User login
Two landmark papers change treatment paradigm for advanced endometrial cancer
I wanted to very briefly highlight a truly extraordinary event in my professional experience as a clinical investigator for almost 40 years in the area of the gynecologic malignancies:
In my career, of course, I’ve treated endometrial cancer, but the paradigm, the algorithms, and the strategies we’ve used have, for the most part, simply followed what we’ve done for ovarian cancer. If platinums worked in ovarian cancer, they probably worked in endometrial cancer, and that was true. If paclitaxel worked and had activity in ovarian cancer, it probably would in endometrial cancer, and that was true. It took some time, but basically, we use the same frontline chemotherapy in advanced or recurrent endometrial cancer as we’ve used in ovarian cancer, and on and on.
That world has changed, very much for the positive. Not only have pharmaceutical companies, academic investigators, and individual investigators in the community setting seen endometrial cancer as a major priority, but we have exciting new developments, and very specifically, of course, the immunotherapeutic agents known as checkpoint inhibitors.
One of these two papers was titled “Pembrolizumab Plus Chemotherapy in Advanced Endometrial Cancer” and the second one was titled “Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer.” Obviously, these were separate studies, but both used checkpoint inhibitor plus the chemotherapeutic agents carboplatin-paclitaxel, compared with chemotherapy alone as frontline therapy for advanced or recurrent ovarian cancer and demonstrated a statistically significant, and in my opinion, highly clinically meaningful improvement, in progression-free survival in favor of the regimen that included the checkpoint inhibitors.
Clearly, we will need longer follow-up to see both the overall magnitude of the effect of these therapies on overall survival and the duration of the effect – the shape of the curve. Do we cure many more people? Do we delay time to progression and death? That remains to be seen.
But the outcomes we have now are remarkably positive for patients and have absolutely changed the standard of care in the management of recurrent or advanced endometrial cancer.
I should note that this includes both patients who have evidence of mismatch repair deficiency and those patients who do not have evidence of deficiency, which is a large patient population. These studies demonstrated the benefit to the entire population of patients.
However, on the basis of the data that we have – not only in endometrial cancer, but in other tumor types – the greatest impact was seen in patients with evidence of mismatch repair deficiency, where the immunotherapy agent has been shown to be most relevant; not exclusively, but most relevant.
These are very important papers. If you have an interest in endometrial cancer or immunotherapy, I would encourage you to read these papers. They change the paradigm of management for advanced endometrial cancer, and they clearly point out directions for future research in the management of this class of gynecologic cancers.
Dr. Markman is a professor in the department of medical oncology and therapeutics research at City of Hope in Duarte, Calif., and the president of Medicine & Science at City of Hope Atlanta, Chicago, and Phoenix. He reported conflicts of interest with AstraZeneca and GlaxoSmithKline.
This transcript has been edited for clarity. A version of this article first appeared on Medscape.com.
I wanted to very briefly highlight a truly extraordinary event in my professional experience as a clinical investigator for almost 40 years in the area of the gynecologic malignancies:
In my career, of course, I’ve treated endometrial cancer, but the paradigm, the algorithms, and the strategies we’ve used have, for the most part, simply followed what we’ve done for ovarian cancer. If platinums worked in ovarian cancer, they probably worked in endometrial cancer, and that was true. If paclitaxel worked and had activity in ovarian cancer, it probably would in endometrial cancer, and that was true. It took some time, but basically, we use the same frontline chemotherapy in advanced or recurrent endometrial cancer as we’ve used in ovarian cancer, and on and on.
That world has changed, very much for the positive. Not only have pharmaceutical companies, academic investigators, and individual investigators in the community setting seen endometrial cancer as a major priority, but we have exciting new developments, and very specifically, of course, the immunotherapeutic agents known as checkpoint inhibitors.
One of these two papers was titled “Pembrolizumab Plus Chemotherapy in Advanced Endometrial Cancer” and the second one was titled “Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer.” Obviously, these were separate studies, but both used checkpoint inhibitor plus the chemotherapeutic agents carboplatin-paclitaxel, compared with chemotherapy alone as frontline therapy for advanced or recurrent ovarian cancer and demonstrated a statistically significant, and in my opinion, highly clinically meaningful improvement, in progression-free survival in favor of the regimen that included the checkpoint inhibitors.
Clearly, we will need longer follow-up to see both the overall magnitude of the effect of these therapies on overall survival and the duration of the effect – the shape of the curve. Do we cure many more people? Do we delay time to progression and death? That remains to be seen.
But the outcomes we have now are remarkably positive for patients and have absolutely changed the standard of care in the management of recurrent or advanced endometrial cancer.
I should note that this includes both patients who have evidence of mismatch repair deficiency and those patients who do not have evidence of deficiency, which is a large patient population. These studies demonstrated the benefit to the entire population of patients.
However, on the basis of the data that we have – not only in endometrial cancer, but in other tumor types – the greatest impact was seen in patients with evidence of mismatch repair deficiency, where the immunotherapy agent has been shown to be most relevant; not exclusively, but most relevant.
These are very important papers. If you have an interest in endometrial cancer or immunotherapy, I would encourage you to read these papers. They change the paradigm of management for advanced endometrial cancer, and they clearly point out directions for future research in the management of this class of gynecologic cancers.
Dr. Markman is a professor in the department of medical oncology and therapeutics research at City of Hope in Duarte, Calif., and the president of Medicine & Science at City of Hope Atlanta, Chicago, and Phoenix. He reported conflicts of interest with AstraZeneca and GlaxoSmithKline.
This transcript has been edited for clarity. A version of this article first appeared on Medscape.com.
I wanted to very briefly highlight a truly extraordinary event in my professional experience as a clinical investigator for almost 40 years in the area of the gynecologic malignancies:
In my career, of course, I’ve treated endometrial cancer, but the paradigm, the algorithms, and the strategies we’ve used have, for the most part, simply followed what we’ve done for ovarian cancer. If platinums worked in ovarian cancer, they probably worked in endometrial cancer, and that was true. If paclitaxel worked and had activity in ovarian cancer, it probably would in endometrial cancer, and that was true. It took some time, but basically, we use the same frontline chemotherapy in advanced or recurrent endometrial cancer as we’ve used in ovarian cancer, and on and on.
That world has changed, very much for the positive. Not only have pharmaceutical companies, academic investigators, and individual investigators in the community setting seen endometrial cancer as a major priority, but we have exciting new developments, and very specifically, of course, the immunotherapeutic agents known as checkpoint inhibitors.
One of these two papers was titled “Pembrolizumab Plus Chemotherapy in Advanced Endometrial Cancer” and the second one was titled “Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer.” Obviously, these were separate studies, but both used checkpoint inhibitor plus the chemotherapeutic agents carboplatin-paclitaxel, compared with chemotherapy alone as frontline therapy for advanced or recurrent ovarian cancer and demonstrated a statistically significant, and in my opinion, highly clinically meaningful improvement, in progression-free survival in favor of the regimen that included the checkpoint inhibitors.
Clearly, we will need longer follow-up to see both the overall magnitude of the effect of these therapies on overall survival and the duration of the effect – the shape of the curve. Do we cure many more people? Do we delay time to progression and death? That remains to be seen.
But the outcomes we have now are remarkably positive for patients and have absolutely changed the standard of care in the management of recurrent or advanced endometrial cancer.
I should note that this includes both patients who have evidence of mismatch repair deficiency and those patients who do not have evidence of deficiency, which is a large patient population. These studies demonstrated the benefit to the entire population of patients.
However, on the basis of the data that we have – not only in endometrial cancer, but in other tumor types – the greatest impact was seen in patients with evidence of mismatch repair deficiency, where the immunotherapy agent has been shown to be most relevant; not exclusively, but most relevant.
These are very important papers. If you have an interest in endometrial cancer or immunotherapy, I would encourage you to read these papers. They change the paradigm of management for advanced endometrial cancer, and they clearly point out directions for future research in the management of this class of gynecologic cancers.
Dr. Markman is a professor in the department of medical oncology and therapeutics research at City of Hope in Duarte, Calif., and the president of Medicine & Science at City of Hope Atlanta, Chicago, and Phoenix. He reported conflicts of interest with AstraZeneca and GlaxoSmithKline.
This transcript has been edited for clarity. A version of this article first appeared on Medscape.com.
Trial halted for bleeding reduction with abelacimab vs. rivaroxaban in AFib
– major and clinically relevant nonmajor bleeding – in patients taking abelacimab versus those on rivaroxaban.
The announcement of topline results of the AZALEA-TIMI 71 trial was made by Anthos Therapeutics, the company developing abelacimab.
“The AZALEA-TIMI 71 study is the largest and longest head-to-head study of a Factor XI inhibitor to provide definitive evidence of a highly significant reduction in bleeding as compared to the standard-of-care anticoagulant,” Marc Sabatine, MD, chair of cardiovascular medicine at Brigham and Women’s Hospital and chair of the TIMI study group, both in Boston, stated in the Anthos press release.
“With a median of 21 months of follow-up, spanning more than 2,000 patient years, AZALEA-TIMI 71 represents a landmark study confirming the promise of Factor XI inhibition as causing substantially less bleeding than a current standard-of-care,” Dr. Sabatine added.
Abelacimab is a novel, highly selective, fully human monoclonal antibody with dual inhibitory activity against factor XI and its active form, factor XIa. At the 150-mg dose given subcutaneously once monthly, the drug maintains around 98% inhibition of factor XI, in line with the benign bleeding profile of patients with genetic factor XI deficiency, the company notes.
The AZALEA-TIMI 71 study is an event-driven, randomized study comparing two blinded doses of abelacimab (90 mg or 150 mg given by subcutaneous injection once-monthly) with rivaroxaban 20 mg daily in 1,287 patients with AFib who are at moderate to high risk for stroke. Full results of the study will be presented at an upcoming scientific congress.
Patients in the rivaroxaban arm can transition to abelacimab in an extension study.
In a previous proof-of-concept study published in The New England Journal of Medicine, a single IV dose of abelacimab achieved a large reduction in venous thromboembolism versus enoxaparin in patients undergoing knee surgery.
A phase 3 trial in AFib patients is now planned. The LILAC-TIMI 76 study is an event-driven, randomized trial to evaluate the efficacy and safety of abelacimab relative to placebo on the rate of ischemic stroke or systemic embolism in AFib patients who have been deemed to be unsuitable for currently available anticoagulation therapy. Patients will be randomized to receive abelacimab 150 mg subcutaneously or matching placebo once monthly. The researchers aim to enroll approximately 1,900 patients from North America, Europe, Latin America, the Middle East, and Asia.
Dan Bloomfield, MD, chief medical officer of Anthos Therapeutics, said that, “Abelacimab embodies its promise as a hemostasis-sparing anticoagulant and represents a paradigm shift in the prevention of stroke and other thrombotic conditions.”
It is estimated that 12.1 million people in the United States will have AFib by 2030, but 40%-60% of patients with AFib are not prescribed anticoagulants today, one of the main reasons being concerns about bleeding, the company notes.
“Abelacimab has the potential to provide a game-changing treatment option for all those patients who live with the daily fear of bleeding while taking current anticoagulants,” said Leslie Lake, president of the National Blood Clot Alliance.
Abelacimab has been granted a fast-track designation by the U.S. Food and Drug Administration for the prevention of stroke and systemic embolism in patients with atrial fibrillation.
Several other Factor XI inhibitors are in development and have also shown promising results in terms of a more benign bleeding profile than current standard-of-care anticoagulants.
A version of this article first appeared on Medscape.com.
– major and clinically relevant nonmajor bleeding – in patients taking abelacimab versus those on rivaroxaban.
The announcement of topline results of the AZALEA-TIMI 71 trial was made by Anthos Therapeutics, the company developing abelacimab.
“The AZALEA-TIMI 71 study is the largest and longest head-to-head study of a Factor XI inhibitor to provide definitive evidence of a highly significant reduction in bleeding as compared to the standard-of-care anticoagulant,” Marc Sabatine, MD, chair of cardiovascular medicine at Brigham and Women’s Hospital and chair of the TIMI study group, both in Boston, stated in the Anthos press release.
“With a median of 21 months of follow-up, spanning more than 2,000 patient years, AZALEA-TIMI 71 represents a landmark study confirming the promise of Factor XI inhibition as causing substantially less bleeding than a current standard-of-care,” Dr. Sabatine added.
Abelacimab is a novel, highly selective, fully human monoclonal antibody with dual inhibitory activity against factor XI and its active form, factor XIa. At the 150-mg dose given subcutaneously once monthly, the drug maintains around 98% inhibition of factor XI, in line with the benign bleeding profile of patients with genetic factor XI deficiency, the company notes.
The AZALEA-TIMI 71 study is an event-driven, randomized study comparing two blinded doses of abelacimab (90 mg or 150 mg given by subcutaneous injection once-monthly) with rivaroxaban 20 mg daily in 1,287 patients with AFib who are at moderate to high risk for stroke. Full results of the study will be presented at an upcoming scientific congress.
Patients in the rivaroxaban arm can transition to abelacimab in an extension study.
In a previous proof-of-concept study published in The New England Journal of Medicine, a single IV dose of abelacimab achieved a large reduction in venous thromboembolism versus enoxaparin in patients undergoing knee surgery.
A phase 3 trial in AFib patients is now planned. The LILAC-TIMI 76 study is an event-driven, randomized trial to evaluate the efficacy and safety of abelacimab relative to placebo on the rate of ischemic stroke or systemic embolism in AFib patients who have been deemed to be unsuitable for currently available anticoagulation therapy. Patients will be randomized to receive abelacimab 150 mg subcutaneously or matching placebo once monthly. The researchers aim to enroll approximately 1,900 patients from North America, Europe, Latin America, the Middle East, and Asia.
Dan Bloomfield, MD, chief medical officer of Anthos Therapeutics, said that, “Abelacimab embodies its promise as a hemostasis-sparing anticoagulant and represents a paradigm shift in the prevention of stroke and other thrombotic conditions.”
It is estimated that 12.1 million people in the United States will have AFib by 2030, but 40%-60% of patients with AFib are not prescribed anticoagulants today, one of the main reasons being concerns about bleeding, the company notes.
“Abelacimab has the potential to provide a game-changing treatment option for all those patients who live with the daily fear of bleeding while taking current anticoagulants,” said Leslie Lake, president of the National Blood Clot Alliance.
Abelacimab has been granted a fast-track designation by the U.S. Food and Drug Administration for the prevention of stroke and systemic embolism in patients with atrial fibrillation.
Several other Factor XI inhibitors are in development and have also shown promising results in terms of a more benign bleeding profile than current standard-of-care anticoagulants.
A version of this article first appeared on Medscape.com.
– major and clinically relevant nonmajor bleeding – in patients taking abelacimab versus those on rivaroxaban.
The announcement of topline results of the AZALEA-TIMI 71 trial was made by Anthos Therapeutics, the company developing abelacimab.
“The AZALEA-TIMI 71 study is the largest and longest head-to-head study of a Factor XI inhibitor to provide definitive evidence of a highly significant reduction in bleeding as compared to the standard-of-care anticoagulant,” Marc Sabatine, MD, chair of cardiovascular medicine at Brigham and Women’s Hospital and chair of the TIMI study group, both in Boston, stated in the Anthos press release.
“With a median of 21 months of follow-up, spanning more than 2,000 patient years, AZALEA-TIMI 71 represents a landmark study confirming the promise of Factor XI inhibition as causing substantially less bleeding than a current standard-of-care,” Dr. Sabatine added.
Abelacimab is a novel, highly selective, fully human monoclonal antibody with dual inhibitory activity against factor XI and its active form, factor XIa. At the 150-mg dose given subcutaneously once monthly, the drug maintains around 98% inhibition of factor XI, in line with the benign bleeding profile of patients with genetic factor XI deficiency, the company notes.
The AZALEA-TIMI 71 study is an event-driven, randomized study comparing two blinded doses of abelacimab (90 mg or 150 mg given by subcutaneous injection once-monthly) with rivaroxaban 20 mg daily in 1,287 patients with AFib who are at moderate to high risk for stroke. Full results of the study will be presented at an upcoming scientific congress.
Patients in the rivaroxaban arm can transition to abelacimab in an extension study.
In a previous proof-of-concept study published in The New England Journal of Medicine, a single IV dose of abelacimab achieved a large reduction in venous thromboembolism versus enoxaparin in patients undergoing knee surgery.
A phase 3 trial in AFib patients is now planned. The LILAC-TIMI 76 study is an event-driven, randomized trial to evaluate the efficacy and safety of abelacimab relative to placebo on the rate of ischemic stroke or systemic embolism in AFib patients who have been deemed to be unsuitable for currently available anticoagulation therapy. Patients will be randomized to receive abelacimab 150 mg subcutaneously or matching placebo once monthly. The researchers aim to enroll approximately 1,900 patients from North America, Europe, Latin America, the Middle East, and Asia.
Dan Bloomfield, MD, chief medical officer of Anthos Therapeutics, said that, “Abelacimab embodies its promise as a hemostasis-sparing anticoagulant and represents a paradigm shift in the prevention of stroke and other thrombotic conditions.”
It is estimated that 12.1 million people in the United States will have AFib by 2030, but 40%-60% of patients with AFib are not prescribed anticoagulants today, one of the main reasons being concerns about bleeding, the company notes.
“Abelacimab has the potential to provide a game-changing treatment option for all those patients who live with the daily fear of bleeding while taking current anticoagulants,” said Leslie Lake, president of the National Blood Clot Alliance.
Abelacimab has been granted a fast-track designation by the U.S. Food and Drug Administration for the prevention of stroke and systemic embolism in patients with atrial fibrillation.
Several other Factor XI inhibitors are in development and have also shown promising results in terms of a more benign bleeding profile than current standard-of-care anticoagulants.
A version of this article first appeared on Medscape.com.
Thyroid ablation safety addressed by expert consensus
“There are no documents to date in the United States focusing primarily on the safe adoption and implementation of ablation techniques, including learning curve considerations and necessary pre-procedural skillsets,” reports the ATA task force in the consensus statement, which was published in Thyroid.
“Although these emerging technologies hold great promise, they are not without risk and require development of a unique skill set and environment for optimal, safe performance and consistent outcomes,” task force co-author Catherine F. Sinclair, MD, an associate professor at the Icahn School of Medicine at Mount Sinai, New York, said in an interview.
Chemical ablation has long been utilized as a nonsurgical option for benign thyroid nodule ablation. However, the current array of treatment options has expanded with thermal ablation. Techniques such as radiofrequency ablation (RFA), laser ablation, microwave ablation, and high-intensity focused ultrasound have gained favor as minimally invasive alternatives to surgery.
Much has been published on indications and outcomes with the use of the techniques. The multidisciplinary global task force was convened to address key issues regarding safety and utilization. The report is directed toward specialists, including surgeons, endocrinologists, and interventional radiologists.
The recommendations cover three broad categories: safety considerations spanning preprocedural to postprocedural periods; necessary skill sets for optimal, safe performance with the approaches; and the expectations for success in the context of risks and benefits.
Ablation methods can depend on nodule type
Among key issues addressed are which ablation methods are most appropriate for which types of nodules. Recommendations include chemical ablation, typically involving the injection of dehydrated ethanol in a target nodule. In solid nodules, diffusion with chemical ablation can be unpredictable, which makes it more appropriate for cystic nodules.
Thermal ablation is considered best suited for patients with compressive and/or cosmetic complaints that clearly involve a single or dominant nodule, as well as for autonomously functioning thyroid nodules that cause subclinical or overt hyperthyroidism.
While ethanol ablation is recommended as a first-line treatment for benign cystic thyroid nodules, its efficacy decreases when there is an increase of more than 20% of the solid component. In such cases, RFA or a combination of ethanol ablation and RFA may be considered, the task force recommends.
Patient counseling – managing expectations
Another key consideration in treatment with thyroid nodule ablation is managing patients’ expectations.
Patients should be advised of benefits, such as the avoidance of surgery and general anesthesia and less recovery time. Risks can include thermal or chemical injury to the recurrent laryngeal nerve and other vital structures. The task force underscores discussion of alternative options with patients.
Alternative management options to ablation, including observation, radioactive iodine for functioning nodules, and surgery should also be discussed, and “their relative advantages and disadvantages should be presented without bias such that the patient can make an informed, individual treatment decision,” the task force recommends.
Patients should be informed that, in contrast to surgical management, the benefits of ablation are not immediate; rather, they accrue over the course of months. Reduction in nodule size within the first month is often limited.
Pain, soreness, and some swelling of the nodule and surrounding tissues are common in the first week. These symptoms usually peak in the first 3-5 days after the procedure. Importantly, patients rarely require opioid medications, and their use should be avoided, the task force recommends.
Patients should also be informed about the possibilities of nodule regrowth following ablation and the possible need for more than one ablation procedure.
“Although regrowth definitions in the literature vary, risk of regrowth after thermal ablation is 5%-40% and increases the larger the baseline nodule volume,” the task force notes.
Of note, most studies on ablation to date have shown that thermal ablation complication rates are low. Twelve months post procedure, volume reductions are typically greater than 50%.
Follow-up
For long-term monitoring following ablation, follow-up neck ultrasound is typically recommended at 1-3 months and at 6 and 12 months post ablation to assess volume reduction, nodule appearance, nodule vascularity, and areas at risk for regrowth, the authors note.
Prolonged serial biochemical evaluation of thyroid function is only recommended in cases of hyperfunctioning thyroid nodules.
Key considerations for additional ablative sessions for nodules greater than 20-30 mL in volume should include a failure to achieve adequate reduction in volume, nodule regrowth in previously untreated peripheral areas, and/or persistent or new compressive symptoms.
Learning curve
Dr. Sinclair underscored that successful thyroid nodule ablation requires skill – and experience.
“Probably the greatest concern shared by the writing group on this statement was the potential for clinicians to start ablation practices without having an appropriate prior skill set,” she said.
“Ablation is an advanced, ultrasound-guided procedure, and clinicians need to be experienced in performing neck ultrasounds and biopsies,” she added. “To consider performing ablations without this skill set is both unrealistic and dangerous.”
RFA, currently the most commonly used thermal ablation method for benign thyroid nodule ablation in the U.S., “has a good safety profile but can have a steep learning curve initially,” she said.
Among the most important recommendations is that for their first 20-60 ablation procedures, clinicians should consider limiting treatment to small- to medium-sized benign nodules rather than large-volume disease, Dr. Sinclair added.
“In addition, prior to starting thyroid ablation practices, clinicians should be proficient in ultrasound imaging and fine-needle biopsies and can gain valuable experience by practicing on phantoms and having expert proctoring for the first few cases,” she said.
For initial ablative cases, the task force recommends that clinicians select moderate-size (< 20-30 mL), nonvascular nodules with favorable characteristics and location. The final volume reduction should be based not only on baseline nodule characteristics, such as volume and vascularity, but also on the practitioner’s skill.
Clinicians furthermore should be board certified or eligible in an appropriate medical specialty, have extensive background knowledge, and “should have clinical experience in the clinical diagnosis and treatment of thyroid nodules; neck imaging anatomy; thyroid ultrasound imaging and fine needle aspiration biopsy procedures; and ultrasound risk stratification for benign and malignant thyroid tumors,” the group recommends.
Importantly, the statement is designed to reflect a consensus opinion of the panel of experts but is not meant to serve as a formal guideline or a standard of care for the clinical practice of thermal ablation, Dr. Sinclair added.
“It is not the intent of the statement to replace individual decision-making, the wishes of the patient or family, or clinical judgment.”
The authors’ disclosures are detailed in the published report.
A version of this article first appeared on Medscape.com.
“There are no documents to date in the United States focusing primarily on the safe adoption and implementation of ablation techniques, including learning curve considerations and necessary pre-procedural skillsets,” reports the ATA task force in the consensus statement, which was published in Thyroid.
“Although these emerging technologies hold great promise, they are not without risk and require development of a unique skill set and environment for optimal, safe performance and consistent outcomes,” task force co-author Catherine F. Sinclair, MD, an associate professor at the Icahn School of Medicine at Mount Sinai, New York, said in an interview.
Chemical ablation has long been utilized as a nonsurgical option for benign thyroid nodule ablation. However, the current array of treatment options has expanded with thermal ablation. Techniques such as radiofrequency ablation (RFA), laser ablation, microwave ablation, and high-intensity focused ultrasound have gained favor as minimally invasive alternatives to surgery.
Much has been published on indications and outcomes with the use of the techniques. The multidisciplinary global task force was convened to address key issues regarding safety and utilization. The report is directed toward specialists, including surgeons, endocrinologists, and interventional radiologists.
The recommendations cover three broad categories: safety considerations spanning preprocedural to postprocedural periods; necessary skill sets for optimal, safe performance with the approaches; and the expectations for success in the context of risks and benefits.
Ablation methods can depend on nodule type
Among key issues addressed are which ablation methods are most appropriate for which types of nodules. Recommendations include chemical ablation, typically involving the injection of dehydrated ethanol in a target nodule. In solid nodules, diffusion with chemical ablation can be unpredictable, which makes it more appropriate for cystic nodules.
Thermal ablation is considered best suited for patients with compressive and/or cosmetic complaints that clearly involve a single or dominant nodule, as well as for autonomously functioning thyroid nodules that cause subclinical or overt hyperthyroidism.
While ethanol ablation is recommended as a first-line treatment for benign cystic thyroid nodules, its efficacy decreases when there is an increase of more than 20% of the solid component. In such cases, RFA or a combination of ethanol ablation and RFA may be considered, the task force recommends.
Patient counseling – managing expectations
Another key consideration in treatment with thyroid nodule ablation is managing patients’ expectations.
Patients should be advised of benefits, such as the avoidance of surgery and general anesthesia and less recovery time. Risks can include thermal or chemical injury to the recurrent laryngeal nerve and other vital structures. The task force underscores discussion of alternative options with patients.
Alternative management options to ablation, including observation, radioactive iodine for functioning nodules, and surgery should also be discussed, and “their relative advantages and disadvantages should be presented without bias such that the patient can make an informed, individual treatment decision,” the task force recommends.
Patients should be informed that, in contrast to surgical management, the benefits of ablation are not immediate; rather, they accrue over the course of months. Reduction in nodule size within the first month is often limited.
Pain, soreness, and some swelling of the nodule and surrounding tissues are common in the first week. These symptoms usually peak in the first 3-5 days after the procedure. Importantly, patients rarely require opioid medications, and their use should be avoided, the task force recommends.
Patients should also be informed about the possibilities of nodule regrowth following ablation and the possible need for more than one ablation procedure.
“Although regrowth definitions in the literature vary, risk of regrowth after thermal ablation is 5%-40% and increases the larger the baseline nodule volume,” the task force notes.
Of note, most studies on ablation to date have shown that thermal ablation complication rates are low. Twelve months post procedure, volume reductions are typically greater than 50%.
Follow-up
For long-term monitoring following ablation, follow-up neck ultrasound is typically recommended at 1-3 months and at 6 and 12 months post ablation to assess volume reduction, nodule appearance, nodule vascularity, and areas at risk for regrowth, the authors note.
Prolonged serial biochemical evaluation of thyroid function is only recommended in cases of hyperfunctioning thyroid nodules.
Key considerations for additional ablative sessions for nodules greater than 20-30 mL in volume should include a failure to achieve adequate reduction in volume, nodule regrowth in previously untreated peripheral areas, and/or persistent or new compressive symptoms.
Learning curve
Dr. Sinclair underscored that successful thyroid nodule ablation requires skill – and experience.
“Probably the greatest concern shared by the writing group on this statement was the potential for clinicians to start ablation practices without having an appropriate prior skill set,” she said.
“Ablation is an advanced, ultrasound-guided procedure, and clinicians need to be experienced in performing neck ultrasounds and biopsies,” she added. “To consider performing ablations without this skill set is both unrealistic and dangerous.”
RFA, currently the most commonly used thermal ablation method for benign thyroid nodule ablation in the U.S., “has a good safety profile but can have a steep learning curve initially,” she said.
Among the most important recommendations is that for their first 20-60 ablation procedures, clinicians should consider limiting treatment to small- to medium-sized benign nodules rather than large-volume disease, Dr. Sinclair added.
“In addition, prior to starting thyroid ablation practices, clinicians should be proficient in ultrasound imaging and fine-needle biopsies and can gain valuable experience by practicing on phantoms and having expert proctoring for the first few cases,” she said.
For initial ablative cases, the task force recommends that clinicians select moderate-size (< 20-30 mL), nonvascular nodules with favorable characteristics and location. The final volume reduction should be based not only on baseline nodule characteristics, such as volume and vascularity, but also on the practitioner’s skill.
Clinicians furthermore should be board certified or eligible in an appropriate medical specialty, have extensive background knowledge, and “should have clinical experience in the clinical diagnosis and treatment of thyroid nodules; neck imaging anatomy; thyroid ultrasound imaging and fine needle aspiration biopsy procedures; and ultrasound risk stratification for benign and malignant thyroid tumors,” the group recommends.
Importantly, the statement is designed to reflect a consensus opinion of the panel of experts but is not meant to serve as a formal guideline or a standard of care for the clinical practice of thermal ablation, Dr. Sinclair added.
“It is not the intent of the statement to replace individual decision-making, the wishes of the patient or family, or clinical judgment.”
The authors’ disclosures are detailed in the published report.
A version of this article first appeared on Medscape.com.
“There are no documents to date in the United States focusing primarily on the safe adoption and implementation of ablation techniques, including learning curve considerations and necessary pre-procedural skillsets,” reports the ATA task force in the consensus statement, which was published in Thyroid.
“Although these emerging technologies hold great promise, they are not without risk and require development of a unique skill set and environment for optimal, safe performance and consistent outcomes,” task force co-author Catherine F. Sinclair, MD, an associate professor at the Icahn School of Medicine at Mount Sinai, New York, said in an interview.
Chemical ablation has long been utilized as a nonsurgical option for benign thyroid nodule ablation. However, the current array of treatment options has expanded with thermal ablation. Techniques such as radiofrequency ablation (RFA), laser ablation, microwave ablation, and high-intensity focused ultrasound have gained favor as minimally invasive alternatives to surgery.
Much has been published on indications and outcomes with the use of the techniques. The multidisciplinary global task force was convened to address key issues regarding safety and utilization. The report is directed toward specialists, including surgeons, endocrinologists, and interventional radiologists.
The recommendations cover three broad categories: safety considerations spanning preprocedural to postprocedural periods; necessary skill sets for optimal, safe performance with the approaches; and the expectations for success in the context of risks and benefits.
Ablation methods can depend on nodule type
Among key issues addressed are which ablation methods are most appropriate for which types of nodules. Recommendations include chemical ablation, typically involving the injection of dehydrated ethanol in a target nodule. In solid nodules, diffusion with chemical ablation can be unpredictable, which makes it more appropriate for cystic nodules.
Thermal ablation is considered best suited for patients with compressive and/or cosmetic complaints that clearly involve a single or dominant nodule, as well as for autonomously functioning thyroid nodules that cause subclinical or overt hyperthyroidism.
While ethanol ablation is recommended as a first-line treatment for benign cystic thyroid nodules, its efficacy decreases when there is an increase of more than 20% of the solid component. In such cases, RFA or a combination of ethanol ablation and RFA may be considered, the task force recommends.
Patient counseling – managing expectations
Another key consideration in treatment with thyroid nodule ablation is managing patients’ expectations.
Patients should be advised of benefits, such as the avoidance of surgery and general anesthesia and less recovery time. Risks can include thermal or chemical injury to the recurrent laryngeal nerve and other vital structures. The task force underscores discussion of alternative options with patients.
Alternative management options to ablation, including observation, radioactive iodine for functioning nodules, and surgery should also be discussed, and “their relative advantages and disadvantages should be presented without bias such that the patient can make an informed, individual treatment decision,” the task force recommends.
Patients should be informed that, in contrast to surgical management, the benefits of ablation are not immediate; rather, they accrue over the course of months. Reduction in nodule size within the first month is often limited.
Pain, soreness, and some swelling of the nodule and surrounding tissues are common in the first week. These symptoms usually peak in the first 3-5 days after the procedure. Importantly, patients rarely require opioid medications, and their use should be avoided, the task force recommends.
Patients should also be informed about the possibilities of nodule regrowth following ablation and the possible need for more than one ablation procedure.
“Although regrowth definitions in the literature vary, risk of regrowth after thermal ablation is 5%-40% and increases the larger the baseline nodule volume,” the task force notes.
Of note, most studies on ablation to date have shown that thermal ablation complication rates are low. Twelve months post procedure, volume reductions are typically greater than 50%.
Follow-up
For long-term monitoring following ablation, follow-up neck ultrasound is typically recommended at 1-3 months and at 6 and 12 months post ablation to assess volume reduction, nodule appearance, nodule vascularity, and areas at risk for regrowth, the authors note.
Prolonged serial biochemical evaluation of thyroid function is only recommended in cases of hyperfunctioning thyroid nodules.
Key considerations for additional ablative sessions for nodules greater than 20-30 mL in volume should include a failure to achieve adequate reduction in volume, nodule regrowth in previously untreated peripheral areas, and/or persistent or new compressive symptoms.
Learning curve
Dr. Sinclair underscored that successful thyroid nodule ablation requires skill – and experience.
“Probably the greatest concern shared by the writing group on this statement was the potential for clinicians to start ablation practices without having an appropriate prior skill set,” she said.
“Ablation is an advanced, ultrasound-guided procedure, and clinicians need to be experienced in performing neck ultrasounds and biopsies,” she added. “To consider performing ablations without this skill set is both unrealistic and dangerous.”
RFA, currently the most commonly used thermal ablation method for benign thyroid nodule ablation in the U.S., “has a good safety profile but can have a steep learning curve initially,” she said.
Among the most important recommendations is that for their first 20-60 ablation procedures, clinicians should consider limiting treatment to small- to medium-sized benign nodules rather than large-volume disease, Dr. Sinclair added.
“In addition, prior to starting thyroid ablation practices, clinicians should be proficient in ultrasound imaging and fine-needle biopsies and can gain valuable experience by practicing on phantoms and having expert proctoring for the first few cases,” she said.
For initial ablative cases, the task force recommends that clinicians select moderate-size (< 20-30 mL), nonvascular nodules with favorable characteristics and location. The final volume reduction should be based not only on baseline nodule characteristics, such as volume and vascularity, but also on the practitioner’s skill.
Clinicians furthermore should be board certified or eligible in an appropriate medical specialty, have extensive background knowledge, and “should have clinical experience in the clinical diagnosis and treatment of thyroid nodules; neck imaging anatomy; thyroid ultrasound imaging and fine needle aspiration biopsy procedures; and ultrasound risk stratification for benign and malignant thyroid tumors,” the group recommends.
Importantly, the statement is designed to reflect a consensus opinion of the panel of experts but is not meant to serve as a formal guideline or a standard of care for the clinical practice of thermal ablation, Dr. Sinclair added.
“It is not the intent of the statement to replace individual decision-making, the wishes of the patient or family, or clinical judgment.”
The authors’ disclosures are detailed in the published report.
A version of this article first appeared on Medscape.com.
FROM THYROID
Laboratory testing: No doctor required?
This transcript has been edited for clarity.
Let’s assume, for the sake of argument, that I am a healthy 43-year old man. Nevertheless, I am interested in getting my vitamin D level checked. My primary care doc says it’s unnecessary, but that doesn’t matter because a variety of direct-to-consumer testing companies will do it without a doctor’s prescription – for a fee of course.
Is that okay? Should I be able to get the test?
What if instead of my vitamin D level, I want to test my testosterone level, or my PSA, or my cadmium level, or my Lyme disease antibodies, or even have a full-body MRI scan?
These questions are becoming more and more common, because the direct-to-consumer testing market is exploding.
We’re talking about direct-to-consumer testing, thanks to this paper: Policies of US Companies Offering Direct-to-Consumer Laboratory Tests, appearing in JAMA Internal Medicine, which characterizes the testing practices of direct-to-consumer testing companies.
But before we get to the study, a word on this market. Direct-to-consumer lab testing is projected to be a $2 billion industry by 2025, and lab testing megacorporations Quest Diagnostics and Labcorp are both jumping headlong into this space.
Why is this happening? A couple of reasons, I think. First, the increasing cost of health care has led payers to place significant restrictions on what tests can be ordered and under what circumstances. Physicians are all too familiar with the “prior authorization” system that seeks to limit even the tests we think would benefit our patients.
Frustrated with such a system, it’s no wonder that patients are increasingly deciding to go it on their own. Sure, insurance won’t cover these tests, but the prices are transparent and competition actually keeps them somewhat reasonable. So, is this a win-win? Shouldn’t we allow people to get the tests they want, at least if they are willing to pay for it?
Of course, it’s not quite that simple. If the tests are normal, or negative, then sure – no harm, no foul. But when they are positive, everything changes. What happens when the PSA test I got myself via a direct-to-consumer testing company comes back elevated? Well, at that point, I am right back into the traditional mode of medicine – seeing my doctor, probably getting repeat testing, biopsies, etc., – and some payer will be on the hook for that, which is to say that all of us will be on the hook for that.
One other reason direct-to-consumer testing is getting more popular is a more difficult-to-characterize phenomenon which I might call postpandemic individualism. I’ve seen this across several domains, but I think in some ways the pandemic led people to focus more attention on themselves, perhaps because we were so isolated from each other. Optimizing health through data – whether using a fitness tracking watch, meticulously counting macronutrient intake, or ordering your own lab tests – may be a form of exerting control over a universe that feels increasingly chaotic. But what do I know? I’m not a psychologist.
The study characterizes a total of 21 direct-to-consumer testing companies. They offer a variety of services, as you can see here, with the majority in the endocrine space: thyroid, diabetes, men’s and women’s health. A smattering of companies offer more esoteric testing, such as heavy metals and Lyme disease.
Who’s in charge of all this? It’s fairly regulated, actually, but perhaps not in the way you think. The FDA uses its CLIA authority to ensure that these tests are accurate. The FTC ensures that the companies do not engage in false advertising. But no one is minding the store as to whether the tests are actually beneficial either to an individual or to society.
The 21 companies varied dramatically in regard to how they handle communicating the risks and results of these tests. All of them had a disclaimer that the information does not represent comprehensive medical advice. Fine. But a minority acknowledged any risks or limitations of the tests. Less than half had a statement of HIPAA compliance. And 17 out of 21 provided no information as to whether customers could request their data to be deleted, while 18 out of 21 stated that there could be follow-up for abnormal results, but often it was unclear exactly how that would work.
So, let’s circle back to the first question: Should a healthy person be able to get a laboratory test simply because they want to? The libertarians among us would argue certainly yes, though perhaps without thinking through the societal implications of abnormal results. The evidence-based medicine folks will, accurately, state that there are no clinical trials to suggest that screening healthy people with tests like these has any benefit.
But we should be cautious here. This question is scienceable; you could design a trial to test whether screening healthy 43-year-olds for testosterone level led to significant improvements in overall mortality. It would just take a few million people and about 40 years of follow-up.
And even if it didn’t help, we let people throw their money away on useless things all the time. The only difference between someone spending money on a useless test or on a useless dietary supplement is that someone has to deal with the result.
So, can you do this right? Can you make a direct-to-consumer testing company that is not essentially a free-rider on the rest of the health care ecosystem?
I think there are ways. You’d need physicians involved at all stages to help interpret the testing and guide next steps. You’d need some transparent guidelines, written in language that patients can understand, for what will happen given any conceivable result – and what costs those results might lead to for them and their insurance company. Most important, you’d need longitudinal follow-up and the ability to recommend changes, retest in the future, and potentially address the cost implications of the downstream findings. In the end, it starts to sound very much like a doctor’s office.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Let’s assume, for the sake of argument, that I am a healthy 43-year old man. Nevertheless, I am interested in getting my vitamin D level checked. My primary care doc says it’s unnecessary, but that doesn’t matter because a variety of direct-to-consumer testing companies will do it without a doctor’s prescription – for a fee of course.
Is that okay? Should I be able to get the test?
What if instead of my vitamin D level, I want to test my testosterone level, or my PSA, or my cadmium level, or my Lyme disease antibodies, or even have a full-body MRI scan?
These questions are becoming more and more common, because the direct-to-consumer testing market is exploding.
We’re talking about direct-to-consumer testing, thanks to this paper: Policies of US Companies Offering Direct-to-Consumer Laboratory Tests, appearing in JAMA Internal Medicine, which characterizes the testing practices of direct-to-consumer testing companies.
But before we get to the study, a word on this market. Direct-to-consumer lab testing is projected to be a $2 billion industry by 2025, and lab testing megacorporations Quest Diagnostics and Labcorp are both jumping headlong into this space.
Why is this happening? A couple of reasons, I think. First, the increasing cost of health care has led payers to place significant restrictions on what tests can be ordered and under what circumstances. Physicians are all too familiar with the “prior authorization” system that seeks to limit even the tests we think would benefit our patients.
Frustrated with such a system, it’s no wonder that patients are increasingly deciding to go it on their own. Sure, insurance won’t cover these tests, but the prices are transparent and competition actually keeps them somewhat reasonable. So, is this a win-win? Shouldn’t we allow people to get the tests they want, at least if they are willing to pay for it?
Of course, it’s not quite that simple. If the tests are normal, or negative, then sure – no harm, no foul. But when they are positive, everything changes. What happens when the PSA test I got myself via a direct-to-consumer testing company comes back elevated? Well, at that point, I am right back into the traditional mode of medicine – seeing my doctor, probably getting repeat testing, biopsies, etc., – and some payer will be on the hook for that, which is to say that all of us will be on the hook for that.
One other reason direct-to-consumer testing is getting more popular is a more difficult-to-characterize phenomenon which I might call postpandemic individualism. I’ve seen this across several domains, but I think in some ways the pandemic led people to focus more attention on themselves, perhaps because we were so isolated from each other. Optimizing health through data – whether using a fitness tracking watch, meticulously counting macronutrient intake, or ordering your own lab tests – may be a form of exerting control over a universe that feels increasingly chaotic. But what do I know? I’m not a psychologist.
The study characterizes a total of 21 direct-to-consumer testing companies. They offer a variety of services, as you can see here, with the majority in the endocrine space: thyroid, diabetes, men’s and women’s health. A smattering of companies offer more esoteric testing, such as heavy metals and Lyme disease.
Who’s in charge of all this? It’s fairly regulated, actually, but perhaps not in the way you think. The FDA uses its CLIA authority to ensure that these tests are accurate. The FTC ensures that the companies do not engage in false advertising. But no one is minding the store as to whether the tests are actually beneficial either to an individual or to society.
The 21 companies varied dramatically in regard to how they handle communicating the risks and results of these tests. All of them had a disclaimer that the information does not represent comprehensive medical advice. Fine. But a minority acknowledged any risks or limitations of the tests. Less than half had a statement of HIPAA compliance. And 17 out of 21 provided no information as to whether customers could request their data to be deleted, while 18 out of 21 stated that there could be follow-up for abnormal results, but often it was unclear exactly how that would work.
So, let’s circle back to the first question: Should a healthy person be able to get a laboratory test simply because they want to? The libertarians among us would argue certainly yes, though perhaps without thinking through the societal implications of abnormal results. The evidence-based medicine folks will, accurately, state that there are no clinical trials to suggest that screening healthy people with tests like these has any benefit.
But we should be cautious here. This question is scienceable; you could design a trial to test whether screening healthy 43-year-olds for testosterone level led to significant improvements in overall mortality. It would just take a few million people and about 40 years of follow-up.
And even if it didn’t help, we let people throw their money away on useless things all the time. The only difference between someone spending money on a useless test or on a useless dietary supplement is that someone has to deal with the result.
So, can you do this right? Can you make a direct-to-consumer testing company that is not essentially a free-rider on the rest of the health care ecosystem?
I think there are ways. You’d need physicians involved at all stages to help interpret the testing and guide next steps. You’d need some transparent guidelines, written in language that patients can understand, for what will happen given any conceivable result – and what costs those results might lead to for them and their insurance company. Most important, you’d need longitudinal follow-up and the ability to recommend changes, retest in the future, and potentially address the cost implications of the downstream findings. In the end, it starts to sound very much like a doctor’s office.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Let’s assume, for the sake of argument, that I am a healthy 43-year old man. Nevertheless, I am interested in getting my vitamin D level checked. My primary care doc says it’s unnecessary, but that doesn’t matter because a variety of direct-to-consumer testing companies will do it without a doctor’s prescription – for a fee of course.
Is that okay? Should I be able to get the test?
What if instead of my vitamin D level, I want to test my testosterone level, or my PSA, or my cadmium level, or my Lyme disease antibodies, or even have a full-body MRI scan?
These questions are becoming more and more common, because the direct-to-consumer testing market is exploding.
We’re talking about direct-to-consumer testing, thanks to this paper: Policies of US Companies Offering Direct-to-Consumer Laboratory Tests, appearing in JAMA Internal Medicine, which characterizes the testing practices of direct-to-consumer testing companies.
But before we get to the study, a word on this market. Direct-to-consumer lab testing is projected to be a $2 billion industry by 2025, and lab testing megacorporations Quest Diagnostics and Labcorp are both jumping headlong into this space.
Why is this happening? A couple of reasons, I think. First, the increasing cost of health care has led payers to place significant restrictions on what tests can be ordered and under what circumstances. Physicians are all too familiar with the “prior authorization” system that seeks to limit even the tests we think would benefit our patients.
Frustrated with such a system, it’s no wonder that patients are increasingly deciding to go it on their own. Sure, insurance won’t cover these tests, but the prices are transparent and competition actually keeps them somewhat reasonable. So, is this a win-win? Shouldn’t we allow people to get the tests they want, at least if they are willing to pay for it?
Of course, it’s not quite that simple. If the tests are normal, or negative, then sure – no harm, no foul. But when they are positive, everything changes. What happens when the PSA test I got myself via a direct-to-consumer testing company comes back elevated? Well, at that point, I am right back into the traditional mode of medicine – seeing my doctor, probably getting repeat testing, biopsies, etc., – and some payer will be on the hook for that, which is to say that all of us will be on the hook for that.
One other reason direct-to-consumer testing is getting more popular is a more difficult-to-characterize phenomenon which I might call postpandemic individualism. I’ve seen this across several domains, but I think in some ways the pandemic led people to focus more attention on themselves, perhaps because we were so isolated from each other. Optimizing health through data – whether using a fitness tracking watch, meticulously counting macronutrient intake, or ordering your own lab tests – may be a form of exerting control over a universe that feels increasingly chaotic. But what do I know? I’m not a psychologist.
The study characterizes a total of 21 direct-to-consumer testing companies. They offer a variety of services, as you can see here, with the majority in the endocrine space: thyroid, diabetes, men’s and women’s health. A smattering of companies offer more esoteric testing, such as heavy metals and Lyme disease.
Who’s in charge of all this? It’s fairly regulated, actually, but perhaps not in the way you think. The FDA uses its CLIA authority to ensure that these tests are accurate. The FTC ensures that the companies do not engage in false advertising. But no one is minding the store as to whether the tests are actually beneficial either to an individual or to society.
The 21 companies varied dramatically in regard to how they handle communicating the risks and results of these tests. All of them had a disclaimer that the information does not represent comprehensive medical advice. Fine. But a minority acknowledged any risks or limitations of the tests. Less than half had a statement of HIPAA compliance. And 17 out of 21 provided no information as to whether customers could request their data to be deleted, while 18 out of 21 stated that there could be follow-up for abnormal results, but often it was unclear exactly how that would work.
So, let’s circle back to the first question: Should a healthy person be able to get a laboratory test simply because they want to? The libertarians among us would argue certainly yes, though perhaps without thinking through the societal implications of abnormal results. The evidence-based medicine folks will, accurately, state that there are no clinical trials to suggest that screening healthy people with tests like these has any benefit.
But we should be cautious here. This question is scienceable; you could design a trial to test whether screening healthy 43-year-olds for testosterone level led to significant improvements in overall mortality. It would just take a few million people and about 40 years of follow-up.
And even if it didn’t help, we let people throw their money away on useless things all the time. The only difference between someone spending money on a useless test or on a useless dietary supplement is that someone has to deal with the result.
So, can you do this right? Can you make a direct-to-consumer testing company that is not essentially a free-rider on the rest of the health care ecosystem?
I think there are ways. You’d need physicians involved at all stages to help interpret the testing and guide next steps. You’d need some transparent guidelines, written in language that patients can understand, for what will happen given any conceivable result – and what costs those results might lead to for them and their insurance company. Most important, you’d need longitudinal follow-up and the ability to recommend changes, retest in the future, and potentially address the cost implications of the downstream findings. In the end, it starts to sound very much like a doctor’s office.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Job-related stressors tied to increased CHD risk in men
TOPLINE:
, and those facing both stressors have double the risk compared with colleagues not suffering from these stressors, new research shows. Results in women were inconclusive, suggesting a more complex relationship of these factors, the researchers noted.
METHODOLOGY:
- Evidence suggests psychosocial stressors at work, from job strain related to level of demand and control in workload and decision-making responsibilities, and an effort-reward imbalance (ERI) in areas such as salary, promotion, and job stability, increase CHD risk, with the effect of both types of stressors together possibly being especially harmful.
- The study, which included 6,465 participants in the cardiovascular component of PROQ, a Canadian prospective cohort of white-collar workers initially free of cardiovascular disease, mean age 45 years, estimated that the separate and combined effect of job strain and ERI on CHD incidence.
- Researchers used the Job Content Questionnaire to assess psychological demands and job control; various measures; scales to determine job strain, reward, and effort at work; and the sum of both effort and reward to calculate the ERI ratio.
- They assessed CHD using medico-administrative databases and an algorithm validated by medical records.
TAKEAWAY:
- After a median follow-up of 18.7 years, there were 571 and 265 incident CHD cases among men and women, respectively.
- Men with either job strain or ERI had a 49% increased risk for CHD (hazard ratio [HR], 1.49; 95% confidence interval [CI], 1.07-2.09), an estimate comparable to that of several lifestyle risk factors for CHD.
- Male workers facing both job strain and ERI had a 103% increased risk for CHD (HR, 2.03; 95% CI, 1.38-2.97), which is comparable to the increased risk associated with obesity.
- Associations were robust to adjustments for demographic, socioeconomic, psychosocial, personality, stressful life events, and biomedical and lifestyle factors.
- Among women, results were inconclusive because the CIs were wide enough to encompass both protective and detrimental effects, suggesting more research is needed into the complex interplay of various stressors and women’s heart health.
IN PRACTICE:
“Integrative and interdisciplinary approaches should be used to tackle psychosocial stressors at work,” the authors wrote, adding this involves “going beyond traditional modifiable individual behaviors” and should include “population-based prevention strategies taking into consideration both the individual and their work environment.”
SOURCE:
The study was conducted by Mathilde Lavigne-Robichaud, Population Health and Optimal Health Practices Research Unit, CHU de Québec-Laval University, Quebec City, Canada. It was published online in Circulation: Cardiovascular Quality and Outcomes.
LIMITATIONS:
There was a risk for chance associations due to multiple testing. The exposure may have changed over the course of the study. Using medical databases for CHD event definition may have led to misclassification and underestimation of outcomes. The study population is limited to white-collar workers.
DISCLOSURES:
The study received funding from the Canadian Institute of Health Research. Lavigne-Robichaud was supported by a PhD grant from les Fonds de Recherche du Québec-Santé. See paper for disclosures of other authors.
A version of this article first appeared on Medscape.com.
TOPLINE:
, and those facing both stressors have double the risk compared with colleagues not suffering from these stressors, new research shows. Results in women were inconclusive, suggesting a more complex relationship of these factors, the researchers noted.
METHODOLOGY:
- Evidence suggests psychosocial stressors at work, from job strain related to level of demand and control in workload and decision-making responsibilities, and an effort-reward imbalance (ERI) in areas such as salary, promotion, and job stability, increase CHD risk, with the effect of both types of stressors together possibly being especially harmful.
- The study, which included 6,465 participants in the cardiovascular component of PROQ, a Canadian prospective cohort of white-collar workers initially free of cardiovascular disease, mean age 45 years, estimated that the separate and combined effect of job strain and ERI on CHD incidence.
- Researchers used the Job Content Questionnaire to assess psychological demands and job control; various measures; scales to determine job strain, reward, and effort at work; and the sum of both effort and reward to calculate the ERI ratio.
- They assessed CHD using medico-administrative databases and an algorithm validated by medical records.
TAKEAWAY:
- After a median follow-up of 18.7 years, there were 571 and 265 incident CHD cases among men and women, respectively.
- Men with either job strain or ERI had a 49% increased risk for CHD (hazard ratio [HR], 1.49; 95% confidence interval [CI], 1.07-2.09), an estimate comparable to that of several lifestyle risk factors for CHD.
- Male workers facing both job strain and ERI had a 103% increased risk for CHD (HR, 2.03; 95% CI, 1.38-2.97), which is comparable to the increased risk associated with obesity.
- Associations were robust to adjustments for demographic, socioeconomic, psychosocial, personality, stressful life events, and biomedical and lifestyle factors.
- Among women, results were inconclusive because the CIs were wide enough to encompass both protective and detrimental effects, suggesting more research is needed into the complex interplay of various stressors and women’s heart health.
IN PRACTICE:
“Integrative and interdisciplinary approaches should be used to tackle psychosocial stressors at work,” the authors wrote, adding this involves “going beyond traditional modifiable individual behaviors” and should include “population-based prevention strategies taking into consideration both the individual and their work environment.”
SOURCE:
The study was conducted by Mathilde Lavigne-Robichaud, Population Health and Optimal Health Practices Research Unit, CHU de Québec-Laval University, Quebec City, Canada. It was published online in Circulation: Cardiovascular Quality and Outcomes.
LIMITATIONS:
There was a risk for chance associations due to multiple testing. The exposure may have changed over the course of the study. Using medical databases for CHD event definition may have led to misclassification and underestimation of outcomes. The study population is limited to white-collar workers.
DISCLOSURES:
The study received funding from the Canadian Institute of Health Research. Lavigne-Robichaud was supported by a PhD grant from les Fonds de Recherche du Québec-Santé. See paper for disclosures of other authors.
A version of this article first appeared on Medscape.com.
TOPLINE:
, and those facing both stressors have double the risk compared with colleagues not suffering from these stressors, new research shows. Results in women were inconclusive, suggesting a more complex relationship of these factors, the researchers noted.
METHODOLOGY:
- Evidence suggests psychosocial stressors at work, from job strain related to level of demand and control in workload and decision-making responsibilities, and an effort-reward imbalance (ERI) in areas such as salary, promotion, and job stability, increase CHD risk, with the effect of both types of stressors together possibly being especially harmful.
- The study, which included 6,465 participants in the cardiovascular component of PROQ, a Canadian prospective cohort of white-collar workers initially free of cardiovascular disease, mean age 45 years, estimated that the separate and combined effect of job strain and ERI on CHD incidence.
- Researchers used the Job Content Questionnaire to assess psychological demands and job control; various measures; scales to determine job strain, reward, and effort at work; and the sum of both effort and reward to calculate the ERI ratio.
- They assessed CHD using medico-administrative databases and an algorithm validated by medical records.
TAKEAWAY:
- After a median follow-up of 18.7 years, there were 571 and 265 incident CHD cases among men and women, respectively.
- Men with either job strain or ERI had a 49% increased risk for CHD (hazard ratio [HR], 1.49; 95% confidence interval [CI], 1.07-2.09), an estimate comparable to that of several lifestyle risk factors for CHD.
- Male workers facing both job strain and ERI had a 103% increased risk for CHD (HR, 2.03; 95% CI, 1.38-2.97), which is comparable to the increased risk associated with obesity.
- Associations were robust to adjustments for demographic, socioeconomic, psychosocial, personality, stressful life events, and biomedical and lifestyle factors.
- Among women, results were inconclusive because the CIs were wide enough to encompass both protective and detrimental effects, suggesting more research is needed into the complex interplay of various stressors and women’s heart health.
IN PRACTICE:
“Integrative and interdisciplinary approaches should be used to tackle psychosocial stressors at work,” the authors wrote, adding this involves “going beyond traditional modifiable individual behaviors” and should include “population-based prevention strategies taking into consideration both the individual and their work environment.”
SOURCE:
The study was conducted by Mathilde Lavigne-Robichaud, Population Health and Optimal Health Practices Research Unit, CHU de Québec-Laval University, Quebec City, Canada. It was published online in Circulation: Cardiovascular Quality and Outcomes.
LIMITATIONS:
There was a risk for chance associations due to multiple testing. The exposure may have changed over the course of the study. Using medical databases for CHD event definition may have led to misclassification and underestimation of outcomes. The study population is limited to white-collar workers.
DISCLOSURES:
The study received funding from the Canadian Institute of Health Research. Lavigne-Robichaud was supported by a PhD grant from les Fonds de Recherche du Québec-Santé. See paper for disclosures of other authors.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION: CARDIOVASCULAR QUALITY AND OUTCOMES
New risk factors for cardiovascular disease in women emerging
Multiple emerging risk factors for cardiovascular disease in women must be recognized and assessed to provide timely diagnosis and treatment, according to Dipti N. Itchhaporia, MD, an interventional cardiologist in southern California. These risk factors include pregnancy complications, autoimmune diseases, depression, breast cancer, and breast arterial calcification.
During the session titled “Cardiac Care in Women: Emerging Risk Factors” at CardioAcademic 2023, the former president of the American College of Cardiology emphasized that gender equity in care for cardiovascular disease will be achieved only when risk factors are evaluated from a gender-dependent perspective and when assessments are broadened to include novel and unrecognized risk factors, not just traditional risk factors.
Dr. Itchhaporia also remarked that
“Cardiovascular disease remains the leading cause of death in women, at least in the United States, and globally the outlook is similar,” she explained. “That’s why we need to provide our patients with guidance and carefully investigate when they experience chest pain. We need to remember that smoking and obesity pose a higher risk for cardiovascular disease in women than in men. Taking these risk factors into account will really make a difference by allowing us to provide more timely and targeted care.”
In her presentation, Dr. Itchhaporia noted that cardiovascular disease accounts for 35% of deaths in women worldwide. She reminded her audience that, according to The Lancet Women and Cardiovascular Disease Commission, heart diseases in this population remain “understudied, underrecognized, underdiagnosed, and undertreated. Furthermore, women are underrepresented in cardiovascular [clinical practice].”
She mentioned this because, despite U.S. legislation enacted between 1980 and 1990 that mandated the inclusion of women in clinical trials, women accounted for less than 39% of participants in cardiovascular clinical trials between 2010 and 2017. According to Dr. Itchhaporia, this situation limits the potential for developing tailored strategies and recommendations to treat the cardiovascular diseases affecting women.
Emerging risk factors
Dr. Itchhaporia pointed out that traditional risk factors have been known for many years. For example, 80% of women aged 75 years or younger have arterial hypertension. Only 29% receive adequate blood pressure control, those living with diabetes have a 45% greater risk of suffering ischemic heart disease, and obesity confers a 64% higher risk of developing ischemic heart disease in women versus 46% in men.
In addition to these factors, she noted that emerging factors must be assessed carefully. For example, women who experience pregnancy complications like gestational diabetes have a higher risk for ischemic heart disease and type 2 diabetes. Women with hypertension and preeclampsia are at a threefold higher risk of developing ischemic heart disease.
“Pregnancy can really be a major stress test for the heart, and I believe that, as health care professionals, we should all be asking women if they have had pregnancy-related complications. I don’t think that’s something we’ve been doing on a regular basis. Statistically, we know that 10%-20% of pregnant women report complications during pregnancy, and strong associations have been shown between gestational hypertension [and] preeclampsia.”
Dr. Itchhaporia explained that depression, a condition that globally affects women twice as much as men, is another emerging factor (though it has received some increased recognition). She explained that, in women, depression is a significant risk factor for developing a major adverse cardiovascular event or a combined event of cardiac death and myocardial infarction related to the target lesion and revascularization of the target lesion because of ischemia. Furthermore, women who have experienced a cardiac-related event are more likely to have depression than men.
“If we look into it in more detail, depression leads to changes in behavioral habits and physiological mechanisms,” she said. “Women living with depression are at higher risk of smoking, not exercising as much, are perhaps less careful with their hygiene, are not likely to adhere to their medications, and don’t sleep as well. All this moves them in the direction of heart disease.”
Added to these factors are autoimmune diseases like rheumatoid arthritis and systemic lupus erythematosus, where the female-to-male ratio for rheumatoid arthritis is 2½:1 and for lupus it’s 9:1. Dr. Itchhaporia explained that patients with rheumatoid arthritis are at two- to threefold greater risk for myocardial infarction and have a 50% higher risk for stroke. In the case of systemic lupus, the risk of myocardial infarction is 7-50 times greater than in the general population. She noted that cardiovascular risk calculators underestimate the burden of risk in patients with these diseases.
Lastly, she brought up breast cancer and breast arterial calcification as additional emerging risk factors. She explained that women with breast cancer are more likely to develop hypertension and diabetes, compared with women without this diagnosis. Women with hypertension or diabetes before developing breast cancer have twice the risk for heart problems after cancer.
She added that 12.7% of women screened for breast cancer have some degree of breast calcification. She explained that this occurs when calcium accumulates in the middle layer of artery walls in the breast, which is linked to aging, type 2 diabetes, or arterial hypertension and may be a marker of arterial stiffening, which is a cardiovascular disease.
“It’s extremely important to take into consideration data suggesting a strong association between breast calcifications and cardiovascular disease, independent of other known risk factors of cardiovascular disease. We need to improve our tests for detecting cardiovascular disease in women and we need to ask specific questions and not overlook these emerging factors,” she noted.
Improving health outcomes
Panelist María Guadalupe Parra Machuca, MD, a cardiologist in Guadalajara, Mexico, specializing in women’s heart disease, agreed that it is high time that clinical practice reflect public health policies, so that efforts to diagnose and treat cardiovascular diseases in women more effectively can transition from theory to reality.
“As physicians, we cannot allow public policy to remain outside of the reality we face,” she stressed. “We need to let it impact the decisions we make. Everything we see day to day, the things we learn at these conferences – let’s put it into practice. Otherwise, all our discussions and all the steps taken to improve care, from primary to highly specialized care and to detect and treat cardiovascular disease in women, will be nothing but rhetoric.”
Clinical cardiology specialist Victor Leal, MD, noted that, according to preliminary results from the national survey of cardiovascular risk factors in Mexican women, Mexico is no exception to these emerging risk factors for cardiovascular disease in women. More than 50% of women in Mexico have traditional risk factors, most notably hypertension, obesity, and diabetes, while hypertensive disorders of pregnancy top the list of other sex-specific risk factors.
“Not only are these factors increasing, but also having them increases the risk of a worse prognosis, leaving us with a very challenging scenario,” said Dr. Leal. “Not only do we need to educate patients about the traditional risk factors, but also about factors that might not be on our radar. We need to get women to link these factors to cardiovascular disease and to the possibility of developing much more adverse outcomes. This will reinforce our diagnosis and treatment.”
In an interview, Dr. Itchhaporia emphasized the changing face of cardiovascular disease for women, who have worse short- and long-term outcomes than men because they are not asked sex-specific questions during initial encounters and they experience greater prehospital delays.
She noted that, while experts need to raise awareness of the emerging risk factors among health care professionals, they also need to use information campaigns to make women aware of what the risks are. Then, if they experience any of the emerging risk factors, they can discuss it with their treating physicians.
“We need to assess both the traditional risk factors and the novel ones, those that are underrecognized. We need to include the history of pregnancy and complications during this period and we need to educate women about symptoms of heart disease like chest pain, difficulty breathing, and increasing fatigue,” she emphasized. “We must also provide guidance as to lifestyle, diet, and levels of physical activity and be aware of stress and symptoms of depression. Only then will we bring greater awareness to the fact that cardiovascular disease is the leading cause of death among women, and then we can reverse these trends.”
Dr. Itchhaporia, Dr. Parra, and Dr. Leal reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Multiple emerging risk factors for cardiovascular disease in women must be recognized and assessed to provide timely diagnosis and treatment, according to Dipti N. Itchhaporia, MD, an interventional cardiologist in southern California. These risk factors include pregnancy complications, autoimmune diseases, depression, breast cancer, and breast arterial calcification.
During the session titled “Cardiac Care in Women: Emerging Risk Factors” at CardioAcademic 2023, the former president of the American College of Cardiology emphasized that gender equity in care for cardiovascular disease will be achieved only when risk factors are evaluated from a gender-dependent perspective and when assessments are broadened to include novel and unrecognized risk factors, not just traditional risk factors.
Dr. Itchhaporia also remarked that
“Cardiovascular disease remains the leading cause of death in women, at least in the United States, and globally the outlook is similar,” she explained. “That’s why we need to provide our patients with guidance and carefully investigate when they experience chest pain. We need to remember that smoking and obesity pose a higher risk for cardiovascular disease in women than in men. Taking these risk factors into account will really make a difference by allowing us to provide more timely and targeted care.”
In her presentation, Dr. Itchhaporia noted that cardiovascular disease accounts for 35% of deaths in women worldwide. She reminded her audience that, according to The Lancet Women and Cardiovascular Disease Commission, heart diseases in this population remain “understudied, underrecognized, underdiagnosed, and undertreated. Furthermore, women are underrepresented in cardiovascular [clinical practice].”
She mentioned this because, despite U.S. legislation enacted between 1980 and 1990 that mandated the inclusion of women in clinical trials, women accounted for less than 39% of participants in cardiovascular clinical trials between 2010 and 2017. According to Dr. Itchhaporia, this situation limits the potential for developing tailored strategies and recommendations to treat the cardiovascular diseases affecting women.
Emerging risk factors
Dr. Itchhaporia pointed out that traditional risk factors have been known for many years. For example, 80% of women aged 75 years or younger have arterial hypertension. Only 29% receive adequate blood pressure control, those living with diabetes have a 45% greater risk of suffering ischemic heart disease, and obesity confers a 64% higher risk of developing ischemic heart disease in women versus 46% in men.
In addition to these factors, she noted that emerging factors must be assessed carefully. For example, women who experience pregnancy complications like gestational diabetes have a higher risk for ischemic heart disease and type 2 diabetes. Women with hypertension and preeclampsia are at a threefold higher risk of developing ischemic heart disease.
“Pregnancy can really be a major stress test for the heart, and I believe that, as health care professionals, we should all be asking women if they have had pregnancy-related complications. I don’t think that’s something we’ve been doing on a regular basis. Statistically, we know that 10%-20% of pregnant women report complications during pregnancy, and strong associations have been shown between gestational hypertension [and] preeclampsia.”
Dr. Itchhaporia explained that depression, a condition that globally affects women twice as much as men, is another emerging factor (though it has received some increased recognition). She explained that, in women, depression is a significant risk factor for developing a major adverse cardiovascular event or a combined event of cardiac death and myocardial infarction related to the target lesion and revascularization of the target lesion because of ischemia. Furthermore, women who have experienced a cardiac-related event are more likely to have depression than men.
“If we look into it in more detail, depression leads to changes in behavioral habits and physiological mechanisms,” she said. “Women living with depression are at higher risk of smoking, not exercising as much, are perhaps less careful with their hygiene, are not likely to adhere to their medications, and don’t sleep as well. All this moves them in the direction of heart disease.”
Added to these factors are autoimmune diseases like rheumatoid arthritis and systemic lupus erythematosus, where the female-to-male ratio for rheumatoid arthritis is 2½:1 and for lupus it’s 9:1. Dr. Itchhaporia explained that patients with rheumatoid arthritis are at two- to threefold greater risk for myocardial infarction and have a 50% higher risk for stroke. In the case of systemic lupus, the risk of myocardial infarction is 7-50 times greater than in the general population. She noted that cardiovascular risk calculators underestimate the burden of risk in patients with these diseases.
Lastly, she brought up breast cancer and breast arterial calcification as additional emerging risk factors. She explained that women with breast cancer are more likely to develop hypertension and diabetes, compared with women without this diagnosis. Women with hypertension or diabetes before developing breast cancer have twice the risk for heart problems after cancer.
She added that 12.7% of women screened for breast cancer have some degree of breast calcification. She explained that this occurs when calcium accumulates in the middle layer of artery walls in the breast, which is linked to aging, type 2 diabetes, or arterial hypertension and may be a marker of arterial stiffening, which is a cardiovascular disease.
“It’s extremely important to take into consideration data suggesting a strong association between breast calcifications and cardiovascular disease, independent of other known risk factors of cardiovascular disease. We need to improve our tests for detecting cardiovascular disease in women and we need to ask specific questions and not overlook these emerging factors,” she noted.
Improving health outcomes
Panelist María Guadalupe Parra Machuca, MD, a cardiologist in Guadalajara, Mexico, specializing in women’s heart disease, agreed that it is high time that clinical practice reflect public health policies, so that efforts to diagnose and treat cardiovascular diseases in women more effectively can transition from theory to reality.
“As physicians, we cannot allow public policy to remain outside of the reality we face,” she stressed. “We need to let it impact the decisions we make. Everything we see day to day, the things we learn at these conferences – let’s put it into practice. Otherwise, all our discussions and all the steps taken to improve care, from primary to highly specialized care and to detect and treat cardiovascular disease in women, will be nothing but rhetoric.”
Clinical cardiology specialist Victor Leal, MD, noted that, according to preliminary results from the national survey of cardiovascular risk factors in Mexican women, Mexico is no exception to these emerging risk factors for cardiovascular disease in women. More than 50% of women in Mexico have traditional risk factors, most notably hypertension, obesity, and diabetes, while hypertensive disorders of pregnancy top the list of other sex-specific risk factors.
“Not only are these factors increasing, but also having them increases the risk of a worse prognosis, leaving us with a very challenging scenario,” said Dr. Leal. “Not only do we need to educate patients about the traditional risk factors, but also about factors that might not be on our radar. We need to get women to link these factors to cardiovascular disease and to the possibility of developing much more adverse outcomes. This will reinforce our diagnosis and treatment.”
In an interview, Dr. Itchhaporia emphasized the changing face of cardiovascular disease for women, who have worse short- and long-term outcomes than men because they are not asked sex-specific questions during initial encounters and they experience greater prehospital delays.
She noted that, while experts need to raise awareness of the emerging risk factors among health care professionals, they also need to use information campaigns to make women aware of what the risks are. Then, if they experience any of the emerging risk factors, they can discuss it with their treating physicians.
“We need to assess both the traditional risk factors and the novel ones, those that are underrecognized. We need to include the history of pregnancy and complications during this period and we need to educate women about symptoms of heart disease like chest pain, difficulty breathing, and increasing fatigue,” she emphasized. “We must also provide guidance as to lifestyle, diet, and levels of physical activity and be aware of stress and symptoms of depression. Only then will we bring greater awareness to the fact that cardiovascular disease is the leading cause of death among women, and then we can reverse these trends.”
Dr. Itchhaporia, Dr. Parra, and Dr. Leal reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Multiple emerging risk factors for cardiovascular disease in women must be recognized and assessed to provide timely diagnosis and treatment, according to Dipti N. Itchhaporia, MD, an interventional cardiologist in southern California. These risk factors include pregnancy complications, autoimmune diseases, depression, breast cancer, and breast arterial calcification.
During the session titled “Cardiac Care in Women: Emerging Risk Factors” at CardioAcademic 2023, the former president of the American College of Cardiology emphasized that gender equity in care for cardiovascular disease will be achieved only when risk factors are evaluated from a gender-dependent perspective and when assessments are broadened to include novel and unrecognized risk factors, not just traditional risk factors.
Dr. Itchhaporia also remarked that
“Cardiovascular disease remains the leading cause of death in women, at least in the United States, and globally the outlook is similar,” she explained. “That’s why we need to provide our patients with guidance and carefully investigate when they experience chest pain. We need to remember that smoking and obesity pose a higher risk for cardiovascular disease in women than in men. Taking these risk factors into account will really make a difference by allowing us to provide more timely and targeted care.”
In her presentation, Dr. Itchhaporia noted that cardiovascular disease accounts for 35% of deaths in women worldwide. She reminded her audience that, according to The Lancet Women and Cardiovascular Disease Commission, heart diseases in this population remain “understudied, underrecognized, underdiagnosed, and undertreated. Furthermore, women are underrepresented in cardiovascular [clinical practice].”
She mentioned this because, despite U.S. legislation enacted between 1980 and 1990 that mandated the inclusion of women in clinical trials, women accounted for less than 39% of participants in cardiovascular clinical trials between 2010 and 2017. According to Dr. Itchhaporia, this situation limits the potential for developing tailored strategies and recommendations to treat the cardiovascular diseases affecting women.
Emerging risk factors
Dr. Itchhaporia pointed out that traditional risk factors have been known for many years. For example, 80% of women aged 75 years or younger have arterial hypertension. Only 29% receive adequate blood pressure control, those living with diabetes have a 45% greater risk of suffering ischemic heart disease, and obesity confers a 64% higher risk of developing ischemic heart disease in women versus 46% in men.
In addition to these factors, she noted that emerging factors must be assessed carefully. For example, women who experience pregnancy complications like gestational diabetes have a higher risk for ischemic heart disease and type 2 diabetes. Women with hypertension and preeclampsia are at a threefold higher risk of developing ischemic heart disease.
“Pregnancy can really be a major stress test for the heart, and I believe that, as health care professionals, we should all be asking women if they have had pregnancy-related complications. I don’t think that’s something we’ve been doing on a regular basis. Statistically, we know that 10%-20% of pregnant women report complications during pregnancy, and strong associations have been shown between gestational hypertension [and] preeclampsia.”
Dr. Itchhaporia explained that depression, a condition that globally affects women twice as much as men, is another emerging factor (though it has received some increased recognition). She explained that, in women, depression is a significant risk factor for developing a major adverse cardiovascular event or a combined event of cardiac death and myocardial infarction related to the target lesion and revascularization of the target lesion because of ischemia. Furthermore, women who have experienced a cardiac-related event are more likely to have depression than men.
“If we look into it in more detail, depression leads to changes in behavioral habits and physiological mechanisms,” she said. “Women living with depression are at higher risk of smoking, not exercising as much, are perhaps less careful with their hygiene, are not likely to adhere to their medications, and don’t sleep as well. All this moves them in the direction of heart disease.”
Added to these factors are autoimmune diseases like rheumatoid arthritis and systemic lupus erythematosus, where the female-to-male ratio for rheumatoid arthritis is 2½:1 and for lupus it’s 9:1. Dr. Itchhaporia explained that patients with rheumatoid arthritis are at two- to threefold greater risk for myocardial infarction and have a 50% higher risk for stroke. In the case of systemic lupus, the risk of myocardial infarction is 7-50 times greater than in the general population. She noted that cardiovascular risk calculators underestimate the burden of risk in patients with these diseases.
Lastly, she brought up breast cancer and breast arterial calcification as additional emerging risk factors. She explained that women with breast cancer are more likely to develop hypertension and diabetes, compared with women without this diagnosis. Women with hypertension or diabetes before developing breast cancer have twice the risk for heart problems after cancer.
She added that 12.7% of women screened for breast cancer have some degree of breast calcification. She explained that this occurs when calcium accumulates in the middle layer of artery walls in the breast, which is linked to aging, type 2 diabetes, or arterial hypertension and may be a marker of arterial stiffening, which is a cardiovascular disease.
“It’s extremely important to take into consideration data suggesting a strong association between breast calcifications and cardiovascular disease, independent of other known risk factors of cardiovascular disease. We need to improve our tests for detecting cardiovascular disease in women and we need to ask specific questions and not overlook these emerging factors,” she noted.
Improving health outcomes
Panelist María Guadalupe Parra Machuca, MD, a cardiologist in Guadalajara, Mexico, specializing in women’s heart disease, agreed that it is high time that clinical practice reflect public health policies, so that efforts to diagnose and treat cardiovascular diseases in women more effectively can transition from theory to reality.
“As physicians, we cannot allow public policy to remain outside of the reality we face,” she stressed. “We need to let it impact the decisions we make. Everything we see day to day, the things we learn at these conferences – let’s put it into practice. Otherwise, all our discussions and all the steps taken to improve care, from primary to highly specialized care and to detect and treat cardiovascular disease in women, will be nothing but rhetoric.”
Clinical cardiology specialist Victor Leal, MD, noted that, according to preliminary results from the national survey of cardiovascular risk factors in Mexican women, Mexico is no exception to these emerging risk factors for cardiovascular disease in women. More than 50% of women in Mexico have traditional risk factors, most notably hypertension, obesity, and diabetes, while hypertensive disorders of pregnancy top the list of other sex-specific risk factors.
“Not only are these factors increasing, but also having them increases the risk of a worse prognosis, leaving us with a very challenging scenario,” said Dr. Leal. “Not only do we need to educate patients about the traditional risk factors, but also about factors that might not be on our radar. We need to get women to link these factors to cardiovascular disease and to the possibility of developing much more adverse outcomes. This will reinforce our diagnosis and treatment.”
In an interview, Dr. Itchhaporia emphasized the changing face of cardiovascular disease for women, who have worse short- and long-term outcomes than men because they are not asked sex-specific questions during initial encounters and they experience greater prehospital delays.
She noted that, while experts need to raise awareness of the emerging risk factors among health care professionals, they also need to use information campaigns to make women aware of what the risks are. Then, if they experience any of the emerging risk factors, they can discuss it with their treating physicians.
“We need to assess both the traditional risk factors and the novel ones, those that are underrecognized. We need to include the history of pregnancy and complications during this period and we need to educate women about symptoms of heart disease like chest pain, difficulty breathing, and increasing fatigue,” she emphasized. “We must also provide guidance as to lifestyle, diet, and levels of physical activity and be aware of stress and symptoms of depression. Only then will we bring greater awareness to the fact that cardiovascular disease is the leading cause of death among women, and then we can reverse these trends.”
Dr. Itchhaporia, Dr. Parra, and Dr. Leal reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CARDIOACADEMIC 2023
Islet, kidney transplants boost survival in type 1 diabetes
TOPLINE:
.
METHODOLOGY:
- Study population was all patients with type 1 diabetes in France who received a kidney transplant between 2000 and 2017.
- Among 2,393 patients, 327 were eligible for islet transplantation, including 47 who were actually transplanted with islets.
- The subjects were matched for factors including year of transplantation, recipient age, kidney function, and hemoglobin A1c.
TAKEAWAY:
- Those receiving islets along with the kidney transplant had a 53% lower risk of graft failure, compared with the kidney-alone group.
- Those receiving islet transplantation had a significantly higher estimated life expectancy during 10-year follow-up (9.61 vs. 8.85 years).
- At 1 year post islet transplant, there was an estimated 89.4% probability of graft survival and a 70.2% probability of achieving independence from insulin.
IN PRACTICE:
“Although islet transplantation has previously been shown to improve glycemic control, compared with conventional insulin therapy in recent clinical trials, little was known about its long-term impact on patient prognosis until now. ... These results are exciting and provide hope for people living with type 1 diabetes and kidney transplants.”
SOURCE:
Presented Sept. 17, 2023, at the European Society for Organ Transplantation (ESOT) Congress 2023 by Mehdi Maanaoui, MD, a nephrologist at the University of Lille (France).
LIMITATIONS:
Observational, potential for residual confounding.
DISCLOSURES:
Dr. Maanaoui reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- Study population was all patients with type 1 diabetes in France who received a kidney transplant between 2000 and 2017.
- Among 2,393 patients, 327 were eligible for islet transplantation, including 47 who were actually transplanted with islets.
- The subjects were matched for factors including year of transplantation, recipient age, kidney function, and hemoglobin A1c.
TAKEAWAY:
- Those receiving islets along with the kidney transplant had a 53% lower risk of graft failure, compared with the kidney-alone group.
- Those receiving islet transplantation had a significantly higher estimated life expectancy during 10-year follow-up (9.61 vs. 8.85 years).
- At 1 year post islet transplant, there was an estimated 89.4% probability of graft survival and a 70.2% probability of achieving independence from insulin.
IN PRACTICE:
“Although islet transplantation has previously been shown to improve glycemic control, compared with conventional insulin therapy in recent clinical trials, little was known about its long-term impact on patient prognosis until now. ... These results are exciting and provide hope for people living with type 1 diabetes and kidney transplants.”
SOURCE:
Presented Sept. 17, 2023, at the European Society for Organ Transplantation (ESOT) Congress 2023 by Mehdi Maanaoui, MD, a nephrologist at the University of Lille (France).
LIMITATIONS:
Observational, potential for residual confounding.
DISCLOSURES:
Dr. Maanaoui reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- Study population was all patients with type 1 diabetes in France who received a kidney transplant between 2000 and 2017.
- Among 2,393 patients, 327 were eligible for islet transplantation, including 47 who were actually transplanted with islets.
- The subjects were matched for factors including year of transplantation, recipient age, kidney function, and hemoglobin A1c.
TAKEAWAY:
- Those receiving islets along with the kidney transplant had a 53% lower risk of graft failure, compared with the kidney-alone group.
- Those receiving islet transplantation had a significantly higher estimated life expectancy during 10-year follow-up (9.61 vs. 8.85 years).
- At 1 year post islet transplant, there was an estimated 89.4% probability of graft survival and a 70.2% probability of achieving independence from insulin.
IN PRACTICE:
“Although islet transplantation has previously been shown to improve glycemic control, compared with conventional insulin therapy in recent clinical trials, little was known about its long-term impact on patient prognosis until now. ... These results are exciting and provide hope for people living with type 1 diabetes and kidney transplants.”
SOURCE:
Presented Sept. 17, 2023, at the European Society for Organ Transplantation (ESOT) Congress 2023 by Mehdi Maanaoui, MD, a nephrologist at the University of Lille (France).
LIMITATIONS:
Observational, potential for residual confounding.
DISCLOSURES:
Dr. Maanaoui reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Diffuse Pruritic Eruption in an Immunocompromised Patient
The Diagnosis: Scabies Infestation
Direct microscopy revealed the presence of a live scabies mite and numerous eggs (Figure), confirming the diagnosis of a scabies infestation. Scabies, caused by the Sarcoptes scabiei var hominis mite, characteristically presents in adults as pruritic hyperkeratotic plaques of the interdigital web spaces of the hands, flexor surfaces of the wrists and elbows, axillae, male genitalia, and breasts; however, an atypical presentation is common in immunocompromised or immunosuppressed individuals, such as our patient. In children, the palms, soles, and head (ie, face, scalp, neck) are common sites of involvement. Although dermatologists generally are familiar with severe atypical presentations such as Norwegian crusted scabies or bullous scabies, it is important that they are aware of other atypical presentations, such as the diffuse papulonodular variant observed in our patient.1 As such, a low threshold of suspicion for scabies infestations should be employed in immunocompromised patients with new-onset pruritic eruptions.
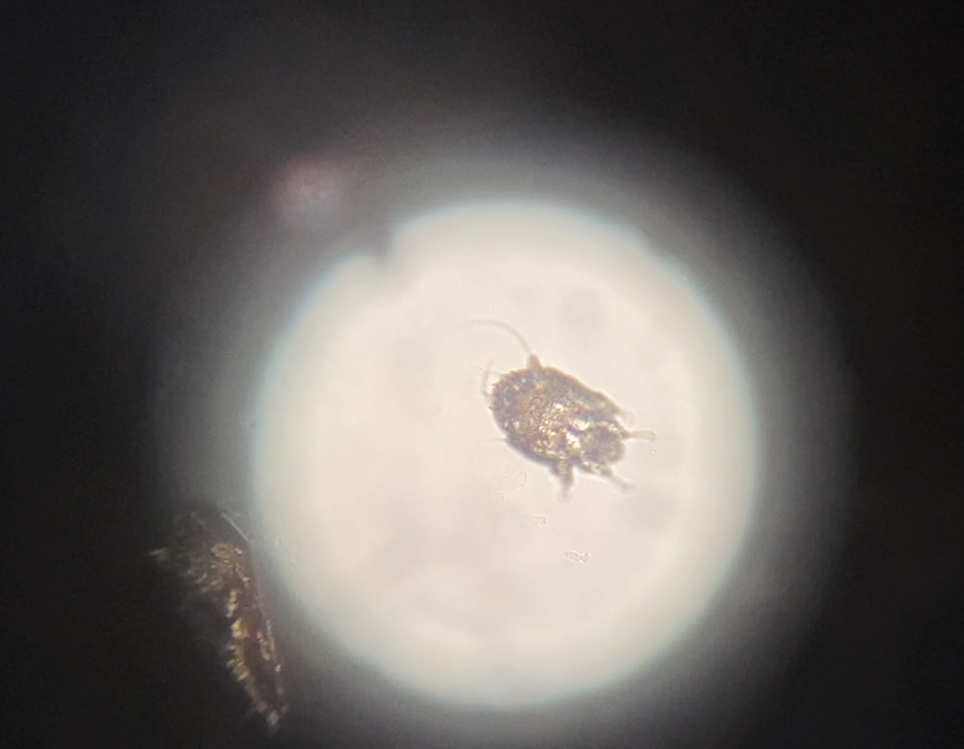
Direct microscopy is widely accepted as the gold standard for the diagnosis of scabies infestations; it is a fast and low-cost diagnostic tool. However, this technique displays variable sensitivity in clinical practice, requiring experience and a skilled hand.1,2 Other more sensitive diagnostic options for suspected scabies infestations include histopathology, serology, and molecular-based techniques such as DNA isolation and polymerase chain reaction. Although these tests do demonstrate greater sensitivity, they also are more invasive, time intensive, and costly.2 Therefore, they typically are not the first choice for a suspected scabies infestation. Dermoscopy has emerged as another tool to aid in the diagnosis of a suspected scabies infestation, enabling visualization of scaly burrows, eggs, and live mites. Classically, findings resembling a delta wing with contrail are seen on dermoscopic examination. The delta wing represents the brown triangular structure of the pigmented scabies mite head and anterior legs; the contrail is the lighter linear structures streaming behind the scabies mite (similar to visible vapor streams occurring behind flying jets), representing the burrow of the mite.
Although treatment of scabies infestations typically can be accomplished with permethrin cream 5%, the diffuse nature of our patient’s lesions in combination with his immunocompromised state made oral therapy a more appropriate choice. Based on Centers for Disease Control and Prevention recommendations, the patient received 2 doses of oral weight-based ivermectin (200 μg/kg per dose) administered 1 week apart.1,3 The initial dose at day 1 serves to eliminate any scabies mites that are present, while the second dose 1 week later eliminates any residual eggs. Our patient experienced complete resolution of the symptoms following this treatment regimen.
It was important to differentiate our patient’s scabies infestation from other intensely pruritic conditions and morphologic mimics including papular urticaria, lichenoid drug eruptions, tinea corporis, and prurigo nodularis. Papular urticaria is an intensely pruritic hypersensitivity reaction to insect bites that commonly affects the extremities or other exposed areas. Visible puncta may be present.4 Our patient’s lesion distribution involved areas covered by clothing, no puncta were present, and he had no history of a recent arthropod assault, making the diagnosis of papular urticaria less likely.
Lichenoid drug eruptions classically present with symmetric, diffuse, pruritic, violaceous, scaling papules and plaques that present 2 to 3 months after exposure to an offending agent.5 Our patient’s eruption was papulonodular with no violaceous plaques, and he did not report changes to his medications, making a lichenoid drug eruption less likely.
Tinea corporis is another intensely pruritic condition that should be considered, especially in immunocompromised patients. It is caused by dermatophytes and classically presents as erythematous pruritic plaques with an annular, advancing, scaling border.6 Although immunocompromised patients may display extensive involvement, our patient’s lesions were papulonodular with no annular morphology or scale, rendering tinea corporis less likely.
Prurigo nodularis is a chronic condition characterized by pruritic, violaceous, dome-shaped, smooth or crusted nodules secondary to repeated scratching or pressure. Although prurigo nodules can develop as a secondary change due to chronic excoriations in scabies infestations, prurigo nodules usually do not develop in areas such as the midline of the back that are not easily reached by the fingernails,7 which made prurigo nodularis less likely in our patient.
This case describes a unique papulonodular variant of scabies presenting in an immunocompromised cancer patient. Timely recognition and diagnosis of atypical scabies infestations can decrease morbidity and improve the quality of life of these patients.
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Siddig EE, Hay R. Laboratory-based diagnosis of scabies: a review of the current status. Trans R Soc Trop Med Hyg. 2022;116:4-9. doi:10.1093/trstmh/trab049
- Centers for Disease Control and Prevention. Parasites—scabies. medications. Accessed September 19, 2023. https://www.cdc.gov/parasites/ scabies/health_professionals/meds.html
- Örnek S, Zuberbier T, Kocatürk E. Annular urticarial lesions. Clin Dermatol. 2022;40:480-504. doi:10.1016/j.clindermatol .2021.12.010
- Cheraghlou S, Levy LL. Fixed drug eruptions, bullous drug eruptions, and lichenoid drug eruptions. Clin Dermatol. 2020;38:679-692. doi:10.1016/j.clindermatol.2020.06.010
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review. Drugs Context. 2020;9:2020-5-6. doi:10.7573/dic.2020-5-6
- Kwon CD, Khanna R, Williams KA, et al. Diagnostic workup and evaluation of patients with prurigo nodularis. Medicines (Basel). 2019;6:97. doi:10.3390/medicines6040097
The Diagnosis: Scabies Infestation
Direct microscopy revealed the presence of a live scabies mite and numerous eggs (Figure), confirming the diagnosis of a scabies infestation. Scabies, caused by the Sarcoptes scabiei var hominis mite, characteristically presents in adults as pruritic hyperkeratotic plaques of the interdigital web spaces of the hands, flexor surfaces of the wrists and elbows, axillae, male genitalia, and breasts; however, an atypical presentation is common in immunocompromised or immunosuppressed individuals, such as our patient. In children, the palms, soles, and head (ie, face, scalp, neck) are common sites of involvement. Although dermatologists generally are familiar with severe atypical presentations such as Norwegian crusted scabies or bullous scabies, it is important that they are aware of other atypical presentations, such as the diffuse papulonodular variant observed in our patient.1 As such, a low threshold of suspicion for scabies infestations should be employed in immunocompromised patients with new-onset pruritic eruptions.

Direct microscopy is widely accepted as the gold standard for the diagnosis of scabies infestations; it is a fast and low-cost diagnostic tool. However, this technique displays variable sensitivity in clinical practice, requiring experience and a skilled hand.1,2 Other more sensitive diagnostic options for suspected scabies infestations include histopathology, serology, and molecular-based techniques such as DNA isolation and polymerase chain reaction. Although these tests do demonstrate greater sensitivity, they also are more invasive, time intensive, and costly.2 Therefore, they typically are not the first choice for a suspected scabies infestation. Dermoscopy has emerged as another tool to aid in the diagnosis of a suspected scabies infestation, enabling visualization of scaly burrows, eggs, and live mites. Classically, findings resembling a delta wing with contrail are seen on dermoscopic examination. The delta wing represents the brown triangular structure of the pigmented scabies mite head and anterior legs; the contrail is the lighter linear structures streaming behind the scabies mite (similar to visible vapor streams occurring behind flying jets), representing the burrow of the mite.
Although treatment of scabies infestations typically can be accomplished with permethrin cream 5%, the diffuse nature of our patient’s lesions in combination with his immunocompromised state made oral therapy a more appropriate choice. Based on Centers for Disease Control and Prevention recommendations, the patient received 2 doses of oral weight-based ivermectin (200 μg/kg per dose) administered 1 week apart.1,3 The initial dose at day 1 serves to eliminate any scabies mites that are present, while the second dose 1 week later eliminates any residual eggs. Our patient experienced complete resolution of the symptoms following this treatment regimen.
It was important to differentiate our patient’s scabies infestation from other intensely pruritic conditions and morphologic mimics including papular urticaria, lichenoid drug eruptions, tinea corporis, and prurigo nodularis. Papular urticaria is an intensely pruritic hypersensitivity reaction to insect bites that commonly affects the extremities or other exposed areas. Visible puncta may be present.4 Our patient’s lesion distribution involved areas covered by clothing, no puncta were present, and he had no history of a recent arthropod assault, making the diagnosis of papular urticaria less likely.
Lichenoid drug eruptions classically present with symmetric, diffuse, pruritic, violaceous, scaling papules and plaques that present 2 to 3 months after exposure to an offending agent.5 Our patient’s eruption was papulonodular with no violaceous plaques, and he did not report changes to his medications, making a lichenoid drug eruption less likely.
Tinea corporis is another intensely pruritic condition that should be considered, especially in immunocompromised patients. It is caused by dermatophytes and classically presents as erythematous pruritic plaques with an annular, advancing, scaling border.6 Although immunocompromised patients may display extensive involvement, our patient’s lesions were papulonodular with no annular morphology or scale, rendering tinea corporis less likely.
Prurigo nodularis is a chronic condition characterized by pruritic, violaceous, dome-shaped, smooth or crusted nodules secondary to repeated scratching or pressure. Although prurigo nodules can develop as a secondary change due to chronic excoriations in scabies infestations, prurigo nodules usually do not develop in areas such as the midline of the back that are not easily reached by the fingernails,7 which made prurigo nodularis less likely in our patient.
This case describes a unique papulonodular variant of scabies presenting in an immunocompromised cancer patient. Timely recognition and diagnosis of atypical scabies infestations can decrease morbidity and improve the quality of life of these patients.
The Diagnosis: Scabies Infestation
Direct microscopy revealed the presence of a live scabies mite and numerous eggs (Figure), confirming the diagnosis of a scabies infestation. Scabies, caused by the Sarcoptes scabiei var hominis mite, characteristically presents in adults as pruritic hyperkeratotic plaques of the interdigital web spaces of the hands, flexor surfaces of the wrists and elbows, axillae, male genitalia, and breasts; however, an atypical presentation is common in immunocompromised or immunosuppressed individuals, such as our patient. In children, the palms, soles, and head (ie, face, scalp, neck) are common sites of involvement. Although dermatologists generally are familiar with severe atypical presentations such as Norwegian crusted scabies or bullous scabies, it is important that they are aware of other atypical presentations, such as the diffuse papulonodular variant observed in our patient.1 As such, a low threshold of suspicion for scabies infestations should be employed in immunocompromised patients with new-onset pruritic eruptions.

Direct microscopy is widely accepted as the gold standard for the diagnosis of scabies infestations; it is a fast and low-cost diagnostic tool. However, this technique displays variable sensitivity in clinical practice, requiring experience and a skilled hand.1,2 Other more sensitive diagnostic options for suspected scabies infestations include histopathology, serology, and molecular-based techniques such as DNA isolation and polymerase chain reaction. Although these tests do demonstrate greater sensitivity, they also are more invasive, time intensive, and costly.2 Therefore, they typically are not the first choice for a suspected scabies infestation. Dermoscopy has emerged as another tool to aid in the diagnosis of a suspected scabies infestation, enabling visualization of scaly burrows, eggs, and live mites. Classically, findings resembling a delta wing with contrail are seen on dermoscopic examination. The delta wing represents the brown triangular structure of the pigmented scabies mite head and anterior legs; the contrail is the lighter linear structures streaming behind the scabies mite (similar to visible vapor streams occurring behind flying jets), representing the burrow of the mite.
Although treatment of scabies infestations typically can be accomplished with permethrin cream 5%, the diffuse nature of our patient’s lesions in combination with his immunocompromised state made oral therapy a more appropriate choice. Based on Centers for Disease Control and Prevention recommendations, the patient received 2 doses of oral weight-based ivermectin (200 μg/kg per dose) administered 1 week apart.1,3 The initial dose at day 1 serves to eliminate any scabies mites that are present, while the second dose 1 week later eliminates any residual eggs. Our patient experienced complete resolution of the symptoms following this treatment regimen.
It was important to differentiate our patient’s scabies infestation from other intensely pruritic conditions and morphologic mimics including papular urticaria, lichenoid drug eruptions, tinea corporis, and prurigo nodularis. Papular urticaria is an intensely pruritic hypersensitivity reaction to insect bites that commonly affects the extremities or other exposed areas. Visible puncta may be present.4 Our patient’s lesion distribution involved areas covered by clothing, no puncta were present, and he had no history of a recent arthropod assault, making the diagnosis of papular urticaria less likely.
Lichenoid drug eruptions classically present with symmetric, diffuse, pruritic, violaceous, scaling papules and plaques that present 2 to 3 months after exposure to an offending agent.5 Our patient’s eruption was papulonodular with no violaceous plaques, and he did not report changes to his medications, making a lichenoid drug eruption less likely.
Tinea corporis is another intensely pruritic condition that should be considered, especially in immunocompromised patients. It is caused by dermatophytes and classically presents as erythematous pruritic plaques with an annular, advancing, scaling border.6 Although immunocompromised patients may display extensive involvement, our patient’s lesions were papulonodular with no annular morphology or scale, rendering tinea corporis less likely.
Prurigo nodularis is a chronic condition characterized by pruritic, violaceous, dome-shaped, smooth or crusted nodules secondary to repeated scratching or pressure. Although prurigo nodules can develop as a secondary change due to chronic excoriations in scabies infestations, prurigo nodules usually do not develop in areas such as the midline of the back that are not easily reached by the fingernails,7 which made prurigo nodularis less likely in our patient.
This case describes a unique papulonodular variant of scabies presenting in an immunocompromised cancer patient. Timely recognition and diagnosis of atypical scabies infestations can decrease morbidity and improve the quality of life of these patients.
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Siddig EE, Hay R. Laboratory-based diagnosis of scabies: a review of the current status. Trans R Soc Trop Med Hyg. 2022;116:4-9. doi:10.1093/trstmh/trab049
- Centers for Disease Control and Prevention. Parasites—scabies. medications. Accessed September 19, 2023. https://www.cdc.gov/parasites/ scabies/health_professionals/meds.html
- Örnek S, Zuberbier T, Kocatürk E. Annular urticarial lesions. Clin Dermatol. 2022;40:480-504. doi:10.1016/j.clindermatol .2021.12.010
- Cheraghlou S, Levy LL. Fixed drug eruptions, bullous drug eruptions, and lichenoid drug eruptions. Clin Dermatol. 2020;38:679-692. doi:10.1016/j.clindermatol.2020.06.010
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review. Drugs Context. 2020;9:2020-5-6. doi:10.7573/dic.2020-5-6
- Kwon CD, Khanna R, Williams KA, et al. Diagnostic workup and evaluation of patients with prurigo nodularis. Medicines (Basel). 2019;6:97. doi:10.3390/medicines6040097
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Siddig EE, Hay R. Laboratory-based diagnosis of scabies: a review of the current status. Trans R Soc Trop Med Hyg. 2022;116:4-9. doi:10.1093/trstmh/trab049
- Centers for Disease Control and Prevention. Parasites—scabies. medications. Accessed September 19, 2023. https://www.cdc.gov/parasites/ scabies/health_professionals/meds.html
- Örnek S, Zuberbier T, Kocatürk E. Annular urticarial lesions. Clin Dermatol. 2022;40:480-504. doi:10.1016/j.clindermatol .2021.12.010
- Cheraghlou S, Levy LL. Fixed drug eruptions, bullous drug eruptions, and lichenoid drug eruptions. Clin Dermatol. 2020;38:679-692. doi:10.1016/j.clindermatol.2020.06.010
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review. Drugs Context. 2020;9:2020-5-6. doi:10.7573/dic.2020-5-6
- Kwon CD, Khanna R, Williams KA, et al. Diagnostic workup and evaluation of patients with prurigo nodularis. Medicines (Basel). 2019;6:97. doi:10.3390/medicines6040097
A 54-year-old man presented to our dermatology clinic for evaluation of a widespread intensely pruritic rash of 4 weeks’ duration. Calamine lotion and oral hydroxyzine provided minimal relief. He was being treated for a myeloproliferative disorder with immunosuppressive therapy consisting of a combination of cladribine, low-dose cytarabine, and fedratinib. Physical examination revealed multiple excoriated papules and indurated nodules on the extensor and flexor surfaces of the arms and legs (top), chest, midline of the back (bottom), and groin. No lesions were noted on the volar aspect of the patient’s wrists or interdigital spaces, and no central puncta or scales were present. He denied any preceding arthropod bites, trauma, new environmental exposures, or changes to his medications. Scrapings from several representative lesions were obtained for mineral oil preparation and microscopic evaluation.
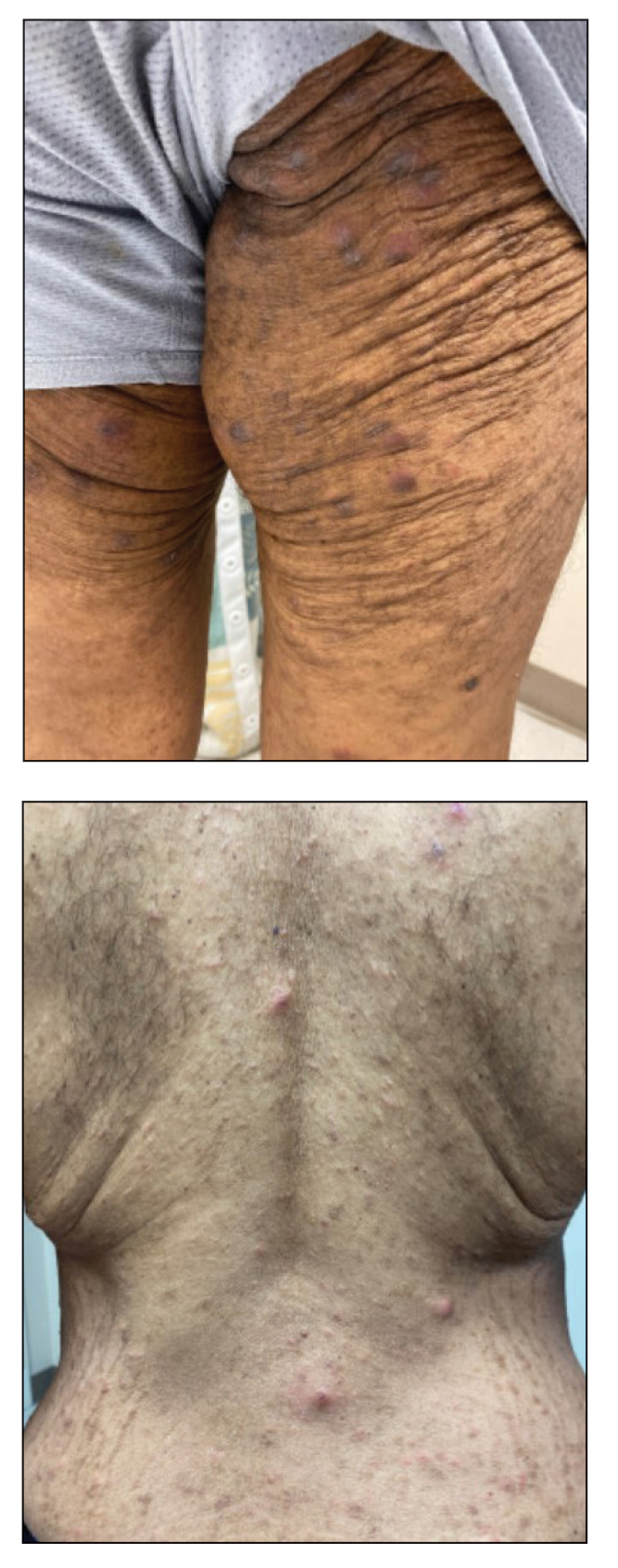
Surgery approach may improve survival in advanced ovarian cancer
TOPLINE:
Neoadjuvant chemotherapy followed by interval cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC) significantly improves progression-free survival (PFS) and overall survival, compared with interval cytoreductive surgery alone, in patients with advanced ovarian cancer, new research shows.
METHODOLOGY:
- Several randomized controlled trials have shown survival benefits with HIPEC followed by interval cytoreductive surgery in advanced ovarian cancer. Despite the data, the use of HIPEC in clinical practice remains limited.
- Potential downsides of HIPEC include longer operative time and treatment-related complications.
- This prospective, multicenter, comparative effectiveness study evaluated the safety and effectiveness of interval cytoreductive surgery with HIPEC versus the surgery alone.
- The study, conducted at seven Korean Gynecologic Oncology Group institutions, included 196 patients (mean age, 58 years) with stage III or IV ovarian cancer who had received at least three cycles of neoadjuvant chemotherapy followed by interval cytoreductive surgery with HIPEC (n = 109) or without HIPEC (n = 87).
- The researchers reported progression-free survival as well as overall survival and treatment-related toxic effects.
TAKEAWAY:
- During a median follow-up of 28.2 months, 128 patients (65%) had a recurrence and 30 died (15.3%) – 8.3% in the HIPEC group and 24.1% in the non-HIPEC group.
- Compared with no HIPEC, interval cytoreductive surgery with HIPEC led to a significant improvement in median PFS (22.9 months vs. 14.2 months; P = .005) and median overall survival (not reached vs. 53 months; P = .002).
- The frequency of grade 3 or 4 postoperative complications was similar in both groups: 2.8% with HIPEC versus 3.4% without HIPEC.
- Among patients with recurrence, the frequency of peritoneal recurrence was significantly lower among those who received HIPEC (32.8% vs. 64.1% without HIPEC; P = .001).
IN PRACTICE:
“We observed a significantly superior survival benefit associated with [interval cytoreductive surgery] with HIPEC, without higher rates of postoperative complications,” the authors concluded, adding that “the survival benefit remained consistent, irrespective of maintenance therapy.”
SOURCE:
The study, led by Jung-Yun Lee, MD, PhD, Yonsei University College of Medicine, Seoul, Korea, was published online in JAMA Surgery.
LIMITATIONS:
The patients were not randomly assigned and the decision to give HIPEC was at the clinician’s discretion, introducing the possibility of selection and treatment bias. The different types of drugs used in HIPEC could result in bias in data interpretation.
DISCLOSURES:
The authors reported no conflicts of interest. The study had no specific funding.
A version of this article first appeared on Medscape.com.
TOPLINE:
Neoadjuvant chemotherapy followed by interval cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC) significantly improves progression-free survival (PFS) and overall survival, compared with interval cytoreductive surgery alone, in patients with advanced ovarian cancer, new research shows.
METHODOLOGY:
- Several randomized controlled trials have shown survival benefits with HIPEC followed by interval cytoreductive surgery in advanced ovarian cancer. Despite the data, the use of HIPEC in clinical practice remains limited.
- Potential downsides of HIPEC include longer operative time and treatment-related complications.
- This prospective, multicenter, comparative effectiveness study evaluated the safety and effectiveness of interval cytoreductive surgery with HIPEC versus the surgery alone.
- The study, conducted at seven Korean Gynecologic Oncology Group institutions, included 196 patients (mean age, 58 years) with stage III or IV ovarian cancer who had received at least three cycles of neoadjuvant chemotherapy followed by interval cytoreductive surgery with HIPEC (n = 109) or without HIPEC (n = 87).
- The researchers reported progression-free survival as well as overall survival and treatment-related toxic effects.
TAKEAWAY:
- During a median follow-up of 28.2 months, 128 patients (65%) had a recurrence and 30 died (15.3%) – 8.3% in the HIPEC group and 24.1% in the non-HIPEC group.
- Compared with no HIPEC, interval cytoreductive surgery with HIPEC led to a significant improvement in median PFS (22.9 months vs. 14.2 months; P = .005) and median overall survival (not reached vs. 53 months; P = .002).
- The frequency of grade 3 or 4 postoperative complications was similar in both groups: 2.8% with HIPEC versus 3.4% without HIPEC.
- Among patients with recurrence, the frequency of peritoneal recurrence was significantly lower among those who received HIPEC (32.8% vs. 64.1% without HIPEC; P = .001).
IN PRACTICE:
“We observed a significantly superior survival benefit associated with [interval cytoreductive surgery] with HIPEC, without higher rates of postoperative complications,” the authors concluded, adding that “the survival benefit remained consistent, irrespective of maintenance therapy.”
SOURCE:
The study, led by Jung-Yun Lee, MD, PhD, Yonsei University College of Medicine, Seoul, Korea, was published online in JAMA Surgery.
LIMITATIONS:
The patients were not randomly assigned and the decision to give HIPEC was at the clinician’s discretion, introducing the possibility of selection and treatment bias. The different types of drugs used in HIPEC could result in bias in data interpretation.
DISCLOSURES:
The authors reported no conflicts of interest. The study had no specific funding.
A version of this article first appeared on Medscape.com.
TOPLINE:
Neoadjuvant chemotherapy followed by interval cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (HIPEC) significantly improves progression-free survival (PFS) and overall survival, compared with interval cytoreductive surgery alone, in patients with advanced ovarian cancer, new research shows.
METHODOLOGY:
- Several randomized controlled trials have shown survival benefits with HIPEC followed by interval cytoreductive surgery in advanced ovarian cancer. Despite the data, the use of HIPEC in clinical practice remains limited.
- Potential downsides of HIPEC include longer operative time and treatment-related complications.
- This prospective, multicenter, comparative effectiveness study evaluated the safety and effectiveness of interval cytoreductive surgery with HIPEC versus the surgery alone.
- The study, conducted at seven Korean Gynecologic Oncology Group institutions, included 196 patients (mean age, 58 years) with stage III or IV ovarian cancer who had received at least three cycles of neoadjuvant chemotherapy followed by interval cytoreductive surgery with HIPEC (n = 109) or without HIPEC (n = 87).
- The researchers reported progression-free survival as well as overall survival and treatment-related toxic effects.
TAKEAWAY:
- During a median follow-up of 28.2 months, 128 patients (65%) had a recurrence and 30 died (15.3%) – 8.3% in the HIPEC group and 24.1% in the non-HIPEC group.
- Compared with no HIPEC, interval cytoreductive surgery with HIPEC led to a significant improvement in median PFS (22.9 months vs. 14.2 months; P = .005) and median overall survival (not reached vs. 53 months; P = .002).
- The frequency of grade 3 or 4 postoperative complications was similar in both groups: 2.8% with HIPEC versus 3.4% without HIPEC.
- Among patients with recurrence, the frequency of peritoneal recurrence was significantly lower among those who received HIPEC (32.8% vs. 64.1% without HIPEC; P = .001).
IN PRACTICE:
“We observed a significantly superior survival benefit associated with [interval cytoreductive surgery] with HIPEC, without higher rates of postoperative complications,” the authors concluded, adding that “the survival benefit remained consistent, irrespective of maintenance therapy.”
SOURCE:
The study, led by Jung-Yun Lee, MD, PhD, Yonsei University College of Medicine, Seoul, Korea, was published online in JAMA Surgery.
LIMITATIONS:
The patients were not randomly assigned and the decision to give HIPEC was at the clinician’s discretion, introducing the possibility of selection and treatment bias. The different types of drugs used in HIPEC could result in bias in data interpretation.
DISCLOSURES:
The authors reported no conflicts of interest. The study had no specific funding.
A version of this article first appeared on Medscape.com.
FROM JAMA SURGERY
Nationwide hematologists shortage: What’s being done?
Over decades, the shrinking pool of CHs – who are compensated far less than hematologist-oncologists – has put patients at risk without access to adequate and timely care. To alleviate this crisis, individual doctors and national organizations are taking action and making more resources available to CHs and their patients.
`Vicious cycle’
The root cause of the CH shortage can be traced to a dramatic reduction in the number of physicians trained in this field, as Leonard Valentino, MD, President of the National Bleeding Disorders Foundation in New York, explained in an interview.
“There is a vicious cycle where there’s not enough classical hematologists to be program directors, and therefore trainees are often steered to fellowships in oncology,” said Dr. Valentino.
According to data published in JAMA, in 1995 there were 74 classical hematology programs in the United States; by 2018, there were only 2, During this same time period, the number of combined hematology/oncology training programs (HOPs) nearly doubled, from 75 to 146. However, it is estimated that less than 5% of graduates of adult HOPs pursued a career in classical hematology, as reported in Blood Advances. This low percentage can be attributed, at least in part, to the emphasis that most HOPs place on oncology.
Dr. Valentino noted that financial pressures are also diverting medical students from becoming CHs, adding that a hematologist-oncologist can make three times the annual salary of a CH.
Furthermore, when CHs treat bleeding and clotting disorders, they often need to meet with a patient for a 60- to 90-minute initial consultation, then they go on to provide a lifetime of labor-intensive care.
“This work is neither verticalized [that is, supported by radiologists, surgeons, and a cadre of nurses], nor is it billable per hour on a scale comparable to what oncologists can charge,” Dr. Valentino explained.
The survey published in Blood Advances illustrates the consequences of such a disparity in income potential: 34% of hematology/oncology fellows surveyed were likely to enter solid tumor oncology, while 20% and 4.6% would proceed to malignant hematology and CH, respectively.
Toll on patients
Primary care doctors treat some common blood disorders, but they almost always refer more difficult or complicated cases to a shrinking population of CHs.
“For many Americans, it is getting more difficult to find providers who subspecialize in hemostasis and thrombosis disorders. Patients can expect prolonged waiting times to get evaluated after a referral” said Mukul Singal, MD, of the Indiana Hemophilia and Thrombosis Center in Indianapolis.
Dr. Singal said the shortage is so acute that “at many institutions, malignant hematologists or oncologists are having to staff in-patient hematology consult services and see outpatient classical hematology patients. General hematologist/oncologists or medical oncologists are often not as comfortable or experienced with dealing with some of the complex CH conditions.”
A working care model, without enough doctors
In 1975, responding to patient advocacy groups, the federal government began funding hemophilia treatment centers (HTCs). Such centers offer a comprehensive care model that gives patients access to practitioners and administrative staff with the expertise to help them stay as healthy as possible. According to the Centers for Disease Control and Prevention, people with hemophilia who used an HTC were 40% less likely to die of a hemophilia-related complication and 40% less likely to be hospitalized for bleeding complications, compared to those who did not receive such specialized care.
“HTCs are effective at keeping patients out of the hospital and engaged in their lives. Between 80% and 95% of hemophilia patients get their care from an HTC and more patients want more services from them,” said Joe Pugliese, president of the Hemophilia Alliance in Lansdale, Pa.
Expanding care to meet patient demand is challenged by the restrictions on doctors’ salaries. All 140 U.S.-based HTCs share a $4.9 million federal grant but, by law, they can’t pay any provider more than $211,000 a year. “These restrictions push many people to industry, leaving too few doctors to meet patient demand,” Mr. Pugliese explained.
The fact that most HTCs are located in or near major cities also presents patients with the challenge of commuting, sometimes across state lines, to see a specialist. However, an uptick in telemedicine has provided one bright spot for many patients, allowing care to be brought to them.
The Hemophilia Alliance is also working on a multifaceted approach to change the rules, so that CHs are offered better compensation. “We have lobbyists in Washington, as well as an advocacy committee and a payer committee working to better support the HTC model,” Mr. Pugliese said.
Beyond the paycheck: Supporting CHs and patients
As market and regulatory restrictions make it difficult to boost the pay of CHs, doctors and nonprofit organizations are collaborating to support young CHs and bring more into the field. The American Society of Hematology has started and fully funded the Hematology Focused Fellowship Training Program (HFFTP). This program pairs comprehensive classical hematology training with education in transfusion medicine, sickle cell disease, hemostasis/thrombosis, systems-based hematology, health equity research, and global health. According to the program’s website, HFFTP’s goal is to add 50 new academic hematologists nationwide by 2030, in an effort to “improve the lives of patients with blood and bone marrow disorders.”
Additionally, classic hematologists are aiming to attract younger physicians and trainees to their field by introducing them to the various rewarding aspects of dealing with patients with inherited, chronic blood diseases. Programs like the Partners Physicians Academy (PPA), a 5-day training course that is specifically designed to encourage and retain young hematology students as classical hematologists, are essential to this effort.
“Along with preparing physicians to work in an HTC, programs like the Hematology Focused Fellowship Training Program and the Partners Physicians Academy are so important because they might convince young doctors to stick with non–oncology-based hematology careers, through the right mix of knowing about exciting research like gene therapy, financial and mentorship support, and a desire to meet unmet medical need,” explained Dr. Valentino.
The next PPA is taking place Sept. 18-22 in Indianapolis.
Dr. Singal, Dr. Valentino, and Mr. Pugliese had no financial disclosures to report.
Over decades, the shrinking pool of CHs – who are compensated far less than hematologist-oncologists – has put patients at risk without access to adequate and timely care. To alleviate this crisis, individual doctors and national organizations are taking action and making more resources available to CHs and their patients.
`Vicious cycle’
The root cause of the CH shortage can be traced to a dramatic reduction in the number of physicians trained in this field, as Leonard Valentino, MD, President of the National Bleeding Disorders Foundation in New York, explained in an interview.
“There is a vicious cycle where there’s not enough classical hematologists to be program directors, and therefore trainees are often steered to fellowships in oncology,” said Dr. Valentino.
According to data published in JAMA, in 1995 there were 74 classical hematology programs in the United States; by 2018, there were only 2, During this same time period, the number of combined hematology/oncology training programs (HOPs) nearly doubled, from 75 to 146. However, it is estimated that less than 5% of graduates of adult HOPs pursued a career in classical hematology, as reported in Blood Advances. This low percentage can be attributed, at least in part, to the emphasis that most HOPs place on oncology.
Dr. Valentino noted that financial pressures are also diverting medical students from becoming CHs, adding that a hematologist-oncologist can make three times the annual salary of a CH.
Furthermore, when CHs treat bleeding and clotting disorders, they often need to meet with a patient for a 60- to 90-minute initial consultation, then they go on to provide a lifetime of labor-intensive care.
“This work is neither verticalized [that is, supported by radiologists, surgeons, and a cadre of nurses], nor is it billable per hour on a scale comparable to what oncologists can charge,” Dr. Valentino explained.
The survey published in Blood Advances illustrates the consequences of such a disparity in income potential: 34% of hematology/oncology fellows surveyed were likely to enter solid tumor oncology, while 20% and 4.6% would proceed to malignant hematology and CH, respectively.
Toll on patients
Primary care doctors treat some common blood disorders, but they almost always refer more difficult or complicated cases to a shrinking population of CHs.
“For many Americans, it is getting more difficult to find providers who subspecialize in hemostasis and thrombosis disorders. Patients can expect prolonged waiting times to get evaluated after a referral” said Mukul Singal, MD, of the Indiana Hemophilia and Thrombosis Center in Indianapolis.
Dr. Singal said the shortage is so acute that “at many institutions, malignant hematologists or oncologists are having to staff in-patient hematology consult services and see outpatient classical hematology patients. General hematologist/oncologists or medical oncologists are often not as comfortable or experienced with dealing with some of the complex CH conditions.”
A working care model, without enough doctors
In 1975, responding to patient advocacy groups, the federal government began funding hemophilia treatment centers (HTCs). Such centers offer a comprehensive care model that gives patients access to practitioners and administrative staff with the expertise to help them stay as healthy as possible. According to the Centers for Disease Control and Prevention, people with hemophilia who used an HTC were 40% less likely to die of a hemophilia-related complication and 40% less likely to be hospitalized for bleeding complications, compared to those who did not receive such specialized care.
“HTCs are effective at keeping patients out of the hospital and engaged in their lives. Between 80% and 95% of hemophilia patients get their care from an HTC and more patients want more services from them,” said Joe Pugliese, president of the Hemophilia Alliance in Lansdale, Pa.
Expanding care to meet patient demand is challenged by the restrictions on doctors’ salaries. All 140 U.S.-based HTCs share a $4.9 million federal grant but, by law, they can’t pay any provider more than $211,000 a year. “These restrictions push many people to industry, leaving too few doctors to meet patient demand,” Mr. Pugliese explained.
The fact that most HTCs are located in or near major cities also presents patients with the challenge of commuting, sometimes across state lines, to see a specialist. However, an uptick in telemedicine has provided one bright spot for many patients, allowing care to be brought to them.
The Hemophilia Alliance is also working on a multifaceted approach to change the rules, so that CHs are offered better compensation. “We have lobbyists in Washington, as well as an advocacy committee and a payer committee working to better support the HTC model,” Mr. Pugliese said.
Beyond the paycheck: Supporting CHs and patients
As market and regulatory restrictions make it difficult to boost the pay of CHs, doctors and nonprofit organizations are collaborating to support young CHs and bring more into the field. The American Society of Hematology has started and fully funded the Hematology Focused Fellowship Training Program (HFFTP). This program pairs comprehensive classical hematology training with education in transfusion medicine, sickle cell disease, hemostasis/thrombosis, systems-based hematology, health equity research, and global health. According to the program’s website, HFFTP’s goal is to add 50 new academic hematologists nationwide by 2030, in an effort to “improve the lives of patients with blood and bone marrow disorders.”
Additionally, classic hematologists are aiming to attract younger physicians and trainees to their field by introducing them to the various rewarding aspects of dealing with patients with inherited, chronic blood diseases. Programs like the Partners Physicians Academy (PPA), a 5-day training course that is specifically designed to encourage and retain young hematology students as classical hematologists, are essential to this effort.
“Along with preparing physicians to work in an HTC, programs like the Hematology Focused Fellowship Training Program and the Partners Physicians Academy are so important because they might convince young doctors to stick with non–oncology-based hematology careers, through the right mix of knowing about exciting research like gene therapy, financial and mentorship support, and a desire to meet unmet medical need,” explained Dr. Valentino.
The next PPA is taking place Sept. 18-22 in Indianapolis.
Dr. Singal, Dr. Valentino, and Mr. Pugliese had no financial disclosures to report.
Over decades, the shrinking pool of CHs – who are compensated far less than hematologist-oncologists – has put patients at risk without access to adequate and timely care. To alleviate this crisis, individual doctors and national organizations are taking action and making more resources available to CHs and their patients.
`Vicious cycle’
The root cause of the CH shortage can be traced to a dramatic reduction in the number of physicians trained in this field, as Leonard Valentino, MD, President of the National Bleeding Disorders Foundation in New York, explained in an interview.
“There is a vicious cycle where there’s not enough classical hematologists to be program directors, and therefore trainees are often steered to fellowships in oncology,” said Dr. Valentino.
According to data published in JAMA, in 1995 there were 74 classical hematology programs in the United States; by 2018, there were only 2, During this same time period, the number of combined hematology/oncology training programs (HOPs) nearly doubled, from 75 to 146. However, it is estimated that less than 5% of graduates of adult HOPs pursued a career in classical hematology, as reported in Blood Advances. This low percentage can be attributed, at least in part, to the emphasis that most HOPs place on oncology.
Dr. Valentino noted that financial pressures are also diverting medical students from becoming CHs, adding that a hematologist-oncologist can make three times the annual salary of a CH.
Furthermore, when CHs treat bleeding and clotting disorders, they often need to meet with a patient for a 60- to 90-minute initial consultation, then they go on to provide a lifetime of labor-intensive care.
“This work is neither verticalized [that is, supported by radiologists, surgeons, and a cadre of nurses], nor is it billable per hour on a scale comparable to what oncologists can charge,” Dr. Valentino explained.
The survey published in Blood Advances illustrates the consequences of such a disparity in income potential: 34% of hematology/oncology fellows surveyed were likely to enter solid tumor oncology, while 20% and 4.6% would proceed to malignant hematology and CH, respectively.
Toll on patients
Primary care doctors treat some common blood disorders, but they almost always refer more difficult or complicated cases to a shrinking population of CHs.
“For many Americans, it is getting more difficult to find providers who subspecialize in hemostasis and thrombosis disorders. Patients can expect prolonged waiting times to get evaluated after a referral” said Mukul Singal, MD, of the Indiana Hemophilia and Thrombosis Center in Indianapolis.
Dr. Singal said the shortage is so acute that “at many institutions, malignant hematologists or oncologists are having to staff in-patient hematology consult services and see outpatient classical hematology patients. General hematologist/oncologists or medical oncologists are often not as comfortable or experienced with dealing with some of the complex CH conditions.”
A working care model, without enough doctors
In 1975, responding to patient advocacy groups, the federal government began funding hemophilia treatment centers (HTCs). Such centers offer a comprehensive care model that gives patients access to practitioners and administrative staff with the expertise to help them stay as healthy as possible. According to the Centers for Disease Control and Prevention, people with hemophilia who used an HTC were 40% less likely to die of a hemophilia-related complication and 40% less likely to be hospitalized for bleeding complications, compared to those who did not receive such specialized care.
“HTCs are effective at keeping patients out of the hospital and engaged in their lives. Between 80% and 95% of hemophilia patients get their care from an HTC and more patients want more services from them,” said Joe Pugliese, president of the Hemophilia Alliance in Lansdale, Pa.
Expanding care to meet patient demand is challenged by the restrictions on doctors’ salaries. All 140 U.S.-based HTCs share a $4.9 million federal grant but, by law, they can’t pay any provider more than $211,000 a year. “These restrictions push many people to industry, leaving too few doctors to meet patient demand,” Mr. Pugliese explained.
The fact that most HTCs are located in or near major cities also presents patients with the challenge of commuting, sometimes across state lines, to see a specialist. However, an uptick in telemedicine has provided one bright spot for many patients, allowing care to be brought to them.
The Hemophilia Alliance is also working on a multifaceted approach to change the rules, so that CHs are offered better compensation. “We have lobbyists in Washington, as well as an advocacy committee and a payer committee working to better support the HTC model,” Mr. Pugliese said.
Beyond the paycheck: Supporting CHs and patients
As market and regulatory restrictions make it difficult to boost the pay of CHs, doctors and nonprofit organizations are collaborating to support young CHs and bring more into the field. The American Society of Hematology has started and fully funded the Hematology Focused Fellowship Training Program (HFFTP). This program pairs comprehensive classical hematology training with education in transfusion medicine, sickle cell disease, hemostasis/thrombosis, systems-based hematology, health equity research, and global health. According to the program’s website, HFFTP’s goal is to add 50 new academic hematologists nationwide by 2030, in an effort to “improve the lives of patients with blood and bone marrow disorders.”
Additionally, classic hematologists are aiming to attract younger physicians and trainees to their field by introducing them to the various rewarding aspects of dealing with patients with inherited, chronic blood diseases. Programs like the Partners Physicians Academy (PPA), a 5-day training course that is specifically designed to encourage and retain young hematology students as classical hematologists, are essential to this effort.
“Along with preparing physicians to work in an HTC, programs like the Hematology Focused Fellowship Training Program and the Partners Physicians Academy are so important because they might convince young doctors to stick with non–oncology-based hematology careers, through the right mix of knowing about exciting research like gene therapy, financial and mentorship support, and a desire to meet unmet medical need,” explained Dr. Valentino.
The next PPA is taking place Sept. 18-22 in Indianapolis.
Dr. Singal, Dr. Valentino, and Mr. Pugliese had no financial disclosures to report.