User login
Imaging suggestive, but symptoms atypical
A 66-year-old man with chronic obstructive pulmonary disease (COPD) was brought to the emergency department with a 2-week history of progressive dyspnea followed by altered mental status. He had no history of diabetes mellitus, hypertension, or drug abuse.
On physical examination, he was stuporous. He had no fever or hypotension, but his pulse and breathing were rapid, and he had central cyanosis, bilateral conjunctival congestion, a puffy face, generalized wheezing, basilar crackles in both lungs, and leg edema.
Laboratory testing showed hypoxia and severe hypercarbia. His hematocrit was 65% (reference range 39–51) and his hemoglobin level was 21.5 g/dL (13–17).
The patient was diagnosed with an exacerbation of COPD. He was intubated, placed on mechanical ventilation, and admitted to the intensive care unit.
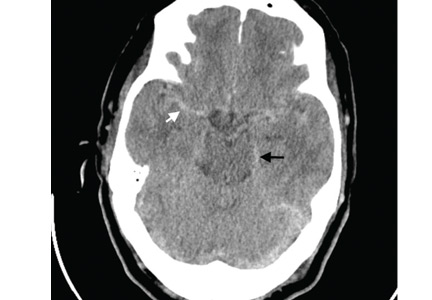
Computed tomography (CT) performed because of his decreased level of consciousness (Figure 1) showed increased attenuation in the ambient cistern and the lateral aspect of the lateral cerebral fissure, suggesting subarachnoid hemorrhage. The attenuation value in these areas was 89 Hounsfield units (typical values for brain tissue are in the 20s to 30s, and for blood in the 30s to 40s). To further evaluate for subarachnoid hemorrhage, lumbar puncture was performed, but analysis of the fluid sample showed normal protein and glucose levels and no cells.
Based on the results of cerebrospinal fluid evaluation and on the CT attenuation value, a diagnosis of pseudosubarachnoid hemorrhage due to polycythemia was made.
SUBARACHNOID VS PSEUDOSUBARACHNOID HEMORRHAGE
Subarachnoid hemorrhage typically begins with a “thunder-clap” headache (beginning suddenly and described by patients as “the worst headache ever.”) While not all patients have this presentation, if imaging suggests subarachnoid hemorrhage but the patient has atypical signs and symptoms (eg, other than headache), then pseudosubarachnoid hemorrhage should be considered.
Brain CT is one of the most reliable tools for diagnosing subarachnoid hemorrhage in the emergency department. Done within 6 hours of symptom onset, it has a sensitivity of 98.7% and a specificity of 99.9%.1 Magnetic resonance imaging can also visualize subarachnoid hemorrhage within the first 12 hours, typically as a hyperintensity in the subarachnoid space on fluid-attenuated inversion-recovery sequences2 and on susceptibility-weighted sequences.
Lumbar puncture is also an important diagnostic tool but carries a risk of brain herniation in patients with brain edema.
Pseudosubarachnoid hemorrhage is an artifact of CT imaging. It is rare, and its prevalence is unknown.3 However, it may be seen in up to 20% of patients after resuscitation, as a result of diffuse cerebral edema that lowers the attenuation of brain tissue on CT, making the vessels relatively conspicuous. It can also be seen in purulent meningitis (due to proteinaceous influx after blood-brain barrier disruption),4 in meningeal leukemia (due to increased cellularity in the leptomeninges), and in severe polycythemia (from a higher concentration of blood and hemoglobin in the vessels).3,5–7
Although the level of attenuation on CT may help distinguish subarachnoid from pseudosubarachnoid hemorrhage, its accuracy has not been defined. Inspecting the CT images may clarify whether areas with high attenuation look like blood vessels vs subarachnoid hemorrhage.
Our patient recovered and had an uneventful follow-up. The cause of his elevated hematocrit was likely chronic hypoxia from COPD.
Acknowledgment: We thank Dr. Saeide Khanbagi and Dr. Azade Nasr-lari for their cooperation.
- Dubosh NM, Bellolio MF, Rabinstein AA, Edlow JA. Sensitivity of early brain computed tomography to exclude aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Stroke 2016; 47:750–755.
- Sohn CH, Baik SK, Lee HJ, et al. MR imaging of hyperacute subarachnoid and intraventricular hemorrhage at 3T: a preliminary report of gradient echo T2*-weighted sequences. AJNR Am J Neuroradiol 2005; 26:662–665.
- Yuzawa H, Higano S, Mugikura S, et al. Pseudo-subarachnoid hemorrhage found in patients with postresuscitation encephalopathy: characteristics of CT findings and clinical importance. AJNR Am J Neuroradiol 2008; 29:1544–1549.
- Given CA 2nd, Burdette JH, Elster AD, Williams DW 3rd. Pseudo-subarachnoid hemorrhage: a potential imaging pitfall associated with diffuse cerebral edema. AJNR Am J Neuroradiol 2003; 24:254–256.
- Avrahami E, Katz R, Rabin A, Friedman V. CT diagnosis of non-traumatic subarachnoid haemorrhage in patients with brain edema. Eur J Radiol 1998; 28:222–225.
- Ben Salem D, Osseby GV, Rezaizadeh-Bourdariat K, et al. Spontaneous hyperdense intracranial vessels seen on CT scan in polycythemia cases. J Radiol 2003; 84:605–608. French.
- Hsieh SW, Khor GT, Chen CN, Huang P. Pseudo subarachnoid hemorrhage in meningeal leukemia. J Emerg Med 2012; 42:e109–e111.
A 66-year-old man with chronic obstructive pulmonary disease (COPD) was brought to the emergency department with a 2-week history of progressive dyspnea followed by altered mental status. He had no history of diabetes mellitus, hypertension, or drug abuse.
On physical examination, he was stuporous. He had no fever or hypotension, but his pulse and breathing were rapid, and he had central cyanosis, bilateral conjunctival congestion, a puffy face, generalized wheezing, basilar crackles in both lungs, and leg edema.
Laboratory testing showed hypoxia and severe hypercarbia. His hematocrit was 65% (reference range 39–51) and his hemoglobin level was 21.5 g/dL (13–17).
The patient was diagnosed with an exacerbation of COPD. He was intubated, placed on mechanical ventilation, and admitted to the intensive care unit.
Computed tomography (CT) performed because of his decreased level of consciousness (Figure 1) showed increased attenuation in the ambient cistern and the lateral aspect of the lateral cerebral fissure, suggesting subarachnoid hemorrhage. The attenuation value in these areas was 89 Hounsfield units (typical values for brain tissue are in the 20s to 30s, and for blood in the 30s to 40s). To further evaluate for subarachnoid hemorrhage, lumbar puncture was performed, but analysis of the fluid sample showed normal protein and glucose levels and no cells.
Based on the results of cerebrospinal fluid evaluation and on the CT attenuation value, a diagnosis of pseudosubarachnoid hemorrhage due to polycythemia was made.
SUBARACHNOID VS PSEUDOSUBARACHNOID HEMORRHAGE
Subarachnoid hemorrhage typically begins with a “thunder-clap” headache (beginning suddenly and described by patients as “the worst headache ever.”) While not all patients have this presentation, if imaging suggests subarachnoid hemorrhage but the patient has atypical signs and symptoms (eg, other than headache), then pseudosubarachnoid hemorrhage should be considered.
Brain CT is one of the most reliable tools for diagnosing subarachnoid hemorrhage in the emergency department. Done within 6 hours of symptom onset, it has a sensitivity of 98.7% and a specificity of 99.9%.1 Magnetic resonance imaging can also visualize subarachnoid hemorrhage within the first 12 hours, typically as a hyperintensity in the subarachnoid space on fluid-attenuated inversion-recovery sequences2 and on susceptibility-weighted sequences.
Lumbar puncture is also an important diagnostic tool but carries a risk of brain herniation in patients with brain edema.
Pseudosubarachnoid hemorrhage is an artifact of CT imaging. It is rare, and its prevalence is unknown.3 However, it may be seen in up to 20% of patients after resuscitation, as a result of diffuse cerebral edema that lowers the attenuation of brain tissue on CT, making the vessels relatively conspicuous. It can also be seen in purulent meningitis (due to proteinaceous influx after blood-brain barrier disruption),4 in meningeal leukemia (due to increased cellularity in the leptomeninges), and in severe polycythemia (from a higher concentration of blood and hemoglobin in the vessels).3,5–7
Although the level of attenuation on CT may help distinguish subarachnoid from pseudosubarachnoid hemorrhage, its accuracy has not been defined. Inspecting the CT images may clarify whether areas with high attenuation look like blood vessels vs subarachnoid hemorrhage.
Our patient recovered and had an uneventful follow-up. The cause of his elevated hematocrit was likely chronic hypoxia from COPD.
Acknowledgment: We thank Dr. Saeide Khanbagi and Dr. Azade Nasr-lari for their cooperation.
A 66-year-old man with chronic obstructive pulmonary disease (COPD) was brought to the emergency department with a 2-week history of progressive dyspnea followed by altered mental status. He had no history of diabetes mellitus, hypertension, or drug abuse.
On physical examination, he was stuporous. He had no fever or hypotension, but his pulse and breathing were rapid, and he had central cyanosis, bilateral conjunctival congestion, a puffy face, generalized wheezing, basilar crackles in both lungs, and leg edema.
Laboratory testing showed hypoxia and severe hypercarbia. His hematocrit was 65% (reference range 39–51) and his hemoglobin level was 21.5 g/dL (13–17).
The patient was diagnosed with an exacerbation of COPD. He was intubated, placed on mechanical ventilation, and admitted to the intensive care unit.
Computed tomography (CT) performed because of his decreased level of consciousness (Figure 1) showed increased attenuation in the ambient cistern and the lateral aspect of the lateral cerebral fissure, suggesting subarachnoid hemorrhage. The attenuation value in these areas was 89 Hounsfield units (typical values for brain tissue are in the 20s to 30s, and for blood in the 30s to 40s). To further evaluate for subarachnoid hemorrhage, lumbar puncture was performed, but analysis of the fluid sample showed normal protein and glucose levels and no cells.
Based on the results of cerebrospinal fluid evaluation and on the CT attenuation value, a diagnosis of pseudosubarachnoid hemorrhage due to polycythemia was made.
SUBARACHNOID VS PSEUDOSUBARACHNOID HEMORRHAGE
Subarachnoid hemorrhage typically begins with a “thunder-clap” headache (beginning suddenly and described by patients as “the worst headache ever.”) While not all patients have this presentation, if imaging suggests subarachnoid hemorrhage but the patient has atypical signs and symptoms (eg, other than headache), then pseudosubarachnoid hemorrhage should be considered.
Brain CT is one of the most reliable tools for diagnosing subarachnoid hemorrhage in the emergency department. Done within 6 hours of symptom onset, it has a sensitivity of 98.7% and a specificity of 99.9%.1 Magnetic resonance imaging can also visualize subarachnoid hemorrhage within the first 12 hours, typically as a hyperintensity in the subarachnoid space on fluid-attenuated inversion-recovery sequences2 and on susceptibility-weighted sequences.
Lumbar puncture is also an important diagnostic tool but carries a risk of brain herniation in patients with brain edema.
Pseudosubarachnoid hemorrhage is an artifact of CT imaging. It is rare, and its prevalence is unknown.3 However, it may be seen in up to 20% of patients after resuscitation, as a result of diffuse cerebral edema that lowers the attenuation of brain tissue on CT, making the vessels relatively conspicuous. It can also be seen in purulent meningitis (due to proteinaceous influx after blood-brain barrier disruption),4 in meningeal leukemia (due to increased cellularity in the leptomeninges), and in severe polycythemia (from a higher concentration of blood and hemoglobin in the vessels).3,5–7
Although the level of attenuation on CT may help distinguish subarachnoid from pseudosubarachnoid hemorrhage, its accuracy has not been defined. Inspecting the CT images may clarify whether areas with high attenuation look like blood vessels vs subarachnoid hemorrhage.
Our patient recovered and had an uneventful follow-up. The cause of his elevated hematocrit was likely chronic hypoxia from COPD.
Acknowledgment: We thank Dr. Saeide Khanbagi and Dr. Azade Nasr-lari for their cooperation.
- Dubosh NM, Bellolio MF, Rabinstein AA, Edlow JA. Sensitivity of early brain computed tomography to exclude aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Stroke 2016; 47:750–755.
- Sohn CH, Baik SK, Lee HJ, et al. MR imaging of hyperacute subarachnoid and intraventricular hemorrhage at 3T: a preliminary report of gradient echo T2*-weighted sequences. AJNR Am J Neuroradiol 2005; 26:662–665.
- Yuzawa H, Higano S, Mugikura S, et al. Pseudo-subarachnoid hemorrhage found in patients with postresuscitation encephalopathy: characteristics of CT findings and clinical importance. AJNR Am J Neuroradiol 2008; 29:1544–1549.
- Given CA 2nd, Burdette JH, Elster AD, Williams DW 3rd. Pseudo-subarachnoid hemorrhage: a potential imaging pitfall associated with diffuse cerebral edema. AJNR Am J Neuroradiol 2003; 24:254–256.
- Avrahami E, Katz R, Rabin A, Friedman V. CT diagnosis of non-traumatic subarachnoid haemorrhage in patients with brain edema. Eur J Radiol 1998; 28:222–225.
- Ben Salem D, Osseby GV, Rezaizadeh-Bourdariat K, et al. Spontaneous hyperdense intracranial vessels seen on CT scan in polycythemia cases. J Radiol 2003; 84:605–608. French.
- Hsieh SW, Khor GT, Chen CN, Huang P. Pseudo subarachnoid hemorrhage in meningeal leukemia. J Emerg Med 2012; 42:e109–e111.
- Dubosh NM, Bellolio MF, Rabinstein AA, Edlow JA. Sensitivity of early brain computed tomography to exclude aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. Stroke 2016; 47:750–755.
- Sohn CH, Baik SK, Lee HJ, et al. MR imaging of hyperacute subarachnoid and intraventricular hemorrhage at 3T: a preliminary report of gradient echo T2*-weighted sequences. AJNR Am J Neuroradiol 2005; 26:662–665.
- Yuzawa H, Higano S, Mugikura S, et al. Pseudo-subarachnoid hemorrhage found in patients with postresuscitation encephalopathy: characteristics of CT findings and clinical importance. AJNR Am J Neuroradiol 2008; 29:1544–1549.
- Given CA 2nd, Burdette JH, Elster AD, Williams DW 3rd. Pseudo-subarachnoid hemorrhage: a potential imaging pitfall associated with diffuse cerebral edema. AJNR Am J Neuroradiol 2003; 24:254–256.
- Avrahami E, Katz R, Rabin A, Friedman V. CT diagnosis of non-traumatic subarachnoid haemorrhage in patients with brain edema. Eur J Radiol 1998; 28:222–225.
- Ben Salem D, Osseby GV, Rezaizadeh-Bourdariat K, et al. Spontaneous hyperdense intracranial vessels seen on CT scan in polycythemia cases. J Radiol 2003; 84:605–608. French.
- Hsieh SW, Khor GT, Chen CN, Huang P. Pseudo subarachnoid hemorrhage in meningeal leukemia. J Emerg Med 2012; 42:e109–e111.
A rose by any other name is still a rose—but why a rose?
Christopher Columbus, age 41, returned to Europe from his first voyage to the New World suffering from what would turn out to be a chronic illness that included recurrent febrile bouts of severe lower-extremity arthritis. For many years he was thought to have had gout, but careful reanalysis led Allison1 and then Arnett et al2 to propose an alternative diagnosis. Based on the pattern of Columbus’s arthritis and the accompanying symptoms, which these clinicians linked to the arthritis, they proposed the diagnosis of reactive arthritis—actually a subset of reactive arthritis, which at the time of their publications was called Reiter’s syndrome. Arnett discussed this extensively at a historic clinicopathologic conference, noting that the bouts of arthritis were prolonged (too long for typical gout) and that the historically described recurrent episodes of red (“bleeding”) eyes with intermittent “blindness” and ultimate immobility due to spine pain (arthritis) would not be typical of gout. Arnett et al recognized the pattern of this arthritis—lower-extremity-predominant with prolonged episodes, associated inflammatory eye disease, spine involvement, and the protracted course of nearly a decade—as far more typical of a reactive arthritis than gout (or ongoing bacterial infection), occurring in a likely HLA-B27-positive individual. They proposed that the probable trigger for the arthritis was an episode of food-borne bacterial infection acquired during the extensive transatlantic journey.
Fast forward about 470 years to an epidemic aboard a US Navy ship of Shigella-associated dysentery that was accompanied by readily diagnosed reactive arthritis.3 Several diarrheal epidemics with associated reactive arthritis aboard cruise ships have been described, and the association is well documented following Shigella, Salmonella, Campylobacter, and nondiarrheal chlamydial infections.
In this issue of the Journal, Gómez-Moyano et al present images of a patient with dermatologic features of reactive arthritis. I have two points to make in introducing this historical background. First, I want to highlight some features of this syndrome that are distinctive enough as a clinical pattern to enable a diagnosis by appearance alone or, as in the case of Columbus, by historical description alone. But second and more important, I wonder, as perhaps you do, why similar inflammatory features occur in patients of seemingly diverse backgrounds, triggered by different organisms that may have infected different mucosal areas. Why does this rose look like a rose and not a gardenia?
Reactive arthritis is classically thought of as self-limited, lasting less than 6 months. But a significant minority of patients may have chronic disease, particularly with spondylitis, which may be more common in patients who have the HLA-B27 haplotype. The peripheral arthritis generally affects relatively few joints, with the larger lower-extremity joints a common target. Enthesitis, especially at the Achilles tendon heel insertion, is common, and dactylitis or “sausage digits” can occur. These features may also be seen in patients with psoriatic or enteropathic arthritis. Inflammation of the skin (as shown by Gómez-Moyano et al), the eye (conjunctivitis, uveitis, episcleritis), oral mucosa, and the genitourinary tract (urethritis, prostatitis) can also occur. Urethritis can occur in patients with enteric diarrhea as the trigger; thus, it is not just associated with genitourinary infections like chlamydia. What all these sites have in common to be targeted following an infection is not known with certainty; relevant bacterial antigens cannot always be detected in the involved tissues. It is intriguing that transgenic rats engineered to contain many copies of the human HLA-B27 gene develop arthritis along with mucosal, eye, and skin inflammation, which can be avoided if the animals are raised in a germ-free environment. But probably fewer than 50% of patients with reactive arthritis have the B27 gene, so other factors must contribute to this uncommon syndrome. B27 is thus not a generally useful diagnostic test.
When a patient presents with acute inflammatory asymmetric oligoarthritis (affecting < 4 joints), infectious arthritis and crystal-induced arthritis need to be excluded. But looking for and finding other features more typical of reactive arthritis may spare the patient a more extensive and expensive inpatient evaluation.
Recognizing a rose can provide an aesthetic moment, even if we can’t define exactly what makes it a rose. Pattern recognition matters in clinical practice, even without full understanding of the molecular backdrop.
- Allison DJ. Christopher Columbus: first case of Reiter’s disease in the Old World? Lancet 1980; 2:1309.
- Arnett FC, Merrill C, Albardaner F, Mackowiak PA. A mariner with crippling arthritis and bleeding eyes. Am J Med Sci 2006; 332:123–130.
- Noer HR. An “experimental” epidemic of Reiter’s syndrome. JAMA 1966; 198:693–698.
Christopher Columbus, age 41, returned to Europe from his first voyage to the New World suffering from what would turn out to be a chronic illness that included recurrent febrile bouts of severe lower-extremity arthritis. For many years he was thought to have had gout, but careful reanalysis led Allison1 and then Arnett et al2 to propose an alternative diagnosis. Based on the pattern of Columbus’s arthritis and the accompanying symptoms, which these clinicians linked to the arthritis, they proposed the diagnosis of reactive arthritis—actually a subset of reactive arthritis, which at the time of their publications was called Reiter’s syndrome. Arnett discussed this extensively at a historic clinicopathologic conference, noting that the bouts of arthritis were prolonged (too long for typical gout) and that the historically described recurrent episodes of red (“bleeding”) eyes with intermittent “blindness” and ultimate immobility due to spine pain (arthritis) would not be typical of gout. Arnett et al recognized the pattern of this arthritis—lower-extremity-predominant with prolonged episodes, associated inflammatory eye disease, spine involvement, and the protracted course of nearly a decade—as far more typical of a reactive arthritis than gout (or ongoing bacterial infection), occurring in a likely HLA-B27-positive individual. They proposed that the probable trigger for the arthritis was an episode of food-borne bacterial infection acquired during the extensive transatlantic journey.
Fast forward about 470 years to an epidemic aboard a US Navy ship of Shigella-associated dysentery that was accompanied by readily diagnosed reactive arthritis.3 Several diarrheal epidemics with associated reactive arthritis aboard cruise ships have been described, and the association is well documented following Shigella, Salmonella, Campylobacter, and nondiarrheal chlamydial infections.
In this issue of the Journal, Gómez-Moyano et al present images of a patient with dermatologic features of reactive arthritis. I have two points to make in introducing this historical background. First, I want to highlight some features of this syndrome that are distinctive enough as a clinical pattern to enable a diagnosis by appearance alone or, as in the case of Columbus, by historical description alone. But second and more important, I wonder, as perhaps you do, why similar inflammatory features occur in patients of seemingly diverse backgrounds, triggered by different organisms that may have infected different mucosal areas. Why does this rose look like a rose and not a gardenia?
Reactive arthritis is classically thought of as self-limited, lasting less than 6 months. But a significant minority of patients may have chronic disease, particularly with spondylitis, which may be more common in patients who have the HLA-B27 haplotype. The peripheral arthritis generally affects relatively few joints, with the larger lower-extremity joints a common target. Enthesitis, especially at the Achilles tendon heel insertion, is common, and dactylitis or “sausage digits” can occur. These features may also be seen in patients with psoriatic or enteropathic arthritis. Inflammation of the skin (as shown by Gómez-Moyano et al), the eye (conjunctivitis, uveitis, episcleritis), oral mucosa, and the genitourinary tract (urethritis, prostatitis) can also occur. Urethritis can occur in patients with enteric diarrhea as the trigger; thus, it is not just associated with genitourinary infections like chlamydia. What all these sites have in common to be targeted following an infection is not known with certainty; relevant bacterial antigens cannot always be detected in the involved tissues. It is intriguing that transgenic rats engineered to contain many copies of the human HLA-B27 gene develop arthritis along with mucosal, eye, and skin inflammation, which can be avoided if the animals are raised in a germ-free environment. But probably fewer than 50% of patients with reactive arthritis have the B27 gene, so other factors must contribute to this uncommon syndrome. B27 is thus not a generally useful diagnostic test.
When a patient presents with acute inflammatory asymmetric oligoarthritis (affecting < 4 joints), infectious arthritis and crystal-induced arthritis need to be excluded. But looking for and finding other features more typical of reactive arthritis may spare the patient a more extensive and expensive inpatient evaluation.
Recognizing a rose can provide an aesthetic moment, even if we can’t define exactly what makes it a rose. Pattern recognition matters in clinical practice, even without full understanding of the molecular backdrop.
Christopher Columbus, age 41, returned to Europe from his first voyage to the New World suffering from what would turn out to be a chronic illness that included recurrent febrile bouts of severe lower-extremity arthritis. For many years he was thought to have had gout, but careful reanalysis led Allison1 and then Arnett et al2 to propose an alternative diagnosis. Based on the pattern of Columbus’s arthritis and the accompanying symptoms, which these clinicians linked to the arthritis, they proposed the diagnosis of reactive arthritis—actually a subset of reactive arthritis, which at the time of their publications was called Reiter’s syndrome. Arnett discussed this extensively at a historic clinicopathologic conference, noting that the bouts of arthritis were prolonged (too long for typical gout) and that the historically described recurrent episodes of red (“bleeding”) eyes with intermittent “blindness” and ultimate immobility due to spine pain (arthritis) would not be typical of gout. Arnett et al recognized the pattern of this arthritis—lower-extremity-predominant with prolonged episodes, associated inflammatory eye disease, spine involvement, and the protracted course of nearly a decade—as far more typical of a reactive arthritis than gout (or ongoing bacterial infection), occurring in a likely HLA-B27-positive individual. They proposed that the probable trigger for the arthritis was an episode of food-borne bacterial infection acquired during the extensive transatlantic journey.
