User login
Psoriasis tied to abdominal aortic aneurysm in nationwide study
Patients with severe psoriasis were nearly 70% more likely to develop abdominal aortic aneurysms compared with the general population, according to a Danish population-based cohort study.
The findings augment existing evidence linking psoriasis and cardiovascular diseases, wrote Dr. Usman Khalid of Copenhagen University Herlev and Gentofte Hospital, Denmark. The report was published online April 14 in Arteriosclerosis, Thrombosis, and Vascular Biology.
While the mechanisms for the link are unclear, “emerging evidence suggests that AAA is a focal representation of a systemic disease with a distinct inflammatory component, rather than a mere consequence of atherosclerosis,” wrote Dr. Khalid and his associates.
Several case series have linked AAA with other autoimmune disorders, including systemic lupus erythematosus and rheumatoid arthritis, they noted. Their study comprised nearly 5.5 million adults in Denmark between 1997 and 2011. The researchers identified 59,423 patients with mild psoriasis and 11,566 patients with severe psoriasis (Arterioscler Thromb Vasc Biol. 2016 April 14. doi: 10.1161/ATVBAHA.116.307449).
The incidence of AAA in the reference population was 3.72 cases per 10,000 person-years, with an average follow-up period of 14.4 years. In contrast, the incidence of AAA in patients with mild psoriasis was 7.30 cases per 10,000 person-years, and the rate in patients with severe psoriasis was 9.87 cases of per 10,000 person-years, with average follow-up periods of 5.7 years. Both mild and severe psoriasis were significantly associated with AAA after the researchers accounted for age, sex, comorbidities, medications, socioeconomic status, and smoking, with adjusted incidence rate ratios of 1.20 (95% confidence interval, 1.03-1.39) and 1.67 (95% CI, 1.21-2.32), respectively.
The historical view that AAA is caused mainly by atherosclerosis has largely been upended, the researchers noted. Instead, AAA appears to be a multifactorial process involving inflammation, matrix degradation, thrombosis, and aortic wall stress. Furthermore, inflammation in both AAA and psoriasis is centrally mediated by T-helper-17 cells and interleukin-17. Together, the data suggest that shared inflammatory mechanisms link psoriasis and AAA, especially because the association correlates with psoriatic disease activity, they said. “This finding clearly requires independent replication, and the clinical consequences are unclear at present.”
The LEO Foundation and the Novo Nordisk Foundation funded the study. Dr. Khalid had no disclosures. Four coinvestigators reported financial ties with Abbott, Pfizer, AstraZeneca, Bayer, and several other pharmaceutical companies.
Patients with severe psoriasis were nearly 70% more likely to develop abdominal aortic aneurysms compared with the general population, according to a Danish population-based cohort study.
The findings augment existing evidence linking psoriasis and cardiovascular diseases, wrote Dr. Usman Khalid of Copenhagen University Herlev and Gentofte Hospital, Denmark. The report was published online April 14 in Arteriosclerosis, Thrombosis, and Vascular Biology.
While the mechanisms for the link are unclear, “emerging evidence suggests that AAA is a focal representation of a systemic disease with a distinct inflammatory component, rather than a mere consequence of atherosclerosis,” wrote Dr. Khalid and his associates.
Several case series have linked AAA with other autoimmune disorders, including systemic lupus erythematosus and rheumatoid arthritis, they noted. Their study comprised nearly 5.5 million adults in Denmark between 1997 and 2011. The researchers identified 59,423 patients with mild psoriasis and 11,566 patients with severe psoriasis (Arterioscler Thromb Vasc Biol. 2016 April 14. doi: 10.1161/ATVBAHA.116.307449).
The incidence of AAA in the reference population was 3.72 cases per 10,000 person-years, with an average follow-up period of 14.4 years. In contrast, the incidence of AAA in patients with mild psoriasis was 7.30 cases per 10,000 person-years, and the rate in patients with severe psoriasis was 9.87 cases of per 10,000 person-years, with average follow-up periods of 5.7 years. Both mild and severe psoriasis were significantly associated with AAA after the researchers accounted for age, sex, comorbidities, medications, socioeconomic status, and smoking, with adjusted incidence rate ratios of 1.20 (95% confidence interval, 1.03-1.39) and 1.67 (95% CI, 1.21-2.32), respectively.
The historical view that AAA is caused mainly by atherosclerosis has largely been upended, the researchers noted. Instead, AAA appears to be a multifactorial process involving inflammation, matrix degradation, thrombosis, and aortic wall stress. Furthermore, inflammation in both AAA and psoriasis is centrally mediated by T-helper-17 cells and interleukin-17. Together, the data suggest that shared inflammatory mechanisms link psoriasis and AAA, especially because the association correlates with psoriatic disease activity, they said. “This finding clearly requires independent replication, and the clinical consequences are unclear at present.”
The LEO Foundation and the Novo Nordisk Foundation funded the study. Dr. Khalid had no disclosures. Four coinvestigators reported financial ties with Abbott, Pfizer, AstraZeneca, Bayer, and several other pharmaceutical companies.
Patients with severe psoriasis were nearly 70% more likely to develop abdominal aortic aneurysms compared with the general population, according to a Danish population-based cohort study.
The findings augment existing evidence linking psoriasis and cardiovascular diseases, wrote Dr. Usman Khalid of Copenhagen University Herlev and Gentofte Hospital, Denmark. The report was published online April 14 in Arteriosclerosis, Thrombosis, and Vascular Biology.
While the mechanisms for the link are unclear, “emerging evidence suggests that AAA is a focal representation of a systemic disease with a distinct inflammatory component, rather than a mere consequence of atherosclerosis,” wrote Dr. Khalid and his associates.
Several case series have linked AAA with other autoimmune disorders, including systemic lupus erythematosus and rheumatoid arthritis, they noted. Their study comprised nearly 5.5 million adults in Denmark between 1997 and 2011. The researchers identified 59,423 patients with mild psoriasis and 11,566 patients with severe psoriasis (Arterioscler Thromb Vasc Biol. 2016 April 14. doi: 10.1161/ATVBAHA.116.307449).
The incidence of AAA in the reference population was 3.72 cases per 10,000 person-years, with an average follow-up period of 14.4 years. In contrast, the incidence of AAA in patients with mild psoriasis was 7.30 cases per 10,000 person-years, and the rate in patients with severe psoriasis was 9.87 cases of per 10,000 person-years, with average follow-up periods of 5.7 years. Both mild and severe psoriasis were significantly associated with AAA after the researchers accounted for age, sex, comorbidities, medications, socioeconomic status, and smoking, with adjusted incidence rate ratios of 1.20 (95% confidence interval, 1.03-1.39) and 1.67 (95% CI, 1.21-2.32), respectively.
The historical view that AAA is caused mainly by atherosclerosis has largely been upended, the researchers noted. Instead, AAA appears to be a multifactorial process involving inflammation, matrix degradation, thrombosis, and aortic wall stress. Furthermore, inflammation in both AAA and psoriasis is centrally mediated by T-helper-17 cells and interleukin-17. Together, the data suggest that shared inflammatory mechanisms link psoriasis and AAA, especially because the association correlates with psoriatic disease activity, they said. “This finding clearly requires independent replication, and the clinical consequences are unclear at present.”
The LEO Foundation and the Novo Nordisk Foundation funded the study. Dr. Khalid had no disclosures. Four coinvestigators reported financial ties with Abbott, Pfizer, AstraZeneca, Bayer, and several other pharmaceutical companies.
FROM ARTERIOSCLEROSIS, THROMBOSIS, AND VASCULAR BIOLOGY
Key clinical point: Psoriasis predicted abdominal aortic aneurysm in a large, population-based study.
Major finding: The adjusted risk of abdominal aortic aneurysm was 1.67 times greater among patients with severe psoriasis than in the reference population.
Data source: A retrospective cohort study of 5.5 million Danish adults, including 59,423 patients with mild psoriasis and 11,566 patients with severe psoriasis.
Disclosures: The LEO Foundation and the Novo Nordisk Foundation funded the study. Dr. Khalid had no disclosures. Four coinvestigators reported financial ties with Abbott, Pfizer, AstraZeneca, Bayer, and several other pharmaceutical companies.
Cyst on the Eyebrow
The best diagnosis is:
a. bronchogenic cyst
b. dermoid cyst
c. epidermal inclusion cyst
d. hidrocystoma
e. steatocystoma
|
|
| H&E, original magnification ×40. |
|
| H&E, original magnification ×100. |
Continue to the next page for the diagnosis >>
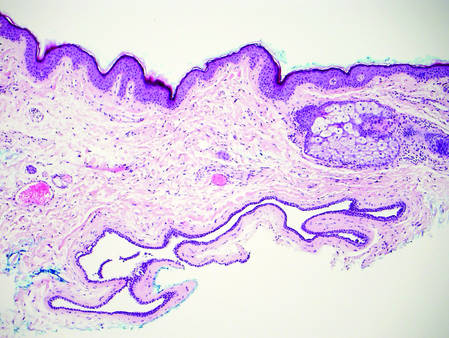
Dermoid Cyst
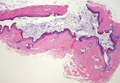
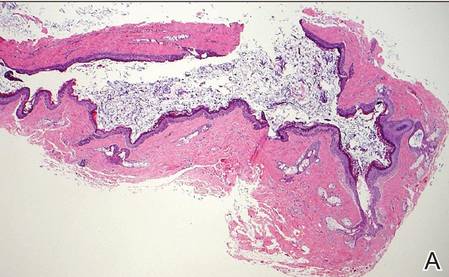
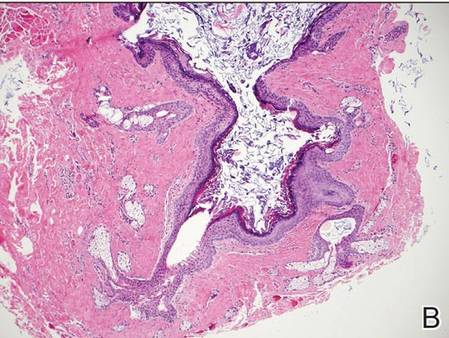
Dermoid cysts often present clinically as firm subcutaneous nodules on the head or neck in young children. They tend to arise along the lateral aspect of the eyebrow but also can occur on the nose, forehead, neck, chest, or scalp.1 Dermoid cysts are thought to arise from the sequestration of ectodermal tissues along the embryonic fusion planes during development.2 As such, they represent congenital defects and often are identified at birth; however, some are not noticed until much later when they enlarge or become inflamed or infected. Midline dermoid cysts may be associated with underlying dysraphism or intracranial extension.3,4 Thus, any midline lesion warrants evaluation that incorporates imaging with computed tomography or magnetic resonance imaging.4,5 Histologically, dermoid cysts are lined by a keratinizing stratified squamous epithelium (quiz image A), but the lining may be brightly eosinophilic and wavy resembling shark teeth.1,3 The wall of a dermoid cyst commonly contains mature adnexal structures such as terminal hair follicles, sebaceous glands, apocrine glands, and/or eccrine glands (quiz image B).1 Smooth muscle also may be seen within the lining; however, bone and cartilage are not commonly reported in dermoid cysts.2 Lamellar keratin is typical of the cyst contents, and terminal hair shafts also are sometimes noted within the cystic space (quiz image B).1,2 Treatment options include excision at the time of diagnosis or close clinical monitoring with subsequent excision if the lesion grows or becomes symptomatic.4,5 Many practitioners opt to excise these cysts at diagnosis, as untreated lesions are at risk for infection and/or inflammation or may be cosmetically deforming.6,7 Surgical resection, including removal of the wall of the cyst, is curative and reoccurrence is rare.5
| |
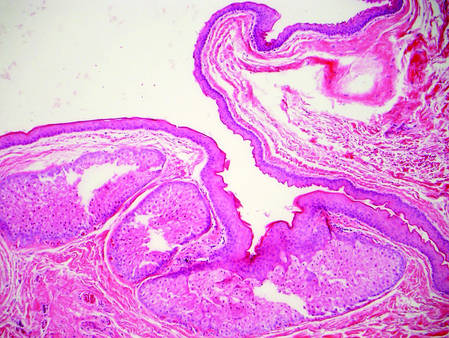
Figure 1. Bronchogenic cyst demonstrating a ciliated pseudostratified epithelial lining encircled by smooth muscle (H&E, original magnification ×200). | |
| |
| Figure 2. Epidermal inclusion cyst containing loose lamellar keratin and a lining that closely resembles the surface epidermis (H&E, original magnification ×40). |
|
Bronchogenic cysts demonstrate an epithelial lining that often is pseudostratified cuboidal or columnar as well as ciliated (Figure 1). Goblet cells are present in the lining in approximately 50% of cases. Smooth muscle may be seen circumferentially surrounding the cyst lining, and rare cases also contain cartilage.1 In contrast to dermoid cysts, other types of adnexal structures are not found within the lining. Bronchogenic cysts that arise in the skin are extremely rare.2 These cysts are thought to arise from respiratory epithelium that has been sequestered during embryologic formation of the tracheobronchial tree. They often are seen overlying the suprasternal notch and occasionally are found on the anterior aspect of the neck or chin. These cysts also are present at birth, similar to dermoid cysts.3
Epidermal inclusion cysts have a lining that histologically bears close resemblance to the surface epidermis. These cysts contain loose lamellar keratin, similar to a dermoid cyst. In contrast, the lining of an epidermal inclusion cyst will lack adnexal structures (Figure 2).1 Clinically, epidermal inclusion cysts often present as smooth, dome-shaped papules and nodules with a central punctum. They are classically found on the face, neck, and trunk. These cysts are thought to arise after a traumatic insult to the pilosebaceous unit.2
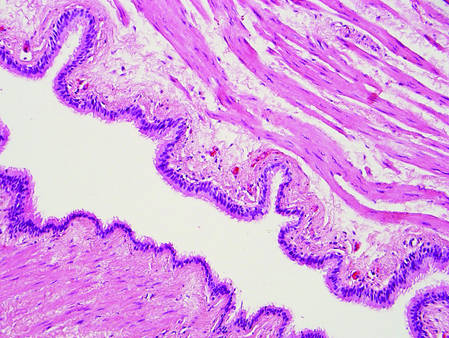
Hidrocystomas can be apocrine or eccrine.3 Eccrine hidrocystomas are unilocular cysts that are lined by 2 layers of flattened to cuboidal epithelial cells (Figure 3). The cysts are filled with clear fluid and often are found adjacent to normal eccrine glands.1 Apocrine hidrocystomas are unilocular or multilocular cysts that are lined by 1 to several layers of epithelial cells. The lining of an apocrine hidrocystoma will often exhibit luminal decapitation secretion.3 Apocrine and eccrine hidrocystomas are clinically identical and appear as blue translucent papules on the cheeks or eyelids of adults.1-3 They usually occur periorbitally but also can be seen on the trunk, popliteal fossa, external ears, or vulva. Eccrine hidrocystomas can wax and wane in accordance with the amount of sweat produced; thus, they often expand in size during the summer months.2
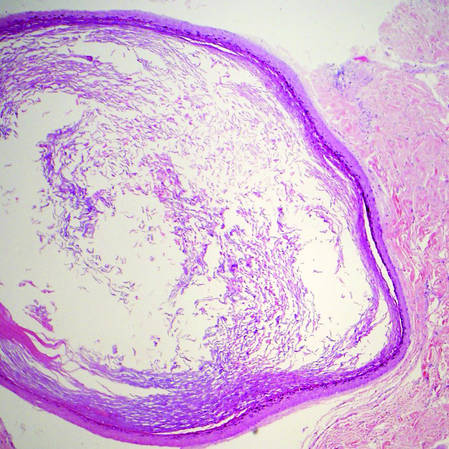
Steatocystomas, or simple sebaceous duct cysts, histologically demonstrate a characteristically wavy and eosinophilic cuticle resembling shark teeth (Figure 4) similar to the lining of the sebaceous duct where it enters the follicle.1 Sebaceous glands are an almost invariable feature, either present within the lining of the cyst (Figure 4) or in the adjacent tissue.2 In comparison, dermoid cysts may have a red wavy cuticle but also will usually have terminal hair follicles or eccrine or apocrine glands within the wall of the cyst. Steatocystomas typically are collapsed and empty or only contain sebaceous debris (Figure 4) rather than the lamellar keratin seen in dermoid and epidermoid inclusion cysts. Steatocystomas can occur as solitary (steatocystoma simplex) or multiple (steatocystoma multiplex) lesions.1,3 They are clinically comprised of small dome-shaped papules that often are translucent and yellow. These cysts are commonly found on the sternum of males and the axillae or groin of females.2
| 
| |
Figure 3. Eccrine hidrocystoma with clear contents and lined by 2 layers of cuboidal epithelial cells (H&E, original magnification ×100). | Figure 4. Steatocystoma with a red wavy cuticle, sparse sebaceous contents, and sebaceous glands within the lining (H&E, original magnification ×100). |
|
1. Elston DM, Ferringer TC, Ko C, et al. Dermatopathology: Requisites in Dermatology. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2014.
2. Calonje JE, Brenn T, Lazar AJ, et al. McKee’s Pathology of the Skin. 4th ed. St Louis, MO: Elsevier/Saunders; 2012.
3. Bolognia JL, Jorizzo JL, Shaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012.
4. Orozco-Covarrubias L, Lara-Carpio R, Saez-De-Ocariz M, et al. Dermoid cysts: a report of 75 pediatric patients. Pediatr Dermatol. 2013;30:706-711.
5. Sorenson EP, Powel JE, Rozzelle CJ, et al. Scalp dermoids: a review of their anatomy, diagnosis, and treatment. Childs Nerv Syst. 2013;29:375-380.
6. Pryor SG, Lewis JE, Weaver AL, et al. Pediatric dermoid cysts of the head and neck. Otolarynol Head Neck Surg. 2005;132:938-942.
7. Abou-Rayyah Y, Rose GE, Konrad H, et al. Clinical, radiological and pathological examination of periocular dermoid cysts: evidence of inflammation from an early age. Eye (Lond). 2002;16:507-512.
The best diagnosis is:
a. bronchogenic cyst
b. dermoid cyst
c. epidermal inclusion cyst
d. hidrocystoma
e. steatocystoma
|

|
| H&E, original magnification ×40. |

|
| H&E, original magnification ×100. |
Continue to the next page for the diagnosis >>
Dermoid Cyst
Dermoid cysts often present clinically as firm subcutaneous nodules on the head or neck in young children. They tend to arise along the lateral aspect of the eyebrow but also can occur on the nose, forehead, neck, chest, or scalp.1 Dermoid cysts are thought to arise from the sequestration of ectodermal tissues along the embryonic fusion planes during development.2 As such, they represent congenital defects and often are identified at birth; however, some are not noticed until much later when they enlarge or become inflamed or infected. Midline dermoid cysts may be associated with underlying dysraphism or intracranial extension.3,4 Thus, any midline lesion warrants evaluation that incorporates imaging with computed tomography or magnetic resonance imaging.4,5 Histologically, dermoid cysts are lined by a keratinizing stratified squamous epithelium (quiz image A), but the lining may be brightly eosinophilic and wavy resembling shark teeth.1,3 The wall of a dermoid cyst commonly contains mature adnexal structures such as terminal hair follicles, sebaceous glands, apocrine glands, and/or eccrine glands (quiz image B).1 Smooth muscle also may be seen within the lining; however, bone and cartilage are not commonly reported in dermoid cysts.2 Lamellar keratin is typical of the cyst contents, and terminal hair shafts also are sometimes noted within the cystic space (quiz image B).1,2 Treatment options include excision at the time of diagnosis or close clinical monitoring with subsequent excision if the lesion grows or becomes symptomatic.4,5 Many practitioners opt to excise these cysts at diagnosis, as untreated lesions are at risk for infection and/or inflammation or may be cosmetically deforming.6,7 Surgical resection, including removal of the wall of the cyst, is curative and reoccurrence is rare.5

| |
Figure 1. Bronchogenic cyst demonstrating a ciliated pseudostratified epithelial lining encircled by smooth muscle (H&E, original magnification ×200). | |

| |
| Figure 2. Epidermal inclusion cyst containing loose lamellar keratin and a lining that closely resembles the surface epidermis (H&E, original magnification ×40). |
|
Bronchogenic cysts demonstrate an epithelial lining that often is pseudostratified cuboidal or columnar as well as ciliated (Figure 1). Goblet cells are present in the lining in approximately 50% of cases. Smooth muscle may be seen circumferentially surrounding the cyst lining, and rare cases also contain cartilage.1 In contrast to dermoid cysts, other types of adnexal structures are not found within the lining. Bronchogenic cysts that arise in the skin are extremely rare.2 These cysts are thought to arise from respiratory epithelium that has been sequestered during embryologic formation of the tracheobronchial tree. They often are seen overlying the suprasternal notch and occasionally are found on the anterior aspect of the neck or chin. These cysts also are present at birth, similar to dermoid cysts.3
Epidermal inclusion cysts have a lining that histologically bears close resemblance to the surface epidermis. These cysts contain loose lamellar keratin, similar to a dermoid cyst. In contrast, the lining of an epidermal inclusion cyst will lack adnexal structures (Figure 2).1 Clinically, epidermal inclusion cysts often present as smooth, dome-shaped papules and nodules with a central punctum. They are classically found on the face, neck, and trunk. These cysts are thought to arise after a traumatic insult to the pilosebaceous unit.2
Hidrocystomas can be apocrine or eccrine.3 Eccrine hidrocystomas are unilocular cysts that are lined by 2 layers of flattened to cuboidal epithelial cells (Figure 3). The cysts are filled with clear fluid and often are found adjacent to normal eccrine glands.1 Apocrine hidrocystomas are unilocular or multilocular cysts that are lined by 1 to several layers of epithelial cells. The lining of an apocrine hidrocystoma will often exhibit luminal decapitation secretion.3 Apocrine and eccrine hidrocystomas are clinically identical and appear as blue translucent papules on the cheeks or eyelids of adults.1-3 They usually occur periorbitally but also can be seen on the trunk, popliteal fossa, external ears, or vulva. Eccrine hidrocystomas can wax and wane in accordance with the amount of sweat produced; thus, they often expand in size during the summer months.2
Steatocystomas, or simple sebaceous duct cysts, histologically demonstrate a characteristically wavy and eosinophilic cuticle resembling shark teeth (Figure 4) similar to the lining of the sebaceous duct where it enters the follicle.1 Sebaceous glands are an almost invariable feature, either present within the lining of the cyst (Figure 4) or in the adjacent tissue.2 In comparison, dermoid cysts may have a red wavy cuticle but also will usually have terminal hair follicles or eccrine or apocrine glands within the wall of the cyst. Steatocystomas typically are collapsed and empty or only contain sebaceous debris (Figure 4) rather than the lamellar keratin seen in dermoid and epidermoid inclusion cysts. Steatocystomas can occur as solitary (steatocystoma simplex) or multiple (steatocystoma multiplex) lesions.1,3 They are clinically comprised of small dome-shaped papules that often are translucent and yellow. These cysts are commonly found on the sternum of males and the axillae or groin of females.2

| 
| |
Figure 3. Eccrine hidrocystoma with clear contents and lined by 2 layers of cuboidal epithelial cells (H&E, original magnification ×100). | Figure 4. Steatocystoma with a red wavy cuticle, sparse sebaceous contents, and sebaceous glands within the lining (H&E, original magnification ×100). |
|
The best diagnosis is:
a. bronchogenic cyst
b. dermoid cyst
c. epidermal inclusion cyst
d. hidrocystoma
e. steatocystoma
|

|
| H&E, original magnification ×40. |

|
| H&E, original magnification ×100. |
Continue to the next page for the diagnosis >>
Dermoid Cyst
Dermoid cysts often present clinically as firm subcutaneous nodules on the head or neck in young children. They tend to arise along the lateral aspect of the eyebrow but also can occur on the nose, forehead, neck, chest, or scalp.1 Dermoid cysts are thought to arise from the sequestration of ectodermal tissues along the embryonic fusion planes during development.2 As such, they represent congenital defects and often are identified at birth; however, some are not noticed until much later when they enlarge or become inflamed or infected. Midline dermoid cysts may be associated with underlying dysraphism or intracranial extension.3,4 Thus, any midline lesion warrants evaluation that incorporates imaging with computed tomography or magnetic resonance imaging.4,5 Histologically, dermoid cysts are lined by a keratinizing stratified squamous epithelium (quiz image A), but the lining may be brightly eosinophilic and wavy resembling shark teeth.1,3 The wall of a dermoid cyst commonly contains mature adnexal structures such as terminal hair follicles, sebaceous glands, apocrine glands, and/or eccrine glands (quiz image B).1 Smooth muscle also may be seen within the lining; however, bone and cartilage are not commonly reported in dermoid cysts.2 Lamellar keratin is typical of the cyst contents, and terminal hair shafts also are sometimes noted within the cystic space (quiz image B).1,2 Treatment options include excision at the time of diagnosis or close clinical monitoring with subsequent excision if the lesion grows or becomes symptomatic.4,5 Many practitioners opt to excise these cysts at diagnosis, as untreated lesions are at risk for infection and/or inflammation or may be cosmetically deforming.6,7 Surgical resection, including removal of the wall of the cyst, is curative and reoccurrence is rare.5

| |
Figure 1. Bronchogenic cyst demonstrating a ciliated pseudostratified epithelial lining encircled by smooth muscle (H&E, original magnification ×200). | |

| |
| Figure 2. Epidermal inclusion cyst containing loose lamellar keratin and a lining that closely resembles the surface epidermis (H&E, original magnification ×40). |
|
Bronchogenic cysts demonstrate an epithelial lining that often is pseudostratified cuboidal or columnar as well as ciliated (Figure 1). Goblet cells are present in the lining in approximately 50% of cases. Smooth muscle may be seen circumferentially surrounding the cyst lining, and rare cases also contain cartilage.1 In contrast to dermoid cysts, other types of adnexal structures are not found within the lining. Bronchogenic cysts that arise in the skin are extremely rare.2 These cysts are thought to arise from respiratory epithelium that has been sequestered during embryologic formation of the tracheobronchial tree. They often are seen overlying the suprasternal notch and occasionally are found on the anterior aspect of the neck or chin. These cysts also are present at birth, similar to dermoid cysts.3
Epidermal inclusion cysts have a lining that histologically bears close resemblance to the surface epidermis. These cysts contain loose lamellar keratin, similar to a dermoid cyst. In contrast, the lining of an epidermal inclusion cyst will lack adnexal structures (Figure 2).1 Clinically, epidermal inclusion cysts often present as smooth, dome-shaped papules and nodules with a central punctum. They are classically found on the face, neck, and trunk. These cysts are thought to arise after a traumatic insult to the pilosebaceous unit.2
Hidrocystomas can be apocrine or eccrine.3 Eccrine hidrocystomas are unilocular cysts that are lined by 2 layers of flattened to cuboidal epithelial cells (Figure 3). The cysts are filled with clear fluid and often are found adjacent to normal eccrine glands.1 Apocrine hidrocystomas are unilocular or multilocular cysts that are lined by 1 to several layers of epithelial cells. The lining of an apocrine hidrocystoma will often exhibit luminal decapitation secretion.3 Apocrine and eccrine hidrocystomas are clinically identical and appear as blue translucent papules on the cheeks or eyelids of adults.1-3 They usually occur periorbitally but also can be seen on the trunk, popliteal fossa, external ears, or vulva. Eccrine hidrocystomas can wax and wane in accordance with the amount of sweat produced; thus, they often expand in size during the summer months.2
Steatocystomas, or simple sebaceous duct cysts, histologically demonstrate a characteristically wavy and eosinophilic cuticle resembling shark teeth (Figure 4) similar to the lining of the sebaceous duct where it enters the follicle.1 Sebaceous glands are an almost invariable feature, either present within the lining of the cyst (Figure 4) or in the adjacent tissue.2 In comparison, dermoid cysts may have a red wavy cuticle but also will usually have terminal hair follicles or eccrine or apocrine glands within the wall of the cyst. Steatocystomas typically are collapsed and empty or only contain sebaceous debris (Figure 4) rather than the lamellar keratin seen in dermoid and epidermoid inclusion cysts. Steatocystomas can occur as solitary (steatocystoma simplex) or multiple (steatocystoma multiplex) lesions.1,3 They are clinically comprised of small dome-shaped papules that often are translucent and yellow. These cysts are commonly found on the sternum of males and the axillae or groin of females.2

| 
| |
Figure 3. Eccrine hidrocystoma with clear contents and lined by 2 layers of cuboidal epithelial cells (H&E, original magnification ×100). | Figure 4. Steatocystoma with a red wavy cuticle, sparse sebaceous contents, and sebaceous glands within the lining (H&E, original magnification ×100). |
|
1. Elston DM, Ferringer TC, Ko C, et al. Dermatopathology: Requisites in Dermatology. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2014.
2. Calonje JE, Brenn T, Lazar AJ, et al. McKee’s Pathology of the Skin. 4th ed. St Louis, MO: Elsevier/Saunders; 2012.
3. Bolognia JL, Jorizzo JL, Shaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012.
4. Orozco-Covarrubias L, Lara-Carpio R, Saez-De-Ocariz M, et al. Dermoid cysts: a report of 75 pediatric patients. Pediatr Dermatol. 2013;30:706-711.
5. Sorenson EP, Powel JE, Rozzelle CJ, et al. Scalp dermoids: a review of their anatomy, diagnosis, and treatment. Childs Nerv Syst. 2013;29:375-380.
6. Pryor SG, Lewis JE, Weaver AL, et al. Pediatric dermoid cysts of the head and neck. Otolarynol Head Neck Surg. 2005;132:938-942.
7. Abou-Rayyah Y, Rose GE, Konrad H, et al. Clinical, radiological and pathological examination of periocular dermoid cysts: evidence of inflammation from an early age. Eye (Lond). 2002;16:507-512.
1. Elston DM, Ferringer TC, Ko C, et al. Dermatopathology: Requisites in Dermatology. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2014.
2. Calonje JE, Brenn T, Lazar AJ, et al. McKee’s Pathology of the Skin. 4th ed. St Louis, MO: Elsevier/Saunders; 2012.
3. Bolognia JL, Jorizzo JL, Shaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012.
4. Orozco-Covarrubias L, Lara-Carpio R, Saez-De-Ocariz M, et al. Dermoid cysts: a report of 75 pediatric patients. Pediatr Dermatol. 2013;30:706-711.
5. Sorenson EP, Powel JE, Rozzelle CJ, et al. Scalp dermoids: a review of their anatomy, diagnosis, and treatment. Childs Nerv Syst. 2013;29:375-380.
6. Pryor SG, Lewis JE, Weaver AL, et al. Pediatric dermoid cysts of the head and neck. Otolarynol Head Neck Surg. 2005;132:938-942.
7. Abou-Rayyah Y, Rose GE, Konrad H, et al. Clinical, radiological and pathological examination of periocular dermoid cysts: evidence of inflammation from an early age. Eye (Lond). 2002;16:507-512.
Breastfeeding reduces infants’ respiratory symptoms early on
Breastfeeding during the first 27 weeks of life had a risk-specific effect on reducing respiratory symptoms in healthy term infants, based on data from a prospective cohort study of 436 children in Switzerland.
“Breastfeeding is generally accepted to be protective against respiratory symptoms in early life,” but most published studies on this topic are cross-sectional and more likely biased, wrote Dr. Olga Gorlanova of the University of Basel (Switzerland) and her colleagues.
The researchers studied infants enrolled in the Bern-Basel Infant Lung Development cohort via weekly telephone interviews during the first year of life. In addition, weekly measurements of environmental particulate matter were collected from local monitoring stations. Risk factors included maternal history of atopy, vaginal vs. cesarean delivery, parents’ level of education, smoking during and after pregnancy, number of older siblings, child care attendance, and housing conditions.
Overall, infants breastfed during the first 27 weeks of life had significantly reduced respiratory symptoms, compared with nonbreastfed infants (risk ratio, .70)
The study “suggests that breastfeeding attenuates the effects of risk factors such as sex, age, gestational age, cesarean delivery, and prenatal maternal tobacco smoking in healthy term infants,” Dr. Gorlanova and her associates wrote. No significant interaction was noted between breastfeeding and child care attendance, number of older siblings, maternal atopy, or environmental particulate matter.
Read the full study here (J Pediatr. 2016. doi: 10.1016/j.jpeds.2016.03.041).
Breastfeeding during the first 27 weeks of life had a risk-specific effect on reducing respiratory symptoms in healthy term infants, based on data from a prospective cohort study of 436 children in Switzerland.
“Breastfeeding is generally accepted to be protective against respiratory symptoms in early life,” but most published studies on this topic are cross-sectional and more likely biased, wrote Dr. Olga Gorlanova of the University of Basel (Switzerland) and her colleagues.
The researchers studied infants enrolled in the Bern-Basel Infant Lung Development cohort via weekly telephone interviews during the first year of life. In addition, weekly measurements of environmental particulate matter were collected from local monitoring stations. Risk factors included maternal history of atopy, vaginal vs. cesarean delivery, parents’ level of education, smoking during and after pregnancy, number of older siblings, child care attendance, and housing conditions.
Overall, infants breastfed during the first 27 weeks of life had significantly reduced respiratory symptoms, compared with nonbreastfed infants (risk ratio, .70)
The study “suggests that breastfeeding attenuates the effects of risk factors such as sex, age, gestational age, cesarean delivery, and prenatal maternal tobacco smoking in healthy term infants,” Dr. Gorlanova and her associates wrote. No significant interaction was noted between breastfeeding and child care attendance, number of older siblings, maternal atopy, or environmental particulate matter.
Read the full study here (J Pediatr. 2016. doi: 10.1016/j.jpeds.2016.03.041).
Breastfeeding during the first 27 weeks of life had a risk-specific effect on reducing respiratory symptoms in healthy term infants, based on data from a prospective cohort study of 436 children in Switzerland.
“Breastfeeding is generally accepted to be protective against respiratory symptoms in early life,” but most published studies on this topic are cross-sectional and more likely biased, wrote Dr. Olga Gorlanova of the University of Basel (Switzerland) and her colleagues.
The researchers studied infants enrolled in the Bern-Basel Infant Lung Development cohort via weekly telephone interviews during the first year of life. In addition, weekly measurements of environmental particulate matter were collected from local monitoring stations. Risk factors included maternal history of atopy, vaginal vs. cesarean delivery, parents’ level of education, smoking during and after pregnancy, number of older siblings, child care attendance, and housing conditions.
Overall, infants breastfed during the first 27 weeks of life had significantly reduced respiratory symptoms, compared with nonbreastfed infants (risk ratio, .70)
The study “suggests that breastfeeding attenuates the effects of risk factors such as sex, age, gestational age, cesarean delivery, and prenatal maternal tobacco smoking in healthy term infants,” Dr. Gorlanova and her associates wrote. No significant interaction was noted between breastfeeding and child care attendance, number of older siblings, maternal atopy, or environmental particulate matter.
Read the full study here (J Pediatr. 2016. doi: 10.1016/j.jpeds.2016.03.041).
FROM THE JOURNAL OF PEDIATRICS
PPI cuts GI events from low- and high-dose aspirin
CHICAGO – Six months of treatment with a proton pump inhibitor (PPI) is a safe way to cut the incidence of major gastrointestinal events in cardiovascular disease patients on dual-antiplatelet therapy regardless of whether they receive low-dose or high-dose aspirin, according to a post-hoc analysis of data from more than 3,700 patients enrolled in the multicenter, randomized COGENT trial.
“Short-term, prophylactic PPI therapy consistently reduced rates of adjudicated upper-gastrointestinal events without increasing cardiovascular events, regardless of the aspirin dose,” Dr. Muthiah Vaduganathan said while presenting his study at the annual meeting of the American College of Cardiology. “Gastroprotection with PPI therapy should be used in appropriately selected patients with coronary artery disease who require dual-antiplatelet therapy even if they are on low-dose aspirin.”
In addition to documenting the safety and efficacy of 6 months of PPI treatment for patients at high risk for cardiovascular events and low or moderate risk for a GI event, the results from the analysis also documented how common GI events are in this population, even when patients receive low-dose aspirin. Nearly two-thirds of the 3,752 patients included in the analysis took low-dose aspirin, either 75 mg or 81 mg per day. Their incidence of an adjudicated upper GI bleed, the study’s primary GI endpoint, occurred in 3.1% of patients on placebo, and in 1.2% of patients taking a prophylactic PPI. Among the other 34% of patients on high-dose aspirin – a daily dosage of at least 150 mg – the rate of adjudicated upper-GI bleeds was 2.6% without a PPI and 0.9% in those on a PPI.
In other words, even among patients deemed to have a relatively low risk for upper GI complications from aspirin (because their entry into this study required no history of major GI bleeds or recent treatment with a gastroprotection agent), treatment with low-dose aspirin resulted in upper-GI bleeds at the same rate, about 3%, as high-dose aspirin. And in both of these aspirin subgroups 6 months of concurrent treatment with a PPI cut the incidence of major GI bleeds by more than half.
The findings are especially notable because the enrollment criteria stacked the deck toward patients with high cardiovascular disease risk and relatively low GI risk. The study enrolled “a unique population at high risk for cardiovascular disease – 71% had previously undergone a percutaneous coronary intervention, and 42% had a history of an acute coronary syndrome – and low GI risk, but even in this population enriched for cardiovascular disease risk, there was no increased rate of cardiovascular disease events” during a median follow-up while on PPI treatment of 110 days, Dr. Vaduganathan said.
Among patients on low-dose aspirin, the rate of cardiovascular death, MI, stroke, or coronary revascularization was 5.6% with PPI treatment and 5.5% without, and in the high-dose aspirin patients the rates were 4.2% with PPI treatment and 5.5% without. Neither of these differences between the subgroups taking or not taking a PPI were statistically significant.
Concurrent with Dr. Vaduganathan’s report at the meeting the results also appeared online (J Am Coll Cardiol. 2016 April 12;67[14]:661-71).
“There appeared to be no adverse clinical effect from PPI treatment. When used short-term, for up to 6 months, PPI treatment appears to be safe in patients with cardiovascular disease,” Dr. Vaduganathan concluded.
The analysis used data collected in COGENT (Clopidogrel and the Optimization of Gastrointestinal Events Trial), a phase 3 study designed to compare a single-pill formulation of 20 mg omeprazole and 75 mg clopidogrel taken orally once daily with 75 mg clopidogrel against a background of all patients taking aspirin. COGENT stopped prematurely in late 2008 as the company developing this formulation and sponsoring the trial, Cogentus Pharmaceuticals, filed for bankruptcy. Despite its abrupt conclusion, the trial had enrolled and followed enough patients to show that treatment with omeprazole plus clopidogrel and aspirin led to a significant reduction in upper GI bleeding without increasing the rate of cardiovascular disease events, compared with clopidogrel plus aspirin (N Engl J Med. 2010 Nov 11;363[20]:1909-17).
The new analysis focused on the greater than 99% of patients in the total COGENT cohort for whom information was available on whether they received high- or low-dose aspirin.
Although the primary findings from COGENT, reported in 2010, documented the safety and efficacy of concomitant PPI treatment during dual-antiplatelet therapy, and despite guidelines revised in 2010 that called for PPI treatment when appropriate, this strategy for preventing GI complications remains underused, Dr. Vaduganathan said. The most recent U.S. recommendations that address this issue called for assessing the potential risk and benefit from PPI treatment in patients receiving dual-antiplatelet therapy: “The risk reduction with PPIs is substantial in patients with risk factors for GI bleeding and may outweigh any potential reduction in the CV efficacy of antiplatelet treatment because of a drug-drug interaction (J Am Coll Cardiol. 2010 Dec;56[24]:2051-66).”
The only caveat Dr. Vaduganathan placed on PPI use was that the COGENT data addressed only 6 months of PPI use; the safety of longer-term use has not been studied. But “the trend is to use PPIs for as short a period as possible,” and the risk for adverse effects from PPI treatment on cardiovascular disease events is likely greatest during the first 6 months of PPI treatment, he noted. If PPI treatment needs to continue beyond 6 months, he suggested systematically reassessing the risk-benefit balance for individual patients from continued PPI treatment every 3 months.*
*Changes were made to this story on 4/20/2016.
On Twitter @mitchelzoler
The new analysis of COGENT provides important insights into patients treated with clopidogrel and aspirin. The data show that patients on low-dose aspirin do not have an increased risk of cardiovascular events, and that patients who take low-dose aspirin still face a significant risk for upper-gastrointestinal events. Patients taking low-dose aspirin have about the same rate of upper-GI events as patients on high-dose aspirin.
The issue of GI safety for patients on low-dose aspirin as part of dual-antiplatelet therapy has been long overshadowed by concern over a hypothetical interaction between clopidogrel and proton pump inhibitors. The issue has also been distorted by a false sense of security that when patients receive low-dose aspirin they do not require protection against GI events.
Treatment of patients taking low-dose aspirin with a PPI is underutilized. The confirmation this analysis provides, that PPI treatment gives GI protection without causing an excess of cardiovascular events, calls for a change in current practice when clinicians prescribe low-dose aspirin. I’m concerned by the apparent lack of enthusiasm by clinicians to prescribe PPIs to their patients on low-dose aspirin despite their significant risk for GI events. The real question is whether all patients on low-dose aspirin should receive a PPI long term or only the subgroup of patients with high risk for an upper-GI bleed.
Dr. Michael E. Farkouh is a cardiologist at Mount Sinai Hospital in Toronto. He has no disclosures. He made these comments in an editorial that accompanied the published report (J Am Coll Cardiol. 2016 April 12;67[14]:1672-3).
The new analysis of COGENT provides important insights into patients treated with clopidogrel and aspirin. The data show that patients on low-dose aspirin do not have an increased risk of cardiovascular events, and that patients who take low-dose aspirin still face a significant risk for upper-gastrointestinal events. Patients taking low-dose aspirin have about the same rate of upper-GI events as patients on high-dose aspirin.
The issue of GI safety for patients on low-dose aspirin as part of dual-antiplatelet therapy has been long overshadowed by concern over a hypothetical interaction between clopidogrel and proton pump inhibitors. The issue has also been distorted by a false sense of security that when patients receive low-dose aspirin they do not require protection against GI events.
Treatment of patients taking low-dose aspirin with a PPI is underutilized. The confirmation this analysis provides, that PPI treatment gives GI protection without causing an excess of cardiovascular events, calls for a change in current practice when clinicians prescribe low-dose aspirin. I’m concerned by the apparent lack of enthusiasm by clinicians to prescribe PPIs to their patients on low-dose aspirin despite their significant risk for GI events. The real question is whether all patients on low-dose aspirin should receive a PPI long term or only the subgroup of patients with high risk for an upper-GI bleed.
Dr. Michael E. Farkouh is a cardiologist at Mount Sinai Hospital in Toronto. He has no disclosures. He made these comments in an editorial that accompanied the published report (J Am Coll Cardiol. 2016 April 12;67[14]:1672-3).
The new analysis of COGENT provides important insights into patients treated with clopidogrel and aspirin. The data show that patients on low-dose aspirin do not have an increased risk of cardiovascular events, and that patients who take low-dose aspirin still face a significant risk for upper-gastrointestinal events. Patients taking low-dose aspirin have about the same rate of upper-GI events as patients on high-dose aspirin.
The issue of GI safety for patients on low-dose aspirin as part of dual-antiplatelet therapy has been long overshadowed by concern over a hypothetical interaction between clopidogrel and proton pump inhibitors. The issue has also been distorted by a false sense of security that when patients receive low-dose aspirin they do not require protection against GI events.
Treatment of patients taking low-dose aspirin with a PPI is underutilized. The confirmation this analysis provides, that PPI treatment gives GI protection without causing an excess of cardiovascular events, calls for a change in current practice when clinicians prescribe low-dose aspirin. I’m concerned by the apparent lack of enthusiasm by clinicians to prescribe PPIs to their patients on low-dose aspirin despite their significant risk for GI events. The real question is whether all patients on low-dose aspirin should receive a PPI long term or only the subgroup of patients with high risk for an upper-GI bleed.
Dr. Michael E. Farkouh is a cardiologist at Mount Sinai Hospital in Toronto. He has no disclosures. He made these comments in an editorial that accompanied the published report (J Am Coll Cardiol. 2016 April 12;67[14]:1672-3).
CHICAGO – Six months of treatment with a proton pump inhibitor (PPI) is a safe way to cut the incidence of major gastrointestinal events in cardiovascular disease patients on dual-antiplatelet therapy regardless of whether they receive low-dose or high-dose aspirin, according to a post-hoc analysis of data from more than 3,700 patients enrolled in the multicenter, randomized COGENT trial.
“Short-term, prophylactic PPI therapy consistently reduced rates of adjudicated upper-gastrointestinal events without increasing cardiovascular events, regardless of the aspirin dose,” Dr. Muthiah Vaduganathan said while presenting his study at the annual meeting of the American College of Cardiology. “Gastroprotection with PPI therapy should be used in appropriately selected patients with coronary artery disease who require dual-antiplatelet therapy even if they are on low-dose aspirin.”
In addition to documenting the safety and efficacy of 6 months of PPI treatment for patients at high risk for cardiovascular events and low or moderate risk for a GI event, the results from the analysis also documented how common GI events are in this population, even when patients receive low-dose aspirin. Nearly two-thirds of the 3,752 patients included in the analysis took low-dose aspirin, either 75 mg or 81 mg per day. Their incidence of an adjudicated upper GI bleed, the study’s primary GI endpoint, occurred in 3.1% of patients on placebo, and in 1.2% of patients taking a prophylactic PPI. Among the other 34% of patients on high-dose aspirin – a daily dosage of at least 150 mg – the rate of adjudicated upper-GI bleeds was 2.6% without a PPI and 0.9% in those on a PPI.
In other words, even among patients deemed to have a relatively low risk for upper GI complications from aspirin (because their entry into this study required no history of major GI bleeds or recent treatment with a gastroprotection agent), treatment with low-dose aspirin resulted in upper-GI bleeds at the same rate, about 3%, as high-dose aspirin. And in both of these aspirin subgroups 6 months of concurrent treatment with a PPI cut the incidence of major GI bleeds by more than half.
The findings are especially notable because the enrollment criteria stacked the deck toward patients with high cardiovascular disease risk and relatively low GI risk. The study enrolled “a unique population at high risk for cardiovascular disease – 71% had previously undergone a percutaneous coronary intervention, and 42% had a history of an acute coronary syndrome – and low GI risk, but even in this population enriched for cardiovascular disease risk, there was no increased rate of cardiovascular disease events” during a median follow-up while on PPI treatment of 110 days, Dr. Vaduganathan said.
Among patients on low-dose aspirin, the rate of cardiovascular death, MI, stroke, or coronary revascularization was 5.6% with PPI treatment and 5.5% without, and in the high-dose aspirin patients the rates were 4.2% with PPI treatment and 5.5% without. Neither of these differences between the subgroups taking or not taking a PPI were statistically significant.
Concurrent with Dr. Vaduganathan’s report at the meeting the results also appeared online (J Am Coll Cardiol. 2016 April 12;67[14]:661-71).
“There appeared to be no adverse clinical effect from PPI treatment. When used short-term, for up to 6 months, PPI treatment appears to be safe in patients with cardiovascular disease,” Dr. Vaduganathan concluded.
The analysis used data collected in COGENT (Clopidogrel and the Optimization of Gastrointestinal Events Trial), a phase 3 study designed to compare a single-pill formulation of 20 mg omeprazole and 75 mg clopidogrel taken orally once daily with 75 mg clopidogrel against a background of all patients taking aspirin. COGENT stopped prematurely in late 2008 as the company developing this formulation and sponsoring the trial, Cogentus Pharmaceuticals, filed for bankruptcy. Despite its abrupt conclusion, the trial had enrolled and followed enough patients to show that treatment with omeprazole plus clopidogrel and aspirin led to a significant reduction in upper GI bleeding without increasing the rate of cardiovascular disease events, compared with clopidogrel plus aspirin (N Engl J Med. 2010 Nov 11;363[20]:1909-17).
The new analysis focused on the greater than 99% of patients in the total COGENT cohort for whom information was available on whether they received high- or low-dose aspirin.
Although the primary findings from COGENT, reported in 2010, documented the safety and efficacy of concomitant PPI treatment during dual-antiplatelet therapy, and despite guidelines revised in 2010 that called for PPI treatment when appropriate, this strategy for preventing GI complications remains underused, Dr. Vaduganathan said. The most recent U.S. recommendations that address this issue called for assessing the potential risk and benefit from PPI treatment in patients receiving dual-antiplatelet therapy: “The risk reduction with PPIs is substantial in patients with risk factors for GI bleeding and may outweigh any potential reduction in the CV efficacy of antiplatelet treatment because of a drug-drug interaction (J Am Coll Cardiol. 2010 Dec;56[24]:2051-66).”
The only caveat Dr. Vaduganathan placed on PPI use was that the COGENT data addressed only 6 months of PPI use; the safety of longer-term use has not been studied. But “the trend is to use PPIs for as short a period as possible,” and the risk for adverse effects from PPI treatment on cardiovascular disease events is likely greatest during the first 6 months of PPI treatment, he noted. If PPI treatment needs to continue beyond 6 months, he suggested systematically reassessing the risk-benefit balance for individual patients from continued PPI treatment every 3 months.*
*Changes were made to this story on 4/20/2016.
On Twitter @mitchelzoler
CHICAGO – Six months of treatment with a proton pump inhibitor (PPI) is a safe way to cut the incidence of major gastrointestinal events in cardiovascular disease patients on dual-antiplatelet therapy regardless of whether they receive low-dose or high-dose aspirin, according to a post-hoc analysis of data from more than 3,700 patients enrolled in the multicenter, randomized COGENT trial.
“Short-term, prophylactic PPI therapy consistently reduced rates of adjudicated upper-gastrointestinal events without increasing cardiovascular events, regardless of the aspirin dose,” Dr. Muthiah Vaduganathan said while presenting his study at the annual meeting of the American College of Cardiology. “Gastroprotection with PPI therapy should be used in appropriately selected patients with coronary artery disease who require dual-antiplatelet therapy even if they are on low-dose aspirin.”
In addition to documenting the safety and efficacy of 6 months of PPI treatment for patients at high risk for cardiovascular events and low or moderate risk for a GI event, the results from the analysis also documented how common GI events are in this population, even when patients receive low-dose aspirin. Nearly two-thirds of the 3,752 patients included in the analysis took low-dose aspirin, either 75 mg or 81 mg per day. Their incidence of an adjudicated upper GI bleed, the study’s primary GI endpoint, occurred in 3.1% of patients on placebo, and in 1.2% of patients taking a prophylactic PPI. Among the other 34% of patients on high-dose aspirin – a daily dosage of at least 150 mg – the rate of adjudicated upper-GI bleeds was 2.6% without a PPI and 0.9% in those on a PPI.
In other words, even among patients deemed to have a relatively low risk for upper GI complications from aspirin (because their entry into this study required no history of major GI bleeds or recent treatment with a gastroprotection agent), treatment with low-dose aspirin resulted in upper-GI bleeds at the same rate, about 3%, as high-dose aspirin. And in both of these aspirin subgroups 6 months of concurrent treatment with a PPI cut the incidence of major GI bleeds by more than half.
The findings are especially notable because the enrollment criteria stacked the deck toward patients with high cardiovascular disease risk and relatively low GI risk. The study enrolled “a unique population at high risk for cardiovascular disease – 71% had previously undergone a percutaneous coronary intervention, and 42% had a history of an acute coronary syndrome – and low GI risk, but even in this population enriched for cardiovascular disease risk, there was no increased rate of cardiovascular disease events” during a median follow-up while on PPI treatment of 110 days, Dr. Vaduganathan said.
Among patients on low-dose aspirin, the rate of cardiovascular death, MI, stroke, or coronary revascularization was 5.6% with PPI treatment and 5.5% without, and in the high-dose aspirin patients the rates were 4.2% with PPI treatment and 5.5% without. Neither of these differences between the subgroups taking or not taking a PPI were statistically significant.
Concurrent with Dr. Vaduganathan’s report at the meeting the results also appeared online (J Am Coll Cardiol. 2016 April 12;67[14]:661-71).
“There appeared to be no adverse clinical effect from PPI treatment. When used short-term, for up to 6 months, PPI treatment appears to be safe in patients with cardiovascular disease,” Dr. Vaduganathan concluded.
The analysis used data collected in COGENT (Clopidogrel and the Optimization of Gastrointestinal Events Trial), a phase 3 study designed to compare a single-pill formulation of 20 mg omeprazole and 75 mg clopidogrel taken orally once daily with 75 mg clopidogrel against a background of all patients taking aspirin. COGENT stopped prematurely in late 2008 as the company developing this formulation and sponsoring the trial, Cogentus Pharmaceuticals, filed for bankruptcy. Despite its abrupt conclusion, the trial had enrolled and followed enough patients to show that treatment with omeprazole plus clopidogrel and aspirin led to a significant reduction in upper GI bleeding without increasing the rate of cardiovascular disease events, compared with clopidogrel plus aspirin (N Engl J Med. 2010 Nov 11;363[20]:1909-17).
The new analysis focused on the greater than 99% of patients in the total COGENT cohort for whom information was available on whether they received high- or low-dose aspirin.
Although the primary findings from COGENT, reported in 2010, documented the safety and efficacy of concomitant PPI treatment during dual-antiplatelet therapy, and despite guidelines revised in 2010 that called for PPI treatment when appropriate, this strategy for preventing GI complications remains underused, Dr. Vaduganathan said. The most recent U.S. recommendations that address this issue called for assessing the potential risk and benefit from PPI treatment in patients receiving dual-antiplatelet therapy: “The risk reduction with PPIs is substantial in patients with risk factors for GI bleeding and may outweigh any potential reduction in the CV efficacy of antiplatelet treatment because of a drug-drug interaction (J Am Coll Cardiol. 2010 Dec;56[24]:2051-66).”
The only caveat Dr. Vaduganathan placed on PPI use was that the COGENT data addressed only 6 months of PPI use; the safety of longer-term use has not been studied. But “the trend is to use PPIs for as short a period as possible,” and the risk for adverse effects from PPI treatment on cardiovascular disease events is likely greatest during the first 6 months of PPI treatment, he noted. If PPI treatment needs to continue beyond 6 months, he suggested systematically reassessing the risk-benefit balance for individual patients from continued PPI treatment every 3 months.*
*Changes were made to this story on 4/20/2016.
On Twitter @mitchelzoler
AT ACC 2016
Key clinical point: In patients at high risk of cardiovascular disease on dual-antiplatelet therapy, concurrent proton pump inhibitor treatment cut gastrointestinal events, regardless of whether patients received a low or high aspirin dosage.
Major finding: Omeprazole cut the rate of upper-GI bleeds by more than half in patients taking low- or high-dose aspirin.
Data source: Post-hoc analysis of data in COGENT, a multicenter, randomized trial with 3,762 patients.
Disclosures: Cogent was sponsored by Cogentus Pharmaceuticals; however, the company went bankrupt and provided no support for the current analysis. Dr. Vaduganathan had no disclosures.
A Practical Overview of Pediatric Atopic Dermatitis, Part 1: Epidemiology and Pathogenesis
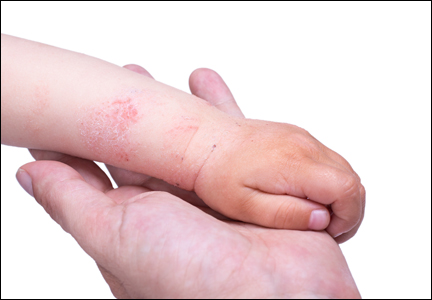
Atopic dermatitis (AD), or eczema, is the leading dermatologic diagnosis worldwide and is vexing to patients due to the itchiness of the rash. It is the leading cause of skin disease burden worldwide with a prevalence of 229,761,000 reported cases in 2010, presenting largely in preadolescence but also persisting through adulthood.1 Using the children’s life quality index, it has been demonstrated that AD has a greater impact on health-related quality of life than renal disease and cystic fibrosis.2 The overall burden of AD includes stress on the patient and his/her family as well as financial burdens that have been estimated to be similar to that of type 1 diabetes mellitus.3
Epidemiology of AD
The worldwide prevalence of AD varies by country and age group surveyed, with a higher prevalence in wealthy developed nations (eg, the United States) compared to poorer developing nations.4 Efforts to identify prevalence data for AD in the United States have been approached through a variety of strategies. A group in Oregon estimated the prevalence of AD in children aged 5 to 9 years to be 17.2% via a survey of parents (N=1465) and 11.8% with doctor-diagnosed eczema. In the same study, the question “Has a doctor ever said that your child has eczema?” was found to have a 91.3% predictive correlation.5 Analysis of the 2003 National Survey of Children’s Health demonstrated the overall US prevalence of pediatric AD to be 10.7% in 102,353 children 17 years or younger, with a range of 8.7% to 18.1% by region.6
In its evaluation of the worldwide prevalence of AD, the International Study of Asthma and Allergies in Childhood ranked the United States 17th.7,8 The prevalence of AD in developed countries such as the United States is fluid and is expected to increase if the trends from the last 20 years remain true. In an assessment of the National Health Interview Survey data from 1997 to 2011 based on responses to the question, “During the past 12 months, has your child had eczema or any kind of skin allergy?”, the Centers for Disease Control and Prevention identified an increase in the prevalence of AD in patients aged 0 to 17 years from 7.4% in 1997-1999 to 12.5% in 2009-2011.9 Rising prevalence seems to be paired with rising incidence in the total number of severe intractable cases, reduced clearance at the approach of grade school, or cases persisting into adulthood.
Racial Disparity in AD
Racial disparity worldwide and migration are thought to contribute to the prevalence of and therapeutic need for AD. For example, in the United Kingdom, the prevalence of AD in London-born Afro-Caribbean children versus white children (total cross-section, N=693 [junior school children]) was 16.3% and 8.7%, respectively.10 In the United States, black children were more likely to have AD than white children (odds ratio, 1.7).6 Asian and black children also were more likely to present to a physician for treatment of AD than white children.6,10-13
Definition and Diagnostic Considerations
According to Hanifin,14 “Eczema represents a family of inflammatory skin conditions characterized by pruritic, papulovesicular, sometimes weeping dermatitis. All demonstrate the histological hallmark of spongiosis, which helps to distinguish the eczemas from papulosquamous diseases such as psoriasis.”14 Atopic dermatitis is a variant of eczema; however, most laymen identify eczema and AD as being one and the same.
The Hanifin and Rajka15 criteria are the major diagnostic criteria for AD but are difficult to use in clinical practice. Three of the following 4 major criteria are needed for diagnosis: (1) pruritus, which is present universally; (2) typical morphology and distribution; (3) chronic or chronically relapsing dermatitis; and (4) personal and/or family history of atopy. Additionally, 3 of the following 23 minor criteria are needed for diagnosis: xerosis; ichthyosis vulgaris, palmar hyperlinearity, or keratosis pilaris; positive skin prick test; elevated serum IgE level; early age of onset; tendency toward cutaneous infections or impaired cell-mediated immunity; tendency toward nonspecific hand or foot dermatitis; nipple eczema; cheilitis; recurrent conjunctivitis; Dennie-Morgan fold (infraorbital fold); keratoconus; anterior subcapsular cataracts; orbital darkening; facial pallor or facial erythema; pityriasis alba; anterior neck folds; itching when sweating; intolerance to wool and lipid solvents; perifollicular accentuation; skin reactions from ingested foods or by food contact; environmental or emotional factors; and lesional/nonlesional white dermographism or delayed blanch.15-17
More pragmatic streamlined diagnostic criteria were established by Eichenfield et al.18 According to these guidelines, essential features for AD include pruritus and eczema. Important features seen in most cases and adding support to the diagnosis include early age of onset, atopy, and xerosis.18 In clinical practice, diagnosis is often made based on a pruritic relapsing condition in typical locations including the face, neck, and extensor surfaces in infants and children.
Age Considerations
Diagnosis of AD is made by 5 years of age in 85% to 90% of children who will develop the disease and by age 1 year in 60% to 65%.6,19,20 Atopic dermatitis will persist into adulthood in up to one-third of children.21,22 Infantile AD is characterized by erythematous, oozing, excoriated plaques on the cheeks (sparing the nose), scalp, trunk, and extensor surfaces. Pruritus is always seen in AD and can be a source of morbidity.16-18 Seborrheic dermatitis may complicate or overlap with AD in infancy.22
By 2 years of age, most children who are going to develop AD begin to show disease signs of childhood AD characterized by flexural lesions and lesions on the neck and in the postauricular area with sparing of the diaper area.23 Adult AD often presents as eczema of the hands and/or feet. Hand eczema in adulthood is correlated with a prior history of childhood hand eczema and/or childhood AD as well as wet work and caring for small children.24 Children with skin of color may manifest with follicular eczema as their primary disease phenotype. Facial and eyelid dermatitis are more common in Asian females, infants, and teenagers.12,25 Other disease phenotypes that are common in patients with skin of color include lichenoid AD and postinflammatory hypopigmentation.12
Pathogenesis of AD
There are 2 theories on the pathogenesis of AD known as the inside-out and outside-in hypotheses.26 The inside-out hypothesis suggests that allergic triggering leads to a weakened skin barrier that furthers allergen introduction and presentation, while the outside-in hypothesis suggests that the skin barrier is weakened in AD and allows for the presentation of allergens. Both theories have validity and biologic basis, and both may in fact be true in certain individuals.26
The Skin Barrier: An Overview
The skin barrier is a complex set of factors present and functional at birth that seal the keratinocytes and the interkeratinocyte space so that the skin can perform key processes and functions including retention of fluid, exclusion of allergens, protection from UV light and solvents, and prevention of pathogen entry (eg, infections).27-29 The superficial stratum corneum or the cornified envelope consists of keratinocytes with intercellular stripes of hydrophobic and hydrophilic substances formed by various intercellular lipids, largely ceramides, cholesterol, and free fatty acids.30,31 Keratinocytes are the first responders to a variety of environmental insults with the production of IL-18, RANTES (regulated on activation, normal T-expressed, and presumably secreted), granulocyte-macrophage colony-stimulating factor, and thymic stromal lymphopoietin. These inflammatory substances produce acute and chronic inflammation, mast cell reactivity, and T-cell activation.14 Corneodesmosins link the keratinocytes. Peptidases released will cleave the corneodesmosins and allow normal desquamation or shedding of surface skin, which is replaced by division of stem cells in the basal layer.29
The stratum granulosum is the layer beneath the stratum corneum that co-contributes to barrier activity. The stratum granulosum is absent or reduced histologically in ichthyosis vulgaris,32 a form of skin dryness linked to filaggrin mutations and AD. Filaggrin breakdown creates natural moisturizing factor, a series of hygroscopic compounds that attract water into the skin.33 Histidine, a filaggrin breakdown product, is used by urocanic acid to process UV light insults.34 Filaggrin also contributes to other barrier functions including pH and stratum corneum cohesion as well as paracellular permeability of the stratum corneum. Tight junctions in the stratum granulosum include claudin-1 and claudin-6 and provide another barrier feature.29
The skin barrier is composed of lipids and keratinocytes. Ceramides, which represent one type of lipids, are reduced in AD, causing alteration in the lamellar pattern35 and increased transepidermal water loss. Furthermore, the stratum corneum is thickened in AD, possibly in response to trauma, and hydration is reduced.36 Filaggrin (chromosome arm 1q21.3) is formed from the 400-kDa+ precursor profilaggrin through dephosphorylation and cleavage, and it performs an essential function in the skin barrier through its differential cleavage and breakdown as well as release of natural moisturizing factor and other compounds.37 Filaggrin mutations are linked to AD and ichthyosis vulgaris; however, barrier defects as evidenced by transepidermal water loss in the absence of filaggrin mutation are sufficient to allow for sensitization to allergens through the skin.29 Filaggrin mutations have been associated with AD development and vary in prevalence worldwide. In the United Kingdom, a prevalence study of filaggrin mutations in patients aged 7 to 9 years (N=792) demonstrated an 18.4% carrier rate in AD patients versus 12.9% in controls.34 A similar study in Sweden (N=3301) showed carrier rates of 13.5% versus 6.5%, respectively.38 Although filaggrin mutations are lower in black patients,39 ceramide content may be reduced in this population, demonstrating that a variety of skin barrier defects can result in AD. Carriers of filaggrin mutations are more likely to have eczema on skin exposed to environmental factors (eg, face, hands).40
Barrier Defects Contributing to AD
The breakdown of the stratum corneum allows for antigen presentation to Langerhans cells, the dendritic antigen-presenting cells of the skin. Breaks in the stratum corneum may occur from scratching. These macroscopic breaks are large, whereas the breaks that otherwise occur due to barrier breakdown may be more microscopic in nature. Scratching causes aggravation of the helper T cell (TH2) response.29 For example, it allows the dendritic ends of Langerhans cells to be exposed to antigens. The dendritic ends capture allergens through IgE (may be elevated in AD29), which is bound to the high-affinity FCER1 receptors on Langerhans cells. Rather than causing a type I hypersensitivity reaction, these Langerhans cells are activated and move to the lymph nodes where they present antigen and initiate a cascade of proinflammatory activity. This TH2 cascade includes release of cytokines such as IL-2, IL-4, IL-8, IL-10, tumor necrosis factor α, and IFN-γ.26,29
Transepidermal water loss and barrier dysfunction contribute to disease activity and facilitate food/environmental allergen sensitization by allowing increased penetration of allergens through the skin to be presented by Langerhans cells to TH1 cells (sensitization phase). The Langerhans cells can reach their dendritic ends through tight junctions and into the stratum corneum, allowing them to reach surface allergens when the barrier is impaired. Ultimate expansion to systemic allergy (effector phase) occurs when dendritic cells move to draining lymph nodes, causing antigen presentation to CD4 and/or CD8 cells. Langerhans cells and dendritic cell sensitization through the weakened skin is believed to be the basis or role of barrier disruption as a trigger of atopic diseases, including AD and food and environmental allergies.
Many different forms of barrier disruption can cause a TH2 response in AD. The TH2 response triggers a constellation of proinflammatory activities including release of IL-4, associated with eosinophilia and elevated IgE levels, the latter being minor criterion in the diagnosis of AD.15 One mechanism by which the TH2 response is elicited may be the release of molecules such as danger-associated molecule patterns that may elicit recruitment of other inflammatory cells. Helper T cell (TH2) activity also can worsen barrier defects through IL-4 and IL-13 release, which can reduce filaggrin expression,29,41 and can aggravate barrier dysfunction in AD.
Inflammatory activation in AD also may involve inflammatory dendritic epidermal cells (IDECs). The IDECs can be tolerogenic or immunogenic mature phenotypes. The IDECs activate helper T cells (TH1), which may contribute to long-term AD activity.
Conclusion
Atopic dermatitis is a common skin condition worldwide and is characterized by the hallmark of pruritus and features that include a typical pattern, history of atopy (personal or family), and usually xerosis and early disease onset. Barrier dysfunction and immune dysregulation are prominent in AD, both of which aggravate the other and may encourage increased development of allergies and other forms of atopy over time.
1. Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
2. Beattie PE, Lewis-Jones MS. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol. 2006;155:145-151.
3. Su JC, Kemp AS, Varigos GA, et al. Atopic eczema: its impact on the family and financial cost. Arch Dis Child. 1997;76:159-162.
4. Garg N, Silverberg JI. Epidemiology of childhood atopic dermatitis. Clin Dermatol. 2015;33:281-288.
5. Laughter D, Istvan JA, Tofte SJ, et al. The prevalence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol. 2000;43:649-655.
6. Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
7. Odhiambo JA, Williams HC, Clayton TO, et al. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251-1258.
8. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet. 1998;351:1225-1232.
9. Hansen TE, Evjenth B, Holt J. Increasing prevalence of asthma, allergic rhinoconjunctivitis and eczema among schoolchildren: three surveys during the period 1985-2008. Acta Paediatr. 2013;102:47-52.
10. Williams HC, Pembroke AC, Forsdyke H, et al. London-born black Caribbean children are at increased risk of atopic dermatitis. J Am Acad Dermatol. 1995;32:212-217.
11. Horii KA, Simon SD, Liu DY, et al. Atopic dermatitis in children in the United States, 1997-2004: visit trends, patient and provider characteristics, and prescribing patterns. Pediatrics. 2007;120:e527-e534.
12. Silverberg NB. Eczematous diseases. In: Silverberg NB. Atlas of Pediatric Cutaneous Biodiversity. New York, NY: Springer; 2012:69-88.
13. Gupta J, Grube E, Ericksen MB, et al. Intrinsically defective skin barrier function in children with atopic dermatitis correlates with disease severity. J Allergy Clin Immunol. 2008;121:725-730.
14. Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
15. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980;92:44-47.
16. Queille-Roussel C, Raynaud F, Saurat JH. A prospective computerized study of 500 cases of atopic dermatitis in childhood. I. Initial analysis of 250 parameters. Acta Derm Venereol Suppl (Stockh). 1985;114:87-92.
17. Böhme M, Svensson A, Kull I, et al. Hanifin’s and Rajka’s minor criteria for atopic dermatitis: which do 2-year-olds exhibit? J Am Acad Dermatol. 2000;43:785-792.
18. Eichenfield LF, Hanifin JM, Luger TA, et al. Consensus conference on pediatric atopic dermatitis. J Am Acad Dermatol. 2003;49:1088-1095.
19. Kay J, Gawkrodger DJ, Mortimer MJ, et al. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol. 1994;30:35-39.
20. Perkin MR, Strachan DP, Williams HC, et al. Natural history of atopic dermatitis and its relationship to serum total immunoglobulin E in a population-based birth cohort study. Pediatr Allergy Immunol. 2004;15:221-229.
21. Ellis CN, Mancini AJ, Paller AS, et al. Understanding and managing atopic dermatitis in adult patients. Semin Cutan Med Surg. 2012;31(suppl 2):S18-S22.
22. Elish D, Silverberg NB. Infantile seborrheic dermatitis. Cutis. 2006;77:297-300.
23. Meding B, Wrangsjö K, Järvholm B. Hand eczema extent and morphology—association and influence on long-term prognosis. J Invest Dermatol. 2007;127:2147-2151.
24. Mortz CG, Bindslev-Jensen C, Andersen KE. Hand eczema in The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis (TOACS): prevalence, incidence and risk factors from adolescence to adulthood [published online August 7, 2014]. Br J Dermatol. 2014;171:313-323.
25. Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
26. Silverberg NB, Silverberg JI. Inside out or outside in: does atopic dermatitis disrupt barrier function or does disruption of barrier function trigger atopic dermatitis? Cutis. 2015;96:359-361.
27. Visscher MO, Adam R, Brink S, et al. Newborn infant skin: physiology, development, and care [published online December 8, 2014]. Clin Dermatol. 2015;33:271-280.
28. Miyagaki T, Sugaya M. Recent advances in atopic dermatitis and psoriasis: genetic background, barrier function, and therapeutic targets. J Dermatol Sci. 2015;78:89-94.
29. De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949-963.
30. Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009;9:437-446.
31. Janssens M, van Smeden J, Gooris GS, et al. Lamellar lipid organization and ceramide composition in the stratum corneum of patients with atopic eczema. J Invest Dermatol. 2011;131:2136-2138.
32. Fitch N, Segool R, Ferenczy A, et al. Dominant ichthyosis vulgaris with an ultrastructurally normal granular layer. Clin Genet. 1976;9:71-76.
33. Chandar P, Nole G, Johnson AW. Understanding natural moisturizing mechanisms: implications for moisturizer technology. Cutis. 2009;84(suppl 1):2-15.
34. Brown SJ, Relton CL, Liao H, et al. Filaggrin null mutations and childhood atopic eczema: a population-based case-control study. J Allergy Clin Immunol. 2008;121:940-946.
35. Marenholz I, Rivera VA, Esparza-Gordillo J, et al. Association screening in the Epidermal Differentiation Complex (EDC) identifies an SPRR3 repeat number variant as a risk factor for eczema. J Invest Dermatol. 2011;131:1644-1649.
36. Nemoto-Hasebe I, Akiyama M, Nomura T, et al. Clinical severity correlates with impaired barrier in filaggrin-related eczema. J Invest Dermatol. 2009;129:682-689.
37. Hoste E, Kemperman P, Devos M, et al. Caspase-14 is required for filaggrin degradation to natural moisturizing factors in the skin. J Invest Dermatol. 2011;131:2233-2241.
38. Ballardini N, Kull I, Söderhäll C, et al. Eczema severity in preadolescent children and its relation to sex, filaggrin mutations, asthma, rhinitis, aggravating factors and topical treatment: a report from the BAMSE birth cohort. Br J Dermatol. 2013;168:588-594.
39. Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912-917.
40. Carson CG, Rasmussen MA, Thyssen JP, et al. Clinical presentation of atopic dermatitis by filaggrin gene mutation status during the first 7 years of life in a prospective cohort study. PLoS One. 2012;7:e48678.
41. Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
Atopic dermatitis (AD), or eczema, is the leading dermatologic diagnosis worldwide and is vexing to patients due to the itchiness of the rash. It is the leading cause of skin disease burden worldwide with a prevalence of 229,761,000 reported cases in 2010, presenting largely in preadolescence but also persisting through adulthood.1 Using the children’s life quality index, it has been demonstrated that AD has a greater impact on health-related quality of life than renal disease and cystic fibrosis.2 The overall burden of AD includes stress on the patient and his/her family as well as financial burdens that have been estimated to be similar to that of type 1 diabetes mellitus.3
Epidemiology of AD
The worldwide prevalence of AD varies by country and age group surveyed, with a higher prevalence in wealthy developed nations (eg, the United States) compared to poorer developing nations.4 Efforts to identify prevalence data for AD in the United States have been approached through a variety of strategies. A group in Oregon estimated the prevalence of AD in children aged 5 to 9 years to be 17.2% via a survey of parents (N=1465) and 11.8% with doctor-diagnosed eczema. In the same study, the question “Has a doctor ever said that your child has eczema?” was found to have a 91.3% predictive correlation.5 Analysis of the 2003 National Survey of Children’s Health demonstrated the overall US prevalence of pediatric AD to be 10.7% in 102,353 children 17 years or younger, with a range of 8.7% to 18.1% by region.6
In its evaluation of the worldwide prevalence of AD, the International Study of Asthma and Allergies in Childhood ranked the United States 17th.7,8 The prevalence of AD in developed countries such as the United States is fluid and is expected to increase if the trends from the last 20 years remain true. In an assessment of the National Health Interview Survey data from 1997 to 2011 based on responses to the question, “During the past 12 months, has your child had eczema or any kind of skin allergy?”, the Centers for Disease Control and Prevention identified an increase in the prevalence of AD in patients aged 0 to 17 years from 7.4% in 1997-1999 to 12.5% in 2009-2011.9 Rising prevalence seems to be paired with rising incidence in the total number of severe intractable cases, reduced clearance at the approach of grade school, or cases persisting into adulthood.
Racial Disparity in AD
Racial disparity worldwide and migration are thought to contribute to the prevalence of and therapeutic need for AD. For example, in the United Kingdom, the prevalence of AD in London-born Afro-Caribbean children versus white children (total cross-section, N=693 [junior school children]) was 16.3% and 8.7%, respectively.10 In the United States, black children were more likely to have AD than white children (odds ratio, 1.7).6 Asian and black children also were more likely to present to a physician for treatment of AD than white children.6,10-13
Definition and Diagnostic Considerations
According to Hanifin,14 “Eczema represents a family of inflammatory skin conditions characterized by pruritic, papulovesicular, sometimes weeping dermatitis. All demonstrate the histological hallmark of spongiosis, which helps to distinguish the eczemas from papulosquamous diseases such as psoriasis.”14 Atopic dermatitis is a variant of eczema; however, most laymen identify eczema and AD as being one and the same.
The Hanifin and Rajka15 criteria are the major diagnostic criteria for AD but are difficult to use in clinical practice. Three of the following 4 major criteria are needed for diagnosis: (1) pruritus, which is present universally; (2) typical morphology and distribution; (3) chronic or chronically relapsing dermatitis; and (4) personal and/or family history of atopy. Additionally, 3 of the following 23 minor criteria are needed for diagnosis: xerosis; ichthyosis vulgaris, palmar hyperlinearity, or keratosis pilaris; positive skin prick test; elevated serum IgE level; early age of onset; tendency toward cutaneous infections or impaired cell-mediated immunity; tendency toward nonspecific hand or foot dermatitis; nipple eczema; cheilitis; recurrent conjunctivitis; Dennie-Morgan fold (infraorbital fold); keratoconus; anterior subcapsular cataracts; orbital darkening; facial pallor or facial erythema; pityriasis alba; anterior neck folds; itching when sweating; intolerance to wool and lipid solvents; perifollicular accentuation; skin reactions from ingested foods or by food contact; environmental or emotional factors; and lesional/nonlesional white dermographism or delayed blanch.15-17
More pragmatic streamlined diagnostic criteria were established by Eichenfield et al.18 According to these guidelines, essential features for AD include pruritus and eczema. Important features seen in most cases and adding support to the diagnosis include early age of onset, atopy, and xerosis.18 In clinical practice, diagnosis is often made based on a pruritic relapsing condition in typical locations including the face, neck, and extensor surfaces in infants and children.
Age Considerations
Diagnosis of AD is made by 5 years of age in 85% to 90% of children who will develop the disease and by age 1 year in 60% to 65%.6,19,20 Atopic dermatitis will persist into adulthood in up to one-third of children.21,22 Infantile AD is characterized by erythematous, oozing, excoriated plaques on the cheeks (sparing the nose), scalp, trunk, and extensor surfaces. Pruritus is always seen in AD and can be a source of morbidity.16-18 Seborrheic dermatitis may complicate or overlap with AD in infancy.22
By 2 years of age, most children who are going to develop AD begin to show disease signs of childhood AD characterized by flexural lesions and lesions on the neck and in the postauricular area with sparing of the diaper area.23 Adult AD often presents as eczema of the hands and/or feet. Hand eczema in adulthood is correlated with a prior history of childhood hand eczema and/or childhood AD as well as wet work and caring for small children.24 Children with skin of color may manifest with follicular eczema as their primary disease phenotype. Facial and eyelid dermatitis are more common in Asian females, infants, and teenagers.12,25 Other disease phenotypes that are common in patients with skin of color include lichenoid AD and postinflammatory hypopigmentation.12
Pathogenesis of AD
There are 2 theories on the pathogenesis of AD known as the inside-out and outside-in hypotheses.26 The inside-out hypothesis suggests that allergic triggering leads to a weakened skin barrier that furthers allergen introduction and presentation, while the outside-in hypothesis suggests that the skin barrier is weakened in AD and allows for the presentation of allergens. Both theories have validity and biologic basis, and both may in fact be true in certain individuals.26
The Skin Barrier: An Overview
The skin barrier is a complex set of factors present and functional at birth that seal the keratinocytes and the interkeratinocyte space so that the skin can perform key processes and functions including retention of fluid, exclusion of allergens, protection from UV light and solvents, and prevention of pathogen entry (eg, infections).27-29 The superficial stratum corneum or the cornified envelope consists of keratinocytes with intercellular stripes of hydrophobic and hydrophilic substances formed by various intercellular lipids, largely ceramides, cholesterol, and free fatty acids.30,31 Keratinocytes are the first responders to a variety of environmental insults with the production of IL-18, RANTES (regulated on activation, normal T-expressed, and presumably secreted), granulocyte-macrophage colony-stimulating factor, and thymic stromal lymphopoietin. These inflammatory substances produce acute and chronic inflammation, mast cell reactivity, and T-cell activation.14 Corneodesmosins link the keratinocytes. Peptidases released will cleave the corneodesmosins and allow normal desquamation or shedding of surface skin, which is replaced by division of stem cells in the basal layer.29
The stratum granulosum is the layer beneath the stratum corneum that co-contributes to barrier activity. The stratum granulosum is absent or reduced histologically in ichthyosis vulgaris,32 a form of skin dryness linked to filaggrin mutations and AD. Filaggrin breakdown creates natural moisturizing factor, a series of hygroscopic compounds that attract water into the skin.33 Histidine, a filaggrin breakdown product, is used by urocanic acid to process UV light insults.34 Filaggrin also contributes to other barrier functions including pH and stratum corneum cohesion as well as paracellular permeability of the stratum corneum. Tight junctions in the stratum granulosum include claudin-1 and claudin-6 and provide another barrier feature.29
The skin barrier is composed of lipids and keratinocytes. Ceramides, which represent one type of lipids, are reduced in AD, causing alteration in the lamellar pattern35 and increased transepidermal water loss. Furthermore, the stratum corneum is thickened in AD, possibly in response to trauma, and hydration is reduced.36 Filaggrin (chromosome arm 1q21.3) is formed from the 400-kDa+ precursor profilaggrin through dephosphorylation and cleavage, and it performs an essential function in the skin barrier through its differential cleavage and breakdown as well as release of natural moisturizing factor and other compounds.37 Filaggrin mutations are linked to AD and ichthyosis vulgaris; however, barrier defects as evidenced by transepidermal water loss in the absence of filaggrin mutation are sufficient to allow for sensitization to allergens through the skin.29 Filaggrin mutations have been associated with AD development and vary in prevalence worldwide. In the United Kingdom, a prevalence study of filaggrin mutations in patients aged 7 to 9 years (N=792) demonstrated an 18.4% carrier rate in AD patients versus 12.9% in controls.34 A similar study in Sweden (N=3301) showed carrier rates of 13.5% versus 6.5%, respectively.38 Although filaggrin mutations are lower in black patients,39 ceramide content may be reduced in this population, demonstrating that a variety of skin barrier defects can result in AD. Carriers of filaggrin mutations are more likely to have eczema on skin exposed to environmental factors (eg, face, hands).40
Barrier Defects Contributing to AD
The breakdown of the stratum corneum allows for antigen presentation to Langerhans cells, the dendritic antigen-presenting cells of the skin. Breaks in the stratum corneum may occur from scratching. These macroscopic breaks are large, whereas the breaks that otherwise occur due to barrier breakdown may be more microscopic in nature. Scratching causes aggravation of the helper T cell (TH2) response.29 For example, it allows the dendritic ends of Langerhans cells to be exposed to antigens. The dendritic ends capture allergens through IgE (may be elevated in AD29), which is bound to the high-affinity FCER1 receptors on Langerhans cells. Rather than causing a type I hypersensitivity reaction, these Langerhans cells are activated and move to the lymph nodes where they present antigen and initiate a cascade of proinflammatory activity. This TH2 cascade includes release of cytokines such as IL-2, IL-4, IL-8, IL-10, tumor necrosis factor α, and IFN-γ.26,29
Transepidermal water loss and barrier dysfunction contribute to disease activity and facilitate food/environmental allergen sensitization by allowing increased penetration of allergens through the skin to be presented by Langerhans cells to TH1 cells (sensitization phase). The Langerhans cells can reach their dendritic ends through tight junctions and into the stratum corneum, allowing them to reach surface allergens when the barrier is impaired. Ultimate expansion to systemic allergy (effector phase) occurs when dendritic cells move to draining lymph nodes, causing antigen presentation to CD4 and/or CD8 cells. Langerhans cells and dendritic cell sensitization through the weakened skin is believed to be the basis or role of barrier disruption as a trigger of atopic diseases, including AD and food and environmental allergies.
Many different forms of barrier disruption can cause a TH2 response in AD. The TH2 response triggers a constellation of proinflammatory activities including release of IL-4, associated with eosinophilia and elevated IgE levels, the latter being minor criterion in the diagnosis of AD.15 One mechanism by which the TH2 response is elicited may be the release of molecules such as danger-associated molecule patterns that may elicit recruitment of other inflammatory cells. Helper T cell (TH2) activity also can worsen barrier defects through IL-4 and IL-13 release, which can reduce filaggrin expression,29,41 and can aggravate barrier dysfunction in AD.
Inflammatory activation in AD also may involve inflammatory dendritic epidermal cells (IDECs). The IDECs can be tolerogenic or immunogenic mature phenotypes. The IDECs activate helper T cells (TH1), which may contribute to long-term AD activity.
Conclusion
Atopic dermatitis is a common skin condition worldwide and is characterized by the hallmark of pruritus and features that include a typical pattern, history of atopy (personal or family), and usually xerosis and early disease onset. Barrier dysfunction and immune dysregulation are prominent in AD, both of which aggravate the other and may encourage increased development of allergies and other forms of atopy over time.
Atopic dermatitis (AD), or eczema, is the leading dermatologic diagnosis worldwide and is vexing to patients due to the itchiness of the rash. It is the leading cause of skin disease burden worldwide with a prevalence of 229,761,000 reported cases in 2010, presenting largely in preadolescence but also persisting through adulthood.1 Using the children’s life quality index, it has been demonstrated that AD has a greater impact on health-related quality of life than renal disease and cystic fibrosis.2 The overall burden of AD includes stress on the patient and his/her family as well as financial burdens that have been estimated to be similar to that of type 1 diabetes mellitus.3
Epidemiology of AD
The worldwide prevalence of AD varies by country and age group surveyed, with a higher prevalence in wealthy developed nations (eg, the United States) compared to poorer developing nations.4 Efforts to identify prevalence data for AD in the United States have been approached through a variety of strategies. A group in Oregon estimated the prevalence of AD in children aged 5 to 9 years to be 17.2% via a survey of parents (N=1465) and 11.8% with doctor-diagnosed eczema. In the same study, the question “Has a doctor ever said that your child has eczema?” was found to have a 91.3% predictive correlation.5 Analysis of the 2003 National Survey of Children’s Health demonstrated the overall US prevalence of pediatric AD to be 10.7% in 102,353 children 17 years or younger, with a range of 8.7% to 18.1% by region.6
In its evaluation of the worldwide prevalence of AD, the International Study of Asthma and Allergies in Childhood ranked the United States 17th.7,8 The prevalence of AD in developed countries such as the United States is fluid and is expected to increase if the trends from the last 20 years remain true. In an assessment of the National Health Interview Survey data from 1997 to 2011 based on responses to the question, “During the past 12 months, has your child had eczema or any kind of skin allergy?”, the Centers for Disease Control and Prevention identified an increase in the prevalence of AD in patients aged 0 to 17 years from 7.4% in 1997-1999 to 12.5% in 2009-2011.9 Rising prevalence seems to be paired with rising incidence in the total number of severe intractable cases, reduced clearance at the approach of grade school, or cases persisting into adulthood.
Racial Disparity in AD
Racial disparity worldwide and migration are thought to contribute to the prevalence of and therapeutic need for AD. For example, in the United Kingdom, the prevalence of AD in London-born Afro-Caribbean children versus white children (total cross-section, N=693 [junior school children]) was 16.3% and 8.7%, respectively.10 In the United States, black children were more likely to have AD than white children (odds ratio, 1.7).6 Asian and black children also were more likely to present to a physician for treatment of AD than white children.6,10-13
Definition and Diagnostic Considerations
According to Hanifin,14 “Eczema represents a family of inflammatory skin conditions characterized by pruritic, papulovesicular, sometimes weeping dermatitis. All demonstrate the histological hallmark of spongiosis, which helps to distinguish the eczemas from papulosquamous diseases such as psoriasis.”14 Atopic dermatitis is a variant of eczema; however, most laymen identify eczema and AD as being one and the same.
The Hanifin and Rajka15 criteria are the major diagnostic criteria for AD but are difficult to use in clinical practice. Three of the following 4 major criteria are needed for diagnosis: (1) pruritus, which is present universally; (2) typical morphology and distribution; (3) chronic or chronically relapsing dermatitis; and (4) personal and/or family history of atopy. Additionally, 3 of the following 23 minor criteria are needed for diagnosis: xerosis; ichthyosis vulgaris, palmar hyperlinearity, or keratosis pilaris; positive skin prick test; elevated serum IgE level; early age of onset; tendency toward cutaneous infections or impaired cell-mediated immunity; tendency toward nonspecific hand or foot dermatitis; nipple eczema; cheilitis; recurrent conjunctivitis; Dennie-Morgan fold (infraorbital fold); keratoconus; anterior subcapsular cataracts; orbital darkening; facial pallor or facial erythema; pityriasis alba; anterior neck folds; itching when sweating; intolerance to wool and lipid solvents; perifollicular accentuation; skin reactions from ingested foods or by food contact; environmental or emotional factors; and lesional/nonlesional white dermographism or delayed blanch.15-17
More pragmatic streamlined diagnostic criteria were established by Eichenfield et al.18 According to these guidelines, essential features for AD include pruritus and eczema. Important features seen in most cases and adding support to the diagnosis include early age of onset, atopy, and xerosis.18 In clinical practice, diagnosis is often made based on a pruritic relapsing condition in typical locations including the face, neck, and extensor surfaces in infants and children.
Age Considerations
Diagnosis of AD is made by 5 years of age in 85% to 90% of children who will develop the disease and by age 1 year in 60% to 65%.6,19,20 Atopic dermatitis will persist into adulthood in up to one-third of children.21,22 Infantile AD is characterized by erythematous, oozing, excoriated plaques on the cheeks (sparing the nose), scalp, trunk, and extensor surfaces. Pruritus is always seen in AD and can be a source of morbidity.16-18 Seborrheic dermatitis may complicate or overlap with AD in infancy.22
By 2 years of age, most children who are going to develop AD begin to show disease signs of childhood AD characterized by flexural lesions and lesions on the neck and in the postauricular area with sparing of the diaper area.23 Adult AD often presents as eczema of the hands and/or feet. Hand eczema in adulthood is correlated with a prior history of childhood hand eczema and/or childhood AD as well as wet work and caring for small children.24 Children with skin of color may manifest with follicular eczema as their primary disease phenotype. Facial and eyelid dermatitis are more common in Asian females, infants, and teenagers.12,25 Other disease phenotypes that are common in patients with skin of color include lichenoid AD and postinflammatory hypopigmentation.12
Pathogenesis of AD
There are 2 theories on the pathogenesis of AD known as the inside-out and outside-in hypotheses.26 The inside-out hypothesis suggests that allergic triggering leads to a weakened skin barrier that furthers allergen introduction and presentation, while the outside-in hypothesis suggests that the skin barrier is weakened in AD and allows for the presentation of allergens. Both theories have validity and biologic basis, and both may in fact be true in certain individuals.26
The Skin Barrier: An Overview
The skin barrier is a complex set of factors present and functional at birth that seal the keratinocytes and the interkeratinocyte space so that the skin can perform key processes and functions including retention of fluid, exclusion of allergens, protection from UV light and solvents, and prevention of pathogen entry (eg, infections).27-29 The superficial stratum corneum or the cornified envelope consists of keratinocytes with intercellular stripes of hydrophobic and hydrophilic substances formed by various intercellular lipids, largely ceramides, cholesterol, and free fatty acids.30,31 Keratinocytes are the first responders to a variety of environmental insults with the production of IL-18, RANTES (regulated on activation, normal T-expressed, and presumably secreted), granulocyte-macrophage colony-stimulating factor, and thymic stromal lymphopoietin. These inflammatory substances produce acute and chronic inflammation, mast cell reactivity, and T-cell activation.14 Corneodesmosins link the keratinocytes. Peptidases released will cleave the corneodesmosins and allow normal desquamation or shedding of surface skin, which is replaced by division of stem cells in the basal layer.29
The stratum granulosum is the layer beneath the stratum corneum that co-contributes to barrier activity. The stratum granulosum is absent or reduced histologically in ichthyosis vulgaris,32 a form of skin dryness linked to filaggrin mutations and AD. Filaggrin breakdown creates natural moisturizing factor, a series of hygroscopic compounds that attract water into the skin.33 Histidine, a filaggrin breakdown product, is used by urocanic acid to process UV light insults.34 Filaggrin also contributes to other barrier functions including pH and stratum corneum cohesion as well as paracellular permeability of the stratum corneum. Tight junctions in the stratum granulosum include claudin-1 and claudin-6 and provide another barrier feature.29
The skin barrier is composed of lipids and keratinocytes. Ceramides, which represent one type of lipids, are reduced in AD, causing alteration in the lamellar pattern35 and increased transepidermal water loss. Furthermore, the stratum corneum is thickened in AD, possibly in response to trauma, and hydration is reduced.36 Filaggrin (chromosome arm 1q21.3) is formed from the 400-kDa+ precursor profilaggrin through dephosphorylation and cleavage, and it performs an essential function in the skin barrier through its differential cleavage and breakdown as well as release of natural moisturizing factor and other compounds.37 Filaggrin mutations are linked to AD and ichthyosis vulgaris; however, barrier defects as evidenced by transepidermal water loss in the absence of filaggrin mutation are sufficient to allow for sensitization to allergens through the skin.29 Filaggrin mutations have been associated with AD development and vary in prevalence worldwide. In the United Kingdom, a prevalence study of filaggrin mutations in patients aged 7 to 9 years (N=792) demonstrated an 18.4% carrier rate in AD patients versus 12.9% in controls.34 A similar study in Sweden (N=3301) showed carrier rates of 13.5% versus 6.5%, respectively.38 Although filaggrin mutations are lower in black patients,39 ceramide content may be reduced in this population, demonstrating that a variety of skin barrier defects can result in AD. Carriers of filaggrin mutations are more likely to have eczema on skin exposed to environmental factors (eg, face, hands).40
Barrier Defects Contributing to AD
The breakdown of the stratum corneum allows for antigen presentation to Langerhans cells, the dendritic antigen-presenting cells of the skin. Breaks in the stratum corneum may occur from scratching. These macroscopic breaks are large, whereas the breaks that otherwise occur due to barrier breakdown may be more microscopic in nature. Scratching causes aggravation of the helper T cell (TH2) response.29 For example, it allows the dendritic ends of Langerhans cells to be exposed to antigens. The dendritic ends capture allergens through IgE (may be elevated in AD29), which is bound to the high-affinity FCER1 receptors on Langerhans cells. Rather than causing a type I hypersensitivity reaction, these Langerhans cells are activated and move to the lymph nodes where they present antigen and initiate a cascade of proinflammatory activity. This TH2 cascade includes release of cytokines such as IL-2, IL-4, IL-8, IL-10, tumor necrosis factor α, and IFN-γ.26,29
Transepidermal water loss and barrier dysfunction contribute to disease activity and facilitate food/environmental allergen sensitization by allowing increased penetration of allergens through the skin to be presented by Langerhans cells to TH1 cells (sensitization phase). The Langerhans cells can reach their dendritic ends through tight junctions and into the stratum corneum, allowing them to reach surface allergens when the barrier is impaired. Ultimate expansion to systemic allergy (effector phase) occurs when dendritic cells move to draining lymph nodes, causing antigen presentation to CD4 and/or CD8 cells. Langerhans cells and dendritic cell sensitization through the weakened skin is believed to be the basis or role of barrier disruption as a trigger of atopic diseases, including AD and food and environmental allergies.
Many different forms of barrier disruption can cause a TH2 response in AD. The TH2 response triggers a constellation of proinflammatory activities including release of IL-4, associated with eosinophilia and elevated IgE levels, the latter being minor criterion in the diagnosis of AD.15 One mechanism by which the TH2 response is elicited may be the release of molecules such as danger-associated molecule patterns that may elicit recruitment of other inflammatory cells. Helper T cell (TH2) activity also can worsen barrier defects through IL-4 and IL-13 release, which can reduce filaggrin expression,29,41 and can aggravate barrier dysfunction in AD.
Inflammatory activation in AD also may involve inflammatory dendritic epidermal cells (IDECs). The IDECs can be tolerogenic or immunogenic mature phenotypes. The IDECs activate helper T cells (TH1), which may contribute to long-term AD activity.
Conclusion
Atopic dermatitis is a common skin condition worldwide and is characterized by the hallmark of pruritus and features that include a typical pattern, history of atopy (personal or family), and usually xerosis and early disease onset. Barrier dysfunction and immune dysregulation are prominent in AD, both of which aggravate the other and may encourage increased development of allergies and other forms of atopy over time.
1. Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
2. Beattie PE, Lewis-Jones MS. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol. 2006;155:145-151.
3. Su JC, Kemp AS, Varigos GA, et al. Atopic eczema: its impact on the family and financial cost. Arch Dis Child. 1997;76:159-162.
4. Garg N, Silverberg JI. Epidemiology of childhood atopic dermatitis. Clin Dermatol. 2015;33:281-288.
5. Laughter D, Istvan JA, Tofte SJ, et al. The prevalence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol. 2000;43:649-655.
6. Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
7. Odhiambo JA, Williams HC, Clayton TO, et al. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251-1258.
8. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet. 1998;351:1225-1232.
9. Hansen TE, Evjenth B, Holt J. Increasing prevalence of asthma, allergic rhinoconjunctivitis and eczema among schoolchildren: three surveys during the period 1985-2008. Acta Paediatr. 2013;102:47-52.
10. Williams HC, Pembroke AC, Forsdyke H, et al. London-born black Caribbean children are at increased risk of atopic dermatitis. J Am Acad Dermatol. 1995;32:212-217.
11. Horii KA, Simon SD, Liu DY, et al. Atopic dermatitis in children in the United States, 1997-2004: visit trends, patient and provider characteristics, and prescribing patterns. Pediatrics. 2007;120:e527-e534.
12. Silverberg NB. Eczematous diseases. In: Silverberg NB. Atlas of Pediatric Cutaneous Biodiversity. New York, NY: Springer; 2012:69-88.
13. Gupta J, Grube E, Ericksen MB, et al. Intrinsically defective skin barrier function in children with atopic dermatitis correlates with disease severity. J Allergy Clin Immunol. 2008;121:725-730.
14. Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
15. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980;92:44-47.
16. Queille-Roussel C, Raynaud F, Saurat JH. A prospective computerized study of 500 cases of atopic dermatitis in childhood. I. Initial analysis of 250 parameters. Acta Derm Venereol Suppl (Stockh). 1985;114:87-92.
17. Böhme M, Svensson A, Kull I, et al. Hanifin’s and Rajka’s minor criteria for atopic dermatitis: which do 2-year-olds exhibit? J Am Acad Dermatol. 2000;43:785-792.
18. Eichenfield LF, Hanifin JM, Luger TA, et al. Consensus conference on pediatric atopic dermatitis. J Am Acad Dermatol. 2003;49:1088-1095.
19. Kay J, Gawkrodger DJ, Mortimer MJ, et al. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol. 1994;30:35-39.
20. Perkin MR, Strachan DP, Williams HC, et al. Natural history of atopic dermatitis and its relationship to serum total immunoglobulin E in a population-based birth cohort study. Pediatr Allergy Immunol. 2004;15:221-229.
21. Ellis CN, Mancini AJ, Paller AS, et al. Understanding and managing atopic dermatitis in adult patients. Semin Cutan Med Surg. 2012;31(suppl 2):S18-S22.
22. Elish D, Silverberg NB. Infantile seborrheic dermatitis. Cutis. 2006;77:297-300.
23. Meding B, Wrangsjö K, Järvholm B. Hand eczema extent and morphology—association and influence on long-term prognosis. J Invest Dermatol. 2007;127:2147-2151.
24. Mortz CG, Bindslev-Jensen C, Andersen KE. Hand eczema in The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis (TOACS): prevalence, incidence and risk factors from adolescence to adulthood [published online August 7, 2014]. Br J Dermatol. 2014;171:313-323.
25. Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
26. Silverberg NB, Silverberg JI. Inside out or outside in: does atopic dermatitis disrupt barrier function or does disruption of barrier function trigger atopic dermatitis? Cutis. 2015;96:359-361.
27. Visscher MO, Adam R, Brink S, et al. Newborn infant skin: physiology, development, and care [published online December 8, 2014]. Clin Dermatol. 2015;33:271-280.
28. Miyagaki T, Sugaya M. Recent advances in atopic dermatitis and psoriasis: genetic background, barrier function, and therapeutic targets. J Dermatol Sci. 2015;78:89-94.
29. De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949-963.
30. Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009;9:437-446.
31. Janssens M, van Smeden J, Gooris GS, et al. Lamellar lipid organization and ceramide composition in the stratum corneum of patients with atopic eczema. J Invest Dermatol. 2011;131:2136-2138.
32. Fitch N, Segool R, Ferenczy A, et al. Dominant ichthyosis vulgaris with an ultrastructurally normal granular layer. Clin Genet. 1976;9:71-76.
33. Chandar P, Nole G, Johnson AW. Understanding natural moisturizing mechanisms: implications for moisturizer technology. Cutis. 2009;84(suppl 1):2-15.
34. Brown SJ, Relton CL, Liao H, et al. Filaggrin null mutations and childhood atopic eczema: a population-based case-control study. J Allergy Clin Immunol. 2008;121:940-946.
35. Marenholz I, Rivera VA, Esparza-Gordillo J, et al. Association screening in the Epidermal Differentiation Complex (EDC) identifies an SPRR3 repeat number variant as a risk factor for eczema. J Invest Dermatol. 2011;131:1644-1649.
36. Nemoto-Hasebe I, Akiyama M, Nomura T, et al. Clinical severity correlates with impaired barrier in filaggrin-related eczema. J Invest Dermatol. 2009;129:682-689.
37. Hoste E, Kemperman P, Devos M, et al. Caspase-14 is required for filaggrin degradation to natural moisturizing factors in the skin. J Invest Dermatol. 2011;131:2233-2241.
38. Ballardini N, Kull I, Söderhäll C, et al. Eczema severity in preadolescent children and its relation to sex, filaggrin mutations, asthma, rhinitis, aggravating factors and topical treatment: a report from the BAMSE birth cohort. Br J Dermatol. 2013;168:588-594.
39. Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912-917.
40. Carson CG, Rasmussen MA, Thyssen JP, et al. Clinical presentation of atopic dermatitis by filaggrin gene mutation status during the first 7 years of life in a prospective cohort study. PLoS One. 2012;7:e48678.
41. Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
1. Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
2. Beattie PE, Lewis-Jones MS. A comparative study of impairment of quality of life in children with skin disease and children with other chronic childhood diseases. Br J Dermatol. 2006;155:145-151.
3. Su JC, Kemp AS, Varigos GA, et al. Atopic eczema: its impact on the family and financial cost. Arch Dis Child. 1997;76:159-162.
4. Garg N, Silverberg JI. Epidemiology of childhood atopic dermatitis. Clin Dermatol. 2015;33:281-288.
5. Laughter D, Istvan JA, Tofte SJ, et al. The prevalence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol. 2000;43:649-655.
6. Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
7. Odhiambo JA, Williams HC, Clayton TO, et al. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251-1258.
8. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet. 1998;351:1225-1232.
9. Hansen TE, Evjenth B, Holt J. Increasing prevalence of asthma, allergic rhinoconjunctivitis and eczema among schoolchildren: three surveys during the period 1985-2008. Acta Paediatr. 2013;102:47-52.
10. Williams HC, Pembroke AC, Forsdyke H, et al. London-born black Caribbean children are at increased risk of atopic dermatitis. J Am Acad Dermatol. 1995;32:212-217.
11. Horii KA, Simon SD, Liu DY, et al. Atopic dermatitis in children in the United States, 1997-2004: visit trends, patient and provider characteristics, and prescribing patterns. Pediatrics. 2007;120:e527-e534.
12. Silverberg NB. Eczematous diseases. In: Silverberg NB. Atlas of Pediatric Cutaneous Biodiversity. New York, NY: Springer; 2012:69-88.
13. Gupta J, Grube E, Ericksen MB, et al. Intrinsically defective skin barrier function in children with atopic dermatitis correlates with disease severity. J Allergy Clin Immunol. 2008;121:725-730.
14. Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
15. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh). 1980;92:44-47.
16. Queille-Roussel C, Raynaud F, Saurat JH. A prospective computerized study of 500 cases of atopic dermatitis in childhood. I. Initial analysis of 250 parameters. Acta Derm Venereol Suppl (Stockh). 1985;114:87-92.
17. Böhme M, Svensson A, Kull I, et al. Hanifin’s and Rajka’s minor criteria for atopic dermatitis: which do 2-year-olds exhibit? J Am Acad Dermatol. 2000;43:785-792.
18. Eichenfield LF, Hanifin JM, Luger TA, et al. Consensus conference on pediatric atopic dermatitis. J Am Acad Dermatol. 2003;49:1088-1095.
19. Kay J, Gawkrodger DJ, Mortimer MJ, et al. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol. 1994;30:35-39.
20. Perkin MR, Strachan DP, Williams HC, et al. Natural history of atopic dermatitis and its relationship to serum total immunoglobulin E in a population-based birth cohort study. Pediatr Allergy Immunol. 2004;15:221-229.
21. Ellis CN, Mancini AJ, Paller AS, et al. Understanding and managing atopic dermatitis in adult patients. Semin Cutan Med Surg. 2012;31(suppl 2):S18-S22.
22. Elish D, Silverberg NB. Infantile seborrheic dermatitis. Cutis. 2006;77:297-300.
23. Meding B, Wrangsjö K, Järvholm B. Hand eczema extent and morphology—association and influence on long-term prognosis. J Invest Dermatol. 2007;127:2147-2151.
24. Mortz CG, Bindslev-Jensen C, Andersen KE. Hand eczema in The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis (TOACS): prevalence, incidence and risk factors from adolescence to adulthood [published online August 7, 2014]. Br J Dermatol. 2014;171:313-323.
25. Kiken DA, Silverberg NB. Atopic dermatitis in children, part 1: epidemiology, clinical features, and complications. Cutis. 2006;78:241-247.
26. Silverberg NB, Silverberg JI. Inside out or outside in: does atopic dermatitis disrupt barrier function or does disruption of barrier function trigger atopic dermatitis? Cutis. 2015;96:359-361.
27. Visscher MO, Adam R, Brink S, et al. Newborn infant skin: physiology, development, and care [published online December 8, 2014]. Clin Dermatol. 2015;33:271-280.
28. Miyagaki T, Sugaya M. Recent advances in atopic dermatitis and psoriasis: genetic background, barrier function, and therapeutic targets. J Dermatol Sci. 2015;78:89-94.
29. De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949-963.
30. Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009;9:437-446.
31. Janssens M, van Smeden J, Gooris GS, et al. Lamellar lipid organization and ceramide composition in the stratum corneum of patients with atopic eczema. J Invest Dermatol. 2011;131:2136-2138.
32. Fitch N, Segool R, Ferenczy A, et al. Dominant ichthyosis vulgaris with an ultrastructurally normal granular layer. Clin Genet. 1976;9:71-76.
33. Chandar P, Nole G, Johnson AW. Understanding natural moisturizing mechanisms: implications for moisturizer technology. Cutis. 2009;84(suppl 1):2-15.
34. Brown SJ, Relton CL, Liao H, et al. Filaggrin null mutations and childhood atopic eczema: a population-based case-control study. J Allergy Clin Immunol. 2008;121:940-946.
35. Marenholz I, Rivera VA, Esparza-Gordillo J, et al. Association screening in the Epidermal Differentiation Complex (EDC) identifies an SPRR3 repeat number variant as a risk factor for eczema. J Invest Dermatol. 2011;131:1644-1649.
36. Nemoto-Hasebe I, Akiyama M, Nomura T, et al. Clinical severity correlates with impaired barrier in filaggrin-related eczema. J Invest Dermatol. 2009;129:682-689.
37. Hoste E, Kemperman P, Devos M, et al. Caspase-14 is required for filaggrin degradation to natural moisturizing factors in the skin. J Invest Dermatol. 2011;131:2233-2241.
38. Ballardini N, Kull I, Söderhäll C, et al. Eczema severity in preadolescent children and its relation to sex, filaggrin mutations, asthma, rhinitis, aggravating factors and topical treatment: a report from the BAMSE birth cohort. Br J Dermatol. 2013;168:588-594.
39. Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912-917.
40. Carson CG, Rasmussen MA, Thyssen JP, et al. Clinical presentation of atopic dermatitis by filaggrin gene mutation status during the first 7 years of life in a prospective cohort study. PLoS One. 2012;7:e48678.
41. Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
Practice Points
- The impact of atopic dermatitis (AD) on health-related quality of life mimics that of chronic childhood illnesses such as cystic fibrosis.
- The prevalence of pediatric AD in the United States is estimated at more than 10% of children, with a 1.7 increased odds ratio in black children.
- Diagnosis generally is made based on the presence of a pruritic eczematous eruption with typical morphology and a personal and/or family history of atopy.
- Atopic dermatitis is caused by a complex interplay of skin barrier dysfunction and immune tendency toward allergy development.
FDA Proposes New Rule to Ban Use of Indoor Tanning Devices by Minors
The US Food and Drug Administration (FDA) has proposed 2 new rules to protect consumers from health risks associated with indoor tanning by banning use of indoor tanning devices by minors and imposing safety measures.
The first proposed rule restricts the use of indoor tanning devices to adults 18 years and older. It also requires indoor tanning facilities to inform adult users about the health risks of indoor tanning and obtain a signed risk acknowledgement from consumers before their first tanning session and every 6 months thereafter.
“Exposure to UV radiation from indoor tanning is a preventable cause of skin cancer,” explained Markham C. Luke, MD, PhD, deputy office director for the Office of Device Evaluation at the FDA’s Center for Devices and Radiological Health. “The FDA is committed to protecting public health by informing consumers of the risks of indoor tanning.”
The second proposed rule addresses performance standards, requiring manufacturers and indoor tanning facilities to take measures to improve the overall safety of tanning devices. Key changes would include:
- Make consumer warnings more prominent and easier to read on tanning devices.
- Require an easily accessible emergency shutoff switch (or panic button) on all tanning devices.
- Add requirements to limit the amount of light allowed through protective eyewear to protect the eyes.
- Improve labeling on replacement bulbs to ensure tanning facility operators use the proper bulbs to reduce risk for accidental burns.
- Prohibit tanning facilities from making dangerous device modifications (eg, installing stronger bulbs) without recertifying and reidentifying the device with the FDA.
The FDA reports that more than 1 million minors use indoor tanning facilities each year. According to the American Academy of Dermatology, consumers younger than 35 years who use indoor tanning facilities are 59% more likely to develop melanoma than those who have never tanned indoors. Because the effects of UV exposure are cumulative and add up over the course of one’s lifetime, minors who use indoor tanning devices are at an increased risk for developing melanoma and nonmelanoma skin cancers later in life.
In 2014 the FDA began requiring tanning devices to be labeled with a visible warning stating that individuals younger than 18 years should not use them. Additionally, several states have already passed laws prohibiting minors from indoor tanning; in Connecticut, New Jersey, New York, and Pennsylvania, tanning devices are banned in individuals younger than 17 years.
Dermatologists are in the position to discuss the health risks of indoor tanning with all patients regardless of age. Patients should be reminded that failure to wear appropriate protective eyewear can lead to short-term and long-term eye injury and that long exposures can lead to burning that may not be recognized until it is too late. It also is important to warn patients that tanning while using certain medications or cosmetics may cause increased sensitivity to UV radiation. Patients can be referred to the FDA website for more consumer updates about indoor tanning and the proposed rules.
The US Food and Drug Administration (FDA) has proposed 2 new rules to protect consumers from health risks associated with indoor tanning by banning use of indoor tanning devices by minors and imposing safety measures.
The first proposed rule restricts the use of indoor tanning devices to adults 18 years and older. It also requires indoor tanning facilities to inform adult users about the health risks of indoor tanning and obtain a signed risk acknowledgement from consumers before their first tanning session and every 6 months thereafter.
“Exposure to UV radiation from indoor tanning is a preventable cause of skin cancer,” explained Markham C. Luke, MD, PhD, deputy office director for the Office of Device Evaluation at the FDA’s Center for Devices and Radiological Health. “The FDA is committed to protecting public health by informing consumers of the risks of indoor tanning.”
The second proposed rule addresses performance standards, requiring manufacturers and indoor tanning facilities to take measures to improve the overall safety of tanning devices. Key changes would include:
- Make consumer warnings more prominent and easier to read on tanning devices.
- Require an easily accessible emergency shutoff switch (or panic button) on all tanning devices.
- Add requirements to limit the amount of light allowed through protective eyewear to protect the eyes.
- Improve labeling on replacement bulbs to ensure tanning facility operators use the proper bulbs to reduce risk for accidental burns.
- Prohibit tanning facilities from making dangerous device modifications (eg, installing stronger bulbs) without recertifying and reidentifying the device with the FDA.
The FDA reports that more than 1 million minors use indoor tanning facilities each year. According to the American Academy of Dermatology, consumers younger than 35 years who use indoor tanning facilities are 59% more likely to develop melanoma than those who have never tanned indoors. Because the effects of UV exposure are cumulative and add up over the course of one’s lifetime, minors who use indoor tanning devices are at an increased risk for developing melanoma and nonmelanoma skin cancers later in life.
In 2014 the FDA began requiring tanning devices to be labeled with a visible warning stating that individuals younger than 18 years should not use them. Additionally, several states have already passed laws prohibiting minors from indoor tanning; in Connecticut, New Jersey, New York, and Pennsylvania, tanning devices are banned in individuals younger than 17 years.
Dermatologists are in the position to discuss the health risks of indoor tanning with all patients regardless of age. Patients should be reminded that failure to wear appropriate protective eyewear can lead to short-term and long-term eye injury and that long exposures can lead to burning that may not be recognized until it is too late. It also is important to warn patients that tanning while using certain medications or cosmetics may cause increased sensitivity to UV radiation. Patients can be referred to the FDA website for more consumer updates about indoor tanning and the proposed rules.
The US Food and Drug Administration (FDA) has proposed 2 new rules to protect consumers from health risks associated with indoor tanning by banning use of indoor tanning devices by minors and imposing safety measures.
The first proposed rule restricts the use of indoor tanning devices to adults 18 years and older. It also requires indoor tanning facilities to inform adult users about the health risks of indoor tanning and obtain a signed risk acknowledgement from consumers before their first tanning session and every 6 months thereafter.
“Exposure to UV radiation from indoor tanning is a preventable cause of skin cancer,” explained Markham C. Luke, MD, PhD, deputy office director for the Office of Device Evaluation at the FDA’s Center for Devices and Radiological Health. “The FDA is committed to protecting public health by informing consumers of the risks of indoor tanning.”
The second proposed rule addresses performance standards, requiring manufacturers and indoor tanning facilities to take measures to improve the overall safety of tanning devices. Key changes would include:
- Make consumer warnings more prominent and easier to read on tanning devices.
- Require an easily accessible emergency shutoff switch (or panic button) on all tanning devices.
- Add requirements to limit the amount of light allowed through protective eyewear to protect the eyes.
- Improve labeling on replacement bulbs to ensure tanning facility operators use the proper bulbs to reduce risk for accidental burns.
- Prohibit tanning facilities from making dangerous device modifications (eg, installing stronger bulbs) without recertifying and reidentifying the device with the FDA.
The FDA reports that more than 1 million minors use indoor tanning facilities each year. According to the American Academy of Dermatology, consumers younger than 35 years who use indoor tanning facilities are 59% more likely to develop melanoma than those who have never tanned indoors. Because the effects of UV exposure are cumulative and add up over the course of one’s lifetime, minors who use indoor tanning devices are at an increased risk for developing melanoma and nonmelanoma skin cancers later in life.
In 2014 the FDA began requiring tanning devices to be labeled with a visible warning stating that individuals younger than 18 years should not use them. Additionally, several states have already passed laws prohibiting minors from indoor tanning; in Connecticut, New Jersey, New York, and Pennsylvania, tanning devices are banned in individuals younger than 17 years.
Dermatologists are in the position to discuss the health risks of indoor tanning with all patients regardless of age. Patients should be reminded that failure to wear appropriate protective eyewear can lead to short-term and long-term eye injury and that long exposures can lead to burning that may not be recognized until it is too late. It also is important to warn patients that tanning while using certain medications or cosmetics may cause increased sensitivity to UV radiation. Patients can be referred to the FDA website for more consumer updates about indoor tanning and the proposed rules.
Shiftless
I drive a 10-year-old pickup truck. The air conditioner no longer works – not a real problem here in Maine. It has hand-operated roll down windows ... a real plus should I back too far down the boat ramp and find myself in the cold waters of the Atlantic. If I ever decide to lock it, I will need to use a real key. But the pile of mismatched work gloves and rusty garden tools stashed behind the front seat hasn’t seemed to attract any burglars. Its dented body sits on a new frame, thanks to a generous recall from the manufacturer.
It’s a four-wheel drive, handy in the winter. But what I really like about it is that my old truck has a standard manual transmission. I still have that boyish enthusiasm for shifting gears. I can imagine myself driving a low-slung sports car or operating some gargantuan piece of heavy machinery.
Driving a stick shift vehicle demands a level of engagement and concentration several levels above that of simply aiming a car equipped with an automatic transmission. While I am sure some have tried, shifting gears is a serious deterrent to texting at the wheel.
The fact that our three children learned to drive on a standard shift station wagon is a tribute to their parents’ ability to tolerate repeated whiplash injuries. But it also may be one of the reasons that they survived those deadly middle teenage years. Nichole Morris, a researcher at the Human FIRST Laboratory of the University of Minnesota has said, “If you are going to have an early, untimely death, the most dangerous 2 years of your life are between 16 and 17, and the reason for that is driving” (“Teenage Drivers? Be Very Afraid,” by Bruce Feiler, New York Times, March 19, 2016). Six teenagers a day die from motor vehicle accident–related injuries, according to the Centers for Disease Control and Prevention. There are more deaths from motor vehicle accidents in this age group than from suicides, cancer, and other accidents.
An unfortunate combination of perceived invincibility and inexperience in an environment richly decorated with distractions makes those first years behind the wheel so dangerous. Charlie Klauer, a researcher at Virginia Tech’s Transportation Institute, believes that one in four teenagers will be involved in a motor vehicle crash in his or her first 6 months of driving. My personal experience supports her observation. Luckily, my daughter’s first accident was a low speed rear-ender into a giant pickup truck that sustained no obvious damage.
Given these frightening statistics, it is surprising that any parents would ever allow their teenage children to start on the path toward a driver’s license. As physicians committed to the health and safety of children, why haven’t pediatricians done more to prevent this tragic loss of life? The honest answer is simply that the motor vehicle is too tightly woven into our culture. We have tried, but we could probably do more.
Technological advances such as self-braking cars that are spinning off from the development of autonomous vehicles may save a few teenage drivers. But, watching your 17-year-old child take the wheel for the first time will continue to be an anxiety-provoking experience for the foreseeable future. We can help by reminding parents that the driving is a privilege that can easily be revoked. We must continue to urge parents to create and enforce rules about the use of cell phones behind the wheel. Many states have enacted laws that restrict teenage drivers from driving with other teens in the car, a well-known and often fatal distraction. But parents must be reminded that they are the first line of enforcement.
Enduring those neck-snapping sessions that are unavoidable when your child is learning to drive a standard shift vehicle was a sacrifice my wife and I made gladly. Manual transmissions aren’t coming back. But there are still plenty of sacrifices for today’s parents to make if they want their children to survive those deadly midteen years.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.”
I drive a 10-year-old pickup truck. The air conditioner no longer works – not a real problem here in Maine. It has hand-operated roll down windows ... a real plus should I back too far down the boat ramp and find myself in the cold waters of the Atlantic. If I ever decide to lock it, I will need to use a real key. But the pile of mismatched work gloves and rusty garden tools stashed behind the front seat hasn’t seemed to attract any burglars. Its dented body sits on a new frame, thanks to a generous recall from the manufacturer.
It’s a four-wheel drive, handy in the winter. But what I really like about it is that my old truck has a standard manual transmission. I still have that boyish enthusiasm for shifting gears. I can imagine myself driving a low-slung sports car or operating some gargantuan piece of heavy machinery.
Driving a stick shift vehicle demands a level of engagement and concentration several levels above that of simply aiming a car equipped with an automatic transmission. While I am sure some have tried, shifting gears is a serious deterrent to texting at the wheel.
The fact that our three children learned to drive on a standard shift station wagon is a tribute to their parents’ ability to tolerate repeated whiplash injuries. But it also may be one of the reasons that they survived those deadly middle teenage years. Nichole Morris, a researcher at the Human FIRST Laboratory of the University of Minnesota has said, “If you are going to have an early, untimely death, the most dangerous 2 years of your life are between 16 and 17, and the reason for that is driving” (“Teenage Drivers? Be Very Afraid,” by Bruce Feiler, New York Times, March 19, 2016). Six teenagers a day die from motor vehicle accident–related injuries, according to the Centers for Disease Control and Prevention. There are more deaths from motor vehicle accidents in this age group than from suicides, cancer, and other accidents.
An unfortunate combination of perceived invincibility and inexperience in an environment richly decorated with distractions makes those first years behind the wheel so dangerous. Charlie Klauer, a researcher at Virginia Tech’s Transportation Institute, believes that one in four teenagers will be involved in a motor vehicle crash in his or her first 6 months of driving. My personal experience supports her observation. Luckily, my daughter’s first accident was a low speed rear-ender into a giant pickup truck that sustained no obvious damage.
Given these frightening statistics, it is surprising that any parents would ever allow their teenage children to start on the path toward a driver’s license. As physicians committed to the health and safety of children, why haven’t pediatricians done more to prevent this tragic loss of life? The honest answer is simply that the motor vehicle is too tightly woven into our culture. We have tried, but we could probably do more.
Technological advances such as self-braking cars that are spinning off from the development of autonomous vehicles may save a few teenage drivers. But, watching your 17-year-old child take the wheel for the first time will continue to be an anxiety-provoking experience for the foreseeable future. We can help by reminding parents that the driving is a privilege that can easily be revoked. We must continue to urge parents to create and enforce rules about the use of cell phones behind the wheel. Many states have enacted laws that restrict teenage drivers from driving with other teens in the car, a well-known and often fatal distraction. But parents must be reminded that they are the first line of enforcement.
Enduring those neck-snapping sessions that are unavoidable when your child is learning to drive a standard shift vehicle was a sacrifice my wife and I made gladly. Manual transmissions aren’t coming back. But there are still plenty of sacrifices for today’s parents to make if they want their children to survive those deadly midteen years.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.”
I drive a 10-year-old pickup truck. The air conditioner no longer works – not a real problem here in Maine. It has hand-operated roll down windows ... a real plus should I back too far down the boat ramp and find myself in the cold waters of the Atlantic. If I ever decide to lock it, I will need to use a real key. But the pile of mismatched work gloves and rusty garden tools stashed behind the front seat hasn’t seemed to attract any burglars. Its dented body sits on a new frame, thanks to a generous recall from the manufacturer.
It’s a four-wheel drive, handy in the winter. But what I really like about it is that my old truck has a standard manual transmission. I still have that boyish enthusiasm for shifting gears. I can imagine myself driving a low-slung sports car or operating some gargantuan piece of heavy machinery.
Driving a stick shift vehicle demands a level of engagement and concentration several levels above that of simply aiming a car equipped with an automatic transmission. While I am sure some have tried, shifting gears is a serious deterrent to texting at the wheel.
The fact that our three children learned to drive on a standard shift station wagon is a tribute to their parents’ ability to tolerate repeated whiplash injuries. But it also may be one of the reasons that they survived those deadly middle teenage years. Nichole Morris, a researcher at the Human FIRST Laboratory of the University of Minnesota has said, “If you are going to have an early, untimely death, the most dangerous 2 years of your life are between 16 and 17, and the reason for that is driving” (“Teenage Drivers? Be Very Afraid,” by Bruce Feiler, New York Times, March 19, 2016). Six teenagers a day die from motor vehicle accident–related injuries, according to the Centers for Disease Control and Prevention. There are more deaths from motor vehicle accidents in this age group than from suicides, cancer, and other accidents.
An unfortunate combination of perceived invincibility and inexperience in an environment richly decorated with distractions makes those first years behind the wheel so dangerous. Charlie Klauer, a researcher at Virginia Tech’s Transportation Institute, believes that one in four teenagers will be involved in a motor vehicle crash in his or her first 6 months of driving. My personal experience supports her observation. Luckily, my daughter’s first accident was a low speed rear-ender into a giant pickup truck that sustained no obvious damage.
Given these frightening statistics, it is surprising that any parents would ever allow their teenage children to start on the path toward a driver’s license. As physicians committed to the health and safety of children, why haven’t pediatricians done more to prevent this tragic loss of life? The honest answer is simply that the motor vehicle is too tightly woven into our culture. We have tried, but we could probably do more.
Technological advances such as self-braking cars that are spinning off from the development of autonomous vehicles may save a few teenage drivers. But, watching your 17-year-old child take the wheel for the first time will continue to be an anxiety-provoking experience for the foreseeable future. We can help by reminding parents that the driving is a privilege that can easily be revoked. We must continue to urge parents to create and enforce rules about the use of cell phones behind the wheel. Many states have enacted laws that restrict teenage drivers from driving with other teens in the car, a well-known and often fatal distraction. But parents must be reminded that they are the first line of enforcement.
Enduring those neck-snapping sessions that are unavoidable when your child is learning to drive a standard shift vehicle was a sacrifice my wife and I made gladly. Manual transmissions aren’t coming back. But there are still plenty of sacrifices for today’s parents to make if they want their children to survive those deadly midteen years.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics including “How to Say No to Your Toddler.”
Physician, know thy patient before recommending treatment
Hollywood, FLA. – Although patients and physicians should always be partners in medical decision making, guiding patients into making medically sound choices may involve a lot of listening and empathy, often followed by a little friendly persuasion, suggests an expert in health care decision making,
“Even when you get to a situation where the clinical practice guideline would point toward shared decision making, we’ve got to do shared decision making right or the guideline just doesn’t do the work for us. We clinicians have to know how to partner with our patients to make decisions when their values matter,” said Dr. Peter A Ubel, professor of business, public policy, and medicine at Duke University, Durham, N.H.
Dr. Ubel discussed how to understand and use patient preferences in cancer treatment decisions at the annual conference of the National Comprehensive Cancer Network.
In the case of early-stage prostate cancer, for example, treatment options include surveillance or active treatment with surgery or radiation, but the risk/benefit trade-offs require careful discussion.
“The different approaches have different pros and cons: If you get active treatment, there’s a pretty good chance you’ll experience incontinence or erectile dysfunction, whereas if you undergo surveillance you won’t experience those as side effects of the surveillance strategy, but you will live with a cancer inside your body, the accompanying anxiety, and wondering every 6 months whether it has advanced,” Dr. Ubel said.
“The question is how do we go figure out which treatment is best for which patient?” he added.
What doctors say not always what patients hear
To examine how decisions are made, Dr. Ubel and his colleagues conducted a study in which patients scheduled for biopsy for suspicion of prostate cancer were approached at the time of their clinic visits and asked to participate in a study about prostate cancer decision making. Patients were given a booklet aimed at the seventh-grade reading level describing prostate cancer and its treatment, and were asked, once they had finished, what course of therapy they might pursue if the diagnosis turned out to be positive, and why. They also were asked if it was important to them to maintain natural sexual function.
The encounter between the patients and their urologists at the time of diagnosis were audio recorded, so that investigators could see whether physicians recommend specific treatments and why.
“One of the things that really jumped out in this study was just how much language, how much explanation urologists use to help patients understand their diagnosis,” Dr. Ubel said.
In one encounter, the urologist explained to the patient that 3 out of 12 biopsy cores had less than 30% cancer involvement, suggesting moderately low-risk disease, but then went on to talk about Gleason scores, tumor grades and patterns, and risk categories.
“When a patient just finds out he has prostate cancer, it’s a tough time to put a whole bunch of information in front of him for him to absorb and make a decision,” Dr. Ubel said. Patients need time to absorb the shock of a cancer diagnosis first – even a diagnosis of an early, easily treated cancer – and information overload may actually reduce their ability to comprehend their choices or retain the information, he added. The urologist in this scenario is making a very earnest effort to tell the patient that he doesn’t have the kind of cancer that’s ever going to kill him, or that it is highly unlikely to cause any problems for the next 10-15 years, and there is ample time to decide how to treat it.
“But the doctor kind of forgets that the patient doesn’t speak medicalese, and the doctor feels like you really have to give them thorough informed consent, after all. So you need to inform the heck out of patients with all of the medical detail you believe is necessary to understand the decision, instead of the translation of the medical detail into terms the patient can understand,” he said.
An important part of shared decision making, therefore, is to make sure that patients can understand their alternatives, but not to overwhelm them with detail, because they may give up and ask the physician, “What do you think I should do?” which can introduce physician bias that may not always lead to the right choice for that patient.
“I actually morally don’t recommend that. I think instead we should give the right amount of information at the right time so they can actually get engaged in the choice,” Dr. Ubel said.
Discussion informs choices
The investigators looked at how prediagnosis education materials and discussions with urologists shaped patient decisions about treatment choice. Patients were called before their appointments to ensure that they had read the booklet, and then just before the appointment were asked which way they were leaning if the diagnosis turned out to be positive.
The investigators found that of 44 patients who expressed a preference for active surveillance before the appointment, 55% actually went on to receive active treatment. Among 119 patients with no expressed preference for surveillance or active therapy, 46% went on to treatment, and of 118 expressing previsit preference only for active surveillance, 54% went on to receive it.
“The leaning that they had before seeing the doctor had no influence on what treatment they got,” Dr. Ubel said. Instead, physicians’ recommendations had a strong influence on treatment choice. Recommendations are an essential part of the discussion, “but I don’t think we often do them well,” he said.
Ask patients to think out loud about what they have read or have been told, and ask them to repeat in their own words what they heard the doctor say, Dr. Ubel suggested. It’s incumbent on the physician to try to understand the patient’s preferences, and say something like, “I’m the expert on medical facts, but you’re the expert on you,” or “What sounds good and bad to you about that treatment alternative?”
Finally, physicians need to make recommendations based on patient preferences, he said.
For example, in one recorded encounter, the physician asked the patient, “Are you the kind of person where the idea of just watching your PSA is that unsettling to you?” When the patient replied “Yeah, I think I would be,” the physician was able to make an informed recommendation, saying “then I don’t think you’d be a good candidate for surveillance.”
“This doctor did not just make a recommendation; he tried to find out something about the patient first, and that’s critical to giving good advice,” Dr. Ubel said.
Hollywood, FLA. – Although patients and physicians should always be partners in medical decision making, guiding patients into making medically sound choices may involve a lot of listening and empathy, often followed by a little friendly persuasion, suggests an expert in health care decision making,
“Even when you get to a situation where the clinical practice guideline would point toward shared decision making, we’ve got to do shared decision making right or the guideline just doesn’t do the work for us. We clinicians have to know how to partner with our patients to make decisions when their values matter,” said Dr. Peter A Ubel, professor of business, public policy, and medicine at Duke University, Durham, N.H.
Dr. Ubel discussed how to understand and use patient preferences in cancer treatment decisions at the annual conference of the National Comprehensive Cancer Network.
In the case of early-stage prostate cancer, for example, treatment options include surveillance or active treatment with surgery or radiation, but the risk/benefit trade-offs require careful discussion.
“The different approaches have different pros and cons: If you get active treatment, there’s a pretty good chance you’ll experience incontinence or erectile dysfunction, whereas if you undergo surveillance you won’t experience those as side effects of the surveillance strategy, but you will live with a cancer inside your body, the accompanying anxiety, and wondering every 6 months whether it has advanced,” Dr. Ubel said.
“The question is how do we go figure out which treatment is best for which patient?” he added.
What doctors say not always what patients hear
To examine how decisions are made, Dr. Ubel and his colleagues conducted a study in which patients scheduled for biopsy for suspicion of prostate cancer were approached at the time of their clinic visits and asked to participate in a study about prostate cancer decision making. Patients were given a booklet aimed at the seventh-grade reading level describing prostate cancer and its treatment, and were asked, once they had finished, what course of therapy they might pursue if the diagnosis turned out to be positive, and why. They also were asked if it was important to them to maintain natural sexual function.
The encounter between the patients and their urologists at the time of diagnosis were audio recorded, so that investigators could see whether physicians recommend specific treatments and why.
“One of the things that really jumped out in this study was just how much language, how much explanation urologists use to help patients understand their diagnosis,” Dr. Ubel said.
In one encounter, the urologist explained to the patient that 3 out of 12 biopsy cores had less than 30% cancer involvement, suggesting moderately low-risk disease, but then went on to talk about Gleason scores, tumor grades and patterns, and risk categories.
“When a patient just finds out he has prostate cancer, it’s a tough time to put a whole bunch of information in front of him for him to absorb and make a decision,” Dr. Ubel said. Patients need time to absorb the shock of a cancer diagnosis first – even a diagnosis of an early, easily treated cancer – and information overload may actually reduce their ability to comprehend their choices or retain the information, he added. The urologist in this scenario is making a very earnest effort to tell the patient that he doesn’t have the kind of cancer that’s ever going to kill him, or that it is highly unlikely to cause any problems for the next 10-15 years, and there is ample time to decide how to treat it.
“But the doctor kind of forgets that the patient doesn’t speak medicalese, and the doctor feels like you really have to give them thorough informed consent, after all. So you need to inform the heck out of patients with all of the medical detail you believe is necessary to understand the decision, instead of the translation of the medical detail into terms the patient can understand,” he said.
An important part of shared decision making, therefore, is to make sure that patients can understand their alternatives, but not to overwhelm them with detail, because they may give up and ask the physician, “What do you think I should do?” which can introduce physician bias that may not always lead to the right choice for that patient.
“I actually morally don’t recommend that. I think instead we should give the right amount of information at the right time so they can actually get engaged in the choice,” Dr. Ubel said.
Discussion informs choices
The investigators looked at how prediagnosis education materials and discussions with urologists shaped patient decisions about treatment choice. Patients were called before their appointments to ensure that they had read the booklet, and then just before the appointment were asked which way they were leaning if the diagnosis turned out to be positive.
The investigators found that of 44 patients who expressed a preference for active surveillance before the appointment, 55% actually went on to receive active treatment. Among 119 patients with no expressed preference for surveillance or active therapy, 46% went on to treatment, and of 118 expressing previsit preference only for active surveillance, 54% went on to receive it.
“The leaning that they had before seeing the doctor had no influence on what treatment they got,” Dr. Ubel said. Instead, physicians’ recommendations had a strong influence on treatment choice. Recommendations are an essential part of the discussion, “but I don’t think we often do them well,” he said.
Ask patients to think out loud about what they have read or have been told, and ask them to repeat in their own words what they heard the doctor say, Dr. Ubel suggested. It’s incumbent on the physician to try to understand the patient’s preferences, and say something like, “I’m the expert on medical facts, but you’re the expert on you,” or “What sounds good and bad to you about that treatment alternative?”
Finally, physicians need to make recommendations based on patient preferences, he said.
For example, in one recorded encounter, the physician asked the patient, “Are you the kind of person where the idea of just watching your PSA is that unsettling to you?” When the patient replied “Yeah, I think I would be,” the physician was able to make an informed recommendation, saying “then I don’t think you’d be a good candidate for surveillance.”
“This doctor did not just make a recommendation; he tried to find out something about the patient first, and that’s critical to giving good advice,” Dr. Ubel said.
Hollywood, FLA. – Although patients and physicians should always be partners in medical decision making, guiding patients into making medically sound choices may involve a lot of listening and empathy, often followed by a little friendly persuasion, suggests an expert in health care decision making,
“Even when you get to a situation where the clinical practice guideline would point toward shared decision making, we’ve got to do shared decision making right or the guideline just doesn’t do the work for us. We clinicians have to know how to partner with our patients to make decisions when their values matter,” said Dr. Peter A Ubel, professor of business, public policy, and medicine at Duke University, Durham, N.H.
Dr. Ubel discussed how to understand and use patient preferences in cancer treatment decisions at the annual conference of the National Comprehensive Cancer Network.
In the case of early-stage prostate cancer, for example, treatment options include surveillance or active treatment with surgery or radiation, but the risk/benefit trade-offs require careful discussion.
“The different approaches have different pros and cons: If you get active treatment, there’s a pretty good chance you’ll experience incontinence or erectile dysfunction, whereas if you undergo surveillance you won’t experience those as side effects of the surveillance strategy, but you will live with a cancer inside your body, the accompanying anxiety, and wondering every 6 months whether it has advanced,” Dr. Ubel said.
“The question is how do we go figure out which treatment is best for which patient?” he added.
What doctors say not always what patients hear
To examine how decisions are made, Dr. Ubel and his colleagues conducted a study in which patients scheduled for biopsy for suspicion of prostate cancer were approached at the time of their clinic visits and asked to participate in a study about prostate cancer decision making. Patients were given a booklet aimed at the seventh-grade reading level describing prostate cancer and its treatment, and were asked, once they had finished, what course of therapy they might pursue if the diagnosis turned out to be positive, and why. They also were asked if it was important to them to maintain natural sexual function.
The encounter between the patients and their urologists at the time of diagnosis were audio recorded, so that investigators could see whether physicians recommend specific treatments and why.
“One of the things that really jumped out in this study was just how much language, how much explanation urologists use to help patients understand their diagnosis,” Dr. Ubel said.
In one encounter, the urologist explained to the patient that 3 out of 12 biopsy cores had less than 30% cancer involvement, suggesting moderately low-risk disease, but then went on to talk about Gleason scores, tumor grades and patterns, and risk categories.
“When a patient just finds out he has prostate cancer, it’s a tough time to put a whole bunch of information in front of him for him to absorb and make a decision,” Dr. Ubel said. Patients need time to absorb the shock of a cancer diagnosis first – even a diagnosis of an early, easily treated cancer – and information overload may actually reduce their ability to comprehend their choices or retain the information, he added. The urologist in this scenario is making a very earnest effort to tell the patient that he doesn’t have the kind of cancer that’s ever going to kill him, or that it is highly unlikely to cause any problems for the next 10-15 years, and there is ample time to decide how to treat it.
“But the doctor kind of forgets that the patient doesn’t speak medicalese, and the doctor feels like you really have to give them thorough informed consent, after all. So you need to inform the heck out of patients with all of the medical detail you believe is necessary to understand the decision, instead of the translation of the medical detail into terms the patient can understand,” he said.
An important part of shared decision making, therefore, is to make sure that patients can understand their alternatives, but not to overwhelm them with detail, because they may give up and ask the physician, “What do you think I should do?” which can introduce physician bias that may not always lead to the right choice for that patient.
“I actually morally don’t recommend that. I think instead we should give the right amount of information at the right time so they can actually get engaged in the choice,” Dr. Ubel said.
Discussion informs choices
The investigators looked at how prediagnosis education materials and discussions with urologists shaped patient decisions about treatment choice. Patients were called before their appointments to ensure that they had read the booklet, and then just before the appointment were asked which way they were leaning if the diagnosis turned out to be positive.
The investigators found that of 44 patients who expressed a preference for active surveillance before the appointment, 55% actually went on to receive active treatment. Among 119 patients with no expressed preference for surveillance or active therapy, 46% went on to treatment, and of 118 expressing previsit preference only for active surveillance, 54% went on to receive it.
“The leaning that they had before seeing the doctor had no influence on what treatment they got,” Dr. Ubel said. Instead, physicians’ recommendations had a strong influence on treatment choice. Recommendations are an essential part of the discussion, “but I don’t think we often do them well,” he said.
Ask patients to think out loud about what they have read or have been told, and ask them to repeat in their own words what they heard the doctor say, Dr. Ubel suggested. It’s incumbent on the physician to try to understand the patient’s preferences, and say something like, “I’m the expert on medical facts, but you’re the expert on you,” or “What sounds good and bad to you about that treatment alternative?”
Finally, physicians need to make recommendations based on patient preferences, he said.
For example, in one recorded encounter, the physician asked the patient, “Are you the kind of person where the idea of just watching your PSA is that unsettling to you?” When the patient replied “Yeah, I think I would be,” the physician was able to make an informed recommendation, saying “then I don’t think you’d be a good candidate for surveillance.”
“This doctor did not just make a recommendation; he tried to find out something about the patient first, and that’s critical to giving good advice,” Dr. Ubel said.
AT THE NCCN ANNUAL CONFERENCE
Key clinical point: Clinicians should understand patients’ coping styles and personalities before making recommendations about comparable treatment options.
Major finding: Physician recommendations were the strongest determining factor in patient choices of prostate cancer therapies.
Data source: Study of 281 prostate cancer patients and their treatment-decision encounters with urologists.
Disclosures: Dr. Ubel reported having no relevant disclosures.
Toe Nodule Obliterating the Nail Bed
The Diagnosis: Superficial Acral Fibromyxoma
Superficial acral fibromyxoma (SAF) was first described in 2001 by Fetsch et al.1 Subsequently, the term digital fibromyxoma was proposed in 2012 by Hollmann et al2 to describe a distinctive, slow-growing, soft-tissue tumor with a predilection for the periungual or subungual regions of the fingers and toes. The benign growth typically presents as a painless or tender nodule in middle-aged adults with a slight male predominance (1.3:1 ratio).1,2 In a case series (N=124) described by Hollmann et al,2 9 of 25 patients (36%) who had imaging studies showed bone involvement by an erosive or lytic lesion. Reports of SAF with bone involvement also have been described in the radiologic and orthopedic surgery literature.3,4 Radiographically, the soft-tissue invasion of the bone is demonstrated by scalloping on plain radiographs (Figure 1).3
Histologically, SAFs are moderately cellular with spindled or stellate fibroblastlike cells within a myxoid or collagenous matrix (Figure 2).1 The vasculature is mildly accentuated and an increase in mast cells usually is observed. The nuclei have a low degree of atypia with few mitotic figures, and the stellate cells exhibit positive immunohistochemical staining for CD34 (Figure 3), epithelial membrane antigen, and CD99.1 Hollmann et al2 found that 66 of 95 tumors (69.5%) infiltrated the dermal collagen, 26 (27.4%) infiltrated fat, and 3 (3.2%) invaded bone. Of the 47 cases that were evaluated on follow-up, 10 tumors (21.3%) recurred locally (all near the nail unit of the fingers or toes) after a mean interval of 27 months. Although invasion of underlying tissues and recurrence of the tumor has been demonstrated, this growth is considered benign. The histologic differential diagnosis includes neurofibroma, myxoma, fibroma, low-grade fibromyxoid sarcoma, dermatofibroma, superficial angiomyxoma, and dermatofibrosarcoma protuberans.2
The primary treatment of SAF is local excision. The incidence of local recurrence found in the case series by Hollmann et al2 was directly linked to positive margins after the first excision (10/47 [21.3%] recurrent lesions had positive margins). To date, there are no known reports of metastatic disease in SAF.2 Our case manifested with a late recurrence of the tumor and bone involvement requiring surgical excision, which illustrates the role of adjuvant imaging and close follow-up following excision of any soft-tissue tumors of the fingers and toes that have been histologically confirmed as SAF, particularly those of the periungual region.
|
|
|
|
1. Fetsch JF, Laskin WB, Miettinen M. Superficial acral fibromyxoma (a clinicopathologic and immunohistochemical analysis of 37 cases of a distinctive soft tissue tumor with a predilection for the fingers and toes.) Hum Pathol. 2001;32:704-714.
2. Hollmann TJ, Bovée JV, Fletcher CD. Digital fibromyxoma (superficial acral fibromyxoma): a detailed characterization of 124 cases. Am J Surg Pathol. 2012;36:789-798.
3. Varikatt W, Soper J, Simmon G, et al. Superficial acral fibromyxoma: a report of two cases with radiological findings. Skeletal Radiol. 2008;37:499-503.
4. Oteo-Alvaro A, Meizoso T, Scarpellini A, et al. Superficial acral fibromyxoma of the toe, with erosion of the distal phalanx. a clinical report. Arch Orthop Trauma Surg. 2008;128:271-274.
The Diagnosis: Superficial Acral Fibromyxoma
Superficial acral fibromyxoma (SAF) was first described in 2001 by Fetsch et al.1 Subsequently, the term digital fibromyxoma was proposed in 2012 by Hollmann et al2 to describe a distinctive, slow-growing, soft-tissue tumor with a predilection for the periungual or subungual regions of the fingers and toes. The benign growth typically presents as a painless or tender nodule in middle-aged adults with a slight male predominance (1.3:1 ratio).1,2 In a case series (N=124) described by Hollmann et al,2 9 of 25 patients (36%) who had imaging studies showed bone involvement by an erosive or lytic lesion. Reports of SAF with bone involvement also have been described in the radiologic and orthopedic surgery literature.3,4 Radiographically, the soft-tissue invasion of the bone is demonstrated by scalloping on plain radiographs (Figure 1).3
Histologically, SAFs are moderately cellular with spindled or stellate fibroblastlike cells within a myxoid or collagenous matrix (Figure 2).1 The vasculature is mildly accentuated and an increase in mast cells usually is observed. The nuclei have a low degree of atypia with few mitotic figures, and the stellate cells exhibit positive immunohistochemical staining for CD34 (Figure 3), epithelial membrane antigen, and CD99.1 Hollmann et al2 found that 66 of 95 tumors (69.5%) infiltrated the dermal collagen, 26 (27.4%) infiltrated fat, and 3 (3.2%) invaded bone. Of the 47 cases that were evaluated on follow-up, 10 tumors (21.3%) recurred locally (all near the nail unit of the fingers or toes) after a mean interval of 27 months. Although invasion of underlying tissues and recurrence of the tumor has been demonstrated, this growth is considered benign. The histologic differential diagnosis includes neurofibroma, myxoma, fibroma, low-grade fibromyxoid sarcoma, dermatofibroma, superficial angiomyxoma, and dermatofibrosarcoma protuberans.2
The primary treatment of SAF is local excision. The incidence of local recurrence found in the case series by Hollmann et al2 was directly linked to positive margins after the first excision (10/47 [21.3%] recurrent lesions had positive margins). To date, there are no known reports of metastatic disease in SAF.2 Our case manifested with a late recurrence of the tumor and bone involvement requiring surgical excision, which illustrates the role of adjuvant imaging and close follow-up following excision of any soft-tissue tumors of the fingers and toes that have been histologically confirmed as SAF, particularly those of the periungual region.

|
|

|
|
The Diagnosis: Superficial Acral Fibromyxoma
Superficial acral fibromyxoma (SAF) was first described in 2001 by Fetsch et al.1 Subsequently, the term digital fibromyxoma was proposed in 2012 by Hollmann et al2 to describe a distinctive, slow-growing, soft-tissue tumor with a predilection for the periungual or subungual regions of the fingers and toes. The benign growth typically presents as a painless or tender nodule in middle-aged adults with a slight male predominance (1.3:1 ratio).1,2 In a case series (N=124) described by Hollmann et al,2 9 of 25 patients (36%) who had imaging studies showed bone involvement by an erosive or lytic lesion. Reports of SAF with bone involvement also have been described in the radiologic and orthopedic surgery literature.3,4 Radiographically, the soft-tissue invasion of the bone is demonstrated by scalloping on plain radiographs (Figure 1).3
Histologically, SAFs are moderately cellular with spindled or stellate fibroblastlike cells within a myxoid or collagenous matrix (Figure 2).1 The vasculature is mildly accentuated and an increase in mast cells usually is observed. The nuclei have a low degree of atypia with few mitotic figures, and the stellate cells exhibit positive immunohistochemical staining for CD34 (Figure 3), epithelial membrane antigen, and CD99.1 Hollmann et al2 found that 66 of 95 tumors (69.5%) infiltrated the dermal collagen, 26 (27.4%) infiltrated fat, and 3 (3.2%) invaded bone. Of the 47 cases that were evaluated on follow-up, 10 tumors (21.3%) recurred locally (all near the nail unit of the fingers or toes) after a mean interval of 27 months. Although invasion of underlying tissues and recurrence of the tumor has been demonstrated, this growth is considered benign. The histologic differential diagnosis includes neurofibroma, myxoma, fibroma, low-grade fibromyxoid sarcoma, dermatofibroma, superficial angiomyxoma, and dermatofibrosarcoma protuberans.2
The primary treatment of SAF is local excision. The incidence of local recurrence found in the case series by Hollmann et al2 was directly linked to positive margins after the first excision (10/47 [21.3%] recurrent lesions had positive margins). To date, there are no known reports of metastatic disease in SAF.2 Our case manifested with a late recurrence of the tumor and bone involvement requiring surgical excision, which illustrates the role of adjuvant imaging and close follow-up following excision of any soft-tissue tumors of the fingers and toes that have been histologically confirmed as SAF, particularly those of the periungual region.

|
|

|
|
1. Fetsch JF, Laskin WB, Miettinen M. Superficial acral fibromyxoma (a clinicopathologic and immunohistochemical analysis of 37 cases of a distinctive soft tissue tumor with a predilection for the fingers and toes.) Hum Pathol. 2001;32:704-714.
2. Hollmann TJ, Bovée JV, Fletcher CD. Digital fibromyxoma (superficial acral fibromyxoma): a detailed characterization of 124 cases. Am J Surg Pathol. 2012;36:789-798.
3. Varikatt W, Soper J, Simmon G, et al. Superficial acral fibromyxoma: a report of two cases with radiological findings. Skeletal Radiol. 2008;37:499-503.
4. Oteo-Alvaro A, Meizoso T, Scarpellini A, et al. Superficial acral fibromyxoma of the toe, with erosion of the distal phalanx. a clinical report. Arch Orthop Trauma Surg. 2008;128:271-274.
1. Fetsch JF, Laskin WB, Miettinen M. Superficial acral fibromyxoma (a clinicopathologic and immunohistochemical analysis of 37 cases of a distinctive soft tissue tumor with a predilection for the fingers and toes.) Hum Pathol. 2001;32:704-714.
2. Hollmann TJ, Bovée JV, Fletcher CD. Digital fibromyxoma (superficial acral fibromyxoma): a detailed characterization of 124 cases. Am J Surg Pathol. 2012;36:789-798.
3. Varikatt W, Soper J, Simmon G, et al. Superficial acral fibromyxoma: a report of two cases with radiological findings. Skeletal Radiol. 2008;37:499-503.
4. Oteo-Alvaro A, Meizoso T, Scarpellini A, et al. Superficial acral fibromyxoma of the toe, with erosion of the distal phalanx. a clinical report. Arch Orthop Trauma Surg. 2008;128:271-274.
A generally healthy 30-year-old man presented with a 3-cm exophytic, yellowish red, subungual nodule of the left great toe of 1 year’s duration that was obliterating the nail plate. Ten years prior, a similar nodule in the same location was removed via laser by a podiatrist. Medical records were not retrievable, but the patient reported that he was told the excised lesion was a benign tumor. Plain radiographs were performed at the current presentation and demonstrated an inferior cortical lucency of the distal phalanx as well as a lucency over the nail bed region with extension of calcification to the soft tissues. Magnetic resonance imaging showed a mass with a proximal to distal maximum dimension of 2.1 cm that involved the dorsal surface of the proximal phalanx. Magnetic resonance imaging also demonstrated bone erosion from the overlying mass. A 4-mm incisional punch biopsy was performed prior to surgical excision.
Patch Testing: Working With Patients to Find a Relevant Allergen
What do your patients need to know at the first visit?
Patients with chronic dermatitis are frequently referred for patch testing. An in-depth conversation reviewing the patch test procedure and the many potential causes of dermatitis (eg, endogenous, allergic, irritant, seborrheic) is needed. Patients should understand the patch test process. The testing extends over a week, requiring 3 days of visits. The patches are applied at day 1 and must be kept dry and in place for 48 hours, then they are removed and evaluated. A second follow-up visit at 96 hours to 1 week after the patches are applied is done to perform a final read, interpret, and explain the final results. The patient needs to know that we are looking for an allergen that might be causing the eruption through contact exposure with the skin. The difference between patch testing and prick testing often needs to be discussed, as patients are not always aware of the difference. Explaining the need to avoid topical steroids at the patch test site, sunburn, or systemic steroids during the patch test period is also important to obtain optimal testing conditions.
Querying all exposures including work, home, personal care products, and hobbies is important to help determine which allergen series should be tested to obtain the best results. Patients need to understand that even small intermittent exposures can cause an ongoing dermatitis. If a causative allergen(s) is identified, strict avoidance can lead to clearance and resolution.
Setting expectations is important, and therefore you should discuss the possibility that no allergen will be identified while letting the patient know that this information is also useful. Also, let patients know there are other things that can be done if patch testing is negative to try and gain control of the dermatitis including laboratory tests and biopsies, which may be needed to help direct future management.
What are your go-to treatments? What are the side effects?
The beauty of patch testing is that finding a relevant allergen and subsequent avoidance of that allergen often is sufficient to improve or clear the dermatitis. Detailed education regarding the allergen, where it is found, and how to avoid it are imperative in patient management. I provide the patient with information sheets or narratives found on the American Contact Dermatitis Society website (http://www.contactderm.org) as well as a list of safe products found on the Contact Allergen Management Program (CAMP) area of the site. These tools help in patient compliance.
Go-to treatments for relevant patch test dermatitis involve topical steroids to calm the acute dermatitis while educating and instituting a personal environment free of the identified allergens. Occasionally, systemic steroids are used to provide relief and calm down an extensive dermatitis while educating, identifying, and eliminating known allergens from the patient’s environment. Identifying and eliminating an allergen can mitigate the need for chronic steroids, and the resultant side effects of hypertension, osteoporosis, avascular necrosis, hyperglycemia, and gastrointestinal tract problems can be avoided. Likewise, avoidance of allergens can lead to the elimination of the need for chronic topical steroids and the resultant atrophy and striae.
Side effects of the patch test procedure itself include an allergic reaction to one of the chemicals tested (eg, gold), which is what you are looking for; persistent reactions; flaring of existing dermatitis; irritation; hyperpigmentation; and rarely anaphylaxis or infection at a patch test site. If no allergy is found, treatment of generalized dermatitis can include topical steroids. Topical calcineurin inhibitors can be useful as well as narrowband UV light. Several oral medications can be used for recalcitrant patch test–negative dermatitis and the selection of the right medication is based on the patient’s comorbidities and extent of dermatitis, including systemic steroids, though long-term use is not recommended. Mycophenolate mofetil, methotrexate, cyclosporine, and azathioprine all have side effects including liver and renal toxicity, immunosuppression, and risk for malignancy and therefore need to be considered on a case-by-case basis.
How do you keep the patient compliant with treatment?
Treating allergic contact dermatitis once an allergen(s) has been identified can be challenging. Education is key so that the patient understands where the allergen is found in his/her environment and how to avoid it. Teaching the patient to read labels also is important. Providing a list of safe products simplifies compliance. Reinforcing the need for ongoing vigilance in allergen avoidance is critical to resolution of the dermatitis. Reinforcing the need for continuous avoidance is imperative, as patients sometimes become less vigilant once the dermatitis resolves and the allergen can sneak back into their environment.
What do I do if a patient refuses treatment?
Sometimes patients are so attached to a product that they do not want to stop using it even though they know it is the cause of their dermatitis. If I can help them identify a comparable product, I introduce them to it, but ultimately they get to decide if they prefer to use a product that they know is the cause of their rash or if they want to avoid it and be clear of the dermatitis. For those who do not have an allergen identified through patch testing, alternative treatments can be used. If they do not want systemic medication, I try and optimize their skin care regimen with mild soaps, bland moisturizing creams, and short lukewarm showers, which often is not enough and eventually due to ongoing itch patients decide to discuss and pursue treatment options.
What do your patients need to know at the first visit?
Patients with chronic dermatitis are frequently referred for patch testing. An in-depth conversation reviewing the patch test procedure and the many potential causes of dermatitis (eg, endogenous, allergic, irritant, seborrheic) is needed. Patients should understand the patch test process. The testing extends over a week, requiring 3 days of visits. The patches are applied at day 1 and must be kept dry and in place for 48 hours, then they are removed and evaluated. A second follow-up visit at 96 hours to 1 week after the patches are applied is done to perform a final read, interpret, and explain the final results. The patient needs to know that we are looking for an allergen that might be causing the eruption through contact exposure with the skin. The difference between patch testing and prick testing often needs to be discussed, as patients are not always aware of the difference. Explaining the need to avoid topical steroids at the patch test site, sunburn, or systemic steroids during the patch test period is also important to obtain optimal testing conditions.
Querying all exposures including work, home, personal care products, and hobbies is important to help determine which allergen series should be tested to obtain the best results. Patients need to understand that even small intermittent exposures can cause an ongoing dermatitis. If a causative allergen(s) is identified, strict avoidance can lead to clearance and resolution.
Setting expectations is important, and therefore you should discuss the possibility that no allergen will be identified while letting the patient know that this information is also useful. Also, let patients know there are other things that can be done if patch testing is negative to try and gain control of the dermatitis including laboratory tests and biopsies, which may be needed to help direct future management.
What are your go-to treatments? What are the side effects?
The beauty of patch testing is that finding a relevant allergen and subsequent avoidance of that allergen often is sufficient to improve or clear the dermatitis. Detailed education regarding the allergen, where it is found, and how to avoid it are imperative in patient management. I provide the patient with information sheets or narratives found on the American Contact Dermatitis Society website (http://www.contactderm.org) as well as a list of safe products found on the Contact Allergen Management Program (CAMP) area of the site. These tools help in patient compliance.
Go-to treatments for relevant patch test dermatitis involve topical steroids to calm the acute dermatitis while educating and instituting a personal environment free of the identified allergens. Occasionally, systemic steroids are used to provide relief and calm down an extensive dermatitis while educating, identifying, and eliminating known allergens from the patient’s environment. Identifying and eliminating an allergen can mitigate the need for chronic steroids, and the resultant side effects of hypertension, osteoporosis, avascular necrosis, hyperglycemia, and gastrointestinal tract problems can be avoided. Likewise, avoidance of allergens can lead to the elimination of the need for chronic topical steroids and the resultant atrophy and striae.
Side effects of the patch test procedure itself include an allergic reaction to one of the chemicals tested (eg, gold), which is what you are looking for; persistent reactions; flaring of existing dermatitis; irritation; hyperpigmentation; and rarely anaphylaxis or infection at a patch test site. If no allergy is found, treatment of generalized dermatitis can include topical steroids. Topical calcineurin inhibitors can be useful as well as narrowband UV light. Several oral medications can be used for recalcitrant patch test–negative dermatitis and the selection of the right medication is based on the patient’s comorbidities and extent of dermatitis, including systemic steroids, though long-term use is not recommended. Mycophenolate mofetil, methotrexate, cyclosporine, and azathioprine all have side effects including liver and renal toxicity, immunosuppression, and risk for malignancy and therefore need to be considered on a case-by-case basis.
How do you keep the patient compliant with treatment?
Treating allergic contact dermatitis once an allergen(s) has been identified can be challenging. Education is key so that the patient understands where the allergen is found in his/her environment and how to avoid it. Teaching the patient to read labels also is important. Providing a list of safe products simplifies compliance. Reinforcing the need for ongoing vigilance in allergen avoidance is critical to resolution of the dermatitis. Reinforcing the need for continuous avoidance is imperative, as patients sometimes become less vigilant once the dermatitis resolves and the allergen can sneak back into their environment.
What do I do if a patient refuses treatment?
Sometimes patients are so attached to a product that they do not want to stop using it even though they know it is the cause of their dermatitis. If I can help them identify a comparable product, I introduce them to it, but ultimately they get to decide if they prefer to use a product that they know is the cause of their rash or if they want to avoid it and be clear of the dermatitis. For those who do not have an allergen identified through patch testing, alternative treatments can be used. If they do not want systemic medication, I try and optimize their skin care regimen with mild soaps, bland moisturizing creams, and short lukewarm showers, which often is not enough and eventually due to ongoing itch patients decide to discuss and pursue treatment options.
What do your patients need to know at the first visit?
Patients with chronic dermatitis are frequently referred for patch testing. An in-depth conversation reviewing the patch test procedure and the many potential causes of dermatitis (eg, endogenous, allergic, irritant, seborrheic) is needed. Patients should understand the patch test process. The testing extends over a week, requiring 3 days of visits. The patches are applied at day 1 and must be kept dry and in place for 48 hours, then they are removed and evaluated. A second follow-up visit at 96 hours to 1 week after the patches are applied is done to perform a final read, interpret, and explain the final results. The patient needs to know that we are looking for an allergen that might be causing the eruption through contact exposure with the skin. The difference between patch testing and prick testing often needs to be discussed, as patients are not always aware of the difference. Explaining the need to avoid topical steroids at the patch test site, sunburn, or systemic steroids during the patch test period is also important to obtain optimal testing conditions.
Querying all exposures including work, home, personal care products, and hobbies is important to help determine which allergen series should be tested to obtain the best results. Patients need to understand that even small intermittent exposures can cause an ongoing dermatitis. If a causative allergen(s) is identified, strict avoidance can lead to clearance and resolution.
Setting expectations is important, and therefore you should discuss the possibility that no allergen will be identified while letting the patient know that this information is also useful. Also, let patients know there are other things that can be done if patch testing is negative to try and gain control of the dermatitis including laboratory tests and biopsies, which may be needed to help direct future management.
What are your go-to treatments? What are the side effects?
The beauty of patch testing is that finding a relevant allergen and subsequent avoidance of that allergen often is sufficient to improve or clear the dermatitis. Detailed education regarding the allergen, where it is found, and how to avoid it are imperative in patient management. I provide the patient with information sheets or narratives found on the American Contact Dermatitis Society website (http://www.contactderm.org) as well as a list of safe products found on the Contact Allergen Management Program (CAMP) area of the site. These tools help in patient compliance.
Go-to treatments for relevant patch test dermatitis involve topical steroids to calm the acute dermatitis while educating and instituting a personal environment free of the identified allergens. Occasionally, systemic steroids are used to provide relief and calm down an extensive dermatitis while educating, identifying, and eliminating known allergens from the patient’s environment. Identifying and eliminating an allergen can mitigate the need for chronic steroids, and the resultant side effects of hypertension, osteoporosis, avascular necrosis, hyperglycemia, and gastrointestinal tract problems can be avoided. Likewise, avoidance of allergens can lead to the elimination of the need for chronic topical steroids and the resultant atrophy and striae.
Side effects of the patch test procedure itself include an allergic reaction to one of the chemicals tested (eg, gold), which is what you are looking for; persistent reactions; flaring of existing dermatitis; irritation; hyperpigmentation; and rarely anaphylaxis or infection at a patch test site. If no allergy is found, treatment of generalized dermatitis can include topical steroids. Topical calcineurin inhibitors can be useful as well as narrowband UV light. Several oral medications can be used for recalcitrant patch test–negative dermatitis and the selection of the right medication is based on the patient’s comorbidities and extent of dermatitis, including systemic steroids, though long-term use is not recommended. Mycophenolate mofetil, methotrexate, cyclosporine, and azathioprine all have side effects including liver and renal toxicity, immunosuppression, and risk for malignancy and therefore need to be considered on a case-by-case basis.
How do you keep the patient compliant with treatment?
Treating allergic contact dermatitis once an allergen(s) has been identified can be challenging. Education is key so that the patient understands where the allergen is found in his/her environment and how to avoid it. Teaching the patient to read labels also is important. Providing a list of safe products simplifies compliance. Reinforcing the need for ongoing vigilance in allergen avoidance is critical to resolution of the dermatitis. Reinforcing the need for continuous avoidance is imperative, as patients sometimes become less vigilant once the dermatitis resolves and the allergen can sneak back into their environment.
What do I do if a patient refuses treatment?
Sometimes patients are so attached to a product that they do not want to stop using it even though they know it is the cause of their dermatitis. If I can help them identify a comparable product, I introduce them to it, but ultimately they get to decide if they prefer to use a product that they know is the cause of their rash or if they want to avoid it and be clear of the dermatitis. For those who do not have an allergen identified through patch testing, alternative treatments can be used. If they do not want systemic medication, I try and optimize their skin care regimen with mild soaps, bland moisturizing creams, and short lukewarm showers, which often is not enough and eventually due to ongoing itch patients decide to discuss and pursue treatment options.