User login
Treatment facility volume linked to survival in MM

Photo courtesy of the CDC
Patients with multiple myeloma (MM) are more likely to live longer if they are treated at a medical center where the staff has more experience with the disease, according to research published in the Journal of Clinical Oncology.
The study showed that patients treated at medical centers seeing 10 new MM patients per year had a 20% higher risk of death than patients treated at centers seeing 40 new MM patients per year.
Most cancer treatment centers in the US see fewer than 10 new MM patients per year.
“It is very difficult to be proficient when doctors are seeing only 1 or 2 new cases of multiple myeloma per year,” said study author Ronald Go, MD, of the Mayo Clinic in Rochester, Minnesota.
“Studies on cancer surgery have shown the more experience the center or practitioner has, the better the outcome. We wanted to see if volume matters when it comes to nonsurgical treatment of rare cancers such as multiple myeloma.”
To investigate, Dr Go and his colleagues used the National Cancer Database, examining outcomes for 94,722 newly diagnosed MM patients treated at 1333 facilities between 2003 and 2011.
The researchers grouped the facilities into quartiles according to the volume of MM patients treated there each year.
The mean number of MM patients treated per year was:
- Less than 3.6 for quartile 1 (Q1)
- 3.6 to 6.1 for Q2
- 6.1 to 10.3 for Q3
- More than 10.3 for Q4.
The majority of patients (60.3%) were treated in Q4 facilities. For all facilities, the median number of new MM patients per year was 6.1 (range, 3.6 to 10.3). The mean was 8.8 ± 9.9.
The researchers calculated the relationship between MM patient volume at these facilities and patient mortality, adjusting for demographic characteristics, socioeconomic factors, geographic factors, comorbidities, and year of diagnosis.
Outcomes
The unadjusted median overall survival was 26.9 months for patients treated at Q1 facilities, 29.1 months for Q2, 31.9 months for Q3, and 49.1 months for Q4 (P<0.001).
The 1-year mortality rate was 33.5% for patients treated at Q1 facilities, 32.3% for Q2, 30.7% for Q3, and 21.9% for Q4.
The researchers’ multivariable analysis showed that facility volume was independently associated with all-cause mortality.
Patients treated at the lower-quartile facilities had a higher risk of death than patients treated at Q4 facilities. The hazard ratios were 1.12 for patients at Q3 facilities, 1.12 for Q2, and 1.22 for Q1.
The researchers performed another analysis in which volume was treated as a continuous variable, and they compared various volume sizes to a reference volume of 10 patients per year.
Compared with facilities treating 10 new MM patients per year, facilities treating 20 MM patients per year had roughly 10% lower overall mortality rates.
Facilities treating 30 MM patients per year had about 15% lower mortality rates. And facilities treating 40 MM patients per year had 20% lower overall mortality rates. ![]()

Photo courtesy of the CDC
Patients with multiple myeloma (MM) are more likely to live longer if they are treated at a medical center where the staff has more experience with the disease, according to research published in the Journal of Clinical Oncology.
The study showed that patients treated at medical centers seeing 10 new MM patients per year had a 20% higher risk of death than patients treated at centers seeing 40 new MM patients per year.
Most cancer treatment centers in the US see fewer than 10 new MM patients per year.
“It is very difficult to be proficient when doctors are seeing only 1 or 2 new cases of multiple myeloma per year,” said study author Ronald Go, MD, of the Mayo Clinic in Rochester, Minnesota.
“Studies on cancer surgery have shown the more experience the center or practitioner has, the better the outcome. We wanted to see if volume matters when it comes to nonsurgical treatment of rare cancers such as multiple myeloma.”
To investigate, Dr Go and his colleagues used the National Cancer Database, examining outcomes for 94,722 newly diagnosed MM patients treated at 1333 facilities between 2003 and 2011.
The researchers grouped the facilities into quartiles according to the volume of MM patients treated there each year.
The mean number of MM patients treated per year was:
- Less than 3.6 for quartile 1 (Q1)
- 3.6 to 6.1 for Q2
- 6.1 to 10.3 for Q3
- More than 10.3 for Q4.
The majority of patients (60.3%) were treated in Q4 facilities. For all facilities, the median number of new MM patients per year was 6.1 (range, 3.6 to 10.3). The mean was 8.8 ± 9.9.
The researchers calculated the relationship between MM patient volume at these facilities and patient mortality, adjusting for demographic characteristics, socioeconomic factors, geographic factors, comorbidities, and year of diagnosis.
Outcomes
The unadjusted median overall survival was 26.9 months for patients treated at Q1 facilities, 29.1 months for Q2, 31.9 months for Q3, and 49.1 months for Q4 (P<0.001).
The 1-year mortality rate was 33.5% for patients treated at Q1 facilities, 32.3% for Q2, 30.7% for Q3, and 21.9% for Q4.
The researchers’ multivariable analysis showed that facility volume was independently associated with all-cause mortality.
Patients treated at the lower-quartile facilities had a higher risk of death than patients treated at Q4 facilities. The hazard ratios were 1.12 for patients at Q3 facilities, 1.12 for Q2, and 1.22 for Q1.
The researchers performed another analysis in which volume was treated as a continuous variable, and they compared various volume sizes to a reference volume of 10 patients per year.
Compared with facilities treating 10 new MM patients per year, facilities treating 20 MM patients per year had roughly 10% lower overall mortality rates.
Facilities treating 30 MM patients per year had about 15% lower mortality rates. And facilities treating 40 MM patients per year had 20% lower overall mortality rates. ![]()

Photo courtesy of the CDC
Patients with multiple myeloma (MM) are more likely to live longer if they are treated at a medical center where the staff has more experience with the disease, according to research published in the Journal of Clinical Oncology.
The study showed that patients treated at medical centers seeing 10 new MM patients per year had a 20% higher risk of death than patients treated at centers seeing 40 new MM patients per year.
Most cancer treatment centers in the US see fewer than 10 new MM patients per year.
“It is very difficult to be proficient when doctors are seeing only 1 or 2 new cases of multiple myeloma per year,” said study author Ronald Go, MD, of the Mayo Clinic in Rochester, Minnesota.
“Studies on cancer surgery have shown the more experience the center or practitioner has, the better the outcome. We wanted to see if volume matters when it comes to nonsurgical treatment of rare cancers such as multiple myeloma.”
To investigate, Dr Go and his colleagues used the National Cancer Database, examining outcomes for 94,722 newly diagnosed MM patients treated at 1333 facilities between 2003 and 2011.
The researchers grouped the facilities into quartiles according to the volume of MM patients treated there each year.
The mean number of MM patients treated per year was:
- Less than 3.6 for quartile 1 (Q1)
- 3.6 to 6.1 for Q2
- 6.1 to 10.3 for Q3
- More than 10.3 for Q4.
The majority of patients (60.3%) were treated in Q4 facilities. For all facilities, the median number of new MM patients per year was 6.1 (range, 3.6 to 10.3). The mean was 8.8 ± 9.9.
The researchers calculated the relationship between MM patient volume at these facilities and patient mortality, adjusting for demographic characteristics, socioeconomic factors, geographic factors, comorbidities, and year of diagnosis.
Outcomes
The unadjusted median overall survival was 26.9 months for patients treated at Q1 facilities, 29.1 months for Q2, 31.9 months for Q3, and 49.1 months for Q4 (P<0.001).
The 1-year mortality rate was 33.5% for patients treated at Q1 facilities, 32.3% for Q2, 30.7% for Q3, and 21.9% for Q4.
The researchers’ multivariable analysis showed that facility volume was independently associated with all-cause mortality.
Patients treated at the lower-quartile facilities had a higher risk of death than patients treated at Q4 facilities. The hazard ratios were 1.12 for patients at Q3 facilities, 1.12 for Q2, and 1.22 for Q1.
The researchers performed another analysis in which volume was treated as a continuous variable, and they compared various volume sizes to a reference volume of 10 patients per year.
Compared with facilities treating 10 new MM patients per year, facilities treating 20 MM patients per year had roughly 10% lower overall mortality rates.
Facilities treating 30 MM patients per year had about 15% lower mortality rates. And facilities treating 40 MM patients per year had 20% lower overall mortality rates. ![]()
Agent could treat hemophilia A and B, team says

A new bypassing agent mimics the pro-clotting activity of factor V Leiden and might prove effective for treating hemophilia A and B, according to preclinical research published in Blood.
“We know that patients who have severe hemophilia and also have mutations that increase clotting, such as factor V Leiden, experience less severe bleeding,” said study author Trevor Baglin, MD, of Addenbrooke’s Hospital in Cambridge, UK.
In patients with factor V Leiden, defects in the anticoagulant activated protein C (APC) mechanism lead to an overactive production of thrombin.
The researchers set out to determine if they could exploit this phenomenon to treat hemophilia by developing a direct inhibitor of APC. They modified serine protease inhibitors, known as serpins, to make them specific and efficient inhibitors of APC.
“We hypothesized that if we targeted the protein C pathway we could prolong thrombin production,” said study author James Huntington, PhD, of the University of Cambridge in the UK.
“We engineered a serpin so that it could selectively prevent APC from shutting down thrombin production before the formation of a stable clot.”
The researchers administered the serpin to mice with hemophilia B and clipped their tails. In this model, the blood loss decreased as the dose increased, with the highest dose reducing bleeding to the level of healthy mice.
Further injury models underscored that the serpin helped the majority of mice form stable clots, with higher doses resulting in quicker clot formation.
The serpin was also able to accelerate clot formation when added to blood samples from patients with hemophilia A.
“It is our understanding that because we are targeting a general anticlotting process, our serpin could effectively treat patients with either hemophilia A or B, including those who develop inhibitors to more traditional therapy,” Dr Huntington said.
“Additionally, we have focused on engineering the serpin to be both subcutaneously delivered and long-acting. This will free patients from the cumbersome thrice-weekly infusions that are necessary under many contemporary therapy regimens.”
“Within 3 years, we hope to be conducting our first-in-man trials of a subcutaneously administered form of our serpin,” Dr Baglin added.
“It is important to remember that the majority of people in the world with hemophilia have no access to therapy. A stable, subcutaneous, long-acting, effective hemostatic agent could bring treatment to a great deal many more hemophilia sufferers.”
This study forms part of a patent application by the authors, and the serpin is being developed into a therapeutic by a start-up company known as ApcinteX, with funding from Medicxi. ![]()

A new bypassing agent mimics the pro-clotting activity of factor V Leiden and might prove effective for treating hemophilia A and B, according to preclinical research published in Blood.
“We know that patients who have severe hemophilia and also have mutations that increase clotting, such as factor V Leiden, experience less severe bleeding,” said study author Trevor Baglin, MD, of Addenbrooke’s Hospital in Cambridge, UK.
In patients with factor V Leiden, defects in the anticoagulant activated protein C (APC) mechanism lead to an overactive production of thrombin.
The researchers set out to determine if they could exploit this phenomenon to treat hemophilia by developing a direct inhibitor of APC. They modified serine protease inhibitors, known as serpins, to make them specific and efficient inhibitors of APC.
“We hypothesized that if we targeted the protein C pathway we could prolong thrombin production,” said study author James Huntington, PhD, of the University of Cambridge in the UK.
“We engineered a serpin so that it could selectively prevent APC from shutting down thrombin production before the formation of a stable clot.”
The researchers administered the serpin to mice with hemophilia B and clipped their tails. In this model, the blood loss decreased as the dose increased, with the highest dose reducing bleeding to the level of healthy mice.
Further injury models underscored that the serpin helped the majority of mice form stable clots, with higher doses resulting in quicker clot formation.
The serpin was also able to accelerate clot formation when added to blood samples from patients with hemophilia A.
“It is our understanding that because we are targeting a general anticlotting process, our serpin could effectively treat patients with either hemophilia A or B, including those who develop inhibitors to more traditional therapy,” Dr Huntington said.
“Additionally, we have focused on engineering the serpin to be both subcutaneously delivered and long-acting. This will free patients from the cumbersome thrice-weekly infusions that are necessary under many contemporary therapy regimens.”
“Within 3 years, we hope to be conducting our first-in-man trials of a subcutaneously administered form of our serpin,” Dr Baglin added.
“It is important to remember that the majority of people in the world with hemophilia have no access to therapy. A stable, subcutaneous, long-acting, effective hemostatic agent could bring treatment to a great deal many more hemophilia sufferers.”
This study forms part of a patent application by the authors, and the serpin is being developed into a therapeutic by a start-up company known as ApcinteX, with funding from Medicxi. ![]()

A new bypassing agent mimics the pro-clotting activity of factor V Leiden and might prove effective for treating hemophilia A and B, according to preclinical research published in Blood.
“We know that patients who have severe hemophilia and also have mutations that increase clotting, such as factor V Leiden, experience less severe bleeding,” said study author Trevor Baglin, MD, of Addenbrooke’s Hospital in Cambridge, UK.
In patients with factor V Leiden, defects in the anticoagulant activated protein C (APC) mechanism lead to an overactive production of thrombin.
The researchers set out to determine if they could exploit this phenomenon to treat hemophilia by developing a direct inhibitor of APC. They modified serine protease inhibitors, known as serpins, to make them specific and efficient inhibitors of APC.
“We hypothesized that if we targeted the protein C pathway we could prolong thrombin production,” said study author James Huntington, PhD, of the University of Cambridge in the UK.
“We engineered a serpin so that it could selectively prevent APC from shutting down thrombin production before the formation of a stable clot.”
The researchers administered the serpin to mice with hemophilia B and clipped their tails. In this model, the blood loss decreased as the dose increased, with the highest dose reducing bleeding to the level of healthy mice.
Further injury models underscored that the serpin helped the majority of mice form stable clots, with higher doses resulting in quicker clot formation.
The serpin was also able to accelerate clot formation when added to blood samples from patients with hemophilia A.
“It is our understanding that because we are targeting a general anticlotting process, our serpin could effectively treat patients with either hemophilia A or B, including those who develop inhibitors to more traditional therapy,” Dr Huntington said.
“Additionally, we have focused on engineering the serpin to be both subcutaneously delivered and long-acting. This will free patients from the cumbersome thrice-weekly infusions that are necessary under many contemporary therapy regimens.”
“Within 3 years, we hope to be conducting our first-in-man trials of a subcutaneously administered form of our serpin,” Dr Baglin added.
“It is important to remember that the majority of people in the world with hemophilia have no access to therapy. A stable, subcutaneous, long-acting, effective hemostatic agent could bring treatment to a great deal many more hemophilia sufferers.”
This study forms part of a patent application by the authors, and the serpin is being developed into a therapeutic by a start-up company known as ApcinteX, with funding from Medicxi. ![]()
NORD publishes physician guide to CTCL

mycosis fungoides
The National Organization for Rare Disorders (NORD) has published a guide for physicians treating patients with cutaneous T-cell lymphoma (CTCL).
