User login
Waist-hip ratio a stronger mortality predictor than BMI
TOPLINE:
Compared with body mass index, waist-hip ratio (WHR) had the strongest and most consistent association with all-cause mortality and was the only measurement unaffected by BMI.
METHODOLOGY:
- Cohort study of incident deaths from the U.K. Biobank (2006-2022), including data from 22 centers across the United Kingdom.
- A total of 387,672 participants were divided into a discovery cohort (n = 337,078) and validation cohort (n = 50,594), with the latter consisting of 25,297 deaths and 2,297 controls.
- The discovery cohort was used to derive genetically determined adiposity measures while the validation cohort was used for analyses.
- Exposure-outcome associations were analyzed through observational and mendelian randomization analyses.
TAKEAWAY:
- In adjusted analysis, a J-shaped association was found for both measured BMI and fat mass index (FMI), whereas the association with WHR was linear (hazard ratio 1.41 per standard deviation increase).
- There was a significant association between all three adiposity measures and all-cause mortality, with odds ratio 1.29 per SD change in genetically determined BMI (P = 1.44×10-13), 1.45 per SD change in genetically determined FMI, 1.45 (P = 6.27×10-30), and 1.51 per SD change in genetically determined WHR (P = 2.11×10-9).
- Compared with BMI, WHR had the stronger association with all-cause mortality, although it was not significantly stronger than FMI.
- The association of genetically determined BMI and FMI with all-cause mortality varied across quantiles of observed BMI, but WHR did not (P = .04, P = .02, and P = .58, for BMI, FMI, and WHR, respectively).
IN PRACTICE:
“Current World Health Organization recommendations for optimal BMI range are inaccurate across individuals with various body compositions and therefore suboptimal for clinical guidelines.”
SOURCE:
Study by Irfan Khan, MSc, of the Population Health Research Institute, David Braley Cardiac, Vascular, and Stroke Research Institute, Hamilton, Ont., and colleagues. Published online in JAMA Network Open.
LIMITATIONS:
Study population was genetically homogeneous, White, and British, so results may not be representative of other racial or ethnic groups.
DISCLOSURES:
Study was funded by, and Irfan Khan received support from, the Ontario Graduate Scholarship–Masters Scholarship, awarded by the government of Ontario.
A version of this article first appeared on Medscape.com.
TOPLINE:
Compared with body mass index, waist-hip ratio (WHR) had the strongest and most consistent association with all-cause mortality and was the only measurement unaffected by BMI.
METHODOLOGY:
- Cohort study of incident deaths from the U.K. Biobank (2006-2022), including data from 22 centers across the United Kingdom.
- A total of 387,672 participants were divided into a discovery cohort (n = 337,078) and validation cohort (n = 50,594), with the latter consisting of 25,297 deaths and 2,297 controls.
- The discovery cohort was used to derive genetically determined adiposity measures while the validation cohort was used for analyses.
- Exposure-outcome associations were analyzed through observational and mendelian randomization analyses.
TAKEAWAY:
- In adjusted analysis, a J-shaped association was found for both measured BMI and fat mass index (FMI), whereas the association with WHR was linear (hazard ratio 1.41 per standard deviation increase).
- There was a significant association between all three adiposity measures and all-cause mortality, with odds ratio 1.29 per SD change in genetically determined BMI (P = 1.44×10-13), 1.45 per SD change in genetically determined FMI, 1.45 (P = 6.27×10-30), and 1.51 per SD change in genetically determined WHR (P = 2.11×10-9).
- Compared with BMI, WHR had the stronger association with all-cause mortality, although it was not significantly stronger than FMI.
- The association of genetically determined BMI and FMI with all-cause mortality varied across quantiles of observed BMI, but WHR did not (P = .04, P = .02, and P = .58, for BMI, FMI, and WHR, respectively).
IN PRACTICE:
“Current World Health Organization recommendations for optimal BMI range are inaccurate across individuals with various body compositions and therefore suboptimal for clinical guidelines.”
SOURCE:
Study by Irfan Khan, MSc, of the Population Health Research Institute, David Braley Cardiac, Vascular, and Stroke Research Institute, Hamilton, Ont., and colleagues. Published online in JAMA Network Open.
LIMITATIONS:
Study population was genetically homogeneous, White, and British, so results may not be representative of other racial or ethnic groups.
DISCLOSURES:
Study was funded by, and Irfan Khan received support from, the Ontario Graduate Scholarship–Masters Scholarship, awarded by the government of Ontario.
A version of this article first appeared on Medscape.com.
TOPLINE:
Compared with body mass index, waist-hip ratio (WHR) had the strongest and most consistent association with all-cause mortality and was the only measurement unaffected by BMI.
METHODOLOGY:
- Cohort study of incident deaths from the U.K. Biobank (2006-2022), including data from 22 centers across the United Kingdom.
- A total of 387,672 participants were divided into a discovery cohort (n = 337,078) and validation cohort (n = 50,594), with the latter consisting of 25,297 deaths and 2,297 controls.
- The discovery cohort was used to derive genetically determined adiposity measures while the validation cohort was used for analyses.
- Exposure-outcome associations were analyzed through observational and mendelian randomization analyses.
TAKEAWAY:
- In adjusted analysis, a J-shaped association was found for both measured BMI and fat mass index (FMI), whereas the association with WHR was linear (hazard ratio 1.41 per standard deviation increase).
- There was a significant association between all three adiposity measures and all-cause mortality, with odds ratio 1.29 per SD change in genetically determined BMI (P = 1.44×10-13), 1.45 per SD change in genetically determined FMI, 1.45 (P = 6.27×10-30), and 1.51 per SD change in genetically determined WHR (P = 2.11×10-9).
- Compared with BMI, WHR had the stronger association with all-cause mortality, although it was not significantly stronger than FMI.
- The association of genetically determined BMI and FMI with all-cause mortality varied across quantiles of observed BMI, but WHR did not (P = .04, P = .02, and P = .58, for BMI, FMI, and WHR, respectively).
IN PRACTICE:
“Current World Health Organization recommendations for optimal BMI range are inaccurate across individuals with various body compositions and therefore suboptimal for clinical guidelines.”
SOURCE:
Study by Irfan Khan, MSc, of the Population Health Research Institute, David Braley Cardiac, Vascular, and Stroke Research Institute, Hamilton, Ont., and colleagues. Published online in JAMA Network Open.
LIMITATIONS:
Study population was genetically homogeneous, White, and British, so results may not be representative of other racial or ethnic groups.
DISCLOSURES:
Study was funded by, and Irfan Khan received support from, the Ontario Graduate Scholarship–Masters Scholarship, awarded by the government of Ontario.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
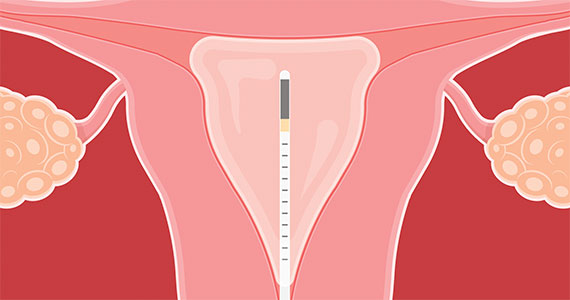
2023 Update on abnormal uterine bleeding
Endometrial ablation continues to be performed in significant numbers in the United States, with an estimated 500,000 cases annually. Several nonresectoscopic endometrial ablation devices have been approved for use, and some are now discontinued. The newest endometrial ablation therapy to gain US Food and Drug Administration (FDA) approval and to have published outcomes is the Cerene cryotherapy ablation device (Channel Medsystems, Inc). The results of 36-month outcomes from the CLARITY study were published last year, and we have chosen to review these long-term data in addition to that of a second study in which investigators assessed the ability to access the endometrial cavity postcryoablation. We believe this is important because of concerns about the inability to access the endometrial cavity after ablation, as well as the potential for delay in the diagnosis of endometrial cancer. It is interesting that 2 publications simultaneously reviewed the incidence of endometrial cancer after endometrial ablation within the past 12 months, and we therefore present those findings as they provide valuable information.
Our second focus in this year’s Update is to provide additional information about the burgeoning data on gonadotropin-releasing hormone (GnRH) antagonists. We review evidence on linzagolix from the PRIMROSE 1 and PRIMROSE 2 trials and longer-term data on relugolix combination therapy for symptomatic uterine fibroids.
Three-year follow-up after endometrial cryoablation with the Cerene device found high patient satisfaction, low hysterectomy rates
Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28;899-908.
Curlin HL, Anderson TL. Endometrial cryoablation for the treatment of heavy menstrual bleeding: 36-month outcomes from the CLARITY study. Int J Womens Health. 2022;14:1083-1092.
The 12-month data on the clinical safety and effectiveness of the Cerene cryoablation device were published in 2021 in the CLARITY trial.1 The 36-month outcomes were published in 2022 and showed sustained clinical effects through month 36 with a low risk of adverse outcomes.2 The interesting aspect of this trial is that although the amenorrhea rate was relatively low at 12 months (6.5%), it continued to remain relatively low compared with rates found with other devices, but the amenorrhea rate increased at 36 months (14.4%). This was the percentage of patients who reported, “I no longer get my period.”
Patient satisfaction was high
Despite a relatively low amenorrhea rate, study participants had a high satisfaction rate and a low 3-year hysterectomy rate. Eighty-five percent of the participants were satisfied or very satisfied, and the cumulative hysterectomy rate was low at 5%.
The overall reintervention rate was 8.7%. Six patients were treated with medications, 2 patients underwent repeat endometrial ablation, 1 received a levonorgestrel-releasing intrauterine device, and 12 underwent hysterectomy.
At 36 months, 201 of the original 242 participants were available for assessment. Unfortunately, 5 pregnancies were reported through the 6-month posttreatment period, which emphasizes the importance of having reliable contraception. However, there were no reports of hematometra or postablation tubal sterilization syndrome (PATSS).
Effect on bleeding was long term
The main finding of the CLARITY study is that the Cerene cryoablation device appears to have a relatively stable effect on bleedingfor the first 3 years after therapy, with minimal risk of hematometra and PATSS. What we find interesting is that despite Cerene cryoablation having one of the lowest amenorrhea rates, it not only had a satisfaction rate in line with that of other devices but also had a low hysterectomy rate—only 5%—at 3 years.
The study authors pointed out that there is a lack of scarring within the endometrial cavity with the Cerene device. Some may find less endometrial scarring worth a low amenorrhea rate in the context of a favorable satisfaction rate. This begs the question, how well can the endometrial cavity be assessed? For answers, keep reading.
Can the endometrial cavity be reliably accessed after Cerene cryoablation?
Endometrial ablation has been associated with intracavitary scarring that results in hematometra, PATSS, and a concern for difficulty in performing an adequate endometrial assessment in patients who develop postablation abnormal uterine bleeding.
In a prospective study, 230 participants (of an initial 242) treated with Cerene cyroablation were studied with hysteroscopic evaluation of the endometrial cavity 12 months after surgery.3 The uterine cavity was accessible in 98.7% of participants. The cavity was not accessible in 3 participants due to pain or cervical stenosis.
Visualization of the uterine cavity was possible by hysteroscopy in 92.7% of study participants (204 of 220), with 1 or both tubal ostia identified in 89.2%. Both tubal ostia were visible in 78.4% and 1 ostium was visible in 10.8%. The cavity was not visualized in 16 of the 220 patients (7.2%) due to intrauterine adhesions, technical difficulties, or menstruation. Also of note, 97 of the 230 participants available at the 12-month follow-up had undergone tubal sterilization before cryoablation and none reported symptoms of PATSS or hematometra, which may be considered surrogate markers for adhesions.
Results of the CLARITY study demonstrated the clinical safety and effectiveness of the Cerene cryoablation device at 12 months, with sustained clinical effects through 36 months and a low risk of adverse outcomes. Patient satisfaction rates were high, and the hysterectomy rate was low. In addition, in a prospective study of patients treated with Cerene cryoablation, hysteroscopic evaluation at 12 months found the uterine cavity accessible in more than 98% of participants, and uterine visualization also was high. Therefore, the Cerene cryoablation device may provide the advantage of an easier evaluation of patients who eventually develop abnormal bleeding after endometrial ablation.
Continue to: Tissue effects differ with ablation technique...
Tissue effects differ with ablation technique
The study authors suggested that different tissue effects occur with freezing compared with heating ablation techniques. With freezing, over weeks to months the chronic inflammatory tissue is eventually replaced by a fibrous scar of collagen, with some preservation of the collagen matrix during tissue repair. This may be different from the charring and boiling of heated tissue that results in architectural tissue loss and may interfere with wound repair and tissue remodeling. Although the incidence of postoperative adhesions after endometrial ablation is not well studied, it is encouraging that most patients who received cryoablation with the Cerene device were able to undergo an evaluation of the endometrium without general anesthesia.
Key takeaway
The main idea from this study is that the endometrium can be assessed by office hysteroscopy in most patients who undergo cryoablation with the Cerene device. This may have advantages in terms of reducing the risk of PATSS and hematometra, and it may allow easier evaluation of the endometrium for patients who have postablation abnormal uterine bleeding. This begs the question, does intrauterine scarring influence the detection of endometrial cancer? For answers, keep reading.
Does endometrial ablation place a patient at higher risk for a delay in the diagnosis of endometrial cancer?
Radestad AF, Dahm-Kahler P, Holmberg E, et al. Long-term incidence of endometrial cancer after endometrial resection and ablation: a population based Swedish gynecologic cancer group (SweGCG) study. Acta Obstet Gynecol Scand. 2022;101:923-930.
Oderkerk TJ, van de Kar MRD, Cornel KMC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555-1560.
The answer to this question appears to be no, based on 2 different types of studies. One study was a 20-year population database review from Sweden,4 and the other was a systematic review of 11 cohort studies.5
Population-based study findings
The data from the Swedish population database is interesting because since 1994 all Swedish citizens have been allocated a unique personal identification number at birth or immigration that enables official registries and research. In reviewing their data from 1997 through 2017, Radestad and colleagues compared transcervical resection of the endometrium (TCRE) and other forms of endometrial ablation against the Swedish National Patient Register data for endometrial cancer.4 They found no increase in the incidence of endometrial cancer after TCRE (0.3%) or after endometrial ablation (0.02%) and suggested a significantly lower incidence of endometrial cancer after endometrial ablation.
This study is beneficial because it is the largest study to explore the long-term incidence of endometrial cancer after TCRE and endometrial ablation. The investigators hypothesized that, as an explanation for the difference between rates, ablation may burn deeper into the myometrium and treat adenomyosis compared with TCRE. However, they also were cautious to note that although this was a 20-year study, the incidence of endometrial carcinoma likely will reach a peak in the next few years.
Systematic review conclusions
In the systematic review, out of 890 publications from the authors’ database search, 11 articles were eventually included for review.5 A total of 29,102 patients with endometrial ablation were followed for a period of up to 25 years, and the incidence of endometrial cancer after endometrial ablation varied from 0.0% to 1.6%. A total of 38 cases of endometrial cancer after endometrial ablation have been described in the literature. Of those cases, bleeding was the most common presenting symptom of the disease. Endometrial sampling was successful in 89% of cases, and in 90% of cases, histological exam showed an early-stage endometrial adenocarcinoma.
Based on their review, the authors concluded that the incidence of endometrial cancer was not increased in patients who received endometrial ablation, and more importantly, there was no apparent delay in the diagnosis of endometrial cancer after endometrial ablation. They further suggested that diagnostic management with endometrial sampling did not appear to be a barrier.
The main findings from these 2 studies by Radestad and colleagues and Oderkerk and associates are that endometrial cancer does not appear to be more common after endometrial ablation, and it appears to be diagnosed with endometrial sampling in most cases.4,5 There may be some protection against endometrial cancer with nonresectoscopic endometrial ablation, although this needs to be verified by additional studies. To juxtapose this information with the prior information about cryotherapy, it emphasizes that the scarring within the endometrium will likely reduce the incidence of PATSS and hematometra, which are relatively low-incidence occurrences at 5% to 7%, but it likely does not affect the detection of endometrial cancer.
Longer-term data for relugolix combination treatment of symptomatic uterine bleeding from fibroids shows sustained efficacy
Al-Hendy A, Lukes AS, Poindexter AN, et al. Long-term relugolix combination therapy for symptomatic uterine leiomyomas. Obstet Gynecol. 2022;140:920-930.
Relugolix combination therapy was previously reported to be effective for the treatment of fibroids based on the 24-week trials LIBERTY 1 and LIBERTY 2. We now have information about longer-term therapy for up to 52 weeks of treatment.6
Relugolix combination therapy is a once-daily single tablet for the treatment of heavy menstrual bleeding thought to be due to uterine fibroids in premenopausal women. It is comprised of relugolix 40 mg (a GnRH antagonist), estradiol 1.0 mg, and norethindrone acetate 0.5 mg.
Continue to: Extension study showed sustained efficacy...
Extension study showed sustained efficacy
The study by Al-Hendy and colleagues showed that the relugolix combination not only was well tolerated but also that there was sustained improvement in heavy bleeding, with the average patient having an approximately 90% decrease in menstrual bleeding from baseline.6 It was noted that 70.6% of patients achieved amenorrhea over the last 35 days of treatment.
Importantly, the treatment effect was independent of race, body mass index, baseline menstrual blood loss, and uterine fibroid volume. The bone mineral density (BMD) change trajectory was similar to what was observed in the pivotal study. No new safety concerns were identified, and BMD generally was preserved.
The extension study by Al-Hendy and colleagues demonstrated that that the reduced fibroid-associated bleeding treated with relugolix combination therapy is sustained throughout the 52-week period, with no new safety concerns.
Linzagolix is the newest GnRH antagonist to be studied in a randomized, placebo-controlled trial
Donnez J, Taylor HS, Stewart EA, et al. Linzagolix with and without hormonal add-back therapy for the treatment of symptomatic uterine fibroids: two randomised, placebo-controlled, phase 3 trials. Lancet. 2022;400:896-907.
At the time of this writing, linzagolix was not approved by the FDA. The results of the PRIMROSE 1 (P1) and PRIMROSE 2 (P2) trials were published last year as 2 identical 52-week randomized, parallel, double-blind, placebo-controlled, phase 3 trials.7 The difference between the development of linzagolix as a GnRH antagonist and other similar medications is the strategy of potential partial hypothalamic pituitary ovarian axis suppression at 100 mg versus complete suppression at 200 mg. In this trial by Donnez and colleagues, both linzagolix doses were evaluated with and without add-back hormonal therapy and also were compared with placebo in a 1:1:1:1:1 ratio.7
Study details and results
To be eligible for this study, participants had to have heavy menstrual bleeding, defined as more than 80 mL for at least 2 cycles, and have at least 1 fibroid that was 2 cm in diameter or multiple small fibroids with the calculated uterine volume of more than 200 cm3. No fibroid larger than 12 cm in diameter was included.
The primary end point was a menstrual blood loss of 80 mL or less and a 50% or more reduction in menstrual blood loss from baseline in the 28 days before week 24. Uterine fibroid volume reduction and a safety assessment, including BMD assessment, also were studied.
In the P1 trial, which was conducted in US sites, the response rate for the primary objective was 56.4% in the linzagolix 100-mg group, 66.4% in the 100-mg plus add-back therapy group, 71.4% in the 200-mg group, and 75.5% in the 200-mg plus add-back group, compared with 35.0% in the placebo group.
In the P2 trial, which included sites in both Europe and the United States, the response rates were 56.7% in the 100-mg group, 77.2% in the 100-mg plus add-back therapy group, 77.7% in the 200-mg group, and 93.9% in the 200-mg plus add-back therapy group, compared with 29.4% in the placebo group. Thus, in both trials a significantly higher proportion of menstrual reduction occurred in all linzagolix treatment groups compared with placebo.
As expected, the incidence of hot flushes was the highest in participants taking the linzagolix 200-mg dose without add-back hormonal therapy, with hot flushes occurring in 35% (P1) and 32% (P2) of patients, compared with all other groups, which was 3% to 14%. All treatment groups showed improvement in quality-of-life scores compared with placebo. Of note, to achieve reduction of fibroid volume in the 40% to 50% range, this was observed consistently only with the linzagolix 200-mg alone dose.
