User login
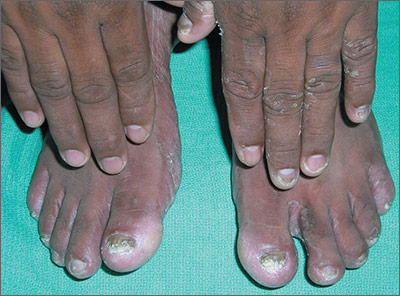
Rash on left hand, feet
The FP diagnosed this patient with two foot one hand syndrome, which is a fungal infection involving both feet and one hand. To be sure of the diagnosis, the FP performed a potassium hydroxide (KOH) preparation from the scale on the left hand and found typical branching hyphae caused by a dermatophyte. (See video on how to perform a KOH preparation.) The dermatophyte was most likely Trichophyton rubrum, but it wasn’t necessary to determine the exact species in order to treat this condition.
Two foot one hand syndrome is not rare, but it is somewhat puzzling as to why it involves only one hand while involving both feet. Also, it does not preferentially involve the dominant hand. In this case, the patient’s 2 large toenails had visible onychomycosis and 3 of the nails on his left hand had fungal changes (his dominant right hand was not affected). Tinea pedis was seen between the toes in a moccasin distribution, as well.
Treatment for this condition usually requires an oral antifungal agent. (If, however, the nails were not involved and there were contraindications to an oral antifungal, then a topical antifungal cream could be tried first.)
The patient described here had no history of liver disease or alcohol abuse. So the FP prescribed terbinafine 250 mg daily for one month to fully clear the skin infection and a follow-up appointment was set for 4 weeks. At that appointment, the FP planned to discuss continuing oral treatment for 2 additional months to clear the fungal infection from the nails. If ongoing oral terbinafine was needed, liver function tests could be performed at that time.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Fungal overview. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill;2013:771-776.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed this patient with two foot one hand syndrome, which is a fungal infection involving both feet and one hand. To be sure of the diagnosis, the FP performed a potassium hydroxide (KOH) preparation from the scale on the left hand and found typical branching hyphae caused by a dermatophyte. (See video on how to perform a KOH preparation.) The dermatophyte was most likely Trichophyton rubrum, but it wasn’t necessary to determine the exact species in order to treat this condition.
Two foot one hand syndrome is not rare, but it is somewhat puzzling as to why it involves only one hand while involving both feet. Also, it does not preferentially involve the dominant hand. In this case, the patient’s 2 large toenails had visible onychomycosis and 3 of the nails on his left hand had fungal changes (his dominant right hand was not affected). Tinea pedis was seen between the toes in a moccasin distribution, as well.
Treatment for this condition usually requires an oral antifungal agent. (If, however, the nails were not involved and there were contraindications to an oral antifungal, then a topical antifungal cream could be tried first.)
The patient described here had no history of liver disease or alcohol abuse. So the FP prescribed terbinafine 250 mg daily for one month to fully clear the skin infection and a follow-up appointment was set for 4 weeks. At that appointment, the FP planned to discuss continuing oral treatment for 2 additional months to clear the fungal infection from the nails. If ongoing oral terbinafine was needed, liver function tests could be performed at that time.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Fungal overview. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill;2013:771-776.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The FP diagnosed this patient with two foot one hand syndrome, which is a fungal infection involving both feet and one hand. To be sure of the diagnosis, the FP performed a potassium hydroxide (KOH) preparation from the scale on the left hand and found typical branching hyphae caused by a dermatophyte. (See video on how to perform a KOH preparation.) The dermatophyte was most likely Trichophyton rubrum, but it wasn’t necessary to determine the exact species in order to treat this condition.
Two foot one hand syndrome is not rare, but it is somewhat puzzling as to why it involves only one hand while involving both feet. Also, it does not preferentially involve the dominant hand. In this case, the patient’s 2 large toenails had visible onychomycosis and 3 of the nails on his left hand had fungal changes (his dominant right hand was not affected). Tinea pedis was seen between the toes in a moccasin distribution, as well.
Treatment for this condition usually requires an oral antifungal agent. (If, however, the nails were not involved and there were contraindications to an oral antifungal, then a topical antifungal cream could be tried first.)
The patient described here had no history of liver disease or alcohol abuse. So the FP prescribed terbinafine 250 mg daily for one month to fully clear the skin infection and a follow-up appointment was set for 4 weeks. At that appointment, the FP planned to discuss continuing oral treatment for 2 additional months to clear the fungal infection from the nails. If ongoing oral terbinafine was needed, liver function tests could be performed at that time.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Usatine R. Fungal overview. In: Usatine R, Smith M, Mayeaux EJ, et al, eds. Color Atlas of Family Medicine. 2nd ed. New York, NY: McGraw-Hill;2013:771-776.
To learn more about the Color Atlas of Family Medicine, see: www.amazon.com/Color-Family-Medicine-Richard-Usatine/dp/0071769641/
You can now get the second edition of the Color Atlas of Family Medicine as an app by clicking on this link: usatinemedia.com
The Complement System in Refractory Myasthenia Gravis
Click here to read the supplement.
Click here to read the supplement.
Click here to read the supplement.
LETTER: Engaging the Next Generation: Hospital Medicine Student Interest Groups
Since the inception of hospital medicine, we are seeing unprecedented levels of reliance on hospitalists for educating and leading the next generation of physicians toward better care. A 2008 survey of internal medicine programs reported that learners gave hospitalists higher scores in the areas of attending rounds quality and the teaching of cost-effective care, in addition to providing an overall better learning experience as compared with non-hospitalist attendings.1
As successful educators, the development of mentorship programs has been shown to improve professional satisfaction and academic productivity in hospitalist communities.2, 3 Unfortunately, most of these programs failed to consider medical schools as valuable targets for outreach, education and support. By limiting vertical integration of training and mentorship, the hospitalist community is keeping itself from realizing its potential in building a pipeline for shaping the leaders of tomorrow’s healthcare sector.
A Hospital Medicine Student Interest Group (HM-SIG) is an organization composed of medical, nurse practitioner or physician assistant students interested in exploring future careers in hospital medicine. The goals of an HM-SIG are multidimensional: (1) introduce students to, or cultivate prior interest in, a career in hospital medicine, (2) provide opportunities for mentorship with faculty, (3) develop a community of future hospitalists, and (4) facilitate student involvement in institutional, local, regional and national hospital medicine projects on patient safety, high-value care and quality improvement.
Since starting the first medical school HM-SIG chapter in 2015, our efforts have led to significant changes in the way students are exposed not only to hospital medicine as a career, but to the tools and the mindset of hospitalists for improving care as well. In the fall of 2015, after brief discussions on the merits of and opportunities in hospital medicine, we selected a dedicated group of individuals and built our executive board. We collectively defined our goals and designed an 18-month plan to create student-led programming, coordinate mentorship opportunities with faculty and build a research pipeline for future medical students to have easier access to quality improvement work within the Loyola University Stritch School of Medicine. Within the first 6 months, we hosted a panel discussion with our hospitalist faculty, facilitated a quality improvement workshop to teach the lean methodology and rolled out a shadowing program that has given students deeper insight into the day in the life of a hospitalist. We’ve also developed a lecture series that has guided curriculum changes in quality improvement, and organized a student-led regional hospital medicine conference for nurse practitioners, physician assistants, medical students and internal medicine residents.
Without any representation from within the medical student community, student exposure to the hospitalist career is entirely dependent on the resources and availability of the affiliate hospital’s department of hospital medicine. With an investment in hospital medicine student interest groups at medical schools, SHM will equip students to become articulate advocates for the profession and SHM as a community.
References
- Goldenberg J, Glasheen JJ. Hospitalist educators: future of inpatient internal medicine training. Mt Sinai J Med 2008;75:430-5.
2. Pane LA, Davis AB, Ottolini MC. Career satisfaction and the role of mentorship: a survey of pediatric hospitalists. Hosp Pediatr. 2012;2(3):141-8.
3. Leary JC, Schainker EG, Leyenaar JK. The unwritten rules of mentorship: Facilitators of and barriers to effective mentorship in pediatric hospital medicine. Hospital Pediatrics. 2016;6(4):219-225; DOI: 10.1542/hpeds.2015-0108
Since the inception of hospital medicine, we are seeing unprecedented levels of reliance on hospitalists for educating and leading the next generation of physicians toward better care. A 2008 survey of internal medicine programs reported that learners gave hospitalists higher scores in the areas of attending rounds quality and the teaching of cost-effective care, in addition to providing an overall better learning experience as compared with non-hospitalist attendings.1
As successful educators, the development of mentorship programs has been shown to improve professional satisfaction and academic productivity in hospitalist communities.2, 3 Unfortunately, most of these programs failed to consider medical schools as valuable targets for outreach, education and support. By limiting vertical integration of training and mentorship, the hospitalist community is keeping itself from realizing its potential in building a pipeline for shaping the leaders of tomorrow’s healthcare sector.
A Hospital Medicine Student Interest Group (HM-SIG) is an organization composed of medical, nurse practitioner or physician assistant students interested in exploring future careers in hospital medicine. The goals of an HM-SIG are multidimensional: (1) introduce students to, or cultivate prior interest in, a career in hospital medicine, (2) provide opportunities for mentorship with faculty, (3) develop a community of future hospitalists, and (4) facilitate student involvement in institutional, local, regional and national hospital medicine projects on patient safety, high-value care and quality improvement.