The guide contains information about disease classification, signs and symptoms of CTCL, methods of diagnosing the disease, standard therapies, and investigational therapies for CTCL.
The guide also includes a list of resources for physicians and patients.
“The NORD Physician Guide to Cutaneous T-Cell Lymphoma (CTCL)” is available for free on the NORD Physician Guides website.
The guide was made possible by an educational grant from Therakos, now a part of Mallinckrodt Pharmaceuticals.
The guide was developed in collaboration with Oleg E. Akilov, MD, PhD, of the University of Pittsburgh School of Medicine in Pennsylvania.
“Eczema and even some cases of psoriasis may look very similar to mycosis fungoides, the most common type of cutaneous T-cell lymphomas,” Dr Akilov noted.
“It is important to be aware of these similarities and to be ready to think about cutaneous lymphoma when a patient with ‘common dermatosis’ does not respond to regular treatments.”
About NORD guides
NORD established its physician guide series as part of a broader strategic initiative to promote earlier diagnosis and state-of-the-art care for people with rare diseases. Each online guide is written or reviewed by a medical professional with expertise on the topic.
Other recent guides in the series include:
- The NORD Physician Guide to Mitochondrial Myopathies
- The NORD Physician Guide to Paroxysmal Nocturnal Hemoglobinuria (PNH)
- The NORD Physician Guide to Atypical Hemolytic Uremic Syndrome (aHUS)
- The NORD Physician Guide to Nontuberculous Mycobacterial Lung Disease.
“People who have rare diseases often go for many years without a diagnosis,” said Marsha Lanes, a genetic counselor in NORD’s Educational Initiatives Department.
“The purpose of NORD’s free online physician guides is to reduce the time to diagnosis and encourage optimal treatment for patients with little-known and little-understood rare diseases.” ![]()

mycosis fungoides
The National Organization for Rare Disorders (NORD) has published a guide for physicians treating patients with cutaneous T-cell lymphoma (CTCL).
The guide contains information about disease classification, signs and symptoms of CTCL, methods of diagnosing the disease, standard therapies, and investigational therapies for CTCL.
The guide also includes a list of resources for physicians and patients.
“The NORD Physician Guide to Cutaneous T-Cell Lymphoma (CTCL)” is available for free on the NORD Physician Guides website.
The guide was made possible by an educational grant from Therakos, now a part of Mallinckrodt Pharmaceuticals.
The guide was developed in collaboration with Oleg E. Akilov, MD, PhD, of the University of Pittsburgh School of Medicine in Pennsylvania.
“Eczema and even some cases of psoriasis may look very similar to mycosis fungoides, the most common type of cutaneous T-cell lymphomas,” Dr Akilov noted.
“It is important to be aware of these similarities and to be ready to think about cutaneous lymphoma when a patient with ‘common dermatosis’ does not respond to regular treatments.”
About NORD guides
NORD established its physician guide series as part of a broader strategic initiative to promote earlier diagnosis and state-of-the-art care for people with rare diseases. Each online guide is written or reviewed by a medical professional with expertise on the topic.
Other recent guides in the series include:
- The NORD Physician Guide to Mitochondrial Myopathies
- The NORD Physician Guide to Paroxysmal Nocturnal Hemoglobinuria (PNH)
- The NORD Physician Guide to Atypical Hemolytic Uremic Syndrome (aHUS)
- The NORD Physician Guide to Nontuberculous Mycobacterial Lung Disease.
“People who have rare diseases often go for many years without a diagnosis,” said Marsha Lanes, a genetic counselor in NORD’s Educational Initiatives Department.
“The purpose of NORD’s free online physician guides is to reduce the time to diagnosis and encourage optimal treatment for patients with little-known and little-understood rare diseases.” ![]()

mycosis fungoides
The National Organization for Rare Disorders (NORD) has published a guide for physicians treating patients with cutaneous T-cell lymphoma (CTCL).
The guide contains information about disease classification, signs and symptoms of CTCL, methods of diagnosing the disease, standard therapies, and investigational therapies for CTCL.
The guide also includes a list of resources for physicians and patients.
“The NORD Physician Guide to Cutaneous T-Cell Lymphoma (CTCL)” is available for free on the NORD Physician Guides website.
The guide was made possible by an educational grant from Therakos, now a part of Mallinckrodt Pharmaceuticals.
The guide was developed in collaboration with Oleg E. Akilov, MD, PhD, of the University of Pittsburgh School of Medicine in Pennsylvania.
“Eczema and even some cases of psoriasis may look very similar to mycosis fungoides, the most common type of cutaneous T-cell lymphomas,” Dr Akilov noted.
“It is important to be aware of these similarities and to be ready to think about cutaneous lymphoma when a patient with ‘common dermatosis’ does not respond to regular treatments.”
About NORD guides
NORD established its physician guide series as part of a broader strategic initiative to promote earlier diagnosis and state-of-the-art care for people with rare diseases. Each online guide is written or reviewed by a medical professional with expertise on the topic.
Other recent guides in the series include:
- The NORD Physician Guide to Mitochondrial Myopathies
- The NORD Physician Guide to Paroxysmal Nocturnal Hemoglobinuria (PNH)
- The NORD Physician Guide to Atypical Hemolytic Uremic Syndrome (aHUS)
- The NORD Physician Guide to Nontuberculous Mycobacterial Lung Disease.
“People who have rare diseases often go for many years without a diagnosis,” said Marsha Lanes, a genetic counselor in NORD’s Educational Initiatives Department.
“The purpose of NORD’s free online physician guides is to reduce the time to diagnosis and encourage optimal treatment for patients with little-known and little-understood rare diseases.” ![]()
Explaining the development of MPNs, leukemia
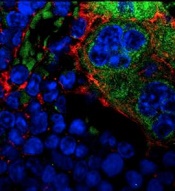
MSPCs with mutant PTPN11
(red) and monocytes (green).
Image courtesy of
Dong et al, Nature 2016
New research published in Nature has shown how certain mutations drive the development of myeloproliferative neoplasms (MPNs) and leukemia.
Investigators discovered that PTPN11 activating mutations promote the development and progression of MPNs through “profound detrimental effects” on hematopoietic stem cells (HSCs).
The team also identified a potential method of treating MPNs in patients with Noonan syndrome.
Noonan syndrome can be caused by mutations in several genes, but the most common is PTPN11. Children with Noonan syndrome are known to have an increased risk of developing MPNs/leukemia.
Previous research had established that mutations in PTPN11 have a conventional cell-autonomous effect on HSC growth.
In the current study, investigators showed that PTPN11 mutations also affect mesenchymal stem/progenitor cells (MSPCs) and osteoprogenitors.
The mutations cause over-production of the CC chemokine CCL3, which attracts monocytes into the HSCs’ niches. The monocytes make inflammatory molecules that stimulate the HSCs to differentiate and proliferate, leading to MPNs and leukemia.
“We have identified CCL3 as a potential therapeutic target for controlling leukemic progression in Noonan syndrome and for improving stem cell transplantation therapy in Noonan syndrome-associated leukemias,” said study author Cheng-Kui Qu, MD, PhD, of Emory University School of Medicine in Atlanta, Georgia.
Dr Qu and his colleagues began this research intending to investigate the effects of PTPN11 mutations in the nervous system. The team developed genetically engineered mice that had altered PTPN11 in neural cells.
The mice all developed a condition resembling an MPN at an early age. It turned out that the mice had changes in the PTPN11 gene in their MSPCs and osteoprogenitors (in addition to their neural cells) but not in their HSCs.
The investigators found the MPN in these PTPN11-mutant mice can be treated in the short-term by HSC transplant, but the condition comes back within months.
However, drugs counteracting CCL3 successfully reversed MPN phenotypes. One of the drugs is the CCR5 antagonist maraviroc, which is approved in the US to combat HIV infection, and another is the CCR1 antagonist BX471.
The investigators noted that other Noonan syndrome mutations, in genes besides PTPN11, need to be assessed for their effects on MPN/leukemia formation. ![]()

MSPCs with mutant PTPN11
(red) and monocytes (green).
Image courtesy of
Dong et al, Nature 2016
New research published in Nature has shown how certain mutations drive the development of myeloproliferative neoplasms (MPNs) and leukemia.
Investigators discovered that PTPN11 activating mutations promote the development and progression of MPNs through “profound detrimental effects” on hematopoietic stem cells (HSCs).
The team also identified a potential method of treating MPNs in patients with Noonan syndrome.
Noonan syndrome can be caused by mutations in several genes, but the most common is PTPN11. Children with Noonan syndrome are known to have an increased risk of developing MPNs/leukemia.
Previous research had established that mutations in PTPN11 have a conventional cell-autonomous effect on HSC growth.
In the current study, investigators showed that PTPN11 mutations also affect mesenchymal stem/progenitor cells (MSPCs) and osteoprogenitors.
The mutations cause over-production of the CC chemokine CCL3, which attracts monocytes into the HSCs’ niches. The monocytes make inflammatory molecules that stimulate the HSCs to differentiate and proliferate, leading to MPNs and leukemia.
“We have identified CCL3 as a potential therapeutic target for controlling leukemic progression in Noonan syndrome and for improving stem cell transplantation therapy in Noonan syndrome-associated leukemias,” said study author Cheng-Kui Qu, MD, PhD, of Emory University School of Medicine in Atlanta, Georgia.
Dr Qu and his colleagues began this research intending to investigate the effects of PTPN11 mutations in the nervous system. The team developed genetically engineered mice that had altered PTPN11 in neural cells.
The mice all developed a condition resembling an MPN at an early age. It turned out that the mice had changes in the PTPN11 gene in their MSPCs and osteoprogenitors (in addition to their neural cells) but not in their HSCs.
The investigators found the MPN in these PTPN11-mutant mice can be treated in the short-term by HSC transplant, but the condition comes back within months.
However, drugs counteracting CCL3 successfully reversed MPN phenotypes. One of the drugs is the CCR5 antagonist maraviroc, which is approved in the US to combat HIV infection, and another is the CCR1 antagonist BX471.
The investigators noted that other Noonan syndrome mutations, in genes besides PTPN11, need to be assessed for their effects on MPN/leukemia formation. ![]()

MSPCs with mutant PTPN11
(red) and monocytes (green).
Image courtesy of
Dong et al, Nature 2016
New research published in Nature has shown how certain mutations drive the development of myeloproliferative neoplasms (MPNs) and leukemia.
Investigators discovered that PTPN11 activating mutations promote the development and progression of MPNs through “profound detrimental effects” on hematopoietic stem cells (HSCs).
The team also identified a potential method of treating MPNs in patients with Noonan syndrome.
Noonan syndrome can be caused by mutations in several genes, but the most common is PTPN11. Children with Noonan syndrome are known to have an increased risk of developing MPNs/leukemia.
Previous research had established that mutations in PTPN11 have a conventional cell-autonomous effect on HSC growth.
In the current study, investigators showed that PTPN11 mutations also affect mesenchymal stem/progenitor cells (MSPCs) and osteoprogenitors.
The mutations cause over-production of the CC chemokine CCL3, which attracts monocytes into the HSCs’ niches. The monocytes make inflammatory molecules that stimulate the HSCs to differentiate and proliferate, leading to MPNs and leukemia.
“We have identified CCL3 as a potential therapeutic target for controlling leukemic progression in Noonan syndrome and for improving stem cell transplantation therapy in Noonan syndrome-associated leukemias,” said study author Cheng-Kui Qu, MD, PhD, of Emory University School of Medicine in Atlanta, Georgia.
Dr Qu and his colleagues began this research intending to investigate the effects of PTPN11 mutations in the nervous system. The team developed genetically engineered mice that had altered PTPN11 in neural cells.
The mice all developed a condition resembling an MPN at an early age. It turned out that the mice had changes in the PTPN11 gene in their MSPCs and osteoprogenitors (in addition to their neural cells) but not in their HSCs.
The investigators found the MPN in these PTPN11-mutant mice can be treated in the short-term by HSC transplant, but the condition comes back within months.
However, drugs counteracting CCL3 successfully reversed MPN phenotypes. One of the drugs is the CCR5 antagonist maraviroc, which is approved in the US to combat HIV infection, and another is the CCR1 antagonist BX471.
The investigators noted that other Noonan syndrome mutations, in genes besides PTPN11, need to be assessed for their effects on MPN/leukemia formation. ![]()
Telehealth has value in Parkinson’s and poststroke care
BALTIMORE – The broadening of access to medical care through telemedicine that’s been occurring for acute neurologic conditions such as stroke has begun to expand to care for more chronic conditions such as Parkinson’s disease and poststroke recovery, according to presentations given at the annual meeting of the American Neurological Association.
Telemedicine care for patients with Parkinson’s appears feasible and acceptable to both patients and clinicians alike, based on recent findings. Ray Dorsey, MD, of the University of Rochester (N.Y.) initiated the Connect.Parkinson study with his colleagues in 2014 to compare usual care enhanced with educational materials against others who received usual care, educational materials, and four virtual sessions with a Parkinson’s disease specialist from 1 of 18 neurology centers nationwide. Some of the participants who lived far away from one of these centers would not otherwise have received such specialized care (Telemed J E Health. 2016;22[7]:590-8).
The participating physicians had concerns about the quality of the video connection, but otherwise were satisfied with the care delivered to the patients. Surveys of participants revealed no differences between the two groups in quality of life and quality of care. About 80% of those who received virtual calls preferred this contact to the regular office visits.
The development of smartphone apps that allow aspects of diseases like Parkinson’s to be monitored are also enabling high-quality, diagnostic telecare. A pilot study of an Android smartphone Parkinson’s disease app by Dr. Dorsey and his colleagues demonstrated its utility in tests of voice, postural sway, gait, finger tapping, and reaction time (Parkinsonism Relat Disord. 2015;21[6]:650-3).
Since that study was completed, an iOS smartphone version of the app, called mPower, has been developed and has enrolled many Parkinson’s disease patients. The technology is being tested to virtually gauge Parkinson’s disease symptoms and the effects of medications on them. This has opened the door to the world of virtual clinical trials and longitudinal studies targeting genetic subpopulations, Dr. Dorsey said at the meeting.
The telehealth portion of COMPASS – in the form of regular phone calls and web-based feedback – enables better stroke care following hospital discharge by keeping track of common complications that are partly responsible for a readmittance rate of around 25% within 90 days of hospital discharge and tracking physiological aspects like blood pressure, diabetes, diet, exercise, and smoking.