Linzagolix effect on bone
Decreases in BMD appeared to be dose dependent, as lumbar spine losses of up to 4% were noted with the linzgolix 200-mg dose, and a 2% loss was observed with the 100-mg dose at 24 weeks. However, these were improved with add-back therapy. There were continued BMD decreases at 52 weeks, with up to 2.4% with 100 mg of linzagolix and up to 1.5% with 100 mg plus add-back therapy, and up to 2% with 200 mg of linzagolix plus add-back therapy. ●
Results of the P1 and P2 trials suggest that there could be a potential niche for linzagolix in patients who need chronic use (> 6 months) without the need for concomitant add-back hormone therapy at lower doses. The non-add-back option may be a possibility for women who have both a contraindication to estrogen and an increased risk for hormone-related adverse events.
- Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28;899-908.
- Curlin HL, Anderson TL. Endometrial cryoablation for the treatment of heavy menstrual bleeding: 36-month outcomes from the CLARITY study. Int J Womens Health. 2022;14:1083-1092.
- Curlin H, Cholkeri-Singh A, Leal JGG, et al. Hysteroscopic access and uterine cavity evaluation 12 months after endometrial ablation with the Cerene cryotherapy device. J Minim Invasive Gynecol. 2022;29:440-447.
- Radestad AF, Dahm-Kahler P, Holmberg E, et al. Longterm incidence of endometrial cancer after endometrial resection and ablation: a population based Swedish gynecologic cancer group (SweGCG) study. Acta Obstet Gynecol Scand. 2022;101:923-930.
- Oderkerk TJ, van de Kar MRD, Cornel KMC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555-1560.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Long-term relugolix combination therapy for symptomatic uterine leiomyomas. Obstet Gynecol. 2022;140:920-930.
- Donnez J, Taylor HS, Stewart EA, et al. Linzagolix with and without hormonal add-back therapy for the treatment of symptomatic uterine fibroids: two randomised, placebo- controlled, phase 3 trials. Lancet. 2022;400:896-907.
Endometrial ablation continues to be performed in significant numbers in the United States, with an estimated 500,000 cases annually. Several nonresectoscopic endometrial ablation devices have been approved for use, and some are now discontinued. The newest endometrial ablation therapy to gain US Food and Drug Administration (FDA) approval and to have published outcomes is the Cerene cryotherapy ablation device (Channel Medsystems, Inc). The results of 36-month outcomes from the CLARITY study were published last year, and we have chosen to review these long-term data in addition to that of a second study in which investigators assessed the ability to access the endometrial cavity postcryoablation. We believe this is important because of concerns about the inability to access the endometrial cavity after ablation, as well as the potential for delay in the diagnosis of endometrial cancer. It is interesting that 2 publications simultaneously reviewed the incidence of endometrial cancer after endometrial ablation within the past 12 months, and we therefore present those findings as they provide valuable information.
Our second focus in this year’s Update is to provide additional information about the burgeoning data on gonadotropin-releasing hormone (GnRH) antagonists. We review evidence on linzagolix from the PRIMROSE 1 and PRIMROSE 2 trials and longer-term data on relugolix combination therapy for symptomatic uterine fibroids.
Three-year follow-up after endometrial cryoablation with the Cerene device found high patient satisfaction, low hysterectomy rates
Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28;899-908.
Curlin HL, Anderson TL. Endometrial cryoablation for the treatment of heavy menstrual bleeding: 36-month outcomes from the CLARITY study. Int J Womens Health. 2022;14:1083-1092.
The 12-month data on the clinical safety and effectiveness of the Cerene cryoablation device were published in 2021 in the CLARITY trial.1 The 36-month outcomes were published in 2022 and showed sustained clinical effects through month 36 with a low risk of adverse outcomes.2 The interesting aspect of this trial is that although the amenorrhea rate was relatively low at 12 months (6.5%), it continued to remain relatively low compared with rates found with other devices, but the amenorrhea rate increased at 36 months (14.4%). This was the percentage of patients who reported, “I no longer get my period.”
Patient satisfaction was high
Despite a relatively low amenorrhea rate, study participants had a high satisfaction rate and a low 3-year hysterectomy rate. Eighty-five percent of the participants were satisfied or very satisfied, and the cumulative hysterectomy rate was low at 5%.
The overall reintervention rate was 8.7%. Six patients were treated with medications, 2 patients underwent repeat endometrial ablation, 1 received a levonorgestrel-releasing intrauterine device, and 12 underwent hysterectomy.
At 36 months, 201 of the original 242 participants were available for assessment. Unfortunately, 5 pregnancies were reported through the 6-month posttreatment period, which emphasizes the importance of having reliable contraception. However, there were no reports of hematometra or postablation tubal sterilization syndrome (PATSS).
Effect on bleeding was long term
The main finding of the CLARITY study is that the Cerene cryoablation device appears to have a relatively stable effect on bleedingfor the first 3 years after therapy, with minimal risk of hematometra and PATSS. What we find interesting is that despite Cerene cryoablation having one of the lowest amenorrhea rates, it not only had a satisfaction rate in line with that of other devices but also had a low hysterectomy rate—only 5%—at 3 years.
The study authors pointed out that there is a lack of scarring within the endometrial cavity with the Cerene device. Some may find less endometrial scarring worth a low amenorrhea rate in the context of a favorable satisfaction rate. This begs the question, how well can the endometrial cavity be assessed? For answers, keep reading.
Can the endometrial cavity be reliably accessed after Cerene cryoablation?
Endometrial ablation has been associated with intracavitary scarring that results in hematometra, PATSS, and a concern for difficulty in performing an adequate endometrial assessment in patients who develop postablation abnormal uterine bleeding.
In a prospective study, 230 participants (of an initial 242) treated with Cerene cyroablation were studied with hysteroscopic evaluation of the endometrial cavity 12 months after surgery.3 The uterine cavity was accessible in 98.7% of participants. The cavity was not accessible in 3 participants due to pain or cervical stenosis.
Visualization of the uterine cavity was possible by hysteroscopy in 92.7% of study participants (204 of 220), with 1 or both tubal ostia identified in 89.2%. Both tubal ostia were visible in 78.4% and 1 ostium was visible in 10.8%. The cavity was not visualized in 16 of the 220 patients (7.2%) due to intrauterine adhesions, technical difficulties, or menstruation. Also of note, 97 of the 230 participants available at the 12-month follow-up had undergone tubal sterilization before cryoablation and none reported symptoms of PATSS or hematometra, which may be considered surrogate markers for adhesions.
Results of the CLARITY study demonstrated the clinical safety and effectiveness of the Cerene cryoablation device at 12 months, with sustained clinical effects through 36 months and a low risk of adverse outcomes. Patient satisfaction rates were high, and the hysterectomy rate was low. In addition, in a prospective study of patients treated with Cerene cryoablation, hysteroscopic evaluation at 12 months found the uterine cavity accessible in more than 98% of participants, and uterine visualization also was high. Therefore, the Cerene cryoablation device may provide the advantage of an easier evaluation of patients who eventually develop abnormal bleeding after endometrial ablation.
Continue to: Tissue effects differ with ablation technique...
Tissue effects differ with ablation technique
The study authors suggested that different tissue effects occur with freezing compared with heating ablation techniques. With freezing, over weeks to months the chronic inflammatory tissue is eventually replaced by a fibrous scar of collagen, with some preservation of the collagen matrix during tissue repair. This may be different from the charring and boiling of heated tissue that results in architectural tissue loss and may interfere with wound repair and tissue remodeling. Although the incidence of postoperative adhesions after endometrial ablation is not well studied, it is encouraging that most patients who received cryoablation with the Cerene device were able to undergo an evaluation of the endometrium without general anesthesia.
Key takeaway
The main idea from this study is that the endometrium can be assessed by office hysteroscopy in most patients who undergo cryoablation with the Cerene device. This may have advantages in terms of reducing the risk of PATSS and hematometra, and it may allow easier evaluation of the endometrium for patients who have postablation abnormal uterine bleeding. This begs the question, does intrauterine scarring influence the detection of endometrial cancer? For answers, keep reading.
Does endometrial ablation place a patient at higher risk for a delay in the diagnosis of endometrial cancer?
Radestad AF, Dahm-Kahler P, Holmberg E, et al. Long-term incidence of endometrial cancer after endometrial resection and ablation: a population based Swedish gynecologic cancer group (SweGCG) study. Acta Obstet Gynecol Scand. 2022;101:923-930.
Oderkerk TJ, van de Kar MRD, Cornel KMC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555-1560.
The answer to this question appears to be no, based on 2 different types of studies. One study was a 20-year population database review from Sweden,4 and the other was a systematic review of 11 cohort studies.5
Population-based study findings
The data from the Swedish population database is interesting because since 1994 all Swedish citizens have been allocated a unique personal identification number at birth or immigration that enables official registries and research. In reviewing their data from 1997 through 2017, Radestad and colleagues compared transcervical resection of the endometrium (TCRE) and other forms of endometrial ablation against the Swedish National Patient Register data for endometrial cancer.4 They found no increase in the incidence of endometrial cancer after TCRE (0.3%) or after endometrial ablation (0.02%) and suggested a significantly lower incidence of endometrial cancer after endometrial ablation.
This study is beneficial because it is the largest study to explore the long-term incidence of endometrial cancer after TCRE and endometrial ablation. The investigators hypothesized that, as an explanation for the difference between rates, ablation may burn deeper into the myometrium and treat adenomyosis compared with TCRE. However, they also were cautious to note that although this was a 20-year study, the incidence of endometrial carcinoma likely will reach a peak in the next few years.
Systematic review conclusions
In the systematic review, out of 890 publications from the authors’ database search, 11 articles were eventually included for review.5 A total of 29,102 patients with endometrial ablation were followed for a period of up to 25 years, and the incidence of endometrial cancer after endometrial ablation varied from 0.0% to 1.6%. A total of 38 cases of endometrial cancer after endometrial ablation have been described in the literature. Of those cases, bleeding was the most common presenting symptom of the disease. Endometrial sampling was successful in 89% of cases, and in 90% of cases, histological exam showed an early-stage endometrial adenocarcinoma.
Based on their review, the authors concluded that the incidence of endometrial cancer was not increased in patients who received endometrial ablation, and more importantly, there was no apparent delay in the diagnosis of endometrial cancer after endometrial ablation. They further suggested that diagnostic management with endometrial sampling did not appear to be a barrier.
The main findings from these 2 studies by Radestad and colleagues and Oderkerk and associates are that endometrial cancer does not appear to be more common after endometrial ablation, and it appears to be diagnosed with endometrial sampling in most cases.4,5 There may be some protection against endometrial cancer with nonresectoscopic endometrial ablation, although this needs to be verified by additional studies. To juxtapose this information with the prior information about cryotherapy, it emphasizes that the scarring within the endometrium will likely reduce the incidence of PATSS and hematometra, which are relatively low-incidence occurrences at 5% to 7%, but it likely does not affect the detection of endometrial cancer.
Longer-term data for relugolix combination treatment of symptomatic uterine bleeding from fibroids shows sustained efficacy
Al-Hendy A, Lukes AS, Poindexter AN, et al. Long-term relugolix combination therapy for symptomatic uterine leiomyomas. Obstet Gynecol. 2022;140:920-930.
Relugolix combination therapy was previously reported to be effective for the treatment of fibroids based on the 24-week trials LIBERTY 1 and LIBERTY 2. We now have information about longer-term therapy for up to 52 weeks of treatment.6
Relugolix combination therapy is a once-daily single tablet for the treatment of heavy menstrual bleeding thought to be due to uterine fibroids in premenopausal women. It is comprised of relugolix 40 mg (a GnRH antagonist), estradiol 1.0 mg, and norethindrone acetate 0.5 mg.
Continue to: Extension study showed sustained efficacy...
Extension study showed sustained efficacy
The study by Al-Hendy and colleagues showed that the relugolix combination not only was well tolerated but also that there was sustained improvement in heavy bleeding, with the average patient having an approximately 90% decrease in menstrual bleeding from baseline.6 It was noted that 70.6% of patients achieved amenorrhea over the last 35 days of treatment.
Importantly, the treatment effect was independent of race, body mass index, baseline menstrual blood loss, and uterine fibroid volume. The bone mineral density (BMD) change trajectory was similar to what was observed in the pivotal study. No new safety concerns were identified, and BMD generally was preserved.
The extension study by Al-Hendy and colleagues demonstrated that that the reduced fibroid-associated bleeding treated with relugolix combination therapy is sustained throughout the 52-week period, with no new safety concerns.
Linzagolix is the newest GnRH antagonist to be studied in a randomized, placebo-controlled trial
Donnez J, Taylor HS, Stewart EA, et al. Linzagolix with and without hormonal add-back therapy for the treatment of symptomatic uterine fibroids: two randomised, placebo-controlled, phase 3 trials. Lancet. 2022;400:896-907.
At the time of this writing, linzagolix was not approved by the FDA. The results of the PRIMROSE 1 (P1) and PRIMROSE 2 (P2) trials were published last year as 2 identical 52-week randomized, parallel, double-blind, placebo-controlled, phase 3 trials.7 The difference between the development of linzagolix as a GnRH antagonist and other similar medications is the strategy of potential partial hypothalamic pituitary ovarian axis suppression at 100 mg versus complete suppression at 200 mg. In this trial by Donnez and colleagues, both linzagolix doses were evaluated with and without add-back hormonal therapy and also were compared with placebo in a 1:1:1:1:1 ratio.7
Study details and results
To be eligible for this study, participants had to have heavy menstrual bleeding, defined as more than 80 mL for at least 2 cycles, and have at least 1 fibroid that was 2 cm in diameter or multiple small fibroids with the calculated uterine volume of more than 200 cm3. No fibroid larger than 12 cm in diameter was included.
The primary end point was a menstrual blood loss of 80 mL or less and a 50% or more reduction in menstrual blood loss from baseline in the 28 days before week 24. Uterine fibroid volume reduction and a safety assessment, including BMD assessment, also were studied.
In the P1 trial, which was conducted in US sites, the response rate for the primary objective was 56.4% in the linzagolix 100-mg group, 66.4% in the 100-mg plus add-back therapy group, 71.4% in the 200-mg group, and 75.5% in the 200-mg plus add-back group, compared with 35.0% in the placebo group.
In the P2 trial, which included sites in both Europe and the United States, the response rates were 56.7% in the 100-mg group, 77.2% in the 100-mg plus add-back therapy group, 77.7% in the 200-mg group, and 93.9% in the 200-mg plus add-back therapy group, compared with 29.4% in the placebo group. Thus, in both trials a significantly higher proportion of menstrual reduction occurred in all linzagolix treatment groups compared with placebo.
As expected, the incidence of hot flushes was the highest in participants taking the linzagolix 200-mg dose without add-back hormonal therapy, with hot flushes occurring in 35% (P1) and 32% (P2) of patients, compared with all other groups, which was 3% to 14%. All treatment groups showed improvement in quality-of-life scores compared with placebo. Of note, to achieve reduction of fibroid volume in the 40% to 50% range, this was observed consistently only with the linzagolix 200-mg alone dose.
Linzagolix effect on bone
Decreases in BMD appeared to be dose dependent, as lumbar spine losses of up to 4% were noted with the linzgolix 200-mg dose, and a 2% loss was observed with the 100-mg dose at 24 weeks. However, these were improved with add-back therapy. There were continued BMD decreases at 52 weeks, with up to 2.4% with 100 mg of linzagolix and up to 1.5% with 100 mg plus add-back therapy, and up to 2% with 200 mg of linzagolix plus add-back therapy. ●
Results of the P1 and P2 trials suggest that there could be a potential niche for linzagolix in patients who need chronic use (> 6 months) without the need for concomitant add-back hormone therapy at lower doses. The non-add-back option may be a possibility for women who have both a contraindication to estrogen and an increased risk for hormone-related adverse events.
Endometrial ablation continues to be performed in significant numbers in the United States, with an estimated 500,000 cases annually. Several nonresectoscopic endometrial ablation devices have been approved for use, and some are now discontinued. The newest endometrial ablation therapy to gain US Food and Drug Administration (FDA) approval and to have published outcomes is the Cerene cryotherapy ablation device (Channel Medsystems, Inc). The results of 36-month outcomes from the CLARITY study were published last year, and we have chosen to review these long-term data in addition to that of a second study in which investigators assessed the ability to access the endometrial cavity postcryoablation. We believe this is important because of concerns about the inability to access the endometrial cavity after ablation, as well as the potential for delay in the diagnosis of endometrial cancer. It is interesting that 2 publications simultaneously reviewed the incidence of endometrial cancer after endometrial ablation within the past 12 months, and we therefore present those findings as they provide valuable information.
Our second focus in this year’s Update is to provide additional information about the burgeoning data on gonadotropin-releasing hormone (GnRH) antagonists. We review evidence on linzagolix from the PRIMROSE 1 and PRIMROSE 2 trials and longer-term data on relugolix combination therapy for symptomatic uterine fibroids.
Three-year follow-up after endometrial cryoablation with the Cerene device found high patient satisfaction, low hysterectomy rates
Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28;899-908.
Curlin HL, Anderson TL. Endometrial cryoablation for the treatment of heavy menstrual bleeding: 36-month outcomes from the CLARITY study. Int J Womens Health. 2022;14:1083-1092.
The 12-month data on the clinical safety and effectiveness of the Cerene cryoablation device were published in 2021 in the CLARITY trial.1 The 36-month outcomes were published in 2022 and showed sustained clinical effects through month 36 with a low risk of adverse outcomes.2 The interesting aspect of this trial is that although the amenorrhea rate was relatively low at 12 months (6.5%), it continued to remain relatively low compared with rates found with other devices, but the amenorrhea rate increased at 36 months (14.4%). This was the percentage of patients who reported, “I no longer get my period.”
Patient satisfaction was high
Despite a relatively low amenorrhea rate, study participants had a high satisfaction rate and a low 3-year hysterectomy rate. Eighty-five percent of the participants were satisfied or very satisfied, and the cumulative hysterectomy rate was low at 5%.
The overall reintervention rate was 8.7%. Six patients were treated with medications, 2 patients underwent repeat endometrial ablation, 1 received a levonorgestrel-releasing intrauterine device, and 12 underwent hysterectomy.
At 36 months, 201 of the original 242 participants were available for assessment. Unfortunately, 5 pregnancies were reported through the 6-month posttreatment period, which emphasizes the importance of having reliable contraception. However, there were no reports of hematometra or postablation tubal sterilization syndrome (PATSS).
Effect on bleeding was long term
The main finding of the CLARITY study is that the Cerene cryoablation device appears to have a relatively stable effect on bleedingfor the first 3 years after therapy, with minimal risk of hematometra and PATSS. What we find interesting is that despite Cerene cryoablation having one of the lowest amenorrhea rates, it not only had a satisfaction rate in line with that of other devices but also had a low hysterectomy rate—only 5%—at 3 years.
The study authors pointed out that there is a lack of scarring within the endometrial cavity with the Cerene device. Some may find less endometrial scarring worth a low amenorrhea rate in the context of a favorable satisfaction rate. This begs the question, how well can the endometrial cavity be assessed? For answers, keep reading.
Can the endometrial cavity be reliably accessed after Cerene cryoablation?
Endometrial ablation has been associated with intracavitary scarring that results in hematometra, PATSS, and a concern for difficulty in performing an adequate endometrial assessment in patients who develop postablation abnormal uterine bleeding.
In a prospective study, 230 participants (of an initial 242) treated with Cerene cyroablation were studied with hysteroscopic evaluation of the endometrial cavity 12 months after surgery.3 The uterine cavity was accessible in 98.7% of participants. The cavity was not accessible in 3 participants due to pain or cervical stenosis.
Visualization of the uterine cavity was possible by hysteroscopy in 92.7% of study participants (204 of 220), with 1 or both tubal ostia identified in 89.2%. Both tubal ostia were visible in 78.4% and 1 ostium was visible in 10.8%. The cavity was not visualized in 16 of the 220 patients (7.2%) due to intrauterine adhesions, technical difficulties, or menstruation. Also of note, 97 of the 230 participants available at the 12-month follow-up had undergone tubal sterilization before cryoablation and none reported symptoms of PATSS or hematometra, which may be considered surrogate markers for adhesions.
