User login
Strategies for prophylactic oophoropexy

Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Tips and tricks for open laparoscopy
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist

Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Tips and tricks for open laparoscopy
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist

Visit the Society of Gynecologic Surgeons online: sgsonline.org
More videos from SGS:
- Tips and tricks for open laparoscopy
- Complete colpectomy & colpocleisis: Model for simulation
- Natural orifice sacral colpopexy
- Alternative options for visualizing ureteral patency during intraoperative cystoscopy
- Use of suprapubic Carter-Thomason needle to assist in cystoscopic excision of an intravesical foreign object
- Uterine artery ligation: Advanced techniques and considerations for the difficult laparoscopic hysterectomy
- Cervical injection of methylene blue for identification of sentinel lymph nodes in cervical cancer
- Misplaced hysteroscopic sterilization micro-insert in the peritoneal cavity: A corpus alienum
- Laparoscopic cystectomy for large, bilateral ovarian dermoids
- Small bowel surgery for the benign gynecologist
This video is brought to you by
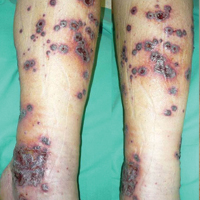
Vascular anomalies often misdiagnosed amidst confusion
CHICAGO – Thanks to convoluted terminology, not to mention confusion in the literature, physicians have been known to frequently misdiagnose vascular malformations as hemangiomas, but an evolving understanding of their differences may lead to more precise diagnoses, according to a report at a symposium on vascular surgery sponsored by Northwestern University.
“Historically there has been a great deal of confusion in the literature when it comes to the nomenclature used to describe vascular anomalies,” said Naiem Nassiri, MD, of Robert Wood Johnson Medical School, New Brunswick, N.J. He pointed out that the term hemangioma “or derivatives thereof” – cavernous hemangioma, cavernous angioma, lymphangioma and cystic hygroma – are “absolute misnomers and continue to be misused and applied almost haphazardly to any anomalous vascular lesion.”
He cited reports that 71% of vascular anomalies have been improperly called hemangiomas, 69% have initially been diagnosed incorrectly, and 21% received the wrong treatment (Pediatr Dermatol. 2008;25[1]:7-12; Plast Reconstr Surg. 2011:127[1]:347-51). “Erroneous terminology has prognostic as well as diagnostic and therapeutic implications, and these can actually be quite devastating for the patient, not only clinically and physically but psychologically as well,” Dr. Nassiri said.
Using the International Society for the Study of Vascular Anomalies classification for hemangiomas and vascular malformations can help physicians make the differential diagnosis, Dr. Nassiri said. Hemangiomas are neoplastic lesions of infancy, though not always congenital, with a finite growth phase, whereas vascular malformations (VMs) are nonneoplastic, congenital lesions that can appear at any age and do not regress spontaneously, he said.
Infantile hemangiomas typically appear as the classic strawberry birthmark in children, whereas VMs tend to appear later in life. “They require some environmental trigger, such as trauma, activity, or changes in the hormonal milieu to manifest onset,” he said of VMs.
Simply put, VMs fall into three broad categories: slow-flow malformations, which include lymphatic and venous malformations; high-flow arteriovenous malformations (AVMs) and fistulas; and congenital mixed syndromes, which can include combinations thereof.
Dr. Nassiri noted that contrast-enhanced MRI is the standard imaging modality for diagnosis of VMs, and can differentiate between slow-flow and high-flow lesions. However, vascular specialists must be vigilant in ordering imaging for slow-flow lesions. “Orders can be changed to MR venography, and I’ve had patients who’ve gone decades with multiple MR venograms and no one can figure out what’s going on as no identifiable lesion is readily detected,” he said. “MR venograms are fantastic for detecting truncular blood flow where there typically are no anomalies in the vast majority of patients with isolated venous malformations, but on contrast-enhanced MRI these convoluted cluster of anomalous veins light up like Christmas trees.”
Lymphatic malformations affect the head and neck more so than the extremities, trunk or viscera, and are prone to infection and bleeding. “You can think of these as fluid-filled balloons, and the goal of treatment is fairly simple: You want to puncture the balloon and drain the fluid inside so as to obtain maximum wall collapse,” Dr. Nassiri said. Infusion of a sclerosant causes an inflammatory reaction leading to fibrosis, which then prevents balloon re-expansion. Surgical excision is best used as a secondary adjunct.
Venous malformations, comprising about 80% of all VMs, typically present as soft, spongy blue or purple compressible masses with associated pain that worsens with exertion, Dr. Nassiri said. “The most dangerous thing that is often overlooked, even by some of the physicians that treat these on a regular basis, is localized intravascular coagulopathy, which if left untreated can progress to fulminant disseminated intravascular coagulopathy,” he said. This tends to occur more in the more widespread varieties of venous malformations.
A common misnomer associated with venous malformations in adults is “liver hemangioma,” owing to the confusing nomenclature, Dr. Nassiri said. “When interrogated angiographically,” he said, “what is often labeled as a hepatic hemangioma is in fact a venous malformation. Natural history of the two entities is completely different.”
Dr. Nassiri described congenital high-flow AVMs as “convoluted networks of blood vessels with poorly differentiated endothelial cells that have neither a venous nor an arterial designation; this entity, otherwise known as a nidus, sits between the feeding arteries and the draining veins.” Treatment aims to eliminate the flow within that nidus.
Super-selective microcatheterization is the best option for nidus access and embolization using liquid embolic agents, preferably those that polymerize when infused. “This is probably the most potent angiogenic entity I’ve ever seen,” Dr. Nassiri said of the nidus.
“It’s like a low-pressure sump and it will recruit collaterals vigorously, so you have to eliminate that nidus.” A variety of different embolic agents, some off label, may be used for high flow AVMs.
For congenital mixed syndromes, the same diagnostic and therapeutic concepts hold true depending on the type of VM involved. Dr. Nassiri advised a multidisciplinary approach, and noted that early trials have investigated the use of sirolimus in severe, life-threatening cases (Br J Clin Pharmacol. 2016;82[5]:1171-9. doi: 10.1111/bcp.13022).
Dr. Nassiri disclosed serving on the speakers bureaus for Boston Scientific, Penumbra, and Merritt Medical, and is a consultant to Merritt Medical.
CHICAGO – Thanks to convoluted terminology, not to mention confusion in the literature, physicians have been known to frequently misdiagnose vascular malformations as hemangiomas, but an evolving understanding of their differences may lead to more precise diagnoses, according to a report at a symposium on vascular surgery sponsored by Northwestern University.
“Historically there has been a great deal of confusion in the literature when it comes to the nomenclature used to describe vascular anomalies,” said Naiem Nassiri, MD, of Robert Wood Johnson Medical School, New Brunswick, N.J. He pointed out that the term hemangioma “or derivatives thereof” – cavernous hemangioma, cavernous angioma, lymphangioma and cystic hygroma – are “absolute misnomers and continue to be misused and applied almost haphazardly to any anomalous vascular lesion.”
He cited reports that 71% of vascular anomalies have been improperly called hemangiomas, 69% have initially been diagnosed incorrectly, and 21% received the wrong treatment (Pediatr Dermatol. 2008;25[1]:7-12; Plast Reconstr Surg. 2011:127[1]:347-51). “Erroneous terminology has prognostic as well as diagnostic and therapeutic implications, and these can actually be quite devastating for the patient, not only clinically and physically but psychologically as well,” Dr. Nassiri said.
Using the International Society for the Study of Vascular Anomalies classification for hemangiomas and vascular malformations can help physicians make the differential diagnosis, Dr. Nassiri said. Hemangiomas are neoplastic lesions of infancy, though not always congenital, with a finite growth phase, whereas vascular malformations (VMs) are nonneoplastic, congenital lesions that can appear at any age and do not regress spontaneously, he said.
Infantile hemangiomas typically appear as the classic strawberry birthmark in children, whereas VMs tend to appear later in life. “They require some environmental trigger, such as trauma, activity, or changes in the hormonal milieu to manifest onset,” he said of VMs.
Simply put, VMs fall into three broad categories: slow-flow malformations, which include lymphatic and venous malformations; high-flow arteriovenous malformations (AVMs) and fistulas; and congenital mixed syndromes, which can include combinations thereof.
Dr. Nassiri noted that contrast-enhanced MRI is the standard imaging modality for diagnosis of VMs, and can differentiate between slow-flow and high-flow lesions. However, vascular specialists must be vigilant in ordering imaging for slow-flow lesions. “Orders can be changed to MR venography, and I’ve had patients who’ve gone decades with multiple MR venograms and no one can figure out what’s going on as no identifiable lesion is readily detected,” he said. “MR venograms are fantastic for detecting truncular blood flow where there typically are no anomalies in the vast majority of patients with isolated venous malformations, but on contrast-enhanced MRI these convoluted cluster of anomalous veins light up like Christmas trees.”
Lymphatic malformations affect the head and neck more so than the extremities, trunk or viscera, and are prone to infection and bleeding. “You can think of these as fluid-filled balloons, and the goal of treatment is fairly simple: You want to puncture the balloon and drain the fluid inside so as to obtain maximum wall collapse,” Dr. Nassiri said. Infusion of a sclerosant causes an inflammatory reaction leading to fibrosis, which then prevents balloon re-expansion. Surgical excision is best used as a secondary adjunct.
Venous malformations, comprising about 80% of all VMs, typically present as soft, spongy blue or purple compressible masses with associated pain that worsens with exertion, Dr. Nassiri said. “The most dangerous thing that is often overlooked, even by some of the physicians that treat these on a regular basis, is localized intravascular coagulopathy, which if left untreated can progress to fulminant disseminated intravascular coagulopathy,” he said. This tends to occur more in the more widespread varieties of venous malformations.
A common misnomer associated with venous malformations in adults is “liver hemangioma,” owing to the confusing nomenclature, Dr. Nassiri said. “When interrogated angiographically,” he said, “what is often labeled as a hepatic hemangioma is in fact a venous malformation. Natural history of the two entities is completely different.”
Dr. Nassiri described congenital high-flow AVMs as “convoluted networks of blood vessels with poorly differentiated endothelial cells that have neither a venous nor an arterial designation; this entity, otherwise known as a nidus, sits between the feeding arteries and the draining veins.” Treatment aims to eliminate the flow within that nidus.
Super-selective microcatheterization is the best option for nidus access and embolization using liquid embolic agents, preferably those that polymerize when infused. “This is probably the most potent angiogenic entity I’ve ever seen,” Dr. Nassiri said of the nidus.
“It’s like a low-pressure sump and it will recruit collaterals vigorously, so you have to eliminate that nidus.” A variety of different embolic agents, some off label, may be used for high flow AVMs.
For congenital mixed syndromes, the same diagnostic and therapeutic concepts hold true depending on the type of VM involved. Dr. Nassiri advised a multidisciplinary approach, and noted that early trials have investigated the use of sirolimus in severe, life-threatening cases (Br J Clin Pharmacol. 2016;82[5]:1171-9. doi: 10.1111/bcp.13022).
Dr. Nassiri disclosed serving on the speakers bureaus for Boston Scientific, Penumbra, and Merritt Medical, and is a consultant to Merritt Medical.
CHICAGO – Thanks to convoluted terminology, not to mention confusion in the literature, physicians have been known to frequently misdiagnose vascular malformations as hemangiomas, but an evolving understanding of their differences may lead to more precise diagnoses, according to a report at a symposium on vascular surgery sponsored by Northwestern University.
“Historically there has been a great deal of confusion in the literature when it comes to the nomenclature used to describe vascular anomalies,” said Naiem Nassiri, MD, of Robert Wood Johnson Medical School, New Brunswick, N.J. He pointed out that the term hemangioma “or derivatives thereof” – cavernous hemangioma, cavernous angioma, lymphangioma and cystic hygroma – are “absolute misnomers and continue to be misused and applied almost haphazardly to any anomalous vascular lesion.”
He cited reports that 71% of vascular anomalies have been improperly called hemangiomas, 69% have initially been diagnosed incorrectly, and 21% received the wrong treatment (Pediatr Dermatol. 2008;25[1]:7-12; Plast Reconstr Surg. 2011:127[1]:347-51). “Erroneous terminology has prognostic as well as diagnostic and therapeutic implications, and these can actually be quite devastating for the patient, not only clinically and physically but psychologically as well,” Dr. Nassiri said.
Using the International Society for the Study of Vascular Anomalies classification for hemangiomas and vascular malformations can help physicians make the differential diagnosis, Dr. Nassiri said. Hemangiomas are neoplastic lesions of infancy, though not always congenital, with a finite growth phase, whereas vascular malformations (VMs) are nonneoplastic, congenital lesions that can appear at any age and do not regress spontaneously, he said.
Infantile hemangiomas typically appear as the classic strawberry birthmark in children, whereas VMs tend to appear later in life. “They require some environmental trigger, such as trauma, activity, or changes in the hormonal milieu to manifest onset,” he said of VMs.
Simply put, VMs fall into three broad categories: slow-flow malformations, which include lymphatic and venous malformations; high-flow arteriovenous malformations (AVMs) and fistulas; and congenital mixed syndromes, which can include combinations thereof.
Dr. Nassiri noted that contrast-enhanced MRI is the standard imaging modality for diagnosis of VMs, and can differentiate between slow-flow and high-flow lesions. However, vascular specialists must be vigilant in ordering imaging for slow-flow lesions. “Orders can be changed to MR venography, and I’ve had patients who’ve gone decades with multiple MR venograms and no one can figure out what’s going on as no identifiable lesion is readily detected,” he said. “MR venograms are fantastic for detecting truncular blood flow where there typically are no anomalies in the vast majority of patients with isolated venous malformations, but on contrast-enhanced MRI these convoluted cluster of anomalous veins light up like Christmas trees.”
Lymphatic malformations affect the head and neck more so than the extremities, trunk or viscera, and are prone to infection and bleeding. “You can think of these as fluid-filled balloons, and the goal of treatment is fairly simple: You want to puncture the balloon and drain the fluid inside so as to obtain maximum wall collapse,” Dr. Nassiri said. Infusion of a sclerosant causes an inflammatory reaction leading to fibrosis, which then prevents balloon re-expansion. Surgical excision is best used as a secondary adjunct.
Venous malformations, comprising about 80% of all VMs, typically present as soft, spongy blue or purple compressible masses with associated pain that worsens with exertion, Dr. Nassiri said. “The most dangerous thing that is often overlooked, even by some of the physicians that treat these on a regular basis, is localized intravascular coagulopathy, which if left untreated can progress to fulminant disseminated intravascular coagulopathy,” he said. This tends to occur more in the more widespread varieties of venous malformations.
A common misnomer associated with venous malformations in adults is “liver hemangioma,” owing to the confusing nomenclature, Dr. Nassiri said. “When interrogated angiographically,” he said, “what is often labeled as a hepatic hemangioma is in fact a venous malformation. Natural history of the two entities is completely different.”
Dr. Nassiri described congenital high-flow AVMs as “convoluted networks of blood vessels with poorly differentiated endothelial cells that have neither a venous nor an arterial designation; this entity, otherwise known as a nidus, sits between the feeding arteries and the draining veins.” Treatment aims to eliminate the flow within that nidus.
