User login
CPSTF: A lesser known, but valuable, resource for FPs
Family physicians have come to rely on the US Preventive Services Task Force (USPSTF) for rigorous, evidence-based recommendations on the use of clinical preventive services. Still, many such services reach too few individuals who need them. And that’s where the less well known Community Preventive Services Task Force comes in. The CPSTF makes recommendations regarding public health interventions and ways to increase the use of preventive services in the clinical setting—eg, means of improving childhood immunization rates or increasing screening for cervical, breast, and colon cancer.
To better understand how the CPSTF can serve as a resource to busy family physicians, it’s helpful to first understand a bit about the inner-workings of the CPSTF itself.
How CPSTF figures out what works
Formed in 1996, the CPSTF consists of 15 independent, nonfederal members with expertise in public health and preventive medicine, appointed by the Director of the Centers for Disease Control and Prevention (CDC). The Task Force makes recommendations and develops guidance on which community-based health promotion and disease-prevention interventions work and which do not, based on available scientific evidence. The Task Force uses an evidence-based methodology similar to that of the USPSTF—ie, assessing systematic reviews of the evidence and tying recommendations to the strength of the evidence. However, the Task Force has only 3 levels of recommendations: recommend for, recommend against, and insufficient evidence to recommend.
Three CPSTF meetings are held each year, and a representative from the American Academy of Family Physicians (AAFP) attends as a liaison, along with liaisons from other organizations with an interest in the methods and recommendations. The CDC provides the CPSTF with technical and administrative support. However, the recommendations developed do not undergo review or approval by the CDC and are the sole responsibility of the Task Force.
The recommendations made are contained in the Guide to Community Preventive Services, often called The Community Guide, which is available on the Task Force’s Web site at www.thecommunityguide.org/index.html. The topics on which the CPSTF currently has recommendations are listed in TABLE 1. (Since community-wide recommendations are rarely subjected to controlled clinical trials, methods of assessing and ranking other forms of evidence are required. To learn more about how the CPSTF approaches this, see: https://www.thecommunityguide.org/about/our-methodology.)

Improving immunization rates
The topic of immunizations is an example of how synergistic the CPSTF recommendations can be with those from clinical organizations. The Advisory Committee on Immunization Practices (ACIP) makes recommendations on the use of vaccines.1 The CPSTF has developed a set of recommendations on how to increase the uptake of vaccines to improve rates of immunization.2 Interventions they recommend include vaccine requirements for attendance at preschool, primary and secondary school, and college; patient reminder and recall systems; patient and family incentives and rewards; providing vaccines at Women, Infants, and Children clinics, schools, work sites, and homes; standing orders for vaccine administration; physician reminders; physician assessments and feedback; reducing out-of-pocket expenses for vaccines; and using immunization registries. Just as important, the CPSTF identifies interventions that lack hard evidence to support their effectiveness.
Cancer screening works, but patient buy-in lags
The USPSTF recommends screening for breast, cervical, and colorectal cancer. And yet, despite the proven effectiveness of these screening tests in decreasing cancer mortality, many people do not get screened. The CPSTF has developed a set of implementation recommendations that are proven to increase the uptake of recommended cancer screening tests.3 These include:
- sending reminders to patients when screening tests are due
- providing one-on-one or group educational sessions
- providing videos and printed materials that describe screening tests and recommendations
- offering testing at locations and times that are convenient for patients
- offering on-site translation, transportation, patient navigators, and other administrative services to facilitate screening
- assessing provider performance and providing feedback.
CPSTF’s range of resources
Resources provided by the CPSTF (TABLE 2) also include the following materials for physicians, patients, and policy makers:
- tools to assist communities in performing a community health assessment and in prioritizing health needs
- fact sheets on what works for specific populations or conditions. (One recently added fact sheet is a description of interventions to address the leading health problems that affect women.4)
- examples of how communities have used CPSTF recommendations to address a major health concern in their populations. (See “An immunization ‘success story’ from the field.”)

Tackling controversial social issues
Public health interventions are often politically charged, and the CPSTF at times makes recommendations that, while supported by evidence, raise objections from certain groups. One example is a recommendation for “comprehensive risk reduction interventions to promote behaviors that prevent or reduce the risk of pregnancy, human immunodeficiency virus (HIV), and other sexually transmitted infections (STIs).”5 These interventions may include a hierarchy of recommended behaviors that identifies abstinence as the best or preferred method, but also provides information about sexual risk reduction strategies. Abstinence-only education initiatives were rated as having insufficient evidence for effectiveness.6
Another example that falls in the controversial realm is a recommendation against “policies facilitating the transfer of juveniles from juvenile to adult criminal justice systems for the purpose of reducing violence, based on strong evidence that these laws and policies are associated with increased subsequent violent behavior among transferred youth.”7
And a third example is a recommendation for “the use of regulatory authority (eg, through licensing and zoning) to limit alcohol outlet density on the basis of sufficient evidence of a positive association between outlet density and excessive alcohol consumption and related harms.”8 The CPSTF also recommends increasing taxes on alcohol products to reduce excess alcohol consumption.9
SIDEBAR
An immunization “success story” from the field
Before 2009, the vaccination completion rates for 2-year-olds in Duval County, Florida, consistently ranked below the national target of 90%, with particularly low rates in Jacksonville. With the aim of improving vaccination rates—and not wanting to waste time “reinventing the wheel”—the Duval County Health Department (DCHD) turned to The Community Guide for interventions proven to work synergistically: system-based efforts (eg, client reminders, standing orders, clinic-based education) and community-based efforts (eg, staff outreach to clients, educational activities).
Checking the Florida Shots Registry, clinic staff identified infants and toddlers who were due for, or had missed, vaccinations. They sent monthly reminders to parents, urging them to make appointments. DCHD also provided parents with educational materials, vaccination schedules, and safety evidence to reinforce awareness of the need for immunizations.
At local clinics, DCHD trained staff to administer vaccines and established standing orders authorizing them to do so even in the absence of a physician or other approving practitioner.
DCHD also formed an immunization task force of community stakeholders that worked with hospitals to send nurses and physicians each week to immunize children at churches and other convenient locations.
Within one year, the rate of complete immunization for 2-year-olds rose from 75% to 90%—the national target. DCHD is now applying interventions from The Community Guide to discourage tobacco use and to prevent sexually transmitted infections.
Read the full story at: https://www.thecommunityguide.org/stories/good-shot-reaching-immunization-targets-duval-county.
Reducing health disparities
The CPSTF places a high priority on interventions that can reduce health disparities. Many of their topics of interest focus on interventions to reduce health inequities among racial and ethnic minorities and low-income populations. For instance, the Task Force recommends early childhood education, all-day kindergarten, and after-school academic programs as ways to improve health and decrease health disparities.10
Social determinants of health for individuals and populations are increasingly appreciated as issues to be addressed by physicians and health systems. The CPSTF can serve as a valuable evidence-based resource in these efforts, and their recommendations complement and build on those of other authoritative groups such as the USPSTF, ACIP, and AAFP.
1. Centers for Disease Control and Prevention. ACIP vaccine recommendations. Available at: http://www.cdc.gov/vaccines/hcp/acip-recs/index.html. Accessed December 6, 2016.
2. Community Preventive Services Task Force. The Community Guide. Vaccination. Available at: https://www.thecommunityguide.org/topic/vaccination. Accessed December 6, 2016.
3. Community Preventive Services Task Force. The Community Guide. Cancer prevention and control: cancer screening [fact sheet]. Available at: https://www.thecommunityguide.org/sites/default/files/assets/What-Works-Cancer-Screening-factsheet-and-insert.pdf. Accessed December 6, 2016.
4. Community Preventive Services Task Fo
5. Community Preventive Services Task Force. The Community Guide. HIV/AIDS, other STIs, and teen pregnancy: group-based comprehensive risk reduction interventions for adolescents. Available at: https://www.thecommunityguide.org/findings/hivaids-other-stis-and-teen-pregnancy-group-based-comprehensive-risk-reduction-interventions. Accessed December 6, 2016.
6. Community Preventive Services Task Force. The Community Guide. HIV/AIDS, STIs and pregnancy. Available at: https://www.thecommunityguide.org/topic/hivaids-stis-and-pregnancy. Accessed December 6, 2016.
7. Community Preventive Services Task Force. The Community Guide. Violence: policies facilitating the transfer of juveniles to adult justice systems. Available at: https://www.thecommunityguide.org/findings/violence-policies-facilitating-transfer-juveniles-adult-justice-systems. Accessed December 6, 2016.
8. Community Preventive Services Task Force. The Community Guide. Alcohol – excessive consumption: regulation of alcohol outlet density. Available at: https://www.thecommunityguide.org/findings/alcohol-excessive-consumption-regulation-alcohol-outlet-density. Accessed December 6, 2016.
9. Community Preventive Services Task Force. The Community Guide. Excessive alcohol consumption. Available at: https://www.thecommunityguide.org/topic/excessive-alcohol-consumption. Accessed December 6, 2016.
10. Community Preventive Services Task Force. The Community Guide. Health equity. Available at: https://www.thecommunityguide.org/topic/health-equity. Accessed December 6, 2016.
Family physicians have come to rely on the US Preventive Services Task Force (USPSTF) for rigorous, evidence-based recommendations on the use of clinical preventive services. Still, many such services reach too few individuals who need them. And that’s where the less well known Community Preventive Services Task Force comes in. The CPSTF makes recommendations regarding public health interventions and ways to increase the use of preventive services in the clinical setting—eg, means of improving childhood immunization rates or increasing screening for cervical, breast, and colon cancer.
To better understand how the CPSTF can serve as a resource to busy family physicians, it’s helpful to first understand a bit about the inner-workings of the CPSTF itself.
How CPSTF figures out what works
Formed in 1996, the CPSTF consists of 15 independent, nonfederal members with expertise in public health and preventive medicine, appointed by the Director of the Centers for Disease Control and Prevention (CDC). The Task Force makes recommendations and develops guidance on which community-based health promotion and disease-prevention interventions work and which do not, based on available scientific evidence. The Task Force uses an evidence-based methodology similar to that of the USPSTF—ie, assessing systematic reviews of the evidence and tying recommendations to the strength of the evidence. However, the Task Force has only 3 levels of recommendations: recommend for, recommend against, and insufficient evidence to recommend.
Three CPSTF meetings are held each year, and a representative from the American Academy of Family Physicians (AAFP) attends as a liaison, along with liaisons from other organizations with an interest in the methods and recommendations. The CDC provides the CPSTF with technical and administrative support. However, the recommendations developed do not undergo review or approval by the CDC and are the sole responsibility of the Task Force.
The recommendations made are contained in the Guide to Community Preventive Services, often called The Community Guide, which is available on the Task Force’s Web site at www.thecommunityguide.org/index.html. The topics on which the CPSTF currently has recommendations are listed in TABLE 1. (Since community-wide recommendations are rarely subjected to controlled clinical trials, methods of assessing and ranking other forms of evidence are required. To learn more about how the CPSTF approaches this, see: https://www.thecommunityguide.org/about/our-methodology.)

Improving immunization rates
The topic of immunizations is an example of how synergistic the CPSTF recommendations can be with those from clinical organizations. The Advisory Committee on Immunization Practices (ACIP) makes recommendations on the use of vaccines.1 The CPSTF has developed a set of recommendations on how to increase the uptake of vaccines to improve rates of immunization.2 Interventions they recommend include vaccine requirements for attendance at preschool, primary and secondary school, and college; patient reminder and recall systems; patient and family incentives and rewards; providing vaccines at Women, Infants, and Children clinics, schools, work sites, and homes; standing orders for vaccine administration; physician reminders; physician assessments and feedback; reducing out-of-pocket expenses for vaccines; and using immunization registries. Just as important, the CPSTF identifies interventions that lack hard evidence to support their effectiveness.
Cancer screening works, but patient buy-in lags
The USPSTF recommends screening for breast, cervical, and colorectal cancer. And yet, despite the proven effectiveness of these screening tests in decreasing cancer mortality, many people do not get screened. The CPSTF has developed a set of implementation recommendations that are proven to increase the uptake of recommended cancer screening tests.3 These include:
- sending reminders to patients when screening tests are due
- providing one-on-one or group educational sessions
- providing videos and printed materials that describe screening tests and recommendations
- offering testing at locations and times that are convenient for patients
- offering on-site translation, transportation, patient navigators, and other administrative services to facilitate screening
- assessing provider performance and providing feedback.
CPSTF’s range of resources
Resources provided by the CPSTF (TABLE 2) also include the following materials for physicians, patients, and policy makers:
- tools to assist communities in performing a community health assessment and in prioritizing health needs
- fact sheets on what works for specific populations or conditions. (One recently added fact sheet is a description of interventions to address the leading health problems that affect women.4)
- examples of how communities have used CPSTF recommendations to address a major health concern in their populations. (See “An immunization ‘success story’ from the field.”)

Tackling controversial social issues
Public health interventions are often politically charged, and the CPSTF at times makes recommendations that, while supported by evidence, raise objections from certain groups. One example is a recommendation for “comprehensive risk reduction interventions to promote behaviors that prevent or reduce the risk of pregnancy, human immunodeficiency virus (HIV), and other sexually transmitted infections (STIs).”5 These interventions may include a hierarchy of recommended behaviors that identifies abstinence as the best or preferred method, but also provides information about sexual risk reduction strategies. Abstinence-only education initiatives were rated as having insufficient evidence for effectiveness.6
Another example that falls in the controversial realm is a recommendation against “policies facilitating the transfer of juveniles from juvenile to adult criminal justice systems for the purpose of reducing violence, based on strong evidence that these laws and policies are associated with increased subsequent violent behavior among transferred youth.”7
And a third example is a recommendation for “the use of regulatory authority (eg, through licensing and zoning) to limit alcohol outlet density on the basis of sufficient evidence of a positive association between outlet density and excessive alcohol consumption and related harms.”8 The CPSTF also recommends increasing taxes on alcohol products to reduce excess alcohol consumption.9
SIDEBAR
An immunization “success story” from the field
Before 2009, the vaccination completion rates for 2-year-olds in Duval County, Florida, consistently ranked below the national target of 90%, with particularly low rates in Jacksonville. With the aim of improving vaccination rates—and not wanting to waste time “reinventing the wheel”—the Duval County Health Department (DCHD) turned to The Community Guide for interventions proven to work synergistically: system-based efforts (eg, client reminders, standing orders, clinic-based education) and community-based efforts (eg, staff outreach to clients, educational activities).
Checking the Florida Shots Registry, clinic staff identified infants and toddlers who were due for, or had missed, vaccinations. They sent monthly reminders to parents, urging them to make appointments. DCHD also provided parents with educational materials, vaccination schedules, and safety evidence to reinforce awareness of the need for immunizations.
At local clinics, DCHD trained staff to administer vaccines and established standing orders authorizing them to do so even in the absence of a physician or other approving practitioner.
DCHD also formed an immunization task force of community stakeholders that worked with hospitals to send nurses and physicians each week to immunize children at churches and other convenient locations.
Within one year, the rate of complete immunization for 2-year-olds rose from 75% to 90%—the national target. DCHD is now applying interventions from The Community Guide to discourage tobacco use and to prevent sexually transmitted infections.
Read the full story at: https://www.thecommunityguide.org/stories/good-shot-reaching-immunization-targets-duval-county.
Reducing health disparities
The CPSTF places a high priority on interventions that can reduce health disparities. Many of their topics of interest focus on interventions to reduce health inequities among racial and ethnic minorities and low-income populations. For instance, the Task Force recommends early childhood education, all-day kindergarten, and after-school academic programs as ways to improve health and decrease health disparities.10
Social determinants of health for individuals and populations are increasingly appreciated as issues to be addressed by physicians and health systems. The CPSTF can serve as a valuable evidence-based resource in these efforts, and their recommendations complement and build on those of other authoritative groups such as the USPSTF, ACIP, and AAFP.
Family physicians have come to rely on the US Preventive Services Task Force (USPSTF) for rigorous, evidence-based recommendations on the use of clinical preventive services. Still, many such services reach too few individuals who need them. And that’s where the less well known Community Preventive Services Task Force comes in. The CPSTF makes recommendations regarding public health interventions and ways to increase the use of preventive services in the clinical setting—eg, means of improving childhood immunization rates or increasing screening for cervical, breast, and colon cancer.
To better understand how the CPSTF can serve as a resource to busy family physicians, it’s helpful to first understand a bit about the inner-workings of the CPSTF itself.
How CPSTF figures out what works
Formed in 1996, the CPSTF consists of 15 independent, nonfederal members with expertise in public health and preventive medicine, appointed by the Director of the Centers for Disease Control and Prevention (CDC). The Task Force makes recommendations and develops guidance on which community-based health promotion and disease-prevention interventions work and which do not, based on available scientific evidence. The Task Force uses an evidence-based methodology similar to that of the USPSTF—ie, assessing systematic reviews of the evidence and tying recommendations to the strength of the evidence. However, the Task Force has only 3 levels of recommendations: recommend for, recommend against, and insufficient evidence to recommend.
Three CPSTF meetings are held each year, and a representative from the American Academy of Family Physicians (AAFP) attends as a liaison, along with liaisons from other organizations with an interest in the methods and recommendations. The CDC provides the CPSTF with technical and administrative support. However, the recommendations developed do not undergo review or approval by the CDC and are the sole responsibility of the Task Force.
The recommendations made are contained in the Guide to Community Preventive Services, often called The Community Guide, which is available on the Task Force’s Web site at www.thecommunityguide.org/index.html. The topics on which the CPSTF currently has recommendations are listed in TABLE 1. (Since community-wide recommendations are rarely subjected to controlled clinical trials, methods of assessing and ranking other forms of evidence are required. To learn more about how the CPSTF approaches this, see: https://www.thecommunityguide.org/about/our-methodology.)

Improving immunization rates
The topic of immunizations is an example of how synergistic the CPSTF recommendations can be with those from clinical organizations. The Advisory Committee on Immunization Practices (ACIP) makes recommendations on the use of vaccines.1 The CPSTF has developed a set of recommendations on how to increase the uptake of vaccines to improve rates of immunization.2 Interventions they recommend include vaccine requirements for attendance at preschool, primary and secondary school, and college; patient reminder and recall systems; patient and family incentives and rewards; providing vaccines at Women, Infants, and Children clinics, schools, work sites, and homes; standing orders for vaccine administration; physician reminders; physician assessments and feedback; reducing out-of-pocket expenses for vaccines; and using immunization registries. Just as important, the CPSTF identifies interventions that lack hard evidence to support their effectiveness.
Cancer screening works, but patient buy-in lags
The USPSTF recommends screening for breast, cervical, and colorectal cancer. And yet, despite the proven effectiveness of these screening tests in decreasing cancer mortality, many people do not get screened. The CPSTF has developed a set of implementation recommendations that are proven to increase the uptake of recommended cancer screening tests.3 These include:
- sending reminders to patients when screening tests are due
- providing one-on-one or group educational sessions
- providing videos and printed materials that describe screening tests and recommendations
- offering testing at locations and times that are convenient for patients
- offering on-site translation, transportation, patient navigators, and other administrative services to facilitate screening
- assessing provider performance and providing feedback.
CPSTF’s range of resources
Resources provided by the CPSTF (TABLE 2) also include the following materials for physicians, patients, and policy makers:
- tools to assist communities in performing a community health assessment and in prioritizing health needs
- fact sheets on what works for specific populations or conditions. (One recently added fact sheet is a description of interventions to address the leading health problems that affect women.4)
- examples of how communities have used CPSTF recommendations to address a major health concern in their populations. (See “An immunization ‘success story’ from the field.”)

