User login
Veteran Homelessness Drops 47% Since 2010
In the latest Department of Housing and Urban Development annual Point-in-Time (PIT) estimate of America’s homeless population, President Barak Obama revealed that since 2010 the rate of homelessness among veterans has dropped 47%. The January count found 13,000 homeless veterans, although the count fell well short of the President’s pledge to completely eliminate homelessness by 2016. “We will not stop until every veteran who fought for America has a home in America,” President Obama told the national Disabled American Veterans (DAV) convention on August 1. “This is something we’ve got to get done.”
“The dramatic decline in veteran homelessness is the result of the Obama administration’s investments in permanent supportive housing solutions, such as HUD-VASH [Housing and Urban Development-VA Supportive Housing] and Supportive Services for Veteran Families programs, extensive community partnerships, coordinated data and outreach, and other proven strategies that put Veterans first,” said VA Secretary Robert A. McDonald in a release. According to the VA, Virginia, Connecticut and 27 cities and towns across the country have “effectively ended” veteran homelessness.
In the DAV speech, President Obama also touted a number of VA health care programs, ranging from rural telehealth initiatives to increased funding for prosthetics and access to women’s health providers at all VA clinics. Pointing out that VA funding has increased during his administration, the President noted that “We’ve made VA benefits available to more than 2 million veterans who didn’t have them before.” The President also pointed out that veterans can apply for VA health care “…from any device, including your smartphone—simple, easy, in as little as 20 minutes.”
The White House also announced that the Million Veteran Program (MVP) had reached a new milestone with 500,000 veterans enrolled. About 62% of MVP enrollees reported a current or past diagnosis of high blood pressure and about one-third reported tinnitus. Also, nearly one-third, or 32%, of veterans present with a history or current diagnosis of cancer. “We believe MVP will accelerate our understanding of disease detection, progression, prevention and treatment by combining this rich clinical, environmental and genomic data,” said VA Under Secretary for Health David J. Shulkin, MD, in a statement. “VA has a deep history of innovation and research. The MVP will allow the nation’s top researchers to perform the most cutting-edge science to treat some of the nation’s most troubling diseases.”
“What this does is it gives us a better understanding of genetics, which will allow us to improve treatments for things like traumatic brain injury and posttraumatic stress, and diabetes, and cancer,” President Obama told the DAV audience. “And that won’t just help veterans. It will help all Americans. And it’s just one more example of how our veterans keep serving our country even after they come home.”
While Acknowledging the VA’s challenges to meet the needs of veterans, President Obama was insistent that VA care was essential for veterans. “Study after study shows that in many areas, like mental health, the quality of care at the VA is often better than in private care,” he told the DAV members. “So let’s listen to our veterans, who are telling us: Don’t destroy VA health care. Fix it and make it work, but don’t break our covenant with our veterans.”
In the latest Department of Housing and Urban Development annual Point-in-Time (PIT) estimate of America’s homeless population, President Barak Obama revealed that since 2010 the rate of homelessness among veterans has dropped 47%. The January count found 13,000 homeless veterans, although the count fell well short of the President’s pledge to completely eliminate homelessness by 2016. “We will not stop until every veteran who fought for America has a home in America,” President Obama told the national Disabled American Veterans (DAV) convention on August 1. “This is something we’ve got to get done.”
“The dramatic decline in veteran homelessness is the result of the Obama administration’s investments in permanent supportive housing solutions, such as HUD-VASH [Housing and Urban Development-VA Supportive Housing] and Supportive Services for Veteran Families programs, extensive community partnerships, coordinated data and outreach, and other proven strategies that put Veterans first,” said VA Secretary Robert A. McDonald in a release. According to the VA, Virginia, Connecticut and 27 cities and towns across the country have “effectively ended” veteran homelessness.
In the DAV speech, President Obama also touted a number of VA health care programs, ranging from rural telehealth initiatives to increased funding for prosthetics and access to women’s health providers at all VA clinics. Pointing out that VA funding has increased during his administration, the President noted that “We’ve made VA benefits available to more than 2 million veterans who didn’t have them before.” The President also pointed out that veterans can apply for VA health care “…from any device, including your smartphone—simple, easy, in as little as 20 minutes.”
The White House also announced that the Million Veteran Program (MVP) had reached a new milestone with 500,000 veterans enrolled. About 62% of MVP enrollees reported a current or past diagnosis of high blood pressure and about one-third reported tinnitus. Also, nearly one-third, or 32%, of veterans present with a history or current diagnosis of cancer. “We believe MVP will accelerate our understanding of disease detection, progression, prevention and treatment by combining this rich clinical, environmental and genomic data,” said VA Under Secretary for Health David J. Shulkin, MD, in a statement. “VA has a deep history of innovation and research. The MVP will allow the nation’s top researchers to perform the most cutting-edge science to treat some of the nation’s most troubling diseases.”
“What this does is it gives us a better understanding of genetics, which will allow us to improve treatments for things like traumatic brain injury and posttraumatic stress, and diabetes, and cancer,” President Obama told the DAV audience. “And that won’t just help veterans. It will help all Americans. And it’s just one more example of how our veterans keep serving our country even after they come home.”
While Acknowledging the VA’s challenges to meet the needs of veterans, President Obama was insistent that VA care was essential for veterans. “Study after study shows that in many areas, like mental health, the quality of care at the VA is often better than in private care,” he told the DAV members. “So let’s listen to our veterans, who are telling us: Don’t destroy VA health care. Fix it and make it work, but don’t break our covenant with our veterans.”
In the latest Department of Housing and Urban Development annual Point-in-Time (PIT) estimate of America’s homeless population, President Barak Obama revealed that since 2010 the rate of homelessness among veterans has dropped 47%. The January count found 13,000 homeless veterans, although the count fell well short of the President’s pledge to completely eliminate homelessness by 2016. “We will not stop until every veteran who fought for America has a home in America,” President Obama told the national Disabled American Veterans (DAV) convention on August 1. “This is something we’ve got to get done.”
“The dramatic decline in veteran homelessness is the result of the Obama administration’s investments in permanent supportive housing solutions, such as HUD-VASH [Housing and Urban Development-VA Supportive Housing] and Supportive Services for Veteran Families programs, extensive community partnerships, coordinated data and outreach, and other proven strategies that put Veterans first,” said VA Secretary Robert A. McDonald in a release. According to the VA, Virginia, Connecticut and 27 cities and towns across the country have “effectively ended” veteran homelessness.
In the DAV speech, President Obama also touted a number of VA health care programs, ranging from rural telehealth initiatives to increased funding for prosthetics and access to women’s health providers at all VA clinics. Pointing out that VA funding has increased during his administration, the President noted that “We’ve made VA benefits available to more than 2 million veterans who didn’t have them before.” The President also pointed out that veterans can apply for VA health care “…from any device, including your smartphone—simple, easy, in as little as 20 minutes.”
The White House also announced that the Million Veteran Program (MVP) had reached a new milestone with 500,000 veterans enrolled. About 62% of MVP enrollees reported a current or past diagnosis of high blood pressure and about one-third reported tinnitus. Also, nearly one-third, or 32%, of veterans present with a history or current diagnosis of cancer. “We believe MVP will accelerate our understanding of disease detection, progression, prevention and treatment by combining this rich clinical, environmental and genomic data,” said VA Under Secretary for Health David J. Shulkin, MD, in a statement. “VA has a deep history of innovation and research. The MVP will allow the nation’s top researchers to perform the most cutting-edge science to treat some of the nation’s most troubling diseases.”
“What this does is it gives us a better understanding of genetics, which will allow us to improve treatments for things like traumatic brain injury and posttraumatic stress, and diabetes, and cancer,” President Obama told the DAV audience. “And that won’t just help veterans. It will help all Americans. And it’s just one more example of how our veterans keep serving our country even after they come home.”
While Acknowledging the VA’s challenges to meet the needs of veterans, President Obama was insistent that VA care was essential for veterans. “Study after study shows that in many areas, like mental health, the quality of care at the VA is often better than in private care,” he told the DAV members. “So let’s listen to our veterans, who are telling us: Don’t destroy VA health care. Fix it and make it work, but don’t break our covenant with our veterans.”
Preorder 2016 State of Hospital Medicine Report
The State of Hospital Medicine (SoHM) report is the most comprehensive survey of hospital medicine in the country and provides current data on hospitalist compensation and productivity, plus covers practice demographics, staffing levels, staff growth, and compensation models.
Order now and be notified directly when the report is released in September 2016 at www.hospitalmedicine.org/survey.
The State of Hospital Medicine (SoHM) report is the most comprehensive survey of hospital medicine in the country and provides current data on hospitalist compensation and productivity, plus covers practice demographics, staffing levels, staff growth, and compensation models.
Order now and be notified directly when the report is released in September 2016 at www.hospitalmedicine.org/survey.
The State of Hospital Medicine (SoHM) report is the most comprehensive survey of hospital medicine in the country and provides current data on hospitalist compensation and productivity, plus covers practice demographics, staffing levels, staff growth, and compensation models.
Order now and be notified directly when the report is released in September 2016 at www.hospitalmedicine.org/survey.
PAs, NPs: Register for 2016 Adult Hospital Medicine Boot Camp
During the course, you will:
- Learn the most current evidence-based clinical practices for key topics in hospital medicine
- Augment your knowledge base to enhance your existing hospital medicine practice
- Expand your knowledge to transition into hospital medicine practice
- Network with like-minded practitioners
To learn more and register, visit www.aapa.org/bootcamp.
During the course, you will:
- Learn the most current evidence-based clinical practices for key topics in hospital medicine
- Augment your knowledge base to enhance your existing hospital medicine practice
- Expand your knowledge to transition into hospital medicine practice
- Network with like-minded practitioners
To learn more and register, visit www.aapa.org/bootcamp.
During the course, you will:
- Learn the most current evidence-based clinical practices for key topics in hospital medicine
- Augment your knowledge base to enhance your existing hospital medicine practice
- Expand your knowledge to transition into hospital medicine practice
- Network with like-minded practitioners
To learn more and register, visit www.aapa.org/bootcamp.
Gene therapy reduces need for FIX prophylaxis

Image courtesy of NIGMS
ORLANDO—The gene therapy AMT-060 can reduce the need for factor IX (FIX) prophylaxis in patients with severe hemophilia B, results of a phase 1/2 study suggest.
All of the patients treated in the low-dose cohort of this study have had sustained improvements in their disease phenotype and continue to maintain durable levels of FIX gene activity for up to 39 weeks post-treatment.
Four of the 5 patients were able to discontinue prophylactic FIX infusions.
In addition, AMT-060 was considered well-tolerated. There were 2 serious adverse events, but both were temporary. And none of the patients developed FIX inhibitors.
These data were presented at the World Federation of Hemophilia 2016 World Congress.* The research is sponsored by uniQure.
“I am very encouraged by the stability of increased FIX activity of AMT-060 and the significant reduction in required infusions of factor replacement,” said study investigator Wolfgang Miesbach, MD, of the University of Frankfurt in Germany.
“This effect is particularly important because it is seen in severe patients with established joint disease who experienced a high frequency of joint bleeds despite intense use of prophylactic FIX prior to study entry.”
Patients and treatment
AMT-060 consists of a codon-optimized wild-type FIX gene cassette, the LP1 liver promoter, and an AAV5 viral vector manufactured by uniQure using its proprietary insect cell-based technology platform.
In this phase 1/2 trial, Dr Miesbach and his colleagues are testing AMT-060 in 10 patients. All patients had severe or moderately severe hemophilia at baseline, including documented FIX levels less than 1% to 2% of normal, and required chronic infusions of prophylactic or on-demand FIX therapy at the time of enrollment.
Each patient received a 1-time, 30-minute, intravenous dose of AMT-060, without the use of corticosteroids. Five patients received AMT-060 at 5x1012 gc/kg, and 5 received AMT-060 at 2x1013 gc/kg.
Dr Miesbach presented results observed in the low-dose cohort. Patients in the high-dose cohort are still in the early stages of follow-up.
Most patients in the low-dose cohort were older than 50 years of age (range, 35-72). Four patients had severe hemophilia B, and 4 had advanced joint disease. All of the patients had frequent bleeding episodes, despite receiving once- or twice-weekly FIX prophylaxis.
Efficacy
For all 5 patients in the low-dose cohort, the mean annualized total FIX usage declined 75% after treatment with AMT-060.
“The majority of patients in this low-dose cohort of AMT-060 are showing FIX activity in the range of 5% of normal, and clinical experience has shown that patients in this range generally do not require prophylactic factor replacement and have a very low frequency of spontaneous joint bleeding episodes,” Dr Miesbach said.
Four patients discontinued prophylactic therapy. The 1 patient who remained on prophylactic therapy has sustained an improved disease phenotype and also required materially less FIX concentrate after treatment with AMT-060.
Through up to 9 months of follow-up, the mean steady-state FIX activity for the 4 patients who discontinued prophylactic FIX therapy was 5.4% of normal, with a range from 3.1% to 6.7% of normal. These patients had a mean reduction in annualized total FIX usage of 82%.
Safety and immunogenicity
Two patients experienced serious adverse events. One patient had self-limiting fever in the first 24 hours after receiving AMT-060.
The other patient had a transient elevation of alanine aminotransferase (ALT) that was responsive to tapering prednisolone (60 mg/day start dose) without loss of FIX activity. At baseline, this patient’s ALT was 26 IU/L. It hit a peak of 61 IU/L at week 10, but values returned to baseline levels within 2 weeks of treatment.
As expected, all of the patients developed anti-AAV5 antibodies after week 1. None of the patients developed inhibitory antibodies against FIX.
There was no evidence of sustained AAV5 capsid-specific T-cell activation, although 1 patient had transient T-cell activation slightly above the positive threshold at 1 time point. This patient did not have ALT elevation. ![]()
*Miesbach W et al, Updated results from a dose escalating study in adult patients with haemophilia B treated with AMT-060 (AAV5-hFIX) gene therapy, WFH 2016 World
Congress, July 2016.

Image courtesy of NIGMS
ORLANDO—The gene therapy AMT-060 can reduce the need for factor IX (FIX) prophylaxis in patients with severe hemophilia B, results of a phase 1/2 study suggest.
All of the patients treated in the low-dose cohort of this study have had sustained improvements in their disease phenotype and continue to maintain durable levels of FIX gene activity for up to 39 weeks post-treatment.
Four of the 5 patients were able to discontinue prophylactic FIX infusions.
In addition, AMT-060 was considered well-tolerated. There were 2 serious adverse events, but both were temporary. And none of the patients developed FIX inhibitors.
These data were presented at the World Federation of Hemophilia 2016 World Congress.* The research is sponsored by uniQure.
“I am very encouraged by the stability of increased FIX activity of AMT-060 and the significant reduction in required infusions of factor replacement,” said study investigator Wolfgang Miesbach, MD, of the University of Frankfurt in Germany.
“This effect is particularly important because it is seen in severe patients with established joint disease who experienced a high frequency of joint bleeds despite intense use of prophylactic FIX prior to study entry.”
Patients and treatment
AMT-060 consists of a codon-optimized wild-type FIX gene cassette, the LP1 liver promoter, and an AAV5 viral vector manufactured by uniQure using its proprietary insect cell-based technology platform.
In this phase 1/2 trial, Dr Miesbach and his colleagues are testing AMT-060 in 10 patients. All patients had severe or moderately severe hemophilia at baseline, including documented FIX levels less than 1% to 2% of normal, and required chronic infusions of prophylactic or on-demand FIX therapy at the time of enrollment.
Each patient received a 1-time, 30-minute, intravenous dose of AMT-060, without the use of corticosteroids. Five patients received AMT-060 at 5x1012 gc/kg, and 5 received AMT-060 at 2x1013 gc/kg.
Dr Miesbach presented results observed in the low-dose cohort. Patients in the high-dose cohort are still in the early stages of follow-up.
Most patients in the low-dose cohort were older than 50 years of age (range, 35-72). Four patients had severe hemophilia B, and 4 had advanced joint disease. All of the patients had frequent bleeding episodes, despite receiving once- or twice-weekly FIX prophylaxis.
Efficacy
For all 5 patients in the low-dose cohort, the mean annualized total FIX usage declined 75% after treatment with AMT-060.
“The majority of patients in this low-dose cohort of AMT-060 are showing FIX activity in the range of 5% of normal, and clinical experience has shown that patients in this range generally do not require prophylactic factor replacement and have a very low frequency of spontaneous joint bleeding episodes,” Dr Miesbach said.
Four patients discontinued prophylactic therapy. The 1 patient who remained on prophylactic therapy has sustained an improved disease phenotype and also required materially less FIX concentrate after treatment with AMT-060.
Through up to 9 months of follow-up, the mean steady-state FIX activity for the 4 patients who discontinued prophylactic FIX therapy was 5.4% of normal, with a range from 3.1% to 6.7% of normal. These patients had a mean reduction in annualized total FIX usage of 82%.
Safety and immunogenicity
Two patients experienced serious adverse events. One patient had self-limiting fever in the first 24 hours after receiving AMT-060.
The other patient had a transient elevation of alanine aminotransferase (ALT) that was responsive to tapering prednisolone (60 mg/day start dose) without loss of FIX activity. At baseline, this patient’s ALT was 26 IU/L. It hit a peak of 61 IU/L at week 10, but values returned to baseline levels within 2 weeks of treatment.
As expected, all of the patients developed anti-AAV5 antibodies after week 1. None of the patients developed inhibitory antibodies against FIX.
There was no evidence of sustained AAV5 capsid-specific T-cell activation, although 1 patient had transient T-cell activation slightly above the positive threshold at 1 time point. This patient did not have ALT elevation. ![]()
*Miesbach W et al, Updated results from a dose escalating study in adult patients with haemophilia B treated with AMT-060 (AAV5-hFIX) gene therapy, WFH 2016 World
Congress, July 2016.

Image courtesy of NIGMS
ORLANDO—The gene therapy AMT-060 can reduce the need for factor IX (FIX) prophylaxis in patients with severe hemophilia B, results of a phase 1/2 study suggest.
All of the patients treated in the low-dose cohort of this study have had sustained improvements in their disease phenotype and continue to maintain durable levels of FIX gene activity for up to 39 weeks post-treatment.
Four of the 5 patients were able to discontinue prophylactic FIX infusions.
In addition, AMT-060 was considered well-tolerated. There were 2 serious adverse events, but both were temporary. And none of the patients developed FIX inhibitors.
These data were presented at the World Federation of Hemophilia 2016 World Congress.* The research is sponsored by uniQure.
“I am very encouraged by the stability of increased FIX activity of AMT-060 and the significant reduction in required infusions of factor replacement,” said study investigator Wolfgang Miesbach, MD, of the University of Frankfurt in Germany.
“This effect is particularly important because it is seen in severe patients with established joint disease who experienced a high frequency of joint bleeds despite intense use of prophylactic FIX prior to study entry.”
Patients and treatment
AMT-060 consists of a codon-optimized wild-type FIX gene cassette, the LP1 liver promoter, and an AAV5 viral vector manufactured by uniQure using its proprietary insect cell-based technology platform.
In this phase 1/2 trial, Dr Miesbach and his colleagues are testing AMT-060 in 10 patients. All patients had severe or moderately severe hemophilia at baseline, including documented FIX levels less than 1% to 2% of normal, and required chronic infusions of prophylactic or on-demand FIX therapy at the time of enrollment.
Each patient received a 1-time, 30-minute, intravenous dose of AMT-060, without the use of corticosteroids. Five patients received AMT-060 at 5x1012 gc/kg, and 5 received AMT-060 at 2x1013 gc/kg.
Dr Miesbach presented results observed in the low-dose cohort. Patients in the high-dose cohort are still in the early stages of follow-up.
Most patients in the low-dose cohort were older than 50 years of age (range, 35-72). Four patients had severe hemophilia B, and 4 had advanced joint disease. All of the patients had frequent bleeding episodes, despite receiving once- or twice-weekly FIX prophylaxis.
Efficacy
For all 5 patients in the low-dose cohort, the mean annualized total FIX usage declined 75% after treatment with AMT-060.
“The majority of patients in this low-dose cohort of AMT-060 are showing FIX activity in the range of 5% of normal, and clinical experience has shown that patients in this range generally do not require prophylactic factor replacement and have a very low frequency of spontaneous joint bleeding episodes,” Dr Miesbach said.
Four patients discontinued prophylactic therapy. The 1 patient who remained on prophylactic therapy has sustained an improved disease phenotype and also required materially less FIX concentrate after treatment with AMT-060.
Through up to 9 months of follow-up, the mean steady-state FIX activity for the 4 patients who discontinued prophylactic FIX therapy was 5.4% of normal, with a range from 3.1% to 6.7% of normal. These patients had a mean reduction in annualized total FIX usage of 82%.
Safety and immunogenicity
Two patients experienced serious adverse events. One patient had self-limiting fever in the first 24 hours after receiving AMT-060.
The other patient had a transient elevation of alanine aminotransferase (ALT) that was responsive to tapering prednisolone (60 mg/day start dose) without loss of FIX activity. At baseline, this patient’s ALT was 26 IU/L. It hit a peak of 61 IU/L at week 10, but values returned to baseline levels within 2 weeks of treatment.
As expected, all of the patients developed anti-AAV5 antibodies after week 1. None of the patients developed inhibitory antibodies against FIX.
There was no evidence of sustained AAV5 capsid-specific T-cell activation, although 1 patient had transient T-cell activation slightly above the positive threshold at 1 time point. This patient did not have ALT elevation. ![]()
*Miesbach W et al, Updated results from a dose escalating study in adult patients with haemophilia B treated with AMT-060 (AAV5-hFIX) gene therapy, WFH 2016 World
Congress, July 2016.
Microneedle system could replace blood draws, team says

Photo courtesy of
Sahan Ranamukhaarachchi
A new microneedle drug monitoring system could one day replace invasive blood draws, according to researchers.
The system consists of a small, thin patch that is pressed against a patient’s arm during medical treatment and measures drugs in the bloodstream painlessly without drawing any blood.
The tiny projections on this patch resemble hollow cones and don’t pierce the skin like a standard hypodermic needle.
The researchers described this system in Scientific Reports.
“Many groups are researching microneedle technology for painless vaccines and drug delivery,” said study author Sahan Ranamukhaarachchi, a PhD student at the University of British Columbia (UBC) in Vancouver, British Columbia, Canada. “Using them to painlessly monitor drugs is a newer idea.”
The microneedle system Ranamukhaarachchi and his colleagues created was developed to monitor the antibiotic vancomycin. Patients taking vancomycin must be closely monitored because the drug can cause life-threatening side effects, so the patients undergo 3 to 4 blood draws per day.
The researchers discovered they could use fluid found just below the outer layer of skin, instead of blood, to monitor levels of vancomycin in the bloodstream.
The microneedle patch collects a tiny amount of the fluid, less than 1 nL, and a reaction occurs on the inside of the microneedles that can be detected using an optical sensor. This allows the user to quickly determine the concentration of vancomycin.
“This is probably one of the smallest probe volumes ever recorded for a medically relevant analysis,” said study author Urs Häfeli, PhD, of UBC.
This microneedle drug monitoring system was developed out of a research collaboration between Dr Häfeli and Boris Stoeber, PhD, also of UBC. The system is being commercialized by the UBC spin-off Microdermics Inc. ![]()

Photo courtesy of
Sahan Ranamukhaarachchi
A new microneedle drug monitoring system could one day replace invasive blood draws, according to researchers.
The system consists of a small, thin patch that is pressed against a patient’s arm during medical treatment and measures drugs in the bloodstream painlessly without drawing any blood.
The tiny projections on this patch resemble hollow cones and don’t pierce the skin like a standard hypodermic needle.
The researchers described this system in Scientific Reports.
“Many groups are researching microneedle technology for painless vaccines and drug delivery,” said study author Sahan Ranamukhaarachchi, a PhD student at the University of British Columbia (UBC) in Vancouver, British Columbia, Canada. “Using them to painlessly monitor drugs is a newer idea.”
The microneedle system Ranamukhaarachchi and his colleagues created was developed to monitor the antibiotic vancomycin. Patients taking vancomycin must be closely monitored because the drug can cause life-threatening side effects, so the patients undergo 3 to 4 blood draws per day.
The researchers discovered they could use fluid found just below the outer layer of skin, instead of blood, to monitor levels of vancomycin in the bloodstream.
The microneedle patch collects a tiny amount of the fluid, less than 1 nL, and a reaction occurs on the inside of the microneedles that can be detected using an optical sensor. This allows the user to quickly determine the concentration of vancomycin.
“This is probably one of the smallest probe volumes ever recorded for a medically relevant analysis,” said study author Urs Häfeli, PhD, of UBC.
This microneedle drug monitoring system was developed out of a research collaboration between Dr Häfeli and Boris Stoeber, PhD, also of UBC. The system is being commercialized by the UBC spin-off Microdermics Inc. ![]()

Photo courtesy of
Sahan Ranamukhaarachchi
A new microneedle drug monitoring system could one day replace invasive blood draws, according to researchers.
The system consists of a small, thin patch that is pressed against a patient’s arm during medical treatment and measures drugs in the bloodstream painlessly without drawing any blood.
The tiny projections on this patch resemble hollow cones and don’t pierce the skin like a standard hypodermic needle.
The researchers described this system in Scientific Reports.
“Many groups are researching microneedle technology for painless vaccines and drug delivery,” said study author Sahan Ranamukhaarachchi, a PhD student at the University of British Columbia (UBC) in Vancouver, British Columbia, Canada. “Using them to painlessly monitor drugs is a newer idea.”
The microneedle system Ranamukhaarachchi and his colleagues created was developed to monitor the antibiotic vancomycin. Patients taking vancomycin must be closely monitored because the drug can cause life-threatening side effects, so the patients undergo 3 to 4 blood draws per day.
The researchers discovered they could use fluid found just below the outer layer of skin, instead of blood, to monitor levels of vancomycin in the bloodstream.
The microneedle patch collects a tiny amount of the fluid, less than 1 nL, and a reaction occurs on the inside of the microneedles that can be detected using an optical sensor. This allows the user to quickly determine the concentration of vancomycin.
“This is probably one of the smallest probe volumes ever recorded for a medically relevant analysis,” said study author Urs Häfeli, PhD, of UBC.
This microneedle drug monitoring system was developed out of a research collaboration between Dr Häfeli and Boris Stoeber, PhD, also of UBC. The system is being commercialized by the UBC spin-off Microdermics Inc. ![]()
FDA approves reconstitution system for FVIII product

Photo courtesy of Baxalta
The US Food and Drug Administration (FDA) has approved the Baxject III reconstitution system for Adynovate, a pegylated recombinant factor VIII (FVIII) product.
The system is designed to mix a FVIII product with a diluent prior to infusion.
The Baxject III reconstitution system was previously FDA-approved for use with Advate, a recombinant FVIII product.
The latest FDA approval means the system will be available with Adynovate as well.
Adynovate and the diluent will come pre-packaged in the reconstitution system.
The Baxject III reconstitution system with Adynovate will be available to most customers in the fourth quarter of 2016, with a 2 mL diluent for the 250, 500, and 1000 IU potencies; and a 5 mL diluent for the 2000 IU potency.
Adynovate was approved by the FDA in 2015 for use in hemophilia A patients age 12 and older for on-demand treatment and control of bleeding and for prophylaxis to reduce the frequency of bleeding episodes. Full prescribing information is available here.
Advate was first approved by the FDA in 2003. The product is indicated for use in children and adults with hemophilia A for the control and prevention of bleeding episodes, perioperative management, and routine prophylaxis to prevent or reduce the frequency of bleeding episodes. Full prescribing information is available here.
The Baxject III reconstitution system, Adynovate, and Advate are all products of Baxalta, which is now a part of Shire. ![]()

Photo courtesy of Baxalta
The US Food and Drug Administration (FDA) has approved the Baxject III reconstitution system for Adynovate, a pegylated recombinant factor VIII (FVIII) product.
The system is designed to mix a FVIII product with a diluent prior to infusion.
The Baxject III reconstitution system was previously FDA-approved for use with Advate, a recombinant FVIII product.
The latest FDA approval means the system will be available with Adynovate as well.
Adynovate and the diluent will come pre-packaged in the reconstitution system.
The Baxject III reconstitution system with Adynovate will be available to most customers in the fourth quarter of 2016, with a 2 mL diluent for the 250, 500, and 1000 IU potencies; and a 5 mL diluent for the 2000 IU potency.
Adynovate was approved by the FDA in 2015 for use in hemophilia A patients age 12 and older for on-demand treatment and control of bleeding and for prophylaxis to reduce the frequency of bleeding episodes. Full prescribing information is available here.
Advate was first approved by the FDA in 2003. The product is indicated for use in children and adults with hemophilia A for the control and prevention of bleeding episodes, perioperative management, and routine prophylaxis to prevent or reduce the frequency of bleeding episodes. Full prescribing information is available here.
The Baxject III reconstitution system, Adynovate, and Advate are all products of Baxalta, which is now a part of Shire. ![]()

Photo courtesy of Baxalta
The US Food and Drug Administration (FDA) has approved the Baxject III reconstitution system for Adynovate, a pegylated recombinant factor VIII (FVIII) product.
The system is designed to mix a FVIII product with a diluent prior to infusion.
The Baxject III reconstitution system was previously FDA-approved for use with Advate, a recombinant FVIII product.
The latest FDA approval means the system will be available with Adynovate as well.
Adynovate and the diluent will come pre-packaged in the reconstitution system.
The Baxject III reconstitution system with Adynovate will be available to most customers in the fourth quarter of 2016, with a 2 mL diluent for the 250, 500, and 1000 IU potencies; and a 5 mL diluent for the 2000 IU potency.
Adynovate was approved by the FDA in 2015 for use in hemophilia A patients age 12 and older for on-demand treatment and control of bleeding and for prophylaxis to reduce the frequency of bleeding episodes. Full prescribing information is available here.
Advate was first approved by the FDA in 2003. The product is indicated for use in children and adults with hemophilia A for the control and prevention of bleeding episodes, perioperative management, and routine prophylaxis to prevent or reduce the frequency of bleeding episodes. Full prescribing information is available here.
The Baxject III reconstitution system, Adynovate, and Advate are all products of Baxalta, which is now a part of Shire. ![]()
HDAC inhibitor granted breakthrough designation

Image by Eric Smith
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for the histone deacetylase (HDAC) inhibitor pracinostat to be used in combination with azacitidine to treat newly diagnosed acute myeloid leukemia (AML) patients who are 75 and older or unfit for intensive chemotherapy.
The FDA’s breakthrough designation is intended to expedite the development and review of new therapies for serious or life-threatening conditions.
To earn the designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
The breakthrough therapy designation for pracinostat is supported by data from a phase 2 study of the HDAC inhibitor in combination with azacitidine in elderly patients with newly diagnosed AML who were not candidates for induction chemotherapy.
Detailed results from this trial were presented at the 20th Congress of the European Hematology Association last year. The research was sponsored by MEI Pharma, the company developing pracinostat.
The study included 50 AML patients who had a median age of 75 (range, 66-84).
The patients received pracinostat at 60 mg orally on days 1, 3, and 5 of each week for 21 days of each 28-day cycle. They received azacitidine subcutaneously or intravenously on days 1-7 or days 1-5 and 8-9 (per site preference) of each 28-day cycle.
According to updated data from MEI Pharma, the complete response rate was 42% (n=21), and the median overall survival was 19.1 months.
The company said these data compare favorably to a phase 3 study of azacitidine (AZA-AML-0011), which showed a median overall survival of 10.4 months with azacitidine alone and a complete response rate of 19.5% in a similar patient population.
The combination of pracinostat and azacitidine was thought to be well tolerated overall, with no unexpected toxicities. The most common grade 3-4 treatment-emergent adverse events included febrile neutropenia, thrombocytopenia, anemia, and fatigue. ![]()

Image by Eric Smith
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for the histone deacetylase (HDAC) inhibitor pracinostat to be used in combination with azacitidine to treat newly diagnosed acute myeloid leukemia (AML) patients who are 75 and older or unfit for intensive chemotherapy.
The FDA’s breakthrough designation is intended to expedite the development and review of new therapies for serious or life-threatening conditions.
To earn the designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
The breakthrough therapy designation for pracinostat is supported by data from a phase 2 study of the HDAC inhibitor in combination with azacitidine in elderly patients with newly diagnosed AML who were not candidates for induction chemotherapy.
Detailed results from this trial were presented at the 20th Congress of the European Hematology Association last year. The research was sponsored by MEI Pharma, the company developing pracinostat.
The study included 50 AML patients who had a median age of 75 (range, 66-84).
The patients received pracinostat at 60 mg orally on days 1, 3, and 5 of each week for 21 days of each 28-day cycle. They received azacitidine subcutaneously or intravenously on days 1-7 or days 1-5 and 8-9 (per site preference) of each 28-day cycle.
According to updated data from MEI Pharma, the complete response rate was 42% (n=21), and the median overall survival was 19.1 months.
The company said these data compare favorably to a phase 3 study of azacitidine (AZA-AML-0011), which showed a median overall survival of 10.4 months with azacitidine alone and a complete response rate of 19.5% in a similar patient population.
The combination of pracinostat and azacitidine was thought to be well tolerated overall, with no unexpected toxicities. The most common grade 3-4 treatment-emergent adverse events included febrile neutropenia, thrombocytopenia, anemia, and fatigue. ![]()

Image by Eric Smith
The US Food and Drug Administration (FDA) has granted breakthrough therapy designation for the histone deacetylase (HDAC) inhibitor pracinostat to be used in combination with azacitidine to treat newly diagnosed acute myeloid leukemia (AML) patients who are 75 and older or unfit for intensive chemotherapy.
The FDA’s breakthrough designation is intended to expedite the development and review of new therapies for serious or life-threatening conditions.
To earn the designation, a treatment must show encouraging early clinical results demonstrating substantial improvement over available therapies with regard to a clinically significant endpoint, or it must fulfill an unmet need.
The breakthrough therapy designation for pracinostat is supported by data from a phase 2 study of the HDAC inhibitor in combination with azacitidine in elderly patients with newly diagnosed AML who were not candidates for induction chemotherapy.
Detailed results from this trial were presented at the 20th Congress of the European Hematology Association last year. The research was sponsored by MEI Pharma, the company developing pracinostat.
The study included 50 AML patients who had a median age of 75 (range, 66-84).
The patients received pracinostat at 60 mg orally on days 1, 3, and 5 of each week for 21 days of each 28-day cycle. They received azacitidine subcutaneously or intravenously on days 1-7 or days 1-5 and 8-9 (per site preference) of each 28-day cycle.
According to updated data from MEI Pharma, the complete response rate was 42% (n=21), and the median overall survival was 19.1 months.
The company said these data compare favorably to a phase 3 study of azacitidine (AZA-AML-0011), which showed a median overall survival of 10.4 months with azacitidine alone and a complete response rate of 19.5% in a similar patient population.
The combination of pracinostat and azacitidine was thought to be well tolerated overall, with no unexpected toxicities. The most common grade 3-4 treatment-emergent adverse events included febrile neutropenia, thrombocytopenia, anemia, and fatigue. ![]()
Overtreatment of Nonpurulent Cellulitis
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 65‐year‐old immunocompetent man with a history of obesity, diabetes, and chronic lower extremity edema presents to the emergency room with a 1‐day history of right lower extremity pain and increased swelling. He reports no antecedent trauma and states he just noticed the symptoms that morning. On examination, he appears generally well. His temperature is 100F, pulse 92 beats per minute, blood pressure 120/60 mm Hg, and respiratory rate 16 breaths per minute. The rest of the exam is notable for right lower extremity erythema and swelling extending from his right shin to his right medial thigh without associated fluctuance or drainage. Labs reveal a mildly elevated white blood cell count of 13,000/L and normal serum creatinine. Are broad‐spectrum antibiotics like vancomycin and piperacillin/tazobactam the preferred regimen?
BACKGROUND
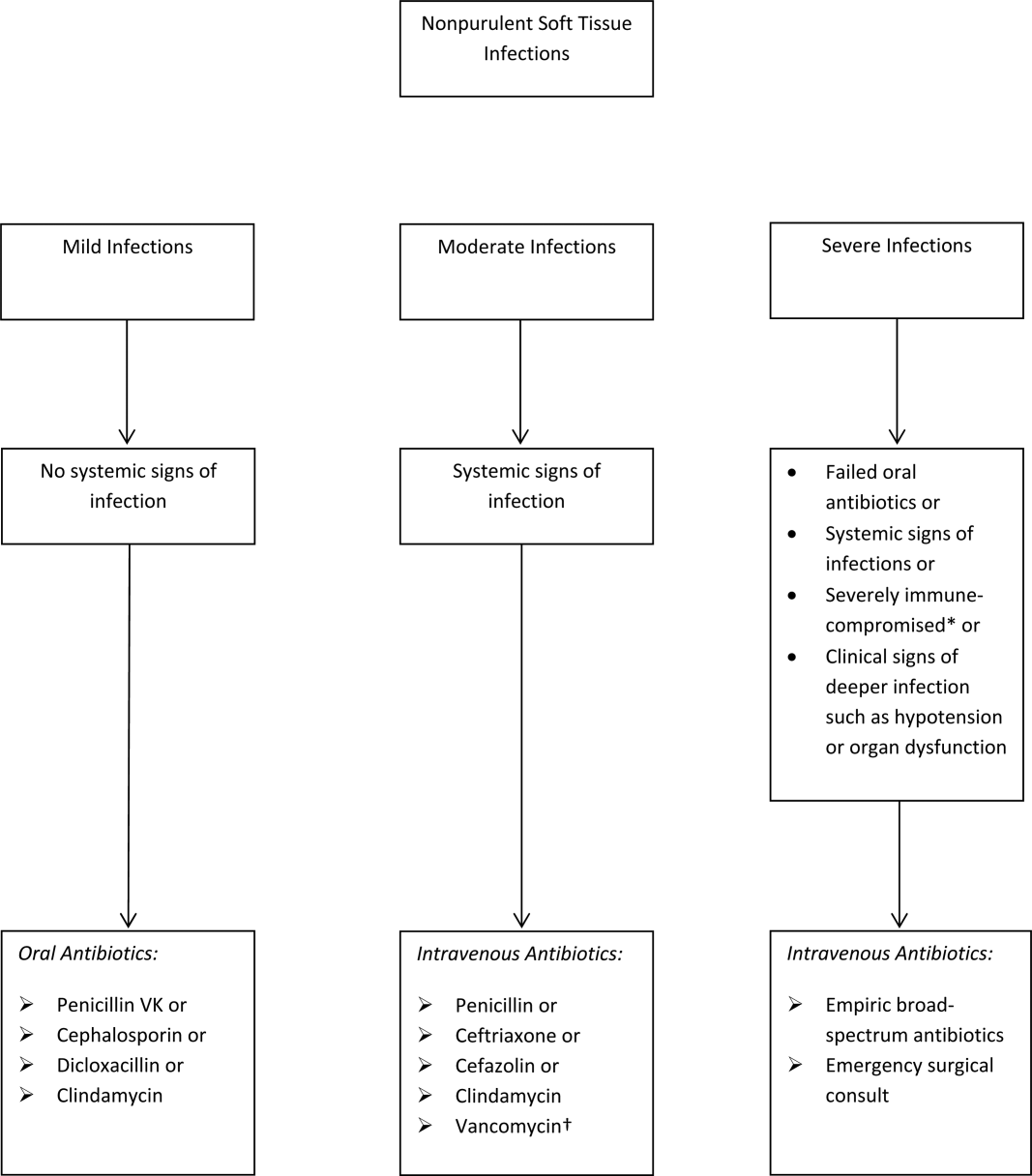
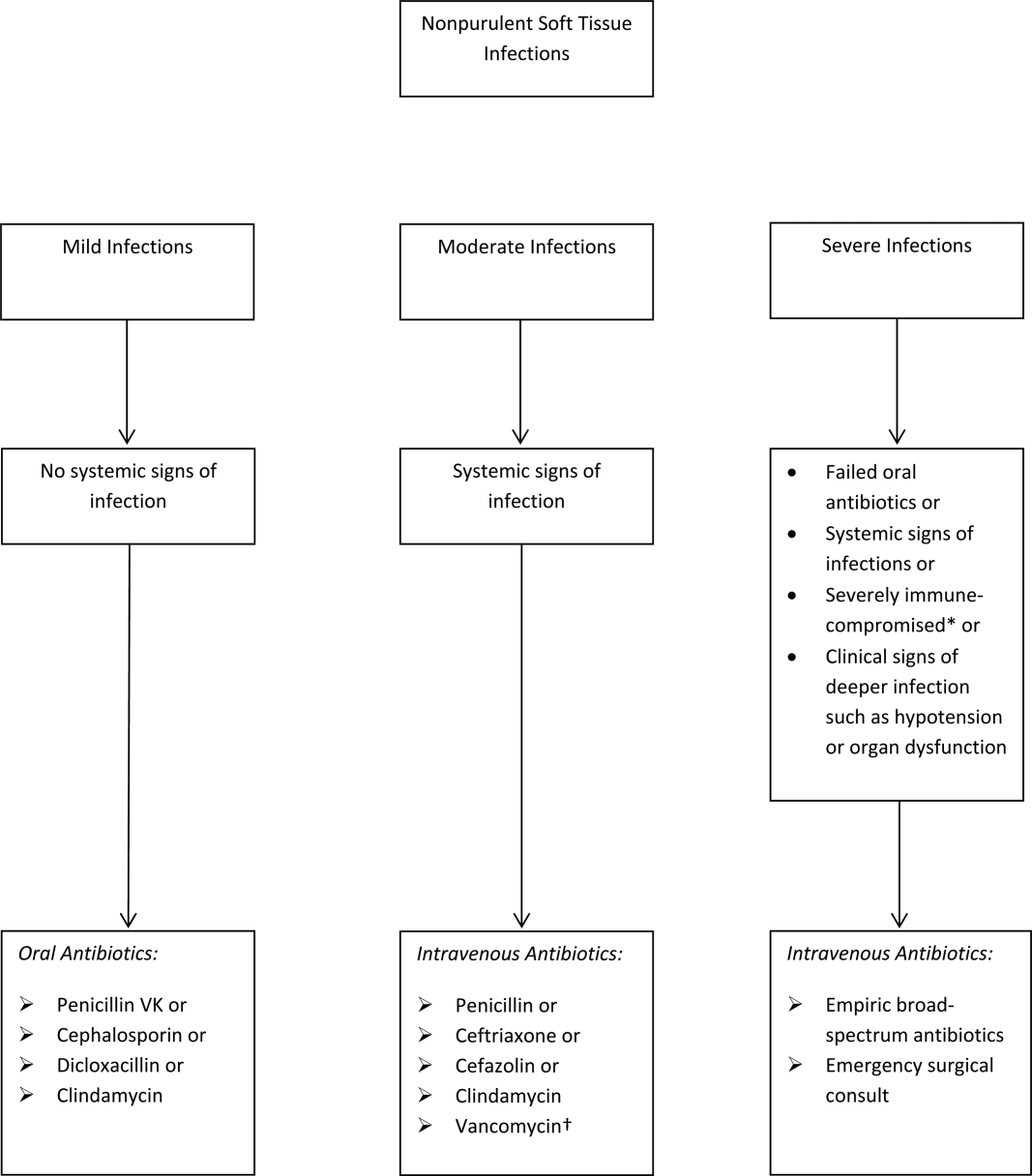
The term skin and soft tissue infection (SSTI) includes a heterogeneous group of infections including cellulitis, cutaneous abscess, diabetic foot infections, surgical site infections, and necrotizing soft tissue infections. As a group, SSTIs are the second most common type of infection in hospitalized adults in the United States behind pneumonia and result in more than 600,000 admissions per year.[1] The current guideline on SSTIs by the Infectious Disease Society of America (IDSA) makes the distinction between purulent and nonpurulent soft tissue infections based on the presence or absence of purulent drainage or abscess and between mild, moderate, and severe infections based on the presence and severity of systemic signs of infection.[2] Figure 1 provides an overview of the IDSA recommendations.
THE PROBLEM: OVERUSE OF BROAD‐SPECTRUM ANTIBIOTICS
Studies over the past decade have shown that the majority of patients hospitalized with SSTI receive broad‐spectrum antibiotics, usually with combinations of antibiotics active against gram‐positive (including methicillin‐resistant Staphylococcus aureus [MRSA]), gram‐negative (often including Pseudomonas aeruginosa), and anaerobic organisms. Broad‐spectrum treatment occurs despite guidelines from the IDSA, which state that the most common pathogens for nonpurulent cellulitis are ‐hemolytic streptococci, which remain susceptible to penicillin.[2, 3] One multicenter study of hospitalized adults with nonpurulent cellulitis, for example, reported that 85% of patients received therapy effective against MRSA (primarily vancomycin), 61% received broad gram‐negative coverage (primarily ‐lactam with ‐lactamase inhibitor), and 74% received anaerobic coverage.[4] Another multicenter study reported that the most common antibiotics given for cellulitis (excluding cases associated with cutaneous abscess) were vancomycin (60%), ‐lactam/‐lactamase combinations (32%), and clindamycin (19%). Only 13% of patients with cellulitis were treated with cefazolin, and only 1.1% of patients were treated with nafcillin or oxacillin.[5] According to the Centers for Disease Control and Prevention, unnecessary antibiotic use is associated with increased cost, development of antibiotic resistance, and increased rates of Clostridium difficile.[6]
The current use of broad‐spectrum antibiotics for nonpurulent cellulitis is likely due to several factors, including the emergence of community‐associated (CA)‐MRSA, confusion due to the heterogeneity of SSTI, and the limited data regarding the microbiology of nonpurulent cellulitis. The resulting uncertainty about cellulitis has been termed an existential crisis for the treating physician and is likely the single biggest factor behind the out‐of‐control prescribing.[7]
The Emergence of CA‐MRSA
Over the past decade, numerous studies have reported the increasing frequency of CA‐MRSA soft tissue infections, predominantly with the pulsed‐field gel electrophoresis type USA‐300. Originally, MRSA infections were limited to nosocomial infections. Subsequent multicenter studies from the United States have shown that CA‐MRSA is the most frequent pathogen isolated from purulent soft tissue infections presenting to emergency rooms[8] and the most frequent pathogen isolated from SSTI specimens in labs.[9] Many authors have therefore concluded that empiric antibiotics for SSTI should include coverage for MRSA.[8, 9]
Heterogeneity of SSTI
As already discussed, the term SSTI is an umbrella term that encompasses several types of clinically distinct infections. The only commonality between the SSTI is that that they all involve the skin and soft tissues in some way. Diabetic foot infections, cutaneous abscesses, surgical site infections, and nonpurulent cellulitis have different hosts, pathophysiology, clinical presentations, and microbiology. At one end of the spectrum is the cutaneous abscess, which is readily culturable through incision and drainage. At the other end of the spectrum is cellulitis, which is typically nonculturable. Unfortunately, studies of SSTI tend to lump all of these entities together when reporting microbiology. The landmark study by Moran et al., for example, described the microbiology of purulent soft tissue infections presenting to a network of emergency rooms across the county. Although all patients had by definition purulent infections, and 81% were abscesses, the authors made broad conclusions about skin and soft tissue infections in general and recommended antimicrobials effective against MRSA for empiric coverage for SSTIs.[8]
Uncertainty About the Microbiology of Nonpurulent Cellulitis
What then is the microbiology of nonpurulent cellulitis? As stated in the 2005 and 2014 IDSA guidelines, traditional teaching remains that nonpurulent cellulitis is primarily due to ‐hemolytic streptococci.[2, 3] Studies using needle aspiration have yielded conflicting results, although a systematic review of these studies concluded that S aureus was the most common pathogen.[10] On the other hand, a systematic review of positive blood cultures of patients identified as having cellulitis found that 61% were due to ‐hemolytic streptococci, and only 15% were due to S aureus.[11] Both reviews, however, comment on the limited quality of the included studies. Ultimately, because nonpurulent soft tissue infections are basically nonculturable, their true microbiologic etiology remains uncertain. Given this uncertainty, as well as the impressive evidence for CA‐MRSA causing cutaneous abscesses, along with the confusion about types of SSTI, it is not surprising that front‐line clinicians have resorted to prescribing broad‐spectrum antibiotics.
THE SOLUTION: NARROW‐SPECTRUM ANTIBIOTICS FOR MOST
Although studies of the microbiology of cellulitis remain inconclusive, several recent clinical trials have indicated that treatment with antimicrobials limited to ‐hemolytic streptococci and methicillin‐susceptible S aureus (MSSA) are as effective as antimicrobials against MRSA. A prospective study from 2010 of consecutive hospitalized adults with nonpurulent cellulitis found that 73% had serologic evidence for streptococcal infection, and overall 95.8% responded to cefazolin monotherapy.[12] More recently, a study of emergency room patients with nonpurulent cellulitis randomized patients to cephalexin alone or cephalexin plus trimethoprim‐sulfamethoxazole. These authors found no difference in response rates and concluded that the addition of anti‐MRSA therapy (trimethoprim‐sulfamethoxazole, in this study) for uncomplicated cellulitis was unnecessary.[13] This later study is the only randomized controlled study to assess the need for MRSA coverage for cellulitis, and the answer for outpatients, at least, is that MRSA coverage is unnecessary. Both of these studies are cited by the IDSA guideline from 2014, which recommends antibiotics for mild‐moderate cellulitis to be limited to antimicrobials effective against ‐hemolytic streptococci and MSSA. The guideline specifically does not recommend routinely treating for MRSA, gram‐negative, or anaerobic organisms citing lack of benefit as well as risks of antibiotic resistance and C difficile infection. A recent study from the University of Utah reported the development of a cellulitis order set, which included a pathway for nonpurulent cellulitis based on the use of cefazolin. These authors reported that the use of the pathway was associated with a 59% decrease in the use of broad‐spectrum antibiotics, a 23% decrease in pharmacy costs, a 13% decrease in total facility cost, with no change in hospital length of stay or readmission rate.[14] One important caveat to the use of clinical pathways is that they are often underused. In the study from the University of Utah, for example, only 55% of eligible patients had the clinical pathway ordered.
WHEN BROAD‐SPECTRUM ANTIBIOTICS ARE RECOMMENDED
The IDSA does recommend empiric broad‐spectrum antibiotics with combination gram‐positive and gram‐negative coverage in several situations, including severe infections in which necrotizing soft tissue infection is suspected, animal bites, immersion injuries, as well as for severely immunocompromised patients or those who have failed limited spectrum antibiotics. Additionally, the IDSA recommends antimicrobials effective against MRSA for purulent infections with systemic signs of inflammation as well as severe nonpurulent infections or those associated with penetrating trauma, injection drug use, and nasal colonization with MRSA (Figure 1).
RECOMMENDATIONS
Our patient has no associated purulence and no abscess and therefore has nonpurulent cellulitis. Based on his mild tachycardia and leukocytosis but intact immune system and lack of suspicion for necrotizing soft tissue infection, he would be classified as moderate‐severity cellulitis by the IDSA. In patients hospitalized with nonpurulent cellulitis who are not severely immunocompromised or severely ill and for whom necrotizing soft tissue infection is not suspected:
- Antibiotics should be directed at ‐hemolytic streptococci and MSSA, with 1 of the suggested antibiotics by the IDSA including penicillin, ceftriaxone, cefazolin, or clindamycin.
- Antibiotics effective against MRSA should be limited to situations described by the IDSA.
- If the cellulitis has not improved within 48 hours, then consider broader‐spectrum antibiotics.
- Hospitals should strongly consider implementation of a cellulitis pathway based on the IDSA recommendations to improve antibiotic stewardship as well as costs.
Disclosure
Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
- , , . Most frequent conditions in U.S. hospitals, 2011. HCUP statistical brief #162. Healthcare Cost and Utilization Project statistical briefs. Rockville, MD: Agency for Health Care Policy and Research; 2013.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–e52.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft‐tissue infections. Clin Infect Dis. 2005;41(10):1373–1406.
- , , , , , . Skin and soft‐tissue infections requiring hospitalization at an academic medical center: opportunities for antimicrobial stewardship. Clin Infect Dis. 2010;51(8):895–903.
- , , , , , . A prospective, multicenter, observational study of complicated skin and soft tissue infections in hospitalized patients: clinical characteristics, medical treatment, and outcomes. BMC Infect Dis. 2012;12:227.
- Centers for Disease Control and Prevention. Overview and evidence to support stewardship. Available at: http://www.cdc.gov/getsmart/healthcare/evidence.html. Accessed March 2, 2016.
- . Cellulitis, by any other name. Clin Infect Dis. 2013;56(12):1763–1764.
- , , , et al. Methicillin‐resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666–674.
- , , , , , . Emergence of community‐acquired methicillin‐resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft‐tissue infections. Ann Intern Med. 2006;144(5):309–317.
- , . Staphylococcus aureus is the most common identified cause of cellulitis: a systematic review. Epidemiol Infect. 2010;138(3):313–317.
- , . A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012;64(2):148–155.
- , , , . The role of beta‐hemolytic streptococci in causing diffuse, nonculturable cellulitis: a prospective investigation. Medicine (Baltimore). 2010;89(4):217–226.
- , , , et al. Clinical trial: comparative effectiveness of cephalexin plus trimethoprim‐sulfamethoxazole versus cephalexin alone for treatment of uncomplicated cellulitis: a randomized controlled trial. Clin Infect Dis. 2013;56(12):1754–1762.
- , , , , . Evidence‐based care pathway for cellulitis improves process, clinical, and cost outcomes. J Hosp Med. 2015;10:780–786.
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 65‐year‐old immunocompetent man with a history of obesity, diabetes, and chronic lower extremity edema presents to the emergency room with a 1‐day history of right lower extremity pain and increased swelling. He reports no antecedent trauma and states he just noticed the symptoms that morning. On examination, he appears generally well. His temperature is 100F, pulse 92 beats per minute, blood pressure 120/60 mm Hg, and respiratory rate 16 breaths per minute. The rest of the exam is notable for right lower extremity erythema and swelling extending from his right shin to his right medial thigh without associated fluctuance or drainage. Labs reveal a mildly elevated white blood cell count of 13,000/L and normal serum creatinine. Are broad‐spectrum antibiotics like vancomycin and piperacillin/tazobactam the preferred regimen?
BACKGROUND
The term skin and soft tissue infection (SSTI) includes a heterogeneous group of infections including cellulitis, cutaneous abscess, diabetic foot infections, surgical site infections, and necrotizing soft tissue infections. As a group, SSTIs are the second most common type of infection in hospitalized adults in the United States behind pneumonia and result in more than 600,000 admissions per year.[1] The current guideline on SSTIs by the Infectious Disease Society of America (IDSA) makes the distinction between purulent and nonpurulent soft tissue infections based on the presence or absence of purulent drainage or abscess and between mild, moderate, and severe infections based on the presence and severity of systemic signs of infection.[2] Figure 1 provides an overview of the IDSA recommendations.

THE PROBLEM: OVERUSE OF BROAD‐SPECTRUM ANTIBIOTICS
Studies over the past decade have shown that the majority of patients hospitalized with SSTI receive broad‐spectrum antibiotics, usually with combinations of antibiotics active against gram‐positive (including methicillin‐resistant Staphylococcus aureus [MRSA]), gram‐negative (often including Pseudomonas aeruginosa), and anaerobic organisms. Broad‐spectrum treatment occurs despite guidelines from the IDSA, which state that the most common pathogens for nonpurulent cellulitis are ‐hemolytic streptococci, which remain susceptible to penicillin.[2, 3] One multicenter study of hospitalized adults with nonpurulent cellulitis, for example, reported that 85% of patients received therapy effective against MRSA (primarily vancomycin), 61% received broad gram‐negative coverage (primarily ‐lactam with ‐lactamase inhibitor), and 74% received anaerobic coverage.[4] Another multicenter study reported that the most common antibiotics given for cellulitis (excluding cases associated with cutaneous abscess) were vancomycin (60%), ‐lactam/‐lactamase combinations (32%), and clindamycin (19%). Only 13% of patients with cellulitis were treated with cefazolin, and only 1.1% of patients were treated with nafcillin or oxacillin.[5] According to the Centers for Disease Control and Prevention, unnecessary antibiotic use is associated with increased cost, development of antibiotic resistance, and increased rates of Clostridium difficile.[6]
The current use of broad‐spectrum antibiotics for nonpurulent cellulitis is likely due to several factors, including the emergence of community‐associated (CA)‐MRSA, confusion due to the heterogeneity of SSTI, and the limited data regarding the microbiology of nonpurulent cellulitis. The resulting uncertainty about cellulitis has been termed an existential crisis for the treating physician and is likely the single biggest factor behind the out‐of‐control prescribing.[7]
The Emergence of CA‐MRSA
Over the past decade, numerous studies have reported the increasing frequency of CA‐MRSA soft tissue infections, predominantly with the pulsed‐field gel electrophoresis type USA‐300. Originally, MRSA infections were limited to nosocomial infections. Subsequent multicenter studies from the United States have shown that CA‐MRSA is the most frequent pathogen isolated from purulent soft tissue infections presenting to emergency rooms[8] and the most frequent pathogen isolated from SSTI specimens in labs.[9] Many authors have therefore concluded that empiric antibiotics for SSTI should include coverage for MRSA.[8, 9]
Heterogeneity of SSTI
As already discussed, the term SSTI is an umbrella term that encompasses several types of clinically distinct infections. The only commonality between the SSTI is that that they all involve the skin and soft tissues in some way. Diabetic foot infections, cutaneous abscesses, surgical site infections, and nonpurulent cellulitis have different hosts, pathophysiology, clinical presentations, and microbiology. At one end of the spectrum is the cutaneous abscess, which is readily culturable through incision and drainage. At the other end of the spectrum is cellulitis, which is typically nonculturable. Unfortunately, studies of SSTI tend to lump all of these entities together when reporting microbiology. The landmark study by Moran et al., for example, described the microbiology of purulent soft tissue infections presenting to a network of emergency rooms across the county. Although all patients had by definition purulent infections, and 81% were abscesses, the authors made broad conclusions about skin and soft tissue infections in general and recommended antimicrobials effective against MRSA for empiric coverage for SSTIs.[8]
Uncertainty About the Microbiology of Nonpurulent Cellulitis
What then is the microbiology of nonpurulent cellulitis? As stated in the 2005 and 2014 IDSA guidelines, traditional teaching remains that nonpurulent cellulitis is primarily due to ‐hemolytic streptococci.[2, 3] Studies using needle aspiration have yielded conflicting results, although a systematic review of these studies concluded that S aureus was the most common pathogen.[10] On the other hand, a systematic review of positive blood cultures of patients identified as having cellulitis found that 61% were due to ‐hemolytic streptococci, and only 15% were due to S aureus.[11] Both reviews, however, comment on the limited quality of the included studies. Ultimately, because nonpurulent soft tissue infections are basically nonculturable, their true microbiologic etiology remains uncertain. Given this uncertainty, as well as the impressive evidence for CA‐MRSA causing cutaneous abscesses, along with the confusion about types of SSTI, it is not surprising that front‐line clinicians have resorted to prescribing broad‐spectrum antibiotics.
THE SOLUTION: NARROW‐SPECTRUM ANTIBIOTICS FOR MOST
Although studies of the microbiology of cellulitis remain inconclusive, several recent clinical trials have indicated that treatment with antimicrobials limited to ‐hemolytic streptococci and methicillin‐susceptible S aureus (MSSA) are as effective as antimicrobials against MRSA. A prospective study from 2010 of consecutive hospitalized adults with nonpurulent cellulitis found that 73% had serologic evidence for streptococcal infection, and overall 95.8% responded to cefazolin monotherapy.[12] More recently, a study of emergency room patients with nonpurulent cellulitis randomized patients to cephalexin alone or cephalexin plus trimethoprim‐sulfamethoxazole. These authors found no difference in response rates and concluded that the addition of anti‐MRSA therapy (trimethoprim‐sulfamethoxazole, in this study) for uncomplicated cellulitis was unnecessary.[13] This later study is the only randomized controlled study to assess the need for MRSA coverage for cellulitis, and the answer for outpatients, at least, is that MRSA coverage is unnecessary. Both of these studies are cited by the IDSA guideline from 2014, which recommends antibiotics for mild‐moderate cellulitis to be limited to antimicrobials effective against ‐hemolytic streptococci and MSSA. The guideline specifically does not recommend routinely treating for MRSA, gram‐negative, or anaerobic organisms citing lack of benefit as well as risks of antibiotic resistance and C difficile infection. A recent study from the University of Utah reported the development of a cellulitis order set, which included a pathway for nonpurulent cellulitis based on the use of cefazolin. These authors reported that the use of the pathway was associated with a 59% decrease in the use of broad‐spectrum antibiotics, a 23% decrease in pharmacy costs, a 13% decrease in total facility cost, with no change in hospital length of stay or readmission rate.[14] One important caveat to the use of clinical pathways is that they are often underused. In the study from the University of Utah, for example, only 55% of eligible patients had the clinical pathway ordered.
WHEN BROAD‐SPECTRUM ANTIBIOTICS ARE RECOMMENDED
The IDSA does recommend empiric broad‐spectrum antibiotics with combination gram‐positive and gram‐negative coverage in several situations, including severe infections in which necrotizing soft tissue infection is suspected, animal bites, immersion injuries, as well as for severely immunocompromised patients or those who have failed limited spectrum antibiotics. Additionally, the IDSA recommends antimicrobials effective against MRSA for purulent infections with systemic signs of inflammation as well as severe nonpurulent infections or those associated with penetrating trauma, injection drug use, and nasal colonization with MRSA (Figure 1).
RECOMMENDATIONS
Our patient has no associated purulence and no abscess and therefore has nonpurulent cellulitis. Based on his mild tachycardia and leukocytosis but intact immune system and lack of suspicion for necrotizing soft tissue infection, he would be classified as moderate‐severity cellulitis by the IDSA. In patients hospitalized with nonpurulent cellulitis who are not severely immunocompromised or severely ill and for whom necrotizing soft tissue infection is not suspected:
- Antibiotics should be directed at ‐hemolytic streptococci and MSSA, with 1 of the suggested antibiotics by the IDSA including penicillin, ceftriaxone, cefazolin, or clindamycin.
- Antibiotics effective against MRSA should be limited to situations described by the IDSA.
- If the cellulitis has not improved within 48 hours, then consider broader‐spectrum antibiotics.
- Hospitals should strongly consider implementation of a cellulitis pathway based on the IDSA recommendations to improve antibiotic stewardship as well as costs.
Disclosure
Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A 65‐year‐old immunocompetent man with a history of obesity, diabetes, and chronic lower extremity edema presents to the emergency room with a 1‐day history of right lower extremity pain and increased swelling. He reports no antecedent trauma and states he just noticed the symptoms that morning. On examination, he appears generally well. His temperature is 100F, pulse 92 beats per minute, blood pressure 120/60 mm Hg, and respiratory rate 16 breaths per minute. The rest of the exam is notable for right lower extremity erythema and swelling extending from his right shin to his right medial thigh without associated fluctuance or drainage. Labs reveal a mildly elevated white blood cell count of 13,000/L and normal serum creatinine. Are broad‐spectrum antibiotics like vancomycin and piperacillin/tazobactam the preferred regimen?
BACKGROUND
The term skin and soft tissue infection (SSTI) includes a heterogeneous group of infections including cellulitis, cutaneous abscess, diabetic foot infections, surgical site infections, and necrotizing soft tissue infections. As a group, SSTIs are the second most common type of infection in hospitalized adults in the United States behind pneumonia and result in more than 600,000 admissions per year.[1] The current guideline on SSTIs by the Infectious Disease Society of America (IDSA) makes the distinction between purulent and nonpurulent soft tissue infections based on the presence or absence of purulent drainage or abscess and between mild, moderate, and severe infections based on the presence and severity of systemic signs of infection.[2] Figure 1 provides an overview of the IDSA recommendations.

THE PROBLEM: OVERUSE OF BROAD‐SPECTRUM ANTIBIOTICS
Studies over the past decade have shown that the majority of patients hospitalized with SSTI receive broad‐spectrum antibiotics, usually with combinations of antibiotics active against gram‐positive (including methicillin‐resistant Staphylococcus aureus [MRSA]), gram‐negative (often including Pseudomonas aeruginosa), and anaerobic organisms. Broad‐spectrum treatment occurs despite guidelines from the IDSA, which state that the most common pathogens for nonpurulent cellulitis are ‐hemolytic streptococci, which remain susceptible to penicillin.[2, 3] One multicenter study of hospitalized adults with nonpurulent cellulitis, for example, reported that 85% of patients received therapy effective against MRSA (primarily vancomycin), 61% received broad gram‐negative coverage (primarily ‐lactam with ‐lactamase inhibitor), and 74% received anaerobic coverage.[4] Another multicenter study reported that the most common antibiotics given for cellulitis (excluding cases associated with cutaneous abscess) were vancomycin (60%), ‐lactam/‐lactamase combinations (32%), and clindamycin (19%). Only 13% of patients with cellulitis were treated with cefazolin, and only 1.1% of patients were treated with nafcillin or oxacillin.[5] According to the Centers for Disease Control and Prevention, unnecessary antibiotic use is associated with increased cost, development of antibiotic resistance, and increased rates of Clostridium difficile.[6]
The current use of broad‐spectrum antibiotics for nonpurulent cellulitis is likely due to several factors, including the emergence of community‐associated (CA)‐MRSA, confusion due to the heterogeneity of SSTI, and the limited data regarding the microbiology of nonpurulent cellulitis. The resulting uncertainty about cellulitis has been termed an existential crisis for the treating physician and is likely the single biggest factor behind the out‐of‐control prescribing.[7]
The Emergence of CA‐MRSA
Over the past decade, numerous studies have reported the increasing frequency of CA‐MRSA soft tissue infections, predominantly with the pulsed‐field gel electrophoresis type USA‐300. Originally, MRSA infections were limited to nosocomial infections. Subsequent multicenter studies from the United States have shown that CA‐MRSA is the most frequent pathogen isolated from purulent soft tissue infections presenting to emergency rooms[8] and the most frequent pathogen isolated from SSTI specimens in labs.[9] Many authors have therefore concluded that empiric antibiotics for SSTI should include coverage for MRSA.[8, 9]
Heterogeneity of SSTI
As already discussed, the term SSTI is an umbrella term that encompasses several types of clinically distinct infections. The only commonality between the SSTI is that that they all involve the skin and soft tissues in some way. Diabetic foot infections, cutaneous abscesses, surgical site infections, and nonpurulent cellulitis have different hosts, pathophysiology, clinical presentations, and microbiology. At one end of the spectrum is the cutaneous abscess, which is readily culturable through incision and drainage. At the other end of the spectrum is cellulitis, which is typically nonculturable. Unfortunately, studies of SSTI tend to lump all of these entities together when reporting microbiology. The landmark study by Moran et al., for example, described the microbiology of purulent soft tissue infections presenting to a network of emergency rooms across the county. Although all patients had by definition purulent infections, and 81% were abscesses, the authors made broad conclusions about skin and soft tissue infections in general and recommended antimicrobials effective against MRSA for empiric coverage for SSTIs.[8]
Uncertainty About the Microbiology of Nonpurulent Cellulitis
What then is the microbiology of nonpurulent cellulitis? As stated in the 2005 and 2014 IDSA guidelines, traditional teaching remains that nonpurulent cellulitis is primarily due to ‐hemolytic streptococci.[2, 3] Studies using needle aspiration have yielded conflicting results, although a systematic review of these studies concluded that S aureus was the most common pathogen.[10] On the other hand, a systematic review of positive blood cultures of patients identified as having cellulitis found that 61% were due to ‐hemolytic streptococci, and only 15% were due to S aureus.[11] Both reviews, however, comment on the limited quality of the included studies. Ultimately, because nonpurulent soft tissue infections are basically nonculturable, their true microbiologic etiology remains uncertain. Given this uncertainty, as well as the impressive evidence for CA‐MRSA causing cutaneous abscesses, along with the confusion about types of SSTI, it is not surprising that front‐line clinicians have resorted to prescribing broad‐spectrum antibiotics.
THE SOLUTION: NARROW‐SPECTRUM ANTIBIOTICS FOR MOST
Although studies of the microbiology of cellulitis remain inconclusive, several recent clinical trials have indicated that treatment with antimicrobials limited to ‐hemolytic streptococci and methicillin‐susceptible S aureus (MSSA) are as effective as antimicrobials against MRSA. A prospective study from 2010 of consecutive hospitalized adults with nonpurulent cellulitis found that 73% had serologic evidence for streptococcal infection, and overall 95.8% responded to cefazolin monotherapy.[12] More recently, a study of emergency room patients with nonpurulent cellulitis randomized patients to cephalexin alone or cephalexin plus trimethoprim‐sulfamethoxazole. These authors found no difference in response rates and concluded that the addition of anti‐MRSA therapy (trimethoprim‐sulfamethoxazole, in this study) for uncomplicated cellulitis was unnecessary.[13] This later study is the only randomized controlled study to assess the need for MRSA coverage for cellulitis, and the answer for outpatients, at least, is that MRSA coverage is unnecessary. Both of these studies are cited by the IDSA guideline from 2014, which recommends antibiotics for mild‐moderate cellulitis to be limited to antimicrobials effective against ‐hemolytic streptococci and MSSA. The guideline specifically does not recommend routinely treating for MRSA, gram‐negative, or anaerobic organisms citing lack of benefit as well as risks of antibiotic resistance and C difficile infection. A recent study from the University of Utah reported the development of a cellulitis order set, which included a pathway for nonpurulent cellulitis based on the use of cefazolin. These authors reported that the use of the pathway was associated with a 59% decrease in the use of broad‐spectrum antibiotics, a 23% decrease in pharmacy costs, a 13% decrease in total facility cost, with no change in hospital length of stay or readmission rate.[14] One important caveat to the use of clinical pathways is that they are often underused. In the study from the University of Utah, for example, only 55% of eligible patients had the clinical pathway ordered.
WHEN BROAD‐SPECTRUM ANTIBIOTICS ARE RECOMMENDED
The IDSA does recommend empiric broad‐spectrum antibiotics with combination gram‐positive and gram‐negative coverage in several situations, including severe infections in which necrotizing soft tissue infection is suspected, animal bites, immersion injuries, as well as for severely immunocompromised patients or those who have failed limited spectrum antibiotics. Additionally, the IDSA recommends antimicrobials effective against MRSA for purulent infections with systemic signs of inflammation as well as severe nonpurulent infections or those associated with penetrating trauma, injection drug use, and nasal colonization with MRSA (Figure 1).
RECOMMENDATIONS
Our patient has no associated purulence and no abscess and therefore has nonpurulent cellulitis. Based on his mild tachycardia and leukocytosis but intact immune system and lack of suspicion for necrotizing soft tissue infection, he would be classified as moderate‐severity cellulitis by the IDSA. In patients hospitalized with nonpurulent cellulitis who are not severely immunocompromised or severely ill and for whom necrotizing soft tissue infection is not suspected:
- Antibiotics should be directed at ‐hemolytic streptococci and MSSA, with 1 of the suggested antibiotics by the IDSA including penicillin, ceftriaxone, cefazolin, or clindamycin.
- Antibiotics effective against MRSA should be limited to situations described by the IDSA.
- If the cellulitis has not improved within 48 hours, then consider broader‐spectrum antibiotics.
- Hospitals should strongly consider implementation of a cellulitis pathway based on the IDSA recommendations to improve antibiotic stewardship as well as costs.
Disclosure
Nothing to report.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing [email protected].
- , , . Most frequent conditions in U.S. hospitals, 2011. HCUP statistical brief #162. Healthcare Cost and Utilization Project statistical briefs. Rockville, MD: Agency for Health Care Policy and Research; 2013.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–e52.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft‐tissue infections. Clin Infect Dis. 2005;41(10):1373–1406.
- , , , , , . Skin and soft‐tissue infections requiring hospitalization at an academic medical center: opportunities for antimicrobial stewardship. Clin Infect Dis. 2010;51(8):895–903.
- , , , , , . A prospective, multicenter, observational study of complicated skin and soft tissue infections in hospitalized patients: clinical characteristics, medical treatment, and outcomes. BMC Infect Dis. 2012;12:227.
- Centers for Disease Control and Prevention. Overview and evidence to support stewardship. Available at: http://www.cdc.gov/getsmart/healthcare/evidence.html. Accessed March 2, 2016.
- . Cellulitis, by any other name. Clin Infect Dis. 2013;56(12):1763–1764.
- , , , et al. Methicillin‐resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666–674.
- , , , , , . Emergence of community‐acquired methicillin‐resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft‐tissue infections. Ann Intern Med. 2006;144(5):309–317.
- , . Staphylococcus aureus is the most common identified cause of cellulitis: a systematic review. Epidemiol Infect. 2010;138(3):313–317.
- , . A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012;64(2):148–155.
- , , , . The role of beta‐hemolytic streptococci in causing diffuse, nonculturable cellulitis: a prospective investigation. Medicine (Baltimore). 2010;89(4):217–226.
- , , , et al. Clinical trial: comparative effectiveness of cephalexin plus trimethoprim‐sulfamethoxazole versus cephalexin alone for treatment of uncomplicated cellulitis: a randomized controlled trial. Clin Infect Dis. 2013;56(12):1754–1762.
- , , , , . Evidence‐based care pathway for cellulitis improves process, clinical, and cost outcomes. J Hosp Med. 2015;10:780–786.
- , , . Most frequent conditions in U.S. hospitals, 2011. HCUP statistical brief #162. Healthcare Cost and Utilization Project statistical briefs. Rockville, MD: Agency for Health Care Policy and Research; 2013.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–e52.
- , , , et al. Practice guidelines for the diagnosis and management of skin and soft‐tissue infections. Clin Infect Dis. 2005;41(10):1373–1406.
- , , , , , . Skin and soft‐tissue infections requiring hospitalization at an academic medical center: opportunities for antimicrobial stewardship. Clin Infect Dis. 2010;51(8):895–903.
- , , , , , . A prospective, multicenter, observational study of complicated skin and soft tissue infections in hospitalized patients: clinical characteristics, medical treatment, and outcomes. BMC Infect Dis. 2012;12:227.
- Centers for Disease Control and Prevention. Overview and evidence to support stewardship. Available at: http://www.cdc.gov/getsmart/healthcare/evidence.html. Accessed March 2, 2016.
- . Cellulitis, by any other name. Clin Infect Dis. 2013;56(12):1763–1764.
- , , , et al. Methicillin‐resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666–674.
- , , , , , . Emergence of community‐acquired methicillin‐resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft‐tissue infections. Ann Intern Med. 2006;144(5):309–317.
- , . Staphylococcus aureus is the most common identified cause of cellulitis: a systematic review. Epidemiol Infect. 2010;138(3):313–317.
- , . A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012;64(2):148–155.
- , , , . The role of beta‐hemolytic streptococci in causing diffuse, nonculturable cellulitis: a prospective investigation. Medicine (Baltimore). 2010;89(4):217–226.
- , , , et al. Clinical trial: comparative effectiveness of cephalexin plus trimethoprim‐sulfamethoxazole versus cephalexin alone for treatment of uncomplicated cellulitis: a randomized controlled trial. Clin Infect Dis. 2013;56(12):1754–1762.
- , , , , . Evidence‐based care pathway for cellulitis improves process, clinical, and cost outcomes. J Hosp Med. 2015;10:780–786.
© 2016 Society of Hospital Medicine
Regular Moderate Exercise Throughout Pregnancy Not Associated with Increased Risk of Preterm Delivery
Study Overview
Objective. To evaluate if exercise during pregnancy has an effect on the risk of preterm birth.
Design. Systematic review and meta-analysis of randomized controlled trials.
Study selection. The authors followed the protocol for conducting meta-analyses recommended by the Cochrane Collaboration. MEDLINE, EMBASE, Web of Science, Scopus, ClinicalTrials.gov, OVID, and the Cochrane Library were searched from the inception of each database to April 2016. Selection criteria included randomized clinical trials that examined the effect of aerobic exercise on preterm birth. Keywords included exercise or physical activity and pregnancy and preterm birth or preterm delivery. Studies were included only if women were randomized to an aerobic exercise program prior to 23 weeks, participants had uncomplicated singleton pregnancies and no contraindication to exercise, and preterm birth was an outcome.
Nine studies met the inclusion criteria and were included in the meta-analysis. The quality of included studies was good overall, with most studies having low risk of selection or attrition bias and low or unclear risk of reporting bias. Most of the studies did not include blinding of participants and research personnel or of the outcome assessment. Sample sizes ranged from 14 to 697, with 2 studies with < 100 participants, 3 with 100 to 200 participants, and 3 with 290 to 687 participants. All of the women randomized to the experimental group began an exercise program by 22 weeks’ gestation. The types of physical activity used in the experimental group included strength and flexibility training, cycling, stretching, resistance, dance, joint mobilization, walking, and toning. Participants engaged in the activity for 35 to 90 minutes (mean, 57 minutes) 3 times a week in 8 studies and 4 times a week in 1 study. The intensity of the aerobic activities ranged from less than 60% to less than 80% of age-predicted maximum heart rate. Participants in 3 control groups were explicitly told not to engage in exercise while those in the others were neither encouraged or discouraged from doing so.
Main outcome measure. Incidence of preterm birth (birth prior to 37 weeks’ gestation).
Main results. A total of 2059 women were included in the meta-analysis, with 1022 in the exercise group and 1037 in the control group. The incidence of preterm birth was similar in the experimental and the control groups (4.5% vs 4.4% respectively, 95% confidence interval [CI], –0.07 to 0.17). The mean gestational age at delivery was also similar, with a mean difference of 0.05 (95% CI, –0.07 to 0.17). Women in the exercise group had a decreased risk of cesarean delivery (0.82%), with 17.9% having a cesarean delivery compared to 22% in the control group ( 95% CI, 0.69 to 0.97).
Conclusion. Exercise during pregnancy in women with singleton, uncomplicated pregnancy is not associated with increased risk of preterm delivery. Additionally, it is associated with a decreased risk of cesarean delivery.
Commentary
Preterm birth accounts for most perinatal deaths in the United States and places surviving infants at risk for serious short- and long-term health problems [1]. Though the rate of preterm births in the United States has been slowly declining in recent years, at 9.57% it continues to be one of the highest among high-income countries [2]. Determining factors that contribute to incidence of preterm birth is critical to reducing this unacceptably high rate. According to the authors of this meta-analysis, the role of exercise related to preterm birth remains controversial due to past beliefs that the increased release of catecholamines during exercise would stimulate myometrial activity and ongoing concerns about possible adverse effects. The health benefits of regular exercise are well-known, including in pregnancy where it has been shown to lower the risk of gestational diabetes and preeclampsia.
Researchers have investigated exercise during pregnancy in earlier reviews; however, this appears to be the first with both preterm birth as the primary outcome and an adequate number of clinical trials in the sample. Prior reviews that examined the effects of exercise on preterm birth, either specifically or as one of a number of pregnancy outcomes, included only 3 to 5 studies pertaining to preterm birth [3–5].
The strengths of this review were the low statistical heterogeneity and high quality of the included studies, lack of publication bias, and the large sample of 2059 participants. As noted by the authors, however, lack of stratification by body mass (underweight, overweight, obese), differences in the types and intensity of exercise among interventions, as well as possible differences in adherence may have affected outcomes. In addition, in 6 studies women in the control group were not specifically instructed to refrain from exercise and there is no information about their exercise habits. The risk of contamination bias exists because some of these women may have engaged in a regular program of exercise. However, considering that levels of regular exercise in pregnant women are low, it is unlikely that this would occur at a rate that would have a significant effect on the outcomes [6].
Applications for Clinical Practice
The results of this meta-analysis provide strong support for the American College of Obstetrics and Gynecology recommendation that women with uncomplicated pregnancies be encouraged to engage in moderate-intensity exercise 20 to 30 minutes per day during pregnancy [7]. Clinicians should advise all women with uncomplicated singleton pregnancies and no medical contraindications to engage in regular aerobic and strength-conditioning exercise throughout their pregnancy.
—Karen Roush, PhD, RN
1. March of Dimes. 2015 Premature birth report cards. Accessed at www.marchofdimes.org/mission/prematurity-reportcard.aspx.
2. CDC. FastStats: Birthweight and gestation. Accessed at www.cdc.gov/nchs/fastats/birthweight.htm.
3. Kramer MS, McDonald SW. Aerobic exercise for women during pregnancy. Cochrane Database Syst Rev 2006;(3):CD000180.
4. Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev 2015;(6):CD007145.
5. Thangaratinam S, Rogozinska E, Jolly K, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: Meta-analysis of randomized evidence. BMJ 2012 May 16;344:e2088.
6. Nascimento SL, Surita FG, Cecatti JG. Physical exercise during pregnancy: a systematic review. Curr Opin Obstet Gynecol 2012 Dec;24:387–94.
7. ACOG Committee Opinion No. 650: Physical activity and exercise during pregnancy and the postpartum period. Obstet Gynecol 2015;126:e135–42.
Study Overview
Objective. To evaluate if exercise during pregnancy has an effect on the risk of preterm birth.
Design. Systematic review and meta-analysis of randomized controlled trials.
Study selection. The authors followed the protocol for conducting meta-analyses recommended by the Cochrane Collaboration. MEDLINE, EMBASE, Web of Science, Scopus, ClinicalTrials.gov, OVID, and the Cochrane Library were searched from the inception of each database to April 2016. Selection criteria included randomized clinical trials that examined the effect of aerobic exercise on preterm birth. Keywords included exercise or physical activity and pregnancy and preterm birth or preterm delivery. Studies were included only if women were randomized to an aerobic exercise program prior to 23 weeks, participants had uncomplicated singleton pregnancies and no contraindication to exercise, and preterm birth was an outcome.
Nine studies met the inclusion criteria and were included in the meta-analysis. The quality of included studies was good overall, with most studies having low risk of selection or attrition bias and low or unclear risk of reporting bias. Most of the studies did not include blinding of participants and research personnel or of the outcome assessment. Sample sizes ranged from 14 to 697, with 2 studies with < 100 participants, 3 with 100 to 200 participants, and 3 with 290 to 687 participants. All of the women randomized to the experimental group began an exercise program by 22 weeks’ gestation. The types of physical activity used in the experimental group included strength and flexibility training, cycling, stretching, resistance, dance, joint mobilization, walking, and toning. Participants engaged in the activity for 35 to 90 minutes (mean, 57 minutes) 3 times a week in 8 studies and 4 times a week in 1 study. The intensity of the aerobic activities ranged from less than 60% to less than 80% of age-predicted maximum heart rate. Participants in 3 control groups were explicitly told not to engage in exercise while those in the others were neither encouraged or discouraged from doing so.
Main outcome measure. Incidence of preterm birth (birth prior to 37 weeks’ gestation).
Main results. A total of 2059 women were included in the meta-analysis, with 1022 in the exercise group and 1037 in the control group. The incidence of preterm birth was similar in the experimental and the control groups (4.5% vs 4.4% respectively, 95% confidence interval [CI], –0.07 to 0.17). The mean gestational age at delivery was also similar, with a mean difference of 0.05 (95% CI, –0.07 to 0.17). Women in the exercise group had a decreased risk of cesarean delivery (0.82%), with 17.9% having a cesarean delivery compared to 22% in the control group ( 95% CI, 0.69 to 0.97).
Conclusion. Exercise during pregnancy in women with singleton, uncomplicated pregnancy is not associated with increased risk of preterm delivery. Additionally, it is associated with a decreased risk of cesarean delivery.
Commentary
Preterm birth accounts for most perinatal deaths in the United States and places surviving infants at risk for serious short- and long-term health problems [1]. Though the rate of preterm births in the United States has been slowly declining in recent years, at 9.57% it continues to be one of the highest among high-income countries [2]. Determining factors that contribute to incidence of preterm birth is critical to reducing this unacceptably high rate. According to the authors of this meta-analysis, the role of exercise related to preterm birth remains controversial due to past beliefs that the increased release of catecholamines during exercise would stimulate myometrial activity and ongoing concerns about possible adverse effects. The health benefits of regular exercise are well-known, including in pregnancy where it has been shown to lower the risk of gestational diabetes and preeclampsia.
Researchers have investigated exercise during pregnancy in earlier reviews; however, this appears to be the first with both preterm birth as the primary outcome and an adequate number of clinical trials in the sample. Prior reviews that examined the effects of exercise on preterm birth, either specifically or as one of a number of pregnancy outcomes, included only 3 to 5 studies pertaining to preterm birth [3–5].
The strengths of this review were the low statistical heterogeneity and high quality of the included studies, lack of publication bias, and the large sample of 2059 participants. As noted by the authors, however, lack of stratification by body mass (underweight, overweight, obese), differences in the types and intensity of exercise among interventions, as well as possible differences in adherence may have affected outcomes. In addition, in 6 studies women in the control group were not specifically instructed to refrain from exercise and there is no information about their exercise habits. The risk of contamination bias exists because some of these women may have engaged in a regular program of exercise. However, considering that levels of regular exercise in pregnant women are low, it is unlikely that this would occur at a rate that would have a significant effect on the outcomes [6].
Applications for Clinical Practice
The results of this meta-analysis provide strong support for the American College of Obstetrics and Gynecology recommendation that women with uncomplicated pregnancies be encouraged to engage in moderate-intensity exercise 20 to 30 minutes per day during pregnancy [7]. Clinicians should advise all women with uncomplicated singleton pregnancies and no medical contraindications to engage in regular aerobic and strength-conditioning exercise throughout their pregnancy.
—Karen Roush, PhD, RN
Study Overview
Objective. To evaluate if exercise during pregnancy has an effect on the risk of preterm birth.
Design. Systematic review and meta-analysis of randomized controlled trials.
Study selection. The authors followed the protocol for conducting meta-analyses recommended by the Cochrane Collaboration. MEDLINE, EMBASE, Web of Science, Scopus, ClinicalTrials.gov, OVID, and the Cochrane Library were searched from the inception of each database to April 2016. Selection criteria included randomized clinical trials that examined the effect of aerobic exercise on preterm birth. Keywords included exercise or physical activity and pregnancy and preterm birth or preterm delivery. Studies were included only if women were randomized to an aerobic exercise program prior to 23 weeks, participants had uncomplicated singleton pregnancies and no contraindication to exercise, and preterm birth was an outcome.
Nine studies met the inclusion criteria and were included in the meta-analysis. The quality of included studies was good overall, with most studies having low risk of selection or attrition bias and low or unclear risk of reporting bias. Most of the studies did not include blinding of participants and research personnel or of the outcome assessment. Sample sizes ranged from 14 to 697, with 2 studies with < 100 participants, 3 with 100 to 200 participants, and 3 with 290 to 687 participants. All of the women randomized to the experimental group began an exercise program by 22 weeks’ gestation. The types of physical activity used in the experimental group included strength and flexibility training, cycling, stretching, resistance, dance, joint mobilization, walking, and toning. Participants engaged in the activity for 35 to 90 minutes (mean, 57 minutes) 3 times a week in 8 studies and 4 times a week in 1 study. The intensity of the aerobic activities ranged from less than 60% to less than 80% of age-predicted maximum heart rate. Participants in 3 control groups were explicitly told not to engage in exercise while those in the others were neither encouraged or discouraged from doing so.
Main outcome measure. Incidence of preterm birth (birth prior to 37 weeks’ gestation).
Main results. A total of 2059 women were included in the meta-analysis, with 1022 in the exercise group and 1037 in the control group. The incidence of preterm birth was similar in the experimental and the control groups (4.5% vs 4.4% respectively, 95% confidence interval [CI], –0.07 to 0.17). The mean gestational age at delivery was also similar, with a mean difference of 0.05 (95% CI, –0.07 to 0.17). Women in the exercise group had a decreased risk of cesarean delivery (0.82%), with 17.9% having a cesarean delivery compared to 22% in the control group ( 95% CI, 0.69 to 0.97).
Conclusion. Exercise during pregnancy in women with singleton, uncomplicated pregnancy is not associated with increased risk of preterm delivery. Additionally, it is associated with a decreased risk of cesarean delivery.
Commentary
Preterm birth accounts for most perinatal deaths in the United States and places surviving infants at risk for serious short- and long-term health problems [1]. Though the rate of preterm births in the United States has been slowly declining in recent years, at 9.57% it continues to be one of the highest among high-income countries [2]. Determining factors that contribute to incidence of preterm birth is critical to reducing this unacceptably high rate. According to the authors of this meta-analysis, the role of exercise related to preterm birth remains controversial due to past beliefs that the increased release of catecholamines during exercise would stimulate myometrial activity and ongoing concerns about possible adverse effects. The health benefits of regular exercise are well-known, including in pregnancy where it has been shown to lower the risk of gestational diabetes and preeclampsia.
Researchers have investigated exercise during pregnancy in earlier reviews; however, this appears to be the first with both preterm birth as the primary outcome and an adequate number of clinical trials in the sample. Prior reviews that examined the effects of exercise on preterm birth, either specifically or as one of a number of pregnancy outcomes, included only 3 to 5 studies pertaining to preterm birth [3–5].
The strengths of this review were the low statistical heterogeneity and high quality of the included studies, lack of publication bias, and the large sample of 2059 participants. As noted by the authors, however, lack of stratification by body mass (underweight, overweight, obese), differences in the types and intensity of exercise among interventions, as well as possible differences in adherence may have affected outcomes. In addition, in 6 studies women in the control group were not specifically instructed to refrain from exercise and there is no information about their exercise habits. The risk of contamination bias exists because some of these women may have engaged in a regular program of exercise. However, considering that levels of regular exercise in pregnant women are low, it is unlikely that this would occur at a rate that would have a significant effect on the outcomes [6].
Applications for Clinical Practice
The results of this meta-analysis provide strong support for the American College of Obstetrics and Gynecology recommendation that women with uncomplicated pregnancies be encouraged to engage in moderate-intensity exercise 20 to 30 minutes per day during pregnancy [7]. Clinicians should advise all women with uncomplicated singleton pregnancies and no medical contraindications to engage in regular aerobic and strength-conditioning exercise throughout their pregnancy.
—Karen Roush, PhD, RN
1. March of Dimes. 2015 Premature birth report cards. Accessed at www.marchofdimes.org/mission/prematurity-reportcard.aspx.
2. CDC. FastStats: Birthweight and gestation. Accessed at www.cdc.gov/nchs/fastats/birthweight.htm.
3. Kramer MS, McDonald SW. Aerobic exercise for women during pregnancy. Cochrane Database Syst Rev 2006;(3):CD000180.
4. Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev 2015;(6):CD007145.
5. Thangaratinam S, Rogozinska E, Jolly K, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: Meta-analysis of randomized evidence. BMJ 2012 May 16;344:e2088.
6. Nascimento SL, Surita FG, Cecatti JG. Physical exercise during pregnancy: a systematic review. Curr Opin Obstet Gynecol 2012 Dec;24:387–94.
7. ACOG Committee Opinion No. 650: Physical activity and exercise during pregnancy and the postpartum period. Obstet Gynecol 2015;126:e135–42.
1. March of Dimes. 2015 Premature birth report cards. Accessed at www.marchofdimes.org/mission/prematurity-reportcard.aspx.
2. CDC. FastStats: Birthweight and gestation. Accessed at www.cdc.gov/nchs/fastats/birthweight.htm.
3. Kramer MS, McDonald SW. Aerobic exercise for women during pregnancy. Cochrane Database Syst Rev 2006;(3):CD000180.
4. Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev 2015;(6):CD007145.
5. Thangaratinam S, Rogozinska E, Jolly K, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: Meta-analysis of randomized evidence. BMJ 2012 May 16;344:e2088.
6. Nascimento SL, Surita FG, Cecatti JG. Physical exercise during pregnancy: a systematic review. Curr Opin Obstet Gynecol 2012 Dec;24:387–94.
7. ACOG Committee Opinion No. 650: Physical activity and exercise during pregnancy and the postpartum period. Obstet Gynecol 2015;126:e135–42.
Can Patient Navigators Increase Cancer Screening Rates in Primary Care Practice?
Study Overview
Objective. To evaluate patient navigation (PN) for breast, cervical, and colorectal cancer (CRC) screening using a population-based information technology (IT) system within a primary care network.
Design. Randomized clinical trial.
Setting and participants. Patients were from 18 primary care practices in the Massachusetts General Primary Care Practice-Based Research Network, which included 4 community health centers. The study used a population health IT application (TopCare [SRG Technology]) to identify patients overdue for breast, cervical and/or CRC screening. Women were deemed eligible and overdue for breast cancer [1] and cervical cancer [2] screening based on United States Preventive Services Task Force (USPSTF) recommendation statements. Patients aged 50 to 75 years without prior total colectomy were considered eligible for CRC screening and overdue if they did not have a colonoscopy in the past 10 years or sigmoidoscopy/barium enema/colonography in the past 5 years.
The study identified patients at high risk for non-adherence via a point system based on history of non-adherence to cancer screening tests, missed appointments, and primary language spoken (non-English speaking). A total of 1956 patients were identified, and after excluding those who were participating in an existing PN program, left the primary care network, died, or were lost to follow-up, the final study population consisted of 1612 patients overdue for at least 1 screening at the start of the study period.
Intervention: The intervention was a PN program comprising 4 part-time patient navigators with at least 2 years’ experience with cancer navigation and who worked 50% of their time in other PN programs. The navigators tracked intervention patients using the IT system, contacted them in their own language, and used extensive outreach efforts to assist them in completing their cancer screening. Most contact with patients took place via phone calls.
Main outcome measures. The primary outcome was the mean cancer screening test completion rate over the follow-up period for each eligible patient, with all eligible cancers combined in intention-to-treat analyses. Secondary outcomes included assessing the proportion of patients completing any and each cancer screening during follow-up among those who were eligible and overdue for at least 1 cancer screening at baseline in intention-to-treat analyses. Additionally, as-treated analyses were conducted, in which patients who left the network or died during follow-up were removed from the intervention and control groups and patients who could not be reached were removed from the intervention group.
Results. A total of 792 patients were randomized to theintervention group (PN) and 820 patients were randomized to usual care. The mean age in the intervention and control groups was 56.9 and 57.1 years, respectively. The intervention and control groups were well-matched in terms of sex, primary language, insurance, proportion of patients connected to a specific physician or seen in a community health center, number of clinic visits over the past 3 years, and risk for nonadherence. Among patients eligible and overdue for cancer screening, mean cancer screening completion rates were higher in the intervention group compared with the control group for all cancers combined (10.2% vs 6.8%; 95% CI [for the difference] 1.5% to 5.2%; P < 0.001) and for breast (14.7% vs 11.0%; 95% CI 0.2% to 7.3%; P = 0.04), cervical (11.1% vs 5.7%; 95% CI 0.8% to 5.2%; P = 0.002), and colon (7.6% vs 4.6%;95% CI 0.8% to 5.2%; P = 0.01) cancer. The secondary outcome, the proportion of overdue patients who completed any cancer screening during follow-up, was higher in the intervention group (25.5% vs 17.0%; 95% CI 4.7% to 12.7%; P < 0.001). More patients in the intervention group completed screening for breast (23.4% vs 16.6%; 95% CI 1.8% to 12.0%; P = 0.009), cervical (14.4% vs 8.6%; 95% CI 1.6% to 10.5%; P = 0.007), and colorectal (13.7% vs 7.0%; 95% CI 3.2% to 10.4%; P < 0.001) cancer. The effect size increased in the as-treated analyses.
Conclusion. PN, using a health IT application, improved cancer screening completion rates among patients at high risk for nonadherence over an 8-month period in an academic primary care network.
Commentary
The potential of PN to help individuals traverse the complexity of the current health care system continues to attract great interest as value-based care becomes a reality for physicians and health systems. Several studies have demonstrated PN to be an effective modality to improve adherence to recommended screenings [3–5]; however, issues surrounding cost, patient perception, and the “outsourcing” of care from the primary care physician to navigators require attention. At this time, the most robust aggregation of data demonstrating benefit outweighing harm for cancer screening is published by the USPSTF [6]. Breast cancer [7], cervical cancer [8], and CRC [9] have the greatest weight of evidence to support screening.
The study was conducted at a single academic medical center with established IT infrastructure and an established PN program, which limits application of the results to large networked organizations and/or private practice settings. One important limitation in the CRC screening component was the lack of alternatives to colonoscopy. Studies have demonstrated greater adherence to CRC screening with methods other than colonoscopy [10], especially among racial/ethnic minorities. Although the authors estimate the intervention cost approximately $100,000, the study does not include the cost of the population health IT solution. The costs associated with both the IT solution in addition to PN may ultimately outweigh the benefits. The short time frame of the study may also limit effect size and add to long-term cost considerations. Lastly, a high percentage of patients randomized to the intervention group were unable to be contacted, declined PN services, had competing comorbidities, or were screened elsewhere. On the other hand, the study has several strengths. Statistically, the study utilized intention-to-treat analyses, where estimate of treatment effect is generally conservative. As compared to the current literature, the authors evaluate 3 different types of cancer—a pragmatic approach from a clinician’s perspective. Additionally, the authors focused efforts on individuals at high risk for nonadherence, a strategy also practicable by clinicians. Another realistic element of the study is that patient navigators had other responsibilities, which implies applicability to resource-limited settings.
Applications for Clinical Practice
PN has been shown to be an effective means of improving population-based health outcomes, and this study demonstrates it improves cancer screening rates, assuming the appropriate IT infrastructure is in place. The costs and benefits of PN should be assessed when considering use of PN in nonadherent populations, and PN interventions should be tailored to available resources and the unique practice environment.
—Ajay Dharod, MD
1. Calonge N, Petitti DB, DeWitt TG, et al. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009;151:716–26.
2. Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012;156:880–91.
3. Phillips CE, Rothstein JD, Beaver K, et al. Patient navigation to increase mammography screening among inner city women. J Gen Intern Med 2011;26:123–9.
4. Jandorf L, Braschi C, Ernstoff E, et al. Culturally targeted patient navigation for increasing African Americans’ adherence to screening colonoscopy: a randomized clinical trial. Cancer Epidemiol Biomarkers Prev 2013;22:1577–87.
5. Braschi CD, Sly JR, Singh S, et al. Increasing colonoscopy screening for Latino Americans through a patient navigation model: a randomized clinical trial. J Immigr Minor Health 2014;16:934–40.
6. US Preventive Services Task Force. Published recommendations. 2016. Accessed 12 July 2016 at www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations.
7. US Preventive Services Task Force. Final recommendation statement: Breast cancer: Screening. 2016. Accessed 12 July 2016 at www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breast-cancer-screening1.
8. US Preventive Services Task Force. Final Recommendation Statement: Cervical cancer: Screening. 2016. Accessed 12 July 2016 at www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/cervical-cancer-screening.
9. US Preventive Services Task Force. Final Recommendation Statement: Colorectal cancer: Screening. 2016. Accessed 12 July 2016 at www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/colorectal-cancer-screening2.
10. Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med 2012;172:575–82.
Study Overview
Objective. To evaluate patient navigation (PN) for breast, cervical, and colorectal cancer (CRC) screening using a population-based information technology (IT) system within a primary care network.
Design. Randomized clinical trial.
Setting and participants. Patients were from 18 primary care practices in the Massachusetts General Primary Care Practice-Based Research Network, which included 4 community health centers. The study used a population health IT application (TopCare [SRG Technology]) to identify patients overdue for breast, cervical and/or CRC screening. Women were deemed eligible and overdue for breast cancer [1] and cervical cancer [2] screening based on United States Preventive Services Task Force (USPSTF) recommendation statements. Patients aged 50 to 75 years without prior total colectomy were considered eligible for CRC screening and overdue if they did not have a colonoscopy in the past 10 years or sigmoidoscopy/barium enema/colonography in the past 5 years.
The study identified patients at high risk for non-adherence via a point system based on history of non-adherence to cancer screening tests, missed appointments, and primary language spoken (non-English speaking). A total of 1956 patients were identified, and after excluding those who were participating in an existing PN program, left the primary care network, died, or were lost to follow-up, the final study population consisted of 1612 patients overdue for at least 1 screening at the start of the study period.
Intervention: The intervention was a PN program comprising 4 part-time patient navigators with at least 2 years’ experience with cancer navigation and who worked 50% of their time in other PN programs. The navigators tracked intervention patients using the IT system, contacted them in their own language, and used extensive outreach efforts to assist them in completing their cancer screening. Most contact with patients took place via phone calls.
Main outcome measures. The primary outcome was the mean cancer screening test completion rate over the follow-up period for each eligible patient, with all eligible cancers combined in intention-to-treat analyses. Secondary outcomes included assessing the proportion of patients completing any and each cancer screening during follow-up among those who were eligible and overdue for at least 1 cancer screening at baseline in intention-to-treat analyses. Additionally, as-treated analyses were conducted, in which patients who left the network or died during follow-up were removed from the intervention and control groups and patients who could not be reached were removed from the intervention group.
Results. A total of 792 patients were randomized to theintervention group (PN) and 820 patients were randomized to usual care. The mean age in the intervention and control groups was 56.9 and 57.1 years, respectively. The intervention and control groups were well-matched in terms of sex, primary language, insurance, proportion of patients connected to a specific physician or seen in a community health center, number of clinic visits over the past 3 years, and risk for nonadherence. Among patients eligible and overdue for cancer screening, mean cancer screening completion rates were higher in the intervention group compared with the control group for all cancers combined (10.2% vs 6.8%; 95% CI [for the difference] 1.5% to 5.2%; P < 0.001) and for breast (14.7% vs 11.0%; 95% CI 0.2% to 7.3%; P = 0.04), cervical (11.1% vs 5.7%; 95% CI 0.8% to 5.2%; P = 0.002), and colon (7.6% vs 4.6%;95% CI 0.8% to 5.2%; P = 0.01) cancer. The secondary outcome, the proportion of overdue patients who completed any cancer screening during follow-up, was higher in the intervention group (25.5% vs 17.0%; 95% CI 4.7% to 12.7%; P < 0.001). More patients in the intervention group completed screening for breast (23.4% vs 16.6%; 95% CI 1.8% to 12.0%; P = 0.009), cervical (14.4% vs 8.6%; 95% CI 1.6% to 10.5%; P = 0.007), and colorectal (13.7% vs 7.0%; 95% CI 3.2% to 10.4%; P < 0.001) cancer. The effect size increased in the as-treated analyses.
Conclusion. PN, using a health IT application, improved cancer screening completion rates among patients at high risk for nonadherence over an 8-month period in an academic primary care network.
Commentary
The potential of PN to help individuals traverse the complexity of the current health care system continues to attract great interest as value-based care becomes a reality for physicians and health systems. Several studies have demonstrated PN to be an effective modality to improve adherence to recommended screenings [3–5]; however, issues surrounding cost, patient perception, and the “outsourcing” of care from the primary care physician to navigators require attention. At this time, the most robust aggregation of data demonstrating benefit outweighing harm for cancer screening is published by the USPSTF [6]. Breast cancer [7], cervical cancer [8], and CRC [9] have the greatest weight of evidence to support screening.
The study was conducted at a single academic medical center with established IT infrastructure and an established PN program, which limits application of the results to large networked organizations and/or private practice settings. One important limitation in the CRC screening component was the lack of alternatives to colonoscopy. Studies have demonstrated greater adherence to CRC screening with methods other than colonoscopy [10], especially among racial/ethnic minorities. Although the authors estimate the intervention cost approximately $100,000, the study does not include the cost of the population health IT solution. The costs associated with both the IT solution in addition to PN may ultimately outweigh the benefits. The short time frame of the study may also limit effect size and add to long-term cost considerations. Lastly, a high percentage of patients randomized to the intervention group were unable to be contacted, declined PN services, had competing comorbidities, or were screened elsewhere. On the other hand, the study has several strengths. Statistically, the study utilized intention-to-treat analyses, where estimate of treatment effect is generally conservative. As compared to the current literature, the authors evaluate 3 different types of cancer—a pragmatic approach from a clinician’s perspective. Additionally, the authors focused efforts on individuals at high risk for nonadherence, a strategy also practicable by clinicians. Another realistic element of the study is that patient navigators had other responsibilities, which implies applicability to resource-limited settings.
Applications for Clinical Practice
PN has been shown to be an effective means of improving population-based health outcomes, and this study demonstrates it improves cancer screening rates, assuming the appropriate IT infrastructure is in place. The costs and benefits of PN should be assessed when considering use of PN in nonadherent populations, and PN interventions should be tailored to available resources and the unique practice environment.
—Ajay Dharod, MD
Study Overview
Objective. To evaluate patient navigation (PN) for breast, cervical, and colorectal cancer (CRC) screening using a population-based information technology (IT) system within a primary care network.
Design. Randomized clinical trial.
Setting and participants. Patients were from 18 primary care practices in the Massachusetts General Primary Care Practice-Based Research Network, which included 4 community health centers. The study used a population health IT application (TopCare [SRG Technology]) to identify patients overdue for breast, cervical and/or CRC screening. Women were deemed eligible and overdue for breast cancer [1] and cervical cancer [2] screening based on United States Preventive Services Task Force (USPSTF) recommendation statements. Patients aged 50 to 75 years without prior total colectomy were considered eligible for CRC screening and overdue if they did not have a colonoscopy in the past 10 years or sigmoidoscopy/barium enema/colonography in the past 5 years.
The study identified patients at high risk for non-adherence via a point system based on history of non-adherence to cancer screening tests, missed appointments, and primary language spoken (non-English speaking). A total of 1956 patients were identified, and after excluding those who were participating in an existing PN program, left the primary care network, died, or were lost to follow-up, the final study population consisted of 1612 patients overdue for at least 1 screening at the start of the study period.
Intervention: The intervention was a PN program comprising 4 part-time patient navigators with at least 2 years’ experience with cancer navigation and who worked 50% of their time in other PN programs. The navigators tracked intervention patients using the IT system, contacted them in their own language, and used extensive outreach efforts to assist them in completing their cancer screening. Most contact with patients took place via phone calls.
Main outcome measures. The primary outcome was the mean cancer screening test completion rate over the follow-up period for each eligible patient, with all eligible cancers combined in intention-to-treat analyses. Secondary outcomes included assessing the proportion of patients completing any and each cancer screening during follow-up among those who were eligible and overdue for at least 1 cancer screening at baseline in intention-to-treat analyses. Additionally, as-treated analyses were conducted, in which patients who left the network or died during follow-up were removed from the intervention and control groups and patients who could not be reached were removed from the intervention group.
Results. A total of 792 patients were randomized to theintervention group (PN) and 820 patients were randomized to usual care. The mean age in the intervention and control groups was 56.9 and 57.1 years, respectively. The intervention and control groups were well-matched in terms of sex, primary language, insurance, proportion of patients connected to a specific physician or seen in a community health center, number of clinic visits over the past 3 years, and risk for nonadherence. Among patients eligible and overdue for cancer screening, mean cancer screening completion rates were higher in the intervention group compared with the control group for all cancers combined (10.2% vs 6.8%; 95% CI [for the difference] 1.5% to 5.2%; P < 0.001) and for breast (14.7% vs 11.0%; 95% CI 0.2% to 7.3%; P = 0.04), cervical (11.1% vs 5.7%; 95% CI 0.8% to 5.2%; P = 0.002), and colon (7.6% vs 4.6%;95% CI 0.8% to 5.2%; P = 0.01) cancer. The secondary outcome, the proportion of overdue patients who completed any cancer screening during follow-up, was higher in the intervention group (25.5% vs 17.0%; 95% CI 4.7% to 12.7%; P < 0.001). More patients in the intervention group completed screening for breast (23.4% vs 16.6%; 95% CI 1.8% to 12.0%; P = 0.009), cervical (14.4% vs 8.6%; 95% CI 1.6% to 10.5%; P = 0.007), and colorectal (13.7% vs 7.0%; 95% CI 3.2% to 10.4%; P < 0.001) cancer. The effect size increased in the as-treated analyses.
Conclusion. PN, using a health IT application, improved cancer screening completion rates among patients at high risk for nonadherence over an 8-month period in an academic primary care network.
Commentary
The potential of PN to help individuals traverse the complexity of the current health care system continues to attract great interest as value-based care becomes a reality for physicians and health systems. Several studies have demonstrated PN to be an effective modality to improve adherence to recommended screenings [3–5]; however, issues surrounding cost, patient perception, and the “outsourcing” of care from the primary care physician to navigators require attention. At this time, the most robust aggregation of data demonstrating benefit outweighing harm for cancer screening is published by the USPSTF [6]. Breast cancer [7], cervical cancer [8], and CRC [9] have the greatest weight of evidence to support screening.
The study was conducted at a single academic medical center with established IT infrastructure and an established PN program, which limits application of the results to large networked organizations and/or private practice settings. One important limitation in the CRC screening component was the lack of alternatives to colonoscopy. Studies have demonstrated greater adherence to CRC screening with methods other than colonoscopy [10], especially among racial/ethnic minorities. Although the authors estimate the intervention cost approximately $100,000, the study does not include the cost of the population health IT solution. The costs associated with both the IT solution in addition to PN may ultimately outweigh the benefits. The short time frame of the study may also limit effect size and add to long-term cost considerations. Lastly, a high percentage of patients randomized to the intervention group were unable to be contacted, declined PN services, had competing comorbidities, or were screened elsewhere. On the other hand, the study has several strengths. Statistically, the study utilized intention-to-treat analyses, where estimate of treatment effect is generally conservative. As compared to the current literature, the authors evaluate 3 different types of cancer—a pragmatic approach from a clinician’s perspective. Additionally, the authors focused efforts on individuals at high risk for nonadherence, a strategy also practicable by clinicians. Another realistic element of the study is that patient navigators had other responsibilities, which implies applicability to resource-limited settings.
Applications for Clinical Practice
PN has been shown to be an effective means of improving population-based health outcomes, and this study demonstrates it improves cancer screening rates, assuming the appropriate IT infrastructure is in place. The costs and benefits of PN should be assessed when considering use of PN in nonadherent populations, and PN interventions should be tailored to available resources and the unique practice environment.
—Ajay Dharod, MD
1. Calonge N, Petitti DB, DeWitt TG, et al. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009;151:716–26.
2. Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012;156:880–91.
3. Phillips CE, Rothstein JD, Beaver K, et al. Patient navigation to increase mammography screening among inner city women. J Gen Intern Med 2011;26:123–9.
4. Jandorf L, Braschi C, Ernstoff E, et al. Culturally targeted patient navigation for increasing African Americans’ adherence to screening colonoscopy: a randomized clinical trial. Cancer Epidemiol Biomarkers Prev 2013;22:1577–87.
5. Braschi CD, Sly JR, Singh S, et al. Increasing colonoscopy screening for Latino Americans through a patient navigation model: a randomized clinical trial. J Immigr Minor Health 2014;16:934–40.
6. US Preventive Services Task Force. Published recommendations. 2016. Accessed 12 July 2016 at www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations.
7. US Preventive Services Task Force. Final recommendation statement: Breast cancer: Screening. 2016. Accessed 12 July 2016 at www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breast-cancer-screening1.
8. US Preventive Services Task Force. Final Recommendation Statement: Cervical cancer: Screening. 2016. Accessed 12 July 2016 at www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/cervical-cancer-screening.
9. US Preventive Services Task Force. Final Recommendation Statement: Colorectal cancer: Screening. 2016. Accessed 12 July 2016 at www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/colorectal-cancer-screening2.
10. Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med 2012;172:575–82.
1. Calonge N, Petitti DB, DeWitt TG, et al. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2009;151:716–26.
2. Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012;156:880–91.
3. Phillips CE, Rothstein JD, Beaver K, et al. Patient navigation to increase mammography screening among inner city women. J Gen Intern Med 2011;26:123–9.
4. Jandorf L, Braschi C, Ernstoff E, et al. Culturally targeted patient navigation for increasing African Americans’ adherence to screening colonoscopy: a randomized clinical trial. Cancer Epidemiol Biomarkers Prev 2013;22:1577–87.
5. Braschi CD, Sly JR, Singh S, et al. Increasing colonoscopy screening for Latino Americans through a patient navigation model: a randomized clinical trial. J Immigr Minor Health 2014;16:934–40.
6. US Preventive Services Task Force. Published recommendations. 2016. Accessed 12 July 2016 at www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations.
7. US Preventive Services Task Force. Final recommendation statement: Breast cancer: Screening. 2016. Accessed 12 July 2016 at www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breast-cancer-screening1.
8. US Preventive Services Task Force. Final Recommendation Statement: Cervical cancer: Screening. 2016. Accessed 12 July 2016 at www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/cervical-cancer-screening.
9. US Preventive Services Task Force. Final Recommendation Statement: Colorectal cancer: Screening. 2016. Accessed 12 July 2016 at www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/colorectal-cancer-screening2.
10. Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med 2012;172:575–82.