 The disease usually starts in proximal regions of the face (that is, surrounding the eyes and mouth), before spreading to muscular groups of the limbs – most prominently, muscles of the scapulae and humeri. Symptoms usually appear in these places initially, but the condition can affect any part of the body. Fifty percent of FSHD patients experience loss of high-frequency hearing and present with retinovasculopathy. Like DMD, FSHD varies in severity, with some forms presenting at birth.
AOC 1020-CS1 is an example of a new FSHD treatment under investigation. The phase 1/2 FORTITUDE trial is a randomized, double-blind, placebo-controlled study exploring the safety, tolerability, pharmacokinetics, pharmacodynamics, and potential efficacy of single- and multiple-dose AOC 1020-CS1 therapy in FSHD.5 The trial began in April 2023; estimated completion date is September 2025.
As with many rare diseases, however, following patients and capturing data that fully narrate their story remains challenging in both DMD and FSHD. Although clinical trials undoubtedly offer hope of expanding treatment options and additional insights into disease-state management, the often insidious, complex nature of some rare diseases, such as DMD and FSHD, presents some limitations.
“Patients are hard to measure,” Dr. Brandsema explained, “because they’re so variable at baseline in history and progress in a different [slower] way than timelines are set up in our system to study drugs.”
Neonatal screening and early diagnosis: Imperative for improving outcomes
Neonatal screening helps with early detection and treatment. Prompt diagnosis does not necessarily prolong a DMD patient’s life, but it can enhance their quality of life.
DNA diagnostics. A critical component of the path to treatment is DNA diagnostics. According to Barry J. Byrne, MD, PhD, chief medical advisor of the Muscular Dystrophy Association, the Human Genome Project conducted by the National Institutes of Health helped make DNA tests affordable; such tests run about $800 today. However, given continuous advancements in sequencing, Dr. Byrne said that whole-exome sequencing for $100 is within reach.
In terms of accessibility, some nations – Canada is an example – include testing as part of national health care services. In the United States, coverage for testing varies by health insurance plan. In addition, some plans have favored rapid diagnostic testing, and the overall cost is often individualized to the patient.
Early diagnosis and supportive care. Early diagnosis can certainly help improve DMD patients’ quality of life; supportive care provides some benefit. Dr. Byrne stressed the importance of managing extraskeletal clinical manifestations in this patient population. A critical area is initiating cardiovascular treatment immediately following diagnosis, even if the patient does not exhibit cardiovascular symptoms.
“Cardiac manifestations are actually the cause of mortality in DMD, and most boys with DMD should begin cardiovascular treatment shortly after diagnosis,” Dr. Byrne told Neurology Reviews 2023 Rare Neurological Disease Special Report. “The message to neurologists is that these patients can benefit from early cardiovascular treatment because we can prevent the complications of DMD-related heart failure until much later in life.”
Historically, clinicians used echocardiography as the mainstay tool to assess cardiovascular function; however, more and more clinicians are turning to magnetic resonance imaging for such investigation. Dr. Byrne, a cardiologist, explained that magnetic resonance imaging identifies cardiovascular dysfunction at earlier stages than echocardiography can. In addition, although DMD patients frequently experience fatigue, Dr. Byrne cautions neurologists that fatigue is usually related to muscle weakness, not necessarily heart failure.
DMD therapies carry a hefty price
Right now, the projected price range of AOC 1044 is $3.2 million to $3.4 million. Akin to the case with onasemnogene abeparvovec-xioi (Zolgensma) for spinal muscular atrophy, the world’s first gene therapy and first seven-figure drug, the manufacturer of AOC 1044 based pricing on the anticipated cost of treating a DMD44 patient throughout the lifespan, according to Dr. Byrne.
Delandistrogene moxeparvovec might come with an even higher price tag. A cost-effectiveness analysis study priced the therapy at $5 million. In a presentation to investors, the manufacturer projected the price in the range of $5 million to $13 million.6,7
‘It takes a village’: Comprehensive care requires a multidisciplinary team
Dr. Brandsema and Dr. Byrne agree: Optimizing outcomes requires ongoing coordinated and collaborative efforts of an interdisciplinary team of health care providers for the duration of DMD and FSHD patients’ lifespan.
A neurologist by training, Dr. Brandsema recognizes the importance of interdisciplinary collaboration in caring for patients with DMD, given the multiorgan manifestations of the disease.
“We have some hope with DMD, and FSHD is right on the cusp of having new therapies approaching ... It is important to recognize that interdisciplinary follow-up and optimized standard of care are important after dosing.”
“I think many patients living with neurological disorders have multiple providers they rely on for care,” Dr. Byrne said, “but cardiovascular and pulmonary care are important because both are affected in the case of DMD – not so much in FSHD.”
Ultimately, advancements in therapy and care give patients living with these disorders, and their caregivers, a renewed sense of hope – hope that their life will be improved by breakthrough therapies that have been approved or will arrive soon.
Dr. Brandsema discloses he is a consultant for Alexion, Audentes, AveXis/Novartis, Biogen, Cytokinetics, Dyne, Edgewise, Fibrogen, Genentech/Roche, Janssen, Marathon, Momenta, NS Pharma, PTC Therapeutics, Sarepta, Scholar Rock, Takeda, and WaVe. He is a speaker for AveXis and Biogen, a medical advisory council member for Cure SMA, and a site investigator for clinical trials with Alexion, Astellas, AveXis/Novartis, Biogen, Catabasis, CSL Behring, Cytokinetics, Fibrogen, Genentech/Roche, Ionis, Lilly, Janssen, Pfizer, PTC Therapeutics, Sarepta, Scholar Rock, Summit, and WaVe. Dr. Byrne has no relevant financial disclosures.
References
1. Centers for Disease Control and Prevention. What is muscular dystrophy? Updated Nov. 21, 2022. Accessed Sept. 3, 2023. https://www.cdc.gov/ncbddd/musculardystrophy/facts.html.
2. FDA approves first gene therapy for treatment of certain patients with Duchenne muscular dystrophy. U.S. Food and Drug Administration. Press release. June 22, 2023. Accessed Sept. 3, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapy-treatment-certain-patients-duchenne-muscular-dystrophy.
3. Study of AOC 1044 in healthy adult volunteers and participants with Duchenne muscular dystrophy (DMD) mutations amenable to exon 44 skipping (EXPLORE44). ClinicalTrials.gov Identifier: NCT05670730. Updated April 4, 2023. Accessed Sep. 3, 2023. https://www.clinicaltrials.gov/study/NCT05670730?cond=DMD&intr=AOC%201044&rank=1.
4. Statland JM, Tawil R. Facioscapulohumeral muscular dystrophy. Continuum (Minneap. Minn). 2016;22(6, Muscle and Neuromuscular Junction Disorders):1916-31. doi: 10.1212/CON.0000000000000399.
5. Phase 1/2 study of AOC 1020 in adults with facioscapulohumeral muscular dystrophy (FSHD) (FORTITUDE). ClinicalTrials.gov Identifier: NCT05747924. Updated Aug. 9, 2023. Accessed Sept. 3, 2023. https://clinicaltrials.gov/study/NCT05747924?term=fORTITUDE&cond=Facioscapulohumeral%20Muscular%20Dystrophy&rank=1.
6. Klimchak AC, Sedita LE, Rodino-Klapac LR, et al. Assessing the value of delandistrogene moxeparvovec (SRP-9001) gene therapy in patients with Duchenne muscular dystrophy in the United States. J Mark Access Health Policy. 2023;11(1):2216518. doi: 10.1080/20016689.2023.2216518.
7. Ingram D. [Investor relations presentation.] Sarepta Therapeutics website. June 22, 2023. Accessed Sept. 3, 2023. https://investorrelations.sarepta.com/static-files/7216948c-f688-4024-922e-39761bc7a984.
“There have been so many breakthroughs recently on the side of genetically targeted treatment [for muscular dystrophy] that supports muscle better,” said John F. Brandsema, MD, a child neurologist and section head at Children’s Hospital of Philadelphia, in an interview with Neurology Reviews 2023 Rare Neurological Disease Special Report. “We’re starting to see clinical response to some things that have been in trials – after decades of banging our heads on the wall trying new therapies, only to see them fail. I think it’s about reframing Duchenne muscular dystrophy [DMD] and facioscapulohumeral muscular dystrophy [FSHD] as treatable by target therapy because previously, they were treated with supportive care.”
DMD: Current and emerging therapies
DMD is caused by a mutation in the dystrophin gene on the X chromosome that inhibits production of dystrophin, a protein that shields muscles from injury during contraction. Dystrophin deficiency prevents muscle recovery, resulting in muscle-cell death and, ultimately, loss of function due to muscle degeneration.
FDA-approved exon-skipping therapies. Treatment modalities for what has historically been an incurable, lifespan-shortening disease involved supportive care that addresses symptoms, not the underlying cause. Consequently, many patients with DMD live only into their 20s and 30s. The tide began to turn in 2016, however, when the U.S. Food and Drug Administration granted accelerated approval for eteplirsen, an exon 51–skipping treatment that was the first RNA-based therapy for DMD to target the underlying cause. Additional exon-skipping therapies followed, including casimersen, which skips exon 45, and golodirsen and viltolarsen, which skip exon 53.
AOC 1044: Novel exon-skipping. In April 2023, the FDA granted orphan-drug designation to the experimental drug antibody oligonucleotide conjugate (AOC) 1044 that skips exon 44. A small interfering RNA (siRNA), AOC 1044 works in patients who have a mutation amenable to exon 44 skipping (a disease type known as DMD44) by delivering phosphorodiamidate morpholino to skeletal muscle and heart tissue that skips exon 44. The process allows for dystrophin production, thereby preventing degradation of muscle tissue.
The orphan drug status of AOC 1044 made it available to the population of patients enrolled in the EXPLORE44 Phase 1/2 trial. However, studies demonstrating effectiveness of the drug – with the hope of, ultimately, providing widespread access to AOC 1044 – are still underway. In one of those studies, investigators expect to enroll approximately 40 healthy volunteers and 24 DMD44 patients 7-27 years of age.3 The study will evaluate the effects of exon skipping and dystrophin protein levels in participants who have DMD44.
Delandistrogene moxeparvovec. Oct. 27, 2021, marked the inception of the phase 3 Multinational, Randomized, Double-Blind, Placebo-Controlled Systemic Gene Delivery Study to Evaluate the Safety and Efficacy of SRP-9001 in Subjects With Duchenne Muscular Dystrophy (EMBARK). The trial is evaluating the safety and efficacy of the gene-therapy agent delandistrogene moxeparvovec in ambulatory boys who were 4 to less than 8 years of age at randomization. The 126 boys enrolled in the trial met the criteria of (1) a diagnosis of DMD confirmed by documented clinical findings and previous genetic testing and (2) a pathogenic frameshift mutation stop codon located between exons 18 and 79 (inclusive), except for a mutation fully contained within exon 45.
Additional inclusion criteria were (1) the ability to cooperate with motor-assessment testing and (2) receiving a steady daily dose of oral corticosteroid for 12 weeks or longer prior to screening, and (3) the expectation of maintaining the study dosage throughout screening. Boys who had previously received gene therapy, investigational medication, or any treatment that could have amplified dystrophin expression within the time limit specified by the protocol were ineligible to participate. Boys were excluded from the study if they presented with any other illness, medical condition, or need for chronic drug treatment.
Exon-skipping therapies in trials. Various biotech and pharmaceutical companies have initiated clinical trials to explore the potential of additional exon-skipping therapies for the DMD population:
ENTR-601-44 is another exon 44–skipping therapy in the pipeline.
On Aug. 22, 2023, the FDA approved delandistrogene moxeparvovec-rokl, a recombinant gene therapy utilizing an adenovirus vector. The product is indicated for ambulatory patients with DMD 4-5 years of age who have a confirmed mutation of the dystrophin gene.
Dyne Therapeutics is actively recruiting participants to investigate Dyne 251, its exon 51–skipping therapy.
Trials are in the works by BioMarin Pharmaceutical for its next-generation peptide-conjugated phosphorodiamidate morpholino oligomers (PPMO) for skipping exon 51.
Despite the prospects of such therapy, therapeutic targeting of exon 44 addresses only patients with DMD44, who account for approximately 10% of the DMD population. Disease involving the most prevalent site of a dystrophin gene mutation, exon 51, affects 13% of the DMD population. This leaves the majority of patients with DMD without gene therapy. Yet Dr. Brandsema is optimistic nevertheless.
“We were just failing over and over again with DMD treatment, but there is some hope now,” Dr. Brandsema said. “Also, FSHD is right on the cusp of having new therapies approaching.”
FSHD: Emerging therapies
The third more common type of muscular dystrophy is not a life-threatening condition. FSHD affects approximately 4 of every 100,000 people.1 An autosomal-dominant condition, FSHD is ultimately caused by inappropriate expression of the DUX4 protein product – a consequence of a complex genetic activity involving DUX4, its chromosomal locus, and the number of repeats of a microsatellite called D4Z4.4 The disease usually starts in proximal regions of the face (that is, surrounding the eyes and mouth), before spreading to muscular groups of the limbs – most prominently, muscles of the scapulae and humeri. Symptoms usually appear in these places initially, but the condition can affect any part of the body. Fifty percent of FSHD patients experience loss of high-frequency hearing and present with retinovasculopathy. Like DMD, FSHD varies in severity, with some forms presenting at birth.
AOC 1020-CS1 is an example of a new FSHD treatment under investigation. The phase 1/2 FORTITUDE trial is a randomized, double-blind, placebo-controlled study exploring the safety, tolerability, pharmacokinetics, pharmacodynamics, and potential efficacy of single- and multiple-dose AOC 1020-CS1 therapy in FSHD.5 The trial began in April 2023; estimated completion date is September 2025.
As with many rare diseases, however, following patients and capturing data that fully narrate their story remains challenging in both DMD and FSHD. Although clinical trials undoubtedly offer hope of expanding treatment options and additional insights into disease-state management, the often insidious, complex nature of some rare diseases, such as DMD and FSHD, presents some limitations.
“Patients are hard to measure,” Dr. Brandsema explained, “because they’re so variable at baseline in history and progress in a different [slower] way than timelines are set up in our system to study drugs.”
Neonatal screening and early diagnosis: Imperative for improving outcomes
Neonatal screening helps with early detection and treatment. Prompt diagnosis does not necessarily prolong a DMD patient’s life, but it can enhance their quality of life.
