User login
United States nears 3,000 Zika-infected pregnancies
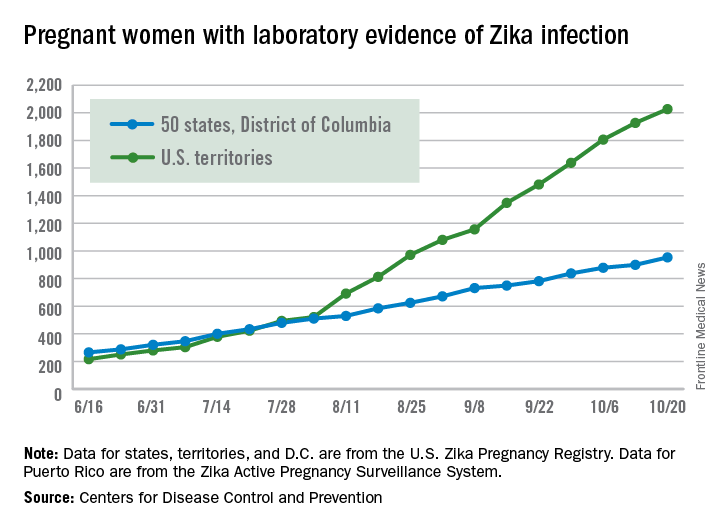
There were 54 new cases of pregnant women with laboratory evidence of Zika virus in the 50 states and the District of Columbia reported during the week ending Oct. 20 – the largest weekly increase in a month, according to the Centers for Disease Control and Prevention.
The number of cases reported in the U.S. territories, 100, was lower for the second week in a row, however, so the U.S. total for the week was a fairly average 154. The total number of pregnant women with laboratory evidence of Zika virus infection is now 2,980 for the year: 953 in the states/D.C. and 2,027 in the territories, the CDC reported.
Among all Americans, the number of cases for 2015-2016 is now up to 32,814, with 1,396 new cases reported for the week ending Oct. 26: 75 in the states/D.C. and 1,321 in the territories. Almost all (98%) of the territorial cases have occurred in Puerto Rico, which continues to retroactively report cases, the CDC Arboviral Disease Branch noted.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
There were 54 new cases of pregnant women with laboratory evidence of Zika virus in the 50 states and the District of Columbia reported during the week ending Oct. 20 – the largest weekly increase in a month, according to the Centers for Disease Control and Prevention.
The number of cases reported in the U.S. territories, 100, was lower for the second week in a row, however, so the U.S. total for the week was a fairly average 154. The total number of pregnant women with laboratory evidence of Zika virus infection is now 2,980 for the year: 953 in the states/D.C. and 2,027 in the territories, the CDC reported.
Among all Americans, the number of cases for 2015-2016 is now up to 32,814, with 1,396 new cases reported for the week ending Oct. 26: 75 in the states/D.C. and 1,321 in the territories. Almost all (98%) of the territorial cases have occurred in Puerto Rico, which continues to retroactively report cases, the CDC Arboviral Disease Branch noted.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
There were 54 new cases of pregnant women with laboratory evidence of Zika virus in the 50 states and the District of Columbia reported during the week ending Oct. 20 – the largest weekly increase in a month, according to the Centers for Disease Control and Prevention.
The number of cases reported in the U.S. territories, 100, was lower for the second week in a row, however, so the U.S. total for the week was a fairly average 154. The total number of pregnant women with laboratory evidence of Zika virus infection is now 2,980 for the year: 953 in the states/D.C. and 2,027 in the territories, the CDC reported.
Among all Americans, the number of cases for 2015-2016 is now up to 32,814, with 1,396 new cases reported for the week ending Oct. 26: 75 in the states/D.C. and 1,321 in the territories. Almost all (98%) of the territorial cases have occurred in Puerto Rico, which continues to retroactively report cases, the CDC Arboviral Disease Branch noted.
Zika-related birth defects reported by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The pregnancy-related figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Updated AAP safe sleep recs for infants reinforce life-saving messages
SAN FRANCISCO – At sleep time, infants should share their parents’ bedroom on a separate sleep surface without bed sharing, should be placed on their backs on a firm surface, and should have a sleep area free of blankets and soft objects, according to updated guidelines from the American Academy of Pediatrics aimed at reducing the risk of sudden infant death syndrome (SIDS) and other sleep-related infant deaths.
Drafted by a multidisciplinary task force, the set of 19 evidence-based recommendations largely reiterate messages that the academy has promoted for years such as “back to sleep for every sleep,” according to task force member Fern R. Hauck, MD, the Spencer P. Bass, MD, Twenty-First Century Professor of Family Medicine at the University of Virginia, Charlottesville. They were unveiled in a press briefing at the academy’s annual meeting and simultaneously published (Pediatrics. 2016;138[5]:e20162938).
Progress, but still a ways to go
Education campaigns that convey these and related messages to new parents and other caregivers have led to a more than halving of the rate of SIDS in recent decades. Yet, 3,500 infants are still lost each year to this syndrome and other sleep-related causes of infant death, such as unintentional suffocation, collectively called sudden unexpected infant death (SUID).
New is a recommendation for skin-to-skin care for at least the first hour of life for healthy newborns, as soon as the mother is alert enough to respond to her infant, according to Dr. Hauck. The aims here are to optimize neurodevelopment and promote temperature regulation.
There is no evidence that swaddling reduces the risk of SIDS, but parents can still use this technique if they wish as long as infants are placed on their back and it is discontinued as soon as they start to show signs of rolling over, she said. Evidence is also lacking for new technologies marketed as protective, for example, crib mattresses designed to reduce re-breathing of carbon dioxide should an infant become prone.
Sleeping in the parents’ room but on a separate surface decreases the risk of SIDS by as much as 50%, according to several studies. Bed sharing is not recommended because of the risk of suffocation, strangulation, and entrapment, the policy states.
The updated recommendations should be followed for every sleep and by every caregiver, until the child reaches 1 year of age, Dr. Hauck stressed. “This includes nap time and bedtime sleep, at home, in day care, or in any other locations where the baby is sleeping.”
“We feel that these messages need to start while the mom’s pregnant because some of the decisions that are made that are not always the best decisions, when the mother is exhausted, can occur spur of the moment,” she added. “As pediatricians, you can set up a prebirth visit to start talking about this, and obstetricians should be doing more as well to bring this up during their prenatal visits.”
Other recommendations include offering a pacifier at nap time and bedtime; avoiding smoke exposure during pregnancy and after birth; and avoiding alcohol and illicit drug use during pregnancy and after birth.
Breastfeeding issues
Although breastfeeding protects against SIDS, it can pose some problems for safe sleep practices, acknowledged Lori B. Feldman-Winter, MD, a liaison from the AAP section on breastfeeding to the task force, as well as head of the division of adolescent medicine and professor of pediatrics at Cooper University Health Care in Camden, N.J.
Bedside sleepers (also called sidecar sleepers) that attach to the parents’ bed may help facilitate the dual aims of breastfeeding and safe sleep, but they have not been formally studied to assess their impact on SIDS risk.
Raising awareness
“As a father and pediatrician, I want parents to know that their baby is safest following the AAP safe sleep recommendations, and spreading this message has become my life’s mission,” said Dr. Samuel P. Hanke, a pediatric cardiologist at the University of Cincinnati, who knows the heartbreak of SIDS firsthand.
“We know practicing safe sleep is hard. We have to be vigilant. We need to start adopting a mentality that safe sleep is not negotiable,” Dr. Hanke asserted. “We cannot emphasize enough that practicing safe sleep for every sleep is as important as buckling your child into a car seat for every drive. And just like car seats, this change won’t occur overnight.”
Federal commitment
Since the 1970s, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) in Bethesda, Md., has been supporting and performing much of the research on which the updated recommendations are based. This research continues to help identify areas where greater efforts are needed, according to acting director Catherine Y. Spong, MD.
NICHD also conducts and collaborates on related education campaigns, such as Safe to Sleep, to disseminate messages such as those in the updated AAP recommendations as widely as possible.
“I encourage all physicians, pediatricians, nurses, and other health care and child care providers to lend their authoritative voices to the Safe to Sleep effort,” Dr. Spong said. “Join us all in sharing safe infant sleep recommendations and in supporting parents and caregivers to make informed decisions that will help keep their baby safe during sleep.”
A closer look at setting
Published in conjunction with the guidelines is a study on risk factors that looked at the role of the setting in which sleep-related infant deaths occur (Pediatrics. 2016 Oct 24:e20161124).
The analysis of nearly 12,000 such deaths found that, relative to counterparts who died in their home, infants who died outside of their home were more likely to be in a stroller or car seat at the time (adjusted odds ratio, 2.6) and in other locations, such as on the floor or a futon (1.9), and to have been placed prone (1.1). They were less likely to have been sharing a bed (0.7).
The groups did not differ in terms of whether the infant was sleeping in an adult bed or on a person, on a couch or chair, or with any objects in their sleep environment.
“Caregivers should be educated on the importance of placing infants to sleep supine in cribs/bassinets to protect against sleep-related deaths, both in and out of the home,” conclude the investigators, one of whom disclosed serving as a paid expert witness in cases of sleep-related infant death.
SAN FRANCISCO – At sleep time, infants should share their parents’ bedroom on a separate sleep surface without bed sharing, should be placed on their backs on a firm surface, and should have a sleep area free of blankets and soft objects, according to updated guidelines from the American Academy of Pediatrics aimed at reducing the risk of sudden infant death syndrome (SIDS) and other sleep-related infant deaths.
Drafted by a multidisciplinary task force, the set of 19 evidence-based recommendations largely reiterate messages that the academy has promoted for years such as “back to sleep for every sleep,” according to task force member Fern R. Hauck, MD, the Spencer P. Bass, MD, Twenty-First Century Professor of Family Medicine at the University of Virginia, Charlottesville. They were unveiled in a press briefing at the academy’s annual meeting and simultaneously published (Pediatrics. 2016;138[5]:e20162938).
Progress, but still a ways to go
Education campaigns that convey these and related messages to new parents and other caregivers have led to a more than halving of the rate of SIDS in recent decades. Yet, 3,500 infants are still lost each year to this syndrome and other sleep-related causes of infant death, such as unintentional suffocation, collectively called sudden unexpected infant death (SUID).
New is a recommendation for skin-to-skin care for at least the first hour of life for healthy newborns, as soon as the mother is alert enough to respond to her infant, according to Dr. Hauck. The aims here are to optimize neurodevelopment and promote temperature regulation.
There is no evidence that swaddling reduces the risk of SIDS, but parents can still use this technique if they wish as long as infants are placed on their back and it is discontinued as soon as they start to show signs of rolling over, she said. Evidence is also lacking for new technologies marketed as protective, for example, crib mattresses designed to reduce re-breathing of carbon dioxide should an infant become prone.
Sleeping in the parents’ room but on a separate surface decreases the risk of SIDS by as much as 50%, according to several studies. Bed sharing is not recommended because of the risk of suffocation, strangulation, and entrapment, the policy states.
The updated recommendations should be followed for every sleep and by every caregiver, until the child reaches 1 year of age, Dr. Hauck stressed. “This includes nap time and bedtime sleep, at home, in day care, or in any other locations where the baby is sleeping.”
“We feel that these messages need to start while the mom’s pregnant because some of the decisions that are made that are not always the best decisions, when the mother is exhausted, can occur spur of the moment,” she added. “As pediatricians, you can set up a prebirth visit to start talking about this, and obstetricians should be doing more as well to bring this up during their prenatal visits.”
Other recommendations include offering a pacifier at nap time and bedtime; avoiding smoke exposure during pregnancy and after birth; and avoiding alcohol and illicit drug use during pregnancy and after birth.
Breastfeeding issues
Although breastfeeding protects against SIDS, it can pose some problems for safe sleep practices, acknowledged Lori B. Feldman-Winter, MD, a liaison from the AAP section on breastfeeding to the task force, as well as head of the division of adolescent medicine and professor of pediatrics at Cooper University Health Care in Camden, N.J.
Bedside sleepers (also called sidecar sleepers) that attach to the parents’ bed may help facilitate the dual aims of breastfeeding and safe sleep, but they have not been formally studied to assess their impact on SIDS risk.
Raising awareness
“As a father and pediatrician, I want parents to know that their baby is safest following the AAP safe sleep recommendations, and spreading this message has become my life’s mission,” said Dr. Samuel P. Hanke, a pediatric cardiologist at the University of Cincinnati, who knows the heartbreak of SIDS firsthand.
“We know practicing safe sleep is hard. We have to be vigilant. We need to start adopting a mentality that safe sleep is not negotiable,” Dr. Hanke asserted. “We cannot emphasize enough that practicing safe sleep for every sleep is as important as buckling your child into a car seat for every drive. And just like car seats, this change won’t occur overnight.”
Federal commitment
Since the 1970s, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) in Bethesda, Md., has been supporting and performing much of the research on which the updated recommendations are based. This research continues to help identify areas where greater efforts are needed, according to acting director Catherine Y. Spong, MD.
NICHD also conducts and collaborates on related education campaigns, such as Safe to Sleep, to disseminate messages such as those in the updated AAP recommendations as widely as possible.
“I encourage all physicians, pediatricians, nurses, and other health care and child care providers to lend their authoritative voices to the Safe to Sleep effort,” Dr. Spong said. “Join us all in sharing safe infant sleep recommendations and in supporting parents and caregivers to make informed decisions that will help keep their baby safe during sleep.”
A closer look at setting
Published in conjunction with the guidelines is a study on risk factors that looked at the role of the setting in which sleep-related infant deaths occur (Pediatrics. 2016 Oct 24:e20161124).
The analysis of nearly 12,000 such deaths found that, relative to counterparts who died in their home, infants who died outside of their home were more likely to be in a stroller or car seat at the time (adjusted odds ratio, 2.6) and in other locations, such as on the floor or a futon (1.9), and to have been placed prone (1.1). They were less likely to have been sharing a bed (0.7).
The groups did not differ in terms of whether the infant was sleeping in an adult bed or on a person, on a couch or chair, or with any objects in their sleep environment.
“Caregivers should be educated on the importance of placing infants to sleep supine in cribs/bassinets to protect against sleep-related deaths, both in and out of the home,” conclude the investigators, one of whom disclosed serving as a paid expert witness in cases of sleep-related infant death.
SAN FRANCISCO – At sleep time, infants should share their parents’ bedroom on a separate sleep surface without bed sharing, should be placed on their backs on a firm surface, and should have a sleep area free of blankets and soft objects, according to updated guidelines from the American Academy of Pediatrics aimed at reducing the risk of sudden infant death syndrome (SIDS) and other sleep-related infant deaths.
Drafted by a multidisciplinary task force, the set of 19 evidence-based recommendations largely reiterate messages that the academy has promoted for years such as “back to sleep for every sleep,” according to task force member Fern R. Hauck, MD, the Spencer P. Bass, MD, Twenty-First Century Professor of Family Medicine at the University of Virginia, Charlottesville. They were unveiled in a press briefing at the academy’s annual meeting and simultaneously published (Pediatrics. 2016;138[5]:e20162938).
Progress, but still a ways to go
Education campaigns that convey these and related messages to new parents and other caregivers have led to a more than halving of the rate of SIDS in recent decades. Yet, 3,500 infants are still lost each year to this syndrome and other sleep-related causes of infant death, such as unintentional suffocation, collectively called sudden unexpected infant death (SUID).
New is a recommendation for skin-to-skin care for at least the first hour of life for healthy newborns, as soon as the mother is alert enough to respond to her infant, according to Dr. Hauck. The aims here are to optimize neurodevelopment and promote temperature regulation.
There is no evidence that swaddling reduces the risk of SIDS, but parents can still use this technique if they wish as long as infants are placed on their back and it is discontinued as soon as they start to show signs of rolling over, she said. Evidence is also lacking for new technologies marketed as protective, for example, crib mattresses designed to reduce re-breathing of carbon dioxide should an infant become prone.
Sleeping in the parents’ room but on a separate surface decreases the risk of SIDS by as much as 50%, according to several studies. Bed sharing is not recommended because of the risk of suffocation, strangulation, and entrapment, the policy states.
The updated recommendations should be followed for every sleep and by every caregiver, until the child reaches 1 year of age, Dr. Hauck stressed. “This includes nap time and bedtime sleep, at home, in day care, or in any other locations where the baby is sleeping.”
“We feel that these messages need to start while the mom’s pregnant because some of the decisions that are made that are not always the best decisions, when the mother is exhausted, can occur spur of the moment,” she added. “As pediatricians, you can set up a prebirth visit to start talking about this, and obstetricians should be doing more as well to bring this up during their prenatal visits.”