Tackling controversial social issues
Public health interventions are often politically charged, and the CPSTF at times makes recommendations that, while supported by evidence, raise objections from certain groups. One example is a recommendation for “comprehensive risk reduction interventions to promote behaviors that prevent or reduce the risk of pregnancy, human immunodeficiency virus (HIV), and other sexually transmitted infections (STIs).”5 These interventions may include a hierarchy of recommended behaviors that identifies abstinence as the best or preferred method, but also provides information about sexual risk reduction strategies. Abstinence-only education initiatives were rated as having insufficient evidence for effectiveness.6
Another example that falls in the controversial realm is a recommendation against “policies facilitating the transfer of juveniles from juvenile to adult criminal justice systems for the purpose of reducing violence, based on strong evidence that these laws and policies are associated with increased subsequent violent behavior among transferred youth.”7
And a third example is a recommendation for “the use of regulatory authority (eg, through licensing and zoning) to limit alcohol outlet density on the basis of sufficient evidence of a positive association between outlet density and excessive alcohol consumption and related harms.”8 The CPSTF also recommends increasing taxes on alcohol products to reduce excess alcohol consumption.9
SIDEBAR
An immunization “success story” from the field
Before 2009, the vaccination completion rates for 2-year-olds in Duval County, Florida, consistently ranked below the national target of 90%, with particularly low rates in Jacksonville. With the aim of improving vaccination rates—and not wanting to waste time “reinventing the wheel”—the Duval County Health Department (DCHD) turned to The Community Guide for interventions proven to work synergistically: system-based efforts (eg, client reminders, standing orders, clinic-based education) and community-based efforts (eg, staff outreach to clients, educational activities).
Checking the Florida Shots Registry, clinic staff identified infants and toddlers who were due for, or had missed, vaccinations. They sent monthly reminders to parents, urging them to make appointments. DCHD also provided parents with educational materials, vaccination schedules, and safety evidence to reinforce awareness of the need for immunizations.
At local clinics, DCHD trained staff to administer vaccines and established standing orders authorizing them to do so even in the absence of a physician or other approving practitioner.
DCHD also formed an immunization task force of community stakeholders that worked with hospitals to send nurses and physicians each week to immunize children at churches and other convenient locations.
Within one year, the rate of complete immunization for 2-year-olds rose from 75% to 90%—the national target. DCHD is now applying interventions from The Community Guide to discourage tobacco use and to prevent sexually transmitted infections.
Read the full story at: https://www.thecommunityguide.org/stories/good-shot-reaching-immunization-targets-duval-county.
Reducing health disparities
The CPSTF places a high priority on interventions that can reduce health disparities. Many of their topics of interest focus on interventions to reduce health inequities among racial and ethnic minorities and low-income populations. For instance, the Task Force recommends early childhood education, all-day kindergarten, and after-school academic programs as ways to improve health and decrease health disparities.10
Social determinants of health for individuals and populations are increasingly appreciated as issues to be addressed by physicians and health systems. The CPSTF can serve as a valuable evidence-based resource in these efforts, and their recommendations complement and build on those of other authoritative groups such as the USPSTF, ACIP, and AAFP.
1. Centers for Disease Control and Prevention. ACIP vaccine recommendations. Available at: http://www.cdc.gov/vaccines/hcp/acip-recs/index.html. Accessed December 6, 2016.
2. Community Preventive Services Task Force. The Community Guide. Vaccination. Available at: https://www.thecommunityguide.org/topic/vaccination. Accessed December 6, 2016.
3. Community Preventive Services Task Force. The Community Guide. Cancer prevention and control: cancer screening [fact sheet]. Available at: https://www.thecommunityguide.org/sites/default/files/assets/What-Works-Cancer-Screening-factsheet-and-insert.pdf. Accessed December 6, 2016.
4. Community Preventive Services Task Fo
5. Community Preventive Services Task Force. The Community Guide. HIV/AIDS, other STIs, and teen pregnancy: group-based comprehensive risk reduction interventions for adolescents. Available at: https://www.thecommunityguide.org/findings/hivaids-other-stis-and-teen-pregnancy-group-based-comprehensive-risk-reduction-interventions. Accessed December 6, 2016.
6. Community Preventive Services Task Force. The Community Guide. HIV/AIDS, STIs and pregnancy. Available at: https://www.thecommunityguide.org/topic/hivaids-stis-and-pregnancy. Accessed December 6, 2016.
7. Community Preventive Services Task Force. The Community Guide. Violence: policies facilitating the transfer of juveniles to adult justice systems. Available at: https://www.thecommunityguide.org/findings/violence-policies-facilitating-transfer-juveniles-adult-justice-systems. Accessed December 6, 2016.
8. Community Preventive Services Task Force. The Community Guide. Alcohol – excessive consumption: regulation of alcohol outlet density. Available at: https://www.thecommunityguide.org/findings/alcohol-excessive-consumption-regulation-alcohol-outlet-density. Accessed December 6, 2016.
9. Community Preventive Services Task Force. The Community Guide. Excessive alcohol consumption. Available at: https://www.thecommunityguide.org/topic/excessive-alcohol-consumption. Accessed December 6, 2016.
10. Community Preventive Services Task Force. The Community Guide. Health equity. Available at: https://www.thecommunityguide.org/topic/health-equity. Accessed December 6, 2016.
1. Centers for Disease Control and Prevention. ACIP vaccine recommendations. Available at: http://www.cdc.gov/vaccines/hcp/acip-recs/index.html. Accessed December 6, 2016.
2. Community Preventive Services Task Force. The Community Guide. Vaccination. Available at: https://www.thecommunityguide.org/topic/vaccination. Accessed December 6, 2016.
3. Community Preventive Services Task Force. The Community Guide. Cancer prevention and control: cancer screening [fact sheet]. Available at: https://www.thecommunityguide.org/sites/default/files/assets/What-Works-Cancer-Screening-factsheet-and-insert.pdf. Accessed December 6, 2016.
4. Community Preventive Services Task Fo
5. Community Preventive Services Task Force. The Community Guide. HIV/AIDS, other STIs, and teen pregnancy: group-based comprehensive risk reduction interventions for adolescents. Available at: https://www.thecommunityguide.org/findings/hivaids-other-stis-and-teen-pregnancy-group-based-comprehensive-risk-reduction-interventions. Accessed December 6, 2016.
6. Community Preventive Services Task Force. The Community Guide. HIV/AIDS, STIs and pregnancy. Available at: https://www.thecommunityguide.org/topic/hivaids-stis-and-pregnancy. Accessed December 6, 2016.
7. Community Preventive Services Task Force. The Community Guide. Violence: policies facilitating the transfer of juveniles to adult justice systems. Available at: https://www.thecommunityguide.org/findings/violence-policies-facilitating-transfer-juveniles-adult-justice-systems. Accessed December 6, 2016.
8. Community Preventive Services Task Force. The Community Guide. Alcohol – excessive consumption: regulation of alcohol outlet density. Available at: https://www.thecommunityguide.org/findings/alcohol-excessive-consumption-regulation-alcohol-outlet-density. Accessed December 6, 2016.
9. Community Preventive Services Task Force. The Community Guide. Excessive alcohol consumption. Available at: https://www.thecommunityguide.org/topic/excessive-alcohol-consumption. Accessed December 6, 2016.
10. Community Preventive Services Task Force. The Community Guide. Health equity. Available at: https://www.thecommunityguide.org/topic/health-equity. Accessed December 6, 2016.
How in-office and ambulatory BP monitoring compare: A systematic review and meta-analysis
ABSTRACT
Purpose We performed a literature review and meta-analysis to ascertain the validity of office blood pressure (BP) measurement in a primary care setting, using ambulatory blood pressure measurement (ABPM) as a benchmark in the monitoring of hypertensive patients receiving treatment.
Methods We conducted a literature search for studies published up to December 2013 that included hypertensive patients receiving treatment in a primary care setting. We compared the mean office BP with readings obtained by ABPM. We summarized the diagnostic accuracy of office BP with respect to ABPM in terms of sensitivity, specificity, and positive and negative likelihood ratios (LR), with a 95% confidence interval (CI).
Results Only 12 studies met the inclusion criteria and contained data to calculate the differences between the means of office and ambulatory BP measurements. Five were suitable for calculating sensitivity, specificity, and likelihood ratios, and 4 contained sufficient extractable data for meta-analysis. Compared with ABPM (thresholds of 140/90 mm Hg for office BP; 130/80 mmHg for ABPM) in diagnosing uncontrolled BP, office BP measurement had a sensitivity of 81.9% (95% CI, 74.8%-87%) and specificity of 41.1% (95% CI, 35.1%-48.4%). Positive LR was 1.35 (95% CI, 1.32-1.38), and the negative LR was 0.44 (95% CI, 0.37-0.53).
Conclusion Likelihood ratios show that isolated BP measurement in the office does not confirm or rule out the presence of poor BP control. Likelihood of underestimating or overestimating BP control is high when relying on in-office BP measurement alone.
A growing body of evidence supports more frequent use of ambulatory blood pressure monitoring (ABPM) to confirm a diagnosis of hypertension1 and to monitor blood pressure (BP) response to treatment.2 The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure has long accepted ABPM for diagnosis of hypertension,3 and many clinicians consider ABPM the reference standard for diagnosing true hypertension and for accurately assessing associated cardiovascular risk in adults, regardless of office BP readings.4 The US Preventive Services Task Force (USPSTF) recommends obtaining BP measurements outside the clinical setting to confirm a diagnosis of hypertension before starting treatment.5 The USPSTF also asserts that elevated 24-hour ambulatory systolic BP is consistently and significantly associated with stroke and other cardiovascular events independent of office BP readings and has greater predictive value than office monitoring.5 The USPSTF concludes that ABPM, because of its large evidence base, is the best confirmatory test for hypertension.6 The recommendation of the American Academy of Family Physicians is similar to that of the USPSTF.7
The challenge. Despite the considerable support for ABPM, this method of BP measurement is still not sufficiently integrated into primary care. And some guidelines, such as those of
But ABPM’s advantages are numerous. Ambulatory monitors, which can record BP for 24 hours, are typically programmed to take readings every 15 to 30 minutes, providing estimates of mean daytime and nighttime BP and revealing an individual’s circadian pattern of BP.8-10 Ambulatory BP values usually considered the uppermost limit of normal are 135/85 mm Hg (day), 120/70 mm Hg (night), and 130/80 mm Hg (24 hour).8
Office BP monitoring, usually performed manually by medical staff, has 2 main drawbacks: the well-known white-coat effect experienced by many patients, and the relatively small number of possible measurements. A more reliable in-office BP estimation of BP would require repeated measurements at each of several visits.
By comparing ABPM and office measurements, 4 clinical findings are possible: isolated clinic or office (white-coat) hypertension (ICH); isolated ambulatory (masked) hypertension (IAH); consistent normotension; or sustained hypertension. With ICH, BP is high in the office and normal with ABPM. With IAH, BP is normal in the office and high with ABPM. With consistent normotension and sustained hypertension, BP readings with both types of measurement agree.8,9
In patients being treated for hypertension, ICH leads to an overestimation of uncontrolled BP and may result in overtreatment. The cardiovascular risk, although controversial, is usually lower than in patients diagnosed with sustained hypertension.11 IAH leads to an underestimation of uncontrolled BP and may result in undertreatment; its associated cardiovascular risk is similar to that of sustained hypertension.12
Our research objective. We recently published a study conducted with 137 hypertensive patients in a primary care center.13 Our conclusion was that in-office measurement of BP had insufficient clinical validity to be recommended as a sole method of monitoring BP control. In accurately classifying BP as controlled or uncontrolled, clinic measurement agreed with 24h-ABPM in just 64.2% of cases.13
In our present study, we performed a literature review and meta-analysis to ascertain the validity of office BP measurement in a primary care setting, using ABPM as a benchmark in the monitoring of hypertensive patients receiving treatment.
METHODS
Most published studies comparing conventional office BP measurement with ABPM have been conducted with patients not taking antihypertensive medication. We excluded these studies and conducted a literature search for studies published up to December 2013 that included hypertensive patients receiving treatment in a primary care setting.
We searched Medline (from 1950 onward) and the Cochrane Database of Systematic Reviews. For the Medline search, we combined keywords for office BP, hypertension, and ambulatory BP with keywords for outpatient setting and primary care, using the following syntax: (((“clinic blood pressure” OR “office blood pressure” OR “casual blood pressure”))) AND (“hypertension” AND ((((“24-h ambulatory blood pressure”) OR “24 h ambulatory blood pressure”) OR “24 hour ambulatory blood pressure”) OR “blood pressure monitoring, ambulatory”[Mesh]) AND ((((((“outpatient setting”) OR “primary care”) OR “family care”) OR “family physician”) OR “family practice”) OR “general practice”)). We chose studies published in English and reviewed the titles and abstracts of identified articles.
With the aim of identifying additional candidate studies, we reviewed the reference lists of eligible primary studies, narrative reviews, and systematic reviews. The studies were generally of good quality and used appropriate statistical methods. Only primary studies qualified for meta-analysis.
Inclusion and exclusion criteria
Acceptable studies had to be conducted in a primary care setting with patients being treated for hypertension, and had to provide data comparing office BP measurement with ABPM. We excluded studies in which participants were treated in the hospital, were untreated, or had not been diagnosed with hypertension.
The quality of the studies included in the meta-analysis was judged by 2 independent observers according to the following criteria: the clear classification and initial comparison of both measurements; explicit and defined diagnostic criteria; compliance with the inclusion/exclusion criteria; and clear and precise definition of outcome variables.
Data extraction
We extracted the following data from each included study: study population, number of patients included, age, gender distribution, number of measurements (ambulatory and office BP), equipment validation, mean office and ambulatory BP, and the period of ambulatory BP measurement. We included adult patients of all ages, and we compared the mean office BP with those obtained by ABPM in hypertensive patients.
STATISTICAL ANALYSIS
For each study, we summarized the diagnostic accuracy of office BP with respect to ABPM in terms of sensitivity, specificity, and positive and negative likelihood ratios (LRs), with the 95% confidence interval (CI), if available. If these rates were not directly reported in the original papers, we used the published data to calculate them.
We used the R v2.15.1 software with the “mada” package for meta-analysis.14 Although a bivariate approach is preferred for the meta-analysis of diagnostic accuracy, it cannot be recommended if the number of primary studies to pool is too small,14 as happened in our case. Therefore, we used a univariate approach and pooled summary statistics for positive LR, negative LR, and the diagnostic odds ratio (DOR) with their 95% confidence intervals. We used the DerSimonian-Laird method to perform a random-effect meta-analysis. To explore heterogeneity between the studies, we used the Cochran’s Q heterogeneity test, I2 index, and Galbraith and L’Abbé plots.
RESULTS
Our search identified 237 studies, only 12 of which met the inclusion criteria and contained data to calculate the differences between the means of office and ambulatory BP measurements (TABLES 1 AND 2).15-26 Of these 12 studies, 5 were suitable for calculating sensitivity, specificity, and LR (TABLE 3),16,18,22,24,26 and 4 contained sufficient extractable data for meta-analysis. The study by Little et al18 was not included in the meta-analysis, as the number of true-positive, true-negative, false-positive, and false-negative results could not be deduced from published data.
The studies differed in sample size (40-31,530), patient ages (mean, 55-72.8 years), sex (percentage of men, 31%-52.9%), and number of measurements for office BP (1-9) and ABPM (32-96) (TABLE 1),15-26 as well as in daytime and nighttime periods for ABPM and BP thresholds, and in differences between the mean office and ambulatory BPs (TABLE 2).15-26
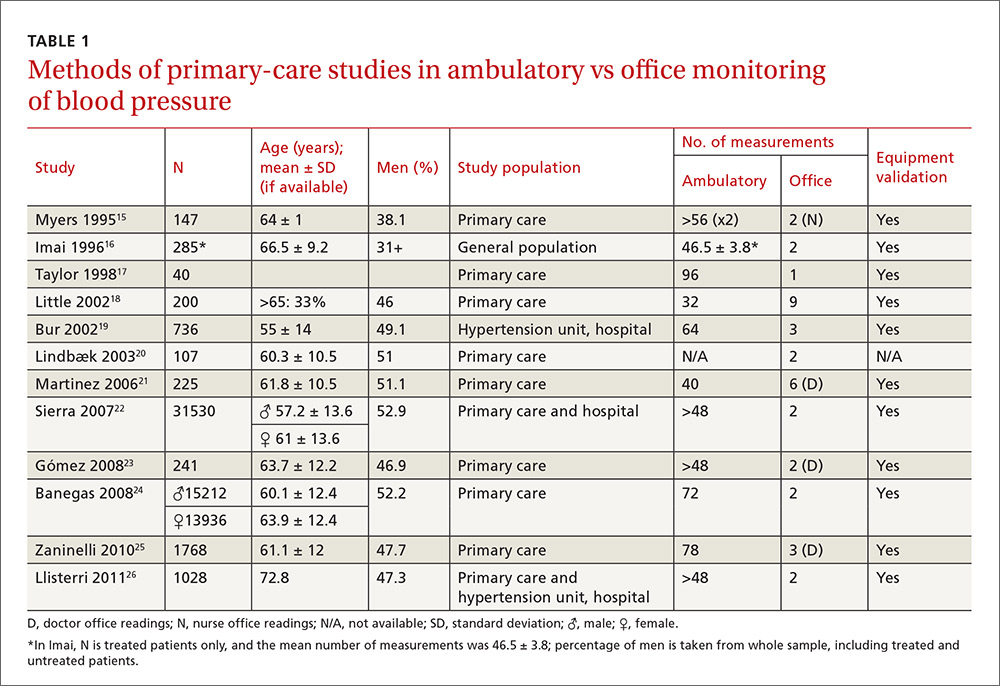
In general, the mean office BP measurements were higher than those obtained with ABPM in any period—from 5/0 mm Hg to 27.4/10.1 mm Hg in the day, and from 7.9/6.3 mm Hg to 31.2/13.7 mm Hg over 24 hours (TABLE 2).15-26
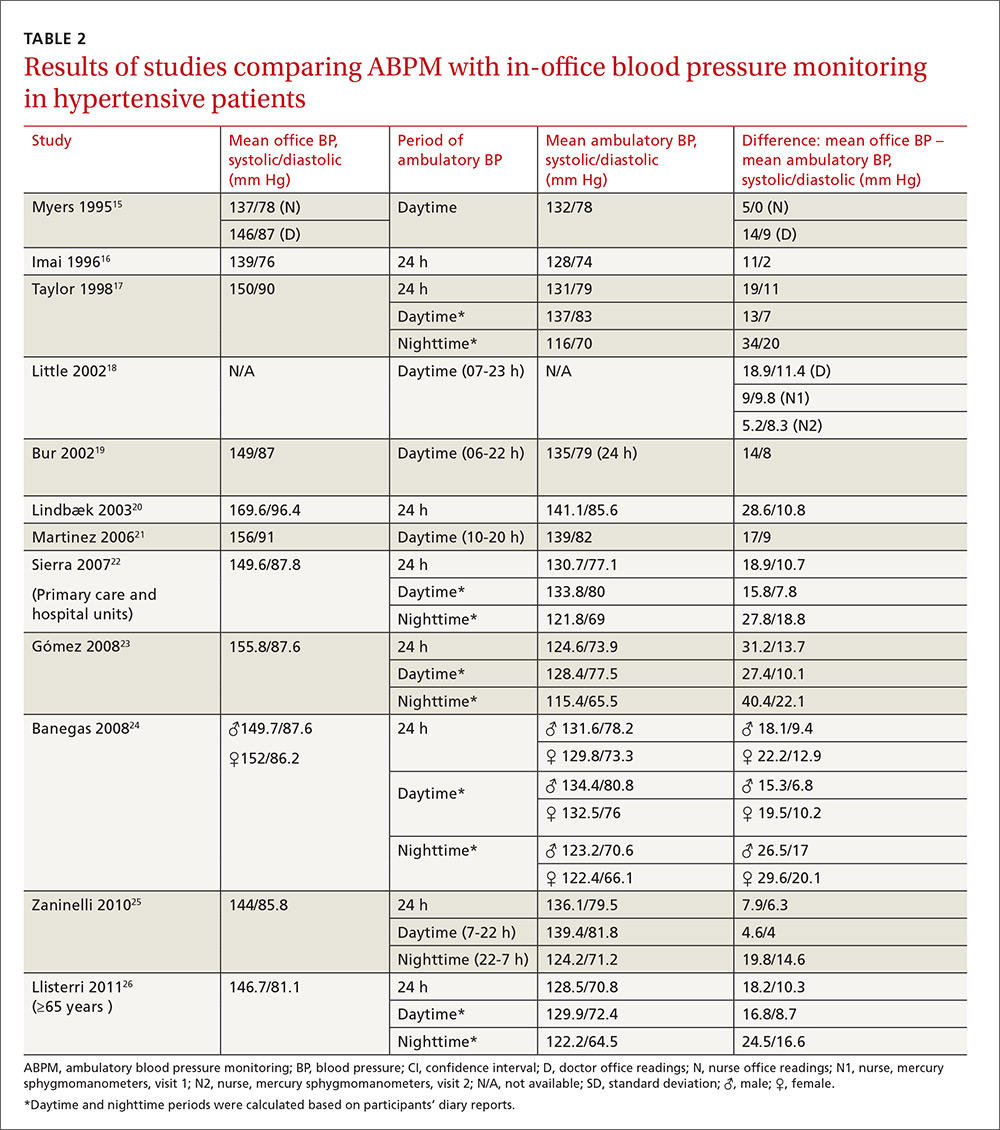
Compared with ABPM in diagnosing uncontrolled BP, office BP measurement had a sensitivity of 55.7% to 91.2% and a specificity of 25.8% to 61.8% (depending on whether the measure was carried out by the doctor or nurse18); positive LR ranged from 1.2 to 1.4, and negative LR from 0.3 to 0.72 (TABLE 3).16,18,22,24,26
For meta-analysis, we pooled studies with the same thresholds (140/90 mm Hg for office BP; 130/80 mm Hg for ABPM), with diagnostic accuracy of office BP expressed as pooled positive and negative LR, and as pooled DOR. The meta-analysis revealed that the pooled positive LR was 1.35 (95% CI, 1.32-1.38), and the pooled negative LR was 0.44 (95% CI, 0.37-0.53). The pooled DOR was 3.47 (95% CI, 3.02-3.98). Sensitivity was 81.9% (95% CI, 74.8%-87%) and specificity was 41.1% (95% CI, 35.1%-48.4%).
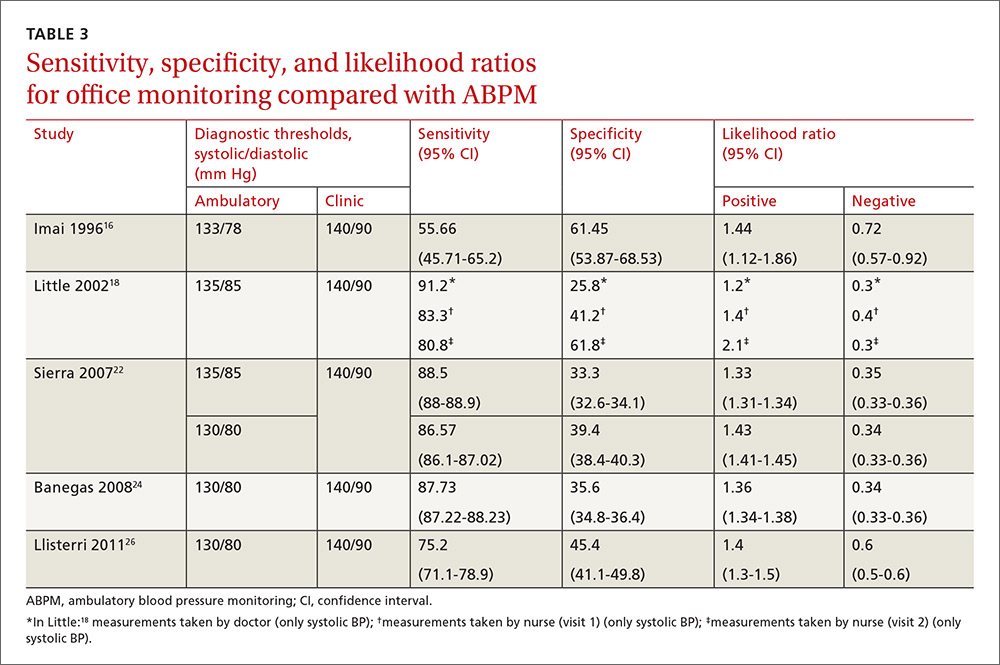
One study16 had a slightly different ambulatory diagnostic threshold (133/78 mm Hg), so we excluded it from a second meta-analysis. Results after the exclusion did not change significantly: positive LR was 1.39 (95% CI, 1.34-1.45); negative LR was 0.38 (95% CI, 0.33-0.44); and DOR was 3.77 (95% CI, 3.31-4.43).
In conclusion, the use of office-based BP readings in the outpatient clinic does not correlate well with ABPM. Therefore, caution must be used when making management decisions based solely on in-office readings of BP.
DISCUSSION
The European Society of Hypertension still regards office BP measurement as the gold standard in screening for, diagnosing, and managing hypertension. As previously mentioned, though, office measurements are usually handled by medical staff and can be compromised by the white-coat effect and a small number of measurements. The USPSTF now considers ABPM the reference standard in primary care to diagnose hypertension in adults, to corroborate or contradict office-based determinations of elevated BP (whether based on single or repeated-interval measurements), and to avoid overtreatment of individuals displaying elevated office BP yet proven normotensive by ABPM.4,7 The recommendation of the American Academy of Family Physicians is similar to that of the USPSTF.7 Therefore, evidence supports ABPM as the reference standard for confirming elevated office BP screening results to avoid misdiagnosis and overtreatment of individuals with isolated clinic hypertension.7
How office measurements stack up against ABPM
Checking the validity of decisions in clinical practice is extremely important for patient management. One of the tools used for decision-making is an estimate of the LR. We used the LR to assess the value of office BP measurement in determining controlled or uncontrolled BP. A high LR (eg, >10) indicates that the office BP can be used to rule in the disease (uncontrolled BP) with a high probability, while a low LR (eg, <0.1) could rule it out. An LR of around one indicates that the office BP measurement cannot rule the diagnosis of uncontrolled BP in or out.27 In our meta-analysis, the positive LR is 1.35 and negative LR is 0.44. Therefore, in treated hypertensive patients, an indication of uncontrolled BP as measured in the clinic does not confirm a diagnosis of uncontrolled BP (as judged by the reference standard of ABPM). On the other hand, the negative LR means that normal office BP does not rule out uncontrolled BP, which may be detected with ABPM. Consequently, the measurement of BP in the office does not change the degree of (un)certainty of adequate control of BP. This knowledge is important, to avoid overtreatment of white coat hypertension and undertreatment of masked cases.
As previously mentioned, we reported similar results in a study designed to determine the validity of office BP measurement in a primary care setting compared with ABPM.13 In that paper, the level of agreement between both methods was poor, indicating that clinic measurements could not be recommended as a single method of BP control in hypertensive patients.
The use of ABPM in diagnosing hypertension is likely to increase as a consequence of some guideline updates.2 Our study emphasizes the importance of their use in the control of hypertensive patients.
Another published meta-analysis1 investigated the validity of office BP for the diagnosis of hypertension in untreated patients, with diagnostic thresholds for arterial hypertension set at 140/90 mm Hg for office measurement, and 135/85 mm Hg for ABPM. In that paper, the sensitivity of office BP was 74.6% (95% CI, 60.7-84.8) and the specificity was 74.6% (95% CI, 47.9-90.4).
In our present study carried out with hypertensive patients receiving treatment, we obtained a slightly higher sensitivity value of 81.9% (within the CI of this meta-analysis) and a lower specificity of 41.1%. Therefore, the discordance between office BP and ABPM seems to be similar for the diagnosis of hypertension and the classification of hypertension as being well or poorly controlled. This confirms the low validity of the office BP, both for diagnosis and monitoring of hypertensive patients.
Strengths of our study. The study focused on (treated) hypertensive patients in a primary care setting, where hypertension is most often managed. It confirms that ABPM is indispensable to a good clinical practice.
Limitations of our study are those inherent to meta-analyses. The main weakness of our study is the paucity of data available regarding the utility of ABPM for monitoring BP control with treatment in a primary care setting. Other limitations are the variability in BP thresholds used, the number of measurements performed, and the ambulatory BP devices used. These differences could contribute to the observed heterogeneity.
Application of our results must take into account that we included only those studies performed in a primary care setting with treated hypertensive patients.
Moreover, this study was not designed to evaluate the consequences of over- and undertreatment of blood pressure, nor to address the accuracy of automated blood pressure machines or newer health and fitness devices.
Implications for practice, policy, or future research. Alternative monitoring methods are home BP self-measurement and automated 30-minute clinic BP measurement.28 However, ABPM provides us with unique information about the BP pattern (dipping or non-dipping), BP variability, and mean nighttime BP. This paper establishes that the measurement of BP in the office is not an accurate method to monitor BP control. ABPM should be incorporated in usual clinical practice in primary care. Although the consequences of ambulatory monitoring are not the focus of this study, we acknowledge that the decision to incorporate ABPM in clinical practice depends on the availability of ambulatory devices, proper training of health care workers, and a cost-effectiveness analysis of its use.
CORRESPONDENCE
Sergio Reino-González, MD, PhD, Adormideras Primary Health Center, Poligono de Adormideras s/n. 15002 A Coruña, Spain; [email protected].
1. Hodgkinson J, Mant J, Martin U, et al. Relative effectiveness of clinic and home blood pressure monitoring compared with ambulatory blood pressure monitoring in diagnosis of hypertension: systematic review. BMJ. 2011;342:d3621.
2. National Institute for Health and Clinical Excellence. Hypertension in adults: diagnosis and management. Available at: http://www.nice.org.uk/guidance/CG127. Accessed November 15, 2016.
3. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.
4. Hermida RC, Smolensky MH, Ayala DE, et al. Ambulatory Blood Pressure Monitoring (ABPM) as the reference standard for diagnosis of hypertension and assessment of vascular risk in adults. Chronobiol Int. 2015;32:1329-1342.
5. Siu AL; U.S. Preventive Services Task Force. Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163:778-786.
6. Piper MA, Evans CV
7. American Academy of Family Physicians. Hypertension. Available at: www.aafp.org/patient-care/clinical-recommendations/all/hypertension.html. Accessed February 10, 2016.
8. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Practice Guidelines for the Management of Arterial Hypertension. Blood Press. 2013;23:3-16.
9. Marin R, de la Sierra A, Armario P, et al. 2005 Spanish guidelines in diagnosis and treatment of arterial hypertension. Medicina Clínica. 2005;125:24-34.
10. Fagard RH, Celis H, Thijs L, et al. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008;51:55-61.
11. Sega R, Trocino G, Lanzarotti A, et al. Alterations of cardiac structure in patients with isolated office, ambulatory, or home hypertension: Data from the general population (Pressione Arteriose Monitorate E Loro Associazioni [PAMELA] Study). Circulation. 2001;104:1385-1392.
12. Verberk WJ, Kessels AG, de Leeuw PW. Prevalence, causes, and consequences of masked hypertension: a meta-analysis. Am J Hypertens. 2008;21:969-975.
13. Reino-González S, Pita-Fernández S, Cibiriain-Sola M, et al. Validity of clinic blood pressure compared to ambulatory monitoring in hypertensive patients in a primary care setting. Blood Press. 2015;24:111-118.
14. Doebler P, Holling H. Meta-analysis of diagnostic accuracy with mada. Available at: https://cran.r-project.org/web/packages/mada/vignettes/mada.pdf. Accessed October 5, 2015.
15. Myers MG, Oh PI, Reeves RA, et al. Prevalence of white coat effect in treated hypertensive patients in the community. Am J Hypertens. 1995;8:591-597.
16. Imai Y, Tsuji I, Nagai K, et al. Ambulatory blood pressure monitoring in evaluating the prevalence of hypertension in adults in Ohasama, a rural Japanese community. Hypertens Res. 1996;19:207-212.
17. Taylor RS, Stockman J, Kernick D, et al. Ambulatory blood pressure monitoring for hypertension in general practice. J R Soc Med. 1998;91:301-304.
18. Little P, Barnett J, Barnsley L, et al. Comparison of agreement between different measures of blood pressure in primary care and daytime ambulatory blood pressure. BMJ. 2002;325:254.
19. Bur A, Herkner H, Vlcek M, et al. Classification of blood pressure levels by ambulatory blood pressure in hypertension. Hypertension. 2002;40:817-822.
20. Lindbaek M, Sandvik E, Liodden K, et al. Predictors for the white coat effect in general practice patients with suspected and treated hypertension. Br J Gen Pract. 2003;53:790-793.
21. Martínez MA, Sancho T, García P, et al. Home blood pressure in poorly controlled hypertension: relationship with ambulatory blood pressure and organ damage. Blood Press Monit. 2006;11:207-213.
22. Sierra BC, de la Sierra IA, Sobrino J, et al. Monitorización ambulatoria de la presión arterial (MAPA): características clínicas de 31.530 pacientes. Medicina Clínica. 2007;129:1-5.
23. Gómez MA, García L, Sánchez Á, et al. Agreement and disagreement between different methods of measuring blood pressure. Hipertensión (Madr). 2008;25:231-239.
24. Banegas JR, Segura J, De la Sierra A, et al. Gender differences in office and ambulatory control of hypertension. Am J Med. 2008;121:1078-1084.
25. Zaninelli A, Parati G, Cricelli C, et al. Office and 24-h ambulatory blood pressure control by treatment in general practice: the ‘Monitoraggio della pressione ARteriosa nella medicina TErritoriale’ study. J Hypertens. 2010;28:910-917.
26. Llisterri JL, Morillas P, Pallarés V, et al. Differences in the degree of control of arterial hypertension according to the measurement procedure of blood pressure in patients ≥ 65 years. FAPRES study. Rev Clin Esp. 2011;211:76-84.
27. Straus SE, Richardson WS, Glasziou P, et al. Evidence-Based Medicine: How to practice and teach it. 4th ed. Edinburgh, Scotland: Churchill Livingstone; 2010.
28. Van der Wel MC, Buunk IE, van Weel C, et al. A novel approach to office blood pressure measurement: 30-minute office blood pressure vs daytime ambulatory blood pressure. Ann Fam Med. 2011;9:128-135.
ABSTRACT
Purpose We performed a literature review and meta-analysis to ascertain the validity of office blood pressure (BP) measurement in a primary care setting, using ambulatory blood pressure measurement (ABPM) as a benchmark in the monitoring of hypertensive patients receiving treatment.
Methods We conducted a literature search for studies published up to December 2013 that included hypertensive patients receiving treatment in a primary care setting. We compared the mean office BP with readings obtained by ABPM. We summarized the diagnostic accuracy of office BP with respect to ABPM in terms of sensitivity, specificity, and positive and negative likelihood ratios (LR), with a 95% confidence interval (CI).
Results Only 12 studies met the inclusion criteria and contained data to calculate the differences between the means of office and ambulatory BP measurements. Five were suitable for calculating sensitivity, specificity, and likelihood ratios, and 4 contained sufficient extractable data for meta-analysis. Compared with ABPM (thresholds of 140/90 mm Hg for office BP; 130/80 mmHg for ABPM) in diagnosing uncontrolled BP, office BP measurement had a sensitivity of 81.9% (95% CI, 74.8%-87%) and specificity of 41.1% (95% CI, 35.1%-48.4%). Positive LR was 1.35 (95% CI, 1.32-1.38), and the negative LR was 0.44 (95% CI, 0.37-0.53).
Conclusion Likelihood ratios show that isolated BP measurement in the office does not confirm or rule out the presence of poor BP control. Likelihood of underestimating or overestimating BP control is high when relying on in-office BP measurement alone.
A growing body of evidence supports more frequent use of ambulatory blood pressure monitoring (ABPM) to confirm a diagnosis of hypertension1 and to monitor blood pressure (BP) response to treatment.2 The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure has long accepted ABPM for diagnosis of hypertension,3 and many clinicians consider ABPM the reference standard for diagnosing true hypertension and for accurately assessing associated cardiovascular risk in adults, regardless of office BP readings.4 The US Preventive Services Task Force (USPSTF) recommends obtaining BP measurements outside the clinical setting to confirm a diagnosis of hypertension before starting treatment.5 The USPSTF also asserts that elevated 24-hour ambulatory systolic BP is consistently and significantly associated with stroke and other cardiovascular events independent of office BP readings and has greater predictive value than office monitoring.5 The USPSTF concludes that ABPM, because of its large evidence base, is the best confirmatory test for hypertension.6 The recommendation of the American Academy of Family Physicians is similar to that of the USPSTF.7
The challenge. Despite the considerable support for ABPM, this method of BP measurement is still not sufficiently integrated into primary care. And some guidelines, such as those of
But ABPM’s advantages are numerous. Ambulatory monitors, which can record BP for 24 hours, are typically programmed to take readings every 15 to 30 minutes, providing estimates of mean daytime and nighttime BP and revealing an individual’s circadian pattern of BP.8-10 Ambulatory BP values usually considered the uppermost limit of normal are 135/85 mm Hg (day), 120/70 mm Hg (night), and 130/80 mm Hg (24 hour).8
Office BP monitoring, usually performed manually by medical staff, has 2 main drawbacks: the well-known white-coat effect experienced by many patients, and the relatively small number of possible measurements. A more reliable in-office BP estimation of BP would require repeated measurements at each of several visits.
By comparing ABPM and office measurements, 4 clinical findings are possible: isolated clinic or office (white-coat) hypertension (ICH); isolated ambulatory (masked) hypertension (IAH); consistent normotension; or sustained hypertension. With ICH, BP is high in the office and normal with ABPM. With IAH, BP is normal in the office and high with ABPM. With consistent normotension and sustained hypertension, BP readings with both types of measurement agree.8,9
In patients being treated for hypertension, ICH leads to an overestimation of uncontrolled BP and may result in overtreatment. The cardiovascular risk, although controversial, is usually lower than in patients diagnosed with sustained hypertension.11 IAH leads to an underestimation of uncontrolled BP and may result in undertreatment; its associated cardiovascular risk is similar to that of sustained hypertension.12
Our research objective. We recently published a study conducted with 137 hypertensive patients in a primary care center.13 Our conclusion was that in-office measurement of BP had insufficient clinical validity to be recommended as a sole method of monitoring BP control. In accurately classifying BP as controlled or uncontrolled, clinic measurement agreed with 24h-ABPM in just 64.2% of cases.13
In our present study, we performed a literature review and meta-analysis to ascertain the validity of office BP measurement in a primary care setting, using ABPM as a benchmark in the monitoring of hypertensive patients receiving treatment.
METHODS
Most published studies comparing conventional office BP measurement with ABPM have been conducted with patients not taking antihypertensive medication. We excluded these studies and conducted a literature search for studies published up to December 2013 that included hypertensive patients receiving treatment in a primary care setting.
We searched Medline (from 1950 onward) and the Cochrane Database of Systematic Reviews. For the Medline search, we combined keywords for office BP, hypertension, and ambulatory BP with keywords for outpatient setting and primary care, using the following syntax: (((“clinic blood pressure” OR “office blood pressure” OR “casual blood pressure”))) AND (“hypertension” AND ((((“24-h ambulatory blood pressure”) OR “24 h ambulatory blood pressure”) OR “24 hour ambulatory blood pressure”) OR “blood pressure monitoring, ambulatory”[Mesh]) AND ((((((“outpatient setting”) OR “primary care”) OR “family care”) OR “family physician”) OR “family practice”) OR “general practice”)). We chose studies published in English and reviewed the titles and abstracts of identified articles.
With the aim of identifying additional candidate studies, we reviewed the reference lists of eligible primary studies, narrative reviews, and systematic reviews. The studies were generally of good quality and used appropriate statistical methods. Only primary studies qualified for meta-analysis.
Inclusion and exclusion criteria
Acceptable studies had to be conducted in a primary care setting with patients being treated for hypertension, and had to provide data comparing office BP measurement with ABPM. We excluded studies in which participants were treated in the hospital, were untreated, or had not been diagnosed with hypertension.
The quality of the studies included in the meta-analysis was judged by 2 independent observers according to the following criteria: the clear classification and initial comparison of both measurements; explicit and defined diagnostic criteria; compliance with the inclusion/exclusion criteria; and clear and precise definition of outcome variables.
Data extraction
We extracted the following data from each included study: study population, number of patients included, age, gender distribution, number of measurements (ambulatory and office BP), equipment validation, mean office and ambulatory BP, and the period of ambulatory BP measurement. We included adult patients of all ages, and we compared the mean office BP with those obtained by ABPM in hypertensive patients.
STATISTICAL ANALYSIS
For each study, we summarized the diagnostic accuracy of office BP with respect to ABPM in terms of sensitivity, specificity, and positive and negative likelihood ratios (LRs), with the 95% confidence interval (CI), if available. If these rates were not directly reported in the original papers, we used the published data to calculate them.
We used the R v2.15.1 software with the “mada” package for meta-analysis.14 Although a bivariate approach is preferred for the meta-analysis of diagnostic accuracy, it cannot be recommended if the number of primary studies to pool is too small,14 as happened in our case. Therefore, we used a univariate approach and pooled summary statistics for positive LR, negative LR, and the diagnostic odds ratio (DOR) with their 95% confidence intervals. We used the DerSimonian-Laird method to perform a random-effect meta-analysis. To explore heterogeneity between the studies, we used the Cochran’s Q heterogeneity test, I2 index, and Galbraith and L’Abbé plots.
RESULTS
Our search identified 237 studies, only 12 of which met the inclusion criteria and contained data to calculate the differences between the means of office and ambulatory BP measurements (TABLES 1 AND 2).15-26 Of these 12 studies, 5 were suitable for calculating sensitivity, specificity, and LR (TABLE 3),16,18,22,24,26 and 4 contained sufficient extractable data for meta-analysis. The study by Little et al18 was not included in the meta-analysis, as the number of true-positive, true-negative, false-positive, and false-negative results could not be deduced from published data.
The studies differed in sample size (40-31,530), patient ages (mean, 55-72.8 years), sex (percentage of men, 31%-52.9%), and number of measurements for office BP (1-9) and ABPM (32-96) (TABLE 1),15-26 as well as in daytime and nighttime periods for ABPM and BP thresholds, and in differences between the mean office and ambulatory BPs (TABLE 2).15-26

In general, the mean office BP measurements were higher than those obtained with ABPM in any period—from 5/0 mm Hg to 27.4/10.1 mm Hg in the day, and from 7.9/6.3 mm Hg to 31.2/13.7 mm Hg over 24 hours (TABLE 2).15-26

Compared with ABPM in diagnosing uncontrolled BP, office BP measurement had a sensitivity of 55.7% to 91.2% and a specificity of 25.8% to 61.8% (depending on whether the measure was carried out by the doctor or nurse18); positive LR ranged from 1.2 to 1.4, and negative LR from 0.3 to 0.72 (TABLE 3).16,18,22,24,26
For meta-analysis, we pooled studies with the same thresholds (140/90 mm Hg for office BP; 130/80 mm Hg for ABPM), with diagnostic accuracy of office BP expressed as pooled positive and negative LR, and as pooled DOR. The meta-analysis revealed that the pooled positive LR was 1.35 (95% CI, 1.32-1.38), and the pooled negative LR was 0.44 (95% CI, 0.37-0.53). The pooled DOR was 3.47 (95% CI, 3.02-3.98). Sensitivity was 81.9% (95% CI, 74.8%-87%) and specificity was 41.1% (95% CI, 35.1%-48.4%).

One study16 had a slightly different ambulatory diagnostic threshold (133/78 mm Hg), so we excluded it from a second meta-analysis. Results after the exclusion did not change significantly: positive LR was 1.39 (95% CI, 1.34-1.45); negative LR was 0.38 (95% CI, 0.33-0.44); and DOR was 3.77 (95% CI, 3.31-4.43).
In conclusion, the use of office-based BP readings in the outpatient clinic does not correlate well with ABPM. Therefore, caution must be used when making management decisions based solely on in-office readings of BP.
DISCUSSION
The European Society of Hypertension still regards office BP measurement as the gold standard in screening for, diagnosing, and managing hypertension. As previously mentioned, though, office measurements are usually handled by medical staff and can be compromised by the white-coat effect and a small number of measurements. The USPSTF now considers ABPM the reference standard in primary care to diagnose hypertension in adults, to corroborate or contradict office-based determinations of elevated BP (whether based on single or repeated-interval measurements), and to avoid overtreatment of individuals displaying elevated office BP yet proven normotensive by ABPM.4,7 The recommendation of the American Academy of Family Physicians is similar to that of the USPSTF.7 Therefore, evidence supports ABPM as the reference standard for confirming elevated office BP screening results to avoid misdiagnosis and overtreatment of individuals with isolated clinic hypertension.7
How office measurements stack up against ABPM
Checking the validity of decisions in clinical practice is extremely important for patient management. One of the tools used for decision-making is an estimate of the LR. We used the LR to assess the value of office BP measurement in determining controlled or uncontrolled BP. A high LR (eg, >10) indicates that the office BP can be used to rule in the disease (uncontrolled BP) with a high probability, while a low LR (eg, <0.1) could rule it out. An LR of around one indicates that the office BP measurement cannot rule the diagnosis of uncontrolled BP in or out.27 In our meta-analysis, the positive LR is 1.35 and negative LR is 0.44. Therefore, in treated hypertensive patients, an indication of uncontrolled BP as measured in the clinic does not confirm a diagnosis of uncontrolled BP (as judged by the reference standard of ABPM). On the other hand, the negative LR means that normal office BP does not rule out uncontrolled BP, which may be detected with ABPM. Consequently, the measurement of BP in the office does not change the degree of (un)certainty of adequate control of BP. This knowledge is important, to avoid overtreatment of white coat hypertension and undertreatment of masked cases.
As previously mentioned, we reported similar results in a study designed to determine the validity of office BP measurement in a primary care setting compared with ABPM.13 In that paper, the level of agreement between both methods was poor, indicating that clinic measurements could not be recommended as a single method of BP control in hypertensive patients.
The use of ABPM in diagnosing hypertension is likely to increase as a consequence of some guideline updates.2 Our study emphasizes the importance of their use in the control of hypertensive patients.
Another published meta-analysis1 investigated the validity of office BP for the diagnosis of hypertension in untreated patients, with diagnostic thresholds for arterial hypertension set at 140/90 mm Hg for office measurement, and 135/85 mm Hg for ABPM. In that paper, the sensitivity of office BP was 74.6% (95% CI, 60.7-84.8) and the specificity was 74.6% (95% CI, 47.9-90.4).
In our present study carried out with hypertensive patients receiving treatment, we obtained a slightly higher sensitivity value of 81.9% (within the CI of this meta-analysis) and a lower specificity of 41.1%. Therefore, the discordance between office BP and ABPM seems to be similar for the diagnosis of hypertension and the classification of hypertension as being well or poorly controlled. This confirms the low validity of the office BP, both for diagnosis and monitoring of hypertensive patients.
Strengths of our study. The study focused on (treated) hypertensive patients in a primary care setting, where hypertension is most often managed. It confirms that ABPM is indispensable to a good clinical practice.
Limitations of our study are those inherent to meta-analyses. The main weakness of our study is the paucity of data available regarding the utility of ABPM for monitoring BP control with treatment in a primary care setting. Other limitations are the variability in BP thresholds used, the number of measurements performed, and the ambulatory BP devices used. These differences could contribute to the observed heterogeneity.
Application of our results must take into account that we included only those studies performed in a primary care setting with treated hypertensive patients.
Moreover, this study was not designed to evaluate the consequences of over- and undertreatment of blood pressure, nor to address the accuracy of automated blood pressure machines or newer health and fitness devices.
Implications for practice, policy, or future research. Alternative monitoring methods are home BP self-measurement and automated 30-minute clinic BP measurement.28 However, ABPM provides us with unique information about the BP pattern (dipping or non-dipping), BP variability, and mean nighttime BP. This paper establishes that the measurement of BP in the office is not an accurate method to monitor BP control. ABPM should be incorporated in usual clinical practice in primary care. Although the consequences of ambulatory monitoring are not the focus of this study, we acknowledge that the decision to incorporate ABPM in clinical practice depends on the availability of ambulatory devices, proper training of health care workers, and a cost-effectiveness analysis of its use.
CORRESPONDENCE
Sergio Reino-González, MD, PhD, Adormideras Primary Health Center, Poligono de Adormideras s/n. 15002 A Coruña, Spain; [email protected].
ABSTRACT
Purpose We performed a literature review and meta-analysis to ascertain the validity of office blood pressure (BP) measurement in a primary care setting, using ambulatory blood pressure measurement (ABPM) as a benchmark in the monitoring of hypertensive patients receiving treatment.
Methods We conducted a literature search for studies published up to December 2013 that included hypertensive patients receiving treatment in a primary care setting. We compared the mean office BP with readings obtained by ABPM. We summarized the diagnostic accuracy of office BP with respect to ABPM in terms of sensitivity, specificity, and positive and negative likelihood ratios (LR), with a 95% confidence interval (CI).
Results Only 12 studies met the inclusion criteria and contained data to calculate the differences between the means of office and ambulatory BP measurements. Five were suitable for calculating sensitivity, specificity, and likelihood ratios, and 4 contained sufficient extractable data for meta-analysis. Compared with ABPM (thresholds of 140/90 mm Hg for office BP; 130/80 mmHg for ABPM) in diagnosing uncontrolled BP, office BP measurement had a sensitivity of 81.9% (95% CI, 74.8%-87%) and specificity of 41.1% (95% CI, 35.1%-48.4%). Positive LR was 1.35 (95% CI, 1.32-1.38), and the negative LR was 0.44 (95% CI, 0.37-0.53).
Conclusion Likelihood ratios show that isolated BP measurement in the office does not confirm or rule out the presence of poor BP control. Likelihood of underestimating or overestimating BP control is high when relying on in-office BP measurement alone.
A growing body of evidence supports more frequent use of ambulatory blood pressure monitoring (ABPM) to confirm a diagnosis of hypertension1 and to monitor blood pressure (BP) response to treatment.2 The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure has long accepted ABPM for diagnosis of hypertension,3 and many clinicians consider ABPM the reference standard for diagnosing true hypertension and for accurately assessing associated cardiovascular risk in adults, regardless of office BP readings.4 The US Preventive Services Task Force (USPSTF) recommends obtaining BP measurements outside the clinical setting to confirm a diagnosis of hypertension before starting treatment.5 The USPSTF also asserts that elevated 24-hour ambulatory systolic BP is consistently and significantly associated with stroke and other cardiovascular events independent of office BP readings and has greater predictive value than office monitoring.5 The USPSTF concludes that ABPM, because of its large evidence base, is the best confirmatory test for hypertension.6 The recommendation of the American Academy of Family Physicians is similar to that of the USPSTF.7
The challenge. Despite the considerable support for ABPM, this method of BP measurement is still not sufficiently integrated into primary care. And some guidelines, such as those of
But ABPM’s advantages are numerous. Ambulatory monitors, which can record BP for 24 hours, are typically programmed to take readings every 15 to 30 minutes, providing estimates of mean daytime and nighttime BP and revealing an individual’s circadian pattern of BP.8-10 Ambulatory BP values usually considered the uppermost limit of normal are 135/85 mm Hg (day), 120/70 mm Hg (night), and 130/80 mm Hg (24 hour).8
Office BP monitoring, usually performed manually by medical staff, has 2 main drawbacks: the well-known white-coat effect experienced by many patients, and the relatively small number of possible measurements. A more reliable in-office BP estimation of BP would require repeated measurements at each of several visits.
By comparing ABPM and office measurements, 4 clinical findings are possible: isolated clinic or office (white-coat) hypertension (ICH); isolated ambulatory (masked) hypertension (IAH); consistent normotension; or sustained hypertension. With ICH, BP is high in the office and normal with ABPM. With IAH, BP is normal in the office and high with ABPM. With consistent normotension and sustained hypertension, BP readings with both types of measurement agree.8,9
In patients being treated for hypertension, ICH leads to an overestimation of uncontrolled BP and may result in overtreatment. The cardiovascular risk, although controversial, is usually lower than in patients diagnosed with sustained hypertension.11 IAH leads to an underestimation of uncontrolled BP and may result in undertreatment; its associated cardiovascular risk is similar to that of sustained hypertension.12
Our research objective. We recently published a study conducted with 137 hypertensive patients in a primary care center.13 Our conclusion was that in-office measurement of BP had insufficient clinical validity to be recommended as a sole method of monitoring BP control. In accurately classifying BP as controlled or uncontrolled, clinic measurement agreed with 24h-ABPM in just 64.2% of cases.13
In our present study, we performed a literature review and meta-analysis to ascertain the validity of office BP measurement in a primary care setting, using ABPM as a benchmark in the monitoring of hypertensive patients receiving treatment.
METHODS
Most published studies comparing conventional office BP measurement with ABPM have been conducted with patients not taking antihypertensive medication. We excluded these studies and conducted a literature search for studies published up to December 2013 that included hypertensive patients receiving treatment in a primary care setting.
We searched Medline (from 1950 onward) and the Cochrane Database of Systematic Reviews. For the Medline search, we combined keywords for office BP, hypertension, and ambulatory BP with keywords for outpatient setting and primary care, using the following syntax: (((“clinic blood pressure” OR “office blood pressure” OR “casual blood pressure”))) AND (“hypertension” AND ((((“24-h ambulatory blood pressure”) OR “24 h ambulatory blood pressure”) OR “24 hour ambulatory blood pressure”) OR “blood pressure monitoring, ambulatory”[Mesh]) AND ((((((“outpatient setting”) OR “primary care”) OR “family care”) OR “family physician”) OR “family practice”) OR “general practice”)). We chose studies published in English and reviewed the titles and abstracts of identified articles.
With the aim of identifying additional candidate studies, we reviewed the reference lists of eligible primary studies, narrative reviews, and systematic reviews. The studies were generally of good quality and used appropriate statistical methods. Only primary studies qualified for meta-analysis.
Inclusion and exclusion criteria
Acceptable studies had to be conducted in a primary care setting with patients being treated for hypertension, and had to provide data comparing office BP measurement with ABPM. We excluded studies in which participants were treated in the hospital, were untreated, or had not been diagnosed with hypertension.
The quality of the studies included in the meta-analysis was judged by 2 independent observers according to the following criteria: the clear classification and initial comparison of both measurements; explicit and defined diagnostic criteria; compliance with the inclusion/exclusion criteria; and clear and precise definition of outcome variables.
Data extraction
We extracted the following data from each included study: study population, number of patients included, age, gender distribution, number of measurements (ambulatory and office BP), equipment validation, mean office and ambulatory BP, and the period of ambulatory BP measurement. We included adult patients of all ages, and we compared the mean office BP with those obtained by ABPM in hypertensive patients.
STATISTICAL ANALYSIS
For each study, we summarized the diagnostic accuracy of office BP with respect to ABPM in terms of sensitivity, specificity, and positive and negative likelihood ratios (LRs), with the 95% confidence interval (CI), if available. If these rates were not directly reported in the original papers, we used the published data to calculate them.
We used the R v2.15.1 software with the “mada” package for meta-analysis.14 Although a bivariate approach is preferred for the meta-analysis of diagnostic accuracy, it cannot be recommended if the number of primary studies to pool is too small,14 as happened in our case. Therefore, we used a univariate approach and pooled summary statistics for positive LR, negative LR, and the diagnostic odds ratio (DOR) with their 95% confidence intervals. We used the DerSimonian-Laird method to perform a random-effect meta-analysis. To explore heterogeneity between the studies, we used the Cochran’s Q heterogeneity test, I2 index, and Galbraith and L’Abbé plots.
RESULTS
Our search identified 237 studies, only 12 of which met the inclusion criteria and contained data to calculate the differences between the means of office and ambulatory BP measurements (TABLES 1 AND 2).15-26 Of these 12 studies, 5 were suitable for calculating sensitivity, specificity, and LR (TABLE 3),16,18,22,24,26 and 4 contained sufficient extractable data for meta-analysis. The study by Little et al18 was not included in the meta-analysis, as the number of true-positive, true-negative, false-positive, and false-negative results could not be deduced from published data.
The studies differed in sample size (40-31,530), patient ages (mean, 55-72.8 years), sex (percentage of men, 31%-52.9%), and number of measurements for office BP (1-9) and ABPM (32-96) (TABLE 1),15-26 as well as in daytime and nighttime periods for ABPM and BP thresholds, and in differences between the mean office and ambulatory BPs (TABLE 2).15-26

In general, the mean office BP measurements were higher than those obtained with ABPM in any period—from 5/0 mm Hg to 27.4/10.1 mm Hg in the day, and from 7.9/6.3 mm Hg to 31.2/13.7 mm Hg over 24 hours (TABLE 2).15-26

Compared with ABPM in diagnosing uncontrolled BP, office BP measurement had a sensitivity of 55.7% to 91.2% and a specificity of 25.8% to 61.8% (depending on whether the measure was carried out by the doctor or nurse18); positive LR ranged from 1.2 to 1.4, and negative LR from 0.3 to 0.72 (TABLE 3).16,18,22,24,26
For meta-analysis, we pooled studies with the same thresholds (140/90 mm Hg for office BP; 130/80 mm Hg for ABPM), with diagnostic accuracy of office BP expressed as pooled positive and negative LR, and as pooled DOR. The meta-analysis revealed that the pooled positive LR was 1.35 (95% CI, 1.32-1.38), and the pooled negative LR was 0.44 (95% CI, 0.37-0.53). The pooled DOR was 3.47 (95% CI, 3.02-3.98). Sensitivity was 81.9% (95% CI, 74.8%-87%) and specificity was 41.1% (95% CI, 35.1%-48.4%).