DNA diagnostics. A critical component of the path to treatment is DNA diagnostics. According to Barry J. Byrne, MD, PhD, chief medical advisor of the Muscular Dystrophy Association, the Human Genome Project conducted by the National Institutes of Health helped make DNA tests affordable; such tests run about $800 today. However, given continuous advancements in sequencing, Dr. Byrne said that whole-exome sequencing for $100 is within reach.
In terms of accessibility, some nations – Canada is an example – include testing as part of national health care services. In the United States, coverage for testing varies by health insurance plan. In addition, some plans have favored rapid diagnostic testing, and the overall cost is often individualized to the patient.
Early diagnosis and supportive care. Early diagnosis can certainly help improve DMD patients’ quality of life; supportive care provides some benefit. Dr. Byrne stressed the importance of managing extraskeletal clinical manifestations in this patient population. A critical area is initiating cardiovascular treatment immediately following diagnosis, even if the patient does not exhibit cardiovascular symptoms.
“Cardiac manifestations are actually the cause of mortality in DMD, and most boys with DMD should begin cardiovascular treatment shortly after diagnosis,” Dr. Byrne told Neurology Reviews 2023 Rare Neurological Disease Special Report. “The message to neurologists is that these patients can benefit from early cardiovascular treatment because we can prevent the complications of DMD-related heart failure until much later in life.”
Historically, clinicians used echocardiography as the mainstay tool to assess cardiovascular function; however, more and more clinicians are turning to magnetic resonance imaging for such investigation. Dr. Byrne, a cardiologist, explained that magnetic resonance imaging identifies cardiovascular dysfunction at earlier stages than echocardiography can. In addition, although DMD patients frequently experience fatigue, Dr. Byrne cautions neurologists that fatigue is usually related to muscle weakness, not necessarily heart failure.
DMD therapies carry a hefty price
Right now, the projected price range of AOC 1044 is $3.2 million to $3.4 million. Akin to the case with onasemnogene abeparvovec-xioi (Zolgensma) for spinal muscular atrophy, the world’s first gene therapy and first seven-figure drug, the manufacturer of AOC 1044 based pricing on the anticipated cost of treating a DMD44 patient throughout the lifespan, according to Dr. Byrne.
Delandistrogene moxeparvovec might come with an even higher price tag. A cost-effectiveness analysis study priced the therapy at $5 million. In a presentation to investors, the manufacturer projected the price in the range of $5 million to $13 million.6,7
‘It takes a village’: Comprehensive care requires a multidisciplinary team
Dr. Brandsema and Dr. Byrne agree: Optimizing outcomes requires ongoing coordinated and collaborative efforts of an interdisciplinary team of health care providers for the duration of DMD and FSHD patients’ lifespan.
A neurologist by training, Dr. Brandsema recognizes the importance of interdisciplinary collaboration in caring for patients with DMD, given the multiorgan manifestations of the disease.
“We have some hope with DMD, and FSHD is right on the cusp of having new therapies approaching ... It is important to recognize that interdisciplinary follow-up and optimized standard of care are important after dosing.”
“I think many patients living with neurological disorders have multiple providers they rely on for care,” Dr. Byrne said, “but cardiovascular and pulmonary care are important because both are affected in the case of DMD – not so much in FSHD.”
Ultimately, advancements in therapy and care give patients living with these disorders, and their caregivers, a renewed sense of hope – hope that their life will be improved by breakthrough therapies that have been approved or will arrive soon.
Dr. Brandsema discloses he is a consultant for Alexion, Audentes, AveXis/Novartis, Biogen, Cytokinetics, Dyne, Edgewise, Fibrogen, Genentech/Roche, Janssen, Marathon, Momenta, NS Pharma, PTC Therapeutics, Sarepta, Scholar Rock, Takeda, and WaVe. He is a speaker for AveXis and Biogen, a medical advisory council member for Cure SMA, and a site investigator for clinical trials with Alexion, Astellas, AveXis/Novartis, Biogen, Catabasis, CSL Behring, Cytokinetics, Fibrogen, Genentech/Roche, Ionis, Lilly, Janssen, Pfizer, PTC Therapeutics, Sarepta, Scholar Rock, Summit, and WaVe. Dr. Byrne has no relevant financial disclosures.
References
1. Centers for Disease Control and Prevention. What is muscular dystrophy? Updated Nov. 21, 2022. Accessed Sept. 3, 2023. https://www.cdc.gov/ncbddd/musculardystrophy/facts.html.
2. FDA approves first gene therapy for treatment of certain patients with Duchenne muscular dystrophy. U.S. Food and Drug Administration. Press release. June 22, 2023. Accessed Sept. 3, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapy-treatment-certain-patients-duchenne-muscular-dystrophy.
3. Study of AOC 1044 in healthy adult volunteers and participants with Duchenne muscular dystrophy (DMD) mutations amenable to exon 44 skipping (EXPLORE44). ClinicalTrials.gov Identifier: NCT05670730. Updated April 4, 2023. Accessed Sep. 3, 2023. https://www.clinicaltrials.gov/study/NCT05670730?cond=DMD&intr=AOC%201044&rank=1.
4. Statland JM, Tawil R. Facioscapulohumeral muscular dystrophy. Continuum (Minneap. Minn). 2016;22(6, Muscle and Neuromuscular Junction Disorders):1916-31. doi: 10.1212/CON.0000000000000399.
5. Phase 1/2 study of AOC 1020 in adults with facioscapulohumeral muscular dystrophy (FSHD) (FORTITUDE). ClinicalTrials.gov Identifier: NCT05747924. Updated Aug. 9, 2023. Accessed Sept. 3, 2023. https://clinicaltrials.gov/study/NCT05747924?term=fORTITUDE&cond=Facioscapulohumeral%20Muscular%20Dystrophy&rank=1.
6. Klimchak AC, Sedita LE, Rodino-Klapac LR, et al. Assessing the value of delandistrogene moxeparvovec (SRP-9001) gene therapy in patients with Duchenne muscular dystrophy in the United States. J Mark Access Health Policy. 2023;11(1):2216518. doi: 10.1080/20016689.2023.2216518.
7. Ingram D. [Investor relations presentation.] Sarepta Therapeutics website. June 22, 2023. Accessed Sept. 3, 2023. https://investorrelations.sarepta.com/static-files/7216948c-f688-4024-922e-39761bc7a984.
“There have been so many breakthroughs recently on the side of genetically targeted treatment [for muscular dystrophy] that supports muscle better,” said John F. Brandsema, MD, a child neurologist and section head at Children’s Hospital of Philadelphia, in an interview with Neurology Reviews 2023 Rare Neurological Disease Special Report. “We’re starting to see clinical response to some things that have been in trials – after decades of banging our heads on the wall trying new therapies, only to see them fail. I think it’s about reframing Duchenne muscular dystrophy [DMD] and facioscapulohumeral muscular dystrophy [FSHD] as treatable by target therapy because previously, they were treated with supportive care.”
DMD: Current and emerging therapies
DMD is caused by a mutation in the dystrophin gene on the X chromosome that inhibits production of dystrophin, a protein that shields muscles from injury during contraction. Dystrophin deficiency prevents muscle recovery, resulting in muscle-cell death and, ultimately, loss of function due to muscle degeneration.
FDA-approved exon-skipping therapies. Treatment modalities for what has historically been an incurable, lifespan-shortening disease involved supportive care that addresses symptoms, not the underlying cause. Consequently, many patients with DMD live only into their 20s and 30s. The tide began to turn in 2016, however, when the U.S. Food and Drug Administration granted accelerated approval for eteplirsen, an exon 51–skipping treatment that was the first RNA-based therapy for DMD to target the underlying cause. Additional exon-skipping therapies followed, including casimersen, which skips exon 45, and golodirsen and viltolarsen, which skip exon 53.
AOC 1044: Novel exon-skipping. In April 2023, the FDA granted orphan-drug designation to the experimental drug antibody oligonucleotide conjugate (AOC) 1044 that skips exon 44. A small interfering RNA (siRNA), AOC 1044 works in patients who have a mutation amenable to exon 44 skipping (a disease type known as DMD44) by delivering phosphorodiamidate morpholino to skeletal muscle and heart tissue that skips exon 44. The process allows for dystrophin production, thereby preventing degradation of muscle tissue.
The orphan drug status of AOC 1044 made it available to the population of patients enrolled in the EXPLORE44 Phase 1/2 trial. However, studies demonstrating effectiveness of the drug – with the hope of, ultimately, providing widespread access to AOC 1044 – are still underway. In one of those studies, investigators expect to enroll approximately 40 healthy volunteers and 24 DMD44 patients 7-27 years of age.3 The study will evaluate the effects of exon skipping and dystrophin protein levels in participants who have DMD44.
Delandistrogene moxeparvovec. Oct. 27, 2021, marked the inception of the phase 3 Multinational, Randomized, Double-Blind, Placebo-Controlled Systemic Gene Delivery Study to Evaluate the Safety and Efficacy of SRP-9001 in Subjects With Duchenne Muscular Dystrophy (EMBARK). The trial is evaluating the safety and efficacy of the gene-therapy agent delandistrogene moxeparvovec in ambulatory boys who were 4 to less than 8 years of age at randomization. The 126 boys enrolled in the trial met the criteria of (1) a diagnosis of DMD confirmed by documented clinical findings and previous genetic testing and (2) a pathogenic frameshift mutation stop codon located between exons 18 and 79 (inclusive), except for a mutation fully contained within exon 45.
Additional inclusion criteria were (1) the ability to cooperate with motor-assessment testing and (2) receiving a steady daily dose of oral corticosteroid for 12 weeks or longer prior to screening, and (3) the expectation of maintaining the study dosage throughout screening. Boys who had previously received gene therapy, investigational medication, or any treatment that could have amplified dystrophin expression within the time limit specified by the protocol were ineligible to participate. Boys were excluded from the study if they presented with any other illness, medical condition, or need for chronic drug treatment.
Exon-skipping therapies in trials. Various biotech and pharmaceutical companies have initiated clinical trials to explore the potential of additional exon-skipping therapies for the DMD population:
ENTR-601-44 is another exon 44–skipping therapy in the pipeline.
On Aug. 22, 2023, the FDA approved delandistrogene moxeparvovec-rokl, a recombinant gene therapy utilizing an adenovirus vector. The product is indicated for ambulatory patients with DMD 4-5 years of age who have a confirmed mutation of the dystrophin gene.
Dyne Therapeutics is actively recruiting participants to investigate Dyne 251, its exon 51–skipping therapy.
Trials are in the works by BioMarin Pharmaceutical for its next-generation peptide-conjugated phosphorodiamidate morpholino oligomers (PPMO) for skipping exon 51.
Despite the prospects of such therapy, therapeutic targeting of exon 44 addresses only patients with DMD44, who account for approximately 10% of the DMD population. Disease involving the most prevalent site of a dystrophin gene mutation, exon 51, affects 13% of the DMD population. This leaves the majority of patients with DMD without gene therapy. Yet Dr. Brandsema is optimistic nevertheless.
“We were just failing over and over again with DMD treatment, but there is some hope now,” Dr. Brandsema said. “Also, FSHD is right on the cusp of having new therapies approaching.”
FSHD: Emerging therapies
The third more common type of muscular dystrophy is not a life-threatening condition. FSHD affects approximately 4 of every 100,000 people.1 An autosomal-dominant condition, FSHD is ultimately caused by inappropriate expression of the DUX4 protein product – a consequence of a complex genetic activity involving DUX4, its chromosomal locus, and the number of repeats of a microsatellite called D4Z4.4 The disease usually starts in proximal regions of the face (that is, surrounding the eyes and mouth), before spreading to muscular groups of the limbs – most prominently, muscles of the scapulae and humeri. Symptoms usually appear in these places initially, but the condition can affect any part of the body. Fifty percent of FSHD patients experience loss of high-frequency hearing and present with retinovasculopathy. Like DMD, FSHD varies in severity, with some forms presenting at birth.
AOC 1020-CS1 is an example of a new FSHD treatment under investigation. The phase 1/2 FORTITUDE trial is a randomized, double-blind, placebo-controlled study exploring the safety, tolerability, pharmacokinetics, pharmacodynamics, and potential efficacy of single- and multiple-dose AOC 1020-CS1 therapy in FSHD.5 The trial began in April 2023; estimated completion date is September 2025.
As with many rare diseases, however, following patients and capturing data that fully narrate their story remains challenging in both DMD and FSHD. Although clinical trials undoubtedly offer hope of expanding treatment options and additional insights into disease-state management, the often insidious, complex nature of some rare diseases, such as DMD and FSHD, presents some limitations.
“Patients are hard to measure,” Dr. Brandsema explained, “because they’re so variable at baseline in history and progress in a different [slower] way than timelines are set up in our system to study drugs.”
Neonatal screening and early diagnosis: Imperative for improving outcomes
Neonatal screening helps with early detection and treatment. Prompt diagnosis does not necessarily prolong a DMD patient’s life, but it can enhance their quality of life.
DNA diagnostics. A critical component of the path to treatment is DNA diagnostics. According to Barry J. Byrne, MD, PhD, chief medical advisor of the Muscular Dystrophy Association, the Human Genome Project conducted by the National Institutes of Health helped make DNA tests affordable; such tests run about $800 today. However, given continuous advancements in sequencing, Dr. Byrne said that whole-exome sequencing for $100 is within reach.
In terms of accessibility, some nations – Canada is an example – include testing as part of national health care services. In the United States, coverage for testing varies by health insurance plan. In addition, some plans have favored rapid diagnostic testing, and the overall cost is often individualized to the patient.
Early diagnosis and supportive care. Early diagnosis can certainly help improve DMD patients’ quality of life; supportive care provides some benefit. Dr. Byrne stressed the importance of managing extraskeletal clinical manifestations in this patient population. A critical area is initiating cardiovascular treatment immediately following diagnosis, even if the patient does not exhibit cardiovascular symptoms.
“Cardiac manifestations are actually the cause of mortality in DMD, and most boys with DMD should begin cardiovascular treatment shortly after diagnosis,” Dr. Byrne told Neurology Reviews 2023 Rare Neurological Disease Special Report. “The message to neurologists is that these patients can benefit from early cardiovascular treatment because we can prevent the complications of DMD-related heart failure until much later in life.”
Historically, clinicians used echocardiography as the mainstay tool to assess cardiovascular function; however, more and more clinicians are turning to magnetic resonance imaging for such investigation. Dr. Byrne, a cardiologist, explained that magnetic resonance imaging identifies cardiovascular dysfunction at earlier stages than echocardiography can. In addition, although DMD patients frequently experience fatigue, Dr. Byrne cautions neurologists that fatigue is usually related to muscle weakness, not necessarily heart failure.