Other recommendations include offering a pacifier at nap time and bedtime; avoiding smoke exposure during pregnancy and after birth; and avoiding alcohol and illicit drug use during pregnancy and after birth.
Breastfeeding issues
Although breastfeeding protects against SIDS, it can pose some problems for safe sleep practices, acknowledged Lori B. Feldman-Winter, MD, a liaison from the AAP section on breastfeeding to the task force, as well as head of the division of adolescent medicine and professor of pediatrics at Cooper University Health Care in Camden, N.J.
Bedside sleepers (also called sidecar sleepers) that attach to the parents’ bed may help facilitate the dual aims of breastfeeding and safe sleep, but they have not been formally studied to assess their impact on SIDS risk.
Raising awareness
“As a father and pediatrician, I want parents to know that their baby is safest following the AAP safe sleep recommendations, and spreading this message has become my life’s mission,” said Dr. Samuel P. Hanke, a pediatric cardiologist at the University of Cincinnati, who knows the heartbreak of SIDS firsthand.
“We know practicing safe sleep is hard. We have to be vigilant. We need to start adopting a mentality that safe sleep is not negotiable,” Dr. Hanke asserted. “We cannot emphasize enough that practicing safe sleep for every sleep is as important as buckling your child into a car seat for every drive. And just like car seats, this change won’t occur overnight.”
Federal commitment
Since the 1970s, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) in Bethesda, Md., has been supporting and performing much of the research on which the updated recommendations are based. This research continues to help identify areas where greater efforts are needed, according to acting director Catherine Y. Spong, MD.
NICHD also conducts and collaborates on related education campaigns, such as Safe to Sleep, to disseminate messages such as those in the updated AAP recommendations as widely as possible.
“I encourage all physicians, pediatricians, nurses, and other health care and child care providers to lend their authoritative voices to the Safe to Sleep effort,” Dr. Spong said. “Join us all in sharing safe infant sleep recommendations and in supporting parents and caregivers to make informed decisions that will help keep their baby safe during sleep.”
A closer look at setting
Published in conjunction with the guidelines is a study on risk factors that looked at the role of the setting in which sleep-related infant deaths occur (Pediatrics. 2016 Oct 24:e20161124).
The analysis of nearly 12,000 such deaths found that, relative to counterparts who died in their home, infants who died outside of their home were more likely to be in a stroller or car seat at the time (adjusted odds ratio, 2.6) and in other locations, such as on the floor or a futon (1.9), and to have been placed prone (1.1). They were less likely to have been sharing a bed (0.7).
The groups did not differ in terms of whether the infant was sleeping in an adult bed or on a person, on a couch or chair, or with any objects in their sleep environment.
“Caregivers should be educated on the importance of placing infants to sleep supine in cribs/bassinets to protect against sleep-related deaths, both in and out of the home,” conclude the investigators, one of whom disclosed serving as a paid expert witness in cases of sleep-related infant death.
EXPERT ANALYSIS FROM AAP 16
Medication-assisted treatment in group settings may result in greater job satisfaction, more reimbursements
WASHINGTON – For practices that offer medication-assisted treatment but perhaps are struggling to balance follow-up appointments with new patient inductions, Leah K. Bauer, MD, has a suggestion: group sessions.
“It’s a lot of fun, and makes my practice more dynamic. It gets me out of the grind of ‘see a patient; write a note; repeat,’ ” Dr. Bauer said at the American Psychiatric Association’s Institute on Psychiatric Services.
Compressing 26.5 hours of individual clinical time into 12 hours of monthly group sessions held twice a week for 90 minutes each, Dr. Bauer said, resulted in an additional $41,000 of revenue annually, with inductions doubling from 8 to 16 per month.
One reason is that despite the sessions taking place in a group setting, she and her staff bill for a series of individual appointments using the CPT code 99212. “It is perfectly legal, and not very recognized,” Dr. Bauer said, noting that the sessions are in a group context, but that she does get to have one-on-one interaction with her patients with the added therapeutic value that peer support brings.
Modeling appropriate behavior is easier in the group setting, she said: “Patients don’t all have to test the same limits.” Instead, they can learn from the interaction of another patient with Dr. Bauer as the therapist. The group setting also helps her deliver more consistent care to all her patients, she said. “I am more conscious of what I am saying.”
A hospitalist and psychiatrist, Dr. Bauer leads group MAT with the help of a clinician cofacilitator who she says reinforces what is being said in the group and acts as a scribe, reducing Dr. Bauer’s administrative burden. “This improves my job satisfaction tremendously,” she said.
Patients sign a “check-in” sheet that also serves as their treatment plan that includes their goals and objectives. It includes the patients’ written self-reflections, what their week was like, and other entries about their mood and struggles with their recovery. The information also is recorded in their patient records. “The sheet is problem focused, and has a lot of counseling and coordination of care built in,” Dr. Bauer said.
If a patient comes to the session late, there is no lost time or productivity for the MAT team, because the group meets regardless of who attends. Patients can come as much or as little as they like every 1-4 weeks. “It’s very flexible,” Dr. Bauer said.
She does not have data on her patient outcomes in the group setting vs. the individual one, but Dr. Bauer said in an interview that she believes it is as effective and allows more people who need MAT to receive it, because few clinics in her state offer it.
The group structure does place more demand on the hospital’s pharmacy, she said, in that, after the sessions, patients arrive en masse to fill their buprenorphine prescriptions.
Questions about confidentiality do arise, although each session begins with a reminder to keep private what is shared during the meetings. However, Dr. Bauer said, she thinks some patients remain reluctant to speak their minds for fear of what they say not remaining confidential. “This can limit the depth of what’s discussed,” she said.
Dr. Bauer said she did not have any relevant financial disclosures.
[email protected]
On Twitter @whitneymcknight
WASHINGTON – For practices that offer medication-assisted treatment but perhaps are struggling to balance follow-up appointments with new patient inductions, Leah K. Bauer, MD, has a suggestion: group sessions.
“It’s a lot of fun, and makes my practice more dynamic. It gets me out of the grind of ‘see a patient; write a note; repeat,’ ” Dr. Bauer said at the American Psychiatric Association’s Institute on Psychiatric Services.
Compressing 26.5 hours of individual clinical time into 12 hours of monthly group sessions held twice a week for 90 minutes each, Dr. Bauer said, resulted in an additional $41,000 of revenue annually, with inductions doubling from 8 to 16 per month.
One reason is that despite the sessions taking place in a group setting, she and her staff bill for a series of individual appointments using the CPT code 99212. “It is perfectly legal, and not very recognized,” Dr. Bauer said, noting that the sessions are in a group context, but that she does get to have one-on-one interaction with her patients with the added therapeutic value that peer support brings.
Modeling appropriate behavior is easier in the group setting, she said: “Patients don’t all have to test the same limits.” Instead, they can learn from the interaction of another patient with Dr. Bauer as the therapist. The group setting also helps her deliver more consistent care to all her patients, she said. “I am more conscious of what I am saying.”
A hospitalist and psychiatrist, Dr. Bauer leads group MAT with the help of a clinician cofacilitator who she says reinforces what is being said in the group and acts as a scribe, reducing Dr. Bauer’s administrative burden. “This improves my job satisfaction tremendously,” she said.
Patients sign a “check-in” sheet that also serves as their treatment plan that includes their goals and objectives. It includes the patients’ written self-reflections, what their week was like, and other entries about their mood and struggles with their recovery. The information also is recorded in their patient records. “The sheet is problem focused, and has a lot of counseling and coordination of care built in,” Dr. Bauer said.
If a patient comes to the session late, there is no lost time or productivity for the MAT team, because the group meets regardless of who attends. Patients can come as much or as little as they like every 1-4 weeks. “It’s very flexible,” Dr. Bauer said.
She does not have data on her patient outcomes in the group setting vs. the individual one, but Dr. Bauer said in an interview that she believes it is as effective and allows more people who need MAT to receive it, because few clinics in her state offer it.
The group structure does place more demand on the hospital’s pharmacy, she said, in that, after the sessions, patients arrive en masse to fill their buprenorphine prescriptions.
Questions about confidentiality do arise, although each session begins with a reminder to keep private what is shared during the meetings. However, Dr. Bauer said, she thinks some patients remain reluctant to speak their minds for fear of what they say not remaining confidential. “This can limit the depth of what’s discussed,” she said.
Dr. Bauer said she did not have any relevant financial disclosures.
[email protected]
On Twitter @whitneymcknight
WASHINGTON – For practices that offer medication-assisted treatment but perhaps are struggling to balance follow-up appointments with new patient inductions, Leah K. Bauer, MD, has a suggestion: group sessions.
“It’s a lot of fun, and makes my practice more dynamic. It gets me out of the grind of ‘see a patient; write a note; repeat,’ ” Dr. Bauer said at the American Psychiatric Association’s Institute on Psychiatric Services.
Compressing 26.5 hours of individual clinical time into 12 hours of monthly group sessions held twice a week for 90 minutes each, Dr. Bauer said, resulted in an additional $41,000 of revenue annually, with inductions doubling from 8 to 16 per month.
One reason is that despite the sessions taking place in a group setting, she and her staff bill for a series of individual appointments using the CPT code 99212. “It is perfectly legal, and not very recognized,” Dr. Bauer said, noting that the sessions are in a group context, but that she does get to have one-on-one interaction with her patients with the added therapeutic value that peer support brings.
Modeling appropriate behavior is easier in the group setting, she said: “Patients don’t all have to test the same limits.” Instead, they can learn from the interaction of another patient with Dr. Bauer as the therapist. The group setting also helps her deliver more consistent care to all her patients, she said. “I am more conscious of what I am saying.”
A hospitalist and psychiatrist, Dr. Bauer leads group MAT with the help of a clinician cofacilitator who she says reinforces what is being said in the group and acts as a scribe, reducing Dr. Bauer’s administrative burden. “This improves my job satisfaction tremendously,” she said.
Patients sign a “check-in” sheet that also serves as their treatment plan that includes their goals and objectives. It includes the patients’ written self-reflections, what their week was like, and other entries about their mood and struggles with their recovery. The information also is recorded in their patient records. “The sheet is problem focused, and has a lot of counseling and coordination of care built in,” Dr. Bauer said.
If a patient comes to the session late, there is no lost time or productivity for the MAT team, because the group meets regardless of who attends. Patients can come as much or as little as they like every 1-4 weeks. “It’s very flexible,” Dr. Bauer said.
She does not have data on her patient outcomes in the group setting vs. the individual one, but Dr. Bauer said in an interview that she believes it is as effective and allows more people who need MAT to receive it, because few clinics in her state offer it.
The group structure does place more demand on the hospital’s pharmacy, she said, in that, after the sessions, patients arrive en masse to fill their buprenorphine prescriptions.
Questions about confidentiality do arise, although each session begins with a reminder to keep private what is shared during the meetings. However, Dr. Bauer said, she thinks some patients remain reluctant to speak their minds for fear of what they say not remaining confidential. “This can limit the depth of what’s discussed,” she said.
Dr. Bauer said she did not have any relevant financial disclosures.
[email protected]
On Twitter @whitneymcknight
Verrucous Plaque on the Leg
Blastomycosis
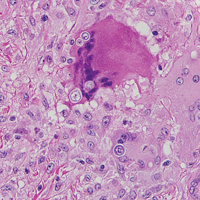
Blastomycosis is caused by Blastomyces dermatitidis, which is endemic in the Midwestern and southeastern United States where it occurs environmentally in wood and soil. Unlike many fungal infections, blastomycosis most often develops in immunocompetent hosts. Infection is usually acquired via inhalation,1 and cutaneous disease typically is secondary to pulmonary infection. Although not common, traumatic inoculation also can cause cutaneous blastomycosis. Skin lesions include crusted verrucous nodules and plaques with elevated borders.1,2 Histologic features include pseudoepitheliomatous hyperplasia with intraepidermal neutrophilic microabscesses (Figure 1), and a neutrophilic and granulomatous dermal infiltrate. Organisms often are found within histiocytes (quiz image) or small abscesses. The yeasts usually are 8 to 15 µm in diameter with a thick cell wall and occasionally display broad-based budding.
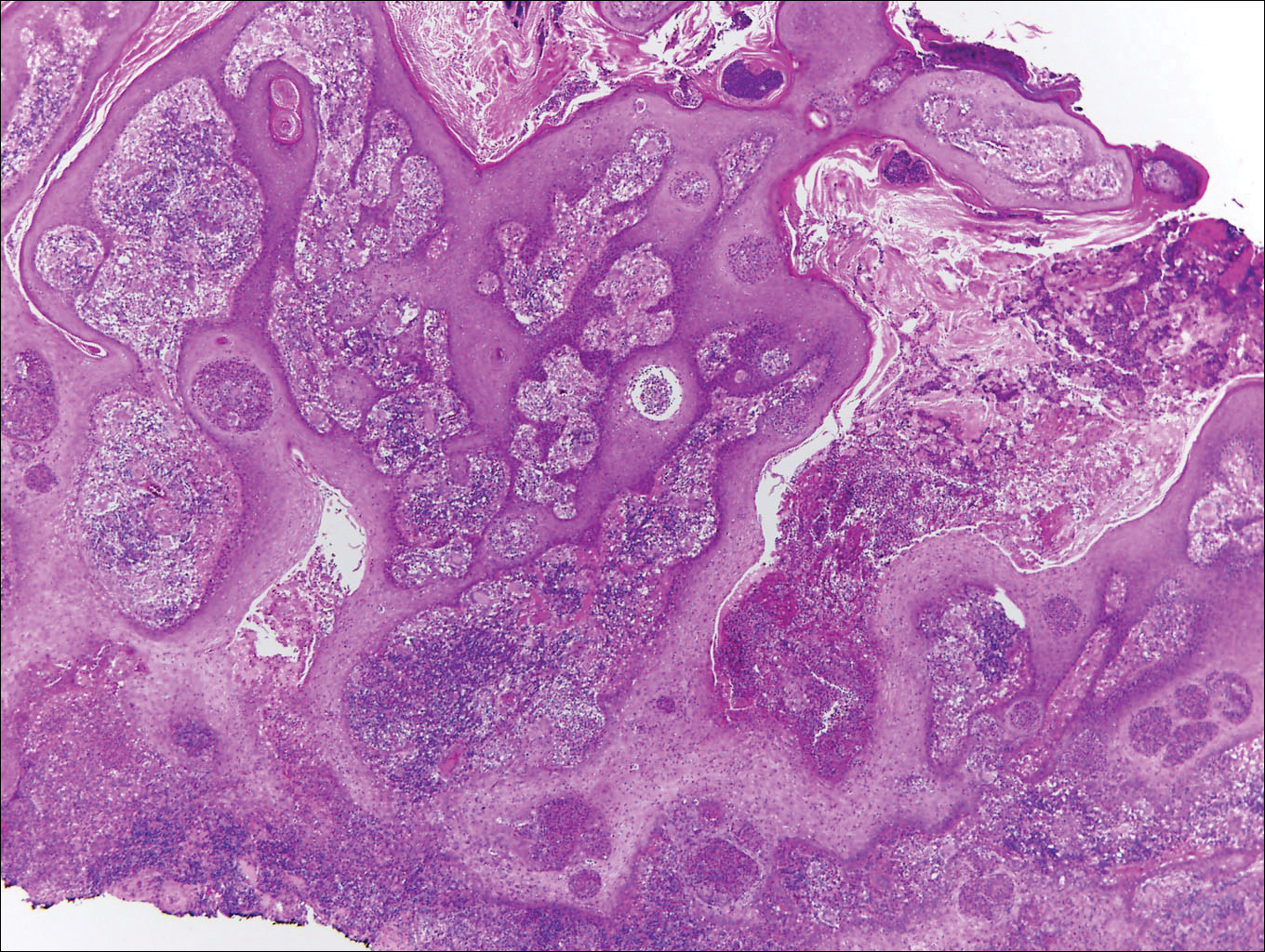
Chromoblastomycosis is caused by dematiaceous (pigmented) fungi, including Fonsecaea, Phialophora, Cladophialophora, and Rhinocladiella species,3 which are present in soil and vegetable debris in tropical and subtropical regions. Infection typically occurs in the foot or lower leg from traumatic inoculation, such as a thorn or splinter injury.2 Histologically, chromoblastomycosis is characterized by pseudoepitheliomatous hyperplasia; suppurative and granulomatous dermatitis; and sclerotic (Medlar) bodies, which are 5 to 12 µm in diameter, round, brown, sometimes septate cells resembling copper pennies (Figure 2).2
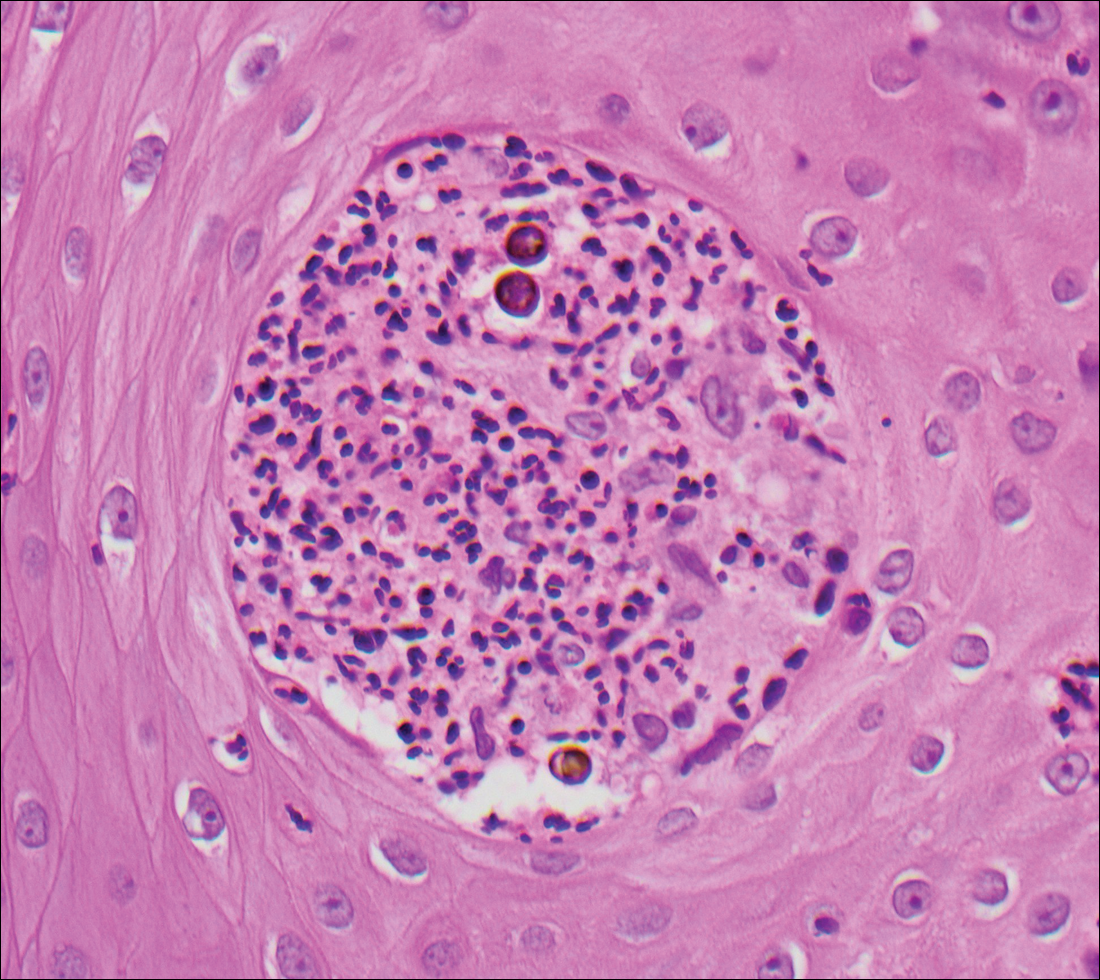
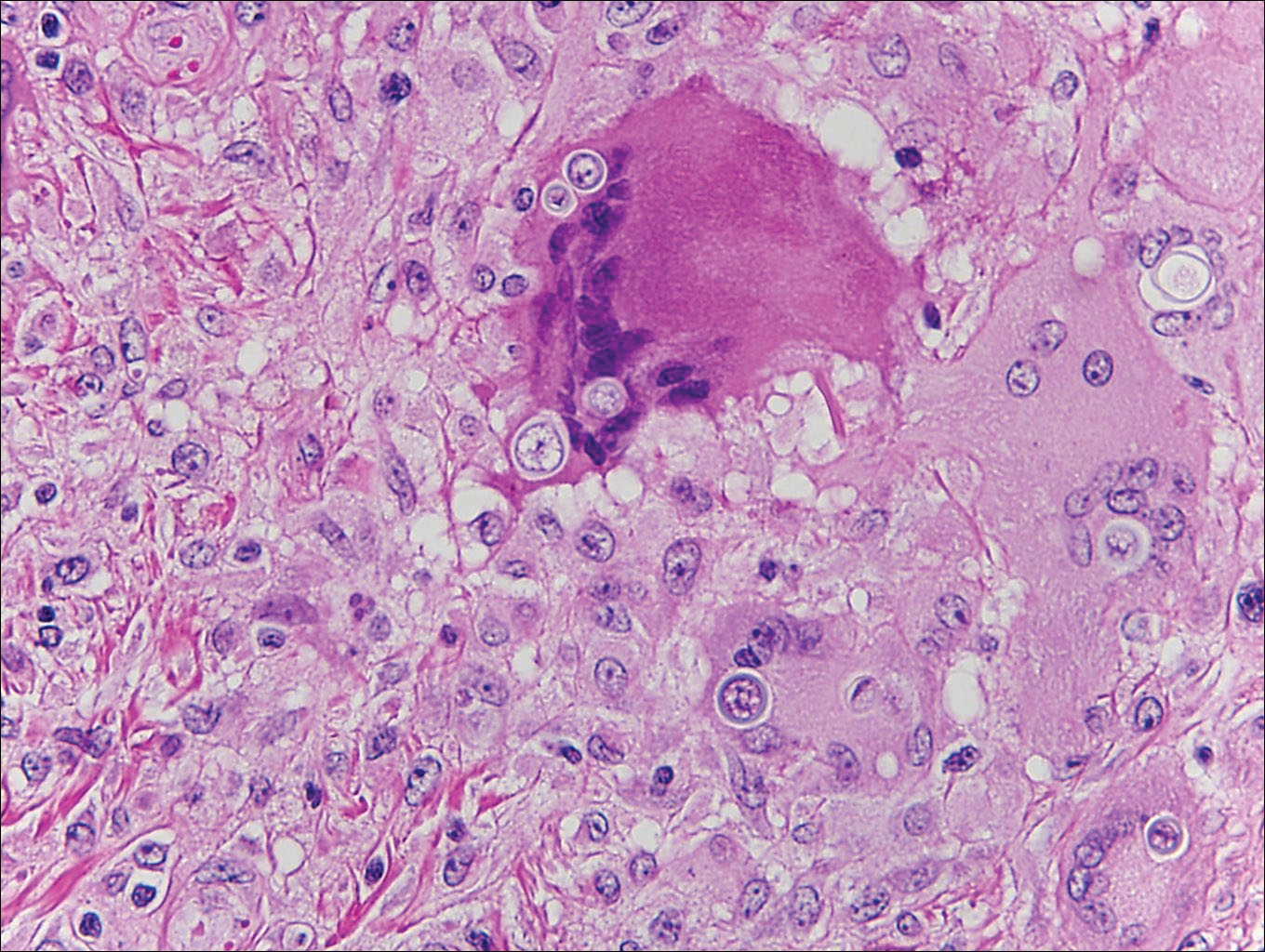
Coccidioidomycosis is caused by Coccidioides immitis, which is found in soil in the southwestern United States. Infection most often occurs via inhalation of airborne arthrospores.2 Cutaneous lesions occasionally are observed following dissemination or rarely following primary inoculation injury. They may present as papules, nodules, pustules, plaques, and ulcers, with the face being the most commonly affected site.1 Histologically, coccidioidomycosis is characterized by pseudoepitheliomatous hyperplasia, suppurative and granulomatous dermatitis, and large spherules (up to 100 µm in diameter) containing numerous small endospores (Figure 3).
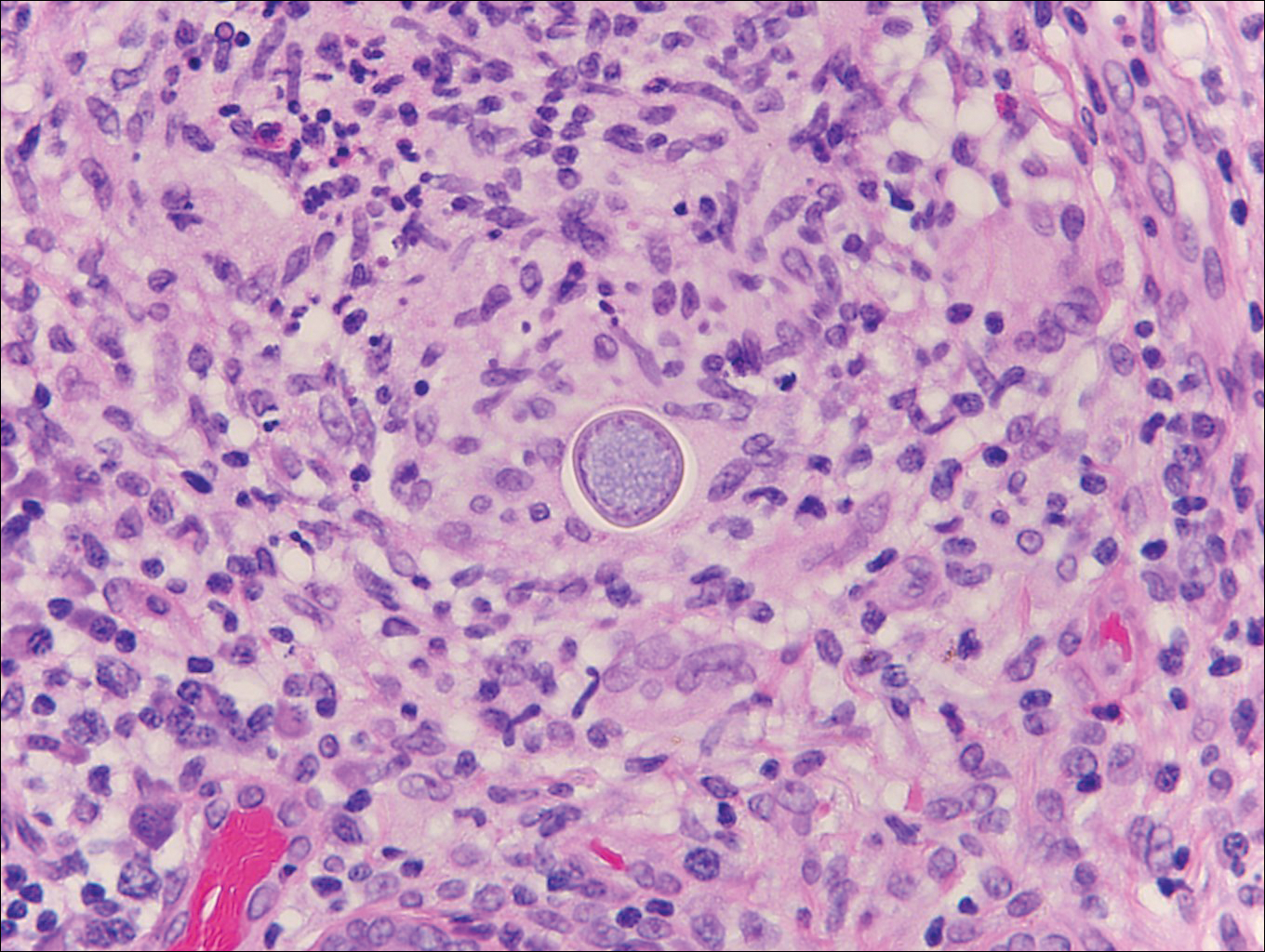
Cryptococcosis is caused by Cryptococcus neoformans, a fungus found in soil, fruit, and pigeon droppings throughout the world.2,3 The most common route of infection is via the respiratory tract. Systemic spread and central nervous system involvement may occur in immunocompromised hosts.2 Skin involvement is uncommon and may present on the head and neck with umbilicated papules, pustules, nodules, plaques, or ulcers. Histologically, Cryptococcus is a spherical yeast, often 4 to 20 µm in diameter. Replication is by narrow-based budding. A characteristic feature is a mucoid capsule, which retracts during processing, leaving a clear space around the yeast (Figure 4). When present, the mucoid capsule can be highlighted on mucicarmine or Alcian blue staining. Histologic variants of cryptococcosis include granulomatous (high host immune response), gelatinous (low host immune response), and suppurative types.3
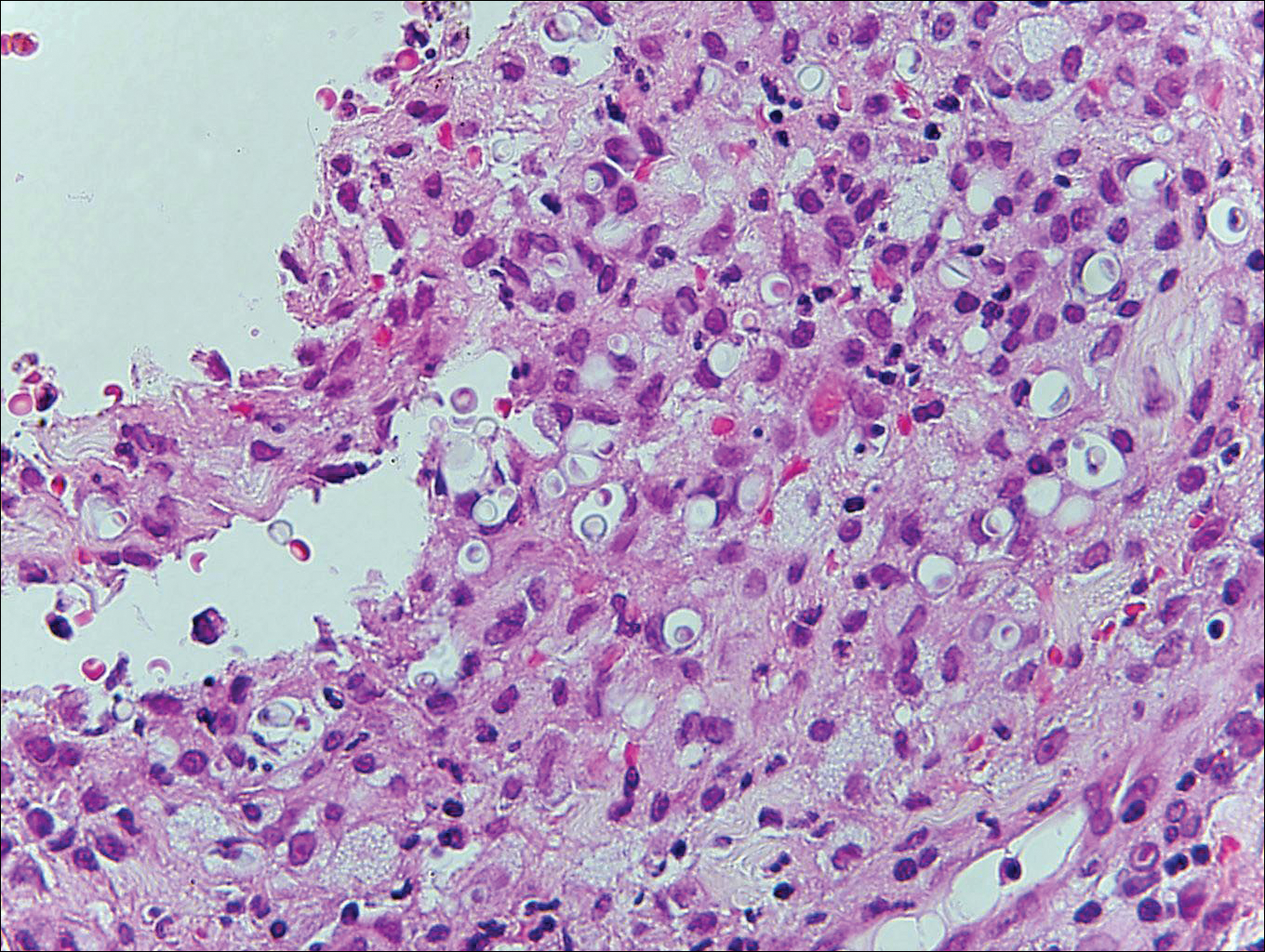
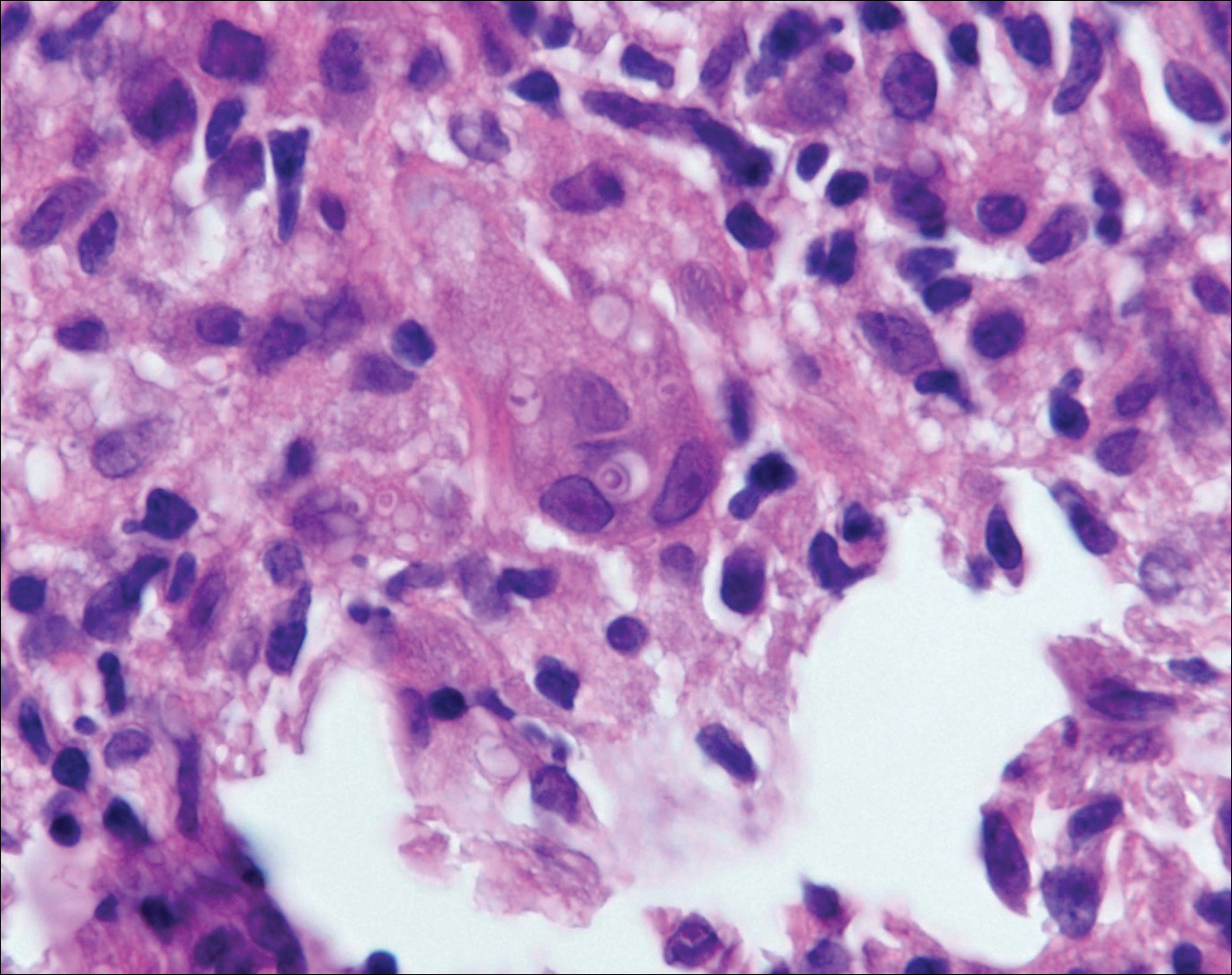
Histoplasmosis is caused by Histoplasma capsulatum, which occurs in soil and bird and bat droppings, with exposure primarily via inhalation. Cutaneous histoplasmosis is almost always a feature of disseminated disease, which occurs most commonly in immunosuppressed individuals.1 Skin lesions may present as macules, papules, indurated plaques, ulcers, purpura, panniculitis, and subcutaneous nodules.2 Histologically, there is a granulomatous and neutrophilic infiltrate within the dermis and subcutis. Yeasts are small (2-4 µm in diameter) and are observed within the cytoplasm of macrophages (Figure 5) where they appear as basophilic dots, sometimes surrounded by an artifactual clear space (pseudocapsule).2
- Bolognia JL, Jorizzo JL, Shaffer JV. Dermatology. 3rd ed. Vol 2. Philadelphia, PA: Elsevier/Saunders; 2012.
- Calonje JE, Brenn T, Lazar AJ, et al. McKee's Pathology of the Skin. 4th ed. St. Louis, MO: Elsevier/Saunders; 2012.
- Schwarzenberger K, Werchniak A, Ko C. Requisites in Dermatology: General Dermatology. Philadelphia, PA: Elsevier/Saunders; 2009.
Blastomycosis
Blastomycosis is caused by Blastomyces dermatitidis, which is endemic in the Midwestern and southeastern United States where it occurs environmentally in wood and soil. Unlike many fungal infections, blastomycosis most often develops in immunocompetent hosts. Infection is usually acquired via inhalation,1 and cutaneous disease typically is secondary to pulmonary infection. Although not common, traumatic inoculation also can cause cutaneous blastomycosis. Skin lesions include crusted verrucous nodules and plaques with elevated borders.1,2 Histologic features include pseudoepitheliomatous hyperplasia with intraepidermal neutrophilic microabscesses (Figure 1), and a neutrophilic and granulomatous dermal infiltrate. Organisms often are found within histiocytes (quiz image) or small abscesses. The yeasts usually are 8 to 15 µm in diameter with a thick cell wall and occasionally display broad-based budding.

Chromoblastomycosis is caused by dematiaceous (pigmented) fungi, including Fonsecaea, Phialophora, Cladophialophora, and Rhinocladiella species,3 which are present in soil and vegetable debris in tropical and subtropical regions. Infection typically occurs in the foot or lower leg from traumatic inoculation, such as a thorn or splinter injury.2 Histologically, chromoblastomycosis is characterized by pseudoepitheliomatous hyperplasia; suppurative and granulomatous dermatitis; and sclerotic (Medlar) bodies, which are 5 to 12 µm in diameter, round, brown, sometimes septate cells resembling copper pennies (Figure 2).2

Coccidioidomycosis is caused by Coccidioides immitis, which is found in soil in the southwestern United States. Infection most often occurs via inhalation of airborne arthrospores.2 Cutaneous lesions occasionally are observed following dissemination or rarely following primary inoculation injury. They may present as papules, nodules, pustules, plaques, and ulcers, with the face being the most commonly affected site.1 Histologically, coccidioidomycosis is characterized by pseudoepitheliomatous hyperplasia, suppurative and granulomatous dermatitis, and large spherules (up to 100 µm in diameter) containing numerous small endospores (Figure 3).

Cryptococcosis is caused by Cryptococcus neoformans, a fungus found in soil, fruit, and pigeon droppings throughout the world.2,3 The most common route of infection is via the respiratory tract. Systemic spread and central nervous system involvement may occur in immunocompromised hosts.2 Skin involvement is uncommon and may present on the head and neck with umbilicated papules, pustules, nodules, plaques, or ulcers. Histologically, Cryptococcus is a spherical yeast, often 4 to 20 µm in diameter. Replication is by narrow-based budding. A characteristic feature is a mucoid capsule, which retracts during processing, leaving a clear space around the yeast (Figure 4). When present, the mucoid capsule can be highlighted on mucicarmine or Alcian blue staining. Histologic variants of cryptococcosis include granulomatous (high host immune response), gelatinous (low host immune response), and suppurative types.3

Histoplasmosis is caused by Histoplasma capsulatum, which occurs in soil and bird and bat droppings, with exposure primarily via inhalation. Cutaneous histoplasmosis is almost always a feature of disseminated disease, which occurs most commonly in immunosuppressed individuals.1 Skin lesions may present as macules, papules, indurated plaques, ulcers, purpura, panniculitis, and subcutaneous nodules.2 Histologically, there is a granulomatous and neutrophilic infiltrate within the dermis and subcutis. Yeasts are small (2-4 µm in diameter) and are observed within the cytoplasm of macrophages (Figure 5) where they appear as basophilic dots, sometimes surrounded by an artifactual clear space (pseudocapsule).2

Blastomycosis
Blastomycosis is caused by Blastomyces dermatitidis, which is endemic in the Midwestern and southeastern United States where it occurs environmentally in wood and soil. Unlike many fungal infections, blastomycosis most often develops in immunocompetent hosts. Infection is usually acquired via inhalation,1 and cutaneous disease typically is secondary to pulmonary infection. Although not common, traumatic inoculation also can cause cutaneous blastomycosis. Skin lesions include crusted verrucous nodules and plaques with elevated borders.1,2 Histologic features include pseudoepitheliomatous hyperplasia with intraepidermal neutrophilic microabscesses (Figure 1), and a neutrophilic and granulomatous dermal infiltrate. Organisms often are found within histiocytes (quiz image) or small abscesses. The yeasts usually are 8 to 15 µm in diameter with a thick cell wall and occasionally display broad-based budding.

Chromoblastomycosis is caused by dematiaceous (pigmented) fungi, including Fonsecaea, Phialophora, Cladophialophora, and Rhinocladiella species,3 which are present in soil and vegetable debris in tropical and subtropical regions. Infection typically occurs in the foot or lower leg from traumatic inoculation, such as a thorn or splinter injury.2 Histologically, chromoblastomycosis is characterized by pseudoepitheliomatous hyperplasia; suppurative and granulomatous dermatitis; and sclerotic (Medlar) bodies, which are 5 to 12 µm in diameter, round, brown, sometimes septate cells resembling copper pennies (Figure 2).2

Coccidioidomycosis is caused by Coccidioides immitis, which is found in soil in the southwestern United States. Infection most often occurs via inhalation of airborne arthrospores.2 Cutaneous lesions occasionally are observed following dissemination or rarely following primary inoculation injury. They may present as papules, nodules, pustules, plaques, and ulcers, with the face being the most commonly affected site.1 Histologically, coccidioidomycosis is characterized by pseudoepitheliomatous hyperplasia, suppurative and granulomatous dermatitis, and large spherules (up to 100 µm in diameter) containing numerous small endospores (Figure 3).

Cryptococcosis is caused by Cryptococcus neoformans, a fungus found in soil, fruit, and pigeon droppings throughout the world.2,3 The most common route of infection is via the respiratory tract. Systemic spread and central nervous system involvement may occur in immunocompromised hosts.2 Skin involvement is uncommon and may present on the head and neck with umbilicated papules, pustules, nodules, plaques, or ulcers. Histologically, Cryptococcus is a spherical yeast, often 4 to 20 µm in diameter. Replication is by narrow-based budding. A characteristic feature is a mucoid capsule, which retracts during processing, leaving a clear space around the yeast (Figure 4). When present, the mucoid capsule can be highlighted on mucicarmine or Alcian blue staining. Histologic variants of cryptococcosis include granulomatous (high host immune response), gelatinous (low host immune response), and suppurative types.3

Histoplasmosis is caused by Histoplasma capsulatum, which occurs in soil and bird and bat droppings, with exposure primarily via inhalation. Cutaneous histoplasmosis is almost always a feature of disseminated disease, which occurs most commonly in immunosuppressed individuals.1 Skin lesions may present as macules, papules, indurated plaques, ulcers, purpura, panniculitis, and subcutaneous nodules.2 Histologically, there is a granulomatous and neutrophilic infiltrate within the dermis and subcutis. Yeasts are small (2-4 µm in diameter) and are observed within the cytoplasm of macrophages (Figure 5) where they appear as basophilic dots, sometimes surrounded by an artifactual clear space (pseudocapsule).2