One study16 had a slightly different ambulatory diagnostic threshold (133/78 mm Hg), so we excluded it from a second meta-analysis. Results after the exclusion did not change significantly: positive LR was 1.39 (95% CI, 1.34-1.45); negative LR was 0.38 (95% CI, 0.33-0.44); and DOR was 3.77 (95% CI, 3.31-4.43).
In conclusion, the use of office-based BP readings in the outpatient clinic does not correlate well with ABPM. Therefore, caution must be used when making management decisions based solely on in-office readings of BP.
DISCUSSION
The European Society of Hypertension still regards office BP measurement as the gold standard in screening for, diagnosing, and managing hypertension. As previously mentioned, though, office measurements are usually handled by medical staff and can be compromised by the white-coat effect and a small number of measurements. The USPSTF now considers ABPM the reference standard in primary care to diagnose hypertension in adults, to corroborate or contradict office-based determinations of elevated BP (whether based on single or repeated-interval measurements), and to avoid overtreatment of individuals displaying elevated office BP yet proven normotensive by ABPM.4,7 The recommendation of the American Academy of Family Physicians is similar to that of the USPSTF.7 Therefore, evidence supports ABPM as the reference standard for confirming elevated office BP screening results to avoid misdiagnosis and overtreatment of individuals with isolated clinic hypertension.7
How office measurements stack up against ABPM
Checking the validity of decisions in clinical practice is extremely important for patient management. One of the tools used for decision-making is an estimate of the LR. We used the LR to assess the value of office BP measurement in determining controlled or uncontrolled BP. A high LR (eg, >10) indicates that the office BP can be used to rule in the disease (uncontrolled BP) with a high probability, while a low LR (eg, <0.1) could rule it out. An LR of around one indicates that the office BP measurement cannot rule the diagnosis of uncontrolled BP in or out.27 In our meta-analysis, the positive LR is 1.35 and negative LR is 0.44. Therefore, in treated hypertensive patients, an indication of uncontrolled BP as measured in the clinic does not confirm a diagnosis of uncontrolled BP (as judged by the reference standard of ABPM). On the other hand, the negative LR means that normal office BP does not rule out uncontrolled BP, which may be detected with ABPM. Consequently, the measurement of BP in the office does not change the degree of (un)certainty of adequate control of BP. This knowledge is important, to avoid overtreatment of white coat hypertension and undertreatment of masked cases.
As previously mentioned, we reported similar results in a study designed to determine the validity of office BP measurement in a primary care setting compared with ABPM.13 In that paper, the level of agreement between both methods was poor, indicating that clinic measurements could not be recommended as a single method of BP control in hypertensive patients.
The use of ABPM in diagnosing hypertension is likely to increase as a consequence of some guideline updates.2 Our study emphasizes the importance of their use in the control of hypertensive patients.
Another published meta-analysis1 investigated the validity of office BP for the diagnosis of hypertension in untreated patients, with diagnostic thresholds for arterial hypertension set at 140/90 mm Hg for office measurement, and 135/85 mm Hg for ABPM. In that paper, the sensitivity of office BP was 74.6% (95% CI, 60.7-84.8) and the specificity was 74.6% (95% CI, 47.9-90.4).
In our present study carried out with hypertensive patients receiving treatment, we obtained a slightly higher sensitivity value of 81.9% (within the CI of this meta-analysis) and a lower specificity of 41.1%. Therefore, the discordance between office BP and ABPM seems to be similar for the diagnosis of hypertension and the classification of hypertension as being well or poorly controlled. This confirms the low validity of the office BP, both for diagnosis and monitoring of hypertensive patients.
Strengths of our study. The study focused on (treated) hypertensive patients in a primary care setting, where hypertension is most often managed. It confirms that ABPM is indispensable to a good clinical practice.
Limitations of our study are those inherent to meta-analyses. The main weakness of our study is the paucity of data available regarding the utility of ABPM for monitoring BP control with treatment in a primary care setting. Other limitations are the variability in BP thresholds used, the number of measurements performed, and the ambulatory BP devices used. These differences could contribute to the observed heterogeneity.
Application of our results must take into account that we included only those studies performed in a primary care setting with treated hypertensive patients.
Moreover, this study was not designed to evaluate the consequences of over- and undertreatment of blood pressure, nor to address the accuracy of automated blood pressure machines or newer health and fitness devices.
Implications for practice, policy, or future research. Alternative monitoring methods are home BP self-measurement and automated 30-minute clinic BP measurement.28 However, ABPM provides us with unique information about the BP pattern (dipping or non-dipping), BP variability, and mean nighttime BP. This paper establishes that the measurement of BP in the office is not an accurate method to monitor BP control. ABPM should be incorporated in usual clinical practice in primary care. Although the consequences of ambulatory monitoring are not the focus of this study, we acknowledge that the decision to incorporate ABPM in clinical practice depends on the availability of ambulatory devices, proper training of health care workers, and a cost-effectiveness analysis of its use.
CORRESPONDENCE
Sergio Reino-González, MD, PhD, Adormideras Primary Health Center, Poligono de Adormideras s/n. 15002 A Coruña, Spain; [email protected].
1. Hodgkinson J, Mant J, Martin U, et al. Relative effectiveness of clinic and home blood pressure monitoring compared with ambulatory blood pressure monitoring in diagnosis of hypertension: systematic review. BMJ. 2011;342:d3621.
2. National Institute for Health and Clinical Excellence. Hypertension in adults: diagnosis and management. Available at: http://www.nice.org.uk/guidance/CG127. Accessed November 15, 2016.
3. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.
4. Hermida RC, Smolensky MH, Ayala DE, et al. Ambulatory Blood Pressure Monitoring (ABPM) as the reference standard for diagnosis of hypertension and assessment of vascular risk in adults. Chronobiol Int. 2015;32:1329-1342.
5. Siu AL; U.S. Preventive Services Task Force. Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163:778-786.
6. Piper MA, Evans CV
7. American Academy of Family Physicians. Hypertension. Available at: www.aafp.org/patient-care/clinical-recommendations/all/hypertension.html. Accessed February 10, 2016.
8. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Practice Guidelines for the Management of Arterial Hypertension. Blood Press. 2013;23:3-16.
9. Marin R, de la Sierra A, Armario P, et al. 2005 Spanish guidelines in diagnosis and treatment of arterial hypertension. Medicina Clínica. 2005;125:24-34.
10. Fagard RH, Celis H, Thijs L, et al. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008;51:55-61.
11. Sega R, Trocino G, Lanzarotti A, et al. Alterations of cardiac structure in patients with isolated office, ambulatory, or home hypertension: Data from the general population (Pressione Arteriose Monitorate E Loro Associazioni [PAMELA] Study). Circulation. 2001;104:1385-1392.
12. Verberk WJ, Kessels AG, de Leeuw PW. Prevalence, causes, and consequences of masked hypertension: a meta-analysis. Am J Hypertens. 2008;21:969-975.
13. Reino-González S, Pita-Fernández S, Cibiriain-Sola M, et al. Validity of clinic blood pressure compared to ambulatory monitoring in hypertensive patients in a primary care setting. Blood Press. 2015;24:111-118.
14. Doebler P, Holling H. Meta-analysis of diagnostic accuracy with mada. Available at: https://cran.r-project.org/web/packages/mada/vignettes/mada.pdf. Accessed October 5, 2015.
15. Myers MG, Oh PI, Reeves RA, et al. Prevalence of white coat effect in treated hypertensive patients in the community. Am J Hypertens. 1995;8:591-597.
16. Imai Y, Tsuji I, Nagai K, et al. Ambulatory blood pressure monitoring in evaluating the prevalence of hypertension in adults in Ohasama, a rural Japanese community. Hypertens Res. 1996;19:207-212.
17. Taylor RS, Stockman J, Kernick D, et al. Ambulatory blood pressure monitoring for hypertension in general practice. J R Soc Med. 1998;91:301-304.
18. Little P, Barnett J, Barnsley L, et al. Comparison of agreement between different measures of blood pressure in primary care and daytime ambulatory blood pressure. BMJ. 2002;325:254.
19. Bur A, Herkner H, Vlcek M, et al. Classification of blood pressure levels by ambulatory blood pressure in hypertension. Hypertension. 2002;40:817-822.
20. Lindbaek M, Sandvik E, Liodden K, et al. Predictors for the white coat effect in general practice patients with suspected and treated hypertension. Br J Gen Pract. 2003;53:790-793.
21. Martínez MA, Sancho T, García P, et al. Home blood pressure in poorly controlled hypertension: relationship with ambulatory blood pressure and organ damage. Blood Press Monit. 2006;11:207-213.
22. Sierra BC, de la Sierra IA, Sobrino J, et al. Monitorización ambulatoria de la presión arterial (MAPA): características clínicas de 31.530 pacientes. Medicina Clínica. 2007;129:1-5.
23. Gómez MA, García L, Sánchez Á, et al. Agreement and disagreement between different methods of measuring blood pressure. Hipertensión (Madr). 2008;25:231-239.
24. Banegas JR, Segura J, De la Sierra A, et al. Gender differences in office and ambulatory control of hypertension. Am J Med. 2008;121:1078-1084.
25. Zaninelli A, Parati G, Cricelli C, et al. Office and 24-h ambulatory blood pressure control by treatment in general practice: the ‘Monitoraggio della pressione ARteriosa nella medicina TErritoriale’ study. J Hypertens. 2010;28:910-917.
26. Llisterri JL, Morillas P, Pallarés V, et al. Differences in the degree of control of arterial hypertension according to the measurement procedure of blood pressure in patients ≥ 65 years. FAPRES study. Rev Clin Esp. 2011;211:76-84.
27. Straus SE, Richardson WS, Glasziou P, et al. Evidence-Based Medicine: How to practice and teach it. 4th ed. Edinburgh, Scotland: Churchill Livingstone; 2010.
28. Van der Wel MC, Buunk IE, van Weel C, et al. A novel approach to office blood pressure measurement: 30-minute office blood pressure vs daytime ambulatory blood pressure. Ann Fam Med. 2011;9:128-135.
1. Hodgkinson J, Mant J, Martin U, et al. Relative effectiveness of clinic and home blood pressure monitoring compared with ambulatory blood pressure monitoring in diagnosis of hypertension: systematic review. BMJ. 2011;342:d3621.
2. National Institute for Health and Clinical Excellence. Hypertension in adults: diagnosis and management. Available at: http://www.nice.org.uk/guidance/CG127. Accessed November 15, 2016.
3. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252.
4. Hermida RC, Smolensky MH, Ayala DE, et al. Ambulatory Blood Pressure Monitoring (ABPM) as the reference standard for diagnosis of hypertension and assessment of vascular risk in adults. Chronobiol Int. 2015;32:1329-1342.
5. Siu AL; U.S. Preventive Services Task Force. Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163:778-786.
6. Piper MA, Evans CV
7. American Academy of Family Physicians. Hypertension. Available at: www.aafp.org/patient-care/clinical-recommendations/all/hypertension.html. Accessed February 10, 2016.
8. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Practice Guidelines for the Management of Arterial Hypertension. Blood Press. 2013;23:3-16.
9. Marin R, de la Sierra A, Armario P, et al. 2005 Spanish guidelines in diagnosis and treatment of arterial hypertension. Medicina Clínica. 2005;125:24-34.
10. Fagard RH, Celis H, Thijs L, et al. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension. 2008;51:55-61.
11. Sega R, Trocino G, Lanzarotti A, et al. Alterations of cardiac structure in patients with isolated office, ambulatory, or home hypertension: Data from the general population (Pressione Arteriose Monitorate E Loro Associazioni [PAMELA] Study). Circulation. 2001;104:1385-1392.
12. Verberk WJ, Kessels AG, de Leeuw PW. Prevalence, causes, and consequences of masked hypertension: a meta-analysis. Am J Hypertens. 2008;21:969-975.
13. Reino-González S, Pita-Fernández S, Cibiriain-Sola M, et al. Validity of clinic blood pressure compared to ambulatory monitoring in hypertensive patients in a primary care setting. Blood Press. 2015;24:111-118.
14. Doebler P, Holling H. Meta-analysis of diagnostic accuracy with mada. Available at: https://cran.r-project.org/web/packages/mada/vignettes/mada.pdf. Accessed October 5, 2015.
15. Myers MG, Oh PI, Reeves RA, et al. Prevalence of white coat effect in treated hypertensive patients in the community. Am J Hypertens. 1995;8:591-597.
16. Imai Y, Tsuji I, Nagai K, et al. Ambulatory blood pressure monitoring in evaluating the prevalence of hypertension in adults in Ohasama, a rural Japanese community. Hypertens Res. 1996;19:207-212.
17. Taylor RS, Stockman J, Kernick D, et al. Ambulatory blood pressure monitoring for hypertension in general practice. J R Soc Med. 1998;91:301-304.
18. Little P, Barnett J, Barnsley L, et al. Comparison of agreement between different measures of blood pressure in primary care and daytime ambulatory blood pressure. BMJ. 2002;325:254.
19. Bur A, Herkner H, Vlcek M, et al. Classification of blood pressure levels by ambulatory blood pressure in hypertension. Hypertension. 2002;40:817-822.
20. Lindbaek M, Sandvik E, Liodden K, et al. Predictors for the white coat effect in general practice patients with suspected and treated hypertension. Br J Gen Pract. 2003;53:790-793.
21. Martínez MA, Sancho T, García P, et al. Home blood pressure in poorly controlled hypertension: relationship with ambulatory blood pressure and organ damage. Blood Press Monit. 2006;11:207-213.
22. Sierra BC, de la Sierra IA, Sobrino J, et al. Monitorización ambulatoria de la presión arterial (MAPA): características clínicas de 31.530 pacientes. Medicina Clínica. 2007;129:1-5.
23. Gómez MA, García L, Sánchez Á, et al. Agreement and disagreement between different methods of measuring blood pressure. Hipertensión (Madr). 2008;25:231-239.
24. Banegas JR, Segura J, De la Sierra A, et al. Gender differences in office and ambulatory control of hypertension. Am J Med. 2008;121:1078-1084.
25. Zaninelli A, Parati G, Cricelli C, et al. Office and 24-h ambulatory blood pressure control by treatment in general practice: the ‘Monitoraggio della pressione ARteriosa nella medicina TErritoriale’ study. J Hypertens. 2010;28:910-917.
26. Llisterri JL, Morillas P, Pallarés V, et al. Differences in the degree of control of arterial hypertension according to the measurement procedure of blood pressure in patients ≥ 65 years. FAPRES study. Rev Clin Esp. 2011;211:76-84.
27. Straus SE, Richardson WS, Glasziou P, et al. Evidence-Based Medicine: How to practice and teach it. 4th ed. Edinburgh, Scotland: Churchill Livingstone; 2010.
28. Van der Wel MC, Buunk IE, van Weel C, et al. A novel approach to office blood pressure measurement: 30-minute office blood pressure vs daytime ambulatory blood pressure. Ann Fam Med. 2011;9:128-135.
Is diabetes distress on your radar screen?
Managing diabetes is a complex undertaking, with an extensive regimen of self-care—including regular exercise, meal planning, blood glucose monitoring, medication scheduling, and multiple visits—that is critically linked to glycemic control and the prevention of complications. Incorporating all of these elements into daily life can be daunting.1-3
In fact, nearly half of US adults with diabetes fail to meet the recommended targets.4 This leads to frustration, which often manifests in psychosocial problems that further hamper efforts to manage the disease.5-10 The most notable is a psychosocial disorder known as diabetes distress, which affects close to 45% of those with diabetes.11,12
It is important to note that diabetes distress is not a psychiatric disorder;13 rather, it is a broad affective reaction to the stress of living with this chronic and complex disease.14,15 By negatively affecting adherence to a self-care regimen, diabetes distress contributes to worsening glycemic control and increasing morbidity.16-18
Recognizing that about 80% of those with diabetes are treated in primary care settings,19 we wrote this review to call your attention to diabetes distress, alert you to brief screening tools that can easily be incorporated into clinic visits, and offer guidance in matching proposed interventions to the aspects of diabetes self-management that cause patients the greatest distress.
Diabetes distress: What it is, what it’s not
For patients with type 2 diabetes, diabetes distress centers around 4 main issues:
- frustration with the demands of self-care;
- apprehension about the future and the possibility of developing serious complications;
- concern about both the quality and the cost of required medical care; and
- perceived lack of support from family and/or friends.11,12,20
As mentioned earlier, diabetes distress is not a psychiatric condition and should not be confused with major depressive disorder (MDD). Here’s help in telling the difference.
For starters, a diagnosis of depression is symptom-based.13 MDD requires the presence of at least 5 of the 9 symptoms defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth ed. (DSM-5)—eg, persistent feelings of worthlessness or guilt, sleep disturbances, lack of interest in normal activities—for at least 2 weeks.21 What’s more, the diagnostic criteria for MDD do not specify a cause or disease process. Nor do they distinguish between a pathological response and an expected reaction to a stressful life event.22 Further, depression measures reflect symptoms (eg, hyperglycemia), as well as stressful experiences resulting from diabetes self-care, which may contribute to the high rate of false positives or incorrect diagnoses of MDD and missed diagnoses of diabetes distress.23
Unlike MDD, diabetes distress has a specific cause—diabetes—and can best be understood as an emotional response to a demanding health condition.13 And, because the source of the problem is identified, diabetes distress can be treated with specific interventions targeting the areas causing the highest levels of stress.
When a psychiatric condition and diabetes distress overlap
MDD, anxiety disorders, and diabetes distress are all common in patients with diabetes,24 and the co-occurrence of a psychiatric disorder and diabetes distress is high.25 Thus, it is important not only to identify cases of diabetes distress but also to consider comorbid depression and/or anxiety in patients with diabetes distress.
More often, though, it is the other way around, according to the Distress and Depression in Diabetes (3D) study. The researchers recently found that 84% of patients with moderate or high diabetes distress did not fulfill the criteria for MDD, but that 67% of diabetes patients with MDD also had moderate or high diabetes distress.13,15,17,25
The data highlight the importance of screening patients with a dual diagnosis of diabetes and MDD for diabetes distress. Keep in mind that individuals diagnosed with both diabetes distress and a comorbid psychiatric condition may require more complex and intensive treatment than those with either diabetes distress or MDD alone.25
Screening for diabetes distress
Diabetes distress can be easily assessed using one of several patient-reported outcome measures. Six validated measures, ranging in length from one to 28 questions, are designed for use in primary care (TABLE).26-30 Some of the measures are easily accessible online; others require subscription to MEDLINE.
Problem Areas in Diabetes (PAID): There are 3 versions of PAID—a 20-item screen assessing a broad range of feelings related to living with diabetes and its treatment, a 5-item version (PAID-5) with high rates of sensitivity (95%) and specificity (89%), and a single-item test (PAID-1) that is highly correlated with the longer version.26,27
Diabetes Distress Scale (DDS): This tool is available in a 17-item measure assessing diabetes distress as it relates to the emotional burden, physician-related distress, regimen-related distress, and interpersonal distress.28 DDS is also available in a short form (DDS-2) with 2 items29 and a 28-item scale specifically for patients with type 1 diabetes.30 T1-DDS, the only diabetes distress measure focused on this particular patient population, assesses the 7 sources of distress found to be common among adults with type 1 diabetes: powerlessness, negative social perceptions, physician distress, friend/family distress, hypoglycemia distress, management distress, and eating distress.
Studies have shown that not only do those with type 1 diabetes experience different stressors compared with their type 2 counterparts, but that they tend to experience distress differently. For patients with type 1 diabetes, for example, powerlessness ranked as the highest source of distress, followed by eating distress and hypoglycemia distress. These sources of distress differ from the regimen distress, emotional burden, interpersonal distress, and physician distress identified by those with type 2 diabetes.30
How to respond to diabetes distress
Diabetes distress is easier to identify than to successfully treat. Few validated treatments for diabetes distress exist and, to our knowledge, only 2 studies have assessed interventions aimed at reduction of such distress.31,32
The REDEEM trial31 recruited adults with type 2 diabetes and diabetes distress to participate in a 12-month randomized controlled trial (RCT). The trial had 3 arms, comparing the effectiveness of a computer-assisted self-management (CASM) program alone, a CASM program plus in-person diabetes distress-specific problem-solving therapy, and a computer-assisted minimally supportive intervention. The main outcomes included diabetes distress (using the DDS scale and subscales), along with self-management behaviors and HbA1c.
Participants in all 3 arms showed significant reductions in total diabetes distress and improvements in self-management behaviors, with no significant differences among the groups. No differences in HbA1c were found. However, those in the CASM program plus distress-specific therapy arm showed a larger reduction in regimen distress compared with the other 2 groups.31
The DIAMOS trial32 recruited adults who had type 1 or type 2 diabetes, diabetes distress, and subclinical depressive symptoms for a 2-arm RCT. One group underwent cognitive behavioral interventions, while the controls had standard group-based diabetes education. The main outcomes included diabetes distress (measured via the PAID scale), depressive symptoms, well-being, diabetes self-care, diabetes acceptance, satisfaction with diabetes treatment, HbA1c, and subclinical inflammation.
The intervention group showed greater improvement in diabetes distress and depressive symptoms compared with the control group, but no differences in well-being, self-care, treatment satisfaction, HbA1c, or subclinical inflammation were observed.32
Both studies support the use of problem-solving therapy and cognitive behavioral interventions for patients with diabetes distress. Future research should evaluate the effectiveness of these interventions in the primary care setting.
What else to offer when challenges mount?
Diabetes is a progressive disease, and most patients experience multiple challenges over time. These typically include complications and comorbidities, physical limitations, polypharmacy, hypoglycemia, and cognitive impairment, as well as changes in everything from medication and lifestyle to insurance coverage and social support.33,34 All increase the risk for diabetes distress, as well as related psychiatric conditions.
Aging and diabetes are independent risk factors for cognitive impairment, for example, and the presence of both increases this risk.35 What’s more, diabetes alone is associated with poorer executive function,36-38 the higher-level cognitive processes that allow individuals to engage in independent, purposeful, and flexible goal-related behaviors. Both poor cognitive function and impaired executive function interfere with the ability to perform self-care behaviors such as adjusting insulin doses, drawing insulin into a syringe, or dialing an insulin dose with an insulin pen.39 This in turn can lead to frustration and increase the likelihood of moderate to high diabetes distress.
Assessing diabetes distress in patients with cognitive impairment, poor executive functioning, or other psychological limitations is particularly difficult, however, as no diabetes distress measures take such deficits into account. Thus, primary care physicians without expertise in neuropsychology should consider referring patients with such problems to specialists for assessment.
The progressive nature of diabetes also highlights the need for primary care physicians to periodically screen for diabetes distress and engage in ongoing discussions about what type of care is best for individual patients, and why. When developing or updating treatment plans and making recommendations, it is crucial to consider the impact the treatment would likely have on the patient’s physical and mental health and to explicitly inquire about and acknowledge his or her values and preferences for care.40-44
It is also important to remain aware of socioeconomic changes—in employment, insurance coverage, and living situations, for example—which are not addressed in the screening tools.
Moderate to high diabetes distress scores, as well as individual items patients identify as “very serious” problems, represent clinical red flags that should be the focus of careful discussion during a medical visit. Patients with moderate to high distress should be referred to a therapist trained in cognitive behavioral therapy or problem-solving therapy. Physicians who lack access to such resources can incorporate cognitive behavioral and problem-solving techniques into patient discussion. (See “Directing help where it’s most needed.”) All patients should be referred to a certified diabetes educator—a key component of diabetes care.45,46
SIDEBAR
Directing help where it's most needed
CASE 1 ›
Conduct a behavioral experiment
Fred J, a 67-year-old diagnosed with type 2 diabetes 6 years ago, comes in for a diabetes check-up. He is a new patient who recently retired from his job as a contractor and was referred by a colleague. In response to a question about his diabetes management, Mr. J tells you he’s having a hard time.
“I get down on myself,” the patient says. “I take my medications every day at the exact same time, but when I test my sugar, it’s 260 or 280. I know I did this to myself. If only I weighed less, ate better, or exercised more.”
At other times, “I think, 'Why bother?'” Mr. J adds. “I feel like there’s nothing I can do to make it better.”
The DDS-2 screen you gave Mr. J bears out his high level of distress and his fear of complications. He tells you about an aunt who “had diabetes like me and had to go on dialysis, then died 2 years later.” When you ask what he fears most, Mr. J says he worries about kidney failure. “I don’t want to go on dialysis,” he insists.
You take the opportunity to point out that nephropathy is not inevitable and that he can perform self-care behaviors now that will prevent or delay kidney complications.
You also decide to try a cognitive behavioral technique in an attempt to change his thought process. You ask Mr. J to agree to a week-long behavioral experiment to examine the effect of walking for 30 minutes each day.
He agrees. You advise him to write down his predictions before he begins the experiment and then to keep a log, checking and recording his glucose levels before and after each walk. You schedule a follow-up visit to discuss the results, hoping that a reduction in blood glucose levels will convince Mr. J that exercise is beneficial to his diabetes.
CASE 2 ›
Identify the problem; brainstorm with the patient
Susan T, a 46-year-old with a husband and 2 teenage children, comes in for her 3-month diabetes check-up. At her last visit, she expressed concerns about her family’s lack of cooperation as she struggled to change her diet. This time, she appears frustrated and distraught.
Your nurse administered the PAID-5 while Ms. T was in the waiting room and entered her score—8, indicating high diabetes distress—in the electronic medical record. You ask Ms. T what’s happening, knowing that encouraging her to verbalize her feelings is a way to increase her trust and help alleviate her concerns.
You also try the following problem-solving technique:
Define the problem. Ms. T is having a hard time maintaining a healthy diet. Her husband and children refuse to eat the healthy meals she prepares and want her to cook separate dinners for them.
Identify challenges. The patient works full-time and does not have the time or energy to cook separate meals. In addition, she is upset by her family’s lack of support in her efforts to control her disease.
Brainstorm multiple solutions:
1) Ms. T can prepare all of her own meals for the work week on Sunday, then cook for the others when she returns from work.
2) Her husband and children can make their own dinner if they do not want to eat the healthier meals she prepares.
3) The patient can join a diabetes support group where she will meet, and possibly learn from, other patients who may be struggling with diabetes self-care.
4) Ms. T can ask her husband and children to come to her next diabetes check-up so they can learn about the importance of family support in diabetes management directly from you.
5) The patient’s family can receive information about a healthy diabetes diet from a certified diabetes educator.
Decide on appropriate solutions. The patient agrees to try and prepare her weekday meals on Sunday so that she is not tempted to eat less healthy options. She also agrees to bring her family to her next diabetes check-up and to diabetes education classes.
CORRESPONDENCE
Elizabeth A. Beverly, PhD, Department of Family Medicine, Ohio University Heritage College of Osteopathic Medicine, 35 W. Green Drive, Athens, OH 45701; [email protected].
1. Gafarian CT, Heiby EM, Blair P, et al. The diabetes time management questionnaire. Diabetes Educator. 1999;25:585-592.
2. Wdowik MJ, Kendall PA, Harris MA. College students with diabetes: using focus groups and interviews to determine psychosocial issues and barriers to control. Diabetes Educator. 1997;23:558-562.
3. Rubin RR. Psychological issues and treatment for people with diabetes. J Clin Psych. 2001;57:457-478.
4. Ali MK, Bullard KM, Gregg EW. Achievement of goals in US diabetes care, 1999-2010. New Engl J Med. 2013;369:287-288.
5. Lloyd CE, Smith J, Weinger K. Stress and diabetes: Review of the links. Diabetes Spectrum. 2005;18:121-127.
6. Weinger K. Psychosocial issues and self-care. Am J Nurs. 2007;107(6 suppl): S34-S38.
7. Weinger K, Jacobson AM. Psychosocial and quality of life correlates of glycemic control during intensive treatment of type 1 diabetes. Patient Education Counseling. 2001;42:123-131.
8. Albright TL, Parchman M, Burge SK. Predictors of self-care behavior in adults with type 2 diabetes: an RRNeST study. Fam Med. 2001;33:354-360.
9. Gonzalez JS, Safren SA, Cagliero E, et al. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care. 2007;30:2222-2227.
10. Gonzalez JS, Safren SA, Delahanty LM, et al. Symptoms of depression prospectively predict poorer self-care in patients with Type 2 diabetes. Diabetic Med. 2008;25:1102-1107.
11. Nicolucci A, Kovacs Burns K, Holt RI, et al. Diabetes Attitudes, Wishes and Needs second study (DAWN2): cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes. Diabetic Med. 2013;30:767-777.
12. Fisher L, Hessler DM, Polonsky W, et al. When is diabetes distress clinically meaningful?: establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35:259-264.
13. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabetic Med. 2014;31:764-772.
14. Fisher L, Mullan JT, Skaff MM, et al. Predicting diabetes distress in patients with Type 2 diabetes: a longitudinal study. Diabetic Med. 2009;26:622-627.
15. Fisher L, Skaff MM, Mullan JT, et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care. 2007;30:542-548.
16. Gonzalez JS, Delahanty LM, Safren SA, et al. Differentiating symptoms of depression from diabetes-specific distress: relationships with self-care in type 2 diabetes. Diabetologia. 2008;51:2822-1825.
17. Fisher L, Mullan JT, Arean P, et al. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33:23-28.
18. Fisher EB, Thorpe CT, Devellis BM, et al. Healthy coping, negative emotions, and diabetes management: a systematic review and appraisal. Diabetes Educator. 2007;33:1080-1103; 1104-1086.
19. Peterson KA, Radosevich DM, O’Connor PJ, et al. Improving diabetes care in practice: findings from the TRANSLATE trial. Diabetes Care. 2008;31:2238-2243.
20. Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care. 2010;33:1034-1036.
21. Cole J, McGuffin P, Farmer AE. The classification of depression: are we still confused? Br J Psychiatr. 2008;192:83-85.
22. Wakefield JC. The concept of mental disorder. On the boundary between biological facts and social values. Am Psychologist. 1992;47:373-388.
23. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabetic Med. 2014;31:764-772.
24. Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278-3285.
25. Fisher L, Skaff MM, Mullan JT, et al. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with Type 2 diabetes. Diabetic Med. 2008;25:1096-1101.
26. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18:754-760.
27. McGuire BE, Morrison TG, Hermanns N, et al. Short-form measures of diabetes-related emotional distress: the Problem Areas in Diabetes Scale (PAID)-5 and PAID-1. Diabetologia. 2010;53:66-69.
28. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28:626-631.
29. Fisher L, Glasgow RE, Mullan JT, et al. Development of a brief diabetes distress screening instrument. Ann Fam Med. 2008;6:246-252.
30. Fisher L, Polonsky WH, Hessler DM, et al. Understanding the sources of diabetes distress in adults with type 1 diabetes. J Diabetes Complications. 2015;29:572-577.
31. Fisher L, Hessler D, Glasgow RE, et al. REDEEM: a pragmatic trial to reduce diabetes distress. Diabetes Care. 2013;36:2551-2558.
32. Hermanns N, Schmitt A, Gahr A, et al. The effect of a Diabetes-Specific Cognitive Behavioral Treatment Program (DIAMOS) for patients with diabetes and subclinical depression: results of a randomized controlled trial. Diabetes Care. 2015;38:551-560.
33. Weinger K, Beverly EA, Smaldone A. Diabetes self-care and the older adult. Western J Nurs Res. 2014;36:1272-1298.
34. Beverly EA, Ritholz MD, Shepherd C, et al. The psychosocial challenges and care of older adults with diabetes: “can’t do what I used to do; can’t be who I once was.” Curr Diabetes Rep. 2016;16:48.
35. Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PloS One. 2009;4:e4144.
36. Thabit H, Kyaw TT, McDermott J, et al. Executive function and diabetes mellitus—a stone left unturned? Curr Diabetes Rev. 2012;8:109-115.
37. McNally K, Rohan J, Pendley JS, et al. Executive functioning, treatment adherence, and glycemic control in children with type 1 diabetes. Diabetes Care. 2010;33:1159-1162.
38. Rucker JL, McDowd JM, Kluding PM. Executive function and type 2 diabetes: putting the pieces together. Phys Ther. 2012;92:454-462.
39. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
40. Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA. 2006;295:1935-1940.
41. Oftedal B, Karlsen B, Bru E. Life values and self-regulation behaviours among adults with type 2 diabetes. J Clin Nurs. 2010;19:2548-2556.
42. Morrow AS, Haidet P, Skinner J, et al. Integrating diabetes self-management with the health goals of older adults: a qualitative exploration. Patient Education Counseling. 2008;72:418-423.
43. Huang ES, Gorawara-Bhat R, Chin MH. Self-reported goals of older patients with type 2 diabetes mellitus. J Am Geriatr Soc. 2005;53:306-311.
44. Beverly EA, Wray LA, LaCoe CL, et al. Listening to older adults’ values and preferences for Type 2 diabetes care: a qualitative study. Diabetes Spectrum. 2014;27:44-49.
45. American Association of Diabetes Educators. Why refer for diabetes education? American Association of Diabetes Educators. Available at: https://www.diabeteseducator.org/practice/provider-resources/why-refer-for-diabetes-education. Accessed August 15, 2016.
46. Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet. 2004;363:1589-1597.
Managing diabetes is a complex undertaking, with an extensive regimen of self-care—including regular exercise, meal planning, blood glucose monitoring, medication scheduling, and multiple visits—that is critically linked to glycemic control and the prevention of complications. Incorporating all of these elements into daily life can be daunting.1-3
In fact, nearly half of US adults with diabetes fail to meet the recommended targets.4 This leads to frustration, which often manifests in psychosocial problems that further hamper efforts to manage the disease.5-10 The most notable is a psychosocial disorder known as diabetes distress, which affects close to 45% of those with diabetes.11,12
It is important to note that diabetes distress is not a psychiatric disorder;13 rather, it is a broad affective reaction to the stress of living with this chronic and complex disease.14,15 By negatively affecting adherence to a self-care regimen, diabetes distress contributes to worsening glycemic control and increasing morbidity.16-18
Recognizing that about 80% of those with diabetes are treated in primary care settings,19 we wrote this review to call your attention to diabetes distress, alert you to brief screening tools that can easily be incorporated into clinic visits, and offer guidance in matching proposed interventions to the aspects of diabetes self-management that cause patients the greatest distress.
Diabetes distress: What it is, what it’s not
For patients with type 2 diabetes, diabetes distress centers around 4 main issues:
- frustration with the demands of self-care;
- apprehension about the future and the possibility of developing serious complications;
- concern about both the quality and the cost of required medical care; and
- perceived lack of support from family and/or friends.11,12,20
As mentioned earlier, diabetes distress is not a psychiatric condition and should not be confused with major depressive disorder (MDD). Here’s help in telling the difference.
For starters, a diagnosis of depression is symptom-based.13 MDD requires the presence of at least 5 of the 9 symptoms defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth ed. (DSM-5)—eg, persistent feelings of worthlessness or guilt, sleep disturbances, lack of interest in normal activities—for at least 2 weeks.21 What’s more, the diagnostic criteria for MDD do not specify a cause or disease process. Nor do they distinguish between a pathological response and an expected reaction to a stressful life event.22 Further, depression measures reflect symptoms (eg, hyperglycemia), as well as stressful experiences resulting from diabetes self-care, which may contribute to the high rate of false positives or incorrect diagnoses of MDD and missed diagnoses of diabetes distress.23
Unlike MDD, diabetes distress has a specific cause—diabetes—and can best be understood as an emotional response to a demanding health condition.13 And, because the source of the problem is identified, diabetes distress can be treated with specific interventions targeting the areas causing the highest levels of stress.
When a psychiatric condition and diabetes distress overlap
MDD, anxiety disorders, and diabetes distress are all common in patients with diabetes,24 and the co-occurrence of a psychiatric disorder and diabetes distress is high.25 Thus, it is important not only to identify cases of diabetes distress but also to consider comorbid depression and/or anxiety in patients with diabetes distress.
More often, though, it is the other way around, according to the Distress and Depression in Diabetes (3D) study. The researchers recently found that 84% of patients with moderate or high diabetes distress did not fulfill the criteria for MDD, but that 67% of diabetes patients with MDD also had moderate or high diabetes distress.13,15,17,25
The data highlight the importance of screening patients with a dual diagnosis of diabetes and MDD for diabetes distress. Keep in mind that individuals diagnosed with both diabetes distress and a comorbid psychiatric condition may require more complex and intensive treatment than those with either diabetes distress or MDD alone.25
Screening for diabetes distress
Diabetes distress can be easily assessed using one of several patient-reported outcome measures. Six validated measures, ranging in length from one to 28 questions, are designed for use in primary care (TABLE).26-30 Some of the measures are easily accessible online; others require subscription to MEDLINE.

