User login
Is SUDEP Preventable in Children?
HOUSTON—Sudden unexpected death in epilepsy (SUDEP) in children is rare, but can it be avoided? The majority of pediatric SUDEP cases may occur in children with global developmental delay, early-onset epilepsy, or with seizures requiring polytherapy, according to research presented at the 70th Annual Meeting of the American Epilepsy Society.
“Every death of a person with epilepsy is devastating, the death of a child even more so. If we can understand who is at higher risk of SUDEP, then our clinical and basic science researchers can work to find preventative techniques, and hopefully we can apply those techniques to patients who are most at risk,” said Elizabeth Donner, MD, Director of the Comprehensive Epilepsy Program at the Hospital for Sick Children and Associate Professor of Pediatrics at the University of Toronto.
The incidence of SUDEP in children is estimated to be 0.43 per 1,000 patient years of epilepsy, more than 10 times the rate of sudden death in children overall. Since the numbers are relatively low, it has been debatable whether doctors should discuss SUDEP with their patients. Recent studies, however, suggest that SUDEP may be more common and potentially avoidable. To determine potential risk factors for pediatric SUDEP, Dr. Donner and her colleagues developed a national, multicenter prospective population registry for SUDEP.
Researchers collected data from the Canadian Pediatric Epilepsy Network, the Canadian Pediatric Surveillance Program, and the Ontario Forensic Pathology Service. They reviewed demographics, clinical features, circumstances surrounding death, and autopsy findings.
Researchers sought to include children with epilepsy with an unexpected death between January 1, 2014, and December 31, 2015. Inclusion criteria were age 18 or younger at death; a history of two or more seizures; and death that was sudden, unexpected, and occurred during normal circumstances. Autopsies, when available, determined that there was no anatomical or toxicological cause of death. Investigators excluded deaths due to trauma and drowning and status epilepticus.
The majority of deaths occurred in children between the ages of 5 and 10, and 52% were boys. In addition, all children had seizure onset before age 5, and median age of seizure onset was about 6 months. Seven of the children had genetic abnormalities. One child was seizure-free for 12 months and was not being treated with antiepileptic medications.
The investigators identified 21 cases of definite, probable, or possible pediatric SUDEP: 10 cases of definite SUDEP, two cases of definite SUDEP plus, six cases of probable SUDEP, and three cases of possible SUDEP (ie, an autopsy was not performed). Additionally, 10 of 12 children were having tonic clonic seizures six months prior to death. Researchers also found that in 10 of 17 cases, the parents reported that their child had a recent infection. Nearly all of the deaths occurred during sleep and were unwitnessed.
“It may be worth looking at whether infection in children with epilepsy changes their risk of sudden death,” said Dr. Donner. “We really have a lot more work to do with this limited number of cases, and we are still identifying more cases and working to better understand the data.”
—Erica Tricarico
Suggested Reading
Donner EJ, Waddell B, Osland K, et al. After sudden unexpected death in epilepsy: Lessons learned and the road forward. Epilepsia. 2016;57(Suppl 1):46-53.
HOUSTON—Sudden unexpected death in epilepsy (SUDEP) in children is rare, but can it be avoided? The majority of pediatric SUDEP cases may occur in children with global developmental delay, early-onset epilepsy, or with seizures requiring polytherapy, according to research presented at the 70th Annual Meeting of the American Epilepsy Society.
“Every death of a person with epilepsy is devastating, the death of a child even more so. If we can understand who is at higher risk of SUDEP, then our clinical and basic science researchers can work to find preventative techniques, and hopefully we can apply those techniques to patients who are most at risk,” said Elizabeth Donner, MD, Director of the Comprehensive Epilepsy Program at the Hospital for Sick Children and Associate Professor of Pediatrics at the University of Toronto.
The incidence of SUDEP in children is estimated to be 0.43 per 1,000 patient years of epilepsy, more than 10 times the rate of sudden death in children overall. Since the numbers are relatively low, it has been debatable whether doctors should discuss SUDEP with their patients. Recent studies, however, suggest that SUDEP may be more common and potentially avoidable. To determine potential risk factors for pediatric SUDEP, Dr. Donner and her colleagues developed a national, multicenter prospective population registry for SUDEP.
Researchers collected data from the Canadian Pediatric Epilepsy Network, the Canadian Pediatric Surveillance Program, and the Ontario Forensic Pathology Service. They reviewed demographics, clinical features, circumstances surrounding death, and autopsy findings.
Researchers sought to include children with epilepsy with an unexpected death between January 1, 2014, and December 31, 2015. Inclusion criteria were age 18 or younger at death; a history of two or more seizures; and death that was sudden, unexpected, and occurred during normal circumstances. Autopsies, when available, determined that there was no anatomical or toxicological cause of death. Investigators excluded deaths due to trauma and drowning and status epilepticus.
The majority of deaths occurred in children between the ages of 5 and 10, and 52% were boys. In addition, all children had seizure onset before age 5, and median age of seizure onset was about 6 months. Seven of the children had genetic abnormalities. One child was seizure-free for 12 months and was not being treated with antiepileptic medications.
The investigators identified 21 cases of definite, probable, or possible pediatric SUDEP: 10 cases of definite SUDEP, two cases of definite SUDEP plus, six cases of probable SUDEP, and three cases of possible SUDEP (ie, an autopsy was not performed). Additionally, 10 of 12 children were having tonic clonic seizures six months prior to death. Researchers also found that in 10 of 17 cases, the parents reported that their child had a recent infection. Nearly all of the deaths occurred during sleep and were unwitnessed.
“It may be worth looking at whether infection in children with epilepsy changes their risk of sudden death,” said Dr. Donner. “We really have a lot more work to do with this limited number of cases, and we are still identifying more cases and working to better understand the data.”
—Erica Tricarico
Suggested Reading
Donner EJ, Waddell B, Osland K, et al. After sudden unexpected death in epilepsy: Lessons learned and the road forward. Epilepsia. 2016;57(Suppl 1):46-53.
HOUSTON—Sudden unexpected death in epilepsy (SUDEP) in children is rare, but can it be avoided? The majority of pediatric SUDEP cases may occur in children with global developmental delay, early-onset epilepsy, or with seizures requiring polytherapy, according to research presented at the 70th Annual Meeting of the American Epilepsy Society.
“Every death of a person with epilepsy is devastating, the death of a child even more so. If we can understand who is at higher risk of SUDEP, then our clinical and basic science researchers can work to find preventative techniques, and hopefully we can apply those techniques to patients who are most at risk,” said Elizabeth Donner, MD, Director of the Comprehensive Epilepsy Program at the Hospital for Sick Children and Associate Professor of Pediatrics at the University of Toronto.
The incidence of SUDEP in children is estimated to be 0.43 per 1,000 patient years of epilepsy, more than 10 times the rate of sudden death in children overall. Since the numbers are relatively low, it has been debatable whether doctors should discuss SUDEP with their patients. Recent studies, however, suggest that SUDEP may be more common and potentially avoidable. To determine potential risk factors for pediatric SUDEP, Dr. Donner and her colleagues developed a national, multicenter prospective population registry for SUDEP.
Researchers collected data from the Canadian Pediatric Epilepsy Network, the Canadian Pediatric Surveillance Program, and the Ontario Forensic Pathology Service. They reviewed demographics, clinical features, circumstances surrounding death, and autopsy findings.
Researchers sought to include children with epilepsy with an unexpected death between January 1, 2014, and December 31, 2015. Inclusion criteria were age 18 or younger at death; a history of two or more seizures; and death that was sudden, unexpected, and occurred during normal circumstances. Autopsies, when available, determined that there was no anatomical or toxicological cause of death. Investigators excluded deaths due to trauma and drowning and status epilepticus.
The majority of deaths occurred in children between the ages of 5 and 10, and 52% were boys. In addition, all children had seizure onset before age 5, and median age of seizure onset was about 6 months. Seven of the children had genetic abnormalities. One child was seizure-free for 12 months and was not being treated with antiepileptic medications.
The investigators identified 21 cases of definite, probable, or possible pediatric SUDEP: 10 cases of definite SUDEP, two cases of definite SUDEP plus, six cases of probable SUDEP, and three cases of possible SUDEP (ie, an autopsy was not performed). Additionally, 10 of 12 children were having tonic clonic seizures six months prior to death. Researchers also found that in 10 of 17 cases, the parents reported that their child had a recent infection. Nearly all of the deaths occurred during sleep and were unwitnessed.
“It may be worth looking at whether infection in children with epilepsy changes their risk of sudden death,” said Dr. Donner. “We really have a lot more work to do with this limited number of cases, and we are still identifying more cases and working to better understand the data.”
—Erica Tricarico
Suggested Reading
Donner EJ, Waddell B, Osland K, et al. After sudden unexpected death in epilepsy: Lessons learned and the road forward. Epilepsia. 2016;57(Suppl 1):46-53.
Watch for cutaneous manifestations of tropical infectious diseases
Treatments designed to combat tropical infectious diseases are lacking, so the best thing travelers to these regions of the world can do is defend themselves against mosquito bites, according to Stephen K. Tyring, MD.
“We have no specific therapies for these infections,” explained Dr. Tyring of the University of Texas in Houston.
“Therefore, the best management is to avoid mosquito bites [by using] DEET, protective clothing, etc.,” he said in an interview prior to the Caribbean Dermatology Symposium.
“Treatment [of Zika virus infections] is supportive,” he said, because currently, there are no vaccine and no antiviral therapy aimed specifically at treating Zika virus infections. It’s also important for clinicians to rule out dengue and chikungunya when testing for Zika virus, and to avoid prescribing NSAIDs and aspirin until a definitive diagnosis is made, to avoid causing hemorrhaging.
Dr. Tyring also advised refraining from sexual contact with any individuals who have been to tropical areas and may have been exposed to the Zika virus.
Dr. Tyring also discussed the cutaneous manifestations and other symptoms of the flavivirus infections dengue and chikungunya.
“[About] 40% of the world’s population live in areas where there is a risk of dengue transmission, [and] the World Health Organization estimates that 50 to 100 million infections occur yearly, including 500,000 DHF [dengue hemorrhagic fever] cases and 22,000 deaths,” mostly in children, he said in his presentation at the meeting provided by Global Academy for Medical Education.
The tourniquet test is a useful tool to determine if a patient has dengue fever. This involves taking the patient’s blood pressure, then inflating the cuff to a point midway between the systolic and diastolic blood pressure, and maintaining it for 5 minutes. Deflate the cuff and wait for 2 minutes; then count the petechiae below the antecubital fossa. A positive test result is 10 or more petechiae per square inch, according to the CDC definition.
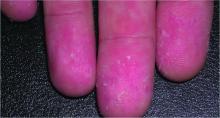
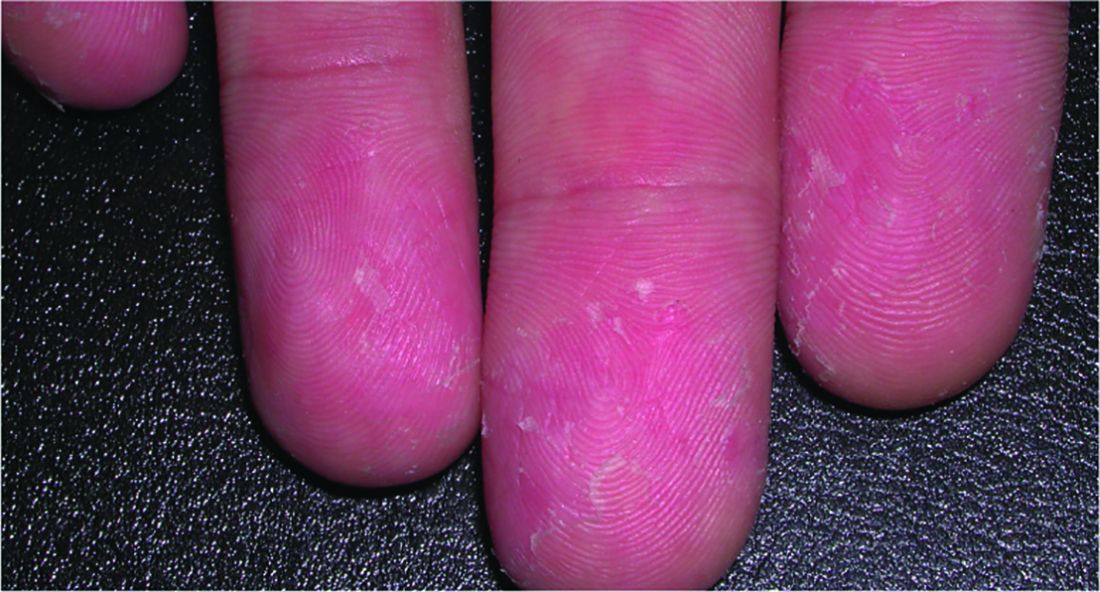
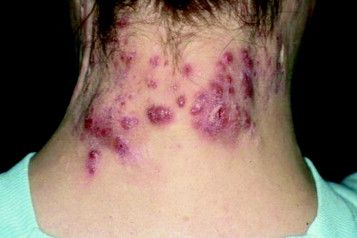
A relative of dengue, the chikungunya virus can present in the form of a morbilliform rash, nasal hyperpigmentation, purpuric macules, and erythema, the latter of which can sometimes be accompanied by ulcers. In addition to dermatologic symptoms (occurring in 40%-75% of patients), joint pain and fever also are associated with a chikungunya virus infection.
“Redness, swelling, and pain of the scrotum and groin region” also can occur, while “ulceration on the vulva in women has occasionally been reported in other outbreaks,” Dr. Tyring explained.
For further reading on this matter, Dr. Tyring recommended a report by Nawas et al. entitled, “Emerging infectious diseases with cutaneous manifestations” (J Am Acad Dermatol. 2016 Jul;75[1]:1-16) and a 2008 JAMA study on dengue and DHF coauthored by Anthony S. Fauci, MD (299[2]:214-6).
Dr. Tyring reported no relevant financial disclosures. Global Academy and this news organization are owned by the same parent company.
Treatments designed to combat tropical infectious diseases are lacking, so the best thing travelers to these regions of the world can do is defend themselves against mosquito bites, according to Stephen K. Tyring, MD.
“We have no specific therapies for these infections,” explained Dr. Tyring of the University of Texas in Houston.
“Therefore, the best management is to avoid mosquito bites [by using] DEET, protective clothing, etc.,” he said in an interview prior to the Caribbean Dermatology Symposium.
“Treatment [of Zika virus infections] is supportive,” he said, because currently, there are no vaccine and no antiviral therapy aimed specifically at treating Zika virus infections. It’s also important for clinicians to rule out dengue and chikungunya when testing for Zika virus, and to avoid prescribing NSAIDs and aspirin until a definitive diagnosis is made, to avoid causing hemorrhaging.
Dr. Tyring also advised refraining from sexual contact with any individuals who have been to tropical areas and may have been exposed to the Zika virus.
Dr. Tyring also discussed the cutaneous manifestations and other symptoms of the flavivirus infections dengue and chikungunya.
“[About] 40% of the world’s population live in areas where there is a risk of dengue transmission, [and] the World Health Organization estimates that 50 to 100 million infections occur yearly, including 500,000 DHF [dengue hemorrhagic fever] cases and 22,000 deaths,” mostly in children, he said in his presentation at the meeting provided by Global Academy for Medical Education.
The tourniquet test is a useful tool to determine if a patient has dengue fever. This involves taking the patient’s blood pressure, then inflating the cuff to a point midway between the systolic and diastolic blood pressure, and maintaining it for 5 minutes. Deflate the cuff and wait for 2 minutes; then count the petechiae below the antecubital fossa. A positive test result is 10 or more petechiae per square inch, according to the CDC definition.
A relative of dengue, the chikungunya virus can present in the form of a morbilliform rash, nasal hyperpigmentation, purpuric macules, and erythema, the latter of which can sometimes be accompanied by ulcers. In addition to dermatologic symptoms (occurring in 40%-75% of patients), joint pain and fever also are associated with a chikungunya virus infection.
“Redness, swelling, and pain of the scrotum and groin region” also can occur, while “ulceration on the vulva in women has occasionally been reported in other outbreaks,” Dr. Tyring explained.
For further reading on this matter, Dr. Tyring recommended a report by Nawas et al. entitled, “Emerging infectious diseases with cutaneous manifestations” (J Am Acad Dermatol. 2016 Jul;75[1]:1-16) and a 2008 JAMA study on dengue and DHF coauthored by Anthony S. Fauci, MD (299[2]:214-6).
Dr. Tyring reported no relevant financial disclosures. Global Academy and this news organization are owned by the same parent company.
Treatments designed to combat tropical infectious diseases are lacking, so the best thing travelers to these regions of the world can do is defend themselves against mosquito bites, according to Stephen K. Tyring, MD.
“We have no specific therapies for these infections,” explained Dr. Tyring of the University of Texas in Houston.
“Therefore, the best management is to avoid mosquito bites [by using] DEET, protective clothing, etc.,” he said in an interview prior to the Caribbean Dermatology Symposium.
“Treatment [of Zika virus infections] is supportive,” he said, because currently, there are no vaccine and no antiviral therapy aimed specifically at treating Zika virus infections. It’s also important for clinicians to rule out dengue and chikungunya when testing for Zika virus, and to avoid prescribing NSAIDs and aspirin until a definitive diagnosis is made, to avoid causing hemorrhaging.
Dr. Tyring also advised refraining from sexual contact with any individuals who have been to tropical areas and may have been exposed to the Zika virus.
Dr. Tyring also discussed the cutaneous manifestations and other symptoms of the flavivirus infections dengue and chikungunya.
“[About] 40% of the world’s population live in areas where there is a risk of dengue transmission, [and] the World Health Organization estimates that 50 to 100 million infections occur yearly, including 500,000 DHF [dengue hemorrhagic fever] cases and 22,000 deaths,” mostly in children, he said in his presentation at the meeting provided by Global Academy for Medical Education.
The tourniquet test is a useful tool to determine if a patient has dengue fever. This involves taking the patient’s blood pressure, then inflating the cuff to a point midway between the systolic and diastolic blood pressure, and maintaining it for 5 minutes. Deflate the cuff and wait for 2 minutes; then count the petechiae below the antecubital fossa. A positive test result is 10 or more petechiae per square inch, according to the CDC definition.