Super-selective microcatheterization is the best option for nidus access and embolization using liquid embolic agents, preferably those that polymerize when infused. “This is probably the most potent angiogenic entity I’ve ever seen,” Dr. Nassiri said of the nidus.
“It’s like a low-pressure sump and it will recruit collaterals vigorously, so you have to eliminate that nidus.” A variety of different embolic agents, some off label, may be used for high flow AVMs.
For congenital mixed syndromes, the same diagnostic and therapeutic concepts hold true depending on the type of VM involved. Dr. Nassiri advised a multidisciplinary approach, and noted that early trials have investigated the use of sirolimus in severe, life-threatening cases (Br J Clin Pharmacol. 2016;82[5]:1171-9. doi: 10.1111/bcp.13022).
Dr. Nassiri disclosed serving on the speakers bureaus for Boston Scientific, Penumbra, and Merritt Medical, and is a consultant to Merritt Medical.
AT THE NORTHWESTERN VASCULAR SYMPOSIUM
Key clinical point:
Major finding: Use of imaging and a clearer understanding of the lack of neoplastic activity are key to more precisely diagnosing vascular malformations.
Data source: Review of literature and center experience.
Disclosure: Dr. Nassiri disclosed serving on the speakers bureaus for Boston Scientific, Penumbra, and Merritt Medical, and is a consultant to Merritt Medical.
But you told me...
“The other doctor I went to told me that the spot he biopsied on my nose was a skin cancer,” Larry said. “But he told me just to keep an eye on it.”
I always try not to roll my eyes when a patient quotes another doctor, especially if the quote doesn’t make much sense. In the first place, it’s bad form to act like you’re smarter than somebody else. In the second place, you probably aren’t.
In the third place, what the patient says the doctor said may not be what the doctor actually said. I have many chances to learn this firsthand, such as when patients quote me incorrectly to myself.
No, I didn’t.
I point out to students that, to patients, calling a mole benign is always provisional. They’re happy that it’s benign today. Tomorrow, who knows?
That’s why when I reassure people about moles I’m not worried about, I say, “It’s benign... and it will always be benign.” When they look startled – as they often do – I elaborate: “Because if I thought it could turn into skin cancer, I would have to remove it right now.” Then they nod, somewhat tentatively. What I just said clearly made sense, only it contradicts what they always assumed was true, which is that you should always keep an eye on things.
Since I thought Steve’s mole was benign, I did not tell him that we need to keep an eye on it, any more than Larry’s previous doctor had told him just to keep an eye on a biopsy-proved skin cancer. Steve just thought that’s what I must have said, because that’s what makes sense to him.
Then there was Amanda, who had stopped her acne gel weeks before. “It was making me worse,” she explained, “and you told me to stop the medicine if anything happened.”
Nope, not even close.
What I did say – what I always say – was this: “These are the reactions you might experience. If you think you’re getting them or any others, call me right away, so I can consider changing to something different.” I never tell patients to just stop treatment and not tell anyone. Who would?
The opposite happens too. Just as some people stop medication without telling their doctors, others find it just as hard to stop treatment even when they’re instructed to.
“When your seborrhea quiets down,” I say, “you can stop the cream. Resume it when you need to, but stop again as soon as you clear up.”
Easy for me to say. But in walks Phillip. He’s been using applying desonide daily for 6 years. “You said I should keep using it,” he explains.
No, I didn’t. “What I was trying to say,” I politely explain, “is that when your skin feels fine, it’s OK to stop. They you can use it again when the rash comes back. Keeping up applying the cream doesn’t stop the rash from coming back if it’s going to.”
Philip nods. I think he understands. But I thought so last time too, didn’t I?
I should also give a shout-out to the patients who say, “I’ve been using the clotrimazole-betamethasone cream you prescribed...”
No, I did not prescribe clotrimazole-betamethasone! I would lose my membership in the dermatologists’ union.
Researchers who study cross-cultural practice look into issues of miscommunication between providers and consumers who come from distant cultures, where basic notions get in the way of each party’s understanding the other. No one seems that interested in studying all the miscommunication that goes on between educated native-English speakers, in medical offices no less than in the halls of the legislature.
I got hold of Larry’s biopsy report, by the way. It was read out as “actinic keratosis,” which is why Larry’s former doctor had told him that they would just watch it.
I called Larry. “It was not an actual cancer,” I told him. “Just precancerous. Come back in 6 months. We’ll keep an eye on it.”
That was clear. I think.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His new book “Act Like a Doctor, Think Like a Patient” is now available at amazon.com and barnesandnoble.com. This is his second book. Write to him at [email protected].
“The other doctor I went to told me that the spot he biopsied on my nose was a skin cancer,” Larry said. “But he told me just to keep an eye on it.”
I always try not to roll my eyes when a patient quotes another doctor, especially if the quote doesn’t make much sense. In the first place, it’s bad form to act like you’re smarter than somebody else. In the second place, you probably aren’t.
In the third place, what the patient says the doctor said may not be what the doctor actually said. I have many chances to learn this firsthand, such as when patients quote me incorrectly to myself.
No, I didn’t.
I point out to students that, to patients, calling a mole benign is always provisional. They’re happy that it’s benign today. Tomorrow, who knows?
That’s why when I reassure people about moles I’m not worried about, I say, “It’s benign... and it will always be benign.” When they look startled – as they often do – I elaborate: “Because if I thought it could turn into skin cancer, I would have to remove it right now.” Then they nod, somewhat tentatively. What I just said clearly made sense, only it contradicts what they always assumed was true, which is that you should always keep an eye on things.
Since I thought Steve’s mole was benign, I did not tell him that we need to keep an eye on it, any more than Larry’s previous doctor had told him just to keep an eye on a biopsy-proved skin cancer. Steve just thought that’s what I must have said, because that’s what makes sense to him.
Then there was Amanda, who had stopped her acne gel weeks before. “It was making me worse,” she explained, “and you told me to stop the medicine if anything happened.”
Nope, not even close.
What I did say – what I always say – was this: “These are the reactions you might experience. If you think you’re getting them or any others, call me right away, so I can consider changing to something different.” I never tell patients to just stop treatment and not tell anyone. Who would?
The opposite happens too. Just as some people stop medication without telling their doctors, others find it just as hard to stop treatment even when they’re instructed to.
“When your seborrhea quiets down,” I say, “you can stop the cream. Resume it when you need to, but stop again as soon as you clear up.”
Easy for me to say. But in walks Phillip. He’s been using applying desonide daily for 6 years. “You said I should keep using it,” he explains.
No, I didn’t. “What I was trying to say,” I politely explain, “is that when your skin feels fine, it’s OK to stop. They you can use it again when the rash comes back. Keeping up applying the cream doesn’t stop the rash from coming back if it’s going to.”
Philip nods. I think he understands. But I thought so last time too, didn’t I?
I should also give a shout-out to the patients who say, “I’ve been using the clotrimazole-betamethasone cream you prescribed...”
No, I did not prescribe clotrimazole-betamethasone! I would lose my membership in the dermatologists’ union.
Researchers who study cross-cultural practice look into issues of miscommunication between providers and consumers who come from distant cultures, where basic notions get in the way of each party’s understanding the other. No one seems that interested in studying all the miscommunication that goes on between educated native-English speakers, in medical offices no less than in the halls of the legislature.
I got hold of Larry’s biopsy report, by the way. It was read out as “actinic keratosis,” which is why Larry’s former doctor had told him that they would just watch it.
I called Larry. “It was not an actual cancer,” I told him. “Just precancerous. Come back in 6 months. We’ll keep an eye on it.”
That was clear. I think.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His new book “Act Like a Doctor, Think Like a Patient” is now available at amazon.com and barnesandnoble.com. This is his second book. Write to him at [email protected].
“The other doctor I went to told me that the spot he biopsied on my nose was a skin cancer,” Larry said. “But he told me just to keep an eye on it.”
I always try not to roll my eyes when a patient quotes another doctor, especially if the quote doesn’t make much sense. In the first place, it’s bad form to act like you’re smarter than somebody else. In the second place, you probably aren’t.
In the third place, what the patient says the doctor said may not be what the doctor actually said. I have many chances to learn this firsthand, such as when patients quote me incorrectly to myself.
No, I didn’t.
I point out to students that, to patients, calling a mole benign is always provisional. They’re happy that it’s benign today. Tomorrow, who knows?
That’s why when I reassure people about moles I’m not worried about, I say, “It’s benign... and it will always be benign.” When they look startled – as they often do – I elaborate: “Because if I thought it could turn into skin cancer, I would have to remove it right now.” Then they nod, somewhat tentatively. What I just said clearly made sense, only it contradicts what they always assumed was true, which is that you should always keep an eye on things.
Since I thought Steve’s mole was benign, I did not tell him that we need to keep an eye on it, any more than Larry’s previous doctor had told him just to keep an eye on a biopsy-proved skin cancer. Steve just thought that’s what I must have said, because that’s what makes sense to him.
Then there was Amanda, who had stopped her acne gel weeks before. “It was making me worse,” she explained, “and you told me to stop the medicine if anything happened.”
Nope, not even close.
What I did say – what I always say – was this: “These are the reactions you might experience. If you think you’re getting them or any others, call me right away, so I can consider changing to something different.” I never tell patients to just stop treatment and not tell anyone. Who would?
The opposite happens too. Just as some people stop medication without telling their doctors, others find it just as hard to stop treatment even when they’re instructed to.
“When your seborrhea quiets down,” I say, “you can stop the cream. Resume it when you need to, but stop again as soon as you clear up.”
Easy for me to say. But in walks Phillip. He’s been using applying desonide daily for 6 years. “You said I should keep using it,” he explains.
No, I didn’t. “What I was trying to say,” I politely explain, “is that when your skin feels fine, it’s OK to stop. They you can use it again when the rash comes back. Keeping up applying the cream doesn’t stop the rash from coming back if it’s going to.”
Philip nods. I think he understands. But I thought so last time too, didn’t I?
I should also give a shout-out to the patients who say, “I’ve been using the clotrimazole-betamethasone cream you prescribed...”
No, I did not prescribe clotrimazole-betamethasone! I would lose my membership in the dermatologists’ union.
Researchers who study cross-cultural practice look into issues of miscommunication between providers and consumers who come from distant cultures, where basic notions get in the way of each party’s understanding the other. No one seems that interested in studying all the miscommunication that goes on between educated native-English speakers, in medical offices no less than in the halls of the legislature.
I got hold of Larry’s biopsy report, by the way. It was read out as “actinic keratosis,” which is why Larry’s former doctor had told him that they would just watch it.
I called Larry. “It was not an actual cancer,” I told him. “Just precancerous. Come back in 6 months. We’ll keep an eye on it.”
That was clear. I think.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years. His new book “Act Like a Doctor, Think Like a Patient” is now available at amazon.com and barnesandnoble.com. This is his second book. Write to him at [email protected].
Ramucirumab benefits gastric cancer patients across age groups
SAN FRANCISCO – Patients with metastatic gastric or gastroesophageal junction adenocarcinoma benefit from treatment with ramucirumab regardless of their age, according to findings from an exploratory subgroup analysis of the phase III RAINBOW and REGARD studies.
The findings, which show at least a trend toward improvements in most age categories, are important given that nearly two-thirds of patients with these cancers are diagnosed at over age 65 years, and more than half of those are over age 75 years, Kei Muro, MD, reported at the symposium sponsored by ASCO, ASTRO, the American Gastroenterological Association, and the Society of Surgical Oncology.
“At the other end of the age spectrum, there is evidence that young age can also be an unfavorable prognostic characteristic for gastric cancer,” said Dr. Muro of Aichi Cancer Center Hospital in Nagoya, Japan.
Both RAINBOW and REGARD demonstrated statistically significant and clinically meaningful overall and progression-free survival benefits and acceptable and manageable toxicity with ramucirumab among patients with advanced gastric cancer who were randomized, in the second-line treatment setting, to receive active treatment with the fully humanized monoclonal antibody directed against vascular endothelial growth factor receptor–2 or placebo.
RAINBOW subjects were randomized 1:1 to receive 8 mg/kg of ramucirumab plus paclitaxel, or placebo plus paclitaxel. Among those aged 45 years or less (37 patients in each group), the median overall survival was 9.0 months for treatment vs. 4.2 months for placebo (hazard ratio, 0.555), and the median progression-free survival was 3.9 vs. 2.8 months (HR, 0.299).
The corresponding median overall survival rates for those aged 45-70 (225 and 230 patients in the groups, respectively), 70 or older (68 patients in each group), and 75 or older (20 and 16 patients in the groups, respectively) were 9.6 vs. 7.6 months (HR, 0.860), 10.8 vs. 8.6 months (HR, 0.881), and 11.0 vs. 11.0 months (HR, 0.971). The corresponding progression-free survival rates for those aged 45-70, 70 or older, and 75 or older were 4.6 vs. 2.8 months (HR, 0.649), 4.7 vs. 2.9 months (HR, 0.676), and 4.2 vs. 2.8 months (HR, 0.330).
REGARD subjects were randomized 2:1 to receive 8 mg/kg of ramucirumab plus best supportive care, or placebo plus best supportive care. Among those aged 45 years or less (37 patients in each group), the median overall survival was 9.0 vs. 4.2 months for treatment vs. placebo (HR, 0.555), and the median progression-free survival was 3.9 vs. 2.8 months (HR, 0.299).
Among REGARD subjects aged 45 years or less (28 and 12 patients in the groups, respectively), the median overall survival was 5.8 vs. 2.9 months for treatment vs. placebo (HR, 0.586), and the median progression-free survival was 1.9 vs. 1.4 months (HR, 0.270).
The corresponding median overall survival rates for those aged 45-70 (166 and 70 patients in the groups, respectively), 70 or older (44 and 35 patients in the groups, respectively), and 75 or older (21 and 13 patients in the groups, respectively) were 4.9 vs. 4.1 months (HR, 0.780), 5.9 vs. 3.8 months (HR, 0.730), and 9.3 vs. 5.1 months (HR, 0.588).
The corresponding progression-free survival rates for those aged 45-70, 70 or older, and 75 or older were 2.2 vs. 1.3 months (HR, 0.451), 2.1 vs. 1.4 months (HR, 0.559), 2.8 vs. 1.4 (HR, 0.420).
Baseline characteristics were generally well balanced between arms in each of the age subgroups, Dr. Muro said, noting that no obvious patterns for differential risks in terms of efficacy and adverse events of any grade or of grade 3 or greater were seen according to age. Discontinuation rates for adverse events were similar across different age groups, and quality of life, as determined by global health status, was satisfactory in all age groups.
“Despite some limitations regarding patient numbers in some age subgroups, this exploratory subgroup analysis supports the use of ramucirumab for the treatment of our patients with gastric cancer irrespective of age,” he concluded.
RAINBOW was funded by Eli Lilly. REGARD was funded by ImClone Systems. Dr. Muro reported receiving honoraria from Chugai Pharma, Merck Serono, Taiho Pharmaceutical, Takeda, Eli Lilly, and Yakult Honsha, as well as serving in a consulting or an advisory role for Ono, Merck Serono, and Eli Lilly, and receiving research funding from MSD, Daiichi Sankyo, Ono, Eisai, Pfizer, Chugai, Dainippon Sumitomo, Merck Serono, Janssen Pharmaceutical K.K., AstraZeneca, GlaxoSmithKline, and Kyowa Hakko Kirin.
