User login
FDA panel rejects implanted GLP1-RA dosing device for T2D
Sept. 21 from an advisory committee of the Food and Drug Administration.
The 19 voting panel members mostly cited concerning signals of both renal toxicity in the form of excess episodes of acute kidney injury (AKI) as well as increased cardiovascular events compared with placebo as their main reasons for voting that the developing company, Intarcia Therapeutics, had not shown adequate evidence that the benefits of the drug-device combination, known as ITCA 650, outweighed its risks for treating people with type 2 diabetes.
“I’m quite uncomfortable with the AKI safety,” said panel member Erica Brittain, PhD, deputy chief of the Biostatistics Research Branch of the National Institute of Allergy and Infectious Diseases in Bethesda, Md.
The case that ITCA 650 is ready for routine use was also undermined by uncertainty documented by FDA staff about the uniformity and reliability of exenatide delivery by the DUROS device, a matchstick-sized reservoir that’s placed subcutaneously and designed to deliver exenatide continuously for 6 months at a time, noted Cecilia C. Low Wang, MD, chair of the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee.
“No evidence of improved adherence”
Another shortcoming was no data on the impact that this form of drug delivery, first developed and FDA approved to treat patients with prostate cancer with leuprolide acetate, really accomplished its goal of improving adherence to a glycemic-control agent. Intarcia Therapeutics presented “no evidence of improved adherence,” said Dr. Low Wang, director of the Glucose Management Team at the University of Colorado Hospital.
However, she and several other panel members acknowledged the compelling comments from several patients and health care professionals experienced in using or administering the device who, during the public comment period, voiced anecdotal testimonials to its positive impact on treatment compliance.
Seven years of FDA review
This review of ITCA 650 capped a nearly 7-year effort by Intarcia Therapeutics to receive marketing approval for ITCA 650 from the FDA, which began with an application filed in November 2016 (and denied by the agency in September 2017). Intarcia resubmitted an amended application in 2019 that the FDA again rejected in 2020. The company’s persistence following that led to the current panel meeting, the first time the ITCA 650 evidence came before an advisory panel.
Committee members in general praised the concept of managing blood glucose by continuous release of a medication 6 months at a time. They also offered ideas on a path forward, such as a study that used an active competitor. Ideally, that could be another agent from the same class of GLP-1 receptor agonists such as Bydureon, an injected formulation of exenatide administered by subcutaneous injection once a week.
But the key, agreed panel members, was to bulk up the evidence that ITCA 650 is safe. “The data show concerning safety signals that need further investigation,” summed up Dr. Low Wong. “There are concerns about overall safety, all-cause mortality, AKI, cardiovascular events, and glycemic excursions.”
All voting members of the advisory committee met the FDA’s standard for having no relevant financial relationships.
A version of this article appeared on Medscape.com.
Sept. 21 from an advisory committee of the Food and Drug Administration.
The 19 voting panel members mostly cited concerning signals of both renal toxicity in the form of excess episodes of acute kidney injury (AKI) as well as increased cardiovascular events compared with placebo as their main reasons for voting that the developing company, Intarcia Therapeutics, had not shown adequate evidence that the benefits of the drug-device combination, known as ITCA 650, outweighed its risks for treating people with type 2 diabetes.
“I’m quite uncomfortable with the AKI safety,” said panel member Erica Brittain, PhD, deputy chief of the Biostatistics Research Branch of the National Institute of Allergy and Infectious Diseases in Bethesda, Md.
The case that ITCA 650 is ready for routine use was also undermined by uncertainty documented by FDA staff about the uniformity and reliability of exenatide delivery by the DUROS device, a matchstick-sized reservoir that’s placed subcutaneously and designed to deliver exenatide continuously for 6 months at a time, noted Cecilia C. Low Wang, MD, chair of the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee.
“No evidence of improved adherence”
Another shortcoming was no data on the impact that this form of drug delivery, first developed and FDA approved to treat patients with prostate cancer with leuprolide acetate, really accomplished its goal of improving adherence to a glycemic-control agent. Intarcia Therapeutics presented “no evidence of improved adherence,” said Dr. Low Wang, director of the Glucose Management Team at the University of Colorado Hospital.
However, she and several other panel members acknowledged the compelling comments from several patients and health care professionals experienced in using or administering the device who, during the public comment period, voiced anecdotal testimonials to its positive impact on treatment compliance.
Seven years of FDA review
This review of ITCA 650 capped a nearly 7-year effort by Intarcia Therapeutics to receive marketing approval for ITCA 650 from the FDA, which began with an application filed in November 2016 (and denied by the agency in September 2017). Intarcia resubmitted an amended application in 2019 that the FDA again rejected in 2020. The company’s persistence following that led to the current panel meeting, the first time the ITCA 650 evidence came before an advisory panel.
Committee members in general praised the concept of managing blood glucose by continuous release of a medication 6 months at a time. They also offered ideas on a path forward, such as a study that used an active competitor. Ideally, that could be another agent from the same class of GLP-1 receptor agonists such as Bydureon, an injected formulation of exenatide administered by subcutaneous injection once a week.
But the key, agreed panel members, was to bulk up the evidence that ITCA 650 is safe. “The data show concerning safety signals that need further investigation,” summed up Dr. Low Wong. “There are concerns about overall safety, all-cause mortality, AKI, cardiovascular events, and glycemic excursions.”
All voting members of the advisory committee met the FDA’s standard for having no relevant financial relationships.
A version of this article appeared on Medscape.com.
Sept. 21 from an advisory committee of the Food and Drug Administration.
The 19 voting panel members mostly cited concerning signals of both renal toxicity in the form of excess episodes of acute kidney injury (AKI) as well as increased cardiovascular events compared with placebo as their main reasons for voting that the developing company, Intarcia Therapeutics, had not shown adequate evidence that the benefits of the drug-device combination, known as ITCA 650, outweighed its risks for treating people with type 2 diabetes.
“I’m quite uncomfortable with the AKI safety,” said panel member Erica Brittain, PhD, deputy chief of the Biostatistics Research Branch of the National Institute of Allergy and Infectious Diseases in Bethesda, Md.
The case that ITCA 650 is ready for routine use was also undermined by uncertainty documented by FDA staff about the uniformity and reliability of exenatide delivery by the DUROS device, a matchstick-sized reservoir that’s placed subcutaneously and designed to deliver exenatide continuously for 6 months at a time, noted Cecilia C. Low Wang, MD, chair of the FDA’s Endocrinologic and Metabolic Drugs Advisory Committee.
“No evidence of improved adherence”
Another shortcoming was no data on the impact that this form of drug delivery, first developed and FDA approved to treat patients with prostate cancer with leuprolide acetate, really accomplished its goal of improving adherence to a glycemic-control agent. Intarcia Therapeutics presented “no evidence of improved adherence,” said Dr. Low Wang, director of the Glucose Management Team at the University of Colorado Hospital.
However, she and several other panel members acknowledged the compelling comments from several patients and health care professionals experienced in using or administering the device who, during the public comment period, voiced anecdotal testimonials to its positive impact on treatment compliance.
Seven years of FDA review
This review of ITCA 650 capped a nearly 7-year effort by Intarcia Therapeutics to receive marketing approval for ITCA 650 from the FDA, which began with an application filed in November 2016 (and denied by the agency in September 2017). Intarcia resubmitted an amended application in 2019 that the FDA again rejected in 2020. The company’s persistence following that led to the current panel meeting, the first time the ITCA 650 evidence came before an advisory panel.
Committee members in general praised the concept of managing blood glucose by continuous release of a medication 6 months at a time. They also offered ideas on a path forward, such as a study that used an active competitor. Ideally, that could be another agent from the same class of GLP-1 receptor agonists such as Bydureon, an injected formulation of exenatide administered by subcutaneous injection once a week.
But the key, agreed panel members, was to bulk up the evidence that ITCA 650 is safe. “The data show concerning safety signals that need further investigation,” summed up Dr. Low Wong. “There are concerns about overall safety, all-cause mortality, AKI, cardiovascular events, and glycemic excursions.”
All voting members of the advisory committee met the FDA’s standard for having no relevant financial relationships.
A version of this article appeared on Medscape.com.
MDMA effective in diverse patients with PTSD
TOPLINE:
METHODOLOGY:
Trauma-focused psychotherapies are the gold standard treatment for PTSD, which affects about 5% of Americans each year. However, many patients have persistent symptoms, and up to 47% don’t respond to the SSRIs sertraline and paroxetine, which are approved for PTSD by the Food and Drug Administration.
Mounting evidence suggests 3,4-methylenedioxymethamphetamine-assisted therapy (MDMA-AT), which promotes monoamine reuptake inhibition and release, simultaneously inducing prosocial feelings and softening responses to emotionally challenging and fearful stimuli, could be an alternative treatment for PTSD, possibly enhancing the benefits of psychotherapy.
A phase 3 study (MAPP1) showed MDMA-AT was generally well-tolerated and met the primary and secondary endpoints of reduced PTSD symptom severity and decreased functional impairment.
This new confirmatory phase 3 study (MAPP2) included 104 patients with PTSD who were randomized to MDMA-AT or placebo with therapy. Participants were a mean age of about 39 years, 71.2% were assigned female sex at birth, 33.7% identified as non-White, and 26.9% identified as Hispanic/Latino.
The mean Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) score at baseline was 39.0 and was similar between groups. Overall, 26.9% and 73.1% of patients had moderate or severe PTSD, respectively.
TAKEAWAY:
Among the 94 participants who completed the study, the least-squares mean change in CAPS-5 total score at 18 weeks was −23.7 (95% confidence interval, −26.9 to −20.4) for MDMA-AT versus −14.8 (95% CI, −18.3 to −11.3) for placebo with therapy (treatment difference: −8.9; 95% CI, −13.7 to −4.1; P < .001).
MDMA-AT significantly mitigated the secondary outcome of clinician-rated functional impairment, as measured by a reduction in the Sheehan Disability Scale score.
About 86.5% of participants treated with MDMA-AT achieved a clinically meaningful benefit, and 71.2% no longer met criteria for PTSD by study end.
Treatment-emergent adverse events were mostly transient and mild or moderate in severity. Although suicidal ideation was reported in both groups, MDMA did not appear to increase the risk, and there were no reports of problematic MDMA abuse or dependence.
IN PRACTICE:
“This confirmatory phase 3 trial showed consistent benefits of MDMA-AT in an ethnoracially diverse group of individuals with long-standing moderate to severe PTSD and numerous comorbidities,” write the authors, noting the dropout rate was low and treatment was generally well tolerated.
SOURCE:
The study was conducted by Jennifer M. Mitchell, PhD, department of neurology and department of psychiatry and behavioral sciences, University of California, San Francisco, and colleagues. It was published online in Nature Medicine.
LIMITATIONS:
The study excluded participants with high suicide risk, comorbid personality disorders, and underlying cardiovascular disease. Effect sizes for MDMA-AT were similar to MAPP1 and, although higher than those observed in SSRI studies, the superiority of MDMA-AT over SSRIs cannot be assumed without a direct comparison.
DISCLOSURES:
The study was funded by the Multidisciplinary Association for Psychedelic Studies, with support from the Steven and Alexandra Cohen Foundation, and organized by the MAPS Public Benefit Corporation. Dr. Mitchell has reported receiving research support from MAPS; grants/contracts from the Veterans Administration and FDA; royalties/licenses from the University of California, Los Angeles; and payment/honoraria from Stanford University and Johns Hopkins. She has been a reviewer for the National Institute on Drug Abuse Clinical Trials Network, a member of the Research Advisory Panel for the California Department of Justice, and a grant reviewer for the Australian National Health and Medical Research Council.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
Trauma-focused psychotherapies are the gold standard treatment for PTSD, which affects about 5% of Americans each year. However, many patients have persistent symptoms, and up to 47% don’t respond to the SSRIs sertraline and paroxetine, which are approved for PTSD by the Food and Drug Administration.
Mounting evidence suggests 3,4-methylenedioxymethamphetamine-assisted therapy (MDMA-AT), which promotes monoamine reuptake inhibition and release, simultaneously inducing prosocial feelings and softening responses to emotionally challenging and fearful stimuli, could be an alternative treatment for PTSD, possibly enhancing the benefits of psychotherapy.
A phase 3 study (MAPP1) showed MDMA-AT was generally well-tolerated and met the primary and secondary endpoints of reduced PTSD symptom severity and decreased functional impairment.
This new confirmatory phase 3 study (MAPP2) included 104 patients with PTSD who were randomized to MDMA-AT or placebo with therapy. Participants were a mean age of about 39 years, 71.2% were assigned female sex at birth, 33.7% identified as non-White, and 26.9% identified as Hispanic/Latino.
The mean Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) score at baseline was 39.0 and was similar between groups. Overall, 26.9% and 73.1% of patients had moderate or severe PTSD, respectively.
TAKEAWAY:
Among the 94 participants who completed the study, the least-squares mean change in CAPS-5 total score at 18 weeks was −23.7 (95% confidence interval, −26.9 to −20.4) for MDMA-AT versus −14.8 (95% CI, −18.3 to −11.3) for placebo with therapy (treatment difference: −8.9; 95% CI, −13.7 to −4.1; P < .001).
MDMA-AT significantly mitigated the secondary outcome of clinician-rated functional impairment, as measured by a reduction in the Sheehan Disability Scale score.
About 86.5% of participants treated with MDMA-AT achieved a clinically meaningful benefit, and 71.2% no longer met criteria for PTSD by study end.
Treatment-emergent adverse events were mostly transient and mild or moderate in severity. Although suicidal ideation was reported in both groups, MDMA did not appear to increase the risk, and there were no reports of problematic MDMA abuse or dependence.
IN PRACTICE:
“This confirmatory phase 3 trial showed consistent benefits of MDMA-AT in an ethnoracially diverse group of individuals with long-standing moderate to severe PTSD and numerous comorbidities,” write the authors, noting the dropout rate was low and treatment was generally well tolerated.
SOURCE:
The study was conducted by Jennifer M. Mitchell, PhD, department of neurology and department of psychiatry and behavioral sciences, University of California, San Francisco, and colleagues. It was published online in Nature Medicine.
LIMITATIONS:
The study excluded participants with high suicide risk, comorbid personality disorders, and underlying cardiovascular disease. Effect sizes for MDMA-AT were similar to MAPP1 and, although higher than those observed in SSRI studies, the superiority of MDMA-AT over SSRIs cannot be assumed without a direct comparison.
DISCLOSURES:
The study was funded by the Multidisciplinary Association for Psychedelic Studies, with support from the Steven and Alexandra Cohen Foundation, and organized by the MAPS Public Benefit Corporation. Dr. Mitchell has reported receiving research support from MAPS; grants/contracts from the Veterans Administration and FDA; royalties/licenses from the University of California, Los Angeles; and payment/honoraria from Stanford University and Johns Hopkins. She has been a reviewer for the National Institute on Drug Abuse Clinical Trials Network, a member of the Research Advisory Panel for the California Department of Justice, and a grant reviewer for the Australian National Health and Medical Research Council.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
Trauma-focused psychotherapies are the gold standard treatment for PTSD, which affects about 5% of Americans each year. However, many patients have persistent symptoms, and up to 47% don’t respond to the SSRIs sertraline and paroxetine, which are approved for PTSD by the Food and Drug Administration.
Mounting evidence suggests 3,4-methylenedioxymethamphetamine-assisted therapy (MDMA-AT), which promotes monoamine reuptake inhibition and release, simultaneously inducing prosocial feelings and softening responses to emotionally challenging and fearful stimuli, could be an alternative treatment for PTSD, possibly enhancing the benefits of psychotherapy.
A phase 3 study (MAPP1) showed MDMA-AT was generally well-tolerated and met the primary and secondary endpoints of reduced PTSD symptom severity and decreased functional impairment.
This new confirmatory phase 3 study (MAPP2) included 104 patients with PTSD who were randomized to MDMA-AT or placebo with therapy. Participants were a mean age of about 39 years, 71.2% were assigned female sex at birth, 33.7% identified as non-White, and 26.9% identified as Hispanic/Latino.
The mean Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) score at baseline was 39.0 and was similar between groups. Overall, 26.9% and 73.1% of patients had moderate or severe PTSD, respectively.
TAKEAWAY:
Among the 94 participants who completed the study, the least-squares mean change in CAPS-5 total score at 18 weeks was −23.7 (95% confidence interval, −26.9 to −20.4) for MDMA-AT versus −14.8 (95% CI, −18.3 to −11.3) for placebo with therapy (treatment difference: −8.9; 95% CI, −13.7 to −4.1; P < .001).
MDMA-AT significantly mitigated the secondary outcome of clinician-rated functional impairment, as measured by a reduction in the Sheehan Disability Scale score.
About 86.5% of participants treated with MDMA-AT achieved a clinically meaningful benefit, and 71.2% no longer met criteria for PTSD by study end.
Treatment-emergent adverse events were mostly transient and mild or moderate in severity. Although suicidal ideation was reported in both groups, MDMA did not appear to increase the risk, and there were no reports of problematic MDMA abuse or dependence.
IN PRACTICE:
“This confirmatory phase 3 trial showed consistent benefits of MDMA-AT in an ethnoracially diverse group of individuals with long-standing moderate to severe PTSD and numerous comorbidities,” write the authors, noting the dropout rate was low and treatment was generally well tolerated.
SOURCE:
The study was conducted by Jennifer M. Mitchell, PhD, department of neurology and department of psychiatry and behavioral sciences, University of California, San Francisco, and colleagues. It was published online in Nature Medicine.
LIMITATIONS:
The study excluded participants with high suicide risk, comorbid personality disorders, and underlying cardiovascular disease. Effect sizes for MDMA-AT were similar to MAPP1 and, although higher than those observed in SSRI studies, the superiority of MDMA-AT over SSRIs cannot be assumed without a direct comparison.
DISCLOSURES:
The study was funded by the Multidisciplinary Association for Psychedelic Studies, with support from the Steven and Alexandra Cohen Foundation, and organized by the MAPS Public Benefit Corporation. Dr. Mitchell has reported receiving research support from MAPS; grants/contracts from the Veterans Administration and FDA; royalties/licenses from the University of California, Los Angeles; and payment/honoraria from Stanford University and Johns Hopkins. She has been a reviewer for the National Institute on Drug Abuse Clinical Trials Network, a member of the Research Advisory Panel for the California Department of Justice, and a grant reviewer for the Australian National Health and Medical Research Council.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE
Hypertensive disorders screening recommended for all pregnant women
Hypertensive disorders of pregnancy in the United States increased from approximately 500 cases per 10,000 deliveries to 1,021 cases per 10,000 deliveries from 1993 to 2016-2017, and remain a leading cause of maternal morbidity and mortality, wrote Task Force Chair Michael J. Barry, MD, of Massachusetts General Hospital, Boston, and colleagues in the final recommendation statement published in JAMA.
The USPSTF commissioned a systematic review to assess the risks and benefits of hypertensive screening for asymptomatic pregnant women. The resulting grade B recommendation indicates that screening for hypertensive disorders in pregnancy using blood pressure measurements yields a substantial net benefit.
The recommendation applies to “all pregnant women and pregnant persons of all genders without a known diagnosis of a hypertensive disorder of pregnancy or chronic hypertension,” the authors said.
The recommendation calls for the use of blood pressure measurements to evaluate hypertensive disorders, with measurements taken at each prenatal visit. A positive result for new-onset hypertension was defined as systolic blood pressure of 140 mm Hg or diastolic blood pressure 90 mm Hg in the absence of chronic hypertension, based on two measurements at least 4 hours apart. Regular review of blood pressure can help identify and manage potentially fatal conditions.
However, screening alone is insufficient to improve inequities in health outcomes associated with hypertensive disorders of pregnancy, the authors emphasized. Data from previous studies have shown that Black patients are at increased risk for hypertensive disorders of pregnancy and severe complications, and that Black and Hispanic patients have twice the risk of stroke with hypertensive disorders of pregnancy as White patients.
In the evidence report that supported the recommendation, Jillian T. Henderson, PhD, of Kaiser Permanente in Portland, Ore., and colleagues reviewed six studies including 10,165 individuals. The studies (five clinical trials and one nonrandomized study) compared changes in prenatal screening with usual care.
Overall, the review yielded no evidence that any other screening strategies were more useful than routine blood pressure measurement to identify hypertensive disorders of pregnancy in asymptomatic women.
The findings cited to support the recommendation were limited by several factors, including the lack of power to detect pregnancy health outcomes and potential harms of different screening programs, and the lack of power to evaluate outcomes for American Indian, Alaska Native, or Black individuals, who have disproportionately high rates of hypertensive disorders of pregnancy, the authors said.
More research is needed to identify which screening approaches may lead to improved disease detection and better health outcomes, but the results of the review support the grade B recommendation for hypertensive screening of all pregnant women, they concluded.
Early identification makes a difference
The new recommendation is important because it can help all moms and babies to be healthier, said Wanda Nicholson, MD, vice chair of the task force, in an interview.
“We are recommending that all pregnant persons have a blood pressure check at every visit throughout pregnancy,” said Dr. Nicholson, an ob.gyn. by training who also serves as professor of prevention and community health at George Washington University in Washington. “We know that there is a maternal health crisis in this country, and we know that hypertensive disorders of pregnancy are one of the key factors related to that,” she said.
Unfortunately, barriers to routine screening for hypertensive disorders of pregnancy persist, said Dr. Nicholson. The incidence of hypertensive disorders of pregnancy is higher in many of the same populations who also have challenges in accessing regular prenatal care, notably those who are Black, Native American, or Alaska Native, she noted.
The new recommendation also serves as an opportunity to call attention to the health care disparities for these populations, not only during pregnancy, but in general, she emphasized.
In clinical practice, the definition of hypertensive disorders of pregnancy involves three different diagnoses – gestational hypertension, preeclampsia, and eclampsia – that can be seen as points on a continuum, said Dr. Nicholson. The sooner patients are identified with hypertensive disorders of pregnancy, the sooner intervention and treatment can begin, she said. To that end, she added the clinical pearl of using a properly sized blood pressure cuff to obtain an accurate reading and avoid missed diagnoses.
The task force also outlined several key areas for additional research, said Dr. Nicholson. First, more research is needed on alternative screening strategies, such as at-home blood pressure monitoring for patients, as well as teleheath visits. Second, more studies are needed to address the disparities in prenatal care and include more diverse populations in clinical research. Third, future studies need to consider social determinants of health and other factors that might impact maternal health outcomes. “These steps will help achieve the larger goal of healthier mothers and babies,” Dr. Nicholson said.
Back to basics to improve women’s health
Some clinicians may be disappointed by the Evidence Report’s primary finding that no alternative screening strategies outperformed routine blood pressure measurement, wrote Anne E. Denoble, MD, and Christian M. Pettker, MD, both of Yale University, New Haven, Conn., in an accompanying editorial.
While potentially frustrating at first glance, the findings of the Evidence Report provide a foundation for improvement and reassurance that the best existing screening methods are basic and fundamental: regular prenatal visits with routine, in-office blood pressure measurements, and urine protein screening when clinically indicated, they said.
However, the USPSTF review also noted persistent research gaps that must be addressed to significantly improve maternal health outcomes, they said. Notable gaps include the disproportionately low numbers of Black patients in current studies, and the need for studies of alternate models of prenatal care, including the use of remote blood pressure monitoring, and the use of biomarkers to screen for and predict hypertensive disorders of pregnancy.
The most striking limitation may be the focus on prenatal care, with lack of attention to postpartum mortality risk, given that more than half of pregnancy-related deaths occur postpartum, the authors noted.
Although current screening tools may be used in practice “with skill and might,” more effort at multiple levels is needed to address the larger maternal health crisis in the United States, they said.
Expand screening, engage primary care for long-term benefits
Screening for hypertensive disorders of pregnancy “can and should be within the purview of internists,” wrote Srilakshmi Mitta, MD; Cary P. Gross, MD; Melissa A. Simon, MD, of Brown University, Yale University, and Northwestern University, respectively, in a separate editorial. The recommendation to extend screening beyond preeclampsia is timely, given the consistent increase in all hypertensive disorders of pregnancy since 1990, the authors said.
Pregnancy is not the only time for screening, counseling, and management of hypertensive disorders, they emphasized. “All persons who have reproductive capacity and/or are planning pregnancy, along with those who are post partum, should be screened for hypertensive disorders, aligning the USPSTF with guidelines from the American College of Obstetricians and Gynecologists, the American College of Cardiology, and the American Heart Association,” they said, and all clinicians should be on board to identify and treat hypertensive disorders of pregnancy, especially in underserved racial and ethnic minorities for whom primary care may be their only source of health care.
“Pregnancy is a window of opportunity to influence current and future life course, not just of the individual, but also of the fetus(es),other children, and family,” and timely intervention has the potential for great public health impact, they said.
Dr. Denoble disclosed grants from the HealthPartners Institute for Education and Research and from the Patient-Centered Outcomes Research Institute. Dr. Simon serves on the Advisory Committee for Research on Women’s Health for the National Institutes of Health Office of Research on Women’s Health and serves as a member of the Centers for Disease Control and Prevention Community Preventive Services Task Force; she was a member of the USPSTF from 2017 to 2020. Dr. Gross disclosed grants from Johnson and Johnson and the National Comprehensive Cancer Network (through a grant to the NCCN from AstraZeneca) and personal fees from Genentech.
Hypertensive disorders of pregnancy in the United States increased from approximately 500 cases per 10,000 deliveries to 1,021 cases per 10,000 deliveries from 1993 to 2016-2017, and remain a leading cause of maternal morbidity and mortality, wrote Task Force Chair Michael J. Barry, MD, of Massachusetts General Hospital, Boston, and colleagues in the final recommendation statement published in JAMA.
The USPSTF commissioned a systematic review to assess the risks and benefits of hypertensive screening for asymptomatic pregnant women. The resulting grade B recommendation indicates that screening for hypertensive disorders in pregnancy using blood pressure measurements yields a substantial net benefit.
The recommendation applies to “all pregnant women and pregnant persons of all genders without a known diagnosis of a hypertensive disorder of pregnancy or chronic hypertension,” the authors said.
The recommendation calls for the use of blood pressure measurements to evaluate hypertensive disorders, with measurements taken at each prenatal visit. A positive result for new-onset hypertension was defined as systolic blood pressure of 140 mm Hg or diastolic blood pressure 90 mm Hg in the absence of chronic hypertension, based on two measurements at least 4 hours apart. Regular review of blood pressure can help identify and manage potentially fatal conditions.
However, screening alone is insufficient to improve inequities in health outcomes associated with hypertensive disorders of pregnancy, the authors emphasized. Data from previous studies have shown that Black patients are at increased risk for hypertensive disorders of pregnancy and severe complications, and that Black and Hispanic patients have twice the risk of stroke with hypertensive disorders of pregnancy as White patients.
In the evidence report that supported the recommendation, Jillian T. Henderson, PhD, of Kaiser Permanente in Portland, Ore., and colleagues reviewed six studies including 10,165 individuals. The studies (five clinical trials and one nonrandomized study) compared changes in prenatal screening with usual care.
Overall, the review yielded no evidence that any other screening strategies were more useful than routine blood pressure measurement to identify hypertensive disorders of pregnancy in asymptomatic women.
The findings cited to support the recommendation were limited by several factors, including the lack of power to detect pregnancy health outcomes and potential harms of different screening programs, and the lack of power to evaluate outcomes for American Indian, Alaska Native, or Black individuals, who have disproportionately high rates of hypertensive disorders of pregnancy, the authors said.
More research is needed to identify which screening approaches may lead to improved disease detection and better health outcomes, but the results of the review support the grade B recommendation for hypertensive screening of all pregnant women, they concluded.
Early identification makes a difference
The new recommendation is important because it can help all moms and babies to be healthier, said Wanda Nicholson, MD, vice chair of the task force, in an interview.
“We are recommending that all pregnant persons have a blood pressure check at every visit throughout pregnancy,” said Dr. Nicholson, an ob.gyn. by training who also serves as professor of prevention and community health at George Washington University in Washington. “We know that there is a maternal health crisis in this country, and we know that hypertensive disorders of pregnancy are one of the key factors related to that,” she said.
Unfortunately, barriers to routine screening for hypertensive disorders of pregnancy persist, said Dr. Nicholson. The incidence of hypertensive disorders of pregnancy is higher in many of the same populations who also have challenges in accessing regular prenatal care, notably those who are Black, Native American, or Alaska Native, she noted.
The new recommendation also serves as an opportunity to call attention to the health care disparities for these populations, not only during pregnancy, but in general, she emphasized.
In clinical practice, the definition of hypertensive disorders of pregnancy involves three different diagnoses – gestational hypertension, preeclampsia, and eclampsia – that can be seen as points on a continuum, said Dr. Nicholson. The sooner patients are identified with hypertensive disorders of pregnancy, the sooner intervention and treatment can begin, she said. To that end, she added the clinical pearl of using a properly sized blood pressure cuff to obtain an accurate reading and avoid missed diagnoses.
The task force also outlined several key areas for additional research, said Dr. Nicholson. First, more research is needed on alternative screening strategies, such as at-home blood pressure monitoring for patients, as well as teleheath visits. Second, more studies are needed to address the disparities in prenatal care and include more diverse populations in clinical research. Third, future studies need to consider social determinants of health and other factors that might impact maternal health outcomes. “These steps will help achieve the larger goal of healthier mothers and babies,” Dr. Nicholson said.
Back to basics to improve women’s health
Some clinicians may be disappointed by the Evidence Report’s primary finding that no alternative screening strategies outperformed routine blood pressure measurement, wrote Anne E. Denoble, MD, and Christian M. Pettker, MD, both of Yale University, New Haven, Conn., in an accompanying editorial.
While potentially frustrating at first glance, the findings of the Evidence Report provide a foundation for improvement and reassurance that the best existing screening methods are basic and fundamental: regular prenatal visits with routine, in-office blood pressure measurements, and urine protein screening when clinically indicated, they said.
However, the USPSTF review also noted persistent research gaps that must be addressed to significantly improve maternal health outcomes, they said. Notable gaps include the disproportionately low numbers of Black patients in current studies, and the need for studies of alternate models of prenatal care, including the use of remote blood pressure monitoring, and the use of biomarkers to screen for and predict hypertensive disorders of pregnancy.
The most striking limitation may be the focus on prenatal care, with lack of attention to postpartum mortality risk, given that more than half of pregnancy-related deaths occur postpartum, the authors noted.
Although current screening tools may be used in practice “with skill and might,” more effort at multiple levels is needed to address the larger maternal health crisis in the United States, they said.
Expand screening, engage primary care for long-term benefits
Screening for hypertensive disorders of pregnancy “can and should be within the purview of internists,” wrote Srilakshmi Mitta, MD; Cary P. Gross, MD; Melissa A. Simon, MD, of Brown University, Yale University, and Northwestern University, respectively, in a separate editorial. The recommendation to extend screening beyond preeclampsia is timely, given the consistent increase in all hypertensive disorders of pregnancy since 1990, the authors said.
Pregnancy is not the only time for screening, counseling, and management of hypertensive disorders, they emphasized. “All persons who have reproductive capacity and/or are planning pregnancy, along with those who are post partum, should be screened for hypertensive disorders, aligning the USPSTF with guidelines from the American College of Obstetricians and Gynecologists, the American College of Cardiology, and the American Heart Association,” they said, and all clinicians should be on board to identify and treat hypertensive disorders of pregnancy, especially in underserved racial and ethnic minorities for whom primary care may be their only source of health care.
“Pregnancy is a window of opportunity to influence current and future life course, not just of the individual, but also of the fetus(es),other children, and family,” and timely intervention has the potential for great public health impact, they said.
Dr. Denoble disclosed grants from the HealthPartners Institute for Education and Research and from the Patient-Centered Outcomes Research Institute. Dr. Simon serves on the Advisory Committee for Research on Women’s Health for the National Institutes of Health Office of Research on Women’s Health and serves as a member of the Centers for Disease Control and Prevention Community Preventive Services Task Force; she was a member of the USPSTF from 2017 to 2020. Dr. Gross disclosed grants from Johnson and Johnson and the National Comprehensive Cancer Network (through a grant to the NCCN from AstraZeneca) and personal fees from Genentech.
Hypertensive disorders of pregnancy in the United States increased from approximately 500 cases per 10,000 deliveries to 1,021 cases per 10,000 deliveries from 1993 to 2016-2017, and remain a leading cause of maternal morbidity and mortality, wrote Task Force Chair Michael J. Barry, MD, of Massachusetts General Hospital, Boston, and colleagues in the final recommendation statement published in JAMA.
The USPSTF commissioned a systematic review to assess the risks and benefits of hypertensive screening for asymptomatic pregnant women. The resulting grade B recommendation indicates that screening for hypertensive disorders in pregnancy using blood pressure measurements yields a substantial net benefit.
The recommendation applies to “all pregnant women and pregnant persons of all genders without a known diagnosis of a hypertensive disorder of pregnancy or chronic hypertension,” the authors said.
The recommendation calls for the use of blood pressure measurements to evaluate hypertensive disorders, with measurements taken at each prenatal visit. A positive result for new-onset hypertension was defined as systolic blood pressure of 140 mm Hg or diastolic blood pressure 90 mm Hg in the absence of chronic hypertension, based on two measurements at least 4 hours apart. Regular review of blood pressure can help identify and manage potentially fatal conditions.
However, screening alone is insufficient to improve inequities in health outcomes associated with hypertensive disorders of pregnancy, the authors emphasized. Data from previous studies have shown that Black patients are at increased risk for hypertensive disorders of pregnancy and severe complications, and that Black and Hispanic patients have twice the risk of stroke with hypertensive disorders of pregnancy as White patients.
In the evidence report that supported the recommendation, Jillian T. Henderson, PhD, of Kaiser Permanente in Portland, Ore., and colleagues reviewed six studies including 10,165 individuals. The studies (five clinical trials and one nonrandomized study) compared changes in prenatal screening with usual care.
Overall, the review yielded no evidence that any other screening strategies were more useful than routine blood pressure measurement to identify hypertensive disorders of pregnancy in asymptomatic women.
The findings cited to support the recommendation were limited by several factors, including the lack of power to detect pregnancy health outcomes and potential harms of different screening programs, and the lack of power to evaluate outcomes for American Indian, Alaska Native, or Black individuals, who have disproportionately high rates of hypertensive disorders of pregnancy, the authors said.
More research is needed to identify which screening approaches may lead to improved disease detection and better health outcomes, but the results of the review support the grade B recommendation for hypertensive screening of all pregnant women, they concluded.
Early identification makes a difference
The new recommendation is important because it can help all moms and babies to be healthier, said Wanda Nicholson, MD, vice chair of the task force, in an interview.
“We are recommending that all pregnant persons have a blood pressure check at every visit throughout pregnancy,” said Dr. Nicholson, an ob.gyn. by training who also serves as professor of prevention and community health at George Washington University in Washington. “We know that there is a maternal health crisis in this country, and we know that hypertensive disorders of pregnancy are one of the key factors related to that,” she said.
Unfortunately, barriers to routine screening for hypertensive disorders of pregnancy persist, said Dr. Nicholson. The incidence of hypertensive disorders of pregnancy is higher in many of the same populations who also have challenges in accessing regular prenatal care, notably those who are Black, Native American, or Alaska Native, she noted.
The new recommendation also serves as an opportunity to call attention to the health care disparities for these populations, not only during pregnancy, but in general, she emphasized.
In clinical practice, the definition of hypertensive disorders of pregnancy involves three different diagnoses – gestational hypertension, preeclampsia, and eclampsia – that can be seen as points on a continuum, said Dr. Nicholson. The sooner patients are identified with hypertensive disorders of pregnancy, the sooner intervention and treatment can begin, she said. To that end, she added the clinical pearl of using a properly sized blood pressure cuff to obtain an accurate reading and avoid missed diagnoses.
The task force also outlined several key areas for additional research, said Dr. Nicholson. First, more research is needed on alternative screening strategies, such as at-home blood pressure monitoring for patients, as well as teleheath visits. Second, more studies are needed to address the disparities in prenatal care and include more diverse populations in clinical research. Third, future studies need to consider social determinants of health and other factors that might impact maternal health outcomes. “These steps will help achieve the larger goal of healthier mothers and babies,” Dr. Nicholson said.
Back to basics to improve women’s health
Some clinicians may be disappointed by the Evidence Report’s primary finding that no alternative screening strategies outperformed routine blood pressure measurement, wrote Anne E. Denoble, MD, and Christian M. Pettker, MD, both of Yale University, New Haven, Conn., in an accompanying editorial.
While potentially frustrating at first glance, the findings of the Evidence Report provide a foundation for improvement and reassurance that the best existing screening methods are basic and fundamental: regular prenatal visits with routine, in-office blood pressure measurements, and urine protein screening when clinically indicated, they said.
However, the USPSTF review also noted persistent research gaps that must be addressed to significantly improve maternal health outcomes, they said. Notable gaps include the disproportionately low numbers of Black patients in current studies, and the need for studies of alternate models of prenatal care, including the use of remote blood pressure monitoring, and the use of biomarkers to screen for and predict hypertensive disorders of pregnancy.
The most striking limitation may be the focus on prenatal care, with lack of attention to postpartum mortality risk, given that more than half of pregnancy-related deaths occur postpartum, the authors noted.
Although current screening tools may be used in practice “with skill and might,” more effort at multiple levels is needed to address the larger maternal health crisis in the United States, they said.
Expand screening, engage primary care for long-term benefits
Screening for hypertensive disorders of pregnancy “can and should be within the purview of internists,” wrote Srilakshmi Mitta, MD; Cary P. Gross, MD; Melissa A. Simon, MD, of Brown University, Yale University, and Northwestern University, respectively, in a separate editorial. The recommendation to extend screening beyond preeclampsia is timely, given the consistent increase in all hypertensive disorders of pregnancy since 1990, the authors said.
Pregnancy is not the only time for screening, counseling, and management of hypertensive disorders, they emphasized. “All persons who have reproductive capacity and/or are planning pregnancy, along with those who are post partum, should be screened for hypertensive disorders, aligning the USPSTF with guidelines from the American College of Obstetricians and Gynecologists, the American College of Cardiology, and the American Heart Association,” they said, and all clinicians should be on board to identify and treat hypertensive disorders of pregnancy, especially in underserved racial and ethnic minorities for whom primary care may be their only source of health care.
“Pregnancy is a window of opportunity to influence current and future life course, not just of the individual, but also of the fetus(es),other children, and family,” and timely intervention has the potential for great public health impact, they said.
Dr. Denoble disclosed grants from the HealthPartners Institute for Education and Research and from the Patient-Centered Outcomes Research Institute. Dr. Simon serves on the Advisory Committee for Research on Women’s Health for the National Institutes of Health Office of Research on Women’s Health and serves as a member of the Centers for Disease Control and Prevention Community Preventive Services Task Force; she was a member of the USPSTF from 2017 to 2020. Dr. Gross disclosed grants from Johnson and Johnson and the National Comprehensive Cancer Network (through a grant to the NCCN from AstraZeneca) and personal fees from Genentech.
FROM JAMA
Creatine may improve key long COVID symptoms: Small study
Taking creatine as a supplement for 6 months appears to significantly improve clinical features of post–COVID-19 fatigue syndrome (PVFS or long COVID), a small randomized, placebo-controlled, double-blinded study suggests.
Researchers, led by Jelena Slankamenac, with Applied Bioenergetics Lab, Faculty of Sport and PE, University of Novi Sad, Serbia, published their findings in Food, Science & Nutrition .
“This is the first human study known to the authors that evaluated the efficacy and safety of supplemental creatine for fatigue, tissue bioenergetics, and patient-reported outcomes in patients with post–COVID-19 fatigue syndrome,” the authors write.
They say the findings may be attributed to creatine’s “energy-replenishing and neuroprotective activity.”
Significant reductions in symptoms
Researchers randomized the 12 participants into two groups of 6 each. The creatine group received 4 g creatine monohydrate per day, while the placebo group received the same amount of inulin.
At 3 months, dietary creatine supplements produced a significant reduction in fatigue, compared with baseline values ( P = .04) and significantly improved scores for several long COVID–related symptoms, including loss of taste, breathing difficulties, body aches, headache, and difficulties concentrating) ( P < .05), the researchers report.
Intervention effect sizes were assessed by Cohen statistics, with a d of at least 0.8 indicating a large effect.
Among highlights of the results were that patients reported a significant 77.8% drop in scores for concentration difficulties at the 3-month follow-up (Cohen’s effect, d = 1.19) and no concentration difficulties at the 6-month follow-up (Cohen’s effect, d = 2.46).
Total creatine levels increased in several locations across the brain (as much as 33% for right parietal white matter). No changes in tissue creatine levels were found in the placebo group during the trial.
“Since PVFS is characterized by impaired tissue bioenergetics ..., supplemental creatine might be an effective dietary intervention to uphold brain creatine in post–COVID-19 fatigue syndrome,” the authors write.
The authors add that creatine supplements for long COVID patients could benefit organs beyond the brain as participants saw “a significant drop in lung and body pain after the intervention.”
Unanswered questions
Some experts said the results should be interpreted with caution.
“This research paper is very interesting,” says Nisha Viswanathan, MD, director of the long COVID program at University of California, Los Angeles, “but the limited number of patients makes the results difficult to generalize.”
Dr. Viswanathan, who was not part of the study, pointed out that the patients included in this study had a recent COVID infection (under 3 months).
“Acute COVID infection can take up to 3 months to resolve,” she says. “We define patients with long COVID as those with symptoms lasting greater than 3 months. Therefore, these patients could have had improvements in their fatigue due to the natural course of the illness rather than creatine supplementation.”
Alba Azola, MD, assistant professor in the department of physical medicine and rehabilitation at Johns Hopkins University, Baltimore, said she also was troubled by the window of 3 months for recent COVID infection.
She said she would like to see results for patients who have ongoing symptoms for at least 6 months after infection, especially given creatine supplements’ history in research.
Creatine supplements for other conditions, such as fibromyalgia and chronic fatigue syndrome, have been tested for nearly 2 decades, she pointed out, with conflicting findings, something the authors acknowledge in the paper.
“I think it’s premature to say (creatine) is the key,” she says. She added that the small sample size is important to consider given the heterogeneity of patients with long COVID.
That said, Dr. Azola says, she applauds all efforts to find treatments for long COVID, especially randomized, controlled studies like this one.
No major side effects
No major side effects were reported for either intervention, except for transient mild nausea reported by one patient after taking creatine.
Compliance with the intervention was 90.6% ± 3.5% in the creatine group and 95.3% ± 5.0% in the control group (P = .04).
Participants were eligible for inclusion if they were 18-65 years old, had a positive COVID test within the last 3 months (documented by a valid polymerase chain reaction [PCR] or antigen test performed in a COVID-19–certified lab); had moderate to severe fatigue; and at least one additional COVID-related symptom, including loss of taste or smell, breathing trouble, lung pain, body aches, headaches, or difficulties concentrating.
The authors acknowledge that they selected a sample of young to middle-aged adults experiencing moderate long COVID symptoms, and it’s unknown whether creatine is equally effective in other PVFS populations, such as elderly people, children, or patients with less or more severe disease.
Senior author Dr. Sergei Ostojic serves as a member of the Scientific Advisory Board on creatine in health and medicine (AlzChem LLC). He co-owns a patent for “Supplements Based on Liquid Creatine” at the European Patent Office. He has received research support related to creatine during the past 36 months from the Serbian Ministry of Education, Science, and Technological Development; Provincial Secretariat for Higher Education and Scientific Research; Alzchem GmbH; ThermoLife International; and Hueston Hennigan LLP. He does not own stocks and shares in any organization. Other authors declare no known relevant financial interests. Dr. Viswanathan and Dr. Azola report no relevant financial relationships.
Taking creatine as a supplement for 6 months appears to significantly improve clinical features of post–COVID-19 fatigue syndrome (PVFS or long COVID), a small randomized, placebo-controlled, double-blinded study suggests.
Researchers, led by Jelena Slankamenac, with Applied Bioenergetics Lab, Faculty of Sport and PE, University of Novi Sad, Serbia, published their findings in Food, Science & Nutrition .
“This is the first human study known to the authors that evaluated the efficacy and safety of supplemental creatine for fatigue, tissue bioenergetics, and patient-reported outcomes in patients with post–COVID-19 fatigue syndrome,” the authors write.
They say the findings may be attributed to creatine’s “energy-replenishing and neuroprotective activity.”
Significant reductions in symptoms
Researchers randomized the 12 participants into two groups of 6 each. The creatine group received 4 g creatine monohydrate per day, while the placebo group received the same amount of inulin.
At 3 months, dietary creatine supplements produced a significant reduction in fatigue, compared with baseline values ( P = .04) and significantly improved scores for several long COVID–related symptoms, including loss of taste, breathing difficulties, body aches, headache, and difficulties concentrating) ( P < .05), the researchers report.
Intervention effect sizes were assessed by Cohen statistics, with a d of at least 0.8 indicating a large effect.
Among highlights of the results were that patients reported a significant 77.8% drop in scores for concentration difficulties at the 3-month follow-up (Cohen’s effect, d = 1.19) and no concentration difficulties at the 6-month follow-up (Cohen’s effect, d = 2.46).
Total creatine levels increased in several locations across the brain (as much as 33% for right parietal white matter). No changes in tissue creatine levels were found in the placebo group during the trial.
“Since PVFS is characterized by impaired tissue bioenergetics ..., supplemental creatine might be an effective dietary intervention to uphold brain creatine in post–COVID-19 fatigue syndrome,” the authors write.
The authors add that creatine supplements for long COVID patients could benefit organs beyond the brain as participants saw “a significant drop in lung and body pain after the intervention.”
Unanswered questions
Some experts said the results should be interpreted with caution.
“This research paper is very interesting,” says Nisha Viswanathan, MD, director of the long COVID program at University of California, Los Angeles, “but the limited number of patients makes the results difficult to generalize.”
Dr. Viswanathan, who was not part of the study, pointed out that the patients included in this study had a recent COVID infection (under 3 months).
“Acute COVID infection can take up to 3 months to resolve,” she says. “We define patients with long COVID as those with symptoms lasting greater than 3 months. Therefore, these patients could have had improvements in their fatigue due to the natural course of the illness rather than creatine supplementation.”
Alba Azola, MD, assistant professor in the department of physical medicine and rehabilitation at Johns Hopkins University, Baltimore, said she also was troubled by the window of 3 months for recent COVID infection.
She said she would like to see results for patients who have ongoing symptoms for at least 6 months after infection, especially given creatine supplements’ history in research.
Creatine supplements for other conditions, such as fibromyalgia and chronic fatigue syndrome, have been tested for nearly 2 decades, she pointed out, with conflicting findings, something the authors acknowledge in the paper.
“I think it’s premature to say (creatine) is the key,” she says. She added that the small sample size is important to consider given the heterogeneity of patients with long COVID.
That said, Dr. Azola says, she applauds all efforts to find treatments for long COVID, especially randomized, controlled studies like this one.
No major side effects
No major side effects were reported for either intervention, except for transient mild nausea reported by one patient after taking creatine.
Compliance with the intervention was 90.6% ± 3.5% in the creatine group and 95.3% ± 5.0% in the control group (P = .04).
Participants were eligible for inclusion if they were 18-65 years old, had a positive COVID test within the last 3 months (documented by a valid polymerase chain reaction [PCR] or antigen test performed in a COVID-19–certified lab); had moderate to severe fatigue; and at least one additional COVID-related symptom, including loss of taste or smell, breathing trouble, lung pain, body aches, headaches, or difficulties concentrating.
The authors acknowledge that they selected a sample of young to middle-aged adults experiencing moderate long COVID symptoms, and it’s unknown whether creatine is equally effective in other PVFS populations, such as elderly people, children, or patients with less or more severe disease.
Senior author Dr. Sergei Ostojic serves as a member of the Scientific Advisory Board on creatine in health and medicine (AlzChem LLC). He co-owns a patent for “Supplements Based on Liquid Creatine” at the European Patent Office. He has received research support related to creatine during the past 36 months from the Serbian Ministry of Education, Science, and Technological Development; Provincial Secretariat for Higher Education and Scientific Research; Alzchem GmbH; ThermoLife International; and Hueston Hennigan LLP. He does not own stocks and shares in any organization. Other authors declare no known relevant financial interests. Dr. Viswanathan and Dr. Azola report no relevant financial relationships.
Taking creatine as a supplement for 6 months appears to significantly improve clinical features of post–COVID-19 fatigue syndrome (PVFS or long COVID), a small randomized, placebo-controlled, double-blinded study suggests.
Researchers, led by Jelena Slankamenac, with Applied Bioenergetics Lab, Faculty of Sport and PE, University of Novi Sad, Serbia, published their findings in Food, Science & Nutrition .
“This is the first human study known to the authors that evaluated the efficacy and safety of supplemental creatine for fatigue, tissue bioenergetics, and patient-reported outcomes in patients with post–COVID-19 fatigue syndrome,” the authors write.
They say the findings may be attributed to creatine’s “energy-replenishing and neuroprotective activity.”
Significant reductions in symptoms
Researchers randomized the 12 participants into two groups of 6 each. The creatine group received 4 g creatine monohydrate per day, while the placebo group received the same amount of inulin.
At 3 months, dietary creatine supplements produced a significant reduction in fatigue, compared with baseline values ( P = .04) and significantly improved scores for several long COVID–related symptoms, including loss of taste, breathing difficulties, body aches, headache, and difficulties concentrating) ( P < .05), the researchers report.
Intervention effect sizes were assessed by Cohen statistics, with a d of at least 0.8 indicating a large effect.
Among highlights of the results were that patients reported a significant 77.8% drop in scores for concentration difficulties at the 3-month follow-up (Cohen’s effect, d = 1.19) and no concentration difficulties at the 6-month follow-up (Cohen’s effect, d = 2.46).
Total creatine levels increased in several locations across the brain (as much as 33% for right parietal white matter). No changes in tissue creatine levels were found in the placebo group during the trial.
“Since PVFS is characterized by impaired tissue bioenergetics ..., supplemental creatine might be an effective dietary intervention to uphold brain creatine in post–COVID-19 fatigue syndrome,” the authors write.
The authors add that creatine supplements for long COVID patients could benefit organs beyond the brain as participants saw “a significant drop in lung and body pain after the intervention.”
Unanswered questions
Some experts said the results should be interpreted with caution.
“This research paper is very interesting,” says Nisha Viswanathan, MD, director of the long COVID program at University of California, Los Angeles, “but the limited number of patients makes the results difficult to generalize.”
Dr. Viswanathan, who was not part of the study, pointed out that the patients included in this study had a recent COVID infection (under 3 months).
“Acute COVID infection can take up to 3 months to resolve,” she says. “We define patients with long COVID as those with symptoms lasting greater than 3 months. Therefore, these patients could have had improvements in their fatigue due to the natural course of the illness rather than creatine supplementation.”
Alba Azola, MD, assistant professor in the department of physical medicine and rehabilitation at Johns Hopkins University, Baltimore, said she also was troubled by the window of 3 months for recent COVID infection.
She said she would like to see results for patients who have ongoing symptoms for at least 6 months after infection, especially given creatine supplements’ history in research.
Creatine supplements for other conditions, such as fibromyalgia and chronic fatigue syndrome, have been tested for nearly 2 decades, she pointed out, with conflicting findings, something the authors acknowledge in the paper.
“I think it’s premature to say (creatine) is the key,” she says. She added that the small sample size is important to consider given the heterogeneity of patients with long COVID.
That said, Dr. Azola says, she applauds all efforts to find treatments for long COVID, especially randomized, controlled studies like this one.
No major side effects
No major side effects were reported for either intervention, except for transient mild nausea reported by one patient after taking creatine.
Compliance with the intervention was 90.6% ± 3.5% in the creatine group and 95.3% ± 5.0% in the control group (P = .04).
Participants were eligible for inclusion if they were 18-65 years old, had a positive COVID test within the last 3 months (documented by a valid polymerase chain reaction [PCR] or antigen test performed in a COVID-19–certified lab); had moderate to severe fatigue; and at least one additional COVID-related symptom, including loss of taste or smell, breathing trouble, lung pain, body aches, headaches, or difficulties concentrating.
The authors acknowledge that they selected a sample of young to middle-aged adults experiencing moderate long COVID symptoms, and it’s unknown whether creatine is equally effective in other PVFS populations, such as elderly people, children, or patients with less or more severe disease.
Senior author Dr. Sergei Ostojic serves as a member of the Scientific Advisory Board on creatine in health and medicine (AlzChem LLC). He co-owns a patent for “Supplements Based on Liquid Creatine” at the European Patent Office. He has received research support related to creatine during the past 36 months from the Serbian Ministry of Education, Science, and Technological Development; Provincial Secretariat for Higher Education and Scientific Research; Alzchem GmbH; ThermoLife International; and Hueston Hennigan LLP. He does not own stocks and shares in any organization. Other authors declare no known relevant financial interests. Dr. Viswanathan and Dr. Azola report no relevant financial relationships.
FROM FOOD, SCIENCE & NUTRITION
Is the U.S. neurologist shortage insurmountable?
, news that comes as no revelation to Thomas Vidic, MD, clinical associate professor of neurology at Indiana University, South Bend.
In 2013, Dr. Vidic and other members of an American Academy of Neurology Workforce Task Force coauthored a report that predicted the demand for neurologists would outstrip supply by 2025. A decade later, it appears the situation is even more dire than anticipated.
While a nationwide physician shortage is affecting all specialties, neurology is facing a particularly difficult confluence of events. Advances in treatments for migraine, epilepsy, multiple sclerosis, and other neurological disorders have created a growing demand for care of pediatric and adult patients.
Over the next 7-27 years, as the number of Americans over age 65 increases, the incidences of Parkinson’s and dementia are set to double, and stroke cases are expected to rise by 20%.
At the same time, physician retirement and burnout are siphoning off neurologists from a workforce that isn’t growing fast enough. The American Medical Association reports the number of neurologists who treat patients in the United States grew by only 598 over the last decade, from 12,761 to 13,359.
This perfect storm has created what another AAN report calls a “grave threat” to patient care. The neurologist shortage “reduces access to care, worsens patient outcomes, and erodes career satisfaction and quality of life for neurologists as they face increasingly insurmountable demands,” write the authors of that 2019 report.
“We’re in trouble,” said Dr. Vidic. “We have a tremendous need for neurologists that we’re just not supporting.”
How did we get here?
Some of the challenges related to neurologist recruitment and retention are similar to those in other specialties. Compensation is certainly a factor, Dr. Vidic said.
Although neurologists’ incomes have increased significantly over the past decade, they still rank in the lower half of all medical specialties. In addition, only 50% of neurologists believe they are fairly compensated.
Burnout is another significant challenge. In 2019, before the pandemic, 53% of neurologists surveyed in Medscape’s National Physician Burnout, Depression, and Suicide Report indicated they were burned out. That percentage increased slightly in 2023, to 55%, with most respondents reporting a strong to severe impact on their lives.
The most common reason for burnout was administration and paperwork that cuts into neurologists’ time with patients. Charting and completing prior authorization and step therapy forms required by most insurers take an average of 17.6 hours a week for neurologists – much longer than the overall physician average and higher than almost all other specialties.
But perhaps the biggest contributor to the nationwide neurologist shortage is a 26-year cap on Medicare funding for medical residency. Enacted as part of the 1997 Balanced Budget Act, the legislation limits Medicare funding for medical residency training at 1996 levels. Most medical residencies are funded by the federal government and Medicare is the largest participating program.
As a result of the cap, the number of total residents in the United States – which grew by 20.6% between 1987 and 1997 – increased by only 8% from 1997 to 2007.
A new study on patients’ long travel times to neurology clinics, published in Neurology, is the latest to illustrate the real-world impact of too few neurologists amid growing caseloads.
Researchers found that 17% of the 563,216 Medicare beneficiaries who visited a neurologist in 2018 had to travel an average of 81 miles one way. Those long distances were endured most often by patients with brain and spinal cord cancers, amyotrophic lateral sclerosis, and multiple sclerosis.
While the neurologist shortage affects every state, a 2020 study suggests rural areas are most affected. This analysis of Medicare recipients showed that just 21% of rural residents with a neurological condition had access to a nearby specialist, compared with 27% of urban dwellers. The findings are similar to those of a 2017 report that identified “neurology deserts” in a number of states across the country.
Wait times for new neurology patients are reported to be among the longest of all specialties, with an average of 30 days for adult patients and 5-6 months for pediatric patients.
More neurology instruction needed
“It’s really hard knowing there are families out there who need the care but can’t get to it in a timely manner,” said Tyler Allison, MD, associate professor of pediatrics at the University of Missouri–Kansas City.
Working in a rural state means Dr. Allison has patients who drive 6 hours or more for an appointment. Although telemedicine has reduced the number of trips for many of his existing pediatric cases, it has had little impact on new patients. This is particularly frustrating, he said, when he sees a new patient with a condition that could have been treated by a primary care physician in their home community.
“One of the biggest problems we have in the child neurology world is that we don’t have enough primary care physicians who feel they are adequately trained to care for these patients,” said Dr. Allison, who also is the program director of the Child Neurology Residency Program at Children’s Mercy Kansas City.
“Sometimes I see patients where, frankly, I only need to see them once to provide reassurance to the family and then they go back to their primary care doctor,” he said. “It’s the kind of thing that if we trained people appropriately from the beginning, it would shorten our wait list.”
Indeed, increasing neurology instruction during medical school is one recommendation offered in a 2019 report that characterized the neurologist shortage as a “grave threat.”
Data from the Association of American Medical Colleges show U.S. medical schools required an average of 4.4 weeks of neurology instruction in 2019-2020. Of the disciplines included in the AAMC report, only radiology and surgical specialties required a shorter clinical course. Many medical schools also require a neurology rotation, usually during the third year.
“There are still medical schools that do not require a neurology rotation,” said Dr. Vidic. Indiana University’s medical school requires a 1-month neurology rotation. “Per capita, we turn out more neurologists than any other medical school in the country because we give the exposure.”
General neurologists needed
The 2019 AAN report also calls for a renewed focus on general neurology in residency training as a way to ease patient wait times.
“General neurologists in the community can care for 75%-85% of patients with neurological disease,” said Michael Markowski, DO, a general neurologist in Cape Cod, Mass., who chaired the AAN’s general neurology task force from 2019 to 2020.
“Our residency training programs aren’t doing anything wrong, but we have data that show we have to start doing something different if we’re going to care for the one in three Americans with neurological disease who deserve care in their community rather than having to travel to subspecialty centers, which are primarily located in larger cities,” he said.
Based on an AAN survey, only about one-third of U.S. neurologists identify as general neurologists; most focus on movement disorders, dementia or Alzheimer’s, epilepsy, or another neurology subspecialty. It’s a sharp contrast from Europe, where the vast majority of neurologists identify as general neurologists.
“It was striking, the difference between the neurologists across Europe who identify as general neurologists, in comparison to the U.S.,” said Dr. Markowski, who was the AAN representative for the European Academy of Neurology General Neurology Task Force. “Close to 28% of U.S. neurologists identify as general neurologists, but across 37 European nations, that [percentage] is 76%.”
In Europe, general neurology rotations make up at least half of the first year of medical residency, Dr. Markowski said, adding that in the United States, there is more focus on inpatient rather than outpatient neurology rotations.
“If you never see that role model during your training who is a general neurologist, who can see the vast majority of all neurology patients, why would you think you could do that when you graduate?” Dr. Markowski said.
A legislative solution
While expanding neurology instruction in medical school and increasing exposure to general neurology rotations in residency could help, the clearest path to increasing the number of neurologists in the United States is to lift the decades-old residency cap.
The Resident Physician Shortage Reduction Act of 2023 would do just that, adding 14,000 new medical residency positions over 7 years. The bill has bipartisan support, with hundreds of cosponsors from both sides of the aisle. Nearly 100 professional societies and medical and hospital groups have submitted testimony in support.
Similar legislation has been introduced at least six times since 2007 and no bill has ever made it out of committee. It’s unclear whether the latest version will meet a similar fate, but its expected price tag of $10-$12 billion over 10 years is a large hurdle to overcome.
Congress did take a small step in 2021 to increase residency spots, with legislation that allocated funding for 1,000 new positions over 5 years. Congress added another 200 spots to that total in a bill passed last year.
Critics say the slots are tied up in Medicare red tape and it’s a far cry from the 14,000 new positions experts say are needed to address the physician shortage.
“We absolutely want the larger bill, and we think that’s the way to go, but we’ll continue to work and try to add as many positions as we can,” said Leonard Marquez, senior director of government relations and legislative advocacy for AAMC.
Congress is also considering legislation to speed up prior reauthorization for Medicare, something the Centers for Medicare & Medicaid Services is also seeking to do through rule changes. Nearly 30 state legislatures are debating similar legislation at the state level. And another bill in Congress would expand the Conrad State 30 program, which allows states to request J-1 visa waivers for international physicians to work in underserved areas.
“The solutions to this problem are multifactorial, and the answer that worked 10 years ago won’t be the right answer today, and the answer that works today won’t be the right answer 10 years from now,” Dr. Vidic said. “All we have to do is keep making changes, keep evolving, and the playing field continually changes.”
A version of this article first appeared on Medscape.com.
, news that comes as no revelation to Thomas Vidic, MD, clinical associate professor of neurology at Indiana University, South Bend.
In 2013, Dr. Vidic and other members of an American Academy of Neurology Workforce Task Force coauthored a report that predicted the demand for neurologists would outstrip supply by 2025. A decade later, it appears the situation is even more dire than anticipated.
While a nationwide physician shortage is affecting all specialties, neurology is facing a particularly difficult confluence of events. Advances in treatments for migraine, epilepsy, multiple sclerosis, and other neurological disorders have created a growing demand for care of pediatric and adult patients.
Over the next 7-27 years, as the number of Americans over age 65 increases, the incidences of Parkinson’s and dementia are set to double, and stroke cases are expected to rise by 20%.
At the same time, physician retirement and burnout are siphoning off neurologists from a workforce that isn’t growing fast enough. The American Medical Association reports the number of neurologists who treat patients in the United States grew by only 598 over the last decade, from 12,761 to 13,359.
This perfect storm has created what another AAN report calls a “grave threat” to patient care. The neurologist shortage “reduces access to care, worsens patient outcomes, and erodes career satisfaction and quality of life for neurologists as they face increasingly insurmountable demands,” write the authors of that 2019 report.
“We’re in trouble,” said Dr. Vidic. “We have a tremendous need for neurologists that we’re just not supporting.”
How did we get here?
Some of the challenges related to neurologist recruitment and retention are similar to those in other specialties. Compensation is certainly a factor, Dr. Vidic said.
Although neurologists’ incomes have increased significantly over the past decade, they still rank in the lower half of all medical specialties. In addition, only 50% of neurologists believe they are fairly compensated.
Burnout is another significant challenge. In 2019, before the pandemic, 53% of neurologists surveyed in Medscape’s National Physician Burnout, Depression, and Suicide Report indicated they were burned out. That percentage increased slightly in 2023, to 55%, with most respondents reporting a strong to severe impact on their lives.
The most common reason for burnout was administration and paperwork that cuts into neurologists’ time with patients. Charting and completing prior authorization and step therapy forms required by most insurers take an average of 17.6 hours a week for neurologists – much longer than the overall physician average and higher than almost all other specialties.
But perhaps the biggest contributor to the nationwide neurologist shortage is a 26-year cap on Medicare funding for medical residency. Enacted as part of the 1997 Balanced Budget Act, the legislation limits Medicare funding for medical residency training at 1996 levels. Most medical residencies are funded by the federal government and Medicare is the largest participating program.
As a result of the cap, the number of total residents in the United States – which grew by 20.6% between 1987 and 1997 – increased by only 8% from 1997 to 2007.
A new study on patients’ long travel times to neurology clinics, published in Neurology, is the latest to illustrate the real-world impact of too few neurologists amid growing caseloads.
Researchers found that 17% of the 563,216 Medicare beneficiaries who visited a neurologist in 2018 had to travel an average of 81 miles one way. Those long distances were endured most often by patients with brain and spinal cord cancers, amyotrophic lateral sclerosis, and multiple sclerosis.
While the neurologist shortage affects every state, a 2020 study suggests rural areas are most affected. This analysis of Medicare recipients showed that just 21% of rural residents with a neurological condition had access to a nearby specialist, compared with 27% of urban dwellers. The findings are similar to those of a 2017 report that identified “neurology deserts” in a number of states across the country.
Wait times for new neurology patients are reported to be among the longest of all specialties, with an average of 30 days for adult patients and 5-6 months for pediatric patients.
More neurology instruction needed
“It’s really hard knowing there are families out there who need the care but can’t get to it in a timely manner,” said Tyler Allison, MD, associate professor of pediatrics at the University of Missouri–Kansas City.
Working in a rural state means Dr. Allison has patients who drive 6 hours or more for an appointment. Although telemedicine has reduced the number of trips for many of his existing pediatric cases, it has had little impact on new patients. This is particularly frustrating, he said, when he sees a new patient with a condition that could have been treated by a primary care physician in their home community.
“One of the biggest problems we have in the child neurology world is that we don’t have enough primary care physicians who feel they are adequately trained to care for these patients,” said Dr. Allison, who also is the program director of the Child Neurology Residency Program at Children’s Mercy Kansas City.
“Sometimes I see patients where, frankly, I only need to see them once to provide reassurance to the family and then they go back to their primary care doctor,” he said. “It’s the kind of thing that if we trained people appropriately from the beginning, it would shorten our wait list.”
Indeed, increasing neurology instruction during medical school is one recommendation offered in a 2019 report that characterized the neurologist shortage as a “grave threat.”
Data from the Association of American Medical Colleges show U.S. medical schools required an average of 4.4 weeks of neurology instruction in 2019-2020. Of the disciplines included in the AAMC report, only radiology and surgical specialties required a shorter clinical course. Many medical schools also require a neurology rotation, usually during the third year.
“There are still medical schools that do not require a neurology rotation,” said Dr. Vidic. Indiana University’s medical school requires a 1-month neurology rotation. “Per capita, we turn out more neurologists than any other medical school in the country because we give the exposure.”
General neurologists needed
The 2019 AAN report also calls for a renewed focus on general neurology in residency training as a way to ease patient wait times.
“General neurologists in the community can care for 75%-85% of patients with neurological disease,” said Michael Markowski, DO, a general neurologist in Cape Cod, Mass., who chaired the AAN’s general neurology task force from 2019 to 2020.
“Our residency training programs aren’t doing anything wrong, but we have data that show we have to start doing something different if we’re going to care for the one in three Americans with neurological disease who deserve care in their community rather than having to travel to subspecialty centers, which are primarily located in larger cities,” he said.
Based on an AAN survey, only about one-third of U.S. neurologists identify as general neurologists; most focus on movement disorders, dementia or Alzheimer’s, epilepsy, or another neurology subspecialty. It’s a sharp contrast from Europe, where the vast majority of neurologists identify as general neurologists.
“It was striking, the difference between the neurologists across Europe who identify as general neurologists, in comparison to the U.S.,” said Dr. Markowski, who was the AAN representative for the European Academy of Neurology General Neurology Task Force. “Close to 28% of U.S. neurologists identify as general neurologists, but across 37 European nations, that [percentage] is 76%.”
In Europe, general neurology rotations make up at least half of the first year of medical residency, Dr. Markowski said, adding that in the United States, there is more focus on inpatient rather than outpatient neurology rotations.
“If you never see that role model during your training who is a general neurologist, who can see the vast majority of all neurology patients, why would you think you could do that when you graduate?” Dr. Markowski said.
A legislative solution
While expanding neurology instruction in medical school and increasing exposure to general neurology rotations in residency could help, the clearest path to increasing the number of neurologists in the United States is to lift the decades-old residency cap.
The Resident Physician Shortage Reduction Act of 2023 would do just that, adding 14,000 new medical residency positions over 7 years. The bill has bipartisan support, with hundreds of cosponsors from both sides of the aisle. Nearly 100 professional societies and medical and hospital groups have submitted testimony in support.
Similar legislation has been introduced at least six times since 2007 and no bill has ever made it out of committee. It’s unclear whether the latest version will meet a similar fate, but its expected price tag of $10-$12 billion over 10 years is a large hurdle to overcome.
Congress did take a small step in 2021 to increase residency spots, with legislation that allocated funding for 1,000 new positions over 5 years. Congress added another 200 spots to that total in a bill passed last year.
Critics say the slots are tied up in Medicare red tape and it’s a far cry from the 14,000 new positions experts say are needed to address the physician shortage.
“We absolutely want the larger bill, and we think that’s the way to go, but we’ll continue to work and try to add as many positions as we can,” said Leonard Marquez, senior director of government relations and legislative advocacy for AAMC.
Congress is also considering legislation to speed up prior reauthorization for Medicare, something the Centers for Medicare & Medicaid Services is also seeking to do through rule changes. Nearly 30 state legislatures are debating similar legislation at the state level. And another bill in Congress would expand the Conrad State 30 program, which allows states to request J-1 visa waivers for international physicians to work in underserved areas.
“The solutions to this problem are multifactorial, and the answer that worked 10 years ago won’t be the right answer today, and the answer that works today won’t be the right answer 10 years from now,” Dr. Vidic said. “All we have to do is keep making changes, keep evolving, and the playing field continually changes.”
A version of this article first appeared on Medscape.com.
, news that comes as no revelation to Thomas Vidic, MD, clinical associate professor of neurology at Indiana University, South Bend.
In 2013, Dr. Vidic and other members of an American Academy of Neurology Workforce Task Force coauthored a report that predicted the demand for neurologists would outstrip supply by 2025. A decade later, it appears the situation is even more dire than anticipated.
While a nationwide physician shortage is affecting all specialties, neurology is facing a particularly difficult confluence of events. Advances in treatments for migraine, epilepsy, multiple sclerosis, and other neurological disorders have created a growing demand for care of pediatric and adult patients.
Over the next 7-27 years, as the number of Americans over age 65 increases, the incidences of Parkinson’s and dementia are set to double, and stroke cases are expected to rise by 20%.
At the same time, physician retirement and burnout are siphoning off neurologists from a workforce that isn’t growing fast enough. The American Medical Association reports the number of neurologists who treat patients in the United States grew by only 598 over the last decade, from 12,761 to 13,359.
This perfect storm has created what another AAN report calls a “grave threat” to patient care. The neurologist shortage “reduces access to care, worsens patient outcomes, and erodes career satisfaction and quality of life for neurologists as they face increasingly insurmountable demands,” write the authors of that 2019 report.
“We’re in trouble,” said Dr. Vidic. “We have a tremendous need for neurologists that we’re just not supporting.”
How did we get here?
Some of the challenges related to neurologist recruitment and retention are similar to those in other specialties. Compensation is certainly a factor, Dr. Vidic said.
Although neurologists’ incomes have increased significantly over the past decade, they still rank in the lower half of all medical specialties. In addition, only 50% of neurologists believe they are fairly compensated.
Burnout is another significant challenge. In 2019, before the pandemic, 53% of neurologists surveyed in Medscape’s National Physician Burnout, Depression, and Suicide Report indicated they were burned out. That percentage increased slightly in 2023, to 55%, with most respondents reporting a strong to severe impact on their lives.
The most common reason for burnout was administration and paperwork that cuts into neurologists’ time with patients. Charting and completing prior authorization and step therapy forms required by most insurers take an average of 17.6 hours a week for neurologists – much longer than the overall physician average and higher than almost all other specialties.
But perhaps the biggest contributor to the nationwide neurologist shortage is a 26-year cap on Medicare funding for medical residency. Enacted as part of the 1997 Balanced Budget Act, the legislation limits Medicare funding for medical residency training at 1996 levels. Most medical residencies are funded by the federal government and Medicare is the largest participating program.
As a result of the cap, the number of total residents in the United States – which grew by 20.6% between 1987 and 1997 – increased by only 8% from 1997 to 2007.
A new study on patients’ long travel times to neurology clinics, published in Neurology, is the latest to illustrate the real-world impact of too few neurologists amid growing caseloads.
Researchers found that 17% of the 563,216 Medicare beneficiaries who visited a neurologist in 2018 had to travel an average of 81 miles one way. Those long distances were endured most often by patients with brain and spinal cord cancers, amyotrophic lateral sclerosis, and multiple sclerosis.
While the neurologist shortage affects every state, a 2020 study suggests rural areas are most affected. This analysis of Medicare recipients showed that just 21% of rural residents with a neurological condition had access to a nearby specialist, compared with 27% of urban dwellers. The findings are similar to those of a 2017 report that identified “neurology deserts” in a number of states across the country.
Wait times for new neurology patients are reported to be among the longest of all specialties, with an average of 30 days for adult patients and 5-6 months for pediatric patients.
More neurology instruction needed
“It’s really hard knowing there are families out there who need the care but can’t get to it in a timely manner,” said Tyler Allison, MD, associate professor of pediatrics at the University of Missouri–Kansas City.
Working in a rural state means Dr. Allison has patients who drive 6 hours or more for an appointment. Although telemedicine has reduced the number of trips for many of his existing pediatric cases, it has had little impact on new patients. This is particularly frustrating, he said, when he sees a new patient with a condition that could have been treated by a primary care physician in their home community.
“One of the biggest problems we have in the child neurology world is that we don’t have enough primary care physicians who feel they are adequately trained to care for these patients,” said Dr. Allison, who also is the program director of the Child Neurology Residency Program at Children’s Mercy Kansas City.
“Sometimes I see patients where, frankly, I only need to see them once to provide reassurance to the family and then they go back to their primary care doctor,” he said. “It’s the kind of thing that if we trained people appropriately from the beginning, it would shorten our wait list.”
Indeed, increasing neurology instruction during medical school is one recommendation offered in a 2019 report that characterized the neurologist shortage as a “grave threat.”
Data from the Association of American Medical Colleges show U.S. medical schools required an average of 4.4 weeks of neurology instruction in 2019-2020. Of the disciplines included in the AAMC report, only radiology and surgical specialties required a shorter clinical course. Many medical schools also require a neurology rotation, usually during the third year.
“There are still medical schools that do not require a neurology rotation,” said Dr. Vidic. Indiana University’s medical school requires a 1-month neurology rotation. “Per capita, we turn out more neurologists than any other medical school in the country because we give the exposure.”
General neurologists needed
The 2019 AAN report also calls for a renewed focus on general neurology in residency training as a way to ease patient wait times.
“General neurologists in the community can care for 75%-85% of patients with neurological disease,” said Michael Markowski, DO, a general neurologist in Cape Cod, Mass., who chaired the AAN’s general neurology task force from 2019 to 2020.
“Our residency training programs aren’t doing anything wrong, but we have data that show we have to start doing something different if we’re going to care for the one in three Americans with neurological disease who deserve care in their community rather than having to travel to subspecialty centers, which are primarily located in larger cities,” he said.
Based on an AAN survey, only about one-third of U.S. neurologists identify as general neurologists; most focus on movement disorders, dementia or Alzheimer’s, epilepsy, or another neurology subspecialty. It’s a sharp contrast from Europe, where the vast majority of neurologists identify as general neurologists.
“It was striking, the difference between the neurologists across Europe who identify as general neurologists, in comparison to the U.S.,” said Dr. Markowski, who was the AAN representative for the European Academy of Neurology General Neurology Task Force. “Close to 28% of U.S. neurologists identify as general neurologists, but across 37 European nations, that [percentage] is 76%.”
In Europe, general neurology rotations make up at least half of the first year of medical residency, Dr. Markowski said, adding that in the United States, there is more focus on inpatient rather than outpatient neurology rotations.
“If you never see that role model during your training who is a general neurologist, who can see the vast majority of all neurology patients, why would you think you could do that when you graduate?” Dr. Markowski said.
A legislative solution
While expanding neurology instruction in medical school and increasing exposure to general neurology rotations in residency could help, the clearest path to increasing the number of neurologists in the United States is to lift the decades-old residency cap.
The Resident Physician Shortage Reduction Act of 2023 would do just that, adding 14,000 new medical residency positions over 7 years. The bill has bipartisan support, with hundreds of cosponsors from both sides of the aisle. Nearly 100 professional societies and medical and hospital groups have submitted testimony in support.
Similar legislation has been introduced at least six times since 2007 and no bill has ever made it out of committee. It’s unclear whether the latest version will meet a similar fate, but its expected price tag of $10-$12 billion over 10 years is a large hurdle to overcome.
Congress did take a small step in 2021 to increase residency spots, with legislation that allocated funding for 1,000 new positions over 5 years. Congress added another 200 spots to that total in a bill passed last year.
Critics say the slots are tied up in Medicare red tape and it’s a far cry from the 14,000 new positions experts say are needed to address the physician shortage.
“We absolutely want the larger bill, and we think that’s the way to go, but we’ll continue to work and try to add as many positions as we can,” said Leonard Marquez, senior director of government relations and legislative advocacy for AAMC.
Congress is also considering legislation to speed up prior reauthorization for Medicare, something the Centers for Medicare & Medicaid Services is also seeking to do through rule changes. Nearly 30 state legislatures are debating similar legislation at the state level. And another bill in Congress would expand the Conrad State 30 program, which allows states to request J-1 visa waivers for international physicians to work in underserved areas.
“The solutions to this problem are multifactorial, and the answer that worked 10 years ago won’t be the right answer today, and the answer that works today won’t be the right answer 10 years from now,” Dr. Vidic said. “All we have to do is keep making changes, keep evolving, and the playing field continually changes.”
A version of this article first appeared on Medscape.com.
Granulomatous Dermatitis in a Patient With Cholangiocarcinoma Treated With BRAF and MEK Inhibitors
To the Editor:
Granulomatous dermatitis (GD) has been described as a rare side effect of MEK and BRAF inhibitor use in the treatment of BRAF V600E mutation–positive metastatic melanoma. As the utilization of BRAF and MEK inhibitors increases for the treatment of a variety of cancers, it is essential that clinicians and pathologists recognize GD as a potential cutaneous manifestation. We present the case of a 52-year-old woman who developed GD while being treated with vemurafenib and cobimetinib for BRAF V600E mutation–positive metastatic cholangiocarcinoma.
A 52-year-old White woman presented with faint patches of nonpalpable violaceous mottling that extended distally to proximally from the ankles to the thighs on the medial aspects of both legs. She was diagnosed with cholangiocarcinoma 10 months prior, with metastases to the lung, liver, and sternum. She underwent treatment with gemcitabine and cisplatin therapy. Computed tomography after several treatment cycles revealed progressive disease with multiple pulmonary nodules as well as metastatic intrathoracic and abdominal adenopathy. Treatment with gemcitabine and cisplatin failed to produce a favorable response and was discontinued after 6 treatment cycles.
Genomic testing performed at the time of diagnosis revealed a positive mutation for BRAF V600E. The patient subsequently enrolled in a clinical trial and started treatment with the BRAF inhibitor vemurafenib and the MEK inhibitor cobimetinib. She developed sun sensitivity and multiple sunburns after starting these therapies. The patient tolerated the next few cycles of therapy well with only moderate concerns of dry sensitive skin.
During the sixth cycle of therapy, she presented to dermatology after developing a rash. Over the next 2 weeks, similar lesions appeared on the arms. The patient denied the use of any new lotions, soaps, or other medications. Punch biopsies of the right forearm and right medial thigh revealed nonnecrotizing granulomas in the superficial dermis that extended into the subcutaneous adipose tissue (Figure 1). Surrounding chronic inflammation was scant, and the presence of rare eosinophils was noted (Figure 2). The histiocytes were highlighted by a CD68 immunohistochemical stain. An auramine-O special stain test was negative for acid-fast bacilli, and a Grocott methenamine-silver special stain test for fungal organisms was negative. These findings were consistent with GD. Computed tomography of the chest performed 2 months prior and 1 month after biopsy of the skin lesions revealed no axillary, mediastinal, or hilar lymphadenopathy. The calcium level at the time of skin biopsy was within reference range.
A topical steroid was prescribed; however, it was not utilized by the patient. Within 2 months of onset, the GD lesions resolved with no treatment. The GD lesions did not affect the patient’s enrollment in the clinical trial, and no dose reductions were made. Due to progressive disease with metastases to the brain, the patient eventually discontinued the clinical trial.
BRAF inhibitors are US Food and Drug Administration approved for the treatment of metastatic melanoma to deactivate the serine-threonine kinase BRAF gene mutation, which leads to decreased generation and survival of melanoma cells.1,2 Vemurafenib, dabrafenib, and encorafenib are the only BRAF inhibitors approved in the United States.3 The most common side effects of vemurafenib include arthralgia, fatigue, rash, and photosensitivity.1,4 There are 4 MEK inhibitors currently available in the United States: cobimetinib, trametinib, selumetinib and binimetinib. The addition of a MEK inhibitor to BRAF inhibitor therapy has shown increased patient response rates and prolonged survival in 3 phase 3 studies.5-10
Response rates remain low in the treatment of advanced cholangiocarcinoma with standard chemotherapy. Recent research has explored if targeted therapies at the molecular level would be of benefit.11 Our patient was enrolled in the American Society of Clinical Oncology Targeted Agent and Profiling Utilization Registry (TAPUR) trial, a phase 2, prospective, nonrandomized trial that matches eligible participants to US Food and Drug Administration–approved study medications based on specific data from their molecular testing results.12 Some of the most common mutations in intrahepatic cholangiocarcinoma include HER2, KRAS, MET, and BRAF.13-17 Our patient’s molecular test results were positive for a BRAF V600E–positive mutation, and she subsequently started therapy with vemurafenib and cobimetinib. The use of personalized genomic treatment approaches for BRAF V600E mutation–positive cholangiocarcinoma has produced a dramatic patient response to BRAF and MEK inhibitor combination therapies.11,18-20
Drug-induced GD most likely is caused by vascular insults that lead to deposition of immune complexes in vessels causing inflammation and a consequent granulomatous infiltrate.21,22 Although cordlike lesions in the subcutaneous tissue on the trunk commonly are reported, the presentation of GD can vary considerably. Other presentations include areas of violaceous or erythematous patches or plaques on the limbs, intertriginous areas, and upper trunk. Diffuse macular erythema or small flesh-colored papules also can be observed.23
Granulomatous dermatitis secondary to drug reactions can have varying morphologies. The infiltrate often can have an interstitial appearance with the presence of lymphocytes, plasma cells, histiocytes, eosinophils, and multinucleated giant cells.24 These findings can be confused with interstitial granuloma annulare. Other cases, such as in our patient, can have discrete granulomata formation with a sarcoidlike appearance. These naked granulomas lack surrounding inflammation and suggest a differential diagnosis of sarcoidosis and infection. Use of immune checkpoint inhibitors (CIs) and kinase inhibitors has been proven to cause sarcoidosislike reactions.25 The development of granulomatous/sarcoidlike lesions associated with the use of BRAF and MEK inhibitors may clinically and radiographically mimic disease recurrence. An awareness of this type of reaction by clinicians and pathologists is important to ensure appropriate management in patients who develop GD.26
Checkpoint inhibitor–induced GD that remains asymptomatic does not necessarily warrant treatment; however, corticosteroid use and elimination of CI therapies have resolved GD in prior cases. Responsiveness of the cancer to CI therapy and severity of GD symptoms should be considered before discontinuation of a CI trial.25
One case report described complete resolution of a GD eruption without interruption of the scheduled BRAF and MEK inhibitor therapies for the treatment of metastatic melanoma. There was no reported use of a steroidal cream or other topical medication to aid in controlling the eruption.27 The exact mechanism of how GD resolves while continuing therapy is unknown; however, it has been suggested that a GD eruption may be the consequence of a BRAF and MEK inhibitor–mediated immune response against a subclinical area of metastatic melanoma.28 If the immune response successfully eliminates the subclinical tumor, one could postulate that the inflammatory response and granulomatous eruption would resolve. Future studies are necessary to further elucidate the exact mechanisms involved.
There have been several case reports of GD with vemurafenib treatment,29,30 1 report of GD and erythema induratum with vemurafenib and cobimetinib treatment,31 2 reports of GD with dabrafenib treatment,27,30 and a few reports of GD with the BRAF inhibitor dabrafenib combined with the MEK inhibitor trametinib,28,32,33 all for the treatment of metastatic melanoma. Additionally, a report described a 3-year-old boy who developed GD secondary to vemurafenib for the treatment of Langerhans cell histiocytosis.34 We present a unique case of BRAF and MEK inhibitor therapy–induced GD in the treatment of metastatic cholangiocarcinoma with vemurafenib and cobimetinib.
BRAF and MEK inhibitor therapy is used in patients with metastatic melanomas with a positive BRAF V600E mutation. Due to advancements in next-generation DNA sequencing, these therapies also are being tested in clinical trials for use in the treatment of other cancers with the same checkpoint mutation, such as metastatic cholangiocarcinoma. Cutaneous reactions frequently are documented side effects that occur during treatment with BRAF and MEK inhibitors; GD is an uncommon finding. As the utilization of BRAF and MEK inhibitors increases for the treatment of a variety of other cancers, it is essential that clinicians and pathologists recognize GD as a potential cutaneous manifestation.
- Mackiewicz J, Mackiewicz A. BRAF and MEK inhibitors in the era of immunotherapy in melanoma patients. Comtemp Oncol (Pozn). 2018;22:68-72.
- Jovanovic B, Krockel D, Linden D, et al. Lack of cytoplasmic ERK activation is an independent adverse prognostic factor in primary cutaneous melanoma. J Invest Dermatol. 2008;128:2696-2704.
- Alqathama A. BRAF in malignant melanoma progression and metastasis: potentials and challenges. Am J Cancer Res. 2020;10:1103-1114.
- Zimmer L, Hillen U, Livingstone E, et al. Atypical melanocytic proliferations and new primary melanomas in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol. 2012;30:2375-2383.
- Casey D, Demko S, Sinha A, et al. FDA approval summary: selumetinib for plexiform neurofibroma. Clin Cancer Res. 2021;27;4142-4146
- Flaherty K, Davies MA, Grob JJ, et al. Genomic analysis and 3-y efficacy and safety update of COMBI-d: a phase 3 study of dabrafenib (D) fl trametinib (T) vs D monotherapy in patients (pts) with unresectable or metastatic BRAF V600E/K-mutant cutaneous melanoma. Abstract presented at: American Society of Clinical Oncology Annual Meeting; June 3-7, 2016; Chicago, IL. P9502.
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
- Robert C, Karaszewska B, Schachter J, et al. Three-year estimate of overall survival in COMBI-v, a randomized phase 3 study evaluating first-line dabrafenib (D) + trametinib (T) in patients (pts) with unresectable or metastatic BRAF V600E/K–mutant cutaneous melanoma. Ann Oncol. 2016;27(suppl 6):vi552-vi587.
- Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867-1876.
- Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advance BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomized, double-blind, phase 3 trial. Lancet Once. 2016;17:1248-1260.
- Kocsis J, Árokszállási A, András C, et al. Combined dabrafenib and trametinib treatment in a case of chemotherapy-refractory extrahepatic BRAF V600E mutant cholangiocarcinoma: dramatic clinical and radiological response with a confusing synchronic new liver lesion. J Gastrointest Oncol. 2017;8:E32-E38.
- Mangat PK, Halabi S, Bruinooge SS, et al. Rationale and design of the Targeted Agent and Profiling Utilization Registry (TAPUR) Study [published online July 11, 2018]. JCO Precis Oncol. doi:10.1200/PO.18.00122
- Terada T, Ashida K, Endo K, et al. c-erbB-2 protein is expressed in hepatolithiasis and cholangiocarcinoma. Histopathology. 1998;33:325-331.
- Tannapfel A, Benicke M, Katalinic A, et al. Frequency of p16INK4A alterations and K-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut. 2000;47:721-727.
- Momoi H, Itoh T, Nozaki Y, et al. Microsatellite instability and alternative genetic pathway in intrahepatic cholangiocarcinoma. J Hepatol. 2001;35:235-244.
- Terada T, Nakanuma Y, Sirica AE. Immunohistochemical demonstration of MET overexpression in human intrahepatic cholangiocarcinoma and in hepatolithiasis. Hum Pathol. 1998;29:175-180.
- Tannapfel A, Sommerer F, Benicke M, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52:706-712.
- Bunyatov T, Zhao A, Kovalenko J, et al. Personalised approach in combined treatment of cholangiocarcinoma: a case report of healing from cholangiocellular carcinoma at stage IV. J Gastrointest Oncol. 2019;10:815-820.
- Lavingia V, Fakih M. Impressive response to dual BRAF and MEK inhibition in patients with BRAF mutant intrahepatic cholangiocarcinoma-2 case reports and a brief review. J Gastrointest Oncol. 2016;7:E98-E102.
- Loaiza-Bonilla A, Clayton E, Furth E, et al. Dramatic response to dabrafenib and trametinib combination in a BRAF V600E-mutated cholangiocarcinoma: implementation of a molecular tumour board and next-generation sequencing for personalized medicine. Ecancermedicalscience. 2014;8:479.
- Rosenbach M, English JC. Reactive granulomatous dermatitis. Dermatol Clin. 2015;33:373-387.
- Tomasini C, Pippione M. Interstitial granulomatous dermatitis with plaques. J Am Acad Dermatol. 2002;46:892-899.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol 2012;166:775-783.
- Calonje JE, Brenn T, Lazar A, Billings S. Lichenoid and interface dermatitis. In: McKee’s Pathology of the Skin. 5th ed. China: Elsevier Limited: 2018;7:241-282.
- Gkiozos I, Kopitopoulou A, Kalkanis A, et al. Sarcoidosis-like reactions induced by checkpoint inhibitors. J Thorac Oncol. 2018;13:1076-1082.
- Tetzlaff MT, Nelson KC, Diab A, et al. Granulomatous/sarcoid-like lesions associated with checkpoint inhibitors: a marker of therapy response in a subset of melanoma patients. J Immunother Cancer. 2018;6:14.
- Garrido MC, Gutiérrez C, Riveiro-Falkenbach E, et al. BRAF inhibitor-induced antitumoral granulomatous dermatitis eruption in advanced melanoma. Am J Dermatopathol. 2015;37:795-798.
- Park JJ, Hawryluk EB, Tahan SR, et al. Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma. JAMA Dermatol. 2014;150:307‐311.
- Ong ELH, Sinha R, Jmor S, et al. BRAF inhibitor-associated granulomatous dermatitis: a report of 3 cases. Am J of Dermatopathol. 2019;41:214-217.
- Wali GN, Stonard C, Espinosa O, et al. Persistent granulomatous cutaneous drug eruption to a BRAF inhibitor. J Am Acad Dermatol. 2017;76(suppl 1):AB195.
- Aj lafolla M, Ramsay J, Wismer J, et al. Cobimetinib- and vemurafenib-induced granulomatous dermatitis and erythema induratum: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847358
- Jansen YJ, Janssens P, Hoorens A, et al. Granulomatous nephritis and dermatitis in a patient with BRAF V600E mutant metastatic melanoma treated with dabrafenib and trametinib. Melanoma Res. 2015;25:550‐554.
- Green JS, Norris DA, Wisell J. Novel cutaneous effects of combination chemotherapy with BRAF and MEK inhibitors: a report of two cases. Br J Dermatol. 2013;169:172-176.
- Chen L, His A, Kothari A, et al. Granulomatous dermatitis secondary to vemurafenib in a child with Langerhans cell histiocytosis. Pediatr Dermatol. 2018;35:E402-E403.
To the Editor:
Granulomatous dermatitis (GD) has been described as a rare side effect of MEK and BRAF inhibitor use in the treatment of BRAF V600E mutation–positive metastatic melanoma. As the utilization of BRAF and MEK inhibitors increases for the treatment of a variety of cancers, it is essential that clinicians and pathologists recognize GD as a potential cutaneous manifestation. We present the case of a 52-year-old woman who developed GD while being treated with vemurafenib and cobimetinib for BRAF V600E mutation–positive metastatic cholangiocarcinoma.
A 52-year-old White woman presented with faint patches of nonpalpable violaceous mottling that extended distally to proximally from the ankles to the thighs on the medial aspects of both legs. She was diagnosed with cholangiocarcinoma 10 months prior, with metastases to the lung, liver, and sternum. She underwent treatment with gemcitabine and cisplatin therapy. Computed tomography after several treatment cycles revealed progressive disease with multiple pulmonary nodules as well as metastatic intrathoracic and abdominal adenopathy. Treatment with gemcitabine and cisplatin failed to produce a favorable response and was discontinued after 6 treatment cycles.
Genomic testing performed at the time of diagnosis revealed a positive mutation for BRAF V600E. The patient subsequently enrolled in a clinical trial and started treatment with the BRAF inhibitor vemurafenib and the MEK inhibitor cobimetinib. She developed sun sensitivity and multiple sunburns after starting these therapies. The patient tolerated the next few cycles of therapy well with only moderate concerns of dry sensitive skin.
During the sixth cycle of therapy, she presented to dermatology after developing a rash. Over the next 2 weeks, similar lesions appeared on the arms. The patient denied the use of any new lotions, soaps, or other medications. Punch biopsies of the right forearm and right medial thigh revealed nonnecrotizing granulomas in the superficial dermis that extended into the subcutaneous adipose tissue (Figure 1). Surrounding chronic inflammation was scant, and the presence of rare eosinophils was noted (Figure 2). The histiocytes were highlighted by a CD68 immunohistochemical stain. An auramine-O special stain test was negative for acid-fast bacilli, and a Grocott methenamine-silver special stain test for fungal organisms was negative. These findings were consistent with GD. Computed tomography of the chest performed 2 months prior and 1 month after biopsy of the skin lesions revealed no axillary, mediastinal, or hilar lymphadenopathy. The calcium level at the time of skin biopsy was within reference range.

A topical steroid was prescribed; however, it was not utilized by the patient. Within 2 months of onset, the GD lesions resolved with no treatment. The GD lesions did not affect the patient’s enrollment in the clinical trial, and no dose reductions were made. Due to progressive disease with metastases to the brain, the patient eventually discontinued the clinical trial.

BRAF inhibitors are US Food and Drug Administration approved for the treatment of metastatic melanoma to deactivate the serine-threonine kinase BRAF gene mutation, which leads to decreased generation and survival of melanoma cells.1,2 Vemurafenib, dabrafenib, and encorafenib are the only BRAF inhibitors approved in the United States.3 The most common side effects of vemurafenib include arthralgia, fatigue, rash, and photosensitivity.1,4 There are 4 MEK inhibitors currently available in the United States: cobimetinib, trametinib, selumetinib and binimetinib. The addition of a MEK inhibitor to BRAF inhibitor therapy has shown increased patient response rates and prolonged survival in 3 phase 3 studies.5-10
Response rates remain low in the treatment of advanced cholangiocarcinoma with standard chemotherapy. Recent research has explored if targeted therapies at the molecular level would be of benefit.11 Our patient was enrolled in the American Society of Clinical Oncology Targeted Agent and Profiling Utilization Registry (TAPUR) trial, a phase 2, prospective, nonrandomized trial that matches eligible participants to US Food and Drug Administration–approved study medications based on specific data from their molecular testing results.12 Some of the most common mutations in intrahepatic cholangiocarcinoma include HER2, KRAS, MET, and BRAF.13-17 Our patient’s molecular test results were positive for a BRAF V600E–positive mutation, and she subsequently started therapy with vemurafenib and cobimetinib. The use of personalized genomic treatment approaches for BRAF V600E mutation–positive cholangiocarcinoma has produced a dramatic patient response to BRAF and MEK inhibitor combination therapies.11,18-20
Drug-induced GD most likely is caused by vascular insults that lead to deposition of immune complexes in vessels causing inflammation and a consequent granulomatous infiltrate.21,22 Although cordlike lesions in the subcutaneous tissue on the trunk commonly are reported, the presentation of GD can vary considerably. Other presentations include areas of violaceous or erythematous patches or plaques on the limbs, intertriginous areas, and upper trunk. Diffuse macular erythema or small flesh-colored papules also can be observed.23
Granulomatous dermatitis secondary to drug reactions can have varying morphologies. The infiltrate often can have an interstitial appearance with the presence of lymphocytes, plasma cells, histiocytes, eosinophils, and multinucleated giant cells.24 These findings can be confused with interstitial granuloma annulare. Other cases, such as in our patient, can have discrete granulomata formation with a sarcoidlike appearance. These naked granulomas lack surrounding inflammation and suggest a differential diagnosis of sarcoidosis and infection. Use of immune checkpoint inhibitors (CIs) and kinase inhibitors has been proven to cause sarcoidosislike reactions.25 The development of granulomatous/sarcoidlike lesions associated with the use of BRAF and MEK inhibitors may clinically and radiographically mimic disease recurrence. An awareness of this type of reaction by clinicians and pathologists is important to ensure appropriate management in patients who develop GD.26
Checkpoint inhibitor–induced GD that remains asymptomatic does not necessarily warrant treatment; however, corticosteroid use and elimination of CI therapies have resolved GD in prior cases. Responsiveness of the cancer to CI therapy and severity of GD symptoms should be considered before discontinuation of a CI trial.25
One case report described complete resolution of a GD eruption without interruption of the scheduled BRAF and MEK inhibitor therapies for the treatment of metastatic melanoma. There was no reported use of a steroidal cream or other topical medication to aid in controlling the eruption.27 The exact mechanism of how GD resolves while continuing therapy is unknown; however, it has been suggested that a GD eruption may be the consequence of a BRAF and MEK inhibitor–mediated immune response against a subclinical area of metastatic melanoma.28 If the immune response successfully eliminates the subclinical tumor, one could postulate that the inflammatory response and granulomatous eruption would resolve. Future studies are necessary to further elucidate the exact mechanisms involved.
There have been several case reports of GD with vemurafenib treatment,29,30 1 report of GD and erythema induratum with vemurafenib and cobimetinib treatment,31 2 reports of GD with dabrafenib treatment,27,30 and a few reports of GD with the BRAF inhibitor dabrafenib combined with the MEK inhibitor trametinib,28,32,33 all for the treatment of metastatic melanoma. Additionally, a report described a 3-year-old boy who developed GD secondary to vemurafenib for the treatment of Langerhans cell histiocytosis.34 We present a unique case of BRAF and MEK inhibitor therapy–induced GD in the treatment of metastatic cholangiocarcinoma with vemurafenib and cobimetinib.
BRAF and MEK inhibitor therapy is used in patients with metastatic melanomas with a positive BRAF V600E mutation. Due to advancements in next-generation DNA sequencing, these therapies also are being tested in clinical trials for use in the treatment of other cancers with the same checkpoint mutation, such as metastatic cholangiocarcinoma. Cutaneous reactions frequently are documented side effects that occur during treatment with BRAF and MEK inhibitors; GD is an uncommon finding. As the utilization of BRAF and MEK inhibitors increases for the treatment of a variety of other cancers, it is essential that clinicians and pathologists recognize GD as a potential cutaneous manifestation.
To the Editor:
Granulomatous dermatitis (GD) has been described as a rare side effect of MEK and BRAF inhibitor use in the treatment of BRAF V600E mutation–positive metastatic melanoma. As the utilization of BRAF and MEK inhibitors increases for the treatment of a variety of cancers, it is essential that clinicians and pathologists recognize GD as a potential cutaneous manifestation. We present the case of a 52-year-old woman who developed GD while being treated with vemurafenib and cobimetinib for BRAF V600E mutation–positive metastatic cholangiocarcinoma.
A 52-year-old White woman presented with faint patches of nonpalpable violaceous mottling that extended distally to proximally from the ankles to the thighs on the medial aspects of both legs. She was diagnosed with cholangiocarcinoma 10 months prior, with metastases to the lung, liver, and sternum. She underwent treatment with gemcitabine and cisplatin therapy. Computed tomography after several treatment cycles revealed progressive disease with multiple pulmonary nodules as well as metastatic intrathoracic and abdominal adenopathy. Treatment with gemcitabine and cisplatin failed to produce a favorable response and was discontinued after 6 treatment cycles.
Genomic testing performed at the time of diagnosis revealed a positive mutation for BRAF V600E. The patient subsequently enrolled in a clinical trial and started treatment with the BRAF inhibitor vemurafenib and the MEK inhibitor cobimetinib. She developed sun sensitivity and multiple sunburns after starting these therapies. The patient tolerated the next few cycles of therapy well with only moderate concerns of dry sensitive skin.
During the sixth cycle of therapy, she presented to dermatology after developing a rash. Over the next 2 weeks, similar lesions appeared on the arms. The patient denied the use of any new lotions, soaps, or other medications. Punch biopsies of the right forearm and right medial thigh revealed nonnecrotizing granulomas in the superficial dermis that extended into the subcutaneous adipose tissue (Figure 1). Surrounding chronic inflammation was scant, and the presence of rare eosinophils was noted (Figure 2). The histiocytes were highlighted by a CD68 immunohistochemical stain. An auramine-O special stain test was negative for acid-fast bacilli, and a Grocott methenamine-silver special stain test for fungal organisms was negative. These findings were consistent with GD. Computed tomography of the chest performed 2 months prior and 1 month after biopsy of the skin lesions revealed no axillary, mediastinal, or hilar lymphadenopathy. The calcium level at the time of skin biopsy was within reference range.