Results of the CLARITY study demonstrated the clinical safety and effectiveness of the Cerene cryoablation device at 12 months, with sustained clinical effects through 36 months and a low risk of adverse outcomes. Patient satisfaction rates were high, and the hysterectomy rate was low. In addition, in a prospective study of patients treated with Cerene cryoablation, hysteroscopic evaluation at 12 months found the uterine cavity accessible in more than 98% of participants, and uterine visualization also was high. Therefore, the Cerene cryoablation device may provide the advantage of an easier evaluation of patients who eventually develop abnormal bleeding after endometrial ablation.
Continue to: Tissue effects differ with ablation technique...
Tissue effects differ with ablation technique
The study authors suggested that different tissue effects occur with freezing compared with heating ablation techniques. With freezing, over weeks to months the chronic inflammatory tissue is eventually replaced by a fibrous scar of collagen, with some preservation of the collagen matrix during tissue repair. This may be different from the charring and boiling of heated tissue that results in architectural tissue loss and may interfere with wound repair and tissue remodeling. Although the incidence of postoperative adhesions after endometrial ablation is not well studied, it is encouraging that most patients who received cryoablation with the Cerene device were able to undergo an evaluation of the endometrium without general anesthesia.
Key takeaway
The main idea from this study is that the endometrium can be assessed by office hysteroscopy in most patients who undergo cryoablation with the Cerene device. This may have advantages in terms of reducing the risk of PATSS and hematometra, and it may allow easier evaluation of the endometrium for patients who have postablation abnormal uterine bleeding. This begs the question, does intrauterine scarring influence the detection of endometrial cancer? For answers, keep reading.
Does endometrial ablation place a patient at higher risk for a delay in the diagnosis of endometrial cancer?
Radestad AF, Dahm-Kahler P, Holmberg E, et al. Long-term incidence of endometrial cancer after endometrial resection and ablation: a population based Swedish gynecologic cancer group (SweGCG) study. Acta Obstet Gynecol Scand. 2022;101:923-930.
Oderkerk TJ, van de Kar MRD, Cornel KMC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555-1560.
The answer to this question appears to be no, based on 2 different types of studies. One study was a 20-year population database review from Sweden,4 and the other was a systematic review of 11 cohort studies.5
Population-based study findings
The data from the Swedish population database is interesting because since 1994 all Swedish citizens have been allocated a unique personal identification number at birth or immigration that enables official registries and research. In reviewing their data from 1997 through 2017, Radestad and colleagues compared transcervical resection of the endometrium (TCRE) and other forms of endometrial ablation against the Swedish National Patient Register data for endometrial cancer.4 They found no increase in the incidence of endometrial cancer after TCRE (0.3%) or after endometrial ablation (0.02%) and suggested a significantly lower incidence of endometrial cancer after endometrial ablation.
This study is beneficial because it is the largest study to explore the long-term incidence of endometrial cancer after TCRE and endometrial ablation. The investigators hypothesized that, as an explanation for the difference between rates, ablation may burn deeper into the myometrium and treat adenomyosis compared with TCRE. However, they also were cautious to note that although this was a 20-year study, the incidence of endometrial carcinoma likely will reach a peak in the next few years.
Systematic review conclusions
In the systematic review, out of 890 publications from the authors’ database search, 11 articles were eventually included for review.5 A total of 29,102 patients with endometrial ablation were followed for a period of up to 25 years, and the incidence of endometrial cancer after endometrial ablation varied from 0.0% to 1.6%. A total of 38 cases of endometrial cancer after endometrial ablation have been described in the literature. Of those cases, bleeding was the most common presenting symptom of the disease. Endometrial sampling was successful in 89% of cases, and in 90% of cases, histological exam showed an early-stage endometrial adenocarcinoma.
Based on their review, the authors concluded that the incidence of endometrial cancer was not increased in patients who received endometrial ablation, and more importantly, there was no apparent delay in the diagnosis of endometrial cancer after endometrial ablation. They further suggested that diagnostic management with endometrial sampling did not appear to be a barrier.
The main findings from these 2 studies by Radestad and colleagues and Oderkerk and associates are that endometrial cancer does not appear to be more common after endometrial ablation, and it appears to be diagnosed with endometrial sampling in most cases.4,5 There may be some protection against endometrial cancer with nonresectoscopic endometrial ablation, although this needs to be verified by additional studies. To juxtapose this information with the prior information about cryotherapy, it emphasizes that the scarring within the endometrium will likely reduce the incidence of PATSS and hematometra, which are relatively low-incidence occurrences at 5% to 7%, but it likely does not affect the detection of endometrial cancer.
Longer-term data for relugolix combination treatment of symptomatic uterine bleeding from fibroids shows sustained efficacy
Al-Hendy A, Lukes AS, Poindexter AN, et al. Long-term relugolix combination therapy for symptomatic uterine leiomyomas. Obstet Gynecol. 2022;140:920-930.
Relugolix combination therapy was previously reported to be effective for the treatment of fibroids based on the 24-week trials LIBERTY 1 and LIBERTY 2. We now have information about longer-term therapy for up to 52 weeks of treatment.6
Relugolix combination therapy is a once-daily single tablet for the treatment of heavy menstrual bleeding thought to be due to uterine fibroids in premenopausal women. It is comprised of relugolix 40 mg (a GnRH antagonist), estradiol 1.0 mg, and norethindrone acetate 0.5 mg.
Continue to: Extension study showed sustained efficacy...
Extension study showed sustained efficacy
The study by Al-Hendy and colleagues showed that the relugolix combination not only was well tolerated but also that there was sustained improvement in heavy bleeding, with the average patient having an approximately 90% decrease in menstrual bleeding from baseline.6 It was noted that 70.6% of patients achieved amenorrhea over the last 35 days of treatment.
Importantly, the treatment effect was independent of race, body mass index, baseline menstrual blood loss, and uterine fibroid volume. The bone mineral density (BMD) change trajectory was similar to what was observed in the pivotal study. No new safety concerns were identified, and BMD generally was preserved.
The extension study by Al-Hendy and colleagues demonstrated that that the reduced fibroid-associated bleeding treated with relugolix combination therapy is sustained throughout the 52-week period, with no new safety concerns.
Linzagolix is the newest GnRH antagonist to be studied in a randomized, placebo-controlled trial
Donnez J, Taylor HS, Stewart EA, et al. Linzagolix with and without hormonal add-back therapy for the treatment of symptomatic uterine fibroids: two randomised, placebo-controlled, phase 3 trials. Lancet. 2022;400:896-907.
At the time of this writing, linzagolix was not approved by the FDA. The results of the PRIMROSE 1 (P1) and PRIMROSE 2 (P2) trials were published last year as 2 identical 52-week randomized, parallel, double-blind, placebo-controlled, phase 3 trials.7 The difference between the development of linzagolix as a GnRH antagonist and other similar medications is the strategy of potential partial hypothalamic pituitary ovarian axis suppression at 100 mg versus complete suppression at 200 mg. In this trial by Donnez and colleagues, both linzagolix doses were evaluated with and without add-back hormonal therapy and also were compared with placebo in a 1:1:1:1:1 ratio.7
Study details and results
To be eligible for this study, participants had to have heavy menstrual bleeding, defined as more than 80 mL for at least 2 cycles, and have at least 1 fibroid that was 2 cm in diameter or multiple small fibroids with the calculated uterine volume of more than 200 cm3. No fibroid larger than 12 cm in diameter was included.
The primary end point was a menstrual blood loss of 80 mL or less and a 50% or more reduction in menstrual blood loss from baseline in the 28 days before week 24. Uterine fibroid volume reduction and a safety assessment, including BMD assessment, also were studied.
In the P1 trial, which was conducted in US sites, the response rate for the primary objective was 56.4% in the linzagolix 100-mg group, 66.4% in the 100-mg plus add-back therapy group, 71.4% in the 200-mg group, and 75.5% in the 200-mg plus add-back group, compared with 35.0% in the placebo group.
In the P2 trial, which included sites in both Europe and the United States, the response rates were 56.7% in the 100-mg group, 77.2% in the 100-mg plus add-back therapy group, 77.7% in the 200-mg group, and 93.9% in the 200-mg plus add-back therapy group, compared with 29.4% in the placebo group. Thus, in both trials a significantly higher proportion of menstrual reduction occurred in all linzagolix treatment groups compared with placebo.
As expected, the incidence of hot flushes was the highest in participants taking the linzagolix 200-mg dose without add-back hormonal therapy, with hot flushes occurring in 35% (P1) and 32% (P2) of patients, compared with all other groups, which was 3% to 14%. All treatment groups showed improvement in quality-of-life scores compared with placebo. Of note, to achieve reduction of fibroid volume in the 40% to 50% range, this was observed consistently only with the linzagolix 200-mg alone dose.
Linzagolix effect on bone
Decreases in BMD appeared to be dose dependent, as lumbar spine losses of up to 4% were noted with the linzgolix 200-mg dose, and a 2% loss was observed with the 100-mg dose at 24 weeks. However, these were improved with add-back therapy. There were continued BMD decreases at 52 weeks, with up to 2.4% with 100 mg of linzagolix and up to 1.5% with 100 mg plus add-back therapy, and up to 2% with 200 mg of linzagolix plus add-back therapy. ●
Results of the P1 and P2 trials suggest that there could be a potential niche for linzagolix in patients who need chronic use (> 6 months) without the need for concomitant add-back hormone therapy at lower doses. The non-add-back option may be a possibility for women who have both a contraindication to estrogen and an increased risk for hormone-related adverse events.
- Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28;899-908.
- Curlin HL, Anderson TL. Endometrial cryoablation for the treatment of heavy menstrual bleeding: 36-month outcomes from the CLARITY study. Int J Womens Health. 2022;14:1083-1092.
- Curlin H, Cholkeri-Singh A, Leal JGG, et al. Hysteroscopic access and uterine cavity evaluation 12 months after endometrial ablation with the Cerene cryotherapy device. J Minim Invasive Gynecol. 2022;29:440-447.
- Radestad AF, Dahm-Kahler P, Holmberg E, et al. Longterm incidence of endometrial cancer after endometrial resection and ablation: a population based Swedish gynecologic cancer group (SweGCG) study. Acta Obstet Gynecol Scand. 2022;101:923-930.
- Oderkerk TJ, van de Kar MRD, Cornel KMC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555-1560.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Long-term relugolix combination therapy for symptomatic uterine leiomyomas. Obstet Gynecol. 2022;140:920-930.
- Donnez J, Taylor HS, Stewart EA, et al. Linzagolix with and without hormonal add-back therapy for the treatment of symptomatic uterine fibroids: two randomised, placebo- controlled, phase 3 trials. Lancet. 2022;400:896-907.
- Curlin HL, Cintron LC, Anderson TL. A prospective, multicenter, clinical trial evaluating the safety and effectiveness of the Cerene device to treat heavy menstrual bleeding. J Minim Invasive Gynecol. 2021;28;899-908.
- Curlin HL, Anderson TL. Endometrial cryoablation for the treatment of heavy menstrual bleeding: 36-month outcomes from the CLARITY study. Int J Womens Health. 2022;14:1083-1092.
- Curlin H, Cholkeri-Singh A, Leal JGG, et al. Hysteroscopic access and uterine cavity evaluation 12 months after endometrial ablation with the Cerene cryotherapy device. J Minim Invasive Gynecol. 2022;29:440-447.
- Radestad AF, Dahm-Kahler P, Holmberg E, et al. Longterm incidence of endometrial cancer after endometrial resection and ablation: a population based Swedish gynecologic cancer group (SweGCG) study. Acta Obstet Gynecol Scand. 2022;101:923-930.
- Oderkerk TJ, van de Kar MRD, Cornel KMC, et al. Endometrial cancer after endometrial ablation: a systematic review. Int J Gynecol Cancer. 2022;32:1555-1560.
- Al-Hendy A, Lukes AS, Poindexter AN, et al. Long-term relugolix combination therapy for symptomatic uterine leiomyomas. Obstet Gynecol. 2022;140:920-930.
- Donnez J, Taylor HS, Stewart EA, et al. Linzagolix with and without hormonal add-back therapy for the treatment of symptomatic uterine fibroids: two randomised, placebo- controlled, phase 3 trials. Lancet. 2022;400:896-907.
News & Perspectives from Ob.Gyn. News
COMMENTARY
Answering the protein question when prescribing plant-based diets
Science supports the use of a whole food, predominantly plant-based dietary pattern for optimal health, including reduced risk for chronic disease, and best practice in treatment of leading chronic disease.
But clinicians who prescribe such eating patterns encounter a common concern from patients whose health may benefit.
“Where will I get my protein?”
We’ve all heard it, and it’s understandable
To ensure that patients have all the facts when making dietary decisions, clinicians need to be prepared to respond to concerns about protein adequacy and quality with evidence-based information. A good starting point for these conversations is to assess how much protein patients are already consuming. A review of the 2015-2016 National Health and Nutrition Examination Survey found that women normally consume an average of 69 g and men an average of 97 g of protein daily.
As a general point of reference, the recommended dietary allowance for protein is about 0.8 g/kg of bodyweight (or 0.36 g/lb), which equates to about 52 g of protein per day for a 145-lb woman and 65 g for a 180-lb man. But for many patients, it may be best to get a more precise recommendation based upon age, gender and physical activity level by using a handy Department of Agriculture tool for health care professionals to calculate daily protein and other nutrient needs. Patients can also use one of countless apps to track their protein and other nutrient intake. By using the tool and a tracking app, both clinician and patients can be fully informed whether protein needs are being met.
LATEST NEWS
Continuous glucose monitors for pregnant patients?
Patients with pregestational diabetes may benefit from use of a continuous subcutaneous insulin infusion pump paired with a continuous glucose monitor. Use of the tools has been associated with a reduction in maternal and neonatal morbidity, a recent study found.
“We were seeing an unacceptable burden of both maternal and fetal disease in our diabetic population,” said Neil Hamill, MD, a maternal-fetal medicine specialist at Methodist Women’s Hospital, Omaha, Neb., and an author of the study. “We thought the success with this technology in the nonpregnant population would and should translate into the pregnant population.”
Dr. Hamill and his colleagues analyzed data from 55 pregnant patients who received care at the Women’s Hospital Perinatal Center at the Nebraska Methodist Health System between October 2019 and October 2022. Everyone in the cohort had pregestational diabetes and required insulin prior to week 20 of pregnancy. They used CGMs for more than 2 weeks. The study set blood glucose levels of less than 140 mg/dL as a healthy benchmark.
Participants who had severe preeclampsia, who had delivered preterm, who had delivered a neonate with respiratory distress syndrome, and/or who had given birth to a larger-than-expected infant spent less time in the safe zone — having a blood glucose level below 140 mg/dL—than women who did not have those risk factors.
“When blood sugar control is better, maternal and fetal outcomes are improved,” Dr. Hamill said.
Neetu Sodhi, MD, an ob.gyn. at Providence Cedars-Sinai Tarzana Medical Center, Los Angeles, expressed optimism that use of blood glucose monitors and insulin pumps can improve outcomes for pregnant patients with pregestational diabetes.
“This is just another case for why it’s so important for patients to have access to these types of devices that really, really improve their outcomes and their health, and now it’s proven in the case of pregnancy outcomes too – or at least suggested strongly with this data,” Dr. Sodhi said.
Continue to: It may be time to pay attention to COVID again...
It may be time to pay attention to COVID again
More than 3 years into the COVID-19 era, most Americans have settled back into their prepandemic lifestyles. But a new dominant variant and rising hospitalization numbers may give way to another summer surge.
Since April, a new COVID variant has cropped up. According to recent Centers for Disease Control and Prevention data, EG.5—from the Omicron family—now makes up 17% of all cases in the United States, up from 7.5% in the first week of July.
A summary from the Center for Infectious Disease Research and Policy at the University of Minnesota says that EG.5, nicknamed “Eris” by health trackers, is nearly the same as its parent strain, XBB.1.9.2, but has one extra spike mutation.
Along with the news of EG.5’s growing prevalence, COVID-related hospitalization rates have increased by 12.5% during the week ending on July 29—the most significant uptick since December. Still, no connection has been made between the new variant and rising hospital admissions. And so far, experts have found no difference in the severity of illness or symptoms between Eris and the strains that came before it.
Cause for concern?
The COVID virus has a great tendency to mutate, said William Schaffner, MD, a professor of infectious diseases at Vanderbilt University, Nashville, Tenn.
“Fortunately, these are relatively minor mutations.” Even so, SARS-CoV-2, the virus that causes COVID-19, continues to be highly contagious. “There isn’t any doubt that it’s spreading—but it’s not more serious.”
So, Dr. Schaffner doesn’t think it’s time to panic. He prefers calling it an “uptick” in cases instead of a “surge,” because a surge “sounds too big.”
While the numbers are still low, compared with 2022’s summer surge, experts still urge people to stay aware of changes in the virus. “I do not think that there is any cause for alarm,” agreed Bernard Camins, MD, an infectious disease specialist at Mount Sinai Hospital, New York.
So why the higher number of cases? “There has been an increase in COVID cases this summer, probably related to travel, socializing, and dwindling masking,” said Anne Liu, MD, an allergy, immunology, and infectious disease specialist at Stanford (Calif.) University. Even so, “because of an existing level of immunity from vaccination and prior infections, it has been limited and case severity has been lower than in prior surges.” ●
COMMENTARY
Answering the protein question when prescribing plant-based diets
Science supports the use of a whole food, predominantly plant-based dietary pattern for optimal health, including reduced risk for chronic disease, and best practice in treatment of leading chronic disease.
But clinicians who prescribe such eating patterns encounter a common concern from patients whose health may benefit.
“Where will I get my protein?”
We’ve all heard it, and it’s understandable
To ensure that patients have all the facts when making dietary decisions, clinicians need to be prepared to respond to concerns about protein adequacy and quality with evidence-based information. A good starting point for these conversations is to assess how much protein patients are already consuming. A review of the 2015-2016 National Health and Nutrition Examination Survey found that women normally consume an average of 69 g and men an average of 97 g of protein daily.
As a general point of reference, the recommended dietary allowance for protein is about 0.8 g/kg of bodyweight (or 0.36 g/lb), which equates to about 52 g of protein per day for a 145-lb woman and 65 g for a 180-lb man. But for many patients, it may be best to get a more precise recommendation based upon age, gender and physical activity level by using a handy Department of Agriculture tool for health care professionals to calculate daily protein and other nutrient needs. Patients can also use one of countless apps to track their protein and other nutrient intake. By using the tool and a tracking app, both clinician and patients can be fully informed whether protein needs are being met.
LATEST NEWS
Continuous glucose monitors for pregnant patients?
Patients with pregestational diabetes may benefit from use of a continuous subcutaneous insulin infusion pump paired with a continuous glucose monitor. Use of the tools has been associated with a reduction in maternal and neonatal morbidity, a recent study found.
“We were seeing an unacceptable burden of both maternal and fetal disease in our diabetic population,” said Neil Hamill, MD, a maternal-fetal medicine specialist at Methodist Women’s Hospital, Omaha, Neb., and an author of the study. “We thought the success with this technology in the nonpregnant population would and should translate into the pregnant population.”
Dr. Hamill and his colleagues analyzed data from 55 pregnant patients who received care at the Women’s Hospital Perinatal Center at the Nebraska Methodist Health System between October 2019 and October 2022. Everyone in the cohort had pregestational diabetes and required insulin prior to week 20 of pregnancy. They used CGMs for more than 2 weeks. The study set blood glucose levels of less than 140 mg/dL as a healthy benchmark.
Participants who had severe preeclampsia, who had delivered preterm, who had delivered a neonate with respiratory distress syndrome, and/or who had given birth to a larger-than-expected infant spent less time in the safe zone — having a blood glucose level below 140 mg/dL—than women who did not have those risk factors.
“When blood sugar control is better, maternal and fetal outcomes are improved,” Dr. Hamill said.
Neetu Sodhi, MD, an ob.gyn. at Providence Cedars-Sinai Tarzana Medical Center, Los Angeles, expressed optimism that use of blood glucose monitors and insulin pumps can improve outcomes for pregnant patients with pregestational diabetes.
“This is just another case for why it’s so important for patients to have access to these types of devices that really, really improve their outcomes and their health, and now it’s proven in the case of pregnancy outcomes too – or at least suggested strongly with this data,” Dr. Sodhi said.