Problem Areas in Diabetes (PAID): There are 3 versions of PAID—a 20-item screen assessing a broad range of feelings related to living with diabetes and its treatment, a 5-item version (PAID-5) with high rates of sensitivity (95%) and specificity (89%), and a single-item test (PAID-1) that is highly correlated with the longer version.26,27
Diabetes Distress Scale (DDS): This tool is available in a 17-item measure assessing diabetes distress as it relates to the emotional burden, physician-related distress, regimen-related distress, and interpersonal distress.28 DDS is also available in a short form (DDS-2) with 2 items29 and a 28-item scale specifically for patients with type 1 diabetes.30 T1-DDS, the only diabetes distress measure focused on this particular patient population, assesses the 7 sources of distress found to be common among adults with type 1 diabetes: powerlessness, negative social perceptions, physician distress, friend/family distress, hypoglycemia distress, management distress, and eating distress.
Studies have shown that not only do those with type 1 diabetes experience different stressors compared with their type 2 counterparts, but that they tend to experience distress differently. For patients with type 1 diabetes, for example, powerlessness ranked as the highest source of distress, followed by eating distress and hypoglycemia distress. These sources of distress differ from the regimen distress, emotional burden, interpersonal distress, and physician distress identified by those with type 2 diabetes.30
How to respond to diabetes distress
Diabetes distress is easier to identify than to successfully treat. Few validated treatments for diabetes distress exist and, to our knowledge, only 2 studies have assessed interventions aimed at reduction of such distress.31,32
The REDEEM trial31 recruited adults with type 2 diabetes and diabetes distress to participate in a 12-month randomized controlled trial (RCT). The trial had 3 arms, comparing the effectiveness of a computer-assisted self-management (CASM) program alone, a CASM program plus in-person diabetes distress-specific problem-solving therapy, and a computer-assisted minimally supportive intervention. The main outcomes included diabetes distress (using the DDS scale and subscales), along with self-management behaviors and HbA1c.
Participants in all 3 arms showed significant reductions in total diabetes distress and improvements in self-management behaviors, with no significant differences among the groups. No differences in HbA1c were found. However, those in the CASM program plus distress-specific therapy arm showed a larger reduction in regimen distress compared with the other 2 groups.31
The DIAMOS trial32 recruited adults who had type 1 or type 2 diabetes, diabetes distress, and subclinical depressive symptoms for a 2-arm RCT. One group underwent cognitive behavioral interventions, while the controls had standard group-based diabetes education. The main outcomes included diabetes distress (measured via the PAID scale), depressive symptoms, well-being, diabetes self-care, diabetes acceptance, satisfaction with diabetes treatment, HbA1c, and subclinical inflammation.
The intervention group showed greater improvement in diabetes distress and depressive symptoms compared with the control group, but no differences in well-being, self-care, treatment satisfaction, HbA1c, or subclinical inflammation were observed.32
Both studies support the use of problem-solving therapy and cognitive behavioral interventions for patients with diabetes distress. Future research should evaluate the effectiveness of these interventions in the primary care setting.
What else to offer when challenges mount?
Diabetes is a progressive disease, and most patients experience multiple challenges over time. These typically include complications and comorbidities, physical limitations, polypharmacy, hypoglycemia, and cognitive impairment, as well as changes in everything from medication and lifestyle to insurance coverage and social support.33,34 All increase the risk for diabetes distress, as well as related psychiatric conditions.
Aging and diabetes are independent risk factors for cognitive impairment, for example, and the presence of both increases this risk.35 What’s more, diabetes alone is associated with poorer executive function,36-38 the higher-level cognitive processes that allow individuals to engage in independent, purposeful, and flexible goal-related behaviors. Both poor cognitive function and impaired executive function interfere with the ability to perform self-care behaviors such as adjusting insulin doses, drawing insulin into a syringe, or dialing an insulin dose with an insulin pen.39 This in turn can lead to frustration and increase the likelihood of moderate to high diabetes distress.
Assessing diabetes distress in patients with cognitive impairment, poor executive functioning, or other psychological limitations is particularly difficult, however, as no diabetes distress measures take such deficits into account. Thus, primary care physicians without expertise in neuropsychology should consider referring patients with such problems to specialists for assessment.
The progressive nature of diabetes also highlights the need for primary care physicians to periodically screen for diabetes distress and engage in ongoing discussions about what type of care is best for individual patients, and why. When developing or updating treatment plans and making recommendations, it is crucial to consider the impact the treatment would likely have on the patient’s physical and mental health and to explicitly inquire about and acknowledge his or her values and preferences for care.40-44
It is also important to remain aware of socioeconomic changes—in employment, insurance coverage, and living situations, for example—which are not addressed in the screening tools.
Moderate to high diabetes distress scores, as well as individual items patients identify as “very serious” problems, represent clinical red flags that should be the focus of careful discussion during a medical visit. Patients with moderate to high distress should be referred to a therapist trained in cognitive behavioral therapy or problem-solving therapy. Physicians who lack access to such resources can incorporate cognitive behavioral and problem-solving techniques into patient discussion. (See “Directing help where it’s most needed.”) All patients should be referred to a certified diabetes educator—a key component of diabetes care.45,46
SIDEBAR
Directing help where it's most needed
CASE 1 ›
Conduct a behavioral experiment
Fred J, a 67-year-old diagnosed with type 2 diabetes 6 years ago, comes in for a diabetes check-up. He is a new patient who recently retired from his job as a contractor and was referred by a colleague. In response to a question about his diabetes management, Mr. J tells you he’s having a hard time.
“I get down on myself,” the patient says. “I take my medications every day at the exact same time, but when I test my sugar, it’s 260 or 280. I know I did this to myself. If only I weighed less, ate better, or exercised more.”
At other times, “I think, 'Why bother?'” Mr. J adds. “I feel like there’s nothing I can do to make it better.”
The DDS-2 screen you gave Mr. J bears out his high level of distress and his fear of complications. He tells you about an aunt who “had diabetes like me and had to go on dialysis, then died 2 years later.” When you ask what he fears most, Mr. J says he worries about kidney failure. “I don’t want to go on dialysis,” he insists.
You take the opportunity to point out that nephropathy is not inevitable and that he can perform self-care behaviors now that will prevent or delay kidney complications.
You also decide to try a cognitive behavioral technique in an attempt to change his thought process. You ask Mr. J to agree to a week-long behavioral experiment to examine the effect of walking for 30 minutes each day.
He agrees. You advise him to write down his predictions before he begins the experiment and then to keep a log, checking and recording his glucose levels before and after each walk. You schedule a follow-up visit to discuss the results, hoping that a reduction in blood glucose levels will convince Mr. J that exercise is beneficial to his diabetes.
CASE 2 ›
Identify the problem; brainstorm with the patient
Susan T, a 46-year-old with a husband and 2 teenage children, comes in for her 3-month diabetes check-up. At her last visit, she expressed concerns about her family’s lack of cooperation as she struggled to change her diet. This time, she appears frustrated and distraught.
Your nurse administered the PAID-5 while Ms. T was in the waiting room and entered her score—8, indicating high diabetes distress—in the electronic medical record. You ask Ms. T what’s happening, knowing that encouraging her to verbalize her feelings is a way to increase her trust and help alleviate her concerns.
You also try the following problem-solving technique:
Define the problem. Ms. T is having a hard time maintaining a healthy diet. Her husband and children refuse to eat the healthy meals she prepares and want her to cook separate dinners for them.
Identify challenges. The patient works full-time and does not have the time or energy to cook separate meals. In addition, she is upset by her family’s lack of support in her efforts to control her disease.
Brainstorm multiple solutions:
1) Ms. T can prepare all of her own meals for the work week on Sunday, then cook for the others when she returns from work.
2) Her husband and children can make their own dinner if they do not want to eat the healthier meals she prepares.
3) The patient can join a diabetes support group where she will meet, and possibly learn from, other patients who may be struggling with diabetes self-care.
4) Ms. T can ask her husband and children to come to her next diabetes check-up so they can learn about the importance of family support in diabetes management directly from you.
5) The patient’s family can receive information about a healthy diabetes diet from a certified diabetes educator.
Decide on appropriate solutions. The patient agrees to try and prepare her weekday meals on Sunday so that she is not tempted to eat less healthy options. She also agrees to bring her family to her next diabetes check-up and to diabetes education classes.
CORRESPONDENCE
Elizabeth A. Beverly, PhD, Department of Family Medicine, Ohio University Heritage College of Osteopathic Medicine, 35 W. Green Drive, Athens, OH 45701; [email protected].
Managing diabetes is a complex undertaking, with an extensive regimen of self-care—including regular exercise, meal planning, blood glucose monitoring, medication scheduling, and multiple visits—that is critically linked to glycemic control and the prevention of complications. Incorporating all of these elements into daily life can be daunting.1-3
In fact, nearly half of US adults with diabetes fail to meet the recommended targets.4 This leads to frustration, which often manifests in psychosocial problems that further hamper efforts to manage the disease.5-10 The most notable is a psychosocial disorder known as diabetes distress, which affects close to 45% of those with diabetes.11,12
It is important to note that diabetes distress is not a psychiatric disorder;13 rather, it is a broad affective reaction to the stress of living with this chronic and complex disease.14,15 By negatively affecting adherence to a self-care regimen, diabetes distress contributes to worsening glycemic control and increasing morbidity.16-18
Recognizing that about 80% of those with diabetes are treated in primary care settings,19 we wrote this review to call your attention to diabetes distress, alert you to brief screening tools that can easily be incorporated into clinic visits, and offer guidance in matching proposed interventions to the aspects of diabetes self-management that cause patients the greatest distress.
Diabetes distress: What it is, what it’s not
For patients with type 2 diabetes, diabetes distress centers around 4 main issues:
- frustration with the demands of self-care;
- apprehension about the future and the possibility of developing serious complications;
- concern about both the quality and the cost of required medical care; and
- perceived lack of support from family and/or friends.11,12,20
As mentioned earlier, diabetes distress is not a psychiatric condition and should not be confused with major depressive disorder (MDD). Here’s help in telling the difference.
For starters, a diagnosis of depression is symptom-based.13 MDD requires the presence of at least 5 of the 9 symptoms defined by the Diagnostic and Statistical Manual of Mental Disorders, Fifth ed. (DSM-5)—eg, persistent feelings of worthlessness or guilt, sleep disturbances, lack of interest in normal activities—for at least 2 weeks.21 What’s more, the diagnostic criteria for MDD do not specify a cause or disease process. Nor do they distinguish between a pathological response and an expected reaction to a stressful life event.22 Further, depression measures reflect symptoms (eg, hyperglycemia), as well as stressful experiences resulting from diabetes self-care, which may contribute to the high rate of false positives or incorrect diagnoses of MDD and missed diagnoses of diabetes distress.23
Unlike MDD, diabetes distress has a specific cause—diabetes—and can best be understood as an emotional response to a demanding health condition.13 And, because the source of the problem is identified, diabetes distress can be treated with specific interventions targeting the areas causing the highest levels of stress.
When a psychiatric condition and diabetes distress overlap
MDD, anxiety disorders, and diabetes distress are all common in patients with diabetes,24 and the co-occurrence of a psychiatric disorder and diabetes distress is high.25 Thus, it is important not only to identify cases of diabetes distress but also to consider comorbid depression and/or anxiety in patients with diabetes distress.
More often, though, it is the other way around, according to the Distress and Depression in Diabetes (3D) study. The researchers recently found that 84% of patients with moderate or high diabetes distress did not fulfill the criteria for MDD, but that 67% of diabetes patients with MDD also had moderate or high diabetes distress.13,15,17,25
The data highlight the importance of screening patients with a dual diagnosis of diabetes and MDD for diabetes distress. Keep in mind that individuals diagnosed with both diabetes distress and a comorbid psychiatric condition may require more complex and intensive treatment than those with either diabetes distress or MDD alone.25
Screening for diabetes distress
Diabetes distress can be easily assessed using one of several patient-reported outcome measures. Six validated measures, ranging in length from one to 28 questions, are designed for use in primary care (TABLE).26-30 Some of the measures are easily accessible online; others require subscription to MEDLINE.

Problem Areas in Diabetes (PAID): There are 3 versions of PAID—a 20-item screen assessing a broad range of feelings related to living with diabetes and its treatment, a 5-item version (PAID-5) with high rates of sensitivity (95%) and specificity (89%), and a single-item test (PAID-1) that is highly correlated with the longer version.26,27
Diabetes Distress Scale (DDS): This tool is available in a 17-item measure assessing diabetes distress as it relates to the emotional burden, physician-related distress, regimen-related distress, and interpersonal distress.28 DDS is also available in a short form (DDS-2) with 2 items29 and a 28-item scale specifically for patients with type 1 diabetes.30 T1-DDS, the only diabetes distress measure focused on this particular patient population, assesses the 7 sources of distress found to be common among adults with type 1 diabetes: powerlessness, negative social perceptions, physician distress, friend/family distress, hypoglycemia distress, management distress, and eating distress.
Studies have shown that not only do those with type 1 diabetes experience different stressors compared with their type 2 counterparts, but that they tend to experience distress differently. For patients with type 1 diabetes, for example, powerlessness ranked as the highest source of distress, followed by eating distress and hypoglycemia distress. These sources of distress differ from the regimen distress, emotional burden, interpersonal distress, and physician distress identified by those with type 2 diabetes.30
How to respond to diabetes distress
Diabetes distress is easier to identify than to successfully treat. Few validated treatments for diabetes distress exist and, to our knowledge, only 2 studies have assessed interventions aimed at reduction of such distress.31,32
The REDEEM trial31 recruited adults with type 2 diabetes and diabetes distress to participate in a 12-month randomized controlled trial (RCT). The trial had 3 arms, comparing the effectiveness of a computer-assisted self-management (CASM) program alone, a CASM program plus in-person diabetes distress-specific problem-solving therapy, and a computer-assisted minimally supportive intervention. The main outcomes included diabetes distress (using the DDS scale and subscales), along with self-management behaviors and HbA1c.
Participants in all 3 arms showed significant reductions in total diabetes distress and improvements in self-management behaviors, with no significant differences among the groups. No differences in HbA1c were found. However, those in the CASM program plus distress-specific therapy arm showed a larger reduction in regimen distress compared with the other 2 groups.31
The DIAMOS trial32 recruited adults who had type 1 or type 2 diabetes, diabetes distress, and subclinical depressive symptoms for a 2-arm RCT. One group underwent cognitive behavioral interventions, while the controls had standard group-based diabetes education. The main outcomes included diabetes distress (measured via the PAID scale), depressive symptoms, well-being, diabetes self-care, diabetes acceptance, satisfaction with diabetes treatment, HbA1c, and subclinical inflammation.
The intervention group showed greater improvement in diabetes distress and depressive symptoms compared with the control group, but no differences in well-being, self-care, treatment satisfaction, HbA1c, or subclinical inflammation were observed.32
Both studies support the use of problem-solving therapy and cognitive behavioral interventions for patients with diabetes distress. Future research should evaluate the effectiveness of these interventions in the primary care setting.
What else to offer when challenges mount?
Diabetes is a progressive disease, and most patients experience multiple challenges over time. These typically include complications and comorbidities, physical limitations, polypharmacy, hypoglycemia, and cognitive impairment, as well as changes in everything from medication and lifestyle to insurance coverage and social support.33,34 All increase the risk for diabetes distress, as well as related psychiatric conditions.
Aging and diabetes are independent risk factors for cognitive impairment, for example, and the presence of both increases this risk.35 What’s more, diabetes alone is associated with poorer executive function,36-38 the higher-level cognitive processes that allow individuals to engage in independent, purposeful, and flexible goal-related behaviors. Both poor cognitive function and impaired executive function interfere with the ability to perform self-care behaviors such as adjusting insulin doses, drawing insulin into a syringe, or dialing an insulin dose with an insulin pen.39 This in turn can lead to frustration and increase the likelihood of moderate to high diabetes distress.
Assessing diabetes distress in patients with cognitive impairment, poor executive functioning, or other psychological limitations is particularly difficult, however, as no diabetes distress measures take such deficits into account. Thus, primary care physicians without expertise in neuropsychology should consider referring patients with such problems to specialists for assessment.
The progressive nature of diabetes also highlights the need for primary care physicians to periodically screen for diabetes distress and engage in ongoing discussions about what type of care is best for individual patients, and why. When developing or updating treatment plans and making recommendations, it is crucial to consider the impact the treatment would likely have on the patient’s physical and mental health and to explicitly inquire about and acknowledge his or her values and preferences for care.40-44
It is also important to remain aware of socioeconomic changes—in employment, insurance coverage, and living situations, for example—which are not addressed in the screening tools.
Moderate to high diabetes distress scores, as well as individual items patients identify as “very serious” problems, represent clinical red flags that should be the focus of careful discussion during a medical visit. Patients with moderate to high distress should be referred to a therapist trained in cognitive behavioral therapy or problem-solving therapy. Physicians who lack access to such resources can incorporate cognitive behavioral and problem-solving techniques into patient discussion. (See “Directing help where it’s most needed.”) All patients should be referred to a certified diabetes educator—a key component of diabetes care.45,46
SIDEBAR
Directing help where it's most needed
CASE 1 ›
Conduct a behavioral experiment
Fred J, a 67-year-old diagnosed with type 2 diabetes 6 years ago, comes in for a diabetes check-up. He is a new patient who recently retired from his job as a contractor and was referred by a colleague. In response to a question about his diabetes management, Mr. J tells you he’s having a hard time.
“I get down on myself,” the patient says. “I take my medications every day at the exact same time, but when I test my sugar, it’s 260 or 280. I know I did this to myself. If only I weighed less, ate better, or exercised more.”
At other times, “I think, 'Why bother?'” Mr. J adds. “I feel like there’s nothing I can do to make it better.”
The DDS-2 screen you gave Mr. J bears out his high level of distress and his fear of complications. He tells you about an aunt who “had diabetes like me and had to go on dialysis, then died 2 years later.” When you ask what he fears most, Mr. J says he worries about kidney failure. “I don’t want to go on dialysis,” he insists.
You take the opportunity to point out that nephropathy is not inevitable and that he can perform self-care behaviors now that will prevent or delay kidney complications.
You also decide to try a cognitive behavioral technique in an attempt to change his thought process. You ask Mr. J to agree to a week-long behavioral experiment to examine the effect of walking for 30 minutes each day.
He agrees. You advise him to write down his predictions before he begins the experiment and then to keep a log, checking and recording his glucose levels before and after each walk. You schedule a follow-up visit to discuss the results, hoping that a reduction in blood glucose levels will convince Mr. J that exercise is beneficial to his diabetes.
CASE 2 ›
Identify the problem; brainstorm with the patient
Susan T, a 46-year-old with a husband and 2 teenage children, comes in for her 3-month diabetes check-up. At her last visit, she expressed concerns about her family’s lack of cooperation as she struggled to change her diet. This time, she appears frustrated and distraught.
Your nurse administered the PAID-5 while Ms. T was in the waiting room and entered her score—8, indicating high diabetes distress—in the electronic medical record. You ask Ms. T what’s happening, knowing that encouraging her to verbalize her feelings is a way to increase her trust and help alleviate her concerns.
You also try the following problem-solving technique:
Define the problem. Ms. T is having a hard time maintaining a healthy diet. Her husband and children refuse to eat the healthy meals she prepares and want her to cook separate dinners for them.
Identify challenges. The patient works full-time and does not have the time or energy to cook separate meals. In addition, she is upset by her family’s lack of support in her efforts to control her disease.
Brainstorm multiple solutions:
1) Ms. T can prepare all of her own meals for the work week on Sunday, then cook for the others when she returns from work.
2) Her husband and children can make their own dinner if they do not want to eat the healthier meals she prepares.
3) The patient can join a diabetes support group where she will meet, and possibly learn from, other patients who may be struggling with diabetes self-care.
4) Ms. T can ask her husband and children to come to her next diabetes check-up so they can learn about the importance of family support in diabetes management directly from you.
5) The patient’s family can receive information about a healthy diabetes diet from a certified diabetes educator.
Decide on appropriate solutions. The patient agrees to try and prepare her weekday meals on Sunday so that she is not tempted to eat less healthy options. She also agrees to bring her family to her next diabetes check-up and to diabetes education classes.
CORRESPONDENCE
Elizabeth A. Beverly, PhD, Department of Family Medicine, Ohio University Heritage College of Osteopathic Medicine, 35 W. Green Drive, Athens, OH 45701; [email protected].
1. Gafarian CT, Heiby EM, Blair P, et al. The diabetes time management questionnaire. Diabetes Educator. 1999;25:585-592.
2. Wdowik MJ, Kendall PA, Harris MA. College students with diabetes: using focus groups and interviews to determine psychosocial issues and barriers to control. Diabetes Educator. 1997;23:558-562.
3. Rubin RR. Psychological issues and treatment for people with diabetes. J Clin Psych. 2001;57:457-478.
4. Ali MK, Bullard KM, Gregg EW. Achievement of goals in US diabetes care, 1999-2010. New Engl J Med. 2013;369:287-288.
5. Lloyd CE, Smith J, Weinger K. Stress and diabetes: Review of the links. Diabetes Spectrum. 2005;18:121-127.
6. Weinger K. Psychosocial issues and self-care. Am J Nurs. 2007;107(6 suppl): S34-S38.
7. Weinger K, Jacobson AM. Psychosocial and quality of life correlates of glycemic control during intensive treatment of type 1 diabetes. Patient Education Counseling. 2001;42:123-131.
8. Albright TL, Parchman M, Burge SK. Predictors of self-care behavior in adults with type 2 diabetes: an RRNeST study. Fam Med. 2001;33:354-360.
9. Gonzalez JS, Safren SA, Cagliero E, et al. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care. 2007;30:2222-2227.
10. Gonzalez JS, Safren SA, Delahanty LM, et al. Symptoms of depression prospectively predict poorer self-care in patients with Type 2 diabetes. Diabetic Med. 2008;25:1102-1107.
11. Nicolucci A, Kovacs Burns K, Holt RI, et al. Diabetes Attitudes, Wishes and Needs second study (DAWN2): cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes. Diabetic Med. 2013;30:767-777.
12. Fisher L, Hessler DM, Polonsky W, et al. When is diabetes distress clinically meaningful?: establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35:259-264.
13. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabetic Med. 2014;31:764-772.
14. Fisher L, Mullan JT, Skaff MM, et al. Predicting diabetes distress in patients with Type 2 diabetes: a longitudinal study. Diabetic Med. 2009;26:622-627.
15. Fisher L, Skaff MM, Mullan JT, et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care. 2007;30:542-548.
16. Gonzalez JS, Delahanty LM, Safren SA, et al. Differentiating symptoms of depression from diabetes-specific distress: relationships with self-care in type 2 diabetes. Diabetologia. 2008;51:2822-1825.
17. Fisher L, Mullan JT, Arean P, et al. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33:23-28.
18. Fisher EB, Thorpe CT, Devellis BM, et al. Healthy coping, negative emotions, and diabetes management: a systematic review and appraisal. Diabetes Educator. 2007;33:1080-1103; 1104-1086.
19. Peterson KA, Radosevich DM, O’Connor PJ, et al. Improving diabetes care in practice: findings from the TRANSLATE trial. Diabetes Care. 2008;31:2238-2243.
20. Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care. 2010;33:1034-1036.
21. Cole J, McGuffin P, Farmer AE. The classification of depression: are we still confused? Br J Psychiatr. 2008;192:83-85.
22. Wakefield JC. The concept of mental disorder. On the boundary between biological facts and social values. Am Psychologist. 1992;47:373-388.
23. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabetic Med. 2014;31:764-772.
24. Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278-3285.
25. Fisher L, Skaff MM, Mullan JT, et al. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with Type 2 diabetes. Diabetic Med. 2008;25:1096-1101.
26. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18:754-760.
27. McGuire BE, Morrison TG, Hermanns N, et al. Short-form measures of diabetes-related emotional distress: the Problem Areas in Diabetes Scale (PAID)-5 and PAID-1. Diabetologia. 2010;53:66-69.
28. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28:626-631.
29. Fisher L, Glasgow RE, Mullan JT, et al. Development of a brief diabetes distress screening instrument. Ann Fam Med. 2008;6:246-252.
30. Fisher L, Polonsky WH, Hessler DM, et al. Understanding the sources of diabetes distress in adults with type 1 diabetes. J Diabetes Complications. 2015;29:572-577.
31. Fisher L, Hessler D, Glasgow RE, et al. REDEEM: a pragmatic trial to reduce diabetes distress. Diabetes Care. 2013;36:2551-2558.
32. Hermanns N, Schmitt A, Gahr A, et al. The effect of a Diabetes-Specific Cognitive Behavioral Treatment Program (DIAMOS) for patients with diabetes and subclinical depression: results of a randomized controlled trial. Diabetes Care. 2015;38:551-560.
33. Weinger K, Beverly EA, Smaldone A. Diabetes self-care and the older adult. Western J Nurs Res. 2014;36:1272-1298.
34. Beverly EA, Ritholz MD, Shepherd C, et al. The psychosocial challenges and care of older adults with diabetes: “can’t do what I used to do; can’t be who I once was.” Curr Diabetes Rep. 2016;16:48.
35. Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PloS One. 2009;4:e4144.
36. Thabit H, Kyaw TT, McDermott J, et al. Executive function and diabetes mellitus—a stone left unturned? Curr Diabetes Rev. 2012;8:109-115.
37. McNally K, Rohan J, Pendley JS, et al. Executive functioning, treatment adherence, and glycemic control in children with type 1 diabetes. Diabetes Care. 2010;33:1159-1162.
38. Rucker JL, McDowd JM, Kluding PM. Executive function and type 2 diabetes: putting the pieces together. Phys Ther. 2012;92:454-462.
39. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
40. Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA. 2006;295:1935-1940.
41. Oftedal B, Karlsen B, Bru E. Life values and self-regulation behaviours among adults with type 2 diabetes. J Clin Nurs. 2010;19:2548-2556.
42. Morrow AS, Haidet P, Skinner J, et al. Integrating diabetes self-management with the health goals of older adults: a qualitative exploration. Patient Education Counseling. 2008;72:418-423.
43. Huang ES, Gorawara-Bhat R, Chin MH. Self-reported goals of older patients with type 2 diabetes mellitus. J Am Geriatr Soc. 2005;53:306-311.
44. Beverly EA, Wray LA, LaCoe CL, et al. Listening to older adults’ values and preferences for Type 2 diabetes care: a qualitative study. Diabetes Spectrum. 2014;27:44-49.
45. American Association of Diabetes Educators. Why refer for diabetes education? American Association of Diabetes Educators. Available at: https://www.diabeteseducator.org/practice/provider-resources/why-refer-for-diabetes-education. Accessed August 15, 2016.
46. Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet. 2004;363:1589-1597.
1. Gafarian CT, Heiby EM, Blair P, et al. The diabetes time management questionnaire. Diabetes Educator. 1999;25:585-592.
2. Wdowik MJ, Kendall PA, Harris MA. College students with diabetes: using focus groups and interviews to determine psychosocial issues and barriers to control. Diabetes Educator. 1997;23:558-562.
3. Rubin RR. Psychological issues and treatment for people with diabetes. J Clin Psych. 2001;57:457-478.
4. Ali MK, Bullard KM, Gregg EW. Achievement of goals in US diabetes care, 1999-2010. New Engl J Med. 2013;369:287-288.
5. Lloyd CE, Smith J, Weinger K. Stress and diabetes: Review of the links. Diabetes Spectrum. 2005;18:121-127.
6. Weinger K. Psychosocial issues and self-care. Am J Nurs. 2007;107(6 suppl): S34-S38.
7. Weinger K, Jacobson AM. Psychosocial and quality of life correlates of glycemic control during intensive treatment of type 1 diabetes. Patient Education Counseling. 2001;42:123-131.
8. Albright TL, Parchman M, Burge SK. Predictors of self-care behavior in adults with type 2 diabetes: an RRNeST study. Fam Med. 2001;33:354-360.
9. Gonzalez JS, Safren SA, Cagliero E, et al. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care. 2007;30:2222-2227.
10. Gonzalez JS, Safren SA, Delahanty LM, et al. Symptoms of depression prospectively predict poorer self-care in patients with Type 2 diabetes. Diabetic Med. 2008;25:1102-1107.
11. Nicolucci A, Kovacs Burns K, Holt RI, et al. Diabetes Attitudes, Wishes and Needs second study (DAWN2): cross-national benchmarking of diabetes-related psychosocial outcomes for people with diabetes. Diabetic Med. 2013;30:767-777.
12. Fisher L, Hessler DM, Polonsky W, et al. When is diabetes distress clinically meaningful?: establishing cut points for the Diabetes Distress Scale. Diabetes Care. 2012;35:259-264.
13. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabetic Med. 2014;31:764-772.
14. Fisher L, Mullan JT, Skaff MM, et al. Predicting diabetes distress in patients with Type 2 diabetes: a longitudinal study. Diabetic Med. 2009;26:622-627.
15. Fisher L, Skaff MM, Mullan JT, et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care. 2007;30:542-548.
16. Gonzalez JS, Delahanty LM, Safren SA, et al. Differentiating symptoms of depression from diabetes-specific distress: relationships with self-care in type 2 diabetes. Diabetologia. 2008;51:2822-1825.
17. Fisher L, Mullan JT, Arean P, et al. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross-sectional and longitudinal analyses. Diabetes Care. 2010;33:23-28.
18. Fisher EB, Thorpe CT, Devellis BM, et al. Healthy coping, negative emotions, and diabetes management: a systematic review and appraisal. Diabetes Educator. 2007;33:1080-1103; 1104-1086.
19. Peterson KA, Radosevich DM, O’Connor PJ, et al. Improving diabetes care in practice: findings from the TRANSLATE trial. Diabetes Care. 2008;31:2238-2243.
20. Fisher L, Glasgow RE, Strycker LA. The relationship between diabetes distress and clinical depression with glycemic control among patients with type 2 diabetes. Diabetes Care. 2010;33:1034-1036.
21. Cole J, McGuffin P, Farmer AE. The classification of depression: are we still confused? Br J Psychiatr. 2008;192:83-85.
22. Wakefield JC. The concept of mental disorder. On the boundary between biological facts and social values. Am Psychologist. 1992;47:373-388.
23. Fisher L, Gonzalez JS, Polonsky WH. The confusing tale of depression and distress in patients with diabetes: a call for greater clarity and precision. Diabetic Med. 2014;31:764-772.
24. Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278-3285.
25. Fisher L, Skaff MM, Mullan JT, et al. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress in adults with Type 2 diabetes. Diabetic Med. 2008;25:1096-1101.
26. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18:754-760.
27. McGuire BE, Morrison TG, Hermanns N, et al. Short-form measures of diabetes-related emotional distress: the Problem Areas in Diabetes Scale (PAID)-5 and PAID-1. Diabetologia. 2010;53:66-69.
28. Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28:626-631.
29. Fisher L, Glasgow RE, Mullan JT, et al. Development of a brief diabetes distress screening instrument. Ann Fam Med. 2008;6:246-252.
30. Fisher L, Polonsky WH, Hessler DM, et al. Understanding the sources of diabetes distress in adults with type 1 diabetes. J Diabetes Complications. 2015;29:572-577.
31. Fisher L, Hessler D, Glasgow RE, et al. REDEEM: a pragmatic trial to reduce diabetes distress. Diabetes Care. 2013;36:2551-2558.
32. Hermanns N, Schmitt A, Gahr A, et al. The effect of a Diabetes-Specific Cognitive Behavioral Treatment Program (DIAMOS) for patients with diabetes and subclinical depression: results of a randomized controlled trial. Diabetes Care. 2015;38:551-560.
33. Weinger K, Beverly EA, Smaldone A. Diabetes self-care and the older adult. Western J Nurs Res. 2014;36:1272-1298.
34. Beverly EA, Ritholz MD, Shepherd C, et al. The psychosocial challenges and care of older adults with diabetes: “can’t do what I used to do; can’t be who I once was.” Curr Diabetes Rep. 2016;16:48.
35. Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PloS One. 2009;4:e4144.
36. Thabit H, Kyaw TT, McDermott J, et al. Executive function and diabetes mellitus—a stone left unturned? Curr Diabetes Rev. 2012;8:109-115.
37. McNally K, Rohan J, Pendley JS, et al. Executive functioning, treatment adherence, and glycemic control in children with type 1 diabetes. Diabetes Care. 2010;33:1159-1162.
38. Rucker JL, McDowd JM, Kluding PM. Executive function and type 2 diabetes: putting the pieces together. Phys Ther. 2012;92:454-462.
39. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650-2664.
40. Durso SC. Using clinical guidelines designed for older adults with diabetes mellitus and complex health status. JAMA. 2006;295:1935-1940.
41. Oftedal B, Karlsen B, Bru E. Life values and self-regulation behaviours among adults with type 2 diabetes. J Clin Nurs. 2010;19:2548-2556.
42. Morrow AS, Haidet P, Skinner J, et al. Integrating diabetes self-management with the health goals of older adults: a qualitative exploration. Patient Education Counseling. 2008;72:418-423.
43. Huang ES, Gorawara-Bhat R, Chin MH. Self-reported goals of older patients with type 2 diabetes mellitus. J Am Geriatr Soc. 2005;53:306-311.
44. Beverly EA, Wray LA, LaCoe CL, et al. Listening to older adults’ values and preferences for Type 2 diabetes care: a qualitative study. Diabetes Spectrum. 2014;27:44-49.
45. American Association of Diabetes Educators. Why refer for diabetes education? American Association of Diabetes Educators. Available at: https://www.diabeteseducator.org/practice/provider-resources/why-refer-for-diabetes-education. Accessed August 15, 2016.
46. Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet. 2004;363:1589-1597.
PRACTICE RECOMMENDATIONS
› Educate patients about diabetes distress, explaining that diabetes is manageable and that neither complications nor diabetes distress is inevitable. C
› Empower patients to take an active role in self-management of diabetes, encouraging them to express their concerns and ask open-ended questions. A
› Support shared decision-making by inquiring about patients’ values and treatment preferences, presenting options, and reviewing the risks and benefits of each. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Atrial fibrillation: Effective strategies using the latest tools
Atrial fibrillation (AF)—the most common supraventricular tachycardia—affects as many as 6.1 million adults in the United States.1 It is associated with a 5-fold increased risk of stroke,2 a 3-fold increased risk of heart failure (HF),3 and about a 2-fold increased risk of dementia4 and mortality.2 The prevalence of AF increases with maturity, from 2% in people <65 years of age to 9% in those ≥65 years,5 and that prevalence is expected to double over the next 25 years as the population ages.1
The primary goals of treatment are to alleviate symptoms and prevent thromboembolism. Strokes related to AF are more likely to result in severe disability or death when compared with those unrelated to AF.6 And yet anticoagulation remains underutilized.7
The net clinical benefit of oral anticoagulation appears to be greatest in patients with the highest risk of bleeding, since these patients are also at the highest risk for stroke.8 Patients at increased risk of stroke are more likely to receive oral anticoagulation; however, for unknown reasons, more than half of people with the highest risk of stroke are not prescribed these important anti-blood-clotting medications.7 One theory is that physicians may be relying on their gut rather than objective risk scores, and underuse of validated schemata leads to poor estimation of risk.
For example, results from the ORBIT-AF (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation) trial, which involved over 10,000 people with AF, found that although 72% (n=7251) had high-risk CHADS2 scores (≥2), only 16% were assessed as having a high risk of stroke by physicians.9 Along the same lines, a recent study of Canadian primary care physicians showed that stroke risk and bleeding risk were not evaluated with validated tools in 58% and 81% of patients, respectively, leading to both significant underestimation and overestimation of risk.10
This review provides the tools to identify when anticoagulation is indicated, reports the advantages and disadvantages of the currently available anticoagulants, and discusses the selection and implementation of rate- vs rhythm-control strategies. But first, a word about the etiology, classification, and diagnosis of AF.
AF: The result of any number of cardiac and non-cardiac causes
AF is characterized by uncoordinated activation of the atria, which results in ineffective atrial contractions and an irregular, often rapid, ventricular response. It is the ultimate clinical manifestation of multiple diseases that alter atrial tissue through inflammation, fibrosis, or hypertrophy.5 The most common causes are hypertension, coronary artery disease, HF, cardiomyopathies, and valvular heart disease, all of which stimulate the renin-angiotensin-aldosterone system, leading to increased susceptibility to arrhythmia.5 Atrial ectopic tachycardia, Wolff-Parkinson-White (WPW) syndrome, and atrioventricular (AV) nodal reentrant tachycardia also may precipitate AF.5 In these cases, AF usually resolves after catheter ablation (CA) of the primary arrhythmia.11 Unrecognized AF may trigger atrial flutter, and more than 80% of patients who undergo radiofrequency ablation for atrial flutter experience AF at some point in the subsequent 5 years.12
Non-cardiac causes of AF include sleep apnea, obesity, hyperthyroidism, drugs, electrocution, pneumonia, and pulmonary embolism.5 An association between binge drinking and AF (“holiday heart syndrome”) has long been recognized. The evidence now suggests that alcohol increases the risk of AF in a dose-dependent manner with intakes of ≥1 drink per day (12 g per drink).13
Classification schema no longer includes “lone AF”
AF is classified in terms of the duration of episodes:5
- Paroxysmal AF is characterized by brief episodes that terminate spontaneously or with intervention within 7 days of onset. These episodes recur with variable frequency.
- Persistent AF refers to AF that is continuously sustained for more than 7 days.
- Longstanding persistent AF refers to continuous AF that lasts longer than 12 months.
- Permanent AF is not an inherent pathophysiologic attribute of AF, but rather an acceptance of AF where the patient and physician abandon further efforts to restore and/or maintain sinus rhythm.
- Nonvalvular AF occurs in the absence of a valve replacement (mechanical or bioprosthetic), rheumatic mitral stenosis, or mitral valve repair.
Although paroxysmal and persistent AF may occur in the same individual, the distinction is still clinically relevant, as outcomes of certain therapies, such as CA, are superior in patients with paroxysmal AF.14 With a more complete understanding of AF pathophysiology, guidelines now discourage use of the potentially confusing term “lone AF,” which has historically been applied to younger patients with no known clinical risk factors or echocardiographic abnormalities. As a result, therapeutic decisions are no longer based on this nomenclature, according to the 2014 AF practice guideline from the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Rhythm Society (HRS).5
Patient complaints—or incidental findings—can prompt a Dx
Fatigue is the most common symptom of AF. Other signs and symptoms include palpitations, dyspnea, HF, hypotension, syncope, chest pain, and stroke. Some patients are asymptomatic, and AF is an incidental finding when an irregular pulse is discovered during a physical examination. The diagnosis is confirmed by electrocardiogram (EKG), telemetry, Holter monitor, event recorder, or an implanted electrocardiographic recording device. A chest x-ray, serum electrolyte levels, a complete blood count, thyroid testing, and renal and hepatic function testing are recommended. Transthoracic echocardiography to measure cardiac function, detect underlying structural heart disease, and evaluate atrial size is essential.5
An electrophysiologic (EP) study may be needed for diagnosis or treatment if another arrhythmia is present. Aberrant conduction may cause AF to present as a wide complex tachycardia and be mislabeled as ventricular tachycardia. The presence of delta waves is an indication for an EP study targeting the WPW accessory pathway. Transesophageal echocardiography (TEE) is the most sensitive and specific test for left atrial thrombi. If you are considering a TEE for a patient with AF of unknown, or >48 hours’, duration who has not been anticoagulated in the preceding 3 weeks, obtain it before performing cardioversion because of the risk of embolism.5
Stroke prevention
The ACC/AHA/HRS AF guideline recommends basing anticoagulation decisions on thromboembolic risk, regardless of AF pattern (paroxysmal, persistent, or permanent) (Class I recommendation).5 For patients with nonvalvular AF and atrial flutter, the guideline recommends using the Birmingham 2009 schema (CHA2DS2-VASc score) (TABLE 115-18) to estimate thromboembolic risk.5,15 CHA2DS2-VASc improves on the older CHADS2 score by significantly reducing the number of patients categorized as having intermediate risk and better identifying truly low-risk patients who are unlikely to benefit from anticoagulation.16,17,19
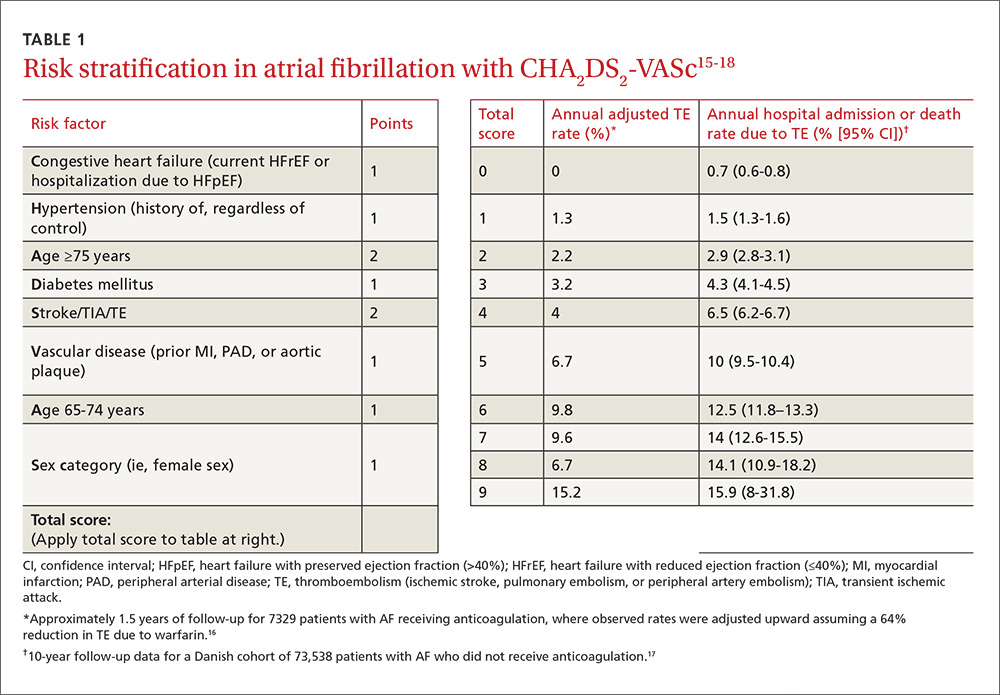
Men with a CHA2DS2-VASc score of zero and women with a score of one do not need anticoagulation.5,20 Discuss the risks and benefits of oral anticoagulation with men who have a score of one. In these intermediate-risk men, antiplatelet therapy with aspirin and/or clopidogrel may be reasonable, especially if there is an indication other than stroke prevention (eg, post-myocardial infarction). Oral anticoagulation is strongly recommended for all patients with a CHA2DS2-VASc score of 2 or higher.5,18,21,22
Anticoagulant considerations: Warfarin vs DOACs
Warfarin was the gold standard for stroke prevention in nonvalvular AF until the direct oral anticoagulants (DOACs) became available in 2010. Guidelines in the United States and the United Kingdom recommend shared decision-making to help patients with AF who do not have a specific indication for warfarin choose between warfarin and the DOACs.5,21 Canadian and European guidelines recommend DOACs as the first-line option for anticoagulation and reserve warfarin for patients who have contraindications to, or are unable to afford, DOACs.18,22 All current guidelines recommend continuing warfarin in patients who are stable, well controlled, and satisfied with warfarin therapy and the monitoring and dietary restrictions it entails.
DOACs are as effective as warfarin. All of the DOACs are approved for stroke prevention based on individual phase III non-inferiority trials in which they were compared to warfarin.23-26 In addition, a meta-analysis of these 4 trials involving a total of 71,683 patients (mean age 70-73 years; median follow-up, 1.8-2.8 years) evaluated the benefits and risks of the 4 DOACs against the former gold standard.27
Higher doses of the DOACs (dabigatran 150 mg BID, rivaroxaban 20 mg/d, edoxaban 60 mg/d, and apixaban 5 mg BID) reduced the rates of stroke or systemic embolism (relative risk [RR]=0.81; 95% confidence interval [CI], 0.73-0.91; P<.0001; number needed to treat [NNT]=147), hemorrhagic stroke (RR=0.49; 95% CI, 0.38-0.64; P<.0001; NNT=219), and all-cause mortality (RR=0.90; 95% CI, 0.85-0.95; P=.0003; NNT=128), compared with warfarin.27 It is important to note that while lower doses of some DOACs (dabigatran 110 mg BID and edoxaban 30 mg/d) were not as effective at preventing ischemic stroke when compared with warfarin (RR=1.3; 95% CI, 1-1.6; P=.045), they still significantly reduced hemorrhagic stroke (RR=0.33; 95% CI, 0.23-0.46; P<.0001) and all-cause mortality (RR=0.89; 95% CI, 0.83-0.96; P=.003).
Of course, the biggest concern is bleeding. In that same meta-analysis, the difference in major bleeding events with DOACs vs warfarin was not statistically significant (RR=0.86; 95% CI, 0.73-1; P=.06). While DOACs likely lower rates of intracranial hemorrhage (RR=0.48; 95% CI, 0.39-0.59; P<.0001; NNT=132), they seem to increase the risk of gastrointestinal (GI) bleeding (RR=1.3; 95% CI, 1-1.6; P=.043; number needed to harm [NNH]=185).27
There was significant heterogeneity in the GI bleeding outcome, however. When compared with warfarin, GI bleeding was increased by dabigatran 150 mg BID (RR=1.5; 95% CI, 1.2-1.9; P<.001) and edoxaban 60 mg/d (HR=1.2; 95% CI, 1.02-1.5; P=.03), but there were no significant differences for dabigatran 110 mg BID or apixaban 5 mg BID.23,25,26
On the other hand, edoxaban 30 mg/d had a lower risk of GI bleeding when compared with warfarin (HR=0.67; 95% CI, 0.53-0.83; P<.001).25 Without head-to-head trials, it is impossible to know if one DOAC is superior to another. Apixaban 5 mg BID appears to offer the best overall balance between efficacy and safety. Other DOACs may be better options for patients who have specific concerns regarding efficacy or safety.28,29
Convenience, interactions, and cost may be the deciding factors. Since all DOACs are fairly comparable in efficacy and safety, other factors such as convenience, interactions with other medications, and cost should be considered when deciding on a medication for an individual patient (TABLE 230,31). The DOACs require no lab monitoring or dose titration, and all 4 have fewer potential drug interactions than warfarin.30 Due to their relatively short half-lives, strict adherence is critical; DOACs are not suitable for patients who frequently miss doses.5 (For more information on starting or switching to DOACs, see, “Is a novel anticoagulant right for your patient?” J Fam Pract. 2014;63:22-28.)

A word about DOACs and renal impairment. Another concern with DOACs is their reliance on renal metabolism and excretion. A meta-analysis of the 4 phase III trials of the DOACs, this time involving 58,338 patients, evaluated DOAC efficacy and safety compared to warfarin in the presence of kidney dysfunction.32 Renal function was categorized as normal (estimated glomerular filtration rate [eGFR] >80 mL/min/1.73 m2), mildly impaired (eGFR 50-80 mL/min/1.73 m2), or moderately impaired (eGFR <50 mL/min/1.73m2). Compared with warfarin, DOACs lowered stroke risk in patients with mild (RR=0.71; 95% CI, 0.62-0.81) or moderate (RR=0.79; 95% CI, 0.66-0.94) renal impairment. DOACs also reduced major bleeding compared to warfarin in patients with mild (RR=0.88; 95% CI, 0.80-0.97) or moderate (RR=0.80; 95% CI, 0.66-0.94) renal impairment. How the DOACs fare in patients with severe renal dysfunction could not be determined because such patients were excluded from the trials.
Keep in mind that the DOACs require dose adjustment at different levels of renal impairment (TABLE 230,31), and warfarin remains the only recommended treatment for patients with severe renal impairment, according to both AHA/ACC/HRS and European Society of Cardiology guidelines.5,18
Tools to help assess patients’ bleeding risk
Of the available scoring mechanisms to identify risk factors for bleeding, 3 have been specifically validated in AF populations (ie, ATRIA,33 HEMORR2HAGES,34 and HAS-BLED35). Of the 3, HAS-BLED is superior,36 the most practical, and recommended by expert guidelines.18,21,22 Additionally, HAS-BLED has good correlation with intracranial hemorrhage risk. The HAS-BLED score ranges from 0 to 9 points with one point assigned for each of the following:35
- Hypertension–uncontrolled with systolic BP >160 mm Hg
- Abnormal liver function–cirrhosis, bilirubin >2× normal, or liver enzymes >3× normal
- Abnormal renal function–dialysis, transplant, or serum creatinine >2.26 mg/dL
- Stroke history–including lacunar infarcts
- Bleeding predisposition–history of major bleeding due to any cause
- Labile international normalized ratio (INR)–time in therapeutic range <60%
- Elderly–age >65 years
- Drug–antiplatelet agents, including nonsteroidal anti-inflammatory drugs
- Alcohol usage–>8 drinks per week.
Patients with a HAS-BLED score ≥3 warrant additional monitoring and attempts to reduce bleeding risk by addressing modifiable risk factors. Bleeding risk scores should not be used to exclude patients from anticoagulation therapy.5 In fact, the British National Institute for Health and Clinical Excellence (NICE) guidelines state that anticoagulation should not be withheld solely due to fall risk.21
Also, anticoagulation with warfarin should not be permanently discontinued because of a single GI bleed, since restarting warfarin is associated with decreased risks of thromboembolism and mortality and a statistically insignificant increase in recurrent GI bleeding.37 Restarting DOAC therapy following a GI bleed has not been evaluated in clinical trials; however, it may be reasonable to use one of the DOAC doses with a lower risk of GI bleeding (dabigatran 110 mg BID, apixaban 5 mg BID, or edoxaban 30 mg/d) in patients who have experienced a GI bleed on warfarin or another DOAC.18,22
An online calculator is available that uses CHA2DS2-VASc and HAS-BLED scores to determine an individual’s risk/benefit profile with the various anticoagulation strategies available (http://www.sparctool.com). Consider percutaneous left atrial appendage occlusion if the risks of anticoagulation truly exceed the benefits.38
Rate control vs rhythm control
Most patients who present with AF require immediate ventricular rate control to reduce symptoms. In the acute setting, this can be accomplished with intravenous (IV) beta-blockers or IV calcium channel antagonists.5,39 If the patient is hemodynamically unstable, urgent direct-current cardioversion is the preferred treatment strategy and should not be delayed pending anticoagulation. IV amiodarone can be used in the ICU patient who does not require cardioversion, but is unable to tolerate beta-blockers or calcium channel antagonists.40 Once the patient is stable, long-term treatment focuses on ventricular rate control or restoration and maintenance of sinus rhythm.
The AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) trial enrolled 4060 patients (mean age 70 years, mean follow-up 3.5 years) with paroxysmal and persistent AF and randomized them to either pharmacologic rate control or rhythm control.41 No significant differences were found in all-cause mortality or in the composite secondary endpoint of death, ischemic stroke, anoxic encephalopathy, major bleeding, or cardiac arrest. In addition, no significant differences emerged in quality of life or global functional status. The number of patients requiring hospitalization during follow-up was significantly lower in the rate-control group vs the rhythm-control group (73% vs 80%; P<.001). Anticoagulation was encouraged but not mandated in the rhythm-control group after 4 weeks in sinus rhythm, and there was a trend toward higher mortality in the rhythm-control group (27% vs 26%; P=.08).
Patients <65 years were excluded from the AFFIRM trial. When younger patients experience significant symptoms, early referral to Cardiology should be considered to discuss the long-term benefits and risks of a rhythm-control strategy. Regardless of age, when patients remain symptomatic despite rate- or rhythm-control management, the strategy should be changed.5
Rate-control targets and options
Target heart rates should be individualized. The 2014 ACC/AHA/HRS guideline recommends a resting target heart rate <80 beats per minute (bpm) in symptomatic patients.5 In patients with permanent AF who remain asymptomatic at higher resting heart rates, a more lenient rate-control strategy (resting heart rate <110 bpm) has demonstrated outcomes equivalent to those of a more strict approach (resting heart rate <80 bpm and heart rate during moderate exercise <110 bpm).42 Pharmacologic rate-control options include beta-blockers, non-dihydropyridine calcium channel antagonists, and digoxin (TABLE 35). Digoxin is associated with increased all-cause mortality in patients with AF regardless of HF status (HR=1.4; 95% CI, 1.2-1.6, P=.0001).43 Digoxin should be reserved for patients who are sedentary or have inadequate control with first-line medications.5
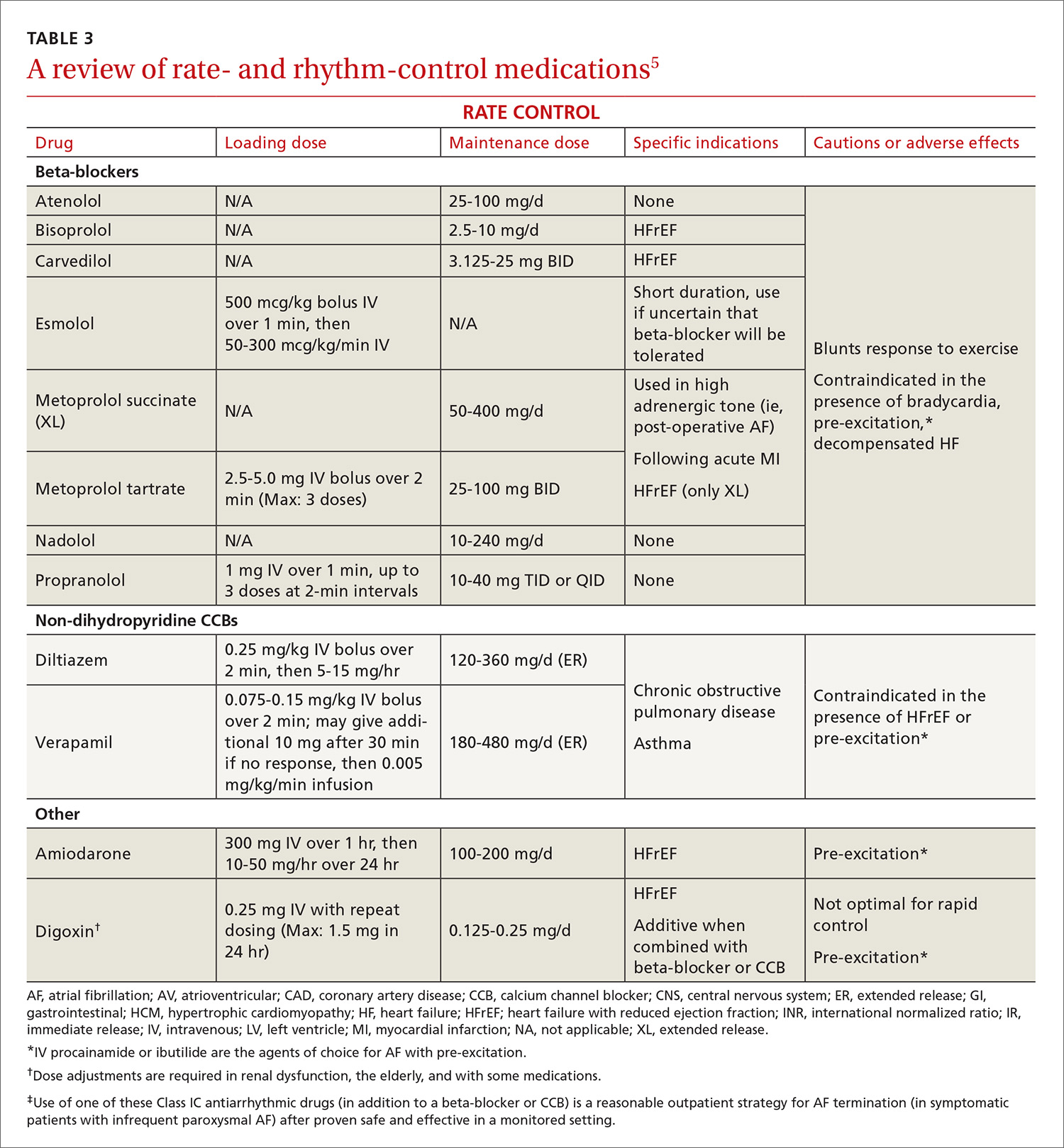
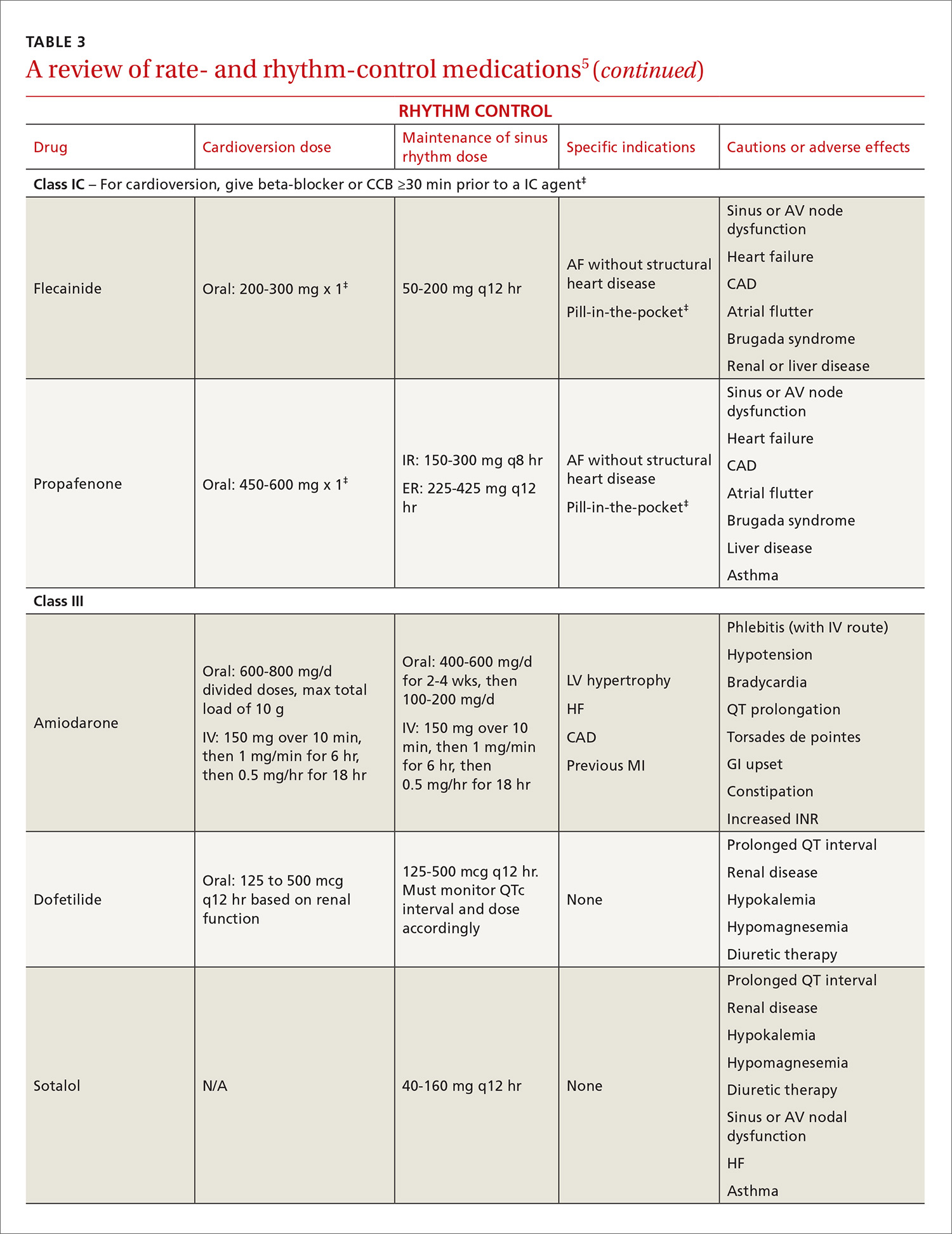
Indications for rhythm control
The NICE guidelines, which are consistent with the ACC/AHA/HRS guidelines, recommend rate control as the first-line strategy for AF management, except in people:21
- whose AF has a reversible cause
- who have HF believed to be primarily caused by AF
- with new-onset AF
- with atrial flutter that is considered suitable for an ablation strategy to restore sinus rhythm
- for whom a rhythm-control strategy would be more suitable based on clinical judgment.
In addition, patients who continue to experience symptomatic AF despite an adequate trial of rate control should be offered rhythm control.5
Pharmacologic rhythm-control strategies. Antiarrhythmic drugs can be used for chemical cardioversion, reduction of paroxysms, and long-term maintenance of sinus rhythm. The most commonly used antiarrhythmic drugs are Class IC and Class III agents (TABLE 3).5 Tailored drug selection for each patient is key. Patients with left atrial diameters >4.5 cm are less likely to remain in sinus rhythm, and patients with left ventricular hypertrophy are at increased risk for proarrhythmic adverse effects.44 Patients with paroxysmal AF may be candidates for a “pill-in-the-pocket” strategy using propafenone or flecainide.5
AF frequently progresses from paroxysmal to persistent and can subsequently result in electrical and structural remodeling that becomes irreversible over time.45 The patient with uncontrolled symptoms despite attempts at rate control and rhythm control should be promptly referred to an electrophysiologist.
Surgical interventions for rate or rhythm control
Electrophysiology interventions include AV nodal ablation with pacemaker placement for rate control, or catheter-directed ablation (radiofrequency or cryotherapy) for rhythm control. CA appears to be more effective than pharmacologic rhythm control.46,47 Treatment with CA is indicated for symptomatic paroxysmal AF when a rhythm-control strategy is desired and the AF is refractory to, or the patient is intolerant of, at least one class I or III antiarrhythmic medication.5 With these same caveats, CA is a reasonable strategy for symptomatic persistent AF.
Consider more invasive interventions, such as an atrial maze procedure, when patients require cardiac surgery for another indication. Patients with an increased risk of thromboembolism (based on CHA2DS2-VASc) remain at high risk even after successful ablation.48 As a result, some guidelines recommend continued long-term anticoagulation following CA.18,22
CORRESPONDENCE
Philip Dooley, MD, University of Kansas School of Medicine–Wichita Family Medicine Residency at Via Christi, 707 North Emporia, Wichita, KS 67207; [email protected].
ACKNOWLEDGMENTS
We thank Professor Anne Walling, MB, ChB, FFPHM, Department of Family and Community Medicine, University of Kansas School of Medicine–Wichita for her suggestions and critical review of an earlier version of this manuscript.
1. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults. National implications for Rhythm Management and Stroke Prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370-2375.
2. Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N-9N.
3. Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba follow-up study. Am J Med. 1995;98:476-484.
4. Ott A, Breteler MMB, de Bruyne MC, et al. Atrial fibrillation and dementia in a population-based study: The Rotterdam Study. Stroke. 1997;28:316-321.
5. January CT, Wann L, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1-e76.
6. Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. Stroke. 1996;27:1760-1764.
7. Hsu JC, Maddox TM, Kennedy KF, et al. Oral anticoagulant therapy prescription in patients with atrial fibrillation across the spectrum of stroke risk: insights from the NCDR PINNACLE registry. JAMA Cardiol. 2016;1:55-62.
8. Olesen JB, Lip GY, Lindhardsen J, et al. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: a net clinical benefit analysis using a ‘real world’ nationwide cohort study. Thromb Haemost. 2011;106:739-749.
9. Steinberg BA, Kim S, Thomas L, et al. Lack of concordance between empirical scores and physician assessments of stroke and bleeding risk in atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Circulation. 2014;129:2005-2012.
10. Angaran P, Dorian P, Tan MK, et al. The risk stratification and stroke prevention therapy care gap in Canadian atrial fibrillation patients. Can J Cardiol. 2016;32:336-343.
11. Waldo AL, Feld GK. Inter-relationships of atrial fibrillation and atrial flutter: mechanisms and clinical implications. J Am Coll Cardiol. 2008;51:779-786.
12. Ellis K, Wazni O, Marrouche N, et al. Incidence of atrial fibrillation post-cavotricuspid isthmus ablation in patients with typical atrial flutter: left-atrial size as an independent predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol. 2007;18:799-802.
13. Larsson SC, Drca N, Wolk A. Alcohol consumption and risk of atrial fibrillation: a prospective study and dose-response meta-analysis. J Am Coll Cardiol. 2014;64:281-289.
14. Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. J Interv Card Electrophysiol. 2012;33:171-257.
15. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263-272.
16. Lip GYH, Frison L, Halperin JL, et al. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010;41:2731-2738.
17. Olesen JB, Lip GYH, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124.
18. Camm AJ, Lip GYH, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719-2747.
19. Olesen JB, Torp-Pedersen C, Hansen ML, et al. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0-1: a nationwide cohort study. Thromb Haemost. 2012;107:1172-1179.
20. Friberg L, Benson L, Rosenqvist M, et al. Assessment of female sex as a risk factor in atrial fibrillation in Sweden: nationwide retrospective cohort study. BMJ. 2012;344:e3522.
21. National Institute for Health and Clinical Excellence (NICE). Atrial fibrillation: the management of atrial fibrillation [CG180]. 2014. Available at: https://www.nice.org.uk/guidance/cg180. Accessed July 31, 2016.
22. Verma A, Cairns JA, Mitchell LB, et al. 2014 focused update of the Canadian Cardiovascular Society Guidelines for the management of atrial fibrillation. Can J Cardiol. 2014;30:1114-1130.
23. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
24. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
25. Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093-2104.
26. Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992.
27. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
28. Morimoto T, Crawford B, Wada K, et al. Comparative efficacy and safety of novel oral anticoagulants in patients with atrial fibrillation: a network meta-analysis with the adjustment for the possible bias from open label studies. J Cardiol. 2015;66:466-474.
29. Verdecchia P, Angeli F, Bartolini C, et al. Safety and efficacy of non-vitamin K oral anticoagulants in non-valvular atrial fibrillation: a Bayesian meta-analysis approach. Expert Opin Drug Saf. 2015;14:7-20.
30. Micromedex® 2.0 (electronic version). Truven Health Analytics, Greenwood Village, Colorado, USA. Available at: http://www.micromedexsolutions.com. Accessed August 18, 2016.
31. GoodRx. Available at: https://www.goodrx.com. Accessed August 18, 2016.
32. Del-Carpio Munoz F, Gharacholou SM, Munger TM, et al. Meta-analysis of renal function on the safety and efficacy of novel oral anticoagulants for atrial fibrillation. Am J Cardiol. 2016;117:69-75.
33. Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58:395-401.
34. Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J. 2006;151:713-719.
35. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093-1100.
36. Zhu W, He W, Guo L, et al. The HAS-BLED Score for predicting major bleeding risk in anticoagulated patients with atrial fibrillation: a systematic review and meta-analysis. Clin Cardiol. 2015;38:555-561.
37. Chai-Adisaksopha C, Hillis C, Monreal M, et al. Thromboembolic events, recurrent bleeding and mortality after resuming anticoagulant following gastrointestinal bleeding. A meta-analysis. Thromb Haemost. 2015;114:819-825.
38. Xu H, Xie X, Wang B, et al. Efficacy and safety of percutaneous left atrial appendage occlusion for stroke prevention in nonvalvular atrial fibrillation: a meta-analysis of contemporary studies. Heart Lung Circ. 2016;25:1107-1117.
39. Siu CW, Lau CP, Lee WL, et al. Intravenous diltiazem is superior to intravenous amiodarone or digoxin for achieving ventricular rate control in patients with acute uncomplicated atrial fibrillation. Crit Care Med. 2009;37:2174-2179.
40. Clemo HF, Wood MA, Gilligan DM, et al. Intravenous amiodarone for acute heart rate control in the critically ill patient with atrial tachyarrhythmias. Am J Cardiol. 1998;81:594-598.
41. The Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
42. Van Gelder IC, Groenveld HF, Crijns HJGM, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363-1373.
43. Wang ZQ, Zhang R, Chen MT, et al. Digoxin is associated with increased all-cause mortality in patients with atrial fibrillation regardless of concomitant heart failure: a meta-analysis. J Cardiovasc Pharmacol. 2015;66:270-275.
44. Olshansky B, Heller EN, Mitchell LB, et al. Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke? Results from the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study. J Am Coll Cardiol. 2005;45:2026-2033.
45. de Vos CB, Pisters R, Nieuwlaat R, et al. Progression from paroxysmal to persistent atrial fibrillation: clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725-731.
46. Cheng X, Li X, He Y, et al. Catheter ablation versus anti-arrhythmic drug therapy for the management of atrial fibrillation: a meta-analysis. J Interv Card Electrophysiol. 2014;41:267-272.
47. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133:1637-1644.
48.
Atrial fibrillation (AF)—the most common supraventricular tachycardia—affects as many as 6.1 million adults in the United States.1 It is associated with a 5-fold increased risk of stroke,2 a 3-fold increased risk of heart failure (HF),3 and about a 2-fold increased risk of dementia4 and mortality.2 The prevalence of AF increases with maturity, from 2% in people <65 years of age to 9% in those ≥65 years,5 and that prevalence is expected to double over the next 25 years as the population ages.1
The primary goals of treatment are to alleviate symptoms and prevent thromboembolism. Strokes related to AF are more likely to result in severe disability or death when compared with those unrelated to AF.6 And yet anticoagulation remains underutilized.7
The net clinical benefit of oral anticoagulation appears to be greatest in patients with the highest risk of bleeding, since these patients are also at the highest risk for stroke.8 Patients at increased risk of stroke are more likely to receive oral anticoagulation; however, for unknown reasons, more than half of people with the highest risk of stroke are not prescribed these important anti-blood-clotting medications.7 One theory is that physicians may be relying on their gut rather than objective risk scores, and underuse of validated schemata leads to poor estimation of risk.

For example, results from the ORBIT-AF (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation) trial, which involved over 10,000 people with AF, found that although 72% (n=7251) had high-risk CHADS2 scores (≥2), only 16% were assessed as having a high risk of stroke by physicians.9 Along the same lines, a recent study of Canadian primary care physicians showed that stroke risk and bleeding risk were not evaluated with validated tools in 58% and 81% of patients, respectively, leading to both significant underestimation and overestimation of risk.10
This review provides the tools to identify when anticoagulation is indicated, reports the advantages and disadvantages of the currently available anticoagulants, and discusses the selection and implementation of rate- vs rhythm-control strategies. But first, a word about the etiology, classification, and diagnosis of AF.
AF: The result of any number of cardiac and non-cardiac causes
AF is characterized by uncoordinated activation of the atria, which results in ineffective atrial contractions and an irregular, often rapid, ventricular response. It is the ultimate clinical manifestation of multiple diseases that alter atrial tissue through inflammation, fibrosis, or hypertrophy.5 The most common causes are hypertension, coronary artery disease, HF, cardiomyopathies, and valvular heart disease, all of which stimulate the renin-angiotensin-aldosterone system, leading to increased susceptibility to arrhythmia.5 Atrial ectopic tachycardia, Wolff-Parkinson-White (WPW) syndrome, and atrioventricular (AV) nodal reentrant tachycardia also may precipitate AF.5 In these cases, AF usually resolves after catheter ablation (CA) of the primary arrhythmia.11 Unrecognized AF may trigger atrial flutter, and more than 80% of patients who undergo radiofrequency ablation for atrial flutter experience AF at some point in the subsequent 5 years.12
Non-cardiac causes of AF include sleep apnea, obesity, hyperthyroidism, drugs, electrocution, pneumonia, and pulmonary embolism.5 An association between binge drinking and AF (“holiday heart syndrome”) has long been recognized. The evidence now suggests that alcohol increases the risk of AF in a dose-dependent manner with intakes of ≥1 drink per day (12 g per drink).13
Classification schema no longer includes “lone AF”
AF is classified in terms of the duration of episodes:5
- Paroxysmal AF is characterized by brief episodes that terminate spontaneously or with intervention within 7 days of onset. These episodes recur with variable frequency.
- Persistent AF refers to AF that is continuously sustained for more than 7 days.
- Longstanding persistent AF refers to continuous AF that lasts longer than 12 months.
- Permanent AF is not an inherent pathophysiologic attribute of AF, but rather an acceptance of AF where the patient and physician abandon further efforts to restore and/or maintain sinus rhythm.
- Nonvalvular AF occurs in the absence of a valve replacement (mechanical or bioprosthetic), rheumatic mitral stenosis, or mitral valve repair.
Although paroxysmal and persistent AF may occur in the same individual, the distinction is still clinically relevant, as outcomes of certain therapies, such as CA, are superior in patients with paroxysmal AF.14 With a more complete understanding of AF pathophysiology, guidelines now discourage use of the potentially confusing term “lone AF,” which has historically been applied to younger patients with no known clinical risk factors or echocardiographic abnormalities. As a result, therapeutic decisions are no longer based on this nomenclature, according to the 2014 AF practice guideline from the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Rhythm Society (HRS).5
Patient complaints—or incidental findings—can prompt a Dx
Fatigue is the most common symptom of AF. Other signs and symptoms include palpitations, dyspnea, HF, hypotension, syncope, chest pain, and stroke. Some patients are asymptomatic, and AF is an incidental finding when an irregular pulse is discovered during a physical examination. The diagnosis is confirmed by electrocardiogram (EKG), telemetry, Holter monitor, event recorder, or an implanted electrocardiographic recording device. A chest x-ray, serum electrolyte levels, a complete blood count, thyroid testing, and renal and hepatic function testing are recommended. Transthoracic echocardiography to measure cardiac function, detect underlying structural heart disease, and evaluate atrial size is essential.5
An electrophysiologic (EP) study may be needed for diagnosis or treatment if another arrhythmia is present. Aberrant conduction may cause AF to present as a wide complex tachycardia and be mislabeled as ventricular tachycardia. The presence of delta waves is an indication for an EP study targeting the WPW accessory pathway. Transesophageal echocardiography (TEE) is the most sensitive and specific test for left atrial thrombi. If you are considering a TEE for a patient with AF of unknown, or >48 hours’, duration who has not been anticoagulated in the preceding 3 weeks, obtain it before performing cardioversion because of the risk of embolism.5
Stroke prevention
The ACC/AHA/HRS AF guideline recommends basing anticoagulation decisions on thromboembolic risk, regardless of AF pattern (paroxysmal, persistent, or permanent) (Class I recommendation).5 For patients with nonvalvular AF and atrial flutter, the guideline recommends using the Birmingham 2009 schema (CHA2DS2-VASc score) (TABLE 115-18) to estimate thromboembolic risk.5,15 CHA2DS2-VASc improves on the older CHADS2 score by significantly reducing the number of patients categorized as having intermediate risk and better identifying truly low-risk patients who are unlikely to benefit from anticoagulation.16,17,19

Men with a CHA2DS2-VASc score of zero and women with a score of one do not need anticoagulation.5,20 Discuss the risks and benefits of oral anticoagulation with men who have a score of one. In these intermediate-risk men, antiplatelet therapy with aspirin and/or clopidogrel may be reasonable, especially if there is an indication other than stroke prevention (eg, post-myocardial infarction). Oral anticoagulation is strongly recommended for all patients with a CHA2DS2-VASc score of 2 or higher.5,18,21,22
Anticoagulant considerations: Warfarin vs DOACs
Warfarin was the gold standard for stroke prevention in nonvalvular AF until the direct oral anticoagulants (DOACs) became available in 2010. Guidelines in the United States and the United Kingdom recommend shared decision-making to help patients with AF who do not have a specific indication for warfarin choose between warfarin and the DOACs.5,21 Canadian and European guidelines recommend DOACs as the first-line option for anticoagulation and reserve warfarin for patients who have contraindications to, or are unable to afford, DOACs.18,22 All current guidelines recommend continuing warfarin in patients who are stable, well controlled, and satisfied with warfarin therapy and the monitoring and dietary restrictions it entails.
DOACs are as effective as warfarin. All of the DOACs are approved for stroke prevention based on individual phase III non-inferiority trials in which they were compared to warfarin.23-26 In addition, a meta-analysis of these 4 trials involving a total of 71,683 patients (mean age 70-73 years; median follow-up, 1.8-2.8 years) evaluated the benefits and risks of the 4 DOACs against the former gold standard.27
Higher doses of the DOACs (dabigatran 150 mg BID, rivaroxaban 20 mg/d, edoxaban 60 mg/d, and apixaban 5 mg BID) reduced the rates of stroke or systemic embolism (relative risk [RR]=0.81; 95% confidence interval [CI], 0.73-0.91; P<.0001; number needed to treat [NNT]=147), hemorrhagic stroke (RR=0.49; 95% CI, 0.38-0.64; P<.0001; NNT=219), and all-cause mortality (RR=0.90; 95% CI, 0.85-0.95; P=.0003; NNT=128), compared with warfarin.27 It is important to note that while lower doses of some DOACs (dabigatran 110 mg BID and edoxaban 30 mg/d) were not as effective at preventing ischemic stroke when compared with warfarin (RR=1.3; 95% CI, 1-1.6; P=.045), they still significantly reduced hemorrhagic stroke (RR=0.33; 95% CI, 0.23-0.46; P<.0001) and all-cause mortality (RR=0.89; 95% CI, 0.83-0.96; P=.003).
Of course, the biggest concern is bleeding. In that same meta-analysis, the difference in major bleeding events with DOACs vs warfarin was not statistically significant (RR=0.86; 95% CI, 0.73-1; P=.06). While DOACs likely lower rates of intracranial hemorrhage (RR=0.48; 95% CI, 0.39-0.59; P<.0001; NNT=132), they seem to increase the risk of gastrointestinal (GI) bleeding (RR=1.3; 95% CI, 1-1.6; P=.043; number needed to harm [NNH]=185).27
There was significant heterogeneity in the GI bleeding outcome, however. When compared with warfarin, GI bleeding was increased by dabigatran 150 mg BID (RR=1.5; 95% CI, 1.2-1.9; P<.001) and edoxaban 60 mg/d (HR=1.2; 95% CI, 1.02-1.5; P=.03), but there were no significant differences for dabigatran 110 mg BID or apixaban 5 mg BID.23,25,26
On the other hand, edoxaban 30 mg/d had a lower risk of GI bleeding when compared with warfarin (HR=0.67; 95% CI, 0.53-0.83; P<.001).25 Without head-to-head trials, it is impossible to know if one DOAC is superior to another. Apixaban 5 mg BID appears to offer the best overall balance between efficacy and safety. Other DOACs may be better options for patients who have specific concerns regarding efficacy or safety.28,29
Convenience, interactions, and cost may be the deciding factors. Since all DOACs are fairly comparable in efficacy and safety, other factors such as convenience, interactions with other medications, and cost should be considered when deciding on a medication for an individual patient (TABLE 230,31). The DOACs require no lab monitoring or dose titration, and all 4 have fewer potential drug interactions than warfarin.30 Due to their relatively short half-lives, strict adherence is critical; DOACs are not suitable for patients who frequently miss doses.5 (For more information on starting or switching to DOACs, see, “Is a novel anticoagulant right for your patient?” J Fam Pract. 2014;63:22-28.)

A word about DOACs and renal impairment. Another concern with DOACs is their reliance on renal metabolism and excretion. A meta-analysis of the 4 phase III trials of the DOACs, this time involving 58,338 patients, evaluated DOAC efficacy and safety compared to warfarin in the presence of kidney dysfunction.32 Renal function was categorized as normal (estimated glomerular filtration rate [eGFR] >80 mL/min/1.73 m2), mildly impaired (eGFR 50-80 mL/min/1.73 m2), or moderately impaired (eGFR <50 mL/min/1.73m2). Compared with warfarin, DOACs lowered stroke risk in patients with mild (RR=0.71; 95% CI, 0.62-0.81) or moderate (RR=0.79; 95% CI, 0.66-0.94) renal impairment. DOACs also reduced major bleeding compared to warfarin in patients with mild (RR=0.88; 95% CI, 0.80-0.97) or moderate (RR=0.80; 95% CI, 0.66-0.94) renal impairment. How the DOACs fare in patients with severe renal dysfunction could not be determined because such patients were excluded from the trials.
Keep in mind that the DOACs require dose adjustment at different levels of renal impairment (TABLE 230,31), and warfarin remains the only recommended treatment for patients with severe renal impairment, according to both AHA/ACC/HRS and European Society of Cardiology guidelines.5,18
Tools to help assess patients’ bleeding risk
Of the available scoring mechanisms to identify risk factors for bleeding, 3 have been specifically validated in AF populations (ie, ATRIA,33 HEMORR2HAGES,34 and HAS-BLED35). Of the 3, HAS-BLED is superior,36 the most practical, and recommended by expert guidelines.18,21,22 Additionally, HAS-BLED has good correlation with intracranial hemorrhage risk. The HAS-BLED score ranges from 0 to 9 points with one point assigned for each of the following:35
- Hypertension–uncontrolled with systolic BP >160 mm Hg
- Abnormal liver function–cirrhosis, bilirubin >2× normal, or liver enzymes >3× normal
- Abnormal renal function–dialysis, transplant, or serum creatinine >2.26 mg/dL
- Stroke history–including lacunar infarcts
- Bleeding predisposition–history of major bleeding due to any cause
- Labile international normalized ratio (INR)–time in therapeutic range <60%
- Elderly–age >65 years
- Drug–antiplatelet agents, including nonsteroidal anti-inflammatory drugs
- Alcohol usage–>8 drinks per week.
Patients with a HAS-BLED score ≥3 warrant additional monitoring and attempts to reduce bleeding risk by addressing modifiable risk factors. Bleeding risk scores should not be used to exclude patients from anticoagulation therapy.5 In fact, the British National Institute for Health and Clinical Excellence (NICE) guidelines state that anticoagulation should not be withheld solely due to fall risk.21
Also, anticoagulation with warfarin should not be permanently discontinued because of a single GI bleed, since restarting warfarin is associated with decreased risks of thromboembolism and mortality and a statistically insignificant increase in recurrent GI bleeding.37 Restarting DOAC therapy following a GI bleed has not been evaluated in clinical trials; however, it may be reasonable to use one of the DOAC doses with a lower risk of GI bleeding (dabigatran 110 mg BID, apixaban 5 mg BID, or edoxaban 30 mg/d) in patients who have experienced a GI bleed on warfarin or another DOAC.18,22
An online calculator is available that uses CHA2DS2-VASc and HAS-BLED scores to determine an individual’s risk/benefit profile with the various anticoagulation strategies available (http://www.sparctool.com). Consider percutaneous left atrial appendage occlusion if the risks of anticoagulation truly exceed the benefits.38
Rate control vs rhythm control
Most patients who present with AF require immediate ventricular rate control to reduce symptoms. In the acute setting, this can be accomplished with intravenous (IV) beta-blockers or IV calcium channel antagonists.5,39 If the patient is hemodynamically unstable, urgent direct-current cardioversion is the preferred treatment strategy and should not be delayed pending anticoagulation. IV amiodarone can be used in the ICU patient who does not require cardioversion, but is unable to tolerate beta-blockers or calcium channel antagonists.40 Once the patient is stable, long-term treatment focuses on ventricular rate control or restoration and maintenance of sinus rhythm.
The AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) trial enrolled 4060 patients (mean age 70 years, mean follow-up 3.5 years) with paroxysmal and persistent AF and randomized them to either pharmacologic rate control or rhythm control.41 No significant differences were found in all-cause mortality or in the composite secondary endpoint of death, ischemic stroke, anoxic encephalopathy, major bleeding, or cardiac arrest. In addition, no significant differences emerged in quality of life or global functional status. The number of patients requiring hospitalization during follow-up was significantly lower in the rate-control group vs the rhythm-control group (73% vs 80%; P<.001). Anticoagulation was encouraged but not mandated in the rhythm-control group after 4 weeks in sinus rhythm, and there was a trend toward higher mortality in the rhythm-control group (27% vs 26%; P=.08).
Patients <65 years were excluded from the AFFIRM trial. When younger patients experience significant symptoms, early referral to Cardiology should be considered to discuss the long-term benefits and risks of a rhythm-control strategy. Regardless of age, when patients remain symptomatic despite rate- or rhythm-control management, the strategy should be changed.5
Rate-control targets and options
Target heart rates should be individualized. The 2014 ACC/AHA/HRS guideline recommends a resting target heart rate <80 beats per minute (bpm) in symptomatic patients.5 In patients with permanent AF who remain asymptomatic at higher resting heart rates, a more lenient rate-control strategy (resting heart rate <110 bpm) has demonstrated outcomes equivalent to those of a more strict approach (resting heart rate <80 bpm and heart rate during moderate exercise <110 bpm).42 Pharmacologic rate-control options include beta-blockers, non-dihydropyridine calcium channel antagonists, and digoxin (TABLE 35). Digoxin is associated with increased all-cause mortality in patients with AF regardless of HF status (HR=1.4; 95% CI, 1.2-1.6, P=.0001).43 Digoxin should be reserved for patients who are sedentary or have inadequate control with first-line medications.5


Indications for rhythm control
The NICE guidelines, which are consistent with the ACC/AHA/HRS guidelines, recommend rate control as the first-line strategy for AF management, except in people:21
- whose AF has a reversible cause
- who have HF believed to be primarily caused by AF
- with new-onset AF
- with atrial flutter that is considered suitable for an ablation strategy to restore sinus rhythm
- for whom a rhythm-control strategy would be more suitable based on clinical judgment.
In addition, patients who continue to experience symptomatic AF despite an adequate trial of rate control should be offered rhythm control.5
Pharmacologic rhythm-control strategies. Antiarrhythmic drugs can be used for chemical cardioversion, reduction of paroxysms, and long-term maintenance of sinus rhythm. The most commonly used antiarrhythmic drugs are Class IC and Class III agents (TABLE 3).5 Tailored drug selection for each patient is key. Patients with left atrial diameters >4.5 cm are less likely to remain in sinus rhythm, and patients with left ventricular hypertrophy are at increased risk for proarrhythmic adverse effects.44 Patients with paroxysmal AF may be candidates for a “pill-in-the-pocket” strategy using propafenone or flecainide.5
AF frequently progresses from paroxysmal to persistent and can subsequently result in electrical and structural remodeling that becomes irreversible over time.45 The patient with uncontrolled symptoms despite attempts at rate control and rhythm control should be promptly referred to an electrophysiologist.
Surgical interventions for rate or rhythm control
Electrophysiology interventions include AV nodal ablation with pacemaker placement for rate control, or catheter-directed ablation (radiofrequency or cryotherapy) for rhythm control. CA appears to be more effective than pharmacologic rhythm control.46,47 Treatment with CA is indicated for symptomatic paroxysmal AF when a rhythm-control strategy is desired and the AF is refractory to, or the patient is intolerant of, at least one class I or III antiarrhythmic medication.5 With these same caveats, CA is a reasonable strategy for symptomatic persistent AF.
Consider more invasive interventions, such as an atrial maze procedure, when patients require cardiac surgery for another indication. Patients with an increased risk of thromboembolism (based on CHA2DS2-VASc) remain at high risk even after successful ablation.48 As a result, some guidelines recommend continued long-term anticoagulation following CA.18,22
CORRESPONDENCE
Philip Dooley, MD, University of Kansas School of Medicine–Wichita Family Medicine Residency at Via Christi, 707 North Emporia, Wichita, KS 67207; [email protected].
ACKNOWLEDGMENTS
We thank Professor Anne Walling, MB, ChB, FFPHM, Department of Family and Community Medicine, University of Kansas School of Medicine–Wichita for her suggestions and critical review of an earlier version of this manuscript.
Atrial fibrillation (AF)—the most common supraventricular tachycardia—affects as many as 6.1 million adults in the United States.1 It is associated with a 5-fold increased risk of stroke,2 a 3-fold increased risk of heart failure (HF),3 and about a 2-fold increased risk of dementia4 and mortality.2 The prevalence of AF increases with maturity, from 2% in people <65 years of age to 9% in those ≥65 years,5 and that prevalence is expected to double over the next 25 years as the population ages.1
The primary goals of treatment are to alleviate symptoms and prevent thromboembolism. Strokes related to AF are more likely to result in severe disability or death when compared with those unrelated to AF.6 And yet anticoagulation remains underutilized.7
The net clinical benefit of oral anticoagulation appears to be greatest in patients with the highest risk of bleeding, since these patients are also at the highest risk for stroke.8 Patients at increased risk of stroke are more likely to receive oral anticoagulation; however, for unknown reasons, more than half of people with the highest risk of stroke are not prescribed these important anti-blood-clotting medications.7 One theory is that physicians may be relying on their gut rather than objective risk scores, and underuse of validated schemata leads to poor estimation of risk.

For example, results from the ORBIT-AF (Outcomes Registry for Better Informed Treatment of Atrial Fibrillation) trial, which involved over 10,000 people with AF, found that although 72% (n=7251) had high-risk CHADS2 scores (≥2), only 16% were assessed as having a high risk of stroke by physicians.9 Along the same lines, a recent study of Canadian primary care physicians showed that stroke risk and bleeding risk were not evaluated with validated tools in 58% and 81% of patients, respectively, leading to both significant underestimation and overestimation of risk.10
This review provides the tools to identify when anticoagulation is indicated, reports the advantages and disadvantages of the currently available anticoagulants, and discusses the selection and implementation of rate- vs rhythm-control strategies. But first, a word about the etiology, classification, and diagnosis of AF.
AF: The result of any number of cardiac and non-cardiac causes
AF is characterized by uncoordinated activation of the atria, which results in ineffective atrial contractions and an irregular, often rapid, ventricular response. It is the ultimate clinical manifestation of multiple diseases that alter atrial tissue through inflammation, fibrosis, or hypertrophy.5 The most common causes are hypertension, coronary artery disease, HF, cardiomyopathies, and valvular heart disease, all of which stimulate the renin-angiotensin-aldosterone system, leading to increased susceptibility to arrhythmia.5 Atrial ectopic tachycardia, Wolff-Parkinson-White (WPW) syndrome, and atrioventricular (AV) nodal reentrant tachycardia also may precipitate AF.5 In these cases, AF usually resolves after catheter ablation (CA) of the primary arrhythmia.11 Unrecognized AF may trigger atrial flutter, and more than 80% of patients who undergo radiofrequency ablation for atrial flutter experience AF at some point in the subsequent 5 years.12
Non-cardiac causes of AF include sleep apnea, obesity, hyperthyroidism, drugs, electrocution, pneumonia, and pulmonary embolism.5 An association between binge drinking and AF (“holiday heart syndrome”) has long been recognized. The evidence now suggests that alcohol increases the risk of AF in a dose-dependent manner with intakes of ≥1 drink per day (12 g per drink).13
Classification schema no longer includes “lone AF”
AF is classified in terms of the duration of episodes:5
- Paroxysmal AF is characterized by brief episodes that terminate spontaneously or with intervention within 7 days of onset. These episodes recur with variable frequency.
- Persistent AF refers to AF that is continuously sustained for more than 7 days.
- Longstanding persistent AF refers to continuous AF that lasts longer than 12 months.
- Permanent AF is not an inherent pathophysiologic attribute of AF, but rather an acceptance of AF where the patient and physician abandon further efforts to restore and/or maintain sinus rhythm.
- Nonvalvular AF occurs in the absence of a valve replacement (mechanical or bioprosthetic), rheumatic mitral stenosis, or mitral valve repair.
Although paroxysmal and persistent AF may occur in the same individual, the distinction is still clinically relevant, as outcomes of certain therapies, such as CA, are superior in patients with paroxysmal AF.14 With a more complete understanding of AF pathophysiology, guidelines now discourage use of the potentially confusing term “lone AF,” which has historically been applied to younger patients with no known clinical risk factors or echocardiographic abnormalities. As a result, therapeutic decisions are no longer based on this nomenclature, according to the 2014 AF practice guideline from the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Rhythm Society (HRS).5
Patient complaints—or incidental findings—can prompt a Dx
Fatigue is the most common symptom of AF. Other signs and symptoms include palpitations, dyspnea, HF, hypotension, syncope, chest pain, and stroke. Some patients are asymptomatic, and AF is an incidental finding when an irregular pulse is discovered during a physical examination. The diagnosis is confirmed by electrocardiogram (EKG), telemetry, Holter monitor, event recorder, or an implanted electrocardiographic recording device. A chest x-ray, serum electrolyte levels, a complete blood count, thyroid testing, and renal and hepatic function testing are recommended. Transthoracic echocardiography to measure cardiac function, detect underlying structural heart disease, and evaluate atrial size is essential.5
An electrophysiologic (EP) study may be needed for diagnosis or treatment if another arrhythmia is present. Aberrant conduction may cause AF to present as a wide complex tachycardia and be mislabeled as ventricular tachycardia. The presence of delta waves is an indication for an EP study targeting the WPW accessory pathway. Transesophageal echocardiography (TEE) is the most sensitive and specific test for left atrial thrombi. If you are considering a TEE for a patient with AF of unknown, or >48 hours’, duration who has not been anticoagulated in the preceding 3 weeks, obtain it before performing cardioversion because of the risk of embolism.5
Stroke prevention
The ACC/AHA/HRS AF guideline recommends basing anticoagulation decisions on thromboembolic risk, regardless of AF pattern (paroxysmal, persistent, or permanent) (Class I recommendation).5 For patients with nonvalvular AF and atrial flutter, the guideline recommends using the Birmingham 2009 schema (CHA2DS2-VASc score) (TABLE 115-18) to estimate thromboembolic risk.5,15 CHA2DS2-VASc improves on the older CHADS2 score by significantly reducing the number of patients categorized as having intermediate risk and better identifying truly low-risk patients who are unlikely to benefit from anticoagulation.16,17,19

Men with a CHA2DS2-VASc score of zero and women with a score of one do not need anticoagulation.5,20 Discuss the risks and benefits of oral anticoagulation with men who have a score of one. In these intermediate-risk men, antiplatelet therapy with aspirin and/or clopidogrel may be reasonable, especially if there is an indication other than stroke prevention (eg, post-myocardial infarction). Oral anticoagulation is strongly recommended for all patients with a CHA2DS2-VASc score of 2 or higher.5,18,21,22
Anticoagulant considerations: Warfarin vs DOACs
Warfarin was the gold standard for stroke prevention in nonvalvular AF until the direct oral anticoagulants (DOACs) became available in 2010. Guidelines in the United States and the United Kingdom recommend shared decision-making to help patients with AF who do not have a specific indication for warfarin choose between warfarin and the DOACs.5,21 Canadian and European guidelines recommend DOACs as the first-line option for anticoagulation and reserve warfarin for patients who have contraindications to, or are unable to afford, DOACs.18,22 All current guidelines recommend continuing warfarin in patients who are stable, well controlled, and satisfied with warfarin therapy and the monitoring and dietary restrictions it entails.
DOACs are as effective as warfarin. All of the DOACs are approved for stroke prevention based on individual phase III non-inferiority trials in which they were compared to warfarin.23-26 In addition, a meta-analysis of these 4 trials involving a total of 71,683 patients (mean age 70-73 years; median follow-up, 1.8-2.8 years) evaluated the benefits and risks of the 4 DOACs against the former gold standard.27
Higher doses of the DOACs (dabigatran 150 mg BID, rivaroxaban 20 mg/d, edoxaban 60 mg/d, and apixaban 5 mg BID) reduced the rates of stroke or systemic embolism (relative risk [RR]=0.81; 95% confidence interval [CI], 0.73-0.91; P<.0001; number needed to treat [NNT]=147), hemorrhagic stroke (RR=0.49; 95% CI, 0.38-0.64; P<.0001; NNT=219), and all-cause mortality (RR=0.90; 95% CI, 0.85-0.95; P=.0003; NNT=128), compared with warfarin.27 It is important to note that while lower doses of some DOACs (dabigatran 110 mg BID and edoxaban 30 mg/d) were not as effective at preventing ischemic stroke when compared with warfarin (RR=1.3; 95% CI, 1-1.6; P=.045), they still significantly reduced hemorrhagic stroke (RR=0.33; 95% CI, 0.23-0.46; P<.0001) and all-cause mortality (RR=0.89; 95% CI, 0.83-0.96; P=.003).
Of course, the biggest concern is bleeding. In that same meta-analysis, the difference in major bleeding events with DOACs vs warfarin was not statistically significant (RR=0.86; 95% CI, 0.73-1; P=.06). While DOACs likely lower rates of intracranial hemorrhage (RR=0.48; 95% CI, 0.39-0.59; P<.0001; NNT=132), they seem to increase the risk of gastrointestinal (GI) bleeding (RR=1.3; 95% CI, 1-1.6; P=.043; number needed to harm [NNH]=185).27
There was significant heterogeneity in the GI bleeding outcome, however. When compared with warfarin, GI bleeding was increased by dabigatran 150 mg BID (RR=1.5; 95% CI, 1.2-1.9; P<.001) and edoxaban 60 mg/d (HR=1.2; 95% CI, 1.02-1.5; P=.03), but there were no significant differences for dabigatran 110 mg BID or apixaban 5 mg BID.23,25,26
On the other hand, edoxaban 30 mg/d had a lower risk of GI bleeding when compared with warfarin (HR=0.67; 95% CI, 0.53-0.83; P<.001).25 Without head-to-head trials, it is impossible to know if one DOAC is superior to another. Apixaban 5 mg BID appears to offer the best overall balance between efficacy and safety. Other DOACs may be better options for patients who have specific concerns regarding efficacy or safety.28,29
Convenience, interactions, and cost may be the deciding factors. Since all DOACs are fairly comparable in efficacy and safety, other factors such as convenience, interactions with other medications, and cost should be considered when deciding on a medication for an individual patient (TABLE 230,31). The DOACs require no lab monitoring or dose titration, and all 4 have fewer potential drug interactions than warfarin.30 Due to their relatively short half-lives, strict adherence is critical; DOACs are not suitable for patients who frequently miss doses.5 (For more information on starting or switching to DOACs, see, “Is a novel anticoagulant right for your patient?” J Fam Pract. 2014;63:22-28.)

A word about DOACs and renal impairment. Another concern with DOACs is their reliance on renal metabolism and excretion. A meta-analysis of the 4 phase III trials of the DOACs, this time involving 58,338 patients, evaluated DOAC efficacy and safety compared to warfarin in the presence of kidney dysfunction.32 Renal function was categorized as normal (estimated glomerular filtration rate [eGFR] >80 mL/min/1.73 m2), mildly impaired (eGFR 50-80 mL/min/1.73 m2), or moderately impaired (eGFR <50 mL/min/1.73m2). Compared with warfarin, DOACs lowered stroke risk in patients with mild (RR=0.71; 95% CI, 0.62-0.81) or moderate (RR=0.79; 95% CI, 0.66-0.94) renal impairment. DOACs also reduced major bleeding compared to warfarin in patients with mild (RR=0.88; 95% CI, 0.80-0.97) or moderate (RR=0.80; 95% CI, 0.66-0.94) renal impairment. How the DOACs fare in patients with severe renal dysfunction could not be determined because such patients were excluded from the trials.
Keep in mind that the DOACs require dose adjustment at different levels of renal impairment (TABLE 230,31), and warfarin remains the only recommended treatment for patients with severe renal impairment, according to both AHA/ACC/HRS and European Society of Cardiology guidelines.5,18
Tools to help assess patients’ bleeding risk
Of the available scoring mechanisms to identify risk factors for bleeding, 3 have been specifically validated in AF populations (ie, ATRIA,33 HEMORR2HAGES,34 and HAS-BLED35). Of the 3, HAS-BLED is superior,36 the most practical, and recommended by expert guidelines.18,21,22 Additionally, HAS-BLED has good correlation with intracranial hemorrhage risk. The HAS-BLED score ranges from 0 to 9 points with one point assigned for each of the following:35
- Hypertension–uncontrolled with systolic BP >160 mm Hg
- Abnormal liver function–cirrhosis, bilirubin >2× normal, or liver enzymes >3× normal
- Abnormal renal function–dialysis, transplant, or serum creatinine >2.26 mg/dL
- Stroke history–including lacunar infarcts
- Bleeding predisposition–history of major bleeding due to any cause
- Labile international normalized ratio (INR)–time in therapeutic range <60%
- Elderly–age >65 years
- Drug–antiplatelet agents, including nonsteroidal anti-inflammatory drugs
- Alcohol usage–>8 drinks per week.
Patients with a HAS-BLED score ≥3 warrant additional monitoring and attempts to reduce bleeding risk by addressing modifiable risk factors. Bleeding risk scores should not be used to exclude patients from anticoagulation therapy.5 In fact, the British National Institute for Health and Clinical Excellence (NICE) guidelines state that anticoagulation should not be withheld solely due to fall risk.21
Also, anticoagulation with warfarin should not be permanently discontinued because of a single GI bleed, since restarting warfarin is associated with decreased risks of thromboembolism and mortality and a statistically insignificant increase in recurrent GI bleeding.37 Restarting DOAC therapy following a GI bleed has not been evaluated in clinical trials; however, it may be reasonable to use one of the DOAC doses with a lower risk of GI bleeding (dabigatran 110 mg BID, apixaban 5 mg BID, or edoxaban 30 mg/d) in patients who have experienced a GI bleed on warfarin or another DOAC.18,22
An online calculator is available that uses CHA2DS2-VASc and HAS-BLED scores to determine an individual’s risk/benefit profile with the various anticoagulation strategies available (http://www.sparctool.com). Consider percutaneous left atrial appendage occlusion if the risks of anticoagulation truly exceed the benefits.38
Rate control vs rhythm control
Most patients who present with AF require immediate ventricular rate control to reduce symptoms. In the acute setting, this can be accomplished with intravenous (IV) beta-blockers or IV calcium channel antagonists.5,39 If the patient is hemodynamically unstable, urgent direct-current cardioversion is the preferred treatment strategy and should not be delayed pending anticoagulation. IV amiodarone can be used in the ICU patient who does not require cardioversion, but is unable to tolerate beta-blockers or calcium channel antagonists.40 Once the patient is stable, long-term treatment focuses on ventricular rate control or restoration and maintenance of sinus rhythm.
The AFFIRM (Atrial Fibrillation Follow-up Investigation of Rhythm Management) trial enrolled 4060 patients (mean age 70 years, mean follow-up 3.5 years) with paroxysmal and persistent AF and randomized them to either pharmacologic rate control or rhythm control.41 No significant differences were found in all-cause mortality or in the composite secondary endpoint of death, ischemic stroke, anoxic encephalopathy, major bleeding, or cardiac arrest. In addition, no significant differences emerged in quality of life or global functional status. The number of patients requiring hospitalization during follow-up was significantly lower in the rate-control group vs the rhythm-control group (73% vs 80%; P<.001). Anticoagulation was encouraged but not mandated in the rhythm-control group after 4 weeks in sinus rhythm, and there was a trend toward higher mortality in the rhythm-control group (27% vs 26%; P=.08).
Patients <65 years were excluded from the AFFIRM trial. When younger patients experience significant symptoms, early referral to Cardiology should be considered to discuss the long-term benefits and risks of a rhythm-control strategy. Regardless of age, when patients remain symptomatic despite rate- or rhythm-control management, the strategy should be changed.5
Rate-control targets and options
Target heart rates should be individualized. The 2014 ACC/AHA/HRS guideline recommends a resting target heart rate <80 beats per minute (bpm) in symptomatic patients.5 In patients with permanent AF who remain asymptomatic at higher resting heart rates, a more lenient rate-control strategy (resting heart rate <110 bpm) has demonstrated outcomes equivalent to those of a more strict approach (resting heart rate <80 bpm and heart rate during moderate exercise <110 bpm).42 Pharmacologic rate-control options include beta-blockers, non-dihydropyridine calcium channel antagonists, and digoxin (TABLE 35). Digoxin is associated with increased all-cause mortality in patients with AF regardless of HF status (HR=1.4; 95% CI, 1.2-1.6, P=.0001).43 Digoxin should be reserved for patients who are sedentary or have inadequate control with first-line medications.5


Indications for rhythm control
The NICE guidelines, which are consistent with the ACC/AHA/HRS guidelines, recommend rate control as the first-line strategy for AF management, except in people:21
- whose AF has a reversible cause
- who have HF believed to be primarily caused by AF
- with new-onset AF
- with atrial flutter that is considered suitable for an ablation strategy to restore sinus rhythm
- for whom a rhythm-control strategy would be more suitable based on clinical judgment.
In addition, patients who continue to experience symptomatic AF despite an adequate trial of rate control should be offered rhythm control.5
Pharmacologic rhythm-control strategies. Antiarrhythmic drugs can be used for chemical cardioversion, reduction of paroxysms, and long-term maintenance of sinus rhythm. The most commonly used antiarrhythmic drugs are Class IC and Class III agents (TABLE 3).5 Tailored drug selection for each patient is key. Patients with left atrial diameters >4.5 cm are less likely to remain in sinus rhythm, and patients with left ventricular hypertrophy are at increased risk for proarrhythmic adverse effects.44 Patients with paroxysmal AF may be candidates for a “pill-in-the-pocket” strategy using propafenone or flecainide.5
AF frequently progresses from paroxysmal to persistent and can subsequently result in electrical and structural remodeling that becomes irreversible over time.45 The patient with uncontrolled symptoms despite attempts at rate control and rhythm control should be promptly referred to an electrophysiologist.
Surgical interventions for rate or rhythm control
Electrophysiology interventions include AV nodal ablation with pacemaker placement for rate control, or catheter-directed ablation (radiofrequency or cryotherapy) for rhythm control. CA appears to be more effective than pharmacologic rhythm control.46,47 Treatment with CA is indicated for symptomatic paroxysmal AF when a rhythm-control strategy is desired and the AF is refractory to, or the patient is intolerant of, at least one class I or III antiarrhythmic medication.5 With these same caveats, CA is a reasonable strategy for symptomatic persistent AF.
Consider more invasive interventions, such as an atrial maze procedure, when patients require cardiac surgery for another indication. Patients with an increased risk of thromboembolism (based on CHA2DS2-VASc) remain at high risk even after successful ablation.48 As a result, some guidelines recommend continued long-term anticoagulation following CA.18,22
CORRESPONDENCE
Philip Dooley, MD, University of Kansas School of Medicine–Wichita Family Medicine Residency at Via Christi, 707 North Emporia, Wichita, KS 67207; [email protected].
ACKNOWLEDGMENTS
We thank Professor Anne Walling, MB, ChB, FFPHM, Department of Family and Community Medicine, University of Kansas School of Medicine–Wichita for her suggestions and critical review of an earlier version of this manuscript.
1. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults. National implications for Rhythm Management and Stroke Prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370-2375.
2. Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N-9N.
3. Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba follow-up study. Am J Med. 1995;98:476-484.
4. Ott A, Breteler MMB, de Bruyne MC, et al. Atrial fibrillation and dementia in a population-based study: The Rotterdam Study. Stroke. 1997;28:316-321.
5. January CT, Wann L, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1-e76.
6. Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. Stroke. 1996;27:1760-1764.
7. Hsu JC, Maddox TM, Kennedy KF, et al. Oral anticoagulant therapy prescription in patients with atrial fibrillation across the spectrum of stroke risk: insights from the NCDR PINNACLE registry. JAMA Cardiol. 2016;1:55-62.
8. Olesen JB, Lip GY, Lindhardsen J, et al. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: a net clinical benefit analysis using a ‘real world’ nationwide cohort study. Thromb Haemost. 2011;106:739-749.
9. Steinberg BA, Kim S, Thomas L, et al. Lack of concordance between empirical scores and physician assessments of stroke and bleeding risk in atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Circulation. 2014;129:2005-2012.
10. Angaran P, Dorian P, Tan MK, et al. The risk stratification and stroke prevention therapy care gap in Canadian atrial fibrillation patients. Can J Cardiol. 2016;32:336-343.
11. Waldo AL, Feld GK. Inter-relationships of atrial fibrillation and atrial flutter: mechanisms and clinical implications. J Am Coll Cardiol. 2008;51:779-786.
12. Ellis K, Wazni O, Marrouche N, et al. Incidence of atrial fibrillation post-cavotricuspid isthmus ablation in patients with typical atrial flutter: left-atrial size as an independent predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol. 2007;18:799-802.
13. Larsson SC, Drca N, Wolk A. Alcohol consumption and risk of atrial fibrillation: a prospective study and dose-response meta-analysis. J Am Coll Cardiol. 2014;64:281-289.
14. Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. J Interv Card Electrophysiol. 2012;33:171-257.
15. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263-272.
16. Lip GYH, Frison L, Halperin JL, et al. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010;41:2731-2738.
17. Olesen JB, Lip GYH, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124.
18. Camm AJ, Lip GYH, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719-2747.
19. Olesen JB, Torp-Pedersen C, Hansen ML, et al. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0-1: a nationwide cohort study. Thromb Haemost. 2012;107:1172-1179.
20. Friberg L, Benson L, Rosenqvist M, et al. Assessment of female sex as a risk factor in atrial fibrillation in Sweden: nationwide retrospective cohort study. BMJ. 2012;344:e3522.
21. National Institute for Health and Clinical Excellence (NICE). Atrial fibrillation: the management of atrial fibrillation [CG180]. 2014. Available at: https://www.nice.org.uk/guidance/cg180. Accessed July 31, 2016.
22. Verma A, Cairns JA, Mitchell LB, et al. 2014 focused update of the Canadian Cardiovascular Society Guidelines for the management of atrial fibrillation. Can J Cardiol. 2014;30:1114-1130.
23. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
24. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
25. Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093-2104.
26. Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992.
27. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
28. Morimoto T, Crawford B, Wada K, et al. Comparative efficacy and safety of novel oral anticoagulants in patients with atrial fibrillation: a network meta-analysis with the adjustment for the possible bias from open label studies. J Cardiol. 2015;66:466-474.
29. Verdecchia P, Angeli F, Bartolini C, et al. Safety and efficacy of non-vitamin K oral anticoagulants in non-valvular atrial fibrillation: a Bayesian meta-analysis approach. Expert Opin Drug Saf. 2015;14:7-20.
30. Micromedex® 2.0 (electronic version). Truven Health Analytics, Greenwood Village, Colorado, USA. Available at: http://www.micromedexsolutions.com. Accessed August 18, 2016.
31. GoodRx. Available at: https://www.goodrx.com. Accessed August 18, 2016.
32. Del-Carpio Munoz F, Gharacholou SM, Munger TM, et al. Meta-analysis of renal function on the safety and efficacy of novel oral anticoagulants for atrial fibrillation. Am J Cardiol. 2016;117:69-75.
33. Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58:395-401.
34. Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J. 2006;151:713-719.
35. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093-1100.
36. Zhu W, He W, Guo L, et al. The HAS-BLED Score for predicting major bleeding risk in anticoagulated patients with atrial fibrillation: a systematic review and meta-analysis. Clin Cardiol. 2015;38:555-561.
37. Chai-Adisaksopha C, Hillis C, Monreal M, et al. Thromboembolic events, recurrent bleeding and mortality after resuming anticoagulant following gastrointestinal bleeding. A meta-analysis. Thromb Haemost. 2015;114:819-825.
38. Xu H, Xie X, Wang B, et al. Efficacy and safety of percutaneous left atrial appendage occlusion for stroke prevention in nonvalvular atrial fibrillation: a meta-analysis of contemporary studies. Heart Lung Circ. 2016;25:1107-1117.
39. Siu CW, Lau CP, Lee WL, et al. Intravenous diltiazem is superior to intravenous amiodarone or digoxin for achieving ventricular rate control in patients with acute uncomplicated atrial fibrillation. Crit Care Med. 2009;37:2174-2179.
40. Clemo HF, Wood MA, Gilligan DM, et al. Intravenous amiodarone for acute heart rate control in the critically ill patient with atrial tachyarrhythmias. Am J Cardiol. 1998;81:594-598.
41. The Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
42. Van Gelder IC, Groenveld HF, Crijns HJGM, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363-1373.
43. Wang ZQ, Zhang R, Chen MT, et al. Digoxin is associated with increased all-cause mortality in patients with atrial fibrillation regardless of concomitant heart failure: a meta-analysis. J Cardiovasc Pharmacol. 2015;66:270-275.
44. Olshansky B, Heller EN, Mitchell LB, et al. Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke? Results from the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study. J Am Coll Cardiol. 2005;45:2026-2033.
45. de Vos CB, Pisters R, Nieuwlaat R, et al. Progression from paroxysmal to persistent atrial fibrillation: clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725-731.
46. Cheng X, Li X, He Y, et al. Catheter ablation versus anti-arrhythmic drug therapy for the management of atrial fibrillation: a meta-analysis. J Interv Card Electrophysiol. 2014;41:267-272.
47. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133:1637-1644.
48.
1. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults. National implications for Rhythm Management and Stroke Prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370-2375.
2. Kannel WB, Wolf PA, Benjamin EJ, et al. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N-9N.
3. Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba follow-up study. Am J Med. 1995;98:476-484.
4. Ott A, Breteler MMB, de Bruyne MC, et al. Atrial fibrillation and dementia in a population-based study: The Rotterdam Study. Stroke. 1997;28:316-321.
5. January CT, Wann L, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1-e76.
6. Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. Stroke. 1996;27:1760-1764.
7. Hsu JC, Maddox TM, Kennedy KF, et al. Oral anticoagulant therapy prescription in patients with atrial fibrillation across the spectrum of stroke risk: insights from the NCDR PINNACLE registry. JAMA Cardiol. 2016;1:55-62.
8. Olesen JB, Lip GY, Lindhardsen J, et al. Risks of thromboembolism and bleeding with thromboprophylaxis in patients with atrial fibrillation: a net clinical benefit analysis using a ‘real world’ nationwide cohort study. Thromb Haemost. 2011;106:739-749.
9. Steinberg BA, Kim S, Thomas L, et al. Lack of concordance between empirical scores and physician assessments of stroke and bleeding risk in atrial fibrillation: results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Circulation. 2014;129:2005-2012.
10. Angaran P, Dorian P, Tan MK, et al. The risk stratification and stroke prevention therapy care gap in Canadian atrial fibrillation patients. Can J Cardiol. 2016;32:336-343.
11. Waldo AL, Feld GK. Inter-relationships of atrial fibrillation and atrial flutter: mechanisms and clinical implications. J Am Coll Cardiol. 2008;51:779-786.
12. Ellis K, Wazni O, Marrouche N, et al. Incidence of atrial fibrillation post-cavotricuspid isthmus ablation in patients with typical atrial flutter: left-atrial size as an independent predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol. 2007;18:799-802.
13. Larsson SC, Drca N, Wolk A. Alcohol consumption and risk of atrial fibrillation: a prospective study and dose-response meta-analysis. J Am Coll Cardiol. 2014;64:281-289.
14. Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. J Interv Card Electrophysiol. 2012;33:171-257.
15. Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263-272.
16. Lip GYH, Frison L, Halperin JL, et al. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010;41:2731-2738.
17. Olesen JB, Lip GYH, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124.
18. Camm AJ, Lip GYH, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719-2747.
19. Olesen JB, Torp-Pedersen C, Hansen ML, et al. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0-1: a nationwide cohort study. Thromb Haemost. 2012;107:1172-1179.
20. Friberg L, Benson L, Rosenqvist M, et al. Assessment of female sex as a risk factor in atrial fibrillation in Sweden: nationwide retrospective cohort study. BMJ. 2012;344:e3522.
21. National Institute for Health and Clinical Excellence (NICE). Atrial fibrillation: the management of atrial fibrillation [CG180]. 2014. Available at: https://www.nice.org.uk/guidance/cg180. Accessed July 31, 2016.
22. Verma A, Cairns JA, Mitchell LB, et al. 2014 focused update of the Canadian Cardiovascular Society Guidelines for the management of atrial fibrillation. Can J Cardiol. 2014;30:1114-1130.
23. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
24. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891.
25. Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093-2104.
26. Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992.
27. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
28. Morimoto T, Crawford B, Wada K, et al. Comparative efficacy and safety of novel oral anticoagulants in patients with atrial fibrillation: a network meta-analysis with the adjustment for the possible bias from open label studies. J Cardiol. 2015;66:466-474.
29. Verdecchia P, Angeli F, Bartolini C, et al. Safety and efficacy of non-vitamin K oral anticoagulants in non-valvular atrial fibrillation: a Bayesian meta-analysis approach. Expert Opin Drug Saf. 2015;14:7-20.
30. Micromedex® 2.0 (electronic version). Truven Health Analytics, Greenwood Village, Colorado, USA. Available at: http://www.micromedexsolutions.com. Accessed August 18, 2016.
31. GoodRx. Available at: https://www.goodrx.com. Accessed August 18, 2016.
32. Del-Carpio Munoz F, Gharacholou SM, Munger TM, et al. Meta-analysis of renal function on the safety and efficacy of novel oral anticoagulants for atrial fibrillation. Am J Cardiol. 2016;117:69-75.
33. Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol. 2011;58:395-401.
34. Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J. 2006;151:713-719.
35. Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093-1100.
36. Zhu W, He W, Guo L, et al. The HAS-BLED Score for predicting major bleeding risk in anticoagulated patients with atrial fibrillation: a systematic review and meta-analysis. Clin Cardiol. 2015;38:555-561.
37. Chai-Adisaksopha C, Hillis C, Monreal M, et al. Thromboembolic events, recurrent bleeding and mortality after resuming anticoagulant following gastrointestinal bleeding. A meta-analysis. Thromb Haemost. 2015;114:819-825.
38. Xu H, Xie X, Wang B, et al. Efficacy and safety of percutaneous left atrial appendage occlusion for stroke prevention in nonvalvular atrial fibrillation: a meta-analysis of contemporary studies. Heart Lung Circ. 2016;25:1107-1117.
39. Siu CW, Lau CP, Lee WL, et al. Intravenous diltiazem is superior to intravenous amiodarone or digoxin for achieving ventricular rate control in patients with acute uncomplicated atrial fibrillation. Crit Care Med. 2009;37:2174-2179.
40. Clemo HF, Wood MA, Gilligan DM, et al. Intravenous amiodarone for acute heart rate control in the critically ill patient with atrial tachyarrhythmias. Am J Cardiol. 1998;81:594-598.
41. The Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Investigators. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825-1833.
42. Van Gelder IC, Groenveld HF, Crijns HJGM, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363-1373.
43. Wang ZQ, Zhang R, Chen MT, et al. Digoxin is associated with increased all-cause mortality in patients with atrial fibrillation regardless of concomitant heart failure: a meta-analysis. J Cardiovasc Pharmacol. 2015;66:270-275.
44. Olshansky B, Heller EN, Mitchell LB, et al. Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke? Results from the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study. J Am Coll Cardiol. 2005;45:2026-2033.
45. de Vos CB, Pisters R, Nieuwlaat R, et al. Progression from paroxysmal to persistent atrial fibrillation: clinical correlates and prognosis. J Am Coll Cardiol. 2010;55:725-731.
46. Cheng X, Li X, He Y, et al. Catheter ablation versus anti-arrhythmic drug therapy for the management of atrial fibrillation: a meta-analysis. J Interv Card Electrophysiol. 2014;41:267-272.
47. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133:1637-1644.
48.
PRACTICE RECOMMENDATIONS
› Use the CHA2DS2-VASc score to assess the risk of thromboembolism, including ischemic stroke. A
› Consider prescribing a direct oral anticoagulant (DOAC) instead of warfarin for patients with nonvalvular atrial fibrillation (AF) because they are superior at preventing strokes and lowering all-cause mortality in this population. B
› Do not use a DOAC in patients with mechanical heart valves, hemodynamically significant mitral stenosis, or severe chronic kidney disease (estimated glomerular filtration rate [eGFR] <30 mL/min/1.73 m2). A
› Pursue a rate-control strategy for most patients with AF, although rhythm control may be preferable for younger (<65 years) symptomatic patients. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
How to provide effective pain treatment
Drugs may be effective against hematologic, other cancers

Image courtesy of PNAS
A diabetes medication and an antihypertensive drug may prove effective in the treatment of hematologic malignancies and other cancers, according to preclinical research published in Science Advances.
Past research has shown that metformin, a drug used to treat type 2 diabetes, has anticancer properties.
However, the usual therapeutic dose is too low to effectively fight cancer, and higher doses of metformin could be too toxic.
With the current study, researchers found that the antihypertensive drug syrosingopine enhances the anticancer efficacy of metformin without harming normal blood cells.
The team screened over a thousand drugs to find one that could boost metformin’s efficacy against cancers.
They identified syrosingopine and tested it in combination with metformin—at concentrations substantially below the drugs’ therapeutic thresholds—on a range of cancer cell lines and in mouse models of liver cancer.
Thirty-five of the 43 cell lines tested were susceptible to both syrosingopine and metformin. This included leukemia, lymphoma, and multiple myeloma cell lines.
In addition, the mice given a short course of syrosingopine and metformin experienced a reduction in the number of visible liver tumors.
The researchers also tested syrosingopine and metformin in peripheral blasts from 12 patients with acute myeloid leukemia and a patient with blast crisis chronic myeloid leukemia. All 13 samples responded to the treatment.
On the other hand, syrosingopine and metformin did not affect peripheral blood cells from healthy subjects.
“[A]lmost all tumor cells were killed by this cocktail and at doses that are actually not toxic to normal cells,” said study author Don Benjamin, of the University of Basel in Switzerland.
“And the effect was exclusively confined to cancer cells, as the blood cells from healthy donors were insensitive to the treatment.”
The researchers believe metformin functions by lowering blood glucose levels for cancer cells, starving them of essential nutrients needed for their survival. However, it is not clear how syrosingopine works in conjunction with metformin.
The team emphasized the need for more research evaluating the drugs in combination.
“We have been able to show that the 2 known drugs lead to more profound effects on cancer cell proliferation than each drug alone,” Dr Benjamin said. “The data from this study support the development of combination approaches for the treatment of cancer patients.” ![]()

Image courtesy of PNAS
A diabetes medication and an antihypertensive drug may prove effective in the treatment of hematologic malignancies and other cancers, according to preclinical research published in Science Advances.
Past research has shown that metformin, a drug used to treat type 2 diabetes, has anticancer properties.
However, the usual therapeutic dose is too low to effectively fight cancer, and higher doses of metformin could be too toxic.
With the current study, researchers found that the antihypertensive drug syrosingopine enhances the anticancer efficacy of metformin without harming normal blood cells.
The team screened over a thousand drugs to find one that could boost metformin’s efficacy against cancers.
They identified syrosingopine and tested it in combination with metformin—at concentrations substantially below the drugs’ therapeutic thresholds—on a range of cancer cell lines and in mouse models of liver cancer.
Thirty-five of the 43 cell lines tested were susceptible to both syrosingopine and metformin. This included leukemia, lymphoma, and multiple myeloma cell lines.
In addition, the mice given a short course of syrosingopine and metformin experienced a reduction in the number of visible liver tumors.
The researchers also tested syrosingopine and metformin in peripheral blasts from 12 patients with acute myeloid leukemia and a patient with blast crisis chronic myeloid leukemia. All 13 samples responded to the treatment.
On the other hand, syrosingopine and metformin did not affect peripheral blood cells from healthy subjects.
“[A]lmost all tumor cells were killed by this cocktail and at doses that are actually not toxic to normal cells,” said study author Don Benjamin, of the University of Basel in Switzerland.
“And the effect was exclusively confined to cancer cells, as the blood cells from healthy donors were insensitive to the treatment.”
The researchers believe metformin functions by lowering blood glucose levels for cancer cells, starving them of essential nutrients needed for their survival. However, it is not clear how syrosingopine works in conjunction with metformin.
The team emphasized the need for more research evaluating the drugs in combination.
“We have been able to show that the 2 known drugs lead to more profound effects on cancer cell proliferation than each drug alone,” Dr Benjamin said. “The data from this study support the development of combination approaches for the treatment of cancer patients.” ![]()

Image courtesy of PNAS
A diabetes medication and an antihypertensive drug may prove effective in the treatment of hematologic malignancies and other cancers, according to preclinical research published in Science Advances.
Past research has shown that metformin, a drug used to treat type 2 diabetes, has anticancer properties.
However, the usual therapeutic dose is too low to effectively fight cancer, and higher doses of metformin could be too toxic.
With the current study, researchers found that the antihypertensive drug syrosingopine enhances the anticancer efficacy of metformin without harming normal blood cells.
The team screened over a thousand drugs to find one that could boost metformin’s efficacy against cancers.
They identified syrosingopine and tested it in combination with metformin—at concentrations substantially below the drugs’ therapeutic thresholds—on a range of cancer cell lines and in mouse models of liver cancer.
Thirty-five of the 43 cell lines tested were susceptible to both syrosingopine and metformin. This included leukemia, lymphoma, and multiple myeloma cell lines.
In addition, the mice given a short course of syrosingopine and metformin experienced a reduction in the number of visible liver tumors.
The researchers also tested syrosingopine and metformin in peripheral blasts from 12 patients with acute myeloid leukemia and a patient with blast crisis chronic myeloid leukemia. All 13 samples responded to the treatment.
On the other hand, syrosingopine and metformin did not affect peripheral blood cells from healthy subjects.
“[A]lmost all tumor cells were killed by this cocktail and at doses that are actually not toxic to normal cells,” said study author Don Benjamin, of the University of Basel in Switzerland.
“And the effect was exclusively confined to cancer cells, as the blood cells from healthy donors were insensitive to the treatment.”
The researchers believe metformin functions by lowering blood glucose levels for cancer cells, starving them of essential nutrients needed for their survival. However, it is not clear how syrosingopine works in conjunction with metformin.
The team emphasized the need for more research evaluating the drugs in combination.
“We have been able to show that the 2 known drugs lead to more profound effects on cancer cell proliferation than each drug alone,” Dr Benjamin said. “The data from this study support the development of combination approaches for the treatment of cancer patients.” ![]()
Shoulder Dislocations
IN THIS ARTICLE
- Types of shoulder dislocations
- Schematics of the shoulder with three types of dislocations
- Association with seizures
CASE A 59-year-old man with a remote history of seizures is transported to the emergency department (ED) by ambulance after a witnessed tonic-clonic seizure. At the time of arrival he is postictal and confused, but his vital signs are stable. A left eyebrow laceration indicating a possible fall is observed on physical exam, as is a left shoulder displacement with no obvious signs of neurovascular compromise. The patient is not currently taking anticonvulsant medication, stating that he has been “seizure free” for five years, and therefore chose to discontinue taking phenytoin against medical advice.
An anteroposterior (AP) bilateral shoulder x-ray is obtained in the ED (see Figures 1a and 1b). The image shows the humeral head to be anteriorly dislocated and reveals a large impaction fracture of the posterior superior humeral head. For a more detailed view of the fracture and to further assess any associated deformities, CT of the left shoulder is performed. The fracture has a depth of 11.6 mm and a length of 24.1 mm, with no additional pathology noted (see Figure 1c).
The shoulder is a large joint capable of moving in many directions and therefore is inherently unstable. The glenoid fossa is shallow, and stability of the joint is provided by both the fibrocartilaginous labrum and varying muscles of the rotator cuff. Because the shoulder joint is poorly supported, dislocations are not uncommon (see the illustrations).
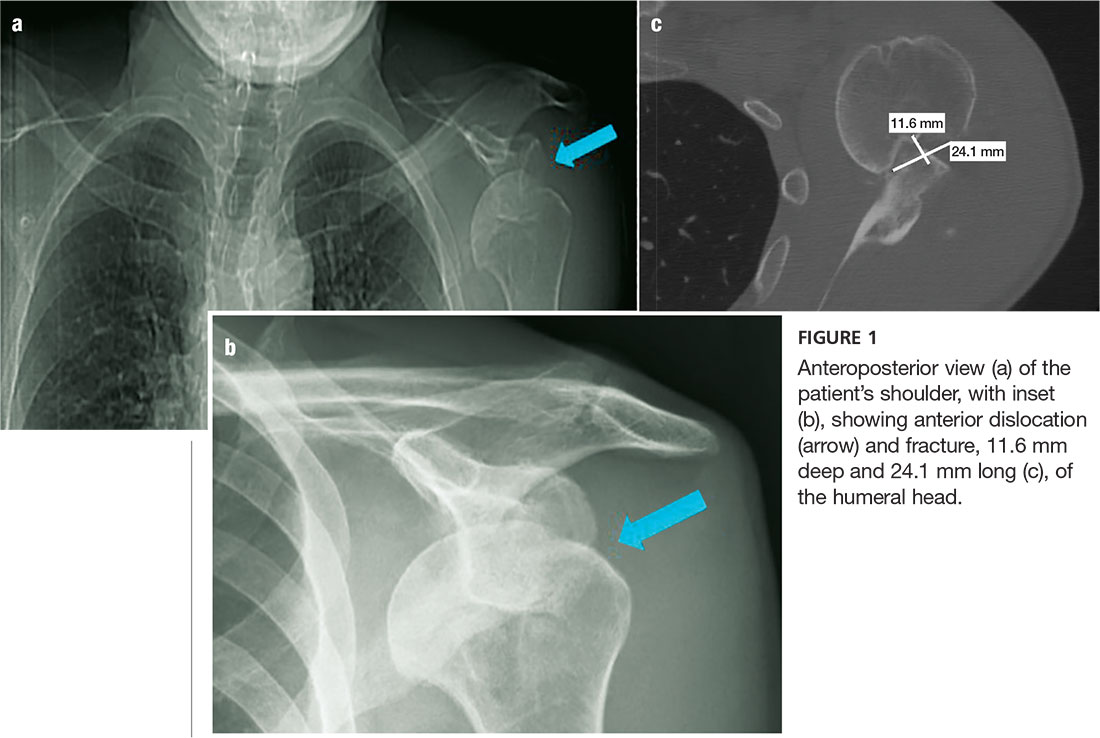
The first step in evaluating a suspected shoulder dislocation is to order an AP radiographic view of the shoulder (known as the Grashey view). A transcapular view (known as the scapular “Y” view) is also sufficient.1 While diagnostic studies, such as CT or MRI arthrography, are excellent for evaluating the glenohumeral ligaments and labrum, they generally are not done in an acute setting.1 For patients who present to the ED, some would recommend taking a CT scan, especially if a posterior dislocation is suspected.2
The three types of shoulder dislocations include anterior, posterior, and inferior.
ANTERIOR
Anterior dislocations account for 95% of all presented cases of shoulder dislocation, making them the most common type.3 They may be caused by a fall on an outstretched arm, trauma to the posterior humerus, or—more frequently—trauma to the arm while it is extended, externally rotated, and abducted (eg, blocking a shot in basketball).
A patient with an anterior dislocation will enter the ED with a slightly abducted and externally rotated arm (see illustration) and will resist any movement by the examiner. Typically, the shoulder loses its rounded appearance, and in thin individuals, the acromion may be prominent. A detailed neurovascular examination of the arm must be performed.
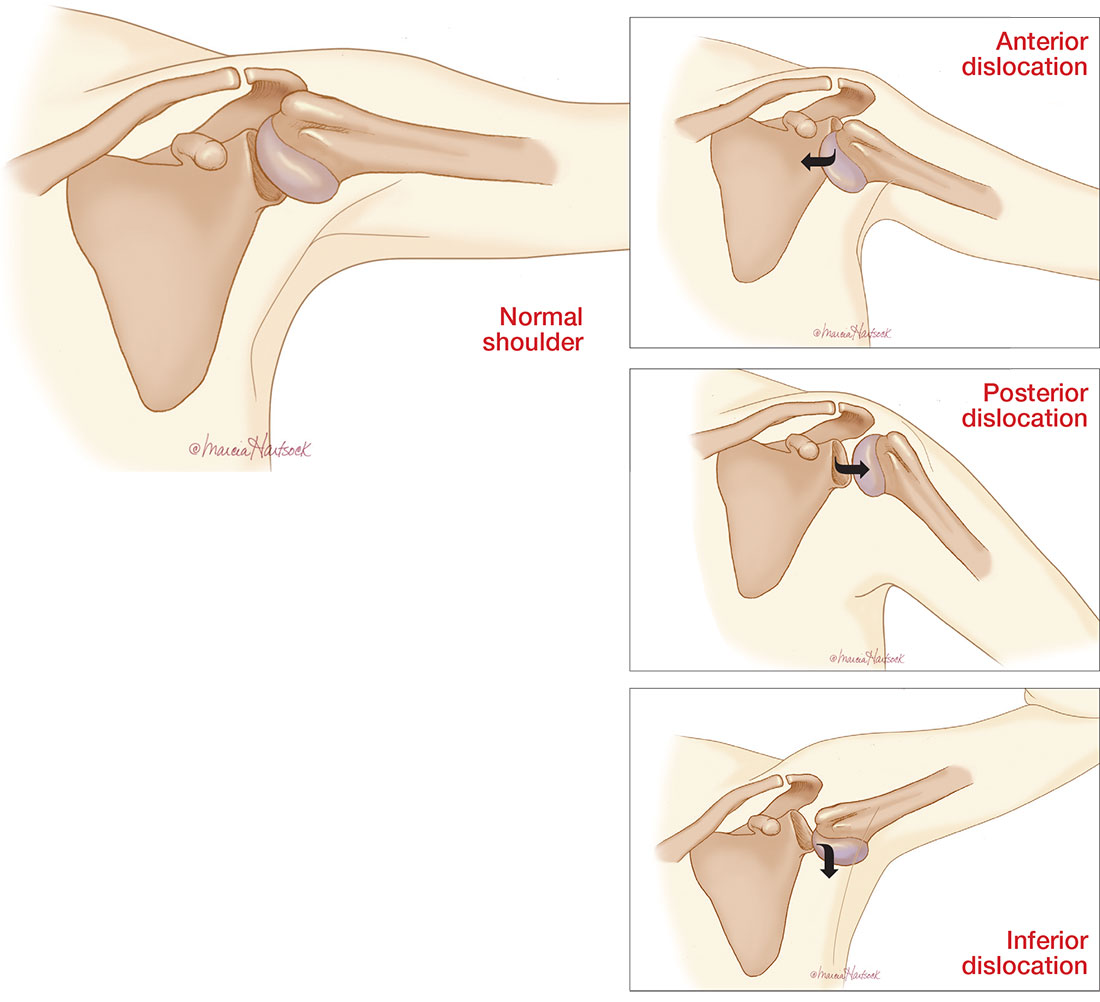
Dislocation of the humerus in any direction may compromise the axillary nerve, artery, or both. The axillary nerve and artery run parallel to each other, beneath and in close proximity to the humeral head. The axillary artery is located upstream from the radial artery; compression of the artery may lead to a diminution or complete absence of the radial pulse and/or coolness of the hand.4 The axillary nerve is both a sensory and motor nerve. If injured, a 2- to 3-cm area over the lateral deltoid may have complete sensory loss, which can be tested for with a light touch and pinprick.5 The patient may also have difficulty abducting the arm, but limitations of movement are difficult to measure with a new dislocation and a patient in pain.4
Any patient presenting with an anterior shoulder dislocation should also be screened for two other potential abnormalities. Hill-Sachs lesion, which occurs in up to 40% of anterior dislocations and 90% of all dislocations, is a cortical depression occurring in the humeral head. Bankart lesions, which occur in less than 5% of all dislocations, are avulsed bone fragments that occur when there is a glenoid labrum disruption.6 Both can be seen on plain films, although Bankart lesions are best seen on CT.4
The combination of an anterior dislocation and a humeral fracture, as seen in this case, is rare.7
POSTERIOR
Posterior shoulder dislocations occur far less frequently than anterior dislocations, representing 2% to 5% of all shoulder dislocations.2 They often result from blows to the anterior portion of the shoulder (ie, motor vehicle accidents or sports-related collisions) or violent muscle contractions (eg, electrocution, electroconvulsive therapy, or seizures).
Unable to externally rotate the shoulder, patients with posterior dislocations present with the arm in adduction and internal rotation, making the coracoid process prominent (see illustration).8 This position is sometimes misdiagnosed as a “frozen shoulder.”2
INFERIOR
Inferior dislocation of the shoulder is the rarest type, accounting for only 0.5% of all cases of shoulder dislocation. The mechanism of injury is forceful hyperabduction and extension of the shoulder during a fall.
Patients present with the affected arm hyperadducted, flexed at the elbow, with the hand positioned above or behind the head in fixed abduction: a “hands up” position of the affected arm (see illustration). These dislocations are best identified via the transcapular “Y” radiographs. Inferior dislocations are often associated with neurovascular compromise, and there are often related tears of the infraspinatus, supraspinatus, and teres minor muscles.9
ASSOCIATION WITH SEIZURES
Any patient who has had a seizure is subject to a variety of injuries, including lacerations, contusions, long bone and skull fractures, and dislocations. Seizures with a fall are associated with a 20% chance of injury.10
Shaw et al were the first to note that, during an active convulsion, the patient’s shoulder is in adduction, internal rotation, and flexion. This positioning predisposes to injury: With sustained contraction of the surrounding shoulder girdle muscles, the humeral head is forced superiorly and posteriorly against the acromion andmedially against the glenoid fossa. The glenoid fossa is shallow; therefore, the humeral head is forced posteriorly and dislocates.11
Researchers at the Mayo Clinic followed 247 patients who were diagnosed with seizures over nine years; 16% of the cohort experienced seizure-related injuries. Of the seizures recorded, 82% were tonic-clonic seizures. The singular predictive factor for injury was seizure frequency: Patients who had more seizures were more susceptible to injury.12
In an evaluation of outpatients with epilepsy, 25% of recorded seizures involved a fall. Among those who sustained an orthopedic injury, one injury occurred for every 178.6 generalized tonic-clonic seizures (0.6%)—a number that doubled for generalized tonic-clonic seizure associated with a fall (1.2%).10
The collective evidence from these and other studies suggests that patients who have poorly controlled tonic-clonic seizures have a higher incidence of seizures and, therefore, falls and injuries.10,12 In the absence of known trauma, a posterior shoulder dislocation is almost pathognomonic of a seizure. In high-risk populations (ie, individuals who have poorly controlled diabetes or who are experiencing alcohol or drug withdrawal), suspicion for posterior shoulder dislocation should be elevated.8
After evaluation in the ED, the patient immediately underwent a nonsurgical closed reduction of the shoulder and suturing of the laceration. He was admitted overnight for further evaluation and was started on an anticonvulsant (levetiracetam). An orthopedic consult was obtained; the dislocation/fracture was managed conservatively with a sling for immobilization. No surgical intervention was recommended, since the patient had a manageable fracture without neurovascular compromise. He was discharged home within 36 hours and scheduled for follow-up appointments with both the neurologist and orthopedic surgeon.
CONCLUSION
This patient had a seizure with an associated fall; both the laceration and the anterior shoulder dislocation with a humeral fracture were associated with the fall and not with tonic-clonic activity from the seizure. Because injuries vary widely from soft tissue to joint dislocations, with possible axillary nerve and/or artery damage, clinicians must do a comprehensive examination of patients entering the ED who have had seizures. Each injury must be addressed individually.
1. Omoumi P, Teixeira P, Lecouvet F, Chung CB. Glenohumeral joint instability. J Magn Reson Imaging. 2010;33(1):2-16.
2. Rouleau DM, Hebert-Davies J. Incidence of associated injury in posterior shoulder dislocation: systematic review of the literature. J Orthop Trauma. 2012;26(4):246-251.
3. Sachit M, Shekhar A, Shekhar S, Joban SH. Acute spontaneous atraumatic bilateral anterior dislocation of the shoulder joint with Hill-Sach’s lesions: a rare case. J Orthop Case Rep. 2015;5(1):55-57.
4. Cutts S, Prempeh M, Drew S. Anterior shoulder dislocation. Ann R Coll Surg Engl. 2009;91(1):2-7.
5. Magee DJ. Orthopedic Physical Assessment. 5th ed. St. Louis, MO. Saunders Elsevier; 2008.
6. Greenspan A. Orthopedic Imaging: A Practical Approach. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
7. Karimi-Nasab MH, Shayesteh-Azar M, Sajjadi-Saravi M, Mehdi Daneshpoor SM. Anterior shoulder dislocation and ipsilateral humeral shaft fracture. Iran J Med Sci. 2012; 37(3):202-204.
8. Robinson CM, Aderinto J. Posterior shoulder dislocations and fracture-dislocations. J Bone Joint Surg Am. 2005; 87(3):639-650.
9. Cacioppo E, Waymack JR. Bilateral inferior shoulder dislocation. West J Emerg Med. 2015;16(1):157.
10. Tiamkao S, Shorvon SD. Seizure-related injury in an adult tertiary epilepsy clinic. Hong Kong Med J. 2006;12(4):260-263.
11. Shaw JL. Bilateral posterior fracture-dislocation of the shoulder and other trauma caused by convulsive seizures. J Bone Joint Surg Am. 1971;53(7):1437-1440.
12. Lawn ND, Bamlet WR, Radhakirshnan K, et al. Injuries due to seizures in persons with epilepsy: a population-based study. Neurology. 2004;63(9):1565-1570.
IN THIS ARTICLE
- Types of shoulder dislocations
- Schematics of the shoulder with three types of dislocations
- Association with seizures
CASE A 59-year-old man with a remote history of seizures is transported to the emergency department (ED) by ambulance after a witnessed tonic-clonic seizure. At the time of arrival he is postictal and confused, but his vital signs are stable. A left eyebrow laceration indicating a possible fall is observed on physical exam, as is a left shoulder displacement with no obvious signs of neurovascular compromise. The patient is not currently taking anticonvulsant medication, stating that he has been “seizure free” for five years, and therefore chose to discontinue taking phenytoin against medical advice.
An anteroposterior (AP) bilateral shoulder x-ray is obtained in the ED (see Figures 1a and 1b). The image shows the humeral head to be anteriorly dislocated and reveals a large impaction fracture of the posterior superior humeral head. For a more detailed view of the fracture and to further assess any associated deformities, CT of the left shoulder is performed. The fracture has a depth of 11.6 mm and a length of 24.1 mm, with no additional pathology noted (see Figure 1c).
The shoulder is a large joint capable of moving in many directions and therefore is inherently unstable. The glenoid fossa is shallow, and stability of the joint is provided by both the fibrocartilaginous labrum and varying muscles of the rotator cuff. Because the shoulder joint is poorly supported, dislocations are not uncommon (see the illustrations).

The first step in evaluating a suspected shoulder dislocation is to order an AP radiographic view of the shoulder (known as the Grashey view). A transcapular view (known as the scapular “Y” view) is also sufficient.1 While diagnostic studies, such as CT or MRI arthrography, are excellent for evaluating the glenohumeral ligaments and labrum, they generally are not done in an acute setting.1 For patients who present to the ED, some would recommend taking a CT scan, especially if a posterior dislocation is suspected.2
The three types of shoulder dislocations include anterior, posterior, and inferior.
ANTERIOR
Anterior dislocations account for 95% of all presented cases of shoulder dislocation, making them the most common type.3 They may be caused by a fall on an outstretched arm, trauma to the posterior humerus, or—more frequently—trauma to the arm while it is extended, externally rotated, and abducted (eg, blocking a shot in basketball).
A patient with an anterior dislocation will enter the ED with a slightly abducted and externally rotated arm (see illustration) and will resist any movement by the examiner. Typically, the shoulder loses its rounded appearance, and in thin individuals, the acromion may be prominent. A detailed neurovascular examination of the arm must be performed.

Dislocation of the humerus in any direction may compromise the axillary nerve, artery, or both. The axillary nerve and artery run parallel to each other, beneath and in close proximity to the humeral head. The axillary artery is located upstream from the radial artery; compression of the artery may lead to a diminution or complete absence of the radial pulse and/or coolness of the hand.4 The axillary nerve is both a sensory and motor nerve. If injured, a 2- to 3-cm area over the lateral deltoid may have complete sensory loss, which can be tested for with a light touch and pinprick.5 The patient may also have difficulty abducting the arm, but limitations of movement are difficult to measure with a new dislocation and a patient in pain.4
Any patient presenting with an anterior shoulder dislocation should also be screened for two other potential abnormalities. Hill-Sachs lesion, which occurs in up to 40% of anterior dislocations and 90% of all dislocations, is a cortical depression occurring in the humeral head. Bankart lesions, which occur in less than 5% of all dislocations, are avulsed bone fragments that occur when there is a glenoid labrum disruption.6 Both can be seen on plain films, although Bankart lesions are best seen on CT.4
The combination of an anterior dislocation and a humeral fracture, as seen in this case, is rare.7
POSTERIOR
Posterior shoulder dislocations occur far less frequently than anterior dislocations, representing 2% to 5% of all shoulder dislocations.2 They often result from blows to the anterior portion of the shoulder (ie, motor vehicle accidents or sports-related collisions) or violent muscle contractions (eg, electrocution, electroconvulsive therapy, or seizures).
Unable to externally rotate the shoulder, patients with posterior dislocations present with the arm in adduction and internal rotation, making the coracoid process prominent (see illustration).8 This position is sometimes misdiagnosed as a “frozen shoulder.”2
INFERIOR
Inferior dislocation of the shoulder is the rarest type, accounting for only 0.5% of all cases of shoulder dislocation. The mechanism of injury is forceful hyperabduction and extension of the shoulder during a fall.
Patients present with the affected arm hyperadducted, flexed at the elbow, with the hand positioned above or behind the head in fixed abduction: a “hands up” position of the affected arm (see illustration). These dislocations are best identified via the transcapular “Y” radiographs. Inferior dislocations are often associated with neurovascular compromise, and there are often related tears of the infraspinatus, supraspinatus, and teres minor muscles.9
ASSOCIATION WITH SEIZURES
Any patient who has had a seizure is subject to a variety of injuries, including lacerations, contusions, long bone and skull fractures, and dislocations. Seizures with a fall are associated with a 20% chance of injury.10
Shaw et al were the first to note that, during an active convulsion, the patient’s shoulder is in adduction, internal rotation, and flexion. This positioning predisposes to injury: With sustained contraction of the surrounding shoulder girdle muscles, the humeral head is forced superiorly and posteriorly against the acromion andmedially against the glenoid fossa. The glenoid fossa is shallow; therefore, the humeral head is forced posteriorly and dislocates.11
Researchers at the Mayo Clinic followed 247 patients who were diagnosed with seizures over nine years; 16% of the cohort experienced seizure-related injuries. Of the seizures recorded, 82% were tonic-clonic seizures. The singular predictive factor for injury was seizure frequency: Patients who had more seizures were more susceptible to injury.12
In an evaluation of outpatients with epilepsy, 25% of recorded seizures involved a fall. Among those who sustained an orthopedic injury, one injury occurred for every 178.6 generalized tonic-clonic seizures (0.6%)—a number that doubled for generalized tonic-clonic seizure associated with a fall (1.2%).10
The collective evidence from these and other studies suggests that patients who have poorly controlled tonic-clonic seizures have a higher incidence of seizures and, therefore, falls and injuries.10,12 In the absence of known trauma, a posterior shoulder dislocation is almost pathognomonic of a seizure. In high-risk populations (ie, individuals who have poorly controlled diabetes or who are experiencing alcohol or drug withdrawal), suspicion for posterior shoulder dislocation should be elevated.8
After evaluation in the ED, the patient immediately underwent a nonsurgical closed reduction of the shoulder and suturing of the laceration. He was admitted overnight for further evaluation and was started on an anticonvulsant (levetiracetam). An orthopedic consult was obtained; the dislocation/fracture was managed conservatively with a sling for immobilization. No surgical intervention was recommended, since the patient had a manageable fracture without neurovascular compromise. He was discharged home within 36 hours and scheduled for follow-up appointments with both the neurologist and orthopedic surgeon.
CONCLUSION
This patient had a seizure with an associated fall; both the laceration and the anterior shoulder dislocation with a humeral fracture were associated with the fall and not with tonic-clonic activity from the seizure. Because injuries vary widely from soft tissue to joint dislocations, with possible axillary nerve and/or artery damage, clinicians must do a comprehensive examination of patients entering the ED who have had seizures. Each injury must be addressed individually.
IN THIS ARTICLE
- Types of shoulder dislocations
- Schematics of the shoulder with three types of dislocations
- Association with seizures
CASE A 59-year-old man with a remote history of seizures is transported to the emergency department (ED) by ambulance after a witnessed tonic-clonic seizure. At the time of arrival he is postictal and confused, but his vital signs are stable. A left eyebrow laceration indicating a possible fall is observed on physical exam, as is a left shoulder displacement with no obvious signs of neurovascular compromise. The patient is not currently taking anticonvulsant medication, stating that he has been “seizure free” for five years, and therefore chose to discontinue taking phenytoin against medical advice.
An anteroposterior (AP) bilateral shoulder x-ray is obtained in the ED (see Figures 1a and 1b). The image shows the humeral head to be anteriorly dislocated and reveals a large impaction fracture of the posterior superior humeral head. For a more detailed view of the fracture and to further assess any associated deformities, CT of the left shoulder is performed. The fracture has a depth of 11.6 mm and a length of 24.1 mm, with no additional pathology noted (see Figure 1c).
The shoulder is a large joint capable of moving in many directions and therefore is inherently unstable. The glenoid fossa is shallow, and stability of the joint is provided by both the fibrocartilaginous labrum and varying muscles of the rotator cuff. Because the shoulder joint is poorly supported, dislocations are not uncommon (see the illustrations).

The first step in evaluating a suspected shoulder dislocation is to order an AP radiographic view of the shoulder (known as the Grashey view). A transcapular view (known as the scapular “Y” view) is also sufficient.1 While diagnostic studies, such as CT or MRI arthrography, are excellent for evaluating the glenohumeral ligaments and labrum, they generally are not done in an acute setting.1 For patients who present to the ED, some would recommend taking a CT scan, especially if a posterior dislocation is suspected.2
The three types of shoulder dislocations include anterior, posterior, and inferior.
ANTERIOR
Anterior dislocations account for 95% of all presented cases of shoulder dislocation, making them the most common type.3 They may be caused by a fall on an outstretched arm, trauma to the posterior humerus, or—more frequently—trauma to the arm while it is extended, externally rotated, and abducted (eg, blocking a shot in basketball).
A patient with an anterior dislocation will enter the ED with a slightly abducted and externally rotated arm (see illustration) and will resist any movement by the examiner. Typically, the shoulder loses its rounded appearance, and in thin individuals, the acromion may be prominent. A detailed neurovascular examination of the arm must be performed.

Dislocation of the humerus in any direction may compromise the axillary nerve, artery, or both. The axillary nerve and artery run parallel to each other, beneath and in close proximity to the humeral head. The axillary artery is located upstream from the radial artery; compression of the artery may lead to a diminution or complete absence of the radial pulse and/or coolness of the hand.4 The axillary nerve is both a sensory and motor nerve. If injured, a 2- to 3-cm area over the lateral deltoid may have complete sensory loss, which can be tested for with a light touch and pinprick.5 The patient may also have difficulty abducting the arm, but limitations of movement are difficult to measure with a new dislocation and a patient in pain.4
Any patient presenting with an anterior shoulder dislocation should also be screened for two other potential abnormalities. Hill-Sachs lesion, which occurs in up to 40% of anterior dislocations and 90% of all dislocations, is a cortical depression occurring in the humeral head. Bankart lesions, which occur in less than 5% of all dislocations, are avulsed bone fragments that occur when there is a glenoid labrum disruption.6 Both can be seen on plain films, although Bankart lesions are best seen on CT.4
The combination of an anterior dislocation and a humeral fracture, as seen in this case, is rare.7
POSTERIOR
Posterior shoulder dislocations occur far less frequently than anterior dislocations, representing 2% to 5% of all shoulder dislocations.2 They often result from blows to the anterior portion of the shoulder (ie, motor vehicle accidents or sports-related collisions) or violent muscle contractions (eg, electrocution, electroconvulsive therapy, or seizures).
Unable to externally rotate the shoulder, patients with posterior dislocations present with the arm in adduction and internal rotation, making the coracoid process prominent (see illustration).8 This position is sometimes misdiagnosed as a “frozen shoulder.”2
INFERIOR
Inferior dislocation of the shoulder is the rarest type, accounting for only 0.5% of all cases of shoulder dislocation. The mechanism of injury is forceful hyperabduction and extension of the shoulder during a fall.
Patients present with the affected arm hyperadducted, flexed at the elbow, with the hand positioned above or behind the head in fixed abduction: a “hands up” position of the affected arm (see illustration). These dislocations are best identified via the transcapular “Y” radiographs. Inferior dislocations are often associated with neurovascular compromise, and there are often related tears of the infraspinatus, supraspinatus, and teres minor muscles.9
ASSOCIATION WITH SEIZURES
Any patient who has had a seizure is subject to a variety of injuries, including lacerations, contusions, long bone and skull fractures, and dislocations. Seizures with a fall are associated with a 20% chance of injury.10
Shaw et al were the first to note that, during an active convulsion, the patient’s shoulder is in adduction, internal rotation, and flexion. This positioning predisposes to injury: With sustained contraction of the surrounding shoulder girdle muscles, the humeral head is forced superiorly and posteriorly against the acromion andmedially against the glenoid fossa. The glenoid fossa is shallow; therefore, the humeral head is forced posteriorly and dislocates.11
Researchers at the Mayo Clinic followed 247 patients who were diagnosed with seizures over nine years; 16% of the cohort experienced seizure-related injuries. Of the seizures recorded, 82% were tonic-clonic seizures. The singular predictive factor for injury was seizure frequency: Patients who had more seizures were more susceptible to injury.12
In an evaluation of outpatients with epilepsy, 25% of recorded seizures involved a fall. Among those who sustained an orthopedic injury, one injury occurred for every 178.6 generalized tonic-clonic seizures (0.6%)—a number that doubled for generalized tonic-clonic seizure associated with a fall (1.2%).10
The collective evidence from these and other studies suggests that patients who have poorly controlled tonic-clonic seizures have a higher incidence of seizures and, therefore, falls and injuries.10,12 In the absence of known trauma, a posterior shoulder dislocation is almost pathognomonic of a seizure. In high-risk populations (ie, individuals who have poorly controlled diabetes or who are experiencing alcohol or drug withdrawal), suspicion for posterior shoulder dislocation should be elevated.8
After evaluation in the ED, the patient immediately underwent a nonsurgical closed reduction of the shoulder and suturing of the laceration. He was admitted overnight for further evaluation and was started on an anticonvulsant (levetiracetam). An orthopedic consult was obtained; the dislocation/fracture was managed conservatively with a sling for immobilization. No surgical intervention was recommended, since the patient had a manageable fracture without neurovascular compromise. He was discharged home within 36 hours and scheduled for follow-up appointments with both the neurologist and orthopedic surgeon.
CONCLUSION
This patient had a seizure with an associated fall; both the laceration and the anterior shoulder dislocation with a humeral fracture were associated with the fall and not with tonic-clonic activity from the seizure. Because injuries vary widely from soft tissue to joint dislocations, with possible axillary nerve and/or artery damage, clinicians must do a comprehensive examination of patients entering the ED who have had seizures. Each injury must be addressed individually.
1. Omoumi P, Teixeira P, Lecouvet F, Chung CB. Glenohumeral joint instability. J Magn Reson Imaging. 2010;33(1):2-16.
2. Rouleau DM, Hebert-Davies J. Incidence of associated injury in posterior shoulder dislocation: systematic review of the literature. J Orthop Trauma. 2012;26(4):246-251.
3. Sachit M, Shekhar A, Shekhar S, Joban SH. Acute spontaneous atraumatic bilateral anterior dislocation of the shoulder joint with Hill-Sach’s lesions: a rare case. J Orthop Case Rep. 2015;5(1):55-57.
4. Cutts S, Prempeh M, Drew S. Anterior shoulder dislocation. Ann R Coll Surg Engl. 2009;91(1):2-7.
5. Magee DJ. Orthopedic Physical Assessment. 5th ed. St. Louis, MO. Saunders Elsevier; 2008.
6. Greenspan A. Orthopedic Imaging: A Practical Approach. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
7. Karimi-Nasab MH, Shayesteh-Azar M, Sajjadi-Saravi M, Mehdi Daneshpoor SM. Anterior shoulder dislocation and ipsilateral humeral shaft fracture. Iran J Med Sci. 2012; 37(3):202-204.
8. Robinson CM, Aderinto J. Posterior shoulder dislocations and fracture-dislocations. J Bone Joint Surg Am. 2005; 87(3):639-650.
9. Cacioppo E, Waymack JR. Bilateral inferior shoulder dislocation. West J Emerg Med. 2015;16(1):157.
10. Tiamkao S, Shorvon SD. Seizure-related injury in an adult tertiary epilepsy clinic. Hong Kong Med J. 2006;12(4):260-263.
11. Shaw JL. Bilateral posterior fracture-dislocation of the shoulder and other trauma caused by convulsive seizures. J Bone Joint Surg Am. 1971;53(7):1437-1440.
12. Lawn ND, Bamlet WR, Radhakirshnan K, et al. Injuries due to seizures in persons with epilepsy: a population-based study. Neurology. 2004;63(9):1565-1570.
1. Omoumi P, Teixeira P, Lecouvet F, Chung CB. Glenohumeral joint instability. J Magn Reson Imaging. 2010;33(1):2-16.
2. Rouleau DM, Hebert-Davies J. Incidence of associated injury in posterior shoulder dislocation: systematic review of the literature. J Orthop Trauma. 2012;26(4):246-251.
3. Sachit M, Shekhar A, Shekhar S, Joban SH. Acute spontaneous atraumatic bilateral anterior dislocation of the shoulder joint with Hill-Sach’s lesions: a rare case. J Orthop Case Rep. 2015;5(1):55-57.
4. Cutts S, Prempeh M, Drew S. Anterior shoulder dislocation. Ann R Coll Surg Engl. 2009;91(1):2-7.
5. Magee DJ. Orthopedic Physical Assessment. 5th ed. St. Louis, MO. Saunders Elsevier; 2008.
6. Greenspan A. Orthopedic Imaging: A Practical Approach. 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
7. Karimi-Nasab MH, Shayesteh-Azar M, Sajjadi-Saravi M, Mehdi Daneshpoor SM. Anterior shoulder dislocation and ipsilateral humeral shaft fracture. Iran J Med Sci. 2012; 37(3):202-204.
8. Robinson CM, Aderinto J. Posterior shoulder dislocations and fracture-dislocations. J Bone Joint Surg Am. 2005; 87(3):639-650.
9. Cacioppo E, Waymack JR. Bilateral inferior shoulder dislocation. West J Emerg Med. 2015;16(1):157.
10. Tiamkao S, Shorvon SD. Seizure-related injury in an adult tertiary epilepsy clinic. Hong Kong Med J. 2006;12(4):260-263.
11. Shaw JL. Bilateral posterior fracture-dislocation of the shoulder and other trauma caused by convulsive seizures. J Bone Joint Surg Am. 1971;53(7):1437-1440.
12. Lawn ND, Bamlet WR, Radhakirshnan K, et al. Injuries due to seizures in persons with epilepsy: a population-based study. Neurology. 2004;63(9):1565-1570.
Pleth Variability Index shows promise for asthma assessments
Clinical question: Does pulse variability on plethysmography, or the Pleth Variability Index (PVI), correlate with disease severity in obstructive airway disease in children?
Background: Asthma is the most common reason for hospitalization in the United S. for children 3-12 years old. Asthma accounts for a quarter of ED visits for children aged 1-9 years old.1 Although systems have been developed to assess asthma exacerbation severity and the need for hospitalization, many of these depend on reassessments over time or have been proven to be invalid in larger studies.2,3,4 Pulsus paradoxus (PP), which is defined as a drop in systolic blood pressure greater than 10 mm Hg, correlates with the severity of obstruction in asthma exacerbations, but it is not practical in the children being evaluated in the ED or hospital.5,6 PP measurement using plethysmography has been found to correlate with measurement by sphygmomanometry.7 Furthermore, PVI, which is derived from amplitude variability in the pulse oximeter waveform, has been found to correlate with fluid responsiveness in mechanically ventilated patients. To this date, no study has assessed the correlation between PVI and exacerbation severity in asthma.
Setting: A 137-bed, tertiary-care children’s hospital.
Synopsis: Over a 6-month period on weekdays, researchers enrolled patients aged 1-18 years evaluated in the ED for asthma exacerbations or reactive airway disease. ED staff diagnosed patients clinically, and other patients with conditions known to affect PP – such as dehydration, croup, and cardiac disease – were excluded. PVI was calculated by measuring the minimum perfusion index (PImin) and the maximum perfusion index (PImax) using the following formula:
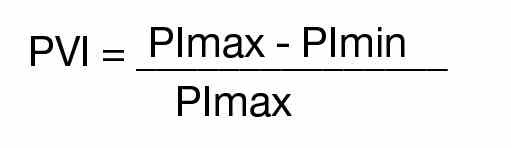
A printout of the first ED pulse oximetry reading was used to obtain the PImax and PImin as below:
Researchers followed patients after the initial evaluation to determine disposition from the ED, which included either discharge to home, admission to a general pediatrics floor, or admission to the PICU. The hospital utilized specific criteria for disposition from the ED (see Table 1).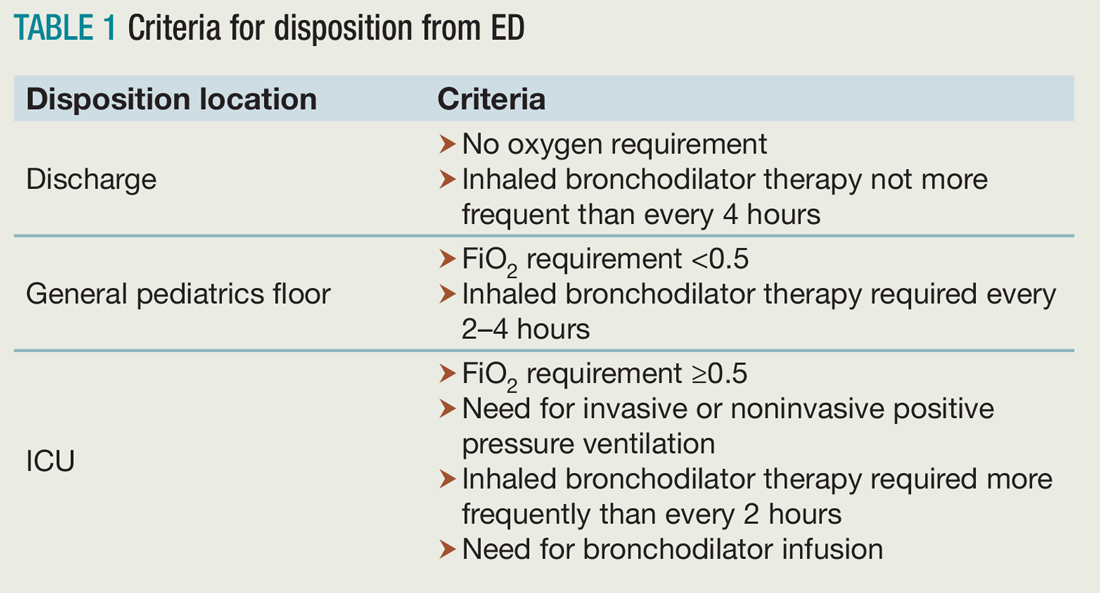
Of the 117 patients who were analyzed after application of exclusion criteria, 48 were discharged to home, 61 were admitted to a general pediatrics floor, and eight were admitted to the PICU. The three groups were found to be demographically similar. Researchers found a significant difference between the PVI of the three groups, but pairwise analysis showed no significant difference between the PVI of patients admitted to the general pediatrics floor versus discharged to home (see Table 2).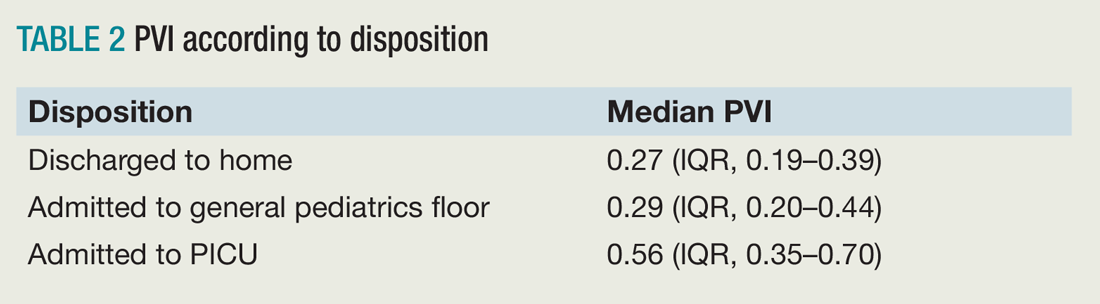
Bottom line: PVI shows promise as a tool to rapidly assess disease severity in pediatric patients being evaluated and treated for asthma, but further studies are needed to validate this in the ED and hospital setting.
Citation: Brandwein A, Patel K, Kline M, Silver P, Gangadharan S. Using pleth variability as a triage tool for children with obstructive airway disease in a pediatric emergency department [published online ahead of print Oct. 6, 2016]. Pediatr Emerg Care. doi: 10.1097/PEC.0000000000000887.
References
1. Care of children and adolescents in U.S. hospitals. Agency for Healthcare Research and Quality website. Available at: https://archive.ahrq.gov/data/hcup/factbk4/factbk4.htm. Accessed Nov. 18, 2016.
2. Kelly AM, Kerr D, Powell C. Is severity assessment after one hour of treatment better for predicting the need for admission in acute asthma? Respir Med. 2004;98(8):777-781.
3. Keogh KA, Macarthur C, Parkin PC, et al. Predictors of hospitalization in children with acute asthma. J Pediatr. 2001;139(2):273-277.
4. Keahey L, Bulloch B, Becker AB, et al. Initial oxygen saturation as a predictor of admission in children presenting to the emergency department with acute asthma. Ann Emerg Med. 2002;40(3):300-307.
5. Guntheroth WG, Morgan BC, Mullins GL. Effect of respiration on venous return and stroke volume in cardiac tamponade. Mechanism of pulsus paradoxus. Circ Res. 1967;20(4):381-390.
6. Frey B, Freezer N. Diagnostic value and pathophysiologic basis of pulsus paradoxus in infants and children with respiratory disease. Pediatr Pulmonol. 2001;31(2):138-143.
7. Clark JA, Lieh-Lai M, Thomas R, Raghavan K, Sarnaik AP. Comparison of traditional and plethysmographic methods for measuring pulsus paradoxus. Arch Pediatr Adolesc Med. 2004;158(1):48-51.
Clinical question: Does pulse variability on plethysmography, or the Pleth Variability Index (PVI), correlate with disease severity in obstructive airway disease in children?
Background: Asthma is the most common reason for hospitalization in the United S. for children 3-12 years old. Asthma accounts for a quarter of ED visits for children aged 1-9 years old.1 Although systems have been developed to assess asthma exacerbation severity and the need for hospitalization, many of these depend on reassessments over time or have been proven to be invalid in larger studies.2,3,4 Pulsus paradoxus (PP), which is defined as a drop in systolic blood pressure greater than 10 mm Hg, correlates with the severity of obstruction in asthma exacerbations, but it is not practical in the children being evaluated in the ED or hospital.5,6 PP measurement using plethysmography has been found to correlate with measurement by sphygmomanometry.7 Furthermore, PVI, which is derived from amplitude variability in the pulse oximeter waveform, has been found to correlate with fluid responsiveness in mechanically ventilated patients. To this date, no study has assessed the correlation between PVI and exacerbation severity in asthma.
Setting: A 137-bed, tertiary-care children’s hospital.
Synopsis: Over a 6-month period on weekdays, researchers enrolled patients aged 1-18 years evaluated in the ED for asthma exacerbations or reactive airway disease. ED staff diagnosed patients clinically, and other patients with conditions known to affect PP – such as dehydration, croup, and cardiac disease – were excluded. PVI was calculated by measuring the minimum perfusion index (PImin) and the maximum perfusion index (PImax) using the following formula:
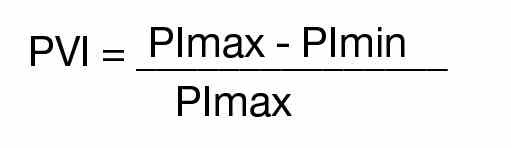
A printout of the first ED pulse oximetry reading was used to obtain the PImax and PImin as below:
Researchers followed patients after the initial evaluation to determine disposition from the ED, which included either discharge to home, admission to a general pediatrics floor, or admission to the PICU. The hospital utilized specific criteria for disposition from the ED (see Table 1).
Of the 117 patients who were analyzed after application of exclusion criteria, 48 were discharged to home, 61 were admitted to a general pediatrics floor, and eight were admitted to the PICU. The three groups were found to be demographically similar. Researchers found a significant difference between the PVI of the three groups, but pairwise analysis showed no significant difference between the PVI of patients admitted to the general pediatrics floor versus discharged to home (see Table 2).
Bottom line: PVI shows promise as a tool to rapidly assess disease severity in pediatric patients being evaluated and treated for asthma, but further studies are needed to validate this in the ED and hospital setting.
Citation: Brandwein A, Patel K, Kline M, Silver P, Gangadharan S. Using pleth variability as a triage tool for children with obstructive airway disease in a pediatric emergency department [published online ahead of print Oct. 6, 2016]. Pediatr Emerg Care. doi: 10.1097/PEC.0000000000000887.
References
1. Care of children and adolescents in U.S. hospitals. Agency for Healthcare Research and Quality website. Available at: https://archive.ahrq.gov/data/hcup/factbk4/factbk4.htm. Accessed Nov. 18, 2016.
2. Kelly AM, Kerr D, Powell C. Is severity assessment after one hour of treatment better for predicting the need for admission in acute asthma? Respir Med. 2004;98(8):777-781.
3. Keogh KA, Macarthur C, Parkin PC, et al. Predictors of hospitalization in children with acute asthma. J Pediatr. 2001;139(2):273-277.
4. Keahey L, Bulloch B, Becker AB, et al. Initial oxygen saturation as a predictor of admission in children presenting to the emergency department with acute asthma. Ann Emerg Med. 2002;40(3):300-307.
5. Guntheroth WG, Morgan BC, Mullins GL. Effect of respiration on venous return and stroke volume in cardiac tamponade. Mechanism of pulsus paradoxus. Circ Res. 1967;20(4):381-390.
6. Frey B, Freezer N. Diagnostic value and pathophysiologic basis of pulsus paradoxus in infants and children with respiratory disease. Pediatr Pulmonol. 2001;31(2):138-143.
7. Clark JA, Lieh-Lai M, Thomas R, Raghavan K, Sarnaik AP. Comparison of traditional and plethysmographic methods for measuring pulsus paradoxus. Arch Pediatr Adolesc Med. 2004;158(1):48-51.
Clinical question: Does pulse variability on plethysmography, or the Pleth Variability Index (PVI), correlate with disease severity in obstructive airway disease in children?
Background: Asthma is the most common reason for hospitalization in the United S. for children 3-12 years old. Asthma accounts for a quarter of ED visits for children aged 1-9 years old.1 Although systems have been developed to assess asthma exacerbation severity and the need for hospitalization, many of these depend on reassessments over time or have been proven to be invalid in larger studies.2,3,4 Pulsus paradoxus (PP), which is defined as a drop in systolic blood pressure greater than 10 mm Hg, correlates with the severity of obstruction in asthma exacerbations, but it is not practical in the children being evaluated in the ED or hospital.5,6 PP measurement using plethysmography has been found to correlate with measurement by sphygmomanometry.7 Furthermore, PVI, which is derived from amplitude variability in the pulse oximeter waveform, has been found to correlate with fluid responsiveness in mechanically ventilated patients. To this date, no study has assessed the correlation between PVI and exacerbation severity in asthma.
Setting: A 137-bed, tertiary-care children’s hospital.
Synopsis: Over a 6-month period on weekdays, researchers enrolled patients aged 1-18 years evaluated in the ED for asthma exacerbations or reactive airway disease. ED staff diagnosed patients clinically, and other patients with conditions known to affect PP – such as dehydration, croup, and cardiac disease – were excluded. PVI was calculated by measuring the minimum perfusion index (PImin) and the maximum perfusion index (PImax) using the following formula:
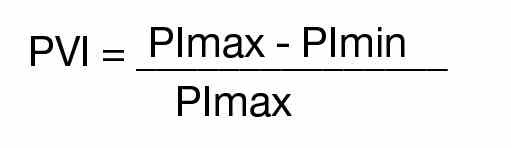
A printout of the first ED pulse oximetry reading was used to obtain the PImax and PImin as below:
Researchers followed patients after the initial evaluation to determine disposition from the ED, which included either discharge to home, admission to a general pediatrics floor, or admission to the PICU. The hospital utilized specific criteria for disposition from the ED (see Table 1).
Of the 117 patients who were analyzed after application of exclusion criteria, 48 were discharged to home, 61 were admitted to a general pediatrics floor, and eight were admitted to the PICU. The three groups were found to be demographically similar. Researchers found a significant difference between the PVI of the three groups, but pairwise analysis showed no significant difference between the PVI of patients admitted to the general pediatrics floor versus discharged to home (see Table 2).
Bottom line: PVI shows promise as a tool to rapidly assess disease severity in pediatric patients being evaluated and treated for asthma, but further studies are needed to validate this in the ED and hospital setting.
Citation: Brandwein A, Patel K, Kline M, Silver P, Gangadharan S. Using pleth variability as a triage tool for children with obstructive airway disease in a pediatric emergency department [published online ahead of print Oct. 6, 2016]. Pediatr Emerg Care. doi: 10.1097/PEC.0000000000000887.
References
1. Care of children and adolescents in U.S. hospitals. Agency for Healthcare Research and Quality website. Available at: https://archive.ahrq.gov/data/hcup/factbk4/factbk4.htm. Accessed Nov. 18, 2016.
2. Kelly AM, Kerr D, Powell C. Is severity assessment after one hour of treatment better for predicting the need for admission in acute asthma? Respir Med. 2004;98(8):777-781.
3. Keogh KA, Macarthur C, Parkin PC, et al. Predictors of hospitalization in children with acute asthma. J Pediatr. 2001;139(2):273-277.
4. Keahey L, Bulloch B, Becker AB, et al. Initial oxygen saturation as a predictor of admission in children presenting to the emergency department with acute asthma. Ann Emerg Med. 2002;40(3):300-307.
5. Guntheroth WG, Morgan BC, Mullins GL. Effect of respiration on venous return and stroke volume in cardiac tamponade. Mechanism of pulsus paradoxus. Circ Res. 1967;20(4):381-390.
6. Frey B, Freezer N. Diagnostic value and pathophysiologic basis of pulsus paradoxus in infants and children with respiratory disease. Pediatr Pulmonol. 2001;31(2):138-143.
7. Clark JA, Lieh-Lai M, Thomas R, Raghavan K, Sarnaik AP. Comparison of traditional and plethysmographic methods for measuring pulsus paradoxus. Arch Pediatr Adolesc Med. 2004;158(1):48-51.
Embrace change as a hospitalist leader
We work in complex environments and in a flawed and rapidly changing health care system. Caregivers, patients, and communities will be led through this complexity by those who embrace change. Last October, I had the privilege of attending and facilitating the SHM Leadership Academy in Orlando, which allowed me the opportunity to meet a group of people who embrace change, including the benefits and challenges that often accompany it.
SHM board member Jeff Glasheen, MD, SFHM, taught one of the first lessons at Leadership Academy, focusing on the importance of meaningful, difficult change. With comparisons to companies that have embraced change, like Apple, and some that have not, like Sears, Jeff summed up how complacency with “good” and a reluctance to tackle the difficulty of change keeps organizations – and people – from becoming great.
“Good is the enemy of great,” Jeff preached.
He largely focused on hospitalists leading organizational change, but the concepts can apply to personal change, too. He explained that “people generally want things to be different, but they don’t want to change.”
Leaders in training
Ten emerging hospitalist leaders sat at my table, soaking in the message. Several of them, like me 8 years ago, had the responsibilities of leadership unexpectedly thrust upon them. Some carried with them the heavy expectations of their colleagues or hospital administration (or both) that by being elevated into a role such as medical director, they would abruptly be able to make improvements in patient care and hospital operations. They had accepted the challenge to change – to move out of purely clinical roles and take on new ones in leadership despite having little or no experience. Doing so, they gingerly but willingly were following in the footsteps of leaders before them, growing their skills, improving their hospitals, and laying a path for future leaders to follow.
A few weeks prior, I had taken a new leadership position myself. The Cleveland Clinic recently acquired a hospital and health system in Akron, Ohio, about 40 miles away from the city. I assumed the role of president of this acquisition, embracing the complex challenge of leading the process of integrating two health systems. After 3 years overseeing a different hospital in the health system, I finally felt I had developed the people, processes, and culture that I had been striving to build. But like the young leaders at Leadership Academy, I had the opportunity to change, grow, develop, take on new risk, and become a stronger leader in this new role. A significant part of the experience of the Leadership Academy involves table exercises. For the first few exercises, the group was quiet, uncertain, tentative. I was struck both by how early these individuals were in their development and by how so much of what is happening today in hospitals and health care is dependent upon the development and success of individuals like these who are enthusiastic and talented but young and overwhelmed.
I believe that successful hospitalists are, through experience, training, and nature, rapid assimilators into their environments. By the third day, the dynamic at my table had gone from tentative and uncertain to much more confident and assertive. To experience this transformation in person at SHM’s Leadership Academy, we welcome you to Scottsdale, Ariz., later this year. Learn more about the program at www.shmleadershipacademy.org.
At Leadership Academy and beyond, I implore hospitalists to look for opportunities to change during this time of New Year’s resolutions and to take the opposite posture and want to change – change how we think, act, and respond; change our roles to take on new, uncomfortable responsibilities; and change how we view change itself.
We will be better for it both personally and professionally, and we will stand out as role models for our colleagues, coworkers, and hospitalists who follow in our footsteps.
Dr. Harte is a practicing hospitalist, president of the Society of Hospital Medicine, and president of Hillcrest Hospital in Mayfield Heights, Ohio, part of the Cleveland Clinic Health System. He is associate professor of medicine at the Cleveland Clinic, Lerner College of Medicine in Cleveland.
We work in complex environments and in a flawed and rapidly changing health care system. Caregivers, patients, and communities will be led through this complexity by those who embrace change. Last October, I had the privilege of attending and facilitating the SHM Leadership Academy in Orlando, which allowed me the opportunity to meet a group of people who embrace change, including the benefits and challenges that often accompany it.
SHM board member Jeff Glasheen, MD, SFHM, taught one of the first lessons at Leadership Academy, focusing on the importance of meaningful, difficult change. With comparisons to companies that have embraced change, like Apple, and some that have not, like Sears, Jeff summed up how complacency with “good” and a reluctance to tackle the difficulty of change keeps organizations – and people – from becoming great.
“Good is the enemy of great,” Jeff preached.
He largely focused on hospitalists leading organizational change, but the concepts can apply to personal change, too. He explained that “people generally want things to be different, but they don’t want to change.”
Leaders in training
Ten emerging hospitalist leaders sat at my table, soaking in the message. Several of them, like me 8 years ago, had the responsibilities of leadership unexpectedly thrust upon them. Some carried with them the heavy expectations of their colleagues or hospital administration (or both) that by being elevated into a role such as medical director, they would abruptly be able to make improvements in patient care and hospital operations. They had accepted the challenge to change – to move out of purely clinical roles and take on new ones in leadership despite having little or no experience. Doing so, they gingerly but willingly were following in the footsteps of leaders before them, growing their skills, improving their hospitals, and laying a path for future leaders to follow.
A few weeks prior, I had taken a new leadership position myself. The Cleveland Clinic recently acquired a hospital and health system in Akron, Ohio, about 40 miles away from the city. I assumed the role of president of this acquisition, embracing the complex challenge of leading the process of integrating two health systems. After 3 years overseeing a different hospital in the health system, I finally felt I had developed the people, processes, and culture that I had been striving to build. But like the young leaders at Leadership Academy, I had the opportunity to change, grow, develop, take on new risk, and become a stronger leader in this new role. A significant part of the experience of the Leadership Academy involves table exercises. For the first few exercises, the group was quiet, uncertain, tentative. I was struck both by how early these individuals were in their development and by how so much of what is happening today in hospitals and health care is dependent upon the development and success of individuals like these who are enthusiastic and talented but young and overwhelmed.
I believe that successful hospitalists are, through experience, training, and nature, rapid assimilators into their environments. By the third day, the dynamic at my table had gone from tentative and uncertain to much more confident and assertive. To experience this transformation in person at SHM’s Leadership Academy, we welcome you to Scottsdale, Ariz., later this year. Learn more about the program at www.shmleadershipacademy.org.
At Leadership Academy and beyond, I implore hospitalists to look for opportunities to change during this time of New Year’s resolutions and to take the opposite posture and want to change – change how we think, act, and respond; change our roles to take on new, uncomfortable responsibilities; and change how we view change itself.
We will be better for it both personally and professionally, and we will stand out as role models for our colleagues, coworkers, and hospitalists who follow in our footsteps.
Dr. Harte is a practicing hospitalist, president of the Society of Hospital Medicine, and president of Hillcrest Hospital in Mayfield Heights, Ohio, part of the Cleveland Clinic Health System. He is associate professor of medicine at the Cleveland Clinic, Lerner College of Medicine in Cleveland.
We work in complex environments and in a flawed and rapidly changing health care system. Caregivers, patients, and communities will be led through this complexity by those who embrace change. Last October, I had the privilege of attending and facilitating the SHM Leadership Academy in Orlando, which allowed me the opportunity to meet a group of people who embrace change, including the benefits and challenges that often accompany it.
SHM board member Jeff Glasheen, MD, SFHM, taught one of the first lessons at Leadership Academy, focusing on the importance of meaningful, difficult change. With comparisons to companies that have embraced change, like Apple, and some that have not, like Sears, Jeff summed up how complacency with “good” and a reluctance to tackle the difficulty of change keeps organizations – and people – from becoming great.
“Good is the enemy of great,” Jeff preached.
He largely focused on hospitalists leading organizational change, but the concepts can apply to personal change, too. He explained that “people generally want things to be different, but they don’t want to change.”
Leaders in training
Ten emerging hospitalist leaders sat at my table, soaking in the message. Several of them, like me 8 years ago, had the responsibilities of leadership unexpectedly thrust upon them. Some carried with them the heavy expectations of their colleagues or hospital administration (or both) that by being elevated into a role such as medical director, they would abruptly be able to make improvements in patient care and hospital operations. They had accepted the challenge to change – to move out of purely clinical roles and take on new ones in leadership despite having little or no experience. Doing so, they gingerly but willingly were following in the footsteps of leaders before them, growing their skills, improving their hospitals, and laying a path for future leaders to follow.
A few weeks prior, I had taken a new leadership position myself. The Cleveland Clinic recently acquired a hospital and health system in Akron, Ohio, about 40 miles away from the city. I assumed the role of president of this acquisition, embracing the complex challenge of leading the process of integrating two health systems. After 3 years overseeing a different hospital in the health system, I finally felt I had developed the people, processes, and culture that I had been striving to build. But like the young leaders at Leadership Academy, I had the opportunity to change, grow, develop, take on new risk, and become a stronger leader in this new role. A significant part of the experience of the Leadership Academy involves table exercises. For the first few exercises, the group was quiet, uncertain, tentative. I was struck both by how early these individuals were in their development and by how so much of what is happening today in hospitals and health care is dependent upon the development and success of individuals like these who are enthusiastic and talented but young and overwhelmed.
I believe that successful hospitalists are, through experience, training, and nature, rapid assimilators into their environments. By the third day, the dynamic at my table had gone from tentative and uncertain to much more confident and assertive. To experience this transformation in person at SHM’s Leadership Academy, we welcome you to Scottsdale, Ariz., later this year. Learn more about the program at www.shmleadershipacademy.org.
At Leadership Academy and beyond, I implore hospitalists to look for opportunities to change during this time of New Year’s resolutions and to take the opposite posture and want to change – change how we think, act, and respond; change our roles to take on new, uncomfortable responsibilities; and change how we view change itself.
We will be better for it both personally and professionally, and we will stand out as role models for our colleagues, coworkers, and hospitalists who follow in our footsteps.
Dr. Harte is a practicing hospitalist, president of the Society of Hospital Medicine, and president of Hillcrest Hospital in Mayfield Heights, Ohio, part of the Cleveland Clinic Health System. He is associate professor of medicine at the Cleveland Clinic, Lerner College of Medicine in Cleveland.
Trending at SHM
Unveiling the hospitalist specialty code
The Centers for Medicare & Medicaid Services announced in November the official implementation date for the Medicare physician specialty code for hospitalists. On April 3, “hospitalist” will be an official specialty designation under Medicare; the code will be C6. Starting on that date, hospitalists can change their specialty designation on the Medicare enrollment application (Form CMS-855I) or through CMS’ online portal (Provider Enrollment, Chain, and Ownership System, or PECOS).
Appropriate use of specialty codes helps distinguish differences among providers and improves the quality of utilization data. SHM applied for a specialty code for hospitalists nearly 3 years ago, and CMS approved the application in February 2016.
Stand with your fellow hospitalists and make sure to declare, “I’m a C6.”
Develop curricula to educate, engage medical students and residents
The ACGME requirements for training in quality and safety are changing – it is no longer an elective. As sponsoring institutions’ residency and fellowship programs mobilize to meet these requirements, leaders may find few faculty members are comfortable enough with the material to teach and create educational content for trainees. These faculty need further development.
Sponsored by SHM, the Quality and Safety Educators Academy (QSEA) responds to that demand by providing medical educators with the knowledge and tools to integrate quality improvement and safety concepts into their curricula. The 2017 meeting is Feb. 26-28 at the Tempe Mission Palms Hotel in Arizona.
This 2½ day meeting aims to fill the current gaps for faculty by offering basic concepts and educational tools in quality improvement and patient safety. Material is presented in an interactive way, providing guidance on career and curriculum development and establishing a national network of quality and safety educators.
For more information and to register, visit www.shmqsea.org.
EHRs: blessing or curse?
SHM’s Health Information Technology (HIT) Committee invited you to participate in a brief survey to inform your experiences with inpatient electronic health record (EHR) systems. The results will serve as a foundation for a white paper to be written by the HIT Committee addressing hospitalists’ attitudes toward EHR systems. It will be released next month, so stay tuned then to view the final paper.
SHM chapters: Your connection to local education, networking, leadership opportunities
SHM offers various opportunities to grow professionally, expand your CV, and engage with other hospitalists. With more than 50 chapters across the country, you can network, learn, teach, and continue to improve patient care at a local level. Find a chapter in your area or start a chapter today by visiting www.hospitalmedicine.org/chapters.
Enhance opioid safety for inpatients
SHM enrolled 10 hospitals into a second mentored implementation cohort around Reducing Adverse Drug Events Related to Opioids (RADEO). The program is now in its second month as the sites work with their mentors to enhance safety for patients in the hospital who are prescribed opioid medications by:
- Developing a needs assessment.
- Putting in place formal selections of data collection measures.
- Beginning to take outcomes and process data collection on intervention units.
- Starting to design and implement key interventions.
Even if you’re not in this mentored implementation cohort, visit www.hospitalmedicine.org/RADEO and view the online toolkit or download the implementation guide.
Earn recognition for your research with SHM’s Junior Investigator Award
The SHM Junior Investigator Award was created for junior/early-stage investigators, defined as faculty in the first 5 years of their most recent position/appointment. Applicants must be a hospitalist or clinician-investigators whose research interests focus on the care of hospitalized patients, the organization of hospitals, or the practice of hospitalists. Applicants must be members of SHM in good standing. Nominations from mentors and self-nominations are both welcome.
The winner will be invited to receive the award during SHM’s annual meeting, HM17, May 1-4, at Mandalay Bay Resort and Casino in Las Vegas. The winner will receive complimentary registration for this meeting as well as a complimentary 1-year membership to SHM.
For more information on the application process, visit www.hospitalmedicine.org/juniorinvestigator.
Unveiling the hospitalist specialty code
The Centers for Medicare & Medicaid Services announced in November the official implementation date for the Medicare physician specialty code for hospitalists. On April 3, “hospitalist” will be an official specialty designation under Medicare; the code will be C6. Starting on that date, hospitalists can change their specialty designation on the Medicare enrollment application (Form CMS-855I) or through CMS’ online portal (Provider Enrollment, Chain, and Ownership System, or PECOS).
Appropriate use of specialty codes helps distinguish differences among providers and improves the quality of utilization data. SHM applied for a specialty code for hospitalists nearly 3 years ago, and CMS approved the application in February 2016.
Stand with your fellow hospitalists and make sure to declare, “I’m a C6.”
Develop curricula to educate, engage medical students and residents
The ACGME requirements for training in quality and safety are changing – it is no longer an elective. As sponsoring institutions’ residency and fellowship programs mobilize to meet these requirements, leaders may find few faculty members are comfortable enough with the material to teach and create educational content for trainees. These faculty need further development.
Sponsored by SHM, the Quality and Safety Educators Academy (QSEA) responds to that demand by providing medical educators with the knowledge and tools to integrate quality improvement and safety concepts into their curricula. The 2017 meeting is Feb. 26-28 at the Tempe Mission Palms Hotel in Arizona.
This 2½ day meeting aims to fill the current gaps for faculty by offering basic concepts and educational tools in quality improvement and patient safety. Material is presented in an interactive way, providing guidance on career and curriculum development and establishing a national network of quality and safety educators.
For more information and to register, visit www.shmqsea.org.
EHRs: blessing or curse?
SHM’s Health Information Technology (HIT) Committee invited you to participate in a brief survey to inform your experiences with inpatient electronic health record (EHR) systems. The results will serve as a foundation for a white paper to be written by the HIT Committee addressing hospitalists’ attitudes toward EHR systems. It will be released next month, so stay tuned then to view the final paper.
SHM chapters: Your connection to local education, networking, leadership opportunities
SHM offers various opportunities to grow professionally, expand your CV, and engage with other hospitalists. With more than 50 chapters across the country, you can network, learn, teach, and continue to improve patient care at a local level. Find a chapter in your area or start a chapter today by visiting www.hospitalmedicine.org/chapters.
Enhance opioid safety for inpatients
SHM enrolled 10 hospitals into a second mentored implementation cohort around Reducing Adverse Drug Events Related to Opioids (RADEO). The program is now in its second month as the sites work with their mentors to enhance safety for patients in the hospital who are prescribed opioid medications by:
- Developing a needs assessment.
- Putting in place formal selections of data collection measures.
- Beginning to take outcomes and process data collection on intervention units.
- Starting to design and implement key interventions.
Even if you’re not in this mentored implementation cohort, visit www.hospitalmedicine.org/RADEO and view the online toolkit or download the implementation guide.
Earn recognition for your research with SHM’s Junior Investigator Award
The SHM Junior Investigator Award was created for junior/early-stage investigators, defined as faculty in the first 5 years of their most recent position/appointment. Applicants must be a hospitalist or clinician-investigators whose research interests focus on the care of hospitalized patients, the organization of hospitals, or the practice of hospitalists. Applicants must be members of SHM in good standing. Nominations from mentors and self-nominations are both welcome.
The winner will be invited to receive the award during SHM’s annual meeting, HM17, May 1-4, at Mandalay Bay Resort and Casino in Las Vegas. The winner will receive complimentary registration for this meeting as well as a complimentary 1-year membership to SHM.
For more information on the application process, visit www.hospitalmedicine.org/juniorinvestigator.
Unveiling the hospitalist specialty code
The Centers for Medicare & Medicaid Services announced in November the official implementation date for the Medicare physician specialty code for hospitalists. On April 3, “hospitalist” will be an official specialty designation under Medicare; the code will be C6. Starting on that date, hospitalists can change their specialty designation on the Medicare enrollment application (Form CMS-855I) or through CMS’ online portal (Provider Enrollment, Chain, and Ownership System, or PECOS).
Appropriate use of specialty codes helps distinguish differences among providers and improves the quality of utilization data. SHM applied for a specialty code for hospitalists nearly 3 years ago, and CMS approved the application in February 2016.
Stand with your fellow hospitalists and make sure to declare, “I’m a C6.”
Develop curricula to educate, engage medical students and residents
The ACGME requirements for training in quality and safety are changing – it is no longer an elective. As sponsoring institutions’ residency and fellowship programs mobilize to meet these requirements, leaders may find few faculty members are comfortable enough with the material to teach and create educational content for trainees. These faculty need further development.
Sponsored by SHM, the Quality and Safety Educators Academy (QSEA) responds to that demand by providing medical educators with the knowledge and tools to integrate quality improvement and safety concepts into their curricula. The 2017 meeting is Feb. 26-28 at the Tempe Mission Palms Hotel in Arizona.
This 2½ day meeting aims to fill the current gaps for faculty by offering basic concepts and educational tools in quality improvement and patient safety. Material is presented in an interactive way, providing guidance on career and curriculum development and establishing a national network of quality and safety educators.
For more information and to register, visit www.shmqsea.org.
EHRs: blessing or curse?
SHM’s Health Information Technology (HIT) Committee invited you to participate in a brief survey to inform your experiences with inpatient electronic health record (EHR) systems. The results will serve as a foundation for a white paper to be written by the HIT Committee addressing hospitalists’ attitudes toward EHR systems. It will be released next month, so stay tuned then to view the final paper.
SHM chapters: Your connection to local education, networking, leadership opportunities
SHM offers various opportunities to grow professionally, expand your CV, and engage with other hospitalists. With more than 50 chapters across the country, you can network, learn, teach, and continue to improve patient care at a local level. Find a chapter in your area or start a chapter today by visiting www.hospitalmedicine.org/chapters.
Enhance opioid safety for inpatients
SHM enrolled 10 hospitals into a second mentored implementation cohort around Reducing Adverse Drug Events Related to Opioids (RADEO). The program is now in its second month as the sites work with their mentors to enhance safety for patients in the hospital who are prescribed opioid medications by:
- Developing a needs assessment.
- Putting in place formal selections of data collection measures.
- Beginning to take outcomes and process data collection on intervention units.
- Starting to design and implement key interventions.
Even if you’re not in this mentored implementation cohort, visit www.hospitalmedicine.org/RADEO and view the online toolkit or download the implementation guide.
Earn recognition for your research with SHM’s Junior Investigator Award
The SHM Junior Investigator Award was created for junior/early-stage investigators, defined as faculty in the first 5 years of their most recent position/appointment. Applicants must be a hospitalist or clinician-investigators whose research interests focus on the care of hospitalized patients, the organization of hospitals, or the practice of hospitalists. Applicants must be members of SHM in good standing. Nominations from mentors and self-nominations are both welcome.
The winner will be invited to receive the award during SHM’s annual meeting, HM17, May 1-4, at Mandalay Bay Resort and Casino in Las Vegas. The winner will receive complimentary registration for this meeting as well as a complimentary 1-year membership to SHM.
For more information on the application process, visit www.hospitalmedicine.org/juniorinvestigator.