SAN FRANCISCO – Patients with metastatic gastric or gastroesophageal junction adenocarcinoma benefit from treatment with ramucirumab regardless of their age, according to findings from an exploratory subgroup analysis of the phase III RAINBOW and REGARD studies.
The findings, which show at least a trend toward improvements in most age categories, are important given that nearly two-thirds of patients with these cancers are diagnosed at over age 65 years, and more than half of those are over age 75 years, Kei Muro, MD, reported at the symposium sponsored by ASCO, ASTRO, the American Gastroenterological Association, and the Society of Surgical Oncology.
“At the other end of the age spectrum, there is evidence that young age can also be an unfavorable prognostic characteristic for gastric cancer,” said Dr. Muro of Aichi Cancer Center Hospital in Nagoya, Japan.
Both RAINBOW and REGARD demonstrated statistically significant and clinically meaningful overall and progression-free survival benefits and acceptable and manageable toxicity with ramucirumab among patients with advanced gastric cancer who were randomized, in the second-line treatment setting, to receive active treatment with the fully humanized monoclonal antibody directed against vascular endothelial growth factor receptor–2 or placebo.
RAINBOW subjects were randomized 1:1 to receive 8 mg/kg of ramucirumab plus paclitaxel, or placebo plus paclitaxel. Among those aged 45 years or less (37 patients in each group), the median overall survival was 9.0 months for treatment vs. 4.2 months for placebo (hazard ratio, 0.555), and the median progression-free survival was 3.9 vs. 2.8 months (HR, 0.299).
The corresponding median overall survival rates for those aged 45-70 (225 and 230 patients in the groups, respectively), 70 or older (68 patients in each group), and 75 or older (20 and 16 patients in the groups, respectively) were 9.6 vs. 7.6 months (HR, 0.860), 10.8 vs. 8.6 months (HR, 0.881), and 11.0 vs. 11.0 months (HR, 0.971). The corresponding progression-free survival rates for those aged 45-70, 70 or older, and 75 or older were 4.6 vs. 2.8 months (HR, 0.649), 4.7 vs. 2.9 months (HR, 0.676), and 4.2 vs. 2.8 months (HR, 0.330).
REGARD subjects were randomized 2:1 to receive 8 mg/kg of ramucirumab plus best supportive care, or placebo plus best supportive care. Among those aged 45 years or less (37 patients in each group), the median overall survival was 9.0 vs. 4.2 months for treatment vs. placebo (HR, 0.555), and the median progression-free survival was 3.9 vs. 2.8 months (HR, 0.299).
Among REGARD subjects aged 45 years or less (28 and 12 patients in the groups, respectively), the median overall survival was 5.8 vs. 2.9 months for treatment vs. placebo (HR, 0.586), and the median progression-free survival was 1.9 vs. 1.4 months (HR, 0.270).
The corresponding median overall survival rates for those aged 45-70 (166 and 70 patients in the groups, respectively), 70 or older (44 and 35 patients in the groups, respectively), and 75 or older (21 and 13 patients in the groups, respectively) were 4.9 vs. 4.1 months (HR, 0.780), 5.9 vs. 3.8 months (HR, 0.730), and 9.3 vs. 5.1 months (HR, 0.588).
The corresponding progression-free survival rates for those aged 45-70, 70 or older, and 75 or older were 2.2 vs. 1.3 months (HR, 0.451), 2.1 vs. 1.4 months (HR, 0.559), 2.8 vs. 1.4 (HR, 0.420).
Baseline characteristics were generally well balanced between arms in each of the age subgroups, Dr. Muro said, noting that no obvious patterns for differential risks in terms of efficacy and adverse events of any grade or of grade 3 or greater were seen according to age. Discontinuation rates for adverse events were similar across different age groups, and quality of life, as determined by global health status, was satisfactory in all age groups.
“Despite some limitations regarding patient numbers in some age subgroups, this exploratory subgroup analysis supports the use of ramucirumab for the treatment of our patients with gastric cancer irrespective of age,” he concluded.
RAINBOW was funded by Eli Lilly. REGARD was funded by ImClone Systems. Dr. Muro reported receiving honoraria from Chugai Pharma, Merck Serono, Taiho Pharmaceutical, Takeda, Eli Lilly, and Yakult Honsha, as well as serving in a consulting or an advisory role for Ono, Merck Serono, and Eli Lilly, and receiving research funding from MSD, Daiichi Sankyo, Ono, Eisai, Pfizer, Chugai, Dainippon Sumitomo, Merck Serono, Janssen Pharmaceutical K.K., AstraZeneca, GlaxoSmithKline, and Kyowa Hakko Kirin.
SAN FRANCISCO – Patients with metastatic gastric or gastroesophageal junction adenocarcinoma benefit from treatment with ramucirumab regardless of their age, according to findings from an exploratory subgroup analysis of the phase III RAINBOW and REGARD studies.
The findings, which show at least a trend toward improvements in most age categories, are important given that nearly two-thirds of patients with these cancers are diagnosed at over age 65 years, and more than half of those are over age 75 years, Kei Muro, MD, reported at the symposium sponsored by ASCO, ASTRO, the American Gastroenterological Association, and the Society of Surgical Oncology.
“At the other end of the age spectrum, there is evidence that young age can also be an unfavorable prognostic characteristic for gastric cancer,” said Dr. Muro of Aichi Cancer Center Hospital in Nagoya, Japan.
Both RAINBOW and REGARD demonstrated statistically significant and clinically meaningful overall and progression-free survival benefits and acceptable and manageable toxicity with ramucirumab among patients with advanced gastric cancer who were randomized, in the second-line treatment setting, to receive active treatment with the fully humanized monoclonal antibody directed against vascular endothelial growth factor receptor–2 or placebo.
RAINBOW subjects were randomized 1:1 to receive 8 mg/kg of ramucirumab plus paclitaxel, or placebo plus paclitaxel. Among those aged 45 years or less (37 patients in each group), the median overall survival was 9.0 months for treatment vs. 4.2 months for placebo (hazard ratio, 0.555), and the median progression-free survival was 3.9 vs. 2.8 months (HR, 0.299).
The corresponding median overall survival rates for those aged 45-70 (225 and 230 patients in the groups, respectively), 70 or older (68 patients in each group), and 75 or older (20 and 16 patients in the groups, respectively) were 9.6 vs. 7.6 months (HR, 0.860), 10.8 vs. 8.6 months (HR, 0.881), and 11.0 vs. 11.0 months (HR, 0.971). The corresponding progression-free survival rates for those aged 45-70, 70 or older, and 75 or older were 4.6 vs. 2.8 months (HR, 0.649), 4.7 vs. 2.9 months (HR, 0.676), and 4.2 vs. 2.8 months (HR, 0.330).
REGARD subjects were randomized 2:1 to receive 8 mg/kg of ramucirumab plus best supportive care, or placebo plus best supportive care. Among those aged 45 years or less (37 patients in each group), the median overall survival was 9.0 vs. 4.2 months for treatment vs. placebo (HR, 0.555), and the median progression-free survival was 3.9 vs. 2.8 months (HR, 0.299).
Among REGARD subjects aged 45 years or less (28 and 12 patients in the groups, respectively), the median overall survival was 5.8 vs. 2.9 months for treatment vs. placebo (HR, 0.586), and the median progression-free survival was 1.9 vs. 1.4 months (HR, 0.270).
The corresponding median overall survival rates for those aged 45-70 (166 and 70 patients in the groups, respectively), 70 or older (44 and 35 patients in the groups, respectively), and 75 or older (21 and 13 patients in the groups, respectively) were 4.9 vs. 4.1 months (HR, 0.780), 5.9 vs. 3.8 months (HR, 0.730), and 9.3 vs. 5.1 months (HR, 0.588).
The corresponding progression-free survival rates for those aged 45-70, 70 or older, and 75 or older were 2.2 vs. 1.3 months (HR, 0.451), 2.1 vs. 1.4 months (HR, 0.559), 2.8 vs. 1.4 (HR, 0.420).
Baseline characteristics were generally well balanced between arms in each of the age subgroups, Dr. Muro said, noting that no obvious patterns for differential risks in terms of efficacy and adverse events of any grade or of grade 3 or greater were seen according to age. Discontinuation rates for adverse events were similar across different age groups, and quality of life, as determined by global health status, was satisfactory in all age groups.
“Despite some limitations regarding patient numbers in some age subgroups, this exploratory subgroup analysis supports the use of ramucirumab for the treatment of our patients with gastric cancer irrespective of age,” he concluded.
RAINBOW was funded by Eli Lilly. REGARD was funded by ImClone Systems. Dr. Muro reported receiving honoraria from Chugai Pharma, Merck Serono, Taiho Pharmaceutical, Takeda, Eli Lilly, and Yakult Honsha, as well as serving in a consulting or an advisory role for Ono, Merck Serono, and Eli Lilly, and receiving research funding from MSD, Daiichi Sankyo, Ono, Eisai, Pfizer, Chugai, Dainippon Sumitomo, Merck Serono, Janssen Pharmaceutical K.K., AstraZeneca, GlaxoSmithKline, and Kyowa Hakko Kirin.
AT THE 2017 GASTROINTESTINAL CANCERS SYMPOSIUM
Key clinical point:
Major finding: Among patients 45-70 years and 70 and older, the hazard ratios for overall survival were 0.860 and 0.881 with ramucirumab vs. placebo in RAINBOW, and 0.780 and 0.730 in REGARD
Data source: The phase III RAINBOW and REGARD trials, including a total of more than 1,000 patients.
Disclosures: Dr. Muro reported receiving honoraria from Chugai Pharma, Merck Serono, Taiho Pharmaceutical, Takeda, Eli Lilly, and Yakult Honsha, as well as serving in a consulting or an advisory role for Ono, Merck Serono, and Eli Lilly, and receiving research funding from MSD, Daiichi Sankyo, Ono, Eisai, Pfizer, Chugai, Dainippon Sumitomo, Merck Serono, Janssen Pharmaceutical K.K., AstraZeneca, GlaxoSmithKline, and Kyowa Hakko Kirin.
Complex congenital heart conditions call for complex care in pregnancy
A new scientific statement from the American Heart Association (AHA) brings together recommendations for management of pregnancy for women with serious congenital heart disease. The 38-page document addresses a wide range of complex congenital heart conditions, presenting a newly unified set of recommendations for care that ranges from preconception counseling, through pregnancy, labor, and delivery, to the postpartum period.
Caring for women with complex congenital heart lesions is becoming more commonplace, as more infants undergo successful repairs of previously-unsurvivable cardiac anomalies. “More moms with congenital heart disease are showing up pregnant, having survived the tumultuous peripartum and neonatal period, and are now facing a new set of risks in pregnancy,” Michael Foley, MD, chair of the department of obstetrics and gynecology at the University of Arizona, Phoenix, said in an interview.
Joseph Kay, MD, a cardiologist and professor of medicine and pediatrics at the University of Colorado, Aurora, said that one big benefit of the new scientific statement is having a single reference point for care of these patients. “The scientific statement brings all of the information about caring for these patients together into one document. This will be a very valuable resource for trainees to get a sense of what’s important; it also represents a platform for new programs to understand the scope of services needed,” said Dr. Kay in an interview.
The document provides a thorough review of the physiologic changes of pregnancy and the intrapartum and postpartum periods, noting that the heterogeneity of congenital heart disease means that women who have different lesions carry different risks in pregnancy.
Examples of lesions presenting intermediate risk include most arrhythmias (category II), hypertrophic cardiomyopathy, and a repaired coarctation (both category II-III). The most severe lesions carry a contraindication for pregnancy; the WHO guidelines suggest discussing termination should women with a category IV lesion become pregnant. Severe mitral stenosis, severe symptomatic aortic stenosis, and severe systemic ventricular dysfunction all place women into category IV.
Beginning with pregnancy risk category III, the WHO guidelines recommend intensive cardiac and obstetric monitoring throughout pregnancy, childbirth, and the puerperium. Several maternal-fetal medicine specialists interviewed all agreed that an interdisciplinary team is a must for good obstetric care in this population.
How interdisciplinary care plays out can depend on geography and facility-dependent resources. Dr. Kay said that his facility is the referral site for pregnant women with complex congenital lesions in an area that spans the Canadian and the Mexican borders from north to south, and ranges from parts of Kansas to eastern Montana from east to west. Still, Dr. Kay said that even for patients with lower-risk lesions, “We will see patients at least once, at approximately the midpoint of pregnancy, and again during the third trimester if possible.” The specifics of care depend on “the nature of the lesion and the complexity of the disease,” said Dr. Kay.
In his facility, said Dr. Kay, telemetry is available for all of the labor and delivery unit beds. This means that the mother and infant can usually stay together and receive postpartum nursing and lactation care from a skilled staff.
In no circumstances should ob.gyns. go it alone, said Dr. Foley. “The conversation with the ob.gyn. needs to be about comanaging these patients, at the very least. Even the most learned maternal-fetal medicine specialist needs to be working with a cardiologist and an anesthesiologist to create a delivery plan that includes pain management, fluid management, and consideration for intrapartum hemodynamic monitoring,” he said.
And the team needs to be in place long before delivery, Dr. Foley pointed out. “In many hospitals, the care delivery gap may be the inability to have this consistent proactive approach. You can’t expect the best outcomes when you have to hurriedly assemble an unfamiliar ad hoc team when a woman with congenital heart disease presents in labor. Despite their best intentions, inconsistent team members may not have the knowledge and experience to provide the safest care for these patients,” he said.
Though an individualized labor and delivery plan is a must, and a multispecialty team should be assembled, maternal congenital heart disease doesn’t necessarily consign a woman to cesarean delivery. “Most women can and should have a vaginal delivery. It’s safer for them. If a natural delivery may increase risk of issues, we may consider a facilitated second stage of labor with epidural anesthesia and forceps- or vacuum-assisted delivery,” said Dr. Kay.
It’s important to understand the nuances of an individual patient’s health and risk status, said Dr. Norton. “A simplified view is often bad. It’s not the case that ‘it’s always better to deliver’ or ‘it’s always better to have a cesarean delivery.’”
Especially for women who need anticoagulation or who may have lesions that put them at great risk should pregnancy occur, preconception counseling is a vital part of their care, and guidance in the scientific statement can help specialists avoid the complications that can occur in the absence of evidence-based treatment. Said Dr. Kay, “I have seen an unfortunate case or two of patients whose anticoagulation was stopped or changed, contrary to guidelines, and who suffered strokes. I hope more people will see this document.”
Ms. Canobbio echoed the sentiment: “You don’t want to have to backpedal once a young woman presents with a pregnancy. Appropriate contraceptive counseling needs to be part of the conversation.”
One key concept underscored in the scientific statement is that elevated risk persists into the postpartum period. “Following delivery, the mother is still at risk for an extended period of time. The greatest risk for mortality in these patients is post delivery, when a large volume of blood is expelled from the uterus back into the maternal circulation,” said Ms. Canobbio. “These women need close follow-up; we can’t say they are home free until several weeks to 2 months after delivery. The need for vigilance and surveillance continues.”
Since the scientific statement is not a new set of guidelines, but rather a compilation of currently existing reference documents, the authors noted that management differences may exist in some cases, but did not assign greater value to one practice than another. “We addressed that there are differences between the European and the American guidelines. For example, with regard to anticoagulation, both would agree to use Lovenox [enoxaparin], but the difference is whether it should be used for the entire pregnancy or for parts of the pregnancy,” said Ms. Canobbio.
Looking forward, more women with complex congenital heart disease will bear children, but their future is not certain. Said Ms. Canobbio: “The data are growing that if the patient is clinically stable at the time of pregnancy, it’s likely we can get them through safely. What’s not yet known is whether the burden of pregnancy in a woman who is otherwise healthy will shorten her lifespan. However, early data are promising, and it’s looking like these women can fare well.”
Topics covered in the scientific statement include:
- Defining which patients are at increased risk in pregnancy.
- Physiological adaptations of pregnancy, the puerperium, and the postpartum period, with an emphasis on hemodynamic changes.
- Assessment and evaluation in the preconception and early prenatal periods.
- Pregnancy management, including appropriate testing.
- Medications in pregnancy, including a table of common cardiac drugs and their pregnancy categories and lactation risks.
- Breakdown of suggested prenatal care by trimester.
- Intrapartum care, including indications for fluid management, ECG and hemodynamic monitoring, and management of the second stage of delivery.
- Postpartum care, with attention to the very rapid increase in blood volume and concomitant leap in stroke volume and cardiac output.
- Considerations when choosing contraceptive method.
- Cardiac complications seen in pregnancy, including arrhythmias, managing mechanical valves and anticoagulation, heart failure, and cyanosis.
- Indications for and risks associated with interventional therapies during pregnancy.
- Detailed discussion of management of pregnancy for women with specific lesions.
None of the members of the writing committee for the scientific statement had relevant disclosures. Dr. Foley and Dr. Kay reported no disclosures. Dr. Norton reported that she has received research funding from Natera and Ultragenyx.
[email protected]
On Twitter @karioakes
A new scientific statement from the American Heart Association (AHA) brings together recommendations for management of pregnancy for women with serious congenital heart disease. The 38-page document addresses a wide range of complex congenital heart conditions, presenting a newly unified set of recommendations for care that ranges from preconception counseling, through pregnancy, labor, and delivery, to the postpartum period.
Caring for women with complex congenital heart lesions is becoming more commonplace, as more infants undergo successful repairs of previously-unsurvivable cardiac anomalies. “More moms with congenital heart disease are showing up pregnant, having survived the tumultuous peripartum and neonatal period, and are now facing a new set of risks in pregnancy,” Michael Foley, MD, chair of the department of obstetrics and gynecology at the University of Arizona, Phoenix, said in an interview.
Joseph Kay, MD, a cardiologist and professor of medicine and pediatrics at the University of Colorado, Aurora, said that one big benefit of the new scientific statement is having a single reference point for care of these patients. “The scientific statement brings all of the information about caring for these patients together into one document. This will be a very valuable resource for trainees to get a sense of what’s important; it also represents a platform for new programs to understand the scope of services needed,” said Dr. Kay in an interview.
The document provides a thorough review of the physiologic changes of pregnancy and the intrapartum and postpartum periods, noting that the heterogeneity of congenital heart disease means that women who have different lesions carry different risks in pregnancy.
Examples of lesions presenting intermediate risk include most arrhythmias (category II), hypertrophic cardiomyopathy, and a repaired coarctation (both category II-III). The most severe lesions carry a contraindication for pregnancy; the WHO guidelines suggest discussing termination should women with a category IV lesion become pregnant. Severe mitral stenosis, severe symptomatic aortic stenosis, and severe systemic ventricular dysfunction all place women into category IV.
Beginning with pregnancy risk category III, the WHO guidelines recommend intensive cardiac and obstetric monitoring throughout pregnancy, childbirth, and the puerperium. Several maternal-fetal medicine specialists interviewed all agreed that an interdisciplinary team is a must for good obstetric care in this population.
How interdisciplinary care plays out can depend on geography and facility-dependent resources. Dr. Kay said that his facility is the referral site for pregnant women with complex congenital lesions in an area that spans the Canadian and the Mexican borders from north to south, and ranges from parts of Kansas to eastern Montana from east to west. Still, Dr. Kay said that even for patients with lower-risk lesions, “We will see patients at least once, at approximately the midpoint of pregnancy, and again during the third trimester if possible.” The specifics of care depend on “the nature of the lesion and the complexity of the disease,” said Dr. Kay.
In his facility, said Dr. Kay, telemetry is available for all of the labor and delivery unit beds. This means that the mother and infant can usually stay together and receive postpartum nursing and lactation care from a skilled staff.
In no circumstances should ob.gyns. go it alone, said Dr. Foley. “The conversation with the ob.gyn. needs to be about comanaging these patients, at the very least. Even the most learned maternal-fetal medicine specialist needs to be working with a cardiologist and an anesthesiologist to create a delivery plan that includes pain management, fluid management, and consideration for intrapartum hemodynamic monitoring,” he said.
And the team needs to be in place long before delivery, Dr. Foley pointed out. “In many hospitals, the care delivery gap may be the inability to have this consistent proactive approach. You can’t expect the best outcomes when you have to hurriedly assemble an unfamiliar ad hoc team when a woman with congenital heart disease presents in labor. Despite their best intentions, inconsistent team members may not have the knowledge and experience to provide the safest care for these patients,” he said.
Though an individualized labor and delivery plan is a must, and a multispecialty team should be assembled, maternal congenital heart disease doesn’t necessarily consign a woman to cesarean delivery. “Most women can and should have a vaginal delivery. It’s safer for them. If a natural delivery may increase risk of issues, we may consider a facilitated second stage of labor with epidural anesthesia and forceps- or vacuum-assisted delivery,” said Dr. Kay.
It’s important to understand the nuances of an individual patient’s health and risk status, said Dr. Norton. “A simplified view is often bad. It’s not the case that ‘it’s always better to deliver’ or ‘it’s always better to have a cesarean delivery.’”
Especially for women who need anticoagulation or who may have lesions that put them at great risk should pregnancy occur, preconception counseling is a vital part of their care, and guidance in the scientific statement can help specialists avoid the complications that can occur in the absence of evidence-based treatment. Said Dr. Kay, “I have seen an unfortunate case or two of patients whose anticoagulation was stopped or changed, contrary to guidelines, and who suffered strokes. I hope more people will see this document.”
Ms. Canobbio echoed the sentiment: “You don’t want to have to backpedal once a young woman presents with a pregnancy. Appropriate contraceptive counseling needs to be part of the conversation.”
One key concept underscored in the scientific statement is that elevated risk persists into the postpartum period. “Following delivery, the mother is still at risk for an extended period of time. The greatest risk for mortality in these patients is post delivery, when a large volume of blood is expelled from the uterus back into the maternal circulation,” said Ms. Canobbio. “These women need close follow-up; we can’t say they are home free until several weeks to 2 months after delivery. The need for vigilance and surveillance continues.”
Since the scientific statement is not a new set of guidelines, but rather a compilation of currently existing reference documents, the authors noted that management differences may exist in some cases, but did not assign greater value to one practice than another. “We addressed that there are differences between the European and the American guidelines. For example, with regard to anticoagulation, both would agree to use Lovenox [enoxaparin], but the difference is whether it should be used for the entire pregnancy or for parts of the pregnancy,” said Ms. Canobbio.
Looking forward, more women with complex congenital heart disease will bear children, but their future is not certain. Said Ms. Canobbio: “The data are growing that if the patient is clinically stable at the time of pregnancy, it’s likely we can get them through safely. What’s not yet known is whether the burden of pregnancy in a woman who is otherwise healthy will shorten her lifespan. However, early data are promising, and it’s looking like these women can fare well.”
Topics covered in the scientific statement include:
- Defining which patients are at increased risk in pregnancy.
- Physiological adaptations of pregnancy, the puerperium, and the postpartum period, with an emphasis on hemodynamic changes.
- Assessment and evaluation in the preconception and early prenatal periods.
- Pregnancy management, including appropriate testing.
- Medications in pregnancy, including a table of common cardiac drugs and their pregnancy categories and lactation risks.
- Breakdown of suggested prenatal care by trimester.
- Intrapartum care, including indications for fluid management, ECG and hemodynamic monitoring, and management of the second stage of delivery.
- Postpartum care, with attention to the very rapid increase in blood volume and concomitant leap in stroke volume and cardiac output.
- Considerations when choosing contraceptive method.
- Cardiac complications seen in pregnancy, including arrhythmias, managing mechanical valves and anticoagulation, heart failure, and cyanosis.
- Indications for and risks associated with interventional therapies during pregnancy.
- Detailed discussion of management of pregnancy for women with specific lesions.
None of the members of the writing committee for the scientific statement had relevant disclosures. Dr. Foley and Dr. Kay reported no disclosures. Dr. Norton reported that she has received research funding from Natera and Ultragenyx.
[email protected]
On Twitter @karioakes
A new scientific statement from the American Heart Association (AHA) brings together recommendations for management of pregnancy for women with serious congenital heart disease. The 38-page document addresses a wide range of complex congenital heart conditions, presenting a newly unified set of recommendations for care that ranges from preconception counseling, through pregnancy, labor, and delivery, to the postpartum period.
Caring for women with complex congenital heart lesions is becoming more commonplace, as more infants undergo successful repairs of previously-unsurvivable cardiac anomalies. “More moms with congenital heart disease are showing up pregnant, having survived the tumultuous peripartum and neonatal period, and are now facing a new set of risks in pregnancy,” Michael Foley, MD, chair of the department of obstetrics and gynecology at the University of Arizona, Phoenix, said in an interview.
Joseph Kay, MD, a cardiologist and professor of medicine and pediatrics at the University of Colorado, Aurora, said that one big benefit of the new scientific statement is having a single reference point for care of these patients. “The scientific statement brings all of the information about caring for these patients together into one document. This will be a very valuable resource for trainees to get a sense of what’s important; it also represents a platform for new programs to understand the scope of services needed,” said Dr. Kay in an interview.
The document provides a thorough review of the physiologic changes of pregnancy and the intrapartum and postpartum periods, noting that the heterogeneity of congenital heart disease means that women who have different lesions carry different risks in pregnancy.
Examples of lesions presenting intermediate risk include most arrhythmias (category II), hypertrophic cardiomyopathy, and a repaired coarctation (both category II-III). The most severe lesions carry a contraindication for pregnancy; the WHO guidelines suggest discussing termination should women with a category IV lesion become pregnant. Severe mitral stenosis, severe symptomatic aortic stenosis, and severe systemic ventricular dysfunction all place women into category IV.
Beginning with pregnancy risk category III, the WHO guidelines recommend intensive cardiac and obstetric monitoring throughout pregnancy, childbirth, and the puerperium. Several maternal-fetal medicine specialists interviewed all agreed that an interdisciplinary team is a must for good obstetric care in this population.
How interdisciplinary care plays out can depend on geography and facility-dependent resources. Dr. Kay said that his facility is the referral site for pregnant women with complex congenital lesions in an area that spans the Canadian and the Mexican borders from north to south, and ranges from parts of Kansas to eastern Montana from east to west. Still, Dr. Kay said that even for patients with lower-risk lesions, “We will see patients at least once, at approximately the midpoint of pregnancy, and again during the third trimester if possible.” The specifics of care depend on “the nature of the lesion and the complexity of the disease,” said Dr. Kay.
In his facility, said Dr. Kay, telemetry is available for all of the labor and delivery unit beds. This means that the mother and infant can usually stay together and receive postpartum nursing and lactation care from a skilled staff.
In no circumstances should ob.gyns. go it alone, said Dr. Foley. “The conversation with the ob.gyn. needs to be about comanaging these patients, at the very least. Even the most learned maternal-fetal medicine specialist needs to be working with a cardiologist and an anesthesiologist to create a delivery plan that includes pain management, fluid management, and consideration for intrapartum hemodynamic monitoring,” he said.
And the team needs to be in place long before delivery, Dr. Foley pointed out. “In many hospitals, the care delivery gap may be the inability to have this consistent proactive approach. You can’t expect the best outcomes when you have to hurriedly assemble an unfamiliar ad hoc team when a woman with congenital heart disease presents in labor. Despite their best intentions, inconsistent team members may not have the knowledge and experience to provide the safest care for these patients,” he said.
Though an individualized labor and delivery plan is a must, and a multispecialty team should be assembled, maternal congenital heart disease doesn’t necessarily consign a woman to cesarean delivery. “Most women can and should have a vaginal delivery. It’s safer for them. If a natural delivery may increase risk of issues, we may consider a facilitated second stage of labor with epidural anesthesia and forceps- or vacuum-assisted delivery,” said Dr. Kay.
It’s important to understand the nuances of an individual patient’s health and risk status, said Dr. Norton. “A simplified view is often bad. It’s not the case that ‘it’s always better to deliver’ or ‘it’s always better to have a cesarean delivery.’”
Especially for women who need anticoagulation or who may have lesions that put them at great risk should pregnancy occur, preconception counseling is a vital part of their care, and guidance in the scientific statement can help specialists avoid the complications that can occur in the absence of evidence-based treatment. Said Dr. Kay, “I have seen an unfortunate case or two of patients whose anticoagulation was stopped or changed, contrary to guidelines, and who suffered strokes. I hope more people will see this document.”
Ms. Canobbio echoed the sentiment: “You don’t want to have to backpedal once a young woman presents with a pregnancy. Appropriate contraceptive counseling needs to be part of the conversation.”
One key concept underscored in the scientific statement is that elevated risk persists into the postpartum period. “Following delivery, the mother is still at risk for an extended period of time. The greatest risk for mortality in these patients is post delivery, when a large volume of blood is expelled from the uterus back into the maternal circulation,” said Ms. Canobbio. “These women need close follow-up; we can’t say they are home free until several weeks to 2 months after delivery. The need for vigilance and surveillance continues.”
Since the scientific statement is not a new set of guidelines, but rather a compilation of currently existing reference documents, the authors noted that management differences may exist in some cases, but did not assign greater value to one practice than another. “We addressed that there are differences between the European and the American guidelines. For example, with regard to anticoagulation, both would agree to use Lovenox [enoxaparin], but the difference is whether it should be used for the entire pregnancy or for parts of the pregnancy,” said Ms. Canobbio.
Looking forward, more women with complex congenital heart disease will bear children, but their future is not certain. Said Ms. Canobbio: “The data are growing that if the patient is clinically stable at the time of pregnancy, it’s likely we can get them through safely. What’s not yet known is whether the burden of pregnancy in a woman who is otherwise healthy will shorten her lifespan. However, early data are promising, and it’s looking like these women can fare well.”
Topics covered in the scientific statement include:
- Defining which patients are at increased risk in pregnancy.
- Physiological adaptations of pregnancy, the puerperium, and the postpartum period, with an emphasis on hemodynamic changes.
- Assessment and evaluation in the preconception and early prenatal periods.
- Pregnancy management, including appropriate testing.
- Medications in pregnancy, including a table of common cardiac drugs and their pregnancy categories and lactation risks.