The pilot demonstration of the potential of the program was pivotal in securing funding for a cluster-randomized pragmatic trial. The trial will randomize hospitals to normal discharge or discharge followed by regular poststroke contact. Patient functional status at 90 days post stroke will be assessed for 1 year. After the year, the hospitals randomized to COMPASS care delivery will continue this care, and the hospitals offering normal discharge will also adopt this poststroke service care. Patient outcome will be followed for another year.
The anticipated patient enrollment is 5,400. Results from the first year of the 2-year study are expected in Spring 2018. “COMPASS will have an impact on the post-acute stroke care pathway. After evaluating the effectiveness, the goal is to disseminate and scale to other settings,” Dr. Bushnell said at the meeting.
Dr. Dorsey receives research support from Excellus BlueCross BlueShield, Google, and the Verizon Foundation. He has received compensation for consulting services for Medtronic and owns stock options in ConsultingMD. Dr. Bushnell acknowledged salary support from the COMPASS program, which receives funding from the Patient-Centered Outcomes Research Institute.
BALTIMORE – The broadening of access to medical care through telemedicine that’s been occurring for acute neurologic conditions such as stroke has begun to expand to care for more chronic conditions such as Parkinson’s disease and poststroke recovery, according to presentations given at the annual meeting of the American Neurological Association.
Telemedicine care for patients with Parkinson’s appears feasible and acceptable to both patients and clinicians alike, based on recent findings. Ray Dorsey, MD, of the University of Rochester (N.Y.) initiated the Connect.Parkinson study with his colleagues in 2014 to compare usual care enhanced with educational materials against others who received usual care, educational materials, and four virtual sessions with a Parkinson’s disease specialist from 1 of 18 neurology centers nationwide. Some of the participants who lived far away from one of these centers would not otherwise have received such specialized care (Telemed J E Health. 2016;22[7]:590-8).
The participating physicians had concerns about the quality of the video connection, but otherwise were satisfied with the care delivered to the patients. Surveys of participants revealed no differences between the two groups in quality of life and quality of care. About 80% of those who received virtual calls preferred this contact to the regular office visits.
The development of smartphone apps that allow aspects of diseases like Parkinson’s to be monitored are also enabling high-quality, diagnostic telecare. A pilot study of an Android smartphone Parkinson’s disease app by Dr. Dorsey and his colleagues demonstrated its utility in tests of voice, postural sway, gait, finger tapping, and reaction time (Parkinsonism Relat Disord. 2015;21[6]:650-3).
Since that study was completed, an iOS smartphone version of the app, called mPower, has been developed and has enrolled many Parkinson’s disease patients. The technology is being tested to virtually gauge Parkinson’s disease symptoms and the effects of medications on them. This has opened the door to the world of virtual clinical trials and longitudinal studies targeting genetic subpopulations, Dr. Dorsey said at the meeting.
The telehealth portion of COMPASS – in the form of regular phone calls and web-based feedback – enables better stroke care following hospital discharge by keeping track of common complications that are partly responsible for a readmittance rate of around 25% within 90 days of hospital discharge and tracking physiological aspects like blood pressure, diabetes, diet, exercise, and smoking.
The pilot demonstration of the potential of the program was pivotal in securing funding for a cluster-randomized pragmatic trial. The trial will randomize hospitals to normal discharge or discharge followed by regular poststroke contact. Patient functional status at 90 days post stroke will be assessed for 1 year. After the year, the hospitals randomized to COMPASS care delivery will continue this care, and the hospitals offering normal discharge will also adopt this poststroke service care. Patient outcome will be followed for another year.
The anticipated patient enrollment is 5,400. Results from the first year of the 2-year study are expected in Spring 2018. “COMPASS will have an impact on the post-acute stroke care pathway. After evaluating the effectiveness, the goal is to disseminate and scale to other settings,” Dr. Bushnell said at the meeting.
Dr. Dorsey receives research support from Excellus BlueCross BlueShield, Google, and the Verizon Foundation. He has received compensation for consulting services for Medtronic and owns stock options in ConsultingMD. Dr. Bushnell acknowledged salary support from the COMPASS program, which receives funding from the Patient-Centered Outcomes Research Institute.
BALTIMORE – The broadening of access to medical care through telemedicine that’s been occurring for acute neurologic conditions such as stroke has begun to expand to care for more chronic conditions such as Parkinson’s disease and poststroke recovery, according to presentations given at the annual meeting of the American Neurological Association.
Telemedicine care for patients with Parkinson’s appears feasible and acceptable to both patients and clinicians alike, based on recent findings. Ray Dorsey, MD, of the University of Rochester (N.Y.) initiated the Connect.Parkinson study with his colleagues in 2014 to compare usual care enhanced with educational materials against others who received usual care, educational materials, and four virtual sessions with a Parkinson’s disease specialist from 1 of 18 neurology centers nationwide. Some of the participants who lived far away from one of these centers would not otherwise have received such specialized care (Telemed J E Health. 2016;22[7]:590-8).
The participating physicians had concerns about the quality of the video connection, but otherwise were satisfied with the care delivered to the patients. Surveys of participants revealed no differences between the two groups in quality of life and quality of care. About 80% of those who received virtual calls preferred this contact to the regular office visits.
The development of smartphone apps that allow aspects of diseases like Parkinson’s to be monitored are also enabling high-quality, diagnostic telecare. A pilot study of an Android smartphone Parkinson’s disease app by Dr. Dorsey and his colleagues demonstrated its utility in tests of voice, postural sway, gait, finger tapping, and reaction time (Parkinsonism Relat Disord. 2015;21[6]:650-3).
Since that study was completed, an iOS smartphone version of the app, called mPower, has been developed and has enrolled many Parkinson’s disease patients. The technology is being tested to virtually gauge Parkinson’s disease symptoms and the effects of medications on them. This has opened the door to the world of virtual clinical trials and longitudinal studies targeting genetic subpopulations, Dr. Dorsey said at the meeting.
The telehealth portion of COMPASS – in the form of regular phone calls and web-based feedback – enables better stroke care following hospital discharge by keeping track of common complications that are partly responsible for a readmittance rate of around 25% within 90 days of hospital discharge and tracking physiological aspects like blood pressure, diabetes, diet, exercise, and smoking.
The pilot demonstration of the potential of the program was pivotal in securing funding for a cluster-randomized pragmatic trial. The trial will randomize hospitals to normal discharge or discharge followed by regular poststroke contact. Patient functional status at 90 days post stroke will be assessed for 1 year. After the year, the hospitals randomized to COMPASS care delivery will continue this care, and the hospitals offering normal discharge will also adopt this poststroke service care. Patient outcome will be followed for another year.
The anticipated patient enrollment is 5,400. Results from the first year of the 2-year study are expected in Spring 2018. “COMPASS will have an impact on the post-acute stroke care pathway. After evaluating the effectiveness, the goal is to disseminate and scale to other settings,” Dr. Bushnell said at the meeting.
Dr. Dorsey receives research support from Excellus BlueCross BlueShield, Google, and the Verizon Foundation. He has received compensation for consulting services for Medtronic and owns stock options in ConsultingMD. Dr. Bushnell acknowledged salary support from the COMPASS program, which receives funding from the Patient-Centered Outcomes Research Institute.
EXPERT ANALYSIS FROM ANA 2016
Genetic Variations May Affect Vitamin D Level and MS Relapse Rate
BALTIMORE—A genetic scoring system for identifying individuals at high risk for low vitamin D levels also detects patients with multiple sclerosis (MS) who have an increased risk for relapse, according to a multicenter cohort study. The findings could have clinical significance in MS treatment and patient counseling, said Jennifer S. Graves, MD, PhD, Assistant Professor of Neurology at the University of California, San Francisco, at the 141st Annual Meeting of the American Neurological Association.
Low vitamin D levels are associated with an increased risk of MS, but whether this association is causal has not been determined. Dr. Graves and her colleagues sought to gain insight into the association by focusing on 29 single nucleotide polymorphisms (SNPs) within genes that previously had been discovered to be involved in the manufacture of 25-OH vitamin D. The investigators analyzed the relationship of these SNPs to relapses in patients with MS.
They compared the SNP profiles of 181 patients with MS or high-risk clinically isolated syndrome (the discovery cohort) with those of a replication cohort of 110 patients of comparable age, race, and median vitamin D serum level. Patients in the discovery cohort were enrolled at two pediatric MS centers in California between 2006 and 2011, and those in the replication cohort were enrolled at nine MS centers in the United States from 2011 to 2015.
Three of the SNPs were strongly associated with the vitamin D levels in the discovery cohort after a statistical correction that revealed individual influences of genes among the 29 different mutations. The researchers used these three SNPs to generate risk scores for vitamin D levels. The lowest and highest risk scores had linear associations with vitamin D levels. The highest scores were associated with serum vitamin D levels that were nearly 15 ng/mL lower in the discovery and replication cohorts. The risk of MS relapse for individuals with the highest risk score in the discovery cohort was nearly twice as high as it was for individuals with the lowest risk score.
“A genetic score of three functional SNPs captures risk of low vitamin D level and identifies those who may be at risk of relapse related to this risk factor. These findings support a causal association of vitamin D with relapse rate,” Dr. Graves said.
The study may potentially be important beyond MS. “This risk score may also have some utility in other disease states where vitamin D deficiency may be contributing to disease course,” she said.
The study was funded by the Race to Erase MS, the National MS Society, and the National Institute of Neurological Disorders and Stroke.
—Brian Hoyle
BALTIMORE—A genetic scoring system for identifying individuals at high risk for low vitamin D levels also detects patients with multiple sclerosis (MS) who have an increased risk for relapse, according to a multicenter cohort study. The findings could have clinical significance in MS treatment and patient counseling, said Jennifer S. Graves, MD, PhD, Assistant Professor of Neurology at the University of California, San Francisco, at the 141st Annual Meeting of the American Neurological Association.
Low vitamin D levels are associated with an increased risk of MS, but whether this association is causal has not been determined. Dr. Graves and her colleagues sought to gain insight into the association by focusing on 29 single nucleotide polymorphisms (SNPs) within genes that previously had been discovered to be involved in the manufacture of 25-OH vitamin D. The investigators analyzed the relationship of these SNPs to relapses in patients with MS.
They compared the SNP profiles of 181 patients with MS or high-risk clinically isolated syndrome (the discovery cohort) with those of a replication cohort of 110 patients of comparable age, race, and median vitamin D serum level. Patients in the discovery cohort were enrolled at two pediatric MS centers in California between 2006 and 2011, and those in the replication cohort were enrolled at nine MS centers in the United States from 2011 to 2015.
Three of the SNPs were strongly associated with the vitamin D levels in the discovery cohort after a statistical correction that revealed individual influences of genes among the 29 different mutations. The researchers used these three SNPs to generate risk scores for vitamin D levels. The lowest and highest risk scores had linear associations with vitamin D levels. The highest scores were associated with serum vitamin D levels that were nearly 15 ng/mL lower in the discovery and replication cohorts. The risk of MS relapse for individuals with the highest risk score in the discovery cohort was nearly twice as high as it was for individuals with the lowest risk score.
“A genetic score of three functional SNPs captures risk of low vitamin D level and identifies those who may be at risk of relapse related to this risk factor. These findings support a causal association of vitamin D with relapse rate,” Dr. Graves said.
The study may potentially be important beyond MS. “This risk score may also have some utility in other disease states where vitamin D deficiency may be contributing to disease course,” she said.
The study was funded by the Race to Erase MS, the National MS Society, and the National Institute of Neurological Disorders and Stroke.
—Brian Hoyle
BALTIMORE—A genetic scoring system for identifying individuals at high risk for low vitamin D levels also detects patients with multiple sclerosis (MS) who have an increased risk for relapse, according to a multicenter cohort study. The findings could have clinical significance in MS treatment and patient counseling, said Jennifer S. Graves, MD, PhD, Assistant Professor of Neurology at the University of California, San Francisco, at the 141st Annual Meeting of the American Neurological Association.
Low vitamin D levels are associated with an increased risk of MS, but whether this association is causal has not been determined. Dr. Graves and her colleagues sought to gain insight into the association by focusing on 29 single nucleotide polymorphisms (SNPs) within genes that previously had been discovered to be involved in the manufacture of 25-OH vitamin D. The investigators analyzed the relationship of these SNPs to relapses in patients with MS.
They compared the SNP profiles of 181 patients with MS or high-risk clinically isolated syndrome (the discovery cohort) with those of a replication cohort of 110 patients of comparable age, race, and median vitamin D serum level. Patients in the discovery cohort were enrolled at two pediatric MS centers in California between 2006 and 2011, and those in the replication cohort were enrolled at nine MS centers in the United States from 2011 to 2015.
Three of the SNPs were strongly associated with the vitamin D levels in the discovery cohort after a statistical correction that revealed individual influences of genes among the 29 different mutations. The researchers used these three SNPs to generate risk scores for vitamin D levels. The lowest and highest risk scores had linear associations with vitamin D levels. The highest scores were associated with serum vitamin D levels that were nearly 15 ng/mL lower in the discovery and replication cohorts. The risk of MS relapse for individuals with the highest risk score in the discovery cohort was nearly twice as high as it was for individuals with the lowest risk score.
“A genetic score of three functional SNPs captures risk of low vitamin D level and identifies those who may be at risk of relapse related to this risk factor. These findings support a causal association of vitamin D with relapse rate,” Dr. Graves said.
The study may potentially be important beyond MS. “This risk score may also have some utility in other disease states where vitamin D deficiency may be contributing to disease course,” she said.
The study was funded by the Race to Erase MS, the National MS Society, and the National Institute of Neurological Disorders and Stroke.
—Brian Hoyle
Early Epilepsy Increases Risk of Later Comorbid ADHD in Autism
VIENNA—Early-onset idiopathic epilepsy occurring before age 7 nearly doubles the likelihood that a child with autism spectrum disorder (ASD) will later develop comorbid ADHD, reported Johnny Downs, MD, a child psychiatrist at King’s College in London, at the 29th Annual Congress of the European College of Neuropsychopharmacology.
Comorbid ADHD is common in the setting of ASD. In a search for risk factors for the comorbid condition, Dr. Downs and his colleagues reviewed the physical health records prior to age 7 of 3,032 patients with ASD referred at ages 3 to 17 to child and adolescent mental health services clinics serving South London. “That’s information that often doesn’t make it into the clinical psychiatric record,” said Dr. Downs.
Half of the 3,032 subjects were diagnosed with ASD at ages 6 to 12 and another 39% at ages 13 to 17. During five years of prospective follow-up after being diagnosed with ASD, 25.5% of patients were diagnosed with comorbid ADHD. When reviewing early physical health records, researchers observed that 114 (3.76%) of study participants had experienced early-onset epilepsy before age 7.
A large sample size allowed for robust multivariate adjustment for potential confounders. In a multivariate analysis, patients with ASD and a history of early-onset epilepsy were at a significant 1.75-fold increased risk for subsequent comorbid ADHD. The analysis was adjusted for family history of epilepsy, sociodemographic factors, intellectual disability, previous head injury, perinatal complications, CNS tumors, early meningitis, and other confounders.
Compared with white subjects with ASD, the risk of developing comorbid ADHD was reduced by 37% in black patients and by 52% in Asian patients with ASD.
“The take-home message would be if you’ve got social and communication difficulties in a young child appearing at the age of 5, 6, or 7, and there’s a history of seizures, we are seeing from observational data that the child is at increased risk of ADHD over the age of 7,” said Dr. Downs.
—Bruce Jancin
VIENNA—Early-onset idiopathic epilepsy occurring before age 7 nearly doubles the likelihood that a child with autism spectrum disorder (ASD) will later develop comorbid ADHD, reported Johnny Downs, MD, a child psychiatrist at King’s College in London, at the 29th Annual Congress of the European College of Neuropsychopharmacology.
Comorbid ADHD is common in the setting of ASD. In a search for risk factors for the comorbid condition, Dr. Downs and his colleagues reviewed the physical health records prior to age 7 of 3,032 patients with ASD referred at ages 3 to 17 to child and adolescent mental health services clinics serving South London. “That’s information that often doesn’t make it into the clinical psychiatric record,” said Dr. Downs.
Half of the 3,032 subjects were diagnosed with ASD at ages 6 to 12 and another 39% at ages 13 to 17. During five years of prospective follow-up after being diagnosed with ASD, 25.5% of patients were diagnosed with comorbid ADHD. When reviewing early physical health records, researchers observed that 114 (3.76%) of study participants had experienced early-onset epilepsy before age 7.
A large sample size allowed for robust multivariate adjustment for potential confounders. In a multivariate analysis, patients with ASD and a history of early-onset epilepsy were at a significant 1.75-fold increased risk for subsequent comorbid ADHD. The analysis was adjusted for family history of epilepsy, sociodemographic factors, intellectual disability, previous head injury, perinatal complications, CNS tumors, early meningitis, and other confounders.
Compared with white subjects with ASD, the risk of developing comorbid ADHD was reduced by 37% in black patients and by 52% in Asian patients with ASD.
“The take-home message would be if you’ve got social and communication difficulties in a young child appearing at the age of 5, 6, or 7, and there’s a history of seizures, we are seeing from observational data that the child is at increased risk of ADHD over the age of 7,” said Dr. Downs.
—Bruce Jancin
VIENNA—Early-onset idiopathic epilepsy occurring before age 7 nearly doubles the likelihood that a child with autism spectrum disorder (ASD) will later develop comorbid ADHD, reported Johnny Downs, MD, a child psychiatrist at King’s College in London, at the 29th Annual Congress of the European College of Neuropsychopharmacology.
Comorbid ADHD is common in the setting of ASD. In a search for risk factors for the comorbid condition, Dr. Downs and his colleagues reviewed the physical health records prior to age 7 of 3,032 patients with ASD referred at ages 3 to 17 to child and adolescent mental health services clinics serving South London. “That’s information that often doesn’t make it into the clinical psychiatric record,” said Dr. Downs.
Half of the 3,032 subjects were diagnosed with ASD at ages 6 to 12 and another 39% at ages 13 to 17. During five years of prospective follow-up after being diagnosed with ASD, 25.5% of patients were diagnosed with comorbid ADHD. When reviewing early physical health records, researchers observed that 114 (3.76%) of study participants had experienced early-onset epilepsy before age 7.
A large sample size allowed for robust multivariate adjustment for potential confounders. In a multivariate analysis, patients with ASD and a history of early-onset epilepsy were at a significant 1.75-fold increased risk for subsequent comorbid ADHD. The analysis was adjusted for family history of epilepsy, sociodemographic factors, intellectual disability, previous head injury, perinatal complications, CNS tumors, early meningitis, and other confounders.
Compared with white subjects with ASD, the risk of developing comorbid ADHD was reduced by 37% in black patients and by 52% in Asian patients with ASD.
“The take-home message would be if you’ve got social and communication difficulties in a young child appearing at the age of 5, 6, or 7, and there’s a history of seizures, we are seeing from observational data that the child is at increased risk of ADHD over the age of 7,” said Dr. Downs.
—Bruce Jancin
Mefloquine labeling falls short on adverse reaction recommendations
The current labeling for the antimalarial mefloquine is inconsistent internationally with medication guides regarding certain adverse reactions, including depression and anxiety, according to a review of drug labels and medication guides from six English-speaking countries.
Neuropsychiatric reactions have been reported by 29% to 77% of mefloquine users at prophylactic doses of 250 mg per week, wrote Remington L. Nevin, MD, MPH, of Johns Hopkins University in Baltimore, and Aricia M. Byrd, an MD student at Trinity School of Medicine, Kingstown, St. Vincent and the Grenadines. “Neuropsychiatric adverse reactions may occur early during use – frequently within the first three doses – and may even occur after only a single dose,” the researchers said (Neurol Ther. 2016 Jun;5[1]:69-83).
In addition, data suggest that the neuropsychiatric adverse reactions, including nightmares and cognitive dysfunction, can last many years after the drug has been discontinued, and a black box warning was added to the U.S. label in 2013 to emphasize the potential long-term impact, Dr. Nevin and Ms. Byrd reported.
In this study, Dr. Nevin and Ms. Byrd compared prescribing information and patient safety guidance in the United States and five other English-speaking countries: the United Kingdom, Ireland, Australia, Canada, and New Zealand.
At the time of the study, mefloquine was licensed in all six countries, but the innovator product was withdrawn from the United States in 2011 and from Canada in 2013.
In addition to the United States, the United Kingdom and Ireland recommended discontinuing mefloquine at the onset of any general neurologic or psychiatric symptoms, the researchers noted.
All six countries were in complete agreement with corresponding medication guides and drug labeling that recommended discontinuing mefloquine or consulting a healthcare provider if adverse reactions occurred within four high level group terms (HLGTs): anxiety disorders and symptoms, changes in physical activity, depressed mood disorders and disturbances, and deliria (including confusion).
Three of the six countries (the United States, the United Kingdom, and Ireland) show partial agreement in medication guides and drug labeling recommendations to discontinue the drug or consult a healthcare provider in the instance of three other HLGTs: disturbances in thinking and perception, personality disorders and disturbances in behavior, and suicidal and self-injurious behaviors not elsewhere classified. The United Kingdom and Ireland also showed partial agreement in corresponding medication guides and drug labeling, drug discontinuation, or consulting a healthcare provider for the following adverse reactions: neuromuscular disorders, sleep disorders and disturbances, and peripheral neuropathies.
In the United States alone, medication guides and drug labeling corresponded in terms of drug discontinuation or healthcare provider consultation in cases of cranial nerve disorders, excluding neoplasms and neurologic disorders not elsewhere classified. For nine other areas of adverse reactions, medication guidelines recommended healthcare provider consultation, but no corresponding guidance was found on the drug labeling.
The review was limited by several factors, including the use of data from only six countries and the subjective interpretation of the language used in the drug labeling and medication guides, the researchers noted.
However, the results “suggest opportunities for physicians in these countries to improve patient counseling by specifically emphasizing the need to discontinue at the onset of these adverse reactions,” they said.
“The results of this analysis also suggest opportunities for international drug regulators to clarify language in future updates to remaining mefloquine drug labels and medication guides to better reflect national risk-benefit considerations for continued use of the drug,” they concluded.
Dr. Nevin disclosed that he has been retained as consultant and expert witness in legal cases involving antimalarial drug toxicity claims. Ms. Byrd had no financial conflicts to disclose.
“The big picture is that it is critically important to fund research on mefloquine, because there has been relatively little postmarketing surveillance,” said Col. (Ret.) Elspeth Cameron Ritchie, MD, MPH, a forensic psychiatrist with expertise in military and veterans’ issues. Although the risk of neuropsychiatric side effects associated with mefloquine has been known, it has become more recognized in the past 15 years in the United States, she said.
“Dr. Nevin has really been a leader in this area for 10 years; he was a major force in putting the black box warning on this drug that led to the U.S. military dramatically decreasing their use of it,” Dr. Ritchie said.
As for further research, Dr. Ritchie said, “I think we need a good definition of mefloquine toxicity. We need to determine the best medication to treat the neuropsychiatric side effects and determine what part of the brain is affected.”
A systematic review of the side effects also is needed to help determine treatment, she added.
Dr. Ritchie retired from the U.S. Army in 2010 after serving for 24 years and holding many leadership positions, including chief of psychiatry. Currently, Dr. Ritchie is chief of mental health for the community-based outpatient clinics at the Washington VA Medical Center. She also serves as professor of psychiatry at the Uniformed Services University of the Health Sciences in Bethesda, Md., and at Georgetown University and Howard University, both in Washington. She had no relevant financial conflicts to disclose.
“The big picture is that it is critically important to fund research on mefloquine, because there has been relatively little postmarketing surveillance,” said Col. (Ret.) Elspeth Cameron Ritchie, MD, MPH, a forensic psychiatrist with expertise in military and veterans’ issues. Although the risk of neuropsychiatric side effects associated with mefloquine has been known, it has become more recognized in the past 15 years in the United States, she said.
“Dr. Nevin has really been a leader in this area for 10 years; he was a major force in putting the black box warning on this drug that led to the U.S. military dramatically decreasing their use of it,” Dr. Ritchie said.
As for further research, Dr. Ritchie said, “I think we need a good definition of mefloquine toxicity. We need to determine the best medication to treat the neuropsychiatric side effects and determine what part of the brain is affected.”
A systematic review of the side effects also is needed to help determine treatment, she added.
Dr. Ritchie retired from the U.S. Army in 2010 after serving for 24 years and holding many leadership positions, including chief of psychiatry. Currently, Dr. Ritchie is chief of mental health for the community-based outpatient clinics at the Washington VA Medical Center. She also serves as professor of psychiatry at the Uniformed Services University of the Health Sciences in Bethesda, Md., and at Georgetown University and Howard University, both in Washington. She had no relevant financial conflicts to disclose.
“The big picture is that it is critically important to fund research on mefloquine, because there has been relatively little postmarketing surveillance,” said Col. (Ret.) Elspeth Cameron Ritchie, MD, MPH, a forensic psychiatrist with expertise in military and veterans’ issues. Although the risk of neuropsychiatric side effects associated with mefloquine has been known, it has become more recognized in the past 15 years in the United States, she said.
“Dr. Nevin has really been a leader in this area for 10 years; he was a major force in putting the black box warning on this drug that led to the U.S. military dramatically decreasing their use of it,” Dr. Ritchie said.
As for further research, Dr. Ritchie said, “I think we need a good definition of mefloquine toxicity. We need to determine the best medication to treat the neuropsychiatric side effects and determine what part of the brain is affected.”
A systematic review of the side effects also is needed to help determine treatment, she added.
Dr. Ritchie retired from the U.S. Army in 2010 after serving for 24 years and holding many leadership positions, including chief of psychiatry. Currently, Dr. Ritchie is chief of mental health for the community-based outpatient clinics at the Washington VA Medical Center. She also serves as professor of psychiatry at the Uniformed Services University of the Health Sciences in Bethesda, Md., and at Georgetown University and Howard University, both in Washington. She had no relevant financial conflicts to disclose.
The current labeling for the antimalarial mefloquine is inconsistent internationally with medication guides regarding certain adverse reactions, including depression and anxiety, according to a review of drug labels and medication guides from six English-speaking countries.
Neuropsychiatric reactions have been reported by 29% to 77% of mefloquine users at prophylactic doses of 250 mg per week, wrote Remington L. Nevin, MD, MPH, of Johns Hopkins University in Baltimore, and Aricia M. Byrd, an MD student at Trinity School of Medicine, Kingstown, St. Vincent and the Grenadines. “Neuropsychiatric adverse reactions may occur early during use – frequently within the first three doses – and may even occur after only a single dose,” the researchers said (Neurol Ther. 2016 Jun;5[1]:69-83).
In addition, data suggest that the neuropsychiatric adverse reactions, including nightmares and cognitive dysfunction, can last many years after the drug has been discontinued, and a black box warning was added to the U.S. label in 2013 to emphasize the potential long-term impact, Dr. Nevin and Ms. Byrd reported.
In this study, Dr. Nevin and Ms. Byrd compared prescribing information and patient safety guidance in the United States and five other English-speaking countries: the United Kingdom, Ireland, Australia, Canada, and New Zealand.
At the time of the study, mefloquine was licensed in all six countries, but the innovator product was withdrawn from the United States in 2011 and from Canada in 2013.
In addition to the United States, the United Kingdom and Ireland recommended discontinuing mefloquine at the onset of any general neurologic or psychiatric symptoms, the researchers noted.
All six countries were in complete agreement with corresponding medication guides and drug labeling that recommended discontinuing mefloquine or consulting a healthcare provider if adverse reactions occurred within four high level group terms (HLGTs): anxiety disorders and symptoms, changes in physical activity, depressed mood disorders and disturbances, and deliria (including confusion).
Three of the six countries (the United States, the United Kingdom, and Ireland) show partial agreement in medication guides and drug labeling recommendations to discontinue the drug or consult a healthcare provider in the instance of three other HLGTs: disturbances in thinking and perception, personality disorders and disturbances in behavior, and suicidal and self-injurious behaviors not elsewhere classified. The United Kingdom and Ireland also showed partial agreement in corresponding medication guides and drug labeling, drug discontinuation, or consulting a healthcare provider for the following adverse reactions: neuromuscular disorders, sleep disorders and disturbances, and peripheral neuropathies.
In the United States alone, medication guides and drug labeling corresponded in terms of drug discontinuation or healthcare provider consultation in cases of cranial nerve disorders, excluding neoplasms and neurologic disorders not elsewhere classified. For nine other areas of adverse reactions, medication guidelines recommended healthcare provider consultation, but no corresponding guidance was found on the drug labeling.
The review was limited by several factors, including the use of data from only six countries and the subjective interpretation of the language used in the drug labeling and medication guides, the researchers noted.
However, the results “suggest opportunities for physicians in these countries to improve patient counseling by specifically emphasizing the need to discontinue at the onset of these adverse reactions,” they said.
“The results of this analysis also suggest opportunities for international drug regulators to clarify language in future updates to remaining mefloquine drug labels and medication guides to better reflect national risk-benefit considerations for continued use of the drug,” they concluded.
Dr. Nevin disclosed that he has been retained as consultant and expert witness in legal cases involving antimalarial drug toxicity claims. Ms. Byrd had no financial conflicts to disclose.
The current labeling for the antimalarial mefloquine is inconsistent internationally with medication guides regarding certain adverse reactions, including depression and anxiety, according to a review of drug labels and medication guides from six English-speaking countries.
Neuropsychiatric reactions have been reported by 29% to 77% of mefloquine users at prophylactic doses of 250 mg per week, wrote Remington L. Nevin, MD, MPH, of Johns Hopkins University in Baltimore, and Aricia M. Byrd, an MD student at Trinity School of Medicine, Kingstown, St. Vincent and the Grenadines. “Neuropsychiatric adverse reactions may occur early during use – frequently within the first three doses – and may even occur after only a single dose,” the researchers said (Neurol Ther. 2016 Jun;5[1]:69-83).
In addition, data suggest that the neuropsychiatric adverse reactions, including nightmares and cognitive dysfunction, can last many years after the drug has been discontinued, and a black box warning was added to the U.S. label in 2013 to emphasize the potential long-term impact, Dr. Nevin and Ms. Byrd reported.
In this study, Dr. Nevin and Ms. Byrd compared prescribing information and patient safety guidance in the United States and five other English-speaking countries: the United Kingdom, Ireland, Australia, Canada, and New Zealand.
At the time of the study, mefloquine was licensed in all six countries, but the innovator product was withdrawn from the United States in 2011 and from Canada in 2013.
In addition to the United States, the United Kingdom and Ireland recommended discontinuing mefloquine at the onset of any general neurologic or psychiatric symptoms, the researchers noted.
All six countries were in complete agreement with corresponding medication guides and drug labeling that recommended discontinuing mefloquine or consulting a healthcare provider if adverse reactions occurred within four high level group terms (HLGTs): anxiety disorders and symptoms, changes in physical activity, depressed mood disorders and disturbances, and deliria (including confusion).
Three of the six countries (the United States, the United Kingdom, and Ireland) show partial agreement in medication guides and drug labeling recommendations to discontinue the drug or consult a healthcare provider in the instance of three other HLGTs: disturbances in thinking and perception, personality disorders and disturbances in behavior, and suicidal and self-injurious behaviors not elsewhere classified. The United Kingdom and Ireland also showed partial agreement in corresponding medication guides and drug labeling, drug discontinuation, or consulting a healthcare provider for the following adverse reactions: neuromuscular disorders, sleep disorders and disturbances, and peripheral neuropathies.
In the United States alone, medication guides and drug labeling corresponded in terms of drug discontinuation or healthcare provider consultation in cases of cranial nerve disorders, excluding neoplasms and neurologic disorders not elsewhere classified. For nine other areas of adverse reactions, medication guidelines recommended healthcare provider consultation, but no corresponding guidance was found on the drug labeling.
The review was limited by several factors, including the use of data from only six countries and the subjective interpretation of the language used in the drug labeling and medication guides, the researchers noted.
However, the results “suggest opportunities for physicians in these countries to improve patient counseling by specifically emphasizing the need to discontinue at the onset of these adverse reactions,” they said.
“The results of this analysis also suggest opportunities for international drug regulators to clarify language in future updates to remaining mefloquine drug labels and medication guides to better reflect national risk-benefit considerations for continued use of the drug,” they concluded.
Dr. Nevin disclosed that he has been retained as consultant and expert witness in legal cases involving antimalarial drug toxicity claims. Ms. Byrd had no financial conflicts to disclose.
FROM NEUROLOGY AND THERAPY
Key clinical point: Drug labeling and medication guides are inconsistent in some aspects of recommendations to discontinue use of the antimalarial drug mefloquine in cases of certain neuropsychiatric adverse reactions.
Major finding: All six countries were in complete agreement with corresponding medication guides and drug labeling that recommended discontinuing mefloquine or consulting a healthcare provider if adverse reactions occurred within four high level group terms (HLGTs): anxiety disorders and symptoms, changes in physical activity, depressed mood disorders and disturbances, and deliria (including confusion).
Data source: A review of drug labeling and medication guides in six countries: the United States, the United Kingdom, Ireland, Australia, Canada, and New Zealand.
Disclosures: Dr. Nevin disclosed that he has been retained as consultant and expert witness in legal cases involving antimalarial drug toxicity claims. Ms. Byrd had no financial conflicts to disclose.
Launching a quality improvement initiative
This article by Adam Weizman and colleagues is the first of a three-part series that will provide practical advice for practices that wish to develop a quality initiative. The first article, “Launching a quality improvement initiative” describes the infrastructure, personnel, and structure needed to approach an identified problem within a practice (variability in adenoma detection rates). This case-based approach helps us understand the step-by-step approach needed to reduce variability and improve quality. The authors present a plan (road map) in a straightforward and practical way that seems simple, but if followed carefully, leads to success. These articles are rich in resources and link to state-of-the-art advice.
John I. Allen, MD, MBA, AGAF, Special Section Editor
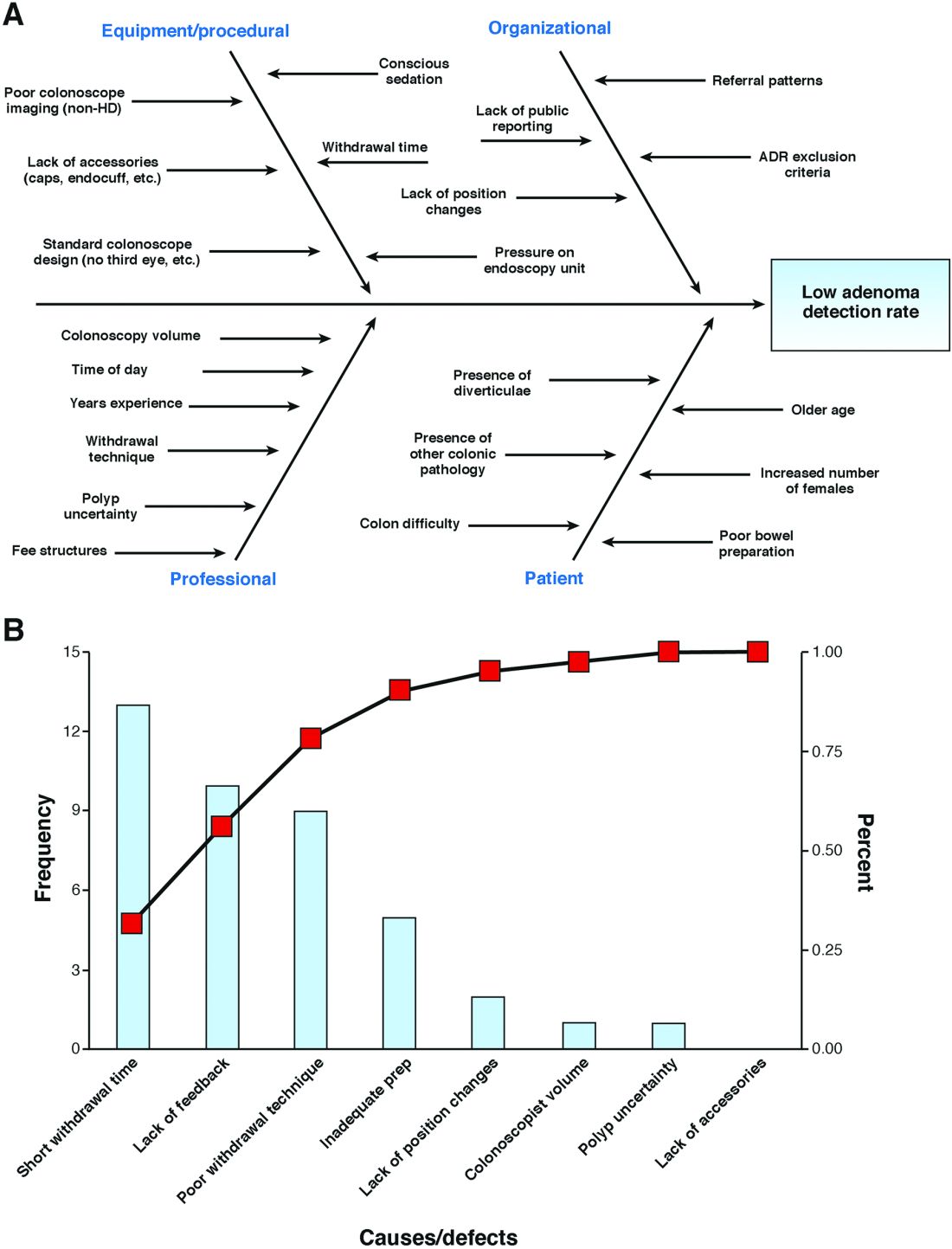
There has been increasing focus on measuring quality indicators in gastroenterology over the past few years. The adenoma detection rate (ADR) has emerged as one of the most important quality indicators because it is supported by robust clinical evidence.1-3 With every 1% increase in ADR, a 3% reduction in interval colorectal cancer has been noted.3 As such, an ADR of 25% has been designated as an important quality target for all endoscopists who perform colorectal cancer screening.1
You work at a community hospital in a large, metropolitan area. Your colleagues in a number of other departments across your hospital have been increasingly interested in quality improvement (QI) and have launched QI interventions, although none in your department. Moreover, there have been reforms in how hospital endoscopy units are funded in your jurisdiction, with a move toward volume-based funding with a quality overlay. In an effort to improve efficiency and better characterize performance, the hospital has been auditing the performance of all endoscopists at your institution over the past year. Among the eight endoscopists who work at your hospital, the overall ADR has been found to be 19%, decreasing to less than the generally accepted benchmark.1
In response to the results of the audit in your unit, you decide that you would like to develop an initiative to improve your group’s ADR.
Forming a quality improvement team
The first step in any QI project is to establish an improvement team. This working group consists of individuals with specific roles who perform interdependent tasks and share a common goal.4 Usually, frontline health care workers who are impacted most by the quality-of-care problem form the foundation of the team. A team lead is identified who will oversee the project. Content experts are also helpful members of the team who may have particular expertise in the clinical domain that will be the focus of the project. In addition, an improvement adviser, an individual with some expertise in QI, is needed on the team. This adviser may be from within your department or from outside. Although they may not possess expertise in the clinical problem you are trying to tackle, they should have skills in QI methodology and process to aid the team. An executive sponsor also needs to be identified. This should be an influential and well-respected individual who holds a senior administrative position at your institution who can help the team overcome barriers and secure resources. Physician engagement is a critical, often-overlooked step in any improvement effort. Regardless of the initiative, physicians continue to have tremendous influence over hospital-based outcomes.5 Identifying a physician champion, a prominent and respected physician at your organization to help spread the importance of your efforts and create a burning platform for change, is helpful. It also is valuable to have a patient on the improvement team to provide unique perspectives that only the end user of health care can convey and to ensure that the project is patient centered, as all improvement efforts should be.6
Improvement framework
Before starting any improvement effort, there are several important considerations that need to be addressed when choosing a quality improvement target.7 It is important to have a good understanding of the burden and severity of the problem. This often requires audit and measuring. For example, although we may think there is a problem with ADR in our endoscopy unit based on a general impression, it is critical to have data to support this suspicion. This is part of a current state analysis (discussed later). It also is important to select a quality-of-care problem that is under you or your group’s direct control. For example, it would be difficult to initiate a quality improvement project aimed at changing the practice of radiology reporting as a gastroenterologist. It is important to pick a problem that is focused and within a narrow scope that is feasible to address and then improve. Consideration of the unintended consequences of an improvement initiative often is overlooked, but needs to be considered because not all that comes out of quality improvement efforts is good. Finally, the likelihood of success of a quality initiative is increased significantly if it can generate momentum and lead to other interventions both within your department and beyond.
There are several specific improvement frameworks that can be used by a team to address a quality-of-care problem and perform a quality improvement project. The framework chosen depends on the type of problem that is being targeted and the training of the individuals on the improvement team. Three of the most commonly used improvement frameworks include the following: 1) Six Sigma; 2) Lean; and 3) Model for Improvement.
Six Sigma
Six Sigma is focused on improvement by reducing variability.8 It is a highly analytic framework relying on statistical analysis and mathematical modeling. It is best suited for projects in which the root cause and contributors to the target problem remain unclear and the aim of the intervention is to reduce variation.
Lean
Lean emphasizes improvement through elimination of waste and classifies all parts of any process as value added and nonvalue added.9 It is estimated that 95% of activities in any health care process are nonvalue added and the objective of Lean is to identify opportunities to simplify and create efficiencies. It is best suited for target problems that directly can be observed and mapped out, for example, process of care, flow, and efficiency of an endoscopy unit.
Model for Improvement
The Model for Improvement has been popularized by the Institute for Healthcare Improvement.10,11 It is well suited for health care teams, and its advantages are its adaptability to many improvement targets and lack of extensive training, consultant support, or statistical training as required by the previous frameworks mentioned earlier. As a result, it is the most commonly used improvement framework.
Using the Model for Improvement
The Model for Improvement is organized around three main questions: 1) What are we trying to accomplish? 2) How will we know that a change is an improvement? and 3) What changes can result in improvement?
Question 1: What are we trying to accomplish?
The first stage using the Model for Improvement is developing a clear project aim. A good aim statement should be specific in defining what measures one is hoping to improve and setting a concrete deadline by which to achieve it.10,11 It should answer the questions of what the team is trying to improve, by how much, and by what date. It is more effective for the target to be an ambitious, stretch goal to ensure the effort is worth the resources and time that will be invested by the team. Not only does a good aim statement serve as the foundation for the project, but it can redirect the team if the improvement effort is getting off track. In the earlier example of improving ADR, an aim statement could be “to increase the ADR of all endoscopists who perform colonoscopy at your hospital to 25% over a 12-month period.”
Question 2: How will we know that a change is an improvement?
This step involves defining measures that will allow you to understand if changes implemented are impacting the system within which your target problem resides and if this represents an improvement. This usually involves continuous, real-time measurement. Outcome measures are clinically relevant outcomes and are the ultimate goal of what the project team is trying to accomplish. In the example of ADR, this could be the proportion of endoscopists at your institution with an ADR greater than 25%. Process measures are relevant to the system within which you are working and your target problem resides. Typically, the intervention that you implement will have impact that is measurable much earlier by process outcomes than outcomes measures, which are usually a downstream effect. As such, an improvement project still may be a success if it shows improvements in process measures only. For example, the proportion of endoscopists measuring withdrawal time would be a process measure in an intervention aimed at improving ADR. In time, improvement in process measures may translate to improvements in the outcome measure. Balancing measures are indicators of unintended consequences of the project. Not all that comes from an improvement effort is necessarily positive. If improvements in certain process measures come at the cost of harms shown by the balancing measures, such as deterioration in staff satisfaction or increase in time per procedure, the improvement project may not be worth continuing.
Importance of understanding the target problem: Current-state analysis
In contrast to classic enumerative research in which the clinical environment can be well controlled, quality improvement work focuses on sampling and intervening upon a less controlled and dynamic process or system with the intent of improving it.10 Just as treatment strategies in clinical medicine are based on diagnostic testing, so too in quality improvement work, the strategy of diagnosing the current state allows for linking the root cause of quality problems with solutions that can induce positive change.
Several common diagnostic tools are used to identify root causes of quality and safety issues. These include the following: 1) process mapping, 2) cause-and-effect diagrams, and 3) Pareto charts.
Process mapping
Process maps are tools used to understand the system that is being studied. A process map is a graphic depiction of the flow through a process, which creates a collaborative awareness of the current state and identifies opportunities for improvement. It is important that multiple individuals who have knowledge of the process in question are involved in its creation. Process maps are created by first establishing the start and end of the process. Second, the high-level steps are included. Third, a more detailed set of steps can be included within each of the high-level steps.
Cause-and-effect diagrams
Cause-and-effect diagrams, also known as Ishikawa or fishbone diagrams, are helpful brainstorming tools used to graphically display and explore potential causes of a target problem. They illustrate that there often are many contributing factors to one underlying problem and the relationship between contributing factors. Classic examples of categories include equipment, environment, materials, methods and process, people, and measurement.10 Figure 1 provides an example of these tools in an effort to improve ADR.
To identify the most important contributors to the target problem and thus where to focus improvement efforts, a Pareto chart, a bar graph that places all defects/causes in the order of the frequency in which they occur, is constructed. The x-axis is a list of possible defects (Figure 1). The y-axis is the frequency with which any one defect is occurring, and the third (x-2) axis is the cumulative frequency. In theory, it is expected that there will be a vital few defects that account for 80% of all occurrences (referred to by some as the 80:20 rule).10, 11 Populating this graph requires measurement, which, as discussed earlier, is the key to understanding any problem. Measurement can be accomplished through direct observation/audit, chart review, and/or multivoting.
Question 3: What changes can result in improvement?
Once the improvement team has defined an aim and established its family of measures, it is time to develop and implement an intervention. Rather than investing time and resources into one intervention that may or may not be successful, it is preferable to perform small change cycles in which the intervention is conducted on a small scale, refined, and either repeated or changed. As a result, most quality improvement projects consist of an iterative process. The Model for Improvement defines four steps that allow the improvement team to perform this: Plan, do, study, act (PDSA).4,10,11 The first two questions listed earlier allowed the improvement team to plan the intervention. The next step, do, involves implementing your project on a small scale, thereby testing your change while collecting continuous measurements. Study involves interpreting your data using both conventional methods and several improvement-specific methods (discussed later) that help answer the question of how will we know that a change is improvement? Finally, act involves making a conclusion about your first PDSA cycle, helping to inform subsequent cycles. This results in a series of small, rapid cycle changes, one building on the next, that lead to implementation of change(s) that ultimately serve to address your improvement problem and your project aim.
A change concept is an approach known to be useful in developing specific changes that result in improvement. Change concepts are used as a starting point to generate change ideas. A number of change concepts spanning nine main categories have been defined by the Associates for Process Improvement,10 including eliminating waste, improving work flow, managing variation, and designing systems to prevent error. For the purpose of improving ADR, your team may choose a few change concepts and ideas based on the diagnostic work-up. For example, the change concept of designing the system to prevent errors through standardizing withdrawal time for all physicians may lead to an improvement in ADR. This then is linked to the change idea of audible timers placed in endoscopy suites to ensure longer withdrawal times.12 The impact of this change would be measured and the next cycle would build on these results.
Summary and next steps
In this first article of the series, the QI team moved forward with their aim to increase ADR. A root cause analysis was undertaken using multiple diagnostic tools including a fishbone diagram and a Pareto chart. Finally, change ideas were generated based on the earlier-described root causes and established change concepts. The next steps involve undertaking PDSA cycles to test change ideas and monitor for improvement.
References
1. Rex, D.K., Schoenfeld, P.S., Cohen, J. et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81:31-53.
2. Rex, D.K., Bond, J.H., Winawer, S. et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296-308.
3. Corley, D., Jensen, C.D., Marks, A.R. et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-306.
4. Kotter, J.P. Leading change. Harvard Business Review Press, Boston; 2012
5. Taitz, J.M., Lee, T.H., and Sequist, T.D. A framework for engaging physicians in quality and safety. BMJ Qual Saf. 2012;21:722-8.
6. Carman, K.L., Dardess, P., Maurer, M. et al. Patient and family engagement: a framework for understanding the elements and developing interventions and policies. Health Aff (Millwood). 2013;33:223-31.
7.Ranji, S.R. and Shojania, S.G. Implementing patient safety interventions in your hospital: what to try and what to avoid. Med Clin North Am. 2008;92:275-93.
8. Antony, J. Six Sigma vs Lean: some perspectives from leading academics and practitioners. Int J Product Perform Manage. 2011;60:185-90.
9. Bercaw, R. Taking improvement from the assembly line to healthcare: the application of lean within the healthcare industry. Taylor and Francis, Boca Raton, FL; 2012
10. Langley, G.J., Nolan, K.M., Nolan, T.W. et al. The improvement guide: a practical approach to enhancing organizational performance. Jossey-Bass, San Francisco; 2009
11. Berwick, D.M. A primer on leading the improvement of systems. BMJ. 1996;312:619-22.
12. Corley, D.A., Jensen, C.D., and Marks, A.R. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointest Endosc. 2011;74:656-65.
Dr. Weizman is at the Mount Sinai Hospital Centre for Inflammatory Bowel Disease, and Institute of Health Policy, Management and Evaluation, department of medicine; Dr. Mosko is in the division of gastroenterology, St. Michael’s Hospital, department of medicine, and Institute of Health Policy, Management and Evaluation; Dr. Bollegala is in the division of gastroenterology, Women’s College Hospital, department of medicine; Dr. Bernstein is in the division of gastroenterology, Sunnybrook Health Sciences Centre, department of medicine; Dr. Brahmania is at the Toronto Center for Liver Diseases, division of gastroenterology, University Health Network, department of medicine; Dr. Liu is in the division of gastroenterology, University Health Network, department of medicine; Dr. Steinhart is at Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine, and Institute of Health Policy, Management and Evaluation; Dr. Silver is in the division of nephrology, St. Michael’s Hospital; Dr. Bell is in the division of internal medicine, Mount Sinai Hospital, department of medicine, and Institute of Health Policy, Management and Evaluation; and Dr. Nguyen is at Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine and Institute of Health Policy, Management and Evaluation; all are at the University of Toronto. Dr. Steinhart is an advisory board member for Abbvie, Janssen, Takeda, Shire, Allergan, Pfizer, Merck, Ferring, and Pharmascience; has received research grants from Abbvie, Amgen, Genentech, Cellgene, Arena Pharmaceuticals, Red Hill Biopharma, Millenium, Roche, and Centocor; and has received speaking honoraria from Abbvie, Janssen, Takeda, Shire, Pfizer, Merck, and Ferring.
The remaining authors declare no conflicts for this article.
This article by Adam Weizman and colleagues is the first of a three-part series that will provide practical advice for practices that wish to develop a quality initiative. The first article, “Launching a quality improvement initiative” describes the infrastructure, personnel, and structure needed to approach an identified problem within a practice (variability in adenoma detection rates). This case-based approach helps us understand the step-by-step approach needed to reduce variability and improve quality. The authors present a plan (road map) in a straightforward and practical way that seems simple, but if followed carefully, leads to success. These articles are rich in resources and link to state-of-the-art advice.
John I. Allen, MD, MBA, AGAF, Special Section Editor
There has been increasing focus on measuring quality indicators in gastroenterology over the past few years. The adenoma detection rate (ADR) has emerged as one of the most important quality indicators because it is supported by robust clinical evidence.1-3 With every 1% increase in ADR, a 3% reduction in interval colorectal cancer has been noted.3 As such, an ADR of 25% has been designated as an important quality target for all endoscopists who perform colorectal cancer screening.1
You work at a community hospital in a large, metropolitan area. Your colleagues in a number of other departments across your hospital have been increasingly interested in quality improvement (QI) and have launched QI interventions, although none in your department. Moreover, there have been reforms in how hospital endoscopy units are funded in your jurisdiction, with a move toward volume-based funding with a quality overlay. In an effort to improve efficiency and better characterize performance, the hospital has been auditing the performance of all endoscopists at your institution over the past year. Among the eight endoscopists who work at your hospital, the overall ADR has been found to be 19%, decreasing to less than the generally accepted benchmark.1
In response to the results of the audit in your unit, you decide that you would like to develop an initiative to improve your group’s ADR.
Forming a quality improvement team
The first step in any QI project is to establish an improvement team. This working group consists of individuals with specific roles who perform interdependent tasks and share a common goal.4 Usually, frontline health care workers who are impacted most by the quality-of-care problem form the foundation of the team. A team lead is identified who will oversee the project. Content experts are also helpful members of the team who may have particular expertise in the clinical domain that will be the focus of the project. In addition, an improvement adviser, an individual with some expertise in QI, is needed on the team. This adviser may be from within your department or from outside. Although they may not possess expertise in the clinical problem you are trying to tackle, they should have skills in QI methodology and process to aid the team. An executive sponsor also needs to be identified. This should be an influential and well-respected individual who holds a senior administrative position at your institution who can help the team overcome barriers and secure resources. Physician engagement is a critical, often-overlooked step in any improvement effort. Regardless of the initiative, physicians continue to have tremendous influence over hospital-based outcomes.5 Identifying a physician champion, a prominent and respected physician at your organization to help spread the importance of your efforts and create a burning platform for change, is helpful. It also is valuable to have a patient on the improvement team to provide unique perspectives that only the end user of health care can convey and to ensure that the project is patient centered, as all improvement efforts should be.6
Improvement framework
Before starting any improvement effort, there are several important considerations that need to be addressed when choosing a quality improvement target.7 It is important to have a good understanding of the burden and severity of the problem. This often requires audit and measuring. For example, although we may think there is a problem with ADR in our endoscopy unit based on a general impression, it is critical to have data to support this suspicion. This is part of a current state analysis (discussed later). It also is important to select a quality-of-care problem that is under you or your group’s direct control. For example, it would be difficult to initiate a quality improvement project aimed at changing the practice of radiology reporting as a gastroenterologist. It is important to pick a problem that is focused and within a narrow scope that is feasible to address and then improve. Consideration of the unintended consequences of an improvement initiative often is overlooked, but needs to be considered because not all that comes out of quality improvement efforts is good. Finally, the likelihood of success of a quality initiative is increased significantly if it can generate momentum and lead to other interventions both within your department and beyond.
There are several specific improvement frameworks that can be used by a team to address a quality-of-care problem and perform a quality improvement project. The framework chosen depends on the type of problem that is being targeted and the training of the individuals on the improvement team. Three of the most commonly used improvement frameworks include the following: 1) Six Sigma; 2) Lean; and 3) Model for Improvement.
Six Sigma
Six Sigma is focused on improvement by reducing variability.8 It is a highly analytic framework relying on statistical analysis and mathematical modeling. It is best suited for projects in which the root cause and contributors to the target problem remain unclear and the aim of the intervention is to reduce variation.
Lean
Lean emphasizes improvement through elimination of waste and classifies all parts of any process as value added and nonvalue added.9 It is estimated that 95% of activities in any health care process are nonvalue added and the objective of Lean is to identify opportunities to simplify and create efficiencies. It is best suited for target problems that directly can be observed and mapped out, for example, process of care, flow, and efficiency of an endoscopy unit.
Model for Improvement
The Model for Improvement has been popularized by the Institute for Healthcare Improvement.10,11 It is well suited for health care teams, and its advantages are its adaptability to many improvement targets and lack of extensive training, consultant support, or statistical training as required by the previous frameworks mentioned earlier. As a result, it is the most commonly used improvement framework.
Using the Model for Improvement
The Model for Improvement is organized around three main questions: 1) What are we trying to accomplish? 2) How will we know that a change is an improvement? and 3) What changes can result in improvement?
Question 1: What are we trying to accomplish?
The first stage using the Model for Improvement is developing a clear project aim. A good aim statement should be specific in defining what measures one is hoping to improve and setting a concrete deadline by which to achieve it.10,11 It should answer the questions of what the team is trying to improve, by how much, and by what date. It is more effective for the target to be an ambitious, stretch goal to ensure the effort is worth the resources and time that will be invested by the team. Not only does a good aim statement serve as the foundation for the project, but it can redirect the team if the improvement effort is getting off track. In the earlier example of improving ADR, an aim statement could be “to increase the ADR of all endoscopists who perform colonoscopy at your hospital to 25% over a 12-month period.”
Question 2: How will we know that a change is an improvement?
This step involves defining measures that will allow you to understand if changes implemented are impacting the system within which your target problem resides and if this represents an improvement. This usually involves continuous, real-time measurement. Outcome measures are clinically relevant outcomes and are the ultimate goal of what the project team is trying to accomplish. In the example of ADR, this could be the proportion of endoscopists at your institution with an ADR greater than 25%. Process measures are relevant to the system within which you are working and your target problem resides. Typically, the intervention that you implement will have impact that is measurable much earlier by process outcomes than outcomes measures, which are usually a downstream effect. As such, an improvement project still may be a success if it shows improvements in process measures only. For example, the proportion of endoscopists measuring withdrawal time would be a process measure in an intervention aimed at improving ADR. In time, improvement in process measures may translate to improvements in the outcome measure. Balancing measures are indicators of unintended consequences of the project. Not all that comes from an improvement effort is necessarily positive. If improvements in certain process measures come at the cost of harms shown by the balancing measures, such as deterioration in staff satisfaction or increase in time per procedure, the improvement project may not be worth continuing.
Importance of understanding the target problem: Current-state analysis
In contrast to classic enumerative research in which the clinical environment can be well controlled, quality improvement work focuses on sampling and intervening upon a less controlled and dynamic process or system with the intent of improving it.10 Just as treatment strategies in clinical medicine are based on diagnostic testing, so too in quality improvement work, the strategy of diagnosing the current state allows for linking the root cause of quality problems with solutions that can induce positive change.
Several common diagnostic tools are used to identify root causes of quality and safety issues. These include the following: 1) process mapping, 2) cause-and-effect diagrams, and 3) Pareto charts.
Process mapping
Process maps are tools used to understand the system that is being studied. A process map is a graphic depiction of the flow through a process, which creates a collaborative awareness of the current state and identifies opportunities for improvement. It is important that multiple individuals who have knowledge of the process in question are involved in its creation. Process maps are created by first establishing the start and end of the process. Second, the high-level steps are included. Third, a more detailed set of steps can be included within each of the high-level steps.
Cause-and-effect diagrams
Cause-and-effect diagrams, also known as Ishikawa or fishbone diagrams, are helpful brainstorming tools used to graphically display and explore potential causes of a target problem. They illustrate that there often are many contributing factors to one underlying problem and the relationship between contributing factors. Classic examples of categories include equipment, environment, materials, methods and process, people, and measurement.10 Figure 1 provides an example of these tools in an effort to improve ADR.
To identify the most important contributors to the target problem and thus where to focus improvement efforts, a Pareto chart, a bar graph that places all defects/causes in the order of the frequency in which they occur, is constructed. The x-axis is a list of possible defects (Figure 1). The y-axis is the frequency with which any one defect is occurring, and the third (x-2) axis is the cumulative frequency. In theory, it is expected that there will be a vital few defects that account for 80% of all occurrences (referred to by some as the 80:20 rule).10, 11 Populating this graph requires measurement, which, as discussed earlier, is the key to understanding any problem. Measurement can be accomplished through direct observation/audit, chart review, and/or multivoting.
Question 3: What changes can result in improvement?
Once the improvement team has defined an aim and established its family of measures, it is time to develop and implement an intervention. Rather than investing time and resources into one intervention that may or may not be successful, it is preferable to perform small change cycles in which the intervention is conducted on a small scale, refined, and either repeated or changed. As a result, most quality improvement projects consist of an iterative process. The Model for Improvement defines four steps that allow the improvement team to perform this: Plan, do, study, act (PDSA).4,10,11 The first two questions listed earlier allowed the improvement team to plan the intervention. The next step, do, involves implementing your project on a small scale, thereby testing your change while collecting continuous measurements. Study involves interpreting your data using both conventional methods and several improvement-specific methods (discussed later) that help answer the question of how will we know that a change is improvement? Finally, act involves making a conclusion about your first PDSA cycle, helping to inform subsequent cycles. This results in a series of small, rapid cycle changes, one building on the next, that lead to implementation of change(s) that ultimately serve to address your improvement problem and your project aim.
A change concept is an approach known to be useful in developing specific changes that result in improvement. Change concepts are used as a starting point to generate change ideas. A number of change concepts spanning nine main categories have been defined by the Associates for Process Improvement,10 including eliminating waste, improving work flow, managing variation, and designing systems to prevent error. For the purpose of improving ADR, your team may choose a few change concepts and ideas based on the diagnostic work-up. For example, the change concept of designing the system to prevent errors through standardizing withdrawal time for all physicians may lead to an improvement in ADR. This then is linked to the change idea of audible timers placed in endoscopy suites to ensure longer withdrawal times.12 The impact of this change would be measured and the next cycle would build on these results.
Summary and next steps
In this first article of the series, the QI team moved forward with their aim to increase ADR. A root cause analysis was undertaken using multiple diagnostic tools including a fishbone diagram and a Pareto chart. Finally, change ideas were generated based on the earlier-described root causes and established change concepts. The next steps involve undertaking PDSA cycles to test change ideas and monitor for improvement.
References
1. Rex, D.K., Schoenfeld, P.S., Cohen, J. et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81:31-53.
2. Rex, D.K., Bond, J.H., Winawer, S. et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296-308.
3. Corley, D., Jensen, C.D., Marks, A.R. et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-306.
4. Kotter, J.P. Leading change. Harvard Business Review Press, Boston; 2012
5. Taitz, J.M., Lee, T.H., and Sequist, T.D. A framework for engaging physicians in quality and safety. BMJ Qual Saf. 2012;21:722-8.
6. Carman, K.L., Dardess, P., Maurer, M. et al. Patient and family engagement: a framework for understanding the elements and developing interventions and policies. Health Aff (Millwood). 2013;33:223-31.
7.Ranji, S.R. and Shojania, S.G. Implementing patient safety interventions in your hospital: what to try and what to avoid. Med Clin North Am. 2008;92:275-93.
8. Antony, J. Six Sigma vs Lean: some perspectives from leading academics and practitioners. Int J Product Perform Manage. 2011;60:185-90.
9. Bercaw, R. Taking improvement from the assembly line to healthcare: the application of lean within the healthcare industry. Taylor and Francis, Boca Raton, FL; 2012
10. Langley, G.J., Nolan, K.M., Nolan, T.W. et al. The improvement guide: a practical approach to enhancing organizational performance. Jossey-Bass, San Francisco; 2009
11. Berwick, D.M. A primer on leading the improvement of systems. BMJ. 1996;312:619-22.
12. Corley, D.A., Jensen, C.D., and Marks, A.R. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointest Endosc. 2011;74:656-65.
Dr. Weizman is at the Mount Sinai Hospital Centre for Inflammatory Bowel Disease, and Institute of Health Policy, Management and Evaluation, department of medicine; Dr. Mosko is in the division of gastroenterology, St. Michael’s Hospital, department of medicine, and Institute of Health Policy, Management and Evaluation; Dr. Bollegala is in the division of gastroenterology, Women’s College Hospital, department of medicine; Dr. Bernstein is in the division of gastroenterology, Sunnybrook Health Sciences Centre, department of medicine; Dr. Brahmania is at the Toronto Center for Liver Diseases, division of gastroenterology, University Health Network, department of medicine; Dr. Liu is in the division of gastroenterology, University Health Network, department of medicine; Dr. Steinhart is at Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine, and Institute of Health Policy, Management and Evaluation; Dr. Silver is in the division of nephrology, St. Michael’s Hospital; Dr. Bell is in the division of internal medicine, Mount Sinai Hospital, department of medicine, and Institute of Health Policy, Management and Evaluation; and Dr. Nguyen is at Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine and Institute of Health Policy, Management and Evaluation; all are at the University of Toronto. Dr. Steinhart is an advisory board member for Abbvie, Janssen, Takeda, Shire, Allergan, Pfizer, Merck, Ferring, and Pharmascience; has received research grants from Abbvie, Amgen, Genentech, Cellgene, Arena Pharmaceuticals, Red Hill Biopharma, Millenium, Roche, and Centocor; and has received speaking honoraria from Abbvie, Janssen, Takeda, Shire, Pfizer, Merck, and Ferring.
The remaining authors declare no conflicts for this article.
This article by Adam Weizman and colleagues is the first of a three-part series that will provide practical advice for practices that wish to develop a quality initiative. The first article, “Launching a quality improvement initiative” describes the infrastructure, personnel, and structure needed to approach an identified problem within a practice (variability in adenoma detection rates). This case-based approach helps us understand the step-by-step approach needed to reduce variability and improve quality. The authors present a plan (road map) in a straightforward and practical way that seems simple, but if followed carefully, leads to success. These articles are rich in resources and link to state-of-the-art advice.
John I. Allen, MD, MBA, AGAF, Special Section Editor
There has been increasing focus on measuring quality indicators in gastroenterology over the past few years. The adenoma detection rate (ADR) has emerged as one of the most important quality indicators because it is supported by robust clinical evidence.1-3 With every 1% increase in ADR, a 3% reduction in interval colorectal cancer has been noted.3 As such, an ADR of 25% has been designated as an important quality target for all endoscopists who perform colorectal cancer screening.1
You work at a community hospital in a large, metropolitan area. Your colleagues in a number of other departments across your hospital have been increasingly interested in quality improvement (QI) and have launched QI interventions, although none in your department. Moreover, there have been reforms in how hospital endoscopy units are funded in your jurisdiction, with a move toward volume-based funding with a quality overlay. In an effort to improve efficiency and better characterize performance, the hospital has been auditing the performance of all endoscopists at your institution over the past year. Among the eight endoscopists who work at your hospital, the overall ADR has been found to be 19%, decreasing to less than the generally accepted benchmark.1
In response to the results of the audit in your unit, you decide that you would like to develop an initiative to improve your group’s ADR.
Forming a quality improvement team
The first step in any QI project is to establish an improvement team. This working group consists of individuals with specific roles who perform interdependent tasks and share a common goal.4 Usually, frontline health care workers who are impacted most by the quality-of-care problem form the foundation of the team. A team lead is identified who will oversee the project. Content experts are also helpful members of the team who may have particular expertise in the clinical domain that will be the focus of the project. In addition, an improvement adviser, an individual with some expertise in QI, is needed on the team. This adviser may be from within your department or from outside. Although they may not possess expertise in the clinical problem you are trying to tackle, they should have skills in QI methodology and process to aid the team. An executive sponsor also needs to be identified. This should be an influential and well-respected individual who holds a senior administrative position at your institution who can help the team overcome barriers and secure resources. Physician engagement is a critical, often-overlooked step in any improvement effort. Regardless of the initiative, physicians continue to have tremendous influence over hospital-based outcomes.5 Identifying a physician champion, a prominent and respected physician at your organization to help spread the importance of your efforts and create a burning platform for change, is helpful. It also is valuable to have a patient on the improvement team to provide unique perspectives that only the end user of health care can convey and to ensure that the project is patient centered, as all improvement efforts should be.6
Improvement framework
Before starting any improvement effort, there are several important considerations that need to be addressed when choosing a quality improvement target.7 It is important to have a good understanding of the burden and severity of the problem. This often requires audit and measuring. For example, although we may think there is a problem with ADR in our endoscopy unit based on a general impression, it is critical to have data to support this suspicion. This is part of a current state analysis (discussed later). It also is important to select a quality-of-care problem that is under you or your group’s direct control. For example, it would be difficult to initiate a quality improvement project aimed at changing the practice of radiology reporting as a gastroenterologist. It is important to pick a problem that is focused and within a narrow scope that is feasible to address and then improve. Consideration of the unintended consequences of an improvement initiative often is overlooked, but needs to be considered because not all that comes out of quality improvement efforts is good. Finally, the likelihood of success of a quality initiative is increased significantly if it can generate momentum and lead to other interventions both within your department and beyond.
There are several specific improvement frameworks that can be used by a team to address a quality-of-care problem and perform a quality improvement project. The framework chosen depends on the type of problem that is being targeted and the training of the individuals on the improvement team. Three of the most commonly used improvement frameworks include the following: 1) Six Sigma; 2) Lean; and 3) Model for Improvement.
Six Sigma
Six Sigma is focused on improvement by reducing variability.8 It is a highly analytic framework relying on statistical analysis and mathematical modeling. It is best suited for projects in which the root cause and contributors to the target problem remain unclear and the aim of the intervention is to reduce variation.
Lean
Lean emphasizes improvement through elimination of waste and classifies all parts of any process as value added and nonvalue added.9 It is estimated that 95% of activities in any health care process are nonvalue added and the objective of Lean is to identify opportunities to simplify and create efficiencies. It is best suited for target problems that directly can be observed and mapped out, for example, process of care, flow, and efficiency of an endoscopy unit.
Model for Improvement
The Model for Improvement has been popularized by the Institute for Healthcare Improvement.10,11 It is well suited for health care teams, and its advantages are its adaptability to many improvement targets and lack of extensive training, consultant support, or statistical training as required by the previous frameworks mentioned earlier. As a result, it is the most commonly used improvement framework.
Using the Model for Improvement
The Model for Improvement is organized around three main questions: 1) What are we trying to accomplish? 2) How will we know that a change is an improvement? and 3) What changes can result in improvement?
Question 1: What are we trying to accomplish?
The first stage using the Model for Improvement is developing a clear project aim. A good aim statement should be specific in defining what measures one is hoping to improve and setting a concrete deadline by which to achieve it.10,11 It should answer the questions of what the team is trying to improve, by how much, and by what date. It is more effective for the target to be an ambitious, stretch goal to ensure the effort is worth the resources and time that will be invested by the team. Not only does a good aim statement serve as the foundation for the project, but it can redirect the team if the improvement effort is getting off track. In the earlier example of improving ADR, an aim statement could be “to increase the ADR of all endoscopists who perform colonoscopy at your hospital to 25% over a 12-month period.”
Question 2: How will we know that a change is an improvement?
This step involves defining measures that will allow you to understand if changes implemented are impacting the system within which your target problem resides and if this represents an improvement. This usually involves continuous, real-time measurement. Outcome measures are clinically relevant outcomes and are the ultimate goal of what the project team is trying to accomplish. In the example of ADR, this could be the proportion of endoscopists at your institution with an ADR greater than 25%. Process measures are relevant to the system within which you are working and your target problem resides. Typically, the intervention that you implement will have impact that is measurable much earlier by process outcomes than outcomes measures, which are usually a downstream effect. As such, an improvement project still may be a success if it shows improvements in process measures only. For example, the proportion of endoscopists measuring withdrawal time would be a process measure in an intervention aimed at improving ADR. In time, improvement in process measures may translate to improvements in the outcome measure. Balancing measures are indicators of unintended consequences of the project. Not all that comes from an improvement effort is necessarily positive. If improvements in certain process measures come at the cost of harms shown by the balancing measures, such as deterioration in staff satisfaction or increase in time per procedure, the improvement project may not be worth continuing.
Importance of understanding the target problem: Current-state analysis
In contrast to classic enumerative research in which the clinical environment can be well controlled, quality improvement work focuses on sampling and intervening upon a less controlled and dynamic process or system with the intent of improving it.10 Just as treatment strategies in clinical medicine are based on diagnostic testing, so too in quality improvement work, the strategy of diagnosing the current state allows for linking the root cause of quality problems with solutions that can induce positive change.
Several common diagnostic tools are used to identify root causes of quality and safety issues. These include the following: 1) process mapping, 2) cause-and-effect diagrams, and 3) Pareto charts.
Process mapping
Process maps are tools used to understand the system that is being studied. A process map is a graphic depiction of the flow through a process, which creates a collaborative awareness of the current state and identifies opportunities for improvement. It is important that multiple individuals who have knowledge of the process in question are involved in its creation. Process maps are created by first establishing the start and end of the process. Second, the high-level steps are included. Third, a more detailed set of steps can be included within each of the high-level steps.
Cause-and-effect diagrams
Cause-and-effect diagrams, also known as Ishikawa or fishbone diagrams, are helpful brainstorming tools used to graphically display and explore potential causes of a target problem. They illustrate that there often are many contributing factors to one underlying problem and the relationship between contributing factors. Classic examples of categories include equipment, environment, materials, methods and process, people, and measurement.10 Figure 1 provides an example of these tools in an effort to improve ADR.
To identify the most important contributors to the target problem and thus where to focus improvement efforts, a Pareto chart, a bar graph that places all defects/causes in the order of the frequency in which they occur, is constructed. The x-axis is a list of possible defects (Figure 1). The y-axis is the frequency with which any one defect is occurring, and the third (x-2) axis is the cumulative frequency. In theory, it is expected that there will be a vital few defects that account for 80% of all occurrences (referred to by some as the 80:20 rule).10, 11 Populating this graph requires measurement, which, as discussed earlier, is the key to understanding any problem. Measurement can be accomplished through direct observation/audit, chart review, and/or multivoting.
Question 3: What changes can result in improvement?