A relative of dengue, the chikungunya virus can present in the form of a morbilliform rash, nasal hyperpigmentation, purpuric macules, and erythema, the latter of which can sometimes be accompanied by ulcers. In addition to dermatologic symptoms (occurring in 40%-75% of patients), joint pain and fever also are associated with a chikungunya virus infection.
“Redness, swelling, and pain of the scrotum and groin region” also can occur, while “ulceration on the vulva in women has occasionally been reported in other outbreaks,” Dr. Tyring explained.
For further reading on this matter, Dr. Tyring recommended a report by Nawas et al. entitled, “Emerging infectious diseases with cutaneous manifestations” (J Am Acad Dermatol. 2016 Jul;75[1]:1-16) and a 2008 JAMA study on dengue and DHF coauthored by Anthony S. Fauci, MD (299[2]:214-6).
Dr. Tyring reported no relevant financial disclosures. Global Academy and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM THE CARIBBEAN DERMATOLOGY SYMPOSIUM
Acute kidney injury in patients treated with vancomycin and piperacillin-tazobactam: A retrospective cohort analysis
Empiric antimicrobial therapy often consists of the combination of gram-positive coverage with vancomycin (VAN) and gram-negative coverage, specifically an antipseudomonal beta-lactam such as piperacillin-tazobactam (PTZ). Literature from a variety of patient populations reports nephrotoxicity associated with VAN, targeting troughs greater than 15 µg/mL, that occur in 5% to 43% of patients.1 In a study of critically ill patients, acute kidney injury (AKI) was found in 21% of patients receiving VAN, with increasing duration of VAN treatment, greater VAN levels, concomitant vasoactive medication administration, and intermittent infusion methods being associated with higher odds of AKI.2 A recent report from adult internal medicine patients estimated the incidence of VAN-associated nephrotoxicity at 13.6% and implicated concomitant PTZ therapy as a key factor in these patients.3
Further studies have explored the interaction between empiric beta-lactam and VAN therapy, showing mixed results. Reports of AKI associated with the combination of VAN and PTZ range from 16.3% to 34.8%,4-8 while the cefepime-VAN combination is reported to range from 12.5% to 13.3%.5,6 While VAN monotherapy groups were well represented, only 1 study7 compared the PTZ-VAN combination to a control group of PTZ monotherapy.
The primary objective of this study was to evaluate the differences in AKI incidence between patients treated with VAN and with PTZ, alone and in combination.
METHODS
This is a retrospective cohort study of adult patients conducted at the University of Kentucky Chandler Medical Center (UKMC) from September 1, 2010 through August 31, 2014. Patients were included if they were at least 18 years of age on admission; remained hospitalized for at least 48 hours; received VAN combined with PTZ (VAN/PTZ), VAN alone, or PTZ alone; and had at least 48 hours of therapy (and 48 hours of overlapping therapy in the VAN/PTZ group). Patients were excluded if they had underlying diagnosis of chronic kidney disease according to the International Classification of Diseases 9 (ICD-9) code, were receiving renal replacement therapy before admission, had a diagnosis of cystic fibrosis, or were pregnant. Additionally, patients were excluded if they presented with AKI, defined as an initial creatinine clearance less than 30 mL/min, or if baseline creatinine clearance was greater than 4 times the standard deviation from the mean; serum creatinine values were not obtained during admission; and if AKI occurred prior to therapy initiation, within 48 hours of initiation, or more than 7 days after treatment was discontinued. Patients were followed throughout their stay until time of discharge.
Data Source
Patient data were collected from the University of Kentucky Center for Clinical and Translational Science Enterprise Data Trust (EDT). The EDT contains clinical data from the inpatient population of UKMC from 2006 to present. Data stored and updated nightly by the EDT includes: demographics, financial classification (Medicare, Medicaid, private insurance), provider-level detail (service line), medical diagnosis (ICD-9 codes), medical procedures (Current Procedural Terminology [CPT] codes), lab tests and results, medication administration details, visit details (age, length of stay, etc), and vital signs. This study was approved by the UKMC Institutional Review Board.
Data collected for each patient included: demographic data, visit details (length of stay, admitting and primary diagnosis codes, etc.), severity of underlying illness as defined by the Charlson Comorbidity Index (CCI), all serum creatinine levels drawn per visit, medication administration information (dose, date, and time administered), all VAN trough levels, receipt of other nephrotoxic agents, blood pressures, and receipt of vasopressors.
Outcome Ascertainment
The definition of AKI was based on the RIFLE (Risk, Injury, Failure, Loss, End-stage) criteria,9 with risk defined as a 25% to 50% decrease in estimated glomerular filtration rate (GFR), injury as a 50% to 75% decrease in estimated GFR, and failure defined as a greater than 75% decrease in estimated GFR. Loss and end-stage classifications were not assessed because of this study’s follow-up period. The adjusted Cockcroft and Gault equation10 was used to estimate GFR due to the inconsistency of weight availability in the dataset and concordance with the institution’s practice. Baseline creatinine clearance was calculated with the first serum creatinine obtained, and the minimum creatinine clearance was calculated using the maximum serum creatinine during each patient’s visit. The percent decrease in creatinine clearance was calculated from these 2 values. AKI status was defined as meeting any of the RIFLE criteria. Mortality was assessed for all patients and defined as the composite of inhospital mortality and discharge or transfer to hospice care.
Exposure Ascertainment
Hypotension exposure was defined as experiencing 1 of the following: mean arterial blood pressure less than 60 mm Hg, a diagnosis of hypotension by a physician, or receipt of vasopressors or inotropic agents. Days of therapy for each drug were obtained and combination days of therapy were calculated by including only those days in which the patient received both medications. Total days of therapy were calculated by the sum of all days receiving at least 1 study agent. Exposure to other nephrotoxic agents (eg, acyclovir, angiotensin converting enzyme [ACE] inhibitors, angiotensin II receptor antagonists, aminoglycosides, amphotericin B, cyclosporine, foscarnet, loop diuretics, nonsteroidal anti-inflammatory drugs, sulfonamides, tacrolimus, and tenofovir) were defined as receipt of at least 1 dose of the agent during hospitalization.
Statistical Analysis
Characteristics between groups were described with basic descriptive statistics. Continuous variables were compared with 1-way analysis of variance (ANOVA) or the Kruskal-Wallis test. Categorical variables were compared with chi-square or Fisher exact test. Yearly AKI trends were assessed with Pearson correlation coefficient. To control for differences in underlying severity of illness between groups, a subanalysis was performed in which the cohort was split into 4 groups (0, 1, 2 to 4, and ≥5 points) based on CCI. Univariate models for all covariates were created with probability of AKI as the outcome. Covariates significant after univariate were incorporated into the multivariate model, which was subsequently adjusted to achieve the highest predictive accuracy by minimizing the Akaike information criterion (AIC). Nephrotoxic agent exposures were included in the final multivariate model regardless of statistical significance in univariate analysis. Model fit was assessed with a standardized Hosmer-Lemeshow goodness-of-fit test.11 All statistical analyses were completed with RStudio v 0.98 running R v 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria).12 All tests were 2-tailed and significance was defined at an alpha of 0.05.
RESULTS
Of 17,879 patients initially screened, 11,650 patients were evaluated, of which 5,497 received VAN and PTZ (VAN/PTZ), 3,055 received VAN alone, and 3,098 received PTZ alone. Table 1 contains basic demographic information. The mean age of patients was 52.5 years ± 16.8 years with 6,242 (53.6%) males. Patients receiving VAN/PTZ had higher CCIs than either monotherapy group and had significantly increased length of hospitalization. While patients in the combination therapy group were more likely to experience hypotension, concomitant nephrotoxic agent exposure was more common in the VAN monotherapy group.
RIFLE-defined AKI occurred in 1,647 (14.1%) across the entire cohort. AKI occurred in 21% of VAN/PTZ patients, 8.3% of VAN patients, and 7.8% of PTZ patients (P < 0.0001). RIFLE-defined risk, injury, and failure occurred more frequently in the VAN/PTZ cohort compared to the VAN and PTZ monotherapy groups (Figure). There were no differences in AKI rates between years studied (r2 = 0.4732, P = 0.2). Patients in the VAN/PTZ group experienced AKI on average of 8.0 days after treatment initiation, compared to 8.7 days and 5.2 days for VAN and PTZ monotherapy groups, respectively. The composite of inhospital mortality and transfer-to-hospice care was more common in VAN/PTZ patients (9.6%) compared to monotherapy groups (VAN, 3.9%; PTZ, 3.4%), most likely due to the increased severity of illness.
In the subgroup analysis of patients with similar CCI, AKI incidence increased with severity of illness. When CCI was 0, 7.5% of patients experienced AKI compared to 11.2%, 16.4%, and 18.9% of patients when CCI was 1, 2 to 4, and ≥5, respectively (P < 0.0001). VAN/PTZ (range = 12.1% to 26.5%) was associated with greater AKI incidence than either VAN (range = 4.8% to 11.5%) or PTZ (range = 3.8% to 10.4%) alone in each subgroup (P < 0.0001 for all subgroups).
Factors associated with AKI in univariate analyses included treatment with VAN/PTZ, days of therapy, baseline creatinine clearance, transfer from outside hospitals, CCI, admission type, length of hospitalization, dehydration exposure, and hypotension exposure. Exposure to aminoglycosides, amphotericin B, ACE inhibitors, nonsteroidal anti-inflammatory drugs, tacrolimus, foscarnet, loop diuretics, sulfonamides, and tenofovir were all associated with increased odds of AKI in simple univariate logistic regression. Gender, age, year of treatment, angiotensin II receptor antagonist exposure, and cyclosporine exposure were not significantly associated with AKI incidence.
After multivariate logistic regression, monotherapy with VAN or PTZ was associated with decreased odds of AKI compared to VAN/PTZ therapy (aORVAN,0.48; 95% CIVAN,0.41-0.57; aORPTZ, 0.43; 95% CIPTZ, 0.37-0.50). No difference in AKI incidence was observed between VAN and PTZ groups (aORPTZ:VAN, 0.88; 95% CI, 0.73-1.08). Table 2 describes the relationship between AKI and other covariates included in the model. Increased odds of AKI were seen with concomitant administration of ACE inhibitors, amphotericin B, tacrolimus, loop diuretics, and tenofovir. Radio-contrast dye administration was associated with lower odds of AKI. Patients admitted urgently and emergently were at higher risk of AKI, while those admitted via the trauma center were less likely to experience AKI compared to patients who were electively admitted. Increased length of stay and duration of therapy were both associated with increased likelihood of AKI, independent of treatment group; however, durations of therapy beyond 12 days was not associated with increased AKI. Hypotension, as defined, and diagnosed dehydration both independently increased AKI odds. Aside from those older than 80 years of age, increasing age was not associated with increased AKI risk. Male gender was associated with a slight decrease in AKI rate. No evidence of overfitting was observed with the standardized Hosmer-Lemeshow P-value of 0.683, and the model provides good predictive accuracy with a C-statistic of 0.788.
CONCLUSIONS
Acute kidney injury secondary to VAN therapy is a well-characterized adverse effect, while AKI incidence secondary to PTZ is less understood. Additionally, there appears to be an additive effect when these agents are used in combination. This is the largest review of AKI in patients receiving VAN,PTZ, or the combination of both agents.
There is increasing evidence suggesting greater nephrotoxicity in patients treated with the combination of VAN and antipseudomonal beta-lactams. The mechanism for the apparent increase in nephrotoxicity with this drug combination is not well understood and needs further study in both animal models and humans.
Acute kidney injury rates related to VAN vary widely, with recent studies in critically ill and internal medicine patients estimated at 21% and 13.6%, respectively.2,3 In our VAN monotherapy cohort, the AKI rate was 8.3%, with 2.3% of patients experiencing a greater than 50% decrease in creatinine clearance. Piperacillin-tazobactam-related AKI rates are not well characterized; however, a small retrospective analysis estimated that 11.1% of PTZ patients experienced acute renal failure (defined as either increase in serum creatinine greater than 0.5 mg/dL or 50% increase from baseline).13 In the present study, we found the PTZ-related AKI rate to be 7.8%, which may be due to a more stringent definition of AKI. Additionally, Hellwig et al13 found that PTZ monotherapy was associated with higher AKI rates compared to VAN monotherapy (11.1% vs 4.9%; P = 0.014). This was not replicated in our study, with VAN and PTZ monotherapy having similar AKI rates (8.3% and 7.8%, respectively) and an adjusted aOR of 0.88 (95% CI 0.0.73-1.08) for AKI in PTZ- compared to VAN-treated patients. The estimated AKI incidence of 21% in the combination therapy group at our institution is consistent with literature that ranges from 16.3% to 34.8%.4-8,13
To control for differences in baseline severity of illness, we performed a subgroup analysis of patients with similar CCI scores. The finding of increased AKI in patients receiving combination VAN and PTZ was consistent in each subgroup, suggesting that the increase in AKI is independent of illness severity.
This study is not without limitations. As with all retrospective studies, it is difficult to determine a causal link between VAN and PTZ combination therapy and increased AKI incidence due to confounding. We employed a rigorous study design that controlled for major confounders of AKI, such as concomitant nephrotoxic exposure, hypotension, and renal disease. Severity of illness was measured with CCI, which may not accurately capture the severity of illness at treatment initiation. Alternatives, such as acute physiology and chronic health evaluation (APACHE) and sequential organ failure assessment (SOFA) scores, may more accurately reflect critical illness on presentation; however, this study was not focused specifically on critically ill patients. In addition to baseline comorbidity, we controlled for hypotension and dehydration as a surrogate marker for critical illness. In the subgroup analysis of patients with similar CCI, the effect of VAN/PTZ on AKI compared to VAN or PTZ monotherapy was consistent in each group. Nephrotoxic potential of agents was assumed to be equal, which is not necessarily true. Additionally, the binary representation of nephrotoxic exposure does not describe the amount of the agent received; as such, our estimations of AKI odds may be artificially elevated. Approximately one-quarter of the patients in this study were transferred from an outside hospital, for which no data regarding initial treatment are available. This may lead to exposure misclassification. We attempted to control for this factor in the regression model and found that, after controlling for other covariates, hospital transfer was associated with increasing odds of AKI. Finally, data were collected retrospectively from the electronic medical record and are subject to inaccuracies documented in the chart; however, any bias introduced should be nondifferential.
In our large retrospective study of combination empiric therapy with VAN and PTZ, we found that combination therapy was associated with more than double the odds of AKI occurring compared to either monotherapy with VAN or PTZ. Increasing duration of therapy was also associated with increases in AKI. These findings demonstrate the need for judicious use of combination therapy and strengthen the need for antimicrobial de-escalation when appropriate to avoid deleterious effects.
Acknowledgments
The authors thank Chantal Le Rutter, MPA, for copyediting services.
Disclosures
This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant numbers UL1TR000117 and UL1TR001998. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors report no conflicts of interest.
1. van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57:734-744. PubMed
2. Hanrahan TP, Harlow G, Hutchinson J, et al. Vancomycin-associated nephrotoxicity in the critically ill: a retrospective multivariate regression analysis. Crit Care Med. 2014;42:2527-2536. PubMed
3. Meaney CJ, Hynicka LM, Tsoukleris MG. Vancomycin-associated nephrotoxicity in adult medicine patients: incidence, outcomes, and risk factors. Pharmacotherapy. 2014;34:653-661. PubMed
4. Burgess LD, Drew RH. Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin-tazobactam. Pharmacotherapy. 2014;34:670-676. PubMed
5. Moenster RP, Linneman TW, Finnegan PM, Hand S, Thomas Z, McDonald JR. Acute renal failure associated with vancomycin and β-lactams for the treatment of osteomyelitis in diabetics: piperacillin-tazobactam as compared with cefepime. Clin Microbiol Infect. 2014;20:O384-O389. PubMed
6. Gomes DM, Smotherman C, Birch A, et al. Comparison of acute kidney injury during treatment with vancomycin in combination with piperacillin-tazobactam or cefepime. Pharmacotherapy. 2014;34:662-669. PubMed
7. Kim T, Kandiah S, Patel M, et al. Risk factors for kidney injury during vancomycin and piperacillin/tazobactam administration, including increased odds of injury with combination therapy. BMC Res Notes. 2015;8:579. PubMed
8. Davies SW, Efird JT, Guidry CA, et al. Top guns: the “Maverick” and “Goose” of empiric therapy. Surg Infect (Larchmt). 2016;17:38-47. PubMed
9. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204-R212. PubMed
10. Wilhelm SM, Kale-Pradhan PB. Estimating creatinine clearance: a meta-analysis. Pharmacotherapy. 2011;31:658-664. PubMed
11. Paul P, Pennell ML, Lemeshow S. Standardizing the power of the Hosmer-Lemeshow goodness of fit test in large data sets. Stat Med. 2013;32:67-80. PubMed
12. R Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: http://www.R-project.org/.
13. Hellwig T, Hammerquist R, Loecker B, Shields J. Retrospective evaluation of the incidence of vancomycin and/or piperacillin-tazobactam induced acute renal failure. Abstracts of the Society of Critical Care Medicine 41st Critical Care Congress. February 4-8, 2012. Houston, Texas. Crit Care Med. 2011;39:1-264.
Empiric antimicrobial therapy often consists of the combination of gram-positive coverage with vancomycin (VAN) and gram-negative coverage, specifically an antipseudomonal beta-lactam such as piperacillin-tazobactam (PTZ). Literature from a variety of patient populations reports nephrotoxicity associated with VAN, targeting troughs greater than 15 µg/mL, that occur in 5% to 43% of patients.1 In a study of critically ill patients, acute kidney injury (AKI) was found in 21% of patients receiving VAN, with increasing duration of VAN treatment, greater VAN levels, concomitant vasoactive medication administration, and intermittent infusion methods being associated with higher odds of AKI.2 A recent report from adult internal medicine patients estimated the incidence of VAN-associated nephrotoxicity at 13.6% and implicated concomitant PTZ therapy as a key factor in these patients.3
Further studies have explored the interaction between empiric beta-lactam and VAN therapy, showing mixed results. Reports of AKI associated with the combination of VAN and PTZ range from 16.3% to 34.8%,4-8 while the cefepime-VAN combination is reported to range from 12.5% to 13.3%.5,6 While VAN monotherapy groups were well represented, only 1 study7 compared the PTZ-VAN combination to a control group of PTZ monotherapy.