Since starting the first medical school HM-SIG chapter in 2015, our efforts have led to significant changes in the way students are exposed not only to hospital medicine as a career, but to the tools and the mindset of hospitalists for improving care as well. In the fall of 2015, after brief discussions on the merits of and opportunities in hospital medicine, we selected a dedicated group of individuals and built our executive board. We collectively defined our goals and designed an 18-month plan to create student-led programming, coordinate mentorship opportunities with faculty and build a research pipeline for future medical students to have easier access to quality improvement work within the Loyola University Stritch School of Medicine. Within the first 6 months, we hosted a panel discussion with our hospitalist faculty, facilitated a quality improvement workshop to teach the lean methodology and rolled out a shadowing program that has given students deeper insight into the day in the life of a hospitalist. We’ve also developed a lecture series that has guided curriculum changes in quality improvement, and organized a student-led regional hospital medicine conference for nurse practitioners, physician assistants, medical students and internal medicine residents.
Without any representation from within the medical student community, student exposure to the hospitalist career is entirely dependent on the resources and availability of the affiliate hospital’s department of hospital medicine. With an investment in hospital medicine student interest groups at medical schools, SHM will equip students to become articulate advocates for the profession and SHM as a community.
References
- Goldenberg J, Glasheen JJ. Hospitalist educators: future of inpatient internal medicine training. Mt Sinai J Med 2008;75:430-5.
2. Pane LA, Davis AB, Ottolini MC. Career satisfaction and the role of mentorship: a survey of pediatric hospitalists. Hosp Pediatr. 2012;2(3):141-8.
3. Leary JC, Schainker EG, Leyenaar JK. The unwritten rules of mentorship: Facilitators of and barriers to effective mentorship in pediatric hospital medicine. Hospital Pediatrics. 2016;6(4):219-225; DOI: 10.1542/hpeds.2015-0108
Since the inception of hospital medicine, we are seeing unprecedented levels of reliance on hospitalists for educating and leading the next generation of physicians toward better care. A 2008 survey of internal medicine programs reported that learners gave hospitalists higher scores in the areas of attending rounds quality and the teaching of cost-effective care, in addition to providing an overall better learning experience as compared with non-hospitalist attendings.1
As successful educators, the development of mentorship programs has been shown to improve professional satisfaction and academic productivity in hospitalist communities.2, 3 Unfortunately, most of these programs failed to consider medical schools as valuable targets for outreach, education and support. By limiting vertical integration of training and mentorship, the hospitalist community is keeping itself from realizing its potential in building a pipeline for shaping the leaders of tomorrow’s healthcare sector.
A Hospital Medicine Student Interest Group (HM-SIG) is an organization composed of medical, nurse practitioner or physician assistant students interested in exploring future careers in hospital medicine. The goals of an HM-SIG are multidimensional: (1) introduce students to, or cultivate prior interest in, a career in hospital medicine, (2) provide opportunities for mentorship with faculty, (3) develop a community of future hospitalists, and (4) facilitate student involvement in institutional, local, regional and national hospital medicine projects on patient safety, high-value care and quality improvement.
Since starting the first medical school HM-SIG chapter in 2015, our efforts have led to significant changes in the way students are exposed not only to hospital medicine as a career, but to the tools and the mindset of hospitalists for improving care as well. In the fall of 2015, after brief discussions on the merits of and opportunities in hospital medicine, we selected a dedicated group of individuals and built our executive board. We collectively defined our goals and designed an 18-month plan to create student-led programming, coordinate mentorship opportunities with faculty and build a research pipeline for future medical students to have easier access to quality improvement work within the Loyola University Stritch School of Medicine. Within the first 6 months, we hosted a panel discussion with our hospitalist faculty, facilitated a quality improvement workshop to teach the lean methodology and rolled out a shadowing program that has given students deeper insight into the day in the life of a hospitalist. We’ve also developed a lecture series that has guided curriculum changes in quality improvement, and organized a student-led regional hospital medicine conference for nurse practitioners, physician assistants, medical students and internal medicine residents.
Without any representation from within the medical student community, student exposure to the hospitalist career is entirely dependent on the resources and availability of the affiliate hospital’s department of hospital medicine. With an investment in hospital medicine student interest groups at medical schools, SHM will equip students to become articulate advocates for the profession and SHM as a community.
References
- Goldenberg J, Glasheen JJ. Hospitalist educators: future of inpatient internal medicine training. Mt Sinai J Med 2008;75:430-5.
2. Pane LA, Davis AB, Ottolini MC. Career satisfaction and the role of mentorship: a survey of pediatric hospitalists. Hosp Pediatr. 2012;2(3):141-8.
3. Leary JC, Schainker EG, Leyenaar JK. The unwritten rules of mentorship: Facilitators of and barriers to effective mentorship in pediatric hospital medicine. Hospital Pediatrics. 2016;6(4):219-225; DOI: 10.1542/hpeds.2015-0108
CBT may be best option for pts with MRD, doc says

Photo courtesy of NHS
A cord blood transplant (CBT) may be the best option for patients with acute leukemia or myelodysplastic syndrome who have minimal residual disease (MRD) and no related donor, according to the senior author of a study published in NEJM.
This retrospective study showed that patients with MRD at the time of transplant were less likely to relapse if they received CBT rather than a graft from an unrelated adult donor, whether HLA-matched or mismatched.
In addition, the risk of death was significantly higher for patients with a mismatched donor than for CBT recipients, although there was no significant difference between those with a matched donor and CBT recipients.
Among patients without MRD, there were no significant differences between the transplant types for the risk of relapse or death.
“This paper shows that if you’ve got high-risk disease and are at high risk for relapse post-transplant, transplant with a cord blood donor may be the best option,” said Colleen Delaney, MD, of Fred Hutchinson Cancer Research Center in Seattle, Washington.
Dr Delaney and her colleagues analyzed data on 582 patients—300 with acute myeloid leukemia, 185 with acute lymphoblastic leukemia, and 97 with myelodysplastic syndromes.
Most patients received a transplant from an HLA-matched unrelated donor (n=344), 140 received a CBT from an unrelated donor, and 98 received a transplant from an HLA-mismatched unrelated donor.
The researchers calculated the relative risks of death and relapse for each transplant group, and they found that a patient’s MRD status prior to transplant played a role.
Presence of MRD
Among patients with MRD, the risk of death was significantly higher for recipients of mismatched grafts than for CBT recipients, with a hazard ratio (HR) of 2.92 (P=0.001).
However, the risk of death was not significantly different for recipients of matched grafts compared to CBT recipients. The HR was 1.69 (P=0.08).
The risk of relapse was about 3 times higher for recipients of mismatched grafts (HR=3.01, P=0.02) or matched grafts (HR=2.92, P=0.007) than for CBT recipients.
No MRD
Among patients without MRD, there was no significant difference in the risk of death for recipients of CBT, mismatched grafts (HR=1.36, P=0.30), or matched grafts (HR=0.78, P=0.33).
And there was no significant difference in the risk of relapse for recipients of CBT, mismatched grafts (HR=1.28, P=0.60), or matched grafts (HR=1.30, P=0.46).
“This brings home the point that cord blood shouldn’t be called an alternative donor,” Dr Delaney said. “The outcomes are the same as a conventional donor.” ![]()

Photo courtesy of NHS
A cord blood transplant (CBT) may be the best option for patients with acute leukemia or myelodysplastic syndrome who have minimal residual disease (MRD) and no related donor, according to the senior author of a study published in NEJM.
This retrospective study showed that patients with MRD at the time of transplant were less likely to relapse if they received CBT rather than a graft from an unrelated adult donor, whether HLA-matched or mismatched.
In addition, the risk of death was significantly higher for patients with a mismatched donor than for CBT recipients, although there was no significant difference between those with a matched donor and CBT recipients.
Among patients without MRD, there were no significant differences between the transplant types for the risk of relapse or death.
“This paper shows that if you’ve got high-risk disease and are at high risk for relapse post-transplant, transplant with a cord blood donor may be the best option,” said Colleen Delaney, MD, of Fred Hutchinson Cancer Research Center in Seattle, Washington.
Dr Delaney and her colleagues analyzed data on 582 patients—300 with acute myeloid leukemia, 185 with acute lymphoblastic leukemia, and 97 with myelodysplastic syndromes.
Most patients received a transplant from an HLA-matched unrelated donor (n=344), 140 received a CBT from an unrelated donor, and 98 received a transplant from an HLA-mismatched unrelated donor.
The researchers calculated the relative risks of death and relapse for each transplant group, and they found that a patient’s MRD status prior to transplant played a role.
Presence of MRD
Among patients with MRD, the risk of death was significantly higher for recipients of mismatched grafts than for CBT recipients, with a hazard ratio (HR) of 2.92 (P=0.001).
However, the risk of death was not significantly different for recipients of matched grafts compared to CBT recipients. The HR was 1.69 (P=0.08).
The risk of relapse was about 3 times higher for recipients of mismatched grafts (HR=3.01, P=0.02) or matched grafts (HR=2.92, P=0.007) than for CBT recipients.
No MRD
Among patients without MRD, there was no significant difference in the risk of death for recipients of CBT, mismatched grafts (HR=1.36, P=0.30), or matched grafts (HR=0.78, P=0.33).
And there was no significant difference in the risk of relapse for recipients of CBT, mismatched grafts (HR=1.28, P=0.60), or matched grafts (HR=1.30, P=0.46).
“This brings home the point that cord blood shouldn’t be called an alternative donor,” Dr Delaney said. “The outcomes are the same as a conventional donor.” ![]()

Photo courtesy of NHS
A cord blood transplant (CBT) may be the best option for patients with acute leukemia or myelodysplastic syndrome who have minimal residual disease (MRD) and no related donor, according to the senior author of a study published in NEJM.
This retrospective study showed that patients with MRD at the time of transplant were less likely to relapse if they received CBT rather than a graft from an unrelated adult donor, whether HLA-matched or mismatched.
In addition, the risk of death was significantly higher for patients with a mismatched donor than for CBT recipients, although there was no significant difference between those with a matched donor and CBT recipients.
Among patients without MRD, there were no significant differences between the transplant types for the risk of relapse or death.
“This paper shows that if you’ve got high-risk disease and are at high risk for relapse post-transplant, transplant with a cord blood donor may be the best option,” said Colleen Delaney, MD, of Fred Hutchinson Cancer Research Center in Seattle, Washington.