Once the improvement team has defined an aim and established its family of measures, it is time to develop and implement an intervention. Rather than investing time and resources into one intervention that may or may not be successful, it is preferable to perform small change cycles in which the intervention is conducted on a small scale, refined, and either repeated or changed. As a result, most quality improvement projects consist of an iterative process. The Model for Improvement defines four steps that allow the improvement team to perform this: Plan, do, study, act (PDSA).4,10,11 The first two questions listed earlier allowed the improvement team to plan the intervention. The next step, do, involves implementing your project on a small scale, thereby testing your change while collecting continuous measurements. Study involves interpreting your data using both conventional methods and several improvement-specific methods (discussed later) that help answer the question of how will we know that a change is improvement? Finally, act involves making a conclusion about your first PDSA cycle, helping to inform subsequent cycles. This results in a series of small, rapid cycle changes, one building on the next, that lead to implementation of change(s) that ultimately serve to address your improvement problem and your project aim.
A change concept is an approach known to be useful in developing specific changes that result in improvement. Change concepts are used as a starting point to generate change ideas. A number of change concepts spanning nine main categories have been defined by the Associates for Process Improvement,10 including eliminating waste, improving work flow, managing variation, and designing systems to prevent error. For the purpose of improving ADR, your team may choose a few change concepts and ideas based on the diagnostic work-up. For example, the change concept of designing the system to prevent errors through standardizing withdrawal time for all physicians may lead to an improvement in ADR. This then is linked to the change idea of audible timers placed in endoscopy suites to ensure longer withdrawal times.12 The impact of this change would be measured and the next cycle would build on these results.
Summary and next steps
In this first article of the series, the QI team moved forward with their aim to increase ADR. A root cause analysis was undertaken using multiple diagnostic tools including a fishbone diagram and a Pareto chart. Finally, change ideas were generated based on the earlier-described root causes and established change concepts. The next steps involve undertaking PDSA cycles to test change ideas and monitor for improvement.
References
1. Rex, D.K., Schoenfeld, P.S., Cohen, J. et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81:31-53.
2. Rex, D.K., Bond, J.H., Winawer, S. et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296-308.
3. Corley, D., Jensen, C.D., Marks, A.R. et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-306.
4. Kotter, J.P. Leading change. Harvard Business Review Press, Boston; 2012
5. Taitz, J.M., Lee, T.H., and Sequist, T.D. A framework for engaging physicians in quality and safety. BMJ Qual Saf. 2012;21:722-8.
6. Carman, K.L., Dardess, P., Maurer, M. et al. Patient and family engagement: a framework for understanding the elements and developing interventions and policies. Health Aff (Millwood). 2013;33:223-31.
7.Ranji, S.R. and Shojania, S.G. Implementing patient safety interventions in your hospital: what to try and what to avoid. Med Clin North Am. 2008;92:275-93.
8. Antony, J. Six Sigma vs Lean: some perspectives from leading academics and practitioners. Int J Product Perform Manage. 2011;60:185-90.
9. Bercaw, R. Taking improvement from the assembly line to healthcare: the application of lean within the healthcare industry. Taylor and Francis, Boca Raton, FL; 2012
10. Langley, G.J., Nolan, K.M., Nolan, T.W. et al. The improvement guide: a practical approach to enhancing organizational performance. Jossey-Bass, San Francisco; 2009
11. Berwick, D.M. A primer on leading the improvement of systems. BMJ. 1996;312:619-22.
12. Corley, D.A., Jensen, C.D., and Marks, A.R. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointest Endosc. 2011;74:656-65.
Dr. Weizman is at the Mount Sinai Hospital Centre for Inflammatory Bowel Disease, and Institute of Health Policy, Management and Evaluation, department of medicine; Dr. Mosko is in the division of gastroenterology, St. Michael’s Hospital, department of medicine, and Institute of Health Policy, Management and Evaluation; Dr. Bollegala is in the division of gastroenterology, Women’s College Hospital, department of medicine; Dr. Bernstein is in the division of gastroenterology, Sunnybrook Health Sciences Centre, department of medicine; Dr. Brahmania is at the Toronto Center for Liver Diseases, division of gastroenterology, University Health Network, department of medicine; Dr. Liu is in the division of gastroenterology, University Health Network, department of medicine; Dr. Steinhart is at Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine, and Institute of Health Policy, Management and Evaluation; Dr. Silver is in the division of nephrology, St. Michael’s Hospital; Dr. Bell is in the division of internal medicine, Mount Sinai Hospital, department of medicine, and Institute of Health Policy, Management and Evaluation; and Dr. Nguyen is at Mount Sinai Hospital Centre for Inflammatory Bowel Disease, department of medicine and Institute of Health Policy, Management and Evaluation; all are at the University of Toronto. Dr. Steinhart is an advisory board member for Abbvie, Janssen, Takeda, Shire, Allergan, Pfizer, Merck, Ferring, and Pharmascience; has received research grants from Abbvie, Amgen, Genentech, Cellgene, Arena Pharmaceuticals, Red Hill Biopharma, Millenium, Roche, and Centocor; and has received speaking honoraria from Abbvie, Janssen, Takeda, Shire, Pfizer, Merck, and Ferring.
The remaining authors declare no conflicts for this article.
Neuronal Protein Could Be a Blood Biomarker of MS
LONDON—Higher levels of a neuronal protein were found in the blood of patients with relapsing-remitting multiple sclerosis (RRMS) than in healthy subjects in a proof-of-concept study reported at the 32nd Annual Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
Blood levels of neurofilament light chain (NfL) were 28.1 and 12.5 pg/mL, respectively, and were also found to be higher in patients with RRMS with greater disease activity seen on MRI.
As the number of gadolinium-enhancing (Gd+) lesions increased, so did the blood concentration of NfL, which was 23.9 pg/mL in patients with no Gd+ lesions, 26.7 pg/mL in those with one Gd+ lesion, 33.4 pg/mL in those with two to three Gd+ lesions, and 55.9 pg/mL in those with more than three Gd+ lesions.
“These findings support a role for NfL as a peripheral biomarker for MS,” said Jens Kuhle, MD, of University Hospital Basel in Switzerland. “There is an urgent unmet need for reliable biomarkers of neurodegeneration, besides efforts that are being done in MRI, optical coherence tomography, and evoked potentials.
“NfL is an exclusively neuronal protein. It is expressed in the cytosol of neurons, it is released upon cell injury into the CSF, and obviously it also appears in the blood circulation.” NfL in the CSF thus reflects nerve damage and had been seen in patients with MS and several other neurologic diseases, including Alzheimer’s disease, Parkinson’s disease, and ALS.
Until recently, however, it was not possible to detect NfL in the blood, as levels are around 50 to 100 times lower than in the CSF, but Dr. Kuhle and his associates have shown that an electrochemiluminescence immunoassay could detect increasing NfL levels with increasing disease activity. In the current study, Dr. Kuhle and colleagues used a Single Molecule Array Immunoassay (Quanterix). This test is based on an enzyme-linked immunoassay with more than 280,000 wells and was developed to be an ultrasensitive diagnostic platform to measure minute quantities of individual proteins. Dr. Kuhle noted that it had “significantly increased sensitivity to measure NfL in blood,” when compared with conventional enzyme-linked immunosorbent assay (ELISA) or electrochemiluminescence.
Two to three consecutive blood samples taken from 149 patients with RRMS participating in the phase III FREEDOMS trial were obtained and compared with samples from 29 similarly aged healthy individuals without MS obtained from a separate biobank.
Patients with two or more relapses in the previous 24 months had significantly higher NfL levels than those with one relapse or no relapses. Serum NfL also significantly increased with the Expanded Disability Status Scale score recorded at the time the blood samples were taken.
Furthermore, “blood NfL levels predicted subsequent brain atrophy rates,” Dr. Kuhle reported, with NfL levels at six months being highly predictive of brain volume changes at 24 months.
And, in this preliminary dataset, patients who had been treated with fingolimod versus placebo during the FREEDOMS trial had lower NfL levels at six, 12, and 24 months. Dr. Kuhle observed that the findings of this study were corroborated by other study data presented at the ECTRIMS meeting.
The FREEDOMS trial was sponsored by Novartis. Dr. Kuhle received research support and consulting fees from Biogen, Novartis, and Protagen. He also disclosed receiving speaker fees and travel expenses from Novartis and several other pharmaceutical companies. Several of Dr. Kuhle’s coinvestigators were employees of Novartis.
—Sara Freeman
LONDON—Higher levels of a neuronal protein were found in the blood of patients with relapsing-remitting multiple sclerosis (RRMS) than in healthy subjects in a proof-of-concept study reported at the 32nd Annual Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
Blood levels of neurofilament light chain (NfL) were 28.1 and 12.5 pg/mL, respectively, and were also found to be higher in patients with RRMS with greater disease activity seen on MRI.
As the number of gadolinium-enhancing (Gd+) lesions increased, so did the blood concentration of NfL, which was 23.9 pg/mL in patients with no Gd+ lesions, 26.7 pg/mL in those with one Gd+ lesion, 33.4 pg/mL in those with two to three Gd+ lesions, and 55.9 pg/mL in those with more than three Gd+ lesions.
“These findings support a role for NfL as a peripheral biomarker for MS,” said Jens Kuhle, MD, of University Hospital Basel in Switzerland. “There is an urgent unmet need for reliable biomarkers of neurodegeneration, besides efforts that are being done in MRI, optical coherence tomography, and evoked potentials.
“NfL is an exclusively neuronal protein. It is expressed in the cytosol of neurons, it is released upon cell injury into the CSF, and obviously it also appears in the blood circulation.” NfL in the CSF thus reflects nerve damage and had been seen in patients with MS and several other neurologic diseases, including Alzheimer’s disease, Parkinson’s disease, and ALS.
Until recently, however, it was not possible to detect NfL in the blood, as levels are around 50 to 100 times lower than in the CSF, but Dr. Kuhle and his associates have shown that an electrochemiluminescence immunoassay could detect increasing NfL levels with increasing disease activity. In the current study, Dr. Kuhle and colleagues used a Single Molecule Array Immunoassay (Quanterix). This test is based on an enzyme-linked immunoassay with more than 280,000 wells and was developed to be an ultrasensitive diagnostic platform to measure minute quantities of individual proteins. Dr. Kuhle noted that it had “significantly increased sensitivity to measure NfL in blood,” when compared with conventional enzyme-linked immunosorbent assay (ELISA) or electrochemiluminescence.
Two to three consecutive blood samples taken from 149 patients with RRMS participating in the phase III FREEDOMS trial were obtained and compared with samples from 29 similarly aged healthy individuals without MS obtained from a separate biobank.
Patients with two or more relapses in the previous 24 months had significantly higher NfL levels than those with one relapse or no relapses. Serum NfL also significantly increased with the Expanded Disability Status Scale score recorded at the time the blood samples were taken.
Furthermore, “blood NfL levels predicted subsequent brain atrophy rates,” Dr. Kuhle reported, with NfL levels at six months being highly predictive of brain volume changes at 24 months.
And, in this preliminary dataset, patients who had been treated with fingolimod versus placebo during the FREEDOMS trial had lower NfL levels at six, 12, and 24 months. Dr. Kuhle observed that the findings of this study were corroborated by other study data presented at the ECTRIMS meeting.
The FREEDOMS trial was sponsored by Novartis. Dr. Kuhle received research support and consulting fees from Biogen, Novartis, and Protagen. He also disclosed receiving speaker fees and travel expenses from Novartis and several other pharmaceutical companies. Several of Dr. Kuhle’s coinvestigators were employees of Novartis.
—Sara Freeman
LONDON—Higher levels of a neuronal protein were found in the blood of patients with relapsing-remitting multiple sclerosis (RRMS) than in healthy subjects in a proof-of-concept study reported at the 32nd Annual Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS).
Blood levels of neurofilament light chain (NfL) were 28.1 and 12.5 pg/mL, respectively, and were also found to be higher in patients with RRMS with greater disease activity seen on MRI.
As the number of gadolinium-enhancing (Gd+) lesions increased, so did the blood concentration of NfL, which was 23.9 pg/mL in patients with no Gd+ lesions, 26.7 pg/mL in those with one Gd+ lesion, 33.4 pg/mL in those with two to three Gd+ lesions, and 55.9 pg/mL in those with more than three Gd+ lesions.
“These findings support a role for NfL as a peripheral biomarker for MS,” said Jens Kuhle, MD, of University Hospital Basel in Switzerland. “There is an urgent unmet need for reliable biomarkers of neurodegeneration, besides efforts that are being done in MRI, optical coherence tomography, and evoked potentials.
“NfL is an exclusively neuronal protein. It is expressed in the cytosol of neurons, it is released upon cell injury into the CSF, and obviously it also appears in the blood circulation.” NfL in the CSF thus reflects nerve damage and had been seen in patients with MS and several other neurologic diseases, including Alzheimer’s disease, Parkinson’s disease, and ALS.
Until recently, however, it was not possible to detect NfL in the blood, as levels are around 50 to 100 times lower than in the CSF, but Dr. Kuhle and his associates have shown that an electrochemiluminescence immunoassay could detect increasing NfL levels with increasing disease activity. In the current study, Dr. Kuhle and colleagues used a Single Molecule Array Immunoassay (Quanterix). This test is based on an enzyme-linked immunoassay with more than 280,000 wells and was developed to be an ultrasensitive diagnostic platform to measure minute quantities of individual proteins. Dr. Kuhle noted that it had “significantly increased sensitivity to measure NfL in blood,” when compared with conventional enzyme-linked immunosorbent assay (ELISA) or electrochemiluminescence.
Two to three consecutive blood samples taken from 149 patients with RRMS participating in the phase III FREEDOMS trial were obtained and compared with samples from 29 similarly aged healthy individuals without MS obtained from a separate biobank.
Patients with two or more relapses in the previous 24 months had significantly higher NfL levels than those with one relapse or no relapses. Serum NfL also significantly increased with the Expanded Disability Status Scale score recorded at the time the blood samples were taken.
Furthermore, “blood NfL levels predicted subsequent brain atrophy rates,” Dr. Kuhle reported, with NfL levels at six months being highly predictive of brain volume changes at 24 months.
And, in this preliminary dataset, patients who had been treated with fingolimod versus placebo during the FREEDOMS trial had lower NfL levels at six, 12, and 24 months. Dr. Kuhle observed that the findings of this study were corroborated by other study data presented at the ECTRIMS meeting.
The FREEDOMS trial was sponsored by Novartis. Dr. Kuhle received research support and consulting fees from Biogen, Novartis, and Protagen. He also disclosed receiving speaker fees and travel expenses from Novartis and several other pharmaceutical companies. Several of Dr. Kuhle’s coinvestigators were employees of Novartis.
—Sara Freeman