The primary objective of this study was to evaluate the differences in AKI incidence between patients treated with VAN and with PTZ, alone and in combination.
METHODS
This is a retrospective cohort study of adult patients conducted at the University of Kentucky Chandler Medical Center (UKMC) from September 1, 2010 through August 31, 2014. Patients were included if they were at least 18 years of age on admission; remained hospitalized for at least 48 hours; received VAN combined with PTZ (VAN/PTZ), VAN alone, or PTZ alone; and had at least 48 hours of therapy (and 48 hours of overlapping therapy in the VAN/PTZ group). Patients were excluded if they had underlying diagnosis of chronic kidney disease according to the International Classification of Diseases 9 (ICD-9) code, were receiving renal replacement therapy before admission, had a diagnosis of cystic fibrosis, or were pregnant. Additionally, patients were excluded if they presented with AKI, defined as an initial creatinine clearance less than 30 mL/min, or if baseline creatinine clearance was greater than 4 times the standard deviation from the mean; serum creatinine values were not obtained during admission; and if AKI occurred prior to therapy initiation, within 48 hours of initiation, or more than 7 days after treatment was discontinued. Patients were followed throughout their stay until time of discharge.
Data Source
Patient data were collected from the University of Kentucky Center for Clinical and Translational Science Enterprise Data Trust (EDT). The EDT contains clinical data from the inpatient population of UKMC from 2006 to present. Data stored and updated nightly by the EDT includes: demographics, financial classification (Medicare, Medicaid, private insurance), provider-level detail (service line), medical diagnosis (ICD-9 codes), medical procedures (Current Procedural Terminology [CPT] codes), lab tests and results, medication administration details, visit details (age, length of stay, etc), and vital signs. This study was approved by the UKMC Institutional Review Board.
Data collected for each patient included: demographic data, visit details (length of stay, admitting and primary diagnosis codes, etc.), severity of underlying illness as defined by the Charlson Comorbidity Index (CCI), all serum creatinine levels drawn per visit, medication administration information (dose, date, and time administered), all VAN trough levels, receipt of other nephrotoxic agents, blood pressures, and receipt of vasopressors.
Outcome Ascertainment
The definition of AKI was based on the RIFLE (Risk, Injury, Failure, Loss, End-stage) criteria,9 with risk defined as a 25% to 50% decrease in estimated glomerular filtration rate (GFR), injury as a 50% to 75% decrease in estimated GFR, and failure defined as a greater than 75% decrease in estimated GFR. Loss and end-stage classifications were not assessed because of this study’s follow-up period. The adjusted Cockcroft and Gault equation10 was used to estimate GFR due to the inconsistency of weight availability in the dataset and concordance with the institution’s practice. Baseline creatinine clearance was calculated with the first serum creatinine obtained, and the minimum creatinine clearance was calculated using the maximum serum creatinine during each patient’s visit. The percent decrease in creatinine clearance was calculated from these 2 values. AKI status was defined as meeting any of the RIFLE criteria. Mortality was assessed for all patients and defined as the composite of inhospital mortality and discharge or transfer to hospice care.
Exposure Ascertainment
Hypotension exposure was defined as experiencing 1 of the following: mean arterial blood pressure less than 60 mm Hg, a diagnosis of hypotension by a physician, or receipt of vasopressors or inotropic agents. Days of therapy for each drug were obtained and combination days of therapy were calculated by including only those days in which the patient received both medications. Total days of therapy were calculated by the sum of all days receiving at least 1 study agent. Exposure to other nephrotoxic agents (eg, acyclovir, angiotensin converting enzyme [ACE] inhibitors, angiotensin II receptor antagonists, aminoglycosides, amphotericin B, cyclosporine, foscarnet, loop diuretics, nonsteroidal anti-inflammatory drugs, sulfonamides, tacrolimus, and tenofovir) were defined as receipt of at least 1 dose of the agent during hospitalization.
Statistical Analysis
Characteristics between groups were described with basic descriptive statistics. Continuous variables were compared with 1-way analysis of variance (ANOVA) or the Kruskal-Wallis test. Categorical variables were compared with chi-square or Fisher exact test. Yearly AKI trends were assessed with Pearson correlation coefficient. To control for differences in underlying severity of illness between groups, a subanalysis was performed in which the cohort was split into 4 groups (0, 1, 2 to 4, and ≥5 points) based on CCI. Univariate models for all covariates were created with probability of AKI as the outcome. Covariates significant after univariate were incorporated into the multivariate model, which was subsequently adjusted to achieve the highest predictive accuracy by minimizing the Akaike information criterion (AIC). Nephrotoxic agent exposures were included in the final multivariate model regardless of statistical significance in univariate analysis. Model fit was assessed with a standardized Hosmer-Lemeshow goodness-of-fit test.11 All statistical analyses were completed with RStudio v 0.98 running R v 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria).12 All tests were 2-tailed and significance was defined at an alpha of 0.05.
RESULTS
Of 17,879 patients initially screened, 11,650 patients were evaluated, of which 5,497 received VAN and PTZ (VAN/PTZ), 3,055 received VAN alone, and 3,098 received PTZ alone. Table 1 contains basic demographic information. The mean age of patients was 52.5 years ± 16.8 years with 6,242 (53.6%) males. Patients receiving VAN/PTZ had higher CCIs than either monotherapy group and had significantly increased length of hospitalization. While patients in the combination therapy group were more likely to experience hypotension, concomitant nephrotoxic agent exposure was more common in the VAN monotherapy group.
RIFLE-defined AKI occurred in 1,647 (14.1%) across the entire cohort. AKI occurred in 21% of VAN/PTZ patients, 8.3% of VAN patients, and 7.8% of PTZ patients (P < 0.0001). RIFLE-defined risk, injury, and failure occurred more frequently in the VAN/PTZ cohort compared to the VAN and PTZ monotherapy groups (Figure). There were no differences in AKI rates between years studied (r2 = 0.4732, P = 0.2). Patients in the VAN/PTZ group experienced AKI on average of 8.0 days after treatment initiation, compared to 8.7 days and 5.2 days for VAN and PTZ monotherapy groups, respectively. The composite of inhospital mortality and transfer-to-hospice care was more common in VAN/PTZ patients (9.6%) compared to monotherapy groups (VAN, 3.9%; PTZ, 3.4%), most likely due to the increased severity of illness.
In the subgroup analysis of patients with similar CCI, AKI incidence increased with severity of illness. When CCI was 0, 7.5% of patients experienced AKI compared to 11.2%, 16.4%, and 18.9% of patients when CCI was 1, 2 to 4, and ≥5, respectively (P < 0.0001). VAN/PTZ (range = 12.1% to 26.5%) was associated with greater AKI incidence than either VAN (range = 4.8% to 11.5%) or PTZ (range = 3.8% to 10.4%) alone in each subgroup (P < 0.0001 for all subgroups).
Factors associated with AKI in univariate analyses included treatment with VAN/PTZ, days of therapy, baseline creatinine clearance, transfer from outside hospitals, CCI, admission type, length of hospitalization, dehydration exposure, and hypotension exposure. Exposure to aminoglycosides, amphotericin B, ACE inhibitors, nonsteroidal anti-inflammatory drugs, tacrolimus, foscarnet, loop diuretics, sulfonamides, and tenofovir were all associated with increased odds of AKI in simple univariate logistic regression. Gender, age, year of treatment, angiotensin II receptor antagonist exposure, and cyclosporine exposure were not significantly associated with AKI incidence.
After multivariate logistic regression, monotherapy with VAN or PTZ was associated with decreased odds of AKI compared to VAN/PTZ therapy (aORVAN,0.48; 95% CIVAN,0.41-0.57; aORPTZ, 0.43; 95% CIPTZ, 0.37-0.50). No difference in AKI incidence was observed between VAN and PTZ groups (aORPTZ:VAN, 0.88; 95% CI, 0.73-1.08). Table 2 describes the relationship between AKI and other covariates included in the model. Increased odds of AKI were seen with concomitant administration of ACE inhibitors, amphotericin B, tacrolimus, loop diuretics, and tenofovir. Radio-contrast dye administration was associated with lower odds of AKI. Patients admitted urgently and emergently were at higher risk of AKI, while those admitted via the trauma center were less likely to experience AKI compared to patients who were electively admitted. Increased length of stay and duration of therapy were both associated with increased likelihood of AKI, independent of treatment group; however, durations of therapy beyond 12 days was not associated with increased AKI. Hypotension, as defined, and diagnosed dehydration both independently increased AKI odds. Aside from those older than 80 years of age, increasing age was not associated with increased AKI risk. Male gender was associated with a slight decrease in AKI rate. No evidence of overfitting was observed with the standardized Hosmer-Lemeshow P-value of 0.683, and the model provides good predictive accuracy with a C-statistic of 0.788.
CONCLUSIONS
Acute kidney injury secondary to VAN therapy is a well-characterized adverse effect, while AKI incidence secondary to PTZ is less understood. Additionally, there appears to be an additive effect when these agents are used in combination. This is the largest review of AKI in patients receiving VAN,PTZ, or the combination of both agents.
There is increasing evidence suggesting greater nephrotoxicity in patients treated with the combination of VAN and antipseudomonal beta-lactams. The mechanism for the apparent increase in nephrotoxicity with this drug combination is not well understood and needs further study in both animal models and humans.
Acute kidney injury rates related to VAN vary widely, with recent studies in critically ill and internal medicine patients estimated at 21% and 13.6%, respectively.2,3 In our VAN monotherapy cohort, the AKI rate was 8.3%, with 2.3% of patients experiencing a greater than 50% decrease in creatinine clearance. Piperacillin-tazobactam-related AKI rates are not well characterized; however, a small retrospective analysis estimated that 11.1% of PTZ patients experienced acute renal failure (defined as either increase in serum creatinine greater than 0.5 mg/dL or 50% increase from baseline).13 In the present study, we found the PTZ-related AKI rate to be 7.8%, which may be due to a more stringent definition of AKI. Additionally, Hellwig et al13 found that PTZ monotherapy was associated with higher AKI rates compared to VAN monotherapy (11.1% vs 4.9%; P = 0.014). This was not replicated in our study, with VAN and PTZ monotherapy having similar AKI rates (8.3% and 7.8%, respectively) and an adjusted aOR of 0.88 (95% CI 0.0.73-1.08) for AKI in PTZ- compared to VAN-treated patients. The estimated AKI incidence of 21% in the combination therapy group at our institution is consistent with literature that ranges from 16.3% to 34.8%.4-8,13
To control for differences in baseline severity of illness, we performed a subgroup analysis of patients with similar CCI scores. The finding of increased AKI in patients receiving combination VAN and PTZ was consistent in each subgroup, suggesting that the increase in AKI is independent of illness severity.
This study is not without limitations. As with all retrospective studies, it is difficult to determine a causal link between VAN and PTZ combination therapy and increased AKI incidence due to confounding. We employed a rigorous study design that controlled for major confounders of AKI, such as concomitant nephrotoxic exposure, hypotension, and renal disease. Severity of illness was measured with CCI, which may not accurately capture the severity of illness at treatment initiation. Alternatives, such as acute physiology and chronic health evaluation (APACHE) and sequential organ failure assessment (SOFA) scores, may more accurately reflect critical illness on presentation; however, this study was not focused specifically on critically ill patients. In addition to baseline comorbidity, we controlled for hypotension and dehydration as a surrogate marker for critical illness. In the subgroup analysis of patients with similar CCI, the effect of VAN/PTZ on AKI compared to VAN or PTZ monotherapy was consistent in each group. Nephrotoxic potential of agents was assumed to be equal, which is not necessarily true. Additionally, the binary representation of nephrotoxic exposure does not describe the amount of the agent received; as such, our estimations of AKI odds may be artificially elevated. Approximately one-quarter of the patients in this study were transferred from an outside hospital, for which no data regarding initial treatment are available. This may lead to exposure misclassification. We attempted to control for this factor in the regression model and found that, after controlling for other covariates, hospital transfer was associated with increasing odds of AKI. Finally, data were collected retrospectively from the electronic medical record and are subject to inaccuracies documented in the chart; however, any bias introduced should be nondifferential.
In our large retrospective study of combination empiric therapy with VAN and PTZ, we found that combination therapy was associated with more than double the odds of AKI occurring compared to either monotherapy with VAN or PTZ. Increasing duration of therapy was also associated with increases in AKI. These findings demonstrate the need for judicious use of combination therapy and strengthen the need for antimicrobial de-escalation when appropriate to avoid deleterious effects.
Acknowledgments
The authors thank Chantal Le Rutter, MPA, for copyediting services.
Disclosures
This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant numbers UL1TR000117 and UL1TR001998. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors report no conflicts of interest.
Empiric antimicrobial therapy often consists of the combination of gram-positive coverage with vancomycin (VAN) and gram-negative coverage, specifically an antipseudomonal beta-lactam such as piperacillin-tazobactam (PTZ). Literature from a variety of patient populations reports nephrotoxicity associated with VAN, targeting troughs greater than 15 µg/mL, that occur in 5% to 43% of patients.1 In a study of critically ill patients, acute kidney injury (AKI) was found in 21% of patients receiving VAN, with increasing duration of VAN treatment, greater VAN levels, concomitant vasoactive medication administration, and intermittent infusion methods being associated with higher odds of AKI.2 A recent report from adult internal medicine patients estimated the incidence of VAN-associated nephrotoxicity at 13.6% and implicated concomitant PTZ therapy as a key factor in these patients.3
Further studies have explored the interaction between empiric beta-lactam and VAN therapy, showing mixed results. Reports of AKI associated with the combination of VAN and PTZ range from 16.3% to 34.8%,4-8 while the cefepime-VAN combination is reported to range from 12.5% to 13.3%.5,6 While VAN monotherapy groups were well represented, only 1 study7 compared the PTZ-VAN combination to a control group of PTZ monotherapy.
The primary objective of this study was to evaluate the differences in AKI incidence between patients treated with VAN and with PTZ, alone and in combination.
METHODS
This is a retrospective cohort study of adult patients conducted at the University of Kentucky Chandler Medical Center (UKMC) from September 1, 2010 through August 31, 2014. Patients were included if they were at least 18 years of age on admission; remained hospitalized for at least 48 hours; received VAN combined with PTZ (VAN/PTZ), VAN alone, or PTZ alone; and had at least 48 hours of therapy (and 48 hours of overlapping therapy in the VAN/PTZ group). Patients were excluded if they had underlying diagnosis of chronic kidney disease according to the International Classification of Diseases 9 (ICD-9) code, were receiving renal replacement therapy before admission, had a diagnosis of cystic fibrosis, or were pregnant. Additionally, patients were excluded if they presented with AKI, defined as an initial creatinine clearance less than 30 mL/min, or if baseline creatinine clearance was greater than 4 times the standard deviation from the mean; serum creatinine values were not obtained during admission; and if AKI occurred prior to therapy initiation, within 48 hours of initiation, or more than 7 days after treatment was discontinued. Patients were followed throughout their stay until time of discharge.
Data Source
Patient data were collected from the University of Kentucky Center for Clinical and Translational Science Enterprise Data Trust (EDT). The EDT contains clinical data from the inpatient population of UKMC from 2006 to present. Data stored and updated nightly by the EDT includes: demographics, financial classification (Medicare, Medicaid, private insurance), provider-level detail (service line), medical diagnosis (ICD-9 codes), medical procedures (Current Procedural Terminology [CPT] codes), lab tests and results, medication administration details, visit details (age, length of stay, etc), and vital signs. This study was approved by the UKMC Institutional Review Board.
Data collected for each patient included: demographic data, visit details (length of stay, admitting and primary diagnosis codes, etc.), severity of underlying illness as defined by the Charlson Comorbidity Index (CCI), all serum creatinine levels drawn per visit, medication administration information (dose, date, and time administered), all VAN trough levels, receipt of other nephrotoxic agents, blood pressures, and receipt of vasopressors.
Outcome Ascertainment
The definition of AKI was based on the RIFLE (Risk, Injury, Failure, Loss, End-stage) criteria,9 with risk defined as a 25% to 50% decrease in estimated glomerular filtration rate (GFR), injury as a 50% to 75% decrease in estimated GFR, and failure defined as a greater than 75% decrease in estimated GFR. Loss and end-stage classifications were not assessed because of this study’s follow-up period. The adjusted Cockcroft and Gault equation10 was used to estimate GFR due to the inconsistency of weight availability in the dataset and concordance with the institution’s practice. Baseline creatinine clearance was calculated with the first serum creatinine obtained, and the minimum creatinine clearance was calculated using the maximum serum creatinine during each patient’s visit. The percent decrease in creatinine clearance was calculated from these 2 values. AKI status was defined as meeting any of the RIFLE criteria. Mortality was assessed for all patients and defined as the composite of inhospital mortality and discharge or transfer to hospice care.
Exposure Ascertainment
Hypotension exposure was defined as experiencing 1 of the following: mean arterial blood pressure less than 60 mm Hg, a diagnosis of hypotension by a physician, or receipt of vasopressors or inotropic agents. Days of therapy for each drug were obtained and combination days of therapy were calculated by including only those days in which the patient received both medications. Total days of therapy were calculated by the sum of all days receiving at least 1 study agent. Exposure to other nephrotoxic agents (eg, acyclovir, angiotensin converting enzyme [ACE] inhibitors, angiotensin II receptor antagonists, aminoglycosides, amphotericin B, cyclosporine, foscarnet, loop diuretics, nonsteroidal anti-inflammatory drugs, sulfonamides, tacrolimus, and tenofovir) were defined as receipt of at least 1 dose of the agent during hospitalization.