Dr Delaney and her colleagues analyzed data on 582 patients—300 with acute myeloid leukemia, 185 with acute lymphoblastic leukemia, and 97 with myelodysplastic syndromes.
Most patients received a transplant from an HLA-matched unrelated donor (n=344), 140 received a CBT from an unrelated donor, and 98 received a transplant from an HLA-mismatched unrelated donor.
The researchers calculated the relative risks of death and relapse for each transplant group, and they found that a patient’s MRD status prior to transplant played a role.
Presence of MRD
Among patients with MRD, the risk of death was significantly higher for recipients of mismatched grafts than for CBT recipients, with a hazard ratio (HR) of 2.92 (P=0.001).
However, the risk of death was not significantly different for recipients of matched grafts compared to CBT recipients. The HR was 1.69 (P=0.08).
The risk of relapse was about 3 times higher for recipients of mismatched grafts (HR=3.01, P=0.02) or matched grafts (HR=2.92, P=0.007) than for CBT recipients.
No MRD
Among patients without MRD, there was no significant difference in the risk of death for recipients of CBT, mismatched grafts (HR=1.36, P=0.30), or matched grafts (HR=0.78, P=0.33).
And there was no significant difference in the risk of relapse for recipients of CBT, mismatched grafts (HR=1.28, P=0.60), or matched grafts (HR=1.30, P=0.46).
“This brings home the point that cord blood shouldn’t be called an alternative donor,” Dr Delaney said. “The outcomes are the same as a conventional donor.” ![]()
Risk factors for early death in older pts with DLBCL

Photo courtesy of CDC
A retrospective study has uncovered potential risk factors for early death in older patients with diffuse large B-cell lymphoma (DLBCL).
Researchers examined electronic health record data for roughly 5500 DLBCL patients over the age of 65 who received contemporary immunochemotherapy.
This revealed 7 factors that were significantly associated with the risk of death within 30 days of treatment initiation.
“The first month of treatment, when patients are compromised both by active lymphoma and toxicities of chemotherapy, is a period of particular concern, as nearly 1 in 4 patients were hospitalized during that time,” said study author Adam J. Olszewski, MD, of Rhode Island Hospital in Providence.
“While comprehensive geriatric assessment remains the gold standard for risk assessment, our study suggests that readily available data from electronic medical records can help identify the high-risk factors in practice.”
Dr Olszewski and his colleagues described their study in JNCCN.
The researchers looked at Medicare claims linked to Surveillance, Epidemiology and End Results registry data for 5530 DLBCL patients who had a median age of 76.
The patients were treated with rituximab, cyclophosphamide, and vincristine in combination with doxorubicin, mitoxantrone, or etoposide from 2003 to 2012.
The cumulative incidence of death at 30 days was 2.2%. The most common causes of death were lymphoma (72%), heart disease (9%), septicemia (3%), and cerebrovascular events (3%).
The researchers created a prediction model based on 7 factors that were significantly associated with early death in multivariate analysis. These include:
- B symptoms
- Chronic kidney disease
- Poor performance status
- Prior use of walking aids or wheelchairs
- Prior hospitalization within the past 12 months
- Upper endoscopy within the past 12 months
- Age 75 or older.
Patients with 0 to 1 of these risk factors were considered low-risk, with a 0.6% chance of early death. Fifty-six percent of the patients studied fit this definition.
Patients with 2 to 3 of the risk factors were intermediate-risk, with a 3.2% chance of early death. Thirty-eight percent of the patients studied fell into this category.
Only 6% of the patients studied were considered high-risk. These patients had 4 or more risk factors and an 8.3% chance of early death.
The researchers also found the administration of prophylactic granulocyte-colony stimulating factor (G-CSF) was associated with lower probability of early death in the high-risk group.
They noted that prophylactic G-CSF was given to 66% of patients in this study, which suggests an opportunity for preventing early deaths.
“It is equally important to realize that a majority of older patients without risk factors can safely receive curative immunochemotherapy,” Dr Olszewski said. “Enhanced supportive care and monitoring should be provided for high-risk groups.” ![]()

Photo courtesy of CDC
A retrospective study has uncovered potential risk factors for early death in older patients with diffuse large B-cell lymphoma (DLBCL).
Researchers examined electronic health record data for roughly 5500 DLBCL patients over the age of 65 who received contemporary immunochemotherapy.
This revealed 7 factors that were significantly associated with the risk of death within 30 days of treatment initiation.
“The first month of treatment, when patients are compromised both by active lymphoma and toxicities of chemotherapy, is a period of particular concern, as nearly 1 in 4 patients were hospitalized during that time,” said study author Adam J. Olszewski, MD, of Rhode Island Hospital in Providence.
“While comprehensive geriatric assessment remains the gold standard for risk assessment, our study suggests that readily available data from electronic medical records can help identify the high-risk factors in practice.”
Dr Olszewski and his colleagues described their study in JNCCN.
The researchers looked at Medicare claims linked to Surveillance, Epidemiology and End Results registry data for 5530 DLBCL patients who had a median age of 76.
The patients were treated with rituximab, cyclophosphamide, and vincristine in combination with doxorubicin, mitoxantrone, or etoposide from 2003 to 2012.
The cumulative incidence of death at 30 days was 2.2%. The most common causes of death were lymphoma (72%), heart disease (9%), septicemia (3%), and cerebrovascular events (3%).
The researchers created a prediction model based on 7 factors that were significantly associated with early death in multivariate analysis. These include:
- B symptoms
- Chronic kidney disease
- Poor performance status
- Prior use of walking aids or wheelchairs
- Prior hospitalization within the past 12 months
- Upper endoscopy within the past 12 months
- Age 75 or older.
Patients with 0 to 1 of these risk factors were considered low-risk, with a 0.6% chance of early death. Fifty-six percent of the patients studied fit this definition.
Patients with 2 to 3 of the risk factors were intermediate-risk, with a 3.2% chance of early death. Thirty-eight percent of the patients studied fell into this category.
Only 6% of the patients studied were considered high-risk. These patients had 4 or more risk factors and an 8.3% chance of early death.
The researchers also found the administration of prophylactic granulocyte-colony stimulating factor (G-CSF) was associated with lower probability of early death in the high-risk group.
They noted that prophylactic G-CSF was given to 66% of patients in this study, which suggests an opportunity for preventing early deaths.
“It is equally important to realize that a majority of older patients without risk factors can safely receive curative immunochemotherapy,” Dr Olszewski said. “Enhanced supportive care and monitoring should be provided for high-risk groups.” ![]()

Photo courtesy of CDC
A retrospective study has uncovered potential risk factors for early death in older patients with diffuse large B-cell lymphoma (DLBCL).
Researchers examined electronic health record data for roughly 5500 DLBCL patients over the age of 65 who received contemporary immunochemotherapy.
This revealed 7 factors that were significantly associated with the risk of death within 30 days of treatment initiation.
“The first month of treatment, when patients are compromised both by active lymphoma and toxicities of chemotherapy, is a period of particular concern, as nearly 1 in 4 patients were hospitalized during that time,” said study author Adam J. Olszewski, MD, of Rhode Island Hospital in Providence.
“While comprehensive geriatric assessment remains the gold standard for risk assessment, our study suggests that readily available data from electronic medical records can help identify the high-risk factors in practice.”
Dr Olszewski and his colleagues described their study in JNCCN.
The researchers looked at Medicare claims linked to Surveillance, Epidemiology and End Results registry data for 5530 DLBCL patients who had a median age of 76.
The patients were treated with rituximab, cyclophosphamide, and vincristine in combination with doxorubicin, mitoxantrone, or etoposide from 2003 to 2012.
The cumulative incidence of death at 30 days was 2.2%. The most common causes of death were lymphoma (72%), heart disease (9%), septicemia (3%), and cerebrovascular events (3%).
The researchers created a prediction model based on 7 factors that were significantly associated with early death in multivariate analysis. These include:
- B symptoms
- Chronic kidney disease
- Poor performance status
- Prior use of walking aids or wheelchairs
- Prior hospitalization within the past 12 months
- Upper endoscopy within the past 12 months
- Age 75 or older.
Patients with 0 to 1 of these risk factors were considered low-risk, with a 0.6% chance of early death. Fifty-six percent of the patients studied fit this definition.
Patients with 2 to 3 of the risk factors were intermediate-risk, with a 3.2% chance of early death. Thirty-eight percent of the patients studied fell into this category.
Only 6% of the patients studied were considered high-risk. These patients had 4 or more risk factors and an 8.3% chance of early death.
The researchers also found the administration of prophylactic granulocyte-colony stimulating factor (G-CSF) was associated with lower probability of early death in the high-risk group.
They noted that prophylactic G-CSF was given to 66% of patients in this study, which suggests an opportunity for preventing early deaths.
“It is equally important to realize that a majority of older patients without risk factors can safely receive curative immunochemotherapy,” Dr Olszewski said. “Enhanced supportive care and monitoring should be provided for high-risk groups.” ![]()
Gene therapy effective against SCD in mice

blood cells from a mouse
Image courtesy of
University of Michigan
Preclinical research suggests a novel gene therapy may be effective against sickle cell disease (SCD).
The therapy is designed to selectively inhibit the fetal hemoglobin repressor BCL11A in erythroid cells.
Researchers found this was sufficient to increase fetal hemoglobin production and reverse the effects of SCD in vivo, without presenting the same problems as ubiquitous BCL11A knockdown.
The team reported these findings in The Journal of Clinical Investigation.
Previous research showed that suppressing BCL11A can replace the defective beta
hemoglobin that causes sickling with healthy fetal hemoglobin.
“BCL11A represses fetal hemoglobin, which does not lead to sickling, and also activates beta hemoglobin, which is affected by the sickle cell mutation,” explained study author David A. Williams, MD, of Boston Children’s Hospital in Massachusetts.
“So when you knock BCL11A down, you simultaneously increase fetal hemoglobin and repress sickling hemoglobin, which is why we think this is the best approach to gene therapy in sickle cell disease.”
However, Dr Williams and his colleagues found that ubiquitous knockdown of BCL11A impaired the engraftment of human and murine hematopoietic stem cells (HSCs).
To circumvent this problem, the researchers set out to silence BCL11A only in erythroid cells.
Selectively knocking down BCL11A involved several layers of engineering. As the core of their gene therapy vector, the researchers used a short hairpin RNA that inactivates BCL11A. To get it into cells, they embedded the short hairpin RNA in a microRNA that cells generally recognize and process.
To make this assembly work in the right place at the right time, the team hooked it to a promoter of beta hemoglobin expression, together with regulatory elements active only in erythroid cells. Finally, they inserted the whole package into a lentivirus.
HSCs from mice and SCD patients were then exposed to the manipulated virus, taking up the new genetic material. The resulting genetically engineered erythroid cells began producing fetal hemoglobin rather than the mutated beta hemoglobin.
When HSCs treated with this gene therapy were transplanted into mice with SCD, the cells engrafted successfully and reduced signs of SCD—namely, hemolytic anemia and increased numbers
of reticulocytes.
Dr Williams believes this approach could substantially increase the ratio of non-sickling to sickling hemoglobin in SCD. He also said the approach could be beneficial in beta-thalassemia. ![]()

blood cells from a mouse
Image courtesy of
University of Michigan
Preclinical research suggests a novel gene therapy may be effective against sickle cell disease (SCD).
The therapy is designed to selectively inhibit the fetal hemoglobin repressor BCL11A in erythroid cells.
Researchers found this was sufficient to increase fetal hemoglobin production and reverse the effects of SCD in vivo, without presenting the same problems as ubiquitous BCL11A knockdown.
The team reported these findings in The Journal of Clinical Investigation.
Previous research showed that suppressing BCL11A can replace the defective beta
hemoglobin that causes sickling with healthy fetal hemoglobin.
“BCL11A represses fetal hemoglobin, which does not lead to sickling, and also activates beta hemoglobin, which is affected by the sickle cell mutation,” explained study author David A. Williams, MD, of Boston Children’s Hospital in Massachusetts.
“So when you knock BCL11A down, you simultaneously increase fetal hemoglobin and repress sickling hemoglobin, which is why we think this is the best approach to gene therapy in sickle cell disease.”
However, Dr Williams and his colleagues found that ubiquitous knockdown of BCL11A impaired the engraftment of human and murine hematopoietic stem cells (HSCs).
To circumvent this problem, the researchers set out to silence BCL11A only in erythroid cells.
Selectively knocking down BCL11A involved several layers of engineering. As the core of their gene therapy vector, the researchers used a short hairpin RNA that inactivates BCL11A. To get it into cells, they embedded the short hairpin RNA in a microRNA that cells generally recognize and process.
To make this assembly work in the right place at the right time, the team hooked it to a promoter of beta hemoglobin expression, together with regulatory elements active only in erythroid cells. Finally, they inserted the whole package into a lentivirus.
HSCs from mice and SCD patients were then exposed to the manipulated virus, taking up the new genetic material. The resulting genetically engineered erythroid cells began producing fetal hemoglobin rather than the mutated beta hemoglobin.
When HSCs treated with this gene therapy were transplanted into mice with SCD, the cells engrafted successfully and reduced signs of SCD—namely, hemolytic anemia and increased numbers
of reticulocytes.
Dr Williams believes this approach could substantially increase the ratio of non-sickling to sickling hemoglobin in SCD. He also said the approach could be beneficial in beta-thalassemia. ![]()

blood cells from a mouse
Image courtesy of
University of Michigan
Preclinical research suggests a novel gene therapy may be effective against sickle cell disease (SCD).
The therapy is designed to selectively inhibit the fetal hemoglobin repressor BCL11A in erythroid cells.
Researchers found this was sufficient to increase fetal hemoglobin production and reverse the effects of SCD in vivo, without presenting the same problems as ubiquitous BCL11A knockdown.
The team reported these findings in The Journal of Clinical Investigation.
Previous research showed that suppressing BCL11A can replace the defective beta
hemoglobin that causes sickling with healthy fetal hemoglobin.
“BCL11A represses fetal hemoglobin, which does not lead to sickling, and also activates beta hemoglobin, which is affected by the sickle cell mutation,” explained study author David A. Williams, MD, of Boston Children’s Hospital in Massachusetts.
“So when you knock BCL11A down, you simultaneously increase fetal hemoglobin and repress sickling hemoglobin, which is why we think this is the best approach to gene therapy in sickle cell disease.”
However, Dr Williams and his colleagues found that ubiquitous knockdown of BCL11A impaired the engraftment of human and murine hematopoietic stem cells (HSCs).
To circumvent this problem, the researchers set out to silence BCL11A only in erythroid cells.
Selectively knocking down BCL11A involved several layers of engineering. As the core of their gene therapy vector, the researchers used a short hairpin RNA that inactivates BCL11A. To get it into cells, they embedded the short hairpin RNA in a microRNA that cells generally recognize and process.
To make this assembly work in the right place at the right time, the team hooked it to a promoter of beta hemoglobin expression, together with regulatory elements active only in erythroid cells. Finally, they inserted the whole package into a lentivirus.
HSCs from mice and SCD patients were then exposed to the manipulated virus, taking up the new genetic material. The resulting genetically engineered erythroid cells began producing fetal hemoglobin rather than the mutated beta hemoglobin.
When HSCs treated with this gene therapy were transplanted into mice with SCD, the cells engrafted successfully and reduced signs of SCD—namely, hemolytic anemia and increased numbers
of reticulocytes.
Dr Williams believes this approach could substantially increase the ratio of non-sickling to sickling hemoglobin in SCD. He also said the approach could be beneficial in beta-thalassemia. ![]()
Optimizing CAR T-cell therapy in NHL

Photo from Fred Hutchinson
Cancer Research Center
Results from a phase 1 study have provided insights that may help researchers optimize treatment with JCAR014, a chimeric antigen receptor (CAR) T-cell therapy, in patients with advanced non-Hodgkin lymphoma (NHL).
Researchers said they identified a lymphodepleting regimen that improved the likelihood of complete response (CR) to JCAR014.
Although the regimen also increased the risk of severe cytokine release syndrome (CRS) and neurotoxicity, the researchers discovered biomarkers that might allow them to identify patients who have a high risk of these events and could potentially benefit from early interventions.
The researchers reported these findings in Science Translational Medicine. The trial (NCT01865617) was funded, in part, by Juno Therapeutics, the company developing JCAR014.
Previous results from this trial, in patients with acute lymphoblastic leukemia, were published in The Journal of Clinical Investigation.
About JCAR014
JCAR014 is a CD19-directed CAR T-cell therapy in which CD4+ and CD8+ cells are administered in equal proportions.
“The idea . . . is that by [controlling the ratio of T cells], we would get more reproducible data around the effects of the cells—both beneficial effects against the cancer and also any side effects they might cause the patient,” said study author Stanley Riddell, MD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington.
“And then, by adjusting the dose, we could improve what we call the therapeutic index—the benefit against the tumor—without too much toxicity.”
Patients and treatment
Dr Riddell and his colleagues reported results with JCAR014, following lymphodepleting chemotherapy, in 32 patients with NHL who had a median age of 58 (range, 36-70).
The patients had de novo large B-cell lymphoma (n=11), transformed de novo large B-cell lymphoma (n=11), mantle cell lymphoma (n=4), and follicular lymphoma (n=6).
They had received a median of 5 prior treatment regimens (range, 2 to 11), including autologous
(n=14) and allogeneic (n=4) transplant. All patients had measurable disease (≥ 2 cm) in the lymph nodes or other extramedullary sites at baseline.
The patients received JCAR014 at 1 of 3 dose levels, given 36 to 96 hours after lymphodepleting chemotherapy.
Twelve patients received cyclophosphamide (Cy) alone or Cy and etoposide (E), and 20 received Cy plus fludarabine (Flu). Five patients received 2 × 105 CAR T cells/kg, 18 received 2 × 106 CAR T cells/kg, and 9 received 2 × 107 CAR T cells/kg.
Efficacy
In the 30 evaluable patients, the overall response rate (ORR) was 63% (n=19), and the CR rate was 33% (n=10).
Among patients who received Cy or Cy/E, the ORR was 50% (n=6), and the CR rate was 8% (n=1). Among patients who received Cy/Flu, the ORR was 72% (n=13), and the CR rate was 50% (n=9).
The patients who received Cy/Flu had better CAR T-cell expansion and persistence than patients who received Cy or Cy/E, which was reflected in the higher response rates.
Higher response rates were also observed in patients who received 2 × 106 CAR T cells/kg rather than the other 2 dose levels.
The researchers noted that, although follow-up is short, patients who received CAR T cells at ≤ 2 × 106/kg after Cy/Flu had better progression-free survival (P=0.008) than patients who received CAR T cells at ≤ 2 × 106/kg after Cy or Cy/E.
Of the 9 patients who achieved a CR after Cy/Flu and JCAR014, 1 has relapsed, with follow-up ranging from 2.3 months to 11.2 months. (Seven of these 9 patients had received 2 × 106 CAR T cells/kg, and 1 patient each had received the other 2 doses.)
“The main message is that you can treat patients with non-Hodgkin’s lymphoma with CAR T cells and get very good response rates with optimization of the CAR T-cell dose and lymphodepletion,” said study author Cameron Turtle, MBBS, PhD, of the Fred Hutchinson Cancer Research Center.
“Strategies like modifying the lymphodepletion in conjunction with suitable CAR T-cell dosing can have a big impact on clinical outcome.”
Safety
Two patients who were treated with the highest CAR T-cell dose (2 × 107 cells/kg) died—1 of pontine hemorrhage and 1 of gastrointestinal hemorrhage associated with a known gastrointestinal invasive lymphomatous mass.
This dose was also associated with an increased risk of severe CRS and neurotoxicity, as was the Cy/Flu regimen.
Twenty patients (62.5%) developed CRS, and 4 (12.5%) had severe CRS. All 4 of these patients had received Cy/Flu.
Nine patients (28%) developed severe neurotoxicity (grade 3 or higher), 7 of whom had received Cy/Flu.
Three of the 6 patients (50%) treated at 2 × 107 CAR-T cells/kg after Cy/Flu developed severe CRS, and 4 of the 6 patients (67%) developed severe neurotoxicity.
The researchers looked for biomarkers of toxicity in serum collected 1 day after CAR T-cell infusion.
They found that high IL-6, IL-8, IL-10, IL-15, and IFN-γ concentrations were associated with subsequent severe CRS and neurotoxicity, and low TGF-Β concentration was associated with neurotoxicity.
The team said these findings provide an opportunity for studying the use of serum cytokine concentrations to identify patients at the highest risk of toxicity who might benefit from early interventions. ![]()

Photo from Fred Hutchinson
Cancer Research Center
Results from a phase 1 study have provided insights that may help researchers optimize treatment with JCAR014, a chimeric antigen receptor (CAR) T-cell therapy, in patients with advanced non-Hodgkin lymphoma (NHL).
Researchers said they identified a lymphodepleting regimen that improved the likelihood of complete response (CR) to JCAR014.
Although the regimen also increased the risk of severe cytokine release syndrome (CRS) and neurotoxicity, the researchers discovered biomarkers that might allow them to identify patients who have a high risk of these events and could potentially benefit from early interventions.
The researchers reported these findings in Science Translational Medicine. The trial (NCT01865617) was funded, in part, by Juno Therapeutics, the company developing JCAR014.
Previous results from this trial, in patients with acute lymphoblastic leukemia, were published in The Journal of Clinical Investigation.
About JCAR014
JCAR014 is a CD19-directed CAR T-cell therapy in which CD4+ and CD8+ cells are administered in equal proportions.
“The idea . . . is that by [controlling the ratio of T cells], we would get more reproducible data around the effects of the cells—both beneficial effects against the cancer and also any side effects they might cause the patient,” said study author Stanley Riddell, MD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington.
“And then, by adjusting the dose, we could improve what we call the therapeutic index—the benefit against the tumor—without too much toxicity.”
Patients and treatment
Dr Riddell and his colleagues reported results with JCAR014, following lymphodepleting chemotherapy, in 32 patients with NHL who had a median age of 58 (range, 36-70).
The patients had de novo large B-cell lymphoma (n=11), transformed de novo large B-cell lymphoma (n=11), mantle cell lymphoma (n=4), and follicular lymphoma (n=6).
They had received a median of 5 prior treatment regimens (range, 2 to 11), including autologous
(n=14) and allogeneic (n=4) transplant. All patients had measurable disease (≥ 2 cm) in the lymph nodes or other extramedullary sites at baseline.
The patients received JCAR014 at 1 of 3 dose levels, given 36 to 96 hours after lymphodepleting chemotherapy.
Twelve patients received cyclophosphamide (Cy) alone or Cy and etoposide (E), and 20 received Cy plus fludarabine (Flu). Five patients received 2 × 105 CAR T cells/kg, 18 received 2 × 106 CAR T cells/kg, and 9 received 2 × 107 CAR T cells/kg.
Efficacy
In the 30 evaluable patients, the overall response rate (ORR) was 63% (n=19), and the CR rate was 33% (n=10).
Among patients who received Cy or Cy/E, the ORR was 50% (n=6), and the CR rate was 8% (n=1). Among patients who received Cy/Flu, the ORR was 72% (n=13), and the CR rate was 50% (n=9).
The patients who received Cy/Flu had better CAR T-cell expansion and persistence than patients who received Cy or Cy/E, which was reflected in the higher response rates.
Higher response rates were also observed in patients who received 2 × 106 CAR T cells/kg rather than the other 2 dose levels.
The researchers noted that, although follow-up is short, patients who received CAR T cells at ≤ 2 × 106/kg after Cy/Flu had better progression-free survival (P=0.008) than patients who received CAR T cells at ≤ 2 × 106/kg after Cy or Cy/E.
Of the 9 patients who achieved a CR after Cy/Flu and JCAR014, 1 has relapsed, with follow-up ranging from 2.3 months to 11.2 months. (Seven of these 9 patients had received 2 × 106 CAR T cells/kg, and 1 patient each had received the other 2 doses.)
“The main message is that you can treat patients with non-Hodgkin’s lymphoma with CAR T cells and get very good response rates with optimization of the CAR T-cell dose and lymphodepletion,” said study author Cameron Turtle, MBBS, PhD, of the Fred Hutchinson Cancer Research Center.
“Strategies like modifying the lymphodepletion in conjunction with suitable CAR T-cell dosing can have a big impact on clinical outcome.”
Safety
Two patients who were treated with the highest CAR T-cell dose (2 × 107 cells/kg) died—1 of pontine hemorrhage and 1 of gastrointestinal hemorrhage associated with a known gastrointestinal invasive lymphomatous mass.
This dose was also associated with an increased risk of severe CRS and neurotoxicity, as was the Cy/Flu regimen.
Twenty patients (62.5%) developed CRS, and 4 (12.5%) had severe CRS. All 4 of these patients had received Cy/Flu.
Nine patients (28%) developed severe neurotoxicity (grade 3 or higher), 7 of whom had received Cy/Flu.
Three of the 6 patients (50%) treated at 2 × 107 CAR-T cells/kg after Cy/Flu developed severe CRS, and 4 of the 6 patients (67%) developed severe neurotoxicity.
The researchers looked for biomarkers of toxicity in serum collected 1 day after CAR T-cell infusion.
They found that high IL-6, IL-8, IL-10, IL-15, and IFN-γ concentrations were associated with subsequent severe CRS and neurotoxicity, and low TGF-Β concentration was associated with neurotoxicity.
The team said these findings provide an opportunity for studying the use of serum cytokine concentrations to identify patients at the highest risk of toxicity who might benefit from early interventions. ![]()

Photo from Fred Hutchinson
Cancer Research Center
Results from a phase 1 study have provided insights that may help researchers optimize treatment with JCAR014, a chimeric antigen receptor (CAR) T-cell therapy, in patients with advanced non-Hodgkin lymphoma (NHL).
Researchers said they identified a lymphodepleting regimen that improved the likelihood of complete response (CR) to JCAR014.
Although the regimen also increased the risk of severe cytokine release syndrome (CRS) and neurotoxicity, the researchers discovered biomarkers that might allow them to identify patients who have a high risk of these events and could potentially benefit from early interventions.
The researchers reported these findings in Science Translational Medicine. The trial (NCT01865617) was funded, in part, by Juno Therapeutics, the company developing JCAR014.
Previous results from this trial, in patients with acute lymphoblastic leukemia, were published in The Journal of Clinical Investigation.
About JCAR014
JCAR014 is a CD19-directed CAR T-cell therapy in which CD4+ and CD8+ cells are administered in equal proportions.
“The idea . . . is that by [controlling the ratio of T cells], we would get more reproducible data around the effects of the cells—both beneficial effects against the cancer and also any side effects they might cause the patient,” said study author Stanley Riddell, MD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington.
“And then, by adjusting the dose, we could improve what we call the therapeutic index—the benefit against the tumor—without too much toxicity.”
Patients and treatment
Dr Riddell and his colleagues reported results with JCAR014, following lymphodepleting chemotherapy, in 32 patients with NHL who had a median age of 58 (range, 36-70).
The patients had de novo large B-cell lymphoma (n=11), transformed de novo large B-cell lymphoma (n=11), mantle cell lymphoma (n=4), and follicular lymphoma (n=6).
They had received a median of 5 prior treatment regimens (range, 2 to 11), including autologous
(n=14) and allogeneic (n=4) transplant. All patients had measurable disease (≥ 2 cm) in the lymph nodes or other extramedullary sites at baseline.
The patients received JCAR014 at 1 of 3 dose levels, given 36 to 96 hours after lymphodepleting chemotherapy.
Twelve patients received cyclophosphamide (Cy) alone or Cy and etoposide (E), and 20 received Cy plus fludarabine (Flu). Five patients received 2 × 105 CAR T cells/kg, 18 received 2 × 106 CAR T cells/kg, and 9 received 2 × 107 CAR T cells/kg.
Efficacy
In the 30 evaluable patients, the overall response rate (ORR) was 63% (n=19), and the CR rate was 33% (n=10).
Among patients who received Cy or Cy/E, the ORR was 50% (n=6), and the CR rate was 8% (n=1). Among patients who received Cy/Flu, the ORR was 72% (n=13), and the CR rate was 50% (n=9).
The patients who received Cy/Flu had better CAR T-cell expansion and persistence than patients who received Cy or Cy/E, which was reflected in the higher response rates.
Higher response rates were also observed in patients who received 2 × 106 CAR T cells/kg rather than the other 2 dose levels.
The researchers noted that, although follow-up is short, patients who received CAR T cells at ≤ 2 × 106/kg after Cy/Flu had better progression-free survival (P=0.008) than patients who received CAR T cells at ≤ 2 × 106/kg after Cy or Cy/E.
Of the 9 patients who achieved a CR after Cy/Flu and JCAR014, 1 has relapsed, with follow-up ranging from 2.3 months to 11.2 months. (Seven of these 9 patients had received 2 × 106 CAR T cells/kg, and 1 patient each had received the other 2 doses.)
“The main message is that you can treat patients with non-Hodgkin’s lymphoma with CAR T cells and get very good response rates with optimization of the CAR T-cell dose and lymphodepletion,” said study author Cameron Turtle, MBBS, PhD, of the Fred Hutchinson Cancer Research Center.