Fast forward about 470 years to an epidemic aboard a US Navy ship of Shigella-associated dysentery that was accompanied by readily diagnosed reactive arthritis.3 Several diarrheal epidemics with associated reactive arthritis aboard cruise ships have been described, and the association is well documented following Shigella, Salmonella, Campylobacter, and nondiarrheal chlamydial infections.
In this issue of the Journal, Gómez-Moyano et al present images of a patient with dermatologic features of reactive arthritis. I have two points to make in introducing this historical background. First, I want to highlight some features of this syndrome that are distinctive enough as a clinical pattern to enable a diagnosis by appearance alone or, as in the case of Columbus, by historical description alone. But second and more important, I wonder, as perhaps you do, why similar inflammatory features occur in patients of seemingly diverse backgrounds, triggered by different organisms that may have infected different mucosal areas. Why does this rose look like a rose and not a gardenia?
Reactive arthritis is classically thought of as self-limited, lasting less than 6 months. But a significant minority of patients may have chronic disease, particularly with spondylitis, which may be more common in patients who have the HLA-B27 haplotype. The peripheral arthritis generally affects relatively few joints, with the larger lower-extremity joints a common target. Enthesitis, especially at the Achilles tendon heel insertion, is common, and dactylitis or “sausage digits” can occur. These features may also be seen in patients with psoriatic or enteropathic arthritis. Inflammation of the skin (as shown by Gómez-Moyano et al), the eye (conjunctivitis, uveitis, episcleritis), oral mucosa, and the genitourinary tract (urethritis, prostatitis) can also occur. Urethritis can occur in patients with enteric diarrhea as the trigger; thus, it is not just associated with genitourinary infections like chlamydia. What all these sites have in common to be targeted following an infection is not known with certainty; relevant bacterial antigens cannot always be detected in the involved tissues. It is intriguing that transgenic rats engineered to contain many copies of the human HLA-B27 gene develop arthritis along with mucosal, eye, and skin inflammation, which can be avoided if the animals are raised in a germ-free environment. But probably fewer than 50% of patients with reactive arthritis have the B27 gene, so other factors must contribute to this uncommon syndrome. B27 is thus not a generally useful diagnostic test.
When a patient presents with acute inflammatory asymmetric oligoarthritis (affecting < 4 joints), infectious arthritis and crystal-induced arthritis need to be excluded. But looking for and finding other features more typical of reactive arthritis may spare the patient a more extensive and expensive inpatient evaluation.
Recognizing a rose can provide an aesthetic moment, even if we can’t define exactly what makes it a rose. Pattern recognition matters in clinical practice, even without full understanding of the molecular backdrop.
- Allison DJ. Christopher Columbus: first case of Reiter’s disease in the Old World? Lancet 1980; 2:1309.
- Arnett FC, Merrill C, Albardaner F, Mackowiak PA. A mariner with crippling arthritis and bleeding eyes. Am J Med Sci 2006; 332:123–130.
- Noer HR. An “experimental” epidemic of Reiter’s syndrome. JAMA 1966; 198:693–698.
- Allison DJ. Christopher Columbus: first case of Reiter’s disease in the Old World? Lancet 1980; 2:1309.
- Arnett FC, Merrill C, Albardaner F, Mackowiak PA. A mariner with crippling arthritis and bleeding eyes. Am J Med Sci 2006; 332:123–130.
- Noer HR. An “experimental” epidemic of Reiter’s syndrome. JAMA 1966; 198:693–698.
Benign prostatic hyperplasia: Evaluation and medical management in primary care
Primary care physicians are uniquely positioned to screen for benign prostatic hyperplasia (BPH) and lower urinary tract symptoms, to perform the initial diagnostic workup, and to start medical therapy in uncomplicated cases. Effective medical therapy is available but underutilized in the primary care setting.1
This overview covers how to identify and evaluate patients with lower urinary tract symptoms, initiate therapy, and identify factors warranting timely urology referral.
TWO MECHANISMS: STATIC, DYNAMIC
BPH is a histologic diagnosis of proliferation of smooth muscle, epithelium, and stromal cells within the transition zone of the prostate,2 which surrounds the proximal urethra.
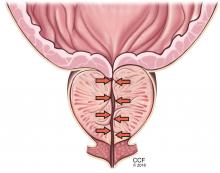
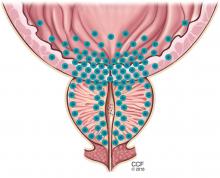
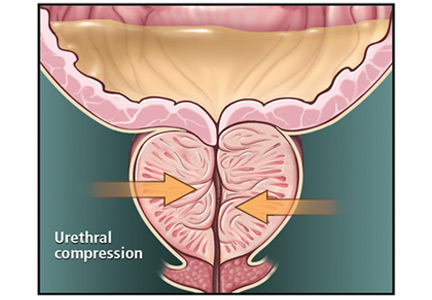
Symptoms arise through two mechanisms: static, in which the hyperplastic prostatic tissue compresses the urethra (Figure 1); and dynamic, with increased adrenergic nervous system and prostatic smooth muscle tone (Figure 2).3 Both mechanisms increase resistance to urinary flow at the level of the bladder outlet.
As an adaptive change to overcome outlet resistance and maintain urinary flow, the detrusor muscles undergo hypertrophy. However, over time the bladder may develop diminished compliance and increased detrusor activity, causing symptoms such as urinary frequency and urgency. Chronic bladder outlet obstruction can lead to bladder decompensation and detrusor underactivity, manifesting as incomplete emptying, urinary hesitancy, intermittency (starting and stopping while voiding), a weakened urinary stream, and urinary retention.
MOST MEN EVENTUALLY DEVELOP BPH
Autopsy studies have shown that BPH increases in prevalence with age beginning around age 30 and reaching a peak prevalence of 88% in men in their 80s.4 This trend parallels those of the incidence and severity of lower urinary tract symptoms.5
In the year 2000 alone, BPH was responsible for 4.5 million physician visits at an estimated direct cost of $1.1 billion, not including the cost of pharmacotherapy.6
OFFICE WORKUP
BPH can cause lower urinary tract symptoms that fall into two categories: storage and emptying. Storage symptoms include urinary frequency, urgency, and nocturia, whereas emptying symptoms include weak stream, hesitancy, intermittency, incomplete emptying, straining, and postvoid dribbling.
History and differential diagnosis
Assessment begins with characterizing the patient’s symptoms and determining those that are most bothersome. Because BPH is just one of many possible causes of lower urinary tract symptoms, a detailed medical history is necessary to evaluate for other conditions that may cause lower urinary tract dysfunction or complicate its treatment.
Obstructive urinary symptoms can arise from BPH or from other conditions, including urethral stricture disease and neurogenic voiding dysfunction.
Irritative voiding symptoms such as urinary urgency and frequency can result from detrusor overactivity secondary to BPH, but can also be caused by neurologic disease, malignancy, initiation of diuretic therapy, high fluid intake, or consumption of bladder irritants such as caffeine, alcohol, and spicy foods.
Urinary frequency is sometimes a presenting symptom of undiagnosed or poorly controlled diabetes mellitus resulting from glucosuria and polyuria. Iatrogenic causes of polyuria include the new hypoglycemic agents canagliflozin and dapagliflozin, which block renal glucose reabsorption, improving glycemic control by inducing urinary
glucose loss.7
Nocturia has many possible nonurologic causes including heart failure (in which excess extravascular fluid shifts to the intravascular space when the patient lies down, resulting in polyuria), obstructive sleep apnea, and behavioral factors such as high evening fluid intake. In these cases, patients usually have nocturnal polyuria (greater than one-third of 24-hour urine output at night) rather than only nocturia (waking at night to void). A fluid diary is a simple tool that can differentiate these two conditions.
Hematuria can develop in patients with BPH with bleeding from congested prostatic or bladder neck vessels; however, hematuria may indicate an underlying malignancy or urolithiasis, for which a urologic workup is indicated.
The broad differential diagnosis for the different lower urinary tract symptoms highlights the importance of obtaining a thorough history.
Physical examination
A general examination should include the following:
Body mass index. Obese patients are at risk of obstructive sleep apnea, which can cause nocturnal polyuria.
Gait. Abnormal gait may suggest a neurologic condition such as Parkinson disease or stroke that can also affect lower urinary tract function.
Lower abdomen. A palpable bladder suggests urinary retention.
External genitalia. Penile causes of urinary obstruction include urethral meatal stenosis or a palpable urethral mass.
Digital rectal examination can reveal benign prostatic enlargement or nodules or firmness, which suggest malignancy and warrant urologic referral.
Neurologic examination, including evaluation of anal sphincter tone and lower extremity sensorimotor function.
Feet. Bilateral lower-extremity edema may be due to heart failure or venous insufficiency.
The International Prostate Symptom Score
All men with lower urinary tract symptoms should complete the International Prostate Symptom Score (IPSS) survey, consisting of seven questions about urinary symptoms plus one about quality of life.8 Specifically, it asks the patient, “Over the past month, how often have you…”
- Had a sensation of not emptying your bladder completely after you finish urinating?
- Had to urinate again less than 2 hours after you finished urinating?
- Found you stopped and started again several times when you urinated?
- Found it difficult to postpone urination?
- Had a weak urinary stream?
- Had to push or strain to begin urination?
Each question above is scored as 0 (not at all), 1 (less than 1 time in 5), 2 (less than half the time), 3 (about half the time), 4 (more than half the time, or 5 (almost always).
- Over the past month, how many times did you most typically get up to urinate from the time you went to bed until the time you got up in the morning?
This question is scored from 0 (none) to 5 (5 times or more).
- If you were to spend the rest of your life with your urinary condition the way it is now, how would you feel about that?
This question is scored as 0 (delighted), 1 (pleased), 2 (mostly satisfied), 3 (mixed: equally satisfied and dissatisfied), 4 (mostly dissatisfied), 5 (unhappy), or 6 (terrible).
A total score of 1 to 7 is categorized as mild, 8 to 19 moderate, and 20 to 35 severe.
The questionnaire can also be used to evaluate for disease progression and response to treatment over time. A change of 3 points is clinically significant, as patients are unable to discern a difference below this threshold.9
Urinalysis
Urinalysis is recommended to assess for urinary tract infection, hematuria, proteinuria, or glucosuria.
Fluid diary
A fluid diary is useful for patients complaining of frequency or nocturia and can help quantify the volume of fluid intake, frequency of urination, and volumes voided. The patient should complete the diary over a 24-hour period, recording the time and volume of fluid intake and each void. This aids in diagnosing polyuria (> 3 L of urine output per 24 hours), nocturnal polyuria, and behavioral causes of symptoms, including excessive total fluid intake or high evening fluid intake contributing to nocturia.
Serum creatinine not recommended
Measuring serum creatinine is not recommended in the initial BPH workup, as men with lower urinary tract symptoms are not at higher risk of renal failure than those without these symptoms.10
Prostate-specific antigen
Prostate-specific antigen (PSA) is a glycoprotein primarily produced by prostatic luminal epithelial cells. It is most commonly discussed in the setting of prostate cancer screening, but its utility extends to guiding the management of BPH.
PSA levels correlate with prostate volume and subsequent growth.11 In addition, the risks of developing acute urinary retention or needing surgical intervention rise with increasing PSA.12 Among men in the Proscar Long-Term Efficacy and Safety Study, the risk of acute urinary retention or BPH-related surgery after 4 years in the watchful-waiting arm was 7.8% in men with a PSA of 1.3 ng/dL or less, compared with 19.9% in men with a PSA greater than 3.2 ng/dL.11 Therefore, men with BPH and an elevated PSA are at higher risk with watchful waiting and may be better served with medical therapy.
In addition, American Urological Association guidelines recommend measuring serum PSA levels in men with a life expectancy greater than 10 years in whom the diagnosis of prostate cancer would alter management.10
Urologic referral
If the initial evaluation reveals hematuria, recurrent urinary tract infection, a palpable bladder, abnormal findings on digital rectal examination suggesting prostate cancer, or a history of or risk factors for urethral stricture or neurologic disease, the patient should be referred to a urologist for further evaluation (Table 1).10 Other patients who should undergo urologic evaluation are those with persistent bothersome symptoms after basic management and those who desire referral.
Adjunctive tests
Patients referred for urologic evaluation may require additional tests for diagnosis and to guide management.
Postvoid residual volume is easily measured with either abdominal ultrasonography or catheterization and is often included in the urologic evaluation of BPH. Patients vary considerably in their residual volume, which correlates poorly with BPH, symptom severity, or surgical success. However, those with a residual volume of more than 100 mL have a slightly higher rate of failure with watchful waiting.13 Postvoid residual volume is not routinely monitored in patients with a low residual volume unless there is a significant change in urinary symptoms. Conversely, patients with a volume greater than 200 mL should be monitored closely for worsening urinary retention, especially if considering anticholinergic therapy.
There is no absolute threshold postvoid residual volume above which therapy is mandatory. Rather, the decision to intervene is based on symptom severity and whether sequelae of urinary retention (eg, incontinence, urinary tract infection, hematuria, hydronephrosis, renal dysfunction) are present.
Uroflowmetry is a noninvasive test measuring the urinary flow rate during voiding and is recommended during specialist evaluation of men with lower urinary tract symptoms and suspected BPH.10 Though a diminished urinary flow rate may be detected in men with bladder outlet obstruction from BPH, it cannot differentiate obstruction from detrusor underactivity, both of which may result in reduced urinary flow. Urodynamic studies can help differentiate between these two mechanisms of lower urinary tract symptoms. Uroflowmetry may be useful in selecting surgical candidates, as patients with a maximum urinary flow rate of 15 mL/second or greater have been shown to have lower rates of surgical success.14
Urodynamic studies. If the diagnosis of bladder outlet obstruction remains in doubt, urodynamic studies can differentiate obstruction from detrusor underactivity. Urodynamic studies allow simultaneous measurement of urinary flow and detrusor pressure, differentiating between obstruction (manifesting as diminished urinary flow with normal or elevated detrusor pressure) and detrusor underactivity (diminished urinary flow with diminished detrusor pressure). Nomograms15 and the easily calculated bladder outlet obstruction index16 are simple tools used to differentiate these two causes of diminished urinary flow.
Cystourethroscopy is not recommended for routine evaluation of BPH. Indications for cystourethroscopy include hematuria and the presence of a risk factor for urethral stricture disease such as urethritis, prior urethral instrumentation, or perineal trauma. Cystourethroscopy can also aid in surgical planning when intervention is considered.
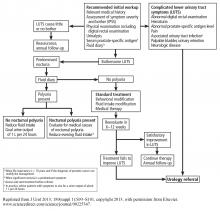
An algorithm for diagnostic workup and management of BPH and lower urinary tract symptoms is shown in Figure 3.17
MANAGEMENT STRATEGIES FOR BPH
While BPH is rarely life-threatening, it can significantly detract from a patient’s quality of life. The goal of treatment is not only to alleviate bothersome symptoms, but also to prevent disease progression and disease-related complications.
BPH tends to progress
Understanding the natural history of BPH is imperative to appropriately counsel patients on management options, which include watchful waiting, behavioral modification, pharmacologic therapy, and surgery.
In a randomized trial,18 men with moderately symptomatic BPH underwent either surgery or, in the control group, watchful waiting. At 5 years, the failure rate was 21% with watchful waiting vs 10% with surgery (P < .0004). (Failure was defined as a composite of death, repeated or intractable urinary retention, residual urine volume > 350 mL, development of bladder calculus, new persistent incontinence requiring use of a pad or other incontinence device, symptom score in the severe range [> 24 at 1 visit or score of 21 or higher at two consecutive visits, with 27 being the maximum score], or a doubling of baseline serum creatinine.) In the watchful-waiting group, 36% of the men crossed over to surgery. Men with more bothersome symptoms at enrollment were at higher risk of progressing to surgery.
In a longitudinal study of men with BPH and mild symptoms (IPSS < 8), the risk of progression to moderate or severe symptoms (IPPS ≥ 8) was 31% at 4 years.19
The Olmsted County Study of Urinary Symptoms and Health Status Among Men20 found that the peak urinary flow rate decreased by a mean of 2.1% per year, declining faster in older men who had a lower peak flow at baseline. In this cohort, the IPSS increased by a mean of 0.18 points per year, with a greater increase in older men.21
Though men managed with watchful waiting are at no higher risk of death or renal failure than men managed surgically,17 population-based studies have demonstrated an overall risk of acute urinary retention of 6.8/1,000 person-years with watchful waiting. Older men with a larger prostate, higher symptom score, and lower peak urinary flow rate are at higher risk of acute urinary retention and progression to needing BPH treatment.22,23
There is evidence that patients progressing to needing surgery after an initial period of watchful waiting have worse surgical outcomes than men managed surgically at the onset.18 This observation must be considered in counseling and selecting patients for watchful waiting. Ideal candidates include patients who have mild or moderate symptoms that cause little bother.10 Patients electing watchful waiting warrant annual follow-up including history, physical examination, and symptom assessment with the IPSS.
Behavioral modification
Behavioral modification should be incorporated into whichever management strategy a patient elects. Such modifications include:
- Reducing total or evening fluid intake for patients with urinary frequency or nocturia.
- Minimizing consumption of bladder irritants such as alcohol and caffeine, which exacerbate storage symptoms.
- Smoking cessation counseling.
- For patients with lower extremity edema who complain of nocturia, using compression stockings or elevating their legs in the afternoon to mobilize lower extremity edema and promote diuresis before going to sleep. If these measures fail, initiating or increasing the dose of a diuretic should be considered. Patients on diuretic therapy with nocturnal lower urinary tract symptoms should be instructed to take diuretics in the morning and early afternoon to avoid diuresis just before bed.
MEDICAL MANAGEMENT
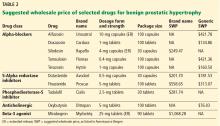
Drugs for BPH include alpha-adrenergic blockers, 5-alpha reductase inhibitors, anticholinergics, beta-3 agonists, and phosphodiesterase-5 inhibitors. Costs of selected agents in these classes are listed in Table 2.
Alpha-adrenergic receptor blockers
Alpha-adrenergic receptors are found throughout the body and modulate smooth muscle tone.24 The alpha-1a receptor is the predominant subtype found in the bladder neck and prostate25 (Figure 2) and is a target of therapy. By antagonizing the alpha-1a receptor, alpha-blockers relax the smooth muscle in the prostate and bladder neck, reduce bladder outlet resistance, and improve urinary flow.26
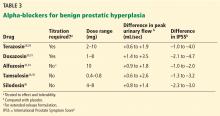
In clinical trials in BPH, alpha-blockers improved the symptom score by 30% to 45% and increased the peak urinary flow rate by 15% to 30% from baseline values.27 These agents have a rapid onset (within a few days) and result in significant symptom improvement. They are all about the same in efficacy (Table 3),28–36 with no strong evidence that any one of them is superior to another; thus, decisions about which agent to use must consider differences in receptor subtype specificity, adverse-effect profile, and tolerability.
In the Medical Therapy of Prostatic Symptoms (MTOPS) trial,37 men randomized to the alpha-blocker doxazosin had a 39% lower risk of BPH progression than with placebo, largely due to symptom score reduction. However, doxazosin failed to reduce the risk of progressing to acute urinary retention or surgical intervention. Though rapidly effective in reducing symptoms, alpha-blocker monotherapy may not be the best option in men at higher risk of BPH progression, as discussed below.
Before starting this therapy, patients must be counseled about common side effects such as dizziness, fatigue, peripheral edema, orthostatic hypotension, and ejaculatory dysfunction. The incidence of adverse effects varies among agents (Table 4).28–30,34,35,38,39
To maximize efficacy of alpha-blocker therapy, it is imperative to understand dosing variations among agents.
Alpha-blockers are classified as uroselective or non-uroselective based on alpha-1a receptor subtype specificity. The non-uroselective alpha-blockers doxazosin and terazosin need to be titrated because the higher the dose the greater the efficacy, but also the greater the blood pressure-lowering effect and other side effects.25 Though non-uroselective, alfuzosin does not affect blood pressure and does not require dose titration. Similarly, the uroselective alpha-blockers tamsulosin and silodosin can be initiated at a therapeutic dose.
Terazosin, a non-uroselective agent, can lower blood pressure and often causes dizziness. It should be started at 2 mg and titrated to side effects, efficacy, or maximum therapeutic dose (10 mg daily).28
Doxazosin has a high, dose-related incidence of dizziness (up to 20%) and must be titrated, starting at 1 mg to a maximum 8 mg.30
Alfuzosin, tamsulosin, and silodosin do not require titration and can be initiated at the therapeutic doses listed in Table 3. Of note, obese patients often require 0.8 mg tamsulosin for maximum efficacy due to a higher volume of distribution.
Before initiating an alpha-blocker, a physician must determine whether a patient plans to undergo cataract surgery, as the use of alpha-blockers is associated with intraoperative floppy iris syndrome. This condition is marked by poor intraoperative pupil dilation, increasing the risk of surgical complications.40 It is unclear whether discontinuing alpha-blockers before cataract surgery reduces the risk of intraoperative floppy iris syndrome. As such, alpha-blocker therapy should be delayed in patients planning to undergo cataract surgery.
5-Alpha reductase inhibitors
Prostate growth is androgen-dependent and mediated predominantly by dihydrotestosterone, which is generated from testosterone by the action of 5-alpha reductase. There are two 5-alpha reductase isoenzymes: type 1, expressed in the liver and skin, and type 2, expressed primarily in the prostate.
There are also two 5-alpha reductase inhibitors: dutasteride and finasteride. Dutasteride inhibits both isoenzymes, while finasteride is selective for type 2. By inhibiting both isoenzymes, dutasteride reduces the serum dihydrotestosterone concentration more than finasteride does (by 95% vs 70%), and also reduces the intraprostatic dihydrotestosterone concentration more (by 94% vs 80%).41–43 Both agents induce apoptosis of prostatic stroma, with a resultant 20% to 25% mean reduction in prostate volume.41,42
Finasteride and dutasteride are believed to mitigate the static obstructive component of BPH, with similar improvements in urinary flow rate (1.6–2.2 mL/sec) and symptom score (–2.7 to – 4.5 points) in men with an enlarged prostate.41,42 Indeed, data from the MTOPS trial showed that men with a prostate volume of 30 grams or greater or a PSA level of 1.5 ng/mL or greater are most likely to benefit from 5-alpha reductase inhibitors.37 Maximum symptomatic improvement is seen after 3 to 6 months of 5-alpha reductase inhibitor therapy.
In addition to improving urinary flow and lower urinary tract symptoms, finasteride has been shown to reduce the risk of disease progression in men with prostates greater than 30 grams.44 Compared with placebo, these drugs significantly reduce the risk of developing acute urinary retention or requiring BPH-related surgery, a benefit not seen with alpha-blockers.37 To estimate prostate volume, most practitioners rely on digital rectal examination. Though less precise than transrectal ultrasonography, digital rectal examination can identify men with significant prostatic enlargement likely to benefit from this therapy.
Before starting 5-alpha reductase inhibitor therapy, patients should be counseled about common adverse effects such as erectile dysfunction (occurring in 5%–8%), decreased libido (5%), ejaculatory dysfunction (1%–5%), and gynecomastia (1%).
Combination therapy
The MTOPS trial37 randomized patients to receive doxazosin, finasteride, both, or placebo. The combination of doxazosin (an alpha-blocker) and finasteride (a 5-alpha reductase inhibitor) reduced the risk of disease progression to a greater extent than doxazosin or finasteride alone. It also reduced the IPSS more and increased the peak urinary flow rate more. Similar results have been seen with the combination of dutasteride and tamsulosin.45
Given its superior efficacy and benefits in preventing disease progression, combination therapy should be considered for men with an enlarged prostate and moderate to severe lower urinary tract symptoms.
Anticholinergic agents
Anticholinergic agents block muscarinic receptors within the detrusor muscle, resulting in relaxation. They are used in the treatment of overactive bladder for symptoms of urinary urgency, frequency, and urge incontinence.
Anticholinergics were historically contraindicated in men with BPH because of concern about urinary retention. However, in men with a postvoid residual volume less than 200 mL, anticholinergics do not increase the risk of urinary retention.46 Further, greater symptom improvement has been demonstrated with the addition of anticholinergics to alpha-blocker therapy for men with BPH, irritative lower urinary tract symptoms, and a low postvoid residual volume.47
Beta-3 agonists
Anticholinergic side effects often limit the use of anticholinergic agents. An alternative in such instances is the beta-3 agonist mirabegron. By activating beta-3 adrenergic receptors in the bladder wall, mirabegron promotes detrusor relaxation and inhibits detrusor overactivity.48 Mirabegron does not have anticholinergic side effects and is generally well tolerated, though poorly controlled hypertension is a contraindication to its use.
Phosphodiesterase-5 inhibitors
Phosphodiesterase-5 (PDE5) inhibitors are a mainstay in the treatment of erectile dysfunction. These agents act within penile corporal smooth muscle cells and antagonize PDE5, resulting in cyclic guanosine monophosphate accumulation and smooth muscle relaxation. PDE5 is also found within the prostate and its inhibition is believed to reduce prostatic smooth muscle tone. Randomized studies have demonstrated significant improvement in lower urinary tract symptoms with PDE5 inhibitors, with an average 2-point IPSS improvement on a PDE5 inhibitor compared with placebo.49
Tadalafil is the only drug of this class approved by the FDA for the treatment of lower urinary tract symptoms, though other agents have demonstrated similar efficacy.
Dual therapy with a PDE5 inhibitor and an alpha-blocker has greater efficacy than either monotherapy alone; however, caution must be exercised as these agents are titrated to avoid symptomatic hypotension. Lower urinary tract symptoms and sexual dysfunction often coexist; PDE5 inhibitors are appropriate in the management of such cases.
SURGERY FOR BPH
Even with effective medical therapy, the disease will progress in some men. In the MTOPS trial,37 the 4-year incidence of disease progression was 10% for men on alpha-blocker or 5-alpha reductase inhibitor monotherapy and 5% for men on combination therapy; from 1% to 3% of those in the various treatment groups needed surgery. With this in mind, patients whose symptoms do not improve with medical therapy, whose symptoms progress, or who simply are interested in surgery should be referred for urologic evaluation.
A number of effective surgical therapies are available for men with BPH (Table 5), providing excellent 1-year outcomes including a mean 70% reduction in IPSS and a mean 12 mL/sec improvement in peak urinary flow.50 Given the efficacy of surgical therapy, men who do not improve with medical therapy who demonstrate any of the findings outlined in Table 1 warrant urologic evaluation.
Acknowledgments: We would like to thank Mary Ellen Amos, PharmD, and Kara Sink, BS, RPh, for their assistance in obtaining the suggested wholesale pricing information included in Table 2.
- Wei JT, Miner MM, Steers WD, et al; BPH Registry Steering Committee. Benign prostatic hyperplasia evaluation and management by urologists and primary care physicians: practice patterns from the observational BPH registry. J Urol 2011; 186:971–976.
- McNeal J. Pathology of benign prostatic hyperplasia. insight into etiology. Urol Clin North Am 1990; 17:477–486.
- Roehrborn CG, Schwinn DA. Alpha1-adrenergic receptors and their inhibitors in lower urinary tract symptoms and benign prostatic hyperplasia. J Urol 2004; 171:1029–1035.
- Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol 1984; 132:474–479.
- Platz EA, Smit E, Curhan GC, Nyberg LM, Giovannucci E. Prevalence of and racial/ethnic variation in lower urinary tract symptoms and noncancer prostate surgery in US men. Urology 2002; 59:877–883.
- Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol 2008; 179(suppl):S75–S80.
- Scheen AJ, Paquot N. Metabolic effects of SGLT-2 inhibitors beyond increased glucosuria: a review of the clinical evidence. Diabetes Metab 2014; 40(suppl 1):S4–S11.
- Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 1992; 148:1549–1564.
- Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol 1995; 154:1770–1774.
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011; 185:1793–1803.
- Roehrborn CG, McConnell J, Bonilla J, et al. Serum prostate specific antigen is a strong predictor of future prostate growth in men with benign prostatic hyperplasia. PROSCAR long-term efficacy and safety study. J Urol 2000; 163:13–20.
- Roehrborn CG, McConnell JD, Lieber M, et al. Serum prostate-specific antigen concentration is a powerful predictor of acute urinary retention and need for surgery in men with clinical benign prostatic hyperplasia. PLESS Study Group. Urology 1999; 53:473–480.
- Wasson JH, Reda DJ, Bruskewitz RC, Elinson J, Keller AM, Henderson WG. A comparison of transurethral surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. The Veterans Affairs Cooperative Study Group on Transurethral Resection of the Prostate. N Engl J Med 1995; 332:75–79.
- Jensen KM, Bruskewitz RC, Iversen P, Madsen PO. Spontaneous uroflowmetry in prostatism. Urology 1984; 24:403–409.
- Abrams PH, Griffiths DJ. The assessment of prostatic obstruction from urodynamic measurements and from residual urine. Br J Urol 1979; 51:129–134.
- Lim CS, Abrams P. The Abrams-Griffiths nomogram. World J Urol 1995; 13:34–39.
- Abrams P, Chapple C, Khoury S, Roehrborn C, de la Rosette J; International Consultation on New Developments in Prostate Cancer and Prostate Diseases. Evaluation and treatment of lower urinary tract symptoms in older men. J Urol 2013; 189(suppl 1):S93–S101.
- Flanigan RC, Reda DJ, Wasson JH, Anderson RJ, Abdellatif M, Bruskewitz RC. 5-year outcome of surgical resection and watchful waiting for men with moderately symptomatic benign prostatic hyperplasia: a Department of Veterans Affairs cooperative study. J Urol 1998; 160:12–17.
- Djavan B, Fong YK, Harik M, et al. Longitudinal study of men with mild symptoms of bladder outlet obstruction treated with watchful waiting for four years. Urology 2004; 64:1144–1148.
- Roberts RO, Jacobsen SJ, Jacobson DJ, Rhodes T, Girman CJ, Lieber MM. Longitudinal changes in peak urinary flow rates in a community based cohort. J Urol 2000; 163:107–113.
- Jacobsen SJ, Girman CJ, Guess HA, Rhodes T, Oesterling JE, Lieber MM. Natural history of prostatism: longitudinal changes in voiding symptoms in community dwelling men. J Urol 1996; 155:595–600.
- Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Treatment for benign prostatic hyperplasia among community dwelling men: the Olmsted County study of urinary symptoms and health status. J Urol 1999; 162:1301–1306.
- Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol 1997; 158:481–487.
- Kobayashi S, Tang R, Shapiro E, Lepor H. Characterization and localization of prostatic alpha 1 adrenoceptors using radioligand receptor binding on slide-mounted tissue section. J Urol 1993; 150:2002–2006.
- Kirby RS, Pool JL. Alpha adrenoceptor blockade in the treatment of benign prostatic hyperplasia: past, present and future. Br J Urol 1997; 80:521–532.
- Kirby RS, Pool JL. Alpha adrenoceptor blockade in the treatment of benign prostatic hyperplasia: past, present and future. Br J Urol 1997; 80:521–532.
- Milani S, Djavan B. Lower urinary tract symptoms suggestive of benign prostatic hyperplasia: latest update on alpha-adrenoceptor antagonists. BJU Int 2005; 95(suppl 4):29–36.
- Lepor H, Auerbach S, Puras-Baez A, et al. A randomized, placebo-controlled multicenter study of the efficacy and safety of terazosin in the treatment of benign prostatic hyperplasia. J Urol 1992; 148:1467–1474.
- Roehrborn CG, Oesterling JE, Auerbach S, et al. The Hytrin Community Assessment Trial study: a one-year study of terazosin versus placebo in the treatment of men with symptomatic benign prostatic hyperplasia. HYCAT Investigator Group. Urology 1996; 47:159–168.
- Gillenwater JY, Conn RL, Chrysant SG, et al. Doxazosin for the treatment of benign prostatic hyperplasia in patients with mild to moderate essential hypertension: a double-blind, placebo-controlled, dose-response multicenter study. J Urol 1995; 154:110–115.
- Chapple CR, Carter P, Christmas TJ, et al. A three month double-blind study of doxazosin as treatment for benign prostatic bladder outlet obstruction. Br J Urol 1994; 74:50–56.
- Buzelin JM, Roth S, Geffriaud-Ricouard C, Delauche-Cavallier MC. Efficacy and safety of sustained-release alfuzosin 5 mg in patients with benign prostatic hyperplasia. ALGEBI Study Group. Eur Urol 1997; 31:190–198.
- van Kerrebroeck P, Jardin A, Laval KU, van Cangh P. Efficacy and safety of a new prolonged release formulation of alfuzosin 10 mg once daily versus alfuzosin 2.5 mg thrice daily and placebo in patients with symptomatic benign prostatic hyperplasia. ALFORTI Study Group. Eur Urol 2000; 37:306–313.
- Narayan P, Tewari A. A second phase III multicenter placebo controlled study of 2 dosages of modified release tamsulosin in patients with symptoms of benign prostatic hyperplasia. United States 93-01 Study Group. J Urol 1998; 160:1701–1706.
- Lepor H. Phase III multicenter placebo-controlled study of tamsulosin in benign prostatic hyperplasia. Tamsulosin Investigator Group. Urology 1998; 51:892–900.
- Ding H, Du W, Hou ZZ, Wang HZ, Wang ZP. Silodosin is effective for treatment of LUTS in men with BPH: a systematic review. Asian J Androl 2013; 15:121–128.
- McConnell JD, Roehrborn CG, Bautista OM, et al; Medical Therapy of Prostatic Symptoms (MTOPS) Research Group. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003; 349:2387–2398.
- Jardin A, Bensadoun H, Delauche-Cavallier MC, Attali P. Alfuzosin for treatment of benign prostatic hypertrophy. The BPH-ALF Group. Lancet 1991; 337:1457–1461.
- Marks LS, Gittelman MC, Hill LA, Volinn W, Hoel G. Rapid efficacy of the highly selective alpha1A-adrenoceptor antagonist silodosin in men with signs and symptoms of benign prostatic hyperplasia: pooled results of 2 phase 3 studies. J Urol 2009; 181:2634–2640.
- Chang DF, Campbell JR. Intraoperative floppy iris syndrome associated with tamsulosin. J Cataract Refract Surg 2005; 31:664–673.
- Gormley GJ, Stoner E, Bruskewitz RC, et al. The effect of finasteride in men with benign prostatic hyperplasia. The Finasteride Study Group. N Engl J Med 1992; 327:1185–1191.
- Roehrborn CG, Boyle P, Nickel JC, Hoefner K, Andriole G; ARIA3001 ARIA3002 and ARIA3003 Study Investigators. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology 2002; 60:434–441.
- Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab 2004; 89:2179–2184.
- Kaplan SA, Lee JY, Meehan AG, Kusek JW; MTOPS Research Group. Long-term treatment with finasteride improves clinical progression of benign prostatic hyperplasia in men with an enlarged versus a smaller prostate: data from the MTOPS trial. J Urol 2011; 185:1369–1373.
- Roehrborn CG, Siami P, Barkin J, et al; CombAT Study Group. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol 2010; 57:123–131.
- Abrams P, Kaplan S, De Koning Gans HJ, Millard R. Safety and tolerability of tolterodine for the treatment of overactive bladder in men with bladder outlet obstruction. J Urol 2006; 175:999–1004.
- Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA 2006; 296:2319–2328.
- Suarez O, Osborn D, Kaufman M, Reynolds WS, Dmochowski R. Mirabegron for male lower urinary tract symptoms. Curr Urol Rep 2013; 14:580–584.
- Oelke M, Giuliano F, Mirone V, Xu L, Cox D, Viktrup L. Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo-controlled clinical trial. Eur Urol 2012; 61:917–925.
- Welliver C, McVary KT. Minimally invasive and endoscopic management of benign prostatic hyperplasia. In: Wein AJ, Kavoussi LR, Partin AW, Peters CA, eds. Campbell-Walsh Urology. 11th ed. Philadelphia, PA: Elsevier; 2016:2504–2534.
Primary care physicians are uniquely positioned to screen for benign prostatic hyperplasia (BPH) and lower urinary tract symptoms, to perform the initial diagnostic workup, and to start medical therapy in uncomplicated cases. Effective medical therapy is available but underutilized in the primary care setting.1
This overview covers how to identify and evaluate patients with lower urinary tract symptoms, initiate therapy, and identify factors warranting timely urology referral.
TWO MECHANISMS: STATIC, DYNAMIC
BPH is a histologic diagnosis of proliferation of smooth muscle, epithelium, and stromal cells within the transition zone of the prostate,2 which surrounds the proximal urethra.
Symptoms arise through two mechanisms: static, in which the hyperplastic prostatic tissue compresses the urethra (Figure 1); and dynamic, with increased adrenergic nervous system and prostatic smooth muscle tone (Figure 2).3 Both mechanisms increase resistance to urinary flow at the level of the bladder outlet.
As an adaptive change to overcome outlet resistance and maintain urinary flow, the detrusor muscles undergo hypertrophy. However, over time the bladder may develop diminished compliance and increased detrusor activity, causing symptoms such as urinary frequency and urgency. Chronic bladder outlet obstruction can lead to bladder decompensation and detrusor underactivity, manifesting as incomplete emptying, urinary hesitancy, intermittency (starting and stopping while voiding), a weakened urinary stream, and urinary retention.
MOST MEN EVENTUALLY DEVELOP BPH
Autopsy studies have shown that BPH increases in prevalence with age beginning around age 30 and reaching a peak prevalence of 88% in men in their 80s.4 This trend parallels those of the incidence and severity of lower urinary tract symptoms.5
In the year 2000 alone, BPH was responsible for 4.5 million physician visits at an estimated direct cost of $1.1 billion, not including the cost of pharmacotherapy.6
OFFICE WORKUP
BPH can cause lower urinary tract symptoms that fall into two categories: storage and emptying. Storage symptoms include urinary frequency, urgency, and nocturia, whereas emptying symptoms include weak stream, hesitancy, intermittency, incomplete emptying, straining, and postvoid dribbling.
History and differential diagnosis
Assessment begins with characterizing the patient’s symptoms and determining those that are most bothersome. Because BPH is just one of many possible causes of lower urinary tract symptoms, a detailed medical history is necessary to evaluate for other conditions that may cause lower urinary tract dysfunction or complicate its treatment.
Obstructive urinary symptoms can arise from BPH or from other conditions, including urethral stricture disease and neurogenic voiding dysfunction.
Irritative voiding symptoms such as urinary urgency and frequency can result from detrusor overactivity secondary to BPH, but can also be caused by neurologic disease, malignancy, initiation of diuretic therapy, high fluid intake, or consumption of bladder irritants such as caffeine, alcohol, and spicy foods.
Urinary frequency is sometimes a presenting symptom of undiagnosed or poorly controlled diabetes mellitus resulting from glucosuria and polyuria. Iatrogenic causes of polyuria include the new hypoglycemic agents canagliflozin and dapagliflozin, which block renal glucose reabsorption, improving glycemic control by inducing urinary
glucose loss.7
Nocturia has many possible nonurologic causes including heart failure (in which excess extravascular fluid shifts to the intravascular space when the patient lies down, resulting in polyuria), obstructive sleep apnea, and behavioral factors such as high evening fluid intake. In these cases, patients usually have nocturnal polyuria (greater than one-third of 24-hour urine output at night) rather than only nocturia (waking at night to void). A fluid diary is a simple tool that can differentiate these two conditions.
Hematuria can develop in patients with BPH with bleeding from congested prostatic or bladder neck vessels; however, hematuria may indicate an underlying malignancy or urolithiasis, for which a urologic workup is indicated.
The broad differential diagnosis for the different lower urinary tract symptoms highlights the importance of obtaining a thorough history.
Physical examination
A general examination should include the following:
Body mass index. Obese patients are at risk of obstructive sleep apnea, which can cause nocturnal polyuria.
Gait. Abnormal gait may suggest a neurologic condition such as Parkinson disease or stroke that can also affect lower urinary tract function.
Lower abdomen. A palpable bladder suggests urinary retention.
External genitalia. Penile causes of urinary obstruction include urethral meatal stenosis or a palpable urethral mass.
Digital rectal examination can reveal benign prostatic enlargement or nodules or firmness, which suggest malignancy and warrant urologic referral.
Neurologic examination, including evaluation of anal sphincter tone and lower extremity sensorimotor function.
Feet. Bilateral lower-extremity edema may be due to heart failure or venous insufficiency.
The International Prostate Symptom Score
All men with lower urinary tract symptoms should complete the International Prostate Symptom Score (IPSS) survey, consisting of seven questions about urinary symptoms plus one about quality of life.8 Specifically, it asks the patient, “Over the past month, how often have you…”
- Had a sensation of not emptying your bladder completely after you finish urinating?
- Had to urinate again less than 2 hours after you finished urinating?
- Found you stopped and started again several times when you urinated?
- Found it difficult to postpone urination?
- Had a weak urinary stream?
- Had to push or strain to begin urination?
Each question above is scored as 0 (not at all), 1 (less than 1 time in 5), 2 (less than half the time), 3 (about half the time), 4 (more than half the time, or 5 (almost always).
- Over the past month, how many times did you most typically get up to urinate from the time you went to bed until the time you got up in the morning?
This question is scored from 0 (none) to 5 (5 times or more).
- If you were to spend the rest of your life with your urinary condition the way it is now, how would you feel about that?
This question is scored as 0 (delighted), 1 (pleased), 2 (mostly satisfied), 3 (mixed: equally satisfied and dissatisfied), 4 (mostly dissatisfied), 5 (unhappy), or 6 (terrible).
A total score of 1 to 7 is categorized as mild, 8 to 19 moderate, and 20 to 35 severe.
The questionnaire can also be used to evaluate for disease progression and response to treatment over time. A change of 3 points is clinically significant, as patients are unable to discern a difference below this threshold.9
Urinalysis
Urinalysis is recommended to assess for urinary tract infection, hematuria, proteinuria, or glucosuria.
Fluid diary
A fluid diary is useful for patients complaining of frequency or nocturia and can help quantify the volume of fluid intake, frequency of urination, and volumes voided. The patient should complete the diary over a 24-hour period, recording the time and volume of fluid intake and each void. This aids in diagnosing polyuria (> 3 L of urine output per 24 hours), nocturnal polyuria, and behavioral causes of symptoms, including excessive total fluid intake or high evening fluid intake contributing to nocturia.
Serum creatinine not recommended
Measuring serum creatinine is not recommended in the initial BPH workup, as men with lower urinary tract symptoms are not at higher risk of renal failure than those without these symptoms.10
Prostate-specific antigen
Prostate-specific antigen (PSA) is a glycoprotein primarily produced by prostatic luminal epithelial cells. It is most commonly discussed in the setting of prostate cancer screening, but its utility extends to guiding the management of BPH.
PSA levels correlate with prostate volume and subsequent growth.11 In addition, the risks of developing acute urinary retention or needing surgical intervention rise with increasing PSA.12 Among men in the Proscar Long-Term Efficacy and Safety Study, the risk of acute urinary retention or BPH-related surgery after 4 years in the watchful-waiting arm was 7.8% in men with a PSA of 1.3 ng/dL or less, compared with 19.9% in men with a PSA greater than 3.2 ng/dL.11 Therefore, men with BPH and an elevated PSA are at higher risk with watchful waiting and may be better served with medical therapy.
In addition, American Urological Association guidelines recommend measuring serum PSA levels in men with a life expectancy greater than 10 years in whom the diagnosis of prostate cancer would alter management.10
Urologic referral
If the initial evaluation reveals hematuria, recurrent urinary tract infection, a palpable bladder, abnormal findings on digital rectal examination suggesting prostate cancer, or a history of or risk factors for urethral stricture or neurologic disease, the patient should be referred to a urologist for further evaluation (Table 1).10 Other patients who should undergo urologic evaluation are those with persistent bothersome symptoms after basic management and those who desire referral.
Adjunctive tests
Patients referred for urologic evaluation may require additional tests for diagnosis and to guide management.
Postvoid residual volume is easily measured with either abdominal ultrasonography or catheterization and is often included in the urologic evaluation of BPH. Patients vary considerably in their residual volume, which correlates poorly with BPH, symptom severity, or surgical success. However, those with a residual volume of more than 100 mL have a slightly higher rate of failure with watchful waiting.13 Postvoid residual volume is not routinely monitored in patients with a low residual volume unless there is a significant change in urinary symptoms. Conversely, patients with a volume greater than 200 mL should be monitored closely for worsening urinary retention, especially if considering anticholinergic therapy.
There is no absolute threshold postvoid residual volume above which therapy is mandatory. Rather, the decision to intervene is based on symptom severity and whether sequelae of urinary retention (eg, incontinence, urinary tract infection, hematuria, hydronephrosis, renal dysfunction) are present.
Uroflowmetry is a noninvasive test measuring the urinary flow rate during voiding and is recommended during specialist evaluation of men with lower urinary tract symptoms and suspected BPH.10 Though a diminished urinary flow rate may be detected in men with bladder outlet obstruction from BPH, it cannot differentiate obstruction from detrusor underactivity, both of which may result in reduced urinary flow. Urodynamic studies can help differentiate between these two mechanisms of lower urinary tract symptoms. Uroflowmetry may be useful in selecting surgical candidates, as patients with a maximum urinary flow rate of 15 mL/second or greater have been shown to have lower rates of surgical success.14
Urodynamic studies. If the diagnosis of bladder outlet obstruction remains in doubt, urodynamic studies can differentiate obstruction from detrusor underactivity. Urodynamic studies allow simultaneous measurement of urinary flow and detrusor pressure, differentiating between obstruction (manifesting as diminished urinary flow with normal or elevated detrusor pressure) and detrusor underactivity (diminished urinary flow with diminished detrusor pressure). Nomograms15 and the easily calculated bladder outlet obstruction index16 are simple tools used to differentiate these two causes of diminished urinary flow.
Cystourethroscopy is not recommended for routine evaluation of BPH. Indications for cystourethroscopy include hematuria and the presence of a risk factor for urethral stricture disease such as urethritis, prior urethral instrumentation, or perineal trauma. Cystourethroscopy can also aid in surgical planning when intervention is considered.
An algorithm for diagnostic workup and management of BPH and lower urinary tract symptoms is shown in Figure 3.17
MANAGEMENT STRATEGIES FOR BPH
While BPH is rarely life-threatening, it can significantly detract from a patient’s quality of life. The goal of treatment is not only to alleviate bothersome symptoms, but also to prevent disease progression and disease-related complications.
BPH tends to progress
Understanding the natural history of BPH is imperative to appropriately counsel patients on management options, which include watchful waiting, behavioral modification, pharmacologic therapy, and surgery.
In a randomized trial,18 men with moderately symptomatic BPH underwent either surgery or, in the control group, watchful waiting. At 5 years, the failure rate was 21% with watchful waiting vs 10% with surgery (P < .0004). (Failure was defined as a composite of death, repeated or intractable urinary retention, residual urine volume > 350 mL, development of bladder calculus, new persistent incontinence requiring use of a pad or other incontinence device, symptom score in the severe range [> 24 at 1 visit or score of 21 or higher at two consecutive visits, with 27 being the maximum score], or a doubling of baseline serum creatinine.) In the watchful-waiting group, 36% of the men crossed over to surgery. Men with more bothersome symptoms at enrollment were at higher risk of progressing to surgery.
In a longitudinal study of men with BPH and mild symptoms (IPSS < 8), the risk of progression to moderate or severe symptoms (IPPS ≥ 8) was 31% at 4 years.19
The Olmsted County Study of Urinary Symptoms and Health Status Among Men20 found that the peak urinary flow rate decreased by a mean of 2.1% per year, declining faster in older men who had a lower peak flow at baseline. In this cohort, the IPSS increased by a mean of 0.18 points per year, with a greater increase in older men.21
Though men managed with watchful waiting are at no higher risk of death or renal failure than men managed surgically,17 population-based studies have demonstrated an overall risk of acute urinary retention of 6.8/1,000 person-years with watchful waiting. Older men with a larger prostate, higher symptom score, and lower peak urinary flow rate are at higher risk of acute urinary retention and progression to needing BPH treatment.22,23
There is evidence that patients progressing to needing surgery after an initial period of watchful waiting have worse surgical outcomes than men managed surgically at the onset.18 This observation must be considered in counseling and selecting patients for watchful waiting. Ideal candidates include patients who have mild or moderate symptoms that cause little bother.10 Patients electing watchful waiting warrant annual follow-up including history, physical examination, and symptom assessment with the IPSS.
