User login
Up in Arms: Bilateral Luxatio Erecta Fracture-Dislocations
Unilateral inferior shoulder dislocation (luxatio erecta) is uncommon, accounting for only 0.5% of all shoulder dislocations.1 Bilateral luxatio erecta is extremely rare, having been described fewer than 20 times in the literature. The most common etiology is hyperabduction causing the humerus to lever on the acromion; less common is axial loading onto a fully abducted arm and an extended elbow.2 Hyperabduction can occur when a person grabs an object in an attempt to stop a fall, as occurred in the present case. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 58-year-old man with a trauma injury presented to our emergency department. For his open right elbow fracture, emergency medical services had given him fentanyl en route, and when he arrived he was less responsive. As the patient reported, he had been on a scaffold 16 feet high when it began to give way. He jumped for another scaffold, 3 to 4 feet away, but came up short and, in an attempt to stop himself from falling, grabbed onto it with arms extended and above his head. His hands and arms were immediately pulled up in full extension. When both shoulders became dislocated, he could not hold on and fell to the ground, landing on a buttock. He did not lose consciousness.
Physical examination revealed both arms abducted at the shoulder, and elbows extended (Figure 1).
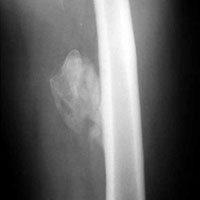
Radiographs confirmed the diagnosis and showed bilateral nondisplaced proximal humeral fractures of the greater tuberosity (Figure 2).
For the shoulder reductions, we administered propofol for conscious sedation and fentanyl for analgesia. Then, a sheet was wrapped supraclavicular and pulled across the torso inferiorly to allow countertraction when pulling the arm superiorly on the axial line. Another countertraction sheet was placed on the opposite side. For each arm, the countertraction was pulled inferiorly when the arm was pulled superiorly, both on the longitudinal plane. The arm was then gently rotated in adduction until reduction was achieved.
The right shoulder reduced relatively easily. The left shoulder reduced into an anterior dislocation—a relatively uncommon outcome in in-line traction attempts.3 (Reduction into anterior dislocation can also be a desired result in a specific technique of 2-step reduction, as described by Nho and colleagues.4) The patient’s anterior dislocation was then easily reduced into anatomical position with use of the Kocher technique of arm adduction with elbow flexion, followed by external rotation, and then finally into anatomical position with internal rotation.5 Both arms were then immobilized in full adduction with bilateral slings. The patient was admitted for further treatment of multiple fractures of the arms and vertebrae.
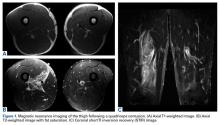
He was discharged in bilateral shoulder slings to an extended-care facility for physical therapy. One month after discharge, he could not elevate his arms and had minimal use of them. Two weeks later, magnetic resonance imaging showed a “comminuted greater tuberosity fracture with new displacement of fragments involving the attachment of the supraspinatus and infraspinatus; posterior subluxation of the glenohumeral joint with evidence of posterior and anterior labral tears; and large glenohumeral joint effusion.” The patient opted for surgical repair and underwent left shoulder arthroscopy with extensive débridement, open rotator cuff repair, open greater tuberosity reduction and internal fixation, and open biceps tenodesis. He was then discharged back to an extended-care facility to continue rehabilitation. One and a half months after surgery, he started the physical therapy phase of the massive rotator cuff repair protocol. He declined reverse total shoulder arthroplasty (RTSA).
Four and a half months after injury (3 months after surgery), the left shoulder demonstrated 20° of flexion and 70° to 110° of abduction (external rotation not tested), and the right shoulder demonstrated 30° of flexion and 70° to 110° of abduction (external rotation not tested). He had no instability and no lag with good external rotation.
Six months after injury, the patient still could not lift his arms above his head. He likely would not be able to do so without RTSA, which he again declined. He continued physical therapy and clinical follow-ups.
Discussion
Although inferior shoulder dislocations are rare, they carry a higher rate of complications, most of which our patient experienced. Our patient had bilateral humeral head fractures, which occur in 80% of cases.6 Postreduction CT showed the degree of his fractures (Figure 3).
Our patient also had reduced sensation in the axillary nerve distribution, which occurs in 60% of inferior dislocations.6 Axillary nerve injuries produce numbness in the lateral arm or posterior shoulder and weakness with shoulder flexion, abduction, and external rotation.7 In our patient’s case, sensation returned after reduction, which is typical (most patients have a positive prognosis).8 As the shoulder dislocates inferiorly, the humeral head tears the glenohumeral capsule inferiorly, which can damage the axillary artery. This artery becomes the brachial and eventually the radial and ulnar arteries, which can have decreased or absent pulses with injury.
Inferior dislocations are also associated with abundant soft-tissue injuries, including torn rotator cuff, shoulder capsule avulsion, and disruption of adjacent muscles (supraspinatus, infraspinatus, teres minor, subscapularis, pectoralis major).9Luxatio erecta is relatively easy to diagnose given the unmistakable arm positioning. The key for the physician is first to assess for the many possible complications, then to administer the proper sedation and analgesia for reduction, and finally to reassess for complications.
Am J Orthop. 2016;45(6):E328-E330. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Camarda L, Martorana U, D’Arienzo M. A case of bilateral luxatio erecta. J Orthop Traumatol. 2009;10(2):97-99.
2. Musmeci E, Gaspari D, Sandri A, Regis D, Bartolozzi P. Bilateral luxatio erecta humeri associated with a unilateral brachial plexus and bilateral rotator cuff injuries: a case report. J Orthop Trauma. 2008;22(7):498-500.
3. Lam AC, Shih RD. Luxatio erecta complicated by anterior shoulder dislocation during reduction. West J Emerg Med. 2010;11(1):28-30.
4. Nho SJ, Dodson CC, Bardzik KF, Brophy RH, Domb BG, MacGillivray JD. The two-step maneuver for closed reduction of inferior glenohumeral dislocation (luxatio erecta to anterior dislocation to reduction). J Orthop Trauma. 2006;20(5):354-357.
5. Beattie TF, Steedman DJ, McGowan A, Robertson CE. A comparison of the Milch and Kocher techniques for acute anterior dislocation of the shoulder. Injury. 1986;17(5):349-352.
6. Mallon WJ, Bassett FH 3rd, Goldner RD. Luxatio erecta: the inferior glenohumeral dislocation. J Orthop Trauma. 1990;4(1):19-24.
7. Miller T. Peripheral nerve injuries at the shoulder. J Manipulative Physiol Ther. 1998;6(4):170-183.
8. Groh GI, Wirth MA, Rockwood CA Jr. Results of treatment of luxatio erecta (inferior shoulder dislocation). J Shoulder Elbow Surg. 2010;19(3):423-426.
9. Garcia R, Ponsky T, Brody F, Long J. Bilateral luxatio erecta complicated by venous thrombosis. J Trauma. 2006;60(5):1132-1134.
Unilateral inferior shoulder dislocation (luxatio erecta) is uncommon, accounting for only 0.5% of all shoulder dislocations.1 Bilateral luxatio erecta is extremely rare, having been described fewer than 20 times in the literature. The most common etiology is hyperabduction causing the humerus to lever on the acromion; less common is axial loading onto a fully abducted arm and an extended elbow.2 Hyperabduction can occur when a person grabs an object in an attempt to stop a fall, as occurred in the present case. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 58-year-old man with a trauma injury presented to our emergency department. For his open right elbow fracture, emergency medical services had given him fentanyl en route, and when he arrived he was less responsive. As the patient reported, he had been on a scaffold 16 feet high when it began to give way. He jumped for another scaffold, 3 to 4 feet away, but came up short and, in an attempt to stop himself from falling, grabbed onto it with arms extended and above his head. His hands and arms were immediately pulled up in full extension. When both shoulders became dislocated, he could not hold on and fell to the ground, landing on a buttock. He did not lose consciousness.
Physical examination revealed both arms abducted at the shoulder, and elbows extended (Figure 1).
Radiographs confirmed the diagnosis and showed bilateral nondisplaced proximal humeral fractures of the greater tuberosity (Figure 2).
For the shoulder reductions, we administered propofol for conscious sedation and fentanyl for analgesia. Then, a sheet was wrapped supraclavicular and pulled across the torso inferiorly to allow countertraction when pulling the arm superiorly on the axial line. Another countertraction sheet was placed on the opposite side. For each arm, the countertraction was pulled inferiorly when the arm was pulled superiorly, both on the longitudinal plane. The arm was then gently rotated in adduction until reduction was achieved.
The right shoulder reduced relatively easily. The left shoulder reduced into an anterior dislocation—a relatively uncommon outcome in in-line traction attempts.3 (Reduction into anterior dislocation can also be a desired result in a specific technique of 2-step reduction, as described by Nho and colleagues.4) The patient’s anterior dislocation was then easily reduced into anatomical position with use of the Kocher technique of arm adduction with elbow flexion, followed by external rotation, and then finally into anatomical position with internal rotation.5 Both arms were then immobilized in full adduction with bilateral slings. The patient was admitted for further treatment of multiple fractures of the arms and vertebrae.
He was discharged in bilateral shoulder slings to an extended-care facility for physical therapy. One month after discharge, he could not elevate his arms and had minimal use of them. Two weeks later, magnetic resonance imaging showed a “comminuted greater tuberosity fracture with new displacement of fragments involving the attachment of the supraspinatus and infraspinatus; posterior subluxation of the glenohumeral joint with evidence of posterior and anterior labral tears; and large glenohumeral joint effusion.” The patient opted for surgical repair and underwent left shoulder arthroscopy with extensive débridement, open rotator cuff repair, open greater tuberosity reduction and internal fixation, and open biceps tenodesis. He was then discharged back to an extended-care facility to continue rehabilitation. One and a half months after surgery, he started the physical therapy phase of the massive rotator cuff repair protocol. He declined reverse total shoulder arthroplasty (RTSA).
Four and a half months after injury (3 months after surgery), the left shoulder demonstrated 20° of flexion and 70° to 110° of abduction (external rotation not tested), and the right shoulder demonstrated 30° of flexion and 70° to 110° of abduction (external rotation not tested). He had no instability and no lag with good external rotation.
Six months after injury, the patient still could not lift his arms above his head. He likely would not be able to do so without RTSA, which he again declined. He continued physical therapy and clinical follow-ups.
Discussion
Although inferior shoulder dislocations are rare, they carry a higher rate of complications, most of which our patient experienced. Our patient had bilateral humeral head fractures, which occur in 80% of cases.6 Postreduction CT showed the degree of his fractures (Figure 3).
Our patient also had reduced sensation in the axillary nerve distribution, which occurs in 60% of inferior dislocations.6 Axillary nerve injuries produce numbness in the lateral arm or posterior shoulder and weakness with shoulder flexion, abduction, and external rotation.7 In our patient’s case, sensation returned after reduction, which is typical (most patients have a positive prognosis).8 As the shoulder dislocates inferiorly, the humeral head tears the glenohumeral capsule inferiorly, which can damage the axillary artery. This artery becomes the brachial and eventually the radial and ulnar arteries, which can have decreased or absent pulses with injury.
Inferior dislocations are also associated with abundant soft-tissue injuries, including torn rotator cuff, shoulder capsule avulsion, and disruption of adjacent muscles (supraspinatus, infraspinatus, teres minor, subscapularis, pectoralis major).9Luxatio erecta is relatively easy to diagnose given the unmistakable arm positioning. The key for the physician is first to assess for the many possible complications, then to administer the proper sedation and analgesia for reduction, and finally to reassess for complications.
Am J Orthop. 2016;45(6):E328-E330. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Unilateral inferior shoulder dislocation (luxatio erecta) is uncommon, accounting for only 0.5% of all shoulder dislocations.1 Bilateral luxatio erecta is extremely rare, having been described fewer than 20 times in the literature. The most common etiology is hyperabduction causing the humerus to lever on the acromion; less common is axial loading onto a fully abducted arm and an extended elbow.2 Hyperabduction can occur when a person grabs an object in an attempt to stop a fall, as occurred in the present case. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 58-year-old man with a trauma injury presented to our emergency department. For his open right elbow fracture, emergency medical services had given him fentanyl en route, and when he arrived he was less responsive. As the patient reported, he had been on a scaffold 16 feet high when it began to give way. He jumped for another scaffold, 3 to 4 feet away, but came up short and, in an attempt to stop himself from falling, grabbed onto it with arms extended and above his head. His hands and arms were immediately pulled up in full extension. When both shoulders became dislocated, he could not hold on and fell to the ground, landing on a buttock. He did not lose consciousness.
Physical examination revealed both arms abducted at the shoulder, and elbows extended (Figure 1).
Radiographs confirmed the diagnosis and showed bilateral nondisplaced proximal humeral fractures of the greater tuberosity (Figure 2).
For the shoulder reductions, we administered propofol for conscious sedation and fentanyl for analgesia. Then, a sheet was wrapped supraclavicular and pulled across the torso inferiorly to allow countertraction when pulling the arm superiorly on the axial line. Another countertraction sheet was placed on the opposite side. For each arm, the countertraction was pulled inferiorly when the arm was pulled superiorly, both on the longitudinal plane. The arm was then gently rotated in adduction until reduction was achieved.
The right shoulder reduced relatively easily. The left shoulder reduced into an anterior dislocation—a relatively uncommon outcome in in-line traction attempts.3 (Reduction into anterior dislocation can also be a desired result in a specific technique of 2-step reduction, as described by Nho and colleagues.4) The patient’s anterior dislocation was then easily reduced into anatomical position with use of the Kocher technique of arm adduction with elbow flexion, followed by external rotation, and then finally into anatomical position with internal rotation.5 Both arms were then immobilized in full adduction with bilateral slings. The patient was admitted for further treatment of multiple fractures of the arms and vertebrae.
He was discharged in bilateral shoulder slings to an extended-care facility for physical therapy. One month after discharge, he could not elevate his arms and had minimal use of them. Two weeks later, magnetic resonance imaging showed a “comminuted greater tuberosity fracture with new displacement of fragments involving the attachment of the supraspinatus and infraspinatus; posterior subluxation of the glenohumeral joint with evidence of posterior and anterior labral tears; and large glenohumeral joint effusion.” The patient opted for surgical repair and underwent left shoulder arthroscopy with extensive débridement, open rotator cuff repair, open greater tuberosity reduction and internal fixation, and open biceps tenodesis. He was then discharged back to an extended-care facility to continue rehabilitation. One and a half months after surgery, he started the physical therapy phase of the massive rotator cuff repair protocol. He declined reverse total shoulder arthroplasty (RTSA).
Four and a half months after injury (3 months after surgery), the left shoulder demonstrated 20° of flexion and 70° to 110° of abduction (external rotation not tested), and the right shoulder demonstrated 30° of flexion and 70° to 110° of abduction (external rotation not tested). He had no instability and no lag with good external rotation.
Six months after injury, the patient still could not lift his arms above his head. He likely would not be able to do so without RTSA, which he again declined. He continued physical therapy and clinical follow-ups.
Discussion
Although inferior shoulder dislocations are rare, they carry a higher rate of complications, most of which our patient experienced. Our patient had bilateral humeral head fractures, which occur in 80% of cases.6 Postreduction CT showed the degree of his fractures (Figure 3).
Our patient also had reduced sensation in the axillary nerve distribution, which occurs in 60% of inferior dislocations.6 Axillary nerve injuries produce numbness in the lateral arm or posterior shoulder and weakness with shoulder flexion, abduction, and external rotation.7 In our patient’s case, sensation returned after reduction, which is typical (most patients have a positive prognosis).8 As the shoulder dislocates inferiorly, the humeral head tears the glenohumeral capsule inferiorly, which can damage the axillary artery. This artery becomes the brachial and eventually the radial and ulnar arteries, which can have decreased or absent pulses with injury.
Inferior dislocations are also associated with abundant soft-tissue injuries, including torn rotator cuff, shoulder capsule avulsion, and disruption of adjacent muscles (supraspinatus, infraspinatus, teres minor, subscapularis, pectoralis major).9Luxatio erecta is relatively easy to diagnose given the unmistakable arm positioning. The key for the physician is first to assess for the many possible complications, then to administer the proper sedation and analgesia for reduction, and finally to reassess for complications.
Am J Orthop. 2016;45(6):E328-E330. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Camarda L, Martorana U, D’Arienzo M. A case of bilateral luxatio erecta. J Orthop Traumatol. 2009;10(2):97-99.
2. Musmeci E, Gaspari D, Sandri A, Regis D, Bartolozzi P. Bilateral luxatio erecta humeri associated with a unilateral brachial plexus and bilateral rotator cuff injuries: a case report. J Orthop Trauma. 2008;22(7):498-500.
3. Lam AC, Shih RD. Luxatio erecta complicated by anterior shoulder dislocation during reduction. West J Emerg Med. 2010;11(1):28-30.
4. Nho SJ, Dodson CC, Bardzik KF, Brophy RH, Domb BG, MacGillivray JD. The two-step maneuver for closed reduction of inferior glenohumeral dislocation (luxatio erecta to anterior dislocation to reduction). J Orthop Trauma. 2006;20(5):354-357.
5. Beattie TF, Steedman DJ, McGowan A, Robertson CE. A comparison of the Milch and Kocher techniques for acute anterior dislocation of the shoulder. Injury. 1986;17(5):349-352.
6. Mallon WJ, Bassett FH 3rd, Goldner RD. Luxatio erecta: the inferior glenohumeral dislocation. J Orthop Trauma. 1990;4(1):19-24.
7. Miller T. Peripheral nerve injuries at the shoulder. J Manipulative Physiol Ther. 1998;6(4):170-183.
8. Groh GI, Wirth MA, Rockwood CA Jr. Results of treatment of luxatio erecta (inferior shoulder dislocation). J Shoulder Elbow Surg. 2010;19(3):423-426.
9. Garcia R, Ponsky T, Brody F, Long J. Bilateral luxatio erecta complicated by venous thrombosis. J Trauma. 2006;60(5):1132-1134.
1. Camarda L, Martorana U, D’Arienzo M. A case of bilateral luxatio erecta. J Orthop Traumatol. 2009;10(2):97-99.
2. Musmeci E, Gaspari D, Sandri A, Regis D, Bartolozzi P. Bilateral luxatio erecta humeri associated with a unilateral brachial plexus and bilateral rotator cuff injuries: a case report. J Orthop Trauma. 2008;22(7):498-500.
3. Lam AC, Shih RD. Luxatio erecta complicated by anterior shoulder dislocation during reduction. West J Emerg Med. 2010;11(1):28-30.
4. Nho SJ, Dodson CC, Bardzik KF, Brophy RH, Domb BG, MacGillivray JD. The two-step maneuver for closed reduction of inferior glenohumeral dislocation (luxatio erecta to anterior dislocation to reduction). J Orthop Trauma. 2006;20(5):354-357.
5. Beattie TF, Steedman DJ, McGowan A, Robertson CE. A comparison of the Milch and Kocher techniques for acute anterior dislocation of the shoulder. Injury. 1986;17(5):349-352.
6. Mallon WJ, Bassett FH 3rd, Goldner RD. Luxatio erecta: the inferior glenohumeral dislocation. J Orthop Trauma. 1990;4(1):19-24.
7. Miller T. Peripheral nerve injuries at the shoulder. J Manipulative Physiol Ther. 1998;6(4):170-183.
8. Groh GI, Wirth MA, Rockwood CA Jr. Results of treatment of luxatio erecta (inferior shoulder dislocation). J Shoulder Elbow Surg. 2010;19(3):423-426.
9. Garcia R, Ponsky T, Brody F, Long J. Bilateral luxatio erecta complicated by venous thrombosis. J Trauma. 2006;60(5):1132-1134.
Historical Patterns and Variation in Treatment of Injuries in NFL (National Football League) Players and NCAA (National Collegiate Athletic Association) Division I Football Players
Among National Football League (NFL) and National Collegiate Athletic Association (NCAA) team physicians, there is no consensus on the management of various injuries. At national and regional meetings, the management of football injuries often is debated.
Given the high level of interest in the treatment of elite football players, we wanted to determine treatment patterns by surveying orthopedic team physicians. We conducted a study to determine the demographics of NFL and NCAA team physicians and to identify patterns and variations in the management of common injuries in these groups of elite football players.
Materials and Methods
The study was reviewed by an Institutional Review Board before data collection and was classified as exempt. The study population consisted of head orthopedic team physicians for NFL teams and NCAA Division I universities. The survey (Appendix),
Chi-square tests were used to determine significant differences between groups. P < .05 was considered statistically significant.
Results
Responses were received from 31 (97%) of the 32 NFL and 111 (93%) of the 119 NCAA team physicians. The 2 groups’ surveys were identical with the exception of question 3, regarding NFL division or NCAA conference.
Team Physician Demographics
All survey respondents were the head orthopedic physicians for their teams. Seventy-one percent were the head team physicians as well; another 25% named a primary care physician as the head team physician. Thirty-nine percent of the NFL team physicians had been a team physician at the NFL level for more than 15 years, and 58% of the NCAA team physicians had been a team physician at the Division I level for more than 15 years. Eighty-one percent of NFL and 66% of NCAA team physicians had fellowship training in sports medicine. For away games, 10% of NFL vs 65% of NCAA teams traveled with 2 physicians; 90% of NFL and 28% of NCAA teams traveled with 3 or more physicians.
Only a small percentage of respondents (NFL, 10%; NCAA, 14%) indicated they had received advertising in exchange for services. Most respondents (NFL, 93%; NCAA, 89%) did not pay to provide team coverage. In contrast, 97% of NFL vs only 31% of NCAA physicians indicated they received a monetary stipend for providing orthopedic coverage.
Anterior Cruciate Ligament Reconstructions
Eighty-seven percent of NFL and 67% of NCAA respondents indicated that patellar tendon autograft was their preferred graft choice (Table 1).
Anterior Shoulder Dislocations (Without Bony Bankart)
Sling use after reduction of anterior shoulder dislocation was varied, with most physicians using a sling 2 weeks or less (Table 2).
Acromioclavicular Joint Injuries
Roughly two-thirds of respondents (NFL, 60%; NCAA, 69%) indicated that, during a game, they managed acute acromioclavicular (AC) joint injuries (type I/II) with injection of a local anesthetic that allowed return to play. In addition, a majority (NFL, 90%; NCAA, 87%) indicated they gave such athletes pregame injections that allowed them to play. About half the physicians (NFL, 57%; NCAA, 52%) injected the AC joint with cortisone during the acute/subacute period (<1 month) to decrease inflammation.
No significant difference was found between the 2 groups in terms of proportion of surgeons electing to treat type III AC joint injuries operatively versus nonoperatively (Table 4).
Medial Collateral Ligament Injuries
There was a significant (P < .0001) difference in use of prophylactic bracing for medial collateral ligament (MCL) injuries (NFL, 28%; NCAA, 89%).
Posterior Cruciate Ligament Injuries
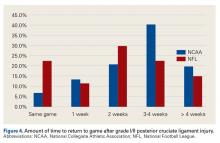
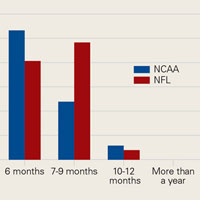
The percentage of physicians who allowed athletes to return to play after a grade I/II posterior cruciate ligament (PCL) injury was significantly (P = .01) higher in NFL physicians (22%) than in NCAA physicians (7%). The amount of time varied up to more than 4 weeks (Figure 4).
Elbow Ulnar Collateral Ligament Tears
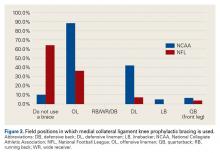
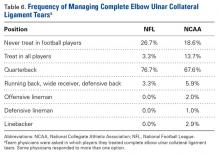
A majority of respondents indicated they would treat a complete elbow ulnar collateral ligament (UCL) tear in a quarterback; a much smaller percentage preferred operative repair in athletes playing other positions (Table 6).
Thumb Ulnar Collateral Ligament Tears
For athletes with in-season thumb UCL tears, 63% of NFL and 54% of NCAA physicians indicated they cast the thumb and allowed return to play. Others recommended operative repair and either cast the thumb and allowed return to play (NFL, 30%; NCAA, 41%) or let the thumb heal before allowing return to play (NFL, 7%; NCAA, 5%).
Fifth Metatarsal Fractures
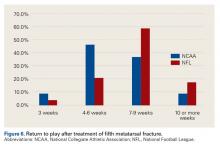
For a large majority of physicians (NFL, 100%; NCAA, 94%), the preferred treatment for fifth metatarsal fractures was screw fixation.
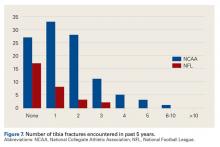
Tibia Fractures
In the 5-year period before the survey, 43% of NFL and 75% of NCAA physicians managed at least one tibia fracture (P < .001) (Figure 7).
Ketorolac Injections
Intramuscular ketorolac injections were frequently given to elite football players, significantly (P < .01) more so in the NFL (93%) than in the NCAA (62%). The average number of injections varied among physicians, though a significantly (P < .0001) higher percentage of NFL (79%) than NCAA (13%) physicians gave 5 or more injections per game.
Discussion
This survey on managing common injuries in elite football players had an overall response rate of 94%. All NFL divisions and NCAA conferences were represented in physicians’ responses. Ninety percent of NFL and 65% of NCAA head team physicians were orthopedists. These findings differ from those of Stockard1 (1997), who surveyed athletic directors at Division I schools and reported 45% of head team physicians were family medicine-trained and 41% were orthopedists.
Given the high visibility of team coverage and the economics of college football’s highest division, one might expect team physicians to receive financial remuneration. This was not the case, according to our survey: Only 30% of physicians received a monetary stipend for team coverage, and only 14% received advertising in exchange for their services. Twelve NCAA team physicians indicated they pay to be allowed to provide team coverage.
Injury Management
Anterior Cruciate Ligament Injuries. For NFL and NCAA team physicians, the preferred graft choice for ACL reconstruction was patellar tendon autograft. This finding is similar to what Erickson and colleagues2 reported from a survey of NFL and NCAA team physicians: 86% of surgeons preferred bone–patellar tendon–bone (BPTB) autograft. However, only 1 surgeon (0.7%) in that study, vs 16% in ours, preferred allograft. Allograft use may be somewhat controversial, as relevant data on competitive athletes are lacking, and it has been shown that the graft rupture rate3 is higher for BPTB allograft than for BPTB autograft in young patients. However, much of the data on higher failure rates with use of allograft in young patients4,5 has appeared since our data were collected.
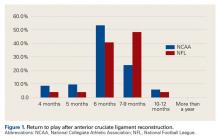
Our return-to-play data are similar to data from other studies.2,6 According to our survey, the most common length of time from ACL reconstruction to return to football was 6 months, and 94% of team physicians allowed return to football by 9 months. In the survey by Erickson and colleagues,2 55% of surgeons waited a minimum of 6 months before returning athletes to play, and only 12% waited at least 9 months. In the study by Bradley and colleagues6 (2002), 84% of surgeons waited at least 6 months before returning athletes to play. Of note, we found a significantly higher percentage of NCAA football players than NFL players returning within 6 months after surgery. The difference may be attributable to a more cautious approach being taken with NFL players, whereas most NCAA players are limited in the time remaining in their football careers and want to return to the playing field as soon as possible.
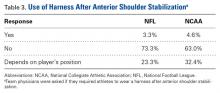
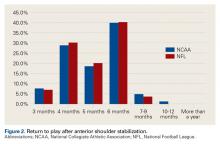
Shoulder Dislocations. Responses to the 5 survey questions on anterior shoulder dislocation showed little consensus with respect to management. The exception pertained to use of a harness for in-season return to play with a dislocation—92% of physicians preferred management with a harness. Of note, 7 of 10 team surgeons performed anterior stabilization through an arthroscopic approach. Despite historical recommendations to perform open anterior stabilization in collision athletes, NFL and NCAA physicians’ practice patterns have evolved.7 Although return to contact activity was varied among responses, 94% of physicians allowed return to contact within 6 months.
Acromioclavicular Joint Injuries. For college football players, AC joint injuries are the most common shoulder injuries.8 In the NFL Combine, the incidence of AC joint injuries was 15.7 per 100 players.8 Several studies have cited favorable results with nonoperative management of type III AC joint injuries.9-12 Nonoperative management was the preferred treatment in our study as well, yet 26% of surgeons still preferred operative treatment in quarterbacks. Opinions about operative repair of type III injuries in overhead athletes vary,13 but nonoperative management clearly is the preferred method for elite football players. A 2013 study by Lynch and colleagues14 found that only 2 of 40 NFL players with type III AC joint injuries underwent surgery.
For type I and II AC joint injuries that occur during a game, more than two-thirds of the NCAA team physicians in our study favored injecting a local anesthetic to reduce pain and allow return to play in the same game. An even larger majority indicated they gave a pregame injection of an anesthetic to allow play. Similar use of injections for AC joint injuries has been reported in Australian-rules football and rugby.15Medial Collateral Ligament Injuries. Whether bracing is prophylactic against MCL injuries is controversial.16 Some studies have found it effective.17,18 According to our survey, 89% of Division I football teams used prophylactic knee bracing, mainly in offensive linemen but frequently in defensive linemen, too. No schools used bracing in athletes who played skill positions, except quarterbacks. Six schools used bracing on a quarterback’s front leg.
The percentage of teams that used prophylactic MCL bracing was significantly higher in the NCAA than in the NFL. NCAA team physicians generally have more control over players and therefore can implement widespread use of this bracing.
Posterior Cruciate Ligament Injuries. These injuries are infrequent. According to Parolie and Bergfeld,19 only 2% of college football players at the NFL Combine had a PCL injury. Treatment in athletes remains controversial. Our survey showed physicians’ willingness to return players to competition within 4 weeks after grade I/II PCL injuries. There is no consensus on management or on postinjury bracing. In operative cases, however, the preferred graft is allograft, and the preferred repair method is the arthroscopic single-bundle technique. These findings mirror those of a 2004 survey of the Herodicus Society by Dennis and colleagues.20 Elbow Ulnar Collateral Ligament Tears. In throwing athletes with UCL tears, operative treatment has been recommended.21,22 A majority of our survey respondents preferred operative treatment for quarterbacks. However, operative treatment is still controversial, and quarterbacks differ from baseball players in their throwing motions and in the stresses acting on the UCLs during throwing. Two systematic reviews of UCL reconstruction have affirmed the positive outcomes of operative treatment in throwing athletes.21,22 However, most of the studies covered by these reviews focused on baseball players. In athletes playing positions other than quarterback, these injuries were typically treated nonoperatively.
Thumb Ulnar Collateral Ligament Tears. Our survey respondents differed in their opinions on treating thumb UCL tears. About half recommended cast treatment, and the other half recommended operative treatment. Previous data suggest that delaying surgical treatment may be deleterious to the eventual outcome.23,24Fifth Metatarsal Fractures. For fifth metatarsal fractures, screw fixation was preferred by 90% of our survey respondents—vs 73% of NFL team physicians in a 2004 study by Low and colleagues.25 What remains controversial is the length of time before return to play. Our most frequent response was 4 to 6 weeks, and 46% of our respondents indicated they would wait 7 weeks or longer. These times differ significantly from what Low and colleagues25 reported: 86% of their physicians allowed return to competition after 6 to 12 weeks.
Tibia Fractures. Management of tibia fractures in US football players has not been reported. Chang and colleagues26 described 24 tibial shaft fractures in UK soccer players. Eleven fractures (~50%) were treated with intramedullary nails, 2 with plating, and 11 with conservative management. All players returned to activity, the operative group at 23.3 weeks and the nonoperative group at 27.6 weeks. Our respondents reported treating at least 150 tibial shaft fractures in the 5-year period before our survey, demonstrating the incidence and importance of this type of injury. A vast majority of team surgeons (96%) opted for treatment with intramedullary nailing. This choice may reflect an ability to return to play earlier—the ability to move the knee and maintain strength in the legs. Some have suggested it is important to remove the nail before the player returns to the football field, but this was not common practice among our groups of team surgeons. Other studies have not found any advantage to tibial nail removal.27Ketorolac Injections. Authors have described using ketorolac for the treatment of acute or pregame pain in professional football players.28-30 According to a 2000 survey, 93% of NFL teams used intramuscular ketorolac, and on average 15 players per team were treated, primarily on game day. Our survey found frequent use of ketorolac, with almost two-thirds of team orthopedists indicating pregame use. Ketorolac use was popular, particularly because of its effect in reducing postoperative pain and its potent effect in reducing pain on game day. However, injections by football team physicians have declined significantly in recent years, ever since an NFL Physician Society task force published recommendations on ketorolac use.31
Conclusion
There is a wide variety of patterns in treating athletes who play football at the highest levels of competition. Our findings can initiate further discussion on these topics and assist orthopedists providing game coverage at all levels of play in their decision-making process by helping to define the standard of care for their injured players.
Am J Orthop. 2016;45(6):E319-E327. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Stockard AR. Team physician preferences at National Collegiate Athletic Association Division I universities. J Am Osteopath Assoc. 1997;97(2):89-95.
2. Erickson BJ, Harris JD, Fillingham YA, et al. Anterior cruciate ligament reconstruction practice patterns by NFL and NCAA football team physicians. Arthroscopy. 2014;30(6):731-738.
3. Kraeutler MJ, Bravman JT, McCarty EC. Bone-patellar tendon-bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: a meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439-2448.
4. Bottoni CR, Smith EL, Shaha J, et al. Autograft versus allograft anterior cruciate ligament reconstruction: a prospective, randomized clinical study with a minimum 10-year follow-up. Am J Sports Med. 2015;43(10):2501-2509.
5. Sun K, Tian S, Zhang J, Xia C, Zhang C, Yu T. Anterior cruciate ligament reconstruction with BPTB autograft, irradiated versus non-irradiated allograft: a prospective randomized clinical study. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):464-474.
6. Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Arthroscopy. 2002;18(5):502-509.
7. Rhee YG, Ha JH, Cho NS. Anterior shoulder stabilization in collision athletes: arthroscopic versus open Bankart repair. Am J Sports Med. 2006;34(6):979-985.
8. Brophy RH, Barnes R, Rodeo SA, Warren RF. Prevalence of musculoskeletal disorders at the NFL Combine—trends from 1987 to 2000. Med Sci Sports Exerc. 2007;39(1):22-27.
9. Bishop JY, Kaeding C. Treatment of the acute traumatic acromioclavicular separation. Sports Med Arthrosc. 2006;14(4):237-245.
10. Mazzocca AD, Arciero RA, Bicos J. Evaluation and treatment of acromioclavicular joint injuries. Am J Sports Med. 2007;35(2):316-329.
11. Schlegel TF, Burks RT, Marcus RL, Dunn HK. A prospective evaluation of untreated acute grade III acromioclavicular separations. Am J Sports Med. 2001;29(6):699-703.
12. Spencer EE Jr. Treatment of grade III acromioclavicular joint injuries: a systematic review. Clin Orthop Relat Res. 2007;(455):38-44.
13. Kraeutler MJ, Williams GR Jr, Cohen SB, et al. Inter- and intraobserver reliability of the radiographic diagnosis and treatment of acromioclavicular joint separations. Orthopedics. 2012;35(10):e1483-e1487.
14. Lynch TS, Saltzman MD, Ghodasra JH, Bilimoria KY, Bowen MK, Nuber GW. Acromioclavicular joint injuries in the National Football League: epidemiology and management. Am J Sports Med. 2013;41(12):2904-2908.
15. Orchard JW. Benefits and risks of using local anaesthetic for pain relief to allow early return to play in professional football. Br J Sports Med. 2002;36(3):209-213.
16. Salata MJ, Gibbs AE, Sekiya JK. The effectiveness of prophylactic knee bracing in American football: a systematic review. Sports Health. 2010;2(5):375-379.
17. Albright JP, Powell JW, Smith W, et al. Medial collateral ligament knee sprains in college football. Effectiveness of preventive braces. Am J Sports Med. 1994;22(1):12-18.
18. Sitler M, Ryan J, Hopkinson W, et al. The efficacy of a prophylactic knee brace to reduce knee injuries in football. A prospective, randomized study at West Point. Am J Sports Med. 1990;18(3):310-315.
19. Parolie JM, Bergfeld JA. Long-term results of nonoperative treatment of isolated posterior cruciate ligament injuries in the athlete. Am J Sports Med. 1986;14(1):35-38.
20. Dennis MG, Fox JA, Alford JW, Hayden JK, Bach BR Jr. Posterior cruciate ligament reconstruction: current trends. J Knee Surg. 2004;17(3):133-139.
21. Purcell DB, Matava MJ, Wright RW. Ulnar collateral ligament reconstruction: a systematic review. Clin Orthop Relat Res. 2007;(455):72-77.
22. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
23. Fricker R, Hintermann B. Skier’s thumb. Treatment, prevention and recommendations. Sports Med. 1995;19(1):73-79.
24. Smith RJ. Post-traumatic instability of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1977;59(1):14-21.
25. Low K, Noblin JD, Browne JE, Barnthouse CD, Scott AR. Jones fractures in the elite football player. J Surg Orthop Adv. 2004;13(3):156-160.
26. Chang WR, Kapasi Z, Daisley S, Leach WJ. Tibial shaft fractures in football players. J Orthop Surg Res. 2007;2:11.
27. Karladani AH, Ericsson PA, Granhed H, Karlsson L, Nyberg P. Tibial intramedullary nails—should they be removed? A retrospective study of 71 patients. Acta Orthop. 2007;78(5):668-671.
28. Eichner ER. Intramuscular ketorolac injections: the pregame Toradol parade. Curr Sports Med Rep. 2012;11(4):169-170.
29. Nepple JJ, Matava MJ. Soft tissue injections in the athlete. Sports Health. 2009;1(5):396-404.
30. Powell ET, Tokish JM, Hawkins RJ. Toradol use in the athletic population. Curr Sports Med Rep. 2002;1(4):191.
31. Matava M, Brater DC, Gritter N, et al. Recommendations of the National Football League physician society task force on the use of toradol® ketorolac in the National Football League. Sports Health. 2012;4(5):377-383.
Among National Football League (NFL) and National Collegiate Athletic Association (NCAA) team physicians, there is no consensus on the management of various injuries. At national and regional meetings, the management of football injuries often is debated.
Given the high level of interest in the treatment of elite football players, we wanted to determine treatment patterns by surveying orthopedic team physicians. We conducted a study to determine the demographics of NFL and NCAA team physicians and to identify patterns and variations in the management of common injuries in these groups of elite football players.
Materials and Methods
The study was reviewed by an Institutional Review Board before data collection and was classified as exempt. The study population consisted of head orthopedic team physicians for NFL teams and NCAA Division I universities. The survey (Appendix),
Chi-square tests were used to determine significant differences between groups. P < .05 was considered statistically significant.
Results
Responses were received from 31 (97%) of the 32 NFL and 111 (93%) of the 119 NCAA team physicians. The 2 groups’ surveys were identical with the exception of question 3, regarding NFL division or NCAA conference.
Team Physician Demographics
All survey respondents were the head orthopedic physicians for their teams. Seventy-one percent were the head team physicians as well; another 25% named a primary care physician as the head team physician. Thirty-nine percent of the NFL team physicians had been a team physician at the NFL level for more than 15 years, and 58% of the NCAA team physicians had been a team physician at the Division I level for more than 15 years. Eighty-one percent of NFL and 66% of NCAA team physicians had fellowship training in sports medicine. For away games, 10% of NFL vs 65% of NCAA teams traveled with 2 physicians; 90% of NFL and 28% of NCAA teams traveled with 3 or more physicians.
Only a small percentage of respondents (NFL, 10%; NCAA, 14%) indicated they had received advertising in exchange for services. Most respondents (NFL, 93%; NCAA, 89%) did not pay to provide team coverage. In contrast, 97% of NFL vs only 31% of NCAA physicians indicated they received a monetary stipend for providing orthopedic coverage.
Anterior Cruciate Ligament Reconstructions
Eighty-seven percent of NFL and 67% of NCAA respondents indicated that patellar tendon autograft was their preferred graft choice (Table 1).
Anterior Shoulder Dislocations (Without Bony Bankart)
Sling use after reduction of anterior shoulder dislocation was varied, with most physicians using a sling 2 weeks or less (Table 2).
Acromioclavicular Joint Injuries
Roughly two-thirds of respondents (NFL, 60%; NCAA, 69%) indicated that, during a game, they managed acute acromioclavicular (AC) joint injuries (type I/II) with injection of a local anesthetic that allowed return to play. In addition, a majority (NFL, 90%; NCAA, 87%) indicated they gave such athletes pregame injections that allowed them to play. About half the physicians (NFL, 57%; NCAA, 52%) injected the AC joint with cortisone during the acute/subacute period (<1 month) to decrease inflammation.
No significant difference was found between the 2 groups in terms of proportion of surgeons electing to treat type III AC joint injuries operatively versus nonoperatively (Table 4).
Medial Collateral Ligament Injuries
There was a significant (P < .0001) difference in use of prophylactic bracing for medial collateral ligament (MCL) injuries (NFL, 28%; NCAA, 89%).
Posterior Cruciate Ligament Injuries
The percentage of physicians who allowed athletes to return to play after a grade I/II posterior cruciate ligament (PCL) injury was significantly (P = .01) higher in NFL physicians (22%) than in NCAA physicians (7%). The amount of time varied up to more than 4 weeks (Figure 4).
Elbow Ulnar Collateral Ligament Tears
A majority of respondents indicated they would treat a complete elbow ulnar collateral ligament (UCL) tear in a quarterback; a much smaller percentage preferred operative repair in athletes playing other positions (Table 6).
Thumb Ulnar Collateral Ligament Tears
For athletes with in-season thumb UCL tears, 63% of NFL and 54% of NCAA physicians indicated they cast the thumb and allowed return to play. Others recommended operative repair and either cast the thumb and allowed return to play (NFL, 30%; NCAA, 41%) or let the thumb heal before allowing return to play (NFL, 7%; NCAA, 5%).
Fifth Metatarsal Fractures
For a large majority of physicians (NFL, 100%; NCAA, 94%), the preferred treatment for fifth metatarsal fractures was screw fixation.
Tibia Fractures
In the 5-year period before the survey, 43% of NFL and 75% of NCAA physicians managed at least one tibia fracture (P < .001) (Figure 7).
Ketorolac Injections
Intramuscular ketorolac injections were frequently given to elite football players, significantly (P < .01) more so in the NFL (93%) than in the NCAA (62%). The average number of injections varied among physicians, though a significantly (P < .0001) higher percentage of NFL (79%) than NCAA (13%) physicians gave 5 or more injections per game.
Discussion
This survey on managing common injuries in elite football players had an overall response rate of 94%. All NFL divisions and NCAA conferences were represented in physicians’ responses. Ninety percent of NFL and 65% of NCAA head team physicians were orthopedists. These findings differ from those of Stockard1 (1997), who surveyed athletic directors at Division I schools and reported 45% of head team physicians were family medicine-trained and 41% were orthopedists.
Given the high visibility of team coverage and the economics of college football’s highest division, one might expect team physicians to receive financial remuneration. This was not the case, according to our survey: Only 30% of physicians received a monetary stipend for team coverage, and only 14% received advertising in exchange for their services. Twelve NCAA team physicians indicated they pay to be allowed to provide team coverage.
Injury Management
Anterior Cruciate Ligament Injuries. For NFL and NCAA team physicians, the preferred graft choice for ACL reconstruction was patellar tendon autograft. This finding is similar to what Erickson and colleagues2 reported from a survey of NFL and NCAA team physicians: 86% of surgeons preferred bone–patellar tendon–bone (BPTB) autograft. However, only 1 surgeon (0.7%) in that study, vs 16% in ours, preferred allograft. Allograft use may be somewhat controversial, as relevant data on competitive athletes are lacking, and it has been shown that the graft rupture rate3 is higher for BPTB allograft than for BPTB autograft in young patients. However, much of the data on higher failure rates with use of allograft in young patients4,5 has appeared since our data were collected.
Our return-to-play data are similar to data from other studies.2,6 According to our survey, the most common length of time from ACL reconstruction to return to football was 6 months, and 94% of team physicians allowed return to football by 9 months. In the survey by Erickson and colleagues,2 55% of surgeons waited a minimum of 6 months before returning athletes to play, and only 12% waited at least 9 months. In the study by Bradley and colleagues6 (2002), 84% of surgeons waited at least 6 months before returning athletes to play. Of note, we found a significantly higher percentage of NCAA football players than NFL players returning within 6 months after surgery. The difference may be attributable to a more cautious approach being taken with NFL players, whereas most NCAA players are limited in the time remaining in their football careers and want to return to the playing field as soon as possible.
Shoulder Dislocations. Responses to the 5 survey questions on anterior shoulder dislocation showed little consensus with respect to management. The exception pertained to use of a harness for in-season return to play with a dislocation—92% of physicians preferred management with a harness. Of note, 7 of 10 team surgeons performed anterior stabilization through an arthroscopic approach. Despite historical recommendations to perform open anterior stabilization in collision athletes, NFL and NCAA physicians’ practice patterns have evolved.7 Although return to contact activity was varied among responses, 94% of physicians allowed return to contact within 6 months.
Acromioclavicular Joint Injuries. For college football players, AC joint injuries are the most common shoulder injuries.8 In the NFL Combine, the incidence of AC joint injuries was 15.7 per 100 players.8 Several studies have cited favorable results with nonoperative management of type III AC joint injuries.9-12 Nonoperative management was the preferred treatment in our study as well, yet 26% of surgeons still preferred operative treatment in quarterbacks. Opinions about operative repair of type III injuries in overhead athletes vary,13 but nonoperative management clearly is the preferred method for elite football players. A 2013 study by Lynch and colleagues14 found that only 2 of 40 NFL players with type III AC joint injuries underwent surgery.
For type I and II AC joint injuries that occur during a game, more than two-thirds of the NCAA team physicians in our study favored injecting a local anesthetic to reduce pain and allow return to play in the same game. An even larger majority indicated they gave a pregame injection of an anesthetic to allow play. Similar use of injections for AC joint injuries has been reported in Australian-rules football and rugby.15Medial Collateral Ligament Injuries. Whether bracing is prophylactic against MCL injuries is controversial.16 Some studies have found it effective.17,18 According to our survey, 89% of Division I football teams used prophylactic knee bracing, mainly in offensive linemen but frequently in defensive linemen, too. No schools used bracing in athletes who played skill positions, except quarterbacks. Six schools used bracing on a quarterback’s front leg.
The percentage of teams that used prophylactic MCL bracing was significantly higher in the NCAA than in the NFL. NCAA team physicians generally have more control over players and therefore can implement widespread use of this bracing.
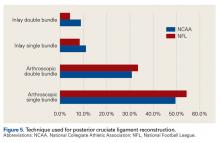
Posterior Cruciate Ligament Injuries. These injuries are infrequent. According to Parolie and Bergfeld,19 only 2% of college football players at the NFL Combine had a PCL injury. Treatment in athletes remains controversial. Our survey showed physicians’ willingness to return players to competition within 4 weeks after grade I/II PCL injuries. There is no consensus on management or on postinjury bracing. In operative cases, however, the preferred graft is allograft, and the preferred repair method is the arthroscopic single-bundle technique. These findings mirror those of a 2004 survey of the Herodicus Society by Dennis and colleagues.20 Elbow Ulnar Collateral Ligament Tears. In throwing athletes with UCL tears, operative treatment has been recommended.21,22 A majority of our survey respondents preferred operative treatment for quarterbacks. However, operative treatment is still controversial, and quarterbacks differ from baseball players in their throwing motions and in the stresses acting on the UCLs during throwing. Two systematic reviews of UCL reconstruction have affirmed the positive outcomes of operative treatment in throwing athletes.21,22 However, most of the studies covered by these reviews focused on baseball players. In athletes playing positions other than quarterback, these injuries were typically treated nonoperatively.
Thumb Ulnar Collateral Ligament Tears. Our survey respondents differed in their opinions on treating thumb UCL tears. About half recommended cast treatment, and the other half recommended operative treatment. Previous data suggest that delaying surgical treatment may be deleterious to the eventual outcome.23,24Fifth Metatarsal Fractures. For fifth metatarsal fractures, screw fixation was preferred by 90% of our survey respondents—vs 73% of NFL team physicians in a 2004 study by Low and colleagues.25 What remains controversial is the length of time before return to play. Our most frequent response was 4 to 6 weeks, and 46% of our respondents indicated they would wait 7 weeks or longer. These times differ significantly from what Low and colleagues25 reported: 86% of their physicians allowed return to competition after 6 to 12 weeks.
Tibia Fractures. Management of tibia fractures in US football players has not been reported. Chang and colleagues26 described 24 tibial shaft fractures in UK soccer players. Eleven fractures (~50%) were treated with intramedullary nails, 2 with plating, and 11 with conservative management. All players returned to activity, the operative group at 23.3 weeks and the nonoperative group at 27.6 weeks. Our respondents reported treating at least 150 tibial shaft fractures in the 5-year period before our survey, demonstrating the incidence and importance of this type of injury. A vast majority of team surgeons (96%) opted for treatment with intramedullary nailing. This choice may reflect an ability to return to play earlier—the ability to move the knee and maintain strength in the legs. Some have suggested it is important to remove the nail before the player returns to the football field, but this was not common practice among our groups of team surgeons. Other studies have not found any advantage to tibial nail removal.27Ketorolac Injections. Authors have described using ketorolac for the treatment of acute or pregame pain in professional football players.28-30 According to a 2000 survey, 93% of NFL teams used intramuscular ketorolac, and on average 15 players per team were treated, primarily on game day. Our survey found frequent use of ketorolac, with almost two-thirds of team orthopedists indicating pregame use. Ketorolac use was popular, particularly because of its effect in reducing postoperative pain and its potent effect in reducing pain on game day. However, injections by football team physicians have declined significantly in recent years, ever since an NFL Physician Society task force published recommendations on ketorolac use.31
Conclusion
There is a wide variety of patterns in treating athletes who play football at the highest levels of competition. Our findings can initiate further discussion on these topics and assist orthopedists providing game coverage at all levels of play in their decision-making process by helping to define the standard of care for their injured players.
Am J Orthop. 2016;45(6):E319-E327. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Among National Football League (NFL) and National Collegiate Athletic Association (NCAA) team physicians, there is no consensus on the management of various injuries. At national and regional meetings, the management of football injuries often is debated.
Given the high level of interest in the treatment of elite football players, we wanted to determine treatment patterns by surveying orthopedic team physicians. We conducted a study to determine the demographics of NFL and NCAA team physicians and to identify patterns and variations in the management of common injuries in these groups of elite football players.
Materials and Methods
The study was reviewed by an Institutional Review Board before data collection and was classified as exempt. The study population consisted of head orthopedic team physicians for NFL teams and NCAA Division I universities. The survey (Appendix),
Chi-square tests were used to determine significant differences between groups. P < .05 was considered statistically significant.
Results
Responses were received from 31 (97%) of the 32 NFL and 111 (93%) of the 119 NCAA team physicians. The 2 groups’ surveys were identical with the exception of question 3, regarding NFL division or NCAA conference.
Team Physician Demographics
All survey respondents were the head orthopedic physicians for their teams. Seventy-one percent were the head team physicians as well; another 25% named a primary care physician as the head team physician. Thirty-nine percent of the NFL team physicians had been a team physician at the NFL level for more than 15 years, and 58% of the NCAA team physicians had been a team physician at the Division I level for more than 15 years. Eighty-one percent of NFL and 66% of NCAA team physicians had fellowship training in sports medicine. For away games, 10% of NFL vs 65% of NCAA teams traveled with 2 physicians; 90% of NFL and 28% of NCAA teams traveled with 3 or more physicians.
Only a small percentage of respondents (NFL, 10%; NCAA, 14%) indicated they had received advertising in exchange for services. Most respondents (NFL, 93%; NCAA, 89%) did not pay to provide team coverage. In contrast, 97% of NFL vs only 31% of NCAA physicians indicated they received a monetary stipend for providing orthopedic coverage.
Anterior Cruciate Ligament Reconstructions
Eighty-seven percent of NFL and 67% of NCAA respondents indicated that patellar tendon autograft was their preferred graft choice (Table 1).
Anterior Shoulder Dislocations (Without Bony Bankart)
Sling use after reduction of anterior shoulder dislocation was varied, with most physicians using a sling 2 weeks or less (Table 2).
Acromioclavicular Joint Injuries
Roughly two-thirds of respondents (NFL, 60%; NCAA, 69%) indicated that, during a game, they managed acute acromioclavicular (AC) joint injuries (type I/II) with injection of a local anesthetic that allowed return to play. In addition, a majority (NFL, 90%; NCAA, 87%) indicated they gave such athletes pregame injections that allowed them to play. About half the physicians (NFL, 57%; NCAA, 52%) injected the AC joint with cortisone during the acute/subacute period (<1 month) to decrease inflammation.
No significant difference was found between the 2 groups in terms of proportion of surgeons electing to treat type III AC joint injuries operatively versus nonoperatively (Table 4).
Medial Collateral Ligament Injuries
There was a significant (P < .0001) difference in use of prophylactic bracing for medial collateral ligament (MCL) injuries (NFL, 28%; NCAA, 89%).
Posterior Cruciate Ligament Injuries
The percentage of physicians who allowed athletes to return to play after a grade I/II posterior cruciate ligament (PCL) injury was significantly (P = .01) higher in NFL physicians (22%) than in NCAA physicians (7%). The amount of time varied up to more than 4 weeks (Figure 4).
Elbow Ulnar Collateral Ligament Tears
A majority of respondents indicated they would treat a complete elbow ulnar collateral ligament (UCL) tear in a quarterback; a much smaller percentage preferred operative repair in athletes playing other positions (Table 6).
Thumb Ulnar Collateral Ligament Tears
For athletes with in-season thumb UCL tears, 63% of NFL and 54% of NCAA physicians indicated they cast the thumb and allowed return to play. Others recommended operative repair and either cast the thumb and allowed return to play (NFL, 30%; NCAA, 41%) or let the thumb heal before allowing return to play (NFL, 7%; NCAA, 5%).
Fifth Metatarsal Fractures
For a large majority of physicians (NFL, 100%; NCAA, 94%), the preferred treatment for fifth metatarsal fractures was screw fixation.
Tibia Fractures
In the 5-year period before the survey, 43% of NFL and 75% of NCAA physicians managed at least one tibia fracture (P < .001) (Figure 7).
Ketorolac Injections
Intramuscular ketorolac injections were frequently given to elite football players, significantly (P < .01) more so in the NFL (93%) than in the NCAA (62%). The average number of injections varied among physicians, though a significantly (P < .0001) higher percentage of NFL (79%) than NCAA (13%) physicians gave 5 or more injections per game.
Discussion
This survey on managing common injuries in elite football players had an overall response rate of 94%. All NFL divisions and NCAA conferences were represented in physicians’ responses. Ninety percent of NFL and 65% of NCAA head team physicians were orthopedists. These findings differ from those of Stockard1 (1997), who surveyed athletic directors at Division I schools and reported 45% of head team physicians were family medicine-trained and 41% were orthopedists.
Given the high visibility of team coverage and the economics of college football’s highest division, one might expect team physicians to receive financial remuneration. This was not the case, according to our survey: Only 30% of physicians received a monetary stipend for team coverage, and only 14% received advertising in exchange for their services. Twelve NCAA team physicians indicated they pay to be allowed to provide team coverage.
Injury Management
Anterior Cruciate Ligament Injuries. For NFL and NCAA team physicians, the preferred graft choice for ACL reconstruction was patellar tendon autograft. This finding is similar to what Erickson and colleagues2 reported from a survey of NFL and NCAA team physicians: 86% of surgeons preferred bone–patellar tendon–bone (BPTB) autograft. However, only 1 surgeon (0.7%) in that study, vs 16% in ours, preferred allograft. Allograft use may be somewhat controversial, as relevant data on competitive athletes are lacking, and it has been shown that the graft rupture rate3 is higher for BPTB allograft than for BPTB autograft in young patients. However, much of the data on higher failure rates with use of allograft in young patients4,5 has appeared since our data were collected.
Our return-to-play data are similar to data from other studies.2,6 According to our survey, the most common length of time from ACL reconstruction to return to football was 6 months, and 94% of team physicians allowed return to football by 9 months. In the survey by Erickson and colleagues,2 55% of surgeons waited a minimum of 6 months before returning athletes to play, and only 12% waited at least 9 months. In the study by Bradley and colleagues6 (2002), 84% of surgeons waited at least 6 months before returning athletes to play. Of note, we found a significantly higher percentage of NCAA football players than NFL players returning within 6 months after surgery. The difference may be attributable to a more cautious approach being taken with NFL players, whereas most NCAA players are limited in the time remaining in their football careers and want to return to the playing field as soon as possible.
Shoulder Dislocations. Responses to the 5 survey questions on anterior shoulder dislocation showed little consensus with respect to management. The exception pertained to use of a harness for in-season return to play with a dislocation—92% of physicians preferred management with a harness. Of note, 7 of 10 team surgeons performed anterior stabilization through an arthroscopic approach. Despite historical recommendations to perform open anterior stabilization in collision athletes, NFL and NCAA physicians’ practice patterns have evolved.7 Although return to contact activity was varied among responses, 94% of physicians allowed return to contact within 6 months.
Acromioclavicular Joint Injuries. For college football players, AC joint injuries are the most common shoulder injuries.8 In the NFL Combine, the incidence of AC joint injuries was 15.7 per 100 players.8 Several studies have cited favorable results with nonoperative management of type III AC joint injuries.9-12 Nonoperative management was the preferred treatment in our study as well, yet 26% of surgeons still preferred operative treatment in quarterbacks. Opinions about operative repair of type III injuries in overhead athletes vary,13 but nonoperative management clearly is the preferred method for elite football players. A 2013 study by Lynch and colleagues14 found that only 2 of 40 NFL players with type III AC joint injuries underwent surgery.
For type I and II AC joint injuries that occur during a game, more than two-thirds of the NCAA team physicians in our study favored injecting a local anesthetic to reduce pain and allow return to play in the same game. An even larger majority indicated they gave a pregame injection of an anesthetic to allow play. Similar use of injections for AC joint injuries has been reported in Australian-rules football and rugby.15Medial Collateral Ligament Injuries. Whether bracing is prophylactic against MCL injuries is controversial.16 Some studies have found it effective.17,18 According to our survey, 89% of Division I football teams used prophylactic knee bracing, mainly in offensive linemen but frequently in defensive linemen, too. No schools used bracing in athletes who played skill positions, except quarterbacks. Six schools used bracing on a quarterback’s front leg.
The percentage of teams that used prophylactic MCL bracing was significantly higher in the NCAA than in the NFL. NCAA team physicians generally have more control over players and therefore can implement widespread use of this bracing.
Posterior Cruciate Ligament Injuries. These injuries are infrequent. According to Parolie and Bergfeld,19 only 2% of college football players at the NFL Combine had a PCL injury. Treatment in athletes remains controversial. Our survey showed physicians’ willingness to return players to competition within 4 weeks after grade I/II PCL injuries. There is no consensus on management or on postinjury bracing. In operative cases, however, the preferred graft is allograft, and the preferred repair method is the arthroscopic single-bundle technique. These findings mirror those of a 2004 survey of the Herodicus Society by Dennis and colleagues.20 Elbow Ulnar Collateral Ligament Tears. In throwing athletes with UCL tears, operative treatment has been recommended.21,22 A majority of our survey respondents preferred operative treatment for quarterbacks. However, operative treatment is still controversial, and quarterbacks differ from baseball players in their throwing motions and in the stresses acting on the UCLs during throwing. Two systematic reviews of UCL reconstruction have affirmed the positive outcomes of operative treatment in throwing athletes.21,22 However, most of the studies covered by these reviews focused on baseball players. In athletes playing positions other than quarterback, these injuries were typically treated nonoperatively.
Thumb Ulnar Collateral Ligament Tears. Our survey respondents differed in their opinions on treating thumb UCL tears. About half recommended cast treatment, and the other half recommended operative treatment. Previous data suggest that delaying surgical treatment may be deleterious to the eventual outcome.23,24Fifth Metatarsal Fractures. For fifth metatarsal fractures, screw fixation was preferred by 90% of our survey respondents—vs 73% of NFL team physicians in a 2004 study by Low and colleagues.25 What remains controversial is the length of time before return to play. Our most frequent response was 4 to 6 weeks, and 46% of our respondents indicated they would wait 7 weeks or longer. These times differ significantly from what Low and colleagues25 reported: 86% of their physicians allowed return to competition after 6 to 12 weeks.
Tibia Fractures. Management of tibia fractures in US football players has not been reported. Chang and colleagues26 described 24 tibial shaft fractures in UK soccer players. Eleven fractures (~50%) were treated with intramedullary nails, 2 with plating, and 11 with conservative management. All players returned to activity, the operative group at 23.3 weeks and the nonoperative group at 27.6 weeks. Our respondents reported treating at least 150 tibial shaft fractures in the 5-year period before our survey, demonstrating the incidence and importance of this type of injury. A vast majority of team surgeons (96%) opted for treatment with intramedullary nailing. This choice may reflect an ability to return to play earlier—the ability to move the knee and maintain strength in the legs. Some have suggested it is important to remove the nail before the player returns to the football field, but this was not common practice among our groups of team surgeons. Other studies have not found any advantage to tibial nail removal.27Ketorolac Injections. Authors have described using ketorolac for the treatment of acute or pregame pain in professional football players.28-30 According to a 2000 survey, 93% of NFL teams used intramuscular ketorolac, and on average 15 players per team were treated, primarily on game day. Our survey found frequent use of ketorolac, with almost two-thirds of team orthopedists indicating pregame use. Ketorolac use was popular, particularly because of its effect in reducing postoperative pain and its potent effect in reducing pain on game day. However, injections by football team physicians have declined significantly in recent years, ever since an NFL Physician Society task force published recommendations on ketorolac use.31
Conclusion
There is a wide variety of patterns in treating athletes who play football at the highest levels of competition. Our findings can initiate further discussion on these topics and assist orthopedists providing game coverage at all levels of play in their decision-making process by helping to define the standard of care for their injured players.
Am J Orthop. 2016;45(6):E319-E327. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Stockard AR. Team physician preferences at National Collegiate Athletic Association Division I universities. J Am Osteopath Assoc. 1997;97(2):89-95.
2. Erickson BJ, Harris JD, Fillingham YA, et al. Anterior cruciate ligament reconstruction practice patterns by NFL and NCAA football team physicians. Arthroscopy. 2014;30(6):731-738.
3. Kraeutler MJ, Bravman JT, McCarty EC. Bone-patellar tendon-bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: a meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439-2448.
4. Bottoni CR, Smith EL, Shaha J, et al. Autograft versus allograft anterior cruciate ligament reconstruction: a prospective, randomized clinical study with a minimum 10-year follow-up. Am J Sports Med. 2015;43(10):2501-2509.
5. Sun K, Tian S, Zhang J, Xia C, Zhang C, Yu T. Anterior cruciate ligament reconstruction with BPTB autograft, irradiated versus non-irradiated allograft: a prospective randomized clinical study. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):464-474.
6. Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Arthroscopy. 2002;18(5):502-509.
7. Rhee YG, Ha JH, Cho NS. Anterior shoulder stabilization in collision athletes: arthroscopic versus open Bankart repair. Am J Sports Med. 2006;34(6):979-985.
8. Brophy RH, Barnes R, Rodeo SA, Warren RF. Prevalence of musculoskeletal disorders at the NFL Combine—trends from 1987 to 2000. Med Sci Sports Exerc. 2007;39(1):22-27.
9. Bishop JY, Kaeding C. Treatment of the acute traumatic acromioclavicular separation. Sports Med Arthrosc. 2006;14(4):237-245.
10. Mazzocca AD, Arciero RA, Bicos J. Evaluation and treatment of acromioclavicular joint injuries. Am J Sports Med. 2007;35(2):316-329.
11. Schlegel TF, Burks RT, Marcus RL, Dunn HK. A prospective evaluation of untreated acute grade III acromioclavicular separations. Am J Sports Med. 2001;29(6):699-703.
12. Spencer EE Jr. Treatment of grade III acromioclavicular joint injuries: a systematic review. Clin Orthop Relat Res. 2007;(455):38-44.
13. Kraeutler MJ, Williams GR Jr, Cohen SB, et al. Inter- and intraobserver reliability of the radiographic diagnosis and treatment of acromioclavicular joint separations. Orthopedics. 2012;35(10):e1483-e1487.
14. Lynch TS, Saltzman MD, Ghodasra JH, Bilimoria KY, Bowen MK, Nuber GW. Acromioclavicular joint injuries in the National Football League: epidemiology and management. Am J Sports Med. 2013;41(12):2904-2908.
15. Orchard JW. Benefits and risks of using local anaesthetic for pain relief to allow early return to play in professional football. Br J Sports Med. 2002;36(3):209-213.
16. Salata MJ, Gibbs AE, Sekiya JK. The effectiveness of prophylactic knee bracing in American football: a systematic review. Sports Health. 2010;2(5):375-379.
17. Albright JP, Powell JW, Smith W, et al. Medial collateral ligament knee sprains in college football. Effectiveness of preventive braces. Am J Sports Med. 1994;22(1):12-18.
18. Sitler M, Ryan J, Hopkinson W, et al. The efficacy of a prophylactic knee brace to reduce knee injuries in football. A prospective, randomized study at West Point. Am J Sports Med. 1990;18(3):310-315.
19. Parolie JM, Bergfeld JA. Long-term results of nonoperative treatment of isolated posterior cruciate ligament injuries in the athlete. Am J Sports Med. 1986;14(1):35-38.
20. Dennis MG, Fox JA, Alford JW, Hayden JK, Bach BR Jr. Posterior cruciate ligament reconstruction: current trends. J Knee Surg. 2004;17(3):133-139.
21. Purcell DB, Matava MJ, Wright RW. Ulnar collateral ligament reconstruction: a systematic review. Clin Orthop Relat Res. 2007;(455):72-77.
22. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
23. Fricker R, Hintermann B. Skier’s thumb. Treatment, prevention and recommendations. Sports Med. 1995;19(1):73-79.
24. Smith RJ. Post-traumatic instability of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1977;59(1):14-21.
25. Low K, Noblin JD, Browne JE, Barnthouse CD, Scott AR. Jones fractures in the elite football player. J Surg Orthop Adv. 2004;13(3):156-160.
26. Chang WR, Kapasi Z, Daisley S, Leach WJ. Tibial shaft fractures in football players. J Orthop Surg Res. 2007;2:11.
27. Karladani AH, Ericsson PA, Granhed H, Karlsson L, Nyberg P. Tibial intramedullary nails—should they be removed? A retrospective study of 71 patients. Acta Orthop. 2007;78(5):668-671.
28. Eichner ER. Intramuscular ketorolac injections: the pregame Toradol parade. Curr Sports Med Rep. 2012;11(4):169-170.
29. Nepple JJ, Matava MJ. Soft tissue injections in the athlete. Sports Health. 2009;1(5):396-404.
30. Powell ET, Tokish JM, Hawkins RJ. Toradol use in the athletic population. Curr Sports Med Rep. 2002;1(4):191.
31. Matava M, Brater DC, Gritter N, et al. Recommendations of the National Football League physician society task force on the use of toradol® ketorolac in the National Football League. Sports Health. 2012;4(5):377-383.
1. Stockard AR. Team physician preferences at National Collegiate Athletic Association Division I universities. J Am Osteopath Assoc. 1997;97(2):89-95.
2. Erickson BJ, Harris JD, Fillingham YA, et al. Anterior cruciate ligament reconstruction practice patterns by NFL and NCAA football team physicians. Arthroscopy. 2014;30(6):731-738.
3. Kraeutler MJ, Bravman JT, McCarty EC. Bone-patellar tendon-bone autograft versus allograft in outcomes of anterior cruciate ligament reconstruction: a meta-analysis of 5182 patients. Am J Sports Med. 2013;41(10):2439-2448.
4. Bottoni CR, Smith EL, Shaha J, et al. Autograft versus allograft anterior cruciate ligament reconstruction: a prospective, randomized clinical study with a minimum 10-year follow-up. Am J Sports Med. 2015;43(10):2501-2509.
5. Sun K, Tian S, Zhang J, Xia C, Zhang C, Yu T. Anterior cruciate ligament reconstruction with BPTB autograft, irradiated versus non-irradiated allograft: a prospective randomized clinical study. Knee Surg Sports Traumatol Arthrosc. 2009;17(5):464-474.
6. Bradley JP, Klimkiewicz JJ, Rytel MJ, Powell JW. Anterior cruciate ligament injuries in the National Football League: epidemiology and current treatment trends among team physicians. Arthroscopy. 2002;18(5):502-509.
7. Rhee YG, Ha JH, Cho NS. Anterior shoulder stabilization in collision athletes: arthroscopic versus open Bankart repair. Am J Sports Med. 2006;34(6):979-985.
8. Brophy RH, Barnes R, Rodeo SA, Warren RF. Prevalence of musculoskeletal disorders at the NFL Combine—trends from 1987 to 2000. Med Sci Sports Exerc. 2007;39(1):22-27.
9. Bishop JY, Kaeding C. Treatment of the acute traumatic acromioclavicular separation. Sports Med Arthrosc. 2006;14(4):237-245.
10. Mazzocca AD, Arciero RA, Bicos J. Evaluation and treatment of acromioclavicular joint injuries. Am J Sports Med. 2007;35(2):316-329.
11. Schlegel TF, Burks RT, Marcus RL, Dunn HK. A prospective evaluation of untreated acute grade III acromioclavicular separations. Am J Sports Med. 2001;29(6):699-703.
12. Spencer EE Jr. Treatment of grade III acromioclavicular joint injuries: a systematic review. Clin Orthop Relat Res. 2007;(455):38-44.
13. Kraeutler MJ, Williams GR Jr, Cohen SB, et al. Inter- and intraobserver reliability of the radiographic diagnosis and treatment of acromioclavicular joint separations. Orthopedics. 2012;35(10):e1483-e1487.
14. Lynch TS, Saltzman MD, Ghodasra JH, Bilimoria KY, Bowen MK, Nuber GW. Acromioclavicular joint injuries in the National Football League: epidemiology and management. Am J Sports Med. 2013;41(12):2904-2908.
15. Orchard JW. Benefits and risks of using local anaesthetic for pain relief to allow early return to play in professional football. Br J Sports Med. 2002;36(3):209-213.
16. Salata MJ, Gibbs AE, Sekiya JK. The effectiveness of prophylactic knee bracing in American football: a systematic review. Sports Health. 2010;2(5):375-379.
17. Albright JP, Powell JW, Smith W, et al. Medial collateral ligament knee sprains in college football. Effectiveness of preventive braces. Am J Sports Med. 1994;22(1):12-18.
18. Sitler M, Ryan J, Hopkinson W, et al. The efficacy of a prophylactic knee brace to reduce knee injuries in football. A prospective, randomized study at West Point. Am J Sports Med. 1990;18(3):310-315.
19. Parolie JM, Bergfeld JA. Long-term results of nonoperative treatment of isolated posterior cruciate ligament injuries in the athlete. Am J Sports Med. 1986;14(1):35-38.
20. Dennis MG, Fox JA, Alford JW, Hayden JK, Bach BR Jr. Posterior cruciate ligament reconstruction: current trends. J Knee Surg. 2004;17(3):133-139.
21. Purcell DB, Matava MJ, Wright RW. Ulnar collateral ligament reconstruction: a systematic review. Clin Orthop Relat Res. 2007;(455):72-77.
22. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
23. Fricker R, Hintermann B. Skier’s thumb. Treatment, prevention and recommendations. Sports Med. 1995;19(1):73-79.
24. Smith RJ. Post-traumatic instability of the metacarpophalangeal joint of the thumb. J Bone Joint Surg Am. 1977;59(1):14-21.
25. Low K, Noblin JD, Browne JE, Barnthouse CD, Scott AR. Jones fractures in the elite football player. J Surg Orthop Adv. 2004;13(3):156-160.
26. Chang WR, Kapasi Z, Daisley S, Leach WJ. Tibial shaft fractures in football players. J Orthop Surg Res. 2007;2:11.
27. Karladani AH, Ericsson PA, Granhed H, Karlsson L, Nyberg P. Tibial intramedullary nails—should they be removed? A retrospective study of 71 patients. Acta Orthop. 2007;78(5):668-671.
28. Eichner ER. Intramuscular ketorolac injections: the pregame Toradol parade. Curr Sports Med Rep. 2012;11(4):169-170.
29. Nepple JJ, Matava MJ. Soft tissue injections in the athlete. Sports Health. 2009;1(5):396-404.
30. Powell ET, Tokish JM, Hawkins RJ. Toradol use in the athletic population. Curr Sports Med Rep. 2002;1(4):191.
31. Matava M, Brater DC, Gritter N, et al. Recommendations of the National Football League physician society task force on the use of toradol® ketorolac in the National Football League. Sports Health. 2012;4(5):377-383.
Finding Balance: New Strategies to Optimize Care for Patients With Parkinson’s Disease Psychosis
This activity is supported by an independent educational grant from ACADIA Pharmaceuticals LLC
After reading this CME supplement, health care providers will have improved their ability to:
|
This CME supplement entitles the reader to 1.0 AMA PRA Category 1 Credit™
Click here to read supplement.
After reading the supplement, click here to access the CME posttest
This activity is supported by an independent educational grant from ACADIA Pharmaceuticals LLC
After reading this CME supplement, health care providers will have improved their ability to:
|
This CME supplement entitles the reader to 1.0 AMA PRA Category 1 Credit™
Click here to read supplement.
After reading the supplement, click here to access the CME posttest
This activity is supported by an independent educational grant from ACADIA Pharmaceuticals LLC
After reading this CME supplement, health care providers will have improved their ability to:
|
This CME supplement entitles the reader to 1.0 AMA PRA Category 1 Credit™
Click here to read supplement.
After reading the supplement, click here to access the CME posttest
Thigh Injuries in American Football
American football has the highest injury rate of any team sport in the United States at the high school, collegiate, and professional levels.1-3 Muscle strains and contusions constitute a large proportion of football injuries. For example, at the high school level, muscle strains comprise 12% to 24% of all injuries;2 at the collegiate level, they account for approximately 20% of all practice injuries, with nearly half of all strains occurring within the thigh.1,4 Among a single National Football League (NFL) team, Feeley and colleagues5 reported that muscle strains accounted for 46% of practice and 22% of preseason game injuries. The hamstrings, followed by the quadriceps, are the most commonly strained muscle groups among both professional and amateur athletes,5,6 with hamstring and quadriceps injuries making up approximately 13% of all injuries among NFL players.7 Given the relatively large surface area and muscle volume of the anterior and posterior thigh, as well as the activities and maneuvers necessitated by the various football positions, it is not surprising that the thigh is frequently involved in football-related injuries.
The purpose of this review is to describe the clinical manifestations of thigh-related soft-tissue injuries seen in football players. Two of these conditions—muscle strains and contusions—are relatively common, while a third condition—the Morel-Lavallée lesion—is a rare, yet relevant injury that warrants discussion.
Quadriceps Contusion
Pathophysiology
Contusion to the quadriceps muscle is a common injury in contact sports generally resulting from a direct blow from a helmet, knee, or shoulder.8 Bleeding within the musculature causes swelling, pain, stiffness, and limitation of quadriceps excursion, ultimately resulting in loss of knee flexion and an inability to run or squat. The injury is typically confined to a single quadriceps muscle.8 The use of thigh padding, though helpful, does not completely eliminate the risk of this injury.
History and Physical Examination
Immediately after injury, the athlete may complain only of thigh pain. However, swelling, pain, and diminished range of knee motion may develop within the first 24 hours depending on the severity of injury and how quickly treatment is instituted.8 Jackson and Feagin9 developed an injury grading system for quadriceps contusions based on the limitation of knee flexion observed (Table 1).
Imaging
A quadriceps contusion is a clinical diagnosis based on a typical history and physical examination; therefore, advanced imaging usually does not need to be obtained except to gauge the severity of injury, to rule out concurrent injuries (ie, tendon rupture), and to identify the presence of a hematoma that may necessitate aspiration. Plain radiographs are typically unremarkable in the acute setting. Appearance on magnetic resonance imaging (MRI) varies by injury severity, with increased signal throughout the affected muscle belly and a diffuse, feathery appearance centered at the point of impact on short TI inversion recovery (STIR) and T2-weighted images reflecting edema and possibly hematoma (Figures 1A-1C).8,11
Treatment
Treatment of a quadriceps contusion is nonoperative and consists of a 3-phase recovery.10 The first phase lasts approximately 2 days and consists of rest, ice, compression, and elevation (RICE) to limit hemorrhage. The knee should be rested in a flexed position to maintain quadriceps muscle fiber length in order to promote muscle compression and limit knee stiffness. For severe contusions in which there is a question of an acute thigh compartment syndrome, compression should be avoided with appropriate treatment based on typical symptoms and intra-compartmental pressure measurement.12 Nonsteroidal anti-inflammatory drugs (NSAIDs) may be administered to diminish pain as well as the risk of myositis ossificans. While there is no data on the efficacy of NSAIDs in preventing myositis ossificans following quadriceps contusions, both COX-2 selective (ie, celecoxib) and nonselective (ie, naproxen, indomethacin) COX inhibitors have been demonstrated to significantly reduce the incidence of heterotopic ossification following hip surgery—a condition occurring from a similar pathophysiologic process as myositis ossificans.13-17 However, this class of drugs should not be given any sooner than 48 to 72 hours after injury to decrease further bleeding risk, given its inhibitory effect on platelet function.18 Narcotic pain medications are rarely required.
The second phase focuses on restoring active and passive knee and hip flexion and begins when permitted by pain.8 Icing, pain control, and physical therapy modalities are also continued in order to reduce pain and swelling as knee motion is progressed. The third phase begins once full range of knee and hip motion is restored and consists of quadriceps strengthening and functional rehabilitation of the lower extremity.8,19 Return to athletic activities and eventually competition should take place when a full, painless range of motion is restored and strength returns to baseline. Isokinetic strength testing may be utilized to more accurately assess strength and endurance. Noncontact, position-specific drills are incorporated as clinical improvement allows. A full recovery should be expected within 4 weeks of injury, with faster resolution and return to play seen in less severe contusions depending on the athlete’s position.8 Continued quadriceps stretching is recommended to prevent recurrence once the athlete returns to play. A protective hard shell may also be utilized both during rehabilitation as well as once the athlete returns to play in order to protect the thigh from reinjury, which may increase the risk of myositis ossificans.8
Complications
A prolonged recovery or persistent symptoms should alert the treating physician to the possibility of complications, including myositis ossificans.8,20 Myositis ossificans typically results from moderate to severe contusions, which may present initially as a painful, indurated mass that later becomes quite firm. This mass may be seen on plain radiographs as early as 2 to 4 weeks following injury if the athlete complains of persistent pain or a palpable thigh mass (Figure 2).9
Mani-Babu and colleagues23 reported a case of a 14-year-old male football player who sustained a quadriceps contusion after a direct blow from an opponent’s helmet to the lateral thigh. Persistent pain and limitation of motion at 2 months follow-up prompted imaging studies that demonstrated myositis ossificans. The patient was treated with intravenous pamidronate (a bisphosphonate) twice over a 3-month period and demonstrated a full recovery within 5 months.
Acute compartment syndrome of the thigh has also been reported following severe quadriceps contusions, with the majority occurring in the anterior compartment.12,24-28 When injury from blunt trauma extends into and disrupts the muscular layer adjacent to the femur, vascular disruption can cause hematoma formation, muscle edema, and significant swelling, thereby increasing intracompartmental pressure. The relatively large volume of the anterior thigh compartment and lack of a rigid deep fascial envelope may be protective from the development of compartment syndrome compared to other sites.28 It can be difficult to distinguish a severe contusion from a compartment syndrome, as both can occur from the same mechanism and have similar presenting signs and symptoms. Signs of a compartment syndrome include pain out of proportion to the injury that is aggravated by passive stretch of the quadriceps muscles, an increasingly firm muscle compartment to palpation, and neurovascular deficits.29 Both acute compartment syndrome and a severe contusion may present with significant pain, inability to bear weight, tense swelling, tenderness to palpation, and pain with passive knee flexion.24 While the successful conservative treatment of athletes with acute compartment syndrome of the thigh has been reported, it is important to closely monitor the patient’s condition and consider intracompartmental pressure monitoring if the patient’s clinical condition deteriorates.12 An acute fasciotomy should be strongly considered when intracompartmental pressures are within 30 mm Hg of diastolic pressure.24-27 Fortunately, it is highly uncommon for thigh compartment pressure to rise to this level. Percutaneous compartment decompression using liposuction equipment or a large cannula has been described to decrease intracompartmental pressure, potentially expediting recovery and minimizing morbidity.18 Interestingly, reports of fasciotomies for acute thigh compartment syndrome following closed athletic injuries have not described necrotic or non-contractile muscle typical of an acute compartment syndrome, calling into question the need for fasciotomy following closed blunt athletic trauma to the thigh.18
Quadriceps Strain
Pathophysiology
Acute quadriceps strains occur during sudden forceful eccentric contraction of the extensor mechanism. Occasionally, in the absence of a clear mechanism, these injuries mistakenly appear as a contusion resulting from a direct blow to the thigh.30,31 The rectus femoris is the most frequently strained quadriceps muscle due, in part, to its superficial location and predominance of type II muscle fibers, which are more likely to be strained.11,32 Although classically described as occurring along the distal portion of the rectus femoris at the musculotendinous junction, quadriceps strains most commonly occur at the mid to proximal aspect of the rectus femoris.30,33 The quadriceps muscle complex crosses 2 joints and, as a result, is more predisposed to eccentric injury than mono-articular muscles.34 We have had a subset of complete myotendinous tears of the rectus femoris that occur in the plant leg of placekickers that result in significant disability.
Risk Factors
Quadriceps and thigh injuries comprise approximately 4.5% of injuries among NFL players.7 Several risk factors for quadriceps strains have been described. In a study of Australian Rules football players, Orchard35 demonstrated that for all muscle strains, the strongest risk factor was a recent history of the same injury, with the next strongest risk factor being a past history of the same injury. Increasing age was found to be a risk factor for hamstring strains but not quadriceps strains. Muscle fatigue may also contribute to injury susceptibility.36
History and Physical Examination
Injuries typically occur during kicking, jumping, or a sudden change in direction while running.30 Athletes may localize pain anywhere along the quadriceps muscle, although strains most commonly occur at the proximal to mid portion of the rectus femoris.30,33 The grading system for quadriceps strains described by Kary30 is based on level of pain, quadriceps strength, and the presence or absence of a palpable defect (Table 2).
The athlete typically walks with an antalgic gait. Visible swelling and/or ecchymosis may be present depending on when the athlete is seen, as ecchymosis may develop within the first 24 hours of injury. The examiner should palpate along the entire length of the injured muscle. High-grade strains or complete tears may present with a bulge or defect in the muscle belly, but in most cases no defect will be palpable. There may be loss of knee flexion similar to a quadriceps contusion. Strength testing should be performed in both the sitting and prone position with the hip both flexed and extended to assess resisted knee extension strength.30 Loss of strength is proportional to the degree of injury.
Imaging
While most quadriceps strains are adequately diagnosed clinically without the need for imaging studies, ultrasound or MRI can be used to evaluate for partial or complete rupture.30,33 In milder cases, MRI usually demonstrates interstitial edema and hemorrhage with a feathery appearance on STIR and T2-weighted imaging (Figures 3A-3C).11
Treatment
Acute treatment of quadriceps strains focuses on minimizing bleeding using the principles of RICE treatment.37 NSAIDs may be used immediately to assist with pain control.30 COX-2-specific NSAIDs are preferred due to their lack of any inhibitory effect on platelet function in order to reduce the risk of further bleeding within the muscle compartment. For the first 24 to 72 hours following injury, the quadriceps should be maintained relatively immobilized to prevent further injury.38 High-grade injuries might necessitate crutches for ambulatory assistance.
Depending on injury severity, the active phase of treatment usually begins within 5 days of injury and consists of stretching and knee/hip range of motion. An active warm-up should precede rehabilitation exercises to activate neural pathways within the muscle and improve muscle elasticity.38 Ballistic stretching should be avoided to prevent additional injury to the muscle fibers. Strengthening should proceed when the athlete recovers a pain-free range of motion. When isometric exercises can be completed at increasing degrees of knee flexion, isotonic exercises may be implemented into the rehabilitation program.30 Return to football can be considered when the athlete has recovered knee and hip range of motion, is pain-free, and has near-normal strength compared to the contralateral side. The athlete should also perform satisfactorily in simulated position-specific activities in a noncontact fashion prior to return to full competition.30
Hamstring Strain
Pathophysiology
Hamstring strains are the most common noncontact injuries in football resulting from excessive muscle stretching during eccentric contraction generally occurring at the musculotendinous junction.5,39 Because the hamstrings cross both the hip and knee, simultaneous hip flexion and knee extension results in maximal lengthening, making them most vulnerable to injury at the terminal swing phase of gait just prior to heel strike.39-42 The long head of the biceps femoris undergoes the greatest stretch, reaching 110% of resting length during terminal swing phase and is the most commonly injured hamstring muscle.43,44 Injury occurs when the force of eccentric contraction, and resulting muscle strain, exceeds the mechanical limits of the tissue.42,45 It remains to be shown whether hamstring strains occur as a result of accumulated microscopic muscle damage or secondary to a single event that exceeds the mechanical limits of the muscle.42
Epidemiology and Risk Factors
The majority of hamstring strains are sustained during noncontact activities, with most athletes citing sprinting as the activity at the time of injury.3 Approximately 93% of injuries occur during noncontact activities among defensive backs and wide receivers.3 Hamstring strains are the second-most common injury among NFL players, comprising approximately 9% of all injuries,5,7 with 16% to 31% of these injuries associated with recurrence.3,5,35,46 Using the NFL’s Injury Surveillance System, Elliott and colleagues3 reported 1716 hamstring strains over a 10-year period (1989-1998). Fifty-one percent of hamstring strains occurred during the 7-week preseason, with a greater than 4-fold increased injury rate noted during the preseason compared to the 16-week regular season. An increased incidence in the preseason is partially attributable to relative deconditioning over the offseason. Defensive backs, wide receivers, and special teams players accounted for the majority of injured players, suggesting that speed position players and those who must “backpedal” (run backwards) are at an increased risk for injury.
Several risk factors for hamstring strain have been described, including prior injury, older age, quadriceps-hamstring strength imbalances, limited hip and knee flexibility, and fatigue.39,42,47 Inadequate rehabilitation and premature return to competition are also likely important factors predisposing to recurrent injury.39,48
History and Physical Examination
The majority of hamstring strains occur in the acute setting when the player experiences the sudden onset of pain in the posterior thigh during strenuous exercise, most commonly while sprinting.39 The injury typically occurs in the early or late stage of practice or competition due, in part, to inadequate warm-up or fatigue. The athlete may describe an audible pop and an inability to continue play, depending on injury severity.
Physical examination may demonstrate palpable induration and tenderness immediately or shortly after injury. In the setting of severe strains, there can be significant thigh swelling and ecchymosis, and in complete ruptures, a palpable defect.39 The affected muscle should be palpated along its entire length, and is best performed prone with the knee flexed to 90° as well as with the knee partially extended to place it under mild tension. Injury severity can be assessed by determining the restriction of passive knee extension while the athlete is lying supine with the hip flexed to 90°. The severity of hamstring strains varies from minor damage of a few myofibers without loss of structural integrity to complete muscle rupture.
Imaging
Similar to other muscle strains, hamstring strains are a clinical diagnosis and generally do not necessitate advanced imaging studies except to assess the degree of damage (ie, partial vs complete rupture) and to rule out other injuries, especially if the athlete fails to respond to treatment. Plain radiographs in acute cases are usually unremarkable. However, more severe injuries may go on to develop myositis ossificans similar to quadriceps soft tissue injuries (Figure 5).
Treatment
Most hamstring strains respond to conservative treatment, with operative intervention rarely indicated except for proximal or distal tendon avulsions.39 Like other muscle strains, initial management consists of RICE. COX-2-selective NSAIDs are preferred initially following injury. During a brief period of immobilization, the leg should be extended as much as tolerated to maximize muscle length, limit hematoma formation, and reduce the risk of contracture.39 Controlled mobilization should begin as soon as tolerated by the athlete.39 Isometric exercises and a stretching program should be started early in the rehabilitation period, with isotonic exercises added as motion and pain improve. Active stretching should be initiated and progressed to passive, static stretching as guided by pain.
The late phase of rehabilitation and long-term conditioning protocols should incorporate eccentric training once the athlete is pain-free, performing isotonic and isokinetic exercises. Eccentric exercises best strengthen the hamstrings at their most susceptible point, prepares the athlete for functional activities, and minimizes the risk of reinjury,3,50,51 Elliot and colleagues3 reported an order of magnitude decrease in hamstring injuries in high-risk athletes with identifiable hamstring muscle weakness after implementing an eccentric strengthening program and progressive sprint training. Similarly, in a large cohort of elite soccer players, correction of strength deficits in players with prior hamstring injuries led to similar rates of injury compared to athletes without strength deficits or prior injury.52 Those athletes with persistent weakness who did not undergo rehabilitation had significantly higher rates of reinjury.
Various injections containing local anesthetics, corticosteroids, platelet-rich plasma (PRP), and other substances have been administered to football players following acute muscle strains in an effort to alleviate pain and safely return the athlete to competition. Some practitioners have been reluctant to administer injections (especially those containing corticosteroids) due to a potentially increased risk of tendinopathy or rupture.31 Drakos and colleagues53 reported their outcomes following muscle and ligament strains treated with combined corticosteroid and local anesthetic injections on one NFL team. While quadriceps and hamstring strains were associated with the most missed games among all muscle strains, these injections resulted in no adverse events or progression of injury severity. Similarly, Levine and colleagues 51 administered intramuscular corticosteroid injections to 58 NFL players with high-grade hamstring injuries that had a palpable defect within the muscle belly. They reported no complications or strength deficits at final examination. In a case-control study, Rettig and colleagues46 administered PRP injections under ultrasound guidance in 5 NFL players with hamstring injuries. Compared to players treated with a focused rehabilitation program only, there were no significant differences in recovery or return to play.
The decision to return to play should be based on a clinical assessment considering pain, strength, motion, and flexibility. Player position should also be considered. Return-to-play guidelines describing the appropriate progression through rehabilitation and return to sport have been described and can be used as a template for the rehabilitation of football players.54 It should be noted that primary hamstring strains are associated with decreased athletic performance and an increased risk of more severe reinjury after return to sport.55,56
Morel-Lavallée Lesion
Pathophysiology
Morel-Lavallée lesions (MLLs) are uncommon football injuries, but often occur in the thigh.57,58 An MLL is a posttraumatic soft tissue injury in which deforming forces of pressure and shear cause a closed, soft tissue degloving injury; in this injury, the skin and subcutaneous tissues are separated from the underlying fascia, disrupting perforating blood vessels. The resulting space between the fascia and subcutaneous tissue fills with blood, lymphatics, and necrotic fat, resulting in a hematoma/seroma that can be a nidus for bacterial infection.58 The most common anatomic regions are the anterior distal thigh and lateral hip. Both of these areas are commonly involved in both direct contact and shear forces following a fall to the ground.
History and Physical Examination
Athletes with MLLs typically present with the insidious onset of a fluid collection within the thigh following a fall to the ground, usually while sliding or diving on the playing surface.57,58 The fluid collection can be associated with thigh tightness and may extend distally into the suprapatellar region or proximally over the greater trochanter. Thigh swelling, ecchymosis, and palpable fluctuance are seen in most cases. Progressive increases in pain and thigh swelling may be seen in severe injuries, but thigh compartments generally remain soft and nontender. Signs and symptoms of an MLL do not typically manifest immediately following the athletic event. Tejwani and colleagues58 reported a case series of MLLs of the knee in 27 NFL players from a single team over a 14-year period, with an average of 3 days between injury and evaluation by the medical staff. The mechanism of injury was a shearing blow from the knee striking the playing surface in 81% of cases and direct contact to the knee from another player in 19% of cases; all cases occurred in game situations. No affected players were wearing kneepads at the time of injury.
Imaging
Plain radiography may reveal a noncalcified soft tissue mass over the involved area and is not usually helpful except to rule out an underlying fracture. The appearance of an MLL on ultrasound is nonspecific and variable, often described as anechoic, hypoechoic, or hyperechoic depending on the presence of hemolymphatic fluid sedimentation and varying amounts of internal fat debris. MRI is the imaging modality of choice and typically shows a well-defined oval or fusiform, fluid-filled mass with tapering margins blending with adjacent fascial planes.
Treatment
Similar to quadriceps contusions, treatment goals for MLLs are evacuation of the fluid collection, prevention of fluid recurrence, a full range of active knee flexion, and prompt return to play.57,58 Initial treatment for smaller lesions consists of cryotherapy, compression wrapping of the involved area, and immediate active and passive range of motion of the hip and knee. While MLLs were traditionally treated with serial open debridements, less invasive approaches—including elastic compression, aspiration, percutaneous irrigation with debridement and suction drainage, or liposuction and drainage followed by suction therapy—have been recently described.57,58,60,61 Less invasive approaches aim to minimize soft tissue dissection and disruption of the vascular supply while accelerating rehabilitation. The presence of a surrounding capsule on MRI makes conservative or minimally invasive approaches less likely to be successful and may necessitate an open procedure.62 Antibiotics should be used preoperatively due to the presence of a dead space containing necrotic debris that makes infection a potential complication. While elite contact athletes can expect to return to competition long before complete resolution of an MLL, there is a risk of further delamination and lesion expansion due to re-injury prior to compete healing.
Tejwani and colleagues58 performed aspiration at the area of palpable fluctuance in the thigh or suprapatellar region using a 14-gauge needle in those athletes who failed to improve with conservative treatments alone. Mean time to resolution of the fluid collection was 16 days following aspiration. Fifty-two percent of the athletes were successfully treated with cryotherapy, compression, and motion exercises alone; 48% were treated with at least one aspiration, with a mean of 2.7 aspirations per knee. In 11% of cases that failed to resolve after multiple aspirations, doxycycline sclerodesis was performed immediately following an aspiration. Patients treated with sclerodesis had no return of the fluid collection and returned to play the following day.
Matava and colleagues57 described the case of an NFL player who sustained a closed MLL of the lateral hip while diving onto an artificial turf surface attempting to catch a pass. Despite immediate thigh pain and swelling, he was able to continue play. Immediately following the game, the player was examined and had a tense thigh with ecchymosis extending into the trochanteric region. Aspiration of the fluctuant area was unsuccessful. Progressive increases in pain and thigh swelling prompted hospital admission. Percutaneous irrigation and debridement was performed as described by Tseng and Tornetta.61 A suction drain was placed within the residual dead space, and constant wall suction was applied in addition to hip compression using a spica
Conclusion
Quadriceps and hamstring injuries occur frequently in football and are generally treated conservatively. While return to competition following hamstring strains is relatively quick, a high rate of injury recurrence highlights the importance of targeted rehabilitation and conditioning. Rarely, complications from quadriceps contusions, including acute compartment syndrome and myositis ossificans, may require operative intervention if unresponsive to conservative treatment. MLLs are rare in sports, but usually involve the thigh when they occur in football players. Team physicians must maintain a heightened degree of awareness of this injury as it may require operative intervention.
Acknowledgements: The authors would like to thank Jonathon Baker, MD and David Rubin, MD for their assistance in providing radiographic images for this paper.
Am J Orthop. 2016;45(6):E308-E318. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
2. Rechel JA, Yard EE, Comstock RD. An epidemiologic comparison of high school sports injuries sustained in practice and competition. J Athl Train. 2008;43(2):197-204.
3. Elliott MC, Zarins B, Powell JW, Kenyon CD. Hamstring muscle strains in professional football players: a 10-year review. Am J Sports Med. 2011;39(4):843-850.
4. Dick R, Ferrara MS, Agel J, et al. Descriptive epidemiology of collegiate men’s football injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athl Train. 2007;42(2):221-233.
5. Feeley BT, Kennelly S, Barnes RP, et al. Epidemiology of National Football League training camp injuries from 1998 to 2007. Am J Sports Med. 2008;36(8):1597-1603.
6. Garrett WE Jr. Muscle strain injuries. Am J Sports Med. 1996;24(6 Suppl):S2-S8.
7. Lawrence DW, Hutchison MG, Comper P. Descriptive epidemiology of musculoskeletal injuries and concussions in the National Football League, 2012-2014. Orthop J Sports Med. 2015;3(5):2325967115583653.
8. Diaz JA, Fischer DA, Rettig AC, Davis TJ, Shelbourne KD. Severe quadriceps muscle contusions in athletes. A report of three cases. Am J Sports Med. 2003;31(2):289-293.
9. Jackson DW, Feagin JA. Quadriceps contusions in young athletes. Relation of severity of injury to treatment and prognosis. J Bone Joint Surg Am. 1973;55(1):95-105.
10. Ryan JB, Wheeler JH, Hopkinson WJ, Arciero RA, Kolakowski KR. Quadriceps contusions. West Point update. Am J Sports Med. 1991;19(3):299-304.
11. Bencardino JT, Rosenberg ZS, Brown RR, Hassankhani A, Lustrin ES, Beltran J. Traumatic musculotendinous injuries of the knee: diagnosis with MR imaging. Radiographics. 2000;20 Spec No:S103-S120.
12. Robinson D, On E, Halperin N. Anterior compartment syndrome of the thigh in athletes--indications for conservative treatment. J Trauma. 1992;32(2):183-186.
13. Beckmann JT, Wylie JD, Kapron AL, Hanson JA, Maak TG, Aoki SK. The effect of NSAID prophylaxis and operative variables on heterotopic ossification after hip arthroscopy. Am J Sports Med. 2014;42(6):1359-1364.
14. Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J Nucl Med. 2002;43(3):346-353.
15. Beckmann JT, Wylie JD, Potter MQ, Maak TG, Greene TH, Aoki SK. Effect of naproxen prophylaxis on heterotopic ossification following hip arthroscopy: a double-blind randomized placebo-controlled trial. J Bone Joint Surg Am. 2015;97(24):2032-2037.
16. Yeung M, Jamshidi S, Horner N, Simunovic N, Karlsson J, Ayeni OR. Efficacy of nonsteroidal anti-inflammatory drug prophylaxis for heterotrophic ossification in hip arthroscopy: a systematic review. Arthroscopy. 2016;32(3):519-525.
17. Goyal K, Pettis CR, Bancroft AE, Wasyliw CW, Scherer KF. Myositis ossificans in the thigh of a lacrosse player. Orthopedics. 2015;38(8):468,515-518.
18. Cooper DE. Severe quadriceps muscle contusions in athletes. Am J Sports Med. 2004;32(3):820.
19. Bonsell S, Freudigman PT, Moore HA. Quadriceps muscle contusion resulting in osteomyelitis of the femur in a high school football player. A case report. Am J Sports Med. 2001;29(6):818-820.
20. Rothwell AG. Quadriceps hematoma. A prospective clinical study. Clin Orthop Relat Res. 1982;(171):97-103.
21. Armfield DR, Kim DH, Towers JD, Bradley JP, Robertson DD. Sports-related muscle injury in the lower extremity. Clin Sports Med. 2006;25(4):803-842.
22. Lipscomb AB, Thomas ED, Johnston RK. Treatment of myositis ossificans traumatica in athletes. Am J Sports Med. 1976;4(3):111-120.
23. Mani-Babu S, Wolman R, Keen R. Quadriceps traumatic myositis ossificans in a football player: management with intravenous pamidronate. Clin J Sport Med. 2014;24(5):e56-e58.
24. McCaffrey DD, Clarke J, Bunn J, McCormack MJ. Acute compartment syndrome of the anterior thigh in the absence of fracture secondary to sporting trauma. J Trauma. 2009;66(4):1238-1242.
25. Klasson SC, Vander Schilden JL. Acute anterior thigh compartment syndrome complicating quadriceps hematoma. Two case reports and review of the literature. Orthop Rev. 1990;19(5):421-427.
26. Rooser B. Quadriceps contusion with compartment syndrome. Evacuation of hematoma in 2 cases. Acta Orthop Scand. 1987;58(2):170-172.
27. Rooser B, Bengtson S, Hagglund G. Acute compartment syndrome from anterior thigh muscle contusion: a report of eight cases. J Orthop Trauma. 1991;5(1):57-59.
28. Schwartz JT Jr, Brumback RJ, Lakatos R, Poka A, Bathon GH, Burgess AR. Acute compartment syndrome of the thigh. A spectrum of injury. J Bone Joint Surg Am. 1989;71(3):392-400.
29. Elliott KG, Johnstone AJ. Diagnosing acute compartment syndrome. J Bone Joint Surg Br. 2003;85(5):625-632.
30. Kary JM. Diagnosis and management of quadriceps strains and contusions. Curr Rev Musculoskelet Med. 2010;3(1-4):26-31.
31. Boublik M, Schlegel TF, Koonce RC, Genuario JW, Kinkartz JD. Quadriceps tendon injuries in national football league players. Am J Sports Med. 2013;41(8):1841-1846.
32. Palmer WE, Kuong SJ, Elmadbouh HM. MR imaging of myotendinous strain. AJR Am J Roentgenol. 1999;173(3):703-709.
33. Cross TM, Gibbs N, Houang MT, Cameron M. Acute quadriceps muscle strains: magnetic resonance imaging features and prognosis. Am J Sports Med. 2004;32(3):710-719.
34. Hughes C 4th, Hasselman CT, Best TM, Martinez S, Garrett WE Jr. Incomplete, intrasubstance strain injuries of the rectus femoris muscle. Am J Sports Med. 1995;23(4):500-506.
35. Orchard JW. Intrinsic and extrinsic risk factors for muscle strains in Australian football. Am J Sports Med. 2001;29(3):300-303.36. Mair SD, Seaber AV, Glisson RR, Garrett WE, Jr. The role of fatigue in susceptibility to acute muscle strain injury. Am J Sports Med. 1996;24(2):137-143.
37. Bleakley C, McDonough S, MacAuley D. The use of ice in the treatment of acute soft-tissue injury: a systematic review of randomized controlled trials. Am J Sports Med. 2004;32(1):251-261.
38. Jarvinen TA, Jarvinen TL, Kaariainen M, Kalimo H, Jarvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33(5):745-764.
39. Clanton TO, Coupe KJ. Hamstring strains in athletes: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(4):237-248.
40. Novacheck TF. The biomechanics of running. Gait Posture. 1998;7(1):77-95.
41. Yu B, Queen RM, Abbey AN, Liu Y, Moorman CT, Garrett WE. Hamstring muscle kinematics and activation during overground sprinting. J Biomech. 2008;41(15):3121-3126.
42. Opar DA, Williams MD, Shield AJ. Hamstring strain injuries: factors that lead to injury and re-injury. Sports Med. 2012;42(3):209-226.
43. Askling CM, Tengvar M, Saartok T, Thorstensson A. Acute first-time hamstring strains during high-speed running: a longitudinal study including clinical and magnetic resonance imaging findings. Am J Sports Med. 2007;35(2):197-206.
44. Thelen DG, Chumanov ES, Hoerth DM, et al. Hamstring muscle kinematics during treadmill sprinting. Med Sci Sports Exerc. 2005;37(1):108-114.
45. Chumanov ES, Heiderscheit BC, Thelen DG. The effect of speed and influence of individual muscles on hamstring mechanics during the swing phase of sprinting. J Biomech. 2007;40(16):3555-3562.
46. Rettig AC, Meyer S, Bhadra AK. Platelet-rich plasma in addition to rehabilitation for acute hamstring injuries in NFL players: clinical effects and time to return to play. Orthop J Sports Med. 2013;1(1):2325967113494354.
47. Zvijac JE, Toriscelli TA, Merrick S, Kiebzak GM. Isokinetic concentric quadriceps and hamstring strength variables from the NFL Scouting Combine are not predictive of hamstring injury in first-year professional football players. Am J Sports Med. 2013;41(7):1511-1518.
48. Arnason A, Sigurdsson SB, Gudmundsson A, Holme I, Engebretsen L, Bahr R. Risk factors for injuries in football. Am J Sports Med. 2004;32(1 Suppl):5S-16S.
49. Zarins B, Ciullo JV. Acute muscle and tendon injuries in athletes. Clin Sports Med. 1983;2(1):167-182.
50. Arnason A, Andersen TE, Holme I, Engebretsen L, Bahr R. Prevention of hamstring strains in elite soccer: an intervention study. Scand J Med Sci Sports. 2008;18(1):40-48.
51. Levine WN, Bergfeld JA, Tessendorf W, Moorman CT 3rd. Intramuscular corticosteroid injection for hamstring injuries. A 13-year experience in the National Football League. Am J Sports Med. 2000;28(3):297-300.
52. Croisier JL, Ganteaume S, Binet J, Genty M, Ferret JM. Strength imbalances and prevention of hamstring injury in professional soccer players: a prospective study. Am J Sports Med. 2008;36(8):1469-1475.
53. Drakos M, Birmingham P, Delos D, et al. Corticosteroid and anesthetic injections for muscle strains and ligament sprains in the NFL. HSS J. 2014;10(2):136-142.
54. Worrell TW. Factors associated with hamstring injuries. An approach to treatment and preventative measures. Sports Med. 1994;17(5):338-345.
55. Brooks JH, Fuller CW, Kemp SP, Reddin DB. Incidence, risk, and prevention of hamstring muscle injuries in professional rugby union. Am J Sports Med. 2006;34(8):1297-1306.
56. Verrall GM, Kalairajah Y, Slavotinek JP, Spriggins AJ. Assessment of player performance following return to sport after hamstring muscle strain injury. J Sci Med Sport. 2006;9(1-2):87-90.
57. Matava MJ, Ellis E, Shah NR, Pogue D, Williams T. Morel-lavallee lesion in a professional american football player. Am J Orthop. 2010;39(3):144-147.
58. Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallee lesion of the knee: twenty-seven cases in the national football league. Am J Sports Med. 2007;35(7):1162-1167.
59. Mellado JM, Bencardino JT. Morel-Lavallee lesion: review with emphasis on MR imaging. Magn Reson Imaging Clin N Am. 2005;13(4):775-782.
60. Harma A, Inan M, Ertem K. [The Morel-Lavallee lesion: a conservative approach to closed degloving injuries]. Acta Orthop Traumatol Turc. 2004;38(4):270-273.
61. Tseng S, Tornetta P 3rd. Percutaneous management of Morel-Lavallee lesions. J Bone Joint Surg Am. 2006;88(1):92-96.
62. Gilbert BC, Bui-Mansfield LT, Dejong S. MRI of a Morel-Lavellee lesion. AJR Am J Roentgenol. 2004;182(5):1347-1348.
American football has the highest injury rate of any team sport in the United States at the high school, collegiate, and professional levels.1-3 Muscle strains and contusions constitute a large proportion of football injuries. For example, at the high school level, muscle strains comprise 12% to 24% of all injuries;2 at the collegiate level, they account for approximately 20% of all practice injuries, with nearly half of all strains occurring within the thigh.1,4 Among a single National Football League (NFL) team, Feeley and colleagues5 reported that muscle strains accounted for 46% of practice and 22% of preseason game injuries. The hamstrings, followed by the quadriceps, are the most commonly strained muscle groups among both professional and amateur athletes,5,6 with hamstring and quadriceps injuries making up approximately 13% of all injuries among NFL players.7 Given the relatively large surface area and muscle volume of the anterior and posterior thigh, as well as the activities and maneuvers necessitated by the various football positions, it is not surprising that the thigh is frequently involved in football-related injuries.
The purpose of this review is to describe the clinical manifestations of thigh-related soft-tissue injuries seen in football players. Two of these conditions—muscle strains and contusions—are relatively common, while a third condition—the Morel-Lavallée lesion—is a rare, yet relevant injury that warrants discussion.
Quadriceps Contusion
Pathophysiology
Contusion to the quadriceps muscle is a common injury in contact sports generally resulting from a direct blow from a helmet, knee, or shoulder.8 Bleeding within the musculature causes swelling, pain, stiffness, and limitation of quadriceps excursion, ultimately resulting in loss of knee flexion and an inability to run or squat. The injury is typically confined to a single quadriceps muscle.8 The use of thigh padding, though helpful, does not completely eliminate the risk of this injury.
History and Physical Examination
Immediately after injury, the athlete may complain only of thigh pain. However, swelling, pain, and diminished range of knee motion may develop within the first 24 hours depending on the severity of injury and how quickly treatment is instituted.8 Jackson and Feagin9 developed an injury grading system for quadriceps contusions based on the limitation of knee flexion observed (Table 1).
Imaging
A quadriceps contusion is a clinical diagnosis based on a typical history and physical examination; therefore, advanced imaging usually does not need to be obtained except to gauge the severity of injury, to rule out concurrent injuries (ie, tendon rupture), and to identify the presence of a hematoma that may necessitate aspiration. Plain radiographs are typically unremarkable in the acute setting. Appearance on magnetic resonance imaging (MRI) varies by injury severity, with increased signal throughout the affected muscle belly and a diffuse, feathery appearance centered at the point of impact on short TI inversion recovery (STIR) and T2-weighted images reflecting edema and possibly hematoma (Figures 1A-1C).8,11
Treatment
Treatment of a quadriceps contusion is nonoperative and consists of a 3-phase recovery.10 The first phase lasts approximately 2 days and consists of rest, ice, compression, and elevation (RICE) to limit hemorrhage. The knee should be rested in a flexed position to maintain quadriceps muscle fiber length in order to promote muscle compression and limit knee stiffness. For severe contusions in which there is a question of an acute thigh compartment syndrome, compression should be avoided with appropriate treatment based on typical symptoms and intra-compartmental pressure measurement.12 Nonsteroidal anti-inflammatory drugs (NSAIDs) may be administered to diminish pain as well as the risk of myositis ossificans. While there is no data on the efficacy of NSAIDs in preventing myositis ossificans following quadriceps contusions, both COX-2 selective (ie, celecoxib) and nonselective (ie, naproxen, indomethacin) COX inhibitors have been demonstrated to significantly reduce the incidence of heterotopic ossification following hip surgery—a condition occurring from a similar pathophysiologic process as myositis ossificans.13-17 However, this class of drugs should not be given any sooner than 48 to 72 hours after injury to decrease further bleeding risk, given its inhibitory effect on platelet function.18 Narcotic pain medications are rarely required.
The second phase focuses on restoring active and passive knee and hip flexion and begins when permitted by pain.8 Icing, pain control, and physical therapy modalities are also continued in order to reduce pain and swelling as knee motion is progressed. The third phase begins once full range of knee and hip motion is restored and consists of quadriceps strengthening and functional rehabilitation of the lower extremity.8,19 Return to athletic activities and eventually competition should take place when a full, painless range of motion is restored and strength returns to baseline. Isokinetic strength testing may be utilized to more accurately assess strength and endurance. Noncontact, position-specific drills are incorporated as clinical improvement allows. A full recovery should be expected within 4 weeks of injury, with faster resolution and return to play seen in less severe contusions depending on the athlete’s position.8 Continued quadriceps stretching is recommended to prevent recurrence once the athlete returns to play. A protective hard shell may also be utilized both during rehabilitation as well as once the athlete returns to play in order to protect the thigh from reinjury, which may increase the risk of myositis ossificans.8
Complications
A prolonged recovery or persistent symptoms should alert the treating physician to the possibility of complications, including myositis ossificans.8,20 Myositis ossificans typically results from moderate to severe contusions, which may present initially as a painful, indurated mass that later becomes quite firm. This mass may be seen on plain radiographs as early as 2 to 4 weeks following injury if the athlete complains of persistent pain or a palpable thigh mass (Figure 2).9
Mani-Babu and colleagues23 reported a case of a 14-year-old male football player who sustained a quadriceps contusion after a direct blow from an opponent’s helmet to the lateral thigh. Persistent pain and limitation of motion at 2 months follow-up prompted imaging studies that demonstrated myositis ossificans. The patient was treated with intravenous pamidronate (a bisphosphonate) twice over a 3-month period and demonstrated a full recovery within 5 months.
Acute compartment syndrome of the thigh has also been reported following severe quadriceps contusions, with the majority occurring in the anterior compartment.12,24-28 When injury from blunt trauma extends into and disrupts the muscular layer adjacent to the femur, vascular disruption can cause hematoma formation, muscle edema, and significant swelling, thereby increasing intracompartmental pressure. The relatively large volume of the anterior thigh compartment and lack of a rigid deep fascial envelope may be protective from the development of compartment syndrome compared to other sites.28 It can be difficult to distinguish a severe contusion from a compartment syndrome, as both can occur from the same mechanism and have similar presenting signs and symptoms. Signs of a compartment syndrome include pain out of proportion to the injury that is aggravated by passive stretch of the quadriceps muscles, an increasingly firm muscle compartment to palpation, and neurovascular deficits.29 Both acute compartment syndrome and a severe contusion may present with significant pain, inability to bear weight, tense swelling, tenderness to palpation, and pain with passive knee flexion.24 While the successful conservative treatment of athletes with acute compartment syndrome of the thigh has been reported, it is important to closely monitor the patient’s condition and consider intracompartmental pressure monitoring if the patient’s clinical condition deteriorates.12 An acute fasciotomy should be strongly considered when intracompartmental pressures are within 30 mm Hg of diastolic pressure.24-27 Fortunately, it is highly uncommon for thigh compartment pressure to rise to this level. Percutaneous compartment decompression using liposuction equipment or a large cannula has been described to decrease intracompartmental pressure, potentially expediting recovery and minimizing morbidity.18 Interestingly, reports of fasciotomies for acute thigh compartment syndrome following closed athletic injuries have not described necrotic or non-contractile muscle typical of an acute compartment syndrome, calling into question the need for fasciotomy following closed blunt athletic trauma to the thigh.18
Quadriceps Strain
Pathophysiology
Acute quadriceps strains occur during sudden forceful eccentric contraction of the extensor mechanism. Occasionally, in the absence of a clear mechanism, these injuries mistakenly appear as a contusion resulting from a direct blow to the thigh.30,31 The rectus femoris is the most frequently strained quadriceps muscle due, in part, to its superficial location and predominance of type II muscle fibers, which are more likely to be strained.11,32 Although classically described as occurring along the distal portion of the rectus femoris at the musculotendinous junction, quadriceps strains most commonly occur at the mid to proximal aspect of the rectus femoris.30,33 The quadriceps muscle complex crosses 2 joints and, as a result, is more predisposed to eccentric injury than mono-articular muscles.34 We have had a subset of complete myotendinous tears of the rectus femoris that occur in the plant leg of placekickers that result in significant disability.
Risk Factors
Quadriceps and thigh injuries comprise approximately 4.5% of injuries among NFL players.7 Several risk factors for quadriceps strains have been described. In a study of Australian Rules football players, Orchard35 demonstrated that for all muscle strains, the strongest risk factor was a recent history of the same injury, with the next strongest risk factor being a past history of the same injury. Increasing age was found to be a risk factor for hamstring strains but not quadriceps strains. Muscle fatigue may also contribute to injury susceptibility.36
History and Physical Examination
Injuries typically occur during kicking, jumping, or a sudden change in direction while running.30 Athletes may localize pain anywhere along the quadriceps muscle, although strains most commonly occur at the proximal to mid portion of the rectus femoris.30,33 The grading system for quadriceps strains described by Kary30 is based on level of pain, quadriceps strength, and the presence or absence of a palpable defect (Table 2).
The athlete typically walks with an antalgic gait. Visible swelling and/or ecchymosis may be present depending on when the athlete is seen, as ecchymosis may develop within the first 24 hours of injury. The examiner should palpate along the entire length of the injured muscle. High-grade strains or complete tears may present with a bulge or defect in the muscle belly, but in most cases no defect will be palpable. There may be loss of knee flexion similar to a quadriceps contusion. Strength testing should be performed in both the sitting and prone position with the hip both flexed and extended to assess resisted knee extension strength.30 Loss of strength is proportional to the degree of injury.
Imaging
While most quadriceps strains are adequately diagnosed clinically without the need for imaging studies, ultrasound or MRI can be used to evaluate for partial or complete rupture.30,33 In milder cases, MRI usually demonstrates interstitial edema and hemorrhage with a feathery appearance on STIR and T2-weighted imaging (Figures 3A-3C).11
Treatment
Acute treatment of quadriceps strains focuses on minimizing bleeding using the principles of RICE treatment.37 NSAIDs may be used immediately to assist with pain control.30 COX-2-specific NSAIDs are preferred due to their lack of any inhibitory effect on platelet function in order to reduce the risk of further bleeding within the muscle compartment. For the first 24 to 72 hours following injury, the quadriceps should be maintained relatively immobilized to prevent further injury.38 High-grade injuries might necessitate crutches for ambulatory assistance.
Depending on injury severity, the active phase of treatment usually begins within 5 days of injury and consists of stretching and knee/hip range of motion. An active warm-up should precede rehabilitation exercises to activate neural pathways within the muscle and improve muscle elasticity.38 Ballistic stretching should be avoided to prevent additional injury to the muscle fibers. Strengthening should proceed when the athlete recovers a pain-free range of motion. When isometric exercises can be completed at increasing degrees of knee flexion, isotonic exercises may be implemented into the rehabilitation program.30 Return to football can be considered when the athlete has recovered knee and hip range of motion, is pain-free, and has near-normal strength compared to the contralateral side. The athlete should also perform satisfactorily in simulated position-specific activities in a noncontact fashion prior to return to full competition.30
Hamstring Strain
Pathophysiology
Hamstring strains are the most common noncontact injuries in football resulting from excessive muscle stretching during eccentric contraction generally occurring at the musculotendinous junction.5,39 Because the hamstrings cross both the hip and knee, simultaneous hip flexion and knee extension results in maximal lengthening, making them most vulnerable to injury at the terminal swing phase of gait just prior to heel strike.39-42 The long head of the biceps femoris undergoes the greatest stretch, reaching 110% of resting length during terminal swing phase and is the most commonly injured hamstring muscle.43,44 Injury occurs when the force of eccentric contraction, and resulting muscle strain, exceeds the mechanical limits of the tissue.42,45 It remains to be shown whether hamstring strains occur as a result of accumulated microscopic muscle damage or secondary to a single event that exceeds the mechanical limits of the muscle.42
Epidemiology and Risk Factors
The majority of hamstring strains are sustained during noncontact activities, with most athletes citing sprinting as the activity at the time of injury.3 Approximately 93% of injuries occur during noncontact activities among defensive backs and wide receivers.3 Hamstring strains are the second-most common injury among NFL players, comprising approximately 9% of all injuries,5,7 with 16% to 31% of these injuries associated with recurrence.3,5,35,46 Using the NFL’s Injury Surveillance System, Elliott and colleagues3 reported 1716 hamstring strains over a 10-year period (1989-1998). Fifty-one percent of hamstring strains occurred during the 7-week preseason, with a greater than 4-fold increased injury rate noted during the preseason compared to the 16-week regular season. An increased incidence in the preseason is partially attributable to relative deconditioning over the offseason. Defensive backs, wide receivers, and special teams players accounted for the majority of injured players, suggesting that speed position players and those who must “backpedal” (run backwards) are at an increased risk for injury.
Several risk factors for hamstring strain have been described, including prior injury, older age, quadriceps-hamstring strength imbalances, limited hip and knee flexibility, and fatigue.39,42,47 Inadequate rehabilitation and premature return to competition are also likely important factors predisposing to recurrent injury.39,48
History and Physical Examination
The majority of hamstring strains occur in the acute setting when the player experiences the sudden onset of pain in the posterior thigh during strenuous exercise, most commonly while sprinting.39 The injury typically occurs in the early or late stage of practice or competition due, in part, to inadequate warm-up or fatigue. The athlete may describe an audible pop and an inability to continue play, depending on injury severity.
Physical examination may demonstrate palpable induration and tenderness immediately or shortly after injury. In the setting of severe strains, there can be significant thigh swelling and ecchymosis, and in complete ruptures, a palpable defect.39 The affected muscle should be palpated along its entire length, and is best performed prone with the knee flexed to 90° as well as with the knee partially extended to place it under mild tension. Injury severity can be assessed by determining the restriction of passive knee extension while the athlete is lying supine with the hip flexed to 90°. The severity of hamstring strains varies from minor damage of a few myofibers without loss of structural integrity to complete muscle rupture.
Imaging
Similar to other muscle strains, hamstring strains are a clinical diagnosis and generally do not necessitate advanced imaging studies except to assess the degree of damage (ie, partial vs complete rupture) and to rule out other injuries, especially if the athlete fails to respond to treatment. Plain radiographs in acute cases are usually unremarkable. However, more severe injuries may go on to develop myositis ossificans similar to quadriceps soft tissue injuries (Figure 5).
Treatment
Most hamstring strains respond to conservative treatment, with operative intervention rarely indicated except for proximal or distal tendon avulsions.39 Like other muscle strains, initial management consists of RICE. COX-2-selective NSAIDs are preferred initially following injury. During a brief period of immobilization, the leg should be extended as much as tolerated to maximize muscle length, limit hematoma formation, and reduce the risk of contracture.39 Controlled mobilization should begin as soon as tolerated by the athlete.39 Isometric exercises and a stretching program should be started early in the rehabilitation period, with isotonic exercises added as motion and pain improve. Active stretching should be initiated and progressed to passive, static stretching as guided by pain.
The late phase of rehabilitation and long-term conditioning protocols should incorporate eccentric training once the athlete is pain-free, performing isotonic and isokinetic exercises. Eccentric exercises best strengthen the hamstrings at their most susceptible point, prepares the athlete for functional activities, and minimizes the risk of reinjury,3,50,51 Elliot and colleagues3 reported an order of magnitude decrease in hamstring injuries in high-risk athletes with identifiable hamstring muscle weakness after implementing an eccentric strengthening program and progressive sprint training. Similarly, in a large cohort of elite soccer players, correction of strength deficits in players with prior hamstring injuries led to similar rates of injury compared to athletes without strength deficits or prior injury.52 Those athletes with persistent weakness who did not undergo rehabilitation had significantly higher rates of reinjury.
Various injections containing local anesthetics, corticosteroids, platelet-rich plasma (PRP), and other substances have been administered to football players following acute muscle strains in an effort to alleviate pain and safely return the athlete to competition. Some practitioners have been reluctant to administer injections (especially those containing corticosteroids) due to a potentially increased risk of tendinopathy or rupture.31 Drakos and colleagues53 reported their outcomes following muscle and ligament strains treated with combined corticosteroid and local anesthetic injections on one NFL team. While quadriceps and hamstring strains were associated with the most missed games among all muscle strains, these injections resulted in no adverse events or progression of injury severity. Similarly, Levine and colleagues 51 administered intramuscular corticosteroid injections to 58 NFL players with high-grade hamstring injuries that had a palpable defect within the muscle belly. They reported no complications or strength deficits at final examination. In a case-control study, Rettig and colleagues46 administered PRP injections under ultrasound guidance in 5 NFL players with hamstring injuries. Compared to players treated with a focused rehabilitation program only, there were no significant differences in recovery or return to play.
The decision to return to play should be based on a clinical assessment considering pain, strength, motion, and flexibility. Player position should also be considered. Return-to-play guidelines describing the appropriate progression through rehabilitation and return to sport have been described and can be used as a template for the rehabilitation of football players.54 It should be noted that primary hamstring strains are associated with decreased athletic performance and an increased risk of more severe reinjury after return to sport.55,56
Morel-Lavallée Lesion
Pathophysiology
Morel-Lavallée lesions (MLLs) are uncommon football injuries, but often occur in the thigh.57,58 An MLL is a posttraumatic soft tissue injury in which deforming forces of pressure and shear cause a closed, soft tissue degloving injury; in this injury, the skin and subcutaneous tissues are separated from the underlying fascia, disrupting perforating blood vessels. The resulting space between the fascia and subcutaneous tissue fills with blood, lymphatics, and necrotic fat, resulting in a hematoma/seroma that can be a nidus for bacterial infection.58 The most common anatomic regions are the anterior distal thigh and lateral hip. Both of these areas are commonly involved in both direct contact and shear forces following a fall to the ground.
History and Physical Examination
Athletes with MLLs typically present with the insidious onset of a fluid collection within the thigh following a fall to the ground, usually while sliding or diving on the playing surface.57,58 The fluid collection can be associated with thigh tightness and may extend distally into the suprapatellar region or proximally over the greater trochanter. Thigh swelling, ecchymosis, and palpable fluctuance are seen in most cases. Progressive increases in pain and thigh swelling may be seen in severe injuries, but thigh compartments generally remain soft and nontender. Signs and symptoms of an MLL do not typically manifest immediately following the athletic event. Tejwani and colleagues58 reported a case series of MLLs of the knee in 27 NFL players from a single team over a 14-year period, with an average of 3 days between injury and evaluation by the medical staff. The mechanism of injury was a shearing blow from the knee striking the playing surface in 81% of cases and direct contact to the knee from another player in 19% of cases; all cases occurred in game situations. No affected players were wearing kneepads at the time of injury.
Imaging
Plain radiography may reveal a noncalcified soft tissue mass over the involved area and is not usually helpful except to rule out an underlying fracture. The appearance of an MLL on ultrasound is nonspecific and variable, often described as anechoic, hypoechoic, or hyperechoic depending on the presence of hemolymphatic fluid sedimentation and varying amounts of internal fat debris. MRI is the imaging modality of choice and typically shows a well-defined oval or fusiform, fluid-filled mass with tapering margins blending with adjacent fascial planes.
Treatment
Similar to quadriceps contusions, treatment goals for MLLs are evacuation of the fluid collection, prevention of fluid recurrence, a full range of active knee flexion, and prompt return to play.57,58 Initial treatment for smaller lesions consists of cryotherapy, compression wrapping of the involved area, and immediate active and passive range of motion of the hip and knee. While MLLs were traditionally treated with serial open debridements, less invasive approaches—including elastic compression, aspiration, percutaneous irrigation with debridement and suction drainage, or liposuction and drainage followed by suction therapy—have been recently described.57,58,60,61 Less invasive approaches aim to minimize soft tissue dissection and disruption of the vascular supply while accelerating rehabilitation. The presence of a surrounding capsule on MRI makes conservative or minimally invasive approaches less likely to be successful and may necessitate an open procedure.62 Antibiotics should be used preoperatively due to the presence of a dead space containing necrotic debris that makes infection a potential complication. While elite contact athletes can expect to return to competition long before complete resolution of an MLL, there is a risk of further delamination and lesion expansion due to re-injury prior to compete healing.
Tejwani and colleagues58 performed aspiration at the area of palpable fluctuance in the thigh or suprapatellar region using a 14-gauge needle in those athletes who failed to improve with conservative treatments alone. Mean time to resolution of the fluid collection was 16 days following aspiration. Fifty-two percent of the athletes were successfully treated with cryotherapy, compression, and motion exercises alone; 48% were treated with at least one aspiration, with a mean of 2.7 aspirations per knee. In 11% of cases that failed to resolve after multiple aspirations, doxycycline sclerodesis was performed immediately following an aspiration. Patients treated with sclerodesis had no return of the fluid collection and returned to play the following day.
Matava and colleagues57 described the case of an NFL player who sustained a closed MLL of the lateral hip while diving onto an artificial turf surface attempting to catch a pass. Despite immediate thigh pain and swelling, he was able to continue play. Immediately following the game, the player was examined and had a tense thigh with ecchymosis extending into the trochanteric region. Aspiration of the fluctuant area was unsuccessful. Progressive increases in pain and thigh swelling prompted hospital admission. Percutaneous irrigation and debridement was performed as described by Tseng and Tornetta.61 A suction drain was placed within the residual dead space, and constant wall suction was applied in addition to hip compression using a spica
Conclusion
Quadriceps and hamstring injuries occur frequently in football and are generally treated conservatively. While return to competition following hamstring strains is relatively quick, a high rate of injury recurrence highlights the importance of targeted rehabilitation and conditioning. Rarely, complications from quadriceps contusions, including acute compartment syndrome and myositis ossificans, may require operative intervention if unresponsive to conservative treatment. MLLs are rare in sports, but usually involve the thigh when they occur in football players. Team physicians must maintain a heightened degree of awareness of this injury as it may require operative intervention.
Acknowledgements: The authors would like to thank Jonathon Baker, MD and David Rubin, MD for their assistance in providing radiographic images for this paper.
Am J Orthop. 2016;45(6):E308-E318. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
American football has the highest injury rate of any team sport in the United States at the high school, collegiate, and professional levels.1-3 Muscle strains and contusions constitute a large proportion of football injuries. For example, at the high school level, muscle strains comprise 12% to 24% of all injuries;2 at the collegiate level, they account for approximately 20% of all practice injuries, with nearly half of all strains occurring within the thigh.1,4 Among a single National Football League (NFL) team, Feeley and colleagues5 reported that muscle strains accounted for 46% of practice and 22% of preseason game injuries. The hamstrings, followed by the quadriceps, are the most commonly strained muscle groups among both professional and amateur athletes,5,6 with hamstring and quadriceps injuries making up approximately 13% of all injuries among NFL players.7 Given the relatively large surface area and muscle volume of the anterior and posterior thigh, as well as the activities and maneuvers necessitated by the various football positions, it is not surprising that the thigh is frequently involved in football-related injuries.
The purpose of this review is to describe the clinical manifestations of thigh-related soft-tissue injuries seen in football players. Two of these conditions—muscle strains and contusions—are relatively common, while a third condition—the Morel-Lavallée lesion—is a rare, yet relevant injury that warrants discussion.
Quadriceps Contusion
Pathophysiology
Contusion to the quadriceps muscle is a common injury in contact sports generally resulting from a direct blow from a helmet, knee, or shoulder.8 Bleeding within the musculature causes swelling, pain, stiffness, and limitation of quadriceps excursion, ultimately resulting in loss of knee flexion and an inability to run or squat. The injury is typically confined to a single quadriceps muscle.8 The use of thigh padding, though helpful, does not completely eliminate the risk of this injury.
History and Physical Examination
Immediately after injury, the athlete may complain only of thigh pain. However, swelling, pain, and diminished range of knee motion may develop within the first 24 hours depending on the severity of injury and how quickly treatment is instituted.8 Jackson and Feagin9 developed an injury grading system for quadriceps contusions based on the limitation of knee flexion observed (Table 1).
Imaging
A quadriceps contusion is a clinical diagnosis based on a typical history and physical examination; therefore, advanced imaging usually does not need to be obtained except to gauge the severity of injury, to rule out concurrent injuries (ie, tendon rupture), and to identify the presence of a hematoma that may necessitate aspiration. Plain radiographs are typically unremarkable in the acute setting. Appearance on magnetic resonance imaging (MRI) varies by injury severity, with increased signal throughout the affected muscle belly and a diffuse, feathery appearance centered at the point of impact on short TI inversion recovery (STIR) and T2-weighted images reflecting edema and possibly hematoma (Figures 1A-1C).8,11
Treatment
Treatment of a quadriceps contusion is nonoperative and consists of a 3-phase recovery.10 The first phase lasts approximately 2 days and consists of rest, ice, compression, and elevation (RICE) to limit hemorrhage. The knee should be rested in a flexed position to maintain quadriceps muscle fiber length in order to promote muscle compression and limit knee stiffness. For severe contusions in which there is a question of an acute thigh compartment syndrome, compression should be avoided with appropriate treatment based on typical symptoms and intra-compartmental pressure measurement.12 Nonsteroidal anti-inflammatory drugs (NSAIDs) may be administered to diminish pain as well as the risk of myositis ossificans. While there is no data on the efficacy of NSAIDs in preventing myositis ossificans following quadriceps contusions, both COX-2 selective (ie, celecoxib) and nonselective (ie, naproxen, indomethacin) COX inhibitors have been demonstrated to significantly reduce the incidence of heterotopic ossification following hip surgery—a condition occurring from a similar pathophysiologic process as myositis ossificans.13-17 However, this class of drugs should not be given any sooner than 48 to 72 hours after injury to decrease further bleeding risk, given its inhibitory effect on platelet function.18 Narcotic pain medications are rarely required.
The second phase focuses on restoring active and passive knee and hip flexion and begins when permitted by pain.8 Icing, pain control, and physical therapy modalities are also continued in order to reduce pain and swelling as knee motion is progressed. The third phase begins once full range of knee and hip motion is restored and consists of quadriceps strengthening and functional rehabilitation of the lower extremity.8,19 Return to athletic activities and eventually competition should take place when a full, painless range of motion is restored and strength returns to baseline. Isokinetic strength testing may be utilized to more accurately assess strength and endurance. Noncontact, position-specific drills are incorporated as clinical improvement allows. A full recovery should be expected within 4 weeks of injury, with faster resolution and return to play seen in less severe contusions depending on the athlete’s position.8 Continued quadriceps stretching is recommended to prevent recurrence once the athlete returns to play. A protective hard shell may also be utilized both during rehabilitation as well as once the athlete returns to play in order to protect the thigh from reinjury, which may increase the risk of myositis ossificans.8
Complications
A prolonged recovery or persistent symptoms should alert the treating physician to the possibility of complications, including myositis ossificans.8,20 Myositis ossificans typically results from moderate to severe contusions, which may present initially as a painful, indurated mass that later becomes quite firm. This mass may be seen on plain radiographs as early as 2 to 4 weeks following injury if the athlete complains of persistent pain or a palpable thigh mass (Figure 2).9
Mani-Babu and colleagues23 reported a case of a 14-year-old male football player who sustained a quadriceps contusion after a direct blow from an opponent’s helmet to the lateral thigh. Persistent pain and limitation of motion at 2 months follow-up prompted imaging studies that demonstrated myositis ossificans. The patient was treated with intravenous pamidronate (a bisphosphonate) twice over a 3-month period and demonstrated a full recovery within 5 months.
Acute compartment syndrome of the thigh has also been reported following severe quadriceps contusions, with the majority occurring in the anterior compartment.12,24-28 When injury from blunt trauma extends into and disrupts the muscular layer adjacent to the femur, vascular disruption can cause hematoma formation, muscle edema, and significant swelling, thereby increasing intracompartmental pressure. The relatively large volume of the anterior thigh compartment and lack of a rigid deep fascial envelope may be protective from the development of compartment syndrome compared to other sites.28 It can be difficult to distinguish a severe contusion from a compartment syndrome, as both can occur from the same mechanism and have similar presenting signs and symptoms. Signs of a compartment syndrome include pain out of proportion to the injury that is aggravated by passive stretch of the quadriceps muscles, an increasingly firm muscle compartment to palpation, and neurovascular deficits.29 Both acute compartment syndrome and a severe contusion may present with significant pain, inability to bear weight, tense swelling, tenderness to palpation, and pain with passive knee flexion.24 While the successful conservative treatment of athletes with acute compartment syndrome of the thigh has been reported, it is important to closely monitor the patient’s condition and consider intracompartmental pressure monitoring if the patient’s clinical condition deteriorates.12 An acute fasciotomy should be strongly considered when intracompartmental pressures are within 30 mm Hg of diastolic pressure.24-27 Fortunately, it is highly uncommon for thigh compartment pressure to rise to this level. Percutaneous compartment decompression using liposuction equipment or a large cannula has been described to decrease intracompartmental pressure, potentially expediting recovery and minimizing morbidity.18 Interestingly, reports of fasciotomies for acute thigh compartment syndrome following closed athletic injuries have not described necrotic or non-contractile muscle typical of an acute compartment syndrome, calling into question the need for fasciotomy following closed blunt athletic trauma to the thigh.18
Quadriceps Strain
Pathophysiology
Acute quadriceps strains occur during sudden forceful eccentric contraction of the extensor mechanism. Occasionally, in the absence of a clear mechanism, these injuries mistakenly appear as a contusion resulting from a direct blow to the thigh.30,31 The rectus femoris is the most frequently strained quadriceps muscle due, in part, to its superficial location and predominance of type II muscle fibers, which are more likely to be strained.11,32 Although classically described as occurring along the distal portion of the rectus femoris at the musculotendinous junction, quadriceps strains most commonly occur at the mid to proximal aspect of the rectus femoris.30,33 The quadriceps muscle complex crosses 2 joints and, as a result, is more predisposed to eccentric injury than mono-articular muscles.34 We have had a subset of complete myotendinous tears of the rectus femoris that occur in the plant leg of placekickers that result in significant disability.
Risk Factors
Quadriceps and thigh injuries comprise approximately 4.5% of injuries among NFL players.7 Several risk factors for quadriceps strains have been described. In a study of Australian Rules football players, Orchard35 demonstrated that for all muscle strains, the strongest risk factor was a recent history of the same injury, with the next strongest risk factor being a past history of the same injury. Increasing age was found to be a risk factor for hamstring strains but not quadriceps strains. Muscle fatigue may also contribute to injury susceptibility.36
History and Physical Examination
Injuries typically occur during kicking, jumping, or a sudden change in direction while running.30 Athletes may localize pain anywhere along the quadriceps muscle, although strains most commonly occur at the proximal to mid portion of the rectus femoris.30,33 The grading system for quadriceps strains described by Kary30 is based on level of pain, quadriceps strength, and the presence or absence of a palpable defect (Table 2).
The athlete typically walks with an antalgic gait. Visible swelling and/or ecchymosis may be present depending on when the athlete is seen, as ecchymosis may develop within the first 24 hours of injury. The examiner should palpate along the entire length of the injured muscle. High-grade strains or complete tears may present with a bulge or defect in the muscle belly, but in most cases no defect will be palpable. There may be loss of knee flexion similar to a quadriceps contusion. Strength testing should be performed in both the sitting and prone position with the hip both flexed and extended to assess resisted knee extension strength.30 Loss of strength is proportional to the degree of injury.
Imaging
While most quadriceps strains are adequately diagnosed clinically without the need for imaging studies, ultrasound or MRI can be used to evaluate for partial or complete rupture.30,33 In milder cases, MRI usually demonstrates interstitial edema and hemorrhage with a feathery appearance on STIR and T2-weighted imaging (Figures 3A-3C).11
Treatment
Acute treatment of quadriceps strains focuses on minimizing bleeding using the principles of RICE treatment.37 NSAIDs may be used immediately to assist with pain control.30 COX-2-specific NSAIDs are preferred due to their lack of any inhibitory effect on platelet function in order to reduce the risk of further bleeding within the muscle compartment. For the first 24 to 72 hours following injury, the quadriceps should be maintained relatively immobilized to prevent further injury.38 High-grade injuries might necessitate crutches for ambulatory assistance.
Depending on injury severity, the active phase of treatment usually begins within 5 days of injury and consists of stretching and knee/hip range of motion. An active warm-up should precede rehabilitation exercises to activate neural pathways within the muscle and improve muscle elasticity.38 Ballistic stretching should be avoided to prevent additional injury to the muscle fibers. Strengthening should proceed when the athlete recovers a pain-free range of motion. When isometric exercises can be completed at increasing degrees of knee flexion, isotonic exercises may be implemented into the rehabilitation program.30 Return to football can be considered when the athlete has recovered knee and hip range of motion, is pain-free, and has near-normal strength compared to the contralateral side. The athlete should also perform satisfactorily in simulated position-specific activities in a noncontact fashion prior to return to full competition.30
Hamstring Strain
Pathophysiology
Hamstring strains are the most common noncontact injuries in football resulting from excessive muscle stretching during eccentric contraction generally occurring at the musculotendinous junction.5,39 Because the hamstrings cross both the hip and knee, simultaneous hip flexion and knee extension results in maximal lengthening, making them most vulnerable to injury at the terminal swing phase of gait just prior to heel strike.39-42 The long head of the biceps femoris undergoes the greatest stretch, reaching 110% of resting length during terminal swing phase and is the most commonly injured hamstring muscle.43,44 Injury occurs when the force of eccentric contraction, and resulting muscle strain, exceeds the mechanical limits of the tissue.42,45 It remains to be shown whether hamstring strains occur as a result of accumulated microscopic muscle damage or secondary to a single event that exceeds the mechanical limits of the muscle.42
Epidemiology and Risk Factors
The majority of hamstring strains are sustained during noncontact activities, with most athletes citing sprinting as the activity at the time of injury.3 Approximately 93% of injuries occur during noncontact activities among defensive backs and wide receivers.3 Hamstring strains are the second-most common injury among NFL players, comprising approximately 9% of all injuries,5,7 with 16% to 31% of these injuries associated with recurrence.3,5,35,46 Using the NFL’s Injury Surveillance System, Elliott and colleagues3 reported 1716 hamstring strains over a 10-year period (1989-1998). Fifty-one percent of hamstring strains occurred during the 7-week preseason, with a greater than 4-fold increased injury rate noted during the preseason compared to the 16-week regular season. An increased incidence in the preseason is partially attributable to relative deconditioning over the offseason. Defensive backs, wide receivers, and special teams players accounted for the majority of injured players, suggesting that speed position players and those who must “backpedal” (run backwards) are at an increased risk for injury.
Several risk factors for hamstring strain have been described, including prior injury, older age, quadriceps-hamstring strength imbalances, limited hip and knee flexibility, and fatigue.39,42,47 Inadequate rehabilitation and premature return to competition are also likely important factors predisposing to recurrent injury.39,48
History and Physical Examination
The majority of hamstring strains occur in the acute setting when the player experiences the sudden onset of pain in the posterior thigh during strenuous exercise, most commonly while sprinting.39 The injury typically occurs in the early or late stage of practice or competition due, in part, to inadequate warm-up or fatigue. The athlete may describe an audible pop and an inability to continue play, depending on injury severity.
Physical examination may demonstrate palpable induration and tenderness immediately or shortly after injury. In the setting of severe strains, there can be significant thigh swelling and ecchymosis, and in complete ruptures, a palpable defect.39 The affected muscle should be palpated along its entire length, and is best performed prone with the knee flexed to 90° as well as with the knee partially extended to place it under mild tension. Injury severity can be assessed by determining the restriction of passive knee extension while the athlete is lying supine with the hip flexed to 90°. The severity of hamstring strains varies from minor damage of a few myofibers without loss of structural integrity to complete muscle rupture.
Imaging
Similar to other muscle strains, hamstring strains are a clinical diagnosis and generally do not necessitate advanced imaging studies except to assess the degree of damage (ie, partial vs complete rupture) and to rule out other injuries, especially if the athlete fails to respond to treatment. Plain radiographs in acute cases are usually unremarkable. However, more severe injuries may go on to develop myositis ossificans similar to quadriceps soft tissue injuries (Figure 5).
Treatment
Most hamstring strains respond to conservative treatment, with operative intervention rarely indicated except for proximal or distal tendon avulsions.39 Like other muscle strains, initial management consists of RICE. COX-2-selective NSAIDs are preferred initially following injury. During a brief period of immobilization, the leg should be extended as much as tolerated to maximize muscle length, limit hematoma formation, and reduce the risk of contracture.39 Controlled mobilization should begin as soon as tolerated by the athlete.39 Isometric exercises and a stretching program should be started early in the rehabilitation period, with isotonic exercises added as motion and pain improve. Active stretching should be initiated and progressed to passive, static stretching as guided by pain.
The late phase of rehabilitation and long-term conditioning protocols should incorporate eccentric training once the athlete is pain-free, performing isotonic and isokinetic exercises. Eccentric exercises best strengthen the hamstrings at their most susceptible point, prepares the athlete for functional activities, and minimizes the risk of reinjury,3,50,51 Elliot and colleagues3 reported an order of magnitude decrease in hamstring injuries in high-risk athletes with identifiable hamstring muscle weakness after implementing an eccentric strengthening program and progressive sprint training. Similarly, in a large cohort of elite soccer players, correction of strength deficits in players with prior hamstring injuries led to similar rates of injury compared to athletes without strength deficits or prior injury.52 Those athletes with persistent weakness who did not undergo rehabilitation had significantly higher rates of reinjury.
Various injections containing local anesthetics, corticosteroids, platelet-rich plasma (PRP), and other substances have been administered to football players following acute muscle strains in an effort to alleviate pain and safely return the athlete to competition. Some practitioners have been reluctant to administer injections (especially those containing corticosteroids) due to a potentially increased risk of tendinopathy or rupture.31 Drakos and colleagues53 reported their outcomes following muscle and ligament strains treated with combined corticosteroid and local anesthetic injections on one NFL team. While quadriceps and hamstring strains were associated with the most missed games among all muscle strains, these injections resulted in no adverse events or progression of injury severity. Similarly, Levine and colleagues 51 administered intramuscular corticosteroid injections to 58 NFL players with high-grade hamstring injuries that had a palpable defect within the muscle belly. They reported no complications or strength deficits at final examination. In a case-control study, Rettig and colleagues46 administered PRP injections under ultrasound guidance in 5 NFL players with hamstring injuries. Compared to players treated with a focused rehabilitation program only, there were no significant differences in recovery or return to play.
The decision to return to play should be based on a clinical assessment considering pain, strength, motion, and flexibility. Player position should also be considered. Return-to-play guidelines describing the appropriate progression through rehabilitation and return to sport have been described and can be used as a template for the rehabilitation of football players.54 It should be noted that primary hamstring strains are associated with decreased athletic performance and an increased risk of more severe reinjury after return to sport.55,56
Morel-Lavallée Lesion
Pathophysiology
Morel-Lavallée lesions (MLLs) are uncommon football injuries, but often occur in the thigh.57,58 An MLL is a posttraumatic soft tissue injury in which deforming forces of pressure and shear cause a closed, soft tissue degloving injury; in this injury, the skin and subcutaneous tissues are separated from the underlying fascia, disrupting perforating blood vessels. The resulting space between the fascia and subcutaneous tissue fills with blood, lymphatics, and necrotic fat, resulting in a hematoma/seroma that can be a nidus for bacterial infection.58 The most common anatomic regions are the anterior distal thigh and lateral hip. Both of these areas are commonly involved in both direct contact and shear forces following a fall to the ground.
History and Physical Examination
Athletes with MLLs typically present with the insidious onset of a fluid collection within the thigh following a fall to the ground, usually while sliding or diving on the playing surface.57,58 The fluid collection can be associated with thigh tightness and may extend distally into the suprapatellar region or proximally over the greater trochanter. Thigh swelling, ecchymosis, and palpable fluctuance are seen in most cases. Progressive increases in pain and thigh swelling may be seen in severe injuries, but thigh compartments generally remain soft and nontender. Signs and symptoms of an MLL do not typically manifest immediately following the athletic event. Tejwani and colleagues58 reported a case series of MLLs of the knee in 27 NFL players from a single team over a 14-year period, with an average of 3 days between injury and evaluation by the medical staff. The mechanism of injury was a shearing blow from the knee striking the playing surface in 81% of cases and direct contact to the knee from another player in 19% of cases; all cases occurred in game situations. No affected players were wearing kneepads at the time of injury.
Imaging
Plain radiography may reveal a noncalcified soft tissue mass over the involved area and is not usually helpful except to rule out an underlying fracture. The appearance of an MLL on ultrasound is nonspecific and variable, often described as anechoic, hypoechoic, or hyperechoic depending on the presence of hemolymphatic fluid sedimentation and varying amounts of internal fat debris. MRI is the imaging modality of choice and typically shows a well-defined oval or fusiform, fluid-filled mass with tapering margins blending with adjacent fascial planes.
Treatment
Similar to quadriceps contusions, treatment goals for MLLs are evacuation of the fluid collection, prevention of fluid recurrence, a full range of active knee flexion, and prompt return to play.57,58 Initial treatment for smaller lesions consists of cryotherapy, compression wrapping of the involved area, and immediate active and passive range of motion of the hip and knee. While MLLs were traditionally treated with serial open debridements, less invasive approaches—including elastic compression, aspiration, percutaneous irrigation with debridement and suction drainage, or liposuction and drainage followed by suction therapy—have been recently described.57,58,60,61 Less invasive approaches aim to minimize soft tissue dissection and disruption of the vascular supply while accelerating rehabilitation. The presence of a surrounding capsule on MRI makes conservative or minimally invasive approaches less likely to be successful and may necessitate an open procedure.62 Antibiotics should be used preoperatively due to the presence of a dead space containing necrotic debris that makes infection a potential complication. While elite contact athletes can expect to return to competition long before complete resolution of an MLL, there is a risk of further delamination and lesion expansion due to re-injury prior to compete healing.
Tejwani and colleagues58 performed aspiration at the area of palpable fluctuance in the thigh or suprapatellar region using a 14-gauge needle in those athletes who failed to improve with conservative treatments alone. Mean time to resolution of the fluid collection was 16 days following aspiration. Fifty-two percent of the athletes were successfully treated with cryotherapy, compression, and motion exercises alone; 48% were treated with at least one aspiration, with a mean of 2.7 aspirations per knee. In 11% of cases that failed to resolve after multiple aspirations, doxycycline sclerodesis was performed immediately following an aspiration. Patients treated with sclerodesis had no return of the fluid collection and returned to play the following day.
Matava and colleagues57 described the case of an NFL player who sustained a closed MLL of the lateral hip while diving onto an artificial turf surface attempting to catch a pass. Despite immediate thigh pain and swelling, he was able to continue play. Immediately following the game, the player was examined and had a tense thigh with ecchymosis extending into the trochanteric region. Aspiration of the fluctuant area was unsuccessful. Progressive increases in pain and thigh swelling prompted hospital admission. Percutaneous irrigation and debridement was performed as described by Tseng and Tornetta.61 A suction drain was placed within the residual dead space, and constant wall suction was applied in addition to hip compression using a spica
Conclusion
Quadriceps and hamstring injuries occur frequently in football and are generally treated conservatively. While return to competition following hamstring strains is relatively quick, a high rate of injury recurrence highlights the importance of targeted rehabilitation and conditioning. Rarely, complications from quadriceps contusions, including acute compartment syndrome and myositis ossificans, may require operative intervention if unresponsive to conservative treatment. MLLs are rare in sports, but usually involve the thigh when they occur in football players. Team physicians must maintain a heightened degree of awareness of this injury as it may require operative intervention.
Acknowledgements: The authors would like to thank Jonathon Baker, MD and David Rubin, MD for their assistance in providing radiographic images for this paper.
Am J Orthop. 2016;45(6):E308-E318. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
2. Rechel JA, Yard EE, Comstock RD. An epidemiologic comparison of high school sports injuries sustained in practice and competition. J Athl Train. 2008;43(2):197-204.
3. Elliott MC, Zarins B, Powell JW, Kenyon CD. Hamstring muscle strains in professional football players: a 10-year review. Am J Sports Med. 2011;39(4):843-850.
4. Dick R, Ferrara MS, Agel J, et al. Descriptive epidemiology of collegiate men’s football injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athl Train. 2007;42(2):221-233.
5. Feeley BT, Kennelly S, Barnes RP, et al. Epidemiology of National Football League training camp injuries from 1998 to 2007. Am J Sports Med. 2008;36(8):1597-1603.
6. Garrett WE Jr. Muscle strain injuries. Am J Sports Med. 1996;24(6 Suppl):S2-S8.
7. Lawrence DW, Hutchison MG, Comper P. Descriptive epidemiology of musculoskeletal injuries and concussions in the National Football League, 2012-2014. Orthop J Sports Med. 2015;3(5):2325967115583653.
8. Diaz JA, Fischer DA, Rettig AC, Davis TJ, Shelbourne KD. Severe quadriceps muscle contusions in athletes. A report of three cases. Am J Sports Med. 2003;31(2):289-293.
9. Jackson DW, Feagin JA. Quadriceps contusions in young athletes. Relation of severity of injury to treatment and prognosis. J Bone Joint Surg Am. 1973;55(1):95-105.
10. Ryan JB, Wheeler JH, Hopkinson WJ, Arciero RA, Kolakowski KR. Quadriceps contusions. West Point update. Am J Sports Med. 1991;19(3):299-304.
11. Bencardino JT, Rosenberg ZS, Brown RR, Hassankhani A, Lustrin ES, Beltran J. Traumatic musculotendinous injuries of the knee: diagnosis with MR imaging. Radiographics. 2000;20 Spec No:S103-S120.
12. Robinson D, On E, Halperin N. Anterior compartment syndrome of the thigh in athletes--indications for conservative treatment. J Trauma. 1992;32(2):183-186.
13. Beckmann JT, Wylie JD, Kapron AL, Hanson JA, Maak TG, Aoki SK. The effect of NSAID prophylaxis and operative variables on heterotopic ossification after hip arthroscopy. Am J Sports Med. 2014;42(6):1359-1364.
14. Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J Nucl Med. 2002;43(3):346-353.
15. Beckmann JT, Wylie JD, Potter MQ, Maak TG, Greene TH, Aoki SK. Effect of naproxen prophylaxis on heterotopic ossification following hip arthroscopy: a double-blind randomized placebo-controlled trial. J Bone Joint Surg Am. 2015;97(24):2032-2037.
16. Yeung M, Jamshidi S, Horner N, Simunovic N, Karlsson J, Ayeni OR. Efficacy of nonsteroidal anti-inflammatory drug prophylaxis for heterotrophic ossification in hip arthroscopy: a systematic review. Arthroscopy. 2016;32(3):519-525.
17. Goyal K, Pettis CR, Bancroft AE, Wasyliw CW, Scherer KF. Myositis ossificans in the thigh of a lacrosse player. Orthopedics. 2015;38(8):468,515-518.
18. Cooper DE. Severe quadriceps muscle contusions in athletes. Am J Sports Med. 2004;32(3):820.
19. Bonsell S, Freudigman PT, Moore HA. Quadriceps muscle contusion resulting in osteomyelitis of the femur in a high school football player. A case report. Am J Sports Med. 2001;29(6):818-820.
20. Rothwell AG. Quadriceps hematoma. A prospective clinical study. Clin Orthop Relat Res. 1982;(171):97-103.
21. Armfield DR, Kim DH, Towers JD, Bradley JP, Robertson DD. Sports-related muscle injury in the lower extremity. Clin Sports Med. 2006;25(4):803-842.
22. Lipscomb AB, Thomas ED, Johnston RK. Treatment of myositis ossificans traumatica in athletes. Am J Sports Med. 1976;4(3):111-120.
23. Mani-Babu S, Wolman R, Keen R. Quadriceps traumatic myositis ossificans in a football player: management with intravenous pamidronate. Clin J Sport Med. 2014;24(5):e56-e58.
24. McCaffrey DD, Clarke J, Bunn J, McCormack MJ. Acute compartment syndrome of the anterior thigh in the absence of fracture secondary to sporting trauma. J Trauma. 2009;66(4):1238-1242.
25. Klasson SC, Vander Schilden JL. Acute anterior thigh compartment syndrome complicating quadriceps hematoma. Two case reports and review of the literature. Orthop Rev. 1990;19(5):421-427.
26. Rooser B. Quadriceps contusion with compartment syndrome. Evacuation of hematoma in 2 cases. Acta Orthop Scand. 1987;58(2):170-172.
27. Rooser B, Bengtson S, Hagglund G. Acute compartment syndrome from anterior thigh muscle contusion: a report of eight cases. J Orthop Trauma. 1991;5(1):57-59.
28. Schwartz JT Jr, Brumback RJ, Lakatos R, Poka A, Bathon GH, Burgess AR. Acute compartment syndrome of the thigh. A spectrum of injury. J Bone Joint Surg Am. 1989;71(3):392-400.
29. Elliott KG, Johnstone AJ. Diagnosing acute compartment syndrome. J Bone Joint Surg Br. 2003;85(5):625-632.
30. Kary JM. Diagnosis and management of quadriceps strains and contusions. Curr Rev Musculoskelet Med. 2010;3(1-4):26-31.
31. Boublik M, Schlegel TF, Koonce RC, Genuario JW, Kinkartz JD. Quadriceps tendon injuries in national football league players. Am J Sports Med. 2013;41(8):1841-1846.
32. Palmer WE, Kuong SJ, Elmadbouh HM. MR imaging of myotendinous strain. AJR Am J Roentgenol. 1999;173(3):703-709.
33. Cross TM, Gibbs N, Houang MT, Cameron M. Acute quadriceps muscle strains: magnetic resonance imaging features and prognosis. Am J Sports Med. 2004;32(3):710-719.
34. Hughes C 4th, Hasselman CT, Best TM, Martinez S, Garrett WE Jr. Incomplete, intrasubstance strain injuries of the rectus femoris muscle. Am J Sports Med. 1995;23(4):500-506.
35. Orchard JW. Intrinsic and extrinsic risk factors for muscle strains in Australian football. Am J Sports Med. 2001;29(3):300-303.36. Mair SD, Seaber AV, Glisson RR, Garrett WE, Jr. The role of fatigue in susceptibility to acute muscle strain injury. Am J Sports Med. 1996;24(2):137-143.
37. Bleakley C, McDonough S, MacAuley D. The use of ice in the treatment of acute soft-tissue injury: a systematic review of randomized controlled trials. Am J Sports Med. 2004;32(1):251-261.
38. Jarvinen TA, Jarvinen TL, Kaariainen M, Kalimo H, Jarvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33(5):745-764.
39. Clanton TO, Coupe KJ. Hamstring strains in athletes: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(4):237-248.
40. Novacheck TF. The biomechanics of running. Gait Posture. 1998;7(1):77-95.
41. Yu B, Queen RM, Abbey AN, Liu Y, Moorman CT, Garrett WE. Hamstring muscle kinematics and activation during overground sprinting. J Biomech. 2008;41(15):3121-3126.
42. Opar DA, Williams MD, Shield AJ. Hamstring strain injuries: factors that lead to injury and re-injury. Sports Med. 2012;42(3):209-226.
43. Askling CM, Tengvar M, Saartok T, Thorstensson A. Acute first-time hamstring strains during high-speed running: a longitudinal study including clinical and magnetic resonance imaging findings. Am J Sports Med. 2007;35(2):197-206.
44. Thelen DG, Chumanov ES, Hoerth DM, et al. Hamstring muscle kinematics during treadmill sprinting. Med Sci Sports Exerc. 2005;37(1):108-114.
45. Chumanov ES, Heiderscheit BC, Thelen DG. The effect of speed and influence of individual muscles on hamstring mechanics during the swing phase of sprinting. J Biomech. 2007;40(16):3555-3562.
46. Rettig AC, Meyer S, Bhadra AK. Platelet-rich plasma in addition to rehabilitation for acute hamstring injuries in NFL players: clinical effects and time to return to play. Orthop J Sports Med. 2013;1(1):2325967113494354.
47. Zvijac JE, Toriscelli TA, Merrick S, Kiebzak GM. Isokinetic concentric quadriceps and hamstring strength variables from the NFL Scouting Combine are not predictive of hamstring injury in first-year professional football players. Am J Sports Med. 2013;41(7):1511-1518.
48. Arnason A, Sigurdsson SB, Gudmundsson A, Holme I, Engebretsen L, Bahr R. Risk factors for injuries in football. Am J Sports Med. 2004;32(1 Suppl):5S-16S.
49. Zarins B, Ciullo JV. Acute muscle and tendon injuries in athletes. Clin Sports Med. 1983;2(1):167-182.
50. Arnason A, Andersen TE, Holme I, Engebretsen L, Bahr R. Prevention of hamstring strains in elite soccer: an intervention study. Scand J Med Sci Sports. 2008;18(1):40-48.
51. Levine WN, Bergfeld JA, Tessendorf W, Moorman CT 3rd. Intramuscular corticosteroid injection for hamstring injuries. A 13-year experience in the National Football League. Am J Sports Med. 2000;28(3):297-300.
52. Croisier JL, Ganteaume S, Binet J, Genty M, Ferret JM. Strength imbalances and prevention of hamstring injury in professional soccer players: a prospective study. Am J Sports Med. 2008;36(8):1469-1475.
53. Drakos M, Birmingham P, Delos D, et al. Corticosteroid and anesthetic injections for muscle strains and ligament sprains in the NFL. HSS J. 2014;10(2):136-142.
54. Worrell TW. Factors associated with hamstring injuries. An approach to treatment and preventative measures. Sports Med. 1994;17(5):338-345.
55. Brooks JH, Fuller CW, Kemp SP, Reddin DB. Incidence, risk, and prevention of hamstring muscle injuries in professional rugby union. Am J Sports Med. 2006;34(8):1297-1306.
56. Verrall GM, Kalairajah Y, Slavotinek JP, Spriggins AJ. Assessment of player performance following return to sport after hamstring muscle strain injury. J Sci Med Sport. 2006;9(1-2):87-90.
57. Matava MJ, Ellis E, Shah NR, Pogue D, Williams T. Morel-lavallee lesion in a professional american football player. Am J Orthop. 2010;39(3):144-147.
58. Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallee lesion of the knee: twenty-seven cases in the national football league. Am J Sports Med. 2007;35(7):1162-1167.
59. Mellado JM, Bencardino JT. Morel-Lavallee lesion: review with emphasis on MR imaging. Magn Reson Imaging Clin N Am. 2005;13(4):775-782.
60. Harma A, Inan M, Ertem K. [The Morel-Lavallee lesion: a conservative approach to closed degloving injuries]. Acta Orthop Traumatol Turc. 2004;38(4):270-273.
61. Tseng S, Tornetta P 3rd. Percutaneous management of Morel-Lavallee lesions. J Bone Joint Surg Am. 2006;88(1):92-96.
62. Gilbert BC, Bui-Mansfield LT, Dejong S. MRI of a Morel-Lavellee lesion. AJR Am J Roentgenol. 2004;182(5):1347-1348.
1. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
2. Rechel JA, Yard EE, Comstock RD. An epidemiologic comparison of high school sports injuries sustained in practice and competition. J Athl Train. 2008;43(2):197-204.
3. Elliott MC, Zarins B, Powell JW, Kenyon CD. Hamstring muscle strains in professional football players: a 10-year review. Am J Sports Med. 2011;39(4):843-850.
4. Dick R, Ferrara MS, Agel J, et al. Descriptive epidemiology of collegiate men’s football injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athl Train. 2007;42(2):221-233.
5. Feeley BT, Kennelly S, Barnes RP, et al. Epidemiology of National Football League training camp injuries from 1998 to 2007. Am J Sports Med. 2008;36(8):1597-1603.
6. Garrett WE Jr. Muscle strain injuries. Am J Sports Med. 1996;24(6 Suppl):S2-S8.
7. Lawrence DW, Hutchison MG, Comper P. Descriptive epidemiology of musculoskeletal injuries and concussions in the National Football League, 2012-2014. Orthop J Sports Med. 2015;3(5):2325967115583653.
8. Diaz JA, Fischer DA, Rettig AC, Davis TJ, Shelbourne KD. Severe quadriceps muscle contusions in athletes. A report of three cases. Am J Sports Med. 2003;31(2):289-293.
9. Jackson DW, Feagin JA. Quadriceps contusions in young athletes. Relation of severity of injury to treatment and prognosis. J Bone Joint Surg Am. 1973;55(1):95-105.
10. Ryan JB, Wheeler JH, Hopkinson WJ, Arciero RA, Kolakowski KR. Quadriceps contusions. West Point update. Am J Sports Med. 1991;19(3):299-304.
11. Bencardino JT, Rosenberg ZS, Brown RR, Hassankhani A, Lustrin ES, Beltran J. Traumatic musculotendinous injuries of the knee: diagnosis with MR imaging. Radiographics. 2000;20 Spec No:S103-S120.
12. Robinson D, On E, Halperin N. Anterior compartment syndrome of the thigh in athletes--indications for conservative treatment. J Trauma. 1992;32(2):183-186.
13. Beckmann JT, Wylie JD, Kapron AL, Hanson JA, Maak TG, Aoki SK. The effect of NSAID prophylaxis and operative variables on heterotopic ossification after hip arthroscopy. Am J Sports Med. 2014;42(6):1359-1364.
14. Shehab D, Elgazzar AH, Collier BD. Heterotopic ossification. J Nucl Med. 2002;43(3):346-353.
15. Beckmann JT, Wylie JD, Potter MQ, Maak TG, Greene TH, Aoki SK. Effect of naproxen prophylaxis on heterotopic ossification following hip arthroscopy: a double-blind randomized placebo-controlled trial. J Bone Joint Surg Am. 2015;97(24):2032-2037.
16. Yeung M, Jamshidi S, Horner N, Simunovic N, Karlsson J, Ayeni OR. Efficacy of nonsteroidal anti-inflammatory drug prophylaxis for heterotrophic ossification in hip arthroscopy: a systematic review. Arthroscopy. 2016;32(3):519-525.