“Strategies like modifying the lymphodepletion in conjunction with suitable CAR T-cell dosing can have a big impact on clinical outcome.”
Safety
Two patients who were treated with the highest CAR T-cell dose (2 × 107 cells/kg) died—1 of pontine hemorrhage and 1 of gastrointestinal hemorrhage associated with a known gastrointestinal invasive lymphomatous mass.
This dose was also associated with an increased risk of severe CRS and neurotoxicity, as was the Cy/Flu regimen.
Twenty patients (62.5%) developed CRS, and 4 (12.5%) had severe CRS. All 4 of these patients had received Cy/Flu.
Nine patients (28%) developed severe neurotoxicity (grade 3 or higher), 7 of whom had received Cy/Flu.
Three of the 6 patients (50%) treated at 2 × 107 CAR-T cells/kg after Cy/Flu developed severe CRS, and 4 of the 6 patients (67%) developed severe neurotoxicity.
The researchers looked for biomarkers of toxicity in serum collected 1 day after CAR T-cell infusion.
They found that high IL-6, IL-8, IL-10, IL-15, and IFN-γ concentrations were associated with subsequent severe CRS and neurotoxicity, and low TGF-Β concentration was associated with neurotoxicity.
The team said these findings provide an opportunity for studying the use of serum cytokine concentrations to identify patients at the highest risk of toxicity who might benefit from early interventions. ![]()
NAMDRC and Partners Focus on CMS Threat to Pulmonary Rehabilitation
In a genuine very good news, very bad news proposal included in the 2017 hospital outpatient regulations, the Centers for Medicare & Medicaid Services (CMS) has proposed a major payment boost for pulmonary rehab services billed through hospital outpatient departments, but, simultaneously, the Agency proposes to preclude certain programs from utilizing that long- standing payment mechanism.
In November 2015, Congress authorized CMS to take action on the growing trend of hospitals purchasing certain physician practices so that the hospital can bill for certain services at a notably higher rate than the same service when provided in the physician office setting. CMS Section 603 pf P.L. 114-74 authorizes such action, and “These proposals are made in accordance with our belief that section 603… is intended to curb the practice of hospital acquisition of physician practices that result in receiving additional Medicare payment for similar services.” While we recognize that the congressional intent has some level of legitimacy, as is often the case, the CMS approach is too inclusive, especially as it applies to pulmonary rehabilitation services billed through HCPCS code G0424.
This problem has evolved because of two distinctly different formulas for determining payment. The physician fee schedule is based on the concept of RVUs, practice expense, and malpractice expense. Hospital outpatient services that may be virtually identical are based on a formula that includes charge data from Medicare claims forms and the annual hospital cost report identifying overhead.
If adopted as proposed, hospital outpatient programs in place on the date of enactment of P.L. 114-74 (early November 2015) are grandfathered into the hospital outpatient methodology. However, new programs that are not part of the main hospital campus (or within 250 yards of the campus) will only be able to bill at the physician office setting rate. Likewise, an existing program that moves to a new location that is beyond the 250-yard threshold will lose its “grandfather” status and be forced to bill at the physician office setting payment rate.
For practical purposes, the 2017 proposed rate for G0424 in the hospital setting is $160+, while the same service in the physician office setting is $30+.
While there is certainly understandable logic in the Congressional mandate, the CMS approach that includes pulmonary rehab is fraught with basic flaws in logic, strongly supported by CMS data. For example, in 2014 only 231 distinct providers billed for a total of 22,603 services. That translates into an outlay of approximately $535,000. Compare that outlay for 2014 with the outlay for hospital outpatient pulmonary rehab at just under $120 million, billed by 1350 distinct providers.
Those data alone strongly support the contention that a business model of pulmonary rehab in a physician office setting is rarely viable. Space, capital investment, and staffing, coupled with low payment, hardly create an incentive for a hospital to purchase a pulmonary practice because of lucrative pulmonary rehab services.
Other Medicare data also work in our favor. An examination of the physician specialties that actually bill G0424 through the physician fee schedule also punches a large hole in the CMS argument. The top five physician specialties that billed G0424 through the physician office setting include:
2012 2013 2014
TOTAL $688,489 $589,116 $535,512
Pulmonary $340,805 $310,065 $229,832
Family Practice $175,788 $116,681 $183,499
Internal Medicine $79,053 $78,211 $52,943
Crit Care (intensivists) $29,964 $29,139 $18,723
Cardiology $31,947 $17,729 $17,242
Source: Physician Supplier Procedure Summary File (PSPS), 2012-2014
These data speak volumes, or perhaps an absolute lack of volume. How does one support the concept that this proposed action is necessary to stem the tide of hospital acquisition of pulmonary practices when the total volume, notably declining over the past 3 years, of actual billings for pulmonary rehab is valued at under $230,000? The comparison with actual hospital billing in 2014 of just under $120 million is critical. There is no rhyme or reason to the CMS proposal as it applies to pulmonary rehabilitation services.
Unintended consequences are not difficult to imagine. With the new payment rates, hospitals may choose to expand their programs but cannot do so unless that physical location in on the main campus or within 250 yards of the campus. An off-site program that must move to accommodate larger space would be precluded from such a move. Likewise, hospitals that may want to open a new program must do so within the confines of the hospital campus/250-yard perimeter. Otherwise, these programs would be required to bill at the physician fee schedule rate.
In a genuine very good news, very bad news proposal included in the 2017 hospital outpatient regulations, the Centers for Medicare & Medicaid Services (CMS) has proposed a major payment boost for pulmonary rehab services billed through hospital outpatient departments, but, simultaneously, the Agency proposes to preclude certain programs from utilizing that long- standing payment mechanism.
In November 2015, Congress authorized CMS to take action on the growing trend of hospitals purchasing certain physician practices so that the hospital can bill for certain services at a notably higher rate than the same service when provided in the physician office setting. CMS Section 603 pf P.L. 114-74 authorizes such action, and “These proposals are made in accordance with our belief that section 603… is intended to curb the practice of hospital acquisition of physician practices that result in receiving additional Medicare payment for similar services.” While we recognize that the congressional intent has some level of legitimacy, as is often the case, the CMS approach is too inclusive, especially as it applies to pulmonary rehabilitation services billed through HCPCS code G0424.
This problem has evolved because of two distinctly different formulas for determining payment. The physician fee schedule is based on the concept of RVUs, practice expense, and malpractice expense. Hospital outpatient services that may be virtually identical are based on a formula that includes charge data from Medicare claims forms and the annual hospital cost report identifying overhead.
If adopted as proposed, hospital outpatient programs in place on the date of enactment of P.L. 114-74 (early November 2015) are grandfathered into the hospital outpatient methodology. However, new programs that are not part of the main hospital campus (or within 250 yards of the campus) will only be able to bill at the physician office setting rate. Likewise, an existing program that moves to a new location that is beyond the 250-yard threshold will lose its “grandfather” status and be forced to bill at the physician office setting payment rate.
For practical purposes, the 2017 proposed rate for G0424 in the hospital setting is $160+, while the same service in the physician office setting is $30+.
While there is certainly understandable logic in the Congressional mandate, the CMS approach that includes pulmonary rehab is fraught with basic flaws in logic, strongly supported by CMS data. For example, in 2014 only 231 distinct providers billed for a total of 22,603 services. That translates into an outlay of approximately $535,000. Compare that outlay for 2014 with the outlay for hospital outpatient pulmonary rehab at just under $120 million, billed by 1350 distinct providers.
Those data alone strongly support the contention that a business model of pulmonary rehab in a physician office setting is rarely viable. Space, capital investment, and staffing, coupled with low payment, hardly create an incentive for a hospital to purchase a pulmonary practice because of lucrative pulmonary rehab services.
Other Medicare data also work in our favor. An examination of the physician specialties that actually bill G0424 through the physician fee schedule also punches a large hole in the CMS argument. The top five physician specialties that billed G0424 through the physician office setting include:
2012 2013 2014
TOTAL $688,489 $589,116 $535,512
Pulmonary $340,805 $310,065 $229,832
Family Practice $175,788 $116,681 $183,499
Internal Medicine $79,053 $78,211 $52,943
Crit Care (intensivists) $29,964 $29,139 $18,723
Cardiology $31,947 $17,729 $17,242
Source: Physician Supplier Procedure Summary File (PSPS), 2012-2014
These data speak volumes, or perhaps an absolute lack of volume. How does one support the concept that this proposed action is necessary to stem the tide of hospital acquisition of pulmonary practices when the total volume, notably declining over the past 3 years, of actual billings for pulmonary rehab is valued at under $230,000? The comparison with actual hospital billing in 2014 of just under $120 million is critical. There is no rhyme or reason to the CMS proposal as it applies to pulmonary rehabilitation services.
Unintended consequences are not difficult to imagine. With the new payment rates, hospitals may choose to expand their programs but cannot do so unless that physical location in on the main campus or within 250 yards of the campus. An off-site program that must move to accommodate larger space would be precluded from such a move. Likewise, hospitals that may want to open a new program must do so within the confines of the hospital campus/250-yard perimeter. Otherwise, these programs would be required to bill at the physician fee schedule rate.
In a genuine very good news, very bad news proposal included in the 2017 hospital outpatient regulations, the Centers for Medicare & Medicaid Services (CMS) has proposed a major payment boost for pulmonary rehab services billed through hospital outpatient departments, but, simultaneously, the Agency proposes to preclude certain programs from utilizing that long- standing payment mechanism.
In November 2015, Congress authorized CMS to take action on the growing trend of hospitals purchasing certain physician practices so that the hospital can bill for certain services at a notably higher rate than the same service when provided in the physician office setting. CMS Section 603 pf P.L. 114-74 authorizes such action, and “These proposals are made in accordance with our belief that section 603… is intended to curb the practice of hospital acquisition of physician practices that result in receiving additional Medicare payment for similar services.” While we recognize that the congressional intent has some level of legitimacy, as is often the case, the CMS approach is too inclusive, especially as it applies to pulmonary rehabilitation services billed through HCPCS code G0424.