- Breakdown of suggested prenatal care by trimester.
- Intrapartum care, including indications for fluid management, ECG and hemodynamic monitoring, and management of the second stage of delivery.
- Postpartum care, with attention to the very rapid increase in blood volume and concomitant leap in stroke volume and cardiac output.
- Considerations when choosing contraceptive method.
- Cardiac complications seen in pregnancy, including arrhythmias, managing mechanical valves and anticoagulation, heart failure, and cyanosis.
- Indications for and risks associated with interventional therapies during pregnancy.
- Detailed discussion of management of pregnancy for women with specific lesions.
None of the members of the writing committee for the scientific statement had relevant disclosures. Dr. Foley and Dr. Kay reported no disclosures. Dr. Norton reported that she has received research funding from Natera and Ultragenyx.
[email protected]
On Twitter @karioakes
Childhood obesity tied to maternal obesity, cesarean birth
NEW ORLEANS – Maternal obesity and cesarean delivery were each independently associated with increased rates of overweight or obesity during childhood in a prospective study of 1,441 mothers and their children.
In addition, these risks for childhood obesity appeared to interact in an additive way, so that women who were both obese and delivered by C-section had a nearly threefold increased rate of having a child who was overweight or obese at about 5 years of age, compared with children born to normal-weight women who delivered vaginally, Noel T. Mueller, PhD, said at the American Heart Association Scientific Sessions.
This finding of a link between maternal overweight and obesity and childhood obesity in the next generation supports results from previously reported studies. The new results “also add to the growing evidence for an association between C-section and obesity [in offspring], as well as C-section and immune-related disorders such as asthma and allergies” in offspring, Dr. Mueller said in an interview.
He hypothesized that delivery mode may contribute to a child’s obesity risk by producing an abnormal gastrointestinal microbiome. For example, vaginal delivery seems to associate with a higher prevalence of Bacteroides species in a child’s gut, bacteria that aid in the digestion of breast milk, Dr. Mueller said.
His study used data collected in the Boston Birth Cohort from 1,441 mothers and their children from full-term, singleton pregnancies born to women with a body mass index of at least 18.5 kg/m2 during 1998-2014. The child’s weight was measured at a median age of 4.8 years, with an interquartile range of 3-6 years. Children were deemed overweight if they were at or above the 85th percentile for weight, according to standards from the Centers for Disease Control and Prevention.
Just under half the women were normal weight, slightly more than a quarter were overweight, and a quarter were obese. The incidence of 5-year-old children who were overweight or obese was 70% higher in children of overweight mothers and 80% higher in those with obese mothers, compared with children with normal-weight mothers in an analysis that adjusted for maternal age at delivery, race or ethnicity, and education. Both were statistically significant differences, Dr. Mueller reported.
Two-thirds of the women had vaginal deliveries and a third had C-sections. Overweight or obesity occurred in 40% more of the children delivered by C-section, compared with children born vaginally, a statistically significant difference in an analysis that controlled for the same three covariates as well as prepregnancy body mass index, pregnancy weight gain, and other variables.
When Dr. Mueller and his associates ran a combined analysis they found that the highest risk for childhood overweight or obesity was in children born to obese mothers by C-section, and it was a 2.8-fold higher rate than that in the children born to normal-weight mothers by vaginal delivery, a statistically significant difference.
Dr. Mueller had no disclosures.
[email protected]
On Twitter @mitchelzoler
NEW ORLEANS – Maternal obesity and cesarean delivery were each independently associated with increased rates of overweight or obesity during childhood in a prospective study of 1,441 mothers and their children.
In addition, these risks for childhood obesity appeared to interact in an additive way, so that women who were both obese and delivered by C-section had a nearly threefold increased rate of having a child who was overweight or obese at about 5 years of age, compared with children born to normal-weight women who delivered vaginally, Noel T. Mueller, PhD, said at the American Heart Association Scientific Sessions.
This finding of a link between maternal overweight and obesity and childhood obesity in the next generation supports results from previously reported studies. The new results “also add to the growing evidence for an association between C-section and obesity [in offspring], as well as C-section and immune-related disorders such as asthma and allergies” in offspring, Dr. Mueller said in an interview.
He hypothesized that delivery mode may contribute to a child’s obesity risk by producing an abnormal gastrointestinal microbiome. For example, vaginal delivery seems to associate with a higher prevalence of Bacteroides species in a child’s gut, bacteria that aid in the digestion of breast milk, Dr. Mueller said.
His study used data collected in the Boston Birth Cohort from 1,441 mothers and their children from full-term, singleton pregnancies born to women with a body mass index of at least 18.5 kg/m2 during 1998-2014. The child’s weight was measured at a median age of 4.8 years, with an interquartile range of 3-6 years. Children were deemed overweight if they were at or above the 85th percentile for weight, according to standards from the Centers for Disease Control and Prevention.
Just under half the women were normal weight, slightly more than a quarter were overweight, and a quarter were obese. The incidence of 5-year-old children who were overweight or obese was 70% higher in children of overweight mothers and 80% higher in those with obese mothers, compared with children with normal-weight mothers in an analysis that adjusted for maternal age at delivery, race or ethnicity, and education. Both were statistically significant differences, Dr. Mueller reported.
Two-thirds of the women had vaginal deliveries and a third had C-sections. Overweight or obesity occurred in 40% more of the children delivered by C-section, compared with children born vaginally, a statistically significant difference in an analysis that controlled for the same three covariates as well as prepregnancy body mass index, pregnancy weight gain, and other variables.
When Dr. Mueller and his associates ran a combined analysis they found that the highest risk for childhood overweight or obesity was in children born to obese mothers by C-section, and it was a 2.8-fold higher rate than that in the children born to normal-weight mothers by vaginal delivery, a statistically significant difference.
Dr. Mueller had no disclosures.
[email protected]
On Twitter @mitchelzoler
NEW ORLEANS – Maternal obesity and cesarean delivery were each independently associated with increased rates of overweight or obesity during childhood in a prospective study of 1,441 mothers and their children.
In addition, these risks for childhood obesity appeared to interact in an additive way, so that women who were both obese and delivered by C-section had a nearly threefold increased rate of having a child who was overweight or obese at about 5 years of age, compared with children born to normal-weight women who delivered vaginally, Noel T. Mueller, PhD, said at the American Heart Association Scientific Sessions.
This finding of a link between maternal overweight and obesity and childhood obesity in the next generation supports results from previously reported studies. The new results “also add to the growing evidence for an association between C-section and obesity [in offspring], as well as C-section and immune-related disorders such as asthma and allergies” in offspring, Dr. Mueller said in an interview.
He hypothesized that delivery mode may contribute to a child’s obesity risk by producing an abnormal gastrointestinal microbiome. For example, vaginal delivery seems to associate with a higher prevalence of Bacteroides species in a child’s gut, bacteria that aid in the digestion of breast milk, Dr. Mueller said.
His study used data collected in the Boston Birth Cohort from 1,441 mothers and their children from full-term, singleton pregnancies born to women with a body mass index of at least 18.5 kg/m2 during 1998-2014. The child’s weight was measured at a median age of 4.8 years, with an interquartile range of 3-6 years. Children were deemed overweight if they were at or above the 85th percentile for weight, according to standards from the Centers for Disease Control and Prevention.
Just under half the women were normal weight, slightly more than a quarter were overweight, and a quarter were obese. The incidence of 5-year-old children who were overweight or obese was 70% higher in children of overweight mothers and 80% higher in those with obese mothers, compared with children with normal-weight mothers in an analysis that adjusted for maternal age at delivery, race or ethnicity, and education. Both were statistically significant differences, Dr. Mueller reported.
Two-thirds of the women had vaginal deliveries and a third had C-sections. Overweight or obesity occurred in 40% more of the children delivered by C-section, compared with children born vaginally, a statistically significant difference in an analysis that controlled for the same three covariates as well as prepregnancy body mass index, pregnancy weight gain, and other variables.
When Dr. Mueller and his associates ran a combined analysis they found that the highest risk for childhood overweight or obesity was in children born to obese mothers by C-section, and it was a 2.8-fold higher rate than that in the children born to normal-weight mothers by vaginal delivery, a statistically significant difference.
Dr. Mueller had no disclosures.
[email protected]
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Children from obese mothers who had cesarean sections had a 2.8-fold higher obesity rate, compared with children from normal-weight mothers who had vaginal deliveries.
Data source: The Boston Birth Cohort, with prospective data from 1,441 pregnant women and their children.
Disclosures: Dr. Mueller had no disclosures.
Observe, assess, intervene
On most days when I walk into the exam room for a well-child visit, I find an anxious mom or a fretful father sitting next to a fearless child. I quickly shove aside my unrealistic expectation of finding both parents together holding the child. During the progression of my day, I see a diversity of parents playing their parts in caring for their children. It is sometimes a single mom, strong and robust, surrounded by a firm aura of principles and rules; she is concealing all signs of weakness, to make sure her child doesn’t cross any line that she has so cautiously made. Sometimes it is a single mom who is nervous and scared with a galaxy of fear in her eyes, desperately seeking for reassurance of her parenting. Fathers also come playing many roles, from someone struggling with tears as his child gets immunizations, to someone who has parenting in his bag, and skillfully plays eeny meeny miny moe with the little ones in the waiting room.
They all have one thing in common: the immense love for their children and the pressure of being a single or separated parent. It is indeed a reality that I see in most of the clinic rooms – that 60%-70% of children are not living with both mom and dad in the same house. Please note that these are raw data based entirely on my observation. While I watch each parent struggling as mentioned above, my mind often wanders to how each young child copes with such a situation.
What is our role as pediatricians as we walk into the exam room, as we encounter these different family dynamics? To simplify it for myself, I divide it into three categories: Observe, assess, and intervene. Most of the time as physicians, our gut feelings and instincts guide us to where help is needed. It is important to anticipate the changes a family might go through as we meet a first-time single mom or a family who has recently been separated. As we anticipate and observe, it also is important to ask specific questions of parents who may not feel comfortable volunteering this information:
• “Are you and your child undergoing any sort of stress?”
• “How do you think your child is coping with the separation?”
• “Do you identify any flaws in how things are going now?”
Of course, we need to ask questions about stress and family dynamics of all parents. We also should maintain a high level of sensitivity as we approach such questions. It is important to identify any changes in a child’s emotional and social development as we see them on every visit. And when we deem the need, to intervene and identify resources for the family. We also can help parents with ideas for communication with the child; anger management; helping parents and children understand changes; and encouraging open discussion when possible, instead of bottling up unsaid feelings and emotions. This is especially true for single-parent families, but two-parent families undergo stresses as well, for which pediatricians should keep an eye out.
While it is extremely important for us on every well-child visit to ensure that a child’s physical health is up to par, it is equally important not to ignore their emotional and social well-being as we walk in the room so we can help them flourish into the best version of themselves.
Dr. Fatima is a first-year pediatric resident at Albert Einstein Medical Center, Philadelphia. Email her at [email protected].
On most days when I walk into the exam room for a well-child visit, I find an anxious mom or a fretful father sitting next to a fearless child. I quickly shove aside my unrealistic expectation of finding both parents together holding the child. During the progression of my day, I see a diversity of parents playing their parts in caring for their children. It is sometimes a single mom, strong and robust, surrounded by a firm aura of principles and rules; she is concealing all signs of weakness, to make sure her child doesn’t cross any line that she has so cautiously made. Sometimes it is a single mom who is nervous and scared with a galaxy of fear in her eyes, desperately seeking for reassurance of her parenting. Fathers also come playing many roles, from someone struggling with tears as his child gets immunizations, to someone who has parenting in his bag, and skillfully plays eeny meeny miny moe with the little ones in the waiting room.
They all have one thing in common: the immense love for their children and the pressure of being a single or separated parent. It is indeed a reality that I see in most of the clinic rooms – that 60%-70% of children are not living with both mom and dad in the same house. Please note that these are raw data based entirely on my observation. While I watch each parent struggling as mentioned above, my mind often wanders to how each young child copes with such a situation.
What is our role as pediatricians as we walk into the exam room, as we encounter these different family dynamics? To simplify it for myself, I divide it into three categories: Observe, assess, and intervene. Most of the time as physicians, our gut feelings and instincts guide us to where help is needed. It is important to anticipate the changes a family might go through as we meet a first-time single mom or a family who has recently been separated. As we anticipate and observe, it also is important to ask specific questions of parents who may not feel comfortable volunteering this information:
• “Are you and your child undergoing any sort of stress?”
• “How do you think your child is coping with the separation?”
• “Do you identify any flaws in how things are going now?”
Of course, we need to ask questions about stress and family dynamics of all parents. We also should maintain a high level of sensitivity as we approach such questions. It is important to identify any changes in a child’s emotional and social development as we see them on every visit. And when we deem the need, to intervene and identify resources for the family. We also can help parents with ideas for communication with the child; anger management; helping parents and children understand changes; and encouraging open discussion when possible, instead of bottling up unsaid feelings and emotions. This is especially true for single-parent families, but two-parent families undergo stresses as well, for which pediatricians should keep an eye out.
While it is extremely important for us on every well-child visit to ensure that a child’s physical health is up to par, it is equally important not to ignore their emotional and social well-being as we walk in the room so we can help them flourish into the best version of themselves.
Dr. Fatima is a first-year pediatric resident at Albert Einstein Medical Center, Philadelphia. Email her at [email protected].
On most days when I walk into the exam room for a well-child visit, I find an anxious mom or a fretful father sitting next to a fearless child. I quickly shove aside my unrealistic expectation of finding both parents together holding the child. During the progression of my day, I see a diversity of parents playing their parts in caring for their children. It is sometimes a single mom, strong and robust, surrounded by a firm aura of principles and rules; she is concealing all signs of weakness, to make sure her child doesn’t cross any line that she has so cautiously made. Sometimes it is a single mom who is nervous and scared with a galaxy of fear in her eyes, desperately seeking for reassurance of her parenting. Fathers also come playing many roles, from someone struggling with tears as his child gets immunizations, to someone who has parenting in his bag, and skillfully plays eeny meeny miny moe with the little ones in the waiting room.
They all have one thing in common: the immense love for their children and the pressure of being a single or separated parent. It is indeed a reality that I see in most of the clinic rooms – that 60%-70% of children are not living with both mom and dad in the same house. Please note that these are raw data based entirely on my observation. While I watch each parent struggling as mentioned above, my mind often wanders to how each young child copes with such a situation.
What is our role as pediatricians as we walk into the exam room, as we encounter these different family dynamics? To simplify it for myself, I divide it into three categories: Observe, assess, and intervene. Most of the time as physicians, our gut feelings and instincts guide us to where help is needed. It is important to anticipate the changes a family might go through as we meet a first-time single mom or a family who has recently been separated. As we anticipate and observe, it also is important to ask specific questions of parents who may not feel comfortable volunteering this information:
• “Are you and your child undergoing any sort of stress?”
• “How do you think your child is coping with the separation?”
• “Do you identify any flaws in how things are going now?”
