User login
The frequency of influenza and bacterial coinfection
Treatment of patients admitted to the hospital with an upper respiratory infection is often complicated by the lack of diagnostics. Even in cases where a patient has a confirmed case of influenza, there is still the possibility that they may have, or become infected by, a secondary bacterial infection. This leads clinicians to treat patients empirically with antibiotics, which can result in unnecessary antibiotic use among patients without a bacterial infection.
Though antibiotics can be a lifesaving drug, their use is not without risks. Estimates suggest that 20% of patients taking common antibiotics experience some side effect. While most side effects are not life-threatening gastrointestinal effects, other nonnegligible side effects include anaphylactic shock, drug‐induced liver injury, increases in the risk of retinal detachment, serious arrhythmias, and superinfection with resistant bacteria. Antibiotics can also lead to secondary infections, such as Clostridium difficile.
Yet, despite these risks, there has been limited research on the percentage of patients with influenza who actually have a bacterial coinfection. A recent systematic review and meta-analysis conducted by my colleagues at Johns Hopkins University and the Center for Disease Dynamics, Economics & Policy examined the frequency of bacterial coinfection among hospitalized patients with influenza and identified the most common infecting bacterial species.
The findings, published in the journal Influenza and Other Respiratory Viruses, found that in the majority of studies, between 11% and 35% of patients with confirmed influenza had a bacterial coinfection. The most common coinfecting bacteria were found to be Streptococcus pneumoniae and Staphylococcus aureus. Combined, S. pneumoniae and S. aureus accounted for more than 60% of the identified coinfecting bacteria; however, many other bacterial species were found to cause infections as well.
The results suggest that while bacterial infection is common in influenza patients, only about a quarter of patients are likely to be infected. However, the studies were widely heterogeneous, both in patient makeup and results. Analyses of age, setting, enrollment year, study type, study size, and bacterial collection methods did not reveal a source for the heterogeneity in results. Thus, additional factors, such as patient comorbidities or prior antibiotic use, which could not be systematically assessed, may affect the likelihood of coinfection.
Given that the symptoms of influenza and bacterial infection often overlap, correctly diagnosing bacterial coinfection without a laboratory culture can present a challenge. This diagnostic uncertainty leads to significant overuse of antibiotics in patients with influenza alone. Most influenza cases will never result in serious bacterial infections (particularly in nonhospitalized patients), and thus a lot of antibiotic use is unnecessary. As mentioned earlier, unnecessary antibiotic use poses a nonnegligible risk to patients, but it also contributes significantly to rising rates of antibiotic resistance, a major public health issue.
The results from this study highlight that the majority of patients hospitalized with influenza are unlikely to be coinfected with a bacterial pathogen. Thus, it is important for clinicians to appropriately treat patients with antiviral drugs, and ensure that bacterial testing is done when presumptively starting patients on antibiotics. Based on the microbiology results, antibiotics can be stopped if no pathogen is identified or altered to be more appropriate depending on the pathogen found.
Although the findings of this study suggest we need a more thorough analysis of the issue, the results should still aid clinicians by improving their understanding of the likelihood of bacterial coinfection in hospitalized patients with influenza, and thus help them balance the need to minimize patient risks as well as the individual and societal risks of nonessential antibiotic use.
Eili Klein, PhD, is assistant professor in the department of emergency medicine at Johns Hopkins Medicine, Baltimore.
Treatment of patients admitted to the hospital with an upper respiratory infection is often complicated by the lack of diagnostics. Even in cases where a patient has a confirmed case of influenza, there is still the possibility that they may have, or become infected by, a secondary bacterial infection. This leads clinicians to treat patients empirically with antibiotics, which can result in unnecessary antibiotic use among patients without a bacterial infection.
Though antibiotics can be a lifesaving drug, their use is not without risks. Estimates suggest that 20% of patients taking common antibiotics experience some side effect. While most side effects are not life-threatening gastrointestinal effects, other nonnegligible side effects include anaphylactic shock, drug‐induced liver injury, increases in the risk of retinal detachment, serious arrhythmias, and superinfection with resistant bacteria. Antibiotics can also lead to secondary infections, such as Clostridium difficile.
Yet, despite these risks, there has been limited research on the percentage of patients with influenza who actually have a bacterial coinfection. A recent systematic review and meta-analysis conducted by my colleagues at Johns Hopkins University and the Center for Disease Dynamics, Economics & Policy examined the frequency of bacterial coinfection among hospitalized patients with influenza and identified the most common infecting bacterial species.
The findings, published in the journal Influenza and Other Respiratory Viruses, found that in the majority of studies, between 11% and 35% of patients with confirmed influenza had a bacterial coinfection. The most common coinfecting bacteria were found to be Streptococcus pneumoniae and Staphylococcus aureus. Combined, S. pneumoniae and S. aureus accounted for more than 60% of the identified coinfecting bacteria; however, many other bacterial species were found to cause infections as well.
The results suggest that while bacterial infection is common in influenza patients, only about a quarter of patients are likely to be infected. However, the studies were widely heterogeneous, both in patient makeup and results. Analyses of age, setting, enrollment year, study type, study size, and bacterial collection methods did not reveal a source for the heterogeneity in results. Thus, additional factors, such as patient comorbidities or prior antibiotic use, which could not be systematically assessed, may affect the likelihood of coinfection.
Given that the symptoms of influenza and bacterial infection often overlap, correctly diagnosing bacterial coinfection without a laboratory culture can present a challenge. This diagnostic uncertainty leads to significant overuse of antibiotics in patients with influenza alone. Most influenza cases will never result in serious bacterial infections (particularly in nonhospitalized patients), and thus a lot of antibiotic use is unnecessary. As mentioned earlier, unnecessary antibiotic use poses a nonnegligible risk to patients, but it also contributes significantly to rising rates of antibiotic resistance, a major public health issue.
The results from this study highlight that the majority of patients hospitalized with influenza are unlikely to be coinfected with a bacterial pathogen. Thus, it is important for clinicians to appropriately treat patients with antiviral drugs, and ensure that bacterial testing is done when presumptively starting patients on antibiotics. Based on the microbiology results, antibiotics can be stopped if no pathogen is identified or altered to be more appropriate depending on the pathogen found.
Although the findings of this study suggest we need a more thorough analysis of the issue, the results should still aid clinicians by improving their understanding of the likelihood of bacterial coinfection in hospitalized patients with influenza, and thus help them balance the need to minimize patient risks as well as the individual and societal risks of nonessential antibiotic use.
Eili Klein, PhD, is assistant professor in the department of emergency medicine at Johns Hopkins Medicine, Baltimore.
Treatment of patients admitted to the hospital with an upper respiratory infection is often complicated by the lack of diagnostics. Even in cases where a patient has a confirmed case of influenza, there is still the possibility that they may have, or become infected by, a secondary bacterial infection. This leads clinicians to treat patients empirically with antibiotics, which can result in unnecessary antibiotic use among patients without a bacterial infection.
Though antibiotics can be a lifesaving drug, their use is not without risks. Estimates suggest that 20% of patients taking common antibiotics experience some side effect. While most side effects are not life-threatening gastrointestinal effects, other nonnegligible side effects include anaphylactic shock, drug‐induced liver injury, increases in the risk of retinal detachment, serious arrhythmias, and superinfection with resistant bacteria. Antibiotics can also lead to secondary infections, such as Clostridium difficile.
Yet, despite these risks, there has been limited research on the percentage of patients with influenza who actually have a bacterial coinfection. A recent systematic review and meta-analysis conducted by my colleagues at Johns Hopkins University and the Center for Disease Dynamics, Economics & Policy examined the frequency of bacterial coinfection among hospitalized patients with influenza and identified the most common infecting bacterial species.
The findings, published in the journal Influenza and Other Respiratory Viruses, found that in the majority of studies, between 11% and 35% of patients with confirmed influenza had a bacterial coinfection. The most common coinfecting bacteria were found to be Streptococcus pneumoniae and Staphylococcus aureus. Combined, S. pneumoniae and S. aureus accounted for more than 60% of the identified coinfecting bacteria; however, many other bacterial species were found to cause infections as well.
The results suggest that while bacterial infection is common in influenza patients, only about a quarter of patients are likely to be infected. However, the studies were widely heterogeneous, both in patient makeup and results. Analyses of age, setting, enrollment year, study type, study size, and bacterial collection methods did not reveal a source for the heterogeneity in results. Thus, additional factors, such as patient comorbidities or prior antibiotic use, which could not be systematically assessed, may affect the likelihood of coinfection.
Given that the symptoms of influenza and bacterial infection often overlap, correctly diagnosing bacterial coinfection without a laboratory culture can present a challenge. This diagnostic uncertainty leads to significant overuse of antibiotics in patients with influenza alone. Most influenza cases will never result in serious bacterial infections (particularly in nonhospitalized patients), and thus a lot of antibiotic use is unnecessary. As mentioned earlier, unnecessary antibiotic use poses a nonnegligible risk to patients, but it also contributes significantly to rising rates of antibiotic resistance, a major public health issue.
The results from this study highlight that the majority of patients hospitalized with influenza are unlikely to be coinfected with a bacterial pathogen. Thus, it is important for clinicians to appropriately treat patients with antiviral drugs, and ensure that bacterial testing is done when presumptively starting patients on antibiotics. Based on the microbiology results, antibiotics can be stopped if no pathogen is identified or altered to be more appropriate depending on the pathogen found.
Although the findings of this study suggest we need a more thorough analysis of the issue, the results should still aid clinicians by improving their understanding of the likelihood of bacterial coinfection in hospitalized patients with influenza, and thus help them balance the need to minimize patient risks as well as the individual and societal risks of nonessential antibiotic use.
Eili Klein, PhD, is assistant professor in the department of emergency medicine at Johns Hopkins Medicine, Baltimore.
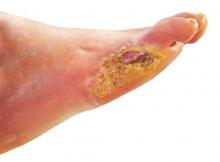
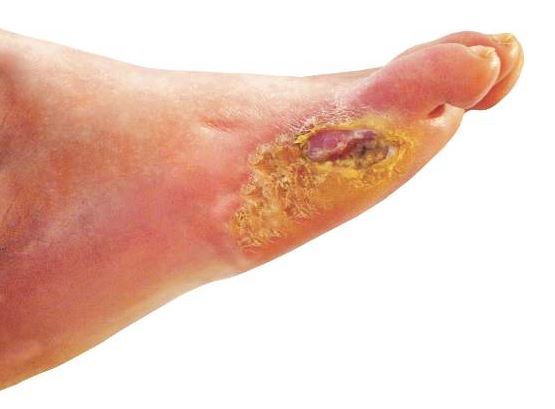
Many patients with diabetic foot infections get unnecessary MRSA treatment
Many patients with diabetic foot infections receive methicillin-resistant Staphylococcus aureus antibiotics unnecessarily, according to Kelly Reveles, PharmD, and her associates.
Among the 318 patients with diabetic foot infections (DFIs) in the study, S. aureus was the most common pathogen, accounting for 146 cases. MRSA accounted for 47 of S. aureus cases, and 15% of overall cases. Although MRSA accounted for a relatively small number of cases, MRSA antibiotics were administered to 86% of all patients, resulting in 71% of all patients receiving the treatment unnecessarily.
Independent risk factors for MRSA DFI were male sex and bone involvement. Other risk factors included previous MRSA infection, more severe infection, and a higher white cell count. The most common comorbidities of DFI were hypertension, dyslipidemia, and obesity.
“The improper use of antibiotics unnecessarily exposes the patient to potential complications of the therapy. Furthermore, the overuse of antibiotics drives antimicrobial resistance and is likely to increase the health care burden,” the investigators wrote.
Find the full study in PLoS One (doi: 10.1371/journal.pone.0161658).
Many patients with diabetic foot infections receive methicillin-resistant Staphylococcus aureus antibiotics unnecessarily, according to Kelly Reveles, PharmD, and her associates.
Among the 318 patients with diabetic foot infections (DFIs) in the study, S. aureus was the most common pathogen, accounting for 146 cases. MRSA accounted for 47 of S. aureus cases, and 15% of overall cases. Although MRSA accounted for a relatively small number of cases, MRSA antibiotics were administered to 86% of all patients, resulting in 71% of all patients receiving the treatment unnecessarily.
Independent risk factors for MRSA DFI were male sex and bone involvement. Other risk factors included previous MRSA infection, more severe infection, and a higher white cell count. The most common comorbidities of DFI were hypertension, dyslipidemia, and obesity.
“The improper use of antibiotics unnecessarily exposes the patient to potential complications of the therapy. Furthermore, the overuse of antibiotics drives antimicrobial resistance and is likely to increase the health care burden,” the investigators wrote.
Find the full study in PLoS One (doi: 10.1371/journal.pone.0161658).
Many patients with diabetic foot infections receive methicillin-resistant Staphylococcus aureus antibiotics unnecessarily, according to Kelly Reveles, PharmD, and her associates.
Among the 318 patients with diabetic foot infections (DFIs) in the study, S. aureus was the most common pathogen, accounting for 146 cases. MRSA accounted for 47 of S. aureus cases, and 15% of overall cases. Although MRSA accounted for a relatively small number of cases, MRSA antibiotics were administered to 86% of all patients, resulting in 71% of all patients receiving the treatment unnecessarily.
Independent risk factors for MRSA DFI were male sex and bone involvement. Other risk factors included previous MRSA infection, more severe infection, and a higher white cell count. The most common comorbidities of DFI were hypertension, dyslipidemia, and obesity.
“The improper use of antibiotics unnecessarily exposes the patient to potential complications of the therapy. Furthermore, the overuse of antibiotics drives antimicrobial resistance and is likely to increase the health care burden,” the investigators wrote.
Find the full study in PLoS One (doi: 10.1371/journal.pone.0161658).
FROM PLOS ONE
Don’t balk at using medical therapy to manage alcohol use disorder
There is ample evidence in the medical literature, as well as clinical experience, that patients seeking help for chemical dependency benefit from pharmacotherapy. It is common, however, for physicians, patients, and family to balk at the idea. Even within the psychiatry community, where there should be better understanding of substance use disorders, many practitioners hesitate to employ medications, especially for alcohol use disorder (AUD).
Efficacy for such FDA-approved medications has been demonstrated in well-designed, randomized controlled trials, but many trainees, and even experienced professionals, have never seen these medications used effectively and appropriately. Medication-assisted treatment (MAT) is not an alternative to biopsychosocial approaches but is an augmentation that can (1) help stabilize the patient until he (she) can be educated in relapse prevention skills and (2) allow the brain to rewire and heal until he regains impulse control.
Diverse presentations
Do you remember that patient who often arrived for appointments intoxicated, promising that he plans to cut down? How about the man you saw in the emergency department with an elevated blood alcohol level, who was constantly endorsing suicidal thoughts that subsided when he reached clinical sobriety? What about the college student who often was treated for alcohol poisoning after binge drinking on weekends, but who never considered this behavior problematic? And, how about the elderly woman who was evaluated for anxiety, but had been drinking 4 beers nightly for the past 30 years?
Despite the diverse presentations, these patients all have a chronic disease and we fail them when we do not apply evidence-based medicine to their treatment.
As psychiatrists, we encounter many patients with AUD as a primary or comorbid diagnosis. This is a global problem associated with significant human and financial cost. With 80% of American adolescents having reported using alcohol in the past year, the problem will continue to grow.1 Furthermore, a greater prevalence of AUD is noted in clinical populations undergoing psychiatric treatment.2 Ongoing alcohol abuse complicates the course of medical and psychiatric conditions and incites significant societal exclusion.
Pharmacotherapy is underutilized
Despite an increase in the use of psychotropic medications for treating psychiatric illness, pharmacotherapy for AUD is underutilized: only 3% of patients have received an FDA-approved treatment.2,3 Nearly one-third of adults are affected by AUD during their lifetime, yet only 20% seek help.3 Management today remains limited to episodic, brief inpatient detoxification and psychosocial therapy.
Recovery rates are highest when addiction treatment that monitors abstinence is continuous; yet, for most part, alcohol addiction is treated in discrete episodes upon relapse. Although MAT is recommended by experts for “moderate” and “severe” substance use disorders, practitioners, in general, have demonstrated considerable resistance to using this modality as part of routine practice.4,5 This is regrettable: Regardless of terminology used to describe their condition, these people suffer a potentially fatal disease characterized by high post-treatment recidivism.
Neuroscience supports the brain disease model of addiction, with neuroplasticity changes being made during phases of drug use. Medications are shown to assist in preventing relapse while the brain is healing and normal emotional and decision-making capacities are being restored.6
Why hesitate to use pharmacotherapeutics?
There are diverse pharmacotherapeutic options that can be pursued for treating AUD with minimal disruption to home and work life. Alarmingly, many trainees have never prescribed or even considered such medications. Despite modest effect sizes in randomized controlled trials, efficacy has been demonstrated in reducing relapse rates and overall severity of drinking days.4,5 So, from where does the ambivalence of patients and providers about using these treatments to achieve lasting recovery stem?
Starting MAT certainly requires both parties to be in agreement. A patient might decline medication because of a fear of dependence or because he overestimates his ability to achieve remission on his own. There also may be financial barriers in a current alcohol treatment system that is traditionally non-medically oriented. Prescribers also fail to offer medications because of:
- lack of familiarity with available agents
- absence of guidelines for use
- disbelief that the condition is treatable.
Given that treatment often is based on a 12-step approach, such as Alcoholics Anonymous (AA), providers might hesitate to prescribe medication for an illness that is thought to be managed through psychosocial interventions, such as group and motivational therapy.
Therapeutic options
Choice of medication depends on the prescriber’s comfort level, reputation of the medication, potential side-effect profile, medical contraindications, and affordability; the most important consideration, however, should be the overall goals and expectations of the patient.
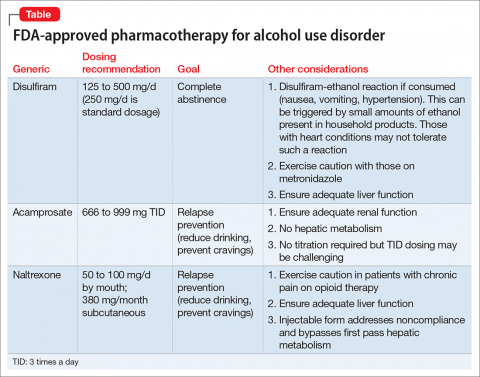
There are 4 FDA-approved medications for AUD (Table); many others are off-label. It is advisable to start with an FDA-approved medication such as disulfiram for the motivated patient who has a collaborator and desires complete abstinence; naltrexone for a patient who wants to cut down on intake (a long-acting formulation can be used for poorly adherent patients); and acamprosate for a patient with at least some established sobriety who needs help with post-withdrawal sleep disturbances.
With regard to off-label medications, topiramate has the highest evidence for efficacy. Gabapentin can augment naltrexone and also helps with sleep, anxiety, withdrawal, and cravings.4,5
Psychosocial interventions
Medications are just 1 tool in recovery; patients should be engaged in a program of counseling. Encourage attendance at AA meetings. An up-and-coming concept is the use of smartphone applications to prevent relapse (or even induce remission); apps that provide an accurate blood alcohol tracking systems and integrated psychosocial therapies are in the pipeline. The novel Reddit online forum r/StopDrinking is a 24-hour peer-support community that relies on
fellowship, accountability, monitoring, and anonymity; the forum can compete with
motivational interviewing for efficacy in increasing abstinence and preventing relapse.
1. Johnson L, O’Malley P, Miech RA, et al. Monitoring the Future national survey results on drug use, 1975-2015: overview, key findings on adolescent drug use. http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2015.pdf. Published February 2016. Accessed January 20, 2016.
2. Substance Abuse and Mental Health Services Administration. Results from the 2013 national survey on drug use and health: mental health findings, NSDUH Series H-49, HHS Publication No. (SMA) 14-4887. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
3. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiological Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
4. Robinson S, Meeks TW, Geniza C. Medication for alcohol use disorder: which agents work best. Current Psychiatry. 2014;13(1):22-29.
5. Substance Abuse and Mental Health Services Administration and National Institute on Alcohol Abuse and Alcoholism. Medication for the treatment of alcohol use disorder: a brief guide. HHS Publication No. (SMA) 15-4907. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
6. Volkow ND, Koob GF, McLellan AT. Neurobiological advances from the brain disease model of addiction. N Engl J Med. 2016;374(4):363-371.
Acknowledgment
The authors would like to thank Thomas M. Penders, MS, MD, Medical Director for Consultation-Liaison Psychiatry at Cape Cod Healthcare, Hyannis, Massachusetts, and Affiliate Professor at East Carolina University, Greenville, North Carolina, for all his guidance, support, and mentorship.
There is ample evidence in the medical literature, as well as clinical experience, that patients seeking help for chemical dependency benefit from pharmacotherapy. It is common, however, for physicians, patients, and family to balk at the idea. Even within the psychiatry community, where there should be better understanding of substance use disorders, many practitioners hesitate to employ medications, especially for alcohol use disorder (AUD).
Efficacy for such FDA-approved medications has been demonstrated in well-designed, randomized controlled trials, but many trainees, and even experienced professionals, have never seen these medications used effectively and appropriately. Medication-assisted treatment (MAT) is not an alternative to biopsychosocial approaches but is an augmentation that can (1) help stabilize the patient until he (she) can be educated in relapse prevention skills and (2) allow the brain to rewire and heal until he regains impulse control.
Diverse presentations
Do you remember that patient who often arrived for appointments intoxicated, promising that he plans to cut down? How about the man you saw in the emergency department with an elevated blood alcohol level, who was constantly endorsing suicidal thoughts that subsided when he reached clinical sobriety? What about the college student who often was treated for alcohol poisoning after binge drinking on weekends, but who never considered this behavior problematic? And, how about the elderly woman who was evaluated for anxiety, but had been drinking 4 beers nightly for the past 30 years?
Despite the diverse presentations, these patients all have a chronic disease and we fail them when we do not apply evidence-based medicine to their treatment.
As psychiatrists, we encounter many patients with AUD as a primary or comorbid diagnosis. This is a global problem associated with significant human and financial cost. With 80% of American adolescents having reported using alcohol in the past year, the problem will continue to grow.1 Furthermore, a greater prevalence of AUD is noted in clinical populations undergoing psychiatric treatment.2 Ongoing alcohol abuse complicates the course of medical and psychiatric conditions and incites significant societal exclusion.
Pharmacotherapy is underutilized
Despite an increase in the use of psychotropic medications for treating psychiatric illness, pharmacotherapy for AUD is underutilized: only 3% of patients have received an FDA-approved treatment.2,3 Nearly one-third of adults are affected by AUD during their lifetime, yet only 20% seek help.3 Management today remains limited to episodic, brief inpatient detoxification and psychosocial therapy.
Recovery rates are highest when addiction treatment that monitors abstinence is continuous; yet, for most part, alcohol addiction is treated in discrete episodes upon relapse. Although MAT is recommended by experts for “moderate” and “severe” substance use disorders, practitioners, in general, have demonstrated considerable resistance to using this modality as part of routine practice.4,5 This is regrettable: Regardless of terminology used to describe their condition, these people suffer a potentially fatal disease characterized by high post-treatment recidivism.
Neuroscience supports the brain disease model of addiction, with neuroplasticity changes being made during phases of drug use. Medications are shown to assist in preventing relapse while the brain is healing and normal emotional and decision-making capacities are being restored.6
Why hesitate to use pharmacotherapeutics?
There are diverse pharmacotherapeutic options that can be pursued for treating AUD with minimal disruption to home and work life. Alarmingly, many trainees have never prescribed or even considered such medications. Despite modest effect sizes in randomized controlled trials, efficacy has been demonstrated in reducing relapse rates and overall severity of drinking days.4,5 So, from where does the ambivalence of patients and providers about using these treatments to achieve lasting recovery stem?
Starting MAT certainly requires both parties to be in agreement. A patient might decline medication because of a fear of dependence or because he overestimates his ability to achieve remission on his own. There also may be financial barriers in a current alcohol treatment system that is traditionally non-medically oriented. Prescribers also fail to offer medications because of:
- lack of familiarity with available agents
- absence of guidelines for use
- disbelief that the condition is treatable.
Given that treatment often is based on a 12-step approach, such as Alcoholics Anonymous (AA), providers might hesitate to prescribe medication for an illness that is thought to be managed through psychosocial interventions, such as group and motivational therapy.
Therapeutic options
Choice of medication depends on the prescriber’s comfort level, reputation of the medication, potential side-effect profile, medical contraindications, and affordability; the most important consideration, however, should be the overall goals and expectations of the patient.
There are 4 FDA-approved medications for AUD (Table); many others are off-label. It is advisable to start with an FDA-approved medication such as disulfiram for the motivated patient who has a collaborator and desires complete abstinence; naltrexone for a patient who wants to cut down on intake (a long-acting formulation can be used for poorly adherent patients); and acamprosate for a patient with at least some established sobriety who needs help with post-withdrawal sleep disturbances.
With regard to off-label medications, topiramate has the highest evidence for efficacy. Gabapentin can augment naltrexone and also helps with sleep, anxiety, withdrawal, and cravings.4,5
Psychosocial interventions
Medications are just 1 tool in recovery; patients should be engaged in a program of counseling. Encourage attendance at AA meetings. An up-and-coming concept is the use of smartphone applications to prevent relapse (or even induce remission); apps that provide an accurate blood alcohol tracking systems and integrated psychosocial therapies are in the pipeline. The novel Reddit online forum r/StopDrinking is a 24-hour peer-support community that relies on
fellowship, accountability, monitoring, and anonymity; the forum can compete with
motivational interviewing for efficacy in increasing abstinence and preventing relapse.
There is ample evidence in the medical literature, as well as clinical experience, that patients seeking help for chemical dependency benefit from pharmacotherapy. It is common, however, for physicians, patients, and family to balk at the idea. Even within the psychiatry community, where there should be better understanding of substance use disorders, many practitioners hesitate to employ medications, especially for alcohol use disorder (AUD).
Efficacy for such FDA-approved medications has been demonstrated in well-designed, randomized controlled trials, but many trainees, and even experienced professionals, have never seen these medications used effectively and appropriately. Medication-assisted treatment (MAT) is not an alternative to biopsychosocial approaches but is an augmentation that can (1) help stabilize the patient until he (she) can be educated in relapse prevention skills and (2) allow the brain to rewire and heal until he regains impulse control.
Diverse presentations
Do you remember that patient who often arrived for appointments intoxicated, promising that he plans to cut down? How about the man you saw in the emergency department with an elevated blood alcohol level, who was constantly endorsing suicidal thoughts that subsided when he reached clinical sobriety? What about the college student who often was treated for alcohol poisoning after binge drinking on weekends, but who never considered this behavior problematic? And, how about the elderly woman who was evaluated for anxiety, but had been drinking 4 beers nightly for the past 30 years?
Despite the diverse presentations, these patients all have a chronic disease and we fail them when we do not apply evidence-based medicine to their treatment.
As psychiatrists, we encounter many patients with AUD as a primary or comorbid diagnosis. This is a global problem associated with significant human and financial cost. With 80% of American adolescents having reported using alcohol in the past year, the problem will continue to grow.1 Furthermore, a greater prevalence of AUD is noted in clinical populations undergoing psychiatric treatment.2 Ongoing alcohol abuse complicates the course of medical and psychiatric conditions and incites significant societal exclusion.
Pharmacotherapy is underutilized
Despite an increase in the use of psychotropic medications for treating psychiatric illness, pharmacotherapy for AUD is underutilized: only 3% of patients have received an FDA-approved treatment.2,3 Nearly one-third of adults are affected by AUD during their lifetime, yet only 20% seek help.3 Management today remains limited to episodic, brief inpatient detoxification and psychosocial therapy.
Recovery rates are highest when addiction treatment that monitors abstinence is continuous; yet, for most part, alcohol addiction is treated in discrete episodes upon relapse. Although MAT is recommended by experts for “moderate” and “severe” substance use disorders, practitioners, in general, have demonstrated considerable resistance to using this modality as part of routine practice.4,5 This is regrettable: Regardless of terminology used to describe their condition, these people suffer a potentially fatal disease characterized by high post-treatment recidivism.
Neuroscience supports the brain disease model of addiction, with neuroplasticity changes being made during phases of drug use. Medications are shown to assist in preventing relapse while the brain is healing and normal emotional and decision-making capacities are being restored.6
Why hesitate to use pharmacotherapeutics?
There are diverse pharmacotherapeutic options that can be pursued for treating AUD with minimal disruption to home and work life. Alarmingly, many trainees have never prescribed or even considered such medications. Despite modest effect sizes in randomized controlled trials, efficacy has been demonstrated in reducing relapse rates and overall severity of drinking days.4,5 So, from where does the ambivalence of patients and providers about using these treatments to achieve lasting recovery stem?
Starting MAT certainly requires both parties to be in agreement. A patient might decline medication because of a fear of dependence or because he overestimates his ability to achieve remission on his own. There also may be financial barriers in a current alcohol treatment system that is traditionally non-medically oriented. Prescribers also fail to offer medications because of:
- lack of familiarity with available agents
- absence of guidelines for use
- disbelief that the condition is treatable.
Given that treatment often is based on a 12-step approach, such as Alcoholics Anonymous (AA), providers might hesitate to prescribe medication for an illness that is thought to be managed through psychosocial interventions, such as group and motivational therapy.
Therapeutic options
Choice of medication depends on the prescriber’s comfort level, reputation of the medication, potential side-effect profile, medical contraindications, and affordability; the most important consideration, however, should be the overall goals and expectations of the patient.
There are 4 FDA-approved medications for AUD (Table); many others are off-label. It is advisable to start with an FDA-approved medication such as disulfiram for the motivated patient who has a collaborator and desires complete abstinence; naltrexone for a patient who wants to cut down on intake (a long-acting formulation can be used for poorly adherent patients); and acamprosate for a patient with at least some established sobriety who needs help with post-withdrawal sleep disturbances.
With regard to off-label medications, topiramate has the highest evidence for efficacy. Gabapentin can augment naltrexone and also helps with sleep, anxiety, withdrawal, and cravings.4,5
Psychosocial interventions
Medications are just 1 tool in recovery; patients should be engaged in a program of counseling. Encourage attendance at AA meetings. An up-and-coming concept is the use of smartphone applications to prevent relapse (or even induce remission); apps that provide an accurate blood alcohol tracking systems and integrated psychosocial therapies are in the pipeline. The novel Reddit online forum r/StopDrinking is a 24-hour peer-support community that relies on
fellowship, accountability, monitoring, and anonymity; the forum can compete with
motivational interviewing for efficacy in increasing abstinence and preventing relapse.
1. Johnson L, O’Malley P, Miech RA, et al. Monitoring the Future national survey results on drug use, 1975-2015: overview, key findings on adolescent drug use. http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2015.pdf. Published February 2016. Accessed January 20, 2016.
2. Substance Abuse and Mental Health Services Administration. Results from the 2013 national survey on drug use and health: mental health findings, NSDUH Series H-49, HHS Publication No. (SMA) 14-4887. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
3. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiological Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
4. Robinson S, Meeks TW, Geniza C. Medication for alcohol use disorder: which agents work best. Current Psychiatry. 2014;13(1):22-29.
5. Substance Abuse and Mental Health Services Administration and National Institute on Alcohol Abuse and Alcoholism. Medication for the treatment of alcohol use disorder: a brief guide. HHS Publication No. (SMA) 15-4907. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
6. Volkow ND, Koob GF, McLellan AT. Neurobiological advances from the brain disease model of addiction. N Engl J Med. 2016;374(4):363-371.
Acknowledgment
The authors would like to thank Thomas M. Penders, MS, MD, Medical Director for Consultation-Liaison Psychiatry at Cape Cod Healthcare, Hyannis, Massachusetts, and Affiliate Professor at East Carolina University, Greenville, North Carolina, for all his guidance, support, and mentorship.
1. Johnson L, O’Malley P, Miech RA, et al. Monitoring the Future national survey results on drug use, 1975-2015: overview, key findings on adolescent drug use. http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2015.pdf. Published February 2016. Accessed January 20, 2016.
2. Substance Abuse and Mental Health Services Administration. Results from the 2013 national survey on drug use and health: mental health findings, NSDUH Series H-49, HHS Publication No. (SMA) 14-4887. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014.
3. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiological Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766.
4. Robinson S, Meeks TW, Geniza C. Medication for alcohol use disorder: which agents work best. Current Psychiatry. 2014;13(1):22-29.
5. Substance Abuse and Mental Health Services Administration and National Institute on Alcohol Abuse and Alcoholism. Medication for the treatment of alcohol use disorder: a brief guide. HHS Publication No. (SMA) 15-4907. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2015.
6. Volkow ND, Koob GF, McLellan AT. Neurobiological advances from the brain disease model of addiction. N Engl J Med. 2016;374(4):363-371.
Acknowledgment
The authors would like to thank Thomas M. Penders, MS, MD, Medical Director for Consultation-Liaison Psychiatry at Cape Cod Healthcare, Hyannis, Massachusetts, and Affiliate Professor at East Carolina University, Greenville, North Carolina, for all his guidance, support, and mentorship.
Ups and Downs of Local Health Departments
Despite staff and budget reductions, local health departments (LHDs) are finding ways to expand services in some areas, according to a survey by the National Association of County and City Health Officials (NACCHO). One-third of LHDs reported reducing services of at least 1 program area, such as immunization, diabetes screening, and high blood pressure screening. However, one-fourth said they were expanding population-based preventive programs for obesity, drug, alcohol, and tobacco.
The association has periodically surveyed LHDs since 2008 to assess the impact of the economic recession. In 2014, NACCHO renamed the survey “The Forces of Change” and expanded it to take in a wider range of factors. The 16-question online survey, distributed during January-February 2015, went to 948 LHDs in the U.S. (excluding Rhode Island and Hawaii, which have no LHDs), representing one-third of all LHDs. Of the 690 top executives who responded, 353 represented small, 271 medium, and 66 large LHDs.
Related: Meta-Analysis Examines Quality of VA Health Care
At the peak of budget cuts, in 2009, 45% reported budget decreases. Since then, about 1 in 4 LHDs is still reporting budget cuts compared with the previous year; 27% expect budget decreases to continue into the next year.
Since 2008, NACCHO says, 51,700 jobs have been lost. More than half the 3,400 lost in 2014 were due to attrition; the rest to layoffs. The number of lost jobs was “most marked” among large LHDs: Sixty-one percent of those reported at least 1 job lost, followed by 41% of medium and 26% of small LHDs.
Related: Pharmacists in the Emergency Department: Feasibility and Cost
For many LHDs, “the cumulative effects of budget cuts and job losses” have not been reversed as the economy recovered. Some are trying creative workarounds, such as collaborations with primary care providers (PCPs). For example, 61% report actively encouraging PCPs to use evidence-based public health services, such as interventions to reduce asthma triggers.
However, < 10% of LHDs were actively engaged in new systems of care with PCPs, such as State Innovation Models (multipayer health care payment and service delivery models), patient-centered medical homes, or accountable care organizations (networks of health care providers voluntarily responsible for providing coordinated care). And < 70% are engaged in or exploring partnerships with nonprofit hospitals, which NACCHO says “might benefit multiple stakeholders and the community at large.”
Related: Implementing the EQUiPPED Medication Management Program at 5 VA Emergency Departments
Despite staff and budget reductions, local health departments (LHDs) are finding ways to expand services in some areas, according to a survey by the National Association of County and City Health Officials (NACCHO). One-third of LHDs reported reducing services of at least 1 program area, such as immunization, diabetes screening, and high blood pressure screening. However, one-fourth said they were expanding population-based preventive programs for obesity, drug, alcohol, and tobacco.
The association has periodically surveyed LHDs since 2008 to assess the impact of the economic recession. In 2014, NACCHO renamed the survey “The Forces of Change” and expanded it to take in a wider range of factors. The 16-question online survey, distributed during January-February 2015, went to 948 LHDs in the U.S. (excluding Rhode Island and Hawaii, which have no LHDs), representing one-third of all LHDs. Of the 690 top executives who responded, 353 represented small, 271 medium, and 66 large LHDs.
Related: Meta-Analysis Examines Quality of VA Health Care
At the peak of budget cuts, in 2009, 45% reported budget decreases. Since then, about 1 in 4 LHDs is still reporting budget cuts compared with the previous year; 27% expect budget decreases to continue into the next year.
Since 2008, NACCHO says, 51,700 jobs have been lost. More than half the 3,400 lost in 2014 were due to attrition; the rest to layoffs. The number of lost jobs was “most marked” among large LHDs: Sixty-one percent of those reported at least 1 job lost, followed by 41% of medium and 26% of small LHDs.
Related: Pharmacists in the Emergency Department: Feasibility and Cost
For many LHDs, “the cumulative effects of budget cuts and job losses” have not been reversed as the economy recovered. Some are trying creative workarounds, such as collaborations with primary care providers (PCPs). For example, 61% report actively encouraging PCPs to use evidence-based public health services, such as interventions to reduce asthma triggers.
However, < 10% of LHDs were actively engaged in new systems of care with PCPs, such as State Innovation Models (multipayer health care payment and service delivery models), patient-centered medical homes, or accountable care organizations (networks of health care providers voluntarily responsible for providing coordinated care). And < 70% are engaged in or exploring partnerships with nonprofit hospitals, which NACCHO says “might benefit multiple stakeholders and the community at large.”
Related: Implementing the EQUiPPED Medication Management Program at 5 VA Emergency Departments
Despite staff and budget reductions, local health departments (LHDs) are finding ways to expand services in some areas, according to a survey by the National Association of County and City Health Officials (NACCHO). One-third of LHDs reported reducing services of at least 1 program area, such as immunization, diabetes screening, and high blood pressure screening. However, one-fourth said they were expanding population-based preventive programs for obesity, drug, alcohol, and tobacco.
The association has periodically surveyed LHDs since 2008 to assess the impact of the economic recession. In 2014, NACCHO renamed the survey “The Forces of Change” and expanded it to take in a wider range of factors. The 16-question online survey, distributed during January-February 2015, went to 948 LHDs in the U.S. (excluding Rhode Island and Hawaii, which have no LHDs), representing one-third of all LHDs. Of the 690 top executives who responded, 353 represented small, 271 medium, and 66 large LHDs.
Related: Meta-Analysis Examines Quality of VA Health Care
At the peak of budget cuts, in 2009, 45% reported budget decreases. Since then, about 1 in 4 LHDs is still reporting budget cuts compared with the previous year; 27% expect budget decreases to continue into the next year.
Since 2008, NACCHO says, 51,700 jobs have been lost. More than half the 3,400 lost in 2014 were due to attrition; the rest to layoffs. The number of lost jobs was “most marked” among large LHDs: Sixty-one percent of those reported at least 1 job lost, followed by 41% of medium and 26% of small LHDs.
Related: Pharmacists in the Emergency Department: Feasibility and Cost
For many LHDs, “the cumulative effects of budget cuts and job losses” have not been reversed as the economy recovered. Some are trying creative workarounds, such as collaborations with primary care providers (PCPs). For example, 61% report actively encouraging PCPs to use evidence-based public health services, such as interventions to reduce asthma triggers.
However, < 10% of LHDs were actively engaged in new systems of care with PCPs, such as State Innovation Models (multipayer health care payment and service delivery models), patient-centered medical homes, or accountable care organizations (networks of health care providers voluntarily responsible for providing coordinated care). And < 70% are engaged in or exploring partnerships with nonprofit hospitals, which NACCHO says “might benefit multiple stakeholders and the community at large.”
Related: Implementing the EQUiPPED Medication Management Program at 5 VA Emergency Departments
LETTER: The Value of a Structured On-Boarding Peer Mentorship Program
To demonstrate the impact of a structured peer mentorship program in a large size service-oriented hospitalist group with 71 full-time hospitalist and 21 full-time APPs serving a daily census of 400 patients, we piloted a structured peer mentorship project from June 2015 until December 2015 with 10 new hospitalist hires. Each new hire was paired with a senior hospitalist colleague for a total of four weeks over a period of two months and the outcomes were measured through a 10-question anonymous survey at the end of 90 days. The survey response rate was 80%. The questions pertained to the effectiveness of mentorship program, practice group culture orientation, adherence to high-yield patient satisfaction behaviors related to Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey, work efficiency, job satisfaction, navigating through various hospital floors, efficient clinical staff communication, hand of care sign-out process, understanding of various hospitalist shifts and open-ended feedback.
Our results revealed that 100% of the new hires recommended to continue the on-boarding mentorship program on a permanent basis and 95% of the responses on the Likert scale were either very positive or positive. The total cost of the mentorship program was estimated to be 2-3 moon-lighting shifts ($2400-$3600) for the group. This cost was mainly associated with extra staffing needed during the first half of the shadowing week since the mentor was carrying half of the daily census. The marginal benefits of the program were far more and long lasting than the short-term cost. The program assisted in early acclimatization to the practice group culture, provider engagement and satisfaction and early productivity. It also has the potential to increase retention in a high-turnover hospitalist work field. We conclude that effective peer mentorship can play an important role in the organizational success of a large hospitalist program. Successful mentoring programs require proper understanding, planning, resource allocation, implementation and evaluation. From increased morale to increased productivity, the benefits are numerous. Mentoring is a tangible way to show employees that they are valued and that the organization’s future includes them.
— Muhammad Nabeel, MD, FACP, Clinical Assistant Professor, College of Human Medicine, Michigan State University, GRMEP; Hospitalist, Spectrum Health Medical Group, Grand Rapids, MI
— Rashelle Ludolph, MHA, MBA (Second Author), Director Operations, Acute Care Medicine, Spectrum Health Medical Group, Grand Rapids, MI
To demonstrate the impact of a structured peer mentorship program in a large size service-oriented hospitalist group with 71 full-time hospitalist and 21 full-time APPs serving a daily census of 400 patients, we piloted a structured peer mentorship project from June 2015 until December 2015 with 10 new hospitalist hires. Each new hire was paired with a senior hospitalist colleague for a total of four weeks over a period of two months and the outcomes were measured through a 10-question anonymous survey at the end of 90 days. The survey response rate was 80%. The questions pertained to the effectiveness of mentorship program, practice group culture orientation, adherence to high-yield patient satisfaction behaviors related to Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey, work efficiency, job satisfaction, navigating through various hospital floors, efficient clinical staff communication, hand of care sign-out process, understanding of various hospitalist shifts and open-ended feedback.
Our results revealed that 100% of the new hires recommended to continue the on-boarding mentorship program on a permanent basis and 95% of the responses on the Likert scale were either very positive or positive. The total cost of the mentorship program was estimated to be 2-3 moon-lighting shifts ($2400-$3600) for the group. This cost was mainly associated with extra staffing needed during the first half of the shadowing week since the mentor was carrying half of the daily census. The marginal benefits of the program were far more and long lasting than the short-term cost. The program assisted in early acclimatization to the practice group culture, provider engagement and satisfaction and early productivity. It also has the potential to increase retention in a high-turnover hospitalist work field. We conclude that effective peer mentorship can play an important role in the organizational success of a large hospitalist program. Successful mentoring programs require proper understanding, planning, resource allocation, implementation and evaluation. From increased morale to increased productivity, the benefits are numerous. Mentoring is a tangible way to show employees that they are valued and that the organization’s future includes them.
— Muhammad Nabeel, MD, FACP, Clinical Assistant Professor, College of Human Medicine, Michigan State University, GRMEP; Hospitalist, Spectrum Health Medical Group, Grand Rapids, MI
— Rashelle Ludolph, MHA, MBA (Second Author), Director Operations, Acute Care Medicine, Spectrum Health Medical Group, Grand Rapids, MI
To demonstrate the impact of a structured peer mentorship program in a large size service-oriented hospitalist group with 71 full-time hospitalist and 21 full-time APPs serving a daily census of 400 patients, we piloted a structured peer mentorship project from June 2015 until December 2015 with 10 new hospitalist hires. Each new hire was paired with a senior hospitalist colleague for a total of four weeks over a period of two months and the outcomes were measured through a 10-question anonymous survey at the end of 90 days. The survey response rate was 80%. The questions pertained to the effectiveness of mentorship program, practice group culture orientation, adherence to high-yield patient satisfaction behaviors related to Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey, work efficiency, job satisfaction, navigating through various hospital floors, efficient clinical staff communication, hand of care sign-out process, understanding of various hospitalist shifts and open-ended feedback.
Our results revealed that 100% of the new hires recommended to continue the on-boarding mentorship program on a permanent basis and 95% of the responses on the Likert scale were either very positive or positive. The total cost of the mentorship program was estimated to be 2-3 moon-lighting shifts ($2400-$3600) for the group. This cost was mainly associated with extra staffing needed during the first half of the shadowing week since the mentor was carrying half of the daily census. The marginal benefits of the program were far more and long lasting than the short-term cost. The program assisted in early acclimatization to the practice group culture, provider engagement and satisfaction and early productivity. It also has the potential to increase retention in a high-turnover hospitalist work field. We conclude that effective peer mentorship can play an important role in the organizational success of a large hospitalist program. Successful mentoring programs require proper understanding, planning, resource allocation, implementation and evaluation. From increased morale to increased productivity, the benefits are numerous. Mentoring is a tangible way to show employees that they are valued and that the organization’s future includes them.
— Muhammad Nabeel, MD, FACP, Clinical Assistant Professor, College of Human Medicine, Michigan State University, GRMEP; Hospitalist, Spectrum Health Medical Group, Grand Rapids, MI
— Rashelle Ludolph, MHA, MBA (Second Author), Director Operations, Acute Care Medicine, Spectrum Health Medical Group, Grand Rapids, MI
Therapy can provide clinical benefit in haplo-HSCT, data suggest

Photo by Chad McNeeley
BARCELONA—Results from 2 studies suggest an immunogene therapy can provide a clinical benefit in adults with high-risk hematologic malignancies undergoing haploidentical hematopoietic stem cell transplant (haplo-HSCT).
When compared to historical controls, patients who received the immunogene therapy, Zalmoxis, had lower rates of non-relapse mortality (NRM) and chronic graft-vs-host disease (GVHD), as well as improved overall survival (OS).
The only adverse event related to Zalmoxis was GVHD, which was resolved.
These data were presented at the EBMT International Transplant Course. The studies were funded by MolMed S.p.A., the company developing Zalmoxis.
About the therapy
Zalmoxis is a treatment consisting of allogeneic, genetically modified T cells. The cells are intended to be given to haplo-HSCT recipients to help fight off infection, enhance the success of the transplant, and support long-lasting anticancer effects.
Because the genetically modified T cells can also cause GVHD, they are equipped with a suicide gene, which makes them susceptible to treatment with ganciclovir or valganciclovir. So if a patient develops GVHD, he or she can receive ganciclovir/valganciclovir, which should kill the modified T cells and prevent further development of GVHD.
Trial data
The data presented at the EBMT International Transplant Course were from the phase 1/2 TK007 trial and the ongoing phase 3 TK008 trial.
The TK007 trial included haplo-HSCT recipients with various high-risk hematologic malignancies, while the TK008 trial is enrolling patients with high-risk acute leukemia who are undergoing haplo-HSCT.
Researchers have compared 37 Zalmoxis-treated patients from these trials to 140 contemporaneous control patients from the database of the EBMT registry.
Results of this pair-matched analysis showed an OS improvement in Zalmoxis-treated patients, which was driven by a reduction in NRM. The OS was 49% in the Zalmoxis group and 37% in controls (P=0.01), and the NRM was 22% and 43%, respectively (P=0.014).
Among controls dying from non-relapse causes, the majority (78%) died from either infection (56%) or GVHD (22%), while the only adverse event related to Zalmoxis treatment was GVHD. And this GVHD was fully resolved by activating the suicide-gene system with ganciclovir treatment, without any GVHD-related death.
Furthermore, the incidence of chronic GVHD was lower in Zalmoxis-treated patients than in controls—6% and 25%, respectively (P=0.04).
Therefore, the researchers concluded that the protective effects of Zalmoxis in controlling infection and GVHD mainly drove the decreased NRM in the Zalmoxis group.
These data supported the European Commission’s recent decision to grant conditional marketing authorization for Zalmoxis. ![]()

Photo by Chad McNeeley
BARCELONA—Results from 2 studies suggest an immunogene therapy can provide a clinical benefit in adults with high-risk hematologic malignancies undergoing haploidentical hematopoietic stem cell transplant (haplo-HSCT).
When compared to historical controls, patients who received the immunogene therapy, Zalmoxis, had lower rates of non-relapse mortality (NRM) and chronic graft-vs-host disease (GVHD), as well as improved overall survival (OS).
The only adverse event related to Zalmoxis was GVHD, which was resolved.
These data were presented at the EBMT International Transplant Course. The studies were funded by MolMed S.p.A., the company developing Zalmoxis.
About the therapy
Zalmoxis is a treatment consisting of allogeneic, genetically modified T cells. The cells are intended to be given to haplo-HSCT recipients to help fight off infection, enhance the success of the transplant, and support long-lasting anticancer effects.
Because the genetically modified T cells can also cause GVHD, they are equipped with a suicide gene, which makes them susceptible to treatment with ganciclovir or valganciclovir. So if a patient develops GVHD, he or she can receive ganciclovir/valganciclovir, which should kill the modified T cells and prevent further development of GVHD.
Trial data
The data presented at the EBMT International Transplant Course were from the phase 1/2 TK007 trial and the ongoing phase 3 TK008 trial.
The TK007 trial included haplo-HSCT recipients with various high-risk hematologic malignancies, while the TK008 trial is enrolling patients with high-risk acute leukemia who are undergoing haplo-HSCT.
Researchers have compared 37 Zalmoxis-treated patients from these trials to 140 contemporaneous control patients from the database of the EBMT registry.
Results of this pair-matched analysis showed an OS improvement in Zalmoxis-treated patients, which was driven by a reduction in NRM. The OS was 49% in the Zalmoxis group and 37% in controls (P=0.01), and the NRM was 22% and 43%, respectively (P=0.014).
Among controls dying from non-relapse causes, the majority (78%) died from either infection (56%) or GVHD (22%), while the only adverse event related to Zalmoxis treatment was GVHD. And this GVHD was fully resolved by activating the suicide-gene system with ganciclovir treatment, without any GVHD-related death.
Furthermore, the incidence of chronic GVHD was lower in Zalmoxis-treated patients than in controls—6% and 25%, respectively (P=0.04).
Therefore, the researchers concluded that the protective effects of Zalmoxis in controlling infection and GVHD mainly drove the decreased NRM in the Zalmoxis group.
These data supported the European Commission’s recent decision to grant conditional marketing authorization for Zalmoxis. ![]()

Photo by Chad McNeeley
BARCELONA—Results from 2 studies suggest an immunogene therapy can provide a clinical benefit in adults with high-risk hematologic malignancies undergoing haploidentical hematopoietic stem cell transplant (haplo-HSCT).
When compared to historical controls, patients who received the immunogene therapy, Zalmoxis, had lower rates of non-relapse mortality (NRM) and chronic graft-vs-host disease (GVHD), as well as improved overall survival (OS).
The only adverse event related to Zalmoxis was GVHD, which was resolved.
These data were presented at the EBMT International Transplant Course. The studies were funded by MolMed S.p.A., the company developing Zalmoxis.
About the therapy
Zalmoxis is a treatment consisting of allogeneic, genetically modified T cells. The cells are intended to be given to haplo-HSCT recipients to help fight off infection, enhance the success of the transplant, and support long-lasting anticancer effects.
Because the genetically modified T cells can also cause GVHD, they are equipped with a suicide gene, which makes them susceptible to treatment with ganciclovir or valganciclovir. So if a patient develops GVHD, he or she can receive ganciclovir/valganciclovir, which should kill the modified T cells and prevent further development of GVHD.
Trial data
The data presented at the EBMT International Transplant Course were from the phase 1/2 TK007 trial and the ongoing phase 3 TK008 trial.
The TK007 trial included haplo-HSCT recipients with various high-risk hematologic malignancies, while the TK008 trial is enrolling patients with high-risk acute leukemia who are undergoing haplo-HSCT.
Researchers have compared 37 Zalmoxis-treated patients from these trials to 140 contemporaneous control patients from the database of the EBMT registry.
Results of this pair-matched analysis showed an OS improvement in Zalmoxis-treated patients, which was driven by a reduction in NRM. The OS was 49% in the Zalmoxis group and 37% in controls (P=0.01), and the NRM was 22% and 43%, respectively (P=0.014).
Among controls dying from non-relapse causes, the majority (78%) died from either infection (56%) or GVHD (22%), while the only adverse event related to Zalmoxis treatment was GVHD. And this GVHD was fully resolved by activating the suicide-gene system with ganciclovir treatment, without any GVHD-related death.
Furthermore, the incidence of chronic GVHD was lower in Zalmoxis-treated patients than in controls—6% and 25%, respectively (P=0.04).
Therefore, the researchers concluded that the protective effects of Zalmoxis in controlling infection and GVHD mainly drove the decreased NRM in the Zalmoxis group.
These data supported the European Commission’s recent decision to grant conditional marketing authorization for Zalmoxis. ![]()
FDA grants drug orphan designation for PNH

The US Food and Drug Administration (FDA) has granted orphan drug designation to Coversin as a treatment for paroxysmal nocturnal hemoglobinuria (PNH).
Coversin is a recombinant small protein (16,740 Da) derived from a native protein found in the saliva of the Ornithodoros moubata tick.
The drug is a second-generation complement inhibitor that acts on complement component C5, preventing release of C5a and formation of C5b-9 (also known as the membrane attack complex).
Coversin is being developed by Akari Therapeutics.
In vitro experiments have shown that Coversin inhibits red blood cell lysis in PNH, and Coversin can achieve full complement inhibition in the blood of PNH patients who are resistant to eculizumab.
In a phase 1a trial of healthy volunteers, Coversin completely inhibited complement C5 activity within 12 hours of administration.
Akari Therapeutics is currently conducting a phase 1b study of Coversin in healthy volunteers and is enrolling patients with eculizumab-resistant PNH in a phase 2 trial.
The company has also been administering Coversin to a patient with eculizumab-resistant PNH. Thus far, Coversin has prevented hemolytic episodes and improved disease symptoms in this patient. The only drug-related adverse event has been occasional local and transient irritation at the injection site.
“We have continued to see complete complement inhibition and symptom control in a PNH patient with resistance to eculizumab, who has been self-administering subcutaneous Coversin for over 7 months,” said Gur Roshwalb, MD, CEO of Akari Therapeutics.
“We believe that Coversin, when approved, could provide important benefits for all patients with PNH.”
Coversin is also being studied in atypical hemolytic uremic syndrome and Guillain Barré syndrome.
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()

The US Food and Drug Administration (FDA) has granted orphan drug designation to Coversin as a treatment for paroxysmal nocturnal hemoglobinuria (PNH).
Coversin is a recombinant small protein (16,740 Da) derived from a native protein found in the saliva of the Ornithodoros moubata tick.
The drug is a second-generation complement inhibitor that acts on complement component C5, preventing release of C5a and formation of C5b-9 (also known as the membrane attack complex).
Coversin is being developed by Akari Therapeutics.
In vitro experiments have shown that Coversin inhibits red blood cell lysis in PNH, and Coversin can achieve full complement inhibition in the blood of PNH patients who are resistant to eculizumab.
In a phase 1a trial of healthy volunteers, Coversin completely inhibited complement C5 activity within 12 hours of administration.
Akari Therapeutics is currently conducting a phase 1b study of Coversin in healthy volunteers and is enrolling patients with eculizumab-resistant PNH in a phase 2 trial.
The company has also been administering Coversin to a patient with eculizumab-resistant PNH. Thus far, Coversin has prevented hemolytic episodes and improved disease symptoms in this patient. The only drug-related adverse event has been occasional local and transient irritation at the injection site.
“We have continued to see complete complement inhibition and symptom control in a PNH patient with resistance to eculizumab, who has been self-administering subcutaneous Coversin for over 7 months,” said Gur Roshwalb, MD, CEO of Akari Therapeutics.
“We believe that Coversin, when approved, could provide important benefits for all patients with PNH.”
Coversin is also being studied in atypical hemolytic uremic syndrome and Guillain Barré syndrome.
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()

The US Food and Drug Administration (FDA) has granted orphan drug designation to Coversin as a treatment for paroxysmal nocturnal hemoglobinuria (PNH).
Coversin is a recombinant small protein (16,740 Da) derived from a native protein found in the saliva of the Ornithodoros moubata tick.
The drug is a second-generation complement inhibitor that acts on complement component C5, preventing release of C5a and formation of C5b-9 (also known as the membrane attack complex).
Coversin is being developed by Akari Therapeutics.
In vitro experiments have shown that Coversin inhibits red blood cell lysis in PNH, and Coversin can achieve full complement inhibition in the blood of PNH patients who are resistant to eculizumab.
In a phase 1a trial of healthy volunteers, Coversin completely inhibited complement C5 activity within 12 hours of administration.
Akari Therapeutics is currently conducting a phase 1b study of Coversin in healthy volunteers and is enrolling patients with eculizumab-resistant PNH in a phase 2 trial.
The company has also been administering Coversin to a patient with eculizumab-resistant PNH. Thus far, Coversin has prevented hemolytic episodes and improved disease symptoms in this patient. The only drug-related adverse event has been occasional local and transient irritation at the injection site.
“We have continued to see complete complement inhibition and symptom control in a PNH patient with resistance to eculizumab, who has been self-administering subcutaneous Coversin for over 7 months,” said Gur Roshwalb, MD, CEO of Akari Therapeutics.
“We believe that Coversin, when approved, could provide important benefits for all patients with PNH.”
Coversin is also being studied in atypical hemolytic uremic syndrome and Guillain Barré syndrome.
About orphan designation
The FDA grants orphan designation to drugs and biologics intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved. ![]()
FDA approves cord blood product

The US Food and Drug Administration (FDA) has issued a biologics license to the Cleveland Cord Blood Center (CCBC) for Clevecord™ (HPC, Cord Blood), a hematopoietic progenitor cell product derived from umbilical cord blood.
Under this license, CCBC is authorized to manufacture Clevecord at its facility in Warrensville Heights, Ohio.
Clevecord is indicated for use in unrelated donor transplant procedures, in conjunction with an appropriate preparative regimen.
The product can be used for hematopoietic and immunologic reconstitution in patients with disorders that affect the hematopoietic system, whether they are inherited, acquired, or result from myeloablative treatment.
Each Clevecord unit contains a minimum of 5 x 108 total nucleated cells with at least 1.25 x 106 viable CD34+ cells at the time of cryopreservation. The recommended minimum dose is 2.5 x 107 nucleated cells/kg at cryopreservation.
Clevecord has been approved with a black box warning, which states that use of the product may result in fatal infusion reactions, graft-vs-host disease, engraftment syndrome, and graft failure.
For more details on Clevecord, see the package insert on the FDA website.
“Obtaining FDA licensure for Clevecord is reflective of the Cleveland Cord Blood Center’s dedication to meeting the highest quality standards in the industry for distribution of our cord blood products to transplant centers throughout the US and around the world,” said Wouter Van’t Hof, cord blood bank director at CCBC.
CCBC collects, processes, stores, and distributes umbilical cord blood units for use in transplants and advanced research in cellular therapy.
The organization says its cord blood collections represent a diverse cross-section of donor ethnicity to support transplant needs, particularly in the underserved African-American population.
“Up to 50% of parents giving birth in our partner hospitals donate their baby’s umbilical cord blood, a rate well above the national average,” said Marcie Finney, executive director of CCBC.
CCBC cord blood units can be searched and accessed through registries, including the National Bone Marrow Donor Program and Bone Marrow Donors Worldwide. ![]()

The US Food and Drug Administration (FDA) has issued a biologics license to the Cleveland Cord Blood Center (CCBC) for Clevecord™ (HPC, Cord Blood), a hematopoietic progenitor cell product derived from umbilical cord blood.
Under this license, CCBC is authorized to manufacture Clevecord at its facility in Warrensville Heights, Ohio.
Clevecord is indicated for use in unrelated donor transplant procedures, in conjunction with an appropriate preparative regimen.
The product can be used for hematopoietic and immunologic reconstitution in patients with disorders that affect the hematopoietic system, whether they are inherited, acquired, or result from myeloablative treatment.
Each Clevecord unit contains a minimum of 5 x 108 total nucleated cells with at least 1.25 x 106 viable CD34+ cells at the time of cryopreservation. The recommended minimum dose is 2.5 x 107 nucleated cells/kg at cryopreservation.
Clevecord has been approved with a black box warning, which states that use of the product may result in fatal infusion reactions, graft-vs-host disease, engraftment syndrome, and graft failure.
For more details on Clevecord, see the package insert on the FDA website.
“Obtaining FDA licensure for Clevecord is reflective of the Cleveland Cord Blood Center’s dedication to meeting the highest quality standards in the industry for distribution of our cord blood products to transplant centers throughout the US and around the world,” said Wouter Van’t Hof, cord blood bank director at CCBC.
CCBC collects, processes, stores, and distributes umbilical cord blood units for use in transplants and advanced research in cellular therapy.
The organization says its cord blood collections represent a diverse cross-section of donor ethnicity to support transplant needs, particularly in the underserved African-American population.
“Up to 50% of parents giving birth in our partner hospitals donate their baby’s umbilical cord blood, a rate well above the national average,” said Marcie Finney, executive director of CCBC.
CCBC cord blood units can be searched and accessed through registries, including the National Bone Marrow Donor Program and Bone Marrow Donors Worldwide. ![]()

The US Food and Drug Administration (FDA) has issued a biologics license to the Cleveland Cord Blood Center (CCBC) for Clevecord™ (HPC, Cord Blood), a hematopoietic progenitor cell product derived from umbilical cord blood.
Under this license, CCBC is authorized to manufacture Clevecord at its facility in Warrensville Heights, Ohio.
Clevecord is indicated for use in unrelated donor transplant procedures, in conjunction with an appropriate preparative regimen.
The product can be used for hematopoietic and immunologic reconstitution in patients with disorders that affect the hematopoietic system, whether they are inherited, acquired, or result from myeloablative treatment.
Each Clevecord unit contains a minimum of 5 x 108 total nucleated cells with at least 1.25 x 106 viable CD34+ cells at the time of cryopreservation. The recommended minimum dose is 2.5 x 107 nucleated cells/kg at cryopreservation.
Clevecord has been approved with a black box warning, which states that use of the product may result in fatal infusion reactions, graft-vs-host disease, engraftment syndrome, and graft failure.
For more details on Clevecord, see the package insert on the FDA website.
“Obtaining FDA licensure for Clevecord is reflective of the Cleveland Cord Blood Center’s dedication to meeting the highest quality standards in the industry for distribution of our cord blood products to transplant centers throughout the US and around the world,” said Wouter Van’t Hof, cord blood bank director at CCBC.
CCBC collects, processes, stores, and distributes umbilical cord blood units for use in transplants and advanced research in cellular therapy.
The organization says its cord blood collections represent a diverse cross-section of donor ethnicity to support transplant needs, particularly in the underserved African-American population.
“Up to 50% of parents giving birth in our partner hospitals donate their baby’s umbilical cord blood, a rate well above the national average,” said Marcie Finney, executive director of CCBC.
CCBC cord blood units can be searched and accessed through registries, including the National Bone Marrow Donor Program and Bone Marrow Donors Worldwide. ![]()
Effects of caring for advanced cancer patients
SAN FRANCISCO—Family caregivers of patients with high-mortality cancers may often experience high levels of depression and anxiety, results of a survey suggest.
The survey showed that caregivers can spend more than 8 hours a day providing care.
And as caregiving time increases, self-care behaviors such as sleep and exercise decline, which may confer poorer mental health.
These findings were presented at the 2016 Palliative Care in Oncology Symposium (abstract 239).
“Caregivers and patients are faced with an enormous physical and emotional toll when dealing with advanced cancer,” said study investigator J. Nicholas Dionne-Odom, PhD, RN, of the University of Alabama at Birmingham.
“When they put their own health and well-being on the back burner, it can affect their care to the patient.”
Dr Dionne-Odom and his colleagues conducted a cross-sectional survey of 294 family caregivers of Medicare beneficiaries diagnosed with pancreatic, lung, brain, ovarian, head and neck, hematologic, or stage IV cancers.
The survey was fielded across 8 cancer centers in Alabama, Florida, and Tennessee. Survey questions explored measures of self-care behaviors and quality of life.
The caregivers had an average age of 66. They were mostly female (72.8%), white (91.2%), retired (54.4%), and the patient’s spouse/partner (60.2%). Nearly half of the caregivers lived in rural areas (46.9%), and more than half had annual incomes less than $50,000 (53.8%).
Most of the caregivers said they provided care 6 to 7 days a week (71%) for more than 1 year (68%).
Twenty-three percent of caregivers reported a high level of depressive symptoms, and 34% reported borderline or high levels of anxiety symptoms, associated with significantly lower scores for self-care.
Lower self-care behavior scores were associated with a longer overall duration of caregiving, more hours in the day spent caregiving, more days of the week spent caregiving, and with fair or poor patient health.
“We hope our research rallies the oncology palliative care communities to develop assessment tools and services that support caregivers,” Dr Dionne-Odom said. “These efforts would help ensure that caregivers are supported and healthy when they take on the important role of caring for an individual with advanced cancer.” ![]()
SAN FRANCISCO—Family caregivers of patients with high-mortality cancers may often experience high levels of depression and anxiety, results of a survey suggest.
The survey showed that caregivers can spend more than 8 hours a day providing care.
And as caregiving time increases, self-care behaviors such as sleep and exercise decline, which may confer poorer mental health.
These findings were presented at the 2016 Palliative Care in Oncology Symposium (abstract 239).
“Caregivers and patients are faced with an enormous physical and emotional toll when dealing with advanced cancer,” said study investigator J. Nicholas Dionne-Odom, PhD, RN, of the University of Alabama at Birmingham.
“When they put their own health and well-being on the back burner, it can affect their care to the patient.”
Dr Dionne-Odom and his colleagues conducted a cross-sectional survey of 294 family caregivers of Medicare beneficiaries diagnosed with pancreatic, lung, brain, ovarian, head and neck, hematologic, or stage IV cancers.
The survey was fielded across 8 cancer centers in Alabama, Florida, and Tennessee. Survey questions explored measures of self-care behaviors and quality of life.
The caregivers had an average age of 66. They were mostly female (72.8%), white (91.2%), retired (54.4%), and the patient’s spouse/partner (60.2%). Nearly half of the caregivers lived in rural areas (46.9%), and more than half had annual incomes less than $50,000 (53.8%).
Most of the caregivers said they provided care 6 to 7 days a week (71%) for more than 1 year (68%).
Twenty-three percent of caregivers reported a high level of depressive symptoms, and 34% reported borderline or high levels of anxiety symptoms, associated with significantly lower scores for self-care.
Lower self-care behavior scores were associated with a longer overall duration of caregiving, more hours in the day spent caregiving, more days of the week spent caregiving, and with fair or poor patient health.
“We hope our research rallies the oncology palliative care communities to develop assessment tools and services that support caregivers,” Dr Dionne-Odom said. “These efforts would help ensure that caregivers are supported and healthy when they take on the important role of caring for an individual with advanced cancer.” ![]()
SAN FRANCISCO—Family caregivers of patients with high-mortality cancers may often experience high levels of depression and anxiety, results of a survey suggest.
The survey showed that caregivers can spend more than 8 hours a day providing care.
And as caregiving time increases, self-care behaviors such as sleep and exercise decline, which may confer poorer mental health.
These findings were presented at the 2016 Palliative Care in Oncology Symposium (abstract 239).
“Caregivers and patients are faced with an enormous physical and emotional toll when dealing with advanced cancer,” said study investigator J. Nicholas Dionne-Odom, PhD, RN, of the University of Alabama at Birmingham.
“When they put their own health and well-being on the back burner, it can affect their care to the patient.”
Dr Dionne-Odom and his colleagues conducted a cross-sectional survey of 294 family caregivers of Medicare beneficiaries diagnosed with pancreatic, lung, brain, ovarian, head and neck, hematologic, or stage IV cancers.
The survey was fielded across 8 cancer centers in Alabama, Florida, and Tennessee. Survey questions explored measures of self-care behaviors and quality of life.
The caregivers had an average age of 66. They were mostly female (72.8%), white (91.2%), retired (54.4%), and the patient’s spouse/partner (60.2%). Nearly half of the caregivers lived in rural areas (46.9%), and more than half had annual incomes less than $50,000 (53.8%).
Most of the caregivers said they provided care 6 to 7 days a week (71%) for more than 1 year (68%).
Twenty-three percent of caregivers reported a high level of depressive symptoms, and 34% reported borderline or high levels of anxiety symptoms, associated with significantly lower scores for self-care.
Lower self-care behavior scores were associated with a longer overall duration of caregiving, more hours in the day spent caregiving, more days of the week spent caregiving, and with fair or poor patient health.
“We hope our research rallies the oncology palliative care communities to develop assessment tools and services that support caregivers,” Dr Dionne-Odom said. “These efforts would help ensure that caregivers are supported and healthy when they take on the important role of caring for an individual with advanced cancer.” ![]()
WHO updates ranking of critically important antimicrobials
In light of increasing antibiotic resistance among pathogens, the World Health Organization has revised its global rankings of critically important antimicrobials used in human medicine, designating quinolones, third- and fourth-generation cephalosporins, macrolides and ketolides, and glycopeptides as among the highest-priority drugs in the world.
Peter C. Collignon, MBBS, of Canberra (Australia) Hospital and his colleagues on the WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance, created the rankings for use in developing risk management strategies related to antimicrobial use in food production animals. According to Dr. Collignon and his coauthors, the rankings are intended to help regulators and other stakeholders know which types of antimicrobials used in animals present potentially higher risks to human populations and help inform how this use might be better managed (e.g. restriction to single-animal therapy or prohibition of mass treatment and extra-label use) to minimize the risk of transmission of resistance to the human population.
WHO studies previously suggested that antimicrobials which currently have no veterinary equivalent (for example, carbapenems) “as well as any new class of antimicrobial developed for human therapy should not be used in animals.” Dr. Collignon’s WHO Advisory Group followed two essential criteria to designate antimicrobials of utmost importance to human health in the new study: 1. antimicrobials that are the sole, or one of limited available therapies, to treat serious bacterial infections in people and 2. antimicrobials used to treat infections in people caused by either (a) bacteria that may be transmitted to humans from nonhuman sources or (b) bacteria that may acquire resistance genes from nonhuman sources.
The highest-priority and most critically important antimicrobials are those which meet the criteria listed above and that are used in greatest volume or highest frequency by humans. Another criteria for prioritization involves antimicrobial classes where evidence suggests that the “transmission of resistant bacteria or resistance genes from nonhuman sources is already occurring, or has occurred previously.” Quinolones, third- and fourth-generation cephalosporins, macrolides and ketolides, and glycopeptides were the only antimicrobials that met all criteria for prioritization.
“Antimicrobial resistance remains a threat to human health and drivers of resistance act in all sectors; human, animal, and the environment,” the WHO Advisory Group concluded. “Prioritizing the antimicrobials that are critically important for humans is a valuable and strategic risk-management tool and will be improved with the evidence-based approach which is currently underway.”
Read the full study in Clinical Infectious Diseases (doi: 10.1093/cid/ciw475).
In light of increasing antibiotic resistance among pathogens, the World Health Organization has revised its global rankings of critically important antimicrobials used in human medicine, designating quinolones, third- and fourth-generation cephalosporins, macrolides and ketolides, and glycopeptides as among the highest-priority drugs in the world.
Peter C. Collignon, MBBS, of Canberra (Australia) Hospital and his colleagues on the WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance, created the rankings for use in developing risk management strategies related to antimicrobial use in food production animals. According to Dr. Collignon and his coauthors, the rankings are intended to help regulators and other stakeholders know which types of antimicrobials used in animals present potentially higher risks to human populations and help inform how this use might be better managed (e.g. restriction to single-animal therapy or prohibition of mass treatment and extra-label use) to minimize the risk of transmission of resistance to the human population.
WHO studies previously suggested that antimicrobials which currently have no veterinary equivalent (for example, carbapenems) “as well as any new class of antimicrobial developed for human therapy should not be used in animals.” Dr. Collignon’s WHO Advisory Group followed two essential criteria to designate antimicrobials of utmost importance to human health in the new study: 1. antimicrobials that are the sole, or one of limited available therapies, to treat serious bacterial infections in people and 2. antimicrobials used to treat infections in people caused by either (a) bacteria that may be transmitted to humans from nonhuman sources or (b) bacteria that may acquire resistance genes from nonhuman sources.
The highest-priority and most critically important antimicrobials are those which meet the criteria listed above and that are used in greatest volume or highest frequency by humans. Another criteria for prioritization involves antimicrobial classes where evidence suggests that the “transmission of resistant bacteria or resistance genes from nonhuman sources is already occurring, or has occurred previously.” Quinolones, third- and fourth-generation cephalosporins, macrolides and ketolides, and glycopeptides were the only antimicrobials that met all criteria for prioritization.
“Antimicrobial resistance remains a threat to human health and drivers of resistance act in all sectors; human, animal, and the environment,” the WHO Advisory Group concluded. “Prioritizing the antimicrobials that are critically important for humans is a valuable and strategic risk-management tool and will be improved with the evidence-based approach which is currently underway.”
Read the full study in Clinical Infectious Diseases (doi: 10.1093/cid/ciw475).
In light of increasing antibiotic resistance among pathogens, the World Health Organization has revised its global rankings of critically important antimicrobials used in human medicine, designating quinolones, third- and fourth-generation cephalosporins, macrolides and ketolides, and glycopeptides as among the highest-priority drugs in the world.
Peter C. Collignon, MBBS, of Canberra (Australia) Hospital and his colleagues on the WHO Advisory Group on Integrated Surveillance of Antimicrobial Resistance, created the rankings for use in developing risk management strategies related to antimicrobial use in food production animals. According to Dr. Collignon and his coauthors, the rankings are intended to help regulators and other stakeholders know which types of antimicrobials used in animals present potentially higher risks to human populations and help inform how this use might be better managed (e.g. restriction to single-animal therapy or prohibition of mass treatment and extra-label use) to minimize the risk of transmission of resistance to the human population.
WHO studies previously suggested that antimicrobials which currently have no veterinary equivalent (for example, carbapenems) “as well as any new class of antimicrobial developed for human therapy should not be used in animals.” Dr. Collignon’s WHO Advisory Group followed two essential criteria to designate antimicrobials of utmost importance to human health in the new study: 1. antimicrobials that are the sole, or one of limited available therapies, to treat serious bacterial infections in people and 2. antimicrobials used to treat infections in people caused by either (a) bacteria that may be transmitted to humans from nonhuman sources or (b) bacteria that may acquire resistance genes from nonhuman sources.
The highest-priority and most critically important antimicrobials are those which meet the criteria listed above and that are used in greatest volume or highest frequency by humans. Another criteria for prioritization involves antimicrobial classes where evidence suggests that the “transmission of resistant bacteria or resistance genes from nonhuman sources is already occurring, or has occurred previously.” Quinolones, third- and fourth-generation cephalosporins, macrolides and ketolides, and glycopeptides were the only antimicrobials that met all criteria for prioritization.
“Antimicrobial resistance remains a threat to human health and drivers of resistance act in all sectors; human, animal, and the environment,” the WHO Advisory Group concluded. “Prioritizing the antimicrobials that are critically important for humans is a valuable and strategic risk-management tool and will be improved with the evidence-based approach which is currently underway.”
Read the full study in Clinical Infectious Diseases (doi: 10.1093/cid/ciw475).
FROM CLINICAL INFECTIOUS DISEASES