A topical steroid was prescribed; however, it was not utilized by the patient. Within 2 months of onset, the GD lesions resolved with no treatment. The GD lesions did not affect the patient’s enrollment in the clinical trial, and no dose reductions were made. Due to progressive disease with metastases to the brain, the patient eventually discontinued the clinical trial.

BRAF inhibitors are US Food and Drug Administration approved for the treatment of metastatic melanoma to deactivate the serine-threonine kinase BRAF gene mutation, which leads to decreased generation and survival of melanoma cells.1,2 Vemurafenib, dabrafenib, and encorafenib are the only BRAF inhibitors approved in the United States.3 The most common side effects of vemurafenib include arthralgia, fatigue, rash, and photosensitivity.1,4 There are 4 MEK inhibitors currently available in the United States: cobimetinib, trametinib, selumetinib and binimetinib. The addition of a MEK inhibitor to BRAF inhibitor therapy has shown increased patient response rates and prolonged survival in 3 phase 3 studies.5-10
Response rates remain low in the treatment of advanced cholangiocarcinoma with standard chemotherapy. Recent research has explored if targeted therapies at the molecular level would be of benefit.11 Our patient was enrolled in the American Society of Clinical Oncology Targeted Agent and Profiling Utilization Registry (TAPUR) trial, a phase 2, prospective, nonrandomized trial that matches eligible participants to US Food and Drug Administration–approved study medications based on specific data from their molecular testing results.12 Some of the most common mutations in intrahepatic cholangiocarcinoma include HER2, KRAS, MET, and BRAF.13-17 Our patient’s molecular test results were positive for a BRAF V600E–positive mutation, and she subsequently started therapy with vemurafenib and cobimetinib. The use of personalized genomic treatment approaches for BRAF V600E mutation–positive cholangiocarcinoma has produced a dramatic patient response to BRAF and MEK inhibitor combination therapies.11,18-20
Drug-induced GD most likely is caused by vascular insults that lead to deposition of immune complexes in vessels causing inflammation and a consequent granulomatous infiltrate.21,22 Although cordlike lesions in the subcutaneous tissue on the trunk commonly are reported, the presentation of GD can vary considerably. Other presentations include areas of violaceous or erythematous patches or plaques on the limbs, intertriginous areas, and upper trunk. Diffuse macular erythema or small flesh-colored papules also can be observed.23
Granulomatous dermatitis secondary to drug reactions can have varying morphologies. The infiltrate often can have an interstitial appearance with the presence of lymphocytes, plasma cells, histiocytes, eosinophils, and multinucleated giant cells.24 These findings can be confused with interstitial granuloma annulare. Other cases, such as in our patient, can have discrete granulomata formation with a sarcoidlike appearance. These naked granulomas lack surrounding inflammation and suggest a differential diagnosis of sarcoidosis and infection. Use of immune checkpoint inhibitors (CIs) and kinase inhibitors has been proven to cause sarcoidosislike reactions.25 The development of granulomatous/sarcoidlike lesions associated with the use of BRAF and MEK inhibitors may clinically and radiographically mimic disease recurrence. An awareness of this type of reaction by clinicians and pathologists is important to ensure appropriate management in patients who develop GD.26
Checkpoint inhibitor–induced GD that remains asymptomatic does not necessarily warrant treatment; however, corticosteroid use and elimination of CI therapies have resolved GD in prior cases. Responsiveness of the cancer to CI therapy and severity of GD symptoms should be considered before discontinuation of a CI trial.25
One case report described complete resolution of a GD eruption without interruption of the scheduled BRAF and MEK inhibitor therapies for the treatment of metastatic melanoma. There was no reported use of a steroidal cream or other topical medication to aid in controlling the eruption.27 The exact mechanism of how GD resolves while continuing therapy is unknown; however, it has been suggested that a GD eruption may be the consequence of a BRAF and MEK inhibitor–mediated immune response against a subclinical area of metastatic melanoma.28 If the immune response successfully eliminates the subclinical tumor, one could postulate that the inflammatory response and granulomatous eruption would resolve. Future studies are necessary to further elucidate the exact mechanisms involved.
There have been several case reports of GD with vemurafenib treatment,29,30 1 report of GD and erythema induratum with vemurafenib and cobimetinib treatment,31 2 reports of GD with dabrafenib treatment,27,30 and a few reports of GD with the BRAF inhibitor dabrafenib combined with the MEK inhibitor trametinib,28,32,33 all for the treatment of metastatic melanoma. Additionally, a report described a 3-year-old boy who developed GD secondary to vemurafenib for the treatment of Langerhans cell histiocytosis.34 We present a unique case of BRAF and MEK inhibitor therapy–induced GD in the treatment of metastatic cholangiocarcinoma with vemurafenib and cobimetinib.
BRAF and MEK inhibitor therapy is used in patients with metastatic melanomas with a positive BRAF V600E mutation. Due to advancements in next-generation DNA sequencing, these therapies also are being tested in clinical trials for use in the treatment of other cancers with the same checkpoint mutation, such as metastatic cholangiocarcinoma. Cutaneous reactions frequently are documented side effects that occur during treatment with BRAF and MEK inhibitors; GD is an uncommon finding. As the utilization of BRAF and MEK inhibitors increases for the treatment of a variety of other cancers, it is essential that clinicians and pathologists recognize GD as a potential cutaneous manifestation.
- Mackiewicz J, Mackiewicz A. BRAF and MEK inhibitors in the era of immunotherapy in melanoma patients. Comtemp Oncol (Pozn). 2018;22:68-72.
- Jovanovic B, Krockel D, Linden D, et al. Lack of cytoplasmic ERK activation is an independent adverse prognostic factor in primary cutaneous melanoma. J Invest Dermatol. 2008;128:2696-2704.
- Alqathama A. BRAF in malignant melanoma progression and metastasis: potentials and challenges. Am J Cancer Res. 2020;10:1103-1114.
- Zimmer L, Hillen U, Livingstone E, et al. Atypical melanocytic proliferations and new primary melanomas in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol. 2012;30:2375-2383.
- Casey D, Demko S, Sinha A, et al. FDA approval summary: selumetinib for plexiform neurofibroma. Clin Cancer Res. 2021;27;4142-4146
- Flaherty K, Davies MA, Grob JJ, et al. Genomic analysis and 3-y efficacy and safety update of COMBI-d: a phase 3 study of dabrafenib (D) fl trametinib (T) vs D monotherapy in patients (pts) with unresectable or metastatic BRAF V600E/K-mutant cutaneous melanoma. Abstract presented at: American Society of Clinical Oncology Annual Meeting; June 3-7, 2016; Chicago, IL. P9502.
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
- Robert C, Karaszewska B, Schachter J, et al. Three-year estimate of overall survival in COMBI-v, a randomized phase 3 study evaluating first-line dabrafenib (D) + trametinib (T) in patients (pts) with unresectable or metastatic BRAF V600E/K–mutant cutaneous melanoma. Ann Oncol. 2016;27(suppl 6):vi552-vi587.
- Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867-1876.
- Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advance BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomized, double-blind, phase 3 trial. Lancet Once. 2016;17:1248-1260.
- Kocsis J, Árokszállási A, András C, et al. Combined dabrafenib and trametinib treatment in a case of chemotherapy-refractory extrahepatic BRAF V600E mutant cholangiocarcinoma: dramatic clinical and radiological response with a confusing synchronic new liver lesion. J Gastrointest Oncol. 2017;8:E32-E38.
- Mangat PK, Halabi S, Bruinooge SS, et al. Rationale and design of the Targeted Agent and Profiling Utilization Registry (TAPUR) Study [published online July 11, 2018]. JCO Precis Oncol. doi:10.1200/PO.18.00122
- Terada T, Ashida K, Endo K, et al. c-erbB-2 protein is expressed in hepatolithiasis and cholangiocarcinoma. Histopathology. 1998;33:325-331.
- Tannapfel A, Benicke M, Katalinic A, et al. Frequency of p16INK4A alterations and K-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut. 2000;47:721-727.
- Momoi H, Itoh T, Nozaki Y, et al. Microsatellite instability and alternative genetic pathway in intrahepatic cholangiocarcinoma. J Hepatol. 2001;35:235-244.
- Terada T, Nakanuma Y, Sirica AE. Immunohistochemical demonstration of MET overexpression in human intrahepatic cholangiocarcinoma and in hepatolithiasis. Hum Pathol. 1998;29:175-180.
- Tannapfel A, Sommerer F, Benicke M, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52:706-712.
- Bunyatov T, Zhao A, Kovalenko J, et al. Personalised approach in combined treatment of cholangiocarcinoma: a case report of healing from cholangiocellular carcinoma at stage IV. J Gastrointest Oncol. 2019;10:815-820.
- Lavingia V, Fakih M. Impressive response to dual BRAF and MEK inhibition in patients with BRAF mutant intrahepatic cholangiocarcinoma-2 case reports and a brief review. J Gastrointest Oncol. 2016;7:E98-E102.
- Loaiza-Bonilla A, Clayton E, Furth E, et al. Dramatic response to dabrafenib and trametinib combination in a BRAF V600E-mutated cholangiocarcinoma: implementation of a molecular tumour board and next-generation sequencing for personalized medicine. Ecancermedicalscience. 2014;8:479.
- Rosenbach M, English JC. Reactive granulomatous dermatitis. Dermatol Clin. 2015;33:373-387.
- Tomasini C, Pippione M. Interstitial granulomatous dermatitis with plaques. J Am Acad Dermatol. 2002;46:892-899.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol 2012;166:775-783.
- Calonje JE, Brenn T, Lazar A, Billings S. Lichenoid and interface dermatitis. In: McKee’s Pathology of the Skin. 5th ed. China: Elsevier Limited: 2018;7:241-282.
- Gkiozos I, Kopitopoulou A, Kalkanis A, et al. Sarcoidosis-like reactions induced by checkpoint inhibitors. J Thorac Oncol. 2018;13:1076-1082.
- Tetzlaff MT, Nelson KC, Diab A, et al. Granulomatous/sarcoid-like lesions associated with checkpoint inhibitors: a marker of therapy response in a subset of melanoma patients. J Immunother Cancer. 2018;6:14.
- Garrido MC, Gutiérrez C, Riveiro-Falkenbach E, et al. BRAF inhibitor-induced antitumoral granulomatous dermatitis eruption in advanced melanoma. Am J Dermatopathol. 2015;37:795-798.
- Park JJ, Hawryluk EB, Tahan SR, et al. Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma. JAMA Dermatol. 2014;150:307‐311.
- Ong ELH, Sinha R, Jmor S, et al. BRAF inhibitor-associated granulomatous dermatitis: a report of 3 cases. Am J of Dermatopathol. 2019;41:214-217.
- Wali GN, Stonard C, Espinosa O, et al. Persistent granulomatous cutaneous drug eruption to a BRAF inhibitor. J Am Acad Dermatol. 2017;76(suppl 1):AB195.
- Aj lafolla M, Ramsay J, Wismer J, et al. Cobimetinib- and vemurafenib-induced granulomatous dermatitis and erythema induratum: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847358
- Jansen YJ, Janssens P, Hoorens A, et al. Granulomatous nephritis and dermatitis in a patient with BRAF V600E mutant metastatic melanoma treated with dabrafenib and trametinib. Melanoma Res. 2015;25:550‐554.
- Green JS, Norris DA, Wisell J. Novel cutaneous effects of combination chemotherapy with BRAF and MEK inhibitors: a report of two cases. Br J Dermatol. 2013;169:172-176.
- Chen L, His A, Kothari A, et al. Granulomatous dermatitis secondary to vemurafenib in a child with Langerhans cell histiocytosis. Pediatr Dermatol. 2018;35:E402-E403.
- Mackiewicz J, Mackiewicz A. BRAF and MEK inhibitors in the era of immunotherapy in melanoma patients. Comtemp Oncol (Pozn). 2018;22:68-72.
- Jovanovic B, Krockel D, Linden D, et al. Lack of cytoplasmic ERK activation is an independent adverse prognostic factor in primary cutaneous melanoma. J Invest Dermatol. 2008;128:2696-2704.
- Alqathama A. BRAF in malignant melanoma progression and metastasis: potentials and challenges. Am J Cancer Res. 2020;10:1103-1114.
- Zimmer L, Hillen U, Livingstone E, et al. Atypical melanocytic proliferations and new primary melanomas in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol. 2012;30:2375-2383.
- Casey D, Demko S, Sinha A, et al. FDA approval summary: selumetinib for plexiform neurofibroma. Clin Cancer Res. 2021;27;4142-4146
- Flaherty K, Davies MA, Grob JJ, et al. Genomic analysis and 3-y efficacy and safety update of COMBI-d: a phase 3 study of dabrafenib (D) fl trametinib (T) vs D monotherapy in patients (pts) with unresectable or metastatic BRAF V600E/K-mutant cutaneous melanoma. Abstract presented at: American Society of Clinical Oncology Annual Meeting; June 3-7, 2016; Chicago, IL. P9502.
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
- Robert C, Karaszewska B, Schachter J, et al. Three-year estimate of overall survival in COMBI-v, a randomized phase 3 study evaluating first-line dabrafenib (D) + trametinib (T) in patients (pts) with unresectable or metastatic BRAF V600E/K–mutant cutaneous melanoma. Ann Oncol. 2016;27(suppl 6):vi552-vi587.
- Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867-1876.
- Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advance BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomized, double-blind, phase 3 trial. Lancet Once. 2016;17:1248-1260.
- Kocsis J, Árokszállási A, András C, et al. Combined dabrafenib and trametinib treatment in a case of chemotherapy-refractory extrahepatic BRAF V600E mutant cholangiocarcinoma: dramatic clinical and radiological response with a confusing synchronic new liver lesion. J Gastrointest Oncol. 2017;8:E32-E38.
- Mangat PK, Halabi S, Bruinooge SS, et al. Rationale and design of the Targeted Agent and Profiling Utilization Registry (TAPUR) Study [published online July 11, 2018]. JCO Precis Oncol. doi:10.1200/PO.18.00122
- Terada T, Ashida K, Endo K, et al. c-erbB-2 protein is expressed in hepatolithiasis and cholangiocarcinoma. Histopathology. 1998;33:325-331.
- Tannapfel A, Benicke M, Katalinic A, et al. Frequency of p16INK4A alterations and K-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut. 2000;47:721-727.
- Momoi H, Itoh T, Nozaki Y, et al. Microsatellite instability and alternative genetic pathway in intrahepatic cholangiocarcinoma. J Hepatol. 2001;35:235-244.
- Terada T, Nakanuma Y, Sirica AE. Immunohistochemical demonstration of MET overexpression in human intrahepatic cholangiocarcinoma and in hepatolithiasis. Hum Pathol. 1998;29:175-180.
- Tannapfel A, Sommerer F, Benicke M, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52:706-712.
- Bunyatov T, Zhao A, Kovalenko J, et al. Personalised approach in combined treatment of cholangiocarcinoma: a case report of healing from cholangiocellular carcinoma at stage IV. J Gastrointest Oncol. 2019;10:815-820.
- Lavingia V, Fakih M. Impressive response to dual BRAF and MEK inhibition in patients with BRAF mutant intrahepatic cholangiocarcinoma-2 case reports and a brief review. J Gastrointest Oncol. 2016;7:E98-E102.
- Loaiza-Bonilla A, Clayton E, Furth E, et al. Dramatic response to dabrafenib and trametinib combination in a BRAF V600E-mutated cholangiocarcinoma: implementation of a molecular tumour board and next-generation sequencing for personalized medicine. Ecancermedicalscience. 2014;8:479.
- Rosenbach M, English JC. Reactive granulomatous dermatitis. Dermatol Clin. 2015;33:373-387.
- Tomasini C, Pippione M. Interstitial granulomatous dermatitis with plaques. J Am Acad Dermatol. 2002;46:892-899.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol 2012;166:775-783.
- Calonje JE, Brenn T, Lazar A, Billings S. Lichenoid and interface dermatitis. In: McKee’s Pathology of the Skin. 5th ed. China: Elsevier Limited: 2018;7:241-282.
- Gkiozos I, Kopitopoulou A, Kalkanis A, et al. Sarcoidosis-like reactions induced by checkpoint inhibitors. J Thorac Oncol. 2018;13:1076-1082.
- Tetzlaff MT, Nelson KC, Diab A, et al. Granulomatous/sarcoid-like lesions associated with checkpoint inhibitors: a marker of therapy response in a subset of melanoma patients. J Immunother Cancer. 2018;6:14.
- Garrido MC, Gutiérrez C, Riveiro-Falkenbach E, et al. BRAF inhibitor-induced antitumoral granulomatous dermatitis eruption in advanced melanoma. Am J Dermatopathol. 2015;37:795-798.
- Park JJ, Hawryluk EB, Tahan SR, et al. Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma. JAMA Dermatol. 2014;150:307‐311.
- Ong ELH, Sinha R, Jmor S, et al. BRAF inhibitor-associated granulomatous dermatitis: a report of 3 cases. Am J of Dermatopathol. 2019;41:214-217.
- Wali GN, Stonard C, Espinosa O, et al. Persistent granulomatous cutaneous drug eruption to a BRAF inhibitor. J Am Acad Dermatol. 2017;76(suppl 1):AB195.
- Aj lafolla M, Ramsay J, Wismer J, et al. Cobimetinib- and vemurafenib-induced granulomatous dermatitis and erythema induratum: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847358
- Jansen YJ, Janssens P, Hoorens A, et al. Granulomatous nephritis and dermatitis in a patient with BRAF V600E mutant metastatic melanoma treated with dabrafenib and trametinib. Melanoma Res. 2015;25:550‐554.
- Green JS, Norris DA, Wisell J. Novel cutaneous effects of combination chemotherapy with BRAF and MEK inhibitors: a report of two cases. Br J Dermatol. 2013;169:172-176.
- Chen L, His A, Kothari A, et al. Granulomatous dermatitis secondary to vemurafenib in a child with Langerhans cell histiocytosis. Pediatr Dermatol. 2018;35:E402-E403.
Practice Points
- Granulomatous dermatitis (GD) is a potential rare side effect of the use of BRAF and MEK inhibitors for the treatment of BRAF V600 mutation–positive cancers, including metastatic cholangiocarcinoma.
- Granulomatous dermatitis can resolve despite continuation of BRAF and MEK inhibitor therapies.
- Histologically, GD can appear similar to disease recurrence. It is imperative that clinicians and pathologists recognize the cutaneous manifestations of BRAF and MEK inhibitors.
Concurrent Atopic Dermatitis and Psoriasis Successfully Treated With Dual Biologic Therapy
Atopic dermatitis (AD) and psoriasis are common skin diseases in which dysfunction of the epidermal barrier leads to skin inflammation and altered expression of proinflammatory cytokines.1 There often is overlap in the clinical and histopathologic features of AD and psoriasis, which can make diagnosis a challenge. Persistent late-stage AD can present with psoriasiform lichenified changes, and psoriasis lesions in the acute stage can have an eczematous appearance.2 Histologically, chronic psoriasis lesions share many overlapping features with AD, and some subsets of AD with IL-17 predominance (ie, intrinsic, pediatric, presentation in Asian patients) exhibit a psoriasiform appearance.3,4
Atopic dermatitis and psoriasis are considered 2 distinct conditions because AD is a helper T cell (TH2)–driven disease with subsequent overproduction of IL-4 and IL-13 and psoriasis is a TH17 cell–driven disease with overproduction of IL-173; however, the shared features of AD and psoriasis represent an underlying immunopathological spectrum2,5,6 in which one condition can develop following treatment of the other condition (immunological shift in pathways), both conditions can occur at different times in a patient’s life with alternating cycles of disease flares, or both conditions can coexist as an overlapping syndrome.1,2 A retrospective study from 2012 to 2019 estimated the prevalence of concomitant AD and psoriasis in the United States at 1.3%, with AD following the diagnosis of psoriasis in 67% of cases.1 Concurrent AD and psoriasis—when both diseases flaresimultaneously—is the rarest scenario.2,5
Treatment modalities for AD include topical corticosteroids, which act on immune cells to suppress the release of proinflammatory cytokines, as well as dupilumab, which offers targeted blockade of involved cytokines IL-4 and IL-13. Psoriasis can be treated with multiple immune modulators, including topical corticosteroids and vitamin D analogs, as well as systemic medications that reduce T-cell activation and inflammatory cytokines through targeting of IFN-γ, IL-2, tumor necrosis factor α, IL-17, and IL-23.7,8
We present the case of a patient with long-standing concurrent, treatment-resistant AD and psoriasis who was successfully treated with dual biologic therapy with guselkumab and dupilumab.
Case Report
A 62-year-old woman presented to our dermatology clinic with red itchy scales and painful fissures on the palms, hands, and soles of more than 12 years’ duration. Her medical history included an allergy to amoxicillin-clavulanate as well as an allergy to both dog and cat dander on prick testing. Her family history included dyshidrotic eczema in her mother. A complete blood cell count with differential was within reference range. A shave biopsy of the right dorsal hand performed at the onset of symptoms at an outside facility revealed hyperkeratotic acanthotic epidermis with a mild perivascular lymphocytic infiltrate.
Results of patch testing indicated contact hypersensitivity to the botanical rosin colophonium (or colophony); carba mix (1, 3-diphenylguanidine, zinc dibutyldithiocarbamate, and zinc diethydithiocarbamate); thiuram mix (tetramethylthiuram disulfide, tetramethylthiuram monosulfide, and tetraethylthiuram disulfide); n,n-diphenylguanidine; and tixocortol-21-pivalate. Our patient was given guidance on avoiding these agents, as it was suspected that exposure may be exacerbating the psoriasis. The psoriasis was treated with topical corticosteroids, keratolytics, and calcineurin inhibitors, all of which offered minimal or no relief. Trials of systemic agents, including methotrexate (discontinued because transaminitis developed), etanercept, adalimumab, and apremilast for 6 to 10 months did not provide improvement.
Two years prior to the current presentation, our patient had been treated with the IL-23 inhibitor guselkumab, which provided moderate improvement. When she presented to our clinic, physical examination while she was taking guselkumab demonstrated prurigo with excoriations of the extremities, hyperkeratosis with scaling and fissures of the soles, erythematous scaly plaques on the palms and dorsal surface of the hands, and mild onycholysis of the nails (Figures 1 and 2). Because we were concerned about concomitant intrinsic AD, dupilumab was initiated in conjunction with guselkumab. A second biopsy was considered but deferred in favor of clinical monitoring.
After 1 year of dual biologic therapy, the patient experienced near-complete resolution of symptoms. The psoriasis completely resolved from an initial body surface area of 5%, and the AD body surface area decreased from 30% to 2% (Figure 3). The patient reported no adverse effects from treatment.
Comment
Atopic dermatitis and psoriasis involve complex immunopathology and a spectrum of cytokines that might explain the overlap in their clinical and histopathologic presentations.
Atopic dermatitis—Atopic dermatitis involves TH1, TH2, TH9, TH17, and TH22 cells; TH2 cells release IL-4, IL-5, and IL-13, all of which are key cytokines in the inflammatory pathway of AD.9,10 Activation of the helper T-cell subset and the release of cytokines differ slightly based on the subcategory of AD and the stage of exacerbation. In addition to TH2-cell activation, TH1 cells and TH22 cells—which release IL-12 and IL-22, respectively—are active in both intrinsic and extrinsic AD. TH17 cells and TH9 cells—which release IL-17 and IL-9, respectively—are more prominent in the intrinsic pathway than in the extrinsic pathway.9 Intrinsic AD is recognized by a lack of eosinophilia, female predominance, and delayed onset compared to extrinsic AD; there also is a lack of history of atopy.1 Extrinsic AD is characterized by eosinophilia as well as a personal and family history of atopy.11 Our patient—a female with onset in older adulthood, lack of eosinophilia, and a family history of atopy—displayed features of both intrinsic and extrinsic AD.
Psoriasis—The immunopathology of psoriasis involves stimulation of dendritic cells, which activate TH17 cells through IL-23. TH17 cells then release IL-17 and IL-22. Therefore, both AD and psoriasis involve activation of TH22 and TH1 cells, with increased IL-17 and IL-22 production.3,10,12 IL-17 and IL-22 induce epidermal hyperplasia; IL-22 also contributes to skin barrier dysfunction.12 Therefore, it might be reasonable to consider psoriasis and AD as diseases that exist across a T-cell axis spectrum, thereby accounting for some overlap in disease characteristics.3
Dual Biologic Therapy—Dupilumab blocks the IL-4 receptor α subunit, a receptor for IL-4 and IL-13, which are key cytokines in the pathogenesis of AD.10 Guselkumab inhibits IL-23, thus blocking the inflammatory cascade of TH17 cell activation and release of IL-17 and IL-22 in the psoriasis pathway.13 Although an immunopathological spectrum exists between the 2 diseases, the continued presence of AD symptoms after blocking the IL-23 cascade suggests that additional blockade of TH2 cells is required to control AD in patients with true concurrent disease.
Accurate diagnosis of AD and/or psoriasis is important when considering targeted treatment of these conditions with biologics. The use of dual biologics is limited by a paucity of data regarding the safety of these agents when given in combination. A recent meta-analysis of dual biologic therapy in patients with inflammatory bowel disease demonstrated acceptable safety results with a pooled adverse reaction rate of 31%.14
Anchoring Bias—Anchoring bias can occur when a clinician’s decisions are influenced by a particular event or reference point, which might cause them to disregard subsequent evidence. Our case illustrates the importance of critically assessing the response to treatment and being mindful of the potential influence of anchoring bias on the differential diagnosis. Although overcoming biases in conditions with clinical overlap can be challenging, it is important to consider coexisting AD and psoriasis in patients with extensive hand involvement when multiple treatments have failed and only a partial response to targeted pathways has been achieved. In our case, the patient also had contact hypersensitivity to tixocortol-21-pivalate, which indicates hypersensitivity to many prescription topical corticosteroids, oral prednisone, and over-the-counter hydrocortisone; however, topical corticosteroids continued to be prescribed for her, which might have contributed to the lack of improvement and even exacerbated the rash.
Future Considerations—A consideration for the future in this case is discontinuing guselkumab to observe whether symptoms recur. We discussed this option with the patient, but she opted to continue treatment with dupilumab and guselkumab because of the symptom resolution.
Conclusion
Concomitant disease can present as an overlapping pattern in the same area, whereas other regions might have geographically isolated disease. Our patient’s overlap of symptoms, the failure of multiple treatments, and the partial improvement she experienced on guselkumab made diagnosis and management challenging; however, dual biologic therapy was successful.
- Barry K, Zancanaro P, Casseres R, et al. Concomitant atopic dermatitis and psoriasis—a retrospective review. J Dermatolog Treat. 2021;32:716-720. doi:10.1080/09546634.2019.1702147
- Bozek A, Zajac M, Krupka M. Atopic dermatitis and psoriasis as overlapping syndromes. Mediators Inflamm. 2020;2020:7527859. doi:10.1155/2020/7527859
- Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:68-73. doi:10.1016/j.coi.2017.08.008
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Docampo A, MJ, I, et al. Response to letter to the editor: ‘psoriasis dermatitis: an overlap condition of psoriasis and atopic dermatitis in children.’ J Eur Acad Dermatol Venereol. 2019;33:E410-E412. doi:10.1111/jdv.15716
- Johnson MC, Bowers NL, Strowd LC. Concurrent atopic dermatitis and psoriasis vulgaris: implications for targeted biologic therapy. Cutis. 2022;109:110-112. doi:10.12788/cutis.0453
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Klonowska J, Glen J, Nowicki RJ, et al. New cytokines in the pathogenesis of atopic dermatitis—new therapeutic targets. Int J Mol Sci. 2018;19:3086. doi:10.3390/ijms19103086
- Ratchataswan T, Banzon TM, Thyssen JP, et al. Biologics for treatment of atopic dermatitis: current status and future prospect. J Allergy Clin Immunol Pract. 2021;9:1053-1065. doi:10.1016/j.jaip.2020.11.034
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Tokuyama M, Mabuchi T. New treatment addressing the pathogenesis of psoriasis. Int J Mol Sci. 2020;21:7488. doi:10.3390/ijms21207488
- Gordon KB, Armstrong AW, Foley P, et al. Guselkumab efficacy after withdrawal is associated with suppression of serum IL-23-regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 study. J Invest Dermatol. 2019;139:2437-2446.e1. doi:10.1016/j.jid.2019.05.016
- Gold SL, Steinlauf AF. Efficacy and safety of dual biologic therapy in patients with inflammatory bowel disease: a review of the literature. Gastroenterol Hepatol (N Y). 2021;17:406-414.
Atopic dermatitis (AD) and psoriasis are common skin diseases in which dysfunction of the epidermal barrier leads to skin inflammation and altered expression of proinflammatory cytokines.1 There often is overlap in the clinical and histopathologic features of AD and psoriasis, which can make diagnosis a challenge. Persistent late-stage AD can present with psoriasiform lichenified changes, and psoriasis lesions in the acute stage can have an eczematous appearance.2 Histologically, chronic psoriasis lesions share many overlapping features with AD, and some subsets of AD with IL-17 predominance (ie, intrinsic, pediatric, presentation in Asian patients) exhibit a psoriasiform appearance.3,4
Atopic dermatitis and psoriasis are considered 2 distinct conditions because AD is a helper T cell (TH2)–driven disease with subsequent overproduction of IL-4 and IL-13 and psoriasis is a TH17 cell–driven disease with overproduction of IL-173; however, the shared features of AD and psoriasis represent an underlying immunopathological spectrum2,5,6 in which one condition can develop following treatment of the other condition (immunological shift in pathways), both conditions can occur at different times in a patient’s life with alternating cycles of disease flares, or both conditions can coexist as an overlapping syndrome.1,2 A retrospective study from 2012 to 2019 estimated the prevalence of concomitant AD and psoriasis in the United States at 1.3%, with AD following the diagnosis of psoriasis in 67% of cases.1 Concurrent AD and psoriasis—when both diseases flaresimultaneously—is the rarest scenario.2,5
Treatment modalities for AD include topical corticosteroids, which act on immune cells to suppress the release of proinflammatory cytokines, as well as dupilumab, which offers targeted blockade of involved cytokines IL-4 and IL-13. Psoriasis can be treated with multiple immune modulators, including topical corticosteroids and vitamin D analogs, as well as systemic medications that reduce T-cell activation and inflammatory cytokines through targeting of IFN-γ, IL-2, tumor necrosis factor α, IL-17, and IL-23.7,8
We present the case of a patient with long-standing concurrent, treatment-resistant AD and psoriasis who was successfully treated with dual biologic therapy with guselkumab and dupilumab.
Case Report
A 62-year-old woman presented to our dermatology clinic with red itchy scales and painful fissures on the palms, hands, and soles of more than 12 years’ duration. Her medical history included an allergy to amoxicillin-clavulanate as well as an allergy to both dog and cat dander on prick testing. Her family history included dyshidrotic eczema in her mother. A complete blood cell count with differential was within reference range. A shave biopsy of the right dorsal hand performed at the onset of symptoms at an outside facility revealed hyperkeratotic acanthotic epidermis with a mild perivascular lymphocytic infiltrate.
Results of patch testing indicated contact hypersensitivity to the botanical rosin colophonium (or colophony); carba mix (1, 3-diphenylguanidine, zinc dibutyldithiocarbamate, and zinc diethydithiocarbamate); thiuram mix (tetramethylthiuram disulfide, tetramethylthiuram monosulfide, and tetraethylthiuram disulfide); n,n-diphenylguanidine; and tixocortol-21-pivalate. Our patient was given guidance on avoiding these agents, as it was suspected that exposure may be exacerbating the psoriasis. The psoriasis was treated with topical corticosteroids, keratolytics, and calcineurin inhibitors, all of which offered minimal or no relief. Trials of systemic agents, including methotrexate (discontinued because transaminitis developed), etanercept, adalimumab, and apremilast for 6 to 10 months did not provide improvement.

Two years prior to the current presentation, our patient had been treated with the IL-23 inhibitor guselkumab, which provided moderate improvement. When she presented to our clinic, physical examination while she was taking guselkumab demonstrated prurigo with excoriations of the extremities, hyperkeratosis with scaling and fissures of the soles, erythematous scaly plaques on the palms and dorsal surface of the hands, and mild onycholysis of the nails (Figures 1 and 2). Because we were concerned about concomitant intrinsic AD, dupilumab was initiated in conjunction with guselkumab. A second biopsy was considered but deferred in favor of clinical monitoring.

After 1 year of dual biologic therapy, the patient experienced near-complete resolution of symptoms. The psoriasis completely resolved from an initial body surface area of 5%, and the AD body surface area decreased from 30% to 2% (Figure 3). The patient reported no adverse effects from treatment.

Comment
Atopic dermatitis and psoriasis involve complex immunopathology and a spectrum of cytokines that might explain the overlap in their clinical and histopathologic presentations.
Atopic dermatitis—Atopic dermatitis involves TH1, TH2, TH9, TH17, and TH22 cells; TH2 cells release IL-4, IL-5, and IL-13, all of which are key cytokines in the inflammatory pathway of AD.9,10 Activation of the helper T-cell subset and the release of cytokines differ slightly based on the subcategory of AD and the stage of exacerbation. In addition to TH2-cell activation, TH1 cells and TH22 cells—which release IL-12 and IL-22, respectively—are active in both intrinsic and extrinsic AD. TH17 cells and TH9 cells—which release IL-17 and IL-9, respectively—are more prominent in the intrinsic pathway than in the extrinsic pathway.9 Intrinsic AD is recognized by a lack of eosinophilia, female predominance, and delayed onset compared to extrinsic AD; there also is a lack of history of atopy.1 Extrinsic AD is characterized by eosinophilia as well as a personal and family history of atopy.11 Our patient—a female with onset in older adulthood, lack of eosinophilia, and a family history of atopy—displayed features of both intrinsic and extrinsic AD.
Psoriasis—The immunopathology of psoriasis involves stimulation of dendritic cells, which activate TH17 cells through IL-23. TH17 cells then release IL-17 and IL-22. Therefore, both AD and psoriasis involve activation of TH22 and TH1 cells, with increased IL-17 and IL-22 production.3,10,12 IL-17 and IL-22 induce epidermal hyperplasia; IL-22 also contributes to skin barrier dysfunction.12 Therefore, it might be reasonable to consider psoriasis and AD as diseases that exist across a T-cell axis spectrum, thereby accounting for some overlap in disease characteristics.3
Dual Biologic Therapy—Dupilumab blocks the IL-4 receptor α subunit, a receptor for IL-4 and IL-13, which are key cytokines in the pathogenesis of AD.10 Guselkumab inhibits IL-23, thus blocking the inflammatory cascade of TH17 cell activation and release of IL-17 and IL-22 in the psoriasis pathway.13 Although an immunopathological spectrum exists between the 2 diseases, the continued presence of AD symptoms after blocking the IL-23 cascade suggests that additional blockade of TH2 cells is required to control AD in patients with true concurrent disease.
Accurate diagnosis of AD and/or psoriasis is important when considering targeted treatment of these conditions with biologics. The use of dual biologics is limited by a paucity of data regarding the safety of these agents when given in combination. A recent meta-analysis of dual biologic therapy in patients with inflammatory bowel disease demonstrated acceptable safety results with a pooled adverse reaction rate of 31%.14
Anchoring Bias—Anchoring bias can occur when a clinician’s decisions are influenced by a particular event or reference point, which might cause them to disregard subsequent evidence. Our case illustrates the importance of critically assessing the response to treatment and being mindful of the potential influence of anchoring bias on the differential diagnosis. Although overcoming biases in conditions with clinical overlap can be challenging, it is important to consider coexisting AD and psoriasis in patients with extensive hand involvement when multiple treatments have failed and only a partial response to targeted pathways has been achieved. In our case, the patient also had contact hypersensitivity to tixocortol-21-pivalate, which indicates hypersensitivity to many prescription topical corticosteroids, oral prednisone, and over-the-counter hydrocortisone; however, topical corticosteroids continued to be prescribed for her, which might have contributed to the lack of improvement and even exacerbated the rash.
Future Considerations—A consideration for the future in this case is discontinuing guselkumab to observe whether symptoms recur. We discussed this option with the patient, but she opted to continue treatment with dupilumab and guselkumab because of the symptom resolution.
Conclusion
Concomitant disease can present as an overlapping pattern in the same area, whereas other regions might have geographically isolated disease. Our patient’s overlap of symptoms, the failure of multiple treatments, and the partial improvement she experienced on guselkumab made diagnosis and management challenging; however, dual biologic therapy was successful.
Atopic dermatitis (AD) and psoriasis are common skin diseases in which dysfunction of the epidermal barrier leads to skin inflammation and altered expression of proinflammatory cytokines.1 There often is overlap in the clinical and histopathologic features of AD and psoriasis, which can make diagnosis a challenge. Persistent late-stage AD can present with psoriasiform lichenified changes, and psoriasis lesions in the acute stage can have an eczematous appearance.2 Histologically, chronic psoriasis lesions share many overlapping features with AD, and some subsets of AD with IL-17 predominance (ie, intrinsic, pediatric, presentation in Asian patients) exhibit a psoriasiform appearance.3,4
Atopic dermatitis and psoriasis are considered 2 distinct conditions because AD is a helper T cell (TH2)–driven disease with subsequent overproduction of IL-4 and IL-13 and psoriasis is a TH17 cell–driven disease with overproduction of IL-173; however, the shared features of AD and psoriasis represent an underlying immunopathological spectrum2,5,6 in which one condition can develop following treatment of the other condition (immunological shift in pathways), both conditions can occur at different times in a patient’s life with alternating cycles of disease flares, or both conditions can coexist as an overlapping syndrome.1,2 A retrospective study from 2012 to 2019 estimated the prevalence of concomitant AD and psoriasis in the United States at 1.3%, with AD following the diagnosis of psoriasis in 67% of cases.1 Concurrent AD and psoriasis—when both diseases flaresimultaneously—is the rarest scenario.2,5
Treatment modalities for AD include topical corticosteroids, which act on immune cells to suppress the release of proinflammatory cytokines, as well as dupilumab, which offers targeted blockade of involved cytokines IL-4 and IL-13. Psoriasis can be treated with multiple immune modulators, including topical corticosteroids and vitamin D analogs, as well as systemic medications that reduce T-cell activation and inflammatory cytokines through targeting of IFN-γ, IL-2, tumor necrosis factor α, IL-17, and IL-23.7,8
We present the case of a patient with long-standing concurrent, treatment-resistant AD and psoriasis who was successfully treated with dual biologic therapy with guselkumab and dupilumab.
Case Report
A 62-year-old woman presented to our dermatology clinic with red itchy scales and painful fissures on the palms, hands, and soles of more than 12 years’ duration. Her medical history included an allergy to amoxicillin-clavulanate as well as an allergy to both dog and cat dander on prick testing. Her family history included dyshidrotic eczema in her mother. A complete blood cell count with differential was within reference range. A shave biopsy of the right dorsal hand performed at the onset of symptoms at an outside facility revealed hyperkeratotic acanthotic epidermis with a mild perivascular lymphocytic infiltrate.
Results of patch testing indicated contact hypersensitivity to the botanical rosin colophonium (or colophony); carba mix (1, 3-diphenylguanidine, zinc dibutyldithiocarbamate, and zinc diethydithiocarbamate); thiuram mix (tetramethylthiuram disulfide, tetramethylthiuram monosulfide, and tetraethylthiuram disulfide); n,n-diphenylguanidine; and tixocortol-21-pivalate. Our patient was given guidance on avoiding these agents, as it was suspected that exposure may be exacerbating the psoriasis. The psoriasis was treated with topical corticosteroids, keratolytics, and calcineurin inhibitors, all of which offered minimal or no relief. Trials of systemic agents, including methotrexate (discontinued because transaminitis developed), etanercept, adalimumab, and apremilast for 6 to 10 months did not provide improvement.

Two years prior to the current presentation, our patient had been treated with the IL-23 inhibitor guselkumab, which provided moderate improvement. When she presented to our clinic, physical examination while she was taking guselkumab demonstrated prurigo with excoriations of the extremities, hyperkeratosis with scaling and fissures of the soles, erythematous scaly plaques on the palms and dorsal surface of the hands, and mild onycholysis of the nails (Figures 1 and 2). Because we were concerned about concomitant intrinsic AD, dupilumab was initiated in conjunction with guselkumab. A second biopsy was considered but deferred in favor of clinical monitoring.

After 1 year of dual biologic therapy, the patient experienced near-complete resolution of symptoms. The psoriasis completely resolved from an initial body surface area of 5%, and the AD body surface area decreased from 30% to 2% (Figure 3). The patient reported no adverse effects from treatment.

Comment
Atopic dermatitis and psoriasis involve complex immunopathology and a spectrum of cytokines that might explain the overlap in their clinical and histopathologic presentations.
Atopic dermatitis—Atopic dermatitis involves TH1, TH2, TH9, TH17, and TH22 cells; TH2 cells release IL-4, IL-5, and IL-13, all of which are key cytokines in the inflammatory pathway of AD.9,10 Activation of the helper T-cell subset and the release of cytokines differ slightly based on the subcategory of AD and the stage of exacerbation. In addition to TH2-cell activation, TH1 cells and TH22 cells—which release IL-12 and IL-22, respectively—are active in both intrinsic and extrinsic AD. TH17 cells and TH9 cells—which release IL-17 and IL-9, respectively—are more prominent in the intrinsic pathway than in the extrinsic pathway.9 Intrinsic AD is recognized by a lack of eosinophilia, female predominance, and delayed onset compared to extrinsic AD; there also is a lack of history of atopy.1 Extrinsic AD is characterized by eosinophilia as well as a personal and family history of atopy.11 Our patient—a female with onset in older adulthood, lack of eosinophilia, and a family history of atopy—displayed features of both intrinsic and extrinsic AD.
Psoriasis—The immunopathology of psoriasis involves stimulation of dendritic cells, which activate TH17 cells through IL-23. TH17 cells then release IL-17 and IL-22. Therefore, both AD and psoriasis involve activation of TH22 and TH1 cells, with increased IL-17 and IL-22 production.3,10,12 IL-17 and IL-22 induce epidermal hyperplasia; IL-22 also contributes to skin barrier dysfunction.12 Therefore, it might be reasonable to consider psoriasis and AD as diseases that exist across a T-cell axis spectrum, thereby accounting for some overlap in disease characteristics.3
Dual Biologic Therapy—Dupilumab blocks the IL-4 receptor α subunit, a receptor for IL-4 and IL-13, which are key cytokines in the pathogenesis of AD.10 Guselkumab inhibits IL-23, thus blocking the inflammatory cascade of TH17 cell activation and release of IL-17 and IL-22 in the psoriasis pathway.13 Although an immunopathological spectrum exists between the 2 diseases, the continued presence of AD symptoms after blocking the IL-23 cascade suggests that additional blockade of TH2 cells is required to control AD in patients with true concurrent disease.
Accurate diagnosis of AD and/or psoriasis is important when considering targeted treatment of these conditions with biologics. The use of dual biologics is limited by a paucity of data regarding the safety of these agents when given in combination. A recent meta-analysis of dual biologic therapy in patients with inflammatory bowel disease demonstrated acceptable safety results with a pooled adverse reaction rate of 31%.14
Anchoring Bias—Anchoring bias can occur when a clinician’s decisions are influenced by a particular event or reference point, which might cause them to disregard subsequent evidence. Our case illustrates the importance of critically assessing the response to treatment and being mindful of the potential influence of anchoring bias on the differential diagnosis. Although overcoming biases in conditions with clinical overlap can be challenging, it is important to consider coexisting AD and psoriasis in patients with extensive hand involvement when multiple treatments have failed and only a partial response to targeted pathways has been achieved. In our case, the patient also had contact hypersensitivity to tixocortol-21-pivalate, which indicates hypersensitivity to many prescription topical corticosteroids, oral prednisone, and over-the-counter hydrocortisone; however, topical corticosteroids continued to be prescribed for her, which might have contributed to the lack of improvement and even exacerbated the rash.
Future Considerations—A consideration for the future in this case is discontinuing guselkumab to observe whether symptoms recur. We discussed this option with the patient, but she opted to continue treatment with dupilumab and guselkumab because of the symptom resolution.
Conclusion
Concomitant disease can present as an overlapping pattern in the same area, whereas other regions might have geographically isolated disease. Our patient’s overlap of symptoms, the failure of multiple treatments, and the partial improvement she experienced on guselkumab made diagnosis and management challenging; however, dual biologic therapy was successful.
- Barry K, Zancanaro P, Casseres R, et al. Concomitant atopic dermatitis and psoriasis—a retrospective review. J Dermatolog Treat. 2021;32:716-720. doi:10.1080/09546634.2019.1702147
- Bozek A, Zajac M, Krupka M. Atopic dermatitis and psoriasis as overlapping syndromes. Mediators Inflamm. 2020;2020:7527859. doi:10.1155/2020/7527859
- Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:68-73. doi:10.1016/j.coi.2017.08.008
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Docampo A, MJ, I, et al. Response to letter to the editor: ‘psoriasis dermatitis: an overlap condition of psoriasis and atopic dermatitis in children.’ J Eur Acad Dermatol Venereol. 2019;33:E410-E412. doi:10.1111/jdv.15716
- Johnson MC, Bowers NL, Strowd LC. Concurrent atopic dermatitis and psoriasis vulgaris: implications for targeted biologic therapy. Cutis. 2022;109:110-112. doi:10.12788/cutis.0453
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Klonowska J, Glen J, Nowicki RJ, et al. New cytokines in the pathogenesis of atopic dermatitis—new therapeutic targets. Int J Mol Sci. 2018;19:3086. doi:10.3390/ijms19103086
- Ratchataswan T, Banzon TM, Thyssen JP, et al. Biologics for treatment of atopic dermatitis: current status and future prospect. J Allergy Clin Immunol Pract. 2021;9:1053-1065. doi:10.1016/j.jaip.2020.11.034
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Tokuyama M, Mabuchi T. New treatment addressing the pathogenesis of psoriasis. Int J Mol Sci. 2020;21:7488. doi:10.3390/ijms21207488
- Gordon KB, Armstrong AW, Foley P, et al. Guselkumab efficacy after withdrawal is associated with suppression of serum IL-23-regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 study. J Invest Dermatol. 2019;139:2437-2446.e1. doi:10.1016/j.jid.2019.05.016
- Gold SL, Steinlauf AF. Efficacy and safety of dual biologic therapy in patients with inflammatory bowel disease: a review of the literature. Gastroenterol Hepatol (N Y). 2021;17:406-414.
- Barry K, Zancanaro P, Casseres R, et al. Concomitant atopic dermatitis and psoriasis—a retrospective review. J Dermatolog Treat. 2021;32:716-720. doi:10.1080/09546634.2019.1702147
- Bozek A, Zajac M, Krupka M. Atopic dermatitis and psoriasis as overlapping syndromes. Mediators Inflamm. 2020;2020:7527859. doi:10.1155/2020/7527859
- Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:68-73. doi:10.1016/j.coi.2017.08.008
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Docampo A, MJ, I, et al. Response to letter to the editor: ‘psoriasis dermatitis: an overlap condition of psoriasis and atopic dermatitis in children.’ J Eur Acad Dermatol Venereol. 2019;33:E410-E412. doi:10.1111/jdv.15716
- Johnson MC, Bowers NL, Strowd LC. Concurrent atopic dermatitis and psoriasis vulgaris: implications for targeted biologic therapy. Cutis. 2022;109:110-112. doi:10.12788/cutis.0453
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Klonowska J, Glen J, Nowicki RJ, et al. New cytokines in the pathogenesis of atopic dermatitis—new therapeutic targets. Int J Mol Sci. 2018;19:3086. doi:10.3390/ijms19103086
- Ratchataswan T, Banzon TM, Thyssen JP, et al. Biologics for treatment of atopic dermatitis: current status and future prospect. J Allergy Clin Immunol Pract. 2021;9:1053-1065. doi:10.1016/j.jaip.2020.11.034
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Tokuyama M, Mabuchi T. New treatment addressing the pathogenesis of psoriasis. Int J Mol Sci. 2020;21:7488. doi:10.3390/ijms21207488
- Gordon KB, Armstrong AW, Foley P, et al. Guselkumab efficacy after withdrawal is associated with suppression of serum IL-23-regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 study. J Invest Dermatol. 2019;139:2437-2446.e1. doi:10.1016/j.jid.2019.05.016
- Gold SL, Steinlauf AF. Efficacy and safety of dual biologic therapy in patients with inflammatory bowel disease: a review of the literature. Gastroenterol Hepatol (N Y). 2021;17:406-414.
Practice Points
- Atopic dermatitis and psoriasis can share clinical and histopathologic features, which represents their underlying immunopathologic spectrum.
- Atopic dermatitis and psoriasis can coexist in a single patient, which may be suspected from a clinical picture of treatment-resistant disease, a partial response to targeted therapies, or extensive hand involvement.
Endocrine Mucin-Producing Sweat Gland Carcinoma and Primary Cutaneous Mucinous Carcinoma: A Case Series
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) and
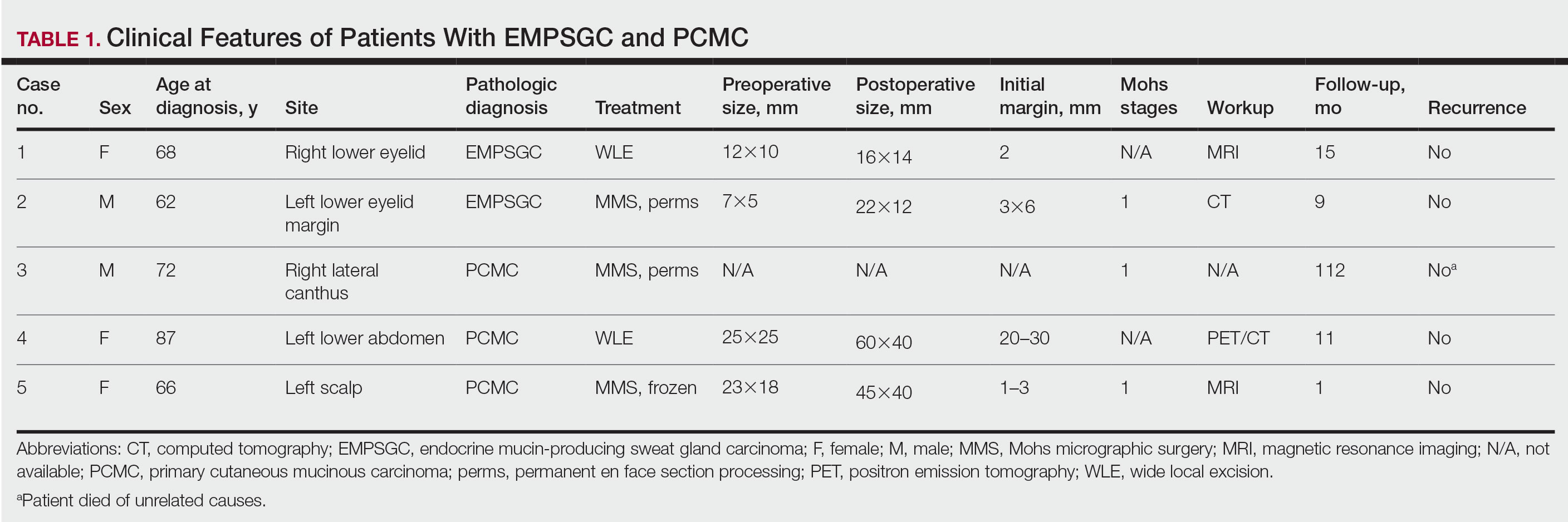
Methods
Following institutional review board approval, we conducted a retrospective, single-institution case series. We searched electronic medical records dating from 2000 to 2019 for tumors diagnosed as PCMC or extramammary Paget disease treated with MMS. We gathered demographic, clinical, pathologic, and follow-up information from the electronic medical records for each case (Tables 1 and 2). Two dermatopathologists (B.P. and B.F.K.) reviewed the hematoxylin and eosin–stained slides of each tumor as well as all available immunohistochemical stains. One of the reviewers (B.F.K.) is a board-certified dermatologist, dermatopathologist, and fellowship-trained Mohs surgeon.
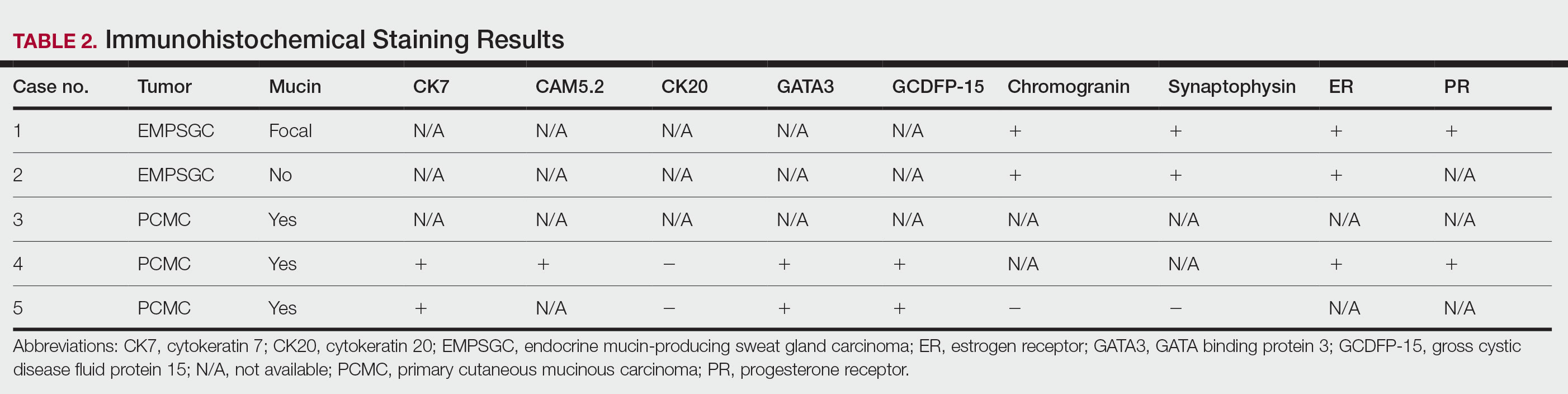
Results
Demographic and Clinical Information—We identified 2 cases of EMPSGC and 3 cases of PCMC diagnosed and treated at our institution; 4 of these cases had been treated within the last 2 years. One had been treated 18 years prior; case information was limited due to planned institutional record destruction. Three of the patients were female and 2 were male. The mean age at presentation was 71 years (range, 62–87 years). None had experienced recurrence or metastases after a mean follow-up of 30 months.
Case 1—A 68-year-old woman noted a slow-growing, flesh-colored papule measuring 12×10 mm on the right lower eyelid. An excisional biopsy was completed with 2-mm clinical margins, and the defect was closed in a linear fashion. Histologic sections demonstrated EMPSGC with uninvolved margins. The patient desired no further intervention and was clinically followed. Magnetic resonance imaging (MRI) of the head and neck found no evidence of metastasis. She has had no recurrence after 15 months.
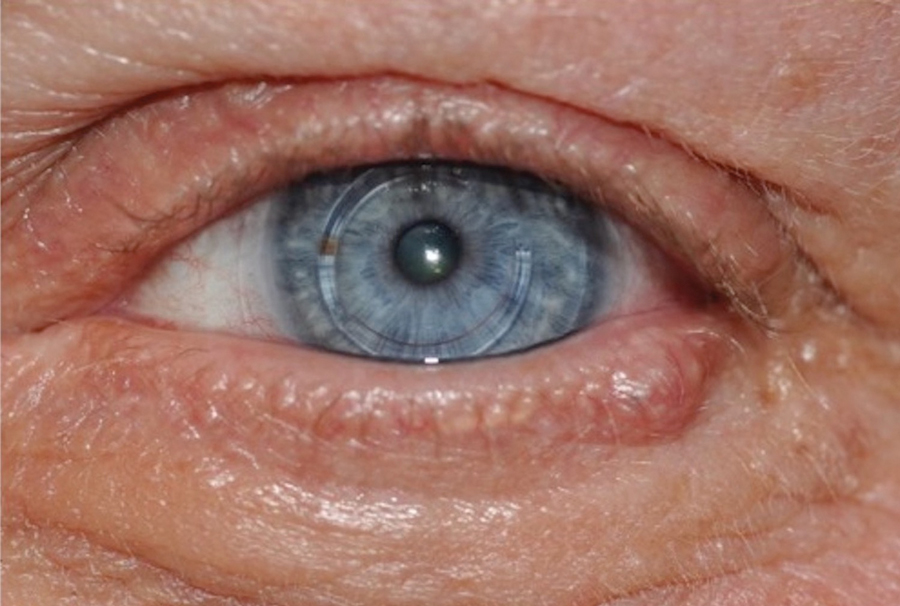
Case 2—A 62-year-old man presented with a 7×5-mm, flesh-colored papule on the left lower eyelid margin (Figure 1). It was previously treated conservatively as a hordeolum but was biopsied after it failed to resolve with 3-mm margins. Histopathology demonstrated an EMPSGC (Figure 2). The lesion was treated with modified MMS with permanent en face section processing and cleared after 1 stage. Computed tomography of the head and neck showed no abnormalities. He has had no recurrence after 9 months.
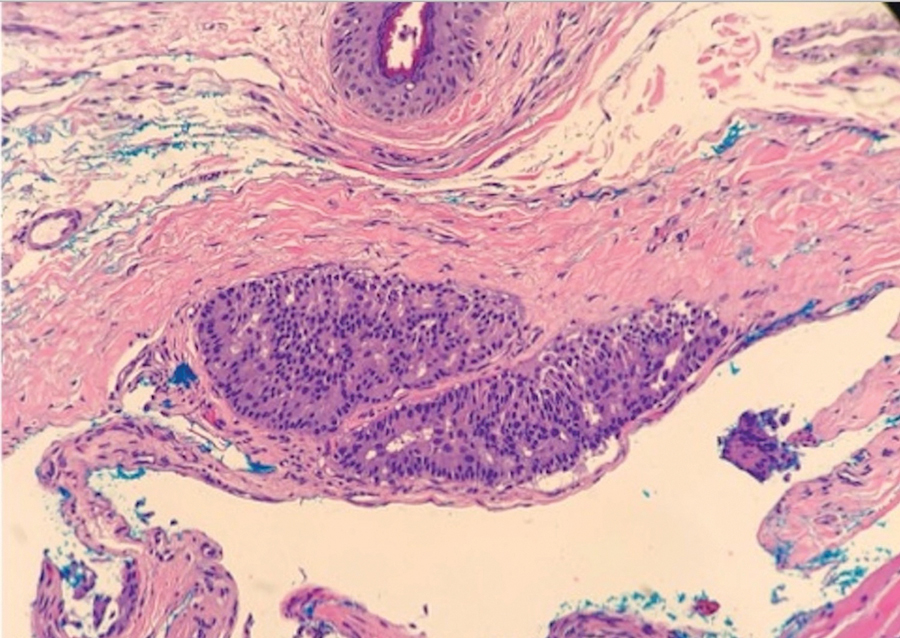
Case 3—A 72-year-old man presented with a nontender papule near the right lateral canthus. A punch biopsy demonstrated PCMC. He was treated via modified MMS with permanent en face section processing. The tumor was cleared in 1 stage. He showed no evidence of recurrence after 112 months and died of unrelated causes. The rest of his clinical information was limited because of planned institutional destruction of records.
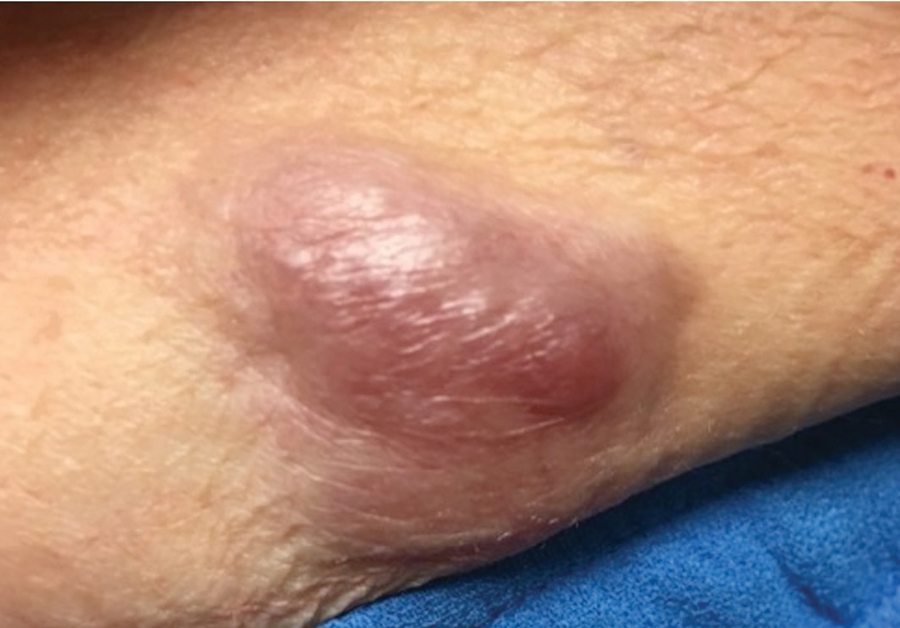
Case 4—An 87-year-old woman presented with a 25×25-mm, slow-growing mass of 12 months’ duration on the left lower abdomen (Figure 3). A biopsy demonstrated PCMC (Figure 4). Because of the size of the lesion, she underwent WLE with 20- to 30-mm margins by a general surgeon under general anesthesia. Positron emission tomography/computed tomography was unremarkable. She has remained disease free for 11 months.
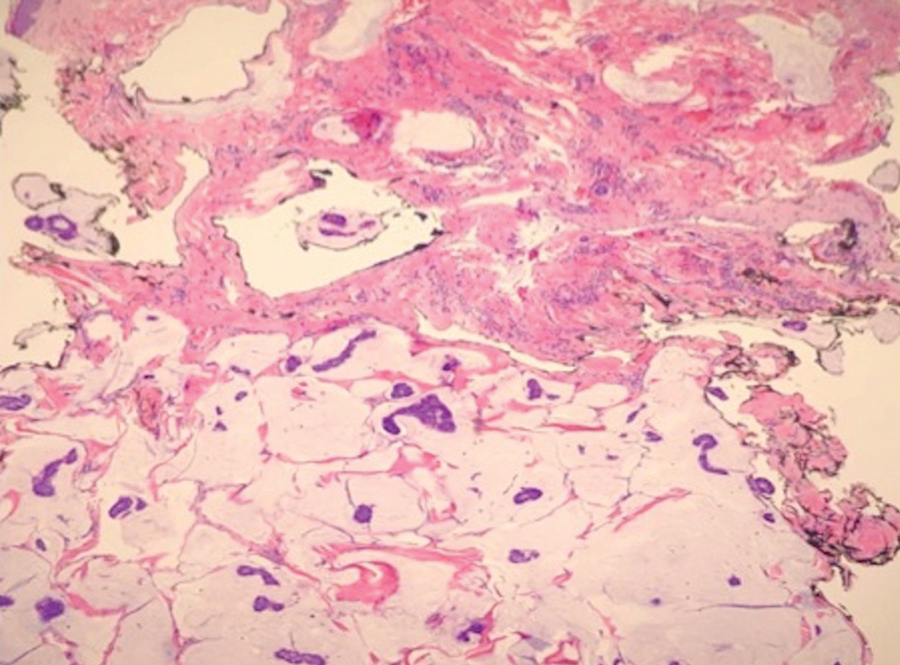
Case 5—A 66-year-old woman presented for evaluation of a posterior scalp mass measuring 23×18 mm that had grown over the last 24 months. Biopsy showed mucinous carcinoma with lymphovascular invasion consistent with PCMC (Figure 5) confirmed on multiple tissue levels and with the aid of immunohistochemistry. She was sent for an MRI of the head, neck, chest, abdomen, and pelvis, which demonstrated 2 enlarged postauricular lymph nodes and raised suspicion for metastatic disease vs reactive lymphadenopathy. Mohs micrographic surgery with frozen sections was performed with 1- to 3-mm margins; the final layer was sent for permanent processing and confirmed negative margins. Sentinel lymph node biopsy and lymphadenectomy of the 2 nodes present on imaging showed no evidence of metastasis. The patient had no recurrence in 1 month.
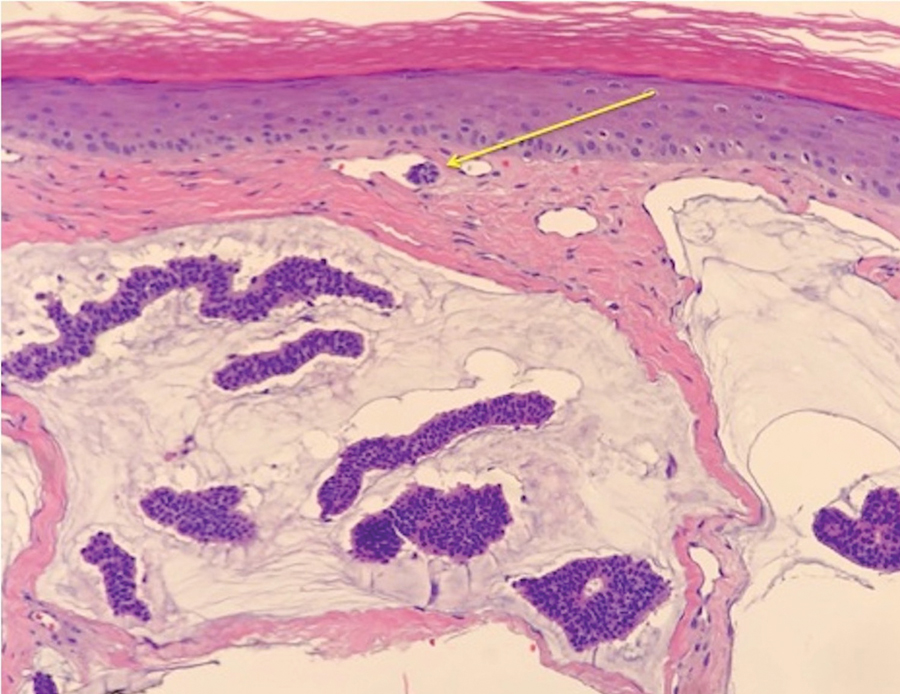
Comment
Endocrine mucin-producing sweat gland carcinoma and PCMC are sweat gland malignancies that carry low metastatic potential but are locally aggressive. Endocrine mucin-producing sweat gland carcinoma has a strong predilection for the periorbital region, especially the lower eyelids of older women.3 Primary cutaneous mucinous carcinoma may arise on the eyelids, scalp, axillae, and trunk and has been reported more often in older men. These slow-growing tumors appear as nonspecific nodules.3 Lesions frequently are asymptomatic but rarely may cause pruritus and bleeding. Histologically, EMPSGC appears as solid or cystic nodules of cells with a papillary, cribriform, or pseudopapillary appearance. Intracellular or extracellular mucin as well as malignant spread of tumor cells along pre-existing ductlike structures make it difficult to histologically distinguish EMPSGC from ductal carcinoma in situ.3
A key histopathologic feature of PCMC is basophilic epithelioid cell nests in mucinous lakes.4 Rosettelike structures are seen within solid areas of the tumor. Fibrous septae separate individual collections of mucin, creating a lobulated appearance. The histopathologic differential diagnosis of EMPSGC and PCMC is broad, including basal cell carcinoma, hidradenoma, hidradenocarcinoma, apocrine adenoma, and dermal duct tumor. Positive expression of at least 1 neuroendocrine marker (ie, synaptophysin, neuron-specific enolase, chromogranin) and low-molecular cytokeratin (cytokeratin 7, CAM5.2, Ber-EP4) can aid in the diagnosis of both EMPSGC and PCMC.4 The use of p63 immunostaining is beneficial in delineating adnexal neoplasms. Adnexal tumors that stain positively with p63 are more likely to be of primary cutaneous origin, whereas lack of p63 staining usually denotes a secondary metastatic process. However, p63 staining is less reliable when distinguishing primary and metastatic mucinous neoplasms. Metastatic mucinous carcinomas often stain positive with p63, while PCMC usually stains negative despite its primary cutaneous origin, decreasing the clinical utility of p63. The tumor may be identical to metastatic mucinous adenocarcinoma of the breast, gastrointestinal tract, lung, ovary, and pancreas. Tumor islands floating in mucin are identified in both primary cutaneous and metastatic disease to the skin.3,6 Areas of tumor necrosis, notable atypia, and perineural or lymphovascular invasion are infrequently reported in EMPSGC or PCMC, though lymphatic invasion was identified in case 5 presented herein.
A metastatic workup is warranted in all cases of PCMC, including a thorough history, review of systems, breast examination, and imaging. A workup may be considered in cases of EMPSGC depending on histologic features or clinical history.
There is uncertainty regarding the optimal management of these slow-growing yet locally destructive tumors.5 The incidence of local recurrence of PCMC after WLE with narrow margins of at least 1 cm can be as high as 30% to 40%, especially on the eyelid.4 There is no consensus on surgical care for either of these tumors.5 Because of the high recurrence rate and the predilection for the eyelid and face, MMS provides an excellent alternative to WLE for tissue preservation and meticulous margin control. We advocate for the use of the Mohs technique with permanent sectioning, which may delay the repair, but reviewing tissue with permanent fixation improves the quality and accuracy of the margin evaluation because these tumors often are infiltrative and difficult to delineate under frozen section processing. Permanent en face sectioning allows the laboratory to utilize the full array of immunohistochemical stains for these tumors, providing accurate and timely results.
Limitations to our retrospective uncontrolled study include missing or incomplete data points and short follow-up time. Additionally, there was no standardization to the margins removed with MMS or WLE because of the limited available data that comment on appropriate margins.
- Held L, Ruetten A, Kutzner H, et al. Endocrine mucin‐producing sweat gland carcinoma: clinicopathologic, immunohistochemical and molecular analysis of 11 cases with emphasis on MYB immunoexpression. J Cutan Pathol. 2018;45:674-680.
- Navrazhina K, Petukhova T, Wildman HF, et al. Endocrine mucin-producing sweat gland carcinoma of the scalp treated with Mohs micrographic surgery. JAAD Case Rep. 2018;4:887-889.
- Scott BL, Anyanwu CO, Vandergriff T, et al. Endocrine mucin–producing sweat gland carcinoma treated with Mohs micrographic surgery. Dermatol Surg. 2017;43:1498-1500.
- Chang S, Shim SH, Joo M, et al. A case of endocrine mucin-producing sweat gland carcinoma co-existing with mucinous carcinoma: a case report. Korean J Pathol. 2010;44:97-100.
- Kamalpour L, Brindise RT, Nodzenski M, et al. Primary cutaneous mucinous carcinoma: a systematic review and meta-analysis of outcomes after surgery. JAMA Dermatol. 2014;150:380-384.
- Bulliard C, Murali R, Maloof A, et al. Endocrine mucin‐producing sweat gland carcinoma: report of a case and review of the literature. J Cutan Pathol. 2006;33:812-816.
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) and

Methods
Following institutional review board approval, we conducted a retrospective, single-institution case series. We searched electronic medical records dating from 2000 to 2019 for tumors diagnosed as PCMC or extramammary Paget disease treated with MMS. We gathered demographic, clinical, pathologic, and follow-up information from the electronic medical records for each case (Tables 1 and 2). Two dermatopathologists (B.P. and B.F.K.) reviewed the hematoxylin and eosin–stained slides of each tumor as well as all available immunohistochemical stains. One of the reviewers (B.F.K.) is a board-certified dermatologist, dermatopathologist, and fellowship-trained Mohs surgeon.

Results
Demographic and Clinical Information—We identified 2 cases of EMPSGC and 3 cases of PCMC diagnosed and treated at our institution; 4 of these cases had been treated within the last 2 years. One had been treated 18 years prior; case information was limited due to planned institutional record destruction. Three of the patients were female and 2 were male. The mean age at presentation was 71 years (range, 62–87 years). None had experienced recurrence or metastases after a mean follow-up of 30 months.
Case 1—A 68-year-old woman noted a slow-growing, flesh-colored papule measuring 12×10 mm on the right lower eyelid. An excisional biopsy was completed with 2-mm clinical margins, and the defect was closed in a linear fashion. Histologic sections demonstrated EMPSGC with uninvolved margins. The patient desired no further intervention and was clinically followed. Magnetic resonance imaging (MRI) of the head and neck found no evidence of metastasis. She has had no recurrence after 15 months.

Case 2—A 62-year-old man presented with a 7×5-mm, flesh-colored papule on the left lower eyelid margin (Figure 1). It was previously treated conservatively as a hordeolum but was biopsied after it failed to resolve with 3-mm margins. Histopathology demonstrated an EMPSGC (Figure 2). The lesion was treated with modified MMS with permanent en face section processing and cleared after 1 stage. Computed tomography of the head and neck showed no abnormalities. He has had no recurrence after 9 months.

Case 3—A 72-year-old man presented with a nontender papule near the right lateral canthus. A punch biopsy demonstrated PCMC. He was treated via modified MMS with permanent en face section processing. The tumor was cleared in 1 stage. He showed no evidence of recurrence after 112 months and died of unrelated causes. The rest of his clinical information was limited because of planned institutional destruction of records.

Case 4—An 87-year-old woman presented with a 25×25-mm, slow-growing mass of 12 months’ duration on the left lower abdomen (Figure 3). A biopsy demonstrated PCMC (Figure 4). Because of the size of the lesion, she underwent WLE with 20- to 30-mm margins by a general surgeon under general anesthesia. Positron emission tomography/computed tomography was unremarkable. She has remained disease free for 11 months.

Case 5—A 66-year-old woman presented for evaluation of a posterior scalp mass measuring 23×18 mm that had grown over the last 24 months. Biopsy showed mucinous carcinoma with lymphovascular invasion consistent with PCMC (Figure 5) confirmed on multiple tissue levels and with the aid of immunohistochemistry. She was sent for an MRI of the head, neck, chest, abdomen, and pelvis, which demonstrated 2 enlarged postauricular lymph nodes and raised suspicion for metastatic disease vs reactive lymphadenopathy. Mohs micrographic surgery with frozen sections was performed with 1- to 3-mm margins; the final layer was sent for permanent processing and confirmed negative margins. Sentinel lymph node biopsy and lymphadenectomy of the 2 nodes present on imaging showed no evidence of metastasis. The patient had no recurrence in 1 month.

Comment
Endocrine mucin-producing sweat gland carcinoma and PCMC are sweat gland malignancies that carry low metastatic potential but are locally aggressive. Endocrine mucin-producing sweat gland carcinoma has a strong predilection for the periorbital region, especially the lower eyelids of older women.3 Primary cutaneous mucinous carcinoma may arise on the eyelids, scalp, axillae, and trunk and has been reported more often in older men. These slow-growing tumors appear as nonspecific nodules.3 Lesions frequently are asymptomatic but rarely may cause pruritus and bleeding. Histologically, EMPSGC appears as solid or cystic nodules of cells with a papillary, cribriform, or pseudopapillary appearance. Intracellular or extracellular mucin as well as malignant spread of tumor cells along pre-existing ductlike structures make it difficult to histologically distinguish EMPSGC from ductal carcinoma in situ.3
A key histopathologic feature of PCMC is basophilic epithelioid cell nests in mucinous lakes.4 Rosettelike structures are seen within solid areas of the tumor. Fibrous septae separate individual collections of mucin, creating a lobulated appearance. The histopathologic differential diagnosis of EMPSGC and PCMC is broad, including basal cell carcinoma, hidradenoma, hidradenocarcinoma, apocrine adenoma, and dermal duct tumor. Positive expression of at least 1 neuroendocrine marker (ie, synaptophysin, neuron-specific enolase, chromogranin) and low-molecular cytokeratin (cytokeratin 7, CAM5.2, Ber-EP4) can aid in the diagnosis of both EMPSGC and PCMC.4 The use of p63 immunostaining is beneficial in delineating adnexal neoplasms. Adnexal tumors that stain positively with p63 are more likely to be of primary cutaneous origin, whereas lack of p63 staining usually denotes a secondary metastatic process. However, p63 staining is less reliable when distinguishing primary and metastatic mucinous neoplasms. Metastatic mucinous carcinomas often stain positive with p63, while PCMC usually stains negative despite its primary cutaneous origin, decreasing the clinical utility of p63. The tumor may be identical to metastatic mucinous adenocarcinoma of the breast, gastrointestinal tract, lung, ovary, and pancreas. Tumor islands floating in mucin are identified in both primary cutaneous and metastatic disease to the skin.3,6 Areas of tumor necrosis, notable atypia, and perineural or lymphovascular invasion are infrequently reported in EMPSGC or PCMC, though lymphatic invasion was identified in case 5 presented herein.
A metastatic workup is warranted in all cases of PCMC, including a thorough history, review of systems, breast examination, and imaging. A workup may be considered in cases of EMPSGC depending on histologic features or clinical history.
There is uncertainty regarding the optimal management of these slow-growing yet locally destructive tumors.5 The incidence of local recurrence of PCMC after WLE with narrow margins of at least 1 cm can be as high as 30% to 40%, especially on the eyelid.4 There is no consensus on surgical care for either of these tumors.5 Because of the high recurrence rate and the predilection for the eyelid and face, MMS provides an excellent alternative to WLE for tissue preservation and meticulous margin control. We advocate for the use of the Mohs technique with permanent sectioning, which may delay the repair, but reviewing tissue with permanent fixation improves the quality and accuracy of the margin evaluation because these tumors often are infiltrative and difficult to delineate under frozen section processing. Permanent en face sectioning allows the laboratory to utilize the full array of immunohistochemical stains for these tumors, providing accurate and timely results.
Limitations to our retrospective uncontrolled study include missing or incomplete data points and short follow-up time. Additionally, there was no standardization to the margins removed with MMS or WLE because of the limited available data that comment on appropriate margins.
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) and

Methods
Following institutional review board approval, we conducted a retrospective, single-institution case series. We searched electronic medical records dating from 2000 to 2019 for tumors diagnosed as PCMC or extramammary Paget disease treated with MMS. We gathered demographic, clinical, pathologic, and follow-up information from the electronic medical records for each case (Tables 1 and 2). Two dermatopathologists (B.P. and B.F.K.) reviewed the hematoxylin and eosin–stained slides of each tumor as well as all available immunohistochemical stains. One of the reviewers (B.F.K.) is a board-certified dermatologist, dermatopathologist, and fellowship-trained Mohs surgeon.

Results
Demographic and Clinical Information—We identified 2 cases of EMPSGC and 3 cases of PCMC diagnosed and treated at our institution; 4 of these cases had been treated within the last 2 years. One had been treated 18 years prior; case information was limited due to planned institutional record destruction. Three of the patients were female and 2 were male. The mean age at presentation was 71 years (range, 62–87 years). None had experienced recurrence or metastases after a mean follow-up of 30 months.
Case 1—A 68-year-old woman noted a slow-growing, flesh-colored papule measuring 12×10 mm on the right lower eyelid. An excisional biopsy was completed with 2-mm clinical margins, and the defect was closed in a linear fashion. Histologic sections demonstrated EMPSGC with uninvolved margins. The patient desired no further intervention and was clinically followed. Magnetic resonance imaging (MRI) of the head and neck found no evidence of metastasis. She has had no recurrence after 15 months.

Case 2—A 62-year-old man presented with a 7×5-mm, flesh-colored papule on the left lower eyelid margin (Figure 1). It was previously treated conservatively as a hordeolum but was biopsied after it failed to resolve with 3-mm margins. Histopathology demonstrated an EMPSGC (Figure 2). The lesion was treated with modified MMS with permanent en face section processing and cleared after 1 stage. Computed tomography of the head and neck showed no abnormalities. He has had no recurrence after 9 months.

Case 3—A 72-year-old man presented with a nontender papule near the right lateral canthus. A punch biopsy demonstrated PCMC. He was treated via modified MMS with permanent en face section processing. The tumor was cleared in 1 stage. He showed no evidence of recurrence after 112 months and died of unrelated causes. The rest of his clinical information was limited because of planned institutional destruction of records.

Case 4—An 87-year-old woman presented with a 25×25-mm, slow-growing mass of 12 months’ duration on the left lower abdomen (Figure 3). A biopsy demonstrated PCMC (Figure 4). Because of the size of the lesion, she underwent WLE with 20- to 30-mm margins by a general surgeon under general anesthesia. Positron emission tomography/computed tomography was unremarkable. She has remained disease free for 11 months.

Case 5—A 66-year-old woman presented for evaluation of a posterior scalp mass measuring 23×18 mm that had grown over the last 24 months. Biopsy showed mucinous carcinoma with lymphovascular invasion consistent with PCMC (Figure 5) confirmed on multiple tissue levels and with the aid of immunohistochemistry. She was sent for an MRI of the head, neck, chest, abdomen, and pelvis, which demonstrated 2 enlarged postauricular lymph nodes and raised suspicion for metastatic disease vs reactive lymphadenopathy. Mohs micrographic surgery with frozen sections was performed with 1- to 3-mm margins; the final layer was sent for permanent processing and confirmed negative margins. Sentinel lymph node biopsy and lymphadenectomy of the 2 nodes present on imaging showed no evidence of metastasis. The patient had no recurrence in 1 month.

Comment
Endocrine mucin-producing sweat gland carcinoma and PCMC are sweat gland malignancies that carry low metastatic potential but are locally aggressive. Endocrine mucin-producing sweat gland carcinoma has a strong predilection for the periorbital region, especially the lower eyelids of older women.3 Primary cutaneous mucinous carcinoma may arise on the eyelids, scalp, axillae, and trunk and has been reported more often in older men. These slow-growing tumors appear as nonspecific nodules.3 Lesions frequently are asymptomatic but rarely may cause pruritus and bleeding. Histologically, EMPSGC appears as solid or cystic nodules of cells with a papillary, cribriform, or pseudopapillary appearance. Intracellular or extracellular mucin as well as malignant spread of tumor cells along pre-existing ductlike structures make it difficult to histologically distinguish EMPSGC from ductal carcinoma in situ.3
A key histopathologic feature of PCMC is basophilic epithelioid cell nests in mucinous lakes.4 Rosettelike structures are seen within solid areas of the tumor. Fibrous septae separate individual collections of mucin, creating a lobulated appearance. The histopathologic differential diagnosis of EMPSGC and PCMC is broad, including basal cell carcinoma, hidradenoma, hidradenocarcinoma, apocrine adenoma, and dermal duct tumor. Positive expression of at least 1 neuroendocrine marker (ie, synaptophysin, neuron-specific enolase, chromogranin) and low-molecular cytokeratin (cytokeratin 7, CAM5.2, Ber-EP4) can aid in the diagnosis of both EMPSGC and PCMC.4 The use of p63 immunostaining is beneficial in delineating adnexal neoplasms. Adnexal tumors that stain positively with p63 are more likely to be of primary cutaneous origin, whereas lack of p63 staining usually denotes a secondary metastatic process. However, p63 staining is less reliable when distinguishing primary and metastatic mucinous neoplasms. Metastatic mucinous carcinomas often stain positive with p63, while PCMC usually stains negative despite its primary cutaneous origin, decreasing the clinical utility of p63. The tumor may be identical to metastatic mucinous adenocarcinoma of the breast, gastrointestinal tract, lung, ovary, and pancreas. Tumor islands floating in mucin are identified in both primary cutaneous and metastatic disease to the skin.3,6 Areas of tumor necrosis, notable atypia, and perineural or lymphovascular invasion are infrequently reported in EMPSGC or PCMC, though lymphatic invasion was identified in case 5 presented herein.
A metastatic workup is warranted in all cases of PCMC, including a thorough history, review of systems, breast examination, and imaging. A workup may be considered in cases of EMPSGC depending on histologic features or clinical history.
There is uncertainty regarding the optimal management of these slow-growing yet locally destructive tumors.5 The incidence of local recurrence of PCMC after WLE with narrow margins of at least 1 cm can be as high as 30% to 40%, especially on the eyelid.4 There is no consensus on surgical care for either of these tumors.5 Because of the high recurrence rate and the predilection for the eyelid and face, MMS provides an excellent alternative to WLE for tissue preservation and meticulous margin control. We advocate for the use of the Mohs technique with permanent sectioning, which may delay the repair, but reviewing tissue with permanent fixation improves the quality and accuracy of the margin evaluation because these tumors often are infiltrative and difficult to delineate under frozen section processing. Permanent en face sectioning allows the laboratory to utilize the full array of immunohistochemical stains for these tumors, providing accurate and timely results.
Limitations to our retrospective uncontrolled study include missing or incomplete data points and short follow-up time. Additionally, there was no standardization to the margins removed with MMS or WLE because of the limited available data that comment on appropriate margins.
- Held L, Ruetten A, Kutzner H, et al. Endocrine mucin‐producing sweat gland carcinoma: clinicopathologic, immunohistochemical and molecular analysis of 11 cases with emphasis on MYB immunoexpression. J Cutan Pathol. 2018;45:674-680.
- Navrazhina K, Petukhova T, Wildman HF, et al. Endocrine mucin-producing sweat gland carcinoma of the scalp treated with Mohs micrographic surgery. JAAD Case Rep. 2018;4:887-889.
- Scott BL, Anyanwu CO, Vandergriff T, et al. Endocrine mucin–producing sweat gland carcinoma treated with Mohs micrographic surgery. Dermatol Surg. 2017;43:1498-1500.
- Chang S, Shim SH, Joo M, et al. A case of endocrine mucin-producing sweat gland carcinoma co-existing with mucinous carcinoma: a case report. Korean J Pathol. 2010;44:97-100.
- Kamalpour L, Brindise RT, Nodzenski M, et al. Primary cutaneous mucinous carcinoma: a systematic review and meta-analysis of outcomes after surgery. JAMA Dermatol. 2014;150:380-384.
- Bulliard C, Murali R, Maloof A, et al. Endocrine mucin‐producing sweat gland carcinoma: report of a case and review of the literature. J Cutan Pathol. 2006;33:812-816.
- Held L, Ruetten A, Kutzner H, et al. Endocrine mucin‐producing sweat gland carcinoma: clinicopathologic, immunohistochemical and molecular analysis of 11 cases with emphasis on MYB immunoexpression. J Cutan Pathol. 2018;45:674-680.
- Navrazhina K, Petukhova T, Wildman HF, et al. Endocrine mucin-producing sweat gland carcinoma of the scalp treated with Mohs micrographic surgery. JAAD Case Rep. 2018;4:887-889.
- Scott BL, Anyanwu CO, Vandergriff T, et al. Endocrine mucin–producing sweat gland carcinoma treated with Mohs micrographic surgery. Dermatol Surg. 2017;43:1498-1500.
- Chang S, Shim SH, Joo M, et al. A case of endocrine mucin-producing sweat gland carcinoma co-existing with mucinous carcinoma: a case report. Korean J Pathol. 2010;44:97-100.
- Kamalpour L, Brindise RT, Nodzenski M, et al. Primary cutaneous mucinous carcinoma: a systematic review and meta-analysis of outcomes after surgery. JAMA Dermatol. 2014;150:380-384.
- Bulliard C, Murali R, Maloof A, et al. Endocrine mucin‐producing sweat gland carcinoma: report of a case and review of the literature. J Cutan Pathol. 2006;33:812-816.
Practice Points
- Endocrine mucin-producing sweat gland carcinoma and primary cutaneous mucinous carcinoma are rare low-grade neoplasms thought to arise from apocrine glands that are morphologically and immunohistochemically analogous to ductal carcinoma in situ and mucinous carcinoma of the breast, respectively.
- Management involves a metastatic workup and either wide local excision with margins greater than 5 mm or Mohs micrographic surgery in anatomically sensitive areas.
Exercise timing may dictate obesity, type 2 diabetes risk
Tongyu Ma, PhD, research assistant professor with the Health Sciences Department, Franklin Pierce University, Rindge, N.H., and colleagues studied data on almost 5,300 individuals, finding a strong association between moderate to vigorous physical activity (MVPA) and obesity.
The research, published in Obesity, showed that people who exercised in the morning had a lower body mass index than that of those who exercised at other times, even though they were more sedentary.
For the second study, Chirag J. Patel, PhD, associate professor of biomedical informatics at Harvard Medical School, Boston, and colleagues examined more than 93,000 individuals and found that morning and afternoon, but not evening, exercise reduced the risk for type 2 diabetes.
However, the results, published in Diabetologia, also indicated that people who undertook at least MVPA were protected against developing type 2 diabetes no matter what time of day they exercised.
Along with considering the timing of exercise, the authors suggest that it is “helpful to include some higher intensity activity to help reduce the risk of developing diabetes and other cardiovascular disease.”
Morning exercisers perform less physical activity
Dr. Ma and colleagues noted that “although a beneficial association among the levels of physical activity with obesity has been frequently reported, the optimal timing of physical activity for decreasing obesity remains controversial.”
The researchers analyzed data from the National Health and Nutrition Examination Survey for the 2003-2004 and 2005-2006 cycles, because accelerometry was implemented in those periods.
They included 5,285 individuals aged ≥ 20 years who had physical activity measured via an accelerometer worn on the right hip during waking hours for 7 consecutive days.
The diurnal pattern of MVPA was classified into three clusters by the established technique of K-means clustering analysis: morning (n = 642), midday (n = 2,456), and evening (n = 2,187).
The association between MVPA, diurnal pattern, and obesity was then assessed in linear regression models taking into account a range of potential confounding factors.
Overall, participants in the morning cluster were older and more likely to be female than those in the other clusters (P < .001 for both). They were also more likely to be nonsmokers (P = .007) and to have less than high school education (P = .0041).
Morning cluster individuals performed less physical activity and were more sedentary than those in the midday and evening groups (P < .001 for both), although they were more likely to be healthy eaters (P = .004), with a lower calorie intake (P < .001).
Individuals in the morning cluster had, on average, a lower body mass index than those in other clusters, at 27.4 vs. 28.4 in the midday cluster and 28.2 in the evening cluster (P for interaction = .02).
Morning cluster participants also had a lower waist circumference than participants in the midday or evening cluster: 95.9 cm, 97.9 cm, and 97.3 cm, respectively (P for interaction = .06).
The team reported that there was a strong linear association between MVPA and obesity in the morning cluster, whereas there was a weaker curvilinear association in the midday and evening clusters.
“This is exciting new research that is consistent with a common tip for meeting exercise goals – that is, schedule exercise in the morning before emails, phone calls, or meetings that might distract you,” Rebecca Krukowski, PhD, professor, public health sciences, University of Virginia, Charlottesville, said in a release.
However, she noted that the cross-sectional nature of the study means that it is “not known whether people who exercise consistently in the morning may be systematically different from those who exercise at other times, in ways that were not measured in this study.
“For example, people who exercise regularly in the morning could have more predictable schedules, such as being less likely to be shift workers or less likely to have caregiving responsibilities that impede morning exercise,” said Dr. Krukowski, who was not involved in the study.
No association between evening activity and type 2 diabetes risk
In the second study, the team studied 93,095 persons in the UK Biobank, with a mean age of 62 years and no history of type 2 diabetes, who wore a wrist accelerometer for 1 week.
The movement data were used to estimate the metabolic equivalent of task, which was then summed into the total physical activity completed in the morning, afternoon, and evening and linked to the development of incident type 2 diabetes.
After adjustment for potential confounding factors, both morning and afternoon physical activity were associated with a reduced risk of developing type 2 diabetes, at hazard ratios of 0.90 (P = 7 × 10-8) and 0.91 (P = 1 × 10-5), respectively.
However, there was no association between evening activity and the risk for type 2 diabetes, at a hazard ratio of 0.95 (P = .07).
The team found, however, that MVPA and vigorous physical activity were associated with a reduced risk for type 2 diabetes at all times of day.
Dr. Patel’s study was supported in part by National Institutes of Health grants. No other funding was declared. No relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
Tongyu Ma, PhD, research assistant professor with the Health Sciences Department, Franklin Pierce University, Rindge, N.H., and colleagues studied data on almost 5,300 individuals, finding a strong association between moderate to vigorous physical activity (MVPA) and obesity.
The research, published in Obesity, showed that people who exercised in the morning had a lower body mass index than that of those who exercised at other times, even though they were more sedentary.
For the second study, Chirag J. Patel, PhD, associate professor of biomedical informatics at Harvard Medical School, Boston, and colleagues examined more than 93,000 individuals and found that morning and afternoon, but not evening, exercise reduced the risk for type 2 diabetes.
However, the results, published in Diabetologia, also indicated that people who undertook at least MVPA were protected against developing type 2 diabetes no matter what time of day they exercised.
Along with considering the timing of exercise, the authors suggest that it is “helpful to include some higher intensity activity to help reduce the risk of developing diabetes and other cardiovascular disease.”
Morning exercisers perform less physical activity
Dr. Ma and colleagues noted that “although a beneficial association among the levels of physical activity with obesity has been frequently reported, the optimal timing of physical activity for decreasing obesity remains controversial.”
The researchers analyzed data from the National Health and Nutrition Examination Survey for the 2003-2004 and 2005-2006 cycles, because accelerometry was implemented in those periods.
They included 5,285 individuals aged ≥ 20 years who had physical activity measured via an accelerometer worn on the right hip during waking hours for 7 consecutive days.
The diurnal pattern of MVPA was classified into three clusters by the established technique of K-means clustering analysis: morning (n = 642), midday (n = 2,456), and evening (n = 2,187).
The association between MVPA, diurnal pattern, and obesity was then assessed in linear regression models taking into account a range of potential confounding factors.
Overall, participants in the morning cluster were older and more likely to be female than those in the other clusters (P < .001 for both). They were also more likely to be nonsmokers (P = .007) and to have less than high school education (P = .0041).
Morning cluster individuals performed less physical activity and were more sedentary than those in the midday and evening groups (P < .001 for both), although they were more likely to be healthy eaters (P = .004), with a lower calorie intake (P < .001).
Individuals in the morning cluster had, on average, a lower body mass index than those in other clusters, at 27.4 vs. 28.4 in the midday cluster and 28.2 in the evening cluster (P for interaction = .02).
Morning cluster participants also had a lower waist circumference than participants in the midday or evening cluster: 95.9 cm, 97.9 cm, and 97.3 cm, respectively (P for interaction = .06).
The team reported that there was a strong linear association between MVPA and obesity in the morning cluster, whereas there was a weaker curvilinear association in the midday and evening clusters.
“This is exciting new research that is consistent with a common tip for meeting exercise goals – that is, schedule exercise in the morning before emails, phone calls, or meetings that might distract you,” Rebecca Krukowski, PhD, professor, public health sciences, University of Virginia, Charlottesville, said in a release.
However, she noted that the cross-sectional nature of the study means that it is “not known whether people who exercise consistently in the morning may be systematically different from those who exercise at other times, in ways that were not measured in this study.
“For example, people who exercise regularly in the morning could have more predictable schedules, such as being less likely to be shift workers or less likely to have caregiving responsibilities that impede morning exercise,” said Dr. Krukowski, who was not involved in the study.
No association between evening activity and type 2 diabetes risk
In the second study, the team studied 93,095 persons in the UK Biobank, with a mean age of 62 years and no history of type 2 diabetes, who wore a wrist accelerometer for 1 week.
The movement data were used to estimate the metabolic equivalent of task, which was then summed into the total physical activity completed in the morning, afternoon, and evening and linked to the development of incident type 2 diabetes.
After adjustment for potential confounding factors, both morning and afternoon physical activity were associated with a reduced risk of developing type 2 diabetes, at hazard ratios of 0.90 (P = 7 × 10-8) and 0.91 (P = 1 × 10-5), respectively.
However, there was no association between evening activity and the risk for type 2 diabetes, at a hazard ratio of 0.95 (P = .07).
The team found, however, that MVPA and vigorous physical activity were associated with a reduced risk for type 2 diabetes at all times of day.
Dr. Patel’s study was supported in part by National Institutes of Health grants. No other funding was declared. No relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
Tongyu Ma, PhD, research assistant professor with the Health Sciences Department, Franklin Pierce University, Rindge, N.H., and colleagues studied data on almost 5,300 individuals, finding a strong association between moderate to vigorous physical activity (MVPA) and obesity.
The research, published in Obesity, showed that people who exercised in the morning had a lower body mass index than that of those who exercised at other times, even though they were more sedentary.
For the second study, Chirag J. Patel, PhD, associate professor of biomedical informatics at Harvard Medical School, Boston, and colleagues examined more than 93,000 individuals and found that morning and afternoon, but not evening, exercise reduced the risk for type 2 diabetes.
However, the results, published in Diabetologia, also indicated that people who undertook at least MVPA were protected against developing type 2 diabetes no matter what time of day they exercised.
Along with considering the timing of exercise, the authors suggest that it is “helpful to include some higher intensity activity to help reduce the risk of developing diabetes and other cardiovascular disease.”
Morning exercisers perform less physical activity
Dr. Ma and colleagues noted that “although a beneficial association among the levels of physical activity with obesity has been frequently reported, the optimal timing of physical activity for decreasing obesity remains controversial.”
The researchers analyzed data from the National Health and Nutrition Examination Survey for the 2003-2004 and 2005-2006 cycles, because accelerometry was implemented in those periods.
They included 5,285 individuals aged ≥ 20 years who had physical activity measured via an accelerometer worn on the right hip during waking hours for 7 consecutive days.
The diurnal pattern of MVPA was classified into three clusters by the established technique of K-means clustering analysis: morning (n = 642), midday (n = 2,456), and evening (n = 2,187).
The association between MVPA, diurnal pattern, and obesity was then assessed in linear regression models taking into account a range of potential confounding factors.
Overall, participants in the morning cluster were older and more likely to be female than those in the other clusters (P < .001 for both). They were also more likely to be nonsmokers (P = .007) and to have less than high school education (P = .0041).
Morning cluster individuals performed less physical activity and were more sedentary than those in the midday and evening groups (P < .001 for both), although they were more likely to be healthy eaters (P = .004), with a lower calorie intake (P < .001).
Individuals in the morning cluster had, on average, a lower body mass index than those in other clusters, at 27.4 vs. 28.4 in the midday cluster and 28.2 in the evening cluster (P for interaction = .02).
Morning cluster participants also had a lower waist circumference than participants in the midday or evening cluster: 95.9 cm, 97.9 cm, and 97.3 cm, respectively (P for interaction = .06).
The team reported that there was a strong linear association between MVPA and obesity in the morning cluster, whereas there was a weaker curvilinear association in the midday and evening clusters.
“This is exciting new research that is consistent with a common tip for meeting exercise goals – that is, schedule exercise in the morning before emails, phone calls, or meetings that might distract you,” Rebecca Krukowski, PhD, professor, public health sciences, University of Virginia, Charlottesville, said in a release.
However, she noted that the cross-sectional nature of the study means that it is “not known whether people who exercise consistently in the morning may be systematically different from those who exercise at other times, in ways that were not measured in this study.
“For example, people who exercise regularly in the morning could have more predictable schedules, such as being less likely to be shift workers or less likely to have caregiving responsibilities that impede morning exercise,” said Dr. Krukowski, who was not involved in the study.
No association between evening activity and type 2 diabetes risk
In the second study, the team studied 93,095 persons in the UK Biobank, with a mean age of 62 years and no history of type 2 diabetes, who wore a wrist accelerometer for 1 week.
The movement data were used to estimate the metabolic equivalent of task, which was then summed into the total physical activity completed in the morning, afternoon, and evening and linked to the development of incident type 2 diabetes.
After adjustment for potential confounding factors, both morning and afternoon physical activity were associated with a reduced risk of developing type 2 diabetes, at hazard ratios of 0.90 (P = 7 × 10-8) and 0.91 (P = 1 × 10-5), respectively.
However, there was no association between evening activity and the risk for type 2 diabetes, at a hazard ratio of 0.95 (P = .07).
The team found, however, that MVPA and vigorous physical activity were associated with a reduced risk for type 2 diabetes at all times of day.
Dr. Patel’s study was supported in part by National Institutes of Health grants. No other funding was declared. No relevant financial relationships were declared.
A version of this article appeared on Medscape.com.
Beyond A1c: Implementing the new ESC 2023 guidelines
A significant mortality gap persists between patients with type 2 diabetes and cardiovascular disease and similarly aged patients with neither condition. Data from the Emerging Risk Factors Collaboration showed that on average, a 60-year-old female patient with type 2 diabetes and a history of myocardial infarction dies around 14 years earlier than a similarly aged patient with neither of these conditions.
Therefore, I was keen to hear the key new recommendations from the 2023 European Society of Cardiology (ESC) guidelines for the management of cardiovascular disease in patients with diabetes. These recommendations were presented at the recent ESC 2023 congress in Amsterdam, which I was fortunate enough to attend.
Of course, good glycemic control remains important to protect against the microvascular complications of diabetes, but glycemic control has only a modest impact on macrovascular complications such as cardiovascular disease.
The updated guideline recommends that all patients with type 2 diabetes without symptomatic atherosclerotic cardiovascular disease or severe target-organ damage be screened for the risk for cardiovascular disease using a new 10-year cardiovascular risk calculator called SCORE2-Diabetes. This calculator extends the well-established SCORE2 cardiovascular risk-prediction tool with added predictors specifically related to type 2 diabetes. It also accounts for variation in risk across Europe.
Using SCORE2 Diabetes will be a change in practice for me, as I have been using QRISK3, which is a United Kingdom–based cardiovascular risk tool that has been less extensively validated in patients with type 2 diabetes. Helpfully, an ESC CVD Risk Calculation app is available and can be tailored to your geographical region to calculate a SCORE2-Diabetes risk score easily. For example, Eastern Europe has a higher cardiovascular risk profile than Western Europe.
Cardiovascular risk categories are now defined on the basis of the presence of atherosclerotic cardiovascular disease, severe target-organ damage, or the 10-year cardiovascular risk using SCORE2-Diabetes.
For patients at very high cardiovascular risk (for example, those with type 2 diabetes and established atherosclerotic cardiovascular disease), the ESC guidance recommends dual therapy with a GLP-1 receptor agonist and an SGLT2 inhibitor to reduce cardiovascular risk independent of glucose control (that is, A1c). This dual therapy is recommended in addition to standard-of-care antiplatelet, antihypertensive, and lipid-lowering therapies.
There is no doubt that the evidence for GLP-1 receptor agonist use and reduction in atherosclerotic cardiovascular disease in type 2 diabetes is compelling, perhaps more so than the evidence for SGLT2 inhibitor use. However, this recommendation will be challenging to implement, given the current global supply issues with GLP-1 receptor agonists, which are driven by the off-label use of these medications for the management of obesity. GLP-1 receptor agonist supplies are not expected to stabilize until mid-2024.
Controversially, the updated ESC guidance suggests the use of metformin only in patients with type 2 diabetes and atherosclerotic cardiovascular disease if additional glucose control is required. This is a misstep, in my opinion, as insulin resistance is one of the key pathophysiologic abnormalities in patients with type 2 diabetes. One of the key advantages of metformin is an improvement in insulin sensitivity. This recommendation will not change my practice, and I will continue to prescribe metformin alongside GLP-1 receptor agonists or SGLT2 inhibitors for my patients at highest cardiovascular risk.
The updated ESC guidance also explicitly reminds healthcare professionals to look for significant comorbidities, such as heart failure of all subtypes and chronic kidney disease.
The ESC guidance recommends a systematic survey for heart failure symptoms and signs at each clinical encounter in all patients with type 2 diabetes. Although I agree that heart failure is underdiagnosed in this population, the recommendation will be challenging to implement and has significant workload implications, as heart failure often presents in insidious, nonspecific ways in primary care.
For patients with type 2 diabetes and heart failure with reduced ejection fraction, SGLT2 inhibitors are recommended to reduce the risk for heart failure hospitalization and cardiovascular death. Again, this recommendation is independent of glycemic control. In addition, for patients with type 2 diabetes and heart failure with mid-range ejection fraction or heart failure with preserved ejection fraction (that is, left ventricular ejection fraction > 40%), SGLT2 inhibitors are also recommended to reduce the risk for heart failure hospitalization or cardiovascular death independent of glycemic control. This recommendation is consistent with other updated global heart failure guidance. Increasingly, the pillars of heart failure therapy are being challenged with the early initiation of SGLT2 inhibitors, given their compelling evidence base, early symptomatic benefit, and ease of use, with less requirement of routine blood monitoring.
Finally, for patients with type 2 diabetes and chronic kidney disease, SGLT2 inhibitors and finerenone are now recommended to reduce the risk for kidney failure and cardiovascular disease, independent of glycemic control and in addition to standard of care.
Finerenone is a nonsteroidal selective mineralocorticoid receptor antagonist with quite different pharmacokinetics and clinical effects, compared with those of spironolactone and eplerenone, which are steroidal MRAs. Specifically, finerenone does not significantly lower blood pressure and has fewer steroid-induced adverse effects such as gynecomastia, impotence, and low libido. However, like steroidal MRAs, finerenone can result in hyperkalemia.
Finerenone has demonstrated significant kidney and cardiovascular benefits across the spectrum of chronic kidney disease in patients with type 2 diabetes. It entails no significant imbalance in adverse events, hence this recommendation. This observation reinforces the importance of measuring urinary albumin–creatinine ratio in patients with type 2 diabetes and preserved kidney function.
In conclusion, the 2023 ESC guidelines for the management of cardiovascular disease in patients with diabetes are forward-thinking recommendations. They look beyond glycemia and reflect the current evidence for newer glucose-lowering therapies with proven cardiorenal benefits. Nevertheless, the implementation of these guidelines will be challenging, given their workload implications, the unstable supply of GLP-1 receptor agonists, and a persisting glucocentric approach to type 2 diabetes care in some areas. Implementation will require ongoing education for health care professionals about the risk-benefit ratios of SGLT2 inhibitors and GLP-1 receptor agonists. It also will require a re-evaluation of workforce strategy to support the development of a skilled and sustainable workforce.
Dr. Fernando is a general practitioner partner with North Berwick (Scotland) Health Centre, with a specialist interest in diabetes; cardiovascular, renal, and metabolic diseases; and medical education. He disclosed receiving speakers’ fees from Eli Lilly and Novo Nordisk.
A version of this article appeared on Medscape.com.
A significant mortality gap persists between patients with type 2 diabetes and cardiovascular disease and similarly aged patients with neither condition. Data from the Emerging Risk Factors Collaboration showed that on average, a 60-year-old female patient with type 2 diabetes and a history of myocardial infarction dies around 14 years earlier than a similarly aged patient with neither of these conditions.
Therefore, I was keen to hear the key new recommendations from the 2023 European Society of Cardiology (ESC) guidelines for the management of cardiovascular disease in patients with diabetes. These recommendations were presented at the recent ESC 2023 congress in Amsterdam, which I was fortunate enough to attend.
Of course, good glycemic control remains important to protect against the microvascular complications of diabetes, but glycemic control has only a modest impact on macrovascular complications such as cardiovascular disease.
The updated guideline recommends that all patients with type 2 diabetes without symptomatic atherosclerotic cardiovascular disease or severe target-organ damage be screened for the risk for cardiovascular disease using a new 10-year cardiovascular risk calculator called SCORE2-Diabetes. This calculator extends the well-established SCORE2 cardiovascular risk-prediction tool with added predictors specifically related to type 2 diabetes. It also accounts for variation in risk across Europe.
Using SCORE2 Diabetes will be a change in practice for me, as I have been using QRISK3, which is a United Kingdom–based cardiovascular risk tool that has been less extensively validated in patients with type 2 diabetes. Helpfully, an ESC CVD Risk Calculation app is available and can be tailored to your geographical region to calculate a SCORE2-Diabetes risk score easily. For example, Eastern Europe has a higher cardiovascular risk profile than Western Europe.
Cardiovascular risk categories are now defined on the basis of the presence of atherosclerotic cardiovascular disease, severe target-organ damage, or the 10-year cardiovascular risk using SCORE2-Diabetes.
For patients at very high cardiovascular risk (for example, those with type 2 diabetes and established atherosclerotic cardiovascular disease), the ESC guidance recommends dual therapy with a GLP-1 receptor agonist and an SGLT2 inhibitor to reduce cardiovascular risk independent of glucose control (that is, A1c). This dual therapy is recommended in addition to standard-of-care antiplatelet, antihypertensive, and lipid-lowering therapies.
There is no doubt that the evidence for GLP-1 receptor agonist use and reduction in atherosclerotic cardiovascular disease in type 2 diabetes is compelling, perhaps more so than the evidence for SGLT2 inhibitor use. However, this recommendation will be challenging to implement, given the current global supply issues with GLP-1 receptor agonists, which are driven by the off-label use of these medications for the management of obesity. GLP-1 receptor agonist supplies are not expected to stabilize until mid-2024.
Controversially, the updated ESC guidance suggests the use of metformin only in patients with type 2 diabetes and atherosclerotic cardiovascular disease if additional glucose control is required. This is a misstep, in my opinion, as insulin resistance is one of the key pathophysiologic abnormalities in patients with type 2 diabetes. One of the key advantages of metformin is an improvement in insulin sensitivity. This recommendation will not change my practice, and I will continue to prescribe metformin alongside GLP-1 receptor agonists or SGLT2 inhibitors for my patients at highest cardiovascular risk.
The updated ESC guidance also explicitly reminds healthcare professionals to look for significant comorbidities, such as heart failure of all subtypes and chronic kidney disease.
The ESC guidance recommends a systematic survey for heart failure symptoms and signs at each clinical encounter in all patients with type 2 diabetes. Although I agree that heart failure is underdiagnosed in this population, the recommendation will be challenging to implement and has significant workload implications, as heart failure often presents in insidious, nonspecific ways in primary care.
For patients with type 2 diabetes and heart failure with reduced ejection fraction, SGLT2 inhibitors are recommended to reduce the risk for heart failure hospitalization and cardiovascular death. Again, this recommendation is independent of glycemic control. In addition, for patients with type 2 diabetes and heart failure with mid-range ejection fraction or heart failure with preserved ejection fraction (that is, left ventricular ejection fraction > 40%), SGLT2 inhibitors are also recommended to reduce the risk for heart failure hospitalization or cardiovascular death independent of glycemic control. This recommendation is consistent with other updated global heart failure guidance. Increasingly, the pillars of heart failure therapy are being challenged with the early initiation of SGLT2 inhibitors, given their compelling evidence base, early symptomatic benefit, and ease of use, with less requirement of routine blood monitoring.
Finally, for patients with type 2 diabetes and chronic kidney disease, SGLT2 inhibitors and finerenone are now recommended to reduce the risk for kidney failure and cardiovascular disease, independent of glycemic control and in addition to standard of care.
Finerenone is a nonsteroidal selective mineralocorticoid receptor antagonist with quite different pharmacokinetics and clinical effects, compared with those of spironolactone and eplerenone, which are steroidal MRAs. Specifically, finerenone does not significantly lower blood pressure and has fewer steroid-induced adverse effects such as gynecomastia, impotence, and low libido. However, like steroidal MRAs, finerenone can result in hyperkalemia.
Finerenone has demonstrated significant kidney and cardiovascular benefits across the spectrum of chronic kidney disease in patients with type 2 diabetes. It entails no significant imbalance in adverse events, hence this recommendation. This observation reinforces the importance of measuring urinary albumin–creatinine ratio in patients with type 2 diabetes and preserved kidney function.
In conclusion, the 2023 ESC guidelines for the management of cardiovascular disease in patients with diabetes are forward-thinking recommendations. They look beyond glycemia and reflect the current evidence for newer glucose-lowering therapies with proven cardiorenal benefits. Nevertheless, the implementation of these guidelines will be challenging, given their workload implications, the unstable supply of GLP-1 receptor agonists, and a persisting glucocentric approach to type 2 diabetes care in some areas. Implementation will require ongoing education for health care professionals about the risk-benefit ratios of SGLT2 inhibitors and GLP-1 receptor agonists. It also will require a re-evaluation of workforce strategy to support the development of a skilled and sustainable workforce.
Dr. Fernando is a general practitioner partner with North Berwick (Scotland) Health Centre, with a specialist interest in diabetes; cardiovascular, renal, and metabolic diseases; and medical education. He disclosed receiving speakers’ fees from Eli Lilly and Novo Nordisk.
A version of this article appeared on Medscape.com.
A significant mortality gap persists between patients with type 2 diabetes and cardiovascular disease and similarly aged patients with neither condition. Data from the Emerging Risk Factors Collaboration showed that on average, a 60-year-old female patient with type 2 diabetes and a history of myocardial infarction dies around 14 years earlier than a similarly aged patient with neither of these conditions.
Therefore, I was keen to hear the key new recommendations from the 2023 European Society of Cardiology (ESC) guidelines for the management of cardiovascular disease in patients with diabetes. These recommendations were presented at the recent ESC 2023 congress in Amsterdam, which I was fortunate enough to attend.
Of course, good glycemic control remains important to protect against the microvascular complications of diabetes, but glycemic control has only a modest impact on macrovascular complications such as cardiovascular disease.
The updated guideline recommends that all patients with type 2 diabetes without symptomatic atherosclerotic cardiovascular disease or severe target-organ damage be screened for the risk for cardiovascular disease using a new 10-year cardiovascular risk calculator called SCORE2-Diabetes. This calculator extends the well-established SCORE2 cardiovascular risk-prediction tool with added predictors specifically related to type 2 diabetes. It also accounts for variation in risk across Europe.
Using SCORE2 Diabetes will be a change in practice for me, as I have been using QRISK3, which is a United Kingdom–based cardiovascular risk tool that has been less extensively validated in patients with type 2 diabetes. Helpfully, an ESC CVD Risk Calculation app is available and can be tailored to your geographical region to calculate a SCORE2-Diabetes risk score easily. For example, Eastern Europe has a higher cardiovascular risk profile than Western Europe.
Cardiovascular risk categories are now defined on the basis of the presence of atherosclerotic cardiovascular disease, severe target-organ damage, or the 10-year cardiovascular risk using SCORE2-Diabetes.
For patients at very high cardiovascular risk (for example, those with type 2 diabetes and established atherosclerotic cardiovascular disease), the ESC guidance recommends dual therapy with a GLP-1 receptor agonist and an SGLT2 inhibitor to reduce cardiovascular risk independent of glucose control (that is, A1c). This dual therapy is recommended in addition to standard-of-care antiplatelet, antihypertensive, and lipid-lowering therapies.
There is no doubt that the evidence for GLP-1 receptor agonist use and reduction in atherosclerotic cardiovascular disease in type 2 diabetes is compelling, perhaps more so than the evidence for SGLT2 inhibitor use. However, this recommendation will be challenging to implement, given the current global supply issues with GLP-1 receptor agonists, which are driven by the off-label use of these medications for the management of obesity. GLP-1 receptor agonist supplies are not expected to stabilize until mid-2024.
Controversially, the updated ESC guidance suggests the use of metformin only in patients with type 2 diabetes and atherosclerotic cardiovascular disease if additional glucose control is required. This is a misstep, in my opinion, as insulin resistance is one of the key pathophysiologic abnormalities in patients with type 2 diabetes. One of the key advantages of metformin is an improvement in insulin sensitivity. This recommendation will not change my practice, and I will continue to prescribe metformin alongside GLP-1 receptor agonists or SGLT2 inhibitors for my patients at highest cardiovascular risk.
The updated ESC guidance also explicitly reminds healthcare professionals to look for significant comorbidities, such as heart failure of all subtypes and chronic kidney disease.
The ESC guidance recommends a systematic survey for heart failure symptoms and signs at each clinical encounter in all patients with type 2 diabetes. Although I agree that heart failure is underdiagnosed in this population, the recommendation will be challenging to implement and has significant workload implications, as heart failure often presents in insidious, nonspecific ways in primary care.
For patients with type 2 diabetes and heart failure with reduced ejection fraction, SGLT2 inhibitors are recommended to reduce the risk for heart failure hospitalization and cardiovascular death. Again, this recommendation is independent of glycemic control. In addition, for patients with type 2 diabetes and heart failure with mid-range ejection fraction or heart failure with preserved ejection fraction (that is, left ventricular ejection fraction > 40%), SGLT2 inhibitors are also recommended to reduce the risk for heart failure hospitalization or cardiovascular death independent of glycemic control. This recommendation is consistent with other updated global heart failure guidance. Increasingly, the pillars of heart failure therapy are being challenged with the early initiation of SGLT2 inhibitors, given their compelling evidence base, early symptomatic benefit, and ease of use, with less requirement of routine blood monitoring.
Finally, for patients with type 2 diabetes and chronic kidney disease, SGLT2 inhibitors and finerenone are now recommended to reduce the risk for kidney failure and cardiovascular disease, independent of glycemic control and in addition to standard of care.
Finerenone is a nonsteroidal selective mineralocorticoid receptor antagonist with quite different pharmacokinetics and clinical effects, compared with those of spironolactone and eplerenone, which are steroidal MRAs. Specifically, finerenone does not significantly lower blood pressure and has fewer steroid-induced adverse effects such as gynecomastia, impotence, and low libido. However, like steroidal MRAs, finerenone can result in hyperkalemia.
Finerenone has demonstrated significant kidney and cardiovascular benefits across the spectrum of chronic kidney disease in patients with type 2 diabetes. It entails no significant imbalance in adverse events, hence this recommendation. This observation reinforces the importance of measuring urinary albumin–creatinine ratio in patients with type 2 diabetes and preserved kidney function.
In conclusion, the 2023 ESC guidelines for the management of cardiovascular disease in patients with diabetes are forward-thinking recommendations. They look beyond glycemia and reflect the current evidence for newer glucose-lowering therapies with proven cardiorenal benefits. Nevertheless, the implementation of these guidelines will be challenging, given their workload implications, the unstable supply of GLP-1 receptor agonists, and a persisting glucocentric approach to type 2 diabetes care in some areas. Implementation will require ongoing education for health care professionals about the risk-benefit ratios of SGLT2 inhibitors and GLP-1 receptor agonists. It also will require a re-evaluation of workforce strategy to support the development of a skilled and sustainable workforce.
Dr. Fernando is a general practitioner partner with North Berwick (Scotland) Health Centre, with a specialist interest in diabetes; cardiovascular, renal, and metabolic diseases; and medical education. He disclosed receiving speakers’ fees from Eli Lilly and Novo Nordisk.
A version of this article appeared on Medscape.com.