- Bolognia JL, Jorizzo JL, Shaffer JV. Dermatology. 3rd ed. Vol 2. Philadelphia, PA: Elsevier/Saunders; 2012.
- Calonje JE, Brenn T, Lazar AJ, et al. McKee's Pathology of the Skin. 4th ed. St. Louis, MO: Elsevier/Saunders; 2012.
- Schwarzenberger K, Werchniak A, Ko C. Requisites in Dermatology: General Dermatology. Philadelphia, PA: Elsevier/Saunders; 2009.
- Bolognia JL, Jorizzo JL, Shaffer JV. Dermatology. 3rd ed. Vol 2. Philadelphia, PA: Elsevier/Saunders; 2012.
- Calonje JE, Brenn T, Lazar AJ, et al. McKee's Pathology of the Skin. 4th ed. St. Louis, MO: Elsevier/Saunders; 2012.
- Schwarzenberger K, Werchniak A, Ko C. Requisites in Dermatology: General Dermatology. Philadelphia, PA: Elsevier/Saunders; 2009.
2016 a big year for mental health legislation
• The Comprehensive Addiction and Recovery Act. Signed into law in July, CARA is primarily intended, as its name indicates, to comprehensively address the opioid addiction crisis. The law includes components aimed at prevention, treatment, recovery, law enforcement, criminal justice reform, and overdose treatment. It also has provisions aimed at expanded prescription drug monitoring programs and other drug abuse prevention mechanisms.
Another important part of this law is the expansion of buprenorphine prescribing rights to nurse practitioners and physician assistants who obtain the necessary training. Public comment is being taken through Nov. 1, on what the requirements should be. This provision will be revisited in 2021.
In all, the law allocates $25 million annually from 2017 through 2021 to expand medication-assisted treatment (MAT). An abundance of evidence shows that MAT – the use of opioid agonists and partial opioid agonists such as methadone, naltrexone, and buprenorphine, in combination with psychosocial therapies – is more effective than either modality alone.
• The Helping Families in Mental Health Crisis Act. Originating in 2013 in response to the Newtown, Conn., massacre, this bill (H.R. 2646) is focused on serious mental illness. It passed the House in July with near unanimous support, but awaits the Senate to sort out its own version of the bill. If this bill is passed, HIPAA laws would loosen, allowing practices to share all but psychotherapy notes with caregivers and guardians of people with serious mental illness. Practices also could bill Medicaid and Medicare for medical and mental health services delivered on the same day, and there would be support for the better integration of medical and mental health electronic medical records. The current 30-day limit on inpatient psychiatric facility services paid for by Medicaid would be lifted, and $20 million in grants would be made available for assisted outpatient treatment of this population. Grants for expanded use of telepsychiatry also would be available.
• The Mental Health Reform Act of 2016. Essentially the companion bill to the one passed in the House, this bill (S. 2680) also calls for the expansion of telepsychiatry, especially for pediatric and adolescent mental and behavioral health needs. If it becomes law, grant money would be made available for better integration of primary and behavioral health care services. This bill calls for strengthening current mental health parity laws by requiring additional federal guidance to help insurance plans comply. The bill currently is stalled on the Senate floor and is not expected to be revisited until after the presidential election.
• The Medication Assisted Treatment for Opioid Use Disorders final rule. As of August, the rule allows addiction medicine specialists to treat up to 275 patients using buprenorphine for substance use disorder annually. Previously, they were held to treating no more than 100 such patients per year. For practices not already licensed to provide MAT, this rule doesn’t have much direct impact. However, for practices with patients on their panels who are struggling with substance use disorders, this could expand available referral resources.
• The Quality Payment Program final rule. While this rule – borne of the MACRA (Medicare Access and CHIP Reauthorization Act) law that is now referred to as the Quality Payment Program – does not directly address mental and behavioral health, it directly affects delivery of these services when it goes into effect in 2017. Because mental and behavioral health outcomes of patient panels that include Medicare populations will be assessed as part of overall patient outcomes, providing effective, integrated services will be imperative. How severely a practice will be penalized for poor outcomes will depend upon how that practice chooses to set up its reimbursement structures and quality metrics over the next few years.
[email protected]
On Twitter @whitneymcknight
• The Comprehensive Addiction and Recovery Act. Signed into law in July, CARA is primarily intended, as its name indicates, to comprehensively address the opioid addiction crisis. The law includes components aimed at prevention, treatment, recovery, law enforcement, criminal justice reform, and overdose treatment. It also has provisions aimed at expanded prescription drug monitoring programs and other drug abuse prevention mechanisms.
Another important part of this law is the expansion of buprenorphine prescribing rights to nurse practitioners and physician assistants who obtain the necessary training. Public comment is being taken through Nov. 1, on what the requirements should be. This provision will be revisited in 2021.
In all, the law allocates $25 million annually from 2017 through 2021 to expand medication-assisted treatment (MAT). An abundance of evidence shows that MAT – the use of opioid agonists and partial opioid agonists such as methadone, naltrexone, and buprenorphine, in combination with psychosocial therapies – is more effective than either modality alone.
• The Helping Families in Mental Health Crisis Act. Originating in 2013 in response to the Newtown, Conn., massacre, this bill (H.R. 2646) is focused on serious mental illness. It passed the House in July with near unanimous support, but awaits the Senate to sort out its own version of the bill. If this bill is passed, HIPAA laws would loosen, allowing practices to share all but psychotherapy notes with caregivers and guardians of people with serious mental illness. Practices also could bill Medicaid and Medicare for medical and mental health services delivered on the same day, and there would be support for the better integration of medical and mental health electronic medical records. The current 30-day limit on inpatient psychiatric facility services paid for by Medicaid would be lifted, and $20 million in grants would be made available for assisted outpatient treatment of this population. Grants for expanded use of telepsychiatry also would be available.
• The Mental Health Reform Act of 2016. Essentially the companion bill to the one passed in the House, this bill (S. 2680) also calls for the expansion of telepsychiatry, especially for pediatric and adolescent mental and behavioral health needs. If it becomes law, grant money would be made available for better integration of primary and behavioral health care services. This bill calls for strengthening current mental health parity laws by requiring additional federal guidance to help insurance plans comply. The bill currently is stalled on the Senate floor and is not expected to be revisited until after the presidential election.
• The Medication Assisted Treatment for Opioid Use Disorders final rule. As of August, the rule allows addiction medicine specialists to treat up to 275 patients using buprenorphine for substance use disorder annually. Previously, they were held to treating no more than 100 such patients per year. For practices not already licensed to provide MAT, this rule doesn’t have much direct impact. However, for practices with patients on their panels who are struggling with substance use disorders, this could expand available referral resources.
• The Quality Payment Program final rule. While this rule – borne of the MACRA (Medicare Access and CHIP Reauthorization Act) law that is now referred to as the Quality Payment Program – does not directly address mental and behavioral health, it directly affects delivery of these services when it goes into effect in 2017. Because mental and behavioral health outcomes of patient panels that include Medicare populations will be assessed as part of overall patient outcomes, providing effective, integrated services will be imperative. How severely a practice will be penalized for poor outcomes will depend upon how that practice chooses to set up its reimbursement structures and quality metrics over the next few years.
[email protected]
On Twitter @whitneymcknight
• The Comprehensive Addiction and Recovery Act. Signed into law in July, CARA is primarily intended, as its name indicates, to comprehensively address the opioid addiction crisis. The law includes components aimed at prevention, treatment, recovery, law enforcement, criminal justice reform, and overdose treatment. It also has provisions aimed at expanded prescription drug monitoring programs and other drug abuse prevention mechanisms.
Another important part of this law is the expansion of buprenorphine prescribing rights to nurse practitioners and physician assistants who obtain the necessary training. Public comment is being taken through Nov. 1, on what the requirements should be. This provision will be revisited in 2021.
In all, the law allocates $25 million annually from 2017 through 2021 to expand medication-assisted treatment (MAT). An abundance of evidence shows that MAT – the use of opioid agonists and partial opioid agonists such as methadone, naltrexone, and buprenorphine, in combination with psychosocial therapies – is more effective than either modality alone.
• The Helping Families in Mental Health Crisis Act. Originating in 2013 in response to the Newtown, Conn., massacre, this bill (H.R. 2646) is focused on serious mental illness. It passed the House in July with near unanimous support, but awaits the Senate to sort out its own version of the bill. If this bill is passed, HIPAA laws would loosen, allowing practices to share all but psychotherapy notes with caregivers and guardians of people with serious mental illness. Practices also could bill Medicaid and Medicare for medical and mental health services delivered on the same day, and there would be support for the better integration of medical and mental health electronic medical records. The current 30-day limit on inpatient psychiatric facility services paid for by Medicaid would be lifted, and $20 million in grants would be made available for assisted outpatient treatment of this population. Grants for expanded use of telepsychiatry also would be available.
• The Mental Health Reform Act of 2016. Essentially the companion bill to the one passed in the House, this bill (S. 2680) also calls for the expansion of telepsychiatry, especially for pediatric and adolescent mental and behavioral health needs. If it becomes law, grant money would be made available for better integration of primary and behavioral health care services. This bill calls for strengthening current mental health parity laws by requiring additional federal guidance to help insurance plans comply. The bill currently is stalled on the Senate floor and is not expected to be revisited until after the presidential election.
• The Medication Assisted Treatment for Opioid Use Disorders final rule. As of August, the rule allows addiction medicine specialists to treat up to 275 patients using buprenorphine for substance use disorder annually. Previously, they were held to treating no more than 100 such patients per year. For practices not already licensed to provide MAT, this rule doesn’t have much direct impact. However, for practices with patients on their panels who are struggling with substance use disorders, this could expand available referral resources.
• The Quality Payment Program final rule. While this rule – borne of the MACRA (Medicare Access and CHIP Reauthorization Act) law that is now referred to as the Quality Payment Program – does not directly address mental and behavioral health, it directly affects delivery of these services when it goes into effect in 2017. Because mental and behavioral health outcomes of patient panels that include Medicare populations will be assessed as part of overall patient outcomes, providing effective, integrated services will be imperative. How severely a practice will be penalized for poor outcomes will depend upon how that practice chooses to set up its reimbursement structures and quality metrics over the next few years.
[email protected]
On Twitter @whitneymcknight
Aquatic Antagonists: Cutaneous Sea Urchin Spine Injury
Sea urchin injuries are commonly seen in coastal regions near both warm and cold salt water with frequent recreational water activities or fishing. Sea urchins belong to the class Echinoidea with approximately 600 species, of which roughly 80 are poisonous to humans.1,2 When a human comes in contact with a sea urchin, the spines of the sea urchin (made of calcium carbonate) can penetrate the skin and break off from the sea urchin, becoming embedded in the skin. Injuries from sea urchin spines are most commonly seen on the hands and feet, as the likelihood of contact with a sea urchin is greater on these sites. The severity of sea urchin spine injuries can vary widely, from minimal local trauma and pain to arthritis, synovitis, and occasionally systemic illness.1,3 It is important to recognize the wide variety of responses to sea urchin spine injuries and the impact of prompt treatment. Many published reports on injuries from sea urchin spines describe arthritis and synovitis from spines in the joints.1,2,4-6 Fewer reports discuss nonjoint injuries and the dermatologic aspects of sea urchin spine injuries.3,7,8 We pre-sent a case of a patient with a puncture injury from sea urchin spines that resulted in painful granulomas.
Case Report
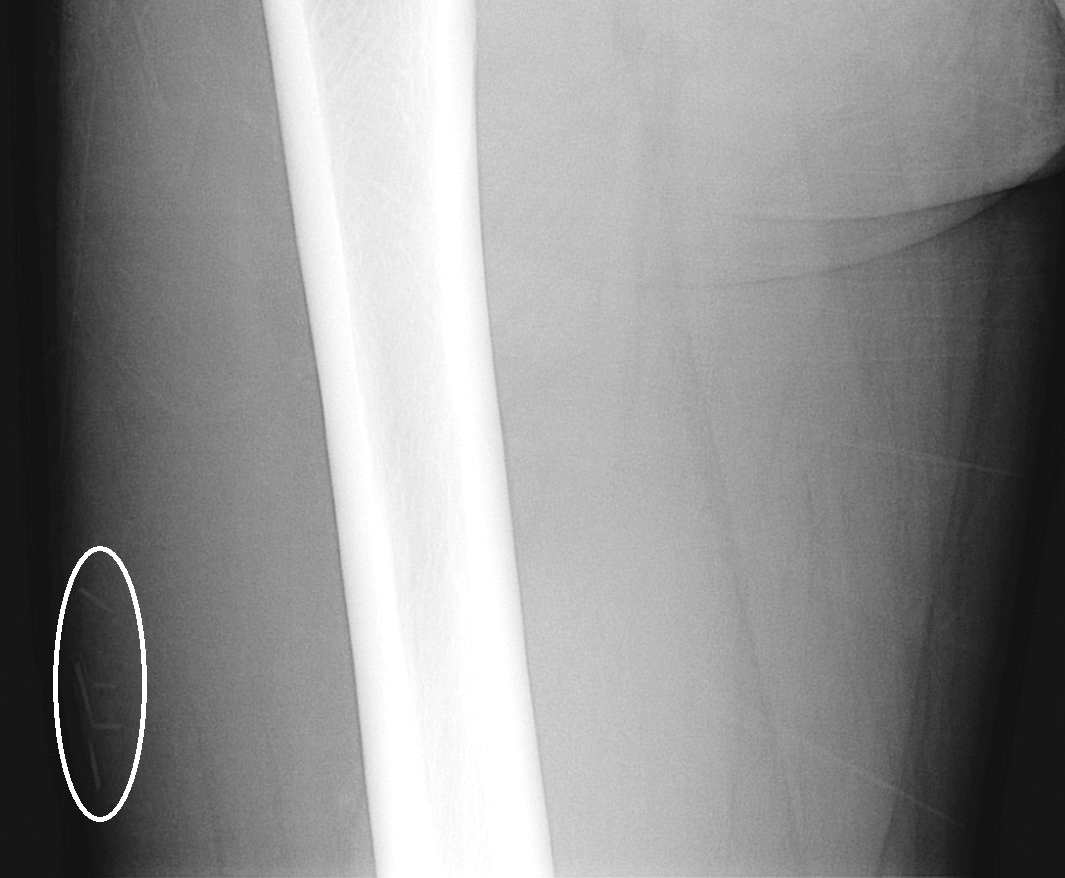
A 29-year-old otherwise healthy man was referred to our dermatology clinic by the university student health center due to continued pain in the right thigh. Five weeks prior to presentation to the student health center, the patient had fallen on a sea urchin while snorkeling in Hawaii. Sea urchin spines became lodged in the right thigh, some of which were removed in a local medical clinic in Hawaii. He was given oral antibiotics prior to his return home. A plain film radiograph of the affected area ordered by the student health center showed several punctate and linear densities in the lateral aspect of the right mid thigh (Figure 1). These findings were consistent with sea urchin spines within the superficial soft tissues of the lateral thigh.
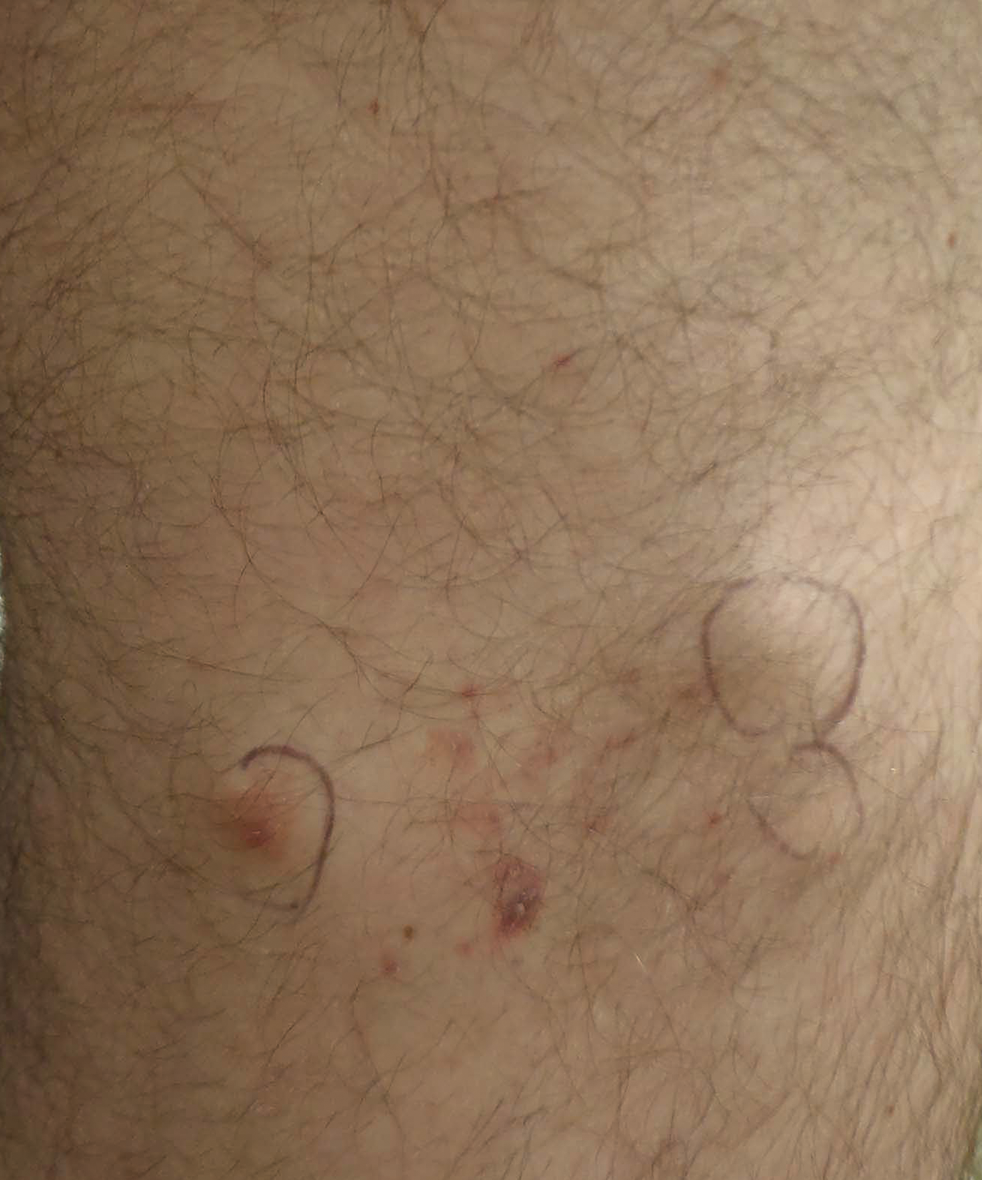
At the time of presentation to our dermatology clinic, the patient reported sharp intermittent pain localized to the right thigh. The patient denied any fever, chills, or pain in the joints. On physical examination, there were several firm nodules on the right thigh, ranging from 4 to 20 mm in diameter (Figure 2). The nodules were tender to palpation with some surrounding edema. Drainage was not noted. Several scars were visible at sites of the original puncture injuries and removal of the spines.
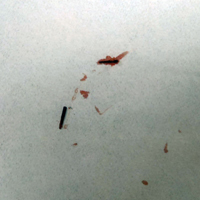
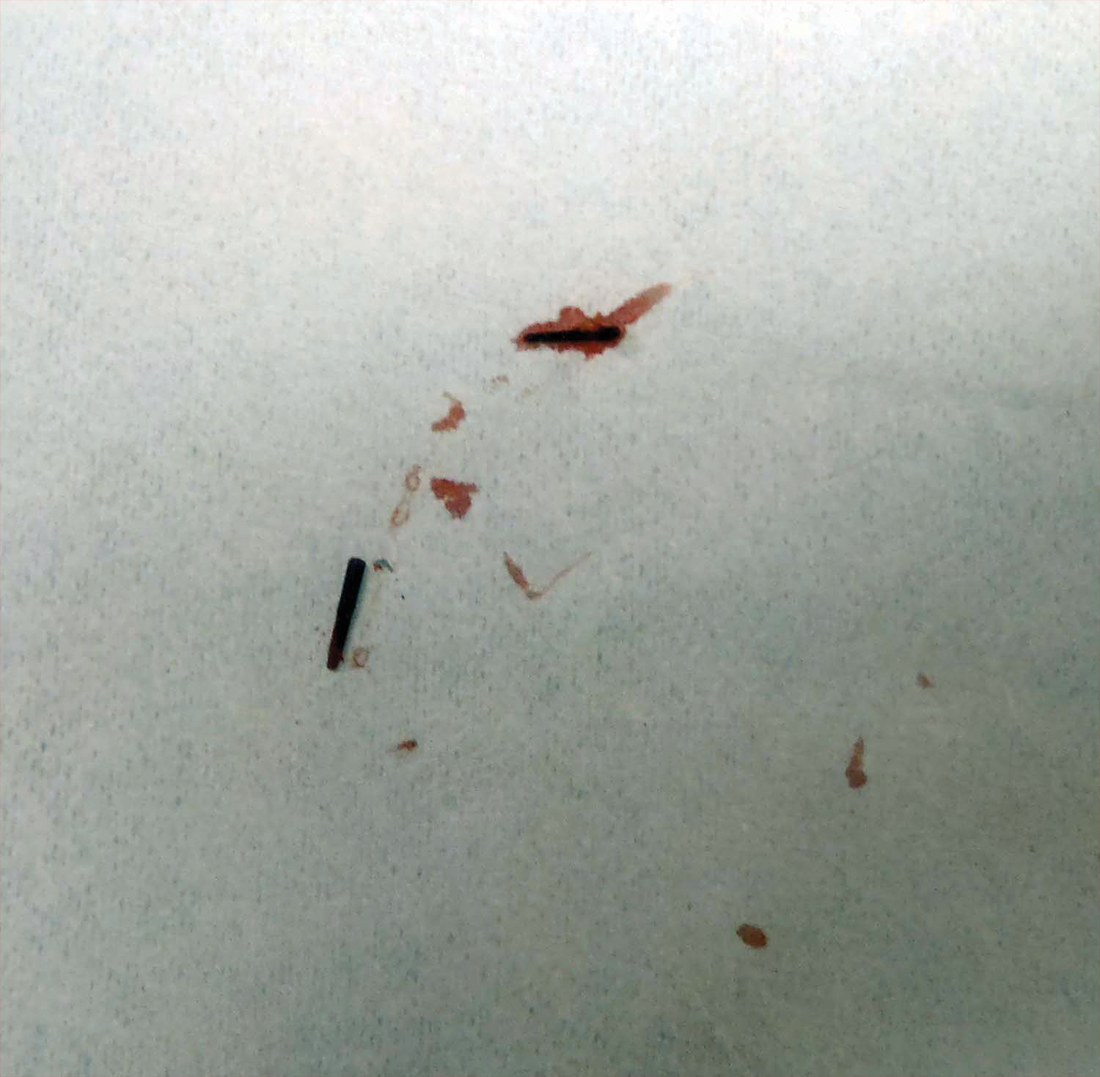
Two 6-mm punch biopsies were performed on representative nodules on the right thigh for histopathologic examination. Along with the biopsy tissue, firm, brown-black, linear foreign bodies consistent with sea urchin spines were extracted with forceps (Figure 3). Histopathologic examination revealed a dense, diffuse, mixed inflammatory cell infiltrate in the dermis predominantly composed of lymphocytes, histiocytes, and numerous eosinophils. Proliferation of small vessels was noted. In one of the biopsies, small fragments of necrotic tissue were present. These findings were consistent with granulomatous inflammation and granulation tissue due to a foreign body.
At the time of suture removal 2 weeks later, the biopsied areas were well healed with minimal erythema. The patient reported decreased pain in the involved areas. He was not seen in clinic again due to resolution of the nodules and associated pain.
Comment
Sea urchin spine injuries are commonly seen in coastal regions with frequent participation in recreational and occupational water activities. A wide variety of responses can be seen in sea urchin spine injuries. There generally are 2 types of cutaneous reaction patterns to sea urchin spines: a primary initial reaction and a secondary delayed/granulomatous reaction. When the spines initially penetrate the skin, the primary initial reaction consists of sharp localized pain that worsens with applied pressure. In addition to pain, bleeding, erythema, edema, and myalgia can occur.3 These symptoms typically subside a few hours after complete removal of the spines from the skin.6 If some spines remain in the skin, a secondary delayed/granulomatous reaction can occur, which can lead to the formation of granulomas that can manifest as nodules or papules and can be diffuse.
Many patients may think their painful encounter with a sea urchin was just an unfortunate event, but depending on the location of the injury, more serious extracutaneous reactions and chronic symptoms may occur. Some cases have described the development of arthritis and synovitis from the implantation of spines into joints.1,2,4-6 Other extracutaneous complications include neuropathy and paresthesia, local bone destruction, radiating pain, muscular weakness, and hypotension.3
The severity of the injury also can depend on the sea urchin species and the number of spines implanted. There are approximately 80 poisonous sea urchin species possessing toxins in venomous spines, resulting in edema and change in the leukocyte-endothelial interaction.9 Substances identified in the spines include proteins, steroids, serotonin, histamine, and glycosides.3,9 The number of spines implanted, particularly the number of venomous spines, can lead to more severe complications. Penetration of 15 or more venomous spines can commonly lead to extracutaneous symptoms.3 Another concern, irrespective of species type, is the potential for secondary infection associated with the spine penetration or implantation into the skin. Mycobacterium marinum infections have been reported in some sea urchin granulomas,10 as well as fungal infection, bacterial infection, and tetanus.3
The diagnosis of sea urchin spine injuries starts with a thorough history and physical examination. A positive history of sea urchin contact suggests the diagnosis, and radiographs can be useful to find the location of the spine(s), especially if there are no visible nodules on the skin. However, small fragments of spine may not be completely observed on plain radiographs. Any signs or symptoms of infection should prompt a culture for confirmation and guidance for management. Cutaneous biopsies can be helpful for both diagnosis confirmation and symptomatic relief. Reported cases have described granulomatous reactions in the vast majority of the histologic specimens, with necrosis an additional common finding.7,8 Sea urchin granulomas can be of varying types, the majority being foreign-body and sarcoid types.3,6,7
Treatment of sea urchin spine injuries primarily involves removal of the spines by a physician. Patients may soak the affected areas in warm water prior to the removal of the spines to aid in pain relief. Surgical removal with local anesthesia and cutaneous extraction is a common treatment method, and more extensive surgical removal of the spines is another option, especially in areas around the joints.2 The use of liquid nitrogen or skin punch biopsy also have been described as possible methods to remove the spines.11,12
Conclusion
Sea urchin spine injuries can result in a wide range of cutaneous and systemic complications. Prompt diagnosis and treatment to remove the sea urchin spines can lessen the associated pain and is important in the prevention of more serious complications.
- Liram N, Gomori M, Perouansky M. Sea urchin puncture resulting in PIP joint synovial arthritis: case report and MRI study. J Travel Med. 2000;7:43-45.
- Dahl WJ, Jebson P, Louis DS. Sea urchin injuries to the hand: a case report and review of the literature. Iowa Orthop J. 2010;30:153-156.
- Rossetto AL, de Macedo Mora J, Haddad Junior V. Sea urchin granuloma. Rev Inst Med Trop Sao Paulo. 2006;48:303-306.
- Ahmad R, McCann PA, Barakat M, et al. Sea urchin spine injuries of the hand. J Hand Surg Eur Vol. 2008;33:670-671.
- Schefflein J, Umans H, Ellenbogen D, et al. Sea urchin spine arthritis in the foot. Skeletal Radiol. 2012;41:1327-1331.
- Wada T, Soma T, Gaman K, et al. Sea urchin spine arthritis of the hand. J Hand Surg. 2008;33:398-401.
- Suárez-Peñaranda JM, Vieites B, Del Río E, et al. Histopathologic and immunohistochemical features of sea urchin granulomas. J Cutan Pathol. 2013;40:550-556.
- De La Torre C, Toribio J. Sea-urchin granuloma: histologic profile. a pathologic study of 50 biopsies. J Cutan Pathol. 2001;28:223-228.
- Sciani JM, Zychar BC, Gonçalves LR, et al. Pro-inflammatory effects of the aqueous extract of Echinometra lucunter sea urchin spines. Exp Biol Med (Maywood). 2011;236:277-280.
- De la Torre C, Vega A, Carracedo A, et al. Identification of Mycobacterium marinum in sea-urchin granulomas. Br J Dermatol. 2001;145:114-116.
- Gargus MD, Morohashi DK. A sea-urchin spine chilling remedy. N Engl J Med. 2012;367:1867-1868.
- Sjøberg T, de Weerd L. The usefulness of a skin biopsy punch to remove sea urchin spines. ANZ J Surg. 2010;80:383.
Sea urchin injuries are commonly seen in coastal regions near both warm and cold salt water with frequent recreational water activities or fishing. Sea urchins belong to the class Echinoidea with approximately 600 species, of which roughly 80 are poisonous to humans.1,2 When a human comes in contact with a sea urchin, the spines of the sea urchin (made of calcium carbonate) can penetrate the skin and break off from the sea urchin, becoming embedded in the skin. Injuries from sea urchin spines are most commonly seen on the hands and feet, as the likelihood of contact with a sea urchin is greater on these sites. The severity of sea urchin spine injuries can vary widely, from minimal local trauma and pain to arthritis, synovitis, and occasionally systemic illness.1,3 It is important to recognize the wide variety of responses to sea urchin spine injuries and the impact of prompt treatment. Many published reports on injuries from sea urchin spines describe arthritis and synovitis from spines in the joints.1,2,4-6 Fewer reports discuss nonjoint injuries and the dermatologic aspects of sea urchin spine injuries.3,7,8 We pre-sent a case of a patient with a puncture injury from sea urchin spines that resulted in painful granulomas.
Case Report
A 29-year-old otherwise healthy man was referred to our dermatology clinic by the university student health center due to continued pain in the right thigh. Five weeks prior to presentation to the student health center, the patient had fallen on a sea urchin while snorkeling in Hawaii. Sea urchin spines became lodged in the right thigh, some of which were removed in a local medical clinic in Hawaii. He was given oral antibiotics prior to his return home. A plain film radiograph of the affected area ordered by the student health center showed several punctate and linear densities in the lateral aspect of the right mid thigh (Figure 1). These findings were consistent with sea urchin spines within the superficial soft tissues of the lateral thigh.

At the time of presentation to our dermatology clinic, the patient reported sharp intermittent pain localized to the right thigh. The patient denied any fever, chills, or pain in the joints. On physical examination, there were several firm nodules on the right thigh, ranging from 4 to 20 mm in diameter (Figure 2). The nodules were tender to palpation with some surrounding edema. Drainage was not noted. Several scars were visible at sites of the original puncture injuries and removal of the spines.

Two 6-mm punch biopsies were performed on representative nodules on the right thigh for histopathologic examination. Along with the biopsy tissue, firm, brown-black, linear foreign bodies consistent with sea urchin spines were extracted with forceps (Figure 3). Histopathologic examination revealed a dense, diffuse, mixed inflammatory cell infiltrate in the dermis predominantly composed of lymphocytes, histiocytes, and numerous eosinophils. Proliferation of small vessels was noted. In one of the biopsies, small fragments of necrotic tissue were present. These findings were consistent with granulomatous inflammation and granulation tissue due to a foreign body.

At the time of suture removal 2 weeks later, the biopsied areas were well healed with minimal erythema. The patient reported decreased pain in the involved areas. He was not seen in clinic again due to resolution of the nodules and associated pain.
Comment
Sea urchin spine injuries are commonly seen in coastal regions with frequent participation in recreational and occupational water activities. A wide variety of responses can be seen in sea urchin spine injuries. There generally are 2 types of cutaneous reaction patterns to sea urchin spines: a primary initial reaction and a secondary delayed/granulomatous reaction. When the spines initially penetrate the skin, the primary initial reaction consists of sharp localized pain that worsens with applied pressure. In addition to pain, bleeding, erythema, edema, and myalgia can occur.3 These symptoms typically subside a few hours after complete removal of the spines from the skin.6 If some spines remain in the skin, a secondary delayed/granulomatous reaction can occur, which can lead to the formation of granulomas that can manifest as nodules or papules and can be diffuse.
Many patients may think their painful encounter with a sea urchin was just an unfortunate event, but depending on the location of the injury, more serious extracutaneous reactions and chronic symptoms may occur. Some cases have described the development of arthritis and synovitis from the implantation of spines into joints.1,2,4-6 Other extracutaneous complications include neuropathy and paresthesia, local bone destruction, radiating pain, muscular weakness, and hypotension.3
The severity of the injury also can depend on the sea urchin species and the number of spines implanted. There are approximately 80 poisonous sea urchin species possessing toxins in venomous spines, resulting in edema and change in the leukocyte-endothelial interaction.9 Substances identified in the spines include proteins, steroids, serotonin, histamine, and glycosides.3,9 The number of spines implanted, particularly the number of venomous spines, can lead to more severe complications. Penetration of 15 or more venomous spines can commonly lead to extracutaneous symptoms.3 Another concern, irrespective of species type, is the potential for secondary infection associated with the spine penetration or implantation into the skin. Mycobacterium marinum infections have been reported in some sea urchin granulomas,10 as well as fungal infection, bacterial infection, and tetanus.3
The diagnosis of sea urchin spine injuries starts with a thorough history and physical examination. A positive history of sea urchin contact suggests the diagnosis, and radiographs can be useful to find the location of the spine(s), especially if there are no visible nodules on the skin. However, small fragments of spine may not be completely observed on plain radiographs. Any signs or symptoms of infection should prompt a culture for confirmation and guidance for management. Cutaneous biopsies can be helpful for both diagnosis confirmation and symptomatic relief. Reported cases have described granulomatous reactions in the vast majority of the histologic specimens, with necrosis an additional common finding.7,8 Sea urchin granulomas can be of varying types, the majority being foreign-body and sarcoid types.3,6,7
Treatment of sea urchin spine injuries primarily involves removal of the spines by a physician. Patients may soak the affected areas in warm water prior to the removal of the spines to aid in pain relief. Surgical removal with local anesthesia and cutaneous extraction is a common treatment method, and more extensive surgical removal of the spines is another option, especially in areas around the joints.2 The use of liquid nitrogen or skin punch biopsy also have been described as possible methods to remove the spines.11,12
Conclusion
Sea urchin spine injuries can result in a wide range of cutaneous and systemic complications. Prompt diagnosis and treatment to remove the sea urchin spines can lessen the associated pain and is important in the prevention of more serious complications.
Sea urchin injuries are commonly seen in coastal regions near both warm and cold salt water with frequent recreational water activities or fishing. Sea urchins belong to the class Echinoidea with approximately 600 species, of which roughly 80 are poisonous to humans.1,2 When a human comes in contact with a sea urchin, the spines of the sea urchin (made of calcium carbonate) can penetrate the skin and break off from the sea urchin, becoming embedded in the skin. Injuries from sea urchin spines are most commonly seen on the hands and feet, as the likelihood of contact with a sea urchin is greater on these sites. The severity of sea urchin spine injuries can vary widely, from minimal local trauma and pain to arthritis, synovitis, and occasionally systemic illness.1,3 It is important to recognize the wide variety of responses to sea urchin spine injuries and the impact of prompt treatment. Many published reports on injuries from sea urchin spines describe arthritis and synovitis from spines in the joints.1,2,4-6 Fewer reports discuss nonjoint injuries and the dermatologic aspects of sea urchin spine injuries.3,7,8 We pre-sent a case of a patient with a puncture injury from sea urchin spines that resulted in painful granulomas.
Case Report
A 29-year-old otherwise healthy man was referred to our dermatology clinic by the university student health center due to continued pain in the right thigh. Five weeks prior to presentation to the student health center, the patient had fallen on a sea urchin while snorkeling in Hawaii. Sea urchin spines became lodged in the right thigh, some of which were removed in a local medical clinic in Hawaii. He was given oral antibiotics prior to his return home. A plain film radiograph of the affected area ordered by the student health center showed several punctate and linear densities in the lateral aspect of the right mid thigh (Figure 1). These findings were consistent with sea urchin spines within the superficial soft tissues of the lateral thigh.

At the time of presentation to our dermatology clinic, the patient reported sharp intermittent pain localized to the right thigh. The patient denied any fever, chills, or pain in the joints. On physical examination, there were several firm nodules on the right thigh, ranging from 4 to 20 mm in diameter (Figure 2). The nodules were tender to palpation with some surrounding edema. Drainage was not noted. Several scars were visible at sites of the original puncture injuries and removal of the spines.

Two 6-mm punch biopsies were performed on representative nodules on the right thigh for histopathologic examination. Along with the biopsy tissue, firm, brown-black, linear foreign bodies consistent with sea urchin spines were extracted with forceps (Figure 3). Histopathologic examination revealed a dense, diffuse, mixed inflammatory cell infiltrate in the dermis predominantly composed of lymphocytes, histiocytes, and numerous eosinophils. Proliferation of small vessels was noted. In one of the biopsies, small fragments of necrotic tissue were present. These findings were consistent with granulomatous inflammation and granulation tissue due to a foreign body.

At the time of suture removal 2 weeks later, the biopsied areas were well healed with minimal erythema. The patient reported decreased pain in the involved areas. He was not seen in clinic again due to resolution of the nodules and associated pain.
Comment
Sea urchin spine injuries are commonly seen in coastal regions with frequent participation in recreational and occupational water activities. A wide variety of responses can be seen in sea urchin spine injuries. There generally are 2 types of cutaneous reaction patterns to sea urchin spines: a primary initial reaction and a secondary delayed/granulomatous reaction. When the spines initially penetrate the skin, the primary initial reaction consists of sharp localized pain that worsens with applied pressure. In addition to pain, bleeding, erythema, edema, and myalgia can occur.3 These symptoms typically subside a few hours after complete removal of the spines from the skin.6 If some spines remain in the skin, a secondary delayed/granulomatous reaction can occur, which can lead to the formation of granulomas that can manifest as nodules or papules and can be diffuse.
Many patients may think their painful encounter with a sea urchin was just an unfortunate event, but depending on the location of the injury, more serious extracutaneous reactions and chronic symptoms may occur. Some cases have described the development of arthritis and synovitis from the implantation of spines into joints.1,2,4-6 Other extracutaneous complications include neuropathy and paresthesia, local bone destruction, radiating pain, muscular weakness, and hypotension.3
The severity of the injury also can depend on the sea urchin species and the number of spines implanted. There are approximately 80 poisonous sea urchin species possessing toxins in venomous spines, resulting in edema and change in the leukocyte-endothelial interaction.9 Substances identified in the spines include proteins, steroids, serotonin, histamine, and glycosides.3,9 The number of spines implanted, particularly the number of venomous spines, can lead to more severe complications. Penetration of 15 or more venomous spines can commonly lead to extracutaneous symptoms.3 Another concern, irrespective of species type, is the potential for secondary infection associated with the spine penetration or implantation into the skin. Mycobacterium marinum infections have been reported in some sea urchin granulomas,10 as well as fungal infection, bacterial infection, and tetanus.3
The diagnosis of sea urchin spine injuries starts with a thorough history and physical examination. A positive history of sea urchin contact suggests the diagnosis, and radiographs can be useful to find the location of the spine(s), especially if there are no visible nodules on the skin. However, small fragments of spine may not be completely observed on plain radiographs. Any signs or symptoms of infection should prompt a culture for confirmation and guidance for management. Cutaneous biopsies can be helpful for both diagnosis confirmation and symptomatic relief. Reported cases have described granulomatous reactions in the vast majority of the histologic specimens, with necrosis an additional common finding.7,8 Sea urchin granulomas can be of varying types, the majority being foreign-body and sarcoid types.3,6,7
Treatment of sea urchin spine injuries primarily involves removal of the spines by a physician. Patients may soak the affected areas in warm water prior to the removal of the spines to aid in pain relief. Surgical removal with local anesthesia and cutaneous extraction is a common treatment method, and more extensive surgical removal of the spines is another option, especially in areas around the joints.2 The use of liquid nitrogen or skin punch biopsy also have been described as possible methods to remove the spines.11,12
Conclusion
Sea urchin spine injuries can result in a wide range of cutaneous and systemic complications. Prompt diagnosis and treatment to remove the sea urchin spines can lessen the associated pain and is important in the prevention of more serious complications.
- Liram N, Gomori M, Perouansky M. Sea urchin puncture resulting in PIP joint synovial arthritis: case report and MRI study. J Travel Med. 2000;7:43-45.
- Dahl WJ, Jebson P, Louis DS. Sea urchin injuries to the hand: a case report and review of the literature. Iowa Orthop J. 2010;30:153-156.
- Rossetto AL, de Macedo Mora J, Haddad Junior V. Sea urchin granuloma. Rev Inst Med Trop Sao Paulo. 2006;48:303-306.
- Ahmad R, McCann PA, Barakat M, et al. Sea urchin spine injuries of the hand. J Hand Surg Eur Vol. 2008;33:670-671.
- Schefflein J, Umans H, Ellenbogen D, et al. Sea urchin spine arthritis in the foot. Skeletal Radiol. 2012;41:1327-1331.
- Wada T, Soma T, Gaman K, et al. Sea urchin spine arthritis of the hand. J Hand Surg. 2008;33:398-401.
- Suárez-Peñaranda JM, Vieites B, Del Río E, et al. Histopathologic and immunohistochemical features of sea urchin granulomas. J Cutan Pathol. 2013;40:550-556.
- De La Torre C, Toribio J. Sea-urchin granuloma: histologic profile. a pathologic study of 50 biopsies. J Cutan Pathol. 2001;28:223-228.
- Sciani JM, Zychar BC, Gonçalves LR, et al. Pro-inflammatory effects of the aqueous extract of Echinometra lucunter sea urchin spines. Exp Biol Med (Maywood). 2011;236:277-280.
- De la Torre C, Vega A, Carracedo A, et al. Identification of Mycobacterium marinum in sea-urchin granulomas. Br J Dermatol. 2001;145:114-116.
- Gargus MD, Morohashi DK. A sea-urchin spine chilling remedy. N Engl J Med. 2012;367:1867-1868.
- Sjøberg T, de Weerd L. The usefulness of a skin biopsy punch to remove sea urchin spines. ANZ J Surg. 2010;80:383.
- Liram N, Gomori M, Perouansky M. Sea urchin puncture resulting in PIP joint synovial arthritis: case report and MRI study. J Travel Med. 2000;7:43-45.
- Dahl WJ, Jebson P, Louis DS. Sea urchin injuries to the hand: a case report and review of the literature. Iowa Orthop J. 2010;30:153-156.
- Rossetto AL, de Macedo Mora J, Haddad Junior V. Sea urchin granuloma. Rev Inst Med Trop Sao Paulo. 2006;48:303-306.
- Ahmad R, McCann PA, Barakat M, et al. Sea urchin spine injuries of the hand. J Hand Surg Eur Vol. 2008;33:670-671.
- Schefflein J, Umans H, Ellenbogen D, et al. Sea urchin spine arthritis in the foot. Skeletal Radiol. 2012;41:1327-1331.
- Wada T, Soma T, Gaman K, et al. Sea urchin spine arthritis of the hand. J Hand Surg. 2008;33:398-401.
- Suárez-Peñaranda JM, Vieites B, Del Río E, et al. Histopathologic and immunohistochemical features of sea urchin granulomas. J Cutan Pathol. 2013;40:550-556.
- De La Torre C, Toribio J. Sea-urchin granuloma: histologic profile. a pathologic study of 50 biopsies. J Cutan Pathol. 2001;28:223-228.
- Sciani JM, Zychar BC, Gonçalves LR, et al. Pro-inflammatory effects of the aqueous extract of Echinometra lucunter sea urchin spines. Exp Biol Med (Maywood). 2011;236:277-280.
- De la Torre C, Vega A, Carracedo A, et al. Identification of Mycobacterium marinum in sea-urchin granulomas. Br J Dermatol. 2001;145:114-116.
- Gargus MD, Morohashi DK. A sea-urchin spine chilling remedy. N Engl J Med. 2012;367:1867-1868.
- Sjøberg T, de Weerd L. The usefulness of a skin biopsy punch to remove sea urchin spines. ANZ J Surg. 2010;80:383.
Practice Points
- Radiographic imaging may aid in the identification of sea urchin spines, especially if there are no visible or palpable skin nodules.
- Treatment of sea urchin spine injuries typically involves surgical removal of the spines with local anesthesia and cutaneous extraction.
- Prompt extraction of sea urchin spines can improve pain symptoms and decrease the likelihood of granuloma formation, infection, and extracutaneous complications.
Efficacy and Safety of New Dermal Fillers
Facial aging is the result of the interplay between loss of skin elasticity, changes in subcutaneous fat and other soft-tissue layers, and skeletal remodeling with chronological age.1 Dermal fillers are effective for the treatment of rhytides, facial scars, and lipoatrophy, as well as facial contouring and augmentation. Given that multiple filler options exist, updated reviews are necessary to inform clinicians of the choices that are available. We provide a detailed review of the clinical efficacy and safety of the dermal fillers with the most recent approvals by the US Food and Drug Administration (FDA).
Polymethylmethacrylate
Polymethylmethacrylate (PMMA) microspheres suspended in bovine collagen and lidocaine 0.3% were approved in 2006 for use in nasolabial folds (NLFs) and in 2014 for acne scars. Now branded as Bellafill (Suneva Medical, Inc), it is the only permanent injectable filler currently available. Once injected, the particles are not reabsorbed and can only be removed by procedural extraction (eg, liposuction of the surrounding fat); however, the permanence of PMMA does not extend to facial rejuvenation, which can last up to 5 years. Prior to use, skin testing for bovine collagen reaction is necessary. In a clinical trial of 147 patients with moderate to severe acne scarring, patients were randomized to receive PMMA in collagen (n=97) or saline (n=50).2 Injections were administered using a linear threading or serial puncture technique, and patients were reevaluated after 4 weeks for touch-up injections. After 6 months, 64% of patients treated with PMMA in collagen achieved improvement in acne scars by 2 points or more on the acne scar rating scale versus 33% of the control group (P=.0005).2
Treatment-related adverse events (AEs) include injection-site pain, bruising, swelling, erythema, and more rarely pruritus and lumps/granulomas.3 A 5-year longitudinal safety investigation of 871 patients initially treated with PMMA in collagen for NLF correction revealed that 17 patients (2.0%) had biopsy-confirmed granulomas with half of these retained at study end.4 Fifteen of these patients were treated with intralesional corticosteroids alone or in combination with intralesional 5-fluorouracil, oral antibiotics, or topical calcineurin inhibitors; 1 patient was untreated and another used topical corticosteroids. The authors noted no correlation between treatment method and granuloma response.4 Polymethylmethacrylate in collagen is contraindicated in patients with lidocaine or bovine collagen sensitivity and is not indicated for use in lip augmentation due to high rates of nodule formation.3
Hyaluronic Acid
Hyaluronic acid (HA) is a naturally occurring glycosaminoglycan polymer found in the extracellular matrix of the dermis. Hyaluronic acid fillers are bacteria derived and come in gel form. A useful advantage of HA fillers compared to other dermal fillers is the commercial availability of hyaluronidase to correct injections. Preinjection skin testing is not necessary.5
This category of nonpermanent dermal fillers has the most robust market choices. Older HA dermal fillers with reliable and proven efficacy are Restylane (Galderma Laboratories, LP)(facial rhytides, lip augmentation), Juvéderm (Ultra/Ultra XC/Ultra Plus/Ultra Plus XC [Allergan, Inc])(facial rhytides, lip augmentation), Hydrelle (Anika Therapeutics, Inc)(facial rhytides), and Prevelle Silk (Mentor Corporation)(facial rhytides); they will not be reviewed here. Newer agents include Belotero Balance (Merz Aesthetics), Juvéderm Voluma XC (Allergan, Inc), Restylane Silk (Galderma Laboratories, LP), and Restylane Lyft (Galderma Laboratories, LP).
Belotero Balance
Belotero Balance is used to treat fine lines and wrinkles, especially NLFs.6 The initial pivotal studies that led to FDA approval in 2011 demonstrated noninferiority and superiority to bovine collagen for use in the treatment of NLFs.7,8 One hundred eighteen patients with bilateral NLFs that were rated as 2 (moderate) or 3 (severe) on the wrinkle severity rating scale (WSRS) were randomized to split-face injection of Belotero Balance in one NLF and bovine collagen in the contralateral NLF.7 An additional injection at week 2 was allowed for optimal correction. Belotero Balance was noninferior to bovine collagen at week 2, with mean improvement in WSRS of 1.52 versus 1.57 (P=.50). Belotero Balance was superior to bovine collagen in mean WSRS improvement at weeks 12 (1.25 vs 0.26; P<.001), 16 (1.09 vs 0.66; P<.001), and 24 (1.08 vs 0.50; P<.001).7 In a subsequent open-label extension study, which included 95 of 118 patients who received Belotero Balance injections in both NLFs at week 24, 80.2% of patients showed sustained improvement in WSRS from baseline for 48 weeks without further injection.8
The first comparative study of Belotero Balance with other established HA fillers at the time—Restylane and Juvéderm Ultra 3/Ultra Plus XC—to treat NLFs demonstrated noninferiority.9 Forty patients with bilateral, moderate to severe NLFs (rated 3 or 4 on the Merz severity scale) were randomized to split-face groups of Belotero Balance versus Restylane or Belotero Balance versus Juvéderm. At 12 months, NLF severity improved from 2.3 to 1.5 in the Restylane group and from 2.3 to 1.6 in the Juvéderm group.9
Belotero Balance has been compared to Juvéderm Ultra XC for use in perioral lines.10 The study included 136 patients with moderate to severe perioral lines, according to the perioral lines severity scale, who were randomized (1:1 ratio) to receive injections of Belotero Balance or Juvéderm Ultra XC to correct upper and lower perioral lines, with assessment at week 2 for optimization. After 6 months, 87% of Juvéderm-treated patients compared to 72% of Belotero Balance–treated patients had 1-point improvement in perioral lines (P<.04). Juvéderm-treated patients also reported significantly less pain than Belotero Balance–treated patients (P<.001).10
Treatment-related AEs are described in the Table, with the majority occurring at lower rates compared to a collagen control group and self-resolving within 2 weeks.7
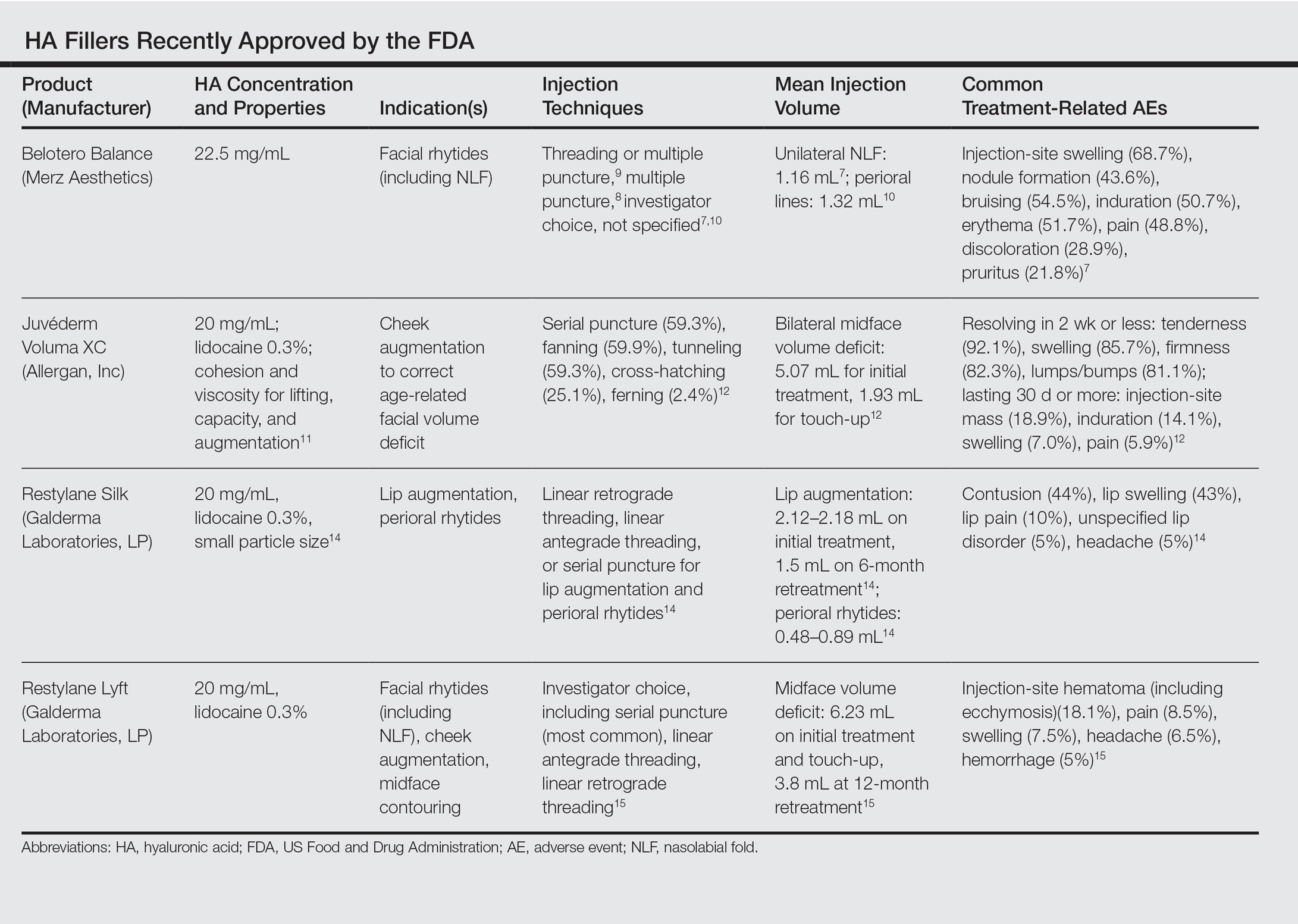
Juvéderm Voluma XC
Juvéderm Voluma XC was FDA approved in 2013 for cheek augmentation to correct age-related volume deficit restoration by subcutaneous or subperiosteal injections. In its landmark multicenter investigation, 282 patients with moderate to severe midface (eg, zygomaticomalar, anteromedial cheek, submalar regions) volume deficit measured on a validated midface volume deficit scale (MFVDS) were treated with Juvéderm Voluma XC (n=235) or control (n=47).11 Patients were reevaluated at 30 days and 81.9% received touch-up injections. At a 6-month primary evaluation, 86% of the Juvéderm-treated patients versus 39% of the control patients showed 1-point improvement on the MFVDS (P<.001). At 24-months’ follow-up, 44.6% of patients sustained efficacy.11 Of these aforementioned patients, 167 received repeat treatment due to lost correction or patient request and 91.1% improved by 1 point or more on the MFVDS on evaluation 12 months after repeat treatment.12 For this same population of patients, a 2-year extended follow-up of patient-reported outcomes revealed that 49% of patients felt fulfilled in their treatment goals 2 years after treatment and 79% of patients rated improvement from baseline based on the global aesthetic improvement scale.13 Efficacy studies involving Juvéderm Voluma XC are currently ongoing for facial temporal aging (registered at www.clinicaltrials.gov with the identifier NCT02437903) and recruiting for mandibular hypoplasia (NCT02330016).
Common treatment-related AEs are detailed in the Table. Two patients required treatment with hyaluronidase for chronic lumpiness and nodularity following non–treatment-related cellulitis.11 The product is contraindicated in patients with allergy to lidocaine.
Restylane Silk
Restylane Silk was approved in 2014 for lip augmentation and perioral rhytides. Efficacy and safety was demonstrated in a large multicenter randomized investigation in which 221 patients seeking lip augmentation received either Restylane Silk (n=177) injected submucosally for treatment of the upper and lower lips and/or intradermally for perioral rhytides or no treatment (n=44).14 Restylane treatment group patients optionally received touch-up at 2 weeks for optimization. All patients, including the control group, received injections at 6 months. At the 2-month primary end point, 80.2% of the treatment group exhibited at least 1-point improvement in upper lip fullness on the Medicis lip fullness scale compared to 11.9% (P<.001) of the control group; response rates for the lower lips were 84.2% versus 18.4% (P<.001). Patients in the treatment group receiving injections for perioral rhytides showed significant improvement in perioral rhytides through week 24 compared to patients treated for lip augmentation only (P<.001).14 Restylane Silk currently is undergoing investigation for cheek rejuvenation (NCT02636894, NCT02679924) and treatment of hand photoaging (NCT02780258).
The most common AEs are listed in the Table. No lip disorders were considered clinically concerning on evaluation. Concomitant lip augmentation and treatment of perioral rhytides yielded similar rates of AEs.14 Restylane Silk is not to be used in patients with known lidocaine allergy.
Restylane Lyft
Restylane Lyft (formerly known as Perlane-L) was approved in 2010 for use in facial rhytides, including NLFs, and gained approval in 2015 for use in cheek augmentation and midface contouring. Only its efficacy and safety for the more recent indication will be reviewed here.
In an evaluator-blinded investigation of 200 patients with mild to substantial bilateral midface deficiency based on the Medicis midface volume scale (MMVS), patients were randomized to receive supraperiosteal and subcutaneous treatment with Restylane Lyft (n=150) or no treatment (n=50).15 Touch-up injections at week 2 or month 12 were available to treatment group patients and all patients were given either an initial treatment or retreatment at 12 months. Primary end point evaluation at week 8 showed that 89% of treatment group patients had at least 1 grade MMVS improvement compared to 16% of the control group (P<.001). Although the percentage of these MMVS responders in the treatment group decreased with each follow-up period to 54.3% at month 12, retreatment was effective in reproducing a similar MMVS response rate as with initial treatment.15 Restylane Lyft is under ongoing investigation for dorsal hand rejuvenation (NCT02650921).
In addition to the common treatment-related AEs listed in the Table, 2 patients reported serious AEs, including bilateral implant-site inflammation and unilateral implant-site hematoma and infection (organism not described), all of which resolved with unspecified treatment.15 Lidocaine allergies are contraindications for use.
Conclusion
Several new options in dermal fillers have been approved in recent years and have demonstrated efficacy and acceptable safety in various cosmetic rejuvenation applications. Restylane Silk and Restylane Lyft are undergoing further studies to evaluate use in hand rejuvenation, an area that currently has few cosmetic filler treatment options. As technology continues to progress and new formulations of dermal fillers with varied properties and benefits are available, clinicians should expect multiple options for use in rhytides, volume deficits, and contouring.
ADDENDUM
After the manuscript was accepted for publication, Juvéderm Volbella XC (Allergan, Inc) was approved by the FDA for use in lip augmentation and thus is not included in this review.
- Fitzgerald R, Graivier MH, Kane M, et al. Update on facial aging. Aesthet Surg J. 2010;30(suppl):S11-S24.
- Karnik J, Baumann L, Bruce S, et al. A double-blind, randomized, multicenter, controlled trial of suspended polymethylmethacrylate microspheres for the correction of atrophic facial acne scars. J Am Acad Dermatol. 2014;71:77-83.
- Bellafill [package insert]. San Diego, CA: Suneva Medical, Inc; 2015.
- Cohen S, Dover J, Monheit G, et al. Five-year safety and satisfaction study of PMMA-collagen in the correction of nasolabial folds. Dermatol Surg. 2015;41(suppl 1):S302-S313.
- Greene JJ, Sidle DM. The hyaluronic acid fillers: current understanding of the tissue device interface. Facial Plast Surg Clin North Am. 2015;23:423-432.
- Lorenc ZP, Fagien S, Flynn TC, et al. Review of key Belotero Balance safety and efficacy trials. Plast Reconstr Surg. 2013;132(4, suppl 2):33S-40S.
- Narins RS, Coleman W, Donofrio L, et al. Nonanimal sourced hyaluronic acid–based dermal filler using a cohesive polydensified matrix technology is superior to bovine collagen in the correction of moderate to severe nasolabial folds: results from a 6-month, randomized, blinded, controlled, multicenter study. Dermatol Surg. 2010;36(suppl 1):730-740.
- Narins RS, Coleman WP 3rd, Donofrio LM, et al. Improvement in nasolabial folds with a hyaluronic acid filler using a cohesive polydensified matrix technology: results from an 18-month open-label extension trial. Dermatol Surg. 2010;36(suppl 3):1800-1808.
- Prager W, Wissmueller E, Havermann I, et al. A prospective, split-face, randomized, comparative study of safety and 12-month longevity of three formulations of hyaluronic acid dermal filler for treatment of nasolabial folds. Dermatol Surg. 2012;38(7, pt 2):1143-1150.
- Butterwick K, Marmur E, Narurkar V, et al. HYC-24L demonstrates greater effectiveness with less pain than CPM-22.5 for treatment of perioral lines in a randomized controlled trial. Dermatol Surg. 2015;41:1351-1360.
- Jones D, Murphy DK. Volumizing hyaluronic acid filler for midface volume deficit: 2-year results from a pivotal single-blind randomized controlled study. Dermatol Surg. 2013;39:1602-1612.
- Baumann L, Narins RS, Beer K, et al. Volumizing hyaluronic acid filler for midface volume deficit: results after repeat treatment. Dermatol Surg. 2015;41(suppl 1):S284-S292.
- Few J, Cox SE, Paradkar-Mitragotri D, et al. A multicenter, single-blind randomized, controlled study of a volumizing hyaluronic acid filler for midface volume deficit: patient-reported outcomes at 2 years. Aesthet Surg J. 2015;35:589-599.
- Beer K, Glogau RG, Dover JS, et al. A randomized, evaluator-blinded, controlled study of effectiveness and safety of small particle hyaluronic acid plus lidocaine for lip augmentation and perioral rhytides. Dermatol Surg. 2015;41(suppl 1):S127-S136.
- Weiss RA, Moradi A, Bank D, et al. Effectiveness and safety of large gel particle hyaluronic acid with lidocaine for correction of midface volume deficit or contour deficiency. Dermatol Surg. 2016;42:699-709.
Facial aging is the result of the interplay between loss of skin elasticity, changes in subcutaneous fat and other soft-tissue layers, and skeletal remodeling with chronological age.1 Dermal fillers are effective for the treatment of rhytides, facial scars, and lipoatrophy, as well as facial contouring and augmentation. Given that multiple filler options exist, updated reviews are necessary to inform clinicians of the choices that are available. We provide a detailed review of the clinical efficacy and safety of the dermal fillers with the most recent approvals by the US Food and Drug Administration (FDA).
Polymethylmethacrylate
Polymethylmethacrylate (PMMA) microspheres suspended in bovine collagen and lidocaine 0.3% were approved in 2006 for use in nasolabial folds (NLFs) and in 2014 for acne scars. Now branded as Bellafill (Suneva Medical, Inc), it is the only permanent injectable filler currently available. Once injected, the particles are not reabsorbed and can only be removed by procedural extraction (eg, liposuction of the surrounding fat); however, the permanence of PMMA does not extend to facial rejuvenation, which can last up to 5 years. Prior to use, skin testing for bovine collagen reaction is necessary. In a clinical trial of 147 patients with moderate to severe acne scarring, patients were randomized to receive PMMA in collagen (n=97) or saline (n=50).2 Injections were administered using a linear threading or serial puncture technique, and patients were reevaluated after 4 weeks for touch-up injections. After 6 months, 64% of patients treated with PMMA in collagen achieved improvement in acne scars by 2 points or more on the acne scar rating scale versus 33% of the control group (P=.0005).2
Treatment-related adverse events (AEs) include injection-site pain, bruising, swelling, erythema, and more rarely pruritus and lumps/granulomas.3 A 5-year longitudinal safety investigation of 871 patients initially treated with PMMA in collagen for NLF correction revealed that 17 patients (2.0%) had biopsy-confirmed granulomas with half of these retained at study end.4 Fifteen of these patients were treated with intralesional corticosteroids alone or in combination with intralesional 5-fluorouracil, oral antibiotics, or topical calcineurin inhibitors; 1 patient was untreated and another used topical corticosteroids. The authors noted no correlation between treatment method and granuloma response.4 Polymethylmethacrylate in collagen is contraindicated in patients with lidocaine or bovine collagen sensitivity and is not indicated for use in lip augmentation due to high rates of nodule formation.3
Hyaluronic Acid
Hyaluronic acid (HA) is a naturally occurring glycosaminoglycan polymer found in the extracellular matrix of the dermis. Hyaluronic acid fillers are bacteria derived and come in gel form. A useful advantage of HA fillers compared to other dermal fillers is the commercial availability of hyaluronidase to correct injections. Preinjection skin testing is not necessary.5
This category of nonpermanent dermal fillers has the most robust market choices. Older HA dermal fillers with reliable and proven efficacy are Restylane (Galderma Laboratories, LP)(facial rhytides, lip augmentation), Juvéderm (Ultra/Ultra XC/Ultra Plus/Ultra Plus XC [Allergan, Inc])(facial rhytides, lip augmentation), Hydrelle (Anika Therapeutics, Inc)(facial rhytides), and Prevelle Silk (Mentor Corporation)(facial rhytides); they will not be reviewed here. Newer agents include Belotero Balance (Merz Aesthetics), Juvéderm Voluma XC (Allergan, Inc), Restylane Silk (Galderma Laboratories, LP), and Restylane Lyft (Galderma Laboratories, LP).
Belotero Balance
Belotero Balance is used to treat fine lines and wrinkles, especially NLFs.6 The initial pivotal studies that led to FDA approval in 2011 demonstrated noninferiority and superiority to bovine collagen for use in the treatment of NLFs.7,8 One hundred eighteen patients with bilateral NLFs that were rated as 2 (moderate) or 3 (severe) on the wrinkle severity rating scale (WSRS) were randomized to split-face injection of Belotero Balance in one NLF and bovine collagen in the contralateral NLF.7 An additional injection at week 2 was allowed for optimal correction. Belotero Balance was noninferior to bovine collagen at week 2, with mean improvement in WSRS of 1.52 versus 1.57 (P=.50). Belotero Balance was superior to bovine collagen in mean WSRS improvement at weeks 12 (1.25 vs 0.26; P<.001), 16 (1.09 vs 0.66; P<.001), and 24 (1.08 vs 0.50; P<.001).7 In a subsequent open-label extension study, which included 95 of 118 patients who received Belotero Balance injections in both NLFs at week 24, 80.2% of patients showed sustained improvement in WSRS from baseline for 48 weeks without further injection.8
The first comparative study of Belotero Balance with other established HA fillers at the time—Restylane and Juvéderm Ultra 3/Ultra Plus XC—to treat NLFs demonstrated noninferiority.9 Forty patients with bilateral, moderate to severe NLFs (rated 3 or 4 on the Merz severity scale) were randomized to split-face groups of Belotero Balance versus Restylane or Belotero Balance versus Juvéderm. At 12 months, NLF severity improved from 2.3 to 1.5 in the Restylane group and from 2.3 to 1.6 in the Juvéderm group.9
Belotero Balance has been compared to Juvéderm Ultra XC for use in perioral lines.10 The study included 136 patients with moderate to severe perioral lines, according to the perioral lines severity scale, who were randomized (1:1 ratio) to receive injections of Belotero Balance or Juvéderm Ultra XC to correct upper and lower perioral lines, with assessment at week 2 for optimization. After 6 months, 87% of Juvéderm-treated patients compared to 72% of Belotero Balance–treated patients had 1-point improvement in perioral lines (P<.04). Juvéderm-treated patients also reported significantly less pain than Belotero Balance–treated patients (P<.001).10
Treatment-related AEs are described in the Table, with the majority occurring at lower rates compared to a collagen control group and self-resolving within 2 weeks.7

Juvéderm Voluma XC
Juvéderm Voluma XC was FDA approved in 2013 for cheek augmentation to correct age-related volume deficit restoration by subcutaneous or subperiosteal injections. In its landmark multicenter investigation, 282 patients with moderate to severe midface (eg, zygomaticomalar, anteromedial cheek, submalar regions) volume deficit measured on a validated midface volume deficit scale (MFVDS) were treated with Juvéderm Voluma XC (n=235) or control (n=47).11 Patients were reevaluated at 30 days and 81.9% received touch-up injections. At a 6-month primary evaluation, 86% of the Juvéderm-treated patients versus 39% of the control patients showed 1-point improvement on the MFVDS (P<.001). At 24-months’ follow-up, 44.6% of patients sustained efficacy.11 Of these aforementioned patients, 167 received repeat treatment due to lost correction or patient request and 91.1% improved by 1 point or more on the MFVDS on evaluation 12 months after repeat treatment.12 For this same population of patients, a 2-year extended follow-up of patient-reported outcomes revealed that 49% of patients felt fulfilled in their treatment goals 2 years after treatment and 79% of patients rated improvement from baseline based on the global aesthetic improvement scale.13 Efficacy studies involving Juvéderm Voluma XC are currently ongoing for facial temporal aging (registered at www.clinicaltrials.gov with the identifier NCT02437903) and recruiting for mandibular hypoplasia (NCT02330016).
Common treatment-related AEs are detailed in the Table. Two patients required treatment with hyaluronidase for chronic lumpiness and nodularity following non–treatment-related cellulitis.11 The product is contraindicated in patients with allergy to lidocaine.
Restylane Silk
Restylane Silk was approved in 2014 for lip augmentation and perioral rhytides. Efficacy and safety was demonstrated in a large multicenter randomized investigation in which 221 patients seeking lip augmentation received either Restylane Silk (n=177) injected submucosally for treatment of the upper and lower lips and/or intradermally for perioral rhytides or no treatment (n=44).14 Restylane treatment group patients optionally received touch-up at 2 weeks for optimization. All patients, including the control group, received injections at 6 months. At the 2-month primary end point, 80.2% of the treatment group exhibited at least 1-point improvement in upper lip fullness on the Medicis lip fullness scale compared to 11.9% (P<.001) of the control group; response rates for the lower lips were 84.2% versus 18.4% (P<.001). Patients in the treatment group receiving injections for perioral rhytides showed significant improvement in perioral rhytides through week 24 compared to patients treated for lip augmentation only (P<.001).14 Restylane Silk currently is undergoing investigation for cheek rejuvenation (NCT02636894, NCT02679924) and treatment of hand photoaging (NCT02780258).
The most common AEs are listed in the Table. No lip disorders were considered clinically concerning on evaluation. Concomitant lip augmentation and treatment of perioral rhytides yielded similar rates of AEs.14 Restylane Silk is not to be used in patients with known lidocaine allergy.
Restylane Lyft
Restylane Lyft (formerly known as Perlane-L) was approved in 2010 for use in facial rhytides, including NLFs, and gained approval in 2015 for use in cheek augmentation and midface contouring. Only its efficacy and safety for the more recent indication will be reviewed here.
In an evaluator-blinded investigation of 200 patients with mild to substantial bilateral midface deficiency based on the Medicis midface volume scale (MMVS), patients were randomized to receive supraperiosteal and subcutaneous treatment with Restylane Lyft (n=150) or no treatment (n=50).15 Touch-up injections at week 2 or month 12 were available to treatment group patients and all patients were given either an initial treatment or retreatment at 12 months. Primary end point evaluation at week 8 showed that 89% of treatment group patients had at least 1 grade MMVS improvement compared to 16% of the control group (P<.001). Although the percentage of these MMVS responders in the treatment group decreased with each follow-up period to 54.3% at month 12, retreatment was effective in reproducing a similar MMVS response rate as with initial treatment.15 Restylane Lyft is under ongoing investigation for dorsal hand rejuvenation (NCT02650921).
In addition to the common treatment-related AEs listed in the Table, 2 patients reported serious AEs, including bilateral implant-site inflammation and unilateral implant-site hematoma and infection (organism not described), all of which resolved with unspecified treatment.15 Lidocaine allergies are contraindications for use.
Conclusion
Several new options in dermal fillers have been approved in recent years and have demonstrated efficacy and acceptable safety in various cosmetic rejuvenation applications. Restylane Silk and Restylane Lyft are undergoing further studies to evaluate use in hand rejuvenation, an area that currently has few cosmetic filler treatment options. As technology continues to progress and new formulations of dermal fillers with varied properties and benefits are available, clinicians should expect multiple options for use in rhytides, volume deficits, and contouring.
ADDENDUM
After the manuscript was accepted for publication, Juvéderm Volbella XC (Allergan, Inc) was approved by the FDA for use in lip augmentation and thus is not included in this review.
Facial aging is the result of the interplay between loss of skin elasticity, changes in subcutaneous fat and other soft-tissue layers, and skeletal remodeling with chronological age.1 Dermal fillers are effective for the treatment of rhytides, facial scars, and lipoatrophy, as well as facial contouring and augmentation. Given that multiple filler options exist, updated reviews are necessary to inform clinicians of the choices that are available. We provide a detailed review of the clinical efficacy and safety of the dermal fillers with the most recent approvals by the US Food and Drug Administration (FDA).
Polymethylmethacrylate
Polymethylmethacrylate (PMMA) microspheres suspended in bovine collagen and lidocaine 0.3% were approved in 2006 for use in nasolabial folds (NLFs) and in 2014 for acne scars. Now branded as Bellafill (Suneva Medical, Inc), it is the only permanent injectable filler currently available. Once injected, the particles are not reabsorbed and can only be removed by procedural extraction (eg, liposuction of the surrounding fat); however, the permanence of PMMA does not extend to facial rejuvenation, which can last up to 5 years. Prior to use, skin testing for bovine collagen reaction is necessary. In a clinical trial of 147 patients with moderate to severe acne scarring, patients were randomized to receive PMMA in collagen (n=97) or saline (n=50).2 Injections were administered using a linear threading or serial puncture technique, and patients were reevaluated after 4 weeks for touch-up injections. After 6 months, 64% of patients treated with PMMA in collagen achieved improvement in acne scars by 2 points or more on the acne scar rating scale versus 33% of the control group (P=.0005).2
Treatment-related adverse events (AEs) include injection-site pain, bruising, swelling, erythema, and more rarely pruritus and lumps/granulomas.3 A 5-year longitudinal safety investigation of 871 patients initially treated with PMMA in collagen for NLF correction revealed that 17 patients (2.0%) had biopsy-confirmed granulomas with half of these retained at study end.4 Fifteen of these patients were treated with intralesional corticosteroids alone or in combination with intralesional 5-fluorouracil, oral antibiotics, or topical calcineurin inhibitors; 1 patient was untreated and another used topical corticosteroids. The authors noted no correlation between treatment method and granuloma response.4 Polymethylmethacrylate in collagen is contraindicated in patients with lidocaine or bovine collagen sensitivity and is not indicated for use in lip augmentation due to high rates of nodule formation.3
Hyaluronic Acid
Hyaluronic acid (HA) is a naturally occurring glycosaminoglycan polymer found in the extracellular matrix of the dermis. Hyaluronic acid fillers are bacteria derived and come in gel form. A useful advantage of HA fillers compared to other dermal fillers is the commercial availability of hyaluronidase to correct injections. Preinjection skin testing is not necessary.5
This category of nonpermanent dermal fillers has the most robust market choices. Older HA dermal fillers with reliable and proven efficacy are Restylane (Galderma Laboratories, LP)(facial rhytides, lip augmentation), Juvéderm (Ultra/Ultra XC/Ultra Plus/Ultra Plus XC [Allergan, Inc])(facial rhytides, lip augmentation), Hydrelle (Anika Therapeutics, Inc)(facial rhytides), and Prevelle Silk (Mentor Corporation)(facial rhytides); they will not be reviewed here. Newer agents include Belotero Balance (Merz Aesthetics), Juvéderm Voluma XC (Allergan, Inc), Restylane Silk (Galderma Laboratories, LP), and Restylane Lyft (Galderma Laboratories, LP).
Belotero Balance
Belotero Balance is used to treat fine lines and wrinkles, especially NLFs.6 The initial pivotal studies that led to FDA approval in 2011 demonstrated noninferiority and superiority to bovine collagen for use in the treatment of NLFs.7,8 One hundred eighteen patients with bilateral NLFs that were rated as 2 (moderate) or 3 (severe) on the wrinkle severity rating scale (WSRS) were randomized to split-face injection of Belotero Balance in one NLF and bovine collagen in the contralateral NLF.7 An additional injection at week 2 was allowed for optimal correction. Belotero Balance was noninferior to bovine collagen at week 2, with mean improvement in WSRS of 1.52 versus 1.57 (P=.50). Belotero Balance was superior to bovine collagen in mean WSRS improvement at weeks 12 (1.25 vs 0.26; P<.001), 16 (1.09 vs 0.66; P<.001), and 24 (1.08 vs 0.50; P<.001).7 In a subsequent open-label extension study, which included 95 of 118 patients who received Belotero Balance injections in both NLFs at week 24, 80.2% of patients showed sustained improvement in WSRS from baseline for 48 weeks without further injection.8
The first comparative study of Belotero Balance with other established HA fillers at the time—Restylane and Juvéderm Ultra 3/Ultra Plus XC—to treat NLFs demonstrated noninferiority.9 Forty patients with bilateral, moderate to severe NLFs (rated 3 or 4 on the Merz severity scale) were randomized to split-face groups of Belotero Balance versus Restylane or Belotero Balance versus Juvéderm. At 12 months, NLF severity improved from 2.3 to 1.5 in the Restylane group and from 2.3 to 1.6 in the Juvéderm group.9
Belotero Balance has been compared to Juvéderm Ultra XC for use in perioral lines.10 The study included 136 patients with moderate to severe perioral lines, according to the perioral lines severity scale, who were randomized (1:1 ratio) to receive injections of Belotero Balance or Juvéderm Ultra XC to correct upper and lower perioral lines, with assessment at week 2 for optimization. After 6 months, 87% of Juvéderm-treated patients compared to 72% of Belotero Balance–treated patients had 1-point improvement in perioral lines (P<.04). Juvéderm-treated patients also reported significantly less pain than Belotero Balance–treated patients (P<.001).10
Treatment-related AEs are described in the Table, with the majority occurring at lower rates compared to a collagen control group and self-resolving within 2 weeks.7

Juvéderm Voluma XC
Juvéderm Voluma XC was FDA approved in 2013 for cheek augmentation to correct age-related volume deficit restoration by subcutaneous or subperiosteal injections. In its landmark multicenter investigation, 282 patients with moderate to severe midface (eg, zygomaticomalar, anteromedial cheek, submalar regions) volume deficit measured on a validated midface volume deficit scale (MFVDS) were treated with Juvéderm Voluma XC (n=235) or control (n=47).11 Patients were reevaluated at 30 days and 81.9% received touch-up injections. At a 6-month primary evaluation, 86% of the Juvéderm-treated patients versus 39% of the control patients showed 1-point improvement on the MFVDS (P<.001). At 24-months’ follow-up, 44.6% of patients sustained efficacy.11 Of these aforementioned patients, 167 received repeat treatment due to lost correction or patient request and 91.1% improved by 1 point or more on the MFVDS on evaluation 12 months after repeat treatment.12 For this same population of patients, a 2-year extended follow-up of patient-reported outcomes revealed that 49% of patients felt fulfilled in their treatment goals 2 years after treatment and 79% of patients rated improvement from baseline based on the global aesthetic improvement scale.13 Efficacy studies involving Juvéderm Voluma XC are currently ongoing for facial temporal aging (registered at www.clinicaltrials.gov with the identifier NCT02437903) and recruiting for mandibular hypoplasia (NCT02330016).
Common treatment-related AEs are detailed in the Table. Two patients required treatment with hyaluronidase for chronic lumpiness and nodularity following non–treatment-related cellulitis.11 The product is contraindicated in patients with allergy to lidocaine.
Restylane Silk
Restylane Silk was approved in 2014 for lip augmentation and perioral rhytides. Efficacy and safety was demonstrated in a large multicenter randomized investigation in which 221 patients seeking lip augmentation received either Restylane Silk (n=177) injected submucosally for treatment of the upper and lower lips and/or intradermally for perioral rhytides or no treatment (n=44).14 Restylane treatment group patients optionally received touch-up at 2 weeks for optimization. All patients, including the control group, received injections at 6 months. At the 2-month primary end point, 80.2% of the treatment group exhibited at least 1-point improvement in upper lip fullness on the Medicis lip fullness scale compared to 11.9% (P<.001) of the control group; response rates for the lower lips were 84.2% versus 18.4% (P<.001). Patients in the treatment group receiving injections for perioral rhytides showed significant improvement in perioral rhytides through week 24 compared to patients treated for lip augmentation only (P<.001).14 Restylane Silk currently is undergoing investigation for cheek rejuvenation (NCT02636894, NCT02679924) and treatment of hand photoaging (NCT02780258).
The most common AEs are listed in the Table. No lip disorders were considered clinically concerning on evaluation. Concomitant lip augmentation and treatment of perioral rhytides yielded similar rates of AEs.14 Restylane Silk is not to be used in patients with known lidocaine allergy.
Restylane Lyft
Restylane Lyft (formerly known as Perlane-L) was approved in 2010 for use in facial rhytides, including NLFs, and gained approval in 2015 for use in cheek augmentation and midface contouring. Only its efficacy and safety for the more recent indication will be reviewed here.
In an evaluator-blinded investigation of 200 patients with mild to substantial bilateral midface deficiency based on the Medicis midface volume scale (MMVS), patients were randomized to receive supraperiosteal and subcutaneous treatment with Restylane Lyft (n=150) or no treatment (n=50).15 Touch-up injections at week 2 or month 12 were available to treatment group patients and all patients were given either an initial treatment or retreatment at 12 months. Primary end point evaluation at week 8 showed that 89% of treatment group patients had at least 1 grade MMVS improvement compared to 16% of the control group (P<.001). Although the percentage of these MMVS responders in the treatment group decreased with each follow-up period to 54.3% at month 12, retreatment was effective in reproducing a similar MMVS response rate as with initial treatment.15 Restylane Lyft is under ongoing investigation for dorsal hand rejuvenation (NCT02650921).
In addition to the common treatment-related AEs listed in the Table, 2 patients reported serious AEs, including bilateral implant-site inflammation and unilateral implant-site hematoma and infection (organism not described), all of which resolved with unspecified treatment.15 Lidocaine allergies are contraindications for use.
Conclusion
Several new options in dermal fillers have been approved in recent years and have demonstrated efficacy and acceptable safety in various cosmetic rejuvenation applications. Restylane Silk and Restylane Lyft are undergoing further studies to evaluate use in hand rejuvenation, an area that currently has few cosmetic filler treatment options. As technology continues to progress and new formulations of dermal fillers with varied properties and benefits are available, clinicians should expect multiple options for use in rhytides, volume deficits, and contouring.
ADDENDUM
After the manuscript was accepted for publication, Juvéderm Volbella XC (Allergan, Inc) was approved by the FDA for use in lip augmentation and thus is not included in this review.
- Fitzgerald R, Graivier MH, Kane M, et al. Update on facial aging. Aesthet Surg J. 2010;30(suppl):S11-S24.
- Karnik J, Baumann L, Bruce S, et al. A double-blind, randomized, multicenter, controlled trial of suspended polymethylmethacrylate microspheres for the correction of atrophic facial acne scars. J Am Acad Dermatol. 2014;71:77-83.
- Bellafill [package insert]. San Diego, CA: Suneva Medical, Inc; 2015.
- Cohen S, Dover J, Monheit G, et al. Five-year safety and satisfaction study of PMMA-collagen in the correction of nasolabial folds. Dermatol Surg. 2015;41(suppl 1):S302-S313.
- Greene JJ, Sidle DM. The hyaluronic acid fillers: current understanding of the tissue device interface. Facial Plast Surg Clin North Am. 2015;23:423-432.
- Lorenc ZP, Fagien S, Flynn TC, et al. Review of key Belotero Balance safety and efficacy trials. Plast Reconstr Surg. 2013;132(4, suppl 2):33S-40S.
- Narins RS, Coleman W, Donofrio L, et al. Nonanimal sourced hyaluronic acid–based dermal filler using a cohesive polydensified matrix technology is superior to bovine collagen in the correction of moderate to severe nasolabial folds: results from a 6-month, randomized, blinded, controlled, multicenter study. Dermatol Surg. 2010;36(suppl 1):730-740.
- Narins RS, Coleman WP 3rd, Donofrio LM, et al. Improvement in nasolabial folds with a hyaluronic acid filler using a cohesive polydensified matrix technology: results from an 18-month open-label extension trial. Dermatol Surg. 2010;36(suppl 3):1800-1808.
- Prager W, Wissmueller E, Havermann I, et al. A prospective, split-face, randomized, comparative study of safety and 12-month longevity of three formulations of hyaluronic acid dermal filler for treatment of nasolabial folds. Dermatol Surg. 2012;38(7, pt 2):1143-1150.
- Butterwick K, Marmur E, Narurkar V, et al. HYC-24L demonstrates greater effectiveness with less pain than CPM-22.5 for treatment of perioral lines in a randomized controlled trial. Dermatol Surg. 2015;41:1351-1360.
- Jones D, Murphy DK. Volumizing hyaluronic acid filler for midface volume deficit: 2-year results from a pivotal single-blind randomized controlled study. Dermatol Surg. 2013;39:1602-1612.
- Baumann L, Narins RS, Beer K, et al. Volumizing hyaluronic acid filler for midface volume deficit: results after repeat treatment. Dermatol Surg. 2015;41(suppl 1):S284-S292.
- Few J, Cox SE, Paradkar-Mitragotri D, et al. A multicenter, single-blind randomized, controlled study of a volumizing hyaluronic acid filler for midface volume deficit: patient-reported outcomes at 2 years. Aesthet Surg J. 2015;35:589-599.
- Beer K, Glogau RG, Dover JS, et al. A randomized, evaluator-blinded, controlled study of effectiveness and safety of small particle hyaluronic acid plus lidocaine for lip augmentation and perioral rhytides. Dermatol Surg. 2015;41(suppl 1):S127-S136.
- Weiss RA, Moradi A, Bank D, et al. Effectiveness and safety of large gel particle hyaluronic acid with lidocaine for correction of midface volume deficit or contour deficiency. Dermatol Surg. 2016;42:699-709.
- Fitzgerald R, Graivier MH, Kane M, et al. Update on facial aging. Aesthet Surg J. 2010;30(suppl):S11-S24.
- Karnik J, Baumann L, Bruce S, et al. A double-blind, randomized, multicenter, controlled trial of suspended polymethylmethacrylate microspheres for the correction of atrophic facial acne scars. J Am Acad Dermatol. 2014;71:77-83.
- Bellafill [package insert]. San Diego, CA: Suneva Medical, Inc; 2015.
- Cohen S, Dover J, Monheit G, et al. Five-year safety and satisfaction study of PMMA-collagen in the correction of nasolabial folds. Dermatol Surg. 2015;41(suppl 1):S302-S313.
- Greene JJ, Sidle DM. The hyaluronic acid fillers: current understanding of the tissue device interface. Facial Plast Surg Clin North Am. 2015;23:423-432.
- Lorenc ZP, Fagien S, Flynn TC, et al. Review of key Belotero Balance safety and efficacy trials. Plast Reconstr Surg. 2013;132(4, suppl 2):33S-40S.
- Narins RS, Coleman W, Donofrio L, et al. Nonanimal sourced hyaluronic acid–based dermal filler using a cohesive polydensified matrix technology is superior to bovine collagen in the correction of moderate to severe nasolabial folds: results from a 6-month, randomized, blinded, controlled, multicenter study. Dermatol Surg. 2010;36(suppl 1):730-740.
- Narins RS, Coleman WP 3rd, Donofrio LM, et al. Improvement in nasolabial folds with a hyaluronic acid filler using a cohesive polydensified matrix technology: results from an 18-month open-label extension trial. Dermatol Surg. 2010;36(suppl 3):1800-1808.
- Prager W, Wissmueller E, Havermann I, et al. A prospective, split-face, randomized, comparative study of safety and 12-month longevity of three formulations of hyaluronic acid dermal filler for treatment of nasolabial folds. Dermatol Surg. 2012;38(7, pt 2):1143-1150.
- Butterwick K, Marmur E, Narurkar V, et al. HYC-24L demonstrates greater effectiveness with less pain than CPM-22.5 for treatment of perioral lines in a randomized controlled trial. Dermatol Surg. 2015;41:1351-1360.
- Jones D, Murphy DK. Volumizing hyaluronic acid filler for midface volume deficit: 2-year results from a pivotal single-blind randomized controlled study. Dermatol Surg. 2013;39:1602-1612.
- Baumann L, Narins RS, Beer K, et al. Volumizing hyaluronic acid filler for midface volume deficit: results after repeat treatment. Dermatol Surg. 2015;41(suppl 1):S284-S292.
- Few J, Cox SE, Paradkar-Mitragotri D, et al. A multicenter, single-blind randomized, controlled study of a volumizing hyaluronic acid filler for midface volume deficit: patient-reported outcomes at 2 years. Aesthet Surg J. 2015;35:589-599.
- Beer K, Glogau RG, Dover JS, et al. A randomized, evaluator-blinded, controlled study of effectiveness and safety of small particle hyaluronic acid plus lidocaine for lip augmentation and perioral rhytides. Dermatol Surg. 2015;41(suppl 1):S127-S136.
- Weiss RA, Moradi A, Bank D, et al. Effectiveness and safety of large gel particle hyaluronic acid with lidocaine for correction of midface volume deficit or contour deficiency. Dermatol Surg. 2016;42:699-709.
Practice Points
- The merits of new dermal fillers approved by the US Food and Drug Administration should be weighed with an understanding of aesthetic indications of use, duration of efficacy, and common adverse effects, in line with patient preference.
- The most common adverse effects are injection-site contusion, swelling, and pain, usually self-resolving within days to 2 weeks. Patient quality of care can be improved with forewarning and emphasis on alleviating symptoms.
Comment on “Merkel Cell Carcinoma in a Vein Graft Donor Site”
To the Editor:
A recent Cutis article, “Merkel Cell Carcinoma in a Vein Graft Donor Site” (Cutis. 2016;97:364-367), highlighted the localization of a Merkel cell carcinoma (MCC) within a well-healed scar resulting from a vein harvesting procedure performed 18 years prior to presentation. Their discussion focused on factors that may have contributed to the development of the MCC at that specific location. As noted by the authors, this case does not classically fit under the umbrella of a Marjolin ulcer given the stable, well-healed clinical appearance of the scar. We agree and believe it is not secondary to chance either but consistent with Wolf isotopic response.
This concept was originally described by Wyburn-Mason in 19551 and later revived by Wolf et al.2 Wolf isotopic response describes the development of dermatologic disorders that localize to a site of another distinct and clinically healed skin disorder. Originally, it was reserved for infections, malignancies, and immune conditions restricted to a site of a prior herpetic infection but recently has been expanded to encompass other primary nonherpesvirus-related skin disorders. The pathophysiology behind this phenomenon is unknown but thought to be the interplay of several key elements including immune dysregulation, neural, vascular, and locus minoris resistentiae (ie, a site of lessened resistance).3 Immunosuppression is a known risk factor in the development of MCCs,4 thus the proposed local immune dysregulation within a scar may alter the virus-host balance and foster the oncogenic nature of the MCC polyomavirus. A recent article describes another case of an MCC arising within a sternotomy scar,5 lending further credibility to a skin vulnerability philosophy. These cases provide further insight into the pathomechanisms involved in the development of this rare and aggressive neoplasm and sheds light on an intriguing dermatologic phenomenon.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. Br Med J. 1955;2:1106-1109.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Liu CI, Hsu CH. Leukaemia cutis at the site of striae distensae: an isotopic response? Acta Derm Venereol. 2010;90:422-423.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
- Grippaudo FR, Costantino B, Santanelli F. Merkel cell carcinoma on a sternotomy scar: atypical clinical presentation. J Clin Oncol. 2015;33:e22-e24.
To the Editor:
A recent Cutis article, “Merkel Cell Carcinoma in a Vein Graft Donor Site” (Cutis. 2016;97:364-367), highlighted the localization of a Merkel cell carcinoma (MCC) within a well-healed scar resulting from a vein harvesting procedure performed 18 years prior to presentation. Their discussion focused on factors that may have contributed to the development of the MCC at that specific location. As noted by the authors, this case does not classically fit under the umbrella of a Marjolin ulcer given the stable, well-healed clinical appearance of the scar. We agree and believe it is not secondary to chance either but consistent with Wolf isotopic response.
This concept was originally described by Wyburn-Mason in 19551 and later revived by Wolf et al.2 Wolf isotopic response describes the development of dermatologic disorders that localize to a site of another distinct and clinically healed skin disorder. Originally, it was reserved for infections, malignancies, and immune conditions restricted to a site of a prior herpetic infection but recently has been expanded to encompass other primary nonherpesvirus-related skin disorders. The pathophysiology behind this phenomenon is unknown but thought to be the interplay of several key elements including immune dysregulation, neural, vascular, and locus minoris resistentiae (ie, a site of lessened resistance).3 Immunosuppression is a known risk factor in the development of MCCs,4 thus the proposed local immune dysregulation within a scar may alter the virus-host balance and foster the oncogenic nature of the MCC polyomavirus. A recent article describes another case of an MCC arising within a sternotomy scar,5 lending further credibility to a skin vulnerability philosophy. These cases provide further insight into the pathomechanisms involved in the development of this rare and aggressive neoplasm and sheds light on an intriguing dermatologic phenomenon.
To the Editor:
A recent Cutis article, “Merkel Cell Carcinoma in a Vein Graft Donor Site” (Cutis. 2016;97:364-367), highlighted the localization of a Merkel cell carcinoma (MCC) within a well-healed scar resulting from a vein harvesting procedure performed 18 years prior to presentation. Their discussion focused on factors that may have contributed to the development of the MCC at that specific location. As noted by the authors, this case does not classically fit under the umbrella of a Marjolin ulcer given the stable, well-healed clinical appearance of the scar. We agree and believe it is not secondary to chance either but consistent with Wolf isotopic response.
This concept was originally described by Wyburn-Mason in 19551 and later revived by Wolf et al.2 Wolf isotopic response describes the development of dermatologic disorders that localize to a site of another distinct and clinically healed skin disorder. Originally, it was reserved for infections, malignancies, and immune conditions restricted to a site of a prior herpetic infection but recently has been expanded to encompass other primary nonherpesvirus-related skin disorders. The pathophysiology behind this phenomenon is unknown but thought to be the interplay of several key elements including immune dysregulation, neural, vascular, and locus minoris resistentiae (ie, a site of lessened resistance).3 Immunosuppression is a known risk factor in the development of MCCs,4 thus the proposed local immune dysregulation within a scar may alter the virus-host balance and foster the oncogenic nature of the MCC polyomavirus. A recent article describes another case of an MCC arising within a sternotomy scar,5 lending further credibility to a skin vulnerability philosophy. These cases provide further insight into the pathomechanisms involved in the development of this rare and aggressive neoplasm and sheds light on an intriguing dermatologic phenomenon.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. Br Med J. 1955;2:1106-1109.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Liu CI, Hsu CH. Leukaemia cutis at the site of striae distensae: an isotopic response? Acta Derm Venereol. 2010;90:422-423.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
- Grippaudo FR, Costantino B, Santanelli F. Merkel cell carcinoma on a sternotomy scar: atypical clinical presentation. J Clin Oncol. 2015;33:e22-e24.
- Wyburn-Mason R. Malignant change arising in tissues affected by herpes. Br Med J. 1955;2:1106-1109.
- Wolf R, Brenner S, Ruocco V, et al. Isotopic response. Int J Dermatol. 1995;34:341-348.
- Liu CI, Hsu CH. Leukaemia cutis at the site of striae distensae: an isotopic response? Acta Derm Venereol. 2010;90:422-423.
- Heath M, Jaimes N, Lemos B, et al. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375-381.
- Grippaudo FR, Costantino B, Santanelli F. Merkel cell carcinoma on a sternotomy scar: atypical clinical presentation. J Clin Oncol. 2015;33:e22-e24.
Finger length ratio identifies women at increased risk for depression and anxiety
VIENNA – They say that in hula dancing, it’s the expressive hands, not the quaking hips, that tell the story.
And in Dutch women, a relatively short index finger on the left hand bespeaks an increased risk for depression and stress.
That’s right: The ratio of the length of the index finger to the ring finger, or 2D:4D digit ratio, of the left hand shows potential as a quick and dirty biomarker that could be used to screen patients for increased risk for depression. But only in women, Deborah De Kruijff reported at the annual congress of the European College of Neuropsychopharmacology.
She and her coinvestigators measured the lengths of the index and ring fingers on both hands of 124 male and 146 female Dutch college students using Vernier calipers accurate to within 0.01 mm. Participants completed the 21-item version of the Depression, Anxiety, and Stress Scale (DASS-21) and correlated the 2D:4D digit ratios with the DASS-21 total scores as well as the scores on the depression, anxiety, and stress subscales.
The 2D:4D digit ratio didn’t correlate with DASS-21 scores in men. But in women, the lower the 2D:4D ratio on the left hand, the higher their overall DASS-21 score as well as their scores on the depression and stress subscales. Each of these associations was highly statistically significant at the P = .002 to .005 level, according to Ms. De Kruijff, a PhD candidate in neuroscience at Utrecht (the Netherlands) University.
Finding correlates between the 2D:4D digit ratio and predispositions to various diseases, personality traits, and other human characteristics was a popular scientific pastime in the 1800s. After a long dry spell, it rebounded as a research area several decades ago. The 2D:4D ratio is a sexually dimorphic trait. It is thought to depend upon prenatal exposure to sex hormones. A low 2D:4D ratio is associated with in utero exposure to relatively higher levels of fetal testosterone than fetal estrogen. Thus, a greater proportion of men than women have index fingers that are shorter than the ring finger.
Other investigators have linked a low 2D:4D ratio to increased risks of prostate cancer, attention-deficit/hyperactivity disorder, and autism spectrum disorder in men, and to greater assertiveness and increased risk of anorexia nervosa in women.
Ms. De Kruijff said more research is needed to understand why only the finger length on the left hand of the women was predictive of increased risk of depression and stress.
She reported having no financial conflicts of interest regarding this university-funded study.
VIENNA – They say that in hula dancing, it’s the expressive hands, not the quaking hips, that tell the story.
And in Dutch women, a relatively short index finger on the left hand bespeaks an increased risk for depression and stress.
That’s right: The ratio of the length of the index finger to the ring finger, or 2D:4D digit ratio, of the left hand shows potential as a quick and dirty biomarker that could be used to screen patients for increased risk for depression. But only in women, Deborah De Kruijff reported at the annual congress of the European College of Neuropsychopharmacology.
She and her coinvestigators measured the lengths of the index and ring fingers on both hands of 124 male and 146 female Dutch college students using Vernier calipers accurate to within 0.01 mm. Participants completed the 21-item version of the Depression, Anxiety, and Stress Scale (DASS-21) and correlated the 2D:4D digit ratios with the DASS-21 total scores as well as the scores on the depression, anxiety, and stress subscales.
The 2D:4D digit ratio didn’t correlate with DASS-21 scores in men. But in women, the lower the 2D:4D ratio on the left hand, the higher their overall DASS-21 score as well as their scores on the depression and stress subscales. Each of these associations was highly statistically significant at the P = .002 to .005 level, according to Ms. De Kruijff, a PhD candidate in neuroscience at Utrecht (the Netherlands) University.
Finding correlates between the 2D:4D digit ratio and predispositions to various diseases, personality traits, and other human characteristics was a popular scientific pastime in the 1800s. After a long dry spell, it rebounded as a research area several decades ago. The 2D:4D ratio is a sexually dimorphic trait. It is thought to depend upon prenatal exposure to sex hormones. A low 2D:4D ratio is associated with in utero exposure to relatively higher levels of fetal testosterone than fetal estrogen. Thus, a greater proportion of men than women have index fingers that are shorter than the ring finger.
Other investigators have linked a low 2D:4D ratio to increased risks of prostate cancer, attention-deficit/hyperactivity disorder, and autism spectrum disorder in men, and to greater assertiveness and increased risk of anorexia nervosa in women.
Ms. De Kruijff said more research is needed to understand why only the finger length on the left hand of the women was predictive of increased risk of depression and stress.
She reported having no financial conflicts of interest regarding this university-funded study.
VIENNA – They say that in hula dancing, it’s the expressive hands, not the quaking hips, that tell the story.
And in Dutch women, a relatively short index finger on the left hand bespeaks an increased risk for depression and stress.
That’s right: The ratio of the length of the index finger to the ring finger, or 2D:4D digit ratio, of the left hand shows potential as a quick and dirty biomarker that could be used to screen patients for increased risk for depression. But only in women, Deborah De Kruijff reported at the annual congress of the European College of Neuropsychopharmacology.
She and her coinvestigators measured the lengths of the index and ring fingers on both hands of 124 male and 146 female Dutch college students using Vernier calipers accurate to within 0.01 mm. Participants completed the 21-item version of the Depression, Anxiety, and Stress Scale (DASS-21) and correlated the 2D:4D digit ratios with the DASS-21 total scores as well as the scores on the depression, anxiety, and stress subscales.
The 2D:4D digit ratio didn’t correlate with DASS-21 scores in men. But in women, the lower the 2D:4D ratio on the left hand, the higher their overall DASS-21 score as well as their scores on the depression and stress subscales. Each of these associations was highly statistically significant at the P = .002 to .005 level, according to Ms. De Kruijff, a PhD candidate in neuroscience at Utrecht (the Netherlands) University.
Finding correlates between the 2D:4D digit ratio and predispositions to various diseases, personality traits, and other human characteristics was a popular scientific pastime in the 1800s. After a long dry spell, it rebounded as a research area several decades ago. The 2D:4D ratio is a sexually dimorphic trait. It is thought to depend upon prenatal exposure to sex hormones. A low 2D:4D ratio is associated with in utero exposure to relatively higher levels of fetal testosterone than fetal estrogen. Thus, a greater proportion of men than women have index fingers that are shorter than the ring finger.
Other investigators have linked a low 2D:4D ratio to increased risks of prostate cancer, attention-deficit/hyperactivity disorder, and autism spectrum disorder in men, and to greater assertiveness and increased risk of anorexia nervosa in women.
Ms. De Kruijff said more research is needed to understand why only the finger length on the left hand of the women was predictive of increased risk of depression and stress.
She reported having no financial conflicts of interest regarding this university-funded study.
AT THE ECNP CONGRESS
Key clinical point:
Major finding: The lower the ratio of the length of the index finger to the ring finger on the left hand in women, the higher they scored on a validated measure of depression and stress.
Data source: A cross-sectional study involving 124 male and 146 female university students who completed the 21-item version of the Depression, Anxiety, and Stress Scale and were measured for the lengths of their index and ring fingers on both hands.
Disclosures: The presenter reported having no financial conflicts of interest regarding this university-funded study.
Evaluation of a Dementia Resource Fair for Veterans, Caregivers, and Staff
Due to the increasing number of older adults, the annual number of new cases of Alzheimer disease and other types of dementia is projected to double by 2050.1 The cost of caring for persons with dementia is rising as well. In 2015, the expected health care cost for persons with dementia in the U.S. is estimated to be $226 billion.1 There is a growing awareness of the needs of persons with dementia and of the importance of providing caregivers with support and education that enables them to keep their loved ones at home as long as possible. Additionally, caregiver stress adversely affects health and increases mortality risk.2-4 Efficacious interventions that teach caregivers to cope with challenging behaviors and functional decline are also available.5,6 Yet many caregivers encounter barriers that prevent access to these interventions. Some may not be able to access interventions due to lack of insurance plan coverage; others may not have the time to participate in these programs.7,8
The VA has requested that its VISNs and VAMCs develop dementia committees so that VA employees can establish goals focused on improving dementia care. The VA Palo Alto Health Care System (VAPAHCS) Dementia Committee determined that veterans, caregivers, and staff needed simple, clear information about dementia, based on consensus opinion. In 2013, one of the committee co-chairs, a clinical nurse specialist in the Geriatric Research Education and Clinical Center (GRECC), introduced the concept of a dementia resource fair. There is evidence supporting the use of interdisciplinary health fairs to educate allied health trainees (eg, nursing students and social workers) through service learning.9 But to the authors’ knowledge, the use of such a fair to provide dementia information has not been evaluated.
The fair drew from the evidence base for formal psychoeducational interventions for caregiversand for those with dementia or cognitive impairment.10,11 The goal of the fair was to provide information about resources for and management of dementia to veterans, families, staff, caregivers, and the community, using printed material and consultation with knowledgeable staff. The GRECC staff also initiated a systematic evaluation of this new initiative and collaborated with the Stanford/VA Alzheimer’s Research Center staff on the evaluation process.
Initial Plan
A subcommittee, composed of interdisciplinary professionals who work with veterans diagnosed with dementia, planned the initial dementia resource fair. The subcommittee representatives included geriatric medicine, nursing, occupational therapy, pharmacy, psychology, recreational therapy, and social work. Subcommittee members were charged with developing VA-branded handouts as educational tools to address key issues related to dementia, such as advance directive planning, behavioral management, home safety, and medication management. The subcommittee met monthly for 6 months and focused on logistics, identification of resource tables, creation of educational materials, advertising, and development of an evaluation. Table 1 provides an overview of the planning time line for the 2013 fair held in San Jose. Findings from a systematic evaluation of the 2013 fair were used to improve the 2015 fair held in Menlo Park. A discussion about the evaluation method and results follows.
Methods
The first fair was held at a VA community-based outpatient clinic in a small conference room with 13 resource tables. Feedback from attendees in 2013 included suggestions for having more tables, larger event space, more publicity, and alternate locations for the fair. In response to the feedback, the 2015 fair was held at a division of the main VAMC in a large conference room and hosted 20 tables arranged in a horseshoe shape. The second fair included an activity table staffed by a psychology fellow and recreation therapist who provided respite to caregivers if their loved one with dementia accompanied them to the event. Both the 2013 and 2015 fairs were 4 hours long.
A 1-page, anonymous survey was developed to assess attendees’ opinions about the fair. The survey included information about whether attendees were caregivers, veterans, or VA staff but did not ask other demographic questions to preserve anonymity. In 2013, the survey asked attendees to choose the category that best described them, but in 2015, the survey asked attendees to indicate the number of individuals from each category in their party. The 2015 survey assessed 2 additional categories (family member, other) and added a question about the number of people in each party to better estimate attendance. Both surveys also asked attendees to check which resource tables they visited.
The following assessment questions were consistent across both fairs to allow for comparisons. The authors assessed attitudes and learning as a result of the fair, using 2 statements that were rated with a 5-point Likert scale. The authors asked 3 open-ended questions to ascertain the helpful aspects of the fair, unmet needs, and suggestions for improvement. The Stanford University Institutional Review Board (IRB) reviewed this program evaluation plan and determined that the program evaluation project did not require IRB approval.
When attendees arrived at the fair, they received a folder containing branded handouts, a reusable bag, and a survey. Committee members asked that 1 person per party complete the survey at the end of the visit. Attendees visited tables, obtained written materials, and spoke with subcommittee members who staffed the tables. Snacks and light refreshments were provided. The reusable bag was provided by the VAMC Suicide Prevention Program to increase awareness of the VAPAHCS Suicide Prevention Program. As attendees were leaving, they were reminded to complete the survey. Attendees deposited completed surveys in a box to ensure anonymity.
Results
Thirty-six individuals attended the 2013 fair, and 138 individuals attended the 2015 fair. Thirty-one surveys were completed in 2013, yielding an 86% response rate. One hundred six surveys were returned and represented responses for 129 individuals in 2015, yielding a 94% response rate in 2015. Most of the 2013 attendees were caregivers, followed by veterans, VA staff, and outside staff (Table 2). In contrast, most of the 2015 attendees were VA staff, followed by veterans, caregivers/family members, outside staff, and others. Distributions of attendees differed significantly across the fairs: χ2(4) = 12.66; P = .01.
The surveys assessed which tables attendees visited and their perceptions of the fair. The most frequently visited resource table for both 2013 and 2015 fairs was the Alzheimer’s Association table. Other popular resource tables were VA Benefits and VA Caregiver Support in 2013 and Home Safety and End of Life Care in 2015. Ninety-six percent of 2013 attendees and 100% of 2015 attendees strongly agreed or agreed that “attending the dementia fair was worth my time and effort.” Eighty-three percent of 2013 attendees and 100% of 2015 attendees felt that they had learned something useful at the fair. The proportion of individuals reporting that they had learned something useful significantly increased from 2013 to 2015: χ2(2) = 18.07; P = .0001.
To summarize the open-ended responses to the question “What was most helpful about the fair?” the authors constructed a word cloud that displays the 75 most frequently used words in attendees’ descriptions of the 2015 fair (Figure). Attendees provided suggestions about additional information and resources they desired, which included VA benefits enrollment, books and movies about dementia (eg, Still Alice), speech and swallowing disorders representatives, varied types of advance directives, class discussion, question-and-answer time with speakers, and resources for nonveteran older adults. General suggestions for future fairs included hosting the fair at the main division of the VA health care system, having more room between tables, inviting more vendors, using more visual posters at the tables, and additional advertising for VA services.
Discussion
Dementia is a costly disease with detrimental health and well-being effects on caregivers. The dementia resource fairs aimed to connect caregivers with resources for veterans with dementia in the VA and in the community. Given that nearly half the 2015 fair attendees were VA staff, there is an apparent need for increasing dementia education and access to care resource for this VAMC’s workforce. The high proportion of staff attendees at the 2015 fair may be attributed to the 2 VA community living centers at the VAMC site where the fair was held. This unexpected finding points to the importance of informal and interactive education opportunities for staff, particularly those working with veterans with dementia. The fair served an important role for VA staff seeking information on dementia for professional and personal reasons. This systematic evaluation of the fair demonstrated a need for improving access to information about dementia.
The idea of hosting a dementia resource fair was met with enthusiasm from attendees and subcommittee members in 2013. Feedback helped refine the second fair. The increase in self-reported learning from 2013 to 2015 suggests improvements may have been made between the first and second fair; however, this must be interpreted in light of the different compositions of the attendees at each fair and the absence of a control group. Attendees desired even more information about dementia at the second fair, as evidenced by suggestions to have presentations, speakers, and class discussions. These responses suggest that other sites may wish to consider holding similar events. Next steps include researching the effectiveness of low-cost, pragmatic educational initiatives for caregivers. In fact, randomized, controlled trials of dementia caregiver education and skill-building interventions are underway at VAPAHCS.
Conclusion
The primary lesson learned from the most recent fair was that marketing is the key to success. The authors created an efficient hospital publicity plan in 2015 that included (1) flyers posted throughout 2 main medical center campuses; (2) announcements on closed-circuit VA waiting room televisions; (3) e-mail announcements sent to staff; and (4) VA social media announcements. Flyers also were mailed to known caregivers, and announcements of the event were provided to local community agencies. This focus on publicity likely contributed to the substantial increase in participation from the 2013 to 2015 fair.
Future fairs may be improved by providing more detailed information about dementia through formal presentations. The authors aim to increase the number of family caregivers in attendance possibly through coordinating the fair to coincide with primary care clinic hours, advertising the availability of brief respite at the fair, and conducting additional outreach to veterans.
This systematic evaluation of the dementia resource fair confirmed that providing resources in a drop-in setting resulted in self-reported learning about resources available for veterans with dementia. VA dementia care providers are encouraged to use the authors’ time line and lessons learned to develop dementia resource fairs for their sites.
Acknowledgments
The authors wish to acknowledge the members of the 2013 and 2015 Dementia Resource Fair Committees, chaired by Betty Wexler and Kathleen McConnell, respectively. Dr. Gould is supported by the U.S. Department of Veterans Affairs (IK2 RX001478) and by Ellen Schapiro & Gerald Axelbaum through a 2014 NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation. Dr. Scanlon is supported by the U.S. Department of Veterans Affairs (IK2 RX001240; I21 RX001710), U.S. Department of Defense (W81XWH-15-1-0246), Sierra-Pacific Mental Illness Research Education and Clinical Center, and Stanford/VA Alzheimer’s Research Center. Drs. Gould and Scanlon also receive support from Palo Alto Veterans Institute for Research.
1. Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11(3)332-384.
2. Schulz R, Beach SR, Cook TB, Martire LM, Tomlinson JM, Monin JK. Predictors and consequences of perceived lack of choice in becoming an informal caregiver. Aging Ment Health. 2012;16(6):712-721.
3. Cooper C, Mukadam N, Katona C, et al; World Federation of Biological Psychiatry – Old Age Taskforce. Systematic review of the effectiveness of non-pharmacological interventions to improve quality of life of people with dementia. Int Psychogeriatr. 2012;24(6):856-870.
4. Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1991;282(23):2215-2219.
5. Brodaty H, Arasaratnam C. Meta-analysis of nonpharmacological interventions for neuropsychiatric symptoms of dementia. Am J Psychiatry. 2012;169(9):946-953.
6. Gitlin LN. Good news for dementia care: caregiver interventions reduce behavioral symptoms in people with dementia and family distress. Am J Psychiatry. 2012;169(9):894-897.
7. Ho A, Collins SR, Davis K, Doty MM. A look at working-age caregivers roles, health concerns, and need for support. Issue Brief (Commonw Fund). 2005;(854):1-12.
8. Joling KJ, van Marwijk HWJ, Smit F, et al. Does a family meetings intervention prevent depression and anxiety in family caregivers of dementia patients? A randomized trial. PLoS One. 2012;7(1):e30936.
9. Kolomer S, Quinn ME, Steele K. Interdisciplinary health fairs for older adults and the value of interprofessional service learning. J Community Pract. 2010;18(2-3):267-279.
10. Jensen M, Agbata IN, Canavan M, McCarthy G. Effectiveness of educational interventions for informal caregivers of individuals with dementia residing in the community: systematic review and meta-analysis of randomized controlled trials. Int J Geriatr Psychiatry. 2015;30(2):130-143.
11. Quinn C, Toms G, Anderson D, Clare L. A review of self-management interventions for people with dementia and mild cognitive impairment. J Appl Gerontol. 2015;pii:0733464814566852.
Due to the increasing number of older adults, the annual number of new cases of Alzheimer disease and other types of dementia is projected to double by 2050.1 The cost of caring for persons with dementia is rising as well. In 2015, the expected health care cost for persons with dementia in the U.S. is estimated to be $226 billion.1 There is a growing awareness of the needs of persons with dementia and of the importance of providing caregivers with support and education that enables them to keep their loved ones at home as long as possible. Additionally, caregiver stress adversely affects health and increases mortality risk.2-4 Efficacious interventions that teach caregivers to cope with challenging behaviors and functional decline are also available.5,6 Yet many caregivers encounter barriers that prevent access to these interventions. Some may not be able to access interventions due to lack of insurance plan coverage; others may not have the time to participate in these programs.7,8
The VA has requested that its VISNs and VAMCs develop dementia committees so that VA employees can establish goals focused on improving dementia care. The VA Palo Alto Health Care System (VAPAHCS) Dementia Committee determined that veterans, caregivers, and staff needed simple, clear information about dementia, based on consensus opinion. In 2013, one of the committee co-chairs, a clinical nurse specialist in the Geriatric Research Education and Clinical Center (GRECC), introduced the concept of a dementia resource fair. There is evidence supporting the use of interdisciplinary health fairs to educate allied health trainees (eg, nursing students and social workers) through service learning.9 But to the authors’ knowledge, the use of such a fair to provide dementia information has not been evaluated.
The fair drew from the evidence base for formal psychoeducational interventions for caregiversand for those with dementia or cognitive impairment.10,11 The goal of the fair was to provide information about resources for and management of dementia to veterans, families, staff, caregivers, and the community, using printed material and consultation with knowledgeable staff. The GRECC staff also initiated a systematic evaluation of this new initiative and collaborated with the Stanford/VA Alzheimer’s Research Center staff on the evaluation process.
Initial Plan
A subcommittee, composed of interdisciplinary professionals who work with veterans diagnosed with dementia, planned the initial dementia resource fair. The subcommittee representatives included geriatric medicine, nursing, occupational therapy, pharmacy, psychology, recreational therapy, and social work. Subcommittee members were charged with developing VA-branded handouts as educational tools to address key issues related to dementia, such as advance directive planning, behavioral management, home safety, and medication management. The subcommittee met monthly for 6 months and focused on logistics, identification of resource tables, creation of educational materials, advertising, and development of an evaluation. Table 1 provides an overview of the planning time line for the 2013 fair held in San Jose. Findings from a systematic evaluation of the 2013 fair were used to improve the 2015 fair held in Menlo Park. A discussion about the evaluation method and results follows.
Methods
The first fair was held at a VA community-based outpatient clinic in a small conference room with 13 resource tables. Feedback from attendees in 2013 included suggestions for having more tables, larger event space, more publicity, and alternate locations for the fair. In response to the feedback, the 2015 fair was held at a division of the main VAMC in a large conference room and hosted 20 tables arranged in a horseshoe shape. The second fair included an activity table staffed by a psychology fellow and recreation therapist who provided respite to caregivers if their loved one with dementia accompanied them to the event. Both the 2013 and 2015 fairs were 4 hours long.
A 1-page, anonymous survey was developed to assess attendees’ opinions about the fair. The survey included information about whether attendees were caregivers, veterans, or VA staff but did not ask other demographic questions to preserve anonymity. In 2013, the survey asked attendees to choose the category that best described them, but in 2015, the survey asked attendees to indicate the number of individuals from each category in their party. The 2015 survey assessed 2 additional categories (family member, other) and added a question about the number of people in each party to better estimate attendance. Both surveys also asked attendees to check which resource tables they visited.
The following assessment questions were consistent across both fairs to allow for comparisons. The authors assessed attitudes and learning as a result of the fair, using 2 statements that were rated with a 5-point Likert scale. The authors asked 3 open-ended questions to ascertain the helpful aspects of the fair, unmet needs, and suggestions for improvement. The Stanford University Institutional Review Board (IRB) reviewed this program evaluation plan and determined that the program evaluation project did not require IRB approval.
When attendees arrived at the fair, they received a folder containing branded handouts, a reusable bag, and a survey. Committee members asked that 1 person per party complete the survey at the end of the visit. Attendees visited tables, obtained written materials, and spoke with subcommittee members who staffed the tables. Snacks and light refreshments were provided. The reusable bag was provided by the VAMC Suicide Prevention Program to increase awareness of the VAPAHCS Suicide Prevention Program. As attendees were leaving, they were reminded to complete the survey. Attendees deposited completed surveys in a box to ensure anonymity.
Results
Thirty-six individuals attended the 2013 fair, and 138 individuals attended the 2015 fair. Thirty-one surveys were completed in 2013, yielding an 86% response rate. One hundred six surveys were returned and represented responses for 129 individuals in 2015, yielding a 94% response rate in 2015. Most of the 2013 attendees were caregivers, followed by veterans, VA staff, and outside staff (Table 2). In contrast, most of the 2015 attendees were VA staff, followed by veterans, caregivers/family members, outside staff, and others. Distributions of attendees differed significantly across the fairs: χ2(4) = 12.66; P = .01.
The surveys assessed which tables attendees visited and their perceptions of the fair. The most frequently visited resource table for both 2013 and 2015 fairs was the Alzheimer’s Association table. Other popular resource tables were VA Benefits and VA Caregiver Support in 2013 and Home Safety and End of Life Care in 2015. Ninety-six percent of 2013 attendees and 100% of 2015 attendees strongly agreed or agreed that “attending the dementia fair was worth my time and effort.” Eighty-three percent of 2013 attendees and 100% of 2015 attendees felt that they had learned something useful at the fair. The proportion of individuals reporting that they had learned something useful significantly increased from 2013 to 2015: χ2(2) = 18.07; P = .0001.
To summarize the open-ended responses to the question “What was most helpful about the fair?” the authors constructed a word cloud that displays the 75 most frequently used words in attendees’ descriptions of the 2015 fair (Figure). Attendees provided suggestions about additional information and resources they desired, which included VA benefits enrollment, books and movies about dementia (eg, Still Alice), speech and swallowing disorders representatives, varied types of advance directives, class discussion, question-and-answer time with speakers, and resources for nonveteran older adults. General suggestions for future fairs included hosting the fair at the main division of the VA health care system, having more room between tables, inviting more vendors, using more visual posters at the tables, and additional advertising for VA services.
Discussion
Dementia is a costly disease with detrimental health and well-being effects on caregivers. The dementia resource fairs aimed to connect caregivers with resources for veterans with dementia in the VA and in the community. Given that nearly half the 2015 fair attendees were VA staff, there is an apparent need for increasing dementia education and access to care resource for this VAMC’s workforce. The high proportion of staff attendees at the 2015 fair may be attributed to the 2 VA community living centers at the VAMC site where the fair was held. This unexpected finding points to the importance of informal and interactive education opportunities for staff, particularly those working with veterans with dementia. The fair served an important role for VA staff seeking information on dementia for professional and personal reasons. This systematic evaluation of the fair demonstrated a need for improving access to information about dementia.
The idea of hosting a dementia resource fair was met with enthusiasm from attendees and subcommittee members in 2013. Feedback helped refine the second fair. The increase in self-reported learning from 2013 to 2015 suggests improvements may have been made between the first and second fair; however, this must be interpreted in light of the different compositions of the attendees at each fair and the absence of a control group. Attendees desired even more information about dementia at the second fair, as evidenced by suggestions to have presentations, speakers, and class discussions. These responses suggest that other sites may wish to consider holding similar events. Next steps include researching the effectiveness of low-cost, pragmatic educational initiatives for caregivers. In fact, randomized, controlled trials of dementia caregiver education and skill-building interventions are underway at VAPAHCS.
Conclusion
The primary lesson learned from the most recent fair was that marketing is the key to success. The authors created an efficient hospital publicity plan in 2015 that included (1) flyers posted throughout 2 main medical center campuses; (2) announcements on closed-circuit VA waiting room televisions; (3) e-mail announcements sent to staff; and (4) VA social media announcements. Flyers also were mailed to known caregivers, and announcements of the event were provided to local community agencies. This focus on publicity likely contributed to the substantial increase in participation from the 2013 to 2015 fair.
Future fairs may be improved by providing more detailed information about dementia through formal presentations. The authors aim to increase the number of family caregivers in attendance possibly through coordinating the fair to coincide with primary care clinic hours, advertising the availability of brief respite at the fair, and conducting additional outreach to veterans.
This systematic evaluation of the dementia resource fair confirmed that providing resources in a drop-in setting resulted in self-reported learning about resources available for veterans with dementia. VA dementia care providers are encouraged to use the authors’ time line and lessons learned to develop dementia resource fairs for their sites.
Acknowledgments
The authors wish to acknowledge the members of the 2013 and 2015 Dementia Resource Fair Committees, chaired by Betty Wexler and Kathleen McConnell, respectively. Dr. Gould is supported by the U.S. Department of Veterans Affairs (IK2 RX001478) and by Ellen Schapiro & Gerald Axelbaum through a 2014 NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation. Dr. Scanlon is supported by the U.S. Department of Veterans Affairs (IK2 RX001240; I21 RX001710), U.S. Department of Defense (W81XWH-15-1-0246), Sierra-Pacific Mental Illness Research Education and Clinical Center, and Stanford/VA Alzheimer’s Research Center. Drs. Gould and Scanlon also receive support from Palo Alto Veterans Institute for Research.
Due to the increasing number of older adults, the annual number of new cases of Alzheimer disease and other types of dementia is projected to double by 2050.1 The cost of caring for persons with dementia is rising as well. In 2015, the expected health care cost for persons with dementia in the U.S. is estimated to be $226 billion.1 There is a growing awareness of the needs of persons with dementia and of the importance of providing caregivers with support and education that enables them to keep their loved ones at home as long as possible. Additionally, caregiver stress adversely affects health and increases mortality risk.2-4 Efficacious interventions that teach caregivers to cope with challenging behaviors and functional decline are also available.5,6 Yet many caregivers encounter barriers that prevent access to these interventions. Some may not be able to access interventions due to lack of insurance plan coverage; others may not have the time to participate in these programs.7,8
The VA has requested that its VISNs and VAMCs develop dementia committees so that VA employees can establish goals focused on improving dementia care. The VA Palo Alto Health Care System (VAPAHCS) Dementia Committee determined that veterans, caregivers, and staff needed simple, clear information about dementia, based on consensus opinion. In 2013, one of the committee co-chairs, a clinical nurse specialist in the Geriatric Research Education and Clinical Center (GRECC), introduced the concept of a dementia resource fair. There is evidence supporting the use of interdisciplinary health fairs to educate allied health trainees (eg, nursing students and social workers) through service learning.9 But to the authors’ knowledge, the use of such a fair to provide dementia information has not been evaluated.
The fair drew from the evidence base for formal psychoeducational interventions for caregiversand for those with dementia or cognitive impairment.10,11 The goal of the fair was to provide information about resources for and management of dementia to veterans, families, staff, caregivers, and the community, using printed material and consultation with knowledgeable staff. The GRECC staff also initiated a systematic evaluation of this new initiative and collaborated with the Stanford/VA Alzheimer’s Research Center staff on the evaluation process.
Initial Plan
A subcommittee, composed of interdisciplinary professionals who work with veterans diagnosed with dementia, planned the initial dementia resource fair. The subcommittee representatives included geriatric medicine, nursing, occupational therapy, pharmacy, psychology, recreational therapy, and social work. Subcommittee members were charged with developing VA-branded handouts as educational tools to address key issues related to dementia, such as advance directive planning, behavioral management, home safety, and medication management. The subcommittee met monthly for 6 months and focused on logistics, identification of resource tables, creation of educational materials, advertising, and development of an evaluation. Table 1 provides an overview of the planning time line for the 2013 fair held in San Jose. Findings from a systematic evaluation of the 2013 fair were used to improve the 2015 fair held in Menlo Park. A discussion about the evaluation method and results follows.
Methods
The first fair was held at a VA community-based outpatient clinic in a small conference room with 13 resource tables. Feedback from attendees in 2013 included suggestions for having more tables, larger event space, more publicity, and alternate locations for the fair. In response to the feedback, the 2015 fair was held at a division of the main VAMC in a large conference room and hosted 20 tables arranged in a horseshoe shape. The second fair included an activity table staffed by a psychology fellow and recreation therapist who provided respite to caregivers if their loved one with dementia accompanied them to the event. Both the 2013 and 2015 fairs were 4 hours long.
A 1-page, anonymous survey was developed to assess attendees’ opinions about the fair. The survey included information about whether attendees were caregivers, veterans, or VA staff but did not ask other demographic questions to preserve anonymity. In 2013, the survey asked attendees to choose the category that best described them, but in 2015, the survey asked attendees to indicate the number of individuals from each category in their party. The 2015 survey assessed 2 additional categories (family member, other) and added a question about the number of people in each party to better estimate attendance. Both surveys also asked attendees to check which resource tables they visited.
The following assessment questions were consistent across both fairs to allow for comparisons. The authors assessed attitudes and learning as a result of the fair, using 2 statements that were rated with a 5-point Likert scale. The authors asked 3 open-ended questions to ascertain the helpful aspects of the fair, unmet needs, and suggestions for improvement. The Stanford University Institutional Review Board (IRB) reviewed this program evaluation plan and determined that the program evaluation project did not require IRB approval.
When attendees arrived at the fair, they received a folder containing branded handouts, a reusable bag, and a survey. Committee members asked that 1 person per party complete the survey at the end of the visit. Attendees visited tables, obtained written materials, and spoke with subcommittee members who staffed the tables. Snacks and light refreshments were provided. The reusable bag was provided by the VAMC Suicide Prevention Program to increase awareness of the VAPAHCS Suicide Prevention Program. As attendees were leaving, they were reminded to complete the survey. Attendees deposited completed surveys in a box to ensure anonymity.
Results
Thirty-six individuals attended the 2013 fair, and 138 individuals attended the 2015 fair. Thirty-one surveys were completed in 2013, yielding an 86% response rate. One hundred six surveys were returned and represented responses for 129 individuals in 2015, yielding a 94% response rate in 2015. Most of the 2013 attendees were caregivers, followed by veterans, VA staff, and outside staff (Table 2). In contrast, most of the 2015 attendees were VA staff, followed by veterans, caregivers/family members, outside staff, and others. Distributions of attendees differed significantly across the fairs: χ2(4) = 12.66; P = .01.
The surveys assessed which tables attendees visited and their perceptions of the fair. The most frequently visited resource table for both 2013 and 2015 fairs was the Alzheimer’s Association table. Other popular resource tables were VA Benefits and VA Caregiver Support in 2013 and Home Safety and End of Life Care in 2015. Ninety-six percent of 2013 attendees and 100% of 2015 attendees strongly agreed or agreed that “attending the dementia fair was worth my time and effort.” Eighty-three percent of 2013 attendees and 100% of 2015 attendees felt that they had learned something useful at the fair. The proportion of individuals reporting that they had learned something useful significantly increased from 2013 to 2015: χ2(2) = 18.07; P = .0001.
To summarize the open-ended responses to the question “What was most helpful about the fair?” the authors constructed a word cloud that displays the 75 most frequently used words in attendees’ descriptions of the 2015 fair (Figure). Attendees provided suggestions about additional information and resources they desired, which included VA benefits enrollment, books and movies about dementia (eg, Still Alice), speech and swallowing disorders representatives, varied types of advance directives, class discussion, question-and-answer time with speakers, and resources for nonveteran older adults. General suggestions for future fairs included hosting the fair at the main division of the VA health care system, having more room between tables, inviting more vendors, using more visual posters at the tables, and additional advertising for VA services.
Discussion
Dementia is a costly disease with detrimental health and well-being effects on caregivers. The dementia resource fairs aimed to connect caregivers with resources for veterans with dementia in the VA and in the community. Given that nearly half the 2015 fair attendees were VA staff, there is an apparent need for increasing dementia education and access to care resource for this VAMC’s workforce. The high proportion of staff attendees at the 2015 fair may be attributed to the 2 VA community living centers at the VAMC site where the fair was held. This unexpected finding points to the importance of informal and interactive education opportunities for staff, particularly those working with veterans with dementia. The fair served an important role for VA staff seeking information on dementia for professional and personal reasons. This systematic evaluation of the fair demonstrated a need for improving access to information about dementia.
The idea of hosting a dementia resource fair was met with enthusiasm from attendees and subcommittee members in 2013. Feedback helped refine the second fair. The increase in self-reported learning from 2013 to 2015 suggests improvements may have been made between the first and second fair; however, this must be interpreted in light of the different compositions of the attendees at each fair and the absence of a control group. Attendees desired even more information about dementia at the second fair, as evidenced by suggestions to have presentations, speakers, and class discussions. These responses suggest that other sites may wish to consider holding similar events. Next steps include researching the effectiveness of low-cost, pragmatic educational initiatives for caregivers. In fact, randomized, controlled trials of dementia caregiver education and skill-building interventions are underway at VAPAHCS.
Conclusion
The primary lesson learned from the most recent fair was that marketing is the key to success. The authors created an efficient hospital publicity plan in 2015 that included (1) flyers posted throughout 2 main medical center campuses; (2) announcements on closed-circuit VA waiting room televisions; (3) e-mail announcements sent to staff; and (4) VA social media announcements. Flyers also were mailed to known caregivers, and announcements of the event were provided to local community agencies. This focus on publicity likely contributed to the substantial increase in participation from the 2013 to 2015 fair.
Future fairs may be improved by providing more detailed information about dementia through formal presentations. The authors aim to increase the number of family caregivers in attendance possibly through coordinating the fair to coincide with primary care clinic hours, advertising the availability of brief respite at the fair, and conducting additional outreach to veterans.
This systematic evaluation of the dementia resource fair confirmed that providing resources in a drop-in setting resulted in self-reported learning about resources available for veterans with dementia. VA dementia care providers are encouraged to use the authors’ time line and lessons learned to develop dementia resource fairs for their sites.
Acknowledgments
The authors wish to acknowledge the members of the 2013 and 2015 Dementia Resource Fair Committees, chaired by Betty Wexler and Kathleen McConnell, respectively. Dr. Gould is supported by the U.S. Department of Veterans Affairs (IK2 RX001478) and by Ellen Schapiro & Gerald Axelbaum through a 2014 NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation. Dr. Scanlon is supported by the U.S. Department of Veterans Affairs (IK2 RX001240; I21 RX001710), U.S. Department of Defense (W81XWH-15-1-0246), Sierra-Pacific Mental Illness Research Education and Clinical Center, and Stanford/VA Alzheimer’s Research Center. Drs. Gould and Scanlon also receive support from Palo Alto Veterans Institute for Research.
1. Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11(3)332-384.
2. Schulz R, Beach SR, Cook TB, Martire LM, Tomlinson JM, Monin JK. Predictors and consequences of perceived lack of choice in becoming an informal caregiver. Aging Ment Health. 2012;16(6):712-721.
3. Cooper C, Mukadam N, Katona C, et al; World Federation of Biological Psychiatry – Old Age Taskforce. Systematic review of the effectiveness of non-pharmacological interventions to improve quality of life of people with dementia. Int Psychogeriatr. 2012;24(6):856-870.
4. Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1991;282(23):2215-2219.
5. Brodaty H, Arasaratnam C. Meta-analysis of nonpharmacological interventions for neuropsychiatric symptoms of dementia. Am J Psychiatry. 2012;169(9):946-953.
6. Gitlin LN. Good news for dementia care: caregiver interventions reduce behavioral symptoms in people with dementia and family distress. Am J Psychiatry. 2012;169(9):894-897.
7. Ho A, Collins SR, Davis K, Doty MM. A look at working-age caregivers roles, health concerns, and need for support. Issue Brief (Commonw Fund). 2005;(854):1-12.
8. Joling KJ, van Marwijk HWJ, Smit F, et al. Does a family meetings intervention prevent depression and anxiety in family caregivers of dementia patients? A randomized trial. PLoS One. 2012;7(1):e30936.
9. Kolomer S, Quinn ME, Steele K. Interdisciplinary health fairs for older adults and the value of interprofessional service learning. J Community Pract. 2010;18(2-3):267-279.
10. Jensen M, Agbata IN, Canavan M, McCarthy G. Effectiveness of educational interventions for informal caregivers of individuals with dementia residing in the community: systematic review and meta-analysis of randomized controlled trials. Int J Geriatr Psychiatry. 2015;30(2):130-143.
11. Quinn C, Toms G, Anderson D, Clare L. A review of self-management interventions for people with dementia and mild cognitive impairment. J Appl Gerontol. 2015;pii:0733464814566852.
1. Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11(3)332-384.
2. Schulz R, Beach SR, Cook TB, Martire LM, Tomlinson JM, Monin JK. Predictors and consequences of perceived lack of choice in becoming an informal caregiver. Aging Ment Health. 2012;16(6):712-721.
3. Cooper C, Mukadam N, Katona C, et al; World Federation of Biological Psychiatry – Old Age Taskforce. Systematic review of the effectiveness of non-pharmacological interventions to improve quality of life of people with dementia. Int Psychogeriatr. 2012;24(6):856-870.
4. Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1991;282(23):2215-2219.
5. Brodaty H, Arasaratnam C. Meta-analysis of nonpharmacological interventions for neuropsychiatric symptoms of dementia. Am J Psychiatry. 2012;169(9):946-953.
6. Gitlin LN. Good news for dementia care: caregiver interventions reduce behavioral symptoms in people with dementia and family distress. Am J Psychiatry. 2012;169(9):894-897.
7. Ho A, Collins SR, Davis K, Doty MM. A look at working-age caregivers roles, health concerns, and need for support. Issue Brief (Commonw Fund). 2005;(854):1-12.
8. Joling KJ, van Marwijk HWJ, Smit F, et al. Does a family meetings intervention prevent depression and anxiety in family caregivers of dementia patients? A randomized trial. PLoS One. 2012;7(1):e30936.
9. Kolomer S, Quinn ME, Steele K. Interdisciplinary health fairs for older adults and the value of interprofessional service learning. J Community Pract. 2010;18(2-3):267-279.
10. Jensen M, Agbata IN, Canavan M, McCarthy G. Effectiveness of educational interventions for informal caregivers of individuals with dementia residing in the community: systematic review and meta-analysis of randomized controlled trials. Int J Geriatr Psychiatry. 2015;30(2):130-143.
11. Quinn C, Toms G, Anderson D, Clare L. A review of self-management interventions for people with dementia and mild cognitive impairment. J Appl Gerontol. 2015;pii:0733464814566852.