Statistical Analysis
Characteristics between groups were described with basic descriptive statistics. Continuous variables were compared with 1-way analysis of variance (ANOVA) or the Kruskal-Wallis test. Categorical variables were compared with chi-square or Fisher exact test. Yearly AKI trends were assessed with Pearson correlation coefficient. To control for differences in underlying severity of illness between groups, a subanalysis was performed in which the cohort was split into 4 groups (0, 1, 2 to 4, and ≥5 points) based on CCI. Univariate models for all covariates were created with probability of AKI as the outcome. Covariates significant after univariate were incorporated into the multivariate model, which was subsequently adjusted to achieve the highest predictive accuracy by minimizing the Akaike information criterion (AIC). Nephrotoxic agent exposures were included in the final multivariate model regardless of statistical significance in univariate analysis. Model fit was assessed with a standardized Hosmer-Lemeshow goodness-of-fit test.11 All statistical analyses were completed with RStudio v 0.98 running R v 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria).12 All tests were 2-tailed and significance was defined at an alpha of 0.05.
RESULTS
Of 17,879 patients initially screened, 11,650 patients were evaluated, of which 5,497 received VAN and PTZ (VAN/PTZ), 3,055 received VAN alone, and 3,098 received PTZ alone. Table 1 contains basic demographic information. The mean age of patients was 52.5 years ± 16.8 years with 6,242 (53.6%) males. Patients receiving VAN/PTZ had higher CCIs than either monotherapy group and had significantly increased length of hospitalization. While patients in the combination therapy group were more likely to experience hypotension, concomitant nephrotoxic agent exposure was more common in the VAN monotherapy group.
RIFLE-defined AKI occurred in 1,647 (14.1%) across the entire cohort. AKI occurred in 21% of VAN/PTZ patients, 8.3% of VAN patients, and 7.8% of PTZ patients (P < 0.0001). RIFLE-defined risk, injury, and failure occurred more frequently in the VAN/PTZ cohort compared to the VAN and PTZ monotherapy groups (Figure). There were no differences in AKI rates between years studied (r2 = 0.4732, P = 0.2). Patients in the VAN/PTZ group experienced AKI on average of 8.0 days after treatment initiation, compared to 8.7 days and 5.2 days for VAN and PTZ monotherapy groups, respectively. The composite of inhospital mortality and transfer-to-hospice care was more common in VAN/PTZ patients (9.6%) compared to monotherapy groups (VAN, 3.9%; PTZ, 3.4%), most likely due to the increased severity of illness.
In the subgroup analysis of patients with similar CCI, AKI incidence increased with severity of illness. When CCI was 0, 7.5% of patients experienced AKI compared to 11.2%, 16.4%, and 18.9% of patients when CCI was 1, 2 to 4, and ≥5, respectively (P < 0.0001). VAN/PTZ (range = 12.1% to 26.5%) was associated with greater AKI incidence than either VAN (range = 4.8% to 11.5%) or PTZ (range = 3.8% to 10.4%) alone in each subgroup (P < 0.0001 for all subgroups).
Factors associated with AKI in univariate analyses included treatment with VAN/PTZ, days of therapy, baseline creatinine clearance, transfer from outside hospitals, CCI, admission type, length of hospitalization, dehydration exposure, and hypotension exposure. Exposure to aminoglycosides, amphotericin B, ACE inhibitors, nonsteroidal anti-inflammatory drugs, tacrolimus, foscarnet, loop diuretics, sulfonamides, and tenofovir were all associated with increased odds of AKI in simple univariate logistic regression. Gender, age, year of treatment, angiotensin II receptor antagonist exposure, and cyclosporine exposure were not significantly associated with AKI incidence.
After multivariate logistic regression, monotherapy with VAN or PTZ was associated with decreased odds of AKI compared to VAN/PTZ therapy (aORVAN,0.48; 95% CIVAN,0.41-0.57; aORPTZ, 0.43; 95% CIPTZ, 0.37-0.50). No difference in AKI incidence was observed between VAN and PTZ groups (aORPTZ:VAN, 0.88; 95% CI, 0.73-1.08). Table 2 describes the relationship between AKI and other covariates included in the model. Increased odds of AKI were seen with concomitant administration of ACE inhibitors, amphotericin B, tacrolimus, loop diuretics, and tenofovir. Radio-contrast dye administration was associated with lower odds of AKI. Patients admitted urgently and emergently were at higher risk of AKI, while those admitted via the trauma center were less likely to experience AKI compared to patients who were electively admitted. Increased length of stay and duration of therapy were both associated with increased likelihood of AKI, independent of treatment group; however, durations of therapy beyond 12 days was not associated with increased AKI. Hypotension, as defined, and diagnosed dehydration both independently increased AKI odds. Aside from those older than 80 years of age, increasing age was not associated with increased AKI risk. Male gender was associated with a slight decrease in AKI rate. No evidence of overfitting was observed with the standardized Hosmer-Lemeshow P-value of 0.683, and the model provides good predictive accuracy with a C-statistic of 0.788.
CONCLUSIONS
Acute kidney injury secondary to VAN therapy is a well-characterized adverse effect, while AKI incidence secondary to PTZ is less understood. Additionally, there appears to be an additive effect when these agents are used in combination. This is the largest review of AKI in patients receiving VAN,PTZ, or the combination of both agents.
There is increasing evidence suggesting greater nephrotoxicity in patients treated with the combination of VAN and antipseudomonal beta-lactams. The mechanism for the apparent increase in nephrotoxicity with this drug combination is not well understood and needs further study in both animal models and humans.
Acute kidney injury rates related to VAN vary widely, with recent studies in critically ill and internal medicine patients estimated at 21% and 13.6%, respectively.2,3 In our VAN monotherapy cohort, the AKI rate was 8.3%, with 2.3% of patients experiencing a greater than 50% decrease in creatinine clearance. Piperacillin-tazobactam-related AKI rates are not well characterized; however, a small retrospective analysis estimated that 11.1% of PTZ patients experienced acute renal failure (defined as either increase in serum creatinine greater than 0.5 mg/dL or 50% increase from baseline).13 In the present study, we found the PTZ-related AKI rate to be 7.8%, which may be due to a more stringent definition of AKI. Additionally, Hellwig et al13 found that PTZ monotherapy was associated with higher AKI rates compared to VAN monotherapy (11.1% vs 4.9%; P = 0.014). This was not replicated in our study, with VAN and PTZ monotherapy having similar AKI rates (8.3% and 7.8%, respectively) and an adjusted aOR of 0.88 (95% CI 0.0.73-1.08) for AKI in PTZ- compared to VAN-treated patients. The estimated AKI incidence of 21% in the combination therapy group at our institution is consistent with literature that ranges from 16.3% to 34.8%.4-8,13
To control for differences in baseline severity of illness, we performed a subgroup analysis of patients with similar CCI scores. The finding of increased AKI in patients receiving combination VAN and PTZ was consistent in each subgroup, suggesting that the increase in AKI is independent of illness severity.
This study is not without limitations. As with all retrospective studies, it is difficult to determine a causal link between VAN and PTZ combination therapy and increased AKI incidence due to confounding. We employed a rigorous study design that controlled for major confounders of AKI, such as concomitant nephrotoxic exposure, hypotension, and renal disease. Severity of illness was measured with CCI, which may not accurately capture the severity of illness at treatment initiation. Alternatives, such as acute physiology and chronic health evaluation (APACHE) and sequential organ failure assessment (SOFA) scores, may more accurately reflect critical illness on presentation; however, this study was not focused specifically on critically ill patients. In addition to baseline comorbidity, we controlled for hypotension and dehydration as a surrogate marker for critical illness. In the subgroup analysis of patients with similar CCI, the effect of VAN/PTZ on AKI compared to VAN or PTZ monotherapy was consistent in each group. Nephrotoxic potential of agents was assumed to be equal, which is not necessarily true. Additionally, the binary representation of nephrotoxic exposure does not describe the amount of the agent received; as such, our estimations of AKI odds may be artificially elevated. Approximately one-quarter of the patients in this study were transferred from an outside hospital, for which no data regarding initial treatment are available. This may lead to exposure misclassification. We attempted to control for this factor in the regression model and found that, after controlling for other covariates, hospital transfer was associated with increasing odds of AKI. Finally, data were collected retrospectively from the electronic medical record and are subject to inaccuracies documented in the chart; however, any bias introduced should be nondifferential.
In our large retrospective study of combination empiric therapy with VAN and PTZ, we found that combination therapy was associated with more than double the odds of AKI occurring compared to either monotherapy with VAN or PTZ. Increasing duration of therapy was also associated with increases in AKI. These findings demonstrate the need for judicious use of combination therapy and strengthen the need for antimicrobial de-escalation when appropriate to avoid deleterious effects.
Acknowledgments
The authors thank Chantal Le Rutter, MPA, for copyediting services.
Disclosures
This project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant numbers UL1TR000117 and UL1TR001998. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors report no conflicts of interest.
1. van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57:734-744. PubMed
2. Hanrahan TP, Harlow G, Hutchinson J, et al. Vancomycin-associated nephrotoxicity in the critically ill: a retrospective multivariate regression analysis. Crit Care Med. 2014;42:2527-2536. PubMed
3. Meaney CJ, Hynicka LM, Tsoukleris MG. Vancomycin-associated nephrotoxicity in adult medicine patients: incidence, outcomes, and risk factors. Pharmacotherapy. 2014;34:653-661. PubMed
4. Burgess LD, Drew RH. Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin-tazobactam. Pharmacotherapy. 2014;34:670-676. PubMed
5. Moenster RP, Linneman TW, Finnegan PM, Hand S, Thomas Z, McDonald JR. Acute renal failure associated with vancomycin and β-lactams for the treatment of osteomyelitis in diabetics: piperacillin-tazobactam as compared with cefepime. Clin Microbiol Infect. 2014;20:O384-O389. PubMed
6. Gomes DM, Smotherman C, Birch A, et al. Comparison of acute kidney injury during treatment with vancomycin in combination with piperacillin-tazobactam or cefepime. Pharmacotherapy. 2014;34:662-669. PubMed
7. Kim T, Kandiah S, Patel M, et al. Risk factors for kidney injury during vancomycin and piperacillin/tazobactam administration, including increased odds of injury with combination therapy. BMC Res Notes. 2015;8:579. PubMed
8. Davies SW, Efird JT, Guidry CA, et al. Top guns: the “Maverick” and “Goose” of empiric therapy. Surg Infect (Larchmt). 2016;17:38-47. PubMed
9. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204-R212. PubMed
10. Wilhelm SM, Kale-Pradhan PB. Estimating creatinine clearance: a meta-analysis. Pharmacotherapy. 2011;31:658-664. PubMed
11. Paul P, Pennell ML, Lemeshow S. Standardizing the power of the Hosmer-Lemeshow goodness of fit test in large data sets. Stat Med. 2013;32:67-80. PubMed
12. R Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: http://www.R-project.org/.
13. Hellwig T, Hammerquist R, Loecker B, Shields J. Retrospective evaluation of the incidence of vancomycin and/or piperacillin-tazobactam induced acute renal failure. Abstracts of the Society of Critical Care Medicine 41st Critical Care Congress. February 4-8, 2012. Houston, Texas. Crit Care Med. 2011;39:1-264.
1. van Hal SJ, Paterson DL, Lodise TP. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother. 2013;57:734-744. PubMed
2. Hanrahan TP, Harlow G, Hutchinson J, et al. Vancomycin-associated nephrotoxicity in the critically ill: a retrospective multivariate regression analysis. Crit Care Med. 2014;42:2527-2536. PubMed
3. Meaney CJ, Hynicka LM, Tsoukleris MG. Vancomycin-associated nephrotoxicity in adult medicine patients: incidence, outcomes, and risk factors. Pharmacotherapy. 2014;34:653-661. PubMed
4. Burgess LD, Drew RH. Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin-tazobactam. Pharmacotherapy. 2014;34:670-676. PubMed
5. Moenster RP, Linneman TW, Finnegan PM, Hand S, Thomas Z, McDonald JR. Acute renal failure associated with vancomycin and β-lactams for the treatment of osteomyelitis in diabetics: piperacillin-tazobactam as compared with cefepime. Clin Microbiol Infect. 2014;20:O384-O389. PubMed
6. Gomes DM, Smotherman C, Birch A, et al. Comparison of acute kidney injury during treatment with vancomycin in combination with piperacillin-tazobactam or cefepime. Pharmacotherapy. 2014;34:662-669. PubMed
7. Kim T, Kandiah S, Patel M, et al. Risk factors for kidney injury during vancomycin and piperacillin/tazobactam administration, including increased odds of injury with combination therapy. BMC Res Notes. 2015;8:579. PubMed
8. Davies SW, Efird JT, Guidry CA, et al. Top guns: the “Maverick” and “Goose” of empiric therapy. Surg Infect (Larchmt). 2016;17:38-47. PubMed
9. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204-R212. PubMed
10. Wilhelm SM, Kale-Pradhan PB. Estimating creatinine clearance: a meta-analysis. Pharmacotherapy. 2011;31:658-664. PubMed
11. Paul P, Pennell ML, Lemeshow S. Standardizing the power of the Hosmer-Lemeshow goodness of fit test in large data sets. Stat Med. 2013;32:67-80. PubMed
12. R Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: http://www.R-project.org/.
13. Hellwig T, Hammerquist R, Loecker B, Shields J. Retrospective evaluation of the incidence of vancomycin and/or piperacillin-tazobactam induced acute renal failure. Abstracts of the Society of Critical Care Medicine 41st Critical Care Congress. February 4-8, 2012. Houston, Texas. Crit Care Med. 2011;39:1-264.
© 2017 Society of Hospital Medicine
Software could improve image analysis, team says
Researchers say they have developed new software that will analyze medical and scientific images faster and more accurately than ever before.
The team says this software, Tracking Equilibrium and Nonequilibrium shifts in Data (TREND), can analyze any series of images, including nuclear magnetic resonance images, computerized tomography scans, ultrasound images, video images, and imaging from scientific equipment of all kinds.
The researchers described the TREND software in Biophysical Journal.
The team said TREND can study sets of images to resolve and track the changes among the images.
And the software can analyze videos to plot and resolve changes as well as reconstruct videos to focus only on the individual processes and changes of interest.
“TREND allows accurate, rapid analysis of incredibly complex and nuanced images, which can potentially save doctors, patients, and scientists countless hours and money,” said Steve Van Doren, PhD, of the University of Missouri in Columbia, Missouri.
“TREND has allowed us to advance our own research into enzyme interactions considerably. Previously, it would take us weeks to analyze a single group of images. With TREND, that analysis now takes only a few minutes and is more accurate and consistent than if a human performed the work.” ![]()

Researchers say they have developed new software that will analyze medical and scientific images faster and more accurately than ever before.
The team says this software, Tracking Equilibrium and Nonequilibrium shifts in Data (TREND), can analyze any series of images, including nuclear magnetic resonance images, computerized tomography scans, ultrasound images, video images, and imaging from scientific equipment of all kinds.
The researchers described the TREND software in Biophysical Journal.
The team said TREND can study sets of images to resolve and track the changes among the images.
And the software can analyze videos to plot and resolve changes as well as reconstruct videos to focus only on the individual processes and changes of interest.
“TREND allows accurate, rapid analysis of incredibly complex and nuanced images, which can potentially save doctors, patients, and scientists countless hours and money,” said Steve Van Doren, PhD, of the University of Missouri in Columbia, Missouri.
“TREND has allowed us to advance our own research into enzyme interactions considerably. Previously, it would take us weeks to analyze a single group of images. With TREND, that analysis now takes only a few minutes and is more accurate and consistent than if a human performed the work.” ![]()

Researchers say they have developed new software that will analyze medical and scientific images faster and more accurately than ever before.
The team says this software, Tracking Equilibrium and Nonequilibrium shifts in Data (TREND), can analyze any series of images, including nuclear magnetic resonance images, computerized tomography scans, ultrasound images, video images, and imaging from scientific equipment of all kinds.
The researchers described the TREND software in Biophysical Journal.
The team said TREND can study sets of images to resolve and track the changes among the images.
And the software can analyze videos to plot and resolve changes as well as reconstruct videos to focus only on the individual processes and changes of interest.
“TREND allows accurate, rapid analysis of incredibly complex and nuanced images, which can potentially save doctors, patients, and scientists countless hours and money,” said Steve Van Doren, PhD, of the University of Missouri in Columbia, Missouri.
“TREND has allowed us to advance our own research into enzyme interactions considerably. Previously, it would take us weeks to analyze a single group of images. With TREND, that analysis now takes only a few minutes and is more accurate and consistent than if a human performed the work.” ![]()
Combo granted orphan designation for DLBCL

The US Food and Drug Administration (FDA) has granted orphan drug designation for the combination of TG-1101 (ublituximab), an anti-CD20 monoclonal antibody, and TGR-1202, a PI3K delta inhibitor, in the treatment of diffuse large B-cell lymphoma (DLBCL).
The combination is currently being evaluated in patients with relapsed or refractory DLBCL in the phase 2b UNITY-DLBCL trial.
Ublituximab and TGR-1202 are both products of TG Therapeutics, Inc.
Updated results from a phase 1 study of ublituximab and TGR-1202 in patients with DLBCL and other malignancies were presented at the 21st Congress of the European Hematology Association.
The data included 165 patients treated with varying doses of TGR-1202 alone (n=90) or in combination with ublituximab (n=75). The patients were heavily pretreated, with the majority having 3 or more prior lines of therapy.
There were 7 evaluable patients with DLBCL who received the combination at the phase 3 doses— ublituximab at 900 mg and TGR-1202 at 800 mg micronized.
The overall response rate for this group was 57%. Of the 4 responders, 1 patient had a complete response, and 3 had a partial response. Two patients had stable disease, and 1 progressed.
In the overall study population, the most common adverse events were diarrhea (47%), nausea (45%), fatigue (37%), vomiting (27%), and neutropenia (21%). The most common grade 3/4 adverse events were neutropenia (18%) and anemia (5%).
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to
treat, diagnose, or prevent rare diseases/disorders affecting fewer than
200,000 people in the US.
Orphan designation provides companies
with certain incentives to develop products for rare diseases. This
includes a 50% tax break on research and development, a fee waiver,
access to federal grants, and 7 years of market exclusivity if the
product is approved. ![]()

The US Food and Drug Administration (FDA) has granted orphan drug designation for the combination of TG-1101 (ublituximab), an anti-CD20 monoclonal antibody, and TGR-1202, a PI3K delta inhibitor, in the treatment of diffuse large B-cell lymphoma (DLBCL).
The combination is currently being evaluated in patients with relapsed or refractory DLBCL in the phase 2b UNITY-DLBCL trial.
Ublituximab and TGR-1202 are both products of TG Therapeutics, Inc.
Updated results from a phase 1 study of ublituximab and TGR-1202 in patients with DLBCL and other malignancies were presented at the 21st Congress of the European Hematology Association.
The data included 165 patients treated with varying doses of TGR-1202 alone (n=90) or in combination with ublituximab (n=75). The patients were heavily pretreated, with the majority having 3 or more prior lines of therapy.
There were 7 evaluable patients with DLBCL who received the combination at the phase 3 doses— ublituximab at 900 mg and TGR-1202 at 800 mg micronized.
The overall response rate for this group was 57%. Of the 4 responders, 1 patient had a complete response, and 3 had a partial response. Two patients had stable disease, and 1 progressed.
In the overall study population, the most common adverse events were diarrhea (47%), nausea (45%), fatigue (37%), vomiting (27%), and neutropenia (21%). The most common grade 3/4 adverse events were neutropenia (18%) and anemia (5%).
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to
treat, diagnose, or prevent rare diseases/disorders affecting fewer than
200,000 people in the US.
Orphan designation provides companies
with certain incentives to develop products for rare diseases. This
includes a 50% tax break on research and development, a fee waiver,
access to federal grants, and 7 years of market exclusivity if the
product is approved. ![]()

The US Food and Drug Administration (FDA) has granted orphan drug designation for the combination of TG-1101 (ublituximab), an anti-CD20 monoclonal antibody, and TGR-1202, a PI3K delta inhibitor, in the treatment of diffuse large B-cell lymphoma (DLBCL).
The combination is currently being evaluated in patients with relapsed or refractory DLBCL in the phase 2b UNITY-DLBCL trial.
Ublituximab and TGR-1202 are both products of TG Therapeutics, Inc.
Updated results from a phase 1 study of ublituximab and TGR-1202 in patients with DLBCL and other malignancies were presented at the 21st Congress of the European Hematology Association.
The data included 165 patients treated with varying doses of TGR-1202 alone (n=90) or in combination with ublituximab (n=75). The patients were heavily pretreated, with the majority having 3 or more prior lines of therapy.
There were 7 evaluable patients with DLBCL who received the combination at the phase 3 doses— ublituximab at 900 mg and TGR-1202 at 800 mg micronized.
The overall response rate for this group was 57%. Of the 4 responders, 1 patient had a complete response, and 3 had a partial response. Two patients had stable disease, and 1 progressed.
In the overall study population, the most common adverse events were diarrhea (47%), nausea (45%), fatigue (37%), vomiting (27%), and neutropenia (21%). The most common grade 3/4 adverse events were neutropenia (18%) and anemia (5%).
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to
treat, diagnose, or prevent rare diseases/disorders affecting fewer than
200,000 people in the US.
Orphan designation provides companies
with certain incentives to develop products for rare diseases. This
includes a 50% tax break on research and development, a fee waiver,
access to federal grants, and 7 years of market exclusivity if the
product is approved. ![]()
Distress linked to higher risk of death from leukemia, other cancers

patient and her father
Photo by Rhoda Baer
A study published in The BMJ suggests that higher levels of psychological distress (anxiety and depression) may be associated with an increased risk of death from leukemia and other cancers.
The findings are observational, so no firm conclusions about cause and effect can be drawn.
However, the researchers said the findings add to the growing evidence that psychological distress could have some predictive capacity for certain physical conditions.
There is some evidence that psychological distress (anxiety and depression) is related to increased rates of cardiovascular disease, but links with different types of cancer are either unclear or untested.
So David Batty, PhD, of University College London in the UK, and his colleagues set out to examine if psychological distress is a potential predictor of site-specific cancer mortality.
The researchers analyzed data from 16 studies (13 from England and 3 from Scotland), which started between 1994 and 2008. The data included 163,363 men and women age 16 or over who were free from cancer at the start of the study.
Psychological distress scores were measured using the general health questionnaire (GHQ-12), and participants were monitored for an average of 9.5 years. During this time, there were 4353 deaths from cancer.
Several factors that could have influenced the results were taken into account, including age, sex, education, socioeconomic status, body mass index, smoking, and alcohol intake.
“After statistical control for these factors, the results show that, compared with people in the least distressed group, death rates in the most distressed group were consistently higher for cancer of the bowel, prostate, pancreas, and esophagus and for leukemia,” Dr Batty said.
He and his colleagues pointed out that this association may also be affected by reverse causality, where undiagnosed (early) cancer might have had an underlying impact on mood.
In a bid to correct for this, the team conducted a further analysis excluding study participants who died in the first 5 years of follow-up, but this made no difference to the findings. The links between distress and cancer remained.
Specifically, compared to people in the least distressed group (GHQ-12 score 0-6), death rates in the most distressed group (score 7-12) were consistently increased for cancer of all sites combined, with a multivariable adjusted hazard ratio (HR) of 1.32.
Death rates were also increased for colorectal (HR=1.84), prostate (HR=2.42), pancreatic (HR=2.76), and esophageal cancers (HR=2.59), as well as for leukemia (HR=3.86).
“Our findings contribute to the evidence that poor mental health might have some predictive capacity for certain physical diseases,” Dr Batty said, “but we are a long way off from knowing if these relationships are truly causal.” ![]()

patient and her father
Photo by Rhoda Baer
A study published in The BMJ suggests that higher levels of psychological distress (anxiety and depression) may be associated with an increased risk of death from leukemia and other cancers.
The findings are observational, so no firm conclusions about cause and effect can be drawn.
However, the researchers said the findings add to the growing evidence that psychological distress could have some predictive capacity for certain physical conditions.
There is some evidence that psychological distress (anxiety and depression) is related to increased rates of cardiovascular disease, but links with different types of cancer are either unclear or untested.
So David Batty, PhD, of University College London in the UK, and his colleagues set out to examine if psychological distress is a potential predictor of site-specific cancer mortality.
The researchers analyzed data from 16 studies (13 from England and 3 from Scotland), which started between 1994 and 2008. The data included 163,363 men and women age 16 or over who were free from cancer at the start of the study.
Psychological distress scores were measured using the general health questionnaire (GHQ-12), and participants were monitored for an average of 9.5 years. During this time, there were 4353 deaths from cancer.
Several factors that could have influenced the results were taken into account, including age, sex, education, socioeconomic status, body mass index, smoking, and alcohol intake.
“After statistical control for these factors, the results show that, compared with people in the least distressed group, death rates in the most distressed group were consistently higher for cancer of the bowel, prostate, pancreas, and esophagus and for leukemia,” Dr Batty said.
He and his colleagues pointed out that this association may also be affected by reverse causality, where undiagnosed (early) cancer might have had an underlying impact on mood.
In a bid to correct for this, the team conducted a further analysis excluding study participants who died in the first 5 years of follow-up, but this made no difference to the findings. The links between distress and cancer remained.
Specifically, compared to people in the least distressed group (GHQ-12 score 0-6), death rates in the most distressed group (score 7-12) were consistently increased for cancer of all sites combined, with a multivariable adjusted hazard ratio (HR) of 1.32.
Death rates were also increased for colorectal (HR=1.84), prostate (HR=2.42), pancreatic (HR=2.76), and esophageal cancers (HR=2.59), as well as for leukemia (HR=3.86).
“Our findings contribute to the evidence that poor mental health might have some predictive capacity for certain physical diseases,” Dr Batty said, “but we are a long way off from knowing if these relationships are truly causal.” ![]()

patient and her father
Photo by Rhoda Baer
A study published in The BMJ suggests that higher levels of psychological distress (anxiety and depression) may be associated with an increased risk of death from leukemia and other cancers.
The findings are observational, so no firm conclusions about cause and effect can be drawn.
However, the researchers said the findings add to the growing evidence that psychological distress could have some predictive capacity for certain physical conditions.
There is some evidence that psychological distress (anxiety and depression) is related to increased rates of cardiovascular disease, but links with different types of cancer are either unclear or untested.
So David Batty, PhD, of University College London in the UK, and his colleagues set out to examine if psychological distress is a potential predictor of site-specific cancer mortality.
The researchers analyzed data from 16 studies (13 from England and 3 from Scotland), which started between 1994 and 2008. The data included 163,363 men and women age 16 or over who were free from cancer at the start of the study.
Psychological distress scores were measured using the general health questionnaire (GHQ-12), and participants were monitored for an average of 9.5 years. During this time, there were 4353 deaths from cancer.
Several factors that could have influenced the results were taken into account, including age, sex, education, socioeconomic status, body mass index, smoking, and alcohol intake.
“After statistical control for these factors, the results show that, compared with people in the least distressed group, death rates in the most distressed group were consistently higher for cancer of the bowel, prostate, pancreas, and esophagus and for leukemia,” Dr Batty said.
He and his colleagues pointed out that this association may also be affected by reverse causality, where undiagnosed (early) cancer might have had an underlying impact on mood.
In a bid to correct for this, the team conducted a further analysis excluding study participants who died in the first 5 years of follow-up, but this made no difference to the findings. The links between distress and cancer remained.
Specifically, compared to people in the least distressed group (GHQ-12 score 0-6), death rates in the most distressed group (score 7-12) were consistently increased for cancer of all sites combined, with a multivariable adjusted hazard ratio (HR) of 1.32.
Death rates were also increased for colorectal (HR=1.84), prostate (HR=2.42), pancreatic (HR=2.76), and esophageal cancers (HR=2.59), as well as for leukemia (HR=3.86).
“Our findings contribute to the evidence that poor mental health might have some predictive capacity for certain physical diseases,” Dr Batty said, “but we are a long way off from knowing if these relationships are truly causal.” ![]()
Recognize, treat, and teach others to spot hidradenitis suppurativa
MIAMI – Clinicians have many options to treat and help people manage hidradenitis suppurativa, but for most patients, an early and accurate diagnosis remains elusive.
“The problem here is because it has so many mimickers, the diagnosis is often delayed, patients can be [treated for an incorrect diagnosis] and in many ways that treatment can be harmful,” said Adam Friedman, MD, of the George Washington University in Washington. Missed or ignored diagnoses can lead to more pain, impaired function, and wasted time and money.
“There are – no question – gaps in clinical care. Patients seek care outside of dermatology and go to urgent care centers, emergency rooms, [and other settings]. That’s why it’s not only important for us to recognize this, but to teach everyone else as well,” Dr. Friedman said at the Orlando Dermatology Aesthetic and Clinical Conference.
Tips for early detection
Look for chronicity in the disease’s presentation, Dr. Friedman said. “Chronicity is key, but the morphology will change, and lesions will look different over time.” Therefore, “the clinical presentation can be challenging, depending on when you catch the patient.”
Hidradenitis suppurativa is characterized by very purulent, indurated, abscesslike structures often on the underarms and groin. Ask patients where and how often they see lesions. Lesions in certain locations can be very disabling for patients not only because of pain, but also from a psychosocial impact. The groin and chest are prime examples. Also, there is a genetic predisposition so it is important to ask patients about family history as well.
Use combination therapy to hit disease ‘from all angles’
It is imperative to treat patients even when they present at a time of mild disease, Dr. Friedman said. “This is a chronic, snowballing disease that will get worse over time, because inflammation begets inflammation. Even if it’s mild disease, you still want to treat. Combinations are king, and we dermatologists are the synergy masters.”
Effective treatment strategies include medications that curtail inflammation and block hormonal influences; dietary changes (a minimal-dairy, low-carbohydrate diet helps some patients, for example); environmental changes and/or eliminating the invasive proliferative gelatinous mass (IPGM). “This is not step therapy,” Dr. Friedman emphasized. “You want to hit all these angles at once.”
In terms of nutritional support, “I usually put my patients on a combination of zinc and vitamin C, both anti-inflammatories, but also good for wound healing,” Dr. Friedman said. “I also get them on board with V-8, which can be a tough sell sometimes.” He recommends patients drink three small cans per week, adding that he has no financial disclosure related to the vegetable juice.
Patient education, smoking cessation, and keeping affected areas dry and cool are other important management strategies. Instruct patients that stress, friction, and obesity can each worsen the condition “I think obesity is an independent risk factor here, like it is in psoriasis, where obesity alone has been shown to increase the risk of psoriasis later on,” he said.
Ease the inflammation
Hidradenitis suppurativa is a disease of “inappropriate inflammation,” Dr. Friedman said, which explains why anti-inflammatory agents remain the mainstay of treatment. These include antibiotics, classic corticosteroids, and biologics, which all can have a role in therapy. He highlighted the potential role of the cutaneous microbiota and possible dysbiosis associated with disease activity. “I also use a lot chlorhexidine washes to wipe the microbial slate clean in high-risk areas; just be careful to avoid the face.”
Intralesional Kenalog (triamcinolone acetonide), in particular, is useful for its rapid results. “This is such a great and easy trick, and it really works quickly,” Dr. Friedman said. He added that a recent case series provides evidence for its efficacy as well (J Amer Acad Dermatol. 2016 Dec;75:1151-5).
Three take-home treatment pointers
Dr. Friedman shared these three take-aways for treatment of hidradenitis suppurativa with antibiotics:
- The combination of oral clindamycin 300 mg twice daily and oral rifampin 300 mg twice daily carries the most evidence for efficacy and safety, with no evidence of resistance.
- Rifampin also acts against Clostridium difficile infections, which decreases the risk of associated colitis.
- Do not give a tetracycline antibiotic as monotherapy.
In terms of retinoids, “I’ve been pretty disappointed. I find them effective [in other conditions], but for hidradenitis suppurativa, I’m just not impressed, unfortunately,” Dr. Friedman said. “Antihormonal therapies such as oral contraceptives and spironolactone for women and finasteride for men have been a useful adjuncts in my practice, with evidence supporting their use in the literature, he added. Biologics are among the new treatment options, but there are cost and insurance coverage issues, Dr. Friedman said. There are small case series evaluating biologics such as infliximab, which are very supportive – and he himself has had good responses – although the Food and Drug Administration indication has been the hurdle.
One exception is the recent FDA approval of adalimumab (Humira) for hidradenitis suppurativa. “Make sure you realize that the dosing is different, more like a ‘whopping’ Crohn’s disease dose. This is a very important medication in our armamentarium – the issue is when you start it,” Dr. Friedman said. He also cautioned, “if a patient has sinus tracts and scarring, it’s not going to be enough. You still need to address that. Adalimumab is only going to get rid of the inflammation. This is why early diagnosis is so important!”
When it comes to surgery, “my philosophy is it’s all or none. If you’re going to do surgery, do surgery. You better cut these things out. Do a wide global excision,” Dr. Friedman emphasized.
Dr. Friedman is a member of the Dermatology News editorial advisory board.
Dr. Friedman had no relevant financial disclosures.
MIAMI – Clinicians have many options to treat and help people manage hidradenitis suppurativa, but for most patients, an early and accurate diagnosis remains elusive.
“The problem here is because it has so many mimickers, the diagnosis is often delayed, patients can be [treated for an incorrect diagnosis] and in many ways that treatment can be harmful,” said Adam Friedman, MD, of the George Washington University in Washington. Missed or ignored diagnoses can lead to more pain, impaired function, and wasted time and money.
“There are – no question – gaps in clinical care. Patients seek care outside of dermatology and go to urgent care centers, emergency rooms, [and other settings]. That’s why it’s not only important for us to recognize this, but to teach everyone else as well,” Dr. Friedman said at the Orlando Dermatology Aesthetic and Clinical Conference.
Tips for early detection
Look for chronicity in the disease’s presentation, Dr. Friedman said. “Chronicity is key, but the morphology will change, and lesions will look different over time.” Therefore, “the clinical presentation can be challenging, depending on when you catch the patient.”
Hidradenitis suppurativa is characterized by very purulent, indurated, abscesslike structures often on the underarms and groin. Ask patients where and how often they see lesions. Lesions in certain locations can be very disabling for patients not only because of pain, but also from a psychosocial impact. The groin and chest are prime examples. Also, there is a genetic predisposition so it is important to ask patients about family history as well.
Use combination therapy to hit disease ‘from all angles’
It is imperative to treat patients even when they present at a time of mild disease, Dr. Friedman said. “This is a chronic, snowballing disease that will get worse over time, because inflammation begets inflammation. Even if it’s mild disease, you still want to treat. Combinations are king, and we dermatologists are the synergy masters.”
Effective treatment strategies include medications that curtail inflammation and block hormonal influences; dietary changes (a minimal-dairy, low-carbohydrate diet helps some patients, for example); environmental changes and/or eliminating the invasive proliferative gelatinous mass (IPGM). “This is not step therapy,” Dr. Friedman emphasized. “You want to hit all these angles at once.”
In terms of nutritional support, “I usually put my patients on a combination of zinc and vitamin C, both anti-inflammatories, but also good for wound healing,” Dr. Friedman said. “I also get them on board with V-8, which can be a tough sell sometimes.” He recommends patients drink three small cans per week, adding that he has no financial disclosure related to the vegetable juice.
Patient education, smoking cessation, and keeping affected areas dry and cool are other important management strategies. Instruct patients that stress, friction, and obesity can each worsen the condition “I think obesity is an independent risk factor here, like it is in psoriasis, where obesity alone has been shown to increase the risk of psoriasis later on,” he said.
Ease the inflammation
Hidradenitis suppurativa is a disease of “inappropriate inflammation,” Dr. Friedman said, which explains why anti-inflammatory agents remain the mainstay of treatment. These include antibiotics, classic corticosteroids, and biologics, which all can have a role in therapy. He highlighted the potential role of the cutaneous microbiota and possible dysbiosis associated with disease activity. “I also use a lot chlorhexidine washes to wipe the microbial slate clean in high-risk areas; just be careful to avoid the face.”
Intralesional Kenalog (triamcinolone acetonide), in particular, is useful for its rapid results. “This is such a great and easy trick, and it really works quickly,” Dr. Friedman said. He added that a recent case series provides evidence for its efficacy as well (J Amer Acad Dermatol. 2016 Dec;75:1151-5).
Three take-home treatment pointers
Dr. Friedman shared these three take-aways for treatment of hidradenitis suppurativa with antibiotics:
- The combination of oral clindamycin 300 mg twice daily and oral rifampin 300 mg twice daily carries the most evidence for efficacy and safety, with no evidence of resistance.
- Rifampin also acts against Clostridium difficile infections, which decreases the risk of associated colitis.
- Do not give a tetracycline antibiotic as monotherapy.
In terms of retinoids, “I’ve been pretty disappointed. I find them effective [in other conditions], but for hidradenitis suppurativa, I’m just not impressed, unfortunately,” Dr. Friedman said. “Antihormonal therapies such as oral contraceptives and spironolactone for women and finasteride for men have been a useful adjuncts in my practice, with evidence supporting their use in the literature, he added. Biologics are among the new treatment options, but there are cost and insurance coverage issues, Dr. Friedman said. There are small case series evaluating biologics such as infliximab, which are very supportive – and he himself has had good responses – although the Food and Drug Administration indication has been the hurdle.
One exception is the recent FDA approval of adalimumab (Humira) for hidradenitis suppurativa. “Make sure you realize that the dosing is different, more like a ‘whopping’ Crohn’s disease dose. This is a very important medication in our armamentarium – the issue is when you start it,” Dr. Friedman said. He also cautioned, “if a patient has sinus tracts and scarring, it’s not going to be enough. You still need to address that. Adalimumab is only going to get rid of the inflammation. This is why early diagnosis is so important!”
When it comes to surgery, “my philosophy is it’s all or none. If you’re going to do surgery, do surgery. You better cut these things out. Do a wide global excision,” Dr. Friedman emphasized.
Dr. Friedman is a member of the Dermatology News editorial advisory board.
Dr. Friedman had no relevant financial disclosures.
MIAMI – Clinicians have many options to treat and help people manage hidradenitis suppurativa, but for most patients, an early and accurate diagnosis remains elusive.
“The problem here is because it has so many mimickers, the diagnosis is often delayed, patients can be [treated for an incorrect diagnosis] and in many ways that treatment can be harmful,” said Adam Friedman, MD, of the George Washington University in Washington. Missed or ignored diagnoses can lead to more pain, impaired function, and wasted time and money.
“There are – no question – gaps in clinical care. Patients seek care outside of dermatology and go to urgent care centers, emergency rooms, [and other settings]. That’s why it’s not only important for us to recognize this, but to teach everyone else as well,” Dr. Friedman said at the Orlando Dermatology Aesthetic and Clinical Conference.
Tips for early detection
Look for chronicity in the disease’s presentation, Dr. Friedman said. “Chronicity is key, but the morphology will change, and lesions will look different over time.” Therefore, “the clinical presentation can be challenging, depending on when you catch the patient.”
Hidradenitis suppurativa is characterized by very purulent, indurated, abscesslike structures often on the underarms and groin. Ask patients where and how often they see lesions. Lesions in certain locations can be very disabling for patients not only because of pain, but also from a psychosocial impact. The groin and chest are prime examples. Also, there is a genetic predisposition so it is important to ask patients about family history as well.
Use combination therapy to hit disease ‘from all angles’
It is imperative to treat patients even when they present at a time of mild disease, Dr. Friedman said. “This is a chronic, snowballing disease that will get worse over time, because inflammation begets inflammation. Even if it’s mild disease, you still want to treat. Combinations are king, and we dermatologists are the synergy masters.”
Effective treatment strategies include medications that curtail inflammation and block hormonal influences; dietary changes (a minimal-dairy, low-carbohydrate diet helps some patients, for example); environmental changes and/or eliminating the invasive proliferative gelatinous mass (IPGM). “This is not step therapy,” Dr. Friedman emphasized. “You want to hit all these angles at once.”
In terms of nutritional support, “I usually put my patients on a combination of zinc and vitamin C, both anti-inflammatories, but also good for wound healing,” Dr. Friedman said. “I also get them on board with V-8, which can be a tough sell sometimes.” He recommends patients drink three small cans per week, adding that he has no financial disclosure related to the vegetable juice.
Patient education, smoking cessation, and keeping affected areas dry and cool are other important management strategies. Instruct patients that stress, friction, and obesity can each worsen the condition “I think obesity is an independent risk factor here, like it is in psoriasis, where obesity alone has been shown to increase the risk of psoriasis later on,” he said.
Ease the inflammation
Hidradenitis suppurativa is a disease of “inappropriate inflammation,” Dr. Friedman said, which explains why anti-inflammatory agents remain the mainstay of treatment. These include antibiotics, classic corticosteroids, and biologics, which all can have a role in therapy. He highlighted the potential role of the cutaneous microbiota and possible dysbiosis associated with disease activity. “I also use a lot chlorhexidine washes to wipe the microbial slate clean in high-risk areas; just be careful to avoid the face.”
Intralesional Kenalog (triamcinolone acetonide), in particular, is useful for its rapid results. “This is such a great and easy trick, and it really works quickly,” Dr. Friedman said. He added that a recent case series provides evidence for its efficacy as well (J Amer Acad Dermatol. 2016 Dec;75:1151-5).
Three take-home treatment pointers
Dr. Friedman shared these three take-aways for treatment of hidradenitis suppurativa with antibiotics:
- The combination of oral clindamycin 300 mg twice daily and oral rifampin 300 mg twice daily carries the most evidence for efficacy and safety, with no evidence of resistance.
- Rifampin also acts against Clostridium difficile infections, which decreases the risk of associated colitis.
- Do not give a tetracycline antibiotic as monotherapy.
In terms of retinoids, “I’ve been pretty disappointed. I find them effective [in other conditions], but for hidradenitis suppurativa, I’m just not impressed, unfortunately,” Dr. Friedman said. “Antihormonal therapies such as oral contraceptives and spironolactone for women and finasteride for men have been a useful adjuncts in my practice, with evidence supporting their use in the literature, he added. Biologics are among the new treatment options, but there are cost and insurance coverage issues, Dr. Friedman said. There are small case series evaluating biologics such as infliximab, which are very supportive – and he himself has had good responses – although the Food and Drug Administration indication has been the hurdle.
One exception is the recent FDA approval of adalimumab (Humira) for hidradenitis suppurativa. “Make sure you realize that the dosing is different, more like a ‘whopping’ Crohn’s disease dose. This is a very important medication in our armamentarium – the issue is when you start it,” Dr. Friedman said. He also cautioned, “if a patient has sinus tracts and scarring, it’s not going to be enough. You still need to address that. Adalimumab is only going to get rid of the inflammation. This is why early diagnosis is so important!”
When it comes to surgery, “my philosophy is it’s all or none. If you’re going to do surgery, do surgery. You better cut these things out. Do a wide global excision,” Dr. Friedman emphasized.
Dr. Friedman is a member of the Dermatology News editorial advisory board.
Dr. Friedman had no relevant financial disclosures.
EXPERT ANALYSIS FROM ODAC 2017
Endoscopic resection alone sufficed in many T1 colorectal cancers
Patients with T1 colorectal cancer might not benefit from additional surgery after endoscopic resection unless they have positive or indeterminate resection margins or high-risk histology, according to a retrospective, population-based study of 1,315 patients.
After a median follow-up of 6.6 years, the rates of colorectal cancer (CRC) recurrence were 6.2% in patients who underwent endoscopic resection only and 6.4% in patients who also had additional surgery (P = .9), reported Tim D.G. Belderbos, MD, of University Medical Center Utrecht (the Netherlands). Rates of local recurrence also were similar between these groups (4.1% and 3.7%, P = .3), he and his associates reported in the March issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2016.08.041).
Among high-risk patients, however, the rates of overall recurrence were 14% with endoscopic resection only and 7% with endoscopic resection plus additional surgery (P = .06), and the rates of local recurrence were 12% and 1%, respectively (P = .004). “Based on our study, we recommend performing additional surgery after initial endoscopic resection in cases of high-risk T1 CRC, determined by high-risk histology and/or positive resection margins,” the researchers concluded. Invasive CRCs confined to the colonic submucosa (T1 CRC) present a treatment dilemma – they are usually cured by complete endoscopic resection, but up to 13% involve lymph node metastases and need additional surgery, the investigators noted. To identify predictors of recurrence and metastasis, they studied all patients diagnosed with T1 CRC in the Southeast Netherlands from 1995 through 2011. A total of 370 patients (28%) underwent endoscopic resection only, 220 (17%) underwent endoscopic resection with additional surgery, and 725 (55%) had an initial surgical resection.
Surgery after endoscopic resection was more likely when patients had positive or doubtful resection margins (P less than .001), and this link remained significant after high-risk histology, tumor location, time period, age, sex, and comorbidities were controlled for. Endoscopic resection plus surgery did not reduce the risk of recurrence, compared with endoscopic resection only (P = .3), after the investigators accounted for age, sex, year of procedure, tumor location, and margin characteristics. Initial surgery was associated with significantly lower rates of overall and local recurrence, compared with endoscopic resection only, but the differences also lost significance in the multivariable analysis (P = .2).
Only the presence of positive resection margins significantly predicted recurrence among patients undergoing endoscopic resection (hazard ratio, 6.9; 95% confidence interval, 2.3-20.9). Positive or doubtful resection margins also predicted recurrence after initial surgery, with hazard ratios of 13.2 and 3.4, respectively. High-risk histology – that is, poor differentiation, deep submucosal invasion, or lymphangioinvasion – was significantly associated with lymph node metastasis (OR, 2.2; 95% CI, 1.3-3.7; P less than .002), but not with recurrence after resection margins were accounted for. This might result from missing histology data or the fact that patients with high-risk histology tended to undergo surgical rather than endoscopic resection, the researchers said.
They noted several other study limitations, including a lack of details about lesions and procedures. Also, endoscopic submucosal resection was not practiced in the Netherlands during the study period, they said.
The investigators did not report funding sources and had no disclosures.
Patients with T1 colorectal cancer might not benefit from additional surgery after endoscopic resection unless they have positive or indeterminate resection margins or high-risk histology, according to a retrospective, population-based study of 1,315 patients.
After a median follow-up of 6.6 years, the rates of colorectal cancer (CRC) recurrence were 6.2% in patients who underwent endoscopic resection only and 6.4% in patients who also had additional surgery (P = .9), reported Tim D.G. Belderbos, MD, of University Medical Center Utrecht (the Netherlands). Rates of local recurrence also were similar between these groups (4.1% and 3.7%, P = .3), he and his associates reported in the March issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2016.08.041).
Among high-risk patients, however, the rates of overall recurrence were 14% with endoscopic resection only and 7% with endoscopic resection plus additional surgery (P = .06), and the rates of local recurrence were 12% and 1%, respectively (P = .004). “Based on our study, we recommend performing additional surgery after initial endoscopic resection in cases of high-risk T1 CRC, determined by high-risk histology and/or positive resection margins,” the researchers concluded. Invasive CRCs confined to the colonic submucosa (T1 CRC) present a treatment dilemma – they are usually cured by complete endoscopic resection, but up to 13% involve lymph node metastases and need additional surgery, the investigators noted. To identify predictors of recurrence and metastasis, they studied all patients diagnosed with T1 CRC in the Southeast Netherlands from 1995 through 2011. A total of 370 patients (28%) underwent endoscopic resection only, 220 (17%) underwent endoscopic resection with additional surgery, and 725 (55%) had an initial surgical resection.
Surgery after endoscopic resection was more likely when patients had positive or doubtful resection margins (P less than .001), and this link remained significant after high-risk histology, tumor location, time period, age, sex, and comorbidities were controlled for. Endoscopic resection plus surgery did not reduce the risk of recurrence, compared with endoscopic resection only (P = .3), after the investigators accounted for age, sex, year of procedure, tumor location, and margin characteristics. Initial surgery was associated with significantly lower rates of overall and local recurrence, compared with endoscopic resection only, but the differences also lost significance in the multivariable analysis (P = .2).
Only the presence of positive resection margins significantly predicted recurrence among patients undergoing endoscopic resection (hazard ratio, 6.9; 95% confidence interval, 2.3-20.9). Positive or doubtful resection margins also predicted recurrence after initial surgery, with hazard ratios of 13.2 and 3.4, respectively. High-risk histology – that is, poor differentiation, deep submucosal invasion, or lymphangioinvasion – was significantly associated with lymph node metastasis (OR, 2.2; 95% CI, 1.3-3.7; P less than .002), but not with recurrence after resection margins were accounted for. This might result from missing histology data or the fact that patients with high-risk histology tended to undergo surgical rather than endoscopic resection, the researchers said.
They noted several other study limitations, including a lack of details about lesions and procedures. Also, endoscopic submucosal resection was not practiced in the Netherlands during the study period, they said.
The investigators did not report funding sources and had no disclosures.
Patients with T1 colorectal cancer might not benefit from additional surgery after endoscopic resection unless they have positive or indeterminate resection margins or high-risk histology, according to a retrospective, population-based study of 1,315 patients.
After a median follow-up of 6.6 years, the rates of colorectal cancer (CRC) recurrence were 6.2% in patients who underwent endoscopic resection only and 6.4% in patients who also had additional surgery (P = .9), reported Tim D.G. Belderbos, MD, of University Medical Center Utrecht (the Netherlands). Rates of local recurrence also were similar between these groups (4.1% and 3.7%, P = .3), he and his associates reported in the March issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2016.08.041).
Among high-risk patients, however, the rates of overall recurrence were 14% with endoscopic resection only and 7% with endoscopic resection plus additional surgery (P = .06), and the rates of local recurrence were 12% and 1%, respectively (P = .004). “Based on our study, we recommend performing additional surgery after initial endoscopic resection in cases of high-risk T1 CRC, determined by high-risk histology and/or positive resection margins,” the researchers concluded. Invasive CRCs confined to the colonic submucosa (T1 CRC) present a treatment dilemma – they are usually cured by complete endoscopic resection, but up to 13% involve lymph node metastases and need additional surgery, the investigators noted. To identify predictors of recurrence and metastasis, they studied all patients diagnosed with T1 CRC in the Southeast Netherlands from 1995 through 2011. A total of 370 patients (28%) underwent endoscopic resection only, 220 (17%) underwent endoscopic resection with additional surgery, and 725 (55%) had an initial surgical resection.
Surgery after endoscopic resection was more likely when patients had positive or doubtful resection margins (P less than .001), and this link remained significant after high-risk histology, tumor location, time period, age, sex, and comorbidities were controlled for. Endoscopic resection plus surgery did not reduce the risk of recurrence, compared with endoscopic resection only (P = .3), after the investigators accounted for age, sex, year of procedure, tumor location, and margin characteristics. Initial surgery was associated with significantly lower rates of overall and local recurrence, compared with endoscopic resection only, but the differences also lost significance in the multivariable analysis (P = .2).
Only the presence of positive resection margins significantly predicted recurrence among patients undergoing endoscopic resection (hazard ratio, 6.9; 95% confidence interval, 2.3-20.9). Positive or doubtful resection margins also predicted recurrence after initial surgery, with hazard ratios of 13.2 and 3.4, respectively. High-risk histology – that is, poor differentiation, deep submucosal invasion, or lymphangioinvasion – was significantly associated with lymph node metastasis (OR, 2.2; 95% CI, 1.3-3.7; P less than .002), but not with recurrence after resection margins were accounted for. This might result from missing histology data or the fact that patients with high-risk histology tended to undergo surgical rather than endoscopic resection, the researchers said.
They noted several other study limitations, including a lack of details about lesions and procedures. Also, endoscopic submucosal resection was not practiced in the Netherlands during the study period, they said.
The investigators did not report funding sources and had no disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point. Patients with T1 colorectal cancer might not benefit from additional surgery after endoscopic resection unless they have positive or indeterminate resection margins or high-risk histology.
Major finding: After a median follow-up of 6.6 years, rates of CRC recurrence were 6.2% in patients who underwent endoscopic resection only, and 6.4% in patients who also had additional surgery (P = .9). Among high-risk patients, these rates were 14% and 7%, respectively (P = .06).
Data source: A retrospective population-based study of 1,315 patients who underwent endoscopic or surgical resection of T1 colorectal cancer.
Disclosures: The investigators did not report funding sources and had no disclosures.
COBRA trial takes the long view of absorbable biosynthetic mesh outcomes
Absorbable, biosynthetic surgical mesh used to repair ventral hernia defects may be a good alternative to biologic and permanent synthetic mesh products both in terms of long-term durability and cost, according to a longitudinal cohort study.
The results of the COBRA (Complex Open Bioabsorbable Reconstruction of the Abdominal Wall) study published in the January issue of Annals of Surgery represent the longest follow-up of patients in whom this product was used. Lead author of the study, Michael J. Rosen, MD, professor of surgery at Case Western Reserve University, Cleveland, and his colleagues wrote: “Absorbable synthetic mesh has the prospective advantages of a reduced cost, minimal constraints in manufacturing alternative sizes (lengths, widths, and thicknesses), informed consent in certain religious or cultural groups, and ability to be iterative in generational improvements in mesh constructs based on outcome studies, compared with allogeneic or xenogenic mesh.”
Contaminated wounds were present in 77% of participants. About one-fourth of patients had concomitant procedures for fistula takedown; a quarter of the cohort also required the removal of infected, previously placed mesh. More than a fifth of patients required a concomitant repair of both a midline and parastomal hernia; the mean size for the hernia defects was 137 cm2, and the average width was 9 cm.
Placement of the biosynthetic mesh was left to the discretion of the surgeon, but 90% chose retrorectus placement. Primary fascial closure using a single unit of the material was successful in all patients, 68 of whom required concomitant component separation; 21 of these had an external oblique release. Another 50 of these had transversus abdominis release.
At 24 months, when 84% of patients completed follow-up, 17% were found to have a hernia recurrence. Intraperitoneal placement of the material was found to significantly increase the risk of hernia recurrence (P less than or equal to .04). Infections at the surgical site were associated with a higher risk of recurrence (P less than .01). Patient-reported physical and mental quality-of-life scores at 24 months improved significantly from baseline (P less than .05), showing sustained improvement at 6 and 12 months post procedure.
While more studies are needed, the COBRA findings suggest absorbable, biosynthetic mesh compares favorably with biologic mesh when it comes to recurrence. “The Repair of Infected and Contaminated Hernias (RICH) trial is the only long-term, multicentered, prospective trial to evaluate biologic mesh in CDC [Centers for Disease Control and Prevention] class II to IV wounds. The RICH trial reported 66% surgical site occurrence and 28% hernia recurrence after 2 years’ follow-up in patients who underwent ventral hernia repair with a non–cross-linked porcine dermis,” the investigators noted.
Cost is another area in which bioabsorbable synthetic mesh compares favorably with the biologics, not only in terms of savings from fewer recurrences but also the cost of the mesh itself. Biologic mesh can cost $10,000 or more while synthetics run about a quarter of that (Clin Colon Rectal Surg. 2014 Dec; 27[4]:140-8).
In conclusion, the investigators commented that despite the lack of a control group and random assignment in the study, the results “should not be underestimated” when considering alternatives to biologic and costlier, permanent synthetic meshes.
The study was funded by W.L. Gore. The authors had no relevant financial disclosures.
Absorbable, biosynthetic surgical mesh used to repair ventral hernia defects may be a good alternative to biologic and permanent synthetic mesh products both in terms of long-term durability and cost, according to a longitudinal cohort study.
The results of the COBRA (Complex Open Bioabsorbable Reconstruction of the Abdominal Wall) study published in the January issue of Annals of Surgery represent the longest follow-up of patients in whom this product was used. Lead author of the study, Michael J. Rosen, MD, professor of surgery at Case Western Reserve University, Cleveland, and his colleagues wrote: “Absorbable synthetic mesh has the prospective advantages of a reduced cost, minimal constraints in manufacturing alternative sizes (lengths, widths, and thicknesses), informed consent in certain religious or cultural groups, and ability to be iterative in generational improvements in mesh constructs based on outcome studies, compared with allogeneic or xenogenic mesh.”
Contaminated wounds were present in 77% of participants. About one-fourth of patients had concomitant procedures for fistula takedown; a quarter of the cohort also required the removal of infected, previously placed mesh. More than a fifth of patients required a concomitant repair of both a midline and parastomal hernia; the mean size for the hernia defects was 137 cm2, and the average width was 9 cm.
Placement of the biosynthetic mesh was left to the discretion of the surgeon, but 90% chose retrorectus placement. Primary fascial closure using a single unit of the material was successful in all patients, 68 of whom required concomitant component separation; 21 of these had an external oblique release. Another 50 of these had transversus abdominis release.
At 24 months, when 84% of patients completed follow-up, 17% were found to have a hernia recurrence. Intraperitoneal placement of the material was found to significantly increase the risk of hernia recurrence (P less than or equal to .04). Infections at the surgical site were associated with a higher risk of recurrence (P less than .01). Patient-reported physical and mental quality-of-life scores at 24 months improved significantly from baseline (P less than .05), showing sustained improvement at 6 and 12 months post procedure.
While more studies are needed, the COBRA findings suggest absorbable, biosynthetic mesh compares favorably with biologic mesh when it comes to recurrence. “The Repair of Infected and Contaminated Hernias (RICH) trial is the only long-term, multicentered, prospective trial to evaluate biologic mesh in CDC [Centers for Disease Control and Prevention] class II to IV wounds. The RICH trial reported 66% surgical site occurrence and 28% hernia recurrence after 2 years’ follow-up in patients who underwent ventral hernia repair with a non–cross-linked porcine dermis,” the investigators noted.
Cost is another area in which bioabsorbable synthetic mesh compares favorably with the biologics, not only in terms of savings from fewer recurrences but also the cost of the mesh itself. Biologic mesh can cost $10,000 or more while synthetics run about a quarter of that (Clin Colon Rectal Surg. 2014 Dec; 27[4]:140-8).
In conclusion, the investigators commented that despite the lack of a control group and random assignment in the study, the results “should not be underestimated” when considering alternatives to biologic and costlier, permanent synthetic meshes.
The study was funded by W.L. Gore. The authors had no relevant financial disclosures.
Absorbable, biosynthetic surgical mesh used to repair ventral hernia defects may be a good alternative to biologic and permanent synthetic mesh products both in terms of long-term durability and cost, according to a longitudinal cohort study.
The results of the COBRA (Complex Open Bioabsorbable Reconstruction of the Abdominal Wall) study published in the January issue of Annals of Surgery represent the longest follow-up of patients in whom this product was used. Lead author of the study, Michael J. Rosen, MD, professor of surgery at Case Western Reserve University, Cleveland, and his colleagues wrote: “Absorbable synthetic mesh has the prospective advantages of a reduced cost, minimal constraints in manufacturing alternative sizes (lengths, widths, and thicknesses), informed consent in certain religious or cultural groups, and ability to be iterative in generational improvements in mesh constructs based on outcome studies, compared with allogeneic or xenogenic mesh.”
Contaminated wounds were present in 77% of participants. About one-fourth of patients had concomitant procedures for fistula takedown; a quarter of the cohort also required the removal of infected, previously placed mesh. More than a fifth of patients required a concomitant repair of both a midline and parastomal hernia; the mean size for the hernia defects was 137 cm2, and the average width was 9 cm.
Placement of the biosynthetic mesh was left to the discretion of the surgeon, but 90% chose retrorectus placement. Primary fascial closure using a single unit of the material was successful in all patients, 68 of whom required concomitant component separation; 21 of these had an external oblique release. Another 50 of these had transversus abdominis release.
At 24 months, when 84% of patients completed follow-up, 17% were found to have a hernia recurrence. Intraperitoneal placement of the material was found to significantly increase the risk of hernia recurrence (P less than or equal to .04). Infections at the surgical site were associated with a higher risk of recurrence (P less than .01). Patient-reported physical and mental quality-of-life scores at 24 months improved significantly from baseline (P less than .05), showing sustained improvement at 6 and 12 months post procedure.
While more studies are needed, the COBRA findings suggest absorbable, biosynthetic mesh compares favorably with biologic mesh when it comes to recurrence. “The Repair of Infected and Contaminated Hernias (RICH) trial is the only long-term, multicentered, prospective trial to evaluate biologic mesh in CDC [Centers for Disease Control and Prevention] class II to IV wounds. The RICH trial reported 66% surgical site occurrence and 28% hernia recurrence after 2 years’ follow-up in patients who underwent ventral hernia repair with a non–cross-linked porcine dermis,” the investigators noted.
Cost is another area in which bioabsorbable synthetic mesh compares favorably with the biologics, not only in terms of savings from fewer recurrences but also the cost of the mesh itself. Biologic mesh can cost $10,000 or more while synthetics run about a quarter of that (Clin Colon Rectal Surg. 2014 Dec; 27[4]:140-8).
In conclusion, the investigators commented that despite the lack of a control group and random assignment in the study, the results “should not be underestimated” when considering alternatives to biologic and costlier, permanent synthetic meshes.
The study was funded by W.L. Gore. The authors had no relevant financial disclosures.
Key clinical point:
Major finding: At 24 months, 17% of patients were found to have a hernia recurrence.
Data source: An international, multisite, prospective, intention-to-treat cohort analysis of 104 patients with contaminated or noncontaminated hernia defects of at least 9 cm2 in size.
Disclosures: The study was funded by W.L. Gore. The authors had no relevant financial disclosures.
FDA opens abbreviated approval pathway for interchangeable biosimilars
The Food and Drug Administration has proposed a regulatory path for biosimilar biologics that are interchangeable with the reference product, paving the way for a new generation of less-expensive versions of these unique drugs.
But bringing an interchangeable biosimilar to market won’t be easy. The bar for interchangeability will be high, requiring that manufacturers prove switching between the new and older products is safe. And clinicians, while cautiously optimistic, aren’t thrilled with the industry payoff that could come with the designation: freedom for insurance companies and pharmacies to switch products at the dispensing level without requiring a new prescription.
The draft FDA guidance for industry, “Considerations in Demonstrating Interchangeability With a Reference Product,” arises from the Biologics Price Competition and Innovation Act of 2009. That section of the Affordable Care Act provides for abbreviated approval pathways for biological products that are demonstrated to be “highly similar” (biosimilar) to or “interchangeable” with an FDA-approved biological product.
The difference between these appellations is subtle but critical to the regulatory process – and perhaps to patient safety. Regulators recognize that the structure of these large, highly complex molecules can never precisely replicate the reference product. But to be labeled a “biosimilar,” developers must prove that the new product functions essentially the same; there can be no clinically meaningful differences in terms of safety, purity, and potency. Unlike a generic medication, a biosimilar can’t be substituted for its reference product at the pharmacy level. If a physician wants the patient on that biosimilar, the script must specify it.
Interchangeables jump a higher regulatory bar
An “interchangeable biosimilar,” though, would have to jump a higher regulatory bar. Not only must it produce the same clinical result as the reference product, it also must be benignly interchangeable with it, conferring no additional risk if a patient switches from the reference to the biosimilar and back again. A pharmacist could, if permitted by state law, substitute an interchangeable product for the reference product without going through the prescriber.
Like biosimilars, interchangeable products need not be tested in every disease for which the reference drug is approved, according to the document. Once they are proved safe for one indication, those data can be extrapolated to allow approval for the other indications as well. Nor do biosimilars need to prove efficacy per se, as their molecular similarity to the reference product ensures that they bind to the same receptor and exert the same therapeutic effect.
The biosimilar/interchangeable market has been slow to take off in the United States. There are no approved interchangeable biosimilars, and only four biosimilars – three of which were approved in 2016:
• Sandoz’ filgrastim-sndz (Zarxio).
• Pfizer’s and Celltrion’s infliximab-dyyb (Inflectra).
• Sandoz’ etanercept-szzs (Erelzi).
• Amgen’s adalimumab-atto (Amjevita).
Switching studies is the key to achieving the interchangeable designation, according to the FDA document. They must include at least two full switches between the candidate product and the reference product, which must be licensed in the United States.
But because these products are so structurally diverse, the FDA isn’t imposing a one-size-fits-all process on them. Instead, the molecular complexity and immunogenicity of each product will dictate its approval requirements.
Those with relatively low structural complexity, high molecular similarity to the reference product, and a low incidence of immunogenic adverse events may only need a single switching study to achieve the “interchangeability” designation.
The bar will be higher for a product with high structural complexity that is not as similar to the reference product, or which has been associated with immunogenic adverse events. For this product, FDA might also require extensive safety postmarketing data for the product as a licensed biosimilar, as well as a switching study.
Pharmacokinetics, pharmacodynamics, immunogenicity, and safety will be the primary endpoints of a switching study. Efficacy data are not necessary but can be used as supportive endpoints. Any safety signals in a switching study would raise regulatory eyebrows whether they came from the candidate product or the reference product. Since the study replicates what could happen if the two were used sequentially, it makes little difference from which product the event might arise.
“If an apparent difference in immune response or adverse events is noticed between the switching and nonswitching arms of the study ... it would raise concerns as to whether the proposed interchangeable product is interchangeable, regardless of whether the proposed interchangeable product or the reference product or the switching of the two products actually caused the event,” the document notes.
The E.U. vs. U.S. experience
The United States is only now getting a taste of what has become common fare in the European Union, said Angus Worthing, MD, chair of the American College of Rheumatology’s Government Affairs Committee. The European Medicines Agency approved its first biosimilar in 2006. Since then, 23 such drugs have come on the market, at an average price of about 30% less than the reference drug. Prices for some drugs have dropped as much as 70% in countries in which national health care systems abandoned the reference product in favor of the competing biosimilar, Dr. Worthing said in an interview.
“But the U.S. doesn’t have a national health care system, so it won’t work like that here.” In fact, he noted, brand-new data show that Medicare actually paid 22% more for the infliximab biosimilar Inflectra than it did for Remicade in the last quarter of 2016.
It’s not immediately apparent why this is the case, but it’s probably related to company discounts and rebates on these very expensive drugs. According to the report in Inside Health Policy, Janssen Biotech may have increased its discount on the drug to compete with Inflectra’s launch price of 15% below Remicade’s wholesale cost. Prices won’t moderate as much in the United States as in the European Union until several biosimilars of the same class appear, Dr. Worthing said.
There have already been allegations that big pharma manipulates international and national pricing to reduce biosimilar competition.
In June, Russian biotech company Biocad filed a lawsuit in New York charging Roche/Genentech with price fixing. The suit alleges that the companies cut the cost of three cancer drugs (Avastin, Herceptin, and Rituxan/MabThera) in Russia, where Biocad markets biosimilars for each. At the same time, Biocad alleges, the companies raised U.S. prices on those drugs to make up for the money they were losing on the Russian market.
“I think most of the cost benefits will accrue to insurance plans and pharmacy managers, but maybe not to the patients themselves,” he said in an interview. “The most important beneficiaries may not see a single penny of benefit.”
It may be difficult to extrapolate the European economic experience into the U.S. health care market, but the safety record of its biosimilar armamentarium is solid. None of the biosimilars approved in the E.U. have ever been recalled or removed from the European market because of regulatory or safety concerns.
Nonmedical switching raises concerns
Academic medical societies and clinicians interviewed for this article view the proposed approval pathway with cautious optimism. While acknowledging the potential benefit of reducing the costs of prohibitively expensive drugs, they uniformly insist that patient safety – not economic pressure – should be the driving force here.
“I was initially skeptical, and I do believe that we need very close pharmacovigilance in monitoring these for safety,” said Gideon Smith, MD, PhD, a dermatologist at Massachusetts General Hospital, Boston. “But there has been huge uptake of these products in the E.U., and the data are so extensive that we can be reasonably confident these drugs are effective, and no good reason to believe the safety will be any different.”
He is not as comfortable with the prospect of pharmacy-level substitution of an interchangeable biosimilar with the reference product – a feeling that other clinicians echoed.
“I think this is a fundamental issue that should have been dealt with on a federal level. Physicians should always be involved in the decision,” said Dr. Smith, who spoke at an FDA advisory committee meeting last summer on behalf of the American Academy of Dermatology (AAD).
“In general, the GI field is OK with the idea of starting someone on a new prescription [of an interchangeable biosimilar], but not so much with the idea of switching around,” said Dr. Hanauer, who is the Clifford Joseph Barborka Professor of Gastroenterology at Northwestern University, Chicago. “In these biologic compounds, very small differences can be amplified” and alter therapeutic response.
The possibility of switching from the reference to the biosimilar and maybe back again worries him. He hearkened back to the approval of Remicade, when patients who had taken it during clinical trials only were finally able to obtain it on the market. Dr. Hanauer explained that, “20% of them developed serum sickness reactions after the reexposure.”
He also expressed some concern about quality control in international manufacturing plants, citing a 2005 epidemic of immune-mediated pure red cell anemia in patients who received an epoetin alfa biosimilar manufactured in Thailand. The prefilled syringes had an uncoated rubber stopper that apparently reacted with polysorbate 60 in the solution – an interaction that increased immunogenicity when the drug was administered subcutaneously.
Dr. Smith concurred. “We know that some patients produce antibodies to biologics if they come on and off, and so we discourage that. The concern is that switching may lead to an increased rate of medication failure, if you have to switch back. This is especially troubling in the case of a hard-to-control patient with severe flares. If they’re being well controlled on a medication, the last thing you want to do is change it for no good clinical reason. And we may well be forced to do that.”
Neither the AAD nor the American College of Gastroenterology has a published stand on the FDA’s proposed guidance for interchangeable biosimilars. The preliminary view of the American College of Rheumatology is a positive one, Dr. Worthing said. However, ACR feels pharmacy-level switching should be a joint, not unilateral, decision.
“Our position statement on biosimilars has been that if it’s legal for a pharmacy to make that switch then we want the doctor and the patient to know, so we can track for safety signals.”
Bringing any biosimilar to market, though, takes a lot of money and a lot of time. And while companies are growing cell lines and producing new molecules that mimic existing drugs, science marches on, said Dr. Smith.
“If we keep dragging our feet on this issue, it might end up being a moot point,” he said. Newer drugs are achieving better results, raising the bar for therapeutic success. An example is the monoclonal antibody secukinumab (Cosentyx), an inhibitor of interleukin 17A. In October 2016, late-breaking data released at the annual meeting of the European Academy of Dermatology and Venereology impressed the dermatology community. In psoriasis patients, the drug maintained 90% skin clearance for 4 years in 66% of patients, and 100% clearance for 4 years in 43%.
Not only does this kind of efficacy provide symptomatic relief, it also prevents the expensive long-term morbidity associated with psoriasis, Dr. Smith said.
“Even if these new medications are considerably more expensive upfront than a biosimilar for an older drug, they may end up being less expensive in the long run.”
Dr. Krant and Dr. Worthing had no financial disclosures. Dr. Smith has received grants from Allergan and Cipher Pharmaceuticals. Dr. Hanauer has received grants from numerous pharmaceutical companies that manufacture biologics.
*This article was updated 1/31/2017.
[email protected]
On Twitter @alz_gal
The Food and Drug Administration has proposed a regulatory path for biosimilar biologics that are interchangeable with the reference product, paving the way for a new generation of less-expensive versions of these unique drugs.
But bringing an interchangeable biosimilar to market won’t be easy. The bar for interchangeability will be high, requiring that manufacturers prove switching between the new and older products is safe. And clinicians, while cautiously optimistic, aren’t thrilled with the industry payoff that could come with the designation: freedom for insurance companies and pharmacies to switch products at the dispensing level without requiring a new prescription.
The draft FDA guidance for industry, “Considerations in Demonstrating Interchangeability With a Reference Product,” arises from the Biologics Price Competition and Innovation Act of 2009. That section of the Affordable Care Act provides for abbreviated approval pathways for biological products that are demonstrated to be “highly similar” (biosimilar) to or “interchangeable” with an FDA-approved biological product.
The difference between these appellations is subtle but critical to the regulatory process – and perhaps to patient safety. Regulators recognize that the structure of these large, highly complex molecules can never precisely replicate the reference product. But to be labeled a “biosimilar,” developers must prove that the new product functions essentially the same; there can be no clinically meaningful differences in terms of safety, purity, and potency. Unlike a generic medication, a biosimilar can’t be substituted for its reference product at the pharmacy level. If a physician wants the patient on that biosimilar, the script must specify it.
Interchangeables jump a higher regulatory bar
An “interchangeable biosimilar,” though, would have to jump a higher regulatory bar. Not only must it produce the same clinical result as the reference product, it also must be benignly interchangeable with it, conferring no additional risk if a patient switches from the reference to the biosimilar and back again. A pharmacist could, if permitted by state law, substitute an interchangeable product for the reference product without going through the prescriber.
Like biosimilars, interchangeable products need not be tested in every disease for which the reference drug is approved, according to the document. Once they are proved safe for one indication, those data can be extrapolated to allow approval for the other indications as well. Nor do biosimilars need to prove efficacy per se, as their molecular similarity to the reference product ensures that they bind to the same receptor and exert the same therapeutic effect.
The biosimilar/interchangeable market has been slow to take off in the United States. There are no approved interchangeable biosimilars, and only four biosimilars – three of which were approved in 2016:
• Sandoz’ filgrastim-sndz (Zarxio).
• Pfizer’s and Celltrion’s infliximab-dyyb (Inflectra).
• Sandoz’ etanercept-szzs (Erelzi).
• Amgen’s adalimumab-atto (Amjevita).
Switching studies is the key to achieving the interchangeable designation, according to the FDA document. They must include at least two full switches between the candidate product and the reference product, which must be licensed in the United States.
But because these products are so structurally diverse, the FDA isn’t imposing a one-size-fits-all process on them. Instead, the molecular complexity and immunogenicity of each product will dictate its approval requirements.
Those with relatively low structural complexity, high molecular similarity to the reference product, and a low incidence of immunogenic adverse events may only need a single switching study to achieve the “interchangeability” designation.
The bar will be higher for a product with high structural complexity that is not as similar to the reference product, or which has been associated with immunogenic adverse events. For this product, FDA might also require extensive safety postmarketing data for the product as a licensed biosimilar, as well as a switching study.
Pharmacokinetics, pharmacodynamics, immunogenicity, and safety will be the primary endpoints of a switching study. Efficacy data are not necessary but can be used as supportive endpoints. Any safety signals in a switching study would raise regulatory eyebrows whether they came from the candidate product or the reference product. Since the study replicates what could happen if the two were used sequentially, it makes little difference from which product the event might arise.
“If an apparent difference in immune response or adverse events is noticed between the switching and nonswitching arms of the study ... it would raise concerns as to whether the proposed interchangeable product is interchangeable, regardless of whether the proposed interchangeable product or the reference product or the switching of the two products actually caused the event,” the document notes.
The E.U. vs. U.S. experience
The United States is only now getting a taste of what has become common fare in the European Union, said Angus Worthing, MD, chair of the American College of Rheumatology’s Government Affairs Committee. The European Medicines Agency approved its first biosimilar in 2006. Since then, 23 such drugs have come on the market, at an average price of about 30% less than the reference drug. Prices for some drugs have dropped as much as 70% in countries in which national health care systems abandoned the reference product in favor of the competing biosimilar, Dr. Worthing said in an interview.
“But the U.S. doesn’t have a national health care system, so it won’t work like that here.” In fact, he noted, brand-new data show that Medicare actually paid 22% more for the infliximab biosimilar Inflectra than it did for Remicade in the last quarter of 2016.
It’s not immediately apparent why this is the case, but it’s probably related to company discounts and rebates on these very expensive drugs. According to the report in Inside Health Policy, Janssen Biotech may have increased its discount on the drug to compete with Inflectra’s launch price of 15% below Remicade’s wholesale cost. Prices won’t moderate as much in the United States as in the European Union until several biosimilars of the same class appear, Dr. Worthing said.
There have already been allegations that big pharma manipulates international and national pricing to reduce biosimilar competition.
In June, Russian biotech company Biocad filed a lawsuit in New York charging Roche/Genentech with price fixing. The suit alleges that the companies cut the cost of three cancer drugs (Avastin, Herceptin, and Rituxan/MabThera) in Russia, where Biocad markets biosimilars for each. At the same time, Biocad alleges, the companies raised U.S. prices on those drugs to make up for the money they were losing on the Russian market.
“I think most of the cost benefits will accrue to insurance plans and pharmacy managers, but maybe not to the patients themselves,” he said in an interview. “The most important beneficiaries may not see a single penny of benefit.”
It may be difficult to extrapolate the European economic experience into the U.S. health care market, but the safety record of its biosimilar armamentarium is solid. None of the biosimilars approved in the E.U. have ever been recalled or removed from the European market because of regulatory or safety concerns.
Nonmedical switching raises concerns
Academic medical societies and clinicians interviewed for this article view the proposed approval pathway with cautious optimism. While acknowledging the potential benefit of reducing the costs of prohibitively expensive drugs, they uniformly insist that patient safety – not economic pressure – should be the driving force here.
“I was initially skeptical, and I do believe that we need very close pharmacovigilance in monitoring these for safety,” said Gideon Smith, MD, PhD, a dermatologist at Massachusetts General Hospital, Boston. “But there has been huge uptake of these products in the E.U., and the data are so extensive that we can be reasonably confident these drugs are effective, and no good reason to believe the safety will be any different.”
He is not as comfortable with the prospect of pharmacy-level substitution of an interchangeable biosimilar with the reference product – a feeling that other clinicians echoed.
“I think this is a fundamental issue that should have been dealt with on a federal level. Physicians should always be involved in the decision,” said Dr. Smith, who spoke at an FDA advisory committee meeting last summer on behalf of the American Academy of Dermatology (AAD).
“In general, the GI field is OK with the idea of starting someone on a new prescription [of an interchangeable biosimilar], but not so much with the idea of switching around,” said Dr. Hanauer, who is the Clifford Joseph Barborka Professor of Gastroenterology at Northwestern University, Chicago. “In these biologic compounds, very small differences can be amplified” and alter therapeutic response.
The possibility of switching from the reference to the biosimilar and maybe back again worries him. He hearkened back to the approval of Remicade, when patients who had taken it during clinical trials only were finally able to obtain it on the market. Dr. Hanauer explained that, “20% of them developed serum sickness reactions after the reexposure.”
He also expressed some concern about quality control in international manufacturing plants, citing a 2005 epidemic of immune-mediated pure red cell anemia in patients who received an epoetin alfa biosimilar manufactured in Thailand. The prefilled syringes had an uncoated rubber stopper that apparently reacted with polysorbate 60 in the solution – an interaction that increased immunogenicity when the drug was administered subcutaneously.
Dr. Smith concurred. “We know that some patients produce antibodies to biologics if they come on and off, and so we discourage that. The concern is that switching may lead to an increased rate of medication failure, if you have to switch back. This is especially troubling in the case of a hard-to-control patient with severe flares. If they’re being well controlled on a medication, the last thing you want to do is change it for no good clinical reason. And we may well be forced to do that.”
Neither the AAD nor the American College of Gastroenterology has a published stand on the FDA’s proposed guidance for interchangeable biosimilars. The preliminary view of the American College of Rheumatology is a positive one, Dr. Worthing said. However, ACR feels pharmacy-level switching should be a joint, not unilateral, decision.
“Our position statement on biosimilars has been that if it’s legal for a pharmacy to make that switch then we want the doctor and the patient to know, so we can track for safety signals.”
Bringing any biosimilar to market, though, takes a lot of money and a lot of time. And while companies are growing cell lines and producing new molecules that mimic existing drugs, science marches on, said Dr. Smith.
“If we keep dragging our feet on this issue, it might end up being a moot point,” he said. Newer drugs are achieving better results, raising the bar for therapeutic success. An example is the monoclonal antibody secukinumab (Cosentyx), an inhibitor of interleukin 17A. In October 2016, late-breaking data released at the annual meeting of the European Academy of Dermatology and Venereology impressed the dermatology community. In psoriasis patients, the drug maintained 90% skin clearance for 4 years in 66% of patients, and 100% clearance for 4 years in 43%.
Not only does this kind of efficacy provide symptomatic relief, it also prevents the expensive long-term morbidity associated with psoriasis, Dr. Smith said.
“Even if these new medications are considerably more expensive upfront than a biosimilar for an older drug, they may end up being less expensive in the long run.”
Dr. Krant and Dr. Worthing had no financial disclosures. Dr. Smith has received grants from Allergan and Cipher Pharmaceuticals. Dr. Hanauer has received grants from numerous pharmaceutical companies that manufacture biologics.
*This article was updated 1/31/2017.
[email protected]
On Twitter @alz_gal
The Food and Drug Administration has proposed a regulatory path for biosimilar biologics that are interchangeable with the reference product, paving the way for a new generation of less-expensive versions of these unique drugs.
But bringing an interchangeable biosimilar to market won’t be easy. The bar for interchangeability will be high, requiring that manufacturers prove switching between the new and older products is safe. And clinicians, while cautiously optimistic, aren’t thrilled with the industry payoff that could come with the designation: freedom for insurance companies and pharmacies to switch products at the dispensing level without requiring a new prescription.
The draft FDA guidance for industry, “Considerations in Demonstrating Interchangeability With a Reference Product,” arises from the Biologics Price Competition and Innovation Act of 2009. That section of the Affordable Care Act provides for abbreviated approval pathways for biological products that are demonstrated to be “highly similar” (biosimilar) to or “interchangeable” with an FDA-approved biological product.
The difference between these appellations is subtle but critical to the regulatory process – and perhaps to patient safety. Regulators recognize that the structure of these large, highly complex molecules can never precisely replicate the reference product. But to be labeled a “biosimilar,” developers must prove that the new product functions essentially the same; there can be no clinically meaningful differences in terms of safety, purity, and potency. Unlike a generic medication, a biosimilar can’t be substituted for its reference product at the pharmacy level. If a physician wants the patient on that biosimilar, the script must specify it.
Interchangeables jump a higher regulatory bar
An “interchangeable biosimilar,” though, would have to jump a higher regulatory bar. Not only must it produce the same clinical result as the reference product, it also must be benignly interchangeable with it, conferring no additional risk if a patient switches from the reference to the biosimilar and back again. A pharmacist could, if permitted by state law, substitute an interchangeable product for the reference product without going through the prescriber.
Like biosimilars, interchangeable products need not be tested in every disease for which the reference drug is approved, according to the document. Once they are proved safe for one indication, those data can be extrapolated to allow approval for the other indications as well. Nor do biosimilars need to prove efficacy per se, as their molecular similarity to the reference product ensures that they bind to the same receptor and exert the same therapeutic effect.
The biosimilar/interchangeable market has been slow to take off in the United States. There are no approved interchangeable biosimilars, and only four biosimilars – three of which were approved in 2016:
• Sandoz’ filgrastim-sndz (Zarxio).
• Pfizer’s and Celltrion’s infliximab-dyyb (Inflectra).
• Sandoz’ etanercept-szzs (Erelzi).
• Amgen’s adalimumab-atto (Amjevita).
Switching studies is the key to achieving the interchangeable designation, according to the FDA document. They must include at least two full switches between the candidate product and the reference product, which must be licensed in the United States.
But because these products are so structurally diverse, the FDA isn’t imposing a one-size-fits-all process on them. Instead, the molecular complexity and immunogenicity of each product will dictate its approval requirements.
Those with relatively low structural complexity, high molecular similarity to the reference product, and a low incidence of immunogenic adverse events may only need a single switching study to achieve the “interchangeability” designation.
The bar will be higher for a product with high structural complexity that is not as similar to the reference product, or which has been associated with immunogenic adverse events. For this product, FDA might also require extensive safety postmarketing data for the product as a licensed biosimilar, as well as a switching study.
Pharmacokinetics, pharmacodynamics, immunogenicity, and safety will be the primary endpoints of a switching study. Efficacy data are not necessary but can be used as supportive endpoints. Any safety signals in a switching study would raise regulatory eyebrows whether they came from the candidate product or the reference product. Since the study replicates what could happen if the two were used sequentially, it makes little difference from which product the event might arise.
“If an apparent difference in immune response or adverse events is noticed between the switching and nonswitching arms of the study ... it would raise concerns as to whether the proposed interchangeable product is interchangeable, regardless of whether the proposed interchangeable product or the reference product or the switching of the two products actually caused the event,” the document notes.
The E.U. vs. U.S. experience
The United States is only now getting a taste of what has become common fare in the European Union, said Angus Worthing, MD, chair of the American College of Rheumatology’s Government Affairs Committee. The European Medicines Agency approved its first biosimilar in 2006. Since then, 23 such drugs have come on the market, at an average price of about 30% less than the reference drug. Prices for some drugs have dropped as much as 70% in countries in which national health care systems abandoned the reference product in favor of the competing biosimilar, Dr. Worthing said in an interview.
“But the U.S. doesn’t have a national health care system, so it won’t work like that here.” In fact, he noted, brand-new data show that Medicare actually paid 22% more for the infliximab biosimilar Inflectra than it did for Remicade in the last quarter of 2016.
It’s not immediately apparent why this is the case, but it’s probably related to company discounts and rebates on these very expensive drugs. According to the report in Inside Health Policy, Janssen Biotech may have increased its discount on the drug to compete with Inflectra’s launch price of 15% below Remicade’s wholesale cost. Prices won’t moderate as much in the United States as in the European Union until several biosimilars of the same class appear, Dr. Worthing said.
There have already been allegations that big pharma manipulates international and national pricing to reduce biosimilar competition.
In June, Russian biotech company Biocad filed a lawsuit in New York charging Roche/Genentech with price fixing. The suit alleges that the companies cut the cost of three cancer drugs (Avastin, Herceptin, and Rituxan/MabThera) in Russia, where Biocad markets biosimilars for each. At the same time, Biocad alleges, the companies raised U.S. prices on those drugs to make up for the money they were losing on the Russian market.
“I think most of the cost benefits will accrue to insurance plans and pharmacy managers, but maybe not to the patients themselves,” he said in an interview. “The most important beneficiaries may not see a single penny of benefit.”
It may be difficult to extrapolate the European economic experience into the U.S. health care market, but the safety record of its biosimilar armamentarium is solid. None of the biosimilars approved in the E.U. have ever been recalled or removed from the European market because of regulatory or safety concerns.
Nonmedical switching raises concerns
Academic medical societies and clinicians interviewed for this article view the proposed approval pathway with cautious optimism. While acknowledging the potential benefit of reducing the costs of prohibitively expensive drugs, they uniformly insist that patient safety – not economic pressure – should be the driving force here.
“I was initially skeptical, and I do believe that we need very close pharmacovigilance in monitoring these for safety,” said Gideon Smith, MD, PhD, a dermatologist at Massachusetts General Hospital, Boston. “But there has been huge uptake of these products in the E.U., and the data are so extensive that we can be reasonably confident these drugs are effective, and no good reason to believe the safety will be any different.”
He is not as comfortable with the prospect of pharmacy-level substitution of an interchangeable biosimilar with the reference product – a feeling that other clinicians echoed.
“I think this is a fundamental issue that should have been dealt with on a federal level. Physicians should always be involved in the decision,” said Dr. Smith, who spoke at an FDA advisory committee meeting last summer on behalf of the American Academy of Dermatology (AAD).
“In general, the GI field is OK with the idea of starting someone on a new prescription [of an interchangeable biosimilar], but not so much with the idea of switching around,” said Dr. Hanauer, who is the Clifford Joseph Barborka Professor of Gastroenterology at Northwestern University, Chicago. “In these biologic compounds, very small differences can be amplified” and alter therapeutic response.
The possibility of switching from the reference to the biosimilar and maybe back again worries him. He hearkened back to the approval of Remicade, when patients who had taken it during clinical trials only were finally able to obtain it on the market. Dr. Hanauer explained that, “20% of them developed serum sickness reactions after the reexposure.”
He also expressed some concern about quality control in international manufacturing plants, citing a 2005 epidemic of immune-mediated pure red cell anemia in patients who received an epoetin alfa biosimilar manufactured in Thailand. The prefilled syringes had an uncoated rubber stopper that apparently reacted with polysorbate 60 in the solution – an interaction that increased immunogenicity when the drug was administered subcutaneously.
Dr. Smith concurred. “We know that some patients produce antibodies to biologics if they come on and off, and so we discourage that. The concern is that switching may lead to an increased rate of medication failure, if you have to switch back. This is especially troubling in the case of a hard-to-control patient with severe flares. If they’re being well controlled on a medication, the last thing you want to do is change it for no good clinical reason. And we may well be forced to do that.”
Neither the AAD nor the American College of Gastroenterology has a published stand on the FDA’s proposed guidance for interchangeable biosimilars. The preliminary view of the American College of Rheumatology is a positive one, Dr. Worthing said. However, ACR feels pharmacy-level switching should be a joint, not unilateral, decision.
“Our position statement on biosimilars has been that if it’s legal for a pharmacy to make that switch then we want the doctor and the patient to know, so we can track for safety signals.”
Bringing any biosimilar to market, though, takes a lot of money and a lot of time. And while companies are growing cell lines and producing new molecules that mimic existing drugs, science marches on, said Dr. Smith.
“If we keep dragging our feet on this issue, it might end up being a moot point,” he said. Newer drugs are achieving better results, raising the bar for therapeutic success. An example is the monoclonal antibody secukinumab (Cosentyx), an inhibitor of interleukin 17A. In October 2016, late-breaking data released at the annual meeting of the European Academy of Dermatology and Venereology impressed the dermatology community. In psoriasis patients, the drug maintained 90% skin clearance for 4 years in 66% of patients, and 100% clearance for 4 years in 43%.
Not only does this kind of efficacy provide symptomatic relief, it also prevents the expensive long-term morbidity associated with psoriasis, Dr. Smith said.
“Even if these new medications are considerably more expensive upfront than a biosimilar for an older drug, they may end up being less expensive in the long run.”
Dr. Krant and Dr. Worthing had no financial disclosures. Dr. Smith has received grants from Allergan and Cipher Pharmaceuticals. Dr. Hanauer has received grants from numerous pharmaceutical companies that manufacture biologics.
*This article was updated 1/31/2017.
[email protected]
On Twitter @alz_gal