This problem has evolved because of two distinctly different formulas for determining payment. The physician fee schedule is based on the concept of RVUs, practice expense, and malpractice expense. Hospital outpatient services that may be virtually identical are based on a formula that includes charge data from Medicare claims forms and the annual hospital cost report identifying overhead.
If adopted as proposed, hospital outpatient programs in place on the date of enactment of P.L. 114-74 (early November 2015) are grandfathered into the hospital outpatient methodology. However, new programs that are not part of the main hospital campus (or within 250 yards of the campus) will only be able to bill at the physician office setting rate. Likewise, an existing program that moves to a new location that is beyond the 250-yard threshold will lose its “grandfather” status and be forced to bill at the physician office setting payment rate.
For practical purposes, the 2017 proposed rate for G0424 in the hospital setting is $160+, while the same service in the physician office setting is $30+.
While there is certainly understandable logic in the Congressional mandate, the CMS approach that includes pulmonary rehab is fraught with basic flaws in logic, strongly supported by CMS data. For example, in 2014 only 231 distinct providers billed for a total of 22,603 services. That translates into an outlay of approximately $535,000. Compare that outlay for 2014 with the outlay for hospital outpatient pulmonary rehab at just under $120 million, billed by 1350 distinct providers.
Those data alone strongly support the contention that a business model of pulmonary rehab in a physician office setting is rarely viable. Space, capital investment, and staffing, coupled with low payment, hardly create an incentive for a hospital to purchase a pulmonary practice because of lucrative pulmonary rehab services.
Other Medicare data also work in our favor. An examination of the physician specialties that actually bill G0424 through the physician fee schedule also punches a large hole in the CMS argument. The top five physician specialties that billed G0424 through the physician office setting include:
2012 2013 2014
TOTAL $688,489 $589,116 $535,512
Pulmonary $340,805 $310,065 $229,832
Family Practice $175,788 $116,681 $183,499
Internal Medicine $79,053 $78,211 $52,943
Crit Care (intensivists) $29,964 $29,139 $18,723
Cardiology $31,947 $17,729 $17,242
Source: Physician Supplier Procedure Summary File (PSPS), 2012-2014
These data speak volumes, or perhaps an absolute lack of volume. How does one support the concept that this proposed action is necessary to stem the tide of hospital acquisition of pulmonary practices when the total volume, notably declining over the past 3 years, of actual billings for pulmonary rehab is valued at under $230,000? The comparison with actual hospital billing in 2014 of just under $120 million is critical. There is no rhyme or reason to the CMS proposal as it applies to pulmonary rehabilitation services.
Unintended consequences are not difficult to imagine. With the new payment rates, hospitals may choose to expand their programs but cannot do so unless that physical location in on the main campus or within 250 yards of the campus. An off-site program that must move to accommodate larger space would be precluded from such a move. Likewise, hospitals that may want to open a new program must do so within the confines of the hospital campus/250-yard perimeter. Otherwise, these programs would be required to bill at the physician fee schedule rate.
Our Staff Matters
The CHEST staff’s monthly e-newsletter, Staff Matters, recently highlighted two examples that demonstrate the passion, talent, and cooperation exhibited by CHEST staff as colleagues working together to advance CHEST’s mission. As the name of the newsletter indicates, our staff really does matter and continually provides opportunities fostering our mission.
Celebrating a CHEST first
Chad Jackson recently earned a designation as Fellow of the American College of Chest Physicians (FCCP). He’s the first nonphysician member of CHEST staff to earn this designation. In light of this great honor, Chad was asked some questions about what this means to him.
Q: What does this honor mean to you?
A: It means a lot. More than I think I can eloquently describe in a few words ...
I think it is important for the employees to know that CHEST is a HUGE name in the medical space of hospitals and health systems. CHEST also has an excellent reputation with advanced practice professionals who work with our CHEST physicians. When I told people at Florida State College of Medicine that I was coming to work for CHEST, they literally were giving me high fives in the hallways of the college.
I think this is an important perspective for employees to realize. We come to work day in and day out, and it is just a job to a lot of folks. But outside of these walls, CHEST is well-known as a leader in the pulmonary, critical care, and sleep space. It was and still is an honor for me to work here, and I am truly blessed by being able to obtain my FCCP.
Q: Why did you choose to pursue obtaining an FCCP?
A: This is a realization of a dream that I had since coming to CHEST more than 8 years ago. Previously, as a nonphysician advanced professional practitioner, registered respiratory therapists (RRTs) like me could apply for membership only after you obtained a PhD. I was working on my PhD studies and had to take a break from my studies when life “intervened” and I had too much going on. At that point, I thought my dream of obtaining my FCCP was out of reach. When the membership model changed, I don’t think anyone was as excited as I was when the board discussed these changes.
I am the perfect use-case for this new membership model. I wanted a “home” for my practice. For years, I have been a member of the American Association of Respiratory Care (AARC), which many hospital-based RRTs call their home. I have also been a member of the Society of Critical Care Medicine (SCCM) and was even a Fundamentals of Critical Care Skills (FCCS) course instructor. But, my passion was educating physicians and other health care practitioners in pulmonary, critical care, and sleep medicine.
Obtaining my FCCP is the ultimate recognition for me and the work I have been doing in this medical education space.
Q: What does it mean, as a nonphysician, to have the opportunity to be recognized for your commitment to advancing chest medicine?
A: It is HUGE! I think that there are many more folks who would like to receive recognition for their work in this field, who don’t feel that their current “home” organizations appreciate their efforts. For me, again, it was a dream now realized, to be able to be recognized for my efforts along with my physician friends who work so hard to provide the best possible education for our members and attendees.
CHEST Staff in Action
In July, as part of our annual staff appreciation day, CHEST staff members were offered the opportunity to visit the “Feed My Starving Children” facility for a few hours in the morning to prepare food portions for needy children in different parts of the world. The staff’s response was tremendous, and even our incoming President, Dr. Gerard Silvestri, joined us, as we took to different stations portioning out dry ingredients for individual food packets. We soon learned that our packets were destined for Haiti’s children! This community outreach event brought our staff together, volunteering time toward a mutual goal of helping others and advancing CHEST’s mission in our own personal way.
Here is what we achieved that morning:
Large cartons packed: 129
Individual meals filled and packed: 27,864
Children fed for 1 year: 76
In the words of our interim CEO, Steve Welch, “As I looked around at everyone at the event, I was so touched by the enthusiasm that you all showed, and the comments I heard afterward, that I’ve asked HR to look into setting up similar things as a regular opportunity for those staff who wish to participate, in order to continue fostering an environment of volunteerism and giving back. What we do every day is incumbent on our volunteers giving their time for CHEST, and it sets a great example when we are also volunteering for causes that are important to us individually.”
The CHEST staff’s monthly e-newsletter, Staff Matters, recently highlighted two examples that demonstrate the passion, talent, and cooperation exhibited by CHEST staff as colleagues working together to advance CHEST’s mission. As the name of the newsletter indicates, our staff really does matter and continually provides opportunities fostering our mission.
Celebrating a CHEST first
Chad Jackson recently earned a designation as Fellow of the American College of Chest Physicians (FCCP). He’s the first nonphysician member of CHEST staff to earn this designation. In light of this great honor, Chad was asked some questions about what this means to him.
Q: What does this honor mean to you?
A: It means a lot. More than I think I can eloquently describe in a few words ...
I think it is important for the employees to know that CHEST is a HUGE name in the medical space of hospitals and health systems. CHEST also has an excellent reputation with advanced practice professionals who work with our CHEST physicians. When I told people at Florida State College of Medicine that I was coming to work for CHEST, they literally were giving me high fives in the hallways of the college.
I think this is an important perspective for employees to realize. We come to work day in and day out, and it is just a job to a lot of folks. But outside of these walls, CHEST is well-known as a leader in the pulmonary, critical care, and sleep space. It was and still is an honor for me to work here, and I am truly blessed by being able to obtain my FCCP.
Q: Why did you choose to pursue obtaining an FCCP?
A: This is a realization of a dream that I had since coming to CHEST more than 8 years ago. Previously, as a nonphysician advanced professional practitioner, registered respiratory therapists (RRTs) like me could apply for membership only after you obtained a PhD. I was working on my PhD studies and had to take a break from my studies when life “intervened” and I had too much going on. At that point, I thought my dream of obtaining my FCCP was out of reach. When the membership model changed, I don’t think anyone was as excited as I was when the board discussed these changes.
I am the perfect use-case for this new membership model. I wanted a “home” for my practice. For years, I have been a member of the American Association of Respiratory Care (AARC), which many hospital-based RRTs call their home. I have also been a member of the Society of Critical Care Medicine (SCCM) and was even a Fundamentals of Critical Care Skills (FCCS) course instructor. But, my passion was educating physicians and other health care practitioners in pulmonary, critical care, and sleep medicine.
Obtaining my FCCP is the ultimate recognition for me and the work I have been doing in this medical education space.
Q: What does it mean, as a nonphysician, to have the opportunity to be recognized for your commitment to advancing chest medicine?
A: It is HUGE! I think that there are many more folks who would like to receive recognition for their work in this field, who don’t feel that their current “home” organizations appreciate their efforts. For me, again, it was a dream now realized, to be able to be recognized for my efforts along with my physician friends who work so hard to provide the best possible education for our members and attendees.
CHEST Staff in Action
In July, as part of our annual staff appreciation day, CHEST staff members were offered the opportunity to visit the “Feed My Starving Children” facility for a few hours in the morning to prepare food portions for needy children in different parts of the world. The staff’s response was tremendous, and even our incoming President, Dr. Gerard Silvestri, joined us, as we took to different stations portioning out dry ingredients for individual food packets. We soon learned that our packets were destined for Haiti’s children! This community outreach event brought our staff together, volunteering time toward a mutual goal of helping others and advancing CHEST’s mission in our own personal way.