DMD therapies carry a hefty price
Right now, the projected price range of AOC 1044 is $3.2 million to $3.4 million. Akin to the case with onasemnogene abeparvovec-xioi (Zolgensma) for spinal muscular atrophy, the world’s first gene therapy and first seven-figure drug, the manufacturer of AOC 1044 based pricing on the anticipated cost of treating a DMD44 patient throughout the lifespan, according to Dr. Byrne.
Delandistrogene moxeparvovec might come with an even higher price tag. A cost-effectiveness analysis study priced the therapy at $5 million. In a presentation to investors, the manufacturer projected the price in the range of $5 million to $13 million.6,7
‘It takes a village’: Comprehensive care requires a multidisciplinary team
Dr. Brandsema and Dr. Byrne agree: Optimizing outcomes requires ongoing coordinated and collaborative efforts of an interdisciplinary team of health care providers for the duration of DMD and FSHD patients’ lifespan.
A neurologist by training, Dr. Brandsema recognizes the importance of interdisciplinary collaboration in caring for patients with DMD, given the multiorgan manifestations of the disease.
“We have some hope with DMD, and FSHD is right on the cusp of having new therapies approaching ... It is important to recognize that interdisciplinary follow-up and optimized standard of care are important after dosing.”
“I think many patients living with neurological disorders have multiple providers they rely on for care,” Dr. Byrne said, “but cardiovascular and pulmonary care are important because both are affected in the case of DMD – not so much in FSHD.”
Ultimately, advancements in therapy and care give patients living with these disorders, and their caregivers, a renewed sense of hope – hope that their life will be improved by breakthrough therapies that have been approved or will arrive soon.
Dr. Brandsema discloses he is a consultant for Alexion, Audentes, AveXis/Novartis, Biogen, Cytokinetics, Dyne, Edgewise, Fibrogen, Genentech/Roche, Janssen, Marathon, Momenta, NS Pharma, PTC Therapeutics, Sarepta, Scholar Rock, Takeda, and WaVe. He is a speaker for AveXis and Biogen, a medical advisory council member for Cure SMA, and a site investigator for clinical trials with Alexion, Astellas, AveXis/Novartis, Biogen, Catabasis, CSL Behring, Cytokinetics, Fibrogen, Genentech/Roche, Ionis, Lilly, Janssen, Pfizer, PTC Therapeutics, Sarepta, Scholar Rock, Summit, and WaVe. Dr. Byrne has no relevant financial disclosures.
References
1. Centers for Disease Control and Prevention. What is muscular dystrophy? Updated Nov. 21, 2022. Accessed Sept. 3, 2023. https://www.cdc.gov/ncbddd/musculardystrophy/facts.html.
2. FDA approves first gene therapy for treatment of certain patients with Duchenne muscular dystrophy. U.S. Food and Drug Administration. Press release. June 22, 2023. Accessed Sept. 3, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapy-treatment-certain-patients-duchenne-muscular-dystrophy.
3. Study of AOC 1044 in healthy adult volunteers and participants with Duchenne muscular dystrophy (DMD) mutations amenable to exon 44 skipping (EXPLORE44). ClinicalTrials.gov Identifier: NCT05670730. Updated April 4, 2023. Accessed Sep. 3, 2023. https://www.clinicaltrials.gov/study/NCT05670730?cond=DMD&intr=AOC%201044&rank=1.
4. Statland JM, Tawil R. Facioscapulohumeral muscular dystrophy. Continuum (Minneap. Minn). 2016;22(6, Muscle and Neuromuscular Junction Disorders):1916-31. doi: 10.1212/CON.0000000000000399.
5. Phase 1/2 study of AOC 1020 in adults with facioscapulohumeral muscular dystrophy (FSHD) (FORTITUDE). ClinicalTrials.gov Identifier: NCT05747924. Updated Aug. 9, 2023. Accessed Sept. 3, 2023. https://clinicaltrials.gov/study/NCT05747924?term=fORTITUDE&cond=Facioscapulohumeral%20Muscular%20Dystrophy&rank=1.
6. Klimchak AC, Sedita LE, Rodino-Klapac LR, et al. Assessing the value of delandistrogene moxeparvovec (SRP-9001) gene therapy in patients with Duchenne muscular dystrophy in the United States. J Mark Access Health Policy. 2023;11(1):2216518. doi: 10.1080/20016689.2023.2216518.
7. Ingram D. [Investor relations presentation.] Sarepta Therapeutics website. June 22, 2023. Accessed Sept. 3, 2023. https://investorrelations.sarepta.com/static-files/7216948c-f688-4024-922e-39761bc7a984.
CPAP in overlap syndrome: Unveiling the evidence
Sleep Medicine Network
Respiratory-Related Sleep Disorders Section
The overlap syndrome (OS), which refers to the co-occurrence of OSA and COPD, was first described by Flenley in 1985 (Flenley DC. Clin Chest Med. 1985;6[4]:651). Over the years, numerous studies have demonstrated an increased risk of hospitalization and mortality in patients with OS (Brennan M, et al. 2022;1-10). Despite these findings, limited evidence exists regarding the optimal treatment approach for individuals with OS.
CPAP therapy has demonstrated various physiologic advantages for patients with OS (Srivali N, et al. Sleep Med. 2023;108:55-60), which contribute to diminished dyspnea symptoms, lowered pro-inflammatory markers, improved arterial blood gases, increased 6-minute walk distance, enhanced FEV1, and decreased mean pulmonary artery pressure (Suri TM, et al. FASEB BioAdv. 2021;3[9]:683-93). CPAP therapy in patients with OS has been linked to a reduction in COPD exacerbations (Voulgaris A, et al. Clin Respir Jour. 2023; 17[3]:165), fewer COPD-related hospitalizations (Marin JM, et al. Am J Respir Crit Care Med. 2010;182[3]:325-31), decreased cardiovascular events (Kendzerska T, et al. Ann ATS. 2019;16[1]:71), and an overall decline in mortality rates (Machado ML, et al. Eur Respir J. 2010;35[1]:132-7).
It is important to acknowledge that, as of now, no randomized clinical trial has specifically addressed the treatment of OS, leaving recommendations largely reliant on observational studies. Conversely, recent guidelines have proposed the utilization of high-intensity noninvasive ventilation (NIV) for hypercapnic patients with COPD. Thus, extensive research is warranted to characterize distinct sleep-related breathing disorders within the OS population and to investigate the effects of CPAP in comparison to other NIV modalities on patients with overlap syndrome.
Solmaz Ehteshami-Afshar, MD
Kirat Gill, MD, Section Member-at-Large
Sleep Medicine Network
Respiratory-Related Sleep Disorders Section
The overlap syndrome (OS), which refers to the co-occurrence of OSA and COPD, was first described by Flenley in 1985 (Flenley DC. Clin Chest Med. 1985;6[4]:651). Over the years, numerous studies have demonstrated an increased risk of hospitalization and mortality in patients with OS (Brennan M, et al. 2022;1-10). Despite these findings, limited evidence exists regarding the optimal treatment approach for individuals with OS.
CPAP therapy has demonstrated various physiologic advantages for patients with OS (Srivali N, et al. Sleep Med. 2023;108:55-60), which contribute to diminished dyspnea symptoms, lowered pro-inflammatory markers, improved arterial blood gases, increased 6-minute walk distance, enhanced FEV1, and decreased mean pulmonary artery pressure (Suri TM, et al. FASEB BioAdv. 2021;3[9]:683-93). CPAP therapy in patients with OS has been linked to a reduction in COPD exacerbations (Voulgaris A, et al. Clin Respir Jour. 2023; 17[3]:165), fewer COPD-related hospitalizations (Marin JM, et al. Am J Respir Crit Care Med. 2010;182[3]:325-31), decreased cardiovascular events (Kendzerska T, et al. Ann ATS. 2019;16[1]:71), and an overall decline in mortality rates (Machado ML, et al. Eur Respir J. 2010;35[1]:132-7).
It is important to acknowledge that, as of now, no randomized clinical trial has specifically addressed the treatment of OS, leaving recommendations largely reliant on observational studies. Conversely, recent guidelines have proposed the utilization of high-intensity noninvasive ventilation (NIV) for hypercapnic patients with COPD. Thus, extensive research is warranted to characterize distinct sleep-related breathing disorders within the OS population and to investigate the effects of CPAP in comparison to other NIV modalities on patients with overlap syndrome.
Solmaz Ehteshami-Afshar, MD
Kirat Gill, MD, Section Member-at-Large
Sleep Medicine Network
Respiratory-Related Sleep Disorders Section
The overlap syndrome (OS), which refers to the co-occurrence of OSA and COPD, was first described by Flenley in 1985 (Flenley DC. Clin Chest Med. 1985;6[4]:651). Over the years, numerous studies have demonstrated an increased risk of hospitalization and mortality in patients with OS (Brennan M, et al. 2022;1-10). Despite these findings, limited evidence exists regarding the optimal treatment approach for individuals with OS.
CPAP therapy has demonstrated various physiologic advantages for patients with OS (Srivali N, et al. Sleep Med. 2023;108:55-60), which contribute to diminished dyspnea symptoms, lowered pro-inflammatory markers, improved arterial blood gases, increased 6-minute walk distance, enhanced FEV1, and decreased mean pulmonary artery pressure (Suri TM, et al. FASEB BioAdv. 2021;3[9]:683-93). CPAP therapy in patients with OS has been linked to a reduction in COPD exacerbations (Voulgaris A, et al. Clin Respir Jour. 2023; 17[3]:165), fewer COPD-related hospitalizations (Marin JM, et al. Am J Respir Crit Care Med. 2010;182[3]:325-31), decreased cardiovascular events (Kendzerska T, et al. Ann ATS. 2019;16[1]:71), and an overall decline in mortality rates (Machado ML, et al. Eur Respir J. 2010;35[1]:132-7).
It is important to acknowledge that, as of now, no randomized clinical trial has specifically addressed the treatment of OS, leaving recommendations largely reliant on observational studies. Conversely, recent guidelines have proposed the utilization of high-intensity noninvasive ventilation (NIV) for hypercapnic patients with COPD. Thus, extensive research is warranted to characterize distinct sleep-related breathing disorders within the OS population and to investigate the effects of CPAP in comparison to other NIV modalities on patients with overlap syndrome.
Solmaz Ehteshami-Afshar, MD
Kirat Gill, MD, Section Member-at-Large
Use of 6-minute walk distance as a clinical trial outcome in interstitial lung disease
Diffuse Lung & Transplant Network
Pulmonary Physiology & Rehabilitation Section
Pulmonary arterial hypertension (PH) and more recently interstitial lung disease (ILD) trials use the 6-minute walk test (6MWT) as a primary outcome due to its ability to conveniently capture a patient’s functional capacity and quality of life. However, interpreting the 6MWT in complex and diverse diseases, such as ILD, presents significant challenges.
A recent article (Harari, et al. Eur Respir Rev. 2022 Aug 23;31(165):220087. doi: 10.1183/16000617.0087-2022) advocates for further research to determine the optimal use of the 6MWT as a clinical endpoint in ILD trials. A decline in 6MWT can represent progression of ILD; ILD-related PH; or musculoskeletal, hematologic, or cardiac etiologies related to the underlying cause of ILD.
To enhance sensitivity, the authors endorse the inclusion of additional parameters in the analysis, possibly as a composite outcome. This would involve integrating the oxygen desaturation profile, dyspnea scores, and heart rate recovery with changes in the 6MWT-distance. They propose this composite measure could serve as a primary endpoint when the study intervention’s impact on clinical performance – either improvement or stabilization of ILD or ILD-related PH – is clearly defined. The prognostic significance of these additional parameters in patients with ILD, however, requires further investigation.
Inter-test reliability requires a standardized 6MWT, as previously proposed for this population (Lancaster, et al. Contemporary Clin Trials. 2021;Nov 25,2020). The standardized test protocol that includes continuous pulse oximetry and heart rate measurement, oxygen titration, and end of test guidelines, will reduce variability and boost reproducibility.
In light of recent advancements in the affordability, convenience, and portability of oxygen consumption (VO2) gas analyzers, we believe that incorporating V
Ruchicka Sangani, MD, Section Fellow-in-Training
Saqib Baig, MD, Section Member-at-Large
Diffuse Lung & Transplant Network
Pulmonary Physiology & Rehabilitation Section
Pulmonary arterial hypertension (PH) and more recently interstitial lung disease (ILD) trials use the 6-minute walk test (6MWT) as a primary outcome due to its ability to conveniently capture a patient’s functional capacity and quality of life. However, interpreting the 6MWT in complex and diverse diseases, such as ILD, presents significant challenges.
A recent article (Harari, et al. Eur Respir Rev. 2022 Aug 23;31(165):220087. doi: 10.1183/16000617.0087-2022) advocates for further research to determine the optimal use of the 6MWT as a clinical endpoint in ILD trials. A decline in 6MWT can represent progression of ILD; ILD-related PH; or musculoskeletal, hematologic, or cardiac etiologies related to the underlying cause of ILD.
To enhance sensitivity, the authors endorse the inclusion of additional parameters in the analysis, possibly as a composite outcome. This would involve integrating the oxygen desaturation profile, dyspnea scores, and heart rate recovery with changes in the 6MWT-distance. They propose this composite measure could serve as a primary endpoint when the study intervention’s impact on clinical performance – either improvement or stabilization of ILD or ILD-related PH – is clearly defined. The prognostic significance of these additional parameters in patients with ILD, however, requires further investigation.
Inter-test reliability requires a standardized 6MWT, as previously proposed for this population (Lancaster, et al. Contemporary Clin Trials. 2021;Nov 25,2020). The standardized test protocol that includes continuous pulse oximetry and heart rate measurement, oxygen titration, and end of test guidelines, will reduce variability and boost reproducibility.
In light of recent advancements in the affordability, convenience, and portability of oxygen consumption (VO2) gas analyzers, we believe that incorporating V
Ruchicka Sangani, MD, Section Fellow-in-Training
Saqib Baig, MD, Section Member-at-Large
Diffuse Lung & Transplant Network
Pulmonary Physiology & Rehabilitation Section
Pulmonary arterial hypertension (PH) and more recently interstitial lung disease (ILD) trials use the 6-minute walk test (6MWT) as a primary outcome due to its ability to conveniently capture a patient’s functional capacity and quality of life. However, interpreting the 6MWT in complex and diverse diseases, such as ILD, presents significant challenges.
A recent article (Harari, et al. Eur Respir Rev. 2022 Aug 23;31(165):220087. doi: 10.1183/16000617.0087-2022) advocates for further research to determine the optimal use of the 6MWT as a clinical endpoint in ILD trials. A decline in 6MWT can represent progression of ILD; ILD-related PH; or musculoskeletal, hematologic, or cardiac etiologies related to the underlying cause of ILD.
To enhance sensitivity, the authors endorse the inclusion of additional parameters in the analysis, possibly as a composite outcome. This would involve integrating the oxygen desaturation profile, dyspnea scores, and heart rate recovery with changes in the 6MWT-distance. They propose this composite measure could serve as a primary endpoint when the study intervention’s impact on clinical performance – either improvement or stabilization of ILD or ILD-related PH – is clearly defined. The prognostic significance of these additional parameters in patients with ILD, however, requires further investigation.
Inter-test reliability requires a standardized 6MWT, as previously proposed for this population (Lancaster, et al. Contemporary Clin Trials. 2021;Nov 25,2020). The standardized test protocol that includes continuous pulse oximetry and heart rate measurement, oxygen titration, and end of test guidelines, will reduce variability and boost reproducibility.
In light of recent advancements in the affordability, convenience, and portability of oxygen consumption (VO2) gas analyzers, we believe that incorporating V
Ruchicka Sangani, MD, Section Fellow-in-Training
Saqib Baig, MD, Section Member-at-Large
Now we have MERCY
Critical Care Network
Sepsis/Shock Section
Beta-lactam antibiotics, including penicillin, carbapenems, and cephalosporins, exhibit time-dependent bacterial eradication. Prolonged infusions are thought to enhance the duration of effective bactericidal antibiotic exposure, decreasing the emergence of drug resistance due to reduced bacterial regrowth between doses – which may lead to cost savings by reducing drug acquisition costs and shortening hospital stays (Lodise TP Jr, et al. Clin Infect Dis. 2007;44[3]:357-63).
The best evidence for these benefits comes from observational studies and meta-analyses. The Defining Antibiotic Levels in Intensive Care Unit Patients (DALI) study emphasized the correlation between achieving target concentrations of beta-lactam antibiotics in critically ill patients and positive clinical outcomes for bloodstream infections but not for lung or intra-abdominal infections (Roberts JA, et al. Clin Infect Dis. 2014;58[8]:1072-83). A meta-analysis of 29 studies suggested that prolonged infusion of piperacillin-tazobactam was associated with a mortality benefit compared with intermittent infusions, but prolonged infusions of cephalosporins or carbapenems resulted in comparable outcomes without mortality benefit (Teo J, et al. Int J Antimicrob Agents. 2014;43[5]:403-11).
MERCY was a multinational, randomized controlled trial investigating the efficacy of continuous vs intermittent administration of meropenem in critically ill patients with sepsis. The primary outcome, a composite of mortality and emergence of resistant bacteria at day 28, showed no significant difference between continuous and intermittent administration (47% vs. 49%). Secondary outcomes and adverse events also did not display significant differences, suggesting that continuous meropenem did not improve outcomes compared with intermittent administration (Monti G, et al. JAMA. 2023;330[2]:141-51).
MERCY adds to the existing body of evidence suggesting that prolonged and intermittent infusion strategies for meropenem are at least equivalent in efficacy. Therefore, the strategy chosen can depend on other individualized factors.
The views expressed are those of the authors and do not reflect the official policy or position of the U.S. Navy, Department of Defense, or the US Government.
Meredith L. Olsen, MD, Section Member-at-Large
Casey Cable, MD, FCCP, Section Member-at-Large
Kathryn Pendleton, MD, FCCP, Section Vice-Chair
Critical Care Network
Sepsis/Shock Section
Beta-lactam antibiotics, including penicillin, carbapenems, and cephalosporins, exhibit time-dependent bacterial eradication. Prolonged infusions are thought to enhance the duration of effective bactericidal antibiotic exposure, decreasing the emergence of drug resistance due to reduced bacterial regrowth between doses – which may lead to cost savings by reducing drug acquisition costs and shortening hospital stays (Lodise TP Jr, et al. Clin Infect Dis. 2007;44[3]:357-63).
The best evidence for these benefits comes from observational studies and meta-analyses. The Defining Antibiotic Levels in Intensive Care Unit Patients (DALI) study emphasized the correlation between achieving target concentrations of beta-lactam antibiotics in critically ill patients and positive clinical outcomes for bloodstream infections but not for lung or intra-abdominal infections (Roberts JA, et al. Clin Infect Dis. 2014;58[8]:1072-83). A meta-analysis of 29 studies suggested that prolonged infusion of piperacillin-tazobactam was associated with a mortality benefit compared with intermittent infusions, but prolonged infusions of cephalosporins or carbapenems resulted in comparable outcomes without mortality benefit (Teo J, et al. Int J Antimicrob Agents. 2014;43[5]:403-11).
MERCY was a multinational, randomized controlled trial investigating the efficacy of continuous vs intermittent administration of meropenem in critically ill patients with sepsis. The primary outcome, a composite of mortality and emergence of resistant bacteria at day 28, showed no significant difference between continuous and intermittent administration (47% vs. 49%). Secondary outcomes and adverse events also did not display significant differences, suggesting that continuous meropenem did not improve outcomes compared with intermittent administration (Monti G, et al. JAMA. 2023;330[2]:141-51).
MERCY adds to the existing body of evidence suggesting that prolonged and intermittent infusion strategies for meropenem are at least equivalent in efficacy. Therefore, the strategy chosen can depend on other individualized factors.
The views expressed are those of the authors and do not reflect the official policy or position of the U.S. Navy, Department of Defense, or the US Government.
Meredith L. Olsen, MD, Section Member-at-Large
Casey Cable, MD, FCCP, Section Member-at-Large
Kathryn Pendleton, MD, FCCP, Section Vice-Chair
Critical Care Network
Sepsis/Shock Section
Beta-lactam antibiotics, including penicillin, carbapenems, and cephalosporins, exhibit time-dependent bacterial eradication. Prolonged infusions are thought to enhance the duration of effective bactericidal antibiotic exposure, decreasing the emergence of drug resistance due to reduced bacterial regrowth between doses – which may lead to cost savings by reducing drug acquisition costs and shortening hospital stays (Lodise TP Jr, et al. Clin Infect Dis. 2007;44[3]:357-63).
The best evidence for these benefits comes from observational studies and meta-analyses. The Defining Antibiotic Levels in Intensive Care Unit Patients (DALI) study emphasized the correlation between achieving target concentrations of beta-lactam antibiotics in critically ill patients and positive clinical outcomes for bloodstream infections but not for lung or intra-abdominal infections (Roberts JA, et al. Clin Infect Dis. 2014;58[8]:1072-83). A meta-analysis of 29 studies suggested that prolonged infusion of piperacillin-tazobactam was associated with a mortality benefit compared with intermittent infusions, but prolonged infusions of cephalosporins or carbapenems resulted in comparable outcomes without mortality benefit (Teo J, et al. Int J Antimicrob Agents. 2014;43[5]:403-11).
MERCY was a multinational, randomized controlled trial investigating the efficacy of continuous vs intermittent administration of meropenem in critically ill patients with sepsis. The primary outcome, a composite of mortality and emergence of resistant bacteria at day 28, showed no significant difference between continuous and intermittent administration (47% vs. 49%). Secondary outcomes and adverse events also did not display significant differences, suggesting that continuous meropenem did not improve outcomes compared with intermittent administration (Monti G, et al. JAMA. 2023;330[2]:141-51).
MERCY adds to the existing body of evidence suggesting that prolonged and intermittent infusion strategies for meropenem are at least equivalent in efficacy. Therefore, the strategy chosen can depend on other individualized factors.
The views expressed are those of the authors and do not reflect the official policy or position of the U.S. Navy, Department of Defense, or the US Government.
Meredith L. Olsen, MD, Section Member-at-Large
Casey Cable, MD, FCCP, Section Member-at-Large
Kathryn Pendleton, MD, FCCP, Section Vice-Chair
Have you asked your patients: What is your ideal outpatient gynecology experience?
There has been increasing awareness of a need for creating a more patient-centered experience with outpatient gynecology; however, very little data exist about what interventions are important to patients. Given social media’s ease of use and ability for widespread access to a diverse group of users, it has the potential to be a powerful tool for qualitative research questions without the difficulties of cost, transportation, transcription, etc. required of a focus group. Crowdsourced public opinion also has the advantage of producing qualitative metrics in the form of “likes” that, at scale, can provide a reliable measure of public support or engagement for a particular concept.1 Particularly for topics that are controversial or novel, X (formerly Twitter, and referred to as Twitter intermittently throughout this article based on the time the study was conducted), with 300 million monthly users,2 has become a popular tool for general and health care ̶ focused content and sentiment analysis.3,4 This study presents a qualitative analysis of themes from a crowdsourced request on Twitter to design the ideal outpatient gynecologic experience that subsequently went “viral”.5,6
When asked to design the optimized outpatient gynecology experience, social media users expressed:
- hospitality, comfort, and pain control as frequent themes
- preserving privacy and acknowledgement of voluntary nulliparity as frequent themes
- a desire for diverse imagery and representation related to race, LGBTQIA+ themes, age, and weight/body type within the office setting
- a call for a sense of psychological safety within gynecology
Why the need for our research question on patient-centered gyn care
While the body of literature on patient-centered health care has grown rapidly in recent years, a patient-centered outpatient gynecology experience has not yet been described in the medical literature.
Patient-centered office design, driven by cultural sensitivity, has been shown in other studies to be both appreciated by established patients and a viable business strategy to attract new patients.7 Topics such as pain control, trauma-informed care in gynecologyclinics,8 and diverse representation in patient materials and illustrations9 have been popular topics in medicine and in the lay press. Our primary aim in our research was to utilize feedback from the question posed to quantify and rank patient-centered interventions in a gynecology office. These themes and others that emerged in our analysis were used to suggest b
What we asked social media users. The survey query to social media users, “I have the opportunity to design my office from scratch. I’m asking women: How would you design/optimize a visit to the gynecologist’s office?” was crowd-sourced via Twitter on December 5, 2021.5 Given a robust response to the query, it provided an opportunity for a qualitative research study exploring social media users’ perspectives on optimizing outpatient gynecologic care, although the original question was not planned for research utilization.
What we found
By December 27, 2021, the original tweet had earned 9,411 likes; 2,143 retweets; and 3,400 replies. Of this group, we analyzed 131 tweets, all of which had 100 or greater likes on Twitter at the time of the review. The majority of analyzed tweets earned between 100 ̶ 500 likes (75/131; 57.3%), while 22.9% (30/131) had 501 ̶ 1,000 likes, 11.5% (15/131) had >2,000 likes, and 8.4% (11/131) had 1,001 ̶ 1,999 likes.
Identified themes within the tweets analyzed included: medical education, comfort improvements, continuity of care, disability accommodations/accessibility, economic accessibility, nonbinary/transgender care and inclusivity, general layout/floorplan, hospitality, aid for intimate partner violence, childcare accessibility, multi-disciplinary care access, pain/anxiety control, sensitivity toward pregnancy loss/fertility issues, privacy issues, professionalism, representation (subdivided into race, LGBTQIA+, age, and weight/body type), trauma-informed care, and acknowledgement of voluntary nulliparity/support for reproductive choices (TABLE 1). TABLE 2 lists examples of popular tweets by selected themes.
Frequent themes. The most frequently occurring themes within the 131 analyzed tweets (FIGURE 1) were:
- hospitality (77 occurrences)
- comfort improvements (75 occurrences)
- general layout/floorplan (75 occurrences)
- pain/anxiety control (55 occurrences)
- representation (53 occurrences).
Popular themes. Defined as those with more than 1,000 likes at the time of analysis (FIGURE 2), the most popular themes included:
- privacy issues (48.5% of related tweets with >1,000 likes)
- voluntary nulliparity (37.0% of related tweets with >1,000 likes)
- general layout/floorplan (33.4% of related tweets with >1,000 likes)
- representation (32.1% of related tweets with >1,000 likes)
- hospitality (31.3% of related tweets with >1,000 likes).
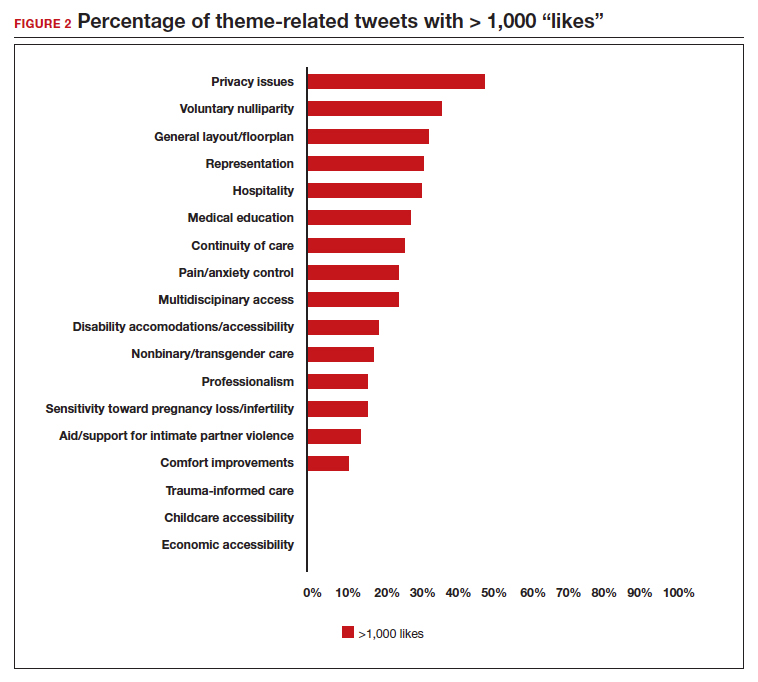
A sub-analysis of themes related to specific types of representation—race, LGBTQIA+, age, and weight/body type was performed. Tweets related to diverse weight/body type representation occurred most frequently (19 code occurrences; FIGURE 3). Similarly, tweets related to the representation of diverse races and the LGBTQIA+ community each comprised 26% of the total representation-based tweets. In terms of popularity as described above, 51.4% of tweets describing racial representation earned >1,000 likes (FIGURE 4).
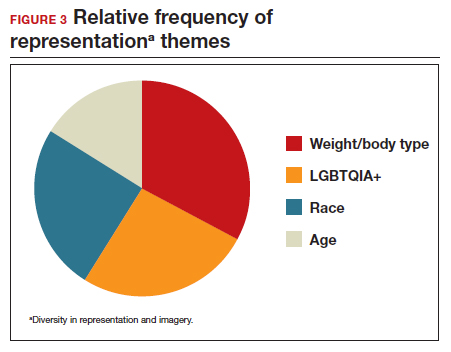
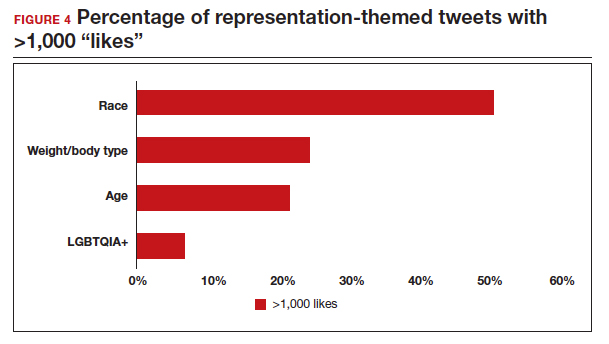
Tweet demographics. Seven (7/131; 5.3%) of the tweet authors were verified Twitter users and 35 (35/131; 26.7%) authors reported working in the health care field within their Twitter profile description.
Continue to: Implementing our feedback can enhance patient experience and care...
Implementing our feedback can enhance patient experience and care
Our study provides a unique view of the patient perspective through analyzed crowdsourced public opinion via Twitter. To our knowledge, an optimized patient-centered outpatient gynecology experience has not previously been described in the medical literature. Optimizing the found domains of hospitality, comfort measures, pain and anxiety control, privacy, and diverse representationin the outpatient gynecologic experience within the outpatient care setting may ultimately result in improved patient satisfaction, patient well-being, and adherence to care through maximizing patient-centered care. We created a checklist of suggestions, including offering analgesics during office-based procedures and tailoring the floorplan to maximize privacy (FIGURE 5), for improving the outpatient gynecology experience based on our findings.

Prior data on patient satisfaction and outcomes
Improving patient satisfaction with health care is a priority for both clinicians and hospital systems. Prior studies have revealed only variable associations between patient satisfaction, safety, and clinical outcomes. One study involving the analysis of clinical and operational data from 171 hospitals found that hospital size, surgical volume, and low mortality rates were associated with higher patient satisfaction, while favorable surgical outcomes did not consistently correlate with higher Hospital Consumer Assessment of Healthcare Provers and Systems (HCAHPS) scores.10 Smaller, lower-volume hospitals earned higher satisfaction scores related to cleanliness, quietness, and receiving help measures.10 It has also been shown that the strongest predictors of patient satisfaction with the hospital childbirth experience included items related to staff communication, compassion, empathy, and respect.11 These data suggest that patient satisfaction is likely more significantly impacted by factors other than patient safety and effectiveness, and this was supported by the findings of our analysis. The growing body of literature associating a sense of psychological and physical safety within the health care system and improved patient outcomes and experience suggests that the data gathered from public commentary such as that presented here is extremely important for galvanizing change within the US health care system.
In one systematic review, the relationship between patient-centered care and clinical outcomes was mixed, although generally the association was positive.12 Additionally, patient-centered care was often associated with increased patient satisfaction and well-being. Some studies suggest that patient well-being and satisfaction also may be associated with improved adherence and self-management behaviors.12,13 Overall, optimizing patient-centered care may lead to improved patient satisfaction and potentially improved clinical outcomes.
Additionally, increasing diverse representation in patient materials and illustrations may help to improve the patient experience. Louie and colleagues found that dark skin tones were represented in only 4.5% of 4,146 images from anatomy texts analyzed in 2018.14 Similarly, a photogrammetric analysis of medical images utilized in New England Journal of Medicine found that only 18% of images depicted non-white skin.15 More recent efforts to create a royalty-free digital gallery of images reflecting bodies with diverse skin tones, body shapes, body hair, and age as well as transgender and nonbinary people have been discussed in the lay press.9 Based on our findings, social media users value and are actively seeking diversity in representation and imagery during their outpatient gynecology experience.
Opportunities for future study
Our research utilized social media as a diverse and accessible source of information; however, there are significant opportunities to refine the methodologic approach to answering the fundamental question of creating the patient-centered gynecologic experience. This type of study has not yet been conducted; however, the richness of the information from this current analysis could be informative to survey creation. Future research on this subject outside of social media could bolster the generalizability of our conclusions and the ability to report on qualitative findings in the setting of known patient demographics.
Social media remains a powerful tool as evidenced by this study, and continued use and observation of trending themes among patients is essential. The influence of social media will remain important for answering questions in gynecology and beyond.
Our work is strengthened by social media’s low threshold for use and the ability for widespread access to a diverse group of users. Additionally, social media allows for many responses to be collected in a timely manner, giving strength to the abstracted themes. The constant production of data by X users and their accessibility provide the opportunity for greater geographic coverage in those surveyed.4 Crowdsourced public opinion also has the advantage of producing qualitative metrics in the form of likes and retweets that may provide a reliable measure of public support or engagement.1
Future studies should examine ways to implement the suggested improvements to the office setting in a cost-effective manner and follow both subjective patient-reported outcomes as well as objective data after implementation, as these changes may have implications for much broader public health crises, such as maternal morbidity and mortality.
Study limitations. Our study is limited by the inherent biases and confounders associated with utilizing data derived from social media. Specifically, not all patients who seek outpatient gynecologic care utilize social media and/or X; using a “like” as a surrogate for endorsement of an idea by an identified party limits the generalizability of the data.
The initial Twitter query specified, “I’m asking women”, which may have altered the intended study population, influenced the analysis, and affected the representativeness of the sample through utilizing non ̶inclusive language. While non-binary/transgender care and inclusivity emerged as a theme discussed with the tweets, it is unclear if this represents an independent theme or rather a reaction to the non–inclusive language within the original tweet. ●
The data abstracted was analyzed with Dedoose1 software using a convenience sample and a mixed-methods analysis. Utilizing X (formerly Twitter and referred here as such given the time the study was conducted) for crowdsourcing functions similarly to an open survey. In the absence of similar analyses, a modified Checklist for Reporting Results of Internet E-Surveys (CHERRIES) checklist was utilized to organize our approach.2
This analysis was comprised of information freely available in the public domain, and the study was classified as IRB exempt. Ethical considerations were made for the fact that this is open access information and participants can reasonably expect their responses to be viewed by the public.3 As this question was not originally intended for research purposes, there was not a formalized development, recruitment, or consent process. The survey was not advertised beyond the original posting on Twitter, and the organic interest that it generated online. No incentives were offered to participants, and all participation was voluntary. There is no mechanism on Twitter for respondents to edit their response, although responses can be deleted. Unique visitors or viewers beyond posted impressions in response to the original tweet could not be determined.
Twitter thread responses were reviewed, and all completed and posted responses to the original Twitter query with 100 or greater “likes” were included in the analysis. These tweets were abstracted from Twitter between December 17, 2021, and December 27, 2021. At the time of tweet abstraction, engagement metrics, including the numbers of likes, retweets, and replies, were recorded. Additionally, author characteristics were abstracted, including author verification status and association with health care, as described in their Twitter profile. Definition of an individual associated with health care was broad and included physicians, advanced practice providers, nurses, first responders, and allied health professionals.
A total of 131 tweets met inclusion criteria and were uploaded for analysis using Dedoose qualitative analytic software.1 Two authors independently utilized a qualitative analysis to code the isolated tweets and identify thematic patterns among them. Uploaded tweets were additionally coded based on ranges of likes: 100-500; 501-1,000; 1,001-1,999; and >2,000. Tweets were coded for author verification status and whether or not the author was associated with the health care field. Themes were identified and defined during the coding process and were shared between the two authors. A total of 18 themes were identified, with 1 theme having 4 subdivisions. Interrater reliability testing was performed using Dedoose1 software and resulted with a pooled Cohen’s Kappa of 0.63, indicating “good” agreement between authors, which is an adequate level of agreement per the Dedoose software guidelines.
References
1. Dedoose website. Accessed July 28, 2022. https://www .dedoose.com/
2. Eysenbach G. Improving the quality of web surveys: the checklist for reporting results of internet e-surveys (CHERRIES) [published correction appears in J Med Internet Res. 2012;14:e8. doi:10.2196/jmir.2042]. J Med Internet Res. 2004;6:e34. doi:10.2196/jmir.6.3.e34
3. Townsend L, Wallace C. Social media research: a guide to ethics [University of Glasgow Information for the Media website]. Accessed March 2, 2023. https://www.gla.ac.uk /media/Media_487729_smxx.pdf
- Garvey MD, Samuel J, Pelaez A. Would you please like my tweet?! An artificially intelligent, generative probabilistic, and econometric based system design for popularity-driven tweet content generation. Decis Support Syst. 2021;144:113497. doi: 10.1016/j.dss.2021.113497
- Twitter Revenue and Usage Statistics (2023). Business of apps. Published August 10, 2023. Accessed September 19, 2023. https://www.businessofapps.com/data/twitter-statistics/
- Doan AE, Bogen KW, Higgins E. A content analysis of twitter backlash to Georgia’s abortion ban. Sex Reprod Healthc. 2022;31:100689. doi:10.1016/j.srhc.2021.100689
- Roberts H, Sadler J, Chapman L. The value of Twitter data for determining the emotional responses of people to urban green spaces: a case study and critical evaluation. Urban Stud. 2019;56:818-835. doi: 10.1177/0042098017748544
- Stewart R [@stuboo]. I have the opportunity to design my office from scratch. I’m asking women. How would you design/optimize a visit to the gynecologist’s office? problems frustrations solutions No detail is too small. If I’ve ever had a tweet worthy of virality, it’s this one. RT. Twitter. Published December 5, 2021. Accessed March 1, 2023. https://twitter .com/stuboo/status/1467522852664532994
- A gynecologist asked Twitter how he should redesign his office. The answers he got were about deeper health care issues. Fortune. Accessed March 2, 2023. https://fortune .com/2021/12/07/gynecologist-twitter-question/
- Anderson GD, Nelson-Becker C, Hannigan EV, et al. A patientcentered health care delivery system by a university obstetrics and gynecology department. Obstet Gynecol. 2005;105:205210. doi:10.1097/01.AOG.0000146288.28195.27
- Ades V, Wu SX, Rabinowitz E, et al. An integrated, traumainformed care model for female survivors of sexual violence: the engage, motivate, protect, organize, self-worth, educate, respect (EMPOWER) clinic. Obstet Gynecol. 2019;133:803809. doi:10.1097/AOG.0000000000003186
- Gordon D. Health equity comes to medical illustrations with launch of new image library. Forbes. Accessed March 2023. https://www.forbes.com/sites/debgordon/2022/05/11 /health-equity-comes-to-medical-illustrations-with-launch -of-new-image-library/
- Kennedy GD, Tevis SE, Kent KC. Is there a relationship between patient satisfaction and favorable outcomes? Ann Surg. 2014;260:592-600. doi:10.1097/SLA.0000000000000932
- Gregory KD, Korst LM, Saeb S, et al. Childbirth-specific patient-reported outcomes as predictors of hospital satisfaction. Am J Obstet Gynecol. 2019;220:201.e1-201.e19. doi:10.1016/j.ajog.2018.10.093
- Rathert C, Wyrwich MD, Boren SA. Patient-centered care and outcomes: a systematic review of the literature. Med Care Res Rev. 2013;70:351-379. doi:10.1177/1077558712465774
- Kahn KL, Schneider EC, Malin JL, et al. Patient-centered experiences in breast cancer: predicting long-term adherence to tamoxifen use. Med Care. 2007;45:431-439. doi:10.1097/01 .mlr.0000257193.10760.7
- Louie P, Wilkes R. Representations of race and skin tone in medical textbook imagery. Soc Sci Med. 2018;202:38-42. doi:10.1016/j.socscimed.2018.02.023
- Massie JP, Cho DY, Kneib CJ, et al. A picture of modern medicine: race and visual representation in medical literature. J Natl Med Assoc. 2021;113:88-94. doi:10.1016/j.jnma.2020.07.013
There has been increasing awareness of a need for creating a more patient-centered experience with outpatient gynecology; however, very little data exist about what interventions are important to patients. Given social media’s ease of use and ability for widespread access to a diverse group of users, it has the potential to be a powerful tool for qualitative research questions without the difficulties of cost, transportation, transcription, etc. required of a focus group. Crowdsourced public opinion also has the advantage of producing qualitative metrics in the form of “likes” that, at scale, can provide a reliable measure of public support or engagement for a particular concept.1 Particularly for topics that are controversial or novel, X (formerly Twitter, and referred to as Twitter intermittently throughout this article based on the time the study was conducted), with 300 million monthly users,2 has become a popular tool for general and health care ̶ focused content and sentiment analysis.3,4 This study presents a qualitative analysis of themes from a crowdsourced request on Twitter to design the ideal outpatient gynecologic experience that subsequently went “viral”.5,6
When asked to design the optimized outpatient gynecology experience, social media users expressed:
- hospitality, comfort, and pain control as frequent themes
- preserving privacy and acknowledgement of voluntary nulliparity as frequent themes
- a desire for diverse imagery and representation related to race, LGBTQIA+ themes, age, and weight/body type within the office setting
- a call for a sense of psychological safety within gynecology
Why the need for our research question on patient-centered gyn care
While the body of literature on patient-centered health care has grown rapidly in recent years, a patient-centered outpatient gynecology experience has not yet been described in the medical literature.
Patient-centered office design, driven by cultural sensitivity, has been shown in other studies to be both appreciated by established patients and a viable business strategy to attract new patients.7 Topics such as pain control, trauma-informed care in gynecologyclinics,8 and diverse representation in patient materials and illustrations9 have been popular topics in medicine and in the lay press. Our primary aim in our research was to utilize feedback from the question posed to quantify and rank patient-centered interventions in a gynecology office. These themes and others that emerged in our analysis were used to suggest b
What we asked social media users. The survey query to social media users, “I have the opportunity to design my office from scratch. I’m asking women: How would you design/optimize a visit to the gynecologist’s office?” was crowd-sourced via Twitter on December 5, 2021.5 Given a robust response to the query, it provided an opportunity for a qualitative research study exploring social media users’ perspectives on optimizing outpatient gynecologic care, although the original question was not planned for research utilization.
What we found
By December 27, 2021, the original tweet had earned 9,411 likes; 2,143 retweets; and 3,400 replies. Of this group, we analyzed 131 tweets, all of which had 100 or greater likes on Twitter at the time of the review. The majority of analyzed tweets earned between 100 ̶ 500 likes (75/131; 57.3%), while 22.9% (30/131) had 501 ̶ 1,000 likes, 11.5% (15/131) had >2,000 likes, and 8.4% (11/131) had 1,001 ̶ 1,999 likes.
Identified themes within the tweets analyzed included: medical education, comfort improvements, continuity of care, disability accommodations/accessibility, economic accessibility, nonbinary/transgender care and inclusivity, general layout/floorplan, hospitality, aid for intimate partner violence, childcare accessibility, multi-disciplinary care access, pain/anxiety control, sensitivity toward pregnancy loss/fertility issues, privacy issues, professionalism, representation (subdivided into race, LGBTQIA+, age, and weight/body type), trauma-informed care, and acknowledgement of voluntary nulliparity/support for reproductive choices (TABLE 1). TABLE 2 lists examples of popular tweets by selected themes.


Frequent themes. The most frequently occurring themes within the 131 analyzed tweets (FIGURE 1) were:
- hospitality (77 occurrences)
- comfort improvements (75 occurrences)
- general layout/floorplan (75 occurrences)
- pain/anxiety control (55 occurrences)
- representation (53 occurrences).

Popular themes. Defined as those with more than 1,000 likes at the time of analysis (FIGURE 2), the most popular themes included:
- privacy issues (48.5% of related tweets with >1,000 likes)
- voluntary nulliparity (37.0% of related tweets with >1,000 likes)
- general layout/floorplan (33.4% of related tweets with >1,000 likes)
- representation (32.1% of related tweets with >1,000 likes)
- hospitality (31.3% of related tweets with >1,000 likes).

A sub-analysis of themes related to specific types of representation—race, LGBTQIA+, age, and weight/body type was performed. Tweets related to diverse weight/body type representation occurred most frequently (19 code occurrences; FIGURE 3). Similarly, tweets related to the representation of diverse races and the LGBTQIA+ community each comprised 26% of the total representation-based tweets. In terms of popularity as described above, 51.4% of tweets describing racial representation earned >1,000 likes (FIGURE 4).


Tweet demographics. Seven (7/131; 5.3%) of the tweet authors were verified Twitter users and 35 (35/131; 26.7%) authors reported working in the health care field within their Twitter profile description.
Continue to: Implementing our feedback can enhance patient experience and care...
Implementing our feedback can enhance patient experience and care
Our study provides a unique view of the patient perspective through analyzed crowdsourced public opinion via Twitter. To our knowledge, an optimized patient-centered outpatient gynecology experience has not previously been described in the medical literature. Optimizing the found domains of hospitality, comfort measures, pain and anxiety control, privacy, and diverse representationin the outpatient gynecologic experience within the outpatient care setting may ultimately result in improved patient satisfaction, patient well-being, and adherence to care through maximizing patient-centered care. We created a checklist of suggestions, including offering analgesics during office-based procedures and tailoring the floorplan to maximize privacy (FIGURE 5), for improving the outpatient gynecology experience based on our findings.

Prior data on patient satisfaction and outcomes
Improving patient satisfaction with health care is a priority for both clinicians and hospital systems. Prior studies have revealed only variable associations between patient satisfaction, safety, and clinical outcomes. One study involving the analysis of clinical and operational data from 171 hospitals found that hospital size, surgical volume, and low mortality rates were associated with higher patient satisfaction, while favorable surgical outcomes did not consistently correlate with higher Hospital Consumer Assessment of Healthcare Provers and Systems (HCAHPS) scores.10 Smaller, lower-volume hospitals earned higher satisfaction scores related to cleanliness, quietness, and receiving help measures.10 It has also been shown that the strongest predictors of patient satisfaction with the hospital childbirth experience included items related to staff communication, compassion, empathy, and respect.11 These data suggest that patient satisfaction is likely more significantly impacted by factors other than patient safety and effectiveness, and this was supported by the findings of our analysis. The growing body of literature associating a sense of psychological and physical safety within the health care system and improved patient outcomes and experience suggests that the data gathered from public commentary such as that presented here is extremely important for galvanizing change within the US health care system.
In one systematic review, the relationship between patient-centered care and clinical outcomes was mixed, although generally the association was positive.12 Additionally, patient-centered care was often associated with increased patient satisfaction and well-being. Some studies suggest that patient well-being and satisfaction also may be associated with improved adherence and self-management behaviors.12,13 Overall, optimizing patient-centered care may lead to improved patient satisfaction and potentially improved clinical outcomes.
Additionally, increasing diverse representation in patient materials and illustrations may help to improve the patient experience. Louie and colleagues found that dark skin tones were represented in only 4.5% of 4,146 images from anatomy texts analyzed in 2018.14 Similarly, a photogrammetric analysis of medical images utilized in New England Journal of Medicine found that only 18% of images depicted non-white skin.15 More recent efforts to create a royalty-free digital gallery of images reflecting bodies with diverse skin tones, body shapes, body hair, and age as well as transgender and nonbinary people have been discussed in the lay press.9 Based on our findings, social media users value and are actively seeking diversity in representation and imagery during their outpatient gynecology experience.
Opportunities for future study
Our research utilized social media as a diverse and accessible source of information; however, there are significant opportunities to refine the methodologic approach to answering the fundamental question of creating the patient-centered gynecologic experience. This type of study has not yet been conducted; however, the richness of the information from this current analysis could be informative to survey creation. Future research on this subject outside of social media could bolster the generalizability of our conclusions and the ability to report on qualitative findings in the setting of known patient demographics.
Social media remains a powerful tool as evidenced by this study, and continued use and observation of trending themes among patients is essential. The influence of social media will remain important for answering questions in gynecology and beyond.
Our work is strengthened by social media’s low threshold for use and the ability for widespread access to a diverse group of users. Additionally, social media allows for many responses to be collected in a timely manner, giving strength to the abstracted themes. The constant production of data by X users and their accessibility provide the opportunity for greater geographic coverage in those surveyed.4 Crowdsourced public opinion also has the advantage of producing qualitative metrics in the form of likes and retweets that may provide a reliable measure of public support or engagement.1
Future studies should examine ways to implement the suggested improvements to the office setting in a cost-effective manner and follow both subjective patient-reported outcomes as well as objective data after implementation, as these changes may have implications for much broader public health crises, such as maternal morbidity and mortality.
Study limitations. Our study is limited by the inherent biases and confounders associated with utilizing data derived from social media. Specifically, not all patients who seek outpatient gynecologic care utilize social media and/or X; using a “like” as a surrogate for endorsement of an idea by an identified party limits the generalizability of the data.
The initial Twitter query specified, “I’m asking women”, which may have altered the intended study population, influenced the analysis, and affected the representativeness of the sample through utilizing non ̶inclusive language. While non-binary/transgender care and inclusivity emerged as a theme discussed with the tweets, it is unclear if this represents an independent theme or rather a reaction to the non–inclusive language within the original tweet. ●
The data abstracted was analyzed with Dedoose1 software using a convenience sample and a mixed-methods analysis. Utilizing X (formerly Twitter and referred here as such given the time the study was conducted) for crowdsourcing functions similarly to an open survey. In the absence of similar analyses, a modified Checklist for Reporting Results of Internet E-Surveys (CHERRIES) checklist was utilized to organize our approach.2
This analysis was comprised of information freely available in the public domain, and the study was classified as IRB exempt. Ethical considerations were made for the fact that this is open access information and participants can reasonably expect their responses to be viewed by the public.3 As this question was not originally intended for research purposes, there was not a formalized development, recruitment, or consent process. The survey was not advertised beyond the original posting on Twitter, and the organic interest that it generated online. No incentives were offered to participants, and all participation was voluntary. There is no mechanism on Twitter for respondents to edit their response, although responses can be deleted. Unique visitors or viewers beyond posted impressions in response to the original tweet could not be determined.
Twitter thread responses were reviewed, and all completed and posted responses to the original Twitter query with 100 or greater “likes” were included in the analysis. These tweets were abstracted from Twitter between December 17, 2021, and December 27, 2021. At the time of tweet abstraction, engagement metrics, including the numbers of likes, retweets, and replies, were recorded. Additionally, author characteristics were abstracted, including author verification status and association with health care, as described in their Twitter profile. Definition of an individual associated with health care was broad and included physicians, advanced practice providers, nurses, first responders, and allied health professionals.
A total of 131 tweets met inclusion criteria and were uploaded for analysis using Dedoose qualitative analytic software.1 Two authors independently utilized a qualitative analysis to code the isolated tweets and identify thematic patterns among them. Uploaded tweets were additionally coded based on ranges of likes: 100-500; 501-1,000; 1,001-1,999; and >2,000. Tweets were coded for author verification status and whether or not the author was associated with the health care field. Themes were identified and defined during the coding process and were shared between the two authors. A total of 18 themes were identified, with 1 theme having 4 subdivisions. Interrater reliability testing was performed using Dedoose1 software and resulted with a pooled Cohen’s Kappa of 0.63, indicating “good” agreement between authors, which is an adequate level of agreement per the Dedoose software guidelines.
References
1. Dedoose website. Accessed July 28, 2022. https://www .dedoose.com/
2. Eysenbach G. Improving the quality of web surveys: the checklist for reporting results of internet e-surveys (CHERRIES) [published correction appears in J Med Internet Res. 2012;14:e8. doi:10.2196/jmir.2042]. J Med Internet Res. 2004;6:e34. doi:10.2196/jmir.6.3.e34
3. Townsend L, Wallace C. Social media research: a guide to ethics [University of Glasgow Information for the Media website]. Accessed March 2, 2023. https://www.gla.ac.uk /media/Media_487729_smxx.pdf
There has been increasing awareness of a need for creating a more patient-centered experience with outpatient gynecology; however, very little data exist about what interventions are important to patients. Given social media’s ease of use and ability for widespread access to a diverse group of users, it has the potential to be a powerful tool for qualitative research questions without the difficulties of cost, transportation, transcription, etc. required of a focus group. Crowdsourced public opinion also has the advantage of producing qualitative metrics in the form of “likes” that, at scale, can provide a reliable measure of public support or engagement for a particular concept.1 Particularly for topics that are controversial or novel, X (formerly Twitter, and referred to as Twitter intermittently throughout this article based on the time the study was conducted), with 300 million monthly users,2 has become a popular tool for general and health care ̶ focused content and sentiment analysis.3,4 This study presents a qualitative analysis of themes from a crowdsourced request on Twitter to design the ideal outpatient gynecologic experience that subsequently went “viral”.5,6
When asked to design the optimized outpatient gynecology experience, social media users expressed:
- hospitality, comfort, and pain control as frequent themes
- preserving privacy and acknowledgement of voluntary nulliparity as frequent themes
- a desire for diverse imagery and representation related to race, LGBTQIA+ themes, age, and weight/body type within the office setting
- a call for a sense of psychological safety within gynecology
Why the need for our research question on patient-centered gyn care
While the body of literature on patient-centered health care has grown rapidly in recent years, a patient-centered outpatient gynecology experience has not yet been described in the medical literature.
Patient-centered office design, driven by cultural sensitivity, has been shown in other studies to be both appreciated by established patients and a viable business strategy to attract new patients.7 Topics such as pain control, trauma-informed care in gynecologyclinics,8 and diverse representation in patient materials and illustrations9 have been popular topics in medicine and in the lay press. Our primary aim in our research was to utilize feedback from the question posed to quantify and rank patient-centered interventions in a gynecology office. These themes and others that emerged in our analysis were used to suggest b
What we asked social media users. The survey query to social media users, “I have the opportunity to design my office from scratch. I’m asking women: How would you design/optimize a visit to the gynecologist’s office?” was crowd-sourced via Twitter on December 5, 2021.5 Given a robust response to the query, it provided an opportunity for a qualitative research study exploring social media users’ perspectives on optimizing outpatient gynecologic care, although the original question was not planned for research utilization.
What we found
By December 27, 2021, the original tweet had earned 9,411 likes; 2,143 retweets; and 3,400 replies. Of this group, we analyzed 131 tweets, all of which had 100 or greater likes on Twitter at the time of the review. The majority of analyzed tweets earned between 100 ̶ 500 likes (75/131; 57.3%), while 22.9% (30/131) had 501 ̶ 1,000 likes, 11.5% (15/131) had >2,000 likes, and 8.4% (11/131) had 1,001 ̶ 1,999 likes.
Identified themes within the tweets analyzed included: medical education, comfort improvements, continuity of care, disability accommodations/accessibility, economic accessibility, nonbinary/transgender care and inclusivity, general layout/floorplan, hospitality, aid for intimate partner violence, childcare accessibility, multi-disciplinary care access, pain/anxiety control, sensitivity toward pregnancy loss/fertility issues, privacy issues, professionalism, representation (subdivided into race, LGBTQIA+, age, and weight/body type), trauma-informed care, and acknowledgement of voluntary nulliparity/support for reproductive choices (TABLE 1). TABLE 2 lists examples of popular tweets by selected themes.


Frequent themes. The most frequently occurring themes within the 131 analyzed tweets (FIGURE 1) were:
- hospitality (77 occurrences)
- comfort improvements (75 occurrences)
- general layout/floorplan (75 occurrences)
- pain/anxiety control (55 occurrences)
- representation (53 occurrences).

Popular themes. Defined as those with more than 1,000 likes at the time of analysis (FIGURE 2), the most popular themes included:
- privacy issues (48.5% of related tweets with >1,000 likes)
- voluntary nulliparity (37.0% of related tweets with >1,000 likes)
- general layout/floorplan (33.4% of related tweets with >1,000 likes)
- representation (32.1% of related tweets with >1,000 likes)
- hospitality (31.3% of related tweets with >1,000 likes).

A sub-analysis of themes related to specific types of representation—race, LGBTQIA+, age, and weight/body type was performed. Tweets related to diverse weight/body type representation occurred most frequently (19 code occurrences; FIGURE 3). Similarly, tweets related to the representation of diverse races and the LGBTQIA+ community each comprised 26% of the total representation-based tweets. In terms of popularity as described above, 51.4% of tweets describing racial representation earned >1,000 likes (FIGURE 4).


Tweet demographics. Seven (7/131; 5.3%) of the tweet authors were verified Twitter users and 35 (35/131; 26.7%) authors reported working in the health care field within their Twitter profile description.
Continue to: Implementing our feedback can enhance patient experience and care...