Continue to: It may be time to pay attention to COVID again...
It may be time to pay attention to COVID again
More than 3 years into the COVID-19 era, most Americans have settled back into their prepandemic lifestyles. But a new dominant variant and rising hospitalization numbers may give way to another summer surge.
Since April, a new COVID variant has cropped up. According to recent Centers for Disease Control and Prevention data, EG.5—from the Omicron family—now makes up 17% of all cases in the United States, up from 7.5% in the first week of July.
A summary from the Center for Infectious Disease Research and Policy at the University of Minnesota says that EG.5, nicknamed “Eris” by health trackers, is nearly the same as its parent strain, XBB.1.9.2, but has one extra spike mutation.
Along with the news of EG.5’s growing prevalence, COVID-related hospitalization rates have increased by 12.5% during the week ending on July 29—the most significant uptick since December. Still, no connection has been made between the new variant and rising hospital admissions. And so far, experts have found no difference in the severity of illness or symptoms between Eris and the strains that came before it.
Cause for concern?
The COVID virus has a great tendency to mutate, said William Schaffner, MD, a professor of infectious diseases at Vanderbilt University, Nashville, Tenn.
“Fortunately, these are relatively minor mutations.” Even so, SARS-CoV-2, the virus that causes COVID-19, continues to be highly contagious. “There isn’t any doubt that it’s spreading—but it’s not more serious.”
So, Dr. Schaffner doesn’t think it’s time to panic. He prefers calling it an “uptick” in cases instead of a “surge,” because a surge “sounds too big.”
While the numbers are still low, compared with 2022’s summer surge, experts still urge people to stay aware of changes in the virus. “I do not think that there is any cause for alarm,” agreed Bernard Camins, MD, an infectious disease specialist at Mount Sinai Hospital, New York.
So why the higher number of cases? “There has been an increase in COVID cases this summer, probably related to travel, socializing, and dwindling masking,” said Anne Liu, MD, an allergy, immunology, and infectious disease specialist at Stanford (Calif.) University. Even so, “because of an existing level of immunity from vaccination and prior infections, it has been limited and case severity has been lower than in prior surges.” ●
COMMENTARY
Answering the protein question when prescribing plant-based diets
Science supports the use of a whole food, predominantly plant-based dietary pattern for optimal health, including reduced risk for chronic disease, and best practice in treatment of leading chronic disease.
But clinicians who prescribe such eating patterns encounter a common concern from patients whose health may benefit.
“Where will I get my protein?”
We’ve all heard it, and it’s understandable
To ensure that patients have all the facts when making dietary decisions, clinicians need to be prepared to respond to concerns about protein adequacy and quality with evidence-based information. A good starting point for these conversations is to assess how much protein patients are already consuming. A review of the 2015-2016 National Health and Nutrition Examination Survey found that women normally consume an average of 69 g and men an average of 97 g of protein daily.
As a general point of reference, the recommended dietary allowance for protein is about 0.8 g/kg of bodyweight (or 0.36 g/lb), which equates to about 52 g of protein per day for a 145-lb woman and 65 g for a 180-lb man. But for many patients, it may be best to get a more precise recommendation based upon age, gender and physical activity level by using a handy Department of Agriculture tool for health care professionals to calculate daily protein and other nutrient needs. Patients can also use one of countless apps to track their protein and other nutrient intake. By using the tool and a tracking app, both clinician and patients can be fully informed whether protein needs are being met.
LATEST NEWS
Continuous glucose monitors for pregnant patients?
Patients with pregestational diabetes may benefit from use of a continuous subcutaneous insulin infusion pump paired with a continuous glucose monitor. Use of the tools has been associated with a reduction in maternal and neonatal morbidity, a recent study found.
“We were seeing an unacceptable burden of both maternal and fetal disease in our diabetic population,” said Neil Hamill, MD, a maternal-fetal medicine specialist at Methodist Women’s Hospital, Omaha, Neb., and an author of the study. “We thought the success with this technology in the nonpregnant population would and should translate into the pregnant population.”
Dr. Hamill and his colleagues analyzed data from 55 pregnant patients who received care at the Women’s Hospital Perinatal Center at the Nebraska Methodist Health System between October 2019 and October 2022. Everyone in the cohort had pregestational diabetes and required insulin prior to week 20 of pregnancy. They used CGMs for more than 2 weeks. The study set blood glucose levels of less than 140 mg/dL as a healthy benchmark.
Participants who had severe preeclampsia, who had delivered preterm, who had delivered a neonate with respiratory distress syndrome, and/or who had given birth to a larger-than-expected infant spent less time in the safe zone — having a blood glucose level below 140 mg/dL—than women who did not have those risk factors.
“When blood sugar control is better, maternal and fetal outcomes are improved,” Dr. Hamill said.
Neetu Sodhi, MD, an ob.gyn. at Providence Cedars-Sinai Tarzana Medical Center, Los Angeles, expressed optimism that use of blood glucose monitors and insulin pumps can improve outcomes for pregnant patients with pregestational diabetes.
“This is just another case for why it’s so important for patients to have access to these types of devices that really, really improve their outcomes and their health, and now it’s proven in the case of pregnancy outcomes too – or at least suggested strongly with this data,” Dr. Sodhi said.
Continue to: It may be time to pay attention to COVID again...
It may be time to pay attention to COVID again
More than 3 years into the COVID-19 era, most Americans have settled back into their prepandemic lifestyles. But a new dominant variant and rising hospitalization numbers may give way to another summer surge.
Since April, a new COVID variant has cropped up. According to recent Centers for Disease Control and Prevention data, EG.5—from the Omicron family—now makes up 17% of all cases in the United States, up from 7.5% in the first week of July.
A summary from the Center for Infectious Disease Research and Policy at the University of Minnesota says that EG.5, nicknamed “Eris” by health trackers, is nearly the same as its parent strain, XBB.1.9.2, but has one extra spike mutation.
Along with the news of EG.5’s growing prevalence, COVID-related hospitalization rates have increased by 12.5% during the week ending on July 29—the most significant uptick since December. Still, no connection has been made between the new variant and rising hospital admissions. And so far, experts have found no difference in the severity of illness or symptoms between Eris and the strains that came before it.
Cause for concern?
The COVID virus has a great tendency to mutate, said William Schaffner, MD, a professor of infectious diseases at Vanderbilt University, Nashville, Tenn.
“Fortunately, these are relatively minor mutations.” Even so, SARS-CoV-2, the virus that causes COVID-19, continues to be highly contagious. “There isn’t any doubt that it’s spreading—but it’s not more serious.”
So, Dr. Schaffner doesn’t think it’s time to panic. He prefers calling it an “uptick” in cases instead of a “surge,” because a surge “sounds too big.”
While the numbers are still low, compared with 2022’s summer surge, experts still urge people to stay aware of changes in the virus. “I do not think that there is any cause for alarm,” agreed Bernard Camins, MD, an infectious disease specialist at Mount Sinai Hospital, New York.
So why the higher number of cases? “There has been an increase in COVID cases this summer, probably related to travel, socializing, and dwindling masking,” said Anne Liu, MD, an allergy, immunology, and infectious disease specialist at Stanford (Calif.) University. Even so, “because of an existing level of immunity from vaccination and prior infections, it has been limited and case severity has been lower than in prior surges.” ●
Commentary: Are "significant" results necessarily clinically meaningful? October 2023
I was excited to see that the study included the use of objective electronic monitoring of sleep quality. This was done using wrist actigraphy, devices on the wrist that measure acceleration movements. What a great tool this could be for measuring how much scratching our patients are doing! With devices like these measuring movements objectively, we wouldn't have to rely on patients' self-report of itch or sleep quality. Sadly, these monitors did not show any meaningful differences between the dupilumab and placebo groups. This technology holds great promise but it isn't yet ready for prime-time assessment of scratching or sleep.
The title of Chiesa Fuxench and colleagues' article, "Risk of Inflammatory Bowel Disease in Patients With Atopic Dermatitis," might be scary to our patients. The authors reported that "children and adults with AD had an increased risk of IBD [inflammatory bowel disease]." The authors concluded, "Clinicians should be aware of these risks, particularly when selecting systemic treatments for AD in patients who may have coincident gastrointestinal symptoms." Bah, humbug, I say!
Be careful when someone tells you there is increased risk. This study was done exceptionally well by an exceptionally good research team. They were working with a huge database and included many controls to ensure that their findings weren't due to chance. And while they did find an "increased risk," they proved — rather conclusively, I believe — that the increased risk is tiny and not something we need to worry about.
The results of this study suggest that there is a scientific link between AD and IBD, probably some genetic inflammatory signaling contributing to both conditions. But even in the highest-risk group, it would take seeing well over 1000 patients for a year to see one more case of IBD due to AD. This article is a good foundation for researchers who want to explore the underlying connection between AD and IBD. The study is an even better foundation for physicians who want to reassure patients that there is little to no meaningful increased risk for IBD in patients with AD.
Am I allowed to just say "Ditto!"? Wan and colleagues' article "Incidence of Cardiovascular Disease and Venous Thromboembolism in Patients with Atopic Dermatitis" does show a statistically significant increased risk for cardiovascular (CV) disease in patients with AD. Is that increase clinically significant? This study was also exceptionally well done by an exceptionally good research team. They concluded, "Atopic dermatitis, particularly when severe, is associated with increased risks of venous thromboembolism and CV disease, which may influence the monitoring of patients and selection of treatments for AD." I look at their findings and conclude that AD, even when severe, is associated with little if any clinically meaningful increased risks for venous thromboembolism or CV disease, and we don't need to add any special CV monitoring of AD patients.
The key data are presented in Table 2 of their manuscript. In children, the risk for deep vein thrombosis (DVT) in those with severe AD was about 3 times (0.16) that of those with no AD (0.05). But those numbers are per 1000 patient-years. Therefore, the increased risk is 0.16 - 0.05 = 0.11/1000 patient-years. Thus, you'd expect to see one more case of DVT per year in every 9000 children with severe AD. Does that mean we need to monitor all 9000 for DVT? Would that be cost-effective? Might the monitoring cause more problems than it would solve?
CV disease is much more common in adults than in children, but still, with a difference in risk of about 0.5-1 per 1000 patient-years, you'd only expect one more event due to AD in every 1000-2000 patients, and even that is assuming that the entire risk difference was due to AD and not to some other variable that wasn't measured.
With so much drug development for AD, I think we are going to be inundated with companies wanting us to hear their message over and over again. One way to do that is to mine clinical trial data for more papers. In Merola and colleagues' article "Safety and Efficacy of Tralokinumab in Older Adults With Moderate-to-Severe Atopic Dermatitis" we see just that. We already know that tralokinumab is effective for moderate to severe AD from past publications of clinical trial data. Here, the investigators report on a subset of the clinical trial data — the data on older adults — and, not surprisingly, the drug worked. The efficacy rate, 17% getting clear or almost clear, doesn't sound particularly exciting compared with the higher rates we've seen for other products, but perhaps that lower rate is due in part to differences in studies. Instead of more cuts of data from the same trials, it would be nice to see how tralokinumab compares with other AD treatments on a head-to-head basis.
I was excited to see that the study included the use of objective electronic monitoring of sleep quality. This was done using wrist actigraphy, devices on the wrist that measure acceleration movements. What a great tool this could be for measuring how much scratching our patients are doing! With devices like these measuring movements objectively, we wouldn't have to rely on patients' self-report of itch or sleep quality. Sadly, these monitors did not show any meaningful differences between the dupilumab and placebo groups. This technology holds great promise but it isn't yet ready for prime-time assessment of scratching or sleep.
The title of Chiesa Fuxench and colleagues' article, "Risk of Inflammatory Bowel Disease in Patients With Atopic Dermatitis," might be scary to our patients. The authors reported that "children and adults with AD had an increased risk of IBD [inflammatory bowel disease]." The authors concluded, "Clinicians should be aware of these risks, particularly when selecting systemic treatments for AD in patients who may have coincident gastrointestinal symptoms." Bah, humbug, I say!
Be careful when someone tells you there is increased risk. This study was done exceptionally well by an exceptionally good research team. They were working with a huge database and included many controls to ensure that their findings weren't due to chance. And while they did find an "increased risk," they proved — rather conclusively, I believe — that the increased risk is tiny and not something we need to worry about.
The results of this study suggest that there is a scientific link between AD and IBD, probably some genetic inflammatory signaling contributing to both conditions. But even in the highest-risk group, it would take seeing well over 1000 patients for a year to see one more case of IBD due to AD. This article is a good foundation for researchers who want to explore the underlying connection between AD and IBD. The study is an even better foundation for physicians who want to reassure patients that there is little to no meaningful increased risk for IBD in patients with AD.
Am I allowed to just say "Ditto!"? Wan and colleagues' article "Incidence of Cardiovascular Disease and Venous Thromboembolism in Patients with Atopic Dermatitis" does show a statistically significant increased risk for cardiovascular (CV) disease in patients with AD. Is that increase clinically significant? This study was also exceptionally well done by an exceptionally good research team. They concluded, "Atopic dermatitis, particularly when severe, is associated with increased risks of venous thromboembolism and CV disease, which may influence the monitoring of patients and selection of treatments for AD." I look at their findings and conclude that AD, even when severe, is associated with little if any clinically meaningful increased risks for venous thromboembolism or CV disease, and we don't need to add any special CV monitoring of AD patients.
The key data are presented in Table 2 of their manuscript. In children, the risk for deep vein thrombosis (DVT) in those with severe AD was about 3 times (0.16) that of those with no AD (0.05). But those numbers are per 1000 patient-years. Therefore, the increased risk is 0.16 - 0.05 = 0.11/1000 patient-years. Thus, you'd expect to see one more case of DVT per year in every 9000 children with severe AD. Does that mean we need to monitor all 9000 for DVT? Would that be cost-effective? Might the monitoring cause more problems than it would solve?
CV disease is much more common in adults than in children, but still, with a difference in risk of about 0.5-1 per 1000 patient-years, you'd only expect one more event due to AD in every 1000-2000 patients, and even that is assuming that the entire risk difference was due to AD and not to some other variable that wasn't measured.
With so much drug development for AD, I think we are going to be inundated with companies wanting us to hear their message over and over again. One way to do that is to mine clinical trial data for more papers. In Merola and colleagues' article "Safety and Efficacy of Tralokinumab in Older Adults With Moderate-to-Severe Atopic Dermatitis" we see just that. We already know that tralokinumab is effective for moderate to severe AD from past publications of clinical trial data. Here, the investigators report on a subset of the clinical trial data — the data on older adults — and, not surprisingly, the drug worked. The efficacy rate, 17% getting clear or almost clear, doesn't sound particularly exciting compared with the higher rates we've seen for other products, but perhaps that lower rate is due in part to differences in studies. Instead of more cuts of data from the same trials, it would be nice to see how tralokinumab compares with other AD treatments on a head-to-head basis.
I was excited to see that the study included the use of objective electronic monitoring of sleep quality. This was done using wrist actigraphy, devices on the wrist that measure acceleration movements. What a great tool this could be for measuring how much scratching our patients are doing! With devices like these measuring movements objectively, we wouldn't have to rely on patients' self-report of itch or sleep quality. Sadly, these monitors did not show any meaningful differences between the dupilumab and placebo groups. This technology holds great promise but it isn't yet ready for prime-time assessment of scratching or sleep.
The title of Chiesa Fuxench and colleagues' article, "Risk of Inflammatory Bowel Disease in Patients With Atopic Dermatitis," might be scary to our patients. The authors reported that "children and adults with AD had an increased risk of IBD [inflammatory bowel disease]." The authors concluded, "Clinicians should be aware of these risks, particularly when selecting systemic treatments for AD in patients who may have coincident gastrointestinal symptoms." Bah, humbug, I say!
Be careful when someone tells you there is increased risk. This study was done exceptionally well by an exceptionally good research team. They were working with a huge database and included many controls to ensure that their findings weren't due to chance. And while they did find an "increased risk," they proved — rather conclusively, I believe — that the increased risk is tiny and not something we need to worry about.
The results of this study suggest that there is a scientific link between AD and IBD, probably some genetic inflammatory signaling contributing to both conditions. But even in the highest-risk group, it would take seeing well over 1000 patients for a year to see one more case of IBD due to AD. This article is a good foundation for researchers who want to explore the underlying connection between AD and IBD. The study is an even better foundation for physicians who want to reassure patients that there is little to no meaningful increased risk for IBD in patients with AD.
Am I allowed to just say "Ditto!"? Wan and colleagues' article "Incidence of Cardiovascular Disease and Venous Thromboembolism in Patients with Atopic Dermatitis" does show a statistically significant increased risk for cardiovascular (CV) disease in patients with AD. Is that increase clinically significant? This study was also exceptionally well done by an exceptionally good research team. They concluded, "Atopic dermatitis, particularly when severe, is associated with increased risks of venous thromboembolism and CV disease, which may influence the monitoring of patients and selection of treatments for AD." I look at their findings and conclude that AD, even when severe, is associated with little if any clinically meaningful increased risks for venous thromboembolism or CV disease, and we don't need to add any special CV monitoring of AD patients.
The key data are presented in Table 2 of their manuscript. In children, the risk for deep vein thrombosis (DVT) in those with severe AD was about 3 times (0.16) that of those with no AD (0.05). But those numbers are per 1000 patient-years. Therefore, the increased risk is 0.16 - 0.05 = 0.11/1000 patient-years. Thus, you'd expect to see one more case of DVT per year in every 9000 children with severe AD. Does that mean we need to monitor all 9000 for DVT? Would that be cost-effective? Might the monitoring cause more problems than it would solve?
CV disease is much more common in adults than in children, but still, with a difference in risk of about 0.5-1 per 1000 patient-years, you'd only expect one more event due to AD in every 1000-2000 patients, and even that is assuming that the entire risk difference was due to AD and not to some other variable that wasn't measured.
With so much drug development for AD, I think we are going to be inundated with companies wanting us to hear their message over and over again. One way to do that is to mine clinical trial data for more papers. In Merola and colleagues' article "Safety and Efficacy of Tralokinumab in Older Adults With Moderate-to-Severe Atopic Dermatitis" we see just that. We already know that tralokinumab is effective for moderate to severe AD from past publications of clinical trial data. Here, the investigators report on a subset of the clinical trial data — the data on older adults — and, not surprisingly, the drug worked. The efficacy rate, 17% getting clear or almost clear, doesn't sound particularly exciting compared with the higher rates we've seen for other products, but perhaps that lower rate is due in part to differences in studies. Instead of more cuts of data from the same trials, it would be nice to see how tralokinumab compares with other AD treatments on a head-to-head basis.
Therapeutic vaccine shows promise in treating lung cancer
SINGAPORE – A few months after releasing its phase 1 and 2 data, OSE Immunotherapeutics, which is based in Nantes, France, has announced positive results for its therapeutic vaccine to treat cancer. Following its promising findings concerning early-stage melanoma, pancreatic cancer, ENT cancers, and HPV-associated anogenital cancer,
The results suggest that Tedopi is the most developmentally advanced therapeutic vaccine for cancer.
The data from Atalante-1 were presented at the World Conference on Lung Cancer and were simultaneously published in Annals of Oncology.
Tedopi is composed of synthetic tumoral neo-epitopes (peptide fragments) that target five tumoral antigens, permitting the activation of tumor-specific T-lymphocytes for patients who are HLA-A2 positive. In 95% of cases, tumors express at least one of these five antigens. The aim of integrating these five antigens is to prevent immune escape. The technology uses the human leukocyte antigen (HLA) system, one of the keys for presenting antigens to T-lymphocytes. The vaccine is effective for patients who express the HLA-A2 gene, which is present in around half of the population. The HLA-A2 biomarker, detected via a blood test, can identify appropriate patients.
Study protocol
In the Atalante-1 trial, participants had locally advanced (unresectable and not eligible for radiotherapy) or metastatic (without alteration of the EGFR and ALK genes) non–small cell lung cancer that was resistant to previous immunotherapy. They had an HLA-A2 phenotype, as determined by a blood draw to determine whether their immune system could respond to the vaccine.
In this trial, 219 patients were randomly assigned in a 2:1 ratio to receive the vaccine or standard-of-care chemotherapy (80% received docetaxel). The vaccine was administered subcutaneously on day 1 every 3 weeks for six cycles. After that point, the vaccine was administered every 8 weeks until 1 year of treatment and every 12 weeks thereafter. The primary endpoint was overall survival.
Secondary resistance
The plan was to enroll 363 patients in the protocol, but the study did not complete its recruitment phase because of the COVID-19 pandemic. As a result, the study was stopped after the enrollment of 219 patients.
“It didn’t have the power we would have liked, but it helped us understand that the people who benefited the most from the vaccine were patients who had responded to immunotherapy in the past. These patients have what is called ‘secondary resistance,’ ” explained Benjamin Besse, MD, PhD, during a press conference organized by OSE Immunotherapeutics. Dr. Besse, the study’s principal investigator, is the director of clinical research at Gustave Roussy, Villejuif, France.
Overall, the results weren’t significant. But the results were positive for patients who had previously responded well to immunotherapy for at least 3 months. Of the 219 patients, 118 (54%) had a positive response.
Among these patients with secondary resistance to immunotherapy, median OS was 11.1 months with Tedopi versus 7.5 months with docetaxel.
For these patients, the risk of death was reduced by 41% with the vaccine, compared with chemotherapy. Overall, 44% of patients lived for another year after receiving Tedopi, versus 27.5% with docetaxel.
“This study is a positive signal for overall survival in the selected population. In this study of 219 patients, we realized that just half of patients really benefited from the vaccine: those who had previously responded to immunotherapy,” said Dr. Besse. “The study needs confirmation from a further, larger phase 3 study in more than 300 patients with secondary resistance to immunotherapy to give us the statistical power we need to convince the regulatory authorities.”
Tolerability profile
Fewer serious adverse effects were reported with the vaccine than with chemotherapy (11.4% with Tedopi and 35.1% with docetaxel).
The vaccine also allowed patients to maintain a better quality of life. Scores from the Quality of Life Questionnaire Core 30, which explores several areas of daily life, were better with the vaccine. Change in patients’ overall well-being was delayed in the vaccine group: 3.3 months in the chemotherapy arm versus 9 months in the vaccine arm.
“The vaccine was well tolerated. It has benefits in terms of controlling disease symptoms and causes few side effects. Chemotherapy with docetaxel, meanwhile, is more toxic and may affect a patient’s overall well-being. It causes hair loss in practically 100% of patients, induces neuropathy, makes hands and feet swell, damages the nails, is associated with nausea and vomiting ...” noted Dr. Besse. He went on to say that after the trial, of the patients who stopped receiving the vaccine or chemotherapy (either for toxicity reasons or for disease progression), those who had been given the vaccine responded better to the subsequent chemotherapy “because their overall health was better.”
Clinical development
The clinical development of Tedopi is ongoing. Three trials are currently taking place. One study is comparing the Tedopi vaccine plus docetaxel with Tedopi plus nivolumab (immunotherapy not used as a first-line treatment) to determine whether the effects of these treatment combinations might might be enhanced for patients with previously treated lung cancer.
Another study relating to ovarian cancer is in the recruitment phase. The researchers seek to evaluate the vaccine alone or in combination with pembrolizumab for patients with platinum-sensitive ovarian cancer. Results from both trials are expected in 2025.
The third trial seeks to assess FOLFIRI as maintenance therapy or FOLFIRI as maintenance plus Tedopi for patients with pancreatic cancer to improve disease management. Efficacy data are expected next year.
OSE Immunotherapeutics is simultaneously working on a companion biomarker, the HLA-A2 test.
The study was funded by OSE Immunotherapeutics. Dr. Besse disclosed the following conflicts of interest (research funding, institution): AbbVie, Amgen, AstraZeneca, Chugai Pharmaceutical, Daiichi-Sankyo, Ellipse Pharma, EISAI, Genmab, Genzyme Corporation, Hedera Dx, Inivata, IPSEN, Janssen, MSD, Pharmamar, Roche-Genentech, Sanofi, Socar Research, Taiho Oncology, and Turning Point Therapeutics.
This article was translated from the Medscape French Edition and a version appeared on Medscape.com.
SINGAPORE – A few months after releasing its phase 1 and 2 data, OSE Immunotherapeutics, which is based in Nantes, France, has announced positive results for its therapeutic vaccine to treat cancer. Following its promising findings concerning early-stage melanoma, pancreatic cancer, ENT cancers, and HPV-associated anogenital cancer,
The results suggest that Tedopi is the most developmentally advanced therapeutic vaccine for cancer.
The data from Atalante-1 were presented at the World Conference on Lung Cancer and were simultaneously published in Annals of Oncology.
Tedopi is composed of synthetic tumoral neo-epitopes (peptide fragments) that target five tumoral antigens, permitting the activation of tumor-specific T-lymphocytes for patients who are HLA-A2 positive. In 95% of cases, tumors express at least one of these five antigens. The aim of integrating these five antigens is to prevent immune escape. The technology uses the human leukocyte antigen (HLA) system, one of the keys for presenting antigens to T-lymphocytes. The vaccine is effective for patients who express the HLA-A2 gene, which is present in around half of the population. The HLA-A2 biomarker, detected via a blood test, can identify appropriate patients.
Study protocol
In the Atalante-1 trial, participants had locally advanced (unresectable and not eligible for radiotherapy) or metastatic (without alteration of the EGFR and ALK genes) non–small cell lung cancer that was resistant to previous immunotherapy. They had an HLA-A2 phenotype, as determined by a blood draw to determine whether their immune system could respond to the vaccine.
In this trial, 219 patients were randomly assigned in a 2:1 ratio to receive the vaccine or standard-of-care chemotherapy (80% received docetaxel). The vaccine was administered subcutaneously on day 1 every 3 weeks for six cycles. After that point, the vaccine was administered every 8 weeks until 1 year of treatment and every 12 weeks thereafter. The primary endpoint was overall survival.
Secondary resistance
The plan was to enroll 363 patients in the protocol, but the study did not complete its recruitment phase because of the COVID-19 pandemic. As a result, the study was stopped after the enrollment of 219 patients.
“It didn’t have the power we would have liked, but it helped us understand that the people who benefited the most from the vaccine were patients who had responded to immunotherapy in the past. These patients have what is called ‘secondary resistance,’ ” explained Benjamin Besse, MD, PhD, during a press conference organized by OSE Immunotherapeutics. Dr. Besse, the study’s principal investigator, is the director of clinical research at Gustave Roussy, Villejuif, France.
Overall, the results weren’t significant. But the results were positive for patients who had previously responded well to immunotherapy for at least 3 months. Of the 219 patients, 118 (54%) had a positive response.
Among these patients with secondary resistance to immunotherapy, median OS was 11.1 months with Tedopi versus 7.5 months with docetaxel.
For these patients, the risk of death was reduced by 41% with the vaccine, compared with chemotherapy. Overall, 44% of patients lived for another year after receiving Tedopi, versus 27.5% with docetaxel.
“This study is a positive signal for overall survival in the selected population. In this study of 219 patients, we realized that just half of patients really benefited from the vaccine: those who had previously responded to immunotherapy,” said Dr. Besse. “The study needs confirmation from a further, larger phase 3 study in more than 300 patients with secondary resistance to immunotherapy to give us the statistical power we need to convince the regulatory authorities.”
Tolerability profile
Fewer serious adverse effects were reported with the vaccine than with chemotherapy (11.4% with Tedopi and 35.1% with docetaxel).
The vaccine also allowed patients to maintain a better quality of life. Scores from the Quality of Life Questionnaire Core 30, which explores several areas of daily life, were better with the vaccine. Change in patients’ overall well-being was delayed in the vaccine group: 3.3 months in the chemotherapy arm versus 9 months in the vaccine arm.
“The vaccine was well tolerated. It has benefits in terms of controlling disease symptoms and causes few side effects. Chemotherapy with docetaxel, meanwhile, is more toxic and may affect a patient’s overall well-being. It causes hair loss in practically 100% of patients, induces neuropathy, makes hands and feet swell, damages the nails, is associated with nausea and vomiting ...” noted Dr. Besse. He went on to say that after the trial, of the patients who stopped receiving the vaccine or chemotherapy (either for toxicity reasons or for disease progression), those who had been given the vaccine responded better to the subsequent chemotherapy “because their overall health was better.”
Clinical development
The clinical development of Tedopi is ongoing. Three trials are currently taking place. One study is comparing the Tedopi vaccine plus docetaxel with Tedopi plus nivolumab (immunotherapy not used as a first-line treatment) to determine whether the effects of these treatment combinations might might be enhanced for patients with previously treated lung cancer.
Another study relating to ovarian cancer is in the recruitment phase. The researchers seek to evaluate the vaccine alone or in combination with pembrolizumab for patients with platinum-sensitive ovarian cancer. Results from both trials are expected in 2025.
The third trial seeks to assess FOLFIRI as maintenance therapy or FOLFIRI as maintenance plus Tedopi for patients with pancreatic cancer to improve disease management. Efficacy data are expected next year.
OSE Immunotherapeutics is simultaneously working on a companion biomarker, the HLA-A2 test.
The study was funded by OSE Immunotherapeutics. Dr. Besse disclosed the following conflicts of interest (research funding, institution): AbbVie, Amgen, AstraZeneca, Chugai Pharmaceutical, Daiichi-Sankyo, Ellipse Pharma, EISAI, Genmab, Genzyme Corporation, Hedera Dx, Inivata, IPSEN, Janssen, MSD, Pharmamar, Roche-Genentech, Sanofi, Socar Research, Taiho Oncology, and Turning Point Therapeutics.
This article was translated from the Medscape French Edition and a version appeared on Medscape.com.
SINGAPORE – A few months after releasing its phase 1 and 2 data, OSE Immunotherapeutics, which is based in Nantes, France, has announced positive results for its therapeutic vaccine to treat cancer. Following its promising findings concerning early-stage melanoma, pancreatic cancer, ENT cancers, and HPV-associated anogenital cancer,
The results suggest that Tedopi is the most developmentally advanced therapeutic vaccine for cancer.
The data from Atalante-1 were presented at the World Conference on Lung Cancer and were simultaneously published in Annals of Oncology.
Tedopi is composed of synthetic tumoral neo-epitopes (peptide fragments) that target five tumoral antigens, permitting the activation of tumor-specific T-lymphocytes for patients who are HLA-A2 positive. In 95% of cases, tumors express at least one of these five antigens. The aim of integrating these five antigens is to prevent immune escape. The technology uses the human leukocyte antigen (HLA) system, one of the keys for presenting antigens to T-lymphocytes. The vaccine is effective for patients who express the HLA-A2 gene, which is present in around half of the population. The HLA-A2 biomarker, detected via a blood test, can identify appropriate patients.
Study protocol
In the Atalante-1 trial, participants had locally advanced (unresectable and not eligible for radiotherapy) or metastatic (without alteration of the EGFR and ALK genes) non–small cell lung cancer that was resistant to previous immunotherapy. They had an HLA-A2 phenotype, as determined by a blood draw to determine whether their immune system could respond to the vaccine.
In this trial, 219 patients were randomly assigned in a 2:1 ratio to receive the vaccine or standard-of-care chemotherapy (80% received docetaxel). The vaccine was administered subcutaneously on day 1 every 3 weeks for six cycles. After that point, the vaccine was administered every 8 weeks until 1 year of treatment and every 12 weeks thereafter. The primary endpoint was overall survival.
Secondary resistance
The plan was to enroll 363 patients in the protocol, but the study did not complete its recruitment phase because of the COVID-19 pandemic. As a result, the study was stopped after the enrollment of 219 patients.
“It didn’t have the power we would have liked, but it helped us understand that the people who benefited the most from the vaccine were patients who had responded to immunotherapy in the past. These patients have what is called ‘secondary resistance,’ ” explained Benjamin Besse, MD, PhD, during a press conference organized by OSE Immunotherapeutics. Dr. Besse, the study’s principal investigator, is the director of clinical research at Gustave Roussy, Villejuif, France.
Overall, the results weren’t significant. But the results were positive for patients who had previously responded well to immunotherapy for at least 3 months. Of the 219 patients, 118 (54%) had a positive response.
Among these patients with secondary resistance to immunotherapy, median OS was 11.1 months with Tedopi versus 7.5 months with docetaxel.
For these patients, the risk of death was reduced by 41% with the vaccine, compared with chemotherapy. Overall, 44% of patients lived for another year after receiving Tedopi, versus 27.5% with docetaxel.
“This study is a positive signal for overall survival in the selected population. In this study of 219 patients, we realized that just half of patients really benefited from the vaccine: those who had previously responded to immunotherapy,” said Dr. Besse. “The study needs confirmation from a further, larger phase 3 study in more than 300 patients with secondary resistance to immunotherapy to give us the statistical power we need to convince the regulatory authorities.”
Tolerability profile
Fewer serious adverse effects were reported with the vaccine than with chemotherapy (11.4% with Tedopi and 35.1% with docetaxel).
The vaccine also allowed patients to maintain a better quality of life. Scores from the Quality of Life Questionnaire Core 30, which explores several areas of daily life, were better with the vaccine. Change in patients’ overall well-being was delayed in the vaccine group: 3.3 months in the chemotherapy arm versus 9 months in the vaccine arm.
“The vaccine was well tolerated. It has benefits in terms of controlling disease symptoms and causes few side effects. Chemotherapy with docetaxel, meanwhile, is more toxic and may affect a patient’s overall well-being. It causes hair loss in practically 100% of patients, induces neuropathy, makes hands and feet swell, damages the nails, is associated with nausea and vomiting ...” noted Dr. Besse. He went on to say that after the trial, of the patients who stopped receiving the vaccine or chemotherapy (either for toxicity reasons or for disease progression), those who had been given the vaccine responded better to the subsequent chemotherapy “because their overall health was better.”
Clinical development
The clinical development of Tedopi is ongoing. Three trials are currently taking place. One study is comparing the Tedopi vaccine plus docetaxel with Tedopi plus nivolumab (immunotherapy not used as a first-line treatment) to determine whether the effects of these treatment combinations might might be enhanced for patients with previously treated lung cancer.
Another study relating to ovarian cancer is in the recruitment phase. The researchers seek to evaluate the vaccine alone or in combination with pembrolizumab for patients with platinum-sensitive ovarian cancer. Results from both trials are expected in 2025.
The third trial seeks to assess FOLFIRI as maintenance therapy or FOLFIRI as maintenance plus Tedopi for patients with pancreatic cancer to improve disease management. Efficacy data are expected next year.
OSE Immunotherapeutics is simultaneously working on a companion biomarker, the HLA-A2 test.
The study was funded by OSE Immunotherapeutics. Dr. Besse disclosed the following conflicts of interest (research funding, institution): AbbVie, Amgen, AstraZeneca, Chugai Pharmaceutical, Daiichi-Sankyo, Ellipse Pharma, EISAI, Genmab, Genzyme Corporation, Hedera Dx, Inivata, IPSEN, Janssen, MSD, Pharmamar, Roche-Genentech, Sanofi, Socar Research, Taiho Oncology, and Turning Point Therapeutics.
This article was translated from the Medscape French Edition and a version appeared on Medscape.com.
AT WCLC 2023
New ‘C word’: Cure should be the goal for patients with lung cancer
This transcript has been edited for clarity.
Hello. It’s Mark Kris from Memorial Sloan-Kettering, still musing on things I learned at ASCO 2023.
I learned that there is a new C word.
People used to be afraid to use the word “cancer,” so they would call it the C word. Hopefully we’ve gotten over that stigma, that cancer is an illness that can be fought like any other illness.
There’s a new C word now that people seem, again, afraid to use, and that word is “cure.” It’s almost a true rarity that – again, I’m talking about the lung cancer world in particular – folks use the word “cure.” I didn’t hear it at ASCO, but the truth of the matter is that’s a word we should be using and be using more.
What do our patients want? I think if you truly ask a patient what their goal of care should be, it would be to cure the illness. What I mean by “cure” is to eradicate the cancer that is in their body, keep the cancer and its effects from interfering with their ability to continue their lives, and to do it for the length of their natural life. That’s what our patients want. Yes, overall survival is important, but not as much as a life free of cancer and the burden that it puts on people having cancer in the body.
When you start thinking about cure and how to make it a goal of care, a number of issues immediately crop up. The first one is defining what is meant by “cure.” We don’t have a strict definition of cure. Again, I would probably go to the patients and ask them what they mean by it. There may be some landmark part of the definition that needs to be discussed and addressed, but again, to me it’s having your life not disturbed by cancer, and that generally comes by eradicating cancer. Living with cancer is harder than the living after cancer has been cured. But we don’t have a good definition.
We also don’t have a good way of designing clinical trials to assess whether the regimen is curative. I don’t think I’ve ever seen a trial in lung cancer that looked at the ability of any given treatment to cure patients. We need to come up with ways to design trials to do that. Now, in addition to clinical trials, we don’t have a good body of evidence to design our preclinical experiments to look for those treatments that can lead to cures, or total eradication of cancer in whatever model system might be used. If we make cure the goal, then we need to find ways preclinically to identify those strategies that could lead to that.
Also in the realm of clinical trials, we need a very clear statistical underpinning to show that one or another treatment has a better chance of cure and to show with scientific rigor that one treatment is better than the other when it comes to cure. I think there needs to be more attention to this, and as we think about revamping the clinical trial process, we need to focus more on cure.
I’m saving the most important step for last. None of this can happen unless we try to make it happen and we say cure is possible. My mentor, George Boswell, always taught us that we would, in every single patient with cancer, try to develop a curative strategy. Is there a curative strategy for this patient? If so, pursue it with all the tools and vigor that we have. We really need to think that way.
Obviously, not every patient with cancer can be cured with our current armamentarium of anticancer treatments, but we need to make sure we put it on the table. We need to [confirm] that a strategy does not currently exist that could lead to cure. And of course, if we do find that strategy, we need to pursue it with all the energy and resources that we have.
Please don’t be afraid to use the word “cure.” Our patients want that. They deserve it. We should work hard to try to provide it and work toward developing strategies that we can propose and cure more patients.
Mark G. Kris, MD, is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York. His research interests include targeted therapies for lung cancer, multimodality therapy, the development of new anticancer drugs, and symptom management with a focus on preventing emesis.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hello. It’s Mark Kris from Memorial Sloan-Kettering, still musing on things I learned at ASCO 2023.
I learned that there is a new C word.
People used to be afraid to use the word “cancer,” so they would call it the C word. Hopefully we’ve gotten over that stigma, that cancer is an illness that can be fought like any other illness.
There’s a new C word now that people seem, again, afraid to use, and that word is “cure.” It’s almost a true rarity that – again, I’m talking about the lung cancer world in particular – folks use the word “cure.” I didn’t hear it at ASCO, but the truth of the matter is that’s a word we should be using and be using more.
What do our patients want? I think if you truly ask a patient what their goal of care should be, it would be to cure the illness. What I mean by “cure” is to eradicate the cancer that is in their body, keep the cancer and its effects from interfering with their ability to continue their lives, and to do it for the length of their natural life. That’s what our patients want. Yes, overall survival is important, but not as much as a life free of cancer and the burden that it puts on people having cancer in the body.
When you start thinking about cure and how to make it a goal of care, a number of issues immediately crop up. The first one is defining what is meant by “cure.” We don’t have a strict definition of cure. Again, I would probably go to the patients and ask them what they mean by it. There may be some landmark part of the definition that needs to be discussed and addressed, but again, to me it’s having your life not disturbed by cancer, and that generally comes by eradicating cancer. Living with cancer is harder than the living after cancer has been cured. But we don’t have a good definition.
We also don’t have a good way of designing clinical trials to assess whether the regimen is curative. I don’t think I’ve ever seen a trial in lung cancer that looked at the ability of any given treatment to cure patients. We need to come up with ways to design trials to do that. Now, in addition to clinical trials, we don’t have a good body of evidence to design our preclinical experiments to look for those treatments that can lead to cures, or total eradication of cancer in whatever model system might be used. If we make cure the goal, then we need to find ways preclinically to identify those strategies that could lead to that.
Also in the realm of clinical trials, we need a very clear statistical underpinning to show that one or another treatment has a better chance of cure and to show with scientific rigor that one treatment is better than the other when it comes to cure. I think there needs to be more attention to this, and as we think about revamping the clinical trial process, we need to focus more on cure.
I’m saving the most important step for last. None of this can happen unless we try to make it happen and we say cure is possible. My mentor, George Boswell, always taught us that we would, in every single patient with cancer, try to develop a curative strategy. Is there a curative strategy for this patient? If so, pursue it with all the tools and vigor that we have. We really need to think that way.
Obviously, not every patient with cancer can be cured with our current armamentarium of anticancer treatments, but we need to make sure we put it on the table. We need to [confirm] that a strategy does not currently exist that could lead to cure. And of course, if we do find that strategy, we need to pursue it with all the energy and resources that we have.
Please don’t be afraid to use the word “cure.” Our patients want that. They deserve it. We should work hard to try to provide it and work toward developing strategies that we can propose and cure more patients.
Mark G. Kris, MD, is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York. His research interests include targeted therapies for lung cancer, multimodality therapy, the development of new anticancer drugs, and symptom management with a focus on preventing emesis.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hello. It’s Mark Kris from Memorial Sloan-Kettering, still musing on things I learned at ASCO 2023.
I learned that there is a new C word.
People used to be afraid to use the word “cancer,” so they would call it the C word. Hopefully we’ve gotten over that stigma, that cancer is an illness that can be fought like any other illness.
There’s a new C word now that people seem, again, afraid to use, and that word is “cure.” It’s almost a true rarity that – again, I’m talking about the lung cancer world in particular – folks use the word “cure.” I didn’t hear it at ASCO, but the truth of the matter is that’s a word we should be using and be using more.
What do our patients want? I think if you truly ask a patient what their goal of care should be, it would be to cure the illness. What I mean by “cure” is to eradicate the cancer that is in their body, keep the cancer and its effects from interfering with their ability to continue their lives, and to do it for the length of their natural life. That’s what our patients want. Yes, overall survival is important, but not as much as a life free of cancer and the burden that it puts on people having cancer in the body.
When you start thinking about cure and how to make it a goal of care, a number of issues immediately crop up. The first one is defining what is meant by “cure.” We don’t have a strict definition of cure. Again, I would probably go to the patients and ask them what they mean by it. There may be some landmark part of the definition that needs to be discussed and addressed, but again, to me it’s having your life not disturbed by cancer, and that generally comes by eradicating cancer. Living with cancer is harder than the living after cancer has been cured. But we don’t have a good definition.
We also don’t have a good way of designing clinical trials to assess whether the regimen is curative. I don’t think I’ve ever seen a trial in lung cancer that looked at the ability of any given treatment to cure patients. We need to come up with ways to design trials to do that. Now, in addition to clinical trials, we don’t have a good body of evidence to design our preclinical experiments to look for those treatments that can lead to cures, or total eradication of cancer in whatever model system might be used. If we make cure the goal, then we need to find ways preclinically to identify those strategies that could lead to that.
Also in the realm of clinical trials, we need a very clear statistical underpinning to show that one or another treatment has a better chance of cure and to show with scientific rigor that one treatment is better than the other when it comes to cure. I think there needs to be more attention to this, and as we think about revamping the clinical trial process, we need to focus more on cure.
I’m saving the most important step for last. None of this can happen unless we try to make it happen and we say cure is possible. My mentor, George Boswell, always taught us that we would, in every single patient with cancer, try to develop a curative strategy. Is there a curative strategy for this patient? If so, pursue it with all the tools and vigor that we have. We really need to think that way.
Obviously, not every patient with cancer can be cured with our current armamentarium of anticancer treatments, but we need to make sure we put it on the table. We need to [confirm] that a strategy does not currently exist that could lead to cure. And of course, if we do find that strategy, we need to pursue it with all the energy and resources that we have.
Please don’t be afraid to use the word “cure.” Our patients want that. They deserve it. We should work hard to try to provide it and work toward developing strategies that we can propose and cure more patients.
Mark G. Kris, MD, is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York. His research interests include targeted therapies for lung cancer, multimodality therapy, the development of new anticancer drugs, and symptom management with a focus on preventing emesis.
A version of this article first appeared on Medscape.com.
Primary care clinicians should spearhead HIV prevention
HIV continues to be a significant public health concern in the United States, with an estimated 1.2 million people currently living with the virus and more than 30,000 new diagnoses in 2020 alone.
Primary care clinicians can help decrease rates of HIV infection by prescribing pre-exposure prophylaxis to people who are sexually active.
But many do not.
“In medical school, we don’t spend much time discussing sexuality, sexual behavior, sexually transmitted infections, and such, so providers may feel uncomfortable asking what kind of sex their patient is having and with whom, whether they use a condom, and other basics,” said Matthew M. Hamill, MBChB, PhD, MPH, a specialist in sexually transmitted diseases at Johns Hopkins Medicine, Baltimore.
PrEP (pre-exposure prophylaxis) is an antiviral medication that cuts the risk of contracting HIV through sex by around 99% when taken as prescribed, according to the Centers for Disease Control and Prevention.
“Many people who would benefit from PrEP are not receiving this highly effective medication,” said John B. Wong, MD, a primary care internist and professor of medicine at Tufts University, Boston. The gap is particularly acute among Black, Hispanic, and Latino people, who are significantly more likely to be diagnosed with HIV but are much less likely than Whites to receive PrEP, he said.
Dr. Wong, a member of the U.S. Preventive Services Task Force, helped write the group’s new PrEP recommendations. Published in August, the guidelines call for clinicians to prescribe the drugs to adolescents and adults who do not have HIV but are at an increased risk for infection.
“Primary care physicians are ideally positioned to prescribe PrEP for their patients because they have longitudinal relationships: They get to know their patients, and hopefully their patients feel comfortable talking with them about their sexual health,” said Brandon Pollak, MD, a primary care physician and HIV specialist at the Ohio State University College of Medicine, Columbus.
Dr. Pollak, who was not involved with the USPSTF recommendations, cares for patients who are heterosexual and living with HIV.
Clinicians should consider PrEP for all patients who have sex with someone who has HIV, do not use condoms, or have had a sexually transmitted infection within the previous 6 months. Men who have sex with men, transgender women who have sex with men, people who inject illicit drugs or engage in transactional sex, and Black, Hispanic, and Latino individuals also are at increased risk for the infection.
“The vast majority of patients on PrEP in any form sail through with no problems; they have regular lab work and can follow up in person or by telemedicine,” Dr. Hamill said. “They tend to be young, fit people without complicated medical histories, and the medications are very well-tolerated, particularly if people expect some short-term side effects.”
What you need to know when prescribing PrEP
Prescribing PrEP is similar in complexity to prescribing hypertension or diabetes medications, Dr. Hamill said.
Because taking the medications while already infected with the virus can lead to the emergence of drug-resistant HIV, patients must have a negative HIV test before starting PrEP. In addition, the USPSTF recommends testing for other sexually transmitted infections and for pregnancy, if appropriate. The task force also recommends conducting kidney function and hepatitis B tests, and a lipid profile before starting specific types of PrEP.
HIV screening is also recommended at 3-month intervals.
“Providers may order labs done at 3- to 4-month intervals but only see patients in clinic once or twice per year, depending on patient needs and risk behaviors,” said Jill S. Blumenthal, MD, associate professor of medicine at UC San Diego Health.
Clinicians should consider medication adherence and whether a patient is likely to take a pill once a day or could benefit from receiving an injection every 2 months. Patients may experience side effects such as diarrhea or headache with oral PrEP or soreness at the injection site. In rare cases, some of the drugs may cause kidney toxicity or bone mineral loss, according to Dr. Hamill.
Three similarly effective forms of PrEP approved by the U.S. Food and Drug Administration enable clinicians to tailor the medications to the specific needs and preferences of each patient. Truvada (emtricitabine and tenofovir disoproxil fumarate) and Descovy (emtricitabine and tenofovir alafenamide) are both daily tablets, although the latter is not advised for people assigned female sex at birth who have receptive vaginal sex. Apretude (cabotegravir), an injectable agent, is not recommended for people who inject illegal drugs.
Patients with renal or bone disease are not good candidates for Truvada.
“Truvada can decrease bone density, so for someone with osteoporosis, you might choose Descovy or Apretude,” Dr. Pollak said. “For someone with chronic kidney disease, consider Descovy or Apretude. “If a patient has hepatitis B, Truvada or Descovy are appropriate, because hepatitis B is treatable.”
Patients taking an injectable PrEP may need more attention, because the concentration of the medication in the body decreases slowly and may linger for many months at low levels that don’t prevent HIV, according to Dr. Hamill. Someone who acquires HIV during that “tail” period might develop resistance to PrEP.
New research also showed that Descovy users were at elevated risk of developing hypertension and statin initiation, especially among those over age 40 years.
Primary care physicians may want to consult with renal specialists about medication safety in patients with severe kidney disease or with rheumatologists or endocrinologists about metabolic bone disease concerns, Dr. Hamill said.
Meanwhile, if a person begins a monogamous relationship and their risk for HIV drops, “it’s fine to stop taking PrEP tablets,” Dr. Pollak said. “I would still recommend routine HIV screening every 6 or 12 months or however often, depending on other risk factors.”
Caring for these patients entails ensuring labs are completed, monitoring adherence, ordering refills, and scheduling regular follow-up visits.
“For the vast majority of patients, the primary care physician is perfectly equipped for their care through the entire PrEP journey, from discussion and initiation to provision of PrEP,” and most cases do not require specialist care, Dr. Hamill said.
However, “if PrEP fails, which is exceedingly rare, primary care physicians should refer patients immediately, preferably with a warm handoff, for linkage to HIV care,” Dr. Blumenthal said.
Talking about PrEP opens the door to conversations with patients about sexual health and broader health issues, Dr. Hamill said. Although these may not come naturally to primary care clinicians, training is available. The National Network of STD Clinical Prevention Training Centers, funded by the CDC, trains providers on how to overcome their anxiety and have open, inclusive conversations about sexuality and sexual behaviors with transgender and gender-diverse, nonbinary people.
“People worry about saying the wrong thing, about causing offense,” Dr. Hamill said. “But once you get comfortable discussing sexuality, you may open conversations around other health issues.”
Barriers for patients
The task force identified several barriers to PrEP access for patients because of lack of trusting relationships with health care, the effects of structural racism on health disparities, and persistent biases within the health care system.
Racial and ethnic disparities in HIV incidence persist, with 42% of new diagnoses occurring among Black people, 27% among Hispanic or Latino people, and 26% among White people in 2020.
Rates of PrEP usage for a year or longer are also low. Sometimes the patient no longer needs PrEP, but barriers often involve the costs of taking time off from work and arranging transportation to clinic visits.
Although nearly all insurance plans and state Medicaid programs cover PrEP, if a patient does not have coverage, the drugs and required tests and office visits can be expensive.
“One of the biggest barriers for all providers is navigating our complicated health system and drug assistance programs,” said Mehri S. McKellar, MD, associate professor of medicine at Duke University School of Medicine, Durham, N.C.
But lower-cost FDA-approved generic emtricitabine/tenofovir disoproxil fumarate is now available, and clinicians can direct patients to programs that help provide the medications at low or no cost.
“Providing PrEP care is straightforward, beneficial, and satisfying,” Dr. Hamill said. “You help people protect themselves from a life-changing diagnosis, and the health system doesn’t need to pay the cost of treating HIV. Everyone wins.”
Dr. Hamill, Dr. McKellar, Dr. Pollak, and Dr. Wong have reported no relevant financial relationships. Dr. Blumenthal has reported a financial relationship with Gilead Sciences.
A version of this article appeared on Medscape.com.
HIV continues to be a significant public health concern in the United States, with an estimated 1.2 million people currently living with the virus and more than 30,000 new diagnoses in 2020 alone.
Primary care clinicians can help decrease rates of HIV infection by prescribing pre-exposure prophylaxis to people who are sexually active.
But many do not.
“In medical school, we don’t spend much time discussing sexuality, sexual behavior, sexually transmitted infections, and such, so providers may feel uncomfortable asking what kind of sex their patient is having and with whom, whether they use a condom, and other basics,” said Matthew M. Hamill, MBChB, PhD, MPH, a specialist in sexually transmitted diseases at Johns Hopkins Medicine, Baltimore.
PrEP (pre-exposure prophylaxis) is an antiviral medication that cuts the risk of contracting HIV through sex by around 99% when taken as prescribed, according to the Centers for Disease Control and Prevention.
“Many people who would benefit from PrEP are not receiving this highly effective medication,” said John B. Wong, MD, a primary care internist and professor of medicine at Tufts University, Boston. The gap is particularly acute among Black, Hispanic, and Latino people, who are significantly more likely to be diagnosed with HIV but are much less likely than Whites to receive PrEP, he said.
Dr. Wong, a member of the U.S. Preventive Services Task Force, helped write the group’s new PrEP recommendations. Published in August, the guidelines call for clinicians to prescribe the drugs to adolescents and adults who do not have HIV but are at an increased risk for infection.
“Primary care physicians are ideally positioned to prescribe PrEP for their patients because they have longitudinal relationships: They get to know their patients, and hopefully their patients feel comfortable talking with them about their sexual health,” said Brandon Pollak, MD, a primary care physician and HIV specialist at the Ohio State University College of Medicine, Columbus.
Dr. Pollak, who was not involved with the USPSTF recommendations, cares for patients who are heterosexual and living with HIV.
Clinicians should consider PrEP for all patients who have sex with someone who has HIV, do not use condoms, or have had a sexually transmitted infection within the previous 6 months. Men who have sex with men, transgender women who have sex with men, people who inject illicit drugs or engage in transactional sex, and Black, Hispanic, and Latino individuals also are at increased risk for the infection.
“The vast majority of patients on PrEP in any form sail through with no problems; they have regular lab work and can follow up in person or by telemedicine,” Dr. Hamill said. “They tend to be young, fit people without complicated medical histories, and the medications are very well-tolerated, particularly if people expect some short-term side effects.”
What you need to know when prescribing PrEP
Prescribing PrEP is similar in complexity to prescribing hypertension or diabetes medications, Dr. Hamill said.
Because taking the medications while already infected with the virus can lead to the emergence of drug-resistant HIV, patients must have a negative HIV test before starting PrEP. In addition, the USPSTF recommends testing for other sexually transmitted infections and for pregnancy, if appropriate. The task force also recommends conducting kidney function and hepatitis B tests, and a lipid profile before starting specific types of PrEP.
HIV screening is also recommended at 3-month intervals.
“Providers may order labs done at 3- to 4-month intervals but only see patients in clinic once or twice per year, depending on patient needs and risk behaviors,” said Jill S. Blumenthal, MD, associate professor of medicine at UC San Diego Health.
Clinicians should consider medication adherence and whether a patient is likely to take a pill once a day or could benefit from receiving an injection every 2 months. Patients may experience side effects such as diarrhea or headache with oral PrEP or soreness at the injection site. In rare cases, some of the drugs may cause kidney toxicity or bone mineral loss, according to Dr. Hamill.
Three similarly effective forms of PrEP approved by the U.S. Food and Drug Administration enable clinicians to tailor the medications to the specific needs and preferences of each patient. Truvada (emtricitabine and tenofovir disoproxil fumarate) and Descovy (emtricitabine and tenofovir alafenamide) are both daily tablets, although the latter is not advised for people assigned female sex at birth who have receptive vaginal sex. Apretude (cabotegravir), an injectable agent, is not recommended for people who inject illegal drugs.
Patients with renal or bone disease are not good candidates for Truvada.
“Truvada can decrease bone density, so for someone with osteoporosis, you might choose Descovy or Apretude,” Dr. Pollak said. “For someone with chronic kidney disease, consider Descovy or Apretude. “If a patient has hepatitis B, Truvada or Descovy are appropriate, because hepatitis B is treatable.”
Patients taking an injectable PrEP may need more attention, because the concentration of the medication in the body decreases slowly and may linger for many months at low levels that don’t prevent HIV, according to Dr. Hamill. Someone who acquires HIV during that “tail” period might develop resistance to PrEP.
New research also showed that Descovy users were at elevated risk of developing hypertension and statin initiation, especially among those over age 40 years.
Primary care physicians may want to consult with renal specialists about medication safety in patients with severe kidney disease or with rheumatologists or endocrinologists about metabolic bone disease concerns, Dr. Hamill said.
Meanwhile, if a person begins a monogamous relationship and their risk for HIV drops, “it’s fine to stop taking PrEP tablets,” Dr. Pollak said. “I would still recommend routine HIV screening every 6 or 12 months or however often, depending on other risk factors.”
Caring for these patients entails ensuring labs are completed, monitoring adherence, ordering refills, and scheduling regular follow-up visits.
“For the vast majority of patients, the primary care physician is perfectly equipped for their care through the entire PrEP journey, from discussion and initiation to provision of PrEP,” and most cases do not require specialist care, Dr. Hamill said.
However, “if PrEP fails, which is exceedingly rare, primary care physicians should refer patients immediately, preferably with a warm handoff, for linkage to HIV care,” Dr. Blumenthal said.
Talking about PrEP opens the door to conversations with patients about sexual health and broader health issues, Dr. Hamill said. Although these may not come naturally to primary care clinicians, training is available. The National Network of STD Clinical Prevention Training Centers, funded by the CDC, trains providers on how to overcome their anxiety and have open, inclusive conversations about sexuality and sexual behaviors with transgender and gender-diverse, nonbinary people.
“People worry about saying the wrong thing, about causing offense,” Dr. Hamill said. “But once you get comfortable discussing sexuality, you may open conversations around other health issues.”
Barriers for patients
The task force identified several barriers to PrEP access for patients because of lack of trusting relationships with health care, the effects of structural racism on health disparities, and persistent biases within the health care system.
Racial and ethnic disparities in HIV incidence persist, with 42% of new diagnoses occurring among Black people, 27% among Hispanic or Latino people, and 26% among White people in 2020.
Rates of PrEP usage for a year or longer are also low. Sometimes the patient no longer needs PrEP, but barriers often involve the costs of taking time off from work and arranging transportation to clinic visits.
Although nearly all insurance plans and state Medicaid programs cover PrEP, if a patient does not have coverage, the drugs and required tests and office visits can be expensive.
“One of the biggest barriers for all providers is navigating our complicated health system and drug assistance programs,” said Mehri S. McKellar, MD, associate professor of medicine at Duke University School of Medicine, Durham, N.C.
But lower-cost FDA-approved generic emtricitabine/tenofovir disoproxil fumarate is now available, and clinicians can direct patients to programs that help provide the medications at low or no cost.
“Providing PrEP care is straightforward, beneficial, and satisfying,” Dr. Hamill said. “You help people protect themselves from a life-changing diagnosis, and the health system doesn’t need to pay the cost of treating HIV. Everyone wins.”
Dr. Hamill, Dr. McKellar, Dr. Pollak, and Dr. Wong have reported no relevant financial relationships. Dr. Blumenthal has reported a financial relationship with Gilead Sciences.
A version of this article appeared on Medscape.com.
HIV continues to be a significant public health concern in the United States, with an estimated 1.2 million people currently living with the virus and more than 30,000 new diagnoses in 2020 alone.
Primary care clinicians can help decrease rates of HIV infection by prescribing pre-exposure prophylaxis to people who are sexually active.
But many do not.
“In medical school, we don’t spend much time discussing sexuality, sexual behavior, sexually transmitted infections, and such, so providers may feel uncomfortable asking what kind of sex their patient is having and with whom, whether they use a condom, and other basics,” said Matthew M. Hamill, MBChB, PhD, MPH, a specialist in sexually transmitted diseases at Johns Hopkins Medicine, Baltimore.
PrEP (pre-exposure prophylaxis) is an antiviral medication that cuts the risk of contracting HIV through sex by around 99% when taken as prescribed, according to the Centers for Disease Control and Prevention.
“Many people who would benefit from PrEP are not receiving this highly effective medication,” said John B. Wong, MD, a primary care internist and professor of medicine at Tufts University, Boston. The gap is particularly acute among Black, Hispanic, and Latino people, who are significantly more likely to be diagnosed with HIV but are much less likely than Whites to receive PrEP, he said.
Dr. Wong, a member of the U.S. Preventive Services Task Force, helped write the group’s new PrEP recommendations. Published in August, the guidelines call for clinicians to prescribe the drugs to adolescents and adults who do not have HIV but are at an increased risk for infection.
“Primary care physicians are ideally positioned to prescribe PrEP for their patients because they have longitudinal relationships: They get to know their patients, and hopefully their patients feel comfortable talking with them about their sexual health,” said Brandon Pollak, MD, a primary care physician and HIV specialist at the Ohio State University College of Medicine, Columbus.
Dr. Pollak, who was not involved with the USPSTF recommendations, cares for patients who are heterosexual and living with HIV.
Clinicians should consider PrEP for all patients who have sex with someone who has HIV, do not use condoms, or have had a sexually transmitted infection within the previous 6 months. Men who have sex with men, transgender women who have sex with men, people who inject illicit drugs or engage in transactional sex, and Black, Hispanic, and Latino individuals also are at increased risk for the infection.
“The vast majority of patients on PrEP in any form sail through with no problems; they have regular lab work and can follow up in person or by telemedicine,” Dr. Hamill said. “They tend to be young, fit people without complicated medical histories, and the medications are very well-tolerated, particularly if people expect some short-term side effects.”
What you need to know when prescribing PrEP
Prescribing PrEP is similar in complexity to prescribing hypertension or diabetes medications, Dr. Hamill said.
Because taking the medications while already infected with the virus can lead to the emergence of drug-resistant HIV, patients must have a negative HIV test before starting PrEP. In addition, the USPSTF recommends testing for other sexually transmitted infections and for pregnancy, if appropriate. The task force also recommends conducting kidney function and hepatitis B tests, and a lipid profile before starting specific types of PrEP.
HIV screening is also recommended at 3-month intervals.
“Providers may order labs done at 3- to 4-month intervals but only see patients in clinic once or twice per year, depending on patient needs and risk behaviors,” said Jill S. Blumenthal, MD, associate professor of medicine at UC San Diego Health.
Clinicians should consider medication adherence and whether a patient is likely to take a pill once a day or could benefit from receiving an injection every 2 months. Patients may experience side effects such as diarrhea or headache with oral PrEP or soreness at the injection site. In rare cases, some of the drugs may cause kidney toxicity or bone mineral loss, according to Dr. Hamill.
Three similarly effective forms of PrEP approved by the U.S. Food and Drug Administration enable clinicians to tailor the medications to the specific needs and preferences of each patient. Truvada (emtricitabine and tenofovir disoproxil fumarate) and Descovy (emtricitabine and tenofovir alafenamide) are both daily tablets, although the latter is not advised for people assigned female sex at birth who have receptive vaginal sex. Apretude (cabotegravir), an injectable agent, is not recommended for people who inject illegal drugs.
Patients with renal or bone disease are not good candidates for Truvada.
“Truvada can decrease bone density, so for someone with osteoporosis, you might choose Descovy or Apretude,” Dr. Pollak said. “For someone with chronic kidney disease, consider Descovy or Apretude. “If a patient has hepatitis B, Truvada or Descovy are appropriate, because hepatitis B is treatable.”
Patients taking an injectable PrEP may need more attention, because the concentration of the medication in the body decreases slowly and may linger for many months at low levels that don’t prevent HIV, according to Dr. Hamill. Someone who acquires HIV during that “tail” period might develop resistance to PrEP.
New research also showed that Descovy users were at elevated risk of developing hypertension and statin initiation, especially among those over age 40 years.
Primary care physicians may want to consult with renal specialists about medication safety in patients with severe kidney disease or with rheumatologists or endocrinologists about metabolic bone disease concerns, Dr. Hamill said.
Meanwhile, if a person begins a monogamous relationship and their risk for HIV drops, “it’s fine to stop taking PrEP tablets,” Dr. Pollak said. “I would still recommend routine HIV screening every 6 or 12 months or however often, depending on other risk factors.”
Caring for these patients entails ensuring labs are completed, monitoring adherence, ordering refills, and scheduling regular follow-up visits.
“For the vast majority of patients, the primary care physician is perfectly equipped for their care through the entire PrEP journey, from discussion and initiation to provision of PrEP,” and most cases do not require specialist care, Dr. Hamill said.
However, “if PrEP fails, which is exceedingly rare, primary care physicians should refer patients immediately, preferably with a warm handoff, for linkage to HIV care,” Dr. Blumenthal said.
Talking about PrEP opens the door to conversations with patients about sexual health and broader health issues, Dr. Hamill said. Although these may not come naturally to primary care clinicians, training is available. The National Network of STD Clinical Prevention Training Centers, funded by the CDC, trains providers on how to overcome their anxiety and have open, inclusive conversations about sexuality and sexual behaviors with transgender and gender-diverse, nonbinary people.
“People worry about saying the wrong thing, about causing offense,” Dr. Hamill said. “But once you get comfortable discussing sexuality, you may open conversations around other health issues.”
Barriers for patients
The task force identified several barriers to PrEP access for patients because of lack of trusting relationships with health care, the effects of structural racism on health disparities, and persistent biases within the health care system.
Racial and ethnic disparities in HIV incidence persist, with 42% of new diagnoses occurring among Black people, 27% among Hispanic or Latino people, and 26% among White people in 2020.
Rates of PrEP usage for a year or longer are also low. Sometimes the patient no longer needs PrEP, but barriers often involve the costs of taking time off from work and arranging transportation to clinic visits.
Although nearly all insurance plans and state Medicaid programs cover PrEP, if a patient does not have coverage, the drugs and required tests and office visits can be expensive.
“One of the biggest barriers for all providers is navigating our complicated health system and drug assistance programs,” said Mehri S. McKellar, MD, associate professor of medicine at Duke University School of Medicine, Durham, N.C.
But lower-cost FDA-approved generic emtricitabine/tenofovir disoproxil fumarate is now available, and clinicians can direct patients to programs that help provide the medications at low or no cost.
“Providing PrEP care is straightforward, beneficial, and satisfying,” Dr. Hamill said. “You help people protect themselves from a life-changing diagnosis, and the health system doesn’t need to pay the cost of treating HIV. Everyone wins.”
Dr. Hamill, Dr. McKellar, Dr. Pollak, and Dr. Wong have reported no relevant financial relationships. Dr. Blumenthal has reported a financial relationship with Gilead Sciences.
A version of this article appeared on Medscape.com.
Insurer’s foray into AI-based ‘shared savings’ program creates ethical problems
Editor’s note: As of this writing, the following proposed health insurance policy from Blue Cross and Blue Shield of North Carolina is still active. The Coalition of State Rheumatology Organizations and other rheumatology advocacy groups are in ongoing discussions with the health insurer and hope to have major changes to this policy implemented.
While AI has been in our world for years, it is expanding by the minute, perhaps by the nanosecond, within the health care sector. The $6.7 billion dollar health care AI market in 2020 is expected to climb to more than $120 billion by 2028. There are many questions regarding the application of AI in our world. Is it a mere instructional algorithm that computes things in a much faster way, or does it create a new story based on the information it has access to? Does it engender excitement or fear ... or both? Remember HAL? As we have seen throughout history with new inventions and technologies, there are risks and rewards. Even the best can have harmful unintended consequences. AI is no different, particularly when it comes to health care. In this case, AI can get a bad name if it is utilized along with biased data input and bad policy.
Shared savings
Here is where “shared savings” comes into play. A shared savings program starts with a baseline cost analysis of a particular care plan and then tracks costs (performance) going forward after certain changes to the original care plan are instituted. If savings are accrued when compared with baseline spending, those savings are shared with the providers of the care. Depending on how the shared savings program is implemented, the optics can be very bad if it appears as though physicians are being paid to reduce care.
‘The volunteer opportunity’
Recently, Blue Cross and Blue Shield of North Carolina, in partnership with Outcomes Matter Innovations, a data analysis company that uses AI/machine-learning technology, offered rheumatologists a new voluntary shared savings, value-based care (VBC) “opportunity.” Rheumatologists would be able to “utilize a web-based machine-learning technology platform that suggests evidence-based care pathways” in the treatment of rheumatoid arthritis and psoriatic arthritis (PsA). The VBC/shared savings model uses the AI platform to propose two different pathways. One model would delay the start of biologics or Janus kinase inhibitors (JAKi), and the second model would taper and/or stop biologics or JAKi altogether.
Delaying the start of biologics/JAKi would be achieved through “methotrexate optimization” and/or the use of triple therapy with methotrexate, sulfasalazine, and hydroxychloroquine. The other model would recommend tapering biologic/JAKi dosing in patients in remission or low disease activity and might even suggest a “medication holiday.”
The intention of this 3-year VBC/shared savings program is to reduce costs and create savings by reducing the use of biologics or JAKi. A tangential question might be, “Reduce costs and create savings for whom?” Apparently, the patients will not reap any of the cost savings, as this is proposed to be a shared savings program with the savings going to the physicians and the insurance company. Perhaps the idea is that patients will benefit by reducing unneeded expensive medications.
How will it work?
A cost baseline will be established on biologic and JAKi use prior to the start of the program. Once started, there will be a calculation of savings based on biologic/JAKi use going forward. It was stated that physicians would receive 22% of the total costs saved. In one flyer, it was estimated that, with methotrexate optimization, rheumatologists could be paid an average of $1,527 a month per patient per month of delay before starting a biologic or JAKi.
The American College of Rheumatology has guidelines for the treatment of RA and PsA, and while optimizing methotrexate and triple therapy is mentioned, tapering or stopping treatment with biologics or JAKi is not. Additionally, after lack of response at 3 months, the standard of care is to change to a more effective treatment, which for most patients is a biologic disease-modifying antirheumatic drug (DMARD). It could be construed that rheumatologists are being monetarily incentivized to reduce the use of expensive medications through ways that are not included in ACR guidelines and are not standard of care.
What if after the medication holiday the patient cannot recapture control of their disease? Is there a liability concern? Remember, there is no institutional review board or informed patient consent for this VBC data gathering model.
How will a patient feel knowing that their physician was paid to withhold care, or even worse, if a patient is not told of this and then finds out later? Not only are the optics for this suboptimal (at best), where does the liability fall if the patient does not do well and it comes out that their rheumatologist was paid to reduce the care, particularly in a way that is not supported in the guideline. Clearly, this appears to be a clinical study without an institutional review board and without patient consent.
There are also the data that are collected from this voluntary “opportunity.” A valid question would be, “What kind of data will this produce if rheumatologists are paid to delay, reduce, or stop the use of biologics/JAKi?” Is it possible that physicians may subconsciously delay putting patients on a biologic and taper more rapidly because of the reimbursement? This could lead to faulty, biased, AI-generated data that erroneously show this type of care is working. It would not be unheard of to wonder whether this once-voluntary opportunity might evolve into mandatory policy because now, they have “data to prove it.” … only this time there is no shared savings.
Low disease activity results in long-term savings
This is not meant to be an indictment of AI in health care, value-based care, or shared savings programs. In reality, AI had very little to do with how poorly this program was presented. Hopefully, it will bring about further discussions on how to achieve savings without sacrificing care. In fact, optimal care in RA and PsA is probably one of the best ways to save money in the long run. Nowhere in this program is there any mention of the high cost associated with uncontrolled disease activity in patients with RA or PsA. The downstream costs can be enormous when long- and short-term sequelae are taken into consideration: joint replacements, cardiovascular disease, certain kinds of malignancies, and all the side effects of increased steroid usage are just a few of the consequences we see with uncontrolled disease activity. It is only recently that we have been able to achieve low disease activity and remission in our patients. The rush to get patients off these medications is not the answer to achieving long-term savings. In addition to the very bad optics of paying rheumatologists to delay, taper, or stop using expensive mediations in their patients, the ultimate data achieved will be biased, and the only real winner will be the health insurance company.
Again, AI machine-learning and shared saving programs are not the guilty parties here. In fact, AI may be helpful in coming up with solutions to long-term health care costs, whether in the realm of economics or scientific research. CSRO and our state member organizations continue to educate the health insurance company on the significant drawbacks to this “volunteer opportunity.” Let’s hope a more reasonable program is put forward with AI-generated data that can be trusted. Hopefully not with a platform named “HAL,” for those of you old enough to remember “2001: A Space Odyssey.”
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of advocacy and government affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
Editor’s note: As of this writing, the following proposed health insurance policy from Blue Cross and Blue Shield of North Carolina is still active. The Coalition of State Rheumatology Organizations and other rheumatology advocacy groups are in ongoing discussions with the health insurer and hope to have major changes to this policy implemented.
While AI has been in our world for years, it is expanding by the minute, perhaps by the nanosecond, within the health care sector. The $6.7 billion dollar health care AI market in 2020 is expected to climb to more than $120 billion by 2028. There are many questions regarding the application of AI in our world. Is it a mere instructional algorithm that computes things in a much faster way, or does it create a new story based on the information it has access to? Does it engender excitement or fear ... or both? Remember HAL? As we have seen throughout history with new inventions and technologies, there are risks and rewards. Even the best can have harmful unintended consequences. AI is no different, particularly when it comes to health care. In this case, AI can get a bad name if it is utilized along with biased data input and bad policy.
Shared savings
Here is where “shared savings” comes into play. A shared savings program starts with a baseline cost analysis of a particular care plan and then tracks costs (performance) going forward after certain changes to the original care plan are instituted. If savings are accrued when compared with baseline spending, those savings are shared with the providers of the care. Depending on how the shared savings program is implemented, the optics can be very bad if it appears as though physicians are being paid to reduce care.
‘The volunteer opportunity’
Recently, Blue Cross and Blue Shield of North Carolina, in partnership with Outcomes Matter Innovations, a data analysis company that uses AI/machine-learning technology, offered rheumatologists a new voluntary shared savings, value-based care (VBC) “opportunity.” Rheumatologists would be able to “utilize a web-based machine-learning technology platform that suggests evidence-based care pathways” in the treatment of rheumatoid arthritis and psoriatic arthritis (PsA). The VBC/shared savings model uses the AI platform to propose two different pathways. One model would delay the start of biologics or Janus kinase inhibitors (JAKi), and the second model would taper and/or stop biologics or JAKi altogether.
Delaying the start of biologics/JAKi would be achieved through “methotrexate optimization” and/or the use of triple therapy with methotrexate, sulfasalazine, and hydroxychloroquine. The other model would recommend tapering biologic/JAKi dosing in patients in remission or low disease activity and might even suggest a “medication holiday.”
The intention of this 3-year VBC/shared savings program is to reduce costs and create savings by reducing the use of biologics or JAKi. A tangential question might be, “Reduce costs and create savings for whom?” Apparently, the patients will not reap any of the cost savings, as this is proposed to be a shared savings program with the savings going to the physicians and the insurance company. Perhaps the idea is that patients will benefit by reducing unneeded expensive medications.
How will it work?
A cost baseline will be established on biologic and JAKi use prior to the start of the program. Once started, there will be a calculation of savings based on biologic/JAKi use going forward. It was stated that physicians would receive 22% of the total costs saved. In one flyer, it was estimated that, with methotrexate optimization, rheumatologists could be paid an average of $1,527 a month per patient per month of delay before starting a biologic or JAKi.
The American College of Rheumatology has guidelines for the treatment of RA and PsA, and while optimizing methotrexate and triple therapy is mentioned, tapering or stopping treatment with biologics or JAKi is not. Additionally, after lack of response at 3 months, the standard of care is to change to a more effective treatment, which for most patients is a biologic disease-modifying antirheumatic drug (DMARD). It could be construed that rheumatologists are being monetarily incentivized to reduce the use of expensive medications through ways that are not included in ACR guidelines and are not standard of care.
What if after the medication holiday the patient cannot recapture control of their disease? Is there a liability concern? Remember, there is no institutional review board or informed patient consent for this VBC data gathering model.
How will a patient feel knowing that their physician was paid to withhold care, or even worse, if a patient is not told of this and then finds out later? Not only are the optics for this suboptimal (at best), where does the liability fall if the patient does not do well and it comes out that their rheumatologist was paid to reduce the care, particularly in a way that is not supported in the guideline. Clearly, this appears to be a clinical study without an institutional review board and without patient consent.
There are also the data that are collected from this voluntary “opportunity.” A valid question would be, “What kind of data will this produce if rheumatologists are paid to delay, reduce, or stop the use of biologics/JAKi?” Is it possible that physicians may subconsciously delay putting patients on a biologic and taper more rapidly because of the reimbursement? This could lead to faulty, biased, AI-generated data that erroneously show this type of care is working. It would not be unheard of to wonder whether this once-voluntary opportunity might evolve into mandatory policy because now, they have “data to prove it.” … only this time there is no shared savings.
Low disease activity results in long-term savings
This is not meant to be an indictment of AI in health care, value-based care, or shared savings programs. In reality, AI had very little to do with how poorly this program was presented. Hopefully, it will bring about further discussions on how to achieve savings without sacrificing care. In fact, optimal care in RA and PsA is probably one of the best ways to save money in the long run. Nowhere in this program is there any mention of the high cost associated with uncontrolled disease activity in patients with RA or PsA. The downstream costs can be enormous when long- and short-term sequelae are taken into consideration: joint replacements, cardiovascular disease, certain kinds of malignancies, and all the side effects of increased steroid usage are just a few of the consequences we see with uncontrolled disease activity. It is only recently that we have been able to achieve low disease activity and remission in our patients. The rush to get patients off these medications is not the answer to achieving long-term savings. In addition to the very bad optics of paying rheumatologists to delay, taper, or stop using expensive mediations in their patients, the ultimate data achieved will be biased, and the only real winner will be the health insurance company.
Again, AI machine-learning and shared saving programs are not the guilty parties here. In fact, AI may be helpful in coming up with solutions to long-term health care costs, whether in the realm of economics or scientific research. CSRO and our state member organizations continue to educate the health insurance company on the significant drawbacks to this “volunteer opportunity.” Let’s hope a more reasonable program is put forward with AI-generated data that can be trusted. Hopefully not with a platform named “HAL,” for those of you old enough to remember “2001: A Space Odyssey.”
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of advocacy and government affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
Editor’s note: As of this writing, the following proposed health insurance policy from Blue Cross and Blue Shield of North Carolina is still active. The Coalition of State Rheumatology Organizations and other rheumatology advocacy groups are in ongoing discussions with the health insurer and hope to have major changes to this policy implemented.
While AI has been in our world for years, it is expanding by the minute, perhaps by the nanosecond, within the health care sector. The $6.7 billion dollar health care AI market in 2020 is expected to climb to more than $120 billion by 2028. There are many questions regarding the application of AI in our world. Is it a mere instructional algorithm that computes things in a much faster way, or does it create a new story based on the information it has access to? Does it engender excitement or fear ... or both? Remember HAL? As we have seen throughout history with new inventions and technologies, there are risks and rewards. Even the best can have harmful unintended consequences. AI is no different, particularly when it comes to health care. In this case, AI can get a bad name if it is utilized along with biased data input and bad policy.
Shared savings
Here is where “shared savings” comes into play. A shared savings program starts with a baseline cost analysis of a particular care plan and then tracks costs (performance) going forward after certain changes to the original care plan are instituted. If savings are accrued when compared with baseline spending, those savings are shared with the providers of the care. Depending on how the shared savings program is implemented, the optics can be very bad if it appears as though physicians are being paid to reduce care.
‘The volunteer opportunity’
Recently, Blue Cross and Blue Shield of North Carolina, in partnership with Outcomes Matter Innovations, a data analysis company that uses AI/machine-learning technology, offered rheumatologists a new voluntary shared savings, value-based care (VBC) “opportunity.” Rheumatologists would be able to “utilize a web-based machine-learning technology platform that suggests evidence-based care pathways” in the treatment of rheumatoid arthritis and psoriatic arthritis (PsA). The VBC/shared savings model uses the AI platform to propose two different pathways. One model would delay the start of biologics or Janus kinase inhibitors (JAKi), and the second model would taper and/or stop biologics or JAKi altogether.
Delaying the start of biologics/JAKi would be achieved through “methotrexate optimization” and/or the use of triple therapy with methotrexate, sulfasalazine, and hydroxychloroquine. The other model would recommend tapering biologic/JAKi dosing in patients in remission or low disease activity and might even suggest a “medication holiday.”
The intention of this 3-year VBC/shared savings program is to reduce costs and create savings by reducing the use of biologics or JAKi. A tangential question might be, “Reduce costs and create savings for whom?” Apparently, the patients will not reap any of the cost savings, as this is proposed to be a shared savings program with the savings going to the physicians and the insurance company. Perhaps the idea is that patients will benefit by reducing unneeded expensive medications.
How will it work?
A cost baseline will be established on biologic and JAKi use prior to the start of the program. Once started, there will be a calculation of savings based on biologic/JAKi use going forward. It was stated that physicians would receive 22% of the total costs saved. In one flyer, it was estimated that, with methotrexate optimization, rheumatologists could be paid an average of $1,527 a month per patient per month of delay before starting a biologic or JAKi.
The American College of Rheumatology has guidelines for the treatment of RA and PsA, and while optimizing methotrexate and triple therapy is mentioned, tapering or stopping treatment with biologics or JAKi is not. Additionally, after lack of response at 3 months, the standard of care is to change to a more effective treatment, which for most patients is a biologic disease-modifying antirheumatic drug (DMARD). It could be construed that rheumatologists are being monetarily incentivized to reduce the use of expensive medications through ways that are not included in ACR guidelines and are not standard of care.
What if after the medication holiday the patient cannot recapture control of their disease? Is there a liability concern? Remember, there is no institutional review board or informed patient consent for this VBC data gathering model.
How will a patient feel knowing that their physician was paid to withhold care, or even worse, if a patient is not told of this and then finds out later? Not only are the optics for this suboptimal (at best), where does the liability fall if the patient does not do well and it comes out that their rheumatologist was paid to reduce the care, particularly in a way that is not supported in the guideline. Clearly, this appears to be a clinical study without an institutional review board and without patient consent.
There are also the data that are collected from this voluntary “opportunity.” A valid question would be, “What kind of data will this produce if rheumatologists are paid to delay, reduce, or stop the use of biologics/JAKi?” Is it possible that physicians may subconsciously delay putting patients on a biologic and taper more rapidly because of the reimbursement? This could lead to faulty, biased, AI-generated data that erroneously show this type of care is working. It would not be unheard of to wonder whether this once-voluntary opportunity might evolve into mandatory policy because now, they have “data to prove it.” … only this time there is no shared savings.
Low disease activity results in long-term savings
This is not meant to be an indictment of AI in health care, value-based care, or shared savings programs. In reality, AI had very little to do with how poorly this program was presented. Hopefully, it will bring about further discussions on how to achieve savings without sacrificing care. In fact, optimal care in RA and PsA is probably one of the best ways to save money in the long run. Nowhere in this program is there any mention of the high cost associated with uncontrolled disease activity in patients with RA or PsA. The downstream costs can be enormous when long- and short-term sequelae are taken into consideration: joint replacements, cardiovascular disease, certain kinds of malignancies, and all the side effects of increased steroid usage are just a few of the consequences we see with uncontrolled disease activity. It is only recently that we have been able to achieve low disease activity and remission in our patients. The rush to get patients off these medications is not the answer to achieving long-term savings. In addition to the very bad optics of paying rheumatologists to delay, taper, or stop using expensive mediations in their patients, the ultimate data achieved will be biased, and the only real winner will be the health insurance company.
Again, AI machine-learning and shared saving programs are not the guilty parties here. In fact, AI may be helpful in coming up with solutions to long-term health care costs, whether in the realm of economics or scientific research. CSRO and our state member organizations continue to educate the health insurance company on the significant drawbacks to this “volunteer opportunity.” Let’s hope a more reasonable program is put forward with AI-generated data that can be trusted. Hopefully not with a platform named “HAL,” for those of you old enough to remember “2001: A Space Odyssey.”
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of advocacy and government affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
Simultaneous marijuana, alcohol use linked to worse outcomes
TOPLINE:
Young adults who simultaneously use alcohol and marijuana (SAM) consume more drinks, are high for more hours in the day, and report more negative alcohol-related consequences.
METHODOLOGY:
- The 2-year study included 409 people aged 18-25 years with a history of simultaneous alcohol and marijuana use (50.9% were women; 48.2% were non-Hispanic White; 48.9% were college students).
- Participants completed daily online surveys about substance use and negative substance-related consequences for 14 continuous days every 4 months.
TAKEAWAY:
- Alcohol use was reported on 36.1% of survey days, marijuana use on 28.0%, and alcohol and marijuana use on 15.0%.
- Negative substance-related consequences were reported on 28.0% of drinking days and 56.4% of marijuana days.
- SAM use was reported in 81.7% of alcohol users and 86.6% of marijuana users.
- On SAM use days, participants consumed an average of 37% more drinks, with 43% more negative alcohol consequences; were high for 10% more hours; and were more likely to feel clumsy or dizzy, compared with non-SAM use days.
IN PRACTICE:
“This finding should be integrated into psychoeducational programs highlighting the risk of combining alcohol and marijuana,” the authors write. “A more nuanced harm-reduction [approach] could also encourage young adults to closely monitor and limit the amount of each substance being used if they choose to combine substances.”
SOURCE:
The study was conducted by Anne M. Fairlie, PhD, University of Washington, Seattle, and colleagues, and funded by the National Institute on Alcohol Abuse and Alcoholism. The study was published online in Alcohol Clinical and Experimental Research.
LIMITATIONS:
Study participants were recruited based on their substance use and lived in a region where recreational marijuana is legal, so the findings may not be generalizable to other populations. Substance use and consequences were self-reported and subject to bias.
DISCLOSURES:
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Young adults who simultaneously use alcohol and marijuana (SAM) consume more drinks, are high for more hours in the day, and report more negative alcohol-related consequences.
METHODOLOGY:
- The 2-year study included 409 people aged 18-25 years with a history of simultaneous alcohol and marijuana use (50.9% were women; 48.2% were non-Hispanic White; 48.9% were college students).
- Participants completed daily online surveys about substance use and negative substance-related consequences for 14 continuous days every 4 months.
TAKEAWAY:
- Alcohol use was reported on 36.1% of survey days, marijuana use on 28.0%, and alcohol and marijuana use on 15.0%.
- Negative substance-related consequences were reported on 28.0% of drinking days and 56.4% of marijuana days.
- SAM use was reported in 81.7% of alcohol users and 86.6% of marijuana users.
- On SAM use days, participants consumed an average of 37% more drinks, with 43% more negative alcohol consequences; were high for 10% more hours; and were more likely to feel clumsy or dizzy, compared with non-SAM use days.
IN PRACTICE:
“This finding should be integrated into psychoeducational programs highlighting the risk of combining alcohol and marijuana,” the authors write. “A more nuanced harm-reduction [approach] could also encourage young adults to closely monitor and limit the amount of each substance being used if they choose to combine substances.”
SOURCE:
The study was conducted by Anne M. Fairlie, PhD, University of Washington, Seattle, and colleagues, and funded by the National Institute on Alcohol Abuse and Alcoholism. The study was published online in Alcohol Clinical and Experimental Research.
LIMITATIONS:
Study participants were recruited based on their substance use and lived in a region where recreational marijuana is legal, so the findings may not be generalizable to other populations. Substance use and consequences were self-reported and subject to bias.
DISCLOSURES:
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
Young adults who simultaneously use alcohol and marijuana (SAM) consume more drinks, are high for more hours in the day, and report more negative alcohol-related consequences.
METHODOLOGY:
- The 2-year study included 409 people aged 18-25 years with a history of simultaneous alcohol and marijuana use (50.9% were women; 48.2% were non-Hispanic White; 48.9% were college students).
- Participants completed daily online surveys about substance use and negative substance-related consequences for 14 continuous days every 4 months.
TAKEAWAY:
- Alcohol use was reported on 36.1% of survey days, marijuana use on 28.0%, and alcohol and marijuana use on 15.0%.
- Negative substance-related consequences were reported on 28.0% of drinking days and 56.4% of marijuana days.
- SAM use was reported in 81.7% of alcohol users and 86.6% of marijuana users.
- On SAM use days, participants consumed an average of 37% more drinks, with 43% more negative alcohol consequences; were high for 10% more hours; and were more likely to feel clumsy or dizzy, compared with non-SAM use days.
IN PRACTICE:
“This finding should be integrated into psychoeducational programs highlighting the risk of combining alcohol and marijuana,” the authors write. “A more nuanced harm-reduction [approach] could also encourage young adults to closely monitor and limit the amount of each substance being used if they choose to combine substances.”
SOURCE:
The study was conducted by Anne M. Fairlie, PhD, University of Washington, Seattle, and colleagues, and funded by the National Institute on Alcohol Abuse and Alcoholism. The study was published online in Alcohol Clinical and Experimental Research.
LIMITATIONS:
Study participants were recruited based on their substance use and lived in a region where recreational marijuana is legal, so the findings may not be generalizable to other populations. Substance use and consequences were self-reported and subject to bias.
DISCLOSURES:
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More on using expired medications
A patient inquires about whether he or she can use an EpiPen after the expiration date. What should you advise?
A. The EpiPen is unlikely to be effective after the expiration date.
B. The EpiPen may be dangerous to use after the expiration date.
C. The EpiPen is likely to be okay up to 2 years past the expiration date.
I think that choice C is the most accurate and will get to all the evidence shortly. It is a date, required by law, that the manufacturer can guarantee greater than 90% original potency of the medication.
Epinephrine is a costly drug and is usually replaced when the Epipen expires. Weir and colleagues studied six epinephrine syringes 30 months past their expiration date.1 Three of the syringes and one control, nonexpired syringe were analyzed using liquid chromatography-mass spectrometry and nuclear magnetic resonance to determine epinephrine content. The contents of the other three syringes of epinephrine were cultured for bacteria and fungus, which yielded no microbial growth. The study showed that the content of epinephrine present in the original sample remained unchanged, compared with the control.
Rachid et al. looked at 35 EpiPens 3-36 months past their expiration dates.2 The percentage of epinephrine found remained 84%-101%, with all EpiPens less than 24 months past expiration having > 90% of the labeled epinephrine dose. Cantrell and colleagues evaluated a combination of 40 EpiPens and Epipen Jrs that were 1-50 months past expiration.3 These pens had not been kept in ideal conditions, as some had been in cars, outdoor cabins, and other environments without temperature control. Sixty-one percent of the Epipens and 56% of the EpiPen Juniors had > 90% of the labeled epinephrine content. I think expired Epipens can be used as a back-up option – that is, they are safe to use if there is not an Epipen available that is not expired.
Shelf life extension program
Lyon and colleagues reported data from the Shelf Life Extension Program (SLEP).4 A total of 122 drugs were studied representing 3,005 lots. Based on testing and stability assessment, 88% of the lots were extended at least 1 year beyond their original expiration date for an average extension of 66 months, but the additional stability period was highly variable. Several antibiotics were studied, including ciprofloxacin (mean extension, 55 months), amoxicillin (mean extension, 23 months), and doxycycline (mean extension, 50 months).
What about other drugs not in pill form?
I am frequently asked about the longevity of medication formulations that are not in pill form. For example, I have been asked about using expired eye drops. There are few data on this. Reis at al. studied whether travoprost that was past the expiration date still lowered intraocular pressures.5 Intraocular pressures in glaucoma patients treated with travoprost 6 weeks after the seal was broken were compared with pressures when drops were used immediately after the container seal was broken. There was no significant difference in intraocular pressure between the two treatment groups during the study.
I found one case report of harm from using expired eye medications. Use of expired eye drops was associated with a case of bilateral toxic epithelial keratopathy.6 Eye drops can be contaminated and cause irritation from the breakdown products of preservatives.
Many people use inhalers for many years. This is especially true for albuterol, which is often used for very intermittent symptoms. I found one recent study on the stability of albuterol. Kutty et al. studied expired albuterol inhalers and solutions up to 20 years past expiration.7 Almost all lots of albuterol maintained > 90% of product (73%-103%), many years past their expiration date. Even at 73% retained activity, the dose would likely be effective.
Pearl: Expired epinephrine and albuterol appear to retain activity several years past expiration.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He has no conflicts of interest. Contact Dr. Paauw at [email protected].
References
1. Weir WB et al. Prehosp Emerg Care. 2018 Jul-Aug;22(4):414-8.
2. Rachid O et al. Ann Allergy Asthma Immunol. 2015 Apr;114(4):354-6.
3. Cantrell FL et al. Ann Intern Med. 2017 Jun 20;166(12):918-9.
4. Lyon RC et al. J Pharmaceut Sci. 2006;95(7):1549-60.
5. Reis R et al. Clin Ther. 2004 Dec;26(12):2121-7.
6. AlGhadeer H, AlHumaiden A. J Clin Pharm Ther. 2022 Dec;47(12):2379-82.
7. Kutty RG et al. Heliyon. 2022 Aug 5;8(8):e10104.
A patient inquires about whether he or she can use an EpiPen after the expiration date. What should you advise?
A. The EpiPen is unlikely to be effective after the expiration date.
B. The EpiPen may be dangerous to use after the expiration date.
C. The EpiPen is likely to be okay up to 2 years past the expiration date.
I think that choice C is the most accurate and will get to all the evidence shortly. It is a date, required by law, that the manufacturer can guarantee greater than 90% original potency of the medication.
Epinephrine is a costly drug and is usually replaced when the Epipen expires. Weir and colleagues studied six epinephrine syringes 30 months past their expiration date.1 Three of the syringes and one control, nonexpired syringe were analyzed using liquid chromatography-mass spectrometry and nuclear magnetic resonance to determine epinephrine content. The contents of the other three syringes of epinephrine were cultured for bacteria and fungus, which yielded no microbial growth. The study showed that the content of epinephrine present in the original sample remained unchanged, compared with the control.
Rachid et al. looked at 35 EpiPens 3-36 months past their expiration dates.2 The percentage of epinephrine found remained 84%-101%, with all EpiPens less than 24 months past expiration having > 90% of the labeled epinephrine dose. Cantrell and colleagues evaluated a combination of 40 EpiPens and Epipen Jrs that were 1-50 months past expiration.3 These pens had not been kept in ideal conditions, as some had been in cars, outdoor cabins, and other environments without temperature control. Sixty-one percent of the Epipens and 56% of the EpiPen Juniors had > 90% of the labeled epinephrine content. I think expired Epipens can be used as a back-up option – that is, they are safe to use if there is not an Epipen available that is not expired.
Shelf life extension program
Lyon and colleagues reported data from the Shelf Life Extension Program (SLEP).4 A total of 122 drugs were studied representing 3,005 lots. Based on testing and stability assessment, 88% of the lots were extended at least 1 year beyond their original expiration date for an average extension of 66 months, but the additional stability period was highly variable. Several antibiotics were studied, including ciprofloxacin (mean extension, 55 months), amoxicillin (mean extension, 23 months), and doxycycline (mean extension, 50 months).
What about other drugs not in pill form?
I am frequently asked about the longevity of medication formulations that are not in pill form. For example, I have been asked about using expired eye drops. There are few data on this. Reis at al. studied whether travoprost that was past the expiration date still lowered intraocular pressures.5 Intraocular pressures in glaucoma patients treated with travoprost 6 weeks after the seal was broken were compared with pressures when drops were used immediately after the container seal was broken. There was no significant difference in intraocular pressure between the two treatment groups during the study.
I found one case report of harm from using expired eye medications. Use of expired eye drops was associated with a case of bilateral toxic epithelial keratopathy.6 Eye drops can be contaminated and cause irritation from the breakdown products of preservatives.
Many people use inhalers for many years. This is especially true for albuterol, which is often used for very intermittent symptoms. I found one recent study on the stability of albuterol. Kutty et al. studied expired albuterol inhalers and solutions up to 20 years past expiration.7 Almost all lots of albuterol maintained > 90% of product (73%-103%), many years past their expiration date. Even at 73% retained activity, the dose would likely be effective.
Pearl: Expired epinephrine and albuterol appear to retain activity several years past expiration.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He has no conflicts of interest. Contact Dr. Paauw at [email protected].
References
1. Weir WB et al. Prehosp Emerg Care. 2018 Jul-Aug;22(4):414-8.
2. Rachid O et al. Ann Allergy Asthma Immunol. 2015 Apr;114(4):354-6.
3. Cantrell FL et al. Ann Intern Med. 2017 Jun 20;166(12):918-9.
4. Lyon RC et al. J Pharmaceut Sci. 2006;95(7):1549-60.
5. Reis R et al. Clin Ther. 2004 Dec;26(12):2121-7.
6. AlGhadeer H, AlHumaiden A. J Clin Pharm Ther. 2022 Dec;47(12):2379-82.
7. Kutty RG et al. Heliyon. 2022 Aug 5;8(8):e10104.
A patient inquires about whether he or she can use an EpiPen after the expiration date. What should you advise?
A. The EpiPen is unlikely to be effective after the expiration date.
B. The EpiPen may be dangerous to use after the expiration date.
C. The EpiPen is likely to be okay up to 2 years past the expiration date.
I think that choice C is the most accurate and will get to all the evidence shortly. It is a date, required by law, that the manufacturer can guarantee greater than 90% original potency of the medication.
Epinephrine is a costly drug and is usually replaced when the Epipen expires. Weir and colleagues studied six epinephrine syringes 30 months past their expiration date.1 Three of the syringes and one control, nonexpired syringe were analyzed using liquid chromatography-mass spectrometry and nuclear magnetic resonance to determine epinephrine content. The contents of the other three syringes of epinephrine were cultured for bacteria and fungus, which yielded no microbial growth. The study showed that the content of epinephrine present in the original sample remained unchanged, compared with the control.
Rachid et al. looked at 35 EpiPens 3-36 months past their expiration dates.2 The percentage of epinephrine found remained 84%-101%, with all EpiPens less than 24 months past expiration having > 90% of the labeled epinephrine dose. Cantrell and colleagues evaluated a combination of 40 EpiPens and Epipen Jrs that were 1-50 months past expiration.3 These pens had not been kept in ideal conditions, as some had been in cars, outdoor cabins, and other environments without temperature control. Sixty-one percent of the Epipens and 56% of the EpiPen Juniors had > 90% of the labeled epinephrine content. I think expired Epipens can be used as a back-up option – that is, they are safe to use if there is not an Epipen available that is not expired.
Shelf life extension program
Lyon and colleagues reported data from the Shelf Life Extension Program (SLEP).4 A total of 122 drugs were studied representing 3,005 lots. Based on testing and stability assessment, 88% of the lots were extended at least 1 year beyond their original expiration date for an average extension of 66 months, but the additional stability period was highly variable. Several antibiotics were studied, including ciprofloxacin (mean extension, 55 months), amoxicillin (mean extension, 23 months), and doxycycline (mean extension, 50 months).
What about other drugs not in pill form?
I am frequently asked about the longevity of medication formulations that are not in pill form. For example, I have been asked about using expired eye drops. There are few data on this. Reis at al. studied whether travoprost that was past the expiration date still lowered intraocular pressures.5 Intraocular pressures in glaucoma patients treated with travoprost 6 weeks after the seal was broken were compared with pressures when drops were used immediately after the container seal was broken. There was no significant difference in intraocular pressure between the two treatment groups during the study.
I found one case report of harm from using expired eye medications. Use of expired eye drops was associated with a case of bilateral toxic epithelial keratopathy.6 Eye drops can be contaminated and cause irritation from the breakdown products of preservatives.
Many people use inhalers for many years. This is especially true for albuterol, which is often used for very intermittent symptoms. I found one recent study on the stability of albuterol. Kutty et al. studied expired albuterol inhalers and solutions up to 20 years past expiration.7 Almost all lots of albuterol maintained > 90% of product (73%-103%), many years past their expiration date. Even at 73% retained activity, the dose would likely be effective.
Pearl: Expired epinephrine and albuterol appear to retain activity several years past expiration.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. He has no conflicts of interest. Contact Dr. Paauw at [email protected].
References
1. Weir WB et al. Prehosp Emerg Care. 2018 Jul-Aug;22(4):414-8.
2. Rachid O et al. Ann Allergy Asthma Immunol. 2015 Apr;114(4):354-6.
3. Cantrell FL et al. Ann Intern Med. 2017 Jun 20;166(12):918-9.
4. Lyon RC et al. J Pharmaceut Sci. 2006;95(7):1549-60.
5. Reis R et al. Clin Ther. 2004 Dec;26(12):2121-7.
6. AlGhadeer H, AlHumaiden A. J Clin Pharm Ther. 2022 Dec;47(12):2379-82.
7. Kutty RG et al. Heliyon. 2022 Aug 5;8(8):e10104.