User login
Biological DMARD equally effective in PsA patients with low or high joint counts
Key clinical point: Biological disease-modifying antirheumatic drugs (bDMARD) showed comparable efficacy in patients with psoriatic arthritis (PsA) with either low joint count (LJC) or high joint count (HJC), which highlights the feasibility of treatment escalation to bDMARD in symptomatic patients with LJC as well as in those with HJC.
Major finding: Patients with LJC (<3 tender or swollen joints) vs HJC (≥3 tender or swollen joints) showed similar treatment efficacy in terms of bDMARD retention (adjusted hazard ratio [aHR] 1.09; P = .63). Treatment discontinuation was unaffected by swollen or tender joint count status (aHR 1.12; P = .47).
Study details: Findings are from an observational cohort study that included 387 patients with PsA who had either LJC (n = 197) or HJC (n = 190) and received bDMARD.
Disclosures: This study was supported by Celgene International Sarl, Boudry, Switzerland. Five authors declared ties with various sources, whereas the other authors declared no conflicts of interest.
Source: Möller B et al for the Swiss Clinical Quality Management (SCQM) physicians, researchers and patients. Biological disease-modifying anti-rheumatic drugs are equally effective in psoriatic arthritis patients with low and high joint counts. Rheumatology (Oxford). 2023 (Sep 7). doi: 10.1093/rheumatology/kead455
Key clinical point: Biological disease-modifying antirheumatic drugs (bDMARD) showed comparable efficacy in patients with psoriatic arthritis (PsA) with either low joint count (LJC) or high joint count (HJC), which highlights the feasibility of treatment escalation to bDMARD in symptomatic patients with LJC as well as in those with HJC.
Major finding: Patients with LJC (<3 tender or swollen joints) vs HJC (≥3 tender or swollen joints) showed similar treatment efficacy in terms of bDMARD retention (adjusted hazard ratio [aHR] 1.09; P = .63). Treatment discontinuation was unaffected by swollen or tender joint count status (aHR 1.12; P = .47).
Study details: Findings are from an observational cohort study that included 387 patients with PsA who had either LJC (n = 197) or HJC (n = 190) and received bDMARD.
Disclosures: This study was supported by Celgene International Sarl, Boudry, Switzerland. Five authors declared ties with various sources, whereas the other authors declared no conflicts of interest.
Source: Möller B et al for the Swiss Clinical Quality Management (SCQM) physicians, researchers and patients. Biological disease-modifying anti-rheumatic drugs are equally effective in psoriatic arthritis patients with low and high joint counts. Rheumatology (Oxford). 2023 (Sep 7). doi: 10.1093/rheumatology/kead455
Key clinical point: Biological disease-modifying antirheumatic drugs (bDMARD) showed comparable efficacy in patients with psoriatic arthritis (PsA) with either low joint count (LJC) or high joint count (HJC), which highlights the feasibility of treatment escalation to bDMARD in symptomatic patients with LJC as well as in those with HJC.
Major finding: Patients with LJC (<3 tender or swollen joints) vs HJC (≥3 tender or swollen joints) showed similar treatment efficacy in terms of bDMARD retention (adjusted hazard ratio [aHR] 1.09; P = .63). Treatment discontinuation was unaffected by swollen or tender joint count status (aHR 1.12; P = .47).
Study details: Findings are from an observational cohort study that included 387 patients with PsA who had either LJC (n = 197) or HJC (n = 190) and received bDMARD.
Disclosures: This study was supported by Celgene International Sarl, Boudry, Switzerland. Five authors declared ties with various sources, whereas the other authors declared no conflicts of interest.
Source: Möller B et al for the Swiss Clinical Quality Management (SCQM) physicians, researchers and patients. Biological disease-modifying anti-rheumatic drugs are equally effective in psoriatic arthritis patients with low and high joint counts. Rheumatology (Oxford). 2023 (Sep 7). doi: 10.1093/rheumatology/kead455
Guselkumab improves effector cytokine levels in PsA patients with inadequate response to TNFi
Key clinical point: Guselkumab led to early reduction of inflammatory cytokines, which was sustained through 48 weeks, with subsequent improvements in clinical response in patients with active psoriatic arthritis (PsA) and an inadequate response to tumor necrosis factor inhibitors (TNFi-IR).
Major finding: Serum levels of interleukin (IL)-17A, IL-17F, IL-22, and serum amyloid A reduced significantly by week 4 and were sustained through week 48 in the guselkumab (P < .05) vs placebo group when compared with control individuals without PsA. Patients who achieved a clinical response to guselkumab at week 24 showed significant reduction in IL-6 levels at week 4 compared with non-responders (P < .05).
Study details: Findings are from the phase 3b COSMOS study including patients with active PsA and TNFi-IR who were randomly assigned to receive either guselkumab (n = 189) or placebo (n = 96) and compared with matched control individuals.
Disclosures: This study was sponsored by Janssen Research & Development (R&D), LLC, USA. Seven authors declared being employees of Janssen R&D, LLC, or others or owning stocks or stock options in Johnson & Johnson. Several authors declared ties with various sources, including Janssen.
Source: Schett G et al. Effect of guselkumab on serum biomarkers in patients with active psoriatic arthritis and inadequate response to tumor necrosis factor inhibitors: Results from the COSMOS phase 3b study. Arthritis Res Ther. 2023;25:150 (Aug 16, corrected Sep 15). doi: 10.1186/s13075-023-03125-4
Key clinical point: Guselkumab led to early reduction of inflammatory cytokines, which was sustained through 48 weeks, with subsequent improvements in clinical response in patients with active psoriatic arthritis (PsA) and an inadequate response to tumor necrosis factor inhibitors (TNFi-IR).
Major finding: Serum levels of interleukin (IL)-17A, IL-17F, IL-22, and serum amyloid A reduced significantly by week 4 and were sustained through week 48 in the guselkumab (P < .05) vs placebo group when compared with control individuals without PsA. Patients who achieved a clinical response to guselkumab at week 24 showed significant reduction in IL-6 levels at week 4 compared with non-responders (P < .05).
Study details: Findings are from the phase 3b COSMOS study including patients with active PsA and TNFi-IR who were randomly assigned to receive either guselkumab (n = 189) or placebo (n = 96) and compared with matched control individuals.
Disclosures: This study was sponsored by Janssen Research & Development (R&D), LLC, USA. Seven authors declared being employees of Janssen R&D, LLC, or others or owning stocks or stock options in Johnson & Johnson. Several authors declared ties with various sources, including Janssen.
Source: Schett G et al. Effect of guselkumab on serum biomarkers in patients with active psoriatic arthritis and inadequate response to tumor necrosis factor inhibitors: Results from the COSMOS phase 3b study. Arthritis Res Ther. 2023;25:150 (Aug 16, corrected Sep 15). doi: 10.1186/s13075-023-03125-4
Key clinical point: Guselkumab led to early reduction of inflammatory cytokines, which was sustained through 48 weeks, with subsequent improvements in clinical response in patients with active psoriatic arthritis (PsA) and an inadequate response to tumor necrosis factor inhibitors (TNFi-IR).
Major finding: Serum levels of interleukin (IL)-17A, IL-17F, IL-22, and serum amyloid A reduced significantly by week 4 and were sustained through week 48 in the guselkumab (P < .05) vs placebo group when compared with control individuals without PsA. Patients who achieved a clinical response to guselkumab at week 24 showed significant reduction in IL-6 levels at week 4 compared with non-responders (P < .05).
Study details: Findings are from the phase 3b COSMOS study including patients with active PsA and TNFi-IR who were randomly assigned to receive either guselkumab (n = 189) or placebo (n = 96) and compared with matched control individuals.
Disclosures: This study was sponsored by Janssen Research & Development (R&D), LLC, USA. Seven authors declared being employees of Janssen R&D, LLC, or others or owning stocks or stock options in Johnson & Johnson. Several authors declared ties with various sources, including Janssen.
Source: Schett G et al. Effect of guselkumab on serum biomarkers in patients with active psoriatic arthritis and inadequate response to tumor necrosis factor inhibitors: Results from the COSMOS phase 3b study. Arthritis Res Ther. 2023;25:150 (Aug 16, corrected Sep 15). doi: 10.1186/s13075-023-03125-4
Can a drug for overactive bladder disease prevent progression to heart failure?
TOPLINE:
(pre-HF) structural heart disease who were at risk of developing or worsening HF.
METHODOLOGY:
- Interventions for patients with asymptomatic pre-HF may be important in reducing the incidence of clinically overt HF, including HF with preserved ejection fraction (HFpEF).
- Mirabegron activates the cardiac beta-3 adrenergic receptor, which may offer an alternative activation of the cyclic guanosine monophosphate protein/kinase G (cGMP/PKG) pathway for patients at risk of or with mild HF and protect against worsening left ventricular hypertrophy (LVH) and/or diastolic dysfunction, but few clinical trials have evaluated the effect of mirabegron on cardiovascular outcomes.
- The phase 2b Beta3_LVH trial included 296 patients, some with and some without HF symptoms (mean age, 63 years), at 10 centers in Europe and the United Kingdom. All had an increased LV mass index (LVMI) (≥ 115 g/m2 for men and ≥ 95 g/m2 for women) or end-diastolic wall thickness of ≥ 13 mm in at least one wall segment.
- Patients, many of whom had risk factors, including hypertension, and were receiving cardiovascular therapies, were randomly assigned to receive mirabegron 50 mg/day or placebo and underwent various tests, including cardiac MRI, Doppler echocardiography, and urine and blood sampling for fasting glucose, insulin, hemoglobin A1c, serum lipids, and other measures.
- The two primary endpoints were change in left ventricular mass index (LVMI), expressed in grams per meters squared, and change in diastolic function, assessed as the ratio of peak early transmitral ventricular filling velocity to early diastolic tissue Doppler velocity (E/e´).
TAKEAWAY:
- Neither primary outcome reached statistical significance at 12 months; adjusted differences between groups included a 1.3g/m2 increase in LVMI (95% confidence interval, −0.15 to 2.74; P = .08) and a −0.15 decrease in E/e´ (95% CI, −0.69 to 0.4; P = .60).
- There was no statistically significant effect of mirabegron, in comparison with placebo, on lipids, glycemic control, or insulin sensitivity.
- The effect of mirabegron remained neutral in exploratory subgroup analyses, including age (≤ 65 years or > 65 years at baseline), sex (men or women), body mass index (≤ 30 kg/m2 or > 30 at baseline), presence of type 2 diabetes, atrial fibrillation, beta-blocker use, and geographic region.
- There were no deaths. There was a total of 428 adverse events (AEs), but there were no statistically significant between-group differences in the occurrence of these AEs.
IN PRACTICE:
While this study showed that mirabegron had a neutral effect on LV mass and diastolic function for patients with pre-HF or mild HF, the researchers suggest that longer-term effects of beta-3 adrenergic stimulation on myocardial remodeling and function “need to be tested in patients with established HFpEF, including with recent, more potent agonists.”
SOURCE:
The study was conducted by Jean-Luc Balligand, MD, Institut de Recherche Expérimentale et Clinique, Université Catholique de Louvain, Brussels, and colleagues. It was published online in JAMA Cardiology.