Implementing our feedback can enhance patient experience and care
Our study provides a unique view of the patient perspective through analyzed crowdsourced public opinion via Twitter. To our knowledge, an optimized patient-centered outpatient gynecology experience has not previously been described in the medical literature. Optimizing the found domains of hospitality, comfort measures, pain and anxiety control, privacy, and diverse representationin the outpatient gynecologic experience within the outpatient care setting may ultimately result in improved patient satisfaction, patient well-being, and adherence to care through maximizing patient-centered care. We created a checklist of suggestions, including offering analgesics during office-based procedures and tailoring the floorplan to maximize privacy (FIGURE 5), for improving the outpatient gynecology experience based on our findings.

Prior data on patient satisfaction and outcomes
Improving patient satisfaction with health care is a priority for both clinicians and hospital systems. Prior studies have revealed only variable associations between patient satisfaction, safety, and clinical outcomes. One study involving the analysis of clinical and operational data from 171 hospitals found that hospital size, surgical volume, and low mortality rates were associated with higher patient satisfaction, while favorable surgical outcomes did not consistently correlate with higher Hospital Consumer Assessment of Healthcare Provers and Systems (HCAHPS) scores.10 Smaller, lower-volume hospitals earned higher satisfaction scores related to cleanliness, quietness, and receiving help measures.10 It has also been shown that the strongest predictors of patient satisfaction with the hospital childbirth experience included items related to staff communication, compassion, empathy, and respect.11 These data suggest that patient satisfaction is likely more significantly impacted by factors other than patient safety and effectiveness, and this was supported by the findings of our analysis. The growing body of literature associating a sense of psychological and physical safety within the health care system and improved patient outcomes and experience suggests that the data gathered from public commentary such as that presented here is extremely important for galvanizing change within the US health care system.
In one systematic review, the relationship between patient-centered care and clinical outcomes was mixed, although generally the association was positive.12 Additionally, patient-centered care was often associated with increased patient satisfaction and well-being. Some studies suggest that patient well-being and satisfaction also may be associated with improved adherence and self-management behaviors.12,13 Overall, optimizing patient-centered care may lead to improved patient satisfaction and potentially improved clinical outcomes.
Additionally, increasing diverse representation in patient materials and illustrations may help to improve the patient experience. Louie and colleagues found that dark skin tones were represented in only 4.5% of 4,146 images from anatomy texts analyzed in 2018.14 Similarly, a photogrammetric analysis of medical images utilized in New England Journal of Medicine found that only 18% of images depicted non-white skin.15 More recent efforts to create a royalty-free digital gallery of images reflecting bodies with diverse skin tones, body shapes, body hair, and age as well as transgender and nonbinary people have been discussed in the lay press.9 Based on our findings, social media users value and are actively seeking diversity in representation and imagery during their outpatient gynecology experience.
Opportunities for future study
Our research utilized social media as a diverse and accessible source of information; however, there are significant opportunities to refine the methodologic approach to answering the fundamental question of creating the patient-centered gynecologic experience. This type of study has not yet been conducted; however, the richness of the information from this current analysis could be informative to survey creation. Future research on this subject outside of social media could bolster the generalizability of our conclusions and the ability to report on qualitative findings in the setting of known patient demographics.
Social media remains a powerful tool as evidenced by this study, and continued use and observation of trending themes among patients is essential. The influence of social media will remain important for answering questions in gynecology and beyond.
Our work is strengthened by social media’s low threshold for use and the ability for widespread access to a diverse group of users. Additionally, social media allows for many responses to be collected in a timely manner, giving strength to the abstracted themes. The constant production of data by X users and their accessibility provide the opportunity for greater geographic coverage in those surveyed.4 Crowdsourced public opinion also has the advantage of producing qualitative metrics in the form of likes and retweets that may provide a reliable measure of public support or engagement.1
Future studies should examine ways to implement the suggested improvements to the office setting in a cost-effective manner and follow both subjective patient-reported outcomes as well as objective data after implementation, as these changes may have implications for much broader public health crises, such as maternal morbidity and mortality.
Study limitations. Our study is limited by the inherent biases and confounders associated with utilizing data derived from social media. Specifically, not all patients who seek outpatient gynecologic care utilize social media and/or X; using a “like” as a surrogate for endorsement of an idea by an identified party limits the generalizability of the data.
The initial Twitter query specified, “I’m asking women”, which may have altered the intended study population, influenced the analysis, and affected the representativeness of the sample through utilizing non ̶inclusive language. While non-binary/transgender care and inclusivity emerged as a theme discussed with the tweets, it is unclear if this represents an independent theme or rather a reaction to the non–inclusive language within the original tweet. ●
The data abstracted was analyzed with Dedoose1 software using a convenience sample and a mixed-methods analysis. Utilizing X (formerly Twitter and referred here as such given the time the study was conducted) for crowdsourcing functions similarly to an open survey. In the absence of similar analyses, a modified Checklist for Reporting Results of Internet E-Surveys (CHERRIES) checklist was utilized to organize our approach.2
This analysis was comprised of information freely available in the public domain, and the study was classified as IRB exempt. Ethical considerations were made for the fact that this is open access information and participants can reasonably expect their responses to be viewed by the public.3 As this question was not originally intended for research purposes, there was not a formalized development, recruitment, or consent process. The survey was not advertised beyond the original posting on Twitter, and the organic interest that it generated online. No incentives were offered to participants, and all participation was voluntary. There is no mechanism on Twitter for respondents to edit their response, although responses can be deleted. Unique visitors or viewers beyond posted impressions in response to the original tweet could not be determined.
Twitter thread responses were reviewed, and all completed and posted responses to the original Twitter query with 100 or greater “likes” were included in the analysis. These tweets were abstracted from Twitter between December 17, 2021, and December 27, 2021. At the time of tweet abstraction, engagement metrics, including the numbers of likes, retweets, and replies, were recorded. Additionally, author characteristics were abstracted, including author verification status and association with health care, as described in their Twitter profile. Definition of an individual associated with health care was broad and included physicians, advanced practice providers, nurses, first responders, and allied health professionals.
A total of 131 tweets met inclusion criteria and were uploaded for analysis using Dedoose qualitative analytic software.1 Two authors independently utilized a qualitative analysis to code the isolated tweets and identify thematic patterns among them. Uploaded tweets were additionally coded based on ranges of likes: 100-500; 501-1,000; 1,001-1,999; and >2,000. Tweets were coded for author verification status and whether or not the author was associated with the health care field. Themes were identified and defined during the coding process and were shared between the two authors. A total of 18 themes were identified, with 1 theme having 4 subdivisions. Interrater reliability testing was performed using Dedoose1 software and resulted with a pooled Cohen’s Kappa of 0.63, indicating “good” agreement between authors, which is an adequate level of agreement per the Dedoose software guidelines.
References
1. Dedoose website. Accessed July 28, 2022. https://www .dedoose.com/
2. Eysenbach G. Improving the quality of web surveys: the checklist for reporting results of internet e-surveys (CHERRIES) [published correction appears in J Med Internet Res. 2012;14:e8. doi:10.2196/jmir.2042]. J Med Internet Res. 2004;6:e34. doi:10.2196/jmir.6.3.e34
3. Townsend L, Wallace C. Social media research: a guide to ethics [University of Glasgow Information for the Media website]. Accessed March 2, 2023. https://www.gla.ac.uk /media/Media_487729_smxx.pdf
- Garvey MD, Samuel J, Pelaez A. Would you please like my tweet?! An artificially intelligent, generative probabilistic, and econometric based system design for popularity-driven tweet content generation. Decis Support Syst. 2021;144:113497. doi: 10.1016/j.dss.2021.113497
- Twitter Revenue and Usage Statistics (2023). Business of apps. Published August 10, 2023. Accessed September 19, 2023. https://www.businessofapps.com/data/twitter-statistics/
- Doan AE, Bogen KW, Higgins E. A content analysis of twitter backlash to Georgia’s abortion ban. Sex Reprod Healthc. 2022;31:100689. doi:10.1016/j.srhc.2021.100689
- Roberts H, Sadler J, Chapman L. The value of Twitter data for determining the emotional responses of people to urban green spaces: a case study and critical evaluation. Urban Stud. 2019;56:818-835. doi: 10.1177/0042098017748544
- Stewart R [@stuboo]. I have the opportunity to design my office from scratch. I’m asking women. How would you design/optimize a visit to the gynecologist’s office? problems frustrations solutions No detail is too small. If I’ve ever had a tweet worthy of virality, it’s this one. RT. Twitter. Published December 5, 2021. Accessed March 1, 2023. https://twitter .com/stuboo/status/1467522852664532994
- A gynecologist asked Twitter how he should redesign his office. The answers he got were about deeper health care issues. Fortune. Accessed March 2, 2023. https://fortune .com/2021/12/07/gynecologist-twitter-question/
- Anderson GD, Nelson-Becker C, Hannigan EV, et al. A patientcentered health care delivery system by a university obstetrics and gynecology department. Obstet Gynecol. 2005;105:205210. doi:10.1097/01.AOG.0000146288.28195.27
- Ades V, Wu SX, Rabinowitz E, et al. An integrated, traumainformed care model for female survivors of sexual violence: the engage, motivate, protect, organize, self-worth, educate, respect (EMPOWER) clinic. Obstet Gynecol. 2019;133:803809. doi:10.1097/AOG.0000000000003186
- Gordon D. Health equity comes to medical illustrations with launch of new image library. Forbes. Accessed March 2023. https://www.forbes.com/sites/debgordon/2022/05/11 /health-equity-comes-to-medical-illustrations-with-launch -of-new-image-library/
- Kennedy GD, Tevis SE, Kent KC. Is there a relationship between patient satisfaction and favorable outcomes? Ann Surg. 2014;260:592-600. doi:10.1097/SLA.0000000000000932
- Gregory KD, Korst LM, Saeb S, et al. Childbirth-specific patient-reported outcomes as predictors of hospital satisfaction. Am J Obstet Gynecol. 2019;220:201.e1-201.e19. doi:10.1016/j.ajog.2018.10.093
- Rathert C, Wyrwich MD, Boren SA. Patient-centered care and outcomes: a systematic review of the literature. Med Care Res Rev. 2013;70:351-379. doi:10.1177/1077558712465774
- Kahn KL, Schneider EC, Malin JL, et al. Patient-centered experiences in breast cancer: predicting long-term adherence to tamoxifen use. Med Care. 2007;45:431-439. doi:10.1097/01 .mlr.0000257193.10760.7
- Louie P, Wilkes R. Representations of race and skin tone in medical textbook imagery. Soc Sci Med. 2018;202:38-42. doi:10.1016/j.socscimed.2018.02.023
- Massie JP, Cho DY, Kneib CJ, et al. A picture of modern medicine: race and visual representation in medical literature. J Natl Med Assoc. 2021;113:88-94. doi:10.1016/j.jnma.2020.07.013
- Garvey MD, Samuel J, Pelaez A. Would you please like my tweet?! An artificially intelligent, generative probabilistic, and econometric based system design for popularity-driven tweet content generation. Decis Support Syst. 2021;144:113497. doi: 10.1016/j.dss.2021.113497
- Twitter Revenue and Usage Statistics (2023). Business of apps. Published August 10, 2023. Accessed September 19, 2023. https://www.businessofapps.com/data/twitter-statistics/
- Doan AE, Bogen KW, Higgins E. A content analysis of twitter backlash to Georgia’s abortion ban. Sex Reprod Healthc. 2022;31:100689. doi:10.1016/j.srhc.2021.100689
- Roberts H, Sadler J, Chapman L. The value of Twitter data for determining the emotional responses of people to urban green spaces: a case study and critical evaluation. Urban Stud. 2019;56:818-835. doi: 10.1177/0042098017748544
- Stewart R [@stuboo]. I have the opportunity to design my office from scratch. I’m asking women. How would you design/optimize a visit to the gynecologist’s office? problems frustrations solutions No detail is too small. If I’ve ever had a tweet worthy of virality, it’s this one. RT. Twitter. Published December 5, 2021. Accessed March 1, 2023. https://twitter .com/stuboo/status/1467522852664532994
- A gynecologist asked Twitter how he should redesign his office. The answers he got were about deeper health care issues. Fortune. Accessed March 2, 2023. https://fortune .com/2021/12/07/gynecologist-twitter-question/
- Anderson GD, Nelson-Becker C, Hannigan EV, et al. A patientcentered health care delivery system by a university obstetrics and gynecology department. Obstet Gynecol. 2005;105:205210. doi:10.1097/01.AOG.0000146288.28195.27
- Ades V, Wu SX, Rabinowitz E, et al. An integrated, traumainformed care model for female survivors of sexual violence: the engage, motivate, protect, organize, self-worth, educate, respect (EMPOWER) clinic. Obstet Gynecol. 2019;133:803809. doi:10.1097/AOG.0000000000003186
- Gordon D. Health equity comes to medical illustrations with launch of new image library. Forbes. Accessed March 2023. https://www.forbes.com/sites/debgordon/2022/05/11 /health-equity-comes-to-medical-illustrations-with-launch -of-new-image-library/
- Kennedy GD, Tevis SE, Kent KC. Is there a relationship between patient satisfaction and favorable outcomes? Ann Surg. 2014;260:592-600. doi:10.1097/SLA.0000000000000932
- Gregory KD, Korst LM, Saeb S, et al. Childbirth-specific patient-reported outcomes as predictors of hospital satisfaction. Am J Obstet Gynecol. 2019;220:201.e1-201.e19. doi:10.1016/j.ajog.2018.10.093
- Rathert C, Wyrwich MD, Boren SA. Patient-centered care and outcomes: a systematic review of the literature. Med Care Res Rev. 2013;70:351-379. doi:10.1177/1077558712465774
- Kahn KL, Schneider EC, Malin JL, et al. Patient-centered experiences in breast cancer: predicting long-term adherence to tamoxifen use. Med Care. 2007;45:431-439. doi:10.1097/01 .mlr.0000257193.10760.7
- Louie P, Wilkes R. Representations of race and skin tone in medical textbook imagery. Soc Sci Med. 2018;202:38-42. doi:10.1016/j.socscimed.2018.02.023
- Massie JP, Cho DY, Kneib CJ, et al. A picture of modern medicine: race and visual representation in medical literature. J Natl Med Assoc. 2021;113:88-94. doi:10.1016/j.jnma.2020.07.013
In-hospital mortality increased in COPD patients with acute exacerbations and high serum phosphate levels
found significantly higher in-hospital mortality among AECOPD patients with high serum phosphate levels. The finding, according to Siqi Li et al. in a preproof HELIYON article, suggests that hyperphosphatemia may be a high-risk factor for AECOPD-related in-hospital mortality.
Phosphorus is key to several physiological processes, among them energy metabolism, bone mineralization, membrane transport, and intracellular signaling. Li et al. pointed out that in patients with multiple diseases, hyperphosphatemia is associated with increased mortality. In the development of COPD specifically, acute exacerbations have been shown in several recent studies to be an important adverse event conferring heightened mortality risk. Despite many efforts, AECOPD mortality rates remain high, making identification of potential factors, Li et al. stated, crucial for improving outcomes in high-risk patients.
The electronic Intensive Care Unit Collaborative Research Database (eICU-CRD) holds data associated with over 200,000 patient stays, providing a large sample size for research studies. To determine the relationship between serum phosphate and in-hospital mortality in AECOPD patients, investigators analyzed data from a total of 1,199 AECOPD patients (mean age, 68 years; ~55% female) enrolled in eICU-CRD and divided them into three groups according to serum phosphate level tertiles: lowest tertile (serum phosphate ≤ 3.0 mg/dL, n = 445), median tertile (serum phosphate > 3.0 mg/dL and ≤ 4.0 mg/dL, n = 378), and highest tertile (serum phosphate > 4.0 mg/dL, n = 376). The Li et al. study’s primary outcome was all-cause in-hospital mortality, defined as survival to hospital discharge. Secondary outcomes included length of stay (LOS) in the intensive care unit (ICU), LOS in the hospital, and all-cause ICU mortality.
The Li et al. analysis of patient characteristics showed that patients in the highest tertile of serum phosphate had significantly higher body mass index (BMI) (P < .001), lower temperature (P < .001), lower heart rate (P < .001), lower mean arterial blood pressure (P = .011), higher creatinine (P < .001), higher potassium (P < .001), higher sequential organ failure assessment (SOFA) (P < .001), higher acute physiology and chronic health evaluation (APACHE IV) (P < .001), and higher ICU mortality (P < .001). Also, patients with higher serum phosphate levels were more likely to receive renal replacement therapy (RRT) (P < .001) and vasoactive drugs (P = .003) than those in the lower serum phosphate group. Such differences were also observed for age (P = .021), calcium level (P = .023), sodium level (P = .039), hypertension (P = .014), coronary artery disease (P = .004), diabetes (P = .017), and chronic kidney disease (P < .001). No significant differences were observed for gender, respiration rate, SpO2, white blood cell count, hemoglobin, platelets, cirrhosis, stroke, ventilation, LOS in ICU, and LOS in hospital (P > .05).
A univariate logistic regression analysis performed to determine the relationship between serum phosphate level and risk of in-hospital mortality revealed that higher serum phosphate level correlated with increased in-hospital mortality (odds ratio, 1.30; 95% confidence interval, 1.16-1.46; P < .001).
Li et al. posited that several mechanisms may explain increased mortality at higher serum phosphate levels in AECOPD patients: increased serum phosphate induces vascular calcification and endothelial dysfunction, leading to organ dysfunction; hyperphosphatemia causes oxidative stress, cell apoptosis, and inflammation, all of which are involved in the pathogenesis of AECOPD, and a higher phosphate diet exacerbates aging and lung emphysema phenotypes; restriction of phosphate intake and absorption relieves these phenotypes and alveolar destruction, which might contribute to the development of AECOPD.
Li et al. concluded: “Reducing serum phosphate levels may be a therapeutic strategy to improve prognosis of AECOPD patients.”
“This large retrospective analysis on eICU database in the U.S. revealed elevated serum phosphate levels with increased in-hospital mortality among patients experiencing acute exacerbation of COPD,” commented Dharani Narendra, MD, assistant professor in medicine, at Baylor College of Medicine, Houston. “This association, previously observed in various chronic conditions including COPD, particularly in men, is now noted to apply to both genders, irrespective of chronic kidney disease. The study also hints at potential mechanisms for elevated phosphate levels, such as inflammation, oxidative stress, and cell apoptosis in AECOPD, as well as a high-phosphate diet.”
She told this news organization also, “It remains imperative to ascertain whether treating hyperphosphatemia or implementing dietary phosphate restrictions can reduce mortality or prevent AECOPD episodes. These demand additional clinical trials to establish a definitive cause-and-effect relationship and to guide potential treatment and prevention strategies.”
Noting study limitations, Li et al. stated that many variables, such as smoking, exacerbation frequency, severity, PH, PaO2, PaCO2, and lactate, were not included in this study owing to more than 20% missing values.
This work was supported by the National Natural Science Foundation of China, Scientific Research Fund of Hunan Provincial Education Department, Hunan Provincial Natural Science Foundation, and Special fund for rehabilitation medicine of the National Clinical Research Center for Geriatric Disorders Clinical Research Fund. The authors declare no competing interests.
found significantly higher in-hospital mortality among AECOPD patients with high serum phosphate levels. The finding, according to Siqi Li et al. in a preproof HELIYON article, suggests that hyperphosphatemia may be a high-risk factor for AECOPD-related in-hospital mortality.
Phosphorus is key to several physiological processes, among them energy metabolism, bone mineralization, membrane transport, and intracellular signaling. Li et al. pointed out that in patients with multiple diseases, hyperphosphatemia is associated with increased mortality. In the development of COPD specifically, acute exacerbations have been shown in several recent studies to be an important adverse event conferring heightened mortality risk. Despite many efforts, AECOPD mortality rates remain high, making identification of potential factors, Li et al. stated, crucial for improving outcomes in high-risk patients.
The electronic Intensive Care Unit Collaborative Research Database (eICU-CRD) holds data associated with over 200,000 patient stays, providing a large sample size for research studies. To determine the relationship between serum phosphate and in-hospital mortality in AECOPD patients, investigators analyzed data from a total of 1,199 AECOPD patients (mean age, 68 years; ~55% female) enrolled in eICU-CRD and divided them into three groups according to serum phosphate level tertiles: lowest tertile (serum phosphate ≤ 3.0 mg/dL, n = 445), median tertile (serum phosphate > 3.0 mg/dL and ≤ 4.0 mg/dL, n = 378), and highest tertile (serum phosphate > 4.0 mg/dL, n = 376). The Li et al. study’s primary outcome was all-cause in-hospital mortality, defined as survival to hospital discharge. Secondary outcomes included length of stay (LOS) in the intensive care unit (ICU), LOS in the hospital, and all-cause ICU mortality.
The Li et al. analysis of patient characteristics showed that patients in the highest tertile of serum phosphate had significantly higher body mass index (BMI) (P < .001), lower temperature (P < .001), lower heart rate (P < .001), lower mean arterial blood pressure (P = .011), higher creatinine (P < .001), higher potassium (P < .001), higher sequential organ failure assessment (SOFA) (P < .001), higher acute physiology and chronic health evaluation (APACHE IV) (P < .001), and higher ICU mortality (P < .001). Also, patients with higher serum phosphate levels were more likely to receive renal replacement therapy (RRT) (P < .001) and vasoactive drugs (P = .003) than those in the lower serum phosphate group. Such differences were also observed for age (P = .021), calcium level (P = .023), sodium level (P = .039), hypertension (P = .014), coronary artery disease (P = .004), diabetes (P = .017), and chronic kidney disease (P < .001). No significant differences were observed for gender, respiration rate, SpO2, white blood cell count, hemoglobin, platelets, cirrhosis, stroke, ventilation, LOS in ICU, and LOS in hospital (P > .05).
A univariate logistic regression analysis performed to determine the relationship between serum phosphate level and risk of in-hospital mortality revealed that higher serum phosphate level correlated with increased in-hospital mortality (odds ratio, 1.30; 95% confidence interval, 1.16-1.46; P < .001).
Li et al. posited that several mechanisms may explain increased mortality at higher serum phosphate levels in AECOPD patients: increased serum phosphate induces vascular calcification and endothelial dysfunction, leading to organ dysfunction; hyperphosphatemia causes oxidative stress, cell apoptosis, and inflammation, all of which are involved in the pathogenesis of AECOPD, and a higher phosphate diet exacerbates aging and lung emphysema phenotypes; restriction of phosphate intake and absorption relieves these phenotypes and alveolar destruction, which might contribute to the development of AECOPD.
Li et al. concluded: “Reducing serum phosphate levels may be a therapeutic strategy to improve prognosis of AECOPD patients.”
“This large retrospective analysis on eICU database in the U.S. revealed elevated serum phosphate levels with increased in-hospital mortality among patients experiencing acute exacerbation of COPD,” commented Dharani Narendra, MD, assistant professor in medicine, at Baylor College of Medicine, Houston. “This association, previously observed in various chronic conditions including COPD, particularly in men, is now noted to apply to both genders, irrespective of chronic kidney disease. The study also hints at potential mechanisms for elevated phosphate levels, such as inflammation, oxidative stress, and cell apoptosis in AECOPD, as well as a high-phosphate diet.”
She told this news organization also, “It remains imperative to ascertain whether treating hyperphosphatemia or implementing dietary phosphate restrictions can reduce mortality or prevent AECOPD episodes. These demand additional clinical trials to establish a definitive cause-and-effect relationship and to guide potential treatment and prevention strategies.”
Noting study limitations, Li et al. stated that many variables, such as smoking, exacerbation frequency, severity, PH, PaO2, PaCO2, and lactate, were not included in this study owing to more than 20% missing values.
This work was supported by the National Natural Science Foundation of China, Scientific Research Fund of Hunan Provincial Education Department, Hunan Provincial Natural Science Foundation, and Special fund for rehabilitation medicine of the National Clinical Research Center for Geriatric Disorders Clinical Research Fund. The authors declare no competing interests.
found significantly higher in-hospital mortality among AECOPD patients with high serum phosphate levels. The finding, according to Siqi Li et al. in a preproof HELIYON article, suggests that hyperphosphatemia may be a high-risk factor for AECOPD-related in-hospital mortality.
Phosphorus is key to several physiological processes, among them energy metabolism, bone mineralization, membrane transport, and intracellular signaling. Li et al. pointed out that in patients with multiple diseases, hyperphosphatemia is associated with increased mortality. In the development of COPD specifically, acute exacerbations have been shown in several recent studies to be an important adverse event conferring heightened mortality risk. Despite many efforts, AECOPD mortality rates remain high, making identification of potential factors, Li et al. stated, crucial for improving outcomes in high-risk patients.
The electronic Intensive Care Unit Collaborative Research Database (eICU-CRD) holds data associated with over 200,000 patient stays, providing a large sample size for research studies. To determine the relationship between serum phosphate and in-hospital mortality in AECOPD patients, investigators analyzed data from a total of 1,199 AECOPD patients (mean age, 68 years; ~55% female) enrolled in eICU-CRD and divided them into three groups according to serum phosphate level tertiles: lowest tertile (serum phosphate ≤ 3.0 mg/dL, n = 445), median tertile (serum phosphate > 3.0 mg/dL and ≤ 4.0 mg/dL, n = 378), and highest tertile (serum phosphate > 4.0 mg/dL, n = 376). The Li et al. study’s primary outcome was all-cause in-hospital mortality, defined as survival to hospital discharge. Secondary outcomes included length of stay (LOS) in the intensive care unit (ICU), LOS in the hospital, and all-cause ICU mortality.
The Li et al. analysis of patient characteristics showed that patients in the highest tertile of serum phosphate had significantly higher body mass index (BMI) (P < .001), lower temperature (P < .001), lower heart rate (P < .001), lower mean arterial blood pressure (P = .011), higher creatinine (P < .001), higher potassium (P < .001), higher sequential organ failure assessment (SOFA) (P < .001), higher acute physiology and chronic health evaluation (APACHE IV) (P < .001), and higher ICU mortality (P < .001). Also, patients with higher serum phosphate levels were more likely to receive renal replacement therapy (RRT) (P < .001) and vasoactive drugs (P = .003) than those in the lower serum phosphate group. Such differences were also observed for age (P = .021), calcium level (P = .023), sodium level (P = .039), hypertension (P = .014), coronary artery disease (P = .004), diabetes (P = .017), and chronic kidney disease (P < .001). No significant differences were observed for gender, respiration rate, SpO2, white blood cell count, hemoglobin, platelets, cirrhosis, stroke, ventilation, LOS in ICU, and LOS in hospital (P > .05).
A univariate logistic regression analysis performed to determine the relationship between serum phosphate level and risk of in-hospital mortality revealed that higher serum phosphate level correlated with increased in-hospital mortality (odds ratio, 1.30; 95% confidence interval, 1.16-1.46; P < .001).
Li et al. posited that several mechanisms may explain increased mortality at higher serum phosphate levels in AECOPD patients: increased serum phosphate induces vascular calcification and endothelial dysfunction, leading to organ dysfunction; hyperphosphatemia causes oxidative stress, cell apoptosis, and inflammation, all of which are involved in the pathogenesis of AECOPD, and a higher phosphate diet exacerbates aging and lung emphysema phenotypes; restriction of phosphate intake and absorption relieves these phenotypes and alveolar destruction, which might contribute to the development of AECOPD.
Li et al. concluded: “Reducing serum phosphate levels may be a therapeutic strategy to improve prognosis of AECOPD patients.”
“This large retrospective analysis on eICU database in the U.S. revealed elevated serum phosphate levels with increased in-hospital mortality among patients experiencing acute exacerbation of COPD,” commented Dharani Narendra, MD, assistant professor in medicine, at Baylor College of Medicine, Houston. “This association, previously observed in various chronic conditions including COPD, particularly in men, is now noted to apply to both genders, irrespective of chronic kidney disease. The study also hints at potential mechanisms for elevated phosphate levels, such as inflammation, oxidative stress, and cell apoptosis in AECOPD, as well as a high-phosphate diet.”
She told this news organization also, “It remains imperative to ascertain whether treating hyperphosphatemia or implementing dietary phosphate restrictions can reduce mortality or prevent AECOPD episodes. These demand additional clinical trials to establish a definitive cause-and-effect relationship and to guide potential treatment and prevention strategies.”
Noting study limitations, Li et al. stated that many variables, such as smoking, exacerbation frequency, severity, PH, PaO2, PaCO2, and lactate, were not included in this study owing to more than 20% missing values.
This work was supported by the National Natural Science Foundation of China, Scientific Research Fund of Hunan Provincial Education Department, Hunan Provincial Natural Science Foundation, and Special fund for rehabilitation medicine of the National Clinical Research Center for Geriatric Disorders Clinical Research Fund. The authors declare no competing interests.
FROM HELIYON