Here is what we achieved that morning:
Large cartons packed: 129
Individual meals filled and packed: 27,864
Children fed for 1 year: 76
In the words of our interim CEO, Steve Welch, “As I looked around at everyone at the event, I was so touched by the enthusiasm that you all showed, and the comments I heard afterward, that I’ve asked HR to look into setting up similar things as a regular opportunity for those staff who wish to participate, in order to continue fostering an environment of volunteerism and giving back. What we do every day is incumbent on our volunteers giving their time for CHEST, and it sets a great example when we are also volunteering for causes that are important to us individually.”
The CHEST staff’s monthly e-newsletter, Staff Matters, recently highlighted two examples that demonstrate the passion, talent, and cooperation exhibited by CHEST staff as colleagues working together to advance CHEST’s mission. As the name of the newsletter indicates, our staff really does matter and continually provides opportunities fostering our mission.
Celebrating a CHEST first
Chad Jackson recently earned a designation as Fellow of the American College of Chest Physicians (FCCP). He’s the first nonphysician member of CHEST staff to earn this designation. In light of this great honor, Chad was asked some questions about what this means to him.
Q: What does this honor mean to you?
A: It means a lot. More than I think I can eloquently describe in a few words ...
I think it is important for the employees to know that CHEST is a HUGE name in the medical space of hospitals and health systems. CHEST also has an excellent reputation with advanced practice professionals who work with our CHEST physicians. When I told people at Florida State College of Medicine that I was coming to work for CHEST, they literally were giving me high fives in the hallways of the college.
I think this is an important perspective for employees to realize. We come to work day in and day out, and it is just a job to a lot of folks. But outside of these walls, CHEST is well-known as a leader in the pulmonary, critical care, and sleep space. It was and still is an honor for me to work here, and I am truly blessed by being able to obtain my FCCP.
Q: Why did you choose to pursue obtaining an FCCP?
A: This is a realization of a dream that I had since coming to CHEST more than 8 years ago. Previously, as a nonphysician advanced professional practitioner, registered respiratory therapists (RRTs) like me could apply for membership only after you obtained a PhD. I was working on my PhD studies and had to take a break from my studies when life “intervened” and I had too much going on. At that point, I thought my dream of obtaining my FCCP was out of reach. When the membership model changed, I don’t think anyone was as excited as I was when the board discussed these changes.
I am the perfect use-case for this new membership model. I wanted a “home” for my practice. For years, I have been a member of the American Association of Respiratory Care (AARC), which many hospital-based RRTs call their home. I have also been a member of the Society of Critical Care Medicine (SCCM) and was even a Fundamentals of Critical Care Skills (FCCS) course instructor. But, my passion was educating physicians and other health care practitioners in pulmonary, critical care, and sleep medicine.
Obtaining my FCCP is the ultimate recognition for me and the work I have been doing in this medical education space.
Q: What does it mean, as a nonphysician, to have the opportunity to be recognized for your commitment to advancing chest medicine?
A: It is HUGE! I think that there are many more folks who would like to receive recognition for their work in this field, who don’t feel that their current “home” organizations appreciate their efforts. For me, again, it was a dream now realized, to be able to be recognized for my efforts along with my physician friends who work so hard to provide the best possible education for our members and attendees.
CHEST Staff in Action
In July, as part of our annual staff appreciation day, CHEST staff members were offered the opportunity to visit the “Feed My Starving Children” facility for a few hours in the morning to prepare food portions for needy children in different parts of the world. The staff’s response was tremendous, and even our incoming President, Dr. Gerard Silvestri, joined us, as we took to different stations portioning out dry ingredients for individual food packets. We soon learned that our packets were destined for Haiti’s children! This community outreach event brought our staff together, volunteering time toward a mutual goal of helping others and advancing CHEST’s mission in our own personal way.
Here is what we achieved that morning:
Large cartons packed: 129
Individual meals filled and packed: 27,864
Children fed for 1 year: 76
In the words of our interim CEO, Steve Welch, “As I looked around at everyone at the event, I was so touched by the enthusiasm that you all showed, and the comments I heard afterward, that I’ve asked HR to look into setting up similar things as a regular opportunity for those staff who wish to participate, in order to continue fostering an environment of volunteerism and giving back. What we do every day is incumbent on our volunteers giving their time for CHEST, and it sets a great example when we are also volunteering for causes that are important to us individually.”
NetWorks
NetWorks Challenge 2016
Which NetWork will champion lung health?
Donate to the CHEST Foundation from now until the CHEST Annual Meeting 2016. Mark your NetWork when making your donation to receive credit. Donate here:
• Online
• By phone: 224/521-9527
• By mail: Download our donation form and mail to CHEST Foundation, 2595 Patriot Boulevard, Glenview, IL 60026
Three ways to win
Round 1
Highest percentage of participation by NetWork Steering Committee
Number of winners: 2
Winning NetWork Steering Committees will receive:
• Additional time at the meeting – 90 minutes total
• Travel grants to CHEST 2016
First Half Winners: Women’s Health NetWork and Occupational and Environmental Health NetWork
Second Half Winners: (announced at the CHEST Annual Meeting)
Round 2
Total amount contributed by NetWork Steering Committee
Number of winners: 2
Winning NetWork Steering Committees will receive:
• One seat (public member) on the CHEST Foundation Awards Committee for the following year
Bonus: The CHEST Foundation will match funds raised by the two winning NetWork Steering Committees that meet a minimum of $15,000, up to $25,000 for a clinical research grant. Winning NetWork Steering Committees will be announced at the CHEST Annual Meeting Monday Opening Session.
Annual Meeting Winners (announced after CHEST Annual Meeting)
Round 3
Highest percentage of participation by a NetWork’s membership
Number of winners: 2 for travel grants, 4 for membership waivers
Winning NetWorks will receive:
• Travel grants to CHEST 2017
• Free CHEST membership for 2017
Note on Rounds 1 and 3: The NetWork Steering Committee will recommend awardee. The recommendations from winning NetWork Steering Committees will be reviewed by the Foundation Awards Committee. In the event of a tie, the NetWork that achieves its percentage of participation earliest will receive the challenge.
NetWorks Challenge 2016
Which NetWork will champion lung health?
Donate to the CHEST Foundation from now until the CHEST Annual Meeting 2016. Mark your NetWork when making your donation to receive credit. Donate here:
• Online
• By phone: 224/521-9527
• By mail: Download our donation form and mail to CHEST Foundation, 2595 Patriot Boulevard, Glenview, IL 60026
Three ways to win
Round 1
Highest percentage of participation by NetWork Steering Committee
Number of winners: 2
Winning NetWork Steering Committees will receive:
• Additional time at the meeting – 90 minutes total
• Travel grants to CHEST 2016
First Half Winners: Women’s Health NetWork and Occupational and Environmental Health NetWork
Second Half Winners: (announced at the CHEST Annual Meeting)
Round 2
Total amount contributed by NetWork Steering Committee
Number of winners: 2
Winning NetWork Steering Committees will receive:
• One seat (public member) on the CHEST Foundation Awards Committee for the following year
Bonus: The CHEST Foundation will match funds raised by the two winning NetWork Steering Committees that meet a minimum of $15,000, up to $25,000 for a clinical research grant. Winning NetWork Steering Committees will be announced at the CHEST Annual Meeting Monday Opening Session.
Annual Meeting Winners (announced after CHEST Annual Meeting)
Round 3
Highest percentage of participation by a NetWork’s membership
Number of winners: 2 for travel grants, 4 for membership waivers
Winning NetWorks will receive:
• Travel grants to CHEST 2017
• Free CHEST membership for 2017
Note on Rounds 1 and 3: The NetWork Steering Committee will recommend awardee. The recommendations from winning NetWork Steering Committees will be reviewed by the Foundation Awards Committee. In the event of a tie, the NetWork that achieves its percentage of participation earliest will receive the challenge.
NetWorks Challenge 2016
Which NetWork will champion lung health?
Donate to the CHEST Foundation from now until the CHEST Annual Meeting 2016. Mark your NetWork when making your donation to receive credit. Donate here:
• Online
• By phone: 224/521-9527
• By mail: Download our donation form and mail to CHEST Foundation, 2595 Patriot Boulevard, Glenview, IL 60026
Three ways to win
Round 1
Highest percentage of participation by NetWork Steering Committee
Number of winners: 2
Winning NetWork Steering Committees will receive:
• Additional time at the meeting – 90 minutes total
• Travel grants to CHEST 2016
First Half Winners: Women’s Health NetWork and Occupational and Environmental Health NetWork
Second Half Winners: (announced at the CHEST Annual Meeting)
Round 2
Total amount contributed by NetWork Steering Committee
Number of winners: 2
Winning NetWork Steering Committees will receive:
• One seat (public member) on the CHEST Foundation Awards Committee for the following year
Bonus: The CHEST Foundation will match funds raised by the two winning NetWork Steering Committees that meet a minimum of $15,000, up to $25,000 for a clinical research grant. Winning NetWork Steering Committees will be announced at the CHEST Annual Meeting Monday Opening Session.
Annual Meeting Winners (announced after CHEST Annual Meeting)
Round 3
Highest percentage of participation by a NetWork’s membership
Number of winners: 2 for travel grants, 4 for membership waivers
Winning NetWorks will receive:
• Travel grants to CHEST 2017
• Free CHEST membership for 2017
Note on Rounds 1 and 3: The NetWork Steering Committee will recommend awardee. The recommendations from winning NetWork Steering Committees will be reviewed by the Foundation Awards Committee. In the event of a tie, the NetWork that achieves its percentage of participation earliest will receive the challenge.