LIMITATIONS:
Inclusion of patients with mild HF and use of a single standard mirabegron dosage (50 mg/day) may have prevented detection of a treatment effect. More advanced techniques than measurements of E/e´, such as cardiac strain, may have been better for assessing early changes in diastolic function. Although missing data and dropouts were relatively infrequent and were compensated for in the study, these remain limitations.
DISCLOSURES:
The study was funded by European Commission Horizon 2020 Framework Programme. Dr. Balligand reported receiving grants from the European Commission during the conduct of the study, grants from Novartis and Daiichi Sankyo outside the submitted work, and consulting fees from Amgen, Novartis, and Daiichi Sankyo outside the submitted work; he also reported being a minor shareholder of Spinovit and serving as a board member for the Wallonia Health and Biotech Cluster, Biowin, and the AstraZeneca Foundation.
A version of this article first appeared on Medscape.com.
TOPLINE:
(pre-HF) structural heart disease who were at risk of developing or worsening HF.
METHODOLOGY:
- Interventions for patients with asymptomatic pre-HF may be important in reducing the incidence of clinically overt HF, including HF with preserved ejection fraction (HFpEF).
- Mirabegron activates the cardiac beta-3 adrenergic receptor, which may offer an alternative activation of the cyclic guanosine monophosphate protein/kinase G (cGMP/PKG) pathway for patients at risk of or with mild HF and protect against worsening left ventricular hypertrophy (LVH) and/or diastolic dysfunction, but few clinical trials have evaluated the effect of mirabegron on cardiovascular outcomes.
- The phase 2b Beta3_LVH trial included 296 patients, some with and some without HF symptoms (mean age, 63 years), at 10 centers in Europe and the United Kingdom. All had an increased LV mass index (LVMI) (≥ 115 g/m2 for men and ≥ 95 g/m2 for women) or end-diastolic wall thickness of ≥ 13 mm in at least one wall segment.
- Patients, many of whom had risk factors, including hypertension, and were receiving cardiovascular therapies, were randomly assigned to receive mirabegron 50 mg/day or placebo and underwent various tests, including cardiac MRI, Doppler echocardiography, and urine and blood sampling for fasting glucose, insulin, hemoglobin A1c, serum lipids, and other measures.
- The two primary endpoints were change in left ventricular mass index (LVMI), expressed in grams per meters squared, and change in diastolic function, assessed as the ratio of peak early transmitral ventricular filling velocity to early diastolic tissue Doppler velocity (E/e´).
TAKEAWAY:
- Neither primary outcome reached statistical significance at 12 months; adjusted differences between groups included a 1.3g/m2 increase in LVMI (95% confidence interval, −0.15 to 2.74; P = .08) and a −0.15 decrease in E/e´ (95% CI, −0.69 to 0.4; P = .60).
- There was no statistically significant effect of mirabegron, in comparison with placebo, on lipids, glycemic control, or insulin sensitivity.
- The effect of mirabegron remained neutral in exploratory subgroup analyses, including age (≤ 65 years or > 65 years at baseline), sex (men or women), body mass index (≤ 30 kg/m2 or > 30 at baseline), presence of type 2 diabetes, atrial fibrillation, beta-blocker use, and geographic region.
- There were no deaths. There was a total of 428 adverse events (AEs), but there were no statistically significant between-group differences in the occurrence of these AEs.
IN PRACTICE:
While this study showed that mirabegron had a neutral effect on LV mass and diastolic function for patients with pre-HF or mild HF, the researchers suggest that longer-term effects of beta-3 adrenergic stimulation on myocardial remodeling and function “need to be tested in patients with established HFpEF, including with recent, more potent agonists.”
SOURCE:
The study was conducted by Jean-Luc Balligand, MD, Institut de Recherche Expérimentale et Clinique, Université Catholique de Louvain, Brussels, and colleagues. It was published online in JAMA Cardiology.
LIMITATIONS:
Inclusion of patients with mild HF and use of a single standard mirabegron dosage (50 mg/day) may have prevented detection of a treatment effect. More advanced techniques than measurements of E/e´, such as cardiac strain, may have been better for assessing early changes in diastolic function. Although missing data and dropouts were relatively infrequent and were compensated for in the study, these remain limitations.
DISCLOSURES:
The study was funded by European Commission Horizon 2020 Framework Programme. Dr. Balligand reported receiving grants from the European Commission during the conduct of the study, grants from Novartis and Daiichi Sankyo outside the submitted work, and consulting fees from Amgen, Novartis, and Daiichi Sankyo outside the submitted work; he also reported being a minor shareholder of Spinovit and serving as a board member for the Wallonia Health and Biotech Cluster, Biowin, and the AstraZeneca Foundation.
A version of this article first appeared on Medscape.com.
TOPLINE:
(pre-HF) structural heart disease who were at risk of developing or worsening HF.
METHODOLOGY:
- Interventions for patients with asymptomatic pre-HF may be important in reducing the incidence of clinically overt HF, including HF with preserved ejection fraction (HFpEF).
- Mirabegron activates the cardiac beta-3 adrenergic receptor, which may offer an alternative activation of the cyclic guanosine monophosphate protein/kinase G (cGMP/PKG) pathway for patients at risk of or with mild HF and protect against worsening left ventricular hypertrophy (LVH) and/or diastolic dysfunction, but few clinical trials have evaluated the effect of mirabegron on cardiovascular outcomes.
- The phase 2b Beta3_LVH trial included 296 patients, some with and some without HF symptoms (mean age, 63 years), at 10 centers in Europe and the United Kingdom. All had an increased LV mass index (LVMI) (≥ 115 g/m2 for men and ≥ 95 g/m2 for women) or end-diastolic wall thickness of ≥ 13 mm in at least one wall segment.
- Patients, many of whom had risk factors, including hypertension, and were receiving cardiovascular therapies, were randomly assigned to receive mirabegron 50 mg/day or placebo and underwent various tests, including cardiac MRI, Doppler echocardiography, and urine and blood sampling for fasting glucose, insulin, hemoglobin A1c, serum lipids, and other measures.
- The two primary endpoints were change in left ventricular mass index (LVMI), expressed in grams per meters squared, and change in diastolic function, assessed as the ratio of peak early transmitral ventricular filling velocity to early diastolic tissue Doppler velocity (E/e´).
TAKEAWAY:
- Neither primary outcome reached statistical significance at 12 months; adjusted differences between groups included a 1.3g/m2 increase in LVMI (95% confidence interval, −0.15 to 2.74; P = .08) and a −0.15 decrease in E/e´ (95% CI, −0.69 to 0.4; P = .60).
- There was no statistically significant effect of mirabegron, in comparison with placebo, on lipids, glycemic control, or insulin sensitivity.
- The effect of mirabegron remained neutral in exploratory subgroup analyses, including age (≤ 65 years or > 65 years at baseline), sex (men or women), body mass index (≤ 30 kg/m2 or > 30 at baseline), presence of type 2 diabetes, atrial fibrillation, beta-blocker use, and geographic region.
- There were no deaths. There was a total of 428 adverse events (AEs), but there were no statistically significant between-group differences in the occurrence of these AEs.
IN PRACTICE:
While this study showed that mirabegron had a neutral effect on LV mass and diastolic function for patients with pre-HF or mild HF, the researchers suggest that longer-term effects of beta-3 adrenergic stimulation on myocardial remodeling and function “need to be tested in patients with established HFpEF, including with recent, more potent agonists.”
SOURCE:
The study was conducted by Jean-Luc Balligand, MD, Institut de Recherche Expérimentale et Clinique, Université Catholique de Louvain, Brussels, and colleagues. It was published online in JAMA Cardiology.
LIMITATIONS:
Inclusion of patients with mild HF and use of a single standard mirabegron dosage (50 mg/day) may have prevented detection of a treatment effect. More advanced techniques than measurements of E/e´, such as cardiac strain, may have been better for assessing early changes in diastolic function. Although missing data and dropouts were relatively infrequent and were compensated for in the study, these remain limitations.
DISCLOSURES:
The study was funded by European Commission Horizon 2020 Framework Programme. Dr. Balligand reported receiving grants from the European Commission during the conduct of the study, grants from Novartis and Daiichi Sankyo outside the submitted work, and consulting fees from Amgen, Novartis, and Daiichi Sankyo outside the submitted work; he also reported being a minor shareholder of Spinovit and serving as a board member for the Wallonia Health and Biotech Cluster, Biowin, and the AstraZeneca Foundation.
A version of this article first appeared on Medscape.com.
FROM JAMA CARDIOLOGY
Anxiety, depression ease after AFib ablation: Clinical or placebo effect?
who had initially tested high for such psychological distress.
The finding, said the researchers, may point to an overlooked potential benefit of ablation that can be discussed with patients considering whether to have the procedure.
Importantly, the 100 adults with symptomatic paroxysmal or persistent AFib in the randomized trial weren’t blinded to treatment assignment, which was either ablation or continued medical therapy.
That leaves open the possibility that psychological distress improved in the ablation group not from any unique effect of ablation itself but because patients expected to benefit from the procedure.
The investigators acknowledged that their trial, called REMEDIAL, can’t rule out a placebo effect as part of the observed benefit. Indeed, studies suggest that there is a substantial placebo component of AFib ablation – which, notably, is usually done to make patients feel better.
But the current findings are more consistent with the conventional view that patients feel better primarily because ablation reduces the AFib causing their symptoms, the group said.
Psychological stress in the study started to fall early after the procedure and continued to decline consistently over the next 6 months (P = .006) and 12 months (P = .005), not a typical pattern for placebo, they wrote.
Moreover, the mental health benefits “correlated very strongly” with less recurrent AFib, reduced AFib burden, and withdrawal of beta-blockers and antiarrhythmic agents, outcomes that might be expected from ablation, said Jonathan M. Kalman, MBBS, PhD.
“Of course, I cannot say there is no placebo effect from having had the procedure, and maybe that something to consider,” but it’s probably not the main driver of benefit, he said in an interview. The relationship between successful AFib ablation “and improvements in physical and now mental health is overwhelming.”
Dr. Kalman, who is affiliated with Royal Melbourne Hospital, is senior author on the study, published in JAMA.
The findings add to “strong, reproducible evidence that ablation is the best way to tackle rhythm control in [AFib] populations” regardless of age, mental health status, or AFib burden, said Auroa Badin, MD, who wasn’t involved in REMEDIAL but has studied the psychological effects of arrhythmia ablation.
For example, there is “very good evidence” from CABANA and other trials that AFib ablation “considerably improves quality of life,” Dr. Badin, of OhioHealth Heart & Vascular Physicians, Columbus, said in an interview. The current study “just emphasizes that there’s also a psychological effect.”
Some of that response could be a placebo or even a nocebo effect. Most of the patients assigned to the medical arm had already been on medications that failed at rhythm control. And their management in the trial, he said, “even if you optimize it, was still drug therapy.”
Patients in the control group, therefore, could have been “disappointed” at the prospect of continued ineffective therapy in a way that influenced their outcomes. “That is another confounding factor,” Dr. Badin said.
But if the psychological results of ablation in the trial were predominantly a placebo effect, early differences in psychological test scores would not have persisted for long, certainly not for a year, he observed. Moreover, the ablation group had better test scores at 12 months than at 6 months, “indicating a likelihood of improvement over time.”
Differences between the groups would probably have been less pronounced if the control group had received a sham procedure, Dr. Badin proposed. That would potentially differentiate ablation’s clinical and placebo contributions to the outcomes.
Still, he said, any observed placebo effect in a sham-controlled trial would probably have been limited. “I think it still would have been a positive trial. It may not show the same difference, but I don’t think you would have a neutral trial just by doing a sham.”
REMEDIAL has “good data,” and its conclusions about ablation’s potential psychological benefits are “reasonable” and worth bringing up when discussing the procedure with patients, Dr. Badin said.
Indeed, psychological distress is “important and often overlooked” in patients with AFib, Dr. Kalman observed. “The dominant indication for atrial fibrillation ablation is symptomatic impact on quality of life. We should think about that broadly, about not just the physical symptoms but the impact on their mental health.”
The trial was conducted at two centers in Australia. It enrolled patients, one-third of whom were women, who were on medical management for AFib. Patients receiving treatment for severe depression were excluded. The included patients were randomly assigned to undergo catheter ablation or to continue on closely managed rhythm-control medication, with cardioversion as indicated.
Psychological distress was measured at baseline and throughout follow-up by a battery of self-administered, validated questionnaires. Baseline test scores for the two groups were similar.
Recurrence and burden of AFib were tracked primarily by daily KardiaMobile (AliveCor) ECG monitoring. A few patients were followed using already implanted cardiac rhythm devices or by 24-hour Holter monitor every 3 months, Dr. Kalman said.
Composite scores on the Hospital Anxiety and Depression Scale (HADS) at 12 months, the primary endpoint, were 7.6 and 11.8 (P = .005) for the ablation and medical groups, respectively. They were 8.2 and 11.9 (P = .006), respectively, at 6 months.
The prevalence of severe psychological distress, defined as a HADS score greater than 15, was lower in the ablation group at 6 months (14.2% vs. 34%; P = .02) and 12 months (10.2% vs. 31.9%; P = .01).
Scores on the Beck Depression Inventory–II questionnaire were also consistently and significantly better for the ablation group at 6 and at 12 months (P = .01 for both).
Monitoring picked up AFib in 47% of the ablation group and 96% of the control group (P < .001) over 12 months. Their median AFib burdens were 0% (interquartile range, 0%-3.2%) and 15.5% (IQR, 1%-46%), respectively (P < .001).
Antiarrhythmic drug use fell from a baseline of 90% to 53% 3 months after ablation and 30% at 12 months (P = .003). Use of these drugs in the control group was 89% at baseline and remained essentially the same, 85%, at 12 months.
AFib symptom severity scores were significantly lower after ablation, compared with medical management at 3, 6, and 12 months.
The observed effect of ablation on psychological stress “clearly speaks in favor of effective rhythm control, and moreover catheter ablation” and is a “novel argument” in support of catheter ablation for AFib, Julia Lurz, MD, Heart Center Leipzig (Germany) at University Leipzig, and Karl-Heinz Ladwig, MD, PhD, Technical University Munich (Germany), wrote in an editorial accompanying publication of REMEDIAL.
But the findings also “raise the question of why rhythm control was so ineffective in the medical treatment group,” they wrote.
They agreed that the randomization process itself may have had its own psychological effects. “Potential disappointment” in the medical group and “high expectations” among patients who received ablation “could have fueled the success of catheter ablation” with respect to mental health endpoints.
Dr. Kalman reported receiving grants from the National Health and Medical Research Council of Australia, Medtronic, Mooney, and Biosense Webster. Dr. Badin, Dr. Lurz, and Dr. Ladwig reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
who had initially tested high for such psychological distress.
The finding, said the researchers, may point to an overlooked potential benefit of ablation that can be discussed with patients considering whether to have the procedure.
Importantly, the 100 adults with symptomatic paroxysmal or persistent AFib in the randomized trial weren’t blinded to treatment assignment, which was either ablation or continued medical therapy.
That leaves open the possibility that psychological distress improved in the ablation group not from any unique effect of ablation itself but because patients expected to benefit from the procedure.
The investigators acknowledged that their trial, called REMEDIAL, can’t rule out a placebo effect as part of the observed benefit. Indeed, studies suggest that there is a substantial placebo component of AFib ablation – which, notably, is usually done to make patients feel better.
But the current findings are more consistent with the conventional view that patients feel better primarily because ablation reduces the AFib causing their symptoms, the group said.
Psychological stress in the study started to fall early after the procedure and continued to decline consistently over the next 6 months (P = .006) and 12 months (P = .005), not a typical pattern for placebo, they wrote.
Moreover, the mental health benefits “correlated very strongly” with less recurrent AFib, reduced AFib burden, and withdrawal of beta-blockers and antiarrhythmic agents, outcomes that might be expected from ablation, said Jonathan M. Kalman, MBBS, PhD.
“Of course, I cannot say there is no placebo effect from having had the procedure, and maybe that something to consider,” but it’s probably not the main driver of benefit, he said in an interview. The relationship between successful AFib ablation “and improvements in physical and now mental health is overwhelming.”
Dr. Kalman, who is affiliated with Royal Melbourne Hospital, is senior author on the study, published in JAMA.
The findings add to “strong, reproducible evidence that ablation is the best way to tackle rhythm control in [AFib] populations” regardless of age, mental health status, or AFib burden, said Auroa Badin, MD, who wasn’t involved in REMEDIAL but has studied the psychological effects of arrhythmia ablation.
For example, there is “very good evidence” from CABANA and other trials that AFib ablation “considerably improves quality of life,” Dr. Badin, of OhioHealth Heart & Vascular Physicians, Columbus, said in an interview. The current study “just emphasizes that there’s also a psychological effect.”
Some of that response could be a placebo or even a nocebo effect. Most of the patients assigned to the medical arm had already been on medications that failed at rhythm control. And their management in the trial, he said, “even if you optimize it, was still drug therapy.”
Patients in the control group, therefore, could have been “disappointed” at the prospect of continued ineffective therapy in a way that influenced their outcomes. “That is another confounding factor,” Dr. Badin said.
But if the psychological results of ablation in the trial were predominantly a placebo effect, early differences in psychological test scores would not have persisted for long, certainly not for a year, he observed. Moreover, the ablation group had better test scores at 12 months than at 6 months, “indicating a likelihood of improvement over time.”
Differences between the groups would probably have been less pronounced if the control group had received a sham procedure, Dr. Badin proposed. That would potentially differentiate ablation’s clinical and placebo contributions to the outcomes.
Still, he said, any observed placebo effect in a sham-controlled trial would probably have been limited. “I think it still would have been a positive trial. It may not show the same difference, but I don’t think you would have a neutral trial just by doing a sham.”
REMEDIAL has “good data,” and its conclusions about ablation’s potential psychological benefits are “reasonable” and worth bringing up when discussing the procedure with patients, Dr. Badin said.
Indeed, psychological distress is “important and often overlooked” in patients with AFib, Dr. Kalman observed. “The dominant indication for atrial fibrillation ablation is symptomatic impact on quality of life. We should think about that broadly, about not just the physical symptoms but the impact on their mental health.”
The trial was conducted at two centers in Australia. It enrolled patients, one-third of whom were women, who were on medical management for AFib. Patients receiving treatment for severe depression were excluded. The included patients were randomly assigned to undergo catheter ablation or to continue on closely managed rhythm-control medication, with cardioversion as indicated.
Psychological distress was measured at baseline and throughout follow-up by a battery of self-administered, validated questionnaires. Baseline test scores for the two groups were similar.
Recurrence and burden of AFib were tracked primarily by daily KardiaMobile (AliveCor) ECG monitoring. A few patients were followed using already implanted cardiac rhythm devices or by 24-hour Holter monitor every 3 months, Dr. Kalman said.
Composite scores on the Hospital Anxiety and Depression Scale (HADS) at 12 months, the primary endpoint, were 7.6 and 11.8 (P = .005) for the ablation and medical groups, respectively. They were 8.2 and 11.9 (P = .006), respectively, at 6 months.
The prevalence of severe psychological distress, defined as a HADS score greater than 15, was lower in the ablation group at 6 months (14.2% vs. 34%; P = .02) and 12 months (10.2% vs. 31.9%; P = .01).
Scores on the Beck Depression Inventory–II questionnaire were also consistently and significantly better for the ablation group at 6 and at 12 months (P = .01 for both).
Monitoring picked up AFib in 47% of the ablation group and 96% of the control group (P < .001) over 12 months. Their median AFib burdens were 0% (interquartile range, 0%-3.2%) and 15.5% (IQR, 1%-46%), respectively (P < .001).
Antiarrhythmic drug use fell from a baseline of 90% to 53% 3 months after ablation and 30% at 12 months (P = .003). Use of these drugs in the control group was 89% at baseline and remained essentially the same, 85%, at 12 months.
AFib symptom severity scores were significantly lower after ablation, compared with medical management at 3, 6, and 12 months.
The observed effect of ablation on psychological stress “clearly speaks in favor of effective rhythm control, and moreover catheter ablation” and is a “novel argument” in support of catheter ablation for AFib, Julia Lurz, MD, Heart Center Leipzig (Germany) at University Leipzig, and Karl-Heinz Ladwig, MD, PhD, Technical University Munich (Germany), wrote in an editorial accompanying publication of REMEDIAL.
But the findings also “raise the question of why rhythm control was so ineffective in the medical treatment group,” they wrote.
They agreed that the randomization process itself may have had its own psychological effects. “Potential disappointment” in the medical group and “high expectations” among patients who received ablation “could have fueled the success of catheter ablation” with respect to mental health endpoints.
Dr. Kalman reported receiving grants from the National Health and Medical Research Council of Australia, Medtronic, Mooney, and Biosense Webster. Dr. Badin, Dr. Lurz, and Dr. Ladwig reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
who had initially tested high for such psychological distress.
The finding, said the researchers, may point to an overlooked potential benefit of ablation that can be discussed with patients considering whether to have the procedure.
Importantly, the 100 adults with symptomatic paroxysmal or persistent AFib in the randomized trial weren’t blinded to treatment assignment, which was either ablation or continued medical therapy.
That leaves open the possibility that psychological distress improved in the ablation group not from any unique effect of ablation itself but because patients expected to benefit from the procedure.
The investigators acknowledged that their trial, called REMEDIAL, can’t rule out a placebo effect as part of the observed benefit. Indeed, studies suggest that there is a substantial placebo component of AFib ablation – which, notably, is usually done to make patients feel better.
But the current findings are more consistent with the conventional view that patients feel better primarily because ablation reduces the AFib causing their symptoms, the group said.
Psychological stress in the study started to fall early after the procedure and continued to decline consistently over the next 6 months (P = .006) and 12 months (P = .005), not a typical pattern for placebo, they wrote.
Moreover, the mental health benefits “correlated very strongly” with less recurrent AFib, reduced AFib burden, and withdrawal of beta-blockers and antiarrhythmic agents, outcomes that might be expected from ablation, said Jonathan M. Kalman, MBBS, PhD.
“Of course, I cannot say there is no placebo effect from having had the procedure, and maybe that something to consider,” but it’s probably not the main driver of benefit, he said in an interview. The relationship between successful AFib ablation “and improvements in physical and now mental health is overwhelming.”
Dr. Kalman, who is affiliated with Royal Melbourne Hospital, is senior author on the study, published in JAMA.
The findings add to “strong, reproducible evidence that ablation is the best way to tackle rhythm control in [AFib] populations” regardless of age, mental health status, or AFib burden, said Auroa Badin, MD, who wasn’t involved in REMEDIAL but has studied the psychological effects of arrhythmia ablation.
For example, there is “very good evidence” from CABANA and other trials that AFib ablation “considerably improves quality of life,” Dr. Badin, of OhioHealth Heart & Vascular Physicians, Columbus, said in an interview. The current study “just emphasizes that there’s also a psychological effect.”
Some of that response could be a placebo or even a nocebo effect. Most of the patients assigned to the medical arm had already been on medications that failed at rhythm control. And their management in the trial, he said, “even if you optimize it, was still drug therapy.”
Patients in the control group, therefore, could have been “disappointed” at the prospect of continued ineffective therapy in a way that influenced their outcomes. “That is another confounding factor,” Dr. Badin said.
But if the psychological results of ablation in the trial were predominantly a placebo effect, early differences in psychological test scores would not have persisted for long, certainly not for a year, he observed. Moreover, the ablation group had better test scores at 12 months than at 6 months, “indicating a likelihood of improvement over time.”
Differences between the groups would probably have been less pronounced if the control group had received a sham procedure, Dr. Badin proposed. That would potentially differentiate ablation’s clinical and placebo contributions to the outcomes.
Still, he said, any observed placebo effect in a sham-controlled trial would probably have been limited. “I think it still would have been a positive trial. It may not show the same difference, but I don’t think you would have a neutral trial just by doing a sham.”
REMEDIAL has “good data,” and its conclusions about ablation’s potential psychological benefits are “reasonable” and worth bringing up when discussing the procedure with patients, Dr. Badin said.
Indeed, psychological distress is “important and often overlooked” in patients with AFib, Dr. Kalman observed. “The dominant indication for atrial fibrillation ablation is symptomatic impact on quality of life. We should think about that broadly, about not just the physical symptoms but the impact on their mental health.”
The trial was conducted at two centers in Australia. It enrolled patients, one-third of whom were women, who were on medical management for AFib. Patients receiving treatment for severe depression were excluded. The included patients were randomly assigned to undergo catheter ablation or to continue on closely managed rhythm-control medication, with cardioversion as indicated.
Psychological distress was measured at baseline and throughout follow-up by a battery of self-administered, validated questionnaires. Baseline test scores for the two groups were similar.
Recurrence and burden of AFib were tracked primarily by daily KardiaMobile (AliveCor) ECG monitoring. A few patients were followed using already implanted cardiac rhythm devices or by 24-hour Holter monitor every 3 months, Dr. Kalman said.
Composite scores on the Hospital Anxiety and Depression Scale (HADS) at 12 months, the primary endpoint, were 7.6 and 11.8 (P = .005) for the ablation and medical groups, respectively. They were 8.2 and 11.9 (P = .006), respectively, at 6 months.
The prevalence of severe psychological distress, defined as a HADS score greater than 15, was lower in the ablation group at 6 months (14.2% vs. 34%; P = .02) and 12 months (10.2% vs. 31.9%; P = .01).
Scores on the Beck Depression Inventory–II questionnaire were also consistently and significantly better for the ablation group at 6 and at 12 months (P = .01 for both).
Monitoring picked up AFib in 47% of the ablation group and 96% of the control group (P < .001) over 12 months. Their median AFib burdens were 0% (interquartile range, 0%-3.2%) and 15.5% (IQR, 1%-46%), respectively (P < .001).
Antiarrhythmic drug use fell from a baseline of 90% to 53% 3 months after ablation and 30% at 12 months (P = .003). Use of these drugs in the control group was 89% at baseline and remained essentially the same, 85%, at 12 months.
AFib symptom severity scores were significantly lower after ablation, compared with medical management at 3, 6, and 12 months.
The observed effect of ablation on psychological stress “clearly speaks in favor of effective rhythm control, and moreover catheter ablation” and is a “novel argument” in support of catheter ablation for AFib, Julia Lurz, MD, Heart Center Leipzig (Germany) at University Leipzig, and Karl-Heinz Ladwig, MD, PhD, Technical University Munich (Germany), wrote in an editorial accompanying publication of REMEDIAL.
But the findings also “raise the question of why rhythm control was so ineffective in the medical treatment group,” they wrote.
They agreed that the randomization process itself may have had its own psychological effects. “Potential disappointment” in the medical group and “high expectations” among patients who received ablation “could have fueled the success of catheter ablation” with respect to mental health endpoints.
Dr. Kalman reported receiving grants from the National Health and Medical Research Council of Australia, Medtronic, Mooney, and Biosense Webster. Dr. Badin, Dr. Lurz, and Dr. Ladwig reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM JAMA
No-biopsy approach to celiac disease diagnosis safe for some
TOPLINE:
For adults with suspected celiac disease who do not have immunoglobulin A (IgA) deficiency, diagnostic bowel biopsy can most likely be avoided if the serum antitissue transglutaminase IgA (tTG-IgA) level is high.
METHODOLOGY:
- Researchers evaluated the reliability of serum tests for diagnosing celiac disease, as defined by duodenal villous atrophy (Marsh type 3 or Corazza-Villanacci grade B).
- The main study cohort included 436 adults with suspected celiac disease who did not have IgA deficiency and who were not on a gluten-free diet (mean age, 40 years; 68% women). The patients were referred by 14 centers across four continents to undergo local endoscopic duodenal biopsy.
- Local serum tTG-IgA was measured with 14 test brands. Concentration was expressed as a multiple of each test’s upper limit of normal (ULN). Tests were defined as positive when they exceeded one times the ULN.
- Histology was assessed by the local pathologist, and discordant cases were reevaluated by a central pathologist.
TAKEAWAY:
- Positive serum tTG-IgA was detected in 363 (83%) participants; negative serum tTG-IgA was detected in 73 (17%).
- After local review, 341 of the participants with positive serum tTG-IgA had positive histology (true positives) and 22 had negative histology (false positives).
- Of the 73 participants with negative serum tTG-IgA, 66 had negative histology (true negatives) and 7 had positive histology (false negatives).
- Central reevaluation of duodenal histology was performed in 29 discordant cases, resulting in 348 true positive cases, 15 false positive cases, 66 true negative cases, and 7 false negative cases – the equivalent of a positive predictive value of 95.9%, a negative predictive value of 90.4%, a sensitivity of 98%, and a specificity of 81.5%.
- The positive predictive value of local serum tTG-IgA increased when the serologic threshold was defined at increasing multiples of the ULN. The test correctly diagnosed duodenal villous atrophy in 97.5% of patients with serum tTG-IgA concentrations greater than 10 times the ULN.
IN PRACTICE:
“The results of this multicentre prospective study indicate that a no-biopsy approach for the diagnosis of coeliac disease is safe and reliable in adult patients without IgA deficiency and with serum tTG-IgA greater than the assay-specific upper limit of normal,” the authors write. “We found no evidence that important comorbidities would be missed by adopting a no-biopsy strategy.”
SOURCE:
The study was led by Carolina Ciacci, Centre for Coeliac Disease, AOU San Giovanni Di Dio e Ruggi d’Aragona, Salerno, Italy, and was published online in The Lancet Gastroenterology and Hepatology.
LIMITATIONS:
Limitations include a lack of data on IgA-deficient participants, a low number of participants in some subgroup analyses, a lack of data for many ethnic groups, limited follow-up information, limited assessments in the central laboratory, and high pretest probability of celiac disease (low number of participants without duodenal villous atrophy).
DISCLOSURES:
The study had no specific funding. One coauthor is an employee of Werfen. The other authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
For adults with suspected celiac disease who do not have immunoglobulin A (IgA) deficiency, diagnostic bowel biopsy can most likely be avoided if the serum antitissue transglutaminase IgA (tTG-IgA) level is high.
METHODOLOGY:
- Researchers evaluated the reliability of serum tests for diagnosing celiac disease, as defined by duodenal villous atrophy (Marsh type 3 or Corazza-Villanacci grade B).
- The main study cohort included 436 adults with suspected celiac disease who did not have IgA deficiency and who were not on a gluten-free diet (mean age, 40 years; 68% women). The patients were referred by 14 centers across four continents to undergo local endoscopic duodenal biopsy.
- Local serum tTG-IgA was measured with 14 test brands. Concentration was expressed as a multiple of each test’s upper limit of normal (ULN). Tests were defined as positive when they exceeded one times the ULN.
- Histology was assessed by the local pathologist, and discordant cases were reevaluated by a central pathologist.
TAKEAWAY:
- Positive serum tTG-IgA was detected in 363 (83%) participants; negative serum tTG-IgA was detected in 73 (17%).
- After local review, 341 of the participants with positive serum tTG-IgA had positive histology (true positives) and 22 had negative histology (false positives).
- Of the 73 participants with negative serum tTG-IgA, 66 had negative histology (true negatives) and 7 had positive histology (false negatives).
- Central reevaluation of duodenal histology was performed in 29 discordant cases, resulting in 348 true positive cases, 15 false positive cases, 66 true negative cases, and 7 false negative cases – the equivalent of a positive predictive value of 95.9%, a negative predictive value of 90.4%, a sensitivity of 98%, and a specificity of 81.5%.
- The positive predictive value of local serum tTG-IgA increased when the serologic threshold was defined at increasing multiples of the ULN. The test correctly diagnosed duodenal villous atrophy in 97.5% of patients with serum tTG-IgA concentrations greater than 10 times the ULN.
IN PRACTICE:
“The results of this multicentre prospective study indicate that a no-biopsy approach for the diagnosis of coeliac disease is safe and reliable in adult patients without IgA deficiency and with serum tTG-IgA greater than the assay-specific upper limit of normal,” the authors write. “We found no evidence that important comorbidities would be missed by adopting a no-biopsy strategy.”
SOURCE:
The study was led by Carolina Ciacci, Centre for Coeliac Disease, AOU San Giovanni Di Dio e Ruggi d’Aragona, Salerno, Italy, and was published online in The Lancet Gastroenterology and Hepatology.
LIMITATIONS:
Limitations include a lack of data on IgA-deficient participants, a low number of participants in some subgroup analyses, a lack of data for many ethnic groups, limited follow-up information, limited assessments in the central laboratory, and high pretest probability of celiac disease (low number of participants without duodenal villous atrophy).
DISCLOSURES:
The study had no specific funding. One coauthor is an employee of Werfen. The other authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
For adults with suspected celiac disease who do not have immunoglobulin A (IgA) deficiency, diagnostic bowel biopsy can most likely be avoided if the serum antitissue transglutaminase IgA (tTG-IgA) level is high.
METHODOLOGY:
- Researchers evaluated the reliability of serum tests for diagnosing celiac disease, as defined by duodenal villous atrophy (Marsh type 3 or Corazza-Villanacci grade B).
- The main study cohort included 436 adults with suspected celiac disease who did not have IgA deficiency and who were not on a gluten-free diet (mean age, 40 years; 68% women). The patients were referred by 14 centers across four continents to undergo local endoscopic duodenal biopsy.
- Local serum tTG-IgA was measured with 14 test brands. Concentration was expressed as a multiple of each test’s upper limit of normal (ULN). Tests were defined as positive when they exceeded one times the ULN.
- Histology was assessed by the local pathologist, and discordant cases were reevaluated by a central pathologist.
TAKEAWAY:
- Positive serum tTG-IgA was detected in 363 (83%) participants; negative serum tTG-IgA was detected in 73 (17%).
- After local review, 341 of the participants with positive serum tTG-IgA had positive histology (true positives) and 22 had negative histology (false positives).
- Of the 73 participants with negative serum tTG-IgA, 66 had negative histology (true negatives) and 7 had positive histology (false negatives).
- Central reevaluation of duodenal histology was performed in 29 discordant cases, resulting in 348 true positive cases, 15 false positive cases, 66 true negative cases, and 7 false negative cases – the equivalent of a positive predictive value of 95.9%, a negative predictive value of 90.4%, a sensitivity of 98%, and a specificity of 81.5%.
- The positive predictive value of local serum tTG-IgA increased when the serologic threshold was defined at increasing multiples of the ULN. The test correctly diagnosed duodenal villous atrophy in 97.5% of patients with serum tTG-IgA concentrations greater than 10 times the ULN.
IN PRACTICE:
“The results of this multicentre prospective study indicate that a no-biopsy approach for the diagnosis of coeliac disease is safe and reliable in adult patients without IgA deficiency and with serum tTG-IgA greater than the assay-specific upper limit of normal,” the authors write. “We found no evidence that important comorbidities would be missed by adopting a no-biopsy strategy.”
SOURCE:
The study was led by Carolina Ciacci, Centre for Coeliac Disease, AOU San Giovanni Di Dio e Ruggi d’Aragona, Salerno, Italy, and was published online in The Lancet Gastroenterology and Hepatology.
LIMITATIONS:
Limitations include a lack of data on IgA-deficient participants, a low number of participants in some subgroup analyses, a lack of data for many ethnic groups, limited follow-up information, limited assessments in the central laboratory, and high pretest probability of celiac disease (low number of participants without duodenal villous atrophy).
DISCLOSURES:
The study had no specific funding. One coauthor is an employee of Werfen. The other authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cat Scratch Disease Presenting With Concurrent Pityriasis Rosea in a 10-Year-Old Girl
To the Editor:
Cat scratch disease (CSD) is caused by Bartonella henselae and Bartonella clarridgeiae bacteria transferred from cats to humans that results in an inflamed inoculation site and tender lymphadenopathy. Pityriasis rosea (PR) and PR-like eruptions are self-limited, acute exanthems that have been associated with infections, vaccinations, and medications. We report a case of PR occurring in a 10-year-old girl with CSD, which may suggest an association between the 2 diseases.

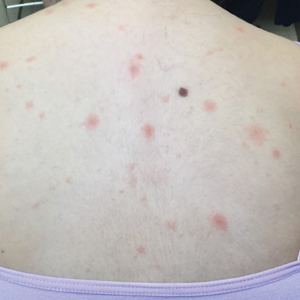
A 10-year-old girl who was otherwise healthy presented in the winter with a rash of 5 days’ duration. Fourteen days prior to the rash, the patient reported being scratched by a new kitten and noted a pinpoint “puncture” on the left forearm that developed into a red papule over the following week. Seven days after the cat scratch, the patient experienced pain and swelling in the left axilla. Approximately 1 week after the onset of lymphadenopathy, the patient developed an asymptomatic rash that started with a large spot on the left chest, followed by smaller spots appearing over the next 2 days and spreading to the rest of the trunk. Four days after the rash onset, the patient experienced a mild headache, low-grade subjective fever, and chills. She denied any recent travel, bug bites, sore throat, and diarrhea. She was up-to-date on all vaccinations and had not received any vaccines preceding the symptoms. Physical examination revealed a 2-cm pink, scaly, thin plaque with a collarette of scale on the left upper chest (herald patch), along with multiple thin pink papules and small plaques with central scale on the trunk (Figure 1). A pustule with adjacent linear erosion was present on the left ventral forearm (Figure 2). The patient had a tender subcutaneous nodule in the left axilla as well as bilateral anterior and posterior cervical-chain subcutaneous tender nodules. There was no involvement of the palms, soles, or mucosae.

The patient was empirically treated for CSD with azithromycin (200 mg/5 mL), 404 mg on day 1 followed by 202 mg daily for 4 days. The rash was treated with hydrocortisone cream 2.5% twice daily for 2 weeks. A wound culture of the pustule on the left forearm was negative for neutrophils and organisms. Antibody serologies obtained 4 weeks after presentation were notable for an elevated B henselae IgG titer of 1:640, confirming the diagnosis of CSD. Following treatment with azithromycin and hydrocortisone, all of the patient’s symptoms resolved after 1 to 2 weeks.
Cat scratch disease is a zoonotic infection caused by the bacteria B henselae and the more recently described pathogen B clarridgeiae. Cat fleas spread these bacteria among cats, which subsequently inoculate the bacteria into humans through bites and scratches. The incidence of CSD in the United States is estimated to be 4.5 to 9.3 per 100,000 individuals in the outpatient setting and 0.19 to 0.86 per 100,000 individuals in the inpatient setting.1 Geographic variance can occur based on flea populations, resulting in higher incidence in warm humid climates and lower incidence in mountainous arid climates. The incidence of CSD in the pediatric population is highest in children aged 5 to 9 years. A national representative survey (N=3011) from 2017 revealed that 37.2% of primary care providers had diagnosed CSD in the prior year.1
Classic CSD presents as an erythematous papule at the inoculation site lasting days to weeks, with progression to tender lymphadenopathy lasting weeks to months. Fever, malaise, and chills also can be seen. Atypical CSD occurs in up to 24% of cases in immunocompetent patients.1 Atypical and systemic presentations are varied and can include fever of unknown origin, neuroretinitis, uveitis, retinal vessel occlusion, encephalitis, hepatosplenic lesions, Parinaud oculoglandular syndrome, osteomyelitis, and endocarditis.1,2 Atypical dermatologic presentations of CSD include maculopapular rash in 7% of cases and erythema nodosum in 2.5% of cases, as well as rare reports of cutaneous vasculitis, urticaria, immune thrombocytopenic purpura, and papuloedematous eruption.3 Treatment guidelines for CSD vary widely depending on the clinical presentation as well as the immunocompetence of the infected individual. Our patient had limited regional lymphadenopathy with no signs of dissemination or neurologic involvement and was successfully treated with a 5-day course of oral azithromycin (weight based, 10 mg/kg). More extensive disease such as hepatosplenic or neurologic CSD may require multiple antibiotics for up to 6 weeks. Alternative or additional antibiotics used for CSD include rifampin, trimethoprim-sulfamethoxazole, ciprofloxacin, doxycycline, gentamicin, and clarithromycin. Opinions vary as to whether all patients or just those with complicated infections warrant antibiotic therapy.4-6
Pityriasis rosea is a self-limited acute exanthematous disease that is classically associated with a systemic reactivation of human herpesvirus (HHV) 6 and/or HHV-7. The incidence of PR is estimated to be 480 per 100,000 dermatologic patients. It is slightly more common in females and occurs most often in patients aged 10 to 35 years.7 Clinically, PR appears with the abrupt onset of a single erythematous scaly patch (termed the herald patch), followed by a secondary eruption of smaller erythematous scaly macules and patches along the trunk’s cleavage lines. The secondary eruption on the back is sometimes termed a Christmas or fir tree pattern.7,8
In addition to the classic presentation of PR, there have been reports of numerous atypical clinical presentations. The herald patch, which classically presents on the trunk, also has been reported to present on the extremities; PR of the extremities is defined by lesions that appear as large scaly plaques on the extremities only. Inverse PR presents with lesions occurring in flexural areas and acral surfaces but not on the trunk. There also is an acral PR variant in which lesions appear only on the palms, wrists, and soles. Purpuric or hemorrhagic PR has been described and presents with purpura and petechiae with or without collarettes of scale in diffuse locations, including the palate. Oral PR presents more commonly in patients of color as erosions, ulcers, hemorrhagic lesions, bullae, or geographic tongue. Erythema multiforme–like PR appears with targetoid lesions on the trunk, face, neck, and arms without a history of herpes simplex virus infection. A large pear-shaped herald patch has been reported and characterizes the gigantea PR of Darier variant. Irritated PR occurs with typical PR findings, but afflicted patients report severe pain and burning with diaphoresis. Relapsing PR can occur within 1 year of a prior episode of PR and presents without a herald patch. Persistent PR is defined by PR lasting more than 3 months, and most reported cases have included oral lesions. Finally, other PR variants that have been described include urticarial, papular, follicular, vesicular, and hypopigmented types.7-9
Furthermore, there have been reports of multiple atypical presentations occurring simultaneously in the same patient.10 Although PR classically has been associated with HHV-6 and/or HHV-7 reactivation, it has been reported with a few other clinical situations and conditions. Pityriasislike eruption specifically refers to an exanthem secondary to drugs or vaccination that resembles PR but shows clinical differences, including diffuse and confluent dusky-red macules and/or plaques with or without desquamation on the trunk, extremities, and face. Drugs that have been implicated as triggers include ACE inhibitors, gold, isotretinoin, nonsteroidal anti-inflammatory agents, omeprazole, terbinafine, and tyrosine kinase inhibitors. Smallpox, tuberculosis, poliomyelitis, influenza, diphtheria, tetanus, hepatitis B virus, pneumococcus, papillomavirus, yellow fever, and pertussis vaccinations also have been associated with PR.7,11,12 Additionally, PR has been reported to occur with active systemic infections, specifically H1N1 influenza, though it is rare.13 Because of its self-limited course, treatment of PR most often involves only reassurance. Topical corticosteroids may be appropriate for pruritus.7,8
Pediatric health care providers including dermatologists should be familiar with both CSD and PR because they are common diseases that more often are encountered in the pediatric population. We present a unique case of CSD presenting with concurrent PR, which highlights a potential new etiology for PR and a rare cutaneous manifestation of CSD. Further investigation into a possible relationship between CSD and PR may be warranted. Patients with any signs and symptoms of fever, tender lymphadenopathy, worsening rash, or exposure to cats warrant a thorough history and physical examination to ensure that neither entity is overlooked.
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge [published online July 14, 2017]. Zoonoses Public Health. 2018;65:67-73. doi:10.1111/zph.12368
- Habot-Wilner Z, Trivizki O, Goldstein M, et al. Cat-scratch disease: ocular manifestations and treatment outcome. Acta Ophthalmol. 2018;96:E524-E532. doi:10.1111/aos.13684
- Schattner A, Uliel L, Dubin I. The cat did it: erythema nodosum and additional atypical presentations of Bartonella henselae infection in immunocompetent hosts [published online February 16, 2018]. BMJ Case Rep. doi:10.1136/bcr-2017-222511
- Shorbatli L, Koranyi K, Nahata M. Effectiveness of antibiotic therapy in pediatric patients with cat scratch disease. Int J Clin Pharm. 2018;40:1458-1461. doi: 10.1007/s11096-018-0746-1
- Bass JW, Freitas BC, Freitas AD, et al. Prospective randomized double blind placebo-controlled evaluation of azithromycin for treatment of cat-scratch disease. Pediatr Infect Dis J. 1998;17:447-452. doi:10.1097/00006454-199806000-00002
- Spach DH, Kaplan SL. Treatment of cat scratch disease. UpToDate. Updated December 9, 2021. Accessed September 12, 2023. https://www.uptodate.com/contents/treatment-of-cat-scratch-disease
- Drago F, Ciccarese G, Rebora A, et al. Pityriasis rosea: a comprehensive classification. Dermatology. 2016;232:431-437. doi:10.1159/000445375
- Urbina F, Das A, Sudy E. Clinical variants of pityriasis rosea. World J Clin Cases. 2017;5:203-211. doi:10.12998/wjcc.v5.i6.203
- Alzahrani NA, Al Jasser MI. Geographic tonguelike presentation in a child with pityriasis rosea: case report and review of oral manifestations of pityriasis rosea. Pediatr Dermatol. 2018;35:E124-E127. doi:10.1111/pde.13417
- Sinha S, Sardana K, Garg V. Coexistence of two atypical variants of pityriasis rosea: a case report and review of literature. Pediatr Dermatol. 2012;29:538-540. doi:10.1111/j.1525-1470.2011.01549.x
- Drago F, Ciccarese G, Parodi A. Pityriasis rosea and pityriasis rosea-like eruptions: how to distinguish them? JAAD Case Rep. 2018;4:800-801. doi:10.1016/j.jdcr.2018.04.002
- Drago F, Ciccarese G, Javor S, et al. Vaccine-induced pityriasis rosea and pityriasis rosea-like eruptions: a review of the literature. J Eur Acad Dermatol Venereol. 2016;30:544-545. doi:10.1111/jdv.12942
- Mubki TF, Bin Dayel SA, Kadry R. A case of pityriasis rosea concurrent with the novel influenza A (H1N1) infection. Pediatr Dermatol. 2011;28:341-342. doi:10.1111/j.1525-1470.2010.01090.x
To the Editor:
Cat scratch disease (CSD) is caused by Bartonella henselae and Bartonella clarridgeiae bacteria transferred from cats to humans that results in an inflamed inoculation site and tender lymphadenopathy. Pityriasis rosea (PR) and PR-like eruptions are self-limited, acute exanthems that have been associated with infections, vaccinations, and medications. We report a case of PR occurring in a 10-year-old girl with CSD, which may suggest an association between the 2 diseases.

A 10-year-old girl who was otherwise healthy presented in the winter with a rash of 5 days’ duration. Fourteen days prior to the rash, the patient reported being scratched by a new kitten and noted a pinpoint “puncture” on the left forearm that developed into a red papule over the following week. Seven days after the cat scratch, the patient experienced pain and swelling in the left axilla. Approximately 1 week after the onset of lymphadenopathy, the patient developed an asymptomatic rash that started with a large spot on the left chest, followed by smaller spots appearing over the next 2 days and spreading to the rest of the trunk. Four days after the rash onset, the patient experienced a mild headache, low-grade subjective fever, and chills. She denied any recent travel, bug bites, sore throat, and diarrhea. She was up-to-date on all vaccinations and had not received any vaccines preceding the symptoms. Physical examination revealed a 2-cm pink, scaly, thin plaque with a collarette of scale on the left upper chest (herald patch), along with multiple thin pink papules and small plaques with central scale on the trunk (Figure 1). A pustule with adjacent linear erosion was present on the left ventral forearm (Figure 2). The patient had a tender subcutaneous nodule in the left axilla as well as bilateral anterior and posterior cervical-chain subcutaneous tender nodules. There was no involvement of the palms, soles, or mucosae.

The patient was empirically treated for CSD with azithromycin (200 mg/5 mL), 404 mg on day 1 followed by 202 mg daily for 4 days. The rash was treated with hydrocortisone cream 2.5% twice daily for 2 weeks. A wound culture of the pustule on the left forearm was negative for neutrophils and organisms. Antibody serologies obtained 4 weeks after presentation were notable for an elevated B henselae IgG titer of 1:640, confirming the diagnosis of CSD. Following treatment with azithromycin and hydrocortisone, all of the patient’s symptoms resolved after 1 to 2 weeks.
Cat scratch disease is a zoonotic infection caused by the bacteria B henselae and the more recently described pathogen B clarridgeiae. Cat fleas spread these bacteria among cats, which subsequently inoculate the bacteria into humans through bites and scratches. The incidence of CSD in the United States is estimated to be 4.5 to 9.3 per 100,000 individuals in the outpatient setting and 0.19 to 0.86 per 100,000 individuals in the inpatient setting.1 Geographic variance can occur based on flea populations, resulting in higher incidence in warm humid climates and lower incidence in mountainous arid climates. The incidence of CSD in the pediatric population is highest in children aged 5 to 9 years. A national representative survey (N=3011) from 2017 revealed that 37.2% of primary care providers had diagnosed CSD in the prior year.1
Classic CSD presents as an erythematous papule at the inoculation site lasting days to weeks, with progression to tender lymphadenopathy lasting weeks to months. Fever, malaise, and chills also can be seen. Atypical CSD occurs in up to 24% of cases in immunocompetent patients.1 Atypical and systemic presentations are varied and can include fever of unknown origin, neuroretinitis, uveitis, retinal vessel occlusion, encephalitis, hepatosplenic lesions, Parinaud oculoglandular syndrome, osteomyelitis, and endocarditis.1,2 Atypical dermatologic presentations of CSD include maculopapular rash in 7% of cases and erythema nodosum in 2.5% of cases, as well as rare reports of cutaneous vasculitis, urticaria, immune thrombocytopenic purpura, and papuloedematous eruption.3 Treatment guidelines for CSD vary widely depending on the clinical presentation as well as the immunocompetence of the infected individual. Our patient had limited regional lymphadenopathy with no signs of dissemination or neurologic involvement and was successfully treated with a 5-day course of oral azithromycin (weight based, 10 mg/kg). More extensive disease such as hepatosplenic or neurologic CSD may require multiple antibiotics for up to 6 weeks. Alternative or additional antibiotics used for CSD include rifampin, trimethoprim-sulfamethoxazole, ciprofloxacin, doxycycline, gentamicin, and clarithromycin. Opinions vary as to whether all patients or just those with complicated infections warrant antibiotic therapy.4-6
Pityriasis rosea is a self-limited acute exanthematous disease that is classically associated with a systemic reactivation of human herpesvirus (HHV) 6 and/or HHV-7. The incidence of PR is estimated to be 480 per 100,000 dermatologic patients. It is slightly more common in females and occurs most often in patients aged 10 to 35 years.7 Clinically, PR appears with the abrupt onset of a single erythematous scaly patch (termed the herald patch), followed by a secondary eruption of smaller erythematous scaly macules and patches along the trunk’s cleavage lines. The secondary eruption on the back is sometimes termed a Christmas or fir tree pattern.7,8
In addition to the classic presentation of PR, there have been reports of numerous atypical clinical presentations. The herald patch, which classically presents on the trunk, also has been reported to present on the extremities; PR of the extremities is defined by lesions that appear as large scaly plaques on the extremities only. Inverse PR presents with lesions occurring in flexural areas and acral surfaces but not on the trunk. There also is an acral PR variant in which lesions appear only on the palms, wrists, and soles. Purpuric or hemorrhagic PR has been described and presents with purpura and petechiae with or without collarettes of scale in diffuse locations, including the palate. Oral PR presents more commonly in patients of color as erosions, ulcers, hemorrhagic lesions, bullae, or geographic tongue. Erythema multiforme–like PR appears with targetoid lesions on the trunk, face, neck, and arms without a history of herpes simplex virus infection. A large pear-shaped herald patch has been reported and characterizes the gigantea PR of Darier variant. Irritated PR occurs with typical PR findings, but afflicted patients report severe pain and burning with diaphoresis. Relapsing PR can occur within 1 year of a prior episode of PR and presents without a herald patch. Persistent PR is defined by PR lasting more than 3 months, and most reported cases have included oral lesions. Finally, other PR variants that have been described include urticarial, papular, follicular, vesicular, and hypopigmented types.7-9
Furthermore, there have been reports of multiple atypical presentations occurring simultaneously in the same patient.10 Although PR classically has been associated with HHV-6 and/or HHV-7 reactivation, it has been reported with a few other clinical situations and conditions. Pityriasislike eruption specifically refers to an exanthem secondary to drugs or vaccination that resembles PR but shows clinical differences, including diffuse and confluent dusky-red macules and/or plaques with or without desquamation on the trunk, extremities, and face. Drugs that have been implicated as triggers include ACE inhibitors, gold, isotretinoin, nonsteroidal anti-inflammatory agents, omeprazole, terbinafine, and tyrosine kinase inhibitors. Smallpox, tuberculosis, poliomyelitis, influenza, diphtheria, tetanus, hepatitis B virus, pneumococcus, papillomavirus, yellow fever, and pertussis vaccinations also have been associated with PR.7,11,12 Additionally, PR has been reported to occur with active systemic infections, specifically H1N1 influenza, though it is rare.13 Because of its self-limited course, treatment of PR most often involves only reassurance. Topical corticosteroids may be appropriate for pruritus.7,8
Pediatric health care providers including dermatologists should be familiar with both CSD and PR because they are common diseases that more often are encountered in the pediatric population. We present a unique case of CSD presenting with concurrent PR, which highlights a potential new etiology for PR and a rare cutaneous manifestation of CSD. Further investigation into a possible relationship between CSD and PR may be warranted. Patients with any signs and symptoms of fever, tender lymphadenopathy, worsening rash, or exposure to cats warrant a thorough history and physical examination to ensure that neither entity is overlooked.
To the Editor:
Cat scratch disease (CSD) is caused by Bartonella henselae and Bartonella clarridgeiae bacteria transferred from cats to humans that results in an inflamed inoculation site and tender lymphadenopathy. Pityriasis rosea (PR) and PR-like eruptions are self-limited, acute exanthems that have been associated with infections, vaccinations, and medications. We report a case of PR occurring in a 10-year-old girl with CSD, which may suggest an association between the 2 diseases.

A 10-year-old girl who was otherwise healthy presented in the winter with a rash of 5 days’ duration. Fourteen days prior to the rash, the patient reported being scratched by a new kitten and noted a pinpoint “puncture” on the left forearm that developed into a red papule over the following week. Seven days after the cat scratch, the patient experienced pain and swelling in the left axilla. Approximately 1 week after the onset of lymphadenopathy, the patient developed an asymptomatic rash that started with a large spot on the left chest, followed by smaller spots appearing over the next 2 days and spreading to the rest of the trunk. Four days after the rash onset, the patient experienced a mild headache, low-grade subjective fever, and chills. She denied any recent travel, bug bites, sore throat, and diarrhea. She was up-to-date on all vaccinations and had not received any vaccines preceding the symptoms. Physical examination revealed a 2-cm pink, scaly, thin plaque with a collarette of scale on the left upper chest (herald patch), along with multiple thin pink papules and small plaques with central scale on the trunk (Figure 1). A pustule with adjacent linear erosion was present on the left ventral forearm (Figure 2). The patient had a tender subcutaneous nodule in the left axilla as well as bilateral anterior and posterior cervical-chain subcutaneous tender nodules. There was no involvement of the palms, soles, or mucosae.

The patient was empirically treated for CSD with azithromycin (200 mg/5 mL), 404 mg on day 1 followed by 202 mg daily for 4 days. The rash was treated with hydrocortisone cream 2.5% twice daily for 2 weeks. A wound culture of the pustule on the left forearm was negative for neutrophils and organisms. Antibody serologies obtained 4 weeks after presentation were notable for an elevated B henselae IgG titer of 1:640, confirming the diagnosis of CSD. Following treatment with azithromycin and hydrocortisone, all of the patient’s symptoms resolved after 1 to 2 weeks.
Cat scratch disease is a zoonotic infection caused by the bacteria B henselae and the more recently described pathogen B clarridgeiae. Cat fleas spread these bacteria among cats, which subsequently inoculate the bacteria into humans through bites and scratches. The incidence of CSD in the United States is estimated to be 4.5 to 9.3 per 100,000 individuals in the outpatient setting and 0.19 to 0.86 per 100,000 individuals in the inpatient setting.1 Geographic variance can occur based on flea populations, resulting in higher incidence in warm humid climates and lower incidence in mountainous arid climates. The incidence of CSD in the pediatric population is highest in children aged 5 to 9 years. A national representative survey (N=3011) from 2017 revealed that 37.2% of primary care providers had diagnosed CSD in the prior year.1
Classic CSD presents as an erythematous papule at the inoculation site lasting days to weeks, with progression to tender lymphadenopathy lasting weeks to months. Fever, malaise, and chills also can be seen. Atypical CSD occurs in up to 24% of cases in immunocompetent patients.1 Atypical and systemic presentations are varied and can include fever of unknown origin, neuroretinitis, uveitis, retinal vessel occlusion, encephalitis, hepatosplenic lesions, Parinaud oculoglandular syndrome, osteomyelitis, and endocarditis.1,2 Atypical dermatologic presentations of CSD include maculopapular rash in 7% of cases and erythema nodosum in 2.5% of cases, as well as rare reports of cutaneous vasculitis, urticaria, immune thrombocytopenic purpura, and papuloedematous eruption.3 Treatment guidelines for CSD vary widely depending on the clinical presentation as well as the immunocompetence of the infected individual. Our patient had limited regional lymphadenopathy with no signs of dissemination or neurologic involvement and was successfully treated with a 5-day course of oral azithromycin (weight based, 10 mg/kg). More extensive disease such as hepatosplenic or neurologic CSD may require multiple antibiotics for up to 6 weeks. Alternative or additional antibiotics used for CSD include rifampin, trimethoprim-sulfamethoxazole, ciprofloxacin, doxycycline, gentamicin, and clarithromycin. Opinions vary as to whether all patients or just those with complicated infections warrant antibiotic therapy.4-6
Pityriasis rosea is a self-limited acute exanthematous disease that is classically associated with a systemic reactivation of human herpesvirus (HHV) 6 and/or HHV-7. The incidence of PR is estimated to be 480 per 100,000 dermatologic patients. It is slightly more common in females and occurs most often in patients aged 10 to 35 years.7 Clinically, PR appears with the abrupt onset of a single erythematous scaly patch (termed the herald patch), followed by a secondary eruption of smaller erythematous scaly macules and patches along the trunk’s cleavage lines. The secondary eruption on the back is sometimes termed a Christmas or fir tree pattern.7,8
In addition to the classic presentation of PR, there have been reports of numerous atypical clinical presentations. The herald patch, which classically presents on the trunk, also has been reported to present on the extremities; PR of the extremities is defined by lesions that appear as large scaly plaques on the extremities only. Inverse PR presents with lesions occurring in flexural areas and acral surfaces but not on the trunk. There also is an acral PR variant in which lesions appear only on the palms, wrists, and soles. Purpuric or hemorrhagic PR has been described and presents with purpura and petechiae with or without collarettes of scale in diffuse locations, including the palate. Oral PR presents more commonly in patients of color as erosions, ulcers, hemorrhagic lesions, bullae, or geographic tongue. Erythema multiforme–like PR appears with targetoid lesions on the trunk, face, neck, and arms without a history of herpes simplex virus infection. A large pear-shaped herald patch has been reported and characterizes the gigantea PR of Darier variant. Irritated PR occurs with typical PR findings, but afflicted patients report severe pain and burning with diaphoresis. Relapsing PR can occur within 1 year of a prior episode of PR and presents without a herald patch. Persistent PR is defined by PR lasting more than 3 months, and most reported cases have included oral lesions. Finally, other PR variants that have been described include urticarial, papular, follicular, vesicular, and hypopigmented types.7-9
Furthermore, there have been reports of multiple atypical presentations occurring simultaneously in the same patient.10 Although PR classically has been associated with HHV-6 and/or HHV-7 reactivation, it has been reported with a few other clinical situations and conditions. Pityriasislike eruption specifically refers to an exanthem secondary to drugs or vaccination that resembles PR but shows clinical differences, including diffuse and confluent dusky-red macules and/or plaques with or without desquamation on the trunk, extremities, and face. Drugs that have been implicated as triggers include ACE inhibitors, gold, isotretinoin, nonsteroidal anti-inflammatory agents, omeprazole, terbinafine, and tyrosine kinase inhibitors. Smallpox, tuberculosis, poliomyelitis, influenza, diphtheria, tetanus, hepatitis B virus, pneumococcus, papillomavirus, yellow fever, and pertussis vaccinations also have been associated with PR.7,11,12 Additionally, PR has been reported to occur with active systemic infections, specifically H1N1 influenza, though it is rare.13 Because of its self-limited course, treatment of PR most often involves only reassurance. Topical corticosteroids may be appropriate for pruritus.7,8
Pediatric health care providers including dermatologists should be familiar with both CSD and PR because they are common diseases that more often are encountered in the pediatric population. We present a unique case of CSD presenting with concurrent PR, which highlights a potential new etiology for PR and a rare cutaneous manifestation of CSD. Further investigation into a possible relationship between CSD and PR may be warranted. Patients with any signs and symptoms of fever, tender lymphadenopathy, worsening rash, or exposure to cats warrant a thorough history and physical examination to ensure that neither entity is overlooked.
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge [published online July 14, 2017]. Zoonoses Public Health. 2018;65:67-73. doi:10.1111/zph.12368
- Habot-Wilner Z, Trivizki O, Goldstein M, et al. Cat-scratch disease: ocular manifestations and treatment outcome. Acta Ophthalmol. 2018;96:E524-E532. doi:10.1111/aos.13684
- Schattner A, Uliel L, Dubin I. The cat did it: erythema nodosum and additional atypical presentations of Bartonella henselae infection in immunocompetent hosts [published online February 16, 2018]. BMJ Case Rep. doi:10.1136/bcr-2017-222511
- Shorbatli L, Koranyi K, Nahata M. Effectiveness of antibiotic therapy in pediatric patients with cat scratch disease. Int J Clin Pharm. 2018;40:1458-1461. doi: 10.1007/s11096-018-0746-1
- Bass JW, Freitas BC, Freitas AD, et al. Prospective randomized double blind placebo-controlled evaluation of azithromycin for treatment of cat-scratch disease. Pediatr Infect Dis J. 1998;17:447-452. doi:10.1097/00006454-199806000-00002
- Spach DH, Kaplan SL. Treatment of cat scratch disease. UpToDate. Updated December 9, 2021. Accessed September 12, 2023. https://www.uptodate.com/contents/treatment-of-cat-scratch-disease
- Drago F, Ciccarese G, Rebora A, et al. Pityriasis rosea: a comprehensive classification. Dermatology. 2016;232:431-437. doi:10.1159/000445375
- Urbina F, Das A, Sudy E. Clinical variants of pityriasis rosea. World J Clin Cases. 2017;5:203-211. doi:10.12998/wjcc.v5.i6.203
- Alzahrani NA, Al Jasser MI. Geographic tonguelike presentation in a child with pityriasis rosea: case report and review of oral manifestations of pityriasis rosea. Pediatr Dermatol. 2018;35:E124-E127. doi:10.1111/pde.13417
- Sinha S, Sardana K, Garg V. Coexistence of two atypical variants of pityriasis rosea: a case report and review of literature. Pediatr Dermatol. 2012;29:538-540. doi:10.1111/j.1525-1470.2011.01549.x
- Drago F, Ciccarese G, Parodi A. Pityriasis rosea and pityriasis rosea-like eruptions: how to distinguish them? JAAD Case Rep. 2018;4:800-801. doi:10.1016/j.jdcr.2018.04.002
- Drago F, Ciccarese G, Javor S, et al. Vaccine-induced pityriasis rosea and pityriasis rosea-like eruptions: a review of the literature. J Eur Acad Dermatol Venereol. 2016;30:544-545. doi:10.1111/jdv.12942
- Mubki TF, Bin Dayel SA, Kadry R. A case of pityriasis rosea concurrent with the novel influenza A (H1N1) infection. Pediatr Dermatol. 2011;28:341-342. doi:10.1111/j.1525-1470.2010.01090.x
- Nelson CA, Moore AR, Perea AE, et al. Cat scratch disease: U.S. clinicians’ experience and knowledge [published online July 14, 2017]. Zoonoses Public Health. 2018;65:67-73. doi:10.1111/zph.12368
- Habot-Wilner Z, Trivizki O, Goldstein M, et al. Cat-scratch disease: ocular manifestations and treatment outcome. Acta Ophthalmol. 2018;96:E524-E532. doi:10.1111/aos.13684
- Schattner A, Uliel L, Dubin I. The cat did it: erythema nodosum and additional atypical presentations of Bartonella henselae infection in immunocompetent hosts [published online February 16, 2018]. BMJ Case Rep. doi:10.1136/bcr-2017-222511
- Shorbatli L, Koranyi K, Nahata M. Effectiveness of antibiotic therapy in pediatric patients with cat scratch disease. Int J Clin Pharm. 2018;40:1458-1461. doi: 10.1007/s11096-018-0746-1
- Bass JW, Freitas BC, Freitas AD, et al. Prospective randomized double blind placebo-controlled evaluation of azithromycin for treatment of cat-scratch disease. Pediatr Infect Dis J. 1998;17:447-452. doi:10.1097/00006454-199806000-00002
- Spach DH, Kaplan SL. Treatment of cat scratch disease. UpToDate. Updated December 9, 2021. Accessed September 12, 2023. https://www.uptodate.com/contents/treatment-of-cat-scratch-disease
- Drago F, Ciccarese G, Rebora A, et al. Pityriasis rosea: a comprehensive classification. Dermatology. 2016;232:431-437. doi:10.1159/000445375
- Urbina F, Das A, Sudy E. Clinical variants of pityriasis rosea. World J Clin Cases. 2017;5:203-211. doi:10.12998/wjcc.v5.i6.203
- Alzahrani NA, Al Jasser MI. Geographic tonguelike presentation in a child with pityriasis rosea: case report and review of oral manifestations of pityriasis rosea. Pediatr Dermatol. 2018;35:E124-E127. doi:10.1111/pde.13417
- Sinha S, Sardana K, Garg V. Coexistence of two atypical variants of pityriasis rosea: a case report and review of literature. Pediatr Dermatol. 2012;29:538-540. doi:10.1111/j.1525-1470.2011.01549.x
- Drago F, Ciccarese G, Parodi A. Pityriasis rosea and pityriasis rosea-like eruptions: how to distinguish them? JAAD Case Rep. 2018;4:800-801. doi:10.1016/j.jdcr.2018.04.002
- Drago F, Ciccarese G, Javor S, et al. Vaccine-induced pityriasis rosea and pityriasis rosea-like eruptions: a review of the literature. J Eur Acad Dermatol Venereol. 2016;30:544-545. doi:10.1111/jdv.12942
- Mubki TF, Bin Dayel SA, Kadry R. A case of pityriasis rosea concurrent with the novel influenza A (H1N1) infection. Pediatr Dermatol. 2011;28:341-342. doi:10.1111/j.1525-1470.2010.01090.x
Practice Points
- Dermatologists should familiarize themselves with the physical examination findings of cat scratch disease.
- There are numerous clinical variants and triggers of pityriasis rosea (PR).
- There may be a new infectious trigger for PR, and exposure to cats prior to a classic PR eruption should raise one’s suspicion as a possible cause.
AVAHO 2023: A New View of Women's Health and Clinician Wellness
Sirisha Manyam, DO, eagerly looks forward to attending Association of VA Hematology and Oncology (AVAHO) 2023 and participating in discussion concerning two central topics: women's health and clinician wellness. Recognizing and meeting the distinctive stressors faced by healthcare workers, which have produced alarming rates of burnout, is an apt priority for Veterans Affairs systems, Dr Maryam suggests, and one which is paralleled by the need to engage the unique challenges faced by women, specifically the cluster of considerations surrounding breast cancer treatment.
Sirisha Manyam, DO, eagerly looks forward to attending Association of VA Hematology and Oncology (AVAHO) 2023 and participating in discussion concerning two central topics: women's health and clinician wellness. Recognizing and meeting the distinctive stressors faced by healthcare workers, which have produced alarming rates of burnout, is an apt priority for Veterans Affairs systems, Dr Maryam suggests, and one which is paralleled by the need to engage the unique challenges faced by women, specifically the cluster of considerations surrounding breast cancer treatment.
Sirisha Manyam, DO, eagerly looks forward to attending Association of VA Hematology and Oncology (AVAHO) 2023 and participating in discussion concerning two central topics: women's health and clinician wellness. Recognizing and meeting the distinctive stressors faced by healthcare workers, which have produced alarming rates of burnout, is an apt priority for Veterans Affairs systems, Dr Maryam suggests, and one which is paralleled by the need to engage the unique challenges faced by women, specifically the cluster of considerations surrounding breast cancer treatment.
AVAHO 2023: Expanding Opportunities for Veteran Care
Timothy O'Brien, MD, shares his expectations for the upcoming 2023 AVAHO conference. Dr O'Brien highlights four key areas of interest: networking with providers from other VA systems; creating more clinical trial opportunities; exploring educational sessions on topics like AI in oncology and geriatric oncology; and fostering team building within the local VA group. With an area of focus in malignant hematology, particularly multiple myeloma, Dr O'Brien sees learning opportunities within the education sessions on geriatric oncology. Considering that the average age of patients with multiple myeloma at his institution is 70 years, he looks forward to gaining valuable strategies for geriatric assessment.
Timothy O'Brien, MD, shares his expectations for the upcoming 2023 AVAHO conference. Dr O'Brien highlights four key areas of interest: networking with providers from other VA systems; creating more clinical trial opportunities; exploring educational sessions on topics like AI in oncology and geriatric oncology; and fostering team building within the local VA group. With an area of focus in malignant hematology, particularly multiple myeloma, Dr O'Brien sees learning opportunities within the education sessions on geriatric oncology. Considering that the average age of patients with multiple myeloma at his institution is 70 years, he looks forward to gaining valuable strategies for geriatric assessment.
Timothy O'Brien, MD, shares his expectations for the upcoming 2023 AVAHO conference. Dr O'Brien highlights four key areas of interest: networking with providers from other VA systems; creating more clinical trial opportunities; exploring educational sessions on topics like AI in oncology and geriatric oncology; and fostering team building within the local VA group. With an area of focus in malignant hematology, particularly multiple myeloma, Dr O'Brien sees learning opportunities within the education sessions on geriatric oncology. Considering that the average age of patients with multiple myeloma at his institution is 70 years, he looks forward to gaining valuable strategies for geriatric assessment.


AVAHO 2023: Exploring AI and Cancer Navigation for Veterans
Soo Park, MD, discusses her expectations for the upcoming 2023 AVAHO meeting in Chicago. Two items on the agenda particularly stand out: the role of artificial intelligence (AI) in oncology and the importance of cancer patient navigation. Dr Park acknowledges AI's potential to transform cancer diagnostics and drug discovery, particularly in aiding molecular profiling. Additionally, she highlights the value of cancer patient navigators in optimizing veteran care, particularly in the setting of geriatric oncology.
Soo Park, MD, discusses her expectations for the upcoming 2023 AVAHO meeting in Chicago. Two items on the agenda particularly stand out: the role of artificial intelligence (AI) in oncology and the importance of cancer patient navigation. Dr Park acknowledges AI's potential to transform cancer diagnostics and drug discovery, particularly in aiding molecular profiling. Additionally, she highlights the value of cancer patient navigators in optimizing veteran care, particularly in the setting of geriatric oncology.
Soo Park, MD, discusses her expectations for the upcoming 2023 AVAHO meeting in Chicago. Two items on the agenda particularly stand out: the role of artificial intelligence (AI) in oncology and the importance of cancer patient navigation. Dr Park acknowledges AI's potential to transform cancer diagnostics and drug discovery, particularly in aiding molecular profiling. Additionally, she highlights the value of cancer patient navigators in optimizing veteran care, particularly in the setting of geriatric oncology.


Severe psoriasis linked to a higher risk for heart disease, study confirms
TOPLINE:
METHODOLOGY:
- Prior studies with small sample sizes have shown that CMD predicts poor cardiovascular outcomes in patients with severe psoriasis.
- In a prospective multicenter study, researchers enrolled 448 patients with moderate to severe psoriasis with no documented clinical cardiovascular disease who underwent transthoracic Doppler echocardiography to evaluate coronary microcirculation.
- The outcome variable of interest was CMD, defined as a coronary flow rate of 2.5 mL or less.
- The researchers used multivariable linear regression to model the associations of the characteristics of patients with psoriasis with CMD.
TAKEAWAY:
- Of the 448 patients, 141 (31.5%) showed CMD.
- Multivariable regression revealed four variables independently associated with CMD: higher Psoriasis Area Severity Index (PASI) score (per unit, odds ratio, 1.058; P < .001), duration of psoriasis (per year; OR, 1.046; P < .001), the presence of psoriatic arthritis (OR, 1.938; P = .015), and hypertension (OR, 2.169; P = .010).
- An increase of 1 point in the PASI score and 1 year of psoriasis duration were associated with a 5.8% and a 4.6% increased risk for CMD, respectively.
IN PRACTICE:
“We should diagnose and actively search for microvascular dysfunction in patients with psoriasis, as this population is at particularly high risk,” the researchers wrote.
SOURCE:
Stefano Piaserico, MD, PhD, of the University of Padova (Italy), led the research. The study was published in the Journal of Investigative Dermatology.
LIMITATIONS:
A small proportion of patients in the study were being treated for psoriasis, and other tools for assessing CMD were not used, such as PET-CT and cardiovascular MRI.
DISCLOSURES:
The authors reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Prior studies with small sample sizes have shown that CMD predicts poor cardiovascular outcomes in patients with severe psoriasis.
- In a prospective multicenter study, researchers enrolled 448 patients with moderate to severe psoriasis with no documented clinical cardiovascular disease who underwent transthoracic Doppler echocardiography to evaluate coronary microcirculation.
- The outcome variable of interest was CMD, defined as a coronary flow rate of 2.5 mL or less.
- The researchers used multivariable linear regression to model the associations of the characteristics of patients with psoriasis with CMD.
TAKEAWAY:
- Of the 448 patients, 141 (31.5%) showed CMD.
- Multivariable regression revealed four variables independently associated with CMD: higher Psoriasis Area Severity Index (PASI) score (per unit, odds ratio, 1.058; P < .001), duration of psoriasis (per year; OR, 1.046; P < .001), the presence of psoriatic arthritis (OR, 1.938; P = .015), and hypertension (OR, 2.169; P = .010).
- An increase of 1 point in the PASI score and 1 year of psoriasis duration were associated with a 5.8% and a 4.6% increased risk for CMD, respectively.
IN PRACTICE:
“We should diagnose and actively search for microvascular dysfunction in patients with psoriasis, as this population is at particularly high risk,” the researchers wrote.
SOURCE:
Stefano Piaserico, MD, PhD, of the University of Padova (Italy), led the research. The study was published in the Journal of Investigative Dermatology.
LIMITATIONS:
A small proportion of patients in the study were being treated for psoriasis, and other tools for assessing CMD were not used, such as PET-CT and cardiovascular MRI.
DISCLOSURES:
The authors reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Prior studies with small sample sizes have shown that CMD predicts poor cardiovascular outcomes in patients with severe psoriasis.
- In a prospective multicenter study, researchers enrolled 448 patients with moderate to severe psoriasis with no documented clinical cardiovascular disease who underwent transthoracic Doppler echocardiography to evaluate coronary microcirculation.
- The outcome variable of interest was CMD, defined as a coronary flow rate of 2.5 mL or less.
- The researchers used multivariable linear regression to model the associations of the characteristics of patients with psoriasis with CMD.
TAKEAWAY:
- Of the 448 patients, 141 (31.5%) showed CMD.
- Multivariable regression revealed four variables independently associated with CMD: higher Psoriasis Area Severity Index (PASI) score (per unit, odds ratio, 1.058; P < .001), duration of psoriasis (per year; OR, 1.046; P < .001), the presence of psoriatic arthritis (OR, 1.938; P = .015), and hypertension (OR, 2.169; P = .010).
- An increase of 1 point in the PASI score and 1 year of psoriasis duration were associated with a 5.8% and a 4.6% increased risk for CMD, respectively.
IN PRACTICE:
“We should diagnose and actively search for microvascular dysfunction in patients with psoriasis, as this population is at particularly high risk,” the researchers wrote.
SOURCE:
Stefano Piaserico, MD, PhD, of the University of Padova (Italy), led the research. The study was published in the Journal of Investigative Dermatology.
LIMITATIONS:
A small proportion of patients in the study were being treated for psoriasis, and other tools for assessing CMD were not used, such as PET-CT and cardiovascular MRI.
DISCLOSURES:
The authors reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY