User login
Surgery may worsen pleural mesothelioma survival outcomes
Pleural mesothelioma is generally treated by extended pleurectomy decortication, and there has been little improvement in systemic treatment of early-stage, resectable mesothelioma, which has led to the recommendations of maximum cytoreduction. U.S. and European guidelines, as well as an international consensus statement, support this approach, but it has never been tested in a randomized, controlled trial.
Now it has, and the result is surprising: The conclusion was uncomfortable for Eric Lim, MD, who presented the results of the MARS2 trial at the annual World Conference on Lung Cancer. “Ladies and gentlemen, as a surgeon standing here, you have no idea how much it pains me to conclude that extended pleurectomy decortication, an operation that we have been offering for over 70 years, has been associated with a higher risk of death, more serious complications, poorer quality of life, and higher costs, compared to mesothelioma patients who were randomized to chemotherapy alone,” said Dr. Lim of the Royal Brompton Hospital, London, during his presentation.
Although the line drew laughter and applause from the audience, Paula Ugalde Figueroa, MD, who served as a discussant, raised some concerns about the study. Disease presence in one hemithorax was assessed only by chest CT scan, which is notorious for underestimating the volume of disease during surgery. There was also no information on pleural effusion or how many patients received it prior to intervention. Existing guidelines suggest staging of mesothelioma should also use PET scans, and invasive mediastinal staging should be assessed with endobronchial ultrasound. “None of these were performed during the trial,” said Dr. Figueroa, who is an associate thoracic surgeon at Brigham and Women’s Hospital, Boston. “At this point, my question is, are the arms of this study well balanced in regard to tumor volume? We don’t know,” she added.
Dr. Figueroa noted that the 90-day mortality seemed higher than that seen in other studies. “So, does the surgeon’s experience and center volume affect the outcome of this study?” she asked. Dr. Figueroa personally made phone calls to the participating centers and found that 45% of the patients in the trial were treated at low-volume centers, defined by her as two to three patients per year. “Should we assume that their surgical outcomes are similar between those centers? In this trial, with approximately half of patients from low-volume centers, extended pleurectomy decortication for mesothelioma had no significant difference when compared to those patients that underwent chemotherapy alone. Would the outcome be different in exclusively high-volume centers?” she concluded.
The study randomized 335 patients to receive surgery and chemotherapy, or chemotherapy alone. They received two cycles of platinum-based chemotherapy and pemetrexed prior to surgery and up to four cycles after surgery. The average age was 69 years; 86.9% were male, and 85.7% of tumors were epithelioid only. Among those in the surgery group, 88.5% underwent extended pleurectomy/decortication, 8.3% underwent pleurectomy decortication, 1.9% underwent partial pleurectomy, 0.6% exploration with no pleurodesis, and 0.6% were classified as “other” surgery. Completeness of resection was R0 in 3.2% of surgeries, R1 in 80.9%, and R2 in 15.9%. In-hospital mortality occurred in 3.8% of patients, and postsurgical 90-day mortality was 8.9%.
Over the first 42 months of follow-up, the hazard ratio for overall survival was 1.28 in the no-surgery group (P = .03). “The survival was so good in this early-stage cohort that we had to extend the trial by 6 months to get the prerequisite number of deaths, underscoring the phenomenal importance of having a randomized comparative cohort for all future studies on surgery for mesothelioma,” said Dr. Lim.
After 42 months, there was no survival difference between the two groups (hazard ratio, 0.48; P = .15). Dr. Lim attributed the change at 42 months to the fact that only 15 patients remained in each arm at that stage. There was no significant difference between the two groups with respect to progression-free survival.
The survival benefit of the no-surgery group was sustained after additional analyses, including adjustment of the number of first-line chemotherapy cycles and immunotherapy received after completion of the trial protocol.
Adverse events were more common in the surgery group (incidence rate ratio, 3.6; P < .001), including any cardiac disorder (IRR, 2.73; 95% confidence interval, 1.11-6.67); any infection or infestation (IRR, 1.99; 95% CI, 1.33-2.99); any respiratory, thoracic, or mediastinal disorder (IRR, 2.40; 95% CI, 1.52-3.80); and any surgical or medical procedure (IRR, 2.23; 95% CI, 1.04-4.78). The EORTC quality of life score favored the nonsurgery group at 6 weeks, but there was no significant difference at other time points.
Dr. Lim and Dr. Figueroa have no relevant financial disclosures.
Pleural mesothelioma is generally treated by extended pleurectomy decortication, and there has been little improvement in systemic treatment of early-stage, resectable mesothelioma, which has led to the recommendations of maximum cytoreduction. U.S. and European guidelines, as well as an international consensus statement, support this approach, but it has never been tested in a randomized, controlled trial.
Now it has, and the result is surprising: The conclusion was uncomfortable for Eric Lim, MD, who presented the results of the MARS2 trial at the annual World Conference on Lung Cancer. “Ladies and gentlemen, as a surgeon standing here, you have no idea how much it pains me to conclude that extended pleurectomy decortication, an operation that we have been offering for over 70 years, has been associated with a higher risk of death, more serious complications, poorer quality of life, and higher costs, compared to mesothelioma patients who were randomized to chemotherapy alone,” said Dr. Lim of the Royal Brompton Hospital, London, during his presentation.
Although the line drew laughter and applause from the audience, Paula Ugalde Figueroa, MD, who served as a discussant, raised some concerns about the study. Disease presence in one hemithorax was assessed only by chest CT scan, which is notorious for underestimating the volume of disease during surgery. There was also no information on pleural effusion or how many patients received it prior to intervention. Existing guidelines suggest staging of mesothelioma should also use PET scans, and invasive mediastinal staging should be assessed with endobronchial ultrasound. “None of these were performed during the trial,” said Dr. Figueroa, who is an associate thoracic surgeon at Brigham and Women’s Hospital, Boston. “At this point, my question is, are the arms of this study well balanced in regard to tumor volume? We don’t know,” she added.
Dr. Figueroa noted that the 90-day mortality seemed higher than that seen in other studies. “So, does the surgeon’s experience and center volume affect the outcome of this study?” she asked. Dr. Figueroa personally made phone calls to the participating centers and found that 45% of the patients in the trial were treated at low-volume centers, defined by her as two to three patients per year. “Should we assume that their surgical outcomes are similar between those centers? In this trial, with approximately half of patients from low-volume centers, extended pleurectomy decortication for mesothelioma had no significant difference when compared to those patients that underwent chemotherapy alone. Would the outcome be different in exclusively high-volume centers?” she concluded.
The study randomized 335 patients to receive surgery and chemotherapy, or chemotherapy alone. They received two cycles of platinum-based chemotherapy and pemetrexed prior to surgery and up to four cycles after surgery. The average age was 69 years; 86.9% were male, and 85.7% of tumors were epithelioid only. Among those in the surgery group, 88.5% underwent extended pleurectomy/decortication, 8.3% underwent pleurectomy decortication, 1.9% underwent partial pleurectomy, 0.6% exploration with no pleurodesis, and 0.6% were classified as “other” surgery. Completeness of resection was R0 in 3.2% of surgeries, R1 in 80.9%, and R2 in 15.9%. In-hospital mortality occurred in 3.8% of patients, and postsurgical 90-day mortality was 8.9%.
Over the first 42 months of follow-up, the hazard ratio for overall survival was 1.28 in the no-surgery group (P = .03). “The survival was so good in this early-stage cohort that we had to extend the trial by 6 months to get the prerequisite number of deaths, underscoring the phenomenal importance of having a randomized comparative cohort for all future studies on surgery for mesothelioma,” said Dr. Lim.
After 42 months, there was no survival difference between the two groups (hazard ratio, 0.48; P = .15). Dr. Lim attributed the change at 42 months to the fact that only 15 patients remained in each arm at that stage. There was no significant difference between the two groups with respect to progression-free survival.
The survival benefit of the no-surgery group was sustained after additional analyses, including adjustment of the number of first-line chemotherapy cycles and immunotherapy received after completion of the trial protocol.
Adverse events were more common in the surgery group (incidence rate ratio, 3.6; P < .001), including any cardiac disorder (IRR, 2.73; 95% confidence interval, 1.11-6.67); any infection or infestation (IRR, 1.99; 95% CI, 1.33-2.99); any respiratory, thoracic, or mediastinal disorder (IRR, 2.40; 95% CI, 1.52-3.80); and any surgical or medical procedure (IRR, 2.23; 95% CI, 1.04-4.78). The EORTC quality of life score favored the nonsurgery group at 6 weeks, but there was no significant difference at other time points.
Dr. Lim and Dr. Figueroa have no relevant financial disclosures.
Pleural mesothelioma is generally treated by extended pleurectomy decortication, and there has been little improvement in systemic treatment of early-stage, resectable mesothelioma, which has led to the recommendations of maximum cytoreduction. U.S. and European guidelines, as well as an international consensus statement, support this approach, but it has never been tested in a randomized, controlled trial.
Now it has, and the result is surprising: The conclusion was uncomfortable for Eric Lim, MD, who presented the results of the MARS2 trial at the annual World Conference on Lung Cancer. “Ladies and gentlemen, as a surgeon standing here, you have no idea how much it pains me to conclude that extended pleurectomy decortication, an operation that we have been offering for over 70 years, has been associated with a higher risk of death, more serious complications, poorer quality of life, and higher costs, compared to mesothelioma patients who were randomized to chemotherapy alone,” said Dr. Lim of the Royal Brompton Hospital, London, during his presentation.
Although the line drew laughter and applause from the audience, Paula Ugalde Figueroa, MD, who served as a discussant, raised some concerns about the study. Disease presence in one hemithorax was assessed only by chest CT scan, which is notorious for underestimating the volume of disease during surgery. There was also no information on pleural effusion or how many patients received it prior to intervention. Existing guidelines suggest staging of mesothelioma should also use PET scans, and invasive mediastinal staging should be assessed with endobronchial ultrasound. “None of these were performed during the trial,” said Dr. Figueroa, who is an associate thoracic surgeon at Brigham and Women’s Hospital, Boston. “At this point, my question is, are the arms of this study well balanced in regard to tumor volume? We don’t know,” she added.
Dr. Figueroa noted that the 90-day mortality seemed higher than that seen in other studies. “So, does the surgeon’s experience and center volume affect the outcome of this study?” she asked. Dr. Figueroa personally made phone calls to the participating centers and found that 45% of the patients in the trial were treated at low-volume centers, defined by her as two to three patients per year. “Should we assume that their surgical outcomes are similar between those centers? In this trial, with approximately half of patients from low-volume centers, extended pleurectomy decortication for mesothelioma had no significant difference when compared to those patients that underwent chemotherapy alone. Would the outcome be different in exclusively high-volume centers?” she concluded.