Behavioral modification
Behavioral modification should be incorporated into whichever management strategy a patient elects. Such modifications include:
- Reducing total or evening fluid intake for patients with urinary frequency or nocturia.
- Minimizing consumption of bladder irritants such as alcohol and caffeine, which exacerbate storage symptoms.
- Smoking cessation counseling.
- For patients with lower extremity edema who complain of nocturia, using compression stockings or elevating their legs in the afternoon to mobilize lower extremity edema and promote diuresis before going to sleep. If these measures fail, initiating or increasing the dose of a diuretic should be considered. Patients on diuretic therapy with nocturnal lower urinary tract symptoms should be instructed to take diuretics in the morning and early afternoon to avoid diuresis just before bed.
MEDICAL MANAGEMENT
Drugs for BPH include alpha-adrenergic blockers, 5-alpha reductase inhibitors, anticholinergics, beta-3 agonists, and phosphodiesterase-5 inhibitors. Costs of selected agents in these classes are listed in Table 2.
Alpha-adrenergic receptor blockers
Alpha-adrenergic receptors are found throughout the body and modulate smooth muscle tone.24 The alpha-1a receptor is the predominant subtype found in the bladder neck and prostate25 (Figure 2) and is a target of therapy. By antagonizing the alpha-1a receptor, alpha-blockers relax the smooth muscle in the prostate and bladder neck, reduce bladder outlet resistance, and improve urinary flow.26
In clinical trials in BPH, alpha-blockers improved the symptom score by 30% to 45% and increased the peak urinary flow rate by 15% to 30% from baseline values.27 These agents have a rapid onset (within a few days) and result in significant symptom improvement. They are all about the same in efficacy (Table 3),28–36 with no strong evidence that any one of them is superior to another; thus, decisions about which agent to use must consider differences in receptor subtype specificity, adverse-effect profile, and tolerability.
In the Medical Therapy of Prostatic Symptoms (MTOPS) trial,37 men randomized to the alpha-blocker doxazosin had a 39% lower risk of BPH progression than with placebo, largely due to symptom score reduction. However, doxazosin failed to reduce the risk of progressing to acute urinary retention or surgical intervention. Though rapidly effective in reducing symptoms, alpha-blocker monotherapy may not be the best option in men at higher risk of BPH progression, as discussed below.
Before starting this therapy, patients must be counseled about common side effects such as dizziness, fatigue, peripheral edema, orthostatic hypotension, and ejaculatory dysfunction. The incidence of adverse effects varies among agents (Table 4).28–30,34,35,38,39
To maximize efficacy of alpha-blocker therapy, it is imperative to understand dosing variations among agents.
Alpha-blockers are classified as uroselective or non-uroselective based on alpha-1a receptor subtype specificity. The non-uroselective alpha-blockers doxazosin and terazosin need to be titrated because the higher the dose the greater the efficacy, but also the greater the blood pressure-lowering effect and other side effects.25 Though non-uroselective, alfuzosin does not affect blood pressure and does not require dose titration. Similarly, the uroselective alpha-blockers tamsulosin and silodosin can be initiated at a therapeutic dose.
Terazosin, a non-uroselective agent, can lower blood pressure and often causes dizziness. It should be started at 2 mg and titrated to side effects, efficacy, or maximum therapeutic dose (10 mg daily).28
Doxazosin has a high, dose-related incidence of dizziness (up to 20%) and must be titrated, starting at 1 mg to a maximum 8 mg.30
Alfuzosin, tamsulosin, and silodosin do not require titration and can be initiated at the therapeutic doses listed in Table 3. Of note, obese patients often require 0.8 mg tamsulosin for maximum efficacy due to a higher volume of distribution.
Before initiating an alpha-blocker, a physician must determine whether a patient plans to undergo cataract surgery, as the use of alpha-blockers is associated with intraoperative floppy iris syndrome. This condition is marked by poor intraoperative pupil dilation, increasing the risk of surgical complications.40 It is unclear whether discontinuing alpha-blockers before cataract surgery reduces the risk of intraoperative floppy iris syndrome. As such, alpha-blocker therapy should be delayed in patients planning to undergo cataract surgery.
5-Alpha reductase inhibitors
Prostate growth is androgen-dependent and mediated predominantly by dihydrotestosterone, which is generated from testosterone by the action of 5-alpha reductase. There are two 5-alpha reductase isoenzymes: type 1, expressed in the liver and skin, and type 2, expressed primarily in the prostate.
There are also two 5-alpha reductase inhibitors: dutasteride and finasteride. Dutasteride inhibits both isoenzymes, while finasteride is selective for type 2. By inhibiting both isoenzymes, dutasteride reduces the serum dihydrotestosterone concentration more than finasteride does (by 95% vs 70%), and also reduces the intraprostatic dihydrotestosterone concentration more (by 94% vs 80%).41–43 Both agents induce apoptosis of prostatic stroma, with a resultant 20% to 25% mean reduction in prostate volume.41,42
Finasteride and dutasteride are believed to mitigate the static obstructive component of BPH, with similar improvements in urinary flow rate (1.6–2.2 mL/sec) and symptom score (–2.7 to – 4.5 points) in men with an enlarged prostate.41,42 Indeed, data from the MTOPS trial showed that men with a prostate volume of 30 grams or greater or a PSA level of 1.5 ng/mL or greater are most likely to benefit from 5-alpha reductase inhibitors.37 Maximum symptomatic improvement is seen after 3 to 6 months of 5-alpha reductase inhibitor therapy.
In addition to improving urinary flow and lower urinary tract symptoms, finasteride has been shown to reduce the risk of disease progression in men with prostates greater than 30 grams.44 Compared with placebo, these drugs significantly reduce the risk of developing acute urinary retention or requiring BPH-related surgery, a benefit not seen with alpha-blockers.37 To estimate prostate volume, most practitioners rely on digital rectal examination. Though less precise than transrectal ultrasonography, digital rectal examination can identify men with significant prostatic enlargement likely to benefit from this therapy.
Before starting 5-alpha reductase inhibitor therapy, patients should be counseled about common adverse effects such as erectile dysfunction (occurring in 5%–8%), decreased libido (5%), ejaculatory dysfunction (1%–5%), and gynecomastia (1%).
Combination therapy
The MTOPS trial37 randomized patients to receive doxazosin, finasteride, both, or placebo. The combination of doxazosin (an alpha-blocker) and finasteride (a 5-alpha reductase inhibitor) reduced the risk of disease progression to a greater extent than doxazosin or finasteride alone. It also reduced the IPSS more and increased the peak urinary flow rate more. Similar results have been seen with the combination of dutasteride and tamsulosin.45
Given its superior efficacy and benefits in preventing disease progression, combination therapy should be considered for men with an enlarged prostate and moderate to severe lower urinary tract symptoms.
Anticholinergic agents
Anticholinergic agents block muscarinic receptors within the detrusor muscle, resulting in relaxation. They are used in the treatment of overactive bladder for symptoms of urinary urgency, frequency, and urge incontinence.
Anticholinergics were historically contraindicated in men with BPH because of concern about urinary retention. However, in men with a postvoid residual volume less than 200 mL, anticholinergics do not increase the risk of urinary retention.46 Further, greater symptom improvement has been demonstrated with the addition of anticholinergics to alpha-blocker therapy for men with BPH, irritative lower urinary tract symptoms, and a low postvoid residual volume.47
Beta-3 agonists
Anticholinergic side effects often limit the use of anticholinergic agents. An alternative in such instances is the beta-3 agonist mirabegron. By activating beta-3 adrenergic receptors in the bladder wall, mirabegron promotes detrusor relaxation and inhibits detrusor overactivity.48 Mirabegron does not have anticholinergic side effects and is generally well tolerated, though poorly controlled hypertension is a contraindication to its use.
Phosphodiesterase-5 inhibitors
Phosphodiesterase-5 (PDE5) inhibitors are a mainstay in the treatment of erectile dysfunction. These agents act within penile corporal smooth muscle cells and antagonize PDE5, resulting in cyclic guanosine monophosphate accumulation and smooth muscle relaxation. PDE5 is also found within the prostate and its inhibition is believed to reduce prostatic smooth muscle tone. Randomized studies have demonstrated significant improvement in lower urinary tract symptoms with PDE5 inhibitors, with an average 2-point IPSS improvement on a PDE5 inhibitor compared with placebo.49
Tadalafil is the only drug of this class approved by the FDA for the treatment of lower urinary tract symptoms, though other agents have demonstrated similar efficacy.
Dual therapy with a PDE5 inhibitor and an alpha-blocker has greater efficacy than either monotherapy alone; however, caution must be exercised as these agents are titrated to avoid symptomatic hypotension. Lower urinary tract symptoms and sexual dysfunction often coexist; PDE5 inhibitors are appropriate in the management of such cases.
SURGERY FOR BPH
Even with effective medical therapy, the disease will progress in some men. In the MTOPS trial,37 the 4-year incidence of disease progression was 10% for men on alpha-blocker or 5-alpha reductase inhibitor monotherapy and 5% for men on combination therapy; from 1% to 3% of those in the various treatment groups needed surgery. With this in mind, patients whose symptoms do not improve with medical therapy, whose symptoms progress, or who simply are interested in surgery should be referred for urologic evaluation.
A number of effective surgical therapies are available for men with BPH (Table 5), providing excellent 1-year outcomes including a mean 70% reduction in IPSS and a mean 12 mL/sec improvement in peak urinary flow.50 Given the efficacy of surgical therapy, men who do not improve with medical therapy who demonstrate any of the findings outlined in Table 1 warrant urologic evaluation.
Acknowledgments: We would like to thank Mary Ellen Amos, PharmD, and Kara Sink, BS, RPh, for their assistance in obtaining the suggested wholesale pricing information included in Table 2.
Primary care physicians are uniquely positioned to screen for benign prostatic hyperplasia (BPH) and lower urinary tract symptoms, to perform the initial diagnostic workup, and to start medical therapy in uncomplicated cases. Effective medical therapy is available but underutilized in the primary care setting.1
This overview covers how to identify and evaluate patients with lower urinary tract symptoms, initiate therapy, and identify factors warranting timely urology referral.
TWO MECHANISMS: STATIC, DYNAMIC
BPH is a histologic diagnosis of proliferation of smooth muscle, epithelium, and stromal cells within the transition zone of the prostate,2 which surrounds the proximal urethra.
Symptoms arise through two mechanisms: static, in which the hyperplastic prostatic tissue compresses the urethra (Figure 1); and dynamic, with increased adrenergic nervous system and prostatic smooth muscle tone (Figure 2).3 Both mechanisms increase resistance to urinary flow at the level of the bladder outlet.
As an adaptive change to overcome outlet resistance and maintain urinary flow, the detrusor muscles undergo hypertrophy. However, over time the bladder may develop diminished compliance and increased detrusor activity, causing symptoms such as urinary frequency and urgency. Chronic bladder outlet obstruction can lead to bladder decompensation and detrusor underactivity, manifesting as incomplete emptying, urinary hesitancy, intermittency (starting and stopping while voiding), a weakened urinary stream, and urinary retention.
MOST MEN EVENTUALLY DEVELOP BPH
Autopsy studies have shown that BPH increases in prevalence with age beginning around age 30 and reaching a peak prevalence of 88% in men in their 80s.4 This trend parallels those of the incidence and severity of lower urinary tract symptoms.5
In the year 2000 alone, BPH was responsible for 4.5 million physician visits at an estimated direct cost of $1.1 billion, not including the cost of pharmacotherapy.6
OFFICE WORKUP
BPH can cause lower urinary tract symptoms that fall into two categories: storage and emptying. Storage symptoms include urinary frequency, urgency, and nocturia, whereas emptying symptoms include weak stream, hesitancy, intermittency, incomplete emptying, straining, and postvoid dribbling.
History and differential diagnosis
Assessment begins with characterizing the patient’s symptoms and determining those that are most bothersome. Because BPH is just one of many possible causes of lower urinary tract symptoms, a detailed medical history is necessary to evaluate for other conditions that may cause lower urinary tract dysfunction or complicate its treatment.
Obstructive urinary symptoms can arise from BPH or from other conditions, including urethral stricture disease and neurogenic voiding dysfunction.
Irritative voiding symptoms such as urinary urgency and frequency can result from detrusor overactivity secondary to BPH, but can also be caused by neurologic disease, malignancy, initiation of diuretic therapy, high fluid intake, or consumption of bladder irritants such as caffeine, alcohol, and spicy foods.
Urinary frequency is sometimes a presenting symptom of undiagnosed or poorly controlled diabetes mellitus resulting from glucosuria and polyuria. Iatrogenic causes of polyuria include the new hypoglycemic agents canagliflozin and dapagliflozin, which block renal glucose reabsorption, improving glycemic control by inducing urinary
glucose loss.7
Nocturia has many possible nonurologic causes including heart failure (in which excess extravascular fluid shifts to the intravascular space when the patient lies down, resulting in polyuria), obstructive sleep apnea, and behavioral factors such as high evening fluid intake. In these cases, patients usually have nocturnal polyuria (greater than one-third of 24-hour urine output at night) rather than only nocturia (waking at night to void). A fluid diary is a simple tool that can differentiate these two conditions.
Hematuria can develop in patients with BPH with bleeding from congested prostatic or bladder neck vessels; however, hematuria may indicate an underlying malignancy or urolithiasis, for which a urologic workup is indicated.
The broad differential diagnosis for the different lower urinary tract symptoms highlights the importance of obtaining a thorough history.
Physical examination
A general examination should include the following:
Body mass index. Obese patients are at risk of obstructive sleep apnea, which can cause nocturnal polyuria.
Gait. Abnormal gait may suggest a neurologic condition such as Parkinson disease or stroke that can also affect lower urinary tract function.
Lower abdomen. A palpable bladder suggests urinary retention.
External genitalia. Penile causes of urinary obstruction include urethral meatal stenosis or a palpable urethral mass.
Digital rectal examination can reveal benign prostatic enlargement or nodules or firmness, which suggest malignancy and warrant urologic referral.
Neurologic examination, including evaluation of anal sphincter tone and lower extremity sensorimotor function.
Feet. Bilateral lower-extremity edema may be due to heart failure or venous insufficiency.
The International Prostate Symptom Score
All men with lower urinary tract symptoms should complete the International Prostate Symptom Score (IPSS) survey, consisting of seven questions about urinary symptoms plus one about quality of life.8 Specifically, it asks the patient, “Over the past month, how often have you…”
- Had a sensation of not emptying your bladder completely after you finish urinating?
- Had to urinate again less than 2 hours after you finished urinating?
- Found you stopped and started again several times when you urinated?
- Found it difficult to postpone urination?
- Had a weak urinary stream?
- Had to push or strain to begin urination?
Each question above is scored as 0 (not at all), 1 (less than 1 time in 5), 2 (less than half the time), 3 (about half the time), 4 (more than half the time, or 5 (almost always).
- Over the past month, how many times did you most typically get up to urinate from the time you went to bed until the time you got up in the morning?
This question is scored from 0 (none) to 5 (5 times or more).
- If you were to spend the rest of your life with your urinary condition the way it is now, how would you feel about that?
This question is scored as 0 (delighted), 1 (pleased), 2 (mostly satisfied), 3 (mixed: equally satisfied and dissatisfied), 4 (mostly dissatisfied), 5 (unhappy), or 6 (terrible).
A total score of 1 to 7 is categorized as mild, 8 to 19 moderate, and 20 to 35 severe.
The questionnaire can also be used to evaluate for disease progression and response to treatment over time. A change of 3 points is clinically significant, as patients are unable to discern a difference below this threshold.9
Urinalysis
Urinalysis is recommended to assess for urinary tract infection, hematuria, proteinuria, or glucosuria.
Fluid diary
A fluid diary is useful for patients complaining of frequency or nocturia and can help quantify the volume of fluid intake, frequency of urination, and volumes voided. The patient should complete the diary over a 24-hour period, recording the time and volume of fluid intake and each void. This aids in diagnosing polyuria (> 3 L of urine output per 24 hours), nocturnal polyuria, and behavioral causes of symptoms, including excessive total fluid intake or high evening fluid intake contributing to nocturia.
Serum creatinine not recommended
Measuring serum creatinine is not recommended in the initial BPH workup, as men with lower urinary tract symptoms are not at higher risk of renal failure than those without these symptoms.10
Prostate-specific antigen
Prostate-specific antigen (PSA) is a glycoprotein primarily produced by prostatic luminal epithelial cells. It is most commonly discussed in the setting of prostate cancer screening, but its utility extends to guiding the management of BPH.
PSA levels correlate with prostate volume and subsequent growth.11 In addition, the risks of developing acute urinary retention or needing surgical intervention rise with increasing PSA.12 Among men in the Proscar Long-Term Efficacy and Safety Study, the risk of acute urinary retention or BPH-related surgery after 4 years in the watchful-waiting arm was 7.8% in men with a PSA of 1.3 ng/dL or less, compared with 19.9% in men with a PSA greater than 3.2 ng/dL.11 Therefore, men with BPH and an elevated PSA are at higher risk with watchful waiting and may be better served with medical therapy.
In addition, American Urological Association guidelines recommend measuring serum PSA levels in men with a life expectancy greater than 10 years in whom the diagnosis of prostate cancer would alter management.10
Urologic referral
If the initial evaluation reveals hematuria, recurrent urinary tract infection, a palpable bladder, abnormal findings on digital rectal examination suggesting prostate cancer, or a history of or risk factors for urethral stricture or neurologic disease, the patient should be referred to a urologist for further evaluation (Table 1).10 Other patients who should undergo urologic evaluation are those with persistent bothersome symptoms after basic management and those who desire referral.
Adjunctive tests
Patients referred for urologic evaluation may require additional tests for diagnosis and to guide management.
Postvoid residual volume is easily measured with either abdominal ultrasonography or catheterization and is often included in the urologic evaluation of BPH. Patients vary considerably in their residual volume, which correlates poorly with BPH, symptom severity, or surgical success. However, those with a residual volume of more than 100 mL have a slightly higher rate of failure with watchful waiting.13 Postvoid residual volume is not routinely monitored in patients with a low residual volume unless there is a significant change in urinary symptoms. Conversely, patients with a volume greater than 200 mL should be monitored closely for worsening urinary retention, especially if considering anticholinergic therapy.
There is no absolute threshold postvoid residual volume above which therapy is mandatory. Rather, the decision to intervene is based on symptom severity and whether sequelae of urinary retention (eg, incontinence, urinary tract infection, hematuria, hydronephrosis, renal dysfunction) are present.
Uroflowmetry is a noninvasive test measuring the urinary flow rate during voiding and is recommended during specialist evaluation of men with lower urinary tract symptoms and suspected BPH.10 Though a diminished urinary flow rate may be detected in men with bladder outlet obstruction from BPH, it cannot differentiate obstruction from detrusor underactivity, both of which may result in reduced urinary flow. Urodynamic studies can help differentiate between these two mechanisms of lower urinary tract symptoms. Uroflowmetry may be useful in selecting surgical candidates, as patients with a maximum urinary flow rate of 15 mL/second or greater have been shown to have lower rates of surgical success.14
Urodynamic studies. If the diagnosis of bladder outlet obstruction remains in doubt, urodynamic studies can differentiate obstruction from detrusor underactivity. Urodynamic studies allow simultaneous measurement of urinary flow and detrusor pressure, differentiating between obstruction (manifesting as diminished urinary flow with normal or elevated detrusor pressure) and detrusor underactivity (diminished urinary flow with diminished detrusor pressure). Nomograms15 and the easily calculated bladder outlet obstruction index16 are simple tools used to differentiate these two causes of diminished urinary flow.
Cystourethroscopy is not recommended for routine evaluation of BPH. Indications for cystourethroscopy include hematuria and the presence of a risk factor for urethral stricture disease such as urethritis, prior urethral instrumentation, or perineal trauma. Cystourethroscopy can also aid in surgical planning when intervention is considered.
An algorithm for diagnostic workup and management of BPH and lower urinary tract symptoms is shown in Figure 3.17
MANAGEMENT STRATEGIES FOR BPH
While BPH is rarely life-threatening, it can significantly detract from a patient’s quality of life. The goal of treatment is not only to alleviate bothersome symptoms, but also to prevent disease progression and disease-related complications.
BPH tends to progress
Understanding the natural history of BPH is imperative to appropriately counsel patients on management options, which include watchful waiting, behavioral modification, pharmacologic therapy, and surgery.
In a randomized trial,18 men with moderately symptomatic BPH underwent either surgery or, in the control group, watchful waiting. At 5 years, the failure rate was 21% with watchful waiting vs 10% with surgery (P < .0004). (Failure was defined as a composite of death, repeated or intractable urinary retention, residual urine volume > 350 mL, development of bladder calculus, new persistent incontinence requiring use of a pad or other incontinence device, symptom score in the severe range [> 24 at 1 visit or score of 21 or higher at two consecutive visits, with 27 being the maximum score], or a doubling of baseline serum creatinine.) In the watchful-waiting group, 36% of the men crossed over to surgery. Men with more bothersome symptoms at enrollment were at higher risk of progressing to surgery.
In a longitudinal study of men with BPH and mild symptoms (IPSS < 8), the risk of progression to moderate or severe symptoms (IPPS ≥ 8) was 31% at 4 years.19
The Olmsted County Study of Urinary Symptoms and Health Status Among Men20 found that the peak urinary flow rate decreased by a mean of 2.1% per year, declining faster in older men who had a lower peak flow at baseline. In this cohort, the IPSS increased by a mean of 0.18 points per year, with a greater increase in older men.21
Though men managed with watchful waiting are at no higher risk of death or renal failure than men managed surgically,17 population-based studies have demonstrated an overall risk of acute urinary retention of 6.8/1,000 person-years with watchful waiting. Older men with a larger prostate, higher symptom score, and lower peak urinary flow rate are at higher risk of acute urinary retention and progression to needing BPH treatment.22,23
There is evidence that patients progressing to needing surgery after an initial period of watchful waiting have worse surgical outcomes than men managed surgically at the onset.18 This observation must be considered in counseling and selecting patients for watchful waiting. Ideal candidates include patients who have mild or moderate symptoms that cause little bother.10 Patients electing watchful waiting warrant annual follow-up including history, physical examination, and symptom assessment with the IPSS.
Behavioral modification
Behavioral modification should be incorporated into whichever management strategy a patient elects. Such modifications include:
- Reducing total or evening fluid intake for patients with urinary frequency or nocturia.
- Minimizing consumption of bladder irritants such as alcohol and caffeine, which exacerbate storage symptoms.
- Smoking cessation counseling.
- For patients with lower extremity edema who complain of nocturia, using compression stockings or elevating their legs in the afternoon to mobilize lower extremity edema and promote diuresis before going to sleep. If these measures fail, initiating or increasing the dose of a diuretic should be considered. Patients on diuretic therapy with nocturnal lower urinary tract symptoms should be instructed to take diuretics in the morning and early afternoon to avoid diuresis just before bed.
MEDICAL MANAGEMENT
Drugs for BPH include alpha-adrenergic blockers, 5-alpha reductase inhibitors, anticholinergics, beta-3 agonists, and phosphodiesterase-5 inhibitors. Costs of selected agents in these classes are listed in Table 2.
Alpha-adrenergic receptor blockers
Alpha-adrenergic receptors are found throughout the body and modulate smooth muscle tone.24 The alpha-1a receptor is the predominant subtype found in the bladder neck and prostate25 (Figure 2) and is a target of therapy. By antagonizing the alpha-1a receptor, alpha-blockers relax the smooth muscle in the prostate and bladder neck, reduce bladder outlet resistance, and improve urinary flow.26
In clinical trials in BPH, alpha-blockers improved the symptom score by 30% to 45% and increased the peak urinary flow rate by 15% to 30% from baseline values.27 These agents have a rapid onset (within a few days) and result in significant symptom improvement. They are all about the same in efficacy (Table 3),28–36 with no strong evidence that any one of them is superior to another; thus, decisions about which agent to use must consider differences in receptor subtype specificity, adverse-effect profile, and tolerability.
In the Medical Therapy of Prostatic Symptoms (MTOPS) trial,37 men randomized to the alpha-blocker doxazosin had a 39% lower risk of BPH progression than with placebo, largely due to symptom score reduction. However, doxazosin failed to reduce the risk of progressing to acute urinary retention or surgical intervention. Though rapidly effective in reducing symptoms, alpha-blocker monotherapy may not be the best option in men at higher risk of BPH progression, as discussed below.