17. Goyal K, Pettis CR, Bancroft AE, Wasyliw CW, Scherer KF. Myositis ossificans in the thigh of a lacrosse player. Orthopedics. 2015;38(8):468,515-518.
18. Cooper DE. Severe quadriceps muscle contusions in athletes. Am J Sports Med. 2004;32(3):820.
19. Bonsell S, Freudigman PT, Moore HA. Quadriceps muscle contusion resulting in osteomyelitis of the femur in a high school football player. A case report. Am J Sports Med. 2001;29(6):818-820.
20. Rothwell AG. Quadriceps hematoma. A prospective clinical study. Clin Orthop Relat Res. 1982;(171):97-103.
21. Armfield DR, Kim DH, Towers JD, Bradley JP, Robertson DD. Sports-related muscle injury in the lower extremity. Clin Sports Med. 2006;25(4):803-842.
22. Lipscomb AB, Thomas ED, Johnston RK. Treatment of myositis ossificans traumatica in athletes. Am J Sports Med. 1976;4(3):111-120.
23. Mani-Babu S, Wolman R, Keen R. Quadriceps traumatic myositis ossificans in a football player: management with intravenous pamidronate. Clin J Sport Med. 2014;24(5):e56-e58.
24. McCaffrey DD, Clarke J, Bunn J, McCormack MJ. Acute compartment syndrome of the anterior thigh in the absence of fracture secondary to sporting trauma. J Trauma. 2009;66(4):1238-1242.
25. Klasson SC, Vander Schilden JL. Acute anterior thigh compartment syndrome complicating quadriceps hematoma. Two case reports and review of the literature. Orthop Rev. 1990;19(5):421-427.
26. Rooser B. Quadriceps contusion with compartment syndrome. Evacuation of hematoma in 2 cases. Acta Orthop Scand. 1987;58(2):170-172.
27. Rooser B, Bengtson S, Hagglund G. Acute compartment syndrome from anterior thigh muscle contusion: a report of eight cases. J Orthop Trauma. 1991;5(1):57-59.
28. Schwartz JT Jr, Brumback RJ, Lakatos R, Poka A, Bathon GH, Burgess AR. Acute compartment syndrome of the thigh. A spectrum of injury. J Bone Joint Surg Am. 1989;71(3):392-400.
29. Elliott KG, Johnstone AJ. Diagnosing acute compartment syndrome. J Bone Joint Surg Br. 2003;85(5):625-632.
30. Kary JM. Diagnosis and management of quadriceps strains and contusions. Curr Rev Musculoskelet Med. 2010;3(1-4):26-31.
31. Boublik M, Schlegel TF, Koonce RC, Genuario JW, Kinkartz JD. Quadriceps tendon injuries in national football league players. Am J Sports Med. 2013;41(8):1841-1846.
32. Palmer WE, Kuong SJ, Elmadbouh HM. MR imaging of myotendinous strain. AJR Am J Roentgenol. 1999;173(3):703-709.
33. Cross TM, Gibbs N, Houang MT, Cameron M. Acute quadriceps muscle strains: magnetic resonance imaging features and prognosis. Am J Sports Med. 2004;32(3):710-719.
34. Hughes C 4th, Hasselman CT, Best TM, Martinez S, Garrett WE Jr. Incomplete, intrasubstance strain injuries of the rectus femoris muscle. Am J Sports Med. 1995;23(4):500-506.
35. Orchard JW. Intrinsic and extrinsic risk factors for muscle strains in Australian football. Am J Sports Med. 2001;29(3):300-303.36. Mair SD, Seaber AV, Glisson RR, Garrett WE, Jr. The role of fatigue in susceptibility to acute muscle strain injury. Am J Sports Med. 1996;24(2):137-143.
37. Bleakley C, McDonough S, MacAuley D. The use of ice in the treatment of acute soft-tissue injury: a systematic review of randomized controlled trials. Am J Sports Med. 2004;32(1):251-261.
38. Jarvinen TA, Jarvinen TL, Kaariainen M, Kalimo H, Jarvinen M. Muscle injuries: biology and treatment. Am J Sports Med. 2005;33(5):745-764.
39. Clanton TO, Coupe KJ. Hamstring strains in athletes: diagnosis and treatment. J Am Acad Orthop Surg. 1998;6(4):237-248.
40. Novacheck TF. The biomechanics of running. Gait Posture. 1998;7(1):77-95.
41. Yu B, Queen RM, Abbey AN, Liu Y, Moorman CT, Garrett WE. Hamstring muscle kinematics and activation during overground sprinting. J Biomech. 2008;41(15):3121-3126.
42. Opar DA, Williams MD, Shield AJ. Hamstring strain injuries: factors that lead to injury and re-injury. Sports Med. 2012;42(3):209-226.
43. Askling CM, Tengvar M, Saartok T, Thorstensson A. Acute first-time hamstring strains during high-speed running: a longitudinal study including clinical and magnetic resonance imaging findings. Am J Sports Med. 2007;35(2):197-206.
44. Thelen DG, Chumanov ES, Hoerth DM, et al. Hamstring muscle kinematics during treadmill sprinting. Med Sci Sports Exerc. 2005;37(1):108-114.
45. Chumanov ES, Heiderscheit BC, Thelen DG. The effect of speed and influence of individual muscles on hamstring mechanics during the swing phase of sprinting. J Biomech. 2007;40(16):3555-3562.
46. Rettig AC, Meyer S, Bhadra AK. Platelet-rich plasma in addition to rehabilitation for acute hamstring injuries in NFL players: clinical effects and time to return to play. Orthop J Sports Med. 2013;1(1):2325967113494354.
47. Zvijac JE, Toriscelli TA, Merrick S, Kiebzak GM. Isokinetic concentric quadriceps and hamstring strength variables from the NFL Scouting Combine are not predictive of hamstring injury in first-year professional football players. Am J Sports Med. 2013;41(7):1511-1518.
48. Arnason A, Sigurdsson SB, Gudmundsson A, Holme I, Engebretsen L, Bahr R. Risk factors for injuries in football. Am J Sports Med. 2004;32(1 Suppl):5S-16S.
49. Zarins B, Ciullo JV. Acute muscle and tendon injuries in athletes. Clin Sports Med. 1983;2(1):167-182.
50. Arnason A, Andersen TE, Holme I, Engebretsen L, Bahr R. Prevention of hamstring strains in elite soccer: an intervention study. Scand J Med Sci Sports. 2008;18(1):40-48.
51. Levine WN, Bergfeld JA, Tessendorf W, Moorman CT 3rd. Intramuscular corticosteroid injection for hamstring injuries. A 13-year experience in the National Football League. Am J Sports Med. 2000;28(3):297-300.
52. Croisier JL, Ganteaume S, Binet J, Genty M, Ferret JM. Strength imbalances and prevention of hamstring injury in professional soccer players: a prospective study. Am J Sports Med. 2008;36(8):1469-1475.
53. Drakos M, Birmingham P, Delos D, et al. Corticosteroid and anesthetic injections for muscle strains and ligament sprains in the NFL. HSS J. 2014;10(2):136-142.
54. Worrell TW. Factors associated with hamstring injuries. An approach to treatment and preventative measures. Sports Med. 1994;17(5):338-345.
55. Brooks JH, Fuller CW, Kemp SP, Reddin DB. Incidence, risk, and prevention of hamstring muscle injuries in professional rugby union. Am J Sports Med. 2006;34(8):1297-1306.
56. Verrall GM, Kalairajah Y, Slavotinek JP, Spriggins AJ. Assessment of player performance following return to sport after hamstring muscle strain injury. J Sci Med Sport. 2006;9(1-2):87-90.
57. Matava MJ, Ellis E, Shah NR, Pogue D, Williams T. Morel-lavallee lesion in a professional american football player. Am J Orthop. 2010;39(3):144-147.
58. Tejwani SG, Cohen SB, Bradley JP. Management of Morel-Lavallee lesion of the knee: twenty-seven cases in the national football league. Am J Sports Med. 2007;35(7):1162-1167.
59. Mellado JM, Bencardino JT. Morel-Lavallee lesion: review with emphasis on MR imaging. Magn Reson Imaging Clin N Am. 2005;13(4):775-782.
60. Harma A, Inan M, Ertem K. [The Morel-Lavallee lesion: a conservative approach to closed degloving injuries]. Acta Orthop Traumatol Turc. 2004;38(4):270-273.
61. Tseng S, Tornetta P 3rd. Percutaneous management of Morel-Lavallee lesions. J Bone Joint Surg Am. 2006;88(1):92-96.
62. Gilbert BC, Bui-Mansfield LT, Dejong S. MRI of a Morel-Lavellee lesion. AJR Am J Roentgenol. 2004;182(5):1347-1348.
Colorectal Screening: Available but Underused
The U.S. health care system has the capacity to screen as many as 80% of eligible adults for colorectal cancer, which would meet national goals for 2018. Unfortunately, not enough people are getting screened: Only half of adults in the U.S. are up-to-date on screening, according to the CDC.
Related: Are Periodic Endoscopies Useful for Patients Under 40?
To find out whether the screening capacity would be up to the task, CDC researchers estimated the number of colonoscopies or fecal immunochemical tests (FITs) that would be necessary every year to screen 80% of adults aged between 50 and 75 years. To accomplish this goal by 2024, the researchers say a national screening program that began in 2014 would need about 47 million FIT procedures and 5.1 million colonoscopies annually, using FIT as the primary screening test. About 11 to 13 million colonoscopies would be needed if a colonoscopy-only screening program were used.
Related: Do Age and Gender Matter in Colorectal Cancer?
The researchers also used data from the 2012 Survey of Endoscopic Capacity, which estimated that about 15 million colonoscopies were performed in 2012 and 10.5 million more could be performed every year.
“Colorectal cancer is the second leading cancer killer for men and women in the U.S.,” said Djenaba Joseph, MD, MPH, medical director of CDC’s colorectal cancer control program and lead author of the study paper, “but it doesn’t have to be.”
The U.S. health care system has the capacity to screen as many as 80% of eligible adults for colorectal cancer, which would meet national goals for 2018. Unfortunately, not enough people are getting screened: Only half of adults in the U.S. are up-to-date on screening, according to the CDC.
Related: Are Periodic Endoscopies Useful for Patients Under 40?
To find out whether the screening capacity would be up to the task, CDC researchers estimated the number of colonoscopies or fecal immunochemical tests (FITs) that would be necessary every year to screen 80% of adults aged between 50 and 75 years. To accomplish this goal by 2024, the researchers say a national screening program that began in 2014 would need about 47 million FIT procedures and 5.1 million colonoscopies annually, using FIT as the primary screening test. About 11 to 13 million colonoscopies would be needed if a colonoscopy-only screening program were used.
Related: Do Age and Gender Matter in Colorectal Cancer?
The researchers also used data from the 2012 Survey of Endoscopic Capacity, which estimated that about 15 million colonoscopies were performed in 2012 and 10.5 million more could be performed every year.
“Colorectal cancer is the second leading cancer killer for men and women in the U.S.,” said Djenaba Joseph, MD, MPH, medical director of CDC’s colorectal cancer control program and lead author of the study paper, “but it doesn’t have to be.”
The U.S. health care system has the capacity to screen as many as 80% of eligible adults for colorectal cancer, which would meet national goals for 2018. Unfortunately, not enough people are getting screened: Only half of adults in the U.S. are up-to-date on screening, according to the CDC.
Related: Are Periodic Endoscopies Useful for Patients Under 40?
To find out whether the screening capacity would be up to the task, CDC researchers estimated the number of colonoscopies or fecal immunochemical tests (FITs) that would be necessary every year to screen 80% of adults aged between 50 and 75 years. To accomplish this goal by 2024, the researchers say a national screening program that began in 2014 would need about 47 million FIT procedures and 5.1 million colonoscopies annually, using FIT as the primary screening test. About 11 to 13 million colonoscopies would be needed if a colonoscopy-only screening program were used.
Related: Do Age and Gender Matter in Colorectal Cancer?
The researchers also used data from the 2012 Survey of Endoscopic Capacity, which estimated that about 15 million colonoscopies were performed in 2012 and 10.5 million more could be performed every year.
“Colorectal cancer is the second leading cancer killer for men and women in the U.S.,” said Djenaba Joseph, MD, MPH, medical director of CDC’s colorectal cancer control program and lead author of the study paper, “but it doesn’t have to be.”
Disordered sleep: Ask the right questions to reveal this hidden confounder
Many patients are sleep deprived but are either unaware of, or unwilling to acknowledge, their problem. Sleep deprivation occurs because of inadequate sleep duration (often caused by insomnia or simply not allowing enough time for sleep), or poor sleep quality (often caused by sleep-disordered breathing). These conditions can be diagnosed by obtaining a thorough sleep history that consists of 5 groups of questions. The specific questions that should be asked in order to make a more accurate diagnosis are included in this article from Current Psychiatry, available at http://www.currentpsychiatry.com/the-publication/issue-single-view/disordered-sleep-ask-the-right-questions-to-reveal-this-hidden-confounder/913369ef53768717303c9cd35a58e790.html.
Many patients are sleep deprived but are either unaware of, or unwilling to acknowledge, their problem. Sleep deprivation occurs because of inadequate sleep duration (often caused by insomnia or simply not allowing enough time for sleep), or poor sleep quality (often caused by sleep-disordered breathing). These conditions can be diagnosed by obtaining a thorough sleep history that consists of 5 groups of questions. The specific questions that should be asked in order to make a more accurate diagnosis are included in this article from Current Psychiatry, available at http://www.currentpsychiatry.com/the-publication/issue-single-view/disordered-sleep-ask-the-right-questions-to-reveal-this-hidden-confounder/913369ef53768717303c9cd35a58e790.html.
Many patients are sleep deprived but are either unaware of, or unwilling to acknowledge, their problem. Sleep deprivation occurs because of inadequate sleep duration (often caused by insomnia or simply not allowing enough time for sleep), or poor sleep quality (often caused by sleep-disordered breathing). These conditions can be diagnosed by obtaining a thorough sleep history that consists of 5 groups of questions. The specific questions that should be asked in order to make a more accurate diagnosis are included in this article from Current Psychiatry, available at http://www.currentpsychiatry.com/the-publication/issue-single-view/disordered-sleep-ask-the-right-questions-to-reveal-this-hidden-confounder/913369ef53768717303c9cd35a58e790.html.
ASCO: Always screen cancer survivors for chronic pain
All adult cancer survivors should be screened for chronic pain at every visit, according to the American Society of Clinical Oncology’s first clinical practice guideline for managing this patient population. Estimates suggest that as many as 40% of the 14 million US adults living with cancer have chronic pain related to their malignancy. Yet most health care providers “haven’t been trained to recognize or treat long-term pain associated with cancer,” said Judith A. Paice, RN, PhD, in a press statement accompanying the release of the new guideline. To learn more about the key recommendations from the guideline, see this article from Family Practice News: http://www.familypracticenews.com/specialty-focus/pain/single-article-page/asco-always-screen-cancer-survivors-for-chronic-pain/5c182fdaacc6b573f00f001f3928749b.html.
All adult cancer survivors should be screened for chronic pain at every visit, according to the American Society of Clinical Oncology’s first clinical practice guideline for managing this patient population. Estimates suggest that as many as 40% of the 14 million US adults living with cancer have chronic pain related to their malignancy. Yet most health care providers “haven’t been trained to recognize or treat long-term pain associated with cancer,” said Judith A. Paice, RN, PhD, in a press statement accompanying the release of the new guideline. To learn more about the key recommendations from the guideline, see this article from Family Practice News: http://www.familypracticenews.com/specialty-focus/pain/single-article-page/asco-always-screen-cancer-survivors-for-chronic-pain/5c182fdaacc6b573f00f001f3928749b.html.
All adult cancer survivors should be screened for chronic pain at every visit, according to the American Society of Clinical Oncology’s first clinical practice guideline for managing this patient population. Estimates suggest that as many as 40% of the 14 million US adults living with cancer have chronic pain related to their malignancy. Yet most health care providers “haven’t been trained to recognize or treat long-term pain associated with cancer,” said Judith A. Paice, RN, PhD, in a press statement accompanying the release of the new guideline. To learn more about the key recommendations from the guideline, see this article from Family Practice News: http://www.familypracticenews.com/specialty-focus/pain/single-article-page/asco-always-screen-cancer-survivors-for-chronic-pain/5c182fdaacc6b573f00f001f3928749b.html.
Renal failure in HCV cirrhosis
A 54-year-old man with a history of cirrhosis secondary to hepatitis C virus (HCV) infection has had a progressive decline in kidney function. He was diagnosed with HCV 15 years ago; he tried interferon treatment, but this failed. He received a transjugular intrahepatic shunt 10 years ago after an episode of esophageal variceal bleeding. He has since been taking furosemide and spironolactone as maintenance treatment for ascites, and he has no other medical concerns, such as hypertension or diabetes. What is the cause of the patient’s renal failure? The answer can be found in this article from Cleveland Clinic Journal of Medicine: http://www.ccjm.org/current-issue/issue-single-view/renal-failure-in-hcv-cirrhosis/6336c91b99df7f186817921a5d5c08ab.html.
A 54-year-old man with a history of cirrhosis secondary to hepatitis C virus (HCV) infection has had a progressive decline in kidney function. He was diagnosed with HCV 15 years ago; he tried interferon treatment, but this failed. He received a transjugular intrahepatic shunt 10 years ago after an episode of esophageal variceal bleeding. He has since been taking furosemide and spironolactone as maintenance treatment for ascites, and he has no other medical concerns, such as hypertension or diabetes. What is the cause of the patient’s renal failure? The answer can be found in this article from Cleveland Clinic Journal of Medicine: http://www.ccjm.org/current-issue/issue-single-view/renal-failure-in-hcv-cirrhosis/6336c91b99df7f186817921a5d5c08ab.html.
A 54-year-old man with a history of cirrhosis secondary to hepatitis C virus (HCV) infection has had a progressive decline in kidney function. He was diagnosed with HCV 15 years ago; he tried interferon treatment, but this failed. He received a transjugular intrahepatic shunt 10 years ago after an episode of esophageal variceal bleeding. He has since been taking furosemide and spironolactone as maintenance treatment for ascites, and he has no other medical concerns, such as hypertension or diabetes. What is the cause of the patient’s renal failure? The answer can be found in this article from Cleveland Clinic Journal of Medicine: http://www.ccjm.org/current-issue/issue-single-view/renal-failure-in-hcv-cirrhosis/6336c91b99df7f186817921a5d5c08ab.html.
VIDEO: ICDs cut mortality in younger, healthier heart failure patients
Implantable cardioverter-defibrillators (ICDs) significantly cut the rate of sudden cardiac death in patients with non-ischemic systolic heart failure, and reduced all-cause mortality in patients younger than 68 years. Results from the Danish Study to Assess the Efficacy of ICDs in Patients with Non-ischemic Systolic Heart Failure on Mortality (DANISH) also highlighted the importance of targeting implantable ICD treatment to the patients with non-ischemic systolic heart failure who are most likely to benefit from it. The DANISH results “tell us ICDs can benefit patients if we can be a little better in selecting the right patients,” said Lars Køber, MD, professor of cardiology at the University of Copenhagen. Read more on which patients would benefit most from ICDs in this article from Cardiology News, available at: http://www.ecardiologynews.com/specialty-focus/heart-failure/single-article-page/video-icds-cut-mortality-in-younger-healthier-heart-failure-patients/8b26c497ed439d4bac87c4086f0f74f1.html#mytake
Implantable cardioverter-defibrillators (ICDs) significantly cut the rate of sudden cardiac death in patients with non-ischemic systolic heart failure, and reduced all-cause mortality in patients younger than 68 years. Results from the Danish Study to Assess the Efficacy of ICDs in Patients with Non-ischemic Systolic Heart Failure on Mortality (DANISH) also highlighted the importance of targeting implantable ICD treatment to the patients with non-ischemic systolic heart failure who are most likely to benefit from it. The DANISH results “tell us ICDs can benefit patients if we can be a little better in selecting the right patients,” said Lars Køber, MD, professor of cardiology at the University of Copenhagen. Read more on which patients would benefit most from ICDs in this article from Cardiology News, available at: http://www.ecardiologynews.com/specialty-focus/heart-failure/single-article-page/video-icds-cut-mortality-in-younger-healthier-heart-failure-patients/8b26c497ed439d4bac87c4086f0f74f1.html#mytake
Implantable cardioverter-defibrillators (ICDs) significantly cut the rate of sudden cardiac death in patients with non-ischemic systolic heart failure, and reduced all-cause mortality in patients younger than 68 years. Results from the Danish Study to Assess the Efficacy of ICDs in Patients with Non-ischemic Systolic Heart Failure on Mortality (DANISH) also highlighted the importance of targeting implantable ICD treatment to the patients with non-ischemic systolic heart failure who are most likely to benefit from it. The DANISH results “tell us ICDs can benefit patients if we can be a little better in selecting the right patients,” said Lars Køber, MD, professor of cardiology at the University of Copenhagen. Read more on which patients would benefit most from ICDs in this article from Cardiology News, available at: http://www.ecardiologynews.com/specialty-focus/heart-failure/single-article-page/video-icds-cut-mortality-in-younger-healthier-heart-failure-patients/8b26c497ed439d4bac87c4086f0f74f1.html#mytake
Rises in LDL and HDL cholesterol, triglycerides tied to lower diabetes risk
Higher levels of LDL cholesterol, HDL cholesterol, and triglycerides over a lifetime are protective against type 2 diabetes, a Mendelian randomization study has shown. Jon White, PhD, of University College London and his co-investigators sought to shed light on the role of the most commonly measured lipid fractions—LDL cholesterol, HDL cholesterol, and triglycerides—in the development of coronary artery disease and diabetes, particularly the observed link between statin therapy and an increased risk of diabetes. More on the study, and how the results can help to identify the potential effects of lipid-modifying drugs, is available at Family Practice News: http://www.familypracticenews.com/specialty-focus/cardiology/single-article-page/rises-in-ldl-and-hdl-cholesterol-triglycerides-tied-to-lower-diabetes-risk/1c99fd4b400f60185df3f4de7d895f5f.html.
Higher levels of LDL cholesterol, HDL cholesterol, and triglycerides over a lifetime are protective against type 2 diabetes, a Mendelian randomization study has shown. Jon White, PhD, of University College London and his co-investigators sought to shed light on the role of the most commonly measured lipid fractions—LDL cholesterol, HDL cholesterol, and triglycerides—in the development of coronary artery disease and diabetes, particularly the observed link between statin therapy and an increased risk of diabetes. More on the study, and how the results can help to identify the potential effects of lipid-modifying drugs, is available at Family Practice News: http://www.familypracticenews.com/specialty-focus/cardiology/single-article-page/rises-in-ldl-and-hdl-cholesterol-triglycerides-tied-to-lower-diabetes-risk/1c99fd4b400f60185df3f4de7d895f5f.html.
Higher levels of LDL cholesterol, HDL cholesterol, and triglycerides over a lifetime are protective against type 2 diabetes, a Mendelian randomization study has shown. Jon White, PhD, of University College London and his co-investigators sought to shed light on the role of the most commonly measured lipid fractions—LDL cholesterol, HDL cholesterol, and triglycerides—in the development of coronary artery disease and diabetes, particularly the observed link between statin therapy and an increased risk of diabetes. More on the study, and how the results can help to identify the potential effects of lipid-modifying drugs, is available at Family Practice News: http://www.familypracticenews.com/specialty-focus/cardiology/single-article-page/rises-in-ldl-and-hdl-cholesterol-triglycerides-tied-to-lower-diabetes-risk/1c99fd4b400f60185df3f4de7d895f5f.html.