User login
Everolimus fails in pretreated gastric cancer
SAN FRANCISCO – Adding everolimus to paclitaxel failed to significantly improve outcomes in pretreated patients with gastric or esophagogastric junction adenocarcinoma in a German randomized phase III study.
Median overall survival in the double-blind multicenter study (RADPAC) was 6.12 months among 150 patients randomized to receive treatment with paclitaxel plus everolimus as 2nd, 3rd, or 4th line therapy, and 5.03 months among those who received paclitaxel and placebo (hazard ratio, 0.93), Salah-Eddin Al-Batran, MD, reported at the symposium sponsored by ASCO, ASTRO, the American Gastroenterological Association, and the Society of Surgical Oncology.
Median progression-free survival was 2.20 vs. 2.07 months in the treatment and placebo groups, respectively (hazard ratio, 0.88), said Dr. Al-Batran of Krankenhaus Nordwest, University Cancer Center, Frankfurt, Germany.
Study subjects had a mean age of 62 years and had progressed after treatment with a fluoropyrimidine/platinum-containing regimen. All had at least one, and a maximum of three prior lines of therapy (median of two in both groups).
Of note, accrual was slow and was stopped early, largely because of the very-high rate of taxane use for first-line treatment, but also because combination ramucirumab/paclitaxel was approved during the course of the study, Dr. Al-Batran said.
The treatment and placebo groups were well balanced. Treatment included 80 mg/m2 of paclitaxel on days 1, 8 and 15, plus placebo or 10 mg of everolimus daily on days 1-28, repeated every 28 days. Dose adjustment was more common in the treatment group (26% vs. 13%) but cumulative doses were similar in the groups.
Also, more patients in the everolimus group discontinued treatment for toxicity (11% vs. 5%). However, the only toxicity that was significantly increased was grade 3-5 oral mucositis in the treatment group (13% vs. 1% in the placebo group).
Gastric cancer is aggressive and difficult to treat, with median survival of only 9-11 months, Dr. Al-Batran said, adding that at the time the RADPAC study was initiated, no treatments had been approved for patients who failed first-line therapy, although agents like paclitaxel and irinotecan were in use.
He and his colleagues sought to evaluate everolimus, because 50%-60% of gastric cancers are driven by dysregulation in the P13k/Akt/mTOR pathway – a key regulator of cell proliferation, growth, survival, metabolism, and angiogenesis, and because everolimus – an oral mTOR inhibitor – showed efficacy in preclinical models of gastric cancer.
In the phase III GRANITE-1 trial, it was associated with trends toward improved progression-free survival and overall survival, compared with best supportive care, he noted.
Subgroup analyses in the current trial suggested that patients with prior taxane use derived greater benefit from everolimus. Overall survival in those patients, who comprised about half of the study population, was 6 months vs. 4 months with placebo; the difference did not reach statistical significance, but showed a strong trend in that direction (P = .072). Progression-free survival was, however, significantly greater with everolimus than with placebo (2.66 vs. 1.81 months; HR, 0.50) in those with prior taxane use.
“Interestingly, the very few patients having ECOG performance status of 2 really performed very poorly,” Dr. Al-Batran said, explaining that those patients had better outcomes with paclitaxel monotherapy.
“So, in conclusion, everolimus combined with paclitaxel improved outcomes as compared with paclitaxel alone in the intention to treat population. However, activity was seen in the taxane pretreated subgroup. Biomarker studies could attempt to identify a subgroup with more benefit, as we see some activity, but this activity is not enough,” he concluded.
Dr. Al-Batran reported receiving honoraria, serving as a consultant or advisor, receiving research funding from, and/or being on the speakers’ bureau for Celgene, Hospira, Lilly, Medac, Merck, Roche, Sanofi, Vifor, and Nordic Bioscience.
SAN FRANCISCO – Adding everolimus to paclitaxel failed to significantly improve outcomes in pretreated patients with gastric or esophagogastric junction adenocarcinoma in a German randomized phase III study.
Median overall survival in the double-blind multicenter study (RADPAC) was 6.12 months among 150 patients randomized to receive treatment with paclitaxel plus everolimus as 2nd, 3rd, or 4th line therapy, and 5.03 months among those who received paclitaxel and placebo (hazard ratio, 0.93), Salah-Eddin Al-Batran, MD, reported at the symposium sponsored by ASCO, ASTRO, the American Gastroenterological Association, and the Society of Surgical Oncology.
Median progression-free survival was 2.20 vs. 2.07 months in the treatment and placebo groups, respectively (hazard ratio, 0.88), said Dr. Al-Batran of Krankenhaus Nordwest, University Cancer Center, Frankfurt, Germany.
Study subjects had a mean age of 62 years and had progressed after treatment with a fluoropyrimidine/platinum-containing regimen. All had at least one, and a maximum of three prior lines of therapy (median of two in both groups).
Of note, accrual was slow and was stopped early, largely because of the very-high rate of taxane use for first-line treatment, but also because combination ramucirumab/paclitaxel was approved during the course of the study, Dr. Al-Batran said.
The treatment and placebo groups were well balanced. Treatment included 80 mg/m2 of paclitaxel on days 1, 8 and 15, plus placebo or 10 mg of everolimus daily on days 1-28, repeated every 28 days. Dose adjustment was more common in the treatment group (26% vs. 13%) but cumulative doses were similar in the groups.
Also, more patients in the everolimus group discontinued treatment for toxicity (11% vs. 5%). However, the only toxicity that was significantly increased was grade 3-5 oral mucositis in the treatment group (13% vs. 1% in the placebo group).
Gastric cancer is aggressive and difficult to treat, with median survival of only 9-11 months, Dr. Al-Batran said, adding that at the time the RADPAC study was initiated, no treatments had been approved for patients who failed first-line therapy, although agents like paclitaxel and irinotecan were in use.
He and his colleagues sought to evaluate everolimus, because 50%-60% of gastric cancers are driven by dysregulation in the P13k/Akt/mTOR pathway – a key regulator of cell proliferation, growth, survival, metabolism, and angiogenesis, and because everolimus – an oral mTOR inhibitor – showed efficacy in preclinical models of gastric cancer.
In the phase III GRANITE-1 trial, it was associated with trends toward improved progression-free survival and overall survival, compared with best supportive care, he noted.
Subgroup analyses in the current trial suggested that patients with prior taxane use derived greater benefit from everolimus. Overall survival in those patients, who comprised about half of the study population, was 6 months vs. 4 months with placebo; the difference did not reach statistical significance, but showed a strong trend in that direction (P = .072). Progression-free survival was, however, significantly greater with everolimus than with placebo (2.66 vs. 1.81 months; HR, 0.50) in those with prior taxane use.
“Interestingly, the very few patients having ECOG performance status of 2 really performed very poorly,” Dr. Al-Batran said, explaining that those patients had better outcomes with paclitaxel monotherapy.
“So, in conclusion, everolimus combined with paclitaxel improved outcomes as compared with paclitaxel alone in the intention to treat population. However, activity was seen in the taxane pretreated subgroup. Biomarker studies could attempt to identify a subgroup with more benefit, as we see some activity, but this activity is not enough,” he concluded.
Dr. Al-Batran reported receiving honoraria, serving as a consultant or advisor, receiving research funding from, and/or being on the speakers’ bureau for Celgene, Hospira, Lilly, Medac, Merck, Roche, Sanofi, Vifor, and Nordic Bioscience.
SAN FRANCISCO – Adding everolimus to paclitaxel failed to significantly improve outcomes in pretreated patients with gastric or esophagogastric junction adenocarcinoma in a German randomized phase III study.
Median overall survival in the double-blind multicenter study (RADPAC) was 6.12 months among 150 patients randomized to receive treatment with paclitaxel plus everolimus as 2nd, 3rd, or 4th line therapy, and 5.03 months among those who received paclitaxel and placebo (hazard ratio, 0.93), Salah-Eddin Al-Batran, MD, reported at the symposium sponsored by ASCO, ASTRO, the American Gastroenterological Association, and the Society of Surgical Oncology.
Median progression-free survival was 2.20 vs. 2.07 months in the treatment and placebo groups, respectively (hazard ratio, 0.88), said Dr. Al-Batran of Krankenhaus Nordwest, University Cancer Center, Frankfurt, Germany.
Study subjects had a mean age of 62 years and had progressed after treatment with a fluoropyrimidine/platinum-containing regimen. All had at least one, and a maximum of three prior lines of therapy (median of two in both groups).
Of note, accrual was slow and was stopped early, largely because of the very-high rate of taxane use for first-line treatment, but also because combination ramucirumab/paclitaxel was approved during the course of the study, Dr. Al-Batran said.
The treatment and placebo groups were well balanced. Treatment included 80 mg/m2 of paclitaxel on days 1, 8 and 15, plus placebo or 10 mg of everolimus daily on days 1-28, repeated every 28 days. Dose adjustment was more common in the treatment group (26% vs. 13%) but cumulative doses were similar in the groups.
Also, more patients in the everolimus group discontinued treatment for toxicity (11% vs. 5%). However, the only toxicity that was significantly increased was grade 3-5 oral mucositis in the treatment group (13% vs. 1% in the placebo group).
Gastric cancer is aggressive and difficult to treat, with median survival of only 9-11 months, Dr. Al-Batran said, adding that at the time the RADPAC study was initiated, no treatments had been approved for patients who failed first-line therapy, although agents like paclitaxel and irinotecan were in use.
He and his colleagues sought to evaluate everolimus, because 50%-60% of gastric cancers are driven by dysregulation in the P13k/Akt/mTOR pathway – a key regulator of cell proliferation, growth, survival, metabolism, and angiogenesis, and because everolimus – an oral mTOR inhibitor – showed efficacy in preclinical models of gastric cancer.
In the phase III GRANITE-1 trial, it was associated with trends toward improved progression-free survival and overall survival, compared with best supportive care, he noted.
Subgroup analyses in the current trial suggested that patients with prior taxane use derived greater benefit from everolimus. Overall survival in those patients, who comprised about half of the study population, was 6 months vs. 4 months with placebo; the difference did not reach statistical significance, but showed a strong trend in that direction (P = .072). Progression-free survival was, however, significantly greater with everolimus than with placebo (2.66 vs. 1.81 months; HR, 0.50) in those with prior taxane use.
“Interestingly, the very few patients having ECOG performance status of 2 really performed very poorly,” Dr. Al-Batran said, explaining that those patients had better outcomes with paclitaxel monotherapy.
“So, in conclusion, everolimus combined with paclitaxel improved outcomes as compared with paclitaxel alone in the intention to treat population. However, activity was seen in the taxane pretreated subgroup. Biomarker studies could attempt to identify a subgroup with more benefit, as we see some activity, but this activity is not enough,” he concluded.
Dr. Al-Batran reported receiving honoraria, serving as a consultant or advisor, receiving research funding from, and/or being on the speakers’ bureau for Celgene, Hospira, Lilly, Medac, Merck, Roche, Sanofi, Vifor, and Nordic Bioscience.
AT THE 2017 GASTROINTESTINAL CANCERS SYMPOSIUM
Key clinical point:
Major finding: Median overall survival was 6.12 vs. 5.03 months with paclitaxel plus everolimus vs. placebo (hazard ratio, 0.93).
Data source: The randomized phase III RADPAC study of 300 patients.
Disclosures: Dr. Al-Batran reported receiving honoraria, serving as a consultant or advisor, receiving research funding from, and/or being on the speakers’ bureau for Celgene, Hospira, Lilly, Medac, Merck, Roche, Sanofi, Vifor, and Nordic Bioscience.
Fresh Press: ACS Surgery News January issue now online
The January issue of ACS Surgery News is available on the website. This month’s issue features a special report on burnout. A new paradigm of burnout is emerging: The roots of the problem may be institutional. Addressing physician burnout must begin with recognition of the challenge and a commitment to change from the top levels of management, according to a study by Tait D. Shanafelt, MD, and John Noseworthy, MD, of the Mayo Clinic.
Don’t miss our annual Meet the Editorial Advisory Board feature. This year, we welcome seven new members: Joshua A. Broghammer, MD, FACS; Samer G. Mattar, MD, FACS; Arden M. Morris, MD, FACS; Rudolfo J. Oviedo, MD, FACS; Kevin M. Reavis, MD, FACS; Michael D. Sarap, MD, FACS; and Gary Timmerman, MD, FACS. On behalf of the editors and our readers, we sincerely thank our members who have finished their term. These colleagues have given of their time and expertise for the benefit of their fellow surgeons. They have earned our admiration and gratitude.
The January issue of ACS Surgery News is available on the website. This month’s issue features a special report on burnout. A new paradigm of burnout is emerging: The roots of the problem may be institutional. Addressing physician burnout must begin with recognition of the challenge and a commitment to change from the top levels of management, according to a study by Tait D. Shanafelt, MD, and John Noseworthy, MD, of the Mayo Clinic.
Don’t miss our annual Meet the Editorial Advisory Board feature. This year, we welcome seven new members: Joshua A. Broghammer, MD, FACS; Samer G. Mattar, MD, FACS; Arden M. Morris, MD, FACS; Rudolfo J. Oviedo, MD, FACS; Kevin M. Reavis, MD, FACS; Michael D. Sarap, MD, FACS; and Gary Timmerman, MD, FACS. On behalf of the editors and our readers, we sincerely thank our members who have finished their term. These colleagues have given of their time and expertise for the benefit of their fellow surgeons. They have earned our admiration and gratitude.
The January issue of ACS Surgery News is available on the website. This month’s issue features a special report on burnout. A new paradigm of burnout is emerging: The roots of the problem may be institutional. Addressing physician burnout must begin with recognition of the challenge and a commitment to change from the top levels of management, according to a study by Tait D. Shanafelt, MD, and John Noseworthy, MD, of the Mayo Clinic.
Don’t miss our annual Meet the Editorial Advisory Board feature. This year, we welcome seven new members: Joshua A. Broghammer, MD, FACS; Samer G. Mattar, MD, FACS; Arden M. Morris, MD, FACS; Rudolfo J. Oviedo, MD, FACS; Kevin M. Reavis, MD, FACS; Michael D. Sarap, MD, FACS; and Gary Timmerman, MD, FACS. On behalf of the editors and our readers, we sincerely thank our members who have finished their term. These colleagues have given of their time and expertise for the benefit of their fellow surgeons. They have earned our admiration and gratitude.
Elements for Success in Managing Type 2 Diabetes With SGLT-2 Inhibitors

Faculty/Faculty Disclosure
Eden M. Miller, DO
Executive Director and Co-founder, Diabetes Nation
High Lakes Health Care
St. Charles Hospital
Bend, Oregon
Competing Interest and Financial Disclosures: Dr. Miller discloses that she is on the advisory boards and speakers’ bureaus for AstraZeneca, Eli Lilly and Company, and Janssen Pharmaceuticals, Inc.

Faculty/Faculty Disclosure
Eden M. Miller, DO
Executive Director and Co-founder, Diabetes Nation
High Lakes Health Care
St. Charles Hospital
Bend, Oregon
Competing Interest and Financial Disclosures: Dr. Miller discloses that she is on the advisory boards and speakers’ bureaus for AstraZeneca, Eli Lilly and Company, and Janssen Pharmaceuticals, Inc.

Faculty/Faculty Disclosure
Eden M. Miller, DO
Executive Director and Co-founder, Diabetes Nation
High Lakes Health Care
St. Charles Hospital
Bend, Oregon
Competing Interest and Financial Disclosures: Dr. Miller discloses that she is on the advisory boards and speakers’ bureaus for AstraZeneca, Eli Lilly and Company, and Janssen Pharmaceuticals, Inc.
Semaglutide compares well with sitagliptin
NEW ORLEANS – Semaglutide, a GLP-1 agonist for type 2 diabetes that’s dosed weekly, was superior to daily sitagliptin in improving glycemic control and reducing body weight in people who are also on metformin and/or thiazolidinediones (TZDs), based on results from a phase III trial. But while the serious adverse event profile was similar for both treatments, far more patients on semaglutide discontinued treatment because of adverse events.
The SUSTAIN study includes more than 8,000 patients with type 2 diabetes. The results are the basis for a new drug application filed in December with the Food and Drug Administration by the investigational drug’s manufacturer, Novo Nordisk, which made the announcement in a press release.
“The SUSTAIN 2 trial has shown that semaglutide at both doses, 0.5 and 1 mg, is superior at improving glycemic control in subjects with type 2 diabetes, compared with sitagliptin, and showed a reduction of 1.3% and 1.6%, respectively, from the baseline HbA1c of 8.1%,” Dr. Ahrén said. For comparison, the sitagliptin group showed an average HbA1c reduction of 0.5%, he said.
The treatments were well tolerated with no new safety concerns, Dr. Ahrén said. “As expected, semaglutide caused more gastrointestinal adverse events, but the frequency was similar to those reported with other GLP-1 receptor agonists,” he said.
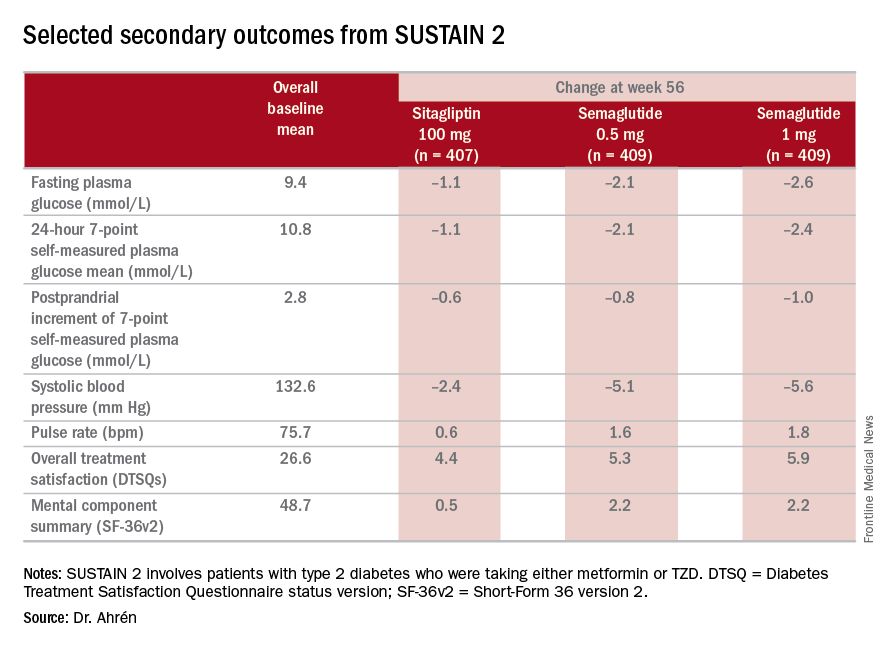
The study’s investigators also looked at a composite endpoint of HbA1c less than 7% without symptomatic hypoglycemia and no weight gain, Dr. Ahrén said, achieved by 63% on 0.5 mg and 74% on 1 mg of semaglutide vs. 27% of the sitagliptin group.
The serious adverse event (AE) profile was similar in all three groups: 7.3% in both semaglutide groups and 7.1% in the sitagliptin group. However, far more patients on semaglutide discontinued treatment because of AEs: 8.1% and 9.5% on 0.5 and 1 mg, respectively, vs. 2.9% on sitagliptin. Gastrointestinal AEs in all groups were 43.5% and 39.9% in the 0.5- and 1-mg semaglutide groups, respectively, and 23.6% in the sitagliptin group.
Six deaths were reported in the study population, Dr. Ahrén said: two on 0.5-mg semaglutide dosing, one on the 1-mg dosing, and three on sitagliptin.
Hypoglycemia rates were also “very low,” he said, with 14 patients overall having reported it; seven on 0.5-mg semaglutide therapy and two in the 1-mg group, and five on sitagliptin, “So there were no increased risks for hypoglycemia with semaglutide.”
Dr. Ahrén disclosed relationships with Novo Nordisk and several other drug companies.
NEW ORLEANS – Semaglutide, a GLP-1 agonist for type 2 diabetes that’s dosed weekly, was superior to daily sitagliptin in improving glycemic control and reducing body weight in people who are also on metformin and/or thiazolidinediones (TZDs), based on results from a phase III trial. But while the serious adverse event profile was similar for both treatments, far more patients on semaglutide discontinued treatment because of adverse events.
The SUSTAIN study includes more than 8,000 patients with type 2 diabetes. The results are the basis for a new drug application filed in December with the Food and Drug Administration by the investigational drug’s manufacturer, Novo Nordisk, which made the announcement in a press release.
“The SUSTAIN 2 trial has shown that semaglutide at both doses, 0.5 and 1 mg, is superior at improving glycemic control in subjects with type 2 diabetes, compared with sitagliptin, and showed a reduction of 1.3% and 1.6%, respectively, from the baseline HbA1c of 8.1%,” Dr. Ahrén said. For comparison, the sitagliptin group showed an average HbA1c reduction of 0.5%, he said.
The treatments were well tolerated with no new safety concerns, Dr. Ahrén said. “As expected, semaglutide caused more gastrointestinal adverse events, but the frequency was similar to those reported with other GLP-1 receptor agonists,” he said.

The study’s investigators also looked at a composite endpoint of HbA1c less than 7% without symptomatic hypoglycemia and no weight gain, Dr. Ahrén said, achieved by 63% on 0.5 mg and 74% on 1 mg of semaglutide vs. 27% of the sitagliptin group.