Before starting this therapy, patients must be counseled about common side effects such as dizziness, fatigue, peripheral edema, orthostatic hypotension, and ejaculatory dysfunction. The incidence of adverse effects varies among agents (Table 4).28–30,34,35,38,39
To maximize efficacy of alpha-blocker therapy, it is imperative to understand dosing variations among agents.
Alpha-blockers are classified as uroselective or non-uroselective based on alpha-1a receptor subtype specificity. The non-uroselective alpha-blockers doxazosin and terazosin need to be titrated because the higher the dose the greater the efficacy, but also the greater the blood pressure-lowering effect and other side effects.25 Though non-uroselective, alfuzosin does not affect blood pressure and does not require dose titration. Similarly, the uroselective alpha-blockers tamsulosin and silodosin can be initiated at a therapeutic dose.
Terazosin, a non-uroselective agent, can lower blood pressure and often causes dizziness. It should be started at 2 mg and titrated to side effects, efficacy, or maximum therapeutic dose (10 mg daily).28
Doxazosin has a high, dose-related incidence of dizziness (up to 20%) and must be titrated, starting at 1 mg to a maximum 8 mg.30
Alfuzosin, tamsulosin, and silodosin do not require titration and can be initiated at the therapeutic doses listed in Table 3. Of note, obese patients often require 0.8 mg tamsulosin for maximum efficacy due to a higher volume of distribution.
Before initiating an alpha-blocker, a physician must determine whether a patient plans to undergo cataract surgery, as the use of alpha-blockers is associated with intraoperative floppy iris syndrome. This condition is marked by poor intraoperative pupil dilation, increasing the risk of surgical complications.40 It is unclear whether discontinuing alpha-blockers before cataract surgery reduces the risk of intraoperative floppy iris syndrome. As such, alpha-blocker therapy should be delayed in patients planning to undergo cataract surgery.
5-Alpha reductase inhibitors
Prostate growth is androgen-dependent and mediated predominantly by dihydrotestosterone, which is generated from testosterone by the action of 5-alpha reductase. There are two 5-alpha reductase isoenzymes: type 1, expressed in the liver and skin, and type 2, expressed primarily in the prostate.
There are also two 5-alpha reductase inhibitors: dutasteride and finasteride. Dutasteride inhibits both isoenzymes, while finasteride is selective for type 2. By inhibiting both isoenzymes, dutasteride reduces the serum dihydrotestosterone concentration more than finasteride does (by 95% vs 70%), and also reduces the intraprostatic dihydrotestosterone concentration more (by 94% vs 80%).41–43 Both agents induce apoptosis of prostatic stroma, with a resultant 20% to 25% mean reduction in prostate volume.41,42
Finasteride and dutasteride are believed to mitigate the static obstructive component of BPH, with similar improvements in urinary flow rate (1.6–2.2 mL/sec) and symptom score (–2.7 to – 4.5 points) in men with an enlarged prostate.41,42 Indeed, data from the MTOPS trial showed that men with a prostate volume of 30 grams or greater or a PSA level of 1.5 ng/mL or greater are most likely to benefit from 5-alpha reductase inhibitors.37 Maximum symptomatic improvement is seen after 3 to 6 months of 5-alpha reductase inhibitor therapy.
In addition to improving urinary flow and lower urinary tract symptoms, finasteride has been shown to reduce the risk of disease progression in men with prostates greater than 30 grams.44 Compared with placebo, these drugs significantly reduce the risk of developing acute urinary retention or requiring BPH-related surgery, a benefit not seen with alpha-blockers.37 To estimate prostate volume, most practitioners rely on digital rectal examination. Though less precise than transrectal ultrasonography, digital rectal examination can identify men with significant prostatic enlargement likely to benefit from this therapy.
Before starting 5-alpha reductase inhibitor therapy, patients should be counseled about common adverse effects such as erectile dysfunction (occurring in 5%–8%), decreased libido (5%), ejaculatory dysfunction (1%–5%), and gynecomastia (1%).
Combination therapy
The MTOPS trial37 randomized patients to receive doxazosin, finasteride, both, or placebo. The combination of doxazosin (an alpha-blocker) and finasteride (a 5-alpha reductase inhibitor) reduced the risk of disease progression to a greater extent than doxazosin or finasteride alone. It also reduced the IPSS more and increased the peak urinary flow rate more. Similar results have been seen with the combination of dutasteride and tamsulosin.45
Given its superior efficacy and benefits in preventing disease progression, combination therapy should be considered for men with an enlarged prostate and moderate to severe lower urinary tract symptoms.
Anticholinergic agents
Anticholinergic agents block muscarinic receptors within the detrusor muscle, resulting in relaxation. They are used in the treatment of overactive bladder for symptoms of urinary urgency, frequency, and urge incontinence.
Anticholinergics were historically contraindicated in men with BPH because of concern about urinary retention. However, in men with a postvoid residual volume less than 200 mL, anticholinergics do not increase the risk of urinary retention.46 Further, greater symptom improvement has been demonstrated with the addition of anticholinergics to alpha-blocker therapy for men with BPH, irritative lower urinary tract symptoms, and a low postvoid residual volume.47
Beta-3 agonists
Anticholinergic side effects often limit the use of anticholinergic agents. An alternative in such instances is the beta-3 agonist mirabegron. By activating beta-3 adrenergic receptors in the bladder wall, mirabegron promotes detrusor relaxation and inhibits detrusor overactivity.48 Mirabegron does not have anticholinergic side effects and is generally well tolerated, though poorly controlled hypertension is a contraindication to its use.
Phosphodiesterase-5 inhibitors
Phosphodiesterase-5 (PDE5) inhibitors are a mainstay in the treatment of erectile dysfunction. These agents act within penile corporal smooth muscle cells and antagonize PDE5, resulting in cyclic guanosine monophosphate accumulation and smooth muscle relaxation. PDE5 is also found within the prostate and its inhibition is believed to reduce prostatic smooth muscle tone. Randomized studies have demonstrated significant improvement in lower urinary tract symptoms with PDE5 inhibitors, with an average 2-point IPSS improvement on a PDE5 inhibitor compared with placebo.49
Tadalafil is the only drug of this class approved by the FDA for the treatment of lower urinary tract symptoms, though other agents have demonstrated similar efficacy.
Dual therapy with a PDE5 inhibitor and an alpha-blocker has greater efficacy than either monotherapy alone; however, caution must be exercised as these agents are titrated to avoid symptomatic hypotension. Lower urinary tract symptoms and sexual dysfunction often coexist; PDE5 inhibitors are appropriate in the management of such cases.
SURGERY FOR BPH
Even with effective medical therapy, the disease will progress in some men. In the MTOPS trial,37 the 4-year incidence of disease progression was 10% for men on alpha-blocker or 5-alpha reductase inhibitor monotherapy and 5% for men on combination therapy; from 1% to 3% of those in the various treatment groups needed surgery. With this in mind, patients whose symptoms do not improve with medical therapy, whose symptoms progress, or who simply are interested in surgery should be referred for urologic evaluation.
A number of effective surgical therapies are available for men with BPH (Table 5), providing excellent 1-year outcomes including a mean 70% reduction in IPSS and a mean 12 mL/sec improvement in peak urinary flow.50 Given the efficacy of surgical therapy, men who do not improve with medical therapy who demonstrate any of the findings outlined in Table 1 warrant urologic evaluation.
Acknowledgments: We would like to thank Mary Ellen Amos, PharmD, and Kara Sink, BS, RPh, for their assistance in obtaining the suggested wholesale pricing information included in Table 2.
- Wei JT, Miner MM, Steers WD, et al; BPH Registry Steering Committee. Benign prostatic hyperplasia evaluation and management by urologists and primary care physicians: practice patterns from the observational BPH registry. J Urol 2011; 186:971–976.
- McNeal J. Pathology of benign prostatic hyperplasia. insight into etiology. Urol Clin North Am 1990; 17:477–486.
- Roehrborn CG, Schwinn DA. Alpha1-adrenergic receptors and their inhibitors in lower urinary tract symptoms and benign prostatic hyperplasia. J Urol 2004; 171:1029–1035.
- Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol 1984; 132:474–479.
- Platz EA, Smit E, Curhan GC, Nyberg LM, Giovannucci E. Prevalence of and racial/ethnic variation in lower urinary tract symptoms and noncancer prostate surgery in US men. Urology 2002; 59:877–883.
- Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol 2008; 179(suppl):S75–S80.
- Scheen AJ, Paquot N. Metabolic effects of SGLT-2 inhibitors beyond increased glucosuria: a review of the clinical evidence. Diabetes Metab 2014; 40(suppl 1):S4–S11.
- Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 1992; 148:1549–1564.
- Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol 1995; 154:1770–1774.
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011; 185:1793–1803.
- Roehrborn CG, McConnell J, Bonilla J, et al. Serum prostate specific antigen is a strong predictor of future prostate growth in men with benign prostatic hyperplasia. PROSCAR long-term efficacy and safety study. J Urol 2000; 163:13–20.
- Roehrborn CG, McConnell JD, Lieber M, et al. Serum prostate-specific antigen concentration is a powerful predictor of acute urinary retention and need for surgery in men with clinical benign prostatic hyperplasia. PLESS Study Group. Urology 1999; 53:473–480.
- Wasson JH, Reda DJ, Bruskewitz RC, Elinson J, Keller AM, Henderson WG. A comparison of transurethral surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. The Veterans Affairs Cooperative Study Group on Transurethral Resection of the Prostate. N Engl J Med 1995; 332:75–79.
- Jensen KM, Bruskewitz RC, Iversen P, Madsen PO. Spontaneous uroflowmetry in prostatism. Urology 1984; 24:403–409.
- Abrams PH, Griffiths DJ. The assessment of prostatic obstruction from urodynamic measurements and from residual urine. Br J Urol 1979; 51:129–134.
- Lim CS, Abrams P. The Abrams-Griffiths nomogram. World J Urol 1995; 13:34–39.
- Abrams P, Chapple C, Khoury S, Roehrborn C, de la Rosette J; International Consultation on New Developments in Prostate Cancer and Prostate Diseases. Evaluation and treatment of lower urinary tract symptoms in older men. J Urol 2013; 189(suppl 1):S93–S101.
- Flanigan RC, Reda DJ, Wasson JH, Anderson RJ, Abdellatif M, Bruskewitz RC. 5-year outcome of surgical resection and watchful waiting for men with moderately symptomatic benign prostatic hyperplasia: a Department of Veterans Affairs cooperative study. J Urol 1998; 160:12–17.
- Djavan B, Fong YK, Harik M, et al. Longitudinal study of men with mild symptoms of bladder outlet obstruction treated with watchful waiting for four years. Urology 2004; 64:1144–1148.
- Roberts RO, Jacobsen SJ, Jacobson DJ, Rhodes T, Girman CJ, Lieber MM. Longitudinal changes in peak urinary flow rates in a community based cohort. J Urol 2000; 163:107–113.
- Jacobsen SJ, Girman CJ, Guess HA, Rhodes T, Oesterling JE, Lieber MM. Natural history of prostatism: longitudinal changes in voiding symptoms in community dwelling men. J Urol 1996; 155:595–600.
- Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Treatment for benign prostatic hyperplasia among community dwelling men: the Olmsted County study of urinary symptoms and health status. J Urol 1999; 162:1301–1306.
- Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol 1997; 158:481–487.
- Kobayashi S, Tang R, Shapiro E, Lepor H. Characterization and localization of prostatic alpha 1 adrenoceptors using radioligand receptor binding on slide-mounted tissue section. J Urol 1993; 150:2002–2006.
- Kirby RS, Pool JL. Alpha adrenoceptor blockade in the treatment of benign prostatic hyperplasia: past, present and future. Br J Urol 1997; 80:521–532.
- Kirby RS, Pool JL. Alpha adrenoceptor blockade in the treatment of benign prostatic hyperplasia: past, present and future. Br J Urol 1997; 80:521–532.
- Milani S, Djavan B. Lower urinary tract symptoms suggestive of benign prostatic hyperplasia: latest update on alpha-adrenoceptor antagonists. BJU Int 2005; 95(suppl 4):29–36.
- Lepor H, Auerbach S, Puras-Baez A, et al. A randomized, placebo-controlled multicenter study of the efficacy and safety of terazosin in the treatment of benign prostatic hyperplasia. J Urol 1992; 148:1467–1474.
- Roehrborn CG, Oesterling JE, Auerbach S, et al. The Hytrin Community Assessment Trial study: a one-year study of terazosin versus placebo in the treatment of men with symptomatic benign prostatic hyperplasia. HYCAT Investigator Group. Urology 1996; 47:159–168.
- Gillenwater JY, Conn RL, Chrysant SG, et al. Doxazosin for the treatment of benign prostatic hyperplasia in patients with mild to moderate essential hypertension: a double-blind, placebo-controlled, dose-response multicenter study. J Urol 1995; 154:110–115.
- Chapple CR, Carter P, Christmas TJ, et al. A three month double-blind study of doxazosin as treatment for benign prostatic bladder outlet obstruction. Br J Urol 1994; 74:50–56.
- Buzelin JM, Roth S, Geffriaud-Ricouard C, Delauche-Cavallier MC. Efficacy and safety of sustained-release alfuzosin 5 mg in patients with benign prostatic hyperplasia. ALGEBI Study Group. Eur Urol 1997; 31:190–198.
- van Kerrebroeck P, Jardin A, Laval KU, van Cangh P. Efficacy and safety of a new prolonged release formulation of alfuzosin 10 mg once daily versus alfuzosin 2.5 mg thrice daily and placebo in patients with symptomatic benign prostatic hyperplasia. ALFORTI Study Group. Eur Urol 2000; 37:306–313.
- Narayan P, Tewari A. A second phase III multicenter placebo controlled study of 2 dosages of modified release tamsulosin in patients with symptoms of benign prostatic hyperplasia. United States 93-01 Study Group. J Urol 1998; 160:1701–1706.
- Lepor H. Phase III multicenter placebo-controlled study of tamsulosin in benign prostatic hyperplasia. Tamsulosin Investigator Group. Urology 1998; 51:892–900.
- Ding H, Du W, Hou ZZ, Wang HZ, Wang ZP. Silodosin is effective for treatment of LUTS in men with BPH: a systematic review. Asian J Androl 2013; 15:121–128.
- McConnell JD, Roehrborn CG, Bautista OM, et al; Medical Therapy of Prostatic Symptoms (MTOPS) Research Group. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003; 349:2387–2398.
- Jardin A, Bensadoun H, Delauche-Cavallier MC, Attali P. Alfuzosin for treatment of benign prostatic hypertrophy. The BPH-ALF Group. Lancet 1991; 337:1457–1461.
- Marks LS, Gittelman MC, Hill LA, Volinn W, Hoel G. Rapid efficacy of the highly selective alpha1A-adrenoceptor antagonist silodosin in men with signs and symptoms of benign prostatic hyperplasia: pooled results of 2 phase 3 studies. J Urol 2009; 181:2634–2640.
- Chang DF, Campbell JR. Intraoperative floppy iris syndrome associated with tamsulosin. J Cataract Refract Surg 2005; 31:664–673.
- Gormley GJ, Stoner E, Bruskewitz RC, et al. The effect of finasteride in men with benign prostatic hyperplasia. The Finasteride Study Group. N Engl J Med 1992; 327:1185–1191.
- Roehrborn CG, Boyle P, Nickel JC, Hoefner K, Andriole G; ARIA3001 ARIA3002 and ARIA3003 Study Investigators. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology 2002; 60:434–441.
- Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab 2004; 89:2179–2184.
- Kaplan SA, Lee JY, Meehan AG, Kusek JW; MTOPS Research Group. Long-term treatment with finasteride improves clinical progression of benign prostatic hyperplasia in men with an enlarged versus a smaller prostate: data from the MTOPS trial. J Urol 2011; 185:1369–1373.
- Roehrborn CG, Siami P, Barkin J, et al; CombAT Study Group. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol 2010; 57:123–131.
- Abrams P, Kaplan S, De Koning Gans HJ, Millard R. Safety and tolerability of tolterodine for the treatment of overactive bladder in men with bladder outlet obstruction. J Urol 2006; 175:999–1004.
- Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA 2006; 296:2319–2328.
- Suarez O, Osborn D, Kaufman M, Reynolds WS, Dmochowski R. Mirabegron for male lower urinary tract symptoms. Curr Urol Rep 2013; 14:580–584.
- Oelke M, Giuliano F, Mirone V, Xu L, Cox D, Viktrup L. Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo-controlled clinical trial. Eur Urol 2012; 61:917–925.
- Welliver C, McVary KT. Minimally invasive and endoscopic management of benign prostatic hyperplasia. In: Wein AJ, Kavoussi LR, Partin AW, Peters CA, eds. Campbell-Walsh Urology. 11th ed. Philadelphia, PA: Elsevier; 2016:2504–2534.
- Wei JT, Miner MM, Steers WD, et al; BPH Registry Steering Committee. Benign prostatic hyperplasia evaluation and management by urologists and primary care physicians: practice patterns from the observational BPH registry. J Urol 2011; 186:971–976.
- McNeal J. Pathology of benign prostatic hyperplasia. insight into etiology. Urol Clin North Am 1990; 17:477–486.
- Roehrborn CG, Schwinn DA. Alpha1-adrenergic receptors and their inhibitors in lower urinary tract symptoms and benign prostatic hyperplasia. J Urol 2004; 171:1029–1035.
- Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol 1984; 132:474–479.
- Platz EA, Smit E, Curhan GC, Nyberg LM, Giovannucci E. Prevalence of and racial/ethnic variation in lower urinary tract symptoms and noncancer prostate surgery in US men. Urology 2002; 59:877–883.
- Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol 2008; 179(suppl):S75–S80.
- Scheen AJ, Paquot N. Metabolic effects of SGLT-2 inhibitors beyond increased glucosuria: a review of the clinical evidence. Diabetes Metab 2014; 40(suppl 1):S4–S11.
- Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol 1992; 148:1549–1564.
- Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol 1995; 154:1770–1774.
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011; 185:1793–1803.
- Roehrborn CG, McConnell J, Bonilla J, et al. Serum prostate specific antigen is a strong predictor of future prostate growth in men with benign prostatic hyperplasia. PROSCAR long-term efficacy and safety study. J Urol 2000; 163:13–20.
- Roehrborn CG, McConnell JD, Lieber M, et al. Serum prostate-specific antigen concentration is a powerful predictor of acute urinary retention and need for surgery in men with clinical benign prostatic hyperplasia. PLESS Study Group. Urology 1999; 53:473–480.
- Wasson JH, Reda DJ, Bruskewitz RC, Elinson J, Keller AM, Henderson WG. A comparison of transurethral surgery with watchful waiting for moderate symptoms of benign prostatic hyperplasia. The Veterans Affairs Cooperative Study Group on Transurethral Resection of the Prostate. N Engl J Med 1995; 332:75–79.
- Jensen KM, Bruskewitz RC, Iversen P, Madsen PO. Spontaneous uroflowmetry in prostatism. Urology 1984; 24:403–409.
- Abrams PH, Griffiths DJ. The assessment of prostatic obstruction from urodynamic measurements and from residual urine. Br J Urol 1979; 51:129–134.
- Lim CS, Abrams P. The Abrams-Griffiths nomogram. World J Urol 1995; 13:34–39.
- Abrams P, Chapple C, Khoury S, Roehrborn C, de la Rosette J; International Consultation on New Developments in Prostate Cancer and Prostate Diseases. Evaluation and treatment of lower urinary tract symptoms in older men. J Urol 2013; 189(suppl 1):S93–S101.
- Flanigan RC, Reda DJ, Wasson JH, Anderson RJ, Abdellatif M, Bruskewitz RC. 5-year outcome of surgical resection and watchful waiting for men with moderately symptomatic benign prostatic hyperplasia: a Department of Veterans Affairs cooperative study. J Urol 1998; 160:12–17.
- Djavan B, Fong YK, Harik M, et al. Longitudinal study of men with mild symptoms of bladder outlet obstruction treated with watchful waiting for four years. Urology 2004; 64:1144–1148.
- Roberts RO, Jacobsen SJ, Jacobson DJ, Rhodes T, Girman CJ, Lieber MM. Longitudinal changes in peak urinary flow rates in a community based cohort. J Urol 2000; 163:107–113.
- Jacobsen SJ, Girman CJ, Guess HA, Rhodes T, Oesterling JE, Lieber MM. Natural history of prostatism: longitudinal changes in voiding symptoms in community dwelling men. J Urol 1996; 155:595–600.
- Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Treatment for benign prostatic hyperplasia among community dwelling men: the Olmsted County study of urinary symptoms and health status. J Urol 1999; 162:1301–1306.
- Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol 1997; 158:481–487.
- Kobayashi S, Tang R, Shapiro E, Lepor H. Characterization and localization of prostatic alpha 1 adrenoceptors using radioligand receptor binding on slide-mounted tissue section. J Urol 1993; 150:2002–2006.
- Kirby RS, Pool JL. Alpha adrenoceptor blockade in the treatment of benign prostatic hyperplasia: past, present and future. Br J Urol 1997; 80:521–532.
- Kirby RS, Pool JL. Alpha adrenoceptor blockade in the treatment of benign prostatic hyperplasia: past, present and future. Br J Urol 1997; 80:521–532.
- Milani S, Djavan B. Lower urinary tract symptoms suggestive of benign prostatic hyperplasia: latest update on alpha-adrenoceptor antagonists. BJU Int 2005; 95(suppl 4):29–36.
- Lepor H, Auerbach S, Puras-Baez A, et al. A randomized, placebo-controlled multicenter study of the efficacy and safety of terazosin in the treatment of benign prostatic hyperplasia. J Urol 1992; 148:1467–1474.
- Roehrborn CG, Oesterling JE, Auerbach S, et al. The Hytrin Community Assessment Trial study: a one-year study of terazosin versus placebo in the treatment of men with symptomatic benign prostatic hyperplasia. HYCAT Investigator Group. Urology 1996; 47:159–168.
- Gillenwater JY, Conn RL, Chrysant SG, et al. Doxazosin for the treatment of benign prostatic hyperplasia in patients with mild to moderate essential hypertension: a double-blind, placebo-controlled, dose-response multicenter study. J Urol 1995; 154:110–115.
- Chapple CR, Carter P, Christmas TJ, et al. A three month double-blind study of doxazosin as treatment for benign prostatic bladder outlet obstruction. Br J Urol 1994; 74:50–56.
- Buzelin JM, Roth S, Geffriaud-Ricouard C, Delauche-Cavallier MC. Efficacy and safety of sustained-release alfuzosin 5 mg in patients with benign prostatic hyperplasia. ALGEBI Study Group. Eur Urol 1997; 31:190–198.
- van Kerrebroeck P, Jardin A, Laval KU, van Cangh P. Efficacy and safety of a new prolonged release formulation of alfuzosin 10 mg once daily versus alfuzosin 2.5 mg thrice daily and placebo in patients with symptomatic benign prostatic hyperplasia. ALFORTI Study Group. Eur Urol 2000; 37:306–313.
- Narayan P, Tewari A. A second phase III multicenter placebo controlled study of 2 dosages of modified release tamsulosin in patients with symptoms of benign prostatic hyperplasia. United States 93-01 Study Group. J Urol 1998; 160:1701–1706.
- Lepor H. Phase III multicenter placebo-controlled study of tamsulosin in benign prostatic hyperplasia. Tamsulosin Investigator Group. Urology 1998; 51:892–900.
- Ding H, Du W, Hou ZZ, Wang HZ, Wang ZP. Silodosin is effective for treatment of LUTS in men with BPH: a systematic review. Asian J Androl 2013; 15:121–128.
- McConnell JD, Roehrborn CG, Bautista OM, et al; Medical Therapy of Prostatic Symptoms (MTOPS) Research Group. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003; 349:2387–2398.
- Jardin A, Bensadoun H, Delauche-Cavallier MC, Attali P. Alfuzosin for treatment of benign prostatic hypertrophy. The BPH-ALF Group. Lancet 1991; 337:1457–1461.
- Marks LS, Gittelman MC, Hill LA, Volinn W, Hoel G. Rapid efficacy of the highly selective alpha1A-adrenoceptor antagonist silodosin in men with signs and symptoms of benign prostatic hyperplasia: pooled results of 2 phase 3 studies. J Urol 2009; 181:2634–2640.