Of course, we need to ask questions about stress and family dynamics of all parents. We also should maintain a high level of sensitivity as we approach such questions. It is important to identify any changes in a child’s emotional and social development as we see them on every visit. And when we deem the need, to intervene and identify resources for the family. We also can help parents with ideas for communication with the child; anger management; helping parents and children understand changes; and encouraging open discussion when possible, instead of bottling up unsaid feelings and emotions. This is especially true for single-parent families, but two-parent families undergo stresses as well, for which pediatricians should keep an eye out.
While it is extremely important for us on every well-child visit to ensure that a child’s physical health is up to par, it is equally important not to ignore their emotional and social well-being as we walk in the room so we can help them flourish into the best version of themselves.
Dr. Fatima is a first-year pediatric resident at Albert Einstein Medical Center, Philadelphia. Email her at [email protected].
Don’t delay pneumococcal conjugate vaccine for preterm infants
There should be no hesitation in administering the routine vaccination schedule for 13-valent pneumococcal conjugate vaccine (PCV13) on account of gestational age or birth weight in preterm infants, researchers concluded.
In a phase IV study, researchers compared 100 term with 100 preterm infants; both groups were vaccinated on the routine schedule at ages 2, 3, 4, and 12 months. After the 12-month (toddler) dose of the PCV13, the infants were evaluated for serum antibody persistence at 12 and 24 months. “To date, no studies have examined the long-term persistence of immune responses to PCV13 in formerly preterm infants,” noted Federico Martinón-Torres, MD, PhD, of Hospital Clínico Universitario de Santiago de Compostela, Spain, and his coauthors.
In the study, at six sites in Spain and five sites in Poland between October 2010 and January 2014, both groups were checked for geometric mean concentrations of serotype-specific anticapsular immunoglobulin G binding antibodies and for opsonophagocytic activity. All 200 subjects were white and were generally healthy; the preterm infants were grouped by gestational age at birth of less than 29 weeks (n = 25), 29 weeks to less than 32 weeks (n = 50), or 32 weeks to less than 37 weeks (n = 25). Twelve subjects dropped out of the study by the first year’s evaluation, and another eight of the term subjects and seven of preterm subjects dropped out by the second year’s evaluation (Ped Infect Dis J. 2017. doi: 10.1097/INF.0000000000001428).
At both follow-up time points, no discernible patterns were observed in IgG GMCs for any serotype or in opsonophagocytic activity geometric mean titers across preterm subgroups based on gestational age.
“The vaccination phase of the study demonstrated that preterm infants are able to generate an immune response to PCV13 that is likely to protect against invasive pneumococcal disease. However, IgG GMCs were lower in preterm than term infants for nearly half of the serotypes at all time points. Antipneumococcal IgG levels in preterm infants were generally lower than in term infants, but fewer differences in opsonophagocytic activity were seen between the groups,” Dr. Martinón-Torres and his associates reported.
Pfizer funded the study. Dr. Martinón-Torres reported receiving research grants and/or honoraria as a consultant/adviser and/or speaker and for conducting vaccine trials for GlaxoSmithKline, MedImmune, Merck, Novartis, Pfizer/Wyeth, Sanofi Pasteur, and the Carlos III Health Institute. Several coauthors disclosed ties with pharmaceutical companies; four are stock-holding employees of Pfizer and another is an employee of a company contracted by Pfizer.
There should be no hesitation in administering the routine vaccination schedule for 13-valent pneumococcal conjugate vaccine (PCV13) on account of gestational age or birth weight in preterm infants, researchers concluded.
In a phase IV study, researchers compared 100 term with 100 preterm infants; both groups were vaccinated on the routine schedule at ages 2, 3, 4, and 12 months. After the 12-month (toddler) dose of the PCV13, the infants were evaluated for serum antibody persistence at 12 and 24 months. “To date, no studies have examined the long-term persistence of immune responses to PCV13 in formerly preterm infants,” noted Federico Martinón-Torres, MD, PhD, of Hospital Clínico Universitario de Santiago de Compostela, Spain, and his coauthors.
In the study, at six sites in Spain and five sites in Poland between October 2010 and January 2014, both groups were checked for geometric mean concentrations of serotype-specific anticapsular immunoglobulin G binding antibodies and for opsonophagocytic activity. All 200 subjects were white and were generally healthy; the preterm infants were grouped by gestational age at birth of less than 29 weeks (n = 25), 29 weeks to less than 32 weeks (n = 50), or 32 weeks to less than 37 weeks (n = 25). Twelve subjects dropped out of the study by the first year’s evaluation, and another eight of the term subjects and seven of preterm subjects dropped out by the second year’s evaluation (Ped Infect Dis J. 2017. doi: 10.1097/INF.0000000000001428).
At both follow-up time points, no discernible patterns were observed in IgG GMCs for any serotype or in opsonophagocytic activity geometric mean titers across preterm subgroups based on gestational age.
“The vaccination phase of the study demonstrated that preterm infants are able to generate an immune response to PCV13 that is likely to protect against invasive pneumococcal disease. However, IgG GMCs were lower in preterm than term infants for nearly half of the serotypes at all time points. Antipneumococcal IgG levels in preterm infants were generally lower than in term infants, but fewer differences in opsonophagocytic activity were seen between the groups,” Dr. Martinón-Torres and his associates reported.
Pfizer funded the study. Dr. Martinón-Torres reported receiving research grants and/or honoraria as a consultant/adviser and/or speaker and for conducting vaccine trials for GlaxoSmithKline, MedImmune, Merck, Novartis, Pfizer/Wyeth, Sanofi Pasteur, and the Carlos III Health Institute. Several coauthors disclosed ties with pharmaceutical companies; four are stock-holding employees of Pfizer and another is an employee of a company contracted by Pfizer.
There should be no hesitation in administering the routine vaccination schedule for 13-valent pneumococcal conjugate vaccine (PCV13) on account of gestational age or birth weight in preterm infants, researchers concluded.
In a phase IV study, researchers compared 100 term with 100 preterm infants; both groups were vaccinated on the routine schedule at ages 2, 3, 4, and 12 months. After the 12-month (toddler) dose of the PCV13, the infants were evaluated for serum antibody persistence at 12 and 24 months. “To date, no studies have examined the long-term persistence of immune responses to PCV13 in formerly preterm infants,” noted Federico Martinón-Torres, MD, PhD, of Hospital Clínico Universitario de Santiago de Compostela, Spain, and his coauthors.
In the study, at six sites in Spain and five sites in Poland between October 2010 and January 2014, both groups were checked for geometric mean concentrations of serotype-specific anticapsular immunoglobulin G binding antibodies and for opsonophagocytic activity. All 200 subjects were white and were generally healthy; the preterm infants were grouped by gestational age at birth of less than 29 weeks (n = 25), 29 weeks to less than 32 weeks (n = 50), or 32 weeks to less than 37 weeks (n = 25). Twelve subjects dropped out of the study by the first year’s evaluation, and another eight of the term subjects and seven of preterm subjects dropped out by the second year’s evaluation (Ped Infect Dis J. 2017. doi: 10.1097/INF.0000000000001428).
At both follow-up time points, no discernible patterns were observed in IgG GMCs for any serotype or in opsonophagocytic activity geometric mean titers across preterm subgroups based on gestational age.
“The vaccination phase of the study demonstrated that preterm infants are able to generate an immune response to PCV13 that is likely to protect against invasive pneumococcal disease. However, IgG GMCs were lower in preterm than term infants for nearly half of the serotypes at all time points. Antipneumococcal IgG levels in preterm infants were generally lower than in term infants, but fewer differences in opsonophagocytic activity were seen between the groups,” Dr. Martinón-Torres and his associates reported.
Pfizer funded the study. Dr. Martinón-Torres reported receiving research grants and/or honoraria as a consultant/adviser and/or speaker and for conducting vaccine trials for GlaxoSmithKline, MedImmune, Merck, Novartis, Pfizer/Wyeth, Sanofi Pasteur, and the Carlos III Health Institute. Several coauthors disclosed ties with pharmaceutical companies; four are stock-holding employees of Pfizer and another is an employee of a company contracted by Pfizer.
Key clinical point:
Major finding: IgG GMCs were lower in preterm than term infants for nearly half of the serotypes at all time points. Antipneumococcal IgG levels in preterm infants were generally lower than in term infants, but fewer differences in opsonophagocytic activity were seen between the groups.
Data source: In a phase IV study, 100 term and 100 preterm infants were evaluated for serum antibody persistence at 12 and 24 months.
Disclosures: Pfizer funded the study. Dr. Martinón-Torres reported receiving research grants and/or honoraria as a consultant/adviser and/or speaker and for conducting vaccine trials for GlaxoSmithKline, MedImmune, Merck, Novartis, Pfizer/Wyeth, Sanofi Pasteur, and the Carlos III Health Institute. Several coauthors disclosed ties with pharmaceutical companies; four are stock-holding employees of Pfizer and another is an employee of a company contracted by Pfizer.
Severe Henoch-Schönlein Purpura Complicating Infliximab Therapy for Ulcerative Colitis
To the Editor:
Anti–tumor necrosis factor (TNF) α treatments have radically improved the management of chronic inflammatory conditions, including rheumatoid arthritis, ankylosing spondylitis, psoriasis and psoriatic arthritis, and bowel diseases (eg, Crohn disease, ulcerative colitis [UC]). Because the number of patients treated with these agents has increased, uncommon adverse reactions have increasingly occurred. Cutaneous adverse reactions that have been reported with anti-TNF agents include immediate injection-site reaction, systemic infusion reactions, and delayed reactions.1 Among the delayed adverse reactions, psoriatic and eczematous eruptions as well as cutaneous infections are the most common, while cutaneous adverse effects related to an immune imbalance syndrome including vasculitis; lupuslike, lichenlike, and granulomatous eruptions; and skin cancer rarely are observed.1 Although most of the cutaneous adverse effects do not require anti-TNF treatment discontinuation and are resolved with symptomatic treatment, anti-TNF therapy must be stopped in more severe cases. We report the case of severe Henoch-Schönlein purpura (HSP) following treatment with infliximab.
A 46-year-old man who was a nonsmoker with quiescent UC on infliximab for 30 months presented with palpable necrotic purpura on both legs (Figure) and arms as well as the abdomen of 10 days’ duration, along with diffuse joint pain and swelling. He had no history of infectious or gastrointestinal symptoms. The last infliximab infusion was performed 6 weeks prior to developing the purpura. His UC was diagnosed 10 years prior to the current presentation and was not associated with any extragastrointestinal manifestations. Since diagnosis, UC had failed to respond to therapies such as azathioprine, cyclosporine, and purinethol. The complete blood cell count was normal. The C-reactive protein level was 18.7 mg/L (reference range, <5 mg/L) and the erythrocyte sedimentation rate was 30 mm/h (reference range, 0–20 mm/h). Electrolytes, urea, creatinine clearance, and liver function were normal, and a chest radiograph and radiographs of the swollen joints were unremarkable. The total IgA level was elevated at 4 g/L (reference range, 0.7–4 g/L), with IgG and IgM levels within reference range. There was no hematuria or proteinuria on urinalysis. Tests for antinuclear antibodies, rheumatoid factor, circulating immune complexes, and antineutrophil cytoplasmic antibody were negative. Total complement, C3, and C4 levels also were normal. A skin biopsy confirmed a leukocytoclastic vasculitis of small vessels with C3 deposition. Serologic tests for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus were negative. Based on these findings, the diagnosis of HSP was made. Systemic corticosteroids—120 mg daily of intravenous methylprednisolone for 3 days, followed by 1 mg/kg daily of oral prednisone for 2 weeks—were then introduced with rapid clinical improvement. Henoch-Schönlein purpura and joint symptoms completely resolved, but UC relapsed with bloody diarrhea and severe abdominal pain. Oral prednisone was maintained (1 mg/kg daily). Because of the severity of cutaneous vasculitis (HSP), a multidisciplinary decision was taken to definitively stop the anti-TNF agents and to first add azathioprine (2 mg/kg daily for 2 months), then subcutaneous methotrexate (25 mg weekly). Colonoscopy did not show any dysplasia or adenocarcinoma and confirmed the diagnosis of UC. After 6 months of combined therapy, UC was still active and we decided to perform a total colectomy with ileostomy formation. Complete remission of UC was obtained and maintained after 28 months of follow-up.
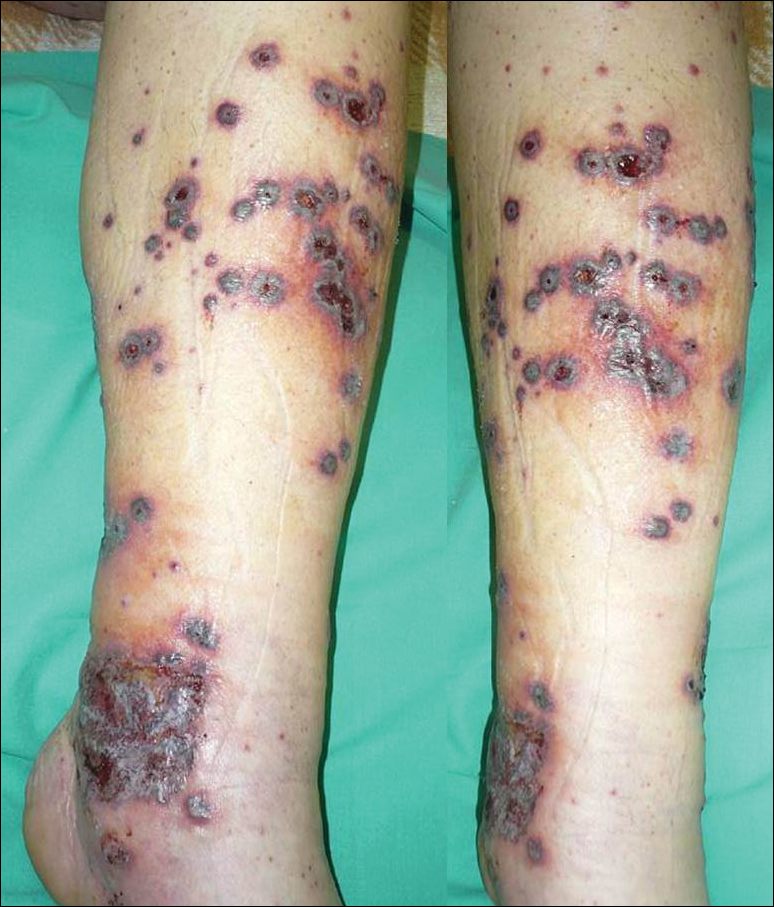
Henoch-Schönlein purpura is a multisystem small vessel leukocytoclastic vasculitis with the deposition of immune complexes containing IgA. Clinical manifestations may include palpable purpura, arthritis, enteritis, and nephritis. Henoch-Schönlein purpura usually affects children. Adult onset is rare but associated with more severe symptoms and a poor prognosis.2 The criteria for HSP, as defined by the American College of Rheumatology,3 include palpable purpura, 20 years or younger at disease onset, bowel angina, and presence of vascular wall granulocytes on biopsy. At least 2 of these criteria are required for HSP diagnosis. Various viral or bacterial infections and drugs can trigger HSP, which also can be associated with autoinflammatory or autoimmune diseases. The association of HSP and UC is a rare event, as demonstrated by de Oliveira et al.4 Although only 2 cases of cutaneous vasculitis mimicking HSP have been described in UC,4 we cannot exclude a possible association between HSP and UC. However, our patient had UC for 10 years and never had clinical manifestations of vasculitis.
There are 5 reports of HSP following etanercept5,6 or adalimumab7-9 therapy and 1 following infliximab therapy.10 In all cases, HSP occurred after several months of anti-TNF therapy. However, there also are reports of cutaneous vasculitis associated with arthralgia and glomerulonephritis that resolved after withdrawal of anti-TNF agents.11,12 It is possible that some of these reactions may have been manifestations of undiagnosed HSP. In a series of 113 patients who developed cutaneous vasculitis after anti-TNF agents, visceral vasculitis was observed in 24% of patients. Treatment of vasculitis involved withdrawal of the anti-TNF therapy in 101 cases (89%).13 In these UC patients with few therapeutic alternatives, the continuation of anti-TNF agents should be discussed. In the previous series,13 of 16 patients who were rechallenged with the same or a different TNF antagonist, 12 (75%) experienced vasculitis relapse, suggesting a class effect of TNF inhibition. Because of the severity of cutaneous vasculitis and as previously suggested in a recent analytical and comprehensive overview on paradoxical reactions under TNF blockers,1 we decided not to re-expose our patient to infliximab or to other anti-TNF agents.
In conclusion, HSP may occur during anti-TNF therapy and physicians need to be aware of this potentially serious complication.
- Toussirot É, Aubin F. Paradoxical reactions under TNF-α blocking agents and other biological agents given for chronic immune-mediated diseases: an analytical and comprehensive overview. RMD Open. 2016;2:e000239.
- Saulsbury FT. Henoch-Schönlein purpura. Curr Opin Rheumatol. 2001;13:35-40.
- Ortiz-Sanjuán F, Blanco R, Hernández JL, et al. Applicability of the 2006 European League Against Rheumatism (EULAR) criteria for the classification ofHenoch-Schönlein purpura. an analysis based on 766 patients with cutaneous vasculitis. Clin Exp Rheumatol. 2015;33(2, suppl 89):S44-S47.
- de Oliveira GT, Martins SS, Deboni M, et al. Cutaneous vasculitis in ulcerative colitis mimicking Henoch-Schönlein purpura [published online May 22, 2012]. J Crohns Colitis. 2013;7:e69-e73.
- Marques I, Lagos A, Reis J, et al. Reversible Henoch-Schönlein purpura complicating adalimumab therapy. J Crohns Colitis. 2012;6:796-799.
- Rahman FZ, Takhar GK, Roy O, et al. Henoch-Schönlein purpura complicating adalimumab therapy for Crohn’s disease. World J Gastrointest Pharmacol Ther. 2010;1:119-122.
- Lee A, Kasama R, Evangelisto A, et al. Henoch-Schönlein purpura after etanercept therapy for psoriasis. J Clin Rheumatol. 2006;12:249-251.
- Duffy TN, Genta M, Moll S, et al. Henoch Schönlein purpura following etanercept treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2006;24(2, suppl 41):S106.
- LaConti JJ, Donet JA, Cho-Vega JH, et al. Henoch-Schönlein purpura with adalimumab therapy for ulcerative colitis: a case report and review of the literature. Case Rep Rheumatol. 2016:2812980.
- Nobile S, Catassi C, Felici L. Herpes zoster infection followed by Henoch-Schönlein purpura in a girl receiving infliximab for ulcerative colitis. J Clin Rheumatol. 2009;15:101.
- Mohan N, Edwards ET, Cupps TR, et al. Leukocytoclastic vasculitis associated with tumor necrosis factor-alpha blocking agents. J Rheumatol. 2004;31:1955-1958.
- Simms R, Kipgen D, Dahill S, et al. ANCA-associated renal vasculitis following anti-tumor necrosis factor alpha therapy. Am J Kidney Dis. 2008;51:e11-e14.
- Ramos-Casals M, Brito-Zerón P, Muñoz S, et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore). 2007;86:242-251.
To the Editor:
Anti–tumor necrosis factor (TNF) α treatments have radically improved the management of chronic inflammatory conditions, including rheumatoid arthritis, ankylosing spondylitis, psoriasis and psoriatic arthritis, and bowel diseases (eg, Crohn disease, ulcerative colitis [UC]). Because the number of patients treated with these agents has increased, uncommon adverse reactions have increasingly occurred. Cutaneous adverse reactions that have been reported with anti-TNF agents include immediate injection-site reaction, systemic infusion reactions, and delayed reactions.1 Among the delayed adverse reactions, psoriatic and eczematous eruptions as well as cutaneous infections are the most common, while cutaneous adverse effects related to an immune imbalance syndrome including vasculitis; lupuslike, lichenlike, and granulomatous eruptions; and skin cancer rarely are observed.1 Although most of the cutaneous adverse effects do not require anti-TNF treatment discontinuation and are resolved with symptomatic treatment, anti-TNF therapy must be stopped in more severe cases. We report the case of severe Henoch-Schönlein purpura (HSP) following treatment with infliximab.
A 46-year-old man who was a nonsmoker with quiescent UC on infliximab for 30 months presented with palpable necrotic purpura on both legs (Figure) and arms as well as the abdomen of 10 days’ duration, along with diffuse joint pain and swelling. He had no history of infectious or gastrointestinal symptoms. The last infliximab infusion was performed 6 weeks prior to developing the purpura. His UC was diagnosed 10 years prior to the current presentation and was not associated with any extragastrointestinal manifestations. Since diagnosis, UC had failed to respond to therapies such as azathioprine, cyclosporine, and purinethol. The complete blood cell count was normal. The C-reactive protein level was 18.7 mg/L (reference range, <5 mg/L) and the erythrocyte sedimentation rate was 30 mm/h (reference range, 0–20 mm/h). Electrolytes, urea, creatinine clearance, and liver function were normal, and a chest radiograph and radiographs of the swollen joints were unremarkable. The total IgA level was elevated at 4 g/L (reference range, 0.7–4 g/L), with IgG and IgM levels within reference range. There was no hematuria or proteinuria on urinalysis. Tests for antinuclear antibodies, rheumatoid factor, circulating immune complexes, and antineutrophil cytoplasmic antibody were negative. Total complement, C3, and C4 levels also were normal. A skin biopsy confirmed a leukocytoclastic vasculitis of small vessels with C3 deposition. Serologic tests for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus were negative. Based on these findings, the diagnosis of HSP was made. Systemic corticosteroids—120 mg daily of intravenous methylprednisolone for 3 days, followed by 1 mg/kg daily of oral prednisone for 2 weeks—were then introduced with rapid clinical improvement. Henoch-Schönlein purpura and joint symptoms completely resolved, but UC relapsed with bloody diarrhea and severe abdominal pain. Oral prednisone was maintained (1 mg/kg daily). Because of the severity of cutaneous vasculitis (HSP), a multidisciplinary decision was taken to definitively stop the anti-TNF agents and to first add azathioprine (2 mg/kg daily for 2 months), then subcutaneous methotrexate (25 mg weekly). Colonoscopy did not show any dysplasia or adenocarcinoma and confirmed the diagnosis of UC. After 6 months of combined therapy, UC was still active and we decided to perform a total colectomy with ileostomy formation. Complete remission of UC was obtained and maintained after 28 months of follow-up.

Henoch-Schönlein purpura is a multisystem small vessel leukocytoclastic vasculitis with the deposition of immune complexes containing IgA. Clinical manifestations may include palpable purpura, arthritis, enteritis, and nephritis. Henoch-Schönlein purpura usually affects children. Adult onset is rare but associated with more severe symptoms and a poor prognosis.2 The criteria for HSP, as defined by the American College of Rheumatology,3 include palpable purpura, 20 years or younger at disease onset, bowel angina, and presence of vascular wall granulocytes on biopsy. At least 2 of these criteria are required for HSP diagnosis. Various viral or bacterial infections and drugs can trigger HSP, which also can be associated with autoinflammatory or autoimmune diseases. The association of HSP and UC is a rare event, as demonstrated by de Oliveira et al.4 Although only 2 cases of cutaneous vasculitis mimicking HSP have been described in UC,4 we cannot exclude a possible association between HSP and UC. However, our patient had UC for 10 years and never had clinical manifestations of vasculitis.
There are 5 reports of HSP following etanercept5,6 or adalimumab7-9 therapy and 1 following infliximab therapy.10 In all cases, HSP occurred after several months of anti-TNF therapy. However, there also are reports of cutaneous vasculitis associated with arthralgia and glomerulonephritis that resolved after withdrawal of anti-TNF agents.11,12 It is possible that some of these reactions may have been manifestations of undiagnosed HSP. In a series of 113 patients who developed cutaneous vasculitis after anti-TNF agents, visceral vasculitis was observed in 24% of patients. Treatment of vasculitis involved withdrawal of the anti-TNF therapy in 101 cases (89%).13 In these UC patients with few therapeutic alternatives, the continuation of anti-TNF agents should be discussed. In the previous series,13 of 16 patients who were rechallenged with the same or a different TNF antagonist, 12 (75%) experienced vasculitis relapse, suggesting a class effect of TNF inhibition. Because of the severity of cutaneous vasculitis and as previously suggested in a recent analytical and comprehensive overview on paradoxical reactions under TNF blockers,1 we decided not to re-expose our patient to infliximab or to other anti-TNF agents.
In conclusion, HSP may occur during anti-TNF therapy and physicians need to be aware of this potentially serious complication.
To the Editor:
Anti–tumor necrosis factor (TNF) α treatments have radically improved the management of chronic inflammatory conditions, including rheumatoid arthritis, ankylosing spondylitis, psoriasis and psoriatic arthritis, and bowel diseases (eg, Crohn disease, ulcerative colitis [UC]). Because the number of patients treated with these agents has increased, uncommon adverse reactions have increasingly occurred. Cutaneous adverse reactions that have been reported with anti-TNF agents include immediate injection-site reaction, systemic infusion reactions, and delayed reactions.1 Among the delayed adverse reactions, psoriatic and eczematous eruptions as well as cutaneous infections are the most common, while cutaneous adverse effects related to an immune imbalance syndrome including vasculitis; lupuslike, lichenlike, and granulomatous eruptions; and skin cancer rarely are observed.1 Although most of the cutaneous adverse effects do not require anti-TNF treatment discontinuation and are resolved with symptomatic treatment, anti-TNF therapy must be stopped in more severe cases. We report the case of severe Henoch-Schönlein purpura (HSP) following treatment with infliximab.
A 46-year-old man who was a nonsmoker with quiescent UC on infliximab for 30 months presented with palpable necrotic purpura on both legs (Figure) and arms as well as the abdomen of 10 days’ duration, along with diffuse joint pain and swelling. He had no history of infectious or gastrointestinal symptoms. The last infliximab infusion was performed 6 weeks prior to developing the purpura. His UC was diagnosed 10 years prior to the current presentation and was not associated with any extragastrointestinal manifestations. Since diagnosis, UC had failed to respond to therapies such as azathioprine, cyclosporine, and purinethol. The complete blood cell count was normal. The C-reactive protein level was 18.7 mg/L (reference range, <5 mg/L) and the erythrocyte sedimentation rate was 30 mm/h (reference range, 0–20 mm/h). Electrolytes, urea, creatinine clearance, and liver function were normal, and a chest radiograph and radiographs of the swollen joints were unremarkable. The total IgA level was elevated at 4 g/L (reference range, 0.7–4 g/L), with IgG and IgM levels within reference range. There was no hematuria or proteinuria on urinalysis. Tests for antinuclear antibodies, rheumatoid factor, circulating immune complexes, and antineutrophil cytoplasmic antibody were negative. Total complement, C3, and C4 levels also were normal. A skin biopsy confirmed a leukocytoclastic vasculitis of small vessels with C3 deposition. Serologic tests for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus were negative. Based on these findings, the diagnosis of HSP was made. Systemic corticosteroids—120 mg daily of intravenous methylprednisolone for 3 days, followed by 1 mg/kg daily of oral prednisone for 2 weeks—were then introduced with rapid clinical improvement. Henoch-Schönlein purpura and joint symptoms completely resolved, but UC relapsed with bloody diarrhea and severe abdominal pain. Oral prednisone was maintained (1 mg/kg daily). Because of the severity of cutaneous vasculitis (HSP), a multidisciplinary decision was taken to definitively stop the anti-TNF agents and to first add azathioprine (2 mg/kg daily for 2 months), then subcutaneous methotrexate (25 mg weekly). Colonoscopy did not show any dysplasia or adenocarcinoma and confirmed the diagnosis of UC. After 6 months of combined therapy, UC was still active and we decided to perform a total colectomy with ileostomy formation. Complete remission of UC was obtained and maintained after 28 months of follow-up.

Henoch-Schönlein purpura is a multisystem small vessel leukocytoclastic vasculitis with the deposition of immune complexes containing IgA. Clinical manifestations may include palpable purpura, arthritis, enteritis, and nephritis. Henoch-Schönlein purpura usually affects children. Adult onset is rare but associated with more severe symptoms and a poor prognosis.2 The criteria for HSP, as defined by the American College of Rheumatology,3 include palpable purpura, 20 years or younger at disease onset, bowel angina, and presence of vascular wall granulocytes on biopsy. At least 2 of these criteria are required for HSP diagnosis. Various viral or bacterial infections and drugs can trigger HSP, which also can be associated with autoinflammatory or autoimmune diseases. The association of HSP and UC is a rare event, as demonstrated by de Oliveira et al.4 Although only 2 cases of cutaneous vasculitis mimicking HSP have been described in UC,4 we cannot exclude a possible association between HSP and UC. However, our patient had UC for 10 years and never had clinical manifestations of vasculitis.
There are 5 reports of HSP following etanercept5,6 or adalimumab7-9 therapy and 1 following infliximab therapy.10 In all cases, HSP occurred after several months of anti-TNF therapy. However, there also are reports of cutaneous vasculitis associated with arthralgia and glomerulonephritis that resolved after withdrawal of anti-TNF agents.11,12 It is possible that some of these reactions may have been manifestations of undiagnosed HSP. In a series of 113 patients who developed cutaneous vasculitis after anti-TNF agents, visceral vasculitis was observed in 24% of patients. Treatment of vasculitis involved withdrawal of the anti-TNF therapy in 101 cases (89%).13 In these UC patients with few therapeutic alternatives, the continuation of anti-TNF agents should be discussed. In the previous series,13 of 16 patients who were rechallenged with the same or a different TNF antagonist, 12 (75%) experienced vasculitis relapse, suggesting a class effect of TNF inhibition. Because of the severity of cutaneous vasculitis and as previously suggested in a recent analytical and comprehensive overview on paradoxical reactions under TNF blockers,1 we decided not to re-expose our patient to infliximab or to other anti-TNF agents.
In conclusion, HSP may occur during anti-TNF therapy and physicians need to be aware of this potentially serious complication.
- Toussirot É, Aubin F. Paradoxical reactions under TNF-α blocking agents and other biological agents given for chronic immune-mediated diseases: an analytical and comprehensive overview. RMD Open. 2016;2:e000239.
- Saulsbury FT. Henoch-Schönlein purpura. Curr Opin Rheumatol. 2001;13:35-40.
- Ortiz-Sanjuán F, Blanco R, Hernández JL, et al. Applicability of the 2006 European League Against Rheumatism (EULAR) criteria for the classification ofHenoch-Schönlein purpura. an analysis based on 766 patients with cutaneous vasculitis. Clin Exp Rheumatol. 2015;33(2, suppl 89):S44-S47.
- de Oliveira GT, Martins SS, Deboni M, et al. Cutaneous vasculitis in ulcerative colitis mimicking Henoch-Schönlein purpura [published online May 22, 2012]. J Crohns Colitis. 2013;7:e69-e73.
- Marques I, Lagos A, Reis J, et al. Reversible Henoch-Schönlein purpura complicating adalimumab therapy. J Crohns Colitis. 2012;6:796-799.
- Rahman FZ, Takhar GK, Roy O, et al. Henoch-Schönlein purpura complicating adalimumab therapy for Crohn’s disease. World J Gastrointest Pharmacol Ther. 2010;1:119-122.
- Lee A, Kasama R, Evangelisto A, et al. Henoch-Schönlein purpura after etanercept therapy for psoriasis. J Clin Rheumatol. 2006;12:249-251.
- Duffy TN, Genta M, Moll S, et al. Henoch Schönlein purpura following etanercept treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2006;24(2, suppl 41):S106.
- LaConti JJ, Donet JA, Cho-Vega JH, et al. Henoch-Schönlein purpura with adalimumab therapy for ulcerative colitis: a case report and review of the literature. Case Rep Rheumatol. 2016:2812980.
- Nobile S, Catassi C, Felici L. Herpes zoster infection followed by Henoch-Schönlein purpura in a girl receiving infliximab for ulcerative colitis. J Clin Rheumatol. 2009;15:101.
- Mohan N, Edwards ET, Cupps TR, et al. Leukocytoclastic vasculitis associated with tumor necrosis factor-alpha blocking agents. J Rheumatol. 2004;31:1955-1958.
- Simms R, Kipgen D, Dahill S, et al. ANCA-associated renal vasculitis following anti-tumor necrosis factor alpha therapy. Am J Kidney Dis. 2008;51:e11-e14.
- Ramos-Casals M, Brito-Zerón P, Muñoz S, et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore). 2007;86:242-251.
- Toussirot É, Aubin F. Paradoxical reactions under TNF-α blocking agents and other biological agents given for chronic immune-mediated diseases: an analytical and comprehensive overview. RMD Open. 2016;2:e000239.
- Saulsbury FT. Henoch-Schönlein purpura. Curr Opin Rheumatol. 2001;13:35-40.
- Ortiz-Sanjuán F, Blanco R, Hernández JL, et al. Applicability of the 2006 European League Against Rheumatism (EULAR) criteria for the classification ofHenoch-Schönlein purpura. an analysis based on 766 patients with cutaneous vasculitis. Clin Exp Rheumatol. 2015;33(2, suppl 89):S44-S47.
- de Oliveira GT, Martins SS, Deboni M, et al. Cutaneous vasculitis in ulcerative colitis mimicking Henoch-Schönlein purpura [published online May 22, 2012]. J Crohns Colitis. 2013;7:e69-e73.
- Marques I, Lagos A, Reis J, et al. Reversible Henoch-Schönlein purpura complicating adalimumab therapy. J Crohns Colitis. 2012;6:796-799.
- Rahman FZ, Takhar GK, Roy O, et al. Henoch-Schönlein purpura complicating adalimumab therapy for Crohn’s disease. World J Gastrointest Pharmacol Ther. 2010;1:119-122.
- Lee A, Kasama R, Evangelisto A, et al. Henoch-Schönlein purpura after etanercept therapy for psoriasis. J Clin Rheumatol. 2006;12:249-251.
- Duffy TN, Genta M, Moll S, et al. Henoch Schönlein purpura following etanercept treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2006;24(2, suppl 41):S106.
- LaConti JJ, Donet JA, Cho-Vega JH, et al. Henoch-Schönlein purpura with adalimumab therapy for ulcerative colitis: a case report and review of the literature. Case Rep Rheumatol. 2016:2812980.
- Nobile S, Catassi C, Felici L. Herpes zoster infection followed by Henoch-Schönlein purpura in a girl receiving infliximab for ulcerative colitis. J Clin Rheumatol. 2009;15:101.
- Mohan N, Edwards ET, Cupps TR, et al. Leukocytoclastic vasculitis associated with tumor necrosis factor-alpha blocking agents. J Rheumatol. 2004;31:1955-1958.
- Simms R, Kipgen D, Dahill S, et al. ANCA-associated renal vasculitis following anti-tumor necrosis factor alpha therapy. Am J Kidney Dis. 2008;51:e11-e14.
- Ramos-Casals M, Brito-Zerón P, Muñoz S, et al. Autoimmune diseases induced by TNF-targeted therapies: analysis of 233 cases. Medicine (Baltimore). 2007;86:242-251.
Practice Points
- Cutaneous adverse effects may occur in approximately 20% of patients treated with anti–tumor necrosis factor (TNF) drugs.
- Henoch-Schönlein purpura (HSP), a small-vessel vasculitis, is an extremely rare complication of anti-TNF treatment.
- Although most cutaneous adverse effects do not require anti-TNF treatment discontinuation and are resolved with symptomatic treatment, anti-TNF therapy must be stopped in more severe cases.
Adjuvant chemotherapy overused in young patients with colon cancer
Adjuvant chemotherapy may be overused among younger patients with colon cancer, without clear evidence of survival benefit over surgery alone, according to a report in JAMA Surgery.
Using data from 3,143 patients with histologically confirmed primary colon adenocarcinoma in the U.S. Department of Defense’s Central Cancer Registry and Military Heath System medical claims databases, researchers compared overall survival in those who underwent surgery and adjuvant chemotherapy to those who underwent surgery alone.
They found patients aged 18-49 years were up to eight times more likely to receive postoperative systemic chemotherapy across all tumor stages compared to patients aged 65-75 years. The odds ratios ranged from 7.98 for stage I tumors to 2.30 for stage III tumors (JAMA Surgery 2017, Jan 25. doi:10.1001/jamasurg.2016.5050).
“Furthermore, young and middle-aged adults were 2.5 times more likely to receive multiagent chemotherapy regimens and most patients with information on chemotherapy regimens underwent multiagent regimens, suggesting a tendency toward more intense treatments,” wrote Janna Manjelievskaia, MPH, of Walter Reed National Military Medical Center, and coauthors.*
However, they found that there was no significant difference in survival between those who had surgery and chemotherapy compared to those who had surgery alone, across age groups and tumor stage.
They did note greater overall survival among middle-aged patients with stage I and stage IV disease who were treated with surgery alone, compared to their older counterparts. Younger patients with stage III disease who received surgery alone also had slightly better survival than did older patients.
“The study suggests that more use of chemotherapy in younger patients did not result in additional survival benefits,” the authors wrote.
While national guidelines advise that selected patients with stage II disease – those with inadequately sampled nodes, T3 lesions or poorly differentiated histology – can be considered for adjuvant chemotherapy, the authors argued there is no solid evidence for the effectiveness of chemotherapy in these patients.
“Patients with cancer who receive chemotherapy are vulnerable to its toxicity and adverse effects and may have reduced quality of life,” they wrote. “As a result, patients may undergo decreased physical, functional, emotional, and social well-being, although these changes might be mitigated over time.”
Given the additional economic and financial cost of adjuvant chemotherapy, the authors called for further research to evaluate the appropriate use of chemotherapy in colon cancer.
The John P. Murtha Cancer Center, Walter Reed National Military Medical Center, and the National Cancer Institute supported the study. No conflicts of interest were declared.
* This story was updated on 2/6/2107
The study by Manjelievskaia et al. is a call for action, and invites contemplation and in-depth study. Appropriate treatment is vital for a patient’s survival, but excess treatment may increase complications and is a poor stewardship of health care funds.
Further investigation of the discrepancies in stage II would be worthwhile, and additional research on the age discrepancies in stage I disease would not only be interesting but also mandatory. Colorectal cancer tumor boards frequently concentrate on the complex care of rectal cancer and metastatic colon cancer. This is also a clear call for improved oversight of chemotherapy for colon cancer.
Tonia M. Young-Fadok, MD, is at the Mayo Clinic, Phoenix, Ariz. These comments are exerpts from an accompanying editorial (JAMA Surg. 2017, Jan 25. doi: 10.1001/jamasurg.2016.5051). No conflicts of interest were declared.
The study by Manjelievskaia et al. is a call for action, and invites contemplation and in-depth study. Appropriate treatment is vital for a patient’s survival, but excess treatment may increase complications and is a poor stewardship of health care funds.
Further investigation of the discrepancies in stage II would be worthwhile, and additional research on the age discrepancies in stage I disease would not only be interesting but also mandatory. Colorectal cancer tumor boards frequently concentrate on the complex care of rectal cancer and metastatic colon cancer. This is also a clear call for improved oversight of chemotherapy for colon cancer.
Tonia M. Young-Fadok, MD, is at the Mayo Clinic, Phoenix, Ariz. These comments are exerpts from an accompanying editorial (JAMA Surg. 2017, Jan 25. doi: 10.1001/jamasurg.2016.5051). No conflicts of interest were declared.
The study by Manjelievskaia et al. is a call for action, and invites contemplation and in-depth study. Appropriate treatment is vital for a patient’s survival, but excess treatment may increase complications and is a poor stewardship of health care funds.
Further investigation of the discrepancies in stage II would be worthwhile, and additional research on the age discrepancies in stage I disease would not only be interesting but also mandatory. Colorectal cancer tumor boards frequently concentrate on the complex care of rectal cancer and metastatic colon cancer. This is also a clear call for improved oversight of chemotherapy for colon cancer.
Tonia M. Young-Fadok, MD, is at the Mayo Clinic, Phoenix, Ariz. These comments are exerpts from an accompanying editorial (JAMA Surg. 2017, Jan 25. doi: 10.1001/jamasurg.2016.5051). No conflicts of interest were declared.
Adjuvant chemotherapy may be overused among younger patients with colon cancer, without clear evidence of survival benefit over surgery alone, according to a report in JAMA Surgery.
Using data from 3,143 patients with histologically confirmed primary colon adenocarcinoma in the U.S. Department of Defense’s Central Cancer Registry and Military Heath System medical claims databases, researchers compared overall survival in those who underwent surgery and adjuvant chemotherapy to those who underwent surgery alone.
They found patients aged 18-49 years were up to eight times more likely to receive postoperative systemic chemotherapy across all tumor stages compared to patients aged 65-75 years. The odds ratios ranged from 7.98 for stage I tumors to 2.30 for stage III tumors (JAMA Surgery 2017, Jan 25. doi:10.1001/jamasurg.2016.5050).
“Furthermore, young and middle-aged adults were 2.5 times more likely to receive multiagent chemotherapy regimens and most patients with information on chemotherapy regimens underwent multiagent regimens, suggesting a tendency toward more intense treatments,” wrote Janna Manjelievskaia, MPH, of Walter Reed National Military Medical Center, and coauthors.*
However, they found that there was no significant difference in survival between those who had surgery and chemotherapy compared to those who had surgery alone, across age groups and tumor stage.
They did note greater overall survival among middle-aged patients with stage I and stage IV disease who were treated with surgery alone, compared to their older counterparts. Younger patients with stage III disease who received surgery alone also had slightly better survival than did older patients.
“The study suggests that more use of chemotherapy in younger patients did not result in additional survival benefits,” the authors wrote.
While national guidelines advise that selected patients with stage II disease – those with inadequately sampled nodes, T3 lesions or poorly differentiated histology – can be considered for adjuvant chemotherapy, the authors argued there is no solid evidence for the effectiveness of chemotherapy in these patients.
“Patients with cancer who receive chemotherapy are vulnerable to its toxicity and adverse effects and may have reduced quality of life,” they wrote. “As a result, patients may undergo decreased physical, functional, emotional, and social well-being, although these changes might be mitigated over time.”
Given the additional economic and financial cost of adjuvant chemotherapy, the authors called for further research to evaluate the appropriate use of chemotherapy in colon cancer.
The John P. Murtha Cancer Center, Walter Reed National Military Medical Center, and the National Cancer Institute supported the study. No conflicts of interest were declared.
* This story was updated on 2/6/2107
Adjuvant chemotherapy may be overused among younger patients with colon cancer, without clear evidence of survival benefit over surgery alone, according to a report in JAMA Surgery.
Using data from 3,143 patients with histologically confirmed primary colon adenocarcinoma in the U.S. Department of Defense’s Central Cancer Registry and Military Heath System medical claims databases, researchers compared overall survival in those who underwent surgery and adjuvant chemotherapy to those who underwent surgery alone.
They found patients aged 18-49 years were up to eight times more likely to receive postoperative systemic chemotherapy across all tumor stages compared to patients aged 65-75 years. The odds ratios ranged from 7.98 for stage I tumors to 2.30 for stage III tumors (JAMA Surgery 2017, Jan 25. doi:10.1001/jamasurg.2016.5050).
“Furthermore, young and middle-aged adults were 2.5 times more likely to receive multiagent chemotherapy regimens and most patients with information on chemotherapy regimens underwent multiagent regimens, suggesting a tendency toward more intense treatments,” wrote Janna Manjelievskaia, MPH, of Walter Reed National Military Medical Center, and coauthors.*
However, they found that there was no significant difference in survival between those who had surgery and chemotherapy compared to those who had surgery alone, across age groups and tumor stage.
They did note greater overall survival among middle-aged patients with stage I and stage IV disease who were treated with surgery alone, compared to their older counterparts. Younger patients with stage III disease who received surgery alone also had slightly better survival than did older patients.
“The study suggests that more use of chemotherapy in younger patients did not result in additional survival benefits,” the authors wrote.
While national guidelines advise that selected patients with stage II disease – those with inadequately sampled nodes, T3 lesions or poorly differentiated histology – can be considered for adjuvant chemotherapy, the authors argued there is no solid evidence for the effectiveness of chemotherapy in these patients.
“Patients with cancer who receive chemotherapy are vulnerable to its toxicity and adverse effects and may have reduced quality of life,” they wrote. “As a result, patients may undergo decreased physical, functional, emotional, and social well-being, although these changes might be mitigated over time.”
Given the additional economic and financial cost of adjuvant chemotherapy, the authors called for further research to evaluate the appropriate use of chemotherapy in colon cancer.
The John P. Murtha Cancer Center, Walter Reed National Military Medical Center, and the National Cancer Institute supported the study. No conflicts of interest were declared.
* This story was updated on 2/6/2107
FROM JAMA SURGERY
Key clinical point: Adjuvant chemotherapy may be overused among younger patients with colon cancer, without clear evidence of a survival benefit over surgery alone.
Major finding: Younger patients with colon cancer are between two and eight times more likely to have adjuvant chemotherapy in addition to surgery compared to older patients with colon cancer.
Data source: A cohort study of 3,143 patients with histologically confirmed primary colon adenocarcinoma.
Disclosures: The John P. Murtha Cancer Center, Walter Reed National Military Medical Center, and the National Cancer Institute supported the study. No conflicts of interest were declared.