The study randomized 335 patients to receive surgery and chemotherapy, or chemotherapy alone. They received two cycles of platinum-based chemotherapy and pemetrexed prior to surgery and up to four cycles after surgery. The average age was 69 years; 86.9% were male, and 85.7% of tumors were epithelioid only. Among those in the surgery group, 88.5% underwent extended pleurectomy/decortication, 8.3% underwent pleurectomy decortication, 1.9% underwent partial pleurectomy, 0.6% exploration with no pleurodesis, and 0.6% were classified as “other” surgery. Completeness of resection was R0 in 3.2% of surgeries, R1 in 80.9%, and R2 in 15.9%. In-hospital mortality occurred in 3.8% of patients, and postsurgical 90-day mortality was 8.9%.
Over the first 42 months of follow-up, the hazard ratio for overall survival was 1.28 in the no-surgery group (P = .03). “The survival was so good in this early-stage cohort that we had to extend the trial by 6 months to get the prerequisite number of deaths, underscoring the phenomenal importance of having a randomized comparative cohort for all future studies on surgery for mesothelioma,” said Dr. Lim.
After 42 months, there was no survival difference between the two groups (hazard ratio, 0.48; P = .15). Dr. Lim attributed the change at 42 months to the fact that only 15 patients remained in each arm at that stage. There was no significant difference between the two groups with respect to progression-free survival.
The survival benefit of the no-surgery group was sustained after additional analyses, including adjustment of the number of first-line chemotherapy cycles and immunotherapy received after completion of the trial protocol.
Adverse events were more common in the surgery group (incidence rate ratio, 3.6; P < .001), including any cardiac disorder (IRR, 2.73; 95% confidence interval, 1.11-6.67); any infection or infestation (IRR, 1.99; 95% CI, 1.33-2.99); any respiratory, thoracic, or mediastinal disorder (IRR, 2.40; 95% CI, 1.52-3.80); and any surgical or medical procedure (IRR, 2.23; 95% CI, 1.04-4.78). The EORTC quality of life score favored the nonsurgery group at 6 weeks, but there was no significant difference at other time points.
Dr. Lim and Dr. Figueroa have no relevant financial disclosures.
FROM WCLC 2023
Heart attack deaths static in those with type 1 diabetes
, new research shows.
Between 2006 and 2020, the annual incidences of overall mortality and major adverse cardiovascular events after a first-time myocardial infarction dropped significantly for people with type 2 diabetes and those without diabetes (controls).
However, the same trend was not seen for people with type 1 diabetes.
“There is an urgent need for further studies understanding cardiovascular disease in people with type 1 diabetes. Clinicians have to be aware of the absence of the declined mortality trend in people with type 1 diabetes having a first-time myocardial infarction,” lead author Thomas Nyström, MD, professor of medicine at the Karolinska Institute, Stockholm, said in an interview.
The findings are scheduled to be presented Oct. 5, 2023, at the annual meeting of the European Association for the Study of Diabetes.
Discussing potential reasons for the findings, the authors say that the standard care after a heart attack has improved with more availability of, for example, percutaneous coronary intervention and better overall medical treatment. However, this standard of care should have improved in all three groups.
“Although glycemic control and diabetes duration were much different between diabetes groups, in that those with type 1 had been exposed for a longer period of glycemia, the current study cannot tell whether glucose control is behind the association between mortality trends observed. Whether this is the case must be investigated with further studies,” Nyström said.
Data from Swedish health care registry
Among people with a first-time MI recorded in national Swedish health care registries between 2006 and 2020, there were 2,527 individuals with type 1 diabetes, 48,321 with type 2 diabetes, and 243,170 controls with neither form of diabetes.
Those with type 1 diabetes were younger than those with type 2 diabetes and controls (62 years vs. 75 and 73 years, respectively). The type 1 diabetes group also had a higher proportion of females (43.6% vs. 38.1% of both the type 2 diabetes and control groups).
The proportions of people with the most severe type of heart attack, ST-elevation MI (STEMI), versus non-STEMI were 29% versus 71% in the type 1 diabetes group, 30% versus 70% in the type 2 diabetes group, and 39% versus 61% in the control group, respectively.
After adjustment for covariates including age, sex, comorbidities, socioeconomic factors, and medication, there was a significant decreased annual incidence trend for all-cause death among the controls (–1.9%) and persons with type 2 diabetes (–1.3%), but there was no such decrease among those with type 1 diabetes.
For cardiovascular deaths, the annual incidence declines were –2.0% and –1.6% in the control group and the type 2 diabetes group, respectively, versus a nonsignificant –0.5% decline in the type 1 diabetes group. Similarly, for major adverse cardiovascular events, those decreases were –2.0% for controls and –1.6% for those with type 2 diabetes, but –0.6% for those with type 1 diabetes – again, a nonsignificant value.
“During the last 15 years, the risk of death and major cardiovascular events in people without diabetes and with type 2 diabetes after having a first-time heart attack has decreased significantly. In contrast, this decreasing trend was absent in people with type 1 diabetes. Our study highlights the urgent need for understanding the cardiovascular risk in people with type 1 diabetes,” the authors conclude.
Dr. Nyström has received honoraria from AstraZeneca, Merck Sharp & Dohme, Novo Nordisk, Eli Lilly , Boehringer Ingelheim, Abbott, and Amgen. The authors acknowledge the ALF agreement between Stockholm County Council and Karolinska Institutet.
A version of this article appeared on Medscape.com.
, new research shows.
Between 2006 and 2020, the annual incidences of overall mortality and major adverse cardiovascular events after a first-time myocardial infarction dropped significantly for people with type 2 diabetes and those without diabetes (controls).
However, the same trend was not seen for people with type 1 diabetes.
“There is an urgent need for further studies understanding cardiovascular disease in people with type 1 diabetes. Clinicians have to be aware of the absence of the declined mortality trend in people with type 1 diabetes having a first-time myocardial infarction,” lead author Thomas Nyström, MD, professor of medicine at the Karolinska Institute, Stockholm, said in an interview.
The findings are scheduled to be presented Oct. 5, 2023, at the annual meeting of the European Association for the Study of Diabetes.
Discussing potential reasons for the findings, the authors say that the standard care after a heart attack has improved with more availability of, for example, percutaneous coronary intervention and better overall medical treatment. However, this standard of care should have improved in all three groups.
“Although glycemic control and diabetes duration were much different between diabetes groups, in that those with type 1 had been exposed for a longer period of glycemia, the current study cannot tell whether glucose control is behind the association between mortality trends observed. Whether this is the case must be investigated with further studies,” Nyström said.
Data from Swedish health care registry
Among people with a first-time MI recorded in national Swedish health care registries between 2006 and 2020, there were 2,527 individuals with type 1 diabetes, 48,321 with type 2 diabetes, and 243,170 controls with neither form of diabetes.
Those with type 1 diabetes were younger than those with type 2 diabetes and controls (62 years vs. 75 and 73 years, respectively). The type 1 diabetes group also had a higher proportion of females (43.6% vs. 38.1% of both the type 2 diabetes and control groups).
The proportions of people with the most severe type of heart attack, ST-elevation MI (STEMI), versus non-STEMI were 29% versus 71% in the type 1 diabetes group, 30% versus 70% in the type 2 diabetes group, and 39% versus 61% in the control group, respectively.
After adjustment for covariates including age, sex, comorbidities, socioeconomic factors, and medication, there was a significant decreased annual incidence trend for all-cause death among the controls (–1.9%) and persons with type 2 diabetes (–1.3%), but there was no such decrease among those with type 1 diabetes.
For cardiovascular deaths, the annual incidence declines were –2.0% and –1.6% in the control group and the type 2 diabetes group, respectively, versus a nonsignificant –0.5% decline in the type 1 diabetes group. Similarly, for major adverse cardiovascular events, those decreases were –2.0% for controls and –1.6% for those with type 2 diabetes, but –0.6% for those with type 1 diabetes – again, a nonsignificant value.
“During the last 15 years, the risk of death and major cardiovascular events in people without diabetes and with type 2 diabetes after having a first-time heart attack has decreased significantly. In contrast, this decreasing trend was absent in people with type 1 diabetes. Our study highlights the urgent need for understanding the cardiovascular risk in people with type 1 diabetes,” the authors conclude.
Dr. Nyström has received honoraria from AstraZeneca, Merck Sharp & Dohme, Novo Nordisk, Eli Lilly , Boehringer Ingelheim, Abbott, and Amgen. The authors acknowledge the ALF agreement between Stockholm County Council and Karolinska Institutet.
A version of this article appeared on Medscape.com.
, new research shows.
Between 2006 and 2020, the annual incidences of overall mortality and major adverse cardiovascular events after a first-time myocardial infarction dropped significantly for people with type 2 diabetes and those without diabetes (controls).
However, the same trend was not seen for people with type 1 diabetes.
“There is an urgent need for further studies understanding cardiovascular disease in people with type 1 diabetes. Clinicians have to be aware of the absence of the declined mortality trend in people with type 1 diabetes having a first-time myocardial infarction,” lead author Thomas Nyström, MD, professor of medicine at the Karolinska Institute, Stockholm, said in an interview.
The findings are scheduled to be presented Oct. 5, 2023, at the annual meeting of the European Association for the Study of Diabetes.
Discussing potential reasons for the findings, the authors say that the standard care after a heart attack has improved with more availability of, for example, percutaneous coronary intervention and better overall medical treatment. However, this standard of care should have improved in all three groups.
“Although glycemic control and diabetes duration were much different between diabetes groups, in that those with type 1 had been exposed for a longer period of glycemia, the current study cannot tell whether glucose control is behind the association between mortality trends observed. Whether this is the case must be investigated with further studies,” Nyström said.
Data from Swedish health care registry
Among people with a first-time MI recorded in national Swedish health care registries between 2006 and 2020, there were 2,527 individuals with type 1 diabetes, 48,321 with type 2 diabetes, and 243,170 controls with neither form of diabetes.
Those with type 1 diabetes were younger than those with type 2 diabetes and controls (62 years vs. 75 and 73 years, respectively). The type 1 diabetes group also had a higher proportion of females (43.6% vs. 38.1% of both the type 2 diabetes and control groups).
The proportions of people with the most severe type of heart attack, ST-elevation MI (STEMI), versus non-STEMI were 29% versus 71% in the type 1 diabetes group, 30% versus 70% in the type 2 diabetes group, and 39% versus 61% in the control group, respectively.
After adjustment for covariates including age, sex, comorbidities, socioeconomic factors, and medication, there was a significant decreased annual incidence trend for all-cause death among the controls (–1.9%) and persons with type 2 diabetes (–1.3%), but there was no such decrease among those with type 1 diabetes.
For cardiovascular deaths, the annual incidence declines were –2.0% and –1.6% in the control group and the type 2 diabetes group, respectively, versus a nonsignificant –0.5% decline in the type 1 diabetes group. Similarly, for major adverse cardiovascular events, those decreases were –2.0% for controls and –1.6% for those with type 2 diabetes, but –0.6% for those with type 1 diabetes – again, a nonsignificant value.
“During the last 15 years, the risk of death and major cardiovascular events in people without diabetes and with type 2 diabetes after having a first-time heart attack has decreased significantly. In contrast, this decreasing trend was absent in people with type 1 diabetes. Our study highlights the urgent need for understanding the cardiovascular risk in people with type 1 diabetes,” the authors conclude.
Dr. Nyström has received honoraria from AstraZeneca, Merck Sharp & Dohme, Novo Nordisk, Eli Lilly , Boehringer Ingelheim, Abbott, and Amgen. The authors acknowledge the ALF agreement between Stockholm County Council and Karolinska Institutet.
A version of this article appeared on Medscape.com.
FROM EASD 2023
12 steps to closing your practice without problems
Whether you’ve decided to retire, relocate, or work for your local hospital, unwinding your practice will take time.
“Doctors shouldn’t assume everything takes care of itself. Many don’t think about compliance issues, patient abandonment, or accounts receivable that they need to keep open to collect from billing, which can occur months after the dates of service,” said David Zetter, president of Zetter HealthCare management consultants in Pennsylvania.
Debra Phairas, president of Practice and Liability Consultants, LLC, in California, suggests doctors start planning for the closing of their practice at least 90-120 days from their closing date.
“Many people and entities need to be notified,” said Ms. Phairas. The list includes patients, payers, vendors, employees, licensing boards, and federal and state agencies.
Medical societies may have specific bylaws that apply; malpractice carriers have rules about how long you should retain medical records; and some state laws require that you communicate that you’re closing in a newspaper, Mr. Zetter added.
Ms. Phairas recommends that physicians decide first whether they will sell their practice or if they’ll just shut it down. If they sell and the buyer is a doctor, they may want to provide transition assistance such as introducing patients and staff, she said. Otherwise, doctors may need to terminate their staff.
After doctors make that decision, Mr. Zetter and Ms. Phairas recommend taking these 12 steps to ensure that the process goes smoothly.
What to do 60-90 days out
1. Check your insurance contracts. The Centers for Medicare & Medicaid Services requires physicians to notify them 90 days after deciding to retire or withdraw from Medicare or Medicaid. Other payers may also require 90 days’ notice to terminate their contracts.
You’ll also need to provide payers with a forwarding address for sending payments after the office closes, and notify your malpractice insurance carrier and any other contracted insurance carriers such as workers’ compensation or employee benefit plans.
2. Buy “tail” coverage. Doctors can be sued for malpractice years after they close their practice so this provides coverage against claims reported after the liability policy expires.
3. Check your hospital contracts. Most hospitals where you have privileges require 90 days’ notice that you are closing the practice.
4. Arrange for safe storage of medical records. If you are selling your practice to another physician, that doctor can take charge of them, as long as you obtain a patient’s consent to transfer the medical records, said Ms. Phairas. Otherwise, the practice is required to make someone the guardian of the records after the practice closes, said Mr. Zetter. This allows patients at a later date to obtain copies of their records at a cost.
“This usually means printing all the records to PDF to be retained; otherwise, doctors have to continue to pay the license fee for the EMR software to access the records, and no practice is going to continue to pay this indefinitely,” said Mr. Zetter.
Check with your malpractice insurance carrier for how long they require medical records to be retained, which may vary for adult and pediatric records.
Ms. Phairas also advises doctors to keep their original records. “The biggest mistake doctors can make is to give patients all their records. Your chart is your best defense weapon in a liability claim.”
What to do 30-60 days out
5. Tell your staff. They should not hear that you’re retiring or leaving the practice from other people, said Ms. Phairas. But timing is important. “If you notify them too soon, they may look for another job. I recommend telling them about 45 days out and just before you notify patients, although you may want to tell the office manager sooner.”
Doctors may need help closing the practice and should consider offering the employees a severance bonus to stay until the end, said Ms. Phairas. If they do leave sooner, then you can hire temporary staff.
6. Notify patients to avoid any claims of abandonment. You should notify all active patients, which, depending on your state, can be any patient the physician has treated sometime in the past 12-36 months.
Some state laws require the notice to be published as an advertisement in the local newspaper and will say how far in advance it needs to be published and how long the ad needs to run. Notification also should be posted throughout the practice, and patients who call or visit should be given oral reminders.
“Your biggest expense will be mailing a letter to all patients,” said Mr. Zetter. The letter should include:
- The date of closing.
- The name(s) of the physicians taking over the practice (if applicable).
- Local physicians who would be willing to accept new patients.
- Instructions for how patients can obtain or transfer medical records (with a deadline for submitting record requests).
- How to contact the practice if patients and families have any concerns about the closing.
7. Notify your professional associations. These include your state medical board, credentialing organizations, and professional memberships. It’s critical to renew your license even if you plan to practice in other states. He recalled that one doctor let his license lapse and the medical board notified Medicaid that he was no longer licensed. “CMS went after him because he didn’t notify them that he was no longer operating in Washington. CMS shut him down in every state/territory. This interventional radiologist spent 3 years with two attorneys to get it resolved,” said Mr. Zetter.
8. Terminate any leases with landlords or try to negotiate renting the office space on a month-to-month basis until you close or sell, suggests Ms. Phairas. If the practice owns the space, the partners will need to decide if the space will be sold or leased to a new business.
What to do 30 days out
9. Notify referring physicians of when you plan to close your practice so they don’t send new patients after that date.
10. Send a letter to the Drug Enforcement Agency to deactivate your license if you plan not to write another prescription and after you have safely disposed of prescription drugs following the federal guidelines. Destroy all prescription pads and contact drug representatives to determine what to do with unused samples, if needed.
11. Notify all vendors. Inform medical suppliers, office suppliers, collection agencies, laundry services, housekeeping services, hazardous waste disposal services, and any other vendors. Make sure to request a final statement from them so you can close out your accounts.
12. Process your accounts receivable to collect money owed to you. Consider employing a collection agency or staff member to reconcile accounts after the practice has closed.
Mr. Zetter also suggested retaining a certified accountant to handle the expenses for shutting down the business and to handle your future tax returns. “If you shut down the practice in 2023, you will still have to file a tax return for that year in 2024,” he said.
A version of this article first appeared on Medscape.com.
Whether you’ve decided to retire, relocate, or work for your local hospital, unwinding your practice will take time.
“Doctors shouldn’t assume everything takes care of itself. Many don’t think about compliance issues, patient abandonment, or accounts receivable that they need to keep open to collect from billing, which can occur months after the dates of service,” said David Zetter, president of Zetter HealthCare management consultants in Pennsylvania.
Debra Phairas, president of Practice and Liability Consultants, LLC, in California, suggests doctors start planning for the closing of their practice at least 90-120 days from their closing date.
“Many people and entities need to be notified,” said Ms. Phairas. The list includes patients, payers, vendors, employees, licensing boards, and federal and state agencies.
Medical societies may have specific bylaws that apply; malpractice carriers have rules about how long you should retain medical records; and some state laws require that you communicate that you’re closing in a newspaper, Mr. Zetter added.
Ms. Phairas recommends that physicians decide first whether they will sell their practice or if they’ll just shut it down. If they sell and the buyer is a doctor, they may want to provide transition assistance such as introducing patients and staff, she said. Otherwise, doctors may need to terminate their staff.
After doctors make that decision, Mr. Zetter and Ms. Phairas recommend taking these 12 steps to ensure that the process goes smoothly.
What to do 60-90 days out
1. Check your insurance contracts. The Centers for Medicare & Medicaid Services requires physicians to notify them 90 days after deciding to retire or withdraw from Medicare or Medicaid. Other payers may also require 90 days’ notice to terminate their contracts.
You’ll also need to provide payers with a forwarding address for sending payments after the office closes, and notify your malpractice insurance carrier and any other contracted insurance carriers such as workers’ compensation or employee benefit plans.
2. Buy “tail” coverage. Doctors can be sued for malpractice years after they close their practice so this provides coverage against claims reported after the liability policy expires.
3. Check your hospital contracts. Most hospitals where you have privileges require 90 days’ notice that you are closing the practice.
4. Arrange for safe storage of medical records. If you are selling your practice to another physician, that doctor can take charge of them, as long as you obtain a patient’s consent to transfer the medical records, said Ms. Phairas. Otherwise, the practice is required to make someone the guardian of the records after the practice closes, said Mr. Zetter. This allows patients at a later date to obtain copies of their records at a cost.
“This usually means printing all the records to PDF to be retained; otherwise, doctors have to continue to pay the license fee for the EMR software to access the records, and no practice is going to continue to pay this indefinitely,” said Mr. Zetter.
Check with your malpractice insurance carrier for how long they require medical records to be retained, which may vary for adult and pediatric records.
Ms. Phairas also advises doctors to keep their original records. “The biggest mistake doctors can make is to give patients all their records. Your chart is your best defense weapon in a liability claim.”
What to do 30-60 days out
5. Tell your staff. They should not hear that you’re retiring or leaving the practice from other people, said Ms. Phairas. But timing is important. “If you notify them too soon, they may look for another job. I recommend telling them about 45 days out and just before you notify patients, although you may want to tell the office manager sooner.”
Doctors may need help closing the practice and should consider offering the employees a severance bonus to stay until the end, said Ms. Phairas. If they do leave sooner, then you can hire temporary staff.
6. Notify patients to avoid any claims of abandonment. You should notify all active patients, which, depending on your state, can be any patient the physician has treated sometime in the past 12-36 months.
Some state laws require the notice to be published as an advertisement in the local newspaper and will say how far in advance it needs to be published and how long the ad needs to run. Notification also should be posted throughout the practice, and patients who call or visit should be given oral reminders.
“Your biggest expense will be mailing a letter to all patients,” said Mr. Zetter. The letter should include:
- The date of closing.
- The name(s) of the physicians taking over the practice (if applicable).
- Local physicians who would be willing to accept new patients.
- Instructions for how patients can obtain or transfer medical records (with a deadline for submitting record requests).
- How to contact the practice if patients and families have any concerns about the closing.
7. Notify your professional associations. These include your state medical board, credentialing organizations, and professional memberships. It’s critical to renew your license even if you plan to practice in other states. He recalled that one doctor let his license lapse and the medical board notified Medicaid that he was no longer licensed. “CMS went after him because he didn’t notify them that he was no longer operating in Washington. CMS shut him down in every state/territory. This interventional radiologist spent 3 years with two attorneys to get it resolved,” said Mr. Zetter.
8. Terminate any leases with landlords or try to negotiate renting the office space on a month-to-month basis until you close or sell, suggests Ms. Phairas. If the practice owns the space, the partners will need to decide if the space will be sold or leased to a new business.
What to do 30 days out
9. Notify referring physicians of when you plan to close your practice so they don’t send new patients after that date.
10. Send a letter to the Drug Enforcement Agency to deactivate your license if you plan not to write another prescription and after you have safely disposed of prescription drugs following the federal guidelines. Destroy all prescription pads and contact drug representatives to determine what to do with unused samples, if needed.
11. Notify all vendors. Inform medical suppliers, office suppliers, collection agencies, laundry services, housekeeping services, hazardous waste disposal services, and any other vendors. Make sure to request a final statement from them so you can close out your accounts.
12. Process your accounts receivable to collect money owed to you. Consider employing a collection agency or staff member to reconcile accounts after the practice has closed.
Mr. Zetter also suggested retaining a certified accountant to handle the expenses for shutting down the business and to handle your future tax returns. “If you shut down the practice in 2023, you will still have to file a tax return for that year in 2024,” he said.
A version of this article first appeared on Medscape.com.
Whether you’ve decided to retire, relocate, or work for your local hospital, unwinding your practice will take time.
“Doctors shouldn’t assume everything takes care of itself. Many don’t think about compliance issues, patient abandonment, or accounts receivable that they need to keep open to collect from billing, which can occur months after the dates of service,” said David Zetter, president of Zetter HealthCare management consultants in Pennsylvania.
Debra Phairas, president of Practice and Liability Consultants, LLC, in California, suggests doctors start planning for the closing of their practice at least 90-120 days from their closing date.
“Many people and entities need to be notified,” said Ms. Phairas. The list includes patients, payers, vendors, employees, licensing boards, and federal and state agencies.
Medical societies may have specific bylaws that apply; malpractice carriers have rules about how long you should retain medical records; and some state laws require that you communicate that you’re closing in a newspaper, Mr. Zetter added.
Ms. Phairas recommends that physicians decide first whether they will sell their practice or if they’ll just shut it down. If they sell and the buyer is a doctor, they may want to provide transition assistance such as introducing patients and staff, she said. Otherwise, doctors may need to terminate their staff.
After doctors make that decision, Mr. Zetter and Ms. Phairas recommend taking these 12 steps to ensure that the process goes smoothly.
What to do 60-90 days out
1. Check your insurance contracts. The Centers for Medicare & Medicaid Services requires physicians to notify them 90 days after deciding to retire or withdraw from Medicare or Medicaid. Other payers may also require 90 days’ notice to terminate their contracts.
You’ll also need to provide payers with a forwarding address for sending payments after the office closes, and notify your malpractice insurance carrier and any other contracted insurance carriers such as workers’ compensation or employee benefit plans.
2. Buy “tail” coverage. Doctors can be sued for malpractice years after they close their practice so this provides coverage against claims reported after the liability policy expires.
3. Check your hospital contracts. Most hospitals where you have privileges require 90 days’ notice that you are closing the practice.
4. Arrange for safe storage of medical records. If you are selling your practice to another physician, that doctor can take charge of them, as long as you obtain a patient’s consent to transfer the medical records, said Ms. Phairas. Otherwise, the practice is required to make someone the guardian of the records after the practice closes, said Mr. Zetter. This allows patients at a later date to obtain copies of their records at a cost.
“This usually means printing all the records to PDF to be retained; otherwise, doctors have to continue to pay the license fee for the EMR software to access the records, and no practice is going to continue to pay this indefinitely,” said Mr. Zetter.
Check with your malpractice insurance carrier for how long they require medical records to be retained, which may vary for adult and pediatric records.
Ms. Phairas also advises doctors to keep their original records. “The biggest mistake doctors can make is to give patients all their records. Your chart is your best defense weapon in a liability claim.”
What to do 30-60 days out
5. Tell your staff. They should not hear that you’re retiring or leaving the practice from other people, said Ms. Phairas. But timing is important. “If you notify them too soon, they may look for another job. I recommend telling them about 45 days out and just before you notify patients, although you may want to tell the office manager sooner.”
Doctors may need help closing the practice and should consider offering the employees a severance bonus to stay until the end, said Ms. Phairas. If they do leave sooner, then you can hire temporary staff.
6. Notify patients to avoid any claims of abandonment. You should notify all active patients, which, depending on your state, can be any patient the physician has treated sometime in the past 12-36 months.
Some state laws require the notice to be published as an advertisement in the local newspaper and will say how far in advance it needs to be published and how long the ad needs to run. Notification also should be posted throughout the practice, and patients who call or visit should be given oral reminders.
“Your biggest expense will be mailing a letter to all patients,” said Mr. Zetter. The letter should include:
- The date of closing.
- The name(s) of the physicians taking over the practice (if applicable).
- Local physicians who would be willing to accept new patients.
- Instructions for how patients can obtain or transfer medical records (with a deadline for submitting record requests).
- How to contact the practice if patients and families have any concerns about the closing.
7. Notify your professional associations. These include your state medical board, credentialing organizations, and professional memberships. It’s critical to renew your license even if you plan to practice in other states. He recalled that one doctor let his license lapse and the medical board notified Medicaid that he was no longer licensed. “CMS went after him because he didn’t notify them that he was no longer operating in Washington. CMS shut him down in every state/territory. This interventional radiologist spent 3 years with two attorneys to get it resolved,” said Mr. Zetter.
8. Terminate any leases with landlords or try to negotiate renting the office space on a month-to-month basis until you close or sell, suggests Ms. Phairas. If the practice owns the space, the partners will need to decide if the space will be sold or leased to a new business.
What to do 30 days out
9. Notify referring physicians of when you plan to close your practice so they don’t send new patients after that date.
10. Send a letter to the Drug Enforcement Agency to deactivate your license if you plan not to write another prescription and after you have safely disposed of prescription drugs following the federal guidelines. Destroy all prescription pads and contact drug representatives to determine what to do with unused samples, if needed.
11. Notify all vendors. Inform medical suppliers, office suppliers, collection agencies, laundry services, housekeeping services, hazardous waste disposal services, and any other vendors. Make sure to request a final statement from them so you can close out your accounts.
12. Process your accounts receivable to collect money owed to you. Consider employing a collection agency or staff member to reconcile accounts after the practice has closed.
Mr. Zetter also suggested retaining a certified accountant to handle the expenses for shutting down the business and to handle your future tax returns. “If you shut down the practice in 2023, you will still have to file a tax return for that year in 2024,” he said.
A version of this article first appeared on Medscape.com.
New co–editors-in-chief named for CMGH
will be taking over their new roles beginning July 1, 2024.
“In my role as co–editor-in-chief of CMGH, I look forward to working with Jonathan Katz to advance its longstanding mission to disseminate rigorous, reproducible, and impactful digestive biology research. I am excited to launch new initiatives including the addition of special topic editors who will cover themes relating to early career investigators and diversity, equity, and inclusion,” said Dr. Battle, who is a professor in the department of cell biology, neurobiology, and anatomy at the Medical College of Wisconsin, Milwaukee.
“I am honored to work with Michele Battle and the rest of our new CMGH board of editors. Some of our goals are to expand CMGH outreach and engagement, incorporate more programs and opportunities for junior investigators, and provide rapid advancements and technical reports that drive research in the field. As such, we will strive to ensure that CMGH remains the preeminent journal focused on high-impact, basic, mechanistic research in GI and hepatology,” said Dr. Katz, an associate professor of medicine in the department of medicine gastroenterology division, director of molecular pathology and imaging care, and director of the undergraduate student scholars program at the University of Pennsylvania, Philadelphia. He is also currently an associate editor for CMGH.
Dr. Battle and Dr. Katz are former AGA Research Foundation awardees, with Dr. Battle receiving an AGA Research Scholar Award in 2009 and Dr. Katz receiving the AGA Astra Merck Advanced Research Training Award in 1998.
Over the next year, they will meet with the current editorial board and start to plan and develop new special sections and initiatives to bring to CMGH during their term.
Please join us in congratulating Dr. Battle and Dr. Katz on their new role as incoming co–editors-in chief.
will be taking over their new roles beginning July 1, 2024.
“In my role as co–editor-in-chief of CMGH, I look forward to working with Jonathan Katz to advance its longstanding mission to disseminate rigorous, reproducible, and impactful digestive biology research. I am excited to launch new initiatives including the addition of special topic editors who will cover themes relating to early career investigators and diversity, equity, and inclusion,” said Dr. Battle, who is a professor in the department of cell biology, neurobiology, and anatomy at the Medical College of Wisconsin, Milwaukee.
“I am honored to work with Michele Battle and the rest of our new CMGH board of editors. Some of our goals are to expand CMGH outreach and engagement, incorporate more programs and opportunities for junior investigators, and provide rapid advancements and technical reports that drive research in the field. As such, we will strive to ensure that CMGH remains the preeminent journal focused on high-impact, basic, mechanistic research in GI and hepatology,” said Dr. Katz, an associate professor of medicine in the department of medicine gastroenterology division, director of molecular pathology and imaging care, and director of the undergraduate student scholars program at the University of Pennsylvania, Philadelphia. He is also currently an associate editor for CMGH.
Dr. Battle and Dr. Katz are former AGA Research Foundation awardees, with Dr. Battle receiving an AGA Research Scholar Award in 2009 and Dr. Katz receiving the AGA Astra Merck Advanced Research Training Award in 1998.
Over the next year, they will meet with the current editorial board and start to plan and develop new special sections and initiatives to bring to CMGH during their term.
Please join us in congratulating Dr. Battle and Dr. Katz on their new role as incoming co–editors-in chief.
will be taking over their new roles beginning July 1, 2024.
“In my role as co–editor-in-chief of CMGH, I look forward to working with Jonathan Katz to advance its longstanding mission to disseminate rigorous, reproducible, and impactful digestive biology research. I am excited to launch new initiatives including the addition of special topic editors who will cover themes relating to early career investigators and diversity, equity, and inclusion,” said Dr. Battle, who is a professor in the department of cell biology, neurobiology, and anatomy at the Medical College of Wisconsin, Milwaukee.
“I am honored to work with Michele Battle and the rest of our new CMGH board of editors. Some of our goals are to expand CMGH outreach and engagement, incorporate more programs and opportunities for junior investigators, and provide rapid advancements and technical reports that drive research in the field. As such, we will strive to ensure that CMGH remains the preeminent journal focused on high-impact, basic, mechanistic research in GI and hepatology,” said Dr. Katz, an associate professor of medicine in the department of medicine gastroenterology division, director of molecular pathology and imaging care, and director of the undergraduate student scholars program at the University of Pennsylvania, Philadelphia. He is also currently an associate editor for CMGH.
Dr. Battle and Dr. Katz are former AGA Research Foundation awardees, with Dr. Battle receiving an AGA Research Scholar Award in 2009 and Dr. Katz receiving the AGA Astra Merck Advanced Research Training Award in 1998.
Over the next year, they will meet with the current editorial board and start to plan and develop new special sections and initiatives to bring to CMGH during their term.
Please join us in congratulating Dr. Battle and Dr. Katz on their new role as incoming co–editors-in chief.
Watch the inaugural Gastro Journal Club
We are delighted to introduce the Gastro Journal Club in which an author of a study published in Gastroenterology will present their paper followed by a Q&A. The inaugural Gastro Journal Club features Loren Laine, MD, professor of medicine (digestive diseases) at Yale University, New Haven, Conn., and was hosted by the McMaster University gastroenterology division. Dr. Laine presented his article “Vonoprazan Versus Lansoprazole for Healing and Maintenance of Healing of Erosive Esophagitis: A Randomized Trial,” published in the January 2023 issue of Gastroenterology.
If you are interested in participating, please contact [email protected].
Watch the inaugural Gastro Journal Club: https://rb.gy/j8mqm
We are delighted to introduce the Gastro Journal Club in which an author of a study published in Gastroenterology will present their paper followed by a Q&A. The inaugural Gastro Journal Club features Loren Laine, MD, professor of medicine (digestive diseases) at Yale University, New Haven, Conn., and was hosted by the McMaster University gastroenterology division. Dr. Laine presented his article “Vonoprazan Versus Lansoprazole for Healing and Maintenance of Healing of Erosive Esophagitis: A Randomized Trial,” published in the January 2023 issue of Gastroenterology.
If you are interested in participating, please contact [email protected].
Watch the inaugural Gastro Journal Club: https://rb.gy/j8mqm
We are delighted to introduce the Gastro Journal Club in which an author of a study published in Gastroenterology will present their paper followed by a Q&A. The inaugural Gastro Journal Club features Loren Laine, MD, professor of medicine (digestive diseases) at Yale University, New Haven, Conn., and was hosted by the McMaster University gastroenterology division. Dr. Laine presented his article “Vonoprazan Versus Lansoprazole for Healing and Maintenance of Healing of Erosive Esophagitis: A Randomized Trial,” published in the January 2023 issue of Gastroenterology.
If you are interested in participating, please contact [email protected].
Watch the inaugural Gastro Journal Club: https://rb.gy/j8mqm
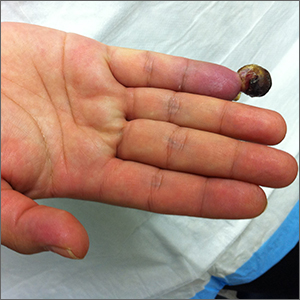
Painful fingertip tumor in pregnancy
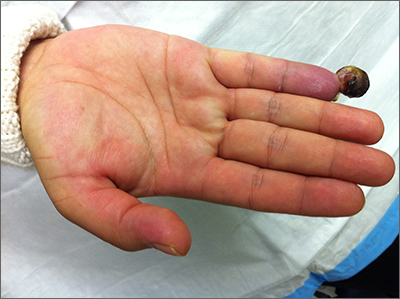
This friable vascular papule was most consistent with a lobular capillary hemangioma (LCH), also called a pyogenic granuloma. A shave biopsy was performed at the base of the tumor to confirm the diagnosis and rule out malignant pedunculated tumors, including nodular melanoma, angiosarcoma, and metastatic carcinoma.
LCHs are benign vascular growths that occur on the skin and mucosa, most often in children and young adults. Growth may occur rapidly over days to weeks and tumors may grow to several centimeters in size. Although LCHs are often painless, they do tend to bleed easily with minor trauma.
While the triggering mechanism is unknown, LCHs have been associated with infection, trauma, hormonal factors (especially in the second and third trimesters of pregnancy), and therapy with retinoids. About 5% of pregnancies are associated with the development of an LCH on the oral mucosa, usually in the second or third trimester.1
Treatment of LCHs is based on small case series and case reports. Individual tumors have a high likelihood of recurrence after a single treatment, so multiple visits for treatment are often recommended. Electrocautery is safe and effective with complete cure occurring after 2 sessions. Similarly, cryotherapy is safe and effective with excellent results after 3 treatment sessions. Cryotherapy may cause depigmentation in patients with darker skin types, so this should be discussed with patients with skin of color. Excision of small lesions is also safe and effective in a single session.2
This patient was treated with light electrodessication and curettage in 2 sessions with complete clearance.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Demir Y, Demir S, Aktepe F. Cutaneous lobular capillary hemangioma induced by pregnancy. J Cutan Pathol. 2004;31:77-80. doi: 10.1046/j.0303-6987.2004.0137.x
2. Lee J, Sinno H, Tahiri Y, et al. Treatment options for cutaneous pyogenic granulomas: a review. J Plast Reconstr Aesthet Surg. 2011;64:1216-1220. doi: 10.1016/j.bjps.2010.12.021

This friable vascular papule was most consistent with a lobular capillary hemangioma (LCH), also called a pyogenic granuloma. A shave biopsy was performed at the base of the tumor to confirm the diagnosis and rule out malignant pedunculated tumors, including nodular melanoma, angiosarcoma, and metastatic carcinoma.
LCHs are benign vascular growths that occur on the skin and mucosa, most often in children and young adults. Growth may occur rapidly over days to weeks and tumors may grow to several centimeters in size. Although LCHs are often painless, they do tend to bleed easily with minor trauma.
While the triggering mechanism is unknown, LCHs have been associated with infection, trauma, hormonal factors (especially in the second and third trimesters of pregnancy), and therapy with retinoids. About 5% of pregnancies are associated with the development of an LCH on the oral mucosa, usually in the second or third trimester.1
Treatment of LCHs is based on small case series and case reports. Individual tumors have a high likelihood of recurrence after a single treatment, so multiple visits for treatment are often recommended. Electrocautery is safe and effective with complete cure occurring after 2 sessions. Similarly, cryotherapy is safe and effective with excellent results after 3 treatment sessions. Cryotherapy may cause depigmentation in patients with darker skin types, so this should be discussed with patients with skin of color. Excision of small lesions is also safe and effective in a single session.2
This patient was treated with light electrodessication and curettage in 2 sessions with complete clearance.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

This friable vascular papule was most consistent with a lobular capillary hemangioma (LCH), also called a pyogenic granuloma. A shave biopsy was performed at the base of the tumor to confirm the diagnosis and rule out malignant pedunculated tumors, including nodular melanoma, angiosarcoma, and metastatic carcinoma.
LCHs are benign vascular growths that occur on the skin and mucosa, most often in children and young adults. Growth may occur rapidly over days to weeks and tumors may grow to several centimeters in size. Although LCHs are often painless, they do tend to bleed easily with minor trauma.
While the triggering mechanism is unknown, LCHs have been associated with infection, trauma, hormonal factors (especially in the second and third trimesters of pregnancy), and therapy with retinoids. About 5% of pregnancies are associated with the development of an LCH on the oral mucosa, usually in the second or third trimester.1
Treatment of LCHs is based on small case series and case reports. Individual tumors have a high likelihood of recurrence after a single treatment, so multiple visits for treatment are often recommended. Electrocautery is safe and effective with complete cure occurring after 2 sessions. Similarly, cryotherapy is safe and effective with excellent results after 3 treatment sessions. Cryotherapy may cause depigmentation in patients with darker skin types, so this should be discussed with patients with skin of color. Excision of small lesions is also safe and effective in a single session.2
This patient was treated with light electrodessication and curettage in 2 sessions with complete clearance.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Demir Y, Demir S, Aktepe F. Cutaneous lobular capillary hemangioma induced by pregnancy. J Cutan Pathol. 2004;31:77-80. doi: 10.1046/j.0303-6987.2004.0137.x
2. Lee J, Sinno H, Tahiri Y, et al. Treatment options for cutaneous pyogenic granulomas: a review. J Plast Reconstr Aesthet Surg. 2011;64:1216-1220. doi: 10.1016/j.bjps.2010.12.021
1. Demir Y, Demir S, Aktepe F. Cutaneous lobular capillary hemangioma induced by pregnancy. J Cutan Pathol. 2004;31:77-80. doi: 10.1046/j.0303-6987.2004.0137.x
2. Lee J, Sinno H, Tahiri Y, et al. Treatment options for cutaneous pyogenic granulomas: a review. J Plast Reconstr Aesthet Surg. 2011;64:1216-1220. doi: 10.1016/j.bjps.2010.12.021
Minimally invasive surfactant shows some benefit in infants’ first 2 years
Results of the OPTIMIST follow-up study were published online in JAMA.
Researchers, led by Peter A. Dargaville, MD, department of paediatrics, Royal Hobart (Australia) Hospital, found that MIST, which involves administering surfactant via a thin catheter, compared with sham treatment, did not reduce the incidence of death or neurodevelopmental disability (NDD) by 2 years of age.
However, infants who received MIST had lower rates of poor respiratory outcomes during those first 2 years of life.
Study spanned 11 countries
The study was conducted in 33 tertiary neonatal intensive care units (NICUs) in 11 countries, including Australia, Canada, Israel, New Zealand, Qatar, Singapore, Slovenia, the Netherlands, Turkey, the United Kingdom, and the United States.
It included 486 infants 25-28 weeks old supported with CPAP; 453 had follow-up data available and data on the key secondary outcome were available for 434 infants.
The sham treatment consisted of only transient repositioning without airway instruments. Treating clinicians, outcome assessors, and parents were blinded to group status.
No significant difference in deaths, NDD
Death or NDD occurred in 36.3% of the patients in the MIST group and 36.1% in the control group (risk difference, 0%; 95% confidence interval, −7.6% to 7.7%; relative risk, 1.0; 95% confidence interval, 0.81-1.24).
Secondary respiratory outcomes were better in the MIST group:
- Hospitalization with respiratory illness occurred in 25.1% in the MIST group versus 38.2% in the control group (RR, 0.66; 95% CI, 0.54-0.81).
- Parent-reported wheezing or breathing difficulty occurred in 40.6% in the MIST group versus 53.6% in controls (RR, 0.76; 95% CI, 0.63-0.90).
- Asthma diagnosed by a physician was reported in 4.4% and 11.9% of MIST and control-group infants, respectively.
- Reported use of inhaled relievers (beta2 agonists) was 23.9% in the MIST group versus 38.7% in controls.
The previous study of early outcomes of deaths or bronchopulmonary dysplasia (BPD; chronic lung injury in preterm infants) was published by the same group of researchers in 2021.
Important benefit for respiratory health
Suhas G. Kallapur, MD, chief of the divisions of neonatology and developmental biology at University of California, Los Angeles, who was not part of either study, said: “This is one of the largest studies to date examining whether the MIST procedure for surfactant is beneficial in preterm babies born at 25-28 weeks’ gestation.”
Overall, when considering the 2021 and 2023 studies together, it appears that the MIST therapy has important benefits for respiratory health during a NICU stay and in early infancy, even though the primary outcome of death or NDD was not different between the treatment and control groups, Dr. Kallapur said.
“The slight (nonsignificant) increase in deaths in the MIST group was confined to the more immature babies – 25-26 weeks’ gestation at birth,” he pointed out. “In the bigger and more mature babies – 27-28 week gestation infants – the benefits of MIST therapy occurred without any increase in mortality, suggesting that this group of babies may be the group that stands to benefit most from this therapy.”
Dr. Kallapur said new data in the developmental origins of health and disease “now show that the trajectory of respiratory health in infancy is an important determinant of respiratory health into adulthood and older age.”
Therefore, the finding of benefit to respiratory health is particularly important, he said.
He noted that MIST or similar therapy is already in use in many NICUs throughout the world and that those already using it will likely feel vindicated by this study.
“Neonatologists who were on the sidelines will likely see these results – especially childhood respiratory outcomes – as a reason to initiate this procedure in all but the most immature preterm infants,” Dr. Kallapur says.
Dr. Dargaville reports personal fees from AbbVie and Chiesi Farmaceutici and provision of surfactant at reduced cost and support for conference travel from Chiesi Farmaceutici during the conduct of the study; in addition, Dr. Dargaville has been issued a patent for a catheter design. One coauthor reports grants from Chiesi Farmaceutici during the conduct of the study. Another coauthor reports serving as chief investigator for OPTI-SURF, an observational study on United Kingdom neonatal surfactant use in respiratory distress syndrome funded by Chiesi UK outside the submitted work. A third coauthor reports personal fees from Chiesi Farmaceutici outside the submitted work. This study was funded by grants from the Royal Hobart Hospital Research Foundation and the Australian National Health and Medical Research Council. Exogenous surfactant was provided at reduced cost by Chiesi Farmaceutici. Dr. Kallapur has no relevant financial relationships.
Results of the OPTIMIST follow-up study were published online in JAMA.
Researchers, led by Peter A. Dargaville, MD, department of paediatrics, Royal Hobart (Australia) Hospital, found that MIST, which involves administering surfactant via a thin catheter, compared with sham treatment, did not reduce the incidence of death or neurodevelopmental disability (NDD) by 2 years of age.
However, infants who received MIST had lower rates of poor respiratory outcomes during those first 2 years of life.
Study spanned 11 countries
The study was conducted in 33 tertiary neonatal intensive care units (NICUs) in 11 countries, including Australia, Canada, Israel, New Zealand, Qatar, Singapore, Slovenia, the Netherlands, Turkey, the United Kingdom, and the United States.
It included 486 infants 25-28 weeks old supported with CPAP; 453 had follow-up data available and data on the key secondary outcome were available for 434 infants.
The sham treatment consisted of only transient repositioning without airway instruments. Treating clinicians, outcome assessors, and parents were blinded to group status.
No significant difference in deaths, NDD
Death or NDD occurred in 36.3% of the patients in the MIST group and 36.1% in the control group (risk difference, 0%; 95% confidence interval, −7.6% to 7.7%; relative risk, 1.0; 95% confidence interval, 0.81-1.24).
Secondary respiratory outcomes were better in the MIST group:
- Hospitalization with respiratory illness occurred in 25.1% in the MIST group versus 38.2% in the control group (RR, 0.66; 95% CI, 0.54-0.81).
- Parent-reported wheezing or breathing difficulty occurred in 40.6% in the MIST group versus 53.6% in controls (RR, 0.76; 95% CI, 0.63-0.90).
- Asthma diagnosed by a physician was reported in 4.4% and 11.9% of MIST and control-group infants, respectively.
- Reported use of inhaled relievers (beta2 agonists) was 23.9% in the MIST group versus 38.7% in controls.
The previous study of early outcomes of deaths or bronchopulmonary dysplasia (BPD; chronic lung injury in preterm infants) was published by the same group of researchers in 2021.
Important benefit for respiratory health
Suhas G. Kallapur, MD, chief of the divisions of neonatology and developmental biology at University of California, Los Angeles, who was not part of either study, said: “This is one of the largest studies to date examining whether the MIST procedure for surfactant is beneficial in preterm babies born at 25-28 weeks’ gestation.”
Overall, when considering the 2021 and 2023 studies together, it appears that the MIST therapy has important benefits for respiratory health during a NICU stay and in early infancy, even though the primary outcome of death or NDD was not different between the treatment and control groups, Dr. Kallapur said.
“The slight (nonsignificant) increase in deaths in the MIST group was confined to the more immature babies – 25-26 weeks’ gestation at birth,” he pointed out. “In the bigger and more mature babies – 27-28 week gestation infants – the benefits of MIST therapy occurred without any increase in mortality, suggesting that this group of babies may be the group that stands to benefit most from this therapy.”
Dr. Kallapur said new data in the developmental origins of health and disease “now show that the trajectory of respiratory health in infancy is an important determinant of respiratory health into adulthood and older age.”
Therefore, the finding of benefit to respiratory health is particularly important, he said.
He noted that MIST or similar therapy is already in use in many NICUs throughout the world and that those already using it will likely feel vindicated by this study.
“Neonatologists who were on the sidelines will likely see these results – especially childhood respiratory outcomes – as a reason to initiate this procedure in all but the most immature preterm infants,” Dr. Kallapur says.
Dr. Dargaville reports personal fees from AbbVie and Chiesi Farmaceutici and provision of surfactant at reduced cost and support for conference travel from Chiesi Farmaceutici during the conduct of the study; in addition, Dr. Dargaville has been issued a patent for a catheter design. One coauthor reports grants from Chiesi Farmaceutici during the conduct of the study. Another coauthor reports serving as chief investigator for OPTI-SURF, an observational study on United Kingdom neonatal surfactant use in respiratory distress syndrome funded by Chiesi UK outside the submitted work. A third coauthor reports personal fees from Chiesi Farmaceutici outside the submitted work. This study was funded by grants from the Royal Hobart Hospital Research Foundation and the Australian National Health and Medical Research Council. Exogenous surfactant was provided at reduced cost by Chiesi Farmaceutici. Dr. Kallapur has no relevant financial relationships.
Results of the OPTIMIST follow-up study were published online in JAMA.
Researchers, led by Peter A. Dargaville, MD, department of paediatrics, Royal Hobart (Australia) Hospital, found that MIST, which involves administering surfactant via a thin catheter, compared with sham treatment, did not reduce the incidence of death or neurodevelopmental disability (NDD) by 2 years of age.
However, infants who received MIST had lower rates of poor respiratory outcomes during those first 2 years of life.
Study spanned 11 countries
The study was conducted in 33 tertiary neonatal intensive care units (NICUs) in 11 countries, including Australia, Canada, Israel, New Zealand, Qatar, Singapore, Slovenia, the Netherlands, Turkey, the United Kingdom, and the United States.
It included 486 infants 25-28 weeks old supported with CPAP; 453 had follow-up data available and data on the key secondary outcome were available for 434 infants.
The sham treatment consisted of only transient repositioning without airway instruments. Treating clinicians, outcome assessors, and parents were blinded to group status.
No significant difference in deaths, NDD
Death or NDD occurred in 36.3% of the patients in the MIST group and 36.1% in the control group (risk difference, 0%; 95% confidence interval, −7.6% to 7.7%; relative risk, 1.0; 95% confidence interval, 0.81-1.24).
Secondary respiratory outcomes were better in the MIST group:
- Hospitalization with respiratory illness occurred in 25.1% in the MIST group versus 38.2% in the control group (RR, 0.66; 95% CI, 0.54-0.81).
- Parent-reported wheezing or breathing difficulty occurred in 40.6% in the MIST group versus 53.6% in controls (RR, 0.76; 95% CI, 0.63-0.90).
- Asthma diagnosed by a physician was reported in 4.4% and 11.9% of MIST and control-group infants, respectively.
- Reported use of inhaled relievers (beta2 agonists) was 23.9% in the MIST group versus 38.7% in controls.
The previous study of early outcomes of deaths or bronchopulmonary dysplasia (BPD; chronic lung injury in preterm infants) was published by the same group of researchers in 2021.
Important benefit for respiratory health
Suhas G. Kallapur, MD, chief of the divisions of neonatology and developmental biology at University of California, Los Angeles, who was not part of either study, said: “This is one of the largest studies to date examining whether the MIST procedure for surfactant is beneficial in preterm babies born at 25-28 weeks’ gestation.”
Overall, when considering the 2021 and 2023 studies together, it appears that the MIST therapy has important benefits for respiratory health during a NICU stay and in early infancy, even though the primary outcome of death or NDD was not different between the treatment and control groups, Dr. Kallapur said.
“The slight (nonsignificant) increase in deaths in the MIST group was confined to the more immature babies – 25-26 weeks’ gestation at birth,” he pointed out. “In the bigger and more mature babies – 27-28 week gestation infants – the benefits of MIST therapy occurred without any increase in mortality, suggesting that this group of babies may be the group that stands to benefit most from this therapy.”
Dr. Kallapur said new data in the developmental origins of health and disease “now show that the trajectory of respiratory health in infancy is an important determinant of respiratory health into adulthood and older age.”
Therefore, the finding of benefit to respiratory health is particularly important, he said.
He noted that MIST or similar therapy is already in use in many NICUs throughout the world and that those already using it will likely feel vindicated by this study.
“Neonatologists who were on the sidelines will likely see these results – especially childhood respiratory outcomes – as a reason to initiate this procedure in all but the most immature preterm infants,” Dr. Kallapur says.
Dr. Dargaville reports personal fees from AbbVie and Chiesi Farmaceutici and provision of surfactant at reduced cost and support for conference travel from Chiesi Farmaceutici during the conduct of the study; in addition, Dr. Dargaville has been issued a patent for a catheter design. One coauthor reports grants from Chiesi Farmaceutici during the conduct of the study. Another coauthor reports serving as chief investigator for OPTI-SURF, an observational study on United Kingdom neonatal surfactant use in respiratory distress syndrome funded by Chiesi UK outside the submitted work. A third coauthor reports personal fees from Chiesi Farmaceutici outside the submitted work. This study was funded by grants from the Royal Hobart Hospital Research Foundation and the Australian National Health and Medical Research Council. Exogenous surfactant was provided at reduced cost by Chiesi Farmaceutici. Dr. Kallapur has no relevant financial relationships.
FROM JAMA
Fighting disparities in palliative and end-of-life care
Palliative care has been shown to improve quality of life, receipt of goal-concordant care, end-of-life decision-making, and improvement in pain and symptoms in individuals with serious illness. However, palliative and end-of-life care remain underutilized in racial and ethnic minorities.1 Health disparities such as access, quality of care, and health outcomes among minority groups exist in delivery and receipt of care within the health care system, and this includes the care of individuals with serious illness and at the end of life.1
Racial and ethnic minorities are less likely to receive goal-concordant care, participate in advance care planning, and have access to palliative care or hospice.2-4 They are more likely to die in a hospital, have inadequate pain and symptom management, and experience poor provider-patient communication.5-7 Other contributing factors include lack of knowledge of hospice and palliative care services, mistrust of the health care system, spiritual and religious beliefs, provider bias, and cultural beliefs.1
Despite these disparities, interventions have had limited success,8 and there are gaps in content, methods, and inclusion of racial and ethnic groups within palliative care research.7
Efforts to improve health equity for people with serious illness have been identified as an “urgent call to action.”1
A few recommended actionable items include delivering culturally competent care by ensuring availability of culturally and linguistically appropriate materials and information, education, and training for providers, and practicing cultural humility; contributing to workforce diversity by hiring and training diverse staff; and partnering with community organizations to build trust and to facilitate dissemination of culturally and linguistically appropriate information to providers in caring for their diverse patient populations.1,9
One of the first steps identified is to recognize that there is a problem and prioritize efforts to understand its “multifaceted nature.”10 This should occur on multiple levels including the individual (patient and caregiver), interpersonal (health care team), organization, and policy levels,10 and be done through clinical, research, and educational platforms.
At the interpersonal level, we as the health care team can start by reflecting, acknowledging biases, seeking educational and training opportunities on cross-cultural interactions, learning about cultural and spiritual beliefs, and developing skills in culturally and linguistically appropriate communication regarding goals of care and advance care planning.1,10
For those seeking resources, organizations such as the Center to Advance Palliative Care’s Project Equity and the American Academy of Hospice and Palliative Medicine have ongoing efforts to educate and train physicians and health care professionals to improve and understand health equity in palliative care by providing resource portals, toolkits, training, and general information.
It is imperative to move forward in actionable ways to address not only racial and ethnic disparities, but advance equity in serious illness care for health care organizations, providers, and policymakers.1
Dr. Kang is in the division of gerontology and geriatric medicine at the University of Washington, Seattle.
References
1. Barrett NJ et al. N C Med J. 2020;81:254-6.
2. Johnson KS et al. J Am Geriatr Soc. 2011;59:732-7.
3. Sharma RK et al. J Clin Oncol. 2015;33:3802-8.
4. Muni S et al. Chest. 2011;139:1025-33.
5. Anderson KO et al. J Pain. 2009;10:1187-204.
6. Mack JW et al. Arch Intern Med. 2010;170:1533-40.
7. Johnson KS. J Palliat Med. 2013;16(11):1329-34.
8. Brown CE et al. J Pain Symptom Manage. 2021;63(5):e465-e71.
9. Chambers B. Center for Advancing Palliative Care. July 9, 2020.
10. Koffman J et al. BMC Palliat Care. 2023;22(64):1-3.
Palliative care has been shown to improve quality of life, receipt of goal-concordant care, end-of-life decision-making, and improvement in pain and symptoms in individuals with serious illness. However, palliative and end-of-life care remain underutilized in racial and ethnic minorities.1 Health disparities such as access, quality of care, and health outcomes among minority groups exist in delivery and receipt of care within the health care system, and this includes the care of individuals with serious illness and at the end of life.1
Racial and ethnic minorities are less likely to receive goal-concordant care, participate in advance care planning, and have access to palliative care or hospice.2-4 They are more likely to die in a hospital, have inadequate pain and symptom management, and experience poor provider-patient communication.5-7 Other contributing factors include lack of knowledge of hospice and palliative care services, mistrust of the health care system, spiritual and religious beliefs, provider bias, and cultural beliefs.1
Despite these disparities, interventions have had limited success,8 and there are gaps in content, methods, and inclusion of racial and ethnic groups within palliative care research.7
Efforts to improve health equity for people with serious illness have been identified as an “urgent call to action.”1
A few recommended actionable items include delivering culturally competent care by ensuring availability of culturally and linguistically appropriate materials and information, education, and training for providers, and practicing cultural humility; contributing to workforce diversity by hiring and training diverse staff; and partnering with community organizations to build trust and to facilitate dissemination of culturally and linguistically appropriate information to providers in caring for their diverse patient populations.1,9
One of the first steps identified is to recognize that there is a problem and prioritize efforts to understand its “multifaceted nature.”10 This should occur on multiple levels including the individual (patient and caregiver), interpersonal (health care team), organization, and policy levels,10 and be done through clinical, research, and educational platforms.
At the interpersonal level, we as the health care team can start by reflecting, acknowledging biases, seeking educational and training opportunities on cross-cultural interactions, learning about cultural and spiritual beliefs, and developing skills in culturally and linguistically appropriate communication regarding goals of care and advance care planning.1,10
For those seeking resources, organizations such as the Center to Advance Palliative Care’s Project Equity and the American Academy of Hospice and Palliative Medicine have ongoing efforts to educate and train physicians and health care professionals to improve and understand health equity in palliative care by providing resource portals, toolkits, training, and general information.
It is imperative to move forward in actionable ways to address not only racial and ethnic disparities, but advance equity in serious illness care for health care organizations, providers, and policymakers.1
Dr. Kang is in the division of gerontology and geriatric medicine at the University of Washington, Seattle.
References
1. Barrett NJ et al. N C Med J. 2020;81:254-6.
2. Johnson KS et al. J Am Geriatr Soc. 2011;59:732-7.
3. Sharma RK et al. J Clin Oncol. 2015;33:3802-8.
4. Muni S et al. Chest. 2011;139:1025-33.
5. Anderson KO et al. J Pain. 2009;10:1187-204.
6. Mack JW et al. Arch Intern Med. 2010;170:1533-40.
7. Johnson KS. J Palliat Med. 2013;16(11):1329-34.
8. Brown CE et al. J Pain Symptom Manage. 2021;63(5):e465-e71.
9. Chambers B. Center for Advancing Palliative Care. July 9, 2020.
10. Koffman J et al. BMC Palliat Care. 2023;22(64):1-3.
Palliative care has been shown to improve quality of life, receipt of goal-concordant care, end-of-life decision-making, and improvement in pain and symptoms in individuals with serious illness. However, palliative and end-of-life care remain underutilized in racial and ethnic minorities.1 Health disparities such as access, quality of care, and health outcomes among minority groups exist in delivery and receipt of care within the health care system, and this includes the care of individuals with serious illness and at the end of life.1
Racial and ethnic minorities are less likely to receive goal-concordant care, participate in advance care planning, and have access to palliative care or hospice.2-4 They are more likely to die in a hospital, have inadequate pain and symptom management, and experience poor provider-patient communication.5-7 Other contributing factors include lack of knowledge of hospice and palliative care services, mistrust of the health care system, spiritual and religious beliefs, provider bias, and cultural beliefs.1
Despite these disparities, interventions have had limited success,8 and there are gaps in content, methods, and inclusion of racial and ethnic groups within palliative care research.7
Efforts to improve health equity for people with serious illness have been identified as an “urgent call to action.”1
A few recommended actionable items include delivering culturally competent care by ensuring availability of culturally and linguistically appropriate materials and information, education, and training for providers, and practicing cultural humility; contributing to workforce diversity by hiring and training diverse staff; and partnering with community organizations to build trust and to facilitate dissemination of culturally and linguistically appropriate information to providers in caring for their diverse patient populations.1,9
One of the first steps identified is to recognize that there is a problem and prioritize efforts to understand its “multifaceted nature.”10 This should occur on multiple levels including the individual (patient and caregiver), interpersonal (health care team), organization, and policy levels,10 and be done through clinical, research, and educational platforms.
At the interpersonal level, we as the health care team can start by reflecting, acknowledging biases, seeking educational and training opportunities on cross-cultural interactions, learning about cultural and spiritual beliefs, and developing skills in culturally and linguistically appropriate communication regarding goals of care and advance care planning.1,10
For those seeking resources, organizations such as the Center to Advance Palliative Care’s Project Equity and the American Academy of Hospice and Palliative Medicine have ongoing efforts to educate and train physicians and health care professionals to improve and understand health equity in palliative care by providing resource portals, toolkits, training, and general information.
It is imperative to move forward in actionable ways to address not only racial and ethnic disparities, but advance equity in serious illness care for health care organizations, providers, and policymakers.1
Dr. Kang is in the division of gerontology and geriatric medicine at the University of Washington, Seattle.
References
1. Barrett NJ et al. N C Med J. 2020;81:254-6.
2. Johnson KS et al. J Am Geriatr Soc. 2011;59:732-7.
3. Sharma RK et al. J Clin Oncol. 2015;33:3802-8.
4. Muni S et al. Chest. 2011;139:1025-33.
5. Anderson KO et al. J Pain. 2009;10:1187-204.
6. Mack JW et al. Arch Intern Med. 2010;170:1533-40.
7. Johnson KS. J Palliat Med. 2013;16(11):1329-34.
8. Brown CE et al. J Pain Symptom Manage. 2021;63(5):e465-e71.
9. Chambers B. Center for Advancing Palliative Care. July 9, 2020.
10. Koffman J et al. BMC Palliat Care. 2023;22(64):1-3.
Cold weather may challenge blood pressure control
A review of electronic health records of more than 60,000 U.S. adults being treated for hypertension found that on average, systolic BP rose by up to 1.7 mm Hg in the cold winter months, compared with the hot summer months.
On a population level, BP control rates decreased by up to 5% during the cold winter months, compared with control rates in the warm summer months.
“Some patients may benefit from increased pharmacological intervention to keep blood pressure controlled during the winter,” Robert Barrett, with the American Medical Association, Greenville, S.C., told this news organization.
“Individuals with hypertension or values near the range of hypertension may benefit from periodic blood pressure monitoring and improvements in physical activity and nutritional patterns during winter months to offset adverse effects from seasonal blood pressure changes,” Mr. Barrett added in a news release.
Mr. Barrett presented the study findings at the American Heart Association Hypertension Scientific Sessions 2023 in Boston.
Supportive data
Mr. Barrett explained that seasonal variation in BP has been previously documented, and as part of the evaluation for the AMA MAP Hypertension program, he and colleagues were interested in the effect of this variation on population control rates under standard metrics (visits with BP < 140/90 mm Hg).
They analyzed data from 60,676 men and women (mean age, 62 years) with hypertension from six health care organizations in the southeastern and midwestern United States that were participating in the quality improvement program.
During the roughly 5-year assessment period, none of the patients had changes in their antihypertensive medication, and all had at least one visit in each temperate season. The researchers estimated the seasonal effect on average systolic BP and BP control (defined as < 140/90 mm Hg).
Across a total of 453,787 visits, systolic BP during the winter averaged 0.47 mm Hg higher (95% confidence interval, 0.364-0.573) than the yearly average, with a significantly lower odds ratio for BP control (OR, 0.92; 95% CI, 0.91-0.94), the researchers report.
In contrast, average systolic BP was 0.92 mm Hg lower during the summer, with a higher likelihood of BP control (OR ,1.10; 95% CI, 1.07-1.12).
“Seasonal variation in blood pressure has a substantial effect on hypertension control, often defined as blood pressure < 140/90,” Barrett told this news organization.
“Patients with hypertension are less likely to have their blood pressure controlled during winter than summer months. If the blood pressure is very well controlled, for example to < 130/80, then seasonal variation will have little effect on control to < 140/90,” Mr. Barrett noted.
“However, if blood pressure is not well controlled, then patients near the 140/90 level could benefit from monitoring their blood pressure regularly, closer medical follow-up, and avoiding decreased physical activity and increased weight toward year end,” he added.
Wanpen Vongpatanasin, MD, clinical chair for the conference, said that it’s “well known that BP tends to lower during summer months and patients may be susceptible to dehydration and acute kidney injury when BP is too low, particularly when treated with certain medication such as diuretics.”
On the flip side, “cold weather predisposes to vasoconstriction as our blood vessel constrict to maintain core temperature and it could be challenging to manage BP. That’s why it is important for high BP patients to monitor home BP regularly,” said Dr. Vongpatanasin, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas.
The study had no commercial funding. Mr. Barrett and Dr. Vongpatanasin have no relevant disclosures.
A version of this article first appeared on Medscape.com.
A review of electronic health records of more than 60,000 U.S. adults being treated for hypertension found that on average, systolic BP rose by up to 1.7 mm Hg in the cold winter months, compared with the hot summer months.
On a population level, BP control rates decreased by up to 5% during the cold winter months, compared with control rates in the warm summer months.
“Some patients may benefit from increased pharmacological intervention to keep blood pressure controlled during the winter,” Robert Barrett, with the American Medical Association, Greenville, S.C., told this news organization.
“Individuals with hypertension or values near the range of hypertension may benefit from periodic blood pressure monitoring and improvements in physical activity and nutritional patterns during winter months to offset adverse effects from seasonal blood pressure changes,” Mr. Barrett added in a news release.
Mr. Barrett presented the study findings at the American Heart Association Hypertension Scientific Sessions 2023 in Boston.
Supportive data
Mr. Barrett explained that seasonal variation in BP has been previously documented, and as part of the evaluation for the AMA MAP Hypertension program, he and colleagues were interested in the effect of this variation on population control rates under standard metrics (visits with BP < 140/90 mm Hg).
They analyzed data from 60,676 men and women (mean age, 62 years) with hypertension from six health care organizations in the southeastern and midwestern United States that were participating in the quality improvement program.
During the roughly 5-year assessment period, none of the patients had changes in their antihypertensive medication, and all had at least one visit in each temperate season. The researchers estimated the seasonal effect on average systolic BP and BP control (defined as < 140/90 mm Hg).
Across a total of 453,787 visits, systolic BP during the winter averaged 0.47 mm Hg higher (95% confidence interval, 0.364-0.573) than the yearly average, with a significantly lower odds ratio for BP control (OR, 0.92; 95% CI, 0.91-0.94), the researchers report.
In contrast, average systolic BP was 0.92 mm Hg lower during the summer, with a higher likelihood of BP control (OR ,1.10; 95% CI, 1.07-1.12).
“Seasonal variation in blood pressure has a substantial effect on hypertension control, often defined as blood pressure < 140/90,” Barrett told this news organization.
“Patients with hypertension are less likely to have their blood pressure controlled during winter than summer months. If the blood pressure is very well controlled, for example to < 130/80, then seasonal variation will have little effect on control to < 140/90,” Mr. Barrett noted.
“However, if blood pressure is not well controlled, then patients near the 140/90 level could benefit from monitoring their blood pressure regularly, closer medical follow-up, and avoiding decreased physical activity and increased weight toward year end,” he added.
Wanpen Vongpatanasin, MD, clinical chair for the conference, said that it’s “well known that BP tends to lower during summer months and patients may be susceptible to dehydration and acute kidney injury when BP is too low, particularly when treated with certain medication such as diuretics.”
On the flip side, “cold weather predisposes to vasoconstriction as our blood vessel constrict to maintain core temperature and it could be challenging to manage BP. That’s why it is important for high BP patients to monitor home BP regularly,” said Dr. Vongpatanasin, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas.
The study had no commercial funding. Mr. Barrett and Dr. Vongpatanasin have no relevant disclosures.
A version of this article first appeared on Medscape.com.
A review of electronic health records of more than 60,000 U.S. adults being treated for hypertension found that on average, systolic BP rose by up to 1.7 mm Hg in the cold winter months, compared with the hot summer months.
On a population level, BP control rates decreased by up to 5% during the cold winter months, compared with control rates in the warm summer months.
“Some patients may benefit from increased pharmacological intervention to keep blood pressure controlled during the winter,” Robert Barrett, with the American Medical Association, Greenville, S.C., told this news organization.
“Individuals with hypertension or values near the range of hypertension may benefit from periodic blood pressure monitoring and improvements in physical activity and nutritional patterns during winter months to offset adverse effects from seasonal blood pressure changes,” Mr. Barrett added in a news release.
Mr. Barrett presented the study findings at the American Heart Association Hypertension Scientific Sessions 2023 in Boston.
Supportive data
Mr. Barrett explained that seasonal variation in BP has been previously documented, and as part of the evaluation for the AMA MAP Hypertension program, he and colleagues were interested in the effect of this variation on population control rates under standard metrics (visits with BP < 140/90 mm Hg).
They analyzed data from 60,676 men and women (mean age, 62 years) with hypertension from six health care organizations in the southeastern and midwestern United States that were participating in the quality improvement program.
During the roughly 5-year assessment period, none of the patients had changes in their antihypertensive medication, and all had at least one visit in each temperate season. The researchers estimated the seasonal effect on average systolic BP and BP control (defined as < 140/90 mm Hg).
Across a total of 453,787 visits, systolic BP during the winter averaged 0.47 mm Hg higher (95% confidence interval, 0.364-0.573) than the yearly average, with a significantly lower odds ratio for BP control (OR, 0.92; 95% CI, 0.91-0.94), the researchers report.
In contrast, average systolic BP was 0.92 mm Hg lower during the summer, with a higher likelihood of BP control (OR ,1.10; 95% CI, 1.07-1.12).
“Seasonal variation in blood pressure has a substantial effect on hypertension control, often defined as blood pressure < 140/90,” Barrett told this news organization.
“Patients with hypertension are less likely to have their blood pressure controlled during winter than summer months. If the blood pressure is very well controlled, for example to < 130/80, then seasonal variation will have little effect on control to < 140/90,” Mr. Barrett noted.
“However, if blood pressure is not well controlled, then patients near the 140/90 level could benefit from monitoring their blood pressure regularly, closer medical follow-up, and avoiding decreased physical activity and increased weight toward year end,” he added.
Wanpen Vongpatanasin, MD, clinical chair for the conference, said that it’s “well known that BP tends to lower during summer months and patients may be susceptible to dehydration and acute kidney injury when BP is too low, particularly when treated with certain medication such as diuretics.”
On the flip side, “cold weather predisposes to vasoconstriction as our blood vessel constrict to maintain core temperature and it could be challenging to manage BP. That’s why it is important for high BP patients to monitor home BP regularly,” said Dr. Vongpatanasin, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas.
The study had no commercial funding. Mr. Barrett and Dr. Vongpatanasin have no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM HYPERTENSION 2023
Landmark obesity legislation reintroduced in Congress
(TROA) (H.R. 4818/S. 2407). This legislation is a vital first step in expanding access to obesity treatment as it would expand Medicare coverage to include screening and treatment of obesity by a diverse range of health care providers who provide obesity care. The bill also includes coverage of behavioral counseling, prescription drugs for long-term weight management, and other prevention and treatment options.
The passage of TROA could lead to improved obesity care options because many private insurance companies model their covered health benefits to reflect Medicare.
You can help lawmakers understand the urgent need for expanded access to affordable, effective obesity treatments and how greater access to these tools will equip you to better care for your patients.
Use the new obesity advocacy toolkit to find the tools and resources you need, including an email template, sample phone script, op-ed template, and more, to assist you in reaching out to your elected officials and urging them to support the passage of TROA.
(TROA) (H.R. 4818/S. 2407). This legislation is a vital first step in expanding access to obesity treatment as it would expand Medicare coverage to include screening and treatment of obesity by a diverse range of health care providers who provide obesity care. The bill also includes coverage of behavioral counseling, prescription drugs for long-term weight management, and other prevention and treatment options.
The passage of TROA could lead to improved obesity care options because many private insurance companies model their covered health benefits to reflect Medicare.
You can help lawmakers understand the urgent need for expanded access to affordable, effective obesity treatments and how greater access to these tools will equip you to better care for your patients.
Use the new obesity advocacy toolkit to find the tools and resources you need, including an email template, sample phone script, op-ed template, and more, to assist you in reaching out to your elected officials and urging them to support the passage of TROA.
(TROA) (H.R. 4818/S. 2407). This legislation is a vital first step in expanding access to obesity treatment as it would expand Medicare coverage to include screening and treatment of obesity by a diverse range of health care providers who provide obesity care. The bill also includes coverage of behavioral counseling, prescription drugs for long-term weight management, and other prevention and treatment options.
The passage of TROA could lead to improved obesity care options because many private insurance companies model their covered health benefits to reflect Medicare.
You can help lawmakers understand the urgent need for expanded access to affordable, effective obesity treatments and how greater access to these tools will equip you to better care for your patients.
Use the new obesity advocacy toolkit to find the tools and resources you need, including an email template, sample phone script, op-ed template, and more, to assist you in reaching out to your elected officials and urging them to support the passage of TROA.