- Chang DF, Campbell JR. Intraoperative floppy iris syndrome associated with tamsulosin. J Cataract Refract Surg 2005; 31:664–673.
- Gormley GJ, Stoner E, Bruskewitz RC, et al. The effect of finasteride in men with benign prostatic hyperplasia. The Finasteride Study Group. N Engl J Med 1992; 327:1185–1191.
- Roehrborn CG, Boyle P, Nickel JC, Hoefner K, Andriole G; ARIA3001 ARIA3002 and ARIA3003 Study Investigators. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology 2002; 60:434–441.
- Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J Clin Endocrinol Metab 2004; 89:2179–2184.
- Kaplan SA, Lee JY, Meehan AG, Kusek JW; MTOPS Research Group. Long-term treatment with finasteride improves clinical progression of benign prostatic hyperplasia in men with an enlarged versus a smaller prostate: data from the MTOPS trial. J Urol 2011; 185:1369–1373.
- Roehrborn CG, Siami P, Barkin J, et al; CombAT Study Group. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT study. Eur Urol 2010; 57:123–131.
- Abrams P, Kaplan S, De Koning Gans HJ, Millard R. Safety and tolerability of tolterodine for the treatment of overactive bladder in men with bladder outlet obstruction. J Urol 2006; 175:999–1004.
- Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA 2006; 296:2319–2328.
- Suarez O, Osborn D, Kaufman M, Reynolds WS, Dmochowski R. Mirabegron for male lower urinary tract symptoms. Curr Urol Rep 2013; 14:580–584.
- Oelke M, Giuliano F, Mirone V, Xu L, Cox D, Viktrup L. Monotherapy with tadalafil or tamsulosin similarly improved lower urinary tract symptoms suggestive of benign prostatic hyperplasia in an international, randomised, parallel, placebo-controlled clinical trial. Eur Urol 2012; 61:917–925.
- Welliver C, McVary KT. Minimally invasive and endoscopic management of benign prostatic hyperplasia. In: Wein AJ, Kavoussi LR, Partin AW, Peters CA, eds. Campbell-Walsh Urology. 11th ed. Philadelphia, PA: Elsevier; 2016:2504–2534.
KEY POINTS
- Watchful waiting is appropriate for patients with mild to moderate symptoms that cause minimal bother.
- Patients with severe or bothersome symptoms should be offered pharmacotherapy, not only to improve symptoms but also to reduce the risk of disease progression.
- Several effective, minimally invasive surgical options are available for patients whose symptoms do not respond to medical therapy. These patients and those with abnormal findings on diagnostic evaluation warrant referral to a urologist for further evaluation.
Fall risk and anticoagulation for atrial fibrillation in the elderly: A delicate balance
An 86-year-old woman with hypertension, osteoporosis, and mild cognitive impairment presents with episodes of palpitations and heart “fluttering.” These episodes occur 1 to 2 times per week, last for up to several hours, and are associated with mild shortness of breath and reduced activity tolerance. She is widowed and lives in a retirement facility, but she is independent in activities of daily living. She has fallen twice in the past year without significant injury.
Physical examination is unremarkable. An electrocardiogram demonstrates sinus rhythm with left ventricular hypertrophy. A 30-day event monitor reveals several episodes of paroxysmal atrial fibrillation that correspond with her symptoms. A subsequent echocardiogram shows normal left ventricular systolic function, mild diastolic dysfunction, and no significant valvular abnormalities. Laboratory studies, including thyroid-stimulating hormone, are normal.
What is this patient’s risk of stroke? What is her risk of major bleeding from anticoagulation? How should fall risk be addressed in the decision-making process? What other factors should be considered?
AGE, ATRIAL FIBRILLATION, AND STROKE RISK
The prevalence of atrial fibrillation increases with age, and nearly half of patients with atrial fibrillation in the United States are 75 or older.1 In addition, older age is an independent risk factor for stroke in patients with atrial fibrillation, and the proportion of strokes attributable to atrial fibrillation increases exponentially with age:
- 1.5% at age 50 to 59
- 2.8% at age 60 to 69
- 9.9% at age 70 to 79
- 23.5% at age 80 to 89.2
Numerous large randomized trials have shown that anticoagulation with warfarin reduces the risk of stroke by about two-thirds in patients with atrial fibrillation, and that this benefit extends to the elderly.
In the Birmingham Atrial Fibrillation Treatment of the Aged trial,3 973 patients at least 75 years old (mean age 81.5, 55% male) were randomized to receive either warfarin with a target international normalized ratio of 2.0 to 3.0 or aspirin 75 mg/day. Over an average follow-up of 2.7 years, the composite outcome of fatal or disabling stroke, arterial embolism, or intracranial hemorrhage occurred in 24 (4.9%) of the 488 patients in the warfarin group and 48 (9.9%) of the 485 patients in the aspirin group (absolute yearly risk reduction 2%, 95% confidence interval 0.7–3.2, number needed to treat 50 for 1 year). Importantly, the benefit of warfarin was similar in men and women, and in patients ages 75 to 79, 80 to 84, and 85 and older.
More recently, the oral anticoagulants dabigatran, rivaroxaban, apixaban, and edoxaban have been shown to be at least as effective as warfarin with respect to both stroke prevention and major bleeding complications, and subgroup analyses have confirmed similar outcomes in older and younger patients.4,5
But despite the proven value of anticoagulation for stroke prevention in older adults, only 40% to 60% of older patients who are suitable candidates for anticoagulation actually receive it.6 Moreover, the proportion of patients who are treated declines progressively with age. The most frequently cited reason for nontreatment is perception of a high risk of falls and associated concerns about bleeding, especially intracranial hemorrhage.7–10
BALANCING STROKE RISK VS BLEEDING RISK
Balancing the risk of stroke against the risk of bleeding related to falls is a commonly encountered conundrum in older patients with atrial fibrillation.
Stroke risk
The CHADS2 score was, until recently, the most widely used method for assessing stroke risk in patients with nonvalvular atrial fibrillation. CHADS2 assigns 1 point each for congestive heart failure, hypertension, age ≥ 75, and diabetes, and 2 points for prior stroke or transient ischemic attack (range 0–6 points). Annual stroke risk based on the CHADS2 score ranges from about 2% to about 18%
(Table 1).11
The CHA2DS2-VASc score,12 a modification of CHADS2, appears to assess the risk of stroke more accurately, especially at the lower end of the scale, and recent guidelines for managing atrial fibrillation recommend using the CHA2DS2-VASc algorithm.13 CHA2DS2-VASc is similar to CHADS2, except that it assigns 1 point for ages 65 to 74, 2 points for ages 75 and older, 1 point for vascular disease (coronary artery disease, peripheral arterial disease, aortic aneurysm), and 1 point for female sex (Table 1).11,12
For both CHADS2 and CHA2DS2-VASc, systemic anticoagulation is recommended for patients who have a score of 2 or higher. Our patient’s CHADS2 score is 2, and her CHA2DS2-VASc score is 4, corresponding to an annual estimated stroke risk of 4% with both scores (Table 1). Note, however, that the CHA2DS2-VASc score provides more information at the lower end of the spectrum.
Bleeding risk
Several scoring systems for assessing bleeding risk have also been developed (Table 2).14–16 Of these, the HAS-BLED score has come to be used more widely in recent years.
Perhaps not surprisingly, some of the same factors associated with risk of stroke also predict increased risk of bleeding (eg, older age, hypertension, prior stroke).14 Note, however, that history of falling or high risk of falling is only included in one of the bleeding risk models (HEMORR2HAGES).15
These tools are somewhat limited by their lack of consideration of concomitant antiplatelet therapy (only included in HAS-BLED) or history of bleeding (only ATRIA16 considers major and minor bleeding, HEMORR2HAGES does not specify bleeding severity, and HAS-BLED only considers major bleeding). The models also fail to include medications such as antibiotics or antiarrhythmic agents, which are commonly used by older patients with atrial fibrillation and may increase bleeding risk. In addition, all bleeding risk scores were developed for warfarin, and their applicability to patients treated with the newer oral anticoagulants has not been established.
At the time of presentation, our patient has a HAS-BLED score of 2 (1 point each for age and hypertension), placing her at intermediate risk of bleeding.14
Fear the clot, not the bleed
So how does one balance the risk of stroke vs the risk of bleeding? An adage from the early days of thrombolytic therapy for acute myocardial infarction was “fear the clot, not the bleed.” In other words, in the present context the consequences of a thrombus embolizing from the heart to the brain are likely to be more devastating and more permanent than the consequences of anticoagulation-associated hemorrhage.
Support for this view is underscored by a 2015 study by Lip et al,17 who examined stroke and bleeding risks and outcomes in a large real-world population of patients age 75 and older. The analysis included 819 patients ages 85 to 89 and 386 patients age 90 and older. The key finding was that the oldest patients derived the greatest net benefit from anticoagulation.
Moreover, the Canadian stroke registry of 3,197 patients, mean age 79, showed that advanced age was a more potent risk factor for ischemic stroke than it was for hemorrhagic stroke.18
Thus, the benefit from anticoagulation in patients with atrial fibrillation does not appear to have an upper age limit.
FALLS AND ANTICOAGULATION
Falls are an important source of morbidity, disability, and activity curtailment in older adults and, like atrial fibrillation, the incidence and prevalence of falls increase with age. In community-dwelling adults age 65 and older, the overall proportion with at least 1 fall in the preceding year ranges from about 30% to 40%.19 However, the rate increases with age and exceeds 50% in nursing home residents.20
Although anticoagulation is associated with a higher risk of bleeding in patients who fall, the absolute risk is small.
In a study of older adults with nonvalvular atrial fibrillation, a history of falls or documented high risk of falling was associated with a risk of intracranial hemorrhage during follow-up that was 1.9 times higher.21 Importantly, however, this risk did not differ among patients treated with warfarin, aspirin, or no antithrombotic therapy. In this analysis, patients with a CHADS2 score of 2 or higher benefited from anticoagulation, whether or not they were considered to be at risk for falls.
In another study,22 it was estimated that an individual would have to fall 295 times in 1 year for the risk of fall-related major bleeding to outweigh the benefit of warfarin in reducing the risk of stroke.
Thus, based on available evidence, perception of a high risk of falling should not be construed as justification for withholding anticoagulation in older patients who are otherwise suitable candidates for such therapy.
AT WHAT POINT DOES BLEEDING RISK OUTWEIGH ANTICOAGULATION BENEFIT?
Absolute contraindications to anticoagulation include an intracranial hemorrhage or neurosurgical procedure with high risk for bleeding within the past 30 days, an intracranial neoplasm or vascular abnormality with high risk of bleeding, recurrent life-threatening gastrointestinal or other bleeding events, and severe bleeding disorders, including severe thrombocytopenia.
In patients with atrial fibrillation at high risk of bleeding as assessed by one of the bleeding risk scores and relatively low risk of ischemic stroke, the risk of anticoagulation may outweigh the benefit, although no studies have specifically addressed this issue.
In patients with frequent falls, including injurious falls, the benefits of anticoagulation usually outweigh the risks of bleeding, but management should incorporate interventions designed to mitigate fall risk.
Finally, in patients with a poor prognosis approaching the end of life, the risks and burdens of anticoagulation may exceed the perceived benefits, in which case discontinuation of anticoagulation may be appropriate.
SHOULD OUR PATIENT RECEIVE ANTICOAGULATION?
As noted above, our patient has a high risk of stroke and a moderate risk of bleeding, and multiple lines of evidence indicate that the benefits of anticoagulation (ie, prevention of stroke and systemic embolization) substantially outweigh the risks of bleeding. Although she has a history of falls, which may seem to muddy the waters, this factor should not play a major role in decision-making. Moreover, her advanced age should, if anything, be considered a point in favor of anticoagulation. So from the scientific standpoint, anticoagulation is the clear winner.
A shared decision
But that is not the end of the story. Since there is tension between benefits and risks with either approach (ie, anticoagulation or no anticoagulation), it is important to discuss the issues and options with the patient and relevant caregivers. Most older adults have witnessed the ravages of stroke in a friend or relative, and a recent study showed that most would be willing to accept a modest risk of bleeding to prevent a stroke.23
However, this is ultimately a personal decision for each patient, and in accordance with the principle of patient autonomy, the patient’s expressed wishes should be honored by using a process of shared decision-making.
Which anticoagulant?
Finally, what about the choice of anticoagulation? The complexities of using warfarin, including its narrow therapeutic range and myriad interactions with other medications and foods, can make it a less appealing option for both patient and provider.
We recommend a novel oral anticoagulant as first-line therapy in the absence of contraindications such as severe renal insufficiency, and prefer apixaban because it is the only agent shown to be superior to warfarin with respect to both stroke prevention and bleeding risk.24
Important disadvantages of the novel oral anticoagulants include their higher cost and lack of an effective antidote in the event of clinically significant bleeding (with the exception of idarucizumab, which was recently approved for reversal of serious bleeding associated with dabigatran), issues that may be of particular concern to older adults. While there is no therapeutic range to monitor for the newer agents, more frequent monitoring for occult anemia may be needed.
Thus, selection of an anticoagulant should also be individualized through shared decision-making.
Is aspirin alone an alternative?
And what if the patient chooses to forgo anticoagulation? In that case, aspirin 75 to 325 mg/day may seem reasonable, but there is scant evidence that aspirin is beneficial for stroke prevention in patients with atrial fibrillation in this age group, and aspirin, too, is associated with an increased risk of bleeding.25
As a result, current US and European guidelines recommend a very limited role for aspirin as a single agent in the management of atrial fibrillation.26 The joint 2014 guidelines of the American Heart Association, American College of Cardiology, and Heart Rhythm Society give aspirin a class IIB recommendation (ie, it “may” be considered), level of evidence C (ie, very limited) for use as an alternative to no antithrombotic therapy or systemic anticoagulation only in patients with a CHA2DS2-VASc score of 1, thereby excluding all patients age 75 and older.13
In most cases, aspirin as sole prophylaxis against stroke in atrial fibrillation should be avoided in the absence of another indication for its use, such as coexisting coronary artery disease or peripheral arterial disease.
A COMPLEX DECISION
In summary, the decisions surrounding anticoagulation of elderly patients with nonvalvular atrial fibrillation are complex. Accurate assessment of stroke risk is key, and although bleeding risk is also an essential consideration, it is important not to overemphasize bleeding and fall risks in the decision-making process.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285:2370–2375.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22:983–988.
- Mant J, Hobbs FD, Fletcher K, et al; BAFTA investigators; Midland Research Practices Network (MidReC). Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007; 370:493–503.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurology 2013; 70:1486–1490.
- Sardar P, Chatterjee S, Chaudhari S, Lip GY. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc 2014; 62:857–864.
- Rich MW. Atrial fibrillation in long term care. J Am Med Dir Assoc 2012; 13:688–691.
- McCrory DC, Matchar DB, Samsa G, Sanders LL, Pritchett EL. Physician attitudes about anticoagulation for nonvalvular atrial fibrillation in the elderly. Arch Intern Med 1995; 155:277–281.
- Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing 2011; 40:675–683.
- Sellers MB, Newby LK. Atrial fibrillation, anticoagulation, fall risk, and outcomes in elderly patients. Am Heart J 2011; 161:241–246.
- Bahri O, Roca F, Lechani T, et al. Underuse of oral anticoagulation for individuals with atrial fibrillation in a nursing home setting in France: comparisons of resident characteristics and physician attitude. J Am Geriatr Soc 2015; 63:71–76.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64:e1–e76.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006; 151:713–719.
- Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol 2011; 58:395–401.
- Lip GY, Clementy N, Pericart L, Banerjee A, Fauchier L. Stroke and major bleeding risk in elderly patients aged ≥ 75 years with atrial fibrillation: the Loire Valley atrial fibrillation project. Stroke 2015; 46:143–150.
- McGrath ER, Kapral MK, Fang J, et al; Investigators of the Registry of the Canadian Stroke Network. Which risk factors are more associated with ischemic stroke than intracerebral hemorrhage in patients with atrial fibrillation? Stroke 2012; 43:2048–2054.
- Phelan EA, Mahoney JE, Voit JC, Stevens JA. Assessment and management of fall risk in primary care settings. Med Clin North Am 2015; 99:281–293.
- Deandrea S, Bravi F, Turati F, Lucenteforte E, La Vecchia C, Negri E. Risk factors for falls in older people in nursing homes and hospitals. A systematic review and meta-analysis. Arch Gerontol Geriatr 2013; 56:407–415.
- Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena DS, Rich MW. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med 2005; 118:612–617.
- Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999; 159:677–685.
- Riva N, Smith DE, Lip GY, Lane DA. Advancing age and bleeding risk are the strongest barriers to anticoagulant prescription in atrial fibrillation. Age Ageing 2011; 40:653–655.
- De Caterina R, Andersson U, Alexander JH, et al; ARISTOTLE Investigators. History of bleeding and outcomes with apixaban versus warfarin in patients with atrial fibrillation in the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation trial. Am Heart J 2016; 175:175–183.
- Ben Freedman S, Gersh BJ, Lip GY. Misperceptions of aspirin efficacy and safety may perpetuate anticoagulant underutilization in atrial fibrillation. Eur Heart J 2015; 36:653–656.
- Camm AJ, Lip GY, De Caterina R, et al; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012; 33:2719–2747.
An 86-year-old woman with hypertension, osteoporosis, and mild cognitive impairment presents with episodes of palpitations and heart “fluttering.” These episodes occur 1 to 2 times per week, last for up to several hours, and are associated with mild shortness of breath and reduced activity tolerance. She is widowed and lives in a retirement facility, but she is independent in activities of daily living. She has fallen twice in the past year without significant injury.
Physical examination is unremarkable. An electrocardiogram demonstrates sinus rhythm with left ventricular hypertrophy. A 30-day event monitor reveals several episodes of paroxysmal atrial fibrillation that correspond with her symptoms. A subsequent echocardiogram shows normal left ventricular systolic function, mild diastolic dysfunction, and no significant valvular abnormalities. Laboratory studies, including thyroid-stimulating hormone, are normal.
What is this patient’s risk of stroke? What is her risk of major bleeding from anticoagulation? How should fall risk be addressed in the decision-making process? What other factors should be considered?
AGE, ATRIAL FIBRILLATION, AND STROKE RISK
The prevalence of atrial fibrillation increases with age, and nearly half of patients with atrial fibrillation in the United States are 75 or older.1 In addition, older age is an independent risk factor for stroke in patients with atrial fibrillation, and the proportion of strokes attributable to atrial fibrillation increases exponentially with age:
- 1.5% at age 50 to 59
- 2.8% at age 60 to 69
- 9.9% at age 70 to 79
- 23.5% at age 80 to 89.2
Numerous large randomized trials have shown that anticoagulation with warfarin reduces the risk of stroke by about two-thirds in patients with atrial fibrillation, and that this benefit extends to the elderly.
In the Birmingham Atrial Fibrillation Treatment of the Aged trial,3 973 patients at least 75 years old (mean age 81.5, 55% male) were randomized to receive either warfarin with a target international normalized ratio of 2.0 to 3.0 or aspirin 75 mg/day. Over an average follow-up of 2.7 years, the composite outcome of fatal or disabling stroke, arterial embolism, or intracranial hemorrhage occurred in 24 (4.9%) of the 488 patients in the warfarin group and 48 (9.9%) of the 485 patients in the aspirin group (absolute yearly risk reduction 2%, 95% confidence interval 0.7–3.2, number needed to treat 50 for 1 year). Importantly, the benefit of warfarin was similar in men and women, and in patients ages 75 to 79, 80 to 84, and 85 and older.
More recently, the oral anticoagulants dabigatran, rivaroxaban, apixaban, and edoxaban have been shown to be at least as effective as warfarin with respect to both stroke prevention and major bleeding complications, and subgroup analyses have confirmed similar outcomes in older and younger patients.4,5
But despite the proven value of anticoagulation for stroke prevention in older adults, only 40% to 60% of older patients who are suitable candidates for anticoagulation actually receive it.6 Moreover, the proportion of patients who are treated declines progressively with age. The most frequently cited reason for nontreatment is perception of a high risk of falls and associated concerns about bleeding, especially intracranial hemorrhage.7–10
BALANCING STROKE RISK VS BLEEDING RISK
Balancing the risk of stroke against the risk of bleeding related to falls is a commonly encountered conundrum in older patients with atrial fibrillation.
Stroke risk
The CHADS2 score was, until recently, the most widely used method for assessing stroke risk in patients with nonvalvular atrial fibrillation. CHADS2 assigns 1 point each for congestive heart failure, hypertension, age ≥ 75, and diabetes, and 2 points for prior stroke or transient ischemic attack (range 0–6 points). Annual stroke risk based on the CHADS2 score ranges from about 2% to about 18%
(Table 1).11
The CHA2DS2-VASc score,12 a modification of CHADS2, appears to assess the risk of stroke more accurately, especially at the lower end of the scale, and recent guidelines for managing atrial fibrillation recommend using the CHA2DS2-VASc algorithm.13 CHA2DS2-VASc is similar to CHADS2, except that it assigns 1 point for ages 65 to 74, 2 points for ages 75 and older, 1 point for vascular disease (coronary artery disease, peripheral arterial disease, aortic aneurysm), and 1 point for female sex (Table 1).11,12
For both CHADS2 and CHA2DS2-VASc, systemic anticoagulation is recommended for patients who have a score of 2 or higher. Our patient’s CHADS2 score is 2, and her CHA2DS2-VASc score is 4, corresponding to an annual estimated stroke risk of 4% with both scores (Table 1). Note, however, that the CHA2DS2-VASc score provides more information at the lower end of the spectrum.
Bleeding risk
Several scoring systems for assessing bleeding risk have also been developed (Table 2).14–16 Of these, the HAS-BLED score has come to be used more widely in recent years.
Perhaps not surprisingly, some of the same factors associated with risk of stroke also predict increased risk of bleeding (eg, older age, hypertension, prior stroke).14 Note, however, that history of falling or high risk of falling is only included in one of the bleeding risk models (HEMORR2HAGES).15
These tools are somewhat limited by their lack of consideration of concomitant antiplatelet therapy (only included in HAS-BLED) or history of bleeding (only ATRIA16 considers major and minor bleeding, HEMORR2HAGES does not specify bleeding severity, and HAS-BLED only considers major bleeding). The models also fail to include medications such as antibiotics or antiarrhythmic agents, which are commonly used by older patients with atrial fibrillation and may increase bleeding risk. In addition, all bleeding risk scores were developed for warfarin, and their applicability to patients treated with the newer oral anticoagulants has not been established.
At the time of presentation, our patient has a HAS-BLED score of 2 (1 point each for age and hypertension), placing her at intermediate risk of bleeding.14
Fear the clot, not the bleed
So how does one balance the risk of stroke vs the risk of bleeding? An adage from the early days of thrombolytic therapy for acute myocardial infarction was “fear the clot, not the bleed.” In other words, in the present context the consequences of a thrombus embolizing from the heart to the brain are likely to be more devastating and more permanent than the consequences of anticoagulation-associated hemorrhage.
Support for this view is underscored by a 2015 study by Lip et al,17 who examined stroke and bleeding risks and outcomes in a large real-world population of patients age 75 and older. The analysis included 819 patients ages 85 to 89 and 386 patients age 90 and older. The key finding was that the oldest patients derived the greatest net benefit from anticoagulation.
Moreover, the Canadian stroke registry of 3,197 patients, mean age 79, showed that advanced age was a more potent risk factor for ischemic stroke than it was for hemorrhagic stroke.18
Thus, the benefit from anticoagulation in patients with atrial fibrillation does not appear to have an upper age limit.
FALLS AND ANTICOAGULATION
Falls are an important source of morbidity, disability, and activity curtailment in older adults and, like atrial fibrillation, the incidence and prevalence of falls increase with age. In community-dwelling adults age 65 and older, the overall proportion with at least 1 fall in the preceding year ranges from about 30% to 40%.19 However, the rate increases with age and exceeds 50% in nursing home residents.20
Although anticoagulation is associated with a higher risk of bleeding in patients who fall, the absolute risk is small.
In a study of older adults with nonvalvular atrial fibrillation, a history of falls or documented high risk of falling was associated with a risk of intracranial hemorrhage during follow-up that was 1.9 times higher.21 Importantly, however, this risk did not differ among patients treated with warfarin, aspirin, or no antithrombotic therapy. In this analysis, patients with a CHADS2 score of 2 or higher benefited from anticoagulation, whether or not they were considered to be at risk for falls.
In another study,22 it was estimated that an individual would have to fall 295 times in 1 year for the risk of fall-related major bleeding to outweigh the benefit of warfarin in reducing the risk of stroke.
Thus, based on available evidence, perception of a high risk of falling should not be construed as justification for withholding anticoagulation in older patients who are otherwise suitable candidates for such therapy.
AT WHAT POINT DOES BLEEDING RISK OUTWEIGH ANTICOAGULATION BENEFIT?
Absolute contraindications to anticoagulation include an intracranial hemorrhage or neurosurgical procedure with high risk for bleeding within the past 30 days, an intracranial neoplasm or vascular abnormality with high risk of bleeding, recurrent life-threatening gastrointestinal or other bleeding events, and severe bleeding disorders, including severe thrombocytopenia.
In patients with atrial fibrillation at high risk of bleeding as assessed by one of the bleeding risk scores and relatively low risk of ischemic stroke, the risk of anticoagulation may outweigh the benefit, although no studies have specifically addressed this issue.
In patients with frequent falls, including injurious falls, the benefits of anticoagulation usually outweigh the risks of bleeding, but management should incorporate interventions designed to mitigate fall risk.
Finally, in patients with a poor prognosis approaching the end of life, the risks and burdens of anticoagulation may exceed the perceived benefits, in which case discontinuation of anticoagulation may be appropriate.
SHOULD OUR PATIENT RECEIVE ANTICOAGULATION?
As noted above, our patient has a high risk of stroke and a moderate risk of bleeding, and multiple lines of evidence indicate that the benefits of anticoagulation (ie, prevention of stroke and systemic embolization) substantially outweigh the risks of bleeding. Although she has a history of falls, which may seem to muddy the waters, this factor should not play a major role in decision-making. Moreover, her advanced age should, if anything, be considered a point in favor of anticoagulation. So from the scientific standpoint, anticoagulation is the clear winner.
A shared decision
But that is not the end of the story. Since there is tension between benefits and risks with either approach (ie, anticoagulation or no anticoagulation), it is important to discuss the issues and options with the patient and relevant caregivers. Most older adults have witnessed the ravages of stroke in a friend or relative, and a recent study showed that most would be willing to accept a modest risk of bleeding to prevent a stroke.23
However, this is ultimately a personal decision for each patient, and in accordance with the principle of patient autonomy, the patient’s expressed wishes should be honored by using a process of shared decision-making.
Which anticoagulant?
Finally, what about the choice of anticoagulation? The complexities of using warfarin, including its narrow therapeutic range and myriad interactions with other medications and foods, can make it a less appealing option for both patient and provider.
We recommend a novel oral anticoagulant as first-line therapy in the absence of contraindications such as severe renal insufficiency, and prefer apixaban because it is the only agent shown to be superior to warfarin with respect to both stroke prevention and bleeding risk.24
Important disadvantages of the novel oral anticoagulants include their higher cost and lack of an effective antidote in the event of clinically significant bleeding (with the exception of idarucizumab, which was recently approved for reversal of serious bleeding associated with dabigatran), issues that may be of particular concern to older adults. While there is no therapeutic range to monitor for the newer agents, more frequent monitoring for occult anemia may be needed.
Thus, selection of an anticoagulant should also be individualized through shared decision-making.
Is aspirin alone an alternative?
And what if the patient chooses to forgo anticoagulation? In that case, aspirin 75 to 325 mg/day may seem reasonable, but there is scant evidence that aspirin is beneficial for stroke prevention in patients with atrial fibrillation in this age group, and aspirin, too, is associated with an increased risk of bleeding.25
As a result, current US and European guidelines recommend a very limited role for aspirin as a single agent in the management of atrial fibrillation.26 The joint 2014 guidelines of the American Heart Association, American College of Cardiology, and Heart Rhythm Society give aspirin a class IIB recommendation (ie, it “may” be considered), level of evidence C (ie, very limited) for use as an alternative to no antithrombotic therapy or systemic anticoagulation only in patients with a CHA2DS2-VASc score of 1, thereby excluding all patients age 75 and older.13
In most cases, aspirin as sole prophylaxis against stroke in atrial fibrillation should be avoided in the absence of another indication for its use, such as coexisting coronary artery disease or peripheral arterial disease.
A COMPLEX DECISION
In summary, the decisions surrounding anticoagulation of elderly patients with nonvalvular atrial fibrillation are complex. Accurate assessment of stroke risk is key, and although bleeding risk is also an essential consideration, it is important not to overemphasize bleeding and fall risks in the decision-making process.
An 86-year-old woman with hypertension, osteoporosis, and mild cognitive impairment presents with episodes of palpitations and heart “fluttering.” These episodes occur 1 to 2 times per week, last for up to several hours, and are associated with mild shortness of breath and reduced activity tolerance. She is widowed and lives in a retirement facility, but she is independent in activities of daily living. She has fallen twice in the past year without significant injury.
Physical examination is unremarkable. An electrocardiogram demonstrates sinus rhythm with left ventricular hypertrophy. A 30-day event monitor reveals several episodes of paroxysmal atrial fibrillation that correspond with her symptoms. A subsequent echocardiogram shows normal left ventricular systolic function, mild diastolic dysfunction, and no significant valvular abnormalities. Laboratory studies, including thyroid-stimulating hormone, are normal.
What is this patient’s risk of stroke? What is her risk of major bleeding from anticoagulation? How should fall risk be addressed in the decision-making process? What other factors should be considered?
AGE, ATRIAL FIBRILLATION, AND STROKE RISK
The prevalence of atrial fibrillation increases with age, and nearly half of patients with atrial fibrillation in the United States are 75 or older.1 In addition, older age is an independent risk factor for stroke in patients with atrial fibrillation, and the proportion of strokes attributable to atrial fibrillation increases exponentially with age:
- 1.5% at age 50 to 59
- 2.8% at age 60 to 69
- 9.9% at age 70 to 79
- 23.5% at age 80 to 89.2
Numerous large randomized trials have shown that anticoagulation with warfarin reduces the risk of stroke by about two-thirds in patients with atrial fibrillation, and that this benefit extends to the elderly.
In the Birmingham Atrial Fibrillation Treatment of the Aged trial,3 973 patients at least 75 years old (mean age 81.5, 55% male) were randomized to receive either warfarin with a target international normalized ratio of 2.0 to 3.0 or aspirin 75 mg/day. Over an average follow-up of 2.7 years, the composite outcome of fatal or disabling stroke, arterial embolism, or intracranial hemorrhage occurred in 24 (4.9%) of the 488 patients in the warfarin group and 48 (9.9%) of the 485 patients in the aspirin group (absolute yearly risk reduction 2%, 95% confidence interval 0.7–3.2, number needed to treat 50 for 1 year). Importantly, the benefit of warfarin was similar in men and women, and in patients ages 75 to 79, 80 to 84, and 85 and older.
More recently, the oral anticoagulants dabigatran, rivaroxaban, apixaban, and edoxaban have been shown to be at least as effective as warfarin with respect to both stroke prevention and major bleeding complications, and subgroup analyses have confirmed similar outcomes in older and younger patients.4,5
But despite the proven value of anticoagulation for stroke prevention in older adults, only 40% to 60% of older patients who are suitable candidates for anticoagulation actually receive it.6 Moreover, the proportion of patients who are treated declines progressively with age. The most frequently cited reason for nontreatment is perception of a high risk of falls and associated concerns about bleeding, especially intracranial hemorrhage.7–10
BALANCING STROKE RISK VS BLEEDING RISK
Balancing the risk of stroke against the risk of bleeding related to falls is a commonly encountered conundrum in older patients with atrial fibrillation.
Stroke risk
The CHADS2 score was, until recently, the most widely used method for assessing stroke risk in patients with nonvalvular atrial fibrillation. CHADS2 assigns 1 point each for congestive heart failure, hypertension, age ≥ 75, and diabetes, and 2 points for prior stroke or transient ischemic attack (range 0–6 points). Annual stroke risk based on the CHADS2 score ranges from about 2% to about 18%
(Table 1).11
The CHA2DS2-VASc score,12 a modification of CHADS2, appears to assess the risk of stroke more accurately, especially at the lower end of the scale, and recent guidelines for managing atrial fibrillation recommend using the CHA2DS2-VASc algorithm.13 CHA2DS2-VASc is similar to CHADS2, except that it assigns 1 point for ages 65 to 74, 2 points for ages 75 and older, 1 point for vascular disease (coronary artery disease, peripheral arterial disease, aortic aneurysm), and 1 point for female sex (Table 1).11,12
For both CHADS2 and CHA2DS2-VASc, systemic anticoagulation is recommended for patients who have a score of 2 or higher. Our patient’s CHADS2 score is 2, and her CHA2DS2-VASc score is 4, corresponding to an annual estimated stroke risk of 4% with both scores (Table 1). Note, however, that the CHA2DS2-VASc score provides more information at the lower end of the spectrum.
Bleeding risk
Several scoring systems for assessing bleeding risk have also been developed (Table 2).14–16 Of these, the HAS-BLED score has come to be used more widely in recent years.
Perhaps not surprisingly, some of the same factors associated with risk of stroke also predict increased risk of bleeding (eg, older age, hypertension, prior stroke).14 Note, however, that history of falling or high risk of falling is only included in one of the bleeding risk models (HEMORR2HAGES).15
These tools are somewhat limited by their lack of consideration of concomitant antiplatelet therapy (only included in HAS-BLED) or history of bleeding (only ATRIA16 considers major and minor bleeding, HEMORR2HAGES does not specify bleeding severity, and HAS-BLED only considers major bleeding). The models also fail to include medications such as antibiotics or antiarrhythmic agents, which are commonly used by older patients with atrial fibrillation and may increase bleeding risk. In addition, all bleeding risk scores were developed for warfarin, and their applicability to patients treated with the newer oral anticoagulants has not been established.
At the time of presentation, our patient has a HAS-BLED score of 2 (1 point each for age and hypertension), placing her at intermediate risk of bleeding.14
Fear the clot, not the bleed
So how does one balance the risk of stroke vs the risk of bleeding? An adage from the early days of thrombolytic therapy for acute myocardial infarction was “fear the clot, not the bleed.” In other words, in the present context the consequences of a thrombus embolizing from the heart to the brain are likely to be more devastating and more permanent than the consequences of anticoagulation-associated hemorrhage.
Support for this view is underscored by a 2015 study by Lip et al,17 who examined stroke and bleeding risks and outcomes in a large real-world population of patients age 75 and older. The analysis included 819 patients ages 85 to 89 and 386 patients age 90 and older. The key finding was that the oldest patients derived the greatest net benefit from anticoagulation.
Moreover, the Canadian stroke registry of 3,197 patients, mean age 79, showed that advanced age was a more potent risk factor for ischemic stroke than it was for hemorrhagic stroke.18
Thus, the benefit from anticoagulation in patients with atrial fibrillation does not appear to have an upper age limit.
FALLS AND ANTICOAGULATION
Falls are an important source of morbidity, disability, and activity curtailment in older adults and, like atrial fibrillation, the incidence and prevalence of falls increase with age. In community-dwelling adults age 65 and older, the overall proportion with at least 1 fall in the preceding year ranges from about 30% to 40%.19 However, the rate increases with age and exceeds 50% in nursing home residents.20
Although anticoagulation is associated with a higher risk of bleeding in patients who fall, the absolute risk is small.
In a study of older adults with nonvalvular atrial fibrillation, a history of falls or documented high risk of falling was associated with a risk of intracranial hemorrhage during follow-up that was 1.9 times higher.21 Importantly, however, this risk did not differ among patients treated with warfarin, aspirin, or no antithrombotic therapy. In this analysis, patients with a CHADS2 score of 2 or higher benefited from anticoagulation, whether or not they were considered to be at risk for falls.
In another study,22 it was estimated that an individual would have to fall 295 times in 1 year for the risk of fall-related major bleeding to outweigh the benefit of warfarin in reducing the risk of stroke.
Thus, based on available evidence, perception of a high risk of falling should not be construed as justification for withholding anticoagulation in older patients who are otherwise suitable candidates for such therapy.
AT WHAT POINT DOES BLEEDING RISK OUTWEIGH ANTICOAGULATION BENEFIT?
Absolute contraindications to anticoagulation include an intracranial hemorrhage or neurosurgical procedure with high risk for bleeding within the past 30 days, an intracranial neoplasm or vascular abnormality with high risk of bleeding, recurrent life-threatening gastrointestinal or other bleeding events, and severe bleeding disorders, including severe thrombocytopenia.
In patients with atrial fibrillation at high risk of bleeding as assessed by one of the bleeding risk scores and relatively low risk of ischemic stroke, the risk of anticoagulation may outweigh the benefit, although no studies have specifically addressed this issue.
In patients with frequent falls, including injurious falls, the benefits of anticoagulation usually outweigh the risks of bleeding, but management should incorporate interventions designed to mitigate fall risk.
Finally, in patients with a poor prognosis approaching the end of life, the risks and burdens of anticoagulation may exceed the perceived benefits, in which case discontinuation of anticoagulation may be appropriate.
SHOULD OUR PATIENT RECEIVE ANTICOAGULATION?
As noted above, our patient has a high risk of stroke and a moderate risk of bleeding, and multiple lines of evidence indicate that the benefits of anticoagulation (ie, prevention of stroke and systemic embolization) substantially outweigh the risks of bleeding. Although she has a history of falls, which may seem to muddy the waters, this factor should not play a major role in decision-making. Moreover, her advanced age should, if anything, be considered a point in favor of anticoagulation. So from the scientific standpoint, anticoagulation is the clear winner.
A shared decision
But that is not the end of the story. Since there is tension between benefits and risks with either approach (ie, anticoagulation or no anticoagulation), it is important to discuss the issues and options with the patient and relevant caregivers. Most older adults have witnessed the ravages of stroke in a friend or relative, and a recent study showed that most would be willing to accept a modest risk of bleeding to prevent a stroke.23
However, this is ultimately a personal decision for each patient, and in accordance with the principle of patient autonomy, the patient’s expressed wishes should be honored by using a process of shared decision-making.
Which anticoagulant?
Finally, what about the choice of anticoagulation? The complexities of using warfarin, including its narrow therapeutic range and myriad interactions with other medications and foods, can make it a less appealing option for both patient and provider.
We recommend a novel oral anticoagulant as first-line therapy in the absence of contraindications such as severe renal insufficiency, and prefer apixaban because it is the only agent shown to be superior to warfarin with respect to both stroke prevention and bleeding risk.24
Important disadvantages of the novel oral anticoagulants include their higher cost and lack of an effective antidote in the event of clinically significant bleeding (with the exception of idarucizumab, which was recently approved for reversal of serious bleeding associated with dabigatran), issues that may be of particular concern to older adults. While there is no therapeutic range to monitor for the newer agents, more frequent monitoring for occult anemia may be needed.
Thus, selection of an anticoagulant should also be individualized through shared decision-making.
Is aspirin alone an alternative?
And what if the patient chooses to forgo anticoagulation? In that case, aspirin 75 to 325 mg/day may seem reasonable, but there is scant evidence that aspirin is beneficial for stroke prevention in patients with atrial fibrillation in this age group, and aspirin, too, is associated with an increased risk of bleeding.25
As a result, current US and European guidelines recommend a very limited role for aspirin as a single agent in the management of atrial fibrillation.26 The joint 2014 guidelines of the American Heart Association, American College of Cardiology, and Heart Rhythm Society give aspirin a class IIB recommendation (ie, it “may” be considered), level of evidence C (ie, very limited) for use as an alternative to no antithrombotic therapy or systemic anticoagulation only in patients with a CHA2DS2-VASc score of 1, thereby excluding all patients age 75 and older.13
In most cases, aspirin as sole prophylaxis against stroke in atrial fibrillation should be avoided in the absence of another indication for its use, such as coexisting coronary artery disease or peripheral arterial disease.
A COMPLEX DECISION
In summary, the decisions surrounding anticoagulation of elderly patients with nonvalvular atrial fibrillation are complex. Accurate assessment of stroke risk is key, and although bleeding risk is also an essential consideration, it is important not to overemphasize bleeding and fall risks in the decision-making process.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285:2370–2375.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22:983–988.
- Mant J, Hobbs FD, Fletcher K, et al; BAFTA investigators; Midland Research Practices Network (MidReC). Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007; 370:493–503.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurology 2013; 70:1486–1490.
- Sardar P, Chatterjee S, Chaudhari S, Lip GY. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc 2014; 62:857–864.
- Rich MW. Atrial fibrillation in long term care. J Am Med Dir Assoc 2012; 13:688–691.
- McCrory DC, Matchar DB, Samsa G, Sanders LL, Pritchett EL. Physician attitudes about anticoagulation for nonvalvular atrial fibrillation in the elderly. Arch Intern Med 1995; 155:277–281.
- Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing 2011; 40:675–683.
- Sellers MB, Newby LK. Atrial fibrillation, anticoagulation, fall risk, and outcomes in elderly patients. Am Heart J 2011; 161:241–246.
- Bahri O, Roca F, Lechani T, et al. Underuse of oral anticoagulation for individuals with atrial fibrillation in a nursing home setting in France: comparisons of resident characteristics and physician attitude. J Am Geriatr Soc 2015; 63:71–76.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64:e1–e76.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006; 151:713–719.
- Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol 2011; 58:395–401.
- Lip GY, Clementy N, Pericart L, Banerjee A, Fauchier L. Stroke and major bleeding risk in elderly patients aged ≥ 75 years with atrial fibrillation: the Loire Valley atrial fibrillation project. Stroke 2015; 46:143–150.
- McGrath ER, Kapral MK, Fang J, et al; Investigators of the Registry of the Canadian Stroke Network. Which risk factors are more associated with ischemic stroke than intracerebral hemorrhage in patients with atrial fibrillation? Stroke 2012; 43:2048–2054.
- Phelan EA, Mahoney JE, Voit JC, Stevens JA. Assessment and management of fall risk in primary care settings. Med Clin North Am 2015; 99:281–293.
- Deandrea S, Bravi F, Turati F, Lucenteforte E, La Vecchia C, Negri E. Risk factors for falls in older people in nursing homes and hospitals. A systematic review and meta-analysis. Arch Gerontol Geriatr 2013; 56:407–415.
- Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena DS, Rich MW. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med 2005; 118:612–617.
- Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999; 159:677–685.
- Riva N, Smith DE, Lip GY, Lane DA. Advancing age and bleeding risk are the strongest barriers to anticoagulant prescription in atrial fibrillation. Age Ageing 2011; 40:653–655.
- De Caterina R, Andersson U, Alexander JH, et al; ARISTOTLE Investigators. History of bleeding and outcomes with apixaban versus warfarin in patients with atrial fibrillation in the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation trial. Am Heart J 2016; 175:175–183.
- Ben Freedman S, Gersh BJ, Lip GY. Misperceptions of aspirin efficacy and safety may perpetuate anticoagulant underutilization in atrial fibrillation. Eur Heart J 2015; 36:653–656.
- Camm AJ, Lip GY, De Caterina R, et al; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012; 33:2719–2747.
- Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001; 285:2370–2375.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991; 22:983–988.
- Mant J, Hobbs FD, Fletcher K, et al; BAFTA investigators; Midland Research Practices Network (MidReC). Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet 2007; 370:493–503.
- Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurology 2013; 70:1486–1490.
- Sardar P, Chatterjee S, Chaudhari S, Lip GY. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc 2014; 62:857–864.
- Rich MW. Atrial fibrillation in long term care. J Am Med Dir Assoc 2012; 13:688–691.
- McCrory DC, Matchar DB, Samsa G, Sanders LL, Pritchett EL. Physician attitudes about anticoagulation for nonvalvular atrial fibrillation in the elderly. Arch Intern Med 1995; 155:277–281.
- Pugh D, Pugh J, Mead GE. Attitudes of physicians regarding anticoagulation for atrial fibrillation: a systematic review. Age Ageing 2011; 40:675–683.
- Sellers MB, Newby LK. Atrial fibrillation, anticoagulation, fall risk, and outcomes in elderly patients. Am Heart J 2011; 161:241–246.
- Bahri O, Roca F, Lechani T, et al. Underuse of oral anticoagulation for individuals with atrial fibrillation in a nursing home setting in France: comparisons of resident characteristics and physician attitude. J Am Geriatr Soc 2015; 63:71–76.
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001; 285:2864–2870.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014; 64:e1–e76.
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010; 138:1093–1100.
- Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J 2006; 151:713–719.
- Fang MC, Go AS, Chang Y, et al. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J Am Coll Cardiol 2011; 58:395–401.
- Lip GY, Clementy N, Pericart L, Banerjee A, Fauchier L. Stroke and major bleeding risk in elderly patients aged ≥ 75 years with atrial fibrillation: the Loire Valley atrial fibrillation project. Stroke 2015; 46:143–150.
- McGrath ER, Kapral MK, Fang J, et al; Investigators of the Registry of the Canadian Stroke Network. Which risk factors are more associated with ischemic stroke than intracerebral hemorrhage in patients with atrial fibrillation? Stroke 2012; 43:2048–2054.
- Phelan EA, Mahoney JE, Voit JC, Stevens JA. Assessment and management of fall risk in primary care settings. Med Clin North Am 2015; 99:281–293.
- Deandrea S, Bravi F, Turati F, Lucenteforte E, La Vecchia C, Negri E. Risk factors for falls in older people in nursing homes and hospitals. A systematic review and meta-analysis. Arch Gerontol Geriatr 2013; 56:407–415.
- Gage BF, Birman-Deych E, Kerzner R, Radford MJ, Nilasena DS, Rich MW. Incidence of intracranial hemorrhage in patients with atrial fibrillation who are prone to fall. Am J Med 2005; 118:612–617.
- Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med 1999; 159:677–685.
- Riva N, Smith DE, Lip GY, Lane DA. Advancing age and bleeding risk are the strongest barriers to anticoagulant prescription in atrial fibrillation. Age Ageing 2011; 40:653–655.
- De Caterina R, Andersson U, Alexander JH, et al; ARISTOTLE Investigators. History of bleeding and outcomes with apixaban versus warfarin in patients with atrial fibrillation in the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation trial. Am Heart J 2016; 175:175–183.
- Ben Freedman S, Gersh BJ, Lip GY. Misperceptions of aspirin efficacy and safety may perpetuate anticoagulant underutilization in atrial fibrillation. Eur Heart J 2015; 36:653–656.
- Camm AJ, Lip GY, De Caterina R, et al; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012; 33:2719–2747.
KEY POINTS
- For most patients in this category, the benefits of anticoagulation outweigh the risks.
- Although they are not perfect, scoring systems have been developed to predict the risk of stroke without anticoagulation and the risk of bleeding with anticoagulation.
- The decision-making process is complex and should be shared with the patient and the patient’s family and caregivers.
January 2017 Digital Edition
Click here to access the January 2017 Digital Edition
Table of Contents
- What’s New for Federal Practitioner in 2017?
- Addressing Sexual Health With Patients
- Leadership Initiatives in Patient-Centered Transgender Care
- Patient Knowledge and Attitudes About Fecal Microbiota Therapy for Clostridium difficile Infection
- Decentralized vs Centralized Pharmacist Treatment of Patients With Atrial Fibrillation Managed With Anticoagulants
- The Importance of Subclavian Angiography in the Evaluation of Chest Pain
- Diabetes: Health Literacy Education Improves Veteran Outcomes
- Torsades de Pointes in Severe Alcohol Withdrawal and Cirrhosis: Implications for Risk Stratification and Management
- Dementia Evaluation, Management, and Outreach

Click here to access the January 2017 Digital Edition
Table of Contents
- What’s New for Federal Practitioner in 2017?
- Addressing Sexual Health With Patients
- Leadership Initiatives in Patient-Centered Transgender Care
- Patient Knowledge and Attitudes About Fecal Microbiota Therapy for Clostridium difficile Infection
- Decentralized vs Centralized Pharmacist Treatment of Patients With Atrial Fibrillation Managed With Anticoagulants
- The Importance of Subclavian Angiography in the Evaluation of Chest Pain
- Diabetes: Health Literacy Education Improves Veteran Outcomes
- Torsades de Pointes in Severe Alcohol Withdrawal and Cirrhosis: Implications for Risk Stratification and Management
- Dementia Evaluation, Management, and Outreach

Click here to access the January 2017 Digital Edition
Table of Contents
- What’s New for Federal Practitioner in 2017?