The serious adverse event (AE) profile was similar in all three groups: 7.3% in both semaglutide groups and 7.1% in the sitagliptin group. However, far more patients on semaglutide discontinued treatment because of AEs: 8.1% and 9.5% on 0.5 and 1 mg, respectively, vs. 2.9% on sitagliptin. Gastrointestinal AEs in all groups were 43.5% and 39.9% in the 0.5- and 1-mg semaglutide groups, respectively, and 23.6% in the sitagliptin group.
Six deaths were reported in the study population, Dr. Ahrén said: two on 0.5-mg semaglutide dosing, one on the 1-mg dosing, and three on sitagliptin.
Hypoglycemia rates were also “very low,” he said, with 14 patients overall having reported it; seven on 0.5-mg semaglutide therapy and two in the 1-mg group, and five on sitagliptin, “So there were no increased risks for hypoglycemia with semaglutide.”
Dr. Ahrén disclosed relationships with Novo Nordisk and several other drug companies.
NEW ORLEANS – Semaglutide, a GLP-1 agonist for type 2 diabetes that’s dosed weekly, was superior to daily sitagliptin in improving glycemic control and reducing body weight in people who are also on metformin and/or thiazolidinediones (TZDs), based on results from a phase III trial. But while the serious adverse event profile was similar for both treatments, far more patients on semaglutide discontinued treatment because of adverse events.
The SUSTAIN study includes more than 8,000 patients with type 2 diabetes. The results are the basis for a new drug application filed in December with the Food and Drug Administration by the investigational drug’s manufacturer, Novo Nordisk, which made the announcement in a press release.
“The SUSTAIN 2 trial has shown that semaglutide at both doses, 0.5 and 1 mg, is superior at improving glycemic control in subjects with type 2 diabetes, compared with sitagliptin, and showed a reduction of 1.3% and 1.6%, respectively, from the baseline HbA1c of 8.1%,” Dr. Ahrén said. For comparison, the sitagliptin group showed an average HbA1c reduction of 0.5%, he said.
The treatments were well tolerated with no new safety concerns, Dr. Ahrén said. “As expected, semaglutide caused more gastrointestinal adverse events, but the frequency was similar to those reported with other GLP-1 receptor agonists,” he said.

The study’s investigators also looked at a composite endpoint of HbA1c less than 7% without symptomatic hypoglycemia and no weight gain, Dr. Ahrén said, achieved by 63% on 0.5 mg and 74% on 1 mg of semaglutide vs. 27% of the sitagliptin group.
The serious adverse event (AE) profile was similar in all three groups: 7.3% in both semaglutide groups and 7.1% in the sitagliptin group. However, far more patients on semaglutide discontinued treatment because of AEs: 8.1% and 9.5% on 0.5 and 1 mg, respectively, vs. 2.9% on sitagliptin. Gastrointestinal AEs in all groups were 43.5% and 39.9% in the 0.5- and 1-mg semaglutide groups, respectively, and 23.6% in the sitagliptin group.
Six deaths were reported in the study population, Dr. Ahrén said: two on 0.5-mg semaglutide dosing, one on the 1-mg dosing, and three on sitagliptin.
Hypoglycemia rates were also “very low,” he said, with 14 patients overall having reported it; seven on 0.5-mg semaglutide therapy and two in the 1-mg group, and five on sitagliptin, “So there were no increased risks for hypoglycemia with semaglutide.”
Dr. Ahrén disclosed relationships with Novo Nordisk and several other drug companies.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: Investigators for a phase III trial have found weekly semaglutide superior to daily sitagliptin as add-on therapy for improving glycemic control and reducing body weight in type 2 diabetes.
Major finding: Semaglutide 0.5 and 1 mg showed a reduction of 1.3% and 1.6%, respectively, from the baseline HbA1c, compared with an average reduction of 0.5% for sitagliptin.
Data source: SUSTAIN 2 double-blind, randomized trial of 1,231 patients with type 2 diabetes taking either metformin or thiazolidinediones.
Disclosures: Dr. Ahrén disclosed relationships with Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Novo Nordisk, and Sanofi-Aventis Deutschland.
Sleep apnea may induce distinct form of atrial fibrillation
ORLANDO – Patients with atrial fibrillation (AF) should be screened for obstructive sleep apnea (OSA), because this information may be useful in guiding ablation strategies, according to results of a prospective study.
The study, which associated OSA in AF with a high relative rate of non–pulmonary vein (PV) triggers, has contributed to the “growing body of evidence implicating sleep apnea in atrial remodeling and promotion of the AF substrate,” Elad Anter, MD, associate director of the clinical electrophysiology laboratory at Beth Israel Deaconess Medical Center, Boston, reported at the annual International AF Symposium.
Despite the close association between OSA and AF, it has been unclear whether OSA is a causative factor. Dr. Anter suggested that mechanistic association is strengthening, however.
It has been hypothesized that OSA generates AF substrate through negative intrathoracic pressure changes and autonomic nervous system activation. But Dr. Anter reported that there is more recent and compelling evidence that the repetitive occlusions produced by OSA result in remodeling of the atria, producing scar tissue that slows conduction and produces susceptibility to reentry AF.
A newly completed prospective multicenter study adds support to this latter hypothesis. In the protocol, patients with paroxysmal AF scheduled for ablation were required to undergo a sleep study, an AF mapping study, and follow-up for at least 12 months. A known history of OSA was an exclusion criterion. To isolate the effect of OSA, there were exclusions for other major etiologies for AF, such as heart failure or coronary artery disease.
The AF mapping was conducted when patients were in sinus rhythm “to evaluate the baseline atrial substrate and avoid measurements related to acute electrical remodeling,” Dr. Anter explained.
Of 172 patients initially enrolled, 133 completed the sleep study, 118 completed the mapping study, and 110 completed both and were followed for at least 12 months. Of these, 43 patients without OSA were compared with 43 patients with OSA defined as an apnea-hypopnea index (AHI) of at least 15. Patients in the two groups did not differ significantly for relevant characteristics, such as body mass index (BMI), age, presence of hypertension, or duration of AF; but the left atrial (LA) volume was significantly greater (P = .01) in those with OSA than those without.
Even though the prevalence of voltage abnormalities was higher in the OSA group for the right (P = .01) and left atria (P = .0001) before ablation, the prevalence of PV triggers (63% vs. 65%), non-PV triggers (19% vs. 12%) and noninducible triggers (19% vs. 23%) were similar.
After ablation, PV triggers were no longer inducible in either group, but there was a striking difference in inducible non-PV triggers. While only 11.6% remained inducible in the non-OSA group, 41.8% (P = .003) remained inducible in the OSA patients.
“AF triggers in OSA were most commonly located at the LA septum, at the zone of low voltage and abnormal electrograms, as determined during sinus rhythm,” Dr. Anter reported. “Ablation of these triggers at the zone of tissue abnormality in the OSA patients resulted in termination of AF in 9 (64.2%) of the 14 patients.”
Overall, at the end of 12 months, 79% of those without OSA remained in arrhythmia-free survival, versus 65.1% of the group with OSA that were treated with PV isolation alone.
The lower rate of success in the OSA group shows the importance of specifically directing ablation to the areas of low voltage and slow conduction in the left anterior septum that Dr. Anter indicated otherwise would be missed.
“These zones are a common source of extra-PV triggers and localized circuits or rotors of AF in OSA patients,” he reported. “Ablation of these low voltage zones is associated with improved clinical outcome in OSA patients with paroxysmal AF.”
The data, which Dr. Anter said are consistent with a growing body of work regarding the relationship of OSA and AF, provided the basis for suggesting that AF patients undergo routine screening for OSA.
In patients with OSA, ablation of PV triggers alone even in paroxysmal PAF “may not be sufficient,” he cautioned. “Evaluation of non-PV triggers should also be performed.”
Dr. Anter reported financial relationships with Biosense Webster and Boston Scientific.
Atrial fibrillation (AF) is the most common cardiac arrhythmia encountered in clinical practice and is associated with increased morbidity and mortality due to thromboembolism, stroke, and worsening of pre-existing heart failure. Both its incidence and prevalence are increasing as AF risk increases with advancing age.1 While the strategies of heart rate control and anticoagulation to lower stroke risk and rhythm control have been found comparable with regard to survival, many patients remain highly symptomatic because of palpitations and reduced cardiac output.1
Structural abnormalities of the atria, including fibrosis and dilation, accompanied by conduction abnormalities, provide the underlying substrate for AF. It is well established that AF episodes perpetuate atrial remodeling leading to more frequent and prolonged AF episodes. Hence, there is the long-standing notion that “AF begets AF.” While a variety of antiarrhythmic drugs have been employed over the years to prevent AF recurrences and to maintain sinus rhythm, their use has decreased over the past 2 decades due to their major side effects and their potential of proarrhythmia.
Since AF patients represent a heterogeneous group of patients with CV diseases of varying type and severity as well as comorbidities, it stands to reason that the pulmonary venous–left atrial junction may not be the sole culprit region of all cases of AF and that other anatomical locations might serve as triggers for AF.
In support of this notion are the results of the prospective multicenter study presented by Dr. Elad Anter at the annual International AF Symposium. This important study is consistent with and expands upon prior studies that have suggested that sites within the atria remote from the pulmonary veins may serve as triggers for AF, rather than lower technical success of pulmonary vein ablation.5 It further highlights the importance of fibrosis and associated electrical dispersion to the pathogenesis of AF.6 However, the recommendation that patients with AF be screened for OSA is not new, as nearly half of patients with AF also have OSA.7 While AF and OSA share common risk factors/comorbidities such as male gender, obesity, hypertension, coronary artery disease, and congestive heart failure, OSA has been found to be an independent risk factor for AF development.
It is important to know whether OSA was treated, as the presence of OSA raises the risk of AF recurrence and OSA treatment decreases AF recurrence after ablation.8,9 Conversely, in the setting of OSA, AF is more resistive to rhythm control. Enhanced vagal activation, elevated sympathetic tone, and oxidative stresses due to oxygen desaturation and left atrial distension have all been implicated in the pathogenesis linking OSA to the development of AF. Repeated increases in upper airway resistance during airway obstruction have been shown to lead to atrial stretch, dilation, and fibrosis.10 Since patients with heart failure, coronary artery disease, and other underlying causes for AF were excluded from the onset, the results may not be applicable to a large segment of AF patients. Exclusion of underlying cardiac conditions potentially raised the yield of patients found to have OSA and the potential value of OSA screening. Of note: Less than half of patients that were enrolled had complete data for analysis, which may further limit applicability of the study findings. All patients had paroxysmal AF and were in sinus rhythm while the mapping procedure was performed, leaving questions as to how to approach patients presenting acutely with persistent or long standing AF, or those recently treated with antiarrhythmic therapy. Also, since arrhythmia-free survival decreases from 1 to 5 years after AF ablation, and short-time success rates do not predict longer success rates, the present study results should be interpreted with cautious optimism.11
However, these limitations should not detract from the major implications of the study. In the setting of AF, OSA should be clinically suspected not only because of the frequent coexistence of the two disorders but because the presence of OSA should prompt electrophysiologists to consider non–pulmonary vein triggers of AF prior to ablation attempts. The consideration of alternative ablation sites might help to explain the lack of ablation procedure endpoints to predict long-term success of ablation and holds promise for increasing technical success rates. Given that airway obstruction may occur in other clinical settings such as seizure-induced laryngospasm and that seizures may induce arrhythmias and sudden death, there is potential for non–pulmonary vein sites to trigger AF and other arrhythmias in settings other than OSA as well.12 Whether other disease states are associated with a higher likelihood of non-pulmonary veins trigger sites also merits further study. Moreover, this study underscores the notion that with regard to AF ablation, “no one site fits all” and “clinical mapping” may serve as a valuable adjunct to anatomical mapping. It also serves as a reminder of the multidisciplinary nature of Chest Medicine and the need of a team oriented approach..
References
1. Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124:2264-74.
2. Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812-22.
3. Kuck KH, Brugada J, Fürnkranz A, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374:2235-45.
4. Calkins H, Reynolds MR, Spector P, et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol. 2009;2:349-61.
5. Narayan SM, Krummen DE, Shivkumar K, et al. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol. 2012;60:628-36.
6. Kottkamp H, Berg J, Bender R, et al. Box Isolation of Fibrotic Areas (BIFA): a patient-tailored substrate modified application approach for ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2016;27:22-30.
7. Stevenson IH, Teichtahl H, Cunnington D, et al. Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. Eur Heart J. 2008;29:1662-9.
8. Fein AS, Shvilkin A, Shah D, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol. 2013;62:300-5.
9. Naruse Y, Tada H, Satoh M, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm. 2013;10:331-7.
10. Otto M, Belohlavek M, Romero-Corral A, et al. Comparison of cardiac structural and functional changes in obese otherwise healthy adults with versus without obstructive sleep apnea. Am J Cardiol. 2007;99:1298-302.
11. Kis Z, Muka T, Franco OH, et al. The short and long-term efficacy of pulmonary vein isolation as a sole treatment strategy for paroxysmal atrial fibrillation: a systematic review and meta-analysis. Curr Cardiol Rev. 2017 Jan 17. [Epub ahead of print].
12. Nakase K, Kollmar R, Lazar J, et al. Laryngospasm, central and obstructive apnea during seizures: defining pathophysiology for sudden death in a rat model. Epilepsy Res. 2016;128:126-39.
Atrial fibrillation (AF) is the most common cardiac arrhythmia encountered in clinical practice and is associated with increased morbidity and mortality due to thromboembolism, stroke, and worsening of pre-existing heart failure. Both its incidence and prevalence are increasing as AF risk increases with advancing age.1 While the strategies of heart rate control and anticoagulation to lower stroke risk and rhythm control have been found comparable with regard to survival, many patients remain highly symptomatic because of palpitations and reduced cardiac output.1
Structural abnormalities of the atria, including fibrosis and dilation, accompanied by conduction abnormalities, provide the underlying substrate for AF. It is well established that AF episodes perpetuate atrial remodeling leading to more frequent and prolonged AF episodes. Hence, there is the long-standing notion that “AF begets AF.” While a variety of antiarrhythmic drugs have been employed over the years to prevent AF recurrences and to maintain sinus rhythm, their use has decreased over the past 2 decades due to their major side effects and their potential of proarrhythmia.
Since AF patients represent a heterogeneous group of patients with CV diseases of varying type and severity as well as comorbidities, it stands to reason that the pulmonary venous–left atrial junction may not be the sole culprit region of all cases of AF and that other anatomical locations might serve as triggers for AF.
In support of this notion are the results of the prospective multicenter study presented by Dr. Elad Anter at the annual International AF Symposium. This important study is consistent with and expands upon prior studies that have suggested that sites within the atria remote from the pulmonary veins may serve as triggers for AF, rather than lower technical success of pulmonary vein ablation.5 It further highlights the importance of fibrosis and associated electrical dispersion to the pathogenesis of AF.6 However, the recommendation that patients with AF be screened for OSA is not new, as nearly half of patients with AF also have OSA.7 While AF and OSA share common risk factors/comorbidities such as male gender, obesity, hypertension, coronary artery disease, and congestive heart failure, OSA has been found to be an independent risk factor for AF development.
It is important to know whether OSA was treated, as the presence of OSA raises the risk of AF recurrence and OSA treatment decreases AF recurrence after ablation.8,9 Conversely, in the setting of OSA, AF is more resistive to rhythm control. Enhanced vagal activation, elevated sympathetic tone, and oxidative stresses due to oxygen desaturation and left atrial distension have all been implicated in the pathogenesis linking OSA to the development of AF. Repeated increases in upper airway resistance during airway obstruction have been shown to lead to atrial stretch, dilation, and fibrosis.10 Since patients with heart failure, coronary artery disease, and other underlying causes for AF were excluded from the onset, the results may not be applicable to a large segment of AF patients. Exclusion of underlying cardiac conditions potentially raised the yield of patients found to have OSA and the potential value of OSA screening. Of note: Less than half of patients that were enrolled had complete data for analysis, which may further limit applicability of the study findings. All patients had paroxysmal AF and were in sinus rhythm while the mapping procedure was performed, leaving questions as to how to approach patients presenting acutely with persistent or long standing AF, or those recently treated with antiarrhythmic therapy. Also, since arrhythmia-free survival decreases from 1 to 5 years after AF ablation, and short-time success rates do not predict longer success rates, the present study results should be interpreted with cautious optimism.11
However, these limitations should not detract from the major implications of the study. In the setting of AF, OSA should be clinically suspected not only because of the frequent coexistence of the two disorders but because the presence of OSA should prompt electrophysiologists to consider non–pulmonary vein triggers of AF prior to ablation attempts. The consideration of alternative ablation sites might help to explain the lack of ablation procedure endpoints to predict long-term success of ablation and holds promise for increasing technical success rates. Given that airway obstruction may occur in other clinical settings such as seizure-induced laryngospasm and that seizures may induce arrhythmias and sudden death, there is potential for non–pulmonary vein sites to trigger AF and other arrhythmias in settings other than OSA as well.12 Whether other disease states are associated with a higher likelihood of non-pulmonary veins trigger sites also merits further study. Moreover, this study underscores the notion that with regard to AF ablation, “no one site fits all” and “clinical mapping” may serve as a valuable adjunct to anatomical mapping. It also serves as a reminder of the multidisciplinary nature of Chest Medicine and the need of a team oriented approach..
References
1. Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124:2264-74.
2. Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812-22.
3. Kuck KH, Brugada J, Fürnkranz A, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374:2235-45.
4. Calkins H, Reynolds MR, Spector P, et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol. 2009;2:349-61.
5. Narayan SM, Krummen DE, Shivkumar K, et al. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol. 2012;60:628-36.
6. Kottkamp H, Berg J, Bender R, et al. Box Isolation of Fibrotic Areas (BIFA): a patient-tailored substrate modified application approach for ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2016;27:22-30.
7. Stevenson IH, Teichtahl H, Cunnington D, et al. Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. Eur Heart J. 2008;29:1662-9.
8. Fein AS, Shvilkin A, Shah D, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol. 2013;62:300-5.
9. Naruse Y, Tada H, Satoh M, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm. 2013;10:331-7.
10. Otto M, Belohlavek M, Romero-Corral A, et al. Comparison of cardiac structural and functional changes in obese otherwise healthy adults with versus without obstructive sleep apnea. Am J Cardiol. 2007;99:1298-302.
11. Kis Z, Muka T, Franco OH, et al. The short and long-term efficacy of pulmonary vein isolation as a sole treatment strategy for paroxysmal atrial fibrillation: a systematic review and meta-analysis. Curr Cardiol Rev. 2017 Jan 17. [Epub ahead of print].
12. Nakase K, Kollmar R, Lazar J, et al. Laryngospasm, central and obstructive apnea during seizures: defining pathophysiology for sudden death in a rat model. Epilepsy Res. 2016;128:126-39.
Atrial fibrillation (AF) is the most common cardiac arrhythmia encountered in clinical practice and is associated with increased morbidity and mortality due to thromboembolism, stroke, and worsening of pre-existing heart failure. Both its incidence and prevalence are increasing as AF risk increases with advancing age.1 While the strategies of heart rate control and anticoagulation to lower stroke risk and rhythm control have been found comparable with regard to survival, many patients remain highly symptomatic because of palpitations and reduced cardiac output.1
Structural abnormalities of the atria, including fibrosis and dilation, accompanied by conduction abnormalities, provide the underlying substrate for AF. It is well established that AF episodes perpetuate atrial remodeling leading to more frequent and prolonged AF episodes. Hence, there is the long-standing notion that “AF begets AF.” While a variety of antiarrhythmic drugs have been employed over the years to prevent AF recurrences and to maintain sinus rhythm, their use has decreased over the past 2 decades due to their major side effects and their potential of proarrhythmia.
Since AF patients represent a heterogeneous group of patients with CV diseases of varying type and severity as well as comorbidities, it stands to reason that the pulmonary venous–left atrial junction may not be the sole culprit region of all cases of AF and that other anatomical locations might serve as triggers for AF.
In support of this notion are the results of the prospective multicenter study presented by Dr. Elad Anter at the annual International AF Symposium. This important study is consistent with and expands upon prior studies that have suggested that sites within the atria remote from the pulmonary veins may serve as triggers for AF, rather than lower technical success of pulmonary vein ablation.5 It further highlights the importance of fibrosis and associated electrical dispersion to the pathogenesis of AF.6 However, the recommendation that patients with AF be screened for OSA is not new, as nearly half of patients with AF also have OSA.7 While AF and OSA share common risk factors/comorbidities such as male gender, obesity, hypertension, coronary artery disease, and congestive heart failure, OSA has been found to be an independent risk factor for AF development.
It is important to know whether OSA was treated, as the presence of OSA raises the risk of AF recurrence and OSA treatment decreases AF recurrence after ablation.8,9 Conversely, in the setting of OSA, AF is more resistive to rhythm control. Enhanced vagal activation, elevated sympathetic tone, and oxidative stresses due to oxygen desaturation and left atrial distension have all been implicated in the pathogenesis linking OSA to the development of AF. Repeated increases in upper airway resistance during airway obstruction have been shown to lead to atrial stretch, dilation, and fibrosis.10 Since patients with heart failure, coronary artery disease, and other underlying causes for AF were excluded from the onset, the results may not be applicable to a large segment of AF patients. Exclusion of underlying cardiac conditions potentially raised the yield of patients found to have OSA and the potential value of OSA screening. Of note: Less than half of patients that were enrolled had complete data for analysis, which may further limit applicability of the study findings. All patients had paroxysmal AF and were in sinus rhythm while the mapping procedure was performed, leaving questions as to how to approach patients presenting acutely with persistent or long standing AF, or those recently treated with antiarrhythmic therapy. Also, since arrhythmia-free survival decreases from 1 to 5 years after AF ablation, and short-time success rates do not predict longer success rates, the present study results should be interpreted with cautious optimism.11
However, these limitations should not detract from the major implications of the study. In the setting of AF, OSA should be clinically suspected not only because of the frequent coexistence of the two disorders but because the presence of OSA should prompt electrophysiologists to consider non–pulmonary vein triggers of AF prior to ablation attempts. The consideration of alternative ablation sites might help to explain the lack of ablation procedure endpoints to predict long-term success of ablation and holds promise for increasing technical success rates. Given that airway obstruction may occur in other clinical settings such as seizure-induced laryngospasm and that seizures may induce arrhythmias and sudden death, there is potential for non–pulmonary vein sites to trigger AF and other arrhythmias in settings other than OSA as well.12 Whether other disease states are associated with a higher likelihood of non-pulmonary veins trigger sites also merits further study. Moreover, this study underscores the notion that with regard to AF ablation, “no one site fits all” and “clinical mapping” may serve as a valuable adjunct to anatomical mapping. It also serves as a reminder of the multidisciplinary nature of Chest Medicine and the need of a team oriented approach..
References
1. Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124:2264-74.
2. Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812-22.
3. Kuck KH, Brugada J, Fürnkranz A, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374:2235-45.
4. Calkins H, Reynolds MR, Spector P, et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol. 2009;2:349-61.
5. Narayan SM, Krummen DE, Shivkumar K, et al. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol. 2012;60:628-36.
6. Kottkamp H, Berg J, Bender R, et al. Box Isolation of Fibrotic Areas (BIFA): a patient-tailored substrate modified application approach for ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2016;27:22-30.
7. Stevenson IH, Teichtahl H, Cunnington D, et al. Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. Eur Heart J. 2008;29:1662-9.
8. Fein AS, Shvilkin A, Shah D, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol. 2013;62:300-5.
9. Naruse Y, Tada H, Satoh M, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm. 2013;10:331-7.
10. Otto M, Belohlavek M, Romero-Corral A, et al. Comparison of cardiac structural and functional changes in obese otherwise healthy adults with versus without obstructive sleep apnea. Am J Cardiol. 2007;99:1298-302.
11. Kis Z, Muka T, Franco OH, et al. The short and long-term efficacy of pulmonary vein isolation as a sole treatment strategy for paroxysmal atrial fibrillation: a systematic review and meta-analysis. Curr Cardiol Rev. 2017 Jan 17. [Epub ahead of print].
12. Nakase K, Kollmar R, Lazar J, et al. Laryngospasm, central and obstructive apnea during seizures: defining pathophysiology for sudden death in a rat model. Epilepsy Res. 2016;128:126-39.
ORLANDO – Patients with atrial fibrillation (AF) should be screened for obstructive sleep apnea (OSA), because this information may be useful in guiding ablation strategies, according to results of a prospective study.
The study, which associated OSA in AF with a high relative rate of non–pulmonary vein (PV) triggers, has contributed to the “growing body of evidence implicating sleep apnea in atrial remodeling and promotion of the AF substrate,” Elad Anter, MD, associate director of the clinical electrophysiology laboratory at Beth Israel Deaconess Medical Center, Boston, reported at the annual International AF Symposium.
Despite the close association between OSA and AF, it has been unclear whether OSA is a causative factor. Dr. Anter suggested that mechanistic association is strengthening, however.
It has been hypothesized that OSA generates AF substrate through negative intrathoracic pressure changes and autonomic nervous system activation. But Dr. Anter reported that there is more recent and compelling evidence that the repetitive occlusions produced by OSA result in remodeling of the atria, producing scar tissue that slows conduction and produces susceptibility to reentry AF.
A newly completed prospective multicenter study adds support to this latter hypothesis. In the protocol, patients with paroxysmal AF scheduled for ablation were required to undergo a sleep study, an AF mapping study, and follow-up for at least 12 months. A known history of OSA was an exclusion criterion. To isolate the effect of OSA, there were exclusions for other major etiologies for AF, such as heart failure or coronary artery disease.
The AF mapping was conducted when patients were in sinus rhythm “to evaluate the baseline atrial substrate and avoid measurements related to acute electrical remodeling,” Dr. Anter explained.
Of 172 patients initially enrolled, 133 completed the sleep study, 118 completed the mapping study, and 110 completed both and were followed for at least 12 months. Of these, 43 patients without OSA were compared with 43 patients with OSA defined as an apnea-hypopnea index (AHI) of at least 15. Patients in the two groups did not differ significantly for relevant characteristics, such as body mass index (BMI), age, presence of hypertension, or duration of AF; but the left atrial (LA) volume was significantly greater (P = .01) in those with OSA than those without.
Even though the prevalence of voltage abnormalities was higher in the OSA group for the right (P = .01) and left atria (P = .0001) before ablation, the prevalence of PV triggers (63% vs. 65%), non-PV triggers (19% vs. 12%) and noninducible triggers (19% vs. 23%) were similar.
After ablation, PV triggers were no longer inducible in either group, but there was a striking difference in inducible non-PV triggers. While only 11.6% remained inducible in the non-OSA group, 41.8% (P = .003) remained inducible in the OSA patients.
“AF triggers in OSA were most commonly located at the LA septum, at the zone of low voltage and abnormal electrograms, as determined during sinus rhythm,” Dr. Anter reported. “Ablation of these triggers at the zone of tissue abnormality in the OSA patients resulted in termination of AF in 9 (64.2%) of the 14 patients.”
Overall, at the end of 12 months, 79% of those without OSA remained in arrhythmia-free survival, versus 65.1% of the group with OSA that were treated with PV isolation alone.
The lower rate of success in the OSA group shows the importance of specifically directing ablation to the areas of low voltage and slow conduction in the left anterior septum that Dr. Anter indicated otherwise would be missed.
“These zones are a common source of extra-PV triggers and localized circuits or rotors of AF in OSA patients,” he reported. “Ablation of these low voltage zones is associated with improved clinical outcome in OSA patients with paroxysmal AF.”
The data, which Dr. Anter said are consistent with a growing body of work regarding the relationship of OSA and AF, provided the basis for suggesting that AF patients undergo routine screening for OSA.
In patients with OSA, ablation of PV triggers alone even in paroxysmal PAF “may not be sufficient,” he cautioned. “Evaluation of non-PV triggers should also be performed.”
Dr. Anter reported financial relationships with Biosense Webster and Boston Scientific.
ORLANDO – Patients with atrial fibrillation (AF) should be screened for obstructive sleep apnea (OSA), because this information may be useful in guiding ablation strategies, according to results of a prospective study.
The study, which associated OSA in AF with a high relative rate of non–pulmonary vein (PV) triggers, has contributed to the “growing body of evidence implicating sleep apnea in atrial remodeling and promotion of the AF substrate,” Elad Anter, MD, associate director of the clinical electrophysiology laboratory at Beth Israel Deaconess Medical Center, Boston, reported at the annual International AF Symposium.
Despite the close association between OSA and AF, it has been unclear whether OSA is a causative factor. Dr. Anter suggested that mechanistic association is strengthening, however.
It has been hypothesized that OSA generates AF substrate through negative intrathoracic pressure changes and autonomic nervous system activation. But Dr. Anter reported that there is more recent and compelling evidence that the repetitive occlusions produced by OSA result in remodeling of the atria, producing scar tissue that slows conduction and produces susceptibility to reentry AF.
A newly completed prospective multicenter study adds support to this latter hypothesis. In the protocol, patients with paroxysmal AF scheduled for ablation were required to undergo a sleep study, an AF mapping study, and follow-up for at least 12 months. A known history of OSA was an exclusion criterion. To isolate the effect of OSA, there were exclusions for other major etiologies for AF, such as heart failure or coronary artery disease.
The AF mapping was conducted when patients were in sinus rhythm “to evaluate the baseline atrial substrate and avoid measurements related to acute electrical remodeling,” Dr. Anter explained.
Of 172 patients initially enrolled, 133 completed the sleep study, 118 completed the mapping study, and 110 completed both and were followed for at least 12 months. Of these, 43 patients without OSA were compared with 43 patients with OSA defined as an apnea-hypopnea index (AHI) of at least 15. Patients in the two groups did not differ significantly for relevant characteristics, such as body mass index (BMI), age, presence of hypertension, or duration of AF; but the left atrial (LA) volume was significantly greater (P = .01) in those with OSA than those without.
Even though the prevalence of voltage abnormalities was higher in the OSA group for the right (P = .01) and left atria (P = .0001) before ablation, the prevalence of PV triggers (63% vs. 65%), non-PV triggers (19% vs. 12%) and noninducible triggers (19% vs. 23%) were similar.
After ablation, PV triggers were no longer inducible in either group, but there was a striking difference in inducible non-PV triggers. While only 11.6% remained inducible in the non-OSA group, 41.8% (P = .003) remained inducible in the OSA patients.
“AF triggers in OSA were most commonly located at the LA septum, at the zone of low voltage and abnormal electrograms, as determined during sinus rhythm,” Dr. Anter reported. “Ablation of these triggers at the zone of tissue abnormality in the OSA patients resulted in termination of AF in 9 (64.2%) of the 14 patients.”
Overall, at the end of 12 months, 79% of those without OSA remained in arrhythmia-free survival, versus 65.1% of the group with OSA that were treated with PV isolation alone.
The lower rate of success in the OSA group shows the importance of specifically directing ablation to the areas of low voltage and slow conduction in the left anterior septum that Dr. Anter indicated otherwise would be missed.
“These zones are a common source of extra-PV triggers and localized circuits or rotors of AF in OSA patients,” he reported. “Ablation of these low voltage zones is associated with improved clinical outcome in OSA patients with paroxysmal AF.”
The data, which Dr. Anter said are consistent with a growing body of work regarding the relationship of OSA and AF, provided the basis for suggesting that AF patients undergo routine screening for OSA.
In patients with OSA, ablation of PV triggers alone even in paroxysmal PAF “may not be sufficient,” he cautioned. “Evaluation of non-PV triggers should also be performed.”
Dr. Anter reported financial relationships with Biosense Webster and Boston Scientific.
Key clinical point: Atrial fibrillation associated with sleep apnea appears to have features that should be addressed specifically for sustained rhythm control.
Major finding: AF patients with sleep apnea have more non–pulmonary vein triggers after ablation than do those without sleep apnea (41.8% vs. 11.6%).
Data source: A prospective multicenter observational study.
Disclosures: Dr. Anter reported financial relationships with Biosense Webster and Boston Scientific.
Localized Pemphigus Foliaceus
To the Editor:
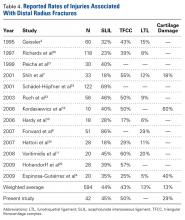
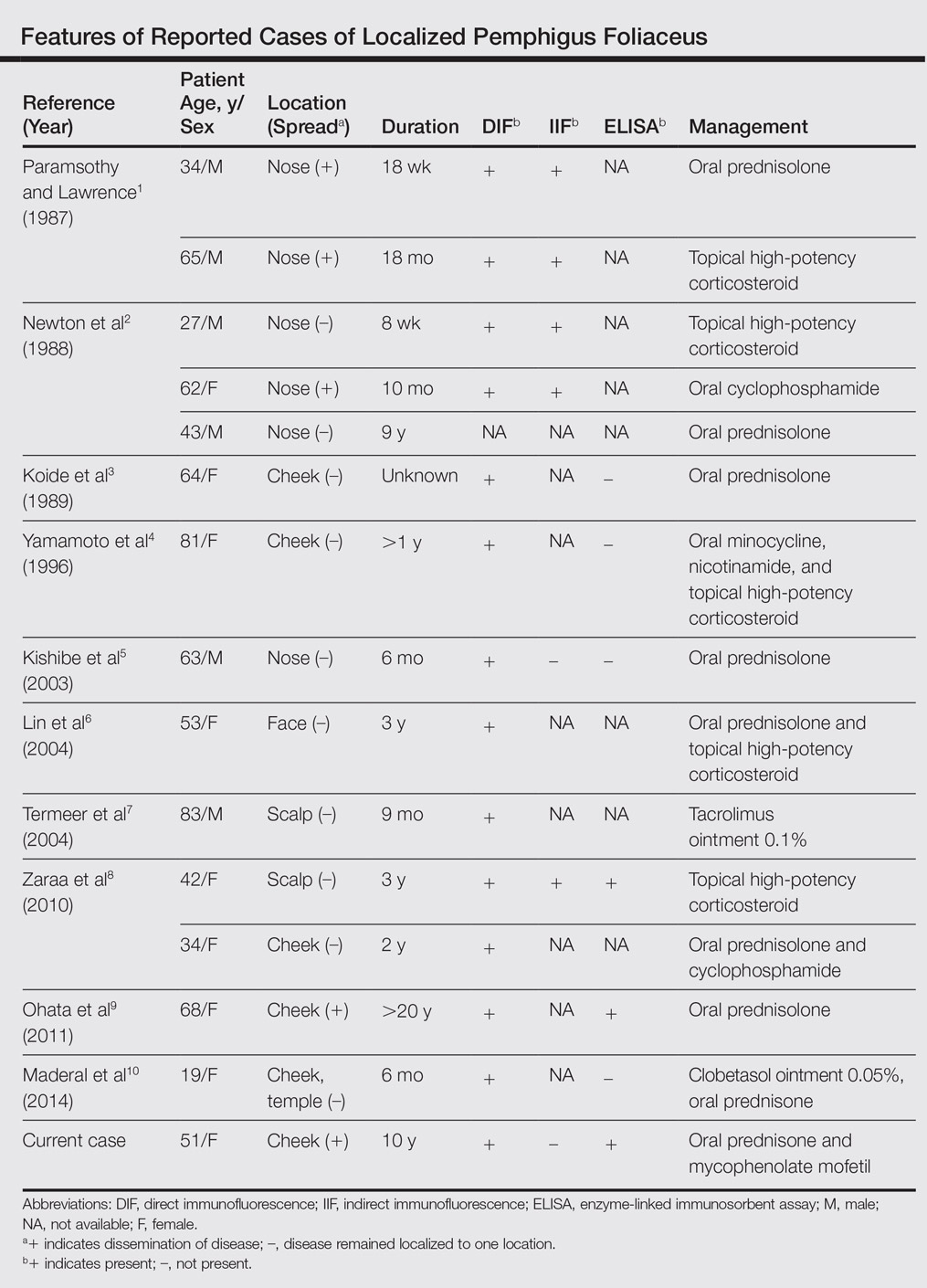
Pemphigus foliaceus is a rare autoimmune blistering disorder that typically presents with crusted scaly erosions in a seborrheic distribution. We describe a case of pemphigus foliaceus localized to the right cheek of 10 years’ duration that spread to other areas. With a PubMed search of articles indexed for MEDLINE yielding only 14 cases of localized pemphigus foliaceus (Table), it represents an extremely rare entity that often is a diagnostic challenge and may be a harbinger for disseminated disease months to years after the inciting lesion appears.
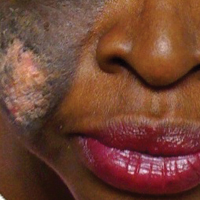
A 51-year-old woman presented with an asymptomatic cutaneous eruption that had remained localized to the right cheek for 10 years before it increased in size and new lesions developed on the left cheek, chest, and upper back. No inciting factors, such as contactants, insect bites, infections, medications, or recent travel were identified. On physical examination a well-demarcated, hypertrophic, verrucouslike plaque with central pink atrophy and exfoliative scale involved the right malar and submalar regions but spared the mucocutaneous junctions of the face (Figure 1). Subtle dark brown papules, some with overlying scale, speckled the left cheek, right jawline, chest, and upper back. The oral cavity was clear.
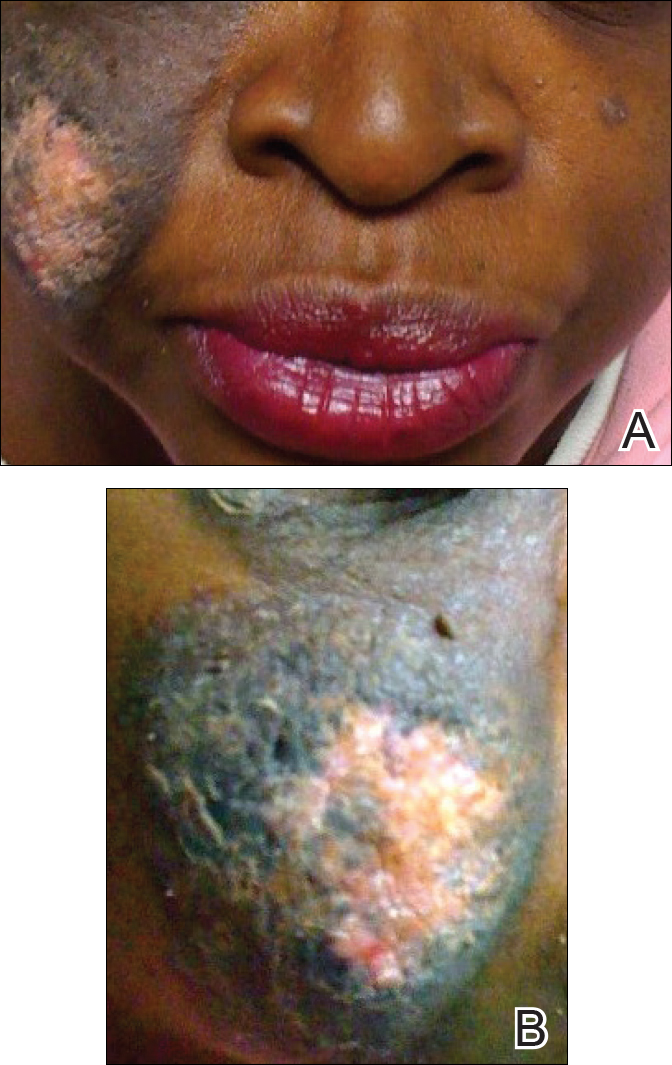
Leading differentials included hypertrophic discoid lupus erythematosus and pemphigus vegetans. Other considerations included sarcoidosis, granuloma faciale, lupus vulgaris, disseminated coccidioidomycosis or blastomycosis, and squamous cell carcinoma.
An initial biopsy revealed a lymphocytic lichenoid dermatitis with epidermal hyperplasia and scattered eosinophils for which the following differentials were provided: insect bite, hypertrophic lichen planus, prurigo nodularis superimposed on rosacea, and allergic contact dermatitis. Under these histologic diagnoses, tacrolimus ointment 0.03%, topical mid-potency corticosteroid, and a combination of oral doxycycline and metronidazole gel 1% were prescribed but failed to ameliorate her condition.
Because the clinical differentials were vast and noncorrelative with the original pathology, additional biopsies were performed: one from the edge of the large malar plaque, which was transected for hematoxylin and eosin (H&E) and tissue cultures; one perilesional to the large malar plaque for direct immunofluorescence (DIF); and one from the papule on the right jawline for H&E. Tissue cultures were negative for fungal and mycobacterial organisms. Both specimens submitted for H&E showed the prominent epidermal hyperplasia and lymphocytic dermal infiltrate noted on the original H&E but also demonstrated intragranular acantholysis (Figure 2). The DIF revealed intercellular IgG and C3 deposition throughout the epidermis (Figure 3). Indirect immunofluorescence was negative, but enzyme-linked immunosorbent assay detected circulating antidesmoglein-1 but not antidesmoglein-3 autoantibodies. Other serologies including antinuclear antibody, anti–double-stranded DNA, antihistone, anti–Sjögren syndrome A, and anti–Sjögren syndrome B antibodies were negative.
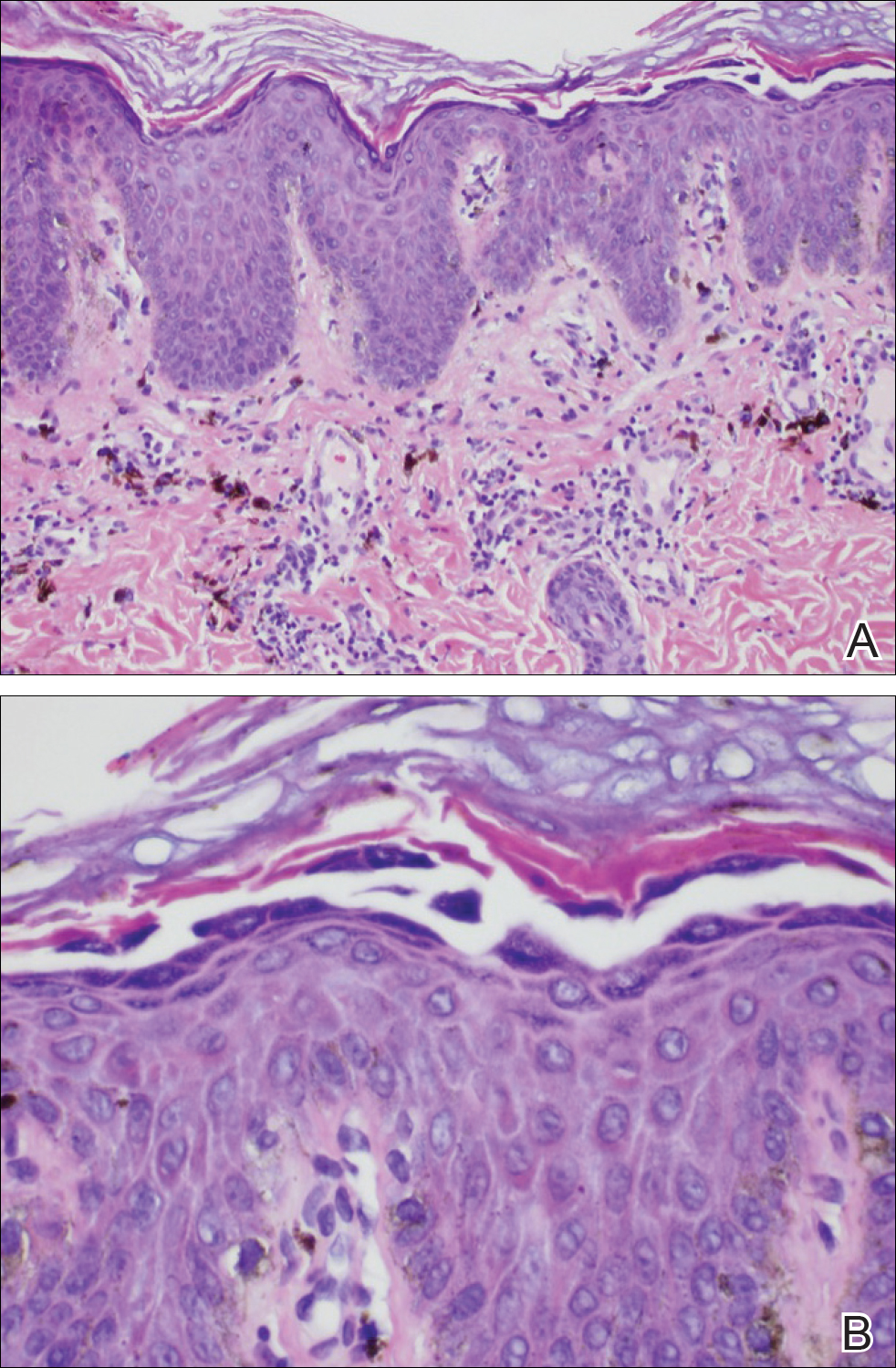

The diagnosis of localized pemphigus foliaceus was made and management with oral prednisone and mycophenolate mofetil resulted in improvement within weeks.
Localized pemphigus foliaceus is extremely rare with only 14 cases reported in the literature (Table).1-10 Its diagnosis is challenging, as the clinical presentation simulates various entities and the histological features and serological markers are difficult to capture.
Localized pemphigus foliaceus typically presents as an isolated, erythematous, scaly, crusted plaque involving the nose, cheek, or scalp and may mimic several conditions including contact dermatitis, seborrheic dermatitis, rosacea, cutaneous sarcoidosis, discoid lupus erythematosus, lupus vulgaris, impetigo contagiosa, solar keratosis, and nonmelanoma skin cancer.1-10
The predilection for sun-exposed areas suggests UV radiation may induce binding of antidesmoglein-1 autoantibodies with subsequent cytokine-mediated inflammation and acantholysis at these sites.11-13 Similarly, the immunomodulatory agent imiquimod has been reported to induce pemphigus foliaceus at its application sites.6
When pemphigus foliaceus is clinically discernible, the histology and DIF are in accordance with the clinical diagnosis 53.8% of the time.13 In cases of localized pemphigus foliaceus in which the diagnosis is more elusive, many biopsies often are needed to capture the characteristic intragranular acantholysis; this feature often is so subtle that unless the diagnosis is suspected, it is underappreciated or undetectable. In chronic lesions, it may be masked by secondary changes such as acanthosis, hyperkeratosis, and parakeratosis.14
In pemphigus foliaceus, detection of circulating antidesmoglein-1 autoantibodies by enzyme-linked immunosorbent assay is slightly more sensitive and specific compared to indirect immunofluorescence, but both correlate with disease activity.15,16 The low or absent autoantibody titers in localized pemphigus foliaceus may reflect its limited involvement, but dissemination of the disease with subsequent elevation of autoantibody titers may occur months to years after initial presentation,1,2,9 as was the case with our patient.
The majority of localized pemphigus foliaceus cases require systemic prednisone, sometimes in conjunction with nonsteroidal immunosuppressants or topical high-potency corticosteroids.1-3,5,6,8-10 One case was efficaciously managed with tacrolimus ointment 0.1%.7
Localized pemphigus foliaceus is a rare and challenging entity that must be a diagnostic consideration for any chronic focal plaque on the face or scalp, as it may herald disseminated disease.
- Paramsothy Y, Lawrence CM. “Tin-tack” sign in localized pemphigus foliaceus. Br J Dermatol. 1987;116:127-129.
- Newton JA, McGibbon DH, Monk B, et al. Pemphigus foliaceus localized to the nose. Br J Dermatol. 1988;118:303-312.
- Koide M, Kokura N, Takano N. Pemphigus foliaceus localized on the face [in Japanese]. Jpn J Dermatol. 1989;97:1262.
- Yamamoto S, Kanekura T, Gushi A, et al. A case of localized pemphigus foliaceus. J Dermatol. 1996;23:893-895.
- Kishibe M, Kinouchi M, Ishida-Yamamoto A, et al. Pemphigus foliaceus localized to the nose. Clin Exp Dermatol. 2003;28:560-562.
- Lin R, Ladd DJ, Powell DJ, et al. Localized pemphigus foliaceus induced by topical imiquimod treatment. Arch Dermatol. 2004;140:889-890.
- Termeer CC, Technau K, Augustin M, et al. Topical tacrolimus (Protopic) for the treatment of a localized pemphigus foliaceus. J Eur Acad Dermatol Venereol. 2004;18:636-637.
- Zaraa I, El Euch D, Kort R, et al. Localized pemphigus: a report of three cases. Int J Dermatol 2010;49:715-716.
- Ohata C, Akamatsu K, Imai N, et al. Localized pemphigus foliaceus exclusively involving the follicular infundibulum: a novel peau d’orange appearance. Eur J Dermatol. 2011;21:392-395.
- Maderal AD, Miner A, Nousari C, et al. Localized pemphigus foliaceus with unilateral facial involvement. Actas Dermosifiliogr. 2014;105:413-417.
- Cram DL, Winkelmann RK. Ultraviolet-induced acantholysis in pemphigus. Arch Dermatol. 1965;92:7-13.
- Kano Y, Shimosegawa M, Mizukawa Y, et al. Pemphigus foliaceus induced by exposure to sunlight. Dermatology. 2000;201:132-138.
- Lebe B, Gül Nıflıoğlu G, Seyrek S, et al. Evaluation of clinical and histopathologic/direct immunofluorescence diagnosis in autoimmune vesiculobullous dermatitis: utility of direct immunofluorescence. Turk Patoloji Derg. 2012;28:11-16.
- Joly P, Litrowski N. Pemphigus group (vulgaris, vegetans, foliaceus, herpetiformis, brasiliensis). Clin Dermatol. 2011;29:432-436.
- Ishii K, Amagai M, Hall RP, et al. Characterization of autoantibodies in pemphigus using antigen specific enzyme-linked immunosorbent assays with baculovirus-expressed recombinant desmogleins. J Immunol. 1997;159:2010-2017.
- Ng PP, Thng ST, Mohamed K, et al. Comparison of desmoglein ELISA and indirect immunofluorescence using two substrates (monkey esophagus and normal human skin) in the diagnosis of pemphigus. Australas J Dermatol. 2005;46:239-241.
To the Editor:
Pemphigus foliaceus is a rare autoimmune blistering disorder that typically presents with crusted scaly erosions in a seborrheic distribution. We describe a case of pemphigus foliaceus localized to the right cheek of 10 years’ duration that spread to other areas. With a PubMed search of articles indexed for MEDLINE yielding only 14 cases of localized pemphigus foliaceus (Table), it represents an extremely rare entity that often is a diagnostic challenge and may be a harbinger for disseminated disease months to years after the inciting lesion appears.
A 51-year-old woman presented with an asymptomatic cutaneous eruption that had remained localized to the right cheek for 10 years before it increased in size and new lesions developed on the left cheek, chest, and upper back. No inciting factors, such as contactants, insect bites, infections, medications, or recent travel were identified. On physical examination a well-demarcated, hypertrophic, verrucouslike plaque with central pink atrophy and exfoliative scale involved the right malar and submalar regions but spared the mucocutaneous junctions of the face (Figure 1). Subtle dark brown papules, some with overlying scale, speckled the left cheek, right jawline, chest, and upper back. The oral cavity was clear.

Leading differentials included hypertrophic discoid lupus erythematosus and pemphigus vegetans. Other considerations included sarcoidosis, granuloma faciale, lupus vulgaris, disseminated coccidioidomycosis or blastomycosis, and squamous cell carcinoma.
An initial biopsy revealed a lymphocytic lichenoid dermatitis with epidermal hyperplasia and scattered eosinophils for which the following differentials were provided: insect bite, hypertrophic lichen planus, prurigo nodularis superimposed on rosacea, and allergic contact dermatitis. Under these histologic diagnoses, tacrolimus ointment 0.03%, topical mid-potency corticosteroid, and a combination of oral doxycycline and metronidazole gel 1% were prescribed but failed to ameliorate her condition.
Because the clinical differentials were vast and noncorrelative with the original pathology, additional biopsies were performed: one from the edge of the large malar plaque, which was transected for hematoxylin and eosin (H&E) and tissue cultures; one perilesional to the large malar plaque for direct immunofluorescence (DIF); and one from the papule on the right jawline for H&E. Tissue cultures were negative for fungal and mycobacterial organisms. Both specimens submitted for H&E showed the prominent epidermal hyperplasia and lymphocytic dermal infiltrate noted on the original H&E but also demonstrated intragranular acantholysis (Figure 2). The DIF revealed intercellular IgG and C3 deposition throughout the epidermis (Figure 3). Indirect immunofluorescence was negative, but enzyme-linked immunosorbent assay detected circulating antidesmoglein-1 but not antidesmoglein-3 autoantibodies. Other serologies including antinuclear antibody, anti–double-stranded DNA, antihistone, anti–Sjögren syndrome A, and anti–Sjögren syndrome B antibodies were negative.


The diagnosis of localized pemphigus foliaceus was made and management with oral prednisone and mycophenolate mofetil resulted in improvement within weeks.
Localized pemphigus foliaceus is extremely rare with only 14 cases reported in the literature (Table).1-10 Its diagnosis is challenging, as the clinical presentation simulates various entities and the histological features and serological markers are difficult to capture.

Localized pemphigus foliaceus typically presents as an isolated, erythematous, scaly, crusted plaque involving the nose, cheek, or scalp and may mimic several conditions including contact dermatitis, seborrheic dermatitis, rosacea, cutaneous sarcoidosis, discoid lupus erythematosus, lupus vulgaris, impetigo contagiosa, solar keratosis, and nonmelanoma skin cancer.1-10
The predilection for sun-exposed areas suggests UV radiation may induce binding of antidesmoglein-1 autoantibodies with subsequent cytokine-mediated inflammation and acantholysis at these sites.11-13 Similarly, the immunomodulatory agent imiquimod has been reported to induce pemphigus foliaceus at its application sites.6
When pemphigus foliaceus is clinically discernible, the histology and DIF are in accordance with the clinical diagnosis 53.8% of the time.13 In cases of localized pemphigus foliaceus in which the diagnosis is more elusive, many biopsies often are needed to capture the characteristic intragranular acantholysis; this feature often is so subtle that unless the diagnosis is suspected, it is underappreciated or undetectable. In chronic lesions, it may be masked by secondary changes such as acanthosis, hyperkeratosis, and parakeratosis.14
In pemphigus foliaceus, detection of circulating antidesmoglein-1 autoantibodies by enzyme-linked immunosorbent assay is slightly more sensitive and specific compared to indirect immunofluorescence, but both correlate with disease activity.15,16 The low or absent autoantibody titers in localized pemphigus foliaceus may reflect its limited involvement, but dissemination of the disease with subsequent elevation of autoantibody titers may occur months to years after initial presentation,1,2,9 as was the case with our patient.
The majority of localized pemphigus foliaceus cases require systemic prednisone, sometimes in conjunction with nonsteroidal immunosuppressants or topical high-potency corticosteroids.1-3,5,6,8-10 One case was efficaciously managed with tacrolimus ointment 0.1%.7
Localized pemphigus foliaceus is a rare and challenging entity that must be a diagnostic consideration for any chronic focal plaque on the face or scalp, as it may herald disseminated disease.
To the Editor:
Pemphigus foliaceus is a rare autoimmune blistering disorder that typically presents with crusted scaly erosions in a seborrheic distribution. We describe a case of pemphigus foliaceus localized to the right cheek of 10 years’ duration that spread to other areas. With a PubMed search of articles indexed for MEDLINE yielding only 14 cases of localized pemphigus foliaceus (Table), it represents an extremely rare entity that often is a diagnostic challenge and may be a harbinger for disseminated disease months to years after the inciting lesion appears.
A 51-year-old woman presented with an asymptomatic cutaneous eruption that had remained localized to the right cheek for 10 years before it increased in size and new lesions developed on the left cheek, chest, and upper back. No inciting factors, such as contactants, insect bites, infections, medications, or recent travel were identified. On physical examination a well-demarcated, hypertrophic, verrucouslike plaque with central pink atrophy and exfoliative scale involved the right malar and submalar regions but spared the mucocutaneous junctions of the face (Figure 1). Subtle dark brown papules, some with overlying scale, speckled the left cheek, right jawline, chest, and upper back. The oral cavity was clear.

Leading differentials included hypertrophic discoid lupus erythematosus and pemphigus vegetans. Other considerations included sarcoidosis, granuloma faciale, lupus vulgaris, disseminated coccidioidomycosis or blastomycosis, and squamous cell carcinoma.
An initial biopsy revealed a lymphocytic lichenoid dermatitis with epidermal hyperplasia and scattered eosinophils for which the following differentials were provided: insect bite, hypertrophic lichen planus, prurigo nodularis superimposed on rosacea, and allergic contact dermatitis. Under these histologic diagnoses, tacrolimus ointment 0.03%, topical mid-potency corticosteroid, and a combination of oral doxycycline and metronidazole gel 1% were prescribed but failed to ameliorate her condition.
Because the clinical differentials were vast and noncorrelative with the original pathology, additional biopsies were performed: one from the edge of the large malar plaque, which was transected for hematoxylin and eosin (H&E) and tissue cultures; one perilesional to the large malar plaque for direct immunofluorescence (DIF); and one from the papule on the right jawline for H&E. Tissue cultures were negative for fungal and mycobacterial organisms. Both specimens submitted for H&E showed the prominent epidermal hyperplasia and lymphocytic dermal infiltrate noted on the original H&E but also demonstrated intragranular acantholysis (Figure 2). The DIF revealed intercellular IgG and C3 deposition throughout the epidermis (Figure 3). Indirect immunofluorescence was negative, but enzyme-linked immunosorbent assay detected circulating antidesmoglein-1 but not antidesmoglein-3 autoantibodies. Other serologies including antinuclear antibody, anti–double-stranded DNA, antihistone, anti–Sjögren syndrome A, and anti–Sjögren syndrome B antibodies were negative.


The diagnosis of localized pemphigus foliaceus was made and management with oral prednisone and mycophenolate mofetil resulted in improvement within weeks.
Localized pemphigus foliaceus is extremely rare with only 14 cases reported in the literature (Table).1-10 Its diagnosis is challenging, as the clinical presentation simulates various entities and the histological features and serological markers are difficult to capture.

Localized pemphigus foliaceus typically presents as an isolated, erythematous, scaly, crusted plaque involving the nose, cheek, or scalp and may mimic several conditions including contact dermatitis, seborrheic dermatitis, rosacea, cutaneous sarcoidosis, discoid lupus erythematosus, lupus vulgaris, impetigo contagiosa, solar keratosis, and nonmelanoma skin cancer.1-10
The predilection for sun-exposed areas suggests UV radiation may induce binding of antidesmoglein-1 autoantibodies with subsequent cytokine-mediated inflammation and acantholysis at these sites.11-13 Similarly, the immunomodulatory agent imiquimod has been reported to induce pemphigus foliaceus at its application sites.6
When pemphigus foliaceus is clinically discernible, the histology and DIF are in accordance with the clinical diagnosis 53.8% of the time.13 In cases of localized pemphigus foliaceus in which the diagnosis is more elusive, many biopsies often are needed to capture the characteristic intragranular acantholysis; this feature often is so subtle that unless the diagnosis is suspected, it is underappreciated or undetectable. In chronic lesions, it may be masked by secondary changes such as acanthosis, hyperkeratosis, and parakeratosis.14
In pemphigus foliaceus, detection of circulating antidesmoglein-1 autoantibodies by enzyme-linked immunosorbent assay is slightly more sensitive and specific compared to indirect immunofluorescence, but both correlate with disease activity.15,16 The low or absent autoantibody titers in localized pemphigus foliaceus may reflect its limited involvement, but dissemination of the disease with subsequent elevation of autoantibody titers may occur months to years after initial presentation,1,2,9 as was the case with our patient.
The majority of localized pemphigus foliaceus cases require systemic prednisone, sometimes in conjunction with nonsteroidal immunosuppressants or topical high-potency corticosteroids.1-3,5,6,8-10 One case was efficaciously managed with tacrolimus ointment 0.1%.7
Localized pemphigus foliaceus is a rare and challenging entity that must be a diagnostic consideration for any chronic focal plaque on the face or scalp, as it may herald disseminated disease.
- Paramsothy Y, Lawrence CM. “Tin-tack” sign in localized pemphigus foliaceus. Br J Dermatol. 1987;116:127-129.
- Newton JA, McGibbon DH, Monk B, et al. Pemphigus foliaceus localized to the nose. Br J Dermatol. 1988;118:303-312.
- Koide M, Kokura N, Takano N. Pemphigus foliaceus localized on the face [in Japanese]. Jpn J Dermatol. 1989;97:1262.
- Yamamoto S, Kanekura T, Gushi A, et al. A case of localized pemphigus foliaceus. J Dermatol. 1996;23:893-895.
- Kishibe M, Kinouchi M, Ishida-Yamamoto A, et al. Pemphigus foliaceus localized to the nose. Clin Exp Dermatol. 2003;28:560-562.
- Lin R, Ladd DJ, Powell DJ, et al. Localized pemphigus foliaceus induced by topical imiquimod treatment. Arch Dermatol. 2004;140:889-890.
- Termeer CC, Technau K, Augustin M, et al. Topical tacrolimus (Protopic) for the treatment of a localized pemphigus foliaceus. J Eur Acad Dermatol Venereol. 2004;18:636-637.
- Zaraa I, El Euch D, Kort R, et al. Localized pemphigus: a report of three cases. Int J Dermatol 2010;49:715-716.
- Ohata C, Akamatsu K, Imai N, et al. Localized pemphigus foliaceus exclusively involving the follicular infundibulum: a novel peau d’orange appearance. Eur J Dermatol. 2011;21:392-395.
- Maderal AD, Miner A, Nousari C, et al. Localized pemphigus foliaceus with unilateral facial involvement. Actas Dermosifiliogr. 2014;105:413-417.
- Cram DL, Winkelmann RK. Ultraviolet-induced acantholysis in pemphigus. Arch Dermatol. 1965;92:7-13.
- Kano Y, Shimosegawa M, Mizukawa Y, et al. Pemphigus foliaceus induced by exposure to sunlight. Dermatology. 2000;201:132-138.
- Lebe B, Gül Nıflıoğlu G, Seyrek S, et al. Evaluation of clinical and histopathologic/direct immunofluorescence diagnosis in autoimmune vesiculobullous dermatitis: utility of direct immunofluorescence. Turk Patoloji Derg. 2012;28:11-16.
- Joly P, Litrowski N. Pemphigus group (vulgaris, vegetans, foliaceus, herpetiformis, brasiliensis). Clin Dermatol. 2011;29:432-436.
- Ishii K, Amagai M, Hall RP, et al. Characterization of autoantibodies in pemphigus using antigen specific enzyme-linked immunosorbent assays with baculovirus-expressed recombinant desmogleins. J Immunol. 1997;159:2010-2017.
- Ng PP, Thng ST, Mohamed K, et al. Comparison of desmoglein ELISA and indirect immunofluorescence using two substrates (monkey esophagus and normal human skin) in the diagnosis of pemphigus. Australas J Dermatol. 2005;46:239-241.
- Paramsothy Y, Lawrence CM. “Tin-tack” sign in localized pemphigus foliaceus. Br J Dermatol. 1987;116:127-129.
- Newton JA, McGibbon DH, Monk B, et al. Pemphigus foliaceus localized to the nose. Br J Dermatol. 1988;118:303-312.
- Koide M, Kokura N, Takano N. Pemphigus foliaceus localized on the face [in Japanese]. Jpn J Dermatol. 1989;97:1262.
- Yamamoto S, Kanekura T, Gushi A, et al. A case of localized pemphigus foliaceus. J Dermatol. 1996;23:893-895.
- Kishibe M, Kinouchi M, Ishida-Yamamoto A, et al. Pemphigus foliaceus localized to the nose. Clin Exp Dermatol. 2003;28:560-562.
- Lin R, Ladd DJ, Powell DJ, et al. Localized pemphigus foliaceus induced by topical imiquimod treatment. Arch Dermatol. 2004;140:889-890.
- Termeer CC, Technau K, Augustin M, et al. Topical tacrolimus (Protopic) for the treatment of a localized pemphigus foliaceus. J Eur Acad Dermatol Venereol. 2004;18:636-637.
- Zaraa I, El Euch D, Kort R, et al. Localized pemphigus: a report of three cases. Int J Dermatol 2010;49:715-716.
- Ohata C, Akamatsu K, Imai N, et al. Localized pemphigus foliaceus exclusively involving the follicular infundibulum: a novel peau d’orange appearance. Eur J Dermatol. 2011;21:392-395.
- Maderal AD, Miner A, Nousari C, et al. Localized pemphigus foliaceus with unilateral facial involvement. Actas Dermosifiliogr. 2014;105:413-417.
- Cram DL, Winkelmann RK. Ultraviolet-induced acantholysis in pemphigus. Arch Dermatol. 1965;92:7-13.
- Kano Y, Shimosegawa M, Mizukawa Y, et al. Pemphigus foliaceus induced by exposure to sunlight. Dermatology. 2000;201:132-138.
- Lebe B, Gül Nıflıoğlu G, Seyrek S, et al. Evaluation of clinical and histopathologic/direct immunofluorescence diagnosis in autoimmune vesiculobullous dermatitis: utility of direct immunofluorescence. Turk Patoloji Derg. 2012;28:11-16.
- Joly P, Litrowski N. Pemphigus group (vulgaris, vegetans, foliaceus, herpetiformis, brasiliensis). Clin Dermatol. 2011;29:432-436.
- Ishii K, Amagai M, Hall RP, et al. Characterization of autoantibodies in pemphigus using antigen specific enzyme-linked immunosorbent assays with baculovirus-expressed recombinant desmogleins. J Immunol. 1997;159:2010-2017.
- Ng PP, Thng ST, Mohamed K, et al. Comparison of desmoglein ELISA and indirect immunofluorescence using two substrates (monkey esophagus and normal human skin) in the diagnosis of pemphigus. Australas J Dermatol. 2005;46:239-241.
Practice Points
- The diagnosis of pemphigus foliceus is challenging, as the clinical presentation simulates various entities.
- Clinicopathological correlation is important. If pathology and other diagnostics do not support clinical findings, trust your clinical assessment and consider repeating or adjusting the workup.
HIV research update: Early January 2017
A great volume of HIV and AIDS research enters the medical literature every month. It’s difficult to monitor everything, so here’s a quick look at some notable news items and journal articles published over the past few weeks.
Immediate antiretroviral therapy reduces the risk of several severe bacterial infections in HIV-positive people with high CD4 cell count, according to a study in The Lancet HIV. The authors said this is partly explained by ART-induced increases in CD4 cell count, but not by increases in neutrophil count.
Postmenopausal status was not associated with a greater risk of unprotected sex in a population of high-risk HIV-positive Kenyan women, a recent study showed.
HIV-infected individuals in South Africa who reported perceived barriers to medical care at diagnosis were more likely to die within 1 year, according to a study in JAIDS.
A study of HIV care among postpartum women in South Africa found evidence of continued care after patients were lost to follow-up, and also identified local and national clinic mobility among women. Researchers said a national health database linked to a unique identifier is necessary to improve reporting and patient care among highly mobile populations.
HBV/HIV coinfection had no adverse influence on main pregnancy outcomes or on HIV viral load suppression in late pregnancy, according to a recent study, but was associated with a significantly reduced CD4 response in pregnancy.
Switching from atazanavir/ritonavir to unboosted atazanavir appears to be safe and effective in selected virologically suppressed HIV-positive patients receiving regimens containing tenofovir disoproxil fumarate (TDF), and may have favorable effects on bilirubin and renal function.
The level of pain is a negative impact on the quality of life of people with HIV/AIDS, according to a study in AIDS Care.
A recent study found children face significant barriers to accessing HIV services in Central Africa, and the HIV epidemic among surviving children in the region has not been adequately evaluated nor addressed.
Less than a quarter of newly HIV-diagnosed patients in Uganda completed antiretroviral therapy assessment, according to a study in HIV Medicine, considerably lower than in other reports from sub-Saharan Africa.
In a study published in Sexually Transmitted Diseases, researchers concluded that current CDC PrEP guidelines should be expanded to incorporate substance use, partner-level, and other syndemic variables that have been shown to contribute to HIV acquisition.
According to a recent review of the Scientific Registry of Transplant Recipients, there was a slight reduction in anti-HBV core antibody positive donor organs from 2005 to 2014, and stable reporting of HCV positive donor organs and HIV positive recipients.
The transmitted drug resistance prevalence in recent HIV infections among notified newly diagnosed HIV patients in Germany was still high (greater than 10%) in 2013 and 2014 and was within the range of other European countries, according to a report in Eurosurveillance.
Higher levels of HIV+ patient engagement in-care are associated with reduced mortality at all stages of infection, including in those who initiate antiretroviral therapy, according to a British study.
A study in the journal AIDS found that HIV/HCV coinfection is associated with the greater homeostasis model assessment of insulin resistance (HOMA-IR), even after researchers controlled for demographic, lifestyle, and metabolic factors.
The Malawi Adult Meningitis Score (MAMS) provides a novel tool for predicting prognosis and improving interpretation of acute bacterial meningitis clinical trials by risk stratification in resource-poor settings, a recent study showed.
[email protected]
On Twitter @richpizzi
A great volume of HIV and AIDS research enters the medical literature every month. It’s difficult to monitor everything, so here’s a quick look at some notable news items and journal articles published over the past few weeks.
Immediate antiretroviral therapy reduces the risk of several severe bacterial infections in HIV-positive people with high CD4 cell count, according to a study in The Lancet HIV. The authors said this is partly explained by ART-induced increases in CD4 cell count, but not by increases in neutrophil count.
Postmenopausal status was not associated with a greater risk of unprotected sex in a population of high-risk HIV-positive Kenyan women, a recent study showed.
HIV-infected individuals in South Africa who reported perceived barriers to medical care at diagnosis were more likely to die within 1 year, according to a study in JAIDS.
A study of HIV care among postpartum women in South Africa found evidence of continued care after patients were lost to follow-up, and also identified local and national clinic mobility among women. Researchers said a national health database linked to a unique identifier is necessary to improve reporting and patient care among highly mobile populations.
HBV/HIV coinfection had no adverse influence on main pregnancy outcomes or on HIV viral load suppression in late pregnancy, according to a recent study, but was associated with a significantly reduced CD4 response in pregnancy.
Switching from atazanavir/ritonavir to unboosted atazanavir appears to be safe and effective in selected virologically suppressed HIV-positive patients receiving regimens containing tenofovir disoproxil fumarate (TDF), and may have favorable effects on bilirubin and renal function.
The level of pain is a negative impact on the quality of life of people with HIV/AIDS, according to a study in AIDS Care.
A recent study found children face significant barriers to accessing HIV services in Central Africa, and the HIV epidemic among surviving children in the region has not been adequately evaluated nor addressed.
Less than a quarter of newly HIV-diagnosed patients in Uganda completed antiretroviral therapy assessment, according to a study in HIV Medicine, considerably lower than in other reports from sub-Saharan Africa.
In a study published in Sexually Transmitted Diseases, researchers concluded that current CDC PrEP guidelines should be expanded to incorporate substance use, partner-level, and other syndemic variables that have been shown to contribute to HIV acquisition.
According to a recent review of the Scientific Registry of Transplant Recipients, there was a slight reduction in anti-HBV core antibody positive donor organs from 2005 to 2014, and stable reporting of HCV positive donor organs and HIV positive recipients.
The transmitted drug resistance prevalence in recent HIV infections among notified newly diagnosed HIV patients in Germany was still high (greater than 10%) in 2013 and 2014 and was within the range of other European countries, according to a report in Eurosurveillance.
Higher levels of HIV+ patient engagement in-care are associated with reduced mortality at all stages of infection, including in those who initiate antiretroviral therapy, according to a British study.
A study in the journal AIDS found that HIV/HCV coinfection is associated with the greater homeostasis model assessment of insulin resistance (HOMA-IR), even after researchers controlled for demographic, lifestyle, and metabolic factors.
The Malawi Adult Meningitis Score (MAMS) provides a novel tool for predicting prognosis and improving interpretation of acute bacterial meningitis clinical trials by risk stratification in resource-poor settings, a recent study showed.
[email protected]
On Twitter @richpizzi
A great volume of HIV and AIDS research enters the medical literature every month. It’s difficult to monitor everything, so here’s a quick look at some notable news items and journal articles published over the past few weeks.
Immediate antiretroviral therapy reduces the risk of several severe bacterial infections in HIV-positive people with high CD4 cell count, according to a study in The Lancet HIV. The authors said this is partly explained by ART-induced increases in CD4 cell count, but not by increases in neutrophil count.
Postmenopausal status was not associated with a greater risk of unprotected sex in a population of high-risk HIV-positive Kenyan women, a recent study showed.
HIV-infected individuals in South Africa who reported perceived barriers to medical care at diagnosis were more likely to die within 1 year, according to a study in JAIDS.
A study of HIV care among postpartum women in South Africa found evidence of continued care after patients were lost to follow-up, and also identified local and national clinic mobility among women. Researchers said a national health database linked to a unique identifier is necessary to improve reporting and patient care among highly mobile populations.
HBV/HIV coinfection had no adverse influence on main pregnancy outcomes or on HIV viral load suppression in late pregnancy, according to a recent study, but was associated with a significantly reduced CD4 response in pregnancy.
Switching from atazanavir/ritonavir to unboosted atazanavir appears to be safe and effective in selected virologically suppressed HIV-positive patients receiving regimens containing tenofovir disoproxil fumarate (TDF), and may have favorable effects on bilirubin and renal function.
The level of pain is a negative impact on the quality of life of people with HIV/AIDS, according to a study in AIDS Care.
A recent study found children face significant barriers to accessing HIV services in Central Africa, and the HIV epidemic among surviving children in the region has not been adequately evaluated nor addressed.
Less than a quarter of newly HIV-diagnosed patients in Uganda completed antiretroviral therapy assessment, according to a study in HIV Medicine, considerably lower than in other reports from sub-Saharan Africa.
In a study published in Sexually Transmitted Diseases, researchers concluded that current CDC PrEP guidelines should be expanded to incorporate substance use, partner-level, and other syndemic variables that have been shown to contribute to HIV acquisition.
According to a recent review of the Scientific Registry of Transplant Recipients, there was a slight reduction in anti-HBV core antibody positive donor organs from 2005 to 2014, and stable reporting of HCV positive donor organs and HIV positive recipients.
The transmitted drug resistance prevalence in recent HIV infections among notified newly diagnosed HIV patients in Germany was still high (greater than 10%) in 2013 and 2014 and was within the range of other European countries, according to a report in Eurosurveillance.
Higher levels of HIV+ patient engagement in-care are associated with reduced mortality at all stages of infection, including in those who initiate antiretroviral therapy, according to a British study.
A study in the journal AIDS found that HIV/HCV coinfection is associated with the greater homeostasis model assessment of insulin resistance (HOMA-IR), even after researchers controlled for demographic, lifestyle, and metabolic factors.
The Malawi Adult Meningitis Score (MAMS) provides a novel tool for predicting prognosis and improving interpretation of acute bacterial meningitis clinical trials by risk stratification in resource-poor settings, a recent study showed.
[email protected]
On Twitter @richpizzi
Heparan sulfate
The discussion this month focuses on an exciting new ingredient that is showing great promise as a topical antiaging agent. For years we have known that aging skin needs more collagen, elastin, and hyaluronic acid. It is time to add a new player to the antiaging team – heparan sulfate (HS). New studies have shown that lower levels of HS are associated with aged skin. This is what you need to know.
Significance of HS
Endogenous HS, an essential glycosaminoglycan (GAG), contributes to skin development and homeostasis, and thus actively promotes skin health.1 GAGs such as HS and hyaluronic acid are well known as endogenous superhydrators that bind and retain water, thus contributing to skin hydration as well as preserving the structural integrity of collagen and elastin fibers. Specifically, HS and its protein-bound forms (HS proteoglycans such as syndecan, glypican, and perlecan) – the most common constituents on the cell surface and in the extracellular matrix (ECM) – play an important role in cutaneous cell proliferation, migration, communication, and activation as well as collagen fiber development, basement membrane regeneration, granulation tissue formation, and cell adhesion related to wound healing.1 This results from their capacity to bind, store, present, degrade, and amplify growth factors and cytokines.
Accordingly, mature skin would be potently activated by its endogenous growth factors (at lower concentration) as cell signal is amplified (lower threshold of activation) by HS analogs. When used topically, HS analogs appear to promote the formation of healthy and functional ECM, resulting in firmer, more elastic, and stronger skin. Augmenting the amount of HS in the skin may yield a rejuvenating effect by expanding the skin’s ability to hold water and restore cutaneous homeostasis. Studies with an HS analog have demonstrated that the formulation penetrates into skin, enhancing hydration, reducing transepidermal water loss, and improving the appearance of wrinkles and skin tone.1 The use of HS analogs or mimics, known as matrix therapy, has been shown to be effective clinically in cutaneous and corneal healing formulations, exhibiting proof of concept in humans, according to Barritault et al.3
Matrix therapy also has been employed successfully in plastic and aesthetic procedures. Zakine et al. assessed the impact of using ReGeneraTing Agents (RGTA) – biodegradable polymers designed to mimic HS in the ECM of damaged tissue – in 17 patients with breast hypertrophy who underwent mammoplasty. Patients received topical treatment on one breast 1, 4, 8, and 11 days after surgery. The investigators also evaluated a different group of 50 patients after centrofacial lifting. These patients received RGTA drops bilaterally in the treatment area after surgery. Inflammation, hypertrophic scars, and pruritus were noted less frequently for the breasts treated with RGTA. Similarly, in patients receiving a centrofacial lift, scar inflammation, edema, and bruising were significantly less frequent in the treated group (10%), compared with the untreated group (90%).4
In 2015, Gallo et al. reported that 15 patients using a new HS analog formulation in an 8-week study displayed improvement in various skin metrics, including hydration, firmness, elasticity, barrier function, and the appearance of fine lines and wrinkles. The investigators concluded that photodamaged skin may benefit from the use of novel topically applied products containing low-molecular-weight HS.1
The synthetic heparan sulfate Cacipliq20 was reported in 2015 to have been used successfully to treat a chronic lower-extremity ulcer in a 22-year-old male patient with Stewart-Bluefarb syndrome,5 and in 2016 to treat a refractory sickle cell ulcer, with the encouraging outcome demonstrated by complete wound coverage and significant improvement in pain score.6 In 2012, Polieri et al. showed that HS 1% cream was comparable or more effective than glycosaminoglycan-polysulphate gel in alleviating the signs and symptoms of hematomas or subcutaneous hematic extravasations in a study with 96 white men and women.7
Conclusion
Heparan sulfate does appear to be a novel viable antiaging option. Endogenous heparan sulfate is involved in skin defense against pathogens, dehydrated/dried skin, redness, and wound healing. Theoretically, then, HS analogs should be able to modulate these processes. More research is necessary to determine if this is the case, however. In the meantime, HS analogs are extremely well tolerated by all patients and especially those with sensitive skin, which is often the case with aging skin. Further, HS analogs promote skin hydration, providing resistance to compressive forces as well as keeping skin looking healthy. Anecdotally, I can report that I have been using the Senté Dermal Repair Cream in my rosacea patients without any problems. I think HS analogs represent a good option for patients who cannot tolerate retinoids.
Resources
1. J Drugs Dermatol. 2015 Jul;14(7):669-74.
2. Proteoglycans in Skin Aging, in “Textbook of Aging Skin” (Heidelberg, Berlin: Springer-Verlag, pp. 109-120).
3. Joint Bone Spine. 2016 Sep 20. doi: 10.1016/j.jbspin.2016.06.012.
4. Ann Chir Plast Esthet. 2010 Oct;55(5):421-8.
5. Int Wound J. 2015 Apr;12(2):169-72.
6. Int Wound J. 2016 Feb;13(1):35-8.
7. ISRN Orthop. 2012 Jan 17. doi: 10.5402/2012/504151.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever. Dr. Baumann also developed and owns the Baumann Skin Type Solution skin typing systems and related products.
The discussion this month focuses on an exciting new ingredient that is showing great promise as a topical antiaging agent. For years we have known that aging skin needs more collagen, elastin, and hyaluronic acid. It is time to add a new player to the antiaging team – heparan sulfate (HS). New studies have shown that lower levels of HS are associated with aged skin. This is what you need to know.
Significance of HS
Endogenous HS, an essential glycosaminoglycan (GAG), contributes to skin development and homeostasis, and thus actively promotes skin health.1 GAGs such as HS and hyaluronic acid are well known as endogenous superhydrators that bind and retain water, thus contributing to skin hydration as well as preserving the structural integrity of collagen and elastin fibers. Specifically, HS and its protein-bound forms (HS proteoglycans such as syndecan, glypican, and perlecan) – the most common constituents on the cell surface and in the extracellular matrix (ECM) – play an important role in cutaneous cell proliferation, migration, communication, and activation as well as collagen fiber development, basement membrane regeneration, granulation tissue formation, and cell adhesion related to wound healing.1 This results from their capacity to bind, store, present, degrade, and amplify growth factors and cytokines.
Accordingly, mature skin would be potently activated by its endogenous growth factors (at lower concentration) as cell signal is amplified (lower threshold of activation) by HS analogs. When used topically, HS analogs appear to promote the formation of healthy and functional ECM, resulting in firmer, more elastic, and stronger skin. Augmenting the amount of HS in the skin may yield a rejuvenating effect by expanding the skin’s ability to hold water and restore cutaneous homeostasis. Studies with an HS analog have demonstrated that the formulation penetrates into skin, enhancing hydration, reducing transepidermal water loss, and improving the appearance of wrinkles and skin tone.1 The use of HS analogs or mimics, known as matrix therapy, has been shown to be effective clinically in cutaneous and corneal healing formulations, exhibiting proof of concept in humans, according to Barritault et al.3
Matrix therapy also has been employed successfully in plastic and aesthetic procedures. Zakine et al. assessed the impact of using ReGeneraTing Agents (RGTA) – biodegradable polymers designed to mimic HS in the ECM of damaged tissue – in 17 patients with breast hypertrophy who underwent mammoplasty. Patients received topical treatment on one breast 1, 4, 8, and 11 days after surgery. The investigators also evaluated a different group of 50 patients after centrofacial lifting. These patients received RGTA drops bilaterally in the treatment area after surgery. Inflammation, hypertrophic scars, and pruritus were noted less frequently for the breasts treated with RGTA. Similarly, in patients receiving a centrofacial lift, scar inflammation, edema, and bruising were significantly less frequent in the treated group (10%), compared with the untreated group (90%).4
In 2015, Gallo et al. reported that 15 patients using a new HS analog formulation in an 8-week study displayed improvement in various skin metrics, including hydration, firmness, elasticity, barrier function, and the appearance of fine lines and wrinkles. The investigators concluded that photodamaged skin may benefit from the use of novel topically applied products containing low-molecular-weight HS.1
The synthetic heparan sulfate Cacipliq20 was reported in 2015 to have been used successfully to treat a chronic lower-extremity ulcer in a 22-year-old male patient with Stewart-Bluefarb syndrome,5 and in 2016 to treat a refractory sickle cell ulcer, with the encouraging outcome demonstrated by complete wound coverage and significant improvement in pain score.6 In 2012, Polieri et al. showed that HS 1% cream was comparable or more effective than glycosaminoglycan-polysulphate gel in alleviating the signs and symptoms of hematomas or subcutaneous hematic extravasations in a study with 96 white men and women.7
Conclusion
Heparan sulfate does appear to be a novel viable antiaging option. Endogenous heparan sulfate is involved in skin defense against pathogens, dehydrated/dried skin, redness, and wound healing. Theoretically, then, HS analogs should be able to modulate these processes. More research is necessary to determine if this is the case, however. In the meantime, HS analogs are extremely well tolerated by all patients and especially those with sensitive skin, which is often the case with aging skin. Further, HS analogs promote skin hydration, providing resistance to compressive forces as well as keeping skin looking healthy. Anecdotally, I can report that I have been using the Senté Dermal Repair Cream in my rosacea patients without any problems. I think HS analogs represent a good option for patients who cannot tolerate retinoids.
Resources
1. J Drugs Dermatol. 2015 Jul;14(7):669-74.
2. Proteoglycans in Skin Aging, in “Textbook of Aging Skin” (Heidelberg, Berlin: Springer-Verlag, pp. 109-120).
3. Joint Bone Spine. 2016 Sep 20. doi: 10.1016/j.jbspin.2016.06.012.
4. Ann Chir Plast Esthet. 2010 Oct;55(5):421-8.
5. Int Wound J. 2015 Apr;12(2):169-72.
6. Int Wound J. 2016 Feb;13(1):35-8.
7. ISRN Orthop. 2012 Jan 17. doi: 10.5402/2012/504151.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever. Dr. Baumann also developed and owns the Baumann Skin Type Solution skin typing systems and related products.
The discussion this month focuses on an exciting new ingredient that is showing great promise as a topical antiaging agent. For years we have known that aging skin needs more collagen, elastin, and hyaluronic acid. It is time to add a new player to the antiaging team – heparan sulfate (HS). New studies have shown that lower levels of HS are associated with aged skin. This is what you need to know.
Significance of HS
Endogenous HS, an essential glycosaminoglycan (GAG), contributes to skin development and homeostasis, and thus actively promotes skin health.1 GAGs such as HS and hyaluronic acid are well known as endogenous superhydrators that bind and retain water, thus contributing to skin hydration as well as preserving the structural integrity of collagen and elastin fibers. Specifically, HS and its protein-bound forms (HS proteoglycans such as syndecan, glypican, and perlecan) – the most common constituents on the cell surface and in the extracellular matrix (ECM) – play an important role in cutaneous cell proliferation, migration, communication, and activation as well as collagen fiber development, basement membrane regeneration, granulation tissue formation, and cell adhesion related to wound healing.1 This results from their capacity to bind, store, present, degrade, and amplify growth factors and cytokines.
Accordingly, mature skin would be potently activated by its endogenous growth factors (at lower concentration) as cell signal is amplified (lower threshold of activation) by HS analogs. When used topically, HS analogs appear to promote the formation of healthy and functional ECM, resulting in firmer, more elastic, and stronger skin. Augmenting the amount of HS in the skin may yield a rejuvenating effect by expanding the skin’s ability to hold water and restore cutaneous homeostasis. Studies with an HS analog have demonstrated that the formulation penetrates into skin, enhancing hydration, reducing transepidermal water loss, and improving the appearance of wrinkles and skin tone.1 The use of HS analogs or mimics, known as matrix therapy, has been shown to be effective clinically in cutaneous and corneal healing formulations, exhibiting proof of concept in humans, according to Barritault et al.3
Matrix therapy also has been employed successfully in plastic and aesthetic procedures. Zakine et al. assessed the impact of using ReGeneraTing Agents (RGTA) – biodegradable polymers designed to mimic HS in the ECM of damaged tissue – in 17 patients with breast hypertrophy who underwent mammoplasty. Patients received topical treatment on one breast 1, 4, 8, and 11 days after surgery. The investigators also evaluated a different group of 50 patients after centrofacial lifting. These patients received RGTA drops bilaterally in the treatment area after surgery. Inflammation, hypertrophic scars, and pruritus were noted less frequently for the breasts treated with RGTA. Similarly, in patients receiving a centrofacial lift, scar inflammation, edema, and bruising were significantly less frequent in the treated group (10%), compared with the untreated group (90%).4
In 2015, Gallo et al. reported that 15 patients using a new HS analog formulation in an 8-week study displayed improvement in various skin metrics, including hydration, firmness, elasticity, barrier function, and the appearance of fine lines and wrinkles. The investigators concluded that photodamaged skin may benefit from the use of novel topically applied products containing low-molecular-weight HS.1
The synthetic heparan sulfate Cacipliq20 was reported in 2015 to have been used successfully to treat a chronic lower-extremity ulcer in a 22-year-old male patient with Stewart-Bluefarb syndrome,5 and in 2016 to treat a refractory sickle cell ulcer, with the encouraging outcome demonstrated by complete wound coverage and significant improvement in pain score.6 In 2012, Polieri et al. showed that HS 1% cream was comparable or more effective than glycosaminoglycan-polysulphate gel in alleviating the signs and symptoms of hematomas or subcutaneous hematic extravasations in a study with 96 white men and women.7
Conclusion
Heparan sulfate does appear to be a novel viable antiaging option. Endogenous heparan sulfate is involved in skin defense against pathogens, dehydrated/dried skin, redness, and wound healing. Theoretically, then, HS analogs should be able to modulate these processes. More research is necessary to determine if this is the case, however. In the meantime, HS analogs are extremely well tolerated by all patients and especially those with sensitive skin, which is often the case with aging skin. Further, HS analogs promote skin hydration, providing resistance to compressive forces as well as keeping skin looking healthy. Anecdotally, I can report that I have been using the Senté Dermal Repair Cream in my rosacea patients without any problems. I think HS analogs represent a good option for patients who cannot tolerate retinoids.
Resources
1. J Drugs Dermatol. 2015 Jul;14(7):669-74.
2. Proteoglycans in Skin Aging, in “Textbook of Aging Skin” (Heidelberg, Berlin: Springer-Verlag, pp. 109-120).
3. Joint Bone Spine. 2016 Sep 20. doi: 10.1016/j.jbspin.2016.06.012.
4. Ann Chir Plast Esthet. 2010 Oct;55(5):421-8.
5. Int Wound J. 2015 Apr;12(2):169-72.
6. Int Wound J. 2016 Feb;13(1):35-8.
7. ISRN Orthop. 2012 Jan 17. doi: 10.5402/2012/504151.
Dr. Baumann is chief executive officer of the Baumann Cosmetic & Research Institute in the Design District in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote the textbook “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and a book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Her latest book, “Cosmeceuticals and Cosmetic Ingredients,” was published in November 2014. Dr. Baumann has received funding for clinical grants from Allergan, Aveeno, Avon Products, Evolus, Galderma, GlaxoSmithKline, Kythera Biopharmaceuticals, Mary Kay, Medicis Pharmaceuticals, Neutrogena, Philosophy, Topix Pharmaceuticals, and Unilever. Dr. Baumann also developed and owns the Baumann Skin Type Solution skin typing systems and related products.
The Effect of Ligament Injuries on Outcomes of Operatively Treated Distal Radius Fractures
Take-Home Points
- Patients sustaining DRFs commonly have associated ligament injuries and chondral damage as well.
- Many of these associated injuries do not seem to affect outcomes up to 1 year after surgery.
- Plain radiographs have a 74% sensitivity and 73% specificity for detecting intra-articular fractures.
- ”Minor” injuries identified incidentally by arthroscopy during fixation of DRFs may not require dedicated treatment.
- The optimal treatment for high-grade ligament or chondral injuries in patients with DRFs remains incompletely understood.
Distal radius fracture (DRF) is one of the most common upper extremity injuries, with up to 20% to 50% requiring surgical fixation.1 With increasing use of wrist arthroscopy to assist in managing these fractures,2-6 it has become easier to accurately assess concomitant wrist ligament injuries. Reported injury rates are 18% to 86% for the scapholunate interosseous ligament (SLIL),7,8 5% to 29% for the lunotriquetral ligament (LTL),8,9 and 17% to 60% for the triangular fibrocartilage complex (TFCC).10,11 Reported chondral injury rates range from 18% to 60%.7,9,12 Despite the common occurrence of these injuries, it is unclear how they affect outcomes and how aggressively they should be treated when detected during fracture surgery.
As the use of arthroscopy in DRF management becomes more common, surgeons often must decide how to treat ligamentous/chondral injuries incidentally discovered during surgery. To date, only 1 study prospectively evaluated how these injuries affect DRF outcomes,8 though it did not use a validated, patient-based outcome measure.
We conducted a study to address a common clinical scenario: When arthroscopy is used to assist with intra-articular reduction during DRF fixation, how should the surgeon respond to incidentally identified ligament and chondral injuries? Specifically, we wanted to address 3 questions: What is the overall incidence of SLIL, TFCC, and chondral surface injuries in patients undergoing operative fracture fixation? On initial injury films, do any radiographic parameters predict specific soft-tissue injuries or ultimate functional outcomes? Do wrist ligament and chondral injuries affect patient-rated outcomes (disability, pain) and objective measures (range of motion [ROM], grip strength, pinch strength) up to 1 year after fracture surgery?
Materials and Methods
Patient Selection/Population
This observational, prognostic study was approved by our Institutional Review Board. Inclusion criteria were age over 18 years, isolated acute operatively treated DRF (surgery within 14 days of injury), and informed consent. All patients were treated by the same surgeon. Exclusion criteria were open DRF, dorsal shear pattern, fractures requiring dorsal arthrotomy for reduction because of significant intra-articular damage, prior ipsilateral DRF, and prior SLIL or TFCC injury.
Surgery was indicated according to general radiographic parameters as measured on postreduction films: radial height, <8 mm; radial inclination, <15°; positive ulnar variance, >3 mm, or 3 mm more than contralateral side; dorsal tilt, >10°; and volar tilt, >15°. With these parameters within acceptable limits, surgery was also indicated when fractures were deemed unstable and likely to displace because of dorsal tilt >20°, dorsal comminution, intra-articular step-off of ≥2 mm on the posterior-anterior (PA) film, associated ulnar fracture, and age >60 years.13Over a 2-year period, 42 patients (12 male, 30 female) met the inclusion criteria and were enrolled in the study. The dominant arm was affected in 17 patients (40%). Mean (SD) age at time of injury was 56.6 (16.4) years (median, 54 years; range, 20-85 years).
Operative Technique
During surgery, damage to the SLIL, the TFCC, and chondral surfaces (scaphoid, lunate, scaphoid fossa, lunate fossa) and to the intra-articular extension of the DRF was assessed and recorded. Wrist arthroscopy was performed with the 3, 4 portal as the primary portal. When significant damage to the TFCC warranted débridement, the 6R (radial) portal was used as an accessory portal. As a midcarpal portal was not used for SLIL assessment, we used a novel classification system: 0 = no injury, normal-appearing ligament without hemorrhage and smooth transition from scaphoid to lunate surface except for slight concave indentation at the ligament; 1 = attenuation, no visible tear with convex shape of ligament with or without hemorrhage; 2 = partial tear with or without step-off at junction between scaphoid and lunate, but 2.7-mm arthroscope cannot “drive through” to midcarpal joint; and 3 = complete tear with positive “drive-through” sign. TFCC injuries were classified according to the system described by Palmer14: Avulsions were central (1A), ulnar (1B), distal (1C), or radial (1D). The trampoline test was performed through a 6R portal by using a probe to evaluate ligament tension/laxity. In some cases, a 6R portal was deemed unnecessary, and a modified trampoline test was performed—tension/laxity/displacement was evaluated by manually palpating at the fovea and observing TFCC motion with the arthroscope. When appropriate, the TFCC was débrided with a shaver through the 6R portal. In cases of significant instability at the SLIL interval, two 0.062-inch K-wires were placed percutaneously through the scaphoid and lunate, and one was placed from the scaphoid to the capitate.
All DRFs underwent internal fixation with a locked volar plate. When necessary, K-wires and/or a locked radial column plate was used for additional fixation. External fixation was not used. The postoperative protocol began with a dorsal wrist splint placed on the patient in the operating room and worn for 10 to 14 days. At the first postoperative visit, the patient received a removable splint that was to be worn at all times except during showers, therapy, and home exercises. Occupational therapy, initiated the week of the first postoperative visit, consisted of active and passive ROM exercises. At 6 weeks, the splint was removed and strengthening initiated.
Outcome Measures
Our primary outcome measure was the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire at 1 year.15 Secondary outcome measures were visual analog scale (VAS) pain rating, ROM, and radiographic measurements. Patients returned for evaluation 2, 6, 12, 24, and 52 weeks after surgery. At each follow-up visit, the DASH questionnaire and the pain VAS were administered, and ROM and strength were measured. Patient-reported pain was recorded on a standard VAS and measured on a scale from 0 (no pain) to 10 (worst possible pain). Wrist flexion and extension and radioulnar deviation were assessed with a goniometer. Forearm supination and pronation were assessed with the elbow flexed 90° at the patient’s side. Grip strength was measured with a calibrated Jamar dynamometer (Sammons Preston Rolyan), and lateral pinch strength was measured with a hydraulic pinch gauge (Sammons Preston Rolyan). The average of 3 trials for both hands was recorded for all strength measurements.
Radiographs were obtained on presentation. When appropriate, the fracture was manually reduced with a hematoma block, and postreduction radiographs were obtained. Then, radiographs were obtained at each postoperative visit until union. Radial height, radial inclination, tilt, and ulnar variance were measured on preoperative and postoperative radiographs according to standard methods.16 Radiographs were used to classify the fracture patterns according to the AO/ASIF (Arbeitsgemeinschaft für Osteosynthesefragen/Association for the Study of Internal Fixation) classification. Union was determined by radiographic healing, absence of tenderness to palpation, absence of pain with motion, and continued functional improvement.
Data Analysis
To evaluate for relationships between patient injury parameters and outcome measures, we used a 1-way analysis of variance seeking statistically significant differences between groups. Patients were divided into 4 groups: no ligament injuries; isolated SLIL injuries; isolated TFCC injuries; and both SLIL and TFCC injuries. These injury classification categories were then evaluated independently against our chosen outcome measures, which included DASH and VAS pain scores, ROM, and grip/pinch strength.
To determine the optimal sample size, we performed a power analysis to estimate the number of patients required to detect a clinically significant difference in DASH scores at 1 year among the 4 groups. According to the literature, standard deviations of DASH scores in healthy volunteers range from 10 to 15,17 consistent with values found in other recent trials of patients with DRFs.18 The recent literature on DASH construct validity has established a DASH score difference of 19 as representing a disability change being “much better or much worse.”19 As such, power analysis for a 1-way analysis of variance among 4 categories, detecting a DASH score difference of 19 with a standard deviation ranging from 10 to 15, would require 28 to 60 patients to detect a difference with an α of 0.05 and a power of 0.8.
In addition, radiographic parameters at time of injury were compared with injury characteristics to assess for significant relationships. Multivariate linear regression analysis was performed to evaluate radial height, radial inclination, and volar tilt as possible predictors of SLIL injury, TFCC injury, and chondral surface damage. A statistically significant result was defined as a correlation with P < .05.
Results
Of the 42 patients included in the study, 11 (26%) had no ligament injuries, 10 (24%) had isolated SLIL injuries, 12 (29%) had isolated TFCC injuries, and 9 (21%) had injuries to both the SLIL and the TFCC. In addition, in 12 patients (29%), the articular cartilage had visible damage (Table 1).
In all patients, bony union occurred. After union, 1 patient underwent hardware removal for hardware-related pain. The same patient had a dorsal ulnar cutaneous nerve neurolysis at the ulnar styloid fixation site. Another patient developed a partial extensor pollicis longus tear from a prominent dorsal screw tip.
All patients returned for their 2- and 6-week follow-ups. At 1 year, 30 patients (71%) returned for follow-up, 11 could not be contacted, and 1 was removed because of an olecranon fracture from a subsequent fall.
Regarding the primary outcome measure, mean DASH score at 1-year follow-up was 30.8 for the group without injuries, 10.8 for the group with SLIL injuries, 14.7 for the group with TFCC injuries, and 21.9 for the group with SLIL and TFCC injuries (Table 2).
Radiographic parameters were restored to acceptable limits in all patients (Table 3).
Discussion
Use of wrist arthroscopy in DRF management has allowed assessment of the incidence of intra-articular injuries, including ligament and chondral surface injuries. Although the literature on the incidence of these injuries has been expanding, their clinical significance remains unclear.
Authors have postulated that some patients do not do well after DRF repair because of undetected ligament injuries. With the current trend of internal fixation, locked plating, and early motion—contrasting with older trends of prolonged immobilization in a cast or external fixation—concerns have been raised that early mobilization results in inadequate treatment of ligament injuries. However, data from the present study suggest no significant morbidity from early mobilization despite the presence of ligament injuries in more than half of all operatively treated DRFs. It is possible morbidity was not appreciated, as most patients with DRFs end up with some stiffness, which masks the effects of ligament injuries during healing.
We found no correlation between injury radiographic parameters, observed soft-tissue injuries, or final subjective outcomes. Interestingly, in this study, there was some discordance between the appearance of intra-articular fractures on radiographs and the direct arthroscopic observation of intra-articular fracture extension. With the present data and with arthroscopic visualization as the gold standard, radiographs had 74% sensitivity and 73% specificity for detecting intra-articular fractures (the corresponding positive predictive value was 83%, and the negative predictive value was 61%). As we typically rely on radiographs as the primary tool in assessing the articular component of a fracture, these results should be taken into account when basing management decisions exclusively on static injury films.
Observational studies of arthroscopy in DRFs have revealed a wide range of injury rates: For SLILs, the average injury rate was 44%; for LTLs, 13%; for TFCCs, 43%; and for chondral surfaces, 32% (Table 4).
This study had several limitations, including loss to follow-up at the primary endpoint (we were unable to contact 29% of patients). In addition, because of resource limitations, we were able to enroll only a limited number of patients, and as a result were able to power the study to detect only major effects on DASH scores. Therefore, although our 32 patients with long-term follow-up are within the range dictated by the power analysis, this study was not powered to capture more subtle differences in disability. Furthermore, because we used 1 year as the longest follow-up point, the long-term sequelae (eg, arthritis) of these injuries may not have been captured. Last, despite the high incidence of soft-tissue injuries overall, the number of patients with severe ligament injuries was relatively low, which makes it difficult to make definitive statements about their contribution to outcomes. A likely explanation is that patients with high-energy injuries and significant intra-articular displacement requiring open arthrotomies were excluded.
At 1-year follow-up, with use of DASH as the gold standard for disability, we found no major difference in subjective or objective outcome measures between patients with and without ligament injuries. Radiographs did not predict soft-tissue injury or ultimate outcome. Rates of ligament injuries in our operatively treated DRFs were similar to those in the literature. Overall, these findings suggest that “minor” injuries incidentally discovered with arthroscopy during DRF surgery may not have a significant effect on outcomes, with the caveat that the significance of very severe injuries (eg, Geissler grade 4 injuries with frank scapholunate diastasis) remains incompletely understood. The decision by the treating surgeon to perform arthroscopy and/or to repair soft-tissue injuries should be made on a case-by-case basis.
Am J Orthop. 2017;46(1):E41-E46. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Róbertsson GO, Jónsson GT, Sigurjónsson K. Epidemiology of distal radius fractures in Iceland in 1985. Acta Orthop Scand. 1990;61(5):457-459.
2. Geissler WB. Arthroscopically assisted reduction of intra-articular fractures of the distal radius. Hand Clin. 1995;11(1):19-29.
3. Trybus M, Guzik P. The economic impact of hand injury [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2003;68(4):269-273.
4. Wolfe SW, Easterling KJ, Yoo HH. Arthroscopic-assisted reduction of distal radius fractures. Arthroscopy. 1995;11(6):706-714.
5. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
6. Doi K, Hattori Y, Otsuka K, Abe Y, Yamamoto H. Intra-articular fractures of the distal aspect of the radius: arthroscopically assisted reduction compared with open reduction and internal fixation. J Bone Joint Surg Am. 1999;81(8):1093-1110.
7. Shih JT, Lee HM, Hou YT, Tan CM. Arthroscopically-assisted reduction of intra-articular fractures and soft tissue management of distal radius. Hand Surg. 2001;6(2):127-135.
8. Forward DP, Lindau TR, Melsom DS. Intercarpal ligament injuries associated with fractures of the distal part of the radius. J Bone Joint Surg Am. 2007;89(11):2334-2340.
9. Espinosa-Gutiérrez A, Rivas-Montero JA, Elias-Escobedo A, Alisedo-Ochoa PG. Wrist arthroscopy for fractures of the distal end of the radius [in Spanish]. Acta Ortop Mex. 2009;23(6):358-365.
10. Hardy P, Gomes N, Chebil M, Bauer T. Wrist arthroscopy and intra-articular fractures of the distal radius in young adults. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1225-1230.
11. Varitimidis SE, Basdekis GK, Dailiana ZH, Hantes ME, Bargiotas K, Malizos K. Treatment of intra-articular fractures of the distal radius: fluoroscopic or arthroscopic reduction? J Bone Joint Surg Br. 2008;90(6):778-785.
12. Kordasiewicz B, Pomianowski S, Rylski W, Antolak L, Marczak D. Intraarticular distal radius fractures—arthroscopic assessment of injuries [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2006;71(2):113-116.
13. Lafontaine M, Hardy D, Delince P. Stability assessment of distal radius fractures. Injury. 1989;20(4):208-210.
14. Palmer AK. Triangular fibrocartilage complex lesions: a classification. J Hand Surg Am. 1989;14(4):594-606.
15. Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (Disabilities of the Arm, Shoulder and Hand) [corrected]. The Upper Extremity Collaborative Group (UECG) [published correction appears in Am J Ind Med. 1996;30(3):372]. Am J Ind Med. 1996;29(6):602-608.
16. Fernandez DL, Jupiter JB. Fractures of the Distal Radius: A Practical Approach to Management. New York, NY: Springer; 1996.
17. Jester A, Harth A, Wind G, Germann G, Sauerbier M. Does the Disability of Shoulder, Arm and Hand questionnaire (DASH) replace grip strength and range of motion in outcome-evaluation? [in German]. Handchir Mikrochir Plast Chir. 2005;37(2):126-130.
18. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
19. Gummesson C, Atroshi I, Ekdahl C. The Disabilities of the Arm, Shoulder and Hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-rated health change after surgery. BMC Musculoskelet Disord. 2003;4:11.
20. Richards RS, Bennett JD, Roth JH, Milne K Jr. Arthroscopic diagnosis of intra-articular soft tissue injuries associated with distal radial fractures. J Hand Surg Am. 1997;22(5):772-776.
21. Peicha G, Seibert F, Fellinger M, Grechenig W. Midterm results of arthroscopic treatment of scapholunate ligament lesions associated with intra-articular distal radius fractures. Knee Surg Sports Traumatol Arthrosc. 1999;7(5):327-333.
22. Schädel-Höpfner M, Böhringer G, Junge A, Celik I, Gotzen L. [Arthroscopic diagnosis of concomitant scapholunate ligament injuries in fractures of the distal radius]. Handchir Mikrochir Plast Chir. 2001;33(4):229-233.
23. Ruch DS, Yang CC, Smith BP. Results of acute arthroscopically repaired triangular fibrocartilage complex injuries associated with intra-articular distal radius fractures. Arthroscopy. 2003;19(5):511-516.
24. Hattori Y, Doi K, Estrella EP, Chen G. Arthroscopically assisted reduction with volar plating or external fixation for displaced intra-articular fractures of the distal radius in the elderly patients. Hand Surg. 2007;12(1):1-12.
25. Hohendorff B, Eck M, Mühldorfer M, Fodor S, Schmitt R, Prommersberger KJ. [Palmar wrist arthroscopy for evaluation of concomitant carpal lesions in operative treatment of distal intraarticular radius fractures]. Handchir Mikrochir Plast Chir. 2009;41(5):295-299.
Take-Home Points
- Patients sustaining DRFs commonly have associated ligament injuries and chondral damage as well.
- Many of these associated injuries do not seem to affect outcomes up to 1 year after surgery.
- Plain radiographs have a 74% sensitivity and 73% specificity for detecting intra-articular fractures.
- ”Minor” injuries identified incidentally by arthroscopy during fixation of DRFs may not require dedicated treatment.
- The optimal treatment for high-grade ligament or chondral injuries in patients with DRFs remains incompletely understood.
Distal radius fracture (DRF) is one of the most common upper extremity injuries, with up to 20% to 50% requiring surgical fixation.1 With increasing use of wrist arthroscopy to assist in managing these fractures,2-6 it has become easier to accurately assess concomitant wrist ligament injuries. Reported injury rates are 18% to 86% for the scapholunate interosseous ligament (SLIL),7,8 5% to 29% for the lunotriquetral ligament (LTL),8,9 and 17% to 60% for the triangular fibrocartilage complex (TFCC).10,11 Reported chondral injury rates range from 18% to 60%.7,9,12 Despite the common occurrence of these injuries, it is unclear how they affect outcomes and how aggressively they should be treated when detected during fracture surgery.
As the use of arthroscopy in DRF management becomes more common, surgeons often must decide how to treat ligamentous/chondral injuries incidentally discovered during surgery. To date, only 1 study prospectively evaluated how these injuries affect DRF outcomes,8 though it did not use a validated, patient-based outcome measure.
We conducted a study to address a common clinical scenario: When arthroscopy is used to assist with intra-articular reduction during DRF fixation, how should the surgeon respond to incidentally identified ligament and chondral injuries? Specifically, we wanted to address 3 questions: What is the overall incidence of SLIL, TFCC, and chondral surface injuries in patients undergoing operative fracture fixation? On initial injury films, do any radiographic parameters predict specific soft-tissue injuries or ultimate functional outcomes? Do wrist ligament and chondral injuries affect patient-rated outcomes (disability, pain) and objective measures (range of motion [ROM], grip strength, pinch strength) up to 1 year after fracture surgery?
Materials and Methods
Patient Selection/Population
This observational, prognostic study was approved by our Institutional Review Board. Inclusion criteria were age over 18 years, isolated acute operatively treated DRF (surgery within 14 days of injury), and informed consent. All patients were treated by the same surgeon. Exclusion criteria were open DRF, dorsal shear pattern, fractures requiring dorsal arthrotomy for reduction because of significant intra-articular damage, prior ipsilateral DRF, and prior SLIL or TFCC injury.
Surgery was indicated according to general radiographic parameters as measured on postreduction films: radial height, <8 mm; radial inclination, <15°; positive ulnar variance, >3 mm, or 3 mm more than contralateral side; dorsal tilt, >10°; and volar tilt, >15°. With these parameters within acceptable limits, surgery was also indicated when fractures were deemed unstable and likely to displace because of dorsal tilt >20°, dorsal comminution, intra-articular step-off of ≥2 mm on the posterior-anterior (PA) film, associated ulnar fracture, and age >60 years.13Over a 2-year period, 42 patients (12 male, 30 female) met the inclusion criteria and were enrolled in the study. The dominant arm was affected in 17 patients (40%). Mean (SD) age at time of injury was 56.6 (16.4) years (median, 54 years; range, 20-85 years).
Operative Technique
During surgery, damage to the SLIL, the TFCC, and chondral surfaces (scaphoid, lunate, scaphoid fossa, lunate fossa) and to the intra-articular extension of the DRF was assessed and recorded. Wrist arthroscopy was performed with the 3, 4 portal as the primary portal. When significant damage to the TFCC warranted débridement, the 6R (radial) portal was used as an accessory portal. As a midcarpal portal was not used for SLIL assessment, we used a novel classification system: 0 = no injury, normal-appearing ligament without hemorrhage and smooth transition from scaphoid to lunate surface except for slight concave indentation at the ligament; 1 = attenuation, no visible tear with convex shape of ligament with or without hemorrhage; 2 = partial tear with or without step-off at junction between scaphoid and lunate, but 2.7-mm arthroscope cannot “drive through” to midcarpal joint; and 3 = complete tear with positive “drive-through” sign. TFCC injuries were classified according to the system described by Palmer14: Avulsions were central (1A), ulnar (1B), distal (1C), or radial (1D). The trampoline test was performed through a 6R portal by using a probe to evaluate ligament tension/laxity. In some cases, a 6R portal was deemed unnecessary, and a modified trampoline test was performed—tension/laxity/displacement was evaluated by manually palpating at the fovea and observing TFCC motion with the arthroscope. When appropriate, the TFCC was débrided with a shaver through the 6R portal. In cases of significant instability at the SLIL interval, two 0.062-inch K-wires were placed percutaneously through the scaphoid and lunate, and one was placed from the scaphoid to the capitate.
All DRFs underwent internal fixation with a locked volar plate. When necessary, K-wires and/or a locked radial column plate was used for additional fixation. External fixation was not used. The postoperative protocol began with a dorsal wrist splint placed on the patient in the operating room and worn for 10 to 14 days. At the first postoperative visit, the patient received a removable splint that was to be worn at all times except during showers, therapy, and home exercises. Occupational therapy, initiated the week of the first postoperative visit, consisted of active and passive ROM exercises. At 6 weeks, the splint was removed and strengthening initiated.
Outcome Measures
Our primary outcome measure was the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire at 1 year.15 Secondary outcome measures were visual analog scale (VAS) pain rating, ROM, and radiographic measurements. Patients returned for evaluation 2, 6, 12, 24, and 52 weeks after surgery. At each follow-up visit, the DASH questionnaire and the pain VAS were administered, and ROM and strength were measured. Patient-reported pain was recorded on a standard VAS and measured on a scale from 0 (no pain) to 10 (worst possible pain). Wrist flexion and extension and radioulnar deviation were assessed with a goniometer. Forearm supination and pronation were assessed with the elbow flexed 90° at the patient’s side. Grip strength was measured with a calibrated Jamar dynamometer (Sammons Preston Rolyan), and lateral pinch strength was measured with a hydraulic pinch gauge (Sammons Preston Rolyan). The average of 3 trials for both hands was recorded for all strength measurements.
Radiographs were obtained on presentation. When appropriate, the fracture was manually reduced with a hematoma block, and postreduction radiographs were obtained. Then, radiographs were obtained at each postoperative visit until union. Radial height, radial inclination, tilt, and ulnar variance were measured on preoperative and postoperative radiographs according to standard methods.16 Radiographs were used to classify the fracture patterns according to the AO/ASIF (Arbeitsgemeinschaft für Osteosynthesefragen/Association for the Study of Internal Fixation) classification. Union was determined by radiographic healing, absence of tenderness to palpation, absence of pain with motion, and continued functional improvement.
Data Analysis
To evaluate for relationships between patient injury parameters and outcome measures, we used a 1-way analysis of variance seeking statistically significant differences between groups. Patients were divided into 4 groups: no ligament injuries; isolated SLIL injuries; isolated TFCC injuries; and both SLIL and TFCC injuries. These injury classification categories were then evaluated independently against our chosen outcome measures, which included DASH and VAS pain scores, ROM, and grip/pinch strength.
To determine the optimal sample size, we performed a power analysis to estimate the number of patients required to detect a clinically significant difference in DASH scores at 1 year among the 4 groups. According to the literature, standard deviations of DASH scores in healthy volunteers range from 10 to 15,17 consistent with values found in other recent trials of patients with DRFs.18 The recent literature on DASH construct validity has established a DASH score difference of 19 as representing a disability change being “much better or much worse.”19 As such, power analysis for a 1-way analysis of variance among 4 categories, detecting a DASH score difference of 19 with a standard deviation ranging from 10 to 15, would require 28 to 60 patients to detect a difference with an α of 0.05 and a power of 0.8.
In addition, radiographic parameters at time of injury were compared with injury characteristics to assess for significant relationships. Multivariate linear regression analysis was performed to evaluate radial height, radial inclination, and volar tilt as possible predictors of SLIL injury, TFCC injury, and chondral surface damage. A statistically significant result was defined as a correlation with P < .05.
Results
Of the 42 patients included in the study, 11 (26%) had no ligament injuries, 10 (24%) had isolated SLIL injuries, 12 (29%) had isolated TFCC injuries, and 9 (21%) had injuries to both the SLIL and the TFCC. In addition, in 12 patients (29%), the articular cartilage had visible damage (Table 1).
In all patients, bony union occurred. After union, 1 patient underwent hardware removal for hardware-related pain. The same patient had a dorsal ulnar cutaneous nerve neurolysis at the ulnar styloid fixation site. Another patient developed a partial extensor pollicis longus tear from a prominent dorsal screw tip.
All patients returned for their 2- and 6-week follow-ups. At 1 year, 30 patients (71%) returned for follow-up, 11 could not be contacted, and 1 was removed because of an olecranon fracture from a subsequent fall.
Regarding the primary outcome measure, mean DASH score at 1-year follow-up was 30.8 for the group without injuries, 10.8 for the group with SLIL injuries, 14.7 for the group with TFCC injuries, and 21.9 for the group with SLIL and TFCC injuries (Table 2).
Radiographic parameters were restored to acceptable limits in all patients (Table 3).
Discussion
Use of wrist arthroscopy in DRF management has allowed assessment of the incidence of intra-articular injuries, including ligament and chondral surface injuries. Although the literature on the incidence of these injuries has been expanding, their clinical significance remains unclear.
Authors have postulated that some patients do not do well after DRF repair because of undetected ligament injuries. With the current trend of internal fixation, locked plating, and early motion—contrasting with older trends of prolonged immobilization in a cast or external fixation—concerns have been raised that early mobilization results in inadequate treatment of ligament injuries. However, data from the present study suggest no significant morbidity from early mobilization despite the presence of ligament injuries in more than half of all operatively treated DRFs. It is possible morbidity was not appreciated, as most patients with DRFs end up with some stiffness, which masks the effects of ligament injuries during healing.
We found no correlation between injury radiographic parameters, observed soft-tissue injuries, or final subjective outcomes. Interestingly, in this study, there was some discordance between the appearance of intra-articular fractures on radiographs and the direct arthroscopic observation of intra-articular fracture extension. With the present data and with arthroscopic visualization as the gold standard, radiographs had 74% sensitivity and 73% specificity for detecting intra-articular fractures (the corresponding positive predictive value was 83%, and the negative predictive value was 61%). As we typically rely on radiographs as the primary tool in assessing the articular component of a fracture, these results should be taken into account when basing management decisions exclusively on static injury films.
Observational studies of arthroscopy in DRFs have revealed a wide range of injury rates: For SLILs, the average injury rate was 44%; for LTLs, 13%; for TFCCs, 43%; and for chondral surfaces, 32% (Table 4).
This study had several limitations, including loss to follow-up at the primary endpoint (we were unable to contact 29% of patients). In addition, because of resource limitations, we were able to enroll only a limited number of patients, and as a result were able to power the study to detect only major effects on DASH scores. Therefore, although our 32 patients with long-term follow-up are within the range dictated by the power analysis, this study was not powered to capture more subtle differences in disability. Furthermore, because we used 1 year as the longest follow-up point, the long-term sequelae (eg, arthritis) of these injuries may not have been captured. Last, despite the high incidence of soft-tissue injuries overall, the number of patients with severe ligament injuries was relatively low, which makes it difficult to make definitive statements about their contribution to outcomes. A likely explanation is that patients with high-energy injuries and significant intra-articular displacement requiring open arthrotomies were excluded.
At 1-year follow-up, with use of DASH as the gold standard for disability, we found no major difference in subjective or objective outcome measures between patients with and without ligament injuries. Radiographs did not predict soft-tissue injury or ultimate outcome. Rates of ligament injuries in our operatively treated DRFs were similar to those in the literature. Overall, these findings suggest that “minor” injuries incidentally discovered with arthroscopy during DRF surgery may not have a significant effect on outcomes, with the caveat that the significance of very severe injuries (eg, Geissler grade 4 injuries with frank scapholunate diastasis) remains incompletely understood. The decision by the treating surgeon to perform arthroscopy and/or to repair soft-tissue injuries should be made on a case-by-case basis.
Am J Orthop. 2017;46(1):E41-E46. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
Take-Home Points
- Patients sustaining DRFs commonly have associated ligament injuries and chondral damage as well.
- Many of these associated injuries do not seem to affect outcomes up to 1 year after surgery.
- Plain radiographs have a 74% sensitivity and 73% specificity for detecting intra-articular fractures.
- ”Minor” injuries identified incidentally by arthroscopy during fixation of DRFs may not require dedicated treatment.
- The optimal treatment for high-grade ligament or chondral injuries in patients with DRFs remains incompletely understood.
Distal radius fracture (DRF) is one of the most common upper extremity injuries, with up to 20% to 50% requiring surgical fixation.1 With increasing use of wrist arthroscopy to assist in managing these fractures,2-6 it has become easier to accurately assess concomitant wrist ligament injuries. Reported injury rates are 18% to 86% for the scapholunate interosseous ligament (SLIL),7,8 5% to 29% for the lunotriquetral ligament (LTL),8,9 and 17% to 60% for the triangular fibrocartilage complex (TFCC).10,11 Reported chondral injury rates range from 18% to 60%.7,9,12 Despite the common occurrence of these injuries, it is unclear how they affect outcomes and how aggressively they should be treated when detected during fracture surgery.
As the use of arthroscopy in DRF management becomes more common, surgeons often must decide how to treat ligamentous/chondral injuries incidentally discovered during surgery. To date, only 1 study prospectively evaluated how these injuries affect DRF outcomes,8 though it did not use a validated, patient-based outcome measure.
We conducted a study to address a common clinical scenario: When arthroscopy is used to assist with intra-articular reduction during DRF fixation, how should the surgeon respond to incidentally identified ligament and chondral injuries? Specifically, we wanted to address 3 questions: What is the overall incidence of SLIL, TFCC, and chondral surface injuries in patients undergoing operative fracture fixation? On initial injury films, do any radiographic parameters predict specific soft-tissue injuries or ultimate functional outcomes? Do wrist ligament and chondral injuries affect patient-rated outcomes (disability, pain) and objective measures (range of motion [ROM], grip strength, pinch strength) up to 1 year after fracture surgery?
Materials and Methods
Patient Selection/Population
This observational, prognostic study was approved by our Institutional Review Board. Inclusion criteria were age over 18 years, isolated acute operatively treated DRF (surgery within 14 days of injury), and informed consent. All patients were treated by the same surgeon. Exclusion criteria were open DRF, dorsal shear pattern, fractures requiring dorsal arthrotomy for reduction because of significant intra-articular damage, prior ipsilateral DRF, and prior SLIL or TFCC injury.
Surgery was indicated according to general radiographic parameters as measured on postreduction films: radial height, <8 mm; radial inclination, <15°; positive ulnar variance, >3 mm, or 3 mm more than contralateral side; dorsal tilt, >10°; and volar tilt, >15°. With these parameters within acceptable limits, surgery was also indicated when fractures were deemed unstable and likely to displace because of dorsal tilt >20°, dorsal comminution, intra-articular step-off of ≥2 mm on the posterior-anterior (PA) film, associated ulnar fracture, and age >60 years.13Over a 2-year period, 42 patients (12 male, 30 female) met the inclusion criteria and were enrolled in the study. The dominant arm was affected in 17 patients (40%). Mean (SD) age at time of injury was 56.6 (16.4) years (median, 54 years; range, 20-85 years).
Operative Technique
During surgery, damage to the SLIL, the TFCC, and chondral surfaces (scaphoid, lunate, scaphoid fossa, lunate fossa) and to the intra-articular extension of the DRF was assessed and recorded. Wrist arthroscopy was performed with the 3, 4 portal as the primary portal. When significant damage to the TFCC warranted débridement, the 6R (radial) portal was used as an accessory portal. As a midcarpal portal was not used for SLIL assessment, we used a novel classification system: 0 = no injury, normal-appearing ligament without hemorrhage and smooth transition from scaphoid to lunate surface except for slight concave indentation at the ligament; 1 = attenuation, no visible tear with convex shape of ligament with or without hemorrhage; 2 = partial tear with or without step-off at junction between scaphoid and lunate, but 2.7-mm arthroscope cannot “drive through” to midcarpal joint; and 3 = complete tear with positive “drive-through” sign. TFCC injuries were classified according to the system described by Palmer14: Avulsions were central (1A), ulnar (1B), distal (1C), or radial (1D). The trampoline test was performed through a 6R portal by using a probe to evaluate ligament tension/laxity. In some cases, a 6R portal was deemed unnecessary, and a modified trampoline test was performed—tension/laxity/displacement was evaluated by manually palpating at the fovea and observing TFCC motion with the arthroscope. When appropriate, the TFCC was débrided with a shaver through the 6R portal. In cases of significant instability at the SLIL interval, two 0.062-inch K-wires were placed percutaneously through the scaphoid and lunate, and one was placed from the scaphoid to the capitate.
All DRFs underwent internal fixation with a locked volar plate. When necessary, K-wires and/or a locked radial column plate was used for additional fixation. External fixation was not used. The postoperative protocol began with a dorsal wrist splint placed on the patient in the operating room and worn for 10 to 14 days. At the first postoperative visit, the patient received a removable splint that was to be worn at all times except during showers, therapy, and home exercises. Occupational therapy, initiated the week of the first postoperative visit, consisted of active and passive ROM exercises. At 6 weeks, the splint was removed and strengthening initiated.
Outcome Measures
Our primary outcome measure was the Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire at 1 year.15 Secondary outcome measures were visual analog scale (VAS) pain rating, ROM, and radiographic measurements. Patients returned for evaluation 2, 6, 12, 24, and 52 weeks after surgery. At each follow-up visit, the DASH questionnaire and the pain VAS were administered, and ROM and strength were measured. Patient-reported pain was recorded on a standard VAS and measured on a scale from 0 (no pain) to 10 (worst possible pain). Wrist flexion and extension and radioulnar deviation were assessed with a goniometer. Forearm supination and pronation were assessed with the elbow flexed 90° at the patient’s side. Grip strength was measured with a calibrated Jamar dynamometer (Sammons Preston Rolyan), and lateral pinch strength was measured with a hydraulic pinch gauge (Sammons Preston Rolyan). The average of 3 trials for both hands was recorded for all strength measurements.
Radiographs were obtained on presentation. When appropriate, the fracture was manually reduced with a hematoma block, and postreduction radiographs were obtained. Then, radiographs were obtained at each postoperative visit until union. Radial height, radial inclination, tilt, and ulnar variance were measured on preoperative and postoperative radiographs according to standard methods.16 Radiographs were used to classify the fracture patterns according to the AO/ASIF (Arbeitsgemeinschaft für Osteosynthesefragen/Association for the Study of Internal Fixation) classification. Union was determined by radiographic healing, absence of tenderness to palpation, absence of pain with motion, and continued functional improvement.
Data Analysis
To evaluate for relationships between patient injury parameters and outcome measures, we used a 1-way analysis of variance seeking statistically significant differences between groups. Patients were divided into 4 groups: no ligament injuries; isolated SLIL injuries; isolated TFCC injuries; and both SLIL and TFCC injuries. These injury classification categories were then evaluated independently against our chosen outcome measures, which included DASH and VAS pain scores, ROM, and grip/pinch strength.
To determine the optimal sample size, we performed a power analysis to estimate the number of patients required to detect a clinically significant difference in DASH scores at 1 year among the 4 groups. According to the literature, standard deviations of DASH scores in healthy volunteers range from 10 to 15,17 consistent with values found in other recent trials of patients with DRFs.18 The recent literature on DASH construct validity has established a DASH score difference of 19 as representing a disability change being “much better or much worse.”19 As such, power analysis for a 1-way analysis of variance among 4 categories, detecting a DASH score difference of 19 with a standard deviation ranging from 10 to 15, would require 28 to 60 patients to detect a difference with an α of 0.05 and a power of 0.8.
In addition, radiographic parameters at time of injury were compared with injury characteristics to assess for significant relationships. Multivariate linear regression analysis was performed to evaluate radial height, radial inclination, and volar tilt as possible predictors of SLIL injury, TFCC injury, and chondral surface damage. A statistically significant result was defined as a correlation with P < .05.
Results
Of the 42 patients included in the study, 11 (26%) had no ligament injuries, 10 (24%) had isolated SLIL injuries, 12 (29%) had isolated TFCC injuries, and 9 (21%) had injuries to both the SLIL and the TFCC. In addition, in 12 patients (29%), the articular cartilage had visible damage (Table 1).
In all patients, bony union occurred. After union, 1 patient underwent hardware removal for hardware-related pain. The same patient had a dorsal ulnar cutaneous nerve neurolysis at the ulnar styloid fixation site. Another patient developed a partial extensor pollicis longus tear from a prominent dorsal screw tip.
All patients returned for their 2- and 6-week follow-ups. At 1 year, 30 patients (71%) returned for follow-up, 11 could not be contacted, and 1 was removed because of an olecranon fracture from a subsequent fall.
Regarding the primary outcome measure, mean DASH score at 1-year follow-up was 30.8 for the group without injuries, 10.8 for the group with SLIL injuries, 14.7 for the group with TFCC injuries, and 21.9 for the group with SLIL and TFCC injuries (Table 2).
Radiographic parameters were restored to acceptable limits in all patients (Table 3).
Discussion
Use of wrist arthroscopy in DRF management has allowed assessment of the incidence of intra-articular injuries, including ligament and chondral surface injuries. Although the literature on the incidence of these injuries has been expanding, their clinical significance remains unclear.
Authors have postulated that some patients do not do well after DRF repair because of undetected ligament injuries. With the current trend of internal fixation, locked plating, and early motion—contrasting with older trends of prolonged immobilization in a cast or external fixation—concerns have been raised that early mobilization results in inadequate treatment of ligament injuries. However, data from the present study suggest no significant morbidity from early mobilization despite the presence of ligament injuries in more than half of all operatively treated DRFs. It is possible morbidity was not appreciated, as most patients with DRFs end up with some stiffness, which masks the effects of ligament injuries during healing.
We found no correlation between injury radiographic parameters, observed soft-tissue injuries, or final subjective outcomes. Interestingly, in this study, there was some discordance between the appearance of intra-articular fractures on radiographs and the direct arthroscopic observation of intra-articular fracture extension. With the present data and with arthroscopic visualization as the gold standard, radiographs had 74% sensitivity and 73% specificity for detecting intra-articular fractures (the corresponding positive predictive value was 83%, and the negative predictive value was 61%). As we typically rely on radiographs as the primary tool in assessing the articular component of a fracture, these results should be taken into account when basing management decisions exclusively on static injury films.
Observational studies of arthroscopy in DRFs have revealed a wide range of injury rates: For SLILs, the average injury rate was 44%; for LTLs, 13%; for TFCCs, 43%; and for chondral surfaces, 32% (Table 4).
This study had several limitations, including loss to follow-up at the primary endpoint (we were unable to contact 29% of patients). In addition, because of resource limitations, we were able to enroll only a limited number of patients, and as a result were able to power the study to detect only major effects on DASH scores. Therefore, although our 32 patients with long-term follow-up are within the range dictated by the power analysis, this study was not powered to capture more subtle differences in disability. Furthermore, because we used 1 year as the longest follow-up point, the long-term sequelae (eg, arthritis) of these injuries may not have been captured. Last, despite the high incidence of soft-tissue injuries overall, the number of patients with severe ligament injuries was relatively low, which makes it difficult to make definitive statements about their contribution to outcomes. A likely explanation is that patients with high-energy injuries and significant intra-articular displacement requiring open arthrotomies were excluded.
At 1-year follow-up, with use of DASH as the gold standard for disability, we found no major difference in subjective or objective outcome measures between patients with and without ligament injuries. Radiographs did not predict soft-tissue injury or ultimate outcome. Rates of ligament injuries in our operatively treated DRFs were similar to those in the literature. Overall, these findings suggest that “minor” injuries incidentally discovered with arthroscopy during DRF surgery may not have a significant effect on outcomes, with the caveat that the significance of very severe injuries (eg, Geissler grade 4 injuries with frank scapholunate diastasis) remains incompletely understood. The decision by the treating surgeon to perform arthroscopy and/or to repair soft-tissue injuries should be made on a case-by-case basis.
Am J Orthop. 2017;46(1):E41-E46. Copyright Frontline Medical Communications Inc. 2017. All rights reserved.
1. Róbertsson GO, Jónsson GT, Sigurjónsson K. Epidemiology of distal radius fractures in Iceland in 1985. Acta Orthop Scand. 1990;61(5):457-459.
2. Geissler WB. Arthroscopically assisted reduction of intra-articular fractures of the distal radius. Hand Clin. 1995;11(1):19-29.
3. Trybus M, Guzik P. The economic impact of hand injury [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2003;68(4):269-273.
4. Wolfe SW, Easterling KJ, Yoo HH. Arthroscopic-assisted reduction of distal radius fractures. Arthroscopy. 1995;11(6):706-714.
5. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
6. Doi K, Hattori Y, Otsuka K, Abe Y, Yamamoto H. Intra-articular fractures of the distal aspect of the radius: arthroscopically assisted reduction compared with open reduction and internal fixation. J Bone Joint Surg Am. 1999;81(8):1093-1110.
7. Shih JT, Lee HM, Hou YT, Tan CM. Arthroscopically-assisted reduction of intra-articular fractures and soft tissue management of distal radius. Hand Surg. 2001;6(2):127-135.
8. Forward DP, Lindau TR, Melsom DS. Intercarpal ligament injuries associated with fractures of the distal part of the radius. J Bone Joint Surg Am. 2007;89(11):2334-2340.
9. Espinosa-Gutiérrez A, Rivas-Montero JA, Elias-Escobedo A, Alisedo-Ochoa PG. Wrist arthroscopy for fractures of the distal end of the radius [in Spanish]. Acta Ortop Mex. 2009;23(6):358-365.
10. Hardy P, Gomes N, Chebil M, Bauer T. Wrist arthroscopy and intra-articular fractures of the distal radius in young adults. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1225-1230.
11. Varitimidis SE, Basdekis GK, Dailiana ZH, Hantes ME, Bargiotas K, Malizos K. Treatment of intra-articular fractures of the distal radius: fluoroscopic or arthroscopic reduction? J Bone Joint Surg Br. 2008;90(6):778-785.
12. Kordasiewicz B, Pomianowski S, Rylski W, Antolak L, Marczak D. Intraarticular distal radius fractures—arthroscopic assessment of injuries [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2006;71(2):113-116.
13. Lafontaine M, Hardy D, Delince P. Stability assessment of distal radius fractures. Injury. 1989;20(4):208-210.
14. Palmer AK. Triangular fibrocartilage complex lesions: a classification. J Hand Surg Am. 1989;14(4):594-606.
15. Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (Disabilities of the Arm, Shoulder and Hand) [corrected]. The Upper Extremity Collaborative Group (UECG) [published correction appears in Am J Ind Med. 1996;30(3):372]. Am J Ind Med. 1996;29(6):602-608.
16. Fernandez DL, Jupiter JB. Fractures of the Distal Radius: A Practical Approach to Management. New York, NY: Springer; 1996.
17. Jester A, Harth A, Wind G, Germann G, Sauerbier M. Does the Disability of Shoulder, Arm and Hand questionnaire (DASH) replace grip strength and range of motion in outcome-evaluation? [in German]. Handchir Mikrochir Plast Chir. 2005;37(2):126-130.
18. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
19. Gummesson C, Atroshi I, Ekdahl C. The Disabilities of the Arm, Shoulder and Hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-rated health change after surgery. BMC Musculoskelet Disord. 2003;4:11.
20. Richards RS, Bennett JD, Roth JH, Milne K Jr. Arthroscopic diagnosis of intra-articular soft tissue injuries associated with distal radial fractures. J Hand Surg Am. 1997;22(5):772-776.
21. Peicha G, Seibert F, Fellinger M, Grechenig W. Midterm results of arthroscopic treatment of scapholunate ligament lesions associated with intra-articular distal radius fractures. Knee Surg Sports Traumatol Arthrosc. 1999;7(5):327-333.
22. Schädel-Höpfner M, Böhringer G, Junge A, Celik I, Gotzen L. [Arthroscopic diagnosis of concomitant scapholunate ligament injuries in fractures of the distal radius]. Handchir Mikrochir Plast Chir. 2001;33(4):229-233.
23. Ruch DS, Yang CC, Smith BP. Results of acute arthroscopically repaired triangular fibrocartilage complex injuries associated with intra-articular distal radius fractures. Arthroscopy. 2003;19(5):511-516.
24. Hattori Y, Doi K, Estrella EP, Chen G. Arthroscopically assisted reduction with volar plating or external fixation for displaced intra-articular fractures of the distal radius in the elderly patients. Hand Surg. 2007;12(1):1-12.
25. Hohendorff B, Eck M, Mühldorfer M, Fodor S, Schmitt R, Prommersberger KJ. [Palmar wrist arthroscopy for evaluation of concomitant carpal lesions in operative treatment of distal intraarticular radius fractures]. Handchir Mikrochir Plast Chir. 2009;41(5):295-299.
1. Róbertsson GO, Jónsson GT, Sigurjónsson K. Epidemiology of distal radius fractures in Iceland in 1985. Acta Orthop Scand. 1990;61(5):457-459.
2. Geissler WB. Arthroscopically assisted reduction of intra-articular fractures of the distal radius. Hand Clin. 1995;11(1):19-29.
3. Trybus M, Guzik P. The economic impact of hand injury [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2003;68(4):269-273.
4. Wolfe SW, Easterling KJ, Yoo HH. Arthroscopic-assisted reduction of distal radius fractures. Arthroscopy. 1995;11(6):706-714.
5. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
6. Doi K, Hattori Y, Otsuka K, Abe Y, Yamamoto H. Intra-articular fractures of the distal aspect of the radius: arthroscopically assisted reduction compared with open reduction and internal fixation. J Bone Joint Surg Am. 1999;81(8):1093-1110.
7. Shih JT, Lee HM, Hou YT, Tan CM. Arthroscopically-assisted reduction of intra-articular fractures and soft tissue management of distal radius. Hand Surg. 2001;6(2):127-135.
8. Forward DP, Lindau TR, Melsom DS. Intercarpal ligament injuries associated with fractures of the distal part of the radius. J Bone Joint Surg Am. 2007;89(11):2334-2340.
9. Espinosa-Gutiérrez A, Rivas-Montero JA, Elias-Escobedo A, Alisedo-Ochoa PG. Wrist arthroscopy for fractures of the distal end of the radius [in Spanish]. Acta Ortop Mex. 2009;23(6):358-365.
10. Hardy P, Gomes N, Chebil M, Bauer T. Wrist arthroscopy and intra-articular fractures of the distal radius in young adults. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1225-1230.
11. Varitimidis SE, Basdekis GK, Dailiana ZH, Hantes ME, Bargiotas K, Malizos K. Treatment of intra-articular fractures of the distal radius: fluoroscopic or arthroscopic reduction? J Bone Joint Surg Br. 2008;90(6):778-785.
12. Kordasiewicz B, Pomianowski S, Rylski W, Antolak L, Marczak D. Intraarticular distal radius fractures—arthroscopic assessment of injuries [in Polish]. Chir Narzadow Ruchu Ortop Pol. 2006;71(2):113-116.
13. Lafontaine M, Hardy D, Delince P. Stability assessment of distal radius fractures. Injury. 1989;20(4):208-210.
14. Palmer AK. Triangular fibrocartilage complex lesions: a classification. J Hand Surg Am. 1989;14(4):594-606.
15. Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (Disabilities of the Arm, Shoulder and Hand) [corrected]. The Upper Extremity Collaborative Group (UECG) [published correction appears in Am J Ind Med. 1996;30(3):372]. Am J Ind Med. 1996;29(6):602-608.
16. Fernandez DL, Jupiter JB. Fractures of the Distal Radius: A Practical Approach to Management. New York, NY: Springer; 1996.
17. Jester A, Harth A, Wind G, Germann G, Sauerbier M. Does the Disability of Shoulder, Arm and Hand questionnaire (DASH) replace grip strength and range of motion in outcome-evaluation? [in German]. Handchir Mikrochir Plast Chir. 2005;37(2):126-130.
18. Wei DH, Raizman NM, Bottino CJ, Jobin CM, Strauch RJ, Rosenwasser MP. Unstable distal radial fractures treated with external fixation, a radial column plate, or a volar plate. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(7):1568-1577.
19. Gummesson C, Atroshi I, Ekdahl C. The Disabilities of the Arm, Shoulder and Hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-rated health change after surgery. BMC Musculoskelet Disord. 2003;4:11.
20. Richards RS, Bennett JD, Roth JH, Milne K Jr. Arthroscopic diagnosis of intra-articular soft tissue injuries associated with distal radial fractures. J Hand Surg Am. 1997;22(5):772-776.
21. Peicha G, Seibert F, Fellinger M, Grechenig W. Midterm results of arthroscopic treatment of scapholunate ligament lesions associated with intra-articular distal radius fractures. Knee Surg Sports Traumatol Arthrosc. 1999;7(5):327-333.
22. Schädel-Höpfner M, Böhringer G, Junge A, Celik I, Gotzen L. [Arthroscopic diagnosis of concomitant scapholunate ligament injuries in fractures of the distal radius]. Handchir Mikrochir Plast Chir. 2001;33(4):229-233.
23. Ruch DS, Yang CC, Smith BP. Results of acute arthroscopically repaired triangular fibrocartilage complex injuries associated with intra-articular distal radius fractures. Arthroscopy. 2003;19(5):511-516.
24. Hattori Y, Doi K, Estrella EP, Chen G. Arthroscopically assisted reduction with volar plating or external fixation for displaced intra-articular fractures of the distal radius in the elderly patients. Hand Surg. 2007;12(1):1-12.
25. Hohendorff B, Eck M, Mühldorfer M, Fodor S, Schmitt R, Prommersberger KJ. [Palmar wrist arthroscopy for evaluation of concomitant carpal lesions in operative treatment of distal intraarticular radius fractures]. Handchir Mikrochir Plast Chir. 2009;41(5):295-299.
Clinicians underusing statins, aspirin in HIV patients
NEW ORLEANS – Clinicians may not be prescribing enough statins and/or aspirin therapy for the HIV-infected population at highest risk for atherosclerotic cardiovascular disease, according to the results of a large retrospective study from an HIV clinic in New York.
“We need to shift our paradigm for care – from ‘take antiretrovirals and that’s it’ – to a focus on the whole patient and chronic conditions,” Emma Kaplan-Lewis, MD, said during a poster presentation at an annual scientific meeting on infectious diseases. Risk of cardiovascular events increases 1.5-fold among people with HIV treated with antiretroviral therapy, compared with the uninfected population, she noted.
The investigators examined prescriptions for a statin or aspirin therapy in three high-risk groups, defined by 2013 American College of Cardiology/American Heart Association guidelines and 2011 AHA/American College of Cardiology Foundation guidelines. They classified patients with an ICD-9 code indicating a history of atherosclerotic cardiovascular disease, 40- to 75-year-olds with diabetes and LDL cholesterol greater than 70 mg/dL, and those with an LDL cholesterol level greater than 190 mg/dL.
Almost two-thirds of the higher-risk patients, 141 (61%), had a history of atherosclerotic cardiovascular disease. In this cohort, 56% were prescribed statin therapy, and 100 (71%) were prescribed aspirin.
Of the 85 high-risk patients with diabetes and an LDL greater than 70 mg/dL, 48 (57%) were on statin therapy (aspirin not indicated), and of the 5 high-risk patients with an LDL cholesterol level greater than 190 mg/dL, 3 (60%) were on statin therapy, and 1 (20%) was on aspirin.
The investigators also found 37% of the higher-risk patients were active cigarette smokers. There was a trend toward lower statin use among smokers, 33% versus 44% for nonsmokers. “Smoking was not significantly associated with statin prescription, but this is a modifiable risk factor – after which they may not need a statin,” Dr. Kaplan-Lewis said.
The findings support risk-reduction interventions for people with HIV infection who are at higher risk for cardiovascular disease, Dr. Kaplan-Lewis said. The results support previous reports in the literature, including a study that found 51% of 13,579 veterans infected with HIV had an indication for statin use, but 22% of this group was not prescribed the therapy (Clin Infect Dis. 2016;63:407-13. doi: 10.1093/cid/ciw289).
In 2017, the investigators plan to share the study data with providers. “Each provider will get a list of their patients who should be on a statin. This is about awareness and making it more of a priority,” Dr. Kaplan-Lewis said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The findings of additional research assessing lower thresholds for statin use among people with HIV infection are still pending, Dr. Kaplan-Lewis added.
The study was supported in part by the New York State Department of Health Empire Clinical Research Investigator Program. Dr. Kaplan-Lewis had no relevant disclosures.
NEW ORLEANS – Clinicians may not be prescribing enough statins and/or aspirin therapy for the HIV-infected population at highest risk for atherosclerotic cardiovascular disease, according to the results of a large retrospective study from an HIV clinic in New York.
“We need to shift our paradigm for care – from ‘take antiretrovirals and that’s it’ – to a focus on the whole patient and chronic conditions,” Emma Kaplan-Lewis, MD, said during a poster presentation at an annual scientific meeting on infectious diseases. Risk of cardiovascular events increases 1.5-fold among people with HIV treated with antiretroviral therapy, compared with the uninfected population, she noted.
The investigators examined prescriptions for a statin or aspirin therapy in three high-risk groups, defined by 2013 American College of Cardiology/American Heart Association guidelines and 2011 AHA/American College of Cardiology Foundation guidelines. They classified patients with an ICD-9 code indicating a history of atherosclerotic cardiovascular disease, 40- to 75-year-olds with diabetes and LDL cholesterol greater than 70 mg/dL, and those with an LDL cholesterol level greater than 190 mg/dL.
Almost two-thirds of the higher-risk patients, 141 (61%), had a history of atherosclerotic cardiovascular disease. In this cohort, 56% were prescribed statin therapy, and 100 (71%) were prescribed aspirin.
Of the 85 high-risk patients with diabetes and an LDL greater than 70 mg/dL, 48 (57%) were on statin therapy (aspirin not indicated), and of the 5 high-risk patients with an LDL cholesterol level greater than 190 mg/dL, 3 (60%) were on statin therapy, and 1 (20%) was on aspirin.
The investigators also found 37% of the higher-risk patients were active cigarette smokers. There was a trend toward lower statin use among smokers, 33% versus 44% for nonsmokers. “Smoking was not significantly associated with statin prescription, but this is a modifiable risk factor – after which they may not need a statin,” Dr. Kaplan-Lewis said.
The findings support risk-reduction interventions for people with HIV infection who are at higher risk for cardiovascular disease, Dr. Kaplan-Lewis said. The results support previous reports in the literature, including a study that found 51% of 13,579 veterans infected with HIV had an indication for statin use, but 22% of this group was not prescribed the therapy (Clin Infect Dis. 2016;63:407-13. doi: 10.1093/cid/ciw289).
In 2017, the investigators plan to share the study data with providers. “Each provider will get a list of their patients who should be on a statin. This is about awareness and making it more of a priority,” Dr. Kaplan-Lewis said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The findings of additional research assessing lower thresholds for statin use among people with HIV infection are still pending, Dr. Kaplan-Lewis added.
The study was supported in part by the New York State Department of Health Empire Clinical Research Investigator Program. Dr. Kaplan-Lewis had no relevant disclosures.
NEW ORLEANS – Clinicians may not be prescribing enough statins and/or aspirin therapy for the HIV-infected population at highest risk for atherosclerotic cardiovascular disease, according to the results of a large retrospective study from an HIV clinic in New York.
“We need to shift our paradigm for care – from ‘take antiretrovirals and that’s it’ – to a focus on the whole patient and chronic conditions,” Emma Kaplan-Lewis, MD, said during a poster presentation at an annual scientific meeting on infectious diseases. Risk of cardiovascular events increases 1.5-fold among people with HIV treated with antiretroviral therapy, compared with the uninfected population, she noted.
The investigators examined prescriptions for a statin or aspirin therapy in three high-risk groups, defined by 2013 American College of Cardiology/American Heart Association guidelines and 2011 AHA/American College of Cardiology Foundation guidelines. They classified patients with an ICD-9 code indicating a history of atherosclerotic cardiovascular disease, 40- to 75-year-olds with diabetes and LDL cholesterol greater than 70 mg/dL, and those with an LDL cholesterol level greater than 190 mg/dL.
Almost two-thirds of the higher-risk patients, 141 (61%), had a history of atherosclerotic cardiovascular disease. In this cohort, 56% were prescribed statin therapy, and 100 (71%) were prescribed aspirin.
Of the 85 high-risk patients with diabetes and an LDL greater than 70 mg/dL, 48 (57%) were on statin therapy (aspirin not indicated), and of the 5 high-risk patients with an LDL cholesterol level greater than 190 mg/dL, 3 (60%) were on statin therapy, and 1 (20%) was on aspirin.
The investigators also found 37% of the higher-risk patients were active cigarette smokers. There was a trend toward lower statin use among smokers, 33% versus 44% for nonsmokers. “Smoking was not significantly associated with statin prescription, but this is a modifiable risk factor – after which they may not need a statin,” Dr. Kaplan-Lewis said.
The findings support risk-reduction interventions for people with HIV infection who are at higher risk for cardiovascular disease, Dr. Kaplan-Lewis said. The results support previous reports in the literature, including a study that found 51% of 13,579 veterans infected with HIV had an indication for statin use, but 22% of this group was not prescribed the therapy (Clin Infect Dis. 2016;63:407-13. doi: 10.1093/cid/ciw289).
In 2017, the investigators plan to share the study data with providers. “Each provider will get a list of their patients who should be on a statin. This is about awareness and making it more of a priority,” Dr. Kaplan-Lewis said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The findings of additional research assessing lower thresholds for statin use among people with HIV infection are still pending, Dr. Kaplan-Lewis added.
The study was supported in part by the New York State Department of Health Empire Clinical Research Investigator Program. Dr. Kaplan-Lewis had no relevant disclosures.
AT IDWEEK 2016
Key clinical point: Statins and aspirin therapy are underutilized in a proportion of the HIV positive population at higher risk for cardiovascular disease.
Major finding: Of the 141 high-risk people with HIV infection and a history of atherosclerotic cardiovascular disease, only 56% were prescribed a statin and 71% were prescribed aspirin.
Data source: Poster presentation at IDWeek 2016.
Disclosures: The study was supported in part by the New York State Department of Health Empire Clinical Research Investigator Program. Dr. Kaplan-Lewis had no relevant disclosures.