- Addressing Sexual Health With Patients
- Leadership Initiatives in Patient-Centered Transgender Care
- Patient Knowledge and Attitudes About Fecal Microbiota Therapy for Clostridium difficile Infection
- Decentralized vs Centralized Pharmacist Treatment of Patients With Atrial Fibrillation Managed With Anticoagulants
- The Importance of Subclavian Angiography in the Evaluation of Chest Pain
- Diabetes: Health Literacy Education Improves Veteran Outcomes
- Torsades de Pointes in Severe Alcohol Withdrawal and Cirrhosis: Implications for Risk Stratification and Management
- Dementia Evaluation, Management, and Outreach

2017 Directory of VA and DoD Facilities
Artificial RBCs show promise in preclinical study

Photo by Aaron Logan
SAN DIEGO—Researchers have developed artificial red blood cells (RBCs) that appear able to emulate functions of natural red blood cells (RBCs), at least in rodents.
The artificial RBCs, known as ErythroMer, are designed to be freeze-dried, stored at ambient temperatures, and reconstituted with water when needed.
If ErythroMer proves safe and effective in humans, it could represent an alternative to blood transfusions that might be useful in situations where donated blood is difficult to obtain or store.
“There are currently no simple, practical means to bring transfusion to most trauma victims outside of hospitals,” said Allan Doctor, MD, of Washington University in Saint Louis, Missouri.
“ErythroMer would be a blood substitute that a medic can carry in his or her pack and literally take it out, add water, and inject it.”
Dr Doctor presented details on ErythroMer at the 2016 ASH Annual Meeting (abstract 1027).
Design
“Due to significant advances in synthetic chemistry and nanomedicine, we’re now able to encapsulate biologics with programmable polymers to generate nanoparticles that can emulate normal cellular physiology,” Dr Doctor noted.
With ErythroMer, he and his colleagues encapsulated human hemoglobin, methylene blue, and 2,3-DPG in an amphiphilic polymer shell. The polymer and its payload components, through microfluidization, self-assemble into toroids that are about one-fiftieth the size of human RBCs.
ErythroMer is designed to be pH-responsive, so that, in areas of high pH, 2,3-DPG is sequestered in the inner surface of the particle shell and does not bind to hemoglobin. In areas of low pH, 2,3-DPG is released from the shell and binds to hemoglobin, facilitating oxygen offloading. The role of methylene blue is to inhibit auto-oxidation of hemoglobin.
The last step in synthesis of the particle is crosslinking of the surface, which neutralizes the surface charge, stabilizes the particle, and generates a selective diffusion barrier to nitric oxide. The particle can be lyophilized for extended storage and later reconstituted.
Testing
Tests showed that ErythroMer matches the oxygen binding feature of human RBCs within 10%, a level researchers say should be sufficient to stabilize a bleeding patient until a blood transfusion can be obtained.
Experiments in mice showed that ErythroMer captures oxygen in the lungs and releases it to tissues in a pattern that is indistinguishable from that seen in a control group of mice injected with their own blood.
In rats, ErythroMer effectively resuscitated animals in shock following acute loss of 40% of their blood volume.
So far, tests suggest ErythroMer has overcome barriers that halted the development of previous blood substitutes.
However, Dr Doctor noted that ErythroMer does have its weaknesses. The particles are cleared rapidly from the bloodstream (in 3 to 7 hours), and hemoglobin sourcing presents a challenge. The researchers are now exploring the possibility of using recombinant hemoglobin genetically engineered in yeast.
The team hopes to further optimize ErythroMer’s shell, extend circulation time, confirm the efficacy of ErythroMer in a larger animal model (rabbits), evaluate the impact of the product on the coagulation and immune systems, and scale up production.
If further testing goes well, the researchers estimate that ErythroMer could be ready for use by field medics and emergency responders within 10 to 12 years.
ErythroMer development has been supported by the Children’s Discovery Institute at Washington University and St. Louis Children’s Hospital, the Skandalaris Center at Washington University, and the BioSTL Fundamentals Program.
This research was funded by the National Institute of General Medical Sciences; the National Heart, Lung, and Blood Institute; the National Institute of Child Health and Human Development, the US Department of Defense; the American Heart Association; Doris Duke Foundation; and Children’s Discovery Institute. ![]()

Photo by Aaron Logan
SAN DIEGO—Researchers have developed artificial red blood cells (RBCs) that appear able to emulate functions of natural red blood cells (RBCs), at least in rodents.
The artificial RBCs, known as ErythroMer, are designed to be freeze-dried, stored at ambient temperatures, and reconstituted with water when needed.
If ErythroMer proves safe and effective in humans, it could represent an alternative to blood transfusions that might be useful in situations where donated blood is difficult to obtain or store.
“There are currently no simple, practical means to bring transfusion to most trauma victims outside of hospitals,” said Allan Doctor, MD, of Washington University in Saint Louis, Missouri.
“ErythroMer would be a blood substitute that a medic can carry in his or her pack and literally take it out, add water, and inject it.”
Dr Doctor presented details on ErythroMer at the 2016 ASH Annual Meeting (abstract 1027).
Design
“Due to significant advances in synthetic chemistry and nanomedicine, we’re now able to encapsulate biologics with programmable polymers to generate nanoparticles that can emulate normal cellular physiology,” Dr Doctor noted.
With ErythroMer, he and his colleagues encapsulated human hemoglobin, methylene blue, and 2,3-DPG in an amphiphilic polymer shell. The polymer and its payload components, through microfluidization, self-assemble into toroids that are about one-fiftieth the size of human RBCs.
ErythroMer is designed to be pH-responsive, so that, in areas of high pH, 2,3-DPG is sequestered in the inner surface of the particle shell and does not bind to hemoglobin. In areas of low pH, 2,3-DPG is released from the shell and binds to hemoglobin, facilitating oxygen offloading. The role of methylene blue is to inhibit auto-oxidation of hemoglobin.
The last step in synthesis of the particle is crosslinking of the surface, which neutralizes the surface charge, stabilizes the particle, and generates a selective diffusion barrier to nitric oxide. The particle can be lyophilized for extended storage and later reconstituted.
Testing
Tests showed that ErythroMer matches the oxygen binding feature of human RBCs within 10%, a level researchers say should be sufficient to stabilize a bleeding patient until a blood transfusion can be obtained.
Experiments in mice showed that ErythroMer captures oxygen in the lungs and releases it to tissues in a pattern that is indistinguishable from that seen in a control group of mice injected with their own blood.
In rats, ErythroMer effectively resuscitated animals in shock following acute loss of 40% of their blood volume.
So far, tests suggest ErythroMer has overcome barriers that halted the development of previous blood substitutes.
However, Dr Doctor noted that ErythroMer does have its weaknesses. The particles are cleared rapidly from the bloodstream (in 3 to 7 hours), and hemoglobin sourcing presents a challenge. The researchers are now exploring the possibility of using recombinant hemoglobin genetically engineered in yeast.
The team hopes to further optimize ErythroMer’s shell, extend circulation time, confirm the efficacy of ErythroMer in a larger animal model (rabbits), evaluate the impact of the product on the coagulation and immune systems, and scale up production.
If further testing goes well, the researchers estimate that ErythroMer could be ready for use by field medics and emergency responders within 10 to 12 years.
ErythroMer development has been supported by the Children’s Discovery Institute at Washington University and St. Louis Children’s Hospital, the Skandalaris Center at Washington University, and the BioSTL Fundamentals Program.
This research was funded by the National Institute of General Medical Sciences; the National Heart, Lung, and Blood Institute; the National Institute of Child Health and Human Development, the US Department of Defense; the American Heart Association; Doris Duke Foundation; and Children’s Discovery Institute. ![]()

Photo by Aaron Logan
SAN DIEGO—Researchers have developed artificial red blood cells (RBCs) that appear able to emulate functions of natural red blood cells (RBCs), at least in rodents.
The artificial RBCs, known as ErythroMer, are designed to be freeze-dried, stored at ambient temperatures, and reconstituted with water when needed.
If ErythroMer proves safe and effective in humans, it could represent an alternative to blood transfusions that might be useful in situations where donated blood is difficult to obtain or store.
“There are currently no simple, practical means to bring transfusion to most trauma victims outside of hospitals,” said Allan Doctor, MD, of Washington University in Saint Louis, Missouri.
“ErythroMer would be a blood substitute that a medic can carry in his or her pack and literally take it out, add water, and inject it.”
Dr Doctor presented details on ErythroMer at the 2016 ASH Annual Meeting (abstract 1027).
Design
“Due to significant advances in synthetic chemistry and nanomedicine, we’re now able to encapsulate biologics with programmable polymers to generate nanoparticles that can emulate normal cellular physiology,” Dr Doctor noted.
With ErythroMer, he and his colleagues encapsulated human hemoglobin, methylene blue, and 2,3-DPG in an amphiphilic polymer shell. The polymer and its payload components, through microfluidization, self-assemble into toroids that are about one-fiftieth the size of human RBCs.
ErythroMer is designed to be pH-responsive, so that, in areas of high pH, 2,3-DPG is sequestered in the inner surface of the particle shell and does not bind to hemoglobin. In areas of low pH, 2,3-DPG is released from the shell and binds to hemoglobin, facilitating oxygen offloading. The role of methylene blue is to inhibit auto-oxidation of hemoglobin.
The last step in synthesis of the particle is crosslinking of the surface, which neutralizes the surface charge, stabilizes the particle, and generates a selective diffusion barrier to nitric oxide. The particle can be lyophilized for extended storage and later reconstituted.
Testing
Tests showed that ErythroMer matches the oxygen binding feature of human RBCs within 10%, a level researchers say should be sufficient to stabilize a bleeding patient until a blood transfusion can be obtained.
Experiments in mice showed that ErythroMer captures oxygen in the lungs and releases it to tissues in a pattern that is indistinguishable from that seen in a control group of mice injected with their own blood.
In rats, ErythroMer effectively resuscitated animals in shock following acute loss of 40% of their blood volume.
So far, tests suggest ErythroMer has overcome barriers that halted the development of previous blood substitutes.
However, Dr Doctor noted that ErythroMer does have its weaknesses. The particles are cleared rapidly from the bloodstream (in 3 to 7 hours), and hemoglobin sourcing presents a challenge. The researchers are now exploring the possibility of using recombinant hemoglobin genetically engineered in yeast.
The team hopes to further optimize ErythroMer’s shell, extend circulation time, confirm the efficacy of ErythroMer in a larger animal model (rabbits), evaluate the impact of the product on the coagulation and immune systems, and scale up production.
If further testing goes well, the researchers estimate that ErythroMer could be ready for use by field medics and emergency responders within 10 to 12 years.
ErythroMer development has been supported by the Children’s Discovery Institute at Washington University and St. Louis Children’s Hospital, the Skandalaris Center at Washington University, and the BioSTL Fundamentals Program.
This research was funded by the National Institute of General Medical Sciences; the National Heart, Lung, and Blood Institute; the National Institute of Child Health and Human Development, the US Department of Defense; the American Heart Association; Doris Duke Foundation; and Children’s Discovery Institute. ![]()
Celebrating 10 years of GI & Hepatology News
Our January 2017 issue marks the 10-year anniversary of GI & Hepatology News (GIHN), the official newspaper of the AGA Institute. In 2007, the AGA created the newspaper with the intent to communicate current news and emerging trends and technologies in GI. I am honored to serve as the third editor of GIHN, following in the esteemed footsteps of Charles J. Lightdale MD, AGAF, and Colin W. Howden MD, AGAF, who worked diligently to establish the publication’s credibility and quality.
The January 2007 issue of GIHN featured current AGA Institute President Timothy C. Wang, MD, AGAF, on its front page. At the time, he served as the chair of the AGA Future Trends Committee, which predicted emerging forces that would alter our practice, including that computed tomographic colonography would likely become an accepted CRC screening option in a few years (the full report of the committee was published in Gastroenterology 2008:134:597-616). For our 2017 10-year anniversary, we will feature a “Flashback” column, written by myself and our associate editors, that highlights and discusses the most impactful GIHN articles from each year of the previous decade.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Our January 2017 issue marks the 10-year anniversary of GI & Hepatology News (GIHN), the official newspaper of the AGA Institute. In 2007, the AGA created the newspaper with the intent to communicate current news and emerging trends and technologies in GI. I am honored to serve as the third editor of GIHN, following in the esteemed footsteps of Charles J. Lightdale MD, AGAF, and Colin W. Howden MD, AGAF, who worked diligently to establish the publication’s credibility and quality.
The January 2007 issue of GIHN featured current AGA Institute President Timothy C. Wang, MD, AGAF, on its front page. At the time, he served as the chair of the AGA Future Trends Committee, which predicted emerging forces that would alter our practice, including that computed tomographic colonography would likely become an accepted CRC screening option in a few years (the full report of the committee was published in Gastroenterology 2008:134:597-616). For our 2017 10-year anniversary, we will feature a “Flashback” column, written by myself and our associate editors, that highlights and discusses the most impactful GIHN articles from each year of the previous decade.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Our January 2017 issue marks the 10-year anniversary of GI & Hepatology News (GIHN), the official newspaper of the AGA Institute. In 2007, the AGA created the newspaper with the intent to communicate current news and emerging trends and technologies in GI. I am honored to serve as the third editor of GIHN, following in the esteemed footsteps of Charles J. Lightdale MD, AGAF, and Colin W. Howden MD, AGAF, who worked diligently to establish the publication’s credibility and quality.
The January 2007 issue of GIHN featured current AGA Institute President Timothy C. Wang, MD, AGAF, on its front page. At the time, he served as the chair of the AGA Future Trends Committee, which predicted emerging forces that would alter our practice, including that computed tomographic colonography would likely become an accepted CRC screening option in a few years (the full report of the committee was published in Gastroenterology 2008:134:597-616). For our 2017 10-year anniversary, we will feature a “Flashback” column, written by myself and our associate editors, that highlights and discusses the most impactful GIHN articles from each year of the previous decade.
John I. Allen, MD, MBA, AGAF
Editor in Chief
AGA Clinical Practice Update: Treatment for severe alcohol hepatitis challenging
Acute alcoholic hepatitis carries a high risk of mortality, yet only a minority of patients admitted to the hospital with the condition receive appropriate treatment, said the authors of an expert review.
Writing in the January 2017 issue of Clinical Gastroenterology and Hepatology, Mack C. Mitchell Jr., MD, of the University of Texas Southwestern Medical Center, Dallas, and Craig J. McClain, MD, of the University of Louisville (Ky.), described the challenges associated with treating acute alcoholic hepatitis and its consequences.
Acute alcohol hepatitis develops in heavy drinkers and presents with rapid onset of malaise, anorexia, tender hepatomegaly, and features of the systemic inflammatory response syndrome. Patients with alcoholic hepatitis also are at high risk of nutritional deficiency, infection, acute kidney injury, and multiorgan failure.
The two most widely used therapies are glucocorticoids – generally considered the standard of care for severe alcoholic hepatitis – and the phosphodiesterase inhibitor pentoxifylline (Clin Gastroenterol Hepatol. 2017. doi: 10.1016/j.cgh.2016.08.047).
“Although in its most severe form AH has a high short-term mortality rate if untreated, in 2011, only 28% of more than 1,600 patients admitted to U.S. hospitals were treated with glucocorticoids and 17% with pentoxifylline (PTX), suggesting a lack of widespread confidence in the two most frequently used therapies for AH,” the authors wrote.
Both drugs work by addressing the underlying inflammation that plays a key role in liver injury, but the evidence for both is mixed: A 2008 Cochrane systematic review of 15 trials concluded there was no benefit from glucocorticoids, largely because of substantial variability in bias across the trials, while two meta-analyses of pentoxifylline trials concluded that there were no differences in short-term mortality between those who received it and those who did not.
Some patients are unsuitable for glucocorticoids and others may develop resistance. There is also the possibility that, while glucocorticoids may improve short-term survival, the associated increase in infection risk removes that advantage at 90 days and 1 year after diagnosis. These infections, in turn, often precede the development of acute kidney injury and multiorgan failure.
The authors, however, did suggest that the approach of very high, short-term bursts of glucocorticoids to induce “immune paralysis” – an approach taken for lupus nephritis – might be considered.
They stressed that abstinence was the cornerstone of treatment for acute alcoholic hepatitis, with studies showing that patients with alcoholic hepatitis who resume heavy drinking have significantly worse outcomes than those who don’t.
“Although abstinence is important at all stages, it is particularly important to emphasize abstinence beyond 90 days when many patients are regaining normal functioning,” Dr. Mitchell and Dr. McClain wrote.
Infection, kidney injury, and malnutrition are all significant concerns in patients with acute alcoholic hepatitis.
With respect to infection, the authors said considerable suspicion is required to pick up bacterial and fungal infections, as patients may not always have a fever and an elevated white blood cell count is an unreliable indicator. Infection also can lead to acute kidney injury.
Malnutrition is not only common in patients with alcohol hepatitis, but it has a significant negative impact on recovery. All patients should be encouraged to meet nutritional goals as early as possible, but just how to achieve this is controversial, the authors stressed.
For example, one study suggested that enteral nutrition was as good as glucocorticoids in reducing 28-day mortality, while another found enteral nutrition via nasogastric tube – in addition to glucocorticoids – was no better than glucocorticoids alone. “Whether [nasogastric] tubes should be used to provide enteral nutrition is a subject of controversy,” the authors wrote. “Normal- to high-protein diets are safe and do not increase the risk of encephalopathy in patients with AH.”
No conflicts of interest were declared.
Acute alcoholic hepatitis carries a high risk of mortality, yet only a minority of patients admitted to the hospital with the condition receive appropriate treatment, said the authors of an expert review.
Writing in the January 2017 issue of Clinical Gastroenterology and Hepatology, Mack C. Mitchell Jr., MD, of the University of Texas Southwestern Medical Center, Dallas, and Craig J. McClain, MD, of the University of Louisville (Ky.), described the challenges associated with treating acute alcoholic hepatitis and its consequences.
Acute alcohol hepatitis develops in heavy drinkers and presents with rapid onset of malaise, anorexia, tender hepatomegaly, and features of the systemic inflammatory response syndrome. Patients with alcoholic hepatitis also are at high risk of nutritional deficiency, infection, acute kidney injury, and multiorgan failure.
The two most widely used therapies are glucocorticoids – generally considered the standard of care for severe alcoholic hepatitis – and the phosphodiesterase inhibitor pentoxifylline (Clin Gastroenterol Hepatol. 2017. doi: 10.1016/j.cgh.2016.08.047).
“Although in its most severe form AH has a high short-term mortality rate if untreated, in 2011, only 28% of more than 1,600 patients admitted to U.S. hospitals were treated with glucocorticoids and 17% with pentoxifylline (PTX), suggesting a lack of widespread confidence in the two most frequently used therapies for AH,” the authors wrote.
Both drugs work by addressing the underlying inflammation that plays a key role in liver injury, but the evidence for both is mixed: A 2008 Cochrane systematic review of 15 trials concluded there was no benefit from glucocorticoids, largely because of substantial variability in bias across the trials, while two meta-analyses of pentoxifylline trials concluded that there were no differences in short-term mortality between those who received it and those who did not.
Some patients are unsuitable for glucocorticoids and others may develop resistance. There is also the possibility that, while glucocorticoids may improve short-term survival, the associated increase in infection risk removes that advantage at 90 days and 1 year after diagnosis. These infections, in turn, often precede the development of acute kidney injury and multiorgan failure.
The authors, however, did suggest that the approach of very high, short-term bursts of glucocorticoids to induce “immune paralysis” – an approach taken for lupus nephritis – might be considered.
They stressed that abstinence was the cornerstone of treatment for acute alcoholic hepatitis, with studies showing that patients with alcoholic hepatitis who resume heavy drinking have significantly worse outcomes than those who don’t.
“Although abstinence is important at all stages, it is particularly important to emphasize abstinence beyond 90 days when many patients are regaining normal functioning,” Dr. Mitchell and Dr. McClain wrote.
Infection, kidney injury, and malnutrition are all significant concerns in patients with acute alcoholic hepatitis.
With respect to infection, the authors said considerable suspicion is required to pick up bacterial and fungal infections, as patients may not always have a fever and an elevated white blood cell count is an unreliable indicator. Infection also can lead to acute kidney injury.
Malnutrition is not only common in patients with alcohol hepatitis, but it has a significant negative impact on recovery. All patients should be encouraged to meet nutritional goals as early as possible, but just how to achieve this is controversial, the authors stressed.
For example, one study suggested that enteral nutrition was as good as glucocorticoids in reducing 28-day mortality, while another found enteral nutrition via nasogastric tube – in addition to glucocorticoids – was no better than glucocorticoids alone. “Whether [nasogastric] tubes should be used to provide enteral nutrition is a subject of controversy,” the authors wrote. “Normal- to high-protein diets are safe and do not increase the risk of encephalopathy in patients with AH.”
No conflicts of interest were declared.
Acute alcoholic hepatitis carries a high risk of mortality, yet only a minority of patients admitted to the hospital with the condition receive appropriate treatment, said the authors of an expert review.
Writing in the January 2017 issue of Clinical Gastroenterology and Hepatology, Mack C. Mitchell Jr., MD, of the University of Texas Southwestern Medical Center, Dallas, and Craig J. McClain, MD, of the University of Louisville (Ky.), described the challenges associated with treating acute alcoholic hepatitis and its consequences.
Acute alcohol hepatitis develops in heavy drinkers and presents with rapid onset of malaise, anorexia, tender hepatomegaly, and features of the systemic inflammatory response syndrome. Patients with alcoholic hepatitis also are at high risk of nutritional deficiency, infection, acute kidney injury, and multiorgan failure.
The two most widely used therapies are glucocorticoids – generally considered the standard of care for severe alcoholic hepatitis – and the phosphodiesterase inhibitor pentoxifylline (Clin Gastroenterol Hepatol. 2017. doi: 10.1016/j.cgh.2016.08.047).
“Although in its most severe form AH has a high short-term mortality rate if untreated, in 2011, only 28% of more than 1,600 patients admitted to U.S. hospitals were treated with glucocorticoids and 17% with pentoxifylline (PTX), suggesting a lack of widespread confidence in the two most frequently used therapies for AH,” the authors wrote.
Both drugs work by addressing the underlying inflammation that plays a key role in liver injury, but the evidence for both is mixed: A 2008 Cochrane systematic review of 15 trials concluded there was no benefit from glucocorticoids, largely because of substantial variability in bias across the trials, while two meta-analyses of pentoxifylline trials concluded that there were no differences in short-term mortality between those who received it and those who did not.
Some patients are unsuitable for glucocorticoids and others may develop resistance. There is also the possibility that, while glucocorticoids may improve short-term survival, the associated increase in infection risk removes that advantage at 90 days and 1 year after diagnosis. These infections, in turn, often precede the development of acute kidney injury and multiorgan failure.
The authors, however, did suggest that the approach of very high, short-term bursts of glucocorticoids to induce “immune paralysis” – an approach taken for lupus nephritis – might be considered.
They stressed that abstinence was the cornerstone of treatment for acute alcoholic hepatitis, with studies showing that patients with alcoholic hepatitis who resume heavy drinking have significantly worse outcomes than those who don’t.
“Although abstinence is important at all stages, it is particularly important to emphasize abstinence beyond 90 days when many patients are regaining normal functioning,” Dr. Mitchell and Dr. McClain wrote.
Infection, kidney injury, and malnutrition are all significant concerns in patients with acute alcoholic hepatitis.
With respect to infection, the authors said considerable suspicion is required to pick up bacterial and fungal infections, as patients may not always have a fever and an elevated white blood cell count is an unreliable indicator. Infection also can lead to acute kidney injury.
Malnutrition is not only common in patients with alcohol hepatitis, but it has a significant negative impact on recovery. All patients should be encouraged to meet nutritional goals as early as possible, but just how to achieve this is controversial, the authors stressed.
For example, one study suggested that enteral nutrition was as good as glucocorticoids in reducing 28-day mortality, while another found enteral nutrition via nasogastric tube – in addition to glucocorticoids – was no better than glucocorticoids alone. “Whether [nasogastric] tubes should be used to provide enteral nutrition is a subject of controversy,” the authors wrote. “Normal- to high-protein diets are safe and do not increase the risk of encephalopathy in patients with AH.”
No conflicts of interest were declared.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY